
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 180
Principles and Methods for Assessing Direct Immunotoxicity
Associated with Exposure to Chemicals
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared at the National Institute of Health Sciences,
Tokyo, Japan, and the Institute of Terrestrial Ecology, Monk's Wood,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Principles and methods for assessing direct immunotoxicity
associated with exposure to chamicals
(Environmental health criteria ; 180)
1.Immunotoxins 2.Immune system 3.Risk assessment I.Series
ISBN 92 4 157180 2 (NLM Classification: QW 630.5.13)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
PREAMBLE
WHO TASK GROUP MEETING ON PRINCIPLES AND METHODS FOR ASSESSING DIRECT
IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
PRINCIPLES AND METHODS
ABBREVIATIONS
SUMMARY AND RECOMMENDATIONS
1. INTRODUCTION TO IMMUNOTOXICOLOGY
1.1. Historical overview
1.2. The immune system; functions, system regulation, and
modifying factors; histophysiology of lymphoid organs
1.2.1. Function of the immune system
1.2.1.1 Encounter and recognition
1.2.1.2 Specificity
1.2.1.3 Choice of effector reaction; diversity
of the answer
1.2.1.4 Immunoregulation
1.2.1.5 Modifying factors outside the immune
system
1.2.1.6 Immunological memory
1.2.2. Histophysiology of lymphoid organs
1.2.2.1 Overview: structure of the immune system
1.2.2.2 Bone marrow
1.2.2.3 Thymus
1.2.2.4 Lymph nodes
1.2.2.5 Spleen
1.2.2.6 Mucosa-associated lymphoid tissue
1.2.2.7 Skin immune system or skin-associated
lymphoid tissue
1.3. Pathophysiology
1.3.1. Susceptibility to toxic action
1.3.2. Regeneration
1.3.3. Changes in lymphoid organs
2. HEALTH IMPACT OF SELECTED IMMUNOTOXIC AGENTS
2.1. Description of consequences on human health
2.1.1. Consequences of immunosuppression
2.1.1.1 Cancer
2.1.1.2 Infectious diseases
2.1.2. Consequences of immunostimulation
2.2. Direct immunotoxicity in laboratory animals
2.2.1. Azathioprine and cyclosporin A
2.2.1.1 Azathioprine
2.2.1.2 Cyclosporin A
2.2.2. Halogenated hydrocarbons
2.2.2.1
2,3,7,8-Tetrachlorodibenzo- para-dioxin
2.2.2.2 Polychlorinated biphenyls
2.2.2.3 Hexachlorobenzene
2.2.3. Pesticides
2.2.3.1 Organochlorine pesticides
2.2.3.2 Organophosphate compounds
2.2.3.3 Pyrethroids
2.2.3.4 Carbamates
2.2.3.5 Dinocap
2.2.4. Polycyclic aromatic hydrocarbons
2.2.5. Solvents
2.2.5.1 Benzene
2.2.5.2 Other solvents
2.2.6. Metals
2.2.6.1 Cadmium
2.2.6.2 Lead
2.2.6.3 Mercury
2.2.6.4 Organotins
2.2.6.5 Gallium arsenide
2.2.6.6 Beryllium
2.2.7. Air pollutants
2.2.8. Mycotoxins
2.2.9. Particles
2.2.9.1 Asbestos
2.2.9.2 Silica
2.2.10. Substances of abuse
2.2.11. Ultraviolet B radiation
2.2.12. Food additives
2.3. Immunotoxicity of environmental chemicals in wildlife and
domesticated species
2.3.1. Fish and other marine species
2.3.1.1 Fish
2.3.1.2 Marine mammals
2.3.2. Cattle and swine
2.3.3. Chickens
2.4. Immunotoxicity of environmental chemicals in humans
2.4.1. Case reports
2.4.2. Air pollutants
2.4.3. Pesticides
2.4.4. Halogenated aromatic hydrocarbons
2.4.5. Metals
2.4.6. Solvents
2.4.7. Ultraviolet radiation
2.4.8. Others
3. STRATEGIES FOR TESTING THE IMMUNOTOXICITY OF CHEMICALS IN ANIMALS
3.1. General testing of the toxicity of chemicals
3.2. Organization of tests in tiers
3.2.1. US National Toxicology Program panel
3.2.2. Dutch National Institute of Public Health and
Environmental Protection panel
3.2.3. US Environmental Protection Agency, Office of
Pesticides panel
3.2.4. US Food and Drug Administration, Center for Food
Safety and Applied Nutrition panel
3.3. Considerations in evaluating systemic and local
immunotoxicity
3.3.1. Species selection
3.3.2. Systemic immunosuppression
3.3.3. Local suppression
4. METHODS OF IMMUNOTOXICOLOGY IN EXPERIMENTAL ANIMALS
4.1. Nonfunctional tests
4.1.1. Organ weights
4.1.2. Pathology
4.1.3. Basal immunoglobulin level
4.1.4. Bone marrow
4.1.5. Enumeration of leukocytes in bronchoalveolar lavage
fluid, peritoneal cavity, and skin
4.1.6. Flow cytometric analysis
4.2. Functional tests
4.2.1. Macrophage activity
4.2.2. Natural killer activity
4.2.3. Antigen-specific antibody responses
4.2.4. Antibody responses to sheep red blood cells
4.2.4.1 Spleen immunoglobulin M and
immunoglobulin G plaque-forming cell
assay to the T-dependent antigen, sheep
red blood cells
4.2.4.2 Enzyme-linked immunosorbent assay of
anti-sheep red blood cell antibodies of
classes M, G, and A in rats
4.2.5. Responsiveness to B-cell mitogens
4.2.6. Responsiveness to T-cell mitogens
4.2.7. Mixed lymphocyte reaction
4.2.8. Cytotoxic T lymphocyte assay
4.2.9. Delayed-type hypersensitivity responses
4.2.10. Host resistance models
4.2.10.1 Listeria monocytogenes
4.2.10.2 Streptococcus infectivity models
4.2.10.3 Viral infection model with mouse and rat
cytomegalovirus
4.2.10.4 Influenza virus model
4.2.10.5 Parasitic infection model with
Trichinella spiralis
4.2.10.6 Plasmodium model
4.2.10.7 B16F10 Melanoma model
4.2.10.8 PYB6 Carcinoma model
4.2.10.9 MADB106 Adenocarcinoma model
4.2.11. Autoimmune models
4.3. Assessment of immunotoxicity in non-rodent species
4.3.1. Non-human primates
4.3.2. Dogs
4.3.3. Non-mammalian species
4.3.3.1 Fish
4.3.3.2 Chickens
4.4. Approaches to assessing immunosuppression in vitro
4.5. Future directions
4.5.1. Molecular approaches in immunotoxicology
4.5.2. Transgenic mice
4.5.3. Severe combined immunodeficient mice
4.6. Biomarkers in epidemiological studies and monitoring
4.7. Quality assurance for immunotoxicology studies
4.8. Validation
5. ESSENTIALS OF IMMUNOTOXICITY ASSESSMENT IN HUMANS
5.1. Introduction: Immunocompetence and immunosuppression
5.2. Considerations in assessing human immune status related to
immunotoxicity
5.3. Confounding variables
5.4. Considerations in the design of epidemiological studies
5.5. Proposed testing regimen
5.6. Assays for assessing immune status
5.6.1. Total blood count and differential
5.6.2. Tests of the antibody-mediated immune system
5.6.2.1 Immunoglobulin concentration
5.6.2.2 Specific antibodies
5.6.3. Tests for inflammation and autoantibodies
5.6.3.1 C-Reactive protein
5.6.3.2 Antinuclear antibody
5.6.3.3 Rheumatoid factor
5.6.3.4 Thyroglobulin antibody
5.6.4. Tests for cellular immunity
5.6.4.1 Flow cytometry
5.6.4.2 Delayed-type hypersensitivity
5.6.4.3 Proliferation of mononuclear cells in
vitro
5.6.5. Tests for nonspecific immunity
5.6.5.1 Natural killer cells
5.6.5.2 Polymorphonuclear granulocytes
5.6.5.3 Complement
5.6.6. Clinical chemistry
5.6.7. Additional confirmatory tests
6. RISK ASSESSMENT
6.1. Introduction
6.2. Complements to extrapolating experimental data
6.2.1. In-vitro approaches
6.2.2. Parallellograms
6.2.3. Severe combined immunodeficient mice
6.3. Host resistance and clinical disease
7. SOME TERMS USED IN IMMUNOTOXICOLOGY
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the Criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 979 9111).
* * *
Funding and support for the preparation and finalization of this
monograph were provided by the United States Environmental Protection
Agency under Cooperative Agreement with the World Health Organization
No. CR 821767-01-0, by the German Federal Ministry for the
Environment, Nature Conservation and Nuclear Safety, and by the
Netherlands National Institute for Public Health and Environmental
Protection.
Environmental Health Criteria
PREAMBLE
Objectives
The WHO Environmental Health Criteria Programme was initiated in
1973, with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976; numerous assessments of chemicals and
of physical effects have since been produced. Many EHC monographs have
been devoted to toxicological methods, e.g. for genetic, neurotoxic,
teratogenic, and nephrotoxic effects. Other publications have been
concerned with e.g. epidemiological guidelines, evaluation of short-
term tests for carcinogens, biomarkers, and effects on the elderly.
Since the time of its inauguration, the EHC Programme has widened
its scope, and the importance of environmental effects has been
increasingly emphasized in the total evaluation of chemicals, in
addition to their health effects.
The original impetus for the Programme came from resolutions of
the World Health Assembly and the recommendations of the 1972 United
Nations Conference on the Human Environment. Subsequently, the work
became an integral part of the International Programme on Chemical
Safety (IPCS), a cooperative programme of UNEP, ILO, and WHO. In this
manner, with the strong support of the new partners, the importance of
occupational health and environmental effects was fully recognized.
The EHC monographs have become widely established, used, and
recognized throughout the world.
The recommendations of the 1992 United Nations Conference on
Environment and Development and the subsequent establishment of the
Intergovernmental Forum on Chemical Safety, with priorities for action
in the six programme areas of Chapter 19, Agenda 21, lend further
weight to the need for EHC assessments of the risks of chemicals.
The Criteria monographs are intended to provide critical reviews
of the effect on human health and the environment of chemicals,
combinations of chemicals, and physical and biological agents. They
include reviews of studies that are of direct relevance for the
evaluation and do not describe every study that has been carried out.
Data obtained worldwide are used, and results are quoted from original
studies, not from abstracts or reviews. Both published and unpublished
reports are considered, and the authors are responsible for assessing
all of the articles cited; however, preference is always given to
published data, and unpublished data are used only when relevant
published data are absent or when the unpublished data are pivotal to
the risk assessment. A detailed policy statement is available that
describes the procedures used for citing unpublished proprietary data,
so that this information can be used in the evaluation without
compromising its confidential nature (WHO, 1990).
In the evaluation of human health risks, sound data on humans,
whenever available, are preferred to data on experimental animals.
Studies of animals and in-vitro systems provide support and are used
mainly to supply evidence missing from human studies. It is mandatory
that research on human subjects be conducted in full accord with
ethical principles, including the provisions of the Helsinki
Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not in any sense recommendations for regulation or
setting standards. The latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary: a review of the salient facts and the risk evaluation of
the chemical
* Identity: physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution, and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in-vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and the field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g. the
International Agency for Research on Cancer, the Joint FAO/WHO
Expert Committee on Food Additives, and the Joint FAO/WHO Meeting
on Pesticide Residues
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of chemicals that are of
priority for subsequent evaluation. Such meetings have been held in
Ispra, Italy (1980); Oxford, United Kingdom (1984); Berlin, Germany
(1987); and North Carolina, United States of America (1995). The
selection of chemicals is based on the following criteria: the
existence of scientific evidence that the substance presents a hazard
to human health and/or the environment; the existence of evidence that
the possible use, persistence, accumulation, or degradation of the
substance involves significant human or environmental exposure; the
existence of evidence that the populations at risk (both human and
other species) and the risks for the environment are of a significant
size and nature; there is international concern, i.e. the substance is
of major interest to several countries; adequate data are available on
the hazards.
If it is proposed to write an EHC monograph on a chemical that is
not on the list of priorities, the IPCS Secretariat first consults
with the cooperating organizations and the participating institutions.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the following flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for the layout and language. The first draft, prepared by
consultants or, more usually, staff at an IPCS participating
institution is based initially on data provided from the International
Register of Potentially Toxic Chemicals and reference data bases such
as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the first draft acceptable,
it is distributed in its unedited form to over 150 EHC contact points
throughout the world for comment on its completeness and accuracy and,
where necessary, to provide additional material. The contact points,
usually designated by governments, may be participating institutions,
IPCS focal points, or individual scientists known for their particular
expertise. Generally, about four months are allowed before the
comments are considered by the RO and author(s). A second draft
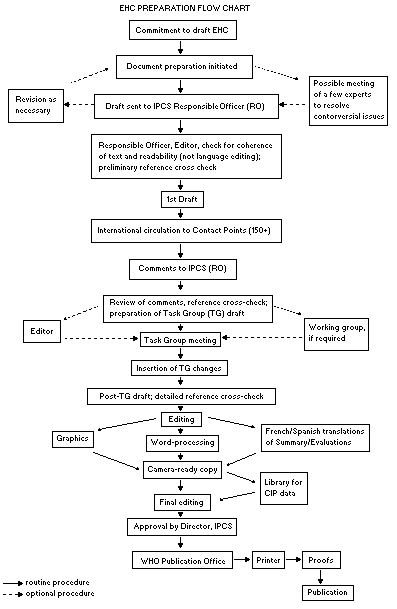 incorporating the comments received and approved by the Director,
IPCS, is then distributed to Task Group members, who carry out a peer
review at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the risks to health and
the environment from exposure to the chemical. A summary and
recommendations for further research and improved safety are also
drawn up. The composition of the Task Group is dictated by the range
of expertise required for the subject of the meeting and by the need
for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations, so that
representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide valuable contributions to the process, they can speak only
at the invitation of the Chairperson. Observers do not participate in
the final evaluation of the chemical, which is the sole responsibility
of the Task Group members. The Task Group may meet in camera when it
considers that to be appropriate.
All individuals who participate in the preparation of an EHC
monograph as authors, consultants, or advisers must, in addition to
serving in their personal capacity as scientists, inform the RO if at
any time a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a statement to that
effect. This procedure ensures the transparency and probity of the
process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it is edited for language, the references are checked, and
camera-ready copy is prepared. After approval by the Director, IPCS,
the monograph is submitted to the WHO Office of Publications for
printing. At this time, a copy of the final draft is also sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern about
health or environmental effects of the agent because of greater
exposure; an appreciable time has elapsed since the last evaluation.
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The chairpersons of task groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON PRINCIPLES AND METHODS FOR ASSESSING DIRECT
IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
Members
Dr A. Emmendörffer, Department of Immunobiology, Fraunhofer Institute
of Toxicology & Aerosol Research, Hanover, Germany
Dr H.S. Koren, Health Effects Research Laboratory, US Environmental
Protection Agency, Chapel Hill, NC, USA (Vice-Chairman)
Dr R.W. Luebke, Immunotoxicology Branch, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, NC, USA (Joint Rapporteur)
Dr M. Luster, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA
Dr C. Madsen, Institute of Toxicology, National Food Agency of
Denmark, Ministry of Health, Soborg, Denmark
Dr P. Ross, Dalhousie University, Halifax, Nova Scotia, Canada
(c/o Seal Rehabilitation and Research Centre, Pieterburen,
Netherlands)
Dr H.J. Schuurman, Preclinical Research/Immunology, Sandoz Pharma Ltd,
Basel, Switzerland
Dr H. Van Loveren, Laboratory for Pathology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Joint Rapporteur)
Dr J.G. Vos, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands (Chairman)
Dr K.L. White, Jr, Immunotoxicology Group, Medical College of
Virginia, Virginia Commonwealth University, Richmond, VA, USA
Observers
IUTOX
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy
ECETOC
Dr R.W.R. Crevel, Environmental Safety Laboratory, Unilever Research
and Engineering, Sharnbrook, Bedfordshire, United Kingdom
Secretariat
Dr J.H. Dean, Sanofi Winthrop, Inc., Sanofi Research Division,
Collegeville, PA, USA
Mr V. Quarg, Federal Ministry for Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA PRINCIPLES AND METHODS FOR ASSESSING
DIRECT IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
A WHO Task Group on Principles and Methods for Assessing Direct
Immunotoxicity Associated with Exposure to Chemicals met at the World
Health Organization, Geneva, from 10 to 14 October 1994. Dr E. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
IPCS, and the cooperating organizations. The Task Group reviewed and
revised the draft monograph and prepared the final text.
The first draft of the monograph was prepared by a group of
authors (listed below) under the coordination of Dr J.G. Vos and
Dr H. Van Loveren of the Dutch National Institute for Public Health
and Environmental Protection (RIVM), an IPCS Collaborating Centre for
Immunotoxicology and Allergic Hypersensitization. The second draft,
incorporating comments received after international circulation to
national experts of the first draft to IPCS contact points for
Environmental Health Criteria monographs, was prepared by Dr J.G.
Vos and Dr H. Van Loveren of the Netherlands and Dr Kimber White, USA.
Dr E. Smith of the IPCS Unit for the Assessment of Risk and
Methods was responsible for the scientific content of the monograph
and Mrs E. Heseltine for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The contributing authors were:
Dr J.H. Dean, Collegeville, PA, USA
Professor J. Descotes, Lyon, France
Dr F. Kuper, Zeist, Netherlands
Dr M. Luster, Research Triangle Park, NC, USA
Dr P.S. Ross, Bilthoven, Netherlands
Dr H.J. Schuurman, Basel, Switzerland
Dr M.J. Selgrade, Research Triangle Park, NC, USA
Dr R.L. de Swart, Bilthoven, Netherlands
Dr H. Van Loveren, Bilthoven, Netherlands
Professor J.G. Vos, Bilthoven, Netherlands
Dr P.W. Wester, Bilthoven, Netherlands
Professor A.G. Zapata, Madrid, Spain
ABBREVIATIONS
ACTH adrenocorticotrophic hormone
Ah aromatic hydrocarbon
AIDS acquired immunodeficiency syndrome
B bursa-dependent
CALLA common acute lymphoblastic leukaemia antigen
CD cluster of differentiation
CEC Commission of the European Communities
CH50 haemolytic complement
CML cell-mediated lympholysis
DMBA 7,12-dimethylbenz[ a]anthracene
DNCB dinitrochlorobenzene
ELISA enzyme-linked immunosorbent assay
EPO erythrocyte lineage differentiation factor
FACS fluorescence activated cell sorter
GALT gut-associated lymphoid tissue
G-CSF granulocyte colony-stimulating factor
GM-CSF granulocyte-macrophage colony-stimulating factor
GVH graft-versus-host
HCB hexaclorobenzene
HEV high endothelial venule
HIV human immunodeficiency virus
HPCA human progenitor cell antigen
HSA heat-stable antigen
ICAM intercellular adhesion molecule
IFN interferon
Ig immunoglobulin
IL interleukin
IPCS International Programme on Chemical Safety
LFA lymphocyte function-related antigen
LIF leukaemia inhibitory factor
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
M microfold
MALT mucosa-associated lymphoid tissue
MARE monoclonal anti-rat immunoglobulin E
MARK monoclonal antibody anti-kappa
M-CSF macrophage colony-stimulating factor
MED minimal erythemal dose
MHC major histocompatibility complex
NCAM neural cell adhesion molecule
NK natural killer
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NTP National Toxicology Program
PAH polycyclic aromatic hydrocarbon
PCB polychlorinated biphenyl
PG prostaglandin
QCA quiescent cell antigen
RIVM Dutch National Institute of Public Health and
Environmental Protection
S9 9000 x g supernatant
SCF stem-cell factor
SCID severe combined immunodeficiency
SIS skin immune system
STM Salmonella typhimurium mitogen
TBTO tri- n-butyltin oxide
Tc cytotoxic T cell
TCDD 2,3,7,8-tetrachlorodibenzo- para-dioxin
TCR T-cell receptor
Tdth delayed-type hypersensitivity T cell
TGF transforming growth factor
Th T helper-inducer cell
THAM T-cell activation molecule
THI 2-acetyl-4(5)-tetrahydroxybutylimidazole
O,O,S-TMP O,O,S-trimethylphosphorothiate
TNF tumour necrosis factor
UVB ultraviolet B
UVR ultraviolet radiation
VCAM vascular cell adhesion molecule
VLA very late antigen
SUMMARY
1. The immune system has evolved to counter challenges to the
integrity of self from either microorganisms or cells that have
escaped the organism's control mechanisms. Recognition that
xenobiotics can impair the function of the immune system has led to
progress in immunotoxicology over the last two decades. Experimental
approaches (mainly in rodent species) have been developed and
validated in multilaboratory studies. In this monograph, the function
and histophysiology of the immune system are reviewed, and the
information necessary to understand and interpret the pathological
changes caused by immunotoxic insults is provided. Emphasis is laid on
the immune systems of humans and rodent species, but reference is made
to other species, including fish, that have been the object of
immunotoxicological studies. The pathophysiology of the immune system,
including the variable susceptibility of its components, alterations
to the lymphoid organs, and the reversibility of changes are important
for understanding the impact of immunotoxicity.
2. Immunosuppression and immunostimulation both have clinical
consequences. Immunodeficiency states and severe immunosuppression,
such as can occur during transplantion and cytostatic therapy, have
both been associated with increased incidences of infectious diseases
(particularly opportunistic ones) and cancer. Exposure to immunotoxic
chemicals in the environment, however, may be expected to result in
more subtle forms of immunosuppression which may be difficult to
detect, leading to increased incidences of infections such as
influenza and the common cold. Studies of experimental animals and
humans have shown that many environmental chemicals suppress the
immune response. Immunotoxic xenobiotics are not restricted to a
particular chemical class. Compounds that adversely affect the immune
system are found among drugs, pesticides, solvents, halogenated and
aromatic hydrocarbons, and metals; ultraviolet radiation can also be
immunotoxic. Therapeutic administration of immunostimulating agents
can have adverse effects, and a few environmental chemicals that have
immunostimulating properties (beryllium, silica, hexachlorobenzene)
can have clinical consequences.
3. The complexity of the immune system results in multiple potential
target sites and pathological sequelae. The initial strategies devised
by immunotoxicologists working in toxicology and safety assessment
were to select and apply a tiered panel of assays to identify
immunosuppressive and immunostimulatory agents in laboratory animals.
Although the configuration of these testing panels may vary depending
on which agency or laboratory is conducting the test and on the animal
species employed, they all include measurement of one or more of the
following: altered lymphoid organ weights and histology; changes in
the cellularity of lymphoid tissue, peripheral blood leukocytes,
and/or bone marrow; impairment of cell function at the effector or
regulatory level; and altered susceptibility to challenge with
infectious agents or tumour cells.
The original test guideline No. 407 of the Organisation for
Economic Co-operation and Development, published in 1981, was not
designed to detect potential immunotoxicity, and modifications have
been proposed to make the guideline more useful for identifying
immunotoxicants. Tiered testing systems have been designed for more
extensive investigation of potential immunotoxicity, by the US
National Toxicology Program, the Dutch National Institute of Public
Health and Environmental Protection, the US Environmental Protection
Agency Office of Pesticides, and the US Food and Drug Administration
Center for Food Safety and Applied Nutrition.
Studies have been conducted in mice, and to a lesser extent in
rats, to investigate the specificity, precision (reproducibility),
sensitivity, accuracy, and relevance for the assessment of risk to
human health of a variety of measures of immune status. International,
interlaboratory validations of methods have been carried out within
the International Collaborative Immunotoxicity Study of IPCS and the
European Union, the Bundesinstitut für Gesundheitlichen
Verbraucherschutz, und Veterinärmedizin, and in studies of cyclosporin
A in Fischer 344 rats.
4. The tests used in the tiered testing schemes are described in
Section 3, which indicates the rationale for their selection and the
complexities involved in their performance. Although these protocols
were designed for studies of rats and mice, some have been applied
successfully for studying immunotoxicity in other animal species,
including non-human primates, marine mammals, dogs, birds, and fish.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to influence the immune
system of experimental animals adversely. These include selection of
the appropriate animal models and exposure variables, inclusion of
general toxicological parameters, an understanding of the biological
relevance of the end-points being measured, use of validated measures,
and quality assurance. The experimental conditions should take into
account the potential route and level of human exposure and any
available information on toxicodynamics and toxicokinetics. The doses
and sample sizes should be selected so as to generate clear dose-
response curves, in addition to no-observed-adverse-effect or
no-observed-effect levels. The strategies are continually refined to
allow better prediction of conditions that may lead to disease. In
addition, techniques should be developed that would help to identify
mechanisms of action; these might include methods in vitro,
examination of local immune responses (such as in the skin, lung, and
intestines), and use of the techniques of molecular biology and
genetically modified animals.
5. The detection of immune changes after exposure to potentially
immunotoxic compounds is more complicated in humans than in
experimental animals. The testing possibilities are limited, levels of
exposure to the agent (i.e. dose) are difficult to establish, and the
immune status of populations is extremely heterogeneous. Age, race,
gender, pregnancy, acute stress and the ability to cope with stress,
coexistent disease and infections, nutritional status, tobacco smoke,
and some medications contribute to this heterogeneity.
An important factor in assessing the usefulness of a particular
study for risk assessment is epidemiological study design. The
commonest design used in immunotoxicity is the cross-sectional study,
in which exposure status and disease status are measured at one time
or over a short period. The immune function of 'exposed' subjects is
then compared with that of a comparable group of 'unexposed'
individuals. There are possible pitfalls in this study design.
Because many of the immune changes seen in humans after exposure
to a chemical may be sporadic and subtle, recently exposed populations
must be studied and sensitive tests be used for assessing the immune
system. Conclusions about immunotoxic effects should be based on
changes not in a single parameter but in the immune profile of an
individual or population.
Most of the tests for specific immunity (cell-mediated and
humoral), nonspecific immunity and inflammation were developed to
detect immune alterations in patients with immunodeficiency disease
and are not always adequate to detect subtle alterations induced by
environmental chemicals. IPCS, the Centers for Disease Control, and
the US National Academy of Sciences have each described procedures for
evaluating changes in the human immune system resulting from exposure
to immunotoxicants, but the tests described require evaluation for
this purpose.
6. Risk assessment is a process in which relevant data on the
biological effects, dose-response relationships, and exposure for a
particular agent are analysed in an attempt to establish qualitative
and quantitative estimates of adverse outcomes. Typically, risk
assessment comprises four major steps: hazard identification, dose-
response assessment, exposure assessment, and risk characterization.
Up until now, immunotoxicology has focused mainly on hazard
identification, and to some extent on dose-response assessment, and
very few studies have included exposure assessment or risk
characterization.
As in other areas of toxicology, uncertainties exist which may
affect the interpretation of data on immunotoxicity with regard to
human health risk. The two most problematic issues -- extrapolating
effects from individual cells to a whole organ or beyond and
extrapolating data from experimental animals to humans -- are common
to most non-cancer end-points. The first issue is due to uncertainties
associated with establishing a quantitative relationship between
changes in individual immune function and altered resistance to
infections and neoplastic disease. The second issue is due to
uncertainties associated with assessing risk to human health on the
basis of studies in laboratory animals.
The ultimate purpose of risk assessment is to protect human
health and the environment. Suitable model systems must therefore be
chosen. The toxicokinetics of the test material and the nature and
magnitude of the immune response generated in the model should be
comparable to that of humans.
Conventionally, empirical uncertainty factors are used in risk
assessment to derive an acceptable exposure limit from experimental
results. This approach does not take into account the functional
reserve or redundancy of the immune system. A more recent development
in risk assessment is use of in-vitro models as an adjunct to studies
of experimental animals. The advantages of this approach are that it
improves the accuracy of extrapolation of data from animals to man and
minimizes the use of animals; it also bridges the gap between those
data, particularly when human experimentation is limited for ethical
considerations. Chapter 6 cites two examples in which in-vitro data
make it possible to reduce the uncertainties in risk assessment
associated with exposure to ozone and ultraviolet radiation. The
difficulty in establishing quantitative relationships between
immunosuppression and clinical disease has limited the use of
immunotoxicological data in risk assessment.
RECOMMENDATIONS
Recommendations for the protection of human health
1. Chemicals should be screened to determine if they are potentially
immunotoxic to humans. If immunotoxicity is detected, the chemicals
should be investigated further as part of the risk assessment process.
2. Chemicals for which little or no information is available on
toxicity should be screened for potential immunotoxicity following a
protocol based on, for example, the revised OECD guideline No. 407.
When some information is available on the test material (e.g.
physicochemical properties, toxicokinetics, structure-activity
relationships), a flexible approach to testing is recommended which
permits a rational selection of test procedures.
3. The immunotoxic risk of mixtures of environmental pollutants, in,
for example, fish, to certain human consumer groups (e.g. fishermen)
should be assessed.
Recommendations for protection of the environment
1. Chemicals should be screened to determine if they are potentially
immunotoxic to wildlife species. If immunotoxicity is detected, the
chemicals should be investigated further as part of the risk
assessment process.
2. The immunotoxic risk of environmental pollution to the health of
the ecosystem should be assessed in laboratory, semi-field, and field
studies of the wildlife occupying high trophic levels or those species
judged to be sensitive.
Recommendations for further research
1. The panels of tests suggested for evaluating xenobiotic-induced
immunotoxicity in humans should be investigated to determine their
ability to detect subtle alterations in immune status.
2. The relationships between alterations in immune function and human
health should be established for use in immunotoxic risk assessment.
Epidemiological studies should be carried out that include assessment
of exposure, in order to establish dose-response relationships.
3. The relationship between immunotoxicity and the development of
neoplasia should be investigated.
4. Baseline immunological data should be established for the general
population and for subpopulations such as ethnic minorities, children,
the aged, and pregnant and lactating women in order to assess their
immune status.
5. Immunotoxicological assessment should be conducted for
subpopulations potentially susceptible to the effects of immunotoxic
compounds, including those at the extremes of age and those with
deficient nutritional status.
6. Biomarkers of exposure, effect, and susceptibility should be
identified, developed, and validated for use in epidemiological
studies of immunotoxicity in both humans and wildlife.
7. The quantitative relationship between immune function and host
resistance in animal models, including the nature, magnitude, and
significance of functional reserve and redundancy, should be explored
for risk assessment.
8. Since chemicals and biological agents enter the body via the
respiratory and alimentary tracts and the skin, more research should
be carried out on local immunity.
9. Preliminary observations in laboratory animals that suggest that
primary immunization does not compromise testing for subacute toxicity
should be substantiated by further research, so that functional
testing can be incorporated into toxicology testing.
10. Methods and reagents should be developed in order to characterize
the immune system of wildlife species and to assess their immune
status for immunotoxicological studies.
11. The mechanisms of the immunotoxic action of xenobiotics in humans
should be elucidated by a combination of studies in laboratory animals
in vivo and experiments with human and animal tissues and cell lines
in vitro.
12. In view of the sensitivity of the developing immune system to
immunotoxic injury, more emphasis should be placed on studies
involving perinatal exposure to a chemical or mixture of chemicals.
13. Studies should be conducted to establish whether exposure to
xenobiotics that are not themselves sensitizing adds to the risk of
allergic disease in general.
14. Autoimmune models in laboratory animals should be used to assess
whether xenobiotics can modulate autoimmune disease in humans.
15. The effects on immune function of confounding factors in humans
and animals, including age, race, sex, gender, nutritional status,
acute stress, and underlying disease, should be evaluated further in
order to determine their effects in tests for the immunotoxicity of
environmental chemicals.
16. Methods for assessing cytokines and their production in different
body compartments, including plasma, bronchoalveolar lavage fluid, and
nasal lavage fluid, and by cells isolated from various anatomical
sites should be validated for humans and laboratory animals, and their
applicability for assessing the risk of chemicals should be
established.
17. Data from clinical trials should be made more widely available;
and patients undergoing therapy with immunomodulatory drugs should be
monitored clinically and immunologically in a systematic way.
18. The toxicokinetics of immunotoxic chemicals should be further
investigated, particularly with regard to whether their concentrations
in human biological fluids indicate levels of environmental exposure.
19. The interactions between the immune system, the nervous system,
and the endocrine system should be further investigated, with
particular emphasis on how xenobiotics adversely affect them.
20. The significance of ultraviolet radiation-induced
immunosuppression for public health and the health of ecosystems
should be evaluated.
1. INTRODUCTION TO IMMUNOTOXICOLOGY
1.1 Historical overview
It is well established that each individual has an intrinsic
capacity to defend itself against pathogens in the environment, with a
defence known as the immune system. By general definition, the immune
system serves the body by neutralizating, inactivating, or eliminating
potentially pathogenic invaders such as microorganisms (bacteria and
viruses); it also guards against uncontrolled growth of cells into
neoplasms, or tumours. The major features of the structure and
function of the immune system have been elucidated over the last three
decades; in parallel, awareness grew of toxicological manifestations
after exposure to xenobiotic chemicals. Recognition of the interplay
between toxicology and immunology is relatively recent: A
comprehensive review, published in 1977 (Vos, 1977), was the first
survey of a large series of xenobiotics that affect immune reactivity
in laboratory animals and hence may influence the health of exposed
individuals. Most research groups focusing on toxicity to the immune
system started their activities during the last decade. Textbooks of
immunotoxicology date only from the early 1980s (Gibson et al., 1983;
Dean et al., 1985; Descotes, 1986), while one on clinical
immunotoxicology is more recent (Newcombe et al., 1992).
Immunotoxicology is the study of the interactions of chemicals
and drugs with the immune system. A major focus of immunotoxicology is
the detection and evaluation of undesired effects of substances by
means of tests on rodents. The prime concern is to assess the
importance of these interactions in regard to human health. Toxic
responses may occur when the immune system is the target of chemical
insults, resulting in altered immune function; this in turn can result
in decreased resistance to infection, certain forms of neoplasia, or
immune dysregulation or stimulation which exacerbates allergy or
autoimmunity. Alternatively, toxicity may arise when the immune system
responds to the antigenic specificity of the chemical as part of a
specific immune response (i.e. allergy or autoimmunity). Certain drugs
induce autoimmunity (Kammüller et al., 1989; Kammüller & Bloksma,
1994). The differentiation between direct toxicity and toxicity due to
an immune response to a compound is to a certain extent artificial.
Some compounds can exert a direct toxic action on the immune system as
well as altering the immune response. Heavy metals like lead and
mercury, for instance, manifest immunosuppressive activity,
hypersensitivity, and autoimmunity (Lawrence et al., 1987).
This monograph is concerned mainly with one aspect of
immunotoxicology: the direct or indirect effect of xenobiotic
compounds (or their biotransformation products) on the immune system.
This effect is usually immunosuppression, or the induction of a state
of deficiency or unresponsiveness. Allergy and autoimmunity will be
dealt with in a future Environmental Health Criteria monograph.
Toxicological research over the past decade has indicated that
the immune system is a potential 'target organ' for toxic damage. This
finding was the basis for a number of large scientific conferences on
immunotoxicology and sparked the active interest of national and
international organizations in this field. One of the milestones in
the development of the discipline was the international seminar on
'The Immunological System as a Target for Toxic Damage', held in
Luxembourg in 1984 and organized by the International Programme on
Chemical Safety (IPCS), and the Commission of the European Communities
(CEC). At the seminar, immunotoxicology was defined as 'the discipline
concerned with the study of the events that can lead to undesired
effects as a result of interaction of xenobiotics with the immune
system. These undesired events may result as a consequence of: (1) a
direct and/or indirect effect of the xenobiotic (and/or its
biotransformation product) on the immune system; or, (2) an
immunologically-based host response to the compound and/or its
metabolite(s) or host antigens modified by the compound or its
metabolites' (Berlin et al., 1987). Recommendations were made
concerning the significance to public health of immunotoxicology,
immunotoxicity testing, research and development in immunotoxicology,
the development of databases, and training and education. A subsequent
workshop on 'Immunotoxicity of Metals and Immunotoxicology', organized
by IPCS and the CEC, in collaboration with the International Union for
Pure and Applied Chemistry and German governmental agencies, was held
in Hanover, Germany, in 1989 (Dayan et al., 1990). A meeting on risk
assessment in immunotoxicology was organized by the United States
National Institute for Environmental Health Sciences in 1990. A
meeting on human immunotoxicology tests was organized by the Agency
for Toxic Substances and Disease Registry and the Centers for Disease
Control, in Atlanta, Georgia, United States of America, in 1992. In
1994, two meetings were held: one in Oxford, United Kingdom, organized
by IPCS, on risk assessment in human imunotoxicity, and one in
Washington DC, United States, organized by the International Life
Sciences Institute, on methods in immunotoxicology.
In parallel to these meetings, activities were started within
IPCS for the development and validation of methods for assessing
toxicity to the immune system. In this regard, a hallmark event was
the meeting in 1986 of a technical review and working group, in
London, United Kingdom (IPCS, 1986).
A number of tiered approaches to immunotoxicity testing have been
proposed, in rats (Vos, 1980; Van Loveren & Vos, 1989) and subsequently
in mice (Luster et al., 1988). These approaches have been evaluated
for their capacity to identify chemicals as immunosuppressive. Of a
group of 18 pesticides evaluated in rats, six were identified as
inducing immunotoxicity at doses similar to those that cause other
toxic effects, and five were immunotoxic at lower doses (Vos & Krajnc,
1983; Vos et al., 1983a). Effects were seen on different parameters
with different compounds and included lymphocytopenia, reduced thymic
and spleen weights, and increased levels of serum immunoglobulin (Ig)
G. One of the compounds identified was hexachlorobenzene (Vos, 1986),
which is further described in Section 2. In mice, the tiered approach
was used to assess the immunotoxicity of 51 chemicals, selected on the
basis of factors including structure-activity relationships with
previously identified immunotoxic substances, and use (Luster et al.,
1992). Of the spectrum of assays applied, the strongest associations
with immunotoxic potential were observed with the splenic IgM antibody
plaque-forming cell response and cell surface marker analysis; somewhat
weaker associations were found for natural killer (NK) cell activity,
cytotoxic T-lymphocyte cytolytic activity, lymphocyte proliferation
in vitro after mitogen stimulation, and thymus:body weight ratio.
The tiered approach in immunotoxicity testing is further described in
Section 3.
Multi-laboratory studies have been initiated to validate the
screening of immunotoxic compounds, including the IPCS-European Union
International Collaborative Immunotoxicity Study, a study in Fischer
344 rats, and the international study of the Bundesinstitut für
Gesundheitlichen Verbraucherschutz, und Veterinärmedizin, which were
designed to determine interlaboratory reproducibility. The
experimental animal used in these studies is the rat, and some
functional tests are included. Test methods are also being developed
and validated within the National Toxicology Program (NTP) in the
United States. This programme includes studies of carcinogenicity in
rats and mice, but because the immune system of mice is better
characterized than that of rats, the NTP chose the mouse as the
experimental animal for immunotoxicity assessment. The immunotoxicity
database of the NTP has been evaluated to determine the predictability
(sensitivity and specificity) of the assays. In the Netherlands, a
Committee for Immunotoxicology of the Dutch Health Council reviewed
methods that could be used to assess the immunotoxic properties of a
compound and for deriving information about risks to humans on the
basis of the results of laboratory experiments. The Committee also
examined the relationship between the immunotoxic properties of a
substance and its mutagenic and carcinogenic properties (Dutch Health
Council, Committee for Immunotoxicology, 1991).
The immune system was reviewed by the United States National
Research Council in order to identify the kinds of basic research that
might reveal markers of environmental exposure and disease. Major
emphasis was placed on biological markers of three types: those
originating from the immune system, those related to exposure to
immunosuppressive toxicants, and those of effects of environmental
pollutants. Markers of susceptibility to environmental materials were
also considered to be important, especially if they are of a genetic
nature and can be used to identify individuals susceptible to
autoimmune diseases. The National Research Council subcommittees on
pulmonary toxicology and on immunotoxicology, have published their
reports (US National Research Council, 1989, 1992).
Interest in immunotoxicology within the scientific community is
reflected by the existence of a special section on immunotoxicology
within the Society of Toxicology. An immunotoxicology discussion group
initiated in the United States has an international composition. The
European Union has a programme on science and technology for
environmental protection that includes immunotoxicology as an
important aspect.
There is growing concern in society about the effects of
xenobiotics, such as environmental pollutants, on public health; the
immune system is one of the targets of such effects. Some chemicals
present in the environment that have been reported to influence the
immune system are listed in Table 1 (IPCS, 1986). Immunotoxicity can
result in e.g. reduced resistance towards infection or generation of
tumours that escape immune surveillance. A number of substances
affect immunological parameters; these include halogenated
hydrocarbons such as polychlorinated biphenyls, polybrominated
biphenyls, polychlorinated dibenzo- para-dioxins, and polychlorinated
dibenzofurans (Elo et al., 1985; Lu & Wu, 1985; Bekesi et al., 1987;
Kimbrough, 1987; Hoffman 1992); pesticides and precursors (Fiore et
al., 1986; Deo et al., 1987; Nigam et al., 1993); organic solvents
(Capurro, 1980; Denkhaus et al., 1986); asbestos (Lew et al., 1986);
silica (Uber & McReynolds, 1982); and metals like lead (Ewers et al.,
1982; Reigart & Graber, 1976). Oxidant air pollutants, like sulfur
dioxide, nitrogen dioxide, and ozone, and particles in airborne dust
may affect immune function (Koren et al., 1989; Van Loveren et al.,
1994).
Immunotoxicity in humans is further discussed in Section 2. Few
epidemiological data have been published that indicate suppression or
altered resistance to infection and tumours. In general, the
usefulness of the epidemiological studies that have been published is
limited by the following: exposure is usually uncontrolled, mainly
occurring during accidents; the magnitude and pattern of exposure are
not known, and the exposure is often too low to alter the immune
system measurably; exposure is often not to one xenobiotic but to a
mixture; it is almost impossible to control for confounding
parameters, such as age, sex, genetic background, health status, and
nutritional status; and it is not always possible to define and
analyse appropriate control groups (US National Research Council,
1992). Environmental pollution and its effect on health status are
currently subjects of concern in eastern European countries and have
generated much interest in the worldwide environmental health science
community. Recent epidemiological studies have compared the possible
relationship between exposure to air pollutants and health effects in
the former German Democratic Republic and Federal Republic of Germany,
Table 1. Examples of compounds that are immunotoxic for humans or
rodents
Chemical Immune toxicity
-------------------
Rodent Human
2,3,7,8-Tetrachlorodibenzo-para-dioxin + +
Polychlorinated biphenyls + +
Polybrominated biphenyls + +
Hexachlorobenzene + Unknown
Lead + Unknown
Cadmium + Unknown
Methyl mercury compounds + Unknown
7,12-Dimethylbenz[a]anthracene + Unknown
Benzo[a]pyrene + Unknown
Di-n-octyltindichloride + Unknown
Di-n-butyltindichloride + Unknown
Benzidine + +
Nitrogen dioxide and ozone + +
Benzene, toluene, and xylene + +
Asbestos + +
N-Nitrosodimethylamine + Unknown
Diethylstilboestrol + +
Vanadium + +
From IPCS (1986)
and some of these studies included immunological data or end-points.
For instance, von Mutius et al. (1992) found a higher prevalence of
asthma among schoolchildren in western than eastern Germany, and
Behrendt et al. (1993) observed, surprisingly, that total serum IgE
levels were higher in schoolchildren in eastern than in western
Germany. Several factors were found to influence total IgE: history of
parasitic disease, number of persons per dwelling, and passive
smoking. Sex and passive smoking were the only variables that had a
significant effect in western German children. Air pollutants and
parasitic infections were suggested to be the major contributing
factors to increased IgE production in children in eastern Germany.
Remarkable differences in air quality were seen between eastern and
western Germany, and Behrendt et al. (1995) distinguished two types of
air pollution: type I, composed of sulfur dioxide particles and dust,
occurring predominantly in eastern Europe, is associated with
respiratory infections and other chronic inflammatory airway
reactions; type II, occurring both indoors and outdoors in the
environment in industrialized western countries, is composed mainly of
nitric oxide, nitrogen dioxide, ozone, volatile organic compounds, and
fine particles. The latter type of air pollution is associated with
allergic diseases and allergic sensitization, indicating that air
pollutants interfere with parameters of allergy at the level of
sensitization, elicitation of symptoms, and exacerbation of disease.
Until further epidemiological studies are conducted in humans,
assessment of immunotoxicity in rodents, with subsequent extrapolation
to the human situation, is still a good indicator of toxicity and can
serve as a basis for subsequent decisions and regulations by
authorities to reduce or prevent the risk of human exposure. This
aspect is discussed further in Section 5.
Humans are exposed to environmental contaminants mainly via food,
water, and air. Open water (e.g. rivers, lakes, and coastal areas) and
sediments often act as sinks for environmental pollution. This global
problem can be deduced from disease manifestations in fish that live
in coastal areas, especially those species that live in close contact
with contaminated silt. High levels of contaminants and the diseases
associated with them are not only of economic importance (i.e. to
fisheries) but also affect people who consume the fish, as seen in
studies showing increased levels of contaminants in people eating fish
from the contaminated Baltic Sea (Svensson et al., 1991) and in Inuit
and Indian populations in Canada who consume large quantities of fish
and marine mammals (DeWailly et al., 1992). There is now some evidence
that wildlife aquatic species have decreased resistance and enhanced
incidences of infection and tumours that may be linked to
environmental pollution (Vos et al., 1989; Wester et al., 1994).
Because the immune system of fish has not been characterized in such
detail as that of mammals, immunotoxicological studies have not been
extensively included in ecotoxicology, although a number of reports of
direct toxic actions of xenobiotics on fish species have been
published in this developing field (Wester & Canton, 1987; Payne &
Fancey, 1989; Anderson, 1990; Wester et al., 1990; Khangarot &
Tripathi, 1991; Secombes et al., 1992; Anderson & Brubacher, 1993;
Faisal & Hugget, 1993).
1.2 The immune system: functions, system regulation, and modifying
factors; histophysiology of lymphoid organs
1.2.1 Function of the immune system
In order to interpret pathological alterations of the immune
system in terms of altered function, the physiology of the system must
be understood. Since knowledge of the structure and function of the
immune system is growing rapidly, a review of this subject, focusing
on histophysiology, is presented. This section is not meant to serve
as a textbook on immunology but to provide sufficient information for
an understanding of pathological changes due to immunotoxic action.
For general textbooks on immunology, reference may be made to Sell
(1987), Klein (1990), Brostoff et al. (1991), Roitt (1991), Paul
(1993), and Roitt et al. (1993). The section covers mainly humans and
rodents, but reference is made to other species that are relevant in
immunotoxicity assessment, e.g. fish in ecotoxicology. It should be
noted that species differences can be large, despite fundamental
similarities between the immune systems of animals. It is therefore
difficult to conduct immunotoxicological studies in immunologically
less well characterized animal species, although comparative studies
that are under way may lessen the problems. Zapata and Cooper (1990)
have written a comprehensive textbook on phylogenetic aspects of
immunology. Phylogenetic data, from primitive fish to mammals, are
presented in Table 2 (Cooper, 1982; Klein, 1986; Du Pasquier, 1989;
Zapata & Cooper, 1990; Sima & Vetvicka, 1992). Relevant phylogenetic
aspects of the immune system are described below.
In mammals, the immune system and its reactions consist of a
finely tuned, complex interplay between various cell types and soluble
mediators secreted by those cells (Figure 1), some of which are listed
in Section 7.
Immune responses can be classified roughly as innate (natural and
nonspecific) and acquired (adaptive) responses, in which the reaction
is directed to a specific determinant (antigenic determinant or
epitope). The nonspecific response involves effector cells such as
macrophages (Vetvicka & Fornusek, 1992), NK cells (Herberman &
Ortaldo, 1981), granulocytes (Ross, 1992), and mediator systems
including the complement system (Tomlinson, 1993). Specificity is
based on recognition by specific receptors on lymphocytes or by
antibodies: The classical reaction to bacterial infection, resulting
in antibacterial antibody formation and antibody-mediated destruction
of the pathogen, is only one part of the intrinsic capacity of the
system. Further attributes of the system (Nossal, 1987) are summarized
below.
1.2.1.1 Encounter and recognition
The initiation of an immune response requires adequate
recognition of the pathogen. This recognition often occurs immediately
after entry, e.g. during or after passage through the epithelial
barrier of the body (skin or mucus-secreting epithelia in the
respiratory and gastrointestinal tract). The first defence includes
nonspecific inactivation, e.g. by nonspecific killer cells,
neutrophilic granulocytes, and cells of the mononuclear phagocyte
system (formerly called the reticuloendothelial system). It also
includes antigen processing and presentation to cells such as
lymphocytes of the T-helper-inducer type (Th) which can generate a
specific response.
Table 2. Evolution of immunologically important traits among vertebrates
A. Major histocompatibility complex (MHC) and transplantation
Species Graft MLR CML MHC control Serologically
rejection and/or of immune detectable
GVH response MHC antigens
Tunicata (sea squirts) + ? ? ? ?
Agnatha
Hagfish (Hyperotreti) + ? - ? ?
Lamprey (Hyperoartii) + ? - ? ?
Chondrichthyes (cartilaginous fish)
Shark, ray + ? + ? +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + ? ? ? ?
Bony fish (Teleostei) + ? + ? +
Lungfish (Dipnoi) + ? ? ? ?
Amphibia (amphibians)
Salamanders (Urodela) + ? ? ? +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + ? ? ?
Lizards, snakes (Squamata) + + + ? ?
Crocodiles, alligators (Crocodilia) + ? ? ? ?
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
B. Complement and immunoglobulins (Ig)
Species Complement Immunoglobulins
IgM IgG-like IgA IgD IgE
Tunicata (sea squirts) ? - - - - -
Agnatha
Hagfish (Hyperotreti) ? ? - - - -
Lamprey (Hyperoartii) ? ? - - - -
Chondrichthyes (cartilaginous fish)
Shark, ray + + + - - -
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + + + - - -
Bony fish (Teleostei) + + + - - -
Lungfish (Dipnoi) + + + - - -
Amphibia (amphibians)
Salamanders (Urodela) + + ? - - -
Frogs, toads (Anura) + + + + - -
Reptilia (reptiles)
Turtles (Chelonia) + + + - - -
Lizards, snakes (Squamata) + + + - - -
Crocodiles, alligators (Crocodilia) + + + ? - -
Aves (birds) + + ? + - -
Mammalia (mammals) + + + + + +
Table 2 (cont'd)
C. Leukocytes
Species Lymphocytes Plasma Macrophages
cells
Small T B
Tunicata (sea squirts) + - - - +
Agnatha
Hagfish (Hyperotreti) + - - + +
Lamprey (Hyperoartii) + - - + +
Chondrichthyes (cartilaginous fish)
Shark, ray + - ? + +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + - ? + +
Bony fish (Teleostei) + + + + +
Lungfish (Dipnoi) + ? ? + +
Amphibia (amphibians)
Salamanders (Urodela) + + + + +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + + + +
Lizards, snakes (Squamata) + + + + +
Crocodiles, alligators (Crocodilia) + + + + +
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
D. Lymphoid organs
Species Bone Thymus Spleen Lymph glands
marrow or nodes
Tunicata (sea squirts) - - - -
Agnatha
Hagfish (Hyperotreti) - - - GALT
Lamprey (Hyperoartii) - - - GALT
Chondrichthyes (cartilaginous fish)
Shark, ray - + + GALT
Osteichthyes (bony fish)
Sturgeon (Chondrostei) - + + GALT
Bony fish (Teleostei) - + + GALT
Lungfish (Dipnoi) - + + GALT
Amphibia (amphibians)
Salamanders (Urodela) + + + -
Frogs, toads (Anura) + + + ?
Reptilia (reptiles)
Turtles (Chelonia) + + + ?
Lizards, snakes (Squamata) + + + ?
Crocodiles, alligators (Crocodilia) + + + ?
Aves (birds) + + + +
Mammalia (mammals) + + + +
+, positive; ±, to be confirmed; -, negative; ?, not investigated.
MLR, mixed leukocyte response; GVH, graft-versus-host; CML, cell-mediated lympholysis;
GALT, gut-associated lymphoid tissue
incorporating the comments received and approved by the Director,
IPCS, is then distributed to Task Group members, who carry out a peer
review at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the risks to health and
the environment from exposure to the chemical. A summary and
recommendations for further research and improved safety are also
drawn up. The composition of the Task Group is dictated by the range
of expertise required for the subject of the meeting and by the need
for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations, so that
representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide valuable contributions to the process, they can speak only
at the invitation of the Chairperson. Observers do not participate in
the final evaluation of the chemical, which is the sole responsibility
of the Task Group members. The Task Group may meet in camera when it
considers that to be appropriate.
All individuals who participate in the preparation of an EHC
monograph as authors, consultants, or advisers must, in addition to
serving in their personal capacity as scientists, inform the RO if at
any time a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a statement to that
effect. This procedure ensures the transparency and probity of the
process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it is edited for language, the references are checked, and
camera-ready copy is prepared. After approval by the Director, IPCS,
the monograph is submitted to the WHO Office of Publications for
printing. At this time, a copy of the final draft is also sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern about
health or environmental effects of the agent because of greater
exposure; an appreciable time has elapsed since the last evaluation.
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The chairpersons of task groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON PRINCIPLES AND METHODS FOR ASSESSING DIRECT
IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
Members
Dr A. Emmendörffer, Department of Immunobiology, Fraunhofer Institute
of Toxicology & Aerosol Research, Hanover, Germany
Dr H.S. Koren, Health Effects Research Laboratory, US Environmental
Protection Agency, Chapel Hill, NC, USA (Vice-Chairman)
Dr R.W. Luebke, Immunotoxicology Branch, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, NC, USA (Joint Rapporteur)
Dr M. Luster, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA
Dr C. Madsen, Institute of Toxicology, National Food Agency of
Denmark, Ministry of Health, Soborg, Denmark
Dr P. Ross, Dalhousie University, Halifax, Nova Scotia, Canada
(c/o Seal Rehabilitation and Research Centre, Pieterburen,
Netherlands)
Dr H.J. Schuurman, Preclinical Research/Immunology, Sandoz Pharma Ltd,
Basel, Switzerland
Dr H. Van Loveren, Laboratory for Pathology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Joint Rapporteur)
Dr J.G. Vos, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands (Chairman)
Dr K.L. White, Jr, Immunotoxicology Group, Medical College of
Virginia, Virginia Commonwealth University, Richmond, VA, USA
Observers
IUTOX
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy
ECETOC
Dr R.W.R. Crevel, Environmental Safety Laboratory, Unilever Research
and Engineering, Sharnbrook, Bedfordshire, United Kingdom
Secretariat
Dr J.H. Dean, Sanofi Winthrop, Inc., Sanofi Research Division,
Collegeville, PA, USA
Mr V. Quarg, Federal Ministry for Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA PRINCIPLES AND METHODS FOR ASSESSING
DIRECT IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
A WHO Task Group on Principles and Methods for Assessing Direct
Immunotoxicity Associated with Exposure to Chemicals met at the World
Health Organization, Geneva, from 10 to 14 October 1994. Dr E. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
IPCS, and the cooperating organizations. The Task Group reviewed and
revised the draft monograph and prepared the final text.
The first draft of the monograph was prepared by a group of
authors (listed below) under the coordination of Dr J.G. Vos and
Dr H. Van Loveren of the Dutch National Institute for Public Health
and Environmental Protection (RIVM), an IPCS Collaborating Centre for
Immunotoxicology and Allergic Hypersensitization. The second draft,
incorporating comments received after international circulation to
national experts of the first draft to IPCS contact points for
Environmental Health Criteria monographs, was prepared by Dr J.G.
Vos and Dr H. Van Loveren of the Netherlands and Dr Kimber White, USA.
Dr E. Smith of the IPCS Unit for the Assessment of Risk and
Methods was responsible for the scientific content of the monograph
and Mrs E. Heseltine for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The contributing authors were:
Dr J.H. Dean, Collegeville, PA, USA
Professor J. Descotes, Lyon, France
Dr F. Kuper, Zeist, Netherlands
Dr M. Luster, Research Triangle Park, NC, USA
Dr P.S. Ross, Bilthoven, Netherlands
Dr H.J. Schuurman, Basel, Switzerland
Dr M.J. Selgrade, Research Triangle Park, NC, USA
Dr R.L. de Swart, Bilthoven, Netherlands
Dr H. Van Loveren, Bilthoven, Netherlands
Professor J.G. Vos, Bilthoven, Netherlands
Dr P.W. Wester, Bilthoven, Netherlands
Professor A.G. Zapata, Madrid, Spain
ABBREVIATIONS
ACTH adrenocorticotrophic hormone
Ah aromatic hydrocarbon
AIDS acquired immunodeficiency syndrome
B bursa-dependent
CALLA common acute lymphoblastic leukaemia antigen
CD cluster of differentiation
CEC Commission of the European Communities
CH50 haemolytic complement
CML cell-mediated lympholysis
DMBA 7,12-dimethylbenz[ a]anthracene
DNCB dinitrochlorobenzene
ELISA enzyme-linked immunosorbent assay
EPO erythrocyte lineage differentiation factor
FACS fluorescence activated cell sorter
GALT gut-associated lymphoid tissue
G-CSF granulocyte colony-stimulating factor
GM-CSF granulocyte-macrophage colony-stimulating factor
GVH graft-versus-host
HCB hexaclorobenzene
HEV high endothelial venule
HIV human immunodeficiency virus
HPCA human progenitor cell antigen
HSA heat-stable antigen
ICAM intercellular adhesion molecule
IFN interferon
Ig immunoglobulin
IL interleukin
IPCS International Programme on Chemical Safety
LFA lymphocyte function-related antigen
LIF leukaemia inhibitory factor
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
M microfold
MALT mucosa-associated lymphoid tissue
MARE monoclonal anti-rat immunoglobulin E
MARK monoclonal antibody anti-kappa
M-CSF macrophage colony-stimulating factor
MED minimal erythemal dose
MHC major histocompatibility complex
NCAM neural cell adhesion molecule
NK natural killer
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NTP National Toxicology Program
PAH polycyclic aromatic hydrocarbon
PCB polychlorinated biphenyl
PG prostaglandin
QCA quiescent cell antigen
RIVM Dutch National Institute of Public Health and
Environmental Protection
S9 9000 x g supernatant
SCF stem-cell factor
SCID severe combined immunodeficiency
SIS skin immune system
STM Salmonella typhimurium mitogen
TBTO tri- n-butyltin oxide
Tc cytotoxic T cell
TCDD 2,3,7,8-tetrachlorodibenzo- para-dioxin
TCR T-cell receptor
Tdth delayed-type hypersensitivity T cell
TGF transforming growth factor
Th T helper-inducer cell
THAM T-cell activation molecule
THI 2-acetyl-4(5)-tetrahydroxybutylimidazole
O,O,S-TMP O,O,S-trimethylphosphorothiate
TNF tumour necrosis factor
UVB ultraviolet B
UVR ultraviolet radiation
VCAM vascular cell adhesion molecule
VLA very late antigen
SUMMARY
1. The immune system has evolved to counter challenges to the
integrity of self from either microorganisms or cells that have
escaped the organism's control mechanisms. Recognition that
xenobiotics can impair the function of the immune system has led to
progress in immunotoxicology over the last two decades. Experimental
approaches (mainly in rodent species) have been developed and
validated in multilaboratory studies. In this monograph, the function
and histophysiology of the immune system are reviewed, and the
information necessary to understand and interpret the pathological
changes caused by immunotoxic insults is provided. Emphasis is laid on
the immune systems of humans and rodent species, but reference is made
to other species, including fish, that have been the object of
immunotoxicological studies. The pathophysiology of the immune system,
including the variable susceptibility of its components, alterations
to the lymphoid organs, and the reversibility of changes are important
for understanding the impact of immunotoxicity.
2. Immunosuppression and immunostimulation both have clinical
consequences. Immunodeficiency states and severe immunosuppression,
such as can occur during transplantion and cytostatic therapy, have
both been associated with increased incidences of infectious diseases
(particularly opportunistic ones) and cancer. Exposure to immunotoxic
chemicals in the environment, however, may be expected to result in
more subtle forms of immunosuppression which may be difficult to
detect, leading to increased incidences of infections such as
influenza and the common cold. Studies of experimental animals and
humans have shown that many environmental chemicals suppress the
immune response. Immunotoxic xenobiotics are not restricted to a
particular chemical class. Compounds that adversely affect the immune
system are found among drugs, pesticides, solvents, halogenated and
aromatic hydrocarbons, and metals; ultraviolet radiation can also be
immunotoxic. Therapeutic administration of immunostimulating agents
can have adverse effects, and a few environmental chemicals that have
immunostimulating properties (beryllium, silica, hexachlorobenzene)
can have clinical consequences.
3. The complexity of the immune system results in multiple potential
target sites and pathological sequelae. The initial strategies devised
by immunotoxicologists working in toxicology and safety assessment
were to select and apply a tiered panel of assays to identify
immunosuppressive and immunostimulatory agents in laboratory animals.
Although the configuration of these testing panels may vary depending
on which agency or laboratory is conducting the test and on the animal
species employed, they all include measurement of one or more of the
following: altered lymphoid organ weights and histology; changes in
the cellularity of lymphoid tissue, peripheral blood leukocytes,
and/or bone marrow; impairment of cell function at the effector or
regulatory level; and altered susceptibility to challenge with
infectious agents or tumour cells.
The original test guideline No. 407 of the Organisation for
Economic Co-operation and Development, published in 1981, was not
designed to detect potential immunotoxicity, and modifications have
been proposed to make the guideline more useful for identifying
immunotoxicants. Tiered testing systems have been designed for more
extensive investigation of potential immunotoxicity, by the US
National Toxicology Program, the Dutch National Institute of Public
Health and Environmental Protection, the US Environmental Protection
Agency Office of Pesticides, and the US Food and Drug Administration
Center for Food Safety and Applied Nutrition.
Studies have been conducted in mice, and to a lesser extent in
rats, to investigate the specificity, precision (reproducibility),
sensitivity, accuracy, and relevance for the assessment of risk to
human health of a variety of measures of immune status. International,
interlaboratory validations of methods have been carried out within
the International Collaborative Immunotoxicity Study of IPCS and the
European Union, the Bundesinstitut für Gesundheitlichen
Verbraucherschutz, und Veterinärmedizin, and in studies of cyclosporin
A in Fischer 344 rats.
4. The tests used in the tiered testing schemes are described in
Section 3, which indicates the rationale for their selection and the
complexities involved in their performance. Although these protocols
were designed for studies of rats and mice, some have been applied
successfully for studying immunotoxicity in other animal species,
including non-human primates, marine mammals, dogs, birds, and fish.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to influence the immune
system of experimental animals adversely. These include selection of
the appropriate animal models and exposure variables, inclusion of
general toxicological parameters, an understanding of the biological
relevance of the end-points being measured, use of validated measures,
and quality assurance. The experimental conditions should take into
account the potential route and level of human exposure and any
available information on toxicodynamics and toxicokinetics. The doses
and sample sizes should be selected so as to generate clear dose-
response curves, in addition to no-observed-adverse-effect or
no-observed-effect levels. The strategies are continually refined to
allow better prediction of conditions that may lead to disease. In
addition, techniques should be developed that would help to identify
mechanisms of action; these might include methods in vitro,
examination of local immune responses (such as in the skin, lung, and
intestines), and use of the techniques of molecular biology and
genetically modified animals.
5. The detection of immune changes after exposure to potentially
immunotoxic compounds is more complicated in humans than in
experimental animals. The testing possibilities are limited, levels of
exposure to the agent (i.e. dose) are difficult to establish, and the
immune status of populations is extremely heterogeneous. Age, race,
gender, pregnancy, acute stress and the ability to cope with stress,
coexistent disease and infections, nutritional status, tobacco smoke,
and some medications contribute to this heterogeneity.
An important factor in assessing the usefulness of a particular
study for risk assessment is epidemiological study design. The
commonest design used in immunotoxicity is the cross-sectional study,
in which exposure status and disease status are measured at one time
or over a short period. The immune function of 'exposed' subjects is
then compared with that of a comparable group of 'unexposed'
individuals. There are possible pitfalls in this study design.
Because many of the immune changes seen in humans after exposure
to a chemical may be sporadic and subtle, recently exposed populations
must be studied and sensitive tests be used for assessing the immune
system. Conclusions about immunotoxic effects should be based on
changes not in a single parameter but in the immune profile of an
individual or population.
Most of the tests for specific immunity (cell-mediated and
humoral), nonspecific immunity and inflammation were developed to
detect immune alterations in patients with immunodeficiency disease
and are not always adequate to detect subtle alterations induced by
environmental chemicals. IPCS, the Centers for Disease Control, and
the US National Academy of Sciences have each described procedures for
evaluating changes in the human immune system resulting from exposure
to immunotoxicants, but the tests described require evaluation for
this purpose.
6. Risk assessment is a process in which relevant data on the
biological effects, dose-response relationships, and exposure for a
particular agent are analysed in an attempt to establish qualitative
and quantitative estimates of adverse outcomes. Typically, risk
assessment comprises four major steps: hazard identification, dose-
response assessment, exposure assessment, and risk characterization.
Up until now, immunotoxicology has focused mainly on hazard
identification, and to some extent on dose-response assessment, and
very few studies have included exposure assessment or risk
characterization.
As in other areas of toxicology, uncertainties exist which may
affect the interpretation of data on immunotoxicity with regard to
human health risk. The two most problematic issues -- extrapolating
effects from individual cells to a whole organ or beyond and
extrapolating data from experimental animals to humans -- are common
to most non-cancer end-points. The first issue is due to uncertainties
associated with establishing a quantitative relationship between
changes in individual immune function and altered resistance to
infections and neoplastic disease. The second issue is due to
uncertainties associated with assessing risk to human health on the
basis of studies in laboratory animals.
The ultimate purpose of risk assessment is to protect human
health and the environment. Suitable model systems must therefore be
chosen. The toxicokinetics of the test material and the nature and
magnitude of the immune response generated in the model should be
comparable to that of humans.
Conventionally, empirical uncertainty factors are used in risk
assessment to derive an acceptable exposure limit from experimental
results. This approach does not take into account the functional
reserve or redundancy of the immune system. A more recent development
in risk assessment is use of in-vitro models as an adjunct to studies
of experimental animals. The advantages of this approach are that it
improves the accuracy of extrapolation of data from animals to man and
minimizes the use of animals; it also bridges the gap between those
data, particularly when human experimentation is limited for ethical
considerations. Chapter 6 cites two examples in which in-vitro data
make it possible to reduce the uncertainties in risk assessment
associated with exposure to ozone and ultraviolet radiation. The
difficulty in establishing quantitative relationships between
immunosuppression and clinical disease has limited the use of
immunotoxicological data in risk assessment.
RECOMMENDATIONS
Recommendations for the protection of human health
1. Chemicals should be screened to determine if they are potentially
immunotoxic to humans. If immunotoxicity is detected, the chemicals
should be investigated further as part of the risk assessment process.
2. Chemicals for which little or no information is available on
toxicity should be screened for potential immunotoxicity following a
protocol based on, for example, the revised OECD guideline No. 407.
When some information is available on the test material (e.g.
physicochemical properties, toxicokinetics, structure-activity
relationships), a flexible approach to testing is recommended which
permits a rational selection of test procedures.
3. The immunotoxic risk of mixtures of environmental pollutants, in,
for example, fish, to certain human consumer groups (e.g. fishermen)
should be assessed.
Recommendations for protection of the environment
1. Chemicals should be screened to determine if they are potentially
immunotoxic to wildlife species. If immunotoxicity is detected, the
chemicals should be investigated further as part of the risk
assessment process.
2. The immunotoxic risk of environmental pollution to the health of
the ecosystem should be assessed in laboratory, semi-field, and field
studies of the wildlife occupying high trophic levels or those species
judged to be sensitive.
Recommendations for further research
1. The panels of tests suggested for evaluating xenobiotic-induced
immunotoxicity in humans should be investigated to determine their
ability to detect subtle alterations in immune status.
2. The relationships between alterations in immune function and human
health should be established for use in immunotoxic risk assessment.
Epidemiological studies should be carried out that include assessment
of exposure, in order to establish dose-response relationships.
3. The relationship between immunotoxicity and the development of
neoplasia should be investigated.
4. Baseline immunological data should be established for the general
population and for subpopulations such as ethnic minorities, children,
the aged, and pregnant and lactating women in order to assess their
immune status.
5. Immunotoxicological assessment should be conducted for
subpopulations potentially susceptible to the effects of immunotoxic
compounds, including those at the extremes of age and those with
deficient nutritional status.
6. Biomarkers of exposure, effect, and susceptibility should be
identified, developed, and validated for use in epidemiological
studies of immunotoxicity in both humans and wildlife.
7. The quantitative relationship between immune function and host
resistance in animal models, including the nature, magnitude, and
significance of functional reserve and redundancy, should be explored
for risk assessment.
8. Since chemicals and biological agents enter the body via the
respiratory and alimentary tracts and the skin, more research should
be carried out on local immunity.
9. Preliminary observations in laboratory animals that suggest that
primary immunization does not compromise testing for subacute toxicity
should be substantiated by further research, so that functional
testing can be incorporated into toxicology testing.
10. Methods and reagents should be developed in order to characterize
the immune system of wildlife species and to assess their immune
status for immunotoxicological studies.
11. The mechanisms of the immunotoxic action of xenobiotics in humans
should be elucidated by a combination of studies in laboratory animals
in vivo and experiments with human and animal tissues and cell lines
in vitro.
12. In view of the sensitivity of the developing immune system to
immunotoxic injury, more emphasis should be placed on studies
involving perinatal exposure to a chemical or mixture of chemicals.
13. Studies should be conducted to establish whether exposure to
xenobiotics that are not themselves sensitizing adds to the risk of
allergic disease in general.
14. Autoimmune models in laboratory animals should be used to assess
whether xenobiotics can modulate autoimmune disease in humans.
15. The effects on immune function of confounding factors in humans
and animals, including age, race, sex, gender, nutritional status,
acute stress, and underlying disease, should be evaluated further in
order to determine their effects in tests for the immunotoxicity of
environmental chemicals.
16. Methods for assessing cytokines and their production in different
body compartments, including plasma, bronchoalveolar lavage fluid, and
nasal lavage fluid, and by cells isolated from various anatomical
sites should be validated for humans and laboratory animals, and their
applicability for assessing the risk of chemicals should be
established.
17. Data from clinical trials should be made more widely available;
and patients undergoing therapy with immunomodulatory drugs should be
monitored clinically and immunologically in a systematic way.
18. The toxicokinetics of immunotoxic chemicals should be further
investigated, particularly with regard to whether their concentrations
in human biological fluids indicate levels of environmental exposure.
19. The interactions between the immune system, the nervous system,
and the endocrine system should be further investigated, with
particular emphasis on how xenobiotics adversely affect them.
20. The significance of ultraviolet radiation-induced
immunosuppression for public health and the health of ecosystems
should be evaluated.
1. INTRODUCTION TO IMMUNOTOXICOLOGY
1.1 Historical overview
It is well established that each individual has an intrinsic
capacity to defend itself against pathogens in the environment, with a
defence known as the immune system. By general definition, the immune
system serves the body by neutralizating, inactivating, or eliminating
potentially pathogenic invaders such as microorganisms (bacteria and
viruses); it also guards against uncontrolled growth of cells into
neoplasms, or tumours. The major features of the structure and
function of the immune system have been elucidated over the last three
decades; in parallel, awareness grew of toxicological manifestations
after exposure to xenobiotic chemicals. Recognition of the interplay
between toxicology and immunology is relatively recent: A
comprehensive review, published in 1977 (Vos, 1977), was the first
survey of a large series of xenobiotics that affect immune reactivity
in laboratory animals and hence may influence the health of exposed
individuals. Most research groups focusing on toxicity to the immune
system started their activities during the last decade. Textbooks of
immunotoxicology date only from the early 1980s (Gibson et al., 1983;
Dean et al., 1985; Descotes, 1986), while one on clinical
immunotoxicology is more recent (Newcombe et al., 1992).
Immunotoxicology is the study of the interactions of chemicals
and drugs with the immune system. A major focus of immunotoxicology is
the detection and evaluation of undesired effects of substances by
means of tests on rodents. The prime concern is to assess the
importance of these interactions in regard to human health. Toxic
responses may occur when the immune system is the target of chemical
insults, resulting in altered immune function; this in turn can result
in decreased resistance to infection, certain forms of neoplasia, or
immune dysregulation or stimulation which exacerbates allergy or
autoimmunity. Alternatively, toxicity may arise when the immune system
responds to the antigenic specificity of the chemical as part of a
specific immune response (i.e. allergy or autoimmunity). Certain drugs
induce autoimmunity (Kammüller et al., 1989; Kammüller & Bloksma,
1994). The differentiation between direct toxicity and toxicity due to
an immune response to a compound is to a certain extent artificial.
Some compounds can exert a direct toxic action on the immune system as
well as altering the immune response. Heavy metals like lead and
mercury, for instance, manifest immunosuppressive activity,
hypersensitivity, and autoimmunity (Lawrence et al., 1987).
This monograph is concerned mainly with one aspect of
immunotoxicology: the direct or indirect effect of xenobiotic
compounds (or their biotransformation products) on the immune system.
This effect is usually immunosuppression, or the induction of a state
of deficiency or unresponsiveness. Allergy and autoimmunity will be
dealt with in a future Environmental Health Criteria monograph.
Toxicological research over the past decade has indicated that
the immune system is a potential 'target organ' for toxic damage. This
finding was the basis for a number of large scientific conferences on
immunotoxicology and sparked the active interest of national and
international organizations in this field. One of the milestones in
the development of the discipline was the international seminar on
'The Immunological System as a Target for Toxic Damage', held in
Luxembourg in 1984 and organized by the International Programme on
Chemical Safety (IPCS), and the Commission of the European Communities
(CEC). At the seminar, immunotoxicology was defined as 'the discipline
concerned with the study of the events that can lead to undesired
effects as a result of interaction of xenobiotics with the immune
system. These undesired events may result as a consequence of: (1) a
direct and/or indirect effect of the xenobiotic (and/or its
biotransformation product) on the immune system; or, (2) an
immunologically-based host response to the compound and/or its
metabolite(s) or host antigens modified by the compound or its
metabolites' (Berlin et al., 1987). Recommendations were made
concerning the significance to public health of immunotoxicology,
immunotoxicity testing, research and development in immunotoxicology,
the development of databases, and training and education. A subsequent
workshop on 'Immunotoxicity of Metals and Immunotoxicology', organized
by IPCS and the CEC, in collaboration with the International Union for
Pure and Applied Chemistry and German governmental agencies, was held
in Hanover, Germany, in 1989 (Dayan et al., 1990). A meeting on risk
assessment in immunotoxicology was organized by the United States
National Institute for Environmental Health Sciences in 1990. A
meeting on human immunotoxicology tests was organized by the Agency
for Toxic Substances and Disease Registry and the Centers for Disease
Control, in Atlanta, Georgia, United States of America, in 1992. In
1994, two meetings were held: one in Oxford, United Kingdom, organized
by IPCS, on risk assessment in human imunotoxicity, and one in
Washington DC, United States, organized by the International Life
Sciences Institute, on methods in immunotoxicology.
In parallel to these meetings, activities were started within
IPCS for the development and validation of methods for assessing
toxicity to the immune system. In this regard, a hallmark event was
the meeting in 1986 of a technical review and working group, in
London, United Kingdom (IPCS, 1986).
A number of tiered approaches to immunotoxicity testing have been
proposed, in rats (Vos, 1980; Van Loveren & Vos, 1989) and subsequently
in mice (Luster et al., 1988). These approaches have been evaluated
for their capacity to identify chemicals as immunosuppressive. Of a
group of 18 pesticides evaluated in rats, six were identified as
inducing immunotoxicity at doses similar to those that cause other
toxic effects, and five were immunotoxic at lower doses (Vos & Krajnc,
1983; Vos et al., 1983a). Effects were seen on different parameters
with different compounds and included lymphocytopenia, reduced thymic
and spleen weights, and increased levels of serum immunoglobulin (Ig)
G. One of the compounds identified was hexachlorobenzene (Vos, 1986),
which is further described in Section 2. In mice, the tiered approach
was used to assess the immunotoxicity of 51 chemicals, selected on the
basis of factors including structure-activity relationships with
previously identified immunotoxic substances, and use (Luster et al.,
1992). Of the spectrum of assays applied, the strongest associations
with immunotoxic potential were observed with the splenic IgM antibody
plaque-forming cell response and cell surface marker analysis; somewhat
weaker associations were found for natural killer (NK) cell activity,
cytotoxic T-lymphocyte cytolytic activity, lymphocyte proliferation
in vitro after mitogen stimulation, and thymus:body weight ratio.
The tiered approach in immunotoxicity testing is further described in
Section 3.
Multi-laboratory studies have been initiated to validate the
screening of immunotoxic compounds, including the IPCS-European Union
International Collaborative Immunotoxicity Study, a study in Fischer
344 rats, and the international study of the Bundesinstitut für
Gesundheitlichen Verbraucherschutz, und Veterinärmedizin, which were
designed to determine interlaboratory reproducibility. The
experimental animal used in these studies is the rat, and some
functional tests are included. Test methods are also being developed
and validated within the National Toxicology Program (NTP) in the
United States. This programme includes studies of carcinogenicity in
rats and mice, but because the immune system of mice is better
characterized than that of rats, the NTP chose the mouse as the
experimental animal for immunotoxicity assessment. The immunotoxicity
database of the NTP has been evaluated to determine the predictability
(sensitivity and specificity) of the assays. In the Netherlands, a
Committee for Immunotoxicology of the Dutch Health Council reviewed
methods that could be used to assess the immunotoxic properties of a
compound and for deriving information about risks to humans on the
basis of the results of laboratory experiments. The Committee also
examined the relationship between the immunotoxic properties of a
substance and its mutagenic and carcinogenic properties (Dutch Health
Council, Committee for Immunotoxicology, 1991).
The immune system was reviewed by the United States National
Research Council in order to identify the kinds of basic research that
might reveal markers of environmental exposure and disease. Major
emphasis was placed on biological markers of three types: those
originating from the immune system, those related to exposure to
immunosuppressive toxicants, and those of effects of environmental
pollutants. Markers of susceptibility to environmental materials were
also considered to be important, especially if they are of a genetic
nature and can be used to identify individuals susceptible to
autoimmune diseases. The National Research Council subcommittees on
pulmonary toxicology and on immunotoxicology, have published their
reports (US National Research Council, 1989, 1992).
Interest in immunotoxicology within the scientific community is
reflected by the existence of a special section on immunotoxicology
within the Society of Toxicology. An immunotoxicology discussion group
initiated in the United States has an international composition. The
European Union has a programme on science and technology for
environmental protection that includes immunotoxicology as an
important aspect.
There is growing concern in society about the effects of
xenobiotics, such as environmental pollutants, on public health; the
immune system is one of the targets of such effects. Some chemicals
present in the environment that have been reported to influence the
immune system are listed in Table 1 (IPCS, 1986). Immunotoxicity can
result in e.g. reduced resistance towards infection or generation of
tumours that escape immune surveillance. A number of substances
affect immunological parameters; these include halogenated
hydrocarbons such as polychlorinated biphenyls, polybrominated
biphenyls, polychlorinated dibenzo- para-dioxins, and polychlorinated
dibenzofurans (Elo et al., 1985; Lu & Wu, 1985; Bekesi et al., 1987;
Kimbrough, 1987; Hoffman 1992); pesticides and precursors (Fiore et
al., 1986; Deo et al., 1987; Nigam et al., 1993); organic solvents
(Capurro, 1980; Denkhaus et al., 1986); asbestos (Lew et al., 1986);
silica (Uber & McReynolds, 1982); and metals like lead (Ewers et al.,
1982; Reigart & Graber, 1976). Oxidant air pollutants, like sulfur
dioxide, nitrogen dioxide, and ozone, and particles in airborne dust
may affect immune function (Koren et al., 1989; Van Loveren et al.,
1994).
Immunotoxicity in humans is further discussed in Section 2. Few
epidemiological data have been published that indicate suppression or
altered resistance to infection and tumours. In general, the
usefulness of the epidemiological studies that have been published is
limited by the following: exposure is usually uncontrolled, mainly
occurring during accidents; the magnitude and pattern of exposure are
not known, and the exposure is often too low to alter the immune
system measurably; exposure is often not to one xenobiotic but to a
mixture; it is almost impossible to control for confounding
parameters, such as age, sex, genetic background, health status, and
nutritional status; and it is not always possible to define and
analyse appropriate control groups (US National Research Council,
1992). Environmental pollution and its effect on health status are
currently subjects of concern in eastern European countries and have
generated much interest in the worldwide environmental health science
community. Recent epidemiological studies have compared the possible
relationship between exposure to air pollutants and health effects in
the former German Democratic Republic and Federal Republic of Germany,
Table 1. Examples of compounds that are immunotoxic for humans or
rodents
Chemical Immune toxicity
-------------------
Rodent Human
2,3,7,8-Tetrachlorodibenzo-para-dioxin + +
Polychlorinated biphenyls + +
Polybrominated biphenyls + +
Hexachlorobenzene + Unknown
Lead + Unknown
Cadmium + Unknown
Methyl mercury compounds + Unknown
7,12-Dimethylbenz[a]anthracene + Unknown
Benzo[a]pyrene + Unknown
Di-n-octyltindichloride + Unknown
Di-n-butyltindichloride + Unknown
Benzidine + +
Nitrogen dioxide and ozone + +
Benzene, toluene, and xylene + +
Asbestos + +
N-Nitrosodimethylamine + Unknown
Diethylstilboestrol + +
Vanadium + +
From IPCS (1986)
and some of these studies included immunological data or end-points.
For instance, von Mutius et al. (1992) found a higher prevalence of
asthma among schoolchildren in western than eastern Germany, and
Behrendt et al. (1993) observed, surprisingly, that total serum IgE
levels were higher in schoolchildren in eastern than in western
Germany. Several factors were found to influence total IgE: history of
parasitic disease, number of persons per dwelling, and passive
smoking. Sex and passive smoking were the only variables that had a
significant effect in western German children. Air pollutants and
parasitic infections were suggested to be the major contributing
factors to increased IgE production in children in eastern Germany.
Remarkable differences in air quality were seen between eastern and
western Germany, and Behrendt et al. (1995) distinguished two types of
air pollution: type I, composed of sulfur dioxide particles and dust,
occurring predominantly in eastern Europe, is associated with
respiratory infections and other chronic inflammatory airway
reactions; type II, occurring both indoors and outdoors in the
environment in industrialized western countries, is composed mainly of
nitric oxide, nitrogen dioxide, ozone, volatile organic compounds, and
fine particles. The latter type of air pollution is associated with
allergic diseases and allergic sensitization, indicating that air
pollutants interfere with parameters of allergy at the level of
sensitization, elicitation of symptoms, and exacerbation of disease.
Until further epidemiological studies are conducted in humans,
assessment of immunotoxicity in rodents, with subsequent extrapolation
to the human situation, is still a good indicator of toxicity and can
serve as a basis for subsequent decisions and regulations by
authorities to reduce or prevent the risk of human exposure. This
aspect is discussed further in Section 5.
Humans are exposed to environmental contaminants mainly via food,
water, and air. Open water (e.g. rivers, lakes, and coastal areas) and
sediments often act as sinks for environmental pollution. This global
problem can be deduced from disease manifestations in fish that live
in coastal areas, especially those species that live in close contact
with contaminated silt. High levels of contaminants and the diseases
associated with them are not only of economic importance (i.e. to
fisheries) but also affect people who consume the fish, as seen in
studies showing increased levels of contaminants in people eating fish
from the contaminated Baltic Sea (Svensson et al., 1991) and in Inuit
and Indian populations in Canada who consume large quantities of fish
and marine mammals (DeWailly et al., 1992). There is now some evidence
that wildlife aquatic species have decreased resistance and enhanced
incidences of infection and tumours that may be linked to
environmental pollution (Vos et al., 1989; Wester et al., 1994).
Because the immune system of fish has not been characterized in such
detail as that of mammals, immunotoxicological studies have not been
extensively included in ecotoxicology, although a number of reports of
direct toxic actions of xenobiotics on fish species have been
published in this developing field (Wester & Canton, 1987; Payne &
Fancey, 1989; Anderson, 1990; Wester et al., 1990; Khangarot &
Tripathi, 1991; Secombes et al., 1992; Anderson & Brubacher, 1993;
Faisal & Hugget, 1993).
1.2 The immune system: functions, system regulation, and modifying
factors; histophysiology of lymphoid organs
1.2.1 Function of the immune system
In order to interpret pathological alterations of the immune
system in terms of altered function, the physiology of the system must
be understood. Since knowledge of the structure and function of the
immune system is growing rapidly, a review of this subject, focusing
on histophysiology, is presented. This section is not meant to serve
as a textbook on immunology but to provide sufficient information for
an understanding of pathological changes due to immunotoxic action.
For general textbooks on immunology, reference may be made to Sell
(1987), Klein (1990), Brostoff et al. (1991), Roitt (1991), Paul
(1993), and Roitt et al. (1993). The section covers mainly humans and
rodents, but reference is made to other species that are relevant in
immunotoxicity assessment, e.g. fish in ecotoxicology. It should be
noted that species differences can be large, despite fundamental
similarities between the immune systems of animals. It is therefore
difficult to conduct immunotoxicological studies in immunologically
less well characterized animal species, although comparative studies
that are under way may lessen the problems. Zapata and Cooper (1990)
have written a comprehensive textbook on phylogenetic aspects of
immunology. Phylogenetic data, from primitive fish to mammals, are
presented in Table 2 (Cooper, 1982; Klein, 1986; Du Pasquier, 1989;
Zapata & Cooper, 1990; Sima & Vetvicka, 1992). Relevant phylogenetic
aspects of the immune system are described below.
In mammals, the immune system and its reactions consist of a
finely tuned, complex interplay between various cell types and soluble
mediators secreted by those cells (Figure 1), some of which are listed
in Section 7.
Immune responses can be classified roughly as innate (natural and
nonspecific) and acquired (adaptive) responses, in which the reaction
is directed to a specific determinant (antigenic determinant or
epitope). The nonspecific response involves effector cells such as
macrophages (Vetvicka & Fornusek, 1992), NK cells (Herberman &
Ortaldo, 1981), granulocytes (Ross, 1992), and mediator systems
including the complement system (Tomlinson, 1993). Specificity is
based on recognition by specific receptors on lymphocytes or by
antibodies: The classical reaction to bacterial infection, resulting
in antibacterial antibody formation and antibody-mediated destruction
of the pathogen, is only one part of the intrinsic capacity of the
system. Further attributes of the system (Nossal, 1987) are summarized
below.
1.2.1.1 Encounter and recognition
The initiation of an immune response requires adequate
recognition of the pathogen. This recognition often occurs immediately
after entry, e.g. during or after passage through the epithelial
barrier of the body (skin or mucus-secreting epithelia in the
respiratory and gastrointestinal tract). The first defence includes
nonspecific inactivation, e.g. by nonspecific killer cells,
neutrophilic granulocytes, and cells of the mononuclear phagocyte
system (formerly called the reticuloendothelial system). It also
includes antigen processing and presentation to cells such as
lymphocytes of the T-helper-inducer type (Th) which can generate a
specific response.
Table 2. Evolution of immunologically important traits among vertebrates
A. Major histocompatibility complex (MHC) and transplantation
Species Graft MLR CML MHC control Serologically
rejection and/or of immune detectable
GVH response MHC antigens
Tunicata (sea squirts) + ? ? ? ?
Agnatha
Hagfish (Hyperotreti) + ? - ? ?
Lamprey (Hyperoartii) + ? - ? ?
Chondrichthyes (cartilaginous fish)
Shark, ray + ? + ? +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + ? ? ? ?
Bony fish (Teleostei) + ? + ? +
Lungfish (Dipnoi) + ? ? ? ?
Amphibia (amphibians)
Salamanders (Urodela) + ? ? ? +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + ? ? ?
Lizards, snakes (Squamata) + + + ? ?
Crocodiles, alligators (Crocodilia) + ? ? ? ?
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
B. Complement and immunoglobulins (Ig)
Species Complement Immunoglobulins
IgM IgG-like IgA IgD IgE
Tunicata (sea squirts) ? - - - - -
Agnatha
Hagfish (Hyperotreti) ? ? - - - -
Lamprey (Hyperoartii) ? ? - - - -
Chondrichthyes (cartilaginous fish)
Shark, ray + + + - - -
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + + + - - -
Bony fish (Teleostei) + + + - - -
Lungfish (Dipnoi) + + + - - -
Amphibia (amphibians)
Salamanders (Urodela) + + ? - - -
Frogs, toads (Anura) + + + + - -
Reptilia (reptiles)
Turtles (Chelonia) + + + - - -
Lizards, snakes (Squamata) + + + - - -
Crocodiles, alligators (Crocodilia) + + + ? - -
Aves (birds) + + ? + - -
Mammalia (mammals) + + + + + +
Table 2 (cont'd)
C. Leukocytes
Species Lymphocytes Plasma Macrophages
cells
Small T B
Tunicata (sea squirts) + - - - +
Agnatha
Hagfish (Hyperotreti) + - - + +
Lamprey (Hyperoartii) + - - + +
Chondrichthyes (cartilaginous fish)
Shark, ray + - ? + +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + - ? + +
Bony fish (Teleostei) + + + + +
Lungfish (Dipnoi) + ? ? + +
Amphibia (amphibians)
Salamanders (Urodela) + + + + +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + + + +
Lizards, snakes (Squamata) + + + + +
Crocodiles, alligators (Crocodilia) + + + + +
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
D. Lymphoid organs
Species Bone Thymus Spleen Lymph glands
marrow or nodes
Tunicata (sea squirts) - - - -
Agnatha
Hagfish (Hyperotreti) - - - GALT
Lamprey (Hyperoartii) - - - GALT
Chondrichthyes (cartilaginous fish)
Shark, ray - + + GALT
Osteichthyes (bony fish)
Sturgeon (Chondrostei) - + + GALT
Bony fish (Teleostei) - + + GALT
Lungfish (Dipnoi) - + + GALT
Amphibia (amphibians)
Salamanders (Urodela) + + + -
Frogs, toads (Anura) + + + ?
Reptilia (reptiles)
Turtles (Chelonia) + + + ?
Lizards, snakes (Squamata) + + + ?
Crocodiles, alligators (Crocodilia) + + + ?
Aves (birds) + + + +
Mammalia (mammals) + + + +
+, positive; ±, to be confirmed; -, negative; ?, not investigated.
MLR, mixed leukocyte response; GVH, graft-versus-host; CML, cell-mediated lympholysis;
GALT, gut-associated lymphoid tissue
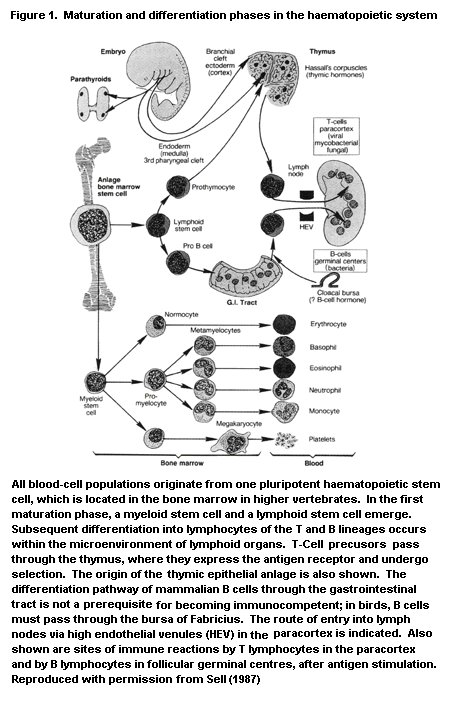 The importance of an epithelial barrier for primitive defence
mechanisms is clear in fish, in which a specific mucosal immune system
with local production of antibodies associated with mucus secretion
has been recognized. The gills are the main entry for both antigens
and pathogens living in water, and both migrating macrophages and gill
epithelial cells are involved in the process.
1.2.1.2 Specificity
The immune system can distinguish one particular determinant in
an immense spectrum of determinants. The discrimination between 'self'
and 'non-self' (i.e. the avoidance of autoreactivity) is an example of
this specificity.
Antigens can be polypeptides, carbohydrates, or lipids (lipopoly-
saccharides and lectins). A polypeptide antigen epitope is made up of
about 10 amino acids. Lymphocytes are central to antigen specificity,
as they express receptors for a single, distinct antigenic determinant
on their surface. On B lymphocytes, this antigen receptor is
essentially an antibody (immunoglobulin) molecule (Hasemann & Capra,
1989). The antigen-binding fragment of the surface receptor and the
antibodies produced after differentiation of B cells into plasma cells
have virtually identical structures -- a quaternary structure
comprising the dual heavy and light chains of the immunoglobulin
molecule (the so-called variable part of these protein chains). B-Cell
surface immunoglobulin and the immunoglobulin product of the plasma
cell progeny may differ in the constant part of the heavy chain. Like
the T-cell receptor (TCR), the B-cell receptor has a hetero-oligomeric
structure. After the antigen is bound to the surface immunoglobulin,
signal transduction occurs, in which one alpha-ß chain is linked to
the surface immunoglobulin. Biological responses involving tyrosine
phosphorylation and calcium mobilization are then induced, including
activation, tolerance, and differentiation, depending on the
differentiation stage of the B cell (Pleiman et al., 1994). On virgin
B cells, the surface receptor is an IgM or IgD molecule (with µ or
delta heavy chains, respectively). After so-called immunoglobulin
class switching (Vercelli & Geha, 1992; Harriman et al., 1993), IgG
(gamma chain), IgA (alpha chain), and IgE (epsilon chain) molecules
can be synthesized. Associated with the immunoglobulin molecule on the
cell surface is a dimeric transmembrane molecule, the Igalpha and
Igßchain, which functions in signal transduction and intracellular
activation of kinases of the src family (Pleiman et al., 1994). This
alpha-ß dimeric molecule is now used in identifying B cells.
For T cells, the antigen receptor is a heterodimeric molecule
(either the alpha-ß or the gamma-delta heterodimer), which has a
constant and a variable part, like those of immunoglobulin molecules
(Hedrick, 1989). Transmembrane signalling (tyrosine phosphorylation)
after antigen contact occurs when this heterodimer is linked on the
cell surface to the T3 (or CD3) molecule, which consists of at least
five invariant chains (Clevers et al., 1988; Chan et al., 1992).
The structural differences between the TCR for antigens and the
B-cell receptor (i.e. antibody) arise from differences in the gene
segments that encode the receptors. It is thus not surprising that
T cells recognize other determinants on the antigenic compound than
those recognized by B cells. For large antigens such as proteins,
distinct T-cell and B-cell epitopes can be identified, as illustrated
by the fact that the alpha-ß heterodimeric receptor on T cells
recognizes the antigenic determinant in the context of polymorphic
determinants of the major histocompatibility complex (MHC) antigens.
The antigenic determinant is either a small peptide, produced during
processing of antigen in the antigen-presenting cell and located in a
'groove' formed by the quaternary structure of the MHC molecule
(Adorini, 1990; Rothbard & Gefter, 1991; Germain & Margulies, 1993),
or a larger molecule associated with the MHC molecule outside the
groove. The latter is found for so-called 'superantigens', like
Staphylococcus enterotoxin A (Herman et al., 1991). Thus, with
either type of antigen, Th cells and delayed-type hypersensitivity
T cells (see below) recognize the antigenic determinant only when it
is presented together with the individual's own (self) determinant of
class II MHC. This phenomenon is called MHC class II restriction. In
contrast, T cells of the suppressor (Ts) and cytotoxic (Tc)
populations are MHC class I restricted. The processing of antigen by
antigen-presenting cells and subsequent complexing with MHC molecules
occur intracellularly, but with different pathways for MHC class
I-associated and MHC class II-associated complexing. In addition, MHC
class II-associated complexing may occur with proteins present at very
high concentrations in the extracellular environment (Neefjes &
Momburg, 1993; Engelhard, 1994). MHC restriction does not necessarily
apply to T-cell subsets within the pool expressing the alpha-ß
heterodimeric TCR (Haas et al., 1993). B Lymphocytes do not function
in an MHC restricted manner but recognize nominal antigen with their
surface immunoglobulin receptor. Therefore, recognition of antigens by
B cells and by most gamma-delta T cells, does not require antigen
presentation on cells carrying their own MHC class I or class II
determinants. The total repertoire of antigen recognition
specificities is about 107 for antibodies and somewhat less for the
TCR (about 106) (Roitt et al., 1993)
Phylogenetically, the capacity to reject an allograft is acquired
very early (e.g. sponges), and this has been interpreted as evidence
for an MHC complex. Information on the molecular features of MHC
antigens (Hughes & Nei, 1993) is available, however, only for the toad
Xenopus (Flajnik et al., 1991; Sato et al., 1993) and for fish
species (Hashimoto et al., 1990; Kasahara et al., 1992; Ono et al.,
1992; Hordvik et al., 1993). All vertebrate species produce specific
antibodies and typical cell-mediated immunity indicative of specific
responses, demonstrating that both T and B cells exist, as in birds
and mammals. Few data are available, however, because there are
virtually no reagents for lymphocyte identification and
classification. In chickens, monoclonal antibodies recognize alpha-ß
and gamma-delta TCR and a third TCR with a configuration ß-ß'; various
T-cell subsets have also been identified in this species with
appropriate antibodies (CD3, CD4, CD8; see below). MHC restriction of
antigen recognition by T cells has been demonstrated in Xenopus. In
fish, as in mammals, antigens are processed and presented by accessory
antigen-presenting cells, such as monocytes, to specific lymphocytes
in a seemingly alloantigen (presumably MHC or MHC-like) restricted
fashion (Vallejo et al., 1990; Stet & Egberts, 1991; Vallejo et al.,
1992). 'B-like' cells expressing immunoglobulins occur in all of the
fish and amphibian species that have been studied so far; however,
there is no IgD molecule in lower vertebrates. Presumably, all B-like
cells express on their surface an immunoglobulin molecule of high
relative molecular mass, similar to IgM in mammals.
All non-mammalian vertebrates produce immunoglobulins of high
relative molecular mass (Fellah et al., 1992; Wilson & Warr, 1992;
Wilson et al., 1992; Litman et al., 1993; Marchalonis et al., 1993).
There is probably also an IgG-like immunoglobulin of low relative
molecular mass that is functionally but not structurally equivalent to
mammalian IgG, but it has been observed in only a few species of bony
fish. There is also evidence for the presence of an IgA-like
immunoglobulin in some lower vertebrates. Non-mammalian vertebrates
have no IgD or IgE. The total repertoire of antigen recognition
specificities for antibodies is smaller in non-mammalian vertebrates
than in mammals.
1.2.1.3 Choice of effector reaction; diversity of the answer
After activation of Th cells, an immune response develops in
order to eliminate the antigen; in practical terms, the response
results in inactivation of the pathogen. The response is humoral
(antibody-mediated) and/or cellular (cell-mediated).
In the humoral response, Th cells together with antigen activate
specified B cells to become antibody-producing plasma cells. The
antibodies produced mediate the subsequent inactivation of the foreign
substance in a number of ways. When present in the form of immune
complexes, IgG and IgM either activate the complement system and
induce complement-mediated cytotoxicity (Hansch, 1992; Tomlinson,
1993) or activate secondary effects, which include: (i)
vasodilatation, increased vascular permeability, and attraction of
granulocytes, with subsequent release of lysosomal proteolytic enzymes
(i.e. components of acute inflammation); (ii) IgG antibody-mediated
cellular cytotoxicity, in which the antibody forms the antigen-
specific bridge between the killer cell (macrophage, binding of the Fc
fragment of IgG) and the target; (iii) IgG- and IgM-induced
opsonization and ingestion by phagocytic cells (granulocytes,
macrophages), with involvement of receptors for immunoglobulin Fc and
the complement split product C3d; (iv) binding of IgE to IgE receptors
on the surface of mast cells and basophilic granulocytes, which
induces degranulation with release of mediators after antigen binding.
Complement is phylogenetically very old, as genes that encode
complement components and complement proteins have been identified in
hagfish (Hanley et al., 1992; Ishiguro et al., 1992), and C3-like
activity exists in invertebrates and in all vertebrates. Complement C3
has been purified from all classes of vertebrates, including fish
(e.g. hagfish); in primitive fish like lampreys, it shows 30% homology
with human C3, whereas that in rodents is about 80% homologous with
that in humans (Lambris, 1993). Complement activation by immune
complexes occurs in most primitive vertebrates, but the secondary
effects emerged later in phylogeny. Antibody-mediated cellular
cytotoxicity involving NK cells has been documented in some bony fish.
Little is known about inflammatory reactions in lower vertebrates,
except for some histopathological data obtained in fish.
In the cellular response, Th cells activate precursors of Tc
cells, which subsequently kill the target after antigen-specific
recognition. Furthermore, precursors of lymphokine-producing cells
(for example, delayed-type hypersensitivity T cells) can be activated,
and the lymphokines thus secreted subsequently activate macrophages to
kill the target. Studies of Th clones in vitro have revealed the
existence of different types (Mosmann & Coffman, 1989). Th1 cells
synthesize interleukin (IL)-2, IL-3, tumour necrosis factors alpha and
ß, and interferon (IFN)gamma(cytokines are discussed below), provide
help to B cells (especially in IgG2a synthesis), activate macrophages,
and initiate delayed-type hypersensitivity reactions. Th2 cells secrete
IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, and tumour necrosis factor alpha,
providing extensive help to B cells (for IgM, IgG1, IgA, and IgE
synthesis), and activate eosinophilic granulocytes. Th2 cells do not
play a role in the initiation of delayed-type hypersensitivity. Various
cytokines are involved in the generation of these Th populations, with
dominant effects of IL-4 (Th2) and IL-12 (Th1). Cytokines produced by
each type of Th cell subpopulation appear to downregulate the activity
of the others. The choice of effector reaction is determined in part
by cooperation between the various populations of Th cells: IFN gamma
from Th1 cells can downregulate Th2 cells, while IL-10 from Th2 cells
can downregulate Th1 cells (Mossman & Coffman, 1989).
Finally, it should be noted that the immune system exerts other
types of responses that do not involve activation of Th cells. These
include T cell-independent activation of B cells, usually when the
antigens consist of repeating polysaccharide units (present on
bacteria such as Escherichia coli and Pneumococcus). They also
include direct activation of T killer cells which bear antigen-
specific receptors comprising the gamma-delta heterodimer (Bell, 1989;
De Weger et al., 1989; Raulet, 1989; Haas et al., 1993).
1.2.1.4 Immunoregulation
The effector reaction is induced by a finely tuned interplay
between cells and soluble mediators (Figure 2) (Van Deuren et al.,
1992). On the one hand, cell-cell contact is required; for instance,
between the antigen-presenting cell with (processed) antigen on its
surface and the Th cell. On the other hand, mediators (cytokines or
interleukins) influence cell function at a distance, after binding to
specific receptors on the target cell and subsequent signal
transduction, resulting in cell activation (Foxwell et al., 1992; Taga
& Kishimoto, 1992). New interleukins and their surface and soluble
receptors are being identified and characterized rapidly: at least 15
different interleukins are known at present, in addition to various
growth factors. As a reflection of their function, these factors are
designated as chemokines (attracting cells) or cytokines (influencing
cell function, such as stimulation). Factors synthesized by
lymphocytes are called lymphokines; those synthesized by monocytes or
macrophages are called monokines. Among the consequences of cytokine-
cell interactions are chemotaxis (Miller & Krangel, 1992), production
of subsequent mediators in the cascade, and down-regulation of
cellular function (Kimchi, 1992). The role of mediators in cell
adhesion to vascular endothelium is described below.
Examples of mediators that influence immune reactions positively
are IL-1 (Dinarello, 1992), which is secreted by antigen-presenting
cells and stimulates Th cells; IL-2, which is secreted by Th cells and
stimulates a variety of T cells to amplify the response; and a number
of B-cell stimulatory cytokines (Figure 2). Surface receptors for
these interleukins are present on certain immune cells (and also in
neuroendocrine tissue, mentioned below) or are expressed during
activation. For instance, IL-2 receptors and HLA-DR molecules are
expressed on T cells as part of the activation process, and their
expression is used to assess the state of cell activation (Shabtai et
al., 1991).
In down-regulation, the immune response manifests active
processes. A number of mediators, such as prostaglandin E2, IL-10, and
transforming growth factor ß (Brigham, 1989; Fontana et al., 1992;
Larrick & Wright, 1992), can suppress subsets of T and B lymphocytes.
After stimulation of Th cells in the initiation of the response,
precursors of Ts cells are activated which subsequently inhibit Th
cells from further amplifying the response, in direct cell-cell
contact or by secretion of soluble inhibitors. The existence of Ts
cells has been disputed, as part of the function of these cells is
cytotoxicity, which is exerted by the closely related MHC class I-
restricted Tc population (Bloom et al., 1992). Immunoregulatory
circuits have also been documented at the antibody level, where a
first antibody generates a second one directed to itself. The
relevance of this antibody-anti-antibody network, the so-called 'anti-
idiotype' network, remains a subject of speculation.
The importance of an epithelial barrier for primitive defence
mechanisms is clear in fish, in which a specific mucosal immune system
with local production of antibodies associated with mucus secretion
has been recognized. The gills are the main entry for both antigens
and pathogens living in water, and both migrating macrophages and gill
epithelial cells are involved in the process.
1.2.1.2 Specificity
The immune system can distinguish one particular determinant in
an immense spectrum of determinants. The discrimination between 'self'
and 'non-self' (i.e. the avoidance of autoreactivity) is an example of
this specificity.
Antigens can be polypeptides, carbohydrates, or lipids (lipopoly-
saccharides and lectins). A polypeptide antigen epitope is made up of
about 10 amino acids. Lymphocytes are central to antigen specificity,
as they express receptors for a single, distinct antigenic determinant
on their surface. On B lymphocytes, this antigen receptor is
essentially an antibody (immunoglobulin) molecule (Hasemann & Capra,
1989). The antigen-binding fragment of the surface receptor and the
antibodies produced after differentiation of B cells into plasma cells
have virtually identical structures -- a quaternary structure
comprising the dual heavy and light chains of the immunoglobulin
molecule (the so-called variable part of these protein chains). B-Cell
surface immunoglobulin and the immunoglobulin product of the plasma
cell progeny may differ in the constant part of the heavy chain. Like
the T-cell receptor (TCR), the B-cell receptor has a hetero-oligomeric
structure. After the antigen is bound to the surface immunoglobulin,
signal transduction occurs, in which one alpha-ß chain is linked to
the surface immunoglobulin. Biological responses involving tyrosine
phosphorylation and calcium mobilization are then induced, including
activation, tolerance, and differentiation, depending on the
differentiation stage of the B cell (Pleiman et al., 1994). On virgin
B cells, the surface receptor is an IgM or IgD molecule (with µ or
delta heavy chains, respectively). After so-called immunoglobulin
class switching (Vercelli & Geha, 1992; Harriman et al., 1993), IgG
(gamma chain), IgA (alpha chain), and IgE (epsilon chain) molecules
can be synthesized. Associated with the immunoglobulin molecule on the
cell surface is a dimeric transmembrane molecule, the Igalpha and
Igßchain, which functions in signal transduction and intracellular
activation of kinases of the src family (Pleiman et al., 1994). This
alpha-ß dimeric molecule is now used in identifying B cells.
For T cells, the antigen receptor is a heterodimeric molecule
(either the alpha-ß or the gamma-delta heterodimer), which has a
constant and a variable part, like those of immunoglobulin molecules
(Hedrick, 1989). Transmembrane signalling (tyrosine phosphorylation)
after antigen contact occurs when this heterodimer is linked on the
cell surface to the T3 (or CD3) molecule, which consists of at least
five invariant chains (Clevers et al., 1988; Chan et al., 1992).
The structural differences between the TCR for antigens and the
B-cell receptor (i.e. antibody) arise from differences in the gene
segments that encode the receptors. It is thus not surprising that
T cells recognize other determinants on the antigenic compound than
those recognized by B cells. For large antigens such as proteins,
distinct T-cell and B-cell epitopes can be identified, as illustrated
by the fact that the alpha-ß heterodimeric receptor on T cells
recognizes the antigenic determinant in the context of polymorphic
determinants of the major histocompatibility complex (MHC) antigens.
The antigenic determinant is either a small peptide, produced during
processing of antigen in the antigen-presenting cell and located in a
'groove' formed by the quaternary structure of the MHC molecule
(Adorini, 1990; Rothbard & Gefter, 1991; Germain & Margulies, 1993),
or a larger molecule associated with the MHC molecule outside the
groove. The latter is found for so-called 'superantigens', like
Staphylococcus enterotoxin A (Herman et al., 1991). Thus, with
either type of antigen, Th cells and delayed-type hypersensitivity
T cells (see below) recognize the antigenic determinant only when it
is presented together with the individual's own (self) determinant of
class II MHC. This phenomenon is called MHC class II restriction. In
contrast, T cells of the suppressor (Ts) and cytotoxic (Tc)
populations are MHC class I restricted. The processing of antigen by
antigen-presenting cells and subsequent complexing with MHC molecules
occur intracellularly, but with different pathways for MHC class
I-associated and MHC class II-associated complexing. In addition, MHC
class II-associated complexing may occur with proteins present at very
high concentrations in the extracellular environment (Neefjes &
Momburg, 1993; Engelhard, 1994). MHC restriction does not necessarily
apply to T-cell subsets within the pool expressing the alpha-ß
heterodimeric TCR (Haas et al., 1993). B Lymphocytes do not function
in an MHC restricted manner but recognize nominal antigen with their
surface immunoglobulin receptor. Therefore, recognition of antigens by
B cells and by most gamma-delta T cells, does not require antigen
presentation on cells carrying their own MHC class I or class II
determinants. The total repertoire of antigen recognition
specificities is about 107 for antibodies and somewhat less for the
TCR (about 106) (Roitt et al., 1993)
Phylogenetically, the capacity to reject an allograft is acquired
very early (e.g. sponges), and this has been interpreted as evidence
for an MHC complex. Information on the molecular features of MHC
antigens (Hughes & Nei, 1993) is available, however, only for the toad
Xenopus (Flajnik et al., 1991; Sato et al., 1993) and for fish
species (Hashimoto et al., 1990; Kasahara et al., 1992; Ono et al.,
1992; Hordvik et al., 1993). All vertebrate species produce specific
antibodies and typical cell-mediated immunity indicative of specific
responses, demonstrating that both T and B cells exist, as in birds
and mammals. Few data are available, however, because there are
virtually no reagents for lymphocyte identification and
classification. In chickens, monoclonal antibodies recognize alpha-ß
and gamma-delta TCR and a third TCR with a configuration ß-ß'; various
T-cell subsets have also been identified in this species with
appropriate antibodies (CD3, CD4, CD8; see below). MHC restriction of
antigen recognition by T cells has been demonstrated in Xenopus. In
fish, as in mammals, antigens are processed and presented by accessory
antigen-presenting cells, such as monocytes, to specific lymphocytes
in a seemingly alloantigen (presumably MHC or MHC-like) restricted
fashion (Vallejo et al., 1990; Stet & Egberts, 1991; Vallejo et al.,
1992). 'B-like' cells expressing immunoglobulins occur in all of the
fish and amphibian species that have been studied so far; however,
there is no IgD molecule in lower vertebrates. Presumably, all B-like
cells express on their surface an immunoglobulin molecule of high
relative molecular mass, similar to IgM in mammals.
All non-mammalian vertebrates produce immunoglobulins of high
relative molecular mass (Fellah et al., 1992; Wilson & Warr, 1992;
Wilson et al., 1992; Litman et al., 1993; Marchalonis et al., 1993).
There is probably also an IgG-like immunoglobulin of low relative
molecular mass that is functionally but not structurally equivalent to
mammalian IgG, but it has been observed in only a few species of bony
fish. There is also evidence for the presence of an IgA-like
immunoglobulin in some lower vertebrates. Non-mammalian vertebrates
have no IgD or IgE. The total repertoire of antigen recognition
specificities for antibodies is smaller in non-mammalian vertebrates
than in mammals.
1.2.1.3 Choice of effector reaction; diversity of the answer
After activation of Th cells, an immune response develops in
order to eliminate the antigen; in practical terms, the response
results in inactivation of the pathogen. The response is humoral
(antibody-mediated) and/or cellular (cell-mediated).
In the humoral response, Th cells together with antigen activate
specified B cells to become antibody-producing plasma cells. The
antibodies produced mediate the subsequent inactivation of the foreign
substance in a number of ways. When present in the form of immune
complexes, IgG and IgM either activate the complement system and
induce complement-mediated cytotoxicity (Hansch, 1992; Tomlinson,
1993) or activate secondary effects, which include: (i)
vasodilatation, increased vascular permeability, and attraction of
granulocytes, with subsequent release of lysosomal proteolytic enzymes
(i.e. components of acute inflammation); (ii) IgG antibody-mediated
cellular cytotoxicity, in which the antibody forms the antigen-
specific bridge between the killer cell (macrophage, binding of the Fc
fragment of IgG) and the target; (iii) IgG- and IgM-induced
opsonization and ingestion by phagocytic cells (granulocytes,
macrophages), with involvement of receptors for immunoglobulin Fc and
the complement split product C3d; (iv) binding of IgE to IgE receptors
on the surface of mast cells and basophilic granulocytes, which
induces degranulation with release of mediators after antigen binding.
Complement is phylogenetically very old, as genes that encode
complement components and complement proteins have been identified in
hagfish (Hanley et al., 1992; Ishiguro et al., 1992), and C3-like
activity exists in invertebrates and in all vertebrates. Complement C3
has been purified from all classes of vertebrates, including fish
(e.g. hagfish); in primitive fish like lampreys, it shows 30% homology
with human C3, whereas that in rodents is about 80% homologous with
that in humans (Lambris, 1993). Complement activation by immune
complexes occurs in most primitive vertebrates, but the secondary
effects emerged later in phylogeny. Antibody-mediated cellular
cytotoxicity involving NK cells has been documented in some bony fish.
Little is known about inflammatory reactions in lower vertebrates,
except for some histopathological data obtained in fish.
In the cellular response, Th cells activate precursors of Tc
cells, which subsequently kill the target after antigen-specific
recognition. Furthermore, precursors of lymphokine-producing cells
(for example, delayed-type hypersensitivity T cells) can be activated,
and the lymphokines thus secreted subsequently activate macrophages to
kill the target. Studies of Th clones in vitro have revealed the
existence of different types (Mosmann & Coffman, 1989). Th1 cells
synthesize interleukin (IL)-2, IL-3, tumour necrosis factors alpha and
ß, and interferon (IFN)gamma(cytokines are discussed below), provide
help to B cells (especially in IgG2a synthesis), activate macrophages,
and initiate delayed-type hypersensitivity reactions. Th2 cells secrete
IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, and tumour necrosis factor alpha,
providing extensive help to B cells (for IgM, IgG1, IgA, and IgE
synthesis), and activate eosinophilic granulocytes. Th2 cells do not
play a role in the initiation of delayed-type hypersensitivity. Various
cytokines are involved in the generation of these Th populations, with
dominant effects of IL-4 (Th2) and IL-12 (Th1). Cytokines produced by
each type of Th cell subpopulation appear to downregulate the activity
of the others. The choice of effector reaction is determined in part
by cooperation between the various populations of Th cells: IFN gamma
from Th1 cells can downregulate Th2 cells, while IL-10 from Th2 cells
can downregulate Th1 cells (Mossman & Coffman, 1989).
Finally, it should be noted that the immune system exerts other
types of responses that do not involve activation of Th cells. These
include T cell-independent activation of B cells, usually when the
antigens consist of repeating polysaccharide units (present on
bacteria such as Escherichia coli and Pneumococcus). They also
include direct activation of T killer cells which bear antigen-
specific receptors comprising the gamma-delta heterodimer (Bell, 1989;
De Weger et al., 1989; Raulet, 1989; Haas et al., 1993).
1.2.1.4 Immunoregulation
The effector reaction is induced by a finely tuned interplay
between cells and soluble mediators (Figure 2) (Van Deuren et al.,
1992). On the one hand, cell-cell contact is required; for instance,
between the antigen-presenting cell with (processed) antigen on its
surface and the Th cell. On the other hand, mediators (cytokines or
interleukins) influence cell function at a distance, after binding to
specific receptors on the target cell and subsequent signal
transduction, resulting in cell activation (Foxwell et al., 1992; Taga
& Kishimoto, 1992). New interleukins and their surface and soluble
receptors are being identified and characterized rapidly: at least 15
different interleukins are known at present, in addition to various
growth factors. As a reflection of their function, these factors are
designated as chemokines (attracting cells) or cytokines (influencing
cell function, such as stimulation). Factors synthesized by
lymphocytes are called lymphokines; those synthesized by monocytes or
macrophages are called monokines. Among the consequences of cytokine-
cell interactions are chemotaxis (Miller & Krangel, 1992), production
of subsequent mediators in the cascade, and down-regulation of
cellular function (Kimchi, 1992). The role of mediators in cell
adhesion to vascular endothelium is described below.
Examples of mediators that influence immune reactions positively
are IL-1 (Dinarello, 1992), which is secreted by antigen-presenting
cells and stimulates Th cells; IL-2, which is secreted by Th cells and
stimulates a variety of T cells to amplify the response; and a number
of B-cell stimulatory cytokines (Figure 2). Surface receptors for
these interleukins are present on certain immune cells (and also in
neuroendocrine tissue, mentioned below) or are expressed during
activation. For instance, IL-2 receptors and HLA-DR molecules are
expressed on T cells as part of the activation process, and their
expression is used to assess the state of cell activation (Shabtai et
al., 1991).
In down-regulation, the immune response manifests active
processes. A number of mediators, such as prostaglandin E2, IL-10, and
transforming growth factor ß (Brigham, 1989; Fontana et al., 1992;
Larrick & Wright, 1992), can suppress subsets of T and B lymphocytes.
After stimulation of Th cells in the initiation of the response,
precursors of Ts cells are activated which subsequently inhibit Th
cells from further amplifying the response, in direct cell-cell
contact or by secretion of soluble inhibitors. The existence of Ts
cells has been disputed, as part of the function of these cells is
cytotoxicity, which is exerted by the closely related MHC class I-
restricted Tc population (Bloom et al., 1992). Immunoregulatory
circuits have also been documented at the antibody level, where a
first antibody generates a second one directed to itself. The
relevance of this antibody-anti-antibody network, the so-called 'anti-
idiotype' network, remains a subject of speculation.

 The immune system is in a continuous state of homoeostatic
equilibrium. The introduction of an antigen (pathogen) disturbs that
balance by activating antigen-specific cell clones of T and B
lymphocyte origin. The system not only allows the proliferation and
amplification of relevant clones to cope with the antigen, but it also
searches for (and reaches) a state of newly defined homeostasis.
Little is known about the phylogenetic development of mechanisms
for regulating immune responses in vertebrates. Helper, cytotoxic, and
even suppressor lymphoid functions have been reported in ectothermic
vertebrates, but the existence of T-cell subpopulations has not been
demonstrated. T and B cells cooperate in teleost fish and in
amphibians, and there is indirect evidence that they do so in
reptiles; however, MHC restriction of these cell interactions has been
demonstrated only in Xenopus. The existence of clusters consisting
of lymphocytes, macrophages, and plasma cells has been documented in
various lower vertebrate species, which indicates the importance of
cell-cell interactions in evoking immune responses. Cytokine activity
resembling that of IL-1 and interferon has been identified in various
fish species. A T-cell growth factor was first characterized in
Xenopus. An idiotype-anti-idiotype network has been proposed to
explain the regulation of antibody production in some bony fish
(Zapata & Cooper, 1990).
1.2.1.5 Modifying factors outside the immune system
Communication with other homeostatic mechanisms in the body is an
important aspect of immunoregulation; mediators of the response have
effects not only on the internal regulatory network but also on
systems outside the immune system. Communication with the clotting and
kallikrein systems by complement components is one example;
communication with the central nervous system is another (Ader et al.,
1990). For instance, IL-1 generated by antigen-presenting cells
affects the temperature regulatory centre (induction of fever) and the
sleep regulatory centre (induction of slow-wave sleep) in the
hypothalamus (Dinarello, 1992).
One example of interaction between the immune system and the
central nervous system is the profound influence of stress on immune
reactivity (Khansari et al., 1990). Both stressful events and the way
in which individuals cope with stress are involved (Bohus et al.,
1991), as documented in studies mainly in rats but also in other
species, including fish (Faisal et al., 1989). In humans, conditions
of acute psychological stress include bereavement (Bartrop et al.,
1977), marital disruption (Kiecolt-Glaser et al., 1987), and
examination periods (Kiecolt-Glaser et al., 1986), and these can be
associated with a decrease in immune status (Kiecolt-Glaser & Glaser,
1986) which can result in increased risk for infection, including
common respiratory tract infections, e.g. influenza (Boyce et al.,
1977; Clover et al., 1989), infectious mononucleosis (Kasl et al.,
1979), and herpes virus reactivation (Glaser et al., 1985).
The hypothalamus-pituitary-adrenal axis is an important pathway
in the communication between the central nervous system and the immune
system, resulting in synthesis of glucocorticosteroid hormone by the
adrenal gland induced by adrenocorticotrophic hormone from the
pituitary gland (Buckingham et al., 1992). Other mechanisms are those
mediated by the direct action of neuropeptides, such as opioid
peptides (van den Bergh et al., 1991), on immune cells; these are
either stimulatory or down-regulatory, depending in part on
experimental design and conditions. In addition, almost all lymphoid
tissues are innervated (Bulloch, 1987; Felten et al., 1987; Kendall &
Al-Shawaf, 1991), although the role of this neuroregulatory pathway is
largely unknown (Freier, 1990). The immune system and the
neuroendocrine system have a number of biologically active mediators
in common, including cytokines and neuromediators (Fabry et al.,
1994), and are strongly interrelated (Weigent & Blalock, 1987; Sibinga
& Goldstein, 1988; Heijnen & Kavelaars, 1991; Heijnen et al., 1991;
Knight et al., 1992). Drugs that act on the central nervous system,
like some tranquillizers, antidepressants, benzodiazepines,
antiepileptics, anaesthetics, and levodopa (an antiparkinson drug),
may cause immunosuppression (Descotes, 1986). Thus, the principles of
immunotoxicology may find application in neurotoxicology and endocrine
toxicology (Snyder, 1989). Some examples of factors that modify the
immune system, based on studies of the thymus of mice during pregnancy
and of birds after hatching, are given below. Exogenous conditions may
have pivotal influences on the structure and function of the immune
system. In ectothermic vertebrates, these include seasonal changes
like temperature and photoperiod and are governed by corticosteroid
and sex hormones. In spring, during the mating period, there is low
immune reactivity, with thymic involution; in the postmating period
and the first part of summer, there is maximal development of lymphoid
tissue and immune reactivity; at the end of summer, activity declines,
and the lymphoid organs undergo pronounced involution, which persists
throughout the autumn and winter (Zapata et al., 1992). The lymphoid
system of ectothermic vertebrates therefore cannot be described in
morpho-functional terms without taking into account the season in
which studies were conducted. Seasonal variation in
immunoresponsiveness is also seen in laboratory animals (Ratajczak et
al., 1993).
1.2.1.6 Immunological memory
A special feature of the immune response is the generation of
memory after initial contact with an antigen (Gray, 1993). The first
response includes activation and amplification of antigen-specific T
or B cells to exert effector reactions; it ends with the return of
antigen-recognizing cells to the normal resting state (small
lymphocytes). The second contact with the antigen results in
recruitment of more antigen-specific cells to give a stronger signal
and more efficient elimination of the antigen or pathogen. The
reaction also occurs faster, and there is a stronger binding of
antibody to antigen. The gradual increase in the binding capacity of
antibodies during the immune response is known as 'affinity
maturation'. The memory pool of lymphocytes mediates a faster response
than the virgin (unprimed) pool.
The memory effect is most evident in the humoral arm of the
immune response. The first response gives rise only to IgM class
antibody, but antibodies of other immunoglobulin classes (especially
IgG in the internal system) are generated after subsequent contact and
immunoglobulin class switch. The antibodies thus formed may show
increased affinity as a result of somatic mutation (Kocks & Rajewski,
1989).
The cellular basis of immunological memory is largely unresolved.
It appears to be located within the T-cell population, because its
presence within the B-cell population is apparently of short duration
and is associated with the presence and stimulatory activity of the
antigen in germinal centres of secondary lymphoid tissue. The
generation of memory is the basis for vaccination, performed to
prevent contracting infectious disease by bringing about contact with
the pathogen in an attenuated or inactivated, nonpathogenic form.
It is not known whether immunological memory exists in primitive
vertebrates. The classical differentiation into primary and secondary
responses does not exist, as it is mainly immunoglobulin of high
relative molecular mass that is present, and the repertoire of
antigen-recognizing specificities is smaller than that in birds and
mammals. Some fish species may have immunological memory.
1.2.2 Histophysiology of lymphoid organs
1.2.2.1 Overview: structure of the immune system
Components of the immune system are present throughout the body
(Figure 3). The lymphocyte compartment is lodged in lymphoid organs,
from which cells can move to sites of infection or inflammation.
Phagocytic cells of the monocyte-macrophage lineage occur in lymphoid
organs and also at extranodal sites, such as Kupffer cells in the
liver, alveolar macrophages in the lung, mesangial macrophages in the
kidney, and glial cells in the brain (Figure 4). Polymorphonuclear
leukocytes are present mainly in blood and bone marrow as mature and
progenitor cells. These cells accumulate at sites of inflammation.
The lymphoid organs can be classified roughly into two types:
primary or central (antigen-independent) and secondary or peripheral
(antigen-dependent). This classification is based on the antigen-
dependence of cell proliferation and differentiation. It does not hold
for lower vertebrates, including fish. The bone marrow in higher
vertebrates is a primary organ, in which are found the pluripotent
haematopoietic stem cells which differentiate into progenitors of
myeloid cells (which in turn differentiate into granulocytes,
monocytes, erythrocytes, and platelets) and lymphoid progenitors
The immune system is in a continuous state of homoeostatic
equilibrium. The introduction of an antigen (pathogen) disturbs that
balance by activating antigen-specific cell clones of T and B
lymphocyte origin. The system not only allows the proliferation and
amplification of relevant clones to cope with the antigen, but it also
searches for (and reaches) a state of newly defined homeostasis.
Little is known about the phylogenetic development of mechanisms
for regulating immune responses in vertebrates. Helper, cytotoxic, and
even suppressor lymphoid functions have been reported in ectothermic
vertebrates, but the existence of T-cell subpopulations has not been
demonstrated. T and B cells cooperate in teleost fish and in
amphibians, and there is indirect evidence that they do so in
reptiles; however, MHC restriction of these cell interactions has been
demonstrated only in Xenopus. The existence of clusters consisting
of lymphocytes, macrophages, and plasma cells has been documented in
various lower vertebrate species, which indicates the importance of
cell-cell interactions in evoking immune responses. Cytokine activity
resembling that of IL-1 and interferon has been identified in various
fish species. A T-cell growth factor was first characterized in
Xenopus. An idiotype-anti-idiotype network has been proposed to
explain the regulation of antibody production in some bony fish
(Zapata & Cooper, 1990).
1.2.1.5 Modifying factors outside the immune system
Communication with other homeostatic mechanisms in the body is an
important aspect of immunoregulation; mediators of the response have
effects not only on the internal regulatory network but also on
systems outside the immune system. Communication with the clotting and
kallikrein systems by complement components is one example;
communication with the central nervous system is another (Ader et al.,
1990). For instance, IL-1 generated by antigen-presenting cells
affects the temperature regulatory centre (induction of fever) and the
sleep regulatory centre (induction of slow-wave sleep) in the
hypothalamus (Dinarello, 1992).
One example of interaction between the immune system and the
central nervous system is the profound influence of stress on immune
reactivity (Khansari et al., 1990). Both stressful events and the way
in which individuals cope with stress are involved (Bohus et al.,
1991), as documented in studies mainly in rats but also in other
species, including fish (Faisal et al., 1989). In humans, conditions
of acute psychological stress include bereavement (Bartrop et al.,
1977), marital disruption (Kiecolt-Glaser et al., 1987), and
examination periods (Kiecolt-Glaser et al., 1986), and these can be
associated with a decrease in immune status (Kiecolt-Glaser & Glaser,
1986) which can result in increased risk for infection, including
common respiratory tract infections, e.g. influenza (Boyce et al.,
1977; Clover et al., 1989), infectious mononucleosis (Kasl et al.,
1979), and herpes virus reactivation (Glaser et al., 1985).
The hypothalamus-pituitary-adrenal axis is an important pathway
in the communication between the central nervous system and the immune
system, resulting in synthesis of glucocorticosteroid hormone by the
adrenal gland induced by adrenocorticotrophic hormone from the
pituitary gland (Buckingham et al., 1992). Other mechanisms are those
mediated by the direct action of neuropeptides, such as opioid
peptides (van den Bergh et al., 1991), on immune cells; these are
either stimulatory or down-regulatory, depending in part on
experimental design and conditions. In addition, almost all lymphoid
tissues are innervated (Bulloch, 1987; Felten et al., 1987; Kendall &
Al-Shawaf, 1991), although the role of this neuroregulatory pathway is
largely unknown (Freier, 1990). The immune system and the
neuroendocrine system have a number of biologically active mediators
in common, including cytokines and neuromediators (Fabry et al.,
1994), and are strongly interrelated (Weigent & Blalock, 1987; Sibinga
& Goldstein, 1988; Heijnen & Kavelaars, 1991; Heijnen et al., 1991;
Knight et al., 1992). Drugs that act on the central nervous system,
like some tranquillizers, antidepressants, benzodiazepines,
antiepileptics, anaesthetics, and levodopa (an antiparkinson drug),
may cause immunosuppression (Descotes, 1986). Thus, the principles of
immunotoxicology may find application in neurotoxicology and endocrine
toxicology (Snyder, 1989). Some examples of factors that modify the
immune system, based on studies of the thymus of mice during pregnancy
and of birds after hatching, are given below. Exogenous conditions may
have pivotal influences on the structure and function of the immune
system. In ectothermic vertebrates, these include seasonal changes
like temperature and photoperiod and are governed by corticosteroid
and sex hormones. In spring, during the mating period, there is low
immune reactivity, with thymic involution; in the postmating period
and the first part of summer, there is maximal development of lymphoid
tissue and immune reactivity; at the end of summer, activity declines,
and the lymphoid organs undergo pronounced involution, which persists
throughout the autumn and winter (Zapata et al., 1992). The lymphoid
system of ectothermic vertebrates therefore cannot be described in
morpho-functional terms without taking into account the season in
which studies were conducted. Seasonal variation in
immunoresponsiveness is also seen in laboratory animals (Ratajczak et
al., 1993).
1.2.1.6 Immunological memory
A special feature of the immune response is the generation of
memory after initial contact with an antigen (Gray, 1993). The first
response includes activation and amplification of antigen-specific T
or B cells to exert effector reactions; it ends with the return of
antigen-recognizing cells to the normal resting state (small
lymphocytes). The second contact with the antigen results in
recruitment of more antigen-specific cells to give a stronger signal
and more efficient elimination of the antigen or pathogen. The
reaction also occurs faster, and there is a stronger binding of
antibody to antigen. The gradual increase in the binding capacity of
antibodies during the immune response is known as 'affinity
maturation'. The memory pool of lymphocytes mediates a faster response
than the virgin (unprimed) pool.
The memory effect is most evident in the humoral arm of the
immune response. The first response gives rise only to IgM class
antibody, but antibodies of other immunoglobulin classes (especially
IgG in the internal system) are generated after subsequent contact and
immunoglobulin class switch. The antibodies thus formed may show
increased affinity as a result of somatic mutation (Kocks & Rajewski,
1989).
The cellular basis of immunological memory is largely unresolved.
It appears to be located within the T-cell population, because its
presence within the B-cell population is apparently of short duration
and is associated with the presence and stimulatory activity of the
antigen in germinal centres of secondary lymphoid tissue. The
generation of memory is the basis for vaccination, performed to
prevent contracting infectious disease by bringing about contact with
the pathogen in an attenuated or inactivated, nonpathogenic form.
It is not known whether immunological memory exists in primitive
vertebrates. The classical differentiation into primary and secondary
responses does not exist, as it is mainly immunoglobulin of high
relative molecular mass that is present, and the repertoire of
antigen-recognizing specificities is smaller than that in birds and
mammals. Some fish species may have immunological memory.
1.2.2 Histophysiology of lymphoid organs
1.2.2.1 Overview: structure of the immune system
Components of the immune system are present throughout the body
(Figure 3). The lymphocyte compartment is lodged in lymphoid organs,
from which cells can move to sites of infection or inflammation.
Phagocytic cells of the monocyte-macrophage lineage occur in lymphoid
organs and also at extranodal sites, such as Kupffer cells in the
liver, alveolar macrophages in the lung, mesangial macrophages in the
kidney, and glial cells in the brain (Figure 4). Polymorphonuclear
leukocytes are present mainly in blood and bone marrow as mature and
progenitor cells. These cells accumulate at sites of inflammation.
The lymphoid organs can be classified roughly into two types:
primary or central (antigen-independent) and secondary or peripheral
(antigen-dependent). This classification is based on the antigen-
dependence of cell proliferation and differentiation. It does not hold
for lower vertebrates, including fish. The bone marrow in higher
vertebrates is a primary organ, in which are found the pluripotent
haematopoietic stem cells which differentiate into progenitors of
myeloid cells (which in turn differentiate into granulocytes,
monocytes, erythrocytes, and platelets) and lymphoid progenitors
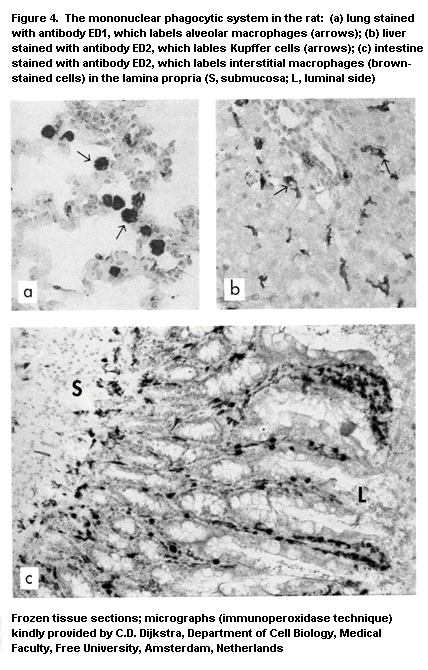 (Figure 1). The differentiation is not antigen-dependent or antigen-
driven, but this does not exclude a role for antigens in the process.
Factors secreted in the periphery during antigen-specific stimulation
can promote haematopoiesis in the bone marrow (Figure 2) (Fletcher &
Williams, 1992; Kincade, 1992; Saito 1992; Williams & Quesenberry,
1992) or inhibit haematopoietic activity (Wright & Pragnell, 1992).
The bone marrow also functions as a secondary lymphoid organ, because
terminal antigen-induced lymphoid cell differentiation can occur in
its microenvironment. For instance, the bone-marrow cells include both
the memory lymphocyte pool and the major plasma cell population, which
contributes to the intravascular pool of immunoglobulins. Normally,
plasma cell differentiation follows antigen presentation at peripheral
sites, and stimulated cells subsequently migrate to the bone marrow
for final differentiation. The secondary or peripheral lymphoid organs
in the body include the lymph nodes, spleen, and lymphoid tissue along
secretory surfaces like the gastrointestinal and respiratory tracts.
A second classification is based on the location of lymphoid
organs, divided into internal organs (some lymph nodes and the spleen,
in addition to thymus and bone marrow) and external organs (lymphoid
tissue along secretory surfaces and the lymph nodes that drain the
mucosa-associated lymphoid tissue (MALT)) (Figure 3). Lymphoid organs
at these two locations behave somewhat independently in host defence,
for instance in immunoglobulin synthesis. The main function of the
external or secretory immune system is to produce (secretory) IgA
antibody, whereas the internal immune system (mainly bone marrow)
produces IgG or IgM antibody; the major site of IgE synthesis in the
body is also along secretory surfaces. The extent of the secretory
immune system should not be underestimated: About half of the body's
lymphocytes are located in the secretory immune system, and its
capacity for immunoglobulin synthesis is about 1.5 times that of the
internal system. In all vertebrates, intraepithelial lymphocytes and
nonencapsulated lymphoid infiltrates occur in the intestine. True
lymphoid organs, such as the tonsils, Peyer's patches, and the
appendix, have been reported only in higher vertebrates. In birds, the
caecal tonsil, a blind appendix of the posterior intestine, becomes an
important peripheral lymphoid organ after involution of the bursa of
Fabricius.
Another organ that contributes to the immune system is the skin.
It does not contain organized lymphoid tissue, but immune components
in skin are interconnected with other immune organs, leading to the
concept of the skin immune system, or skin-associated lymphoid tissue
(Stingl & Steiner, 1989; Bos, 1990; Nickoloff, 1993; see section
1.2.2.7).
Immune cells and cellular products are transported between
lymphoid organs by blood and lymph vessels (Duijvestijn & Hamann,
1989). For example, Langerhans cells on their way from the skin to a
lymph node are present in lymph as 'veiled macrophages'. The blood
circulation contains only a minor part of the body's total pool of
lymphocytes (estimated at about 1%) and only a selected population,
i.e. the recirculating lymphocyte pool (Figure 5). Therefore,
assessment of only the blood lymphoid compartment does not give a
complete inventory of the body's immune system, as it ignores the
activities of the non-recirculating cells. In general terms, the blood
lymphoid compartment does not include cells that are in a state of
activation, proliferation, or differentiation; such cells are
typically tissue-bound. This rule is not absolute. For instance, in
the case of a highly activated B-cell system with hyperplasia of
germinal centres in lymph nodes, activated B cells may occur in the
circulation. These cells are normally not part of the recirculating
pool. With regard to the non-lymphoid cells of the immune system,
macrophages are tissue-bound (histiocytes), and monocytes are their
counterpart in blood. Dendritic cells, which are present in very low
proportions in blood, are the counterparts of Langerhans cells in
skin, veiled macrophages in lymph, and interdigitating dendritic cells
in lymphoid tissue. The follicular dendritic cells, i.e. the antigen-
presenting cells in follicles in lymphoid tissue (see below), do not
exist in the circulation. Within the myeloid series, the mast cell is
typically tissue-bound (Figure 6).
The endothelium lining the blood vasculature has a major role in
the passage of cells from blood to tissue parenchyma. Adhesion
molecules on circulating cells and endothelium are involved in this
process (Patarroyo, 1991; Gahmberg et al., 1992; Gumbiner & Yamada,
1992). These glycoproteins belong to three families of molecules, the
immunoglobulin supergene family, the integrins, and the selectins
(Springer, 1990; Lasky, 1992; Bevilacqua, 1993). They may be present
on endothelium in the resting state but often require alteration to
become biologically active in inflammatory processes (e.g. under the
influence of mediators like IFN gamma). In lymphoid tissue, they are
called addressins, to indicate their role in the selective homing of
passenger lymphocytes into internal or external lymphoid tissue.
The structure and histophysiology of the bone marrow, thymus,
lymph node, spleen, and MALT are presented in more detail in the
following sections. The different microenvironments of these organs
are summarized in Table 3. A detailed description of the histological
and pathological aspects of rat lymphoid tissue is given by Jones et
al. (1990).
(Figure 1). The differentiation is not antigen-dependent or antigen-
driven, but this does not exclude a role for antigens in the process.
Factors secreted in the periphery during antigen-specific stimulation
can promote haematopoiesis in the bone marrow (Figure 2) (Fletcher &
Williams, 1992; Kincade, 1992; Saito 1992; Williams & Quesenberry,
1992) or inhibit haematopoietic activity (Wright & Pragnell, 1992).
The bone marrow also functions as a secondary lymphoid organ, because
terminal antigen-induced lymphoid cell differentiation can occur in
its microenvironment. For instance, the bone-marrow cells include both
the memory lymphocyte pool and the major plasma cell population, which
contributes to the intravascular pool of immunoglobulins. Normally,
plasma cell differentiation follows antigen presentation at peripheral
sites, and stimulated cells subsequently migrate to the bone marrow
for final differentiation. The secondary or peripheral lymphoid organs
in the body include the lymph nodes, spleen, and lymphoid tissue along
secretory surfaces like the gastrointestinal and respiratory tracts.
A second classification is based on the location of lymphoid
organs, divided into internal organs (some lymph nodes and the spleen,
in addition to thymus and bone marrow) and external organs (lymphoid
tissue along secretory surfaces and the lymph nodes that drain the
mucosa-associated lymphoid tissue (MALT)) (Figure 3). Lymphoid organs
at these two locations behave somewhat independently in host defence,
for instance in immunoglobulin synthesis. The main function of the
external or secretory immune system is to produce (secretory) IgA
antibody, whereas the internal immune system (mainly bone marrow)
produces IgG or IgM antibody; the major site of IgE synthesis in the
body is also along secretory surfaces. The extent of the secretory
immune system should not be underestimated: About half of the body's
lymphocytes are located in the secretory immune system, and its
capacity for immunoglobulin synthesis is about 1.5 times that of the
internal system. In all vertebrates, intraepithelial lymphocytes and
nonencapsulated lymphoid infiltrates occur in the intestine. True
lymphoid organs, such as the tonsils, Peyer's patches, and the
appendix, have been reported only in higher vertebrates. In birds, the
caecal tonsil, a blind appendix of the posterior intestine, becomes an
important peripheral lymphoid organ after involution of the bursa of
Fabricius.
Another organ that contributes to the immune system is the skin.
It does not contain organized lymphoid tissue, but immune components
in skin are interconnected with other immune organs, leading to the
concept of the skin immune system, or skin-associated lymphoid tissue
(Stingl & Steiner, 1989; Bos, 1990; Nickoloff, 1993; see section
1.2.2.7).
Immune cells and cellular products are transported between
lymphoid organs by blood and lymph vessels (Duijvestijn & Hamann,
1989). For example, Langerhans cells on their way from the skin to a
lymph node are present in lymph as 'veiled macrophages'. The blood
circulation contains only a minor part of the body's total pool of
lymphocytes (estimated at about 1%) and only a selected population,
i.e. the recirculating lymphocyte pool (Figure 5). Therefore,
assessment of only the blood lymphoid compartment does not give a
complete inventory of the body's immune system, as it ignores the
activities of the non-recirculating cells. In general terms, the blood
lymphoid compartment does not include cells that are in a state of
activation, proliferation, or differentiation; such cells are
typically tissue-bound. This rule is not absolute. For instance, in
the case of a highly activated B-cell system with hyperplasia of
germinal centres in lymph nodes, activated B cells may occur in the
circulation. These cells are normally not part of the recirculating
pool. With regard to the non-lymphoid cells of the immune system,
macrophages are tissue-bound (histiocytes), and monocytes are their
counterpart in blood. Dendritic cells, which are present in very low
proportions in blood, are the counterparts of Langerhans cells in
skin, veiled macrophages in lymph, and interdigitating dendritic cells
in lymphoid tissue. The follicular dendritic cells, i.e. the antigen-
presenting cells in follicles in lymphoid tissue (see below), do not
exist in the circulation. Within the myeloid series, the mast cell is
typically tissue-bound (Figure 6).
The endothelium lining the blood vasculature has a major role in
the passage of cells from blood to tissue parenchyma. Adhesion
molecules on circulating cells and endothelium are involved in this
process (Patarroyo, 1991; Gahmberg et al., 1992; Gumbiner & Yamada,
1992). These glycoproteins belong to three families of molecules, the
immunoglobulin supergene family, the integrins, and the selectins
(Springer, 1990; Lasky, 1992; Bevilacqua, 1993). They may be present
on endothelium in the resting state but often require alteration to
become biologically active in inflammatory processes (e.g. under the
influence of mediators like IFN gamma). In lymphoid tissue, they are
called addressins, to indicate their role in the selective homing of
passenger lymphocytes into internal or external lymphoid tissue.
The structure and histophysiology of the bone marrow, thymus,
lymph node, spleen, and MALT are presented in more detail in the
following sections. The different microenvironments of these organs
are summarized in Table 3. A detailed description of the histological
and pathological aspects of rat lymphoid tissue is given by Jones et
al. (1990).
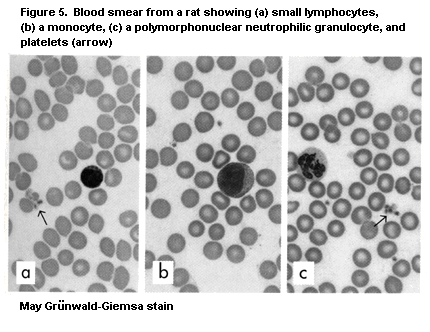 Not only conventional histological features but also the
expression of cell surface markers (immunological phenotype) are
emphasized. Monoclonal antibodies to marker substances
(differentiation antigens) on cell populations are used widely in the
identification of leukocyte populations, especially in cell
suspensions (flow cytometry) and tissue sections (immuno-
histochemistry). The wide range of monoclonal antibodies that currently
exists have been grouped according to the 'cluster of differentiation'
(CD) nomenclature, in which they are classified according to their
reactivity to the same cell marker molecule (but not the same epitope
on those molecules) (Clark & Lanier, 1989; Knapp et al., 1989;
Schlossman et al., 1994, 1995). The CD nomenclature has been adapted
for species other than man (Holmes & Morse, 1988; Jefferies, 1988;
Schuurman et al., 1992a). Some monoclonal antibodies that can be used
in the identification of leukocytes and stromal cells in tissue sections
and cell suspension are described in section 4.1.2.
Not only conventional histological features but also the
expression of cell surface markers (immunological phenotype) are
emphasized. Monoclonal antibodies to marker substances
(differentiation antigens) on cell populations are used widely in the
identification of leukocyte populations, especially in cell
suspensions (flow cytometry) and tissue sections (immuno-
histochemistry). The wide range of monoclonal antibodies that currently
exists have been grouped according to the 'cluster of differentiation'
(CD) nomenclature, in which they are classified according to their
reactivity to the same cell marker molecule (but not the same epitope
on those molecules) (Clark & Lanier, 1989; Knapp et al., 1989;
Schlossman et al., 1994, 1995). The CD nomenclature has been adapted
for species other than man (Holmes & Morse, 1988; Jefferies, 1988;
Schuurman et al., 1992a). Some monoclonal antibodies that can be used
in the identification of leukocytes and stromal cells in tissue sections
and cell suspension are described in section 4.1.2.
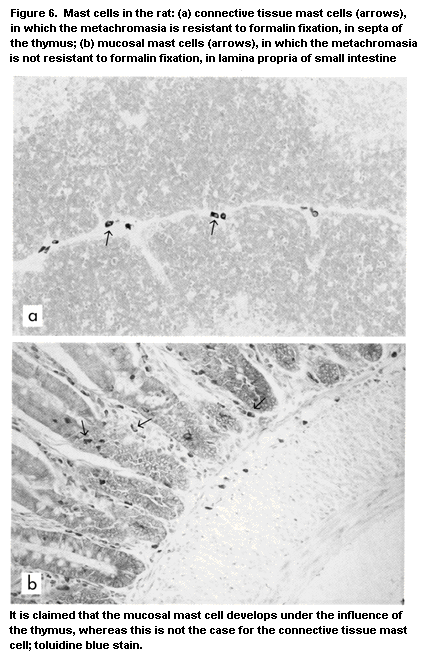 Table 3. Microenvironments in lymphoid tissue
Microenvironment Cells present Function
Bone marrow Haematopoietic cells organized as Differentiation of stem cells into cells
islands within fatty tissue, mature of the erythroid, myeloid/monocytoid,
leukocytes, plasma cells platelet and lymphoid lineage; antibody
synthesis, memory cells
Thymus
Cortex Reticular epithelium, immature Generation of T-cell competence,
T cells T-cell receptor rearrangement, positive
selection (MHC restriction), negative
selection (autoreactive cells), phenotypic
changes
Medulla Reticular epithelium, dendritic cells, Final generation of T-cell competence
T lymphocytes (negative selection), thymic hormone
synthesis, antigen presentation
Lymph node and spleen
Paracortex (lymph node), Interdigitating cells, Th and Ts cells Lymphocyte entry through high periarteriolar
lymphocyte sheath lymphoendothelial venules (lymph node) or
central arteriole (spleen), antigen
presentation to Th cells, T-cell proliferation,
differentiation, and regulation (Ts cells)
Primary follicles, follicular mantle Dendritic cells (subtype of follicular Storage of virgin and memory B cells,
of secondary follicles dendritic cells), dendritic macrophages, recirculating B cells (surface IgM+IgD+)
B cells, small number of T cells
Table 3 (cont'd)
Microenvironment Cells present Function
Germinal centre Follicular dendritic cells, dendritic T Cell-dependent B-lymphocyte
macrophages (starry-sky macrophages), differentiation, antigen presentation in the
B cells (centrocytes, centroblasts), form of immune complexes (with/without
Th cells complement C3)
Medulla (lymph node), Plasma cells, T effector cells, Termination of antigen-specific reaction:
red pulp (spleen) reticular cells, polymorphonuclear antibody synthesis and immune
granulocytes complex-mediated clearance, Tdth and
Tc cell response
Marginal zone (spleen) Marginal zone macrophages, T Cell-independent B-lymphocyte
marginal metallophilic cells, proliferation and differentiation, e.g. to
marginal zone B cells bacterial polysaccharides, B-cell memory
(surface IgM+IgD- cells)
Mucosa-associated lymphoid tissue
Epithelium covering lymphoid M (microfold) cells Transport (uptake) of exogenous
tissue (e.g. Peyer's patches) substances
Follicles and interfollicular See 'lymph node and spleen' For antibody synthesis: precursors of IgA
areas plasma cells
Mucosal epithelium Epithelial cells, Tc cells, natural First line of defence, synthesis of secretory
killer cells, gamma-delta T cells component, transport of IgA (IgM) to
lumen
Lamina propria Plasma cells, macrophages Synthesis of IgA antibody, phagocytosis
and killing
MHC, major histocompatibility complex; Th, T helper; Ts, T suppressor; Ig, immunoglobulin; Tdth, delayed-type hypersensitivity T cells;
Tc, T cytotoxic
Typing of cells in the T lymphocyte lineage is a good
illustration of immunological phenotyping. Subsets with other
functions usually cannot be identified by conventional cytology but
can be recognized by immunological phenotyping. For example, T cells
with a helper-inducer function are labelled by antibodies to CD4
antigens, and cells with a cytotoxic function are labelled by
antibodies to CD8. The reverse does not hold, however; for example,
not all CD4+ cells are helper-inducer cells, because T cells in the
separate subset effecting delayed-type hypersensitivity and some
macrophage populations are also CD4+*CD8+ T cells are in either
the cytotoxic or the suppressor subset. Thus, there is no unequivocal
relationship between CD4 or CD8 expression and cell function, because
these cell-surface molecules are expressed in relation to the way in
which antigen is recognized: T cells recognize antigen in the context
of MHC molecules -- MHC class II molecules for CD4+ cells and MHC
class I antigens for CD8+ cells (Engleman et al., 1981; Meuer et
al., 1983). This phenomenon is further discussed in sections 1.2.2.3
and 1.2.2.4.
Monoclonal antibodies have been developed to most subtypes of
leukocytes, including NK cells. The specificity of antibody 3.2.3,
anti-NKR-P1, for identifying these cells was described by Chambers et
al. (1989). Immunological phenotyping of these cells in rat spleen and
lung is illustrated in Figure 14d.
NK cells and their activities are an example of the differences
between cell function, cytology, and immunological phenotype. NK cells
are characterized by their killer function against selected targets
in vitro. Cells with cytological features that include cytoplasmic
granules, called large-granular lymphocytes, presumably have a natural
killer function, as do cells with an immunophenotype defined by
certain antibodies. The cells identified in these ways are by no means
identical and belong to different, overlapping subpopulations.
1.2.2.2 Bone marrow
The microenvironment of the bone marrow is found in all of the
hollow bones of the body and occupies the medullary space of the
skeleton (Figure 7). Blood enters the marrow from nutrient arteries at
the place where they bifurcate to form the central artery of the
medullary canal. Branches of the central artery terminate in
capillaries within the medullary space or penetrate the endosteum,
where arterial blood from the nutrient artery mixes with blood from
muscular arteries in the periosteal capillaries. The blood returns via
the vascular sinuses to the central sinus and vein. Blood cells in
various stages of development (Figure 1) intermingle with their
microenvironment, which is composed of reticular cells (adventitial
and fibroblastic), adipocytes, endothelial cells, and extracellular
matrix. These, together with passenger accessory cells (macrophages,
monocytes, and lymphocytes, especially T cells), support the
haematopoietic process (Lichtman, 1981; Weiss & Sakai, 1984; Chanarin,
1985; Weiss & Geduldig, 1991; Mayani et al., 1992).
In many mammals during ontogenesis, haematopoiesis is first
located in the yolk sac and fetal liver, until the bone marrow
develops and becomes functional. In rodents, haematopoiesis also
occurs in splenic red pulp later in life. This effect is less
pronounced in other species, but haematopoiesis can occur at other
sites in the body, e.g. the thymus. In lower vertebrates, a bone
marrow containing haematopoietic stem cells occurs firstly in
amphibians. It contains only granulopoietic and lymphoid cells;
erythropoiesis and thrombopoiesis usually occur in the blood sinusoids
of the splenic red pulp. In fish and more primitive vertebrates,
lymphoid tissue is found at many ectopic locations, such as intestine,
brain, heart, gonads, pro- and mesonephrons, and liver and is
functionally and structurally similar to the bone marrow of higher
vertebrates. In birds, the thymus is the main site of erythropoiesis
during certain periods of life (Kendall & Ward, 1974; Kendall &
Frazier, 1979).
Haematopoiesis: The pluripotent haematopoietic stem cells in
the bone marrow proliferate and differentiate to progenitors of
myeloid, erythroid, and lymphoid cells. Some further differentiation
occurs in the marrow, but the final maturation mainly occurs elsewhere
(Figure 1). The common lymphoid progenitor cell differentiates into a
T- and a B-lymphoid progenitor. Progenitor T cells then move to the
thymus for further maturation. B-Lymphoid progenitors mature via the
pre-B cell stage into virgin B cells, which then leave the
microenvironment of the bone marrow and lodge in the periphery. In
birds, this function in B-cell maturation is performed by the bursa of
Fabricius, an epithelial organ located adjacent to the termination of
the gastrointestinal tract (B stands for bursa-dependent) (Cooper,
1982; Du Pasquier, 1989; Zapata & Cooper, 1990). Although the bursa is
considered essential for the production of B lymphocytes, chickens
bursectomized early in embryonic life have B cells in their peripheral
lymphoid organs and can produce antibodies, even if their repertoire
is very limited. Thus, the bursa seems to be involved in the
generation of immunoglobulin diversity rather than just in B
lymphatopoiesis.
The development of stem cells into more mature cells requires the
presence of a microenvironment, as described above, and is regulated
by haematopoietic cytokines (also called haematopoietins) (Fletcher &
Williams, 1992; Kincade, 1992; Quesniaux, 1992; Saito, 1992; Williams
& Quesenberry, 1992; Wright & Pragnell, 1992; see also Figure 2).
Unmyelinated nerve fibres may terminate in the haematopoietic spaces
(Lichtman, 1981), and neuropeptides may play a role in the regulation
of haematopoiesis. A microgeographical distribution of the various
Table 3. Microenvironments in lymphoid tissue
Microenvironment Cells present Function
Bone marrow Haematopoietic cells organized as Differentiation of stem cells into cells
islands within fatty tissue, mature of the erythroid, myeloid/monocytoid,
leukocytes, plasma cells platelet and lymphoid lineage; antibody
synthesis, memory cells
Thymus
Cortex Reticular epithelium, immature Generation of T-cell competence,
T cells T-cell receptor rearrangement, positive
selection (MHC restriction), negative
selection (autoreactive cells), phenotypic
changes
Medulla Reticular epithelium, dendritic cells, Final generation of T-cell competence
T lymphocytes (negative selection), thymic hormone
synthesis, antigen presentation
Lymph node and spleen
Paracortex (lymph node), Interdigitating cells, Th and Ts cells Lymphocyte entry through high periarteriolar
lymphocyte sheath lymphoendothelial venules (lymph node) or
central arteriole (spleen), antigen
presentation to Th cells, T-cell proliferation,
differentiation, and regulation (Ts cells)
Primary follicles, follicular mantle Dendritic cells (subtype of follicular Storage of virgin and memory B cells,
of secondary follicles dendritic cells), dendritic macrophages, recirculating B cells (surface IgM+IgD+)
B cells, small number of T cells
Table 3 (cont'd)
Microenvironment Cells present Function
Germinal centre Follicular dendritic cells, dendritic T Cell-dependent B-lymphocyte
macrophages (starry-sky macrophages), differentiation, antigen presentation in the
B cells (centrocytes, centroblasts), form of immune complexes (with/without
Th cells complement C3)
Medulla (lymph node), Plasma cells, T effector cells, Termination of antigen-specific reaction:
red pulp (spleen) reticular cells, polymorphonuclear antibody synthesis and immune
granulocytes complex-mediated clearance, Tdth and
Tc cell response
Marginal zone (spleen) Marginal zone macrophages, T Cell-independent B-lymphocyte
marginal metallophilic cells, proliferation and differentiation, e.g. to
marginal zone B cells bacterial polysaccharides, B-cell memory
(surface IgM+IgD- cells)
Mucosa-associated lymphoid tissue
Epithelium covering lymphoid M (microfold) cells Transport (uptake) of exogenous
tissue (e.g. Peyer's patches) substances
Follicles and interfollicular See 'lymph node and spleen' For antibody synthesis: precursors of IgA
areas plasma cells
Mucosal epithelium Epithelial cells, Tc cells, natural First line of defence, synthesis of secretory
killer cells, gamma-delta T cells component, transport of IgA (IgM) to
lumen
Lamina propria Plasma cells, macrophages Synthesis of IgA antibody, phagocytosis
and killing
MHC, major histocompatibility complex; Th, T helper; Ts, T suppressor; Ig, immunoglobulin; Tdth, delayed-type hypersensitivity T cells;
Tc, T cytotoxic
Typing of cells in the T lymphocyte lineage is a good
illustration of immunological phenotyping. Subsets with other
functions usually cannot be identified by conventional cytology but
can be recognized by immunological phenotyping. For example, T cells
with a helper-inducer function are labelled by antibodies to CD4
antigens, and cells with a cytotoxic function are labelled by
antibodies to CD8. The reverse does not hold, however; for example,
not all CD4+ cells are helper-inducer cells, because T cells in the
separate subset effecting delayed-type hypersensitivity and some
macrophage populations are also CD4+*CD8+ T cells are in either
the cytotoxic or the suppressor subset. Thus, there is no unequivocal
relationship between CD4 or CD8 expression and cell function, because
these cell-surface molecules are expressed in relation to the way in
which antigen is recognized: T cells recognize antigen in the context
of MHC molecules -- MHC class II molecules for CD4+ cells and MHC
class I antigens for CD8+ cells (Engleman et al., 1981; Meuer et
al., 1983). This phenomenon is further discussed in sections 1.2.2.3
and 1.2.2.4.
Monoclonal antibodies have been developed to most subtypes of
leukocytes, including NK cells. The specificity of antibody 3.2.3,
anti-NKR-P1, for identifying these cells was described by Chambers et
al. (1989). Immunological phenotyping of these cells in rat spleen and
lung is illustrated in Figure 14d.
NK cells and their activities are an example of the differences
between cell function, cytology, and immunological phenotype. NK cells
are characterized by their killer function against selected targets
in vitro. Cells with cytological features that include cytoplasmic
granules, called large-granular lymphocytes, presumably have a natural
killer function, as do cells with an immunophenotype defined by
certain antibodies. The cells identified in these ways are by no means
identical and belong to different, overlapping subpopulations.
1.2.2.2 Bone marrow
The microenvironment of the bone marrow is found in all of the
hollow bones of the body and occupies the medullary space of the
skeleton (Figure 7). Blood enters the marrow from nutrient arteries at
the place where they bifurcate to form the central artery of the
medullary canal. Branches of the central artery terminate in
capillaries within the medullary space or penetrate the endosteum,
where arterial blood from the nutrient artery mixes with blood from
muscular arteries in the periosteal capillaries. The blood returns via
the vascular sinuses to the central sinus and vein. Blood cells in
various stages of development (Figure 1) intermingle with their
microenvironment, which is composed of reticular cells (adventitial
and fibroblastic), adipocytes, endothelial cells, and extracellular
matrix. These, together with passenger accessory cells (macrophages,
monocytes, and lymphocytes, especially T cells), support the
haematopoietic process (Lichtman, 1981; Weiss & Sakai, 1984; Chanarin,
1985; Weiss & Geduldig, 1991; Mayani et al., 1992).
In many mammals during ontogenesis, haematopoiesis is first
located in the yolk sac and fetal liver, until the bone marrow
develops and becomes functional. In rodents, haematopoiesis also
occurs in splenic red pulp later in life. This effect is less
pronounced in other species, but haematopoiesis can occur at other
sites in the body, e.g. the thymus. In lower vertebrates, a bone
marrow containing haematopoietic stem cells occurs firstly in
amphibians. It contains only granulopoietic and lymphoid cells;
erythropoiesis and thrombopoiesis usually occur in the blood sinusoids
of the splenic red pulp. In fish and more primitive vertebrates,
lymphoid tissue is found at many ectopic locations, such as intestine,
brain, heart, gonads, pro- and mesonephrons, and liver and is
functionally and structurally similar to the bone marrow of higher
vertebrates. In birds, the thymus is the main site of erythropoiesis
during certain periods of life (Kendall & Ward, 1974; Kendall &
Frazier, 1979).
Haematopoiesis: The pluripotent haematopoietic stem cells in
the bone marrow proliferate and differentiate to progenitors of
myeloid, erythroid, and lymphoid cells. Some further differentiation
occurs in the marrow, but the final maturation mainly occurs elsewhere
(Figure 1). The common lymphoid progenitor cell differentiates into a
T- and a B-lymphoid progenitor. Progenitor T cells then move to the
thymus for further maturation. B-Lymphoid progenitors mature via the
pre-B cell stage into virgin B cells, which then leave the
microenvironment of the bone marrow and lodge in the periphery. In
birds, this function in B-cell maturation is performed by the bursa of
Fabricius, an epithelial organ located adjacent to the termination of
the gastrointestinal tract (B stands for bursa-dependent) (Cooper,
1982; Du Pasquier, 1989; Zapata & Cooper, 1990). Although the bursa is
considered essential for the production of B lymphocytes, chickens
bursectomized early in embryonic life have B cells in their peripheral
lymphoid organs and can produce antibodies, even if their repertoire
is very limited. Thus, the bursa seems to be involved in the
generation of immunoglobulin diversity rather than just in B
lymphatopoiesis.
The development of stem cells into more mature cells requires the
presence of a microenvironment, as described above, and is regulated
by haematopoietic cytokines (also called haematopoietins) (Fletcher &
Williams, 1992; Kincade, 1992; Quesniaux, 1992; Saito, 1992; Williams
& Quesenberry, 1992; Wright & Pragnell, 1992; see also Figure 2).
Unmyelinated nerve fibres may terminate in the haematopoietic spaces
(Lichtman, 1981), and neuropeptides may play a role in the regulation
of haematopoiesis. A microgeographical distribution of the various
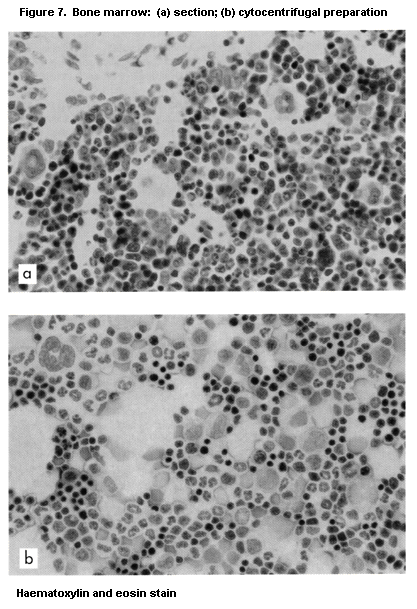 cell lineages in microenvironments has been suggested, such as the
preferential associations between fibroblasts and granulocytes,
megakaryocytes, and sinus endothelial and adventitial cells and
between macrophages and erythroid cells.
The classical bioassay for haematopoietic stem cell activity is
measurement of colony-forming units in the spleen of irradiated mice
after injection of bone-marrow cells. More differentiated progenitors
can be cultured in vitro to provide information on the specific
lineage of the colony-forming cell, to evaluate the progenitor
activity of macrophages, granulocytes, megakaryocytes, eosinophilic
granulocytes, and mast cells, as well as that of several cell
lineages, such as macrophages and granulocytes. Progenitors of
erythrocytes are assayed as early progenitors or as erythroid burst-
forming units. Studies of the mechanisms involved have revealed a
number of factors that promote the differentiation of progenitor
cells, like granulocyte-macrophage colony-stimulating factor,
macrophage colony-stimulating factor, granulocyte colony-stimulating
factor, and c-kit ligand. These mediators are produced by various cell
types, mainly after activation, and promote haematopoiesis in vivo
in conditions of e.g. infection and inflammation. Other soluble
mediators produced by T cells and by other cells involved in the
antigen-driven immune response also promote haematopoiesis; these
include IL-1, IL-3, IL-6, IL-11, interferon and tumour necrosis
factor. Haematopoiesis in the bone marrow is thus not independent of
antigenic stimulation and exposure but is not typically antigen-
driven. For instance, in the case of acute infection, the bone marrow
produces a large proportion of polymorphonuclear granulocytes within a
short time, manifested in blood as leukocytosis and a shift in the
differential count to band-type immature granulocytes. This shift may
be related to migration of T lymphocytes into the bone marrow
microenvironment, where they activate haematopoiesis. Antigen-induced
lymphoid cell differentiation also occurs in the bone marrow; for
instance, bone marrow cells include the memory lymphocyte pool and the
major plasma cell population, which contribute to the intravascular
pool of immunoglobulins.
Development and aging: The bone marrow is the primary site of
haematopoiesis throughout life, generating over 95% of the
haematopoietic activity in adult mammals (Mayani et al., 1992).
Essentially all of the medullary space in the bones is occupied by
haematopoietic tissue in mice and rats; in contrast, in adult humans,
dogs, and rabbits, haematopoietic tissue is restricted to the proximal
epiphyses of the long bones, the central skeleton, and the skull; most
bone marrow is gradually replaced by fat cells. The normal mean
proportions of bone marrow cells and changes with age in rats were
reported by Valli et al. (1990).
1.2.2.3 Thymus
The thymus is a two-lobed organ located in the mediastinum,
anterior to the major vessels of the heart (Kendall, 1991; Von
Gaudecker, 1991; Schuurman et al., 1993), although it is located in
the neck region in the guinea-pig. Its anatomical location complicates
complete thymectomy in vivo. The two independent lobes, attached to
each other only by connective tissue, consist of smaller lobules,
which have basically the same architecture, with a subcapsular and
outer cortical area, a cortex, and a medulla (Figure 8). With
conventional histological stains, the cortex is strongly stained,
owing to a dense population of small lymphocytes, and the outer cortex
and medulla show a paler colouring.
Blood vessels enter the lobules at the cortico-medullary junction
and extend radially into the cortex. Nerves course along the blood
vasculature. Fenestrated capillaries are very infrequent in the
cortex. The thymus is unique among the lymphoid organs in that its
microenvironment consists of a reticular epithelium (in birds, the
bursa of Fabricius also has an epithelial framework). Macrophages
derived from the bone marrow are found in the cortex and medulla as a
transient population. Dendritic cells in the medulla, resembling
interdigitating dendritic cells in lymph nodes, have a major function
in antigen presentation and strongly express MHC class II antigen.
The thymus appears for the first time in vertebrate phylogeny in
cartilaginous fish (sharks and rays). More primitive vertebrates lack
a thymus, although they can manifest cellular immune responses. Except
in bony fish, the thymus is histologically similar in all vertebrates,
although it is derived from distinct pharyngeal pouches in different
species. The classical cortex-medulla demarcation is not present in
fish thymus, and most thymocytes seem to occupy the central part of
the organ. In all species, epithelial cells organize a supporting
network in both cortex and medulla. Hassall's corpuscles, which are
epithelial aggregates with centrally located cell debris, occur in the
medulla of the human thymus but are scarce or absent in the rodent
thymus and in the thymus of ectothermic vertebrates. In the latter,
epithelial cysts are frequent. The thymus of lower vertebrates, in
contrast to that of mammals, contains numerous myoid cells; in avian
thymus, significant erythropoiesis has been documented (Kendall &
Ward, 1974; Kendall & Frazier, 1979), which may also occur in mammals
(Kendall, 1980; Kendall & Singh, 1980).
T-Cell maturation: selection in the thymus: T Cells reside in
the thymus during their maturation from progenitor cells to
immunocompetent T cells. This gland has a privileged function in
promoting the maturation process (Brekelmans & Van Ewijk, 1990;
Shortman et al., 1990; Van Ewijk, 1991; Boyd et al., 1992). In
congenitally athymic, 'nude' animals (Schuurman et al., 1992b,c) and
in thymic aplasia in children with complete Di George's syndrome, the
cell lineages in microenvironments has been suggested, such as the
preferential associations between fibroblasts and granulocytes,
megakaryocytes, and sinus endothelial and adventitial cells and
between macrophages and erythroid cells.
The classical bioassay for haematopoietic stem cell activity is
measurement of colony-forming units in the spleen of irradiated mice
after injection of bone-marrow cells. More differentiated progenitors
can be cultured in vitro to provide information on the specific
lineage of the colony-forming cell, to evaluate the progenitor
activity of macrophages, granulocytes, megakaryocytes, eosinophilic
granulocytes, and mast cells, as well as that of several cell
lineages, such as macrophages and granulocytes. Progenitors of
erythrocytes are assayed as early progenitors or as erythroid burst-
forming units. Studies of the mechanisms involved have revealed a
number of factors that promote the differentiation of progenitor
cells, like granulocyte-macrophage colony-stimulating factor,
macrophage colony-stimulating factor, granulocyte colony-stimulating
factor, and c-kit ligand. These mediators are produced by various cell
types, mainly after activation, and promote haematopoiesis in vivo
in conditions of e.g. infection and inflammation. Other soluble
mediators produced by T cells and by other cells involved in the
antigen-driven immune response also promote haematopoiesis; these
include IL-1, IL-3, IL-6, IL-11, interferon and tumour necrosis
factor. Haematopoiesis in the bone marrow is thus not independent of
antigenic stimulation and exposure but is not typically antigen-
driven. For instance, in the case of acute infection, the bone marrow
produces a large proportion of polymorphonuclear granulocytes within a
short time, manifested in blood as leukocytosis and a shift in the
differential count to band-type immature granulocytes. This shift may
be related to migration of T lymphocytes into the bone marrow
microenvironment, where they activate haematopoiesis. Antigen-induced
lymphoid cell differentiation also occurs in the bone marrow; for
instance, bone marrow cells include the memory lymphocyte pool and the
major plasma cell population, which contribute to the intravascular
pool of immunoglobulins.
Development and aging: The bone marrow is the primary site of
haematopoiesis throughout life, generating over 95% of the
haematopoietic activity in adult mammals (Mayani et al., 1992).
Essentially all of the medullary space in the bones is occupied by
haematopoietic tissue in mice and rats; in contrast, in adult humans,
dogs, and rabbits, haematopoietic tissue is restricted to the proximal
epiphyses of the long bones, the central skeleton, and the skull; most
bone marrow is gradually replaced by fat cells. The normal mean
proportions of bone marrow cells and changes with age in rats were
reported by Valli et al. (1990).
1.2.2.3 Thymus
The thymus is a two-lobed organ located in the mediastinum,
anterior to the major vessels of the heart (Kendall, 1991; Von
Gaudecker, 1991; Schuurman et al., 1993), although it is located in
the neck region in the guinea-pig. Its anatomical location complicates
complete thymectomy in vivo. The two independent lobes, attached to
each other only by connective tissue, consist of smaller lobules,
which have basically the same architecture, with a subcapsular and
outer cortical area, a cortex, and a medulla (Figure 8). With
conventional histological stains, the cortex is strongly stained,
owing to a dense population of small lymphocytes, and the outer cortex
and medulla show a paler colouring.
Blood vessels enter the lobules at the cortico-medullary junction
and extend radially into the cortex. Nerves course along the blood
vasculature. Fenestrated capillaries are very infrequent in the
cortex. The thymus is unique among the lymphoid organs in that its
microenvironment consists of a reticular epithelium (in birds, the
bursa of Fabricius also has an epithelial framework). Macrophages
derived from the bone marrow are found in the cortex and medulla as a
transient population. Dendritic cells in the medulla, resembling
interdigitating dendritic cells in lymph nodes, have a major function
in antigen presentation and strongly express MHC class II antigen.
The thymus appears for the first time in vertebrate phylogeny in
cartilaginous fish (sharks and rays). More primitive vertebrates lack
a thymus, although they can manifest cellular immune responses. Except
in bony fish, the thymus is histologically similar in all vertebrates,
although it is derived from distinct pharyngeal pouches in different
species. The classical cortex-medulla demarcation is not present in
fish thymus, and most thymocytes seem to occupy the central part of
the organ. In all species, epithelial cells organize a supporting
network in both cortex and medulla. Hassall's corpuscles, which are
epithelial aggregates with centrally located cell debris, occur in the
medulla of the human thymus but are scarce or absent in the rodent
thymus and in the thymus of ectothermic vertebrates. In the latter,
epithelial cysts are frequent. The thymus of lower vertebrates, in
contrast to that of mammals, contains numerous myoid cells; in avian
thymus, significant erythropoiesis has been documented (Kendall &
Ward, 1974; Kendall & Frazier, 1979), which may also occur in mammals
(Kendall, 1980; Kendall & Singh, 1980).
T-Cell maturation: selection in the thymus: T Cells reside in
the thymus during their maturation from progenitor cells to
immunocompetent T cells. This gland has a privileged function in
promoting the maturation process (Brekelmans & Van Ewijk, 1990;
Shortman et al., 1990; Van Ewijk, 1991; Boyd et al., 1992). In
congenitally athymic, 'nude' animals (Schuurman et al., 1992b,c) and
in thymic aplasia in children with complete Di George's syndrome, the
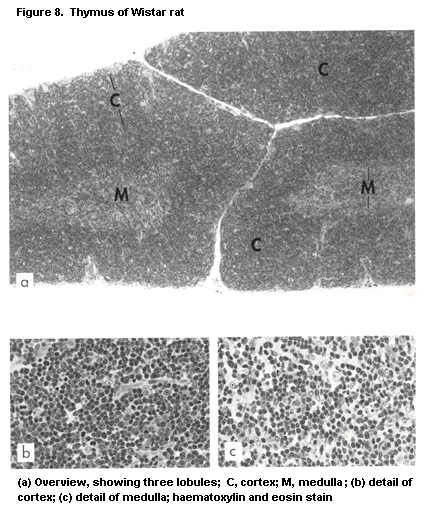 absence of a functionally active T-cell system is causally related to
the aplasia of the organ. The process of T-cell maturation includes a
number of steps located in different microenvironments (Schuurman et
al., 1993): The least mature cells, which enter the lobules from the
bloodstream at the cortico-medullary junction, first move to the outer
subcapsular cortex, where they appear as large lymphoblasts. They then
pass through the cortex, where they become small lymphocytes with a
scanty cytoplasm. Finally, they move to the medulla, where they appear
as medium-sized lymphocytes. These translocational stages in
development are monitored on the basis of the immunological phenotype.
For the CD4-CD8 phenotype, the cells change from CD-CD8- (so-called
double-negative) at a very immature stage, then change to a CD4-CD8+
stage and into a CD4+CD8+ (double-positive) phenotype, which is
found on almost all lymphocytes in the cortex. In the medulla, T cells
have the phenotype of mature cells, with distinct CD4+CD8- (about
two-thirds) and CD4-CD8+ (about one-third) populations.
This phenotypic change is accompanied by a crucial aspect of
intrathymic T-cell maturation: genesis of the TCR, which consists of
the alpha-ß heterodimer. Initially, the DNA genomic organization that
encodes these chains is in germline configuration, with a variety of
gene segments encoding the variable part of the receptor molecule.
Before transcription and translation into TCR becomes possible,
combinations have to be made of the gene segments that encode the
variable and constant parts of the TCR. This process of gene
rearrangement requires the thymic microenvironment, and only after it
is completed can the cell synthesize the receptor. The receptor is
then expressed on the cell membrane together with the CD3 molecule,
which may act as the transmembrane signal transducing molecule after
TCR stimulation; the CD3 molecule is already present in the cytoplasm
of the cell even before the TCR has been synthesized. T Cells at this
stage of maturation can be recognized by cytoplasmic staining with CD3
reagents.
The TCR gene rearrangement is similar to the rearrangement of
genes that encode immunoglobulin heavy and light chains, which takes
place in the bone-marrow microenvironment. Once TCR has been expressed
at the surface, however, the cell undergoes a process unique to T
cells, namely, specific selection on the basis of recognition
specificity (Blackmann et al., 1990; Sprent et al., 1990; Von Boehmer,
1990). First, the cell is examined for its capacity to recognize an
antigen in the context of its own MHC (self-restriction); then it is
allowed to expand (positive selection). Second, the cell is examined
for its capacity to recognize a self-antigen (autoreactivity). If it
recognizes a self-antigen, it is blocked from further differentiation
(negative selection). In this way, the random pool of antigen
recognition specificities of T cells is adapted to the host's
situation; the total repertoire of the alpha-ß T-cell population
(estimated at 1012 different epitopes) changes into the potentially
available repertoire (estimated at recognition of about 106
epitopes). Current theories of negative selection state that this step
is not feasible for all putative autoantigens in the body. Rather, it
applies to a selection of potentially harmful specificities (in
particular MHC antigens). If a cell is not selected during positive or
negative selection, it dies, possibly by suicide or apoptosis
(McDonald & Lees, 1990). A hallmark of apoptosis is endonuclease-
induced chromosomal fragmentation into 200 base-pair fragments
(McConkey et al., 1990). Histologically, apoptosis is recognized by
the presence of condensed, sometimes fragmented nuclei, which can be
found in phagocytic macrophages ('tingible body' or 'starry-sky'
macrophages) (Kendall, 1991).
Function of the microenvironment: It is generally accepted that
the epithelial microenvironment of the thymic cortex plays a major
role in positive selection. This microenvironment expresses MHC class
I and class II products and shows close interactions with lymphocytes
morphologically (at the electron microscopic level). This close
interaction is reflected in the complete inclusion of lymphocytes
inside the epithelial cytoplasm ('thymic nurse cells') (Van Ewijk,
1988). Negative selection has been ascribed to either the epithelial
compartment or the medullary dendritic cells. The different processes
occurring in early (cortical) and late (medullary) maturation are
associated with differences in the microenvironment. Epithelial cells
in the cortex and medulla differ in antigen expression,
ultrastructural characteristics, and their capacity to synthesize
thymic hormones such as thymulin, thymic humoral factor, thymosin, and
thymopoietin. These hormones have a major function late in intrathymic
T-cell maturation, and the major site of thymic hormone synthesis is
the medullary epithelium (Dabrowski & Dabrowski-Bernstein, 1990).
The cortex can be considered a primary lymphoid organ because it
is an antigen-free microenvironment with a blood-thymus barrier. In
contrast, antigens can move relatively freely into the medulla and
encounter antigen-presenting dendritic cells as well as antigen-
reactive T cells. Thus the medulla has the properties of a secondary
lymphoid organ (Van Ewijk, 1988; Kendall, 1991).
Ontogeny, growth, and involution: The thymus in rodents reaches
full development at around day 17 of gestation, that is about five
days before birth. In humans, a fully developed thymus is first seen
at the 16th to 17th week of gestation (Von Gaudecker, 1986), which is
relatively earlier in the gestation period than in rodents, since the
human immune system is more mature at birth than that of rodents.
Nevertheless, a thymus that appears histologically to be fully
developed may be functionally somewhat immature. For instance, fetal
thymus from humans (McCune et al., 1988; Namikawa et al., 1990) or
rats (De Heer et al., 1993) can be transplanted into mice with the
severe combined immunodeficiency (scid) mutation and grow for longer
than tissue obtained postnatally (see also section 4.5.3). After
birth, when the individual first comes into contact with exogenous
antigens, the thymus is called upon to provide large numbers of T
cells to the periphery, and the organ grows in a relatively short time
-- in humans within three to four weeks, from about 15 to 50 g. In
rats, the organ reaches it largest relative size about one week after
birth; the absolute weight is greatest at about six to eight weeks of
age. After adulthood is reached, the thymus starts to involute
(Steinmann, 1986; Kuper et al., 1990a; Schuurman et al., 1991a; Kuper
et al., 1992a), a process that may be related to changes in the
hormonal status of the individual; circulating thymic hormone is
reduced to very low levels in adults. The underlying mechanisms are
not fully understood. The consequences of age-associated involution
are obvious: emigration of lymphocytes from the thymus decreases
dramatically, from, for instance, 1.6 × 106/day in one-month-old
mice to 4 × 104/day in one-year-old mice (Stutman, 1986; Shortman et
al., 1990). Apparently, the persistent generation of a new antigen-
recognition repertoire in the T-cell population of adults is not
needed. Instead, the body can defend itself using the established
repertoire and extrathymic self-renewal of the T cells. Similar
processes may occur after artificial involution of the thymus caused
by toxic compounds or acute stress (including acute disease); this
aspect is further discussed below.
It should be emphasized that the basic architecture of the thymus
is not a fixed histological entity; its features depend on the age and
the stress hormone status of the individual. A 'normal' architecture
can be expected only between the late gestational period and young
adulthood, before the start of age-associated involution. This
phenomenon has important implications for the selection of rodents
according to age for studies of immune function and in the
interpretation of studies of immunotoxicity.
In mice, severe but reversible changes occur in the thymus during
pregnancy (Clarke, 1984; Clarke & Kendall, 1989; see also Figure 9).
The weight of the thymus shows an initial small rise in early
pregnancy, from about 35 to 40 mg, and dramatically decreases to 15 mg
or less at day 17 of pregnancy (Clarke, 1984). This involution is
associated with severe lymphodepletion of the cortex. Cell death is
seen by the presence of apoptotic figures and phagocytosis in
macrophages and epithelial cells. Remarkably, the large lymphoblasts
in the outer cortex remain relatively unattached, and the same applies
for thymocytes in thymic nurse cells. In birds, changes with breeding
sessions have been found (Kendall & Ward, 1974; Ward & Kendall, 1975).
In a study of a wild population of adult red-billed queleas, cyclical
enlargement and regression of the thymus were documented; at the time
of mating and laying, most birds, irrespective of sex, showed an
involuted thymus; on subsequent egg incubation the thymus size
increased, with a decline in the latter half of the rearing period.
1.2.2.4 Lymph nodes
A finely branched lymph vessel system (lymphatics) is involved in
the return of interstitial fluid in tissue to the blood circulation,
with lymph nodes spaced at regular intervals (Dunn, 1954; Tilney,
1971). The major sites of lymph nodes (or groups of lymph nodes) are
shown in Figure 3; a scheme of the areas drained by distinct lymph
nodes or lymph node groups is given in Figure 10, a schematic
presentation of individual lymph node architecture is presented in
Figure 11, and the histology of a lymph node is shown in Figure 12.
The main functions of lymph nodes are to filter pathogens from the
afferent lymph and then to initialize immune reactions. The afferent
lymphatics penetrate the lymph node capsule and connect with the
subcapsular sinus, which in turn connects with the cortical and
medullary sinuses. On the basis of the lymph flow through the node,
basic units can be recognized, each of which is supplied by its own
afferent lymph vessel and which comprise part of the paracortex
(Bélisle & Sainte-Marie, 1981; Sainte-Marie et al., 1990). Afferent
and efferent blood vessels are connected to the organ at the hilus,
where the lymph leaves the node via the efferent lymphatic(s). The
efferent lymphatics drain into other lymph nodes or directly into the
thoracic duct, which enters the bloodstream, in the rat via the left
subclavian vein.
Lymph vessels and lymph nodes occur only in mammals. In some
birds and monotremes (primitive mammals), primitive lymph nodes
directly interposed in the lymph circulation have been described.
Ectothermic vertebrates do not have lymph nodes. Some small lymphoid
organs such as lymph glands and jugular bodies have been described in
some species of frogs but not in others. These organs presumably
filter antigens from both blood and lymph and have been claimed to be
phylogenetic precursors of mammalian lymph nodes. Likewise, small
lymphoid aggregates associated with the cardinal veins occur in some
reptiles.
Lymph nodes are surrounded by a connective tissue capsule. The
nodes comprise various compartments or microenvironments: (i) the
outer cortex, with follicles and interfollicular areas; (ii) the inner
cortex or paracortex; and (iii) the medulla, with medullary cords and
medullary sinuses. These compartments are easily differentiated into
sections after conventional histological staining. In the cortex,
interfollicular areas and the paracortex (T-lymphocyte area) are
differentiated from follicles by the presence of blood vessels lined
by high endothelium (high endothelial postcapillary venules, discussed
below). Follicles (B lymphocyte area) are rounded structures, located
absence of a functionally active T-cell system is causally related to
the aplasia of the organ. The process of T-cell maturation includes a
number of steps located in different microenvironments (Schuurman et
al., 1993): The least mature cells, which enter the lobules from the
bloodstream at the cortico-medullary junction, first move to the outer
subcapsular cortex, where they appear as large lymphoblasts. They then
pass through the cortex, where they become small lymphocytes with a
scanty cytoplasm. Finally, they move to the medulla, where they appear
as medium-sized lymphocytes. These translocational stages in
development are monitored on the basis of the immunological phenotype.
For the CD4-CD8 phenotype, the cells change from CD-CD8- (so-called
double-negative) at a very immature stage, then change to a CD4-CD8+
stage and into a CD4+CD8+ (double-positive) phenotype, which is
found on almost all lymphocytes in the cortex. In the medulla, T cells
have the phenotype of mature cells, with distinct CD4+CD8- (about
two-thirds) and CD4-CD8+ (about one-third) populations.
This phenotypic change is accompanied by a crucial aspect of
intrathymic T-cell maturation: genesis of the TCR, which consists of
the alpha-ß heterodimer. Initially, the DNA genomic organization that
encodes these chains is in germline configuration, with a variety of
gene segments encoding the variable part of the receptor molecule.
Before transcription and translation into TCR becomes possible,
combinations have to be made of the gene segments that encode the
variable and constant parts of the TCR. This process of gene
rearrangement requires the thymic microenvironment, and only after it
is completed can the cell synthesize the receptor. The receptor is
then expressed on the cell membrane together with the CD3 molecule,
which may act as the transmembrane signal transducing molecule after
TCR stimulation; the CD3 molecule is already present in the cytoplasm
of the cell even before the TCR has been synthesized. T Cells at this
stage of maturation can be recognized by cytoplasmic staining with CD3
reagents.
The TCR gene rearrangement is similar to the rearrangement of
genes that encode immunoglobulin heavy and light chains, which takes
place in the bone-marrow microenvironment. Once TCR has been expressed
at the surface, however, the cell undergoes a process unique to T
cells, namely, specific selection on the basis of recognition
specificity (Blackmann et al., 1990; Sprent et al., 1990; Von Boehmer,
1990). First, the cell is examined for its capacity to recognize an
antigen in the context of its own MHC (self-restriction); then it is
allowed to expand (positive selection). Second, the cell is examined
for its capacity to recognize a self-antigen (autoreactivity). If it
recognizes a self-antigen, it is blocked from further differentiation
(negative selection). In this way, the random pool of antigen
recognition specificities of T cells is adapted to the host's
situation; the total repertoire of the alpha-ß T-cell population
(estimated at 1012 different epitopes) changes into the potentially
available repertoire (estimated at recognition of about 106
epitopes). Current theories of negative selection state that this step
is not feasible for all putative autoantigens in the body. Rather, it
applies to a selection of potentially harmful specificities (in
particular MHC antigens). If a cell is not selected during positive or
negative selection, it dies, possibly by suicide or apoptosis
(McDonald & Lees, 1990). A hallmark of apoptosis is endonuclease-
induced chromosomal fragmentation into 200 base-pair fragments
(McConkey et al., 1990). Histologically, apoptosis is recognized by
the presence of condensed, sometimes fragmented nuclei, which can be
found in phagocytic macrophages ('tingible body' or 'starry-sky'
macrophages) (Kendall, 1991).
Function of the microenvironment: It is generally accepted that
the epithelial microenvironment of the thymic cortex plays a major
role in positive selection. This microenvironment expresses MHC class
I and class II products and shows close interactions with lymphocytes
morphologically (at the electron microscopic level). This close
interaction is reflected in the complete inclusion of lymphocytes
inside the epithelial cytoplasm ('thymic nurse cells') (Van Ewijk,
1988). Negative selection has been ascribed to either the epithelial
compartment or the medullary dendritic cells. The different processes
occurring in early (cortical) and late (medullary) maturation are
associated with differences in the microenvironment. Epithelial cells
in the cortex and medulla differ in antigen expression,
ultrastructural characteristics, and their capacity to synthesize
thymic hormones such as thymulin, thymic humoral factor, thymosin, and
thymopoietin. These hormones have a major function late in intrathymic
T-cell maturation, and the major site of thymic hormone synthesis is
the medullary epithelium (Dabrowski & Dabrowski-Bernstein, 1990).
The cortex can be considered a primary lymphoid organ because it
is an antigen-free microenvironment with a blood-thymus barrier. In
contrast, antigens can move relatively freely into the medulla and
encounter antigen-presenting dendritic cells as well as antigen-
reactive T cells. Thus the medulla has the properties of a secondary
lymphoid organ (Van Ewijk, 1988; Kendall, 1991).
Ontogeny, growth, and involution: The thymus in rodents reaches
full development at around day 17 of gestation, that is about five
days before birth. In humans, a fully developed thymus is first seen
at the 16th to 17th week of gestation (Von Gaudecker, 1986), which is
relatively earlier in the gestation period than in rodents, since the
human immune system is more mature at birth than that of rodents.
Nevertheless, a thymus that appears histologically to be fully
developed may be functionally somewhat immature. For instance, fetal
thymus from humans (McCune et al., 1988; Namikawa et al., 1990) or
rats (De Heer et al., 1993) can be transplanted into mice with the
severe combined immunodeficiency (scid) mutation and grow for longer
than tissue obtained postnatally (see also section 4.5.3). After
birth, when the individual first comes into contact with exogenous
antigens, the thymus is called upon to provide large numbers of T
cells to the periphery, and the organ grows in a relatively short time
-- in humans within three to four weeks, from about 15 to 50 g. In
rats, the organ reaches it largest relative size about one week after
birth; the absolute weight is greatest at about six to eight weeks of
age. After adulthood is reached, the thymus starts to involute
(Steinmann, 1986; Kuper et al., 1990a; Schuurman et al., 1991a; Kuper
et al., 1992a), a process that may be related to changes in the
hormonal status of the individual; circulating thymic hormone is
reduced to very low levels in adults. The underlying mechanisms are
not fully understood. The consequences of age-associated involution
are obvious: emigration of lymphocytes from the thymus decreases
dramatically, from, for instance, 1.6 × 106/day in one-month-old
mice to 4 × 104/day in one-year-old mice (Stutman, 1986; Shortman et
al., 1990). Apparently, the persistent generation of a new antigen-
recognition repertoire in the T-cell population of adults is not
needed. Instead, the body can defend itself using the established
repertoire and extrathymic self-renewal of the T cells. Similar
processes may occur after artificial involution of the thymus caused
by toxic compounds or acute stress (including acute disease); this
aspect is further discussed below.
It should be emphasized that the basic architecture of the thymus
is not a fixed histological entity; its features depend on the age and
the stress hormone status of the individual. A 'normal' architecture
can be expected only between the late gestational period and young
adulthood, before the start of age-associated involution. This
phenomenon has important implications for the selection of rodents
according to age for studies of immune function and in the
interpretation of studies of immunotoxicity.
In mice, severe but reversible changes occur in the thymus during
pregnancy (Clarke, 1984; Clarke & Kendall, 1989; see also Figure 9).
The weight of the thymus shows an initial small rise in early
pregnancy, from about 35 to 40 mg, and dramatically decreases to 15 mg
or less at day 17 of pregnancy (Clarke, 1984). This involution is
associated with severe lymphodepletion of the cortex. Cell death is
seen by the presence of apoptotic figures and phagocytosis in
macrophages and epithelial cells. Remarkably, the large lymphoblasts
in the outer cortex remain relatively unattached, and the same applies
for thymocytes in thymic nurse cells. In birds, changes with breeding
sessions have been found (Kendall & Ward, 1974; Ward & Kendall, 1975).
In a study of a wild population of adult red-billed queleas, cyclical
enlargement and regression of the thymus were documented; at the time
of mating and laying, most birds, irrespective of sex, showed an
involuted thymus; on subsequent egg incubation the thymus size
increased, with a decline in the latter half of the rearing period.
1.2.2.4 Lymph nodes
A finely branched lymph vessel system (lymphatics) is involved in
the return of interstitial fluid in tissue to the blood circulation,
with lymph nodes spaced at regular intervals (Dunn, 1954; Tilney,
1971). The major sites of lymph nodes (or groups of lymph nodes) are
shown in Figure 3; a scheme of the areas drained by distinct lymph
nodes or lymph node groups is given in Figure 10, a schematic
presentation of individual lymph node architecture is presented in
Figure 11, and the histology of a lymph node is shown in Figure 12.
The main functions of lymph nodes are to filter pathogens from the
afferent lymph and then to initialize immune reactions. The afferent
lymphatics penetrate the lymph node capsule and connect with the
subcapsular sinus, which in turn connects with the cortical and
medullary sinuses. On the basis of the lymph flow through the node,
basic units can be recognized, each of which is supplied by its own
afferent lymph vessel and which comprise part of the paracortex
(Bélisle & Sainte-Marie, 1981; Sainte-Marie et al., 1990). Afferent
and efferent blood vessels are connected to the organ at the hilus,
where the lymph leaves the node via the efferent lymphatic(s). The
efferent lymphatics drain into other lymph nodes or directly into the
thoracic duct, which enters the bloodstream, in the rat via the left
subclavian vein.
Lymph vessels and lymph nodes occur only in mammals. In some
birds and monotremes (primitive mammals), primitive lymph nodes
directly interposed in the lymph circulation have been described.
Ectothermic vertebrates do not have lymph nodes. Some small lymphoid
organs such as lymph glands and jugular bodies have been described in
some species of frogs but not in others. These organs presumably
filter antigens from both blood and lymph and have been claimed to be
phylogenetic precursors of mammalian lymph nodes. Likewise, small
lymphoid aggregates associated with the cardinal veins occur in some
reptiles.
Lymph nodes are surrounded by a connective tissue capsule. The
nodes comprise various compartments or microenvironments: (i) the
outer cortex, with follicles and interfollicular areas; (ii) the inner
cortex or paracortex; and (iii) the medulla, with medullary cords and
medullary sinuses. These compartments are easily differentiated into
sections after conventional histological staining. In the cortex,
interfollicular areas and the paracortex (T-lymphocyte area) are
differentiated from follicles by the presence of blood vessels lined
by high endothelium (high endothelial postcapillary venules, discussed
below). Follicles (B lymphocyte area) are rounded structures, located
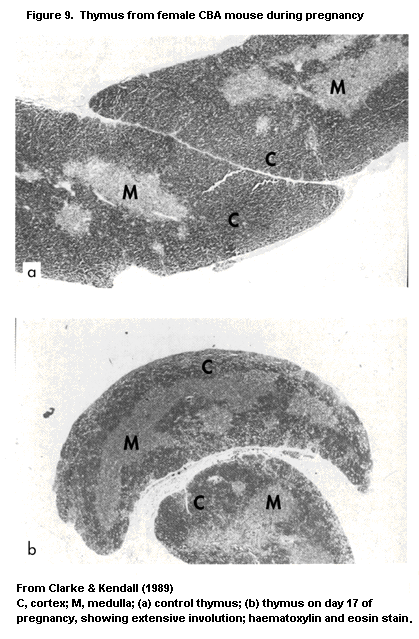
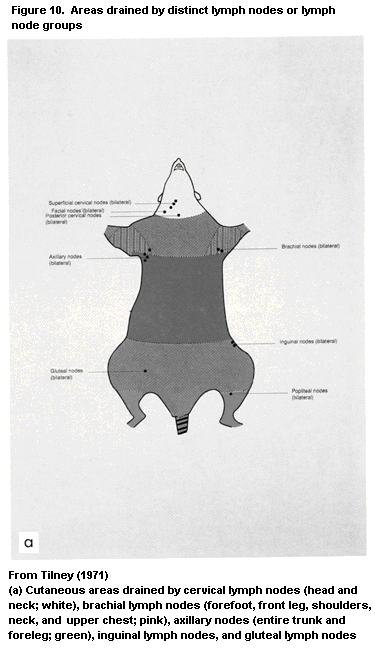


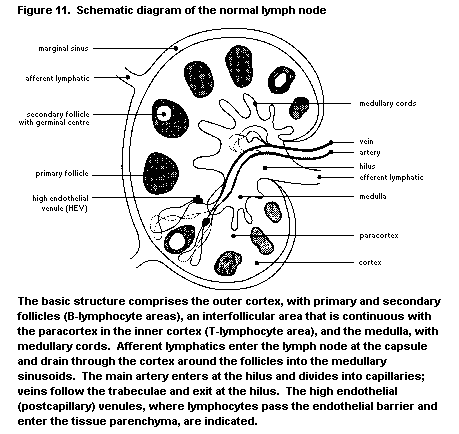
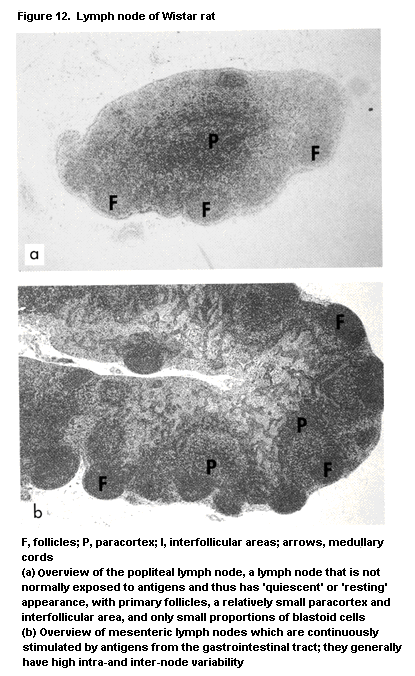

 mainly immediately underneath the capsule; they present as
accumulations of small lymphocytes (primary follicle) or a pale-
stained centre with large lymphoid cells (centrocytes, centroblasts)
and tingible-body macro-phages surrounded by a mantle with small
lymphocytes (secondary follicles). The interfollicular areas are
continuous with the paracortex, and the latter is continuous with the
medullary cords.
The arterial blood supply enters the node at the medulla and ends
in the paracortex as arteriolar capillaries, with branches in the
follicles. The capillaries feed venules that are lined with high
endothelial cells. The high endothelial venules run from the
paracortex into the medullary cords and then leave the node via the
vein in the hilus. Lymphocytes migrate through the high endothelial
venules after adhering to the endothelium by specific receptor-ligand
interactions (Picker & Butcher, 1992). The adhesion molecules on
lymphocytes and endothelium that are involved in this binding and
subsequent passage thought the endothelial layer are the addressins,
reflecting the difference in receptor-ligand interactions that exists
between lymph nodes of the internal and external lymphoid systems.
Subsequently, lymphocytes can specifically reach the various nodes,
using the same route (the blood). After the lymphocytes have migrated
into the parenchyma, they move into their microenvironment or
compartment.
Antigen encounter and immune reactivity: The main route of
access for antigens and pathogens is the afferent lymph flow; antigens
can also come into contact with the lymph node tissues via the blood.
Antigens in the lymph, either free or processed by veiled macrophages,
enter the node through the subcapsular sinus, which is rich in
macrophages that can phagocytose free antigen. From there, antigens
move to the paracortex, where they are presented to CD4+ Th cells by
the antigen-presenting cells for initiation of the immune response.
The main antigen-presenting cells in the lymph node are the
interdigitating dendritic cells, the tissue equivalents of veiled
macrophages, which can arise from Langerhans cells in the skin (for
e.g. lymph nodes draining the skin). The antigen-presenting cells
express MHC class II antigens in high density, enabling the alpha-ß
TCR of the Th cells to recognize the antigenic determinant complexed
with the polymorphic ('self') MHC class II molecule. The CD4+
molecule on the Th cell surface binds to a non-polymorphic determinant
of MHC class II molecules and strengthens the binding between Th and
antigen-presenting cells (Janeway, 1992). The cellular interaction
triggers the synthesis of cytokines like IL-1 and IL-2. This process
is down-regulated by Ts cells in a way that is not yet completely
understood.
Follicles are involved in antigen-driven B-cell activation,
somatic mutation, positive and negative selection, and memory and
plasma cell development (Szakal et al., 1989; Kroese et al., 1990; Liu
et al., 1992) and are known as primary follicles in the resting state.
They contain small, IgM+IgD+ virgin B cells in a framework of
follicular dendritic cells. During stimulation by antigens, the
follicles change into secondary follicles consisting of a germinal
centre surrounded by a mantle. Antigen may be transported into the
follicle by immune (T) cells, but this route is not yet fully
established. Antigen is presented to B cells in immune complexes, with
complement split products like C3b, which are trapped in cytoplasmic
extensions of the follicular dendritic cells. The interaction between
complement and complement receptors on these cells has a pivotal role
in the adherence of antigen to them. Fc receptors also play a role,
but only in rodents. The complement split products in the trapped
immune complexes have an accessory function in antigen presentation.
Local CD4+ Th cells assist in B-cell activation. Antigen-driven
B-cell activation and proliferation in the germinal centre are
accompanied by an isotype switch of the immunoglobulin class
synthesized by the B cell. In addition, the affinity of the antibody
increases as a result of somatic mutation (Kocks & Rajewski, 1989). A
kind of selection mechanism has been proposed in this antigen-driven
process, in which cells that produce antibody of higher affinity are
selected preferentially, and cells that produce antibody of lower
affinity are not selected and subsequently die, perhaps by apoptosis
(programmed cell death) (Liu et al., 1989). This selection resembles
that of developing T lymphocytes in the thymus; it differs from T-cell
selection by the absence of negative selection and the occurrence of
somatic mutation. Antigen may remain in the follicular compartment for
quite some time, thereby causing persistent activation of B cells,
related to the state of immunological memory within the B-cell
population. After the antigen disappears, the immunological memory in
the B cells is short-lived and disappears. B-Cell activation in
germinal centres leads to activated cells with a specific morphology,
the so-called centrocytes and centroblasts. Finally, B-cell activation
leads to the formation of plasma cells, both in the periphery of
germinal centres but more predominantly in the medullary cords of the
lymph nodes.
The main site of effector immune reactions is the medulla. The
medullary cords house macrophages, granulocytes, activated effector T
cells, and plasma cells. The effector T cells include CD4+ delayed-
type hypersensitivity T cells (mediator-producing cells) and CD8+ Tc
cells. The Tc cells bear an alpha-ß TCR that recognizes antigen in the
context of the polymorphic determinant of MHC class I molecules. The
CD8 molecule has an accessory function in this process, since it binds
a non-polymorphic class I determinant (Janeway, 1992). Antigen
recognition by Tc cells is thus different from that by Th cells. The
reaction products of the effector cells, such as lymphokines, and
effector cells like plasma cell precursors leave the lymph nodes via
the efferent lymph or blood circulation to go to other sites of the
body.
Development and aging: Lymph node morphology is dynamic: its
appearance throughout life is directly related to the type and amount
of antigenic stimulation. After antigenic contact, the organ increases
in size within a relatively short time, with high proliferative
activity of lymphocytes and germinal centre formation, depending on
the type of reaction and the choice of immunological reaction. In the
case of B-lymphocyte reactions, hyperplasia of follicles is seen (e.g.
after bacterial infection); in the case of T-cell reactions, the
interfollicular areas or paracortex become enlarged (e.g. in viral
infection). After the reaction is terminated or is transferred to the
next draining lymph node, it regains its normal small size. Germinal
centres with an interfollicular microenvironment can develop
extranodally, especially at sites of chronic inflammation.
The dynamics of the lymph node can be illustrated by several
examples. After immunization of the footpad with antigen mixed with an
adjuvant, such as Freund's complete adjuvant, containing killed
mycobacteria, the draining popliteal lymph node becomes enlarged (in
rats, from about 5 mg to more than 100 mg), and granulomatous
reactions (epitheloid-cell granuloma) can be seen histologically. The
swelling of lymph nodes is used to assess reactivity towards chemicals
and in the evaluation of immunomodulatory drugs (Gleichmann et al.,
1989). In the popliteal lymph node assay, a test substance is injected
subcutaneously into one footpad and the contralateral side is left
untreated or injected with vehicle only. The effect of the substance
is subsequently estimated from the difference in weight between the
popliteal lymph nodes. Further evidence for immunostimulatory activity
in vivo is obtained by histological appearance, often manifested as
follicular hyperplasia.
Lymph nodes develop relatively late in fetal life: At birth, the
anlage of unstimulated lymph nodes is present, containing few lymph
cells. The lymph nodes develop quickly after exposure to many new
(exogenous) antigens. In adults, they may become relatively quiescent
and small, with virgin T and B cells and primary follicles. The lymph
nodes in aged rats are capable of the same degree of activity as those
in young individuals upon antigenic stimuli. The state and type of
activation in the various nodes of adult and aged animals differ under
normal housing conditions and are a reflection of the absence or
existence of continuous local stimulation with antigens or disease
processes, like inflammation and the presence of a tumour in the
drained area (Ward, 1990). The central nodes of the mandibular and
superficial cervical group, which are continuously exposed to
(aero)antigens via the oronasopharynx, may contain a considerable
number of plasma cells and precursors in the medullary cords and
relatively well-developed germinal centres. Sinal histiocytosis (that
is, considerable numbers of macrophages in the sinuses) and
accumulations of pigmented macrophages are often present in the
mesenteric lymph nodes, which are continuously exposed to antigens via
the digestive tract. An extensive review of lymph node development and
aging is given by Losco & Harleman (1992).
1.2.2.5 Spleen
The spleen consists of two main compartments: the red and white
pulp (Van Rooijen et al., 1989; Dijkstra & Sminia, 1990; Laman et al.,
1992; Van den Eertwegh et al., 1992). A schematic drawing of the
spleen is presented in Figure 13 and histological views in Figure 14.
The red pulp consists of blood-filled sinusoids and Bilroth's cords
containing macrophages, lymphocytes, plasma cells, and NK cells.
Macrophages perform major functions in clearing blood cells (for
instance, old red blood cells) and in phagocytosis, especially of non-
opsonized particles. This high-volume filter function is made possible
by two factors: the direct contact, unobstructed by blood-vessel
walls, between phagocytic cells and blood-borne particles; and the
large blood supply, estimated at about 5% of the total blood volume
per minute. There are no lymphatics in the spleen.
The phagocytic function is especially important in the case of
intravascular pathogenic microorganisms, before antibody formation and
subsequent opsonization occur (early bacterial septicaemia). The
mononuclear phagocyte system of the liver (Kupffer cells) plays a
major role in the removal of opsonized particles. Together with the
hepatic phagocytic system, splenic macrophages synthesize complement
components, although this is done mainly by hepatocytes. In rats and
mice, the red pulp contains nests of (extramedullary) haematopoiesis,
characterized histologically by megakaryocytes and normoblasts. In the
case of systemic septicaemia, when pathogenic microorganisms have
reached the blood either directly or after inadequate filtering
through lymph nodes, the red pulp increases and contains large
proportions of (immature) granulocytes. Differentiation between
septicaemia and extramedullary haematopoiesis is not always easy; the
decreased or absent white pulp in septicaemia can be helpful in making
this differentiation.
Phylogenetically, cartilaginous fish are the first species that
have a spleen, which consists of lymphoid follicles and a red pulp
that generally houses developing erythroid cells (Zapata & Cooper,
1990). The lympho-haematopoietic masses of the intestine that are seen
in some lower vertebrates (e.g. lampreys) are not primitive spleens
but rather primitive lymphohaematopoietic organs equivalent to
mammalian bone marrow. In most bony fishes, the white pulp is poorly
developed, probably reflecting the existence of other peripheral
lymphoid organs, e.g. the kidney, which participate actively in the
mainly immediately underneath the capsule; they present as
accumulations of small lymphocytes (primary follicle) or a pale-
stained centre with large lymphoid cells (centrocytes, centroblasts)
and tingible-body macro-phages surrounded by a mantle with small
lymphocytes (secondary follicles). The interfollicular areas are
continuous with the paracortex, and the latter is continuous with the
medullary cords.
The arterial blood supply enters the node at the medulla and ends
in the paracortex as arteriolar capillaries, with branches in the
follicles. The capillaries feed venules that are lined with high
endothelial cells. The high endothelial venules run from the
paracortex into the medullary cords and then leave the node via the
vein in the hilus. Lymphocytes migrate through the high endothelial
venules after adhering to the endothelium by specific receptor-ligand
interactions (Picker & Butcher, 1992). The adhesion molecules on
lymphocytes and endothelium that are involved in this binding and
subsequent passage thought the endothelial layer are the addressins,
reflecting the difference in receptor-ligand interactions that exists
between lymph nodes of the internal and external lymphoid systems.
Subsequently, lymphocytes can specifically reach the various nodes,
using the same route (the blood). After the lymphocytes have migrated
into the parenchyma, they move into their microenvironment or
compartment.
Antigen encounter and immune reactivity: The main route of
access for antigens and pathogens is the afferent lymph flow; antigens
can also come into contact with the lymph node tissues via the blood.
Antigens in the lymph, either free or processed by veiled macrophages,
enter the node through the subcapsular sinus, which is rich in
macrophages that can phagocytose free antigen. From there, antigens
move to the paracortex, where they are presented to CD4+ Th cells by
the antigen-presenting cells for initiation of the immune response.
The main antigen-presenting cells in the lymph node are the
interdigitating dendritic cells, the tissue equivalents of veiled
macrophages, which can arise from Langerhans cells in the skin (for
e.g. lymph nodes draining the skin). The antigen-presenting cells
express MHC class II antigens in high density, enabling the alpha-ß
TCR of the Th cells to recognize the antigenic determinant complexed
with the polymorphic ('self') MHC class II molecule. The CD4+
molecule on the Th cell surface binds to a non-polymorphic determinant
of MHC class II molecules and strengthens the binding between Th and
antigen-presenting cells (Janeway, 1992). The cellular interaction
triggers the synthesis of cytokines like IL-1 and IL-2. This process
is down-regulated by Ts cells in a way that is not yet completely
understood.
Follicles are involved in antigen-driven B-cell activation,
somatic mutation, positive and negative selection, and memory and
plasma cell development (Szakal et al., 1989; Kroese et al., 1990; Liu
et al., 1992) and are known as primary follicles in the resting state.
They contain small, IgM+IgD+ virgin B cells in a framework of
follicular dendritic cells. During stimulation by antigens, the
follicles change into secondary follicles consisting of a germinal
centre surrounded by a mantle. Antigen may be transported into the
follicle by immune (T) cells, but this route is not yet fully
established. Antigen is presented to B cells in immune complexes, with
complement split products like C3b, which are trapped in cytoplasmic
extensions of the follicular dendritic cells. The interaction between
complement and complement receptors on these cells has a pivotal role
in the adherence of antigen to them. Fc receptors also play a role,
but only in rodents. The complement split products in the trapped
immune complexes have an accessory function in antigen presentation.
Local CD4+ Th cells assist in B-cell activation. Antigen-driven
B-cell activation and proliferation in the germinal centre are
accompanied by an isotype switch of the immunoglobulin class
synthesized by the B cell. In addition, the affinity of the antibody
increases as a result of somatic mutation (Kocks & Rajewski, 1989). A
kind of selection mechanism has been proposed in this antigen-driven
process, in which cells that produce antibody of higher affinity are
selected preferentially, and cells that produce antibody of lower
affinity are not selected and subsequently die, perhaps by apoptosis
(programmed cell death) (Liu et al., 1989). This selection resembles
that of developing T lymphocytes in the thymus; it differs from T-cell
selection by the absence of negative selection and the occurrence of
somatic mutation. Antigen may remain in the follicular compartment for
quite some time, thereby causing persistent activation of B cells,
related to the state of immunological memory within the B-cell
population. After the antigen disappears, the immunological memory in
the B cells is short-lived and disappears. B-Cell activation in
germinal centres leads to activated cells with a specific morphology,
the so-called centrocytes and centroblasts. Finally, B-cell activation
leads to the formation of plasma cells, both in the periphery of
germinal centres but more predominantly in the medullary cords of the
lymph nodes.
The main site of effector immune reactions is the medulla. The
medullary cords house macrophages, granulocytes, activated effector T
cells, and plasma cells. The effector T cells include CD4+ delayed-
type hypersensitivity T cells (mediator-producing cells) and CD8+ Tc
cells. The Tc cells bear an alpha-ß TCR that recognizes antigen in the
context of the polymorphic determinant of MHC class I molecules. The
CD8 molecule has an accessory function in this process, since it binds
a non-polymorphic class I determinant (Janeway, 1992). Antigen
recognition by Tc cells is thus different from that by Th cells. The
reaction products of the effector cells, such as lymphokines, and
effector cells like plasma cell precursors leave the lymph nodes via
the efferent lymph or blood circulation to go to other sites of the
body.
Development and aging: Lymph node morphology is dynamic: its
appearance throughout life is directly related to the type and amount
of antigenic stimulation. After antigenic contact, the organ increases
in size within a relatively short time, with high proliferative
activity of lymphocytes and germinal centre formation, depending on
the type of reaction and the choice of immunological reaction. In the
case of B-lymphocyte reactions, hyperplasia of follicles is seen (e.g.
after bacterial infection); in the case of T-cell reactions, the
interfollicular areas or paracortex become enlarged (e.g. in viral
infection). After the reaction is terminated or is transferred to the
next draining lymph node, it regains its normal small size. Germinal
centres with an interfollicular microenvironment can develop
extranodally, especially at sites of chronic inflammation.
The dynamics of the lymph node can be illustrated by several
examples. After immunization of the footpad with antigen mixed with an
adjuvant, such as Freund's complete adjuvant, containing killed
mycobacteria, the draining popliteal lymph node becomes enlarged (in
rats, from about 5 mg to more than 100 mg), and granulomatous
reactions (epitheloid-cell granuloma) can be seen histologically. The
swelling of lymph nodes is used to assess reactivity towards chemicals
and in the evaluation of immunomodulatory drugs (Gleichmann et al.,
1989). In the popliteal lymph node assay, a test substance is injected
subcutaneously into one footpad and the contralateral side is left
untreated or injected with vehicle only. The effect of the substance
is subsequently estimated from the difference in weight between the
popliteal lymph nodes. Further evidence for immunostimulatory activity
in vivo is obtained by histological appearance, often manifested as
follicular hyperplasia.
Lymph nodes develop relatively late in fetal life: At birth, the
anlage of unstimulated lymph nodes is present, containing few lymph
cells. The lymph nodes develop quickly after exposure to many new
(exogenous) antigens. In adults, they may become relatively quiescent
and small, with virgin T and B cells and primary follicles. The lymph
nodes in aged rats are capable of the same degree of activity as those
in young individuals upon antigenic stimuli. The state and type of
activation in the various nodes of adult and aged animals differ under
normal housing conditions and are a reflection of the absence or
existence of continuous local stimulation with antigens or disease
processes, like inflammation and the presence of a tumour in the
drained area (Ward, 1990). The central nodes of the mandibular and
superficial cervical group, which are continuously exposed to
(aero)antigens via the oronasopharynx, may contain a considerable
number of plasma cells and precursors in the medullary cords and
relatively well-developed germinal centres. Sinal histiocytosis (that
is, considerable numbers of macrophages in the sinuses) and
accumulations of pigmented macrophages are often present in the
mesenteric lymph nodes, which are continuously exposed to antigens via
the digestive tract. An extensive review of lymph node development and
aging is given by Losco & Harleman (1992).
1.2.2.5 Spleen
The spleen consists of two main compartments: the red and white
pulp (Van Rooijen et al., 1989; Dijkstra & Sminia, 1990; Laman et al.,
1992; Van den Eertwegh et al., 1992). A schematic drawing of the
spleen is presented in Figure 13 and histological views in Figure 14.
The red pulp consists of blood-filled sinusoids and Bilroth's cords
containing macrophages, lymphocytes, plasma cells, and NK cells.
Macrophages perform major functions in clearing blood cells (for
instance, old red blood cells) and in phagocytosis, especially of non-
opsonized particles. This high-volume filter function is made possible
by two factors: the direct contact, unobstructed by blood-vessel
walls, between phagocytic cells and blood-borne particles; and the
large blood supply, estimated at about 5% of the total blood volume
per minute. There are no lymphatics in the spleen.
The phagocytic function is especially important in the case of
intravascular pathogenic microorganisms, before antibody formation and
subsequent opsonization occur (early bacterial septicaemia). The
mononuclear phagocyte system of the liver (Kupffer cells) plays a
major role in the removal of opsonized particles. Together with the
hepatic phagocytic system, splenic macrophages synthesize complement
components, although this is done mainly by hepatocytes. In rats and
mice, the red pulp contains nests of (extramedullary) haematopoiesis,
characterized histologically by megakaryocytes and normoblasts. In the
case of systemic septicaemia, when pathogenic microorganisms have
reached the blood either directly or after inadequate filtering
through lymph nodes, the red pulp increases and contains large
proportions of (immature) granulocytes. Differentiation between
septicaemia and extramedullary haematopoiesis is not always easy; the
decreased or absent white pulp in septicaemia can be helpful in making
this differentiation.
Phylogenetically, cartilaginous fish are the first species that
have a spleen, which consists of lymphoid follicles and a red pulp
that generally houses developing erythroid cells (Zapata & Cooper,
1990). The lympho-haematopoietic masses of the intestine that are seen
in some lower vertebrates (e.g. lampreys) are not primitive spleens
but rather primitive lymphohaematopoietic organs equivalent to
mammalian bone marrow. In most bony fishes, the white pulp is poorly
developed, probably reflecting the existence of other peripheral
lymphoid organs, e.g. the kidney, which participate actively in the
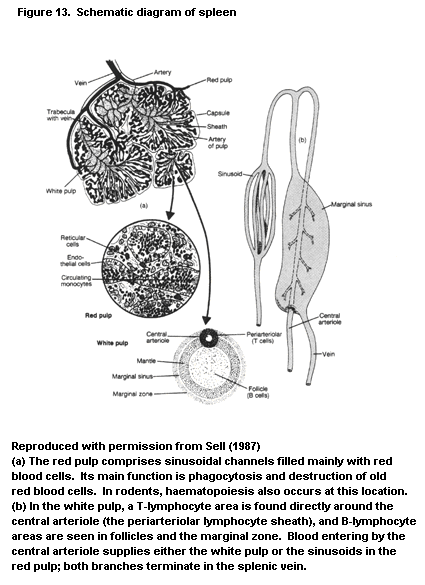
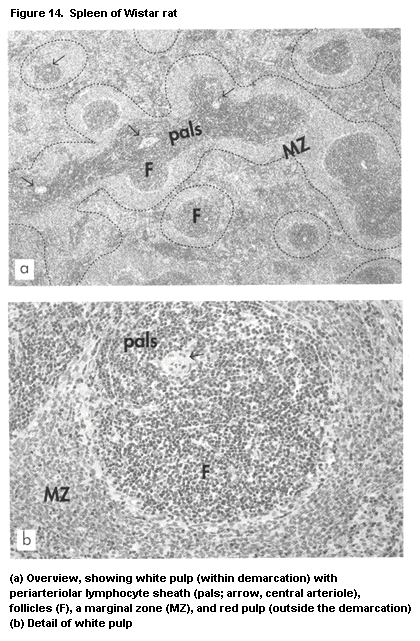
 immune response. After antigenic stimulation, the amount of splenic
lymphoid tissue increases considerably in all lower vertebrates,
although germinal centres do not occur. At the cellular level,
however, antigen-presenting cells and cells retaining immune complexes
on their surface have been described in the spleen of some bony
fishes, anurous species, and reptiles.
White pulp: The spleen contains about one-quarter of the body's
total lymphocyte population; during lymphocyte recirculation, more
cells pass through the spleen than through all the lymph nodes.
Lymphocytes in the spleen reside in the white pulp, which consists of
a central arteriole surrounded by the periarteriolar lymphocyte
sheath, a T-lymphocyte area. The outer sheath contains B lymphocytes
and, after antigenic stimulation, plasma cells. Adjacent follicles
contain B cells. Around the periarteriolar lymphocyte sheath and
follicles is a corona containing B cells, called the marginal zone;
this region is easily distinguished, especially in rats. The
periarteriolar lymphocyte sheath has a microenvironment and a
passenger leukocyte content similar to that of the lymph node
paracortex. Some sources claim that the spleen is a rich source of Ts
cell activity, exceeding that of lymph nodes. The follicles are not
essentially different in structure and function from those of lymph
nodes. The spleen performs a major function in humoral immunity by
synthesizing IgM class antibodies, especially to blood-borne antigens.
The microenvironment of the marginal zone is unique to the
spleen. Histologically, the B cells at this site are of medium size;
on histological staining, they are larger and paler than B cells in
primary follicles and in the follicular mantle of secondary follicles
(Figure 14). In addition, they do not show the morphology of the
centrocytes or centroblasts found in germinal centres, and the
phenotypic expression (surface IgM+IgD-) indicates that the B
cells in the marginal zone are a separate population. Special
macrophage types are present, which are known as marginal zone
macrophages and marginal metallophilic macrophages. The latter are
located at the periphery of the white pulp, along the inner border of
the marginal sinus, and can be stained by silver impregnation.
The physiological function of the marginal zone has been
characterized recently. First, the site retains B-lymphocyte memory;
second, it mediates humoral responses that do not directly involve
T cells. These T-independent responses are elicited by polysaccharide
antigens of encapsulated bacteria, which are present in repeating
units on the microorganism and are presented to the B cells by
marginal zone macrophages. The response may not be completely T cell-
independent in all cases, as T cell-derived factors enhance the
response to some of these antigens. The antibodies generated are
mainly of the IgM class, as T-cell help is required for an isotype
switch.
In conclusion, the main immunological function of the spleen is
to defend the body's vascular compartment by generating T cell-
independent IgM antibody responses to bacterial polysaccharides and by
exerting an enormous phagocytic power. This function is lost after
splenectomy, when reduced nonspecific phagocytosis of non-opsonized
particles, lowered serum IgM levels, and increased susceptibility to
infections by encapsulated bacteria have been described.
1.2.2.6 Mucosa-associated lymphoid tissue
The secretory epithelial surfaces of the body form a major route
of entry for potentially pathogenic substances. These surfaces include
the epithelia of the gastrointestinal, upper and lower respiratory,
and urogenital tracts (Miller & Nicklin, 1987; Sminia et al., 1989).
The host response at these locations ranges from nonspecific
constituents, such as a physical or mechanical component (epithelial
barrier, motility of the gastrointestinal tract, and the mucociliary
escalator in the respiratory tract), to a chemical component (low
gastric pH, mucus, lysosomal and digestive enzymes), and antigen-
specific components of the immune system.
Nonspecific killer cells are found in significant numbers in the
lungs and along the epithelium of the gastrointestinal tract, where
lymphocyte-like cells have been found to kill pathogens, presumably
without prior sensitization (Hanglow et al., 1990). In mice, these
cells have been characterized as T cells with a gamma-delta TCR,
which, in contrast to Tc cells that express alpha-ß TCR, kill targets
in an MHC-nonrestricted manner (Raulet, 1989). The cells have antigen
specificity that is encoded at the DNA level by variable gene
segments, but the repertoire appears to be smaller than that which
encodes TCR alpha or ß chains. These gamma-delta T killer cells are
not generated under the strict influence of the thymus, as are their
alpha-ß T-cell counterparts (Bell, 1989; Haas et al., 1993). Apart
from their killer activity, these cells may serve as inducing elements
for the response mediated by alpha-ß TCR-expressing T subsets. In this
initiating activity, gamma-delta TCR molecules shed from the
lymphocyte surface may act as antigen-specific factors (De Weger et
al., 1989).
Lymphoid tissue: Lymphoid tissue occurs just underneath the
secretory epithelium, in the duodenum and jejunum as Peyer's patches
(Figure 15), in the appendix of the large intestine, along the bronchi
(Sminia et al., 1989), and in the oro- and nasopharyngeal regions
(Kuper et al., 1992b). These mucosal lymphoid tissues share structural
and functional characteristics and are strongly interrelated. The
common designation 'mucosa-associated lymphoid tissue' (MALT) is
therefore used to refer to bronchus-associated, gut-associated, and
nasal-associated (nasal cavity and nasopharynx) lymphoid tissue.
Nasal-associated lymphoid tissue has been identified in horses,
monkeys, and rats (Figure 16) (Kuper et al., 1990b). In humans and
domestic animals, the larger lymphoid nodules in the pharyngeal region
are called tonsils. Together with the intermediate lymphoid tissue,
the tonsils form Waldeyer's tonsillar ring.
The organization of MALT is similar to that of lymph nodes, with
B cell-containing follicles and T cell-containing interfollicular
areas. Afferent lymph vessels are lacking, because pathogens can enter
the tissue through the covering epithelial layer. The epithelial cells
at this location (the 'M' or microfold cells) are often thinner than
those at other secretory sites, in order to enable efficient passage
of antigens. Stimulated gut-associated lymphoid tissue and human
tonsils often have prominent follicles with germinal centres. In
contrast, germinal centres are scarce in stimulated bronchus-
associated and nasal-associated lymphoid tissue in rodents, due to the
fact that immunological reactions occur mainly in the draining
cervical lymph node. Th medulla-like areas seen in lymph nodes are
absent in MALT. Lymphocytes and NK cells are found in the lymphoid
tissue, in interstitial tissue in the lung, and in the lamina propria
along the gastrointestinal tract.
The homing specificity of lymphocytes into lymphoid tissue by
migration through high endothelial venules is described above. The
specificity of the homing phenomenon to MALT has the advantage that
the same circulation pathway (i.e. the blood) is used by the secretory
and internal immune system (with the intrinsic possibility of mutual
contact). In addition, the antigen message received at one secretory
site is followed by effects at all secretory surfaces. Thus, after
antigen presentation in the gastrointestinal tract, effector cells
(e.g. IgA antibody-synthesizing plasma cells, see below) are found at
the site of stimulation and at other secretory sites (e.g. the
respiratory tract). Thus, the major function of MALT is to initiate
immune responses, which are then passed on to draining lymph nodes,
such as mesenteric lymph nodes in the gastrointestinal tract.
The secretory IgA antibody response: The immune response in
MALT differs from that at other sites of the body in that it is
devoted to the generation of an IgA antibody response. Thus, MALT
contains precursors of IgA antibody plasma cells and populations of T
cells capable of promoting a B-cell immunoglobulin class switch into
IgA-producing B cells or plasma cells. B-Cell differentiation into
IgA-producing plasma cells after local antigen presentation is
accompanied by lymphocyte migration and specific homing. Precursors
move through draining lymph nodes into the blood and from there to the
secretory surface, where they lodge as IgA plasma cells in the lamina
propria. Specific homing mechanisms exist by which these cells are
able to select the secretory surface of their final mucosal
destination.
immune response. After antigenic stimulation, the amount of splenic
lymphoid tissue increases considerably in all lower vertebrates,
although germinal centres do not occur. At the cellular level,
however, antigen-presenting cells and cells retaining immune complexes
on their surface have been described in the spleen of some bony
fishes, anurous species, and reptiles.
White pulp: The spleen contains about one-quarter of the body's
total lymphocyte population; during lymphocyte recirculation, more
cells pass through the spleen than through all the lymph nodes.
Lymphocytes in the spleen reside in the white pulp, which consists of
a central arteriole surrounded by the periarteriolar lymphocyte
sheath, a T-lymphocyte area. The outer sheath contains B lymphocytes
and, after antigenic stimulation, plasma cells. Adjacent follicles
contain B cells. Around the periarteriolar lymphocyte sheath and
follicles is a corona containing B cells, called the marginal zone;
this region is easily distinguished, especially in rats. The
periarteriolar lymphocyte sheath has a microenvironment and a
passenger leukocyte content similar to that of the lymph node
paracortex. Some sources claim that the spleen is a rich source of Ts
cell activity, exceeding that of lymph nodes. The follicles are not
essentially different in structure and function from those of lymph
nodes. The spleen performs a major function in humoral immunity by
synthesizing IgM class antibodies, especially to blood-borne antigens.
The microenvironment of the marginal zone is unique to the
spleen. Histologically, the B cells at this site are of medium size;
on histological staining, they are larger and paler than B cells in
primary follicles and in the follicular mantle of secondary follicles
(Figure 14). In addition, they do not show the morphology of the
centrocytes or centroblasts found in germinal centres, and the
phenotypic expression (surface IgM+IgD-) indicates that the B
cells in the marginal zone are a separate population. Special
macrophage types are present, which are known as marginal zone
macrophages and marginal metallophilic macrophages. The latter are
located at the periphery of the white pulp, along the inner border of
the marginal sinus, and can be stained by silver impregnation.
The physiological function of the marginal zone has been
characterized recently. First, the site retains B-lymphocyte memory;
second, it mediates humoral responses that do not directly involve
T cells. These T-independent responses are elicited by polysaccharide
antigens of encapsulated bacteria, which are present in repeating
units on the microorganism and are presented to the B cells by
marginal zone macrophages. The response may not be completely T cell-
independent in all cases, as T cell-derived factors enhance the
response to some of these antigens. The antibodies generated are
mainly of the IgM class, as T-cell help is required for an isotype
switch.
In conclusion, the main immunological function of the spleen is
to defend the body's vascular compartment by generating T cell-
independent IgM antibody responses to bacterial polysaccharides and by
exerting an enormous phagocytic power. This function is lost after
splenectomy, when reduced nonspecific phagocytosis of non-opsonized
particles, lowered serum IgM levels, and increased susceptibility to
infections by encapsulated bacteria have been described.
1.2.2.6 Mucosa-associated lymphoid tissue
The secretory epithelial surfaces of the body form a major route
of entry for potentially pathogenic substances. These surfaces include
the epithelia of the gastrointestinal, upper and lower respiratory,
and urogenital tracts (Miller & Nicklin, 1987; Sminia et al., 1989).
The host response at these locations ranges from nonspecific
constituents, such as a physical or mechanical component (epithelial
barrier, motility of the gastrointestinal tract, and the mucociliary
escalator in the respiratory tract), to a chemical component (low
gastric pH, mucus, lysosomal and digestive enzymes), and antigen-
specific components of the immune system.
Nonspecific killer cells are found in significant numbers in the
lungs and along the epithelium of the gastrointestinal tract, where
lymphocyte-like cells have been found to kill pathogens, presumably
without prior sensitization (Hanglow et al., 1990). In mice, these
cells have been characterized as T cells with a gamma-delta TCR,
which, in contrast to Tc cells that express alpha-ß TCR, kill targets
in an MHC-nonrestricted manner (Raulet, 1989). The cells have antigen
specificity that is encoded at the DNA level by variable gene
segments, but the repertoire appears to be smaller than that which
encodes TCR alpha or ß chains. These gamma-delta T killer cells are
not generated under the strict influence of the thymus, as are their
alpha-ß T-cell counterparts (Bell, 1989; Haas et al., 1993). Apart
from their killer activity, these cells may serve as inducing elements
for the response mediated by alpha-ß TCR-expressing T subsets. In this
initiating activity, gamma-delta TCR molecules shed from the
lymphocyte surface may act as antigen-specific factors (De Weger et
al., 1989).
Lymphoid tissue: Lymphoid tissue occurs just underneath the
secretory epithelium, in the duodenum and jejunum as Peyer's patches
(Figure 15), in the appendix of the large intestine, along the bronchi
(Sminia et al., 1989), and in the oro- and nasopharyngeal regions
(Kuper et al., 1992b). These mucosal lymphoid tissues share structural
and functional characteristics and are strongly interrelated. The
common designation 'mucosa-associated lymphoid tissue' (MALT) is
therefore used to refer to bronchus-associated, gut-associated, and
nasal-associated (nasal cavity and nasopharynx) lymphoid tissue.
Nasal-associated lymphoid tissue has been identified in horses,
monkeys, and rats (Figure 16) (Kuper et al., 1990b). In humans and
domestic animals, the larger lymphoid nodules in the pharyngeal region
are called tonsils. Together with the intermediate lymphoid tissue,
the tonsils form Waldeyer's tonsillar ring.
The organization of MALT is similar to that of lymph nodes, with
B cell-containing follicles and T cell-containing interfollicular
areas. Afferent lymph vessels are lacking, because pathogens can enter
the tissue through the covering epithelial layer. The epithelial cells
at this location (the 'M' or microfold cells) are often thinner than
those at other secretory sites, in order to enable efficient passage
of antigens. Stimulated gut-associated lymphoid tissue and human
tonsils often have prominent follicles with germinal centres. In
contrast, germinal centres are scarce in stimulated bronchus-
associated and nasal-associated lymphoid tissue in rodents, due to the
fact that immunological reactions occur mainly in the draining
cervical lymph node. Th medulla-like areas seen in lymph nodes are
absent in MALT. Lymphocytes and NK cells are found in the lymphoid
tissue, in interstitial tissue in the lung, and in the lamina propria
along the gastrointestinal tract.
The homing specificity of lymphocytes into lymphoid tissue by
migration through high endothelial venules is described above. The
specificity of the homing phenomenon to MALT has the advantage that
the same circulation pathway (i.e. the blood) is used by the secretory
and internal immune system (with the intrinsic possibility of mutual
contact). In addition, the antigen message received at one secretory
site is followed by effects at all secretory surfaces. Thus, after
antigen presentation in the gastrointestinal tract, effector cells
(e.g. IgA antibody-synthesizing plasma cells, see below) are found at
the site of stimulation and at other secretory sites (e.g. the
respiratory tract). Thus, the major function of MALT is to initiate
immune responses, which are then passed on to draining lymph nodes,
such as mesenteric lymph nodes in the gastrointestinal tract.
The secretory IgA antibody response: The immune response in
MALT differs from that at other sites of the body in that it is
devoted to the generation of an IgA antibody response. Thus, MALT
contains precursors of IgA antibody plasma cells and populations of T
cells capable of promoting a B-cell immunoglobulin class switch into
IgA-producing B cells or plasma cells. B-Cell differentiation into
IgA-producing plasma cells after local antigen presentation is
accompanied by lymphocyte migration and specific homing. Precursors
move through draining lymph nodes into the blood and from there to the
secretory surface, where they lodge as IgA plasma cells in the lamina
propria. Specific homing mechanisms exist by which these cells are
able to select the secretory surface of their final mucosal
destination.
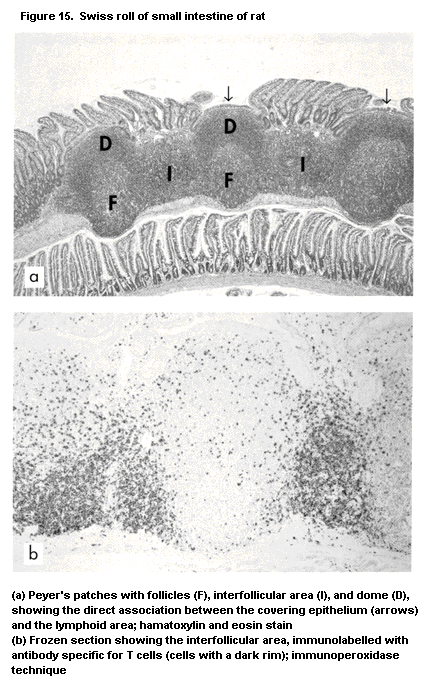
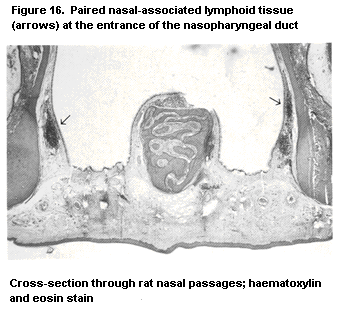 In contrast to IgA produced by the bone marrow and circulating in
blood, IgA synthesized by plasma cells of MALT consists of a dimeric
immunoglobulin subunit. The two monomers are linked by a polypeptide
called the J chain (about 15 kDa). These IgA antibodies have their
main effect outside the body itself, for example in salivary and
gastrointestinal secretions. The transport from the site of synthesis
across the epithelial barrier is specifically adapted for dimeric IgA,
and to a lesser extent for pentameric IgM. Epithelial cells express a
receptor for these immunoglobulins, called secretory component
(a polypeptide of about 70 kDa). After binding to this receptor, the
molecule is transported through the epithelium, possibly through its
cytoplasm, and excreted on the luminal surface. During this process,
secretory component attaches to the immunoglobulin molecule (coiled
around the Fc fragments); the composite molecule, comprising dimeric
IgA, the J chain, and secretory component, is called secretory IgA. In
rodents, a similar secretory component-mediated transport occurs in
the liver (Brown & Kloppel, 1989). Here, a secretory component on the
hepatocyte surface mediates the passage of dimeric IgA from the
sinusoids to the bile canaliculi. In this way, dimeric IgA entering
the liver by the portal vein efficiently recirculates to the bile and
from there into the gastrointestinal lumen. Secretory IgA is more
resistant to luminal conditions (especially proteolytic enzymes) than
dimeric IgA and is thus better able to function there.
IgA lacks the effector reactivity of IgM and IgG in complement
activation by the classical cascade, opsonization and phagocytosis, or
antibody-mediated cellular cytotoxicity. This lack appears to be
related to the absence of effector systems (complement, phagocytes) in
secretory fluid. The main function of IgA is to prevent the entry of
potentially pathogenic substances into the body, by a specific antigen
exclusion function in which the epithelium is coated with 'antiseptic
paint'.
Induction of immunological tolerance: A final feature of MALT
is its capacity to generate immunological tolerance. After antigenic
priming at secretory surfaces, subsequent systemic antigenic challenge
often results in nonresponsiveness (Strobel & Ferguson, 1984;
Challacombe, 1987; Holt & Sedgwick, 1987; Mowat, 1987). Suppressor
cells have been found in the spleen and suppressor factors in the
circulation after local immunization. The induction of tolerance
pertains primarily to dead microorganisms and inactivated proteins
which come into contact with the MALT. The mechanism of tolerance
induction and different responses to live and dead microorganisms is
not completely defined but is important in tolerance to food antigens
and the development of food allergies.
In summary, the host's defence in MALT is different from the
response of the internal immune system. The responses at this first
line of defence range from NK cell activity in the epithelium to
specific IgA antibody-mediated exclusion in the secretory fluid.
Immune responses to antigens entering the body at secretory sites are
initiated by lymphocytes in the epithelium, in lymphoid tissue
immediately underneath the epithelium, and in the lymph nodes that
drain the site (e.g. the mesenteric node). The liver may also
contribute to the response, as antigens passing directly into the
portal vein are efficiently removed and processed by the hepatic
mononuclear phagocyte system (Kupffer cells). Because MALT can
function independently of the internal immune system, blood analysis
alone may not provide complete information on MALT. Instead, analysis
of secretory fluids, such as saliva (for IgA antibody), bronchoalveolar
lavage fluid, and jejunal fluid, or direct investigation of the tissue
itself, are more appropriate approaches.
1.2.2.7 Skin immune system or skin-associated lymphoid tissue
As the skin is the largest organ of the body, its principal
physical function is to act as a barrier to water-soluble compounds,
to mechanical trauma, and to trauma caused by potentially pathogenic
microorganisms and the photons of sunlight. The physicochemical
characteristics of the outermost layer, the corneal or horny substance
of the epidermis, underlie the resistance to exogenous pathogenic
substances. The skin also has a host defence function that can be
designated as immunological. Some studies have suggested that the skin
might function as a primary organ (Fichtelius et al., 1970; Bos &
Kapsenberg, 1986), but most of the relevant immune reactions in the
skin appear to be antigen driven. The components of the skin immune
system, or skin-associated lymphoid tissue, are the following
(Streilein, 1983, 1990) (Figure 17): (i) Langerhans cells in the
epidermis, which are adapted for processing antigen and transporting
it to the draining lymph node, where they are called interdigitating
cells and present the antigen to lymphocytes; (ii) epider-motrophic
recirculating T lymphocyte subpopulations (homing T lymphocytes);
(iii) keratinocytes, which can synthesize cytokines after activation,
thereby influencing T-cell differentiation and haematopoiesis; they
can have an antigen-presenting function, especially after activation
resulting in MHC class II expression; (iv) Thy-1+ dendritic epidermal
cells, described in rodent skin epidermis: a special T cell that bears
the gamma-delta TCR and has an antigen-presenting function; and (v)
skin-draining lymph nodes comprising high endothelial venules through
which lymphocytes enter from the blood circulation.
In contrast to IgA produced by the bone marrow and circulating in
blood, IgA synthesized by plasma cells of MALT consists of a dimeric
immunoglobulin subunit. The two monomers are linked by a polypeptide
called the J chain (about 15 kDa). These IgA antibodies have their
main effect outside the body itself, for example in salivary and
gastrointestinal secretions. The transport from the site of synthesis
across the epithelial barrier is specifically adapted for dimeric IgA,
and to a lesser extent for pentameric IgM. Epithelial cells express a
receptor for these immunoglobulins, called secretory component
(a polypeptide of about 70 kDa). After binding to this receptor, the
molecule is transported through the epithelium, possibly through its
cytoplasm, and excreted on the luminal surface. During this process,
secretory component attaches to the immunoglobulin molecule (coiled
around the Fc fragments); the composite molecule, comprising dimeric
IgA, the J chain, and secretory component, is called secretory IgA. In
rodents, a similar secretory component-mediated transport occurs in
the liver (Brown & Kloppel, 1989). Here, a secretory component on the
hepatocyte surface mediates the passage of dimeric IgA from the
sinusoids to the bile canaliculi. In this way, dimeric IgA entering
the liver by the portal vein efficiently recirculates to the bile and
from there into the gastrointestinal lumen. Secretory IgA is more
resistant to luminal conditions (especially proteolytic enzymes) than
dimeric IgA and is thus better able to function there.
IgA lacks the effector reactivity of IgM and IgG in complement
activation by the classical cascade, opsonization and phagocytosis, or
antibody-mediated cellular cytotoxicity. This lack appears to be
related to the absence of effector systems (complement, phagocytes) in
secretory fluid. The main function of IgA is to prevent the entry of
potentially pathogenic substances into the body, by a specific antigen
exclusion function in which the epithelium is coated with 'antiseptic
paint'.
Induction of immunological tolerance: A final feature of MALT
is its capacity to generate immunological tolerance. After antigenic
priming at secretory surfaces, subsequent systemic antigenic challenge
often results in nonresponsiveness (Strobel & Ferguson, 1984;
Challacombe, 1987; Holt & Sedgwick, 1987; Mowat, 1987). Suppressor
cells have been found in the spleen and suppressor factors in the
circulation after local immunization. The induction of tolerance
pertains primarily to dead microorganisms and inactivated proteins
which come into contact with the MALT. The mechanism of tolerance
induction and different responses to live and dead microorganisms is
not completely defined but is important in tolerance to food antigens
and the development of food allergies.
In summary, the host's defence in MALT is different from the
response of the internal immune system. The responses at this first
line of defence range from NK cell activity in the epithelium to
specific IgA antibody-mediated exclusion in the secretory fluid.
Immune responses to antigens entering the body at secretory sites are
initiated by lymphocytes in the epithelium, in lymphoid tissue
immediately underneath the epithelium, and in the lymph nodes that
drain the site (e.g. the mesenteric node). The liver may also
contribute to the response, as antigens passing directly into the
portal vein are efficiently removed and processed by the hepatic
mononuclear phagocyte system (Kupffer cells). Because MALT can
function independently of the internal immune system, blood analysis
alone may not provide complete information on MALT. Instead, analysis
of secretory fluids, such as saliva (for IgA antibody), bronchoalveolar
lavage fluid, and jejunal fluid, or direct investigation of the tissue
itself, are more appropriate approaches.
1.2.2.7 Skin immune system or skin-associated lymphoid tissue
As the skin is the largest organ of the body, its principal
physical function is to act as a barrier to water-soluble compounds,
to mechanical trauma, and to trauma caused by potentially pathogenic
microorganisms and the photons of sunlight. The physicochemical
characteristics of the outermost layer, the corneal or horny substance
of the epidermis, underlie the resistance to exogenous pathogenic
substances. The skin also has a host defence function that can be
designated as immunological. Some studies have suggested that the skin
might function as a primary organ (Fichtelius et al., 1970; Bos &
Kapsenberg, 1986), but most of the relevant immune reactions in the
skin appear to be antigen driven. The components of the skin immune
system, or skin-associated lymphoid tissue, are the following
(Streilein, 1983, 1990) (Figure 17): (i) Langerhans cells in the
epidermis, which are adapted for processing antigen and transporting
it to the draining lymph node, where they are called interdigitating
cells and present the antigen to lymphocytes; (ii) epider-motrophic
recirculating T lymphocyte subpopulations (homing T lymphocytes);
(iii) keratinocytes, which can synthesize cytokines after activation,
thereby influencing T-cell differentiation and haematopoiesis; they
can have an antigen-presenting function, especially after activation
resulting in MHC class II expression; (iv) Thy-1+ dendritic epidermal
cells, described in rodent skin epidermis: a special T cell that bears
the gamma-delta TCR and has an antigen-presenting function; and (v)
skin-draining lymph nodes comprising high endothelial venules through
which lymphocytes enter from the blood circulation.
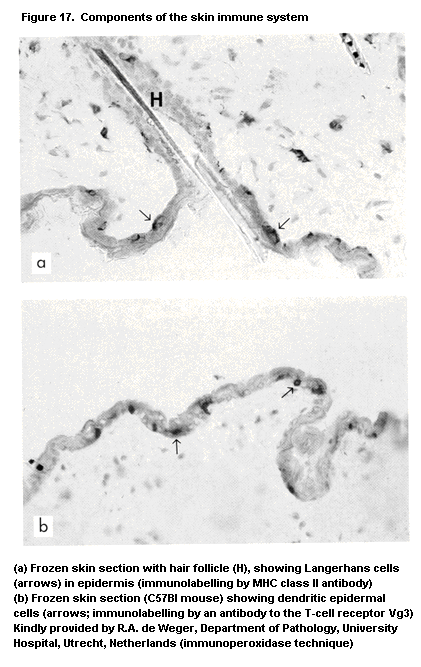 Immune components exist not only in the epidermis but also in the
dermis. At this location, T cells and macrophages have preferential
distributions, especially in the papillary region. T Cells,
macrophages, mast cells, endothelial cells, and dendritic cells are
found in the connective tissue of the dermis, as in connective tissue
at other locations in the body. The reactivity of these cells in the
dermis may differ, however, from those at other locations. For
instance, skin mast cells (Van Loveren et al., 1990a) behave
differently from mast cells at other places. These immunological
components cannot always be recognized in conventional histological
preparations of skin. For instance, Langerhans cells and dendritic
epidermal cells require special immunohistological staining (Figure
17).
Various inflammatory and immune mediators are also present in
skin. These include antimicrobial peptides, complement components,
immunoglobulins, cytokines, fibrinolysins, eicosanoids, and
neuropeptides. They are partly derived from the blood circulation and
are partly of local origin (Bos, 1990). Streilein (1990) described the
function of the skin-associated lymphoid tissue as follows: induction
of primary immune responses to new cutaneous antigens, expression of
immunity to previously encountered antigens, and avoidance of
deleterious immune responses to non-threatening cutaneous antigens.
1.3 Pathophysiology
1.3.1 Susceptibility to toxic action
The dynamic nature of the immune system renders it especially
vulnerable to toxic influences. The major target sites of the immune
system for toxicity are presented in Figure 18. The reactions of
lymphoid cells are associated with gene amplification, transcription,
and translation, and compounds that affect these processes of cell
proliferation and differentiation are especially immunotoxic,
particularly to the rapidly dividing thymocytes and haematopoietic
cells of the bone marrow. Thus, the disappearance of lymphoid cells
from bone marrow, blood, and tissue, and thymus weight may be the
first and most obvious signs of toxicity, as was seen in application
of the tiered approach to assessing the immunotoxicity of pesticides,
mentioned previously (Vos & Krajnc, 1983; Vos et al., 1983a). Effects
on the constituents of the framework (stationary stroma), which
support and steer the activation, proliferation, and differentiation
of lymphoid cells, are observed less often (Krajnc-Franken et al.,
1990; Schuurman et al., 1991b). Such effects result mostly in
degeneration, ending in atrophy and fibrosis. Alternatively, framework
cells and passenger leukocytes may persist but are rendered unable to
function by the toxic insult, and the delicate interactions between
these cells may be affected. This result of toxicity is not always
observed by conventional histology; it may be visualized by changes in
immunolabelling for markers that have functional significance.
Otherwise, functional assessment in vivo or in vitro is required.
As shown in Figure 18, the effects on specific cells in lymphoid
tissues are generally reflected in altered histology of lymphoid
organs, but this is not always the case for effects on responses.
The skin, respiratory tract, and gastrointestinal tract together
form an enormous surface that is in close contact with the outside
world and is potentially exposed to a vast magnitude of microbial
agents and potential toxicants. The toxic effects on these components
of the immune system can differ in (histo)pathological manifestation
from those on internal lymphoid organs. In the human respiratory
tract, these effects include asthma, fibrosis, and pulmonary
infections. Examples of inhaled pollutants that may induce these
effects are oxidant gases and particulates such as silica, asbestos,
and coal dust. The cellular and biochemical profiles of
bronchoalveolar lavage constituents after exposure of experimental
animals and humans by inhalation (Koren et al., 1989) are valuable for
screening immune-mediated lung injury. The products of pulmonary
epithelial cells and alveolar macrophages appear to be key factors. A
number of studies have indicated that lung disease progresses with
postactivational release of cytokines, such as IL-1, tumour necrosis
factor, platelet-derived growth factor, and transforming growth
factors. Alveolar macrophages secrete not only cytokines but also a
variety of short-lived products that may contribute to altered
resistance to pulmonary infections and inflammation; these include
reactive oxygen species, such as superoxide, nitric oxide, and
hydrogen peroxide, and arachidonic acid metabolites. The overall
suppression of these humoral systems, in combination with effects on
e.g. NK cells, may predispose individuals to infectious agents or
tumour development or may alter the inflammatory and degenerative
response (Van Loveren et al., 1990b; Khan & Gupta, 1991; Denis, 1992;
Denis et al., 1993).
The skin is an important target in immunotoxicology, for instance
for chemical allergens (Kimber & Cumberbatch, 1992) and ultraviolet B
(UVB) radiation (Goettsch et al., 1993). The skin can respond to many
xenobiotics by a specific immune response (contact hypersensitivity)
or by a nonspecific inflammatory response (contact irritancy); both
responses are associated with the induction of pro-inflammatory
cytokines. The cells of the immune system are readily recruited from
the circulation to the skin in response to dermal stimulation by
xenobiotics. In addition, various resident immune cells can be
activated, for instance Langerhans cells during the induction of the
Immune components exist not only in the epidermis but also in the
dermis. At this location, T cells and macrophages have preferential
distributions, especially in the papillary region. T Cells,
macrophages, mast cells, endothelial cells, and dendritic cells are
found in the connective tissue of the dermis, as in connective tissue
at other locations in the body. The reactivity of these cells in the
dermis may differ, however, from those at other locations. For
instance, skin mast cells (Van Loveren et al., 1990a) behave
differently from mast cells at other places. These immunological
components cannot always be recognized in conventional histological
preparations of skin. For instance, Langerhans cells and dendritic
epidermal cells require special immunohistological staining (Figure
17).
Various inflammatory and immune mediators are also present in
skin. These include antimicrobial peptides, complement components,
immunoglobulins, cytokines, fibrinolysins, eicosanoids, and
neuropeptides. They are partly derived from the blood circulation and
are partly of local origin (Bos, 1990). Streilein (1990) described the
function of the skin-associated lymphoid tissue as follows: induction
of primary immune responses to new cutaneous antigens, expression of
immunity to previously encountered antigens, and avoidance of
deleterious immune responses to non-threatening cutaneous antigens.
1.3 Pathophysiology
1.3.1 Susceptibility to toxic action
The dynamic nature of the immune system renders it especially
vulnerable to toxic influences. The major target sites of the immune
system for toxicity are presented in Figure 18. The reactions of
lymphoid cells are associated with gene amplification, transcription,
and translation, and compounds that affect these processes of cell
proliferation and differentiation are especially immunotoxic,
particularly to the rapidly dividing thymocytes and haematopoietic
cells of the bone marrow. Thus, the disappearance of lymphoid cells
from bone marrow, blood, and tissue, and thymus weight may be the
first and most obvious signs of toxicity, as was seen in application
of the tiered approach to assessing the immunotoxicity of pesticides,
mentioned previously (Vos & Krajnc, 1983; Vos et al., 1983a). Effects
on the constituents of the framework (stationary stroma), which
support and steer the activation, proliferation, and differentiation
of lymphoid cells, are observed less often (Krajnc-Franken et al.,
1990; Schuurman et al., 1991b). Such effects result mostly in
degeneration, ending in atrophy and fibrosis. Alternatively, framework
cells and passenger leukocytes may persist but are rendered unable to
function by the toxic insult, and the delicate interactions between
these cells may be affected. This result of toxicity is not always
observed by conventional histology; it may be visualized by changes in
immunolabelling for markers that have functional significance.
Otherwise, functional assessment in vivo or in vitro is required.
As shown in Figure 18, the effects on specific cells in lymphoid
tissues are generally reflected in altered histology of lymphoid
organs, but this is not always the case for effects on responses.
The skin, respiratory tract, and gastrointestinal tract together
form an enormous surface that is in close contact with the outside
world and is potentially exposed to a vast magnitude of microbial
agents and potential toxicants. The toxic effects on these components
of the immune system can differ in (histo)pathological manifestation
from those on internal lymphoid organs. In the human respiratory
tract, these effects include asthma, fibrosis, and pulmonary
infections. Examples of inhaled pollutants that may induce these
effects are oxidant gases and particulates such as silica, asbestos,
and coal dust. The cellular and biochemical profiles of
bronchoalveolar lavage constituents after exposure of experimental
animals and humans by inhalation (Koren et al., 1989) are valuable for
screening immune-mediated lung injury. The products of pulmonary
epithelial cells and alveolar macrophages appear to be key factors. A
number of studies have indicated that lung disease progresses with
postactivational release of cytokines, such as IL-1, tumour necrosis
factor, platelet-derived growth factor, and transforming growth
factors. Alveolar macrophages secrete not only cytokines but also a
variety of short-lived products that may contribute to altered
resistance to pulmonary infections and inflammation; these include
reactive oxygen species, such as superoxide, nitric oxide, and
hydrogen peroxide, and arachidonic acid metabolites. The overall
suppression of these humoral systems, in combination with effects on
e.g. NK cells, may predispose individuals to infectious agents or
tumour development or may alter the inflammatory and degenerative
response (Van Loveren et al., 1990b; Khan & Gupta, 1991; Denis, 1992;
Denis et al., 1993).
The skin is an important target in immunotoxicology, for instance
for chemical allergens (Kimber & Cumberbatch, 1992) and ultraviolet B
(UVB) radiation (Goettsch et al., 1993). The skin can respond to many
xenobiotics by a specific immune response (contact hypersensitivity)
or by a nonspecific inflammatory response (contact irritancy); both
responses are associated with the induction of pro-inflammatory
cytokines. The cells of the immune system are readily recruited from
the circulation to the skin in response to dermal stimulation by
xenobiotics. In addition, various resident immune cells can be
activated, for instance Langerhans cells during the induction of the
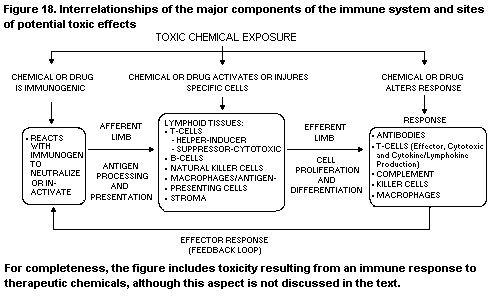 contact hypersensitivity response. Soluble mediators can be produced
locally, and antigen-antibody complexes can be formed at the site of
inflammation. Exogenous factors such as ultraviolet light (Applegate
et al., 1989) and 7,12-dimethylbenz[ a]anthracene (DMBA) (Halliday et
al., 1988) can cause the disappearance of Langerhans cells from the
skin (or the loss of their function), with consequent disturbance or
dysregulation of the skin's immune function. Keratinocytes, which
comprise the vast majority of cells in the epidermis, have an
important role in immune and inflammatory responses, serving as a
significant source of cytokines, which contribute either
quantitatively or qualitatively to the nature of the response of the
skin to exogenous stimulation.
Reactions to drugs illustrate the skin's susceptibility to toxic
influences. Cytostatic drugs used in cancer therapy often induce bone-
marrow depression as a major side-effect, resulting in an increased
risk for infections, so that blood leukocyte counts, which reflect
bone-marrow depression, must be monitored during administration.
Conversely, a number of cytotostatic drugs are immunosuppressive. A
well-known example is azathioprine (see section 2.2.1.1). A number of
new immunosuppressive drugs inhibit DNA synthesis, including
Mizoribine (Bredinin, an imidazole nucleoside), Brequinar sodium (a
quinoline carboxylic acid derivative), and RS61443 (morpholino-
ethylester of mycophenolic acid) (Thomson, 1992). Their specificity to
cells of the immune system may be related to the distinct pathways in
purine and pyrimidine metabolism that are preferentially used by
lymphocytes, such as guanine nucleotide synthesis promoted by inosine
monophosphate dehydrogenase in the case of Mizoribine and RS61443.
Another example of the particular sensitivity of the immune system to
toxic damage is its response to irradiation. Ionizing radiation is
commonly used in cancer therapy. Of the body's constituents, the
haematopoietic system is particularly sensitive to irradiation; when
pluripotent stem cells are affected, regenerative activity is lost.
Other systems destroyed by this treatment, like the intestinal
epithelium, have an intrinsic self-renewal capacity and do not need
replacement therapy. The lymphoid constituents of the immune system
differ in radiosensitivity. The dose of radiation that causes a
reduction in the cell population by a factor of 1/e (or 0.37), the
D0, has been estimated at about 0.9 Gy for bone-marrow lymphoid stem
cells, 0.4-0.9 Gy for pre-B cells, and 0.7 Gy for peripheral
lymphocytes (Anderson & Williams, 1977; Anderson et al., 1977). The
thymus is very sensitive to irradiation (Sharp & Watkins, 1981;
Anderson et al., 1986; Adkins et al., 1988). Cortical lymphocytes
manifest a D0 value of about 0.6 Gy. The intrathymic T-cell
precursor has a D0 value of about 1.4 Gy. A refractory population
(about 20-30%) is present within the medulla, but most cells are
radiosensitive (D0, about 0.7 Gy). The capacity of thymic stroma to
support precursor T-cell processing is radioresistant (Huiskamp et
al., 1988). In addition to cell depletion, peripheral lymphoid tissues
manifest decreased lymphocyte recirculation at a dose of only 0.5 Gy
(Anderson et al., 1977), suggesting that high endothelial venules, or
cell surface molecules involved in lymphocyte migration through these
venules, are radiosensitive. The sensitivity of lymphocytes to
ionizing radiation cannot be ascribed solely to their susceptibility
to death during proliferation. Cells in the resting state disappear
after irradiation, at a time that does not correspond to their
physiological half-life. Apparently, the death of lymphocytes occurs
at phases between cell division. This phenomenon, known as interphase
death, appears to be a distinct characteristic of lymphocytes and
sensitizes the immune system to radiation. Thus, only peripheral blood
lymphocytes need be assessed as dosimeters after accidental
irradiation (such as at Chernobyl in 1986).
Toxicity to the thymus: Of the lymphoid cells of the body, the
lymphocytes of the thymus (thymocytes) are especially susceptible to
the action of toxic compounds (Schuurman et al., 1992d). Table 4
presents data on the susceptibility of various thymic components to
toxic damage, illustrating the particular vulnerability of the
passenger lymphocyte population. The microenvironment appears to be
more resistant, mainly on the basis of histopathological assessment.
Thymocyte depletion, suggestive of toxicity towards this population,
may actually be an indirect effect, in that the microenvironment is
damaged and unable to support thymocyte growth. This situation may
exist in the thymus after exposure to 2,3,7,8-tetrachlorodibenzo-
para-dioxin (TCDD), which preferentially attacks the thymic
reticular epithelium, resulting in lymphocyte depletion histologically
(see section 2.2.2.1). Similarly, the reduction in the cellularity of
the medulla of the thymus after treatment with cyclosporin A is due to
the absence of the medullary lymphocyte population, but the basis of
the reduction is the disappearance of dendritic cells (as seen by
immunohistochemistry; see section 2.2.1.2).
The susceptibility of thymocytes to toxicity is also related to
their fragile composition, especially cortical thymocytes, and to the
delicate interactions between them and their microenvironment. For
instance, they are programmed to enter apoptosis when activated during
the physiological process of selection. A decrease in size or
involution of the organ may thus be the first manifestation of
toxicity. It should be noted that stress itself can induce thymic
involution; furthermore, thymic status is dependent on nutritional
status and age. The main function of the thymus is to generate the
T-cell repertoire during fetal and early postnatal life. Its
susceptibility to toxic compounds and the subsequent effects on the
cell-mediated immune system are most evident during this period of
life.
Table 4. Sensitivity of cell populations in the thymus to toxic chemicals
Cells Location Compound
Lymphocytes
Immature lymphoblasts Outer cortex Some organotin compounds,
2,3,7,8-tetrachlorodibenzo-para-dioxin
Small cells Cortex Glucocorticosteroids, cytostatic drugs
(e.g. azathioprine)
Intermediate-sized cells Medulla Ammonia caramel (THI)
Epithelial cells Cortex 2,3,7,8-Tetrachlorodibenzo-para-dioxin
Medulla Cyclosporin, FK-506
Dendritic cells Medulla Cyclosporin, FK-506
Macrophages Cortex Ammonia caramel (THI)
THI, 2-acetyl-4(5)-tetrahydroxybutylimidazole; caramel colour III
An ever-increasing number of toxic compounds has been shown to
affect the immune system. Induced and spontaneous immunopathology in
rodents have been described in reviews and textbooks (Dean et al.,
1982, 1985; Irons, 1985; Descotes, 1986; Lebish et al., 1986; Gopinath
et al., 1987; Luster et al., 1989; Vos & Luster, 1989; Jones et al.,
1990; Krajnc-Franken et al., 1990; Vos & Krajnc-Franken, 1990;
Schuurman et al., 1991b; Dean et al., 1994; Frith et al., in press).
The weight and histology of lymphoid organs are the main parameters in
evaluating toxicity. The examples given in section 2.2 illustrate the
various ways in which the immune system is injured.
1.3.2 Regeneration
The dynamic nature of the immune system provides it with a strong
regenerative capacity. In principle, the leukocyte population is
generated by a single pluripotent progenitor cell in the bone marrow.
In the thymus, single thymocyte precursors have an enormous capacity
for expansion (Ezine et al., 1984). Thus, after exposure to xenobiotic
compounds that destroy the immune system, regeneration can occur
within a relatively short time: The original architecture is restored
within three to four weeks after involution caused by radiation or
treatment with glucocorticosteroid or organotin compounds. The
restoration process can occur in waves, depending on the sensitivity
of the precursor T cells in the thymus and bone marrow (Huiskamp et
al., 1983; Penit & Ezine, 1989). Regeneration does not occur after
destruction of the white blood cell system, e.g. by sublethal
irradiation, when the stem cells in the bone marrow are affected. In
such cases, bone-marrow transplantation is required, and the anlage of
the lymphoid organs then supports generation of the newly built immune
system. After bone-marrow transplantation, polymorphonuclear
granulocytes are the first cells to appear in the circulation, within
two to three weeks; lymphocytes appear after three to four weeks;
however, establishment of a fully developed T-cell system takes about
six months (De Gast et al., 1985). These examples illustrate the
vulnerability of the bone marrow-derived component to toxic action;
regeneration requires substitution of this component and not the
relatively more resistant stromal component in lymphoid tissue.
1.3.3 Changes in lymphoid organs
The weight and gross morphology of lymphoid organs are the first
parameters studied in assessing toxicity, as the response to injury is
often expressed as changes in tissue or organ weight, size, colour,
and gross appearance. These observations are combined with leukocyte
counts, studies of differentiation, and the results of
histopathological evaluations of lymphoid organs and tissues.
Conventional histopathology allows evaluation of the effects of
xenobiotics on the main cell subsets on the basis of their distinct
morphology, tissue location, and density. In this way, the effects on
lymphocytes, lymphoblasts, and stromal cells can be evaluated
(Schuurman et al., 1994; Kuper et al., 1995). The disappearance of
lymphocytes from blood and tissues and the decrease in size and weight
are often first seen in the thymus, as this organ is very sensitive to
toxicity (see above). Evaluations of effects on distinct
subpopulations must be confirmed by immunohistochemical
characterization (Schuurman et al., 1992a). The sensitivity of
histopathology can be increased by quantitative microscopy and
morphometry and organ cell counts.
During microscopic examination, important aspects to be
considered are the cell density and the size of the various
compartments of the lymphoid organs, as well as qualitative changes
like germinal centre development; however, microscopic examination of
lymphoid organs reveals these highly dynamic and complex processes
only at a static stage. Changes in the number of cells are best
reported by descriptive terms like 'increases' or 'reductions' in
cellularity rather than by interpretive terms like 'involution',
'atrophy', or 'hyperplasia'. The pathology working group of the IPCS-
European Union international collaborative immunotoxicity study has
started to develop such a descriptive approach. A window of control
values and gross and microscopic appearance must be established for
recognition of deviations from normal; however, normal and control
values and histological features are influenced by various endogenous
and exogenous factors, including the age and hormonal (especially sex
hormone) status of the animal (Kammüller et al., 1989; Goonewardene &
Murasko, 1992; Kuper et al., 1992a; Losco, 1992; Losco & Harleman,
1992). The influence of sex hormonal status on the histology and
histophysiology of lymphoid organs is illustrated by the following
examples. Considerable changes are seen in the lymphoid organs of mice
during pregnancy (Clarke, 1984), in birds during hatching (see
Section 2), and in ectothermic vertebrates during different seasons of
the year (see section 1.2.1.5). Castration results in an increase in
thymic size in old animals (Kendall et al., 1990), as has been
observed in old rats with an involuted thymus after treatment with an
analogue of luteinizing hormone-releasing hormone (Greenstein et al.,
1987). Figure 19 shows the wet thymic weight of groups of male rats in
order to illustrate these phenomena: the mean values were about 500 mg
in young male rats at 2.5 months of age, about 125 mg in rats at 12-15
months of age, about 250 mg in aged rats after surgical castration,
and about 300 mg in aged rats after treatment with the hormone
(Kendall et al., 1990).
The neuroendocrine system and the sex steroid hormone balance may
also underlie the changes in lymphoid organs of ectothermic
vertebrates with seasonal changes like temperature and photoperiod
(mentioned above). The thymic status is also dependent on nutritional
status (Van Logten et al., 1981; Corman, 1985; Mittal et al., 1988;
Good & Lorenz, 1992), and stressful conditions influence the
appearance of lymphoid organs, especially the thymus. Finally, housing
conditions, diet, and microbiological status are important. For
instance, the presence of gamma-delta T cells in the rodent intestinal
epithelium is dependent on the presence or absence of microbiological
stimulation (that is, whether the animals are bred and maintained
under specified pathogen-free conditions) (Bandeira et al., 1990;
Dobber et al., 1992; Umesaki et al., 1993). The role of genetic
factors is reflected in strain differences in lymphoid organ
histology, e.g. in rats (Losco & Harleman, 1992; Schuurman et al.,
1992e), seen on examination of data on concurrent and historical
controls.
Genetic factors may also contribute to the individual variability
in response to a given compound. This variability can be relatively
high, even among random-bred and inbred rats, as illustrated in the
following example (Figure 20), derived from a study of the toxicity of
the immunosuppressive drug azathioprine in random-bred Wistar rats.
The data on haematological and histopathological parameters were
subjected to factor analysis in order to facilitate examination of
individual and group responses to the drug. Factor analysis involves
clustering of parameters into composite groups, or 'factors', by
arithmetic manipulation of the data, so that the parameters within a
cluster are related mathematically. It is up to the investigator to
decide whether the clustering is meaningful biologically. Figure 20
shows that not all of the 10 animals that received the highest dose of
azathioprine had the same response: three were considered to be high
responders, six low or medium responders, and one a non-responder. The
high individual variability in response illustrates the significance
of outliers in studies with a limited number of animals.
The relevance of changes observed in a study of exposure in vivo
must be based on knowledge of the nature of the response. Current
understanding of the relationship between the structure and function
of the lymphoid organs and their components often allows only a
provisional hypothesis to be made about the mechanisms of toxicity.
Moreover, different mechanisms of tissue injury can yield similar
histopathological features. Therefore, in-depth, specific histological
examination of tissue response and immune function are indispensable
for interpreting changes and for risk assessment. In interpreting
quantitative changes, like changes in cellularity, it should be noted
that particular components of the immune system may be decreased in
number or size (suppressed or involuted) or increased (stimulated or
expanded), but this does not necessarily reflect the overall effect on
the immune system or lymphoid organs.
The presence of tissue damage, protein complex deposits, and
inflammatory cell infiltrates may indicate the induction of
autoimmunity or the presence of allergy or hypersensitivity. The site
at which such responses are seen is often not a lymphoid organ, but
blood vessels, renal glomeruli, synovial membranes, thyroid, skin,
liver, or lung, which are well-known sites of autoimmunity and
hypersensitivity. Non-lymphoid organs should also be examined in order
to determine whether the effects on the lymphoid system are secondary,
for instance mediated by acute stress. This aspect is not considered
in detail in this monograph, which concerns mainly the direct toxic
action of xenobiotics on the immune system.
contact hypersensitivity response. Soluble mediators can be produced
locally, and antigen-antibody complexes can be formed at the site of
inflammation. Exogenous factors such as ultraviolet light (Applegate
et al., 1989) and 7,12-dimethylbenz[ a]anthracene (DMBA) (Halliday et
al., 1988) can cause the disappearance of Langerhans cells from the
skin (or the loss of their function), with consequent disturbance or
dysregulation of the skin's immune function. Keratinocytes, which
comprise the vast majority of cells in the epidermis, have an
important role in immune and inflammatory responses, serving as a
significant source of cytokines, which contribute either
quantitatively or qualitatively to the nature of the response of the
skin to exogenous stimulation.
Reactions to drugs illustrate the skin's susceptibility to toxic
influences. Cytostatic drugs used in cancer therapy often induce bone-
marrow depression as a major side-effect, resulting in an increased
risk for infections, so that blood leukocyte counts, which reflect
bone-marrow depression, must be monitored during administration.
Conversely, a number of cytotostatic drugs are immunosuppressive. A
well-known example is azathioprine (see section 2.2.1.1). A number of
new immunosuppressive drugs inhibit DNA synthesis, including
Mizoribine (Bredinin, an imidazole nucleoside), Brequinar sodium (a
quinoline carboxylic acid derivative), and RS61443 (morpholino-
ethylester of mycophenolic acid) (Thomson, 1992). Their specificity to
cells of the immune system may be related to the distinct pathways in
purine and pyrimidine metabolism that are preferentially used by
lymphocytes, such as guanine nucleotide synthesis promoted by inosine
monophosphate dehydrogenase in the case of Mizoribine and RS61443.
Another example of the particular sensitivity of the immune system to
toxic damage is its response to irradiation. Ionizing radiation is
commonly used in cancer therapy. Of the body's constituents, the
haematopoietic system is particularly sensitive to irradiation; when
pluripotent stem cells are affected, regenerative activity is lost.
Other systems destroyed by this treatment, like the intestinal
epithelium, have an intrinsic self-renewal capacity and do not need
replacement therapy. The lymphoid constituents of the immune system
differ in radiosensitivity. The dose of radiation that causes a
reduction in the cell population by a factor of 1/e (or 0.37), the
D0, has been estimated at about 0.9 Gy for bone-marrow lymphoid stem
cells, 0.4-0.9 Gy for pre-B cells, and 0.7 Gy for peripheral
lymphocytes (Anderson & Williams, 1977; Anderson et al., 1977). The
thymus is very sensitive to irradiation (Sharp & Watkins, 1981;
Anderson et al., 1986; Adkins et al., 1988). Cortical lymphocytes
manifest a D0 value of about 0.6 Gy. The intrathymic T-cell
precursor has a D0 value of about 1.4 Gy. A refractory population
(about 20-30%) is present within the medulla, but most cells are
radiosensitive (D0, about 0.7 Gy). The capacity of thymic stroma to
support precursor T-cell processing is radioresistant (Huiskamp et
al., 1988). In addition to cell depletion, peripheral lymphoid tissues
manifest decreased lymphocyte recirculation at a dose of only 0.5 Gy
(Anderson et al., 1977), suggesting that high endothelial venules, or
cell surface molecules involved in lymphocyte migration through these
venules, are radiosensitive. The sensitivity of lymphocytes to
ionizing radiation cannot be ascribed solely to their susceptibility
to death during proliferation. Cells in the resting state disappear
after irradiation, at a time that does not correspond to their
physiological half-life. Apparently, the death of lymphocytes occurs
at phases between cell division. This phenomenon, known as interphase
death, appears to be a distinct characteristic of lymphocytes and
sensitizes the immune system to radiation. Thus, only peripheral blood
lymphocytes need be assessed as dosimeters after accidental
irradiation (such as at Chernobyl in 1986).
Toxicity to the thymus: Of the lymphoid cells of the body, the
lymphocytes of the thymus (thymocytes) are especially susceptible to
the action of toxic compounds (Schuurman et al., 1992d). Table 4
presents data on the susceptibility of various thymic components to
toxic damage, illustrating the particular vulnerability of the
passenger lymphocyte population. The microenvironment appears to be
more resistant, mainly on the basis of histopathological assessment.
Thymocyte depletion, suggestive of toxicity towards this population,
may actually be an indirect effect, in that the microenvironment is
damaged and unable to support thymocyte growth. This situation may
exist in the thymus after exposure to 2,3,7,8-tetrachlorodibenzo-
para-dioxin (TCDD), which preferentially attacks the thymic
reticular epithelium, resulting in lymphocyte depletion histologically
(see section 2.2.2.1). Similarly, the reduction in the cellularity of
the medulla of the thymus after treatment with cyclosporin A is due to
the absence of the medullary lymphocyte population, but the basis of
the reduction is the disappearance of dendritic cells (as seen by
immunohistochemistry; see section 2.2.1.2).
The susceptibility of thymocytes to toxicity is also related to
their fragile composition, especially cortical thymocytes, and to the
delicate interactions between them and their microenvironment. For
instance, they are programmed to enter apoptosis when activated during
the physiological process of selection. A decrease in size or
involution of the organ may thus be the first manifestation of
toxicity. It should be noted that stress itself can induce thymic
involution; furthermore, thymic status is dependent on nutritional
status and age. The main function of the thymus is to generate the
T-cell repertoire during fetal and early postnatal life. Its
susceptibility to toxic compounds and the subsequent effects on the
cell-mediated immune system are most evident during this period of
life.
Table 4. Sensitivity of cell populations in the thymus to toxic chemicals
Cells Location Compound
Lymphocytes
Immature lymphoblasts Outer cortex Some organotin compounds,
2,3,7,8-tetrachlorodibenzo-para-dioxin
Small cells Cortex Glucocorticosteroids, cytostatic drugs
(e.g. azathioprine)
Intermediate-sized cells Medulla Ammonia caramel (THI)
Epithelial cells Cortex 2,3,7,8-Tetrachlorodibenzo-para-dioxin
Medulla Cyclosporin, FK-506
Dendritic cells Medulla Cyclosporin, FK-506
Macrophages Cortex Ammonia caramel (THI)
THI, 2-acetyl-4(5)-tetrahydroxybutylimidazole; caramel colour III
An ever-increasing number of toxic compounds has been shown to
affect the immune system. Induced and spontaneous immunopathology in
rodents have been described in reviews and textbooks (Dean et al.,
1982, 1985; Irons, 1985; Descotes, 1986; Lebish et al., 1986; Gopinath
et al., 1987; Luster et al., 1989; Vos & Luster, 1989; Jones et al.,
1990; Krajnc-Franken et al., 1990; Vos & Krajnc-Franken, 1990;
Schuurman et al., 1991b; Dean et al., 1994; Frith et al., in press).
The weight and histology of lymphoid organs are the main parameters in
evaluating toxicity. The examples given in section 2.2 illustrate the
various ways in which the immune system is injured.
1.3.2 Regeneration
The dynamic nature of the immune system provides it with a strong
regenerative capacity. In principle, the leukocyte population is
generated by a single pluripotent progenitor cell in the bone marrow.
In the thymus, single thymocyte precursors have an enormous capacity
for expansion (Ezine et al., 1984). Thus, after exposure to xenobiotic
compounds that destroy the immune system, regeneration can occur
within a relatively short time: The original architecture is restored
within three to four weeks after involution caused by radiation or
treatment with glucocorticosteroid or organotin compounds. The
restoration process can occur in waves, depending on the sensitivity
of the precursor T cells in the thymus and bone marrow (Huiskamp et
al., 1983; Penit & Ezine, 1989). Regeneration does not occur after
destruction of the white blood cell system, e.g. by sublethal
irradiation, when the stem cells in the bone marrow are affected. In
such cases, bone-marrow transplantation is required, and the anlage of
the lymphoid organs then supports generation of the newly built immune
system. After bone-marrow transplantation, polymorphonuclear
granulocytes are the first cells to appear in the circulation, within
two to three weeks; lymphocytes appear after three to four weeks;
however, establishment of a fully developed T-cell system takes about
six months (De Gast et al., 1985). These examples illustrate the
vulnerability of the bone marrow-derived component to toxic action;
regeneration requires substitution of this component and not the
relatively more resistant stromal component in lymphoid tissue.
1.3.3 Changes in lymphoid organs
The weight and gross morphology of lymphoid organs are the first
parameters studied in assessing toxicity, as the response to injury is
often expressed as changes in tissue or organ weight, size, colour,
and gross appearance. These observations are combined with leukocyte
counts, studies of differentiation, and the results of
histopathological evaluations of lymphoid organs and tissues.
Conventional histopathology allows evaluation of the effects of
xenobiotics on the main cell subsets on the basis of their distinct
morphology, tissue location, and density. In this way, the effects on
lymphocytes, lymphoblasts, and stromal cells can be evaluated
(Schuurman et al., 1994; Kuper et al., 1995). The disappearance of
lymphocytes from blood and tissues and the decrease in size and weight
are often first seen in the thymus, as this organ is very sensitive to
toxicity (see above). Evaluations of effects on distinct
subpopulations must be confirmed by immunohistochemical
characterization (Schuurman et al., 1992a). The sensitivity of
histopathology can be increased by quantitative microscopy and
morphometry and organ cell counts.
During microscopic examination, important aspects to be
considered are the cell density and the size of the various
compartments of the lymphoid organs, as well as qualitative changes
like germinal centre development; however, microscopic examination of
lymphoid organs reveals these highly dynamic and complex processes
only at a static stage. Changes in the number of cells are best
reported by descriptive terms like 'increases' or 'reductions' in
cellularity rather than by interpretive terms like 'involution',
'atrophy', or 'hyperplasia'. The pathology working group of the IPCS-
European Union international collaborative immunotoxicity study has
started to develop such a descriptive approach. A window of control
values and gross and microscopic appearance must be established for
recognition of deviations from normal; however, normal and control
values and histological features are influenced by various endogenous
and exogenous factors, including the age and hormonal (especially sex
hormone) status of the animal (Kammüller et al., 1989; Goonewardene &
Murasko, 1992; Kuper et al., 1992a; Losco, 1992; Losco & Harleman,
1992). The influence of sex hormonal status on the histology and
histophysiology of lymphoid organs is illustrated by the following
examples. Considerable changes are seen in the lymphoid organs of mice
during pregnancy (Clarke, 1984), in birds during hatching (see
Section 2), and in ectothermic vertebrates during different seasons of
the year (see section 1.2.1.5). Castration results in an increase in
thymic size in old animals (Kendall et al., 1990), as has been
observed in old rats with an involuted thymus after treatment with an
analogue of luteinizing hormone-releasing hormone (Greenstein et al.,
1987). Figure 19 shows the wet thymic weight of groups of male rats in
order to illustrate these phenomena: the mean values were about 500 mg
in young male rats at 2.5 months of age, about 125 mg in rats at 12-15
months of age, about 250 mg in aged rats after surgical castration,
and about 300 mg in aged rats after treatment with the hormone
(Kendall et al., 1990).
The neuroendocrine system and the sex steroid hormone balance may
also underlie the changes in lymphoid organs of ectothermic
vertebrates with seasonal changes like temperature and photoperiod
(mentioned above). The thymic status is also dependent on nutritional
status (Van Logten et al., 1981; Corman, 1985; Mittal et al., 1988;
Good & Lorenz, 1992), and stressful conditions influence the
appearance of lymphoid organs, especially the thymus. Finally, housing
conditions, diet, and microbiological status are important. For
instance, the presence of gamma-delta T cells in the rodent intestinal
epithelium is dependent on the presence or absence of microbiological
stimulation (that is, whether the animals are bred and maintained
under specified pathogen-free conditions) (Bandeira et al., 1990;
Dobber et al., 1992; Umesaki et al., 1993). The role of genetic
factors is reflected in strain differences in lymphoid organ
histology, e.g. in rats (Losco & Harleman, 1992; Schuurman et al.,
1992e), seen on examination of data on concurrent and historical
controls.
Genetic factors may also contribute to the individual variability
in response to a given compound. This variability can be relatively
high, even among random-bred and inbred rats, as illustrated in the
following example (Figure 20), derived from a study of the toxicity of
the immunosuppressive drug azathioprine in random-bred Wistar rats.
The data on haematological and histopathological parameters were
subjected to factor analysis in order to facilitate examination of
individual and group responses to the drug. Factor analysis involves
clustering of parameters into composite groups, or 'factors', by
arithmetic manipulation of the data, so that the parameters within a
cluster are related mathematically. It is up to the investigator to
decide whether the clustering is meaningful biologically. Figure 20
shows that not all of the 10 animals that received the highest dose of
azathioprine had the same response: three were considered to be high
responders, six low or medium responders, and one a non-responder. The
high individual variability in response illustrates the significance
of outliers in studies with a limited number of animals.
The relevance of changes observed in a study of exposure in vivo
must be based on knowledge of the nature of the response. Current
understanding of the relationship between the structure and function
of the lymphoid organs and their components often allows only a
provisional hypothesis to be made about the mechanisms of toxicity.
Moreover, different mechanisms of tissue injury can yield similar
histopathological features. Therefore, in-depth, specific histological
examination of tissue response and immune function are indispensable
for interpreting changes and for risk assessment. In interpreting
quantitative changes, like changes in cellularity, it should be noted
that particular components of the immune system may be decreased in
number or size (suppressed or involuted) or increased (stimulated or
expanded), but this does not necessarily reflect the overall effect on
the immune system or lymphoid organs.
The presence of tissue damage, protein complex deposits, and
inflammatory cell infiltrates may indicate the induction of
autoimmunity or the presence of allergy or hypersensitivity. The site
at which such responses are seen is often not a lymphoid organ, but
blood vessels, renal glomeruli, synovial membranes, thyroid, skin,
liver, or lung, which are well-known sites of autoimmunity and
hypersensitivity. Non-lymphoid organs should also be examined in order
to determine whether the effects on the lymphoid system are secondary,
for instance mediated by acute stress. This aspect is not considered
in detail in this monograph, which concerns mainly the direct toxic
action of xenobiotics on the immune system.
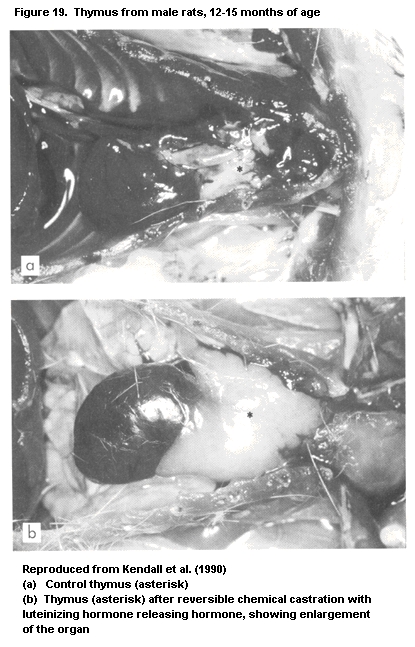
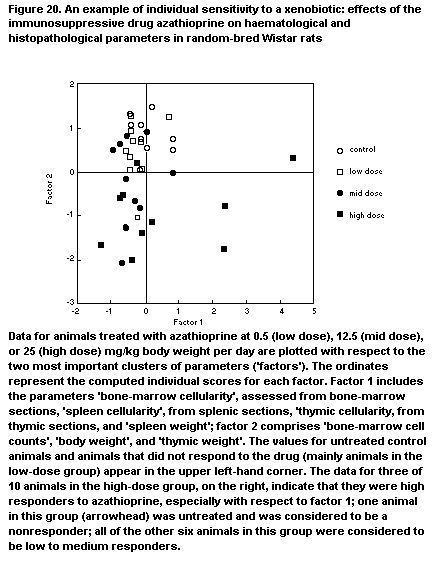 2. HEALTH IMPACT OF SELECTED IMMUNOTOXIC AGENTS
2.1 Description of consequences on human health
2.1.1 Consequences of immunosuppression
Since immunosuppressive drugs were introduced clinically to
prevent allograft rejection more than 25 years ago, the human
consequences of immunosuppression are well known. Infections and
cancers are the main consequences of immunosuppressive therapy, as
exemplified by a number of isolated case reports and epidemiological
studies (IARC, 1987; Descotes, 1990; IARC, 1990). The cancers are
often lymphomas and carcinomas, which are likely to be of viral
origin, especially in immunosuppressed patients. Recurrent respiratory
viral infections should also be considered as sentinel conditions for
immunotoxicity, both in individuals and in community-based epidemics,
including, but not confined to, opportunistic infections. Additional
evidence that immunosuppression can enhance the risk of cancer is the
increased incidence of an atypical form of Kaposi's sarcoma and of
lymphomas frequently located in the brains of patients with AIDS. It
is important to distinguish between profound immunodepression (mainly
seen clinically, e.g. after renal transplantation or cytotoxic therapy
for neoplasia) and the less severe suppression of immune function that
is more likely to be associated with exposure to an environmental
immunotoxic agent.
2.1.1.1 Cancer
Evidence from three sources, namely cancer patients on
chemotherapy, organ transplant patients, and patients with autoimmune
disorders undergoing long-term immunosuppressive therapy, demonstrates
that immunosuppressed patients are at a higher risk than others of
developing malignancies (Boyle et al., 1984; Penn, 1988; IARC, 1990;
Barrett et al., 1993; Bouwes Bavinck et al., 1993; Penn, 1993a,b;
Descotes & Vial, 1994), although not all immunosuppressive drugs have
been shown to be carcinogenic, e.g. prednisone and methotrexate (IARC,
1987). Immunotoxic effects might result in tumour formation through
reduced immune surveillance, i.e. tumours might escape the guard of
the immune system. Reduced immune surveillance can thus be regarded as
tumour promotion.
The risk for second malignancies after prolonged cancer
chemotherapy has been shown in numerous case reports and
epidemiological studies (Henne & Schmähl, 1985; Boivin, 1990; Blatt et
al., 1992). Acute leukaemia is the most frequently reported second
cancer (Kyle, 1984); overall, iatrogenic leukaemias account for 10% of
all leukaemias, with an incidence 5-100 times higher than in the
general population. Non-Hodgkin's lymphoma develops in 0.5-4.5% of
patients within 10 years after cytotoxic therapy. The risk for solid
tumours, e.g. carcinomas of the lung, skin, breast, colon, and
pancreas, is also increased after cytotoxic therapy but with a
different trend: the increase in risk is more prolonged and slower
(Swerdlow et al., 1992).
Although most cytotoxic drugs are genotoxic, their
immunosuppressive effects may also account for the increased risk of
second cancers, as indicated by results obtained in organ transplant
recipients. The Cincinnati Transplant Tumour Registry collected data
on more than 3600 cases of cancer in transplant patients up to June
1988 (Penn, 1988). Large numbers of cancers were also included in the
Australian and New Zealand Combined Dialysis and Transplant Registry
(Sheil et al., 1991). Overall, 1-15% of organ transplant patients
developed cancer within the first five years after transplantation;
whatever the therapeutic regimen, the incidence of cancer was at least
three times that of the general population and increased
logarithmically with the length of follow-up to reach more than 50%
after 20 years in some series. Cancers of the skin and lips were
reported in 18% of patients after 10 years of immunosuppressive
therapy. Squamous-cell carcinoma was the most frequent skin cancer and
was about 250 times more frequent in transplant patients than in the
general population (Hartevelt et al., 1990). Lymphomas accounted for
14-18% of neoplasms in transplant patients, and high-grade non-
Hodgkin's lymphomas accounted for 95% of these lymphomas. The
incidence of Kaposi's sarcoma was 400-500 times more frequent in
transplant patients than in the general population.
A similar pattern of neoplasias was observed in patients on
immunosuppressive therapy for autoimmune diseases (Sela & Shoenfeld,
1988). Non-Hodgkin's lymphomas were 11 times more frequent than in the
general population, and other cancers, namely leukaemias, primary
liver cancers, and squamous-cell carcinomas, were also found to be
more frequent (Penn, 1988; Descotes & Vial, 1994). The respective
roles of immunosuppressive therapy and of the underlying disease
remain to be established, however.
Cancers have been reported to occur after immunosuppressive
therapy with both cytotoxic and noncytotoxic drugs. Even though the
respective roles of genotoxicity and immunosuppression are difficult
to ascertain, cancers have been described in patients on azathioprine
after organ transplantation (Wessel et al., 1988; Singh et al., 1989)
or on low-dose methotrexate for autoimmune disorders (Ellman et al.,
1991; Shiroky et al., 1991; Kingsmore et al., 1992; Kamel et al.,
1993); immunosuppressive drugs increased the risk of malignancies
(especially lymphomas) in the treated patients. Interestingly, all of
the noncytotoxic immunosuppressive drugs were reportedly associated
with a variety of malignancies presumably related to immuno-
suppression. Whatever the type of tumour, the time to tumour
development after treatment with cyclosporin A was shorter (26 months
on average; 14 months for lymphomas) than after conventional
immunosuppressive therapy (60 months on average) (Penn, 1988). The
murine monoclonal antibody OKT3 was also shown to increase the risk
for lymphoproliferative disorders (Swinnen et al., 1990): Lymphomas
developed 1-18 months after starting OKT3, and a correlation was found
between the dose and time to neoplasm development. Lymphoproliferative
disorders were also shown to occur following treatment with FK 506
(Reyes et al., 1991). There are insufficient data to conclude a direct
causal relationship between immunosuppression induced by environmental
chemicals and the development of cancer; however, there is
epidemiological evidence that exposure to various potentially
immunotoxic chemicals (e.g. pesticides, benzene) is associated with
increased risks for cancers that also occur in immunosuppressed
patients (e.g. non-Hodgkin's lymphoma and leukaemia).
No marked difference was found in the relative risks for
lymphoproliferative disorders associated with the various
immunosuppressive drugs currently used, suggesting that
immunosuppression is the causative factor, particularly when account
is taken of the different carcinogenic potentials of azathioprine and
cyclosporin A, both clinical reference immunosuppressive agents
(Ryffel, 1992). Reactivation of latent viruses, e.g. Epstein-Barr
virus, due to immunosuppression was suggested to be involved. Indeed,
most lymphoproliferative disorders induced with cyclosporin A or
methotrexate were B-cell malignant lymphomas associated with this
viral infection (Starzl et al., 1984; Kamel et al., 1993). Uninhibited
proliferation of Epstein-Barr virus in B lymphocytes caused by the
efficient immunosuppression of one or more kinds of controls by
T lymphocytes is the commonly accepted mechanism. Interestingly,
Epstein-Barr virus-associated lymphomas in patients on
immunosuppressive therapy are usually reversible upon cessation of
treatment (Starzl et al., 1984).
2.1.1.2 Infectious diseases
Whatever the primary cause of the immune deficiency, patients
develop more frequent, more severe, recurring, and often atypical
infections, depending on the type and severity of the deficiency. The
complications associated with severe immunosuppression include
bacterial, viral, fungal, and parasitic infections (Waldman, 1988;
Barr et al., 1989; Mandell, 1990; Tieben et al., 1994). The pathogens
most frequently encountered in immunodeficient patients include the
bacterial agents Staphylococcus aureus, Streptococci, Escherichia
coli, Pseudomonas aeruginosa, Listeria monocytogenes, Mycobacterium
tuberculosis, and atypical mycobacteria. Herpes virus,
cytomegalovirus, Epstein-Barr virus, and human papillomavirus are the
leading causes of viral infections in immunosuppressed patients.
Fungal opportunistic infections include those induced by Candida,
Aspergillus, and Cryptococcus species. The immunotoxicity induced
by environmental chemicals often results in subtle changes in the
immune system, which have been suggested to result in increased
incidences of common infections like influenza and the common cold
(see section 2.4).
The respiratory tract is a primary target for infectious
pathogens, especially in immunosuppressed patients. Pulmonary
infections and infections of the upper respiratory tract are the most
common (Frattini & Trulock, 1993). Cytomegalovirus infections, often
asymptomatic, are particularly frequent in renal transplant patients
(Rubin, 1990). In addition, Pneumocystis carinii can cause a
particular form of pneumonia in immunosuppressed patients. Even though
respiratory diseases usually predominate, gastrointestinal infectious
diseases may constitute the leading consequences of immunosuppression
(Bodey et al., 1986). Infections of the central nervous system and
isolated fever are also extremely frequent. Interestingly, the type of
infection that develops in immunosuppressed patients is largely
dependent on the type of immune defect, as illustrated in Table 5.
Infectious complications have been commonly described in patients
treated with various cytotoxic drugs for cancer and with
immunosuppressants, such as cyclosporin A, for the prevention of
allograft rejection or the treatment of autoimmune disorders (Kim,
1989; Descotes & Vial, 1994).
Table 5. Pathogens frequently associated with immune defects
Humoral Cellular Neutrophil Complement
immunity immunity functions system
Campylobacter Candida Aspergillus Neisseria
Echovirus Coccidioides Bacteroides Staphylococci
Gardia Cryptococci Escherichia coli Streptococci
Haemophilus Cytomegalovirus Klebsiella
Pneumococci Herpes virus Pseudomonas
Salmonella
Legionella
Mycobacteria
Staphylococci
Histoplasma
Toxoplasma
Pneumocystis
Human papillomavirus
2.1.2 Consequences of immunostimulation
The health consequences of immunostimulation are less well
established than those associated with immunosuppression. A number of
adverse effects have, however, been reported after treatment with
immunostimulating drugs, including influenza-like reactions,
facilitation or exacerbation of underlying diseases, and inhibition of
hepatic drug metabolism (Descotes, 1992).
Patients with influenza-like reactions present with mild to
moderate fever associated with chills, malaise, and hypotension. The
reaction usually develops within hours after taking an
immunostimulating drug, and the patient recovers uneventfully within a
few hours. Such reactions are relatively uncommon with most
immunomodulating agents but have been shown to limit treatment with
several recombinant cytokines, e.g. IL-1 and tumour necrosis factor.
Facilitation and/or exacerbation of underlying diseases have been
ascribed to most immunostimulating drugs, but the incidence of this
adverse event differs markedly from one drug to another. Exacerbation
of chronic infections, psoriasis, and Crohn's disease have been
reported. More interestingly, several autoimmune diseases are more
frequent in patients treated with various (recombinant) cytokines,
e.g. autoimmunity treated with IFN gamma (Jacob et al., 1987),
thyroiditis with IL-2 (Vial & Descotes, 1993), and lupus erythematosus
with IFN alpha (Vial & Descotes, 1993). Exacerbation or facilitation
of allergic reactions to unrelated allergens has also been reported:
starting an immunostimulating treatment has been associated with
exacerbation of underlying eczema, asthma, or rhinitis. Allergic
reactions to radiological contrast media have been shown to be more
frequent in IL-2-treated patients than controls (Vial & Descotes,
1992).
Oxidative drug metabolism by the hepatic cytochrome P450 system
has been shown to be inhibited by immunostimulating drugs (Descotes,
1985) and by administration of bacillus Calmette-Guérin (BCG) vaccine
or interferon (Vial & Descotes, 1993). Although the mechanism of this
inhibition is still unknown, activation of macrophages resulting in
the release of IL-1 and IL-6 has been suggested to be involved.
Likewise, vaccination has been shown to compromise drug metabolism to
a sufficient extent that normally therapeutic doses of theophylline
caused acute toxicity in humans (Renton et al., 1980). Stimulation of
the immune system has also been shown to alter drug metabolism in
humans (Renton, 1986). A similar effect of infection has been reported
in laboratory animals (Selgrade et al., 1984); infection with mouse
cytomegalovirus before exposure to the insecticide parathion reduced
the total P450 concentration and dramatically increased the toxicity
of parathion.
So far, only a few environmental chemicals have been shown to
exert immunostimulating properties, e.g. hexachlorobenzene and
selenium. There have been no reports of clinical reactions to such
chemicals that are similar to the adverse effects seen with
immunostimulating drugs.
2.2 Direct immunotoxicity in laboratory animals
The following are some illustrative examples of immunotoxic
chemicals.
2.2.1 Azathioprine and cyclosporin A
The immunosuppressive effects of azathioprine and cyclosporin A
are considered because they can shed light on the direct
immunotoxicity of environmental chemicals.
2.2.1.1 Azathioprine
Azathioprine is a thiopurine that is used as cytostatic drug in
the treatment of leukaemias and as an immunosuppressant in patients
who have received allogeneic organ transplants or who have autoimmune
diseases. When used as an immunosuppressant, its main side-effect is
bone-marrow depression, reflected in blood leukocytopenia; its
administration must therefore be monitored through blood leukocyte
counts. Another side-effect, especially after long-term
administration, is tumour formation (IARC, 1987).
In rats, azathioprine is cytotoxic for all cell lineages in the
bone marrow, and strong cellular depletion is observed histologically.
It decreases the cellularity in thymus, blood, and peripheral lymphoid
organs, but it is mainly in the thymus that the immature lymphocyte
population of the cortex is affected. This effect is a general feature
of most cytostatic drugs. A similar effect is seen in the thymus after
treatment with glucocorticosteroids, but the molecular mechanism
resulting in lymphocyte depletion is obviously different: interference
with DNA synthesis resulting in lymphocyte proliferation in contrast
to binding to glucocorticosteroid receptors and cell down-modulation.
Azathioprine affects a number of indicators of immune function, like
macrophage cytotoxicity (Spreafico et al., 1987), lymphocyte
proliferation in vitro after mitogen stimulation (Weissgarten et
al., 1989) and in the mixed leukocyte reaction (Mellert et al., 1989),
and cytotoxicity by NK cells (Pedersen & Beyer, 1986; Spreafico et
al., 1987; Versluis et al., 1989). Both stimulation and suppression of
these functions have been found in experimental animals, depending on
the dosage and the time of testing after exposure. These findings are
in accordance with those in azathioprine-treated patients, who showed
no change in primary antibody response, a decrease in secondary
antibody response, and some or no effect on lymphocyte proliferation
in vitro after mitogen stimulation. The time of testing after the
start of exposure to azathioprine was a crucial factor in the
detection of effects. Azathioprine was tested in the IPCS-European
Union international collaborative immunotoxicity study (see section
1.1) and showed a significant strain-dependent sensitivity.
2.2.1.2 Cyclosporin A
Cyclosporin A is one of the most powerful immunosuppressive drugs
(Kahan, 1989). It is a neutral lipophilic cyclic peptide consisting of
11 amino acids (relative molecular mass, 1203 Da) isolated from the
fungus Tolypocladium inflatum. Its main use is in bone-marrow
transplantation to prevent transplant rejection and graft-versus-host
reactions. It is also used in the therapy of various autoimmune
diseases.
A complication of cyclosporin A treatment is nephrotoxicity.
Another side-effect, especially after long-term administration, is
tumour formation (IARC, 1987). In its immunosuppressive action,
cyclosporin A does not affect resting lymphocytes but blocks the
events occurring after stimulation, particularly the synthesis of
lymphokines, including IL-1 and IL-2, and IL-2 receptors. The
synthesis of IL-1 by antigen-presenting cells and of IL-2 by Th cells
is inhibited, and the synthesis of IFN gamma and tumour necrosis
factor is blocked. These events occur inside the cell at the
transcriptional level. Cyclosporin A binds to an intracellular
receptor, cyclophilin, forming a complex with calcineurin; this
complex in turn interferes with the activation of genes, resulting in
inhibition of lymphokine gene transcription (Baumann et al., 1992;
Sigal & Dumont, 1992).
An interesting feature of cyclosporin A is its specific action on
the thymus and the induction of autoimmune phenomena. Rats treated
with total body irradiation and syngeneic or autologous bone-marrow
transplantation, followed by treatment with cyclosporin A at a dose of
about 10 mg/kg body weight per day subcutaneously for four weeks,
developed signs of acute graft-versus-host reactions, with lymphocytic
infiltration at multiple epithelial sites (Glazier et al., 1983). A
similar pseudo-graft-versus-host reaction has also been evoked in
mice. It is associated with thymic changes, because it can be
transferred in whole thymus or thymocytes (Sakaguchi & Sakaguchi,
1988). Histologically, the medullary area is diminished (Beschorner et
al., 1987a; Schuurman et al., 1990; see also Figure 21). The medullary
stroma shows a decrease in MHC class II expression, indicating a loss
of dendritic cells, which has been confirmed by electron microscopy
(De Waal et al., 1992a). As these cells normally contribute to the
negative selection process, their depletion (or reduced MHC class II
expression) may be related to an absence of negative selection. The
autoreactive T cells may even attack the medullary epithelium.
2. HEALTH IMPACT OF SELECTED IMMUNOTOXIC AGENTS
2.1 Description of consequences on human health
2.1.1 Consequences of immunosuppression
Since immunosuppressive drugs were introduced clinically to
prevent allograft rejection more than 25 years ago, the human
consequences of immunosuppression are well known. Infections and
cancers are the main consequences of immunosuppressive therapy, as
exemplified by a number of isolated case reports and epidemiological
studies (IARC, 1987; Descotes, 1990; IARC, 1990). The cancers are
often lymphomas and carcinomas, which are likely to be of viral
origin, especially in immunosuppressed patients. Recurrent respiratory
viral infections should also be considered as sentinel conditions for
immunotoxicity, both in individuals and in community-based epidemics,
including, but not confined to, opportunistic infections. Additional
evidence that immunosuppression can enhance the risk of cancer is the
increased incidence of an atypical form of Kaposi's sarcoma and of
lymphomas frequently located in the brains of patients with AIDS. It
is important to distinguish between profound immunodepression (mainly
seen clinically, e.g. after renal transplantation or cytotoxic therapy
for neoplasia) and the less severe suppression of immune function that
is more likely to be associated with exposure to an environmental
immunotoxic agent.
2.1.1.1 Cancer
Evidence from three sources, namely cancer patients on
chemotherapy, organ transplant patients, and patients with autoimmune
disorders undergoing long-term immunosuppressive therapy, demonstrates
that immunosuppressed patients are at a higher risk than others of
developing malignancies (Boyle et al., 1984; Penn, 1988; IARC, 1990;
Barrett et al., 1993; Bouwes Bavinck et al., 1993; Penn, 1993a,b;
Descotes & Vial, 1994), although not all immunosuppressive drugs have
been shown to be carcinogenic, e.g. prednisone and methotrexate (IARC,
1987). Immunotoxic effects might result in tumour formation through
reduced immune surveillance, i.e. tumours might escape the guard of
the immune system. Reduced immune surveillance can thus be regarded as
tumour promotion.
The risk for second malignancies after prolonged cancer
chemotherapy has been shown in numerous case reports and
epidemiological studies (Henne & Schmähl, 1985; Boivin, 1990; Blatt et
al., 1992). Acute leukaemia is the most frequently reported second
cancer (Kyle, 1984); overall, iatrogenic leukaemias account for 10% of
all leukaemias, with an incidence 5-100 times higher than in the
general population. Non-Hodgkin's lymphoma develops in 0.5-4.5% of
patients within 10 years after cytotoxic therapy. The risk for solid
tumours, e.g. carcinomas of the lung, skin, breast, colon, and
pancreas, is also increased after cytotoxic therapy but with a
different trend: the increase in risk is more prolonged and slower
(Swerdlow et al., 1992).
Although most cytotoxic drugs are genotoxic, their
immunosuppressive effects may also account for the increased risk of
second cancers, as indicated by results obtained in organ transplant
recipients. The Cincinnati Transplant Tumour Registry collected data
on more than 3600 cases of cancer in transplant patients up to June
1988 (Penn, 1988). Large numbers of cancers were also included in the
Australian and New Zealand Combined Dialysis and Transplant Registry
(Sheil et al., 1991). Overall, 1-15% of organ transplant patients
developed cancer within the first five years after transplantation;
whatever the therapeutic regimen, the incidence of cancer was at least
three times that of the general population and increased
logarithmically with the length of follow-up to reach more than 50%
after 20 years in some series. Cancers of the skin and lips were
reported in 18% of patients after 10 years of immunosuppressive
therapy. Squamous-cell carcinoma was the most frequent skin cancer and
was about 250 times more frequent in transplant patients than in the
general population (Hartevelt et al., 1990). Lymphomas accounted for
14-18% of neoplasms in transplant patients, and high-grade non-
Hodgkin's lymphomas accounted for 95% of these lymphomas. The
incidence of Kaposi's sarcoma was 400-500 times more frequent in
transplant patients than in the general population.
A similar pattern of neoplasias was observed in patients on
immunosuppressive therapy for autoimmune diseases (Sela & Shoenfeld,
1988). Non-Hodgkin's lymphomas were 11 times more frequent than in the
general population, and other cancers, namely leukaemias, primary
liver cancers, and squamous-cell carcinomas, were also found to be
more frequent (Penn, 1988; Descotes & Vial, 1994). The respective
roles of immunosuppressive therapy and of the underlying disease
remain to be established, however.
Cancers have been reported to occur after immunosuppressive
therapy with both cytotoxic and noncytotoxic drugs. Even though the
respective roles of genotoxicity and immunosuppression are difficult
to ascertain, cancers have been described in patients on azathioprine
after organ transplantation (Wessel et al., 1988; Singh et al., 1989)
or on low-dose methotrexate for autoimmune disorders (Ellman et al.,
1991; Shiroky et al., 1991; Kingsmore et al., 1992; Kamel et al.,
1993); immunosuppressive drugs increased the risk of malignancies
(especially lymphomas) in the treated patients. Interestingly, all of
the noncytotoxic immunosuppressive drugs were reportedly associated
with a variety of malignancies presumably related to immuno-
suppression. Whatever the type of tumour, the time to tumour
development after treatment with cyclosporin A was shorter (26 months
on average; 14 months for lymphomas) than after conventional
immunosuppressive therapy (60 months on average) (Penn, 1988). The
murine monoclonal antibody OKT3 was also shown to increase the risk
for lymphoproliferative disorders (Swinnen et al., 1990): Lymphomas
developed 1-18 months after starting OKT3, and a correlation was found
between the dose and time to neoplasm development. Lymphoproliferative
disorders were also shown to occur following treatment with FK 506
(Reyes et al., 1991). There are insufficient data to conclude a direct
causal relationship between immunosuppression induced by environmental
chemicals and the development of cancer; however, there is
epidemiological evidence that exposure to various potentially
immunotoxic chemicals (e.g. pesticides, benzene) is associated with
increased risks for cancers that also occur in immunosuppressed
patients (e.g. non-Hodgkin's lymphoma and leukaemia).
No marked difference was found in the relative risks for
lymphoproliferative disorders associated with the various
immunosuppressive drugs currently used, suggesting that
immunosuppression is the causative factor, particularly when account
is taken of the different carcinogenic potentials of azathioprine and
cyclosporin A, both clinical reference immunosuppressive agents
(Ryffel, 1992). Reactivation of latent viruses, e.g. Epstein-Barr
virus, due to immunosuppression was suggested to be involved. Indeed,
most lymphoproliferative disorders induced with cyclosporin A or
methotrexate were B-cell malignant lymphomas associated with this
viral infection (Starzl et al., 1984; Kamel et al., 1993). Uninhibited
proliferation of Epstein-Barr virus in B lymphocytes caused by the
efficient immunosuppression of one or more kinds of controls by
T lymphocytes is the commonly accepted mechanism. Interestingly,
Epstein-Barr virus-associated lymphomas in patients on
immunosuppressive therapy are usually reversible upon cessation of
treatment (Starzl et al., 1984).
2.1.1.2 Infectious diseases
Whatever the primary cause of the immune deficiency, patients
develop more frequent, more severe, recurring, and often atypical
infections, depending on the type and severity of the deficiency. The
complications associated with severe immunosuppression include
bacterial, viral, fungal, and parasitic infections (Waldman, 1988;
Barr et al., 1989; Mandell, 1990; Tieben et al., 1994). The pathogens
most frequently encountered in immunodeficient patients include the
bacterial agents Staphylococcus aureus, Streptococci, Escherichia
coli, Pseudomonas aeruginosa, Listeria monocytogenes, Mycobacterium
tuberculosis, and atypical mycobacteria. Herpes virus,
cytomegalovirus, Epstein-Barr virus, and human papillomavirus are the
leading causes of viral infections in immunosuppressed patients.
Fungal opportunistic infections include those induced by Candida,
Aspergillus, and Cryptococcus species. The immunotoxicity induced
by environmental chemicals often results in subtle changes in the
immune system, which have been suggested to result in increased
incidences of common infections like influenza and the common cold
(see section 2.4).
The respiratory tract is a primary target for infectious
pathogens, especially in immunosuppressed patients. Pulmonary
infections and infections of the upper respiratory tract are the most
common (Frattini & Trulock, 1993). Cytomegalovirus infections, often
asymptomatic, are particularly frequent in renal transplant patients
(Rubin, 1990). In addition, Pneumocystis carinii can cause a
particular form of pneumonia in immunosuppressed patients. Even though
respiratory diseases usually predominate, gastrointestinal infectious
diseases may constitute the leading consequences of immunosuppression
(Bodey et al., 1986). Infections of the central nervous system and
isolated fever are also extremely frequent. Interestingly, the type of
infection that develops in immunosuppressed patients is largely
dependent on the type of immune defect, as illustrated in Table 5.
Infectious complications have been commonly described in patients
treated with various cytotoxic drugs for cancer and with
immunosuppressants, such as cyclosporin A, for the prevention of
allograft rejection or the treatment of autoimmune disorders (Kim,
1989; Descotes & Vial, 1994).
Table 5. Pathogens frequently associated with immune defects
Humoral Cellular Neutrophil Complement
immunity immunity functions system
Campylobacter Candida Aspergillus Neisseria
Echovirus Coccidioides Bacteroides Staphylococci
Gardia Cryptococci Escherichia coli Streptococci
Haemophilus Cytomegalovirus Klebsiella
Pneumococci Herpes virus Pseudomonas
Salmonella
Legionella
Mycobacteria
Staphylococci
Histoplasma
Toxoplasma
Pneumocystis
Human papillomavirus
2.1.2 Consequences of immunostimulation
The health consequences of immunostimulation are less well
established than those associated with immunosuppression. A number of
adverse effects have, however, been reported after treatment with
immunostimulating drugs, including influenza-like reactions,
facilitation or exacerbation of underlying diseases, and inhibition of
hepatic drug metabolism (Descotes, 1992).
Patients with influenza-like reactions present with mild to
moderate fever associated with chills, malaise, and hypotension. The
reaction usually develops within hours after taking an
immunostimulating drug, and the patient recovers uneventfully within a
few hours. Such reactions are relatively uncommon with most
immunomodulating agents but have been shown to limit treatment with
several recombinant cytokines, e.g. IL-1 and tumour necrosis factor.
Facilitation and/or exacerbation of underlying diseases have been
ascribed to most immunostimulating drugs, but the incidence of this
adverse event differs markedly from one drug to another. Exacerbation
of chronic infections, psoriasis, and Crohn's disease have been
reported. More interestingly, several autoimmune diseases are more
frequent in patients treated with various (recombinant) cytokines,
e.g. autoimmunity treated with IFN gamma (Jacob et al., 1987),
thyroiditis with IL-2 (Vial & Descotes, 1993), and lupus erythematosus
with IFN alpha (Vial & Descotes, 1993). Exacerbation or facilitation
of allergic reactions to unrelated allergens has also been reported:
starting an immunostimulating treatment has been associated with
exacerbation of underlying eczema, asthma, or rhinitis. Allergic
reactions to radiological contrast media have been shown to be more
frequent in IL-2-treated patients than controls (Vial & Descotes,
1992).
Oxidative drug metabolism by the hepatic cytochrome P450 system
has been shown to be inhibited by immunostimulating drugs (Descotes,
1985) and by administration of bacillus Calmette-Guérin (BCG) vaccine
or interferon (Vial & Descotes, 1993). Although the mechanism of this
inhibition is still unknown, activation of macrophages resulting in
the release of IL-1 and IL-6 has been suggested to be involved.
Likewise, vaccination has been shown to compromise drug metabolism to
a sufficient extent that normally therapeutic doses of theophylline
caused acute toxicity in humans (Renton et al., 1980). Stimulation of
the immune system has also been shown to alter drug metabolism in
humans (Renton, 1986). A similar effect of infection has been reported
in laboratory animals (Selgrade et al., 1984); infection with mouse
cytomegalovirus before exposure to the insecticide parathion reduced
the total P450 concentration and dramatically increased the toxicity
of parathion.
So far, only a few environmental chemicals have been shown to
exert immunostimulating properties, e.g. hexachlorobenzene and
selenium. There have been no reports of clinical reactions to such
chemicals that are similar to the adverse effects seen with
immunostimulating drugs.
2.2 Direct immunotoxicity in laboratory animals
The following are some illustrative examples of immunotoxic
chemicals.
2.2.1 Azathioprine and cyclosporin A
The immunosuppressive effects of azathioprine and cyclosporin A
are considered because they can shed light on the direct
immunotoxicity of environmental chemicals.
2.2.1.1 Azathioprine
Azathioprine is a thiopurine that is used as cytostatic drug in
the treatment of leukaemias and as an immunosuppressant in patients
who have received allogeneic organ transplants or who have autoimmune
diseases. When used as an immunosuppressant, its main side-effect is
bone-marrow depression, reflected in blood leukocytopenia; its
administration must therefore be monitored through blood leukocyte
counts. Another side-effect, especially after long-term
administration, is tumour formation (IARC, 1987).
In rats, azathioprine is cytotoxic for all cell lineages in the
bone marrow, and strong cellular depletion is observed histologically.
It decreases the cellularity in thymus, blood, and peripheral lymphoid
organs, but it is mainly in the thymus that the immature lymphocyte
population of the cortex is affected. This effect is a general feature
of most cytostatic drugs. A similar effect is seen in the thymus after
treatment with glucocorticosteroids, but the molecular mechanism
resulting in lymphocyte depletion is obviously different: interference
with DNA synthesis resulting in lymphocyte proliferation in contrast
to binding to glucocorticosteroid receptors and cell down-modulation.
Azathioprine affects a number of indicators of immune function, like
macrophage cytotoxicity (Spreafico et al., 1987), lymphocyte
proliferation in vitro after mitogen stimulation (Weissgarten et
al., 1989) and in the mixed leukocyte reaction (Mellert et al., 1989),
and cytotoxicity by NK cells (Pedersen & Beyer, 1986; Spreafico et
al., 1987; Versluis et al., 1989). Both stimulation and suppression of
these functions have been found in experimental animals, depending on
the dosage and the time of testing after exposure. These findings are
in accordance with those in azathioprine-treated patients, who showed
no change in primary antibody response, a decrease in secondary
antibody response, and some or no effect on lymphocyte proliferation
in vitro after mitogen stimulation. The time of testing after the
start of exposure to azathioprine was a crucial factor in the
detection of effects. Azathioprine was tested in the IPCS-European
Union international collaborative immunotoxicity study (see section
1.1) and showed a significant strain-dependent sensitivity.
2.2.1.2 Cyclosporin A
Cyclosporin A is one of the most powerful immunosuppressive drugs
(Kahan, 1989). It is a neutral lipophilic cyclic peptide consisting of
11 amino acids (relative molecular mass, 1203 Da) isolated from the
fungus Tolypocladium inflatum. Its main use is in bone-marrow
transplantation to prevent transplant rejection and graft-versus-host
reactions. It is also used in the therapy of various autoimmune
diseases.
A complication of cyclosporin A treatment is nephrotoxicity.
Another side-effect, especially after long-term administration, is
tumour formation (IARC, 1987). In its immunosuppressive action,
cyclosporin A does not affect resting lymphocytes but blocks the
events occurring after stimulation, particularly the synthesis of
lymphokines, including IL-1 and IL-2, and IL-2 receptors. The
synthesis of IL-1 by antigen-presenting cells and of IL-2 by Th cells
is inhibited, and the synthesis of IFN gamma and tumour necrosis
factor is blocked. These events occur inside the cell at the
transcriptional level. Cyclosporin A binds to an intracellular
receptor, cyclophilin, forming a complex with calcineurin; this
complex in turn interferes with the activation of genes, resulting in
inhibition of lymphokine gene transcription (Baumann et al., 1992;
Sigal & Dumont, 1992).
An interesting feature of cyclosporin A is its specific action on
the thymus and the induction of autoimmune phenomena. Rats treated
with total body irradiation and syngeneic or autologous bone-marrow
transplantation, followed by treatment with cyclosporin A at a dose of
about 10 mg/kg body weight per day subcutaneously for four weeks,
developed signs of acute graft-versus-host reactions, with lymphocytic
infiltration at multiple epithelial sites (Glazier et al., 1983). A
similar pseudo-graft-versus-host reaction has also been evoked in
mice. It is associated with thymic changes, because it can be
transferred in whole thymus or thymocytes (Sakaguchi & Sakaguchi,
1988). Histologically, the medullary area is diminished (Beschorner et
al., 1987a; Schuurman et al., 1990; see also Figure 21). The medullary
stroma shows a decrease in MHC class II expression, indicating a loss
of dendritic cells, which has been confirmed by electron microscopy
(De Waal et al., 1992a). As these cells normally contribute to the
negative selection process, their depletion (or reduced MHC class II
expression) may be related to an absence of negative selection. The
autoreactive T cells may even attack the medullary epithelium.
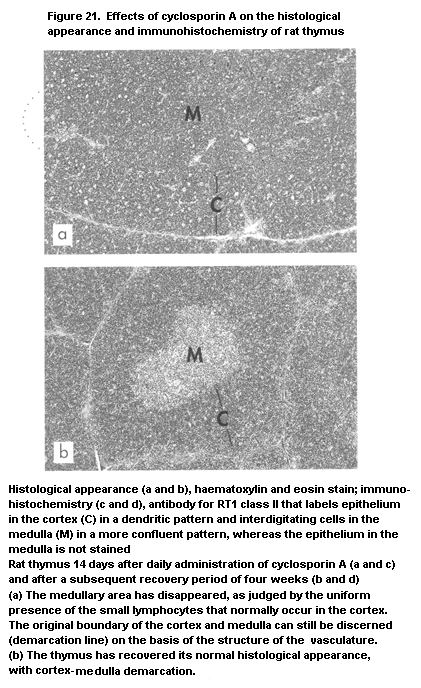
 The effect of cyclosporin A on thymic functions, i.e. the
induction of 'leakiness', with export of T cells that have not been
negatively selected, has not yet been studied for other drugs, but may
not be specific to cyclosporin A. It represents a distinct mechanism
of autoimmunity induced by the action of toxic compounds on the immune
system, mediated via thymic selection. Although the medullary area is
reduced in young rats after treatment with cyclosporin A, this is not
the case in one-year-old rats, which presumably have a lesser output
of mature T cells because of thymic involution (Beschorner et al.,
1987b).
The effect of cyclosporin A in inducing syngeneic graft-versus-
host disease in rodents has an application in clinical medicine:
Patients treated for cancer with high-dose chemotherapy and/or total
body irradiation, followed by autologous bone-marrow transplantation,
develop a recurrence of the original tumour at a higher incidence than
patients who receive an allogeneic bone-marrow transplant. This
difference has been ascribed to the addition of a graft-versus-tumour
effect to the graft-versus-host reaction. Trials have now been
initiated to induce a graft-versus-host reaction with cyclosporin A
treatment after autologous bone-marrow transplantation, in order to
reduce tumour recurrence. The initial results are promising (Hess et
al., 1992; Yeager et al., 1993; Kennedy et al., 1994).
Interestingly, two other immunosuppressive drugs, FK-506 and
rapamycin, which also interfere with gene activation in T lymphocytes,
do not bind to cyclophilin but to another intracellular receptor, the
FK-binding protein. The effect of FK-506 on the thymus is similar to
that of cyclosporin A, i.e. a decrease in the medulla (Pugh-Humphreys
et al., 1990), and rapamycin causes severe acute involution with
disappearance of lymphocytes from the cortex (Zheng et al., 1991).
These findings indicate that the two compounds have different
molecular mechanisms of action on the thymus from those of cyclosporin
A, which have not yet been elucidated.
2.2.2 Halogenated hydrocarbons
2.2.2.1 2,3,7,8-Tetrachlorodibenzo-para-dioxin
The halogenated hydrocarbon most closely studied for its
immunotoxic effects is TCDD. It has a variety of toxic effects, with a
remarkable interspecies variation; however, it causes atrophy of the
thymus and immunotoxicity in all species investigated (Vos & Luster,
1989; Holsapple et al., 1991; Neubert, 1992; Kerkvliet & Burleson,
1994). Atrophy of the thymus is reflected histologically by lymphocyte
depletion of the cortex (Figure 22). Functionally, cell-mediated
immunity appears to be suppressed in a dose-dependent fashion, as
manifested in delayed-type hypersensitivity responses, rejection of
allogeneic skin transplants, graft-versus-host reactivity, and
lymphocyte proliferation in vitro after mitogen stimulation. This
immune suppression is age-related: more severe immunotoxic effects are
observed after perinatal administration than after administration in
adulthood (Vos & Moore, 1974; Thomas & Hinsdill, 1979). TCDD can also
impair antibody-mediated immunity after primary or secondary
immunization. A sensitive parameter of the immunotoxicity of TCDD and
TCDD congeners in mice is suppression of the T cell-dependent antibody
response to sheep red blood cells in mice (Vecchi et al., 1980; Davis
& Safe, 1988; Kerkvliet et al., 1990). No effects have been observed
on classical macrophage functions.
In mice, susceptibility to TCDD is genetically determined and is
segregated at the locus that encodes a cytosolic protein which
mediates aryl hydrocarbon hydroxylase activity (Poland & Knutson,
1982). This Ah (aromatic hydrocarbon) receptor has a high affinity for
TCDD and is strongly active in mouse and rat thymus (Gasiewicz &
Rucci, 1984), particularly in epithelial cells (Greenlee et al., 1985;
Cook et al., 1987). Ah receptor-dependent immunotoxicity has been
demonstrated in mice for thymic atrophy and the antibody response to
sheep red blood cells (Tucker et al., 1986; Kerkvliet & Burleson,
1994); however, the importance of Ah receptor-mediated events in
chronic, low-level TCDD immunotoxicity is controversial (Morris et
al., 1992).
Immunosuppression in adult mice manifests almost exclusively as
suppressed antibody responses and does not appear to be related to
thymic atrophy in experiments in thymectomized (Tucker et al., 1986)
and nude (Kerkvliet & Brauner, 1987) mice. Both T and B lymphocytes
involved in antibody responses can, however, be affected by TCDD. For
example, exposure to TCDD in vivo alters regulatory lymphocyte
function (Kerkvliet & Brauner, 1987) and antigen-specific T lymphocyte
activation (Lundberg et al., 1992). TCDD also inhibits T-independent
antigen responses (Vecchi et al., 1983) and T-dependent responses when
only B cells are treated (Dooley & Holsapple, 1988). Studies of the
effects of TCDD on enriched B-cell populations in vitro have shown
that it selectively inhibits late stages of the cell cycle and the
development of B cells into plasma cells after antigen-specific
activation (Luster et al., 1988). The molecular events responsible for
TCDD immunosuppression have not been examined in detail. While early
events in B-cell maturation, such as inositol phosphate accumulation,
are not affected (Luster et al. 1988), activation of protein kinase
(Kramer et al., 1987) and tyrosine kinase (Clark et al., 1991) have
been observed.
A consequence of TCDD-induced immunosuppression is impaired
resistance to infection by bacterial, viral, and protozoan
microorganisms (Vos et al., 1991). In various mouse strains with
different treatment schedules, TCDD suppressed resistance to models
of infectious diseases with Salmonella bern, S. typhimurium,
Streptococcus pneumoniae, herpes II, Plasmodium yoelli and influenza
viruses. Various effects have been reported on resistance to
The effect of cyclosporin A on thymic functions, i.e. the
induction of 'leakiness', with export of T cells that have not been
negatively selected, has not yet been studied for other drugs, but may
not be specific to cyclosporin A. It represents a distinct mechanism
of autoimmunity induced by the action of toxic compounds on the immune
system, mediated via thymic selection. Although the medullary area is
reduced in young rats after treatment with cyclosporin A, this is not
the case in one-year-old rats, which presumably have a lesser output
of mature T cells because of thymic involution (Beschorner et al.,
1987b).
The effect of cyclosporin A in inducing syngeneic graft-versus-
host disease in rodents has an application in clinical medicine:
Patients treated for cancer with high-dose chemotherapy and/or total
body irradiation, followed by autologous bone-marrow transplantation,
develop a recurrence of the original tumour at a higher incidence than
patients who receive an allogeneic bone-marrow transplant. This
difference has been ascribed to the addition of a graft-versus-tumour
effect to the graft-versus-host reaction. Trials have now been
initiated to induce a graft-versus-host reaction with cyclosporin A
treatment after autologous bone-marrow transplantation, in order to
reduce tumour recurrence. The initial results are promising (Hess et
al., 1992; Yeager et al., 1993; Kennedy et al., 1994).
Interestingly, two other immunosuppressive drugs, FK-506 and
rapamycin, which also interfere with gene activation in T lymphocytes,
do not bind to cyclophilin but to another intracellular receptor, the
FK-binding protein. The effect of FK-506 on the thymus is similar to
that of cyclosporin A, i.e. a decrease in the medulla (Pugh-Humphreys
et al., 1990), and rapamycin causes severe acute involution with
disappearance of lymphocytes from the cortex (Zheng et al., 1991).
These findings indicate that the two compounds have different
molecular mechanisms of action on the thymus from those of cyclosporin
A, which have not yet been elucidated.
2.2.2 Halogenated hydrocarbons
2.2.2.1 2,3,7,8-Tetrachlorodibenzo-para-dioxin
The halogenated hydrocarbon most closely studied for its
immunotoxic effects is TCDD. It has a variety of toxic effects, with a
remarkable interspecies variation; however, it causes atrophy of the
thymus and immunotoxicity in all species investigated (Vos & Luster,
1989; Holsapple et al., 1991; Neubert, 1992; Kerkvliet & Burleson,
1994). Atrophy of the thymus is reflected histologically by lymphocyte
depletion of the cortex (Figure 22). Functionally, cell-mediated
immunity appears to be suppressed in a dose-dependent fashion, as
manifested in delayed-type hypersensitivity responses, rejection of
allogeneic skin transplants, graft-versus-host reactivity, and
lymphocyte proliferation in vitro after mitogen stimulation. This
immune suppression is age-related: more severe immunotoxic effects are
observed after perinatal administration than after administration in
adulthood (Vos & Moore, 1974; Thomas & Hinsdill, 1979). TCDD can also
impair antibody-mediated immunity after primary or secondary
immunization. A sensitive parameter of the immunotoxicity of TCDD and
TCDD congeners in mice is suppression of the T cell-dependent antibody
response to sheep red blood cells in mice (Vecchi et al., 1980; Davis
& Safe, 1988; Kerkvliet et al., 1990). No effects have been observed
on classical macrophage functions.
In mice, susceptibility to TCDD is genetically determined and is
segregated at the locus that encodes a cytosolic protein which
mediates aryl hydrocarbon hydroxylase activity (Poland & Knutson,
1982). This Ah (aromatic hydrocarbon) receptor has a high affinity for
TCDD and is strongly active in mouse and rat thymus (Gasiewicz &
Rucci, 1984), particularly in epithelial cells (Greenlee et al., 1985;
Cook et al., 1987). Ah receptor-dependent immunotoxicity has been
demonstrated in mice for thymic atrophy and the antibody response to
sheep red blood cells (Tucker et al., 1986; Kerkvliet & Burleson,
1994); however, the importance of Ah receptor-mediated events in
chronic, low-level TCDD immunotoxicity is controversial (Morris et
al., 1992).
Immunosuppression in adult mice manifests almost exclusively as
suppressed antibody responses and does not appear to be related to
thymic atrophy in experiments in thymectomized (Tucker et al., 1986)
and nude (Kerkvliet & Brauner, 1987) mice. Both T and B lymphocytes
involved in antibody responses can, however, be affected by TCDD. For
example, exposure to TCDD in vivo alters regulatory lymphocyte
function (Kerkvliet & Brauner, 1987) and antigen-specific T lymphocyte
activation (Lundberg et al., 1992). TCDD also inhibits T-independent
antigen responses (Vecchi et al., 1983) and T-dependent responses when
only B cells are treated (Dooley & Holsapple, 1988). Studies of the
effects of TCDD on enriched B-cell populations in vitro have shown
that it selectively inhibits late stages of the cell cycle and the
development of B cells into plasma cells after antigen-specific
activation (Luster et al., 1988). The molecular events responsible for
TCDD immunosuppression have not been examined in detail. While early
events in B-cell maturation, such as inositol phosphate accumulation,
are not affected (Luster et al. 1988), activation of protein kinase
(Kramer et al., 1987) and tyrosine kinase (Clark et al., 1991) have
been observed.
A consequence of TCDD-induced immunosuppression is impaired
resistance to infection by bacterial, viral, and protozoan
microorganisms (Vos et al., 1991). In various mouse strains with
different treatment schedules, TCDD suppressed resistance to models
of infectious diseases with Salmonella bern, S. typhimurium,
Streptococcus pneumoniae, herpes II, Plasmodium yoelli and influenza
viruses. Various effects have been reported on resistance to

 L. monocytogenes. TCDD had no effect on the mortality of mice
infected with Herpes suis (pseudorabies), whereas the mortality of
mice infected with influenza virus was enhanced by a single oral dose
of TCDD as low as 10 ng/kg body weight (Burleson et al., in press).
Many studies have been performed to investigate the mechanisms of
TCDD-induced thymic atrophy, and a number have presented evidence that
the effect may occur through an action on epithelial cells:
1. The enhanced lymphoproliferation of thymocytes after coculture
with cultured mouse and human epithelial cells was reduced when
the epithelial cells were pretreated with TCDD (Greenlee et al.,
1985; Cook et al., 1987).
2. In mouse radiation chimaeras, TCDD-induced suppression of Tc
lymphocytes is determined by the host (epithelium) and not the
donor (bone marrow, subsequently thymocytes) (Nagarkatti et al.,
1984).
3. Histological and electron microscopy studies of TCDD-exposed rats
reveal formation of epithelial aggregates and a more
differentiated state of cortical epithelium, indicating that TCDD
acts on the thymic epithelium (De Waal et al., 1992b, 1993).
A direct action of TCDD on rat thymocytes has also been
documented in vitro as cell death due to apoptosis (McConkey et al.,
1988), but this effect requires higher concentrations than those that
affect epithelial cell function in vitro. In bone marrow, TCDD
affects myelopoiesis (Luster et al., 1985a) but may be more selective
for prothymocytes (Fine et al., 1989, 1990; Holladay et al., 1991;
Blaylock et al., 1992), thus indirectly affecting thymic function.
2.2.2.2 Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are important environmental
chemicals shown in numerous studies to have immunotoxic properties.
PCB mixtures alter several morphological and functional aspects of the
immune system in rodents, guinea-pigs, rabbits, and chickens (Vos &
Luster, 1989). The first suggestion that PCBs might affect the immune
system came from observations on the weight and histology of lymphoid
organs. Oral exposure of chickens to PCBs resulted in small spleens
(Flick et al., 1965) and atrophy of lymphoid tissue (Vos & Koeman,
1970). Similar effects were noted in rabbits and guinea-pigs
(Figure 23). Dermal application of PCBs to rabbits caused lymphopenia,
atrophy of the thymic cortex, and a reduced number of germinal centres
in spleen and lymph nodes (Vos & Beems, 1971). Oral exposure of guinea-
pigs significantly reduced the number of circulating lymphocytes
and the relative thymus weight (Vos & Van Driel-Grootenhuis, 1972).
L. monocytogenes. TCDD had no effect on the mortality of mice
infected with Herpes suis (pseudorabies), whereas the mortality of
mice infected with influenza virus was enhanced by a single oral dose
of TCDD as low as 10 ng/kg body weight (Burleson et al., in press).
Many studies have been performed to investigate the mechanisms of
TCDD-induced thymic atrophy, and a number have presented evidence that
the effect may occur through an action on epithelial cells:
1. The enhanced lymphoproliferation of thymocytes after coculture
with cultured mouse and human epithelial cells was reduced when
the epithelial cells were pretreated with TCDD (Greenlee et al.,
1985; Cook et al., 1987).
2. In mouse radiation chimaeras, TCDD-induced suppression of Tc
lymphocytes is determined by the host (epithelium) and not the
donor (bone marrow, subsequently thymocytes) (Nagarkatti et al.,
1984).
3. Histological and electron microscopy studies of TCDD-exposed rats
reveal formation of epithelial aggregates and a more
differentiated state of cortical epithelium, indicating that TCDD
acts on the thymic epithelium (De Waal et al., 1992b, 1993).
A direct action of TCDD on rat thymocytes has also been
documented in vitro as cell death due to apoptosis (McConkey et al.,
1988), but this effect requires higher concentrations than those that
affect epithelial cell function in vitro. In bone marrow, TCDD
affects myelopoiesis (Luster et al., 1985a) but may be more selective
for prothymocytes (Fine et al., 1989, 1990; Holladay et al., 1991;
Blaylock et al., 1992), thus indirectly affecting thymic function.
2.2.2.2 Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are important environmental
chemicals shown in numerous studies to have immunotoxic properties.
PCB mixtures alter several morphological and functional aspects of the
immune system in rodents, guinea-pigs, rabbits, and chickens (Vos &
Luster, 1989). The first suggestion that PCBs might affect the immune
system came from observations on the weight and histology of lymphoid
organs. Oral exposure of chickens to PCBs resulted in small spleens
(Flick et al., 1965) and atrophy of lymphoid tissue (Vos & Koeman,
1970). Similar effects were noted in rabbits and guinea-pigs
(Figure 23). Dermal application of PCBs to rabbits caused lymphopenia,
atrophy of the thymic cortex, and a reduced number of germinal centres
in spleen and lymph nodes (Vos & Beems, 1971). Oral exposure of guinea-
pigs significantly reduced the number of circulating lymphocytes
and the relative thymus weight (Vos & Van Driel-Grootenhuis, 1972).
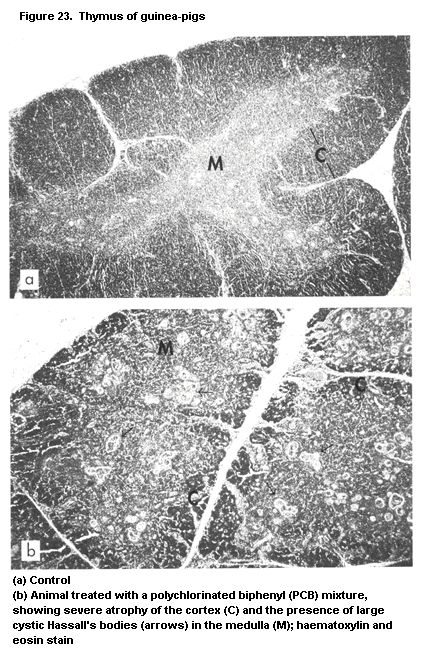 Functional tests have been focused on humoral immune responses.
Exposure of guinea-pigs, rabbits, mice, and rats to PCBs at different
regimens reduced antibody production to foreign antigens, including
tetanus toxoid, pseudorabies virus, sheep red blood cells, and keyhole
limpet haemocyanin (Vos & Van Driel-Grootenhuis, 1972; Koller &
Thigpen, 1973; Loose et al., 1977; Wierda et al., 1981; Exon, 1985;
Kunita et al., 1985). These data are in line with the observations of
Loose et al. (1977) and Thomas & Hinsdill (1978) that exposure to PCBs
lowered circulating immunoglobulin levels in mice. No reduction was
reported in antibody responses to bovine serum albumin (Talcott &
Koller, 1983).
The response to sheep red blood cells in the plaque-forming cell
assay has been used to establish dose-response relationships for
several potentially immunotoxic Aroclors in mice given a single
intraperitoneal injection of PCB mixtures (Davis & Safe, 1989). These
studies indicate that the higher chlorinated PCB mixtures are more
immunotoxic than the lower chlorinated Aroclors (Allen & Abrahamson,
1973; Loose et al., 1978; Tryphonas, in press). Data on the effects of
PCBs on total serum immunoglobulin levels have not been reported in
non-immunized animals.
While the suppressive effects of PCBs on humoral immunity are
well documented, the effects on cell-mediated immune parameters are
less clear. The delayed-type hypersensitivity reaction to tuberculin
was suppressed in guinea-pigs (Vos & Van Driel-Grootenhuis, 1972) but
not in rabbits treated with PCBs (Street & Sharma, 1975). Decreased
delayed-type hypersensitivity reactions were reported in mice by Smith
et al. (1978) but not by others (Talcott & Koller, 1983). Kerkvliet &
Baecher-Steppan (1988) reported that 3,4,5,3',4',5'-hexachlorobiphenyl
reduced Tc lymphocyte activity in the spleens of mice. In contrast,
the graft-versus-host reaction was increased following PCB treatment
(Carter & Clancy, 1980). Studies on the mitogen-induced responses of
splenic mononuclear leukocytes from PCB-treated mice in vitro
resulted in either enhanced or unaltered responses (Bonnyns &
Bastomsky, 1976; Wierda et al., 1981; Davis & Safe, 1989; Smialowicz
et al., 1989).
Functional impairment of the non-specific resistance of macro-
phages has been reported, including reduced phagocytic activity and
clearance of pathogenic bacteria by the spleens and livers of PCB-
exposed animals (Smith et al., 1978) and decreased NK cell activity
(Talcott et al., 1985; Smialowicz et al., 1989). Exposure of mice to
PCBs also enhanced their sensitivity to endotoxin shock (Loose et al.,
1978; Thomas & Hinsdill, 1978).
PCB treatment was shown to protect mice and rats against
Ehrlich's tumour (Keck, 1981) and Walker 256 tumours (Kerkvliet &
Kimeldorf, 1977), shown as reduced tumour growth and metastasis after
transplantation; in other studies, however, no influence of PCB on
tumour-cell implants was reported (Koller, 1977; Loose et al., 1981).
PCBs also affect the resistance of animals to infectious diseases.
Thus, ducklings exposed to low levels of PCBs were more susceptible to
challenge with duck hepatitis virus (Friend & Trainer, 1970), and mice
were more susceptible to challenge with Moloney leukaemia virus
(Koller, 1977), Plasmodium berghei (Loose et al., 1978),
S. typhimurium (Loose et al., 1978), L. monocytogenes (Thomas &
Hinsdill, 1978), and Herpes simplex and Ectromelia viruses
(Imanishi et al., 1980).
The immunotoxic effects of PCBs have also been investigated in a
number of studies with non-human primates. Decreased titres of anti-
sheep red blood cells have been observed in PCB-exposed rhesus (Thomas
& Hinsdill, 1978) and cynomolgus monkeys (Hori et al., 1982; Truelove
et al., 1982; Kunita et al., 1985). Immunotoxic effects were also
reported in adult female rhesus monkeys and their infants (exposed
in utero and through lactation) after low-level exposure (Tryphonas
et al., 1989, 1991a,b). In this study, five groups of female rhesus
monkeys were administered PCB (Aroclor 1254) at 0, 5.0, 20.0, 40.0, or
80.0 µg/kg body weight per day orally. Immunological effects were
reported after both 23 and 55 months of exposure and comprised
significantly decreased IgM and IgG responses to sheep red blood cells
at the lowest dose. Alterations in T-cell subsets were reported in the
group receiving the high dose in comparison with the controls, which
were characterized by an increase in Ts/Tc (CD8) cells and a reduction
in the relative numbers of Th/inducer cells (CD4) and in the CD4:CD8
ratio. No effects were seen on total lymphocytes or on B cells or on
total serum IgG, IgM, and IgA levels. A further study indicated that
Aroclor 1254 had no effect on B lymphocytes, since antibody responses
to T-independent pneumoccocal antigen were not significantly affected.
A trend for reduced incorporation of 3H-thymidine by mitogen-induced
lymphocyte proliferation was noted only for the T mitogens
phytohaemagglutinin and concanavalin A and not for the B pokeweed
mitogen. A significant augmentation of NK cell activity was noted at
the highest dose. Total serum complement activity (CH50) was also
increased. The serum levels of corticosteroids (hydrocortisone), which
were measured throughout the study, were not affected by treatment
(Loo et al., 1989), clearly indicating that the changes in several of
the immune parameters were direct effects of Aroclor 1254 on the
immune system.
2.2.2.3 Hexachlorobenzene
Hexachlorobenzene (HCB) is a highly persistent chemical which was
used in the past as a fungicide. Emissions to the environment now
occur owing to its use as a chemical intermediate and its presence as
a by-product in several chemical processes. It is an immunotoxic
compound (Vos, 1986), with different effects in rats and mice. In
rats, the main changes seen after subacute exposure are increased
weights of spleen and lymph nodes; the serum levels of IgM are also
increased. Histologically, the spleen shows hyperplasia of follicles
and the marginal zone (Figure 24); the lymph nodes have more follicles
with germinal centres and greater proportions of high endothelial
venules, indicating activation (Figures 25 and 26). High endothelial-
type venules are also induced in the lung (Figure 27), and macrophages
accumulate in lung alveoli (Kitchin et al., 1982; Vos, 1986; see also
Figure 28).
Functional assessment showed an increase in cell-mediated
immunity (delayed-type hypersensitivity) and an even greater increase
in antibody-mediated immunity (primary and secondary antibody response
to tetanus toxoid). Macrophage functions were unaltered. Stimulation
of immune reactivity occurs at dietary levels as low as 4 mg/kg after
combined pre- and postnatal exposure for six weeks, whereas the
conventional parameters of hepatotoxicity are not altered at this dose
(Vos et al., 1979a; Vos, 1986). The developing immune system of the
rat therefore seems to be particularly vulnerable to the immunotoxic
action of HCB. Reduced NK cell activity has also been found in the
lung after oral exposure to 150-450 mg/kg HCB in the diet (Van Loveren
et al., 1990c).
Studies on the mechanism of action of HCB indicate a role for
T cells: congenitally athymic rnu/rnu rats, which lack T cells, do
not manifest the hyperplasia of B lymphocytes in splenic follicles and
the marginal zone after administration of the compound; but
endothelial cell proliferation and macrophage accumulation in the lung
are apparently T cell-independent, as these effects were seen in
athymic animals (Vos et al., 1990b). In contrast to the
immunostimulatory effect in rats, HCB suppresses cell-mediated and
antibody-mediated immunity in mice, as well as their resistance to
protozoan infections ( Leishmania and Plasmodium berghei) and to
inoculated tumours (Loose et al., 1977, 1978, 1981). The
susceptibility of mice to HCB is also higher after pre-or perinatal
administration (Barnett et al., 1987). Recent studies indicate that
the immunostimulatory effect of HCB in rats may be related to
autoimmunity:
Functional tests have been focused on humoral immune responses.
Exposure of guinea-pigs, rabbits, mice, and rats to PCBs at different
regimens reduced antibody production to foreign antigens, including
tetanus toxoid, pseudorabies virus, sheep red blood cells, and keyhole
limpet haemocyanin (Vos & Van Driel-Grootenhuis, 1972; Koller &
Thigpen, 1973; Loose et al., 1977; Wierda et al., 1981; Exon, 1985;
Kunita et al., 1985). These data are in line with the observations of
Loose et al. (1977) and Thomas & Hinsdill (1978) that exposure to PCBs
lowered circulating immunoglobulin levels in mice. No reduction was
reported in antibody responses to bovine serum albumin (Talcott &
Koller, 1983).
The response to sheep red blood cells in the plaque-forming cell
assay has been used to establish dose-response relationships for
several potentially immunotoxic Aroclors in mice given a single
intraperitoneal injection of PCB mixtures (Davis & Safe, 1989). These
studies indicate that the higher chlorinated PCB mixtures are more
immunotoxic than the lower chlorinated Aroclors (Allen & Abrahamson,
1973; Loose et al., 1978; Tryphonas, in press). Data on the effects of
PCBs on total serum immunoglobulin levels have not been reported in
non-immunized animals.
While the suppressive effects of PCBs on humoral immunity are
well documented, the effects on cell-mediated immune parameters are
less clear. The delayed-type hypersensitivity reaction to tuberculin
was suppressed in guinea-pigs (Vos & Van Driel-Grootenhuis, 1972) but
not in rabbits treated with PCBs (Street & Sharma, 1975). Decreased
delayed-type hypersensitivity reactions were reported in mice by Smith
et al. (1978) but not by others (Talcott & Koller, 1983). Kerkvliet &
Baecher-Steppan (1988) reported that 3,4,5,3',4',5'-hexachlorobiphenyl
reduced Tc lymphocyte activity in the spleens of mice. In contrast,
the graft-versus-host reaction was increased following PCB treatment
(Carter & Clancy, 1980). Studies on the mitogen-induced responses of
splenic mononuclear leukocytes from PCB-treated mice in vitro
resulted in either enhanced or unaltered responses (Bonnyns &
Bastomsky, 1976; Wierda et al., 1981; Davis & Safe, 1989; Smialowicz
et al., 1989).
Functional impairment of the non-specific resistance of macro-
phages has been reported, including reduced phagocytic activity and
clearance of pathogenic bacteria by the spleens and livers of PCB-
exposed animals (Smith et al., 1978) and decreased NK cell activity
(Talcott et al., 1985; Smialowicz et al., 1989). Exposure of mice to
PCBs also enhanced their sensitivity to endotoxin shock (Loose et al.,
1978; Thomas & Hinsdill, 1978).
PCB treatment was shown to protect mice and rats against
Ehrlich's tumour (Keck, 1981) and Walker 256 tumours (Kerkvliet &
Kimeldorf, 1977), shown as reduced tumour growth and metastasis after
transplantation; in other studies, however, no influence of PCB on
tumour-cell implants was reported (Koller, 1977; Loose et al., 1981).
PCBs also affect the resistance of animals to infectious diseases.
Thus, ducklings exposed to low levels of PCBs were more susceptible to
challenge with duck hepatitis virus (Friend & Trainer, 1970), and mice
were more susceptible to challenge with Moloney leukaemia virus
(Koller, 1977), Plasmodium berghei (Loose et al., 1978),
S. typhimurium (Loose et al., 1978), L. monocytogenes (Thomas &
Hinsdill, 1978), and Herpes simplex and Ectromelia viruses
(Imanishi et al., 1980).
The immunotoxic effects of PCBs have also been investigated in a
number of studies with non-human primates. Decreased titres of anti-
sheep red blood cells have been observed in PCB-exposed rhesus (Thomas
& Hinsdill, 1978) and cynomolgus monkeys (Hori et al., 1982; Truelove
et al., 1982; Kunita et al., 1985). Immunotoxic effects were also
reported in adult female rhesus monkeys and their infants (exposed
in utero and through lactation) after low-level exposure (Tryphonas
et al., 1989, 1991a,b). In this study, five groups of female rhesus
monkeys were administered PCB (Aroclor 1254) at 0, 5.0, 20.0, 40.0, or
80.0 µg/kg body weight per day orally. Immunological effects were
reported after both 23 and 55 months of exposure and comprised
significantly decreased IgM and IgG responses to sheep red blood cells
at the lowest dose. Alterations in T-cell subsets were reported in the
group receiving the high dose in comparison with the controls, which
were characterized by an increase in Ts/Tc (CD8) cells and a reduction
in the relative numbers of Th/inducer cells (CD4) and in the CD4:CD8
ratio. No effects were seen on total lymphocytes or on B cells or on
total serum IgG, IgM, and IgA levels. A further study indicated that
Aroclor 1254 had no effect on B lymphocytes, since antibody responses
to T-independent pneumoccocal antigen were not significantly affected.
A trend for reduced incorporation of 3H-thymidine by mitogen-induced
lymphocyte proliferation was noted only for the T mitogens
phytohaemagglutinin and concanavalin A and not for the B pokeweed
mitogen. A significant augmentation of NK cell activity was noted at
the highest dose. Total serum complement activity (CH50) was also
increased. The serum levels of corticosteroids (hydrocortisone), which
were measured throughout the study, were not affected by treatment
(Loo et al., 1989), clearly indicating that the changes in several of
the immune parameters were direct effects of Aroclor 1254 on the
immune system.
2.2.2.3 Hexachlorobenzene
Hexachlorobenzene (HCB) is a highly persistent chemical which was
used in the past as a fungicide. Emissions to the environment now
occur owing to its use as a chemical intermediate and its presence as
a by-product in several chemical processes. It is an immunotoxic
compound (Vos, 1986), with different effects in rats and mice. In
rats, the main changes seen after subacute exposure are increased
weights of spleen and lymph nodes; the serum levels of IgM are also
increased. Histologically, the spleen shows hyperplasia of follicles
and the marginal zone (Figure 24); the lymph nodes have more follicles
with germinal centres and greater proportions of high endothelial
venules, indicating activation (Figures 25 and 26). High endothelial-
type venules are also induced in the lung (Figure 27), and macrophages
accumulate in lung alveoli (Kitchin et al., 1982; Vos, 1986; see also
Figure 28).
Functional assessment showed an increase in cell-mediated
immunity (delayed-type hypersensitivity) and an even greater increase
in antibody-mediated immunity (primary and secondary antibody response
to tetanus toxoid). Macrophage functions were unaltered. Stimulation
of immune reactivity occurs at dietary levels as low as 4 mg/kg after
combined pre- and postnatal exposure for six weeks, whereas the
conventional parameters of hepatotoxicity are not altered at this dose
(Vos et al., 1979a; Vos, 1986). The developing immune system of the
rat therefore seems to be particularly vulnerable to the immunotoxic
action of HCB. Reduced NK cell activity has also been found in the
lung after oral exposure to 150-450 mg/kg HCB in the diet (Van Loveren
et al., 1990c).
Studies on the mechanism of action of HCB indicate a role for
T cells: congenitally athymic rnu/rnu rats, which lack T cells, do
not manifest the hyperplasia of B lymphocytes in splenic follicles and
the marginal zone after administration of the compound; but
endothelial cell proliferation and macrophage accumulation in the lung
are apparently T cell-independent, as these effects were seen in
athymic animals (Vos et al., 1990b). In contrast to the
immunostimulatory effect in rats, HCB suppresses cell-mediated and
antibody-mediated immunity in mice, as well as their resistance to
protozoan infections ( Leishmania and Plasmodium berghei) and to
inoculated tumours (Loose et al., 1977, 1978, 1981). The
susceptibility of mice to HCB is also higher after pre-or perinatal
administration (Barnett et al., 1987). Recent studies indicate that
the immunostimulatory effect of HCB in rats may be related to
autoimmunity:
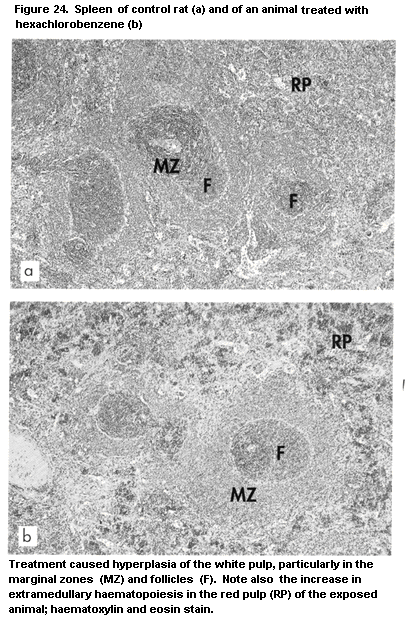
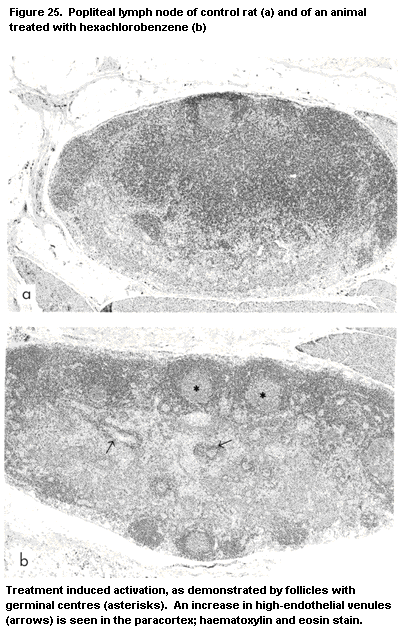
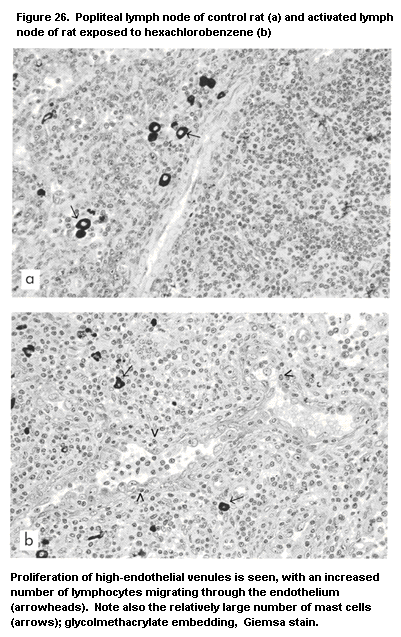
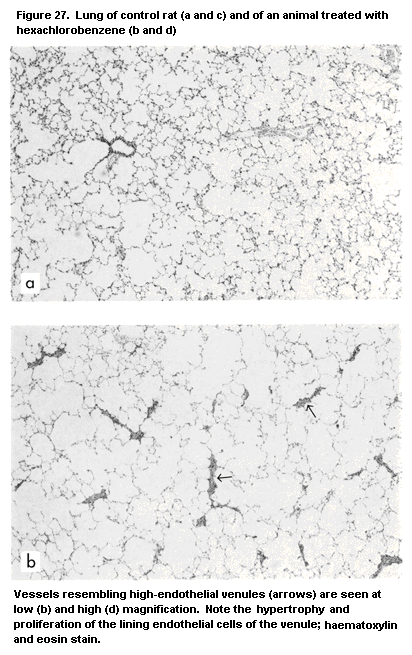
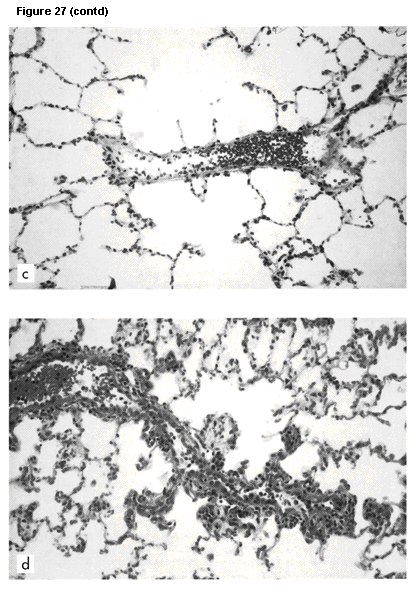
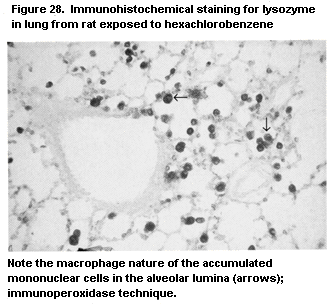 1. Exposure to HCB of Lewis rats, which develop autoimmune disease
after sensitization with complete Freund's adjuvant (adjuvant
arthritis) or with guinea-pig myelin (experimental allergic
encephalomyelitis), had clear effects (Van Loveren et al., 1990c):
Whereas the allergic encephalomyelitis response was severely enhanced,
the arthritic lesions were strongly suppressed.
2. Wistar rats treated with HCB produce antibodies to autoantigens;
thus, IgM, but not IgG, levels against single-stranded DNA, native
DNA, rat IgG (representing rheumatoid factor), and bromelain-treated
mouse erythrocytes (indicating that phosphatidylcholine is a major
autoantigen) were elevated. It has been suggested that HCB activates a
B-cell subset committed to the production of these autoantibodies and
associated with various systemic autoimmune diseases (Schielen et al.,
1993).
2.2.3 Pesticides
A large number of studies have focused on the immunotoxicity of
pesticides. Because of the chemical heterogeneity of these compounds
as a class, the reported effects vary widely (Barnett & Rodgers,
1994).
2.2.3.1 Organochlorine pesticides
The evidence for the immunotoxicity of organochlorine pesticides
as a class is inconclusive.
DDT: Wistar rats treated with 40 mg/kg body weight per day DDT
orally for 60 days showed increased anti-bovine serum albumin titres
(Lukic et al., 1973). Studies by Vos et al. (Vos & Krajnc, 1983, Vos
et al., 1983a), however, showed no changes in thymus or spleen
weights, leukocyte counts, or total serum IgG and IgM levels at doses
up to 800 mg/kg body weight per day.
Chlordane: Prenatal exposure of mice to chlordane was reported
to reduce contact and delayed hypersensitivity responses, suggesting
an effect on T-cell responses. Attempts to elucidate the mechanism
have, however, been unsuccessful. Johnson et al. (1986) observed
increased lymphocyte proliferation only at a dose of 8 mg/kg body
weight in B6C3F1 mice and concluded that chlordane has no significant
immunotoxicity in this model.
Chlordecone: Chlordecone reduced thymus and spleen weights by
40% in Fischer rats at at a dose of 10 mg/kg body weight per day but
had no significant effect at or below 5 mg/kg body weight per day.
T-Lymphocyte proliferation was unaffected at all doses (Smialowicz et
al., 1985a).
Lindane: Lindane had various effects on the anti-sheep red
blood cell response, depending on the immunization protocol. Specific
IgM levels were unchanged by parenteral immunization after four weeks'
treatment with 150 mg/kg in the diet, but specific IgG2b levels were
raised after intragastric immunization (André et al., 1983); however,
the duration of Giardia muris infection was significantly prolonged.
Five weeks' oral treatment with up to 12 mg/kg of diet decreased the
antibody titre to TY3 vaccine in rabbits in a dose-dependent manner
(Desi et al., 1978).
Toxaphene: Toxaphene given at 100 or 200 mg/kg of diet
decreased anti-bovine serum albumin antibody titres in Swiss mice
treated for eight weeks and in the offspring of dams given the same
diet. Macrophage activity was also reduced in these offspring, but
there were no changes in the delayed-type hypersensitivity response to
purified protein derivative (Allen et al., 1983).
Endosulfan: Endosulfan had no immunotoxic effect in Wistar rats
(Vos & Krajnc, 1983; Vos et al., 1983a).
2.2.3.2 Organophosphorus compounds
Single doses of the insecticides parathion, malathion, and
dichlorvos cause significant reductions in anti-sheep red blood cell
plaque-forming cell responses (Casale et al., 1983, 1984). The
relevance of these findings is questionable, however, as they occurred
only if cholinergic or parathion symptoms were also induced.
Administration of multiple doses of malathion resulted in conflicting
findings: C57Bl6 mice given four doses of 240 mg/kg body weight over
eight days had unchanged plaque-forming cell responses to sheep red
blood cells. In contrast, rabbits treated with 5-10 mg/kg body weight
per day over 5-6 weeks had reduced antibody titres after vaccination
with S. typhimurium. Parathion also failed to suppress anti-sheep
red blood cell plaque-forming cell formation when given as four doses
of 4 mg/kg body weight (Casale et al., 1983). Parathion-methyl given
to rabbits for four weeks did not affect immune responses (Desi et
al., 1978). In mice, however, both cellular and humoral responses were
reported to be suppressed by subacute administration of parathion
(Wiltrout et al., 1978).
The immunotoxicity of MPT-IP (the industrial compound for the
production of Wofatox EC50, containing 60% parathion-methyl) was
studied in mice given single oral doses of 8.9 mg/kg body weight or
repeated doses of 0.890 or 0.445 mg/kg body weight for four weeks.
Depending on the day of administration, the single dose increased the
IgM plaque-forming cell content of the spleen and the serum anti-sheep
red blood cell antibody titre. In the subacute system, the smaller
dose (0.445 mg/kg) increased the splenic plaque-forming cell content
and serum antibody titre (Institoris et al., 1992).
Dimethoate was administered by gavage to three generations of
Wistar rats at doses of 14.1, 9.39, and 7.04 mg/kg body weight
(equivalent to 1/50, 1/75, and 1/100 of the LD50), and parathion-
methyl was administered at doses of 0.436, 0.291, a,d 0.218 mg/kg body
weight. The highest dose of dimethoate significantly decreased the
plaque-forming capacity of spleen cells in the first generation and
increased thymic weight in the second and third generations. All three
doses of parathion-methyl decreased the number of red blood cells and
the haematocrit value, and the two highest doses decreased the
leukocyte count. The nucleated cell content of the bone marrow was
increased in the second and third generations, and decreased relative
thymic weight was seen at all three doses in the third generation
(Institoris et al., 1995). In a similar experiment, dichlorvos was
administered at doses of 1.85, 1.24, or 0.972 mg/kg body weight. A
significant decrease in leukocyte count, lowered spleen cellularity,
and decreased plaque-forming capacity were seen with the highest dose
in the second generation. In the third generation, there was a dose-
dependent decrease in femoral bone-marrow cellularity (Institoris et
al., in press). In vitro, 250 µmol/litre of paraoxon, a parathion
metabolite, suppressed mitogenic lymphocyte proliferation in spleen
cells from Sprague-Dawley rats (Pruett & Chambers, 1988).
Reduction of antibody titre against Ty3 vaccine was observed by
the end of six weeks' oral treatment of rabbits with 5-100 mg/kg body
weight of malathion or with 1.25 or 2.5 mg/kg body weight of
dichlorphos (Desi et al., 1978). In the same system, a dose-dependent
decrease was observed in the tuberculin skin reaction after
administration of 0.31, 0.62, or 1.25 mg/kg body weight of
dichlorphos. The cholinesterase activity of red blood cells was
decreased only by the two higher doses.
Convincing evidence for immunotoxicity has been obtained only for
O,O,S-trimethylphosphorothiate ( O,O,S-TMP), a contaminant of
various commercial organophosphorus formulations, such as malathion,
fenitrothion, and acephate. This compound was shown to suppress
humoral and cellular immunity in mice exposed to 10 mg/kg body weight
orally (Devens et al., 1985). Several organophosphorus derivatives can
alter some immune functions in vitro, including mitogen-induced
lymphocyte proliferation (Pruett & Chambers, 1988), T-lymphocyte
cytotoxicity, and production of hydrogen peroxide by macrophages
(Pruett, 1992), at concentrations that can theoretically be attained
in vivo.
Several mechanisms have been proposed to explain organo-
phosphorus-induced immunosuppression (Pruett, 1992). A direct
cholinergic mechanism is unlikely to be involved, as the addition of
various cholinergic agonists does not suppress immune responses in
vitro. In addition, O,O,S-trimethylphosphorodithioate, a structural
analogue of O,O,S-TMP, modulates cholinesterase activity but does
not alter immune competence. An indirect mechanism involving
stress caused by neurotoxicity has also been proposed. Finally, a
direct action on cells of the immune system, and particularly
macrophages, has been suggested to be involved. Mice treated with
O,O,S-TMP, which is not neurotoxic, generate a population of
macrophages, contraindicating lymphocyte proliferation. Antigen
processing and presentation by these highly activated (inflammatory)
macrophages are severely impaired; however, the changes in macrophage
function are not correlated with suppression of humoral or cellular
immunity. While there is no direct evidence that B and T lymphocytes
are the predominant targets of organophosphorus compounds, their
mechanisms of action on macrophages are largely unknown.
2.2.3.3 Pyrethroids
Dose-dependent decreases in the serum anti- S. typhimurium
antibody titre and in the tuberculin skin reaction were observed in
rabbits fed 25, 12.5, or 6.25 mg/kg body weight of technical-grade
cypermethrin (93.5%) for seven weeks (Desi et al., 1985). Single oral
doses (23.5, 20.7, or 18.7 mg/kg body weight) of supermethrin, the
active substance of the pyrethroid pesticide Neramethrin EC 50,
decreased the number of IgM plaque-forming cells in the spleens of
mice but had no effect on the delayed-type hypersensitivity reaction.
Repeated doses of 2.97, 1.49, and 0.743 mg/kg body weight caused only
slight changes in the leukocyte count and in the nucleated cell
content of femoral bone marrow (Siroki et al., 1994).
2.2.3.4 Carbamates
Carbaryl induced marked increases in serum IgG1 and IgG2, but not
IgA, IgG3, or IgM, levels of mice exposed to 150 mg/kg of diet for one
month (André et al., 1983). Rabbits given carbaryl at 4-150 mg/kg of
diet for four weeks had no changes in anti-sheep red blood cell
haemolysin or haemagglutinin titres or in the delayed-type
hypersensitivity response to tuberculin, whereas oral treatment with
carbofuran at 0.5-20 mg/kg of diet for four weeks induced a 60-75%
decrease in the delayed-type hypersensitivity response (Street &
Sharma, 1975). Aldicarb induced no changes in a large battery of
assays for immune function and host resistance in B6C3F1 mice exposed
to 0.1-1000 mg/litre of drinking-water daily for 34 days (Thomas et
al., 1987).
2.2.3.5 Dinocap
Dinocap is a dinitrophenol compound used as a fungicide. Female
C57Bl/6J mice were given doses of 12.5-50 mg/kg body weight per day by
gavage for 7 or 12 days. All mice given the highest dose died after
four days. Mice given 25 mg/kg for 12 days had decreased thymus
weights and cellularity and increased spleen weights but no changes in
body weight, leukocyte count, lymphoproliferative response to B- or
T-cell mitogens, mixed lymphocyte reaction, or NK cell activity of
spleen cells; lymphoproliferative responses to concanavalin A and
phytohaemagglutinin in thymocytes were reduced. In mice exposed for
seven days to 25 mg/kg body weight per day, the cytotoxic T lymphocyte
response to P815 mastocytoma cells was enhanced, and there was a
significant reduction in the IgM and IgC plaque-forming cell response
to sheep red blood cells. In vitro in murine thymocytes, a
concentration of 10 µg/ml dinocap for 72h suppressed the proliferative
response to concanavalin A and phytohaemagglutinin; exposure for as
little as 30 min suppressed the mitogen-stimulated response with no
direct cytotoxicity (Smialowicz et al., 1992a).
2.2.4 Polycyclic aromatic hydrocarbons
A major concern for human health is the carcinogenic potential of
most polycyclic aromatic hydrocarbons (PAHs). Interestingly, those
which are carcinogenic also have potent immunosuppressive properties,
whereas those which are not carcinogenic lack marked immunotoxic
effects (Ward et al., 1985; White, 1986). Suppression of humoral
immunity has been observed frequently after exposure to a number of
PAHs, including benzo[ a]pyrene, DMBA, and 3-methylcholanthrene (Ward
et al., 1985). Structure-activity studies by White et al. (1985), in
which the antibody-forming cell response was used to evaluate 10 PAHs
in B6C3F1 and DBA/2 mice, demonstrated a wide spectrum of activity:
compounds like benzo[ e]pyrene and perylene were not immunotoxic,
whereas dibenz[ a,h)anthracene and DMBA were potent immunosuppressors
of the plaque-forming cell response. Interestingly, the DBA/2 mice
were more susceptible to the immunosuppressive effects than the B6C3F1
mice.
PAHs also suppress cell-mediated immunity. T-Lymphocyte
cytotoxicity and mixed lymphocyte responsiveness were found to be
impaired by most PAHs. Differences between PAHs are seen, however, in
that benzo[ a]pyrene may be less suppressive of cell-mediated
immunity than DMBA, accounting for the greater host susceptibility to
L. monocytogenes and PYB6 sarcoma challenges in DMBA- than in
benzo[ a]pyrene-treated rodents (Ward et al., 1985). Thurmond et al.
(1987) evaluated immunosuppression in B6C3F1 ( Ah-responsive) and
DBA/2 ( Ah-nonresponsive) mice and in Ah-congenic C57Bl/6J
(responsive B6-AhbAhd and nonresponsive B6-AhdAhd) mice after
exposure to DMBA in a battery of immunological assays, including
evaluation of organ weights, plaque-forming cell response, mitogen
responses, and mixed lymphocyte responses. The authors concluded that
the immunosuppressive action of DMBA was independent of the Ah locus
and associated induction of cytochrome P1-450 metabolizing enzymes.
The mechanisms of PAH-mediated immunosuppression remain to be
elucidated. PAHs may exert their immunotoxic effects as the parent
compound or as metabolites. In vitro many of the metabolites of
benzo[ a]pyrene and DMBA are immunosuppressive, the diol metabolites
being the most potent (Kawabata & White, 1987; Ladics et al., 1991).
Several possible mechanisms of action have been proposed, including
altered interleukin levels (Lyte & Bick, 1986; Pallardy et al., 1989),
a direct effect on transmembrane signalling (Pallardy et al., 1992),
and alterations in intracellular calcium mobilization (Burchiel et
al., 1991; Davis & Burchiel, 1992).
Earlier studies suggested that Th cells or faulty antigen
recognition by T cells were possible mechanisms of DMBA-induced
immunosuppression (House et al., 1987, 1989). Myers et al. (1987) also
reported that benzo[ a]pyrene alters macrophage antigen presentation.
Studies by Ladics et al. (1992) demonstrated that the only splenic
cell type capable of metabolizing benzo[ a]pyrene was the macrophage
and that the predominant immunosuppressive metabolite formed was the
benzo[ a]pyrene-7,8 diol epoxide, which is also believed to be the
ultimate carcinogenic metabolite of benzo[ a]pyrene.
2.2.5 Solvents
2.2.5.1 Benzene
Exposure to benzene is associated with myelotoxicity, and a
strong correlation was noted between lymphocytopenia and abnormal
immunological parameters. The myelotoxicity may be due, in part, to
altered differentiation of marrow lymphoid cells, as suggested by the
finding that acute exposure of IgM+ cell-depleted marrow cultures to
hydroquinone, an oxidative metabolite of benzene, blocked the final
maturation stages of B-cell differentiation (King et al., 1987). In
addition, it was shown that the hydroquinone metabolite inhibits
lectin-stimulated lymphocyte agglutination and mitogenesis by reacting
with intracellular sulfhydryl groups (Pfeifer & Irons, 1981).
Immunosuppression associated with exposure to benzene was found
in rabbits to be an impaired antibody response together with an
increased susceptibility to tuberculosis and pneumonia. Similarly,
C57Bl/6 mice exposed to benzene had a lower antibody response and
reduced mitogen-induced lymphocyte proliferation (Wierda et al.,
1981). Chronic inhalation of concentrations as low as 30 ppm impaired
resistance to L. monocytogenes (Rosenthal & Snyder, 1985).
Similarly, increased susceptibility to PYB6 tumour cell challenge was
seen at concentrations that also impaired Tc lymphocyte function.
The mechanism of benzene-induced immunosuppression is unclear.
Cellular depletion may be the major effect, although B- and T-cell
dysfunction may also be involved. The antiproliferative effects of
benzene may be related to its ability to alter cytoskeletal
development through inhibition of microtubule assembly. Polyhydroxy
metabolites of benzene ( para-benzoquinone and hydroquinone) have
been shown to bind to sulfhydryl groups on the proteins necessary for
the integrity and polymerization of microtubules. This effect may
alter cell membrane fluidity and may explain the sublethal effect of
benzene on lymphocyte function.
2.2.5.2 Other solvents
Hexanediol (1.2 mg/kg per day for seven days) decreased thymus
and spleen weights, antibody production, and delayed-type
hypersensitivity in mice (Kannan et al., 1985). Humoral immunity was
suppressed to a greater extent in female than in male mice after a
four-month exposure to trichloroethylene in the drinking-water at
doses of 0.1, 1.0, 2.5, or 5.0 mg/ml; cell-mediated immunity and bone-
marrow stem-cell colonization were inhibited only in females (Sanders
et al., 1982). The immunotoxicity of glycol ethers and some of their
metabolites has been studied in rats by measuring the plaque-forming
cell response to trinitrophenyl lipopolysaccaride. The glycol ethers
2-methoxyethanol and 2-methoxyethylacetate were immunosuppressive, as
was the principal metabolite of the latter, 2-methoxyacetic acid. The
glycol ethers 2-(2-methoxyethoxy)ethanol, bis(2-methoxyethyl) ether,
2-ethoxyethanol and its principal metabolite 2-ethoxyacetic acid,
2-ethoxyethyl acetate, and 2-butoxyethanol were not immunosuppressive
(Smialowicz et al., 1991, 1992b, 1993)
Dichloroethylene did not induce immunotoxic changes in mice given
up to 2 mg/litre per day for 90 days (Shopp et al., 1985). Similarly
negative findings were obtained with trichloroethane (Sanders et al.,
1985).
2.2.6 Metals
Heavy metals have been shown to alter immune responsiveness in
laboratory animals (Koller, 1980). Alterations in B lymphocyte
function have been observed most frequently after exposure to lead and
cadmium, but T-cell and macrophage changes have also been described.
In addition, exposure to metals is correlated better with impaired
resistance to experimental infections than with changes in B-
or T-cell functions. Interestingly, immunostimulation has been
shown to occur at levels of exposure lower than those associated with
immunosuppression. Metals have also been shown to induce immuno-
potentiation, at lower doses than those that cause immunosuppression.
2.2.6.1 Cadmium
Conflicting results have been obtained with regard to the effect
of cadmium on humoral immunity in animals (Descotes et al., 1990).
Cell-mediated immunity, however, is consistently depressed after both
short- and long-term exposure, and phagocytosis and NK cell activity
are found to be depressed. Susceptibility to L. monocytogenes, Herpes
simplex 1 and 2, and influenza virus was increased in B6C3F1 mice
exposed for long periods (Thomas et al., 1985a).
2.2.6.2 Lead
Experimental studies suggest that lead has immunosuppressive
effects in rodents (Lawrence, 1985; Descotes et al., 1990; Koller,
1990). Early studies demonstrated that lead can suppress the humoral
immune response of mice exposed as adults (Koller & Kovacic, 1974) and
of rats exposed pre- and postnatally (Luster et al., 1978). In
contrast, no change in humoral immunity was found in mice exposed to
0.08-10 mmol/litre in drinking-water (Lawrence, 1981) or after a
10-week oral treatment with 13, 130, or 1300 mg/kg of diet (as lead
acetate) (Koller & Roan, 1980). Delayed-type hypersensitivity was
found to be depressed by lead acetate and lead chloride but not in
mice treated with lead oxide, nitrate, or carbonate. The most
consistent finding in experimental studies of the effects of lead on
host resistance, however, is increased susceptibility to infectious
agents (McCabe, 1994).
With respect to nonspecific host defence mechanisms, mice treated
with lead at doses of 5, 10, or 20 µg/kg body weight given intra-
peritoneally once or at doses of 25, 50, or 100 µg/kg body weight
given orally once showed increased clearance of colloidal carbon
(Schlick & Friedberg, 1981). Furthermore, treatment of mice with 130
or 1300 ppm of lead orally for 10 weeks impaired the phagocytosis of
sheep red blood cells (Koller & Roan, 1977). Lead also has consistent
overall effects on host resistance to infection. Thus, treatment
resulted in significantly decreased resistance of mice to Klebsiella
pneumoniae (Hemphill et al., 1971) and S. typhimurium, and decreased
resistance of rats to a bacterial endotoxin and to a challenge with
E. coli, S. epidermidis, or S. enteritidis. The increased
susceptibility of rodents to Gram-negative bacteria after exposure
to lead is likely to be due to hypersensitivity to an endotoxin of
bacterial origin (Cook et al., 1974, 1975)
Organolead compounds, such as tetrethyllead, can also be
immunotoxic (Luster et al., 1992).
2.2.6.3 Mercury
Mercuric salts have been shown repeatedly to depress both humoral
and cellular immunity and nonspecific host defences in animals. For
instance, B6C3F1 mice given mercuric chloride orally for seven weeks
had decreased thymus and spleen weights, an impaired plaque-forming
cell response, and inhibition of lymphocyte proliferation at a daily
dose of 75 mg/litre of drinking-water (Dieter et al., 1983).
Methylmercury was reported to decrease humoral immunity in mice
treated orally for three weeks with 0.5, 2, or 10 mg/litre drinking-
water (Blackley et al., 1980).
2.2.6.4 Organotins
Several organotins have been shown to be markedly immunotoxic and
are considered as prototype immunotoxicants (Penninks et al., 1990),
even though no human data are available.
Di- n-octyltin dichloride at 50 or 150 mg/kg of diet for six
weeks induced a dramatic, dose-related decrease in the weight of the
thymus in rats, associated with a less severe decrease in spleen and
lymph node weights (Seinen & Willems, 1976). The numbers of cells in
the thymus and spleen, but not the bone marrow, were decreased.
Histologically, lymphocyte depletion was seen in the thymus and in
thymus-dependent areas of the spleen. Interestingly, thymic atrophy
recovered quickly after cessation of exposure (Seinen et al., 1977).
It was later shown to be associated with a 25% decrease in peripheral
blood lymphocytes, with a preferential loss of Th lymphocytes. As
expected, T-cell functions, such as the delayed-type hypersensitivity
response and T-lymphocyte proliferation, were depressed. Inhibition of
humoral immunity was also seen, with reduced numbers of plaque-forming
cells and decreased circulating antibody titres. NK cell activity was
not affected, whereas susceptibility to L. monocytogenes infection
was markedly increased.
Immune function is not impaired in guinea-pigs or mice fed
di- n-octyltin dichloride, which correlates with the absence of
thymic atrophy (Seinen & Penninks, 1979). Mice treated intravenously
or intraperitoneally develop thymic atrophy, however, suggesting
interspecies variability in the disposition of dialkyltins after oral
intake, although other, poorly understood mechanisms may account for
this variability (Penninks et al., 1990). No interspecies differences
in lymphocyte functions were noted after exposure in vitro.
Generally similar findings were made with the trialkylorganotin,
tri- n-butyltin oxide (TBTO). As trisubstituted organotins are
rapidly metabolized to disubstituted derivatives, the latter are
considered to be involved in the reported thymic effects (Snoeij et
al., 1988). In a short-term study in rats, pronounced effects were
found on the lymphoid organs: thymus (Figure 29), spleen, and lymph
nodes. These effects were most pronounced in thymus-dependent areas
(Figure 30) (Krajnc et al., 1984). Interestingly, thymus atrophy also
occurred in fish, as guppies exposed to organotin compounds showed
severe thymic atrophy (Figure 31). In function tests (Vos et al.,
1984), rats that were exposed to TBTO for six weeks after weaning had
suppressed delayed-type hypersensitivity responses to ovalbumin and
tuberculin and suppressed IgG responses to sheep erythrocytes. In
vitro mitogen responses to concanavalin A in thymus and spleen and
NK cell activity in both the spleen and the lungs were decreased (Van
Loveren et al., 1990b). Exposure to TBTO at 20 or 80 mg/kg of diet for
six weeks led to decreased resistance to infection with
L. monocytogenes or Trichinella spiralis. The latter effect was
evidenced by increased numbers of adult worms in the gut as a result
of impaired worm expulsion, increased numbers of muscle larvae in the
striated tissue, decreased inflammatory responses around these larvae,
and decreased antibody responses to T. spiralis, especially in the
IgE class (Vos et al., 1984). After long-term exposure (15-17 months)
to 5 or 50 mg/kg of diet, delayed-type hypersensitivity was not
suppressed, but assays for NK cell activity and resistance to
infection indicated suppression.
As the immune responsiveness of older animals can be expected to
be less strong than that of younger rats, the effects of exposure to
immunotoxic chemicals may become evident less easily; however, tests
for function still indicated immunotoxicity. In experiments in which
exposure to TBTO was begun only at 12 months of age, both infection
models showed immunotoxicity to TBTO. Very few studies have focused on
the immunotoxic effects of chemicals on the gut immune system, but the
studies of TBTO showed both a decreased capacity of the host to expel
adult T. spiralis worms from the gut and increased production of
serum IgA specific for this parasite (Vos et al., 1990a).
The mechanism of the immunotoxicity of organotin compounds has
been investigated extensively (Penninks et al., 1990). A direct
influence on the synthesis of thymic hormones is uncertain, as
conflicting results have been obtained in different experiments.
Interference with the influx of prothymocytes can be ruled out, as
thymic atrophy develops too rapidly. Interestingly, organotins reduced
the proliferative activity of thymocytes and the number of
proliferating thymoblasts within 24h after exposure was begun, at a
time when thymic atrophy was not evident. This selective effect on
thymoblasts and the physiological destruction of most cortical
thymocytes would result in marked depletion and, finally, in thymic
atrophy.
2.2.6.5 Gallium arsenide
Gallium arsenide is an intermetallic compound used widely in the
electronics industry, primarily in the manufacture of transistors and
light-emitting diodes. A single intratracheal instillation of 50, 100,
or 200 mg/kg body weight into female B6C3F1 mice resulted in a dose-
related decrease in the IgM and IgG antibody response to sheep
erythrocytes. Similarly, cell-mediated immunity, as evaluated by the
delayed-type hypersensitivity reaction to keyhole limpet haemocyanin
and the mixed lymphocyte response, was also decreased in a dose-
dependent way. Increases were observed in complement C3 levels,
mitogen response to lipopolysaccharide, and NK cell activity. No
effects were observed on response to T-cell mitogens, total complement
CH50 activity, or host resistance to Plasmodium yoelii or
Streptococcus pneumoniae; however, a significant decrease in host
resistance was observed to L. monocytogenes and B16F10 tumour
challenge (Sikorski et al., 1989).
1. Exposure to HCB of Lewis rats, which develop autoimmune disease
after sensitization with complete Freund's adjuvant (adjuvant
arthritis) or with guinea-pig myelin (experimental allergic
encephalomyelitis), had clear effects (Van Loveren et al., 1990c):
Whereas the allergic encephalomyelitis response was severely enhanced,
the arthritic lesions were strongly suppressed.
2. Wistar rats treated with HCB produce antibodies to autoantigens;
thus, IgM, but not IgG, levels against single-stranded DNA, native
DNA, rat IgG (representing rheumatoid factor), and bromelain-treated
mouse erythrocytes (indicating that phosphatidylcholine is a major
autoantigen) were elevated. It has been suggested that HCB activates a
B-cell subset committed to the production of these autoantibodies and
associated with various systemic autoimmune diseases (Schielen et al.,
1993).
2.2.3 Pesticides
A large number of studies have focused on the immunotoxicity of
pesticides. Because of the chemical heterogeneity of these compounds
as a class, the reported effects vary widely (Barnett & Rodgers,
1994).
2.2.3.1 Organochlorine pesticides
The evidence for the immunotoxicity of organochlorine pesticides
as a class is inconclusive.
DDT: Wistar rats treated with 40 mg/kg body weight per day DDT
orally for 60 days showed increased anti-bovine serum albumin titres
(Lukic et al., 1973). Studies by Vos et al. (Vos & Krajnc, 1983, Vos
et al., 1983a), however, showed no changes in thymus or spleen
weights, leukocyte counts, or total serum IgG and IgM levels at doses
up to 800 mg/kg body weight per day.
Chlordane: Prenatal exposure of mice to chlordane was reported
to reduce contact and delayed hypersensitivity responses, suggesting
an effect on T-cell responses. Attempts to elucidate the mechanism
have, however, been unsuccessful. Johnson et al. (1986) observed
increased lymphocyte proliferation only at a dose of 8 mg/kg body
weight in B6C3F1 mice and concluded that chlordane has no significant
immunotoxicity in this model.
Chlordecone: Chlordecone reduced thymus and spleen weights by
40% in Fischer rats at at a dose of 10 mg/kg body weight per day but
had no significant effect at or below 5 mg/kg body weight per day.
T-Lymphocyte proliferation was unaffected at all doses (Smialowicz et
al., 1985a).
Lindane: Lindane had various effects on the anti-sheep red
blood cell response, depending on the immunization protocol. Specific
IgM levels were unchanged by parenteral immunization after four weeks'
treatment with 150 mg/kg in the diet, but specific IgG2b levels were
raised after intragastric immunization (André et al., 1983); however,
the duration of Giardia muris infection was significantly prolonged.
Five weeks' oral treatment with up to 12 mg/kg of diet decreased the
antibody titre to TY3 vaccine in rabbits in a dose-dependent manner
(Desi et al., 1978).
Toxaphene: Toxaphene given at 100 or 200 mg/kg of diet
decreased anti-bovine serum albumin antibody titres in Swiss mice
treated for eight weeks and in the offspring of dams given the same
diet. Macrophage activity was also reduced in these offspring, but
there were no changes in the delayed-type hypersensitivity response to
purified protein derivative (Allen et al., 1983).
Endosulfan: Endosulfan had no immunotoxic effect in Wistar rats
(Vos & Krajnc, 1983; Vos et al., 1983a).
2.2.3.2 Organophosphorus compounds
Single doses of the insecticides parathion, malathion, and
dichlorvos cause significant reductions in anti-sheep red blood cell
plaque-forming cell responses (Casale et al., 1983, 1984). The
relevance of these findings is questionable, however, as they occurred
only if cholinergic or parathion symptoms were also induced.
Administration of multiple doses of malathion resulted in conflicting
findings: C57Bl6 mice given four doses of 240 mg/kg body weight over
eight days had unchanged plaque-forming cell responses to sheep red
blood cells. In contrast, rabbits treated with 5-10 mg/kg body weight
per day over 5-6 weeks had reduced antibody titres after vaccination
with S. typhimurium. Parathion also failed to suppress anti-sheep
red blood cell plaque-forming cell formation when given as four doses
of 4 mg/kg body weight (Casale et al., 1983). Parathion-methyl given
to rabbits for four weeks did not affect immune responses (Desi et
al., 1978). In mice, however, both cellular and humoral responses were
reported to be suppressed by subacute administration of parathion
(Wiltrout et al., 1978).
The immunotoxicity of MPT-IP (the industrial compound for the
production of Wofatox EC50, containing 60% parathion-methyl) was
studied in mice given single oral doses of 8.9 mg/kg body weight or
repeated doses of 0.890 or 0.445 mg/kg body weight for four weeks.
Depending on the day of administration, the single dose increased the
IgM plaque-forming cell content of the spleen and the serum anti-sheep
red blood cell antibody titre. In the subacute system, the smaller
dose (0.445 mg/kg) increased the splenic plaque-forming cell content
and serum antibody titre (Institoris et al., 1992).
Dimethoate was administered by gavage to three generations of
Wistar rats at doses of 14.1, 9.39, and 7.04 mg/kg body weight
(equivalent to 1/50, 1/75, and 1/100 of the LD50), and parathion-
methyl was administered at doses of 0.436, 0.291, a,d 0.218 mg/kg body
weight. The highest dose of dimethoate significantly decreased the
plaque-forming capacity of spleen cells in the first generation and
increased thymic weight in the second and third generations. All three
doses of parathion-methyl decreased the number of red blood cells and
the haematocrit value, and the two highest doses decreased the
leukocyte count. The nucleated cell content of the bone marrow was
increased in the second and third generations, and decreased relative
thymic weight was seen at all three doses in the third generation
(Institoris et al., 1995). In a similar experiment, dichlorvos was
administered at doses of 1.85, 1.24, or 0.972 mg/kg body weight. A
significant decrease in leukocyte count, lowered spleen cellularity,
and decreased plaque-forming capacity were seen with the highest dose
in the second generation. In the third generation, there was a dose-
dependent decrease in femoral bone-marrow cellularity (Institoris et
al., in press). In vitro, 250 µmol/litre of paraoxon, a parathion
metabolite, suppressed mitogenic lymphocyte proliferation in spleen
cells from Sprague-Dawley rats (Pruett & Chambers, 1988).
Reduction of antibody titre against Ty3 vaccine was observed by
the end of six weeks' oral treatment of rabbits with 5-100 mg/kg body
weight of malathion or with 1.25 or 2.5 mg/kg body weight of
dichlorphos (Desi et al., 1978). In the same system, a dose-dependent
decrease was observed in the tuberculin skin reaction after
administration of 0.31, 0.62, or 1.25 mg/kg body weight of
dichlorphos. The cholinesterase activity of red blood cells was
decreased only by the two higher doses.
Convincing evidence for immunotoxicity has been obtained only for
O,O,S-trimethylphosphorothiate ( O,O,S-TMP), a contaminant of
various commercial organophosphorus formulations, such as malathion,
fenitrothion, and acephate. This compound was shown to suppress
humoral and cellular immunity in mice exposed to 10 mg/kg body weight
orally (Devens et al., 1985). Several organophosphorus derivatives can
alter some immune functions in vitro, including mitogen-induced
lymphocyte proliferation (Pruett & Chambers, 1988), T-lymphocyte
cytotoxicity, and production of hydrogen peroxide by macrophages
(Pruett, 1992), at concentrations that can theoretically be attained
in vivo.
Several mechanisms have been proposed to explain organo-
phosphorus-induced immunosuppression (Pruett, 1992). A direct
cholinergic mechanism is unlikely to be involved, as the addition of
various cholinergic agonists does not suppress immune responses in
vitro. In addition, O,O,S-trimethylphosphorodithioate, a structural
analogue of O,O,S-TMP, modulates cholinesterase activity but does
not alter immune competence. An indirect mechanism involving
stress caused by neurotoxicity has also been proposed. Finally, a
direct action on cells of the immune system, and particularly
macrophages, has been suggested to be involved. Mice treated with
O,O,S-TMP, which is not neurotoxic, generate a population of
macrophages, contraindicating lymphocyte proliferation. Antigen
processing and presentation by these highly activated (inflammatory)
macrophages are severely impaired; however, the changes in macrophage
function are not correlated with suppression of humoral or cellular
immunity. While there is no direct evidence that B and T lymphocytes
are the predominant targets of organophosphorus compounds, their
mechanisms of action on macrophages are largely unknown.
2.2.3.3 Pyrethroids
Dose-dependent decreases in the serum anti- S. typhimurium
antibody titre and in the tuberculin skin reaction were observed in
rabbits fed 25, 12.5, or 6.25 mg/kg body weight of technical-grade
cypermethrin (93.5%) for seven weeks (Desi et al., 1985). Single oral
doses (23.5, 20.7, or 18.7 mg/kg body weight) of supermethrin, the
active substance of the pyrethroid pesticide Neramethrin EC 50,
decreased the number of IgM plaque-forming cells in the spleens of
mice but had no effect on the delayed-type hypersensitivity reaction.
Repeated doses of 2.97, 1.49, and 0.743 mg/kg body weight caused only
slight changes in the leukocyte count and in the nucleated cell
content of femoral bone marrow (Siroki et al., 1994).
2.2.3.4 Carbamates
Carbaryl induced marked increases in serum IgG1 and IgG2, but not
IgA, IgG3, or IgM, levels of mice exposed to 150 mg/kg of diet for one
month (André et al., 1983). Rabbits given carbaryl at 4-150 mg/kg of
diet for four weeks had no changes in anti-sheep red blood cell
haemolysin or haemagglutinin titres or in the delayed-type
hypersensitivity response to tuberculin, whereas oral treatment with
carbofuran at 0.5-20 mg/kg of diet for four weeks induced a 60-75%
decrease in the delayed-type hypersensitivity response (Street &
Sharma, 1975). Aldicarb induced no changes in a large battery of
assays for immune function and host resistance in B6C3F1 mice exposed
to 0.1-1000 mg/litre of drinking-water daily for 34 days (Thomas et
al., 1987).
2.2.3.5 Dinocap
Dinocap is a dinitrophenol compound used as a fungicide. Female
C57Bl/6J mice were given doses of 12.5-50 mg/kg body weight per day by
gavage for 7 or 12 days. All mice given the highest dose died after
four days. Mice given 25 mg/kg for 12 days had decreased thymus
weights and cellularity and increased spleen weights but no changes in
body weight, leukocyte count, lymphoproliferative response to B- or
T-cell mitogens, mixed lymphocyte reaction, or NK cell activity of
spleen cells; lymphoproliferative responses to concanavalin A and
phytohaemagglutinin in thymocytes were reduced. In mice exposed for
seven days to 25 mg/kg body weight per day, the cytotoxic T lymphocyte
response to P815 mastocytoma cells was enhanced, and there was a
significant reduction in the IgM and IgC plaque-forming cell response
to sheep red blood cells. In vitro in murine thymocytes, a
concentration of 10 µg/ml dinocap for 72h suppressed the proliferative
response to concanavalin A and phytohaemagglutinin; exposure for as
little as 30 min suppressed the mitogen-stimulated response with no
direct cytotoxicity (Smialowicz et al., 1992a).
2.2.4 Polycyclic aromatic hydrocarbons
A major concern for human health is the carcinogenic potential of
most polycyclic aromatic hydrocarbons (PAHs). Interestingly, those
which are carcinogenic also have potent immunosuppressive properties,
whereas those which are not carcinogenic lack marked immunotoxic
effects (Ward et al., 1985; White, 1986). Suppression of humoral
immunity has been observed frequently after exposure to a number of
PAHs, including benzo[ a]pyrene, DMBA, and 3-methylcholanthrene (Ward
et al., 1985). Structure-activity studies by White et al. (1985), in
which the antibody-forming cell response was used to evaluate 10 PAHs
in B6C3F1 and DBA/2 mice, demonstrated a wide spectrum of activity:
compounds like benzo[ e]pyrene and perylene were not immunotoxic,
whereas dibenz[ a,h)anthracene and DMBA were potent immunosuppressors
of the plaque-forming cell response. Interestingly, the DBA/2 mice
were more susceptible to the immunosuppressive effects than the B6C3F1
mice.
PAHs also suppress cell-mediated immunity. T-Lymphocyte
cytotoxicity and mixed lymphocyte responsiveness were found to be
impaired by most PAHs. Differences between PAHs are seen, however, in
that benzo[ a]pyrene may be less suppressive of cell-mediated
immunity than DMBA, accounting for the greater host susceptibility to
L. monocytogenes and PYB6 sarcoma challenges in DMBA- than in
benzo[ a]pyrene-treated rodents (Ward et al., 1985). Thurmond et al.
(1987) evaluated immunosuppression in B6C3F1 ( Ah-responsive) and
DBA/2 ( Ah-nonresponsive) mice and in Ah-congenic C57Bl/6J
(responsive B6-AhbAhd and nonresponsive B6-AhdAhd) mice after
exposure to DMBA in a battery of immunological assays, including
evaluation of organ weights, plaque-forming cell response, mitogen
responses, and mixed lymphocyte responses. The authors concluded that
the immunosuppressive action of DMBA was independent of the Ah locus
and associated induction of cytochrome P1-450 metabolizing enzymes.
The mechanisms of PAH-mediated immunosuppression remain to be
elucidated. PAHs may exert their immunotoxic effects as the parent
compound or as metabolites. In vitro many of the metabolites of
benzo[ a]pyrene and DMBA are immunosuppressive, the diol metabolites
being the most potent (Kawabata & White, 1987; Ladics et al., 1991).
Several possible mechanisms of action have been proposed, including
altered interleukin levels (Lyte & Bick, 1986; Pallardy et al., 1989),
a direct effect on transmembrane signalling (Pallardy et al., 1992),
and alterations in intracellular calcium mobilization (Burchiel et
al., 1991; Davis & Burchiel, 1992).
Earlier studies suggested that Th cells or faulty antigen
recognition by T cells were possible mechanisms of DMBA-induced
immunosuppression (House et al., 1987, 1989). Myers et al. (1987) also
reported that benzo[ a]pyrene alters macrophage antigen presentation.
Studies by Ladics et al. (1992) demonstrated that the only splenic
cell type capable of metabolizing benzo[ a]pyrene was the macrophage
and that the predominant immunosuppressive metabolite formed was the
benzo[ a]pyrene-7,8 diol epoxide, which is also believed to be the
ultimate carcinogenic metabolite of benzo[ a]pyrene.
2.2.5 Solvents
2.2.5.1 Benzene
Exposure to benzene is associated with myelotoxicity, and a
strong correlation was noted between lymphocytopenia and abnormal
immunological parameters. The myelotoxicity may be due, in part, to
altered differentiation of marrow lymphoid cells, as suggested by the
finding that acute exposure of IgM+ cell-depleted marrow cultures to
hydroquinone, an oxidative metabolite of benzene, blocked the final
maturation stages of B-cell differentiation (King et al., 1987). In
addition, it was shown that the hydroquinone metabolite inhibits
lectin-stimulated lymphocyte agglutination and mitogenesis by reacting
with intracellular sulfhydryl groups (Pfeifer & Irons, 1981).
Immunosuppression associated with exposure to benzene was found
in rabbits to be an impaired antibody response together with an
increased susceptibility to tuberculosis and pneumonia. Similarly,
C57Bl/6 mice exposed to benzene had a lower antibody response and
reduced mitogen-induced lymphocyte proliferation (Wierda et al.,
1981). Chronic inhalation of concentrations as low as 30 ppm impaired
resistance to L. monocytogenes (Rosenthal & Snyder, 1985).
Similarly, increased susceptibility to PYB6 tumour cell challenge was
seen at concentrations that also impaired Tc lymphocyte function.
The mechanism of benzene-induced immunosuppression is unclear.
Cellular depletion may be the major effect, although B- and T-cell
dysfunction may also be involved. The antiproliferative effects of
benzene may be related to its ability to alter cytoskeletal
development through inhibition of microtubule assembly. Polyhydroxy
metabolites of benzene ( para-benzoquinone and hydroquinone) have
been shown to bind to sulfhydryl groups on the proteins necessary for
the integrity and polymerization of microtubules. This effect may
alter cell membrane fluidity and may explain the sublethal effect of
benzene on lymphocyte function.
2.2.5.2 Other solvents
Hexanediol (1.2 mg/kg per day for seven days) decreased thymus
and spleen weights, antibody production, and delayed-type
hypersensitivity in mice (Kannan et al., 1985). Humoral immunity was
suppressed to a greater extent in female than in male mice after a
four-month exposure to trichloroethylene in the drinking-water at
doses of 0.1, 1.0, 2.5, or 5.0 mg/ml; cell-mediated immunity and bone-
marrow stem-cell colonization were inhibited only in females (Sanders
et al., 1982). The immunotoxicity of glycol ethers and some of their
metabolites has been studied in rats by measuring the plaque-forming
cell response to trinitrophenyl lipopolysaccaride. The glycol ethers
2-methoxyethanol and 2-methoxyethylacetate were immunosuppressive, as
was the principal metabolite of the latter, 2-methoxyacetic acid. The
glycol ethers 2-(2-methoxyethoxy)ethanol, bis(2-methoxyethyl) ether,
2-ethoxyethanol and its principal metabolite 2-ethoxyacetic acid,
2-ethoxyethyl acetate, and 2-butoxyethanol were not immunosuppressive
(Smialowicz et al., 1991, 1992b, 1993)
Dichloroethylene did not induce immunotoxic changes in mice given
up to 2 mg/litre per day for 90 days (Shopp et al., 1985). Similarly
negative findings were obtained with trichloroethane (Sanders et al.,
1985).
2.2.6 Metals
Heavy metals have been shown to alter immune responsiveness in
laboratory animals (Koller, 1980). Alterations in B lymphocyte
function have been observed most frequently after exposure to lead and
cadmium, but T-cell and macrophage changes have also been described.
In addition, exposure to metals is correlated better with impaired
resistance to experimental infections than with changes in B-
or T-cell functions. Interestingly, immunostimulation has been
shown to occur at levels of exposure lower than those associated with
immunosuppression. Metals have also been shown to induce immuno-
potentiation, at lower doses than those that cause immunosuppression.
2.2.6.1 Cadmium
Conflicting results have been obtained with regard to the effect
of cadmium on humoral immunity in animals (Descotes et al., 1990).
Cell-mediated immunity, however, is consistently depressed after both
short- and long-term exposure, and phagocytosis and NK cell activity
are found to be depressed. Susceptibility to L. monocytogenes, Herpes
simplex 1 and 2, and influenza virus was increased in B6C3F1 mice
exposed for long periods (Thomas et al., 1985a).
2.2.6.2 Lead
Experimental studies suggest that lead has immunosuppressive
effects in rodents (Lawrence, 1985; Descotes et al., 1990; Koller,
1990). Early studies demonstrated that lead can suppress the humoral
immune response of mice exposed as adults (Koller & Kovacic, 1974) and
of rats exposed pre- and postnatally (Luster et al., 1978). In
contrast, no change in humoral immunity was found in mice exposed to
0.08-10 mmol/litre in drinking-water (Lawrence, 1981) or after a
10-week oral treatment with 13, 130, or 1300 mg/kg of diet (as lead
acetate) (Koller & Roan, 1980). Delayed-type hypersensitivity was
found to be depressed by lead acetate and lead chloride but not in
mice treated with lead oxide, nitrate, or carbonate. The most
consistent finding in experimental studies of the effects of lead on
host resistance, however, is increased susceptibility to infectious
agents (McCabe, 1994).
With respect to nonspecific host defence mechanisms, mice treated
with lead at doses of 5, 10, or 20 µg/kg body weight given intra-
peritoneally once or at doses of 25, 50, or 100 µg/kg body weight
given orally once showed increased clearance of colloidal carbon
(Schlick & Friedberg, 1981). Furthermore, treatment of mice with 130
or 1300 ppm of lead orally for 10 weeks impaired the phagocytosis of
sheep red blood cells (Koller & Roan, 1977). Lead also has consistent
overall effects on host resistance to infection. Thus, treatment
resulted in significantly decreased resistance of mice to Klebsiella
pneumoniae (Hemphill et al., 1971) and S. typhimurium, and decreased
resistance of rats to a bacterial endotoxin and to a challenge with
E. coli, S. epidermidis, or S. enteritidis. The increased
susceptibility of rodents to Gram-negative bacteria after exposure
to lead is likely to be due to hypersensitivity to an endotoxin of
bacterial origin (Cook et al., 1974, 1975)
Organolead compounds, such as tetrethyllead, can also be
immunotoxic (Luster et al., 1992).
2.2.6.3 Mercury
Mercuric salts have been shown repeatedly to depress both humoral
and cellular immunity and nonspecific host defences in animals. For
instance, B6C3F1 mice given mercuric chloride orally for seven weeks
had decreased thymus and spleen weights, an impaired plaque-forming
cell response, and inhibition of lymphocyte proliferation at a daily
dose of 75 mg/litre of drinking-water (Dieter et al., 1983).
Methylmercury was reported to decrease humoral immunity in mice
treated orally for three weeks with 0.5, 2, or 10 mg/litre drinking-
water (Blackley et al., 1980).
2.2.6.4 Organotins
Several organotins have been shown to be markedly immunotoxic and
are considered as prototype immunotoxicants (Penninks et al., 1990),
even though no human data are available.
Di- n-octyltin dichloride at 50 or 150 mg/kg of diet for six
weeks induced a dramatic, dose-related decrease in the weight of the
thymus in rats, associated with a less severe decrease in spleen and
lymph node weights (Seinen & Willems, 1976). The numbers of cells in
the thymus and spleen, but not the bone marrow, were decreased.
Histologically, lymphocyte depletion was seen in the thymus and in
thymus-dependent areas of the spleen. Interestingly, thymic atrophy
recovered quickly after cessation of exposure (Seinen et al., 1977).
It was later shown to be associated with a 25% decrease in peripheral
blood lymphocytes, with a preferential loss of Th lymphocytes. As
expected, T-cell functions, such as the delayed-type hypersensitivity
response and T-lymphocyte proliferation, were depressed. Inhibition of
humoral immunity was also seen, with reduced numbers of plaque-forming
cells and decreased circulating antibody titres. NK cell activity was
not affected, whereas susceptibility to L. monocytogenes infection
was markedly increased.
Immune function is not impaired in guinea-pigs or mice fed
di- n-octyltin dichloride, which correlates with the absence of
thymic atrophy (Seinen & Penninks, 1979). Mice treated intravenously
or intraperitoneally develop thymic atrophy, however, suggesting
interspecies variability in the disposition of dialkyltins after oral
intake, although other, poorly understood mechanisms may account for
this variability (Penninks et al., 1990). No interspecies differences
in lymphocyte functions were noted after exposure in vitro.
Generally similar findings were made with the trialkylorganotin,
tri- n-butyltin oxide (TBTO). As trisubstituted organotins are
rapidly metabolized to disubstituted derivatives, the latter are
considered to be involved in the reported thymic effects (Snoeij et
al., 1988). In a short-term study in rats, pronounced effects were
found on the lymphoid organs: thymus (Figure 29), spleen, and lymph
nodes. These effects were most pronounced in thymus-dependent areas
(Figure 30) (Krajnc et al., 1984). Interestingly, thymus atrophy also
occurred in fish, as guppies exposed to organotin compounds showed
severe thymic atrophy (Figure 31). In function tests (Vos et al.,
1984), rats that were exposed to TBTO for six weeks after weaning had
suppressed delayed-type hypersensitivity responses to ovalbumin and
tuberculin and suppressed IgG responses to sheep erythrocytes. In
vitro mitogen responses to concanavalin A in thymus and spleen and
NK cell activity in both the spleen and the lungs were decreased (Van
Loveren et al., 1990b). Exposure to TBTO at 20 or 80 mg/kg of diet for
six weeks led to decreased resistance to infection with
L. monocytogenes or Trichinella spiralis. The latter effect was
evidenced by increased numbers of adult worms in the gut as a result
of impaired worm expulsion, increased numbers of muscle larvae in the
striated tissue, decreased inflammatory responses around these larvae,
and decreased antibody responses to T. spiralis, especially in the
IgE class (Vos et al., 1984). After long-term exposure (15-17 months)
to 5 or 50 mg/kg of diet, delayed-type hypersensitivity was not
suppressed, but assays for NK cell activity and resistance to
infection indicated suppression.
As the immune responsiveness of older animals can be expected to
be less strong than that of younger rats, the effects of exposure to
immunotoxic chemicals may become evident less easily; however, tests
for function still indicated immunotoxicity. In experiments in which
exposure to TBTO was begun only at 12 months of age, both infection
models showed immunotoxicity to TBTO. Very few studies have focused on
the immunotoxic effects of chemicals on the gut immune system, but the
studies of TBTO showed both a decreased capacity of the host to expel
adult T. spiralis worms from the gut and increased production of
serum IgA specific for this parasite (Vos et al., 1990a).
The mechanism of the immunotoxicity of organotin compounds has
been investigated extensively (Penninks et al., 1990). A direct
influence on the synthesis of thymic hormones is uncertain, as
conflicting results have been obtained in different experiments.
Interference with the influx of prothymocytes can be ruled out, as
thymic atrophy develops too rapidly. Interestingly, organotins reduced
the proliferative activity of thymocytes and the number of
proliferating thymoblasts within 24h after exposure was begun, at a
time when thymic atrophy was not evident. This selective effect on
thymoblasts and the physiological destruction of most cortical
thymocytes would result in marked depletion and, finally, in thymic
atrophy.
2.2.6.5 Gallium arsenide
Gallium arsenide is an intermetallic compound used widely in the
electronics industry, primarily in the manufacture of transistors and
light-emitting diodes. A single intratracheal instillation of 50, 100,
or 200 mg/kg body weight into female B6C3F1 mice resulted in a dose-
related decrease in the IgM and IgG antibody response to sheep
erythrocytes. Similarly, cell-mediated immunity, as evaluated by the
delayed-type hypersensitivity reaction to keyhole limpet haemocyanin
and the mixed lymphocyte response, was also decreased in a dose-
dependent way. Increases were observed in complement C3 levels,
mitogen response to lipopolysaccharide, and NK cell activity. No
effects were observed on response to T-cell mitogens, total complement
CH50 activity, or host resistance to Plasmodium yoelii or
Streptococcus pneumoniae; however, a significant decrease in host
resistance was observed to L. monocytogenes and B16F10 tumour
challenge (Sikorski et al., 1989).
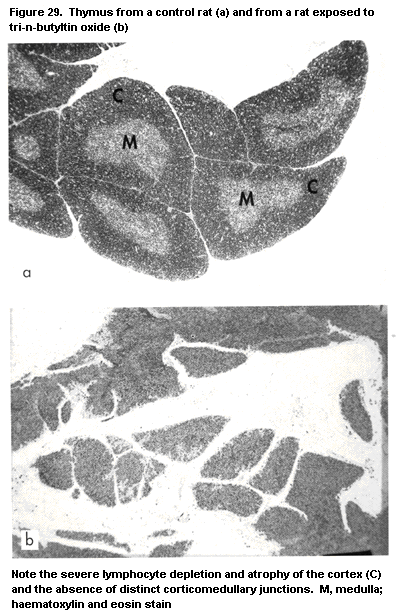
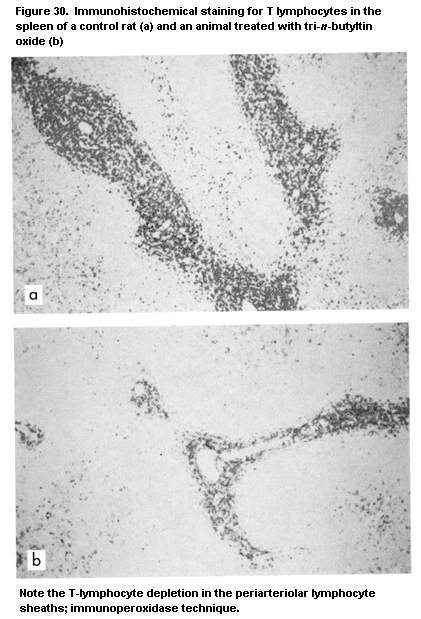
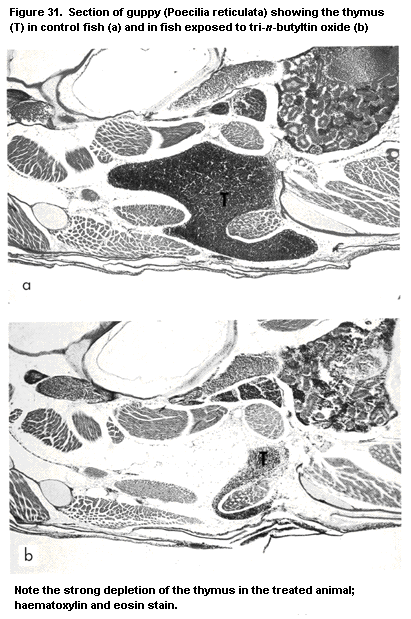 2.2.6.6 Beryllium
Beryllium induces a variety of diseases, including granulomatous
lung (chronic beryllium disease) and skin conditions. These
granulomatous reactions involve a lymphocyte response to beryllium
salts. The major lymphocyte population consists of Th cells (CD4). The
T-cell response to beryllium is IL2-dependent (Saltini et al., 1989).
The antigen has not been identified, but may be a beryllium-protein
complex. There appears to be a genetic predisposition, as the majority
of patients with beryllium lung disease share a particular HLA-Dp
allele (HLA-DpB1) (Richeldi et al., 1992). The development and
maintenance of lung and skin granulomas depend on the presence of
antigen, antigen-presenting cells, and memory T lymphocytes and the
release of proinflammatory cytokines by macrophages and lymphocytes
(Boros, 1988; Kunkel et al., 1989).
2.2.7 Air pollutants
Pollutants characteristic of occupational and urban environments
may cause or aggravate pulmonary diseases. Pulmonary defence
mechanisms to pathogens comprise mechanical defences, nonspecific
defences (ingestion by phagocytic cells, lysis of virus-infected
cells), and specific immunity. A number of studies in experimental
animals have shown that exposure to air pollutants, including ozone,
nitrogen dioxide, sulfur dioxide, some volatile organic compounds, and
metal particulates, adversely affects pulmonary defences, and
primarily nonspecific defences important in clearing certain Gram-
positive bacteria from the lung (Graham & Gardner, 1985; Jakab &
Hmieleski, 1988; Selgrade & Gilmour, 1994).
In dogs, exposure to ozone at 3 ppm for 2 h per day for three
days markedly increased the number of epithelial neutrophils, whereas
the number of circulating neutrophils was decreased (O'Byrne et al.,
1984). A significant decrease in absolute thymocyte numbers was also
observed in mice continuously exposed to 0.7ppm ozone for three to
seven days (Li & Richters, 1991). Decreased spleen and thymus weights
were reported in mice exposed to ozone alone or in combination with
nitrogen dioxide (Fujimaki, 1989; Goodman et al., 1989). The numbers
of neutrophils and alveolar macrophages in bronchoalveolar lavage
fluid were found to be increased in rats, and T-lymphocyte
infiltrations were seen in ozone-induced lesions of mice.
Accumulations of macrophages are located mainly at the bronchoalveolar
junction and in alveoli (Figures 32 and 33).
2.2.6.6 Beryllium
Beryllium induces a variety of diseases, including granulomatous
lung (chronic beryllium disease) and skin conditions. These
granulomatous reactions involve a lymphocyte response to beryllium
salts. The major lymphocyte population consists of Th cells (CD4). The
T-cell response to beryllium is IL2-dependent (Saltini et al., 1989).
The antigen has not been identified, but may be a beryllium-protein
complex. There appears to be a genetic predisposition, as the majority
of patients with beryllium lung disease share a particular HLA-Dp
allele (HLA-DpB1) (Richeldi et al., 1992). The development and
maintenance of lung and skin granulomas depend on the presence of
antigen, antigen-presenting cells, and memory T lymphocytes and the
release of proinflammatory cytokines by macrophages and lymphocytes
(Boros, 1988; Kunkel et al., 1989).
2.2.7 Air pollutants
Pollutants characteristic of occupational and urban environments
may cause or aggravate pulmonary diseases. Pulmonary defence
mechanisms to pathogens comprise mechanical defences, nonspecific
defences (ingestion by phagocytic cells, lysis of virus-infected
cells), and specific immunity. A number of studies in experimental
animals have shown that exposure to air pollutants, including ozone,
nitrogen dioxide, sulfur dioxide, some volatile organic compounds, and
metal particulates, adversely affects pulmonary defences, and
primarily nonspecific defences important in clearing certain Gram-
positive bacteria from the lung (Graham & Gardner, 1985; Jakab &
Hmieleski, 1988; Selgrade & Gilmour, 1994).
In dogs, exposure to ozone at 3 ppm for 2 h per day for three
days markedly increased the number of epithelial neutrophils, whereas
the number of circulating neutrophils was decreased (O'Byrne et al.,
1984). A significant decrease in absolute thymocyte numbers was also
observed in mice continuously exposed to 0.7ppm ozone for three to
seven days (Li & Richters, 1991). Decreased spleen and thymus weights
were reported in mice exposed to ozone alone or in combination with
nitrogen dioxide (Fujimaki, 1989; Goodman et al., 1989). The numbers
of neutrophils and alveolar macrophages in bronchoalveolar lavage
fluid were found to be increased in rats, and T-lymphocyte
infiltrations were seen in ozone-induced lesions of mice.
Accumulations of macrophages are located mainly at the bronchoalveolar
junction and in alveoli (Figures 32 and 33).
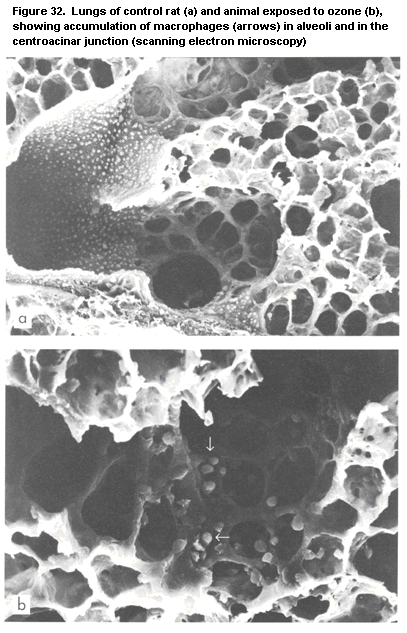
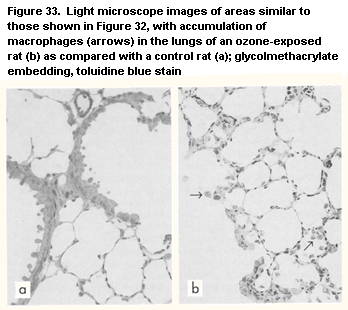 Modulation of nonspecific defence mechanisms by ozone has also
been described (Goldstein et al., 1971; Holt & Keast, 1977; Van
Loveren et al., 1988a, 1990b). Thus, phagocytic activity in alveolar
macrophages is suppressed, but this depends on the concentration and
duration of exposure; enhanced phagocytic activity was also observed.
Alterations in the macrophage production of arachidonic acid
metabolites, resulting in increased prostaglandin 2 production, have
been suggested to be involved (Gilmour et al., 1993). NK cell activity
is either unaffected or stimulated by low ozone concentrations,
whereas high concentrations decreased both the number and the activity
of splenic and pulmonary NK cells (Burleson et al., 1989; Van Loveren
et al., 1990b). Ozone also affects T cells (Dziedzic & White, 1986;
Van Loveren et al., 1988a; Bleavins & Dziedzic, 1990; Dziedzic et al.,
1990). Ozone-induced systemic dysfunction has been reported in animals
and probably contributes to impaired host defences (Aranyi et al.,
1983). Humoral immunity, e.g. circulating antibody titres to a variety
of antigens and the plaque-forming cell response to sheep
erythrocytes, is depressed after exposure to ozone; cellular immunity
is also inhibited. The numbers of all major T lymphocyte subsets,
mitogen-induced T lymphocyte proliferation, and delayed-type
hypersensitivity responses were all shown to be decreased. Numerous
studies with infectivity models show that exposure to ozone has an
adverse influence on the host defences to respiratory infections (Van
Loveren et al., 1994), and most of the studies demonstrate that the
primary targets are the alveolar macrophages (Selgrade & Gilmour,
1994).
Although the influence of other air pollutants such as nitrogen
dioxide and sulfuric acid on host defences has been the subject of
fewer studies, the available data suggest that they have similar
adverse effects (Graham & Gardner, 1985). In view of the numerous
possible targets of air pollutants on respiratory defences and because
of the intricate mechanisms involved, infectivity models in animals
are particularly relevant for ascertaining the likely consequences of
air pollution for exposed human populations.
2.2.8 Mycotoxins
Mycotoxins are structurally diverse secondary metabolites of
fungi that grow on feed. Mycotoxin-induced immunosuppression may be
manifested as depressed T- or B-lymphocyte function, decreased
antibody production, or impaired macrophage activity. Immuno-
stimulation may also be observed with the tricothecenes under
some experimental conditions. Similar effects have been found on the
proliferative responses of human and rodent lymphocytes in vitro
(Lang et al., 1993). Most of the data have been obtained in vivo or
in vitro in animal systems, and there is only limited evidence that
mycotoxins are immunosuppressive in humans (Lea et al., 1989).
Dietary exposure to various mycotoxins resulted in decreased
antibody production, T-lymphocyte proliferative response, delayed-type
hypersensitivity, and NK cell activity (Pestka & Bondy, 1990).
Interestingly, dietary intake was associated with increased
susceptibility to experimental infections.
Aflatoxin is markedly immunosuppressive in cattle and poultry
(see below). Thymic atrophy, suppression of mitogen-induced T- and
B-lymphocyte proliferation, and decreased antibody responses to
various microbial antigens and sheep erythrocytes have been observed
(Corrier, 1992). Cell-mediated immune responses appear to be affected
at lower concentrations than antibody responses. The mechanism of
action seems to be related to impaired protein synthesis.
Ochratoxin, a mycotoxin produced by several species of
Aspergillus and Penicillium, causes depletion of lymphoid cells in
the spleen and lymph nodes of dogs, swine, and mice (Corrier, 1992).
The dose, the route of administration, and the animal species appear
to be critical factors, however; for instance, administration of 13 mg
of ochratoxin to mice in six intraperitoneal injections did not impair
T-lymphocyte proliferation (Luster et al., 1987), whereas
intraperitoneal injections of 5 mg/kg body weight for 50 days did
(Prior & Sisodia, 1982). Ochratoxin also impairs NK cell activity and
increases tumour cell growth in mice (Luster et al., 1987).
The trichothecenes, including T-2 toxin and deoxynivalenol
(vomitoxin), are a structurally related group of mycotoxins produced
by Fusarium. T-2 toxin has been studied extensively and has been
shown to induce lymphoid depletion in the thymus, spleen, and lymph
nodes of numerous laboratory animals (Pestka & Bondy, 1990; Corrier,
1992). In addition, mitogen-induced T- or B-lymphocyte proliferation,
antibody production, and macrophage activation have been found to be
depressed after exposure to either T-2 toxin or vomitoxin. The
impaired immune responsiveness is associated with increased
susceptibility to a variety of experimental infections. As the
tricothecenes are currently considered to be the most potent small-
molecule inhibitors of protein synthesis in eukaryotic cells, the
immunosuppression associated with exposure to these mycotoxins is
likely to be directly or indirectly linked to inhibition of protein
synthesis.
2.2.9 Particles
2.2.9.1 Asbestos
Exposure to asbestos is associated with the development of
inflammatory, fibrotic, and malignant (i.e. pleural mesothelioma and
bronchogenic carcinoma) diseases in humans. Although the pathogenesis
of asbestos-induced lung diseases is complex, a number of observations
indicate that immune processes influence the development and
resolution of both the inflammatory response and fibrotic lesions. For
example, exposure to asbestos is associated with alterations in
cellular and humoral-mediated immunity, including reduction of
lymphocyte mitogenesis, delayed hypersensitivity responses, and
primary antibody responses (Hartmann et al., 1984; Hartmann, 1985;
Bissonette et al., 1989; Miller, 1992). In addition, immunodeficient
mice resolve asbestos-induced inflammatory and fibrotic responses only
with difficulty (Corsini et al., 1994), suggesting that immune
mediators with anti-inflammatory or anti-fibrotic activity (e.g. IL-4
or INF gamma) are involved. Furthermore, it is well established that
alveolar macrophages and type II epithelial cells secrete inflammatory
cytokines, chemokines, and growth factors in response to asbestos
(Driscoll et al., 1990; Rosenthal et al., 1994), and these mediators
are directly involved in the inflammatory responses (e.g. inflammatory
cell recruitment) and fibrogenesis (e.g. fibroblast proliferation).
2.2.9.2 Silica
Experimental animals have been used extensively to define the
pathogenesis of silicosis (Uber & McReynolds, 1982). Several immune
changes have been demonstrated in guinea-pigs, including depression of
humoral and cellular immunity and increased susceptibility to
infectious agents. Similarly, mice exposed to silica showed decreased
lipopolysaccharide-induced proliferation of B lymphocytes and
depressed plaque-forming cell responses (Scheuchenzuber et al., 1985).
Antibody responses to T-independent antigens, however, were less
markedly depressed than responses to T-dependent antigens, suggesting
an additional effect on T-cell control of humoral immunity. The
effects of silica on cellular immunity depend on the dose and route of
entry of antigens. The concanavalin A-induced proliferation response
of spleen T lymphocytes was increased, whereas that of mesenteric
lymph node T lymphocytes was depressed. The aberrations of humoral and
cellular immunity induced by silica are thus complex, and it remains
to be established how these immune changes correlate with the
induction of lung fibrosis or autoantibodies, the major adverse
consequences of exposure to silica. In addition, silica is markedly
toxic to macrophages and activates alveolar macrophages, granulocytes,
and monocytes (Gusev et al., 1993). Infectivity models consistently
show an increased susceptibility of silica-exposed rodents to
infectious pathogens.
2.2.10 Substances of abuse
The immunotoxic consequences of exposure to substances of abuse
are difficult to ascertain in most instances as confounding factors,
such as intercurrent infections secondary to intravenous injection,
may contribute to the observed changes. Recent research has provided
evidence, however, that substances of abuse can directly affect the
immune system (Descotes, 1986; Watson, 1990; Friedman et al., 1991a;
Watson, 1993).
In rodent lymphocytes in vitro, D9-tetrahydrocannabinol
depressed the proliferative responses of T lymphocytes in a dose-
dependent manner (Friedman et al., 1991b). Further to the early
findings that opiates adversely affect immune competence (Cantacuzene,
1898), an increasing body of evidence shows that exogenous opioids
have a variety of effects on cells of the immune system (Rouveix,
1993). At pharmacological concentrations, opiates suppress antibody
production, lymphocyte proliferation, and delayed-type
hypersensitivity and decrease NK cell activity in various animal
models. In addition, phagocytosis is impaired. Opioid peptides can,
however, also have a stimulatory effect on the immune system,
depending on the experimental conditions. ß-Endorphin affects cytokine
production in rat and mouse T-cell cultures in vitro; e.g. it
stimulates the synthesis of IL-2, IL-4, and INF gamma, thereby
inducing MHC class II expression on B cells (van den Bergh et al.,
1993a,b, 1994).
In general, short-term exposure of mice, rats, and guinea-pigs to
mainstream tobacco smoke either produces no significant immuno-
modulatory effect or a slight immunostimulation, which returns
to normal shortly after cessation of exposure (Johnson et al., 1990).
In contrast, subchronic or chronic (more than one year) exposure is
generally immunosuppressive: cellular immunity, e.g. mitogen-induced
lymphocyte proliferative response, and NK cell activity are impaired
after long-term exposure to tobacco smoke. The humoral immune response
is also depressed, as shown by decreased antibody titres, and animals
exposed to cigarette smoke for extended periods are more susceptible
to tumour and infectious challenge than naive animals.
2.2.11 Ultraviolet radiation
The earliest indication that ultraviolet radiation (UVR) affects
the immune system came from studies of host resistance to UVR-induced
tumours in mice (Kripke, 1974). Subsequent studies showed that low
doses of UVR suppress contact hypersensitivity responses to chemical
sensitizers (Toews et al., 1980) and that systemic immunosuppression
(depressed contact hypersensitivity in unirradiated skin) occurs after
exposure to higher doses (Jessup et al., 1978). Irradiated mice were
also found to be less resistant to infection (Giannini, 1990). Other
studies (Noonan & De Fabo, 1990) have determined that systemic
suppression of immunoreactivity is not a function of the dose of UVR
but rather of the interval between irradiation and immunization of the
mice. Thus, induction of contact hypersensitivity responses in mice
exposed to low doses of UVR was not affected when the animals were
immunized through unirradiated skin immediately after exposure to UVR;
however, sensitization was suppressed if three days were allowed to
elapse between irradiation and immunization. It has also been shown
that the dose of UVR required to induce 50% suppression of the immune
response depends on the strain of mouse and the type of antigen used
(Noonan & De Fabo, 1990; Noonan & Hoffman, 1994).
The mechanism of UVR-induced suppression of cellular immunity has
not been elucidated, nor has a single initial event been identified
that leads to suppression of immunoreactivity. Currently, induction of
pyrimidine dimers in DNA (Kripke et al., 1992) and isomerization of
urocanic acid (Noonan & De Fabo, 1992) are the leading contenders.
Increased suppressor cell activity (Brodie & Halliday, 1991) and
efferent lymphatic blockade, which inhibits lymphocyte homing, may be
responsible for the UVR-associated accumulation of lymphocytes in
lymph nodes in UVR-exposed areas (Spangrude et al., 1983) and have
been proposed as possible causes of immunosuppression. Exposure to UVR
has also been shown to alter the pattern of cytokine production by
T cells, from a response dominated by Th1 (i.e. favouring delayed
hypersensitivity responses) to one dominated by Th2 (i.e. favouring
antibody production) (Araneo et al., 1989; Simon et al., 1990).
Exposure to UVR has been reported to affect Langerhans cells directly,
such that their interaction with T cells induces specific antigen
tolerance in the Th1 subpopulation (Simon et al., 1990) and
preferential activation of the Th2 population (Simon et al., 1991).
This may be the reason that mice exposed to UVR are more susceptible
to infection with the protozoan Leishmania major (Giannini, 1992),
since resistance to infection with this intracellular parasite is
dependent on the magnitude of the Th1 response of the host (Reed &
Scott, 1993). In addition, reduced resistance to T. spiralis was
found in rats exposed to UVR on days 5-10 of infection (Goettsch et
al., 1993). Altered cytokine production profiles may also be
responsible for increased sensitivity to Mycobacterium lepraemurium,
an intracellular pathogen that induces a chronic and eventually fatal
infection in susceptible mice. In a comparison of susceptible (BALB/c)
and resistant (C57Bl/6J) mice, Brett & Butler (1986) determined that
resistance to infection is correlated with the ability of mouse
lymphocytes to elaborate cytokines that activate macrophages, rather
than with the actual development of a delayed hypersensitivity
response to bacterial antigens. Jeevan & Kripke (1990) and Jeevan et
al. (1992) reported that irradiation of BALB/c mice resulted in
decreased resistance to infection, as measured by bacterial counts and
length of survival after infection. Elevated bacterial counts were
seen in animals exposed to doses of UVR that did not suppress the
delayed hypersensitivity response to bacterial antigens, suggesting
that the underlying mechanism of UVR-induced suppression of resistance
to infection is independent of suppressed delayed hypersensitivity.
2.2.12 Food additives
There is little information about the effects of food additives
on the immune system. An early study showed that the preservative
methylparaben and the antioxidants butylated hydroxyanisole, butylated
hydroxytoluene, and propylgallate suppress the in-vitro T-dependent
antibody response, whereas vanillin and vanillic acid stimulate it
(Archer et al., 1978). The significance of these findings in vivo
has yet to be established.
The immunotoxicity of 'caramel colour', which covers a large
number of complex products used as food colorants, has been
investigated. One of the compounds in this group, 2-acetyl-4(5)-
tetrahydroxybutylimidazole (THI) (caramel colour III), has been found
to be immunotoxic in rodents (Kroplien et al., 1985). THI induces a
rapid reduction in the number of B and T cells in blood, spleen, and
lymph nodes and morphological changes in the thymus of rats, with an
increased number of mature medullary thymocytes and a decreased number
of cortical macrophages. THI might reduce the migration of mature
thymocytes into the periphery, as a decrease in the number of recent
ER4+ thymic emigrants was found in the spleens of exposed rats
(Houben et al., 1992). Functional studies indicate changes in Th cell
function, an increased capacity to clear the Gram-positive bacterium
L. monocytogenes, and modulation of the activity of adherent splenic
cells (Houben et al., 1993). It has been hypothesized that THI exerts
an antivitamin B6 action by competing with pyridoxal 5'-phosphate for
binding to the cofactor site of one or more pyridoxal 5'-phosphate-
dependent enzymes.
2.3 Immunotoxicity of environmental chemicals in wildlife and
domesticated species
Most of the concern about chemical contamination of wildlife
populations has been focused on the aquatic ecosystem, and a growing
body of literature has appeared on the effects of pollution on the
health status of aquatic life. These studies deal mainly with the
occurrence of tumours and infectious diseases in fish and marine
mammals. These are multifactorial diseases in which perturbations of
the immune system may play a part.
2.3.1 Fish and other marine species
2.3.1.1 Fish
Fish diseases are being monitored on a routine basis at various
sites in North America and Europe. In Europe, most of the programmes
are carried out under the auspices of the International Council for
Exploration of the Sea. National and local studies have been directed
to estuarine, marine, and brackish waters suspected of being polluted,
such as in the vicinity of industrial areas and after major oil
spills. The common diseases that are discussed in relation to
pollution are certain skin diseases, such as lymphocystis, papillomas,
fin rotting, and skin ulcers (Vethaak & ap Rheinallt, 1992), as they
are easily identified grossly and are therefore potentially useful for
biomonitoring. Since most diseases of fish have a viral or bacterial
etiology, and elevated incidences have been correlated with chemical
pollution, immunotoxicity may play a role. A causal relationship
between chemical pollution and a disease state induced by
immunosuppression cannot be finally established, however, since many
confounding factors exist in the natural environment. Liver neoplasia
and precursor lesions have been used to biomonitor environmental
pollution in flatfish (Malins et al., 1988; Vethaak & ap Rheinallt,
1992; Vethaak, 1993); however, the role of the immune system is not
evident.
Effects observed in field studies are modified or confounded by
numerous factors, in particular for feral fish, for which there are
deficient case histories and limited knowledge of their migratory
patterns and biology (Vethaak, 1993). Extensive epidemiological
surveys are required that include specific parameters that have been
validated in experiments under (more) controlled conditions (in
mesocosms or the laboratory). Changes in disease patterns may suggest
immune alterations, but this should be demonstrated. Since most
diseases have a complex etiology, it will be difficult to establish
the role of immunotoxic effects under field conditions. Circumstantial
evidence can be obtained in these instances, although in the case of
feral animals mesocosm or laboratory experiments must carried out in
order to reach a final conclusion (Secombes et al., 1992; Vethaak,
1993).
The etiological components and their role in the pathogenesis of
many diseases in fish in the field are, as yet, poorly understood, and
laboratory experiments are often indispensable for background
knowledge. Even when such scientific deficiencies are resolved,
laboratory studies will still be needed, since function tests under
controlled conditions yield the most reliable and sensitive methods of
assessing immunological stress and must often accompany field studies,
as mentioned above. Findings from laboratory situations do not
necessarily imply effects in the field, however; in particular, when
results from the laboratory are extrapolated to field situations,
there is often a discrepancy between the levels of exposure.
2.3.1.2 Marine mammals
Marine mammals are of special interest to the discipline of
immunotoxicology. As the highest predators in highly contaminated
marine environments, these animals are exposed to a large number of
environmental chemicals, some of which have been identified as
potentially immunotoxic. Persistent lipophilic halogenated compounds
such as PCBs, polychlorinted dibenzodioxins, polychlorinated
dibenzofurans, and pesticides accumulate in the marine food chain and
are thus biomagnified in marine mammals. The concentrations of PCBs in
the blubber layer of marine mammals are higher than in any other
wildlife species measured (Table 6). In times of food shortage and
other stressful circumstances, these lipids are mobilized, thereby
releasing their toxic burden.
Table 6. Concentrations of polychlorinated biphenyls (PCBs) in herring and the blubber
layer of marine mammals
Species Source Total PCBs Reference
(µg/g lipid)
Herring Atlantic Ocean 0.0003-0.001 De Swart et al.
(United Kingdom) (1994)
Herring Baltic Sea 0.0035-0.0045 De Swart et
(Sweden) al. (1993)
Weddell seal Weddell Sea 0.07-0.09 Luckas et al.
(Leptonychotes wedelli) (Antarctic) (1990)
Harbour seal Atlantic Ocean 1-13 Luckas et al.
(Phoca vitulina) (Iceland) (1990)
Harbour seal Baltic Sea 21-140 Luckas et al.
(Phoca vitulina) (Sweden) (1990)
Beluga whale St Lawrence 15-700 Muir et. al.
(Delphinapterus leucas) River (Canada) (1990);
Martineau et al.
(1987)
Striped dolphin Mediterranean 100-2600 Kannan et al.
(Stenella coeruleoalba) Sea (Spain) (1993)
Because of the high level of exposure of marine mammals, they may
be expected to be the first wild animals to suffer from
immunosuppression due to chronic exposure to environmental
contaminants. Toxicological research over the last 20 years has
identified environmental chemicals as the source of many disorders in
marine mammals. In both field studies and controlled experiments, PCBs
have been linked to reproductive problems. As early as 1976, a high
incidence of premature parturition was seen in California sea-lions
(Zalophus californianus), which was caused by a viral infection and
was suggested to be linked to higher levels of pollutants in animals
that aborted as compared with mothers that gave birth to healthy pups
(Gilmartin et al., 1976). Pathological changes in the uteri of seals
in the highly polluted Baltic Sea, in some cases leading to sterility,
could be correlated with increased levels of PCBs (Helle et al.,
1976). In addition, several studies have linked skeletal deformities
in grey seals (Halichoerus grypus) and harbour seals (Phoca
vitulina) in the Baltic Sea to high levels of organochlorines in
their environment (Bergman et al., 1992; Mortensen et al., 1992). In
porpoises (Phocoenoides dalli) living in the north-western part of
the North Pacific, a negative correlation was found between serum
testosterone levels and DDE concentrations in blubber (Subramanian et
al., 1987). In an experimental situation, two groups of harbour seals
were fed fish containing different levels of pollutants. Seals that
were fed fish from the heavily polluted western part of the Dutch
Wadden Sea had significantly lower pup production than seals feeding
on less polluted fish (Reijnders, 1986). In the same study, it was
shown that the seals fed polluted fish also had significantly reduced
levels of vitamin A and thyroid hormone in their serum (Brouwer et
al., 1989). No parameters of immune function were studied.
Such correlative observations in the natural environment, in
combination with the results of semi-field studies, suggest that the
current levels of contaminants may be adversely affecting certain
marine mammal populations. The occurrence of a large number of
epizootics among seals and dolphins inhabiting polluted coastal areas
-- among bottlenose dolphins (Tursiops truncatus) on the Atlantic
coast of the United States in 1987 and in the Gulf of Mexico in 1990,
striped dolphins (Stenella coeruleoalba) on the coast of France in
1989 and in the Mediterranean Sea in 1990-92, Baikal seals (Phoca
sibirica) in Lake Baikal in 1987, and harbour seals in north-west
Europe in 1988 -- led to both public and scientific discussions about
the possible contribution of environmental pollutants to these disease
outbreaks. Owing to the complexities of the relationships between
toxicants and the immune system and the difficulties in obtaining
samples fit for use in immunotoxicological studies, it has been
impossible thus far to conclusively demonstrate immunosuppression in
free-ranging marine mammals.
Another immunotoxicological experiment was carried out in which
two groups of juvenile harbour seals (Phoca vitulina) were fed fish
from the Baltic Sea or from the relatively unpolluted Atlantic Ocean.
The diets were analysed for concentrations of potential immunotoxic
chemicals: the estimated daily intakes of TCDD-like organochlorines
were 270 and 35 ng/day for the two groups, respectively. Immunological
function in the two groups was examined by measuring mitogen- and
antigen-induced proliferative responses of lymphocytes, NK cell
activity, serum antibody levels after immunization with primary
antigens, and delayed-type hypersensitivity reactions. These
techniques had to be validated for application to seals, as virtually
nothing was known about the cellular immune system of marine mammals
(De Swart et al., 1993, 1994). Seals fed herring from the contaminated
Baltic Sea had significantly depressed immune function, as measured by
decreased NK cell activity (Ross et al., in press) and T-cell mitogen-
induced lymphocyte proliferation (De Swart et al., 1994), and
significantly lower delayed-type hypersensitivity and antibody
responses to immunization with ovalbumin (Ross et al., 1995). The
functional changes were accompanied by increased numbers of
neutrophils in the peripheral blood (De Swart et al., 1994). Since NK
cells are an important line of defence against viruses, and
lymphocytes (especially T cells) play a major role in the clearance of
viral infections, functional suppression of these leukocytes may
contribute to the severity of epizootic episodes among marine mammals.
These experiments not only provide the first demonstration of
pollution-induced impairment of immune function in marine mammals but
indicate that mammals in general can undergo such impairment as a
consequence of chronic exposure to the levels of pollution found in
their natural habitats. It is still difficult, however, to link the
disease outbreaks among marine mammals directly to pollution-induced
impairment of immune function.
2.3.2 Cattle and swine
The effects of antimicrobial, corticosteroid, and hormonal
compounds have been investigated in cattle, mainly as lymphocyte
proliferative responses and neutrophil functions in vitro. The
results are in keeping with those obtained in humans (Black et al.,
1992).
Few studies have dealt specifically with the direct immunotoxic
effects of pesticides and environmental pollutants. No statistical
difference was found between control and polybrominated biphenyl-
exposed animals in the numbers of circulating total, T, and B
lymphocytes, serum immunoglobulin levels, mitogen-induced
proliferative responses of lymphocytes, antibody response to keyhole
limpet mitogen, or cell-mediated response to purified protein
derivative (Kateley & Bazzell, 1978). In contrast, peripheral blood
lymphocytes from sows fed polybrominated biphenyls for 12weeks had
significantly decreased responses to mitogens (Howard et al., 1980).
The mycotoxin tricothecene produced by Fusarium and several
other fungi was shown to reduce lymphoid tissue cellularity and serum
IgA, IgG, and IgM concentrations and to impair neutrophil migration,
chemotaxis, and phagocytosis in cattle exposed to high doses (Buening
et al., 1982; Mann et al., 1983). Similarly, aflatoxin was reported to
suppress the mitogen-induced proliferative response of bovine
lymphocytes (Paul et al., 1977). Other mycotoxins, e.g. ochratoxin A
and zearalenone, have been suggested to be immunosuppressive in cattle
(Black et al., 1992).
2.3.3 Chickens
Chickens have been used in a number of immunological studies, as
the unique bursa of Fabricius, the avian organ for B-cell development,
underlies the need for a separate avian model in immunotoxicology.
Exposure of adolescent chickens to cyclophosphamide decreased the
levels of antibodies to various antigens without decreasing graft-
versus-host reactivity (Lerman & Weidanz, 1970). Nutritional
deficiencies in selenium or vitamin E have also been shown to impair
the humoral immune responses of adolescent chickens (Marsh et al.,
1981). Exposure to cyclophosphamide in ovo results in decreased
antibody forming capacity, decreased responsiveness to
phytohaemagglutinin and concanavalin A, and decreased weights and
altered morphology of the thymus, spleen, and bursa of Fabricius
(Eskola & Toivanen, 1974). Peritoneal macrophages were not affected
after exposure to cyclophosphamide in ovo, as judged by their
number, superoxide anion production, and surface expression of Ia
antigen and transferrin receptor (Golemboski et al., 1992). Dietert et
al. (1985) showed that exposure to aflatoxin B1 in ovo did not alter
humoral immunity; however, two parameters of cell-mediated, graft-
versus-host, and cutaneous basophil hypersensitivity reactions were
depressed. Methyl methanesulfonate decreased the bactericidal action
of peritoneal macrophages for E. coli after exposure in vitro
(Qureshi et al., 1989). TCDD impairs B-cell development in the bursa
of Fabricius in chicken embryos (Nikolaidos et al., 1990).
2.4 Immunotoxicity of environmental chemicals in humans
Although limited, various lines of evidence derived from case
reports, clinical studies, and well-designed longitudinal studies
imply that environmental agents can affect the human immune system.
While these data raise concern about potential health effects, they
rarely refer to clinical disease, for a number of reasons. For
example, there may be sufficient redundancy or reserve in the immune
system that 'moderate' levels of immunosuppression do not result in
disease. Alternatively, the clinical changes most likely to be
associated with moderate immunosuppression, e.g. increased severity or
frequency of pulmonary infections, do not occur. Well-designed
clinical studies with adequate populations and appropriate monitoring,
follow-up, and documentation of exposure have rarely been conducted.
Examples of published reports that attribute immune changes in human
populations exposed to environmental agents are summarized below. As
the reader will note, these studies range from poorly defined to
relatively large longitudinal studies.
2.4.1 Case reports
In an unsubstantiated study, a cluster of cases of Hodgkin's
disease reported in a small town in Michigan (United States) was
ascribed to chronic immune stimulation by mitogenic substances in the
environment (Schwartz et al., 1978). Immunological studies of family
members revealed a large number of individuals with altered ratios of
T-lymphocyte subpopulations, autoantibodies, infections, recurrent
rashes, and NK cell function. A report of a four-year study of workers
engaged in the manufacture of benzidine, a human bladder carcinogen,
suggested that individuals with depressed cell-mediated immunity (as
judged by skin testing) had precancerous conditions and subsequent
neoplasms (Gorodilova & Mandrik, 1978); no cases of neoplastic disease
were registered in workers with normal immunological responses.
2.4.2 Air pollutants
The association betweeen changes in immunological parameters and
host resistance and inhalation of particulate materials and oxidant
gases is well established (Folinsbee, 1992). For example, decreases in
delayed-type hypersensitivity response, circulating T-cell numbers,
and T-cell proliferation have been observed with, and sometimes
preceding, asbestos-related diseases, i.e. fibrosis, asbestosis, and
mesothelioma (Kagan et al., 1977a; Gaumer et al., 1981; Lew et al.,
1986; Tsang et al., 1988). B-Cell responses are increased, however, as
evidenced by increased serum and secretory (primarily IgA)
immunoglobulins (Kagan et al., 1977b). Kagan et al. (1979) also
reported an association between exposure to asbestos and B-cell
lymphoproliferative disorders. Several studies have shown altered NK
cell activity after exposure to asbestos (Kubota et al., 1985; Tsang
et al., 1988). In studies of NK cell responses in asbestos workers,
Lew et al. (1986) found that immune changes may occur independently of
any early neoplastic process. Similarly, there have been multiple
observations of abnormal antibody production, decreased cell-mediated
immune responses, and decreased resistance to disease in people
occupationally exposed to silica (Uber & McReynolds, 1982).
Oxidant gases have been associated with an increased prevalence
of respiratory infections, particularly bacterial, and potential
immune effects, but the data are less convincing than those from
studies of rodents. The association between air pollution in
industrialized areas and altered health status has been well
established in epidemiological studies (French et al., 1973). A number
of studies have linked exposure to air pollutants (ozone, nitrogen
dioxide, sulfur dioxide, environmental tobacco smoke) with an
increased incidence, severity, or duration of symptoms associated with
respiratory infections (Lunn et al., 1967; French et al., 1973;
Durham, 1974; Harrington & Krupnick, 1985; Neas et al., 1991; Schwartz
et al., 1991; Schwartz, 1992; US Environmental Protection Agency,
1990), although several studies of nitrogen dioxide failed to show
such an association (Speizer et al., 1980; Ware et al., 1984; Samet et
al., 1993). In Ontario, Canada, increased air pollution from sulfur
dioxide and ozone during the summer was directly related to hospital
admissions for acute respiratory symptoms (Bates & Sizto, 1983).
Goings et al. (1989) subjected young adult volunteers to 1, 2, or
3 ppm nitrogen dioxide for 2h per day on two consecutive days and then
administered influenza virus intranasally. Although no statistical
differences were observed, the frequencies of infections in exposed
volunteers (91%) were higher than the 56-73% in healthy individuals,
suggesting that nitrogen dioxide may play a role in increasing
susceptibility to infection. In assessments of air pollution at home,
young children in households with gas stoves had a higher incidence of
respiratory disease and decreased pulmonary function than children in
households with electric stoves. This difference was related to
increased levels of nitrogen dioxide in homes with gas stoves, which
reached peak values of > 1100 mg/m3 (Melia et al., 1977; Speizer et
al., 1980). In contrast, Samet (1994) found no association between
indoor levels of nitrogen dioxide and respiratory infections in
children. A study of schoolchildren in Chattanooga (United States)
showed an increased incidence of respiratory illness associated with
atmospheric nitrogen dioxide levels (Shy et al., 1970).
Examination of hospital admissions in Massachusetts (United
States) in 1980 and 1982 revealed a positive association between 1-h
maximum ozone levels in the summer months and daily admissions for
pneumonia and influenza (Ozkaynak et al., 1990). An effect of
atmospheric pollution, including oxidant gases, was also seen on the
number of influenza cases in Sofia, Bulgaria (Kalpazanov et al.,
1976); however, no demonstrable adverse effect on the course of a
rhinoviral infection was seen in young adult male volunteers after
exposure to moderate levels of ozone (0.3ppm for 6 h/day over a period
of five days) (Henderson et al., 1988), and children living in areas
with high ozone concentrations had lowered CD4:CD8 ratios of
peripheral lymphocytes but no higher incidence of chest colds (Zwick
et al., 1991). The phagocytic activity of alveolar macrophages
(obtained by bronchoalveolar lavage) and other functions were impaired
in human volunteers exposed to ozone (Devlin et al., 1991). The
sensitivity of human and mouse macrophages to the effects of ozone is
similar (Selgrade et al., 1995).
Not all epidemiological studies have showed a correlation between
air pollution and respiratory disease. Some of the discrepancies from
experimental studies may be due to the parameters used to assess
enhancement of infection. In experimental studies, increases in viral
titres in the respiratory tract tend to be taken as an indication that
the exposure enhances infection, whereas epidemiological studies rely
on symptoms, many of which could be related to enhanced inflammatory
or even allergic responses to the virus in the absence of viral
replication.
Controlled studies (in an environmental chamber) showed that
acute exposure to ozone causes an inflammatory response in the lower
airways of human subjects (Koren et al., 1989; Devlin et al., 1991).
The inflammatory response was manifested by increases in various
inflammatory indicators including polymorphonuclear neutrophils
(Figure 34), proteins, fibronectin, IL-6, and tryptase (Koren et al.,
1989, 1994).
Modulation of nonspecific defence mechanisms by ozone has also
been described (Goldstein et al., 1971; Holt & Keast, 1977; Van
Loveren et al., 1988a, 1990b). Thus, phagocytic activity in alveolar
macrophages is suppressed, but this depends on the concentration and
duration of exposure; enhanced phagocytic activity was also observed.
Alterations in the macrophage production of arachidonic acid
metabolites, resulting in increased prostaglandin 2 production, have
been suggested to be involved (Gilmour et al., 1993). NK cell activity
is either unaffected or stimulated by low ozone concentrations,
whereas high concentrations decreased both the number and the activity
of splenic and pulmonary NK cells (Burleson et al., 1989; Van Loveren
et al., 1990b). Ozone also affects T cells (Dziedzic & White, 1986;
Van Loveren et al., 1988a; Bleavins & Dziedzic, 1990; Dziedzic et al.,
1990). Ozone-induced systemic dysfunction has been reported in animals
and probably contributes to impaired host defences (Aranyi et al.,
1983). Humoral immunity, e.g. circulating antibody titres to a variety
of antigens and the plaque-forming cell response to sheep
erythrocytes, is depressed after exposure to ozone; cellular immunity
is also inhibited. The numbers of all major T lymphocyte subsets,
mitogen-induced T lymphocyte proliferation, and delayed-type
hypersensitivity responses were all shown to be decreased. Numerous
studies with infectivity models show that exposure to ozone has an
adverse influence on the host defences to respiratory infections (Van
Loveren et al., 1994), and most of the studies demonstrate that the
primary targets are the alveolar macrophages (Selgrade & Gilmour,
1994).
Although the influence of other air pollutants such as nitrogen
dioxide and sulfuric acid on host defences has been the subject of
fewer studies, the available data suggest that they have similar
adverse effects (Graham & Gardner, 1985). In view of the numerous
possible targets of air pollutants on respiratory defences and because
of the intricate mechanisms involved, infectivity models in animals
are particularly relevant for ascertaining the likely consequences of
air pollution for exposed human populations.
2.2.8 Mycotoxins
Mycotoxins are structurally diverse secondary metabolites of
fungi that grow on feed. Mycotoxin-induced immunosuppression may be
manifested as depressed T- or B-lymphocyte function, decreased
antibody production, or impaired macrophage activity. Immuno-
stimulation may also be observed with the tricothecenes under
some experimental conditions. Similar effects have been found on the
proliferative responses of human and rodent lymphocytes in vitro
(Lang et al., 1993). Most of the data have been obtained in vivo or
in vitro in animal systems, and there is only limited evidence that
mycotoxins are immunosuppressive in humans (Lea et al., 1989).
Dietary exposure to various mycotoxins resulted in decreased
antibody production, T-lymphocyte proliferative response, delayed-type
hypersensitivity, and NK cell activity (Pestka & Bondy, 1990).
Interestingly, dietary intake was associated with increased
susceptibility to experimental infections.
Aflatoxin is markedly immunosuppressive in cattle and poultry
(see below). Thymic atrophy, suppression of mitogen-induced T- and
B-lymphocyte proliferation, and decreased antibody responses to
various microbial antigens and sheep erythrocytes have been observed
(Corrier, 1992). Cell-mediated immune responses appear to be affected
at lower concentrations than antibody responses. The mechanism of
action seems to be related to impaired protein synthesis.
Ochratoxin, a mycotoxin produced by several species of
Aspergillus and Penicillium, causes depletion of lymphoid cells in
the spleen and lymph nodes of dogs, swine, and mice (Corrier, 1992).
The dose, the route of administration, and the animal species appear
to be critical factors, however; for instance, administration of 13 mg
of ochratoxin to mice in six intraperitoneal injections did not impair
T-lymphocyte proliferation (Luster et al., 1987), whereas
intraperitoneal injections of 5 mg/kg body weight for 50 days did
(Prior & Sisodia, 1982). Ochratoxin also impairs NK cell activity and
increases tumour cell growth in mice (Luster et al., 1987).
The trichothecenes, including T-2 toxin and deoxynivalenol
(vomitoxin), are a structurally related group of mycotoxins produced
by Fusarium. T-2 toxin has been studied extensively and has been
shown to induce lymphoid depletion in the thymus, spleen, and lymph
nodes of numerous laboratory animals (Pestka & Bondy, 1990; Corrier,
1992). In addition, mitogen-induced T- or B-lymphocyte proliferation,
antibody production, and macrophage activation have been found to be
depressed after exposure to either T-2 toxin or vomitoxin. The
impaired immune responsiveness is associated with increased
susceptibility to a variety of experimental infections. As the
tricothecenes are currently considered to be the most potent small-
molecule inhibitors of protein synthesis in eukaryotic cells, the
immunosuppression associated with exposure to these mycotoxins is
likely to be directly or indirectly linked to inhibition of protein
synthesis.
2.2.9 Particles
2.2.9.1 Asbestos
Exposure to asbestos is associated with the development of
inflammatory, fibrotic, and malignant (i.e. pleural mesothelioma and
bronchogenic carcinoma) diseases in humans. Although the pathogenesis
of asbestos-induced lung diseases is complex, a number of observations
indicate that immune processes influence the development and
resolution of both the inflammatory response and fibrotic lesions. For
example, exposure to asbestos is associated with alterations in
cellular and humoral-mediated immunity, including reduction of
lymphocyte mitogenesis, delayed hypersensitivity responses, and
primary antibody responses (Hartmann et al., 1984; Hartmann, 1985;
Bissonette et al., 1989; Miller, 1992). In addition, immunodeficient
mice resolve asbestos-induced inflammatory and fibrotic responses only
with difficulty (Corsini et al., 1994), suggesting that immune
mediators with anti-inflammatory or anti-fibrotic activity (e.g. IL-4
or INF gamma) are involved. Furthermore, it is well established that
alveolar macrophages and type II epithelial cells secrete inflammatory
cytokines, chemokines, and growth factors in response to asbestos
(Driscoll et al., 1990; Rosenthal et al., 1994), and these mediators
are directly involved in the inflammatory responses (e.g. inflammatory
cell recruitment) and fibrogenesis (e.g. fibroblast proliferation).
2.2.9.2 Silica
Experimental animals have been used extensively to define the
pathogenesis of silicosis (Uber & McReynolds, 1982). Several immune
changes have been demonstrated in guinea-pigs, including depression of
humoral and cellular immunity and increased susceptibility to
infectious agents. Similarly, mice exposed to silica showed decreased
lipopolysaccharide-induced proliferation of B lymphocytes and
depressed plaque-forming cell responses (Scheuchenzuber et al., 1985).
Antibody responses to T-independent antigens, however, were less
markedly depressed than responses to T-dependent antigens, suggesting
an additional effect on T-cell control of humoral immunity. The
effects of silica on cellular immunity depend on the dose and route of
entry of antigens. The concanavalin A-induced proliferation response
of spleen T lymphocytes was increased, whereas that of mesenteric
lymph node T lymphocytes was depressed. The aberrations of humoral and
cellular immunity induced by silica are thus complex, and it remains
to be established how these immune changes correlate with the
induction of lung fibrosis or autoantibodies, the major adverse
consequences of exposure to silica. In addition, silica is markedly
toxic to macrophages and activates alveolar macrophages, granulocytes,
and monocytes (Gusev et al., 1993). Infectivity models consistently
show an increased susceptibility of silica-exposed rodents to
infectious pathogens.
2.2.10 Substances of abuse
The immunotoxic consequences of exposure to substances of abuse
are difficult to ascertain in most instances as confounding factors,
such as intercurrent infections secondary to intravenous injection,
may contribute to the observed changes. Recent research has provided
evidence, however, that substances of abuse can directly affect the
immune system (Descotes, 1986; Watson, 1990; Friedman et al., 1991a;
Watson, 1993).
In rodent lymphocytes in vitro, D9-tetrahydrocannabinol
depressed the proliferative responses of T lymphocytes in a dose-
dependent manner (Friedman et al., 1991b). Further to the early
findings that opiates adversely affect immune competence (Cantacuzene,
1898), an increasing body of evidence shows that exogenous opioids
have a variety of effects on cells of the immune system (Rouveix,
1993). At pharmacological concentrations, opiates suppress antibody
production, lymphocyte proliferation, and delayed-type
hypersensitivity and decrease NK cell activity in various animal
models. In addition, phagocytosis is impaired. Opioid peptides can,
however, also have a stimulatory effect on the immune system,
depending on the experimental conditions. ß-Endorphin affects cytokine
production in rat and mouse T-cell cultures in vitro; e.g. it
stimulates the synthesis of IL-2, IL-4, and INF gamma, thereby
inducing MHC class II expression on B cells (van den Bergh et al.,
1993a,b, 1994).
In general, short-term exposure of mice, rats, and guinea-pigs to
mainstream tobacco smoke either produces no significant immuno-
modulatory effect or a slight immunostimulation, which returns
to normal shortly after cessation of exposure (Johnson et al., 1990).
In contrast, subchronic or chronic (more than one year) exposure is
generally immunosuppressive: cellular immunity, e.g. mitogen-induced
lymphocyte proliferative response, and NK cell activity are impaired
after long-term exposure to tobacco smoke. The humoral immune response
is also depressed, as shown by decreased antibody titres, and animals
exposed to cigarette smoke for extended periods are more susceptible
to tumour and infectious challenge than naive animals.
2.2.11 Ultraviolet radiation
The earliest indication that ultraviolet radiation (UVR) affects
the immune system came from studies of host resistance to UVR-induced
tumours in mice (Kripke, 1974). Subsequent studies showed that low
doses of UVR suppress contact hypersensitivity responses to chemical
sensitizers (Toews et al., 1980) and that systemic immunosuppression
(depressed contact hypersensitivity in unirradiated skin) occurs after
exposure to higher doses (Jessup et al., 1978). Irradiated mice were
also found to be less resistant to infection (Giannini, 1990). Other
studies (Noonan & De Fabo, 1990) have determined that systemic
suppression of immunoreactivity is not a function of the dose of UVR
but rather of the interval between irradiation and immunization of the
mice. Thus, induction of contact hypersensitivity responses in mice
exposed to low doses of UVR was not affected when the animals were
immunized through unirradiated skin immediately after exposure to UVR;
however, sensitization was suppressed if three days were allowed to
elapse between irradiation and immunization. It has also been shown
that the dose of UVR required to induce 50% suppression of the immune
response depends on the strain of mouse and the type of antigen used
(Noonan & De Fabo, 1990; Noonan & Hoffman, 1994).
The mechanism of UVR-induced suppression of cellular immunity has
not been elucidated, nor has a single initial event been identified
that leads to suppression of immunoreactivity. Currently, induction of
pyrimidine dimers in DNA (Kripke et al., 1992) and isomerization of
urocanic acid (Noonan & De Fabo, 1992) are the leading contenders.
Increased suppressor cell activity (Brodie & Halliday, 1991) and
efferent lymphatic blockade, which inhibits lymphocyte homing, may be
responsible for the UVR-associated accumulation of lymphocytes in
lymph nodes in UVR-exposed areas (Spangrude et al., 1983) and have
been proposed as possible causes of immunosuppression. Exposure to UVR
has also been shown to alter the pattern of cytokine production by
T cells, from a response dominated by Th1 (i.e. favouring delayed
hypersensitivity responses) to one dominated by Th2 (i.e. favouring
antibody production) (Araneo et al., 1989; Simon et al., 1990).
Exposure to UVR has been reported to affect Langerhans cells directly,
such that their interaction with T cells induces specific antigen
tolerance in the Th1 subpopulation (Simon et al., 1990) and
preferential activation of the Th2 population (Simon et al., 1991).
This may be the reason that mice exposed to UVR are more susceptible
to infection with the protozoan Leishmania major (Giannini, 1992),
since resistance to infection with this intracellular parasite is
dependent on the magnitude of the Th1 response of the host (Reed &
Scott, 1993). In addition, reduced resistance to T. spiralis was
found in rats exposed to UVR on days 5-10 of infection (Goettsch et
al., 1993). Altered cytokine production profiles may also be
responsible for increased sensitivity to Mycobacterium lepraemurium,
an intracellular pathogen that induces a chronic and eventually fatal
infection in susceptible mice. In a comparison of susceptible (BALB/c)
and resistant (C57Bl/6J) mice, Brett & Butler (1986) determined that
resistance to infection is correlated with the ability of mouse
lymphocytes to elaborate cytokines that activate macrophages, rather
than with the actual development of a delayed hypersensitivity
response to bacterial antigens. Jeevan & Kripke (1990) and Jeevan et
al. (1992) reported that irradiation of BALB/c mice resulted in
decreased resistance to infection, as measured by bacterial counts and
length of survival after infection. Elevated bacterial counts were
seen in animals exposed to doses of UVR that did not suppress the
delayed hypersensitivity response to bacterial antigens, suggesting
that the underlying mechanism of UVR-induced suppression of resistance
to infection is independent of suppressed delayed hypersensitivity.
2.2.12 Food additives
There is little information about the effects of food additives
on the immune system. An early study showed that the preservative
methylparaben and the antioxidants butylated hydroxyanisole, butylated
hydroxytoluene, and propylgallate suppress the in-vitro T-dependent
antibody response, whereas vanillin and vanillic acid stimulate it
(Archer et al., 1978). The significance of these findings in vivo
has yet to be established.
The immunotoxicity of 'caramel colour', which covers a large
number of complex products used as food colorants, has been
investigated. One of the compounds in this group, 2-acetyl-4(5)-
tetrahydroxybutylimidazole (THI) (caramel colour III), has been found
to be immunotoxic in rodents (Kroplien et al., 1985). THI induces a
rapid reduction in the number of B and T cells in blood, spleen, and
lymph nodes and morphological changes in the thymus of rats, with an
increased number of mature medullary thymocytes and a decreased number
of cortical macrophages. THI might reduce the migration of mature
thymocytes into the periphery, as a decrease in the number of recent
ER4+ thymic emigrants was found in the spleens of exposed rats
(Houben et al., 1992). Functional studies indicate changes in Th cell
function, an increased capacity to clear the Gram-positive bacterium
L. monocytogenes, and modulation of the activity of adherent splenic
cells (Houben et al., 1993). It has been hypothesized that THI exerts
an antivitamin B6 action by competing with pyridoxal 5'-phosphate for
binding to the cofactor site of one or more pyridoxal 5'-phosphate-
dependent enzymes.
2.3 Immunotoxicity of environmental chemicals in wildlife and
domesticated species
Most of the concern about chemical contamination of wildlife
populations has been focused on the aquatic ecosystem, and a growing
body of literature has appeared on the effects of pollution on the
health status of aquatic life. These studies deal mainly with the
occurrence of tumours and infectious diseases in fish and marine
mammals. These are multifactorial diseases in which perturbations of
the immune system may play a part.
2.3.1 Fish and other marine species
2.3.1.1 Fish
Fish diseases are being monitored on a routine basis at various
sites in North America and Europe. In Europe, most of the programmes
are carried out under the auspices of the International Council for
Exploration of the Sea. National and local studies have been directed
to estuarine, marine, and brackish waters suspected of being polluted,
such as in the vicinity of industrial areas and after major oil
spills. The common diseases that are discussed in relation to
pollution are certain skin diseases, such as lymphocystis, papillomas,
fin rotting, and skin ulcers (Vethaak & ap Rheinallt, 1992), as they
are easily identified grossly and are therefore potentially useful for
biomonitoring. Since most diseases of fish have a viral or bacterial
etiology, and elevated incidences have been correlated with chemical
pollution, immunotoxicity may play a role. A causal relationship
between chemical pollution and a disease state induced by
immunosuppression cannot be finally established, however, since many
confounding factors exist in the natural environment. Liver neoplasia
and precursor lesions have been used to biomonitor environmental
pollution in flatfish (Malins et al., 1988; Vethaak & ap Rheinallt,
1992; Vethaak, 1993); however, the role of the immune system is not
evident.
Effects observed in field studies are modified or confounded by
numerous factors, in particular for feral fish, for which there are
deficient case histories and limited knowledge of their migratory
patterns and biology (Vethaak, 1993). Extensive epidemiological
surveys are required that include specific parameters that have been
validated in experiments under (more) controlled conditions (in
mesocosms or the laboratory). Changes in disease patterns may suggest
immune alterations, but this should be demonstrated. Since most
diseases have a complex etiology, it will be difficult to establish
the role of immunotoxic effects under field conditions. Circumstantial
evidence can be obtained in these instances, although in the case of
feral animals mesocosm or laboratory experiments must carried out in
order to reach a final conclusion (Secombes et al., 1992; Vethaak,
1993).
The etiological components and their role in the pathogenesis of
many diseases in fish in the field are, as yet, poorly understood, and
laboratory experiments are often indispensable for background
knowledge. Even when such scientific deficiencies are resolved,
laboratory studies will still be needed, since function tests under
controlled conditions yield the most reliable and sensitive methods of
assessing immunological stress and must often accompany field studies,
as mentioned above. Findings from laboratory situations do not
necessarily imply effects in the field, however; in particular, when
results from the laboratory are extrapolated to field situations,
there is often a discrepancy between the levels of exposure.
2.3.1.2 Marine mammals
Marine mammals are of special interest to the discipline of
immunotoxicology. As the highest predators in highly contaminated
marine environments, these animals are exposed to a large number of
environmental chemicals, some of which have been identified as
potentially immunotoxic. Persistent lipophilic halogenated compounds
such as PCBs, polychlorinted dibenzodioxins, polychlorinated
dibenzofurans, and pesticides accumulate in the marine food chain and
are thus biomagnified in marine mammals. The concentrations of PCBs in
the blubber layer of marine mammals are higher than in any other
wildlife species measured (Table 6). In times of food shortage and
other stressful circumstances, these lipids are mobilized, thereby
releasing their toxic burden.
Table 6. Concentrations of polychlorinated biphenyls (PCBs) in herring and the blubber
layer of marine mammals
Species Source Total PCBs Reference
(µg/g lipid)
Herring Atlantic Ocean 0.0003-0.001 De Swart et al.
(United Kingdom) (1994)
Herring Baltic Sea 0.0035-0.0045 De Swart et
(Sweden) al. (1993)
Weddell seal Weddell Sea 0.07-0.09 Luckas et al.
(Leptonychotes wedelli) (Antarctic) (1990)
Harbour seal Atlantic Ocean 1-13 Luckas et al.
(Phoca vitulina) (Iceland) (1990)
Harbour seal Baltic Sea 21-140 Luckas et al.
(Phoca vitulina) (Sweden) (1990)
Beluga whale St Lawrence 15-700 Muir et. al.
(Delphinapterus leucas) River (Canada) (1990);
Martineau et al.
(1987)
Striped dolphin Mediterranean 100-2600 Kannan et al.
(Stenella coeruleoalba) Sea (Spain) (1993)
Because of the high level of exposure of marine mammals, they may
be expected to be the first wild animals to suffer from
immunosuppression due to chronic exposure to environmental
contaminants. Toxicological research over the last 20 years has
identified environmental chemicals as the source of many disorders in
marine mammals. In both field studies and controlled experiments, PCBs
have been linked to reproductive problems. As early as 1976, a high
incidence of premature parturition was seen in California sea-lions
(Zalophus californianus), which was caused by a viral infection and
was suggested to be linked to higher levels of pollutants in animals
that aborted as compared with mothers that gave birth to healthy pups
(Gilmartin et al., 1976). Pathological changes in the uteri of seals
in the highly polluted Baltic Sea, in some cases leading to sterility,
could be correlated with increased levels of PCBs (Helle et al.,
1976). In addition, several studies have linked skeletal deformities
in grey seals (Halichoerus grypus) and harbour seals (Phoca
vitulina) in the Baltic Sea to high levels of organochlorines in
their environment (Bergman et al., 1992; Mortensen et al., 1992). In
porpoises (Phocoenoides dalli) living in the north-western part of
the North Pacific, a negative correlation was found between serum
testosterone levels and DDE concentrations in blubber (Subramanian et
al., 1987). In an experimental situation, two groups of harbour seals
were fed fish containing different levels of pollutants. Seals that
were fed fish from the heavily polluted western part of the Dutch
Wadden Sea had significantly lower pup production than seals feeding
on less polluted fish (Reijnders, 1986). In the same study, it was
shown that the seals fed polluted fish also had significantly reduced
levels of vitamin A and thyroid hormone in their serum (Brouwer et
al., 1989). No parameters of immune function were studied.
Such correlative observations in the natural environment, in
combination with the results of semi-field studies, suggest that the
current levels of contaminants may be adversely affecting certain
marine mammal populations. The occurrence of a large number of
epizootics among seals and dolphins inhabiting polluted coastal areas
-- among bottlenose dolphins (Tursiops truncatus) on the Atlantic
coast of the United States in 1987 and in the Gulf of Mexico in 1990,
striped dolphins (Stenella coeruleoalba) on the coast of France in
1989 and in the Mediterranean Sea in 1990-92, Baikal seals (Phoca
sibirica) in Lake Baikal in 1987, and harbour seals in north-west
Europe in 1988 -- led to both public and scientific discussions about
the possible contribution of environmental pollutants to these disease
outbreaks. Owing to the complexities of the relationships between
toxicants and the immune system and the difficulties in obtaining
samples fit for use in immunotoxicological studies, it has been
impossible thus far to conclusively demonstrate immunosuppression in
free-ranging marine mammals.
Another immunotoxicological experiment was carried out in which
two groups of juvenile harbour seals (Phoca vitulina) were fed fish
from the Baltic Sea or from the relatively unpolluted Atlantic Ocean.
The diets were analysed for concentrations of potential immunotoxic
chemicals: the estimated daily intakes of TCDD-like organochlorines
were 270 and 35 ng/day for the two groups, respectively. Immunological
function in the two groups was examined by measuring mitogen- and
antigen-induced proliferative responses of lymphocytes, NK cell
activity, serum antibody levels after immunization with primary
antigens, and delayed-type hypersensitivity reactions. These
techniques had to be validated for application to seals, as virtually
nothing was known about the cellular immune system of marine mammals
(De Swart et al., 1993, 1994). Seals fed herring from the contaminated
Baltic Sea had significantly depressed immune function, as measured by
decreased NK cell activity (Ross et al., in press) and T-cell mitogen-
induced lymphocyte proliferation (De Swart et al., 1994), and
significantly lower delayed-type hypersensitivity and antibody
responses to immunization with ovalbumin (Ross et al., 1995). The
functional changes were accompanied by increased numbers of
neutrophils in the peripheral blood (De Swart et al., 1994). Since NK
cells are an important line of defence against viruses, and
lymphocytes (especially T cells) play a major role in the clearance of
viral infections, functional suppression of these leukocytes may
contribute to the severity of epizootic episodes among marine mammals.
These experiments not only provide the first demonstration of
pollution-induced impairment of immune function in marine mammals but
indicate that mammals in general can undergo such impairment as a
consequence of chronic exposure to the levels of pollution found in
their natural habitats. It is still difficult, however, to link the
disease outbreaks among marine mammals directly to pollution-induced
impairment of immune function.
2.3.2 Cattle and swine
The effects of antimicrobial, corticosteroid, and hormonal
compounds have been investigated in cattle, mainly as lymphocyte
proliferative responses and neutrophil functions in vitro. The
results are in keeping with those obtained in humans (Black et al.,
1992).
Few studies have dealt specifically with the direct immunotoxic
effects of pesticides and environmental pollutants. No statistical
difference was found between control and polybrominated biphenyl-
exposed animals in the numbers of circulating total, T, and B
lymphocytes, serum immunoglobulin levels, mitogen-induced
proliferative responses of lymphocytes, antibody response to keyhole
limpet mitogen, or cell-mediated response to purified protein
derivative (Kateley & Bazzell, 1978). In contrast, peripheral blood
lymphocytes from sows fed polybrominated biphenyls for 12weeks had
significantly decreased responses to mitogens (Howard et al., 1980).
The mycotoxin tricothecene produced by Fusarium and several
other fungi was shown to reduce lymphoid tissue cellularity and serum
IgA, IgG, and IgM concentrations and to impair neutrophil migration,
chemotaxis, and phagocytosis in cattle exposed to high doses (Buening
et al., 1982; Mann et al., 1983). Similarly, aflatoxin was reported to
suppress the mitogen-induced proliferative response of bovine
lymphocytes (Paul et al., 1977). Other mycotoxins, e.g. ochratoxin A
and zearalenone, have been suggested to be immunosuppressive in cattle
(Black et al., 1992).
2.3.3 Chickens
Chickens have been used in a number of immunological studies, as
the unique bursa of Fabricius, the avian organ for B-cell development,
underlies the need for a separate avian model in immunotoxicology.
Exposure of adolescent chickens to cyclophosphamide decreased the
levels of antibodies to various antigens without decreasing graft-
versus-host reactivity (Lerman & Weidanz, 1970). Nutritional
deficiencies in selenium or vitamin E have also been shown to impair
the humoral immune responses of adolescent chickens (Marsh et al.,
1981). Exposure to cyclophosphamide in ovo results in decreased
antibody forming capacity, decreased responsiveness to
phytohaemagglutinin and concanavalin A, and decreased weights and
altered morphology of the thymus, spleen, and bursa of Fabricius
(Eskola & Toivanen, 1974). Peritoneal macrophages were not affected
after exposure to cyclophosphamide in ovo, as judged by their
number, superoxide anion production, and surface expression of Ia
antigen and transferrin receptor (Golemboski et al., 1992). Dietert et
al. (1985) showed that exposure to aflatoxin B1 in ovo did not alter
humoral immunity; however, two parameters of cell-mediated, graft-
versus-host, and cutaneous basophil hypersensitivity reactions were
depressed. Methyl methanesulfonate decreased the bactericidal action
of peritoneal macrophages for E. coli after exposure in vitro
(Qureshi et al., 1989). TCDD impairs B-cell development in the bursa
of Fabricius in chicken embryos (Nikolaidos et al., 1990).
2.4 Immunotoxicity of environmental chemicals in humans
Although limited, various lines of evidence derived from case
reports, clinical studies, and well-designed longitudinal studies
imply that environmental agents can affect the human immune system.
While these data raise concern about potential health effects, they
rarely refer to clinical disease, for a number of reasons. For
example, there may be sufficient redundancy or reserve in the immune
system that 'moderate' levels of immunosuppression do not result in
disease. Alternatively, the clinical changes most likely to be
associated with moderate immunosuppression, e.g. increased severity or
frequency of pulmonary infections, do not occur. Well-designed
clinical studies with adequate populations and appropriate monitoring,
follow-up, and documentation of exposure have rarely been conducted.
Examples of published reports that attribute immune changes in human
populations exposed to environmental agents are summarized below. As
the reader will note, these studies range from poorly defined to
relatively large longitudinal studies.
2.4.1 Case reports
In an unsubstantiated study, a cluster of cases of Hodgkin's
disease reported in a small town in Michigan (United States) was
ascribed to chronic immune stimulation by mitogenic substances in the
environment (Schwartz et al., 1978). Immunological studies of family
members revealed a large number of individuals with altered ratios of
T-lymphocyte subpopulations, autoantibodies, infections, recurrent
rashes, and NK cell function. A report of a four-year study of workers
engaged in the manufacture of benzidine, a human bladder carcinogen,
suggested that individuals with depressed cell-mediated immunity (as
judged by skin testing) had precancerous conditions and subsequent
neoplasms (Gorodilova & Mandrik, 1978); no cases of neoplastic disease
were registered in workers with normal immunological responses.
2.4.2 Air pollutants
The association betweeen changes in immunological parameters and
host resistance and inhalation of particulate materials and oxidant
gases is well established (Folinsbee, 1992). For example, decreases in
delayed-type hypersensitivity response, circulating T-cell numbers,
and T-cell proliferation have been observed with, and sometimes
preceding, asbestos-related diseases, i.e. fibrosis, asbestosis, and
mesothelioma (Kagan et al., 1977a; Gaumer et al., 1981; Lew et al.,
1986; Tsang et al., 1988). B-Cell responses are increased, however, as
evidenced by increased serum and secretory (primarily IgA)
immunoglobulins (Kagan et al., 1977b). Kagan et al. (1979) also
reported an association between exposure to asbestos and B-cell
lymphoproliferative disorders. Several studies have shown altered NK
cell activity after exposure to asbestos (Kubota et al., 1985; Tsang
et al., 1988). In studies of NK cell responses in asbestos workers,
Lew et al. (1986) found that immune changes may occur independently of
any early neoplastic process. Similarly, there have been multiple
observations of abnormal antibody production, decreased cell-mediated
immune responses, and decreased resistance to disease in people
occupationally exposed to silica (Uber & McReynolds, 1982).
Oxidant gases have been associated with an increased prevalence
of respiratory infections, particularly bacterial, and potential
immune effects, but the data are less convincing than those from
studies of rodents. The association between air pollution in
industrialized areas and altered health status has been well
established in epidemiological studies (French et al., 1973). A number
of studies have linked exposure to air pollutants (ozone, nitrogen
dioxide, sulfur dioxide, environmental tobacco smoke) with an
increased incidence, severity, or duration of symptoms associated with
respiratory infections (Lunn et al., 1967; French et al., 1973;
Durham, 1974; Harrington & Krupnick, 1985; Neas et al., 1991; Schwartz
et al., 1991; Schwartz, 1992; US Environmental Protection Agency,
1990), although several studies of nitrogen dioxide failed to show
such an association (Speizer et al., 1980; Ware et al., 1984; Samet et
al., 1993). In Ontario, Canada, increased air pollution from sulfur
dioxide and ozone during the summer was directly related to hospital
admissions for acute respiratory symptoms (Bates & Sizto, 1983).
Goings et al. (1989) subjected young adult volunteers to 1, 2, or
3 ppm nitrogen dioxide for 2h per day on two consecutive days and then
administered influenza virus intranasally. Although no statistical
differences were observed, the frequencies of infections in exposed
volunteers (91%) were higher than the 56-73% in healthy individuals,
suggesting that nitrogen dioxide may play a role in increasing
susceptibility to infection. In assessments of air pollution at home,
young children in households with gas stoves had a higher incidence of
respiratory disease and decreased pulmonary function than children in
households with electric stoves. This difference was related to
increased levels of nitrogen dioxide in homes with gas stoves, which
reached peak values of > 1100 mg/m3 (Melia et al., 1977; Speizer et
al., 1980). In contrast, Samet (1994) found no association between
indoor levels of nitrogen dioxide and respiratory infections in
children. A study of schoolchildren in Chattanooga (United States)
showed an increased incidence of respiratory illness associated with
atmospheric nitrogen dioxide levels (Shy et al., 1970).
Examination of hospital admissions in Massachusetts (United
States) in 1980 and 1982 revealed a positive association between 1-h
maximum ozone levels in the summer months and daily admissions for
pneumonia and influenza (Ozkaynak et al., 1990). An effect of
atmospheric pollution, including oxidant gases, was also seen on the
number of influenza cases in Sofia, Bulgaria (Kalpazanov et al.,
1976); however, no demonstrable adverse effect on the course of a
rhinoviral infection was seen in young adult male volunteers after
exposure to moderate levels of ozone (0.3ppm for 6 h/day over a period
of five days) (Henderson et al., 1988), and children living in areas
with high ozone concentrations had lowered CD4:CD8 ratios of
peripheral lymphocytes but no higher incidence of chest colds (Zwick
et al., 1991). The phagocytic activity of alveolar macrophages
(obtained by bronchoalveolar lavage) and other functions were impaired
in human volunteers exposed to ozone (Devlin et al., 1991). The
sensitivity of human and mouse macrophages to the effects of ozone is
similar (Selgrade et al., 1995).
Not all epidemiological studies have showed a correlation between
air pollution and respiratory disease. Some of the discrepancies from
experimental studies may be due to the parameters used to assess
enhancement of infection. In experimental studies, increases in viral
titres in the respiratory tract tend to be taken as an indication that
the exposure enhances infection, whereas epidemiological studies rely
on symptoms, many of which could be related to enhanced inflammatory
or even allergic responses to the virus in the absence of viral
replication.
Controlled studies (in an environmental chamber) showed that
acute exposure to ozone causes an inflammatory response in the lower
airways of human subjects (Koren et al., 1989; Devlin et al., 1991).
The inflammatory response was manifested by increases in various
inflammatory indicators including polymorphonuclear neutrophils
(Figure 34), proteins, fibronectin, IL-6, and tryptase (Koren et al.,
1989, 1994).
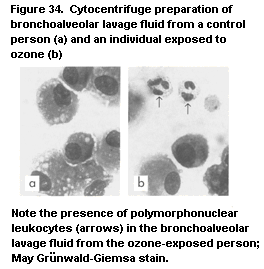 The proinflammatory effects of ozone raise the possibility that
it can increase the sensitivity of people with atopic asthma to
challenge with a specific allergen. Several studies have investigated
the effect of exposure to pollutants on subsequent reactivity to
antigen in atopic human volunteers. Molfino et al. (1991) reported
that exposure to 0.12 ppm ozone significantly increased bronchial
responsiveness to antigen in some individuals. Although they
acknowledged weaknesses in the design of the experiment and
recommended that the findings be confirmed, their observations are in
accordance with those of the majority of epidemiological studies. In
contrast, Bascom et al. (1990) found no alteration in the acute
response to nasal antigen challenge in allergic patients pre-exposed
to ozone in comparison with exposure to air; however, they did report
increased upper respiratory tract inflammation after exposure to ozone
in these patients in the absence of antigen challenge. Similarly, the
bronchial response to inhaled grass pollen was unaffected by prior
exposure to 0.1 ppm nitrogen dioxide (Orehek et al., 1981). The topic
of sensitization and the role of ozone in exacerbating asthma has been
reviewed (Koren & Blomberg, in press).
2.4.3 Pesticides
Pesticides can alter the human immune system. For example, women
chronically exposed to low levels of aldicarb-contaminated groundwater
had altered numbers of T cells and decreased CD4:CD8 ratios (Fiore et
al., 1986). While this finding was not confirmed in studies in animals
(see section 2.2.3.4), follow-up studies by Mirkin et al. (1990)
confirmed the immune changes in those individuals still available for
study, although the population was considerably smaller.
2.4.4 Halogenated aromatic hydrocarbons
A number of chemical accidents have resulted in human exposure to
halogenated aromatic hydrocarbons. A feed supplement for lactating
cows, inadvertently contaminated with polybrominated biphenyls, was
used in more than 500 dairy herds and poultry farms in Michigan
(United States) in 1973. Diary farm residents had reduced proportions
of circulating T Iymphocytes and reducedlymphoproliferative
responsiveness in vitro (Bekesi et al., 1978); these changes
persisted during follow-up (Bekesi et al., 1987). Silva et al. (1979),
however, were unable to detect any immune abnormalities in a similarly
exposed cohort.
In Taiwan, more than 2000 people were exposed in 1979 to rice oil
contaminated with PCBs and polychlorinated dibenzofurans. The clinical
features in many of the exposed individuals included chloracne,
pigmentation of skin, liver disease, and respiratory infections. When
the immune status of the exposed individuals was examined one year
after exposure, decreased concentrations of serum IgM and IgA, but not
IgG, were reported, in addition to decreased numbers of circulating Th
cells. The proportions of Ts/Tc cells and B lymphocytes were within
the control values. Suppression of delayed-type hypersensitivity to
recall antigens (streptokinase, streptodornase, tuberculin), enhanced
mitogen-induced lymphocyte proliferation, and increases in
sinopulmonary infections have been reported in this population (Chang
et al., 1981, 1982; Lu & Wu, 1985). Many of the effects were
transient, since two years after exposure most of the clinical
abnormalities and laboratory parameters had returned to normal. A
similar incident occurred in Japan in 1978, resulting in the 'yusho'
syndrome. The immune system was assumed to be affected because of an
increased frequency of respiratory infections and lowered serum IgM
and IgA concentrations (Shigematsu et al., 1978). Another incident of
intoxication with PCBs and polychlorinated dibenzofurans occurred
after exposure to contaminated soot of fires in electrical equipment
(Elo et al., 1985). The exposed people had serum PCB concentrations up
to 30 mg/litre. The number of blood T cells was lower five weeks after
exposure but had returned to normal values seven weeks later. Lowered
CD4:CD8 ratios and lymphocyte proliferation after mitogen stimulation
were also seen. Nine of the 15 most heavily exposed persons suffered
from at least one infection of the upper respiratory tract. No overt
long-term effects or chloracne were observed.
The existing data also suggest that neonates are particularly
sensitive to the immunotoxic effects of PCBs. Thus, higher incidences
of colds and gastrointestinal (vomiting, abdominal pain) and
dermatological (eczema, itchy skin) manifestations were observed in
breast-fed infants of women occupationally exposed to the PCBs
Kanechlor 500 and 300 than in infants born to unexposed women. The
incidence of these symptoms increased with increasing length of
breast-feeding (Hara, 1985). Epidemiological studies of women who
consumed contaminated fish from the Great Lakes indicated that the
maternal serum PCB level during pregnancy was positively associated
with the number and type of infectious illnesses suffered by their
breast-fed infants, especially during the first four months of life.
The incidence of infections in the infant was correlated strongly with
the highest rate of maternal fish consumption (Swain, 1991).
A number of studies have been conducted of the immune status of
people exposed to TCDD. In 1976, an accident occurred at a chemical
plant in Seveso, Italy, in which high concentrations of TCDD were
released into the local environment. An evaluation of 44 exposed
children (20 with chloracne) showed no overt changes in immune status
(Reggiani, 1980), although the adequacy of the control populations
used has been questioned. In a study of residents of an area of
Missouri (United States) with long-term exposure (average, three
years) to low levels of TCDD in contaminated soil, no clinical
symptoms were recorded, although a number of individuals showed
changes in cell-mediated immunity, manifested as altered delayed-type
hypersensitivity (Hoffman et al., 1986). In the follow-up
investigation, however, the skin anergy was not confirmed (Evans et
al., 1988). The serum concentration of the thymic hormone thymosin-a1,
which has been related to the toxic action of this compound on the
thymus, was reduced (Stehr-Green et al., 1989; Hoffman, 1992).
Jennings et al. (1988) found an increased frequency of circulating
antinuclear antibodies and immune complexes in TCDD-exposed workers.
2.4.5 Metals
Exposure to metals may also affect the immune system. Workers
with elevated blood lead levels (30-90 µg/100 ml) had increased
suppressor cell activity (Cohen et al., 1989), lowered lymphocyte
proliferation after mitogen stimulation in vitro (Jaremin, 1983),
decreased IgA concentrations in saliva, and lowered complement C3
levels (Ewers et al., 1982). These individuals also had an enhanced
prevalence of respiratory infection (Ewers et al., 1982). The
immunotoxic effects of lead may be dose-dependent, since neither
humoral nor cellular parameters were affected after long-term, low-
level exposure (Reigart & Graber, 1976; Kimber et al., 1986). In
unrelated studies, the cationic heavy metal mercury was associated
with immune complex disease in humans (Makker & Aikawa, 1979).
In contrast to the database on the immunotoxic effects of
cadmium, lead, and mercury in experimental animals in vivo and the
results of mechanistic studies in vitro, the data on the effects of
heavy metals on the human immune system are scanty and refer mainly to
occupational exposure. These studies nevertheless provide evidence
that at least mercury and lead affect the immune system (Moszczynski
et al., 1990a; Bernier et al., in press).
Significantly decreased levels of serum IgG and IgA, but not IgM,
IgD, or IgE, were reported in workers occupationally exposed to
metallic mercury vapours for 20 years in comparison with unexposed
controls (Moszczynski et al., 1990b). These workers had blood mercury
levels of < 50 µg/litre. Similarly, significantly decreased IgG and
IgA levels were observed in workers with urinary mercury levels of
0.029-0.545 mg/litre (Bencko et al., 1990). Studies of a small number
of people exposed to mercury in dental amalgams have shown increased
levels of IgE (Anneroth et al., 1992), an increased incidence of
asthma (Drouet et al., 1990), and development of contact dermatitis
(Gonçalo et al., 1992). Total lymphocyte, CD4, and CD8 levels were
higher in exposed people than in controls (Eedy et al., 1990).
Epidemiological data indicate that the main effects of
occupational exposure to lead are on cellular aspects of the immune
system and that humoral parameters remain relatively insensitive to
such exposure. Thus, serum IgG, IgM, and IgA levels remained within
the normal range in workers exposed for 4-30years, with a mean blood
lead level of 38.4 µg/dl, in comparison with a mean control level of
11.8 µg/dl (Kimber et al., 1986). Similarly, no effects were noted on
serum IgG or IgA levels in a cohort exposed to lead oxides at a
reported concentration within the plant of 266 µg/m3, who had an
estimated blood lead level of 64 µg/dl; however, the response of
lymphocytes from the exposed group to stimulation with
phytohaemagglutinin and concanavalin A in vitro was significantly
lower than that of controls (Alomran & Shleamoon, 1988). Decreased
serum Ig levels were reported in occupationally exposed workers with a
mean blood lead level of 46.9 µg/dl, but the duration of exposure was
not reported (Castillo-Mendez et al., 1991). In another study, no
significant effects were noted on serum immunoglobulin levels after
exposure to lead, but the levels of secretory IgA, which plays a major
role in the defence against respiratory and gastrointestinal
infections, was significantly decreased in workers with blood lead
levels of 21-90 µg/dl. The incidence of influenza infections per year
was significantly higher in these workers than in the control group
(Ewers et al., 1982).
Studies on the effects of lead on lymphocyte levels in
occupationally exposed workers have produced inconclusive results. One
study found an increase in absolute B lymphocyte counts and CD8 cells
(Coscia et al., 1987), while a decrease in total T lymphocytes and the
CD4 subset was reported in another set of workers (Fischbein et al.,
1993).
2.4.6 Solvents
Certain organic solvents may induce immune changes in humans.
Benzene-induced pancytopenia with associated bone-marrow hypoplasia, a
classical sign of chronic exposure to benzene, results in an
immunodeficiency state due to the reduced numbers of immunocompetent
cells (Goldstein, 1977). Alterations in the numbers of certain
lymphocyte subsets, e.g. CD3 and CD4 lymphocytes, have also been
reported in workers exposed to solvents (Denkhaus et al., 1986),
suggesting that the effects may be somewhat specific.
2.4.7 Ultraviolet radiation
Numerous reports have shown that UVR inhibits contact
hypersensitivity of the skin to sensitizers such as
dinitrochlorobenzene (DNCB) (O'Dell et al., 1980; Halprin et al.,
1981; Hersey et al., 1983a,b; Kalimo et al., 1983; Sjovall et al.,
1985; Friedmann et al., 1989; Yoshikawa et al., 1990; Vermeer et al.,
1991; Cooper et al., 1992). The dose required to produce
immunosuppressive effects in humans is similar to that in C57Bl/6
mice, the strain phenotypically most sensitive to UVR-induced immune
suppression (Noonan & Hoffman, 1994).
Human buttock skin was exposed to four daily doses of
144 mJ/cm2 UVR, and the irradiated site was sensitized with DNCB
immediately after the last exposure; the inner surface of the forearm
was challenged 30days later with DNCB and contact hypersensitivity
assessed. Forty percent of the volunteers failed to develop contact
hypersensitivity and were designated sensitive, suggesting that, as in
mice, susceptibility to UVR is genetically controlled. The sensitive
phenotype also appeared to be a risk factor for the development of
skin cancer (Yoshikawa et al., 1990). Suppression of contact
hypersensitivity is seen in a similar percentage of black-skinned
individuals, indicating that melanin cannot protect against this
phenomenon (Vermeer et al., 1991). In another study, human buttock
skin was exposed to 0.75 or two minimal erythemal doses (MED) of UVB
(1 MED = 29.1-32.5mJ/cm2, depending on the individual) for four days
and sensitized through irradiated skin immediately after the last
exposure to DNCB; subjects were challenged with diluted DNCB at a
distal site three weeks later. Some subjects were also exposed to four
MED (moderate sunburn) and sensitized three days later with DNCB.
Analysis of overall individual responses revealed decreased
frequencies of fully successful immunizations in all UVB-exposed
groups. The rate of immunological tolerance to DNCB (lasting up to
four months) in the groups that were initially sensitized on skin that
had received erythemagenic doses of UVB was 31%, whereas it was 7% in
unirradiated controls (Cooper et al., 1992).
A dose-response relationship was established in the studies of
Cooper et al. (1992) in a comparison of subjects with types I-III skin
(fair to moderately fair) who received various doses or schedules of
UVR from a bank of FS20 fluorescent sun lamps (rich in UVB) with
respect to their ability to mount an immune response to DNCB. A linear
inhibition of immune responsiveness was seen, the first detectable
decrease occurring at 0.75 of the individual's MED, reaching complete
inhibition of responsiveness for 95% of subjects when two MED were
administered every day for four days before immunization. Similar
inhibition occurred when DNCB was administered through skin that had
been exposed to a single dose of fourMED three days earlier. A dose-
response curve for fair-skinned subjects was constructed by plotting
the dose in total MED administered against the degree of immune
response to DNCB (Figure 35). The 50% immune suppressive dose was
calculated to be about 100mJ/cm2 of UVB.
Unresponsiveness to a contact sensitizer applied to UV-irradiated
skin can thus be induced in a proportion of individuals after exposure
to moderate levels of UVR, and at least some individuals become
immunologically tolerant in a manner similar to experimental animals.
Taken together, these data suggest that the systemic immunosuppression
induced in mice by UVR also occurs in humans, possibly through a
similar mechanism. UVR appears to alter antigen presentation and the
expression of Langerhans (CDla+DR+) cells, which is followed by an
influx of CDla+DR+ monocytes that preferentially activate CD4+
(suppressor-inducer) cells, which induce maturation of CD8+ Tc
Iymphocytes (Cooper et al., 1986; Baadsgaard et al., 1990). The UVR
wavelengths responsible for induction of CDla-DR+ cells are
predominantly within the UVB band and to a lesser extent in the C band
(Baadsgaard et al., 1987, 1989).
UVR from solarium lamps also suppressed NK cell activity in the
blood of subjects exposed for 1h per day for 12 days and tested one
and seven days after exposure; the activity returned to normal by 21
days after exposure (Hersey et al., 1983a). The effects of UVR on NK
cell activity are attributed to A radiation (Hersey et al., 1983b).
The depressed immune function observed in irradiated rodents
reflects anecdotal observations in humans, i.e. that exposure to
sunlight exacerbates certain infectious diseases, particularly those
involving the skin. For example, it was noted at the turn of the
century that smallpox lesions were worsened by exposure to sunlight
(Finsen, 1901), and herpetic lesions and viral warts may be
reactivated or exacerbated by sunlight (Giannini, 1990). It has also
been hypothesized that sunlight affects susceptibility to infection
with the bacteria that cause leprosy (Patki, 1991). Lesions of Herpes
simplex virus type I and type II can be reactivated by exposure to
UVR (Spruanoe, 1985; Klein, 1986). Using the criteria established by
The proinflammatory effects of ozone raise the possibility that
it can increase the sensitivity of people with atopic asthma to
challenge with a specific allergen. Several studies have investigated
the effect of exposure to pollutants on subsequent reactivity to
antigen in atopic human volunteers. Molfino et al. (1991) reported
that exposure to 0.12 ppm ozone significantly increased bronchial
responsiveness to antigen in some individuals. Although they
acknowledged weaknesses in the design of the experiment and
recommended that the findings be confirmed, their observations are in
accordance with those of the majority of epidemiological studies. In
contrast, Bascom et al. (1990) found no alteration in the acute
response to nasal antigen challenge in allergic patients pre-exposed
to ozone in comparison with exposure to air; however, they did report
increased upper respiratory tract inflammation after exposure to ozone
in these patients in the absence of antigen challenge. Similarly, the
bronchial response to inhaled grass pollen was unaffected by prior
exposure to 0.1 ppm nitrogen dioxide (Orehek et al., 1981). The topic
of sensitization and the role of ozone in exacerbating asthma has been
reviewed (Koren & Blomberg, in press).
2.4.3 Pesticides
Pesticides can alter the human immune system. For example, women
chronically exposed to low levels of aldicarb-contaminated groundwater
had altered numbers of T cells and decreased CD4:CD8 ratios (Fiore et
al., 1986). While this finding was not confirmed in studies in animals
(see section 2.2.3.4), follow-up studies by Mirkin et al. (1990)
confirmed the immune changes in those individuals still available for
study, although the population was considerably smaller.
2.4.4 Halogenated aromatic hydrocarbons
A number of chemical accidents have resulted in human exposure to
halogenated aromatic hydrocarbons. A feed supplement for lactating
cows, inadvertently contaminated with polybrominated biphenyls, was
used in more than 500 dairy herds and poultry farms in Michigan
(United States) in 1973. Diary farm residents had reduced proportions
of circulating T Iymphocytes and reducedlymphoproliferative
responsiveness in vitro (Bekesi et al., 1978); these changes
persisted during follow-up (Bekesi et al., 1987). Silva et al. (1979),
however, were unable to detect any immune abnormalities in a similarly
exposed cohort.
In Taiwan, more than 2000 people were exposed in 1979 to rice oil
contaminated with PCBs and polychlorinated dibenzofurans. The clinical
features in many of the exposed individuals included chloracne,
pigmentation of skin, liver disease, and respiratory infections. When
the immune status of the exposed individuals was examined one year
after exposure, decreased concentrations of serum IgM and IgA, but not
IgG, were reported, in addition to decreased numbers of circulating Th
cells. The proportions of Ts/Tc cells and B lymphocytes were within
the control values. Suppression of delayed-type hypersensitivity to
recall antigens (streptokinase, streptodornase, tuberculin), enhanced
mitogen-induced lymphocyte proliferation, and increases in
sinopulmonary infections have been reported in this population (Chang
et al., 1981, 1982; Lu & Wu, 1985). Many of the effects were
transient, since two years after exposure most of the clinical
abnormalities and laboratory parameters had returned to normal. A
similar incident occurred in Japan in 1978, resulting in the 'yusho'
syndrome. The immune system was assumed to be affected because of an
increased frequency of respiratory infections and lowered serum IgM
and IgA concentrations (Shigematsu et al., 1978). Another incident of
intoxication with PCBs and polychlorinated dibenzofurans occurred
after exposure to contaminated soot of fires in electrical equipment
(Elo et al., 1985). The exposed people had serum PCB concentrations up
to 30 mg/litre. The number of blood T cells was lower five weeks after
exposure but had returned to normal values seven weeks later. Lowered
CD4:CD8 ratios and lymphocyte proliferation after mitogen stimulation
were also seen. Nine of the 15 most heavily exposed persons suffered
from at least one infection of the upper respiratory tract. No overt
long-term effects or chloracne were observed.
The existing data also suggest that neonates are particularly
sensitive to the immunotoxic effects of PCBs. Thus, higher incidences
of colds and gastrointestinal (vomiting, abdominal pain) and
dermatological (eczema, itchy skin) manifestations were observed in
breast-fed infants of women occupationally exposed to the PCBs
Kanechlor 500 and 300 than in infants born to unexposed women. The
incidence of these symptoms increased with increasing length of
breast-feeding (Hara, 1985). Epidemiological studies of women who
consumed contaminated fish from the Great Lakes indicated that the
maternal serum PCB level during pregnancy was positively associated
with the number and type of infectious illnesses suffered by their
breast-fed infants, especially during the first four months of life.
The incidence of infections in the infant was correlated strongly with
the highest rate of maternal fish consumption (Swain, 1991).
A number of studies have been conducted of the immune status of
people exposed to TCDD. In 1976, an accident occurred at a chemical
plant in Seveso, Italy, in which high concentrations of TCDD were
released into the local environment. An evaluation of 44 exposed
children (20 with chloracne) showed no overt changes in immune status
(Reggiani, 1980), although the adequacy of the control populations
used has been questioned. In a study of residents of an area of
Missouri (United States) with long-term exposure (average, three
years) to low levels of TCDD in contaminated soil, no clinical
symptoms were recorded, although a number of individuals showed
changes in cell-mediated immunity, manifested as altered delayed-type
hypersensitivity (Hoffman et al., 1986). In the follow-up
investigation, however, the skin anergy was not confirmed (Evans et
al., 1988). The serum concentration of the thymic hormone thymosin-a1,
which has been related to the toxic action of this compound on the
thymus, was reduced (Stehr-Green et al., 1989; Hoffman, 1992).
Jennings et al. (1988) found an increased frequency of circulating
antinuclear antibodies and immune complexes in TCDD-exposed workers.
2.4.5 Metals
Exposure to metals may also affect the immune system. Workers
with elevated blood lead levels (30-90 µg/100 ml) had increased
suppressor cell activity (Cohen et al., 1989), lowered lymphocyte
proliferation after mitogen stimulation in vitro (Jaremin, 1983),
decreased IgA concentrations in saliva, and lowered complement C3
levels (Ewers et al., 1982). These individuals also had an enhanced
prevalence of respiratory infection (Ewers et al., 1982). The
immunotoxic effects of lead may be dose-dependent, since neither
humoral nor cellular parameters were affected after long-term, low-
level exposure (Reigart & Graber, 1976; Kimber et al., 1986). In
unrelated studies, the cationic heavy metal mercury was associated
with immune complex disease in humans (Makker & Aikawa, 1979).
In contrast to the database on the immunotoxic effects of
cadmium, lead, and mercury in experimental animals in vivo and the
results of mechanistic studies in vitro, the data on the effects of
heavy metals on the human immune system are scanty and refer mainly to
occupational exposure. These studies nevertheless provide evidence
that at least mercury and lead affect the immune system (Moszczynski
et al., 1990a; Bernier et al., in press).
Significantly decreased levels of serum IgG and IgA, but not IgM,
IgD, or IgE, were reported in workers occupationally exposed to
metallic mercury vapours for 20 years in comparison with unexposed
controls (Moszczynski et al., 1990b). These workers had blood mercury
levels of < 50 µg/litre. Similarly, significantly decreased IgG and
IgA levels were observed in workers with urinary mercury levels of
0.029-0.545 mg/litre (Bencko et al., 1990). Studies of a small number
of people exposed to mercury in dental amalgams have shown increased
levels of IgE (Anneroth et al., 1992), an increased incidence of
asthma (Drouet et al., 1990), and development of contact dermatitis
(Gonçalo et al., 1992). Total lymphocyte, CD4, and CD8 levels were
higher in exposed people than in controls (Eedy et al., 1990).
Epidemiological data indicate that the main effects of
occupational exposure to lead are on cellular aspects of the immune
system and that humoral parameters remain relatively insensitive to
such exposure. Thus, serum IgG, IgM, and IgA levels remained within
the normal range in workers exposed for 4-30years, with a mean blood
lead level of 38.4 µg/dl, in comparison with a mean control level of
11.8 µg/dl (Kimber et al., 1986). Similarly, no effects were noted on
serum IgG or IgA levels in a cohort exposed to lead oxides at a
reported concentration within the plant of 266 µg/m3, who had an
estimated blood lead level of 64 µg/dl; however, the response of
lymphocytes from the exposed group to stimulation with
phytohaemagglutinin and concanavalin A in vitro was significantly
lower than that of controls (Alomran & Shleamoon, 1988). Decreased
serum Ig levels were reported in occupationally exposed workers with a
mean blood lead level of 46.9 µg/dl, but the duration of exposure was
not reported (Castillo-Mendez et al., 1991). In another study, no
significant effects were noted on serum immunoglobulin levels after
exposure to lead, but the levels of secretory IgA, which plays a major
role in the defence against respiratory and gastrointestinal
infections, was significantly decreased in workers with blood lead
levels of 21-90 µg/dl. The incidence of influenza infections per year
was significantly higher in these workers than in the control group
(Ewers et al., 1982).
Studies on the effects of lead on lymphocyte levels in
occupationally exposed workers have produced inconclusive results. One
study found an increase in absolute B lymphocyte counts and CD8 cells
(Coscia et al., 1987), while a decrease in total T lymphocytes and the
CD4 subset was reported in another set of workers (Fischbein et al.,
1993).
2.4.6 Solvents
Certain organic solvents may induce immune changes in humans.
Benzene-induced pancytopenia with associated bone-marrow hypoplasia, a
classical sign of chronic exposure to benzene, results in an
immunodeficiency state due to the reduced numbers of immunocompetent
cells (Goldstein, 1977). Alterations in the numbers of certain
lymphocyte subsets, e.g. CD3 and CD4 lymphocytes, have also been
reported in workers exposed to solvents (Denkhaus et al., 1986),
suggesting that the effects may be somewhat specific.
2.4.7 Ultraviolet radiation
Numerous reports have shown that UVR inhibits contact
hypersensitivity of the skin to sensitizers such as
dinitrochlorobenzene (DNCB) (O'Dell et al., 1980; Halprin et al.,
1981; Hersey et al., 1983a,b; Kalimo et al., 1983; Sjovall et al.,
1985; Friedmann et al., 1989; Yoshikawa et al., 1990; Vermeer et al.,
1991; Cooper et al., 1992). The dose required to produce
immunosuppressive effects in humans is similar to that in C57Bl/6
mice, the strain phenotypically most sensitive to UVR-induced immune
suppression (Noonan & Hoffman, 1994).
Human buttock skin was exposed to four daily doses of
144 mJ/cm2 UVR, and the irradiated site was sensitized with DNCB
immediately after the last exposure; the inner surface of the forearm
was challenged 30days later with DNCB and contact hypersensitivity
assessed. Forty percent of the volunteers failed to develop contact
hypersensitivity and were designated sensitive, suggesting that, as in
mice, susceptibility to UVR is genetically controlled. The sensitive
phenotype also appeared to be a risk factor for the development of
skin cancer (Yoshikawa et al., 1990). Suppression of contact
hypersensitivity is seen in a similar percentage of black-skinned
individuals, indicating that melanin cannot protect against this
phenomenon (Vermeer et al., 1991). In another study, human buttock
skin was exposed to 0.75 or two minimal erythemal doses (MED) of UVB
(1 MED = 29.1-32.5mJ/cm2, depending on the individual) for four days
and sensitized through irradiated skin immediately after the last
exposure to DNCB; subjects were challenged with diluted DNCB at a
distal site three weeks later. Some subjects were also exposed to four
MED (moderate sunburn) and sensitized three days later with DNCB.
Analysis of overall individual responses revealed decreased
frequencies of fully successful immunizations in all UVB-exposed
groups. The rate of immunological tolerance to DNCB (lasting up to
four months) in the groups that were initially sensitized on skin that
had received erythemagenic doses of UVB was 31%, whereas it was 7% in
unirradiated controls (Cooper et al., 1992).
A dose-response relationship was established in the studies of
Cooper et al. (1992) in a comparison of subjects with types I-III skin
(fair to moderately fair) who received various doses or schedules of
UVR from a bank of FS20 fluorescent sun lamps (rich in UVB) with
respect to their ability to mount an immune response to DNCB. A linear
inhibition of immune responsiveness was seen, the first detectable
decrease occurring at 0.75 of the individual's MED, reaching complete
inhibition of responsiveness for 95% of subjects when two MED were
administered every day for four days before immunization. Similar
inhibition occurred when DNCB was administered through skin that had
been exposed to a single dose of fourMED three days earlier. A dose-
response curve for fair-skinned subjects was constructed by plotting
the dose in total MED administered against the degree of immune
response to DNCB (Figure 35). The 50% immune suppressive dose was
calculated to be about 100mJ/cm2 of UVB.
Unresponsiveness to a contact sensitizer applied to UV-irradiated
skin can thus be induced in a proportion of individuals after exposure
to moderate levels of UVR, and at least some individuals become
immunologically tolerant in a manner similar to experimental animals.
Taken together, these data suggest that the systemic immunosuppression
induced in mice by UVR also occurs in humans, possibly through a
similar mechanism. UVR appears to alter antigen presentation and the
expression of Langerhans (CDla+DR+) cells, which is followed by an
influx of CDla+DR+ monocytes that preferentially activate CD4+
(suppressor-inducer) cells, which induce maturation of CD8+ Tc
Iymphocytes (Cooper et al., 1986; Baadsgaard et al., 1990). The UVR
wavelengths responsible for induction of CDla-DR+ cells are
predominantly within the UVB band and to a lesser extent in the C band
(Baadsgaard et al., 1987, 1989).
UVR from solarium lamps also suppressed NK cell activity in the
blood of subjects exposed for 1h per day for 12 days and tested one
and seven days after exposure; the activity returned to normal by 21
days after exposure (Hersey et al., 1983a). The effects of UVR on NK
cell activity are attributed to A radiation (Hersey et al., 1983b).
The depressed immune function observed in irradiated rodents
reflects anecdotal observations in humans, i.e. that exposure to
sunlight exacerbates certain infectious diseases, particularly those
involving the skin. For example, it was noted at the turn of the
century that smallpox lesions were worsened by exposure to sunlight
(Finsen, 1901), and herpetic lesions and viral warts may be
reactivated or exacerbated by sunlight (Giannini, 1990). It has also
been hypothesized that sunlight affects susceptibility to infection
with the bacteria that cause leprosy (Patki, 1991). Lesions of Herpes
simplex virus type I and type II can be reactivated by exposure to
UVR (Spruanoe, 1985; Klein, 1986). Using the criteria established by
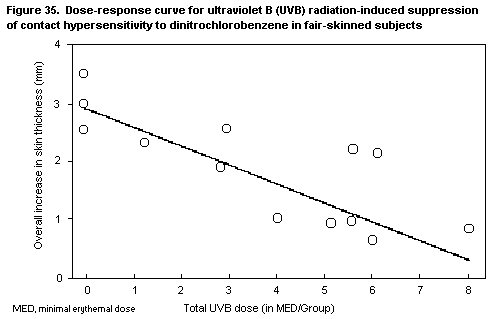 Yoshida & Streilin (1990) for the UVB-sensitive phenotype, Taylor et
al. (1994) reported that 66% of individuals who have a history of
herpes lip lesions provoked by exposure to sunlight were sensitive to
UVB, in comparison with 40-45% of the general population and 92% of
skin cancer patients. Exposure of immunosuppressed patients to
sunlight can increase the incidence of viral warts caused by
papillomavirus (Boyle et al., 1984; Dyall-Smith & Varigos, 1985). It
is also known that UVR exacerbates the clinical course of systemic
lupus erythematosus, an autoimmune disease (Epstein et al., 1965). The
effects of UVR on the risk of infectious disease have been reviewed by
Koren et al. (1994). In contrast, certain infectious diseases appear
to be cured by sunlight; the most notable are erysipelas (Giannini,
1990), a skin disease caused by Streptococcus, and skin lesions
caused by Herpes zoster virus.
2.4.8 Others
A large number of therapeutic drugs and drugs of abuse may also
alter human immune function in humans. These include:
Therapeutic agents
Alkylating agents
Nitrogen mustards: cyclophosphamide, L-phenylalanine mustard,
chlorambucil
Alkyl sulfonates: busulfan
Nitrosoureas: carmustine (BCNU), lomustine (CCNU)
Triazenes: dimethyltriazenoimidazolecarboxamide (DTIC)
Anti-inflammatory agents
Aspirin, indomethacin, penicillamine, gold salts
Adrenocorticosteroids: prednisone
Antimetabolites
Purine antagonists: 6-mercaptopurine, azathioprine, 6-thioguanine
Pyrimidine antagonists: 5-fluorouracil, cytosine arabinoside,
bromodeoxyuridine
Folic acid antagonists: methotrexate (amethopterine)
Natural products
Vinca alkaloids: vinblastine, vincristine, procarbazine
Antibiotics: actinomycin D, adriablastine, bleomycin, daunomycin,
puromycin, mitomycin C, mithramycin
Antifungal agents: griseofulvin
Enzymes: L-asparaginase
Cyclosporin A
Estrogens: diethylstilbestrol, ethinylestradiol
Substances of abuse
Ethanol
Cannabinoids
Cocaine
Opiates
Adapted from Dean & Murray (1990)
3. STRATEGIES FOR TESTING THE IMMUNOTOXICITY OF CHEMICALS IN ANIMALS
3.1 General testing of the toxicity of chemicals
The fact that substances used in various aspects of modern life
can be simultaneously beneficial and harmful to human life creates a
legislative and regulatory dilemma. In order to balance the desire to
use the many new substances that enter the market every year and the
economic benefit that is associated with their use on the one hand
with the health and safety of the population on the other is an
important challenge to governmental authorities. Legislative and
regulatory efforts to minimize and control the risk of adverse effects
on human health has resulted in a system for assessing and classifying
the potential risk of exposure to chemicals. Potential adverse effects
can be assessed in studies with experimental animals. In conducting
such studies, attention must be paid to ethical and regulatory
requirements for animal welfare and to good laboratory practice.
In assessing and evaluating the toxic characteristics of a
chemical, its oral toxicity may be determined once initial information
has been obtained by acute testing. Toxicity is routinely tested
according to guidelines, one of which is guideline No. 407 of the
Organisation for Economic Co-operation and Development (OECD) for
testing of chemicals, the 'Repeated Dose Oral Toxicity - Rodent:
28-day or 14-day Study' (Organisation for Economic Co-operation and
Development, 1981). This guideline has undergone three revisions, the
most recent of which (January 1994) includes parameters of
immunotoxicological relevance (see Table 7). Depending on the amount
of a chemical to be produced and the expected exposure to the
chemical, testing according to this guideline may in many countries
provide the only information on its safety, including potential
toxicity to the immune system. The information yielded by this type of
testing is therefore decisive in determining how chemicals are used in
society. Subsequent guidelines have been defined for use in follow-up
studies if more exposure is expected or if there is a suspicion of
toxicity on the basis of structural analogy with other known
compounds. These include 90-day studies of oral toxicity, long-term
studies, and studies of reproductive effects. Although such guidelines
include more parameters of the immune system than OECD test guideline
No. 407, detection of potential immunotoxicity may still not be
adequately addressed. In practice, the best procedure is to carry out
appropriate tests on a case-by-case basis, at increasing levels of
complexity, when concerns are raised in more general toxicological
studies.
Table 7. Parameters of OECD Test Guideline 407 that relate to the immune system
Current Guideline 407 Proposal for updating Proposal for updating Proposal for updating
(adopted 21 May 1981) Guideline 406 Guideline 407 (revision of Guideline 407 (revision of
(February 1991) January 1993) January 1994)
Total and differential Total and differential Total and differential Total and differential
leukocyte count leukocyte count leukocyte count leukocyte count
Weight of spleen and thymus Weight of spleen and thymus Weight of spleen and thymus
Histopathology of spleen Histopathology of spleen, Histopathology of spleen, Histopathology of spleen, thymus,
thymus, lymph node, and thymus, lymph nodes (one lymph nodes (one relevant to the
bone marrow relevant to route of route of administration and a
administration and a distant distant one to cover systemic
one to cover systemic effects), effects), small intestine (including
and bone marrow Peyer's patches), and bone marrow
Histopathology of target Histopathology of target Histopathology of target Histopathology of target
organs organs organs organs
OECD, Organisation for Economic Co-operation and Development
An insight into the type of information that the OECD test
guideline No. 407 yields is given below. In this test, the substance
is administered orally in daily graduated doses to groups of
experimental animals, one dose per group for 28 or 14 days. The
preferred rodent species for this test is the rat, although others may
be used. At least three doses and a control should be used. The
highest dose should result in toxic effects but not produce an
incidence of fatalities which would prevent a meaningful evaluation;
the lowest dose should not produce any evidence of toxicity and should
exceed a usable estimate of human exposure, when available. Ideally,
the intermediate dose level(s) should produce minimal observable toxic
effects.
In compliance with the guideline, the following examinations are
carried out:
(a) haematology, including haematocrit, haemoglobin concentration,
erythrocyte count, total and differential leukocyte count, and a
measure of clotting potential such as clotting time, prothrombin
time, thromboplastin time, or platelet count;
(b) clinical biochemistry of blood, including blood parameters of
liver and kidney function. The selection of specific tests is
influenced by observations on the mode of action of the
substance. Suggested determinations are: calcium, phosphorus,
chloride, sodium, potassium, fasting glucose, serum alanine
aminotransferase, serum aspartate aminotransferase, ornithine
decarboxylase, gamma-glutamyl transpeptidase, urea nitrogen,
albumin, blood creatinine, total bilirubin, and total serum
protein. Other determinations that may be necessary for an
adequate toxicological evaluation include analyses of lipids,
hormones, acid-base balance, methaemoglobin, and cholinesterase
activity. Additional clinical biochemistry may be used when
necessary, to extend the investigation of any observed effects.
(c) pathology, including gross necropsy, with examination of the
external surface of the body, all orifices, and the cranial,
thoracic, and abdominal cavities and their contents. The liver,
kidneys, adrenal glands, and testes are weighed wet as soon as
possible after dissection to avoid drying. Liver, kidney, spleen,
adrenal glands, heart, and target organs showing gross lesions or
changes in size are preserved in a suitable medium for possible
future histopathological examination. Histopathological
examination is performed on the preserved organs or tissues of
the group given the high dose and the control group. These
examinations may be extended to animals in other dosage groups,
if considered necessary to further investigate changes observed
in the high-dose group.
A properly conducted 28- or 14-day study will provide information
on the effects of repeated doses and can indicate the need for
further, longer-term studies. It can also provide information on the
selection of doses for longer-term studies.
It is clear that the 1994 guideline is not suitable for adequate
assessment of the potential adverse effects of exposure to a test
chemical on the immune system, since the immunological parameters are
restricted to total and differential leukocyte counts and the
histopathology of the spleen. An evaluation of this test (Van Loveren
& Vos, 1992) indicated that over 50% of the immunotoxic chemicals in a
series of about 20 chemicals would not have been identified as such if
the tests had strictly adhered to the guideline. In fact, it is even
doubtful if chemicals indicated as immunotoxic only on the basis of
guideline No. 407 would in practice have been picked up: For instance,
in a toxicological experiment, a small but significant change in the
percentage of basophilic leukocytes would by itself probably not be
considered to be biologically relevant in the absence of any other
parameter to suggest that an effect on the immune system might have
been present.
These data indicate that extension of OECD test guideline No. 407
is necessary in order adequately to assess potential immunotoxicity.
It is recommended that additional immunological parameters be included
in this guideline in order to increase its power (Vos & Van Loveren,
1987; Basketter et al., 1994).
Guidelines also exist for follow-up studies if greater exposure
is expected or if there is a suspicion of toxicity on the basis of
structural analogy with other compounds. In these studies, potential
toxicity to the immune system is generally addressed somewhat more
extensively than in guideline No. 407. For instance, in a 90-day study
of oral toxicity, the OECD guidelines prescribe that histopathological
examination be done on the thymus, a representative lymph node, and
the sternum with bone marrow, in addition to the spleen. Even with
these additional parameters, it is highly questionable whether
potential immunotoxicity is adequately assessed. For this purpose, a
variety of tests is available, which are described in section 4.
Depending on what is already known about the toxicity of the test
compound, different panels of tests (also referred to as tiers) are
selected for immunotoxicological evaluation. Usually, if little or no
information is available, a dose range including high doses is used;
lower, overtly nontoxic doses are chosen if some knowledge is
available about the physical and chemical properties, toxicokinetics,
structure-activity relationships, and intended use.
3.2 Organization of tests in tiers
Immunotoxicity can be assessed in a tiered approach (Luster et
al., 1988; Van Loveren & Vos, 1989). Generally, the objective of the
first tier is to identify potentially hazardous compounds (hazard
identification). If potential immunotoxicity is identified, a second
tier of tests is carried out to confirm and further characterize the
immunotoxicity.
Various approaches have been suggested for evaluating the
potential immunotoxicity of compounds. Most are similar in design, in
that the first tier is usually a screen for immunotoxicity and the
second tier consists of a more specific confirmatory set of studies or
in-depth mechanistic studies. Since the use of the tiers is usually
tailored to the goals or objectives of the organization that proposes
them, they differ in respect of the specific assays recommended and
the organization of the assays into tiers.
3.2.1 United States National Toxicology Program panel
The tiers and assays originally adopted by the NTP, based on the
proposed guidelines for immunotoxicity evaluation in mice reported by
Luster et al. (1988), are shown in Table 8.
Table 8. Panel adopted by the US National Toxicology Program for detecting immune
alterations after exposure of rodentsa to chemicals or drugs
Parameter Procedures
Screen (Tier I)
Immunopathology Haematology: complete and differential blood count
Weights: body, spleen, thymus, kidney, liver
Cellularity: spleen
Histology: spleen, thymus, lymph node
Humoral immunity Enumerate IgM antibody plaque-forming cells to
T-dependent antigen (sheep red blood cells)
Lipopolysaccharide mitogen response
Cell-medicated Lymphocyte blastogenesis to mitogens
immunity (concanavalin A)
Mixed leukocyte response to allogeneic leukocytes
Nonspecific immunity Natural killer cell activity
Comprehensive (Tier II)
Immunopathology Quantification of splenic B and T cell numbers
Humoral-mediated Enumeration of IgG antibody response to sheep red
immunity blood cells
Cell-medicated Cytotoxic T lymphocyte cytolysis; delayed
immunity hypersensitivity response
Nonspecific immunity Macrophage function: quantification of resident
peritoneal cells, and phagocytic ability (basal and
activated by macrophage activating factor)
Host resistance Syngeneic tumour cells
challenge models PYB6 sarcoma (tumour incidence)
(end-points)b B16F10 melanoma (lung burden)
Bacterial models: Listeria monocytogenes (mortality);
Streptococcus species (mortality)
Viral models: influenza (mortality)
Parasite models: Plasmodium yoelii (parasitaemia)
a The testing panel was developed using B6C3F1 female mice.
b For any particular chemical tested, only two or three host resistance models
are selected for examination.
In this approach, the tier 1 assay is limited; it includes assays
for both cell-mediated and humoral-mediated immunity and for innate
(nonspecific) immunity with the inclusion of NK cell assays. It also
includes immunohistopathology, which is part of the standard protocol
for studies of subchronic toxicity and carcinogenicity conducted by
the NTP. Tier II represents a more extensive evaluation and includes
additional assays for assessing effects on cell-mediated, humoral, and
innate immunity, in addition to host resistance. In this approach,
animals are usually evaluated at only one time, so that the
possibility for recovery or reversibility of immunological changes is
not evaluated. A 14-day exposure period is employed routinely;
however, 30- and 90-day exposures have been used, depending on the
pharmacokinetic properties of the chemical being tested. The dose used
in this tier system tends to be lower than those in several of the
other approaches followed. In the NTP approach, dose levels are
selected whenever possible that have no effect on body weight or other
toxicological end-points. The approach has therefore focused on
compounds for which the immune system is the most sensitive target.
This is in marked contrast to other approaches, in which the highest
dose is usually the maximum tolerated dose.
The assays that make up the NTP tier approach have undergone
various revisions, partly on the basis of an immunotoxicological
review of compounds evaluated in this tier structure (Luster et al.,
1992). The mitogen assays were first moved from tier I to tier II and
have now been dropped altogether: They were found to be insensitive
and to add little when run in conjunction with other assays that have
a proliferative component, such as the mixed leukocyte response and
the plaque assay. Furthermore, the only macrophage phagocytic assay
routinely carried out in immunotoxicological studies conducted for the
NTP is evaluation of the functional activity of the mononuclear
phagocyte system, which is an in-vivo assay for phagocytosis.
Studies by Luster et al. (1992) show that the potential
immunotoxicity of a compound can be reasonably predicted with a few
selected assays. As additional data become available, further changes
to the NTP tiers will most likely be forthcoming.
3.2.2 Dutch National Institute of Public Health and Environmental
Protection panel
The tier approach for immunotoxicological evaluation followed at
the National Institute of Public Health and Environmental Protection
(RIVM) in the Netherlands (Vos & Van Loveren, 1987) is shown in
Table 9. This approach is based essentially on OECD test guideline
No. 407, which suggests that the maximum tolerated dose be used as the
high dose in the study. As a result, significantly higher doses are
used than in the NTP approach in evaluating compounds for
immunotoxicity. Additionally, the standard exposure period is 28 days,
and the animal species routinely used is the rat instead of the mouse.
This type of testing can therefore be performed in the context of
studies in rats to determine the toxicological profile of a compound.
At least three doses should be used, the highest having a toxic effect
(but not mortality) and the lowest producing no evidence of toxicity.
Moreover, immunotoxicity tests carried out in the context of such
testing should not in any way influence the toxicity of the chemical
(e.g. immunization or challenge with an infectious agent). In the NTP
panel, the highest dose to which mice are exposed is chosen so that no
overt toxicity, i.e. changes in body weight or gross pathological
effects, is observed. As tests for immunotoxicity must be fairly
sensitive in order to preclude false negatives, the NTP tier I
includes functional assays. With a broader dose range that includes
overt toxicity, potential immunotoxicity is more likely to be
observed, without the inclusion of functional tests. If functional
assays are to be included in the first tier, those tests that require
sensitization of animals would require inclusion of satellite groups.
In OECD test guideline No. 407 for testing chemicals, none of the
other systems is approached functionally.
It has been suggested that the NK cell assay be added to tier 1 (Van
Loveren & Vos, 1992). Since the assay does not require animals to be
sensitized or challenged, the same animals can be used without
affecting other toxicological parameters, and thus an additional
satellite group of animals is unnecessary.
3.2.3 United States Environmental Protection Agency, Office of
Pesticides panel
The United States Environmental Protection Agency has proposed a
tiered approach to the evaluation of biochemical pest control agents,
which fall under the subdivision M guidelines for pesticides (Sjoblad,
1988). The proposed tiers are shown below. Tier 1 of this approach
includes functional assays for evaluating humoral immunity, cell-
mediated immunity, and innate immunity. Thus, while Tier 1 is
considered by the Agency to be an immunotoxicity screen, it is much
more encompassing than the first tier of the other approaches. By
providing options in the selection of assays for tier 1, the approach
can easily accommodate both the rat and the mouse as the laboratory
animal species used. In this approach, the tier 2 studies provide
information sufficient for risk evaluation, including information on
the time course of recovery from immunotoxic effects and host
resistance to infectious agents and tumour models. Additional
functional tests would be required if a dysfunction were observed in
tier 1 tests or if data from other sources indicated the compound
could produce an adverse effect on the immune response.
Table 9. Methods for detecting immunotoxic alterations in the rat evaluated by the
Dutch National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Parameters Procedures
Tier 1
Nonfunctional Routine haematology, including differential cell counts
Serum IgM, IgG, IgA, IgE determination; lymphoid organ
weights (spleen, thymus, local and distant lymph nodes)
Histopathology of lymphoid tissues, including mucosa-
associated lymphoid tissue
Bone-marrow cellularity
Analysis of lymphocyte subpopulations in spleen by flow
cytometry
Tier 2
Cell-medicated Sensitization to T-cell dependent antigens (e.g. ovalbumin,
immunity tuberculin, Listeria), and skin test challenge
Lymphoproliferative response to specific antigens (Listeria)
Mitogen responses (concanavalin A, phytohaemagglutinin)
Humoral Serum titration of IgM, IgG, IgA, IgE responses to
immunity T-dependent antigens (ovalbumin, tetanus toxoid,
Trichinella spiralis, sheep red blood cells) by ELISA
Serum titration of T-cell-independent IgM response to
lipopolysaccharide by ELISA
Mitogen response to lipopolysaccharide
Macrophaand Phagocytosis and killing of Listeria by adherent spleen
function and peritoneal cells in vitro
Cytolysis of YAC-1 lymphoma cells by adherent spleen
and peritoneal cells
Natural killer Cytolysis of YAC-1 lymphoma cells by non-adherent
function spleen and peritoneal cells.
Host resistance Trichinella spiralis challenge (muscle larvae counts and
worm expulsion)
Listeria challenge (spleen and lung clearance)
Cytomegalovirus challenge (clearance from salivary gland)
Endotoxin hypersensitivity;
Autoimmune models (adjuvant arthritis, experimental
allergic encephalomyelitis)
Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay
Subdivision M guidelines: proposed revised requirements by the US
Environmental Protection Agency for testing the immunotoxicity of
biochemical pest control agents
AI. Tier 1
A. Spleen, thymus, and bone-marrow cellularity
B. Humoral immunity (one of the following)
1. Primary and secondary IgG and IgM responses to antigen; or,
2. Antibody plaque-forming cell assay
C. Specific cell-mediated immunity (one of the following)
1. One-way mixed lymphocyte reaction assay; or,
2. Effect of agent on normal delayed-type hypersensitivity
response; or,
3. Effect of agent on generation of cytotoxic T-lymphocyte
response
D. Nonspecific cell-mediated immunity
1. Natural killer cell activity and
2. Macrophage function
II. Tier 2
A. Required if:
1. Dysfunction is observed in tier 1 tests
2. Tier 1 test results cannot be definitively interpreted
3. Data from other sources indicate immunotoxicity
B. General testing features:
1. Evaluate time course for recovery from immunotoxic effects.
2. Determine whether observed effects impair host resistance to
infectious agents or to tumour cell challenge.
3. Perform additional specific, but appropriate, testing
essential for evaluation of potential risks.
This Agency has also suggested that immunotoxicological screening
be conducted in evaluating conventional chemical pesticides
(subdivision F guidelines; see below); however, unlike those of
subdivision M, these guidelines are not designed as a tiered testing
scheme. If the immunotoxicity screen listed in subheading I were added
to subchronic and/or chronic studies in subdivision F, it would be a
more effective screen for immunotoxicity than is currently available.
If this proposed screen indicates that the immune system is a
sensitive target, the Agency considers that it may be necessary to
evaluate the risk for immunotoxic effects as under subheading
II. Currently, these suggestions have not been promulgated as official
guidelines or regulations.
Evaluations suggested by the US Environmental Protection Agency as
appropriate additions to Subdivision F guidelines for immunotoxicity
screening (subheading I) and possible additional data appropriate for
risk evaluation of chemical pesticides (subheading II)
I. Immunotoxicity screen
A. Serum immunoglobulin levels (e.g. IgG, IgM, and IgA)
B. Spleen, thymus, and lymph node weights
C. Spleen, thymus, and bone-marrow cellularity and cell
viability
D. Special histopathology (e.g. enzyme histochemistry,
immunohistochemistry)
E. More complete evaluation of 'premature' mortality of test
animals, as possibly related to immunosuppressive effects
II. Immunotoxicity risk evaluation
A. Host resistance to challenge with infectious agent and/or
tumour cells
B. Specific cell-mediated immune responses (e.g. mixed
leukocyte response, delayed-type hypersensitivity
response,cytotoxic T lymphocyte assays)a
C. Nonspecific cell-mediated immune responses (i.e. natural
killer cell activity, macrophage function)a
D. Time course for recovery from adverse immunological effects
a Measures of specific and nonspecific cell-mediated immune
responses that also may be considered useful in an immunotoxicity
screen
3.2.4 United States Food and Drug Administration, Center for Food
Safety and Applied Nutrition panel
The United States Food and Drug Administration is considering
testing guidelines for evaluating the immunotoxic potential of direct
food additives (Hinton, 1992). The multifaceted approach is included
in the draft revision of the Toxicological Principles for the Safety
Assessment of Direct Food Additives and Color Additives Used in Food
is (US Food and Drug Administration, 1993). The testing requirements
are based on the 'concern level' of the substance, assigned on the
basis of the available toxicological information or the substance's
structural similarity to known toxicants and on estimated human
exposure from its proposed use. A compound with high toxic potential
and high exposure would be assigned a high initial 'concern level'
(3), and one with low toxic potential and low exposure would be
assigned a low initial level (1).
In general, substances will be evaluated for immunotoxic
potential on a case-by-case basis. Two types of immunotoxicity tests
and procedures are defined in this approach: Type 1 tests are those
that do not involve perturbation of the test animal (i.e.
sensitization or challenge). These are further divided into 'basic'
tests, which include haematology and serum chemistry, routine
histopathological examination, and determination of organ and body
weights, and 'expanded' tests, which are logical extensions of the
'basic' tests and include those that can be performed retrospectively.
Type 2 tests include injection of or exposure to antigens, infectious
agents, vaccines, or tumour cells. In general, type 2 tests require a
satellite group of animals for immunological evaluation. The sets of
'basic' and 'expanded' type 1 tests are defined as level-I
immunotoxicity tests, and the sets of type 2 tests are defined as
level-II tests. Some level-I tests can be used to screen for
immunotoxic effects, while others focus on the mechanism of action or
the cell types affected by the test substance. Level-II tests are
conducted to define the immunotoxic effects of food and colour
additives more specifically. The recommended testing scheme is shown
below.
The functional tests generally require sensitization of exposed
rats and controls and subsequent analysis of the responses to the
sensitizing antigens. For this reason, functional tests are not
readily conducted in the first tier of immunotoxicity testing. As
guidelines for routine toxicology experiments preclude compromising
the experiment by any agent other than the test chemical, the second
tier of immunotoxicity testing, with immune function tests, requires a
separate set of experiments. The antigens that are used to sensitize
the exposed and control rats may be relatively simple antigens, such
as ovalbumin or tetanus toxoid, or more complex antigens, such as
sheep red blood cells, bacteria, or parasites. The responses can occur
in various arms of the immune system, which consequently must be
measured with different assays. For instance, humoral responses can be
measured by determination of specific antibodies in serum; the
appropriate tests for cellular responses are proliferative responses
of lymphocytes to the specific antigens ex vivo/in vitro or delayed-
type hypersensitivity responses to injection with antigen in vivo.
Recommendations of the United States Food and Drug Administration for
testing the immunotoxicity of direct food additives
Basic testing (rat model)
Complete blood count, differential white blood cell count;
Total serum protein, albumin:globulin ratio;
Histopathology, gross and microscopic (spleen, thymus, lymph
nodes, Peyer's patches, and bone marrow);
Lymphoid organ and body weights
Retrospective level-I testing (possible in a standard toxicology
study)
Electrophoretic analysis of serum proteinsa (when positive or
marginal effect is noted in basic testing);
Immunostaining of spleen and lymph nodes for B and T cellsa
(quantification of total immunoglobulins);
Serum autoantibody screen and deposition of immunoglobulins
(micrometry for semiquantification of the proliferative
response)
Enhanced level-I testing (possible for more complete screening in the
standard toxicology study core group, with a satellite animal group,
or in a follow-up study)
Cellularity of spleen (lymph nodes and thymus when indicated);
Quantification of total B and T cells (blood and/or spleen);
Mitogen stimulation assays for B and T cells (spleen);
Natural killer cell functional analysis (spleen);
Macrophage quantification and functional analysis (spleen);
Interleukin-2 functional analysis (spleen);
When indicated or for more complete analysis, other end-points
such as total haemolytic complement activity assay in serum
Level-II testing with a satellite group or follow-up study for
screening of functional immune effects
Kinetic evaluation of humoral response to T-dependent antigen
(primary and secondary responses with sheep red blood cells,
tetanus toxoid, or other);
Kinetic evaluation of primary humoral response to a
T-independent antigen such as pneumococcal polysaccharides,
trinitrophenyl-lipopolysaccharide, or other recognized
antigens;
Delayed-type hypersensitivity response to known sensitizer of
known T effector cell;
Reversibility evaluation
Recommendations (cont'd)
Enhanced level-II testing with a satellite group or follow-up study
for evaluation of potential immunotoxic risk
Tumour challenge (MADB106 or other in rat);
PYB6 sarcoma (in mouse);
Infectivity challenge (Trichinella, Candida or other in rat;
Listeria or other in mouse) a Recommended for inclusion in
basic testing
a Recommended for inclusion in basic testing
Not all functional assays require prior sensitization of the test
animals, e.g. proliferative responses of lymphocytes ex vivo/in vitro
to mitogens which are either specific for T cells, giving information
on cellular immunity, or for B cells, providing data on humoral
immunity. The phagocytic and lytic activity of macrophages and the
nonspecific cytotoxic activity of NK cells can also be measured
ex vivo/in vitro, without prior sensitization of the test animals.
Both types of activity are examples of nonspecific defence mechanisms,
directed to bacteria and certain tumour cells and to tumour cells and
virally infected cells, respectively. Since measurement of these types
of activity does not require prior sensitization of the host, such
functional tests can be considered for inclusion in the first tier of
testing for immunotoxicity in routine toxicology.
3.3 Considerations in evaluating systemic and local immunotoxicity
3.3.1 Species selection
Selection of the most appropriate animal model for
immunotoxicology studies has been a matter of great concern. Ideally,
toxicity testing should be performed with a species that responds to a
test chemical in a pharmacologically and toxicological manner similar
to that anticipated in humans, i.e. the test animals and humans
metabolize the chemical similarly and have identical target organs and
toxic responses. Toxicological studies are often conducted in several
animal species, since it is assumed that the more species that show a
specific toxic response, the more likely it is that the response will
occur in humans. Data from studies in rodents on target organ toxicity
at immunosuppressive doses for most immunosuppressive therapeutic
agents have generally been predictive of later clinical observations.
Exceptions to the predictive value of rodent toxicological data are
infrequent but occurred in studies of glucocorticoids, which are
lympholytic in rodents but not in primates (Haynes & Murad, 1985).
Although certain compounds exhibit different pharmacokinetic
properties in rodents and in humans, rodents still appear to be the
most appropriate animal model for examining the non-species-specific
immunotoxicity of compounds, because of established toxicological
knowledge, including similarities of toxicological profiles, and the
relative ease of generating data on host resistance and immune
function in rodents. Comparative toxicological studies should be
continued and expanded, however, as novel recombinant biological
compounds and natural products that enter safety testing will probably
have species-specific host interactions and toxicological profiles.
The quantitative and possibly the qualitative susceptibility of
an individual animal to the immunotoxicity of a selected agent can be
influenced by its genetic characteristics, indicating not only a need
to consider species but also strain. Rao et al. (1988) described two
approaches for selecting appropriate genotypes for toxicity studies.
The first is to select genotypes that are representative of an animal
species, which by virtue of similar metabolic profiles may also
exhibit a sensitivity similar to that of man, such as random-bred
mice. A second approach is to attempt to identify genotypes that are
uniquely suitable for evaluating a specific class of chemicals, such
as the use of Ah-responsive rodent strains in studies with
polyhalogenated aromatic hydrocarbons. In many cases, however, this
approach requires considerable knowledge of the mechanisms of toxicity
of the compound, which may not be available. One compromise has been
to use Fl hybrids which have the stability, phenotypic uniformity, and
known genetic traits of an inbred animal, yet have the vigour
associated with heterozygosity. The description of the genetic
relationships between inbred mouse strains on the basis of the
distribution of alleles at 16 loci (Taylor, 1972) has made possible
rational selection of appropriate Fl hybrids, such as the B6C3Fl
mouse.
3.3.2 Systemic immunosuppression
Because of this complexity, the initial strategies devised by
immunologists working in toxicology and safety assessment were to
select and apply a tiered panel of assays in order to identify
immunosuppressive or, in rare instances, immunostimulatory agents in
laboratory animals (US National Research Council, 1992). Although the
configuration of these testing panels varies according to the
laboratory conducting the test and the animal species used, they
include measurements of one or more of the following: (i) altered
lymphoid organ weights and histology, including immunohistology; (ii)
quantitative changes in the cellularity of lymphoid tissue, peripheral
blood leukocytes, and/or bone marrow; (iii) impairment of cell
function at the effector or regulatory level; and/or (iv) increased
susceptibility to infectious agents or transplantable tumours.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to adversely influence the
immune system. These include appropriate selection of animal models
and exposure variables, consideration of general toxicological
parameters and mechanisms of action, as well as an understanding of
the biological relevance of the end-points to be measured. Treatment
conditions should be based on the potential route, level, and duration
of human exposure, the biophysical properties of the agent, and any
available information on the mechanism of action. Moreover,
toxicokinetic parameters, such as bioavailability, distribution
volume, clearance, and half-life, should be measured. Doses should be
selected that will allow establishment of a clear dose-response curve
and a no-observed-effect level NOEL). Although, for reasons explained
earlier, it is beneficial to include a dose that induces overt
toxicity, any immune change observed at that dose should not
necessarily be considered to be biologically significant, since severe
stress and malnutrition are known to impair immune responsiveness.
Many laboratories routinely use three doses but generally conduct
studies to define the range of doses before a full-scale
immunotoxicological evaluation. If studies are being designed
specifically to establish reference doses for toxic chemicals,
additional exposure levels are advisable. In addition, inclusion of a
'positive control' group, treated with an agent that shares some of
the characteristics of the test compound, may be advantageous when
experimental and fiscal constraints permit.
The selection of the exposure route should reflect the most
probable route of human exposure, which is most often oral,
respiratory, or dermal. If it is necessary to deliver an accurate
dose, a parenteral exposure route may be required; however, this may
significantly change the metabolism or distribution of the agent from
that which would occur following natural exposure.
3.3.3 Local suppression
Local immune suppression has received less attention than
systemic immune suppression, and this is noteworthy, since the surface
that is exposed to the environment, i.e. the skin, the respiratory
tract, and the gastrointestinal tract, are the major ports of entry of
antigens and pathogens. While a variety of validated methods are
currently available to detect chemical skin sensitizers in humans and
experimental animals, there is no standard method to assess the
potential of chemicals to induce local immunosuppression in the skin.
Furthermore, although increasing evidence suggests that the
consequence of skin immunosuppression would be an increase in
neoplastic and infectious diseases of the skin, definitive data are
still lacking. In contrast, considerable efforts are being deployed to
develop sensitive models for monitoring skin irritants. For example,
human keratinocyte cultures and keratinocyte-fibroblast co-cultures
have been examined for end-points ranging from changes in cell
viability to production and loss of various bioactive products. Few
test systems are available for the gut and the respiratory tract.
4. METHODS OF IMMUNOTOXICOLOGY IN EXPERIMENTAL ANIMALS
This section comprises general descriptions of methods used for
evaluating immunotoxicity.
4.1 Nonfunctional tests
4.1.1 Organ weights
It is routine practice in toxicology to weigh organs that are
potentially affected by the compound that is being investigated. The
immunological organs that are suitable for weighing in screening for
potential immunotoxicity are: the thymus, which plays a decisive role
in the development of the immune system and which is affected by many
immunotoxicants; the spleen, which is the repository for many
recirculating lymphocytes; and the lymph nodes, which are important
for the induction of immune processes. Determination of the weight of
draining lymph nodes (depending on the route of exposure, i.e.
mesenteric nodes for oral exposure and bronchial nodes for inhalation)
in addition to distant lymph nodes (such as popliteal lymph nodes for
determining systemic effects) is the best. Mesenteric nodes, in
particular, occur in a string within non-lymphoid fatty tissue, and
care must be taken to remove this non-lymphoid tissue so that the
weight can be adequately determined. The cellularity of these organs
is another indication of the effects of chemicals on the immune
system. Furthermore, cell suspensions can be prepared from lymphoid
organs in order to assess the distribution of subpopulations of
lymphoid cells and to test their functionality within the organs.
Under OECD guideline No. 407, histopathological examination of
lymphoid organs and tissues is crucial for detecting the effects of
chemicals on the immune system. Therefore, upon termination of
exposure to a compound in a toxicological experiment, organs such as
the spleen should first be weighed; subsequently, they are divided
into parts which are also weighed, and one or more parts are used for
histopathological examination and the remainder to prepare cell
suspensions that can be evaluated for distribution of lymphocyte
subpopulations or can be assessed functionally.
4.1.2 Pathology
The histopathology of the thymus, spleen, and draining and
distant lymph nodes, of the mucosal immune system (Peyer's patches in
the gut or bronchus and nose-associated lymphoid tissue in the
respiratory tract), and of the skin immune system should be evaluated,
depending on the route of exposure. The first level of evaluation
should be of haematoxylin-eosin stained, paraffin-embedded slides. A
more sophisticated level of evaluation is immunoperoxidase staining of
special cell types.
Many monoclonal antibodies are available for mice, rats, and
humans to detect differentiation antigens, cell adhesion molecules,
and activation markers on haematolymphoid and stromal cells involved
in immune responses. A list of some monoclonal antibodies that can be
used in the identification of leukocytes and stromal cells in (frozen)
sections of lymphoid tissue is presented in Table 10; a selection of
these is reviewed below.
For a further description of these markers, and the cells that
express them, reference may be made to the introductory section and to
descriptions in the literature (Brideau et al., 1980; Bazin et al.,
1984; Dallman et al., 1984; Dijkstra et al., 1985; Joling et al.,
1985; Vaessen et al., 1985; Joling, 1987; Hünig et al., 1989; Kampinga
et al., 1989; Portoles et al., 1989; Schuurman et al., 1991a).
These markers are usually stained in frozen tissue sections of
6-8 µm, fixed in acetone. A three-step immunoperoxidase procedure is
most suitable: the first step includes the monoclonal antibody
specific for the determinants to be studied (see above), the second
step, rabbit anti-mouse immunoglobulin, and the third step, swine
anti-rabbit immunoglobulin, the latter two antibodies conjugated to
horseradish peroxidase. The peroxidase activity can be developed by
3,3-diaminobenzidine tetrahydrochloride with hydrogen peroxide as
substrate, and the sections can be counterstained with Mayer's
haematoxylin to facilitate evaluation. Negative controls are prepared
by omitting the antibody in the first step or replacing it with an
irrelevant one. Under these conditions, only the peroxidase activity
of polymorphonuclear cells, when present, is visualized, and no
immunolabelling is found.
In general, histopathological evaluation provides a semi-
quantitative estimation of effects. The experienced pathologist can do
this adequately in studies carried out 'blind', especially if the
effects are clear. For more subtle effects, morphometric analysis is a
valuable addition, especially when supported by software for assessing
the values of parameters such as size, surface, and intensity of
staining. The compartments of the immune system, i.e. specific T and B
lymphocyte areas in spleen and lymph nodes and cortical and medullary
areas of immature and differentiated thymocytes within the thymus, and
the numbers of specialized cells per surface unit are parameters that
are well suited for morphometric analysis.
4.1.3 Basal immunoglobulin level
Serum immunoglobulin levels are often altered after exposure of
rats to immunotoxic chemicals (Vos, 1980; Vos et al., 1982, 1984,
1990a; Van Loveren et al., 1993a). This is not surprising, as the
total levels measured are a function of the humoral aspects of the
immune system, which react to the antigens that the host encounters.
For this reason, measurement of antibody levels is potentially
valuable in screening for immunotoxicity. Since the amount of antibody
Table 10. Some monoclonal antibodies to leukocytes and stromal cells used in immunohistochemical studies of tissue sections and flow
cytofluorography on cell suspensions
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells
CD1 gp43,45, Ly-38 OKT6, Lymphocytes in thymic cortex, Langerhans cells in skin,
49,12 a-Leu-6 interdigitating cells
CD2 gp50 Ly-37, MRC OX-34, a-Leu-5, All T cells in thymus and peripheral lymphoid organs, subset
NSM46.7, MRC OX-54, OKT11 of macrophages (rat). Sheep erythrocyte receptor, leukocyte
RM2-5 MRC OX-55 function antigen:-2 (LFA-2). Ligand f or LFA-3 (CD58)
CD3 gp19-29 CD3-1,KT3, IF4, G4.18 a-Leu-4, T Cells in thymic medulla and peripheral lymphoid organs
145-2C11 OKT3 (T-cell receptor-associated, cytoplasmic in precursor T cells
in thymus)
CD4 gp65 Ly-4, L3T4, MRC OX-35, a-Leu-3, Lymphocytes in thymic cortex, about two-thirds of T cells in
YTS 177.9 MRC OX-38, OKT4 peripheral lymphoid organs, subset macrophages, microglia
(ER2), W3/25 T helper/inducer and delayed-hypersensitivity phenotype.
MHC class II binding, receptor for human immunodeficiency
virus
CD5 gp65-62 Ly-1,Lyt-1 MRC OX-19, a-Leu-1 Lymphocytes in thymic cortex (faint). All T cells in thymic
HIS47 medulla and peripheral lymphoid tissue, subset of B cells
CD6 gp120 Tü 33 T Cells in thymic medulla and peripheral lymphoid organs
CD Relative Mouse Rat Human Reactivity
mol. mass
CD7 gp41 WT1,B-F12, Prethymic T-cell precursors, all T cells in thymus and fewer
a-Leu-9 in peripheral lymphoid organs
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
CD8 gp32-33 Ly-2,3,Lyt-2,3, MRC OX-8 a-Leu-2, Lymphocytes in thymic cortex, about one-third of T cells in
YTS 105.8 OKT8 peripheral lymphoid organs, splenic sinusoids (T cytotoxic/
suppressor phenotype, NK cells). MHC class I binding
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells,
thymic cortex cells (rodents). Heat-stable antigen (HSA)
CD43 gp115 Ly-48 W3/13,HIS17 DFT-1, (Pro)thymocytes, T cells, plasma cells, cells in bone
WR-14 marrow, polymorphonuclear granulocytes, brain cells.
Leukosialin, sialophorin
CDw p25-30 Thy-1 (ER4), 5F10 Thymocytes, T lymphocytes, connective tissue structures,
90 MRC OX-7, epithelial cells, fibroblasts, neurons, subset of bone-marrow
HIS51 cells, plasma cells, stem cells (T-activation molecule)
Thy-2 Thymocytes
p40-55 H57-597 R73,HIS42 WT31, T-Cell receptor a-b chain. Mature T cells in thymic medulla
TalphaF1, and peripheral lymphoid tissue
TßF1
p40-55 GL3,GL4, V65 CgammaM1, T-Cell receptor gamma-delta chain
UC7-13D5 11F2,
TCR delta1,
deltaTCS1
p41-55 MRC OX-44 Prothymocytes, lymphocytes in thymic medulla, T and B
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
p41,47 MRC OX-2 Thymocytes, dendritic cells, B cells, brain cells
ER3,ER7, Subset of thymocytes and peripheral T cells, subset of
ER9,ER10 myeloid cells
HIS44 Most lymphocytes in thymic cortex, small subset of
medullary lymphocytes, erythroid cells, cells in germinal
centre
HIS45 Some lymphocytes in thymic cortex, most medullary
thymocytes and peripheral T cells, subset of B cells.
Quiescent cell antigen (QCA-1)
MHC class I (Various antibodies to polymorphic and All nucleated cells, including leukocytes and stromal
non-polymorphic epitopes) cells; for T cells absent on thymic cortex cells (human)
B Cells
CD9 gp24 BA-2 Germinal centres (faint); some cells in thymic cortex. Late
pre-B cells
CD10 gp100 BA-3, Germinal centres (faint); some cells in thymic cortex
W8E7 Common acute lymphoblastic leukaemia antigen (CALLA)
CD19 gp95 a-Leu-12, B Cells in germinal centres and mantles, follicular dendritic
B4, FMC63 cells
CD20 p35 Ly-44 B1, a-Leu-16 B Cells in germinal centres and mantles, follicular dendritic
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
CD21 gp140 B2,BL13, B Cells in germinal centres and mantles (faint), follicular
HB-5 dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus)
CD22 gp135 Lyb-8.2, a-Leu-14,To B Cells in germinal centres and mantles, cytoplasmic in
Cy34.1 To 15,RFB4, precursor B cells
SHCL-1
CD23 p45 Ly-42 a-Leu-20, Some B cells in marginal centres and mantles, activated B
Tü 1 cells, subset of follicular dendritic cells (IgE Fc receptor)
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells, thymic
cortex cells (rodents). Heat-stable antigen (HSA)
CD37 gp40-45 BL14 B Cells in germinal centres and mantles
CD38 gp45 a-Leu-17, Lymphocytes in thymic cortex, cells in germinal centres,
OKT10 plasma cells (immature lymphoid cells, plasma cells)
CDw75 p53? LN1, OKB4 B Cells in germinal centre, in corona (faint), macrophages,
epithelium
CD79a p33,40 mb-1 B Cells, Ig alpha chain
CD79b p33,40 B29 B Cells, Igß chain
p200 (HIS14) All B cells, including TdT+ precursors
p200 (HIS22) All B cells in corona, pre-B cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
MHC class II (Various antibodies to polymorphic and B Lymphocytes, activated T cells, monocytes/macrophages,
non-polymorphic epitopes) interdigitating cells, Langerhans cells, epithelia, endothelia
B Cells (surface); in germinal center IgM+IgG+IgA+ and in
anti-immunoglobulin corona IgM+IgD+, plasma cells
(cytoplasmic)
Monocytes/macrophages, myeloid cells
CD13 p130-150 ER-BMDM-1 a-Leu-M7, Monocytes, granulocytes, dendritic reticulum cells
My7 (aminopeptidase N)
CD14 p55 ED9 UCH-M1, B-A8, Monocytes, some granulocytes and macrophages
a-Leu-M3
CD15 p170-190 a-Leu-M1 Granulocytes, some monocytes (lacto-N-fucose pentaosyl)
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes,
activated macrophages. IgG-FcRIII, low affinity, complexed IgG
CD33 p67 a-Leu-M9, (Precursor) granulocytes, macrophages, Langerhans cells.
My9 Myelin-associated protein
CD68 p110 Ki-M6,Ki-M7 Macrophages (specific)
p160 F4/80 Monocytes-macrophages
p32 Mac-2 Thioglycollate-elicited peritoneal macrophages
p92-110 Mac-3 Peritoneal macrophages
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Monocytes/macrophages, myeloid cells (contd)
4F7 Dendritic cells in skin, bone marrow
ED1 Monocytes/macrophages
ED2,HIS36 Subset of macrophages (F4/80-like)
ED3 Subset of macrophages, restricted, negative in thymus
MRC OX-41 Granulocytes, macrophages, dendritic cells
MRC OX-62 Dendritic cells (integrin-like)
(IF119) Dendritic cells
HIS48 Granulocytes
Mac-387 Macrophages
Natural killer cells
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages. IgG-FcRIII, low affinity, complexed IgG
CD56 p220/135 a-Leu-19, NK cells, monocytes, neuroectodermal cells NKH-1, isoform of
B-A19 neural cell adhesion molecule (NCAM)
CD57 p110 a-Leu-7, NK cells, subset of T cells, some B cells, some epithelial cells,
VC1.1 monocytes, neuroendocrine cells, NKH-1
a-asialo-GM1 NK cells, stromal components
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Natural killer cells (contd)
NK-1.1,2B4, 3.2.3 NK cells (NKR-P1 gene family)
3A4, 5E6
Follicular dendritic cells
ED5,ED6, Ki-M4,DRC-1 Follicular dendritic cells
MRC OX-2
Epithelial cells (thymus)
(Various anti-keratin antibodies) Epithelium
(ER-TR4),4F1 HIS38 TE-3,(MR3, Thymic cortex epithelium
MR6)
(ER-TR5),IVC4 (HIS39) TE-4,(MR19), Thymic subcapsular or medullary epithelium
RFD4
Complement receptors
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells, C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD21 gp140 B2,BL13 B Cells in germinal centres and mantles (faint), follicular
dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus
CD35 p220 To 5 Follicular dendritic cells, macrophages, B cells in corona
(faint), renal glomerular epithelium. C3bR, CR1
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
IgG-Fc receptors
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages; IgG-FcRIII, low affinity, complexed IgG
CD32 gp140 Ly-17 3E1,CIKM5 B Cells, myeloid cells, macrophages; IgGFcRII, low affinity,
complexed IgG
CD64 p75 32.2 Monocytes; IgG-FcRI, high affinity, monomeric IgG
ß1-Integrin (CD29-CD49) family
CD29 p130 9EG7 B-D15 Ubiquitous, not on erythrocytes; ß1 chain of all CD49 antigens
CD49a p200 TS2/7 Activated T cells, monocytes, smooth muscle cells. Very late
antigen-1 (VLA-1), ligand of collagen, laminin
CD49b p155 31H4,AK7, T Cells, B cells, thrombocytes, fibroblasts, endothelium.
P1E6 Very late antigen-2 (VLA-2), ligand of collagen I, II, III,
and IV, laminin
CD49c p145 11G5,P1B5 B Cells, renal glomeruli, basal membranes. Very late antigen-3
(VLA-3), ligand of collagen, laminin, fibronectin, and invasin
CD49d p150 R1-2, P12520, HP2/1,44H6, Thymocytes, lymphocytes, monocytes, NK cells, eosinophilic
MFR4.B MR?4 L25.3 granulocytes, erythroblasts. Very late antigen-4 (VLA-4),
ligand of VCAM-1, fibronectin
CD49e p160 MFR5, SAM-1 Monocytes, leukocytes, memory T cells, fibroblasts,
P12750 thrombocytes and muscle cells. Very late antigen-5 (VLA-5),
ligand of fibronectin
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
ß1-Integrin (CD29-CD49) family (contd)
CD49f p150 GoH3 Go-H3,4F10 T Cells, thymocytes, monocytes, thrombocytes. Very late
antigen-6 (VLA-6), ligand of laminin and invasin
ß2-Integrin (CD11-CD18) family
CD11a p180 Ly-15, WT.1 YTH-81.5, T and B cells, NK cells, erythroid and myeloid stem cells.
2D7, B-B15, Leukocyte function-associated antigen-1 (LFA-1) involved in cell
M17/4 G-25.2 adhesion, ligand for intercellular adhesion molecule (ICAM)-1
(CD54), ICAM-2 (CD102), ICAM-3 (CD50)
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells. C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD11c p150 a-Leu-M5, Monocytes, macrophages, granulocytes (faint), activated
S-HCL-3 lymphocytes. CR4
CD18 p95 YTS213.1, WT.3 BL5 All lymphocytes. ß-Chain of CD11 antigens
C71/16,
M18/2
p160-95 ED7,ED8 CD11-CD18 molecule
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others
Terminal deoxynucleotidyl transferase (TdT) Immature (lymphoid) cells in bone marrow and thymic cortex
(nuclear staining)
CD25 p55 Ly-43, AMT13, MRC OX-39 Tac,a-IL2-R Activated lymphocytes at scattered locations in thymus and
7D4,3C7 T-cell areas in peripheral lymphoid organs. Interleukin-2
receptor alpha chain
CD122 p75 5H4, CF1,Mik-ß2, NK cells, T cells, B cells, monocytes. Interleukin-2 receptor
TM-ß1 Mik-ß3 ß chain
CD26 p120 H194-112 MRC OX-61 134-2C2 (Activated) T cells. Dipeptidyl peptidase IV, in mouse T-cell
activation molecule (THAM)
CD30 p105 Ki-1,Ber-H2 Sporadic cells in thymic (cortex) and T cell areas in
peripheral lymphoid organs, some plasma cells. Activated
lymphocytes,Hodgkin cells
CD34 MY 10,8G12 Haematopoietic progenitor cells, capillary endothelium
QBEND/10 Human progenitor cell antigen (HPCA)
CD44 p65-85 Ly-24 MRC OX-49, a-Leu-44, Prothymocytes, T cells, small B cells. Lymphocyte homing
IM7 MRC OX-50 F10-44-2 receptor. Phagocytic glycoprotein-1 (PgP-1), HCAM
CD45 p180-210 Ly-5 MRC OX-1, T29/33 All leukocytes. Common leukocyte antigen
HIS41
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others (contd)
CD45R p190-220 B220 MRC OX-22, a-Leu-18, All B cells, subset of T cells. Common leukocyte antigen.
MRC OX-32, MB1,MT2 HIS24 restricted to strains of the RT7.2 allotype and labels
HIS24 all peripheral B cells except cells in marginal zone, pre-B
cells
CD45RA p205-220 14.8 MRC OX-33 HI100 B Cells, T cytotoxic-suppressor cells (faint), subset of
thymocytes.In humans, also CD4+ subset (naive-virgin
T-cells)
CD45RO p190-220 UCH-L1 T Cells in immature and memory stage. Common leukocyte
antigen
CD54 p90 KAT-1 1A29 84H10,B-C14 Endothelial cells, many activated cell types. Intercellular
3E2 HA58 adhesion molecule-1 (ICAM-1)
CD71 p95 YTA74.4,C2 MRC OX-26 B3/25 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs, stromal
cells. Transferrin receptor
Ki-67 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs. Proliferation
antigen present in late G1, S, G2, and M phases
PCNA PCNA PCNA Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs
MEL14 Recirculating T and B cells. Lymphocyte homing receptor
MHC, major histocompatibility complex; NK, natural killer; Ig, immunoglobulin
CD nomenclature from: Clark & Lanier (1989); Knapp et al. (1989); Schlossman et al. (1994, 1995)
Antibodies within parentheses are not commercially available.
in serum is a function of the antibody's half-life, the longer the
study the more likely it is that an effect will be observed.
Immunoglobulin levels can be influenced by the cleanness of the
facility: studies conducted in facilities with excellent husbandry
will have lower basal levels than those conducted in 'dirty'
facilities where animals are constantly exposed to foreign antigens,
including pathogens.
Measurement of basal immunoglobulin levels is useful only after
subchronic or chronic exposure, i.e. with sufficient time for normal
metabolic elimination. Basal levels of immunoglobulin decrease only
when synthesis is reduced or prevented such that metabolized
immunoglobulins are not replaced. The parameter therefore yields
little information about possible mechanisms of immunotoxicity, and
should rather be regarded as a screening parameter; this is in fact
true for most non-functional tests. The IgM and G classes have usually
been measured; however, since the two other classes (A and E) are
biologically very important (for instance in mucosal immunity and
allergic manifestations), they should also be measured.
Total IgM and IgG concentrations in serum can be analysed by
means of a 'sandwich' enzyme linked immunosorbent assay (ELISA), as
described by Vos et al. (1982). Total IgA and IgE concentrations can
be analysed in an essentially similar way, except that the microtitre
plates are coated with monoclonal anti-rat IgA (Van Loveren et al.,
1988b) or monoclonal anti-rat IgE antibodies (MARE-1), respectively,
and immunoglobulins bound to these antibodies in serum samples are
detected by sheep anti-rat IgA or monoclonal anti-kappa chains of rat
immunoglobulins (MARK-1), conjugated with peroxidase.
Data from the ELISA are usually reported as percentages of
control values, and a titration curve based on pooled sera is
prepared; optimal dilutions of exposed and unexposed groups are then
plotted from this curve. The deviation of the dilution of the test
groups from the control groups is expressed, with the dilution in the
control group set at 100%. While studies in rats indicate that
measurement of basal immunoglobulin levels is useful in predicting the
immunotoxic effects of compounds, studies conducted in mice at the NTP
do not, and measurement of basal immunoglobulins is not included in
either tier of their testing panel (Luster et al., 1988). There are
several possible reasons for the difference in the usefulness of basal
immunoglobulin levels in rats and mice. First, in the studies of Vos
and colleagues, cited above, the exposure period was routinely longer
than the 14-day studies conducted within the NTP; since serum antibody
level is a function of the antibody half-life, longer studies are more
likely to detect an effect. Furthermore, the doses used at the NTP
were often lower, by design, than those used in rats. A final possible
explanation, which remains to be confirmed, is that immunoglobulin
synthesis in rats is more sensitive than that in mice.
4.1.4 Bone marrow
Bone marrow is an important haematopoietic organ and a source of
precursors for lymphocytes and other leukocytes. Changes in the bone
marrow are therefore likely to result in alterations of
immunocompetent cell populations, which may be long lasting or
permanent and thus serve as an indicator of potential immunotoxicity.
In a study to validate immunotoxicological parameters, bone-marrow
cellularity was shown to be an indicator of the immunotoxicity of
cyclosporin A, used as the model compound (Van Loveren et al., 1993a).
Determination of cellularity in stained slides of bone marrow,
evaluation of smears, and actual counts of the numbers of cells within
bone marrow are practical. For this purpose, both ends of a femur are
cut off, and bone-marrow cells are collected by flushing balanced salt
solution through the femur with a 21-gauge needle. The concentration
of nucleated cells is determined in a Coulter counter; a differential
count of cells can be done visually in May-Grunvald Giemsa-stained
cytospin preparations.
4.1.5 Enumeration of leukocytes in bronchoalveolar lavage fluid,
peritoneal cavity, and skin
Mononuclear phagocytes in the alveoli of the lung play an
important role in clearing inhaled particles, including
microorganisms, from the lung. The numbers of cells and alterations in
their function can be end-points of the toxicity of inhaled chemicals.
In order to study these parameters, methods for harvesting the cells
from the lungs should be easy to perform, guarantee the sterility of
the cell harvest, and be standardized. Methods involve use of either a
syringe (Blusse Van Oud Alblas & Van Furth, 1979) or a complex system
of syringes, tubes, and valves (Moolenbeek, 1982). These methods often
result in contamination of the harvested cell population; moreover,
they are laborious and cannot easily be standardized since the syringe
is operated manually. In a more recently developed method (Van
Soolingen et al., 1990), an excised lung is placed in a pressure
chamber and connected to a cannula through which lavage fluid can be
introduced into the lung and transferred from the lung into a test
tube. This procedure is repeated several times to obtain an optimal
yield.
Enumeration of mononuclear cells in the peritoneal cavity can
also best be performed by harvesting these cells by lavage. Because
of the architecture of this organ, three or four cycles of
intraperitoneal injections of lavage fluid, followed by gentle
massaging of the abdomen, and aspiration of the fluid with the syringe
that was also used for injection suffice.
Langerhans cells in the skin can be enumerated with
histopathological techniques. Frozen tissue sections are used, stained
with immunoperoxidase techniques including markers for MHC class II
antigens or specific markers, as indicated above. Morphometric
analysis may provide a quantitative basis for this type of evaluation.
4.1.6 Flow cytometric analysis
Evaluation of phenotypic markers has proved to be one of the most
sensitive indicators of immunotoxic compounds. The availability of
fluorescent activated cell sorter (FACS) analysis units and
fluorescent cell counter units in immunotoxicology laboratories has
made analysis of cell populations routine. Determination of the
phenotype of lymphoid cells is a non-functional assay, although it has
often been inappropriately grouped with functional tests. The presence
or absence of a particular marker on the surface of a cell does not
reveal the functional capability of the cell. The usefulness of
surface marker analysis for predicting potential immunotoxicity has
been demonstrated. In studies conducted by Luster et al. (1992), a 91%
concordance was found for correct identification of immunotoxic
compounds on the basis of studies of surface markers alone.
As indicated above (section 4.1.2), numerous markers are
expressed on the cells of the immune system. Essentially, the same
reagents as used on tissue sections are applied on cells that have
been isolated from tissues, body fluids, or lavage fluids in
suspension (see above). Furthermore, both polyclonal and monoclonal
antibodies are available for detecting these surface markers. While
many of the markers have been used in immunological investigations,
very few have been evaluated with a large number of immunosuppressive
compounds. The markers that have been routinely used in studies of
immunotoxicity conducted for the NTP in mice and the cell types they
identify are shown in Table 11. The CD4:CD8 ratio in spleen has been
shown to concord best with the immunotoxicity of these surface markers
(Luster et al., 1992).
Table 11. Phenotypic markers on lymphocyte subpopulations used in
studies of immunotoxicity by the United States National
Toxicology Program
Surface marker Cell type
sIg+ Pan B cells
Thy 1.2+ or CD3+ Pan T cells
CD4+CD8- T Helper/delayed-type
hypersensitivity cells
CD8+CD4- T Suppressor-cytotoxic cells
CD8+CD4+ Immature T cells
The identification of phenotypic markers in rats has not
developed as rapidly as in mice; however, antibodies to rat cell
surface markers are now becoming available commercially and are being
used in immunotoxicological assessments (Smialowicz et al., 1990). The
monoclonal antibodies currently used for this purpose are: OX4 or
MARK-1 for B cells, W3/13 or OX 19 for T cells, R79 for the T cell
receptor, W3/25 for CD4 cells, and OX 8 for CD8 cells.
In enumerating the cell types in lymphoid tissue, both
percentages and absolute cell numbers should be reported. Of the two,
absolute cell numbers are by far the most meaningful. Compounds that
affect all populations equally and thus do not change the relative
percentages of the various cell types may be missed if only
percentages are evaluated. In addition, significant differences in the
magnitude of an effect on one or more of the populations can be
observed when the data are evaluated as absolute numbers and not as
percentages. As indicated above, the absolute changes more closely
reflect the events occurring in the animal and should thus be given
priority in interpreting data.
FACS analysis is also being used to determine the activation
state of various cell types, on the basis of changes in detectable
activation markers. Some of the activation markers that have been
studied are F4/80 (Austyn & Gordon, 1981), Mac-1 (Springer et al.,
1979), Mac-2 (Ho & Springer, 1984), transferrin receptor (Neckers &
Cossman, 1983), and IL-2 receptor (Cantrell et al., 1988). While
activation markers are of value in studying the mechanism of action of
compounds, their usefulness as predictors of immunotoxicity has yet to
be firmly established.
4.2 Functional tests
4.2.1 Macrophage activity
Phagocytic activity is the first line of defence against many
pathogens. Macrophages can phagocytose many particles, including
bacteria, and can lyse and inactivate them. Alterations in phagocytic
activity are therefore important potentially adverse effects of
chemicals on the immune system. The capacity to ingest particles
in vitro can be measured, and activity in vivo can be measured by
determining the clearance of bacteria, such as L. monocytogenes.
This test is dealt with in section 4.2.10.1.
Several assays have been developed for evaluating various types
of phagocytosis in mice and can also be used in rats, with slight
modifications. Innate and non-immune-mediated phagocytosis by
macrophages can be evaluated by determining the uptake of fluorescent
latex covaspheres (Duke et al., 1985). Macrophages and peritoneal
exudate cells are placed on a tissue culture slide and incubated with
the covaspheres for 24h on a rocking platform. The slides are then
fixed with methanol. The slide chambers are evaluated under a
fluorescent microscope, and macrophages with more than five latex
covaspheres are counted as positive for phagocytosis. The results are
expressed as percentage of phagocytosis, which is calculated as the
ratio of macrophages positive for phagocytosis to total macrophages
counted. In order to distinguish phagocytosed latex covaspheres from
those that are merely associated with the macrophage surface, the
cells are exposed for 30-60s to methylene chloride vapour. By
immersing the slides in this manner, the covaspheres that have not
been phagocytosed are dissolved, while those inside the macrophage
remain intact (Burleson et al., 1987). Phagocytosed fluorescent latex
particles can easily be quantified under the fluorescence microscope.
While this assay is straightforward, it is labour intensive, and
reading the slides, shifting back and forth from the fluorescent
field, and counting the macrophages is time-consuming.
A radioisotopic procedure, the chicken erythrocyte assay, can be
used to evaluate both adherence to and phagocytosis of particles by
macrophages. The phagocytic capacity is measured as an immunologically
mediated (Fc receptor) response. Macrophages are added to each well of
a 24-well tissue dish and allowed to adhere for a 2-3-h incubation
period. Nonadherent cells are washed, and chicken erythrocytes
labelled with 51Cr are added to each well; then a subagglutinating
dilution of antisera to chicken erythrocytes is added to each well and
the plate incubated for 1h. The plates are then washed to remove
unbound erythrocytes; an ammonium chloride solution is added to lyse
adhered erythrocytes, and the supernatant is collected and counted to
determine adherence of the erythrocytes to the macrophages. Next, both
the macrophages and the phagocytosed chicken erythrocytes are lysed by
addition of 0.1 N sodium hydroxide, and the solution is counted to
determine the amount of phagocytosis. Three to six wells in each group
do not receive 51Cr and are used to evaluate the DNA content
(Labarca & Paigen, 1980). The data are expressed as adherence counts
per minute, phagocytosed counts per minute, and specific activity for
adherence and phagocytosis. Specific activity is determined by
dividing the number of adhered or phagocytosed counts per minute by
the DNA content per well. The data must be expressed in terms of
specific activity, since compounds that affect the macrophages'
ability to adhere to the 24-well culture dish will significantly alter
the results obtained.
While both the nonspecific and immune-mediated phagocytosis
assays are useful for understanding the potential mechanisms of action
of compounds, changes in phagocytic activity in these in-vitro assays
have not been found to be predictive of immunotoxicity. For example, a
single intratracheal exposure to gallium arsenide resulted in
increased adherence and phagocytosis by chicken erythrocytes but
decreased phagocytosis of latex covaspheres (Sikorski et al., 1989).
The phagocytosis assay that is most predictive of altered
macrophage function is evaluation of the functional ability of the
mononuclear phagocyte system. This is a holistic assay for measuring
the capacity of the fixed macrophages of the mononuclear phagocyte
system, where macrophages provide the first line of defence against
both pathogenic and non-pathogenic blood-borne particles. The fixed
macrophages of the mononuclear phagocyte system line the liver
endothelium (Kupffer cells), the spleen, the lymph nodes (reticular
cells), the lung (interstitial macrophages), and other organs such as
the thymus and bone marrow. When the assay is conducted in mice, the
animals are injected intravenously with 51Cr-labelled sheep
erythrocytes, and a 5-µl blood sample is taken from the clipped tail
at 3-min intervals over a 15-min period. A final 30-min blood sample
is taken, and 1h after injection the animals are sacrificed and the
liver, spleen, lungs, thymus, and kidneys are removed, weighed, and
counted in a gamma counter. The 60-min time interval after injection
of sheep erythrocytes was selected as the time of sacrifice since it
represents the plateau for particle uptake by the selected organs
(White et al., 1985). Blood clearance of the radiolabelled cells is
expressed as vascular half-life and as a phagocytic index, which is
determined by the slope of the clearance curve. Organ distribution is
expressed as percent organ uptake and counts per minute per milligram
of tissue (specific activity). The assay can detect both stimulation
and inhibition of the mononuclear phagocyte system. Bick et al. (1984)
reported marked stimulation of the mononuclear phagocyte system after
treatment with diethylstilbestrol; more recently, morphine sulfate was
shown to decrease vascular clearance and hepatic and splenic
phagocytosis significantly (LeVier et al., 1993).
4.2.2 Natural killer activity
NK activity against neoplastic and virus-infected targets has
been clearly demonstrated in vitro and is thought to play an
important role in vivo in providing surveillance against neoplastic
cells and as a first line of defence against viruses (Herberman &
Ortaldo, 1981). In humans, rats, and mice, most cells with NK activity
can be identified by morphological (although the definition is not
morphological) and functional characteristics (Timonen et al., 1981).
Most of the cells that show NK activity are nonadherent, non-
phagocytic lymphocytes and are morphologically associated with large
granular lymphocytes (Timonen et al., 1982). Although cells with NK
activity do not strictly belong to the T-cell lineage, they can
express T cell-associated markers and express surface receptors, such
as those for the Fc portion of IgG and the ganglioside asialo GM1.
Some of these markers are also expressed by monocytes, macrophages,
and polymorphonuclear leukocytes (Herberman & Ortaldo, 1981). Within
4h, the cells can show nonantigen-specific cytotoxic activity
in vitro and in vivo against certain (NK-sensitive) tumour cell
lines and virus-infected cells.
The cells have enhanced cytolytic function after activation with
a variety of stimuli, including viral infection (Stein-Streinlein et
al., 1983), BCG (Tracey et al., 1977), IL-2 (Henney et al., 1981;
Domzig et al., 1983; Lanier et al., 1985; Malkovsky et al., 1987),
interferon, and interferon inducers (polyI:C) (Tracey et al., 1977;
Oehler & Herberman, 1978; Djeu et al., 1979a,b). NK activity in vitro
can be stimulated with IL-2 and interferon (Tracey et al., 1977; Djeu
et al., 1979b). Anti-asialo GM1 antibody can strongly inhibit
cytotoxic NK activity both in vitro and in vivo (Kasai et al.,
1980, 1981; Yosioka et al., 1986). This antibody binds to the cell
surface glycolipid GM1 and suppresses the lytic activity of effector
cells. Large granular lymphocytes are found in several lymphoid
organs. Many cells with high NK activity are found in spleen and
peripheral blood (Rolstad et al., 1986); lymph nodes have less NK
activity, and thymus and bone marrow show only marginal activity. NK
activity can also be demonstrated in the bronchus-associated lymphoid
tissue in the lungs. Moreover, large granular lymphocytes can migrate
from the circulation into the extravascular tissue and can even be in
contact with the lumen of the alveoli (Timonen et al., 1982; Reynolds
et al., 1984; Rolstad et al., 1986; Prichard et al., 1987). The
presence of large granular lymphocytes associated with NK activity in
the lungs is probably of great importance, because the lungs
constitute a major site for neoplastic disease (metastatic spread) and
viral infections. NK cells may also operate in certain types of
bacterial infections. In experimental animals, suppression of NK cell
activity increased the numbers of metastases after transplantation of
tumours.
The clinical significance of altered NK cell activity in humans
has not clearly been established. Asymptomatic individuals with low NK
cell responses may be at some risk for developing upper respiratory
infections and for increased morbidity (Levy et al., 1991); and
extreme susceptibility to severe and repeated herpes virus infection
was reported in an individual without NK cells (Biron et al., 1989).
It is obvious therefore that exposure to toxic substances that alter
NK activity can have biological consequences, and testing this
activity is important in assessing potential immunotoxicity.
The procedure for determining NK activity is as follows: cell
populations that exert NK activity (usually enriched peripheral blood
mononuclear cells or spleen cells) are cultured with NK-sensitive
target cells. A cell type frequently used for this purpose is the YAC
lymphoma cell line, which has been applied to mice, rats, humans, and
even seals. YAC lymphoma target cells are radiolabelled with 51 Cr,
and lysis of the cells, resulting in release of chromium, within 4h is
used to estimate the cytolytic activity of the NK cells within the
cell population. This assay has been used to demonstrate the effects
of numerous compounds on NK activity in rats (e.g. TBTO, ozone, and
HCB: Vos et al., 1984; Van Loveren et al., 1990c), mice (Luster et
al., 1992), and harbour seals (Ross et al., in press).
4.2.3 Antigen-specific antibody responses
Most antibody responses require not only B cells, which, after
maturation into plasma cells, produce antibodies, but also the help of
T lymphocytes. A variety of T cell-dependent antigens can be used for
this purpose, and an excellent one is tetanus toxoid. A typical
immunization schedule in rats comprises intravenous immunization on
day 0 followed by a booster on day 10. Primary and secondary IgG and
IgM responses can then be measured in serum, taken on day 10 (just
before the booster) and day 21, respectively. The primary IgM response
is the immunoglobulin response that is least under the control of T
cells. As tetanus toxoid is also used for human immunization, the
responses to this antigen may be useful in extrapolating experimental
data to humans. The responses can be determined in an ELISA (Vos et
al., 1979b).
Another widely used T cell-dependent antigen is ovalbumin. This
antigen can be and has been used to induce all classes of antibody
responses, i.e. IgM, IgG, IgA, and IgE, that can be measured with the
ELISA (Vos et al., 1980; Van Loveren et al., 1988b). The classical
assay of specific IgE responses is the passive cutaneous anaphylaxis
reaction. Serial dilutions are injected into the skin of rats,
sensitizing local mast cells; the specific antigen is then injected
intravenously, simultaneously with Evans blue. Mast cell products are
released where IgE meets the antigen, and IgE is cross-linked on the
membranes of the mast cells, leading to extravasation of Evans blue.
The titre can be determined from the magnitude of the reaction at each
dilution of IgE. ELISA techniques and the specific reagents to detect
IgE in an ELISA that are now available make this test preferable.
Ovalbumin induces not only humoral responses but also delayed-
type hypersensitivity. Sensitization to ovalbumin in Freund's complete
adjuvant enhances responses and makes it possibile to assay both
responses in one animal. Delayed-type hypersensitivity can also be
directed to purified protein derivative, with responses induced by the
adjuvant (Vos et al., 1980). At least in mice, however, immunization
in complete adjuvant skews responses in the direction of Th1
responses, i.e. delayed hypersensitivity, and hence suppresses Th2-,
IgE-, and IgA-dependent immune responses.
A few antigens can induce humoral immune responses without
involvement of T lymphocytes. One example is trinitrophenol-Ficoll
(lipopolysaccharide). Sensitization of animals to this antigen yields
immunoglobulin responses that can be measured in an ELISA. This is a
useful test for use in mechanistic studies to separate the effects of
compounds on B and T cells.
4.2.4 Antibody responses to sheep red blood cells
4.2.4.1 Spleen immunoglobulin M and immunoglobulin G plaque-forming
cell assay to the T-dependent antigen, sheep red blood cells
A widely used particulate T cell-dependent antigen is sheep red
blood cells. Antibody titres induced after sensitization can be
assayed with various techniques; one that is widely used is the
plaque-forming cell assay, or antibody-forming cell response. This
assay is relatively simple and can be conducted with inexpensive
equipment found in most laboratories, but the optimal concentration of
sheep red blood cells must be injected. As the antigenicity of red
blood cells varies significantly from sheep to sheep, time must be
invested to obtain cells from a sheep that repeatedly gives a high
response (>= 1500 plaque-forming cells/106 spleen cells). The
number of cells administered (about 2 × 108) should also be
optimized for both mice and rats in the laboratory conducting the
assay. The intravenous route is that preferred for sensitization;
intraperitoneal injections can be used but significantly increase the
potential for nonresponding animals as a result of a poor injection.
Animals are sacrificed on day 4 after injection, and spleen cells are
prepared by mincing the spleen between two frosted microscope slides,
teasing it apart with forceps, or passing it through a small mesh
screen; all of these methods are satisfactory, and that used to
prepare single splenocyte cultures varies from laboratory to
laboratory. An aliquot of cells is added to sheep erythrocytes and
guinea-pig complement; these are placed in a microscope slide chamber
when the Cunningham assay method is used (Cunningham & Szenberg,
1968), or, in the Jerne method, cells and guinea-pig complement are
added to a test tube containing warm agar and after thorough mixing
the test tube mixture is plated in a petri dish and covered with a
microscope cover slip (Jerne et al., 1974). In either case, the
preparations are then incubated at 37°C for 3-4h to allow plaques to
develop. The plaques are counted under a Bellco plaque viewer. A
plaque results from the lysis of sheep erythrocytes and is elicited as
a result of the interaction of complement and antibodies directed
against sheep erythrocytes, which are produced in response to the
intravenous sensitization. As each plaque is generated from a single
IgM antibody-producing plasma cell, the number of IgM plaque-forming
cells present in the whole spleen can be calculated. The data are
expressed as specific activity (IgM plaque-forming cells/106 spleen
cells) and IgM plaque-forming cells per spleen.
By incorporating rabbit anti-mouse or anti-rat IgG antibody into
the preparation of spleen cells, complement, and sheep red blood
cells, the number of IgG antibody-forming cells present in the spleen
can also be determined. This number is calculated by subtracting the
number of IgM plaque-forming cells from the total number of both IgM
and IgG plaque-forming cells. The optimal IgG primary response is
observed five days after sensitization (Sikorski et al., 1989).
The T-dependent IgM response to sheep red blood cells is one of
the most sensitive immunotoxicological assays currently in use. Luster
et al. (1992) reported that the individual concordance of the plaque-
forming cell assay for predicting immunotoxicity was the highest of
all the functional assays (78%). Furthermore, use of this assay in
combination with either NK cell activity or surface marker analysis
resulted in pairwise concordances for predictability of more than 90%.
While the plaque-forming cell assay has been shown to be
sensitive and predictive, the procedure does have its limitations. As
indicated earlier, the effect of the test compound on the immune
system is evaluated only in spleen cells, and effects on other
antibody-producing organs and tissues are not determined. The assay is
somewhat laborious, and it is preferable that several people
participate, to help in removing spleens, preparing cell preparations,
counting cells, and adding preparations to either microscope slide
chambers or agar dishes. An additional drawback is that the assay must
be conducted on the same day as the animals are sacrificed. This is in
marked contrast to the ELISA, in which sera can be frozen and
evaluated at a later date. While the slides and petri dishes can be
placed in a cold room or refrigerator and counted the next day, this
procedure is not recommended, as they tend to dry out to some extent,
making viewing and discerning plaques more difficult.
4.2.4.2 Enzyme-linked immunosorbent assay of anti-sheep red blood
cell antibodies of classes M, G, and A in rats
An alternative to the plaque-forming cell assay is ELISA of anti-
sheep red blood cell antibody titres in serum. Antigen preparations
made from ghosts of sheep erythrocytes by extraction with potassium
chloride are used to coat the bottoms of the wells of 96-well
microtitre plates. Serum samples from rats immunized with sheep
erythrocytes are titrated onto these plates using specific polyclonal
antibodies to rat IgM or IgG, to which peroxidase is conjugated. IgA
has also been assayed, using monoclonal anti-rat IgA antibodies and
polyclonal rat anti-mouse IgG conjugated with peroxidase. The ELISA of
serum titres of IgM, IgG, and IgA to sheep erythrocytes is an easy,
reliable method that can be used to detect the effects of chemicals on
the immune system of the rat (Van Loveren et al., 1991; Ladics et al.,
1995).
The assay measures titres of specific antibodies, in contrast to
the plaque-forming cell assay which determines the number of cells
that are actually responsible for production. The ELISA assesses the
production of antibodies, either per cell or in terms of the total
capacity of the host to produce these antibodies in vivo. In
interpreting the effects of exposure to chemicals, account must be
taken of the fact that the cells used in the assay are derived from
specialized parts of the body, such as the spleen, and alterations in
the numbers of antibody-producing cells in such an organ in rats
immunized with sheep red blood cells cannot give information on other,
inaccessible pools of antibody-producing cells. In the ELISA,
alterations in titres due to exposure to chemicals indicate changes in
the immune potential of the exposed animals. In screening for the
effects of chemicals on the immune system, therefore, ELISAs may be
preferable, but for studies on specific immunosuppressive mechanisms,
the plaque-forming cell assay, although labour- and time-intensive, is
a powerful tool for obtaining information complementary to the data
provided by the ELISA. Unfortunately, it is not always possible to
perform the two assays with material from the same animal. The peak
response in the plaque-forming cell assay in both rats (Fischer 344)
and mice (B6C3F1) occurs on day 4 after sensitization, while the peak
response in the ELISA occurs on day 6 for rats and day 4-5 for mice
(Temple et al., 1993). In order to detect the effects of chemicals on
the immune response to sheep red blood cells, it is preferable to
choose the optimal conditions, or to follow the kinetics, of the
response.
4.2.5 Responsiveness to B-cell mitogens
Responsiveness to lipopolysaccharide is another estimate of
humoral immune response, as solely B cells respond to this mitogen.
Although the responses of rats to this mitogen are less pronounced
than those of mice, good results can be obtained, and the
immunosuppressive effects of chemicals can be detected (Vos et al.,
1984).
An alternative B-cell mitogen is S. typhimurium mitogen (STM),
a water-soluble, proteinaceous extract derived from the cell walls of
S. typhimurium; it is a more potent mitogen for rat B lymphocytes
than lipopolysaccharide (Minchin et al., 1990). In both mice and rats,
the polyclonal activation of B lymphocytes is a multistep process. In
mice, mitogens alone can provide all the signals necessary for
proliferation and differentiation; in the rat, STM stimulation induces
B lymphocytes to proliferate without differentiating. The addition of
lymphokines to STM-stimulated B cells also failed to stimulate them to
differentiate (Stunz & Feldbush, 1986). Nevertheless, this mitogen is
useful for evaluating effects on the proliferative ability of rat B
lymphocytes. Smialowicz et al. (1991) showed a decrease in the STM
response in Fischer 344 rats after oral exposure to 2-methoxyethanol.
Unlike the bell-shaped mitogen dose-response curves observed with
T-cell mitogens, the proliferative response of B lymphocytes to both
lipopolysaccharide and STM rises quickly at low concentrations of the
mitogens and plateaus at higher concentrations. As a result, a single
concentration on the plateau phase of the mitogen response curve is
sufficient to evaluate the effects of a test compound on B-cell
mitogen-driven proliferation. One of the reasons that the mitogen
assays appear to be insensitive is that the cells must remain in
culture for several days in order to obtain a peak response. As a
result, they may recover from the immunomodulatory effects of the test
compounds during this in-vitro phase. This is a common problem with
many ex-vivo/in-vitro assays, including the cytotoxic T lymphocyte and
mixed leukocyte response assays; because of the short, 4-h period of
the NK cell assay, this is less of a concern.
4.2.6 Responsiveness to T-cell mitogens
The proliferative ability of T lymphocytes after stimulation with
mitogens can be measured by the uptake of 3H-thymidine in a manner
similar to that used to measure B-cell proliferation (Anderson et al.,
1972). Concanavalin A and phytohaemagglutinin are T-cell mitogens in
both rats and mice; pokeweed mitogen stimulates the proliferation of
both T and B cells and thus lacks specificity. Although both
concanavalin A and phytohaemagglutinin stimulate T lymphocytes,
T cells responsive to concanavalin A have been reported to be less
mature than those responsive to phytohaemagglutinin (Stobo & Paul,
1973). Multiple concentrations of these mitogens should be used to
ensure that a peak response is obtained: both produce a bell-shaped
dose-response curve, and too high a concentration can result in a
suboptimal response.
Historically, mitogens have been included in the battery of tests
for evaluating potential immunotoxicity, because the assay is one that
can also be carried out in humans. Human studies, however, are
conducted on peripheral blood, while most studies of rodent lymphocyte
transformation are conducted using spleen or lymph node cells. Thus,
the argument that the assay has clinical relevance is not well
founded. Furthermore, as the response of lymphocytes is extremely
robust, the assay lacks sensitivity. After a significant number of
compounds were evaluated for potential immunotoxicity in mitogen
assays, use of this assay was shifted from the tier 1 screen
originally described by Luster et al. (1988) to the tier 2
comprehensive evaluation. Use of the mitogen assay has now been
removed completely from studies conducted for the NTP, since other
assays in which cellular proliferation is required (e.g. plaque-
forming cell assay, mixed leukocyte reaction) were considered to be
more sensitive, and the data obtained from the mitogen assays add
little if any to an evaluation of the potential immunotoxicity of test
compounds.
4.2.7 Mixed lymphocyte reaction
In the mixed lymphocyte reaction (also known as mixed lymphocyte
culture), suspensions of responder T lymphocytes from spleen or lymph
nodes are co-cultured with allogeneic stimulator cells. The foreign
histocompatibility antigen (MHC class I or class II molecules)
expressed on the allogeneic stimulator cells serves as the activating
stimulus for inbred populations. In noninbred populations, a pool of
allogeneic cells can be used as stimulators. The assay analyses the
ability of T cells to recognize allogenic cells as 'non-self' as a
result of the presence of different MHC class II antigens on their
surface. In response to the class II antigens, the spleen or node
cells proliferate. Because a sufficiently large number of T cells in
the mixed lymphocyte population respond to the stimulator population,
the responder T cells need not be primed. Proliferation of the
responder cells is one of the parameters for T-cell responsiveness to
cellular antigens. If the allogeneic stimulator cell suspension
contains T cells, their uptake of 3H-thymidine must be prevented by
gamma-irradiation or mitomycin C, in order to preclude background
thymidine uptake.
4.2.8 Cytotoxic T lymphocyte assay
The Tc lymphocyte assay is a continuation of the mixed lymphocyte
reaction response in which the T lymphocytes further differentiate
into cytotoxic effector cells under the influence of various
cytokines. In mice, the assay is usually conducted using P815
mastocytoma cells as the sensitizer and target cell (Murray et al.,
1985). Mice are exposed in vivo to the test agent, and spleen cells
are then removed and placed in culture flasks with the P815
mastocytoma cells. After a five-day co-culture period, the spleen
cells are harvested and added to fresh P815 mastocytoma cells which
have been radiolabelled with 51Cr as sodium chromate. After a 4-h
incubation, the percentage cytotoxicity is determined by measuring the
specific release of 51Cr into the supernatant. The five days of
culture are necessary for the T lymphocytes to differentiate into
cytotoxic effector cells. Unfortunately, this extended period in
culture may give the spleen cells sufficient time to recover from any
adverse effects of the test compound, although such effects may have
been present at the time the spleen cells were removed from the
animal. This inherent limitation of the assay detracts from its
usefulness in assessing the immunotoxicity of test compounds.
A holistic Tc lymphocyte assay has been described, in which the
animal is sensitized after injection of the irradiated target cells
(Devens et al., 1985). Inhibiting the ability of the sensitizing cells
to proliferate either through irradiation or mitomycin C treatment
before injection prevents development of Tc lymphocytes in the animal.
Smialowicz et al. (1989) developed an assay in rats in which effector
cells are generated in culture by incubating cells with lymph node
cells from Wistar/Furth rats, and 51Cr-labelled W/Fu-G1 tumour cells
are used as the target cells. The assay requires four days in culture
and can be run simultaneously with the rat mixed lymphocyte reaction,
thus providing information on the test compound's ability to affect
proliferation and differentiation into effector cells.
4.2.9 Delayed-type hypersensitivity responses
Delayed-type hypersensitivity responsiveness is a reflection of
the capacity of the cellular immune system to execute immune responses
and especially those dependent on IL-2 and INF gamma, which include
attraction and activation of nonspecific mononuclear leukocytes
(macrophages-monocytes). Many systems can be used, depending on the
antigen. One is sensitization to BCG, followed by challenge with
purified protein derivative, to which sensitivity is induced. Another
example is ovalbumin, to which sensitization is most efficient if the
ovalbumin is emulsified in complete Freund's adjuvant. In this system,
delayed hypersensitivity can be measured to both purified protein
derivative and ovalbumin (Vos et al., 1980). Another antigen is
L. monocytogenes: This system is particularly interesting since it
can be used in the context of experiments in which host resistance to
this pathogen is also measured (Van Loveren et al., 1988a).
Delayed hypersensitivity responses can be measured after
sensitization to Listeria by subcutaneous injection of the test
antigen into the ears. Prior to and 24 and/or 48h after challenge, the
increment in ear thickness can be measured with a micrometer by a
person unaware of the experimental group. The background ear swelling
responses of similar, unimmunized control animals are subtracted from
the swelling responses found in immunized animals.
Several delayed-type hypersensitivity assays have been developed
and used for evaluating immunotoxicity in the mouse. Most have
involved measuring swelling in either the footpad or the ear after
sensitization and challenge with a protein antigen. Studies by
LaGrange et al. (1974) demonstrated that sheep erythrocytes could
elicit a delayed-type hypersensitivity response after a single
injection into the foot pad; however, more sheep erythrocytes were
needed to elicit the delayed-type hypersensitivity response than to
produce the optimal humoral immune response. Foot pad swelling can be
measured with a micrometer, as described for rats or by a more
objective, isotopic procedure, as described by Paranjpe & Boone (1972)
and Munson et al. (1982). The delayed-type hypersensitivity response
to sheep erythrocytes was previously considered to be a good assay for
detecting effects on cell-mediated immunity; however, the lack of
persistence of the response (LaGrange et al., 1974; Askenase et al.,
1977) and the possible contribution of antibody to the response raised
concern about the specificity of the assay when sheep erythrocytes are
used as the eliciting antigen. Benzo[ a]pyrene, a compound that
selectively affects humoral but not cell-mediated immunity in adult
mice, appears to decrease cell-mediated immunity when measured in the
sheep erythrocyte assay but has no effect on delayed-type
hypersensitivity when evaluated in the keyhole limpet haemocyanin
assay. The effect in the sheep erythrocyte assay is observed at doses
of benzo[ a]pyrene that decrease antibody production, suggesting a
significant antibody component of the swelling observed (White, 1992).
Keyhole limpet haemocyanin is another protein antigen used in
evaluating delayed-type hypersensitivity responses. Holsapple et al.
(1984) characterized the response to this antigen in the mouse,
showing that it produced the classical delayed-type hypersensitivity
response both with and without adjuvant. Two immunizations with
keyhole limpet haemocyanin were required, however, to produce a
response equivalent to one obtained with complete Freund's adjuvant.
In these studies, animals were sensitized with subcutaneous injections
of keyhole limpet haemocyanin in the shoulder area, with seven days
between the sensitizations. They were then challenged with the same
antigen injected intradermally into the central portion of the pinna
of one of the ears. Increases in ear thickness were evaluated by both
micrometer readings and radioisotopically. The unchallenged ear was
used as an individual control for each animal, and a group of
unsensitized but challenged animals was used to control for
nonspecific and background effects. The results indicated that,
whenever possible, the use of adjuvant in delayed-type
hypersensitivity studies should be avoided. Despite the fact that
complete Freund's adjuvant boosted the responses to keyhole limpet
haemocyanin, it partially masked the dexamethasone-induced suppression
of the response. In some cases, however, delayed-type hypersensitivity
responses are difficult to induce without adjuvant.
The studies currently conducted in mice and rats with this assay
are holistic assays for evaluating cell-mediated immunity. Since
sensitization and challenge occur in the intact animal, all components
of the immune system are present to respond in a physiologically
relevant manner. This type of assay is much more valuable for
evaluating the effects of compounds on cell-mediated immunity than are
in-vitro assays such as the mixed leukocyte response or Tc cell assay.
Luster et al. (1992) reported that the delayed-type hypersensitivity
response assay in mice was highly predictive (100% concordance) of
immunotoxicity when used in combination with the NK cell assay and the
plaque-forming cell assay.
4.2.10 Host resistance models
4.2.10.1 Listeria monocytogenes
Relevant mechanisms of defence against L. monocytogenes include
phagocytosis by macrophages and T cell-dependent lymphokine production
which enhances phagocytosis (Mackaness, 1969; McGregor et al., 1973;
Takeya et al., 1977; Pennington, 1985; Van Loveren et al., 1987).
Humoral immunity is not relevant in protection against infection in
this model. Clearance of Listeria after infection by, for instance,
the intravenous or the intratracheal route can be assessed at various
times after infection by determining the numbers of colony forming
units in the spleen or lungs, respectively. This can be done by
classical methods (Reynolds & Thomson, 1973) that involve the
following steps: Serial dilutions of homogenates of the organs,
prepared in mortars with sterile sea sand, are plated onto sheep blood
agar plates; after a 24-h incubation at 37°C, the colonies are counted
to determine the number of viable bacteria in the organ. Differences
in the numbers of bacteria retrieved from the organs are an indication
of the clearance of the bacteria, i.e. the rate at which the host
disposes of the bacteria after infection.
Histopathology after a Listeria infection can also be valuable.
For instance, exposure to ozone before an intratracheal infection with
Listeria affects pathological lesions due to the infection (Van
Loveren et al., 1988a): Pulmonary infection with Listeria induces
histopathological lesions characterized by foci of inflammatory cells,
such as lymphoid and histiocytic cells, accompanied by local cell
degeneration and influx of granulocytes. If rats are exposed to ozone
for one week before infection, the lesions are much more severe than
in unexposed animals and persist at times when either ozone-associated
or infection-associated effects alone would have resolved. The quality
of the lesions is also influenced by prior exposure to ozone: mature
granulomas were found in Listeria-infected rats that were also
exposed to ozone.
When mice are challenged with Listeria, mortality is the usual
end-point monitored; however, clearance and organ bacterial colony
counts can also be determined. L. monocytogenes is a Gram-positive
bacterium. The resistance of mice to the organism is genetically
regulated, and the susceptibility of the B6C3F1 strain, the strain
designated by the NTP for immunotoxicity studies, comes from the C3H
parent, since the C57Bl/6 mouse is resistant (Kongshavn et al., 1980).
Listeria can easily be stored at -70°C at a stock concentration of
approximately 108 colony forming units per ml. In studies in mice,
three challenge levels are routinely selected to produce 20, 50, and
80% mortality in the vehicle control animals. Mortality is recorded
daily for 14 days. Treatment groups consisting of 12 mice per group
have been found to be useful for obtaining statistically meaningful
data on host resistance. This assay is extremely reproducible when the
organism is administered intravenously.
The Listeria assay can detect both protection from and
increased susceptibility to chemicals and drugs. Morahan et al. (1979)
used the model to demonstrate a dose-dependent decrease in host
resistance after exposure to delta-9-tetrahydrocannabinol, the major
psychoactive constituent of marijuana. The Listeria host resistance
assay is that most often used in immunotoxicological assessment of
compounds, and numerous examples of its use can be found in the
literature. It is one of the primary models used by the NTP for
evaluating immunosuppression. Since Listeria is a human pathogen,
appropriate precautions are needed in conducting the assay.
4.2.10.2 Streptococcus infectivity models
Two species of Streptococcus have been used widely in bacterial
host resistance models for immunotoxicological assessment.
S. pneumoniae has been used primarily for evaluating systemic
immunity. S. zooepidemicus has also been used to evaluate systemic
immunity but is used extensively to evaluate the effects of drugs and
chemicals on the local immunity of the pulmonary system.
S. pneumoniae is a Gram-positive coccus to which host
resistance is multifaceted (Winkelstein, 1981). The first line of
defence against this organism is the complement system. Activation of
the complement system can result in direct lysis of certain strains of
S. pneumoniae; however, owing to the nature of their cell wall, some
strains are resistant to lysis by complement. Complement can still
participate directly in the removal of these bacteria as a result of
deposition of complement component C3 on their surface, which
facilitates phagocytosis by polymorphonuclear leukocytes and
macrophages. In the later stages of the infection, antigen-specific
antibody plays a major role in controlling the infection. Thus,
compounds that affect complement, polymorphonuclear leukocytes, B-cell
maturation and proliferation, or the production of antibody can be
evaluated in this system. S. pneumoniae is an excellent model for
evaluating immunotoxicity, since it elicits multiple immune components
which participate in host resistance, each of which can be a potential
target for an adverse effect of a xenobiotic. To date, this model has
had limited success in rats.
Preparation of S. pneumoniaefor challenge is slightly more
complicated than the procedures used for Listeria; however, the
potential of the model for detecting immunotoxic compounds makes the
additional steps worthwhile. Stock preparations of S. pneumoniae
(ATCC 6314) are easily maintained at -70°C in defibrinated rabbit
blood, and aliquots of the stock preparation can be removed and grown
in culture at various dilutions to obtain the desired challenge
concentration. An alternative approach is to grow the organism in
culture and to monitor the bacterial concentrations by measuring the
turbidity of the culture. A 5-µl aliquot of the stock preparation is
used to inoculate 50ml of brain-heart infusion broth, which is
incubated at 37°C, and the turbidity of the overnight culture is
determined with an Abbott Biochromatic analyser system or another
instrument that can sensitively measure changes in culture turbidity.
The overnight culture is diluted with fresh brain-heart infusion broth
to yield an absorbence difference of 0.020-0.025. The turbidity of the
subculture is monitored periodically, and when the optimal density
reaches an absorbence difference of 0.080, the subculture is rapidly
cooled in an ice bath and diluted to the desired inoculum level. The
turbidity of each inoculum is checked in the analyser, and adjustments
are made to obtain the preselected differences in absorbence.
Routinely, one day after the last exposure, female mice are challenged
intraperitoneally with 0.2ml of the S. pneumoniae inoculum. If the
inoculum is administered intravenously, extremely high challenge
levels must be used, which may reflect the efficiency of the
mononuclear phagocyte system to clear and kill the organism. Three
innocula, each at a different concentration, are prepared to give a
range of lethality (e.g. 20, 50, and 80%), and a sample of each is
serially diluted and placed on blood agar plates to determine the
number of colony-forming units administered to the animals. Owing to
the rapid onset of infection, mortality is recorded twice daily for
seven days. In studies by White et al. (1986), when female B6C3F1 mice
were exposed daily for 14 days to 1,2,3,6,7,8-hexachlorodibenzo-
para-dioxin or TCDD by gavage, they were found to have decreased
host resistance to S. pneumoniae, which is consistent with the
decrease in complement activity caused by these compounds.
Another species of Streptococcus that has been used as a host
resistance model is S. zooepidemicus, a group C streptococcus
(Fugmann et al., 1983). Exposure to N-nitrosodimethylamine was shown
to decrease host resistance to this strain significantly. Infection
with S. zooepidemicus may be dependent on an antibody-mediated
response, since the time to death after challenge is considerably
longer than with S. pneumoniae. Numerous studies have demonstrated
that aerosolized S. zooepidemicus is one of the most sensitive
indicators of the toxicity of air pollution: Mice exposed for short
periods to single or mixed pollutants before infection with an aerosol
of S. zooepidemicus and then assessed for mortality over 20 days had
increased mortality with increasing concentrations of ozone (Coffin &
Gardner, 1972; Ehrlich et al., 1977), nitrogen dioxide (Ehrlich &
Henry, 1968; Sherwood et al., 1981), sulfur dioxide (Selgrade et al.,
1989), metal particulates (Gardner et al., 1977; Adkins et al., 1979,
1980; Aranyi et al., 1985), phosgene (Selgrade et al., 1989), and
other volatile organic compounds (Aranyi et al., 1986). With many of
these compounds, enhanced susceptibility to infection has been
demonstrated at concentrations at or below the United States national
ambient air quality standards or threshold limit values. With all of
these compounds, enhanced mortality has been associated with failure
to clear bacteria from the lung and suppression of alveolar macrophage
phagocytic function. This model has recently been adapted to rats. In
this species, both ozone (Gilmour & Selgrade, 1993) and phosgene (Yang
et al., 1995) delayed clearance of bacteria from the lungs and
enhanced the inflammatory response (polymorphonuclear leukocytes in
lavage fluid) at concentrations that do not themselves produce
inflammation; however, mortality does not occur in this species. While
the bacteria have generally been administered as aerosols, Sherwood et
al. (1988) showed that similar results could be obtained when they
were administered intratracheally or intranasally. Since some strains
of Streptococcus are pathogenic to humans, appropriate precautions
must be taken when using this host resistance model.
4.2.10.3 Viral infection model with mouse and rat cytomegalovirus
Cytomegalovirus infections are widely distributed in humans, with
about 60-90% of the population infected. Human cytomegalovirus
infections occur in several forms, the most serious being congenital
and perinatal infection and infection of immunosuppressed individuals.
Less serious forms include post-perfusion syndrome and some cases of
infectious mononucleosis; however, the vast majority of postnatal
infections in immunocompetent individuals are clinically asymptomatic.
More severe disease may occur in immunodeficient hosts, such as
transplant patients (Naraqi et al., 1977; Pass et al., 1978; Marker et
al., 1981; Rubin et al., 1981). Primarily on the basis of
morphological considerations, cytomegaloviruses are classified as
members of the family Herpesviridae. Because these viruses have a
relatively protracted replication cycle, a slowly developing
cytopathology characterized by cytomegaly, and a relatively restricted
host range, they are grouped into the beta Herpesviridae subfamily
(Roizman et al., 1981; Roizman, 1982). The roles of several arms of
the immune system in resistance to cytomegalovirus have been studied
extensively in mice. The role of humoral immunity is not well
understood. It was suggested initially that neutralizing antibodies do
not play a pivotal role in recovery from cytomegalovirus infection in
mice (Osborn et al., 1968; Tonari & Minamishima, 1983); however, the
role of antibodies in neutralization of murine cytomegalovirus and in
antibody-dependent cell-mediated cytotoxicity is now recognized
(Manischewitz & Quinnan, 1980; Quinnan et al., 1980; Farrell &
Shellam, 1991). Cytomegalovirus-specific Tc cells can be detected in
cytomegalovirus-infected mice (Ho, 1980; Quinnan et al., 1980). NK
cell activity appeared to be the most effective, especially during the
initial stages of infection (Bancroft et al., 1981; Selgrade et al.,
1982; Bukowski et al., 1984). Enhanced susceptibility to infection has
been demonstrated in mice when macrophage function was blocked by
silica, and transfer of syngeneic adult macrophages to suckling mice
significantly increased their resistance to mouse cytomegalovirus
infection (Selgrade & Osborn, 1974). An inverse correlation is seen
between the virulence of mouse cytomegalovirus and its infectivity for
peritoneal macrophages (Inada & Mims, 1985), suggesting that
attenuated virus may be controlled, in part, by macrophages. Since the
rat virus acts very much like the attenuated mouse virus, macrophages
may be even more important in rats. Macrophages may facilitate the
generation of latent infection (Booss, 1980; Yamaguchi et al., 1988).
Enhanced susceptibility to mouse cytomegalovirus has been
demonstrated after treatment of mice with cyclophosphamide,
cyclosporin A, nickel chloride, or DMBA. Treatment with benzo[ a]-
pyrene or TCDD did not affect susceptibility to this infection
(Selgrade et al., 1982). Enhanced susceptibility was correlated with
chemical suppression of virus-augmented NK cell activity during the
first week of infection. In rats, exposure to immunotoxic agents such
as organotin compounds led to altered resistance to rat
cytomegalovirus (Garssen et al., 1995).
Experimentally, rodents can be inoculated intraperitoneally with
a species-specific cytomegalovirus, and the concentration of the virus
in tissue can be determined in a plaque-forming assay, which is a
modification of the method described by Bruggeman et al. (1983, 1985).
Rat embryo-cell monolayers are prepared in 24-well plates. Different
organs (salivary gland, lung, kidney, liver, spleen), obtained at
various times after infection, are homogenized in a tissue grinder and
stored as 10% weight/volume samples at -135°C until use. The confluent
monolayers are then infected with 10-fold serial dilutions of the
organ suspensions. After centrifugation, the suspension is removed,
and 1 or 0.6% agarose is added. After incubation at 37°C in 5% carbon
dioxide for seven days, the cells are fixed in 3.7% formaldehyde
solution, the agarose layer is removed, and the monolayer is stained
with 1% aqueous methylene blue. Plaques are counted under a
stereoscopic microscope.
In PVG rats, cytomegalovirus is detectable eight days after
infection, although the virus load is much higher on days 15-20. The
viral load in the salivary gland is higher than that in other organs,
i.e. spleen, lung, kidney, and liver. In contrast, in Lewis rats and
BN rats the viral load in e.g. the kidney was higher than that in the
salivary gland during the first week after infection (Bruning, 1985),
perhaps due to a strain difference (Bruggeman et al., 1983, 1985).
Total body irradiation of PVG rats with 60Co one day before
infection with cytomegalovirus increased the viral load in the
salivary gland, lung, kidney, spleen, and liver over that in
unirradiated PVG rats; histological analysis also indicated a higher
viral load in the salivary gland of infected rats. The mucosal
epithelium of the salivary gland contains enlarged cells with nuclear
inclusion bodies; these could be detected in irradiated, infected rats
only if the salivary gland was dissected and fixed 15 days after
infection. These results are in agreement with those of Bruggeman et
al. (1983), who found that gamma irradiation also induced higher viral
loads in the salivary gland of BN rats. Taken together these results
indicate a role for cellular immunity in resistance to this virus in
rats.
4.2.10.4 Influenza virus model
Influenza virus A2/Taiwan H2N2 has been used as a viral
challenge in evaluating alterations in host resistance of mice after
exposure to various compounds. Compounds that decrease host resistance
to the virus are N-nitrosodimethylamine (Thomas et al., 1985b) and
TCDD (House et al., 1990a). Compounds that do not to alter host
resistance to this pathogen include ozone (Selgrade et al., 1988),
benzo[ a]pyrene, benzo[ e]pyrene (Munson & White, 1990), methyl
isocyanate (Luster et al., 1986), and Pyrexol (House et al., 1990b).
Mortality is the end-point routinely measured in evaluating decreased
host resistance to influenza virus, which is usually instilled
intranasally (Fenters et al., 1979). Host resistance to this virus has
been reported to be mediated by cell-mediated immunity (Ada et al.,
1981), interferon (Hoshino et al., 1983), and antibody (Vireligier,
1975). This model had been suggested for use in evaluating compounds
that affect humoral immunity; however, its inability to detect such
compounds indicates that it is not suitable. A possible explanation
for the discrepancy is that administration of the virus by intranasal
instillation may invoke local immune mechanisms in the lung and may
not adequately reflect systemic immunocompetence. In several cases,
enhanced mortality has been demonstrated in the absence of effects on
viral titres in the lung (Selgrade et al., 1988; Burleson et al., in
press), indicating that enhanced mortality does not always reflect
effects on virus-specific immune defences.
Influenza virus has been used in evaluating immunotoxicity in
rats, after adaptation. Studies by Ehrlich & Burleson (1991) showed
that rats exposed to phosgene had significantly decreased host
resistance. TCDD was shown to affect the resistance of rats to the
adapted influenza virus RAIV (Yang et al., 1994).
As influenza virus is a human pathogen, appropriate precautions
must be taken.
4.2.10.5 Parasitic infection model with Trichinella spiralis
Resistance to infection with the helminth T. spiralis has been
evaluated in both mice and rats after exposure to a variety of
chemicals. In humans, as in other carnivores, infection occurs by
eating meat containing infectious larvae. The life cycle of the worm
is as follows: Infectious larvae excyst in the acid-pepsin environment
of the stomach, rapidly migrate to the jejunum, and penetrate host
intestinal epithelial cells. Sexually mature parasites are present
within three to four days after infection. The viviparous females
produce larvae that migrate via the lymphatic and blood vessels to
host muscle, where they encyst and are encapsulated within a host-
derived structure. Encapsulated muscle larvae can survive for years
within this structure.
An intense inflammatory response, comprised mainly of mast cells
and eosinophils, accompanies intracellular infection in the intestine.
T Cell-dependent immunity plays a crucial role in this inflammatory
response (Manson-Smith et al., 1979; Vos et al., 1983b; Wakelin,
1993), which is responsible for the expulsion of adult parasites.
Antibodies damage the reproductive structures of the female parasite
(Love et al., 1976), have a major role in the rapid elimination of
subsequent infections in rats (Appleton & McGregor, 1984), and
sensitize migrating newborn larvae for destruction by granulocytes
(Ruitenberg et al., 1983).
The number of encysted muscle larvae is typically much higher in
immunosuppressed animals than in immunocompetent animals, due perhaps
to delayed expulsion of adult worms from the intestine, decreased host
control of parasite fecundity, decreased destruction of migrating
larvae, or a combination of resistance defects. These end-points of
host resistance to T. spiralis infection and class-specific antibody
titres can be measured by standard techniques (Van Loveren et al.,
1994). Histological evaluation of the inflammatory infiltrate
surrounding encysted muscle larvae has also been described (Van
Loveren et al., 1993b).
It should be noted that direct effects of the chemical under
study can affect the outcome of infection. For example Bolas-Fernandez
et al. (1988) determined that cyclosporin A delays expulsion of adult
parasites from the intestines of rats but does not increase the number
of larvae encysted in host muscle. This was determined to be a direct
effect of cyclosporin A on the fecundity of female parasites rather
than on immunity to infection. Animals are infected by oral gavage
with known numbers of larvae, isolated from infected donor muscle.
Because infection is spread only by consumption of infected meat or
freshly isolated larvae, there is little danger of the infection
spreading to other animals housed in the same room. T. spiralis is a
human pathogen and must be handled as such; normal laboratory
practices are sufficient to prevent accidental infection.
T. spiralis infection has been used as a host resistance model
in both rats and mice. In general, chemicals that suppress T-cell
function suppress resistance to T. spiralis infection. Thus, TBTO
(Vos et al., 1990b), diethylstilbestrol (Luebke et al., 1984), TCDD
(Luebke et al., 1994, 1995), and the virustatic agent acyclovir
(Stahlmann et al., 1992) had deleterious effects on resistance.
4.2.10.6 Plasmodium model
Two strains of Plasmodium have been used to evaluate the
potential immunotoxicity of compounds. P. yoelii (17XNL) is a
nonlethal strain that produces a self-limiting parasitaemia in mice.
Resistance to this organism is multifaceted and includes specific
antibody, macrophage involvement, and T cell-mediated functions
(Luster et al., 1986). In this assay, animals are injected with 106
parasitized erythrocytes, and the degree of parasitaemia is monitored
over the course of the infection by taking blood samples. In control
animals, the peak response usually occurs 10-14 days after injection.
The degree of parasitaemia can be evaluated by a variety of methods,
e.g. manually, by counting parasitized erythrocytes in blood smears
(Luebke et al., 1991). Host resistance to P. yoelii has been used to
assess the immunotoxicity of benzidine (Luster et al., 1985b),
diphenylhydantoin (Tucker et al., 1985), TCDD (Tucker et al., 1986),
pyran copolymer (Krishna et al., 1989), gallium arsenide (Sikorski et
al., 1989), and 2'-deoxycoformycin (Luebke et al., 1991).
P. berghei is lethal to mice and certain strains of rats and
has been used in assessing immunotoxicity (Loose et al., 1978). Host
resistance depends on specific antibody production and ingestion and
destruction of antibody-coated Plasmodium by phagocytic cells such
as macrophages. T Lymphocytes may also be involved in host resistance
to the organism (Bradley & Morahan, 1982). Mortality has been
evaluated after injection of 106 Plasmodium-infected erythrocytes.
Compounds that have been evaluated for immunotoxicity in this model
system include 4,4'-thiobis(6- tert-butyl- meta-cresol) (Holsapple
et al., 1988), dietary fish-oil supplement (Blok et al., 1992), and
styrene (Dogra et al., 1992). Neither P. yoelii nor P. berghei is
infectious in humans; infection of animals can occur only through
parenteral injection of contaminated blood.
4.2.10.7 B16F10 Melanoma model
The B16F10 tumour cell line is a malignant melanoma that is
syngeneic with the C57Bl/6 mouse, which is one of the parents of the
B6C3F1 mouse. This tumour line was selected for its propensity to
metastasize to the lung. The assay is an outgrowth of the work of
Fidler and colleagues (Fidler, 1973; Fidler et al., 1978). NK cells
and macrophages have been proposed to be involved in host resistance
to this metastasizing tumour; however, T lymphocytes have also been
shown to play a role (Parhar & Lala, 1987). This host resistance assay
is referred to as an artificial metastasis model, since the tumour
cells are administered by intravenous injection, usually into the tail
vein, and lodge in the lung, which is the first capillary bed they
encounter. The B16F10 tumour cells can be stored frozen and can easily
be grown in culture before use. Routinely, 1-5 × 105 cells are
injected intravenously into sentinel mice (i.e. untreated mice
injected with the highest challenge level of tumour cells), and the
tumour burden is monitored in order to select the optimal day of
assay.
Two parameters are routinely used to assess tumour burden. One is
DNA synthesis in the lungs of mice bearing tumours. Since background
DNA synthesis in the lungs of mice without tumours is extremely low,
any detectable rate is a result of the presence of a tumour. In order
to measure synthesis, mice are pulsed intraperitoneally one day before
sacrifice with 0.2 ml of 10-6 mol/litre of 5-fluorodeoxyuridine,
followed 30 min later by 2 µCi of 125I-iododeoxyuridine administered
by the intravenous route. After sacrifice, the lungs are removed,
placed in Bouin's fixative solution, and counted with a gamma counter.
A second indicator of tumour burden is visual enumeration of tumour
nodules after fixation in Bouin's solution. The visibility of the
black nodules of the melanin-producing B16F10 tumour cells on the
yellow background of the fixed lung tissue allows enumeration of up to
200-250 nodules on the surface of the lungs. A good correlation has
been shown between number of tumour nodules and radioactivity present
in the lungs (White, 1992). Thus, if the tumour nodules become too
numerous to count, the results of the study can still be determined
from the radioassay. This system has been useful in demonstrating
decreased host resistance after systemic exposure to the tumour
promoter phorbol myristate acetate (Murray et al., 1985),
intratracheal exposure to gallium arsenide (Sikorski et al., 1989),
and exposure to nickel chloride (Smialowicz et al., 1985b); it has
also been used to show enhanced host resistance after exposure to
manganese chloride (Smialowicz et al., 1984) and 4,4'-thiobis(6- tert-
butyl- meta-cresol) (Holsapple et al., 1988).
4.2.10.8 PYB6 Carcinoma model
The PYB6 tumour cell line is a fibrosarcoma originally induced
with a polyoma virus in C57Bl/6 mice. Host resistance to the tumour
includes NK cell activity and T cell-mediated killing (Urban et al.,
1982). While PYB6 cells can easily be grown in culture, they should be
passed through an animal before use in challenge studies for
immunotoxicity (Luster et al., 1988). In studies with the PYB6 line,
mice are injected in the thigh with 1-5 × 103 viable tumour cells
and are then palpated weekly to detect the development of tumours at
the injection site. The end-points evaluated include the incidence of
tumours and time to tumour appearance; tumour size can also be
measured. This assay has been useful in detecting decreased host
resistance to many compounds, including Aroclor 1254 (Lubet et al.,
1986), DMBA (Dean et al., 1986), and benzene (Rosenthal & Snyder,
1987).
4.2.10.9 MADB106 Adenocarcinoma model
A tumour model used to evaluate host resistance in rats is the
MADB106 rat mammary adenocarcinoma, which is syngeneic with the
Fischer 344 rat. NK cells appear to play a major role in host defence
to this tumour (Barlozzari et al., 1985). In this model, survival time
after injection of the cells is the usual end-point monitored.
Compounds that decrease host resistance to the tumour can decrease
both the percentage survival and the survival time of treated animals.
Control rats begin to succumb to the adenocarcinoma two to three weeks
after an intravenous injection of 2 × 106 tumour cells. Smialowicz
et al. (1985b) showed a significant decrease in the survival of
animals treated with a single intramuscular dose of nickel chloride,
which was correlated with a decrease in NK cell activity.
4.2.11 Autoimmune models
Autoimmune models can also be used to investigate whether a
compound exacerbates induced or genetically predisposed auto-immunity.
These models are used mainly to elucidate the pathogenesis of
autoimmunity and the effect of immunosuppression in
immunopharmacology. Few studies have been reported, although the
relevance of the model for extrapolation to humans may be good. A
number of autoimmune models are available in rats and mice. Autoimmune
phenomena can be either induced or occur spontaneously. In induced
models, an autoantigen is isolated from a target organ obtained from
another species (generally bovine), and the animal is immunized with
this purified antigen in adjuvant. Examples in the rat (Calder &
Lightman, 1992) are experimental encephalomyelitis elicited by bovine
spinal cord antigen (Stanley & Pender, 1991), experimental uveitis
elicited by bovine retinal S-antigen (Fox et al., 1987), and adjuvant
arthritis elicited by Mycobacterium containing H37RA adjuvant
(adjuvant arthritis) or collagen (Holmdahl et al., 1990; Klareskog &
Olsson, 1990; Wooley, 1991). Autoimmune phenomena and associated organ
pathology normally emerge in almost all immunized animals within two
to three weeks. Depending on the effector reaction and the
reversibility of the damage, the disease either stops when the damage
is complete (e.g. uveitis, resulting in blindness of the animal) or
the autoimmune reaction is transient and animals recover (adjuvant
arthritis). In some models, animals subsequently experience a relapse
around day 30 (experimental encephalomyelitis). The induction and
development of autoimmunity in these animals are mediated by T cells
that show the cytokine expression pattern (IL-2, INF gamma) of the Th1
subset. The effector phase of disease symptoms is also mainly a T
cell-mediated process, to which CD4+ cells, CD8+ cells, and
macrophages contribute. Lewis (RT11) rats are particularly
susceptible. These autoimmune models can be induced in other species,
such as mice (Baker et al., 1990) and rhesus monkeys (Rose et al.,
1991). They are generally accepted as models of human (organ-specific)
autoimmune diseases, e.g. experimental allergic encephalomyelitis as a
model of multiple sclerosis, experimental allergic uveitis as a model
of idiopathic posterior uveitis, and adjuvant arthritis as a model of
rheumatoid arthritis.
Autoimmunity can also be induced by metals (Druet et al., 1989;
Bigazzi, 1992). A well-known example is glomerulopathy induced by
mercuric chloride in BN (RT1n) rats. The process is initiated by T
cells with a cytokine synthesis pattern (IL-4) of the Th2 subset. The
relative incidence of these cells is much higher in BB rats than in
other strains. After the cells of the Th2 subset have been stimulated,
there is polyclonal stimulation of B lymphocytes, leading to synthesis
of antibodies (including pathogenic antibodies) to the glomerular
basement membrane. These antibodies subsequently mediate autoimmune
destruction of renal glomeruli. This model is the best studied model
of 'drug'-induced autoimmunity. Mercuric chloride elicits
glomerulonephritis in other rat strains, in which glomerular
destruction is not due to anti-glomerular autoantibodies but is
mediated by immune complexes deposited in the glomerulus (Druet et
al., 1989).
In spontaneous models, predisposition to the development of
autoimmune phenomena and disease is determined by the genetic
composition of the animal strain. Well-known examples are BB rats
(Like et al., 1982; Guberski, 1994) and NOD mice (Lampeter et al.,
1989; Leiter, 1993), which develop autoimmune pancreatitis and
subsequently diabetes. Within the pancreas, the islets of Langerhans
are infiltrated by T lymphocytes and macrophages; subsequent
destruction of the islets results in diabetes. These spontaneous
models are considered to be animal models of human diabetes (Dotta &
Eisenbarth, 1989; Lampeter et al., 1989; Riley, 1989). Other examples
are the systemic autoimmunity that emerges in certain mouse strains
(Guttierez-Ramos et al., 1990) like (NZB × NZW)F1 mice (Theofilopoulos
& Dixon, 1985) and the mixed lymphocyte reaction in lpr (Matsuzawa
et al., 1990) and gld mice (Roths et al., 1984). The spontaneous
pathology in these animals resembles various disease manifestations in
human systemic lupus erythematosus and is similarly mediated by immune
complexes that deposit in tissue. In (NZB × NZW)F1 mice, mainly lupus
nephritis is induced by immune complexes; in the mixed lymphocyte
reaction in lpr mice, both joint manifestations and
glomerulonephritis are seen. The genes associated with autoimmune and
immune complex disease are not known. In comparison with induced
models, these models have the advantage of spontaneous, gradual
development of autoimmune disease symptoms; however, this is a
disadvantage in experimental design, as not all animals develop
disease, and emerging disease develops at different times or ages.
In general, treatment of animals with immunosuppressive drugs
that interfere with signal transduction (cyclosporin, FK-506,
rapamycin) or cell proliferation (cytostatics like azathioprine,
mizoribine, and brequinar), and anti-inflammatory agents
(corticosteroids) inhibit the development of symptoms in these models.
Exposure to immunotoxic chemicals may also lead to alterations in the
course of disease emergence. For instance, HCB, which leads to
immunoenhancement in rats, markedly enhanced the severity of allergic
encephalomyelitis (Van Loveren at al., 1990c). In contrast, arthritic
lesions were strongly suppressed in HCB-exposed Lewis rats, indicating
that HCB has biologically significant immunotoxic effects. Although
the contrasting effects in the two autoimmune models are not yet
understood, and clear dose-effect relationships have yet to be
established, this type of information should be obtained for risk
assessment.
4.3 Assessment of immunotoxicity in non-rodent species
While most immunotoxicological evaluations are conducted in mice
or rats, use of other species is increasing.
4.3.1 Non-human primates
Various non-human primates, including Macaca mulatta (rhesus
macaques), M. nemestrina (pig-tailed macaques), Cercocebus atys
(sooty mangabeys), M. fasicularis (cynomolgus monkeys), and
marmosets have been used in immunotoxicological studies. Many of the
assays carried out in mice or rats can be adapted for use with non-
human primates. Strategies and methods used in studies of humans have
also been introduced in studies on non-human primates. Monoclonal
antibodies generated to human leukocyte subsets can be used in
phenotyping blood mononuclear cells of e.g. marmoset monkeys
(Callithrix jacchus) (Neubert et al., 1990, 1991), although the
possibility of such use differs, depending on the evolutionary
distance of the non-human primate from humans. While most of the
assays conducted in non-human primates involve serum or peripheral
blood, some assays, such as those used to measure delayed-type
hypersensitivity, are holistic, in that the animals are sensitized
in vivo and then evaluated in vivo at the challenge site (Bugelski
et al., 1990; Bleavins & Alvey, 1991).
The effects of chronic exposure to the PCB Arochlor 1254 on the
immune response of rhesus monkeys have been evaluated by Tryphonas et
al. (1989). In these studies, the lymphocyte response to concanavalin
A and phytohaemagglutinin was evaluated, as were total serum
immunoglobulin levels, antibodies to sheep red blood cells, and
numbers of T and B cells in peripheral blood. In later studies
(Tryphonas et al., 1991a,b), one-way mixed lymphocyte cultures,
antibodies to pneumococcal antigens, phagocytic mononuclear cell
function, NK function, haemolytic complement activity, and production
of IL-1, tumour necrosis factor, thymosins, and interferon were
evaluated in monkeys exposed to Arocolor 1254. Ahmed-Ansari et al.
(1989) evaluated phenotypic markers and function in three species of
non-human primate. The functional assays included NK cell activity,
lymphocyte transformation, and antigen presentation. Extensive studies
included the evaluation of more than 20 phenotypic markers or
combinations of markers for each of the three monkey species. Use of
the monkey as a test species is likely to increase as more and more
biotechnology and recombinant products are produced.
4.3.2 Dogs
While dogs are not the species of choice for immunotoxicological
studies, they are used predominantly in assessing toxicological
safety, and virtually all of the assays used for assessing immunotoxic
potential have been adapted for use in dogs. These include evaluation
of basal levels of IgA, IgG, and IgM (Glickman et al., 1988),
allergen-specific serum IgE (Kleinbeck et al., 1989), mononuclear
phagocyte function (Thiem et al., 1988), NK cell activity (Raskin et
al., 1989), Tc cell activity (Holmes et al., 1989), and mitogen-and
cell-mediated immune responses (Nimmo Wilkie et al., 1992).
4.3.3 Non-mammalian species
Non-mammalian species are also used extensively for evaluating
the potential adverse effects of compounds and agents on the immune
system.
4.3.3.1 Fish
Because of their environment, fish are an excellent model for
studying the effects of water-and sediment-borne pollutants. There are
several other good reasons for studying immunotoxicity in fish: many
of their diseases are related to environmental quality, various
environmental pollutants have immunotoxic potential, and many of the
diseases have an immune component. Moreover, there is concern about
the health status of aquatic ecosystems in relation to pollution, and
fish will be useful target species for developing biomarkers (see
box). Fish are easy to obtain, there is an extensive body of
knowledge, and their economic interest (aquaculture) facilitates the
finding of research resources. At present, immunotoxicology in fish is
not as sophisticated as that in mammals. Screening and functional
tests are being developed in the laboratory but cannot yet be applied
in the field.
A wide range of species is used for field and laboratory studies.
The choice of species depends on its biology (migratory or local,
marine or freshwater; sediment-dwelling or pelagic) and on experience
in the laboratory. A lack of consistency, e.g. becuse of a limited
number of species (as in mammalian immunotoxicology) makes this field
of research diffuse and extended and may result in limited progress;
nevertheless, a variable or consistent effect over a variety of
species is certainly a valuable observation. Some species seem to be
preferred, such as trout, salmon, and carp, which are practical, owing
to their size, for sampling blood and tissues for laboratory studies.
Smaller species, such as guppies (Poecilia reticulata) and medaka
(Oryzias latipes), have secured a niche in aquatic toxicology owing
to the ease of husbandry and relatively low cost; moreover, because of
their small size, whole animals can be used for histopathological
examination (Wester & Canton, 1991), but their application in
immunotoxicology may be limited because of difficulty in obtaining
adequate blood and tissue samples. For studies of saltwater species,
bottom-dwelling flatfish are commonly used in field studies and, to a
certain extent, in studies of mesocosms and in the laboratory. In
Europe, the flounder (Platichthys flesus) and dab (Limanda limanda)
are popular target species since they are susceptible to certain
recognizable diseases and are commonly available.
A compehensive variety of parameters is listed by Anderson (1990)
and Weeks et al. (1992). A modified set based on those lists and
assays used in rodent immunotoxicology is presented in the box above
and classified as tier 1 (screening tests) and tier 2 (functional
assays). Parameters commonly mentioned in the literature are discussed
below.
Blood cell counts and differential counts: As leukocytes play a
major role in specific and nonspecific humoral and cellular immune
responses, this parameter is used as a measure of the status of the
defence system, in particular in tier 1 testing. It is relatively easy
to test blood samples drawn from live animals, but many environmental
factors unrelated to defence may modify leukocyte status (Anderson,
1990). The use of monoclonal antibodies directed against individual
cell types may improve their identification (Bly et al., 1990; Van
Diepen et al., 1991). Another possible parameter is the haematocrit;
however, it has no known specificity for any immune function, although
it may be considered as a general indicator of stress.
Candidate biomarkers for immunotoxicity in fish
Tier 1: Screening tests
* Conventional haematology, including total and differential blood
cell counts, surface markers (flow cytometry), and macrophage
density and morphology: easy, nonspecific
* Serum immunoglobulin concentrations in naive (unstimulated) fish:
easy, limited specificity
* Lymphoid organ weight (mainly spleen, occasionally thymus):
impracticable
* Histopathology of the thymus, spleen, and kidney: possible, can
be specific
Tier 2: Functional assays
* Humoral immune response (agglutination, enzyme-linked
immunosorbent assay): possible, can be specific
* Cellular immune response (allograft rejection in scale, skin, or
eye): possible, can be specific
* Macrophage functions (phagocytosis, bacterial killing, migration,
chemiluminescence): limited specificity
* Host resistance (bacterial infections): possible, can be
specific, relevant
Nonspecific defence: Other indicators of nonspecific defence
have been proposed as indicators of immunological stress. These
include acute-phase proteins (Fletcher, 1986), the levels of which
appear to be stress hormone-dependent; and lysozyme and ceruloplasmin
activity, which are reduced in carp exposed to trichlorphon in vivo
(Siwicki et al., 1990).
Morphology: The spleen is easy to excise and weigh in animals
of adequate size and could thus serve as a biomarker, although it is
not commonly reported in the literature. One reason may be that a
major and variable portion of the spleen consists of storage blood or
erythropoietic tissue (Fänge & Nilsson, 1985); the lymphoid tissue is
poorly developed and is mainly associated with melanomacrophage
centres (Zapata, 1982, 1983; Fänge & Nilsson, 1985; Van Muiswinkel et
al., 1991); and, after immunization, only a small proportion of the
plaque-forming cells is found in the spleen, in contrast to the kidney
pronephros (Van Muiswinkel et al., 1991). The role of the spleen in
most fish species thus seems to be limited, although melanomacrophage
centres are abundant.
Experimental immunotoxicology in mammals has demonstrated that
the weight of the thymus, a primary and exclusively immunological
organ, is a sensitive indicator of thymic effects. This parameter is
not commonly used in fish. One reason is that the thymus has a complex
location in some species, which makes clean dissection nearly
impossible. Other reasons are inconsistencies in thymic morphology,
histopathology, and morphometry (Ghoneum et al., 1986; Wester &
Canton, 1987). The latter paper described studies in which guppies
exposed to TBTO showed dose-dependent atrophy of the thymus, as seen
in rats (Krajnc et al., 1984). Both species also showed a concomitant
increase in 'neutrophils', which suggests functional compensation.
This response could not be reproduced in medaka (Wester et al., 1990),
stickleback, or flounder (P. Wester, unpublished results), probably
indicating species specificity. Thymic lymphocyte function can also be
tested in vitro.
Macrophage function tests: Macrophages are an important cell
population for both specific (antigen processing and presentation) and
nonspecific (phagocytosis and destruction) defence. They are
considered to be a relatively primitive defence mechanism and are
therefore of major importance to lower animals (Ratcliffe & Rowly,
1981). Much effort has been devoted to establishing macrophage
parameters as biomarkers for immune effects in fish; a possible reason
for this preference is the fact that these cells are fairly easy to
obtain, e.g. by peritoneal washing or removal of kidney pronephros,
and many function tests do not require sophisticated techniques or
species-specific reagents or markers (Mathews et al., 1990). The tests
include determination of chemotaxis, phagocytosis, pinocytosis, and
chemiluminescence. Zelikoff et al. (1991) studied the applicability of
trout peritoneal macrophages for immunotoxicology, stressing the need
for systematic baseline information. In addition to the tests listed
above, they studied the morphology and spread of resident and
stimulated peritoneal macrophages and concluded that these cells share
many morphological and functional properties with their mammalian
counterparts and may thus be useful indicators in immunotoxicology.
Many case studies in fish species have been published, demonstrating
the sensitivity of one or more parameters to chemicals, including PAHs
(Weeks & Warinner, 1986; Zelikoff et al., 1991) and pentachlorophenol,
in vitro (Anderson & Brubacher, 1993).
Melanomacrophage centres: Melanomacrophage centres, or
macrophage aggregates, are widely distributed throughout the fish
body, in particular in spleen, liver, and kidney. They are composed of
clusters of swollen, rounded cells (macrophages) that stain pale-tan
to black. This parameter must be determined histopathologically. Their
occurrence and morphology have been described (Agius, 1985), but their
function is not yet fully understood. The presence of pigments
(haemosiderin, lipofuscin, and ceroid) indicates storage of effete
biological material (erythrocytes, biomembranes) (Wolke, 1992). The
melanin present may be a generator of the bactericidal hydrogen
peroxide (Roberts, 1975), and the presence of antigens indicates a
role in immune reactions, e.g. antigen presentation. An increase in
melanomacrophage centres can be found with age and after stress
(Blazer et al., 1987), as confirmed in field studies (Vethaak &
Wester, 1993). Moreover, a large number of relatively small, pale
centres was seen in animals caught in late winter, when conditions are
more stressful, including spawning with associated migration and
starvation (Vethaak & Wester, 1993). The presumption that the small
size and pale appearance are indicators of recent development is
supported by the observation that in liver tumours composed of
relatively young, fast-growing tissue melanomacrophage centres are
usually absent or definitely smaller. As a consequence, when these
centres are used as general parameters of stress, the study groups
must be matched for age.
Because they are characteristic for fish and because of the
multiplicity of their functions, these structures deserve special
attention in the context of defining biomarkers for immunotoxicity. In
addition, they are easy to monitor, since they do not require special
preparation other than routine histological procedures, including
morphometry. Since melanomacrophage centres can be considered
primitive analogues of the mammalian lymph follicle (Payne & Fancey,
1989), it has been suggested that their presence indicates immune
capacity or function, although their role in this context has not yet
been established and the implications of a change in this parameter
for the integrity of the defence systems remains unclear. The density
of melanomacrophage centres in liver or spleen has been successfully
correlated with environmental sediment (Payne & Fancey, 1989) and
along a gradient of pollution in the North Sea (Bucke et al., 1992).
Other studies have reported an increase in melanomacrophage centre
density after contact with chemical contaminants (Blazer et al., 1987;
Secombes et al., 1992), which may indicate accumulation of cytotoxic
waste or immune stimulation.
At present, macrophage function and melanomacrophage centres are
the most widely used and promising indicators of the effects of
environmental stress (Blazer et al., 1987). Their relationship to
other components of the immune system remains to be clarified,
however, in tests with immunotoxicants.
Humoral immune response: Determination of circulating
immunoglobulin levels in serum is useful for testing the net result on
an immunological pathway in vivo. The response can be measured in
'naive' animals (total immunoglobulin) or after exposure to an
antigen, e.g. to verify the efficacy of vaccination in aquaculture.
Sheep red blood cells can be used as a standard antigen, and the
immune response can be measured by agglutination tests. ELISA tests,
which are sensitive and specific, can also be used (Arkoosh &
Kaattari, 1990). A related test is the haemolytic plaque assay which
identifies antibody-producing cells (splenic lymphocytes) (Anderson,
1990), but which has been used to only a limited extent in fish.
Specific lymphocyte stimulation tests: Functional tests widely
used in mammalian immunotoxicology, in which lymphocytes are
stimulated in vitro by exposure to mitogens such as
lipopolysaccharide, phytohaemagglutinin, and concanavalin A, can also
be used in fish. Proliferation is monitored by measuring the
incorporation of 3H-thymidine into DNA. The test is not antigen-
specific but provides information on the capacity of the entire B
(lipopolysaccharide) or T (phytohaemagglutinin, concanavalin A) cell
population. It is used to only a limited extent in fish
immunotoxicology, although Faisal & Hugget (1993) gave an elegant
demonstration of significant suppression of this parameter in spot
(Leiostomus xanthurus) under field conditions; this was shown to be
related to site and pollution in controlled laboratory experiments.
Specific cellular immune responses: Tests described in the
literature to measure cellular immune responses are scale or skin
allograft rejection, a relatively simple test (Zeeman & Brindley,
1981), and eye allograft rejection (Khangarot & Tripathi, 1991);
delayed rejection was seen in carp after exposure to copper. These
tests are applied to only a limited extent.
The tests described above are mainly tier 2 tests. The tests most
often used in immunotoxicology, however, are those for host resistance
(challenge by infections or tumours). The results of such tests are
rarely reported in the literature and have not been validated. For
ultimate proof of immunotoxicity, all phases of a test (maintainence,
exposure, and infection) must be conducted under strictly controlled
laboratory conditions. When suitable (often species-specific)
pathogens are standardized, such tests are valuable and necessary for
estimating the practical consequences of suspected immunotoxicity.
Although the incentive for undertaking immunotoxicological studies in
fish is usually epidemiological observation of a suspected toxic
component, ultimate challenge experiments must be carried out before a
final conclusion about immunotoxic mechanisms can be drawn.
More emphasis has been given to the development of biomarkers
than to their application in the field, for several reasons, including
the lack of specificity and the lack of association between effects at
the level of the biomarker and the population (Mayer et al., 1992).
Some comments on and some needs in this field are as follows:
* Immunological biomarkers in fish have great potential: many have
not yet been fully explored, probably owing to practical
limitations of lack of specificity and predictivity.
* The number of animal species should be limited in order to
concentrate research, which often requires species-specific
knowledge and reagents. Standardization could be achieved by
choosing well-defined inbred strains of fish (e.g. carp or
trout).
* A tiered approach is highly recommended for obtaining knowledge
on the specificity of the biomarker.
* More knowledge is needed on the epidemiology, mechanisms, and
etiology of diseases in fish, and particularly the predictive
value of immune parameters and the influence of hormesis.
* In terms of relevance for the organism, a test that monitors the
net result of a cascade of reactions (e.g. specific antibody
production, host resistance) is more predictive than a single,
nonspecific cell parameter (e.g. macrophage activity in
vitro).
* In identifying potential biomarkers for immunotoxicity, evidence
should be available that the levels tested in the laboratory are
relevant for field conditions and that the effect is directly
related to the immune system.
4.3.3.2 Chickens
Another non-mammalian species that has been studied extensively
with regard to the structure and function of its immune response is
the chicken. It is therefore not surprising that the chicken has
emerged as the predominant avian model for assessing compounds for
potential immunotoxicity. Humoral responses to different antigens have
been assessed routinely (Lerman & Weidanz, 1970; Marsh et al., 1981).
The weights of the thymus, spleen, and bursa of Fabricius have been
used, in combination with decreased antibody responses in vivo and
lymphocyte responses to phytohaemagglutinin and concanavalin A
in vitro (Eskola & Toivanen, 1974). Graft-versus-host and
cutaneous basophil hypersensitivity have also been used to detect
immunosuppression in chickens (Dietert et al., 1985). The availability
of chicken cell lines (Sung et al., 1992) will facilitate studies of
the mechanisms of action of compounds on the immune responses of this
avian model.
4.4 Approaches to assessing immunosuppression in vitro
The complexity of the immune system and the requirement of many
agents for metabolism and distribution to produce an immunotoxic
response has resulted in the almost exclusive use of animal models
in vivo for immunotoxicity assessment. Culture systems have been
used extensively, however, to study the mechanisms by which agents
produce immunosuppression.
Since most of the assays used for assessing immunotoxicity are
ex-vivo/in-vitro tests, they are easily adapted to completely in-vitro
assays for assessing immunosuppression. The direct addition of
compounds in various assays, including those involving NK cells,
lymphocyte proliferation, mixed leukocytes, and Tc lymphocytes, has
been used to determine the mechanisms by which compounds alter the
immune response at the cellular and subcellular level. Similarly, the
action of benzene and its metabolites on bone marrow has been studied
extensively in vitro (Gaido & Wierda, 1987), and the effects of TCDD
on thymocytes have been well studied in thymic epithelium co-cultures
(Greenlee et al, 1985). One of the most useful in-vitro assays for
studying immunosuppression is the T-dependent antibody response to
sheep erythrocytes. This assay, also known as the Mishell-Dutton assay
(Mishell & Dutton, 1967), has been used extensively in studying the
cellular target of immunotoxicants. It is the in-vitro counterpart of
the in-vivo plaque-forming cell assay, but sensitization with sheep
erythrocytes takes place in splenic cell culture and the plaque
response is measured on day 5 after addition of the erythrocytes. The
Mishell-Dutton assay has been used to study the structure - activity
relationships of various immunosuppressive compounds (Kawabata &
White, 1987; Davis & Safe, 1991). Since T cells, B cells, and
macrophages are needed for the response and an adverse effect on any
of these cell types can produce immunomodulation, it has proved
to be a sensitive assay for evaluating compounds in vitro for
immunosuppressive activity. Furthermore, since the various cell types
that participate in the response can easily be separated, individually
treated, and then reconstituted in the culture system, it is an
excellent assay for determining which cell type is adversely affected
by the compound. Using this approach, White & Munson (1986)
demonstrated that asbestos suppresses the response by affecting
macrophages; Shopp & Munson (1985) showed that the primary action of
phorbol ester on the antibody response occurs through an effect on B
cells; and Johnson et al. (1987) found that N-nitrosodimethylamine
affects primarily B cells.
As indicated above, one of the limitations of in-vitro systems is
that exogenous metabolic activation systems are often required. While
lymphocytes can metabolize some compounds, such as benzo[ a]pyrene,
to active metabolites (Ladics et al., 1992), other potent
immunosuppressive compounds such as cyclophosphamide require a
metabolic activation system. Such preparations usually consist of a
9000 x g supernatant of liver (S9). Using S9 preparations, Tucker &
Munson (1981) showed that cyclophosphamide could be activated to an
immunosuppressive form in vitro. Similarly, naphthalene could be
metabolized to an immunosuppressive metabolite (Kawabata & White,
1990). An alternative approach to the S9 activation system is a
hepatocyte co-culture system, which has been shown to be capable of
activating several parent compounds to their immunosuppressive
metabolites (Yang et al., 1986).
Predictive in-vitro systems based on immune cells of human origin
are particularly attractive, given the uncertainties of extrapolating
the results of experimental studies to humans and the accessibility of
immune cells in human peripheral blood. Although many of the immune
cells obtained from human blood are immature forms, the large numbers
and diverse populations (i.e. polymorphonuclear leukocytes, monocytes,
NK cells, T cells, and B cells) that can be obtained provide an
attractive alternative or adjunct to conventional studies in
experimental animals. As a consequence, a number of studies have been
conducted to compare the functional response of human and rodent
lymphocytes to putative immunosuppressive agents in vitro (Cornacoff
et al., 1988; Luo et al., 1992; Wood et al., 1992; Lang et al., 1993).
Although these studies were hampered by the lack of assays to assess
primary antigen-specific immune responses in human lymphocytes, a
relatively good interspecies correlation has been observed in the
limited responses available. Furthermore, several of these assays have
been successfully modified to include co-culture with primary
hepatocytes (Kim et al., 1987) to allow for chemical metabolism.
4.5 Future directions
4.5.1 Molecular approaches in immunotoxicology
A promising avenue for early detection of immunotoxicity may be
measurement of the expression of various interleukins. Cytokines are
involved both in regulation of the immune system and in pathological
phenomena, hence alterations in their pattern of expression may be
early indicators of immunotoxicity. Such testing can be done at the
level of mRNA expression, on mRNA extracted from lymphoid tissue taken
from exposed animals, or in tissue sections, so that the alterations
can be evaluated in the context of morphological indications of the
toxic effects. The signal of the cytokine that is being tested must
therefore be strong enough to be picked up in material from exposed
animals whose immune system has not received other stimuli, i.e.
sensitization or infection. This may not be true for all cytokines;
ex-vivo stimulation of cells that are part of the immune system may be
necessary, although the tests then become more laborious and must in
fact be considered functional assays, like tests for mitogen
responsiveness.
Very sensitive analysis can be done with the semiquantitative
polymerase chain reaction, which is a powerful technique for
elucidating early kinetic changes of cytokine expression, before
translation and secretion (Saiki et al., 1985). In addition, since
immunosuppressive agents can enhance or inhibit the ultimate
production and secretion of cytokines at various stages such as
transcription, the splicing of mRNA, translation of mRNA into
polypeptides on ribosomes, post-translational processing, and
secretion, potential molecular targets can be dissected by such
techniques. Several other molecular approaches may be used, including
northern blotting, dot-slot blotting, in-situ hybridization, and
antisense oligonucleotides for inhibiting the translation of specific
mRNAs.
4.5.2 Transgenic mice
The development of molecular genetic techniques has allowed not
only the isolation and analysis of specific genes but also the
manipulation of embryonic genes. Transgenic technology can be used in
immunology to generate mice that lack virtually any genetic control
mechanism or specific cell subpopulations. As a consequence, complex
systemic responses can be dissected into individual components, and
the mechanism by which immunosuppressive agents exert their affects
can be better understood. Two strategies are used to induce genetic
aberrations in transgenic mice (Bernstein & Breitman, 1989). One
involves the introduction of genes that produce toxins, such as
diphtheria toxin or the A subunit of ricin, into targeted cell
subpopulations. The second strategy involves the thymidine kinase
(tk) gene from Herpes simplex virus: When certain nucleotide
analogues are administered and are metabolized exclusively by viral
thymidine kinase, the metabolites are lethal only to cell
subpopulations that express the tk gene. Both approaches are
inducible systems for killing cells in vivo. Although gene ablation
techniques can be used to generate mutant animals that lack specific
cells in vivo, a small proportion of cells appeared to escape from
targeted cell death in virtually every study using bacterial toxins or
viral tk genes. While this may cause problems in determining the
qualitative roles of ablated cell populations, these techniques hold
promise for understanding the selective toxicity of drugs and
environmental agents on the immune system.
Other promising avenues are the use of animals transgenic with
respect to certain specificities of the TCR. If a gene that encodes
for a certain antigen specificity is introduced into the genome, that
specificity may be the only one that is expressed by the T cells. The
effects of immunotoxicants that affect the (positive and/or negative)
selection process that takes place in the thymus could be studied
elegantly with such models, when either undesired specificities (which
should be negatively selected) or desired specificities (which should
be positively selected) are introduced.
4.5.3 Severe combined immunodeficient mice
Another approach that may warrant further exploration is the use
of severe combined immunodeficient CB-17 scid/scid (SCID) mice
grafted with human immune cells. Xenogeneic lymphoid cells and/or
tissues can be successfully transferred to SCID mice (McCune et al.,
1988; Namikawa et al., 1990; Barry et al., 1991; Greiner et al., 1991;
Surhe & Sprent, 1991). SCID mice have been grafted with human fetal
lymphoid tissue in order to study human haematopoiesis (McCune et al.,
1988) or with human peripheral blood lymphocytes to allow production
of human immunoglobulins, including secondary antibody responses
(Mosier, 1990). SCID mice have also been used to study autoimmunity
and potential antiviral therapeutics. While these animal models still
have limitations (Pollock et al., 1994), they may ultimately provide
predictive models for examining potential immunosuppressive agents.
In particular, SCID mice co-implanted with human fetal thymus and
liver tissue fragments (SCID/hu mice) offer the possibility of
studying the human thymus in vivo in an isolated xenogeneic
environment (McCune et al., 1988; Namikawa et al., 1990) and the
effects of immunotoxicants on these grafts. This system is
particularly interesting with regard to those immunotoxicants for
which the thymus is one locus of action. The placement of human fetal
thymus under the SCID mouse renal capsule, followed by an intravenous
injection of fetal liver cells (McCune et al., 1988), and
co-implantation of human fetal liver and fetal thymus under the renal
capsule of SCID mice (Namikawa et al., 1990) have resulted in
reconstitution of SCID/hu mice; the fetal thymic implants increased in
size, and were found to be vascularized. The architecture and
antigenic distribution of these thymic grafts were virtually
indistinguishable from those of normal, age-matched human thymus.
Human stem cells were found to home to and differentiate within the
grafted human thymus, and phenotypically mature and functional human
T cells were found in the peripheral circulation of these mice (McCune
et al., 1988; Krowka et al., 1991; Vandekerckhove et al., 1991). As
such, the SCID/hu model can be helpful in immunotoxicological research
on the human thymus. When data obtained in experimental animals are
extrapolated to the human situation, a 'control' model, between the
SCID/hu mouse model and the intact laboratory animal (rat), is
desirable in order to test for possible differences in thymic
behaviour, because of its location under the kidney capsule: Thymic
blood flow and therefore the toxicokinetic behaviour of the thymus may
differ. For this reason the SCID/ra model was developed, by implanting
rat fetal thymus and liver tissue fragments under the SCID mouse renal
capsule. The outcomes of exposure of rats and SCID/ra mice can be
compared and the influence of thymus location and mouse metabolism on
extrapolation from SCID/hu to humans can then be determined.
Implantation of fetal rat thymus and liver tissue yields thymic
grafts that are virtually indistinguishable from normal, age-matched
rat thymus (De Heer et al., 1993). After implantation of rat fetal
thymus and liver tissue, the thymic grafts increase considerably in
size. Histologically, the SCID/ra thymic graft bears a close
resemblance to normal rat thymus, and the (immuno)histology of the
SCID/hu and SCID/ra mouse thymic grafts is comparable. Differences are
found, however, in peripheral reconstitutions of SCID/hu and SCID/ra
mice: Whereas large numbers of circulating donor rat T cells are found
in the blood and peripheral lymphoid organs of SCID/ra mice, only a
small number of donor T cells are found in the SCID/hu. This implies
that the data for extrapolating immunotoxic data from rats to humans
must be confined to thymic effects. With this restriction in mind, the
outcome of experiments with SCID/hu and SCID/ra mice can be used to
compare the sensitivity of the human and rat thymus and can thus yield
important information for the process of human risk assessment.
4.6 Biomarkers in epidemiological studies and monitoring
There is a difference between assays of the immune system and
biomarkers. Many validated tests can indicate alterations to the
immune system, including its function, so that most assays can be
helpful for hazard identification. Not every validated assay of the
immune system is a biomarker, however. The IPCS (1994a) definition of
a biomarker is one that indicates exposure (and is specific for
exposure), indicates susceptibility to adverse effects, and/or is
predictive of disease associated with exposure. Biomarkers should be
used to characterize risk due to exposure, on the basis of
identification of the hazard.
Within this strict definition, it is clear that not many
biomarkers are available for immunotoxicity (as is true for other
systems), especially for assessing immunotoxicity or individual
susceptibility to immunotoxicity. Some assays may be useful in
epidemiological studies. In any event, more epidemiological studies
are needed to obtain a better view of the usefulness of biomarkers for
detecting immunotoxic events and hence the possible health risks that
may be associated with exposure to immunotoxicants.
4.7 Quality assurance for immunotoxicology studies
In many countries, studies to support the safety of a compound or
drug must be conducted in accordance with the requirements for 'good
laboratory practice' of the agency that is evaluating the material.
Immunotoxicological studies conducted to support the safety of a new
drug or chemical should follow at least the 'spirit' of good
laboratory practice. The OECD has published their principles of good
laboratory practice, with supporting publications on their
application, and these have been adopted into legislation in a number
of countries. IPCS (1992) has published a monograph, Quality
Management for Chemical Safety Testing, covering the important
aspects of good laboratory practice in a nonregulatory context and
quality control of chemical analyses.
In the United States, good laboratory practice for conducting
nonclinical laboratory studies for submission to the Food and Drug
Administration has been detailed. The standards for studies on
pesticides submitted to the Environmental Protection Agency were also
published, as were the procedures to be followed in conducting studies
submitted on compounds covered by the Toxic Substance Control Act.
Each of these sets of standards is periodically updated by the
respective agencies, and studies must be conducted in accordance with
the most recent updates. While there are some differences in the
wording of the standards, they are generally similar.
Good laboratory practice includes written protocols for
evaluating potential immunotoxicants and the establishment of standard
operating procedures for assays. Each laboratory must run the assays
frequently enough to establish historical control values, and
the results of any study conducted to evaluate a compound for
immunotoxicity must be judged in the context of the historical control
values for the laboratory and appropriate controls. Incorporation of
positive control compounds in the study design provides additional
confidence that the assays are being conducted correctly, particularly
when the tested compound shows no effect.
The selection of assays to be used in evaluating compounds for
immunotoxicity remains a subject of active discussion. Other parts of
this document address this issue in detail. Regardless of which assays
are used, however, they must be standardized and be recognized as
validated and meaningful. Significant advances have been made in the
standardization and harmonization of assay procedures for assessing
immunotoxicity, mainly as a result of the willingness of leading
laboratories in the field to share their standard operating procedures
openly with other laboratories. Published papers and books on
immunotoxicity test methods also contribute to the standardization
process. As a result of studies by Luster et al. (1988), the assays
used by the NTP for evaluating potential immunotoxicants have been
accepted as validated assays in mice.
4.8 Validation
An important requirement of tests for evaluating immunotoxicity
is that they be validated. While there is no agreed definition of
validation, tests must meet certain requirements. In toxicology,
validation is the process by which the reliability and relevance of a
test to identify human health risk is established (Balls et al.,
1990). A flow diagram of a proposed validation process and its end-
point, the acceptance of a method by regulatory authorities for
submission of toxicological data, is presented in Figure 36.
Four parameters must be considered in determining the validity of
a testing method: specificity, sensitivity, accuracy, and precision.
Specificity is based on the rate of false-positive results generated.
Sensitivity is determined by the ability to identify true-positive
results. These two parameters determine the level of predictability or
relevance. In order to determine specificity and sensitivity, the
method is evaluated with a set of compounds of known positive and
negative immunosuppressiveness. This approach was used by the NTP in
Yoshida & Streilin (1990) for the UVB-sensitive phenotype, Taylor et
al. (1994) reported that 66% of individuals who have a history of
herpes lip lesions provoked by exposure to sunlight were sensitive to
UVB, in comparison with 40-45% of the general population and 92% of
skin cancer patients. Exposure of immunosuppressed patients to
sunlight can increase the incidence of viral warts caused by
papillomavirus (Boyle et al., 1984; Dyall-Smith & Varigos, 1985). It
is also known that UVR exacerbates the clinical course of systemic
lupus erythematosus, an autoimmune disease (Epstein et al., 1965). The
effects of UVR on the risk of infectious disease have been reviewed by
Koren et al. (1994). In contrast, certain infectious diseases appear
to be cured by sunlight; the most notable are erysipelas (Giannini,
1990), a skin disease caused by Streptococcus, and skin lesions
caused by Herpes zoster virus.
2.4.8 Others
A large number of therapeutic drugs and drugs of abuse may also
alter human immune function in humans. These include:
Therapeutic agents
Alkylating agents
Nitrogen mustards: cyclophosphamide, L-phenylalanine mustard,
chlorambucil
Alkyl sulfonates: busulfan
Nitrosoureas: carmustine (BCNU), lomustine (CCNU)
Triazenes: dimethyltriazenoimidazolecarboxamide (DTIC)
Anti-inflammatory agents
Aspirin, indomethacin, penicillamine, gold salts
Adrenocorticosteroids: prednisone
Antimetabolites
Purine antagonists: 6-mercaptopurine, azathioprine, 6-thioguanine
Pyrimidine antagonists: 5-fluorouracil, cytosine arabinoside,
bromodeoxyuridine
Folic acid antagonists: methotrexate (amethopterine)
Natural products
Vinca alkaloids: vinblastine, vincristine, procarbazine
Antibiotics: actinomycin D, adriablastine, bleomycin, daunomycin,
puromycin, mitomycin C, mithramycin
Antifungal agents: griseofulvin
Enzymes: L-asparaginase
Cyclosporin A
Estrogens: diethylstilbestrol, ethinylestradiol
Substances of abuse
Ethanol
Cannabinoids
Cocaine
Opiates
Adapted from Dean & Murray (1990)
3. STRATEGIES FOR TESTING THE IMMUNOTOXICITY OF CHEMICALS IN ANIMALS
3.1 General testing of the toxicity of chemicals
The fact that substances used in various aspects of modern life
can be simultaneously beneficial and harmful to human life creates a
legislative and regulatory dilemma. In order to balance the desire to
use the many new substances that enter the market every year and the
economic benefit that is associated with their use on the one hand
with the health and safety of the population on the other is an
important challenge to governmental authorities. Legislative and
regulatory efforts to minimize and control the risk of adverse effects
on human health has resulted in a system for assessing and classifying
the potential risk of exposure to chemicals. Potential adverse effects
can be assessed in studies with experimental animals. In conducting
such studies, attention must be paid to ethical and regulatory
requirements for animal welfare and to good laboratory practice.
In assessing and evaluating the toxic characteristics of a
chemical, its oral toxicity may be determined once initial information
has been obtained by acute testing. Toxicity is routinely tested
according to guidelines, one of which is guideline No. 407 of the
Organisation for Economic Co-operation and Development (OECD) for
testing of chemicals, the 'Repeated Dose Oral Toxicity - Rodent:
28-day or 14-day Study' (Organisation for Economic Co-operation and
Development, 1981). This guideline has undergone three revisions, the
most recent of which (January 1994) includes parameters of
immunotoxicological relevance (see Table 7). Depending on the amount
of a chemical to be produced and the expected exposure to the
chemical, testing according to this guideline may in many countries
provide the only information on its safety, including potential
toxicity to the immune system. The information yielded by this type of
testing is therefore decisive in determining how chemicals are used in
society. Subsequent guidelines have been defined for use in follow-up
studies if more exposure is expected or if there is a suspicion of
toxicity on the basis of structural analogy with other known
compounds. These include 90-day studies of oral toxicity, long-term
studies, and studies of reproductive effects. Although such guidelines
include more parameters of the immune system than OECD test guideline
No. 407, detection of potential immunotoxicity may still not be
adequately addressed. In practice, the best procedure is to carry out
appropriate tests on a case-by-case basis, at increasing levels of
complexity, when concerns are raised in more general toxicological
studies.
Table 7. Parameters of OECD Test Guideline 407 that relate to the immune system
Current Guideline 407 Proposal for updating Proposal for updating Proposal for updating
(adopted 21 May 1981) Guideline 406 Guideline 407 (revision of Guideline 407 (revision of
(February 1991) January 1993) January 1994)
Total and differential Total and differential Total and differential Total and differential
leukocyte count leukocyte count leukocyte count leukocyte count
Weight of spleen and thymus Weight of spleen and thymus Weight of spleen and thymus
Histopathology of spleen Histopathology of spleen, Histopathology of spleen, Histopathology of spleen, thymus,
thymus, lymph node, and thymus, lymph nodes (one lymph nodes (one relevant to the
bone marrow relevant to route of route of administration and a
administration and a distant distant one to cover systemic
one to cover systemic effects), effects), small intestine (including
and bone marrow Peyer's patches), and bone marrow
Histopathology of target Histopathology of target Histopathology of target Histopathology of target
organs organs organs organs
OECD, Organisation for Economic Co-operation and Development
An insight into the type of information that the OECD test
guideline No. 407 yields is given below. In this test, the substance
is administered orally in daily graduated doses to groups of
experimental animals, one dose per group for 28 or 14 days. The
preferred rodent species for this test is the rat, although others may
be used. At least three doses and a control should be used. The
highest dose should result in toxic effects but not produce an
incidence of fatalities which would prevent a meaningful evaluation;
the lowest dose should not produce any evidence of toxicity and should
exceed a usable estimate of human exposure, when available. Ideally,
the intermediate dose level(s) should produce minimal observable toxic
effects.
In compliance with the guideline, the following examinations are
carried out:
(a) haematology, including haematocrit, haemoglobin concentration,
erythrocyte count, total and differential leukocyte count, and a
measure of clotting potential such as clotting time, prothrombin
time, thromboplastin time, or platelet count;
(b) clinical biochemistry of blood, including blood parameters of
liver and kidney function. The selection of specific tests is
influenced by observations on the mode of action of the
substance. Suggested determinations are: calcium, phosphorus,
chloride, sodium, potassium, fasting glucose, serum alanine
aminotransferase, serum aspartate aminotransferase, ornithine
decarboxylase, gamma-glutamyl transpeptidase, urea nitrogen,
albumin, blood creatinine, total bilirubin, and total serum
protein. Other determinations that may be necessary for an
adequate toxicological evaluation include analyses of lipids,
hormones, acid-base balance, methaemoglobin, and cholinesterase
activity. Additional clinical biochemistry may be used when
necessary, to extend the investigation of any observed effects.
(c) pathology, including gross necropsy, with examination of the
external surface of the body, all orifices, and the cranial,
thoracic, and abdominal cavities and their contents. The liver,
kidneys, adrenal glands, and testes are weighed wet as soon as
possible after dissection to avoid drying. Liver, kidney, spleen,
adrenal glands, heart, and target organs showing gross lesions or
changes in size are preserved in a suitable medium for possible
future histopathological examination. Histopathological
examination is performed on the preserved organs or tissues of
the group given the high dose and the control group. These
examinations may be extended to animals in other dosage groups,
if considered necessary to further investigate changes observed
in the high-dose group.
A properly conducted 28- or 14-day study will provide information
on the effects of repeated doses and can indicate the need for
further, longer-term studies. It can also provide information on the
selection of doses for longer-term studies.
It is clear that the 1994 guideline is not suitable for adequate
assessment of the potential adverse effects of exposure to a test
chemical on the immune system, since the immunological parameters are
restricted to total and differential leukocyte counts and the
histopathology of the spleen. An evaluation of this test (Van Loveren
& Vos, 1992) indicated that over 50% of the immunotoxic chemicals in a
series of about 20 chemicals would not have been identified as such if
the tests had strictly adhered to the guideline. In fact, it is even
doubtful if chemicals indicated as immunotoxic only on the basis of
guideline No. 407 would in practice have been picked up: For instance,
in a toxicological experiment, a small but significant change in the
percentage of basophilic leukocytes would by itself probably not be
considered to be biologically relevant in the absence of any other
parameter to suggest that an effect on the immune system might have
been present.
These data indicate that extension of OECD test guideline No. 407
is necessary in order adequately to assess potential immunotoxicity.
It is recommended that additional immunological parameters be included
in this guideline in order to increase its power (Vos & Van Loveren,
1987; Basketter et al., 1994).
Guidelines also exist for follow-up studies if greater exposure
is expected or if there is a suspicion of toxicity on the basis of
structural analogy with other compounds. In these studies, potential
toxicity to the immune system is generally addressed somewhat more
extensively than in guideline No. 407. For instance, in a 90-day study
of oral toxicity, the OECD guidelines prescribe that histopathological
examination be done on the thymus, a representative lymph node, and
the sternum with bone marrow, in addition to the spleen. Even with
these additional parameters, it is highly questionable whether
potential immunotoxicity is adequately assessed. For this purpose, a
variety of tests is available, which are described in section 4.
Depending on what is already known about the toxicity of the test
compound, different panels of tests (also referred to as tiers) are
selected for immunotoxicological evaluation. Usually, if little or no
information is available, a dose range including high doses is used;
lower, overtly nontoxic doses are chosen if some knowledge is
available about the physical and chemical properties, toxicokinetics,
structure-activity relationships, and intended use.
3.2 Organization of tests in tiers
Immunotoxicity can be assessed in a tiered approach (Luster et
al., 1988; Van Loveren & Vos, 1989). Generally, the objective of the
first tier is to identify potentially hazardous compounds (hazard
identification). If potential immunotoxicity is identified, a second
tier of tests is carried out to confirm and further characterize the
immunotoxicity.
Various approaches have been suggested for evaluating the
potential immunotoxicity of compounds. Most are similar in design, in
that the first tier is usually a screen for immunotoxicity and the
second tier consists of a more specific confirmatory set of studies or
in-depth mechanistic studies. Since the use of the tiers is usually
tailored to the goals or objectives of the organization that proposes
them, they differ in respect of the specific assays recommended and
the organization of the assays into tiers.
3.2.1 United States National Toxicology Program panel
The tiers and assays originally adopted by the NTP, based on the
proposed guidelines for immunotoxicity evaluation in mice reported by
Luster et al. (1988), are shown in Table 8.
Table 8. Panel adopted by the US National Toxicology Program for detecting immune
alterations after exposure of rodentsa to chemicals or drugs
Parameter Procedures
Screen (Tier I)
Immunopathology Haematology: complete and differential blood count
Weights: body, spleen, thymus, kidney, liver
Cellularity: spleen
Histology: spleen, thymus, lymph node
Humoral immunity Enumerate IgM antibody plaque-forming cells to
T-dependent antigen (sheep red blood cells)
Lipopolysaccharide mitogen response
Cell-medicated Lymphocyte blastogenesis to mitogens
immunity (concanavalin A)
Mixed leukocyte response to allogeneic leukocytes
Nonspecific immunity Natural killer cell activity
Comprehensive (Tier II)
Immunopathology Quantification of splenic B and T cell numbers
Humoral-mediated Enumeration of IgG antibody response to sheep red
immunity blood cells
Cell-medicated Cytotoxic T lymphocyte cytolysis; delayed
immunity hypersensitivity response
Nonspecific immunity Macrophage function: quantification of resident
peritoneal cells, and phagocytic ability (basal and
activated by macrophage activating factor)
Host resistance Syngeneic tumour cells
challenge models PYB6 sarcoma (tumour incidence)
(end-points)b B16F10 melanoma (lung burden)
Bacterial models: Listeria monocytogenes (mortality);
Streptococcus species (mortality)
Viral models: influenza (mortality)
Parasite models: Plasmodium yoelii (parasitaemia)
a The testing panel was developed using B6C3F1 female mice.
b For any particular chemical tested, only two or three host resistance models
are selected for examination.
In this approach, the tier 1 assay is limited; it includes assays
for both cell-mediated and humoral-mediated immunity and for innate
(nonspecific) immunity with the inclusion of NK cell assays. It also
includes immunohistopathology, which is part of the standard protocol
for studies of subchronic toxicity and carcinogenicity conducted by
the NTP. Tier II represents a more extensive evaluation and includes
additional assays for assessing effects on cell-mediated, humoral, and
innate immunity, in addition to host resistance. In this approach,
animals are usually evaluated at only one time, so that the
possibility for recovery or reversibility of immunological changes is
not evaluated. A 14-day exposure period is employed routinely;
however, 30- and 90-day exposures have been used, depending on the
pharmacokinetic properties of the chemical being tested. The dose used
in this tier system tends to be lower than those in several of the
other approaches followed. In the NTP approach, dose levels are
selected whenever possible that have no effect on body weight or other
toxicological end-points. The approach has therefore focused on
compounds for which the immune system is the most sensitive target.
This is in marked contrast to other approaches, in which the highest
dose is usually the maximum tolerated dose.
The assays that make up the NTP tier approach have undergone
various revisions, partly on the basis of an immunotoxicological
review of compounds evaluated in this tier structure (Luster et al.,
1992). The mitogen assays were first moved from tier I to tier II and
have now been dropped altogether: They were found to be insensitive
and to add little when run in conjunction with other assays that have
a proliferative component, such as the mixed leukocyte response and
the plaque assay. Furthermore, the only macrophage phagocytic assay
routinely carried out in immunotoxicological studies conducted for the
NTP is evaluation of the functional activity of the mononuclear
phagocyte system, which is an in-vivo assay for phagocytosis.
Studies by Luster et al. (1992) show that the potential
immunotoxicity of a compound can be reasonably predicted with a few
selected assays. As additional data become available, further changes
to the NTP tiers will most likely be forthcoming.
3.2.2 Dutch National Institute of Public Health and Environmental
Protection panel
The tier approach for immunotoxicological evaluation followed at
the National Institute of Public Health and Environmental Protection
(RIVM) in the Netherlands (Vos & Van Loveren, 1987) is shown in
Table 9. This approach is based essentially on OECD test guideline
No. 407, which suggests that the maximum tolerated dose be used as the
high dose in the study. As a result, significantly higher doses are
used than in the NTP approach in evaluating compounds for
immunotoxicity. Additionally, the standard exposure period is 28 days,
and the animal species routinely used is the rat instead of the mouse.
This type of testing can therefore be performed in the context of
studies in rats to determine the toxicological profile of a compound.
At least three doses should be used, the highest having a toxic effect
(but not mortality) and the lowest producing no evidence of toxicity.
Moreover, immunotoxicity tests carried out in the context of such
testing should not in any way influence the toxicity of the chemical
(e.g. immunization or challenge with an infectious agent). In the NTP
panel, the highest dose to which mice are exposed is chosen so that no
overt toxicity, i.e. changes in body weight or gross pathological
effects, is observed. As tests for immunotoxicity must be fairly
sensitive in order to preclude false negatives, the NTP tier I
includes functional assays. With a broader dose range that includes
overt toxicity, potential immunotoxicity is more likely to be
observed, without the inclusion of functional tests. If functional
assays are to be included in the first tier, those tests that require
sensitization of animals would require inclusion of satellite groups.
In OECD test guideline No. 407 for testing chemicals, none of the
other systems is approached functionally.
It has been suggested that the NK cell assay be added to tier 1 (Van
Loveren & Vos, 1992). Since the assay does not require animals to be
sensitized or challenged, the same animals can be used without
affecting other toxicological parameters, and thus an additional
satellite group of animals is unnecessary.
3.2.3 United States Environmental Protection Agency, Office of
Pesticides panel
The United States Environmental Protection Agency has proposed a
tiered approach to the evaluation of biochemical pest control agents,
which fall under the subdivision M guidelines for pesticides (Sjoblad,
1988). The proposed tiers are shown below. Tier 1 of this approach
includes functional assays for evaluating humoral immunity, cell-
mediated immunity, and innate immunity. Thus, while Tier 1 is
considered by the Agency to be an immunotoxicity screen, it is much
more encompassing than the first tier of the other approaches. By
providing options in the selection of assays for tier 1, the approach
can easily accommodate both the rat and the mouse as the laboratory
animal species used. In this approach, the tier 2 studies provide
information sufficient for risk evaluation, including information on
the time course of recovery from immunotoxic effects and host
resistance to infectious agents and tumour models. Additional
functional tests would be required if a dysfunction were observed in
tier 1 tests or if data from other sources indicated the compound
could produce an adverse effect on the immune response.
Table 9. Methods for detecting immunotoxic alterations in the rat evaluated by the
Dutch National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Parameters Procedures
Tier 1
Nonfunctional Routine haematology, including differential cell counts
Serum IgM, IgG, IgA, IgE determination; lymphoid organ
weights (spleen, thymus, local and distant lymph nodes)
Histopathology of lymphoid tissues, including mucosa-
associated lymphoid tissue
Bone-marrow cellularity
Analysis of lymphocyte subpopulations in spleen by flow
cytometry
Tier 2
Cell-medicated Sensitization to T-cell dependent antigens (e.g. ovalbumin,
immunity tuberculin, Listeria), and skin test challenge
Lymphoproliferative response to specific antigens (Listeria)
Mitogen responses (concanavalin A, phytohaemagglutinin)
Humoral Serum titration of IgM, IgG, IgA, IgE responses to
immunity T-dependent antigens (ovalbumin, tetanus toxoid,
Trichinella spiralis, sheep red blood cells) by ELISA
Serum titration of T-cell-independent IgM response to
lipopolysaccharide by ELISA
Mitogen response to lipopolysaccharide
Macrophaand Phagocytosis and killing of Listeria by adherent spleen
function and peritoneal cells in vitro
Cytolysis of YAC-1 lymphoma cells by adherent spleen
and peritoneal cells
Natural killer Cytolysis of YAC-1 lymphoma cells by non-adherent
function spleen and peritoneal cells.
Host resistance Trichinella spiralis challenge (muscle larvae counts and
worm expulsion)
Listeria challenge (spleen and lung clearance)
Cytomegalovirus challenge (clearance from salivary gland)
Endotoxin hypersensitivity;
Autoimmune models (adjuvant arthritis, experimental
allergic encephalomyelitis)
Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay
Subdivision M guidelines: proposed revised requirements by the US
Environmental Protection Agency for testing the immunotoxicity of
biochemical pest control agents
AI. Tier 1
A. Spleen, thymus, and bone-marrow cellularity
B. Humoral immunity (one of the following)
1. Primary and secondary IgG and IgM responses to antigen; or,
2. Antibody plaque-forming cell assay
C. Specific cell-mediated immunity (one of the following)
1. One-way mixed lymphocyte reaction assay; or,
2. Effect of agent on normal delayed-type hypersensitivity
response; or,
3. Effect of agent on generation of cytotoxic T-lymphocyte
response
D. Nonspecific cell-mediated immunity
1. Natural killer cell activity and
2. Macrophage function
II. Tier 2
A. Required if:
1. Dysfunction is observed in tier 1 tests
2. Tier 1 test results cannot be definitively interpreted
3. Data from other sources indicate immunotoxicity
B. General testing features:
1. Evaluate time course for recovery from immunotoxic effects.
2. Determine whether observed effects impair host resistance to
infectious agents or to tumour cell challenge.
3. Perform additional specific, but appropriate, testing
essential for evaluation of potential risks.
This Agency has also suggested that immunotoxicological screening
be conducted in evaluating conventional chemical pesticides
(subdivision F guidelines; see below); however, unlike those of
subdivision M, these guidelines are not designed as a tiered testing
scheme. If the immunotoxicity screen listed in subheading I were added
to subchronic and/or chronic studies in subdivision F, it would be a
more effective screen for immunotoxicity than is currently available.
If this proposed screen indicates that the immune system is a
sensitive target, the Agency considers that it may be necessary to
evaluate the risk for immunotoxic effects as under subheading
II. Currently, these suggestions have not been promulgated as official
guidelines or regulations.
Evaluations suggested by the US Environmental Protection Agency as
appropriate additions to Subdivision F guidelines for immunotoxicity
screening (subheading I) and possible additional data appropriate for
risk evaluation of chemical pesticides (subheading II)
I. Immunotoxicity screen
A. Serum immunoglobulin levels (e.g. IgG, IgM, and IgA)
B. Spleen, thymus, and lymph node weights
C. Spleen, thymus, and bone-marrow cellularity and cell
viability
D. Special histopathology (e.g. enzyme histochemistry,
immunohistochemistry)
E. More complete evaluation of 'premature' mortality of test
animals, as possibly related to immunosuppressive effects
II. Immunotoxicity risk evaluation
A. Host resistance to challenge with infectious agent and/or
tumour cells
B. Specific cell-mediated immune responses (e.g. mixed
leukocyte response, delayed-type hypersensitivity
response,cytotoxic T lymphocyte assays)a
C. Nonspecific cell-mediated immune responses (i.e. natural
killer cell activity, macrophage function)a
D. Time course for recovery from adverse immunological effects
a Measures of specific and nonspecific cell-mediated immune
responses that also may be considered useful in an immunotoxicity
screen
3.2.4 United States Food and Drug Administration, Center for Food
Safety and Applied Nutrition panel
The United States Food and Drug Administration is considering
testing guidelines for evaluating the immunotoxic potential of direct
food additives (Hinton, 1992). The multifaceted approach is included
in the draft revision of the Toxicological Principles for the Safety
Assessment of Direct Food Additives and Color Additives Used in Food
is (US Food and Drug Administration, 1993). The testing requirements
are based on the 'concern level' of the substance, assigned on the
basis of the available toxicological information or the substance's
structural similarity to known toxicants and on estimated human
exposure from its proposed use. A compound with high toxic potential
and high exposure would be assigned a high initial 'concern level'
(3), and one with low toxic potential and low exposure would be
assigned a low initial level (1).
In general, substances will be evaluated for immunotoxic
potential on a case-by-case basis. Two types of immunotoxicity tests
and procedures are defined in this approach: Type 1 tests are those
that do not involve perturbation of the test animal (i.e.
sensitization or challenge). These are further divided into 'basic'
tests, which include haematology and serum chemistry, routine
histopathological examination, and determination of organ and body
weights, and 'expanded' tests, which are logical extensions of the
'basic' tests and include those that can be performed retrospectively.
Type 2 tests include injection of or exposure to antigens, infectious
agents, vaccines, or tumour cells. In general, type 2 tests require a
satellite group of animals for immunological evaluation. The sets of
'basic' and 'expanded' type 1 tests are defined as level-I
immunotoxicity tests, and the sets of type 2 tests are defined as
level-II tests. Some level-I tests can be used to screen for
immunotoxic effects, while others focus on the mechanism of action or
the cell types affected by the test substance. Level-II tests are
conducted to define the immunotoxic effects of food and colour
additives more specifically. The recommended testing scheme is shown
below.
The functional tests generally require sensitization of exposed
rats and controls and subsequent analysis of the responses to the
sensitizing antigens. For this reason, functional tests are not
readily conducted in the first tier of immunotoxicity testing. As
guidelines for routine toxicology experiments preclude compromising
the experiment by any agent other than the test chemical, the second
tier of immunotoxicity testing, with immune function tests, requires a
separate set of experiments. The antigens that are used to sensitize
the exposed and control rats may be relatively simple antigens, such
as ovalbumin or tetanus toxoid, or more complex antigens, such as
sheep red blood cells, bacteria, or parasites. The responses can occur
in various arms of the immune system, which consequently must be
measured with different assays. For instance, humoral responses can be
measured by determination of specific antibodies in serum; the
appropriate tests for cellular responses are proliferative responses
of lymphocytes to the specific antigens ex vivo/in vitro or delayed-
type hypersensitivity responses to injection with antigen in vivo.
Recommendations of the United States Food and Drug Administration for
testing the immunotoxicity of direct food additives
Basic testing (rat model)
Complete blood count, differential white blood cell count;
Total serum protein, albumin:globulin ratio;
Histopathology, gross and microscopic (spleen, thymus, lymph
nodes, Peyer's patches, and bone marrow);
Lymphoid organ and body weights
Retrospective level-I testing (possible in a standard toxicology
study)
Electrophoretic analysis of serum proteinsa (when positive or
marginal effect is noted in basic testing);
Immunostaining of spleen and lymph nodes for B and T cellsa
(quantification of total immunoglobulins);
Serum autoantibody screen and deposition of immunoglobulins
(micrometry for semiquantification of the proliferative
response)
Enhanced level-I testing (possible for more complete screening in the
standard toxicology study core group, with a satellite animal group,
or in a follow-up study)
Cellularity of spleen (lymph nodes and thymus when indicated);
Quantification of total B and T cells (blood and/or spleen);
Mitogen stimulation assays for B and T cells (spleen);
Natural killer cell functional analysis (spleen);
Macrophage quantification and functional analysis (spleen);
Interleukin-2 functional analysis (spleen);
When indicated or for more complete analysis, other end-points
such as total haemolytic complement activity assay in serum
Level-II testing with a satellite group or follow-up study for
screening of functional immune effects
Kinetic evaluation of humoral response to T-dependent antigen
(primary and secondary responses with sheep red blood cells,
tetanus toxoid, or other);
Kinetic evaluation of primary humoral response to a
T-independent antigen such as pneumococcal polysaccharides,
trinitrophenyl-lipopolysaccharide, or other recognized
antigens;
Delayed-type hypersensitivity response to known sensitizer of
known T effector cell;
Reversibility evaluation
Recommendations (cont'd)
Enhanced level-II testing with a satellite group or follow-up study
for evaluation of potential immunotoxic risk
Tumour challenge (MADB106 or other in rat);
PYB6 sarcoma (in mouse);
Infectivity challenge (Trichinella, Candida or other in rat;
Listeria or other in mouse) a Recommended for inclusion in
basic testing
a Recommended for inclusion in basic testing
Not all functional assays require prior sensitization of the test
animals, e.g. proliferative responses of lymphocytes ex vivo/in vitro
to mitogens which are either specific for T cells, giving information
on cellular immunity, or for B cells, providing data on humoral
immunity. The phagocytic and lytic activity of macrophages and the
nonspecific cytotoxic activity of NK cells can also be measured
ex vivo/in vitro, without prior sensitization of the test animals.
Both types of activity are examples of nonspecific defence mechanisms,
directed to bacteria and certain tumour cells and to tumour cells and
virally infected cells, respectively. Since measurement of these types
of activity does not require prior sensitization of the host, such
functional tests can be considered for inclusion in the first tier of
testing for immunotoxicity in routine toxicology.
3.3 Considerations in evaluating systemic and local immunotoxicity
3.3.1 Species selection
Selection of the most appropriate animal model for
immunotoxicology studies has been a matter of great concern. Ideally,
toxicity testing should be performed with a species that responds to a
test chemical in a pharmacologically and toxicological manner similar
to that anticipated in humans, i.e. the test animals and humans
metabolize the chemical similarly and have identical target organs and
toxic responses. Toxicological studies are often conducted in several
animal species, since it is assumed that the more species that show a
specific toxic response, the more likely it is that the response will
occur in humans. Data from studies in rodents on target organ toxicity
at immunosuppressive doses for most immunosuppressive therapeutic
agents have generally been predictive of later clinical observations.
Exceptions to the predictive value of rodent toxicological data are
infrequent but occurred in studies of glucocorticoids, which are
lympholytic in rodents but not in primates (Haynes & Murad, 1985).
Although certain compounds exhibit different pharmacokinetic
properties in rodents and in humans, rodents still appear to be the
most appropriate animal model for examining the non-species-specific
immunotoxicity of compounds, because of established toxicological
knowledge, including similarities of toxicological profiles, and the
relative ease of generating data on host resistance and immune
function in rodents. Comparative toxicological studies should be
continued and expanded, however, as novel recombinant biological
compounds and natural products that enter safety testing will probably
have species-specific host interactions and toxicological profiles.
The quantitative and possibly the qualitative susceptibility of
an individual animal to the immunotoxicity of a selected agent can be
influenced by its genetic characteristics, indicating not only a need
to consider species but also strain. Rao et al. (1988) described two
approaches for selecting appropriate genotypes for toxicity studies.
The first is to select genotypes that are representative of an animal
species, which by virtue of similar metabolic profiles may also
exhibit a sensitivity similar to that of man, such as random-bred
mice. A second approach is to attempt to identify genotypes that are
uniquely suitable for evaluating a specific class of chemicals, such
as the use of Ah-responsive rodent strains in studies with
polyhalogenated aromatic hydrocarbons. In many cases, however, this
approach requires considerable knowledge of the mechanisms of toxicity
of the compound, which may not be available. One compromise has been
to use Fl hybrids which have the stability, phenotypic uniformity, and
known genetic traits of an inbred animal, yet have the vigour
associated with heterozygosity. The description of the genetic
relationships between inbred mouse strains on the basis of the
distribution of alleles at 16 loci (Taylor, 1972) has made possible
rational selection of appropriate Fl hybrids, such as the B6C3Fl
mouse.
3.3.2 Systemic immunosuppression
Because of this complexity, the initial strategies devised by
immunologists working in toxicology and safety assessment were to
select and apply a tiered panel of assays in order to identify
immunosuppressive or, in rare instances, immunostimulatory agents in
laboratory animals (US National Research Council, 1992). Although the
configuration of these testing panels varies according to the
laboratory conducting the test and the animal species used, they
include measurements of one or more of the following: (i) altered
lymphoid organ weights and histology, including immunohistology; (ii)
quantitative changes in the cellularity of lymphoid tissue, peripheral
blood leukocytes, and/or bone marrow; (iii) impairment of cell
function at the effector or regulatory level; and/or (iv) increased
susceptibility to infectious agents or transplantable tumours.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to adversely influence the
immune system. These include appropriate selection of animal models
and exposure variables, consideration of general toxicological
parameters and mechanisms of action, as well as an understanding of
the biological relevance of the end-points to be measured. Treatment
conditions should be based on the potential route, level, and duration
of human exposure, the biophysical properties of the agent, and any
available information on the mechanism of action. Moreover,
toxicokinetic parameters, such as bioavailability, distribution
volume, clearance, and half-life, should be measured. Doses should be
selected that will allow establishment of a clear dose-response curve
and a no-observed-effect level NOEL). Although, for reasons explained
earlier, it is beneficial to include a dose that induces overt
toxicity, any immune change observed at that dose should not
necessarily be considered to be biologically significant, since severe
stress and malnutrition are known to impair immune responsiveness.
Many laboratories routinely use three doses but generally conduct
studies to define the range of doses before a full-scale
immunotoxicological evaluation. If studies are being designed
specifically to establish reference doses for toxic chemicals,
additional exposure levels are advisable. In addition, inclusion of a
'positive control' group, treated with an agent that shares some of
the characteristics of the test compound, may be advantageous when
experimental and fiscal constraints permit.
The selection of the exposure route should reflect the most
probable route of human exposure, which is most often oral,
respiratory, or dermal. If it is necessary to deliver an accurate
dose, a parenteral exposure route may be required; however, this may
significantly change the metabolism or distribution of the agent from
that which would occur following natural exposure.
3.3.3 Local suppression
Local immune suppression has received less attention than
systemic immune suppression, and this is noteworthy, since the surface
that is exposed to the environment, i.e. the skin, the respiratory
tract, and the gastrointestinal tract, are the major ports of entry of
antigens and pathogens. While a variety of validated methods are
currently available to detect chemical skin sensitizers in humans and
experimental animals, there is no standard method to assess the
potential of chemicals to induce local immunosuppression in the skin.
Furthermore, although increasing evidence suggests that the
consequence of skin immunosuppression would be an increase in
neoplastic and infectious diseases of the skin, definitive data are
still lacking. In contrast, considerable efforts are being deployed to
develop sensitive models for monitoring skin irritants. For example,
human keratinocyte cultures and keratinocyte-fibroblast co-cultures
have been examined for end-points ranging from changes in cell
viability to production and loss of various bioactive products. Few
test systems are available for the gut and the respiratory tract.
4. METHODS OF IMMUNOTOXICOLOGY IN EXPERIMENTAL ANIMALS
This section comprises general descriptions of methods used for
evaluating immunotoxicity.
4.1 Nonfunctional tests
4.1.1 Organ weights
It is routine practice in toxicology to weigh organs that are
potentially affected by the compound that is being investigated. The
immunological organs that are suitable for weighing in screening for
potential immunotoxicity are: the thymus, which plays a decisive role
in the development of the immune system and which is affected by many
immunotoxicants; the spleen, which is the repository for many
recirculating lymphocytes; and the lymph nodes, which are important
for the induction of immune processes. Determination of the weight of
draining lymph nodes (depending on the route of exposure, i.e.
mesenteric nodes for oral exposure and bronchial nodes for inhalation)
in addition to distant lymph nodes (such as popliteal lymph nodes for
determining systemic effects) is the best. Mesenteric nodes, in
particular, occur in a string within non-lymphoid fatty tissue, and
care must be taken to remove this non-lymphoid tissue so that the
weight can be adequately determined. The cellularity of these organs
is another indication of the effects of chemicals on the immune
system. Furthermore, cell suspensions can be prepared from lymphoid
organs in order to assess the distribution of subpopulations of
lymphoid cells and to test their functionality within the organs.
Under OECD guideline No. 407, histopathological examination of
lymphoid organs and tissues is crucial for detecting the effects of
chemicals on the immune system. Therefore, upon termination of
exposure to a compound in a toxicological experiment, organs such as
the spleen should first be weighed; subsequently, they are divided
into parts which are also weighed, and one or more parts are used for
histopathological examination and the remainder to prepare cell
suspensions that can be evaluated for distribution of lymphocyte
subpopulations or can be assessed functionally.
4.1.2 Pathology
The histopathology of the thymus, spleen, and draining and
distant lymph nodes, of the mucosal immune system (Peyer's patches in
the gut or bronchus and nose-associated lymphoid tissue in the
respiratory tract), and of the skin immune system should be evaluated,
depending on the route of exposure. The first level of evaluation
should be of haematoxylin-eosin stained, paraffin-embedded slides. A
more sophisticated level of evaluation is immunoperoxidase staining of
special cell types.
Many monoclonal antibodies are available for mice, rats, and
humans to detect differentiation antigens, cell adhesion molecules,
and activation markers on haematolymphoid and stromal cells involved
in immune responses. A list of some monoclonal antibodies that can be
used in the identification of leukocytes and stromal cells in (frozen)
sections of lymphoid tissue is presented in Table 10; a selection of
these is reviewed below.
For a further description of these markers, and the cells that
express them, reference may be made to the introductory section and to
descriptions in the literature (Brideau et al., 1980; Bazin et al.,
1984; Dallman et al., 1984; Dijkstra et al., 1985; Joling et al.,
1985; Vaessen et al., 1985; Joling, 1987; Hünig et al., 1989; Kampinga
et al., 1989; Portoles et al., 1989; Schuurman et al., 1991a).
These markers are usually stained in frozen tissue sections of
6-8 µm, fixed in acetone. A three-step immunoperoxidase procedure is
most suitable: the first step includes the monoclonal antibody
specific for the determinants to be studied (see above), the second
step, rabbit anti-mouse immunoglobulin, and the third step, swine
anti-rabbit immunoglobulin, the latter two antibodies conjugated to
horseradish peroxidase. The peroxidase activity can be developed by
3,3-diaminobenzidine tetrahydrochloride with hydrogen peroxide as
substrate, and the sections can be counterstained with Mayer's
haematoxylin to facilitate evaluation. Negative controls are prepared
by omitting the antibody in the first step or replacing it with an
irrelevant one. Under these conditions, only the peroxidase activity
of polymorphonuclear cells, when present, is visualized, and no
immunolabelling is found.
In general, histopathological evaluation provides a semi-
quantitative estimation of effects. The experienced pathologist can do
this adequately in studies carried out 'blind', especially if the
effects are clear. For more subtle effects, morphometric analysis is a
valuable addition, especially when supported by software for assessing
the values of parameters such as size, surface, and intensity of
staining. The compartments of the immune system, i.e. specific T and B
lymphocyte areas in spleen and lymph nodes and cortical and medullary
areas of immature and differentiated thymocytes within the thymus, and
the numbers of specialized cells per surface unit are parameters that
are well suited for morphometric analysis.
4.1.3 Basal immunoglobulin level
Serum immunoglobulin levels are often altered after exposure of
rats to immunotoxic chemicals (Vos, 1980; Vos et al., 1982, 1984,
1990a; Van Loveren et al., 1993a). This is not surprising, as the
total levels measured are a function of the humoral aspects of the
immune system, which react to the antigens that the host encounters.
For this reason, measurement of antibody levels is potentially
valuable in screening for immunotoxicity. Since the amount of antibody
Table 10. Some monoclonal antibodies to leukocytes and stromal cells used in immunohistochemical studies of tissue sections and flow
cytofluorography on cell suspensions
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells
CD1 gp43,45, Ly-38 OKT6, Lymphocytes in thymic cortex, Langerhans cells in skin,
49,12 a-Leu-6 interdigitating cells
CD2 gp50 Ly-37, MRC OX-34, a-Leu-5, All T cells in thymus and peripheral lymphoid organs, subset
NSM46.7, MRC OX-54, OKT11 of macrophages (rat). Sheep erythrocyte receptor, leukocyte
RM2-5 MRC OX-55 function antigen:-2 (LFA-2). Ligand f or LFA-3 (CD58)
CD3 gp19-29 CD3-1,KT3, IF4, G4.18 a-Leu-4, T Cells in thymic medulla and peripheral lymphoid organs
145-2C11 OKT3 (T-cell receptor-associated, cytoplasmic in precursor T cells
in thymus)
CD4 gp65 Ly-4, L3T4, MRC OX-35, a-Leu-3, Lymphocytes in thymic cortex, about two-thirds of T cells in
YTS 177.9 MRC OX-38, OKT4 peripheral lymphoid organs, subset macrophages, microglia
(ER2), W3/25 T helper/inducer and delayed-hypersensitivity phenotype.
MHC class II binding, receptor for human immunodeficiency
virus
CD5 gp65-62 Ly-1,Lyt-1 MRC OX-19, a-Leu-1 Lymphocytes in thymic cortex (faint). All T cells in thymic
HIS47 medulla and peripheral lymphoid tissue, subset of B cells
CD6 gp120 Tü 33 T Cells in thymic medulla and peripheral lymphoid organs
CD Relative Mouse Rat Human Reactivity
mol. mass
CD7 gp41 WT1,B-F12, Prethymic T-cell precursors, all T cells in thymus and fewer
a-Leu-9 in peripheral lymphoid organs
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
CD8 gp32-33 Ly-2,3,Lyt-2,3, MRC OX-8 a-Leu-2, Lymphocytes in thymic cortex, about one-third of T cells in
YTS 105.8 OKT8 peripheral lymphoid organs, splenic sinusoids (T cytotoxic/
suppressor phenotype, NK cells). MHC class I binding
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells,
thymic cortex cells (rodents). Heat-stable antigen (HSA)
CD43 gp115 Ly-48 W3/13,HIS17 DFT-1, (Pro)thymocytes, T cells, plasma cells, cells in bone
WR-14 marrow, polymorphonuclear granulocytes, brain cells.
Leukosialin, sialophorin
CDw p25-30 Thy-1 (ER4), 5F10 Thymocytes, T lymphocytes, connective tissue structures,
90 MRC OX-7, epithelial cells, fibroblasts, neurons, subset of bone-marrow
HIS51 cells, plasma cells, stem cells (T-activation molecule)
Thy-2 Thymocytes
p40-55 H57-597 R73,HIS42 WT31, T-Cell receptor a-b chain. Mature T cells in thymic medulla
TalphaF1, and peripheral lymphoid tissue
TßF1
p40-55 GL3,GL4, V65 CgammaM1, T-Cell receptor gamma-delta chain
UC7-13D5 11F2,
TCR delta1,
deltaTCS1
p41-55 MRC OX-44 Prothymocytes, lymphocytes in thymic medulla, T and B
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
p41,47 MRC OX-2 Thymocytes, dendritic cells, B cells, brain cells
ER3,ER7, Subset of thymocytes and peripheral T cells, subset of
ER9,ER10 myeloid cells
HIS44 Most lymphocytes in thymic cortex, small subset of
medullary lymphocytes, erythroid cells, cells in germinal
centre
HIS45 Some lymphocytes in thymic cortex, most medullary
thymocytes and peripheral T cells, subset of B cells.
Quiescent cell antigen (QCA-1)
MHC class I (Various antibodies to polymorphic and All nucleated cells, including leukocytes and stromal
non-polymorphic epitopes) cells; for T cells absent on thymic cortex cells (human)
B Cells
CD9 gp24 BA-2 Germinal centres (faint); some cells in thymic cortex. Late
pre-B cells
CD10 gp100 BA-3, Germinal centres (faint); some cells in thymic cortex
W8E7 Common acute lymphoblastic leukaemia antigen (CALLA)
CD19 gp95 a-Leu-12, B Cells in germinal centres and mantles, follicular dendritic
B4, FMC63 cells
CD20 p35 Ly-44 B1, a-Leu-16 B Cells in germinal centres and mantles, follicular dendritic
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
CD21 gp140 B2,BL13, B Cells in germinal centres and mantles (faint), follicular
HB-5 dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus)
CD22 gp135 Lyb-8.2, a-Leu-14,To B Cells in germinal centres and mantles, cytoplasmic in
Cy34.1 To 15,RFB4, precursor B cells
SHCL-1
CD23 p45 Ly-42 a-Leu-20, Some B cells in marginal centres and mantles, activated B
Tü 1 cells, subset of follicular dendritic cells (IgE Fc receptor)
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells, thymic
cortex cells (rodents). Heat-stable antigen (HSA)
CD37 gp40-45 BL14 B Cells in germinal centres and mantles
CD38 gp45 a-Leu-17, Lymphocytes in thymic cortex, cells in germinal centres,
OKT10 plasma cells (immature lymphoid cells, plasma cells)
CDw75 p53? LN1, OKB4 B Cells in germinal centre, in corona (faint), macrophages,
epithelium
CD79a p33,40 mb-1 B Cells, Ig alpha chain
CD79b p33,40 B29 B Cells, Igß chain
p200 (HIS14) All B cells, including TdT+ precursors
p200 (HIS22) All B cells in corona, pre-B cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
MHC class II (Various antibodies to polymorphic and B Lymphocytes, activated T cells, monocytes/macrophages,
non-polymorphic epitopes) interdigitating cells, Langerhans cells, epithelia, endothelia
B Cells (surface); in germinal center IgM+IgG+IgA+ and in
anti-immunoglobulin corona IgM+IgD+, plasma cells
(cytoplasmic)
Monocytes/macrophages, myeloid cells
CD13 p130-150 ER-BMDM-1 a-Leu-M7, Monocytes, granulocytes, dendritic reticulum cells
My7 (aminopeptidase N)
CD14 p55 ED9 UCH-M1, B-A8, Monocytes, some granulocytes and macrophages
a-Leu-M3
CD15 p170-190 a-Leu-M1 Granulocytes, some monocytes (lacto-N-fucose pentaosyl)
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes,
activated macrophages. IgG-FcRIII, low affinity, complexed IgG
CD33 p67 a-Leu-M9, (Precursor) granulocytes, macrophages, Langerhans cells.
My9 Myelin-associated protein
CD68 p110 Ki-M6,Ki-M7 Macrophages (specific)
p160 F4/80 Monocytes-macrophages
p32 Mac-2 Thioglycollate-elicited peritoneal macrophages
p92-110 Mac-3 Peritoneal macrophages
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Monocytes/macrophages, myeloid cells (contd)
4F7 Dendritic cells in skin, bone marrow
ED1 Monocytes/macrophages
ED2,HIS36 Subset of macrophages (F4/80-like)
ED3 Subset of macrophages, restricted, negative in thymus
MRC OX-41 Granulocytes, macrophages, dendritic cells
MRC OX-62 Dendritic cells (integrin-like)
(IF119) Dendritic cells
HIS48 Granulocytes
Mac-387 Macrophages
Natural killer cells
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages. IgG-FcRIII, low affinity, complexed IgG
CD56 p220/135 a-Leu-19, NK cells, monocytes, neuroectodermal cells NKH-1, isoform of
B-A19 neural cell adhesion molecule (NCAM)
CD57 p110 a-Leu-7, NK cells, subset of T cells, some B cells, some epithelial cells,
VC1.1 monocytes, neuroendocrine cells, NKH-1
a-asialo-GM1 NK cells, stromal components
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Natural killer cells (contd)
NK-1.1,2B4, 3.2.3 NK cells (NKR-P1 gene family)
3A4, 5E6
Follicular dendritic cells
ED5,ED6, Ki-M4,DRC-1 Follicular dendritic cells
MRC OX-2
Epithelial cells (thymus)
(Various anti-keratin antibodies) Epithelium
(ER-TR4),4F1 HIS38 TE-3,(MR3, Thymic cortex epithelium
MR6)
(ER-TR5),IVC4 (HIS39) TE-4,(MR19), Thymic subcapsular or medullary epithelium
RFD4
Complement receptors
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells, C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD21 gp140 B2,BL13 B Cells in germinal centres and mantles (faint), follicular
dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus
CD35 p220 To 5 Follicular dendritic cells, macrophages, B cells in corona
(faint), renal glomerular epithelium. C3bR, CR1
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
IgG-Fc receptors
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages; IgG-FcRIII, low affinity, complexed IgG
CD32 gp140 Ly-17 3E1,CIKM5 B Cells, myeloid cells, macrophages; IgGFcRII, low affinity,
complexed IgG
CD64 p75 32.2 Monocytes; IgG-FcRI, high affinity, monomeric IgG
ß1-Integrin (CD29-CD49) family
CD29 p130 9EG7 B-D15 Ubiquitous, not on erythrocytes; ß1 chain of all CD49 antigens
CD49a p200 TS2/7 Activated T cells, monocytes, smooth muscle cells. Very late
antigen-1 (VLA-1), ligand of collagen, laminin
CD49b p155 31H4,AK7, T Cells, B cells, thrombocytes, fibroblasts, endothelium.
P1E6 Very late antigen-2 (VLA-2), ligand of collagen I, II, III,
and IV, laminin
CD49c p145 11G5,P1B5 B Cells, renal glomeruli, basal membranes. Very late antigen-3
(VLA-3), ligand of collagen, laminin, fibronectin, and invasin
CD49d p150 R1-2, P12520, HP2/1,44H6, Thymocytes, lymphocytes, monocytes, NK cells, eosinophilic
MFR4.B MR?4 L25.3 granulocytes, erythroblasts. Very late antigen-4 (VLA-4),
ligand of VCAM-1, fibronectin
CD49e p160 MFR5, SAM-1 Monocytes, leukocytes, memory T cells, fibroblasts,
P12750 thrombocytes and muscle cells. Very late antigen-5 (VLA-5),
ligand of fibronectin
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
ß1-Integrin (CD29-CD49) family (contd)
CD49f p150 GoH3 Go-H3,4F10 T Cells, thymocytes, monocytes, thrombocytes. Very late
antigen-6 (VLA-6), ligand of laminin and invasin
ß2-Integrin (CD11-CD18) family
CD11a p180 Ly-15, WT.1 YTH-81.5, T and B cells, NK cells, erythroid and myeloid stem cells.
2D7, B-B15, Leukocyte function-associated antigen-1 (LFA-1) involved in cell
M17/4 G-25.2 adhesion, ligand for intercellular adhesion molecule (ICAM)-1
(CD54), ICAM-2 (CD102), ICAM-3 (CD50)
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells. C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD11c p150 a-Leu-M5, Monocytes, macrophages, granulocytes (faint), activated
S-HCL-3 lymphocytes. CR4
CD18 p95 YTS213.1, WT.3 BL5 All lymphocytes. ß-Chain of CD11 antigens
C71/16,
M18/2
p160-95 ED7,ED8 CD11-CD18 molecule
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others
Terminal deoxynucleotidyl transferase (TdT) Immature (lymphoid) cells in bone marrow and thymic cortex
(nuclear staining)
CD25 p55 Ly-43, AMT13, MRC OX-39 Tac,a-IL2-R Activated lymphocytes at scattered locations in thymus and
7D4,3C7 T-cell areas in peripheral lymphoid organs. Interleukin-2
receptor alpha chain
CD122 p75 5H4, CF1,Mik-ß2, NK cells, T cells, B cells, monocytes. Interleukin-2 receptor
TM-ß1 Mik-ß3 ß chain
CD26 p120 H194-112 MRC OX-61 134-2C2 (Activated) T cells. Dipeptidyl peptidase IV, in mouse T-cell
activation molecule (THAM)
CD30 p105 Ki-1,Ber-H2 Sporadic cells in thymic (cortex) and T cell areas in
peripheral lymphoid organs, some plasma cells. Activated
lymphocytes,Hodgkin cells
CD34 MY 10,8G12 Haematopoietic progenitor cells, capillary endothelium
QBEND/10 Human progenitor cell antigen (HPCA)
CD44 p65-85 Ly-24 MRC OX-49, a-Leu-44, Prothymocytes, T cells, small B cells. Lymphocyte homing
IM7 MRC OX-50 F10-44-2 receptor. Phagocytic glycoprotein-1 (PgP-1), HCAM
CD45 p180-210 Ly-5 MRC OX-1, T29/33 All leukocytes. Common leukocyte antigen
HIS41
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others (contd)
CD45R p190-220 B220 MRC OX-22, a-Leu-18, All B cells, subset of T cells. Common leukocyte antigen.
MRC OX-32, MB1,MT2 HIS24 restricted to strains of the RT7.2 allotype and labels
HIS24 all peripheral B cells except cells in marginal zone, pre-B
cells
CD45RA p205-220 14.8 MRC OX-33 HI100 B Cells, T cytotoxic-suppressor cells (faint), subset of
thymocytes.In humans, also CD4+ subset (naive-virgin
T-cells)
CD45RO p190-220 UCH-L1 T Cells in immature and memory stage. Common leukocyte
antigen
CD54 p90 KAT-1 1A29 84H10,B-C14 Endothelial cells, many activated cell types. Intercellular
3E2 HA58 adhesion molecule-1 (ICAM-1)
CD71 p95 YTA74.4,C2 MRC OX-26 B3/25 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs, stromal
cells. Transferrin receptor
Ki-67 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs. Proliferation
antigen present in late G1, S, G2, and M phases
PCNA PCNA PCNA Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs
MEL14 Recirculating T and B cells. Lymphocyte homing receptor
MHC, major histocompatibility complex; NK, natural killer; Ig, immunoglobulin
CD nomenclature from: Clark & Lanier (1989); Knapp et al. (1989); Schlossman et al. (1994, 1995)
Antibodies within parentheses are not commercially available.
in serum is a function of the antibody's half-life, the longer the
study the more likely it is that an effect will be observed.
Immunoglobulin levels can be influenced by the cleanness of the
facility: studies conducted in facilities with excellent husbandry
will have lower basal levels than those conducted in 'dirty'
facilities where animals are constantly exposed to foreign antigens,
including pathogens.
Measurement of basal immunoglobulin levels is useful only after
subchronic or chronic exposure, i.e. with sufficient time for normal
metabolic elimination. Basal levels of immunoglobulin decrease only
when synthesis is reduced or prevented such that metabolized
immunoglobulins are not replaced. The parameter therefore yields
little information about possible mechanisms of immunotoxicity, and
should rather be regarded as a screening parameter; this is in fact
true for most non-functional tests. The IgM and G classes have usually
been measured; however, since the two other classes (A and E) are
biologically very important (for instance in mucosal immunity and
allergic manifestations), they should also be measured.
Total IgM and IgG concentrations in serum can be analysed by
means of a 'sandwich' enzyme linked immunosorbent assay (ELISA), as
described by Vos et al. (1982). Total IgA and IgE concentrations can
be analysed in an essentially similar way, except that the microtitre
plates are coated with monoclonal anti-rat IgA (Van Loveren et al.,
1988b) or monoclonal anti-rat IgE antibodies (MARE-1), respectively,
and immunoglobulins bound to these antibodies in serum samples are
detected by sheep anti-rat IgA or monoclonal anti-kappa chains of rat
immunoglobulins (MARK-1), conjugated with peroxidase.
Data from the ELISA are usually reported as percentages of
control values, and a titration curve based on pooled sera is
prepared; optimal dilutions of exposed and unexposed groups are then
plotted from this curve. The deviation of the dilution of the test
groups from the control groups is expressed, with the dilution in the
control group set at 100%. While studies in rats indicate that
measurement of basal immunoglobulin levels is useful in predicting the
immunotoxic effects of compounds, studies conducted in mice at the NTP
do not, and measurement of basal immunoglobulins is not included in
either tier of their testing panel (Luster et al., 1988). There are
several possible reasons for the difference in the usefulness of basal
immunoglobulin levels in rats and mice. First, in the studies of Vos
and colleagues, cited above, the exposure period was routinely longer
than the 14-day studies conducted within the NTP; since serum antibody
level is a function of the antibody half-life, longer studies are more
likely to detect an effect. Furthermore, the doses used at the NTP
were often lower, by design, than those used in rats. A final possible
explanation, which remains to be confirmed, is that immunoglobulin
synthesis in rats is more sensitive than that in mice.
4.1.4 Bone marrow
Bone marrow is an important haematopoietic organ and a source of
precursors for lymphocytes and other leukocytes. Changes in the bone
marrow are therefore likely to result in alterations of
immunocompetent cell populations, which may be long lasting or
permanent and thus serve as an indicator of potential immunotoxicity.
In a study to validate immunotoxicological parameters, bone-marrow
cellularity was shown to be an indicator of the immunotoxicity of
cyclosporin A, used as the model compound (Van Loveren et al., 1993a).
Determination of cellularity in stained slides of bone marrow,
evaluation of smears, and actual counts of the numbers of cells within
bone marrow are practical. For this purpose, both ends of a femur are
cut off, and bone-marrow cells are collected by flushing balanced salt
solution through the femur with a 21-gauge needle. The concentration
of nucleated cells is determined in a Coulter counter; a differential
count of cells can be done visually in May-Grunvald Giemsa-stained
cytospin preparations.
4.1.5 Enumeration of leukocytes in bronchoalveolar lavage fluid,
peritoneal cavity, and skin
Mononuclear phagocytes in the alveoli of the lung play an
important role in clearing inhaled particles, including
microorganisms, from the lung. The numbers of cells and alterations in
their function can be end-points of the toxicity of inhaled chemicals.
In order to study these parameters, methods for harvesting the cells
from the lungs should be easy to perform, guarantee the sterility of
the cell harvest, and be standardized. Methods involve use of either a
syringe (Blusse Van Oud Alblas & Van Furth, 1979) or a complex system
of syringes, tubes, and valves (Moolenbeek, 1982). These methods often
result in contamination of the harvested cell population; moreover,
they are laborious and cannot easily be standardized since the syringe
is operated manually. In a more recently developed method (Van
Soolingen et al., 1990), an excised lung is placed in a pressure
chamber and connected to a cannula through which lavage fluid can be
introduced into the lung and transferred from the lung into a test
tube. This procedure is repeated several times to obtain an optimal
yield.
Enumeration of mononuclear cells in the peritoneal cavity can
also best be performed by harvesting these cells by lavage. Because
of the architecture of this organ, three or four cycles of
intraperitoneal injections of lavage fluid, followed by gentle
massaging of the abdomen, and aspiration of the fluid with the syringe
that was also used for injection suffice.
Langerhans cells in the skin can be enumerated with
histopathological techniques. Frozen tissue sections are used, stained
with immunoperoxidase techniques including markers for MHC class II
antigens or specific markers, as indicated above. Morphometric
analysis may provide a quantitative basis for this type of evaluation.
4.1.6 Flow cytometric analysis
Evaluation of phenotypic markers has proved to be one of the most
sensitive indicators of immunotoxic compounds. The availability of
fluorescent activated cell sorter (FACS) analysis units and
fluorescent cell counter units in immunotoxicology laboratories has
made analysis of cell populations routine. Determination of the
phenotype of lymphoid cells is a non-functional assay, although it has
often been inappropriately grouped with functional tests. The presence
or absence of a particular marker on the surface of a cell does not
reveal the functional capability of the cell. The usefulness of
surface marker analysis for predicting potential immunotoxicity has
been demonstrated. In studies conducted by Luster et al. (1992), a 91%
concordance was found for correct identification of immunotoxic
compounds on the basis of studies of surface markers alone.
As indicated above (section 4.1.2), numerous markers are
expressed on the cells of the immune system. Essentially, the same
reagents as used on tissue sections are applied on cells that have
been isolated from tissues, body fluids, or lavage fluids in
suspension (see above). Furthermore, both polyclonal and monoclonal
antibodies are available for detecting these surface markers. While
many of the markers have been used in immunological investigations,
very few have been evaluated with a large number of immunosuppressive
compounds. The markers that have been routinely used in studies of
immunotoxicity conducted for the NTP in mice and the cell types they
identify are shown in Table 11. The CD4:CD8 ratio in spleen has been
shown to concord best with the immunotoxicity of these surface markers
(Luster et al., 1992).
Table 11. Phenotypic markers on lymphocyte subpopulations used in
studies of immunotoxicity by the United States National
Toxicology Program
Surface marker Cell type
sIg+ Pan B cells
Thy 1.2+ or CD3+ Pan T cells
CD4+CD8- T Helper/delayed-type
hypersensitivity cells
CD8+CD4- T Suppressor-cytotoxic cells
CD8+CD4+ Immature T cells
The identification of phenotypic markers in rats has not
developed as rapidly as in mice; however, antibodies to rat cell
surface markers are now becoming available commercially and are being
used in immunotoxicological assessments (Smialowicz et al., 1990). The
monoclonal antibodies currently used for this purpose are: OX4 or
MARK-1 for B cells, W3/13 or OX 19 for T cells, R79 for the T cell
receptor, W3/25 for CD4 cells, and OX 8 for CD8 cells.
In enumerating the cell types in lymphoid tissue, both
percentages and absolute cell numbers should be reported. Of the two,
absolute cell numbers are by far the most meaningful. Compounds that
affect all populations equally and thus do not change the relative
percentages of the various cell types may be missed if only
percentages are evaluated. In addition, significant differences in the
magnitude of an effect on one or more of the populations can be
observed when the data are evaluated as absolute numbers and not as
percentages. As indicated above, the absolute changes more closely
reflect the events occurring in the animal and should thus be given
priority in interpreting data.
FACS analysis is also being used to determine the activation
state of various cell types, on the basis of changes in detectable
activation markers. Some of the activation markers that have been
studied are F4/80 (Austyn & Gordon, 1981), Mac-1 (Springer et al.,
1979), Mac-2 (Ho & Springer, 1984), transferrin receptor (Neckers &
Cossman, 1983), and IL-2 receptor (Cantrell et al., 1988). While
activation markers are of value in studying the mechanism of action of
compounds, their usefulness as predictors of immunotoxicity has yet to
be firmly established.
4.2 Functional tests
4.2.1 Macrophage activity
Phagocytic activity is the first line of defence against many
pathogens. Macrophages can phagocytose many particles, including
bacteria, and can lyse and inactivate them. Alterations in phagocytic
activity are therefore important potentially adverse effects of
chemicals on the immune system. The capacity to ingest particles
in vitro can be measured, and activity in vivo can be measured by
determining the clearance of bacteria, such as L. monocytogenes.
This test is dealt with in section 4.2.10.1.
Several assays have been developed for evaluating various types
of phagocytosis in mice and can also be used in rats, with slight
modifications. Innate and non-immune-mediated phagocytosis by
macrophages can be evaluated by determining the uptake of fluorescent
latex covaspheres (Duke et al., 1985). Macrophages and peritoneal
exudate cells are placed on a tissue culture slide and incubated with
the covaspheres for 24h on a rocking platform. The slides are then
fixed with methanol. The slide chambers are evaluated under a
fluorescent microscope, and macrophages with more than five latex
covaspheres are counted as positive for phagocytosis. The results are
expressed as percentage of phagocytosis, which is calculated as the
ratio of macrophages positive for phagocytosis to total macrophages
counted. In order to distinguish phagocytosed latex covaspheres from
those that are merely associated with the macrophage surface, the
cells are exposed for 30-60s to methylene chloride vapour. By
immersing the slides in this manner, the covaspheres that have not
been phagocytosed are dissolved, while those inside the macrophage
remain intact (Burleson et al., 1987). Phagocytosed fluorescent latex
particles can easily be quantified under the fluorescence microscope.
While this assay is straightforward, it is labour intensive, and
reading the slides, shifting back and forth from the fluorescent
field, and counting the macrophages is time-consuming.
A radioisotopic procedure, the chicken erythrocyte assay, can be
used to evaluate both adherence to and phagocytosis of particles by
macrophages. The phagocytic capacity is measured as an immunologically
mediated (Fc receptor) response. Macrophages are added to each well of
a 24-well tissue dish and allowed to adhere for a 2-3-h incubation
period. Nonadherent cells are washed, and chicken erythrocytes
labelled with 51Cr are added to each well; then a subagglutinating
dilution of antisera to chicken erythrocytes is added to each well and
the plate incubated for 1h. The plates are then washed to remove
unbound erythrocytes; an ammonium chloride solution is added to lyse
adhered erythrocytes, and the supernatant is collected and counted to
determine adherence of the erythrocytes to the macrophages. Next, both
the macrophages and the phagocytosed chicken erythrocytes are lysed by
addition of 0.1 N sodium hydroxide, and the solution is counted to
determine the amount of phagocytosis. Three to six wells in each group
do not receive 51Cr and are used to evaluate the DNA content
(Labarca & Paigen, 1980). The data are expressed as adherence counts
per minute, phagocytosed counts per minute, and specific activity for
adherence and phagocytosis. Specific activity is determined by
dividing the number of adhered or phagocytosed counts per minute by
the DNA content per well. The data must be expressed in terms of
specific activity, since compounds that affect the macrophages'
ability to adhere to the 24-well culture dish will significantly alter
the results obtained.
While both the nonspecific and immune-mediated phagocytosis
assays are useful for understanding the potential mechanisms of action
of compounds, changes in phagocytic activity in these in-vitro assays
have not been found to be predictive of immunotoxicity. For example, a
single intratracheal exposure to gallium arsenide resulted in
increased adherence and phagocytosis by chicken erythrocytes but
decreased phagocytosis of latex covaspheres (Sikorski et al., 1989).
The phagocytosis assay that is most predictive of altered
macrophage function is evaluation of the functional ability of the
mononuclear phagocyte system. This is a holistic assay for measuring
the capacity of the fixed macrophages of the mononuclear phagocyte
system, where macrophages provide the first line of defence against
both pathogenic and non-pathogenic blood-borne particles. The fixed
macrophages of the mononuclear phagocyte system line the liver
endothelium (Kupffer cells), the spleen, the lymph nodes (reticular
cells), the lung (interstitial macrophages), and other organs such as
the thymus and bone marrow. When the assay is conducted in mice, the
animals are injected intravenously with 51Cr-labelled sheep
erythrocytes, and a 5-µl blood sample is taken from the clipped tail
at 3-min intervals over a 15-min period. A final 30-min blood sample
is taken, and 1h after injection the animals are sacrificed and the
liver, spleen, lungs, thymus, and kidneys are removed, weighed, and
counted in a gamma counter. The 60-min time interval after injection
of sheep erythrocytes was selected as the time of sacrifice since it
represents the plateau for particle uptake by the selected organs
(White et al., 1985). Blood clearance of the radiolabelled cells is
expressed as vascular half-life and as a phagocytic index, which is
determined by the slope of the clearance curve. Organ distribution is
expressed as percent organ uptake and counts per minute per milligram
of tissue (specific activity). The assay can detect both stimulation
and inhibition of the mononuclear phagocyte system. Bick et al. (1984)
reported marked stimulation of the mononuclear phagocyte system after
treatment with diethylstilbestrol; more recently, morphine sulfate was
shown to decrease vascular clearance and hepatic and splenic
phagocytosis significantly (LeVier et al., 1993).
4.2.2 Natural killer activity
NK activity against neoplastic and virus-infected targets has
been clearly demonstrated in vitro and is thought to play an
important role in vivo in providing surveillance against neoplastic
cells and as a first line of defence against viruses (Herberman &
Ortaldo, 1981). In humans, rats, and mice, most cells with NK activity
can be identified by morphological (although the definition is not
morphological) and functional characteristics (Timonen et al., 1981).
Most of the cells that show NK activity are nonadherent, non-
phagocytic lymphocytes and are morphologically associated with large
granular lymphocytes (Timonen et al., 1982). Although cells with NK
activity do not strictly belong to the T-cell lineage, they can
express T cell-associated markers and express surface receptors, such
as those for the Fc portion of IgG and the ganglioside asialo GM1.
Some of these markers are also expressed by monocytes, macrophages,
and polymorphonuclear leukocytes (Herberman & Ortaldo, 1981). Within
4h, the cells can show nonantigen-specific cytotoxic activity
in vitro and in vivo against certain (NK-sensitive) tumour cell
lines and virus-infected cells.
The cells have enhanced cytolytic function after activation with
a variety of stimuli, including viral infection (Stein-Streinlein et
al., 1983), BCG (Tracey et al., 1977), IL-2 (Henney et al., 1981;
Domzig et al., 1983; Lanier et al., 1985; Malkovsky et al., 1987),
interferon, and interferon inducers (polyI:C) (Tracey et al., 1977;
Oehler & Herberman, 1978; Djeu et al., 1979a,b). NK activity in vitro
can be stimulated with IL-2 and interferon (Tracey et al., 1977; Djeu
et al., 1979b). Anti-asialo GM1 antibody can strongly inhibit
cytotoxic NK activity both in vitro and in vivo (Kasai et al.,
1980, 1981; Yosioka et al., 1986). This antibody binds to the cell
surface glycolipid GM1 and suppresses the lytic activity of effector
cells. Large granular lymphocytes are found in several lymphoid
organs. Many cells with high NK activity are found in spleen and
peripheral blood (Rolstad et al., 1986); lymph nodes have less NK
activity, and thymus and bone marrow show only marginal activity. NK
activity can also be demonstrated in the bronchus-associated lymphoid
tissue in the lungs. Moreover, large granular lymphocytes can migrate
from the circulation into the extravascular tissue and can even be in
contact with the lumen of the alveoli (Timonen et al., 1982; Reynolds
et al., 1984; Rolstad et al., 1986; Prichard et al., 1987). The
presence of large granular lymphocytes associated with NK activity in
the lungs is probably of great importance, because the lungs
constitute a major site for neoplastic disease (metastatic spread) and
viral infections. NK cells may also operate in certain types of
bacterial infections. In experimental animals, suppression of NK cell
activity increased the numbers of metastases after transplantation of
tumours.
The clinical significance of altered NK cell activity in humans
has not clearly been established. Asymptomatic individuals with low NK
cell responses may be at some risk for developing upper respiratory
infections and for increased morbidity (Levy et al., 1991); and
extreme susceptibility to severe and repeated herpes virus infection
was reported in an individual without NK cells (Biron et al., 1989).
It is obvious therefore that exposure to toxic substances that alter
NK activity can have biological consequences, and testing this
activity is important in assessing potential immunotoxicity.
The procedure for determining NK activity is as follows: cell
populations that exert NK activity (usually enriched peripheral blood
mononuclear cells or spleen cells) are cultured with NK-sensitive
target cells. A cell type frequently used for this purpose is the YAC
lymphoma cell line, which has been applied to mice, rats, humans, and
even seals. YAC lymphoma target cells are radiolabelled with 51 Cr,
and lysis of the cells, resulting in release of chromium, within 4h is
used to estimate the cytolytic activity of the NK cells within the
cell population. This assay has been used to demonstrate the effects
of numerous compounds on NK activity in rats (e.g. TBTO, ozone, and
HCB: Vos et al., 1984; Van Loveren et al., 1990c), mice (Luster et
al., 1992), and harbour seals (Ross et al., in press).
4.2.3 Antigen-specific antibody responses
Most antibody responses require not only B cells, which, after
maturation into plasma cells, produce antibodies, but also the help of
T lymphocytes. A variety of T cell-dependent antigens can be used for
this purpose, and an excellent one is tetanus toxoid. A typical
immunization schedule in rats comprises intravenous immunization on
day 0 followed by a booster on day 10. Primary and secondary IgG and
IgM responses can then be measured in serum, taken on day 10 (just
before the booster) and day 21, respectively. The primary IgM response
is the immunoglobulin response that is least under the control of T
cells. As tetanus toxoid is also used for human immunization, the
responses to this antigen may be useful in extrapolating experimental
data to humans. The responses can be determined in an ELISA (Vos et
al., 1979b).
Another widely used T cell-dependent antigen is ovalbumin. This
antigen can be and has been used to induce all classes of antibody
responses, i.e. IgM, IgG, IgA, and IgE, that can be measured with the
ELISA (Vos et al., 1980; Van Loveren et al., 1988b). The classical
assay of specific IgE responses is the passive cutaneous anaphylaxis
reaction. Serial dilutions are injected into the skin of rats,
sensitizing local mast cells; the specific antigen is then injected
intravenously, simultaneously with Evans blue. Mast cell products are
released where IgE meets the antigen, and IgE is cross-linked on the
membranes of the mast cells, leading to extravasation of Evans blue.
The titre can be determined from the magnitude of the reaction at each
dilution of IgE. ELISA techniques and the specific reagents to detect
IgE in an ELISA that are now available make this test preferable.
Ovalbumin induces not only humoral responses but also delayed-
type hypersensitivity. Sensitization to ovalbumin in Freund's complete
adjuvant enhances responses and makes it possibile to assay both
responses in one animal. Delayed-type hypersensitivity can also be
directed to purified protein derivative, with responses induced by the
adjuvant (Vos et al., 1980). At least in mice, however, immunization
in complete adjuvant skews responses in the direction of Th1
responses, i.e. delayed hypersensitivity, and hence suppresses Th2-,
IgE-, and IgA-dependent immune responses.
A few antigens can induce humoral immune responses without
involvement of T lymphocytes. One example is trinitrophenol-Ficoll
(lipopolysaccharide). Sensitization of animals to this antigen yields
immunoglobulin responses that can be measured in an ELISA. This is a
useful test for use in mechanistic studies to separate the effects of
compounds on B and T cells.
4.2.4 Antibody responses to sheep red blood cells
4.2.4.1 Spleen immunoglobulin M and immunoglobulin G plaque-forming
cell assay to the T-dependent antigen, sheep red blood cells
A widely used particulate T cell-dependent antigen is sheep red
blood cells. Antibody titres induced after sensitization can be
assayed with various techniques; one that is widely used is the
plaque-forming cell assay, or antibody-forming cell response. This
assay is relatively simple and can be conducted with inexpensive
equipment found in most laboratories, but the optimal concentration of
sheep red blood cells must be injected. As the antigenicity of red
blood cells varies significantly from sheep to sheep, time must be
invested to obtain cells from a sheep that repeatedly gives a high
response (>= 1500 plaque-forming cells/106 spleen cells). The
number of cells administered (about 2 × 108) should also be
optimized for both mice and rats in the laboratory conducting the
assay. The intravenous route is that preferred for sensitization;
intraperitoneal injections can be used but significantly increase the
potential for nonresponding animals as a result of a poor injection.
Animals are sacrificed on day 4 after injection, and spleen cells are
prepared by mincing the spleen between two frosted microscope slides,
teasing it apart with forceps, or passing it through a small mesh
screen; all of these methods are satisfactory, and that used to
prepare single splenocyte cultures varies from laboratory to
laboratory. An aliquot of cells is added to sheep erythrocytes and
guinea-pig complement; these are placed in a microscope slide chamber
when the Cunningham assay method is used (Cunningham & Szenberg,
1968), or, in the Jerne method, cells and guinea-pig complement are
added to a test tube containing warm agar and after thorough mixing
the test tube mixture is plated in a petri dish and covered with a
microscope cover slip (Jerne et al., 1974). In either case, the
preparations are then incubated at 37°C for 3-4h to allow plaques to
develop. The plaques are counted under a Bellco plaque viewer. A
plaque results from the lysis of sheep erythrocytes and is elicited as
a result of the interaction of complement and antibodies directed
against sheep erythrocytes, which are produced in response to the
intravenous sensitization. As each plaque is generated from a single
IgM antibody-producing plasma cell, the number of IgM plaque-forming
cells present in the whole spleen can be calculated. The data are
expressed as specific activity (IgM plaque-forming cells/106 spleen
cells) and IgM plaque-forming cells per spleen.
By incorporating rabbit anti-mouse or anti-rat IgG antibody into
the preparation of spleen cells, complement, and sheep red blood
cells, the number of IgG antibody-forming cells present in the spleen
can also be determined. This number is calculated by subtracting the
number of IgM plaque-forming cells from the total number of both IgM
and IgG plaque-forming cells. The optimal IgG primary response is
observed five days after sensitization (Sikorski et al., 1989).
The T-dependent IgM response to sheep red blood cells is one of
the most sensitive immunotoxicological assays currently in use. Luster
et al. (1992) reported that the individual concordance of the plaque-
forming cell assay for predicting immunotoxicity was the highest of
all the functional assays (78%). Furthermore, use of this assay in
combination with either NK cell activity or surface marker analysis
resulted in pairwise concordances for predictability of more than 90%.
While the plaque-forming cell assay has been shown to be
sensitive and predictive, the procedure does have its limitations. As
indicated earlier, the effect of the test compound on the immune
system is evaluated only in spleen cells, and effects on other
antibody-producing organs and tissues are not determined. The assay is
somewhat laborious, and it is preferable that several people
participate, to help in removing spleens, preparing cell preparations,
counting cells, and adding preparations to either microscope slide
chambers or agar dishes. An additional drawback is that the assay must
be conducted on the same day as the animals are sacrificed. This is in
marked contrast to the ELISA, in which sera can be frozen and
evaluated at a later date. While the slides and petri dishes can be
placed in a cold room or refrigerator and counted the next day, this
procedure is not recommended, as they tend to dry out to some extent,
making viewing and discerning plaques more difficult.
4.2.4.2 Enzyme-linked immunosorbent assay of anti-sheep red blood
cell antibodies of classes M, G, and A in rats
An alternative to the plaque-forming cell assay is ELISA of anti-
sheep red blood cell antibody titres in serum. Antigen preparations
made from ghosts of sheep erythrocytes by extraction with potassium
chloride are used to coat the bottoms of the wells of 96-well
microtitre plates. Serum samples from rats immunized with sheep
erythrocytes are titrated onto these plates using specific polyclonal
antibodies to rat IgM or IgG, to which peroxidase is conjugated. IgA
has also been assayed, using monoclonal anti-rat IgA antibodies and
polyclonal rat anti-mouse IgG conjugated with peroxidase. The ELISA of
serum titres of IgM, IgG, and IgA to sheep erythrocytes is an easy,
reliable method that can be used to detect the effects of chemicals on
the immune system of the rat (Van Loveren et al., 1991; Ladics et al.,
1995).
The assay measures titres of specific antibodies, in contrast to
the plaque-forming cell assay which determines the number of cells
that are actually responsible for production. The ELISA assesses the
production of antibodies, either per cell or in terms of the total
capacity of the host to produce these antibodies in vivo. In
interpreting the effects of exposure to chemicals, account must be
taken of the fact that the cells used in the assay are derived from
specialized parts of the body, such as the spleen, and alterations in
the numbers of antibody-producing cells in such an organ in rats
immunized with sheep red blood cells cannot give information on other,
inaccessible pools of antibody-producing cells. In the ELISA,
alterations in titres due to exposure to chemicals indicate changes in
the immune potential of the exposed animals. In screening for the
effects of chemicals on the immune system, therefore, ELISAs may be
preferable, but for studies on specific immunosuppressive mechanisms,
the plaque-forming cell assay, although labour- and time-intensive, is
a powerful tool for obtaining information complementary to the data
provided by the ELISA. Unfortunately, it is not always possible to
perform the two assays with material from the same animal. The peak
response in the plaque-forming cell assay in both rats (Fischer 344)
and mice (B6C3F1) occurs on day 4 after sensitization, while the peak
response in the ELISA occurs on day 6 for rats and day 4-5 for mice
(Temple et al., 1993). In order to detect the effects of chemicals on
the immune response to sheep red blood cells, it is preferable to
choose the optimal conditions, or to follow the kinetics, of the
response.
4.2.5 Responsiveness to B-cell mitogens
Responsiveness to lipopolysaccharide is another estimate of
humoral immune response, as solely B cells respond to this mitogen.
Although the responses of rats to this mitogen are less pronounced
than those of mice, good results can be obtained, and the
immunosuppressive effects of chemicals can be detected (Vos et al.,
1984).
An alternative B-cell mitogen is S. typhimurium mitogen (STM),
a water-soluble, proteinaceous extract derived from the cell walls of
S. typhimurium; it is a more potent mitogen for rat B lymphocytes
than lipopolysaccharide (Minchin et al., 1990). In both mice and rats,
the polyclonal activation of B lymphocytes is a multistep process. In
mice, mitogens alone can provide all the signals necessary for
proliferation and differentiation; in the rat, STM stimulation induces
B lymphocytes to proliferate without differentiating. The addition of
lymphokines to STM-stimulated B cells also failed to stimulate them to
differentiate (Stunz & Feldbush, 1986). Nevertheless, this mitogen is
useful for evaluating effects on the proliferative ability of rat B
lymphocytes. Smialowicz et al. (1991) showed a decrease in the STM
response in Fischer 344 rats after oral exposure to 2-methoxyethanol.
Unlike the bell-shaped mitogen dose-response curves observed with
T-cell mitogens, the proliferative response of B lymphocytes to both
lipopolysaccharide and STM rises quickly at low concentrations of the
mitogens and plateaus at higher concentrations. As a result, a single
concentration on the plateau phase of the mitogen response curve is
sufficient to evaluate the effects of a test compound on B-cell
mitogen-driven proliferation. One of the reasons that the mitogen
assays appear to be insensitive is that the cells must remain in
culture for several days in order to obtain a peak response. As a
result, they may recover from the immunomodulatory effects of the test
compounds during this in-vitro phase. This is a common problem with
many ex-vivo/in-vitro assays, including the cytotoxic T lymphocyte and
mixed leukocyte response assays; because of the short, 4-h period of
the NK cell assay, this is less of a concern.
4.2.6 Responsiveness to T-cell mitogens
The proliferative ability of T lymphocytes after stimulation with
mitogens can be measured by the uptake of 3H-thymidine in a manner
similar to that used to measure B-cell proliferation (Anderson et al.,
1972). Concanavalin A and phytohaemagglutinin are T-cell mitogens in
both rats and mice; pokeweed mitogen stimulates the proliferation of
both T and B cells and thus lacks specificity. Although both
concanavalin A and phytohaemagglutinin stimulate T lymphocytes,
T cells responsive to concanavalin A have been reported to be less
mature than those responsive to phytohaemagglutinin (Stobo & Paul,
1973). Multiple concentrations of these mitogens should be used to
ensure that a peak response is obtained: both produce a bell-shaped
dose-response curve, and too high a concentration can result in a
suboptimal response.
Historically, mitogens have been included in the battery of tests
for evaluating potential immunotoxicity, because the assay is one that
can also be carried out in humans. Human studies, however, are
conducted on peripheral blood, while most studies of rodent lymphocyte
transformation are conducted using spleen or lymph node cells. Thus,
the argument that the assay has clinical relevance is not well
founded. Furthermore, as the response of lymphocytes is extremely
robust, the assay lacks sensitivity. After a significant number of
compounds were evaluated for potential immunotoxicity in mitogen
assays, use of this assay was shifted from the tier 1 screen
originally described by Luster et al. (1988) to the tier 2
comprehensive evaluation. Use of the mitogen assay has now been
removed completely from studies conducted for the NTP, since other
assays in which cellular proliferation is required (e.g. plaque-
forming cell assay, mixed leukocyte reaction) were considered to be
more sensitive, and the data obtained from the mitogen assays add
little if any to an evaluation of the potential immunotoxicity of test
compounds.
4.2.7 Mixed lymphocyte reaction
In the mixed lymphocyte reaction (also known as mixed lymphocyte
culture), suspensions of responder T lymphocytes from spleen or lymph
nodes are co-cultured with allogeneic stimulator cells. The foreign
histocompatibility antigen (MHC class I or class II molecules)
expressed on the allogeneic stimulator cells serves as the activating
stimulus for inbred populations. In noninbred populations, a pool of
allogeneic cells can be used as stimulators. The assay analyses the
ability of T cells to recognize allogenic cells as 'non-self' as a
result of the presence of different MHC class II antigens on their
surface. In response to the class II antigens, the spleen or node
cells proliferate. Because a sufficiently large number of T cells in
the mixed lymphocyte population respond to the stimulator population,
the responder T cells need not be primed. Proliferation of the
responder cells is one of the parameters for T-cell responsiveness to
cellular antigens. If the allogeneic stimulator cell suspension
contains T cells, their uptake of 3H-thymidine must be prevented by
gamma-irradiation or mitomycin C, in order to preclude background
thymidine uptake.
4.2.8 Cytotoxic T lymphocyte assay
The Tc lymphocyte assay is a continuation of the mixed lymphocyte
reaction response in which the T lymphocytes further differentiate
into cytotoxic effector cells under the influence of various
cytokines. In mice, the assay is usually conducted using P815
mastocytoma cells as the sensitizer and target cell (Murray et al.,
1985). Mice are exposed in vivo to the test agent, and spleen cells
are then removed and placed in culture flasks with the P815
mastocytoma cells. After a five-day co-culture period, the spleen
cells are harvested and added to fresh P815 mastocytoma cells which
have been radiolabelled with 51Cr as sodium chromate. After a 4-h
incubation, the percentage cytotoxicity is determined by measuring the
specific release of 51Cr into the supernatant. The five days of
culture are necessary for the T lymphocytes to differentiate into
cytotoxic effector cells. Unfortunately, this extended period in
culture may give the spleen cells sufficient time to recover from any
adverse effects of the test compound, although such effects may have
been present at the time the spleen cells were removed from the
animal. This inherent limitation of the assay detracts from its
usefulness in assessing the immunotoxicity of test compounds.
A holistic Tc lymphocyte assay has been described, in which the
animal is sensitized after injection of the irradiated target cells
(Devens et al., 1985). Inhibiting the ability of the sensitizing cells
to proliferate either through irradiation or mitomycin C treatment
before injection prevents development of Tc lymphocytes in the animal.
Smialowicz et al. (1989) developed an assay in rats in which effector
cells are generated in culture by incubating cells with lymph node
cells from Wistar/Furth rats, and 51Cr-labelled W/Fu-G1 tumour cells
are used as the target cells. The assay requires four days in culture
and can be run simultaneously with the rat mixed lymphocyte reaction,
thus providing information on the test compound's ability to affect
proliferation and differentiation into effector cells.
4.2.9 Delayed-type hypersensitivity responses
Delayed-type hypersensitivity responsiveness is a reflection of
the capacity of the cellular immune system to execute immune responses
and especially those dependent on IL-2 and INF gamma, which include
attraction and activation of nonspecific mononuclear leukocytes
(macrophages-monocytes). Many systems can be used, depending on the
antigen. One is sensitization to BCG, followed by challenge with
purified protein derivative, to which sensitivity is induced. Another
example is ovalbumin, to which sensitization is most efficient if the
ovalbumin is emulsified in complete Freund's adjuvant. In this system,
delayed hypersensitivity can be measured to both purified protein
derivative and ovalbumin (Vos et al., 1980). Another antigen is
L. monocytogenes: This system is particularly interesting since it
can be used in the context of experiments in which host resistance to
this pathogen is also measured (Van Loveren et al., 1988a).
Delayed hypersensitivity responses can be measured after
sensitization to Listeria by subcutaneous injection of the test
antigen into the ears. Prior to and 24 and/or 48h after challenge, the
increment in ear thickness can be measured with a micrometer by a
person unaware of the experimental group. The background ear swelling
responses of similar, unimmunized control animals are subtracted from
the swelling responses found in immunized animals.
Several delayed-type hypersensitivity assays have been developed
and used for evaluating immunotoxicity in the mouse. Most have
involved measuring swelling in either the footpad or the ear after
sensitization and challenge with a protein antigen. Studies by
LaGrange et al. (1974) demonstrated that sheep erythrocytes could
elicit a delayed-type hypersensitivity response after a single
injection into the foot pad; however, more sheep erythrocytes were
needed to elicit the delayed-type hypersensitivity response than to
produce the optimal humoral immune response. Foot pad swelling can be
measured with a micrometer, as described for rats or by a more
objective, isotopic procedure, as described by Paranjpe & Boone (1972)
and Munson et al. (1982). The delayed-type hypersensitivity response
to sheep erythrocytes was previously considered to be a good assay for
detecting effects on cell-mediated immunity; however, the lack of
persistence of the response (LaGrange et al., 1974; Askenase et al.,
1977) and the possible contribution of antibody to the response raised
concern about the specificity of the assay when sheep erythrocytes are
used as the eliciting antigen. Benzo[ a]pyrene, a compound that
selectively affects humoral but not cell-mediated immunity in adult
mice, appears to decrease cell-mediated immunity when measured in the
sheep erythrocyte assay but has no effect on delayed-type
hypersensitivity when evaluated in the keyhole limpet haemocyanin
assay. The effect in the sheep erythrocyte assay is observed at doses
of benzo[ a]pyrene that decrease antibody production, suggesting a
significant antibody component of the swelling observed (White, 1992).
Keyhole limpet haemocyanin is another protein antigen used in
evaluating delayed-type hypersensitivity responses. Holsapple et al.
(1984) characterized the response to this antigen in the mouse,
showing that it produced the classical delayed-type hypersensitivity
response both with and without adjuvant. Two immunizations with
keyhole limpet haemocyanin were required, however, to produce a
response equivalent to one obtained with complete Freund's adjuvant.
In these studies, animals were sensitized with subcutaneous injections
of keyhole limpet haemocyanin in the shoulder area, with seven days
between the sensitizations. They were then challenged with the same
antigen injected intradermally into the central portion of the pinna
of one of the ears. Increases in ear thickness were evaluated by both
micrometer readings and radioisotopically. The unchallenged ear was
used as an individual control for each animal, and a group of
unsensitized but challenged animals was used to control for
nonspecific and background effects. The results indicated that,
whenever possible, the use of adjuvant in delayed-type
hypersensitivity studies should be avoided. Despite the fact that
complete Freund's adjuvant boosted the responses to keyhole limpet
haemocyanin, it partially masked the dexamethasone-induced suppression
of the response. In some cases, however, delayed-type hypersensitivity
responses are difficult to induce without adjuvant.
The studies currently conducted in mice and rats with this assay
are holistic assays for evaluating cell-mediated immunity. Since
sensitization and challenge occur in the intact animal, all components
of the immune system are present to respond in a physiologically
relevant manner. This type of assay is much more valuable for
evaluating the effects of compounds on cell-mediated immunity than are
in-vitro assays such as the mixed leukocyte response or Tc cell assay.
Luster et al. (1992) reported that the delayed-type hypersensitivity
response assay in mice was highly predictive (100% concordance) of
immunotoxicity when used in combination with the NK cell assay and the
plaque-forming cell assay.
4.2.10 Host resistance models
4.2.10.1 Listeria monocytogenes
Relevant mechanisms of defence against L. monocytogenes include
phagocytosis by macrophages and T cell-dependent lymphokine production
which enhances phagocytosis (Mackaness, 1969; McGregor et al., 1973;
Takeya et al., 1977; Pennington, 1985; Van Loveren et al., 1987).
Humoral immunity is not relevant in protection against infection in
this model. Clearance of Listeria after infection by, for instance,
the intravenous or the intratracheal route can be assessed at various
times after infection by determining the numbers of colony forming
units in the spleen or lungs, respectively. This can be done by
classical methods (Reynolds & Thomson, 1973) that involve the
following steps: Serial dilutions of homogenates of the organs,
prepared in mortars with sterile sea sand, are plated onto sheep blood
agar plates; after a 24-h incubation at 37°C, the colonies are counted
to determine the number of viable bacteria in the organ. Differences
in the numbers of bacteria retrieved from the organs are an indication
of the clearance of the bacteria, i.e. the rate at which the host
disposes of the bacteria after infection.
Histopathology after a Listeria infection can also be valuable.
For instance, exposure to ozone before an intratracheal infection with
Listeria affects pathological lesions due to the infection (Van
Loveren et al., 1988a): Pulmonary infection with Listeria induces
histopathological lesions characterized by foci of inflammatory cells,
such as lymphoid and histiocytic cells, accompanied by local cell
degeneration and influx of granulocytes. If rats are exposed to ozone
for one week before infection, the lesions are much more severe than
in unexposed animals and persist at times when either ozone-associated
or infection-associated effects alone would have resolved. The quality
of the lesions is also influenced by prior exposure to ozone: mature
granulomas were found in Listeria-infected rats that were also
exposed to ozone.
When mice are challenged with Listeria, mortality is the usual
end-point monitored; however, clearance and organ bacterial colony
counts can also be determined. L. monocytogenes is a Gram-positive
bacterium. The resistance of mice to the organism is genetically
regulated, and the susceptibility of the B6C3F1 strain, the strain
designated by the NTP for immunotoxicity studies, comes from the C3H
parent, since the C57Bl/6 mouse is resistant (Kongshavn et al., 1980).
Listeria can easily be stored at -70°C at a stock concentration of
approximately 108 colony forming units per ml. In studies in mice,
three challenge levels are routinely selected to produce 20, 50, and
80% mortality in the vehicle control animals. Mortality is recorded
daily for 14 days. Treatment groups consisting of 12 mice per group
have been found to be useful for obtaining statistically meaningful
data on host resistance. This assay is extremely reproducible when the
organism is administered intravenously.
The Listeria assay can detect both protection from and
increased susceptibility to chemicals and drugs. Morahan et al. (1979)
used the model to demonstrate a dose-dependent decrease in host
resistance after exposure to delta-9-tetrahydrocannabinol, the major
psychoactive constituent of marijuana. The Listeria host resistance
assay is that most often used in immunotoxicological assessment of
compounds, and numerous examples of its use can be found in the
literature. It is one of the primary models used by the NTP for
evaluating immunosuppression. Since Listeria is a human pathogen,
appropriate precautions are needed in conducting the assay.
4.2.10.2 Streptococcus infectivity models
Two species of Streptococcus have been used widely in bacterial
host resistance models for immunotoxicological assessment.
S. pneumoniae has been used primarily for evaluating systemic
immunity. S. zooepidemicus has also been used to evaluate systemic
immunity but is used extensively to evaluate the effects of drugs and
chemicals on the local immunity of the pulmonary system.
S. pneumoniae is a Gram-positive coccus to which host
resistance is multifaceted (Winkelstein, 1981). The first line of
defence against this organism is the complement system. Activation of
the complement system can result in direct lysis of certain strains of
S. pneumoniae; however, owing to the nature of their cell wall, some
strains are resistant to lysis by complement. Complement can still
participate directly in the removal of these bacteria as a result of
deposition of complement component C3 on their surface, which
facilitates phagocytosis by polymorphonuclear leukocytes and
macrophages. In the later stages of the infection, antigen-specific
antibody plays a major role in controlling the infection. Thus,
compounds that affect complement, polymorphonuclear leukocytes, B-cell
maturation and proliferation, or the production of antibody can be
evaluated in this system. S. pneumoniae is an excellent model for
evaluating immunotoxicity, since it elicits multiple immune components
which participate in host resistance, each of which can be a potential
target for an adverse effect of a xenobiotic. To date, this model has
had limited success in rats.
Preparation of S. pneumoniaefor challenge is slightly more
complicated than the procedures used for Listeria; however, the
potential of the model for detecting immunotoxic compounds makes the
additional steps worthwhile. Stock preparations of S. pneumoniae
(ATCC 6314) are easily maintained at -70°C in defibrinated rabbit
blood, and aliquots of the stock preparation can be removed and grown
in culture at various dilutions to obtain the desired challenge
concentration. An alternative approach is to grow the organism in
culture and to monitor the bacterial concentrations by measuring the
turbidity of the culture. A 5-µl aliquot of the stock preparation is
used to inoculate 50ml of brain-heart infusion broth, which is
incubated at 37°C, and the turbidity of the overnight culture is
determined with an Abbott Biochromatic analyser system or another
instrument that can sensitively measure changes in culture turbidity.
The overnight culture is diluted with fresh brain-heart infusion broth
to yield an absorbence difference of 0.020-0.025. The turbidity of the
subculture is monitored periodically, and when the optimal density
reaches an absorbence difference of 0.080, the subculture is rapidly
cooled in an ice bath and diluted to the desired inoculum level. The
turbidity of each inoculum is checked in the analyser, and adjustments
are made to obtain the preselected differences in absorbence.
Routinely, one day after the last exposure, female mice are challenged
intraperitoneally with 0.2ml of the S. pneumoniae inoculum. If the
inoculum is administered intravenously, extremely high challenge
levels must be used, which may reflect the efficiency of the
mononuclear phagocyte system to clear and kill the organism. Three
innocula, each at a different concentration, are prepared to give a
range of lethality (e.g. 20, 50, and 80%), and a sample of each is
serially diluted and placed on blood agar plates to determine the
number of colony-forming units administered to the animals. Owing to
the rapid onset of infection, mortality is recorded twice daily for
seven days. In studies by White et al. (1986), when female B6C3F1 mice
were exposed daily for 14 days to 1,2,3,6,7,8-hexachlorodibenzo-
para-dioxin or TCDD by gavage, they were found to have decreased
host resistance to S. pneumoniae, which is consistent with the
decrease in complement activity caused by these compounds.
Another species of Streptococcus that has been used as a host
resistance model is S. zooepidemicus, a group C streptococcus
(Fugmann et al., 1983). Exposure to N-nitrosodimethylamine was shown
to decrease host resistance to this strain significantly. Infection
with S. zooepidemicus may be dependent on an antibody-mediated
response, since the time to death after challenge is considerably
longer than with S. pneumoniae. Numerous studies have demonstrated
that aerosolized S. zooepidemicus is one of the most sensitive
indicators of the toxicity of air pollution: Mice exposed for short
periods to single or mixed pollutants before infection with an aerosol
of S. zooepidemicus and then assessed for mortality over 20 days had
increased mortality with increasing concentrations of ozone (Coffin &
Gardner, 1972; Ehrlich et al., 1977), nitrogen dioxide (Ehrlich &
Henry, 1968; Sherwood et al., 1981), sulfur dioxide (Selgrade et al.,
1989), metal particulates (Gardner et al., 1977; Adkins et al., 1979,
1980; Aranyi et al., 1985), phosgene (Selgrade et al., 1989), and
other volatile organic compounds (Aranyi et al., 1986). With many of
these compounds, enhanced susceptibility to infection has been
demonstrated at concentrations at or below the United States national
ambient air quality standards or threshold limit values. With all of
these compounds, enhanced mortality has been associated with failure
to clear bacteria from the lung and suppression of alveolar macrophage
phagocytic function. This model has recently been adapted to rats. In
this species, both ozone (Gilmour & Selgrade, 1993) and phosgene (Yang
et al., 1995) delayed clearance of bacteria from the lungs and
enhanced the inflammatory response (polymorphonuclear leukocytes in
lavage fluid) at concentrations that do not themselves produce
inflammation; however, mortality does not occur in this species. While
the bacteria have generally been administered as aerosols, Sherwood et
al. (1988) showed that similar results could be obtained when they
were administered intratracheally or intranasally. Since some strains
of Streptococcus are pathogenic to humans, appropriate precautions
must be taken when using this host resistance model.
4.2.10.3 Viral infection model with mouse and rat cytomegalovirus
Cytomegalovirus infections are widely distributed in humans, with
about 60-90% of the population infected. Human cytomegalovirus
infections occur in several forms, the most serious being congenital
and perinatal infection and infection of immunosuppressed individuals.
Less serious forms include post-perfusion syndrome and some cases of
infectious mononucleosis; however, the vast majority of postnatal
infections in immunocompetent individuals are clinically asymptomatic.
More severe disease may occur in immunodeficient hosts, such as
transplant patients (Naraqi et al., 1977; Pass et al., 1978; Marker et
al., 1981; Rubin et al., 1981). Primarily on the basis of
morphological considerations, cytomegaloviruses are classified as
members of the family Herpesviridae. Because these viruses have a
relatively protracted replication cycle, a slowly developing
cytopathology characterized by cytomegaly, and a relatively restricted
host range, they are grouped into the beta Herpesviridae subfamily
(Roizman et al., 1981; Roizman, 1982). The roles of several arms of
the immune system in resistance to cytomegalovirus have been studied
extensively in mice. The role of humoral immunity is not well
understood. It was suggested initially that neutralizing antibodies do
not play a pivotal role in recovery from cytomegalovirus infection in
mice (Osborn et al., 1968; Tonari & Minamishima, 1983); however, the
role of antibodies in neutralization of murine cytomegalovirus and in
antibody-dependent cell-mediated cytotoxicity is now recognized
(Manischewitz & Quinnan, 1980; Quinnan et al., 1980; Farrell &
Shellam, 1991). Cytomegalovirus-specific Tc cells can be detected in
cytomegalovirus-infected mice (Ho, 1980; Quinnan et al., 1980). NK
cell activity appeared to be the most effective, especially during the
initial stages of infection (Bancroft et al., 1981; Selgrade et al.,
1982; Bukowski et al., 1984). Enhanced susceptibility to infection has
been demonstrated in mice when macrophage function was blocked by
silica, and transfer of syngeneic adult macrophages to suckling mice
significantly increased their resistance to mouse cytomegalovirus
infection (Selgrade & Osborn, 1974). An inverse correlation is seen
between the virulence of mouse cytomegalovirus and its infectivity for
peritoneal macrophages (Inada & Mims, 1985), suggesting that
attenuated virus may be controlled, in part, by macrophages. Since the
rat virus acts very much like the attenuated mouse virus, macrophages
may be even more important in rats. Macrophages may facilitate the
generation of latent infection (Booss, 1980; Yamaguchi et al., 1988).
Enhanced susceptibility to mouse cytomegalovirus has been
demonstrated after treatment of mice with cyclophosphamide,
cyclosporin A, nickel chloride, or DMBA. Treatment with benzo[ a]-
pyrene or TCDD did not affect susceptibility to this infection
(Selgrade et al., 1982). Enhanced susceptibility was correlated with
chemical suppression of virus-augmented NK cell activity during the
first week of infection. In rats, exposure to immunotoxic agents such
as organotin compounds led to altered resistance to rat
cytomegalovirus (Garssen et al., 1995).
Experimentally, rodents can be inoculated intraperitoneally with
a species-specific cytomegalovirus, and the concentration of the virus
in tissue can be determined in a plaque-forming assay, which is a
modification of the method described by Bruggeman et al. (1983, 1985).
Rat embryo-cell monolayers are prepared in 24-well plates. Different
organs (salivary gland, lung, kidney, liver, spleen), obtained at
various times after infection, are homogenized in a tissue grinder and
stored as 10% weight/volume samples at -135°C until use. The confluent
monolayers are then infected with 10-fold serial dilutions of the
organ suspensions. After centrifugation, the suspension is removed,
and 1 or 0.6% agarose is added. After incubation at 37°C in 5% carbon
dioxide for seven days, the cells are fixed in 3.7% formaldehyde
solution, the agarose layer is removed, and the monolayer is stained
with 1% aqueous methylene blue. Plaques are counted under a
stereoscopic microscope.
In PVG rats, cytomegalovirus is detectable eight days after
infection, although the virus load is much higher on days 15-20. The
viral load in the salivary gland is higher than that in other organs,
i.e. spleen, lung, kidney, and liver. In contrast, in Lewis rats and
BN rats the viral load in e.g. the kidney was higher than that in the
salivary gland during the first week after infection (Bruning, 1985),
perhaps due to a strain difference (Bruggeman et al., 1983, 1985).
Total body irradiation of PVG rats with 60Co one day before
infection with cytomegalovirus increased the viral load in the
salivary gland, lung, kidney, spleen, and liver over that in
unirradiated PVG rats; histological analysis also indicated a higher
viral load in the salivary gland of infected rats. The mucosal
epithelium of the salivary gland contains enlarged cells with nuclear
inclusion bodies; these could be detected in irradiated, infected rats
only if the salivary gland was dissected and fixed 15 days after
infection. These results are in agreement with those of Bruggeman et
al. (1983), who found that gamma irradiation also induced higher viral
loads in the salivary gland of BN rats. Taken together these results
indicate a role for cellular immunity in resistance to this virus in
rats.
4.2.10.4 Influenza virus model
Influenza virus A2/Taiwan H2N2 has been used as a viral
challenge in evaluating alterations in host resistance of mice after
exposure to various compounds. Compounds that decrease host resistance
to the virus are N-nitrosodimethylamine (Thomas et al., 1985b) and
TCDD (House et al., 1990a). Compounds that do not to alter host
resistance to this pathogen include ozone (Selgrade et al., 1988),
benzo[ a]pyrene, benzo[ e]pyrene (Munson & White, 1990), methyl
isocyanate (Luster et al., 1986), and Pyrexol (House et al., 1990b).
Mortality is the end-point routinely measured in evaluating decreased
host resistance to influenza virus, which is usually instilled
intranasally (Fenters et al., 1979). Host resistance to this virus has
been reported to be mediated by cell-mediated immunity (Ada et al.,
1981), interferon (Hoshino et al., 1983), and antibody (Vireligier,
1975). This model had been suggested for use in evaluating compounds
that affect humoral immunity; however, its inability to detect such
compounds indicates that it is not suitable. A possible explanation
for the discrepancy is that administration of the virus by intranasal
instillation may invoke local immune mechanisms in the lung and may
not adequately reflect systemic immunocompetence. In several cases,
enhanced mortality has been demonstrated in the absence of effects on
viral titres in the lung (Selgrade et al., 1988; Burleson et al., in
press), indicating that enhanced mortality does not always reflect
effects on virus-specific immune defences.
Influenza virus has been used in evaluating immunotoxicity in
rats, after adaptation. Studies by Ehrlich & Burleson (1991) showed
that rats exposed to phosgene had significantly decreased host
resistance. TCDD was shown to affect the resistance of rats to the
adapted influenza virus RAIV (Yang et al., 1994).
As influenza virus is a human pathogen, appropriate precautions
must be taken.
4.2.10.5 Parasitic infection model with Trichinella spiralis
Resistance to infection with the helminth T. spiralis has been
evaluated in both mice and rats after exposure to a variety of
chemicals. In humans, as in other carnivores, infection occurs by
eating meat containing infectious larvae. The life cycle of the worm
is as follows: Infectious larvae excyst in the acid-pepsin environment
of the stomach, rapidly migrate to the jejunum, and penetrate host
intestinal epithelial cells. Sexually mature parasites are present
within three to four days after infection. The viviparous females
produce larvae that migrate via the lymphatic and blood vessels to
host muscle, where they encyst and are encapsulated within a host-
derived structure. Encapsulated muscle larvae can survive for years
within this structure.
An intense inflammatory response, comprised mainly of mast cells
and eosinophils, accompanies intracellular infection in the intestine.
T Cell-dependent immunity plays a crucial role in this inflammatory
response (Manson-Smith et al., 1979; Vos et al., 1983b; Wakelin,
1993), which is responsible for the expulsion of adult parasites.
Antibodies damage the reproductive structures of the female parasite
(Love et al., 1976), have a major role in the rapid elimination of
subsequent infections in rats (Appleton & McGregor, 1984), and
sensitize migrating newborn larvae for destruction by granulocytes
(Ruitenberg et al., 1983).
The number of encysted muscle larvae is typically much higher in
immunosuppressed animals than in immunocompetent animals, due perhaps
to delayed expulsion of adult worms from the intestine, decreased host
control of parasite fecundity, decreased destruction of migrating
larvae, or a combination of resistance defects. These end-points of
host resistance to T. spiralis infection and class-specific antibody
titres can be measured by standard techniques (Van Loveren et al.,
1994). Histological evaluation of the inflammatory infiltrate
surrounding encysted muscle larvae has also been described (Van
Loveren et al., 1993b).
It should be noted that direct effects of the chemical under
study can affect the outcome of infection. For example Bolas-Fernandez
et al. (1988) determined that cyclosporin A delays expulsion of adult
parasites from the intestines of rats but does not increase the number
of larvae encysted in host muscle. This was determined to be a direct
effect of cyclosporin A on the fecundity of female parasites rather
than on immunity to infection. Animals are infected by oral gavage
with known numbers of larvae, isolated from infected donor muscle.
Because infection is spread only by consumption of infected meat or
freshly isolated larvae, there is little danger of the infection
spreading to other animals housed in the same room. T. spiralis is a
human pathogen and must be handled as such; normal laboratory
practices are sufficient to prevent accidental infection.
T. spiralis infection has been used as a host resistance model
in both rats and mice. In general, chemicals that suppress T-cell
function suppress resistance to T. spiralis infection. Thus, TBTO
(Vos et al., 1990b), diethylstilbestrol (Luebke et al., 1984), TCDD
(Luebke et al., 1994, 1995), and the virustatic agent acyclovir
(Stahlmann et al., 1992) had deleterious effects on resistance.
4.2.10.6 Plasmodium model
Two strains of Plasmodium have been used to evaluate the
potential immunotoxicity of compounds. P. yoelii (17XNL) is a
nonlethal strain that produces a self-limiting parasitaemia in mice.
Resistance to this organism is multifaceted and includes specific
antibody, macrophage involvement, and T cell-mediated functions
(Luster et al., 1986). In this assay, animals are injected with 106
parasitized erythrocytes, and the degree of parasitaemia is monitored
over the course of the infection by taking blood samples. In control
animals, the peak response usually occurs 10-14 days after injection.
The degree of parasitaemia can be evaluated by a variety of methods,
e.g. manually, by counting parasitized erythrocytes in blood smears
(Luebke et al., 1991). Host resistance to P. yoelii has been used to
assess the immunotoxicity of benzidine (Luster et al., 1985b),
diphenylhydantoin (Tucker et al., 1985), TCDD (Tucker et al., 1986),
pyran copolymer (Krishna et al., 1989), gallium arsenide (Sikorski et
al., 1989), and 2'-deoxycoformycin (Luebke et al., 1991).
P. berghei is lethal to mice and certain strains of rats and
has been used in assessing immunotoxicity (Loose et al., 1978). Host
resistance depends on specific antibody production and ingestion and
destruction of antibody-coated Plasmodium by phagocytic cells such
as macrophages. T Lymphocytes may also be involved in host resistance
to the organism (Bradley & Morahan, 1982). Mortality has been
evaluated after injection of 106 Plasmodium-infected erythrocytes.
Compounds that have been evaluated for immunotoxicity in this model
system include 4,4'-thiobis(6- tert-butyl- meta-cresol) (Holsapple
et al., 1988), dietary fish-oil supplement (Blok et al., 1992), and
styrene (Dogra et al., 1992). Neither P. yoelii nor P. berghei is
infectious in humans; infection of animals can occur only through
parenteral injection of contaminated blood.
4.2.10.7 B16F10 Melanoma model
The B16F10 tumour cell line is a malignant melanoma that is
syngeneic with the C57Bl/6 mouse, which is one of the parents of the
B6C3F1 mouse. This tumour line was selected for its propensity to
metastasize to the lung. The assay is an outgrowth of the work of
Fidler and colleagues (Fidler, 1973; Fidler et al., 1978). NK cells
and macrophages have been proposed to be involved in host resistance
to this metastasizing tumour; however, T lymphocytes have also been
shown to play a role (Parhar & Lala, 1987). This host resistance assay
is referred to as an artificial metastasis model, since the tumour
cells are administered by intravenous injection, usually into the tail
vein, and lodge in the lung, which is the first capillary bed they
encounter. The B16F10 tumour cells can be stored frozen and can easily
be grown in culture before use. Routinely, 1-5 × 105 cells are
injected intravenously into sentinel mice (i.e. untreated mice
injected with the highest challenge level of tumour cells), and the
tumour burden is monitored in order to select the optimal day of
assay.
Two parameters are routinely used to assess tumour burden. One is
DNA synthesis in the lungs of mice bearing tumours. Since background
DNA synthesis in the lungs of mice without tumours is extremely low,
any detectable rate is a result of the presence of a tumour. In order
to measure synthesis, mice are pulsed intraperitoneally one day before
sacrifice with 0.2 ml of 10-6 mol/litre of 5-fluorodeoxyuridine,
followed 30 min later by 2 µCi of 125I-iododeoxyuridine administered
by the intravenous route. After sacrifice, the lungs are removed,
placed in Bouin's fixative solution, and counted with a gamma counter.
A second indicator of tumour burden is visual enumeration of tumour
nodules after fixation in Bouin's solution. The visibility of the
black nodules of the melanin-producing B16F10 tumour cells on the
yellow background of the fixed lung tissue allows enumeration of up to
200-250 nodules on the surface of the lungs. A good correlation has
been shown between number of tumour nodules and radioactivity present
in the lungs (White, 1992). Thus, if the tumour nodules become too
numerous to count, the results of the study can still be determined
from the radioassay. This system has been useful in demonstrating
decreased host resistance after systemic exposure to the tumour
promoter phorbol myristate acetate (Murray et al., 1985),
intratracheal exposure to gallium arsenide (Sikorski et al., 1989),
and exposure to nickel chloride (Smialowicz et al., 1985b); it has
also been used to show enhanced host resistance after exposure to
manganese chloride (Smialowicz et al., 1984) and 4,4'-thiobis(6- tert-
butyl- meta-cresol) (Holsapple et al., 1988).
4.2.10.8 PYB6 Carcinoma model
The PYB6 tumour cell line is a fibrosarcoma originally induced
with a polyoma virus in C57Bl/6 mice. Host resistance to the tumour
includes NK cell activity and T cell-mediated killing (Urban et al.,
1982). While PYB6 cells can easily be grown in culture, they should be
passed through an animal before use in challenge studies for
immunotoxicity (Luster et al., 1988). In studies with the PYB6 line,
mice are injected in the thigh with 1-5 × 103 viable tumour cells
and are then palpated weekly to detect the development of tumours at
the injection site. The end-points evaluated include the incidence of
tumours and time to tumour appearance; tumour size can also be
measured. This assay has been useful in detecting decreased host
resistance to many compounds, including Aroclor 1254 (Lubet et al.,
1986), DMBA (Dean et al., 1986), and benzene (Rosenthal & Snyder,
1987).
4.2.10.9 MADB106 Adenocarcinoma model
A tumour model used to evaluate host resistance in rats is the
MADB106 rat mammary adenocarcinoma, which is syngeneic with the
Fischer 344 rat. NK cells appear to play a major role in host defence
to this tumour (Barlozzari et al., 1985). In this model, survival time
after injection of the cells is the usual end-point monitored.
Compounds that decrease host resistance to the tumour can decrease
both the percentage survival and the survival time of treated animals.
Control rats begin to succumb to the adenocarcinoma two to three weeks
after an intravenous injection of 2 × 106 tumour cells. Smialowicz
et al. (1985b) showed a significant decrease in the survival of
animals treated with a single intramuscular dose of nickel chloride,
which was correlated with a decrease in NK cell activity.
4.2.11 Autoimmune models
Autoimmune models can also be used to investigate whether a
compound exacerbates induced or genetically predisposed auto-immunity.
These models are used mainly to elucidate the pathogenesis of
autoimmunity and the effect of immunosuppression in
immunopharmacology. Few studies have been reported, although the
relevance of the model for extrapolation to humans may be good. A
number of autoimmune models are available in rats and mice. Autoimmune
phenomena can be either induced or occur spontaneously. In induced
models, an autoantigen is isolated from a target organ obtained from
another species (generally bovine), and the animal is immunized with
this purified antigen in adjuvant. Examples in the rat (Calder &
Lightman, 1992) are experimental encephalomyelitis elicited by bovine
spinal cord antigen (Stanley & Pender, 1991), experimental uveitis
elicited by bovine retinal S-antigen (Fox et al., 1987), and adjuvant
arthritis elicited by Mycobacterium containing H37RA adjuvant
(adjuvant arthritis) or collagen (Holmdahl et al., 1990; Klareskog &
Olsson, 1990; Wooley, 1991). Autoimmune phenomena and associated organ
pathology normally emerge in almost all immunized animals within two
to three weeks. Depending on the effector reaction and the
reversibility of the damage, the disease either stops when the damage
is complete (e.g. uveitis, resulting in blindness of the animal) or
the autoimmune reaction is transient and animals recover (adjuvant
arthritis). In some models, animals subsequently experience a relapse
around day 30 (experimental encephalomyelitis). The induction and
development of autoimmunity in these animals are mediated by T cells
that show the cytokine expression pattern (IL-2, INF gamma) of the Th1
subset. The effector phase of disease symptoms is also mainly a T
cell-mediated process, to which CD4+ cells, CD8+ cells, and
macrophages contribute. Lewis (RT11) rats are particularly
susceptible. These autoimmune models can be induced in other species,
such as mice (Baker et al., 1990) and rhesus monkeys (Rose et al.,
1991). They are generally accepted as models of human (organ-specific)
autoimmune diseases, e.g. experimental allergic encephalomyelitis as a
model of multiple sclerosis, experimental allergic uveitis as a model
of idiopathic posterior uveitis, and adjuvant arthritis as a model of
rheumatoid arthritis.
Autoimmunity can also be induced by metals (Druet et al., 1989;
Bigazzi, 1992). A well-known example is glomerulopathy induced by
mercuric chloride in BN (RT1n) rats. The process is initiated by T
cells with a cytokine synthesis pattern (IL-4) of the Th2 subset. The
relative incidence of these cells is much higher in BB rats than in
other strains. After the cells of the Th2 subset have been stimulated,
there is polyclonal stimulation of B lymphocytes, leading to synthesis
of antibodies (including pathogenic antibodies) to the glomerular
basement membrane. These antibodies subsequently mediate autoimmune
destruction of renal glomeruli. This model is the best studied model
of 'drug'-induced autoimmunity. Mercuric chloride elicits
glomerulonephritis in other rat strains, in which glomerular
destruction is not due to anti-glomerular autoantibodies but is
mediated by immune complexes deposited in the glomerulus (Druet et
al., 1989).
In spontaneous models, predisposition to the development of
autoimmune phenomena and disease is determined by the genetic
composition of the animal strain. Well-known examples are BB rats
(Like et al., 1982; Guberski, 1994) and NOD mice (Lampeter et al.,
1989; Leiter, 1993), which develop autoimmune pancreatitis and
subsequently diabetes. Within the pancreas, the islets of Langerhans
are infiltrated by T lymphocytes and macrophages; subsequent
destruction of the islets results in diabetes. These spontaneous
models are considered to be animal models of human diabetes (Dotta &
Eisenbarth, 1989; Lampeter et al., 1989; Riley, 1989). Other examples
are the systemic autoimmunity that emerges in certain mouse strains
(Guttierez-Ramos et al., 1990) like (NZB × NZW)F1 mice (Theofilopoulos
& Dixon, 1985) and the mixed lymphocyte reaction in lpr (Matsuzawa
et al., 1990) and gld mice (Roths et al., 1984). The spontaneous
pathology in these animals resembles various disease manifestations in
human systemic lupus erythematosus and is similarly mediated by immune
complexes that deposit in tissue. In (NZB × NZW)F1 mice, mainly lupus
nephritis is induced by immune complexes; in the mixed lymphocyte
reaction in lpr mice, both joint manifestations and
glomerulonephritis are seen. The genes associated with autoimmune and
immune complex disease are not known. In comparison with induced
models, these models have the advantage of spontaneous, gradual
development of autoimmune disease symptoms; however, this is a
disadvantage in experimental design, as not all animals develop
disease, and emerging disease develops at different times or ages.
In general, treatment of animals with immunosuppressive drugs
that interfere with signal transduction (cyclosporin, FK-506,
rapamycin) or cell proliferation (cytostatics like azathioprine,
mizoribine, and brequinar), and anti-inflammatory agents
(corticosteroids) inhibit the development of symptoms in these models.
Exposure to immunotoxic chemicals may also lead to alterations in the
course of disease emergence. For instance, HCB, which leads to
immunoenhancement in rats, markedly enhanced the severity of allergic
encephalomyelitis (Van Loveren at al., 1990c). In contrast, arthritic
lesions were strongly suppressed in HCB-exposed Lewis rats, indicating
that HCB has biologically significant immunotoxic effects. Although
the contrasting effects in the two autoimmune models are not yet
understood, and clear dose-effect relationships have yet to be
established, this type of information should be obtained for risk
assessment.
4.3 Assessment of immunotoxicity in non-rodent species
While most immunotoxicological evaluations are conducted in mice
or rats, use of other species is increasing.
4.3.1 Non-human primates
Various non-human primates, including Macaca mulatta (rhesus
macaques), M. nemestrina (pig-tailed macaques), Cercocebus atys
(sooty mangabeys), M. fasicularis (cynomolgus monkeys), and
marmosets have been used in immunotoxicological studies. Many of the
assays carried out in mice or rats can be adapted for use with non-
human primates. Strategies and methods used in studies of humans have
also been introduced in studies on non-human primates. Monoclonal
antibodies generated to human leukocyte subsets can be used in
phenotyping blood mononuclear cells of e.g. marmoset monkeys
(Callithrix jacchus) (Neubert et al., 1990, 1991), although the
possibility of such use differs, depending on the evolutionary
distance of the non-human primate from humans. While most of the
assays conducted in non-human primates involve serum or peripheral
blood, some assays, such as those used to measure delayed-type
hypersensitivity, are holistic, in that the animals are sensitized
in vivo and then evaluated in vivo at the challenge site (Bugelski
et al., 1990; Bleavins & Alvey, 1991).
The effects of chronic exposure to the PCB Arochlor 1254 on the
immune response of rhesus monkeys have been evaluated by Tryphonas et
al. (1989). In these studies, the lymphocyte response to concanavalin
A and phytohaemagglutinin was evaluated, as were total serum
immunoglobulin levels, antibodies to sheep red blood cells, and
numbers of T and B cells in peripheral blood. In later studies
(Tryphonas et al., 1991a,b), one-way mixed lymphocyte cultures,
antibodies to pneumococcal antigens, phagocytic mononuclear cell
function, NK function, haemolytic complement activity, and production
of IL-1, tumour necrosis factor, thymosins, and interferon were
evaluated in monkeys exposed to Arocolor 1254. Ahmed-Ansari et al.
(1989) evaluated phenotypic markers and function in three species of
non-human primate. The functional assays included NK cell activity,
lymphocyte transformation, and antigen presentation. Extensive studies
included the evaluation of more than 20 phenotypic markers or
combinations of markers for each of the three monkey species. Use of
the monkey as a test species is likely to increase as more and more
biotechnology and recombinant products are produced.
4.3.2 Dogs
While dogs are not the species of choice for immunotoxicological
studies, they are used predominantly in assessing toxicological
safety, and virtually all of the assays used for assessing immunotoxic
potential have been adapted for use in dogs. These include evaluation
of basal levels of IgA, IgG, and IgM (Glickman et al., 1988),
allergen-specific serum IgE (Kleinbeck et al., 1989), mononuclear
phagocyte function (Thiem et al., 1988), NK cell activity (Raskin et
al., 1989), Tc cell activity (Holmes et al., 1989), and mitogen-and
cell-mediated immune responses (Nimmo Wilkie et al., 1992).
4.3.3 Non-mammalian species
Non-mammalian species are also used extensively for evaluating
the potential adverse effects of compounds and agents on the immune
system.
4.3.3.1 Fish
Because of their environment, fish are an excellent model for
studying the effects of water-and sediment-borne pollutants. There are
several other good reasons for studying immunotoxicity in fish: many
of their diseases are related to environmental quality, various
environmental pollutants have immunotoxic potential, and many of the
diseases have an immune component. Moreover, there is concern about
the health status of aquatic ecosystems in relation to pollution, and
fish will be useful target species for developing biomarkers (see
box). Fish are easy to obtain, there is an extensive body of
knowledge, and their economic interest (aquaculture) facilitates the
finding of research resources. At present, immunotoxicology in fish is
not as sophisticated as that in mammals. Screening and functional
tests are being developed in the laboratory but cannot yet be applied
in the field.
A wide range of species is used for field and laboratory studies.
The choice of species depends on its biology (migratory or local,
marine or freshwater; sediment-dwelling or pelagic) and on experience
in the laboratory. A lack of consistency, e.g. becuse of a limited
number of species (as in mammalian immunotoxicology) makes this field
of research diffuse and extended and may result in limited progress;
nevertheless, a variable or consistent effect over a variety of
species is certainly a valuable observation. Some species seem to be
preferred, such as trout, salmon, and carp, which are practical, owing
to their size, for sampling blood and tissues for laboratory studies.
Smaller species, such as guppies (Poecilia reticulata) and medaka
(Oryzias latipes), have secured a niche in aquatic toxicology owing
to the ease of husbandry and relatively low cost; moreover, because of
their small size, whole animals can be used for histopathological
examination (Wester & Canton, 1991), but their application in
immunotoxicology may be limited because of difficulty in obtaining
adequate blood and tissue samples. For studies of saltwater species,
bottom-dwelling flatfish are commonly used in field studies and, to a
certain extent, in studies of mesocosms and in the laboratory. In
Europe, the flounder (Platichthys flesus) and dab (Limanda limanda)
are popular target species since they are susceptible to certain
recognizable diseases and are commonly available.
A compehensive variety of parameters is listed by Anderson (1990)
and Weeks et al. (1992). A modified set based on those lists and
assays used in rodent immunotoxicology is presented in the box above
and classified as tier 1 (screening tests) and tier 2 (functional
assays). Parameters commonly mentioned in the literature are discussed
below.
Blood cell counts and differential counts: As leukocytes play a
major role in specific and nonspecific humoral and cellular immune
responses, this parameter is used as a measure of the status of the
defence system, in particular in tier 1 testing. It is relatively easy
to test blood samples drawn from live animals, but many environmental
factors unrelated to defence may modify leukocyte status (Anderson,
1990). The use of monoclonal antibodies directed against individual
cell types may improve their identification (Bly et al., 1990; Van
Diepen et al., 1991). Another possible parameter is the haematocrit;
however, it has no known specificity for any immune function, although
it may be considered as a general indicator of stress.
Candidate biomarkers for immunotoxicity in fish
Tier 1: Screening tests
* Conventional haematology, including total and differential blood
cell counts, surface markers (flow cytometry), and macrophage
density and morphology: easy, nonspecific
* Serum immunoglobulin concentrations in naive (unstimulated) fish:
easy, limited specificity
* Lymphoid organ weight (mainly spleen, occasionally thymus):
impracticable
* Histopathology of the thymus, spleen, and kidney: possible, can
be specific
Tier 2: Functional assays
* Humoral immune response (agglutination, enzyme-linked
immunosorbent assay): possible, can be specific
* Cellular immune response (allograft rejection in scale, skin, or
eye): possible, can be specific
* Macrophage functions (phagocytosis, bacterial killing, migration,
chemiluminescence): limited specificity
* Host resistance (bacterial infections): possible, can be
specific, relevant
Nonspecific defence: Other indicators of nonspecific defence
have been proposed as indicators of immunological stress. These
include acute-phase proteins (Fletcher, 1986), the levels of which
appear to be stress hormone-dependent; and lysozyme and ceruloplasmin
activity, which are reduced in carp exposed to trichlorphon in vivo
(Siwicki et al., 1990).
Morphology: The spleen is easy to excise and weigh in animals
of adequate size and could thus serve as a biomarker, although it is
not commonly reported in the literature. One reason may be that a
major and variable portion of the spleen consists of storage blood or
erythropoietic tissue (Fänge & Nilsson, 1985); the lymphoid tissue is
poorly developed and is mainly associated with melanomacrophage
centres (Zapata, 1982, 1983; Fänge & Nilsson, 1985; Van Muiswinkel et
al., 1991); and, after immunization, only a small proportion of the
plaque-forming cells is found in the spleen, in contrast to the kidney
pronephros (Van Muiswinkel et al., 1991). The role of the spleen in
most fish species thus seems to be limited, although melanomacrophage
centres are abundant.
Experimental immunotoxicology in mammals has demonstrated that
the weight of the thymus, a primary and exclusively immunological
organ, is a sensitive indicator of thymic effects. This parameter is
not commonly used in fish. One reason is that the thymus has a complex
location in some species, which makes clean dissection nearly
impossible. Other reasons are inconsistencies in thymic morphology,
histopathology, and morphometry (Ghoneum et al., 1986; Wester &
Canton, 1987). The latter paper described studies in which guppies
exposed to TBTO showed dose-dependent atrophy of the thymus, as seen
in rats (Krajnc et al., 1984). Both species also showed a concomitant
increase in 'neutrophils', which suggests functional compensation.
This response could not be reproduced in medaka (Wester et al., 1990),
stickleback, or flounder (P. Wester, unpublished results), probably
indicating species specificity. Thymic lymphocyte function can also be
tested in vitro.
Macrophage function tests: Macrophages are an important cell
population for both specific (antigen processing and presentation) and
nonspecific (phagocytosis and destruction) defence. They are
considered to be a relatively primitive defence mechanism and are
therefore of major importance to lower animals (Ratcliffe & Rowly,
1981). Much effort has been devoted to establishing macrophage
parameters as biomarkers for immune effects in fish; a possible reason
for this preference is the fact that these cells are fairly easy to
obtain, e.g. by peritoneal washing or removal of kidney pronephros,
and many function tests do not require sophisticated techniques or
species-specific reagents or markers (Mathews et al., 1990). The tests
include determination of chemotaxis, phagocytosis, pinocytosis, and
chemiluminescence. Zelikoff et al. (1991) studied the applicability of
trout peritoneal macrophages for immunotoxicology, stressing the need
for systematic baseline information. In addition to the tests listed
above, they studied the morphology and spread of resident and
stimulated peritoneal macrophages and concluded that these cells share
many morphological and functional properties with their mammalian
counterparts and may thus be useful indicators in immunotoxicology.
Many case studies in fish species have been published, demonstrating
the sensitivity of one or more parameters to chemicals, including PAHs
(Weeks & Warinner, 1986; Zelikoff et al., 1991) and pentachlorophenol,
in vitro (Anderson & Brubacher, 1993).
Melanomacrophage centres: Melanomacrophage centres, or
macrophage aggregates, are widely distributed throughout the fish
body, in particular in spleen, liver, and kidney. They are composed of
clusters of swollen, rounded cells (macrophages) that stain pale-tan
to black. This parameter must be determined histopathologically. Their
occurrence and morphology have been described (Agius, 1985), but their
function is not yet fully understood. The presence of pigments
(haemosiderin, lipofuscin, and ceroid) indicates storage of effete
biological material (erythrocytes, biomembranes) (Wolke, 1992). The
melanin present may be a generator of the bactericidal hydrogen
peroxide (Roberts, 1975), and the presence of antigens indicates a
role in immune reactions, e.g. antigen presentation. An increase in
melanomacrophage centres can be found with age and after stress
(Blazer et al., 1987), as confirmed in field studies (Vethaak &
Wester, 1993). Moreover, a large number of relatively small, pale
centres was seen in animals caught in late winter, when conditions are
more stressful, including spawning with associated migration and
starvation (Vethaak & Wester, 1993). The presumption that the small
size and pale appearance are indicators of recent development is
supported by the observation that in liver tumours composed of
relatively young, fast-growing tissue melanomacrophage centres are
usually absent or definitely smaller. As a consequence, when these
centres are used as general parameters of stress, the study groups
must be matched for age.
Because they are characteristic for fish and because of the
multiplicity of their functions, these structures deserve special
attention in the context of defining biomarkers for immunotoxicity. In
addition, they are easy to monitor, since they do not require special
preparation other than routine histological procedures, including
morphometry. Since melanomacrophage centres can be considered
primitive analogues of the mammalian lymph follicle (Payne & Fancey,
1989), it has been suggested that their presence indicates immune
capacity or function, although their role in this context has not yet
been established and the implications of a change in this parameter
for the integrity of the defence systems remains unclear. The density
of melanomacrophage centres in liver or spleen has been successfully
correlated with environmental sediment (Payne & Fancey, 1989) and
along a gradient of pollution in the North Sea (Bucke et al., 1992).
Other studies have reported an increase in melanomacrophage centre
density after contact with chemical contaminants (Blazer et al., 1987;
Secombes et al., 1992), which may indicate accumulation of cytotoxic
waste or immune stimulation.
At present, macrophage function and melanomacrophage centres are
the most widely used and promising indicators of the effects of
environmental stress (Blazer et al., 1987). Their relationship to
other components of the immune system remains to be clarified,
however, in tests with immunotoxicants.
Humoral immune response: Determination of circulating
immunoglobulin levels in serum is useful for testing the net result on
an immunological pathway in vivo. The response can be measured in
'naive' animals (total immunoglobulin) or after exposure to an
antigen, e.g. to verify the efficacy of vaccination in aquaculture.
Sheep red blood cells can be used as a standard antigen, and the
immune response can be measured by agglutination tests. ELISA tests,
which are sensitive and specific, can also be used (Arkoosh &
Kaattari, 1990). A related test is the haemolytic plaque assay which
identifies antibody-producing cells (splenic lymphocytes) (Anderson,
1990), but which has been used to only a limited extent in fish.
Specific lymphocyte stimulation tests: Functional tests widely
used in mammalian immunotoxicology, in which lymphocytes are
stimulated in vitro by exposure to mitogens such as
lipopolysaccharide, phytohaemagglutinin, and concanavalin A, can also
be used in fish. Proliferation is monitored by measuring the
incorporation of 3H-thymidine into DNA. The test is not antigen-
specific but provides information on the capacity of the entire B
(lipopolysaccharide) or T (phytohaemagglutinin, concanavalin A) cell
population. It is used to only a limited extent in fish
immunotoxicology, although Faisal & Hugget (1993) gave an elegant
demonstration of significant suppression of this parameter in spot
(Leiostomus xanthurus) under field conditions; this was shown to be
related to site and pollution in controlled laboratory experiments.
Specific cellular immune responses: Tests described in the
literature to measure cellular immune responses are scale or skin
allograft rejection, a relatively simple test (Zeeman & Brindley,
1981), and eye allograft rejection (Khangarot & Tripathi, 1991);
delayed rejection was seen in carp after exposure to copper. These
tests are applied to only a limited extent.
The tests described above are mainly tier 2 tests. The tests most
often used in immunotoxicology, however, are those for host resistance
(challenge by infections or tumours). The results of such tests are
rarely reported in the literature and have not been validated. For
ultimate proof of immunotoxicity, all phases of a test (maintainence,
exposure, and infection) must be conducted under strictly controlled
laboratory conditions. When suitable (often species-specific)
pathogens are standardized, such tests are valuable and necessary for
estimating the practical consequences of suspected immunotoxicity.
Although the incentive for undertaking immunotoxicological studies in
fish is usually epidemiological observation of a suspected toxic
component, ultimate challenge experiments must be carried out before a
final conclusion about immunotoxic mechanisms can be drawn.
More emphasis has been given to the development of biomarkers
than to their application in the field, for several reasons, including
the lack of specificity and the lack of association between effects at
the level of the biomarker and the population (Mayer et al., 1992).
Some comments on and some needs in this field are as follows:
* Immunological biomarkers in fish have great potential: many have
not yet been fully explored, probably owing to practical
limitations of lack of specificity and predictivity.
* The number of animal species should be limited in order to
concentrate research, which often requires species-specific
knowledge and reagents. Standardization could be achieved by
choosing well-defined inbred strains of fish (e.g. carp or
trout).
* A tiered approach is highly recommended for obtaining knowledge
on the specificity of the biomarker.
* More knowledge is needed on the epidemiology, mechanisms, and
etiology of diseases in fish, and particularly the predictive
value of immune parameters and the influence of hormesis.
* In terms of relevance for the organism, a test that monitors the
net result of a cascade of reactions (e.g. specific antibody
production, host resistance) is more predictive than a single,
nonspecific cell parameter (e.g. macrophage activity in
vitro).
* In identifying potential biomarkers for immunotoxicity, evidence
should be available that the levels tested in the laboratory are
relevant for field conditions and that the effect is directly
related to the immune system.
4.3.3.2 Chickens
Another non-mammalian species that has been studied extensively
with regard to the structure and function of its immune response is
the chicken. It is therefore not surprising that the chicken has
emerged as the predominant avian model for assessing compounds for
potential immunotoxicity. Humoral responses to different antigens have
been assessed routinely (Lerman & Weidanz, 1970; Marsh et al., 1981).
The weights of the thymus, spleen, and bursa of Fabricius have been
used, in combination with decreased antibody responses in vivo and
lymphocyte responses to phytohaemagglutinin and concanavalin A
in vitro (Eskola & Toivanen, 1974). Graft-versus-host and
cutaneous basophil hypersensitivity have also been used to detect
immunosuppression in chickens (Dietert et al., 1985). The availability
of chicken cell lines (Sung et al., 1992) will facilitate studies of
the mechanisms of action of compounds on the immune responses of this
avian model.
4.4 Approaches to assessing immunosuppression in vitro
The complexity of the immune system and the requirement of many
agents for metabolism and distribution to produce an immunotoxic
response has resulted in the almost exclusive use of animal models
in vivo for immunotoxicity assessment. Culture systems have been
used extensively, however, to study the mechanisms by which agents
produce immunosuppression.
Since most of the assays used for assessing immunotoxicity are
ex-vivo/in-vitro tests, they are easily adapted to completely in-vitro
assays for assessing immunosuppression. The direct addition of
compounds in various assays, including those involving NK cells,
lymphocyte proliferation, mixed leukocytes, and Tc lymphocytes, has
been used to determine the mechanisms by which compounds alter the
immune response at the cellular and subcellular level. Similarly, the
action of benzene and its metabolites on bone marrow has been studied
extensively in vitro (Gaido & Wierda, 1987), and the effects of TCDD
on thymocytes have been well studied in thymic epithelium co-cultures
(Greenlee et al, 1985). One of the most useful in-vitro assays for
studying immunosuppression is the T-dependent antibody response to
sheep erythrocytes. This assay, also known as the Mishell-Dutton assay
(Mishell & Dutton, 1967), has been used extensively in studying the
cellular target of immunotoxicants. It is the in-vitro counterpart of
the in-vivo plaque-forming cell assay, but sensitization with sheep
erythrocytes takes place in splenic cell culture and the plaque
response is measured on day 5 after addition of the erythrocytes. The
Mishell-Dutton assay has been used to study the structure - activity
relationships of various immunosuppressive compounds (Kawabata &
White, 1987; Davis & Safe, 1991). Since T cells, B cells, and
macrophages are needed for the response and an adverse effect on any
of these cell types can produce immunomodulation, it has proved
to be a sensitive assay for evaluating compounds in vitro for
immunosuppressive activity. Furthermore, since the various cell types
that participate in the response can easily be separated, individually
treated, and then reconstituted in the culture system, it is an
excellent assay for determining which cell type is adversely affected
by the compound. Using this approach, White & Munson (1986)
demonstrated that asbestos suppresses the response by affecting
macrophages; Shopp & Munson (1985) showed that the primary action of
phorbol ester on the antibody response occurs through an effect on B
cells; and Johnson et al. (1987) found that N-nitrosodimethylamine
affects primarily B cells.
As indicated above, one of the limitations of in-vitro systems is
that exogenous metabolic activation systems are often required. While
lymphocytes can metabolize some compounds, such as benzo[ a]pyrene,
to active metabolites (Ladics et al., 1992), other potent
immunosuppressive compounds such as cyclophosphamide require a
metabolic activation system. Such preparations usually consist of a
9000 x g supernatant of liver (S9). Using S9 preparations, Tucker &
Munson (1981) showed that cyclophosphamide could be activated to an
immunosuppressive form in vitro. Similarly, naphthalene could be
metabolized to an immunosuppressive metabolite (Kawabata & White,
1990). An alternative approach to the S9 activation system is a
hepatocyte co-culture system, which has been shown to be capable of
activating several parent compounds to their immunosuppressive
metabolites (Yang et al., 1986).
Predictive in-vitro systems based on immune cells of human origin
are particularly attractive, given the uncertainties of extrapolating
the results of experimental studies to humans and the accessibility of
immune cells in human peripheral blood. Although many of the immune
cells obtained from human blood are immature forms, the large numbers
and diverse populations (i.e. polymorphonuclear leukocytes, monocytes,
NK cells, T cells, and B cells) that can be obtained provide an
attractive alternative or adjunct to conventional studies in
experimental animals. As a consequence, a number of studies have been
conducted to compare the functional response of human and rodent
lymphocytes to putative immunosuppressive agents in vitro (Cornacoff
et al., 1988; Luo et al., 1992; Wood et al., 1992; Lang et al., 1993).
Although these studies were hampered by the lack of assays to assess
primary antigen-specific immune responses in human lymphocytes, a
relatively good interspecies correlation has been observed in the
limited responses available. Furthermore, several of these assays have
been successfully modified to include co-culture with primary
hepatocytes (Kim et al., 1987) to allow for chemical metabolism.
4.5 Future directions
4.5.1 Molecular approaches in immunotoxicology
A promising avenue for early detection of immunotoxicity may be
measurement of the expression of various interleukins. Cytokines are
involved both in regulation of the immune system and in pathological
phenomena, hence alterations in their pattern of expression may be
early indicators of immunotoxicity. Such testing can be done at the
level of mRNA expression, on mRNA extracted from lymphoid tissue taken
from exposed animals, or in tissue sections, so that the alterations
can be evaluated in the context of morphological indications of the
toxic effects. The signal of the cytokine that is being tested must
therefore be strong enough to be picked up in material from exposed
animals whose immune system has not received other stimuli, i.e.
sensitization or infection. This may not be true for all cytokines;
ex-vivo stimulation of cells that are part of the immune system may be
necessary, although the tests then become more laborious and must in
fact be considered functional assays, like tests for mitogen
responsiveness.
Very sensitive analysis can be done with the semiquantitative
polymerase chain reaction, which is a powerful technique for
elucidating early kinetic changes of cytokine expression, before
translation and secretion (Saiki et al., 1985). In addition, since
immunosuppressive agents can enhance or inhibit the ultimate
production and secretion of cytokines at various stages such as
transcription, the splicing of mRNA, translation of mRNA into
polypeptides on ribosomes, post-translational processing, and
secretion, potential molecular targets can be dissected by such
techniques. Several other molecular approaches may be used, including
northern blotting, dot-slot blotting, in-situ hybridization, and
antisense oligonucleotides for inhibiting the translation of specific
mRNAs.
4.5.2 Transgenic mice
The development of molecular genetic techniques has allowed not
only the isolation and analysis of specific genes but also the
manipulation of embryonic genes. Transgenic technology can be used in
immunology to generate mice that lack virtually any genetic control
mechanism or specific cell subpopulations. As a consequence, complex
systemic responses can be dissected into individual components, and
the mechanism by which immunosuppressive agents exert their affects
can be better understood. Two strategies are used to induce genetic
aberrations in transgenic mice (Bernstein & Breitman, 1989). One
involves the introduction of genes that produce toxins, such as
diphtheria toxin or the A subunit of ricin, into targeted cell
subpopulations. The second strategy involves the thymidine kinase
(tk) gene from Herpes simplex virus: When certain nucleotide
analogues are administered and are metabolized exclusively by viral
thymidine kinase, the metabolites are lethal only to cell
subpopulations that express the tk gene. Both approaches are
inducible systems for killing cells in vivo. Although gene ablation
techniques can be used to generate mutant animals that lack specific
cells in vivo, a small proportion of cells appeared to escape from
targeted cell death in virtually every study using bacterial toxins or
viral tk genes. While this may cause problems in determining the
qualitative roles of ablated cell populations, these techniques hold
promise for understanding the selective toxicity of drugs and
environmental agents on the immune system.
Other promising avenues are the use of animals transgenic with
respect to certain specificities of the TCR. If a gene that encodes
for a certain antigen specificity is introduced into the genome, that
specificity may be the only one that is expressed by the T cells. The
effects of immunotoxicants that affect the (positive and/or negative)
selection process that takes place in the thymus could be studied
elegantly with such models, when either undesired specificities (which
should be negatively selected) or desired specificities (which should
be positively selected) are introduced.
4.5.3 Severe combined immunodeficient mice
Another approach that may warrant further exploration is the use
of severe combined immunodeficient CB-17 scid/scid (SCID) mice
grafted with human immune cells. Xenogeneic lymphoid cells and/or
tissues can be successfully transferred to SCID mice (McCune et al.,
1988; Namikawa et al., 1990; Barry et al., 1991; Greiner et al., 1991;
Surhe & Sprent, 1991). SCID mice have been grafted with human fetal
lymphoid tissue in order to study human haematopoiesis (McCune et al.,
1988) or with human peripheral blood lymphocytes to allow production
of human immunoglobulins, including secondary antibody responses
(Mosier, 1990). SCID mice have also been used to study autoimmunity
and potential antiviral therapeutics. While these animal models still
have limitations (Pollock et al., 1994), they may ultimately provide
predictive models for examining potential immunosuppressive agents.
In particular, SCID mice co-implanted with human fetal thymus and
liver tissue fragments (SCID/hu mice) offer the possibility of
studying the human thymus in vivo in an isolated xenogeneic
environment (McCune et al., 1988; Namikawa et al., 1990) and the
effects of immunotoxicants on these grafts. This system is
particularly interesting with regard to those immunotoxicants for
which the thymus is one locus of action. The placement of human fetal
thymus under the SCID mouse renal capsule, followed by an intravenous
injection of fetal liver cells (McCune et al., 1988), and
co-implantation of human fetal liver and fetal thymus under the renal
capsule of SCID mice (Namikawa et al., 1990) have resulted in
reconstitution of SCID/hu mice; the fetal thymic implants increased in
size, and were found to be vascularized. The architecture and
antigenic distribution of these thymic grafts were virtually
indistinguishable from those of normal, age-matched human thymus.
Human stem cells were found to home to and differentiate within the
grafted human thymus, and phenotypically mature and functional human
T cells were found in the peripheral circulation of these mice (McCune
et al., 1988; Krowka et al., 1991; Vandekerckhove et al., 1991). As
such, the SCID/hu model can be helpful in immunotoxicological research
on the human thymus. When data obtained in experimental animals are
extrapolated to the human situation, a 'control' model, between the
SCID/hu mouse model and the intact laboratory animal (rat), is
desirable in order to test for possible differences in thymic
behaviour, because of its location under the kidney capsule: Thymic
blood flow and therefore the toxicokinetic behaviour of the thymus may
differ. For this reason the SCID/ra model was developed, by implanting
rat fetal thymus and liver tissue fragments under the SCID mouse renal
capsule. The outcomes of exposure of rats and SCID/ra mice can be
compared and the influence of thymus location and mouse metabolism on
extrapolation from SCID/hu to humans can then be determined.
Implantation of fetal rat thymus and liver tissue yields thymic
grafts that are virtually indistinguishable from normal, age-matched
rat thymus (De Heer et al., 1993). After implantation of rat fetal
thymus and liver tissue, the thymic grafts increase considerably in
size. Histologically, the SCID/ra thymic graft bears a close
resemblance to normal rat thymus, and the (immuno)histology of the
SCID/hu and SCID/ra mouse thymic grafts is comparable. Differences are
found, however, in peripheral reconstitutions of SCID/hu and SCID/ra
mice: Whereas large numbers of circulating donor rat T cells are found
in the blood and peripheral lymphoid organs of SCID/ra mice, only a
small number of donor T cells are found in the SCID/hu. This implies
that the data for extrapolating immunotoxic data from rats to humans
must be confined to thymic effects. With this restriction in mind, the
outcome of experiments with SCID/hu and SCID/ra mice can be used to
compare the sensitivity of the human and rat thymus and can thus yield
important information for the process of human risk assessment.
4.6 Biomarkers in epidemiological studies and monitoring
There is a difference between assays of the immune system and
biomarkers. Many validated tests can indicate alterations to the
immune system, including its function, so that most assays can be
helpful for hazard identification. Not every validated assay of the
immune system is a biomarker, however. The IPCS (1994a) definition of
a biomarker is one that indicates exposure (and is specific for
exposure), indicates susceptibility to adverse effects, and/or is
predictive of disease associated with exposure. Biomarkers should be
used to characterize risk due to exposure, on the basis of
identification of the hazard.
Within this strict definition, it is clear that not many
biomarkers are available for immunotoxicity (as is true for other
systems), especially for assessing immunotoxicity or individual
susceptibility to immunotoxicity. Some assays may be useful in
epidemiological studies. In any event, more epidemiological studies
are needed to obtain a better view of the usefulness of biomarkers for
detecting immunotoxic events and hence the possible health risks that
may be associated with exposure to immunotoxicants.
4.7 Quality assurance for immunotoxicology studies
In many countries, studies to support the safety of a compound or
drug must be conducted in accordance with the requirements for 'good
laboratory practice' of the agency that is evaluating the material.
Immunotoxicological studies conducted to support the safety of a new
drug or chemical should follow at least the 'spirit' of good
laboratory practice. The OECD has published their principles of good
laboratory practice, with supporting publications on their
application, and these have been adopted into legislation in a number
of countries. IPCS (1992) has published a monograph, Quality
Management for Chemical Safety Testing, covering the important
aspects of good laboratory practice in a nonregulatory context and
quality control of chemical analyses.
In the United States, good laboratory practice for conducting
nonclinical laboratory studies for submission to the Food and Drug
Administration has been detailed. The standards for studies on
pesticides submitted to the Environmental Protection Agency were also
published, as were the procedures to be followed in conducting studies
submitted on compounds covered by the Toxic Substance Control Act.
Each of these sets of standards is periodically updated by the
respective agencies, and studies must be conducted in accordance with
the most recent updates. While there are some differences in the
wording of the standards, they are generally similar.
Good laboratory practice includes written protocols for
evaluating potential immunotoxicants and the establishment of standard
operating procedures for assays. Each laboratory must run the assays
frequently enough to establish historical control values, and
the results of any study conducted to evaluate a compound for
immunotoxicity must be judged in the context of the historical control
values for the laboratory and appropriate controls. Incorporation of
positive control compounds in the study design provides additional
confidence that the assays are being conducted correctly, particularly
when the tested compound shows no effect.
The selection of assays to be used in evaluating compounds for
immunotoxicity remains a subject of active discussion. Other parts of
this document address this issue in detail. Regardless of which assays
are used, however, they must be standardized and be recognized as
validated and meaningful. Significant advances have been made in the
standardization and harmonization of assay procedures for assessing
immunotoxicity, mainly as a result of the willingness of leading
laboratories in the field to share their standard operating procedures
openly with other laboratories. Published papers and books on
immunotoxicity test methods also contribute to the standardization
process. As a result of studies by Luster et al. (1988), the assays
used by the NTP for evaluating potential immunotoxicants have been
accepted as validated assays in mice.
4.8 Validation
An important requirement of tests for evaluating immunotoxicity
is that they be validated. While there is no agreed definition of
validation, tests must meet certain requirements. In toxicology,
validation is the process by which the reliability and relevance of a
test to identify human health risk is established (Balls et al.,
1990). A flow diagram of a proposed validation process and its end-
point, the acceptance of a method by regulatory authorities for
submission of toxicological data, is presented in Figure 36.
Four parameters must be considered in determining the validity of
a testing method: specificity, sensitivity, accuracy, and precision.
Specificity is based on the rate of false-positive results generated.
Sensitivity is determined by the ability to identify true-positive
results. These two parameters determine the level of predictability or
relevance. In order to determine specificity and sensitivity, the
method is evaluated with a set of compounds of known positive and
negative immunosuppressiveness. This approach was used by the NTP in
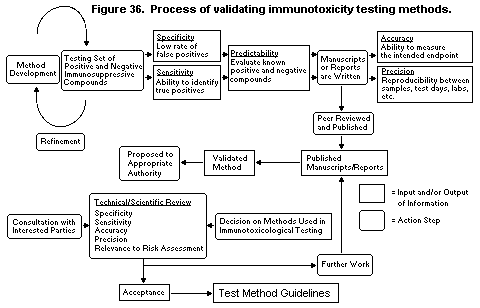 evaluating the predictability of various assays (Luster et al., 1988).
In a subsequent study, the potential immunotoxicity (defined as a
dose-related effect on any of two immunotoxicological parameters with
no effect on body weight) was determined for 51 chemicals in mice,
using a variety of general and functional immunological parameters
(Luster et al., 1992).
Accuracy is determined by the ability to measure the intended
end-point truly. Precision is the ability to reproduce results from
experiment to experiment or between laboratories. In the NTP studies
in mice (Luster et al., 1988), four laboratories participated in the
inter-laboratory validation process. A number of international studies
are in progress on the precision of several assays, using the rat as a
model for immunotoxicological evaluations, in an attempt to bring the
level of acceptance of immunotoxicological studies in rats to the
level that has been achieved for mice. Most of these studies are
multinational and represent an interaction of industry, government,
and academia to achieve this common goal.
A comparative study in Fischer 344 rats with cyclosporin A (White
et al., 1994) encompasses nine laboratories in Canada, Europe, and the
United States. The primary focus of the study is on the use of
functional assays for detecting immunosuppression; lymphoid organs and
tissue are weighed and examined histopathologically in several of the
laboratories. The functional assays used in this protocol include the
plaque-forming cell assay or ELISA to sheep erythrocytes, splenocyte
proliferative assays to concanavalin A and STM, the NK cell assay, and
the mixed leukocyte response. Splenocyte surface markers were also
analysed. The study design was similar to that used by the NTP, with a
14-day exposure and administration by oral gavage. The preliminary
results demonstrated excellent reproducibility of the results for the
plaque-forming cell assay, splenocyte proliferative assays to
concanavalin A and STM, and the mixed leukocyte response. Differences
were observed between the laboratories in the results of the test for
NK cell activity.
The IPCS-European Union international collaborative
immunotoxicity study in rats is also in progress. The study involves
20 laboratories in Canada, Europe, Japan, and the United States; its
design is based on the OECD test guideline No. 407 for a 28-day
toxicity study. The study focuses primarily on the ability to detect
immunotoxic compounds on the basis of organ weights, pathological
findings, and 'enhanced pathology', which includes additional
evaluation of lymphoid tissues not currently required by test
guideline No. 407. Functional assays were also conducted; the core
assays included the plaque-forming cell assay or ELISA to sheep
erythrocytes, splenocyte proliferative assays (concanavalin A and
STM), and the NK cell assay. The study was conducted in two phases. In
the first phase, azathioprine was used as the test compound, various
strains of rat were used, and each laboratory established its own
doses on the basis of a predetermined maximum tolerated dose. In the
second phase, cyclosporin A was the test compound, only three strains
of rat were used, and a more structured protocol was followed. A
report on phase I of the study has been drafted, and the data from
phase II are currently being analysed. All of the laboratories found
that azathioprine is immunosuppressive, even though several strains of
rats were used, different dose levels were administered, and no
standard protocol was followed. The preliminary results with
cyclosporin A show good agreement between the laboratories for the
plaque-forming cell assay but some differences for the splenocyte
proliferative assays (concanavalin A and STM) and the NK cell assay.
A third interlaboratory study in rats has been organized by the
German Bundesgesundheitsamt, in Berlin, with German and French
participants. The design is also based on OECD test guideline No. 407
for a 28-day repeated-dose study in Wistar rats. Cyclosporin A was
selected as the test substance. The live phase of this study has been
completed, the data are being analysed, and the final report is being
prepared.
Information obtained from studies of predictability, accuracy,
and precision, such as those described above, must undergo peer review
before publication. A major goal of the validation process is to
determine which methods should be recommended in the testing
guidelines of regulatory agencies. Figure 36 shows each input and
output of information and the action steps. The process of developing
and obtaining acceptance of testing guidelines is based on three major
inputs: (1) publication of reports in peer-reviewed journals;
(2) guidelines for deciding whether a method is valid; and
(3) implementation of test methods that are interpretable by
scientists involved in assessing biologically relevant risks and the
results of which can be incorporated into quantitative dose-response
analyses. The proposed process is built around the generation of these
major inputs. Two of the issues that will arise in the development of
guidelines are: 'How many and what type of compounds should be
included in the validation process?' and 'Should the compounds be
shown to be immunosuppressive in both humans and mice?'
5. ESSENTIALS OF IMMUNOTOXICITY ASSESSMENT IN HUMANS
5.1 Introduction: immunocompetence and immunosuppression
An immune response in the fully mature, immunologically competent
individual provides protection against a myriad of infectious agents
and environmental hazards. The immune system acts as a self-restoring
(homeostatic) system which can quickly return to normal levels of
function after periods of marked stimulation and response. This self-
regulation allows the individual to recover from or circumvent the
toxic effects of many potentially damaging environmental hazards.
There are many well-known clinical conditions of inherited deficiency
in immunological function; some result in specific defects in antibody
formation, others consist of T-cell and/or metabolic defects, while
others include impairment of both B- and T-cell function. These
conditions, known as primary immunodeficiency disorders, are due to
definable, inheritable genetic defects. Clinical studies of these
disorders have demonstrated the importance of the immune system to
host defence and individual survival, showing that individuals with
partial or absolute defects in T-cell function rarely survive beyond
infancy or early childhood. In contrast, individuals with defects in
B-cell function, resulting in a deficiency in antibody formation, may
suffer from a variety of chronic, recurrent infectious diseases and
diminished health but can survive with appropriate therapy when the
underlying disorder is recognized. Study of these genetic
immunodeficiency states has also provided considerable information
about the functions of human B and T cells which would otherwise not
have been determined.
Impairment of the function of a key component of the immune
system results in a diminished immune response (immunosuppression) or
immunodeficiency. Acquired immunodeficiency states were recognized
only sporadically until the late 1970s, when a syndrome appeared that
spread rapidly through certain groups and produced a generalized type
of immunosuppression known as acquired immunodeficiency syndrome
(AIDS). AIDS was found to be due to retroviruses that infect and
destroy Th (CD4+) cells in humans (Fauci et al., 1991). CD+
lymphocytes have been identified in experimental studies as the key
cells in the recognition and secondary processing of antigens. Thus,
progression of AIDS is associated with progressive loss of Th cells
and increased frequencies of infection by bacterial, fungal, viral,
and parasitic agents and of certain types of neoplasms.
5.2 Considerations in assessing human immune status related to
immunotoxicity
The assessment of immunotoxicity in humans exposed to potentially
immunotoxic compounds is much more complicated than in experimental
animals. Issues such as logistics, appropriate controls, magnitude and
pattern of exposure, and confounding parameters such as medication,
drug abuse, and illness must be considered. Other considerations that
should be taken into account in comparing human immune status with
that in laboratory animals in relation to immunotoxicity are as
follows:
(1) The human population is heterogeneous and genetically
disparate; it can be considered as 'wildlife'. Inbred laboratory
animals are, by definition, genetically identical; outbred laboratory
animals typically have a larger genetic variability than inbred
animals but a variability that is much smaller than that in wildlife
populations. Genetic constitution, which accounts for the variability,
has consequences for the antigen recognition capacity of the immune
system, especially for the T-lymphocyte population. Antigen
recognition by T cells is restricted to the MHC haplotype of the
individual and therefore differs between (allogeneic) individuals.
Inbred, and most outbred, laboratory animals are much more alike in
antigen recognition capacity than wildlife populations. For instance,
the repertoire of certain inbred mouse strains lacks part of the
spectrum of T-cell specificity, as seen by the absence of T cells that
express distinct 'variable gene families' in the repertoire of TCR
specificities. Such 'gaps' in the repertoire have thus far not been
detected in the outbred human population by similar methods of
detection (Hu et al., 1993), perhaps because each individual in an
outbred population expresses the MHC products of both parents and can
in principle multiply the repertoire of MHC-restricted reactions by a
factor of 2 (including MHC I and MHC II).
Interindividual variability has obvious consequences for
immunotoxicity, in which the response to the chemical or drug
underlies the mechanism of toxicity. Its effect on direct toxicity is
presumably less, but the manifestation of toxicity is often reduced
immune reactivity (e.g. increased incidence of infections) and hence
determined by individual reactions to antigens.
(2) The human population, like populations of 'wildlife' animals,
is continuously exposed to environmental stimuli. It is well known
that it is not necessary that each member of a population be protected
('immune') but that a certain proportion of protected individuals must
be reached in order to achieve 'herd immunity'. That is to say, the
whole population is protected when a certain percentage of individuals
is immune. In contrast, when this percentage falls below the required
incidence (which differs for different infectious agents), the
population as a whole loses its protected state, and an infectious
epidemic can result. This situation easily arises in small groups in a
country where vaccination is minimal. Well-known examples are the
outbreaks of poliomyelitis in the Netherlands and the hepatitis A
virus outbreaks in China. This phenomenon should be taken into account
in epidemiological studies and associated laboratory investigations
(e.g. antibody levels to microorganisms) in assessing immunotoxicity
in human populations.
(3) Most of the human population is continuously exposed to
environmental stimuli and maintains its ability to respond to foreign
material from the pool of immunological memory. From the first
postnatal period through to adulthood, the T-cell repertoire is
generated by the thymus; later, this generation of cells is reduced to
a low level. Strict MHC restriction implies that the T-cell population
cannot easily change specificity, e.g. by somatic mutation of
the genes that encode the TCR. There is some evidence from
immunophenotyping, both in mice and humans, that the T-cell population
shifts gradually during life, from naive (committed) T cells to memory
T cells. Within the B-cell population, the situation is different.
Here, the repertoire changes continuously, due to somatic mutation
presumably associated with 'affinity maturation' in lymph nodes. This
phenomenon may result in the emergence of B cells with a strong
affinity for stimuli and the disappearance of low-affinity cells. It
is not known whether neoantigens or pathogens are recognized by
'affinity matured' or memory B cells or by naive B cells. In the
absence of information on this aspect, it can be suggested that most
of the immune capacity of adults is deployed for memory reactions,
whereas in young people the contribution from the naive pool is
higher. This is reflected in infectious epidemics, when microorganisms
like influenza change to phenotypes that cannot be recognized by the
memory pool, an aspect to be kept in mind when choosing immune tests
to be used in evaluating immunotoxicity. Primary responses, like those
to keyhole limpet haemocyanin antigen, are considered to be more
sensitive than secondary responses, like those to tetanus toxoid.
Another example of this effect is the composition of the recall
antigens used in testing delayed-type hypersensitivity (Borleffs et
al., 1993). Both primary and secondary antibody responses, however,
are valuable for evaluating the intrinsic naive and memory immune
capacity of individuals, although secondary responses are less
sensitive to immunological insults.
(4) For a number of infectious microorganisms, the immune
response does not result in complete elimination of the invader but
rather in its 'silent' integration into the genome. Certain viruses,
like herpes viruses, cytomegalovirus, and Epstein-Barr virus, are
dealt with in this way. An individual is considered to be a carrier of
the virus (postinfection status) on the basis of the presence of
antibodies. In other words, individual postinfection is a continuous
defence against these viruses, often with sufficient capacity to keep
the virus in a silent form. In diminished immune capacity, this
natural protection can be lost, and infections can re-occur after
viral reactivation. Primary infection and reactivation therefore have
different pathogeneses, although the subsequent disease may be
characterized by the same symptoms. This situation is well known
clinically, when high doses of immunosuppressive drugs are given for
long periods. The relevance of this observation in immunotoxicity
testing must be established.
(5) Ex-vivo diagnosis in humans is often restricted to
haematological investigations, so that only information on the
circulatory pool of cells and plasma factors is obtained. For example,
the distribution of immunoglobulins differs in the intravascular and
extravascular spaces. Only about 1% of the total lymphocyte pool is
present in blood (1010 cells out of the total of about 1012), and
this population represents only the recirculating pool of cells and
not the tissue-bound cells that participate actively in immunological
responses. Investigations of peripheral blood cells can be somewhat
misleading: for instance, patients infected with the human
immunodeficiency virus (HIV)-1 may show severe depression of CD+
cells in blood but less reduction in CD cells in lymphoid tissue
(Schuurman et al., 1985).
It is considerably more complex to establish immune changes in
humans than in animals, since noninvasive tests are limited, the
levels of exposure to an agent (i.e. dose) are difficult to establish,
and the responses in the population are extremely heterogeneous. With
respect to the latter, the variation in immune responses (genetic or
environmental) can exceed a coefficient of variation greater than
20-30%. Because many of the immune changes in humans that follow
exposure to chemicals may be sporadic and subtle, recently exposed
populations must be studied and sensitive tests for assessing the
immune system be performed. Since many of the immune tests used in
humans have a certain degree of overlap (redundancy), it is also
important that a positive diagnosis not be based on a change in one
test but on a profile (pattern) of changes, similar to that observed
in primary or secondary immunodeficiency diseases. For example, low
CD:CD8 ratios are often accompanied by changes in skin reactions to
recall antigens. The Clinical Immunology Subcommittee of WHO and the
International Union of Immunological Societies published methods for
examining changes in the human immune system and described their
pitfalls (Bentwich et al., 1982, 1988); however, most of the tests
were selected on the basis of observations in patients with primary
immunodeficiency diseases. Such individuals suffer from severe
recurring infections, and their degree of immunosuppression is
probably considerably greater than that induced by chemicals. Thus,
some of the methods may be of limited value for examining potential
chemical-induced immunosuppression, and further evaluation of methods
is needed.
In view of the difficulties in identifying chemical-induced
immunosuppression in humans, establishment of exposure levels (e.g. in
blood or tissue) would not only be useful but would in many instances
be essential for determining a cause-effect relationship. Clinical
disease may not necessarily have to be present in order for
immunosuppression to be meaningful, for several reasons. Firstly,
there are uncertainties about the extent of the reserve capacity of
the immune system and whether the relationship between immune function
and clinical disease shows a linear or a threshold response. In a
linear relationship, even minor changes in immune function would be
related to an increase in disease, if the population examined is large
enough. While the relationship at the low end of the dose-response
curve is unclear, at the high end of the curve (i.e. severe
immunosuppression), clinical disease is readily apparent. This is
exemplified by increased incidences of the opportunistic infections
that occur in AIDS patients. Secondly, clinical disease may be
difficult to establish, as neoplastic diseases may involve a
10-20-year latency before tumour appearance, and increased infection
rates are difficult to ascertain in epidemiological surveys (e.g.
increased numbers of cases of severe common cold).
5.3 Confounding variables
The normal population has a wide range of immunological
responses, with no apparent health impact. In addition to the
underlying population variability, certain host characteristics and
common exposures may be associated with significant, predictable
alterations in immunological parameters. If not recognized and
effectively addressed in study design or statistical analysis, these
confounding factors may severely alter the results of population
studies.
Examples of factors associated with measurable alterations in
immunological parameters are age, race, sex, pregnancy status, acute
stress and the ability to cope with stress, coexistent disease or
infection, nutritional status, lifestyle, tobacco smoking, and some
medications. The effect of acute stress on the immune system is
mentioned in section 1.2.1.5. Protein calorie restriction and
deficiencies of trace elements such as zinc have also been associated
with immune deficiency (Chandra, 1992; Good & Lorenz, 1992; Chandra,
1993). Periodic influences, ranging from daily to seasonal, also
exist; some are relatively minor, but others are of a magnitude that
may rival the expected effect of a low-level exposure to a toxic
agent. They are therefore of primary concern in large epidemiological
studies. For example, African Americans have, on average, serum IgG
levels that are 15-20% higher, neutrophil counts that are 10-15%
lower, and a proportion of circulating B cells that is significantly
higher than those of Caucasians, with no discernible health
implications. Cigarette smoking is associated with a significant
decrease in IgG level and an increase in leukocyte count, independent
of ethnic differences. Therefore, it is imperative that population
norms and reference ranges be supplemented by detailed analysis of
potential confounding factors. Study designs should include
considerations of matching, stratification, and subgroup analysis to
control for these potential effects. As new immunological assays are
developed, normative data will be required, particularly for ethnic
minorities, children, the aged, and certain groups potentially at high
risk, such as pregnant and lactating women.
Certain endocrine diseases and conditions may be associated with
significant alterations in immune function (e.g. adrenal dysfunction).
Some medications, such as corticosteroids, phenytoin, and nonsteroidal
anti-inflammatory agents, may depress a variety of immune functions.
Questionnaires and population surveys should allow the collection of
sufficient information to make it possible to consider these factors
in data analysis and interpretation.
An increasingly important consideration in any analysis of immune
function, particularly in relation to immune deficiency, is the
potential presence of HIV infection, which causes widespread
alterations in virtually all elements of the immune system. Even a
small proportion of unrecognized HIV-infected individuals in a study
population may significantly affect the results and the interpretation
of data. When immunological studies indicate decreased immune
parameters consistent with HIV infection, testing for the virus should
be considered; otherwise, interpretation of the results of
immunological tests of immune dysfunction, particularly among
populations with potentially high rates of HIV infection, may be
severely limited.
In assessing human immunotoxicity, it is useful to establish the
presence of infectious, allergic, or autoimmune diseases in order to
ensure completeness and to rule out additional confounding variables.
A clue to the type of immunological defect is often provided by the
kind of infection observed. For example, patients with impaired
humoral immunity have an increased incidence of recurrent infections
with encapsulated bacterial pathogens (e.g. Pneumococcus and
Haemophilus influenzae), which can induce chronic sinopulmonary
infection, bacteraemia, and meningitis. In contrast, if cellular
immunity is intact, the patients will have less severe infections with
fungal and viral agents. Abnormalities of T cells and impairment of
cell-mediated immunity predispose individuals to infections with a
wide variety of agents, including viruses that cause disseminated
infections (e.g. Herpes simplex virus, varicella-zoster virus, and
cytomegalovirus), fungi that cause mucocutaneous candidiasis, and
parasitic organisms including the protozoan Pneumocystis carinii.
5.4 Considerations in the design of epidemiological studies
An important factor in assessing the usefulness of an
epidemiological study for risk assessment is its design. The commonest
design used in immunotoxicology is the cross-sectional study, in which
exposure and disease status (in this case, changes in immunological
function) are measured at one time or over a short period. The immune
function of 'exposed' subjects is compared with that of a comparable
group of 'unexposed' individuals. Important considerations in using
the data provided by such studies in risk assessment have been
discussed (E. Ward, unpublished manuscript):
(1) What is the relationship between changes in immune function and
human health risk?
(2) Are the selection procedures for study subjects adequately
documented?
(3) Is there evidence that the exposed group was actually exposed to
the substance of interest?
(4) Has the possibility that other exposures, of either the entire
population or individuals, been accounted for?
(5) Are the 'exposed' and 'unexposed' populations comparable with
respect to factors other than the exposure of interest?
(6) Have major individual aspects (such as illness and use of
medications) that may influence the outcome of tests for immune
function been accounted for?
(7) Has inter- or intralaboratory variability been controlled for?
Was the laboratory that ran the tests for immune function able to
distinguish between samples from 'exposed' and 'unexposed'
individuals?
5.5 Proposed testing regimen
Biological research involving human subjects must be conducted in
accordance with ethical standards and involve scientific procedures
designed to ensure the safety of the subjects (Council for
International Organizations of Medical Sciences, 1993). Below are
shown a testing scheme proposed by WHO for preliminary evaluation of
individuals exposed to immunotoxicants, an approach developed by a
working group organized by the United States Centers for Disease
Control and Agency for Toxic Substances and Disease Registry, and that
proposed by a panel of the United States National Academy of Science
(US National Research Council, 1992). The three approaches have many
similarities.
5.6 Assays for assessing immune status
A plethora of tests has been developed to assess immunity in
humans (Bentwich et al., 1982, 1988), which are described in
laboratory manuals (Lawlor & Fischer, 1988; Miller et al., 1991;
Coligan et al., 1994). Many of these tests are now commercially
available in kits. A systematic approach to the evaluation of immune
function, which is based on simple screening procedures, followed by
appropriate specialized tests of immune function, usually permits the
definition of an immune alteration. The tests should include
evaluation of the B-cell system, of the T-cell system, and of
nonspecific resistance (polymorphonuclear leukocytes, monocytes and
macrophages, NK cells, the complement system). Although some exogenous
Assays suggested by WHO for assessing immunotoxicity in all persons
exposed to immunotoxicants
1. Complete blood count with differential counts
2. Antibody-mediated immunity (one or more of following):
* Primary antibody response to protein antigen (e.g. epitope-
labelled influenza vaccine)
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
* Secondary antibody response to protein antigen (diphtheria,
tetanus, or poliomyelitis)
* Natural immunity to blood-group antigens (e.g. anti-A and
anti-B)
3. Phenotypic analysis of lymphocytes by flow cytometry
* Surface analysis of CD3, CD4, CD8, CD20
4. Cellular immunity
* Delayed-type hypersensitivity skin testing using Multitest
Biomerieux(R)
* Primary delayed-type hypersensitivity reaction to protein
(keyhole limpet haemocyanin)
* Proliferation to recall antigens
5. Autoantibodies and inflammation
* C-Reactive protein
* Autoantibody titres to nuclei, DNA, mitochondria and IgE
(rheumatoid factor)
* IgE to allergens
6. Measure of nonspecific immunity
* Numbers of natural killer cells (CD56 or CD60) or cytolytic
activity against K562
* Phagocytosis (nitroblue tetrazolium or chemiluminescence)
7. Clinical chemistry
Screening panel recommended for human studies by the United States Centers for
Disease Control and Agency for Toxic Substances and Disease Registry
* Complete blood count with differential counts
- absolute lymphocyte count
- granulocyte count
- platelet count
- absolute eosinophil count
- examination of peripheral smear
* Immunoglobulins
- IgG
- IgA (optional)
- IgM (optional)
* C-Reactive protein
* Autoantibody screening panel
- Antinuclear antibody
- Rheumatoid factor
- Anti-thyroglobulin antibody
* Peripheral blood leukocyte surface markers
- CD2/CD3
- CD4/CD8
- CD8/CD3
- CD19/CD20
* Clinical chemistry in serum
- Blood urea nitrogen
- Cholesterol
- Creatinine
- Total bilirubin
- Alkaline transaminase
- Alkaline phosphatase
- Total protein (albumin:globulin ratio)
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
Tier 1 (all persons exposed to immunotoxicants)
I. Humoral immunity
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
and immunofixation electrophoresis
* Natural immunity: Antibody levels to ubiquitous antigens
(e.g. anti-A and anti-B group substances in individuals of
non-AB blood type)
* Secondary antibody responses to proteins (e.g. diphtheria,
tetanus, poliomyelitis) and polysaccharides (e.g.
pneumococcal, meningococcal)
Note: In immunization studies, live microorganisms should not be
given to persons suspected of being severely immunocompromised.
II. Lymphocytes
* Enumeration of B and T cells in blood
* Surface analysis of CD3, CD4, CD8, CD20
* Secondary delayed-type hypersensitivity reaction (e.g.
candida, diphtheria, tetanus)
* Alternative: Multiple antigen skin test kit
III. Autoantibody titres (to red blood cells, nuclei, DNA,
mitochondria, IgE (rheumatoid factor)
Tier 2 (all persons with abnormal Tier 1 test results and
a fraction of the total exposed population to be determined
by a statistician)
I. Humoral immunity
* Primary antibody response to protein and polysaccharide
antigens
Note: A panel of antigens should be developed that can be used
in sequential studies on a given individual, since a particular
antigen can be used only once to assess a primary response.
II. Cellular immunity
* Proliferative response to mitogens (phytohaemagglutinin,
concanavalin A) and possible antigens such as tetanus;
primary delayed-type hypersensitivity reaction to keyhole
limpet haemocyanin
Note: Here, too, a panel of standard antigens is needed for
sequential testing; these could be the same as those used to
assess primary antibody responses.
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
III. Natural killer cells, monocytes, and other T- and B-cell markers
* CD5, CD11, CD16, CD19, CD23, CD64; class II MHC on T cells
by two-colour flow cytometry for co-expression of class II
and a T-cell marker such as CD3
IV. Serum levels of cytokines (e.g. IL-1, IL-2, IL-6) and of shed or
secreted cellular activation markers and receptors (e.g. CD25).
V. Class I and II MHC antigen typing
Tier 3 (to be considered for persons with abnormalities
in Tier 2 tests or for a random fraction of the entire
population in Tier 2)
* If a proportion of CD16 cells of Tier 2, III, is abnormal:
nonspecific killing of a tumour cell line to test for natural
killer function
* If primary delayed-type hypersensitivity reaction in Tier 2, II,
is abnormal:cell proliferation in response to phorbol ester and
calcium ionophore,anti-CD3 antibody, and a staphylococcal
enterotoxin B (experimental)
* Generation of secondary cell-mediated immune reactions
(proliferation and MHC-restricted cytotoxicity) in vivo, e.g.
with influenza virus (experimental)
* Immunoglobulin subclass levels in serum (IgA1, IgA2, IgG1-4)
* Antiviral titres (e.g. influenza, parainfluenza,
cytomegalovirus, human immunodeficiency virus) in serum (no
deliberate immunization)
agents can alter several elements of the human immune system, others
have a primary effect on a single element. For example, low doses of
cyclosporin A selectively affect T cells by acting on the production
of IL2 and IL2 receptors. Conversely, the anticonvulsive drug
phenytoin acts primarily on the humoral immune system, leading to a
selective deficiency of IgA.
A number of immune function assays recommended for inclusion in
a simple screening panel for assessing human immune function after
potential exposure to xenobiotics believed to affect the immune system
are described below. It should be noted that there are many indicators
of altered immune function in humans which may not be specific markers
for exposure, immune disease or susceptibility (IPCS, 1994a;
IPCS/Department of Health, 1995).
5.6.1 Total blood count and differential
A complete blood count, with differential absolute counts of
lymphocytes, granulocytes, eosinophils, and platelets, are basic
components of immunotoxicology. These tests are useful in defining the
general health status of a population, since they are relatively well
standardized over most age, sex, and race groups. Such counts are also
essential for interpreting the results of ex-vivo/in-vitro functional
tests, described below, since functional tests reflect a combination
of numbers of cell types and activity per cell.
The absolute lymphocyte count is critical: Higher absolute
counts are found in children than in adults, but lymphocyte counts
consistently below 1500/mm3 are indicative of lymphocytopenia, and a
higher count signals a defect in the T-cell system or effects on the
bone marrow. Lymphocytopenia can occur not only in primary immune
deficiency but also as a result of viral infections, malnutrition,
stress, autoimmune diseases, and haematopoietic malignancies.
Examination of bone marrow may be indicated to exclude some other
factors once lymphocytopenia has been confirmed. Additional assessment
of cell mediated immunity and direct measurement of T-cell parameters,
such as lymphocyte phenotypic markers, may also be indicated.
Review of peripheral smears for morphological abnormalities of
the white and red cells adds useful information for interpreting raw
cell counts, such as the atypical lymphocytosis of many acute viral
infections. The absolute eosinophil count can be very helpful in
delineating allergic disorders, vascular collagen diseases, and
parasitic manifestations.
5.6.2 Tests of the antibody-mediated immune system
Evaluation of antibody-mediated immunity involves measurement of
serum concentrations of immunoglobulins and assessment of antibody
formation after immunization or measurement of 'natural antibodies'.
5.6.2.1 Immunoglobulin concentration
Several methods are available for measuring the concentrations
of the five major classes of immunoglobulin, IgM, IgG, IgA, IgD, and
IgE, in serum, including single radial diffusion, double diffusion in
agar gel, immunoelectrodiffusion, radioimmunoassay, ELISA, and
automated laser nephelometry. Single radial diffusion is widely used.
Gel diffusion methods are very sensitive to differences in diffusion
constants and thus to differences in molecular size.
The serum concentration of each of the major immunoglobulins
should be determined, with the exception of IgD (which occurs
predominantly on the cell surface). The determinations must be well
standardized because antisera vary in quality. Since serum
immunoglobulin concentrations vary with age and environment,
appropriate norms must be used. Patients can manifest a deficiency in
all immunoglobulin classes (common variable hypogammaglobulinaemia),
or they may have a deficiency in only a single class (IgA deficiency
as a primary defect or after phenytoin therapy).
The concentration of immunoglobulins cannot be used as the
sole criterion for a diagnosis of immunodeficiency. Diminished
immunoglobulin concentrations can result from loss into the
gastrointestinal tract as well as from decreased synthesis. An
indication of loss can be obtained by measuring serum albumin, which
is usually lost concomitantly.
5.6.2.2 Specific antibodies
Antibody-mediated immunity can be assessed from antibody
responses to common specific antigens (basal levels). Humoral immunity
after immunization can be assessed in the same way. The response to
antigenic stimulation with both protein and polysaccharide antigens
must be defined if immunodeficiency is strongly suspected. Failure to
respond to one or more classes of antigen has been observed in
patients with normal or high levels of immunoglobulins, regardless of
whether they have an isolated immunoglobulin class or subclass
deficiency. Specifically, patients with the Wiskott-Aldrich syndrome
may have normal or even elevated immunoglobulin concentrations, yet
have multiple infections because of their failure to mount a specific
immune response, especially when they are exposed predominantly to
polysaccharide antigens.
Natural antibodies: Isohaemagglutinins are naturally occurring
antibodies to blood group A and B antigens found in all normal
individuals except those with type AB red cells. By three years of
age, 98% of normal persons with type A, B, or O blood have
isohaemagglutinin titres of at least 1:16. Patients with the Wiskott-
Aldrich syndrome may have normal immunoglobulin levels yet lack
isohaemagglutinins as an indicator of their antibody-deficient state.
Other natural antibodies that can be assayed include heterolysins
(e.g. against sheep or rabbit red blood cells) and antistreptolysin.
Antibody response to immunization: In order to test for
T cell-dependent antibody responses, commercially available
diphtheria-tetanus vaccine can be given in recommended doses. Blood is
taken two weeks after each injection and tetanus and diphtheria
antibodies are determined. In patients who have been immunized with
diphtheria-tetanus or diphtheria-pertussis-tetanus vaccine, one
booster injection is given before determination of antibodies. In
testing for T cell-independent antibody responses, commercially
available pneumococcal vaccine can be given in recommended doses.
Three doses of killed poliomyelitis vaccine (1.0 ml intramuscularly,
at intervals of two weeks) can also be used as the immunogen. Blood is
taken two weeks after the last injection, and antibody is usually
determined by virus neutralization. There is strong consensus that
quantification of a primary immune response (antibody and/or cellular)
after immunization is not only a very relevant test but also very
sensitive. Although such tests are not routine in clinical immunology,
they have been used successfully for determining immune integrity.
While keyhole limpet haemocyanin has been used as the antigen,
beneficial immunizations such as influenza vaccine linked to a marker
epitope can also be used.
5.6.3 Tests for inflammation and autoantibodies
5.6.3.1 C-Reactive protein
Inclusion of an acute-phase reactant marker is helpful for
clinical interpretation of other laboratory biomarkers such as
autoantibodies. The concentration of C-reactive protein rises and
falls to baseline values in direct proportion to tissue damage and is
thus a sensitive indicator of the presence of a generalized
inflammatory state. It offers the best example of an accepted,
standardized procedure that can be used in large population studies,
because it is less subject to transport variables than other
procedures.
5.6.3.2 Antinuclear antibody
Antinuclear antibody is a common autoantibody that may be
associated with various rheumatic disorders and, classically, systemic
lupus erythematosus. Several commercial kits are available to detect
the presence of autoantibodies. Progressively greater titres increase
the specificity for a disease. Positive sera should be titred at
dilutions of > 1:40 to 1:640.
5.6.3.3 Rheumatoid factor
Rheumatoid factor is an autoantibody to immunoglobulin (usually
IgM) that occurs in a high percentage (50-70%) of individuals with
classical rheumatoid arthritis but may also develop in a variety of
other disorders, including infections and immunological diseases.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.3.4 Thyroglobulin antibody
This antibody occurs in association with a variety of thyroid
disorders but may be found without detectable thyroid dysfunction.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.4 Tests for cellular immunity
A variety of tests are commonly used to assess cell-mediated
immunity, including enumeration of T cells and T-cell subsets,
identification of delayed skin reactions, and measurement in vitro
of stimulation of lymphocytes to proliferate and form blast cells.
Other tests are available to measure T-cell effector or regulatory
function in vitro. As for humoral immunity, a series of simple tests
is available to screen for defects in cell-mediated immunity. The
proportion of circulating T cells in a mononuclear cell preparation
can be determined by immunofluorescence with CD2 or CD3 monoclonal
antibodies. Normally, T cells constitute 55-80% of peripheral blood
lymphocytes. The normal values reported for absolute numbers of
circulating T cells are 1620-4320/mm3 during the first week up to
18 months of life and 590-3090/mm3 after 18 months of age (Fleisher
et al., 1975).
5.6.4.1 Flow cytometry
Antibodies that may be used in immunological phenotyping are
listed in Table 10 in section 4.1.2. Subsets of T cells have been
defined with monoclonal antibodies specific to cell-surface antigens.
The association of a particular T-cell subset with a given function
has caused some confusion in the analysis of immunological data for
patients with immunodeficiency states, as discussed in more detail in
section 1.2.2.1. For example, CD-positive cells have commonly been
associated with helper functions, and CD8 cells have been associated
with cytotoxic functions. This approach is an oversimplification,
which became evident with the finding that CD and CD8 cells recognize
foreign antigens in the context of MHC class II and class I antigens,
respectively. Thus, the CD population contains helper cells, memory
cells, and cytotoxic cells for targets bearing MHC class II molecules.
The CD8 population contains cytotoxic cells and also cells that can
recognize antigens presented by macrophages and cells that can augment
the interaction of CD-positive cells with B cells. Abnormalities in
the number of CD or CD8 cells, or their ratio, can be associated with
abnormalities in the ability to recognize antigens and regulate T-cell
function that can lead to immune incompetence or autoimmunity.
5.6.4.2 Delayed-type hypersensitivity
The ability of patients to manifest pre-existing T-cell immunity
has been evaluated in vivo with a series of skin antigens that
normally produce a delayed-type cutaneous hypersensitivity response.
Because delayed cutaneous hypersensitivity, a localized immunological
skin response, depends on functional thymus-derived lymphocytes, it is
used in detecting T cell-mediated immunodeficiency. A device
(Multitest(R), Institut Merieux, Lyon, France) is available that
enables the simultaneous intradermal injection of seven standardized
antigens and so overcomes the inconvenience of giving seven separate
injections of antigens and the technical difficulty of ensuring their
intradermal rather than subcutaneous injection. The Multitest(R),
consisting of eight tined, preloaded heads, delivers a glycerol
control and the seven test antigens dissolved in glycerol. The size of
the induration produced by each antigen should be measured at 48h in
two diameters: reactions of less than 2 mm are scored as negative. No
reaction should be seen with the glycerol control. The test includes
as antigens: tetanus, diphtheria, streptococcus, tuberculin, Candida,
Trichophyton, and Proteus.
Recall of delayed-type hypersensitivity as a test for cell-
mediated immune competence was assessed with the Multitest(R) device
in 254 subjects. When 77 subjects were tested concurrently with the
Multitest(R) and a conventional panel of six antigens (Frazer et
al., 1985), similar results ( r = 0.65) were obtained with the two
systems. The reproducibility of the Multitest(R) among three
observers, who assessed the aggregate size of reactions in 45 subjects
independently, was high ( r = 0.89); the correlation for the reaction
score in 24 subjects tested twice, three months apart, was also high
( r = 0.88), demonstrating the suitability of the test for serial
studies of immune function.
5.6.4.3 Proliferation of mononuclear cells in vitro
A common test of lymphocyte function is measurement of the
capacity of lymphocyte subsets to enlarge and convert into blast-like
cells that synthesize DNA and incorporate thymidine after stimulation
in vitro. In this test, lymphocytes can be activated by antigens
(e.g. purified protein derivative, Candida, streptokinase, tetanus,
or diphtheria); allogeneic cells are also used in the one-way mixed
lymphocyte culture to stimulate T-cell proliferation. T-Cell blast
transformation can be assessed directly by measuring blastogenesis and
proliferation of cells, expression of activation antigens (e.g. CD69
or CD25 and HLA DR), and release of mediators. The blastogenic
response is assessed as incorporation of 3H-thymidine, usually for
16-24h, followed by cell precipitation on filter paper and liquid
scintillation counting. Non-isotope assay alternatives are also
available. The responses to various antigenic stimuli by different
types of responding cells must be interpreted with caution. The mixed
leukocyte reaction is the result of T-cell reactivity to MHC-encoded
peptides displayed on the surface of B cells and monocytes. The
T cells in the population of normal irradiated or mitomycin C-treated
lymphocytes used as the stimulators can secrete factors that induce
blastogenesis in the patient's lymphocytes. As this can be misleading,
it is preferable to use B-cell lines or T cell-depleted normal cells
as the stimulators.
5.6.5 Tests for nonspecific immunity
5.6.5.1 Natural killer cells
The differences between NK cell function, phenotyping for NK
cells, and the cytology of large granular lymphocytes are described in
section 1.2.2.1. The identification of NK cells remains problematic
owing to this apparent heterogeneity. The cells can be evaluated in
ex-vivo/in-vivo tests with enriched peripheral blood mononuclear
cells. The proportion of NK cells can be identified with appropriate
monoclonal antibodies (see Table 10). Functional assays of NK activity
involve the ability of the appropriate mononuclear cells to kill
specific NK targets, such as the K562 cell, in which cell-mediated
cytolysis in vitro is quantified by release of 51Cr from the
target cells.
5.6.5.2 Polymorphonuclear granulocytes
Some defects of phagocytic function affect polymorphonuclear
phagocytes. Neutrophil function depends on movement in response to
chemotactic stimulus, adherence, endocytosis, and destruction of the
ingested particles. Phagocyte mobility depends on the integrity of the
cytoskeleton and contractile system. Defects in intracellular killing
of ingested microorganisms usually result from failure of the
'respiratory burst' that is critical to production of superoxide
radicals and hydrogen peroxide. The organisms cultured from lesions of
patients with this type of defect generally contain catalase and
include staphylococci, E. coli, Serratia marcescens, fungi, and
Nocardia. Patients with defects in mobility, adherence, and
endocytosis usually have infections of the skin, periodontitis, and
intestinal or perianal fistulae; patients with normal endocytosis and
defective killing tend to have chronic granulomas. Measurement of
nitroblue tetrazolium dye reduction or chemiluminescence by actively
phagocytosing leukocytes has been accepted as a standard measure for
the adequacy of the respiratory burst. Recently, methods have been
developed to measure the production of reactive oxygen intermediates
by flow cytometry (Emmendorfer et al., 1994). Kits are commercially
available for assessing phagocytic capacities and the production of
reactive oxygen intermediates by phagocytes. Assays for bacterial
killing yield highly variable results, depending on the bacterial
species used in the assay, and are not recommended for routine use.
The activation state of neutrophilic granulocytes can be assessed by
flow cytometry with the antibodies CD11b, CD14, CD16, CD54, and CD64
(Spiekermann et al., 1994). Activation of platelets can also be
assessed by flow cytometry (Tschöpe et al., 1990)
5.6.5.3 Complement
The classic complement system consists of nine components (C1-9)
and a series of regulatory proteins (C1 inhibitor, C4 binding protein,
and properdin factors). Many biological activities important in the
inflammatory response and in host resistance to infection occur at
various points in the classical and alternative pathways of complement
activation. Three clinical states should raise a suspicion of
deficiency in a complement component: systemic lupus erythematosus,
recurrent infections of the type seen in hypogammaglobulinaemia in
patients with normal immunoglobulin levels, and severe Neisseria
infection. Laboratory measurement of serum haemolytic complement
(CH50) is a useful test. Serum haemolytic complement is usually absent
and rarely above 10% of the normal value in inherited complement
deficiencies, with the exception of hypercatabolism of C3. More
detailed analysis of the complement system requires functional and
antigenic measurements of the individual components, usually best
performed in laboratories that specialize in the complement system.
5.6.6 Clinical chemistry
Assessment of clinical abnormalities in standard serum
chemistry, such as liver function, renal function, glucose, and
albumin, is indicated to facilitate reasonable interpretation of
specific changes in the immune system as secondary to another
condition.
5.6.7 Additional confirmatory tests
After activation, mononuclear cells from peripheral blood
express the genes that encode a series of interleukins and colony-
stimulating factors. Activated T cells and monocytes synthesize and
secrete IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, interferon, other
cytokines such as tumour necrosis factor, and cytokine receptors.
These cytokines are involved in the growth and differentiation of
T and B cells, eosinophils, and basophils. The supernatants of
mononuclear cells from peripheral blood stimulated by soluble
phytohaemagglutinin can be assessed for IL-2 by determining their
capacity to stimulate 3H-thymidine uptake by mouse IL-2-dependent,
cultured T-cell lines. Other biological assays, radioimmunoassays, and
ELISAs have been developed to quantify the production of lymphokines
and colony-stimulating factors. With molecular cloning techniques,
messenger RNA transcription by each of the lymphokines can be
quantified after appropriate lymphocyte activation. In the assessment
of lymphokines, T cells or peripheral blood mononuclear cells are
usually activated with concanavalin A, pokeweed mitogen, or
insolubilized CD3 antibodies; then, the appropriate assays are
performed to quantify the specific lymphokine(s) produced in the
culture media. Different patterns of lymphokine secretion have been
observed with different subsets of T cells: INF gamma and IL-2 are
produced by the Th1 subset, while IL-4, IL-5, and IL-10 are produced
by the Th2 subset, as originally documented for cloned CD T-cell lines
from mice (Mosmann & Coffman, 1989). Thus, an assay of the pattern of
lymphokine production could be used in pinpointing the action of an
immunotoxicant on a particular subset of immune cells.
6. RISK ASSESSMENT
6.1 Introduction
Publications on immunotoxicology published by IPCS and the
European Union (Berlin et al., 1987; Dayan et al., 1990), the United
States Office of Technology Assessment (1991), and the United States
National Research Council (1992) demonstrate the growing interest and
concern within scientific and public communities on the capacity of
environmental agents to perturb normal immune processes. The
incorporation of experimental data on toxicant-induced alterations in
the immune system into evaluations of drugs, chemicals, and biological
agents for human risk assessment has thus become increasingly common.
For example, in the United States, the Environmental Protection Agency
(Sjoblad, 1988) and Food and Drug Administration (Hoyle & Cooper,
1990; Hinton, 1992) indicate the benefits of testing the
immunosuppressive potential of biochemical pest control agents,
antiviral drugs, and food additives. Furthermore, the Environmental
Protection Agency has established reference doses (an estimate of the
daily exposure of the human population that is likely to have no
appreciable risk of deleterious effects during a lifetime), on the
basis of data on the immunotoxicity of several compounds, including
1,1,2-trichloroethane, 2,4-dichlorophenol, and dibutyltin oxide. The
United States Agency for Toxic Substances and Disease Registry has
derived 'minimal risk levels' for arsenic, dieldrin, nickel,
1,2-dichloroethane, and 2,4-dichlorophenol on the basis of immune
end-points.
Risk assessment is a process whereby relevant biological, dose-
response, and exposure data for a particular agent are analysed in an
attempt to establish qualitative and quantitative estimates of adverse
outcomes (Scala, 1991). Such data are sometimes used in the
development of standards for regulating the manufacture, use, and
release of chemicals into the environment (Kimmel, 1990). As defined
by the United States National Academy of Science (US National Research
Council, 1983), risk assessment comprises four steps: hazard
identification, dose-response assessment, exposure assessment, and
risk characterization. The process of assessing the risk of both
cancer and non-cancer end-points, including immunotoxicity, may be
adapted to this form.
The first step, hazard identification, involves a qualitative
evaluation of available human and animal data to determine whether a
chemical agent poses a potential hazard. Consideration is given to
dose, route, and duration of exposure. The precise quantitative
relationships between changes in immune function or in the
histological appearance of lymphoid organs and host resistance to
infectious agents or neoplastic diseases are unclear. It can be
assumed that any significant difference from appropriate controls in
the ability of the immune system to respond to a challenge may evolve
into an adverse effect and thus present a potential hazard. This
applies to adaptive as well as nonadaptive responses.
After hazard identification, 'dose-response' is assessed. For
non-cancer toxicity, a no-observed-adverse-effect-level (NOAEL) is
established for an adverse response of interest. This process is no
different in immunotoxicology and is the same as for the other target
organ systems. The NOAEL value is either obtained from the dose-
response curve or is estimated from the lowest-observed-adverse-
effect-level (LOAEL). Once the NOAEL has been determined, safety and
uncertainty factors can be applied to allow for various uncertainties,
such as species or interspecies variability, irreversible effects, and
chronic exposure. The use of safety factors, however, should be
flexible and should allow incorporation of any relevant information on
the mechanism of action of the chemical under review. Ideally,
however, dose-response relationships should be established from human
epidemiological data that include the exposure levels expected on the
basis of human contact with the agent in the environment. As
illustrated in an assessment of developmental toxicity (Kimmel &
Gaylor, 1988), use of the risk assessment and management paradigm of
the United States National Academy of Sciences (US National Research
Council, 1983) to non-cancer end-points such as immunotoxicity, offers
some serious challenges. For example, since the presence or absence of
an effect is based upon whether a statistically and/or biologically
significant response is observed at a certain dose or doses, and since
multiple assays are routinely conducted, the NOAEL will depend heavily
on the sensitivity of the assay. The differences may be particularly
exaggerated when continuous responses (i.e. the results of most immune
function tests) are compared with categorical data, the latter being
routinely expressed as proportions. For example, in experimental
animals, changes in many immune functions may be statistically
significant when they vary by as little as 15-25% from the control
values. In contrast, host resistance, when expressed categorically
(e.g. tumour frequency), must change by 80% to reach a comparable
degree of significance, assuming group sizes of about 15 animals and
effective doses of 20% in the control group. Furthermore, immune tests
are often, but not always, interdependent (Luster et al., 1992), and
individual or combinations of tests might have to be ranked in order
of sensitivity and degree of interdependence before dose-response is
assessed. This has not been done in the past.
Exposure assessment (step 3) is done in parallel with hazard
identification and dose-response assessment (Scala, 1991). It often
involves field measurements and other estimates of human exposure,
such as the composition and size of the population, biological or
clinical effects and types, and the magnitude, frequency, and duration
of exposure to the agent. Many of these parameters are difficult to
determine accurately in a longitudinal study; even measurements of
body burden can be misleading since the concentrations at the target
organ (e.g. lymphoid tissue) are not determined. The problems in
immune testing in humans are similar to those in testing other organs
and systems and include differences in individual responses due to
unique sensitivities (e.g. age, pregnancy, genetics) and confounding
factors (e.g. smoking, stress, drugs).
Risk characterization (step 4) is the aggregation of the three
previous processes. It provides an estimate of the incidence of
adverse effects in a population and the potential health problems. As
part of risk characterization, the strengths and weaknesses of each
component of the assessment are considered, with assumptions,
scientific judgements, and, to the extent possible, estimated
uncertainties. Most assessments of the risks presented by chemical
agents have focused on the estimated incidence of cancer after
lifetime exposure to a chemical at some unit dose, assuming that there
is essentially no threshold for carcinogenicity. The assessment of
non-cancer end-points, such as disorders of the neurological,
developmental, and reproductive systems, is somewhat similar to that
of cancer, in that it involves calculations that include both
assumptions and uncertainties. For example, considerations in risk
assessment include ranking the value of epidemiological against
experimental data, extrapolations from high to low doses, from
subchronic to chronic exposure, and from animals to humans, and
appropriate use of mechanistic and pharmacokinetic data. Data from
immunotoxicology, like those from developmental toxicology (Schwetz &
Tyl, 1987), do not easily lend themselves to the mathematical models
used in cancer risk assessment, which usually involve non-threshold
models for genotoxic carcinogens. For more accurate assessment,
mechanistic models will be required which include the concept of
'threshold'. It is assumed that threshold levels exist below which no
adverse immunological effect can be demonstrated. Complex mixtures of
chemicals, in which each chemical occurs at a subthreshold
concentration, may reach or exceed a threshold for immunotoxicity
(Germolec et al., 1989), although problems associated with mixtures of
compounds are not unique to the field of immunotoxicology.
The approaches currently used by the US Environmental Protection
Agency (1986) in extrapolating the risk for developmental toxicity
have been outlined, and similar guidelines have been developed by IPCS
(1994b). One method is to apply uncertainty factors to the NOEL or
NOAEL for the most sensitive animal species tested. The uncertainty
factor is usually composed of a 10-fold factor to account for
interspecies differences and a 10-fold factor for intraspecies
variability. If no NOEL is available, an additional 10-fold factor may
be applied to the lowest-observed-effect level (LOEL). Another
approach is to calculate a margin of safety, which is the NOEL divided
by the estimated level of human exposure from all potential sources.
The margin of safety can then be evaluated for adequacy to protect
human health. There are several drawbacks to both of these approaches,
the primary one being that they use only one point on the dose-
response curve (NOEL or LOEL) and ignore the rest of the data. Also,
since the variability around the NOEL and LOEL is usually not taken
into account, these approaches may rely on poor studies, i.e. studies
that result in a higher NOEL because of their limited ability to
detect small changes over the background.
Since the purpose of risk assessment is to make inferences about
potential risk to human health, the most appropriate data are those
derived from studies of humans; however, adequate data are seldom
available, and most risk assessments are based on results obtained in
experimental animals. In order to use these results, a number of
assumptions must be made about their relevance to potential human
health risk. Firstly, it is assumed that experimental animals respond
to the agent of interest in a pharmacological and toxicological manner
similar to that anticipated in humans (i.e. the test animals and
humans metabolize the chemical similarly and have identical responses
and toxicity at the target organ). Secondly, the immune system of the
experimental species must be very similar to that of humans: the
vertebrate immune system is highly conserved among higher vertebrate
species, and the immune components and their interactions in mice,
rats, and humans are closely similar. Thus, if toxicokinetic
properties are similar, it is reasonable to test for potential adverse
effects in humans using laboratory rodents.
As immunosuppressive agents cannot ethically be administered to
humans, quantitative comparisons of dose-responses in humans and
experimental animals are limited (although it is possible to do so in
hypersensitivity tests). Nonetheless, controlled human exposures have
been studied and the results compared with the immune effects observed
in animals. As an example, the immune effects of cyclosporin A in
various species are compiled in Table 12, which shows that the mouse
is much less sensitive to cyclosporin A than other species, in
particular the rat, and that humans are slightly more sensitive than
other species (Dean & Thurmond, 1987); however, for the most part,
there was good qualitative and quantitative agreement between the
species examined. Selgrade et al. (1995) compared phagocytosis by
human and murine alveolar macrophages after exposure to ozone
in vitro and in vivo (Table 13): The effects of ozone on alveolar
macrophage function in murine species are predictive of effects that
occur in humans, and the effects on macrophage phagocytosis seen
in vitro are predictive of those that occur in vivo. Quantitative
comparisons have also been made in mice and humans for the ability of
UVR to inhibit the contact hypersensitivity response (Table 14). As
described in section 2.2.11, exposure to UVB inhibits delayed-type
hypersensitivity (Kripke et al., 1979). Noonan & Hoffman (1994)
described three strains of mice with low, intermediate, and high
susceptibility to UVR-induced immunosuppression, and Oberhelman et al.
(1992) reported that suppression of the hypersensitivity response also
occurs in humans and that the dose of radiation required to induce 50%
suppression in fair-and dark-skinned individuals is similar to that
required to inhibit the response in mice with high and intermediate
susceptibility, respectively.
Table 12. Comparison of doses of cyclosporin A that suppress
the immune response in various species
Species Response Cyclosporin A
(mg/kg body
weight)
Mouse Antibody production 50-300
Cell-mediated immunity 100-300
(delayed-type hypersensitivity)
Graft-versus-host reaction 50-250
Rat Antibody production 20-50
Graft-versus-host reaction 10-60
Guinea-pig Cell-mediated immunity 10-100
(delayed-type hypersensitivity)
Dog Cell-mediated immunity 15-30
(delayed-type hypersensitivity)
Rhesus monkey Antibody production 50-250
Human Cell-mediated immunity 10-20
Adapted from Dean & Thurmond (1987); White et al. (1994)
Data from immunotoxicology also differ from those for
carcinogenicity and possibly other non-cancer end-points because the
immune system contains components with overlapping functions. For
example, when an individual is exposed to an infectious agent,
multiple immune components may work either independently or in concert
to help defend the host; i.e. there is redundancy between immune
functions. Furthermore, while a significant change in any immune
function can be considered potentially deleterious, in that it may
increase the risk of developing clinical disease, a change in immune
function does not necessarily precipitate a disease or clinical health
affect. That is, immunocompromised individuals function normally in
the absence of infectious agents. Thus, immune function reserve and
redundancy are relative terms, depending on the dose of infectious
agent. This complicates dose-response assessment, and models should be
developed that incorporate available information on the quantitative
relationship between immune function and clinical disease and
potential redundancy.
Table 13. Effect of exposure to ozone on phagocytosis by alveolar
macrophages
Treatment Phagocytic index (no. fluorescent
particles ingested/100 macrophages)
--------------------------------------
Mice Humans
---------------- -------------
Mean SE Mean SE
Air in vitro 369.2 26.4 386.7 50.5
(n = 6) (n = 6)
Ozone in vitro 291.7 17.4* 275.0 45.1*
(n = 6) (n = 6)
Suppression 21% 29%
Air in vivo 330.6 10.4 714.9 46.1
(n = 4) (n = 10)
Ozone in vivoa 194.0 19.7* 539.2 22.3*
(n = 4) (n = 10)
Suppression 42% 25%
Suppression corrected 28% 25%
for dosimetric differenceb
Adapted from Selgrade et al. (1995)
* Significantly different from air control (P < 0.05; Student's
t test)
a Mice were exposed to 0.8 ppm for 3 h; humans were exposed to
0.08 ppm for 6.6 h while undergoing intermittent exercise.
b On the basis of studies using 18O, alveolar macrophages of
mice exposed to 0.8 ppm ozone for 3 h receive roughly 1.5 times
more ozone than those of humans exposed to 0.08 ppm ozone for 6.6 h
while exercising moderately.
Table 14. Comparison of doses of ultraviolet radiation that cause
50% suppression of contact sensitivity in mice and humans
Mousea Humanb
--------------------------------------------- --------------
Sensitivity kJ/m2 mJ/cm2 Skin type
mJ/cm2
(phenotype)
High (C57Bl) 0.7-2.3 70-230 Fair 100
Intermediate (C3H) 4.7-6.9 470-690 Dark 225
Low (BALB/c) 9.6-12.3 960-1230
Adapted from Selgrade et al. (1995)
a Data from Noonan & Hoffman (1994)
b Data from Oberhelman et al. (1992)
The increasing evidence that environmental contaminants affect
wildlife populations has led to risk assessment at the level of the
ecosystem; however, the limited evidence for immunotoxic effects in
wildlife precludes an adequate understanding of the risks posed by
current levels of environmental pollution. The demonstration of
immunosuppression in harbour seals fed herring from the contaminated
Baltic Sea in a semi-field experiment (De Swart et al., 1994; Ross et
al., 1995) provided a first indication that ambient levels of
contaminants in certain areas present an immunotoxic risk to mammalian
wildlife occupying a high trophic level. These results may partially
explain the severity of a series of unrelated epizootic viral episodes
in various marine mammalian populations in coastal areas of Europe and
North America (Dietz et al., 1989; Van Bressem et al., 1991). In
similar semi-field experiments in a bottom-dwelling fish species,
flounder exposed to contaminated sediments had more viral lymphocytic
infection and liver tumours than controls (Vethaak & Wester, 1993).
While the difficulties in conducting adequate immunological studies
with wildlife may preclude an approach as comprehensive as that in
humans, such semi-field strategies may provide the best available
direction.
Should the application of field studies be possible, correlative
approaches to immunotoxicology may substantiate an effect on the
ecosystem. Such an approach was used to establish a correlation
between the induction of mixed-function oxidases and pollutant burden
(as measured by the toxic equivalence of organochlorine chemicals) in
cormorant (Phalocrocorax carbo) chicks collected from various sites
in the Netherlands (van den Berg et al., 1994). A combination of
laboratory experiments under controlled conditions, semi-field
experiments under controlled conditions with exposure to environmental
mixtures of pollutants, and correlative field studies is necessary to
understand immunotoxicity in wildlife populations.
6.2 Complements to extrapolating experimental data
6.2.1 In-vitro approaches
The complexities of the immune system and the requirement of
many agents for metabolism and distribution in order to produce an
immunotoxic response have resulted in the almost exclusive use of
animal models in vivo for assessing immunotoxicity. Culture systems
have been used extensively, however, to study the mechanisms by which
agents induce immunosuppression. In-vitro test systems with immune
cells of human origin are particularly attractive, given the
uncertainties in extrapolating the results of studies in experimental
animals to humans and the accessibility of human peripheral blood
cells. Although many of the immune cells obtained from human blood are
immature forms, the large numbers and diverse populations (i.e.
polymorphonuclear granulocytes, monocytes, NK cells, T cells, and
B cells) that can be obtained and the ease of conducting challenge
assays in vitro provide an attractive alternative (or, preferably,
adjunct) to more conventional studies in animals. Surprisingly few
laboratories have conducted studies with immunosuppressive agents in
which immune function responses in human immune cells are compared
with thosein rodents in vivo (Cornacoff et al., 1988; Luo et al.,
1992; Wood et al., 1992; Lang et al., 1993). Althoughstudies in human
cells in vitro have been hampered by a lack of assays to assess
primary antigen-specific immune responses, a relatively good
interspecies correlation has been observed in the limited responses
examined. Furthermore, some of these assays have been successfully
modified to include metabolic fractions of liver homogenates (Shand,
1975) or co-culture with primary hepatocytes (Kim et al., 1987) to
allow for chemical metabolism.
6.2.2 Parallelograms
Interpretation of studies in experimental animals or in vitro
can be improved when even limited data on human exposure in vivo are
available, using a 'parallelogram' approach. In general, if a
parallelogram can be constructed in which data are available for five
of the six angles (Figure 37), it may be easier to predict the outcome
at the remaining angle, at least qualitatively. For example, cytokine
and phagocytic responses of alveolar macrophages or pulmonary
epithelial cells after exposure to ozone in vitro can be compared
with the responses to ozone after exposure of humans and animals
evaluating the predictability of various assays (Luster et al., 1988).
In a subsequent study, the potential immunotoxicity (defined as a
dose-related effect on any of two immunotoxicological parameters with
no effect on body weight) was determined for 51 chemicals in mice,
using a variety of general and functional immunological parameters
(Luster et al., 1992).
Accuracy is determined by the ability to measure the intended
end-point truly. Precision is the ability to reproduce results from
experiment to experiment or between laboratories. In the NTP studies
in mice (Luster et al., 1988), four laboratories participated in the
inter-laboratory validation process. A number of international studies
are in progress on the precision of several assays, using the rat as a
model for immunotoxicological evaluations, in an attempt to bring the
level of acceptance of immunotoxicological studies in rats to the
level that has been achieved for mice. Most of these studies are
multinational and represent an interaction of industry, government,
and academia to achieve this common goal.
A comparative study in Fischer 344 rats with cyclosporin A (White
et al., 1994) encompasses nine laboratories in Canada, Europe, and the
United States. The primary focus of the study is on the use of
functional assays for detecting immunosuppression; lymphoid organs and
tissue are weighed and examined histopathologically in several of the
laboratories. The functional assays used in this protocol include the
plaque-forming cell assay or ELISA to sheep erythrocytes, splenocyte
proliferative assays to concanavalin A and STM, the NK cell assay, and
the mixed leukocyte response. Splenocyte surface markers were also
analysed. The study design was similar to that used by the NTP, with a
14-day exposure and administration by oral gavage. The preliminary
results demonstrated excellent reproducibility of the results for the
plaque-forming cell assay, splenocyte proliferative assays to
concanavalin A and STM, and the mixed leukocyte response. Differences
were observed between the laboratories in the results of the test for
NK cell activity.
The IPCS-European Union international collaborative
immunotoxicity study in rats is also in progress. The study involves
20 laboratories in Canada, Europe, Japan, and the United States; its
design is based on the OECD test guideline No. 407 for a 28-day
toxicity study. The study focuses primarily on the ability to detect
immunotoxic compounds on the basis of organ weights, pathological
findings, and 'enhanced pathology', which includes additional
evaluation of lymphoid tissues not currently required by test
guideline No. 407. Functional assays were also conducted; the core
assays included the plaque-forming cell assay or ELISA to sheep
erythrocytes, splenocyte proliferative assays (concanavalin A and
STM), and the NK cell assay. The study was conducted in two phases. In
the first phase, azathioprine was used as the test compound, various
strains of rat were used, and each laboratory established its own
doses on the basis of a predetermined maximum tolerated dose. In the
second phase, cyclosporin A was the test compound, only three strains
of rat were used, and a more structured protocol was followed. A
report on phase I of the study has been drafted, and the data from
phase II are currently being analysed. All of the laboratories found
that azathioprine is immunosuppressive, even though several strains of
rats were used, different dose levels were administered, and no
standard protocol was followed. The preliminary results with
cyclosporin A show good agreement between the laboratories for the
plaque-forming cell assay but some differences for the splenocyte
proliferative assays (concanavalin A and STM) and the NK cell assay.
A third interlaboratory study in rats has been organized by the
German Bundesgesundheitsamt, in Berlin, with German and French
participants. The design is also based on OECD test guideline No. 407
for a 28-day repeated-dose study in Wistar rats. Cyclosporin A was
selected as the test substance. The live phase of this study has been
completed, the data are being analysed, and the final report is being
prepared.
Information obtained from studies of predictability, accuracy,
and precision, such as those described above, must undergo peer review
before publication. A major goal of the validation process is to
determine which methods should be recommended in the testing
guidelines of regulatory agencies. Figure 36 shows each input and
output of information and the action steps. The process of developing
and obtaining acceptance of testing guidelines is based on three major
inputs: (1) publication of reports in peer-reviewed journals;
(2) guidelines for deciding whether a method is valid; and
(3) implementation of test methods that are interpretable by
scientists involved in assessing biologically relevant risks and the
results of which can be incorporated into quantitative dose-response
analyses. The proposed process is built around the generation of these
major inputs. Two of the issues that will arise in the development of
guidelines are: 'How many and what type of compounds should be
included in the validation process?' and 'Should the compounds be
shown to be immunosuppressive in both humans and mice?'
5. ESSENTIALS OF IMMUNOTOXICITY ASSESSMENT IN HUMANS
5.1 Introduction: immunocompetence and immunosuppression
An immune response in the fully mature, immunologically competent
individual provides protection against a myriad of infectious agents
and environmental hazards. The immune system acts as a self-restoring
(homeostatic) system which can quickly return to normal levels of
function after periods of marked stimulation and response. This self-
regulation allows the individual to recover from or circumvent the
toxic effects of many potentially damaging environmental hazards.
There are many well-known clinical conditions of inherited deficiency
in immunological function; some result in specific defects in antibody
formation, others consist of T-cell and/or metabolic defects, while
others include impairment of both B- and T-cell function. These
conditions, known as primary immunodeficiency disorders, are due to
definable, inheritable genetic defects. Clinical studies of these
disorders have demonstrated the importance of the immune system to
host defence and individual survival, showing that individuals with
partial or absolute defects in T-cell function rarely survive beyond
infancy or early childhood. In contrast, individuals with defects in
B-cell function, resulting in a deficiency in antibody formation, may
suffer from a variety of chronic, recurrent infectious diseases and
diminished health but can survive with appropriate therapy when the
underlying disorder is recognized. Study of these genetic
immunodeficiency states has also provided considerable information
about the functions of human B and T cells which would otherwise not
have been determined.
Impairment of the function of a key component of the immune
system results in a diminished immune response (immunosuppression) or
immunodeficiency. Acquired immunodeficiency states were recognized
only sporadically until the late 1970s, when a syndrome appeared that
spread rapidly through certain groups and produced a generalized type
of immunosuppression known as acquired immunodeficiency syndrome
(AIDS). AIDS was found to be due to retroviruses that infect and
destroy Th (CD4+) cells in humans (Fauci et al., 1991). CD+
lymphocytes have been identified in experimental studies as the key
cells in the recognition and secondary processing of antigens. Thus,
progression of AIDS is associated with progressive loss of Th cells
and increased frequencies of infection by bacterial, fungal, viral,
and parasitic agents and of certain types of neoplasms.
5.2 Considerations in assessing human immune status related to
immunotoxicity
The assessment of immunotoxicity in humans exposed to potentially
immunotoxic compounds is much more complicated than in experimental
animals. Issues such as logistics, appropriate controls, magnitude and
pattern of exposure, and confounding parameters such as medication,
drug abuse, and illness must be considered. Other considerations that
should be taken into account in comparing human immune status with
that in laboratory animals in relation to immunotoxicity are as
follows:
(1) The human population is heterogeneous and genetically
disparate; it can be considered as 'wildlife'. Inbred laboratory
animals are, by definition, genetically identical; outbred laboratory
animals typically have a larger genetic variability than inbred
animals but a variability that is much smaller than that in wildlife
populations. Genetic constitution, which accounts for the variability,
has consequences for the antigen recognition capacity of the immune
system, especially for the T-lymphocyte population. Antigen
recognition by T cells is restricted to the MHC haplotype of the
individual and therefore differs between (allogeneic) individuals.
Inbred, and most outbred, laboratory animals are much more alike in
antigen recognition capacity than wildlife populations. For instance,
the repertoire of certain inbred mouse strains lacks part of the
spectrum of T-cell specificity, as seen by the absence of T cells that
express distinct 'variable gene families' in the repertoire of TCR
specificities. Such 'gaps' in the repertoire have thus far not been
detected in the outbred human population by similar methods of
detection (Hu et al., 1993), perhaps because each individual in an
outbred population expresses the MHC products of both parents and can
in principle multiply the repertoire of MHC-restricted reactions by a
factor of 2 (including MHC I and MHC II).
Interindividual variability has obvious consequences for
immunotoxicity, in which the response to the chemical or drug
underlies the mechanism of toxicity. Its effect on direct toxicity is
presumably less, but the manifestation of toxicity is often reduced
immune reactivity (e.g. increased incidence of infections) and hence
determined by individual reactions to antigens.
(2) The human population, like populations of 'wildlife' animals,
is continuously exposed to environmental stimuli. It is well known
that it is not necessary that each member of a population be protected
('immune') but that a certain proportion of protected individuals must
be reached in order to achieve 'herd immunity'. That is to say, the
whole population is protected when a certain percentage of individuals
is immune. In contrast, when this percentage falls below the required
incidence (which differs for different infectious agents), the
population as a whole loses its protected state, and an infectious
epidemic can result. This situation easily arises in small groups in a
country where vaccination is minimal. Well-known examples are the
outbreaks of poliomyelitis in the Netherlands and the hepatitis A
virus outbreaks in China. This phenomenon should be taken into account
in epidemiological studies and associated laboratory investigations
(e.g. antibody levels to microorganisms) in assessing immunotoxicity
in human populations.
(3) Most of the human population is continuously exposed to
environmental stimuli and maintains its ability to respond to foreign
material from the pool of immunological memory. From the first
postnatal period through to adulthood, the T-cell repertoire is
generated by the thymus; later, this generation of cells is reduced to
a low level. Strict MHC restriction implies that the T-cell population
cannot easily change specificity, e.g. by somatic mutation of
the genes that encode the TCR. There is some evidence from
immunophenotyping, both in mice and humans, that the T-cell population
shifts gradually during life, from naive (committed) T cells to memory
T cells. Within the B-cell population, the situation is different.
Here, the repertoire changes continuously, due to somatic mutation
presumably associated with 'affinity maturation' in lymph nodes. This
phenomenon may result in the emergence of B cells with a strong
affinity for stimuli and the disappearance of low-affinity cells. It
is not known whether neoantigens or pathogens are recognized by
'affinity matured' or memory B cells or by naive B cells. In the
absence of information on this aspect, it can be suggested that most
of the immune capacity of adults is deployed for memory reactions,
whereas in young people the contribution from the naive pool is
higher. This is reflected in infectious epidemics, when microorganisms
like influenza change to phenotypes that cannot be recognized by the
memory pool, an aspect to be kept in mind when choosing immune tests
to be used in evaluating immunotoxicity. Primary responses, like those
to keyhole limpet haemocyanin antigen, are considered to be more
sensitive than secondary responses, like those to tetanus toxoid.
Another example of this effect is the composition of the recall
antigens used in testing delayed-type hypersensitivity (Borleffs et
al., 1993). Both primary and secondary antibody responses, however,
are valuable for evaluating the intrinsic naive and memory immune
capacity of individuals, although secondary responses are less
sensitive to immunological insults.
(4) For a number of infectious microorganisms, the immune
response does not result in complete elimination of the invader but
rather in its 'silent' integration into the genome. Certain viruses,
like herpes viruses, cytomegalovirus, and Epstein-Barr virus, are
dealt with in this way. An individual is considered to be a carrier of
the virus (postinfection status) on the basis of the presence of
antibodies. In other words, individual postinfection is a continuous
defence against these viruses, often with sufficient capacity to keep
the virus in a silent form. In diminished immune capacity, this
natural protection can be lost, and infections can re-occur after
viral reactivation. Primary infection and reactivation therefore have
different pathogeneses, although the subsequent disease may be
characterized by the same symptoms. This situation is well known
clinically, when high doses of immunosuppressive drugs are given for
long periods. The relevance of this observation in immunotoxicity
testing must be established.
(5) Ex-vivo diagnosis in humans is often restricted to
haematological investigations, so that only information on the
circulatory pool of cells and plasma factors is obtained. For example,
the distribution of immunoglobulins differs in the intravascular and
extravascular spaces. Only about 1% of the total lymphocyte pool is
present in blood (1010 cells out of the total of about 1012), and
this population represents only the recirculating pool of cells and
not the tissue-bound cells that participate actively in immunological
responses. Investigations of peripheral blood cells can be somewhat
misleading: for instance, patients infected with the human
immunodeficiency virus (HIV)-1 may show severe depression of CD+
cells in blood but less reduction in CD cells in lymphoid tissue
(Schuurman et al., 1985).
It is considerably more complex to establish immune changes in
humans than in animals, since noninvasive tests are limited, the
levels of exposure to an agent (i.e. dose) are difficult to establish,
and the responses in the population are extremely heterogeneous. With
respect to the latter, the variation in immune responses (genetic or
environmental) can exceed a coefficient of variation greater than
20-30%. Because many of the immune changes in humans that follow
exposure to chemicals may be sporadic and subtle, recently exposed
populations must be studied and sensitive tests for assessing the
immune system be performed. Since many of the immune tests used in
humans have a certain degree of overlap (redundancy), it is also
important that a positive diagnosis not be based on a change in one
test but on a profile (pattern) of changes, similar to that observed
in primary or secondary immunodeficiency diseases. For example, low
CD:CD8 ratios are often accompanied by changes in skin reactions to
recall antigens. The Clinical Immunology Subcommittee of WHO and the
International Union of Immunological Societies published methods for
examining changes in the human immune system and described their
pitfalls (Bentwich et al., 1982, 1988); however, most of the tests
were selected on the basis of observations in patients with primary
immunodeficiency diseases. Such individuals suffer from severe
recurring infections, and their degree of immunosuppression is
probably considerably greater than that induced by chemicals. Thus,
some of the methods may be of limited value for examining potential
chemical-induced immunosuppression, and further evaluation of methods
is needed.
In view of the difficulties in identifying chemical-induced
immunosuppression in humans, establishment of exposure levels (e.g. in
blood or tissue) would not only be useful but would in many instances
be essential for determining a cause-effect relationship. Clinical
disease may not necessarily have to be present in order for
immunosuppression to be meaningful, for several reasons. Firstly,
there are uncertainties about the extent of the reserve capacity of
the immune system and whether the relationship between immune function
and clinical disease shows a linear or a threshold response. In a
linear relationship, even minor changes in immune function would be
related to an increase in disease, if the population examined is large
enough. While the relationship at the low end of the dose-response
curve is unclear, at the high end of the curve (i.e. severe
immunosuppression), clinical disease is readily apparent. This is
exemplified by increased incidences of the opportunistic infections
that occur in AIDS patients. Secondly, clinical disease may be
difficult to establish, as neoplastic diseases may involve a
10-20-year latency before tumour appearance, and increased infection
rates are difficult to ascertain in epidemiological surveys (e.g.
increased numbers of cases of severe common cold).
5.3 Confounding variables
The normal population has a wide range of immunological
responses, with no apparent health impact. In addition to the
underlying population variability, certain host characteristics and
common exposures may be associated with significant, predictable
alterations in immunological parameters. If not recognized and
effectively addressed in study design or statistical analysis, these
confounding factors may severely alter the results of population
studies.
Examples of factors associated with measurable alterations in
immunological parameters are age, race, sex, pregnancy status, acute
stress and the ability to cope with stress, coexistent disease or
infection, nutritional status, lifestyle, tobacco smoking, and some
medications. The effect of acute stress on the immune system is
mentioned in section 1.2.1.5. Protein calorie restriction and
deficiencies of trace elements such as zinc have also been associated
with immune deficiency (Chandra, 1992; Good & Lorenz, 1992; Chandra,
1993). Periodic influences, ranging from daily to seasonal, also
exist; some are relatively minor, but others are of a magnitude that
may rival the expected effect of a low-level exposure to a toxic
agent. They are therefore of primary concern in large epidemiological
studies. For example, African Americans have, on average, serum IgG
levels that are 15-20% higher, neutrophil counts that are 10-15%
lower, and a proportion of circulating B cells that is significantly
higher than those of Caucasians, with no discernible health
implications. Cigarette smoking is associated with a significant
decrease in IgG level and an increase in leukocyte count, independent
of ethnic differences. Therefore, it is imperative that population
norms and reference ranges be supplemented by detailed analysis of
potential confounding factors. Study designs should include
considerations of matching, stratification, and subgroup analysis to
control for these potential effects. As new immunological assays are
developed, normative data will be required, particularly for ethnic
minorities, children, the aged, and certain groups potentially at high
risk, such as pregnant and lactating women.
Certain endocrine diseases and conditions may be associated with
significant alterations in immune function (e.g. adrenal dysfunction).
Some medications, such as corticosteroids, phenytoin, and nonsteroidal
anti-inflammatory agents, may depress a variety of immune functions.
Questionnaires and population surveys should allow the collection of
sufficient information to make it possible to consider these factors
in data analysis and interpretation.
An increasingly important consideration in any analysis of immune
function, particularly in relation to immune deficiency, is the
potential presence of HIV infection, which causes widespread
alterations in virtually all elements of the immune system. Even a
small proportion of unrecognized HIV-infected individuals in a study
population may significantly affect the results and the interpretation
of data. When immunological studies indicate decreased immune
parameters consistent with HIV infection, testing for the virus should
be considered; otherwise, interpretation of the results of
immunological tests of immune dysfunction, particularly among
populations with potentially high rates of HIV infection, may be
severely limited.
In assessing human immunotoxicity, it is useful to establish the
presence of infectious, allergic, or autoimmune diseases in order to
ensure completeness and to rule out additional confounding variables.
A clue to the type of immunological defect is often provided by the
kind of infection observed. For example, patients with impaired
humoral immunity have an increased incidence of recurrent infections
with encapsulated bacterial pathogens (e.g. Pneumococcus and
Haemophilus influenzae), which can induce chronic sinopulmonary
infection, bacteraemia, and meningitis. In contrast, if cellular
immunity is intact, the patients will have less severe infections with
fungal and viral agents. Abnormalities of T cells and impairment of
cell-mediated immunity predispose individuals to infections with a
wide variety of agents, including viruses that cause disseminated
infections (e.g. Herpes simplex virus, varicella-zoster virus, and
cytomegalovirus), fungi that cause mucocutaneous candidiasis, and
parasitic organisms including the protozoan Pneumocystis carinii.
5.4 Considerations in the design of epidemiological studies
An important factor in assessing the usefulness of an
epidemiological study for risk assessment is its design. The commonest
design used in immunotoxicology is the cross-sectional study, in which
exposure and disease status (in this case, changes in immunological
function) are measured at one time or over a short period. The immune
function of 'exposed' subjects is compared with that of a comparable
group of 'unexposed' individuals. Important considerations in using
the data provided by such studies in risk assessment have been
discussed (E. Ward, unpublished manuscript):
(1) What is the relationship between changes in immune function and
human health risk?
(2) Are the selection procedures for study subjects adequately
documented?
(3) Is there evidence that the exposed group was actually exposed to
the substance of interest?
(4) Has the possibility that other exposures, of either the entire
population or individuals, been accounted for?
(5) Are the 'exposed' and 'unexposed' populations comparable with
respect to factors other than the exposure of interest?
(6) Have major individual aspects (such as illness and use of
medications) that may influence the outcome of tests for immune
function been accounted for?
(7) Has inter- or intralaboratory variability been controlled for?
Was the laboratory that ran the tests for immune function able to
distinguish between samples from 'exposed' and 'unexposed'
individuals?
5.5 Proposed testing regimen
Biological research involving human subjects must be conducted in
accordance with ethical standards and involve scientific procedures
designed to ensure the safety of the subjects (Council for
International Organizations of Medical Sciences, 1993). Below are
shown a testing scheme proposed by WHO for preliminary evaluation of
individuals exposed to immunotoxicants, an approach developed by a
working group organized by the United States Centers for Disease
Control and Agency for Toxic Substances and Disease Registry, and that
proposed by a panel of the United States National Academy of Science
(US National Research Council, 1992). The three approaches have many
similarities.
5.6 Assays for assessing immune status
A plethora of tests has been developed to assess immunity in
humans (Bentwich et al., 1982, 1988), which are described in
laboratory manuals (Lawlor & Fischer, 1988; Miller et al., 1991;
Coligan et al., 1994). Many of these tests are now commercially
available in kits. A systematic approach to the evaluation of immune
function, which is based on simple screening procedures, followed by
appropriate specialized tests of immune function, usually permits the
definition of an immune alteration. The tests should include
evaluation of the B-cell system, of the T-cell system, and of
nonspecific resistance (polymorphonuclear leukocytes, monocytes and
macrophages, NK cells, the complement system). Although some exogenous
Assays suggested by WHO for assessing immunotoxicity in all persons
exposed to immunotoxicants
1. Complete blood count with differential counts
2. Antibody-mediated immunity (one or more of following):
* Primary antibody response to protein antigen (e.g. epitope-
labelled influenza vaccine)
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
* Secondary antibody response to protein antigen (diphtheria,
tetanus, or poliomyelitis)
* Natural immunity to blood-group antigens (e.g. anti-A and
anti-B)
3. Phenotypic analysis of lymphocytes by flow cytometry
* Surface analysis of CD3, CD4, CD8, CD20
4. Cellular immunity
* Delayed-type hypersensitivity skin testing using Multitest
Biomerieux(R)
* Primary delayed-type hypersensitivity reaction to protein
(keyhole limpet haemocyanin)
* Proliferation to recall antigens
5. Autoantibodies and inflammation
* C-Reactive protein
* Autoantibody titres to nuclei, DNA, mitochondria and IgE
(rheumatoid factor)
* IgE to allergens
6. Measure of nonspecific immunity
* Numbers of natural killer cells (CD56 or CD60) or cytolytic
activity against K562
* Phagocytosis (nitroblue tetrazolium or chemiluminescence)
7. Clinical chemistry
Screening panel recommended for human studies by the United States Centers for
Disease Control and Agency for Toxic Substances and Disease Registry
* Complete blood count with differential counts
- absolute lymphocyte count
- granulocyte count
- platelet count
- absolute eosinophil count
- examination of peripheral smear
* Immunoglobulins
- IgG
- IgA (optional)
- IgM (optional)
* C-Reactive protein
* Autoantibody screening panel
- Antinuclear antibody
- Rheumatoid factor
- Anti-thyroglobulin antibody
* Peripheral blood leukocyte surface markers
- CD2/CD3
- CD4/CD8
- CD8/CD3
- CD19/CD20
* Clinical chemistry in serum
- Blood urea nitrogen
- Cholesterol
- Creatinine
- Total bilirubin
- Alkaline transaminase
- Alkaline phosphatase
- Total protein (albumin:globulin ratio)
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
Tier 1 (all persons exposed to immunotoxicants)
I. Humoral immunity
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
and immunofixation electrophoresis
* Natural immunity: Antibody levels to ubiquitous antigens
(e.g. anti-A and anti-B group substances in individuals of
non-AB blood type)
* Secondary antibody responses to proteins (e.g. diphtheria,
tetanus, poliomyelitis) and polysaccharides (e.g.
pneumococcal, meningococcal)
Note: In immunization studies, live microorganisms should not be
given to persons suspected of being severely immunocompromised.
II. Lymphocytes
* Enumeration of B and T cells in blood
* Surface analysis of CD3, CD4, CD8, CD20
* Secondary delayed-type hypersensitivity reaction (e.g.
candida, diphtheria, tetanus)
* Alternative: Multiple antigen skin test kit
III. Autoantibody titres (to red blood cells, nuclei, DNA,
mitochondria, IgE (rheumatoid factor)
Tier 2 (all persons with abnormal Tier 1 test results and
a fraction of the total exposed population to be determined
by a statistician)
I. Humoral immunity
* Primary antibody response to protein and polysaccharide
antigens
Note: A panel of antigens should be developed that can be used
in sequential studies on a given individual, since a particular
antigen can be used only once to assess a primary response.
II. Cellular immunity
* Proliferative response to mitogens (phytohaemagglutinin,
concanavalin A) and possible antigens such as tetanus;
primary delayed-type hypersensitivity reaction to keyhole
limpet haemocyanin
Note: Here, too, a panel of standard antigens is needed for
sequential testing; these could be the same as those used to
assess primary antibody responses.
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
III. Natural killer cells, monocytes, and other T- and B-cell markers
* CD5, CD11, CD16, CD19, CD23, CD64; class II MHC on T cells
by two-colour flow cytometry for co-expression of class II
and a T-cell marker such as CD3
IV. Serum levels of cytokines (e.g. IL-1, IL-2, IL-6) and of shed or
secreted cellular activation markers and receptors (e.g. CD25).
V. Class I and II MHC antigen typing
Tier 3 (to be considered for persons with abnormalities
in Tier 2 tests or for a random fraction of the entire
population in Tier 2)
* If a proportion of CD16 cells of Tier 2, III, is abnormal:
nonspecific killing of a tumour cell line to test for natural
killer function
* If primary delayed-type hypersensitivity reaction in Tier 2, II,
is abnormal:cell proliferation in response to phorbol ester and
calcium ionophore,anti-CD3 antibody, and a staphylococcal
enterotoxin B (experimental)
* Generation of secondary cell-mediated immune reactions
(proliferation and MHC-restricted cytotoxicity) in vivo, e.g.
with influenza virus (experimental)
* Immunoglobulin subclass levels in serum (IgA1, IgA2, IgG1-4)
* Antiviral titres (e.g. influenza, parainfluenza,
cytomegalovirus, human immunodeficiency virus) in serum (no
deliberate immunization)
agents can alter several elements of the human immune system, others
have a primary effect on a single element. For example, low doses of
cyclosporin A selectively affect T cells by acting on the production
of IL2 and IL2 receptors. Conversely, the anticonvulsive drug
phenytoin acts primarily on the humoral immune system, leading to a
selective deficiency of IgA.
A number of immune function assays recommended for inclusion in
a simple screening panel for assessing human immune function after
potential exposure to xenobiotics believed to affect the immune system
are described below. It should be noted that there are many indicators
of altered immune function in humans which may not be specific markers
for exposure, immune disease or susceptibility (IPCS, 1994a;
IPCS/Department of Health, 1995).
5.6.1 Total blood count and differential
A complete blood count, with differential absolute counts of
lymphocytes, granulocytes, eosinophils, and platelets, are basic
components of immunotoxicology. These tests are useful in defining the
general health status of a population, since they are relatively well
standardized over most age, sex, and race groups. Such counts are also
essential for interpreting the results of ex-vivo/in-vitro functional
tests, described below, since functional tests reflect a combination
of numbers of cell types and activity per cell.
The absolute lymphocyte count is critical: Higher absolute
counts are found in children than in adults, but lymphocyte counts
consistently below 1500/mm3 are indicative of lymphocytopenia, and a
higher count signals a defect in the T-cell system or effects on the
bone marrow. Lymphocytopenia can occur not only in primary immune
deficiency but also as a result of viral infections, malnutrition,
stress, autoimmune diseases, and haematopoietic malignancies.
Examination of bone marrow may be indicated to exclude some other
factors once lymphocytopenia has been confirmed. Additional assessment
of cell mediated immunity and direct measurement of T-cell parameters,
such as lymphocyte phenotypic markers, may also be indicated.
Review of peripheral smears for morphological abnormalities of
the white and red cells adds useful information for interpreting raw
cell counts, such as the atypical lymphocytosis of many acute viral
infections. The absolute eosinophil count can be very helpful in
delineating allergic disorders, vascular collagen diseases, and
parasitic manifestations.
5.6.2 Tests of the antibody-mediated immune system
Evaluation of antibody-mediated immunity involves measurement of
serum concentrations of immunoglobulins and assessment of antibody
formation after immunization or measurement of 'natural antibodies'.
5.6.2.1 Immunoglobulin concentration
Several methods are available for measuring the concentrations
of the five major classes of immunoglobulin, IgM, IgG, IgA, IgD, and
IgE, in serum, including single radial diffusion, double diffusion in
agar gel, immunoelectrodiffusion, radioimmunoassay, ELISA, and
automated laser nephelometry. Single radial diffusion is widely used.
Gel diffusion methods are very sensitive to differences in diffusion
constants and thus to differences in molecular size.
The serum concentration of each of the major immunoglobulins
should be determined, with the exception of IgD (which occurs
predominantly on the cell surface). The determinations must be well
standardized because antisera vary in quality. Since serum
immunoglobulin concentrations vary with age and environment,
appropriate norms must be used. Patients can manifest a deficiency in
all immunoglobulin classes (common variable hypogammaglobulinaemia),
or they may have a deficiency in only a single class (IgA deficiency
as a primary defect or after phenytoin therapy).
The concentration of immunoglobulins cannot be used as the
sole criterion for a diagnosis of immunodeficiency. Diminished
immunoglobulin concentrations can result from loss into the
gastrointestinal tract as well as from decreased synthesis. An
indication of loss can be obtained by measuring serum albumin, which
is usually lost concomitantly.
5.6.2.2 Specific antibodies
Antibody-mediated immunity can be assessed from antibody
responses to common specific antigens (basal levels). Humoral immunity
after immunization can be assessed in the same way. The response to
antigenic stimulation with both protein and polysaccharide antigens
must be defined if immunodeficiency is strongly suspected. Failure to
respond to one or more classes of antigen has been observed in
patients with normal or high levels of immunoglobulins, regardless of
whether they have an isolated immunoglobulin class or subclass
deficiency. Specifically, patients with the Wiskott-Aldrich syndrome
may have normal or even elevated immunoglobulin concentrations, yet
have multiple infections because of their failure to mount a specific
immune response, especially when they are exposed predominantly to
polysaccharide antigens.
Natural antibodies: Isohaemagglutinins are naturally occurring
antibodies to blood group A and B antigens found in all normal
individuals except those with type AB red cells. By three years of
age, 98% of normal persons with type A, B, or O blood have
isohaemagglutinin titres of at least 1:16. Patients with the Wiskott-
Aldrich syndrome may have normal immunoglobulin levels yet lack
isohaemagglutinins as an indicator of their antibody-deficient state.
Other natural antibodies that can be assayed include heterolysins
(e.g. against sheep or rabbit red blood cells) and antistreptolysin.
Antibody response to immunization: In order to test for
T cell-dependent antibody responses, commercially available
diphtheria-tetanus vaccine can be given in recommended doses. Blood is
taken two weeks after each injection and tetanus and diphtheria
antibodies are determined. In patients who have been immunized with
diphtheria-tetanus or diphtheria-pertussis-tetanus vaccine, one
booster injection is given before determination of antibodies. In
testing for T cell-independent antibody responses, commercially
available pneumococcal vaccine can be given in recommended doses.
Three doses of killed poliomyelitis vaccine (1.0 ml intramuscularly,
at intervals of two weeks) can also be used as the immunogen. Blood is
taken two weeks after the last injection, and antibody is usually
determined by virus neutralization. There is strong consensus that
quantification of a primary immune response (antibody and/or cellular)
after immunization is not only a very relevant test but also very
sensitive. Although such tests are not routine in clinical immunology,
they have been used successfully for determining immune integrity.
While keyhole limpet haemocyanin has been used as the antigen,
beneficial immunizations such as influenza vaccine linked to a marker
epitope can also be used.
5.6.3 Tests for inflammation and autoantibodies
5.6.3.1 C-Reactive protein
Inclusion of an acute-phase reactant marker is helpful for
clinical interpretation of other laboratory biomarkers such as
autoantibodies. The concentration of C-reactive protein rises and
falls to baseline values in direct proportion to tissue damage and is
thus a sensitive indicator of the presence of a generalized
inflammatory state. It offers the best example of an accepted,
standardized procedure that can be used in large population studies,
because it is less subject to transport variables than other
procedures.
5.6.3.2 Antinuclear antibody
Antinuclear antibody is a common autoantibody that may be
associated with various rheumatic disorders and, classically, systemic
lupus erythematosus. Several commercial kits are available to detect
the presence of autoantibodies. Progressively greater titres increase
the specificity for a disease. Positive sera should be titred at
dilutions of > 1:40 to 1:640.
5.6.3.3 Rheumatoid factor
Rheumatoid factor is an autoantibody to immunoglobulin (usually
IgM) that occurs in a high percentage (50-70%) of individuals with
classical rheumatoid arthritis but may also develop in a variety of
other disorders, including infections and immunological diseases.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.3.4 Thyroglobulin antibody
This antibody occurs in association with a variety of thyroid
disorders but may be found without detectable thyroid dysfunction.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.4 Tests for cellular immunity
A variety of tests are commonly used to assess cell-mediated
immunity, including enumeration of T cells and T-cell subsets,
identification of delayed skin reactions, and measurement in vitro
of stimulation of lymphocytes to proliferate and form blast cells.
Other tests are available to measure T-cell effector or regulatory
function in vitro. As for humoral immunity, a series of simple tests
is available to screen for defects in cell-mediated immunity. The
proportion of circulating T cells in a mononuclear cell preparation
can be determined by immunofluorescence with CD2 or CD3 monoclonal
antibodies. Normally, T cells constitute 55-80% of peripheral blood
lymphocytes. The normal values reported for absolute numbers of
circulating T cells are 1620-4320/mm3 during the first week up to
18 months of life and 590-3090/mm3 after 18 months of age (Fleisher
et al., 1975).
5.6.4.1 Flow cytometry
Antibodies that may be used in immunological phenotyping are
listed in Table 10 in section 4.1.2. Subsets of T cells have been
defined with monoclonal antibodies specific to cell-surface antigens.
The association of a particular T-cell subset with a given function
has caused some confusion in the analysis of immunological data for
patients with immunodeficiency states, as discussed in more detail in
section 1.2.2.1. For example, CD-positive cells have commonly been
associated with helper functions, and CD8 cells have been associated
with cytotoxic functions. This approach is an oversimplification,
which became evident with the finding that CD and CD8 cells recognize
foreign antigens in the context of MHC class II and class I antigens,
respectively. Thus, the CD population contains helper cells, memory
cells, and cytotoxic cells for targets bearing MHC class II molecules.
The CD8 population contains cytotoxic cells and also cells that can
recognize antigens presented by macrophages and cells that can augment
the interaction of CD-positive cells with B cells. Abnormalities in
the number of CD or CD8 cells, or their ratio, can be associated with
abnormalities in the ability to recognize antigens and regulate T-cell
function that can lead to immune incompetence or autoimmunity.
5.6.4.2 Delayed-type hypersensitivity
The ability of patients to manifest pre-existing T-cell immunity
has been evaluated in vivo with a series of skin antigens that
normally produce a delayed-type cutaneous hypersensitivity response.
Because delayed cutaneous hypersensitivity, a localized immunological
skin response, depends on functional thymus-derived lymphocytes, it is
used in detecting T cell-mediated immunodeficiency. A device
(Multitest(R), Institut Merieux, Lyon, France) is available that
enables the simultaneous intradermal injection of seven standardized
antigens and so overcomes the inconvenience of giving seven separate
injections of antigens and the technical difficulty of ensuring their
intradermal rather than subcutaneous injection. The Multitest(R),
consisting of eight tined, preloaded heads, delivers a glycerol
control and the seven test antigens dissolved in glycerol. The size of
the induration produced by each antigen should be measured at 48h in
two diameters: reactions of less than 2 mm are scored as negative. No
reaction should be seen with the glycerol control. The test includes
as antigens: tetanus, diphtheria, streptococcus, tuberculin, Candida,
Trichophyton, and Proteus.
Recall of delayed-type hypersensitivity as a test for cell-
mediated immune competence was assessed with the Multitest(R) device
in 254 subjects. When 77 subjects were tested concurrently with the
Multitest(R) and a conventional panel of six antigens (Frazer et
al., 1985), similar results ( r = 0.65) were obtained with the two
systems. The reproducibility of the Multitest(R) among three
observers, who assessed the aggregate size of reactions in 45 subjects
independently, was high ( r = 0.89); the correlation for the reaction
score in 24 subjects tested twice, three months apart, was also high
( r = 0.88), demonstrating the suitability of the test for serial
studies of immune function.
5.6.4.3 Proliferation of mononuclear cells in vitro
A common test of lymphocyte function is measurement of the
capacity of lymphocyte subsets to enlarge and convert into blast-like
cells that synthesize DNA and incorporate thymidine after stimulation
in vitro. In this test, lymphocytes can be activated by antigens
(e.g. purified protein derivative, Candida, streptokinase, tetanus,
or diphtheria); allogeneic cells are also used in the one-way mixed
lymphocyte culture to stimulate T-cell proliferation. T-Cell blast
transformation can be assessed directly by measuring blastogenesis and
proliferation of cells, expression of activation antigens (e.g. CD69
or CD25 and HLA DR), and release of mediators. The blastogenic
response is assessed as incorporation of 3H-thymidine, usually for
16-24h, followed by cell precipitation on filter paper and liquid
scintillation counting. Non-isotope assay alternatives are also
available. The responses to various antigenic stimuli by different
types of responding cells must be interpreted with caution. The mixed
leukocyte reaction is the result of T-cell reactivity to MHC-encoded
peptides displayed on the surface of B cells and monocytes. The
T cells in the population of normal irradiated or mitomycin C-treated
lymphocytes used as the stimulators can secrete factors that induce
blastogenesis in the patient's lymphocytes. As this can be misleading,
it is preferable to use B-cell lines or T cell-depleted normal cells
as the stimulators.
5.6.5 Tests for nonspecific immunity
5.6.5.1 Natural killer cells
The differences between NK cell function, phenotyping for NK
cells, and the cytology of large granular lymphocytes are described in
section 1.2.2.1. The identification of NK cells remains problematic
owing to this apparent heterogeneity. The cells can be evaluated in
ex-vivo/in-vivo tests with enriched peripheral blood mononuclear
cells. The proportion of NK cells can be identified with appropriate
monoclonal antibodies (see Table 10). Functional assays of NK activity
involve the ability of the appropriate mononuclear cells to kill
specific NK targets, such as the K562 cell, in which cell-mediated
cytolysis in vitro is quantified by release of 51Cr from the
target cells.
5.6.5.2 Polymorphonuclear granulocytes
Some defects of phagocytic function affect polymorphonuclear
phagocytes. Neutrophil function depends on movement in response to
chemotactic stimulus, adherence, endocytosis, and destruction of the
ingested particles. Phagocyte mobility depends on the integrity of the
cytoskeleton and contractile system. Defects in intracellular killing
of ingested microorganisms usually result from failure of the
'respiratory burst' that is critical to production of superoxide
radicals and hydrogen peroxide. The organisms cultured from lesions of
patients with this type of defect generally contain catalase and
include staphylococci, E. coli, Serratia marcescens, fungi, and
Nocardia. Patients with defects in mobility, adherence, and
endocytosis usually have infections of the skin, periodontitis, and
intestinal or perianal fistulae; patients with normal endocytosis and
defective killing tend to have chronic granulomas. Measurement of
nitroblue tetrazolium dye reduction or chemiluminescence by actively
phagocytosing leukocytes has been accepted as a standard measure for
the adequacy of the respiratory burst. Recently, methods have been
developed to measure the production of reactive oxygen intermediates
by flow cytometry (Emmendorfer et al., 1994). Kits are commercially
available for assessing phagocytic capacities and the production of
reactive oxygen intermediates by phagocytes. Assays for bacterial
killing yield highly variable results, depending on the bacterial
species used in the assay, and are not recommended for routine use.
The activation state of neutrophilic granulocytes can be assessed by
flow cytometry with the antibodies CD11b, CD14, CD16, CD54, and CD64
(Spiekermann et al., 1994). Activation of platelets can also be
assessed by flow cytometry (Tschöpe et al., 1990)
5.6.5.3 Complement
The classic complement system consists of nine components (C1-9)
and a series of regulatory proteins (C1 inhibitor, C4 binding protein,
and properdin factors). Many biological activities important in the
inflammatory response and in host resistance to infection occur at
various points in the classical and alternative pathways of complement
activation. Three clinical states should raise a suspicion of
deficiency in a complement component: systemic lupus erythematosus,
recurrent infections of the type seen in hypogammaglobulinaemia in
patients with normal immunoglobulin levels, and severe Neisseria
infection. Laboratory measurement of serum haemolytic complement
(CH50) is a useful test. Serum haemolytic complement is usually absent
and rarely above 10% of the normal value in inherited complement
deficiencies, with the exception of hypercatabolism of C3. More
detailed analysis of the complement system requires functional and
antigenic measurements of the individual components, usually best
performed in laboratories that specialize in the complement system.
5.6.6 Clinical chemistry
Assessment of clinical abnormalities in standard serum
chemistry, such as liver function, renal function, glucose, and
albumin, is indicated to facilitate reasonable interpretation of
specific changes in the immune system as secondary to another
condition.
5.6.7 Additional confirmatory tests
After activation, mononuclear cells from peripheral blood
express the genes that encode a series of interleukins and colony-
stimulating factors. Activated T cells and monocytes synthesize and
secrete IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, interferon, other
cytokines such as tumour necrosis factor, and cytokine receptors.
These cytokines are involved in the growth and differentiation of
T and B cells, eosinophils, and basophils. The supernatants of
mononuclear cells from peripheral blood stimulated by soluble
phytohaemagglutinin can be assessed for IL-2 by determining their
capacity to stimulate 3H-thymidine uptake by mouse IL-2-dependent,
cultured T-cell lines. Other biological assays, radioimmunoassays, and
ELISAs have been developed to quantify the production of lymphokines
and colony-stimulating factors. With molecular cloning techniques,
messenger RNA transcription by each of the lymphokines can be
quantified after appropriate lymphocyte activation. In the assessment
of lymphokines, T cells or peripheral blood mononuclear cells are
usually activated with concanavalin A, pokeweed mitogen, or
insolubilized CD3 antibodies; then, the appropriate assays are
performed to quantify the specific lymphokine(s) produced in the
culture media. Different patterns of lymphokine secretion have been
observed with different subsets of T cells: INF gamma and IL-2 are
produced by the Th1 subset, while IL-4, IL-5, and IL-10 are produced
by the Th2 subset, as originally documented for cloned CD T-cell lines
from mice (Mosmann & Coffman, 1989). Thus, an assay of the pattern of
lymphokine production could be used in pinpointing the action of an
immunotoxicant on a particular subset of immune cells.
6. RISK ASSESSMENT
6.1 Introduction
Publications on immunotoxicology published by IPCS and the
European Union (Berlin et al., 1987; Dayan et al., 1990), the United
States Office of Technology Assessment (1991), and the United States
National Research Council (1992) demonstrate the growing interest and
concern within scientific and public communities on the capacity of
environmental agents to perturb normal immune processes. The
incorporation of experimental data on toxicant-induced alterations in
the immune system into evaluations of drugs, chemicals, and biological
agents for human risk assessment has thus become increasingly common.
For example, in the United States, the Environmental Protection Agency
(Sjoblad, 1988) and Food and Drug Administration (Hoyle & Cooper,
1990; Hinton, 1992) indicate the benefits of testing the
immunosuppressive potential of biochemical pest control agents,
antiviral drugs, and food additives. Furthermore, the Environmental
Protection Agency has established reference doses (an estimate of the
daily exposure of the human population that is likely to have no
appreciable risk of deleterious effects during a lifetime), on the
basis of data on the immunotoxicity of several compounds, including
1,1,2-trichloroethane, 2,4-dichlorophenol, and dibutyltin oxide. The
United States Agency for Toxic Substances and Disease Registry has
derived 'minimal risk levels' for arsenic, dieldrin, nickel,
1,2-dichloroethane, and 2,4-dichlorophenol on the basis of immune
end-points.
Risk assessment is a process whereby relevant biological, dose-
response, and exposure data for a particular agent are analysed in an
attempt to establish qualitative and quantitative estimates of adverse
outcomes (Scala, 1991). Such data are sometimes used in the
development of standards for regulating the manufacture, use, and
release of chemicals into the environment (Kimmel, 1990). As defined
by the United States National Academy of Science (US National Research
Council, 1983), risk assessment comprises four steps: hazard
identification, dose-response assessment, exposure assessment, and
risk characterization. The process of assessing the risk of both
cancer and non-cancer end-points, including immunotoxicity, may be
adapted to this form.
The first step, hazard identification, involves a qualitative
evaluation of available human and animal data to determine whether a
chemical agent poses a potential hazard. Consideration is given to
dose, route, and duration of exposure. The precise quantitative
relationships between changes in immune function or in the
histological appearance of lymphoid organs and host resistance to
infectious agents or neoplastic diseases are unclear. It can be
assumed that any significant difference from appropriate controls in
the ability of the immune system to respond to a challenge may evolve
into an adverse effect and thus present a potential hazard. This
applies to adaptive as well as nonadaptive responses.
After hazard identification, 'dose-response' is assessed. For
non-cancer toxicity, a no-observed-adverse-effect-level (NOAEL) is
established for an adverse response of interest. This process is no
different in immunotoxicology and is the same as for the other target
organ systems. The NOAEL value is either obtained from the dose-
response curve or is estimated from the lowest-observed-adverse-
effect-level (LOAEL). Once the NOAEL has been determined, safety and
uncertainty factors can be applied to allow for various uncertainties,
such as species or interspecies variability, irreversible effects, and
chronic exposure. The use of safety factors, however, should be
flexible and should allow incorporation of any relevant information on
the mechanism of action of the chemical under review. Ideally,
however, dose-response relationships should be established from human
epidemiological data that include the exposure levels expected on the
basis of human contact with the agent in the environment. As
illustrated in an assessment of developmental toxicity (Kimmel &
Gaylor, 1988), use of the risk assessment and management paradigm of
the United States National Academy of Sciences (US National Research
Council, 1983) to non-cancer end-points such as immunotoxicity, offers
some serious challenges. For example, since the presence or absence of
an effect is based upon whether a statistically and/or biologically
significant response is observed at a certain dose or doses, and since
multiple assays are routinely conducted, the NOAEL will depend heavily
on the sensitivity of the assay. The differences may be particularly
exaggerated when continuous responses (i.e. the results of most immune
function tests) are compared with categorical data, the latter being
routinely expressed as proportions. For example, in experimental
animals, changes in many immune functions may be statistically
significant when they vary by as little as 15-25% from the control
values. In contrast, host resistance, when expressed categorically
(e.g. tumour frequency), must change by 80% to reach a comparable
degree of significance, assuming group sizes of about 15 animals and
effective doses of 20% in the control group. Furthermore, immune tests
are often, but not always, interdependent (Luster et al., 1992), and
individual or combinations of tests might have to be ranked in order
of sensitivity and degree of interdependence before dose-response is
assessed. This has not been done in the past.
Exposure assessment (step 3) is done in parallel with hazard
identification and dose-response assessment (Scala, 1991). It often
involves field measurements and other estimates of human exposure,
such as the composition and size of the population, biological or
clinical effects and types, and the magnitude, frequency, and duration
of exposure to the agent. Many of these parameters are difficult to
determine accurately in a longitudinal study; even measurements of
body burden can be misleading since the concentrations at the target
organ (e.g. lymphoid tissue) are not determined. The problems in
immune testing in humans are similar to those in testing other organs
and systems and include differences in individual responses due to
unique sensitivities (e.g. age, pregnancy, genetics) and confounding
factors (e.g. smoking, stress, drugs).
Risk characterization (step 4) is the aggregation of the three
previous processes. It provides an estimate of the incidence of
adverse effects in a population and the potential health problems. As
part of risk characterization, the strengths and weaknesses of each
component of the assessment are considered, with assumptions,
scientific judgements, and, to the extent possible, estimated
uncertainties. Most assessments of the risks presented by chemical
agents have focused on the estimated incidence of cancer after
lifetime exposure to a chemical at some unit dose, assuming that there
is essentially no threshold for carcinogenicity. The assessment of
non-cancer end-points, such as disorders of the neurological,
developmental, and reproductive systems, is somewhat similar to that
of cancer, in that it involves calculations that include both
assumptions and uncertainties. For example, considerations in risk
assessment include ranking the value of epidemiological against
experimental data, extrapolations from high to low doses, from
subchronic to chronic exposure, and from animals to humans, and
appropriate use of mechanistic and pharmacokinetic data. Data from
immunotoxicology, like those from developmental toxicology (Schwetz &
Tyl, 1987), do not easily lend themselves to the mathematical models
used in cancer risk assessment, which usually involve non-threshold
models for genotoxic carcinogens. For more accurate assessment,
mechanistic models will be required which include the concept of
'threshold'. It is assumed that threshold levels exist below which no
adverse immunological effect can be demonstrated. Complex mixtures of
chemicals, in which each chemical occurs at a subthreshold
concentration, may reach or exceed a threshold for immunotoxicity
(Germolec et al., 1989), although problems associated with mixtures of
compounds are not unique to the field of immunotoxicology.
The approaches currently used by the US Environmental Protection
Agency (1986) in extrapolating the risk for developmental toxicity
have been outlined, and similar guidelines have been developed by IPCS
(1994b). One method is to apply uncertainty factors to the NOEL or
NOAEL for the most sensitive animal species tested. The uncertainty
factor is usually composed of a 10-fold factor to account for
interspecies differences and a 10-fold factor for intraspecies
variability. If no NOEL is available, an additional 10-fold factor may
be applied to the lowest-observed-effect level (LOEL). Another
approach is to calculate a margin of safety, which is the NOEL divided
by the estimated level of human exposure from all potential sources.
The margin of safety can then be evaluated for adequacy to protect
human health. There are several drawbacks to both of these approaches,
the primary one being that they use only one point on the dose-
response curve (NOEL or LOEL) and ignore the rest of the data. Also,
since the variability around the NOEL and LOEL is usually not taken
into account, these approaches may rely on poor studies, i.e. studies
that result in a higher NOEL because of their limited ability to
detect small changes over the background.
Since the purpose of risk assessment is to make inferences about
potential risk to human health, the most appropriate data are those
derived from studies of humans; however, adequate data are seldom
available, and most risk assessments are based on results obtained in
experimental animals. In order to use these results, a number of
assumptions must be made about their relevance to potential human
health risk. Firstly, it is assumed that experimental animals respond
to the agent of interest in a pharmacological and toxicological manner
similar to that anticipated in humans (i.e. the test animals and
humans metabolize the chemical similarly and have identical responses
and toxicity at the target organ). Secondly, the immune system of the
experimental species must be very similar to that of humans: the
vertebrate immune system is highly conserved among higher vertebrate
species, and the immune components and their interactions in mice,
rats, and humans are closely similar. Thus, if toxicokinetic
properties are similar, it is reasonable to test for potential adverse
effects in humans using laboratory rodents.
As immunosuppressive agents cannot ethically be administered to
humans, quantitative comparisons of dose-responses in humans and
experimental animals are limited (although it is possible to do so in
hypersensitivity tests). Nonetheless, controlled human exposures have
been studied and the results compared with the immune effects observed
in animals. As an example, the immune effects of cyclosporin A in
various species are compiled in Table 12, which shows that the mouse
is much less sensitive to cyclosporin A than other species, in
particular the rat, and that humans are slightly more sensitive than
other species (Dean & Thurmond, 1987); however, for the most part,
there was good qualitative and quantitative agreement between the
species examined. Selgrade et al. (1995) compared phagocytosis by
human and murine alveolar macrophages after exposure to ozone
in vitro and in vivo (Table 13): The effects of ozone on alveolar
macrophage function in murine species are predictive of effects that
occur in humans, and the effects on macrophage phagocytosis seen
in vitro are predictive of those that occur in vivo. Quantitative
comparisons have also been made in mice and humans for the ability of
UVR to inhibit the contact hypersensitivity response (Table 14). As
described in section 2.2.11, exposure to UVB inhibits delayed-type
hypersensitivity (Kripke et al., 1979). Noonan & Hoffman (1994)
described three strains of mice with low, intermediate, and high
susceptibility to UVR-induced immunosuppression, and Oberhelman et al.
(1992) reported that suppression of the hypersensitivity response also
occurs in humans and that the dose of radiation required to induce 50%
suppression in fair-and dark-skinned individuals is similar to that
required to inhibit the response in mice with high and intermediate
susceptibility, respectively.
Table 12. Comparison of doses of cyclosporin A that suppress
the immune response in various species
Species Response Cyclosporin A
(mg/kg body
weight)
Mouse Antibody production 50-300
Cell-mediated immunity 100-300
(delayed-type hypersensitivity)
Graft-versus-host reaction 50-250
Rat Antibody production 20-50
Graft-versus-host reaction 10-60
Guinea-pig Cell-mediated immunity 10-100
(delayed-type hypersensitivity)
Dog Cell-mediated immunity 15-30
(delayed-type hypersensitivity)
Rhesus monkey Antibody production 50-250
Human Cell-mediated immunity 10-20
Adapted from Dean & Thurmond (1987); White et al. (1994)
Data from immunotoxicology also differ from those for
carcinogenicity and possibly other non-cancer end-points because the
immune system contains components with overlapping functions. For
example, when an individual is exposed to an infectious agent,
multiple immune components may work either independently or in concert
to help defend the host; i.e. there is redundancy between immune
functions. Furthermore, while a significant change in any immune
function can be considered potentially deleterious, in that it may
increase the risk of developing clinical disease, a change in immune
function does not necessarily precipitate a disease or clinical health
affect. That is, immunocompromised individuals function normally in
the absence of infectious agents. Thus, immune function reserve and
redundancy are relative terms, depending on the dose of infectious
agent. This complicates dose-response assessment, and models should be
developed that incorporate available information on the quantitative
relationship between immune function and clinical disease and
potential redundancy.
Table 13. Effect of exposure to ozone on phagocytosis by alveolar
macrophages
Treatment Phagocytic index (no. fluorescent
particles ingested/100 macrophages)
--------------------------------------
Mice Humans
---------------- -------------
Mean SE Mean SE
Air in vitro 369.2 26.4 386.7 50.5
(n = 6) (n = 6)
Ozone in vitro 291.7 17.4* 275.0 45.1*
(n = 6) (n = 6)
Suppression 21% 29%
Air in vivo 330.6 10.4 714.9 46.1
(n = 4) (n = 10)
Ozone in vivoa 194.0 19.7* 539.2 22.3*
(n = 4) (n = 10)
Suppression 42% 25%
Suppression corrected 28% 25%
for dosimetric differenceb
Adapted from Selgrade et al. (1995)
* Significantly different from air control (P < 0.05; Student's
t test)
a Mice were exposed to 0.8 ppm for 3 h; humans were exposed to
0.08 ppm for 6.6 h while undergoing intermittent exercise.
b On the basis of studies using 18O, alveolar macrophages of
mice exposed to 0.8 ppm ozone for 3 h receive roughly 1.5 times
more ozone than those of humans exposed to 0.08 ppm ozone for 6.6 h
while exercising moderately.
Table 14. Comparison of doses of ultraviolet radiation that cause
50% suppression of contact sensitivity in mice and humans
Mousea Humanb
--------------------------------------------- --------------
Sensitivity kJ/m2 mJ/cm2 Skin type
mJ/cm2
(phenotype)
High (C57Bl) 0.7-2.3 70-230 Fair 100
Intermediate (C3H) 4.7-6.9 470-690 Dark 225
Low (BALB/c) 9.6-12.3 960-1230
Adapted from Selgrade et al. (1995)
a Data from Noonan & Hoffman (1994)
b Data from Oberhelman et al. (1992)
The increasing evidence that environmental contaminants affect
wildlife populations has led to risk assessment at the level of the
ecosystem; however, the limited evidence for immunotoxic effects in
wildlife precludes an adequate understanding of the risks posed by
current levels of environmental pollution. The demonstration of
immunosuppression in harbour seals fed herring from the contaminated
Baltic Sea in a semi-field experiment (De Swart et al., 1994; Ross et
al., 1995) provided a first indication that ambient levels of
contaminants in certain areas present an immunotoxic risk to mammalian
wildlife occupying a high trophic level. These results may partially
explain the severity of a series of unrelated epizootic viral episodes
in various marine mammalian populations in coastal areas of Europe and
North America (Dietz et al., 1989; Van Bressem et al., 1991). In
similar semi-field experiments in a bottom-dwelling fish species,
flounder exposed to contaminated sediments had more viral lymphocytic
infection and liver tumours than controls (Vethaak & Wester, 1993).
While the difficulties in conducting adequate immunological studies
with wildlife may preclude an approach as comprehensive as that in
humans, such semi-field strategies may provide the best available
direction.
Should the application of field studies be possible, correlative
approaches to immunotoxicology may substantiate an effect on the
ecosystem. Such an approach was used to establish a correlation
between the induction of mixed-function oxidases and pollutant burden
(as measured by the toxic equivalence of organochlorine chemicals) in
cormorant (Phalocrocorax carbo) chicks collected from various sites
in the Netherlands (van den Berg et al., 1994). A combination of
laboratory experiments under controlled conditions, semi-field
experiments under controlled conditions with exposure to environmental
mixtures of pollutants, and correlative field studies is necessary to
understand immunotoxicity in wildlife populations.
6.2 Complements to extrapolating experimental data
6.2.1 In-vitro approaches
The complexities of the immune system and the requirement of
many agents for metabolism and distribution in order to produce an
immunotoxic response have resulted in the almost exclusive use of
animal models in vivo for assessing immunotoxicity. Culture systems
have been used extensively, however, to study the mechanisms by which
agents induce immunosuppression. In-vitro test systems with immune
cells of human origin are particularly attractive, given the
uncertainties in extrapolating the results of studies in experimental
animals to humans and the accessibility of human peripheral blood
cells. Although many of the immune cells obtained from human blood are
immature forms, the large numbers and diverse populations (i.e.
polymorphonuclear granulocytes, monocytes, NK cells, T cells, and
B cells) that can be obtained and the ease of conducting challenge
assays in vitro provide an attractive alternative (or, preferably,
adjunct) to more conventional studies in animals. Surprisingly few
laboratories have conducted studies with immunosuppressive agents in
which immune function responses in human immune cells are compared
with thosein rodents in vivo (Cornacoff et al., 1988; Luo et al.,
1992; Wood et al., 1992; Lang et al., 1993). Althoughstudies in human
cells in vitro have been hampered by a lack of assays to assess
primary antigen-specific immune responses, a relatively good
interspecies correlation has been observed in the limited responses
examined. Furthermore, some of these assays have been successfully
modified to include metabolic fractions of liver homogenates (Shand,
1975) or co-culture with primary hepatocytes (Kim et al., 1987) to
allow for chemical metabolism.
6.2.2 Parallelograms
Interpretation of studies in experimental animals or in vitro
can be improved when even limited data on human exposure in vivo are
available, using a 'parallelogram' approach. In general, if a
parallelogram can be constructed in which data are available for five
of the six angles (Figure 37), it may be easier to predict the outcome
at the remaining angle, at least qualitatively. For example, cytokine
and phagocytic responses of alveolar macrophages or pulmonary
epithelial cells after exposure to ozone in vitro can be compared
with the responses to ozone after exposure of humans and animals
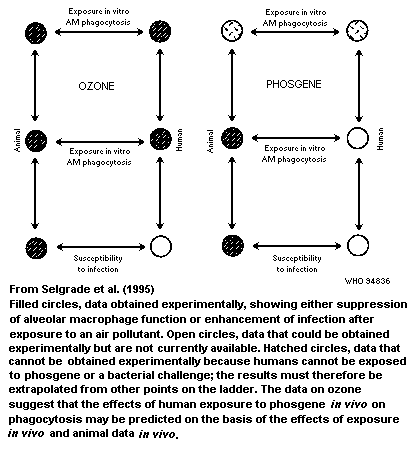 in vivo. If the in-vitro data prove to be predictive indicators of
the in-vivo effects in humans, more weight can be given to in-vitro
studies with similar agents or with compounds that are too toxic to be
assessed in clinical studies but for which data are available on both
animals in vivo and animals and humans in vitro. A similar
approach can be used to establish the relationship between acute and
subchronic effects as a means of extrapolating from acute effects in
humans to chronic effects, for which few data are usually available.
Another situation in which this approach may be applicable is in
extrapolating deficits in immune function to increased susceptibility
to disease in animal models, as a means of interpreting the risk of
disease in humans, for whom data on immune function but not infectious
disease may be available.
6.2.3 Severe combined immunodeficient mice
Another approach, which warrants further exploration, is the use
of SCID mice grafted with human immune cells. This model is described
in section 4.5.3. In short, SCID mice have been successfully
grafted with human fetal lymphoid tissue in order to study human
haematopoiesis (McCune et al., 1988) or with human peripheral blood
lymphocytes, which allow production of human immunoglobulins, to study
secondary antibody responses (Mosier, 1990). Reconstituted mice have
been used to study autoimmunity and the efficacy of antiviral
therapeutic agents. There are still limitations to the use of these
animals for immunotoxicology (Pollock et al., 1994).
6.3 Host resistance and clinical disease
A major limitation in assessing the risk of immunotoxicity is
the difficulty in establishing quantitative relationships between
immunosuppression and clinical diseases. The diseases are usually
manifested as increases in the frequency, duration, or severity of
infections, increased incidences of certain cancers, such as Kaposi's
sarcoma and non-Hodgkin's lymphoma (malignancies often observed in
immunosuppressed individuals), or an increased incidence of autoimmune
diseases.
Despite overwhelming experimental and clinical evidence that
increases in the incidences of neoplastic and/or infectious diseases
occur in animals and individuals with secondary immunodeficiency
(Austin et al., 1989; Ehrke et al., 1989), neither the most
appropriate immune end-points for predicting clinical disease nor the
quantitative relationship between changes in immune function and
impairment of host defence are clearly defined. For example, it would
be useful to determine whether certain immune end-points (or quantity
of changes) predict certain outcomes (e.g. increased susceptibility to
influenza and decreased antibody responses). A better understanding of
these relationships would be particularly beneficial for risk
assessment, since changes in immune function are more readily
quantifiable in populations at risk than are changes in the frequency
or severity of infections. A particularly relevant question for risk
estimation is whether increases in host susceptibility to challenge
agents follow linear or 'threshold-like' models as a function of
increased immunosuppression. While terms such as 'immune reserve' and
'immunological redundancy' are applicable for individual responses, it
is unclear how they would be applied to large populations. Since the
potential outcomes of immunosuppression are increases in infections or
neoplastic diseases and there is already a background incidence of
these diseases in the population (Centers for Disease Control, 1991),
it would be helpful to determine the additional frequency of disease
that is associated with increased loss of immune function. Qualitative
relationships are well established, but the quantitative relationship
between immune function and clinical disease in humans has proved
difficult to explore, owing, in part, to the complexity of the immune
system, overlapping (i.e. redundant) immune responses, and variability
in the virulence of infectious agents. Nonetheless, several studies
have shown quantitative relationships in humans. For example, in
longitudinal studies of a relatively large population, asymptomatic
individuals with low NK cell activity were found to be at risk for
developing upper respiratory infections (Levy et al., 1991). Larger
population studies have been conducted in AIDS patients, as the
depletion of CD4+ cells following HIV-1 infection is a clinical
hallmark of the disease. The normal human range of CD4+ cells is
800-1200 cells/µl, but this level generally declines to less than 500
cells/µl within three to four years after HIV-1 infection and to 200
cells/µl before overt opportunistic infections are seen (Masur et al.,
1989; Phair et al., 1990). It has also been shown in seropositive
individuals that a drop in CD4+ cells by 7% or more in a year
increases the relative risk of developing AIDS (Burcham et al., 1991).
Because of the uncertainties about the quantitative relationship
between immune function and disease, there has been continuing
interest in developing sensitive, reproducible experimental models of
host resistance to define altered immune function after exposure to
environmental agents. Most of these models were developed in mice and,
to a lesser extent, in rats; they include bacterial, viral, protozoan,
fungal, and syngeneic or semisyngeneic transplantable tumour cell
models. Although the target organs and general host defence activities
have been defined for most of these models, multiple immune and
nonimmune, mechanisms are involved in resistance, making it difficult
to determine the exact defect without assessing immune function
responses to the challenge agent. For example, defence against
extracellular organisms involves the interactions of T lymphocytes,
B lymphocytes, macrophages, and polymorphonuclear granulocytes, in
addition to a variety of cell-secreted products, whereas resistance to
generalized infection from intracellular pathogens and neoplastic
diseases is likely to involve macrophages, NK cells, and T cell-
mediated immune processes.
Although many host resistance assays are relatively simple to
perform, they normally require large numbers of animals and appear to
be less sensitive than immune function tests (Luster et al., 1993).
Other studies have shown that host resistance assays are more
sensitive than immune function tests (Vos et al., 1991; Burleson et
al., in press). The dose of challenge agent used in experimental
studies is important, since too low or too high a dose will not allow
detection of changes in immunocompromised groups in comparison with
controls (Selgrade et al., 1982; Luster et al., 1993). The sensitivity
of a host resistance assay also depends on the end-point measured. For
example, tests involving survival or tumour models (i.e. dichotomous)
are by nature less sensitive than those with end-points that provide
continuous data, such as enumeration of tumours, bacteria, or soluble
immune activation markers, and several end-points in one model of
infection, such as T. spiralis. This is attributable partly to
differences in the types of statistical analyses used to establish
group differences. With dichotomous data, two approaches can be used.
Some laboratories use a challenge dose that produces a response in
10-30% of animals in the control group. An alternative is to use a
dose slightly below that which would induce the desired response in
any of the animals in the control group. The latter design increases
the statistical power of data analysis. In most cases, extreme
accuracy is needed in the delivery of the agent, to ensure that the
administered dose of agent is only slightly smaller than that which
will give the desired response. In either approach, statistical
significance is heavily dependent on the dose of challenge agent and
the number of animals in each treatment group: Table 15 shows that
doubling the number of animals in a study greatly increases the
ability to detect a significant change. Even more obvious is the
increased ability to detect significant differences when the dose of
the agent in the control group is lowered to a subclinical
concentration. These hypothetical values demonstrate how statistically
significant changes can be obtained in susceptibility assays by
modifying the experimental design. In the first design, an effective
dose of 30% is used in the control group (i.e. a concentration of
agent that produces a response in 30% of normal animals) with 15
animals per treatment group. In the second design, increasing the
group size to 30 allows for even greater statistical significance. In
the third design, a challenge inoculation is given which produces no
effect in the control group (effective dose, 0), allowing for greater
statistical significance.
Two variables that influence the quantitative relationship
between immune function and disease are the virulence and amount of
the infectious agent. These remain a constant in most experimental
studies but may vary between experiments as well as in the human
population. For example, in the general population one can assume that
an infectious disease such as influenza can develop in any individual,
independently of their immune capacity or prior immunization, provided
that the virulence or quantity of the challenging agent is sufficient
to overwhelm the individual's defensive capacities. In Figure 38,
in vivo. If the in-vitro data prove to be predictive indicators of
the in-vivo effects in humans, more weight can be given to in-vitro
studies with similar agents or with compounds that are too toxic to be
assessed in clinical studies but for which data are available on both
animals in vivo and animals and humans in vitro. A similar
approach can be used to establish the relationship between acute and
subchronic effects as a means of extrapolating from acute effects in
humans to chronic effects, for which few data are usually available.
Another situation in which this approach may be applicable is in
extrapolating deficits in immune function to increased susceptibility
to disease in animal models, as a means of interpreting the risk of
disease in humans, for whom data on immune function but not infectious
disease may be available.
6.2.3 Severe combined immunodeficient mice
Another approach, which warrants further exploration, is the use
of SCID mice grafted with human immune cells. This model is described
in section 4.5.3. In short, SCID mice have been successfully
grafted with human fetal lymphoid tissue in order to study human
haematopoiesis (McCune et al., 1988) or with human peripheral blood
lymphocytes, which allow production of human immunoglobulins, to study
secondary antibody responses (Mosier, 1990). Reconstituted mice have
been used to study autoimmunity and the efficacy of antiviral
therapeutic agents. There are still limitations to the use of these
animals for immunotoxicology (Pollock et al., 1994).
6.3 Host resistance and clinical disease
A major limitation in assessing the risk of immunotoxicity is
the difficulty in establishing quantitative relationships between
immunosuppression and clinical diseases. The diseases are usually
manifested as increases in the frequency, duration, or severity of
infections, increased incidences of certain cancers, such as Kaposi's
sarcoma and non-Hodgkin's lymphoma (malignancies often observed in
immunosuppressed individuals), or an increased incidence of autoimmune
diseases.
Despite overwhelming experimental and clinical evidence that
increases in the incidences of neoplastic and/or infectious diseases
occur in animals and individuals with secondary immunodeficiency
(Austin et al., 1989; Ehrke et al., 1989), neither the most
appropriate immune end-points for predicting clinical disease nor the
quantitative relationship between changes in immune function and
impairment of host defence are clearly defined. For example, it would
be useful to determine whether certain immune end-points (or quantity
of changes) predict certain outcomes (e.g. increased susceptibility to
influenza and decreased antibody responses). A better understanding of
these relationships would be particularly beneficial for risk
assessment, since changes in immune function are more readily
quantifiable in populations at risk than are changes in the frequency
or severity of infections. A particularly relevant question for risk
estimation is whether increases in host susceptibility to challenge
agents follow linear or 'threshold-like' models as a function of
increased immunosuppression. While terms such as 'immune reserve' and
'immunological redundancy' are applicable for individual responses, it
is unclear how they would be applied to large populations. Since the
potential outcomes of immunosuppression are increases in infections or
neoplastic diseases and there is already a background incidence of
these diseases in the population (Centers for Disease Control, 1991),
it would be helpful to determine the additional frequency of disease
that is associated with increased loss of immune function. Qualitative
relationships are well established, but the quantitative relationship
between immune function and clinical disease in humans has proved
difficult to explore, owing, in part, to the complexity of the immune
system, overlapping (i.e. redundant) immune responses, and variability
in the virulence of infectious agents. Nonetheless, several studies
have shown quantitative relationships in humans. For example, in
longitudinal studies of a relatively large population, asymptomatic
individuals with low NK cell activity were found to be at risk for
developing upper respiratory infections (Levy et al., 1991). Larger
population studies have been conducted in AIDS patients, as the
depletion of CD4+ cells following HIV-1 infection is a clinical
hallmark of the disease. The normal human range of CD4+ cells is
800-1200 cells/µl, but this level generally declines to less than 500
cells/µl within three to four years after HIV-1 infection and to 200
cells/µl before overt opportunistic infections are seen (Masur et al.,
1989; Phair et al., 1990). It has also been shown in seropositive
individuals that a drop in CD4+ cells by 7% or more in a year
increases the relative risk of developing AIDS (Burcham et al., 1991).
Because of the uncertainties about the quantitative relationship
between immune function and disease, there has been continuing
interest in developing sensitive, reproducible experimental models of
host resistance to define altered immune function after exposure to
environmental agents. Most of these models were developed in mice and,
to a lesser extent, in rats; they include bacterial, viral, protozoan,
fungal, and syngeneic or semisyngeneic transplantable tumour cell
models. Although the target organs and general host defence activities
have been defined for most of these models, multiple immune and
nonimmune, mechanisms are involved in resistance, making it difficult
to determine the exact defect without assessing immune function
responses to the challenge agent. For example, defence against
extracellular organisms involves the interactions of T lymphocytes,
B lymphocytes, macrophages, and polymorphonuclear granulocytes, in
addition to a variety of cell-secreted products, whereas resistance to
generalized infection from intracellular pathogens and neoplastic
diseases is likely to involve macrophages, NK cells, and T cell-
mediated immune processes.
Although many host resistance assays are relatively simple to
perform, they normally require large numbers of animals and appear to
be less sensitive than immune function tests (Luster et al., 1993).
Other studies have shown that host resistance assays are more
sensitive than immune function tests (Vos et al., 1991; Burleson et
al., in press). The dose of challenge agent used in experimental
studies is important, since too low or too high a dose will not allow
detection of changes in immunocompromised groups in comparison with
controls (Selgrade et al., 1982; Luster et al., 1993). The sensitivity
of a host resistance assay also depends on the end-point measured. For
example, tests involving survival or tumour models (i.e. dichotomous)
are by nature less sensitive than those with end-points that provide
continuous data, such as enumeration of tumours, bacteria, or soluble
immune activation markers, and several end-points in one model of
infection, such as T. spiralis. This is attributable partly to
differences in the types of statistical analyses used to establish
group differences. With dichotomous data, two approaches can be used.
Some laboratories use a challenge dose that produces a response in
10-30% of animals in the control group. An alternative is to use a
dose slightly below that which would induce the desired response in
any of the animals in the control group. The latter design increases
the statistical power of data analysis. In most cases, extreme
accuracy is needed in the delivery of the agent, to ensure that the
administered dose of agent is only slightly smaller than that which
will give the desired response. In either approach, statistical
significance is heavily dependent on the dose of challenge agent and
the number of animals in each treatment group: Table 15 shows that
doubling the number of animals in a study greatly increases the
ability to detect a significant change. Even more obvious is the
increased ability to detect significant differences when the dose of
the agent in the control group is lowered to a subclinical
concentration. These hypothetical values demonstrate how statistically
significant changes can be obtained in susceptibility assays by
modifying the experimental design. In the first design, an effective
dose of 30% is used in the control group (i.e. a concentration of
agent that produces a response in 30% of normal animals) with 15
animals per treatment group. In the second design, increasing the
group size to 30 allows for even greater statistical significance. In
the third design, a challenge inoculation is given which produces no
effect in the control group (effective dose, 0), allowing for greater
statistical significance.
Two variables that influence the quantitative relationship
between immune function and disease are the virulence and amount of
the infectious agent. These remain a constant in most experimental
studies but may vary between experiments as well as in the human
population. For example, in the general population one can assume that
an infectious disease such as influenza can develop in any individual,
independently of their immune capacity or prior immunization, provided
that the virulence or quantity of the challenging agent is sufficient
to overwhelm the individual's defensive capacities. In Figure 38,
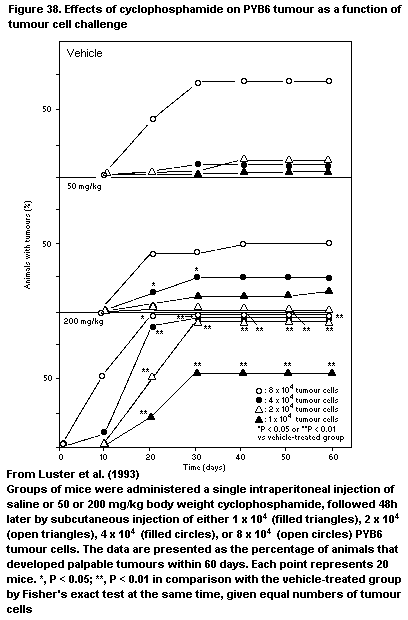 Table 15. Chi-squared values (hypothetical)
Treatment Design 1 Design 2 Design 3
------------------------------- ------------------------------- --------------------------------
No. affected/ One-tailed No. affected/ One-tailed No. affected/ One-tailed
no. tested P value no. tested P value no. tested P value
Control 3/15 - 6/30 - 0/15 -
Dose 1 4//15 0.532 8/30 0.428 1/15 0.516
2 5/15 0.411 10/30 0.273 2/15 0.274
3 6/15 0.312 12/30 0.165 3/15 0.150
4 7/15 0.233 14/30 0.096 4/15 0.084
5 8/15 0.173 16/30 0.055 5/15 0.048*
6 9/15 0.128 18/30 0.031* 6/15 0.028*
7 10/15 0.094 20/30 0.017* 7/15 0.017*
8 11/15 0.069 22/30 0.009* 8/15 0.010*
9 12/15 0.051 24/30 0.005* 9/15 0.006*
10 13/15 0.038* 26/30 0.003* 10/15 0.004*
11 14/15 0.028* 28/30 0.002* 11/15 0.002*
12 15/15 0.021* 30/30 0.001* 12/15 0.002*
groups of mice pretreated with either vehicle (saline), 50 mg/kg
cyclophosphamide (causing minimal immunosuppression), or 200 mg/kg
cyclophosphamide (causing severe immunosuppression) were administered
various numbers of PYB6 tumour cells. Even vehicle-treated mice
developed a high frequency of tumours, provided that the challenge was
sufficiently high (i.e. 8 × 104 tumour cells). In contrast, severely
immunosuppressed mice (high dose of cyclophosphamide) developed an
increased tumour frequency at all challenge levels of PYB6 tumour
cells. The groups treated with the low dose of cyclophosphamide were
of particular interest, since evidence of increased susceptibility
appeared but only as a function of the tumour cell concentration.
Assuming that these observations are applicable to human populations,
even small changes in immune function could increase the likelihood of
disease.
As indicated earlier, while experimental data have been used
occasionally in risk assessment, most immunotoxicological data have
focused on hazard identification. Although comparative data on the
effects of specific immunotoxic agents in humans and animals are
limited, other factors contribute to the minimal use of these data in
risk assessment, including the concern that immunotoxicity testing has
often been conducted without full knowledge of its predictive value in
humans or its quantitative relationship to immune-mediated diseases.
Luster et al. (1988) reported on the design and content of a
screening battery involving a tier approach for detecting potential
immunosuppressive compounds in mice. This battery has been used to
examine a variety of compounds, and the database, generated on over 50
compounds, has been analysed in an attempt to improve the accuracy and
efficiency of tests for screening chemicals for immunosuppression and
to identify better those tests that predict experimentally induced
immune-mediated diseases (Luster et al., 1992, 1993). Specifically,
attempts have been made to develop a 'streamlined' test configuration
for accurately predicting immunotoxic agents and to establish models
that could be used to provide insight into the qualitative and
quantitative relationships between the immune and host resistance
assays commonly used to examine potential immunotoxic chemicals in
experimental animals. While the analyses had a number of limitations,
several conclusions can be drawn from the results:
(1) With this particular testing configuration, examination of
only two or three immune parameters was needed in order to identify
potential immunotoxicants. Lymphocyte enumeration and quantification
of the T cell-dependent antibody response appeared to be particularly
useful. Furthermore, some commonly employed measures (e.g. leukocyte
counts, lymphoid organ weights), while probably good predictors of
immunotoxicity, are apparently not as sensitive as the other tests.
Obviously, inclusion of additional tests that are not part of the
original battery may improve the prediction of immunotoxicity.
(2) A good correlation was found between changes in immune tests
and altered host resistance, in that there was no instance in which
host resistance was altered without a significant change in the immune
test(s). In many instances, however, immune changes were observed in
the absence of detectable changes in host resistance (Table 16),
indicating that immune tests are generally more sensitive than host
resistance assays.
(3) No single immune test could be considered highly predictive
for altered host resistance; however, many of the tests were good
indicators, while others, such as leukocyte counts and proliferative
response to lipopolysaccharide, were relatively poor indicators of a
change in host resistance. Some of the tests that showed the highest
association with host resistance were those described previously as
the best indicators of immunotoxicity, such as the plaque-forming
assay and surface markers, but also included tests such as delayed-
type hypersensitivity and thymic weight.
(4) Regression modelling, using a large data set on one chemical
agent, indicated that most, but not all, of the immune function-host
resistance relationships follow a linear model. It was not possible,
however, to establish linear or threshold models for most of the
chemicals studied when the data from all 50 chemicals were combined;
thus, a more mechanistically based mathematical model will have to be
developed. A similar conclusion was drawn on the basis of a limited
data set collected by the Environmental Protection Agency (Selgrade et
al., 1992), in which changes in NK cell activity were correlated with
changes in susceptibility to cytomegalovirus in a murine model. It is
impossible, at present, to determine how applicable these analyses
will be for immunotoxic compounds with different immune profiles;
however, as more analyses become available, the ability to estimate
potential clinical effects accurately from the results of
immunological tests should increase.
Table 16. Association between the results of host resistance models and immune tests
Challenge No. of Frequencya
agent tests
Specificity Sensitivity Concordance
(-/-) (+/+) (Total)
Listeria monocytogenes 34 100 52 65**
PYB6 tumour 24 100 39 54
Streptococcus pneumoniae 19 100 38 58
B16F10 melanoma 19 100 40 68
Plasmodium yoelii 11 100 38 55
Influenza 9 100 17 44
Any of the aboveb 46 100 68 78*
From Luster et al. (1993)
* Agreement statistically significant at P < 0.05
a Frequency is defined as: specificity, the percentage of non-immunotoxic
chemicals with no effect on the host resistance models; sensitivity,
the percentage of potentially immunotoxic chemicals causing a change
in a host resistance model; concordance, percentage of qualitative agreement
b Frequency calculated on the basis of all of the host resistance models used to
study an agent
7. SOME TERMS USED IN IMMUNOTOXICOLOGY
Accessory cell. Passenger cell (leukocyte, mainly monocytes) or
stationary cell (reticulum cell, epithelial cell, endothelial cell)
that aids T or B lymphocytes in inducing immunological reactions,
either by direct contact or by releasing factors; normally expresses
MHC class II molecules
Acquired immunity. A state of protection against pathogen-induced
injury, with rapid immune elimination of pathogenic invaders; due to
previous immunization or vaccination
Activation. The process of going from a resting or inactive state to
a functionally active state, of leukocytes (lymphocytes, monocytes) or
proteins (complement, coagulation)
Acute-phase protein. Non-antibody humoral factor that emerges in
increasing amounts in the circulation shortly after induction of an
inflammatory response; e.g. alpha 2-macroglobulin, C-reactive protein,
fibrinogen, alpha 1-antitrypsin, and complement components
Adaptive immunity. A state of specific acquired protection against
pathogenic invaders, induced by immunization or vaccination
Addressin. Receptor for lymphocytes on endothelial cells of venules,
involved in homing of cells in lymphoid tissue; belongs to the
immunoglobulin gene superfamily, integrins, and selectins
Adenoid. See Tonsil
Adhesion receptors Molecule involved in cellular adhesion between
passenger cells and the extracellular stationary matrix (endothelium,
connective tissue); comprises three main families; member of the
immunoglobulin gene superfamily, integrins, and selectins
Adjuvant. Material that enhances an immune response
Adoptive immunity or tolerance. Transfer of a state of immunity or
tolerance via cells or serum from an immune or tolerant individual to
a naive individual
Affinity. Binding strength of an antibody-combining site to an
antigenic determinant (epitope); expressed as an association constant
(Ka)
Agglutination. Process of aggregation of visible antigenic particles
(e.g. erythrocytes) mediated by antibodies directed towards the
particles
Allele. One or more genes at the same chromosomal locus which
control alternative forms (phenotypes) of a particular inherited
characteristic
Allergen. Antigen that induces an allergic or hypersensitivity
reaction, resulting in immune-mediated or nonimmune-mediated tissue
damage; restricted mainly to immediate hypersensitivity or
anaphylactic reactions
Allergy. State of altered immunity, resulting in hypersensitivity
reaction on contact with antigen or allergen; often restricted to
immediate hypersensitivity or anaphylaxis
Alloantigen. Antigen that differs between different (not inbred)
individuals within one species
Allogeneic. Genetically different phenotypes in different (not
inbred) individuals within one species; opposite of isogeneic, or
xenogeneic
Allotype. Genetically different antigenic determinants on protein of
(not inbred) individuals within one species
alpha Chain. First chain of a multimeric receptor molecule: in
immunoglobulin molecules, the a heavy chain forming the IgA class; in
T-cell receptor molecules, one of the chains forming the dimeric
alpha-ß receptor molecule; in MHC class I molecules, the main
polypeptide chain associated with the ß2-microglobulin molecule; in
MHC class II molecules, one of the chains in the dimeric molecule
Alternative pathway of complement activation. Activation of
complement pathway by substances other than antigen-antibody
complexes; involves factor B, properdin, and complement component C3
Anaphylatoxin. Activated components of complement components C3 and
C5 (C3a and C5a, respectively), which induce anaphylactic reactions by
activating mast cells and basophilic granulocytes
Anaphylaxis or anaphylactic reaction. Local or systemic immediate
hypersensitivity reaction initiated by mediators released after
immunological stimulation; symptoms can be a drop in blood pressure
related to vascular permeability and vascular dilatation, and
obstruction of airways related to smooth muscle contraction or
bronchoconstriction
Anergy. State of unresponsiveness to antigenic stimulation, due to
the absence of responding elements or the loss of capacity of existing
elements to mount a reaction; synonym for tolerance
Antibody. Immunoglobulin molecule produced in response to immunization
or sensitization, which specifically reacts with antigen Antibody-dependent
cell-mediated cytotoxicity. Cytotoxic reaction in which an antibody forms
the bridge between the cytotoxic cell (lymphocyte, macrophage) and the
target cell
Antigen. Any substance that induces a specific immunological
response
Antigen-binding site (paratope). Part on an antibody molecule that
binds antigen (antigenic determinant, or epitope); part of the T-cell
receptor that binds the complex of antigen and MHC molecule
Antigenic determinant (epitope). Part of an antigen that binds to
antibody or T-cell receptor (the latter in combination with MHC
molecule)
Antigenicity. Capacity to react with components of the specific
immune system (antibody, receptors on lymphocytes)
Antigen presentation. Process of enabling lymphocytes to recognize
antigen on a specific receptor on the cell surface. For presentation
to T lymphocytes, includes intracellular processing and complexing of
processed peptides with MHC molecule on the cell membrane of the
antigen-presenting cell. For presentation to B lymphocytes, can
include formation of immune complexes (in germinal centres)
Antigen-presenting cell. Cell that presents antigen to lymphocytes,
making possible specific recognition by receptors on the cell surface.
In a more restricted way, used to describe MHC class II-positive
(accessory) cells which can present (processed) antigenic peptides
complexed with MHC class II molecules to T helper-inducer lymphocytes.
Includes macrophage populations (in particular, Langerhans cells and
dendritic or interdigitating cells), B lymphocytes, activated T
lymphocytes, certain epithelia (after MHC class II antigen induction
by e.g. interferon gamma); others are follicular dendritic cells, not
of bone-marrow origin, which present antigen in the form of immune
complexes to B cells in germinal centres of peripheral lymphoid
tissue; marginal zone macrophages in splenic marginal zone, which
present antigen, without contact with T helper cells, to B cells at
this location (T cell-independent response, e.g. to bacterial
polysaccharide). In rodent skin epidermis, a dendritic epidermal cell,
of T-cell origin, has an antigen-presenting function.
Antigen receptor. Multichain molecule on lymphocytes, to which
antigens bind. For B lymphocytes, an immunoglobulin molecule that
recognizes nominal antigen; for T lymphocytes, a T-cell receptor
molecule that recognizes antigenic peptide in combination with the
polymorphic determinant of an MHC molecule
Antinuclear antibody. Antibody directed to nuclear antigen; can have
various specificities (e.g. to single- or double-stranded DNA or
histone proteins); frequently observed in patients with rheumatoid
arthritis, scleroderma, Sjögren's syndrome, systemic lupus
erythematosus, and mixed connective tissue disease. Also called
antinuclear factor
Antiserum. Serum from an individual that contains antibodies to a
given antigen
Aplasia. Absence of tissue structure or cellular component, either
congenital or acquired
Apoptosis (programmed cell death). Process whereby the cell kills
itself after activation, by Ca2+-dependent endonuclease-induced
chromosomal fragmentation into fragments of about 200 base-pairs
Appendix. Lymphoid organ in the gastrointestinal tract, at the
junction of ileum and caecum; forms part of gut-associated lymphoid
tissue
Arthus reaction. Inflammatory response, generally in skin, induced
by immune complexes formed after injection of antigen into an
individual that contains antibodies
Asthma. Chronic inflammatory disease characterized by bronchial
hyperresponsiveness to various stimuli
Atopy. In general terms, 'unwanted reactivity'; used mostly to
describe the state of general systemic or local hypersensitivity
reactions related to genetic predisposition
Auto-antibody. Antibody to component in the individual itself
Auto-antigen. Antigen to which an autoimmune reaction is directed
Autoimmunity. A state of immune reactivity towards self
Autologous. Derived from self; components of an immunological
reaction (e.g. antibody, lymphocytes, grafted tissue) from the same
individual; opposite of heterologous
Avidity. Binding strength between antibody and antigen, or receptor
and ligand; for antibody, represents the product of more than one
interaction between antigen-binding site and antigenic determinant
Bacteraemia. Presence of bacteria in blood
Basophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with acid glycoproteins stained by basic (blue) dyes; after
release, glycoproteins induce anaphylactic reactions
B-Cell growth and differentiation factor, B-cell growth factor, and
B-cell stimulating factor. See Interleukin-4 and -5
ß Chain. In T-cell receptor molecules, one of the chains forming the
dimeric alpha-ß receptor molecule; in MHC class II molecules, one of
the chains in the dimeric molecule
ß2-Microglobulin. A peptide of 12 kDa, which forms part of MHC class
I molecule
B Lymphocyte or cell. Lymphocytes that recognize nominal antigen by
immunoglobulin (antibody) surface receptor (on virgin B cell, IgM and
IgD) and, after activation, proliferate and differentiate into
antibody-producing plasma cells. During a T-dependent process, there
is immunoglobulin class switch (IgM into IgG, IgA, IgD, or IgE), with
maintenance of the antigen-combining structure. For T-independent
antigens, cells differentiate only in IgM-producing plasma cells. B
Lymphocytes originate from precursor cells in bone marrow; in avian
species, they undergo maturation in the bursa of Fabricius (B, bursa-
dependent); in mammals, in the bone marrow
B Lymphocyte area. That part of an lymphoid organ or tissue that is
occupied by B lymphocytes, e.g. follicles in peripheral lymphoid
tissue, marginal zone in spleen
Birbeck granule. Rod-shaped structure with rounded end,
approximately 6 nm thick, found in the cytoplasm of Langerhans cells
in the epidermis, and interdigitating dendritic cells in T-lymphocyte
area of lymphoid tissues
Blast cell. Large cell (about 15 µm or more) with dispersed nuclear
chromatin and cytoplasm rich in ribosomes; in an active stage of the
cell cycle before mitosis
Blast transformation. Process of activation of lymphocytes into cell
cycle and to form blastoid cells before mitosis
Blocking antibody. Antibody that can interfere with another antibody
or with reactive cells in binding antigen, thereby preventing effector
reactions (often used in association with an allergic reaction or
tissue damage)
Bone marrow. Soft tissue in hollow bones, containing haematopoietic
stem cells and precursor cells of all blood cell subpopulations
(primary lymphoid organ); major site of plasma cell and antibody
production (secondary lymphoid organ)
Booster. Dose of antigen given after immunization or sensitization
to evoke a secondary response
Bradykinin. Peptide of nine amino acids split from alpha 2-
macroglobulin by the enzyme kallikrein; causes contraction of smooth
muscle
Bronchus-associated lymphoid tissue. Lymphoid tissue located along
the bronchi, considered to represent the location of presentation of
antigens entering the airways; contributes to mucosa-associated
lymphoid tissue
Bursa equivalent. Site where B-cell precursors undergo maturation
into immunocompetent cells in non-avian species; bone marrow in adult
mammals
Bursa of Fabricius. Primary lymphoid organ in avian species, located
in cloaca, with an epithelial reticulum, where precursors of B
lymphocytes from the bone marrow undergo maturation into
immunocompetent cells and then move to peripheral lymphoid organs
C (constant) gene. Gene that encodes the constant part of
immunoglobulin chains or T-cell receptor chains (e.g. Cµ, Cdelta
for immunoglobulin heavy chains, Ckappa for immunoglobulin kappa
light chain, Calpha for T-cell receptor alpha chain)
C (constant) region. Region at carboxy terminal of immunoglobulin
chains or T-cell receptor chains, identical for a given immunoglobulin
class or subclass or for a given T-cell receptor chain; encoded by C
genes in DNA
Cachectin. See Tumour necrosis factor
CD (cluster of differentiation). Group of (monoclonal mouse)
antibodies that react to identical leukocyte surface molecules in
humans (but not necessarily to identical epitopes), on the basis of
comparative evaluations during international workshops and transferred
to other species by analogy. Does not include MHC or immunoglobulin
molecules
CD3 molecule. Molecule consisting of at least four invariant
polypeptide chains, present on the surface of T lymphocytes associated
with the T-cell receptor; mediates transmembrane signalling (tyrosine
phosphorylation) after antigen binding to T-cell receptor
CD4 molecule. Glycoprotein of 55 kDa on the surface of T lymphocytes
and part of monocytes and macrophages. On mature T cells, restricted
to T helper (inducer) cells; has an accessory function to antigen
binding by T-cell receptors, by binding to a non-polymorphic
determinant of MHC class II molecule
CD8 molecule. Complex of dimers or higher multimers of 32-34 kDa
glycosated polypeptides linked by disulfide bridges, on the surface of
T lymphocytes. On mature T cells, the presence is restricted to
T cytotoxic-suppressor cells; has an accessory function to antigen
binding by the T-cell receptor, by binding to a non-polymorphic
determinant of MHC class I molecule
Cell-mediated immunity. Immunological reactivity mediated by
T lymphocytes
Central (primary) lymphoid organ. Lymphoid organ in which precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment, to form immunocompetent cells; not antigen-driven
but can be influenced by mediators produced as a result of antigen
stimulation
Centroblast. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; a medium to large,
12-18-µm cell, with a round to ovoid nucleus that has moderately
condensed heterochromatin and medium-sized nucleoli close to the
nuclear membrane, medium-sized to broad cytoplasm containing many
polyribosomes and a variable amount of rough endoplasmic reticulum
Centrocyte. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; medium-sized,
8-12-µm lymphoid cell with irregular nucleus with condensed
heterochromatin; small cytoplasm containing a few organelles
CH50. The amount of serum (or dilution of serum) that is required to
lyse 50% of erythrocytes in a standard haemolytic complement assay
Chemiluminescence. Luminescence produced by direct transformation of
chemical energy into light energy
Chemotactic factor. Substance that attracts cells to inflammatory
lesions
Chemotaxis. Process of attracting cells to a given location, where
they contribute to an inflammatory lesion
Class I MHC molecule. Molecule coded by the A, B, or C locus in the
HLA complex, the K and D locus in the mouse H2 complex, and less well
defined MHC I gene loci in other species, in association with the
ß2-microglobulin molecule. Two-chain molecule occurring on all
nucleated cells, without allelic exclusion
Class II MHC molecule. Molecule coded by the D (DR, DP, DQ) locus in
the HLA complex, the I-A and I-E locus in the mouse H2 complex, and
less well defined MHC II gene loci in other species, comprising an
alpha and a ß chain (intracellular, associated with an 'invariant'
chain). Two-chain molecule occurring, without allelic exclusion, on B
lymphocytes, activated T lymphocytes, monocytes-macrophages,
interdigitating dendritic cells, some epithelial and endothelial cells
(variable, dependent on species and state of activation); antigen-
presenting cells
Class switch. See Immunoglobulin class switch
Classical pathway of complement activation. Activation of complement
pathway by antigen-antibody complexes, starting with complement
component C1 and ending with complement component C3
Clone. Population of cells that emerge from a single precursor cell;
within T or B lymphocytes, cells with a fixed rearrangement of genes
coding for T-cell receptor or immunoglobulin
Clonal expansion. Proliferation of cells that have a genetically
identical constitution; when uncontrolled, may result in tumour
formation
Colony-stimulating factor. Substance that supports clonal cell
growth of haematopoietic cells
Complement. Group of about 20 proteinase precursors that activate
and split each other, in sequential order. The various components are
present in inactive (precursor) form, except for C3, which in a normal
state shows a low turnover (major split products C3a and C3b). The
split products are either bound to the activating substance (immune
complex or antibody-coated cell) or are released as active mediators.
The classical cascade starts by activation of component C1,
subsequently C4, C2, and C3, and is initiated by (IgG/IgM) immune
complexes. The alternative cascade starts by activation of C3b and
factor B, subsequently factors D and C3, and is initiated by nonimmune
specific activators like microbial polysaccharides and some
'allergens'. C3 split products (C3b) activate the amplification loop,
in which factors D and B are also used, activate C5, and thereafter
the terminal cascade C6, C7, C8, and C9, which attack the cell
membrane and kill the cell (microorganism). The cascade is under the
control of various inhibitors. Major effects of complement split
products are adherence to receptors on phagocytes (C3b, C3d); mediator
activity, like chemoattraction of inflammatory cells, vasodilatation,
increased vascular permeability, and smooth muscle contraction
(C3a, C5a); cell lysis by membrane lesions (C6-C9)
Complement fixation. Binding and consumption of complement by
antigen-antibody complexes; often used in association with assays for
complement activity
Complement receptor (CR). Cell surface molecule that can bind
activated complement components in e.g. antigen-antibody complexes;
CR1 (CD35) is a receptor for C3b, present on B lymphocytes, monocytes
and macrophages, granulocytes, and erythrocytes; CR2 (CD21) is a
receptor for C3d, present on mature B lymphocytes; CR3 (CD11b/CD18) is
a receptor for C3b present on macrophages, granulocytes, natural
killer cells, and a subset of CD5+ B lymphocytes; CR4 (CD11c/CD18)
is a receptor for C3b present on monocytes, macrophages, granulocytes,
and natural killer cells
Contact sensitivity. Hypersensitivity reaction evoked in skin by
placing sensitizing agents or substances on skin
Cords of Billroth. Medullary cords in spleen
Corona. See Mantle
Cortex. Outer parenchymal layer of organs
Cross-reactivity. Reactivity of antigen-specific elements (T lymphocytes
sensitized by T-cell receptor, B lymphocytes by antibody; antibody
molecules) towards antigens other than those used in original
sensitization, owing to shared antigenic epitopes on different
antigenic molecules; also used to describe reactions towards antigenic
determinants other than those originally used in sensitization, due to
similarities in structure
Cytokine. Biologically active peptide, synthesized mainly by
lymphocytes (lymphokines) or monocytes and macrophages (monokines);
modulates the function of cells in immunological reactions; include
interleukins. Some (pleotrophic cytokines) have a broad spectrum of
biological action, including neuromodulation, growth factor activity,
and proinflammatory activity
Cytokine receptor. Ligand for cytokine on target cell, acts in
signal transduction through the cell membrane; many are multichain
molecules belonging to different receptor families
Cytolytic antibody. Antibody that can mediate lysis of the target to
which it is directed, either in combination with complement or as
bridge between cytotoxic cell and target
Cytotoxic cell (killer cell). Effector T cell, natural killer cell,
or activated macrophage; kills target cells and tissue extracellularly
after binding; mediated by release of substances from cytolytic
granules (including serine esterase, cytolysin, and perforins)
Cytotoxic reaction. Effector reaction of antibody or cells, resulting
in lysis of target cell or tissue
Cytotoxic T lymphocyte. Subpopulation of T lymphocytes with CD8
phenotype; after recognition of antigen in an MHC class I-restricted
manner, differentiates from precursor to effector cytotoxic cell and
subsequently kills target cells
Degranulation. Process of fusion of cytoplasmic granules with cell
membrane, whereby the content of the granules is released into the
extracellular space; mainly used in association with immediate
hypersensitivity reactions
Delayed-type hypersensitivity. Inflammatory lesion mediated by
effector T lymphocytes or their products, with attraction mainly of
macrophages towards the inflammatory lesion. Term originates from the
classical skin reaction after challenge of a sensitized individual;
maximal (wheal and flare) response reached within 24-72 h
Delayed-type hypersensitivity T cell. Subpopulation of T lymphocytes
with CD4 phenotype; after recognition of antigen in an MHC class
II-restricted manner, secretes mediators involved in inflammatory
responses, e.g. INF gamma and tumour necrosis factor
delta Chain. In immunoglobulin molecules, delta heavy chain forming
the IgD immunoglobulin class; in T-cell receptor molecules, one of the
chains forming the dimeric gamma-delta receptor molecule; one of the
chains in the CD3 molecule associated with the T-cell receptor
Dendritic cell. Cell in tissue that shows elongations or protrusions
of cytoplasm into the parenchyma. Often used in a restricted manner to
designate a type of antigen-presenting cell, of which two categories
exist: one of bone-marrow origin belonging to the macrophage lineage,
including Langerhans cells in skin and interdigitating dendritic cells
in T-cell areas of lymphoid tissue and a very small leukocyte
population in blood; the second of tissue parenchymal origin
(presumably pericytes around blood vessels), the follicular dendritic
cells in B-cell areas (follicles) of lymphoid tissue
Dendritic epidermal cell. T Cell in the epidermis that has dendritic
morphology; has antigen-presenting function but is not a (macrophage-
related) Langerhans cell. Occurs in rodents but not in humans;
contributes to skin immune system
Dermal immune system. See Skin immune system
Desensitization. Induction of anergy or tolerance to allergic
substances by active intervention in immune reactivity (exhaustion of
reactive elements or induction of blocking phenomena)
Diapedesis. Passage of cells through blood vessel walls into tissue
parenchyma, mediated by constriction of endothelial cells
D (diversity) gene. Gene that encodes the variable part of
immunoglobulin heavy chains, or T-cell receptor alpha, ß, or gamma
chain (e.g. DH1-DHn for immunoglobulin heavy chains, Ddelta
1-Vdelta n for T-cell receptor alpha or delta chain)
Domain. Part of polypeptide chain folded to a relatively rigid
globular tertiary structure fixed by disulfide bonds. Molecules of the
immunoglobulin gene superfamily have a tertiary domain-like structure:
each domain is about 110 amino acids long and arranged in a sandwich
of two sheets of anti-parallel ß strands. See also Homologous,
Homology
Ectoderm. Outermost of the three cellular layers of the embryo;
produces the epidermis and neuronal tissue
Eczema. Superficial inflammation in skin, involving primarily the
epidermis; characterized by redness, itching, minute papules and
vesicles, weeping, oozing, and crusting. Histological changes include
microvesiculation and oedema of the epidermis and an infiltrate of
lymphocytes and macrophages in the dermis
Effector cell. General term to describe a cell that mediates a
function after a stage of activation, differentiation, and
proliferation
Endocytosis. Process of uptake of material by a cell; special forms
are phagocytosis and pinocytosis
Endoderm. Innermost of the three cellular layers of the embryo;
produces the gastrointestinal lining and some internal organs, such as
liver and pancreas
Endoplasmic reticulum. Membrane-like structure in cell cytoplasm;
site of protein synthesis
Endothelium. Cells that line blood vessels; exert a major function
in traffic of leukocytes from blood into tissue, by altered expression
of adhesion molecules (modulation of numbers of receptors; maturation
and activation resulting in altered glycosylation, expression of new
ligands or altered ligand binding affinity; change in cytoskeleton
organization). A special endothelial cell type occurs in T-lymphocyte
areas of lymphoid tissue in the high endothelial (postcapillary)
venule.
Endotoxin. Lipopolysaccharide from the cell wall of Gram-negative
bacteria; has toxic, pyrogenic, and immunoactivating effects
Enzyme-linked immunosorbent assay. Immunoenzymetric assay based on
the use of antigens or antibodies labelled with a specific enzyme;
combines the virtues of solid-phase technology and enzyme-labelled
immunoreagents. The antigen-antibody complex is determined by an
enzyme assay involving the incubation of the complex with an
appropriate substrate of the enzyme.
Eosinophil chemotactic factor. Acidic tetrapeptide of 0.5 kDa
produced by mast cells (preformed mediator); attracts eosinophils to
the site of inflammation
Eosinophilia. State of increased proportions of eosinophilic
granulocytes in blood
Eosinophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with basic proteins stained by acidophilic (red) dyes; after
release, the proteins modulate inflammatory reactions
Epithelium. Cells covering the surface of the body and forming the
first line of defence against pathogenic invaders. Reticular
epithelium forming the stroma of tissue occurs in thymus (in avian
species in the bursa of Fabricius); these cells have a major function
in processing precursor cells to immunocompetent lymphocytes
Epithelioid cell. Cell of macrophage origin in chronic inflammatory
lesions, which resembles an epithelial cell morphologically
Epitope (antigenic determinant). Part of antigen that binds to
antibody or T-cell receptors (the latter in combination with MHC
molecule)
epsilon Chain (see also alpha Chain). In immunoglobulin molecules,
the epsilon heavy chain forming the IgE immunoglobulin class; one of
the chains in the CD3 molecule associated with the T-cell receptor
Erythema. Redness of skin produced by congestion of blood
capillaries due to dermal arterial vasodilatation
Erythrocyte. Red blood cell; a bone marrow-derived blood cell
component involved in oxygen transport to tissue; contains a nucleus
in distinct avian species like chickens but does not have a nucleus in
mammals
Exudation. Inflammation in tissue; contains blood cells and fluid
comprising serum proteins of high relative molecular mass
Ex vivo/in vitro. An assay method in which the effects of a
xenobiotic are evaluated in vitro in cells isolated from an animal
or human exposed to the compound of interest
Fab fragment. Part of an antibody molecule in which monovalent
binding of an antigenic determinant occurs; formed by the three-
dimensional structure of variable parts (domain) of one heavy and one
light chain and the adjacent part of the constant part (constant
domain); ab, antigen binding
F(ab')2 fragment. Part of an antibody molecule in which divalent
binding of antigenic determinants occurs; formed by the
three-dimensional structure of both Fab fragments
Fc fragment. Part of an antibody molecule formed by the three-
dimensional structure of the constant part (constant domains) of the
heavy chains (except that adjacent to the variable domain), involved
in antibody effector functions; c, crystallizable
Fc receptor. Structure on leukocytes (lymphocytes, monocytes,
macrophages, granulocytes) that mediates binding of immunoglobulin or
antibody, alone or after forming aggregates in antigen-antibody
complexes. Receptors for IgE (Fc epsilon) occur on mast cells and
basophilic granulocytes and are involved in immediate hypersensitivity
reactions; receptors for IgG are of three classes: low-affinity FcR
III, CD16, on natural killer cells, monocytes, macrophages, and
granulocytes; low-affinity FcR II, CD32, on B cells, myeloid cells,
Langerhans cells, and interdigitating dendritic cells; high-affinity
FcR III, CD64, on monocytes and macrophages
Follicle. Round to oval structure in lymphoid tissue, where B cells
are lodged. Primary follicles contain only small resting B cells;
secondary follicles comprise a pale-stained germinal centre, with
centrocytes and centroblast, and contain B lymphocytes in a state of
activation or proliferation and macrophages, the stroma consisting of
follicular dendritic cells. The germinal centre is surrounded by a
mantle with small B lymphocytes
Follicular dendritic cell. Cell forming the stationary micro-
environment of germinal centres of follicles in lymphoid tissue;
elongated, often binucleated cell with long branches extending between
germinal centre cells and forming a labyrinth-like structure; linked
by desmosomes. Of local parenchymal origin, presumably from pericytes
surrounding blood vessels. Its main function is presentation of
antigen, trapped as immune complex in the labyrinth, to B lymphocytes.
gamma Chain (see also alpha Chain). In immunoglobulin molecules,
the gamma heavy chain forming the IgG immunoglobulin class; in T-cell
receptor molecules, one of the chains forming the dimeric gamma-delta
receptor molecule; one of the chains in the CD3 molecule associated
with the T-cell receptor
gamma-delta T cell. T Lymphocyte with an antigen receptor composed
of a gamma and a delta chain associated with CD3 transmembrane
molecule; develops in part inside the thymus (including intrathymic
selection), in part outside the thymus. Has a major role as a
cytotoxic cell in the first phase of the (innate) immune response,
e.g. in rodents in the mucosal epithelium
Gammaglobulin. Part of serum proteins that move towards the negative
electrode (gamma fraction) upon electrophoresis; contains
immunoglobulins
Gene rearrangement. For immunoglobulin and T-cell receptor, the
process whereby the germline chromosomal genomic structure of variable
(diversity), joining, and constant segments recombine to form a
specific V-(D-)J-C combination, enabling transcription into mRNA and
translation into protein. The V-(D-)J combination of different chains
determines the specificity of the receptor (immunoglobulin or T-cell
receptor).
Germinal centre. The pale-staining centre in follicles of lymphoid
tissue, where B lymphocytes are activated by antigen in a
T lymphocyte-dependent manner and subsequently go into proliferation
and differentiation, acquiring the morphology of centroblasts,
centrocytes, and plasma cells. Has a special microenvironment made up
of follicular dendritic cells and large macrophages (tingible body or
starry-sky macrophages)
Glomerulonephritis. Inflammation of glomeruli in kidney, often
associated with deposition of immune complexes along the glomerular
basement membrane or in the mesangium, and influx of polymorpho-
nuclear granulocytes
Golgi apparatus. Tubular structures in cytoplasm, involved in
secretion of synthesized proteins
Granulocyte colony-stimulating factor. Synthesized by T lymphocytes
and macrophages, epithelial cells, fibroblasts, and endothelial cells;
supports growth of granulocyte progenitors, in synergism with IL-3 and
granulocyte-macrophage colony-stimulating factor of monocyte-
macrophage progenitors
Granulocyte-macrophage colony-stimulating factor. Produced by T
lymphocytes, endothelial cells, macrophages, and lung cells; supports
growth and differentiation of macrophages and granulocyte progenitors;
activates macrophages and polymorphonuclear macrophages to become
tumoricidal and produce superoxide anion
Granuloma. Chronic inflammatory reaction in tissue comprising macro-
phages (epitheloid cells), lymphocytes, and fibroblasts; formed in a
cell-mediated response towards poorly degradable material, in
immunological reactions as part of a delayed-type hypersensitivity
reaction
Gut-associated lymphoid tissue. Lymphoid organs and tissue located
along the gastrointestinal tract, presumed to be a first location of
presentation of antigens entering through the digestive tract;
comprises Peyer's patches, appendix, in part mesenteric lymph nodes,
adenoids, and tonsils; contributes to the mucosa-associated lymphoid
tissue
H-2. Major histocompatibility complex of mouse
Haemagglutinin. Antibody or substance that induces agglutination of
erythrocytes
Haematopoiesis. Production of cells of blood; subdivided into
erythropoiesis, lymphopoiesis, and myelopoiesis
Haematopoietic malignancy. Malignancy of blood-forming cells
Haemolysis. Process of lysis of erythrocytes, with release of
haemoglobin
Haemolytic agent (haemolysin). Antibody or substance that induces
lysis of erythrocytes
Haemopoietin. Growth factor that induces production of distinct
types of blood cells; also enhances the function of the mature cells
Haplotype. Phenotype of inherited characteristic on closely linked
genes on one chromosome
Hapten. Structure around one antigenic determinant, which itself
does not evoke an immune response unless coupled to a carrier
substance but can react with the products (antibodies, cells) of an
immune response
Hassall's corpuscle. Epithelial aggregate in onion-like structure,
often with debris of other cells; in the medulla of the thymus,
surrounded by large epithelial cells secreting thymic hormones; does
not occur in rodent thymus
Heat-shock protein. Family of proteins (60-90 kDa) with conserved
sequence in evolution; play a prime role in regulation and transport
of intracellular proteins. Expression is upregulated when cells are
under 'stress' (originally induced by heating), such as inflammatory
conditions, and may act as autoantigen in triggering and perpetuating
an auto-immune response
H (heavy) chain. One of the 45-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and three
constant domains (four constant domains in the 55-kDa µ chain). The
combination of the constant part of two heavy chains (alpha, ß, delta,
gamma, or µ) forms the immunoglobulin class-associated part of the
molecule (IgA, IgD, IgE, IgG, or IgM class)
Helper (inducer) T cell. Cell in a subpopulation of T lymphocytes,
with CD4 phenotype; after recognizing antigen in an MHC class
II-restricted manner, induces immunological reactions, secretes
interleukins, and cooperates (supports) B lymphocytes, cytotoxic
T-cell precursors, and macrophages
Helper T cell subpopulations. Th1 and Th2: Th1 cells produce
interleukin-2 and interleukin-3, interferon gamma, tumour necrosis
factor alpha and ß, and granulocyte-macrophage colony stimulating
factor, and function in induction of delayed-type hypersensitivity,
macrophage activation, and IgG2a synthesis. Th2 cells produce
interleukin-3, interleukin-4, and interleukin-5, tumour necrosis
factor alpha and granulocyte-macrophage colony stimulating factor, and
function in induction of IgG1, IgA, and IgE synthesis and induction of
eosinophilic granulocytes
Heterologous. Derived from foreign source or species; components of
an immunological reaction (e.g. antibody, lymphocytes, grafted tissue)
derived from another individual of the same species or another
species; opposite of autologous
Heterophilic antigen. Antigen in unrelated species; can be directed
towards xenogeneic immune reactivity; often has carbohydrate
structure; opposite of homocytotropic antibody
High endothelial (postcapillary) venule. Specialized blood vessels
in T-lymphocyte area of lymphoid tissue, through which circulating
lymphocytes pass into the parenchyma
Hinge region. Stretch in immunoglobulin molecule between Fab and Fc
fragments (first constant domain and other constant domains of the
heavy chain), where the quaternary structure of the molecule is not
rigid but flexible; bending of the hinge region after antigen binding
serves as a signal transduction, resulting in effector reactions
Histamine. ß-Imidazolylethylamine; component of granules in mast
cells and basophilic granulocytes that is released upon activation and
induces immediate hypersensitivity reaction, e.g. vasodilatation,
vascular permeability, smooth muscle contraction, and broncho-
constriction
Histiocyte (histiocytic reticulum cell). Monocyte in tissue. See
Macrophage
Histiocytosis. Increase in proportion of macrophages in tissue
HLA, human leukocyte antigen. Major histocompatibility complex of
humans
Homocytotropic antibody. Antibody that binds preferentially to cells
from the same species rather than to cells from other species;
opposite of heterologous antibody
Homologous, Homology. Similarity in primary structure between
substances; homology region is a synonym for domain
Host defence. Ability of an individual or species to protect itself
against opportunistic agents and to eliminate certain tumours and
exogenous agents such as (micro)organisms, viruses, and particles that
can cause disease
Hot spot. See Hypervariable region
Humoral immunity. Immunological reactivity mediated by antibody
Hybridoma. Transformed cell line or cell clone formed by fusion of
two different parental cell lines or clones
Hyperplasia. Reversible increase in cell number, usually as the
result of a physiological stimulus or persistent cell injury due to
irritating compounds
Hypersensitivity. Increased reactivity or sensitivity; in
immunological reactions, often associated with tissue destruction
Hypervariable region. Amino acid sequences in the variable regions
of antibody molecules or T-cell receptor chains where variability is
highest and which together form the antigen-binding site. Synonym for
hot spot
Hypoplasia. Reversible decrease in cell number, usually as a result
of a physiological stimulus
Ia antigen. MHC class II cell surface molecule
Idiotype. Antigenic determinant of variable domain of immunoglobulin
molecules or T-cell receptor
Immediate hypersensitivity. Inflammatory response that occurs within
minutes after exposure to allergen; caused by physical or
immunological stimulus, with vascular dilatation, increased vascular
permeability, and oedema as the main effects. Term originates from the
classical skin reaction after challenge of a sensitized individual in
skin, which takes 20-30 min to reach maximal (wheal and flare)
response and is mimicked by injection of mediator only (histamine)
Immune adherence. Binding of antigen-antibody complexes (antibody-
coated particles) to erythrocytes, platelets, or leukocytes; mediated
by activation of complement C3
Immune complex. Complex between antigen and antibody
Immune elimination. Rapid clearance of pathogen from the circulation
by components of the immune system; often used in association with
antibody molecules (removal by immune complex formation and
phagocytosis)
Immune exclusion. Process whereby entry of pathogens at mucosal
surfaces is prevented by the action of specific (secretory IgA)
antibody
Immune interferon. Former name for interferon gamma
Immune surveillance. Function (still hypothetical) of the immune
system in preventing or eliminating cells after malignant
transformation to a neoplastic process
Immunity. State of protection against pathogen-induced injury, with
fast immune elimination of pathogenic invaders due to previous antigen
contact or a special acquired state of responsiveness
Immunization (vaccination). Active intervention resulting in
immunity; used mainly in the context of presentation of (inactivated
or attenuated, nonpathogenic) substance to induce immunological
memory. Passive immunization is the adoptive transfer of immune system
components after previous contact with the pathogen and is performed
mainly with antibodies
Immunoblast. Intermediately differentiated B lymphocyte in lymphoid
tissue; a large, 15-20 µm, round-to-spherical cell with a rounded
euchromatic nucleus. The abundant cytoplasm contains many ribosomes,
well-developed rough endoplasmic reticulum and Golgi complex
Immunocompetence. Capacity of B or T lymphocytes to specifically
recognize antigen, resulting in a specific immunological reaction
Immunodeficiency. Defects in the immune system resulting in
decreased or absent reactivity to pathogens. Primary immunodeficiency
is mainly intrinsic defects in the differentiation of T or B
lymphocytes and can be congenital or acquired. Secondary
immunodeficiency is defects of which the cause is outside the immune
system (malnutrition; stress; protein loss after burns, nephrotic
syndrome, or intestinal bleeding; viral infection; therapy with
immunosuppressive or cytostatic drugs; irradiation).
Immunogen. A substance that can induce an immunological reaction
Immunogenicity. Capacity to evoke an immune response
Immunoglobulin. Formerly the electrophoretically-defined
gammaglobulin (in blood) but is also present in the ß fraction;
synthesized by plasma cells. The basic subunit consists of two
identical heavy chains (about 500 amino acid residues, organized into
four homologous domains; for µ chain in IgM, about 600 amino acid
residues, organized into five homologous domains) and two identical
light chains (about 250 amino acid residues organized into two
homologous domains), each consisting of a variable domain and one to
three constant domains (in the µ chain, four constant domains). The
antigen-binding fragment (Fab) consists of variable domains of heavy
and light chains (two per basic subunit). Five classes of
immunoglobulins exist, which differ according to heavy chain type
(constant domains): IgG (major immunoglobulin in blood), IgM
(pentamer, consisting of five basic units), IgA (major immunoglobulin
in secretions; present mainly as a dimeric molecule), IgD (major
function, receptor on B lymphocytes), and IgE. Effector functions
after antigen binding are mediated by constant domains of the heavy
chain (Fc part of the molecule) and include complement activation
(IgG, IgM), binding to phagocytic cells (IgG), sensitization and
antibody-dependent cell-mediated cytotoxicity (IgG), adherence to
platelets (IgG), sensitization and degranulation of mast cells and
basophils (IgE). IgA lacks these effector functions and acts mainly in
immune exclusion (prevention of entry in the body) at secretory
surfaces ('antiseptic paint').
I mmunoglobulin class. Subfamily of immunoglobulins, based on
difference in heavy chain. Five classes exist: IgA, secretory
immunoglobulin, dimeric; IgD, immunoglobulin on B cells that acts as
antigen receptor; IgE, immunoglobulin fixed to mast cells and
basophilic granulocytes, involved in immediate hypersensitivity
reactions; IgG, main immunoglobulin in circulation; IgM, pentameric
immunoglobulin with optimal agglutinating capacity, produced on first
antigen contact
Immunoglobulin class switch. Process whereby synthesis of IgM
antibody changes into synthesis of antibody of another immunoglobulin
class, with maintenance of the same variable part of the
immunoglobulin molecule. At the genomic level, this includes gene
rearrangement, with an exchange of a constant gene segment to a fixed
V-D-J gene segment combination. This switch is thought to occur in
germinal centres of follicles in lymphoid tissue, during the change of
a primary into a secondary response, and is under the control of
cytokines (switch factors)
Immunoglobulin gene superfamily. Group of molecules including
immunoglobulins, T-cell receptors, MHC molecules, and others, like the
lymphocyte function-related antigens LFA-2 (CD2) and LFA-3 (CD58), the
intercellular adhesion molecules ICAM-1 (CD54) and ICAM-2, the
vascular cell adhesion molecule VCAM-1, the neural cell adhesion
molecule NCAM (CD56), and the CD4 and CD8 molecules, which have a
similar tertiary basic domain-like structure, in which each domain is
about 110 amino acids long and stabilized by a disulfide bridge. These
molecules are known to be important for specific recognition and
adhesion functions
Immunoglobulin light chain type. Defines the light chain in the
immunoglobulin unit, either kappa or lamda, each defined at the
germline DNA level by individual constant (C), joining (J), and
variable (V) gene segments
Immunoglobulin subclass. Subfamily within a distinct immunoglobulin
class, based on subtle differences in heavy chain. For instance, in
humans there are two IgA subclasses, IgA1 (alpha1 heavy chain) and
IgA2 (alpha 2 heavy chain), and four IgG subclasses, IgG1-IgG4
(gamma 1-gamma 4 heavy chain). In rodents, these are designated IgG1
(gamma 1), IgG2a (gamma 2a), IgG2b (gamma 2b), and IgG3 (gamma 3)
Immunological memory. Acquired state of the immune system after
first contact with antigen, whereby the reaction upon subsequent
contact is faster, more intense, and of higher affinity. For antibody
response, associated with an immunoglobulin class switch and 'affinity
maturation' (by somatic mutation)
Immunosuppression. Prevention or diminution of the immune response
by administration of antineoplastic or antimetabolic drugs,
antilymphocyte serum, or exposure to e.g. environmental chemicals or
microorganisms (viruses)
Immunotoxicant. Drug, chemical, or other agent that is toxic to
cells or other components of the immune system
Inducer (helper) T cell. See Helper (inducer) T cell
Inflammation. Process whereby blood proteins or leukocytes enter
tissue in response to or in association with infection or tissue
injury
Inflammatory cell. General description of cells in an inflammatory
infiltrate; in acute inflammation, mainly polymorphonuclear
leukocytes; in chronic inflammation, mainly lymphocytes and
macrophages
Innate immunity. State of protection against pathogen-induced
injury; does not require previous immunization or vaccination
Innocent bystander. Cell or tissue component that is destroyed by an
immunological reaction specifically directed against a unrelated
antigen
Integrin. Family of heterodimeric molecules sharing a ß chain (ß1,
ß2, ß3, about 750 amino acids long), each with a different alpha chain
(about 1100 amino acids long), with a major function in cell adhesion
and migration. Form a protein family rather than a superfamily on the
basis of strong structural and functional similarities. Examples:
leukocyte function-related antigen LFA-1 (alpha L/ß1, CD11a/CD18;
receptor for intercellular adhesion molecules ICAM-1, ICAM-2, and
ICAM-3); Mac-1 (alpha M/ß2, CD11b/CD18; complement C3 receptor, CR3);
p150,95 (alpha X/ß2, CD11c/CD18); very late antigens VLA-1
(alpha 1/ß1, CD49a/CD29; laminin, collagen receptor), VLA-2
(alpha 2/ß1, CD49b/CD29; laminin, collagen receptor), VLA-3
(alpha 3/ß1, CD49c/CD29; laminin, collagen, fibronectin receptor),
VLA-4/LPAM-1 (alpha 4/ß1, CD49d/CD29; receptor for fibronectin and
VCAM-1), VLA-5 (alpha 5/ß1, CD49e/CD29, fibronectin receptor), and
VLA-6 (alpha 6/ß1, CD49f/CD29; laminin receptor, and alpha V/ß1,
CD51/CD29; vitronectin receptor); LPAM-2 (alpha 4/ßp, CD49d/.., or
alpha 4/ß7)
Interdigitating dendritic cell. Leukocyte belonging to the monocyte-
macrophage cell lineage, present in T-cell areas in lymphoid organs;
has a major function in presentation of antigen (MHC class
II-restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures called Birbeck granules. Its
equivalent in epidermis is the Langerhans cell, and that in lymph, the
veiled macrophage.
Interferon. Low-relative-molecular-mass substance produced mainly
during viral infection by leukocytes (IFN alpha), fibroblasts (IFNß),
and lymphocytes (IFN gamma); has a major function in interfering with
viral replication
Interferon-alpha. Produced by leukocytes; stimulates B cells to
proliferate and differentiate; stimulates natural killer cells and
increases cytotoxic T cell generation, but blocks T-cell proliferation
and lymphokine-activated killer activity; stimulates macrophage
accessory activity and enhances Fc receptor expression and MHC class I
and II expression on various cell types; induces antiviral state in
cells and is cytostatic for tumour cells, inhibits fibroblast and
adipocyte differentiation, and enhances promyelocytic and monoblastic
cell differentiation
Interferon-ß. Produced by fibroblasts and epithelia; activity
similar to that of IFN alpha
Interferon-gamma. Produced by T cells; induces antiviral state and
is cytostatic for tumour cells; enhances MHC class I and II expression
on various cell types, is antagonistic to interleukin-4 in IgE/IgG1
synthesis, and stimulates IgG2a synthesis; activates macrophages to
become cytolytic and enhances natural killer and lymphokine-activated
killer activity.
Interfollicular area. Area between follicles in lymphoid tissue,
where mainly small T lymphocytes are lodged; recognized by presence of
high endothelial venules. In lymph nodes, located in the outer cortex
and continuous with the paracortex
Interleukin. Immunoregulatory protein, also known as lymphokine,
monokine, interferon, or cytokine. Generally, low relative molecular
mass (< 80 kDa) and frequent glycosylation; regulates immune cell
function and inflammation by binding to specific cell surface
receptors; transient and local production; acts in paracrine or
autocrine manner, with stimulatory or blocking effect on growth and
differentiation; very potent, functions at picomolar concentrations.
Represents an extensive series of mediators (interleukins 1-12), with
a wide range of overlapping functions. Other mediators in this series
are c-kit ligand, interferon, tumour necrosis factor, and transforming
growth factor
Interleukin 1. Comprises two forms, IL-1 alpha and IL-1ß; produced
mainly by cells of the mononuclear phagocytic system (macrophages),
astrocytes, endothelium, and some epithelia, following stimulation by
e.g. microorganisms, immune complexes, or particulate compounds.
IL-1 alpha is mainly cell-associated; IL-1ß is released. IL-1 has
(together with IL-6 and tumour necrosis factor) multiple effects in
the systemic acute-phase response and in local acute and chronic
inflammation: it stimulates T (helper) cells to synthesize IL-2 and
IL-2 receptors, interferon gamma, and other lymphokines, B cells
(proliferation and differentiation), neutrophils, and natural killer
cells; stimulates monocytes and macrophages to produce IL-1, IL-6, and
tumour necrosis factor; acts in the acute-phase response by inducing
synthesis of acute-phase proteins in liver and reducing cytochrome
P450 synthesis; induces natriuresis in kidney, insulin production in
pancreas ß cells, muscular proteolysis ('easy' energy generation) in
muscle cells, slow-wave sleep in cerebral cortex; raises the
temperature set-point (fever) in hypothalamus; stimulates
haematopoiesis and prostaglandin synthesis by various cell types
(fibroblasts, macrophages, endothelium); inhibits gastric motility
in vitro ; induces collagenase production by synovial cells and
osteoclasts, and antiviral state; inhibits gastric smooth muscle
in vitro ; is cytostatic for tumour cells and activates endothelium
Interleukin 2. Synthesized by T helper cells after activation;
stimulates (autocrine) T cells to divide and release mediators,
B cells to proliferate and differentiate; activates monocytes and
natural killer cells; stimulates lymphokine-activated killer cells;
promotes generation of T helper 1 cells.
Interleukin 3. Formerly called multi-colony-stimulating factor;
synthesized by T helper cells; promotes growth of pluripotent
haematopoietic progenitor cells to granulocytes (eosinophilic,
basophilic, neutrophilic), mast cells, macrophages, megakaryocytes,
and, together with erythropoietin, to normoblasts and erythrocytes;
activates eosinophils and mast cells; stimulates haematopoiesis and
B-cell differentiation; blocks lymphokine-activated killer cells
Interleukin 4. Formerly called B-cell growth factor or B-cell
stimulating factor; synthesized by T helper and B cells; stimulates
IgE and IgG1 production by B cells and enhances MHC class II and IgE
receptor expression on B cells; acts in synergism with IL-2 in killer
cell generation, is mitogenic for T cells, and activates macrophages.
It is the dominant interleukin in generating T helper 2 cells, with a
negative feedback on the generation of T helper 1 cells.
Interleukin 5. Formerly called T-cell replacing factor or B-cell
growth and differentiation factor II; synthesized by T helper cells;
activates B cells and eosinophils, and stimulates IgA production by
B cells
Interleukin 6. Formerly called interferon ß2; synthesized by T
cells, monocytes, endothelial cells, fibroblasts, and smooth muscle
cells, among others, during inflammatory reactions; stimulates T and B
cells to proliferate and differentiate; has properties similar to IL-1
and acts synergistically with it in the acute-phase response (fever,
synthesis of acute-phase proteins); synergizes with IL-3 in promoting
haematopoietic progenitor cell proliferation; inhibits production of
IL-1 and tumour necrosis factor by monocytes
Interleukin 7. Formerly called lymphopoietin; synthesized by bone-
marrow stroma; induces growth of immature T and B lymphocytes
Interleukin 8. Formerly called neutrophil-activating protein;
synthesized by monocytes and various tissue cells in response to
inflammatory stimuli; performs chemotaxis of neutrophilic granulocytes
and subsequent granule exocytosis and respiratory burst; induces
increased expression of adhesion molecules CD11b/CD18 (complement C3
receptor CR3) and promotes vascular leakage. Endothelium-derived IL-8
inhibits adhesion of neutrophilic granulocytes induced by IL-1
Interleukin 9. Synthesized by T lymphocytes; stimulates growth of
erythroid and megakaryocyte precursors and promotes (mucosal) mast-
cell growth; acts synergistically with IL-4 in modulating IgE and IgG
production
Interleukin 10. Synthesized by T and B lymphocytes; inhibits
mediator synthesis (IL-2, IL-3, tumour necrosis factor, interferon
gamma, granulocyte-macrophage colony-stimulating factor) by T helper
1 cells, inhibits mediator synthesis (IL-1 alpha, IL-1ß, IL-6, IL-8,
and tumour necrosis factor alpha) by monocytes; stimulates IL-2-
dependent growth and cytotoxicity of cytotoxic T cells and stimulates
mast cell growth together with IL-2 or IL-3 and IL-4; induces MHC
class II antigen expression on B cells, but down-regulates MHC class
II on monocytes; promotes generation of T helper 2 cells
Interleukin 11. Synthesized by fibroblasts and bone-marrow stromal
cells; resembles IL-6 in function: stimulates haematopoietic cell
growth and differentiation (myeloid, erythroid, megakaryocyte
lineage); enhances T-cell-dependent antibody response; and suppresses
adipocyte differentiation and lipoprotein lipase production
Interleukin 12. Also called natural killer cell stimulatory factor;
synthesized by monocytes-macrophages, B cells, and accessory cells, in
response to bacteria or parasites; stimulates T-lymphocyte
proliferation, activates natural killer cells, and stimulates
lymphokine-activated killer activity; synergizes with IL-2 in
activation of cytotoxic lymphocytes; induces production of interferon
gamma and other cytokines by lymphocytes. It is the dominant
interleukin in generating T helper 1 cells and has a negative feedback
on the generation of T helper 2 cells.
in vitro. In the context of this book, exposure of cells or cell
systems to the immunotoxic agent in vitro. If the donors of cells or
cell systems are exposed but these are analysed in vitro, the term
ex vivo/in vitro is used
Isohaemagglutinin. Antibodies mainly of the IgM class that react
with (carbohydrate) antigens on erythrocytes from individuals of the
same species, resulting in agglutination in vitro
Isologous. Synonym for isogeneic
Isotype. Antigenic determinant that defines class or subclass of
immunoglobulin molecules
J (joining) chain. A 15-kDa polypeptide chain that acts
intracellularly to combine (identical) IgA or IgM immunoglobulin
units, consisting of two heavy and two light chains, to form a dimeric
IgA or a pentameric IgM molecule
J (joining) genes. Genes that encode the variable part of
immunoglobulin or T-cell receptor chains (e.g. JH1-JHn for
immunoglobulin heavy chains, Jkappa 1-Jkappa n for immunoglobulin
kappa light chain, Jalpha 1-Jalpha n for T-cell receptor alpha
chain)
Kallikrein (kininogenase). Arises in tissue fluids after cleavage of
prekallikrein; acts on kininogen to produce kinins, resulting in
immediate hypersensitivity reaction, e.g. vasodilatation and oedema.
It is a preformed mediator present in mast cell granules
kappa Chain. In immunoglobulin molecules, the kappa light chain
forms the light chain type of the molecule
Keratinocyte. Epithelial cell in the epidermis; in some
circumstances, can manifest antigen presentation and produce
immunoregulatory cytokines; hence belongs to the skin immune system
Killer cell (K cell). See Cytotoxic cell
Kinin system. Humoral amplification system involved in inflammation,
whereby substrate proteins become active after enzymatic cleavage;
cause vasodilatation, increased vascular permeability, hypotension,
and contraction of smooth muscle
c-Kit ligand. Also called stem cell growth factor or mast cell
growth factor; synthesized by various stromal cells, fibroblasts, and
liver cells; stimulates growth of early pluripotent progenitor cells
and that of myeloid, erythroid, and lymphoid progenitors in synergy
with interleukin-1, -3, -6, -7, and granulocyte-macrophage colony-
stimulating factor; promotes growth of mast cells
Kupffer cells. Macrophages on or between endothelial cells lining
the sinusoids of the liver
lamba Chain. In immunoglobulin molecules, the lamba light chain
forms the light chain type of the molecule
Lamina propria. Thin layer of connective tissue under the villous
epithelium of the gastrointestinal tract; site of plasma cells,
producing mainly dimeric IgA, including J chain
Langerhans cell. Leukocyte belonging to the monocyte-macrophage cell
lineage, present in skin epidermis; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II
restricted) to T helper lymphocytes. Cytoplasm contains characteristic
rod-like structures, Birbeck granules. Its equivalent in lymphoid
tissue is the interdigitating dendritic cell, and that in lymph,
veiled macrophage; forms part of the skin immune system
Large granular lymphocyte. Intermediate-sized, 10-12-µm lymphocyte
with a kidney-shaped nucleus and prominent, large, azurophilic
granules in the cytoplasm; occurs in the circulation and in tissue and
has a major function as a natural killer cell; forms a heterogeneous
population with either T markers or monocyte-macrophage markers.
Lectin. Plant-derived substance that binds to lymphocytes and can
induce cell proliferation; some also bind to other haematopoietic
cells
Leukaemia. Neoplasia of lymphoid cells in blood or bone marrow
Leukocyte. Bone marrow-derived white blood cell, including cells in
the lymphoid, myeloid, and monocyte lineages; sometimes used to
describe only granulocytes
Leukocytosis. Increased proportion of leukocytes in blood
Leukopenia, leukocytopenia. Reduced proportion of leukocytes in
blood
Leukotriene. Formerly called slow-reacting substance of anaphylaxis;
products of arachidonic acid metabolism following the lipoxygenase
pathway, which act as mediators in the immediate hypersensitivity
reaction, mainly as chemoattractants for granulocytes and monocytes,
and in smooth muscle contraction; newly synthesized by mast cells upon
activation
L (light) chain. One of the 22-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and a
constant domain. The light chain, either kappa or lamba, determines
the light chain type of the immunoglobulin molecule
Ly antigen. T Lymphocyte antigen in mice
Lymph. Fluid in lymphatic vessels
Lymph node. Secondary (peripheral) lymphoid organ, the main function
of which is to filter lymphatic vessels. Present throughout the body,
at connecting places of lymphatics and blood vessels; forms a major
site of encounter between pathogenic substances in the lymph and
lymphocytes entering from blood vessels, and subsequent initiation of
antigen-specific immunological reactions
Lymphatic. Vessel that collects fluid from interstitial spaces and
goes via lymph nodes (filtering) to the thoracic duct and blood
Lymphocyte. Cell belonging to the lymphoid lineage of bone marrow-
derived haematopoietic cells. In a restricted way, the designation of
a small, resting or recirculating mononuclear cell in blood or
lymphoid tissue, which measures about 7-8 µm, has a round nucleus
containing densely aggregated chromatin, and little cytoplasm. Plays a
key role in immune reactions by specific recognition of antigens
Lymphocytosis. Increased proportions of lymphocytes in blood
Lymphoid organ. Tissue in the body where cells of the immune system,
mainly lymphocytes, are lodged in an organized microenvironment,
either in a resting state or in a state of activation,
differentiation, or proliferation. Includes bone marrow, thymus, lymph
nodes, spleen, and mucosa-associated lymphoid tissue
Lymphokine. Hormonal substance synthesized by lymphocytes, which
modulates the function of cells in immunological reactions
Lymphoma. Neoplasia of lymphoid cells in tissue
Lymphopenia, lymphocytopenia. Reduced proportions of lymphocytes in
blood
Lymphotoxin. Former name for tumour necrosis factor ß; lymphokine
synthesized by T lymphocytes, which kills selected target cells
Lysosome. Granule present in many cell types that contain hydrolytic
enzymes; also performs intracellular degradation of pathogens after
phagocytosis
Lysozyme (muramidase). A low-relative-molecular-mass, cationic
enzyme present in tissue fluids and secretions, which degrades
mucopeptides of bacterial cell walls
Macroglobulin. Glycoprotein of relative molecular mass > 200 kDa
Macrophage. Large, 12-20-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in tissue, and forming the
mononuclear phagocytic system. Its reniform nucleus usually has
pronounced peripheral condensation of nuclear chromatin; its cytoplasm
contains a great variety of cell organelles, including rough
endoplasmic reticulum, mitochondria, ribosomes, lysosomes, and Golgi
complex. Has a major function in (chronic) inflammatory reactions, by
virtue of its phagocytic capacity, with immunoglobulin Fc and
complement C3 receptors which bind to immune complexes. Macrophages
develop into killer cells after activation by e.g. T-cell factors and
can mediate antibody-dependent cell-mediated cytotoxicity; also
functions as an accessory cell in induction of immune responses
(antigen presentation, mediator secretion). Macrophages in blood are
called monocytes. Subtypes with special functions are interdigitating
dendritic cells: T-cell area of lymphoid tissue, Langerhans cell
(skin), Kupffer cells (liver), metallophilic macrophages (spleen),
microglia (brain), osteoclasts (bone), tingible body macrophage
(starry-sky macrophage), veiled macrophage (lymph).
Macrophage colony-stimulating factor. Synthesized mainly by
endothelial cells and fibroblasts, and possibly macrophages; supports
growth of monocyte-macrophage progenitors.
Major histocompatibility complex (MHC). Set of genes that codes for
tissue compatibility markers, which are targets in allograft rejection
and hence determine the fate of allografts; plays a central role in
control of cellular interactions during immunological reactions.
Tissue compatibility is coded by classes I and II loci (see Class I
and Class II MHC molecule). Genes within or closely linked to MHC
control certain complement components (MHC class III genes). The MHC
complex of humans is HLA, that of mice H-2, and that of rats, RT-1.
Mantle (corona). Zone in secondary follicles surrounding the central
germinal centre, densely packed with small resting B lymphocytes
Marginal zone. Outer layer of white pulp in spleen, surrounding
follicles, and periarteriolar lymphocyte sheath; separated from these
by the marginal sinus; populated by intermediate-sized, slightly
pyroninophilic B cells which have a major function in the T cell-
independent antibody response. The microenvironment manifests a
special type of macrophage, the marginal metallophilic macrophage
Margination. Adherence of blood leukocytes to endothelium during
inflammatory reactions
Mast cell. A bone marrow-derived polymorphonuclear leukocyte present
in tissue; has a major function in immediate hypersensitivity
reactions; has a round or oval nucleus and abundant cytoplasm with
basophilic (blue) granules stained by metachromatic dyes; granules
contain mediators of immediate hypersensitivity reactions, e.g.
heparin, histamine, serotonin, tryptase, kallikrein, and
chemoattractants for neutrophilic and eosinophilic granulocytes; has a
high-affinity receptor for IgE. After activation (physical stimuli,
cross-linking via allergen-IgE-IgE receptor), there is immediate
granule release and synthesis of other mediators, including
prostaglandins, thromboxanes, leukotrienes, and platelet-activating
factor. The cell can exert modulatory activity by secreting cytokines
such as IL-3, IL-6, and tumour necrosis factor. Two subtypes exist, in
the mucosa and in connective tissue; the equivalent in the circulation
is the basophilic granulocyte
Mast cell growth factor. See Interleukin 9
Medulla. Inner parenchymal layer of organs
Medullary cord. Parenchyma in medulla of lymph nodes separating
lymphatic sinusoids
Megakaryocyte. Large, multinucleated giant cell, precursor of blood
platelets, formed by separation of portions of membrane-bound
cytoplasm; occurs in haematopoietic tissue, including bone marrow
Memory. See Immunological memory
Mesoderm. Middle of the three cellular layers of the embryo;
produces connective tissue and blood cells
Metallophilic macrophage. Subtype of macrophage identified by silver
impregnation, present at the inner border of the marginal zone in
spleen
MHC restriction. Immunological reactions can occur only in
associated recognition with the polymorphic determinant of a given MHC
molecule and not with that of another MHC molecule. Applies to T
lymphocytes with an alpha-ß T-cell receptor, which recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules, and part of the T cell population with the gamma-delta
T-cell receptor.
Microglia. Macrophages in central nervous system
Migration inhibitory factor. A lymphokine that inhibits the movement
of macrophages
Milky spot. Aggregate of lymphoid cells in omentum, macroscopically
visible as a small white spot; not organized tissue but rather the
product of immune stimulation in that area of the body
Minor histocompatibility antigen. Ill-defined histocompatibility
marker not encoded by the MHC, which is a target in allograft
reactions (apart from products of the MHC)
Mitogen. Substance that activates resting cells to transform and
proliferate
Monoclonal. Derived from a single clone. For T and B lymphocytes, a
cell population in which all cells have a distinct V-D-J gene
rearrangement product (as seen in lymphoma and leukaemia). Monoclonal
antibodies are products of hybridomas prepared after fusion of
antibody-producing cells and a transformed (non-producing)
plasmacytoid cell line.
Monocyte. Large, 10-15-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in the blood and in lymphatic
vessels
Monokine. Hormonal substance synthesized by monocytes-macrophages;
modulates the function of cells in immunological reactions
Mononuclear cell. Leukocyte with a single rounded nucleus, e.g.
lymphocytes and monocytes-macrophages
Mononuclear phagocytic system. Formerly called reticuloendothelial
system; composite of phagocytic cells in the body, including monocytes
and tissue macrophages. Main populations are Kupffer cells in liver,
microglia in the central nervous system, macrophages in red pulp of
spleen, alveolar macrophages in lung, and, after induction, peritoneal
macrophages in the peritoneal cavity; others are mesangial macrophages
in kidney and osteoclasts in bone
µ Chain. In immunoglobulin molecules, the µ heavy chain forming the
IgM immunoglobulin class
Mucosa-associated lymphoid tissue. Lymphoid tissue or organs in
immediate contact with the mucous-secreting mucosal layer in nasal
cavity and nasopharynx (nasal-associated lymphoid tissue), airways
(bronchus-associated lymphoid tissue), and intestinal tract (gut-
associated lymphoid tissue). Serves as the immunological defence at
secretory surfaces, to some extent independent of the systemic
(internal) response; includes IgA synthesis by plasma cells in the
lamina propria and excretion into the lumen
Multiple myeloma. Tumour of plasma cells in bone marrow
Muramidase. See Lysozyme
Myeloblast. Immature precursor cell in the lineage of polymorpho-
nuclear cells (granulocytes, mast cells)
Nasal-associated lymphoid tissue. Lymphoid organs or tissue located
in the nasal cavity and nasopharynx, presumed to be a first location
for presentation of antigens entering through the nose; contributes to
the mucosa-associated lymphoid tissue
Natural antibody. Antibody in serum of individuals with no previous
exposure to the corresponding antigen; often generated by contact with
cross-reacting agents, e.g. bacterial products; often restricted to
antibodies that react to xenogeneic antigens
Natural killer cell. Leukocyte with a limited repertoire to
recognize antigen; can kill target cells without prior sensitization;
can be of lymphoid or monocyte-macrophage origin. Large granular
lymphocytes are the main population
Necrosis. Death of tissue and cells
Neoantigen. New antigen appearing on cells or tissue during
malignant transformation or (viral) infection
Neoplasia. Uncontrolled malignant transformation of cells resulting
in tumour formation
Neutralization. Process whereby a pathogenic substance becomes
inactivated by effector components (antibodies) of the immunological
reaction
Neutropenia. Reduced proportions of neutrophilic granulocytes in
blood
Neutrophil chemotactic factor. Preformed mediator with a relative
molecular mass > 750 kDa, present in granules of mast cells and
basophilic granulocytes; released after activation and attracts
neutrophilic granulocytes to the site of inflammation or
hypersensitivity
Neutrophilic granulocyte. Polymorphonuclear leukocyte that contains
granules stained by neither acidophilic nor basophilic dyes; can
phagocytose immune complexes by receptor-mediated endocytosis,
followed by intracellular degradation in lysosomes. Degranulation
releases catepsins and lysosomal enzymes, resulting in tissue damage.
Nonspecific immunity. Immunity induced by non-immunological
mechanisms, for instance by action of complement, lysozyme,
phagocytosis, or interferon
Oedema. Swelling of tissue due to extravasation of fluid from the
intravascular space following increase in vascular permeability
Ontogeny. Life cycle of an organism; in immunological terms, often
used to describe the process whereby the immune system develops
immunocompetence
Opsonization. Adherence of pathogen to phagocytic cell due to action
of antibody or activated complement
Osteoclast. Macrophage in bony tissue involved in bone resorption
Paracortex. Area in the inner cortex of lymph node where T
lymphocytes are lodged; recognized by the presence of high endothelial
venules; continuous with interfollicular areas in the outer cortex on
one side and with the medullary cords on the other
Paratope (antigen-binding site). Part of antibody that binds antigen
(antigenic determinant, or epitope); part of T-cell receptor that
binds the complex of antigen and MHC molecule
Periarteriolar lymphocyte sheath. Area in white pulp of spleen
surrounding the central artery, populated mainly by small
T lymphocytes
Peripheral (secondary) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of these
reactions
Peyer's patch. Lymphoid tissue in wall of small intestine,
particularly ileum, separated from the gut lumen by a domed area and
an epithelial layer ('dome' epithelium); forms part of gut-associated
lymphoid tissue; main function is initiation of immunological
reactions towards pathogens entering through dome epithelium
Phagocytosis. Uptake of material >1 µm by cells, by receptor-
mediated endocytosis, by cells of the mononuclear phagocytic system;
requires Fc receptors, with accessory help of complement receptors; is
blocked by cytochalasins. Occurs via a 'zipper' mechanism, in which
the opsonized particle (coated with antibody or complement) becomes
enclosed by the cell membrane of the phagocyte; a second mechanism
involves oxidative burst, with formation of superoxide anion, peroxide
anion, and hydroxyl radicals, which kill or degrade the phagocytosed
particle
Phagolysosome. Membrane-bound cytoplasmic vesicle formed by fusion
of a phagosome and a lysosome
Phagosome. Membrane-bound vesicle in phagocytic cells containing
phagocytosed material
Pharmacokinetics. Fate of drugs or chemicals in the body over time,
including the processes of absorption and distribution in tissues,
biotransformation, and excretion
Phenotype. Characteristic of a distinct cell or individual,
reflecting the expression of a genetically determined property
Phenotypic marker. Expressed characteristic(s), for instance an
antigenic determinant, of a given cell or molecule, associated with
function or specificity
Phylogeny. Evolutionary history of a particular species
Pinocytosis. Uptake of material < 1 µm by cells; often restricted
to a receptor-mediated process in leukocytes of the monocyte-
macrophage series, e.g. uptake of lipoproteins and viruses into
clathrin-coated vesicles
Plasma. Fluid of uncoagulated blood after removal of cells
Plasma cell. Terminally differentiated B lymphocyte that synthesizes
and secretes immunoglobulin; these medium-sized, 10-15-µm cells have a
small excentric nucleus, with heterochromatin organized in a
'cartwheel'-like structure, and abundant cytoplasm filled with rough
endoplasmic reticulum.
Platelet. Small bone marrow-derived cytoplasmic fragment in blood
responsible for coagulation; main role is to block damaged vessel
walls and prevent haemorrhage, by clumping and aggregation; contains
heparin and serotonin, which contribute after release to the acute
vascular response in hypersensitivity reactions and produce oxygen
radicals
Platelet-activating factor. Low-relative-molecular-mass phospholipid
generated from alkyl phospholipids in mast cells, basophilic and
neutrophilic granulocytes, and monocytes-macrophages, which mediates
microthrombus formation of platelets in hypersensitivity reactions
Polyclonal. Derived from many different clones; for T and B
lymphocytes, cell populations in which the cells have different V-D-J
gene rearrangement products. Polyclonal activation is stimulation of
multiple lymphocyte clones, resulting in a heterogeneous response
Polymorphonuclear granulocyte. Leukocyte of bone-marrow origin, with
a lobulated nucleus, involved in acute inflammatory reactions. Main
subsets are basophilic, eosinophilic, and neutrophilic granulocytes
(different cytoplasmic granule colours after haematological staining).
Contributes to (acute) inflammatory reactions after attraction by
specific (immune complex-mediated) or nonspecific stimuli (including
complement components); after activation, releases granules containing
various hydrolytic enzymes
Postcapillary venule. Small blood vessel though which blood flows
after leaving the capillaries before reaching veins; often the site
where inflammatory cells leave the circulation and enter the tissue
Primary (central) lymphoid organ. Lymphoid organ where precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment to form immunocompetent cells; not antigen-driven but
can be influenced by mediators produced as a result of antigen
stimulation
Primary follicle. See Follicle
Primary response. Immunological reaction after first contact with
antigen, resulting in generation of immunological memory
Programmed cell death. See Apoptosis
Prostaglandin. Aliphatic acid produced by arachidonic acid
metabolism following the cyclo-oxygenase pathway; synthesized by mast
cells after activation; mediates immediate hypersensitivity reactions,
mainly smooth muscle contraction or bronchoconstriction; also
decreases the threshold for pain
Prothymocyte. Precursor of T lymphocytes in bone marrow before
moving to the thymus, or present in the thymus just before intrathymic
processing
Pyrogen. Substance that increases the temperature in the central
nervous system, resulting in fever; examples are bacterial endotoxin
and IL-1
Reactive oxygen intermediate. Reactive species of oxygen produced
e.g. by phagocytes (granulocytes and monocyte-macrophages) in response
to phagocytic stimuli like bacteria
Reagin. Former designation of IgE class antibody
Recall antigen. Antigen used to elicit a response from an individual
already sensitized to that antigen; may be one that the host has
knowingly been sensitized to or, in humans, one that it is assumed
that most individuals have been sensitized to
Red pulp. Area in spleen comprising venous sinuses filled with blood
and splenic cords; venous sinuses mainly contain erythrocytes
surrounded by endothelial cells; cords comprise macrophages,
lymphocytes, and occasionally megakaryocytes, but other types of blood
cells can also be present. Main function is phagocytosis of
particulate material and removal of old erythrocytes from blood. In
rodents, the red pulp can also be a site of haematopoiesis.
Reticuloendothelial system. See Mononuclear phagocytic system
Repertoire. All specific antigen-recognizing capacities (diversity)
within a population of T or B lymphocytes
RT-1. The major histocompatibility complex of rats
Secretory immunoglobulin. Immunoglobulin encountered in secretions
like tears, saliva, and jejunal juice; concerns mainly secretory IgA,
a dimer of the basic four-chain immunoglobulin structure, linked by a
J chain and surrounded by a secretory piece molecule.
Secretory piece. A 70-kDa molecule produced by epithelial cells
covering mucosa-associated lymphoid tissue; functions as a receptor
for IgA or IgM, thereby facilitating intercellular transport of these
molecules into the lumen. During this process, the secretory piece
becomes associated with the immunoglobulin, thereby enhancing its
stability in nonphysiological conditions of secretory fluid
Secondary follicle. See Follicle
Secondary (peripheral) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of those
reactions
Secondary response. Response after first contact (immunization,
primary response) with an antigen, based on the presence of
immunological memory; characterized as faster, more intense, and of
higher affinity; for the antibody response, associated with an
immunoglobulin class switch
Selectin. Cell surface glycoprotein that has a prominent function in
the interaction between lymphocytes, monocytes, neutrophilic
granulocytes, and endothelium. They share an N-terminal domain of
approximately 120 amino acids that is homologous to many Ca2+-
dependent animal lectins and binds to carbohydrates. Examples are
L-selectin (MEL-14, LAM-1, present on leukocytes; adherence of
endothelial cells, role in lymphocyte recirculation and neutrophil and
leukocyte inflammation); E-selectin (ELAM-1, present on endothelium;
adherence of monocytes, neutrophils, and T cells; role in
inflammation); P-selectin (PADGEM, GMP-140, CD62, present on platelets
and endothelium; adherence of monocytes, neutrophils, and T cells;
role in inflammation).
Self-MHC restriction. See MHC restriction; applies to MHC
molecules of the individual
Sensitization. Induction of specialized immunological memory in an
individual by exposure to antigen
Serotonin. 5-Hydroxytryptamine; catecholamine with relative molecular
mass of 176 Da; preformed mediator of immediate hypersensitivity
reactions, present in granules of mast cells and in platelets. After
activation, is released and mediates vasodilatation and increased
vascular permeability
Serum. Fluid of blood after coagulation (removal of fibrinogen) and
removal of cells
Serum sickness. Systemic vasculitis, glomerulonephritis, or arthritis
due to immune complex formation after the reaction between antibody and
injected foreign antigen (serum)
Skin-associated lymphoid tissue. See Skin immune system
Skin immune system. Combination of immune system components and
their function, present in skin; antigen presentation by Langerhans
cells, by dendritic epidermal cells, and in some conditions by
keratinocytes; immunoregulation by e.g. keratinocyte-derived
cytokines, and distinct dermatotropic T-cell populations
Slow-reacting substance of anaphylaxis. See Leukotriene
Somatic mutation. Small changes in genes resulting in alterations in
amino acids built into protein chains. For immunoglobulin molecules,
changes in diversity of antigen-binding site (variable region)
Spleen. Lymphoid organ in the left abdominal cavity, for filtering
blood; main function is phagocytosis of particles from blood, removal
of old erythrocytes in red pulp, and initiation of immunological
reactions in white pulp. The marginal zone of the white pulp serves as
the main site of T cell-independent antibody formation.
Starry-sky macrophage. See Tingible body macrophage
Stem cell. Multipotential, self-renewing precursor cell of all
haematopoietic cell lineages, present in bone marrow
Stem-cell growth factor (synonym for c-Kit ligand). An interleukin
that supports continuous growth of mast cells and augments the
response of progenitor cells to stem growth factors; interacts via
c-kit proto-oncogene
Subcapsular sinus. Area in lymph node just under the capsule and
surrounding the cortex, which is connected with afferent lymphatics,
and through cortical (peritrabecular) sinuses with medullary sinuses;
contains dendritic macrophages
Superantigen. Antigenic moiety that, in MHC-restricted presentation
to T lymphocytes, is not present in the 'groove' made by the
quaternary structure of the MHC molecule but is complexed with the MHC
molecule. Examples are the endogenous viral-encoded Mlsa (minor
lymphocyte stimulatory) antigen, which is present in certain mouse
strains, and Staphylococcus enterotoxin A
Suppressor T cell. Subpopulation of T lymphocytes with CD8 phenotype;
after recognition of antigen in an MHC class I-restricted manner,
suppresses immunological reactions, in part by cytotoxic activity
Systemic lupus erythematosus. Chronic, remitting, relapsing,
inflammatory, and often febrile multisystemic disorder of connective
tissues, with possible involvement of the central nervous system,
skin, joints, kidneys, and serosal membranes; can be acute or
insidious in onset. The etiology is unknown but is thought to follow a
failure of the regulatory mechanisms of the immune system that sustain
self-tolerance. Many drugs and chemicals can induce lupus-like
symptoms (drug-induced lupus erythematosus)
T-Cell receptor. Heterodimeric molecule on the surface of the T
lymphocyte that recognizes antigen. The polypeptide chains have a
variable and a constant part, and can be alpha, ß, gamma, or delta.
The alpha-ß T-cell receptor occurs on most T cells and recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules (self-MHC restricted). The gamma-delta T-cell receptor
occurs on a small subpopulation, e.g. in mucosal epithelium, and can
recognize antigen in a non-MHC restricted manner. The T-cell receptor
occurs exclusively with the CD3 molecule, which is thought to mediate
transmembrane signalling.
T-Dependent antigen. Antigen for which antibody formation requires
T cells.
Terminal pathway of complement activation. Activation of complement
components C6-C9, with formation of the membrane attack complex and
subsequent lysis of the cell
Tingible body macrophage (starry-sky macrophage). Large macrophage
in cortex of thymus and germinal centres of follicles in lymphoid
tissue, filled with condensed nuclear material with high affinity for
dyes; has a major function in phagocytosis, presumably of apoptotic
cells
T Lymphocyte or cell. Lymphocyte that induces, regulates, and
effects specific immunological reactions after stimulation by antigen,
mostly in the form of processed antigen complexed with MHC product on
an antigen-presenting cell. They originate from precursors in the bone
marrow and undergo maturation in the thymus (T, thymus-dependent).
Most T lymphocytes recognize antigen by a heterodimeric alpha-ß
surface receptor molecule associated with the CD3 molecule, which
mediates transmembrane signalling. Subsets include helper-inducer and
suppressor-cytotoxic cells.
T-Lymphocyte area. That part of a lymphoid organ or tissue that is
occupied by T lymphocytes, e.g. paracortex or interfollicular area in
lymph node, periarteriolar lymphocyte sheath in spleen
Thrombocyte. See Platelet
Thrombocytopenia. Reduced proportion of platelets in blood
Thromboxane. Product of arachidonic acid following the cyclo-
oxygenase pathway; synthesized by mast cells after activation and
mediates immediate hypersensitivity reactions, mainly smooth muscle
contraction, bronchoconstriction, and platelet aggregation
Thymocyte. Lymphocyte in the thymus
Thymoma. Tumour of the thymus; neoplastic cell is an epithelial cell
Thymus. Central lymphoid organ located dorsal to the cranial part of
the sternum in the thorax, comprising two lobes, each consisting of
many lobules. Its main function is generation of immunocompetent
T lymphocytes from prothymocytes from the bone marrow
Tolerance. State of unresponsiveness to antigenic stimulation, due
to the absence of responding elements or loss of capacity of existing
elements to mount a reaction. Synonym for anergy
Tolerogen. Antigen that evokes tolerance
Tonsil. Organized mucosa-associated lymphoid tissue in
oronasopharynx. Adenoids strictu sensu are also tonsils. The main
function is initiation of immunological reactions towards pathogens
entering through the mouth. Contributes in part to the gut-associated
lymphoid tissue. Together with lymphoid aggregates in oronasopharynx,
these tissues form the ring of Waldeyer
Transforming growth factor ß. Mediator synthesized by lymphocytes or
macrophages, with a function in down-regulation of immune reactions;
suppresses T- and B-lymphocyte growth, IgM and IgG production, and
down-regulates MHC class II expression; interferes with production of
tumour necrosis factor and adhesion of granulocytes to endothelial
cells; is chemotactic for monocytes and induces interleukin-1 and
interleukin-6 expression
Transudation. Transfer of fluid and low-relative-molecular-mass
proteins from intravascular to extravascular tissue during
inflammatory processes
Tryptase. Proteolytic enzyme present in granules of mast cells;
released after activation and activates complement component C3, with
formation of the anaphylatoxin C3a
Tumour necrosis factor. General mediator of inflammation and septic
shock; formerly named cachectin and lymphotoxin. Two forms: alpha and
ß, both produced by monocytes-macrophages, TNF-ß also by T lymphocytes
and natural killer cells. Has activity similar to interleukin-1 and
acts synergistically with it; promotes antiviral state and is
cytotoxic for tumour cells; stimulates granulocytes and eosinophils,
activates macrophages to interleukin-1 synthesis, stimulates B cells
to proliferate and differentiate, T cells to proliferate,interleukin-2
receptor synthesis, and interferon gamma synthesis; induces
fibroblasts to synthesize prostaglandin and proliferate; induces fever
and synthesis of acute-phase proteins; reduces cytochrome P450
synthesis; activates endothelium and promotes adherence of
neutrophilic granulocytes to endothelium; induces cell adhesion
molecules like lymphocyte function-associated antigens LFA-1 and
LFA-3, ICAM-1, and ELAM-1; inhibits gastric motility in vitro;
reduces lipoprotein lipase synthesis by adipocytes; and activates
osteoclasts to bone resorption
Urticaria. Transient eruption of skin characterized by erythematous
or oedematous swelling (wheal) of the dermis or subcutaneous tissue
Vaccination. See Immunization
Valency. Number of antigenic determinants or ligands that can bind
to one antibody molecule or receptor
V (variable) gene. Gene that encodes the variable part of
immunoglobulin or T-cell receptor chains (e.g. VH1-VHn for
immunoglobulin heavy chains, Vkappa 1-Vkappa n for immunoglobulin
kappa light chain, Valpha 1-Valpha n for T-cell receptor alpha
chain)
Variable gene family. Groups of germline V genes (which encode
immunoglobulin chains or T-cell receptor genes) that have more than
about 80% nucleotide sequence identity
V (variable) region. Region at the amino terminal of immunoglobulin
or T-cell receptor chains, which contributes to the antigen-binding
site of the molecule. Encoded by V (variable), D (diversity), and J
(joining) genes in DNA
Vasoconstriction. Contraction of capillary venules, resulting in
decreased blood flow
Vasodilatation. Dilatation of capillary venules, resulting in
increased blood flow through capillaries and lowering of local blood
pressure
Veiled macrophage. Leukocyte belonging to the monocyte-macrophage
lineage, present in lymph; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II-
restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures, Birbeck granules. Its equivalent
in lymphoid tissue is the interdigitating dendritic cell, and that in
skin is the Langerhans cell
Waldeyer's ring. Lymphoid tissue of tonsils and adenoids located
around the junction of the pharynx and oral cavity in humans and
domestic animals. Main function is initiation of immunological
reactions towards pathogens entering through the mouth. Contributes to
the gut-associated lymphoid tissue
White blood cell. Polymorphonuclear leukocyte, lymphocyte, or
monocyte in peripheral blood
White pulp. Area in spleen around central arterioles where lymphoid
cells reside. Comprises three major compartments: the periarteriolar
lymphocyte sheath, follicles, and marginal zone
Xenobiotic. Chemical or substance that is foreign to the biological
system
Xenogeneic. Genetically different phenotypes in individuals of
different species; opposite of allogeneic, or isogeneic
zeta Chain (see epsilon Chain). One of the chains in the CD3
molecule associated with the T-cell receptor
REFERENCES
Ada GL, Leung KN, & Ertl H (1981) An analysis of effector T-cell
generation and function in mice exposed to influenza A or Sendai
virus. Immunol Rev, 50: 5-24.
Ader, R, Felten D, & Cohen, N (1990) Interactions between the brain
and the immune system. Ann Rev Pharmacol Toxicol, 30: 561-602.
Adkins B, Richards JH, & Gardner DE (1979) Enhancement of experimental
respiratory infection following nickel inhalation. Environ Res,
20: 33-42.
Adkins B, Luginbuhl GH, Miller, FJ, & Gardner DE (1980) Increased
pulmonary susceptibility to Streptococcal infection following
inhalation of manganese oxide. Environ Res, 23: 110-120.
Adkins B, Gandour D, Strober S & Weissman I (1988) Total lymphoid
irradiation leads to transient depletion of the mouse thymic medulla
and persistent abnormalities among medullary stromal cells. J Immunol,
140: 3373-3379.
Adorini L ed. (1990) The molecular basis of antigen presentation to
T lymphocytes: Novel possibilities for immunointervention. Int Rev
Immunol, 6: 1-88.
Agius C (1985) The melanomacrophage centers of fish; a review.
In: Manning MJ & Tatner MF, eds, Fish Immunology. London, Academic
Press, pp 85-105.
Ahmed-Ansari A, Brodie AR, Fultz PN, Anderson DC, Sell KW, & McClure
HM (1989) Flow microfluorometric analysis of peripheral blood
mononuclear cells from nonhuman primates: Correlation of phenotype
with immune function. Am J Primatol, 17: 107-131.
Allen JR, & Abrahamson LJ (1973) Morphological and biochemical changes
in the liver of rats fed polychlorinated biphenyls. Arch Environ
Contam Toxicol, 1: 265-280.
Allen AL, Koller LD, & Pollock GA (1983) Effect of toxaphen exposure
on immune responses in mice. J Toxicol Environ Health, 11: 61-69.
Alomran, AH & Shleamoon, MN (1988) The influence of chronic lead
exposure on lymphocyte proliferative response and immunoglobulin
levels in storage battery workers. J Biol Sci Res, 19: 575-585.
Anderson DP (1990) Immunological indicators: Effects of environmental
stress on immune protection and disease outbreaks. Am Fish Soc Symp
8: 38-50.
Anderson RS & Brubacher LL (1993) Inhibition by pentachlorophenol of
production of reactive-oxygen intermediates by medaka phagocytic blood
cells. Marine Environ Res, 35: 125-129.
Anderson RE & Williams WL (1977) Radiosensitivity of T and B
lymphocytes. V. Effects of whole-body irradiation on numbers of
recirculating T cells and sensitization to primary skin grafts in
mice. Am J Pathol, 89: 367-378.
Anderson J, Moller G & Sjorberg O (1972) Selective induction of DNA
synthesis in T and B lymphocytes. Cell Immunol, 4: 381-393.
Anderson RE, Olson GB, Autry JR, Howarth JL, Troup GM ,& Bartels PH
(1977) Radiosensitivity of T and B lymphocytes. IV. Effect of whole
body irradiation upon various lymphoid tissues and numbers of
recirculating lymphocytes. J Immunol, 118: 1191-1200.
Anderson RE, Standefer JC, & Tokuda S (1986) The structural and
functional assessment of cytotoxic injury of the immune system with
particular reference to the effects of ionizing radiation and
cyclophosphamide. Br J Cancer, 53 (Suppl 7): 140-160.
André F, Gillon F, André C, Lafont S, & Jourdan G (1983) Pesticide-
containing diet augments anti-sheep red blood cell non reagenic
antibody responses in mice but may prolong murine infection with
Giardia muris. Environ Res 32: 145-150.
Anneroth G, Ericson T, Johannson I, Mornstad H, Ryberg M, Skoglund A,
& Stegmayr B (1992) Comprehensive medical examination of a group of
patients with alleged adverse effects from dental amalgam. Acta
Odontol Scand, 50: 101-107.
Applegate LA, Ley RD, Alcalay J, & Kripke ML (1989) Identification of
the molecular target for the suppression of contact hypersensitivity
by UV radiation. J Exp Med, 170: 1117-1131.
Appleton JA & McGregor DD (1984) Rapid expulsion of Trichinella
spiralisin suckling rats. Science, 226: 70-72.
Araneo BAT, Dowell T, Moon HB; & Daynes RA (1989) Regulation of murine
lymphokine production in vivo: Ultraviolet radiation exposure
depresses IL-2 and enhances IL-4 production by T cells through an IL-1
independent mechanism. J Immunol, 143: 1737-1745.
Aranyi C, Vana SC, Thomas PT, Bradof JN, Fenters JD, Graham JA, &
Miller FJ (1983) Effects of subchronic exposure to a mixture of O3,
SO2 and (NH4)SO4 on host defenses in mice. Environ Res,
12: 55-71.
Aranyi C, Bradof JN, O'Shea WJ, Graham JA, & Miller FJ (1985) Effects
of arsenic trioxide inhalation exposure on pulmonary antibacterial
defenses in mice. J Toxicol Environ Health, 15: 163-172.
Aranyi C, O'Shea WJ, Graham JA & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6: 713-720.
Archer DL, Smith BG, & Bukovic-Wess JA (1978) Use of an in vitro
antibody producing system for recognizing potentially
immunosuppressive compounds. Int Arch Allergy Appl Immunol, 56: 90-93.
Arkoosh MR & Kaattari SL (1990) Quantitation of fish antibody to a
specific antigen by an enzyme-linked immunosorbent assay (ELISA).
In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS & van Muiswinkel
WB ed. Techniques in Fish Immunology. Fair Haven, NJ, SOS
Publications, Vol 1, pp 15-24.
Askenase PW, Hayden B, & Gershon RK (1977) Evanescent delayed
hypersensitivity: Mediation by effector cells with a short life span.
J Immunol, 119: 1830-1835.
Austin JH, Schulman LL, & Mastrobattista JD (1989) Pulmonary infection
after cardiac transplantation: Clinical and radiologic correlations.
Radiology, 172: 259-265.
Austyn JM & Gordon S (1981) F4/80, a monoclonal antibody directed
specifically against the mouse macrophage. Eur J Immunol, 11: 805-815.
Baadsgaard O, Wulf HC, Wantzin GL, & Cooper KD (1987) UVB and UVC, but
not UVA, potently induce the appearance of T6-DR+ antigen-presenting
cells in human epidermis. J Invest Dermatol, 89: 113-118.
Baadsgaard O, Lisby S, Wulf HC, Wantzin GL, & Cooper KD (1989) Rapid
recovery of Langerhans cell alloreactivity, without induction of
autoreactivity, after in vivo ultraviolet A, but not ultraviolet
B exposure of human skin. J Immunol, 142: 4213-4218.
Baadsgaard O, Salvo B, Mannie A, Dass B, Fox DA, & Cooper KD (1990)
In vivo ultraviolet-exposed human epidermal cells activate T
suppressor cell pathways that involve CD4+CD45RA+ suppressor-inducer
T cells. J Immunol, 145: 2854-2861.
Baker D, O'Neill JK, Gschmeissner SE, Wilcox CE, Butter C, & Turk JL
(1990) Induction of chronic relapsing experimental allergic
encephalomyelitis in Biozzi mice. J Neuroimmunol, 28: 261-270.
Balls M, Blaauboer B, Brusick D, Frazier J, Lamb D, Pemberton M,
Reinhardt C, Roberfroid M, Rosenkranz H, Schmid B, Spielmann H,
Stammati A-L, & Walum E (1990) Report and recommendations of the
CAAT/ERGATT workshop on the validation of toxicity test procedures.
ATLA, 18: 313-337.
Bancroft GJ, Shellam GR, & Chalmer JE (1981) Genetic influences on the
augmentation of natural killer (NK) cells during murine
cytomegalovirus infection: Correlation with patterns of resistance. J
Immunol, 126: 988-994.
Bandeira A, Mota-Santos T, Otohara S, Degermann S, Heusser C, Tonegawa
S, & Coutinho A (1990) Localization of gamma/delta cells to the
intestinal epithelium is independent of normal microbial colonization.
J Exp Med, 172: 239-244. 306
Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, & Reynolds CW
(1985) Direct evidence for the role of LGL in the inhibition of
experimental tumor metastases. J Immunol, 134: 2783-2789.
Barnett JB & Rodgers KE (1994) Pesticides. In: Dean JH, Luster MI,
Munson AE, & Kimber I ed. Immunotoxicity and immunopharmacology, 2nd
Ed. New York, Raven Press, pp 191-212.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LS
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39: 263-274.
Barr BBB, Benton EC, McLaren K, Bunney MH, Smith IW, Blessing K,
& Hunter JAA (1989) Human papilloma virus infection and skin cancer in
renal allograft recipients. Lancet, i: 124-129.
Barrett WL, First MR, Aron BS, & Penn I (1993) Clinical course of
malignancies in renal transplant recipients. Cancer, 72: 2186-2189.
Barry TS, Jones DM, Richter CB, & Haynes BF (1991) Successful
engraftment of human postnatal thymus in severe combined immune
deficient (SCID) mice: Differential engraftment of thymic components
with irradiation versus anti-asialo GM-1 immunosuppressive regimens.
J Exp Med, 173: 167-180.
Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, & Penny R (1977)
Depressed lymphocyte function after bereavement. Lancet, i: 834-836.
Bascom R, Naclerio RM, Fitzgerald TK, Kagey-Sobotka A, & Proud D
(1990) Effect of ozone inhalation on the response to nasal challenge
of allergic subjects. Am Rev Respir Dis, 142: 594-601.
Basketter DA, Bremmer JN, Kammuller ME, Kawabata T, Kimber I, Loveless
SE, Magda S, Pal THM, Stringer DA, & Vohr HW (1994) The identification
of chemicals with sensitizing or immunosuppressive properties in
routine toxicology. Food Chem Toxicol, 32: 289-296.
Bates DV & Sizto R (1983) Relationship between air pollutant levels
and hospital admissions in southern Ontario. Can J Public Health,
74: 117-122.
Baumann G, Zenke G, Wenger R, Hiestand P, Quesniaux V, Andersen E, &
Schreier MH (1992) Molecular mechanisms of immunosuppression. J
Autoimmunol, 5 (Suppl A): 67-72.
Bazin H, Xhurdebise LM, Burtonboy G, Lebacq AM, de Clerq L, & Cormont
F (1984) Rat monoclonal antibodies. I. Rapid purification from
in vitro culture supernatants. J Immunol Meth 66: 261-269.
Behrendt H, Krämer U, Dolgner R, Hinrinchs J, Willer H, Hagenbeck H, &
Schlipköter HW (1993) Elevated levels of total serum IgE in East
German children: Atopy, parasites and pollutants. J Allergol, 2:31.
Behrendt H, Friedrichs K-H, Krämer U, Hitzfeld B, Becker W-M, & Ring J
(1994) The role of indoor and outdoor air pollution in allergic
diseases. In: Vos JG, Younes M, & Smith E ed. Allergic
hypersensitivities induced by chemicals: Recommendations for
prevention. Boca Raton, Florida, CRC Press, pp 173-182.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Roboz JP, Fischbein A, & Mason P (1987) Immunotoxicology,
environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the State of Michigan. Cancer Detect
Prev, Suppl 1: 29-37.
Bélisle C & Sainte-Marie G (1981) Tridimensional study of the deep
cortex of the rat lymph node. II. Relation of deep cortex units to
afferent lymphatic vessels. Anat Rec, 199: 61-72.
Bell EB (1989) Thymus-derived and non-thymus-derived T-like cells. The
origin and function of cells bearing gd receptors. Thymus, 14: 3-17.
Bencko V, Wagner V, Wagnerova M, & Ondrejcak K (1990) Immunological
profiles in workers occupationally exposed to inorganic mercury. J Hyg
Epidemiol Microbiol Immunol, 34: 9-14.
Bentwich Z, Bianco N, Jager L, Houba V, Lambert PH, Knaap W, Rose N,
Seligman M, Thompson R, Torrigiani G, & de Weck A (1982) Use and abuse
of laboratory tests in clinical immunology: Critical considerations of
eight widely used diagnostic procedures. report of a Joint IUIS/WHO
Meeting on Assessment of Tests Used in Clinical Immunology. Clin
Immunol Immunopathol, 24: 122-138.
Bentwich Z, Beverley PCL, Hammarstrom L, Kalden JR, Lambert PH, Rose
NR, & Thompson RA (1988) Laboratory investigations in clinical
immunology: Methods, pitfalls, and clinical indications. Clin Immunol
Immunopathol 49: 478-497.
van den Bergh P, Rozing J, & Nagelkerken L (1991) Opioid peptides as
cytokines in T cell activation. Adv Neuroimmunol, 1: 189-203.
van den Bergh P, Rozing J, & Nagelkerken L (1993a) Identification of
two moieties of ß-endorphin with opposing effects on rat T-cell
proliferation. Immunology, 79: 18-23.
van den Bergh P, Rozing J, & Nagelkerken L (1993b) b-Endorphin
stimulates Ia expression on mouse B cells by inducing interleukin-4
secretion by CD4+ cells. Cell Immunol, 149: 180-192
van den Bergh P, Dobber R, Ramlal S, Rozing J, & Nagelkerken L (1994)
Role of opioid peptides in the regulation of cytokine production by
murine CD4+ T cells. Cell Immunol, 154: 109-122.
Bergman A, Olsson M, & Reiland S (1992) Skull-bone lesions in the
Baltic grey seal (Halichoerus grypus). Ambio, 21: 517-519.
Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F ed. (1987)
Immunotoxicology. Dordrecht, Martinus Nijhoff Publishers, pp xi-xxvii.
Bernier J, Brousseau P, Krzystyniak K, Tryphonas H, & Fournier M (in
press) Immunotoxicity of heavy metals in relation to Great Lakes.
Environ Health Perspectives, Suppl 9.
Bernstein A & Breitman M (1989) Genetic ablation in transgenic mice.
Mol Biol Med, 6: 523-530.
Beschorner WE, Namnoun JD, Hess AD, Shinn CA, & Santos GW (1987a)
Cyclosporin A and the thymus immunopathology. Am J Pathol,
126: 487-496.
Beschorner, WE, Di Gennaro KA, Hess AD, & Santos GW (1987b)
Cyclosporine and the thymus: Influence of irradiation and age on
thymic immunopathology and recovery. Cell Immunol, 110: 350-364.
Bevilacqua MP (1993) Endothelial-leukocyte adhesion molecules. Ann Rev
Immunol, 11: 767-804.
Bick PH, Tucker AN, White KL, & Holsapple MP (1984) Effects of
subchronic exposure to diethylstilbestrol on humoral immune function
in adult female (C3B6)F1 mice. Immunopharmacology, 7: 27-39.
Bigazzi PE (1992) Lessons from animal models: The scope of mercury-
induced autoimmunity. Clin Immunol Immunopathol 65: 81-84.
Biron CA, Byron KS, & Sullivan JL (1989) Severe herpesvirus infection
in an adolescent without natural killer cells. New Engl J Med,
320: 1731-1735.
Bissonette E, Dubois C, & Rola-Pleszczynski M (1989) Changes in
lymphocyte function and lung histology during the development of
asbestosis and silicosis in the mouse. Res Commun Chem Pathol
Pharmacol, 65: 211-227.
Black RD, McVey DS, & Oehme FW (1992) Immunotoxicity in the bovine
animal: A review. Vet Hum Toxicol, 34: 438-442.
Blackley BR, Sisodia CS, & Mukkur TK (1980) The effects of
methylmercury, tetraethyllead and sodium arsenite on the humoral
immune response in mice. Toxicol Appl Pharmacol, 52: 245-254.
Blackmann M, Kappler J, & Marrack P (1990) The role of the T cell
receptor in positive and negative selection of developing T cells.
Science, 248: 1335-1341.
Blatt J, Olshan A, Gula MJ, Dickman PS, & Zarenek B (1992) Second
malignancies in very long-term survivors of childhood cancer. Am J
Med, 93: 57-60.
Blaylock BL, Holladay SD, Comment CE, Heindel JJ, & Luster MI (1992)
Exposure to tetrachlorodibenzo- p-dioxin (TCDD) alters fetal
thymocyte maturation. Toxicol Appl Pharmacol, 112: 207-213.
Blazer VS, Wolke RE, Brown J, & Powell CA (1987) Piscine macrophage
aggregate parameters as health monitors: Effects of age, sex, relative
weight, season and site quality in largemouth bass (Micropterus
salmoides). Aquat Toxicol, 10: 199-215.
Bleavins MR & Alvey JD (1991) Immunotoxicologic evaluation of a purine
nucleoside phosphorylase inhibitor in the cynomolgus monkey.
Toxicologist, 11: 205.
Bleavins MR & Dziedzic D (1990) An immunofluorescence study of T and B
lymphocytes in ozone-induced pulmonary lesions in the mouse. Toxicol
Appl Pharmacol, 105: 93-102.
Blok WL, Vogels MT, Curfs JH, Eling WM, Buurman WA, & Van Der Meer JW
(1992) Dietary fish-oil supplementation in experimental Gram negative
infection and in cerebral malaria in mice. J Infect Dis, 165: 898-903.
Bloom BR, Salgame P, & Diamond B (1992) Revisiting and revising
suppressor T cells. Immunol Today, 131: 131-136.
Blusse Van Oud Alblas A, & Van Furth R (1979) Origin, kinetics and
characteristics of pulmonary macrophages in the normal steady state.
J Exp Med, 149: 1504-1518.
Bly JE, Miller NW, & Clem LW (1990) A monoclonal antibody specific for
neutrophils in normal and stressed channel catfish. Dev Comp Immunol,
14: 211-221.
Bodey GP, Fainstein V, & Guerrant R (1986) Infections of the
gastrointestinal tract in the immunocompromised patient Ann Rev Med,
37: 271-281.
Bohus B, Koolhaas JM, De Ruiter AJH, & Heijnen CJ (1991) Stress and
differential alterations in immune system functions: Conclusions from
social stress studies in animals. Neth J Med, 39: 306-315.
Boivin JF (1990) Second cancers and other late side-effects of cancer
treatment: A review. Cancer, 65: 770-775.
Bolas-Fernandez F, Grencis RK & Wakelin D (1988) Cyclosporin A and
Trichinella spiralis: Anthelminthic effects in immunosuppressed
mice. Parasite Immunol, 10: 111-116.
Bonnyns M & Bastomsky CH (1976) Polychlorinated biphenyl-induced
modifica-tion of lymphocyte response to plant mitogens in rats.
Experientia, 15: 522-523.
Booss J (1980) Establishment of cytomegalovirus infection in mice:
Role of a macrophage-enriched subpopulation. J Infect Dis,
141: 466-472.
Borleffs JCC, Schuurman H-J, Vrehen HM, Van Schaik M, & Bast EJEG
(1993) in vitro and in vivo measurement of cell-mediated immunity
in patients with HIV-1-infection. Scand J Immunol, 37: 634-636.
Boros DL (1988) Mechanisms of granuloma formation in the lung.
In: Daniele RP ed. Immunology and immunologic diseases of the lung.
Boston, Blackwell Scientific Publications, pp 263-287.
Bos J ed. (1990) The skin immune system. Boca Raton, Florida, CRC
Press.
Bos JD & Kapsenberg ML (1986) The skin immune system (SIS): Its
cellular constituents and their interactions. Immunol Today,
7: 235-240.
Bouwes Bavinck JN, de Boer A, Vermeer BJ, Hartevelt MM, van der Woude
FJ, Claas FHJ, Wolterbeek R, & Vandenbroucke JP (1993) Sunlight,
keratotic skin lesions and skin cancer in renal transplant recipients.
Br J Dermatol, 129: 242-249.
Boyce WT, Jensen EW, Cassel JC, Collier AM, Smith AH, & Ramey CT
(1977) Influence of life events and family routines on childhood
respiratory tract illness. Pediatrics, 60: 609-615.
Boyd RL, Wilson TJ, Bean AG, Ward HA, & Gershwin ME (1992) Phenotypic
characterization of chicken thymic stromal elements. Dev Immunol,
2: 51-66.
Boyle J, Mackie RM, Briggs JD, Junor BJR, & Aitchison TC (1984)
Cancer, warts, and sunshine in renal transplant patients: A case-
control study. Lancet, i: 702-705.
Bradley SG & Morahan PS (1982) Approaches to assessing host
resistance. Environ Health Perspectives, 43: 61-69.
Brekelmans P & Van Ewijk W (1990) Phenotypic characterization of
murine thymic microenvironments. Sem Immunol, 2: 13-24.
Brett SJ & Butler R (1986) Resistance to Mycobacterium lepraemurium
is correlated with the capacity to generate macrophage activating
factor(s) in response to mycobacterial antigens in vitro.
Immunology, 59: 339-345.
Brideau RJ, Carter PB, McMaster WR, Mason DW, & Williams AF (1980) Two
subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J
Immunol, 10: 609-615.
Brigham KL (1989) Mediators in inflammation. In: Henderson PM & Murphy
RC ed. Handbook of inflammation, Vol. 6: Mediators of the inflammatory
process. Amsterdam, Elsevier Science Publishers, pp 1-14.
Brodie AM & Halliday WJ (1991) Suppressor cells and soluble factors
from the spleens of UV-irradiated mice. Immunol Cell Biol,
69: 199-204.
Brostoff J, Scadding GK, Male D, & Roitt IM (1991) Clinical
immunology. London, Gower Medical Publishers.
Brouwer A, Reijnders PJH, & Koeman JH (1989) Polychlorinated biphenyl
(PCB)-contaminated fish induces vitamin A and thyroid hormone
deficiency in the common seal (Phoca vitulina). Aquat Toxicol,
15: 99-106.
Brown WR & Kloppel TM (1989) The liver and IgA: Immunological, cell
biological and clinical implications. Hepatology, 9: 763-778.
Bruggeman CA, Debie WMH, Grauls G, Majoor G, & Boven CPA (1983)
Infection of laboratory rats with a new cytomegalo-like virus. Arch
Virol, 76: 189-199.
Bruggeman CA, Meijer H, Bosman F, & Boven CPA (1985) Biology of rat
cytomegalovirus. Intervirology, 24: 1-9.
Bruning JH (1985) Cytomegalovirus infections in renal transplantation;
study in a rat model. PhD thesis, Utrecht, Utrecht University.
Bucke D, Vethaak AD, & Lang T (1992) Quantitative assessment of
melanomacrophage centers (MMC's) in dab (Limanda limanda) along a
pollution transect in the German Bight. Mar Ecol Prog Ser,
91: 193-196.
Buckingham JC, Safieh B, Singh S, Arduino LA, Cover PO, & Kendall MD
(1992) Interactions between hypothalamo-pituitary adrenal axis and the
thymus in the rat: A role for corticotropin in the control of thymulin
release. J Neuroendocrinol, 4: 295-301.
Buening GM, Mann DD, Hook B, & Osweiler BP (1982) The effect of T-2
toxin on the bovine immunity system: Cellular factors. Vet Immunol
Immunopathol, 3: 411-417.
Bugelski PJ, Thiem PA, Solleveld HA, & Morgan DG (1990) Effects of
sensitization to dinitrochlorobenzene (DNCB) on clinical pathology
parameters and mitogen-mediated blastogenesis in cynomolgus monkeys
(Macaca fascicularis). Toxicol Pathol, 18: 643-650.
Bukowski JF, Woda BA, & Welsh RM (1984) Pathogenesis of murine
cytomegalovirus infection in natural killer cell-depleted mice.
J Virol, 52: 119-128.
Bulloch K (1987) The innervation of immune system tissues and organs.
In: Cotman CW, Brinton RE, Galaburda A, McEwen B, & Schneider DM ed.
The neuro-immune-endocrine connection. New York, Raven Press,
pp 33-47.
Burcham J, Marmor M, Dubin N, Tindall B, Cooper DA, Berry G, & Penny R
(1991) CD4+ is the best predictor of development of AIDS in a cohort
of HIV-infected homosexual men. AIDS, 5: 365-372.
Burchiel SW, Thompson TA, & Davis AP (1991) Alterations in mitogen-
induced calcium mobilization and intracellular free calcium produced
by 7,12-dimethylbenz (a)anthracene in the Jurkat human T cell line.
Int J Immunopharmacol, 13: 109-115.
Burleson GR, Fuller LB, Menache MG, & Graham JA (1987) Poly(I):
poly(C)-enhanced alveolar and peritoneal macrophage phagocytosis:
Quantification by a new method utilizing fluorescent beads. Proc Soc
Exp Biol Med, 184: 468-476.
Burleson GR, Keyes LL, & Stutzman JD (1989) Immunosuppression of
pulmonary natural killer activity by exposure to ozone.
Immunopharmacol Immunotoxicol, 11: 715-735.
Burleson GR, Lebrec H, Yang YG, lbanes JD, Pennington KN, & Birnbaum
LS (in press) Effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)
on influenza virus host resistance in mice. Fundam Appl Toxicol.
Calder VL & Lightman SL (1992) Experimental autoimmune uveoretinitis
(EAU) versus experimental allergic encephalomyelitis (EAE): A
comparison of T cell-mediated mechanisms. Clin Exp Immunol,
89: 165-169.
Cantacuzene MJ (1898) Nouvelles recherches sur le mode de destruction
des virions dans l'organisme. Ann Inst Pasteur, 12: 274-300.
Cantrell DA, Collins MKL, & Crumpton DA (1988) Autocrine regulation of
T-lymphocyte proliferation: Differential induction of IL-2 and IL-2
receptor. Immunology, 65: 343-349.
Capurro PU (1980) The effects of solvents and pesticides on health.
Clin Toxicol, 16: 549-553.
Carter JW & Clancy J (1980) Acutely administered polychlorinated
biphenyls (PCBs) decrease splenic cellularity but increase its
ability to cause graft-versus-host reactions in BALB/c mice.
Immunopharmacology, 2: 341-347.
Casale GP, Cohen SD, & DiCapua RA (1983) The effects of
organophosphate-induced cholinergic stimulation on the antibody
response to sheep erythrocytes in inbred mice. Toxicol Appl
Pharmacol, 68: 198-205.
Casale GP, Cohen SD, & DiCapua RA (1984) Parathion-induced suppression
of humoral immunity in inbred mice. Toxicol Lett, 23: 239-247.
Castillo-Mendez A, Rodriguez-Diaz T, Leon-Lobeck A, & Gravalosa-Cruz
AJ (1991) Influence of occupational lead exposure on the concentration
of immunoglobins and immune cellular functions in human. Rev Allergol
Mex, 38: 69.
Centers for Disease Control (1991) Summary of notifiable diseases,
United States, 1990. Morb Mortal Weekly Rep, 39 (53).
Challacombe SJ (1987) The investigation of secretory and systemic
immune responses to ingested material in animal models. In: Miller K
& Nicklin S ed. Immunology of the gastrointestinal tract, Vol 2. Boca
Raton, Florida, CRC Press, pp 99-124.
Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, &
Hiseroth JC (1989) Monoclonal antibody to a triggering structure
expressed on rat natural killer cells and adherent lymphokine-
activated killer cells. J Exp Med 169: 1373-1389.
Chan AC, Irving BA, & Weiss A (1992) New insights into T-cell antigen
receptor structure and signal transduction. Curr Opin Immunol,
4: 246-251.
Chanarin I (1985) Structure and function of the bone marrow. In: Irons
RD ed. Toxicology of the blood and bone marrow. New York, Raven Press,
pp 1-16.
Chandra RK (1992) Nutrition and immunoregulation. Significance of host
resistance to tumors and infectious diseases in humans and rodents.
J Nutr, 122 (Suppl): 754-757.
Chandra RK (1993) Nutrition and the immune system. Proc Nutr Soc,
52: 77-84.
Chang KH, Hsieh KH, Lee TP, Tang SY, & Tung TC (1981) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Determination of lymphocyte subpopulations. Toxicol Appl Pharmacol,
61: 58-63.
Chang KH, Hsieh KH, Tang SY, Tung TC, & Lee TP (1982) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Evaluation of delayed-type skin hypersensitivity response and its
relation to clinical studies. J Toxicol Environ Health, 9: 217-223.
Clark EA & Lanier LL (1989) Report from Vienna: In search of all
surface molecules expressed on human leukocytes. J Clin Immunol,
9: 265-272.
Clark GC, Blank JA, Germolec DR & Luster MI (1991)
2,3,7,8-Tetrachlorodibenzo- p-dioxin stimulation of tyrosine
phosphorylation in B lymphocytes: Potential role in
immunosuppression. Mol Pharmacol, 39: 495-501.
Clarke AG (1984) Immunological studies on pregnancy in the mouse.
In: Crighton DB ed. Immunological aspects of reproduction in mammals.
London, Butterworths, pp 153-182.
Clarke AG & Kendall MD (1989) Histological changes in the thymus
during mouse pregnancy. Thymus, 14: 65-78.
Clevers H, Alarcon B, Wileman T, & Terhorst C (1988) The T cell
receptor/CD3 complex: A dynamic protein ensemble. Ann Rev Immunol,
6: 629-662.
Clover RD, Abell T, Becker LA, Crawford S, & Ramsey CN (1989) Family
functioning and stress as predictors of influenza B infection.
J Family Practice, 28: 535-539.
Coffin DL & Gardner DE (1972) Interaction of biological agents and
chemical air pollutants. Ann Occup Hyg, 15: 219-234.
Cohen N, Modai D, Golik A, Weissgarten J, Peller S, Katz A, Averbuhk
Z, & Shaked U (l989) Increased concanavalin A-induced suppressor cell
activity in humans with occupational lead exposure. Environ Res,
48: 1-6.
Coligan JE, Kruisbeek, AM, Margulies DH, Shevach EM, & Strober W ed.
(1994) Current protocols in immunology, Vols 1 and 2. New York, John
Wiley & Sons.
Cook JA, Marconi EA, & DiLuzio NR (1974) Lead, cadmium, endotoxin
interaction: Effect on mortality and hepatic function. Toxicol Appl
Pharmacol, 28: 292.
Cook JA, Hoffman EO, & DiLuzio NR (1975) Influence of lead and cadmium
on the susceptibility of rats to bacterial challenge (39117). Proc Soc
Exp Biol Med, 150: 741.
Cook JC, Gaido KW, receptor: & Greenlee WF Relevance of (1987) Ah
mechanistic studies to human risk assessment. Environ Health
Perspectives, 76: 71-77.
Cooper EL (1982) How the immune system evolved. In: General
immunology. Oxford, Pergamon Press, pp 279-323.
Cooper KD, Neises GR, & Katz SI (1986) Antigen-presenting OKM5+
melanophages appear in human epidermis after ultraviolet radiation.
J Invest Dermatol, 86: 363-370.
Cooper KD, Oberhelman BS, Hamilton MS, Baadsgaard O, Terhune M, Levee
G, Anderson T, & Koren H (1992) UV exposure reduces immunization rates
and promotes tolerance to epicutaneous antigens in humans;
relationship to dose, CDla DR+ epidermal macrophage inductlon and
Langerhans cell depletion. Proc Natl Acad Sci USA, 89: 8497-8501.
Corman LC (1985) Effects of specific nutrients on the immune response.
Selected clinical applications. Med Clin North Am, 69: 759-791.
Cornacoff JB, Tucker AN, & Dean JH (1988) Development of a human
peripheral blood mononuclear leucocyte model for immunotoxicity
evaluation. In Vitro Toxicol, 2: 81-89.
Corrier DE (1992) Mycotoxicosis: Mechanisms of immunosuppression. Vet
Immunol Immunopathol, 30: 73-87.
Corsini E, Luster MI, Mahler J, Craig WA, Blazka M, & Rosenthal GJ
(1994) A protective role for T-lymphocytes in asbestos-induced
pulmonary inflammation and collagen deposition. Am J Respir Cell Mol
Biol, 7: 60-82.
Coscia GC, Discalzi G, & Ponzetti C (1987) Immunological aspects of
occupational lead exposure. Med Lav, 78: 360-364.
Council for International Organizations of Medical Sciences (1993)
International ethical guidelines for biomedical research involving
human subjects. Geneva, 63 pp.
Cunningham AJ & Szenberg A (1968) Further improvements in the plaque
tech-nique for detecting single antibody-forming cells. Immunology,
14: 559-600.
Dabrowski MP & Dabrowski-Bernstein BK (1990) Immunoregulatory role of
the thymus. Boca Raton, Florida, CRC Press.
Dallman MJ, Thomas ML, & Green JR (1984) MRC OX-19: A monoclonal
antibody that labels rat T lymphocytes and augments in
vitroproliferative responses. Eur J Immunol, 14: 260-267.
Davis AP & Burchiel SW (1992) Inhibition of calcium-dependent pathways
of B-cell activation by DMBA. Toxicol Appl Pharmacol, 116: 202-208.
Davis D & Safe S (1988) Immunosuppressive activities of
polychlorinated dibenzofuran congeners: Quantitative structure-
activity relationships and interactive effects. Toxicol Appl
Pharmacol, 94: 141-149.
Davis D & Safe S (1989) Dose-response immunotoxicities of commercial
polychlorinated biphenyls (PCBs) and their interaction with
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol Lett, 48: 35-43.
Davis D & Safe S (1991) Halogenated aryl hydrocarbon-induced
suppression of the in vitro plaque-forming cell response to
sheep red blood cells is not dependent on the Ah receptor.
Immunopharmacology, 21: 183-190.
Dayan AD, Hertel RF, Heseltine E, Kazantis G, Smith EM, & Van der
Venne MT ed. (1990) Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press.
Dean JH & Murray MJ (1990) Toxic responses of the immune system.
In: Klaassen CD, Amdur MO, & Doull J ed. Toxicology: The basic science
of poisons, Vol 4. New York, MacMillan Press, pp 282-333.
Dean JH & Thurmond LM (1987) Immunotoxicology: An overview. Toxicol
Pathol, 15: 265-271.
Dean JH, Luster MI, & Boorman GA (1982) Immunotoxicology. In: Sirois
P, & Pleszczynski MR ed. Immunopharmacology. Amsterdam, Elsevier
Science Publishers, pp 349-397.
Dean JH, Luster MI, Munson AE, & Amos HA (1985) Immunotoxicology and
immunopharmacology. New York, Raven Press.
Dean JH, Ward EC, Murray MJ, Lauer LD, & House RV (1986)
Immunosuppression following 7,12-dimethylbenz-a-anthracene exposure
in B6C3F1 mice. II. Altered cell-mediated immunity and tumor
resistance. Int J Immunopharmacol, 8: 189-198.
Dean JH, Luster MI, Munson AE, & Kimber I (1994) Immunotoxicology and
immunopharmacology, 2nd Ed. New York, Raven Press.
De Gast GC, Verdonck LF, Middeldorp JM, The TH, Hekker A, Van der
Linden JA, Kreeft HA, & Bast BJ (1985) Recovery of T cell subsets
after autologous bone marrow transplantation is mainly due to
proliferation of mature T cells in the graft. Blood, 66: 428-431.
De Heer C, Verlaan APJ, Penninks AH, Schuurman HJ, & Van Loveren H
(1993) The SCID-RA mouse: Rat T cell differentiation in the severe
combined immunodeficient mouse. APMIS, 101: 467-479.
Denis M (1992) Mouse hypersensitivity pneumonitis: Depletion of NK
cells abrogates the spontaneous regression phase and leads to massive
fibrosis. Exp Lung Res, 18: 761-773.
Denis M, Bisson D, & Ghardirian E (1993) Cellular and cytokine
profiles in spontaneous regression phase of hypersensitivity
pneumonitis. Exp Lung Res, 19: 257-271.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
Deo MG, Gangal S, Bhisey AN, Somasundaram R, Balsara Gulwani B,
Darbaru BS, Bhiide S, & Maru B (1987) Immunological, mutagenic and
genotoxic investigations in gas exposed population of Bhopal. Indian
J Med Res, 86 (Suppl): 63-76.
Descotes J (1985) Immunomodulating agents and hepatic drug metabolism.
Drug Metab Rev, 16: 175-183.
Descotes J (1986) Immunotoxicology of drugs and chemicals. Amsterdam,
Elsevier Science Publishers.
Descotes J (1990) Drug-induced immune diseases. Amsterdam, Elsevier
Science Publishers.
Descotes J (1992) Immunomodulating agents. In: Dukes MNG ed. Meyler's
side-effects of drugs, 12th Ed. Amsterdam, Elsevier Science
Publishers, pp 954-958.
Descotes J & Vial T (1994) Cytoreductive drugs. In: Dean JH, Luster
MI, Munson AE, & White K ed. Immunotoxicology and immunopharmacology,
2nd Ed. New York, Raven Press, pp 293-301.
Descotes J, Verdier F, Brouland JP, & Pulce C (1990) Immunotoxicity of
lead, cadmium and arsenic: Experimental data and their relevance to
man. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM, &
Van der Venne, M-T ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, pp 209-213.
Desi L, Varga L, & Farkas I (1978) Studies on the immunosuppressive
effect of organochlorine and organophosporic pesticides in subacute
experiments. J Hyg Epidemiol Microbiol Immunol, 22: 115-122.
Desi L, Varga L, Dobronyi L, & Szklenarik G (1985) Immunotoxicological
investigation of the effects of a pesticide; cypermethrin. Arch
Toxicol, Suppl 8: 305-309.
De Swart RL, Kluten RMG, Huizing CJ, Vedder LJ, Reijnders PJH, Visser
IKG, UytdeHaag FGCM, & Osterhaus ADME (1993) Mitogen and antigen
induced B and T cell responses of peripheral blood mononuclear cells
from the harbour seal (Phoca vitulina). Vet Immunol Immunopathol,
37: 217-230.
De Swart RL, Ross PS, Vedder EJ, Timmerman HH, Heisterkamp SH, Van
Loveren H, Vos JG, Reijnders PJH, & Osterhaus ADME (1994) Impairment
of immune function in harbour seals (Phoca vitulina) feeding on fish
from polluted waters. Ambio, 23: 155-159.
Devens BH, Grayson MH, Imamura IK, & Rodgers KE (1985)
O,O,S-Trimethyl-phosphorothioate effect on immunocompetence.
Pestic Biochem Physiol, 24: 251-259.
Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers
D, & Koren H (1991) Exposure of humans to ambient levels of ozone for
6.6 hours causes cellular and biochemical changes in the lung.
Am J Resp Cell Mol Biol, 4: 72-81.
De Waal EJ, Rademakers LHPM, Schuurman H-J, & Van Loveren H (1992a)
Interdigitating cells in the rat thymus during cyclosporin A
treatment: Ultrastructural observations. Thymus, 20: 163-170.
De Waal EJ, Schuurman H-J, Loeber JG, Van Loveren H, & Vos JG (1992b)
Alterations in the cortical thymic epithelium of rats after
in vivo exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD):
An (immuno)histological study. Toxicol Appl Pharmacol, 115: 80-88.
De Waal EJ, Rademakers LHPM, Schuurman H-J, Van Loveren H, & Vos JG
(1993) The cortical epithelium of the rat thymus after in vivo
exposure to bis(tri-n-butyltin)oxide (TBTO). Arch Toxicol,
67: 186-192.
DeWailly E, Nantel A, Brunean S, Laliberte C, Ferron L, & Gingras S
(1992) Breast milk contamination by PCDDs, PCDFs, and PCBs in Arctic
Quebec: A preliminary assessment. Chemosphere, 25: 1245-1249.
De Weger RA, Van Loveren H, Vandebriel RJ, & Parmentier HK (1989).
Sequential activity of gamma-delta T-cell receptor bearing
lymphocytes, specific T-cell factors, and alpha-ß T-cell receptor
bearing T-cells: A hypothesis. Thymus, 14: 19-30.
Dieter MP, Luster MI, Boorman GA, Jameson CW, Dean JH, & Cox JW (1983)
Immunological and biochemical responses in mice treated with mercuric
chloride Toxicol Appl Pharmacol, 68: 218-228.
Dietert RR, Qureshi MA, Nanna UC, & Bloom SE (1985) Embryonic exposure
to aflatoxin-B1: Mutagenicity and influence on development and
immunity. Environ Mutag, 7: 715-725.
Dietz R, Heide-Jorgensen MP, & Härkönen T (1989) Mass deaths of harbor
seals (Phoca vitulina) in Europe. Ambio, 18: 258-264.
Dijkstra CD & Sminia T (1990) Normal anatomy, histology,
immunohistology, ultrastructure, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI monograph). Berlin, Springer
Verlag, pp 185-193.
Dijkstra CD, Döpp EA, Joling P, & Kraal G (1985) The heterogeneity of
mononuclear phagocytes in lymphoid organs: Distinct macrophage
subpopu-lations in rat recognized by monoclonal antibodies ED1, ED2
and ED3. Immunology, 54: 589-599.
Dinarello CA (1992) Role of interleukin-1 in infectious diseases.
Immunol Rev, 127: 119-146.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979a) Role of
macrophages in the augmentation of mouse natural killer activity by
polyI:C and interferon. J Immunol, 122: 182-188.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979b) Augmentation
of mouse natural killer cell activity by interferon and interferon
inducers. J Immunol, 122: 175-181.
Dobber R, Hertog-Huijbregts A, Rozing J, Bottomly K, & Nagelkerken L
(1992) The involvement of the intestinal microflora in the expansion
of CD4+ T cells with a naive phenotype in the periphery. Dev
Immunol, 2: 141-150.
Dogra RK, Chandra K, Chandra S, Gupta S, Khanna S, Srivastava SN,
Shukla LJ, Katiyar JC, & Shanker R (1992) Host resistance assays as
predictive models in styrene immunomodulation. Int J Immunopharmacol,
14: 1003-1009.
Domzig W, Stadler BM, & Herberman RB (1983) Interleukin-2 dependence
of human natural killer (NK) cell activity. J Immunol, 130: 1970-1973.
Dooley RK & Holsapple MP (1988) Elucidation of cellular targets
responsible for tetrachlorodibenzo- p-dioxin (TCDD)-induced
suppression of antibody responses: I. The role of the B lymphocyte.
Immunopharmacology, 16: 167-180.
Dotta F & Eisenbarth GS (1989) Type I diabetes mellitus: A predictable
autoimmune disease with interindividual variation in the rate of
ß cell destruction. Clin Immunol Immunopathol, 50: S85-S95.
Driscoll KE, Higgins JM, Leytart MJ, & Corsby LL (1990) Differential
effects of mineral dusts on the in vitro activation of alveolar
macrophage eicosanoid and cytokine release. Toxicity in vitro,
4, 284-288.
Drouet M, Le-Sellin J, Bonneau JC, & Sabbah A(1990) Is mercury an
allergen of the respiratory tract. Allerg Immunol (Paris),
22: 81, 84-85, 87.
Druet P, Pelletier L, Rossert J, Druet E, Hirsch F, & Sapin C (1989)
Autoimmune reactions induced by metals. In: Kammüller ME, Bloksma N,
& Seinen W ed. Autoimmunity and toxicology. Immune disregulation
induced by drugs and chemicals. Amsterdam, Elsevier Science
Publishers, pp 347-361.
Duijvestijn A & Hamann A (1989) Mechanisms and regulation of
lymphocyte migration. Immunol Today, 10: 23-28.
Duke SS, Schook LB, & Holsapple MP (1985) Effects of
N-nitrosodimethylamine on tumor susceptibility. J Leukocyte
Biol, 37: 383-394.
Dunn TB (1954) Normal and pathologic anatomy of the reticular tissue
in laboratory mice, with a classification and discussion of neoplasms.
J Natl Cancer Inst, 14: 1281-1433.
Du Pasquier L (1989) Evolution of the immune system. In: Paul WE ed.
Fundamental immunology, 2nd Ed. New York, Raven Press, pp 139-165.
Durham WH (1974) Air pollution and student health. Arch Environ
Health, 28: 241-254.
Dutch Health Council, Committee for Immunotoxicology (1991)
Immunotoxicity of compounds. The Hague.
Dyall-Smith C & Varigos G (1985) The malignant potential of
papillomavirus. Aust J Dermatol, 26: 102-107.
Dziedzic D & White HJ (1986) T-Cell activation in pulmonary lymph
nodes of mice exposed to ozone. Environ Res, 41: 610-622.
Dziedzic D, Wright ES, & Sargent HE (1990) Pulmonary response to
ozone. Reaction of bronchus-associated lymphoid tissue and lymph node
lymphocytes in the rat. Environ Res, 51: 194-208.
Eedy DJ, Burrows D, Clifford T, & Fay A (1990) Elevated T cell
subpopulations in dental students. J Prosthet Dent, 63: 593.
Ehrke MJ, Mihich E, Berd D, & Mastrangelo MJ (1989) Effects of
anticancer drugs on the immune system in humans. Semin Oncol,
16: 230-253.
Ehrlich JP & Burleson GR (1991) Enhanced and prolonged pulmonary
influenza virus infection following phosgene inhalation. J Toxicol
Environ Health, 34: 259-273.
Ehrlich R & Henry MC (1968) Chronic toxicity of nitrogen dioxide.
I. Effect on resistance to bacterial pneumonia. Arch Environ Health,
17: 860-865.
Ehrlich R, Findlay JC, Fonters JD, & Gardner DE (1977) Health effects
of short-term inhalation of nitrogen dioxide and ozone mixtures.
Environ Res, 4: 223-231.
Ellman MH, Hurwitz H, Thomas C, & Kozloff M (1991) Lymphoma developing
in a patient with rheumatoid arthritis taking low dose weekly
methotrexate. J Rheumatol, 18: 1741-1743.
Elo O, Vuojolahti P, Janhunen R, & Rantanen J (1985) Recent PCB
accidents in Finland. Environ Health Perspectives, 60: 315-319.
Emmendorfer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML,
& Roesler J (1994) Evaluation of flow cytometric methods for diagnosis
of chronic granulomatous disease variants under routine laboratory
conditions. Cytometry, 18: 147-155.
Engelhard VH (1994) Structure of peptides associated with class I and
class II MHC molecules. Ann Rev Immunol, 12: 181-207.
Engleman EG, Benike CJ, Grumet FC, & Evans RL (1981) Activation of
human T lymphocyte subsets: Helper and suppressor/cytotoxic T cells
recognize and respond to distinct histocompatibility antigens.
J Immunol, 127: 2124-2129.
Epstein JH, Tuffanelli DL, & Dubois EL (1965) Light sensitivity and
lupus erythematosus. Arch Dermatol, 91: 483-485.
Eskola J & Toivanen P (1974) Effect of in ovo treatment with
cyclophosphamide on lymphoid system in chicken. Cell Immunol,
13: 459-471.
Evans RG, Webb KB, Knutsen AP, Roodman ST, Roberts DW, Bagby JR,
Garrett WA, & Andrews JS (1988) A medical follow-up of the health
effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. Arch Environ Health, 43: 273-278.
Ewers U, Stiller-Winkler R, & Idel H (1982) Serum immunoglobulin,
complement C3, and salivary IgA levels in lead workers. Environ Res,
29: 351-357.
Exon JR (1985) Effect of lead, polychlorinated biphenyls and
cyclophosphamide on rat natural killer cells, interleukin 2, and
antibody synthesis. Fundam Appl Toxicol, 5: 158-164.
Ezine S, Weissman IL, & Rouse RV (1984) Bone marrow cells give rise to
distinct cell clones within the thymus. Nature, 309: 629-631.
Fabry Z, Raine CS, & Hart MN (1994) Nervous tissue as an immune
compartment: Dialect of the immune response in the CNS. Immunol Today,
15: 218-224.
Faisal M & Hugget RJ (1993) Effects of polycyclic aromatic
hydrocarbons on the lymphocyte mitogenic response in spot, Leiostomus
xanthurus. Mar Environ Res, 35: 121-124.
Faisal M, Chiapelli F, Ahmed II, Cooper EL, & Weiner H (1989) Social
confrontation stress in aggressive fish is associated with an
endogenous opioid-mediated suppression of proliferative response to
mitogens and nonspecific cytotoxicity. Brain Behav Immunol,
3: 223-233.
Fänge R & Nilsson S (1985) The fish spleen: Structure and function.
Experientia, 41: 152-158.
Farrell HE & Shellam GR (1991) Protection against murine
cytomegalovirus infection by passive transfer of neutralizing and non-
neutralizing monoclonal antibodies. Infect Immunol, 72: 149-156.
Fauci A, Schnittman SM, & Poli G (1991) Immunopathologenic mechanisms
in human immunodeficiency virus (HIV) infection. Ann Intern Med.,
114: 678-693.
Fellah JS, Wiles MV, Charlemagne J, & Scwager J (1992) Evolution of
vertebrate IgM: Complete amino acid sequence of the constant region of
Ambystoma mexicanum µ chain deduced from cDNA sequence. Eur J
Immunol, 22: 2595-2601.
Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden
KS, Olschowka JA, & Livnat S (1987) Noradrenergic sympathetic neural
interaction with the immune system: Structure and function. Immunol
Rev, 100: 225-260.
Fenters J, Bradof J, Aranyi C, Ketels K, Ehrlich R, & Gardner D (1979)
Health effects of long-term inhalation of sulfuric acid mist-carbon
particle mixtures. Environ Res, 19: 244-257.
Fichtelius KE, Groth O, & Liden S (1970) The skin, a first level
lymphoid organ? Int Arch Allergy, 37: 607-620.
Fidler IJ (1973) Selection of successive tumor lines for metastasis.
Nature New Biol, 242: 148-149.
Fidler IJ, Gersten DM, & Hart IR (1978) The biology of cancer invasion
and metastases. Adv Cancer Res, 28: 149-250.
Fine JS, Gasiewicz TA, & Silverstone AE (1989) Lymphocyte
stem cell alterations following perinatal exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol Pharmacol, 35: 18-25.
Fine JS, Gasiewicz TA, Fiore NC, & Silverstone AE (1990) Prothymocyte
activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo- p-dioxin
exposure. J Exp Pharmacol Ther, 255: 1-5.
Finsen NR (1901) The treatment of lupus vulgaris by concentrated rays.
In: Phototherapy. London, Edward Arnold, pp 73-75.
Fiore MC, Anderson HA, Hong R, Golubjatnokov R, Seiser JE, Nordstrom
D, Hanrahan L, & Belluck D (1986) Chronic exposure to aldicarb-
contaminated groundwater and human immune function. Environ Res,
41: 633-645.
Fischbein A, Tsang P, Luo JC, Roboz JP, Jiang JD, & Bekesi JG (1993)
Phenotypic aberrations of CD3+ and CD4+ cells and functional
impairments of lymphocytes at low-level occupational exposure to lead.
Clin Immunol Immunopathol, 66: 163-171.
Flajnik MF, Canel C, Kramer J, & Kasahara M (1991) Evolution of the
major histocompatibility complex class I from the amphibian Xenopus.
Proc Natl Acad Sci USA, 88: 537-541.
Fleisher TA, Luckasen JR, Sabad A, Gehrtz RC, & Kersey JH (1975) T and
B lymphocyte subpopulations in children. Pediatrics, 55: 162-165.
Fletcher TC (1986) Modulation of non specific host defences in fish.
Vet Immunol Immunopathol, 12: 59-67.
Fletcher FA & Williams DE (1992) Recent progress in the discovery and
invention of novel hematopoietic cytokines. Crit Rev Oncol Hematol,
13: 1-15.
Flick DF, O'Dell RG, & Childs VA (1965) Studies on the chick edema
disease. 3. Similarity of symptoms produced by feeding chlorinated
biphenyls. Poultry Sci, 44: 1460-1465.
Folinsbee LJ (1992) Human health effects of air pollution. Environ
Health Perspectives, 100: 45-56.
Fontana A, Constamm DB, Frei K, Malipiero U, & Pfister HW (1992)
Modulation of the immune response by transforming growth factor beta.
Int Arch Allergy Immunol, 99: 1-7.
Food Safety Council (1978) Proposed system for food safety assessment.
Food Cosmet Toxicol, 16 (Suppl 1): 1-136.
Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, & Gery
I (1987) Experimental autoimmune uveoretinitis (EAU) induced by
retinal interphotoreceptor retinoid-binding protein (IRBP):
Differences between EAU induced by IRBP and by S-antigen. Clin Immunol
Immunopathol, 43: 256-264.
Foxwell BM, Barrett K, & Feldmann M (1992) Cytokine receptors:
Structure and signal transduction. Clin Exp Immunol, 90: 161-169.
Frasca D, Adorini L, & Doria G (1987) Enhanced frequency of mitogen-
responsive T cell precursors in old mice injected with thymosin alpha
1. Eur J Immunol, 17: 727-730.
Frattini JE & Trulock EP (1993) Respiratory infections in
immunocompromised patients. Immunol Allergy Clin North Am,
13: 193-204.
Frazer IH, Collins EJ, Fox JS, Jones B, Oliphant RC, & Mackay IR
(1985) Assessment of delayed-type hypersensitivity in man: A
comparison of the 'Multitest' and conventional intradermal injection
of six antigens. Clin Immunol Immunopathol, 35: 182-190.
Freier S, ed. (1990) The neuroendocrine-immune network. Boca Raton,
Florida, CRC Press.
French JG, Lowrimore G, Nelson WC, Finklea JF, English T, & Hertz M
(1973) The effect of sulfur dioxide and suspended sulfates on acute
respiratory disease. Arch Environ Health, 27: 129-133.
Friedman H, Klein T, & Specter S (1991a) Immunosuppression by
marijuana and its components. In: Ader R, Felten DL, & Cohen N ed.
Psychoneuroimmunology, 2nd Ed. San Diego, Academic Press, pp 931-953.
Friedman H, Specter S, & Klein TW (1991b) Drugs of abuse, immunity and
immunodeficiency (Advances in Experimental Biology and Medicine,
Volume 288). New York, Plenum Press.
Friedmann PS, White SI, Parker S, Moss C, & Mattews JNS (1989)
Antigenic stimulation during ultraviolet therapy in man does not
result in immunological tolerance. Clin Exp Immunol, 76: 68-72.
Friend M & Trainer DO (1970) Polychlorinated biphenyl: Interaction
with duck hepatitis virus. Science, 170: 1314-1316.
Frith CH, Ward JM, Brown RH, Tyler RD, Chandra M, & Stromberg PC (in
press) Proliferative lesions of the hematopoietic system in rats. In:
Streett CS, Burek JD, Hardisty JF, Garner FM, Leiniger JR, Pletcher
JM, & Moch RW ed. Standardized system of nomenclature and diagnostic
criteria. Guides for toxicologic pathology. Washington DC, Society of
Toxicologic Pathologists, Armed Forces Institute of Pathology,
American Registry of Pathology.
Fugmann RA, Aranyi C, Barbera PW, Bradof JN, Gibbons RD, & Fenters JD
(1983) The effects of DES as measured by host resistance and tumor
susceptibility assays in mice. J Toxicol Environ Health, 11: 827-841.
Fujimaki H (1989) Impairment of humoral responses in mice exposed to
nitrogen dioxide and ozone mixtures. Environ Res, 48: 211-217.
Gahmberg CG, Nortamo P, Li R, & Valmu L (1992) Leukocyte cell adhesion
proteins: From molecular dissection to clinical applications. Ann Med,
24: 329-335.
Gaido KW & Wierda D (1987) Suppression of bone marrow stromal cell
function by benzene and hydroquinone is ameliorated by indomethacin.
Toxicol Appl Pharmacol, 89: 378-390.
Gardner DE, Miller FJ, Illing JW, & Kirtz JM (1977) Alterations in
bacterial defense mechanisms of the lung induced by inhalation of
cadmium. Bull Eur Physiopathol Resp, 13: 157-174.
Garssen J, Van der Vliet H, De Klerk A, Dormans JAMA, & Bruggeman CA
(1995) A rat cytomegalovirus infection model as a tool for
immunotoxicity testing. Eur J Pharmacol, 292: 233-281.
Gasiewicz TA & Rucci G (1984) Cytosolic receptor for
2,3,7,8-tetrachloro-dibenzo- p-dioxin Evidence for a homologous
nature among various mammalian species. Mol Pharmacol, 26: 90-98.
Gaumer HS, Doll NJ, Kaimal J, Schuyler M, & Salvaggio JE (1981)
Diminished suppressor cell function in patients with asbestosis.
Clin Exp Immunol, 44: 108.
Germain RN & Margulies DH (1993) The biochemistry and cell biology of
antigen processing and presentation. Ann Rev Immunol, 11: 403-450.
Germolec DR, Yang RSH, Ackermann MF, Rosenthal GJ, Boorman GA, Blair
P, & Luster MI (1989) Toxicology studies of a chemical mixture of 25
groundwater contaminants. II. Immunosuppression in B6C3F1 mice.
Fundam Appl Toxicol, 13: 377-387.
Ghoneum MH, Egami N, Ljiri K & Cooper EL (1986) Effect of
corticosteroids on the thymus of the fish Oryzias latipes. Dev Comp
Immunol, 10: 35-44.
Giannini SH (1990) Effects of UVB on infectious diseases. In: White JC
ed. Global atmospheric change and public health. Amsterdam, Elsevier
Science Publishers, pp 33-45.
Giannini SH (1992) Effects of UV radiation on cutaneous leishmaniasis.
Parasitol Today, 8: 44-48.
Gibson GG, Hubbard R, & Parke DV (1983) Immunotoxicology. London,
Academic Press.
Gilmartin WG, Delong RL, Smith AW, Sweeney JC, De Lappe BW, Risebrough
RW, Griner LA, Dailey MD, & Peakall DB (1976) Premature parturition in
the California sea lion. J Wildl Dis, 12: 104-115.
Gilmour MI & Selgrade MJK (1993) A comparison of the pulmonary
defenses against streptococcal infection in rats and mice following
O3exposures: A possible disease resistance in rats. Toxicol Appl
Pharmacol, 1233: 211-218.
Gilmour MI, Park P, Doefler D, & Selgrade MJK (1993) Factors which
influence the suppression of pulmonary anti-bacterial defenses in mice
exposed to ozone. Exp Lung Res, 9: 229-314.
Glaser R, Kiecolt-Glaser JK, Speicher CE, & Holliday JE (1985) Stress,
loneliness, and changes in herpesvirus latency. J Behav Med,
8: 249-250.
Glazier A, Tutschka PJ, Farmer ER, & Santos GW (1983) Graft-versus-
host disease in cyclosporin A-treated rats after syngeneic and
autologous bone marrow reconstitution J Exp Med, 158: 1-8.
Gleichmann E, Vohr, H-W, Stringer C, Nuyens J, & Gleichmann H (1989)
Testing the sensitization of T cell to chemicals. From murine graft-
versus-host (GVH) reactions to chemical-induced GVH-like immunological
diseases. In: Kammüller ME, Bloksma N, & Seinen W ed. Autoimmunity and
toxicology: Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers, pp 364-390.
Glickman LT, Shofer FS, Payton AJ, Laster LL, & Felsburg PJ (1988)
Survey of serum IgA, IgG, and IgM concentrations in a large beagle
population in which IgA deficiency had been identified. Am J Vet Res,
49: 1240-1245.
Goettsch W, Garssen J, De Gruijl FR, & Van Loveren H (1993) UV-B and
the immune system: A review with special emphasis on T cell-mediated
immunity. Thymus, 21: 93-114.
Goings SAJ, Kulle TJ, Bascom R, Sauder LR, Green DJ, Hebel R, &
Clements ML (1989) Effects of nitrogen dioxide exposure on
susceptibility to influenza A virus infection in healthy adults.
Am Rev Respir Dis, 139: 1075-1081.
Goldstein BD (1977) Hematotoxicity in humans. J Toxicol Environ
Health, 2(Suppl): 69-106.
Goldstein A, Lowney LI, & Pal BK (1971) Stereospecific and nonspecific
interactions of the morphine congener levorphanol in subcellular
fractions of mouse brain. Proc Natl Acad Sci USA, 68: 1742-1752.
Golemboski KA, Bloom SE, & Dietert RR (1992) Assessment of neonatal
avian inflammatory macrophage function following embryonic
cyclophospha-mide exposure. Int J Immunopharmacol, 14: 19-26.
Gonçalo S, Gonçalo M, Azenha A, Barros MA, Bastos AS, Brandao FM,
Faria A, Marques MSJ, Pecegueiro M, Rodrigues JB, Salgueiro E, &
Torres V (1992) Allergic contact dermatitis in children. Contact Derm,
26: 112.
Good RA & Lorenz E (1992) Nutrition and cellular immunity. Int J
Immunopharmacol, 14: 361-366.
Goodman JW, Peter-Fizaine FE, Shinpock SG, Hall EA, & Famie DJ (1989)
Immunologic and hematologic consequences in mice of exposure to ozone.
JEPTO, 9: 243-252.
Goonewardene IM & Murasko DM (1992) Changes in the immune response.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat. Washington DC, ILSI Press, pp 3-7.
Gopinath C, Prentice DE, & Lewis DJ (1987) The lymphoid system.
In: Atlas of experimental toxicologic pathology. Boston, MTP Press,
pp 122-137.
Gorodilova VV & Mandrik EV (1978) The use of some immunological
reactions for studying the immune response in persons presenting a
high oncological risk. Sov Med, 8: 50-53.
Graham JA & Gardner DE (1985) Immunotoxicity of air pollutants.
In: Dean JH, Luster MI, & Munson AE ed. Immunotoxicology and
Immunopharmacology. New York, Raven Press, pp 367-380.
Gray, D (1993) Immunological memory. Ann Rev Immunol, 11: 47-77.
Greenlee WF, Dold KM, Irons RD, & Osborne R (1985) Evidence for direct
action of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) on thymic
epithelium. Toxicol Appl Pharmacol, 79: 112--120.
Greenstein BD, Fitzpatrick FTA, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 112: 345-350.
Greiner DL, Shultz LD, Rossini AA, Mordes JP, Handler ES, & Rajan TV
(1991) Recapitulation of normal and abnormal BB rat immune system
development in SCID mouse/rat lymphohemopoietic chimeras. J Clin
Invest, 88: 717-719.
Guberski DL (1994) Diabetes-prone and diabetes-resistant BB rats:
Animal models of spontaneous and virally induced diabetes mellitus,
lymphocytic thyroiditis, and collagen-induced arthritis. ILAR News,
35: 29-37.
Gumbiner BM & Yamada KM ed. (1992) Cell-to-cell contact and
extracellular matrix. Curr Opin Cell Biol, 4: 757-874.
Gusev VA, Danilovskaya YV, Vatolkina OY, Lomonosova OS, & Velichkowsky
BT (1993) Effect of quartz and alumina dust on generation of
superoxide radicals and hydrogen peroxide by alveolar macrophages,
granulocytes, and monocytes. Br J Ind Med, 50: 732-735.
Gutierrez-Ramos JC, Andreu JL, Moreno de Alboran I, Rodriguez J,
Leonardo E, Kroemer G, Marcos MAR, & Martinez AC (1990) Insights into
autoimmunity: From classical models to current perspectives. Immunol
Rev, 118: 73-101.
Haas W, Pereira P, & Tonegawa S (1993) Gamma/delta cells. Ann Rev
Immunol, 11: 637-685.
Halliday GM, Cavanaugh LL, & Muller HK (1988) Antigen present in local
lymph node by cells from dimethylbenzanthracene-treated murine
epidermis activate suppressor cells. Cell Immunol, 109: 206-221.
Halprin KM, Comerford M, Presser SE, & Taylor JR (1981) Ultraviolet
light treatment delays contact sensitization to nitrogen mustard.
Br J Dermatol, 105: 71-76.
Hanglow AC, Ernst PB, & Bienenstock J (1990) Intraepithelial
leukocytes, murine. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed.
Hemopoietic system (ILSI Monograph). Berlin, Springer Verlag,
pp 315-322.
Hanley PJ, Hook JW, Raftos DA, Gooley AA, Trent R, & Raison RL (1992)
Hagfish humoral defense protein exhibits structural and functional
homology with mammalian complement components. Proc Natl Acad Sci USA,
89: 7910-7914.
Hansch GM (1992) The complement attack phase: Control of lysis and
non-lethal effects of C5b-9. Immunopharmacology, 24: 107-117.
Hara I (1985) Health status and PCBs in blood of workers exposed to
PCBs and of their children. Environ Health Perspectives, 59: 85-90.
Harriman W, Völk H, Defranoux N, & Wabl M (1993) Immunoglobulin class
switch recombination. Ann Rev Immunol, 11: 361-384.
Harrington W & Krupnick AJ (1985) Short-term nitrogen dioxide exposure
and acute respiratory disease in children. J Air Pollut Control Assoc,
35: 1061-1067.
Hartevelt MM, Bouwes Bavinck JN, Kootte AMM, Vermeer BJ, &
Vandenbroucke JP (1990) Incidence of skin cancer after renal
transplantation in the Netherlands. Transplantation, 49: 506-509.
Hartmann DP (1985) Immunological consequences of asbestos exposure.
Surv Immunol Res, 4: 65-68.
Hartmann DP, Georgian MM, Oghiso Y, & Kagan E (1984) Enhanced
interleukin activity following asbestos inhalation. Clin Exp Immunol,
55: 643-650.
Hasemann CA & Capra JD (1989) Immunoglobulins: Structure and function.
In: Paul WE ed. Fundamental immunology, 2nd Ed. New York, Raven Press,
pp 209-233.
Hashimoto K, Nakanishi T, & Kurosawa Y (1990) Isolation of carp genes
encoding major histocompatibility complex antigens. Proc Natl Acad Sci
USA, 87: 6863-6867.
Haynes RC & Murad F (1985) Adrenocortical steroids and their synthetic
analogs: Inhibitors of adrenocortical steroid biosynthesis. In: Gilman
AG, Goodman LS, Rall TW, & Murad F ed. Goodman and Gilman's
pharmacological basis of therapeutics. New York, Macmillan, p 1459.
Hedrick SM (1989) T Lymphocyte receptors. In: Paul WE ed. Fundamental
immunology, 2nd Ed. New York, Raven Press, pp 291-313.
Heijnen CJ & Kavelaars A (1991) The contribution of neuroendocrine
substances to the immune response. Neth J Med, 39: 281-294.
Heijnen CJ, Kavelaars A, & Ballieux RE (1991) ß-Endorphin: Cytokine
and neuropeptide. Immunol Rev, 119: 41-63.
Helle E, Olsson M, & Jensen S (1976) PCB levels correlated with
pathological changes in seal uteri. Ambio, 5: 261-263.
Hemphill FE, Kaeberle ML, & Buck WB (1971) Lead suppression of mouse
resistance to Salmonella typhimurium. Science, 172: 1031.
Henderson FW, Dubovy EJ, Harder S, Seal E, & Graham D (1988)
Experimental rhinovirus infection in human volunteers exposed to
ozone. Am Rev Respir Dis, 137: 124-127.
Henne T & Schmähl D (1985) Occurrence of second primary malignancies
in man: A second look. Cancer Treat Rev, 12: 77-94.
Henney CS, Kuriayashi K, Kern BE, & Gillis S (1981) Interleukin-2
augments natural killer cell activity. Nature, 291: 335-338.
Herberman RB & Ortaldo JR (1981) Natural killer cells: Their role in
defenses against disease. Science, 214: 24-30.
Herman A, Kappler JW, Marrack P, & Pullen AM (1991) Superantigens:
Mechanism of T-cell stimulation and role in immune responses. Ann Rev
Immunol, 9: 745-772.
Hersey P, Hasic E, Edwards A, Bradley M, Haran G, & McCarthy WH
(1983a) Immunological effects of solarium exposure. Lancet,
i: 545-548.
Hersey P, Haran G, Hasic E, & Edwards A (1983b) Alteration of T cell
subsets and induction of suppressor T cell activity in normal subjects
after exposure to sunlight. J Immunol, 31: 171-174.
Hess AD, Jones RJ, Morris LE, Noga SJ, Vogelsang GB, & Santos GW
(1992) Autologous graft-versus-host disease: A novel approach for
antitumor immunotherapy. Hum Immunol 34: 219-224.
Hinton DM (1992) Testing guidelines for evaluation of the immunotoxic
potential of direct food additives. Crit Rev Food Sci Nutr,
32: 173-190.
Ho M (1980) Role of specific cytotoxic lymphocytes in cellular
immunity against murine cytomegalovirus. Infect Immunol, 27: 767-776.
Ho MK & Springer TA (1984) Preparation and use of monoclonal
antimacrophage antibodies. Methods Enzymol, 108: 313-324.
Hoffman RE (1992) Recent studies of the human immunotoxic effects of
2,3,7,8-tetrachlorodibenzo- p-dioxin. In: Newcombe DS, Rose NR, &
Bloom JC ed. Clinical immunotoxicology. New York, Raven Press,
pp 339-347.
Hoffman RE, Stehr-Green PA, Webb KB, Evans G, Knutsen AP, Schramm WF,
Staake JL, Gibson BG, & Steinberg KK (1986) Health effects of long-
term exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin. J Am Med
Assoc, 255: 2031-2038.
Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR,
Heindell JJ, & Luster MI (1991) Perinatal thymocyte antigen expression
and postnatal immune development altered by gestational exposure to
tetrachlorodibenzo- p-dioxin (TCDD). Teratology, 44: 385-393.
Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, & Mo
JA (1990) Type II collagen autoimmunity in animals and provocations
leading to arthritis. Immunol Rev, 118: 193-232.
Holmes KL & Morse HC (1988) Murine hematopoetic cell surface antigen
expression. Immunol Today, 9: 344-350.
Holmes MA, Duffus WP, & Gorman NT (1989) Natural cytotoxicity in the
dog: Description of two new allogeneic tumour targets. Vet Immunol
Immunopathol, 23: 161-170.
Holsapple MP, Page DG, Shopp GM, & Bick PH (1984) Characterization of
the delayed hypersensitivity response to a protein antigen in the
mouse. Int J Immunopharmacol, 6: 399-405.
Holsapple MP, White KL, McCay JA, Bradley SG, & Munson AE (1988) An
immunotoxicological evaluation of 4,4'-thiobis-(6- t-butyl- m-cresol)
in female B6C3F1 mice. Fundam Appl Toxicol, 10: 701-716.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo- p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-251.
Holt PG & Keast D (1977) Environmentally induced changes in
immunological function: Acute and chronic effects of inhalation of
tobacco smoke and other atmospheric contaminants in man and
experimental animals. Bacteriol Rev, 41: 205-216.
Holt PG & Sedgwick JD (1987) Suppression of IgE responses following
inhalation of antigen. A natural homeostatic mechanism which limits
sensitization to aeroallergens. Immunol Today, 8: 14-15.
Hordvik I, Grimholt U, Fosse VM, Lie O, & Endresen C (1993) Cloning
and sequence analysis of cDNAs encoding the MHC class II ß chain in
Atlantic salmon (Salmo salar). Immunogenetics, 37: 437-441.
Hori S, Obana H, Kashimoto T, Otake T, Nishimura H, Ikegami N, Kunita
N, & Uda H (1982) Effect of polychlorinated biphenyls and
polychlorinated quaterphenyls in cynomolgus monkey (Macaca
fascicularis). Toxicology, 24: 123-139.
Hoshino A, Takenaka H, Mizukoshi O, Imanishi J, Kishida T, & Tovey M
(1983) Effect of anti-interferon serum of influenza virus infection in
mice. Antiviral Res, 3: 59-65.
Houben GF, Van Dokkum W, Van Loveren H, Penninks AH, Seinen W,
Spanhaak S, & Ockhuizen T (1992) Effects of caramel colour III on the
number of blood lymphocytes: A human study on caramel colour III
immunotoxicity and a comparison of the results with data from rat
studies. Food Chem Toxicol, 5: 427-430.
Houben GF, Penninks AH, Seinen W, Vos JG, & Van Loveren H (1993)
Immunotoxic effects of the color additive caramel color III: Immune
function studies in rats. Fundam Appl Toxicol, 20: 30-37.
House RV, Lauer LD, Murray MJ, & Dean JH (1987) Suppression of
T-helper cell function in mice following exposure to the carcinogen
7,12-dimethylbenz[ a]-anthracene and its restoration by
interleukin-2. Int J Immunopharmacol, 9: 89-97.
House RV, Pallardy MJ & Dean JH (1989) Suppression of murine
cytotoxic T-lymphocyte induction following exposure to
7,12-dimethylbenz[ a]anthracene: Dysfunction of antigen recognition.
Int J Immunopharmacol, 11: 207-215.
House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR,
& Dean JH (1990a) Examination of immune parameters and host
resistance mechanisms in B6C3F1 mice following adult exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. J Toxicol Environ Health,
31: 203-215.
House RV, Pearson FC, & Thomas PT (1990b) Selective potentiation of
host resistance in mice following treatment with pyrexol. Mol Biother,
2: 175-178.
Howard SK, Werner PR, & Sleigh SD (1980) Polybrominated biphenyl
toxicosis in swine: Effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol,
55: 146-153.
Hoyle PC & Cooper EC (1990) Nonclinical toxicity studies of antiviral
drugs indicated for the treatment of non-life threatening diseases:
Evaluation of drug toxicity prior to phase I clinical studies. Regul
Toxicol Pharmacol, 11: 81-89.
Hu HZ, Queirò MR, Tilanus MGJ, De Weger RA, & Schuurman H-J (1993)
Expression of T-cell receptor aand b variable genes in normal and
malignant human T cells. Br J Haematol, 84: 39-48.
Hughes & Nei (1993) Evolutionary relationships of the classes of major
histocompatibility complex genes. Immunogenetics, 93: 337-346.
Huiskamp R, Davids JAG, & Vos O (1983) Short- and long-term effects of
whole-body irradiation with fission neutrons or X rays on the thymus
in CBA mice. Radiat Res, 95: 370-381.
Huiskamp R, Davids JAG, & Van Ewijk W (1988) The effect of graded
doses of fission neutrons or X rays on the stromal compartment of the
thymus in mice. Radiat Res, 113: 25-39.
Hünig T, Wallny HJ, Hastley JK, Lawetzky A, & Tiefenthaler G (1989) A
monoclonal antibody to a constant determinant of the rat T cell
antigen receptor that induces T cell activation. Differential
reactivity with subsets of immature and mature T lymphocytes. J Exp
Med, 169: 73-86.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42 (IARC monographs on the evaluation
of carcinogenic risks to humans, Suppl 7). Lyon.
IARC (1990) Ciclosporin. In: Pharmaceutical drugs (IARC monographs on
the avaluation of carcinogenic risks to humans, Vol. 50). Lyon,
pp 77-114.
Imanishi J, Nomura H, Matsubara M, Kita M, Won S-J, Mizutani T, &
Kishida T (1980) Effect of polychlorinated biphenyl on viral
infections in mice. Infect Immunol, 29: 275-277.
Inada T & Mims CA (1985) Association of virulence of murine
cytomegalovirus with macrophage susceptibility and with virion-bound
non-neutralizing antibody. J Gen Virol, 66: 879-882.
Institoris L, Siroki O, Toth S, & Desi I (1992) Immunotoxic effects of
MTP-IP containing 60% methylparathion in mice. Hum Exp Toxicol,
11: 11-16.
Institoris L, Siroki O, & Desi I (1995) Immunotoxicity study of
repeated small doses of dimethoate and methylparathion administered to
rats over three generations. Hum Exp Toxicol, 14: 879-883.
Institoris L, Siroki O, Fekete K, & Desi I (in press)
Immunotoxicological investigation of repeated small doses of
dichlorvos (DDPV) in three generations of rats. Int J Environ Health
Res.
IPCS (1978) Environmental health criteria 6: Principles and methods
for evaluating the toxicity of chemicals. Part I. Geneva, WHO.
IPCS (1986) Immunotoxicology. Development of predictive testing for
determining the immunotoxic potential of chemicals. Report of a
technical review meeting. London, November 3-5, 1986. Geneva, WHO.
IPCS (1992) Environmental health criteria 141: Quality management for
chemical safety testing. Geneva, WHO.
IPCS (1994a) Environmental health criteria 155: Biomarkers and risk
assessment: Concepts and principles. Geneva, WHO.
IPCS (1994b) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO.
IPCS/Department of Health (1995) Proceedings of International
Programme on Chemical Safety and UK Department of Health International
Workshop on Environmental Immunotoxicology and Human Health, Oxford,
22-25 March, 1994. Hum Exp Toxicol, 14: 77-154.
Irons RD (1985) Histology of the immune system: Structure and
function. In: Dean J, Luster MI, Munson AE, & Amos H ed.
Immunotoxicology and immunopharmacology. New York, Raven Press,
pp 11-22.
Ishiguro H, Kobayashi K Suzuki M, Titani K, Tomonaga S, & Kurosawa Y
(1992) Isolation of a hagfish gene that encodes a complement
component. EMBO J, 11: 829-837.
Jacob CO, Van Der Meide PH, & McDevitt HO (1987) In vivo treatment of
(NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma-
interferon. J Exp Med, 166: 798-803.
Jakab GJ & Hmieleski RR (1988) Reduction of influenza virus
pathogenesis by exposure to 0.5 ppm ozone. J Toxicol Environ Health,
23: 455-472.
Janeway CA (1992) The T-cell receptor as a multicomponent signalling
machine: CD4/CD8 coreceptors and CD45 in T-cell activation. Ann Rev
Immunol, 10: 645-674.
Jaremin B (1983) Blast lymphocyte transformation (LTT), rosette
(E-RFC) and leukocyte migration inhibition (MIF) tests in persons
exposed to the action of lead during work. Report II. Bull Inst Mar
Trop Med (Gdynia), 34: 187-197.
Jeevan A & Kripke ML (1990) Alteration of the immune response to
Mycobacterium bovis BCG in mice exposed chronically to low doses of
UV radiation. Cell Immunol, 130: 32-41.
Jeevan A, Gilliam K, Heard H, & Kripke ML (1992) Effects of
ultraviolet radiation on the pathogenesis of Mycobacterium
lepraemurium infection in mice. Exp Dermatol, 1: 152-160
Jefferies WA (1988) Hemopoietic and T-lymphocyte marker antigens of
the rat characterized with monoclonal antibodies. In: Miyasaka M &
Trnka Z ed. Differentiation antigens in lymphohemopoietic tissues. New
York, Marcel Dekker, pp 173-247.
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunological
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachloro- p-dioxin. Br J Ind Med, 45: 701-704.
Jerne NK, Henry C, Nordin AA, Fun H, Koros MC, & Lefkovits I (1974)
Plaque forming cells: Methodology and theory. Transplant Rev,
18: 130-191.
Jessup JM, Hanna N, Palaszynski E, & Kripke ML (1978) Mechanisms of
depressed reactivity to dinitrochlorobenzene and ultraviolet-induced
tumours during ultraviolet carcinogenesis in BALB/c mice. Cell
Immunol, 38: 105-115.
Johnson KW, Holsapple MP, & Munson AE (1986) An immunotoxicological
evaluation of g-chlordane. Fundam Appl Toxicol, 6: 317.
Johnson KW, Munson AE, Kim DH, & Holsapple MP (1987) Role of
reactive metabolites in suppression of humoral immunity by
N-nitrosodimethylamine. J Pharmacol Exp Ther, 240: 847-855.
Johnson JD, Houchens DP, De Kluwe WM, Craig DK, & Fisher GL (1990)
Effects of mainstream and environmental tobacco smoke on the immune
system of animals and humans: A review. CRC Crit Rev Toxicol,
20: 369-395.
Joling P, Tielen FJ, Vaessen LMB, Hesse CJ, & Rozing J (1985)
Intrathymic differentiation in the rat. Adv Exp Med Biol,
186: 235-244.
Joling P (1987) Allografting and the T cell system. A multiparameter
analysis of rejection in the rat. PhD Thesis, Rotterdam, Erasmus
University.
Jones TC, Ward JM, Mohr U, & Hunt RD (1990) Hemopoietic system (ILSI
monograph). Berlin, Springer Verlag.
Kafafi SA, Afeefy HY, Ali AH, Said HK, & Kafafi AG (1993) Binding of
polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ
Health Perspectives, 101: 422-425.
Kagan E, Solomon A, Cochrane JC, Beissner EI, Gluckman J, Rocks PH, &
Webster I (1977a) Immunological studies of patients with asbestosis.
I. Studies of cell-mediated immunity. Clin Exp Immunol, 28: 261-267.
Kagan E, Solomon A, Cochrane JC, Kuba P, Rocks PH, & Webster I (1977b)
Immunological studies of patients with asbestosis. II. Studies of
circulating lymphoid numbers and humoral immunity. Clin Exp Immunol,
28: 268-275.
Kagan E, Jacobson RJ, Yeung KY, Haidak DJ, & Nachnani GH (1979)
Asbestos-associated neoplasms of B cell lineage. Am J Med,
67: 325-330.
Kahan BD (1989) Cyclosporine. New Engl J Med, 321: 1725-1738.
Kalimo K, Koulu L, & Jansen CT (1983) UV exposure and
dinitrochlorobenzene (DNCB). Arch Dermatol Res, 275: 374-378.
Kalpazanov Y, Stamenova M, & Kurchatova G (1976) Air pollution and the
1974 - 1975 influenza epidemic in Sofia. Environ Res, 12: 1-8.
Kamel OW, Van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA,
Montgomery PG, Warnke RA, & Dorfman RF (1993) Reversible lymphoma
associated with Epstein-Barr virus occurring during methotrexate
therapy for rheumatoid arthritis and dermatomyositis. New Engl J Med,
328: 1317-1321.
Kammüller ME & Bloksma N (1994) Drug-induced autoimmunity. In: Dean J,
Luster MI, Munson AE, & Kimber I ed. Immunotoxicology and
immunopharmacology. New York, Raven Press, pp 573-588.
Kammüller ME, Bloksma N, & Seinen W (1989) Autoimmunity and
toxicology. Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers.
Kampinga J, Berges S, Boyd RL, Brekelmans P, Colic M, Van Ewijk W,
Kendall M, Ladyman H, Nieuwenhuis P, Ritter MA, Schuurman HJ, &
Tournefier A (1989) Thymic epithelial antibodies: Immunohistological
analysis and introduction of nomenclature. Thymus, 13: 165-173.
Kannan K, Singh KP, Goel SK, & Shanker R (1985) Effect of
2,5-hexanediol on immunocompetence of mice. Environ Res, 36: 14.
Kannan K, Tanabe S, Borrell A, Aguilar A, Focardi S, & Tatsukawa R
(1993) Isomer-specific analysis and toxic evaluation of
polychlorinated biphenyls in striped dolphins affected by an epizootic
in the western Mediterranean Sea. Arch Environ Contam Toxicol,
25: 227-233.
Kasahara M, Vazquez M, Sato K, McKinney EC, & Flajnik MF (1992)
Evolution of the major histocompatibility complex: Isolation of class
II A cDNA clones from the cartilagenous fish. Proc Natl Acad Sci USA,
89: 6688-6692.
Kasai M, Iwamari M, Nagai T, Okumura K, & Tada T (1980) A glycolipid
on the surface of mouse natural killer cells. Eur J Immunol,
10: 175-180.
Kasai M, Yoneda T, Habu S, Maruyana Y, Okumura K, & Tokunaga T (1981)
In vivo effect of anti-asialo GM-1 antibody on natural killer cell
activity. Nature, 291: 334-335.
Kasl SV, Evans AS, & Neiderman JC (1979) Psychosocial risk factors
in the development of infectious mononucleosis. Psychosom Med,
41: 445-466.
Kateley JR & Bazzell SJ (1978) Immunological studies in cattle exposed
to polybrominated biphenyls. Environ Health Perspectives, 23: 75-82.
Kawabata TT & White KL (1987) Suppression of the in vitro humoral
immune response of mouse splenocytes by benzo( a)pyrene metabolites
and inhibition of benzo( a)pyrene-induced immunosuppression by
a-naphthoflavone. Cancer Res, 47: 2317-2322.
Kawabata TT & White KL (1990) Effects of naphthalene and naphthalene
metabolites on the in vitro humoral immune response. J Toxicol
Environ Health, 30: 53-67.
Keck G (1981) Effets de la contamination par les polychlorobiphenyles
(PCB) sur le developpement de la tumeur d'Ehrlich chez la souris
Swiss. Toxicol Eur Res, 3: 229-236.
Kendall MD (1980) The occurrence of erythropoiesis in the thymus of
the bank vole (Clethrionomys glareolus). Cell Tissue Res,
212: 307-314.
Kendall MD (1991) Functional anatomy of the thymic microenvironment.
J Anat, 177: 1-29.
Kendall MD & Al-Shawaf AA (1991) Innervation of the thymus gland.
Brain Behav Immunol, 5: 9-28.
Kendall MD & Frazier JA (1979) Ultrastructural studies on
erythropoiesis in the avian thymus. I. Description of cell types. Cell
Tissue Res, 199: 37-61.
Kendall MD & Singh J (1980) The presence of erythroid cells in the
thymus gland of man. J Anat, 130: 183-189.
Kendall MD & Ward P (1974) Erythropoiesis in an avian thymus. Nature,
249: 3 66-367.
Kendall MD, Fitzpatrick FTA, Greenstein BD, Khoylou F, Safieh B, &
Hamblin A (1990) Reversal of ageing changes in the thymus of rats by
chemical or surgical castration. Cell Tissue Res, 261: 555-564.
Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V,
Huelskamp AM, & Davidson NE (1994) Phase I trial of interferon gamma
to potentiate cyclosporine-induced graft-versus-host disease in women
undergoing autologous bone marrow transplantation for breast cancer.
J Clin Oncol, 12: 249-257.
Kerkvliet NI & Baecher-Steppan L (1988) Suppression of allograft
immunity by 3,4,5,3 ',4 ',5 '-hexachlorobiphenyl. I. Effects of
exposure on tumor rejection and cytotoxic T cell activity.
Immunopharmacology, 16: 1-12.
Kerkvliet NI & Brauner JA (1987) Mechanisms of 1,2,3,4,6,7,8-
heptachlorodi-benzo- p-dioxin (HpCDD)-induced humoral suppression:
Evidence of primary defect in T-cell regulation. Toxicol Appl
Pharmacol, 87: 18-31.
Kerkvliet NI & Burleson GR (1994) Immunotoxicity of TCDD and related
halogenated aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE,
& Kimber I ed. Immunotoxicity and immunopharmacology, 2nd Ed. New
York, Raven Press, pp 97-121.
Kerkvliet NI & Kimeldorf DJ (1977) Antitumor activity of a
polychlorinated biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J Natl Cancer Inst, 59: 951-955.
Kerkvliet NI, Steppan LB, Smith BB, Youngberg JA, Henderson MC, &
Buhler DR (1990) Role of the Ah locus in suppression of cytotoxic T
lymphocyte (CTL) activity by halogenated aromatic hydrocarbons (PCBs
and TCDD): Structure-activity relationships and effects in C57Bl/6
mice. Fundam Appl Toxicol, 14: 532-541.
Khan MF & Gupta GS (1991) Cellular and biochemical indices of
bronchoalveolar lavage for detection of lung injury following insult
by airborne toxicants. Toxicol Lett, 58: 239-255
Khangarot BS & Tripathi DM (1991) Changes in humoral and cell-mediated
immune responses and in skin and respiratory surfaces of catfish,
Saccobranchus fossilis, following copper exposure. Ecotox Environ
Saf, 22: 291-308.
Khansari DN, Murgo AJ, & Faith RE (1990) Effects of stress on the
immune system. Immunol Today, 11: 170-175.
Kiecolt-Glaser JK & Glaser R (1986) Psychological influences on
immunity. Psychonomatics, 27: 621-624.
Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday
JE, & Speicher CE (1986) Modulation of cellular immunity in medical
students. J Behav Med, 9: 5-21.
Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, &
Glaser R (1987) Marital quality, marital disruption, and immune
function. Psychosom Med, 49: 13-33.
Kim JH (1989) Infection and cyclosporin. Rev Infect Dis, 11: 677-690.
Kim DH, Yang KH, Johnson KW, & Holsapple MP (1987) Suppression of
in vitro antibody production by dimethylnitrosamine in mixed
cultures of mouse primary hepatocytes and mouse splenocytes. Toxicol
Appl Pharmacol, 87: 32-42.
Kimber I & Cumberbatch M (1992) Dendritic cells and cutaneous immune
responses to chemical allergens. Toxicol Appl Pharmacol, 117: 137-146.
Kimber I, Stonard MD, Gidlow DA, & Niewola Z (1986) Influence of
chronic low-level exposure to lead on plasma immunoglobulin
concentration and cellular immune function in man. Int Arch Occup
Environ Health, 57: 117-125.
Kimbrough RD (1987) Human health effects of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls (PBBs). Ann Rev Pharmacol Toxicol,
27: 87-11.
Kimchi A (1992) Cytokine triggered molecular pathways that control
cell cycle arrest. J Cell Biochem, 50: 1-9.
Kimmel CA (1990) Quantitative approaches to human risk assessment for
noncancer health effects. Neurotoxicology, 11: 189-198.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Kincade PW (1992) Cell interaction molecules and cytokines which
participate in B lymphopoiesis. Baillieres Clin Haematol, 5: 575-598.
King AG, Landbreth KS, & Wierda D (1987) Hydroquinone inhibits bone
marrow pre-B cell maturation in vitro. Mol Pharmacol, 32: 807-812.
Kingsmore SF, Hall BD, Alen NB, Rice JR, & Caldwell DS (1992)
Association of methotrexate, rheumatoid arthritis and lymphoma: Report
of 2 cases and literature review. J Rheumatol, 19: 1462-1465.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Klareskog L & Olsson T (1990) Autoimmunity to collagen II and myelin
basic protein: Comparative studies in humans and rodents. Immunol Rev,
118: 285-310.
Klein J (1986) Natural history of the major histocompatibility
complex. New York, John Wiley and Sons.
Klein J (1990) Immunology. Oxford, Blackwell Scientific Publications.
Kleinbeck ML, Hites MJ, Loker JL, Halliwell RE,& Lee KW (1989) Enzyme-
linked immunosorbent assay for measurement of allergen-specific IgE
antibodies in canine serum. Am J Vet Res, 50: 1831-1839.
Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, & Von dem
Borne AEGK ed. (1989) Leucocyte typing IV: White cell differentiation
antigens. Oxford, Oxford University Press.
Knight R, Sarlis N, & Stephanou A (1992) Interleukins and
neurohormones: A common language. Postgrad Med J, 68: 603-605.
Kocks C & Rajewski K (1989) Stable expression and somatic
hypermutation of antibody V regions in B cell developmental pathways.
Ann Rev Immunol, 7: 537-559.
Koller LD (1977) Enhanced polychlorinated biphenyl lesions in Moloney
leukemia virus-infected mice. Clin Toxicol, 11: 107-116.
Koller LD (1980) Immunotoxicology of heavy metals. Int J
Immunopharmacol, 2: 269-279.
Koller LD (1990) The immunotoxic effects of lead in lead-exposed
laboratory animals. Ann NY Acad Sci, 587: 160-167.
Koller LD & Kovacic S (1974) Decreased antibody formation in mice
exposed to lead. Nature, 250: 148-150.
Koller LD & Roan JG (1977) Effects of lead and cadmium on mouse
peritoneal macrophages. J Reticuloendothel Soc, 21: 7.
Koller LD & Roan JG (1980) Effects of lead, cadmium and methylmercury
on immunological memory. J Reticuloendothel Soc, 21: 7-12.
Koller LD & Thigpen JE (1973) Biphenyl-exposed rabbits. Am J Vet Res,
34: 1605-1606.
Kongshavn PAL, Sadarangani C, & Skamene E (1980) Cellular mechanisms
of genetically-determined resistance to Listeria monocytogenes.
In: Skamene E ed. Genetic control of natural resistance. New York,
Academic Press, pp 149-163.
Koren HS & Blomberg PA (in press) Respiratory responses of asthmatics
to ozone. Int Arch Allergy.
Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo
WJ, Becker S, House DE, McDonnel WF, & Bromberg PA (1989) Ozone-
induced inflammation in the lower airways of human subjects. Am Rev
Respir Dis, 139: 407-415.
Koren HS, Devlin RB, & Becker S (1994) Ozone-induced inflammatory
response in pulmonary cells. In: Schook LB & Laskin DL ed. Xenobiotics
and inflammation: Roles of cytokines and growth factors. New York,
Academic Press, pp 249-281.
Krajnc EI, Wester PW, Loeber JG, Van Leeuwen FXR, Vos JG, Vaessen
HAMG, & Van der Heijden CA (1984) Toxicity of bis(tri-n-butyltin)oxide
in the rat. I. Short-term effects on general parameters and on the
endocrine and lymphoid systems. Toxicol Appl Pharmacol, 75: 363-386.
Krajnc-Franken MAM, Van Loveren H, Schuurman HJ, & Vos JG (1990) The
immune system as a target for toxicity. A tiered approach of testing,
with special emphasis on histopathology. In: Dayan AD, Hertel RF,
Heseltine E, Kazantis G, Smith EM, & Van der Venne MT ed.
Immunotoxicity of metals and immunotoxicology. New York, Plenum Press,
pp 241-264.
Kramer CM, Johnson KW, Dooley RK, & Holsapple MP (1987) 2,3,7,8-
Tetrachlorodibenzo- p-dioxin (TCDD) enhances antibody production and
protein kinase activity in murine B cells. Biochem Biophys Res Commun,
145: 25-33.
Kripke ML (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J Natl Cancer Inst, 53: 1333-1336.
Kripke ML, Thorn RM, Lill PH, Civin CI, Pazmino NH, & Fisher MS (1979)
Further characterization of immunological unresponsiveness induced in
mice by ultraviolet radiation. Transplantation, 28: 212-217.
Kripke ML, Cox PA, Alas LG, & Yarosh DB (1992) Pyrimidine dimers in
DNA initiate systemic immunosuppression in UV-irradiated mice. Proc
Natl Acad Sci USA, 89: 7516-7520.
Krishna VR, Fouant MM, & Bradley SG (1989) Effects of pyran copolymer
on host resistance of mice to Plasmodium yoelii. J Parasitol,
75: 405-410.
Kroese FGM, Timens W, & Nieuwenhuis P (1990) Germinal center reaction
and B lymphocytes: Morphology and function. In: Grundmann E & Vollmer
E ed. Reaction patterns of the lymph node. Part 1. Cell types and
functions. Berlin, Springer Verlag, pp 103-148.
Kroplien U, Rosdorfer J, van der Greef J, Long RC, & Goldstein JH
(1985) 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)imidazole: Detection
in commercial caramel color III and preparation by a model browning
reaction. J Org Chem, 50: 1131-1133.
Krowka JF, Sarin S, Namikawa R, McCune JM, & Kaneshima H (1991) Human
T cells in the SCID-hu mouse are phenotypically normal and
functionally competent. J Immunol, 146: 3751-3756.
Kubota M, Kagamimori S, Yokoyama K, & Okada A (1985) Reduced killer
cell activity of lymphocytes from patients with asbestosis. Br J Ind
Med, 42: 276-280.
Kunita N, Hori S, Obana H, Otake T, Nishimura H, Kashimoto T, &
Ikegami N (1985) Biological effect of PCBS, PCQs and PCDFs present in
the oil causing yusho and yu-cheng. Environ Health Perspectives,
59: 79-84.
Kunkel SL, Chensue SW, Strieter RM, Lynch JP, & Remick DG (1989)
Cellular and molecular aspects of granulomatous inflammation. Am J
Respir Cell Mol Biol, 1: 439-447.
Kuper CF, Beems RB, & Hollanders VMH (1990a) Development and aging;
thymus, rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic
system (ILSI Monograph). Berlin, Springer Verlag, pp 257-263.
Kuper CF, Hameleers DMH, Bruijntjes JP, Van der Ven I, Biewenga J, &
Sminia T (1990b) Lymphoid and non-lymphoid cells in nasal-associated
lymphoid tissue (NALT) in the rat. An immuno- and enzymehistochemical
study. Cell Tissue Res, 259: 371-377.
Kuper CF, Beems RB, Bruijntjes JP, Schuurman H-J, & Vos JG (1992a)
Normal development, growth and aging of the thymus. In: Mohr U,
Dungworth DL, & Capen CC ed. Pathobiology of the aging rat, Vol. 1.
Washington DC, ILSI Press, pp 25-49.
Kuper CF, Koornstra PJ, Hameleers DMH, Biewenga J, Spit BJ,
Duijvestijn AM, Van Breda Vriesman PJC, & Sminia T (1992b) The role of
nasopharyngeal lymphoid tissue. Immunol Today, 13: 219-224.
Kuper CF, Schuurman H-J, & Vos JG (1995) Pathology in immunotoxicology.
In: Burleson G, Munson A, & Dean J ed. Modern methods in immuno-
toxicology. New York, John Wiley & Sons, pp 397-436.
Kyle RA (1984) Second malignancies associated with chemotherapy. In:
Perry MC & Yarbro JW ed. Toxicity of chemotherapy. New York, Grune &
Stratton, pp 479-506.
Labarca C & Paigen KA (1980) A simple, rapid, and sensitive DNA assay
procedure. Anal Biochem, 102: 344-352.
Ladics GS, Kawabata TT, & White KL (1991) Suppression of the
in vitro humoral immune response of mouse splenocytes by
7,12-dimethylbenz[ a]anthracene metabolites and inhibition of
immunosuppression by a-naphthflavone. Toxicol Appl Pharmacol,
110: 31-44.
Ladics GS, Kawabata TT, Munson AE, & White KL (1992) Metabolism of
benzo(a)pyrene by murine splenic cell types. Toxicol Appl Pharmacol,
116: 248-257.
Ladics GS, Smith C, Heaps K, Elliott GS, Slone TW, & Loveless SE
(1995) Possible incorporation of an immunotoxicological functional
assay for assessing humoral immunity for hazard identification
purposes in rats on standard toxicology study. Toxicology,
96: 225-238.
LaGrange PH, Mackaness GB, & Miller TE (1974) Influence of dose and
route of antigen injection on the immunological induction of T cells.
J Exp Med, 139: 528-542.
Laman JD, Van den Eertwegh AJM, Claassen E, & Van Rooijen N (1992)
Cell-cell interactions: In situ studies of splenic humoral immune
responses. In: Fornusek L & Vetvicka V ed. Immune system accessory
cells. Boca Raton, Florida, CRC Press, pp 201-231.
Lambris JD (1993) The chemistry, biology and phylogeny of C3.
In: Cruse JM & Lewis RE ed. Complement today, Vol. 1. Basel, Karger,
pp 16-45.
Lampeter EF, Signore A, Gale EAM, & Pozzilli P (1989) Lesson from the
NOD mouse for the pathogenesis and immunotherapy of human type 1
(insulin-dependent) diabetes mellitus. Diabetologia, 32: 703-708.
Lang D, Meier KL, & Luster MI (1993) Comparative effects of
immunotoxic chemicals on in vitro proliferative responses of human
and rodent lymphocytes. Fundam Appl Toxicol, 21: 535-545.
Lanier LL, Benike CJ, Phillips JH, & Engleman EG (1985) Recombinant
interleukin-2 enhanced natural killer cell-mediated cytotoxicity in
human lymphocyte subpopulations expressing the leu-7 and leu-11
antigens. J Immunol, 2: 794-801.
Larrick JW & Wright SC (1992) Native cytokine antagonists. Baillieres
Clin Haematol, 5: 681-702.
Lasky LA (1992) Selectins: Interpreters of cell-specific carbohydrate
information during inflammation. Science, 258: 964-969.
Lawlor GJ Jr & Fischer TJ ed. (1988) Manual of allergy and immunology.
Diagnosis and therapy, 2nd Ed. Boston, Little, Brown & Co.
Lawrence DH (1981) In vivo and in vitro effects of lead on humoral
and cell-mediated immunity. Infect Immun, 31: 136-143.
Lawrence DA (1985) Immunotoxicity of heavy metals. In: Dean JH, Luster
MI, Munson AE, & Amos H ed. Immunotoxicology and immunopharmacology.
New York, Raven Press, p 341.
Lawrence D, Mudzinski S, Rudofsky U, & Warner A (1987) Mechanism of
metal-induced immunotoxicity. In: Berlin A, Dean J, Draper MH, Smith
EMB, & Spreafico F ed. Immunotoxicology. Dordrecht, Martinus Nijhoff,
pp 293-307.
Lea T, Stelen K, & Stormer FC (1989) Mechanism of ochratoxin A-induced
immunosuppression. Mycopatholgia, 107: 153-159.
Lebish IJ, Hurvitz A, Lewis RM, Cramer DV, & Krakowka S (1986)
Immunopathology of laboratory animals. Toxicol Pathol, 14: 129-134.
Leiter EH (1993) The NOD mouse: A model for analyzing the interplay
between heredity and environment in development of autoimmune disease.
ILAR News, 35: 4-14.
Lerman SP & Weidanz WP (1970) The effect of cyclophosphamide on the
ontogeny of the humoral immune response in chickens. J Immunol,
105: 614-619.
LeVier DG, Brown RD, McCay JA, Fuchs BA, Harris LS, & Munson AE (1993)
Hepatic and splenic phagocytosis in female B6C3F1 mice implanted with
morphine sulfate pellets. J Pharmacol Exp Ther, 267: 357-363.
Levy SM, Herberman RB, Lee J, Whiteside T, Beadle M, Heiden L ,&
Simons A (1991) Persistently low natural killer cell activity, age,
and environmental stress as predictors of infectious morbidity. Nat
Immun Cell Growth Regul, 10: 289-307.
Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, & Bekesi JG (1986)
High frequency of immune dysfunctions in asbestos workers and in
patients with malignant mesothelioma. J Clin Immunol, 6: 225-233.
Li FY & Richters A (1991) Effects of 0.7 ppm ozone exposure on
thymocytes: In vivo and in vitro studies. Inhal Toxicol, 3: 61-71.
Lichtman MA (1981) The ultrastructure of the hemopoietic environment
of the marrow: A review. Exp Hematol, 9: 391-410.
Like AA, Butler L, Williams M, Appel MC, Weringer EJ, & Rossini AA
(1982) Spontaneous autoimmune diabetes mellitus in the BB rat.
Diabetes, 31(Suppl 1): 7-13.
Litman GW, Rast JP, Shamblott MJ, Haire RN, Hulst M, Roess W, Litman
RT, Hind-Frey KR, Zilch A, & Amemiya CT (1993) Phylogenetic
diversification of immunoglobulin genes and the antibody repertoire.
Mol Biol Evol, 10: 60-72.
Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, & MacLennan ICM
(1989) Mechanism of antigen-driven selection in germinal centres.
Nature, 342: 929-931.
Liu Y-J, Johnson GD, Gordon J, & MacLennan ICM (1992) Germinal centres
in T-cell-dependent antibody responses. Immunol Today, 13: 17-21.
Loo JCK, Tryphonas H, Jordan N, Brien R, Karpinski KF, & Arnold DL
(1989) Effects of Aroclor 1254 on hydrocortisone levels in adult
rhesus monkeys (Macaca mulatta). Bull Environ Contam Toxicol,
43: 667-669.
Loose LD, Pittman KA, Benitz K-F, & Silkworth JB (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J Reticuloendothel Soc, 22: 253-271.
Loose LD, Silkworth JB, Pittman KA, Benitz K-F, & Mueller W (1978)
Impaired host resistance to endotoxin and malaria in polychlorinated
biphenyl- and hexachlorobenzene-treated mice. Infect Immun, 20: 30-35.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspectives, 39: 79-91.
Losco P (1992) Normal development, growth and aging of the spleen.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat, Vol. 1. Washington DC, ILSI Press, pp 75-95.
Losco P & Harleman H (1992) Normal development, growth, and aging of
the lymph node. In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology
of the aging rat, Vol. 1. Washington DC, ILSI Press, pp 49-75.
Love RJ, Ogilvie BM, & McLaren DJ (1976) The immune mechanism which
expels the intestinal stage of Trichinella spiralis from rats.
Immunology, 30: 7-13.
Lu YC & Wu YC (1985) Clinical findings and immunological abnormalities
in yu-cheng patients. Environ Health Perspectives, 59: 17-29.
Lubet RA, Lemaire BN, Avery D, & Kouri RE (1986) Induction of
immunotoxicity in mice by polyhalogenated biphenyls. Arch Toxicol,
59: 71-77.
Luckas B, Vetter W, Fischer P, Heidemann G, & Plötz J (1990)
Characteristic chlorinated hydrocarbon patterns in the blubber of
seals from different marine regions. Chemosphere, 21: 13-19.
Luebke RW, Luster MI, Dean JH, & Hayes HT (1984) Altered host
resistance to Trichinella spiralis infection following subchronic
exposure to diethylstilbestrol. Int J Immunopharmacol, 6: 609-617.
Luebke RW, Andrews DL, Copeland CB, Riddle MM, Rogers RR, & Smialowicz
RJ (1991) Host resistance to murine malaria in mice exposed to the
adenosine deaminase inhibitor, 2'-deoxycoformycin. Int J
Immunopharmacol, 13: 987-997.
Luebke RW, Copeland CB, Diliberto JJ, Akubue PI, Andrews DL,
Riddle MM, Williams WC, & Birnbaum LS (1994) Assessment of host
resistance to Trichinella spiralis in mice following preinfection
exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol, 125: 7-16.
Luebke RW, Copeland CB, & Andrews DL (1995) Host resistance
to Trichinella spiralis infection in rats exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Fundam Appl Toxicol,
24: 285-289.
Lukic ML, Popeskovic L, & Jankovic BD (1973) Potentiation of immune
responsiveness in rats treated with DDT. Fed Proc, 32: 1037.
Lundberg K, Dencker L, & Gronvik KO (1992) 2,3,7,8-Tetrachlorodibenzo-
p-dioxin (TCDD) inhibits the activation of antigen-specific T cells
in mice. Int J Immunopharmacol, 14: 699-705.
Lunn JE Knowelden J, & Handyside AJ (1967) Patterns of respiratory
illness in Sheffield infant schoolchildren. Br J Prev Soc Med,
21: 7-16.
Luo YD, Patel MK, Wiederholt MD, & Ou DW (1992) Effects of
cannabinoids and cocaine on the mitogen-induced transformations of
lymphocytes of human and mouse origins. Int J Immunopharmacol,
14: 49-56.
Luster MI, Faith RE, & Moore JA (1978) Depression of humoral immunity
in rats following chronic developmental lead exposure. J Environ
Pathol Toxicol, 1: 397-402.
Luster MI, Hong LH, Boorman GH, Clark G, Hayes HT, Greenlee WF, Dold
K, & Tucker AN (1985a) Acute myelotoxic responses in mice exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Toxicol Appl Pharmacol,
81: 151-165.
Luster MI, Tucker AN, Hayes HT, Pung OJ, Burka T, McMillan R, & Eling
T (1985b) Immunosuppressive effects of benzidine in mice: Evidence of
alterations in arachidonic acid metabolism. J Immunol, 135: 2754-2761.
Luster MI, Tucker AN, Germolec DR, Silver MT, Thomas PT, Vore SJ, &
Bucher JR (1986) Immunotoxicity studies in mice exposed to methyl
isocyanate. Toxicol Appl Pharmacol, 86: 140-144.
Luster MI, Germolec DR, Burleson GR, Jameson CW, Ackerman MF, Lamm KR,
& Hayes HT (1987) Selective immunosuppression in mice of natural
killer cell activity by ochratoxin A. Cancer Res, 47: 2259-2263.
Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL,
Lauer LD, Germolec DR, Rosenthal GJ, & Dean JH (1988) Development of a
testing battery to assess chemical-induced immunotoxicity: National
Toxicology Program's guidelines for immunotoxicity evaluation in mice.
Fundam Appl Toxicol, 10: 2-19.
Luster MI, Ackermann MF, Germolec DR, & Rosenthal GJ (1989)
Perturbations of the immune system by xenobiotics. Environ Health
Perspectives, 81: 157-162.
Luster MI, Portier C, Pait DG, White KL, Gennings C, Munson AE, &
Rosenthal GJ (1992) Risk assessment in immunotoxicology I. Sensitivity
and predictability of immune tests. Fundam Appl Toxicol., 18: 200-210.
Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E,
Blaylock BL, Pollock P, Kouchi Y, Craig W, White KL, Munson AE, &
Comment CE (1993) Risk assessment in immunotoxicology. II.
Relationship between immune and host resistance tests. Fundam Appl
Toxicol, 21: 71-82.
Lyte M & Bick PH (1986) Modulation of interleukin-1 production by
macrophages following benzo( a)pyrene exposure. Int J
Immunopharmacol, 8: 377-381.
Mackaness GB (1969) The influence of immunologically committed
lymphoid cells on macrophage activity in vivo. J Exp Med,
129: 973-992.
Makker SP & Aikawa M (1979) Mesangial glomerulonephropathy with
deposition of IgG, IgM, and C3 induced by mercuric chloride. Lab
Invest, 41: 45-49.
Malins DC, McClain BB, Landahl JT, Meyers MS, Krahn MM, Brow DW, Chan
SL, & Roubal WT (1988) Neoplasia and other diseases in fish in
relation to toxic chemicals: An overview. Aquat Toxicol, 11: 43-67.
Malkovsky M, Loveland B, North M, Asherson GL, Gao L, Word P, & Fiers
W (1987) Recombinant Interleukin-2 directly augments the cytotoxicity
of human monocytes. Nature, 325: 262-265.
Mandell LA (1990) Infections in the immunocompromised host. J Intern
Med Res, 18: 177-190.
Manischewitz JE & Quinnan GV (1980) Antivirus antibody-dependent cell-
mediated immunity during murine cytomegalovirus infection. Infect
Immun, 29: 1050-1054.
Mann DD, Buening GM, Hook B, & Osweiler GD (1983) Effects of T-2 toxin
on bovine serum proteins. Am J Vet Res, 44: 1751-1759.
Manson-Smith DF, Bruce RG, & Parrott DMV (1979) Villous atrophy and
expul-sion of intestinal Trichinella spiralis are mediated by T
cells. Cell Immunol, 47: 285-292.
Marchalonis JJ, Hohman VS, Thomas C, & Schluter SF (1993) Antibody
production in sharks and humans: A role for natural antibodies. Dev
Comp Immunol, 17: 41-53.
Marker SC, Howard RJ, Simmons RL, Kalis JM, Conelly DP, Najarian JS, &
Balfour HH (1981) Cytomegalovirus infection: A quantitative
prospective study of 320 consecutive renal transplants. Surgery,
89: 660-671.
Marsh JA, Dietert RR, & Combs GF (1981) Influence of dietary selenium
and vitamin E on the humoral immune response of the chick. Proc Soc
Exp Biol Med, 166: 228-236.
Martineau D, Béland P, Desjardins C, & Lagacé A (1987) Levels of
organochlorine chemicals in tissues of beluga whales (Delphinapterus
leucas) from the St Lawrence Estuary, Québec, Canada. Arch Environ
Contam Toxicol, 16: 137-147.
Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird FF, Travis W,
Suffredini AF, Deyton l, Kovacs JA, Falloon J, Davey R, Polis M,
Metcalf J, Baseler M, Wesley R, Gill VJ, Fauci AS, & Lane HC (1989)
CD4 counts as predictors of opportunistic pneumonias in human
immunodeficiency virus (HIV) infection. Ann Intern Med, 111: 223-231.
Mathews SE, Warinner JE, & Weeks BA (1990) Assays of immune function
in fish macrophages. In: Stolen JS, Fletcher TC, Anderson DP, Roberson
BS, & van Muiswinkel WB ed. Techniques in fish immunology, Vol. 1.
Fair Haven, New Jersey, SOS Publications, pp 155-163.
Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, &
Katagiri T (1990) A new allele of the lpr locus, lprcg, that
complements the gld gene in induction of lymphadenopathy in the mouse.
J Exp Med, 171: 519-531.
Mayani H, Guilbert LJ, & Janowska-Wieczorek A (1992) Biology of the
hemopoietic microenvironment. Eur J Haematol, 49: 225-233.
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, &
Rattner BA (1992) Physiological and nonspecific biomarkers. In:
Biomarkers, biochemical, physiological and histological markers of
anthropogenic stress. London, Lewis Publishers, pp 5-85.
McCabe MJ (1994) Mechanisms and consequences of immunomodulation by
lead. In: Dean JH, Luster MI, Munson AE, & Kimber I ed. Immunotoxicity
and immunopharmacology, 2nd Ed. New York, Raven Press, pp 143-162.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo- p-dioxin kills immature thymocytes by
Ca2+-mediated endonuclease activation. Science, 242: 256-259.
McConkey DJ, Orrenius S, & Jondal M (1990) Cellular signalling in
programmed cell death (apoptosis). Immunol Today, 11: 120-121.
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, & Weissman
IL (1988) The SCID-hu mouse: Murine model for the analysis of human
hematolymphoid differentiation and function. Science, 241: 1632-1639.
McDonald HR & Lees RK (1990) Programmed death of autoreactive
thymocytes. Nature, 343: 642-644.
McGregor DD, Hahn HH, & Mackaness GB (1973) The mediator of cellular
immunity. V. Development of cellular resistance to infection in
thymectomized irradiated rats. Cell Immunol, 6: 186-199.
Melia RJW, Du V, Florey C, Altman DG, & Swan AV (1977) Association
between gas cooking and respiratory disease in children. Br Med J,
ii: 149-152.
Mellert J, Hopt UT, Erath F, & Holzer H (1989) Differential effects of
azathioprine (aza), cyclosporin A (CSA) and dexamethasone (DEXA) on
lymphokine mediated inflammation (LMI) in rejecting allografts.
Transplant Proc, 21: 98-99.
Meuer SC, Fitzgerald KA, Hussey RE, Hodgdon JC, Schlossman SF, &
Reinherz EL (1983) Clonotypic structures involved in antigen-specific
human T cell function. Relationship to the T3 molecular complex. J Exp
Med, 157: 705-719.
Miller K (1992) Mineral dusts: Asbestos, silica and others.
In: Miller K, Turk JL ,& Nicklin S ed. Principles and practice of
immunotoxicology. Oxford, Blackwell Scientific Publications,
pp 217-227.
Miller MD & Krangel MS (1992) Biology and biochemistry of the
chemokines: A family of chemotactic and inflammatory cytokines. Crit
Rev Immunol, 12: 17-46.
Miller K, & Nicklin S, eds (1987) Immunology of the gastrointestinal
tract. Boca Raton, Florida, CRC Press.
Miller LE, Ludke HR, Peacock JE, & Tomar RH (1991) Manual of
laboratory immunology, 2nd Ed. Philadelphia, Lea & Febiger.
Minchin SA, Leitenberg D, Stunz LL, & Feldbush TL (1990) Polyclonal
activation of rat B cells. II. Dextran sulfate as a cofactor in
mitogen-induced and antigen-induced differentiation of rat B
lymphocytes. J Immunol, 145: 2427-2433.
Mirkin IR, Anderson HA, Handaran L, Hong R, Gilubjatnikov R, & Belluck
D (1990) Changes in T-lymphocyte distribution associated with
ingestion of aldi-carb-contaminated drinking water: A follow-up study.
Environ Res, 51: 35-50.
Mishell RI & Dutton RW (1967) Immunization of dissociated spleen cell
cultures from normal mice. J Exp Med, 126: 423-442.
Mittal A, Woodward B, & Chandra RK (1988) Involution of thymic
epithelium and low serum thymulin bioactivity in weanling mice
subjected to severe food intake restriction or severe protein
deficiency. Exp Mol Pathol, 48: 226-235.
Molfino NA, Wright SC, Katz I, Tarlo S, Silverman F, McClean PA,
Szalai JP, Raizenne M, Slutski AS, & Zamel N (1991) Effect of low
concentrations of ozone on inhaled allergen responses in asthmatic
subjects. Lancet, 338: 199-203.
Moolenbeek C (1982) Obtaining alveolar macrophages from small
laboratory animals. Lab Anim, 16: 56-58.
Morahan PS, Klykken PC, Smith SH, Harris LS, & Munson AE (1979)
Effects of cannabinoids on host resistance to Listeria
monocytogenesand herpes simplex virus. Infect Immun, 23: 670-674.
Morris DL, Snyder NK, Gokani V, Blair RE, & Holsapple MP (1992)
Enhanced suppression of humoral immunity in DBA/2 mice following
subchronic exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD).
Toxicol Appl Pharmacol, 112: 128-132.
Mortensen P, Bergman A, Bignert A, Hansen H-J, Harkonen T, & Olsson
M (1992) Prevalence of skull lesions in harbor seals (Phoca vitulina)
in Swedish and Danish museum collections: 1835-1988. Ambio,
21: 520-524.
Mosier DE (1990) Immunodeficient mice xenografted with human lymphoid
cells: New models for in vivo studies of human immunobiology and
infectious diseases. J Clin Immunol, 10: 185-191.
Mosmann TR & Coffman RL (1989) Heterogeneity and cytokine secretion
patterns and functions of helper T cells. Adv Immunol, 46: 111-147.
Moszczynski P, Bem S, Moszczynski P, & Bartus R (1990a) Indicators of
immunity and acute phase reaction in men in relation to the duration
of exposure to mercury vapors. Med Pract, 41: 169.
Moszczynski P, Lisiewicz J, Bartus R, & Bem S (1990b) The serum
immunoglobulins in workers after prolonged occupational exposure to
the mercury vapors. Rev Roum Méd Intern, 28: 25-30.
Mowat AM (1987) The regulation of immune responses to dietary protein
antigens. Immunol Today, 8: 93-98.
Muir DCG, Ford CA, Stewart REA, Smith TG, Addison RF, Zinck ME, &
Béland P (1990) Organochlorine contaminants in belugas
(Delphinapterus leucas), from Canadian waters. Can Bull Fish Aquat
Sci, 224: 165-190.
Munson AE & White KL (1990) Immunotoxicology. In: Clayson DB, Munro
IC, Shubik P, & Swenberg JA ed. Progress in predictive toxicology.
Amsterdam, Elsevier Scientific Publishers, pp 285-313.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspectives, 43: 41-52.
Murray MJ, Lauer LD, Luster MI, Luebke RW, Adams DO, & Dean JH (1985)
Correlation of murine susceptibility to tumor, parasite and bacterial
challenge with altered cell-mediated immunity following systemic
exposure to the tumor promoter phorbol myristate acetate. Int J
Immunopharmacol, 7: 491-500.
Myers MJ, Schook LB, & Bick PH (1987) Mechanisms of benzo( a)pyrene-
induced modulation of antigen presentation. J Pharmacol Exp Ther,
242: 399-404.
Nagarkatti PS, Sweeney GC, Gauldie J, & Clark DA (1984) Sensitivity to
suppression of cytotoxic T-cell generation by 2,3,7,8-tetra-
chlorodibenzo- p-dioxin (TCDD) is dependent on the Ah genotype of the
murine host. Toxicol Appl Pharmacol, 72: 169-176.
Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, & McCune JM (1990)
Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med,
172: 1055-1063.
Naraqi S, Jackson GG, Jonasson O, & Yamashiroya HJ (1977) Prospective
study of prevalence, incidence and source of herpesvirus infections in
patients with renal allografts. J Infect Dis, 136: 531.
Neas LM, Dockery DD, Ware JH, Spengler JD, Speizer FE, & Ferris BG
(1991) Association of indoor nitrogen dioxide with respiratory
symptoms and pulmonary function in children. Am J Epidemiol,
134: 204-218
Neckers LM & Cossman J (1983) Transferrin receptor induction in
mitogen-stimulated human T lymphocytes is required for DNA synthesis
and cell division and is regulated by interleukin 2. Proc Natl Acad
Sci USA, 80: 3494-3498.
Neefjes JJ & Momburg F (1993) Cell biology of antigen processing. Curr
Opin Immunol, 5: 27-34.
Neubert D (1992) Evaluation of toxicity of TCDD in animals as a basis
for human risk assessment. Toxic Subst J, 12: 237-276.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1990)
Polyhalo-genated dibenzo- p-dioxins and dibenzofurans and the immune
system. 1. Effects on peripheral lymphocyte subpopulations of a
non-human primate (Callitrix jacchus) after treatment with
2,3,7,8-tetrachlorodibenzo-p -dioxin (TCDD). Arch Toxicol,
64: 345-359.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1991)
Polyhalogenated dibenzo- p-dioxins and dibenzofurans and the immune
system. 2. In vitro effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) on lymphocytes of venous blood from man and a non-human primate
(Callitrix jacchus). Arch Toxicol, 65: 213-219.
Newcombe DS, Rose NR, & Bloom JC (1992) Clinical immunotoxicology. New
York, Raven Press.
Nickoloff BJ ed. (1993) Heterogeneity among CD36+ cells in normal
diseased human skin. In: Dermal immune system. Boca Raton, Florida,
CRC Press, pp 245-275.
Nigam SK, Karnik AB, Chattopadhyay P, Lakkad K, Venkaiah K,
& Kashyap SK (1993) Clinical and biochemical investigations to
evolve early diagnosis in workers involved in the manufacture of
hexachlorocyclohexane. Int Arch Occup Environ Health, 65: S193-S196.
Nikolaidos E, Brunström B, Dencker L, & Veromaa T (1990) TCDD inhibits
the support of B-cell development by the bursa of Fabricius. Pharmacol
Toxicol, 67: 22-26.
Nimmo Wilkie JS, Yager JA, Wilkie BN, & Pascoe PJ (1992) Changes in
cell-mediated immune responses after experimentally-induced
anaphylaxis in dogs. Vet Immunol Immunopathol, 32: 325-338.
Noonan FP & De Fabo EC (1990) Ultraviolet-B-dose-response curves for
local and systemic immunosuppression are identical. Photochem
Photobiol, 52: 801-810.
Noonan FP & De Fabo EC (1992) Immunosuppression by ultraviolet-B
radiation: Initiation by urocanic acid. Immunol Today, 13: 250-254.
Noonan FP & Hoffman HA (1994) Susceptibility to immunosuppression by
ultraviolet B radiation in the mouse. Immunogenetics, 39: 29-39.
Nossal GJV (1987) Immunology: The basic components of the immune
system. New Engl J Med, 316: 1320-1325.
Oberhelman L, Koren H, & Cooper KD (1992) Depressed capacity to
contact sensitize women at the onset of menses; elevated contact
sensitization capacity during mid cycle can be inhibited by UV.
Clin Res, 40: 542A.
O'Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE,
Nadel JA, & Holtzman (1984) Neutrophil depletion inhibits airway
hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis,
130: 214-219.
O'Dell BL, Jessen RT, Becker LE, Jackson RT, & Smith EB (1980)
Diminished immune response in sun-damaged skin. Arch Dermatol,
116: 559-561.
Oehler R & Herberman RB (1978) Natural cell-mediated cytotoxicity in
rats. III. Effects of immunopharmacologic treatments on natural
reactivity and reactivity augmented by plyinosinic-polycytidylicacid.
Int J Cancer, 21: 221-229.
Ono H, Klein D, Vincek V, Figueroa F, O'Huigin C, Tichy H, & Klein J
(1992) Major histocompatibility complex class II genes of zebra fish.
Proc Natl Acad Sci USA, 89: 11886-11890.
Orehek J, Grimaldi F, Muls E, Durand JP, Viala A, & Charpin J (1981)
Bronchial response to allergens after controlled NO2exposure. Bull Eur
Physiopathol Respir, 17: 911-915.
Organisation for Economic Co-operation and Development (1981) OECD
guidelines for testing chemicals. Repeated dose oral toxicity -
Rodent: 28-day or 14-day study (Guideline 407). Paris.
Osborn JE, Blazkovec AA, & Walker DL (1968) Immunosuppression during
acute cytomegalovirus infection. J Immunol, 100: 835-844.
Ozkaynak H, Kinney PL, & Burbank B (1990) Recent epidemiological
findings on morbidity and mortality effects of ozone. Air Waste Manage
Assoc Rep, 6: 90-150.
Pallardy MJ, House RV, & Dean JH (1989) Molecular mechanism of
7,12-dimethylbenz[ a]anthracene-induced immunosuppression: Evidence
for action via the interleukin-2 pathway. Mol Pharmacol, 36: 128-133.
Pallardy MJ, Mishal Z, Lebrec H, & Bohuon C (1992) Immune
modification due to chemical interference with transmembrane
signalling: Applications to polycyclic aromatic hydrocarbons.
Int J Immunopharmacol, 14: 377-382.
Paranjpe MS & Boone CW (1972) Delayed hypersensitivity to simian virus
40 tumor cells in BALB/c mice demonstrated by a radioisotopic foot-pad
assay. J Natl Cancer Inst, 48: 563-566.
Parhar RS & Lala PK (1987) Amelioration of B16F10 melanoma lung
metastasis in mice by a combination therapy with indomethacin and
interleukin 2. J Exp Med, 165: 14-28.
Pass RF, Long WK, Whitley RJ, Soong S, Diethelm A, Reynolds DW, &
Alford CA (1978) Productive infection with CMV and HSV in renal
transplant recipients: Role of source of kidney. J Infect Dis,
137: 556.
Patarroyo M (1991) Leukocyte adhesion in host defence and tissue
injury. Clin Immunol Immunopathol, 60: 333-348.
Patki AH (1991) Hypothesis: Solar ultraviolet radiation and the
initial skin lesion of leprosy. Int J Leprosy, 59: 492-493.
Paul WE (1993) Fundamental immunology, 3rd Ed. New York, Raven Press.
Paul PS, Johnson DW, Mirocha CJ, Soper FF, Thoen CO, Muscoplat CC, &
Weber AF (1977) In vitro stimulation of bovine peripheral blood
lymphocytes: Suppression of phytomitogen and specific antigen
lymphocyte responses by aflatoxin. Am J Vet Res, 38: 2033-2035.
Payne JF & Fancey LF (1989) Effect of polycyclic aromatic hydrocarbons
on immune response in fish: Change in melanomacrophage centers in
flounder (Pseudopleuronectes americanus) exposed to hydrocarbon-
contaminated sediments. Mar Environ Res, 28: 431-435.
Pedersen BK & Beyer JM (1986) A longitudinal study of the influence of
azathioprine on natural killer cell activity. Allergy, 41: 286-289.
Penit C & Ezine S (1989) Cell proliferation and thymocyte subset
reconstitution in sublethally irradiated mice: Compared kinetics of
endogenous and intrathymically transferred progenitors. Proc Natl Acad
Sci USA, 86: 5547-5551.
Penn I (1988) Cancer is a long-term hazard of immunosuppressive
therapy. J Autoimmun, 1: 545-558.
Penn I (1993a) Incidence and treatment of neoplasia after
transplantation. J Heart Lung Transplant, 12: 328-336.
Penn I (1993b) Tumors after renal and cardiac transplantation. Hematol
Oncol Clin North Am, 7: 431-445.
Pennington JE (1985) Immunosuppression of pulmonary host defense. Adv
Host Def Mech, 4: 141-164.
Penninks AH, Snoeij NJ, Peiters RHH, & Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: An
overview. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM,
& Van der Venne MT ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, p 191.
Pestka JJ & Bondy GS (1990) Alteration of immune function following
dietary mycotoxin exposure. Can J Physiol Pharmacol, 68: 1009-1016.
Pfeifer R & Irons R (1981) Inhibition of lecithin-stimulated
lymphocyte agglutination and mitogenesis by hydroquinone; reactivity
with sulfhydyl groups. Exp Mol Pathol, 35: 189-198.
Phair J, Munoz A, Detels RI, Kaslow R, Rinaldo C, & Saah A (1990) The
risk of Pneumocystis carinii pneumonia among men infected with human
immunodeficiency virus type 1. New Engl J Med, 322: 161-165.
Picker LJ & Butcher EC (1992) Physiological and molecular mechanisms
of lymphocyte homing. Ann Rev Immunol, 10: 561-591.
Pleiman CM, D'Ambrosio D, & Cambier JC (1994) The B-cell antigen
receptor complex: Structure and signal transduction. Immunol Today,
15: 393-399.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p -dioxin and
related halogenated aromatic hydrocarbons: Examination of the
mechanism of toxicity. Ann Rev Pharmacol Toxicol, 22: 517-554.
Pollock PL, Germolec DR, Comment CE, Rosenthal GJ, & Luster MI (1994)
Development of human lymphocyte engrafted SCID mice as a model for
immunotoxicity assessment. Fundam Appl Toxicol., 22: 130-138.
Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L,
Janeway CA, Murphy DB, & Bottomly K (1989) Monoclonal antibodies to
murine CD3e defined distinct epitopes, one of which may interact with
CD4 during T cell activation. J Immunol, 142: 4169-4175.
Prichard MG, Boerth LW, & Pennington JE (1987) Compartmental analysis
of resting and activated pulmonary natural killer cells. Exp Lung Res,
12: 239-251.
Prior AC & Sisodia CS (1982) The effects of ochratoxin A on the immune
responses of Swiss mice. Can J Comp Med, 46: 91-96.
Pruett SB (1992) Immunotoxicity of organophosphorus compounds.
In: Chambers JE & Levi PE ed. Organophosphates. Chemistry, fate and
effects. New York, Academic Press, pp 367-385.
Pruett SB & Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin
2 system. Toxicol Lett, 40: 11-20.
Pugh-Humphreys RGP, Ross CSK, & Thomson AW (1990) The influence of
FK-506 on the thymus. An immunophenotypic and structural analysis.
Immunology, 70: 398-404.
Quesniaux VFJ (1992) Interleukins 9, 10, 11 and 12 and kit ligand:
A brief overview. Res Immunol, 143: 385-400.
Quinnan GV, Manischewitz JE, & Ennis FA (1980) Role of cytotoxic
T-lymphocytes in murine cytomegalovirus infection. J Gen Virol,
47: 503-508.
Qureshi MA, Bloom SE, Hamilton JW, & Dietert RR (1989) Toxic effects
of methyl methanesulfonate (MMS) on activated macrophages from
chickens. Environ Mol Mutag, 13: 253-262
Rao GN, Bimbaurn LS, Collins JJ, Tennant RW, & Skow LC (1988) Mouse
strains for chemical carcinogenicity studies: Overview of a workshop.
Fundam Appl Toxicol, 10: 385-394.
Raskin RE, Tvedten HW, Bull RW, Crow SE, Dunstan RW, & Krehbiel JD
(1989) Natural killer cell activity in untreated and treated dogs with
lymphoma. Am J Vet Res, 4: 483-487.
Ratajczak HV, Thomas PT, Sothern RB, Vollmuth T, & Heck D (1993)
Evidence for genetic basis of seasonal differences in antibody
formation between two mouse strains. Chronobiol Int, 10: 383-394.
Ratcliffe NA & Rowly AF (1981) Intervertebrate blood cells. London,
Academic Press.
Raulet DH (1989) The structure, function, and molecular genetics of
the gamma/delta T cell receptor. Ann Rev Immunol, 7: 175-207.
Reed SG & Scott P (1993) T-Cell and cytokine responses in
leishmaniasis. Curr Opin Immunol, 5: 524-531.
Reggiani G (1980) Acute human exposure to TCDD in Seveso, Italy.
J Toxicol Environ Health, 6: 27-43.
Reigart JR & Graber CD (1976) Evaluation of the humoral immune
response of children with low lead exposure. Bull Environ Contam
Toxicol, 16: 112-117.
Reijnders PJH (1986) Reproductive failure in common seals feeding on
fish from polluted coastal waters. Nature, 324: 456-457.
Renton KW (1986) Factors affecting drug biotransformation. Clin
Biochem, 19: 72-75.
Renton KW, Gray JD, & Hall RI (1980) Decreased elimination of
theophylline after influenza vaccination. Can Med Assoc J,
123: 288-290.
Reyes J, Tzakis A, Green M, Nour B, Nalesnik M, Van Thiel D, Martin M,
Breinig MK, Fung LL, Cooper M, & Starzl TE (1991) Post-transplant
lymphoproliferative disorders under primary FK506 immunosuppression.
Transplant Proc, 23: 3044-3046
Reynolds HY & Thomson RE (1973) Pulmonary host defenses.
II. Interaction of respiratory antibodies with Pseudomonas aeruginosa
and alveolar macrophages. J Immunol, 111: 369-380.
Reynolds CW, Wenn AC, Barlozzari T, Wiltrout T, Reichardt WA, &
Herberman RB (1984) Natural killer activity in the rat. IV.
Distribution of large granular lymohocytes (LGL) following intravenous
and intraperitoneal transfer. Cell Immunol, 86: 371-380.
Richeldi L, Persichini T, Ferrara GB, Crystal RG, Markham T, Luisetti
M, Bertrorelli G, Guerritore D, & Saltini C (1992) Chronic beryllium
disease association with the HLA-DP 2.1 allele. Am Rev Respir Dis,
145 (Suppl): A324.
Riley WJ (1989) Insulin dependent diabetes mellitus, an autoimmune
disorder? Clin Immunol Immunopathol, 53: S92-S98.
Roberts RJ (1975) Melanin-containing cells of the teleost fish and
their relation to disease. In: Ribelin WE & Migaki G ed. The pathology
of fishes. Madison, Wisconsin, University of Wisconsin Press,
pp 399-428.
Roitt IM (1991) Essential immunology, 7th Ed. Oxford, Blackwell
Scientific Publications.
Roitt IM, Brostoff J & Male DK (1993) Immunology, 3rd Ed. London,
Gower Medical Publications.
Roizman B (1982) The family Herpesviridae. General description,
taxonomy, and classification. In: Roizman B ed. The viruses, Vol 1:
Herpesviruses. New York, Plenum Press, pp 1-23.
Roizman B, Carmichael LE, Deinhardt F, de The G, Nahmias AJ, Plowright
W, Rapp F, Sheldrick P, Takahashi M, & Wolf K (1981) Herpesviridae.
Definition, provisional nomenclature and taxonomy. Intervirology,
16: 201-217.
Rolstad B, Herberman RB, & Reynolds GW (1986) Natural killer activity
in the rat: V. The circulation patterns and tissue localization of
peripheral blood lymphocytes. J Immunol, 136: 2600-2808.
Rose LM, Richards TL, Petersen R, Peterson J, Hruby S, & Alvord EC Jr
(1991) Remitting-relapsing EAE in nonhuman primates: A valid model of
multiple sclerosis. Clin Immunol Immunopathol, 59: 1-15.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502-510.
Rosenthal GJ & Snyder CA (1987) Inhaled benzene reduces aspects of
cell-mediated tumor surveillance in mice. Toxicol Appl Pharmacol,
88: 35-43.
Rosenthal GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock
P, Ling-Yuan K, Kwon J, & Luster MI (1994) Asbestos stimulates
IL-8 production from human lung epithelial cells. J Immunol,
153: 3237-3244.
Ross GD (1992) Function of neutrophils in host defence and immunity.
In: Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca
Raton, Florida, CRC Press, pp 263-286.
Ross PS, De Swart RL, Reijnders PJH, Van Loveren H, Vos JG, &
Osterhaus ADME (1995) Contaminant-related suppression of delayed-type
hypersensitivity and antibody responses in harbor seals fed herring
from the Baltic Sea. Environ Health Perspectives, 103: 162-167.
Ross PS, De Swart RL, Timmerman HH, Reijnders PJH, Vos JG, Van Loveren
H, & Osterhaus ADME (in press) Suppression of natural killer activity
in harbour seals (Phoca virulina) fed Baltic Sea herring. Aquat
Toxicol.
Rothbard JB & Gefter ML (1991) Interactions between immunogenic
peptides and MHC molecules. Ann Rev Immunol, 9: 527-565.
Roths JB, Murphy ED, & Eicher EM (1984) A new mutation, gld, that
produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp
Med, 159: 1-20.
Rouveix B(1993) Opiates and immune function. Thérapie, 47: 503-512.
Rubin RH (1990) Impact of cytomegalovirus infection in organ
transplant recipients. Rev Infect Dis, 12: S754-S766.
Rubin RH, Cosimi AB, Hirsch MS, Herrin JT, Russell PS, & Tolkoff Rubin
NE (1981) Effects of antithymocyte globulin on CMV infection in renal
transplant recipients. Transplantation, 31: 143-145.
Ruitenberg EJ, Buys J, Teppema JS, & Elgersma A (1983) Rat mononuclear
cells and neutrophils are more effective than eosinophils in antibody-
mediated stage-specific killing of Trichinella spiralis in vitro.
Parasitenkunde, 69: 807-815.
Ryffel B (1992) The carcinogenicity of ciclosporin. Toxicology,
73: 1-22.
Saiki RK, Scharf SJ, Faloona FA, Mullis KB, Hom GT, Erlich HA, &
Arnheim N (1985) Enzymatic amplification of ß-globulin genomic
sequences and restriction site analysis for diagnosis of sickle cell
anemia. Science, 230: 1350-1354.
Sainte-Marie G, Bélisle C, & Peng FS (1990) The deep cortex of the
lymph node: Morphological variations and functional aspects. In:
Grundmann E & Vollmer E ed. Reaction patterns of the lymph node, Part
1. Cell types and functions. Berlin, Springer Verlag, pp 33-63.
Saito M (1992) Recent trends in research on differentiation of
hematopoietic cells and lymphokines (hemopoietins). Hum Cell,
5: 54-69.
Sakaguchi S & Sakaguchi N (1988) Thymus and autoimmunity. Transplanta-
tion of the thymus from cyclosporin A-treated mice causes organ-
specific autoimmune disease in athymic nude mice. J Exp Med,
167: 1479-1485.
Saltini C, Winestock K, Kirby M, Pinkston P, & Crystal RB (1989)
Maintenance of alveolitis in patients with chronic beryllium disease
by beryllium-specific helper T cells. New Engl J Med, 320: 1103-1109.
Samet JM (1994) Nitrogen dioxide and respiratory infection. Am Rev
Respir Dis, 139: 1073-1074.
Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA,
McLaren LC, Schwab M, & Spengler JD (1993) Nitrogen dioxide and
respiratory illnesses in infants. Am Rev Respir Dis, 148: 1258-1265.
Sanders VM, Tucker AN, White KL, Kauffmann BM, Hallett P, Carchman RA,
Borzelleca JF, & Munson AE (1982) Humoral and cell-mediated immune
status in mice exposed to trichloroethylene in the drinking water.
Toxicol Appl Pharmacol, 62: 358-368.
Sanders VM, White KL, Shopp GM, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to 1,1,2-trichloroethane. Drug
Chem Toxicol, 8: 357.
Sato K, Flajnik MF, Du Pasquier L, Katagiri M, & Kasahara M (1993)
Evolution of the MHC: Isolation of class II ß-chain cDNA clones from
the amphibian Xenopus laevis. J Immunol, 150: 2831-2843.
Scala R (1991) Risk assessment. In: Amdur M, Doull J, & Klaasen C ed.
Casarett and Doull's Toxicology, 3rd Ed. Elmsford, New York, Pergamon
Press, pp 985-996.
Scheuchenzuber WJ, Eskew ML, & Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environ Res, 38: 389-399.
Schielen P, Schoo W, Tekstra J, Oostermeijer HH, Seinen W, & Bloksma N
(1993) Autoimmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schlick E & Friedberg KD (1981) The influence of low lead doses on the
reticulo-endothelial system and leukocytes of mice. Arch Toxicol,
47: 197.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
C, Ritz J, Shaw S, Silverstein RL, Springer TA, Tedder TF, & Todd RF
(1994) CD antigens 1993. Immunol Today, 15: 98-99.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
T, Ritz J, Shaw S, Springer TA, Tedder TF, & Todd RF (1995) Leucocyte
typing, V: White cell differentiation antigens. Oxford, Oxford
University Press.
Schouk LB & Laskin DL (1994) Xenobiotics and inflammation. San Diego,
California, Academic Press.
Schuurman H-J, Kluin PM, Gmelig-Meyling FHJ, Van Unnik JAM, & Kater L
(1985) Lymphocyte status of lymph node and blood in acquired
immunodeficien-cy syndrome (AIDS) and AIDS-related complex disease.
J Pathol, 147: 269-280.
Schuurman H-J, Van Loveren H, Rozing J, Van Dijk A, Loeber JG, & Vos
JG (1990) Cyclosporin and the rat thymus. An immunohistochemical
study. Thymus, 16: 235-254.
Schuurman H-J, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1991a) Immune system. In: Haschek WM & Rousseaux CG ed. Handbook of
toxicologic pathology. San Diego, Academic Press, pp 421-487.
Schuurman H-J, Nagelkerken L, De Weger RA, & Rozing J (1991b) Age-
associated involution: Significance of a physiologic process. Thymus,
18: 1-6.
Schuurman H-J, De Weger RA, Van Loveren H, Krajnc-Franken MAM, & Vos
JG (1992a) Histopathological approaches. In: Miller K, Turk JL, &
Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 279-303.
Schuurman H-J, Hougen HP, & Van Loveren H (1992b) The rnu (Rowett
nude) and rnuN ( nznu, New Zealand nude) rat: An update. ILAR
News, 34: 3-12.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijt BC,
De Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Pass D, Vankerkom
J, Boot R, Broekhuizen R, Dormans J, van Herck H, Hermans PGC, Hoekman
J, van der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992c)
Comparative evaluation of the immune status of congenitally athymic
and euthymic rat strains bred and maintained at different institutes:
2. Athymic rats. J Exp Anim Sci, 35: 33-48.
Schuurman H-J, Van Loveren H, Rozing J, & Vos JG (1992d) Chemicals
trophic for the thymus: Risk for immunodeficiency and autoimmunity.
Int J Immunopharmacol, 14: 369-375.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijht BC,
de Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Vankerkom J, Boot
R, Broekhuizen R, Dormans J, van Herck H, Hermans GC, Hoekman J, van
der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992e) Comparative
evaluation of the immune status of congenitally athymic and euthymic
rat strains bred and maintained at different institutes: 1. Euthymic
rats. J Exp Anim Sci, 35: 16-32.
Schuurman H-J, Hu HZ, De Weger RA, & Clevers HC (1993) Thoughts on
thymus and T-lymphocyte repertoire. Relevance for tolerance of the
immune response. Neth J Med, 43: 38-54.
Schuurman H-J, Kuper CF, & Vos JG (1994) Histopathology of the immune
system as a tool to assess immunotoxicity. Toxicology, 86: 197-212.
Schwartz J (1992) Air pollution and the duration of acute respiratory
symptoms. Arch Environ Health, 47: 116-122.
Schwartz RS, Callen JP, & Silva JR (1978) A cluster of Hodgkin's
disease in a small community: Evidence for environmental factors.
Am J Epidemiol, 108: 19-26.
Schwartz J, Spix C, Wichmann HE, & Malin E (1991) Air pollution and
acute respiratory illness in five German communities. Environ Res,
56: 1-14.
Schwetz BA & Tyl RW (1987) Consensus workshop on the evaluation of
maternal and developmental toxicity. Workgroup III report. Low dose
extrapolation and other considerations for risk assessment - models
and applications. Teratog Carcinog Mutag, 1: 1-7.
Secombes CJ, Fletcher TC, White A, Costello MJ, Stagg R, & Houlihan DF
(1992) Effects of sewage sludge on immune response in the dab,
Limanda limanda. Aquat Toxicol, 23: 217-230.
Seinen W & Penninks AH (1979) Immune suppression as a consequence of a
selective cytotoxic activity of certain organometallic compounds on
thymus and thymus-dependent lymphocytes. Ann NY Acad Sci,
320: 499-517.
Seinen W & Willems MI (1976) Toxicity of organotin compounds.
I. Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di- n-octyltin dichloride. Toxicol Appl Pharmacol, 35: 63-75.
Seinen W, Vos JG, Van Krieken R, Penninks AH, Brands R, & Hooykaas H
(1977) Toxicity of organotin compounds. II. Comparative in vivo and
in vitro studies with various organotin and organolead compounds in
different animal species with special emphasis on lymphocyte
cytotoxicity. Toxicol Appl Pharmacol, 42: 197-212.
Sela O & Shoenfeld Y (1988) Cancer in autoimmune diseases. Semin Arthr
Rheum, 18: 78-87.
Selgrade MJK & Gilmour MI (1994) Effects of gaseous air pollutants on
immune responses and susceptibility to infectious and allergic
disease. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicity and immunopharmacology, 2nd Ed. New York, Raven Press,
pp 395-411.
Selgrade MJK & Osborn TE (1974) Role of macrophages in resistance to
murine cytomegalovirus. Infect Immun, 10: 1383-1390.
Selgrade MJK, Daniels MJ, Hu PC, Miller FJ, & Graham JA (1982) Effects
of immunosuppression with cyclophosphamide on acute murine
cytomegalovirus infection and virus-augmented natural killer cell
activity. Infect Immun, 38: 1046-1055.
Selgrade MK, Daniels MJ, Illing JW, Ralston AL, Grady MA, Chartlet E,
& Graham JA (1984) Increased susceptibility to parathion poisoning
following murine cytomegalovirus infection. Toxicol Appl Pharmacol,
76: 356-364.
Selgrade MJK, Illing JW, Starnes DM, Stead AG, Menache MG, & Stevens
MA (1988) Evaluation of effects of ozone exposure on influenza
infection in mice using several indicators of susceptibility. Fundam
Appl Toxicol, 11: 169-180.
Selgrade MJK, Starnes DM, Illing JW, Daniels MJ, & Graham JA (1989)
Effects of phosgene exposure on bacterial, viral, and neoplastic lung
disease susceptibility in mice. Inhal Toxicol, 1: 243-259.
Selgrade MJK, Daniels MJ, & Dean JH (1992) Correlation between
chemical suppression of natural killer cell activity in mice and
susceptibility to cytomegalovirus: Rationale for applying murine
cytomegalovirus as a host resistance model and for interpreting
immunotoxicity testing in terms of risk of disease. J Toxicol Environ
Health, 37: 123-137.
Selgrade MJK, Cooper KD, Devlin RB, Van Loveren H, Biagini RE, &
Luster MI (1995) Immunotoxicity - Bridging the gap between animal
research and human health effects. Fundam Appl Toxicol, 24: 13-21.
Sell S (1987) Immunology, immunopathology and immunity, 4th Ed.
Norwalk, Connecticut, Appleton & Lange.
Shabtai M, Malinowski K, Waltzer WC, Pullis C, Raisbeck AP, & Rapaport
FT (1991) Quantitative analysis of surface marker densities after
exposure of T cells to concanavalin A (ConA): A sensitive early index
of cellular activation. Cell Immunol, 133: 519-525.
Shand FL (1975) Review/commentary: The immunopharmacology of
cyclophosphamide. Int J Immunopharmacol, 1: 165-171.
Sharp JG & Watkins EB (1981) Cellular and immunological consequences
of thymic irradiation. In: Dubois JB, Serrou B, & Rosenfeld C ed.
Immunopharmacologic effects of radiation therapy. New York, Raven
Press, pp 137-179.
Sheil AG, Disney AP, Mathew TH, Amiss N, & Excell L (1991) Cancer
development in cadaveric donor renal allograft recipients treated with
azathioprine (AZA) or cyclosporine (CyA) or AZA/CyA. Transplant Proc,
23: 1111-1112.
Sherwood RL, Tarkington B, Lippert WE, & Goldstein E (1981) Effect of
ferrous sulphate aerosols and nitrogen dioxide aerosols on murine
pulmonary defense. Arch Environ Health, 36: 130-135.
Sherwood RL, Thomas PT, Kawanishi CY, & Fenters JD (1988) Comparison
of Streptococcus zooepidemicus and influenza virus pathogenicity in
mice by three pulmonary exposure routes. Appl Environ Microbiol,
54: 1744-1751.
Shigematsu N, Ishimaru S, Saito R, Ikeda T, Matsuba K, Sigiyama K, &
Masuda Y (1978) Respiratory involvement in polychlorinated biphenyls
poisoning. Environ Res, 16: 92-100.
Shiroky JB, Frosp A, Skelton JD, Haegert DG, Newkirk MM, & Neville
C (1991) Complications of immunosuppression associated with weekly low
dose methrotrexate. J Rheum, 18: 1172-1175.
Shopp GM & Munson AE (1985) Suppression of the antibody response by
phorbol esters in the mouse is due to an effect on the nylon wool
adherent cell population. Agents Actions, 6: 535-541.
Shopp GM, Sanders VM, White KL, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to trans-12-dichlorethylene.
Drug Chem Toxicol, 8: 393.
Shortman K, Egerton M, Spangrude GJ, & Scollay R (1990) The generation
and fate of thymocytes. Sem Immunol, 2: 3-13.
Shy CM, Creason JP, Pearlman ME, McClain KE, & Benson FB (1970) The
Chattanooga study: Effects of community exposure to nitrogen dioxide.
II. Incidence of acute respiratory illness. J Air Pollut Control
Assoc, 20: 582-588.
Sibinga NES & Goldstein A (1988) Opioid peptides and opioid receptors
in cells of the immune system. Ann Rev Immunol, 6: 219-249.
Sigal NH & Dumont FJ (1992) Cyclosporin A, FK-506, and Rapamycin:
Pharmacologic probes of lymphocyte signal transduction. Ann Rev
Immunol, 10: 519-560.
Sikorski EE, McCay JA, White KL, Bradley SG, & Munson AE (1989)
Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1
mice. Fundam Appl Toxicol, 13: 843-858.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW,
Wilcox KR, VanAmburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls.
J Reticuloendothel Soc, 26: 341.
Sima P & Vetvicka V (1992) Evolution of immune accessory function. In:
Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca Raton,
Florida, CRC Press, pp 1-56.
Simon JC, Cruz PD, Bergstresser PR, & Tigelaar RE (1990) Low dose
ultraviolet B-irradiated Langerhans cells preferentially activate
CD4+ cells of the T helper 2 subset. J Immunol, 145: 2087-2094.
Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, & Cruz PD (1991)
Ultraviolet B radiation converts Langerhans cells from immunogenic to
tolerogenic antigen-presenting cells. Induction of specific clonal
anergy in CD4+ T helper 1 cells. J Immunol, 146: 485-491.
Singh G, Fries JF, Spitz P, & Williams CA (1989) Toxic effects of
azathioprine in rheumatoid arthritis patients. A national post-
marketing perspective. Arthr Rheum, 32: 837-843.
Siroki O, Institoris L, Tatar F, & Desi I (1994) Immunotoxicological
investigation of SCMF, a new pyrethroid pesticide in mice. Hum Exp
Toxicol, 13: 337-343.
Siwicki AK, Cossarini-Dunier M, Studnicka M, & Demael A (1990)
In vivo effect of the organophosphorus insecticide trichlorphon on
immune response of carp (Cyprinus carpio). Ecotoxicol Environ Saf,
19: 99-105.
Sjoblad RD (1988) Potential future requirements for immunotoxicity
testing of pesticides. Toxicol Ind Health, 4: 391-394.
Sjovall P, Christensen OB, & Moller H (1985) Single exposure to
ultraviolet irradiation and elicitation of human allergic contact
dermatitis. Acta Dermatol Venereol, 65: 93-96.
Smialowicz RJ, Rogers RR, Riddle MM, Luebke RW, Rowe DG, & Garner RJ
(1984) Manganese chloride enhances murine cell-mediated cytotoxicity:
Effects on natural killer cells. J Immunopharmacol, 6: 1-23.
Smialowicz R, Luebke RW, Riddle MM, Rogers RR, & Rowe DG (1985a)
Evaluation of the immunotoxic potential of chlordecone with comparison
to cyclophosphamide. J Toxicol Environ Health, 15: 561-574.
Smialowicz RJ, Luebke RW, Rogers RR, Riddle MM, & Rowe DG (1985b)
Evaluation of immune function in mice exposed to ordram. Toxicology,
37: 307-314.
Smialowicz RJ, Rogers RR, Riddle MM, Garner RJ, Rowe DG, & Luebke RW
(1985c) Immunologic effects of nickel. II. Suppression of natural
killer cell activity. Environ Res, 36: 56-66.
Smialowicz RJ, Andrews JE, Riddle MM, Rogers RR, Luebke RW, & Copeland
CB (1989) Evaluation of the immunotoxicity of low level PCB exposure
in the rat. Toxicology, 56: 197-211.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB, & Ernest
GG (1990) Immune alterations in rats following subacute exposure to
tributyltin oxide. Toxicology, 64: 169-178.
Smialowicz RJ, Riddle MM, Luebke RW, Copeland CB, Andrews D, Rogers
RR, Gray LE, & Laskey JW (1991) Immunotoxicity of 2-methoxyethanol
following oral administration in Fischer 344 rats. Toxicol Appl
Pharmacol, 109: 494-506.
Smialowicz RJ, Luebke RW, & Riddle MM (1992a) Assessment of the
immunotoxic potential of the fungicide dinocap in mice. Toxicology,
75: 235-247.
Smialowicz RJ, Williams WC, Riddle MM, Andrews DL, Luebke RW, &
Copeland CB (1992b) Comparative immunosuppression of various glycol
ethers orally administered to Fischer 344 rats. Fundam Appl Toxicol,
18: 621-627.
Smialowicz RJ, Riddle MM, & Williams WC (1993) Methoxyacetaldehyde, an
intermediate metabolite of 2-methoxyethanol, is immunosuppressive in
the rat. Fundam Appl Toxicol, 21: 1-7.
Sminia T, Van der Brugge-Gamelkoorn GJ, & Jeurissen SHM (1989)
Structure and function of bronchus-associated lymphoid tissue (BALT).
Crit Rev Immunol, 9: 119-150.
Smith SH, Sanders VM, Barrett BA, Borzellaca JF, & Munson AE (1978)
Immunotoxicological evaluation on mice exposed to polychlorinated
biphenyls. Toxicol Appl Pharmacol, 45: 330.
Snoeij NJ, Penninks AH, & Seinen W (1988) Dibutyltin and tributyltin
compounds induce thymic atrophy in rats due to a selective action on
thymic lymphoblasts. Int J Immunopharmacol, 10: 889-891.
Snyder CA (1989) The neuroendocrine system, an opportunity for
immunotoxicologists. Environ Health Perspectives, 81: 165-166.
Spangrude GJ, Berhard EJ, Ajoika RS, & Daynes RA (1983) Alterations in
lymphocyte homing patterns within mice exposed to ultraviolet
radiation. J Immunol, 130: 2974-2981.
Speizer FE, Ferris B, Bishop YMM, & Spengler J (1980) Respiratory
disease rates and pulmonary function in children associated with NO2
exposure. Am Rev Respir Dis, 121: 3-10.
Spiekermann K, Emmendoerfer A, Elsner J, Raeder E, Lohmann-Matthes ML,
Prahst A, Link H, Freund M, Welte K, & Roesler J (1994) Altered
surface marker expression and function of G-CSF induced neutrophils
from test subjects and patients under chemotherapy. Br J Haematol,
87: 31-38.
Spreafico F, Merendino A, Braceschi L, & Sozanni S (1987)
Immunodepressive drugs as prototype immunotoxicants. In: Berlin A,
Dean J, Draper MH, Smith EMB, & Van Der Venne, M-T ed.
Immunotoxicology. Dordrecht, Martinus Nijhoff, pp 192-207.
Sprent J, Gao EK, & Webb SR (1990) T Cell reactivity to MHC molecules:
Immunity versus tolerance. Science, 248: 1357-1363.
Springer TA (1990) Adhesion receptors of the immune system. Nature,
346: 425-434.
Springer T, Galfré G, Secher DS, & Milstein C (1979) Mac-1: A
macrophage differentiation antigen identified by monclonal antibody.
Eur J Immunol, 9: 301-306.
Spruanoe SL (1985) Pathogenesis of herpes simplex labialis:
Experimental induction of lesions with UV light. J Clin Microbiol,
22: 366-368.
Stahlmann R, Korte M, Van Loveren H, Vos JG, Thiel R, & Neubert D
(1992) Abnormal thymus development and impaired function of the immune
system in rats after prenatal exposure to aciclovir. Arch Toxicol,
66: 551-559.
Stanley GP & Pender MP (1991) The pathophysiology of chronic relapsing
experimental allergic encephalomyelitis in the Lewis rat. Brain,
114: 1827-1853.
Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP,
Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL, Atchison RW, Jaffe R, &
Bahnson HT (1984) Reversibility of lymphomas and lymphoproliferative
lesions developing under cyclosporin-steroid therapy. Lancet,
i: 583-587.
Stehr-Green PA, Naylor PH, & Hoffman RE (1989) Diminished thymosin
alpha-1 levels in persons exposed to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. J Toxicol Environ Health, 28: 285-295.
Steinmann GG (1986) Changes in the human thymus during aging. Curr
Topics Pathol, 75: 43-88.
Stein-Streinlein J, Bennett M, Mann D, & Kumar V (1983) NK cells in
mouse lungs: Surface phenotype, target preference and response to
local influenza virus infection. J Immunol, 131: 2699-2704.
Stet RJM & Egberts E (1991) The histocompatibility system in
teleostean fishes: From multiple histocompatibility loci to a major
histocompatibility complex. Fish Shellfish Immunol, 1: 1-16.
Stingl G & Steiner G (1989) Immunological host defence of the skin.
Curr Prob Dermatol, 18: 22-30.
Stobo JD & Paul WE (1973) Functional heterogeneity of murine
lymphoid cells. III. Differential responsiveness of T cells to
phytohemagglutinin and concanavalin A for T cell subsets. J Immunol,
110: 362-369.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral responses by pesticides and chemicals of environmental
concern: Quantitative studies of immunosuppression by DDT, Aroclor,
carbaryl, carbofuran and methylparathion. Toxicol Appl Pharmacol,
32: 587-602.
Streilein JW (1983) Skin associated lymphoid tissues (SALT): Origins
and functions. J Invest Dermatol, 80 (Suppl): 12s-16s.
Streilein JW (1990) Skin-associated lymphoid tissues (SALT): The next
generation. In: Bos J ed. The skin immune system. Boca Raton, Florida,
CRC Press, pp 25-48.
Strobel S & Ferguson A (1984) Immune responses to fed protein antigens
in mice. 3. Systemic tolerance or priming is related to age at which
antigen is first encountered. Ped Res, 18: 588-594.
Stunz LL & Feldbush TL (1986) Polyclonal activation of rat B
lymphocytes. I. A single mitogenic signal can stimulate proliferation,
but three signals are required for differentiation. J Immunol,
136: 4006-4012.
Stutman O (1986) Postthymic T-cell development. Immunol Rev,
91: 160-194.
Subramanian A, Tanabe S, Tatsukawa R, Saito S, & Miyazaki N (1987)
Reduction in the testosterone levels by PCBs and DDE in Dall's
porpoises of northwestern North Pacific. Mar Pollut Bull, 18: 643-646.
Sung YJ, Hotchkiss JH, Austic RE, & Dietert RR (1992) Direct
measurement of nitric oxide in headspace gas produced by a chicken
macrophage cell line in a closed culture system. Biochem Biophys Res
Commun, 184: 36-42.
Surhe CD & Sprent J (1991) Long-term xenogeneic chimeras. Full
differentiation of rat T and B cells in SCID mice. J Immunol,
147: 2148-2154.
Svensson BG, Nilsson A, Hansson M, Rappe C, Åkesson B, & Skerving S
(1991) Exposure to dioxins and dibenzofurans through the consumption
of fish. New Engl J Med, 324: 8-12.
Swain WR (1991) Effects of organochlorine chemicals on the
reproductive outcome of humans who consumed contaminated Great Lakes
fish: An epidemiologic consideration. J Toxicol Environ Health,
33: 587-639.
Swerdlow AJ, Douglas AJ, Vaughan Hudson G, Vaughan Hudson B, Bennett
MH, & MacLennan KA (1992) Risk of second primary cancers after
Hodgkin's disease by type of treatment: Analysis of 2846 patients in
the British National Lymphoma Investigation. Br Med J, 304: 1137-1143.
Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR,
Heroux AL, Dizikes GJ, Pifarre R, & Fisher RI (1990) Increased
incidence of lymphoproliferative disorder after immunosuppression with
the monoclonal antibody OKT3 in cardiac-transplant recipient. New Engl
J Med, 323: 1723-1728.
Szakal AK, Kosco MH, & Tew JG (1989) Microanatomy of lymphoid tissue
during humoral immune responses: Structure function relationships. Ann
Rev Immunol, 7: 91-109.
Taga T, & Kishimoto T (1992) Cytokine receptors and signal
transduction. FASEB J, 6: 3387-3396.
Takeya U, Shimotori S, Tanaguchi T, & Nomoto K (1977) Cellular
mechanisms in the protection against infection by Listeria
monocytogenesin mice. J Gen Microbiol, 100: 373-379.
Talcott PA & Koller LD (1983) The effect of inorganic lead and/or a
polychlorinated biphenyl on the developing immune system of mice. J
Toxicol Environ Health, 12: 337-352.
Talcott PA, Koller LD, & Exon JH (1985) The effect of lead and
polychlorinated biphenyl exposure on rat natural killer cell
cytotoxicity. Int J Immunopharmacol, 7: 255-261.
Taylor BA (1972) Genetic relationships between inbred strains of mice.
J Hered, 63: 83-86.
Taylor JR, Schmieder GF, Shimizu T, Tie C, & Streilin JW (1994)
Interrelationship between ultraviolet light and recurrent herpes
simplex infections in man. J Dermatol Sci, 8: 224-232.
Temple L, Kawabata TT, Munson AE, & White KL (1993) Comparison of
ELISA and plaque-forming cell assays for measuring the humoral immune
response to SRBC in rats and mice treated with benzo[ a]pyrene or
cyclophosphamide. Fundam Appl Toxicol, 21: 412-419.
Theofilopoulos AN & Dixon FJ (1985) Murine models of systemic lupus
erythematosus. Adv Immunol, 37: 269-390.
Thiem PA, Halper LK, & Bloom JC (1988) Techniques for assessing canine
mononuclear phagocytic function as part of an immunotoxicologic
evaluation. Int J Immunopharmacol, 10: 765-771.
Thomas PT & Hinsdill RD (1978) Effect of polychlorinated biphenyls on
the immune responses of rhesus monkeys and mice. Toxicol Appl
Pharmacol, 44: 41-51.
Thomas PT & Hinsdill RD (1979) The effect of perinatal exposure to
tetrachlorodibenzo- p-dioxin on the immune response of young mice.
Drug Chem Toxicol, 2: 77-98.
Thomas PT, Ratajczak HV, Aranyi C, Gibbons R, & Fenters JD (1985a)
Evaluation of host resistance and immune function in cadmium-exposed
mice. Toxicol Appl Pharmacol, 80: 446-456.
Thomas PT, Fugmann R, Aranyi C, Barbera P, Gibbons R, & Fenters J
(1985b) Effect of dimethylnitrosamine on host resistance and immunity.
Toxicol Appl Pharmacol, 77: 219-229.
Thomas PT, Ratajczak HV, Eisenberg WC, Furedi-Machacek M, Ketels K, &
Barbera PW (1987) Evaluation of host resistance and immunity in mice
exposed to the carbamate pesticide aldicarb. Fundam Appl Toxicol,
9: 82-89.
Thomson AW (1992) The spectrum of action of new immunosuppressive
drugs. Clin Exp Immunol, 89: 170-173.
Thurmond LM, Lauer LD, House RV, Cook JC, & Dean JH (1987) Immuno-
suppression following exposure to 7,12-dimethylbenz[ a]anthracene
(DMBA) in Ah-responsive and Ah-nonresponsive mice. Toxicol
Appl Pharmacol, 91: 450-460.
Tieben LM, Berkhout RJM, Smits HL, Bouwes Bavinck JN, Vermeer BJ,
Bruijn JA, van der Woude FJ, & ter Schegget J (1994) Detection of
epidermodysplasia verruciformis-like human papillomavirus types in
renal allograft recipients. Br J Dermatol, 131: 226-230.
Tilney NL (1971) Patterns of lymphatic drainage in the adult
laboratory rat. J Anat, 109: 369-383.
Timonen T, Ortaldo JR, & Herberman RB (1981) Characteristics of human
large granular lymphocytes and relationship to natural killer and K
cells. J Exp Med, 153: 569-582.
Timonen T, Ortaldo JR, & Herberman RB (1982) Isolation of human and
rat natural killer cells. J Immunol Meth, 51: 269-277.
Toews GB, Bergstresser PR, Streilein JW, & Sullivan S (1980) Epidermal
Langerhans cell density determines whether contact hypersensitivity or
unresponsiveness follows skin painting with DNFB. J Immunol,
124: 445-453.
Tomlinson S (1993) Complement defense mechanisms. Curr Opin Immunol,
5: 83-89.
Tonari Y, & Minamishima Y (1983) Pathogenicity and immunogenicity of
temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol,
64: 1983-1990.
Tracey DE, Walfe SA, Durdik JM ,& Henney CS (1977) BCG-induced murine
effector cells; cytolytic activity in peritoneal exudates: An early
response to BCG. J Immunol, 119: 1145-1151.
Truelove J, Grant D, Mes J, Tryphonas H, Tryphonas L, & Zawidzka Z
(1982) Polychlorinated biphenyl toxicity in the pregnant cynomolgus
monkey: A pilot study. Arch Environ Contam Toxicol, 11: 583-588.
Tryphonas H (in press) Immunotoxicity of PCBs (Aroclors) in relation
to Great Lakes. Environ Health Perspectives, Suppl 9.
Tryphonas H, Hayward S, O'Grady L, Loo JCK, Arnold DL, Bryce F, &
Zawidzka ZZ (1989) Immunotoxicity studies of PCB (Aroclor 1254) in the
adult rhesus (Macaca mulatta) monkey--Preliminary report. Int J
Immunopharmacol, 11: 199-206.
Tryphonas H, Luster MI, White KL, Naylor PH, Erdos MR, Burleson GR,
Germolec D, Hodgen M, Hayward S, & Arnold DL (1991a) Effects of PCB
(Aroclor 1254) on non-specific immune parameters in rhesus (Macaca
mulatta) monkeys. Int J Immunopharmacol, 13: 639-648.
Tryphonas H, Luster MI, Schiffman G, Dawson L-L, Hodgen M, Germolec D,
Hayward S, Bryce, F, Loo JCK, Mandy F, & Arnold DL (1991b) Effects of
chronic exposure of PCB (Aroclor 1254) on specific and non-specific
immune parameters in the rhesus (Macaca mulatta) monkey. Fundam Appl
Toxicol, 16: 773-786.
Tsang PH, Chu FN, Fischbein A, & Bekesi JG (1988) Impairments in
functional subsets of T-suppressor (CD8) lymphocytes, monocytes, and
natural killer cells among asbestos-exposed workers. Clin Immunol
Immunopathol, 47: 323-332.
Tschöpe D, Roesen B, Scwippert B, Kehrel B, Scauseil S, Esser J, &
Gries FA (1990) Platelet analysis using flow cytometry procedures.
Platelets, 1: 127-133.
Tucker AN & Munson AE (1981) In vitro inhibition of the primary
antibody response to sheep erythrocytes by cyclophosphamide. Toxicol
Appl Pharmacol, 59: 617-619.
Tucker AN, Hong L, Boorman GA, Pung O, & Luster MI (1985) Alteration
of bone marrow cell cycle kinetics by diphenylhydantoin: Relationship
to folate utilization and immune function. J Pharmacol Exp Ther,
234: 57-62.
Tucker AN, Vore SJ, & Luster MI (1986) Suppression of B cell
differentiation by 2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol
Pharmacol, 29: 372-377.
Uber CL & McReynolds RA (1982) Immunotoxicology of silica. CRC Crit
Rev Toxicol, 10: 303-319.
Umesaki Y, Setoyama H, Matsumoto S, & Okada Y (1993) Expansion of
alpha ß T-cell receptor-bearing intestinal intraepithelial lymphocytes
after microbial colonization in germ-free mice and its independence
from thymus. Immunology, 79: 32-37.
Urban JL, Burton RC, Holland JM, Kripke ML, & Schreiber H (1982)
Mechanisms of syngeneic tumor rejection, susceptibility of host-
selected progressor variants to various immunological effector cells.
J Exp Med, 155: 557-573.
US Environmental Protection Agency (1986) Guidelines for the health
assessment of suspect developmental toxicants. Fed Regist,
51: 34027-34040.
US Environmental Protection Agency (1990) Respiratory health effects
of passive smoking: Lung cancer and other disorders
(EPA/600/6-90/006F). Cincinnati, Ohio, Center for Environmental
Research Information.
US Environmental Protection Agency (1991) Workshop report on toxicity
equivalency factors for polychlorinated biphenyl congeners. Washington
DC, Risk Assessment Forum.
US Food and Drug Administration (1993) Draft toxicological principles
for the safety assessment of direct food additives and color additives
used in food ('Redbook II'). Washington DC, Center for Food Safety and
Applied Nutrition.
US National Academy of Sciences (1977) Committee for the revision of
NAS publication No. 1138: Principles and procedures for evaluating the
toxicity of household substances. Washington DC.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Washington DC, National Academy
Press, pp 1-50.
US National Research Council (1989) Biologic markers in pulmonary
toxicology. Washington DC, National Academy Press.
US National Research Council (1992) Biologic markers in
immunotoxicology. Washington DC, National Academy Press.
US Office of Technology Assessment (1991) Identifying and controlling
immunotoxic substances: Background paper (OTA-BP-BA-75). Washington
DC, Congress of the United States, US Government Printing Office.
Vaessen LMB, Joling P, Nieuwenhuis P, Van Haperen MJ, & Rozing J
(1985) T Cell markers on pre-B cells in the rat. Adv Exp Med Biol,
186: 17-26.
Vallejo AN, Miller NW, Jorgensen T, & Clem LW (1990) Phylogeny of
immune recognition: Antigen processing/presentation in channel
catfish; immune responses to hemocyanins. Cell Immunol, 130: 364-377.
Vallejo AN, Miller NW, & Clem LW (1992) Antigen processing and
presentation in teleost immune responses. Ann Rev Fish Dis,
2: 167-183.
Valli VE, Villeneuve DC, Reed B, Barsoum N, & Smith G (1990)
Evaluation of blood and bone marrow, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI Monograph). Berlin, Springer
Verlag, pp 9-26.
Van Bressem MF, Visser IKG, Van de Bildt MWG, Teppema JS, Raga JA, &
Osterhaus ADME (1991) Morbillivirus infection in Mediterranean striped
dolphins (Stenella coeruleoalba). Vet Rec, 129: 471-472.
Vandekerckhove BAE, Krowka JF, McCune JM, De Vries JE, Spits H, &
Roncarolo MG (1991) Clonal analysis of the peripheral T cell
compartment of the SCID-hu mouse. J Immunol, 146: 4173-4179.
Van den Eertwegh AJM, Boersma WJA, & Claassen E (1992) Immunological
functions and in vivo cell-cell interactions of T cells in the
spleen. Crit Rev Immunol, 11: 337-380.
Van Deuren M, Dofferhoff AS, & Van der Meer JW (1992) Cytokines and
the response to infection. J Pathol, 168: 349-356.
Van Diepen JCE, Wagenaar GTM, & Rombout JHWM (1991) Immunocytochemical
detection of membrane antigens of carp leukocytes using light and
electron microscopy. Fish Shellfish Immunol, 1: 47-57.
Van Ewijk W (1988) Cell surface topography of thymic microenvironments.
Lab Invest, 59: 579-590.
Van Ewijk W (1991) T-Cell differentiation is influenced by thymic
microenvironments. Ann Rev Immunol, 9: 591-615.
Van Logten MJ, Gupta BN, McConnell EE, & Moore JA (1981) The influence
of malnutrition on the toxicity of tetrachlorodibenzo- p-dioxin
(TCDD) in rats. Toxicology, 21: 77-88.
Van Loveren H & Vos JG (1989) Immunotoxicological considerations: A
practical approach to immunotoxicity testing in the rat. In: Dayan AD
& Paine AJ ed. Advances in applied toxicology. London, Taylor &
Francis, pp 143-165.
Van Loveren H & Vos JG (1992) Evaluation of OECD guideline # 407 for
assessment of toxicity of chemicals with respect to potential adverse
effects to the immune system (RIVM report no. 158801001). Bilthoven,
National Institute of Public Health and Environmental Protection.
Van Loveren H, Postma GW, Van Soolingen D, Kruizinga W, Groothuis D, &
Vos JG (1987) Enhanced macrophage activity and deficient acquired
resistance to Listeria monocytogenes in nude rats. In: Rygaard J,
Brunner N, Graem N, & Spang-Thomson M ed. Immune-deficient animals in
biomedical research. Basel, Karger.
Van Loveren H, Rombout PJA, Wagenaar SS, Walvoort HC, & Vos JG (1988a)
Effects of ozone on the defence to a respiratory Listeria
monocytogenes infection in the rat. Suppression of macrophage
function and cellular immunity and aggravation of histopathology in
lung and liver during infection. Toxicol Appl Pharmacol, 94: 374-393.
Van Loveren H, Osterhaus ADME, Nagel J, Schurrman HJ, & Vos JG (1988b)
Quantification of IgA responses in the rat. Detection of IgA
antibodies and enumeration of IgA antibody producing cells specific
for ovalbumin or Trichinella spiralis. Scand J Immunol, 28: 377-381.
Van Loveren H, Teppema JS, & Askenase PW (1990a) Skin mast cells.
In: Bos J ed. The skin immune system. Boca Raton, Florida, CRC Press,
pp 171-193.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, & Vos JG (1990b)
Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Loveren H, Van Eden W, Krajnc EI, Krajnc-Franken MAM, De Kort W, &
Vos JG (1990c) Hexachlorobenzene interferes with the development of
experimental autoimmune disease in the Lewis rat. Toxicologist,
10: 221.
Van Loveren H, Verlaan APJ, & Vos JG (1991) An enzyme-linked
immunosorbent assay of anti-sheep red blood cell antibodies of the
classes M, G and A in the rat. Int J Immunopharmacol, 13: 689-695.
Van Loveren H, Timmerman H, Dortant P, & De Waal E (1993a) Validation
of a screening battery for immunotoxicity of drugs within a 28 day
oral toxicity study, using cyclosporin as a model compound (RIVM
report no. 312620001). Bilthoven, National Institute of Public Health
and Environmental Protection.
Van Loveren H Rombout PJA, Fischer P, Lebret E, & Van Bree L (1993b)
Effects of oxidant air pollution on resistance to respiratory
infection, a review (RIVM report no. 619002003). Bilthoven, National
Institute of Public Health and Environmental Protection.
Van Loveren H, Luebke RL, & Vos JG (1994) Assessment of immunotoxicity
using the host infectivity model Trichinella spiralis. In: Burleson
G, Munson A, & Dean J ed. Methods in immunotoxicology. Chicago,
Wiley-Liss.
Van Muiswinkel WB, Lamers CHJ, & Rombout JHWM (1991) Structural and
functional aspects of the spleen in bony fish. Res Immunol,
142: 362-366.
Van Rooijen N, Claassen E, Kraal G, & Dijkstra CD (1989) Cytological
basis of immune functions of the spleen. Immunocytochemical
characterization of lymphoid and non-lymphoid cells involved in the
'in situ' immune response. Progr Histochem Cytochem, 19: 1-71.
Van Soolingen D, Moolenbeek C, & Van Loveren H (1990) An improved
method of bronchoalveolar lavage of lungs of small laboratory animals:
Short report. Lab Anim, 24: 197-199.
Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, & Garattini S
(1980) Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin on humoral antibody production. Chem-Biol Interactions,
30: 337-342.
Vecchi A, Sironi M, Canegrail MA, Recchia M, & Garattini S (1983)
Immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo- p-dioxin in
strains of mice with different susceptibility to induction of aryl
hydrocarbon hydroxylase. Toxicol Appl Pharmacol, 68: 434-441.
Vercelli D & Geha RS (1992) Regulation of isotype switching. Curr Opin
Immunol, 4: 794-797.
Vermeer M, Schmieder GJ, Yoshikawa T, Van Den Berg J-W, Metzman MS,
Taylor JR, & Streilin JW (1991) Effects of ultraviolet B light on
cutaneous immune responses of humans with deeply pigmented skin. J
Invest Dermatol, 97: 729-734.
Versluis DJ, Bijma AM, Vaessen LMB, & Weimar W (1989) Changes in
immunological parameters after conversion from cyclosporin A to
azathioprine in renal transplant recipients. Int J Immunopharmacol,
11: 157-164.
Vethaak D (1993) A large-scale mesocosm study of the effects of marine
pollution on disease development in flounder (Platichthys flesus).
In: Fish disease and marine pollution: A case study of the flounder
(Platichthys flesus) in Dutch coastal and estuarine waters. Thesis
(Meetkundige Dienst RWS, ISBN 90-369-0413-7), Amsterdam, University of
Amsterdam, pp 107-127.
Vethaak AD & ap Rheinallt T (1992) Fish disease as a monitor for
marine pollution: The case of the North Sea. Rev Fish Biol Fish,
2: 1-32.
Vethaak AD & Wester PW (1993) Diseases of flounder (Platichthys
flesus) in Dutch coastal waters, with particular reference to
environmental stress factors. Part II. Liver histopathology. In: Fish
disease and marine pollution, Thesis (Meetkundige Dienst RWS, ISBN
90-369-0413-7). Amsterdam, University of Amsterdam, pp 60-78.
Vetvicka V & Fornusek L (1992) Macrophages. In: Fornusek L & Vetvicka
V ed. Immune system accessory cells. Boca Raton, Florida, CRC Press,
pp 95-108.
Vial T & Descotes J (1992) Clinical toxicity of interleukin-2. Drug
Saf, 7: 417-433.
Vial T & Descotes J (1994) Clinical toxicity of the interferons. Drug
Saf, 10: 115-150.
Vireligier J (1975) Host defenses against influenza virus: The role of
anti-hemagglutinin antibody. J Immunol, 115: 434-439.
Von Boehmer H (1990) Developmental biology of T cells in T cell-
receptor transgenic mice. Ann Rev Immunol, 8: 531-536.
Von Gaudecker B (1986) The development of the human thymus
microenvironment. Curr Topics Pathol, 75: 1-41.
Von Gaudecker B (1991) Functional histology of the thymus. Anat
Embryol, 183: 1-15.
Von Mutius E, Fritzsch C, Weiland SK, Roll G, & Magnussen H (1992)
Prevalence of asthma and allergic disorders among children in united
Germany. Br Med J, 305: 1395-1399.
Vos JG (1977) Immune suppression as related to toxicology. CRC Crit
Rev Toxicol, 5: 67-101.
Vos JG (1980) Immunotoxicity assessment: Screening and function
studies. Arch Toxicol, 4 (Suppl): 95-108.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an international
symposium (IARC Scientific Publications No 77). Lyon, International
Agency for Research on Cancer, pp 347-356.
Vos JG & Beems RB (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits. Toxicol
Appl Pharmacol, 19: 617-633.
Vos JG & Koeman TH (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis, and tissue residues.
Toxicol Appl Pharmacol, 17: 656-668.
Vos JG & Krajnc EI (1983) Immunotoxicology of pesticides. In: Hayes
AW, Schnell RC, & Miya TS ed. Developments in the science and practice
of toxicology. Amsterdam, Elsevier Science Publishers, pp 229-240.
Vos JG & Krajnc-Franken MAM (1990) Toxic effects on the immune system:
Rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system.
Berlin, Springer Verlag, pp 168-181.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Elsevier Science
Publishers, pp 295-322.
Vos JG & Moore JA (1974) Suppression of cellular immunity in rats and
mice by maternal treatment with 2,3,7,8-tetrachlorobenzo- p-dioxin.
Int Arch Allergy Appl Immunol, 47: 777-794.
Vos JG & Van Driel-Grootenhuis, L (1972) PCB-induced suppression of
the humoral and cell-mediated immunity in guinea pigs. Sci Total
Environ, 1: 289-302.
Vos JG & Van Genderen H (1973) Toxicological aspects of
immunosuppression. In: Pesticides and the environment: A continuing
controversy. North Miami, Florida, Symposia Specialists, pp 527-545.
Vos JG & Van Loveren H (1987) Immunotoxicity testing in the rat. In:
Burger EJ, Tardiff RG, & Bellanti JA ed. Advances in modern
environmental toxicology, Vol 13: Environmental chemical exposures and
immune system integrity. Princeton, New Jersey, Princeton Scientific
Publishing, pp 167-180.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979a) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Buys J, Hanstede JG, & Hagenaars AM (1979b) Comparison of
enzyme-linked immunosorbent assay and passive haemagglutination method
for quantification of antibodies to lipopolysaccharide and tetanus
toxoid in rats. Infect Immun, 24: 798-803.
Vos JG, Boerkamp J, Buys J, & Steerenberg PA (1980) Ovalbumin immunity
in the rat: Simultaneous testing of IgM, IgG and IgE response measured
by ELISA and delayed-type hypersensitivity. Scand J Immunol,
12: 289-295.
Vos JG, Krajnc EI, & Beekhof P (1982) Use of the enzyme-linked
immunosorbent assy (elisa) in immunotoxicity testing. Environ Health
Perspectives, 43: 115-121.
Vos JG, Krajnc EI, Beekhof PK, & Van Logten MJ (1983a) Methods for
testing immune effects of toxic chemicals: Evaluation of the
immunotoxicity of various pesticides in the rat. In: Miyamoto T ed.
IUPAC pesticide chemistry: Human welfare and the environment. Oxford,
Pergamon Press, pp 497-504.
Vos JG, Ruitenberg EJ, Van Basten N, Buys J, Elgersma A, & Kruizinga W
(1983b) The athymic nude rat. IV. Immunocytochemical study to detect
T cells, and immunological and histopathological reactions against
Trichinella spiralis. Parasite Immunol, 5: 195-215.
Vos JG, De Klerk A, Krajnc EI, Kruizinga W, Van Ommen B, & Rozing J
(1984) Toxicity of bis(tri-n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicol Appl
Pharmacol, 75: 387-408.
Vos JG, Van Loveren H, Wester P, & Vethaak D (1989) Toxic effects of
environmental chemicals on the immune system. TiPs, 10: 289-292.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rozing J (1990a)
Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on
thymus-dependent-immunity and non-specific resistance following long-
term exposure in young versus aged rats. Toxicol Appl Pharmacol,
105: 144-155.
Vos JG, Dormans JAMA, Kraal G, Krajnc EI, Krajnc-Franken MAM, & Van
Loveren H (1990b) Immunotoxicity of hexachlorobenzene in the rat is
thymus-dependent. Toxicologist, 10: 221.
Vos JG, Van Loveren H, & Schuurman H-J (1991) Immunotoxicity of
dioxin: Immune function and host resistance in laboratory animals and
humans. In: Biological basis for risk assessment of dioxins and
related compounds (Banbury report 35). Cold Spring Harbor, New York,
CSH Press, pp 79-93.
Wakelin D (1993) Allergic inflammation as a hypothesis for the
expulsion of worms from tissues. Parasitol Today, 9: 115-116.
Waldman TA (1988) Immunodeficiency diseases: Primary and acquired.
In: Immunological diseases. Boston, Little Brown Co, pp 441-466.
Ward JM (1990) Classification of reactive lesions of lymph nodes.
In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system (ILSI
Monograph). Berlin, Springer Verlag, pp 155-162.
Ward P & Kendall MD (1975) Morphological changes in the thymus of
young and adult red-billed queleas Quelea quelea (aves). Phil Trans
R Soc Lond B, 273: 55-64.
Ward EC, Murray MJ, & Dean JH (1985) Immunotoxicity of nonhalogenated
polycyclic aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE, &
Amos H ed. Immunotoxicology and immunopharmacology. New York, Raven
Press, pp 291-304.
Ware JH, Dockery DW, Spiro A, Speizer FE, & Ferris BG (1984) Passive
smoking, gas cooking, and respiratory health of children living in six
cities. Am Rev Respir Dis, 129: 366-374.
Watson RR (1990) Drugs of abuse and immune function. Boca Raton,
Florida, CRC Press.
Watson RR (1993) Alcohol, drugs of abuse and immunomodulation
(Advances in the Biosciences, Vol 86). Oxford, Pergamon Press.
Weeks BA & Warinner JE (1986) Functional evaluation of macrophages in
fish from a polluted estuary. Vet Immunol Immunopathol, 12: 313-320.
Weeks BA, Anderson DP, Dufour AP, Fairbrother A, Goven AJ, Lahvis GP,
& Peters G (1992) Immunological biomarkers to assess environmental
stress. In: Biomarkers, biochemical, physiological and histological
markers of anthropo-genic stress. London, Lewis Publishers, pp 5-85.
Weigent DA & Blalock JE (1987) Interactions between the neuroendocrine
and immune systems: Common hormones and receptors. Immunol Rev,
100: 79-108.
Weiss L & Geduldig U (1991) Barrier cells: Stromal regulation of
hematopoiesis and blood release in normal and stressed murine bone
marrow. Blood, 78: 975-990.
Weiss L & Sakai H (1984) The hematopoietic stroma. Am J Anat,
170: 447-463.
Weissgarten J, Freidman CNGE, & Cardella CJ (1989) B Lymphocytes from
stable renal transplant patients do not respond to exogenous
lymphokines: Comparison of azathioprine and cyclosporin treated
patients. Transplant Proc, 21: 393-394.
Weissler JC, Nicod LP, & Toews GB (1987) Pulmonary natural killer cell
activity is reduced in patients with bronchogenic carcinoma. Am Rev
Respir Dis, 135: 1353-1357.
Wessel G, Abenroth K, & Wisheit M (1988) Malignant transformation
during immunosuppressive therapy (azathioprine) of rheumatoid
arthritis and systemic lupus erythematosus. Scand J Rheumatol, 67
(Suppl): 73-75.
Wester PW, & Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to bis(tri- n-
butyltin)oxide (TBTO) and di- n-butyltindichloride (DBTC). Aquat
Toxicol 10: 143-165.
Wester PW & Canton JH (1991) The usefulness of histopathology in
aquatic toxicity studies. Comp Biochem Physiol, 100C: 115-117.
Wester PW, Canton JH, Van Iersel AAJ, Krajnc EI ,& Vaessen HAMG
(1990) The toxicity of bis(tri- n-butyltin)oxide (TBTO) and
di- n-butyltindichloride (DBTC) in the small fish species Oryzias
latipes (medaka) and Poecilia reticulata (guppy). Aquat Toxicol,
16: 53-72.
Wester PW, Vethaak AD, & Van Muiswinkel WB (1994) Fish as biomarkers
in immunotoxicology. Toxicology, 86: 213-232.
White KL (1986) An overview of immunotoxicology and carcinogenic
polycyclic aromatic hydrocarbons. J Environ Health Sci, C4: 163-202.
White KL (1992) Specific immune function assays. In: Miller K, Turk J,
& Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 304-323.
White KL & Munson AE (1986) Suppression of the in vitro humoral
immune response by chrysotile asbestos. Toxicol Appl Pharmacol,
82: 493-504.
White KL, Sander VM, Barnes DW, Shopp GM, & Munson AE (1985)
Immunotoxicological investigations in the mouse: General approach and
methods. Drug Chem Toxicol, 8: 299-331.
White KL, Lysy HH, McCay JA, & Anderson AC (1986) Modulation of serum
complement levels following exposure to polychlorinated dibenzo- p-
dioxins. Toxicol Appl Pharmacol, 84: 209-219.
White KL, Gennings C, Murray MJ, & Dean JH (1994) Summary of
international methods: Validation study, carried out in nine
laboratories, on immunological assessment of cyclosporin A in the rat.
In Vitro Toxicity, 8: 957-961.
WHO (1990) Revised guidelines for preparation of Environmental Health
Criteria monographs (PCS/90.69), Geneva
Wierda D, Irons RD, & Greenlee WF (1981) Immunotoxicity in C57Bl/6
mice exposed to benzene and Aroclor 1254. Toxicol Appl Pharmacol,
60: 410-417.
Williams ME & Quesenberry PJ (1992) Hematopoietic growth factors.
Hematol Pathol, 6: 105-124.
Wilson MR & Warr GW (1992) Fish immunoglobulins and the genes that
encode them. Ann Rev Fish Dis, 201-221.
Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, & Steinberg C
(1992) What limits affinity maturation of antibodies in Xenopus: The
rate of somatic mutation or the ability to select mutants? EMBO J,
11: 4337-4347.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity in
mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20: 423.
Winkelstein JA (1981) The role of complement in the host defense
against Streptococcus pneumoniae. Rev Infect Dis, 3: 289-298.
Wolke RE (1992) Piscine macrophage aggregates: A review. Ann Rev Fish
Dis, 91-108.
Wood SC, Karras JG, & Holsapple MP (1992) Integration of the human
lymphocyte into immunotoxicological investigations. Fundam Appl
Toxicol, 18: 450-459.
Wooley PH (1991) Animal models of rheumatoid arthritis. Curr Opin
Rheumatol, 3: 407-420.
Wright EG & Pragnell IB (1992) Stem cell proliferation inhibitors.
Bailliere's Clin Haematol, 5: 723-739.
Yamaguchi T, Shinagawal Y, & Pollard RB (1988) Relationship between
the production of murine cytomegalovirus and interferon in
macrophages. J Gen Virol, 69: 2961-2971.
Yang KH, Kim BS, Munson AE, & Holsapple MP (1986) Immunosuppression
induced by chemicals requiring metabolic activation in mixed cultures
of rat hepatocytes and murine splenocytes. Toxicol Appl Pharmacol,
83: 420-429.
Yang YG, Lebrec H, & Burleson GR (1994) Effect of 2,3,7,8
tetrachlorodibenzo- p-dioxin (TCDD) on viral replication and natural
killer (NK) activity in rats. Fundam Appl Toxicol, 23: 125-131.
Yang YG, Gilmour MI, Lange R, Burleson GR, & Selgrade MJK (1995)
Effects of acute exposure to phosgene on pulmonary host defenses and
resistance to infection. Inhal Toxicol, 7: 393-404.
Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, & Santos GW
(1993) Cyclosporine-induced graft-versus-host disease after autologous
bone marrow transplantation for acute myeloid leukemia. Leukemia
Lymphoma, 11: 215-220.
Yoshida T & Streilin JW (1990) On the genetic basis of the effects of
ultraviolet B on cutaneous immunity. Evidence that polymorphisms at
the TNFa and Lps loci govern susceptibility. Immunogenetics,
32: 398-405.
Yoshikawa T, Rae V, Bruins-Slot W, Van Den Berg J-W, Taylor JR, &
Streilin JW (1990) Susceptibility to effects of UVB radiation on
induction of contact hypersensitivity as a risk factor for skin cancer
in humans. J Invest Dermatol, 95: 530-536.
Yosioka T, Sato S, Fujiwana H, & Hamacka T (1986) The role of GM-1
antibody-sensitive cells in the implementation of tumor-specific
T-cell mediated immunity in vivo. Jpn J Cancer Res, 77: 825-832.
Zapata A (1982) Lymphoid organs of teleost fish III. Splenic lymphoid
tissue of Rutilus rutilus and Gobio gobio. Dev Comp Immunol,
6: 87-94.
Zapata A (1983) Phylogeny of the fish immune system. Bull Inst
Pasteur, 81: 185-186.
Zapata AG & Cooper EL (1990) The immune system: Comparative
histophysiology. New York, John Wiley & Sons.
Zapata AG, Varas A, & Torroba M (1992) Seasonal variations in the
immune system of lower vertebrates. Immunol Today, 13: 142-147.
Zeeman MG & Brindley WA (1981) Effects of toxic agents upon fish
immune systems: A review. In: Sharma RP, ed. Immunologic
considerations in toxicology, Vol II. Boca Raton, Florida, CRC Press,
pp 1-60.
Zelikoff JT, Enane NA, Bowser D, Squibb KS, & Frenkel K (1991)
Development of fish peritoneal macrophages as a model for higher
vertebrates in immunotoxicological studies. Fundam Appl Toxicol,
16: 576-589.
Zheng B, Shorthouse R, Masek MA, Berry G, Billingham ME, & Morris RE
(1991) Effect of the new and highly active immunosuppressant,
Rapamycin, on lymphoid tissues and cells in vivo. Transplant Proc,
23: 851-855.
Zwick H, Popp W, Wagner C, Reiser K, Schmoger J, Bock A, Herkner K, &
Radunsky K (1991) Effects of ozone on the respiratory health allergic
sensitization, and cellular immune system in children. Am Rev Respir
Dis, 144: 1075-1079.
1. RESUME
1. Le système immunitaire a évolué de manière à contrer les
atteintes que peuvent porter à l'intégrité du soi des microorganismes
ou des cellules ayant échappé au contrôle de l'organisme. Une
intrusion xénobiotique peut perturber le fonctionnement du système
immunitaire et c'est la reconnaissance de cet état de choses qui a
permis les progrès de ces vingt dernières années. Toute une
méthodologie expérimentale a été développée et validée
(essentiellement sur des rongeurs) par des études impliquant une
multitude de laboratoires. La présente monographie examine les
fonctions et l'histophysiologie du système immunitaire et présente les
données nécessaires à la compréhension et à l'interprétation des
modifications pathologiques provoquées par les agressions
immmunotoxiques. L'accent est mis sur le système immunitaire de
l'homme et des rongeurs, mais sans négliger d'autres espèces, les
poissons en particulier, auxquelles des études immunotoxicologiques
ont été consacrées. Il importe, pour comprendre l'impact des effets
immunotoxiques, de connaître la pathophysiologie du système
immunitaire, et notamment la sensibilité variable de ses constituants,
les modifications subies par les organes lymphoïdes et la réversibilité
de ces modifications.
2. L'immunosuppression et l'immunostimulation ont toutes deux des
conséquences sur le plan clinique. On a établi une corrélation entre
les états d'immunodéficience ou d'immunodépression grave que l'on
observe par exemple chez des patients greffés ou sous traitement
cytostatique, et l'accroissement de l'incidence des maladies
infectieuses (opportunistes en particulier) et du cancer. L'exposition
à des substances chimiques immunotoxiques présentes dans l'environnement
peut toutefois donner lieu à des formes plus subtiles d'immunodépression,
difficiles à déceler, qui entraînent une augmentation de l'incidence de
maladies infectieuses comme la grippe ou le rhume. Des travaux effectués
sur des animaux de laboratoire et sur l'homme montrent que de nombreux
produits chimiques présents dans l'environnement dépriment la réponse
immunitaire. Ces substances xénobiotiques immunotoxiques ne se limitent
pas à untype particulier de composé chimique ou d'agent physique. Il y
a, parmi les médicaments, les pesticides, les solvants, les hydro-
carbures halogénés, les hydrocarbures aromatiques et les métaux, des
substances susceptibles d'altérer le système immunitaire et le
rayonnement ultra-violet a également cette propriété. L'administration,
à des fins thérapeutiques, d'agents immunostimulants, peut avoir des
effets nocifs et certaines substances (béryllium, silice, hexa-
chlorobenzène) présentes dans l'environnement, ont des propriétés
immunostimulantes qui peuvent se traduire par des effets cliniques.
3. Du fait de sa complexité, le système immunitaire offre une
multiplicité de cibles potentielles à l'action de ces agents avec
toutes sortes de séquelles pathologiques. Les premières stratégies
élaborées par les immunotoxicologues ont consisté à choisir et à
mettre en oeuvre une batterie de tests multiphasiques sur animaux de
laboratoire afin d'identifier les agents immunosuppresseurs ou
immunostimulants. Ces batteries de tests peuvent varier selon
l'organisme ou le laboratoire qui les mettent en oeuvre ou encore
selon l'espèce animale utilisée, mais elles comportent toutes un ou
plusieurs des éléments suivants: modification du poids ou de
l'histologie des organes lymphoïdes; examen des leucocytes du sang
périphérique, des cellules du tissu lymphoïde ou de la moelle osseuse
à la recherche de modifications; intégrité de la fonction des cellules
effectrices et régulatrices et modification éventuelle de la
sensibilité à une exposition à des agents infectieux ou à des cellules
tumorales.
La directive expérimentale originale No 407 de l'Organisation de
coopération et de développement économiques, publiée en 1981, n'avait
pas pour but la mise en évidence d'une immunotoxicité potentielle,
aussi a-t-elle été modifiée pour la rendre plus adaptée à
l'identification des substances immunotoxiques. Des systèmes d'épreuve
multiphasiques permettant une investigation plus large de
l'immunotoxicité ont été conçus par l'US National Toxicology Program,
l'Institut Néerlandais de la Santé Publique et de la Protection de
l'Environnement, l'US Environmental Protection Agency (Office of
Pesticides), ainsi que par le Center for Food Safety andApplied
Nutrition de l'US Food and Drug Administration. Des études ont été
effectuées sur des souris et, à un moindre degré, sur des rats afin
d'évaluer la spécificité, la précision (reproductibilité), la
sensibilité, l'exactitude et la pertinence, pour l'appréciation du
risque sanitaire, de diverses mesures de l'état immunitaire. On a
entrepris la validation interlaboratoires, au niveau international, de
toutes ces méthodes, dans le cadre de l'Etude collective
internationale sur l'immunotoxicité organisée par le PISC, l'Union
européenne et le Bundesinstitut für Gesundheitllichen
Verbraucherschutz und Veterinärmedizin. Des études analogues ont été
menées sur des rats Fischer 344 à propos de la cyclosporine A.
4. Les épreuves utilisées dans le système multiphasique sont
décrites à la section 3 avec les raisons qui ont guidé ce choix et un
exposé des difficultés que l'on peut rencontrer dans leur mise en
oeuvre. Bien que conçus à l'origine pour des études sur des rats et
des souris, certains de ces protocoles ont pu être utilisés avec fruit
pour des travaux d'immunotoxicologie portant sur d'autres espèces,
notamment des primates non humains, des mammifères marins, des chiens,
des oiseaux et des poissons.
Lorsqu'on se propose de déterminer dans quelle mesure un agent
environnemental ou un médicament est susceptible d'avoir une influence
nocive sur le système immunitaire, il faut prendre en considération un
certain nombre de facteurs. Il s'agit notamment du choix d'un modèle
animal et de variables d'exposition appropriés, de la prise en compte
des paramètres toxicologiques généraux, de la pertinence biologique
des points d'aboutissement retenus, de la mesure de grandeurs dûment
validées et de la mise en oeuvre d'un système d'assurance de la
qualité. Les conditions expérimentales doivent tenir compte des
modalités de l'exposition humaine (voie de pénétration et
concentration ou intensité) et de toute information disponible d'ordre
toxicodynamique ou toxicocinétique. Les doses et la taille des
échantillons doivent être choisies de manière à permettre l'obtention
de bonnes courbes dose-réponse et la détermination de la dose sans
effet (nocif ou non) observable. Cesstratégies sont améliorées en
permanence afin de permettre une meilleure prévision des situations
susceptibles d'entraîner une pathologie. En outre, on devrait pouvoir
disposer de techniques qui faciliteraient l'étude du mode d'action des
agents en cause. Il pourrait s'agir de méthodes in vitro, de l'étude
des réponses immunitaires locales (par exemple au niveau de la peau,
des poumons et de l'intestin), de techniques de biologie moléculaire
et de l'utilisation d'animaux génétiquement modifiés.
5. Mettre en évidence des modifications d'ordre immunologique après
une exposition à des composés potentiellement immunotoxiques est plus
complexe chez l'homme que chez l'animal de laboratoire. Les
possibilités d'expérimentation sont limitées, il est difficile
d'établir le niveau d'exposition à l'agent en cause (c'est-à-dire la
dose) et de plus, l'état immunitaire des populations est extrêmement
hétérogène. Cette hétérogénéité trouve son origine dans un certain
nombre de facteurs: âge, sexe, race, gravidité, stress et aptitude à y
faire face, pathologies et états infectieux cocomitants, état
nutritionnel, tabagisme et prise de certains médicaments. L'intérêt de
telle ou telle étude pour l'évaluation du risque est conditionné par
un élément important, à savoir le concept épidémiologique qui la sous-
tend. La plupart du temps on procède à une étude transversale, qui
consiste à déterminer l'exposition et la morbidité à un moment donné
ou sur une courte période. On compare ensuite la fonction immunitaire
des sujets exposés à celle d'un groupe analogue de sujets non exposés.
Ce genre d'étude peut receler un certain nombre de pièges.
Nombre des altérations que produit l'exposition à une substance
chimique ne se manifestent chez l'homme que de manière subtile et
sporadique, aussi faut-il étudier des populations récemment exposées
et utiliser des épreuves sensibles.
La plupart des épreuves concernant l'immunité spécifique (à
médiation cellulaire ou humorale), l'immunité non spécifique et les
processus inflammatoires ont été conçues pour rechercher des anomalies
chez des patients atteints d'une déficience immunitaire et ne sont pas
forcément capables de déceler les modifications subtiles provoquées
par les substances chimiques. Le PISC, les Centers for Disease Control
et l'Académie des Sciences des Etats-Unis ont, chacun de leur côté,
défini une méthodologie pour évaluer les modifications du système
immunitaire quipourraient résulter d'une exposition à des produits
immunotoxiques mais les épreuves proposées demandent encore à être
validées.
6. L'évaluation du risque consiste à analyser les données
pertinentes sur un agent donné (effets biologiques, relations dose-
réponse et exposition) pour tenter d'obtenir une estimation
qualitative et quantitative des diverses conséquences nocives de la
présence de cet agent. Elle se caractérise par quatre phases
principales: reconnaître le danger, établir la relation dose-réponse,
évaluer l'exposition et caractériser le risque. Jusqu'ici,
l'immunotoxicologie s'est essentiellement attachée à reconnaître le
danger et, dans une certaine mesure, à établir des relations dose-
réponse, mais très peu d'études ont été consacrées à l'évaluation du
risque ou à sa caractérisation.
Comme dans d'autres domaines de la toxicologie, des incertitudes
demeurent qui sont susceptibles de gêner l'interprétation des données
immunotoxicologiques dans l'optique du risque pour la santé humaine.
Les deux questions qui sont actuellement les plus problématiques -
l'extrapolation à l'organe dans son ensemble des effets constatés sur
la cellule et l'extrapolation à l'homme des données obtenues sur
l'animal - valent pour la plupart des points d'aboutissement des
effets, cancer excepté. Le premier problème tient aux incertitudes que
comporte l'établissement d'une relation quatitative entre les
modifications subies par la fonction immunitaire d'un individu et
l'altération de sa résistance aux maladies infectieuses et
néoplasiques. Le deuxième est lié à l'incertitude qui entache
l'évaluation du risque pour la santé humaine à partir des résultats
obtenus sur des animaux de laboratoire.
L'évaluation du risque a pour finalité de protéger la santé
humaine et l'environnement. Il faut donc que le choix des modéles
expérimentaux soit judicieux. La toxicocinétique de la substance
étudiée et la réponse immunitaire suscitée dans le modèle doivent
pouvoir être comparées à celles qu'on observerait chez l'homme.
Pour tirer une limite d'exposition des résultats expérimentaux,
il est convenu d'appliquer un facteur d'incertitude aux données
d'évaluation du risque. Cette convention ne tient pas compte de la
réserve fonctionnelle ou de la redondance du système immunitaire. Une
méthode plus récente d'évaluation du risque consiste à ultiliser des
modèles in vitro en complément aux études sur animaux de laboratoire.
Cette méthode a l'avantage de permettre une extrapolation plus fidèle
de l'animal à l'homme et de ne nécessiter qu'un minimum d'animaux.
Elle permet également de pallier l'absence de données dans les cas où
des considérations d'éthique limitent l'expérimentation sur l'homme.
Le Chapitre 6 donne deux exemples de situation où les données obtenues
in vitro permettent de lever en partie les incertitudes dans
l'évaluation du risque dû à l'exposition à l'ozone et au rayonnement
ultra-violet. L'utilisation des données immunotoxicologiques dans
l'évaluation du risque reste limitée par la difficulté d'établir des
relations quantitatives entre l'immunodépression et les manifestations
cliniques d'une pathologie donnée.
RESUMEN
1. El sistema inmunitario ha evolucionado para hacer frente a las
amenazas a la integridad del organismo vivo provenientes de
microorganismos o de células que han escapado a los mecanismos de
control del organismo. El reconocimiento de que las sustancias
xenobióticas pueden trastornar el funcionamiento del sistema
inmunitario ha llevado a avances en el campo de la inmunotoxicología
durante los dos últimos decenios. Se han formulado métodos
experimentales (empleando principalmente especies de roedores), que
han sido validados en estudios multilaboratorio. En esta monografía se
examinan la función y la histofisiología del sistema inmunitario,
presentándose la información necesaria para la comprensión e
interpretación de los cambios patológicos causados por las agresiones
inmunotóxicas. Si bien se hace hincapié en los sistemas inmunitarios
del ser humano y de las especies de roedores, se hace referencia a
otras especies, incluidos los peces, que han sido objeto de estudios
inmunotoxicológicos. La fisiopatología del sistema inmunitario,
incluidas la sensibilidad variable de sus componentes, las
alteraciones de los órganos linfoides y la reversibilidad de los
cambios, es importante para comprender las repercusiones de la
inmunotoxicidad.
2. Tanto la inmunosupresión como la inmunoestimulación tienen
consecuencias clínicas. Se ha observado que los estados de
inmunodeficiencia y de inmunosupresión grave, como los que se pueden
presentar en casos de trasplante y de terapia citostática, van
acompañados de mayor incidencia de enfermedades infecciosas
(especialmente las oportunistas) y de cáncer. Con todo, cabe prever
que la exposición a los productos químicos inmunotóxicos enel medio
ambiente dará origen a formas más sutiles de inmunosupresión cuya
detección podría resultar difícil, lo que se traduciría en una mayor
incidencia de infecciones tales como la gripe y el resfriado común.
Estudios realizados con animales de laboratorio y seres humanos han
mostrado que muchos de los productos químicos presentes en el medio
ambiente provocan la supresión de la respuesta inmunitaria. Las
sustancias xenobióticas inmunotóxicas no se limitan a una clase
determinada de productos químicos. Entre los compuestos que tienen
efectos nocivos para el sistema inmunitario se cuentan fármacos,
plaguicidas, disolventes, hidrocarburos halogenados y aromáticos y
metales; la radiación ultravioleta puede resultar también
inmunotóxica. La administración terapéutica de agentes
inmunoestimulantes puede provocar reacciones adversas; asimismo,
algunos de los productos químicos presentes en el medio ambiente que
poseen propiedades inmunoestimulantes (berilio, sílice,
hexaclorobenceno) pueden tener consecuencias clínicas.
3. La complejidad del sistema inmunitario lleva aparejadas
multiplicidad de posibles puntos vulnerables y secuelas patológicas.
Los métodos iniciales ideados por los inmunotoxicólogos que realizan
investigaciones sobre toxicología y evaluación de la inocuidad
consistían en seleccionar y aplicar una serie de valoraciones
escalonadas para identificar los agentes inmunosupresores e
inmunoestimulantes en animales de laboratorio. Si bien la
configuración de esas series de análisis puede variar según la
institución o el laboratorio en que se llevan a cabo, así como según
las especies de animales empleadas, en todas se mide unoo más de los
siguientes parámetros: alteraciones del peso y de la histología de los
órganos linfoides; cambios en la celularidad del tejido linfoide, de
los leucocitos en la sangre periférica y/o de la médula ósea;
trastornos de la función celular a nivel de los efectores o de la
regulación, y alteración de la sensibilidad a la amenaza que presentan
los agentes infecciosos o las células tumorales.
La Directriz de pruebas inicial No 407 de la Organización de
Cooperación y Desarrollo Económicos, publicada en 1981, no preveía la
detección de los riesgos de inmunotoxicidad, y se han propuesto
modificaciones destinadas a aumentar la utilidad de esa Directriz para
la identificación de las sustancias inmunotóxicas. El Programa
Nacional de Toxicología de los Estados Unidos, el Instituto Nacional
de Salud Pública y Protección del Medio Ambiente de los Países Bajos,
la Oficina de Plaguicidas de la Agencia para la Protección del Medio
Ambiente de los Estados Unidos y el Centro de Seguridad de los
Alimentos y Nutrición Aplicada de la Administración de Alimentos y
Medicamentos de los Estados Unidos han elaborado sistemas de pruebas
escalonadas para la investigación en mayor escala de los riesgos de
inmunotoxicidad. Se han realizado estudios con ratones, y en menor
medida con ratas, de diferentes indicadores del estado inmunológico
con el fin de determinar su especificidad, precisión (reproducibilidad),
sensibilidad, exactitud y pertinencia para la evaluación de los riesgos
para la salud humana. Los métodos han sido objeto de validaciones
internacionales interlaboratorios en el marco del Estudio Internacional
en Colaboración sobre Inmunotoxicidad del IPCS, la Unión Europea y el
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinärmedizin, yen estudios sobre la ciclosporina A en ratas
Fisher 344.
4. Las pruebas empleadas en los programas de verificaciones
escalonadas se describen en la Sección 3, en la que se indican la
razón de ser de su selección y las complejidades que entraña su
realización. Si bien esos protocolos fueron diseñados para estudios
realizados con ratas y ratones, algunos de ellos se han aplicado con
buenos resultados al estudio de la inmunotoxicidad en otras especies
animales, incluidos primates no humanos, mamíferos marinos, perros,
aves y peces.
A la hora de evaluar las posibles repercusiones negativas de un
agente ambiental o fármaco sobre el sistema inmunitario de los
animales experimentales deberán considerarse diversos factores. Entre
ellos cabe señalar: la selección de los modelos y las variables de
exposición apropiados para los animales, la inclusión de parámetros
toxicológicos generales, la comprensión de la importancia de los
parámetros objeto de medición, el empleo de medidas validadas y el
control de la calidad. En las condiciones experimentales deberán
tenerse en cuenta las vías y el nivel posibles de exposición del ser
humano, así como toda la información disponible sobre toxicodinámica y
toxicocinética. Las dosis y el tamaño de las muestras deberán
seleccionarse de manera que se puedan obtener curvas de dosis-
respuesta bien definidas, además del nivel sin efectos adversos
observados y del nivel sin efectos observados. Los métodos se
perfeccionan continuamente para poder predecir mejor las condiciones
que podrían ser causantes de enfermedades. Además, deberán elaborarse
técnicas que contribuyana la identificación de mecanismos de acción;
éstos podrían incluir los métodos in vitro, el examen de las
respuestas inmunitarias locales (por ejemplo, en la piel, los pulmones
y los intestinos), y el empleo de las técnicas de la biología
molecular y de animales modificados genéticamente.
5. La detección de los cambios inmunológicos ocurridos tras la
exposición a compuestos que podrían ser inmunotóxicos resulta más
complicada en el ser humano que en los animales de laboratorio. Las
posibilidades de realizar pruebas son limitadas; los niveles de
exposición al agente (es decir, la dosis) son difíciles de establecer
y el estado inmunitario de las poblaciones es sumamente heterogéneo.
La edad, la raza, el sexo, la gestación, el estrés agudo y la
capacidad para hacerle frente, las enfermedades e infecciones
coexistentes, el estado nutricional, el humo del tabaco y algunos
medicamentos se cuentan entre los factores que contribuyen a esa
heterogeneidad.
El diseño de los estudios epidemiológicos es un factor importante
para determinar la utilidad de un estudio determinado para la
evaluación de riesgos. El tipo de diseño empleado más frecuentemente
en materia de inmunotoxicidad son los estudios transversales, en los
que se miden las condiciones de exposición y el estado de la
enfermedad en un momento dado, o durante un periodo breve. A
continuación se compara la función inmunitaria de los sujetos
«expuestos» con la de un grupo comparable de individuos
«no expuestos». Ese tipo de diseño lleva aparejados posibles escollos.
Como muchos de los cambios observados en la respuesta inmunitaria
de los seres humanos tras su exposición a un productoquímico podrían
ser esporádicos y sutiles, habrá que estudiar las poblaciones que han
estado expuestas recientemente empleando pruebas de gran sensibilidad
para la evaluación del sistema inmunitario. Las conclusiones sobre los
efectos inmunotóxicos deberán estar basadas en las variaciones
detectadas no en un parámetro aislado sino en el perfil inmunológico
del individuo o de la población.
La mayoría de las pruebas existentes para determinar la inmunidad
específica (de base celular y humoral), la inmunidad no específica y
la inflamación han sido concebidas para detectar las alteraciones
inmunitarias en pacientes que padecen de inmunodeficiencia y no
siempre resultan adecuadas para detectar las alteraciones sutiles
provocadas por los productos químicos presentes en el medio ambiente.
El IPCS, los Centros de Control de Enfermedades y la Academia de
Ciencias de los Estados Unidos han descrito procedimientos para
evaluar los cambios que ocurren en el sistema inmunitario del ser
humano como consecuencia de la exposición a sustancias inmunotóxicas;
con todo, las pruebas descritas deberán ser evaluadas con este fin.
6. La evaluación de riesgos es un proceso en el que se analizan la
información pertinente sobre los efectos biológicos, las relaciones
dosis-respuesta y la exposición a un agente determinado con miras a
establecer estimaciones cualitativas y cuantitativas de los resultados
adversos. Por regla general, la evaluación de los riesgos supone
cuatro pasos fundamentales: identificación de los riesgos; evaluación
de la relación dosis-respuesta; evaluación de la exposición y
caracterización de los riesgos. Hasta ahora, lainmunotoxicología se ha
centrado principalmente en la identificación de los riesgos y, en
cierta medida, en la evaluación de las relaciones dosis-respuesta; muy
contados han sido los estudios que han incluido la evaluación de la
exposición o la caracterización de los riesgos.
Al igual que ocurre en otros campos de la toxicología, existen
incertidumbres que podrían afectar a la interpretación de los datos
sobre inmunotoxicidad en cuanto a los riesgos para la salud humana.
Las dos cuestiones más problemáticas - la extrapolación de los efectos
observados en células individuales a todo un órgano, o a niveles
superiores, y la extrapolación al ser humano de los resultados
obtenidos en experimentos con animales - son comunes a la mayoría de
los parámetros de valoración no relacionados con el cáncer. La primera
obedece a las incertidumbres vinculadas al establecimiento de una
relación cuantitativa entre los cambios observados en la función
inmunitaria del individuo y la perturbación de la resistencia a las
infecciones y enfermedades neoplásicas. La segunda cuestión es
consecuencia de las incertidumbres que lleva aparejadas la evaluación
de los riesgos para la salud humana basándose en los estudios
realizados con animales de laboratorio.
El objetivo fundamental de la evaluación de los riesgos es la
protección de la salud de los seres humanos y del medio ambiente. Por
lo tanto, deberán seleccionarse sistemas modelo idóneos. La
toxicocinética del material de prueba y la índole y magnitud de la
respuesta inmunitaria generada en el modelo deberán ser comparables a
la de los seres humanos.
Habitualmente, en la evaluación de los riesgos se emplean
factores empíricos de incertidumbre para determinar el límite de
exposición aceptable a partir de los resultados experimentales. Ese
procedimiento no toma en cuenta la reserva funcional ni la redundancia
del sistema inmunitario. Un adelanto más reciente en materia de
evaluación de riesgos es el empleo de modelos in vitro como
complemento de los estudios realizados con animales de laboratorio.
Ese procedimiento tiene la ventaja de que permite aumentar la
exactitud de la extrapolación al ser humano de los resultados
obtenidos en los experimentos realizados con animales, reduciendo al
mínimo el número de animales necesarios; asimismo, permite colmar la
brecha entre ambos tipos de información, sobre todo en los casos en
que los experimentos con seres humanos se ven limitados por
consideraciones de índole ética. En el capítulo 6 se presentan dos
ejemplos de cómo la información in vitro permite reducir las
incertidumbres en materia de evaluación de riesgos relacionadas con la
exposición al ozono y a la radiación ultravioleta. La dificultad para
establecer relaciones cuantitativas entre la inmunosupresión y las
enfermedades clínicas ha limitado el empleo de los datos
inmunotoxicológicos en la evaluación de los riesgos.
Table 15. Chi-squared values (hypothetical)
Treatment Design 1 Design 2 Design 3
------------------------------- ------------------------------- --------------------------------
No. affected/ One-tailed No. affected/ One-tailed No. affected/ One-tailed
no. tested P value no. tested P value no. tested P value
Control 3/15 - 6/30 - 0/15 -
Dose 1 4//15 0.532 8/30 0.428 1/15 0.516
2 5/15 0.411 10/30 0.273 2/15 0.274
3 6/15 0.312 12/30 0.165 3/15 0.150
4 7/15 0.233 14/30 0.096 4/15 0.084
5 8/15 0.173 16/30 0.055 5/15 0.048*
6 9/15 0.128 18/30 0.031* 6/15 0.028*
7 10/15 0.094 20/30 0.017* 7/15 0.017*
8 11/15 0.069 22/30 0.009* 8/15 0.010*
9 12/15 0.051 24/30 0.005* 9/15 0.006*
10 13/15 0.038* 26/30 0.003* 10/15 0.004*
11 14/15 0.028* 28/30 0.002* 11/15 0.002*
12 15/15 0.021* 30/30 0.001* 12/15 0.002*
groups of mice pretreated with either vehicle (saline), 50 mg/kg
cyclophosphamide (causing minimal immunosuppression), or 200 mg/kg
cyclophosphamide (causing severe immunosuppression) were administered
various numbers of PYB6 tumour cells. Even vehicle-treated mice
developed a high frequency of tumours, provided that the challenge was
sufficiently high (i.e. 8 × 104 tumour cells). In contrast, severely
immunosuppressed mice (high dose of cyclophosphamide) developed an
increased tumour frequency at all challenge levels of PYB6 tumour
cells. The groups treated with the low dose of cyclophosphamide were
of particular interest, since evidence of increased susceptibility
appeared but only as a function of the tumour cell concentration.
Assuming that these observations are applicable to human populations,
even small changes in immune function could increase the likelihood of
disease.
As indicated earlier, while experimental data have been used
occasionally in risk assessment, most immunotoxicological data have
focused on hazard identification. Although comparative data on the
effects of specific immunotoxic agents in humans and animals are
limited, other factors contribute to the minimal use of these data in
risk assessment, including the concern that immunotoxicity testing has
often been conducted without full knowledge of its predictive value in
humans or its quantitative relationship to immune-mediated diseases.
Luster et al. (1988) reported on the design and content of a
screening battery involving a tier approach for detecting potential
immunosuppressive compounds in mice. This battery has been used to
examine a variety of compounds, and the database, generated on over 50
compounds, has been analysed in an attempt to improve the accuracy and
efficiency of tests for screening chemicals for immunosuppression and
to identify better those tests that predict experimentally induced
immune-mediated diseases (Luster et al., 1992, 1993). Specifically,
attempts have been made to develop a 'streamlined' test configuration
for accurately predicting immunotoxic agents and to establish models
that could be used to provide insight into the qualitative and
quantitative relationships between the immune and host resistance
assays commonly used to examine potential immunotoxic chemicals in
experimental animals. While the analyses had a number of limitations,
several conclusions can be drawn from the results:
(1) With this particular testing configuration, examination of
only two or three immune parameters was needed in order to identify
potential immunotoxicants. Lymphocyte enumeration and quantification
of the T cell-dependent antibody response appeared to be particularly
useful. Furthermore, some commonly employed measures (e.g. leukocyte
counts, lymphoid organ weights), while probably good predictors of
immunotoxicity, are apparently not as sensitive as the other tests.
Obviously, inclusion of additional tests that are not part of the
original battery may improve the prediction of immunotoxicity.
(2) A good correlation was found between changes in immune tests
and altered host resistance, in that there was no instance in which
host resistance was altered without a significant change in the immune
test(s). In many instances, however, immune changes were observed in
the absence of detectable changes in host resistance (Table 16),
indicating that immune tests are generally more sensitive than host
resistance assays.
(3) No single immune test could be considered highly predictive
for altered host resistance; however, many of the tests were good
indicators, while others, such as leukocyte counts and proliferative
response to lipopolysaccharide, were relatively poor indicators of a
change in host resistance. Some of the tests that showed the highest
association with host resistance were those described previously as
the best indicators of immunotoxicity, such as the plaque-forming
assay and surface markers, but also included tests such as delayed-
type hypersensitivity and thymic weight.
(4) Regression modelling, using a large data set on one chemical
agent, indicated that most, but not all, of the immune function-host
resistance relationships follow a linear model. It was not possible,
however, to establish linear or threshold models for most of the
chemicals studied when the data from all 50 chemicals were combined;
thus, a more mechanistically based mathematical model will have to be
developed. A similar conclusion was drawn on the basis of a limited
data set collected by the Environmental Protection Agency (Selgrade et
al., 1992), in which changes in NK cell activity were correlated with
changes in susceptibility to cytomegalovirus in a murine model. It is
impossible, at present, to determine how applicable these analyses
will be for immunotoxic compounds with different immune profiles;
however, as more analyses become available, the ability to estimate
potential clinical effects accurately from the results of
immunological tests should increase.
Table 16. Association between the results of host resistance models and immune tests
Challenge No. of Frequencya
agent tests
Specificity Sensitivity Concordance
(-/-) (+/+) (Total)
Listeria monocytogenes 34 100 52 65**
PYB6 tumour 24 100 39 54
Streptococcus pneumoniae 19 100 38 58
B16F10 melanoma 19 100 40 68
Plasmodium yoelii 11 100 38 55
Influenza 9 100 17 44
Any of the aboveb 46 100 68 78*
From Luster et al. (1993)
* Agreement statistically significant at P < 0.05
a Frequency is defined as: specificity, the percentage of non-immunotoxic
chemicals with no effect on the host resistance models; sensitivity,
the percentage of potentially immunotoxic chemicals causing a change
in a host resistance model; concordance, percentage of qualitative agreement
b Frequency calculated on the basis of all of the host resistance models used to
study an agent
7. SOME TERMS USED IN IMMUNOTOXICOLOGY
Accessory cell. Passenger cell (leukocyte, mainly monocytes) or
stationary cell (reticulum cell, epithelial cell, endothelial cell)
that aids T or B lymphocytes in inducing immunological reactions,
either by direct contact or by releasing factors; normally expresses
MHC class II molecules
Acquired immunity. A state of protection against pathogen-induced
injury, with rapid immune elimination of pathogenic invaders; due to
previous immunization or vaccination
Activation. The process of going from a resting or inactive state to
a functionally active state, of leukocytes (lymphocytes, monocytes) or
proteins (complement, coagulation)
Acute-phase protein. Non-antibody humoral factor that emerges in
increasing amounts in the circulation shortly after induction of an
inflammatory response; e.g. alpha 2-macroglobulin, C-reactive protein,
fibrinogen, alpha 1-antitrypsin, and complement components
Adaptive immunity. A state of specific acquired protection against
pathogenic invaders, induced by immunization or vaccination
Addressin. Receptor for lymphocytes on endothelial cells of venules,
involved in homing of cells in lymphoid tissue; belongs to the
immunoglobulin gene superfamily, integrins, and selectins
Adenoid. See Tonsil
Adhesion receptors Molecule involved in cellular adhesion between
passenger cells and the extracellular stationary matrix (endothelium,
connective tissue); comprises three main families; member of the
immunoglobulin gene superfamily, integrins, and selectins
Adjuvant. Material that enhances an immune response
Adoptive immunity or tolerance. Transfer of a state of immunity or
tolerance via cells or serum from an immune or tolerant individual to
a naive individual
Affinity. Binding strength of an antibody-combining site to an
antigenic determinant (epitope); expressed as an association constant
(Ka)
Agglutination. Process of aggregation of visible antigenic particles
(e.g. erythrocytes) mediated by antibodies directed towards the
particles
Allele. One or more genes at the same chromosomal locus which
control alternative forms (phenotypes) of a particular inherited
characteristic
Allergen. Antigen that induces an allergic or hypersensitivity
reaction, resulting in immune-mediated or nonimmune-mediated tissue
damage; restricted mainly to immediate hypersensitivity or
anaphylactic reactions
Allergy. State of altered immunity, resulting in hypersensitivity
reaction on contact with antigen or allergen; often restricted to
immediate hypersensitivity or anaphylaxis
Alloantigen. Antigen that differs between different (not inbred)
individuals within one species
Allogeneic. Genetically different phenotypes in different (not
inbred) individuals within one species; opposite of isogeneic, or
xenogeneic
Allotype. Genetically different antigenic determinants on protein of
(not inbred) individuals within one species
alpha Chain. First chain of a multimeric receptor molecule: in
immunoglobulin molecules, the a heavy chain forming the IgA class; in
T-cell receptor molecules, one of the chains forming the dimeric
alpha-ß receptor molecule; in MHC class I molecules, the main
polypeptide chain associated with the ß2-microglobulin molecule; in
MHC class II molecules, one of the chains in the dimeric molecule
Alternative pathway of complement activation. Activation of
complement pathway by substances other than antigen-antibody
complexes; involves factor B, properdin, and complement component C3
Anaphylatoxin. Activated components of complement components C3 and
C5 (C3a and C5a, respectively), which induce anaphylactic reactions by
activating mast cells and basophilic granulocytes
Anaphylaxis or anaphylactic reaction. Local or systemic immediate
hypersensitivity reaction initiated by mediators released after
immunological stimulation; symptoms can be a drop in blood pressure
related to vascular permeability and vascular dilatation, and
obstruction of airways related to smooth muscle contraction or
bronchoconstriction
Anergy. State of unresponsiveness to antigenic stimulation, due to
the absence of responding elements or the loss of capacity of existing
elements to mount a reaction; synonym for tolerance
Antibody. Immunoglobulin molecule produced in response to immunization
or sensitization, which specifically reacts with antigen Antibody-dependent
cell-mediated cytotoxicity. Cytotoxic reaction in which an antibody forms
the bridge between the cytotoxic cell (lymphocyte, macrophage) and the
target cell
Antigen. Any substance that induces a specific immunological
response
Antigen-binding site (paratope). Part on an antibody molecule that
binds antigen (antigenic determinant, or epitope); part of the T-cell
receptor that binds the complex of antigen and MHC molecule
Antigenic determinant (epitope). Part of an antigen that binds to
antibody or T-cell receptor (the latter in combination with MHC
molecule)
Antigenicity. Capacity to react with components of the specific
immune system (antibody, receptors on lymphocytes)
Antigen presentation. Process of enabling lymphocytes to recognize
antigen on a specific receptor on the cell surface. For presentation
to T lymphocytes, includes intracellular processing and complexing of
processed peptides with MHC molecule on the cell membrane of the
antigen-presenting cell. For presentation to B lymphocytes, can
include formation of immune complexes (in germinal centres)
Antigen-presenting cell. Cell that presents antigen to lymphocytes,
making possible specific recognition by receptors on the cell surface.
In a more restricted way, used to describe MHC class II-positive
(accessory) cells which can present (processed) antigenic peptides
complexed with MHC class II molecules to T helper-inducer lymphocytes.
Includes macrophage populations (in particular, Langerhans cells and
dendritic or interdigitating cells), B lymphocytes, activated T
lymphocytes, certain epithelia (after MHC class II antigen induction
by e.g. interferon gamma); others are follicular dendritic cells, not
of bone-marrow origin, which present antigen in the form of immune
complexes to B cells in germinal centres of peripheral lymphoid
tissue; marginal zone macrophages in splenic marginal zone, which
present antigen, without contact with T helper cells, to B cells at
this location (T cell-independent response, e.g. to bacterial
polysaccharide). In rodent skin epidermis, a dendritic epidermal cell,
of T-cell origin, has an antigen-presenting function.
Antigen receptor. Multichain molecule on lymphocytes, to which
antigens bind. For B lymphocytes, an immunoglobulin molecule that
recognizes nominal antigen; for T lymphocytes, a T-cell receptor
molecule that recognizes antigenic peptide in combination with the
polymorphic determinant of an MHC molecule
Antinuclear antibody. Antibody directed to nuclear antigen; can have
various specificities (e.g. to single- or double-stranded DNA or
histone proteins); frequently observed in patients with rheumatoid
arthritis, scleroderma, Sjögren's syndrome, systemic lupus
erythematosus, and mixed connective tissue disease. Also called
antinuclear factor
Antiserum. Serum from an individual that contains antibodies to a
given antigen
Aplasia. Absence of tissue structure or cellular component, either
congenital or acquired
Apoptosis (programmed cell death). Process whereby the cell kills
itself after activation, by Ca2+-dependent endonuclease-induced
chromosomal fragmentation into fragments of about 200 base-pairs
Appendix. Lymphoid organ in the gastrointestinal tract, at the
junction of ileum and caecum; forms part of gut-associated lymphoid
tissue
Arthus reaction. Inflammatory response, generally in skin, induced
by immune complexes formed after injection of antigen into an
individual that contains antibodies
Asthma. Chronic inflammatory disease characterized by bronchial
hyperresponsiveness to various stimuli
Atopy. In general terms, 'unwanted reactivity'; used mostly to
describe the state of general systemic or local hypersensitivity
reactions related to genetic predisposition
Auto-antibody. Antibody to component in the individual itself
Auto-antigen. Antigen to which an autoimmune reaction is directed
Autoimmunity. A state of immune reactivity towards self
Autologous. Derived from self; components of an immunological
reaction (e.g. antibody, lymphocytes, grafted tissue) from the same
individual; opposite of heterologous
Avidity. Binding strength between antibody and antigen, or receptor
and ligand; for antibody, represents the product of more than one
interaction between antigen-binding site and antigenic determinant
Bacteraemia. Presence of bacteria in blood
Basophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with acid glycoproteins stained by basic (blue) dyes; after
release, glycoproteins induce anaphylactic reactions
B-Cell growth and differentiation factor, B-cell growth factor, and
B-cell stimulating factor. See Interleukin-4 and -5
ß Chain. In T-cell receptor molecules, one of the chains forming the
dimeric alpha-ß receptor molecule; in MHC class II molecules, one of
the chains in the dimeric molecule
ß2-Microglobulin. A peptide of 12 kDa, which forms part of MHC class
I molecule
B Lymphocyte or cell. Lymphocytes that recognize nominal antigen by
immunoglobulin (antibody) surface receptor (on virgin B cell, IgM and
IgD) and, after activation, proliferate and differentiate into
antibody-producing plasma cells. During a T-dependent process, there
is immunoglobulin class switch (IgM into IgG, IgA, IgD, or IgE), with
maintenance of the antigen-combining structure. For T-independent
antigens, cells differentiate only in IgM-producing plasma cells. B
Lymphocytes originate from precursor cells in bone marrow; in avian
species, they undergo maturation in the bursa of Fabricius (B, bursa-
dependent); in mammals, in the bone marrow
B Lymphocyte area. That part of an lymphoid organ or tissue that is
occupied by B lymphocytes, e.g. follicles in peripheral lymphoid
tissue, marginal zone in spleen
Birbeck granule. Rod-shaped structure with rounded end,
approximately 6 nm thick, found in the cytoplasm of Langerhans cells
in the epidermis, and interdigitating dendritic cells in T-lymphocyte
area of lymphoid tissues
Blast cell. Large cell (about 15 µm or more) with dispersed nuclear
chromatin and cytoplasm rich in ribosomes; in an active stage of the
cell cycle before mitosis
Blast transformation. Process of activation of lymphocytes into cell
cycle and to form blastoid cells before mitosis
Blocking antibody. Antibody that can interfere with another antibody
or with reactive cells in binding antigen, thereby preventing effector
reactions (often used in association with an allergic reaction or
tissue damage)
Bone marrow. Soft tissue in hollow bones, containing haematopoietic
stem cells and precursor cells of all blood cell subpopulations
(primary lymphoid organ); major site of plasma cell and antibody
production (secondary lymphoid organ)
Booster. Dose of antigen given after immunization or sensitization
to evoke a secondary response
Bradykinin. Peptide of nine amino acids split from alpha 2-
macroglobulin by the enzyme kallikrein; causes contraction of smooth
muscle
Bronchus-associated lymphoid tissue. Lymphoid tissue located along
the bronchi, considered to represent the location of presentation of
antigens entering the airways; contributes to mucosa-associated
lymphoid tissue
Bursa equivalent. Site where B-cell precursors undergo maturation
into immunocompetent cells in non-avian species; bone marrow in adult
mammals
Bursa of Fabricius. Primary lymphoid organ in avian species, located
in cloaca, with an epithelial reticulum, where precursors of B
lymphocytes from the bone marrow undergo maturation into
immunocompetent cells and then move to peripheral lymphoid organs
C (constant) gene. Gene that encodes the constant part of
immunoglobulin chains or T-cell receptor chains (e.g. Cµ, Cdelta
for immunoglobulin heavy chains, Ckappa for immunoglobulin kappa
light chain, Calpha for T-cell receptor alpha chain)
C (constant) region. Region at carboxy terminal of immunoglobulin
chains or T-cell receptor chains, identical for a given immunoglobulin
class or subclass or for a given T-cell receptor chain; encoded by C
genes in DNA
Cachectin. See Tumour necrosis factor
CD (cluster of differentiation). Group of (monoclonal mouse)
antibodies that react to identical leukocyte surface molecules in
humans (but not necessarily to identical epitopes), on the basis of
comparative evaluations during international workshops and transferred
to other species by analogy. Does not include MHC or immunoglobulin
molecules
CD3 molecule. Molecule consisting of at least four invariant
polypeptide chains, present on the surface of T lymphocytes associated
with the T-cell receptor; mediates transmembrane signalling (tyrosine
phosphorylation) after antigen binding to T-cell receptor
CD4 molecule. Glycoprotein of 55 kDa on the surface of T lymphocytes
and part of monocytes and macrophages. On mature T cells, restricted
to T helper (inducer) cells; has an accessory function to antigen
binding by T-cell receptors, by binding to a non-polymorphic
determinant of MHC class II molecule
CD8 molecule. Complex of dimers or higher multimers of 32-34 kDa
glycosated polypeptides linked by disulfide bridges, on the surface of
T lymphocytes. On mature T cells, the presence is restricted to
T cytotoxic-suppressor cells; has an accessory function to antigen
binding by the T-cell receptor, by binding to a non-polymorphic
determinant of MHC class I molecule
Cell-mediated immunity. Immunological reactivity mediated by
T lymphocytes
Central (primary) lymphoid organ. Lymphoid organ in which precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment, to form immunocompetent cells; not antigen-driven
but can be influenced by mediators produced as a result of antigen
stimulation
Centroblast. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; a medium to large,
12-18-µm cell, with a round to ovoid nucleus that has moderately
condensed heterochromatin and medium-sized nucleoli close to the
nuclear membrane, medium-sized to broad cytoplasm containing many
polyribosomes and a variable amount of rough endoplasmic reticulum
Centrocyte. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; medium-sized,
8-12-µm lymphoid cell with irregular nucleus with condensed
heterochromatin; small cytoplasm containing a few organelles
CH50. The amount of serum (or dilution of serum) that is required to
lyse 50% of erythrocytes in a standard haemolytic complement assay
Chemiluminescence. Luminescence produced by direct transformation of
chemical energy into light energy
Chemotactic factor. Substance that attracts cells to inflammatory
lesions
Chemotaxis. Process of attracting cells to a given location, where
they contribute to an inflammatory lesion
Class I MHC molecule. Molecule coded by the A, B, or C locus in the
HLA complex, the K and D locus in the mouse H2 complex, and less well
defined MHC I gene loci in other species, in association with the
ß2-microglobulin molecule. Two-chain molecule occurring on all
nucleated cells, without allelic exclusion
Class II MHC molecule. Molecule coded by the D (DR, DP, DQ) locus in
the HLA complex, the I-A and I-E locus in the mouse H2 complex, and
less well defined MHC II gene loci in other species, comprising an
alpha and a ß chain (intracellular, associated with an 'invariant'
chain). Two-chain molecule occurring, without allelic exclusion, on B
lymphocytes, activated T lymphocytes, monocytes-macrophages,
interdigitating dendritic cells, some epithelial and endothelial cells
(variable, dependent on species and state of activation); antigen-
presenting cells
Class switch. See Immunoglobulin class switch
Classical pathway of complement activation. Activation of complement
pathway by antigen-antibody complexes, starting with complement
component C1 and ending with complement component C3
Clone. Population of cells that emerge from a single precursor cell;
within T or B lymphocytes, cells with a fixed rearrangement of genes
coding for T-cell receptor or immunoglobulin
Clonal expansion. Proliferation of cells that have a genetically
identical constitution; when uncontrolled, may result in tumour
formation
Colony-stimulating factor. Substance that supports clonal cell
growth of haematopoietic cells
Complement. Group of about 20 proteinase precursors that activate
and split each other, in sequential order. The various components are
present in inactive (precursor) form, except for C3, which in a normal
state shows a low turnover (major split products C3a and C3b). The
split products are either bound to the activating substance (immune
complex or antibody-coated cell) or are released as active mediators.
The classical cascade starts by activation of component C1,
subsequently C4, C2, and C3, and is initiated by (IgG/IgM) immune
complexes. The alternative cascade starts by activation of C3b and
factor B, subsequently factors D and C3, and is initiated by nonimmune
specific activators like microbial polysaccharides and some
'allergens'. C3 split products (C3b) activate the amplification loop,
in which factors D and B are also used, activate C5, and thereafter
the terminal cascade C6, C7, C8, and C9, which attack the cell
membrane and kill the cell (microorganism). The cascade is under the
control of various inhibitors. Major effects of complement split
products are adherence to receptors on phagocytes (C3b, C3d); mediator
activity, like chemoattraction of inflammatory cells, vasodilatation,
increased vascular permeability, and smooth muscle contraction
(C3a, C5a); cell lysis by membrane lesions (C6-C9)
Complement fixation. Binding and consumption of complement by
antigen-antibody complexes; often used in association with assays for
complement activity
Complement receptor (CR). Cell surface molecule that can bind
activated complement components in e.g. antigen-antibody complexes;
CR1 (CD35) is a receptor for C3b, present on B lymphocytes, monocytes
and macrophages, granulocytes, and erythrocytes; CR2 (CD21) is a
receptor for C3d, present on mature B lymphocytes; CR3 (CD11b/CD18) is
a receptor for C3b present on macrophages, granulocytes, natural
killer cells, and a subset of CD5+ B lymphocytes; CR4 (CD11c/CD18)
is a receptor for C3b present on monocytes, macrophages, granulocytes,
and natural killer cells
Contact sensitivity. Hypersensitivity reaction evoked in skin by
placing sensitizing agents or substances on skin
Cords of Billroth. Medullary cords in spleen
Corona. See Mantle
Cortex. Outer parenchymal layer of organs
Cross-reactivity. Reactivity of antigen-specific elements (T lymphocytes
sensitized by T-cell receptor, B lymphocytes by antibody; antibody
molecules) towards antigens other than those used in original
sensitization, owing to shared antigenic epitopes on different
antigenic molecules; also used to describe reactions towards antigenic
determinants other than those originally used in sensitization, due to
similarities in structure
Cytokine. Biologically active peptide, synthesized mainly by
lymphocytes (lymphokines) or monocytes and macrophages (monokines);
modulates the function of cells in immunological reactions; include
interleukins. Some (pleotrophic cytokines) have a broad spectrum of
biological action, including neuromodulation, growth factor activity,
and proinflammatory activity
Cytokine receptor. Ligand for cytokine on target cell, acts in
signal transduction through the cell membrane; many are multichain
molecules belonging to different receptor families
Cytolytic antibody. Antibody that can mediate lysis of the target to
which it is directed, either in combination with complement or as
bridge between cytotoxic cell and target
Cytotoxic cell (killer cell). Effector T cell, natural killer cell,
or activated macrophage; kills target cells and tissue extracellularly
after binding; mediated by release of substances from cytolytic
granules (including serine esterase, cytolysin, and perforins)
Cytotoxic reaction. Effector reaction of antibody or cells, resulting
in lysis of target cell or tissue
Cytotoxic T lymphocyte. Subpopulation of T lymphocytes with CD8
phenotype; after recognition of antigen in an MHC class I-restricted
manner, differentiates from precursor to effector cytotoxic cell and
subsequently kills target cells
Degranulation. Process of fusion of cytoplasmic granules with cell
membrane, whereby the content of the granules is released into the
extracellular space; mainly used in association with immediate
hypersensitivity reactions
Delayed-type hypersensitivity. Inflammatory lesion mediated by
effector T lymphocytes or their products, with attraction mainly of
macrophages towards the inflammatory lesion. Term originates from the
classical skin reaction after challenge of a sensitized individual;
maximal (wheal and flare) response reached within 24-72 h
Delayed-type hypersensitivity T cell. Subpopulation of T lymphocytes
with CD4 phenotype; after recognition of antigen in an MHC class
II-restricted manner, secretes mediators involved in inflammatory
responses, e.g. INF gamma and tumour necrosis factor
delta Chain. In immunoglobulin molecules, delta heavy chain forming
the IgD immunoglobulin class; in T-cell receptor molecules, one of the
chains forming the dimeric gamma-delta receptor molecule; one of the
chains in the CD3 molecule associated with the T-cell receptor
Dendritic cell. Cell in tissue that shows elongations or protrusions
of cytoplasm into the parenchyma. Often used in a restricted manner to
designate a type of antigen-presenting cell, of which two categories
exist: one of bone-marrow origin belonging to the macrophage lineage,
including Langerhans cells in skin and interdigitating dendritic cells
in T-cell areas of lymphoid tissue and a very small leukocyte
population in blood; the second of tissue parenchymal origin
(presumably pericytes around blood vessels), the follicular dendritic
cells in B-cell areas (follicles) of lymphoid tissue
Dendritic epidermal cell. T Cell in the epidermis that has dendritic
morphology; has antigen-presenting function but is not a (macrophage-
related) Langerhans cell. Occurs in rodents but not in humans;
contributes to skin immune system
Dermal immune system. See Skin immune system
Desensitization. Induction of anergy or tolerance to allergic
substances by active intervention in immune reactivity (exhaustion of
reactive elements or induction of blocking phenomena)
Diapedesis. Passage of cells through blood vessel walls into tissue
parenchyma, mediated by constriction of endothelial cells
D (diversity) gene. Gene that encodes the variable part of
immunoglobulin heavy chains, or T-cell receptor alpha, ß, or gamma
chain (e.g. DH1-DHn for immunoglobulin heavy chains, Ddelta
1-Vdelta n for T-cell receptor alpha or delta chain)
Domain. Part of polypeptide chain folded to a relatively rigid
globular tertiary structure fixed by disulfide bonds. Molecules of the
immunoglobulin gene superfamily have a tertiary domain-like structure:
each domain is about 110 amino acids long and arranged in a sandwich
of two sheets of anti-parallel ß strands. See also Homologous,
Homology
Ectoderm. Outermost of the three cellular layers of the embryo;
produces the epidermis and neuronal tissue
Eczema. Superficial inflammation in skin, involving primarily the
epidermis; characterized by redness, itching, minute papules and
vesicles, weeping, oozing, and crusting. Histological changes include
microvesiculation and oedema of the epidermis and an infiltrate of
lymphocytes and macrophages in the dermis
Effector cell. General term to describe a cell that mediates a
function after a stage of activation, differentiation, and
proliferation
Endocytosis. Process of uptake of material by a cell; special forms
are phagocytosis and pinocytosis
Endoderm. Innermost of the three cellular layers of the embryo;
produces the gastrointestinal lining and some internal organs, such as
liver and pancreas
Endoplasmic reticulum. Membrane-like structure in cell cytoplasm;
site of protein synthesis
Endothelium. Cells that line blood vessels; exert a major function
in traffic of leukocytes from blood into tissue, by altered expression
of adhesion molecules (modulation of numbers of receptors; maturation
and activation resulting in altered glycosylation, expression of new
ligands or altered ligand binding affinity; change in cytoskeleton
organization). A special endothelial cell type occurs in T-lymphocyte
areas of lymphoid tissue in the high endothelial (postcapillary)
venule.
Endotoxin. Lipopolysaccharide from the cell wall of Gram-negative
bacteria; has toxic, pyrogenic, and immunoactivating effects
Enzyme-linked immunosorbent assay. Immunoenzymetric assay based on
the use of antigens or antibodies labelled with a specific enzyme;
combines the virtues of solid-phase technology and enzyme-labelled
immunoreagents. The antigen-antibody complex is determined by an
enzyme assay involving the incubation of the complex with an
appropriate substrate of the enzyme.
Eosinophil chemotactic factor. Acidic tetrapeptide of 0.5 kDa
produced by mast cells (preformed mediator); attracts eosinophils to
the site of inflammation
Eosinophilia. State of increased proportions of eosinophilic
granulocytes in blood
Eosinophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with basic proteins stained by acidophilic (red) dyes; after
release, the proteins modulate inflammatory reactions
Epithelium. Cells covering the surface of the body and forming the
first line of defence against pathogenic invaders. Reticular
epithelium forming the stroma of tissue occurs in thymus (in avian
species in the bursa of Fabricius); these cells have a major function
in processing precursor cells to immunocompetent lymphocytes
Epithelioid cell. Cell of macrophage origin in chronic inflammatory
lesions, which resembles an epithelial cell morphologically
Epitope (antigenic determinant). Part of antigen that binds to
antibody or T-cell receptors (the latter in combination with MHC
molecule)
epsilon Chain (see also alpha Chain). In immunoglobulin molecules,
the epsilon heavy chain forming the IgE immunoglobulin class; one of
the chains in the CD3 molecule associated with the T-cell receptor
Erythema. Redness of skin produced by congestion of blood
capillaries due to dermal arterial vasodilatation
Erythrocyte. Red blood cell; a bone marrow-derived blood cell
component involved in oxygen transport to tissue; contains a nucleus
in distinct avian species like chickens but does not have a nucleus in
mammals
Exudation. Inflammation in tissue; contains blood cells and fluid
comprising serum proteins of high relative molecular mass
Ex vivo/in vitro. An assay method in which the effects of a
xenobiotic are evaluated in vitro in cells isolated from an animal
or human exposed to the compound of interest
Fab fragment. Part of an antibody molecule in which monovalent
binding of an antigenic determinant occurs; formed by the three-
dimensional structure of variable parts (domain) of one heavy and one
light chain and the adjacent part of the constant part (constant
domain); ab, antigen binding
F(ab')2 fragment. Part of an antibody molecule in which divalent
binding of antigenic determinants occurs; formed by the
three-dimensional structure of both Fab fragments
Fc fragment. Part of an antibody molecule formed by the three-
dimensional structure of the constant part (constant domains) of the
heavy chains (except that adjacent to the variable domain), involved
in antibody effector functions; c, crystallizable
Fc receptor. Structure on leukocytes (lymphocytes, monocytes,
macrophages, granulocytes) that mediates binding of immunoglobulin or
antibody, alone or after forming aggregates in antigen-antibody
complexes. Receptors for IgE (Fc epsilon) occur on mast cells and
basophilic granulocytes and are involved in immediate hypersensitivity
reactions; receptors for IgG are of three classes: low-affinity FcR
III, CD16, on natural killer cells, monocytes, macrophages, and
granulocytes; low-affinity FcR II, CD32, on B cells, myeloid cells,
Langerhans cells, and interdigitating dendritic cells; high-affinity
FcR III, CD64, on monocytes and macrophages
Follicle. Round to oval structure in lymphoid tissue, where B cells
are lodged. Primary follicles contain only small resting B cells;
secondary follicles comprise a pale-stained germinal centre, with
centrocytes and centroblast, and contain B lymphocytes in a state of
activation or proliferation and macrophages, the stroma consisting of
follicular dendritic cells. The germinal centre is surrounded by a
mantle with small B lymphocytes
Follicular dendritic cell. Cell forming the stationary micro-
environment of germinal centres of follicles in lymphoid tissue;
elongated, often binucleated cell with long branches extending between
germinal centre cells and forming a labyrinth-like structure; linked
by desmosomes. Of local parenchymal origin, presumably from pericytes
surrounding blood vessels. Its main function is presentation of
antigen, trapped as immune complex in the labyrinth, to B lymphocytes.
gamma Chain (see also alpha Chain). In immunoglobulin molecules,
the gamma heavy chain forming the IgG immunoglobulin class; in T-cell
receptor molecules, one of the chains forming the dimeric gamma-delta
receptor molecule; one of the chains in the CD3 molecule associated
with the T-cell receptor
gamma-delta T cell. T Lymphocyte with an antigen receptor composed
of a gamma and a delta chain associated with CD3 transmembrane
molecule; develops in part inside the thymus (including intrathymic
selection), in part outside the thymus. Has a major role as a
cytotoxic cell in the first phase of the (innate) immune response,
e.g. in rodents in the mucosal epithelium
Gammaglobulin. Part of serum proteins that move towards the negative
electrode (gamma fraction) upon electrophoresis; contains
immunoglobulins
Gene rearrangement. For immunoglobulin and T-cell receptor, the
process whereby the germline chromosomal genomic structure of variable
(diversity), joining, and constant segments recombine to form a
specific V-(D-)J-C combination, enabling transcription into mRNA and
translation into protein. The V-(D-)J combination of different chains
determines the specificity of the receptor (immunoglobulin or T-cell
receptor).
Germinal centre. The pale-staining centre in follicles of lymphoid
tissue, where B lymphocytes are activated by antigen in a
T lymphocyte-dependent manner and subsequently go into proliferation
and differentiation, acquiring the morphology of centroblasts,
centrocytes, and plasma cells. Has a special microenvironment made up
of follicular dendritic cells and large macrophages (tingible body or
starry-sky macrophages)
Glomerulonephritis. Inflammation of glomeruli in kidney, often
associated with deposition of immune complexes along the glomerular
basement membrane or in the mesangium, and influx of polymorpho-
nuclear granulocytes
Golgi apparatus. Tubular structures in cytoplasm, involved in
secretion of synthesized proteins
Granulocyte colony-stimulating factor. Synthesized by T lymphocytes
and macrophages, epithelial cells, fibroblasts, and endothelial cells;
supports growth of granulocyte progenitors, in synergism with IL-3 and
granulocyte-macrophage colony-stimulating factor of monocyte-
macrophage progenitors
Granulocyte-macrophage colony-stimulating factor. Produced by T
lymphocytes, endothelial cells, macrophages, and lung cells; supports
growth and differentiation of macrophages and granulocyte progenitors;
activates macrophages and polymorphonuclear macrophages to become
tumoricidal and produce superoxide anion
Granuloma. Chronic inflammatory reaction in tissue comprising macro-
phages (epitheloid cells), lymphocytes, and fibroblasts; formed in a
cell-mediated response towards poorly degradable material, in
immunological reactions as part of a delayed-type hypersensitivity
reaction
Gut-associated lymphoid tissue. Lymphoid organs and tissue located
along the gastrointestinal tract, presumed to be a first location of
presentation of antigens entering through the digestive tract;
comprises Peyer's patches, appendix, in part mesenteric lymph nodes,
adenoids, and tonsils; contributes to the mucosa-associated lymphoid
tissue
H-2. Major histocompatibility complex of mouse
Haemagglutinin. Antibody or substance that induces agglutination of
erythrocytes
Haematopoiesis. Production of cells of blood; subdivided into
erythropoiesis, lymphopoiesis, and myelopoiesis
Haematopoietic malignancy. Malignancy of blood-forming cells
Haemolysis. Process of lysis of erythrocytes, with release of
haemoglobin
Haemolytic agent (haemolysin). Antibody or substance that induces
lysis of erythrocytes
Haemopoietin. Growth factor that induces production of distinct
types of blood cells; also enhances the function of the mature cells
Haplotype. Phenotype of inherited characteristic on closely linked
genes on one chromosome
Hapten. Structure around one antigenic determinant, which itself
does not evoke an immune response unless coupled to a carrier
substance but can react with the products (antibodies, cells) of an
immune response
Hassall's corpuscle. Epithelial aggregate in onion-like structure,
often with debris of other cells; in the medulla of the thymus,
surrounded by large epithelial cells secreting thymic hormones; does
not occur in rodent thymus
Heat-shock protein. Family of proteins (60-90 kDa) with conserved
sequence in evolution; play a prime role in regulation and transport
of intracellular proteins. Expression is upregulated when cells are
under 'stress' (originally induced by heating), such as inflammatory
conditions, and may act as autoantigen in triggering and perpetuating
an auto-immune response
H (heavy) chain. One of the 45-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and three
constant domains (four constant domains in the 55-kDa µ chain). The
combination of the constant part of two heavy chains (alpha, ß, delta,
gamma, or µ) forms the immunoglobulin class-associated part of the
molecule (IgA, IgD, IgE, IgG, or IgM class)
Helper (inducer) T cell. Cell in a subpopulation of T lymphocytes,
with CD4 phenotype; after recognizing antigen in an MHC class
II-restricted manner, induces immunological reactions, secretes
interleukins, and cooperates (supports) B lymphocytes, cytotoxic
T-cell precursors, and macrophages
Helper T cell subpopulations. Th1 and Th2: Th1 cells produce
interleukin-2 and interleukin-3, interferon gamma, tumour necrosis
factor alpha and ß, and granulocyte-macrophage colony stimulating
factor, and function in induction of delayed-type hypersensitivity,
macrophage activation, and IgG2a synthesis. Th2 cells produce
interleukin-3, interleukin-4, and interleukin-5, tumour necrosis
factor alpha and granulocyte-macrophage colony stimulating factor, and
function in induction of IgG1, IgA, and IgE synthesis and induction of
eosinophilic granulocytes
Heterologous. Derived from foreign source or species; components of
an immunological reaction (e.g. antibody, lymphocytes, grafted tissue)
derived from another individual of the same species or another
species; opposite of autologous
Heterophilic antigen. Antigen in unrelated species; can be directed
towards xenogeneic immune reactivity; often has carbohydrate
structure; opposite of homocytotropic antibody
High endothelial (postcapillary) venule. Specialized blood vessels
in T-lymphocyte area of lymphoid tissue, through which circulating
lymphocytes pass into the parenchyma
Hinge region. Stretch in immunoglobulin molecule between Fab and Fc
fragments (first constant domain and other constant domains of the
heavy chain), where the quaternary structure of the molecule is not
rigid but flexible; bending of the hinge region after antigen binding
serves as a signal transduction, resulting in effector reactions
Histamine. ß-Imidazolylethylamine; component of granules in mast
cells and basophilic granulocytes that is released upon activation and
induces immediate hypersensitivity reaction, e.g. vasodilatation,
vascular permeability, smooth muscle contraction, and broncho-
constriction
Histiocyte (histiocytic reticulum cell). Monocyte in tissue. See
Macrophage
Histiocytosis. Increase in proportion of macrophages in tissue
HLA, human leukocyte antigen. Major histocompatibility complex of
humans
Homocytotropic antibody. Antibody that binds preferentially to cells
from the same species rather than to cells from other species;
opposite of heterologous antibody
Homologous, Homology. Similarity in primary structure between
substances; homology region is a synonym for domain
Host defence. Ability of an individual or species to protect itself
against opportunistic agents and to eliminate certain tumours and
exogenous agents such as (micro)organisms, viruses, and particles that
can cause disease
Hot spot. See Hypervariable region
Humoral immunity. Immunological reactivity mediated by antibody
Hybridoma. Transformed cell line or cell clone formed by fusion of
two different parental cell lines or clones
Hyperplasia. Reversible increase in cell number, usually as the
result of a physiological stimulus or persistent cell injury due to
irritating compounds
Hypersensitivity. Increased reactivity or sensitivity; in
immunological reactions, often associated with tissue destruction
Hypervariable region. Amino acid sequences in the variable regions
of antibody molecules or T-cell receptor chains where variability is
highest and which together form the antigen-binding site. Synonym for
hot spot
Hypoplasia. Reversible decrease in cell number, usually as a result
of a physiological stimulus
Ia antigen. MHC class II cell surface molecule
Idiotype. Antigenic determinant of variable domain of immunoglobulin
molecules or T-cell receptor
Immediate hypersensitivity. Inflammatory response that occurs within
minutes after exposure to allergen; caused by physical or
immunological stimulus, with vascular dilatation, increased vascular
permeability, and oedema as the main effects. Term originates from the
classical skin reaction after challenge of a sensitized individual in
skin, which takes 20-30 min to reach maximal (wheal and flare)
response and is mimicked by injection of mediator only (histamine)
Immune adherence. Binding of antigen-antibody complexes (antibody-
coated particles) to erythrocytes, platelets, or leukocytes; mediated
by activation of complement C3
Immune complex. Complex between antigen and antibody
Immune elimination. Rapid clearance of pathogen from the circulation
by components of the immune system; often used in association with
antibody molecules (removal by immune complex formation and
phagocytosis)
Immune exclusion. Process whereby entry of pathogens at mucosal
surfaces is prevented by the action of specific (secretory IgA)
antibody
Immune interferon. Former name for interferon gamma
Immune surveillance. Function (still hypothetical) of the immune
system in preventing or eliminating cells after malignant
transformation to a neoplastic process
Immunity. State of protection against pathogen-induced injury, with
fast immune elimination of pathogenic invaders due to previous antigen
contact or a special acquired state of responsiveness
Immunization (vaccination). Active intervention resulting in
immunity; used mainly in the context of presentation of (inactivated
or attenuated, nonpathogenic) substance to induce immunological
memory. Passive immunization is the adoptive transfer of immune system
components after previous contact with the pathogen and is performed
mainly with antibodies
Immunoblast. Intermediately differentiated B lymphocyte in lymphoid
tissue; a large, 15-20 µm, round-to-spherical cell with a rounded
euchromatic nucleus. The abundant cytoplasm contains many ribosomes,
well-developed rough endoplasmic reticulum and Golgi complex
Immunocompetence. Capacity of B or T lymphocytes to specifically
recognize antigen, resulting in a specific immunological reaction
Immunodeficiency. Defects in the immune system resulting in
decreased or absent reactivity to pathogens. Primary immunodeficiency
is mainly intrinsic defects in the differentiation of T or B
lymphocytes and can be congenital or acquired. Secondary
immunodeficiency is defects of which the cause is outside the immune
system (malnutrition; stress; protein loss after burns, nephrotic
syndrome, or intestinal bleeding; viral infection; therapy with
immunosuppressive or cytostatic drugs; irradiation).
Immunogen. A substance that can induce an immunological reaction
Immunogenicity. Capacity to evoke an immune response
Immunoglobulin. Formerly the electrophoretically-defined
gammaglobulin (in blood) but is also present in the ß fraction;
synthesized by plasma cells. The basic subunit consists of two
identical heavy chains (about 500 amino acid residues, organized into
four homologous domains; for µ chain in IgM, about 600 amino acid
residues, organized into five homologous domains) and two identical
light chains (about 250 amino acid residues organized into two
homologous domains), each consisting of a variable domain and one to
three constant domains (in the µ chain, four constant domains). The
antigen-binding fragment (Fab) consists of variable domains of heavy
and light chains (two per basic subunit). Five classes of
immunoglobulins exist, which differ according to heavy chain type
(constant domains): IgG (major immunoglobulin in blood), IgM
(pentamer, consisting of five basic units), IgA (major immunoglobulin
in secretions; present mainly as a dimeric molecule), IgD (major
function, receptor on B lymphocytes), and IgE. Effector functions
after antigen binding are mediated by constant domains of the heavy
chain (Fc part of the molecule) and include complement activation
(IgG, IgM), binding to phagocytic cells (IgG), sensitization and
antibody-dependent cell-mediated cytotoxicity (IgG), adherence to
platelets (IgG), sensitization and degranulation of mast cells and
basophils (IgE). IgA lacks these effector functions and acts mainly in
immune exclusion (prevention of entry in the body) at secretory
surfaces ('antiseptic paint').
I mmunoglobulin class. Subfamily of immunoglobulins, based on
difference in heavy chain. Five classes exist: IgA, secretory
immunoglobulin, dimeric; IgD, immunoglobulin on B cells that acts as
antigen receptor; IgE, immunoglobulin fixed to mast cells and
basophilic granulocytes, involved in immediate hypersensitivity
reactions; IgG, main immunoglobulin in circulation; IgM, pentameric
immunoglobulin with optimal agglutinating capacity, produced on first
antigen contact
Immunoglobulin class switch. Process whereby synthesis of IgM
antibody changes into synthesis of antibody of another immunoglobulin
class, with maintenance of the same variable part of the
immunoglobulin molecule. At the genomic level, this includes gene
rearrangement, with an exchange of a constant gene segment to a fixed
V-D-J gene segment combination. This switch is thought to occur in
germinal centres of follicles in lymphoid tissue, during the change of
a primary into a secondary response, and is under the control of
cytokines (switch factors)
Immunoglobulin gene superfamily. Group of molecules including
immunoglobulins, T-cell receptors, MHC molecules, and others, like the
lymphocyte function-related antigens LFA-2 (CD2) and LFA-3 (CD58), the
intercellular adhesion molecules ICAM-1 (CD54) and ICAM-2, the
vascular cell adhesion molecule VCAM-1, the neural cell adhesion
molecule NCAM (CD56), and the CD4 and CD8 molecules, which have a
similar tertiary basic domain-like structure, in which each domain is
about 110 amino acids long and stabilized by a disulfide bridge. These
molecules are known to be important for specific recognition and
adhesion functions
Immunoglobulin light chain type. Defines the light chain in the
immunoglobulin unit, either kappa or lamda, each defined at the
germline DNA level by individual constant (C), joining (J), and
variable (V) gene segments
Immunoglobulin subclass. Subfamily within a distinct immunoglobulin
class, based on subtle differences in heavy chain. For instance, in
humans there are two IgA subclasses, IgA1 (alpha1 heavy chain) and
IgA2 (alpha 2 heavy chain), and four IgG subclasses, IgG1-IgG4
(gamma 1-gamma 4 heavy chain). In rodents, these are designated IgG1
(gamma 1), IgG2a (gamma 2a), IgG2b (gamma 2b), and IgG3 (gamma 3)
Immunological memory. Acquired state of the immune system after
first contact with antigen, whereby the reaction upon subsequent
contact is faster, more intense, and of higher affinity. For antibody
response, associated with an immunoglobulin class switch and 'affinity
maturation' (by somatic mutation)
Immunosuppression. Prevention or diminution of the immune response
by administration of antineoplastic or antimetabolic drugs,
antilymphocyte serum, or exposure to e.g. environmental chemicals or
microorganisms (viruses)
Immunotoxicant. Drug, chemical, or other agent that is toxic to
cells or other components of the immune system
Inducer (helper) T cell. See Helper (inducer) T cell
Inflammation. Process whereby blood proteins or leukocytes enter
tissue in response to or in association with infection or tissue
injury
Inflammatory cell. General description of cells in an inflammatory
infiltrate; in acute inflammation, mainly polymorphonuclear
leukocytes; in chronic inflammation, mainly lymphocytes and
macrophages
Innate immunity. State of protection against pathogen-induced
injury; does not require previous immunization or vaccination
Innocent bystander. Cell or tissue component that is destroyed by an
immunological reaction specifically directed against a unrelated
antigen
Integrin. Family of heterodimeric molecules sharing a ß chain (ß1,
ß2, ß3, about 750 amino acids long), each with a different alpha chain
(about 1100 amino acids long), with a major function in cell adhesion
and migration. Form a protein family rather than a superfamily on the
basis of strong structural and functional similarities. Examples:
leukocyte function-related antigen LFA-1 (alpha L/ß1, CD11a/CD18;
receptor for intercellular adhesion molecules ICAM-1, ICAM-2, and
ICAM-3); Mac-1 (alpha M/ß2, CD11b/CD18; complement C3 receptor, CR3);
p150,95 (alpha X/ß2, CD11c/CD18); very late antigens VLA-1
(alpha 1/ß1, CD49a/CD29; laminin, collagen receptor), VLA-2
(alpha 2/ß1, CD49b/CD29; laminin, collagen receptor), VLA-3
(alpha 3/ß1, CD49c/CD29; laminin, collagen, fibronectin receptor),
VLA-4/LPAM-1 (alpha 4/ß1, CD49d/CD29; receptor for fibronectin and
VCAM-1), VLA-5 (alpha 5/ß1, CD49e/CD29, fibronectin receptor), and
VLA-6 (alpha 6/ß1, CD49f/CD29; laminin receptor, and alpha V/ß1,
CD51/CD29; vitronectin receptor); LPAM-2 (alpha 4/ßp, CD49d/.., or
alpha 4/ß7)
Interdigitating dendritic cell. Leukocyte belonging to the monocyte-
macrophage cell lineage, present in T-cell areas in lymphoid organs;
has a major function in presentation of antigen (MHC class
II-restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures called Birbeck granules. Its
equivalent in epidermis is the Langerhans cell, and that in lymph, the
veiled macrophage.
Interferon. Low-relative-molecular-mass substance produced mainly
during viral infection by leukocytes (IFN alpha), fibroblasts (IFNß),
and lymphocytes (IFN gamma); has a major function in interfering with
viral replication
Interferon-alpha. Produced by leukocytes; stimulates B cells to
proliferate and differentiate; stimulates natural killer cells and
increases cytotoxic T cell generation, but blocks T-cell proliferation
and lymphokine-activated killer activity; stimulates macrophage
accessory activity and enhances Fc receptor expression and MHC class I
and II expression on various cell types; induces antiviral state in
cells and is cytostatic for tumour cells, inhibits fibroblast and
adipocyte differentiation, and enhances promyelocytic and monoblastic
cell differentiation
Interferon-ß. Produced by fibroblasts and epithelia; activity
similar to that of IFN alpha
Interferon-gamma. Produced by T cells; induces antiviral state and
is cytostatic for tumour cells; enhances MHC class I and II expression
on various cell types, is antagonistic to interleukin-4 in IgE/IgG1
synthesis, and stimulates IgG2a synthesis; activates macrophages to
become cytolytic and enhances natural killer and lymphokine-activated
killer activity.
Interfollicular area. Area between follicles in lymphoid tissue,
where mainly small T lymphocytes are lodged; recognized by presence of
high endothelial venules. In lymph nodes, located in the outer cortex
and continuous with the paracortex
Interleukin. Immunoregulatory protein, also known as lymphokine,
monokine, interferon, or cytokine. Generally, low relative molecular
mass (< 80 kDa) and frequent glycosylation; regulates immune cell
function and inflammation by binding to specific cell surface
receptors; transient and local production; acts in paracrine or
autocrine manner, with stimulatory or blocking effect on growth and
differentiation; very potent, functions at picomolar concentrations.
Represents an extensive series of mediators (interleukins 1-12), with
a wide range of overlapping functions. Other mediators in this series
are c-kit ligand, interferon, tumour necrosis factor, and transforming
growth factor
Interleukin 1. Comprises two forms, IL-1 alpha and IL-1ß; produced
mainly by cells of the mononuclear phagocytic system (macrophages),
astrocytes, endothelium, and some epithelia, following stimulation by
e.g. microorganisms, immune complexes, or particulate compounds.
IL-1 alpha is mainly cell-associated; IL-1ß is released. IL-1 has
(together with IL-6 and tumour necrosis factor) multiple effects in
the systemic acute-phase response and in local acute and chronic
inflammation: it stimulates T (helper) cells to synthesize IL-2 and
IL-2 receptors, interferon gamma, and other lymphokines, B cells
(proliferation and differentiation), neutrophils, and natural killer
cells; stimulates monocytes and macrophages to produce IL-1, IL-6, and
tumour necrosis factor; acts in the acute-phase response by inducing
synthesis of acute-phase proteins in liver and reducing cytochrome
P450 synthesis; induces natriuresis in kidney, insulin production in
pancreas ß cells, muscular proteolysis ('easy' energy generation) in
muscle cells, slow-wave sleep in cerebral cortex; raises the
temperature set-point (fever) in hypothalamus; stimulates
haematopoiesis and prostaglandin synthesis by various cell types
(fibroblasts, macrophages, endothelium); inhibits gastric motility
in vitro ; induces collagenase production by synovial cells and
osteoclasts, and antiviral state; inhibits gastric smooth muscle
in vitro ; is cytostatic for tumour cells and activates endothelium
Interleukin 2. Synthesized by T helper cells after activation;
stimulates (autocrine) T cells to divide and release mediators,
B cells to proliferate and differentiate; activates monocytes and
natural killer cells; stimulates lymphokine-activated killer cells;
promotes generation of T helper 1 cells.
Interleukin 3. Formerly called multi-colony-stimulating factor;
synthesized by T helper cells; promotes growth of pluripotent
haematopoietic progenitor cells to granulocytes (eosinophilic,
basophilic, neutrophilic), mast cells, macrophages, megakaryocytes,
and, together with erythropoietin, to normoblasts and erythrocytes;
activates eosinophils and mast cells; stimulates haematopoiesis and
B-cell differentiation; blocks lymphokine-activated killer cells
Interleukin 4. Formerly called B-cell growth factor or B-cell
stimulating factor; synthesized by T helper and B cells; stimulates
IgE and IgG1 production by B cells and enhances MHC class II and IgE
receptor expression on B cells; acts in synergism with IL-2 in killer
cell generation, is mitogenic for T cells, and activates macrophages.
It is the dominant interleukin in generating T helper 2 cells, with a
negative feedback on the generation of T helper 1 cells.
Interleukin 5. Formerly called T-cell replacing factor or B-cell
growth and differentiation factor II; synthesized by T helper cells;
activates B cells and eosinophils, and stimulates IgA production by
B cells
Interleukin 6. Formerly called interferon ß2; synthesized by T
cells, monocytes, endothelial cells, fibroblasts, and smooth muscle
cells, among others, during inflammatory reactions; stimulates T and B
cells to proliferate and differentiate; has properties similar to IL-1
and acts synergistically with it in the acute-phase response (fever,
synthesis of acute-phase proteins); synergizes with IL-3 in promoting
haematopoietic progenitor cell proliferation; inhibits production of
IL-1 and tumour necrosis factor by monocytes
Interleukin 7. Formerly called lymphopoietin; synthesized by bone-
marrow stroma; induces growth of immature T and B lymphocytes
Interleukin 8. Formerly called neutrophil-activating protein;
synthesized by monocytes and various tissue cells in response to
inflammatory stimuli; performs chemotaxis of neutrophilic granulocytes
and subsequent granule exocytosis and respiratory burst; induces
increased expression of adhesion molecules CD11b/CD18 (complement C3
receptor CR3) and promotes vascular leakage. Endothelium-derived IL-8
inhibits adhesion of neutrophilic granulocytes induced by IL-1
Interleukin 9. Synthesized by T lymphocytes; stimulates growth of
erythroid and megakaryocyte precursors and promotes (mucosal) mast-
cell growth; acts synergistically with IL-4 in modulating IgE and IgG
production
Interleukin 10. Synthesized by T and B lymphocytes; inhibits
mediator synthesis (IL-2, IL-3, tumour necrosis factor, interferon
gamma, granulocyte-macrophage colony-stimulating factor) by T helper
1 cells, inhibits mediator synthesis (IL-1 alpha, IL-1ß, IL-6, IL-8,
and tumour necrosis factor alpha) by monocytes; stimulates IL-2-
dependent growth and cytotoxicity of cytotoxic T cells and stimulates
mast cell growth together with IL-2 or IL-3 and IL-4; induces MHC
class II antigen expression on B cells, but down-regulates MHC class
II on monocytes; promotes generation of T helper 2 cells
Interleukin 11. Synthesized by fibroblasts and bone-marrow stromal
cells; resembles IL-6 in function: stimulates haematopoietic cell
growth and differentiation (myeloid, erythroid, megakaryocyte
lineage); enhances T-cell-dependent antibody response; and suppresses
adipocyte differentiation and lipoprotein lipase production
Interleukin 12. Also called natural killer cell stimulatory factor;
synthesized by monocytes-macrophages, B cells, and accessory cells, in
response to bacteria or parasites; stimulates T-lymphocyte
proliferation, activates natural killer cells, and stimulates
lymphokine-activated killer activity; synergizes with IL-2 in
activation of cytotoxic lymphocytes; induces production of interferon
gamma and other cytokines by lymphocytes. It is the dominant
interleukin in generating T helper 1 cells and has a negative feedback
on the generation of T helper 2 cells.
in vitro. In the context of this book, exposure of cells or cell
systems to the immunotoxic agent in vitro. If the donors of cells or
cell systems are exposed but these are analysed in vitro, the term
ex vivo/in vitro is used
Isohaemagglutinin. Antibodies mainly of the IgM class that react
with (carbohydrate) antigens on erythrocytes from individuals of the
same species, resulting in agglutination in vitro
Isologous. Synonym for isogeneic
Isotype. Antigenic determinant that defines class or subclass of
immunoglobulin molecules
J (joining) chain. A 15-kDa polypeptide chain that acts
intracellularly to combine (identical) IgA or IgM immunoglobulin
units, consisting of two heavy and two light chains, to form a dimeric
IgA or a pentameric IgM molecule
J (joining) genes. Genes that encode the variable part of
immunoglobulin or T-cell receptor chains (e.g. JH1-JHn for
immunoglobulin heavy chains, Jkappa 1-Jkappa n for immunoglobulin
kappa light chain, Jalpha 1-Jalpha n for T-cell receptor alpha
chain)
Kallikrein (kininogenase). Arises in tissue fluids after cleavage of
prekallikrein; acts on kininogen to produce kinins, resulting in
immediate hypersensitivity reaction, e.g. vasodilatation and oedema.
It is a preformed mediator present in mast cell granules
kappa Chain. In immunoglobulin molecules, the kappa light chain
forms the light chain type of the molecule
Keratinocyte. Epithelial cell in the epidermis; in some
circumstances, can manifest antigen presentation and produce
immunoregulatory cytokines; hence belongs to the skin immune system
Killer cell (K cell). See Cytotoxic cell
Kinin system. Humoral amplification system involved in inflammation,
whereby substrate proteins become active after enzymatic cleavage;
cause vasodilatation, increased vascular permeability, hypotension,
and contraction of smooth muscle
c-Kit ligand. Also called stem cell growth factor or mast cell
growth factor; synthesized by various stromal cells, fibroblasts, and
liver cells; stimulates growth of early pluripotent progenitor cells
and that of myeloid, erythroid, and lymphoid progenitors in synergy
with interleukin-1, -3, -6, -7, and granulocyte-macrophage colony-
stimulating factor; promotes growth of mast cells
Kupffer cells. Macrophages on or between endothelial cells lining
the sinusoids of the liver
lamba Chain. In immunoglobulin molecules, the lamba light chain
forms the light chain type of the molecule
Lamina propria. Thin layer of connective tissue under the villous
epithelium of the gastrointestinal tract; site of plasma cells,
producing mainly dimeric IgA, including J chain
Langerhans cell. Leukocyte belonging to the monocyte-macrophage cell
lineage, present in skin epidermis; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II
restricted) to T helper lymphocytes. Cytoplasm contains characteristic
rod-like structures, Birbeck granules. Its equivalent in lymphoid
tissue is the interdigitating dendritic cell, and that in lymph,
veiled macrophage; forms part of the skin immune system
Large granular lymphocyte. Intermediate-sized, 10-12-µm lymphocyte
with a kidney-shaped nucleus and prominent, large, azurophilic
granules in the cytoplasm; occurs in the circulation and in tissue and
has a major function as a natural killer cell; forms a heterogeneous
population with either T markers or monocyte-macrophage markers.
Lectin. Plant-derived substance that binds to lymphocytes and can
induce cell proliferation; some also bind to other haematopoietic
cells
Leukaemia. Neoplasia of lymphoid cells in blood or bone marrow
Leukocyte. Bone marrow-derived white blood cell, including cells in
the lymphoid, myeloid, and monocyte lineages; sometimes used to
describe only granulocytes
Leukocytosis. Increased proportion of leukocytes in blood
Leukopenia, leukocytopenia. Reduced proportion of leukocytes in
blood
Leukotriene. Formerly called slow-reacting substance of anaphylaxis;
products of arachidonic acid metabolism following the lipoxygenase
pathway, which act as mediators in the immediate hypersensitivity
reaction, mainly as chemoattractants for granulocytes and monocytes,
and in smooth muscle contraction; newly synthesized by mast cells upon
activation
L (light) chain. One of the 22-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and a
constant domain. The light chain, either kappa or lamba, determines
the light chain type of the immunoglobulin molecule
Ly antigen. T Lymphocyte antigen in mice
Lymph. Fluid in lymphatic vessels
Lymph node. Secondary (peripheral) lymphoid organ, the main function
of which is to filter lymphatic vessels. Present throughout the body,
at connecting places of lymphatics and blood vessels; forms a major
site of encounter between pathogenic substances in the lymph and
lymphocytes entering from blood vessels, and subsequent initiation of
antigen-specific immunological reactions
Lymphatic. Vessel that collects fluid from interstitial spaces and
goes via lymph nodes (filtering) to the thoracic duct and blood
Lymphocyte. Cell belonging to the lymphoid lineage of bone marrow-
derived haematopoietic cells. In a restricted way, the designation of
a small, resting or recirculating mononuclear cell in blood or
lymphoid tissue, which measures about 7-8 µm, has a round nucleus
containing densely aggregated chromatin, and little cytoplasm. Plays a
key role in immune reactions by specific recognition of antigens
Lymphocytosis. Increased proportions of lymphocytes in blood
Lymphoid organ. Tissue in the body where cells of the immune system,
mainly lymphocytes, are lodged in an organized microenvironment,
either in a resting state or in a state of activation,
differentiation, or proliferation. Includes bone marrow, thymus, lymph
nodes, spleen, and mucosa-associated lymphoid tissue
Lymphokine. Hormonal substance synthesized by lymphocytes, which
modulates the function of cells in immunological reactions
Lymphoma. Neoplasia of lymphoid cells in tissue
Lymphopenia, lymphocytopenia. Reduced proportions of lymphocytes in
blood
Lymphotoxin. Former name for tumour necrosis factor ß; lymphokine
synthesized by T lymphocytes, which kills selected target cells
Lysosome. Granule present in many cell types that contain hydrolytic
enzymes; also performs intracellular degradation of pathogens after
phagocytosis
Lysozyme (muramidase). A low-relative-molecular-mass, cationic
enzyme present in tissue fluids and secretions, which degrades
mucopeptides of bacterial cell walls
Macroglobulin. Glycoprotein of relative molecular mass > 200 kDa
Macrophage. Large, 12-20-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in tissue, and forming the
mononuclear phagocytic system. Its reniform nucleus usually has
pronounced peripheral condensation of nuclear chromatin; its cytoplasm
contains a great variety of cell organelles, including rough
endoplasmic reticulum, mitochondria, ribosomes, lysosomes, and Golgi
complex. Has a major function in (chronic) inflammatory reactions, by
virtue of its phagocytic capacity, with immunoglobulin Fc and
complement C3 receptors which bind to immune complexes. Macrophages
develop into killer cells after activation by e.g. T-cell factors and
can mediate antibody-dependent cell-mediated cytotoxicity; also
functions as an accessory cell in induction of immune responses
(antigen presentation, mediator secretion). Macrophages in blood are
called monocytes. Subtypes with special functions are interdigitating
dendritic cells: T-cell area of lymphoid tissue, Langerhans cell
(skin), Kupffer cells (liver), metallophilic macrophages (spleen),
microglia (brain), osteoclasts (bone), tingible body macrophage
(starry-sky macrophage), veiled macrophage (lymph).
Macrophage colony-stimulating factor. Synthesized mainly by
endothelial cells and fibroblasts, and possibly macrophages; supports
growth of monocyte-macrophage progenitors.
Major histocompatibility complex (MHC). Set of genes that codes for
tissue compatibility markers, which are targets in allograft rejection
and hence determine the fate of allografts; plays a central role in
control of cellular interactions during immunological reactions.
Tissue compatibility is coded by classes I and II loci (see Class I
and Class II MHC molecule). Genes within or closely linked to MHC
control certain complement components (MHC class III genes). The MHC
complex of humans is HLA, that of mice H-2, and that of rats, RT-1.
Mantle (corona). Zone in secondary follicles surrounding the central
germinal centre, densely packed with small resting B lymphocytes
Marginal zone. Outer layer of white pulp in spleen, surrounding
follicles, and periarteriolar lymphocyte sheath; separated from these
by the marginal sinus; populated by intermediate-sized, slightly
pyroninophilic B cells which have a major function in the T cell-
independent antibody response. The microenvironment manifests a
special type of macrophage, the marginal metallophilic macrophage
Margination. Adherence of blood leukocytes to endothelium during
inflammatory reactions
Mast cell. A bone marrow-derived polymorphonuclear leukocyte present
in tissue; has a major function in immediate hypersensitivity
reactions; has a round or oval nucleus and abundant cytoplasm with
basophilic (blue) granules stained by metachromatic dyes; granules
contain mediators of immediate hypersensitivity reactions, e.g.
heparin, histamine, serotonin, tryptase, kallikrein, and
chemoattractants for neutrophilic and eosinophilic granulocytes; has a
high-affinity receptor for IgE. After activation (physical stimuli,
cross-linking via allergen-IgE-IgE receptor), there is immediate
granule release and synthesis of other mediators, including
prostaglandins, thromboxanes, leukotrienes, and platelet-activating
factor. The cell can exert modulatory activity by secreting cytokines
such as IL-3, IL-6, and tumour necrosis factor. Two subtypes exist, in
the mucosa and in connective tissue; the equivalent in the circulation
is the basophilic granulocyte
Mast cell growth factor. See Interleukin 9
Medulla. Inner parenchymal layer of organs
Medullary cord. Parenchyma in medulla of lymph nodes separating
lymphatic sinusoids
Megakaryocyte. Large, multinucleated giant cell, precursor of blood
platelets, formed by separation of portions of membrane-bound
cytoplasm; occurs in haematopoietic tissue, including bone marrow
Memory. See Immunological memory
Mesoderm. Middle of the three cellular layers of the embryo;
produces connective tissue and blood cells
Metallophilic macrophage. Subtype of macrophage identified by silver
impregnation, present at the inner border of the marginal zone in
spleen
MHC restriction. Immunological reactions can occur only in
associated recognition with the polymorphic determinant of a given MHC
molecule and not with that of another MHC molecule. Applies to T
lymphocytes with an alpha-ß T-cell receptor, which recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules, and part of the T cell population with the gamma-delta
T-cell receptor.
Microglia. Macrophages in central nervous system
Migration inhibitory factor. A lymphokine that inhibits the movement
of macrophages
Milky spot. Aggregate of lymphoid cells in omentum, macroscopically
visible as a small white spot; not organized tissue but rather the
product of immune stimulation in that area of the body
Minor histocompatibility antigen. Ill-defined histocompatibility
marker not encoded by the MHC, which is a target in allograft
reactions (apart from products of the MHC)
Mitogen. Substance that activates resting cells to transform and
proliferate
Monoclonal. Derived from a single clone. For T and B lymphocytes, a
cell population in which all cells have a distinct V-D-J gene
rearrangement product (as seen in lymphoma and leukaemia). Monoclonal
antibodies are products of hybridomas prepared after fusion of
antibody-producing cells and a transformed (non-producing)
plasmacytoid cell line.
Monocyte. Large, 10-15-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in the blood and in lymphatic
vessels
Monokine. Hormonal substance synthesized by monocytes-macrophages;
modulates the function of cells in immunological reactions
Mononuclear cell. Leukocyte with a single rounded nucleus, e.g.
lymphocytes and monocytes-macrophages
Mononuclear phagocytic system. Formerly called reticuloendothelial
system; composite of phagocytic cells in the body, including monocytes
and tissue macrophages. Main populations are Kupffer cells in liver,
microglia in the central nervous system, macrophages in red pulp of
spleen, alveolar macrophages in lung, and, after induction, peritoneal
macrophages in the peritoneal cavity; others are mesangial macrophages
in kidney and osteoclasts in bone
µ Chain. In immunoglobulin molecules, the µ heavy chain forming the
IgM immunoglobulin class
Mucosa-associated lymphoid tissue. Lymphoid tissue or organs in
immediate contact with the mucous-secreting mucosal layer in nasal
cavity and nasopharynx (nasal-associated lymphoid tissue), airways
(bronchus-associated lymphoid tissue), and intestinal tract (gut-
associated lymphoid tissue). Serves as the immunological defence at
secretory surfaces, to some extent independent of the systemic
(internal) response; includes IgA synthesis by plasma cells in the
lamina propria and excretion into the lumen
Multiple myeloma. Tumour of plasma cells in bone marrow
Muramidase. See Lysozyme
Myeloblast. Immature precursor cell in the lineage of polymorpho-
nuclear cells (granulocytes, mast cells)
Nasal-associated lymphoid tissue. Lymphoid organs or tissue located
in the nasal cavity and nasopharynx, presumed to be a first location
for presentation of antigens entering through the nose; contributes to
the mucosa-associated lymphoid tissue
Natural antibody. Antibody in serum of individuals with no previous
exposure to the corresponding antigen; often generated by contact with
cross-reacting agents, e.g. bacterial products; often restricted to
antibodies that react to xenogeneic antigens
Natural killer cell. Leukocyte with a limited repertoire to
recognize antigen; can kill target cells without prior sensitization;
can be of lymphoid or monocyte-macrophage origin. Large granular
lymphocytes are the main population
Necrosis. Death of tissue and cells
Neoantigen. New antigen appearing on cells or tissue during
malignant transformation or (viral) infection
Neoplasia. Uncontrolled malignant transformation of cells resulting
in tumour formation
Neutralization. Process whereby a pathogenic substance becomes
inactivated by effector components (antibodies) of the immunological
reaction
Neutropenia. Reduced proportions of neutrophilic granulocytes in
blood
Neutrophil chemotactic factor. Preformed mediator with a relative
molecular mass > 750 kDa, present in granules of mast cells and
basophilic granulocytes; released after activation and attracts
neutrophilic granulocytes to the site of inflammation or
hypersensitivity
Neutrophilic granulocyte. Polymorphonuclear leukocyte that contains
granules stained by neither acidophilic nor basophilic dyes; can
phagocytose immune complexes by receptor-mediated endocytosis,
followed by intracellular degradation in lysosomes. Degranulation
releases catepsins and lysosomal enzymes, resulting in tissue damage.
Nonspecific immunity. Immunity induced by non-immunological
mechanisms, for instance by action of complement, lysozyme,
phagocytosis, or interferon
Oedema. Swelling of tissue due to extravasation of fluid from the
intravascular space following increase in vascular permeability
Ontogeny. Life cycle of an organism; in immunological terms, often
used to describe the process whereby the immune system develops
immunocompetence
Opsonization. Adherence of pathogen to phagocytic cell due to action
of antibody or activated complement
Osteoclast. Macrophage in bony tissue involved in bone resorption
Paracortex. Area in the inner cortex of lymph node where T
lymphocytes are lodged; recognized by the presence of high endothelial
venules; continuous with interfollicular areas in the outer cortex on
one side and with the medullary cords on the other
Paratope (antigen-binding site). Part of antibody that binds antigen
(antigenic determinant, or epitope); part of T-cell receptor that
binds the complex of antigen and MHC molecule
Periarteriolar lymphocyte sheath. Area in white pulp of spleen
surrounding the central artery, populated mainly by small
T lymphocytes
Peripheral (secondary) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of these
reactions
Peyer's patch. Lymphoid tissue in wall of small intestine,
particularly ileum, separated from the gut lumen by a domed area and
an epithelial layer ('dome' epithelium); forms part of gut-associated
lymphoid tissue; main function is initiation of immunological
reactions towards pathogens entering through dome epithelium
Phagocytosis. Uptake of material >1 µm by cells, by receptor-
mediated endocytosis, by cells of the mononuclear phagocytic system;
requires Fc receptors, with accessory help of complement receptors; is
blocked by cytochalasins. Occurs via a 'zipper' mechanism, in which
the opsonized particle (coated with antibody or complement) becomes
enclosed by the cell membrane of the phagocyte; a second mechanism
involves oxidative burst, with formation of superoxide anion, peroxide
anion, and hydroxyl radicals, which kill or degrade the phagocytosed
particle
Phagolysosome. Membrane-bound cytoplasmic vesicle formed by fusion
of a phagosome and a lysosome
Phagosome. Membrane-bound vesicle in phagocytic cells containing
phagocytosed material
Pharmacokinetics. Fate of drugs or chemicals in the body over time,
including the processes of absorption and distribution in tissues,
biotransformation, and excretion
Phenotype. Characteristic of a distinct cell or individual,
reflecting the expression of a genetically determined property
Phenotypic marker. Expressed characteristic(s), for instance an
antigenic determinant, of a given cell or molecule, associated with
function or specificity
Phylogeny. Evolutionary history of a particular species
Pinocytosis. Uptake of material < 1 µm by cells; often restricted
to a receptor-mediated process in leukocytes of the monocyte-
macrophage series, e.g. uptake of lipoproteins and viruses into
clathrin-coated vesicles
Plasma. Fluid of uncoagulated blood after removal of cells
Plasma cell. Terminally differentiated B lymphocyte that synthesizes
and secretes immunoglobulin; these medium-sized, 10-15-µm cells have a
small excentric nucleus, with heterochromatin organized in a
'cartwheel'-like structure, and abundant cytoplasm filled with rough
endoplasmic reticulum.
Platelet. Small bone marrow-derived cytoplasmic fragment in blood
responsible for coagulation; main role is to block damaged vessel
walls and prevent haemorrhage, by clumping and aggregation; contains
heparin and serotonin, which contribute after release to the acute
vascular response in hypersensitivity reactions and produce oxygen
radicals
Platelet-activating factor. Low-relative-molecular-mass phospholipid
generated from alkyl phospholipids in mast cells, basophilic and
neutrophilic granulocytes, and monocytes-macrophages, which mediates
microthrombus formation of platelets in hypersensitivity reactions
Polyclonal. Derived from many different clones; for T and B
lymphocytes, cell populations in which the cells have different V-D-J
gene rearrangement products. Polyclonal activation is stimulation of
multiple lymphocyte clones, resulting in a heterogeneous response
Polymorphonuclear granulocyte. Leukocyte of bone-marrow origin, with
a lobulated nucleus, involved in acute inflammatory reactions. Main
subsets are basophilic, eosinophilic, and neutrophilic granulocytes
(different cytoplasmic granule colours after haematological staining).
Contributes to (acute) inflammatory reactions after attraction by
specific (immune complex-mediated) or nonspecific stimuli (including
complement components); after activation, releases granules containing
various hydrolytic enzymes
Postcapillary venule. Small blood vessel though which blood flows
after leaving the capillaries before reaching veins; often the site
where inflammatory cells leave the circulation and enter the tissue
Primary (central) lymphoid organ. Lymphoid organ where precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment to form immunocompetent cells; not antigen-driven but
can be influenced by mediators produced as a result of antigen
stimulation
Primary follicle. See Follicle
Primary response. Immunological reaction after first contact with
antigen, resulting in generation of immunological memory
Programmed cell death. See Apoptosis
Prostaglandin. Aliphatic acid produced by arachidonic acid
metabolism following the cyclo-oxygenase pathway; synthesized by mast
cells after activation; mediates immediate hypersensitivity reactions,
mainly smooth muscle contraction or bronchoconstriction; also
decreases the threshold for pain
Prothymocyte. Precursor of T lymphocytes in bone marrow before
moving to the thymus, or present in the thymus just before intrathymic
processing
Pyrogen. Substance that increases the temperature in the central
nervous system, resulting in fever; examples are bacterial endotoxin
and IL-1
Reactive oxygen intermediate. Reactive species of oxygen produced
e.g. by phagocytes (granulocytes and monocyte-macrophages) in response
to phagocytic stimuli like bacteria
Reagin. Former designation of IgE class antibody
Recall antigen. Antigen used to elicit a response from an individual
already sensitized to that antigen; may be one that the host has
knowingly been sensitized to or, in humans, one that it is assumed
that most individuals have been sensitized to
Red pulp. Area in spleen comprising venous sinuses filled with blood
and splenic cords; venous sinuses mainly contain erythrocytes
surrounded by endothelial cells; cords comprise macrophages,
lymphocytes, and occasionally megakaryocytes, but other types of blood
cells can also be present. Main function is phagocytosis of
particulate material and removal of old erythrocytes from blood. In
rodents, the red pulp can also be a site of haematopoiesis.
Reticuloendothelial system. See Mononuclear phagocytic system
Repertoire. All specific antigen-recognizing capacities (diversity)
within a population of T or B lymphocytes
RT-1. The major histocompatibility complex of rats
Secretory immunoglobulin. Immunoglobulin encountered in secretions
like tears, saliva, and jejunal juice; concerns mainly secretory IgA,
a dimer of the basic four-chain immunoglobulin structure, linked by a
J chain and surrounded by a secretory piece molecule.
Secretory piece. A 70-kDa molecule produced by epithelial cells
covering mucosa-associated lymphoid tissue; functions as a receptor
for IgA or IgM, thereby facilitating intercellular transport of these
molecules into the lumen. During this process, the secretory piece
becomes associated with the immunoglobulin, thereby enhancing its
stability in nonphysiological conditions of secretory fluid
Secondary follicle. See Follicle
Secondary (peripheral) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of those
reactions
Secondary response. Response after first contact (immunization,
primary response) with an antigen, based on the presence of
immunological memory; characterized as faster, more intense, and of
higher affinity; for the antibody response, associated with an
immunoglobulin class switch
Selectin. Cell surface glycoprotein that has a prominent function in
the interaction between lymphocytes, monocytes, neutrophilic
granulocytes, and endothelium. They share an N-terminal domain of
approximately 120 amino acids that is homologous to many Ca2+-
dependent animal lectins and binds to carbohydrates. Examples are
L-selectin (MEL-14, LAM-1, present on leukocytes; adherence of
endothelial cells, role in lymphocyte recirculation and neutrophil and
leukocyte inflammation); E-selectin (ELAM-1, present on endothelium;
adherence of monocytes, neutrophils, and T cells; role in
inflammation); P-selectin (PADGEM, GMP-140, CD62, present on platelets
and endothelium; adherence of monocytes, neutrophils, and T cells;
role in inflammation).
Self-MHC restriction. See MHC restriction; applies to MHC
molecules of the individual
Sensitization. Induction of specialized immunological memory in an
individual by exposure to antigen
Serotonin. 5-Hydroxytryptamine; catecholamine with relative molecular
mass of 176 Da; preformed mediator of immediate hypersensitivity
reactions, present in granules of mast cells and in platelets. After
activation, is released and mediates vasodilatation and increased
vascular permeability
Serum. Fluid of blood after coagulation (removal of fibrinogen) and
removal of cells
Serum sickness. Systemic vasculitis, glomerulonephritis, or arthritis
due to immune complex formation after the reaction between antibody and
injected foreign antigen (serum)
Skin-associated lymphoid tissue. See Skin immune system
Skin immune system. Combination of immune system components and
their function, present in skin; antigen presentation by Langerhans
cells, by dendritic epidermal cells, and in some conditions by
keratinocytes; immunoregulation by e.g. keratinocyte-derived
cytokines, and distinct dermatotropic T-cell populations
Slow-reacting substance of anaphylaxis. See Leukotriene
Somatic mutation. Small changes in genes resulting in alterations in
amino acids built into protein chains. For immunoglobulin molecules,
changes in diversity of antigen-binding site (variable region)
Spleen. Lymphoid organ in the left abdominal cavity, for filtering
blood; main function is phagocytosis of particles from blood, removal
of old erythrocytes in red pulp, and initiation of immunological
reactions in white pulp. The marginal zone of the white pulp serves as
the main site of T cell-independent antibody formation.
Starry-sky macrophage. See Tingible body macrophage
Stem cell. Multipotential, self-renewing precursor cell of all
haematopoietic cell lineages, present in bone marrow
Stem-cell growth factor (synonym for c-Kit ligand). An interleukin
that supports continuous growth of mast cells and augments the
response of progenitor cells to stem growth factors; interacts via
c-kit proto-oncogene
Subcapsular sinus. Area in lymph node just under the capsule and
surrounding the cortex, which is connected with afferent lymphatics,
and through cortical (peritrabecular) sinuses with medullary sinuses;
contains dendritic macrophages
Superantigen. Antigenic moiety that, in MHC-restricted presentation
to T lymphocytes, is not present in the 'groove' made by the
quaternary structure of the MHC molecule but is complexed with the MHC
molecule. Examples are the endogenous viral-encoded Mlsa (minor
lymphocyte stimulatory) antigen, which is present in certain mouse
strains, and Staphylococcus enterotoxin A
Suppressor T cell. Subpopulation of T lymphocytes with CD8 phenotype;
after recognition of antigen in an MHC class I-restricted manner,
suppresses immunological reactions, in part by cytotoxic activity
Systemic lupus erythematosus. Chronic, remitting, relapsing,
inflammatory, and often febrile multisystemic disorder of connective
tissues, with possible involvement of the central nervous system,
skin, joints, kidneys, and serosal membranes; can be acute or
insidious in onset. The etiology is unknown but is thought to follow a
failure of the regulatory mechanisms of the immune system that sustain
self-tolerance. Many drugs and chemicals can induce lupus-like
symptoms (drug-induced lupus erythematosus)
T-Cell receptor. Heterodimeric molecule on the surface of the T
lymphocyte that recognizes antigen. The polypeptide chains have a
variable and a constant part, and can be alpha, ß, gamma, or delta.
The alpha-ß T-cell receptor occurs on most T cells and recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules (self-MHC restricted). The gamma-delta T-cell receptor
occurs on a small subpopulation, e.g. in mucosal epithelium, and can
recognize antigen in a non-MHC restricted manner. The T-cell receptor
occurs exclusively with the CD3 molecule, which is thought to mediate
transmembrane signalling.
T-Dependent antigen. Antigen for which antibody formation requires
T cells.
Terminal pathway of complement activation. Activation of complement
components C6-C9, with formation of the membrane attack complex and
subsequent lysis of the cell
Tingible body macrophage (starry-sky macrophage). Large macrophage
in cortex of thymus and germinal centres of follicles in lymphoid
tissue, filled with condensed nuclear material with high affinity for
dyes; has a major function in phagocytosis, presumably of apoptotic
cells
T Lymphocyte or cell. Lymphocyte that induces, regulates, and
effects specific immunological reactions after stimulation by antigen,
mostly in the form of processed antigen complexed with MHC product on
an antigen-presenting cell. They originate from precursors in the bone
marrow and undergo maturation in the thymus (T, thymus-dependent).
Most T lymphocytes recognize antigen by a heterodimeric alpha-ß
surface receptor molecule associated with the CD3 molecule, which
mediates transmembrane signalling. Subsets include helper-inducer and
suppressor-cytotoxic cells.
T-Lymphocyte area. That part of a lymphoid organ or tissue that is
occupied by T lymphocytes, e.g. paracortex or interfollicular area in
lymph node, periarteriolar lymphocyte sheath in spleen
Thrombocyte. See Platelet
Thrombocytopenia. Reduced proportion of platelets in blood
Thromboxane. Product of arachidonic acid following the cyclo-
oxygenase pathway; synthesized by mast cells after activation and
mediates immediate hypersensitivity reactions, mainly smooth muscle
contraction, bronchoconstriction, and platelet aggregation
Thymocyte. Lymphocyte in the thymus
Thymoma. Tumour of the thymus; neoplastic cell is an epithelial cell
Thymus. Central lymphoid organ located dorsal to the cranial part of
the sternum in the thorax, comprising two lobes, each consisting of
many lobules. Its main function is generation of immunocompetent
T lymphocytes from prothymocytes from the bone marrow
Tolerance. State of unresponsiveness to antigenic stimulation, due
to the absence of responding elements or loss of capacity of existing
elements to mount a reaction. Synonym for anergy
Tolerogen. Antigen that evokes tolerance
Tonsil. Organized mucosa-associated lymphoid tissue in
oronasopharynx. Adenoids strictu sensu are also tonsils. The main
function is initiation of immunological reactions towards pathogens
entering through the mouth. Contributes in part to the gut-associated
lymphoid tissue. Together with lymphoid aggregates in oronasopharynx,
these tissues form the ring of Waldeyer
Transforming growth factor ß. Mediator synthesized by lymphocytes or
macrophages, with a function in down-regulation of immune reactions;
suppresses T- and B-lymphocyte growth, IgM and IgG production, and
down-regulates MHC class II expression; interferes with production of
tumour necrosis factor and adhesion of granulocytes to endothelial
cells; is chemotactic for monocytes and induces interleukin-1 and
interleukin-6 expression
Transudation. Transfer of fluid and low-relative-molecular-mass
proteins from intravascular to extravascular tissue during
inflammatory processes
Tryptase. Proteolytic enzyme present in granules of mast cells;
released after activation and activates complement component C3, with
formation of the anaphylatoxin C3a
Tumour necrosis factor. General mediator of inflammation and septic
shock; formerly named cachectin and lymphotoxin. Two forms: alpha and
ß, both produced by monocytes-macrophages, TNF-ß also by T lymphocytes
and natural killer cells. Has activity similar to interleukin-1 and
acts synergistically with it; promotes antiviral state and is
cytotoxic for tumour cells; stimulates granulocytes and eosinophils,
activates macrophages to interleukin-1 synthesis, stimulates B cells
to proliferate and differentiate, T cells to proliferate,interleukin-2
receptor synthesis, and interferon gamma synthesis; induces
fibroblasts to synthesize prostaglandin and proliferate; induces fever
and synthesis of acute-phase proteins; reduces cytochrome P450
synthesis; activates endothelium and promotes adherence of
neutrophilic granulocytes to endothelium; induces cell adhesion
molecules like lymphocyte function-associated antigens LFA-1 and
LFA-3, ICAM-1, and ELAM-1; inhibits gastric motility in vitro;
reduces lipoprotein lipase synthesis by adipocytes; and activates
osteoclasts to bone resorption
Urticaria. Transient eruption of skin characterized by erythematous
or oedematous swelling (wheal) of the dermis or subcutaneous tissue
Vaccination. See Immunization
Valency. Number of antigenic determinants or ligands that can bind
to one antibody molecule or receptor
V (variable) gene. Gene that encodes the variable part of
immunoglobulin or T-cell receptor chains (e.g. VH1-VHn for
immunoglobulin heavy chains, Vkappa 1-Vkappa n for immunoglobulin
kappa light chain, Valpha 1-Valpha n for T-cell receptor alpha
chain)
Variable gene family. Groups of germline V genes (which encode
immunoglobulin chains or T-cell receptor genes) that have more than
about 80% nucleotide sequence identity
V (variable) region. Region at the amino terminal of immunoglobulin
or T-cell receptor chains, which contributes to the antigen-binding
site of the molecule. Encoded by V (variable), D (diversity), and J
(joining) genes in DNA
Vasoconstriction. Contraction of capillary venules, resulting in
decreased blood flow
Vasodilatation. Dilatation of capillary venules, resulting in
increased blood flow through capillaries and lowering of local blood
pressure
Veiled macrophage. Leukocyte belonging to the monocyte-macrophage
lineage, present in lymph; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II-
restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures, Birbeck granules. Its equivalent
in lymphoid tissue is the interdigitating dendritic cell, and that in
skin is the Langerhans cell
Waldeyer's ring. Lymphoid tissue of tonsils and adenoids located
around the junction of the pharynx and oral cavity in humans and
domestic animals. Main function is initiation of immunological
reactions towards pathogens entering through the mouth. Contributes to
the gut-associated lymphoid tissue
White blood cell. Polymorphonuclear leukocyte, lymphocyte, or
monocyte in peripheral blood
White pulp. Area in spleen around central arterioles where lymphoid
cells reside. Comprises three major compartments: the periarteriolar
lymphocyte sheath, follicles, and marginal zone
Xenobiotic. Chemical or substance that is foreign to the biological
system
Xenogeneic. Genetically different phenotypes in individuals of
different species; opposite of allogeneic, or isogeneic
zeta Chain (see epsilon Chain). One of the chains in the CD3
molecule associated with the T-cell receptor
REFERENCES
Ada GL, Leung KN, & Ertl H (1981) An analysis of effector T-cell
generation and function in mice exposed to influenza A or Sendai
virus. Immunol Rev, 50: 5-24.
Ader, R, Felten D, & Cohen, N (1990) Interactions between the brain
and the immune system. Ann Rev Pharmacol Toxicol, 30: 561-602.
Adkins B, Richards JH, & Gardner DE (1979) Enhancement of experimental
respiratory infection following nickel inhalation. Environ Res,
20: 33-42.
Adkins B, Luginbuhl GH, Miller, FJ, & Gardner DE (1980) Increased
pulmonary susceptibility to Streptococcal infection following
inhalation of manganese oxide. Environ Res, 23: 110-120.
Adkins B, Gandour D, Strober S & Weissman I (1988) Total lymphoid
irradiation leads to transient depletion of the mouse thymic medulla
and persistent abnormalities among medullary stromal cells. J Immunol,
140: 3373-3379.
Adorini L ed. (1990) The molecular basis of antigen presentation to
T lymphocytes: Novel possibilities for immunointervention. Int Rev
Immunol, 6: 1-88.
Agius C (1985) The melanomacrophage centers of fish; a review.
In: Manning MJ & Tatner MF, eds, Fish Immunology. London, Academic
Press, pp 85-105.
Ahmed-Ansari A, Brodie AR, Fultz PN, Anderson DC, Sell KW, & McClure
HM (1989) Flow microfluorometric analysis of peripheral blood
mononuclear cells from nonhuman primates: Correlation of phenotype
with immune function. Am J Primatol, 17: 107-131.
Allen JR, & Abrahamson LJ (1973) Morphological and biochemical changes
in the liver of rats fed polychlorinated biphenyls. Arch Environ
Contam Toxicol, 1: 265-280.
Allen AL, Koller LD, & Pollock GA (1983) Effect of toxaphen exposure
on immune responses in mice. J Toxicol Environ Health, 11: 61-69.
Alomran, AH & Shleamoon, MN (1988) The influence of chronic lead
exposure on lymphocyte proliferative response and immunoglobulin
levels in storage battery workers. J Biol Sci Res, 19: 575-585.
Anderson DP (1990) Immunological indicators: Effects of environmental
stress on immune protection and disease outbreaks. Am Fish Soc Symp
8: 38-50.
Anderson RS & Brubacher LL (1993) Inhibition by pentachlorophenol of
production of reactive-oxygen intermediates by medaka phagocytic blood
cells. Marine Environ Res, 35: 125-129.
Anderson RE & Williams WL (1977) Radiosensitivity of T and B
lymphocytes. V. Effects of whole-body irradiation on numbers of
recirculating T cells and sensitization to primary skin grafts in
mice. Am J Pathol, 89: 367-378.
Anderson J, Moller G & Sjorberg O (1972) Selective induction of DNA
synthesis in T and B lymphocytes. Cell Immunol, 4: 381-393.
Anderson RE, Olson GB, Autry JR, Howarth JL, Troup GM ,& Bartels PH
(1977) Radiosensitivity of T and B lymphocytes. IV. Effect of whole
body irradiation upon various lymphoid tissues and numbers of
recirculating lymphocytes. J Immunol, 118: 1191-1200.
Anderson RE, Standefer JC, & Tokuda S (1986) The structural and
functional assessment of cytotoxic injury of the immune system with
particular reference to the effects of ionizing radiation and
cyclophosphamide. Br J Cancer, 53 (Suppl 7): 140-160.
André F, Gillon F, André C, Lafont S, & Jourdan G (1983) Pesticide-
containing diet augments anti-sheep red blood cell non reagenic
antibody responses in mice but may prolong murine infection with
Giardia muris. Environ Res 32: 145-150.
Anneroth G, Ericson T, Johannson I, Mornstad H, Ryberg M, Skoglund A,
& Stegmayr B (1992) Comprehensive medical examination of a group of
patients with alleged adverse effects from dental amalgam. Acta
Odontol Scand, 50: 101-107.
Applegate LA, Ley RD, Alcalay J, & Kripke ML (1989) Identification of
the molecular target for the suppression of contact hypersensitivity
by UV radiation. J Exp Med, 170: 1117-1131.
Appleton JA & McGregor DD (1984) Rapid expulsion of Trichinella
spiralisin suckling rats. Science, 226: 70-72.
Araneo BAT, Dowell T, Moon HB; & Daynes RA (1989) Regulation of murine
lymphokine production in vivo: Ultraviolet radiation exposure
depresses IL-2 and enhances IL-4 production by T cells through an IL-1
independent mechanism. J Immunol, 143: 1737-1745.
Aranyi C, Vana SC, Thomas PT, Bradof JN, Fenters JD, Graham JA, &
Miller FJ (1983) Effects of subchronic exposure to a mixture of O3,
SO2 and (NH4)SO4 on host defenses in mice. Environ Res,
12: 55-71.
Aranyi C, Bradof JN, O'Shea WJ, Graham JA, & Miller FJ (1985) Effects
of arsenic trioxide inhalation exposure on pulmonary antibacterial
defenses in mice. J Toxicol Environ Health, 15: 163-172.
Aranyi C, O'Shea WJ, Graham JA & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6: 713-720.
Archer DL, Smith BG, & Bukovic-Wess JA (1978) Use of an in vitro
antibody producing system for recognizing potentially
immunosuppressive compounds. Int Arch Allergy Appl Immunol, 56: 90-93.
Arkoosh MR & Kaattari SL (1990) Quantitation of fish antibody to a
specific antigen by an enzyme-linked immunosorbent assay (ELISA).
In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS & van Muiswinkel
WB ed. Techniques in Fish Immunology. Fair Haven, NJ, SOS
Publications, Vol 1, pp 15-24.
Askenase PW, Hayden B, & Gershon RK (1977) Evanescent delayed
hypersensitivity: Mediation by effector cells with a short life span.
J Immunol, 119: 1830-1835.
Austin JH, Schulman LL, & Mastrobattista JD (1989) Pulmonary infection
after cardiac transplantation: Clinical and radiologic correlations.
Radiology, 172: 259-265.
Austyn JM & Gordon S (1981) F4/80, a monoclonal antibody directed
specifically against the mouse macrophage. Eur J Immunol, 11: 805-815.
Baadsgaard O, Wulf HC, Wantzin GL, & Cooper KD (1987) UVB and UVC, but
not UVA, potently induce the appearance of T6-DR+ antigen-presenting
cells in human epidermis. J Invest Dermatol, 89: 113-118.
Baadsgaard O, Lisby S, Wulf HC, Wantzin GL, & Cooper KD (1989) Rapid
recovery of Langerhans cell alloreactivity, without induction of
autoreactivity, after in vivo ultraviolet A, but not ultraviolet
B exposure of human skin. J Immunol, 142: 4213-4218.
Baadsgaard O, Salvo B, Mannie A, Dass B, Fox DA, & Cooper KD (1990)
In vivo ultraviolet-exposed human epidermal cells activate T
suppressor cell pathways that involve CD4+CD45RA+ suppressor-inducer
T cells. J Immunol, 145: 2854-2861.
Baker D, O'Neill JK, Gschmeissner SE, Wilcox CE, Butter C, & Turk JL
(1990) Induction of chronic relapsing experimental allergic
encephalomyelitis in Biozzi mice. J Neuroimmunol, 28: 261-270.
Balls M, Blaauboer B, Brusick D, Frazier J, Lamb D, Pemberton M,
Reinhardt C, Roberfroid M, Rosenkranz H, Schmid B, Spielmann H,
Stammati A-L, & Walum E (1990) Report and recommendations of the
CAAT/ERGATT workshop on the validation of toxicity test procedures.
ATLA, 18: 313-337.
Bancroft GJ, Shellam GR, & Chalmer JE (1981) Genetic influences on the
augmentation of natural killer (NK) cells during murine
cytomegalovirus infection: Correlation with patterns of resistance. J
Immunol, 126: 988-994.
Bandeira A, Mota-Santos T, Otohara S, Degermann S, Heusser C, Tonegawa
S, & Coutinho A (1990) Localization of gamma/delta cells to the
intestinal epithelium is independent of normal microbial colonization.
J Exp Med, 172: 239-244. 306
Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, & Reynolds CW
(1985) Direct evidence for the role of LGL in the inhibition of
experimental tumor metastases. J Immunol, 134: 2783-2789.
Barnett JB & Rodgers KE (1994) Pesticides. In: Dean JH, Luster MI,
Munson AE, & Kimber I ed. Immunotoxicity and immunopharmacology, 2nd
Ed. New York, Raven Press, pp 191-212.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LS
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39: 263-274.
Barr BBB, Benton EC, McLaren K, Bunney MH, Smith IW, Blessing K,
& Hunter JAA (1989) Human papilloma virus infection and skin cancer in
renal allograft recipients. Lancet, i: 124-129.
Barrett WL, First MR, Aron BS, & Penn I (1993) Clinical course of
malignancies in renal transplant recipients. Cancer, 72: 2186-2189.
Barry TS, Jones DM, Richter CB, & Haynes BF (1991) Successful
engraftment of human postnatal thymus in severe combined immune
deficient (SCID) mice: Differential engraftment of thymic components
with irradiation versus anti-asialo GM-1 immunosuppressive regimens.
J Exp Med, 173: 167-180.
Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, & Penny R (1977)
Depressed lymphocyte function after bereavement. Lancet, i: 834-836.
Bascom R, Naclerio RM, Fitzgerald TK, Kagey-Sobotka A, & Proud D
(1990) Effect of ozone inhalation on the response to nasal challenge
of allergic subjects. Am Rev Respir Dis, 142: 594-601.
Basketter DA, Bremmer JN, Kammuller ME, Kawabata T, Kimber I, Loveless
SE, Magda S, Pal THM, Stringer DA, & Vohr HW (1994) The identification
of chemicals with sensitizing or immunosuppressive properties in
routine toxicology. Food Chem Toxicol, 32: 289-296.
Bates DV & Sizto R (1983) Relationship between air pollutant levels
and hospital admissions in southern Ontario. Can J Public Health,
74: 117-122.
Baumann G, Zenke G, Wenger R, Hiestand P, Quesniaux V, Andersen E, &
Schreier MH (1992) Molecular mechanisms of immunosuppression. J
Autoimmunol, 5 (Suppl A): 67-72.
Bazin H, Xhurdebise LM, Burtonboy G, Lebacq AM, de Clerq L, & Cormont
F (1984) Rat monoclonal antibodies. I. Rapid purification from
in vitro culture supernatants. J Immunol Meth 66: 261-269.
Behrendt H, Krämer U, Dolgner R, Hinrinchs J, Willer H, Hagenbeck H, &
Schlipköter HW (1993) Elevated levels of total serum IgE in East
German children: Atopy, parasites and pollutants. J Allergol, 2:31.
Behrendt H, Friedrichs K-H, Krämer U, Hitzfeld B, Becker W-M, & Ring J
(1994) The role of indoor and outdoor air pollution in allergic
diseases. In: Vos JG, Younes M, & Smith E ed. Allergic
hypersensitivities induced by chemicals: Recommendations for
prevention. Boca Raton, Florida, CRC Press, pp 173-182.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Roboz JP, Fischbein A, & Mason P (1987) Immunotoxicology,
environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the State of Michigan. Cancer Detect
Prev, Suppl 1: 29-37.
Bélisle C & Sainte-Marie G (1981) Tridimensional study of the deep
cortex of the rat lymph node. II. Relation of deep cortex units to
afferent lymphatic vessels. Anat Rec, 199: 61-72.
Bell EB (1989) Thymus-derived and non-thymus-derived T-like cells. The
origin and function of cells bearing gd receptors. Thymus, 14: 3-17.
Bencko V, Wagner V, Wagnerova M, & Ondrejcak K (1990) Immunological
profiles in workers occupationally exposed to inorganic mercury. J Hyg
Epidemiol Microbiol Immunol, 34: 9-14.
Bentwich Z, Bianco N, Jager L, Houba V, Lambert PH, Knaap W, Rose N,
Seligman M, Thompson R, Torrigiani G, & de Weck A (1982) Use and abuse
of laboratory tests in clinical immunology: Critical considerations of
eight widely used diagnostic procedures. report of a Joint IUIS/WHO
Meeting on Assessment of Tests Used in Clinical Immunology. Clin
Immunol Immunopathol, 24: 122-138.
Bentwich Z, Beverley PCL, Hammarstrom L, Kalden JR, Lambert PH, Rose
NR, & Thompson RA (1988) Laboratory investigations in clinical
immunology: Methods, pitfalls, and clinical indications. Clin Immunol
Immunopathol 49: 478-497.
van den Bergh P, Rozing J, & Nagelkerken L (1991) Opioid peptides as
cytokines in T cell activation. Adv Neuroimmunol, 1: 189-203.
van den Bergh P, Rozing J, & Nagelkerken L (1993a) Identification of
two moieties of ß-endorphin with opposing effects on rat T-cell
proliferation. Immunology, 79: 18-23.
van den Bergh P, Rozing J, & Nagelkerken L (1993b) b-Endorphin
stimulates Ia expression on mouse B cells by inducing interleukin-4
secretion by CD4+ cells. Cell Immunol, 149: 180-192
van den Bergh P, Dobber R, Ramlal S, Rozing J, & Nagelkerken L (1994)
Role of opioid peptides in the regulation of cytokine production by
murine CD4+ T cells. Cell Immunol, 154: 109-122.
Bergman A, Olsson M, & Reiland S (1992) Skull-bone lesions in the
Baltic grey seal (Halichoerus grypus). Ambio, 21: 517-519.
Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F ed. (1987)
Immunotoxicology. Dordrecht, Martinus Nijhoff Publishers, pp xi-xxvii.
Bernier J, Brousseau P, Krzystyniak K, Tryphonas H, & Fournier M (in
press) Immunotoxicity of heavy metals in relation to Great Lakes.
Environ Health Perspectives, Suppl 9.
Bernstein A & Breitman M (1989) Genetic ablation in transgenic mice.
Mol Biol Med, 6: 523-530.
Beschorner WE, Namnoun JD, Hess AD, Shinn CA, & Santos GW (1987a)
Cyclosporin A and the thymus immunopathology. Am J Pathol,
126: 487-496.
Beschorner, WE, Di Gennaro KA, Hess AD, & Santos GW (1987b)
Cyclosporine and the thymus: Influence of irradiation and age on
thymic immunopathology and recovery. Cell Immunol, 110: 350-364.
Bevilacqua MP (1993) Endothelial-leukocyte adhesion molecules. Ann Rev
Immunol, 11: 767-804.
Bick PH, Tucker AN, White KL, & Holsapple MP (1984) Effects of
subchronic exposure to diethylstilbestrol on humoral immune function
in adult female (C3B6)F1 mice. Immunopharmacology, 7: 27-39.
Bigazzi PE (1992) Lessons from animal models: The scope of mercury-
induced autoimmunity. Clin Immunol Immunopathol 65: 81-84.
Biron CA, Byron KS, & Sullivan JL (1989) Severe herpesvirus infection
in an adolescent without natural killer cells. New Engl J Med,
320: 1731-1735.
Bissonette E, Dubois C, & Rola-Pleszczynski M (1989) Changes in
lymphocyte function and lung histology during the development of
asbestosis and silicosis in the mouse. Res Commun Chem Pathol
Pharmacol, 65: 211-227.
Black RD, McVey DS, & Oehme FW (1992) Immunotoxicity in the bovine
animal: A review. Vet Hum Toxicol, 34: 438-442.
Blackley BR, Sisodia CS, & Mukkur TK (1980) The effects of
methylmercury, tetraethyllead and sodium arsenite on the humoral
immune response in mice. Toxicol Appl Pharmacol, 52: 245-254.
Blackmann M, Kappler J, & Marrack P (1990) The role of the T cell
receptor in positive and negative selection of developing T cells.
Science, 248: 1335-1341.
Blatt J, Olshan A, Gula MJ, Dickman PS, & Zarenek B (1992) Second
malignancies in very long-term survivors of childhood cancer. Am J
Med, 93: 57-60.
Blaylock BL, Holladay SD, Comment CE, Heindel JJ, & Luster MI (1992)
Exposure to tetrachlorodibenzo- p-dioxin (TCDD) alters fetal
thymocyte maturation. Toxicol Appl Pharmacol, 112: 207-213.
Blazer VS, Wolke RE, Brown J, & Powell CA (1987) Piscine macrophage
aggregate parameters as health monitors: Effects of age, sex, relative
weight, season and site quality in largemouth bass (Micropterus
salmoides). Aquat Toxicol, 10: 199-215.
Bleavins MR & Alvey JD (1991) Immunotoxicologic evaluation of a purine
nucleoside phosphorylase inhibitor in the cynomolgus monkey.
Toxicologist, 11: 205.
Bleavins MR & Dziedzic D (1990) An immunofluorescence study of T and B
lymphocytes in ozone-induced pulmonary lesions in the mouse. Toxicol
Appl Pharmacol, 105: 93-102.
Blok WL, Vogels MT, Curfs JH, Eling WM, Buurman WA, & Van Der Meer JW
(1992) Dietary fish-oil supplementation in experimental Gram negative
infection and in cerebral malaria in mice. J Infect Dis, 165: 898-903.
Bloom BR, Salgame P, & Diamond B (1992) Revisiting and revising
suppressor T cells. Immunol Today, 131: 131-136.
Blusse Van Oud Alblas A, & Van Furth R (1979) Origin, kinetics and
characteristics of pulmonary macrophages in the normal steady state.
J Exp Med, 149: 1504-1518.
Bly JE, Miller NW, & Clem LW (1990) A monoclonal antibody specific for
neutrophils in normal and stressed channel catfish. Dev Comp Immunol,
14: 211-221.
Bodey GP, Fainstein V, & Guerrant R (1986) Infections of the
gastrointestinal tract in the immunocompromised patient Ann Rev Med,
37: 271-281.
Bohus B, Koolhaas JM, De Ruiter AJH, & Heijnen CJ (1991) Stress and
differential alterations in immune system functions: Conclusions from
social stress studies in animals. Neth J Med, 39: 306-315.
Boivin JF (1990) Second cancers and other late side-effects of cancer
treatment: A review. Cancer, 65: 770-775.
Bolas-Fernandez F, Grencis RK & Wakelin D (1988) Cyclosporin A and
Trichinella spiralis: Anthelminthic effects in immunosuppressed
mice. Parasite Immunol, 10: 111-116.
Bonnyns M & Bastomsky CH (1976) Polychlorinated biphenyl-induced
modifica-tion of lymphocyte response to plant mitogens in rats.
Experientia, 15: 522-523.
Booss J (1980) Establishment of cytomegalovirus infection in mice:
Role of a macrophage-enriched subpopulation. J Infect Dis,
141: 466-472.
Borleffs JCC, Schuurman H-J, Vrehen HM, Van Schaik M, & Bast EJEG
(1993) in vitro and in vivo measurement of cell-mediated immunity
in patients with HIV-1-infection. Scand J Immunol, 37: 634-636.
Boros DL (1988) Mechanisms of granuloma formation in the lung.
In: Daniele RP ed. Immunology and immunologic diseases of the lung.
Boston, Blackwell Scientific Publications, pp 263-287.
Bos J ed. (1990) The skin immune system. Boca Raton, Florida, CRC
Press.
Bos JD & Kapsenberg ML (1986) The skin immune system (SIS): Its
cellular constituents and their interactions. Immunol Today,
7: 235-240.
Bouwes Bavinck JN, de Boer A, Vermeer BJ, Hartevelt MM, van der Woude
FJ, Claas FHJ, Wolterbeek R, & Vandenbroucke JP (1993) Sunlight,
keratotic skin lesions and skin cancer in renal transplant recipients.
Br J Dermatol, 129: 242-249.
Boyce WT, Jensen EW, Cassel JC, Collier AM, Smith AH, & Ramey CT
(1977) Influence of life events and family routines on childhood
respiratory tract illness. Pediatrics, 60: 609-615.
Boyd RL, Wilson TJ, Bean AG, Ward HA, & Gershwin ME (1992) Phenotypic
characterization of chicken thymic stromal elements. Dev Immunol,
2: 51-66.
Boyle J, Mackie RM, Briggs JD, Junor BJR, & Aitchison TC (1984)
Cancer, warts, and sunshine in renal transplant patients: A case-
control study. Lancet, i: 702-705.
Bradley SG & Morahan PS (1982) Approaches to assessing host
resistance. Environ Health Perspectives, 43: 61-69.
Brekelmans P & Van Ewijk W (1990) Phenotypic characterization of
murine thymic microenvironments. Sem Immunol, 2: 13-24.
Brett SJ & Butler R (1986) Resistance to Mycobacterium lepraemurium
is correlated with the capacity to generate macrophage activating
factor(s) in response to mycobacterial antigens in vitro.
Immunology, 59: 339-345.
Brideau RJ, Carter PB, McMaster WR, Mason DW, & Williams AF (1980) Two
subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J
Immunol, 10: 609-615.
Brigham KL (1989) Mediators in inflammation. In: Henderson PM & Murphy
RC ed. Handbook of inflammation, Vol. 6: Mediators of the inflammatory
process. Amsterdam, Elsevier Science Publishers, pp 1-14.
Brodie AM & Halliday WJ (1991) Suppressor cells and soluble factors
from the spleens of UV-irradiated mice. Immunol Cell Biol,
69: 199-204.
Brostoff J, Scadding GK, Male D, & Roitt IM (1991) Clinical
immunology. London, Gower Medical Publishers.
Brouwer A, Reijnders PJH, & Koeman JH (1989) Polychlorinated biphenyl
(PCB)-contaminated fish induces vitamin A and thyroid hormone
deficiency in the common seal (Phoca vitulina). Aquat Toxicol,
15: 99-106.
Brown WR & Kloppel TM (1989) The liver and IgA: Immunological, cell
biological and clinical implications. Hepatology, 9: 763-778.
Bruggeman CA, Debie WMH, Grauls G, Majoor G, & Boven CPA (1983)
Infection of laboratory rats with a new cytomegalo-like virus. Arch
Virol, 76: 189-199.
Bruggeman CA, Meijer H, Bosman F, & Boven CPA (1985) Biology of rat
cytomegalovirus. Intervirology, 24: 1-9.
Bruning JH (1985) Cytomegalovirus infections in renal transplantation;
study in a rat model. PhD thesis, Utrecht, Utrecht University.
Bucke D, Vethaak AD, & Lang T (1992) Quantitative assessment of
melanomacrophage centers (MMC's) in dab (Limanda limanda) along a
pollution transect in the German Bight. Mar Ecol Prog Ser,
91: 193-196.
Buckingham JC, Safieh B, Singh S, Arduino LA, Cover PO, & Kendall MD
(1992) Interactions between hypothalamo-pituitary adrenal axis and the
thymus in the rat: A role for corticotropin in the control of thymulin
release. J Neuroendocrinol, 4: 295-301.
Buening GM, Mann DD, Hook B, & Osweiler BP (1982) The effect of T-2
toxin on the bovine immunity system: Cellular factors. Vet Immunol
Immunopathol, 3: 411-417.
Bugelski PJ, Thiem PA, Solleveld HA, & Morgan DG (1990) Effects of
sensitization to dinitrochlorobenzene (DNCB) on clinical pathology
parameters and mitogen-mediated blastogenesis in cynomolgus monkeys
(Macaca fascicularis). Toxicol Pathol, 18: 643-650.
Bukowski JF, Woda BA, & Welsh RM (1984) Pathogenesis of murine
cytomegalovirus infection in natural killer cell-depleted mice.
J Virol, 52: 119-128.
Bulloch K (1987) The innervation of immune system tissues and organs.
In: Cotman CW, Brinton RE, Galaburda A, McEwen B, & Schneider DM ed.
The neuro-immune-endocrine connection. New York, Raven Press,
pp 33-47.
Burcham J, Marmor M, Dubin N, Tindall B, Cooper DA, Berry G, & Penny R
(1991) CD4+ is the best predictor of development of AIDS in a cohort
of HIV-infected homosexual men. AIDS, 5: 365-372.
Burchiel SW, Thompson TA, & Davis AP (1991) Alterations in mitogen-
induced calcium mobilization and intracellular free calcium produced
by 7,12-dimethylbenz (a)anthracene in the Jurkat human T cell line.
Int J Immunopharmacol, 13: 109-115.
Burleson GR, Fuller LB, Menache MG, & Graham JA (1987) Poly(I):
poly(C)-enhanced alveolar and peritoneal macrophage phagocytosis:
Quantification by a new method utilizing fluorescent beads. Proc Soc
Exp Biol Med, 184: 468-476.
Burleson GR, Keyes LL, & Stutzman JD (1989) Immunosuppression of
pulmonary natural killer activity by exposure to ozone.
Immunopharmacol Immunotoxicol, 11: 715-735.
Burleson GR, Lebrec H, Yang YG, lbanes JD, Pennington KN, & Birnbaum
LS (in press) Effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)
on influenza virus host resistance in mice. Fundam Appl Toxicol.
Calder VL & Lightman SL (1992) Experimental autoimmune uveoretinitis
(EAU) versus experimental allergic encephalomyelitis (EAE): A
comparison of T cell-mediated mechanisms. Clin Exp Immunol,
89: 165-169.
Cantacuzene MJ (1898) Nouvelles recherches sur le mode de destruction
des virions dans l'organisme. Ann Inst Pasteur, 12: 274-300.
Cantrell DA, Collins MKL, & Crumpton DA (1988) Autocrine regulation of
T-lymphocyte proliferation: Differential induction of IL-2 and IL-2
receptor. Immunology, 65: 343-349.
Capurro PU (1980) The effects of solvents and pesticides on health.
Clin Toxicol, 16: 549-553.
Carter JW & Clancy J (1980) Acutely administered polychlorinated
biphenyls (PCBs) decrease splenic cellularity but increase its
ability to cause graft-versus-host reactions in BALB/c mice.
Immunopharmacology, 2: 341-347.
Casale GP, Cohen SD, & DiCapua RA (1983) The effects of
organophosphate-induced cholinergic stimulation on the antibody
response to sheep erythrocytes in inbred mice. Toxicol Appl
Pharmacol, 68: 198-205.
Casale GP, Cohen SD, & DiCapua RA (1984) Parathion-induced suppression
of humoral immunity in inbred mice. Toxicol Lett, 23: 239-247.
Castillo-Mendez A, Rodriguez-Diaz T, Leon-Lobeck A, & Gravalosa-Cruz
AJ (1991) Influence of occupational lead exposure on the concentration
of immunoglobins and immune cellular functions in human. Rev Allergol
Mex, 38: 69.
Centers for Disease Control (1991) Summary of notifiable diseases,
United States, 1990. Morb Mortal Weekly Rep, 39 (53).
Challacombe SJ (1987) The investigation of secretory and systemic
immune responses to ingested material in animal models. In: Miller K
& Nicklin S ed. Immunology of the gastrointestinal tract, Vol 2. Boca
Raton, Florida, CRC Press, pp 99-124.
Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, &
Hiseroth JC (1989) Monoclonal antibody to a triggering structure
expressed on rat natural killer cells and adherent lymphokine-
activated killer cells. J Exp Med 169: 1373-1389.
Chan AC, Irving BA, & Weiss A (1992) New insights into T-cell antigen
receptor structure and signal transduction. Curr Opin Immunol,
4: 246-251.
Chanarin I (1985) Structure and function of the bone marrow. In: Irons
RD ed. Toxicology of the blood and bone marrow. New York, Raven Press,
pp 1-16.
Chandra RK (1992) Nutrition and immunoregulation. Significance of host
resistance to tumors and infectious diseases in humans and rodents.
J Nutr, 122 (Suppl): 754-757.
Chandra RK (1993) Nutrition and the immune system. Proc Nutr Soc,
52: 77-84.
Chang KH, Hsieh KH, Lee TP, Tang SY, & Tung TC (1981) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Determination of lymphocyte subpopulations. Toxicol Appl Pharmacol,
61: 58-63.
Chang KH, Hsieh KH, Tang SY, Tung TC, & Lee TP (1982) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Evaluation of delayed-type skin hypersensitivity response and its
relation to clinical studies. J Toxicol Environ Health, 9: 217-223.
Clark EA & Lanier LL (1989) Report from Vienna: In search of all
surface molecules expressed on human leukocytes. J Clin Immunol,
9: 265-272.
Clark GC, Blank JA, Germolec DR & Luster MI (1991)
2,3,7,8-Tetrachlorodibenzo- p-dioxin stimulation of tyrosine
phosphorylation in B lymphocytes: Potential role in
immunosuppression. Mol Pharmacol, 39: 495-501.
Clarke AG (1984) Immunological studies on pregnancy in the mouse.
In: Crighton DB ed. Immunological aspects of reproduction in mammals.
London, Butterworths, pp 153-182.
Clarke AG & Kendall MD (1989) Histological changes in the thymus
during mouse pregnancy. Thymus, 14: 65-78.
Clevers H, Alarcon B, Wileman T, & Terhorst C (1988) The T cell
receptor/CD3 complex: A dynamic protein ensemble. Ann Rev Immunol,
6: 629-662.
Clover RD, Abell T, Becker LA, Crawford S, & Ramsey CN (1989) Family
functioning and stress as predictors of influenza B infection.
J Family Practice, 28: 535-539.
Coffin DL & Gardner DE (1972) Interaction of biological agents and
chemical air pollutants. Ann Occup Hyg, 15: 219-234.
Cohen N, Modai D, Golik A, Weissgarten J, Peller S, Katz A, Averbuhk
Z, & Shaked U (l989) Increased concanavalin A-induced suppressor cell
activity in humans with occupational lead exposure. Environ Res,
48: 1-6.
Coligan JE, Kruisbeek, AM, Margulies DH, Shevach EM, & Strober W ed.
(1994) Current protocols in immunology, Vols 1 and 2. New York, John
Wiley & Sons.
Cook JA, Marconi EA, & DiLuzio NR (1974) Lead, cadmium, endotoxin
interaction: Effect on mortality and hepatic function. Toxicol Appl
Pharmacol, 28: 292.
Cook JA, Hoffman EO, & DiLuzio NR (1975) Influence of lead and cadmium
on the susceptibility of rats to bacterial challenge (39117). Proc Soc
Exp Biol Med, 150: 741.
Cook JC, Gaido KW, receptor: & Greenlee WF Relevance of (1987) Ah
mechanistic studies to human risk assessment. Environ Health
Perspectives, 76: 71-77.
Cooper EL (1982) How the immune system evolved. In: General
immunology. Oxford, Pergamon Press, pp 279-323.
Cooper KD, Neises GR, & Katz SI (1986) Antigen-presenting OKM5+
melanophages appear in human epidermis after ultraviolet radiation.
J Invest Dermatol, 86: 363-370.
Cooper KD, Oberhelman BS, Hamilton MS, Baadsgaard O, Terhune M, Levee
G, Anderson T, & Koren H (1992) UV exposure reduces immunization rates
and promotes tolerance to epicutaneous antigens in humans;
relationship to dose, CDla DR+ epidermal macrophage inductlon and
Langerhans cell depletion. Proc Natl Acad Sci USA, 89: 8497-8501.
Corman LC (1985) Effects of specific nutrients on the immune response.
Selected clinical applications. Med Clin North Am, 69: 759-791.
Cornacoff JB, Tucker AN, & Dean JH (1988) Development of a human
peripheral blood mononuclear leucocyte model for immunotoxicity
evaluation. In Vitro Toxicol, 2: 81-89.
Corrier DE (1992) Mycotoxicosis: Mechanisms of immunosuppression. Vet
Immunol Immunopathol, 30: 73-87.
Corsini E, Luster MI, Mahler J, Craig WA, Blazka M, & Rosenthal GJ
(1994) A protective role for T-lymphocytes in asbestos-induced
pulmonary inflammation and collagen deposition. Am J Respir Cell Mol
Biol, 7: 60-82.
Coscia GC, Discalzi G, & Ponzetti C (1987) Immunological aspects of
occupational lead exposure. Med Lav, 78: 360-364.
Council for International Organizations of Medical Sciences (1993)
International ethical guidelines for biomedical research involving
human subjects. Geneva, 63 pp.
Cunningham AJ & Szenberg A (1968) Further improvements in the plaque
tech-nique for detecting single antibody-forming cells. Immunology,
14: 559-600.
Dabrowski MP & Dabrowski-Bernstein BK (1990) Immunoregulatory role of
the thymus. Boca Raton, Florida, CRC Press.
Dallman MJ, Thomas ML, & Green JR (1984) MRC OX-19: A monoclonal
antibody that labels rat T lymphocytes and augments in
vitroproliferative responses. Eur J Immunol, 14: 260-267.
Davis AP & Burchiel SW (1992) Inhibition of calcium-dependent pathways
of B-cell activation by DMBA. Toxicol Appl Pharmacol, 116: 202-208.
Davis D & Safe S (1988) Immunosuppressive activities of
polychlorinated dibenzofuran congeners: Quantitative structure-
activity relationships and interactive effects. Toxicol Appl
Pharmacol, 94: 141-149.
Davis D & Safe S (1989) Dose-response immunotoxicities of commercial
polychlorinated biphenyls (PCBs) and their interaction with
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol Lett, 48: 35-43.
Davis D & Safe S (1991) Halogenated aryl hydrocarbon-induced
suppression of the in vitro plaque-forming cell response to
sheep red blood cells is not dependent on the Ah receptor.
Immunopharmacology, 21: 183-190.
Dayan AD, Hertel RF, Heseltine E, Kazantis G, Smith EM, & Van der
Venne MT ed. (1990) Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press.
Dean JH & Murray MJ (1990) Toxic responses of the immune system.
In: Klaassen CD, Amdur MO, & Doull J ed. Toxicology: The basic science
of poisons, Vol 4. New York, MacMillan Press, pp 282-333.
Dean JH & Thurmond LM (1987) Immunotoxicology: An overview. Toxicol
Pathol, 15: 265-271.
Dean JH, Luster MI, & Boorman GA (1982) Immunotoxicology. In: Sirois
P, & Pleszczynski MR ed. Immunopharmacology. Amsterdam, Elsevier
Science Publishers, pp 349-397.
Dean JH, Luster MI, Munson AE, & Amos HA (1985) Immunotoxicology and
immunopharmacology. New York, Raven Press.
Dean JH, Ward EC, Murray MJ, Lauer LD, & House RV (1986)
Immunosuppression following 7,12-dimethylbenz-a-anthracene exposure
in B6C3F1 mice. II. Altered cell-mediated immunity and tumor
resistance. Int J Immunopharmacol, 8: 189-198.
Dean JH, Luster MI, Munson AE, & Kimber I (1994) Immunotoxicology and
immunopharmacology, 2nd Ed. New York, Raven Press.
De Gast GC, Verdonck LF, Middeldorp JM, The TH, Hekker A, Van der
Linden JA, Kreeft HA, & Bast BJ (1985) Recovery of T cell subsets
after autologous bone marrow transplantation is mainly due to
proliferation of mature T cells in the graft. Blood, 66: 428-431.
De Heer C, Verlaan APJ, Penninks AH, Schuurman HJ, & Van Loveren H
(1993) The SCID-RA mouse: Rat T cell differentiation in the severe
combined immunodeficient mouse. APMIS, 101: 467-479.
Denis M (1992) Mouse hypersensitivity pneumonitis: Depletion of NK
cells abrogates the spontaneous regression phase and leads to massive
fibrosis. Exp Lung Res, 18: 761-773.
Denis M, Bisson D, & Ghardirian E (1993) Cellular and cytokine
profiles in spontaneous regression phase of hypersensitivity
pneumonitis. Exp Lung Res, 19: 257-271.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
Deo MG, Gangal S, Bhisey AN, Somasundaram R, Balsara Gulwani B,
Darbaru BS, Bhiide S, & Maru B (1987) Immunological, mutagenic and
genotoxic investigations in gas exposed population of Bhopal. Indian
J Med Res, 86 (Suppl): 63-76.
Descotes J (1985) Immunomodulating agents and hepatic drug metabolism.
Drug Metab Rev, 16: 175-183.
Descotes J (1986) Immunotoxicology of drugs and chemicals. Amsterdam,
Elsevier Science Publishers.
Descotes J (1990) Drug-induced immune diseases. Amsterdam, Elsevier
Science Publishers.
Descotes J (1992) Immunomodulating agents. In: Dukes MNG ed. Meyler's
side-effects of drugs, 12th Ed. Amsterdam, Elsevier Science
Publishers, pp 954-958.
Descotes J & Vial T (1994) Cytoreductive drugs. In: Dean JH, Luster
MI, Munson AE, & White K ed. Immunotoxicology and immunopharmacology,
2nd Ed. New York, Raven Press, pp 293-301.
Descotes J, Verdier F, Brouland JP, & Pulce C (1990) Immunotoxicity of
lead, cadmium and arsenic: Experimental data and their relevance to
man. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM, &
Van der Venne, M-T ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, pp 209-213.
Desi L, Varga L, & Farkas I (1978) Studies on the immunosuppressive
effect of organochlorine and organophosporic pesticides in subacute
experiments. J Hyg Epidemiol Microbiol Immunol, 22: 115-122.
Desi L, Varga L, Dobronyi L, & Szklenarik G (1985) Immunotoxicological
investigation of the effects of a pesticide; cypermethrin. Arch
Toxicol, Suppl 8: 305-309.
De Swart RL, Kluten RMG, Huizing CJ, Vedder LJ, Reijnders PJH, Visser
IKG, UytdeHaag FGCM, & Osterhaus ADME (1993) Mitogen and antigen
induced B and T cell responses of peripheral blood mononuclear cells
from the harbour seal (Phoca vitulina). Vet Immunol Immunopathol,
37: 217-230.
De Swart RL, Ross PS, Vedder EJ, Timmerman HH, Heisterkamp SH, Van
Loveren H, Vos JG, Reijnders PJH, & Osterhaus ADME (1994) Impairment
of immune function in harbour seals (Phoca vitulina) feeding on fish
from polluted waters. Ambio, 23: 155-159.
Devens BH, Grayson MH, Imamura IK, & Rodgers KE (1985)
O,O,S-Trimethyl-phosphorothioate effect on immunocompetence.
Pestic Biochem Physiol, 24: 251-259.
Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers
D, & Koren H (1991) Exposure of humans to ambient levels of ozone for
6.6 hours causes cellular and biochemical changes in the lung.
Am J Resp Cell Mol Biol, 4: 72-81.
De Waal EJ, Rademakers LHPM, Schuurman H-J, & Van Loveren H (1992a)
Interdigitating cells in the rat thymus during cyclosporin A
treatment: Ultrastructural observations. Thymus, 20: 163-170.
De Waal EJ, Schuurman H-J, Loeber JG, Van Loveren H, & Vos JG (1992b)
Alterations in the cortical thymic epithelium of rats after
in vivo exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD):
An (immuno)histological study. Toxicol Appl Pharmacol, 115: 80-88.
De Waal EJ, Rademakers LHPM, Schuurman H-J, Van Loveren H, & Vos JG
(1993) The cortical epithelium of the rat thymus after in vivo
exposure to bis(tri-n-butyltin)oxide (TBTO). Arch Toxicol,
67: 186-192.
DeWailly E, Nantel A, Brunean S, Laliberte C, Ferron L, & Gingras S
(1992) Breast milk contamination by PCDDs, PCDFs, and PCBs in Arctic
Quebec: A preliminary assessment. Chemosphere, 25: 1245-1249.
De Weger RA, Van Loveren H, Vandebriel RJ, & Parmentier HK (1989).
Sequential activity of gamma-delta T-cell receptor bearing
lymphocytes, specific T-cell factors, and alpha-ß T-cell receptor
bearing T-cells: A hypothesis. Thymus, 14: 19-30.
Dieter MP, Luster MI, Boorman GA, Jameson CW, Dean JH, & Cox JW (1983)
Immunological and biochemical responses in mice treated with mercuric
chloride Toxicol Appl Pharmacol, 68: 218-228.
Dietert RR, Qureshi MA, Nanna UC, & Bloom SE (1985) Embryonic exposure
to aflatoxin-B1: Mutagenicity and influence on development and
immunity. Environ Mutag, 7: 715-725.
Dietz R, Heide-Jorgensen MP, & Härkönen T (1989) Mass deaths of harbor
seals (Phoca vitulina) in Europe. Ambio, 18: 258-264.
Dijkstra CD & Sminia T (1990) Normal anatomy, histology,
immunohistology, ultrastructure, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI monograph). Berlin, Springer
Verlag, pp 185-193.
Dijkstra CD, Döpp EA, Joling P, & Kraal G (1985) The heterogeneity of
mononuclear phagocytes in lymphoid organs: Distinct macrophage
subpopu-lations in rat recognized by monoclonal antibodies ED1, ED2
and ED3. Immunology, 54: 589-599.
Dinarello CA (1992) Role of interleukin-1 in infectious diseases.
Immunol Rev, 127: 119-146.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979a) Role of
macrophages in the augmentation of mouse natural killer activity by
polyI:C and interferon. J Immunol, 122: 182-188.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979b) Augmentation
of mouse natural killer cell activity by interferon and interferon
inducers. J Immunol, 122: 175-181.
Dobber R, Hertog-Huijbregts A, Rozing J, Bottomly K, & Nagelkerken L
(1992) The involvement of the intestinal microflora in the expansion
of CD4+ T cells with a naive phenotype in the periphery. Dev
Immunol, 2: 141-150.
Dogra RK, Chandra K, Chandra S, Gupta S, Khanna S, Srivastava SN,
Shukla LJ, Katiyar JC, & Shanker R (1992) Host resistance assays as
predictive models in styrene immunomodulation. Int J Immunopharmacol,
14: 1003-1009.
Domzig W, Stadler BM, & Herberman RB (1983) Interleukin-2 dependence
of human natural killer (NK) cell activity. J Immunol, 130: 1970-1973.
Dooley RK & Holsapple MP (1988) Elucidation of cellular targets
responsible for tetrachlorodibenzo- p-dioxin (TCDD)-induced
suppression of antibody responses: I. The role of the B lymphocyte.
Immunopharmacology, 16: 167-180.
Dotta F & Eisenbarth GS (1989) Type I diabetes mellitus: A predictable
autoimmune disease with interindividual variation in the rate of
ß cell destruction. Clin Immunol Immunopathol, 50: S85-S95.
Driscoll KE, Higgins JM, Leytart MJ, & Corsby LL (1990) Differential
effects of mineral dusts on the in vitro activation of alveolar
macrophage eicosanoid and cytokine release. Toxicity in vitro,
4, 284-288.
Drouet M, Le-Sellin J, Bonneau JC, & Sabbah A(1990) Is mercury an
allergen of the respiratory tract. Allerg Immunol (Paris),
22: 81, 84-85, 87.
Druet P, Pelletier L, Rossert J, Druet E, Hirsch F, & Sapin C (1989)
Autoimmune reactions induced by metals. In: Kammüller ME, Bloksma N,
& Seinen W ed. Autoimmunity and toxicology. Immune disregulation
induced by drugs and chemicals. Amsterdam, Elsevier Science
Publishers, pp 347-361.
Duijvestijn A & Hamann A (1989) Mechanisms and regulation of
lymphocyte migration. Immunol Today, 10: 23-28.
Duke SS, Schook LB, & Holsapple MP (1985) Effects of
N-nitrosodimethylamine on tumor susceptibility. J Leukocyte
Biol, 37: 383-394.
Dunn TB (1954) Normal and pathologic anatomy of the reticular tissue
in laboratory mice, with a classification and discussion of neoplasms.
J Natl Cancer Inst, 14: 1281-1433.
Du Pasquier L (1989) Evolution of the immune system. In: Paul WE ed.
Fundamental immunology, 2nd Ed. New York, Raven Press, pp 139-165.
Durham WH (1974) Air pollution and student health. Arch Environ
Health, 28: 241-254.
Dutch Health Council, Committee for Immunotoxicology (1991)
Immunotoxicity of compounds. The Hague.
Dyall-Smith C & Varigos G (1985) The malignant potential of
papillomavirus. Aust J Dermatol, 26: 102-107.
Dziedzic D & White HJ (1986) T-Cell activation in pulmonary lymph
nodes of mice exposed to ozone. Environ Res, 41: 610-622.
Dziedzic D, Wright ES, & Sargent HE (1990) Pulmonary response to
ozone. Reaction of bronchus-associated lymphoid tissue and lymph node
lymphocytes in the rat. Environ Res, 51: 194-208.
Eedy DJ, Burrows D, Clifford T, & Fay A (1990) Elevated T cell
subpopulations in dental students. J Prosthet Dent, 63: 593.
Ehrke MJ, Mihich E, Berd D, & Mastrangelo MJ (1989) Effects of
anticancer drugs on the immune system in humans. Semin Oncol,
16: 230-253.
Ehrlich JP & Burleson GR (1991) Enhanced and prolonged pulmonary
influenza virus infection following phosgene inhalation. J Toxicol
Environ Health, 34: 259-273.
Ehrlich R & Henry MC (1968) Chronic toxicity of nitrogen dioxide.
I. Effect on resistance to bacterial pneumonia. Arch Environ Health,
17: 860-865.
Ehrlich R, Findlay JC, Fonters JD, & Gardner DE (1977) Health effects
of short-term inhalation of nitrogen dioxide and ozone mixtures.
Environ Res, 4: 223-231.
Ellman MH, Hurwitz H, Thomas C, & Kozloff M (1991) Lymphoma developing
in a patient with rheumatoid arthritis taking low dose weekly
methotrexate. J Rheumatol, 18: 1741-1743.
Elo O, Vuojolahti P, Janhunen R, & Rantanen J (1985) Recent PCB
accidents in Finland. Environ Health Perspectives, 60: 315-319.
Emmendorfer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML,
& Roesler J (1994) Evaluation of flow cytometric methods for diagnosis
of chronic granulomatous disease variants under routine laboratory
conditions. Cytometry, 18: 147-155.
Engelhard VH (1994) Structure of peptides associated with class I and
class II MHC molecules. Ann Rev Immunol, 12: 181-207.
Engleman EG, Benike CJ, Grumet FC, & Evans RL (1981) Activation of
human T lymphocyte subsets: Helper and suppressor/cytotoxic T cells
recognize and respond to distinct histocompatibility antigens.
J Immunol, 127: 2124-2129.
Epstein JH, Tuffanelli DL, & Dubois EL (1965) Light sensitivity and
lupus erythematosus. Arch Dermatol, 91: 483-485.
Eskola J & Toivanen P (1974) Effect of in ovo treatment with
cyclophosphamide on lymphoid system in chicken. Cell Immunol,
13: 459-471.
Evans RG, Webb KB, Knutsen AP, Roodman ST, Roberts DW, Bagby JR,
Garrett WA, & Andrews JS (1988) A medical follow-up of the health
effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. Arch Environ Health, 43: 273-278.
Ewers U, Stiller-Winkler R, & Idel H (1982) Serum immunoglobulin,
complement C3, and salivary IgA levels in lead workers. Environ Res,
29: 351-357.
Exon JR (1985) Effect of lead, polychlorinated biphenyls and
cyclophosphamide on rat natural killer cells, interleukin 2, and
antibody synthesis. Fundam Appl Toxicol, 5: 158-164.
Ezine S, Weissman IL, & Rouse RV (1984) Bone marrow cells give rise to
distinct cell clones within the thymus. Nature, 309: 629-631.
Fabry Z, Raine CS, & Hart MN (1994) Nervous tissue as an immune
compartment: Dialect of the immune response in the CNS. Immunol Today,
15: 218-224.
Faisal M & Hugget RJ (1993) Effects of polycyclic aromatic
hydrocarbons on the lymphocyte mitogenic response in spot, Leiostomus
xanthurus. Mar Environ Res, 35: 121-124.
Faisal M, Chiapelli F, Ahmed II, Cooper EL, & Weiner H (1989) Social
confrontation stress in aggressive fish is associated with an
endogenous opioid-mediated suppression of proliferative response to
mitogens and nonspecific cytotoxicity. Brain Behav Immunol,
3: 223-233.
Fänge R & Nilsson S (1985) The fish spleen: Structure and function.
Experientia, 41: 152-158.
Farrell HE & Shellam GR (1991) Protection against murine
cytomegalovirus infection by passive transfer of neutralizing and non-
neutralizing monoclonal antibodies. Infect Immunol, 72: 149-156.
Fauci A, Schnittman SM, & Poli G (1991) Immunopathologenic mechanisms
in human immunodeficiency virus (HIV) infection. Ann Intern Med.,
114: 678-693.
Fellah JS, Wiles MV, Charlemagne J, & Scwager J (1992) Evolution of
vertebrate IgM: Complete amino acid sequence of the constant region of
Ambystoma mexicanum µ chain deduced from cDNA sequence. Eur J
Immunol, 22: 2595-2601.
Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden
KS, Olschowka JA, & Livnat S (1987) Noradrenergic sympathetic neural
interaction with the immune system: Structure and function. Immunol
Rev, 100: 225-260.
Fenters J, Bradof J, Aranyi C, Ketels K, Ehrlich R, & Gardner D (1979)
Health effects of long-term inhalation of sulfuric acid mist-carbon
particle mixtures. Environ Res, 19: 244-257.
Fichtelius KE, Groth O, & Liden S (1970) The skin, a first level
lymphoid organ? Int Arch Allergy, 37: 607-620.
Fidler IJ (1973) Selection of successive tumor lines for metastasis.
Nature New Biol, 242: 148-149.
Fidler IJ, Gersten DM, & Hart IR (1978) The biology of cancer invasion
and metastases. Adv Cancer Res, 28: 149-250.
Fine JS, Gasiewicz TA, & Silverstone AE (1989) Lymphocyte
stem cell alterations following perinatal exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol Pharmacol, 35: 18-25.
Fine JS, Gasiewicz TA, Fiore NC, & Silverstone AE (1990) Prothymocyte
activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo- p-dioxin
exposure. J Exp Pharmacol Ther, 255: 1-5.
Finsen NR (1901) The treatment of lupus vulgaris by concentrated rays.
In: Phototherapy. London, Edward Arnold, pp 73-75.
Fiore MC, Anderson HA, Hong R, Golubjatnokov R, Seiser JE, Nordstrom
D, Hanrahan L, & Belluck D (1986) Chronic exposure to aldicarb-
contaminated groundwater and human immune function. Environ Res,
41: 633-645.
Fischbein A, Tsang P, Luo JC, Roboz JP, Jiang JD, & Bekesi JG (1993)
Phenotypic aberrations of CD3+ and CD4+ cells and functional
impairments of lymphocytes at low-level occupational exposure to lead.
Clin Immunol Immunopathol, 66: 163-171.
Flajnik MF, Canel C, Kramer J, & Kasahara M (1991) Evolution of the
major histocompatibility complex class I from the amphibian Xenopus.
Proc Natl Acad Sci USA, 88: 537-541.
Fleisher TA, Luckasen JR, Sabad A, Gehrtz RC, & Kersey JH (1975) T and
B lymphocyte subpopulations in children. Pediatrics, 55: 162-165.
Fletcher TC (1986) Modulation of non specific host defences in fish.
Vet Immunol Immunopathol, 12: 59-67.
Fletcher FA & Williams DE (1992) Recent progress in the discovery and
invention of novel hematopoietic cytokines. Crit Rev Oncol Hematol,
13: 1-15.
Flick DF, O'Dell RG, & Childs VA (1965) Studies on the chick edema
disease. 3. Similarity of symptoms produced by feeding chlorinated
biphenyls. Poultry Sci, 44: 1460-1465.
Folinsbee LJ (1992) Human health effects of air pollution. Environ
Health Perspectives, 100: 45-56.
Fontana A, Constamm DB, Frei K, Malipiero U, & Pfister HW (1992)
Modulation of the immune response by transforming growth factor beta.
Int Arch Allergy Immunol, 99: 1-7.
Food Safety Council (1978) Proposed system for food safety assessment.
Food Cosmet Toxicol, 16 (Suppl 1): 1-136.
Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, & Gery
I (1987) Experimental autoimmune uveoretinitis (EAU) induced by
retinal interphotoreceptor retinoid-binding protein (IRBP):
Differences between EAU induced by IRBP and by S-antigen. Clin Immunol
Immunopathol, 43: 256-264.
Foxwell BM, Barrett K, & Feldmann M (1992) Cytokine receptors:
Structure and signal transduction. Clin Exp Immunol, 90: 161-169.
Frasca D, Adorini L, & Doria G (1987) Enhanced frequency of mitogen-
responsive T cell precursors in old mice injected with thymosin alpha
1. Eur J Immunol, 17: 727-730.
Frattini JE & Trulock EP (1993) Respiratory infections in
immunocompromised patients. Immunol Allergy Clin North Am,
13: 193-204.
Frazer IH, Collins EJ, Fox JS, Jones B, Oliphant RC, & Mackay IR
(1985) Assessment of delayed-type hypersensitivity in man: A
comparison of the 'Multitest' and conventional intradermal injection
of six antigens. Clin Immunol Immunopathol, 35: 182-190.
Freier S, ed. (1990) The neuroendocrine-immune network. Boca Raton,
Florida, CRC Press.
French JG, Lowrimore G, Nelson WC, Finklea JF, English T, & Hertz M
(1973) The effect of sulfur dioxide and suspended sulfates on acute
respiratory disease. Arch Environ Health, 27: 129-133.
Friedman H, Klein T, & Specter S (1991a) Immunosuppression by
marijuana and its components. In: Ader R, Felten DL, & Cohen N ed.
Psychoneuroimmunology, 2nd Ed. San Diego, Academic Press, pp 931-953.
Friedman H, Specter S, & Klein TW (1991b) Drugs of abuse, immunity and
immunodeficiency (Advances in Experimental Biology and Medicine,
Volume 288). New York, Plenum Press.
Friedmann PS, White SI, Parker S, Moss C, & Mattews JNS (1989)
Antigenic stimulation during ultraviolet therapy in man does not
result in immunological tolerance. Clin Exp Immunol, 76: 68-72.
Friend M & Trainer DO (1970) Polychlorinated biphenyl: Interaction
with duck hepatitis virus. Science, 170: 1314-1316.
Frith CH, Ward JM, Brown RH, Tyler RD, Chandra M, & Stromberg PC (in
press) Proliferative lesions of the hematopoietic system in rats. In:
Streett CS, Burek JD, Hardisty JF, Garner FM, Leiniger JR, Pletcher
JM, & Moch RW ed. Standardized system of nomenclature and diagnostic
criteria. Guides for toxicologic pathology. Washington DC, Society of
Toxicologic Pathologists, Armed Forces Institute of Pathology,
American Registry of Pathology.
Fugmann RA, Aranyi C, Barbera PW, Bradof JN, Gibbons RD, & Fenters JD
(1983) The effects of DES as measured by host resistance and tumor
susceptibility assays in mice. J Toxicol Environ Health, 11: 827-841.
Fujimaki H (1989) Impairment of humoral responses in mice exposed to
nitrogen dioxide and ozone mixtures. Environ Res, 48: 211-217.
Gahmberg CG, Nortamo P, Li R, & Valmu L (1992) Leukocyte cell adhesion
proteins: From molecular dissection to clinical applications. Ann Med,
24: 329-335.
Gaido KW & Wierda D (1987) Suppression of bone marrow stromal cell
function by benzene and hydroquinone is ameliorated by indomethacin.
Toxicol Appl Pharmacol, 89: 378-390.
Gardner DE, Miller FJ, Illing JW, & Kirtz JM (1977) Alterations in
bacterial defense mechanisms of the lung induced by inhalation of
cadmium. Bull Eur Physiopathol Resp, 13: 157-174.
Garssen J, Van der Vliet H, De Klerk A, Dormans JAMA, & Bruggeman CA
(1995) A rat cytomegalovirus infection model as a tool for
immunotoxicity testing. Eur J Pharmacol, 292: 233-281.
Gasiewicz TA & Rucci G (1984) Cytosolic receptor for
2,3,7,8-tetrachloro-dibenzo- p-dioxin Evidence for a homologous
nature among various mammalian species. Mol Pharmacol, 26: 90-98.
Gaumer HS, Doll NJ, Kaimal J, Schuyler M, & Salvaggio JE (1981)
Diminished suppressor cell function in patients with asbestosis.
Clin Exp Immunol, 44: 108.
Germain RN & Margulies DH (1993) The biochemistry and cell biology of
antigen processing and presentation. Ann Rev Immunol, 11: 403-450.
Germolec DR, Yang RSH, Ackermann MF, Rosenthal GJ, Boorman GA, Blair
P, & Luster MI (1989) Toxicology studies of a chemical mixture of 25
groundwater contaminants. II. Immunosuppression in B6C3F1 mice.
Fundam Appl Toxicol, 13: 377-387.
Ghoneum MH, Egami N, Ljiri K & Cooper EL (1986) Effect of
corticosteroids on the thymus of the fish Oryzias latipes. Dev Comp
Immunol, 10: 35-44.
Giannini SH (1990) Effects of UVB on infectious diseases. In: White JC
ed. Global atmospheric change and public health. Amsterdam, Elsevier
Science Publishers, pp 33-45.
Giannini SH (1992) Effects of UV radiation on cutaneous leishmaniasis.
Parasitol Today, 8: 44-48.
Gibson GG, Hubbard R, & Parke DV (1983) Immunotoxicology. London,
Academic Press.
Gilmartin WG, Delong RL, Smith AW, Sweeney JC, De Lappe BW, Risebrough
RW, Griner LA, Dailey MD, & Peakall DB (1976) Premature parturition in
the California sea lion. J Wildl Dis, 12: 104-115.
Gilmour MI & Selgrade MJK (1993) A comparison of the pulmonary
defenses against streptococcal infection in rats and mice following
O3exposures: A possible disease resistance in rats. Toxicol Appl
Pharmacol, 1233: 211-218.
Gilmour MI, Park P, Doefler D, & Selgrade MJK (1993) Factors which
influence the suppression of pulmonary anti-bacterial defenses in mice
exposed to ozone. Exp Lung Res, 9: 229-314.
Glaser R, Kiecolt-Glaser JK, Speicher CE, & Holliday JE (1985) Stress,
loneliness, and changes in herpesvirus latency. J Behav Med,
8: 249-250.
Glazier A, Tutschka PJ, Farmer ER, & Santos GW (1983) Graft-versus-
host disease in cyclosporin A-treated rats after syngeneic and
autologous bone marrow reconstitution J Exp Med, 158: 1-8.
Gleichmann E, Vohr, H-W, Stringer C, Nuyens J, & Gleichmann H (1989)
Testing the sensitization of T cell to chemicals. From murine graft-
versus-host (GVH) reactions to chemical-induced GVH-like immunological
diseases. In: Kammüller ME, Bloksma N, & Seinen W ed. Autoimmunity and
toxicology: Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers, pp 364-390.
Glickman LT, Shofer FS, Payton AJ, Laster LL, & Felsburg PJ (1988)
Survey of serum IgA, IgG, and IgM concentrations in a large beagle
population in which IgA deficiency had been identified. Am J Vet Res,
49: 1240-1245.
Goettsch W, Garssen J, De Gruijl FR, & Van Loveren H (1993) UV-B and
the immune system: A review with special emphasis on T cell-mediated
immunity. Thymus, 21: 93-114.
Goings SAJ, Kulle TJ, Bascom R, Sauder LR, Green DJ, Hebel R, &
Clements ML (1989) Effects of nitrogen dioxide exposure on
susceptibility to influenza A virus infection in healthy adults.
Am Rev Respir Dis, 139: 1075-1081.
Goldstein BD (1977) Hematotoxicity in humans. J Toxicol Environ
Health, 2(Suppl): 69-106.
Goldstein A, Lowney LI, & Pal BK (1971) Stereospecific and nonspecific
interactions of the morphine congener levorphanol in subcellular
fractions of mouse brain. Proc Natl Acad Sci USA, 68: 1742-1752.
Golemboski KA, Bloom SE, & Dietert RR (1992) Assessment of neonatal
avian inflammatory macrophage function following embryonic
cyclophospha-mide exposure. Int J Immunopharmacol, 14: 19-26.
Gonçalo S, Gonçalo M, Azenha A, Barros MA, Bastos AS, Brandao FM,
Faria A, Marques MSJ, Pecegueiro M, Rodrigues JB, Salgueiro E, &
Torres V (1992) Allergic contact dermatitis in children. Contact Derm,
26: 112.
Good RA & Lorenz E (1992) Nutrition and cellular immunity. Int J
Immunopharmacol, 14: 361-366.
Goodman JW, Peter-Fizaine FE, Shinpock SG, Hall EA, & Famie DJ (1989)
Immunologic and hematologic consequences in mice of exposure to ozone.
JEPTO, 9: 243-252.
Goonewardene IM & Murasko DM (1992) Changes in the immune response.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat. Washington DC, ILSI Press, pp 3-7.
Gopinath C, Prentice DE, & Lewis DJ (1987) The lymphoid system.
In: Atlas of experimental toxicologic pathology. Boston, MTP Press,
pp 122-137.
Gorodilova VV & Mandrik EV (1978) The use of some immunological
reactions for studying the immune response in persons presenting a
high oncological risk. Sov Med, 8: 50-53.
Graham JA & Gardner DE (1985) Immunotoxicity of air pollutants.
In: Dean JH, Luster MI, & Munson AE ed. Immunotoxicology and
Immunopharmacology. New York, Raven Press, pp 367-380.
Gray, D (1993) Immunological memory. Ann Rev Immunol, 11: 47-77.
Greenlee WF, Dold KM, Irons RD, & Osborne R (1985) Evidence for direct
action of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) on thymic
epithelium. Toxicol Appl Pharmacol, 79: 112--120.
Greenstein BD, Fitzpatrick FTA, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 112: 345-350.
Greiner DL, Shultz LD, Rossini AA, Mordes JP, Handler ES, & Rajan TV
(1991) Recapitulation of normal and abnormal BB rat immune system
development in SCID mouse/rat lymphohemopoietic chimeras. J Clin
Invest, 88: 717-719.
Guberski DL (1994) Diabetes-prone and diabetes-resistant BB rats:
Animal models of spontaneous and virally induced diabetes mellitus,
lymphocytic thyroiditis, and collagen-induced arthritis. ILAR News,
35: 29-37.
Gumbiner BM & Yamada KM ed. (1992) Cell-to-cell contact and
extracellular matrix. Curr Opin Cell Biol, 4: 757-874.
Gusev VA, Danilovskaya YV, Vatolkina OY, Lomonosova OS, & Velichkowsky
BT (1993) Effect of quartz and alumina dust on generation of
superoxide radicals and hydrogen peroxide by alveolar macrophages,
granulocytes, and monocytes. Br J Ind Med, 50: 732-735.
Gutierrez-Ramos JC, Andreu JL, Moreno de Alboran I, Rodriguez J,
Leonardo E, Kroemer G, Marcos MAR, & Martinez AC (1990) Insights into
autoimmunity: From classical models to current perspectives. Immunol
Rev, 118: 73-101.
Haas W, Pereira P, & Tonegawa S (1993) Gamma/delta cells. Ann Rev
Immunol, 11: 637-685.
Halliday GM, Cavanaugh LL, & Muller HK (1988) Antigen present in local
lymph node by cells from dimethylbenzanthracene-treated murine
epidermis activate suppressor cells. Cell Immunol, 109: 206-221.
Halprin KM, Comerford M, Presser SE, & Taylor JR (1981) Ultraviolet
light treatment delays contact sensitization to nitrogen mustard.
Br J Dermatol, 105: 71-76.
Hanglow AC, Ernst PB, & Bienenstock J (1990) Intraepithelial
leukocytes, murine. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed.
Hemopoietic system (ILSI Monograph). Berlin, Springer Verlag,
pp 315-322.
Hanley PJ, Hook JW, Raftos DA, Gooley AA, Trent R, & Raison RL (1992)
Hagfish humoral defense protein exhibits structural and functional
homology with mammalian complement components. Proc Natl Acad Sci USA,
89: 7910-7914.
Hansch GM (1992) The complement attack phase: Control of lysis and
non-lethal effects of C5b-9. Immunopharmacology, 24: 107-117.
Hara I (1985) Health status and PCBs in blood of workers exposed to
PCBs and of their children. Environ Health Perspectives, 59: 85-90.
Harriman W, Völk H, Defranoux N, & Wabl M (1993) Immunoglobulin class
switch recombination. Ann Rev Immunol, 11: 361-384.
Harrington W & Krupnick AJ (1985) Short-term nitrogen dioxide exposure
and acute respiratory disease in children. J Air Pollut Control Assoc,
35: 1061-1067.
Hartevelt MM, Bouwes Bavinck JN, Kootte AMM, Vermeer BJ, &
Vandenbroucke JP (1990) Incidence of skin cancer after renal
transplantation in the Netherlands. Transplantation, 49: 506-509.
Hartmann DP (1985) Immunological consequences of asbestos exposure.
Surv Immunol Res, 4: 65-68.
Hartmann DP, Georgian MM, Oghiso Y, & Kagan E (1984) Enhanced
interleukin activity following asbestos inhalation. Clin Exp Immunol,
55: 643-650.
Hasemann CA & Capra JD (1989) Immunoglobulins: Structure and function.
In: Paul WE ed. Fundamental immunology, 2nd Ed. New York, Raven Press,
pp 209-233.
Hashimoto K, Nakanishi T, & Kurosawa Y (1990) Isolation of carp genes
encoding major histocompatibility complex antigens. Proc Natl Acad Sci
USA, 87: 6863-6867.
Haynes RC & Murad F (1985) Adrenocortical steroids and their synthetic
analogs: Inhibitors of adrenocortical steroid biosynthesis. In: Gilman
AG, Goodman LS, Rall TW, & Murad F ed. Goodman and Gilman's
pharmacological basis of therapeutics. New York, Macmillan, p 1459.
Hedrick SM (1989) T Lymphocyte receptors. In: Paul WE ed. Fundamental
immunology, 2nd Ed. New York, Raven Press, pp 291-313.
Heijnen CJ & Kavelaars A (1991) The contribution of neuroendocrine
substances to the immune response. Neth J Med, 39: 281-294.
Heijnen CJ, Kavelaars A, & Ballieux RE (1991) ß-Endorphin: Cytokine
and neuropeptide. Immunol Rev, 119: 41-63.
Helle E, Olsson M, & Jensen S (1976) PCB levels correlated with
pathological changes in seal uteri. Ambio, 5: 261-263.
Hemphill FE, Kaeberle ML, & Buck WB (1971) Lead suppression of mouse
resistance to Salmonella typhimurium. Science, 172: 1031.
Henderson FW, Dubovy EJ, Harder S, Seal E, & Graham D (1988)
Experimental rhinovirus infection in human volunteers exposed to
ozone. Am Rev Respir Dis, 137: 124-127.
Henne T & Schmähl D (1985) Occurrence of second primary malignancies
in man: A second look. Cancer Treat Rev, 12: 77-94.
Henney CS, Kuriayashi K, Kern BE, & Gillis S (1981) Interleukin-2
augments natural killer cell activity. Nature, 291: 335-338.
Herberman RB & Ortaldo JR (1981) Natural killer cells: Their role in
defenses against disease. Science, 214: 24-30.
Herman A, Kappler JW, Marrack P, & Pullen AM (1991) Superantigens:
Mechanism of T-cell stimulation and role in immune responses. Ann Rev
Immunol, 9: 745-772.
Hersey P, Hasic E, Edwards A, Bradley M, Haran G, & McCarthy WH
(1983a) Immunological effects of solarium exposure. Lancet,
i: 545-548.
Hersey P, Haran G, Hasic E, & Edwards A (1983b) Alteration of T cell
subsets and induction of suppressor T cell activity in normal subjects
after exposure to sunlight. J Immunol, 31: 171-174.
Hess AD, Jones RJ, Morris LE, Noga SJ, Vogelsang GB, & Santos GW
(1992) Autologous graft-versus-host disease: A novel approach for
antitumor immunotherapy. Hum Immunol 34: 219-224.
Hinton DM (1992) Testing guidelines for evaluation of the immunotoxic
potential of direct food additives. Crit Rev Food Sci Nutr,
32: 173-190.
Ho M (1980) Role of specific cytotoxic lymphocytes in cellular
immunity against murine cytomegalovirus. Infect Immunol, 27: 767-776.
Ho MK & Springer TA (1984) Preparation and use of monoclonal
antimacrophage antibodies. Methods Enzymol, 108: 313-324.
Hoffman RE (1992) Recent studies of the human immunotoxic effects of
2,3,7,8-tetrachlorodibenzo- p-dioxin. In: Newcombe DS, Rose NR, &
Bloom JC ed. Clinical immunotoxicology. New York, Raven Press,
pp 339-347.
Hoffman RE, Stehr-Green PA, Webb KB, Evans G, Knutsen AP, Schramm WF,
Staake JL, Gibson BG, & Steinberg KK (1986) Health effects of long-
term exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin. J Am Med
Assoc, 255: 2031-2038.
Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR,
Heindell JJ, & Luster MI (1991) Perinatal thymocyte antigen expression
and postnatal immune development altered by gestational exposure to
tetrachlorodibenzo- p-dioxin (TCDD). Teratology, 44: 385-393.
Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, & Mo
JA (1990) Type II collagen autoimmunity in animals and provocations
leading to arthritis. Immunol Rev, 118: 193-232.
Holmes KL & Morse HC (1988) Murine hematopoetic cell surface antigen
expression. Immunol Today, 9: 344-350.
Holmes MA, Duffus WP, & Gorman NT (1989) Natural cytotoxicity in the
dog: Description of two new allogeneic tumour targets. Vet Immunol
Immunopathol, 23: 161-170.
Holsapple MP, Page DG, Shopp GM, & Bick PH (1984) Characterization of
the delayed hypersensitivity response to a protein antigen in the
mouse. Int J Immunopharmacol, 6: 399-405.
Holsapple MP, White KL, McCay JA, Bradley SG, & Munson AE (1988) An
immunotoxicological evaluation of 4,4'-thiobis-(6- t-butyl- m-cresol)
in female B6C3F1 mice. Fundam Appl Toxicol, 10: 701-716.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo- p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-251.
Holt PG & Keast D (1977) Environmentally induced changes in
immunological function: Acute and chronic effects of inhalation of
tobacco smoke and other atmospheric contaminants in man and
experimental animals. Bacteriol Rev, 41: 205-216.
Holt PG & Sedgwick JD (1987) Suppression of IgE responses following
inhalation of antigen. A natural homeostatic mechanism which limits
sensitization to aeroallergens. Immunol Today, 8: 14-15.
Hordvik I, Grimholt U, Fosse VM, Lie O, & Endresen C (1993) Cloning
and sequence analysis of cDNAs encoding the MHC class II ß chain in
Atlantic salmon (Salmo salar). Immunogenetics, 37: 437-441.
Hori S, Obana H, Kashimoto T, Otake T, Nishimura H, Ikegami N, Kunita
N, & Uda H (1982) Effect of polychlorinated biphenyls and
polychlorinated quaterphenyls in cynomolgus monkey (Macaca
fascicularis). Toxicology, 24: 123-139.
Hoshino A, Takenaka H, Mizukoshi O, Imanishi J, Kishida T, & Tovey M
(1983) Effect of anti-interferon serum of influenza virus infection in
mice. Antiviral Res, 3: 59-65.
Houben GF, Van Dokkum W, Van Loveren H, Penninks AH, Seinen W,
Spanhaak S, & Ockhuizen T (1992) Effects of caramel colour III on the
number of blood lymphocytes: A human study on caramel colour III
immunotoxicity and a comparison of the results with data from rat
studies. Food Chem Toxicol, 5: 427-430.
Houben GF, Penninks AH, Seinen W, Vos JG, & Van Loveren H (1993)
Immunotoxic effects of the color additive caramel color III: Immune
function studies in rats. Fundam Appl Toxicol, 20: 30-37.
House RV, Lauer LD, Murray MJ, & Dean JH (1987) Suppression of
T-helper cell function in mice following exposure to the carcinogen
7,12-dimethylbenz[ a]-anthracene and its restoration by
interleukin-2. Int J Immunopharmacol, 9: 89-97.
House RV, Pallardy MJ & Dean JH (1989) Suppression of murine
cytotoxic T-lymphocyte induction following exposure to
7,12-dimethylbenz[ a]anthracene: Dysfunction of antigen recognition.
Int J Immunopharmacol, 11: 207-215.
House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR,
& Dean JH (1990a) Examination of immune parameters and host
resistance mechanisms in B6C3F1 mice following adult exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. J Toxicol Environ Health,
31: 203-215.
House RV, Pearson FC, & Thomas PT (1990b) Selective potentiation of
host resistance in mice following treatment with pyrexol. Mol Biother,
2: 175-178.
Howard SK, Werner PR, & Sleigh SD (1980) Polybrominated biphenyl
toxicosis in swine: Effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol,
55: 146-153.
Hoyle PC & Cooper EC (1990) Nonclinical toxicity studies of antiviral
drugs indicated for the treatment of non-life threatening diseases:
Evaluation of drug toxicity prior to phase I clinical studies. Regul
Toxicol Pharmacol, 11: 81-89.
Hu HZ, Queirò MR, Tilanus MGJ, De Weger RA, & Schuurman H-J (1993)
Expression of T-cell receptor aand b variable genes in normal and
malignant human T cells. Br J Haematol, 84: 39-48.
Hughes & Nei (1993) Evolutionary relationships of the classes of major
histocompatibility complex genes. Immunogenetics, 93: 337-346.
Huiskamp R, Davids JAG, & Vos O (1983) Short- and long-term effects of
whole-body irradiation with fission neutrons or X rays on the thymus
in CBA mice. Radiat Res, 95: 370-381.
Huiskamp R, Davids JAG, & Van Ewijk W (1988) The effect of graded
doses of fission neutrons or X rays on the stromal compartment of the
thymus in mice. Radiat Res, 113: 25-39.
Hünig T, Wallny HJ, Hastley JK, Lawetzky A, & Tiefenthaler G (1989) A
monoclonal antibody to a constant determinant of the rat T cell
antigen receptor that induces T cell activation. Differential
reactivity with subsets of immature and mature T lymphocytes. J Exp
Med, 169: 73-86.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42 (IARC monographs on the evaluation
of carcinogenic risks to humans, Suppl 7). Lyon.
IARC (1990) Ciclosporin. In: Pharmaceutical drugs (IARC monographs on
the avaluation of carcinogenic risks to humans, Vol. 50). Lyon,
pp 77-114.
Imanishi J, Nomura H, Matsubara M, Kita M, Won S-J, Mizutani T, &
Kishida T (1980) Effect of polychlorinated biphenyl on viral
infections in mice. Infect Immunol, 29: 275-277.
Inada T & Mims CA (1985) Association of virulence of murine
cytomegalovirus with macrophage susceptibility and with virion-bound
non-neutralizing antibody. J Gen Virol, 66: 879-882.
Institoris L, Siroki O, Toth S, & Desi I (1992) Immunotoxic effects of
MTP-IP containing 60% methylparathion in mice. Hum Exp Toxicol,
11: 11-16.
Institoris L, Siroki O, & Desi I (1995) Immunotoxicity study of
repeated small doses of dimethoate and methylparathion administered to
rats over three generations. Hum Exp Toxicol, 14: 879-883.
Institoris L, Siroki O, Fekete K, & Desi I (in press)
Immunotoxicological investigation of repeated small doses of
dichlorvos (DDPV) in three generations of rats. Int J Environ Health
Res.
IPCS (1978) Environmental health criteria 6: Principles and methods
for evaluating the toxicity of chemicals. Part I. Geneva, WHO.
IPCS (1986) Immunotoxicology. Development of predictive testing for
determining the immunotoxic potential of chemicals. Report of a
technical review meeting. London, November 3-5, 1986. Geneva, WHO.
IPCS (1992) Environmental health criteria 141: Quality management for
chemical safety testing. Geneva, WHO.
IPCS (1994a) Environmental health criteria 155: Biomarkers and risk
assessment: Concepts and principles. Geneva, WHO.
IPCS (1994b) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO.
IPCS/Department of Health (1995) Proceedings of International
Programme on Chemical Safety and UK Department of Health International
Workshop on Environmental Immunotoxicology and Human Health, Oxford,
22-25 March, 1994. Hum Exp Toxicol, 14: 77-154.
Irons RD (1985) Histology of the immune system: Structure and
function. In: Dean J, Luster MI, Munson AE, & Amos H ed.
Immunotoxicology and immunopharmacology. New York, Raven Press,
pp 11-22.
Ishiguro H, Kobayashi K Suzuki M, Titani K, Tomonaga S, & Kurosawa Y
(1992) Isolation of a hagfish gene that encodes a complement
component. EMBO J, 11: 829-837.
Jacob CO, Van Der Meide PH, & McDevitt HO (1987) In vivo treatment of
(NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma-
interferon. J Exp Med, 166: 798-803.
Jakab GJ & Hmieleski RR (1988) Reduction of influenza virus
pathogenesis by exposure to 0.5 ppm ozone. J Toxicol Environ Health,
23: 455-472.
Janeway CA (1992) The T-cell receptor as a multicomponent signalling
machine: CD4/CD8 coreceptors and CD45 in T-cell activation. Ann Rev
Immunol, 10: 645-674.
Jaremin B (1983) Blast lymphocyte transformation (LTT), rosette
(E-RFC) and leukocyte migration inhibition (MIF) tests in persons
exposed to the action of lead during work. Report II. Bull Inst Mar
Trop Med (Gdynia), 34: 187-197.
Jeevan A & Kripke ML (1990) Alteration of the immune response to
Mycobacterium bovis BCG in mice exposed chronically to low doses of
UV radiation. Cell Immunol, 130: 32-41.
Jeevan A, Gilliam K, Heard H, & Kripke ML (1992) Effects of
ultraviolet radiation on the pathogenesis of Mycobacterium
lepraemurium infection in mice. Exp Dermatol, 1: 152-160
Jefferies WA (1988) Hemopoietic and T-lymphocyte marker antigens of
the rat characterized with monoclonal antibodies. In: Miyasaka M &
Trnka Z ed. Differentiation antigens in lymphohemopoietic tissues. New
York, Marcel Dekker, pp 173-247.
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunological
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachloro- p-dioxin. Br J Ind Med, 45: 701-704.
Jerne NK, Henry C, Nordin AA, Fun H, Koros MC, & Lefkovits I (1974)
Plaque forming cells: Methodology and theory. Transplant Rev,
18: 130-191.
Jessup JM, Hanna N, Palaszynski E, & Kripke ML (1978) Mechanisms of
depressed reactivity to dinitrochlorobenzene and ultraviolet-induced
tumours during ultraviolet carcinogenesis in BALB/c mice. Cell
Immunol, 38: 105-115.
Johnson KW, Holsapple MP, & Munson AE (1986) An immunotoxicological
evaluation of g-chlordane. Fundam Appl Toxicol, 6: 317.
Johnson KW, Munson AE, Kim DH, & Holsapple MP (1987) Role of
reactive metabolites in suppression of humoral immunity by
N-nitrosodimethylamine. J Pharmacol Exp Ther, 240: 847-855.
Johnson JD, Houchens DP, De Kluwe WM, Craig DK, & Fisher GL (1990)
Effects of mainstream and environmental tobacco smoke on the immune
system of animals and humans: A review. CRC Crit Rev Toxicol,
20: 369-395.
Joling P, Tielen FJ, Vaessen LMB, Hesse CJ, & Rozing J (1985)
Intrathymic differentiation in the rat. Adv Exp Med Biol,
186: 235-244.
Joling P (1987) Allografting and the T cell system. A multiparameter
analysis of rejection in the rat. PhD Thesis, Rotterdam, Erasmus
University.
Jones TC, Ward JM, Mohr U, & Hunt RD (1990) Hemopoietic system (ILSI
monograph). Berlin, Springer Verlag.
Kafafi SA, Afeefy HY, Ali AH, Said HK, & Kafafi AG (1993) Binding of
polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ
Health Perspectives, 101: 422-425.
Kagan E, Solomon A, Cochrane JC, Beissner EI, Gluckman J, Rocks PH, &
Webster I (1977a) Immunological studies of patients with asbestosis.
I. Studies of cell-mediated immunity. Clin Exp Immunol, 28: 261-267.
Kagan E, Solomon A, Cochrane JC, Kuba P, Rocks PH, & Webster I (1977b)
Immunological studies of patients with asbestosis. II. Studies of
circulating lymphoid numbers and humoral immunity. Clin Exp Immunol,
28: 268-275.
Kagan E, Jacobson RJ, Yeung KY, Haidak DJ, & Nachnani GH (1979)
Asbestos-associated neoplasms of B cell lineage. Am J Med,
67: 325-330.
Kahan BD (1989) Cyclosporine. New Engl J Med, 321: 1725-1738.
Kalimo K, Koulu L, & Jansen CT (1983) UV exposure and
dinitrochlorobenzene (DNCB). Arch Dermatol Res, 275: 374-378.
Kalpazanov Y, Stamenova M, & Kurchatova G (1976) Air pollution and the
1974 - 1975 influenza epidemic in Sofia. Environ Res, 12: 1-8.
Kamel OW, Van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA,
Montgomery PG, Warnke RA, & Dorfman RF (1993) Reversible lymphoma
associated with Epstein-Barr virus occurring during methotrexate
therapy for rheumatoid arthritis and dermatomyositis. New Engl J Med,
328: 1317-1321.
Kammüller ME & Bloksma N (1994) Drug-induced autoimmunity. In: Dean J,
Luster MI, Munson AE, & Kimber I ed. Immunotoxicology and
immunopharmacology. New York, Raven Press, pp 573-588.
Kammüller ME, Bloksma N, & Seinen W (1989) Autoimmunity and
toxicology. Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers.
Kampinga J, Berges S, Boyd RL, Brekelmans P, Colic M, Van Ewijk W,
Kendall M, Ladyman H, Nieuwenhuis P, Ritter MA, Schuurman HJ, &
Tournefier A (1989) Thymic epithelial antibodies: Immunohistological
analysis and introduction of nomenclature. Thymus, 13: 165-173.
Kannan K, Singh KP, Goel SK, & Shanker R (1985) Effect of
2,5-hexanediol on immunocompetence of mice. Environ Res, 36: 14.
Kannan K, Tanabe S, Borrell A, Aguilar A, Focardi S, & Tatsukawa R
(1993) Isomer-specific analysis and toxic evaluation of
polychlorinated biphenyls in striped dolphins affected by an epizootic
in the western Mediterranean Sea. Arch Environ Contam Toxicol,
25: 227-233.
Kasahara M, Vazquez M, Sato K, McKinney EC, & Flajnik MF (1992)
Evolution of the major histocompatibility complex: Isolation of class
II A cDNA clones from the cartilagenous fish. Proc Natl Acad Sci USA,
89: 6688-6692.
Kasai M, Iwamari M, Nagai T, Okumura K, & Tada T (1980) A glycolipid
on the surface of mouse natural killer cells. Eur J Immunol,
10: 175-180.
Kasai M, Yoneda T, Habu S, Maruyana Y, Okumura K, & Tokunaga T (1981)
In vivo effect of anti-asialo GM-1 antibody on natural killer cell
activity. Nature, 291: 334-335.
Kasl SV, Evans AS, & Neiderman JC (1979) Psychosocial risk factors
in the development of infectious mononucleosis. Psychosom Med,
41: 445-466.
Kateley JR & Bazzell SJ (1978) Immunological studies in cattle exposed
to polybrominated biphenyls. Environ Health Perspectives, 23: 75-82.
Kawabata TT & White KL (1987) Suppression of the in vitro humoral
immune response of mouse splenocytes by benzo( a)pyrene metabolites
and inhibition of benzo( a)pyrene-induced immunosuppression by
a-naphthoflavone. Cancer Res, 47: 2317-2322.
Kawabata TT & White KL (1990) Effects of naphthalene and naphthalene
metabolites on the in vitro humoral immune response. J Toxicol
Environ Health, 30: 53-67.
Keck G (1981) Effets de la contamination par les polychlorobiphenyles
(PCB) sur le developpement de la tumeur d'Ehrlich chez la souris
Swiss. Toxicol Eur Res, 3: 229-236.
Kendall MD (1980) The occurrence of erythropoiesis in the thymus of
the bank vole (Clethrionomys glareolus). Cell Tissue Res,
212: 307-314.
Kendall MD (1991) Functional anatomy of the thymic microenvironment.
J Anat, 177: 1-29.
Kendall MD & Al-Shawaf AA (1991) Innervation of the thymus gland.
Brain Behav Immunol, 5: 9-28.
Kendall MD & Frazier JA (1979) Ultrastructural studies on
erythropoiesis in the avian thymus. I. Description of cell types. Cell
Tissue Res, 199: 37-61.
Kendall MD & Singh J (1980) The presence of erythroid cells in the
thymus gland of man. J Anat, 130: 183-189.
Kendall MD & Ward P (1974) Erythropoiesis in an avian thymus. Nature,
249: 3 66-367.
Kendall MD, Fitzpatrick FTA, Greenstein BD, Khoylou F, Safieh B, &
Hamblin A (1990) Reversal of ageing changes in the thymus of rats by
chemical or surgical castration. Cell Tissue Res, 261: 555-564.
Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V,
Huelskamp AM, & Davidson NE (1994) Phase I trial of interferon gamma
to potentiate cyclosporine-induced graft-versus-host disease in women
undergoing autologous bone marrow transplantation for breast cancer.
J Clin Oncol, 12: 249-257.
Kerkvliet NI & Baecher-Steppan L (1988) Suppression of allograft
immunity by 3,4,5,3 ',4 ',5 '-hexachlorobiphenyl. I. Effects of
exposure on tumor rejection and cytotoxic T cell activity.
Immunopharmacology, 16: 1-12.
Kerkvliet NI & Brauner JA (1987) Mechanisms of 1,2,3,4,6,7,8-
heptachlorodi-benzo- p-dioxin (HpCDD)-induced humoral suppression:
Evidence of primary defect in T-cell regulation. Toxicol Appl
Pharmacol, 87: 18-31.
Kerkvliet NI & Burleson GR (1994) Immunotoxicity of TCDD and related
halogenated aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE,
& Kimber I ed. Immunotoxicity and immunopharmacology, 2nd Ed. New
York, Raven Press, pp 97-121.
Kerkvliet NI & Kimeldorf DJ (1977) Antitumor activity of a
polychlorinated biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J Natl Cancer Inst, 59: 951-955.
Kerkvliet NI, Steppan LB, Smith BB, Youngberg JA, Henderson MC, &
Buhler DR (1990) Role of the Ah locus in suppression of cytotoxic T
lymphocyte (CTL) activity by halogenated aromatic hydrocarbons (PCBs
and TCDD): Structure-activity relationships and effects in C57Bl/6
mice. Fundam Appl Toxicol, 14: 532-541.
Khan MF & Gupta GS (1991) Cellular and biochemical indices of
bronchoalveolar lavage for detection of lung injury following insult
by airborne toxicants. Toxicol Lett, 58: 239-255
Khangarot BS & Tripathi DM (1991) Changes in humoral and cell-mediated
immune responses and in skin and respiratory surfaces of catfish,
Saccobranchus fossilis, following copper exposure. Ecotox Environ
Saf, 22: 291-308.
Khansari DN, Murgo AJ, & Faith RE (1990) Effects of stress on the
immune system. Immunol Today, 11: 170-175.
Kiecolt-Glaser JK & Glaser R (1986) Psychological influences on
immunity. Psychonomatics, 27: 621-624.
Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday
JE, & Speicher CE (1986) Modulation of cellular immunity in medical
students. J Behav Med, 9: 5-21.
Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, &
Glaser R (1987) Marital quality, marital disruption, and immune
function. Psychosom Med, 49: 13-33.
Kim JH (1989) Infection and cyclosporin. Rev Infect Dis, 11: 677-690.
Kim DH, Yang KH, Johnson KW, & Holsapple MP (1987) Suppression of
in vitro antibody production by dimethylnitrosamine in mixed
cultures of mouse primary hepatocytes and mouse splenocytes. Toxicol
Appl Pharmacol, 87: 32-42.
Kimber I & Cumberbatch M (1992) Dendritic cells and cutaneous immune
responses to chemical allergens. Toxicol Appl Pharmacol, 117: 137-146.
Kimber I, Stonard MD, Gidlow DA, & Niewola Z (1986) Influence of
chronic low-level exposure to lead on plasma immunoglobulin
concentration and cellular immune function in man. Int Arch Occup
Environ Health, 57: 117-125.
Kimbrough RD (1987) Human health effects of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls (PBBs). Ann Rev Pharmacol Toxicol,
27: 87-11.
Kimchi A (1992) Cytokine triggered molecular pathways that control
cell cycle arrest. J Cell Biochem, 50: 1-9.
Kimmel CA (1990) Quantitative approaches to human risk assessment for
noncancer health effects. Neurotoxicology, 11: 189-198.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Kincade PW (1992) Cell interaction molecules and cytokines which
participate in B lymphopoiesis. Baillieres Clin Haematol, 5: 575-598.
King AG, Landbreth KS, & Wierda D (1987) Hydroquinone inhibits bone
marrow pre-B cell maturation in vitro. Mol Pharmacol, 32: 807-812.
Kingsmore SF, Hall BD, Alen NB, Rice JR, & Caldwell DS (1992)
Association of methotrexate, rheumatoid arthritis and lymphoma: Report
of 2 cases and literature review. J Rheumatol, 19: 1462-1465.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Klareskog L & Olsson T (1990) Autoimmunity to collagen II and myelin
basic protein: Comparative studies in humans and rodents. Immunol Rev,
118: 285-310.
Klein J (1986) Natural history of the major histocompatibility
complex. New York, John Wiley and Sons.
Klein J (1990) Immunology. Oxford, Blackwell Scientific Publications.
Kleinbeck ML, Hites MJ, Loker JL, Halliwell RE,& Lee KW (1989) Enzyme-
linked immunosorbent assay for measurement of allergen-specific IgE
antibodies in canine serum. Am J Vet Res, 50: 1831-1839.
Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, & Von dem
Borne AEGK ed. (1989) Leucocyte typing IV: White cell differentiation
antigens. Oxford, Oxford University Press.
Knight R, Sarlis N, & Stephanou A (1992) Interleukins and
neurohormones: A common language. Postgrad Med J, 68: 603-605.
Kocks C & Rajewski K (1989) Stable expression and somatic
hypermutation of antibody V regions in B cell developmental pathways.
Ann Rev Immunol, 7: 537-559.
Koller LD (1977) Enhanced polychlorinated biphenyl lesions in Moloney
leukemia virus-infected mice. Clin Toxicol, 11: 107-116.
Koller LD (1980) Immunotoxicology of heavy metals. Int J
Immunopharmacol, 2: 269-279.
Koller LD (1990) The immunotoxic effects of lead in lead-exposed
laboratory animals. Ann NY Acad Sci, 587: 160-167.
Koller LD & Kovacic S (1974) Decreased antibody formation in mice
exposed to lead. Nature, 250: 148-150.
Koller LD & Roan JG (1977) Effects of lead and cadmium on mouse
peritoneal macrophages. J Reticuloendothel Soc, 21: 7.
Koller LD & Roan JG (1980) Effects of lead, cadmium and methylmercury
on immunological memory. J Reticuloendothel Soc, 21: 7-12.
Koller LD & Thigpen JE (1973) Biphenyl-exposed rabbits. Am J Vet Res,
34: 1605-1606.
Kongshavn PAL, Sadarangani C, & Skamene E (1980) Cellular mechanisms
of genetically-determined resistance to Listeria monocytogenes.
In: Skamene E ed. Genetic control of natural resistance. New York,
Academic Press, pp 149-163.
Koren HS & Blomberg PA (in press) Respiratory responses of asthmatics
to ozone. Int Arch Allergy.
Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo
WJ, Becker S, House DE, McDonnel WF, & Bromberg PA (1989) Ozone-
induced inflammation in the lower airways of human subjects. Am Rev
Respir Dis, 139: 407-415.
Koren HS, Devlin RB, & Becker S (1994) Ozone-induced inflammatory
response in pulmonary cells. In: Schook LB & Laskin DL ed. Xenobiotics
and inflammation: Roles of cytokines and growth factors. New York,
Academic Press, pp 249-281.
Krajnc EI, Wester PW, Loeber JG, Van Leeuwen FXR, Vos JG, Vaessen
HAMG, & Van der Heijden CA (1984) Toxicity of bis(tri-n-butyltin)oxide
in the rat. I. Short-term effects on general parameters and on the
endocrine and lymphoid systems. Toxicol Appl Pharmacol, 75: 363-386.
Krajnc-Franken MAM, Van Loveren H, Schuurman HJ, & Vos JG (1990) The
immune system as a target for toxicity. A tiered approach of testing,
with special emphasis on histopathology. In: Dayan AD, Hertel RF,
Heseltine E, Kazantis G, Smith EM, & Van der Venne MT ed.
Immunotoxicity of metals and immunotoxicology. New York, Plenum Press,
pp 241-264.
Kramer CM, Johnson KW, Dooley RK, & Holsapple MP (1987) 2,3,7,8-
Tetrachlorodibenzo- p-dioxin (TCDD) enhances antibody production and
protein kinase activity in murine B cells. Biochem Biophys Res Commun,
145: 25-33.
Kripke ML (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J Natl Cancer Inst, 53: 1333-1336.
Kripke ML, Thorn RM, Lill PH, Civin CI, Pazmino NH, & Fisher MS (1979)
Further characterization of immunological unresponsiveness induced in
mice by ultraviolet radiation. Transplantation, 28: 212-217.
Kripke ML, Cox PA, Alas LG, & Yarosh DB (1992) Pyrimidine dimers in
DNA initiate systemic immunosuppression in UV-irradiated mice. Proc
Natl Acad Sci USA, 89: 7516-7520.
Krishna VR, Fouant MM, & Bradley SG (1989) Effects of pyran copolymer
on host resistance of mice to Plasmodium yoelii. J Parasitol,
75: 405-410.
Kroese FGM, Timens W, & Nieuwenhuis P (1990) Germinal center reaction
and B lymphocytes: Morphology and function. In: Grundmann E & Vollmer
E ed. Reaction patterns of the lymph node. Part 1. Cell types and
functions. Berlin, Springer Verlag, pp 103-148.
Kroplien U, Rosdorfer J, van der Greef J, Long RC, & Goldstein JH
(1985) 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)imidazole: Detection
in commercial caramel color III and preparation by a model browning
reaction. J Org Chem, 50: 1131-1133.
Krowka JF, Sarin S, Namikawa R, McCune JM, & Kaneshima H (1991) Human
T cells in the SCID-hu mouse are phenotypically normal and
functionally competent. J Immunol, 146: 3751-3756.
Kubota M, Kagamimori S, Yokoyama K, & Okada A (1985) Reduced killer
cell activity of lymphocytes from patients with asbestosis. Br J Ind
Med, 42: 276-280.
Kunita N, Hori S, Obana H, Otake T, Nishimura H, Kashimoto T, &
Ikegami N (1985) Biological effect of PCBS, PCQs and PCDFs present in
the oil causing yusho and yu-cheng. Environ Health Perspectives,
59: 79-84.
Kunkel SL, Chensue SW, Strieter RM, Lynch JP, & Remick DG (1989)
Cellular and molecular aspects of granulomatous inflammation. Am J
Respir Cell Mol Biol, 1: 439-447.
Kuper CF, Beems RB, & Hollanders VMH (1990a) Development and aging;
thymus, rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic
system (ILSI Monograph). Berlin, Springer Verlag, pp 257-263.
Kuper CF, Hameleers DMH, Bruijntjes JP, Van der Ven I, Biewenga J, &
Sminia T (1990b) Lymphoid and non-lymphoid cells in nasal-associated
lymphoid tissue (NALT) in the rat. An immuno- and enzymehistochemical
study. Cell Tissue Res, 259: 371-377.
Kuper CF, Beems RB, Bruijntjes JP, Schuurman H-J, & Vos JG (1992a)
Normal development, growth and aging of the thymus. In: Mohr U,
Dungworth DL, & Capen CC ed. Pathobiology of the aging rat, Vol. 1.
Washington DC, ILSI Press, pp 25-49.
Kuper CF, Koornstra PJ, Hameleers DMH, Biewenga J, Spit BJ,
Duijvestijn AM, Van Breda Vriesman PJC, & Sminia T (1992b) The role of
nasopharyngeal lymphoid tissue. Immunol Today, 13: 219-224.
Kuper CF, Schuurman H-J, & Vos JG (1995) Pathology in immunotoxicology.
In: Burleson G, Munson A, & Dean J ed. Modern methods in immuno-
toxicology. New York, John Wiley & Sons, pp 397-436.
Kyle RA (1984) Second malignancies associated with chemotherapy. In:
Perry MC & Yarbro JW ed. Toxicity of chemotherapy. New York, Grune &
Stratton, pp 479-506.
Labarca C & Paigen KA (1980) A simple, rapid, and sensitive DNA assay
procedure. Anal Biochem, 102: 344-352.
Ladics GS, Kawabata TT, & White KL (1991) Suppression of the
in vitro humoral immune response of mouse splenocytes by
7,12-dimethylbenz[ a]anthracene metabolites and inhibition of
immunosuppression by a-naphthflavone. Toxicol Appl Pharmacol,
110: 31-44.
Ladics GS, Kawabata TT, Munson AE, & White KL (1992) Metabolism of
benzo(a)pyrene by murine splenic cell types. Toxicol Appl Pharmacol,
116: 248-257.
Ladics GS, Smith C, Heaps K, Elliott GS, Slone TW, & Loveless SE
(1995) Possible incorporation of an immunotoxicological functional
assay for assessing humoral immunity for hazard identification
purposes in rats on standard toxicology study. Toxicology,
96: 225-238.
LaGrange PH, Mackaness GB, & Miller TE (1974) Influence of dose and
route of antigen injection on the immunological induction of T cells.
J Exp Med, 139: 528-542.
Laman JD, Van den Eertwegh AJM, Claassen E, & Van Rooijen N (1992)
Cell-cell interactions: In situ studies of splenic humoral immune
responses. In: Fornusek L & Vetvicka V ed. Immune system accessory
cells. Boca Raton, Florida, CRC Press, pp 201-231.
Lambris JD (1993) The chemistry, biology and phylogeny of C3.
In: Cruse JM & Lewis RE ed. Complement today, Vol. 1. Basel, Karger,
pp 16-45.
Lampeter EF, Signore A, Gale EAM, & Pozzilli P (1989) Lesson from the
NOD mouse for the pathogenesis and immunotherapy of human type 1
(insulin-dependent) diabetes mellitus. Diabetologia, 32: 703-708.
Lang D, Meier KL, & Luster MI (1993) Comparative effects of
immunotoxic chemicals on in vitro proliferative responses of human
and rodent lymphocytes. Fundam Appl Toxicol, 21: 535-545.
Lanier LL, Benike CJ, Phillips JH, & Engleman EG (1985) Recombinant
interleukin-2 enhanced natural killer cell-mediated cytotoxicity in
human lymphocyte subpopulations expressing the leu-7 and leu-11
antigens. J Immunol, 2: 794-801.
Larrick JW & Wright SC (1992) Native cytokine antagonists. Baillieres
Clin Haematol, 5: 681-702.
Lasky LA (1992) Selectins: Interpreters of cell-specific carbohydrate
information during inflammation. Science, 258: 964-969.
Lawlor GJ Jr & Fischer TJ ed. (1988) Manual of allergy and immunology.
Diagnosis and therapy, 2nd Ed. Boston, Little, Brown & Co.
Lawrence DH (1981) In vivo and in vitro effects of lead on humoral
and cell-mediated immunity. Infect Immun, 31: 136-143.
Lawrence DA (1985) Immunotoxicity of heavy metals. In: Dean JH, Luster
MI, Munson AE, & Amos H ed. Immunotoxicology and immunopharmacology.
New York, Raven Press, p 341.
Lawrence D, Mudzinski S, Rudofsky U, & Warner A (1987) Mechanism of
metal-induced immunotoxicity. In: Berlin A, Dean J, Draper MH, Smith
EMB, & Spreafico F ed. Immunotoxicology. Dordrecht, Martinus Nijhoff,
pp 293-307.
Lea T, Stelen K, & Stormer FC (1989) Mechanism of ochratoxin A-induced
immunosuppression. Mycopatholgia, 107: 153-159.
Lebish IJ, Hurvitz A, Lewis RM, Cramer DV, & Krakowka S (1986)
Immunopathology of laboratory animals. Toxicol Pathol, 14: 129-134.
Leiter EH (1993) The NOD mouse: A model for analyzing the interplay
between heredity and environment in development of autoimmune disease.
ILAR News, 35: 4-14.
Lerman SP & Weidanz WP (1970) The effect of cyclophosphamide on the
ontogeny of the humoral immune response in chickens. J Immunol,
105: 614-619.
LeVier DG, Brown RD, McCay JA, Fuchs BA, Harris LS, & Munson AE (1993)
Hepatic and splenic phagocytosis in female B6C3F1 mice implanted with
morphine sulfate pellets. J Pharmacol Exp Ther, 267: 357-363.
Levy SM, Herberman RB, Lee J, Whiteside T, Beadle M, Heiden L ,&
Simons A (1991) Persistently low natural killer cell activity, age,
and environmental stress as predictors of infectious morbidity. Nat
Immun Cell Growth Regul, 10: 289-307.
Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, & Bekesi JG (1986)
High frequency of immune dysfunctions in asbestos workers and in
patients with malignant mesothelioma. J Clin Immunol, 6: 225-233.
Li FY & Richters A (1991) Effects of 0.7 ppm ozone exposure on
thymocytes: In vivo and in vitro studies. Inhal Toxicol, 3: 61-71.
Lichtman MA (1981) The ultrastructure of the hemopoietic environment
of the marrow: A review. Exp Hematol, 9: 391-410.
Like AA, Butler L, Williams M, Appel MC, Weringer EJ, & Rossini AA
(1982) Spontaneous autoimmune diabetes mellitus in the BB rat.
Diabetes, 31(Suppl 1): 7-13.
Litman GW, Rast JP, Shamblott MJ, Haire RN, Hulst M, Roess W, Litman
RT, Hind-Frey KR, Zilch A, & Amemiya CT (1993) Phylogenetic
diversification of immunoglobulin genes and the antibody repertoire.
Mol Biol Evol, 10: 60-72.
Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, & MacLennan ICM
(1989) Mechanism of antigen-driven selection in germinal centres.
Nature, 342: 929-931.
Liu Y-J, Johnson GD, Gordon J, & MacLennan ICM (1992) Germinal centres
in T-cell-dependent antibody responses. Immunol Today, 13: 17-21.
Loo JCK, Tryphonas H, Jordan N, Brien R, Karpinski KF, & Arnold DL
(1989) Effects of Aroclor 1254 on hydrocortisone levels in adult
rhesus monkeys (Macaca mulatta). Bull Environ Contam Toxicol,
43: 667-669.
Loose LD, Pittman KA, Benitz K-F, & Silkworth JB (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J Reticuloendothel Soc, 22: 253-271.
Loose LD, Silkworth JB, Pittman KA, Benitz K-F, & Mueller W (1978)
Impaired host resistance to endotoxin and malaria in polychlorinated
biphenyl- and hexachlorobenzene-treated mice. Infect Immun, 20: 30-35.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspectives, 39: 79-91.
Losco P (1992) Normal development, growth and aging of the spleen.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat, Vol. 1. Washington DC, ILSI Press, pp 75-95.
Losco P & Harleman H (1992) Normal development, growth, and aging of
the lymph node. In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology
of the aging rat, Vol. 1. Washington DC, ILSI Press, pp 49-75.
Love RJ, Ogilvie BM, & McLaren DJ (1976) The immune mechanism which
expels the intestinal stage of Trichinella spiralis from rats.
Immunology, 30: 7-13.
Lu YC & Wu YC (1985) Clinical findings and immunological abnormalities
in yu-cheng patients. Environ Health Perspectives, 59: 17-29.
Lubet RA, Lemaire BN, Avery D, & Kouri RE (1986) Induction of
immunotoxicity in mice by polyhalogenated biphenyls. Arch Toxicol,
59: 71-77.
Luckas B, Vetter W, Fischer P, Heidemann G, & Plötz J (1990)
Characteristic chlorinated hydrocarbon patterns in the blubber of
seals from different marine regions. Chemosphere, 21: 13-19.
Luebke RW, Luster MI, Dean JH, & Hayes HT (1984) Altered host
resistance to Trichinella spiralis infection following subchronic
exposure to diethylstilbestrol. Int J Immunopharmacol, 6: 609-617.
Luebke RW, Andrews DL, Copeland CB, Riddle MM, Rogers RR, & Smialowicz
RJ (1991) Host resistance to murine malaria in mice exposed to the
adenosine deaminase inhibitor, 2'-deoxycoformycin. Int J
Immunopharmacol, 13: 987-997.
Luebke RW, Copeland CB, Diliberto JJ, Akubue PI, Andrews DL,
Riddle MM, Williams WC, & Birnbaum LS (1994) Assessment of host
resistance to Trichinella spiralis in mice following preinfection
exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol, 125: 7-16.
Luebke RW, Copeland CB, & Andrews DL (1995) Host resistance
to Trichinella spiralis infection in rats exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Fundam Appl Toxicol,
24: 285-289.
Lukic ML, Popeskovic L, & Jankovic BD (1973) Potentiation of immune
responsiveness in rats treated with DDT. Fed Proc, 32: 1037.
Lundberg K, Dencker L, & Gronvik KO (1992) 2,3,7,8-Tetrachlorodibenzo-
p-dioxin (TCDD) inhibits the activation of antigen-specific T cells
in mice. Int J Immunopharmacol, 14: 699-705.
Lunn JE Knowelden J, & Handyside AJ (1967) Patterns of respiratory
illness in Sheffield infant schoolchildren. Br J Prev Soc Med,
21: 7-16.
Luo YD, Patel MK, Wiederholt MD, & Ou DW (1992) Effects of
cannabinoids and cocaine on the mitogen-induced transformations of
lymphocytes of human and mouse origins. Int J Immunopharmacol,
14: 49-56.
Luster MI, Faith RE, & Moore JA (1978) Depression of humoral immunity
in rats following chronic developmental lead exposure. J Environ
Pathol Toxicol, 1: 397-402.
Luster MI, Hong LH, Boorman GH, Clark G, Hayes HT, Greenlee WF, Dold
K, & Tucker AN (1985a) Acute myelotoxic responses in mice exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Toxicol Appl Pharmacol,
81: 151-165.
Luster MI, Tucker AN, Hayes HT, Pung OJ, Burka T, McMillan R, & Eling
T (1985b) Immunosuppressive effects of benzidine in mice: Evidence of
alterations in arachidonic acid metabolism. J Immunol, 135: 2754-2761.
Luster MI, Tucker AN, Germolec DR, Silver MT, Thomas PT, Vore SJ, &
Bucher JR (1986) Immunotoxicity studies in mice exposed to methyl
isocyanate. Toxicol Appl Pharmacol, 86: 140-144.
Luster MI, Germolec DR, Burleson GR, Jameson CW, Ackerman MF, Lamm KR,
& Hayes HT (1987) Selective immunosuppression in mice of natural
killer cell activity by ochratoxin A. Cancer Res, 47: 2259-2263.
Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL,
Lauer LD, Germolec DR, Rosenthal GJ, & Dean JH (1988) Development of a
testing battery to assess chemical-induced immunotoxicity: National
Toxicology Program's guidelines for immunotoxicity evaluation in mice.
Fundam Appl Toxicol, 10: 2-19.
Luster MI, Ackermann MF, Germolec DR, & Rosenthal GJ (1989)
Perturbations of the immune system by xenobiotics. Environ Health
Perspectives, 81: 157-162.
Luster MI, Portier C, Pait DG, White KL, Gennings C, Munson AE, &
Rosenthal GJ (1992) Risk assessment in immunotoxicology I. Sensitivity
and predictability of immune tests. Fundam Appl Toxicol., 18: 200-210.
Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E,
Blaylock BL, Pollock P, Kouchi Y, Craig W, White KL, Munson AE, &
Comment CE (1993) Risk assessment in immunotoxicology. II.
Relationship between immune and host resistance tests. Fundam Appl
Toxicol, 21: 71-82.
Lyte M & Bick PH (1986) Modulation of interleukin-1 production by
macrophages following benzo( a)pyrene exposure. Int J
Immunopharmacol, 8: 377-381.
Mackaness GB (1969) The influence of immunologically committed
lymphoid cells on macrophage activity in vivo. J Exp Med,
129: 973-992.
Makker SP & Aikawa M (1979) Mesangial glomerulonephropathy with
deposition of IgG, IgM, and C3 induced by mercuric chloride. Lab
Invest, 41: 45-49.
Malins DC, McClain BB, Landahl JT, Meyers MS, Krahn MM, Brow DW, Chan
SL, & Roubal WT (1988) Neoplasia and other diseases in fish in
relation to toxic chemicals: An overview. Aquat Toxicol, 11: 43-67.
Malkovsky M, Loveland B, North M, Asherson GL, Gao L, Word P, & Fiers
W (1987) Recombinant Interleukin-2 directly augments the cytotoxicity
of human monocytes. Nature, 325: 262-265.
Mandell LA (1990) Infections in the immunocompromised host. J Intern
Med Res, 18: 177-190.
Manischewitz JE & Quinnan GV (1980) Antivirus antibody-dependent cell-
mediated immunity during murine cytomegalovirus infection. Infect
Immun, 29: 1050-1054.
Mann DD, Buening GM, Hook B, & Osweiler GD (1983) Effects of T-2 toxin
on bovine serum proteins. Am J Vet Res, 44: 1751-1759.
Manson-Smith DF, Bruce RG, & Parrott DMV (1979) Villous atrophy and
expul-sion of intestinal Trichinella spiralis are mediated by T
cells. Cell Immunol, 47: 285-292.
Marchalonis JJ, Hohman VS, Thomas C, & Schluter SF (1993) Antibody
production in sharks and humans: A role for natural antibodies. Dev
Comp Immunol, 17: 41-53.
Marker SC, Howard RJ, Simmons RL, Kalis JM, Conelly DP, Najarian JS, &
Balfour HH (1981) Cytomegalovirus infection: A quantitative
prospective study of 320 consecutive renal transplants. Surgery,
89: 660-671.
Marsh JA, Dietert RR, & Combs GF (1981) Influence of dietary selenium
and vitamin E on the humoral immune response of the chick. Proc Soc
Exp Biol Med, 166: 228-236.
Martineau D, Béland P, Desjardins C, & Lagacé A (1987) Levels of
organochlorine chemicals in tissues of beluga whales (Delphinapterus
leucas) from the St Lawrence Estuary, Québec, Canada. Arch Environ
Contam Toxicol, 16: 137-147.
Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird FF, Travis W,
Suffredini AF, Deyton l, Kovacs JA, Falloon J, Davey R, Polis M,
Metcalf J, Baseler M, Wesley R, Gill VJ, Fauci AS, & Lane HC (1989)
CD4 counts as predictors of opportunistic pneumonias in human
immunodeficiency virus (HIV) infection. Ann Intern Med, 111: 223-231.
Mathews SE, Warinner JE, & Weeks BA (1990) Assays of immune function
in fish macrophages. In: Stolen JS, Fletcher TC, Anderson DP, Roberson
BS, & van Muiswinkel WB ed. Techniques in fish immunology, Vol. 1.
Fair Haven, New Jersey, SOS Publications, pp 155-163.
Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, &
Katagiri T (1990) A new allele of the lpr locus, lprcg, that
complements the gld gene in induction of lymphadenopathy in the mouse.
J Exp Med, 171: 519-531.
Mayani H, Guilbert LJ, & Janowska-Wieczorek A (1992) Biology of the
hemopoietic microenvironment. Eur J Haematol, 49: 225-233.
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, &
Rattner BA (1992) Physiological and nonspecific biomarkers. In:
Biomarkers, biochemical, physiological and histological markers of
anthropogenic stress. London, Lewis Publishers, pp 5-85.
McCabe MJ (1994) Mechanisms and consequences of immunomodulation by
lead. In: Dean JH, Luster MI, Munson AE, & Kimber I ed. Immunotoxicity
and immunopharmacology, 2nd Ed. New York, Raven Press, pp 143-162.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo- p-dioxin kills immature thymocytes by
Ca2+-mediated endonuclease activation. Science, 242: 256-259.
McConkey DJ, Orrenius S, & Jondal M (1990) Cellular signalling in
programmed cell death (apoptosis). Immunol Today, 11: 120-121.
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, & Weissman
IL (1988) The SCID-hu mouse: Murine model for the analysis of human
hematolymphoid differentiation and function. Science, 241: 1632-1639.
McDonald HR & Lees RK (1990) Programmed death of autoreactive
thymocytes. Nature, 343: 642-644.
McGregor DD, Hahn HH, & Mackaness GB (1973) The mediator of cellular
immunity. V. Development of cellular resistance to infection in
thymectomized irradiated rats. Cell Immunol, 6: 186-199.
Melia RJW, Du V, Florey C, Altman DG, & Swan AV (1977) Association
between gas cooking and respiratory disease in children. Br Med J,
ii: 149-152.
Mellert J, Hopt UT, Erath F, & Holzer H (1989) Differential effects of
azathioprine (aza), cyclosporin A (CSA) and dexamethasone (DEXA) on
lymphokine mediated inflammation (LMI) in rejecting allografts.
Transplant Proc, 21: 98-99.
Meuer SC, Fitzgerald KA, Hussey RE, Hodgdon JC, Schlossman SF, &
Reinherz EL (1983) Clonotypic structures involved in antigen-specific
human T cell function. Relationship to the T3 molecular complex. J Exp
Med, 157: 705-719.
Miller K (1992) Mineral dusts: Asbestos, silica and others.
In: Miller K, Turk JL ,& Nicklin S ed. Principles and practice of
immunotoxicology. Oxford, Blackwell Scientific Publications,
pp 217-227.
Miller MD & Krangel MS (1992) Biology and biochemistry of the
chemokines: A family of chemotactic and inflammatory cytokines. Crit
Rev Immunol, 12: 17-46.
Miller K, & Nicklin S, eds (1987) Immunology of the gastrointestinal
tract. Boca Raton, Florida, CRC Press.
Miller LE, Ludke HR, Peacock JE, & Tomar RH (1991) Manual of
laboratory immunology, 2nd Ed. Philadelphia, Lea & Febiger.
Minchin SA, Leitenberg D, Stunz LL, & Feldbush TL (1990) Polyclonal
activation of rat B cells. II. Dextran sulfate as a cofactor in
mitogen-induced and antigen-induced differentiation of rat B
lymphocytes. J Immunol, 145: 2427-2433.
Mirkin IR, Anderson HA, Handaran L, Hong R, Gilubjatnikov R, & Belluck
D (1990) Changes in T-lymphocyte distribution associated with
ingestion of aldi-carb-contaminated drinking water: A follow-up study.
Environ Res, 51: 35-50.
Mishell RI & Dutton RW (1967) Immunization of dissociated spleen cell
cultures from normal mice. J Exp Med, 126: 423-442.
Mittal A, Woodward B, & Chandra RK (1988) Involution of thymic
epithelium and low serum thymulin bioactivity in weanling mice
subjected to severe food intake restriction or severe protein
deficiency. Exp Mol Pathol, 48: 226-235.
Molfino NA, Wright SC, Katz I, Tarlo S, Silverman F, McClean PA,
Szalai JP, Raizenne M, Slutski AS, & Zamel N (1991) Effect of low
concentrations of ozone on inhaled allergen responses in asthmatic
subjects. Lancet, 338: 199-203.
Moolenbeek C (1982) Obtaining alveolar macrophages from small
laboratory animals. Lab Anim, 16: 56-58.
Morahan PS, Klykken PC, Smith SH, Harris LS, & Munson AE (1979)
Effects of cannabinoids on host resistance to Listeria
monocytogenesand herpes simplex virus. Infect Immun, 23: 670-674.
Morris DL, Snyder NK, Gokani V, Blair RE, & Holsapple MP (1992)
Enhanced suppression of humoral immunity in DBA/2 mice following
subchronic exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD).
Toxicol Appl Pharmacol, 112: 128-132.
Mortensen P, Bergman A, Bignert A, Hansen H-J, Harkonen T, & Olsson
M (1992) Prevalence of skull lesions in harbor seals (Phoca vitulina)
in Swedish and Danish museum collections: 1835-1988. Ambio,
21: 520-524.
Mosier DE (1990) Immunodeficient mice xenografted with human lymphoid
cells: New models for in vivo studies of human immunobiology and
infectious diseases. J Clin Immunol, 10: 185-191.
Mosmann TR & Coffman RL (1989) Heterogeneity and cytokine secretion
patterns and functions of helper T cells. Adv Immunol, 46: 111-147.
Moszczynski P, Bem S, Moszczynski P, & Bartus R (1990a) Indicators of
immunity and acute phase reaction in men in relation to the duration
of exposure to mercury vapors. Med Pract, 41: 169.
Moszczynski P, Lisiewicz J, Bartus R, & Bem S (1990b) The serum
immunoglobulins in workers after prolonged occupational exposure to
the mercury vapors. Rev Roum Méd Intern, 28: 25-30.
Mowat AM (1987) The regulation of immune responses to dietary protein
antigens. Immunol Today, 8: 93-98.
Muir DCG, Ford CA, Stewart REA, Smith TG, Addison RF, Zinck ME, &
Béland P (1990) Organochlorine contaminants in belugas
(Delphinapterus leucas), from Canadian waters. Can Bull Fish Aquat
Sci, 224: 165-190.
Munson AE & White KL (1990) Immunotoxicology. In: Clayson DB, Munro
IC, Shubik P, & Swenberg JA ed. Progress in predictive toxicology.
Amsterdam, Elsevier Scientific Publishers, pp 285-313.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspectives, 43: 41-52.
Murray MJ, Lauer LD, Luster MI, Luebke RW, Adams DO, & Dean JH (1985)
Correlation of murine susceptibility to tumor, parasite and bacterial
challenge with altered cell-mediated immunity following systemic
exposure to the tumor promoter phorbol myristate acetate. Int J
Immunopharmacol, 7: 491-500.
Myers MJ, Schook LB, & Bick PH (1987) Mechanisms of benzo( a)pyrene-
induced modulation of antigen presentation. J Pharmacol Exp Ther,
242: 399-404.
Nagarkatti PS, Sweeney GC, Gauldie J, & Clark DA (1984) Sensitivity to
suppression of cytotoxic T-cell generation by 2,3,7,8-tetra-
chlorodibenzo- p-dioxin (TCDD) is dependent on the Ah genotype of the
murine host. Toxicol Appl Pharmacol, 72: 169-176.
Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, & McCune JM (1990)
Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med,
172: 1055-1063.
Naraqi S, Jackson GG, Jonasson O, & Yamashiroya HJ (1977) Prospective
study of prevalence, incidence and source of herpesvirus infections in
patients with renal allografts. J Infect Dis, 136: 531.
Neas LM, Dockery DD, Ware JH, Spengler JD, Speizer FE, & Ferris BG
(1991) Association of indoor nitrogen dioxide with respiratory
symptoms and pulmonary function in children. Am J Epidemiol,
134: 204-218
Neckers LM & Cossman J (1983) Transferrin receptor induction in
mitogen-stimulated human T lymphocytes is required for DNA synthesis
and cell division and is regulated by interleukin 2. Proc Natl Acad
Sci USA, 80: 3494-3498.
Neefjes JJ & Momburg F (1993) Cell biology of antigen processing. Curr
Opin Immunol, 5: 27-34.
Neubert D (1992) Evaluation of toxicity of TCDD in animals as a basis
for human risk assessment. Toxic Subst J, 12: 237-276.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1990)
Polyhalo-genated dibenzo- p-dioxins and dibenzofurans and the immune
system. 1. Effects on peripheral lymphocyte subpopulations of a
non-human primate (Callitrix jacchus) after treatment with
2,3,7,8-tetrachlorodibenzo-p -dioxin (TCDD). Arch Toxicol,
64: 345-359.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1991)
Polyhalogenated dibenzo- p-dioxins and dibenzofurans and the immune
system. 2. In vitro effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) on lymphocytes of venous blood from man and a non-human primate
(Callitrix jacchus). Arch Toxicol, 65: 213-219.
Newcombe DS, Rose NR, & Bloom JC (1992) Clinical immunotoxicology. New
York, Raven Press.
Nickoloff BJ ed. (1993) Heterogeneity among CD36+ cells in normal
diseased human skin. In: Dermal immune system. Boca Raton, Florida,
CRC Press, pp 245-275.
Nigam SK, Karnik AB, Chattopadhyay P, Lakkad K, Venkaiah K,
& Kashyap SK (1993) Clinical and biochemical investigations to
evolve early diagnosis in workers involved in the manufacture of
hexachlorocyclohexane. Int Arch Occup Environ Health, 65: S193-S196.
Nikolaidos E, Brunström B, Dencker L, & Veromaa T (1990) TCDD inhibits
the support of B-cell development by the bursa of Fabricius. Pharmacol
Toxicol, 67: 22-26.
Nimmo Wilkie JS, Yager JA, Wilkie BN, & Pascoe PJ (1992) Changes in
cell-mediated immune responses after experimentally-induced
anaphylaxis in dogs. Vet Immunol Immunopathol, 32: 325-338.
Noonan FP & De Fabo EC (1990) Ultraviolet-B-dose-response curves for
local and systemic immunosuppression are identical. Photochem
Photobiol, 52: 801-810.
Noonan FP & De Fabo EC (1992) Immunosuppression by ultraviolet-B
radiation: Initiation by urocanic acid. Immunol Today, 13: 250-254.
Noonan FP & Hoffman HA (1994) Susceptibility to immunosuppression by
ultraviolet B radiation in the mouse. Immunogenetics, 39: 29-39.
Nossal GJV (1987) Immunology: The basic components of the immune
system. New Engl J Med, 316: 1320-1325.
Oberhelman L, Koren H, & Cooper KD (1992) Depressed capacity to
contact sensitize women at the onset of menses; elevated contact
sensitization capacity during mid cycle can be inhibited by UV.
Clin Res, 40: 542A.
O'Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE,
Nadel JA, & Holtzman (1984) Neutrophil depletion inhibits airway
hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis,
130: 214-219.
O'Dell BL, Jessen RT, Becker LE, Jackson RT, & Smith EB (1980)
Diminished immune response in sun-damaged skin. Arch Dermatol,
116: 559-561.
Oehler R & Herberman RB (1978) Natural cell-mediated cytotoxicity in
rats. III. Effects of immunopharmacologic treatments on natural
reactivity and reactivity augmented by plyinosinic-polycytidylicacid.
Int J Cancer, 21: 221-229.
Ono H, Klein D, Vincek V, Figueroa F, O'Huigin C, Tichy H, & Klein J
(1992) Major histocompatibility complex class II genes of zebra fish.
Proc Natl Acad Sci USA, 89: 11886-11890.
Orehek J, Grimaldi F, Muls E, Durand JP, Viala A, & Charpin J (1981)
Bronchial response to allergens after controlled NO2exposure. Bull Eur
Physiopathol Respir, 17: 911-915.
Organisation for Economic Co-operation and Development (1981) OECD
guidelines for testing chemicals. Repeated dose oral toxicity -
Rodent: 28-day or 14-day study (Guideline 407). Paris.
Osborn JE, Blazkovec AA, & Walker DL (1968) Immunosuppression during
acute cytomegalovirus infection. J Immunol, 100: 835-844.
Ozkaynak H, Kinney PL, & Burbank B (1990) Recent epidemiological
findings on morbidity and mortality effects of ozone. Air Waste Manage
Assoc Rep, 6: 90-150.
Pallardy MJ, House RV, & Dean JH (1989) Molecular mechanism of
7,12-dimethylbenz[ a]anthracene-induced immunosuppression: Evidence
for action via the interleukin-2 pathway. Mol Pharmacol, 36: 128-133.
Pallardy MJ, Mishal Z, Lebrec H, & Bohuon C (1992) Immune
modification due to chemical interference with transmembrane
signalling: Applications to polycyclic aromatic hydrocarbons.
Int J Immunopharmacol, 14: 377-382.
Paranjpe MS & Boone CW (1972) Delayed hypersensitivity to simian virus
40 tumor cells in BALB/c mice demonstrated by a radioisotopic foot-pad
assay. J Natl Cancer Inst, 48: 563-566.
Parhar RS & Lala PK (1987) Amelioration of B16F10 melanoma lung
metastasis in mice by a combination therapy with indomethacin and
interleukin 2. J Exp Med, 165: 14-28.
Pass RF, Long WK, Whitley RJ, Soong S, Diethelm A, Reynolds DW, &
Alford CA (1978) Productive infection with CMV and HSV in renal
transplant recipients: Role of source of kidney. J Infect Dis,
137: 556.
Patarroyo M (1991) Leukocyte adhesion in host defence and tissue
injury. Clin Immunol Immunopathol, 60: 333-348.
Patki AH (1991) Hypothesis: Solar ultraviolet radiation and the
initial skin lesion of leprosy. Int J Leprosy, 59: 492-493.
Paul WE (1993) Fundamental immunology, 3rd Ed. New York, Raven Press.
Paul PS, Johnson DW, Mirocha CJ, Soper FF, Thoen CO, Muscoplat CC, &
Weber AF (1977) In vitro stimulation of bovine peripheral blood
lymphocytes: Suppression of phytomitogen and specific antigen
lymphocyte responses by aflatoxin. Am J Vet Res, 38: 2033-2035.
Payne JF & Fancey LF (1989) Effect of polycyclic aromatic hydrocarbons
on immune response in fish: Change in melanomacrophage centers in
flounder (Pseudopleuronectes americanus) exposed to hydrocarbon-
contaminated sediments. Mar Environ Res, 28: 431-435.
Pedersen BK & Beyer JM (1986) A longitudinal study of the influence of
azathioprine on natural killer cell activity. Allergy, 41: 286-289.
Penit C & Ezine S (1989) Cell proliferation and thymocyte subset
reconstitution in sublethally irradiated mice: Compared kinetics of
endogenous and intrathymically transferred progenitors. Proc Natl Acad
Sci USA, 86: 5547-5551.
Penn I (1988) Cancer is a long-term hazard of immunosuppressive
therapy. J Autoimmun, 1: 545-558.
Penn I (1993a) Incidence and treatment of neoplasia after
transplantation. J Heart Lung Transplant, 12: 328-336.
Penn I (1993b) Tumors after renal and cardiac transplantation. Hematol
Oncol Clin North Am, 7: 431-445.
Pennington JE (1985) Immunosuppression of pulmonary host defense. Adv
Host Def Mech, 4: 141-164.
Penninks AH, Snoeij NJ, Peiters RHH, & Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: An
overview. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM,
& Van der Venne MT ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, p 191.
Pestka JJ & Bondy GS (1990) Alteration of immune function following
dietary mycotoxin exposure. Can J Physiol Pharmacol, 68: 1009-1016.
Pfeifer R & Irons R (1981) Inhibition of lecithin-stimulated
lymphocyte agglutination and mitogenesis by hydroquinone; reactivity
with sulfhydyl groups. Exp Mol Pathol, 35: 189-198.
Phair J, Munoz A, Detels RI, Kaslow R, Rinaldo C, & Saah A (1990) The
risk of Pneumocystis carinii pneumonia among men infected with human
immunodeficiency virus type 1. New Engl J Med, 322: 161-165.
Picker LJ & Butcher EC (1992) Physiological and molecular mechanisms
of lymphocyte homing. Ann Rev Immunol, 10: 561-591.
Pleiman CM, D'Ambrosio D, & Cambier JC (1994) The B-cell antigen
receptor complex: Structure and signal transduction. Immunol Today,
15: 393-399.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p -dioxin and
related halogenated aromatic hydrocarbons: Examination of the
mechanism of toxicity. Ann Rev Pharmacol Toxicol, 22: 517-554.
Pollock PL, Germolec DR, Comment CE, Rosenthal GJ, & Luster MI (1994)
Development of human lymphocyte engrafted SCID mice as a model for
immunotoxicity assessment. Fundam Appl Toxicol., 22: 130-138.
Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L,
Janeway CA, Murphy DB, & Bottomly K (1989) Monoclonal antibodies to
murine CD3e defined distinct epitopes, one of which may interact with
CD4 during T cell activation. J Immunol, 142: 4169-4175.
Prichard MG, Boerth LW, & Pennington JE (1987) Compartmental analysis
of resting and activated pulmonary natural killer cells. Exp Lung Res,
12: 239-251.
Prior AC & Sisodia CS (1982) The effects of ochratoxin A on the immune
responses of Swiss mice. Can J Comp Med, 46: 91-96.
Pruett SB (1992) Immunotoxicity of organophosphorus compounds.
In: Chambers JE & Levi PE ed. Organophosphates. Chemistry, fate and
effects. New York, Academic Press, pp 367-385.
Pruett SB & Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin
2 system. Toxicol Lett, 40: 11-20.
Pugh-Humphreys RGP, Ross CSK, & Thomson AW (1990) The influence of
FK-506 on the thymus. An immunophenotypic and structural analysis.
Immunology, 70: 398-404.
Quesniaux VFJ (1992) Interleukins 9, 10, 11 and 12 and kit ligand:
A brief overview. Res Immunol, 143: 385-400.
Quinnan GV, Manischewitz JE, & Ennis FA (1980) Role of cytotoxic
T-lymphocytes in murine cytomegalovirus infection. J Gen Virol,
47: 503-508.
Qureshi MA, Bloom SE, Hamilton JW, & Dietert RR (1989) Toxic effects
of methyl methanesulfonate (MMS) on activated macrophages from
chickens. Environ Mol Mutag, 13: 253-262
Rao GN, Bimbaurn LS, Collins JJ, Tennant RW, & Skow LC (1988) Mouse
strains for chemical carcinogenicity studies: Overview of a workshop.
Fundam Appl Toxicol, 10: 385-394.
Raskin RE, Tvedten HW, Bull RW, Crow SE, Dunstan RW, & Krehbiel JD
(1989) Natural killer cell activity in untreated and treated dogs with
lymphoma. Am J Vet Res, 4: 483-487.
Ratajczak HV, Thomas PT, Sothern RB, Vollmuth T, & Heck D (1993)
Evidence for genetic basis of seasonal differences in antibody
formation between two mouse strains. Chronobiol Int, 10: 383-394.
Ratcliffe NA & Rowly AF (1981) Intervertebrate blood cells. London,
Academic Press.
Raulet DH (1989) The structure, function, and molecular genetics of
the gamma/delta T cell receptor. Ann Rev Immunol, 7: 175-207.
Reed SG & Scott P (1993) T-Cell and cytokine responses in
leishmaniasis. Curr Opin Immunol, 5: 524-531.
Reggiani G (1980) Acute human exposure to TCDD in Seveso, Italy.
J Toxicol Environ Health, 6: 27-43.
Reigart JR & Graber CD (1976) Evaluation of the humoral immune
response of children with low lead exposure. Bull Environ Contam
Toxicol, 16: 112-117.
Reijnders PJH (1986) Reproductive failure in common seals feeding on
fish from polluted coastal waters. Nature, 324: 456-457.
Renton KW (1986) Factors affecting drug biotransformation. Clin
Biochem, 19: 72-75.
Renton KW, Gray JD, & Hall RI (1980) Decreased elimination of
theophylline after influenza vaccination. Can Med Assoc J,
123: 288-290.
Reyes J, Tzakis A, Green M, Nour B, Nalesnik M, Van Thiel D, Martin M,
Breinig MK, Fung LL, Cooper M, & Starzl TE (1991) Post-transplant
lymphoproliferative disorders under primary FK506 immunosuppression.
Transplant Proc, 23: 3044-3046
Reynolds HY & Thomson RE (1973) Pulmonary host defenses.
II. Interaction of respiratory antibodies with Pseudomonas aeruginosa
and alveolar macrophages. J Immunol, 111: 369-380.
Reynolds CW, Wenn AC, Barlozzari T, Wiltrout T, Reichardt WA, &
Herberman RB (1984) Natural killer activity in the rat. IV.
Distribution of large granular lymohocytes (LGL) following intravenous
and intraperitoneal transfer. Cell Immunol, 86: 371-380.
Richeldi L, Persichini T, Ferrara GB, Crystal RG, Markham T, Luisetti
M, Bertrorelli G, Guerritore D, & Saltini C (1992) Chronic beryllium
disease association with the HLA-DP 2.1 allele. Am Rev Respir Dis,
145 (Suppl): A324.
Riley WJ (1989) Insulin dependent diabetes mellitus, an autoimmune
disorder? Clin Immunol Immunopathol, 53: S92-S98.
Roberts RJ (1975) Melanin-containing cells of the teleost fish and
their relation to disease. In: Ribelin WE & Migaki G ed. The pathology
of fishes. Madison, Wisconsin, University of Wisconsin Press,
pp 399-428.
Roitt IM (1991) Essential immunology, 7th Ed. Oxford, Blackwell
Scientific Publications.
Roitt IM, Brostoff J & Male DK (1993) Immunology, 3rd Ed. London,
Gower Medical Publications.
Roizman B (1982) The family Herpesviridae. General description,
taxonomy, and classification. In: Roizman B ed. The viruses, Vol 1:
Herpesviruses. New York, Plenum Press, pp 1-23.
Roizman B, Carmichael LE, Deinhardt F, de The G, Nahmias AJ, Plowright
W, Rapp F, Sheldrick P, Takahashi M, & Wolf K (1981) Herpesviridae.
Definition, provisional nomenclature and taxonomy. Intervirology,
16: 201-217.
Rolstad B, Herberman RB, & Reynolds GW (1986) Natural killer activity
in the rat: V. The circulation patterns and tissue localization of
peripheral blood lymphocytes. J Immunol, 136: 2600-2808.
Rose LM, Richards TL, Petersen R, Peterson J, Hruby S, & Alvord EC Jr
(1991) Remitting-relapsing EAE in nonhuman primates: A valid model of
multiple sclerosis. Clin Immunol Immunopathol, 59: 1-15.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502-510.
Rosenthal GJ & Snyder CA (1987) Inhaled benzene reduces aspects of
cell-mediated tumor surveillance in mice. Toxicol Appl Pharmacol,
88: 35-43.
Rosenthal GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock
P, Ling-Yuan K, Kwon J, & Luster MI (1994) Asbestos stimulates
IL-8 production from human lung epithelial cells. J Immunol,
153: 3237-3244.
Ross GD (1992) Function of neutrophils in host defence and immunity.
In: Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca
Raton, Florida, CRC Press, pp 263-286.
Ross PS, De Swart RL, Reijnders PJH, Van Loveren H, Vos JG, &
Osterhaus ADME (1995) Contaminant-related suppression of delayed-type
hypersensitivity and antibody responses in harbor seals fed herring
from the Baltic Sea. Environ Health Perspectives, 103: 162-167.
Ross PS, De Swart RL, Timmerman HH, Reijnders PJH, Vos JG, Van Loveren
H, & Osterhaus ADME (in press) Suppression of natural killer activity
in harbour seals (Phoca virulina) fed Baltic Sea herring. Aquat
Toxicol.
Rothbard JB & Gefter ML (1991) Interactions between immunogenic
peptides and MHC molecules. Ann Rev Immunol, 9: 527-565.
Roths JB, Murphy ED, & Eicher EM (1984) A new mutation, gld, that
produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp
Med, 159: 1-20.
Rouveix B(1993) Opiates and immune function. Thérapie, 47: 503-512.
Rubin RH (1990) Impact of cytomegalovirus infection in organ
transplant recipients. Rev Infect Dis, 12: S754-S766.
Rubin RH, Cosimi AB, Hirsch MS, Herrin JT, Russell PS, & Tolkoff Rubin
NE (1981) Effects of antithymocyte globulin on CMV infection in renal
transplant recipients. Transplantation, 31: 143-145.
Ruitenberg EJ, Buys J, Teppema JS, & Elgersma A (1983) Rat mononuclear
cells and neutrophils are more effective than eosinophils in antibody-
mediated stage-specific killing of Trichinella spiralis in vitro.
Parasitenkunde, 69: 807-815.
Ryffel B (1992) The carcinogenicity of ciclosporin. Toxicology,
73: 1-22.
Saiki RK, Scharf SJ, Faloona FA, Mullis KB, Hom GT, Erlich HA, &
Arnheim N (1985) Enzymatic amplification of ß-globulin genomic
sequences and restriction site analysis for diagnosis of sickle cell
anemia. Science, 230: 1350-1354.
Sainte-Marie G, Bélisle C, & Peng FS (1990) The deep cortex of the
lymph node: Morphological variations and functional aspects. In:
Grundmann E & Vollmer E ed. Reaction patterns of the lymph node, Part
1. Cell types and functions. Berlin, Springer Verlag, pp 33-63.
Saito M (1992) Recent trends in research on differentiation of
hematopoietic cells and lymphokines (hemopoietins). Hum Cell,
5: 54-69.
Sakaguchi S & Sakaguchi N (1988) Thymus and autoimmunity. Transplanta-
tion of the thymus from cyclosporin A-treated mice causes organ-
specific autoimmune disease in athymic nude mice. J Exp Med,
167: 1479-1485.
Saltini C, Winestock K, Kirby M, Pinkston P, & Crystal RB (1989)
Maintenance of alveolitis in patients with chronic beryllium disease
by beryllium-specific helper T cells. New Engl J Med, 320: 1103-1109.
Samet JM (1994) Nitrogen dioxide and respiratory infection. Am Rev
Respir Dis, 139: 1073-1074.
Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA,
McLaren LC, Schwab M, & Spengler JD (1993) Nitrogen dioxide and
respiratory illnesses in infants. Am Rev Respir Dis, 148: 1258-1265.
Sanders VM, Tucker AN, White KL, Kauffmann BM, Hallett P, Carchman RA,
Borzelleca JF, & Munson AE (1982) Humoral and cell-mediated immune
status in mice exposed to trichloroethylene in the drinking water.
Toxicol Appl Pharmacol, 62: 358-368.
Sanders VM, White KL, Shopp GM, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to 1,1,2-trichloroethane. Drug
Chem Toxicol, 8: 357.
Sato K, Flajnik MF, Du Pasquier L, Katagiri M, & Kasahara M (1993)
Evolution of the MHC: Isolation of class II ß-chain cDNA clones from
the amphibian Xenopus laevis. J Immunol, 150: 2831-2843.
Scala R (1991) Risk assessment. In: Amdur M, Doull J, & Klaasen C ed.
Casarett and Doull's Toxicology, 3rd Ed. Elmsford, New York, Pergamon
Press, pp 985-996.
Scheuchenzuber WJ, Eskew ML, & Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environ Res, 38: 389-399.
Schielen P, Schoo W, Tekstra J, Oostermeijer HH, Seinen W, & Bloksma N
(1993) Autoimmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schlick E & Friedberg KD (1981) The influence of low lead doses on the
reticulo-endothelial system and leukocytes of mice. Arch Toxicol,
47: 197.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
C, Ritz J, Shaw S, Silverstein RL, Springer TA, Tedder TF, & Todd RF
(1994) CD antigens 1993. Immunol Today, 15: 98-99.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
T, Ritz J, Shaw S, Springer TA, Tedder TF, & Todd RF (1995) Leucocyte
typing, V: White cell differentiation antigens. Oxford, Oxford
University Press.
Schouk LB & Laskin DL (1994) Xenobiotics and inflammation. San Diego,
California, Academic Press.
Schuurman H-J, Kluin PM, Gmelig-Meyling FHJ, Van Unnik JAM, & Kater L
(1985) Lymphocyte status of lymph node and blood in acquired
immunodeficien-cy syndrome (AIDS) and AIDS-related complex disease.
J Pathol, 147: 269-280.
Schuurman H-J, Van Loveren H, Rozing J, Van Dijk A, Loeber JG, & Vos
JG (1990) Cyclosporin and the rat thymus. An immunohistochemical
study. Thymus, 16: 235-254.
Schuurman H-J, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1991a) Immune system. In: Haschek WM & Rousseaux CG ed. Handbook of
toxicologic pathology. San Diego, Academic Press, pp 421-487.
Schuurman H-J, Nagelkerken L, De Weger RA, & Rozing J (1991b) Age-
associated involution: Significance of a physiologic process. Thymus,
18: 1-6.
Schuurman H-J, De Weger RA, Van Loveren H, Krajnc-Franken MAM, & Vos
JG (1992a) Histopathological approaches. In: Miller K, Turk JL, &
Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 279-303.
Schuurman H-J, Hougen HP, & Van Loveren H (1992b) The rnu (Rowett
nude) and rnuN ( nznu, New Zealand nude) rat: An update. ILAR
News, 34: 3-12.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijt BC,
De Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Pass D, Vankerkom
J, Boot R, Broekhuizen R, Dormans J, van Herck H, Hermans PGC, Hoekman
J, van der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992c)
Comparative evaluation of the immune status of congenitally athymic
and euthymic rat strains bred and maintained at different institutes:
2. Athymic rats. J Exp Anim Sci, 35: 33-48.
Schuurman H-J, Van Loveren H, Rozing J, & Vos JG (1992d) Chemicals
trophic for the thymus: Risk for immunodeficiency and autoimmunity.
Int J Immunopharmacol, 14: 369-375.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijht BC,
de Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Vankerkom J, Boot
R, Broekhuizen R, Dormans J, van Herck H, Hermans GC, Hoekman J, van
der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992e) Comparative
evaluation of the immune status of congenitally athymic and euthymic
rat strains bred and maintained at different institutes: 1. Euthymic
rats. J Exp Anim Sci, 35: 16-32.
Schuurman H-J, Hu HZ, De Weger RA, & Clevers HC (1993) Thoughts on
thymus and T-lymphocyte repertoire. Relevance for tolerance of the
immune response. Neth J Med, 43: 38-54.
Schuurman H-J, Kuper CF, & Vos JG (1994) Histopathology of the immune
system as a tool to assess immunotoxicity. Toxicology, 86: 197-212.
Schwartz J (1992) Air pollution and the duration of acute respiratory
symptoms. Arch Environ Health, 47: 116-122.
Schwartz RS, Callen JP, & Silva JR (1978) A cluster of Hodgkin's
disease in a small community: Evidence for environmental factors.
Am J Epidemiol, 108: 19-26.
Schwartz J, Spix C, Wichmann HE, & Malin E (1991) Air pollution and
acute respiratory illness in five German communities. Environ Res,
56: 1-14.
Schwetz BA & Tyl RW (1987) Consensus workshop on the evaluation of
maternal and developmental toxicity. Workgroup III report. Low dose
extrapolation and other considerations for risk assessment - models
and applications. Teratog Carcinog Mutag, 1: 1-7.
Secombes CJ, Fletcher TC, White A, Costello MJ, Stagg R, & Houlihan DF
(1992) Effects of sewage sludge on immune response in the dab,
Limanda limanda. Aquat Toxicol, 23: 217-230.
Seinen W & Penninks AH (1979) Immune suppression as a consequence of a
selective cytotoxic activity of certain organometallic compounds on
thymus and thymus-dependent lymphocytes. Ann NY Acad Sci,
320: 499-517.
Seinen W & Willems MI (1976) Toxicity of organotin compounds.
I. Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di- n-octyltin dichloride. Toxicol Appl Pharmacol, 35: 63-75.
Seinen W, Vos JG, Van Krieken R, Penninks AH, Brands R, & Hooykaas H
(1977) Toxicity of organotin compounds. II. Comparative in vivo and
in vitro studies with various organotin and organolead compounds in
different animal species with special emphasis on lymphocyte
cytotoxicity. Toxicol Appl Pharmacol, 42: 197-212.
Sela O & Shoenfeld Y (1988) Cancer in autoimmune diseases. Semin Arthr
Rheum, 18: 78-87.
Selgrade MJK & Gilmour MI (1994) Effects of gaseous air pollutants on
immune responses and susceptibility to infectious and allergic
disease. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicity and immunopharmacology, 2nd Ed. New York, Raven Press,
pp 395-411.
Selgrade MJK & Osborn TE (1974) Role of macrophages in resistance to
murine cytomegalovirus. Infect Immun, 10: 1383-1390.
Selgrade MJK, Daniels MJ, Hu PC, Miller FJ, & Graham JA (1982) Effects
of immunosuppression with cyclophosphamide on acute murine
cytomegalovirus infection and virus-augmented natural killer cell
activity. Infect Immun, 38: 1046-1055.
Selgrade MK, Daniels MJ, Illing JW, Ralston AL, Grady MA, Chartlet E,
& Graham JA (1984) Increased susceptibility to parathion poisoning
following murine cytomegalovirus infection. Toxicol Appl Pharmacol,
76: 356-364.
Selgrade MJK, Illing JW, Starnes DM, Stead AG, Menache MG, & Stevens
MA (1988) Evaluation of effects of ozone exposure on influenza
infection in mice using several indicators of susceptibility. Fundam
Appl Toxicol, 11: 169-180.
Selgrade MJK, Starnes DM, Illing JW, Daniels MJ, & Graham JA (1989)
Effects of phosgene exposure on bacterial, viral, and neoplastic lung
disease susceptibility in mice. Inhal Toxicol, 1: 243-259.
Selgrade MJK, Daniels MJ, & Dean JH (1992) Correlation between
chemical suppression of natural killer cell activity in mice and
susceptibility to cytomegalovirus: Rationale for applying murine
cytomegalovirus as a host resistance model and for interpreting
immunotoxicity testing in terms of risk of disease. J Toxicol Environ
Health, 37: 123-137.
Selgrade MJK, Cooper KD, Devlin RB, Van Loveren H, Biagini RE, &
Luster MI (1995) Immunotoxicity - Bridging the gap between animal
research and human health effects. Fundam Appl Toxicol, 24: 13-21.
Sell S (1987) Immunology, immunopathology and immunity, 4th Ed.
Norwalk, Connecticut, Appleton & Lange.
Shabtai M, Malinowski K, Waltzer WC, Pullis C, Raisbeck AP, & Rapaport
FT (1991) Quantitative analysis of surface marker densities after
exposure of T cells to concanavalin A (ConA): A sensitive early index
of cellular activation. Cell Immunol, 133: 519-525.
Shand FL (1975) Review/commentary: The immunopharmacology of
cyclophosphamide. Int J Immunopharmacol, 1: 165-171.
Sharp JG & Watkins EB (1981) Cellular and immunological consequences
of thymic irradiation. In: Dubois JB, Serrou B, & Rosenfeld C ed.
Immunopharmacologic effects of radiation therapy. New York, Raven
Press, pp 137-179.
Sheil AG, Disney AP, Mathew TH, Amiss N, & Excell L (1991) Cancer
development in cadaveric donor renal allograft recipients treated with
azathioprine (AZA) or cyclosporine (CyA) or AZA/CyA. Transplant Proc,
23: 1111-1112.
Sherwood RL, Tarkington B, Lippert WE, & Goldstein E (1981) Effect of
ferrous sulphate aerosols and nitrogen dioxide aerosols on murine
pulmonary defense. Arch Environ Health, 36: 130-135.
Sherwood RL, Thomas PT, Kawanishi CY, & Fenters JD (1988) Comparison
of Streptococcus zooepidemicus and influenza virus pathogenicity in
mice by three pulmonary exposure routes. Appl Environ Microbiol,
54: 1744-1751.
Shigematsu N, Ishimaru S, Saito R, Ikeda T, Matsuba K, Sigiyama K, &
Masuda Y (1978) Respiratory involvement in polychlorinated biphenyls
poisoning. Environ Res, 16: 92-100.
Shiroky JB, Frosp A, Skelton JD, Haegert DG, Newkirk MM, & Neville
C (1991) Complications of immunosuppression associated with weekly low
dose methrotrexate. J Rheum, 18: 1172-1175.
Shopp GM & Munson AE (1985) Suppression of the antibody response by
phorbol esters in the mouse is due to an effect on the nylon wool
adherent cell population. Agents Actions, 6: 535-541.
Shopp GM, Sanders VM, White KL, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to trans-12-dichlorethylene.
Drug Chem Toxicol, 8: 393.
Shortman K, Egerton M, Spangrude GJ, & Scollay R (1990) The generation
and fate of thymocytes. Sem Immunol, 2: 3-13.
Shy CM, Creason JP, Pearlman ME, McClain KE, & Benson FB (1970) The
Chattanooga study: Effects of community exposure to nitrogen dioxide.
II. Incidence of acute respiratory illness. J Air Pollut Control
Assoc, 20: 582-588.
Sibinga NES & Goldstein A (1988) Opioid peptides and opioid receptors
in cells of the immune system. Ann Rev Immunol, 6: 219-249.
Sigal NH & Dumont FJ (1992) Cyclosporin A, FK-506, and Rapamycin:
Pharmacologic probes of lymphocyte signal transduction. Ann Rev
Immunol, 10: 519-560.
Sikorski EE, McCay JA, White KL, Bradley SG, & Munson AE (1989)
Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1
mice. Fundam Appl Toxicol, 13: 843-858.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW,
Wilcox KR, VanAmburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls.
J Reticuloendothel Soc, 26: 341.
Sima P & Vetvicka V (1992) Evolution of immune accessory function. In:
Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca Raton,
Florida, CRC Press, pp 1-56.
Simon JC, Cruz PD, Bergstresser PR, & Tigelaar RE (1990) Low dose
ultraviolet B-irradiated Langerhans cells preferentially activate
CD4+ cells of the T helper 2 subset. J Immunol, 145: 2087-2094.
Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, & Cruz PD (1991)
Ultraviolet B radiation converts Langerhans cells from immunogenic to
tolerogenic antigen-presenting cells. Induction of specific clonal
anergy in CD4+ T helper 1 cells. J Immunol, 146: 485-491.
Singh G, Fries JF, Spitz P, & Williams CA (1989) Toxic effects of
azathioprine in rheumatoid arthritis patients. A national post-
marketing perspective. Arthr Rheum, 32: 837-843.
Siroki O, Institoris L, Tatar F, & Desi I (1994) Immunotoxicological
investigation of SCMF, a new pyrethroid pesticide in mice. Hum Exp
Toxicol, 13: 337-343.
Siwicki AK, Cossarini-Dunier M, Studnicka M, & Demael A (1990)
In vivo effect of the organophosphorus insecticide trichlorphon on
immune response of carp (Cyprinus carpio). Ecotoxicol Environ Saf,
19: 99-105.
Sjoblad RD (1988) Potential future requirements for immunotoxicity
testing of pesticides. Toxicol Ind Health, 4: 391-394.
Sjovall P, Christensen OB, & Moller H (1985) Single exposure to
ultraviolet irradiation and elicitation of human allergic contact
dermatitis. Acta Dermatol Venereol, 65: 93-96.
Smialowicz RJ, Rogers RR, Riddle MM, Luebke RW, Rowe DG, & Garner RJ
(1984) Manganese chloride enhances murine cell-mediated cytotoxicity:
Effects on natural killer cells. J Immunopharmacol, 6: 1-23.
Smialowicz R, Luebke RW, Riddle MM, Rogers RR, & Rowe DG (1985a)
Evaluation of the immunotoxic potential of chlordecone with comparison
to cyclophosphamide. J Toxicol Environ Health, 15: 561-574.
Smialowicz RJ, Luebke RW, Rogers RR, Riddle MM, & Rowe DG (1985b)
Evaluation of immune function in mice exposed to ordram. Toxicology,
37: 307-314.
Smialowicz RJ, Rogers RR, Riddle MM, Garner RJ, Rowe DG, & Luebke RW
(1985c) Immunologic effects of nickel. II. Suppression of natural
killer cell activity. Environ Res, 36: 56-66.
Smialowicz RJ, Andrews JE, Riddle MM, Rogers RR, Luebke RW, & Copeland
CB (1989) Evaluation of the immunotoxicity of low level PCB exposure
in the rat. Toxicology, 56: 197-211.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB, & Ernest
GG (1990) Immune alterations in rats following subacute exposure to
tributyltin oxide. Toxicology, 64: 169-178.
Smialowicz RJ, Riddle MM, Luebke RW, Copeland CB, Andrews D, Rogers
RR, Gray LE, & Laskey JW (1991) Immunotoxicity of 2-methoxyethanol
following oral administration in Fischer 344 rats. Toxicol Appl
Pharmacol, 109: 494-506.
Smialowicz RJ, Luebke RW, & Riddle MM (1992a) Assessment of the
immunotoxic potential of the fungicide dinocap in mice. Toxicology,
75: 235-247.
Smialowicz RJ, Williams WC, Riddle MM, Andrews DL, Luebke RW, &
Copeland CB (1992b) Comparative immunosuppression of various glycol
ethers orally administered to Fischer 344 rats. Fundam Appl Toxicol,
18: 621-627.
Smialowicz RJ, Riddle MM, & Williams WC (1993) Methoxyacetaldehyde, an
intermediate metabolite of 2-methoxyethanol, is immunosuppressive in
the rat. Fundam Appl Toxicol, 21: 1-7.
Sminia T, Van der Brugge-Gamelkoorn GJ, & Jeurissen SHM (1989)
Structure and function of bronchus-associated lymphoid tissue (BALT).
Crit Rev Immunol, 9: 119-150.
Smith SH, Sanders VM, Barrett BA, Borzellaca JF, & Munson AE (1978)
Immunotoxicological evaluation on mice exposed to polychlorinated
biphenyls. Toxicol Appl Pharmacol, 45: 330.
Snoeij NJ, Penninks AH, & Seinen W (1988) Dibutyltin and tributyltin
compounds induce thymic atrophy in rats due to a selective action on
thymic lymphoblasts. Int J Immunopharmacol, 10: 889-891.
Snyder CA (1989) The neuroendocrine system, an opportunity for
immunotoxicologists. Environ Health Perspectives, 81: 165-166.
Spangrude GJ, Berhard EJ, Ajoika RS, & Daynes RA (1983) Alterations in
lymphocyte homing patterns within mice exposed to ultraviolet
radiation. J Immunol, 130: 2974-2981.
Speizer FE, Ferris B, Bishop YMM, & Spengler J (1980) Respiratory
disease rates and pulmonary function in children associated with NO2
exposure. Am Rev Respir Dis, 121: 3-10.
Spiekermann K, Emmendoerfer A, Elsner J, Raeder E, Lohmann-Matthes ML,
Prahst A, Link H, Freund M, Welte K, & Roesler J (1994) Altered
surface marker expression and function of G-CSF induced neutrophils
from test subjects and patients under chemotherapy. Br J Haematol,
87: 31-38.
Spreafico F, Merendino A, Braceschi L, & Sozanni S (1987)
Immunodepressive drugs as prototype immunotoxicants. In: Berlin A,
Dean J, Draper MH, Smith EMB, & Van Der Venne, M-T ed.
Immunotoxicology. Dordrecht, Martinus Nijhoff, pp 192-207.
Sprent J, Gao EK, & Webb SR (1990) T Cell reactivity to MHC molecules:
Immunity versus tolerance. Science, 248: 1357-1363.
Springer TA (1990) Adhesion receptors of the immune system. Nature,
346: 425-434.
Springer T, Galfré G, Secher DS, & Milstein C (1979) Mac-1: A
macrophage differentiation antigen identified by monclonal antibody.
Eur J Immunol, 9: 301-306.
Spruanoe SL (1985) Pathogenesis of herpes simplex labialis:
Experimental induction of lesions with UV light. J Clin Microbiol,
22: 366-368.
Stahlmann R, Korte M, Van Loveren H, Vos JG, Thiel R, & Neubert D
(1992) Abnormal thymus development and impaired function of the immune
system in rats after prenatal exposure to aciclovir. Arch Toxicol,
66: 551-559.
Stanley GP & Pender MP (1991) The pathophysiology of chronic relapsing
experimental allergic encephalomyelitis in the Lewis rat. Brain,
114: 1827-1853.
Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP,
Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL, Atchison RW, Jaffe R, &
Bahnson HT (1984) Reversibility of lymphomas and lymphoproliferative
lesions developing under cyclosporin-steroid therapy. Lancet,
i: 583-587.
Stehr-Green PA, Naylor PH, & Hoffman RE (1989) Diminished thymosin
alpha-1 levels in persons exposed to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. J Toxicol Environ Health, 28: 285-295.
Steinmann GG (1986) Changes in the human thymus during aging. Curr
Topics Pathol, 75: 43-88.
Stein-Streinlein J, Bennett M, Mann D, & Kumar V (1983) NK cells in
mouse lungs: Surface phenotype, target preference and response to
local influenza virus infection. J Immunol, 131: 2699-2704.
Stet RJM & Egberts E (1991) The histocompatibility system in
teleostean fishes: From multiple histocompatibility loci to a major
histocompatibility complex. Fish Shellfish Immunol, 1: 1-16.
Stingl G & Steiner G (1989) Immunological host defence of the skin.
Curr Prob Dermatol, 18: 22-30.
Stobo JD & Paul WE (1973) Functional heterogeneity of murine
lymphoid cells. III. Differential responsiveness of T cells to
phytohemagglutinin and concanavalin A for T cell subsets. J Immunol,
110: 362-369.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral responses by pesticides and chemicals of environmental
concern: Quantitative studies of immunosuppression by DDT, Aroclor,
carbaryl, carbofuran and methylparathion. Toxicol Appl Pharmacol,
32: 587-602.
Streilein JW (1983) Skin associated lymphoid tissues (SALT): Origins
and functions. J Invest Dermatol, 80 (Suppl): 12s-16s.
Streilein JW (1990) Skin-associated lymphoid tissues (SALT): The next
generation. In: Bos J ed. The skin immune system. Boca Raton, Florida,
CRC Press, pp 25-48.
Strobel S & Ferguson A (1984) Immune responses to fed protein antigens
in mice. 3. Systemic tolerance or priming is related to age at which
antigen is first encountered. Ped Res, 18: 588-594.
Stunz LL & Feldbush TL (1986) Polyclonal activation of rat B
lymphocytes. I. A single mitogenic signal can stimulate proliferation,
but three signals are required for differentiation. J Immunol,
136: 4006-4012.
Stutman O (1986) Postthymic T-cell development. Immunol Rev,
91: 160-194.
Subramanian A, Tanabe S, Tatsukawa R, Saito S, & Miyazaki N (1987)
Reduction in the testosterone levels by PCBs and DDE in Dall's
porpoises of northwestern North Pacific. Mar Pollut Bull, 18: 643-646.
Sung YJ, Hotchkiss JH, Austic RE, & Dietert RR (1992) Direct
measurement of nitric oxide in headspace gas produced by a chicken
macrophage cell line in a closed culture system. Biochem Biophys Res
Commun, 184: 36-42.
Surhe CD & Sprent J (1991) Long-term xenogeneic chimeras. Full
differentiation of rat T and B cells in SCID mice. J Immunol,
147: 2148-2154.
Svensson BG, Nilsson A, Hansson M, Rappe C, Åkesson B, & Skerving S
(1991) Exposure to dioxins and dibenzofurans through the consumption
of fish. New Engl J Med, 324: 8-12.
Swain WR (1991) Effects of organochlorine chemicals on the
reproductive outcome of humans who consumed contaminated Great Lakes
fish: An epidemiologic consideration. J Toxicol Environ Health,
33: 587-639.
Swerdlow AJ, Douglas AJ, Vaughan Hudson G, Vaughan Hudson B, Bennett
MH, & MacLennan KA (1992) Risk of second primary cancers after
Hodgkin's disease by type of treatment: Analysis of 2846 patients in
the British National Lymphoma Investigation. Br Med J, 304: 1137-1143.
Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR,
Heroux AL, Dizikes GJ, Pifarre R, & Fisher RI (1990) Increased
incidence of lymphoproliferative disorder after immunosuppression with
the monoclonal antibody OKT3 in cardiac-transplant recipient. New Engl
J Med, 323: 1723-1728.
Szakal AK, Kosco MH, & Tew JG (1989) Microanatomy of lymphoid tissue
during humoral immune responses: Structure function relationships. Ann
Rev Immunol, 7: 91-109.
Taga T, & Kishimoto T (1992) Cytokine receptors and signal
transduction. FASEB J, 6: 3387-3396.
Takeya U, Shimotori S, Tanaguchi T, & Nomoto K (1977) Cellular
mechanisms in the protection against infection by Listeria
monocytogenesin mice. J Gen Microbiol, 100: 373-379.
Talcott PA & Koller LD (1983) The effect of inorganic lead and/or a
polychlorinated biphenyl on the developing immune system of mice. J
Toxicol Environ Health, 12: 337-352.
Talcott PA, Koller LD, & Exon JH (1985) The effect of lead and
polychlorinated biphenyl exposure on rat natural killer cell
cytotoxicity. Int J Immunopharmacol, 7: 255-261.
Taylor BA (1972) Genetic relationships between inbred strains of mice.
J Hered, 63: 83-86.
Taylor JR, Schmieder GF, Shimizu T, Tie C, & Streilin JW (1994)
Interrelationship between ultraviolet light and recurrent herpes
simplex infections in man. J Dermatol Sci, 8: 224-232.
Temple L, Kawabata TT, Munson AE, & White KL (1993) Comparison of
ELISA and plaque-forming cell assays for measuring the humoral immune
response to SRBC in rats and mice treated with benzo[ a]pyrene or
cyclophosphamide. Fundam Appl Toxicol, 21: 412-419.
Theofilopoulos AN & Dixon FJ (1985) Murine models of systemic lupus
erythematosus. Adv Immunol, 37: 269-390.
Thiem PA, Halper LK, & Bloom JC (1988) Techniques for assessing canine
mononuclear phagocytic function as part of an immunotoxicologic
evaluation. Int J Immunopharmacol, 10: 765-771.
Thomas PT & Hinsdill RD (1978) Effect of polychlorinated biphenyls on
the immune responses of rhesus monkeys and mice. Toxicol Appl
Pharmacol, 44: 41-51.
Thomas PT & Hinsdill RD (1979) The effect of perinatal exposure to
tetrachlorodibenzo- p-dioxin on the immune response of young mice.
Drug Chem Toxicol, 2: 77-98.
Thomas PT, Ratajczak HV, Aranyi C, Gibbons R, & Fenters JD (1985a)
Evaluation of host resistance and immune function in cadmium-exposed
mice. Toxicol Appl Pharmacol, 80: 446-456.
Thomas PT, Fugmann R, Aranyi C, Barbera P, Gibbons R, & Fenters J
(1985b) Effect of dimethylnitrosamine on host resistance and immunity.
Toxicol Appl Pharmacol, 77: 219-229.
Thomas PT, Ratajczak HV, Eisenberg WC, Furedi-Machacek M, Ketels K, &
Barbera PW (1987) Evaluation of host resistance and immunity in mice
exposed to the carbamate pesticide aldicarb. Fundam Appl Toxicol,
9: 82-89.
Thomson AW (1992) The spectrum of action of new immunosuppressive
drugs. Clin Exp Immunol, 89: 170-173.
Thurmond LM, Lauer LD, House RV, Cook JC, & Dean JH (1987) Immuno-
suppression following exposure to 7,12-dimethylbenz[ a]anthracene
(DMBA) in Ah-responsive and Ah-nonresponsive mice. Toxicol
Appl Pharmacol, 91: 450-460.
Tieben LM, Berkhout RJM, Smits HL, Bouwes Bavinck JN, Vermeer BJ,
Bruijn JA, van der Woude FJ, & ter Schegget J (1994) Detection of
epidermodysplasia verruciformis-like human papillomavirus types in
renal allograft recipients. Br J Dermatol, 131: 226-230.
Tilney NL (1971) Patterns of lymphatic drainage in the adult
laboratory rat. J Anat, 109: 369-383.
Timonen T, Ortaldo JR, & Herberman RB (1981) Characteristics of human
large granular lymphocytes and relationship to natural killer and K
cells. J Exp Med, 153: 569-582.
Timonen T, Ortaldo JR, & Herberman RB (1982) Isolation of human and
rat natural killer cells. J Immunol Meth, 51: 269-277.
Toews GB, Bergstresser PR, Streilein JW, & Sullivan S (1980) Epidermal
Langerhans cell density determines whether contact hypersensitivity or
unresponsiveness follows skin painting with DNFB. J Immunol,
124: 445-453.
Tomlinson S (1993) Complement defense mechanisms. Curr Opin Immunol,
5: 83-89.
Tonari Y, & Minamishima Y (1983) Pathogenicity and immunogenicity of
temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol,
64: 1983-1990.
Tracey DE, Walfe SA, Durdik JM ,& Henney CS (1977) BCG-induced murine
effector cells; cytolytic activity in peritoneal exudates: An early
response to BCG. J Immunol, 119: 1145-1151.
Truelove J, Grant D, Mes J, Tryphonas H, Tryphonas L, & Zawidzka Z
(1982) Polychlorinated biphenyl toxicity in the pregnant cynomolgus
monkey: A pilot study. Arch Environ Contam Toxicol, 11: 583-588.
Tryphonas H (in press) Immunotoxicity of PCBs (Aroclors) in relation
to Great Lakes. Environ Health Perspectives, Suppl 9.
Tryphonas H, Hayward S, O'Grady L, Loo JCK, Arnold DL, Bryce F, &
Zawidzka ZZ (1989) Immunotoxicity studies of PCB (Aroclor 1254) in the
adult rhesus (Macaca mulatta) monkey--Preliminary report. Int J
Immunopharmacol, 11: 199-206.
Tryphonas H, Luster MI, White KL, Naylor PH, Erdos MR, Burleson GR,
Germolec D, Hodgen M, Hayward S, & Arnold DL (1991a) Effects of PCB
(Aroclor 1254) on non-specific immune parameters in rhesus (Macaca
mulatta) monkeys. Int J Immunopharmacol, 13: 639-648.
Tryphonas H, Luster MI, Schiffman G, Dawson L-L, Hodgen M, Germolec D,
Hayward S, Bryce, F, Loo JCK, Mandy F, & Arnold DL (1991b) Effects of
chronic exposure of PCB (Aroclor 1254) on specific and non-specific
immune parameters in the rhesus (Macaca mulatta) monkey. Fundam Appl
Toxicol, 16: 773-786.
Tsang PH, Chu FN, Fischbein A, & Bekesi JG (1988) Impairments in
functional subsets of T-suppressor (CD8) lymphocytes, monocytes, and
natural killer cells among asbestos-exposed workers. Clin Immunol
Immunopathol, 47: 323-332.
Tschöpe D, Roesen B, Scwippert B, Kehrel B, Scauseil S, Esser J, &
Gries FA (1990) Platelet analysis using flow cytometry procedures.
Platelets, 1: 127-133.
Tucker AN & Munson AE (1981) In vitro inhibition of the primary
antibody response to sheep erythrocytes by cyclophosphamide. Toxicol
Appl Pharmacol, 59: 617-619.
Tucker AN, Hong L, Boorman GA, Pung O, & Luster MI (1985) Alteration
of bone marrow cell cycle kinetics by diphenylhydantoin: Relationship
to folate utilization and immune function. J Pharmacol Exp Ther,
234: 57-62.
Tucker AN, Vore SJ, & Luster MI (1986) Suppression of B cell
differentiation by 2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol
Pharmacol, 29: 372-377.
Uber CL & McReynolds RA (1982) Immunotoxicology of silica. CRC Crit
Rev Toxicol, 10: 303-319.
Umesaki Y, Setoyama H, Matsumoto S, & Okada Y (1993) Expansion of
alpha ß T-cell receptor-bearing intestinal intraepithelial lymphocytes
after microbial colonization in germ-free mice and its independence
from thymus. Immunology, 79: 32-37.
Urban JL, Burton RC, Holland JM, Kripke ML, & Schreiber H (1982)
Mechanisms of syngeneic tumor rejection, susceptibility of host-
selected progressor variants to various immunological effector cells.
J Exp Med, 155: 557-573.
US Environmental Protection Agency (1986) Guidelines for the health
assessment of suspect developmental toxicants. Fed Regist,
51: 34027-34040.
US Environmental Protection Agency (1990) Respiratory health effects
of passive smoking: Lung cancer and other disorders
(EPA/600/6-90/006F). Cincinnati, Ohio, Center for Environmental
Research Information.
US Environmental Protection Agency (1991) Workshop report on toxicity
equivalency factors for polychlorinated biphenyl congeners. Washington
DC, Risk Assessment Forum.
US Food and Drug Administration (1993) Draft toxicological principles
for the safety assessment of direct food additives and color additives
used in food ('Redbook II'). Washington DC, Center for Food Safety and
Applied Nutrition.
US National Academy of Sciences (1977) Committee for the revision of
NAS publication No. 1138: Principles and procedures for evaluating the
toxicity of household substances. Washington DC.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Washington DC, National Academy
Press, pp 1-50.
US National Research Council (1989) Biologic markers in pulmonary
toxicology. Washington DC, National Academy Press.
US National Research Council (1992) Biologic markers in
immunotoxicology. Washington DC, National Academy Press.
US Office of Technology Assessment (1991) Identifying and controlling
immunotoxic substances: Background paper (OTA-BP-BA-75). Washington
DC, Congress of the United States, US Government Printing Office.
Vaessen LMB, Joling P, Nieuwenhuis P, Van Haperen MJ, & Rozing J
(1985) T Cell markers on pre-B cells in the rat. Adv Exp Med Biol,
186: 17-26.
Vallejo AN, Miller NW, Jorgensen T, & Clem LW (1990) Phylogeny of
immune recognition: Antigen processing/presentation in channel
catfish; immune responses to hemocyanins. Cell Immunol, 130: 364-377.
Vallejo AN, Miller NW, & Clem LW (1992) Antigen processing and
presentation in teleost immune responses. Ann Rev Fish Dis,
2: 167-183.
Valli VE, Villeneuve DC, Reed B, Barsoum N, & Smith G (1990)
Evaluation of blood and bone marrow, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI Monograph). Berlin, Springer
Verlag, pp 9-26.
Van Bressem MF, Visser IKG, Van de Bildt MWG, Teppema JS, Raga JA, &
Osterhaus ADME (1991) Morbillivirus infection in Mediterranean striped
dolphins (Stenella coeruleoalba). Vet Rec, 129: 471-472.
Vandekerckhove BAE, Krowka JF, McCune JM, De Vries JE, Spits H, &
Roncarolo MG (1991) Clonal analysis of the peripheral T cell
compartment of the SCID-hu mouse. J Immunol, 146: 4173-4179.
Van den Eertwegh AJM, Boersma WJA, & Claassen E (1992) Immunological
functions and in vivo cell-cell interactions of T cells in the
spleen. Crit Rev Immunol, 11: 337-380.
Van Deuren M, Dofferhoff AS, & Van der Meer JW (1992) Cytokines and
the response to infection. J Pathol, 168: 349-356.
Van Diepen JCE, Wagenaar GTM, & Rombout JHWM (1991) Immunocytochemical
detection of membrane antigens of carp leukocytes using light and
electron microscopy. Fish Shellfish Immunol, 1: 47-57.
Van Ewijk W (1988) Cell surface topography of thymic microenvironments.
Lab Invest, 59: 579-590.
Van Ewijk W (1991) T-Cell differentiation is influenced by thymic
microenvironments. Ann Rev Immunol, 9: 591-615.
Van Logten MJ, Gupta BN, McConnell EE, & Moore JA (1981) The influence
of malnutrition on the toxicity of tetrachlorodibenzo- p-dioxin
(TCDD) in rats. Toxicology, 21: 77-88.
Van Loveren H & Vos JG (1989) Immunotoxicological considerations: A
practical approach to immunotoxicity testing in the rat. In: Dayan AD
& Paine AJ ed. Advances in applied toxicology. London, Taylor &
Francis, pp 143-165.
Van Loveren H & Vos JG (1992) Evaluation of OECD guideline # 407 for
assessment of toxicity of chemicals with respect to potential adverse
effects to the immune system (RIVM report no. 158801001). Bilthoven,
National Institute of Public Health and Environmental Protection.
Van Loveren H, Postma GW, Van Soolingen D, Kruizinga W, Groothuis D, &
Vos JG (1987) Enhanced macrophage activity and deficient acquired
resistance to Listeria monocytogenes in nude rats. In: Rygaard J,
Brunner N, Graem N, & Spang-Thomson M ed. Immune-deficient animals in
biomedical research. Basel, Karger.
Van Loveren H, Rombout PJA, Wagenaar SS, Walvoort HC, & Vos JG (1988a)
Effects of ozone on the defence to a respiratory Listeria
monocytogenes infection in the rat. Suppression of macrophage
function and cellular immunity and aggravation of histopathology in
lung and liver during infection. Toxicol Appl Pharmacol, 94: 374-393.
Van Loveren H, Osterhaus ADME, Nagel J, Schurrman HJ, & Vos JG (1988b)
Quantification of IgA responses in the rat. Detection of IgA
antibodies and enumeration of IgA antibody producing cells specific
for ovalbumin or Trichinella spiralis. Scand J Immunol, 28: 377-381.
Van Loveren H, Teppema JS, & Askenase PW (1990a) Skin mast cells.
In: Bos J ed. The skin immune system. Boca Raton, Florida, CRC Press,
pp 171-193.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, & Vos JG (1990b)
Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Loveren H, Van Eden W, Krajnc EI, Krajnc-Franken MAM, De Kort W, &
Vos JG (1990c) Hexachlorobenzene interferes with the development of
experimental autoimmune disease in the Lewis rat. Toxicologist,
10: 221.
Van Loveren H, Verlaan APJ, & Vos JG (1991) An enzyme-linked
immunosorbent assay of anti-sheep red blood cell antibodies of the
classes M, G and A in the rat. Int J Immunopharmacol, 13: 689-695.
Van Loveren H, Timmerman H, Dortant P, & De Waal E (1993a) Validation
of a screening battery for immunotoxicity of drugs within a 28 day
oral toxicity study, using cyclosporin as a model compound (RIVM
report no. 312620001). Bilthoven, National Institute of Public Health
and Environmental Protection.
Van Loveren H Rombout PJA, Fischer P, Lebret E, & Van Bree L (1993b)
Effects of oxidant air pollution on resistance to respiratory
infection, a review (RIVM report no. 619002003). Bilthoven, National
Institute of Public Health and Environmental Protection.
Van Loveren H, Luebke RL, & Vos JG (1994) Assessment of immunotoxicity
using the host infectivity model Trichinella spiralis. In: Burleson
G, Munson A, & Dean J ed. Methods in immunotoxicology. Chicago,
Wiley-Liss.
Van Muiswinkel WB, Lamers CHJ, & Rombout JHWM (1991) Structural and
functional aspects of the spleen in bony fish. Res Immunol,
142: 362-366.
Van Rooijen N, Claassen E, Kraal G, & Dijkstra CD (1989) Cytological
basis of immune functions of the spleen. Immunocytochemical
characterization of lymphoid and non-lymphoid cells involved in the
'in situ' immune response. Progr Histochem Cytochem, 19: 1-71.
Van Soolingen D, Moolenbeek C, & Van Loveren H (1990) An improved
method of bronchoalveolar lavage of lungs of small laboratory animals:
Short report. Lab Anim, 24: 197-199.
Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, & Garattini S
(1980) Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin on humoral antibody production. Chem-Biol Interactions,
30: 337-342.
Vecchi A, Sironi M, Canegrail MA, Recchia M, & Garattini S (1983)
Immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo- p-dioxin in
strains of mice with different susceptibility to induction of aryl
hydrocarbon hydroxylase. Toxicol Appl Pharmacol, 68: 434-441.
Vercelli D & Geha RS (1992) Regulation of isotype switching. Curr Opin
Immunol, 4: 794-797.
Vermeer M, Schmieder GJ, Yoshikawa T, Van Den Berg J-W, Metzman MS,
Taylor JR, & Streilin JW (1991) Effects of ultraviolet B light on
cutaneous immune responses of humans with deeply pigmented skin. J
Invest Dermatol, 97: 729-734.
Versluis DJ, Bijma AM, Vaessen LMB, & Weimar W (1989) Changes in
immunological parameters after conversion from cyclosporin A to
azathioprine in renal transplant recipients. Int J Immunopharmacol,
11: 157-164.
Vethaak D (1993) A large-scale mesocosm study of the effects of marine
pollution on disease development in flounder (Platichthys flesus).
In: Fish disease and marine pollution: A case study of the flounder
(Platichthys flesus) in Dutch coastal and estuarine waters. Thesis
(Meetkundige Dienst RWS, ISBN 90-369-0413-7), Amsterdam, University of
Amsterdam, pp 107-127.
Vethaak AD & ap Rheinallt T (1992) Fish disease as a monitor for
marine pollution: The case of the North Sea. Rev Fish Biol Fish,
2: 1-32.
Vethaak AD & Wester PW (1993) Diseases of flounder (Platichthys
flesus) in Dutch coastal waters, with particular reference to
environmental stress factors. Part II. Liver histopathology. In: Fish
disease and marine pollution, Thesis (Meetkundige Dienst RWS, ISBN
90-369-0413-7). Amsterdam, University of Amsterdam, pp 60-78.
Vetvicka V & Fornusek L (1992) Macrophages. In: Fornusek L & Vetvicka
V ed. Immune system accessory cells. Boca Raton, Florida, CRC Press,
pp 95-108.
Vial T & Descotes J (1992) Clinical toxicity of interleukin-2. Drug
Saf, 7: 417-433.
Vial T & Descotes J (1994) Clinical toxicity of the interferons. Drug
Saf, 10: 115-150.
Vireligier J (1975) Host defenses against influenza virus: The role of
anti-hemagglutinin antibody. J Immunol, 115: 434-439.
Von Boehmer H (1990) Developmental biology of T cells in T cell-
receptor transgenic mice. Ann Rev Immunol, 8: 531-536.
Von Gaudecker B (1986) The development of the human thymus
microenvironment. Curr Topics Pathol, 75: 1-41.
Von Gaudecker B (1991) Functional histology of the thymus. Anat
Embryol, 183: 1-15.
Von Mutius E, Fritzsch C, Weiland SK, Roll G, & Magnussen H (1992)
Prevalence of asthma and allergic disorders among children in united
Germany. Br Med J, 305: 1395-1399.
Vos JG (1977) Immune suppression as related to toxicology. CRC Crit
Rev Toxicol, 5: 67-101.
Vos JG (1980) Immunotoxicity assessment: Screening and function
studies. Arch Toxicol, 4 (Suppl): 95-108.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an international
symposium (IARC Scientific Publications No 77). Lyon, International
Agency for Research on Cancer, pp 347-356.
Vos JG & Beems RB (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits. Toxicol
Appl Pharmacol, 19: 617-633.
Vos JG & Koeman TH (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis, and tissue residues.
Toxicol Appl Pharmacol, 17: 656-668.
Vos JG & Krajnc EI (1983) Immunotoxicology of pesticides. In: Hayes
AW, Schnell RC, & Miya TS ed. Developments in the science and practice
of toxicology. Amsterdam, Elsevier Science Publishers, pp 229-240.
Vos JG & Krajnc-Franken MAM (1990) Toxic effects on the immune system:
Rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system.
Berlin, Springer Verlag, pp 168-181.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Elsevier Science
Publishers, pp 295-322.
Vos JG & Moore JA (1974) Suppression of cellular immunity in rats and
mice by maternal treatment with 2,3,7,8-tetrachlorobenzo- p-dioxin.
Int Arch Allergy Appl Immunol, 47: 777-794.
Vos JG & Van Driel-Grootenhuis, L (1972) PCB-induced suppression of
the humoral and cell-mediated immunity in guinea pigs. Sci Total
Environ, 1: 289-302.
Vos JG & Van Genderen H (1973) Toxicological aspects of
immunosuppression. In: Pesticides and the environment: A continuing
controversy. North Miami, Florida, Symposia Specialists, pp 527-545.
Vos JG & Van Loveren H (1987) Immunotoxicity testing in the rat. In:
Burger EJ, Tardiff RG, & Bellanti JA ed. Advances in modern
environmental toxicology, Vol 13: Environmental chemical exposures and
immune system integrity. Princeton, New Jersey, Princeton Scientific
Publishing, pp 167-180.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979a) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Buys J, Hanstede JG, & Hagenaars AM (1979b) Comparison of
enzyme-linked immunosorbent assay and passive haemagglutination method
for quantification of antibodies to lipopolysaccharide and tetanus
toxoid in rats. Infect Immun, 24: 798-803.
Vos JG, Boerkamp J, Buys J, & Steerenberg PA (1980) Ovalbumin immunity
in the rat: Simultaneous testing of IgM, IgG and IgE response measured
by ELISA and delayed-type hypersensitivity. Scand J Immunol,
12: 289-295.
Vos JG, Krajnc EI, & Beekhof P (1982) Use of the enzyme-linked
immunosorbent assy (elisa) in immunotoxicity testing. Environ Health
Perspectives, 43: 115-121.
Vos JG, Krajnc EI, Beekhof PK, & Van Logten MJ (1983a) Methods for
testing immune effects of toxic chemicals: Evaluation of the
immunotoxicity of various pesticides in the rat. In: Miyamoto T ed.
IUPAC pesticide chemistry: Human welfare and the environment. Oxford,
Pergamon Press, pp 497-504.
Vos JG, Ruitenberg EJ, Van Basten N, Buys J, Elgersma A, & Kruizinga W
(1983b) The athymic nude rat. IV. Immunocytochemical study to detect
T cells, and immunological and histopathological reactions against
Trichinella spiralis. Parasite Immunol, 5: 195-215.
Vos JG, De Klerk A, Krajnc EI, Kruizinga W, Van Ommen B, & Rozing J
(1984) Toxicity of bis(tri-n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicol Appl
Pharmacol, 75: 387-408.
Vos JG, Van Loveren H, Wester P, & Vethaak D (1989) Toxic effects of
environmental chemicals on the immune system. TiPs, 10: 289-292.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rozing J (1990a)
Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on
thymus-dependent-immunity and non-specific resistance following long-
term exposure in young versus aged rats. Toxicol Appl Pharmacol,
105: 144-155.
Vos JG, Dormans JAMA, Kraal G, Krajnc EI, Krajnc-Franken MAM, & Van
Loveren H (1990b) Immunotoxicity of hexachlorobenzene in the rat is
thymus-dependent. Toxicologist, 10: 221.
Vos JG, Van Loveren H, & Schuurman H-J (1991) Immunotoxicity of
dioxin: Immune function and host resistance in laboratory animals and
humans. In: Biological basis for risk assessment of dioxins and
related compounds (Banbury report 35). Cold Spring Harbor, New York,
CSH Press, pp 79-93.
Wakelin D (1993) Allergic inflammation as a hypothesis for the
expulsion of worms from tissues. Parasitol Today, 9: 115-116.
Waldman TA (1988) Immunodeficiency diseases: Primary and acquired.
In: Immunological diseases. Boston, Little Brown Co, pp 441-466.
Ward JM (1990) Classification of reactive lesions of lymph nodes.
In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system (ILSI
Monograph). Berlin, Springer Verlag, pp 155-162.
Ward P & Kendall MD (1975) Morphological changes in the thymus of
young and adult red-billed queleas Quelea quelea (aves). Phil Trans
R Soc Lond B, 273: 55-64.
Ward EC, Murray MJ, & Dean JH (1985) Immunotoxicity of nonhalogenated
polycyclic aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE, &
Amos H ed. Immunotoxicology and immunopharmacology. New York, Raven
Press, pp 291-304.
Ware JH, Dockery DW, Spiro A, Speizer FE, & Ferris BG (1984) Passive
smoking, gas cooking, and respiratory health of children living in six
cities. Am Rev Respir Dis, 129: 366-374.
Watson RR (1990) Drugs of abuse and immune function. Boca Raton,
Florida, CRC Press.
Watson RR (1993) Alcohol, drugs of abuse and immunomodulation
(Advances in the Biosciences, Vol 86). Oxford, Pergamon Press.
Weeks BA & Warinner JE (1986) Functional evaluation of macrophages in
fish from a polluted estuary. Vet Immunol Immunopathol, 12: 313-320.
Weeks BA, Anderson DP, Dufour AP, Fairbrother A, Goven AJ, Lahvis GP,
& Peters G (1992) Immunological biomarkers to assess environmental
stress. In: Biomarkers, biochemical, physiological and histological
markers of anthropo-genic stress. London, Lewis Publishers, pp 5-85.
Weigent DA & Blalock JE (1987) Interactions between the neuroendocrine
and immune systems: Common hormones and receptors. Immunol Rev,
100: 79-108.
Weiss L & Geduldig U (1991) Barrier cells: Stromal regulation of
hematopoiesis and blood release in normal and stressed murine bone
marrow. Blood, 78: 975-990.
Weiss L & Sakai H (1984) The hematopoietic stroma. Am J Anat,
170: 447-463.
Weissgarten J, Freidman CNGE, & Cardella CJ (1989) B Lymphocytes from
stable renal transplant patients do not respond to exogenous
lymphokines: Comparison of azathioprine and cyclosporin treated
patients. Transplant Proc, 21: 393-394.
Weissler JC, Nicod LP, & Toews GB (1987) Pulmonary natural killer cell
activity is reduced in patients with bronchogenic carcinoma. Am Rev
Respir Dis, 135: 1353-1357.
Wessel G, Abenroth K, & Wisheit M (1988) Malignant transformation
during immunosuppressive therapy (azathioprine) of rheumatoid
arthritis and systemic lupus erythematosus. Scand J Rheumatol, 67
(Suppl): 73-75.
Wester PW, & Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to bis(tri- n-
butyltin)oxide (TBTO) and di- n-butyltindichloride (DBTC). Aquat
Toxicol 10: 143-165.
Wester PW & Canton JH (1991) The usefulness of histopathology in
aquatic toxicity studies. Comp Biochem Physiol, 100C: 115-117.
Wester PW, Canton JH, Van Iersel AAJ, Krajnc EI ,& Vaessen HAMG
(1990) The toxicity of bis(tri- n-butyltin)oxide (TBTO) and
di- n-butyltindichloride (DBTC) in the small fish species Oryzias
latipes (medaka) and Poecilia reticulata (guppy). Aquat Toxicol,
16: 53-72.
Wester PW, Vethaak AD, & Van Muiswinkel WB (1994) Fish as biomarkers
in immunotoxicology. Toxicology, 86: 213-232.
White KL (1986) An overview of immunotoxicology and carcinogenic
polycyclic aromatic hydrocarbons. J Environ Health Sci, C4: 163-202.
White KL (1992) Specific immune function assays. In: Miller K, Turk J,
& Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 304-323.
White KL & Munson AE (1986) Suppression of the in vitro humoral
immune response by chrysotile asbestos. Toxicol Appl Pharmacol,
82: 493-504.
White KL, Sander VM, Barnes DW, Shopp GM, & Munson AE (1985)
Immunotoxicological investigations in the mouse: General approach and
methods. Drug Chem Toxicol, 8: 299-331.
White KL, Lysy HH, McCay JA, & Anderson AC (1986) Modulation of serum
complement levels following exposure to polychlorinated dibenzo- p-
dioxins. Toxicol Appl Pharmacol, 84: 209-219.
White KL, Gennings C, Murray MJ, & Dean JH (1994) Summary of
international methods: Validation study, carried out in nine
laboratories, on immunological assessment of cyclosporin A in the rat.
In Vitro Toxicity, 8: 957-961.
WHO (1990) Revised guidelines for preparation of Environmental Health
Criteria monographs (PCS/90.69), Geneva
Wierda D, Irons RD, & Greenlee WF (1981) Immunotoxicity in C57Bl/6
mice exposed to benzene and Aroclor 1254. Toxicol Appl Pharmacol,
60: 410-417.
Williams ME & Quesenberry PJ (1992) Hematopoietic growth factors.
Hematol Pathol, 6: 105-124.
Wilson MR & Warr GW (1992) Fish immunoglobulins and the genes that
encode them. Ann Rev Fish Dis, 201-221.
Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, & Steinberg C
(1992) What limits affinity maturation of antibodies in Xenopus: The
rate of somatic mutation or the ability to select mutants? EMBO J,
11: 4337-4347.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity in
mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20: 423.
Winkelstein JA (1981) The role of complement in the host defense
against Streptococcus pneumoniae. Rev Infect Dis, 3: 289-298.
Wolke RE (1992) Piscine macrophage aggregates: A review. Ann Rev Fish
Dis, 91-108.
Wood SC, Karras JG, & Holsapple MP (1992) Integration of the human
lymphocyte into immunotoxicological investigations. Fundam Appl
Toxicol, 18: 450-459.
Wooley PH (1991) Animal models of rheumatoid arthritis. Curr Opin
Rheumatol, 3: 407-420.
Wright EG & Pragnell IB (1992) Stem cell proliferation inhibitors.
Bailliere's Clin Haematol, 5: 723-739.
Yamaguchi T, Shinagawal Y, & Pollard RB (1988) Relationship between
the production of murine cytomegalovirus and interferon in
macrophages. J Gen Virol, 69: 2961-2971.
Yang KH, Kim BS, Munson AE, & Holsapple MP (1986) Immunosuppression
induced by chemicals requiring metabolic activation in mixed cultures
of rat hepatocytes and murine splenocytes. Toxicol Appl Pharmacol,
83: 420-429.
Yang YG, Lebrec H, & Burleson GR (1994) Effect of 2,3,7,8
tetrachlorodibenzo- p-dioxin (TCDD) on viral replication and natural
killer (NK) activity in rats. Fundam Appl Toxicol, 23: 125-131.
Yang YG, Gilmour MI, Lange R, Burleson GR, & Selgrade MJK (1995)
Effects of acute exposure to phosgene on pulmonary host defenses and
resistance to infection. Inhal Toxicol, 7: 393-404.
Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, & Santos GW
(1993) Cyclosporine-induced graft-versus-host disease after autologous
bone marrow transplantation for acute myeloid leukemia. Leukemia
Lymphoma, 11: 215-220.
Yoshida T & Streilin JW (1990) On the genetic basis of the effects of
ultraviolet B on cutaneous immunity. Evidence that polymorphisms at
the TNFa and Lps loci govern susceptibility. Immunogenetics,
32: 398-405.
Yoshikawa T, Rae V, Bruins-Slot W, Van Den Berg J-W, Taylor JR, &
Streilin JW (1990) Susceptibility to effects of UVB radiation on
induction of contact hypersensitivity as a risk factor for skin cancer
in humans. J Invest Dermatol, 95: 530-536.
Yosioka T, Sato S, Fujiwana H, & Hamacka T (1986) The role of GM-1
antibody-sensitive cells in the implementation of tumor-specific
T-cell mediated immunity in vivo. Jpn J Cancer Res, 77: 825-832.
Zapata A (1982) Lymphoid organs of teleost fish III. Splenic lymphoid
tissue of Rutilus rutilus and Gobio gobio. Dev Comp Immunol,
6: 87-94.
Zapata A (1983) Phylogeny of the fish immune system. Bull Inst
Pasteur, 81: 185-186.
Zapata AG & Cooper EL (1990) The immune system: Comparative
histophysiology. New York, John Wiley & Sons.
Zapata AG, Varas A, & Torroba M (1992) Seasonal variations in the
immune system of lower vertebrates. Immunol Today, 13: 142-147.
Zeeman MG & Brindley WA (1981) Effects of toxic agents upon fish
immune systems: A review. In: Sharma RP, ed. Immunologic
considerations in toxicology, Vol II. Boca Raton, Florida, CRC Press,
pp 1-60.
Zelikoff JT, Enane NA, Bowser D, Squibb KS, & Frenkel K (1991)
Development of fish peritoneal macrophages as a model for higher
vertebrates in immunotoxicological studies. Fundam Appl Toxicol,
16: 576-589.
Zheng B, Shorthouse R, Masek MA, Berry G, Billingham ME, & Morris RE
(1991) Effect of the new and highly active immunosuppressant,
Rapamycin, on lymphoid tissues and cells in vivo. Transplant Proc,
23: 851-855.
Zwick H, Popp W, Wagner C, Reiser K, Schmoger J, Bock A, Herkner K, &
Radunsky K (1991) Effects of ozone on the respiratory health allergic
sensitization, and cellular immune system in children. Am Rev Respir
Dis, 144: 1075-1079.
1. RESUME
1. Le système immunitaire a évolué de manière à contrer les
atteintes que peuvent porter à l'intégrité du soi des microorganismes
ou des cellules ayant échappé au contrôle de l'organisme. Une
intrusion xénobiotique peut perturber le fonctionnement du système
immunitaire et c'est la reconnaissance de cet état de choses qui a
permis les progrès de ces vingt dernières années. Toute une
méthodologie expérimentale a été développée et validée
(essentiellement sur des rongeurs) par des études impliquant une
multitude de laboratoires. La présente monographie examine les
fonctions et l'histophysiologie du système immunitaire et présente les
données nécessaires à la compréhension et à l'interprétation des
modifications pathologiques provoquées par les agressions
immmunotoxiques. L'accent est mis sur le système immunitaire de
l'homme et des rongeurs, mais sans négliger d'autres espèces, les
poissons en particulier, auxquelles des études immunotoxicologiques
ont été consacrées. Il importe, pour comprendre l'impact des effets
immunotoxiques, de connaître la pathophysiologie du système
immunitaire, et notamment la sensibilité variable de ses constituants,
les modifications subies par les organes lymphoïdes et la réversibilité
de ces modifications.
2. L'immunosuppression et l'immunostimulation ont toutes deux des
conséquences sur le plan clinique. On a établi une corrélation entre
les états d'immunodéficience ou d'immunodépression grave que l'on
observe par exemple chez des patients greffés ou sous traitement
cytostatique, et l'accroissement de l'incidence des maladies
infectieuses (opportunistes en particulier) et du cancer. L'exposition
à des substances chimiques immunotoxiques présentes dans l'environnement
peut toutefois donner lieu à des formes plus subtiles d'immunodépression,
difficiles à déceler, qui entraînent une augmentation de l'incidence de
maladies infectieuses comme la grippe ou le rhume. Des travaux effectués
sur des animaux de laboratoire et sur l'homme montrent que de nombreux
produits chimiques présents dans l'environnement dépriment la réponse
immunitaire. Ces substances xénobiotiques immunotoxiques ne se limitent
pas à untype particulier de composé chimique ou d'agent physique. Il y
a, parmi les médicaments, les pesticides, les solvants, les hydro-
carbures halogénés, les hydrocarbures aromatiques et les métaux, des
substances susceptibles d'altérer le système immunitaire et le
rayonnement ultra-violet a également cette propriété. L'administration,
à des fins thérapeutiques, d'agents immunostimulants, peut avoir des
effets nocifs et certaines substances (béryllium, silice, hexa-
chlorobenzène) présentes dans l'environnement, ont des propriétés
immunostimulantes qui peuvent se traduire par des effets cliniques.
3. Du fait de sa complexité, le système immunitaire offre une
multiplicité de cibles potentielles à l'action de ces agents avec
toutes sortes de séquelles pathologiques. Les premières stratégies
élaborées par les immunotoxicologues ont consisté à choisir et à
mettre en oeuvre une batterie de tests multiphasiques sur animaux de
laboratoire afin d'identifier les agents immunosuppresseurs ou
immunostimulants. Ces batteries de tests peuvent varier selon
l'organisme ou le laboratoire qui les mettent en oeuvre ou encore
selon l'espèce animale utilisée, mais elles comportent toutes un ou
plusieurs des éléments suivants: modification du poids ou de
l'histologie des organes lymphoïdes; examen des leucocytes du sang
périphérique, des cellules du tissu lymphoïde ou de la moelle osseuse
à la recherche de modifications; intégrité de la fonction des cellules
effectrices et régulatrices et modification éventuelle de la
sensibilité à une exposition à des agents infectieux ou à des cellules
tumorales.
La directive expérimentale originale No 407 de l'Organisation de
coopération et de développement économiques, publiée en 1981, n'avait
pas pour but la mise en évidence d'une immunotoxicité potentielle,
aussi a-t-elle été modifiée pour la rendre plus adaptée à
l'identification des substances immunotoxiques. Des systèmes d'épreuve
multiphasiques permettant une investigation plus large de
l'immunotoxicité ont été conçus par l'US National Toxicology Program,
l'Institut Néerlandais de la Santé Publique et de la Protection de
l'Environnement, l'US Environmental Protection Agency (Office of
Pesticides), ainsi que par le Center for Food Safety andApplied
Nutrition de l'US Food and Drug Administration. Des études ont été
effectuées sur des souris et, à un moindre degré, sur des rats afin
d'évaluer la spécificité, la précision (reproductibilité), la
sensibilité, l'exactitude et la pertinence, pour l'appréciation du
risque sanitaire, de diverses mesures de l'état immunitaire. On a
entrepris la validation interlaboratoires, au niveau international, de
toutes ces méthodes, dans le cadre de l'Etude collective
internationale sur l'immunotoxicité organisée par le PISC, l'Union
européenne et le Bundesinstitut für Gesundheitllichen
Verbraucherschutz und Veterinärmedizin. Des études analogues ont été
menées sur des rats Fischer 344 à propos de la cyclosporine A.
4. Les épreuves utilisées dans le système multiphasique sont
décrites à la section 3 avec les raisons qui ont guidé ce choix et un
exposé des difficultés que l'on peut rencontrer dans leur mise en
oeuvre. Bien que conçus à l'origine pour des études sur des rats et
des souris, certains de ces protocoles ont pu être utilisés avec fruit
pour des travaux d'immunotoxicologie portant sur d'autres espèces,
notamment des primates non humains, des mammifères marins, des chiens,
des oiseaux et des poissons.
Lorsqu'on se propose de déterminer dans quelle mesure un agent
environnemental ou un médicament est susceptible d'avoir une influence
nocive sur le système immunitaire, il faut prendre en considération un
certain nombre de facteurs. Il s'agit notamment du choix d'un modèle
animal et de variables d'exposition appropriés, de la prise en compte
des paramètres toxicologiques généraux, de la pertinence biologique
des points d'aboutissement retenus, de la mesure de grandeurs dûment
validées et de la mise en oeuvre d'un système d'assurance de la
qualité. Les conditions expérimentales doivent tenir compte des
modalités de l'exposition humaine (voie de pénétration et
concentration ou intensité) et de toute information disponible d'ordre
toxicodynamique ou toxicocinétique. Les doses et la taille des
échantillons doivent être choisies de manière à permettre l'obtention
de bonnes courbes dose-réponse et la détermination de la dose sans
effet (nocif ou non) observable. Cesstratégies sont améliorées en
permanence afin de permettre une meilleure prévision des situations
susceptibles d'entraîner une pathologie. En outre, on devrait pouvoir
disposer de techniques qui faciliteraient l'étude du mode d'action des
agents en cause. Il pourrait s'agir de méthodes in vitro, de l'étude
des réponses immunitaires locales (par exemple au niveau de la peau,
des poumons et de l'intestin), de techniques de biologie moléculaire
et de l'utilisation d'animaux génétiquement modifiés.
5. Mettre en évidence des modifications d'ordre immunologique après
une exposition à des composés potentiellement immunotoxiques est plus
complexe chez l'homme que chez l'animal de laboratoire. Les
possibilités d'expérimentation sont limitées, il est difficile
d'établir le niveau d'exposition à l'agent en cause (c'est-à-dire la
dose) et de plus, l'état immunitaire des populations est extrêmement
hétérogène. Cette hétérogénéité trouve son origine dans un certain
nombre de facteurs: âge, sexe, race, gravidité, stress et aptitude à y
faire face, pathologies et états infectieux cocomitants, état
nutritionnel, tabagisme et prise de certains médicaments. L'intérêt de
telle ou telle étude pour l'évaluation du risque est conditionné par
un élément important, à savoir le concept épidémiologique qui la sous-
tend. La plupart du temps on procède à une étude transversale, qui
consiste à déterminer l'exposition et la morbidité à un moment donné
ou sur une courte période. On compare ensuite la fonction immunitaire
des sujets exposés à celle d'un groupe analogue de sujets non exposés.
Ce genre d'étude peut receler un certain nombre de pièges.
Nombre des altérations que produit l'exposition à une substance
chimique ne se manifestent chez l'homme que de manière subtile et
sporadique, aussi faut-il étudier des populations récemment exposées
et utiliser des épreuves sensibles.
La plupart des épreuves concernant l'immunité spécifique (à
médiation cellulaire ou humorale), l'immunité non spécifique et les
processus inflammatoires ont été conçues pour rechercher des anomalies
chez des patients atteints d'une déficience immunitaire et ne sont pas
forcément capables de déceler les modifications subtiles provoquées
par les substances chimiques. Le PISC, les Centers for Disease Control
et l'Académie des Sciences des Etats-Unis ont, chacun de leur côté,
défini une méthodologie pour évaluer les modifications du système
immunitaire quipourraient résulter d'une exposition à des produits
immunotoxiques mais les épreuves proposées demandent encore à être
validées.
6. L'évaluation du risque consiste à analyser les données
pertinentes sur un agent donné (effets biologiques, relations dose-
réponse et exposition) pour tenter d'obtenir une estimation
qualitative et quantitative des diverses conséquences nocives de la
présence de cet agent. Elle se caractérise par quatre phases
principales: reconnaître le danger, établir la relation dose-réponse,
évaluer l'exposition et caractériser le risque. Jusqu'ici,
l'immunotoxicologie s'est essentiellement attachée à reconnaître le
danger et, dans une certaine mesure, à établir des relations dose-
réponse, mais très peu d'études ont été consacrées à l'évaluation du
risque ou à sa caractérisation.
Comme dans d'autres domaines de la toxicologie, des incertitudes
demeurent qui sont susceptibles de gêner l'interprétation des données
immunotoxicologiques dans l'optique du risque pour la santé humaine.
Les deux questions qui sont actuellement les plus problématiques -
l'extrapolation à l'organe dans son ensemble des effets constatés sur
la cellule et l'extrapolation à l'homme des données obtenues sur
l'animal - valent pour la plupart des points d'aboutissement des
effets, cancer excepté. Le premier problème tient aux incertitudes que
comporte l'établissement d'une relation quatitative entre les
modifications subies par la fonction immunitaire d'un individu et
l'altération de sa résistance aux maladies infectieuses et
néoplasiques. Le deuxième est lié à l'incertitude qui entache
l'évaluation du risque pour la santé humaine à partir des résultats
obtenus sur des animaux de laboratoire.
L'évaluation du risque a pour finalité de protéger la santé
humaine et l'environnement. Il faut donc que le choix des modéles
expérimentaux soit judicieux. La toxicocinétique de la substance
étudiée et la réponse immunitaire suscitée dans le modèle doivent
pouvoir être comparées à celles qu'on observerait chez l'homme.
Pour tirer une limite d'exposition des résultats expérimentaux,
il est convenu d'appliquer un facteur d'incertitude aux données
d'évaluation du risque. Cette convention ne tient pas compte de la
réserve fonctionnelle ou de la redondance du système immunitaire. Une
méthode plus récente d'évaluation du risque consiste à ultiliser des
modèles in vitro en complément aux études sur animaux de laboratoire.
Cette méthode a l'avantage de permettre une extrapolation plus fidèle
de l'animal à l'homme et de ne nécessiter qu'un minimum d'animaux.
Elle permet également de pallier l'absence de données dans les cas où
des considérations d'éthique limitent l'expérimentation sur l'homme.
Le Chapitre 6 donne deux exemples de situation où les données obtenues
in vitro permettent de lever en partie les incertitudes dans
l'évaluation du risque dû à l'exposition à l'ozone et au rayonnement
ultra-violet. L'utilisation des données immunotoxicologiques dans
l'évaluation du risque reste limitée par la difficulté d'établir des
relations quantitatives entre l'immunodépression et les manifestations
cliniques d'une pathologie donnée.
RESUMEN
1. El sistema inmunitario ha evolucionado para hacer frente a las
amenazas a la integridad del organismo vivo provenientes de
microorganismos o de células que han escapado a los mecanismos de
control del organismo. El reconocimiento de que las sustancias
xenobióticas pueden trastornar el funcionamiento del sistema
inmunitario ha llevado a avances en el campo de la inmunotoxicología
durante los dos últimos decenios. Se han formulado métodos
experimentales (empleando principalmente especies de roedores), que
han sido validados en estudios multilaboratorio. En esta monografía se
examinan la función y la histofisiología del sistema inmunitario,
presentándose la información necesaria para la comprensión e
interpretación de los cambios patológicos causados por las agresiones
inmunotóxicas. Si bien se hace hincapié en los sistemas inmunitarios
del ser humano y de las especies de roedores, se hace referencia a
otras especies, incluidos los peces, que han sido objeto de estudios
inmunotoxicológicos. La fisiopatología del sistema inmunitario,
incluidas la sensibilidad variable de sus componentes, las
alteraciones de los órganos linfoides y la reversibilidad de los
cambios, es importante para comprender las repercusiones de la
inmunotoxicidad.
2. Tanto la inmunosupresión como la inmunoestimulación tienen
consecuencias clínicas. Se ha observado que los estados de
inmunodeficiencia y de inmunosupresión grave, como los que se pueden
presentar en casos de trasplante y de terapia citostática, van
acompañados de mayor incidencia de enfermedades infecciosas
(especialmente las oportunistas) y de cáncer. Con todo, cabe prever
que la exposición a los productos químicos inmunotóxicos enel medio
ambiente dará origen a formas más sutiles de inmunosupresión cuya
detección podría resultar difícil, lo que se traduciría en una mayor
incidencia de infecciones tales como la gripe y el resfriado común.
Estudios realizados con animales de laboratorio y seres humanos han
mostrado que muchos de los productos químicos presentes en el medio
ambiente provocan la supresión de la respuesta inmunitaria. Las
sustancias xenobióticas inmunotóxicas no se limitan a una clase
determinada de productos químicos. Entre los compuestos que tienen
efectos nocivos para el sistema inmunitario se cuentan fármacos,
plaguicidas, disolventes, hidrocarburos halogenados y aromáticos y
metales; la radiación ultravioleta puede resultar también
inmunotóxica. La administración terapéutica de agentes
inmunoestimulantes puede provocar reacciones adversas; asimismo,
algunos de los productos químicos presentes en el medio ambiente que
poseen propiedades inmunoestimulantes (berilio, sílice,
hexaclorobenceno) pueden tener consecuencias clínicas.
3. La complejidad del sistema inmunitario lleva aparejadas
multiplicidad de posibles puntos vulnerables y secuelas patológicas.
Los métodos iniciales ideados por los inmunotoxicólogos que realizan
investigaciones sobre toxicología y evaluación de la inocuidad
consistían en seleccionar y aplicar una serie de valoraciones
escalonadas para identificar los agentes inmunosupresores e
inmunoestimulantes en animales de laboratorio. Si bien la
configuración de esas series de análisis puede variar según la
institución o el laboratorio en que se llevan a cabo, así como según
las especies de animales empleadas, en todas se mide unoo más de los
siguientes parámetros: alteraciones del peso y de la histología de los
órganos linfoides; cambios en la celularidad del tejido linfoide, de
los leucocitos en la sangre periférica y/o de la médula ósea;
trastornos de la función celular a nivel de los efectores o de la
regulación, y alteración de la sensibilidad a la amenaza que presentan
los agentes infecciosos o las células tumorales.
La Directriz de pruebas inicial No 407 de la Organización de
Cooperación y Desarrollo Económicos, publicada en 1981, no preveía la
detección de los riesgos de inmunotoxicidad, y se han propuesto
modificaciones destinadas a aumentar la utilidad de esa Directriz para
la identificación de las sustancias inmunotóxicas. El Programa
Nacional de Toxicología de los Estados Unidos, el Instituto Nacional
de Salud Pública y Protección del Medio Ambiente de los Países Bajos,
la Oficina de Plaguicidas de la Agencia para la Protección del Medio
Ambiente de los Estados Unidos y el Centro de Seguridad de los
Alimentos y Nutrición Aplicada de la Administración de Alimentos y
Medicamentos de los Estados Unidos han elaborado sistemas de pruebas
escalonadas para la investigación en mayor escala de los riesgos de
inmunotoxicidad. Se han realizado estudios con ratones, y en menor
medida con ratas, de diferentes indicadores del estado inmunológico
con el fin de determinar su especificidad, precisión (reproducibilidad),
sensibilidad, exactitud y pertinencia para la evaluación de los riesgos
para la salud humana. Los métodos han sido objeto de validaciones
internacionales interlaboratorios en el marco del Estudio Internacional
en Colaboración sobre Inmunotoxicidad del IPCS, la Unión Europea y el
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinärmedizin, yen estudios sobre la ciclosporina A en ratas
Fisher 344.
4. Las pruebas empleadas en los programas de verificaciones
escalonadas se describen en la Sección 3, en la que se indican la
razón de ser de su selección y las complejidades que entraña su
realización. Si bien esos protocolos fueron diseñados para estudios
realizados con ratas y ratones, algunos de ellos se han aplicado con
buenos resultados al estudio de la inmunotoxicidad en otras especies
animales, incluidos primates no humanos, mamíferos marinos, perros,
aves y peces.
A la hora de evaluar las posibles repercusiones negativas de un
agente ambiental o fármaco sobre el sistema inmunitario de los
animales experimentales deberán considerarse diversos factores. Entre
ellos cabe señalar: la selección de los modelos y las variables de
exposición apropiados para los animales, la inclusión de parámetros
toxicológicos generales, la comprensión de la importancia de los
parámetros objeto de medición, el empleo de medidas validadas y el
control de la calidad. En las condiciones experimentales deberán
tenerse en cuenta las vías y el nivel posibles de exposición del ser
humano, así como toda la información disponible sobre toxicodinámica y
toxicocinética. Las dosis y el tamaño de las muestras deberán
seleccionarse de manera que se puedan obtener curvas de dosis-
respuesta bien definidas, además del nivel sin efectos adversos
observados y del nivel sin efectos observados. Los métodos se
perfeccionan continuamente para poder predecir mejor las condiciones
que podrían ser causantes de enfermedades. Además, deberán elaborarse
técnicas que contribuyana la identificación de mecanismos de acción;
éstos podrían incluir los métodos in vitro, el examen de las
respuestas inmunitarias locales (por ejemplo, en la piel, los pulmones
y los intestinos), y el empleo de las técnicas de la biología
molecular y de animales modificados genéticamente.
5. La detección de los cambios inmunológicos ocurridos tras la
exposición a compuestos que podrían ser inmunotóxicos resulta más
complicada en el ser humano que en los animales de laboratorio. Las
posibilidades de realizar pruebas son limitadas; los niveles de
exposición al agente (es decir, la dosis) son difíciles de establecer
y el estado inmunitario de las poblaciones es sumamente heterogéneo.
La edad, la raza, el sexo, la gestación, el estrés agudo y la
capacidad para hacerle frente, las enfermedades e infecciones
coexistentes, el estado nutricional, el humo del tabaco y algunos
medicamentos se cuentan entre los factores que contribuyen a esa
heterogeneidad.
El diseño de los estudios epidemiológicos es un factor importante
para determinar la utilidad de un estudio determinado para la
evaluación de riesgos. El tipo de diseño empleado más frecuentemente
en materia de inmunotoxicidad son los estudios transversales, en los
que se miden las condiciones de exposición y el estado de la
enfermedad en un momento dado, o durante un periodo breve. A
continuación se compara la función inmunitaria de los sujetos
«expuestos» con la de un grupo comparable de individuos
«no expuestos». Ese tipo de diseño lleva aparejados posibles escollos.
Como muchos de los cambios observados en la respuesta inmunitaria
de los seres humanos tras su exposición a un productoquímico podrían
ser esporádicos y sutiles, habrá que estudiar las poblaciones que han
estado expuestas recientemente empleando pruebas de gran sensibilidad
para la evaluación del sistema inmunitario. Las conclusiones sobre los
efectos inmunotóxicos deberán estar basadas en las variaciones
detectadas no en un parámetro aislado sino en el perfil inmunológico
del individuo o de la población.
La mayoría de las pruebas existentes para determinar la inmunidad
específica (de base celular y humoral), la inmunidad no específica y
la inflamación han sido concebidas para detectar las alteraciones
inmunitarias en pacientes que padecen de inmunodeficiencia y no
siempre resultan adecuadas para detectar las alteraciones sutiles
provocadas por los productos químicos presentes en el medio ambiente.
El IPCS, los Centros de Control de Enfermedades y la Academia de
Ciencias de los Estados Unidos han descrito procedimientos para
evaluar los cambios que ocurren en el sistema inmunitario del ser
humano como consecuencia de la exposición a sustancias inmunotóxicas;
con todo, las pruebas descritas deberán ser evaluadas con este fin.
6. La evaluación de riesgos es un proceso en el que se analizan la
información pertinente sobre los efectos biológicos, las relaciones
dosis-respuesta y la exposición a un agente determinado con miras a
establecer estimaciones cualitativas y cuantitativas de los resultados
adversos. Por regla general, la evaluación de los riesgos supone
cuatro pasos fundamentales: identificación de los riesgos; evaluación
de la relación dosis-respuesta; evaluación de la exposición y
caracterización de los riesgos. Hasta ahora, lainmunotoxicología se ha
centrado principalmente en la identificación de los riesgos y, en
cierta medida, en la evaluación de las relaciones dosis-respuesta; muy
contados han sido los estudios que han incluido la evaluación de la
exposición o la caracterización de los riesgos.
Al igual que ocurre en otros campos de la toxicología, existen
incertidumbres que podrían afectar a la interpretación de los datos
sobre inmunotoxicidad en cuanto a los riesgos para la salud humana.
Las dos cuestiones más problemáticas - la extrapolación de los efectos
observados en células individuales a todo un órgano, o a niveles
superiores, y la extrapolación al ser humano de los resultados
obtenidos en experimentos con animales - son comunes a la mayoría de
los parámetros de valoración no relacionados con el cáncer. La primera
obedece a las incertidumbres vinculadas al establecimiento de una
relación cuantitativa entre los cambios observados en la función
inmunitaria del individuo y la perturbación de la resistencia a las
infecciones y enfermedades neoplásicas. La segunda cuestión es
consecuencia de las incertidumbres que lleva aparejadas la evaluación
de los riesgos para la salud humana basándose en los estudios
realizados con animales de laboratorio.
El objetivo fundamental de la evaluación de los riesgos es la
protección de la salud de los seres humanos y del medio ambiente. Por
lo tanto, deberán seleccionarse sistemas modelo idóneos. La
toxicocinética del material de prueba y la índole y magnitud de la
respuesta inmunitaria generada en el modelo deberán ser comparables a
la de los seres humanos.
Habitualmente, en la evaluación de los riesgos se emplean
factores empíricos de incertidumbre para determinar el límite de
exposición aceptable a partir de los resultados experimentales. Ese
procedimiento no toma en cuenta la reserva funcional ni la redundancia
del sistema inmunitario. Un adelanto más reciente en materia de
evaluación de riesgos es el empleo de modelos in vitro como
complemento de los estudios realizados con animales de laboratorio.
Ese procedimiento tiene la ventaja de que permite aumentar la
exactitud de la extrapolación al ser humano de los resultados
obtenidos en los experimentos realizados con animales, reduciendo al
mínimo el número de animales necesarios; asimismo, permite colmar la
brecha entre ambos tipos de información, sobre todo en los casos en
que los experimentos con seres humanos se ven limitados por
consideraciones de índole ética. En el capítulo 6 se presentan dos
ejemplos de cómo la información in vitro permite reducir las
incertidumbres en materia de evaluación de riesgos relacionadas con la
exposición al ozono y a la radiación ultravioleta. La dificultad para
establecer relaciones cuantitativas entre la inmunosupresión y las
enfermedades clínicas ha limitado el empleo de los datos
inmunotoxicológicos en la evaluación de los riesgos.
 incorporating the comments received and approved by the Director,
IPCS, is then distributed to Task Group members, who carry out a peer
review at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the risks to health and
the environment from exposure to the chemical. A summary and
recommendations for further research and improved safety are also
drawn up. The composition of the Task Group is dictated by the range
of expertise required for the subject of the meeting and by the need
for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations, so that
representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide valuable contributions to the process, they can speak only
at the invitation of the Chairperson. Observers do not participate in
the final evaluation of the chemical, which is the sole responsibility
of the Task Group members. The Task Group may meet in camera when it
considers that to be appropriate.
All individuals who participate in the preparation of an EHC
monograph as authors, consultants, or advisers must, in addition to
serving in their personal capacity as scientists, inform the RO if at
any time a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a statement to that
effect. This procedure ensures the transparency and probity of the
process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it is edited for language, the references are checked, and
camera-ready copy is prepared. After approval by the Director, IPCS,
the monograph is submitted to the WHO Office of Publications for
printing. At this time, a copy of the final draft is also sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern about
health or environmental effects of the agent because of greater
exposure; an appreciable time has elapsed since the last evaluation.
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The chairpersons of task groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON PRINCIPLES AND METHODS FOR ASSESSING DIRECT
IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
Members
Dr A. Emmendörffer, Department of Immunobiology, Fraunhofer Institute
of Toxicology & Aerosol Research, Hanover, Germany
Dr H.S. Koren, Health Effects Research Laboratory, US Environmental
Protection Agency, Chapel Hill, NC, USA (Vice-Chairman)
Dr R.W. Luebke, Immunotoxicology Branch, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, NC, USA (Joint Rapporteur)
Dr M. Luster, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA
Dr C. Madsen, Institute of Toxicology, National Food Agency of
Denmark, Ministry of Health, Soborg, Denmark
Dr P. Ross, Dalhousie University, Halifax, Nova Scotia, Canada
(c/o Seal Rehabilitation and Research Centre, Pieterburen,
Netherlands)
Dr H.J. Schuurman, Preclinical Research/Immunology, Sandoz Pharma Ltd,
Basel, Switzerland
Dr H. Van Loveren, Laboratory for Pathology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Joint Rapporteur)
Dr J.G. Vos, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands (Chairman)
Dr K.L. White, Jr, Immunotoxicology Group, Medical College of
Virginia, Virginia Commonwealth University, Richmond, VA, USA
Observers
IUTOX
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy
ECETOC
Dr R.W.R. Crevel, Environmental Safety Laboratory, Unilever Research
and Engineering, Sharnbrook, Bedfordshire, United Kingdom
Secretariat
Dr J.H. Dean, Sanofi Winthrop, Inc., Sanofi Research Division,
Collegeville, PA, USA
Mr V. Quarg, Federal Ministry for Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA PRINCIPLES AND METHODS FOR ASSESSING
DIRECT IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
A WHO Task Group on Principles and Methods for Assessing Direct
Immunotoxicity Associated with Exposure to Chemicals met at the World
Health Organization, Geneva, from 10 to 14 October 1994. Dr E. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
IPCS, and the cooperating organizations. The Task Group reviewed and
revised the draft monograph and prepared the final text.
The first draft of the monograph was prepared by a group of
authors (listed below) under the coordination of Dr J.G. Vos and
Dr H. Van Loveren of the Dutch National Institute for Public Health
and Environmental Protection (RIVM), an IPCS Collaborating Centre for
Immunotoxicology and Allergic Hypersensitization. The second draft,
incorporating comments received after international circulation to
national experts of the first draft to IPCS contact points for
Environmental Health Criteria monographs, was prepared by Dr J.G.
Vos and Dr H. Van Loveren of the Netherlands and Dr Kimber White, USA.
Dr E. Smith of the IPCS Unit for the Assessment of Risk and
Methods was responsible for the scientific content of the monograph
and Mrs E. Heseltine for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The contributing authors were:
Dr J.H. Dean, Collegeville, PA, USA
Professor J. Descotes, Lyon, France
Dr F. Kuper, Zeist, Netherlands
Dr M. Luster, Research Triangle Park, NC, USA
Dr P.S. Ross, Bilthoven, Netherlands
Dr H.J. Schuurman, Basel, Switzerland
Dr M.J. Selgrade, Research Triangle Park, NC, USA
Dr R.L. de Swart, Bilthoven, Netherlands
Dr H. Van Loveren, Bilthoven, Netherlands
Professor J.G. Vos, Bilthoven, Netherlands
Dr P.W. Wester, Bilthoven, Netherlands
Professor A.G. Zapata, Madrid, Spain
ABBREVIATIONS
ACTH adrenocorticotrophic hormone
Ah aromatic hydrocarbon
AIDS acquired immunodeficiency syndrome
B bursa-dependent
CALLA common acute lymphoblastic leukaemia antigen
CD cluster of differentiation
CEC Commission of the European Communities
CH50 haemolytic complement
CML cell-mediated lympholysis
DMBA 7,12-dimethylbenz[ a]anthracene
DNCB dinitrochlorobenzene
ELISA enzyme-linked immunosorbent assay
EPO erythrocyte lineage differentiation factor
FACS fluorescence activated cell sorter
GALT gut-associated lymphoid tissue
G-CSF granulocyte colony-stimulating factor
GM-CSF granulocyte-macrophage colony-stimulating factor
GVH graft-versus-host
HCB hexaclorobenzene
HEV high endothelial venule
HIV human immunodeficiency virus
HPCA human progenitor cell antigen
HSA heat-stable antigen
ICAM intercellular adhesion molecule
IFN interferon
Ig immunoglobulin
IL interleukin
IPCS International Programme on Chemical Safety
LFA lymphocyte function-related antigen
LIF leukaemia inhibitory factor
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
M microfold
MALT mucosa-associated lymphoid tissue
MARE monoclonal anti-rat immunoglobulin E
MARK monoclonal antibody anti-kappa
M-CSF macrophage colony-stimulating factor
MED minimal erythemal dose
MHC major histocompatibility complex
NCAM neural cell adhesion molecule
NK natural killer
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NTP National Toxicology Program
PAH polycyclic aromatic hydrocarbon
PCB polychlorinated biphenyl
PG prostaglandin
QCA quiescent cell antigen
RIVM Dutch National Institute of Public Health and
Environmental Protection
S9 9000 x g supernatant
SCF stem-cell factor
SCID severe combined immunodeficiency
SIS skin immune system
STM Salmonella typhimurium mitogen
TBTO tri- n-butyltin oxide
Tc cytotoxic T cell
TCDD 2,3,7,8-tetrachlorodibenzo- para-dioxin
TCR T-cell receptor
Tdth delayed-type hypersensitivity T cell
TGF transforming growth factor
Th T helper-inducer cell
THAM T-cell activation molecule
THI 2-acetyl-4(5)-tetrahydroxybutylimidazole
O,O,S-TMP O,O,S-trimethylphosphorothiate
TNF tumour necrosis factor
UVB ultraviolet B
UVR ultraviolet radiation
VCAM vascular cell adhesion molecule
VLA very late antigen
SUMMARY
1. The immune system has evolved to counter challenges to the
integrity of self from either microorganisms or cells that have
escaped the organism's control mechanisms. Recognition that
xenobiotics can impair the function of the immune system has led to
progress in immunotoxicology over the last two decades. Experimental
approaches (mainly in rodent species) have been developed and
validated in multilaboratory studies. In this monograph, the function
and histophysiology of the immune system are reviewed, and the
information necessary to understand and interpret the pathological
changes caused by immunotoxic insults is provided. Emphasis is laid on
the immune systems of humans and rodent species, but reference is made
to other species, including fish, that have been the object of
immunotoxicological studies. The pathophysiology of the immune system,
including the variable susceptibility of its components, alterations
to the lymphoid organs, and the reversibility of changes are important
for understanding the impact of immunotoxicity.
2. Immunosuppression and immunostimulation both have clinical
consequences. Immunodeficiency states and severe immunosuppression,
such as can occur during transplantion and cytostatic therapy, have
both been associated with increased incidences of infectious diseases
(particularly opportunistic ones) and cancer. Exposure to immunotoxic
chemicals in the environment, however, may be expected to result in
more subtle forms of immunosuppression which may be difficult to
detect, leading to increased incidences of infections such as
influenza and the common cold. Studies of experimental animals and
humans have shown that many environmental chemicals suppress the
immune response. Immunotoxic xenobiotics are not restricted to a
particular chemical class. Compounds that adversely affect the immune
system are found among drugs, pesticides, solvents, halogenated and
aromatic hydrocarbons, and metals; ultraviolet radiation can also be
immunotoxic. Therapeutic administration of immunostimulating agents
can have adverse effects, and a few environmental chemicals that have
immunostimulating properties (beryllium, silica, hexachlorobenzene)
can have clinical consequences.
3. The complexity of the immune system results in multiple potential
target sites and pathological sequelae. The initial strategies devised
by immunotoxicologists working in toxicology and safety assessment
were to select and apply a tiered panel of assays to identify
immunosuppressive and immunostimulatory agents in laboratory animals.
Although the configuration of these testing panels may vary depending
on which agency or laboratory is conducting the test and on the animal
species employed, they all include measurement of one or more of the
following: altered lymphoid organ weights and histology; changes in
the cellularity of lymphoid tissue, peripheral blood leukocytes,
and/or bone marrow; impairment of cell function at the effector or
regulatory level; and altered susceptibility to challenge with
infectious agents or tumour cells.
The original test guideline No. 407 of the Organisation for
Economic Co-operation and Development, published in 1981, was not
designed to detect potential immunotoxicity, and modifications have
been proposed to make the guideline more useful for identifying
immunotoxicants. Tiered testing systems have been designed for more
extensive investigation of potential immunotoxicity, by the US
National Toxicology Program, the Dutch National Institute of Public
Health and Environmental Protection, the US Environmental Protection
Agency Office of Pesticides, and the US Food and Drug Administration
Center for Food Safety and Applied Nutrition.
Studies have been conducted in mice, and to a lesser extent in
rats, to investigate the specificity, precision (reproducibility),
sensitivity, accuracy, and relevance for the assessment of risk to
human health of a variety of measures of immune status. International,
interlaboratory validations of methods have been carried out within
the International Collaborative Immunotoxicity Study of IPCS and the
European Union, the Bundesinstitut für Gesundheitlichen
Verbraucherschutz, und Veterinärmedizin, and in studies of cyclosporin
A in Fischer 344 rats.
4. The tests used in the tiered testing schemes are described in
Section 3, which indicates the rationale for their selection and the
complexities involved in their performance. Although these protocols
were designed for studies of rats and mice, some have been applied
successfully for studying immunotoxicity in other animal species,
including non-human primates, marine mammals, dogs, birds, and fish.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to influence the immune
system of experimental animals adversely. These include selection of
the appropriate animal models and exposure variables, inclusion of
general toxicological parameters, an understanding of the biological
relevance of the end-points being measured, use of validated measures,
and quality assurance. The experimental conditions should take into
account the potential route and level of human exposure and any
available information on toxicodynamics and toxicokinetics. The doses
and sample sizes should be selected so as to generate clear dose-
response curves, in addition to no-observed-adverse-effect or
no-observed-effect levels. The strategies are continually refined to
allow better prediction of conditions that may lead to disease. In
addition, techniques should be developed that would help to identify
mechanisms of action; these might include methods in vitro,
examination of local immune responses (such as in the skin, lung, and
intestines), and use of the techniques of molecular biology and
genetically modified animals.
5. The detection of immune changes after exposure to potentially
immunotoxic compounds is more complicated in humans than in
experimental animals. The testing possibilities are limited, levels of
exposure to the agent (i.e. dose) are difficult to establish, and the
immune status of populations is extremely heterogeneous. Age, race,
gender, pregnancy, acute stress and the ability to cope with stress,
coexistent disease and infections, nutritional status, tobacco smoke,
and some medications contribute to this heterogeneity.
An important factor in assessing the usefulness of a particular
study for risk assessment is epidemiological study design. The
commonest design used in immunotoxicity is the cross-sectional study,
in which exposure status and disease status are measured at one time
or over a short period. The immune function of 'exposed' subjects is
then compared with that of a comparable group of 'unexposed'
individuals. There are possible pitfalls in this study design.
Because many of the immune changes seen in humans after exposure
to a chemical may be sporadic and subtle, recently exposed populations
must be studied and sensitive tests be used for assessing the immune
system. Conclusions about immunotoxic effects should be based on
changes not in a single parameter but in the immune profile of an
individual or population.
Most of the tests for specific immunity (cell-mediated and
humoral), nonspecific immunity and inflammation were developed to
detect immune alterations in patients with immunodeficiency disease
and are not always adequate to detect subtle alterations induced by
environmental chemicals. IPCS, the Centers for Disease Control, and
the US National Academy of Sciences have each described procedures for
evaluating changes in the human immune system resulting from exposure
to immunotoxicants, but the tests described require evaluation for
this purpose.
6. Risk assessment is a process in which relevant data on the
biological effects, dose-response relationships, and exposure for a
particular agent are analysed in an attempt to establish qualitative
and quantitative estimates of adverse outcomes. Typically, risk
assessment comprises four major steps: hazard identification, dose-
response assessment, exposure assessment, and risk characterization.
Up until now, immunotoxicology has focused mainly on hazard
identification, and to some extent on dose-response assessment, and
very few studies have included exposure assessment or risk
characterization.
As in other areas of toxicology, uncertainties exist which may
affect the interpretation of data on immunotoxicity with regard to
human health risk. The two most problematic issues -- extrapolating
effects from individual cells to a whole organ or beyond and
extrapolating data from experimental animals to humans -- are common
to most non-cancer end-points. The first issue is due to uncertainties
associated with establishing a quantitative relationship between
changes in individual immune function and altered resistance to
infections and neoplastic disease. The second issue is due to
uncertainties associated with assessing risk to human health on the
basis of studies in laboratory animals.
The ultimate purpose of risk assessment is to protect human
health and the environment. Suitable model systems must therefore be
chosen. The toxicokinetics of the test material and the nature and
magnitude of the immune response generated in the model should be
comparable to that of humans.
Conventionally, empirical uncertainty factors are used in risk
assessment to derive an acceptable exposure limit from experimental
results. This approach does not take into account the functional
reserve or redundancy of the immune system. A more recent development
in risk assessment is use of in-vitro models as an adjunct to studies
of experimental animals. The advantages of this approach are that it
improves the accuracy of extrapolation of data from animals to man and
minimizes the use of animals; it also bridges the gap between those
data, particularly when human experimentation is limited for ethical
considerations. Chapter 6 cites two examples in which in-vitro data
make it possible to reduce the uncertainties in risk assessment
associated with exposure to ozone and ultraviolet radiation. The
difficulty in establishing quantitative relationships between
immunosuppression and clinical disease has limited the use of
immunotoxicological data in risk assessment.
RECOMMENDATIONS
Recommendations for the protection of human health
1. Chemicals should be screened to determine if they are potentially
immunotoxic to humans. If immunotoxicity is detected, the chemicals
should be investigated further as part of the risk assessment process.
2. Chemicals for which little or no information is available on
toxicity should be screened for potential immunotoxicity following a
protocol based on, for example, the revised OECD guideline No. 407.
When some information is available on the test material (e.g.
physicochemical properties, toxicokinetics, structure-activity
relationships), a flexible approach to testing is recommended which
permits a rational selection of test procedures.
3. The immunotoxic risk of mixtures of environmental pollutants, in,
for example, fish, to certain human consumer groups (e.g. fishermen)
should be assessed.
Recommendations for protection of the environment
1. Chemicals should be screened to determine if they are potentially
immunotoxic to wildlife species. If immunotoxicity is detected, the
chemicals should be investigated further as part of the risk
assessment process.
2. The immunotoxic risk of environmental pollution to the health of
the ecosystem should be assessed in laboratory, semi-field, and field
studies of the wildlife occupying high trophic levels or those species
judged to be sensitive.
Recommendations for further research
1. The panels of tests suggested for evaluating xenobiotic-induced
immunotoxicity in humans should be investigated to determine their
ability to detect subtle alterations in immune status.
2. The relationships between alterations in immune function and human
health should be established for use in immunotoxic risk assessment.
Epidemiological studies should be carried out that include assessment
of exposure, in order to establish dose-response relationships.
3. The relationship between immunotoxicity and the development of
neoplasia should be investigated.
4. Baseline immunological data should be established for the general
population and for subpopulations such as ethnic minorities, children,
the aged, and pregnant and lactating women in order to assess their
immune status.
5. Immunotoxicological assessment should be conducted for
subpopulations potentially susceptible to the effects of immunotoxic
compounds, including those at the extremes of age and those with
deficient nutritional status.
6. Biomarkers of exposure, effect, and susceptibility should be
identified, developed, and validated for use in epidemiological
studies of immunotoxicity in both humans and wildlife.
7. The quantitative relationship between immune function and host
resistance in animal models, including the nature, magnitude, and
significance of functional reserve and redundancy, should be explored
for risk assessment.
8. Since chemicals and biological agents enter the body via the
respiratory and alimentary tracts and the skin, more research should
be carried out on local immunity.
9. Preliminary observations in laboratory animals that suggest that
primary immunization does not compromise testing for subacute toxicity
should be substantiated by further research, so that functional
testing can be incorporated into toxicology testing.
10. Methods and reagents should be developed in order to characterize
the immune system of wildlife species and to assess their immune
status for immunotoxicological studies.
11. The mechanisms of the immunotoxic action of xenobiotics in humans
should be elucidated by a combination of studies in laboratory animals
in vivo and experiments with human and animal tissues and cell lines
in vitro.
12. In view of the sensitivity of the developing immune system to
immunotoxic injury, more emphasis should be placed on studies
involving perinatal exposure to a chemical or mixture of chemicals.
13. Studies should be conducted to establish whether exposure to
xenobiotics that are not themselves sensitizing adds to the risk of
allergic disease in general.
14. Autoimmune models in laboratory animals should be used to assess
whether xenobiotics can modulate autoimmune disease in humans.
15. The effects on immune function of confounding factors in humans
and animals, including age, race, sex, gender, nutritional status,
acute stress, and underlying disease, should be evaluated further in
order to determine their effects in tests for the immunotoxicity of
environmental chemicals.
16. Methods for assessing cytokines and their production in different
body compartments, including plasma, bronchoalveolar lavage fluid, and
nasal lavage fluid, and by cells isolated from various anatomical
sites should be validated for humans and laboratory animals, and their
applicability for assessing the risk of chemicals should be
established.
17. Data from clinical trials should be made more widely available;
and patients undergoing therapy with immunomodulatory drugs should be
monitored clinically and immunologically in a systematic way.
18. The toxicokinetics of immunotoxic chemicals should be further
investigated, particularly with regard to whether their concentrations
in human biological fluids indicate levels of environmental exposure.
19. The interactions between the immune system, the nervous system,
and the endocrine system should be further investigated, with
particular emphasis on how xenobiotics adversely affect them.
20. The significance of ultraviolet radiation-induced
immunosuppression for public health and the health of ecosystems
should be evaluated.
1. INTRODUCTION TO IMMUNOTOXICOLOGY
1.1 Historical overview
It is well established that each individual has an intrinsic
capacity to defend itself against pathogens in the environment, with a
defence known as the immune system. By general definition, the immune
system serves the body by neutralizating, inactivating, or eliminating
potentially pathogenic invaders such as microorganisms (bacteria and
viruses); it also guards against uncontrolled growth of cells into
neoplasms, or tumours. The major features of the structure and
function of the immune system have been elucidated over the last three
decades; in parallel, awareness grew of toxicological manifestations
after exposure to xenobiotic chemicals. Recognition of the interplay
between toxicology and immunology is relatively recent: A
comprehensive review, published in 1977 (Vos, 1977), was the first
survey of a large series of xenobiotics that affect immune reactivity
in laboratory animals and hence may influence the health of exposed
individuals. Most research groups focusing on toxicity to the immune
system started their activities during the last decade. Textbooks of
immunotoxicology date only from the early 1980s (Gibson et al., 1983;
Dean et al., 1985; Descotes, 1986), while one on clinical
immunotoxicology is more recent (Newcombe et al., 1992).
Immunotoxicology is the study of the interactions of chemicals
and drugs with the immune system. A major focus of immunotoxicology is
the detection and evaluation of undesired effects of substances by
means of tests on rodents. The prime concern is to assess the
importance of these interactions in regard to human health. Toxic
responses may occur when the immune system is the target of chemical
insults, resulting in altered immune function; this in turn can result
in decreased resistance to infection, certain forms of neoplasia, or
immune dysregulation or stimulation which exacerbates allergy or
autoimmunity. Alternatively, toxicity may arise when the immune system
responds to the antigenic specificity of the chemical as part of a
specific immune response (i.e. allergy or autoimmunity). Certain drugs
induce autoimmunity (Kammüller et al., 1989; Kammüller & Bloksma,
1994). The differentiation between direct toxicity and toxicity due to
an immune response to a compound is to a certain extent artificial.
Some compounds can exert a direct toxic action on the immune system as
well as altering the immune response. Heavy metals like lead and
mercury, for instance, manifest immunosuppressive activity,
hypersensitivity, and autoimmunity (Lawrence et al., 1987).
This monograph is concerned mainly with one aspect of
immunotoxicology: the direct or indirect effect of xenobiotic
compounds (or their biotransformation products) on the immune system.
This effect is usually immunosuppression, or the induction of a state
of deficiency or unresponsiveness. Allergy and autoimmunity will be
dealt with in a future Environmental Health Criteria monograph.
Toxicological research over the past decade has indicated that
the immune system is a potential 'target organ' for toxic damage. This
finding was the basis for a number of large scientific conferences on
immunotoxicology and sparked the active interest of national and
international organizations in this field. One of the milestones in
the development of the discipline was the international seminar on
'The Immunological System as a Target for Toxic Damage', held in
Luxembourg in 1984 and organized by the International Programme on
Chemical Safety (IPCS), and the Commission of the European Communities
(CEC). At the seminar, immunotoxicology was defined as 'the discipline
concerned with the study of the events that can lead to undesired
effects as a result of interaction of xenobiotics with the immune
system. These undesired events may result as a consequence of: (1) a
direct and/or indirect effect of the xenobiotic (and/or its
biotransformation product) on the immune system; or, (2) an
immunologically-based host response to the compound and/or its
metabolite(s) or host antigens modified by the compound or its
metabolites' (Berlin et al., 1987). Recommendations were made
concerning the significance to public health of immunotoxicology,
immunotoxicity testing, research and development in immunotoxicology,
the development of databases, and training and education. A subsequent
workshop on 'Immunotoxicity of Metals and Immunotoxicology', organized
by IPCS and the CEC, in collaboration with the International Union for
Pure and Applied Chemistry and German governmental agencies, was held
in Hanover, Germany, in 1989 (Dayan et al., 1990). A meeting on risk
assessment in immunotoxicology was organized by the United States
National Institute for Environmental Health Sciences in 1990. A
meeting on human immunotoxicology tests was organized by the Agency
for Toxic Substances and Disease Registry and the Centers for Disease
Control, in Atlanta, Georgia, United States of America, in 1992. In
1994, two meetings were held: one in Oxford, United Kingdom, organized
by IPCS, on risk assessment in human imunotoxicity, and one in
Washington DC, United States, organized by the International Life
Sciences Institute, on methods in immunotoxicology.
In parallel to these meetings, activities were started within
IPCS for the development and validation of methods for assessing
toxicity to the immune system. In this regard, a hallmark event was
the meeting in 1986 of a technical review and working group, in
London, United Kingdom (IPCS, 1986).
A number of tiered approaches to immunotoxicity testing have been
proposed, in rats (Vos, 1980; Van Loveren & Vos, 1989) and subsequently
in mice (Luster et al., 1988). These approaches have been evaluated
for their capacity to identify chemicals as immunosuppressive. Of a
group of 18 pesticides evaluated in rats, six were identified as
inducing immunotoxicity at doses similar to those that cause other
toxic effects, and five were immunotoxic at lower doses (Vos & Krajnc,
1983; Vos et al., 1983a). Effects were seen on different parameters
with different compounds and included lymphocytopenia, reduced thymic
and spleen weights, and increased levels of serum immunoglobulin (Ig)
G. One of the compounds identified was hexachlorobenzene (Vos, 1986),
which is further described in Section 2. In mice, the tiered approach
was used to assess the immunotoxicity of 51 chemicals, selected on the
basis of factors including structure-activity relationships with
previously identified immunotoxic substances, and use (Luster et al.,
1992). Of the spectrum of assays applied, the strongest associations
with immunotoxic potential were observed with the splenic IgM antibody
plaque-forming cell response and cell surface marker analysis; somewhat
weaker associations were found for natural killer (NK) cell activity,
cytotoxic T-lymphocyte cytolytic activity, lymphocyte proliferation
in vitro after mitogen stimulation, and thymus:body weight ratio.
The tiered approach in immunotoxicity testing is further described in
Section 3.
Multi-laboratory studies have been initiated to validate the
screening of immunotoxic compounds, including the IPCS-European Union
International Collaborative Immunotoxicity Study, a study in Fischer
344 rats, and the international study of the Bundesinstitut für
Gesundheitlichen Verbraucherschutz, und Veterinärmedizin, which were
designed to determine interlaboratory reproducibility. The
experimental animal used in these studies is the rat, and some
functional tests are included. Test methods are also being developed
and validated within the National Toxicology Program (NTP) in the
United States. This programme includes studies of carcinogenicity in
rats and mice, but because the immune system of mice is better
characterized than that of rats, the NTP chose the mouse as the
experimental animal for immunotoxicity assessment. The immunotoxicity
database of the NTP has been evaluated to determine the predictability
(sensitivity and specificity) of the assays. In the Netherlands, a
Committee for Immunotoxicology of the Dutch Health Council reviewed
methods that could be used to assess the immunotoxic properties of a
compound and for deriving information about risks to humans on the
basis of the results of laboratory experiments. The Committee also
examined the relationship between the immunotoxic properties of a
substance and its mutagenic and carcinogenic properties (Dutch Health
Council, Committee for Immunotoxicology, 1991).
The immune system was reviewed by the United States National
Research Council in order to identify the kinds of basic research that
might reveal markers of environmental exposure and disease. Major
emphasis was placed on biological markers of three types: those
originating from the immune system, those related to exposure to
immunosuppressive toxicants, and those of effects of environmental
pollutants. Markers of susceptibility to environmental materials were
also considered to be important, especially if they are of a genetic
nature and can be used to identify individuals susceptible to
autoimmune diseases. The National Research Council subcommittees on
pulmonary toxicology and on immunotoxicology, have published their
reports (US National Research Council, 1989, 1992).
Interest in immunotoxicology within the scientific community is
reflected by the existence of a special section on immunotoxicology
within the Society of Toxicology. An immunotoxicology discussion group
initiated in the United States has an international composition. The
European Union has a programme on science and technology for
environmental protection that includes immunotoxicology as an
important aspect.
There is growing concern in society about the effects of
xenobiotics, such as environmental pollutants, on public health; the
immune system is one of the targets of such effects. Some chemicals
present in the environment that have been reported to influence the
immune system are listed in Table 1 (IPCS, 1986). Immunotoxicity can
result in e.g. reduced resistance towards infection or generation of
tumours that escape immune surveillance. A number of substances
affect immunological parameters; these include halogenated
hydrocarbons such as polychlorinated biphenyls, polybrominated
biphenyls, polychlorinated dibenzo- para-dioxins, and polychlorinated
dibenzofurans (Elo et al., 1985; Lu & Wu, 1985; Bekesi et al., 1987;
Kimbrough, 1987; Hoffman 1992); pesticides and precursors (Fiore et
al., 1986; Deo et al., 1987; Nigam et al., 1993); organic solvents
(Capurro, 1980; Denkhaus et al., 1986); asbestos (Lew et al., 1986);
silica (Uber & McReynolds, 1982); and metals like lead (Ewers et al.,
1982; Reigart & Graber, 1976). Oxidant air pollutants, like sulfur
dioxide, nitrogen dioxide, and ozone, and particles in airborne dust
may affect immune function (Koren et al., 1989; Van Loveren et al.,
1994).
Immunotoxicity in humans is further discussed in Section 2. Few
epidemiological data have been published that indicate suppression or
altered resistance to infection and tumours. In general, the
usefulness of the epidemiological studies that have been published is
limited by the following: exposure is usually uncontrolled, mainly
occurring during accidents; the magnitude and pattern of exposure are
not known, and the exposure is often too low to alter the immune
system measurably; exposure is often not to one xenobiotic but to a
mixture; it is almost impossible to control for confounding
parameters, such as age, sex, genetic background, health status, and
nutritional status; and it is not always possible to define and
analyse appropriate control groups (US National Research Council,
1992). Environmental pollution and its effect on health status are
currently subjects of concern in eastern European countries and have
generated much interest in the worldwide environmental health science
community. Recent epidemiological studies have compared the possible
relationship between exposure to air pollutants and health effects in
the former German Democratic Republic and Federal Republic of Germany,
Table 1. Examples of compounds that are immunotoxic for humans or
rodents
Chemical Immune toxicity
-------------------
Rodent Human
2,3,7,8-Tetrachlorodibenzo-para-dioxin + +
Polychlorinated biphenyls + +
Polybrominated biphenyls + +
Hexachlorobenzene + Unknown
Lead + Unknown
Cadmium + Unknown
Methyl mercury compounds + Unknown
7,12-Dimethylbenz[a]anthracene + Unknown
Benzo[a]pyrene + Unknown
Di-n-octyltindichloride + Unknown
Di-n-butyltindichloride + Unknown
Benzidine + +
Nitrogen dioxide and ozone + +
Benzene, toluene, and xylene + +
Asbestos + +
N-Nitrosodimethylamine + Unknown
Diethylstilboestrol + +
Vanadium + +
From IPCS (1986)
and some of these studies included immunological data or end-points.
For instance, von Mutius et al. (1992) found a higher prevalence of
asthma among schoolchildren in western than eastern Germany, and
Behrendt et al. (1993) observed, surprisingly, that total serum IgE
levels were higher in schoolchildren in eastern than in western
Germany. Several factors were found to influence total IgE: history of
parasitic disease, number of persons per dwelling, and passive
smoking. Sex and passive smoking were the only variables that had a
significant effect in western German children. Air pollutants and
parasitic infections were suggested to be the major contributing
factors to increased IgE production in children in eastern Germany.
Remarkable differences in air quality were seen between eastern and
western Germany, and Behrendt et al. (1995) distinguished two types of
air pollution: type I, composed of sulfur dioxide particles and dust,
occurring predominantly in eastern Europe, is associated with
respiratory infections and other chronic inflammatory airway
reactions; type II, occurring both indoors and outdoors in the
environment in industrialized western countries, is composed mainly of
nitric oxide, nitrogen dioxide, ozone, volatile organic compounds, and
fine particles. The latter type of air pollution is associated with
allergic diseases and allergic sensitization, indicating that air
pollutants interfere with parameters of allergy at the level of
sensitization, elicitation of symptoms, and exacerbation of disease.
Until further epidemiological studies are conducted in humans,
assessment of immunotoxicity in rodents, with subsequent extrapolation
to the human situation, is still a good indicator of toxicity and can
serve as a basis for subsequent decisions and regulations by
authorities to reduce or prevent the risk of human exposure. This
aspect is discussed further in Section 5.
Humans are exposed to environmental contaminants mainly via food,
water, and air. Open water (e.g. rivers, lakes, and coastal areas) and
sediments often act as sinks for environmental pollution. This global
problem can be deduced from disease manifestations in fish that live
in coastal areas, especially those species that live in close contact
with contaminated silt. High levels of contaminants and the diseases
associated with them are not only of economic importance (i.e. to
fisheries) but also affect people who consume the fish, as seen in
studies showing increased levels of contaminants in people eating fish
from the contaminated Baltic Sea (Svensson et al., 1991) and in Inuit
and Indian populations in Canada who consume large quantities of fish
and marine mammals (DeWailly et al., 1992). There is now some evidence
that wildlife aquatic species have decreased resistance and enhanced
incidences of infection and tumours that may be linked to
environmental pollution (Vos et al., 1989; Wester et al., 1994).
Because the immune system of fish has not been characterized in such
detail as that of mammals, immunotoxicological studies have not been
extensively included in ecotoxicology, although a number of reports of
direct toxic actions of xenobiotics on fish species have been
published in this developing field (Wester & Canton, 1987; Payne &
Fancey, 1989; Anderson, 1990; Wester et al., 1990; Khangarot &
Tripathi, 1991; Secombes et al., 1992; Anderson & Brubacher, 1993;
Faisal & Hugget, 1993).
1.2 The immune system: functions, system regulation, and modifying
factors; histophysiology of lymphoid organs
1.2.1 Function of the immune system
In order to interpret pathological alterations of the immune
system in terms of altered function, the physiology of the system must
be understood. Since knowledge of the structure and function of the
immune system is growing rapidly, a review of this subject, focusing
on histophysiology, is presented. This section is not meant to serve
as a textbook on immunology but to provide sufficient information for
an understanding of pathological changes due to immunotoxic action.
For general textbooks on immunology, reference may be made to Sell
(1987), Klein (1990), Brostoff et al. (1991), Roitt (1991), Paul
(1993), and Roitt et al. (1993). The section covers mainly humans and
rodents, but reference is made to other species that are relevant in
immunotoxicity assessment, e.g. fish in ecotoxicology. It should be
noted that species differences can be large, despite fundamental
similarities between the immune systems of animals. It is therefore
difficult to conduct immunotoxicological studies in immunologically
less well characterized animal species, although comparative studies
that are under way may lessen the problems. Zapata and Cooper (1990)
have written a comprehensive textbook on phylogenetic aspects of
immunology. Phylogenetic data, from primitive fish to mammals, are
presented in Table 2 (Cooper, 1982; Klein, 1986; Du Pasquier, 1989;
Zapata & Cooper, 1990; Sima & Vetvicka, 1992). Relevant phylogenetic
aspects of the immune system are described below.
In mammals, the immune system and its reactions consist of a
finely tuned, complex interplay between various cell types and soluble
mediators secreted by those cells (Figure 1), some of which are listed
in Section 7.
Immune responses can be classified roughly as innate (natural and
nonspecific) and acquired (adaptive) responses, in which the reaction
is directed to a specific determinant (antigenic determinant or
epitope). The nonspecific response involves effector cells such as
macrophages (Vetvicka & Fornusek, 1992), NK cells (Herberman &
Ortaldo, 1981), granulocytes (Ross, 1992), and mediator systems
including the complement system (Tomlinson, 1993). Specificity is
based on recognition by specific receptors on lymphocytes or by
antibodies: The classical reaction to bacterial infection, resulting
in antibacterial antibody formation and antibody-mediated destruction
of the pathogen, is only one part of the intrinsic capacity of the
system. Further attributes of the system (Nossal, 1987) are summarized
below.
1.2.1.1 Encounter and recognition
The initiation of an immune response requires adequate
recognition of the pathogen. This recognition often occurs immediately
after entry, e.g. during or after passage through the epithelial
barrier of the body (skin or mucus-secreting epithelia in the
respiratory and gastrointestinal tract). The first defence includes
nonspecific inactivation, e.g. by nonspecific killer cells,
neutrophilic granulocytes, and cells of the mononuclear phagocyte
system (formerly called the reticuloendothelial system). It also
includes antigen processing and presentation to cells such as
lymphocytes of the T-helper-inducer type (Th) which can generate a
specific response.
Table 2. Evolution of immunologically important traits among vertebrates
A. Major histocompatibility complex (MHC) and transplantation
Species Graft MLR CML MHC control Serologically
rejection and/or of immune detectable
GVH response MHC antigens
Tunicata (sea squirts) + ? ? ? ?
Agnatha
Hagfish (Hyperotreti) + ? - ? ?
Lamprey (Hyperoartii) + ? - ? ?
Chondrichthyes (cartilaginous fish)
Shark, ray + ? + ? +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + ? ? ? ?
Bony fish (Teleostei) + ? + ? +
Lungfish (Dipnoi) + ? ? ? ?
Amphibia (amphibians)
Salamanders (Urodela) + ? ? ? +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + ? ? ?
Lizards, snakes (Squamata) + + + ? ?
Crocodiles, alligators (Crocodilia) + ? ? ? ?
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
B. Complement and immunoglobulins (Ig)
Species Complement Immunoglobulins
IgM IgG-like IgA IgD IgE
Tunicata (sea squirts) ? - - - - -
Agnatha
Hagfish (Hyperotreti) ? ? - - - -
Lamprey (Hyperoartii) ? ? - - - -
Chondrichthyes (cartilaginous fish)
Shark, ray + + + - - -
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + + + - - -
Bony fish (Teleostei) + + + - - -
Lungfish (Dipnoi) + + + - - -
Amphibia (amphibians)
Salamanders (Urodela) + + ? - - -
Frogs, toads (Anura) + + + + - -
Reptilia (reptiles)
Turtles (Chelonia) + + + - - -
Lizards, snakes (Squamata) + + + - - -
Crocodiles, alligators (Crocodilia) + + + ? - -
Aves (birds) + + ? + - -
Mammalia (mammals) + + + + + +
Table 2 (cont'd)
C. Leukocytes
Species Lymphocytes Plasma Macrophages
cells
Small T B
Tunicata (sea squirts) + - - - +
Agnatha
Hagfish (Hyperotreti) + - - + +
Lamprey (Hyperoartii) + - - + +
Chondrichthyes (cartilaginous fish)
Shark, ray + - ? + +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + - ? + +
Bony fish (Teleostei) + + + + +
Lungfish (Dipnoi) + ? ? + +
Amphibia (amphibians)
Salamanders (Urodela) + + + + +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + + + +
Lizards, snakes (Squamata) + + + + +
Crocodiles, alligators (Crocodilia) + + + + +
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
D. Lymphoid organs
Species Bone Thymus Spleen Lymph glands
marrow or nodes
Tunicata (sea squirts) - - - -
Agnatha
Hagfish (Hyperotreti) - - - GALT
Lamprey (Hyperoartii) - - - GALT
Chondrichthyes (cartilaginous fish)
Shark, ray - + + GALT
Osteichthyes (bony fish)
Sturgeon (Chondrostei) - + + GALT
Bony fish (Teleostei) - + + GALT
Lungfish (Dipnoi) - + + GALT
Amphibia (amphibians)
Salamanders (Urodela) + + + -
Frogs, toads (Anura) + + + ?
Reptilia (reptiles)
Turtles (Chelonia) + + + ?
Lizards, snakes (Squamata) + + + ?
Crocodiles, alligators (Crocodilia) + + + ?
Aves (birds) + + + +
Mammalia (mammals) + + + +
+, positive; ±, to be confirmed; -, negative; ?, not investigated.
MLR, mixed leukocyte response; GVH, graft-versus-host; CML, cell-mediated lympholysis;
GALT, gut-associated lymphoid tissue
incorporating the comments received and approved by the Director,
IPCS, is then distributed to Task Group members, who carry out a peer
review at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the risks to health and
the environment from exposure to the chemical. A summary and
recommendations for further research and improved safety are also
drawn up. The composition of the Task Group is dictated by the range
of expertise required for the subject of the meeting and by the need
for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations, so that
representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide valuable contributions to the process, they can speak only
at the invitation of the Chairperson. Observers do not participate in
the final evaluation of the chemical, which is the sole responsibility
of the Task Group members. The Task Group may meet in camera when it
considers that to be appropriate.
All individuals who participate in the preparation of an EHC
monograph as authors, consultants, or advisers must, in addition to
serving in their personal capacity as scientists, inform the RO if at
any time a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a statement to that
effect. This procedure ensures the transparency and probity of the
process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it is edited for language, the references are checked, and
camera-ready copy is prepared. After approval by the Director, IPCS,
the monograph is submitted to the WHO Office of Publications for
printing. At this time, a copy of the final draft is also sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern about
health or environmental effects of the agent because of greater
exposure; an appreciable time has elapsed since the last evaluation.
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The chairpersons of task groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON PRINCIPLES AND METHODS FOR ASSESSING DIRECT
IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
Members
Dr A. Emmendörffer, Department of Immunobiology, Fraunhofer Institute
of Toxicology & Aerosol Research, Hanover, Germany
Dr H.S. Koren, Health Effects Research Laboratory, US Environmental
Protection Agency, Chapel Hill, NC, USA (Vice-Chairman)
Dr R.W. Luebke, Immunotoxicology Branch, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, NC, USA (Joint Rapporteur)
Dr M. Luster, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA
Dr C. Madsen, Institute of Toxicology, National Food Agency of
Denmark, Ministry of Health, Soborg, Denmark
Dr P. Ross, Dalhousie University, Halifax, Nova Scotia, Canada
(c/o Seal Rehabilitation and Research Centre, Pieterburen,
Netherlands)
Dr H.J. Schuurman, Preclinical Research/Immunology, Sandoz Pharma Ltd,
Basel, Switzerland
Dr H. Van Loveren, Laboratory for Pathology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Joint Rapporteur)
Dr J.G. Vos, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands (Chairman)
Dr K.L. White, Jr, Immunotoxicology Group, Medical College of
Virginia, Virginia Commonwealth University, Richmond, VA, USA
Observers
IUTOX
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy
ECETOC
Dr R.W.R. Crevel, Environmental Safety Laboratory, Unilever Research
and Engineering, Sharnbrook, Bedfordshire, United Kingdom
Secretariat
Dr J.H. Dean, Sanofi Winthrop, Inc., Sanofi Research Division,
Collegeville, PA, USA
Mr V. Quarg, Federal Ministry for Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA PRINCIPLES AND METHODS FOR ASSESSING
DIRECT IMMUNOTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
A WHO Task Group on Principles and Methods for Assessing Direct
Immunotoxicity Associated with Exposure to Chemicals met at the World
Health Organization, Geneva, from 10 to 14 October 1994. Dr E. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
IPCS, and the cooperating organizations. The Task Group reviewed and
revised the draft monograph and prepared the final text.
The first draft of the monograph was prepared by a group of
authors (listed below) under the coordination of Dr J.G. Vos and
Dr H. Van Loveren of the Dutch National Institute for Public Health
and Environmental Protection (RIVM), an IPCS Collaborating Centre for
Immunotoxicology and Allergic Hypersensitization. The second draft,
incorporating comments received after international circulation to
national experts of the first draft to IPCS contact points for
Environmental Health Criteria monographs, was prepared by Dr J.G.
Vos and Dr H. Van Loveren of the Netherlands and Dr Kimber White, USA.
Dr E. Smith of the IPCS Unit for the Assessment of Risk and
Methods was responsible for the scientific content of the monograph
and Mrs E. Heseltine for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The contributing authors were:
Dr J.H. Dean, Collegeville, PA, USA
Professor J. Descotes, Lyon, France
Dr F. Kuper, Zeist, Netherlands
Dr M. Luster, Research Triangle Park, NC, USA
Dr P.S. Ross, Bilthoven, Netherlands
Dr H.J. Schuurman, Basel, Switzerland
Dr M.J. Selgrade, Research Triangle Park, NC, USA
Dr R.L. de Swart, Bilthoven, Netherlands
Dr H. Van Loveren, Bilthoven, Netherlands
Professor J.G. Vos, Bilthoven, Netherlands
Dr P.W. Wester, Bilthoven, Netherlands
Professor A.G. Zapata, Madrid, Spain
ABBREVIATIONS
ACTH adrenocorticotrophic hormone
Ah aromatic hydrocarbon
AIDS acquired immunodeficiency syndrome
B bursa-dependent
CALLA common acute lymphoblastic leukaemia antigen
CD cluster of differentiation
CEC Commission of the European Communities
CH50 haemolytic complement
CML cell-mediated lympholysis
DMBA 7,12-dimethylbenz[ a]anthracene
DNCB dinitrochlorobenzene
ELISA enzyme-linked immunosorbent assay
EPO erythrocyte lineage differentiation factor
FACS fluorescence activated cell sorter
GALT gut-associated lymphoid tissue
G-CSF granulocyte colony-stimulating factor
GM-CSF granulocyte-macrophage colony-stimulating factor
GVH graft-versus-host
HCB hexaclorobenzene
HEV high endothelial venule
HIV human immunodeficiency virus
HPCA human progenitor cell antigen
HSA heat-stable antigen
ICAM intercellular adhesion molecule
IFN interferon
Ig immunoglobulin
IL interleukin
IPCS International Programme on Chemical Safety
LFA lymphocyte function-related antigen
LIF leukaemia inhibitory factor
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
M microfold
MALT mucosa-associated lymphoid tissue
MARE monoclonal anti-rat immunoglobulin E
MARK monoclonal antibody anti-kappa
M-CSF macrophage colony-stimulating factor
MED minimal erythemal dose
MHC major histocompatibility complex
NCAM neural cell adhesion molecule
NK natural killer
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NTP National Toxicology Program
PAH polycyclic aromatic hydrocarbon
PCB polychlorinated biphenyl
PG prostaglandin
QCA quiescent cell antigen
RIVM Dutch National Institute of Public Health and
Environmental Protection
S9 9000 x g supernatant
SCF stem-cell factor
SCID severe combined immunodeficiency
SIS skin immune system
STM Salmonella typhimurium mitogen
TBTO tri- n-butyltin oxide
Tc cytotoxic T cell
TCDD 2,3,7,8-tetrachlorodibenzo- para-dioxin
TCR T-cell receptor
Tdth delayed-type hypersensitivity T cell
TGF transforming growth factor
Th T helper-inducer cell
THAM T-cell activation molecule
THI 2-acetyl-4(5)-tetrahydroxybutylimidazole
O,O,S-TMP O,O,S-trimethylphosphorothiate
TNF tumour necrosis factor
UVB ultraviolet B
UVR ultraviolet radiation
VCAM vascular cell adhesion molecule
VLA very late antigen
SUMMARY
1. The immune system has evolved to counter challenges to the
integrity of self from either microorganisms or cells that have
escaped the organism's control mechanisms. Recognition that
xenobiotics can impair the function of the immune system has led to
progress in immunotoxicology over the last two decades. Experimental
approaches (mainly in rodent species) have been developed and
validated in multilaboratory studies. In this monograph, the function
and histophysiology of the immune system are reviewed, and the
information necessary to understand and interpret the pathological
changes caused by immunotoxic insults is provided. Emphasis is laid on
the immune systems of humans and rodent species, but reference is made
to other species, including fish, that have been the object of
immunotoxicological studies. The pathophysiology of the immune system,
including the variable susceptibility of its components, alterations
to the lymphoid organs, and the reversibility of changes are important
for understanding the impact of immunotoxicity.
2. Immunosuppression and immunostimulation both have clinical
consequences. Immunodeficiency states and severe immunosuppression,
such as can occur during transplantion and cytostatic therapy, have
both been associated with increased incidences of infectious diseases
(particularly opportunistic ones) and cancer. Exposure to immunotoxic
chemicals in the environment, however, may be expected to result in
more subtle forms of immunosuppression which may be difficult to
detect, leading to increased incidences of infections such as
influenza and the common cold. Studies of experimental animals and
humans have shown that many environmental chemicals suppress the
immune response. Immunotoxic xenobiotics are not restricted to a
particular chemical class. Compounds that adversely affect the immune
system are found among drugs, pesticides, solvents, halogenated and
aromatic hydrocarbons, and metals; ultraviolet radiation can also be
immunotoxic. Therapeutic administration of immunostimulating agents
can have adverse effects, and a few environmental chemicals that have
immunostimulating properties (beryllium, silica, hexachlorobenzene)
can have clinical consequences.
3. The complexity of the immune system results in multiple potential
target sites and pathological sequelae. The initial strategies devised
by immunotoxicologists working in toxicology and safety assessment
were to select and apply a tiered panel of assays to identify
immunosuppressive and immunostimulatory agents in laboratory animals.
Although the configuration of these testing panels may vary depending
on which agency or laboratory is conducting the test and on the animal
species employed, they all include measurement of one or more of the
following: altered lymphoid organ weights and histology; changes in
the cellularity of lymphoid tissue, peripheral blood leukocytes,
and/or bone marrow; impairment of cell function at the effector or
regulatory level; and altered susceptibility to challenge with
infectious agents or tumour cells.
The original test guideline No. 407 of the Organisation for
Economic Co-operation and Development, published in 1981, was not
designed to detect potential immunotoxicity, and modifications have
been proposed to make the guideline more useful for identifying
immunotoxicants. Tiered testing systems have been designed for more
extensive investigation of potential immunotoxicity, by the US
National Toxicology Program, the Dutch National Institute of Public
Health and Environmental Protection, the US Environmental Protection
Agency Office of Pesticides, and the US Food and Drug Administration
Center for Food Safety and Applied Nutrition.
Studies have been conducted in mice, and to a lesser extent in
rats, to investigate the specificity, precision (reproducibility),
sensitivity, accuracy, and relevance for the assessment of risk to
human health of a variety of measures of immune status. International,
interlaboratory validations of methods have been carried out within
the International Collaborative Immunotoxicity Study of IPCS and the
European Union, the Bundesinstitut für Gesundheitlichen
Verbraucherschutz, und Veterinärmedizin, and in studies of cyclosporin
A in Fischer 344 rats.
4. The tests used in the tiered testing schemes are described in
Section 3, which indicates the rationale for their selection and the
complexities involved in their performance. Although these protocols
were designed for studies of rats and mice, some have been applied
successfully for studying immunotoxicity in other animal species,
including non-human primates, marine mammals, dogs, birds, and fish.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to influence the immune
system of experimental animals adversely. These include selection of
the appropriate animal models and exposure variables, inclusion of
general toxicological parameters, an understanding of the biological
relevance of the end-points being measured, use of validated measures,
and quality assurance. The experimental conditions should take into
account the potential route and level of human exposure and any
available information on toxicodynamics and toxicokinetics. The doses
and sample sizes should be selected so as to generate clear dose-
response curves, in addition to no-observed-adverse-effect or
no-observed-effect levels. The strategies are continually refined to
allow better prediction of conditions that may lead to disease. In
addition, techniques should be developed that would help to identify
mechanisms of action; these might include methods in vitro,
examination of local immune responses (such as in the skin, lung, and
intestines), and use of the techniques of molecular biology and
genetically modified animals.
5. The detection of immune changes after exposure to potentially
immunotoxic compounds is more complicated in humans than in
experimental animals. The testing possibilities are limited, levels of
exposure to the agent (i.e. dose) are difficult to establish, and the
immune status of populations is extremely heterogeneous. Age, race,
gender, pregnancy, acute stress and the ability to cope with stress,
coexistent disease and infections, nutritional status, tobacco smoke,
and some medications contribute to this heterogeneity.
An important factor in assessing the usefulness of a particular
study for risk assessment is epidemiological study design. The
commonest design used in immunotoxicity is the cross-sectional study,
in which exposure status and disease status are measured at one time
or over a short period. The immune function of 'exposed' subjects is
then compared with that of a comparable group of 'unexposed'
individuals. There are possible pitfalls in this study design.
Because many of the immune changes seen in humans after exposure
to a chemical may be sporadic and subtle, recently exposed populations
must be studied and sensitive tests be used for assessing the immune
system. Conclusions about immunotoxic effects should be based on
changes not in a single parameter but in the immune profile of an
individual or population.
Most of the tests for specific immunity (cell-mediated and
humoral), nonspecific immunity and inflammation were developed to
detect immune alterations in patients with immunodeficiency disease
and are not always adequate to detect subtle alterations induced by
environmental chemicals. IPCS, the Centers for Disease Control, and
the US National Academy of Sciences have each described procedures for
evaluating changes in the human immune system resulting from exposure
to immunotoxicants, but the tests described require evaluation for
this purpose.
6. Risk assessment is a process in which relevant data on the
biological effects, dose-response relationships, and exposure for a
particular agent are analysed in an attempt to establish qualitative
and quantitative estimates of adverse outcomes. Typically, risk
assessment comprises four major steps: hazard identification, dose-
response assessment, exposure assessment, and risk characterization.
Up until now, immunotoxicology has focused mainly on hazard
identification, and to some extent on dose-response assessment, and
very few studies have included exposure assessment or risk
characterization.
As in other areas of toxicology, uncertainties exist which may
affect the interpretation of data on immunotoxicity with regard to
human health risk. The two most problematic issues -- extrapolating
effects from individual cells to a whole organ or beyond and
extrapolating data from experimental animals to humans -- are common
to most non-cancer end-points. The first issue is due to uncertainties
associated with establishing a quantitative relationship between
changes in individual immune function and altered resistance to
infections and neoplastic disease. The second issue is due to
uncertainties associated with assessing risk to human health on the
basis of studies in laboratory animals.
The ultimate purpose of risk assessment is to protect human
health and the environment. Suitable model systems must therefore be
chosen. The toxicokinetics of the test material and the nature and
magnitude of the immune response generated in the model should be
comparable to that of humans.
Conventionally, empirical uncertainty factors are used in risk
assessment to derive an acceptable exposure limit from experimental
results. This approach does not take into account the functional
reserve or redundancy of the immune system. A more recent development
in risk assessment is use of in-vitro models as an adjunct to studies
of experimental animals. The advantages of this approach are that it
improves the accuracy of extrapolation of data from animals to man and
minimizes the use of animals; it also bridges the gap between those
data, particularly when human experimentation is limited for ethical
considerations. Chapter 6 cites two examples in which in-vitro data
make it possible to reduce the uncertainties in risk assessment
associated with exposure to ozone and ultraviolet radiation. The
difficulty in establishing quantitative relationships between
immunosuppression and clinical disease has limited the use of
immunotoxicological data in risk assessment.
RECOMMENDATIONS
Recommendations for the protection of human health
1. Chemicals should be screened to determine if they are potentially
immunotoxic to humans. If immunotoxicity is detected, the chemicals
should be investigated further as part of the risk assessment process.
2. Chemicals for which little or no information is available on
toxicity should be screened for potential immunotoxicity following a
protocol based on, for example, the revised OECD guideline No. 407.
When some information is available on the test material (e.g.
physicochemical properties, toxicokinetics, structure-activity
relationships), a flexible approach to testing is recommended which
permits a rational selection of test procedures.
3. The immunotoxic risk of mixtures of environmental pollutants, in,
for example, fish, to certain human consumer groups (e.g. fishermen)
should be assessed.
Recommendations for protection of the environment
1. Chemicals should be screened to determine if they are potentially
immunotoxic to wildlife species. If immunotoxicity is detected, the
chemicals should be investigated further as part of the risk
assessment process.
2. The immunotoxic risk of environmental pollution to the health of
the ecosystem should be assessed in laboratory, semi-field, and field
studies of the wildlife occupying high trophic levels or those species
judged to be sensitive.
Recommendations for further research
1. The panels of tests suggested for evaluating xenobiotic-induced
immunotoxicity in humans should be investigated to determine their
ability to detect subtle alterations in immune status.
2. The relationships between alterations in immune function and human
health should be established for use in immunotoxic risk assessment.
Epidemiological studies should be carried out that include assessment
of exposure, in order to establish dose-response relationships.
3. The relationship between immunotoxicity and the development of
neoplasia should be investigated.
4. Baseline immunological data should be established for the general
population and for subpopulations such as ethnic minorities, children,
the aged, and pregnant and lactating women in order to assess their
immune status.
5. Immunotoxicological assessment should be conducted for
subpopulations potentially susceptible to the effects of immunotoxic
compounds, including those at the extremes of age and those with
deficient nutritional status.
6. Biomarkers of exposure, effect, and susceptibility should be
identified, developed, and validated for use in epidemiological
studies of immunotoxicity in both humans and wildlife.
7. The quantitative relationship between immune function and host
resistance in animal models, including the nature, magnitude, and
significance of functional reserve and redundancy, should be explored
for risk assessment.
8. Since chemicals and biological agents enter the body via the
respiratory and alimentary tracts and the skin, more research should
be carried out on local immunity.
9. Preliminary observations in laboratory animals that suggest that
primary immunization does not compromise testing for subacute toxicity
should be substantiated by further research, so that functional
testing can be incorporated into toxicology testing.
10. Methods and reagents should be developed in order to characterize
the immune system of wildlife species and to assess their immune
status for immunotoxicological studies.
11. The mechanisms of the immunotoxic action of xenobiotics in humans
should be elucidated by a combination of studies in laboratory animals
in vivo and experiments with human and animal tissues and cell lines
in vitro.
12. In view of the sensitivity of the developing immune system to
immunotoxic injury, more emphasis should be placed on studies
involving perinatal exposure to a chemical or mixture of chemicals.
13. Studies should be conducted to establish whether exposure to
xenobiotics that are not themselves sensitizing adds to the risk of
allergic disease in general.
14. Autoimmune models in laboratory animals should be used to assess
whether xenobiotics can modulate autoimmune disease in humans.
15. The effects on immune function of confounding factors in humans
and animals, including age, race, sex, gender, nutritional status,
acute stress, and underlying disease, should be evaluated further in
order to determine their effects in tests for the immunotoxicity of
environmental chemicals.
16. Methods for assessing cytokines and their production in different
body compartments, including plasma, bronchoalveolar lavage fluid, and
nasal lavage fluid, and by cells isolated from various anatomical
sites should be validated for humans and laboratory animals, and their
applicability for assessing the risk of chemicals should be
established.
17. Data from clinical trials should be made more widely available;
and patients undergoing therapy with immunomodulatory drugs should be
monitored clinically and immunologically in a systematic way.
18. The toxicokinetics of immunotoxic chemicals should be further
investigated, particularly with regard to whether their concentrations
in human biological fluids indicate levels of environmental exposure.
19. The interactions between the immune system, the nervous system,
and the endocrine system should be further investigated, with
particular emphasis on how xenobiotics adversely affect them.
20. The significance of ultraviolet radiation-induced
immunosuppression for public health and the health of ecosystems
should be evaluated.
1. INTRODUCTION TO IMMUNOTOXICOLOGY
1.1 Historical overview
It is well established that each individual has an intrinsic
capacity to defend itself against pathogens in the environment, with a
defence known as the immune system. By general definition, the immune
system serves the body by neutralizating, inactivating, or eliminating
potentially pathogenic invaders such as microorganisms (bacteria and
viruses); it also guards against uncontrolled growth of cells into
neoplasms, or tumours. The major features of the structure and
function of the immune system have been elucidated over the last three
decades; in parallel, awareness grew of toxicological manifestations
after exposure to xenobiotic chemicals. Recognition of the interplay
between toxicology and immunology is relatively recent: A
comprehensive review, published in 1977 (Vos, 1977), was the first
survey of a large series of xenobiotics that affect immune reactivity
in laboratory animals and hence may influence the health of exposed
individuals. Most research groups focusing on toxicity to the immune
system started their activities during the last decade. Textbooks of
immunotoxicology date only from the early 1980s (Gibson et al., 1983;
Dean et al., 1985; Descotes, 1986), while one on clinical
immunotoxicology is more recent (Newcombe et al., 1992).
Immunotoxicology is the study of the interactions of chemicals
and drugs with the immune system. A major focus of immunotoxicology is
the detection and evaluation of undesired effects of substances by
means of tests on rodents. The prime concern is to assess the
importance of these interactions in regard to human health. Toxic
responses may occur when the immune system is the target of chemical
insults, resulting in altered immune function; this in turn can result
in decreased resistance to infection, certain forms of neoplasia, or
immune dysregulation or stimulation which exacerbates allergy or
autoimmunity. Alternatively, toxicity may arise when the immune system
responds to the antigenic specificity of the chemical as part of a
specific immune response (i.e. allergy or autoimmunity). Certain drugs
induce autoimmunity (Kammüller et al., 1989; Kammüller & Bloksma,
1994). The differentiation between direct toxicity and toxicity due to
an immune response to a compound is to a certain extent artificial.
Some compounds can exert a direct toxic action on the immune system as
well as altering the immune response. Heavy metals like lead and
mercury, for instance, manifest immunosuppressive activity,
hypersensitivity, and autoimmunity (Lawrence et al., 1987).
This monograph is concerned mainly with one aspect of
immunotoxicology: the direct or indirect effect of xenobiotic
compounds (or their biotransformation products) on the immune system.
This effect is usually immunosuppression, or the induction of a state
of deficiency or unresponsiveness. Allergy and autoimmunity will be
dealt with in a future Environmental Health Criteria monograph.
Toxicological research over the past decade has indicated that
the immune system is a potential 'target organ' for toxic damage. This
finding was the basis for a number of large scientific conferences on
immunotoxicology and sparked the active interest of national and
international organizations in this field. One of the milestones in
the development of the discipline was the international seminar on
'The Immunological System as a Target for Toxic Damage', held in
Luxembourg in 1984 and organized by the International Programme on
Chemical Safety (IPCS), and the Commission of the European Communities
(CEC). At the seminar, immunotoxicology was defined as 'the discipline
concerned with the study of the events that can lead to undesired
effects as a result of interaction of xenobiotics with the immune
system. These undesired events may result as a consequence of: (1) a
direct and/or indirect effect of the xenobiotic (and/or its
biotransformation product) on the immune system; or, (2) an
immunologically-based host response to the compound and/or its
metabolite(s) or host antigens modified by the compound or its
metabolites' (Berlin et al., 1987). Recommendations were made
concerning the significance to public health of immunotoxicology,
immunotoxicity testing, research and development in immunotoxicology,
the development of databases, and training and education. A subsequent
workshop on 'Immunotoxicity of Metals and Immunotoxicology', organized
by IPCS and the CEC, in collaboration with the International Union for
Pure and Applied Chemistry and German governmental agencies, was held
in Hanover, Germany, in 1989 (Dayan et al., 1990). A meeting on risk
assessment in immunotoxicology was organized by the United States
National Institute for Environmental Health Sciences in 1990. A
meeting on human immunotoxicology tests was organized by the Agency
for Toxic Substances and Disease Registry and the Centers for Disease
Control, in Atlanta, Georgia, United States of America, in 1992. In
1994, two meetings were held: one in Oxford, United Kingdom, organized
by IPCS, on risk assessment in human imunotoxicity, and one in
Washington DC, United States, organized by the International Life
Sciences Institute, on methods in immunotoxicology.
In parallel to these meetings, activities were started within
IPCS for the development and validation of methods for assessing
toxicity to the immune system. In this regard, a hallmark event was
the meeting in 1986 of a technical review and working group, in
London, United Kingdom (IPCS, 1986).
A number of tiered approaches to immunotoxicity testing have been
proposed, in rats (Vos, 1980; Van Loveren & Vos, 1989) and subsequently
in mice (Luster et al., 1988). These approaches have been evaluated
for their capacity to identify chemicals as immunosuppressive. Of a
group of 18 pesticides evaluated in rats, six were identified as
inducing immunotoxicity at doses similar to those that cause other
toxic effects, and five were immunotoxic at lower doses (Vos & Krajnc,
1983; Vos et al., 1983a). Effects were seen on different parameters
with different compounds and included lymphocytopenia, reduced thymic
and spleen weights, and increased levels of serum immunoglobulin (Ig)
G. One of the compounds identified was hexachlorobenzene (Vos, 1986),
which is further described in Section 2. In mice, the tiered approach
was used to assess the immunotoxicity of 51 chemicals, selected on the
basis of factors including structure-activity relationships with
previously identified immunotoxic substances, and use (Luster et al.,
1992). Of the spectrum of assays applied, the strongest associations
with immunotoxic potential were observed with the splenic IgM antibody
plaque-forming cell response and cell surface marker analysis; somewhat
weaker associations were found for natural killer (NK) cell activity,
cytotoxic T-lymphocyte cytolytic activity, lymphocyte proliferation
in vitro after mitogen stimulation, and thymus:body weight ratio.
The tiered approach in immunotoxicity testing is further described in
Section 3.
Multi-laboratory studies have been initiated to validate the
screening of immunotoxic compounds, including the IPCS-European Union
International Collaborative Immunotoxicity Study, a study in Fischer
344 rats, and the international study of the Bundesinstitut für
Gesundheitlichen Verbraucherschutz, und Veterinärmedizin, which were
designed to determine interlaboratory reproducibility. The
experimental animal used in these studies is the rat, and some
functional tests are included. Test methods are also being developed
and validated within the National Toxicology Program (NTP) in the
United States. This programme includes studies of carcinogenicity in
rats and mice, but because the immune system of mice is better
characterized than that of rats, the NTP chose the mouse as the
experimental animal for immunotoxicity assessment. The immunotoxicity
database of the NTP has been evaluated to determine the predictability
(sensitivity and specificity) of the assays. In the Netherlands, a
Committee for Immunotoxicology of the Dutch Health Council reviewed
methods that could be used to assess the immunotoxic properties of a
compound and for deriving information about risks to humans on the
basis of the results of laboratory experiments. The Committee also
examined the relationship between the immunotoxic properties of a
substance and its mutagenic and carcinogenic properties (Dutch Health
Council, Committee for Immunotoxicology, 1991).
The immune system was reviewed by the United States National
Research Council in order to identify the kinds of basic research that
might reveal markers of environmental exposure and disease. Major
emphasis was placed on biological markers of three types: those
originating from the immune system, those related to exposure to
immunosuppressive toxicants, and those of effects of environmental
pollutants. Markers of susceptibility to environmental materials were
also considered to be important, especially if they are of a genetic
nature and can be used to identify individuals susceptible to
autoimmune diseases. The National Research Council subcommittees on
pulmonary toxicology and on immunotoxicology, have published their
reports (US National Research Council, 1989, 1992).
Interest in immunotoxicology within the scientific community is
reflected by the existence of a special section on immunotoxicology
within the Society of Toxicology. An immunotoxicology discussion group
initiated in the United States has an international composition. The
European Union has a programme on science and technology for
environmental protection that includes immunotoxicology as an
important aspect.
There is growing concern in society about the effects of
xenobiotics, such as environmental pollutants, on public health; the
immune system is one of the targets of such effects. Some chemicals
present in the environment that have been reported to influence the
immune system are listed in Table 1 (IPCS, 1986). Immunotoxicity can
result in e.g. reduced resistance towards infection or generation of
tumours that escape immune surveillance. A number of substances
affect immunological parameters; these include halogenated
hydrocarbons such as polychlorinated biphenyls, polybrominated
biphenyls, polychlorinated dibenzo- para-dioxins, and polychlorinated
dibenzofurans (Elo et al., 1985; Lu & Wu, 1985; Bekesi et al., 1987;
Kimbrough, 1987; Hoffman 1992); pesticides and precursors (Fiore et
al., 1986; Deo et al., 1987; Nigam et al., 1993); organic solvents
(Capurro, 1980; Denkhaus et al., 1986); asbestos (Lew et al., 1986);
silica (Uber & McReynolds, 1982); and metals like lead (Ewers et al.,
1982; Reigart & Graber, 1976). Oxidant air pollutants, like sulfur
dioxide, nitrogen dioxide, and ozone, and particles in airborne dust
may affect immune function (Koren et al., 1989; Van Loveren et al.,
1994).
Immunotoxicity in humans is further discussed in Section 2. Few
epidemiological data have been published that indicate suppression or
altered resistance to infection and tumours. In general, the
usefulness of the epidemiological studies that have been published is
limited by the following: exposure is usually uncontrolled, mainly
occurring during accidents; the magnitude and pattern of exposure are
not known, and the exposure is often too low to alter the immune
system measurably; exposure is often not to one xenobiotic but to a
mixture; it is almost impossible to control for confounding
parameters, such as age, sex, genetic background, health status, and
nutritional status; and it is not always possible to define and
analyse appropriate control groups (US National Research Council,
1992). Environmental pollution and its effect on health status are
currently subjects of concern in eastern European countries and have
generated much interest in the worldwide environmental health science
community. Recent epidemiological studies have compared the possible
relationship between exposure to air pollutants and health effects in
the former German Democratic Republic and Federal Republic of Germany,
Table 1. Examples of compounds that are immunotoxic for humans or
rodents
Chemical Immune toxicity
-------------------
Rodent Human
2,3,7,8-Tetrachlorodibenzo-para-dioxin + +
Polychlorinated biphenyls + +
Polybrominated biphenyls + +
Hexachlorobenzene + Unknown
Lead + Unknown
Cadmium + Unknown
Methyl mercury compounds + Unknown
7,12-Dimethylbenz[a]anthracene + Unknown
Benzo[a]pyrene + Unknown
Di-n-octyltindichloride + Unknown
Di-n-butyltindichloride + Unknown
Benzidine + +
Nitrogen dioxide and ozone + +
Benzene, toluene, and xylene + +
Asbestos + +
N-Nitrosodimethylamine + Unknown
Diethylstilboestrol + +
Vanadium + +
From IPCS (1986)
and some of these studies included immunological data or end-points.
For instance, von Mutius et al. (1992) found a higher prevalence of
asthma among schoolchildren in western than eastern Germany, and
Behrendt et al. (1993) observed, surprisingly, that total serum IgE
levels were higher in schoolchildren in eastern than in western
Germany. Several factors were found to influence total IgE: history of
parasitic disease, number of persons per dwelling, and passive
smoking. Sex and passive smoking were the only variables that had a
significant effect in western German children. Air pollutants and
parasitic infections were suggested to be the major contributing
factors to increased IgE production in children in eastern Germany.
Remarkable differences in air quality were seen between eastern and
western Germany, and Behrendt et al. (1995) distinguished two types of
air pollution: type I, composed of sulfur dioxide particles and dust,
occurring predominantly in eastern Europe, is associated with
respiratory infections and other chronic inflammatory airway
reactions; type II, occurring both indoors and outdoors in the
environment in industrialized western countries, is composed mainly of
nitric oxide, nitrogen dioxide, ozone, volatile organic compounds, and
fine particles. The latter type of air pollution is associated with
allergic diseases and allergic sensitization, indicating that air
pollutants interfere with parameters of allergy at the level of
sensitization, elicitation of symptoms, and exacerbation of disease.
Until further epidemiological studies are conducted in humans,
assessment of immunotoxicity in rodents, with subsequent extrapolation
to the human situation, is still a good indicator of toxicity and can
serve as a basis for subsequent decisions and regulations by
authorities to reduce or prevent the risk of human exposure. This
aspect is discussed further in Section 5.
Humans are exposed to environmental contaminants mainly via food,
water, and air. Open water (e.g. rivers, lakes, and coastal areas) and
sediments often act as sinks for environmental pollution. This global
problem can be deduced from disease manifestations in fish that live
in coastal areas, especially those species that live in close contact
with contaminated silt. High levels of contaminants and the diseases
associated with them are not only of economic importance (i.e. to
fisheries) but also affect people who consume the fish, as seen in
studies showing increased levels of contaminants in people eating fish
from the contaminated Baltic Sea (Svensson et al., 1991) and in Inuit
and Indian populations in Canada who consume large quantities of fish
and marine mammals (DeWailly et al., 1992). There is now some evidence
that wildlife aquatic species have decreased resistance and enhanced
incidences of infection and tumours that may be linked to
environmental pollution (Vos et al., 1989; Wester et al., 1994).
Because the immune system of fish has not been characterized in such
detail as that of mammals, immunotoxicological studies have not been
extensively included in ecotoxicology, although a number of reports of
direct toxic actions of xenobiotics on fish species have been
published in this developing field (Wester & Canton, 1987; Payne &
Fancey, 1989; Anderson, 1990; Wester et al., 1990; Khangarot &
Tripathi, 1991; Secombes et al., 1992; Anderson & Brubacher, 1993;
Faisal & Hugget, 1993).
1.2 The immune system: functions, system regulation, and modifying
factors; histophysiology of lymphoid organs
1.2.1 Function of the immune system
In order to interpret pathological alterations of the immune
system in terms of altered function, the physiology of the system must
be understood. Since knowledge of the structure and function of the
immune system is growing rapidly, a review of this subject, focusing
on histophysiology, is presented. This section is not meant to serve
as a textbook on immunology but to provide sufficient information for
an understanding of pathological changes due to immunotoxic action.
For general textbooks on immunology, reference may be made to Sell
(1987), Klein (1990), Brostoff et al. (1991), Roitt (1991), Paul
(1993), and Roitt et al. (1993). The section covers mainly humans and
rodents, but reference is made to other species that are relevant in
immunotoxicity assessment, e.g. fish in ecotoxicology. It should be
noted that species differences can be large, despite fundamental
similarities between the immune systems of animals. It is therefore
difficult to conduct immunotoxicological studies in immunologically
less well characterized animal species, although comparative studies
that are under way may lessen the problems. Zapata and Cooper (1990)
have written a comprehensive textbook on phylogenetic aspects of
immunology. Phylogenetic data, from primitive fish to mammals, are
presented in Table 2 (Cooper, 1982; Klein, 1986; Du Pasquier, 1989;
Zapata & Cooper, 1990; Sima & Vetvicka, 1992). Relevant phylogenetic
aspects of the immune system are described below.
In mammals, the immune system and its reactions consist of a
finely tuned, complex interplay between various cell types and soluble
mediators secreted by those cells (Figure 1), some of which are listed
in Section 7.
Immune responses can be classified roughly as innate (natural and
nonspecific) and acquired (adaptive) responses, in which the reaction
is directed to a specific determinant (antigenic determinant or
epitope). The nonspecific response involves effector cells such as
macrophages (Vetvicka & Fornusek, 1992), NK cells (Herberman &
Ortaldo, 1981), granulocytes (Ross, 1992), and mediator systems
including the complement system (Tomlinson, 1993). Specificity is
based on recognition by specific receptors on lymphocytes or by
antibodies: The classical reaction to bacterial infection, resulting
in antibacterial antibody formation and antibody-mediated destruction
of the pathogen, is only one part of the intrinsic capacity of the
system. Further attributes of the system (Nossal, 1987) are summarized
below.
1.2.1.1 Encounter and recognition
The initiation of an immune response requires adequate
recognition of the pathogen. This recognition often occurs immediately
after entry, e.g. during or after passage through the epithelial
barrier of the body (skin or mucus-secreting epithelia in the
respiratory and gastrointestinal tract). The first defence includes
nonspecific inactivation, e.g. by nonspecific killer cells,
neutrophilic granulocytes, and cells of the mononuclear phagocyte
system (formerly called the reticuloendothelial system). It also
includes antigen processing and presentation to cells such as
lymphocytes of the T-helper-inducer type (Th) which can generate a
specific response.
Table 2. Evolution of immunologically important traits among vertebrates
A. Major histocompatibility complex (MHC) and transplantation
Species Graft MLR CML MHC control Serologically
rejection and/or of immune detectable
GVH response MHC antigens
Tunicata (sea squirts) + ? ? ? ?
Agnatha
Hagfish (Hyperotreti) + ? - ? ?
Lamprey (Hyperoartii) + ? - ? ?
Chondrichthyes (cartilaginous fish)
Shark, ray + ? + ? +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + ? ? ? ?
Bony fish (Teleostei) + ? + ? +
Lungfish (Dipnoi) + ? ? ? ?
Amphibia (amphibians)
Salamanders (Urodela) + ? ? ? +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + ? ? ?
Lizards, snakes (Squamata) + + + ? ?
Crocodiles, alligators (Crocodilia) + ? ? ? ?
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
B. Complement and immunoglobulins (Ig)
Species Complement Immunoglobulins
IgM IgG-like IgA IgD IgE
Tunicata (sea squirts) ? - - - - -
Agnatha
Hagfish (Hyperotreti) ? ? - - - -
Lamprey (Hyperoartii) ? ? - - - -
Chondrichthyes (cartilaginous fish)
Shark, ray + + + - - -
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + + + - - -
Bony fish (Teleostei) + + + - - -
Lungfish (Dipnoi) + + + - - -
Amphibia (amphibians)
Salamanders (Urodela) + + ? - - -
Frogs, toads (Anura) + + + + - -
Reptilia (reptiles)
Turtles (Chelonia) + + + - - -
Lizards, snakes (Squamata) + + + - - -
Crocodiles, alligators (Crocodilia) + + + ? - -
Aves (birds) + + ? + - -
Mammalia (mammals) + + + + + +
Table 2 (cont'd)
C. Leukocytes
Species Lymphocytes Plasma Macrophages
cells
Small T B
Tunicata (sea squirts) + - - - +
Agnatha
Hagfish (Hyperotreti) + - - + +
Lamprey (Hyperoartii) + - - + +
Chondrichthyes (cartilaginous fish)
Shark, ray + - ? + +
Osteichthyes (bony fish)
Sturgeon (Chondrostei) + - ? + +
Bony fish (Teleostei) + + + + +
Lungfish (Dipnoi) + ? ? + +
Amphibia (amphibians)
Salamanders (Urodela) + + + + +
Frogs, toads (Anura) + + + + +
Reptilia (reptiles)
Turtles (Chelonia) + + + + +
Lizards, snakes (Squamata) + + + + +
Crocodiles, alligators (Crocodilia) + + + + +
Aves (birds) + + + + +
Mammalia (mammals) + + + + +
Table 2 (cont'd)
D. Lymphoid organs
Species Bone Thymus Spleen Lymph glands
marrow or nodes
Tunicata (sea squirts) - - - -
Agnatha
Hagfish (Hyperotreti) - - - GALT
Lamprey (Hyperoartii) - - - GALT
Chondrichthyes (cartilaginous fish)
Shark, ray - + + GALT
Osteichthyes (bony fish)
Sturgeon (Chondrostei) - + + GALT
Bony fish (Teleostei) - + + GALT
Lungfish (Dipnoi) - + + GALT
Amphibia (amphibians)
Salamanders (Urodela) + + + -
Frogs, toads (Anura) + + + ?
Reptilia (reptiles)
Turtles (Chelonia) + + + ?
Lizards, snakes (Squamata) + + + ?
Crocodiles, alligators (Crocodilia) + + + ?
Aves (birds) + + + +
Mammalia (mammals) + + + +
+, positive; ±, to be confirmed; -, negative; ?, not investigated.
MLR, mixed leukocyte response; GVH, graft-versus-host; CML, cell-mediated lympholysis;
GALT, gut-associated lymphoid tissue
 The importance of an epithelial barrier for primitive defence
mechanisms is clear in fish, in which a specific mucosal immune system
with local production of antibodies associated with mucus secretion
has been recognized. The gills are the main entry for both antigens
and pathogens living in water, and both migrating macrophages and gill
epithelial cells are involved in the process.
1.2.1.2 Specificity
The immune system can distinguish one particular determinant in
an immense spectrum of determinants. The discrimination between 'self'
and 'non-self' (i.e. the avoidance of autoreactivity) is an example of
this specificity.
Antigens can be polypeptides, carbohydrates, or lipids (lipopoly-
saccharides and lectins). A polypeptide antigen epitope is made up of
about 10 amino acids. Lymphocytes are central to antigen specificity,
as they express receptors for a single, distinct antigenic determinant
on their surface. On B lymphocytes, this antigen receptor is
essentially an antibody (immunoglobulin) molecule (Hasemann & Capra,
1989). The antigen-binding fragment of the surface receptor and the
antibodies produced after differentiation of B cells into plasma cells
have virtually identical structures -- a quaternary structure
comprising the dual heavy and light chains of the immunoglobulin
molecule (the so-called variable part of these protein chains). B-Cell
surface immunoglobulin and the immunoglobulin product of the plasma
cell progeny may differ in the constant part of the heavy chain. Like
the T-cell receptor (TCR), the B-cell receptor has a hetero-oligomeric
structure. After the antigen is bound to the surface immunoglobulin,
signal transduction occurs, in which one alpha-ß chain is linked to
the surface immunoglobulin. Biological responses involving tyrosine
phosphorylation and calcium mobilization are then induced, including
activation, tolerance, and differentiation, depending on the
differentiation stage of the B cell (Pleiman et al., 1994). On virgin
B cells, the surface receptor is an IgM or IgD molecule (with µ or
delta heavy chains, respectively). After so-called immunoglobulin
class switching (Vercelli & Geha, 1992; Harriman et al., 1993), IgG
(gamma chain), IgA (alpha chain), and IgE (epsilon chain) molecules
can be synthesized. Associated with the immunoglobulin molecule on the
cell surface is a dimeric transmembrane molecule, the Igalpha and
Igßchain, which functions in signal transduction and intracellular
activation of kinases of the src family (Pleiman et al., 1994). This
alpha-ß dimeric molecule is now used in identifying B cells.
For T cells, the antigen receptor is a heterodimeric molecule
(either the alpha-ß or the gamma-delta heterodimer), which has a
constant and a variable part, like those of immunoglobulin molecules
(Hedrick, 1989). Transmembrane signalling (tyrosine phosphorylation)
after antigen contact occurs when this heterodimer is linked on the
cell surface to the T3 (or CD3) molecule, which consists of at least
five invariant chains (Clevers et al., 1988; Chan et al., 1992).
The structural differences between the TCR for antigens and the
B-cell receptor (i.e. antibody) arise from differences in the gene
segments that encode the receptors. It is thus not surprising that
T cells recognize other determinants on the antigenic compound than
those recognized by B cells. For large antigens such as proteins,
distinct T-cell and B-cell epitopes can be identified, as illustrated
by the fact that the alpha-ß heterodimeric receptor on T cells
recognizes the antigenic determinant in the context of polymorphic
determinants of the major histocompatibility complex (MHC) antigens.
The antigenic determinant is either a small peptide, produced during
processing of antigen in the antigen-presenting cell and located in a
'groove' formed by the quaternary structure of the MHC molecule
(Adorini, 1990; Rothbard & Gefter, 1991; Germain & Margulies, 1993),
or a larger molecule associated with the MHC molecule outside the
groove. The latter is found for so-called 'superantigens', like
Staphylococcus enterotoxin A (Herman et al., 1991). Thus, with
either type of antigen, Th cells and delayed-type hypersensitivity
T cells (see below) recognize the antigenic determinant only when it
is presented together with the individual's own (self) determinant of
class II MHC. This phenomenon is called MHC class II restriction. In
contrast, T cells of the suppressor (Ts) and cytotoxic (Tc)
populations are MHC class I restricted. The processing of antigen by
antigen-presenting cells and subsequent complexing with MHC molecules
occur intracellularly, but with different pathways for MHC class
I-associated and MHC class II-associated complexing. In addition, MHC
class II-associated complexing may occur with proteins present at very
high concentrations in the extracellular environment (Neefjes &
Momburg, 1993; Engelhard, 1994). MHC restriction does not necessarily
apply to T-cell subsets within the pool expressing the alpha-ß
heterodimeric TCR (Haas et al., 1993). B Lymphocytes do not function
in an MHC restricted manner but recognize nominal antigen with their
surface immunoglobulin receptor. Therefore, recognition of antigens by
B cells and by most gamma-delta T cells, does not require antigen
presentation on cells carrying their own MHC class I or class II
determinants. The total repertoire of antigen recognition
specificities is about 107 for antibodies and somewhat less for the
TCR (about 106) (Roitt et al., 1993)
Phylogenetically, the capacity to reject an allograft is acquired
very early (e.g. sponges), and this has been interpreted as evidence
for an MHC complex. Information on the molecular features of MHC
antigens (Hughes & Nei, 1993) is available, however, only for the toad
Xenopus (Flajnik et al., 1991; Sato et al., 1993) and for fish
species (Hashimoto et al., 1990; Kasahara et al., 1992; Ono et al.,
1992; Hordvik et al., 1993). All vertebrate species produce specific
antibodies and typical cell-mediated immunity indicative of specific
responses, demonstrating that both T and B cells exist, as in birds
and mammals. Few data are available, however, because there are
virtually no reagents for lymphocyte identification and
classification. In chickens, monoclonal antibodies recognize alpha-ß
and gamma-delta TCR and a third TCR with a configuration ß-ß'; various
T-cell subsets have also been identified in this species with
appropriate antibodies (CD3, CD4, CD8; see below). MHC restriction of
antigen recognition by T cells has been demonstrated in Xenopus. In
fish, as in mammals, antigens are processed and presented by accessory
antigen-presenting cells, such as monocytes, to specific lymphocytes
in a seemingly alloantigen (presumably MHC or MHC-like) restricted
fashion (Vallejo et al., 1990; Stet & Egberts, 1991; Vallejo et al.,
1992). 'B-like' cells expressing immunoglobulins occur in all of the
fish and amphibian species that have been studied so far; however,
there is no IgD molecule in lower vertebrates. Presumably, all B-like
cells express on their surface an immunoglobulin molecule of high
relative molecular mass, similar to IgM in mammals.
All non-mammalian vertebrates produce immunoglobulins of high
relative molecular mass (Fellah et al., 1992; Wilson & Warr, 1992;
Wilson et al., 1992; Litman et al., 1993; Marchalonis et al., 1993).
There is probably also an IgG-like immunoglobulin of low relative
molecular mass that is functionally but not structurally equivalent to
mammalian IgG, but it has been observed in only a few species of bony
fish. There is also evidence for the presence of an IgA-like
immunoglobulin in some lower vertebrates. Non-mammalian vertebrates
have no IgD or IgE. The total repertoire of antigen recognition
specificities for antibodies is smaller in non-mammalian vertebrates
than in mammals.
1.2.1.3 Choice of effector reaction; diversity of the answer
After activation of Th cells, an immune response develops in
order to eliminate the antigen; in practical terms, the response
results in inactivation of the pathogen. The response is humoral
(antibody-mediated) and/or cellular (cell-mediated).
In the humoral response, Th cells together with antigen activate
specified B cells to become antibody-producing plasma cells. The
antibodies produced mediate the subsequent inactivation of the foreign
substance in a number of ways. When present in the form of immune
complexes, IgG and IgM either activate the complement system and
induce complement-mediated cytotoxicity (Hansch, 1992; Tomlinson,
1993) or activate secondary effects, which include: (i)
vasodilatation, increased vascular permeability, and attraction of
granulocytes, with subsequent release of lysosomal proteolytic enzymes
(i.e. components of acute inflammation); (ii) IgG antibody-mediated
cellular cytotoxicity, in which the antibody forms the antigen-
specific bridge between the killer cell (macrophage, binding of the Fc
fragment of IgG) and the target; (iii) IgG- and IgM-induced
opsonization and ingestion by phagocytic cells (granulocytes,
macrophages), with involvement of receptors for immunoglobulin Fc and
the complement split product C3d; (iv) binding of IgE to IgE receptors
on the surface of mast cells and basophilic granulocytes, which
induces degranulation with release of mediators after antigen binding.
Complement is phylogenetically very old, as genes that encode
complement components and complement proteins have been identified in
hagfish (Hanley et al., 1992; Ishiguro et al., 1992), and C3-like
activity exists in invertebrates and in all vertebrates. Complement C3
has been purified from all classes of vertebrates, including fish
(e.g. hagfish); in primitive fish like lampreys, it shows 30% homology
with human C3, whereas that in rodents is about 80% homologous with
that in humans (Lambris, 1993). Complement activation by immune
complexes occurs in most primitive vertebrates, but the secondary
effects emerged later in phylogeny. Antibody-mediated cellular
cytotoxicity involving NK cells has been documented in some bony fish.
Little is known about inflammatory reactions in lower vertebrates,
except for some histopathological data obtained in fish.
In the cellular response, Th cells activate precursors of Tc
cells, which subsequently kill the target after antigen-specific
recognition. Furthermore, precursors of lymphokine-producing cells
(for example, delayed-type hypersensitivity T cells) can be activated,
and the lymphokines thus secreted subsequently activate macrophages to
kill the target. Studies of Th clones in vitro have revealed the
existence of different types (Mosmann & Coffman, 1989). Th1 cells
synthesize interleukin (IL)-2, IL-3, tumour necrosis factors alpha and
ß, and interferon (IFN)gamma(cytokines are discussed below), provide
help to B cells (especially in IgG2a synthesis), activate macrophages,
and initiate delayed-type hypersensitivity reactions. Th2 cells secrete
IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, and tumour necrosis factor alpha,
providing extensive help to B cells (for IgM, IgG1, IgA, and IgE
synthesis), and activate eosinophilic granulocytes. Th2 cells do not
play a role in the initiation of delayed-type hypersensitivity. Various
cytokines are involved in the generation of these Th populations, with
dominant effects of IL-4 (Th2) and IL-12 (Th1). Cytokines produced by
each type of Th cell subpopulation appear to downregulate the activity
of the others. The choice of effector reaction is determined in part
by cooperation between the various populations of Th cells: IFN gamma
from Th1 cells can downregulate Th2 cells, while IL-10 from Th2 cells
can downregulate Th1 cells (Mossman & Coffman, 1989).
Finally, it should be noted that the immune system exerts other
types of responses that do not involve activation of Th cells. These
include T cell-independent activation of B cells, usually when the
antigens consist of repeating polysaccharide units (present on
bacteria such as Escherichia coli and Pneumococcus). They also
include direct activation of T killer cells which bear antigen-
specific receptors comprising the gamma-delta heterodimer (Bell, 1989;
De Weger et al., 1989; Raulet, 1989; Haas et al., 1993).
1.2.1.4 Immunoregulation
The effector reaction is induced by a finely tuned interplay
between cells and soluble mediators (Figure 2) (Van Deuren et al.,
1992). On the one hand, cell-cell contact is required; for instance,
between the antigen-presenting cell with (processed) antigen on its
surface and the Th cell. On the other hand, mediators (cytokines or
interleukins) influence cell function at a distance, after binding to
specific receptors on the target cell and subsequent signal
transduction, resulting in cell activation (Foxwell et al., 1992; Taga
& Kishimoto, 1992). New interleukins and their surface and soluble
receptors are being identified and characterized rapidly: at least 15
different interleukins are known at present, in addition to various
growth factors. As a reflection of their function, these factors are
designated as chemokines (attracting cells) or cytokines (influencing
cell function, such as stimulation). Factors synthesized by
lymphocytes are called lymphokines; those synthesized by monocytes or
macrophages are called monokines. Among the consequences of cytokine-
cell interactions are chemotaxis (Miller & Krangel, 1992), production
of subsequent mediators in the cascade, and down-regulation of
cellular function (Kimchi, 1992). The role of mediators in cell
adhesion to vascular endothelium is described below.
Examples of mediators that influence immune reactions positively
are IL-1 (Dinarello, 1992), which is secreted by antigen-presenting
cells and stimulates Th cells; IL-2, which is secreted by Th cells and
stimulates a variety of T cells to amplify the response; and a number
of B-cell stimulatory cytokines (Figure 2). Surface receptors for
these interleukins are present on certain immune cells (and also in
neuroendocrine tissue, mentioned below) or are expressed during
activation. For instance, IL-2 receptors and HLA-DR molecules are
expressed on T cells as part of the activation process, and their
expression is used to assess the state of cell activation (Shabtai et
al., 1991).
In down-regulation, the immune response manifests active
processes. A number of mediators, such as prostaglandin E2, IL-10, and
transforming growth factor ß (Brigham, 1989; Fontana et al., 1992;
Larrick & Wright, 1992), can suppress subsets of T and B lymphocytes.
After stimulation of Th cells in the initiation of the response,
precursors of Ts cells are activated which subsequently inhibit Th
cells from further amplifying the response, in direct cell-cell
contact or by secretion of soluble inhibitors. The existence of Ts
cells has been disputed, as part of the function of these cells is
cytotoxicity, which is exerted by the closely related MHC class I-
restricted Tc population (Bloom et al., 1992). Immunoregulatory
circuits have also been documented at the antibody level, where a
first antibody generates a second one directed to itself. The
relevance of this antibody-anti-antibody network, the so-called 'anti-
idiotype' network, remains a subject of speculation.
The importance of an epithelial barrier for primitive defence
mechanisms is clear in fish, in which a specific mucosal immune system
with local production of antibodies associated with mucus secretion
has been recognized. The gills are the main entry for both antigens
and pathogens living in water, and both migrating macrophages and gill
epithelial cells are involved in the process.
1.2.1.2 Specificity
The immune system can distinguish one particular determinant in
an immense spectrum of determinants. The discrimination between 'self'
and 'non-self' (i.e. the avoidance of autoreactivity) is an example of
this specificity.
Antigens can be polypeptides, carbohydrates, or lipids (lipopoly-
saccharides and lectins). A polypeptide antigen epitope is made up of
about 10 amino acids. Lymphocytes are central to antigen specificity,
as they express receptors for a single, distinct antigenic determinant
on their surface. On B lymphocytes, this antigen receptor is
essentially an antibody (immunoglobulin) molecule (Hasemann & Capra,
1989). The antigen-binding fragment of the surface receptor and the
antibodies produced after differentiation of B cells into plasma cells
have virtually identical structures -- a quaternary structure
comprising the dual heavy and light chains of the immunoglobulin
molecule (the so-called variable part of these protein chains). B-Cell
surface immunoglobulin and the immunoglobulin product of the plasma
cell progeny may differ in the constant part of the heavy chain. Like
the T-cell receptor (TCR), the B-cell receptor has a hetero-oligomeric
structure. After the antigen is bound to the surface immunoglobulin,
signal transduction occurs, in which one alpha-ß chain is linked to
the surface immunoglobulin. Biological responses involving tyrosine
phosphorylation and calcium mobilization are then induced, including
activation, tolerance, and differentiation, depending on the
differentiation stage of the B cell (Pleiman et al., 1994). On virgin
B cells, the surface receptor is an IgM or IgD molecule (with µ or
delta heavy chains, respectively). After so-called immunoglobulin
class switching (Vercelli & Geha, 1992; Harriman et al., 1993), IgG
(gamma chain), IgA (alpha chain), and IgE (epsilon chain) molecules
can be synthesized. Associated with the immunoglobulin molecule on the
cell surface is a dimeric transmembrane molecule, the Igalpha and
Igßchain, which functions in signal transduction and intracellular
activation of kinases of the src family (Pleiman et al., 1994). This
alpha-ß dimeric molecule is now used in identifying B cells.
For T cells, the antigen receptor is a heterodimeric molecule
(either the alpha-ß or the gamma-delta heterodimer), which has a
constant and a variable part, like those of immunoglobulin molecules
(Hedrick, 1989). Transmembrane signalling (tyrosine phosphorylation)
after antigen contact occurs when this heterodimer is linked on the
cell surface to the T3 (or CD3) molecule, which consists of at least
five invariant chains (Clevers et al., 1988; Chan et al., 1992).
The structural differences between the TCR for antigens and the
B-cell receptor (i.e. antibody) arise from differences in the gene
segments that encode the receptors. It is thus not surprising that
T cells recognize other determinants on the antigenic compound than
those recognized by B cells. For large antigens such as proteins,
distinct T-cell and B-cell epitopes can be identified, as illustrated
by the fact that the alpha-ß heterodimeric receptor on T cells
recognizes the antigenic determinant in the context of polymorphic
determinants of the major histocompatibility complex (MHC) antigens.
The antigenic determinant is either a small peptide, produced during
processing of antigen in the antigen-presenting cell and located in a
'groove' formed by the quaternary structure of the MHC molecule
(Adorini, 1990; Rothbard & Gefter, 1991; Germain & Margulies, 1993),
or a larger molecule associated with the MHC molecule outside the
groove. The latter is found for so-called 'superantigens', like
Staphylococcus enterotoxin A (Herman et al., 1991). Thus, with
either type of antigen, Th cells and delayed-type hypersensitivity
T cells (see below) recognize the antigenic determinant only when it
is presented together with the individual's own (self) determinant of
class II MHC. This phenomenon is called MHC class II restriction. In
contrast, T cells of the suppressor (Ts) and cytotoxic (Tc)
populations are MHC class I restricted. The processing of antigen by
antigen-presenting cells and subsequent complexing with MHC molecules
occur intracellularly, but with different pathways for MHC class
I-associated and MHC class II-associated complexing. In addition, MHC
class II-associated complexing may occur with proteins present at very
high concentrations in the extracellular environment (Neefjes &
Momburg, 1993; Engelhard, 1994). MHC restriction does not necessarily
apply to T-cell subsets within the pool expressing the alpha-ß
heterodimeric TCR (Haas et al., 1993). B Lymphocytes do not function
in an MHC restricted manner but recognize nominal antigen with their
surface immunoglobulin receptor. Therefore, recognition of antigens by
B cells and by most gamma-delta T cells, does not require antigen
presentation on cells carrying their own MHC class I or class II
determinants. The total repertoire of antigen recognition
specificities is about 107 for antibodies and somewhat less for the
TCR (about 106) (Roitt et al., 1993)
Phylogenetically, the capacity to reject an allograft is acquired
very early (e.g. sponges), and this has been interpreted as evidence
for an MHC complex. Information on the molecular features of MHC
antigens (Hughes & Nei, 1993) is available, however, only for the toad
Xenopus (Flajnik et al., 1991; Sato et al., 1993) and for fish
species (Hashimoto et al., 1990; Kasahara et al., 1992; Ono et al.,
1992; Hordvik et al., 1993). All vertebrate species produce specific
antibodies and typical cell-mediated immunity indicative of specific
responses, demonstrating that both T and B cells exist, as in birds
and mammals. Few data are available, however, because there are
virtually no reagents for lymphocyte identification and
classification. In chickens, monoclonal antibodies recognize alpha-ß
and gamma-delta TCR and a third TCR with a configuration ß-ß'; various
T-cell subsets have also been identified in this species with
appropriate antibodies (CD3, CD4, CD8; see below). MHC restriction of
antigen recognition by T cells has been demonstrated in Xenopus. In
fish, as in mammals, antigens are processed and presented by accessory
antigen-presenting cells, such as monocytes, to specific lymphocytes
in a seemingly alloantigen (presumably MHC or MHC-like) restricted
fashion (Vallejo et al., 1990; Stet & Egberts, 1991; Vallejo et al.,
1992). 'B-like' cells expressing immunoglobulins occur in all of the
fish and amphibian species that have been studied so far; however,
there is no IgD molecule in lower vertebrates. Presumably, all B-like
cells express on their surface an immunoglobulin molecule of high
relative molecular mass, similar to IgM in mammals.
All non-mammalian vertebrates produce immunoglobulins of high
relative molecular mass (Fellah et al., 1992; Wilson & Warr, 1992;
Wilson et al., 1992; Litman et al., 1993; Marchalonis et al., 1993).
There is probably also an IgG-like immunoglobulin of low relative
molecular mass that is functionally but not structurally equivalent to
mammalian IgG, but it has been observed in only a few species of bony
fish. There is also evidence for the presence of an IgA-like
immunoglobulin in some lower vertebrates. Non-mammalian vertebrates
have no IgD or IgE. The total repertoire of antigen recognition
specificities for antibodies is smaller in non-mammalian vertebrates
than in mammals.
1.2.1.3 Choice of effector reaction; diversity of the answer
After activation of Th cells, an immune response develops in
order to eliminate the antigen; in practical terms, the response
results in inactivation of the pathogen. The response is humoral
(antibody-mediated) and/or cellular (cell-mediated).
In the humoral response, Th cells together with antigen activate
specified B cells to become antibody-producing plasma cells. The
antibodies produced mediate the subsequent inactivation of the foreign
substance in a number of ways. When present in the form of immune
complexes, IgG and IgM either activate the complement system and
induce complement-mediated cytotoxicity (Hansch, 1992; Tomlinson,
1993) or activate secondary effects, which include: (i)
vasodilatation, increased vascular permeability, and attraction of
granulocytes, with subsequent release of lysosomal proteolytic enzymes
(i.e. components of acute inflammation); (ii) IgG antibody-mediated
cellular cytotoxicity, in which the antibody forms the antigen-
specific bridge between the killer cell (macrophage, binding of the Fc
fragment of IgG) and the target; (iii) IgG- and IgM-induced
opsonization and ingestion by phagocytic cells (granulocytes,
macrophages), with involvement of receptors for immunoglobulin Fc and
the complement split product C3d; (iv) binding of IgE to IgE receptors
on the surface of mast cells and basophilic granulocytes, which
induces degranulation with release of mediators after antigen binding.
Complement is phylogenetically very old, as genes that encode
complement components and complement proteins have been identified in
hagfish (Hanley et al., 1992; Ishiguro et al., 1992), and C3-like
activity exists in invertebrates and in all vertebrates. Complement C3
has been purified from all classes of vertebrates, including fish
(e.g. hagfish); in primitive fish like lampreys, it shows 30% homology
with human C3, whereas that in rodents is about 80% homologous with
that in humans (Lambris, 1993). Complement activation by immune
complexes occurs in most primitive vertebrates, but the secondary
effects emerged later in phylogeny. Antibody-mediated cellular
cytotoxicity involving NK cells has been documented in some bony fish.
Little is known about inflammatory reactions in lower vertebrates,
except for some histopathological data obtained in fish.
In the cellular response, Th cells activate precursors of Tc
cells, which subsequently kill the target after antigen-specific
recognition. Furthermore, precursors of lymphokine-producing cells
(for example, delayed-type hypersensitivity T cells) can be activated,
and the lymphokines thus secreted subsequently activate macrophages to
kill the target. Studies of Th clones in vitro have revealed the
existence of different types (Mosmann & Coffman, 1989). Th1 cells
synthesize interleukin (IL)-2, IL-3, tumour necrosis factors alpha and
ß, and interferon (IFN)gamma(cytokines are discussed below), provide
help to B cells (especially in IgG2a synthesis), activate macrophages,
and initiate delayed-type hypersensitivity reactions. Th2 cells secrete
IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, and tumour necrosis factor alpha,
providing extensive help to B cells (for IgM, IgG1, IgA, and IgE
synthesis), and activate eosinophilic granulocytes. Th2 cells do not
play a role in the initiation of delayed-type hypersensitivity. Various
cytokines are involved in the generation of these Th populations, with
dominant effects of IL-4 (Th2) and IL-12 (Th1). Cytokines produced by
each type of Th cell subpopulation appear to downregulate the activity
of the others. The choice of effector reaction is determined in part
by cooperation between the various populations of Th cells: IFN gamma
from Th1 cells can downregulate Th2 cells, while IL-10 from Th2 cells
can downregulate Th1 cells (Mossman & Coffman, 1989).
Finally, it should be noted that the immune system exerts other
types of responses that do not involve activation of Th cells. These
include T cell-independent activation of B cells, usually when the
antigens consist of repeating polysaccharide units (present on
bacteria such as Escherichia coli and Pneumococcus). They also
include direct activation of T killer cells which bear antigen-
specific receptors comprising the gamma-delta heterodimer (Bell, 1989;
De Weger et al., 1989; Raulet, 1989; Haas et al., 1993).
1.2.1.4 Immunoregulation
The effector reaction is induced by a finely tuned interplay
between cells and soluble mediators (Figure 2) (Van Deuren et al.,
1992). On the one hand, cell-cell contact is required; for instance,
between the antigen-presenting cell with (processed) antigen on its
surface and the Th cell. On the other hand, mediators (cytokines or
interleukins) influence cell function at a distance, after binding to
specific receptors on the target cell and subsequent signal
transduction, resulting in cell activation (Foxwell et al., 1992; Taga
& Kishimoto, 1992). New interleukins and their surface and soluble
receptors are being identified and characterized rapidly: at least 15
different interleukins are known at present, in addition to various
growth factors. As a reflection of their function, these factors are
designated as chemokines (attracting cells) or cytokines (influencing
cell function, such as stimulation). Factors synthesized by
lymphocytes are called lymphokines; those synthesized by monocytes or
macrophages are called monokines. Among the consequences of cytokine-
cell interactions are chemotaxis (Miller & Krangel, 1992), production
of subsequent mediators in the cascade, and down-regulation of
cellular function (Kimchi, 1992). The role of mediators in cell
adhesion to vascular endothelium is described below.
Examples of mediators that influence immune reactions positively
are IL-1 (Dinarello, 1992), which is secreted by antigen-presenting
cells and stimulates Th cells; IL-2, which is secreted by Th cells and
stimulates a variety of T cells to amplify the response; and a number
of B-cell stimulatory cytokines (Figure 2). Surface receptors for
these interleukins are present on certain immune cells (and also in
neuroendocrine tissue, mentioned below) or are expressed during
activation. For instance, IL-2 receptors and HLA-DR molecules are
expressed on T cells as part of the activation process, and their
expression is used to assess the state of cell activation (Shabtai et
al., 1991).
In down-regulation, the immune response manifests active
processes. A number of mediators, such as prostaglandin E2, IL-10, and
transforming growth factor ß (Brigham, 1989; Fontana et al., 1992;
Larrick & Wright, 1992), can suppress subsets of T and B lymphocytes.
After stimulation of Th cells in the initiation of the response,
precursors of Ts cells are activated which subsequently inhibit Th
cells from further amplifying the response, in direct cell-cell
contact or by secretion of soluble inhibitors. The existence of Ts
cells has been disputed, as part of the function of these cells is
cytotoxicity, which is exerted by the closely related MHC class I-
restricted Tc population (Bloom et al., 1992). Immunoregulatory
circuits have also been documented at the antibody level, where a
first antibody generates a second one directed to itself. The
relevance of this antibody-anti-antibody network, the so-called 'anti-
idiotype' network, remains a subject of speculation.


 The immune system is in a continuous state of homoeostatic
equilibrium. The introduction of an antigen (pathogen) disturbs that
balance by activating antigen-specific cell clones of T and B
lymphocyte origin. The system not only allows the proliferation and
amplification of relevant clones to cope with the antigen, but it also
searches for (and reaches) a state of newly defined homeostasis.
Little is known about the phylogenetic development of mechanisms
for regulating immune responses in vertebrates. Helper, cytotoxic, and
even suppressor lymphoid functions have been reported in ectothermic
vertebrates, but the existence of T-cell subpopulations has not been
demonstrated. T and B cells cooperate in teleost fish and in
amphibians, and there is indirect evidence that they do so in
reptiles; however, MHC restriction of these cell interactions has been
demonstrated only in Xenopus. The existence of clusters consisting
of lymphocytes, macrophages, and plasma cells has been documented in
various lower vertebrate species, which indicates the importance of
cell-cell interactions in evoking immune responses. Cytokine activity
resembling that of IL-1 and interferon has been identified in various
fish species. A T-cell growth factor was first characterized in
Xenopus. An idiotype-anti-idiotype network has been proposed to
explain the regulation of antibody production in some bony fish
(Zapata & Cooper, 1990).
1.2.1.5 Modifying factors outside the immune system
Communication with other homeostatic mechanisms in the body is an
important aspect of immunoregulation; mediators of the response have
effects not only on the internal regulatory network but also on
systems outside the immune system. Communication with the clotting and
kallikrein systems by complement components is one example;
communication with the central nervous system is another (Ader et al.,
1990). For instance, IL-1 generated by antigen-presenting cells
affects the temperature regulatory centre (induction of fever) and the
sleep regulatory centre (induction of slow-wave sleep) in the
hypothalamus (Dinarello, 1992).
One example of interaction between the immune system and the
central nervous system is the profound influence of stress on immune
reactivity (Khansari et al., 1990). Both stressful events and the way
in which individuals cope with stress are involved (Bohus et al.,
1991), as documented in studies mainly in rats but also in other
species, including fish (Faisal et al., 1989). In humans, conditions
of acute psychological stress include bereavement (Bartrop et al.,
1977), marital disruption (Kiecolt-Glaser et al., 1987), and
examination periods (Kiecolt-Glaser et al., 1986), and these can be
associated with a decrease in immune status (Kiecolt-Glaser & Glaser,
1986) which can result in increased risk for infection, including
common respiratory tract infections, e.g. influenza (Boyce et al.,
1977; Clover et al., 1989), infectious mononucleosis (Kasl et al.,
1979), and herpes virus reactivation (Glaser et al., 1985).
The hypothalamus-pituitary-adrenal axis is an important pathway
in the communication between the central nervous system and the immune
system, resulting in synthesis of glucocorticosteroid hormone by the
adrenal gland induced by adrenocorticotrophic hormone from the
pituitary gland (Buckingham et al., 1992). Other mechanisms are those
mediated by the direct action of neuropeptides, such as opioid
peptides (van den Bergh et al., 1991), on immune cells; these are
either stimulatory or down-regulatory, depending in part on
experimental design and conditions. In addition, almost all lymphoid
tissues are innervated (Bulloch, 1987; Felten et al., 1987; Kendall &
Al-Shawaf, 1991), although the role of this neuroregulatory pathway is
largely unknown (Freier, 1990). The immune system and the
neuroendocrine system have a number of biologically active mediators
in common, including cytokines and neuromediators (Fabry et al.,
1994), and are strongly interrelated (Weigent & Blalock, 1987; Sibinga
& Goldstein, 1988; Heijnen & Kavelaars, 1991; Heijnen et al., 1991;
Knight et al., 1992). Drugs that act on the central nervous system,
like some tranquillizers, antidepressants, benzodiazepines,
antiepileptics, anaesthetics, and levodopa (an antiparkinson drug),
may cause immunosuppression (Descotes, 1986). Thus, the principles of
immunotoxicology may find application in neurotoxicology and endocrine
toxicology (Snyder, 1989). Some examples of factors that modify the
immune system, based on studies of the thymus of mice during pregnancy
and of birds after hatching, are given below. Exogenous conditions may
have pivotal influences on the structure and function of the immune
system. In ectothermic vertebrates, these include seasonal changes
like temperature and photoperiod and are governed by corticosteroid
and sex hormones. In spring, during the mating period, there is low
immune reactivity, with thymic involution; in the postmating period
and the first part of summer, there is maximal development of lymphoid
tissue and immune reactivity; at the end of summer, activity declines,
and the lymphoid organs undergo pronounced involution, which persists
throughout the autumn and winter (Zapata et al., 1992). The lymphoid
system of ectothermic vertebrates therefore cannot be described in
morpho-functional terms without taking into account the season in
which studies were conducted. Seasonal variation in
immunoresponsiveness is also seen in laboratory animals (Ratajczak et
al., 1993).
1.2.1.6 Immunological memory
A special feature of the immune response is the generation of
memory after initial contact with an antigen (Gray, 1993). The first
response includes activation and amplification of antigen-specific T
or B cells to exert effector reactions; it ends with the return of
antigen-recognizing cells to the normal resting state (small
lymphocytes). The second contact with the antigen results in
recruitment of more antigen-specific cells to give a stronger signal
and more efficient elimination of the antigen or pathogen. The
reaction also occurs faster, and there is a stronger binding of
antibody to antigen. The gradual increase in the binding capacity of
antibodies during the immune response is known as 'affinity
maturation'. The memory pool of lymphocytes mediates a faster response
than the virgin (unprimed) pool.
The memory effect is most evident in the humoral arm of the
immune response. The first response gives rise only to IgM class
antibody, but antibodies of other immunoglobulin classes (especially
IgG in the internal system) are generated after subsequent contact and
immunoglobulin class switch. The antibodies thus formed may show
increased affinity as a result of somatic mutation (Kocks & Rajewski,
1989).
The cellular basis of immunological memory is largely unresolved.
It appears to be located within the T-cell population, because its
presence within the B-cell population is apparently of short duration
and is associated with the presence and stimulatory activity of the
antigen in germinal centres of secondary lymphoid tissue. The
generation of memory is the basis for vaccination, performed to
prevent contracting infectious disease by bringing about contact with
the pathogen in an attenuated or inactivated, nonpathogenic form.
It is not known whether immunological memory exists in primitive
vertebrates. The classical differentiation into primary and secondary
responses does not exist, as it is mainly immunoglobulin of high
relative molecular mass that is present, and the repertoire of
antigen-recognizing specificities is smaller than that in birds and
mammals. Some fish species may have immunological memory.
1.2.2 Histophysiology of lymphoid organs
1.2.2.1 Overview: structure of the immune system
Components of the immune system are present throughout the body
(Figure 3). The lymphocyte compartment is lodged in lymphoid organs,
from which cells can move to sites of infection or inflammation.
Phagocytic cells of the monocyte-macrophage lineage occur in lymphoid
organs and also at extranodal sites, such as Kupffer cells in the
liver, alveolar macrophages in the lung, mesangial macrophages in the
kidney, and glial cells in the brain (Figure 4). Polymorphonuclear
leukocytes are present mainly in blood and bone marrow as mature and
progenitor cells. These cells accumulate at sites of inflammation.
The lymphoid organs can be classified roughly into two types:
primary or central (antigen-independent) and secondary or peripheral
(antigen-dependent). This classification is based on the antigen-
dependence of cell proliferation and differentiation. It does not hold
for lower vertebrates, including fish. The bone marrow in higher
vertebrates is a primary organ, in which are found the pluripotent
haematopoietic stem cells which differentiate into progenitors of
myeloid cells (which in turn differentiate into granulocytes,
monocytes, erythrocytes, and platelets) and lymphoid progenitors
The immune system is in a continuous state of homoeostatic
equilibrium. The introduction of an antigen (pathogen) disturbs that
balance by activating antigen-specific cell clones of T and B
lymphocyte origin. The system not only allows the proliferation and
amplification of relevant clones to cope with the antigen, but it also
searches for (and reaches) a state of newly defined homeostasis.
Little is known about the phylogenetic development of mechanisms
for regulating immune responses in vertebrates. Helper, cytotoxic, and
even suppressor lymphoid functions have been reported in ectothermic
vertebrates, but the existence of T-cell subpopulations has not been
demonstrated. T and B cells cooperate in teleost fish and in
amphibians, and there is indirect evidence that they do so in
reptiles; however, MHC restriction of these cell interactions has been
demonstrated only in Xenopus. The existence of clusters consisting
of lymphocytes, macrophages, and plasma cells has been documented in
various lower vertebrate species, which indicates the importance of
cell-cell interactions in evoking immune responses. Cytokine activity
resembling that of IL-1 and interferon has been identified in various
fish species. A T-cell growth factor was first characterized in
Xenopus. An idiotype-anti-idiotype network has been proposed to
explain the regulation of antibody production in some bony fish
(Zapata & Cooper, 1990).
1.2.1.5 Modifying factors outside the immune system
Communication with other homeostatic mechanisms in the body is an
important aspect of immunoregulation; mediators of the response have
effects not only on the internal regulatory network but also on
systems outside the immune system. Communication with the clotting and
kallikrein systems by complement components is one example;
communication with the central nervous system is another (Ader et al.,
1990). For instance, IL-1 generated by antigen-presenting cells
affects the temperature regulatory centre (induction of fever) and the
sleep regulatory centre (induction of slow-wave sleep) in the
hypothalamus (Dinarello, 1992).
One example of interaction between the immune system and the
central nervous system is the profound influence of stress on immune
reactivity (Khansari et al., 1990). Both stressful events and the way
in which individuals cope with stress are involved (Bohus et al.,
1991), as documented in studies mainly in rats but also in other
species, including fish (Faisal et al., 1989). In humans, conditions
of acute psychological stress include bereavement (Bartrop et al.,
1977), marital disruption (Kiecolt-Glaser et al., 1987), and
examination periods (Kiecolt-Glaser et al., 1986), and these can be
associated with a decrease in immune status (Kiecolt-Glaser & Glaser,
1986) which can result in increased risk for infection, including
common respiratory tract infections, e.g. influenza (Boyce et al.,
1977; Clover et al., 1989), infectious mononucleosis (Kasl et al.,
1979), and herpes virus reactivation (Glaser et al., 1985).
The hypothalamus-pituitary-adrenal axis is an important pathway
in the communication between the central nervous system and the immune
system, resulting in synthesis of glucocorticosteroid hormone by the
adrenal gland induced by adrenocorticotrophic hormone from the
pituitary gland (Buckingham et al., 1992). Other mechanisms are those
mediated by the direct action of neuropeptides, such as opioid
peptides (van den Bergh et al., 1991), on immune cells; these are
either stimulatory or down-regulatory, depending in part on
experimental design and conditions. In addition, almost all lymphoid
tissues are innervated (Bulloch, 1987; Felten et al., 1987; Kendall &
Al-Shawaf, 1991), although the role of this neuroregulatory pathway is
largely unknown (Freier, 1990). The immune system and the
neuroendocrine system have a number of biologically active mediators
in common, including cytokines and neuromediators (Fabry et al.,
1994), and are strongly interrelated (Weigent & Blalock, 1987; Sibinga
& Goldstein, 1988; Heijnen & Kavelaars, 1991; Heijnen et al., 1991;
Knight et al., 1992). Drugs that act on the central nervous system,
like some tranquillizers, antidepressants, benzodiazepines,
antiepileptics, anaesthetics, and levodopa (an antiparkinson drug),
may cause immunosuppression (Descotes, 1986). Thus, the principles of
immunotoxicology may find application in neurotoxicology and endocrine
toxicology (Snyder, 1989). Some examples of factors that modify the
immune system, based on studies of the thymus of mice during pregnancy
and of birds after hatching, are given below. Exogenous conditions may
have pivotal influences on the structure and function of the immune
system. In ectothermic vertebrates, these include seasonal changes
like temperature and photoperiod and are governed by corticosteroid
and sex hormones. In spring, during the mating period, there is low
immune reactivity, with thymic involution; in the postmating period
and the first part of summer, there is maximal development of lymphoid
tissue and immune reactivity; at the end of summer, activity declines,
and the lymphoid organs undergo pronounced involution, which persists
throughout the autumn and winter (Zapata et al., 1992). The lymphoid
system of ectothermic vertebrates therefore cannot be described in
morpho-functional terms without taking into account the season in
which studies were conducted. Seasonal variation in
immunoresponsiveness is also seen in laboratory animals (Ratajczak et
al., 1993).
1.2.1.6 Immunological memory
A special feature of the immune response is the generation of
memory after initial contact with an antigen (Gray, 1993). The first
response includes activation and amplification of antigen-specific T
or B cells to exert effector reactions; it ends with the return of
antigen-recognizing cells to the normal resting state (small
lymphocytes). The second contact with the antigen results in
recruitment of more antigen-specific cells to give a stronger signal
and more efficient elimination of the antigen or pathogen. The
reaction also occurs faster, and there is a stronger binding of
antibody to antigen. The gradual increase in the binding capacity of
antibodies during the immune response is known as 'affinity
maturation'. The memory pool of lymphocytes mediates a faster response
than the virgin (unprimed) pool.
The memory effect is most evident in the humoral arm of the
immune response. The first response gives rise only to IgM class
antibody, but antibodies of other immunoglobulin classes (especially
IgG in the internal system) are generated after subsequent contact and
immunoglobulin class switch. The antibodies thus formed may show
increased affinity as a result of somatic mutation (Kocks & Rajewski,
1989).
The cellular basis of immunological memory is largely unresolved.
It appears to be located within the T-cell population, because its
presence within the B-cell population is apparently of short duration
and is associated with the presence and stimulatory activity of the
antigen in germinal centres of secondary lymphoid tissue. The
generation of memory is the basis for vaccination, performed to
prevent contracting infectious disease by bringing about contact with
the pathogen in an attenuated or inactivated, nonpathogenic form.
It is not known whether immunological memory exists in primitive
vertebrates. The classical differentiation into primary and secondary
responses does not exist, as it is mainly immunoglobulin of high
relative molecular mass that is present, and the repertoire of
antigen-recognizing specificities is smaller than that in birds and
mammals. Some fish species may have immunological memory.
1.2.2 Histophysiology of lymphoid organs
1.2.2.1 Overview: structure of the immune system
Components of the immune system are present throughout the body
(Figure 3). The lymphocyte compartment is lodged in lymphoid organs,
from which cells can move to sites of infection or inflammation.
Phagocytic cells of the monocyte-macrophage lineage occur in lymphoid
organs and also at extranodal sites, such as Kupffer cells in the
liver, alveolar macrophages in the lung, mesangial macrophages in the
kidney, and glial cells in the brain (Figure 4). Polymorphonuclear
leukocytes are present mainly in blood and bone marrow as mature and
progenitor cells. These cells accumulate at sites of inflammation.
The lymphoid organs can be classified roughly into two types:
primary or central (antigen-independent) and secondary or peripheral
(antigen-dependent). This classification is based on the antigen-
dependence of cell proliferation and differentiation. It does not hold
for lower vertebrates, including fish. The bone marrow in higher
vertebrates is a primary organ, in which are found the pluripotent
haematopoietic stem cells which differentiate into progenitors of
myeloid cells (which in turn differentiate into granulocytes,
monocytes, erythrocytes, and platelets) and lymphoid progenitors

 (Figure 1). The differentiation is not antigen-dependent or antigen-
driven, but this does not exclude a role for antigens in the process.
Factors secreted in the periphery during antigen-specific stimulation
can promote haematopoiesis in the bone marrow (Figure 2) (Fletcher &
Williams, 1992; Kincade, 1992; Saito 1992; Williams & Quesenberry,
1992) or inhibit haematopoietic activity (Wright & Pragnell, 1992).
The bone marrow also functions as a secondary lymphoid organ, because
terminal antigen-induced lymphoid cell differentiation can occur in
its microenvironment. For instance, the bone-marrow cells include both
the memory lymphocyte pool and the major plasma cell population, which
contributes to the intravascular pool of immunoglobulins. Normally,
plasma cell differentiation follows antigen presentation at peripheral
sites, and stimulated cells subsequently migrate to the bone marrow
for final differentiation. The secondary or peripheral lymphoid organs
in the body include the lymph nodes, spleen, and lymphoid tissue along
secretory surfaces like the gastrointestinal and respiratory tracts.
A second classification is based on the location of lymphoid
organs, divided into internal organs (some lymph nodes and the spleen,
in addition to thymus and bone marrow) and external organs (lymphoid
tissue along secretory surfaces and the lymph nodes that drain the
mucosa-associated lymphoid tissue (MALT)) (Figure 3). Lymphoid organs
at these two locations behave somewhat independently in host defence,
for instance in immunoglobulin synthesis. The main function of the
external or secretory immune system is to produce (secretory) IgA
antibody, whereas the internal immune system (mainly bone marrow)
produces IgG or IgM antibody; the major site of IgE synthesis in the
body is also along secretory surfaces. The extent of the secretory
immune system should not be underestimated: About half of the body's
lymphocytes are located in the secretory immune system, and its
capacity for immunoglobulin synthesis is about 1.5 times that of the
internal system. In all vertebrates, intraepithelial lymphocytes and
nonencapsulated lymphoid infiltrates occur in the intestine. True
lymphoid organs, such as the tonsils, Peyer's patches, and the
appendix, have been reported only in higher vertebrates. In birds, the
caecal tonsil, a blind appendix of the posterior intestine, becomes an
important peripheral lymphoid organ after involution of the bursa of
Fabricius.
Another organ that contributes to the immune system is the skin.
It does not contain organized lymphoid tissue, but immune components
in skin are interconnected with other immune organs, leading to the
concept of the skin immune system, or skin-associated lymphoid tissue
(Stingl & Steiner, 1989; Bos, 1990; Nickoloff, 1993; see section
1.2.2.7).
Immune cells and cellular products are transported between
lymphoid organs by blood and lymph vessels (Duijvestijn & Hamann,
1989). For example, Langerhans cells on their way from the skin to a
lymph node are present in lymph as 'veiled macrophages'. The blood
circulation contains only a minor part of the body's total pool of
lymphocytes (estimated at about 1%) and only a selected population,
i.e. the recirculating lymphocyte pool (Figure 5). Therefore,
assessment of only the blood lymphoid compartment does not give a
complete inventory of the body's immune system, as it ignores the
activities of the non-recirculating cells. In general terms, the blood
lymphoid compartment does not include cells that are in a state of
activation, proliferation, or differentiation; such cells are
typically tissue-bound. This rule is not absolute. For instance, in
the case of a highly activated B-cell system with hyperplasia of
germinal centres in lymph nodes, activated B cells may occur in the
circulation. These cells are normally not part of the recirculating
pool. With regard to the non-lymphoid cells of the immune system,
macrophages are tissue-bound (histiocytes), and monocytes are their
counterpart in blood. Dendritic cells, which are present in very low
proportions in blood, are the counterparts of Langerhans cells in
skin, veiled macrophages in lymph, and interdigitating dendritic cells
in lymphoid tissue. The follicular dendritic cells, i.e. the antigen-
presenting cells in follicles in lymphoid tissue (see below), do not
exist in the circulation. Within the myeloid series, the mast cell is
typically tissue-bound (Figure 6).
The endothelium lining the blood vasculature has a major role in
the passage of cells from blood to tissue parenchyma. Adhesion
molecules on circulating cells and endothelium are involved in this
process (Patarroyo, 1991; Gahmberg et al., 1992; Gumbiner & Yamada,
1992). These glycoproteins belong to three families of molecules, the
immunoglobulin supergene family, the integrins, and the selectins
(Springer, 1990; Lasky, 1992; Bevilacqua, 1993). They may be present
on endothelium in the resting state but often require alteration to
become biologically active in inflammatory processes (e.g. under the
influence of mediators like IFN gamma). In lymphoid tissue, they are
called addressins, to indicate their role in the selective homing of
passenger lymphocytes into internal or external lymphoid tissue.
The structure and histophysiology of the bone marrow, thymus,
lymph node, spleen, and MALT are presented in more detail in the
following sections. The different microenvironments of these organs
are summarized in Table 3. A detailed description of the histological
and pathological aspects of rat lymphoid tissue is given by Jones et
al. (1990).
(Figure 1). The differentiation is not antigen-dependent or antigen-
driven, but this does not exclude a role for antigens in the process.
Factors secreted in the periphery during antigen-specific stimulation
can promote haematopoiesis in the bone marrow (Figure 2) (Fletcher &
Williams, 1992; Kincade, 1992; Saito 1992; Williams & Quesenberry,
1992) or inhibit haematopoietic activity (Wright & Pragnell, 1992).
The bone marrow also functions as a secondary lymphoid organ, because
terminal antigen-induced lymphoid cell differentiation can occur in
its microenvironment. For instance, the bone-marrow cells include both
the memory lymphocyte pool and the major plasma cell population, which
contributes to the intravascular pool of immunoglobulins. Normally,
plasma cell differentiation follows antigen presentation at peripheral
sites, and stimulated cells subsequently migrate to the bone marrow
for final differentiation. The secondary or peripheral lymphoid organs
in the body include the lymph nodes, spleen, and lymphoid tissue along
secretory surfaces like the gastrointestinal and respiratory tracts.
A second classification is based on the location of lymphoid
organs, divided into internal organs (some lymph nodes and the spleen,
in addition to thymus and bone marrow) and external organs (lymphoid
tissue along secretory surfaces and the lymph nodes that drain the
mucosa-associated lymphoid tissue (MALT)) (Figure 3). Lymphoid organs
at these two locations behave somewhat independently in host defence,
for instance in immunoglobulin synthesis. The main function of the
external or secretory immune system is to produce (secretory) IgA
antibody, whereas the internal immune system (mainly bone marrow)
produces IgG or IgM antibody; the major site of IgE synthesis in the
body is also along secretory surfaces. The extent of the secretory
immune system should not be underestimated: About half of the body's
lymphocytes are located in the secretory immune system, and its
capacity for immunoglobulin synthesis is about 1.5 times that of the
internal system. In all vertebrates, intraepithelial lymphocytes and
nonencapsulated lymphoid infiltrates occur in the intestine. True
lymphoid organs, such as the tonsils, Peyer's patches, and the
appendix, have been reported only in higher vertebrates. In birds, the
caecal tonsil, a blind appendix of the posterior intestine, becomes an
important peripheral lymphoid organ after involution of the bursa of
Fabricius.
Another organ that contributes to the immune system is the skin.
It does not contain organized lymphoid tissue, but immune components
in skin are interconnected with other immune organs, leading to the
concept of the skin immune system, or skin-associated lymphoid tissue
(Stingl & Steiner, 1989; Bos, 1990; Nickoloff, 1993; see section
1.2.2.7).
Immune cells and cellular products are transported between
lymphoid organs by blood and lymph vessels (Duijvestijn & Hamann,
1989). For example, Langerhans cells on their way from the skin to a
lymph node are present in lymph as 'veiled macrophages'. The blood
circulation contains only a minor part of the body's total pool of
lymphocytes (estimated at about 1%) and only a selected population,
i.e. the recirculating lymphocyte pool (Figure 5). Therefore,
assessment of only the blood lymphoid compartment does not give a
complete inventory of the body's immune system, as it ignores the
activities of the non-recirculating cells. In general terms, the blood
lymphoid compartment does not include cells that are in a state of
activation, proliferation, or differentiation; such cells are
typically tissue-bound. This rule is not absolute. For instance, in
the case of a highly activated B-cell system with hyperplasia of
germinal centres in lymph nodes, activated B cells may occur in the
circulation. These cells are normally not part of the recirculating
pool. With regard to the non-lymphoid cells of the immune system,
macrophages are tissue-bound (histiocytes), and monocytes are their
counterpart in blood. Dendritic cells, which are present in very low
proportions in blood, are the counterparts of Langerhans cells in
skin, veiled macrophages in lymph, and interdigitating dendritic cells
in lymphoid tissue. The follicular dendritic cells, i.e. the antigen-
presenting cells in follicles in lymphoid tissue (see below), do not
exist in the circulation. Within the myeloid series, the mast cell is
typically tissue-bound (Figure 6).
The endothelium lining the blood vasculature has a major role in
the passage of cells from blood to tissue parenchyma. Adhesion
molecules on circulating cells and endothelium are involved in this
process (Patarroyo, 1991; Gahmberg et al., 1992; Gumbiner & Yamada,
1992). These glycoproteins belong to three families of molecules, the
immunoglobulin supergene family, the integrins, and the selectins
(Springer, 1990; Lasky, 1992; Bevilacqua, 1993). They may be present
on endothelium in the resting state but often require alteration to
become biologically active in inflammatory processes (e.g. under the
influence of mediators like IFN gamma). In lymphoid tissue, they are
called addressins, to indicate their role in the selective homing of
passenger lymphocytes into internal or external lymphoid tissue.
The structure and histophysiology of the bone marrow, thymus,
lymph node, spleen, and MALT are presented in more detail in the
following sections. The different microenvironments of these organs
are summarized in Table 3. A detailed description of the histological
and pathological aspects of rat lymphoid tissue is given by Jones et
al. (1990).
 Not only conventional histological features but also the
expression of cell surface markers (immunological phenotype) are
emphasized. Monoclonal antibodies to marker substances
(differentiation antigens) on cell populations are used widely in the
identification of leukocyte populations, especially in cell
suspensions (flow cytometry) and tissue sections (immuno-
histochemistry). The wide range of monoclonal antibodies that currently
exists have been grouped according to the 'cluster of differentiation'
(CD) nomenclature, in which they are classified according to their
reactivity to the same cell marker molecule (but not the same epitope
on those molecules) (Clark & Lanier, 1989; Knapp et al., 1989;
Schlossman et al., 1994, 1995). The CD nomenclature has been adapted
for species other than man (Holmes & Morse, 1988; Jefferies, 1988;
Schuurman et al., 1992a). Some monoclonal antibodies that can be used
in the identification of leukocytes and stromal cells in tissue sections
and cell suspension are described in section 4.1.2.
Not only conventional histological features but also the
expression of cell surface markers (immunological phenotype) are
emphasized. Monoclonal antibodies to marker substances
(differentiation antigens) on cell populations are used widely in the
identification of leukocyte populations, especially in cell
suspensions (flow cytometry) and tissue sections (immuno-
histochemistry). The wide range of monoclonal antibodies that currently
exists have been grouped according to the 'cluster of differentiation'
(CD) nomenclature, in which they are classified according to their
reactivity to the same cell marker molecule (but not the same epitope
on those molecules) (Clark & Lanier, 1989; Knapp et al., 1989;
Schlossman et al., 1994, 1995). The CD nomenclature has been adapted
for species other than man (Holmes & Morse, 1988; Jefferies, 1988;
Schuurman et al., 1992a). Some monoclonal antibodies that can be used
in the identification of leukocytes and stromal cells in tissue sections
and cell suspension are described in section 4.1.2.
 Table 3. Microenvironments in lymphoid tissue
Microenvironment Cells present Function
Bone marrow Haematopoietic cells organized as Differentiation of stem cells into cells
islands within fatty tissue, mature of the erythroid, myeloid/monocytoid,
leukocytes, plasma cells platelet and lymphoid lineage; antibody
synthesis, memory cells
Thymus
Cortex Reticular epithelium, immature Generation of T-cell competence,
T cells T-cell receptor rearrangement, positive
selection (MHC restriction), negative
selection (autoreactive cells), phenotypic
changes
Medulla Reticular epithelium, dendritic cells, Final generation of T-cell competence
T lymphocytes (negative selection), thymic hormone
synthesis, antigen presentation
Lymph node and spleen
Paracortex (lymph node), Interdigitating cells, Th and Ts cells Lymphocyte entry through high periarteriolar
lymphocyte sheath lymphoendothelial venules (lymph node) or
central arteriole (spleen), antigen
presentation to Th cells, T-cell proliferation,
differentiation, and regulation (Ts cells)
Primary follicles, follicular mantle Dendritic cells (subtype of follicular Storage of virgin and memory B cells,
of secondary follicles dendritic cells), dendritic macrophages, recirculating B cells (surface IgM+IgD+)
B cells, small number of T cells
Table 3 (cont'd)
Microenvironment Cells present Function
Germinal centre Follicular dendritic cells, dendritic T Cell-dependent B-lymphocyte
macrophages (starry-sky macrophages), differentiation, antigen presentation in the
B cells (centrocytes, centroblasts), form of immune complexes (with/without
Th cells complement C3)
Medulla (lymph node), Plasma cells, T effector cells, Termination of antigen-specific reaction:
red pulp (spleen) reticular cells, polymorphonuclear antibody synthesis and immune
granulocytes complex-mediated clearance, Tdth and
Tc cell response
Marginal zone (spleen) Marginal zone macrophages, T Cell-independent B-lymphocyte
marginal metallophilic cells, proliferation and differentiation, e.g. to
marginal zone B cells bacterial polysaccharides, B-cell memory
(surface IgM+IgD- cells)
Mucosa-associated lymphoid tissue
Epithelium covering lymphoid M (microfold) cells Transport (uptake) of exogenous
tissue (e.g. Peyer's patches) substances
Follicles and interfollicular See 'lymph node and spleen' For antibody synthesis: precursors of IgA
areas plasma cells
Mucosal epithelium Epithelial cells, Tc cells, natural First line of defence, synthesis of secretory
killer cells, gamma-delta T cells component, transport of IgA (IgM) to
lumen
Lamina propria Plasma cells, macrophages Synthesis of IgA antibody, phagocytosis
and killing
MHC, major histocompatibility complex; Th, T helper; Ts, T suppressor; Ig, immunoglobulin; Tdth, delayed-type hypersensitivity T cells;
Tc, T cytotoxic
Typing of cells in the T lymphocyte lineage is a good
illustration of immunological phenotyping. Subsets with other
functions usually cannot be identified by conventional cytology but
can be recognized by immunological phenotyping. For example, T cells
with a helper-inducer function are labelled by antibodies to CD4
antigens, and cells with a cytotoxic function are labelled by
antibodies to CD8. The reverse does not hold, however; for example,
not all CD4+ cells are helper-inducer cells, because T cells in the
separate subset effecting delayed-type hypersensitivity and some
macrophage populations are also CD4+*CD8+ T cells are in either
the cytotoxic or the suppressor subset. Thus, there is no unequivocal
relationship between CD4 or CD8 expression and cell function, because
these cell-surface molecules are expressed in relation to the way in
which antigen is recognized: T cells recognize antigen in the context
of MHC molecules -- MHC class II molecules for CD4+ cells and MHC
class I antigens for CD8+ cells (Engleman et al., 1981; Meuer et
al., 1983). This phenomenon is further discussed in sections 1.2.2.3
and 1.2.2.4.
Monoclonal antibodies have been developed to most subtypes of
leukocytes, including NK cells. The specificity of antibody 3.2.3,
anti-NKR-P1, for identifying these cells was described by Chambers et
al. (1989). Immunological phenotyping of these cells in rat spleen and
lung is illustrated in Figure 14d.
NK cells and their activities are an example of the differences
between cell function, cytology, and immunological phenotype. NK cells
are characterized by their killer function against selected targets
in vitro. Cells with cytological features that include cytoplasmic
granules, called large-granular lymphocytes, presumably have a natural
killer function, as do cells with an immunophenotype defined by
certain antibodies. The cells identified in these ways are by no means
identical and belong to different, overlapping subpopulations.
1.2.2.2 Bone marrow
The microenvironment of the bone marrow is found in all of the
hollow bones of the body and occupies the medullary space of the
skeleton (Figure 7). Blood enters the marrow from nutrient arteries at
the place where they bifurcate to form the central artery of the
medullary canal. Branches of the central artery terminate in
capillaries within the medullary space or penetrate the endosteum,
where arterial blood from the nutrient artery mixes with blood from
muscular arteries in the periosteal capillaries. The blood returns via
the vascular sinuses to the central sinus and vein. Blood cells in
various stages of development (Figure 1) intermingle with their
microenvironment, which is composed of reticular cells (adventitial
and fibroblastic), adipocytes, endothelial cells, and extracellular
matrix. These, together with passenger accessory cells (macrophages,
monocytes, and lymphocytes, especially T cells), support the
haematopoietic process (Lichtman, 1981; Weiss & Sakai, 1984; Chanarin,
1985; Weiss & Geduldig, 1991; Mayani et al., 1992).
In many mammals during ontogenesis, haematopoiesis is first
located in the yolk sac and fetal liver, until the bone marrow
develops and becomes functional. In rodents, haematopoiesis also
occurs in splenic red pulp later in life. This effect is less
pronounced in other species, but haematopoiesis can occur at other
sites in the body, e.g. the thymus. In lower vertebrates, a bone
marrow containing haematopoietic stem cells occurs firstly in
amphibians. It contains only granulopoietic and lymphoid cells;
erythropoiesis and thrombopoiesis usually occur in the blood sinusoids
of the splenic red pulp. In fish and more primitive vertebrates,
lymphoid tissue is found at many ectopic locations, such as intestine,
brain, heart, gonads, pro- and mesonephrons, and liver and is
functionally and structurally similar to the bone marrow of higher
vertebrates. In birds, the thymus is the main site of erythropoiesis
during certain periods of life (Kendall & Ward, 1974; Kendall &
Frazier, 1979).
Haematopoiesis: The pluripotent haematopoietic stem cells in
the bone marrow proliferate and differentiate to progenitors of
myeloid, erythroid, and lymphoid cells. Some further differentiation
occurs in the marrow, but the final maturation mainly occurs elsewhere
(Figure 1). The common lymphoid progenitor cell differentiates into a
T- and a B-lymphoid progenitor. Progenitor T cells then move to the
thymus for further maturation. B-Lymphoid progenitors mature via the
pre-B cell stage into virgin B cells, which then leave the
microenvironment of the bone marrow and lodge in the periphery. In
birds, this function in B-cell maturation is performed by the bursa of
Fabricius, an epithelial organ located adjacent to the termination of
the gastrointestinal tract (B stands for bursa-dependent) (Cooper,
1982; Du Pasquier, 1989; Zapata & Cooper, 1990). Although the bursa is
considered essential for the production of B lymphocytes, chickens
bursectomized early in embryonic life have B cells in their peripheral
lymphoid organs and can produce antibodies, even if their repertoire
is very limited. Thus, the bursa seems to be involved in the
generation of immunoglobulin diversity rather than just in B
lymphatopoiesis.
The development of stem cells into more mature cells requires the
presence of a microenvironment, as described above, and is regulated
by haematopoietic cytokines (also called haematopoietins) (Fletcher &
Williams, 1992; Kincade, 1992; Quesniaux, 1992; Saito, 1992; Williams
& Quesenberry, 1992; Wright & Pragnell, 1992; see also Figure 2).
Unmyelinated nerve fibres may terminate in the haematopoietic spaces
(Lichtman, 1981), and neuropeptides may play a role in the regulation
of haematopoiesis. A microgeographical distribution of the various
Table 3. Microenvironments in lymphoid tissue
Microenvironment Cells present Function
Bone marrow Haematopoietic cells organized as Differentiation of stem cells into cells
islands within fatty tissue, mature of the erythroid, myeloid/monocytoid,
leukocytes, plasma cells platelet and lymphoid lineage; antibody
synthesis, memory cells
Thymus
Cortex Reticular epithelium, immature Generation of T-cell competence,
T cells T-cell receptor rearrangement, positive
selection (MHC restriction), negative
selection (autoreactive cells), phenotypic
changes
Medulla Reticular epithelium, dendritic cells, Final generation of T-cell competence
T lymphocytes (negative selection), thymic hormone
synthesis, antigen presentation
Lymph node and spleen
Paracortex (lymph node), Interdigitating cells, Th and Ts cells Lymphocyte entry through high periarteriolar
lymphocyte sheath lymphoendothelial venules (lymph node) or
central arteriole (spleen), antigen
presentation to Th cells, T-cell proliferation,
differentiation, and regulation (Ts cells)
Primary follicles, follicular mantle Dendritic cells (subtype of follicular Storage of virgin and memory B cells,
of secondary follicles dendritic cells), dendritic macrophages, recirculating B cells (surface IgM+IgD+)
B cells, small number of T cells
Table 3 (cont'd)
Microenvironment Cells present Function
Germinal centre Follicular dendritic cells, dendritic T Cell-dependent B-lymphocyte
macrophages (starry-sky macrophages), differentiation, antigen presentation in the
B cells (centrocytes, centroblasts), form of immune complexes (with/without
Th cells complement C3)
Medulla (lymph node), Plasma cells, T effector cells, Termination of antigen-specific reaction:
red pulp (spleen) reticular cells, polymorphonuclear antibody synthesis and immune
granulocytes complex-mediated clearance, Tdth and
Tc cell response
Marginal zone (spleen) Marginal zone macrophages, T Cell-independent B-lymphocyte
marginal metallophilic cells, proliferation and differentiation, e.g. to
marginal zone B cells bacterial polysaccharides, B-cell memory
(surface IgM+IgD- cells)
Mucosa-associated lymphoid tissue
Epithelium covering lymphoid M (microfold) cells Transport (uptake) of exogenous
tissue (e.g. Peyer's patches) substances
Follicles and interfollicular See 'lymph node and spleen' For antibody synthesis: precursors of IgA
areas plasma cells
Mucosal epithelium Epithelial cells, Tc cells, natural First line of defence, synthesis of secretory
killer cells, gamma-delta T cells component, transport of IgA (IgM) to
lumen
Lamina propria Plasma cells, macrophages Synthesis of IgA antibody, phagocytosis
and killing
MHC, major histocompatibility complex; Th, T helper; Ts, T suppressor; Ig, immunoglobulin; Tdth, delayed-type hypersensitivity T cells;
Tc, T cytotoxic
Typing of cells in the T lymphocyte lineage is a good
illustration of immunological phenotyping. Subsets with other
functions usually cannot be identified by conventional cytology but
can be recognized by immunological phenotyping. For example, T cells
with a helper-inducer function are labelled by antibodies to CD4
antigens, and cells with a cytotoxic function are labelled by
antibodies to CD8. The reverse does not hold, however; for example,
not all CD4+ cells are helper-inducer cells, because T cells in the
separate subset effecting delayed-type hypersensitivity and some
macrophage populations are also CD4+*CD8+ T cells are in either
the cytotoxic or the suppressor subset. Thus, there is no unequivocal
relationship between CD4 or CD8 expression and cell function, because
these cell-surface molecules are expressed in relation to the way in
which antigen is recognized: T cells recognize antigen in the context
of MHC molecules -- MHC class II molecules for CD4+ cells and MHC
class I antigens for CD8+ cells (Engleman et al., 1981; Meuer et
al., 1983). This phenomenon is further discussed in sections 1.2.2.3
and 1.2.2.4.
Monoclonal antibodies have been developed to most subtypes of
leukocytes, including NK cells. The specificity of antibody 3.2.3,
anti-NKR-P1, for identifying these cells was described by Chambers et
al. (1989). Immunological phenotyping of these cells in rat spleen and
lung is illustrated in Figure 14d.
NK cells and their activities are an example of the differences
between cell function, cytology, and immunological phenotype. NK cells
are characterized by their killer function against selected targets
in vitro. Cells with cytological features that include cytoplasmic
granules, called large-granular lymphocytes, presumably have a natural
killer function, as do cells with an immunophenotype defined by
certain antibodies. The cells identified in these ways are by no means
identical and belong to different, overlapping subpopulations.
1.2.2.2 Bone marrow
The microenvironment of the bone marrow is found in all of the
hollow bones of the body and occupies the medullary space of the
skeleton (Figure 7). Blood enters the marrow from nutrient arteries at
the place where they bifurcate to form the central artery of the
medullary canal. Branches of the central artery terminate in
capillaries within the medullary space or penetrate the endosteum,
where arterial blood from the nutrient artery mixes with blood from
muscular arteries in the periosteal capillaries. The blood returns via
the vascular sinuses to the central sinus and vein. Blood cells in
various stages of development (Figure 1) intermingle with their
microenvironment, which is composed of reticular cells (adventitial
and fibroblastic), adipocytes, endothelial cells, and extracellular
matrix. These, together with passenger accessory cells (macrophages,
monocytes, and lymphocytes, especially T cells), support the
haematopoietic process (Lichtman, 1981; Weiss & Sakai, 1984; Chanarin,
1985; Weiss & Geduldig, 1991; Mayani et al., 1992).
In many mammals during ontogenesis, haematopoiesis is first
located in the yolk sac and fetal liver, until the bone marrow
develops and becomes functional. In rodents, haematopoiesis also
occurs in splenic red pulp later in life. This effect is less
pronounced in other species, but haematopoiesis can occur at other
sites in the body, e.g. the thymus. In lower vertebrates, a bone
marrow containing haematopoietic stem cells occurs firstly in
amphibians. It contains only granulopoietic and lymphoid cells;
erythropoiesis and thrombopoiesis usually occur in the blood sinusoids
of the splenic red pulp. In fish and more primitive vertebrates,
lymphoid tissue is found at many ectopic locations, such as intestine,
brain, heart, gonads, pro- and mesonephrons, and liver and is
functionally and structurally similar to the bone marrow of higher
vertebrates. In birds, the thymus is the main site of erythropoiesis
during certain periods of life (Kendall & Ward, 1974; Kendall &
Frazier, 1979).
Haematopoiesis: The pluripotent haematopoietic stem cells in
the bone marrow proliferate and differentiate to progenitors of
myeloid, erythroid, and lymphoid cells. Some further differentiation
occurs in the marrow, but the final maturation mainly occurs elsewhere
(Figure 1). The common lymphoid progenitor cell differentiates into a
T- and a B-lymphoid progenitor. Progenitor T cells then move to the
thymus for further maturation. B-Lymphoid progenitors mature via the
pre-B cell stage into virgin B cells, which then leave the
microenvironment of the bone marrow and lodge in the periphery. In
birds, this function in B-cell maturation is performed by the bursa of
Fabricius, an epithelial organ located adjacent to the termination of
the gastrointestinal tract (B stands for bursa-dependent) (Cooper,
1982; Du Pasquier, 1989; Zapata & Cooper, 1990). Although the bursa is
considered essential for the production of B lymphocytes, chickens
bursectomized early in embryonic life have B cells in their peripheral
lymphoid organs and can produce antibodies, even if their repertoire
is very limited. Thus, the bursa seems to be involved in the
generation of immunoglobulin diversity rather than just in B
lymphatopoiesis.
The development of stem cells into more mature cells requires the
presence of a microenvironment, as described above, and is regulated
by haematopoietic cytokines (also called haematopoietins) (Fletcher &
Williams, 1992; Kincade, 1992; Quesniaux, 1992; Saito, 1992; Williams
& Quesenberry, 1992; Wright & Pragnell, 1992; see also Figure 2).
Unmyelinated nerve fibres may terminate in the haematopoietic spaces
(Lichtman, 1981), and neuropeptides may play a role in the regulation
of haematopoiesis. A microgeographical distribution of the various
 cell lineages in microenvironments has been suggested, such as the
preferential associations between fibroblasts and granulocytes,
megakaryocytes, and sinus endothelial and adventitial cells and
between macrophages and erythroid cells.
The classical bioassay for haematopoietic stem cell activity is
measurement of colony-forming units in the spleen of irradiated mice
after injection of bone-marrow cells. More differentiated progenitors
can be cultured in vitro to provide information on the specific
lineage of the colony-forming cell, to evaluate the progenitor
activity of macrophages, granulocytes, megakaryocytes, eosinophilic
granulocytes, and mast cells, as well as that of several cell
lineages, such as macrophages and granulocytes. Progenitors of
erythrocytes are assayed as early progenitors or as erythroid burst-
forming units. Studies of the mechanisms involved have revealed a
number of factors that promote the differentiation of progenitor
cells, like granulocyte-macrophage colony-stimulating factor,
macrophage colony-stimulating factor, granulocyte colony-stimulating
factor, and c-kit ligand. These mediators are produced by various cell
types, mainly after activation, and promote haematopoiesis in vivo
in conditions of e.g. infection and inflammation. Other soluble
mediators produced by T cells and by other cells involved in the
antigen-driven immune response also promote haematopoiesis; these
include IL-1, IL-3, IL-6, IL-11, interferon and tumour necrosis
factor. Haematopoiesis in the bone marrow is thus not independent of
antigenic stimulation and exposure but is not typically antigen-
driven. For instance, in the case of acute infection, the bone marrow
produces a large proportion of polymorphonuclear granulocytes within a
short time, manifested in blood as leukocytosis and a shift in the
differential count to band-type immature granulocytes. This shift may
be related to migration of T lymphocytes into the bone marrow
microenvironment, where they activate haematopoiesis. Antigen-induced
lymphoid cell differentiation also occurs in the bone marrow; for
instance, bone marrow cells include the memory lymphocyte pool and the
major plasma cell population, which contribute to the intravascular
pool of immunoglobulins.
Development and aging: The bone marrow is the primary site of
haematopoiesis throughout life, generating over 95% of the
haematopoietic activity in adult mammals (Mayani et al., 1992).
Essentially all of the medullary space in the bones is occupied by
haematopoietic tissue in mice and rats; in contrast, in adult humans,
dogs, and rabbits, haematopoietic tissue is restricted to the proximal
epiphyses of the long bones, the central skeleton, and the skull; most
bone marrow is gradually replaced by fat cells. The normal mean
proportions of bone marrow cells and changes with age in rats were
reported by Valli et al. (1990).
1.2.2.3 Thymus
The thymus is a two-lobed organ located in the mediastinum,
anterior to the major vessels of the heart (Kendall, 1991; Von
Gaudecker, 1991; Schuurman et al., 1993), although it is located in
the neck region in the guinea-pig. Its anatomical location complicates
complete thymectomy in vivo. The two independent lobes, attached to
each other only by connective tissue, consist of smaller lobules,
which have basically the same architecture, with a subcapsular and
outer cortical area, a cortex, and a medulla (Figure 8). With
conventional histological stains, the cortex is strongly stained,
owing to a dense population of small lymphocytes, and the outer cortex
and medulla show a paler colouring.
Blood vessels enter the lobules at the cortico-medullary junction
and extend radially into the cortex. Nerves course along the blood
vasculature. Fenestrated capillaries are very infrequent in the
cortex. The thymus is unique among the lymphoid organs in that its
microenvironment consists of a reticular epithelium (in birds, the
bursa of Fabricius also has an epithelial framework). Macrophages
derived from the bone marrow are found in the cortex and medulla as a
transient population. Dendritic cells in the medulla, resembling
interdigitating dendritic cells in lymph nodes, have a major function
in antigen presentation and strongly express MHC class II antigen.
The thymus appears for the first time in vertebrate phylogeny in
cartilaginous fish (sharks and rays). More primitive vertebrates lack
a thymus, although they can manifest cellular immune responses. Except
in bony fish, the thymus is histologically similar in all vertebrates,
although it is derived from distinct pharyngeal pouches in different
species. The classical cortex-medulla demarcation is not present in
fish thymus, and most thymocytes seem to occupy the central part of
the organ. In all species, epithelial cells organize a supporting
network in both cortex and medulla. Hassall's corpuscles, which are
epithelial aggregates with centrally located cell debris, occur in the
medulla of the human thymus but are scarce or absent in the rodent
thymus and in the thymus of ectothermic vertebrates. In the latter,
epithelial cysts are frequent. The thymus of lower vertebrates, in
contrast to that of mammals, contains numerous myoid cells; in avian
thymus, significant erythropoiesis has been documented (Kendall &
Ward, 1974; Kendall & Frazier, 1979), which may also occur in mammals
(Kendall, 1980; Kendall & Singh, 1980).
T-Cell maturation: selection in the thymus: T Cells reside in
the thymus during their maturation from progenitor cells to
immunocompetent T cells. This gland has a privileged function in
promoting the maturation process (Brekelmans & Van Ewijk, 1990;
Shortman et al., 1990; Van Ewijk, 1991; Boyd et al., 1992). In
congenitally athymic, 'nude' animals (Schuurman et al., 1992b,c) and
in thymic aplasia in children with complete Di George's syndrome, the
cell lineages in microenvironments has been suggested, such as the
preferential associations between fibroblasts and granulocytes,
megakaryocytes, and sinus endothelial and adventitial cells and
between macrophages and erythroid cells.
The classical bioassay for haematopoietic stem cell activity is
measurement of colony-forming units in the spleen of irradiated mice
after injection of bone-marrow cells. More differentiated progenitors
can be cultured in vitro to provide information on the specific
lineage of the colony-forming cell, to evaluate the progenitor
activity of macrophages, granulocytes, megakaryocytes, eosinophilic
granulocytes, and mast cells, as well as that of several cell
lineages, such as macrophages and granulocytes. Progenitors of
erythrocytes are assayed as early progenitors or as erythroid burst-
forming units. Studies of the mechanisms involved have revealed a
number of factors that promote the differentiation of progenitor
cells, like granulocyte-macrophage colony-stimulating factor,
macrophage colony-stimulating factor, granulocyte colony-stimulating
factor, and c-kit ligand. These mediators are produced by various cell
types, mainly after activation, and promote haematopoiesis in vivo
in conditions of e.g. infection and inflammation. Other soluble
mediators produced by T cells and by other cells involved in the
antigen-driven immune response also promote haematopoiesis; these
include IL-1, IL-3, IL-6, IL-11, interferon and tumour necrosis
factor. Haematopoiesis in the bone marrow is thus not independent of
antigenic stimulation and exposure but is not typically antigen-
driven. For instance, in the case of acute infection, the bone marrow
produces a large proportion of polymorphonuclear granulocytes within a
short time, manifested in blood as leukocytosis and a shift in the
differential count to band-type immature granulocytes. This shift may
be related to migration of T lymphocytes into the bone marrow
microenvironment, where they activate haematopoiesis. Antigen-induced
lymphoid cell differentiation also occurs in the bone marrow; for
instance, bone marrow cells include the memory lymphocyte pool and the
major plasma cell population, which contribute to the intravascular
pool of immunoglobulins.
Development and aging: The bone marrow is the primary site of
haematopoiesis throughout life, generating over 95% of the
haematopoietic activity in adult mammals (Mayani et al., 1992).
Essentially all of the medullary space in the bones is occupied by
haematopoietic tissue in mice and rats; in contrast, in adult humans,
dogs, and rabbits, haematopoietic tissue is restricted to the proximal
epiphyses of the long bones, the central skeleton, and the skull; most
bone marrow is gradually replaced by fat cells. The normal mean
proportions of bone marrow cells and changes with age in rats were
reported by Valli et al. (1990).
1.2.2.3 Thymus
The thymus is a two-lobed organ located in the mediastinum,
anterior to the major vessels of the heart (Kendall, 1991; Von
Gaudecker, 1991; Schuurman et al., 1993), although it is located in
the neck region in the guinea-pig. Its anatomical location complicates
complete thymectomy in vivo. The two independent lobes, attached to
each other only by connective tissue, consist of smaller lobules,
which have basically the same architecture, with a subcapsular and
outer cortical area, a cortex, and a medulla (Figure 8). With
conventional histological stains, the cortex is strongly stained,
owing to a dense population of small lymphocytes, and the outer cortex
and medulla show a paler colouring.
Blood vessels enter the lobules at the cortico-medullary junction
and extend radially into the cortex. Nerves course along the blood
vasculature. Fenestrated capillaries are very infrequent in the
cortex. The thymus is unique among the lymphoid organs in that its
microenvironment consists of a reticular epithelium (in birds, the
bursa of Fabricius also has an epithelial framework). Macrophages
derived from the bone marrow are found in the cortex and medulla as a
transient population. Dendritic cells in the medulla, resembling
interdigitating dendritic cells in lymph nodes, have a major function
in antigen presentation and strongly express MHC class II antigen.
The thymus appears for the first time in vertebrate phylogeny in
cartilaginous fish (sharks and rays). More primitive vertebrates lack
a thymus, although they can manifest cellular immune responses. Except
in bony fish, the thymus is histologically similar in all vertebrates,
although it is derived from distinct pharyngeal pouches in different
species. The classical cortex-medulla demarcation is not present in
fish thymus, and most thymocytes seem to occupy the central part of
the organ. In all species, epithelial cells organize a supporting
network in both cortex and medulla. Hassall's corpuscles, which are
epithelial aggregates with centrally located cell debris, occur in the
medulla of the human thymus but are scarce or absent in the rodent
thymus and in the thymus of ectothermic vertebrates. In the latter,
epithelial cysts are frequent. The thymus of lower vertebrates, in
contrast to that of mammals, contains numerous myoid cells; in avian
thymus, significant erythropoiesis has been documented (Kendall &
Ward, 1974; Kendall & Frazier, 1979), which may also occur in mammals
(Kendall, 1980; Kendall & Singh, 1980).
T-Cell maturation: selection in the thymus: T Cells reside in
the thymus during their maturation from progenitor cells to
immunocompetent T cells. This gland has a privileged function in
promoting the maturation process (Brekelmans & Van Ewijk, 1990;
Shortman et al., 1990; Van Ewijk, 1991; Boyd et al., 1992). In
congenitally athymic, 'nude' animals (Schuurman et al., 1992b,c) and
in thymic aplasia in children with complete Di George's syndrome, the
 absence of a functionally active T-cell system is causally related to
the aplasia of the organ. The process of T-cell maturation includes a
number of steps located in different microenvironments (Schuurman et
al., 1993): The least mature cells, which enter the lobules from the
bloodstream at the cortico-medullary junction, first move to the outer
subcapsular cortex, where they appear as large lymphoblasts. They then
pass through the cortex, where they become small lymphocytes with a
scanty cytoplasm. Finally, they move to the medulla, where they appear
as medium-sized lymphocytes. These translocational stages in
development are monitored on the basis of the immunological phenotype.
For the CD4-CD8 phenotype, the cells change from CD-CD8- (so-called
double-negative) at a very immature stage, then change to a CD4-CD8+
stage and into a CD4+CD8+ (double-positive) phenotype, which is
found on almost all lymphocytes in the cortex. In the medulla, T cells
have the phenotype of mature cells, with distinct CD4+CD8- (about
two-thirds) and CD4-CD8+ (about one-third) populations.
This phenotypic change is accompanied by a crucial aspect of
intrathymic T-cell maturation: genesis of the TCR, which consists of
the alpha-ß heterodimer. Initially, the DNA genomic organization that
encodes these chains is in germline configuration, with a variety of
gene segments encoding the variable part of the receptor molecule.
Before transcription and translation into TCR becomes possible,
combinations have to be made of the gene segments that encode the
variable and constant parts of the TCR. This process of gene
rearrangement requires the thymic microenvironment, and only after it
is completed can the cell synthesize the receptor. The receptor is
then expressed on the cell membrane together with the CD3 molecule,
which may act as the transmembrane signal transducing molecule after
TCR stimulation; the CD3 molecule is already present in the cytoplasm
of the cell even before the TCR has been synthesized. T Cells at this
stage of maturation can be recognized by cytoplasmic staining with CD3
reagents.
The TCR gene rearrangement is similar to the rearrangement of
genes that encode immunoglobulin heavy and light chains, which takes
place in the bone-marrow microenvironment. Once TCR has been expressed
at the surface, however, the cell undergoes a process unique to T
cells, namely, specific selection on the basis of recognition
specificity (Blackmann et al., 1990; Sprent et al., 1990; Von Boehmer,
1990). First, the cell is examined for its capacity to recognize an
antigen in the context of its own MHC (self-restriction); then it is
allowed to expand (positive selection). Second, the cell is examined
for its capacity to recognize a self-antigen (autoreactivity). If it
recognizes a self-antigen, it is blocked from further differentiation
(negative selection). In this way, the random pool of antigen
recognition specificities of T cells is adapted to the host's
situation; the total repertoire of the alpha-ß T-cell population
(estimated at 1012 different epitopes) changes into the potentially
available repertoire (estimated at recognition of about 106
epitopes). Current theories of negative selection state that this step
is not feasible for all putative autoantigens in the body. Rather, it
applies to a selection of potentially harmful specificities (in
particular MHC antigens). If a cell is not selected during positive or
negative selection, it dies, possibly by suicide or apoptosis
(McDonald & Lees, 1990). A hallmark of apoptosis is endonuclease-
induced chromosomal fragmentation into 200 base-pair fragments
(McConkey et al., 1990). Histologically, apoptosis is recognized by
the presence of condensed, sometimes fragmented nuclei, which can be
found in phagocytic macrophages ('tingible body' or 'starry-sky'
macrophages) (Kendall, 1991).
Function of the microenvironment: It is generally accepted that
the epithelial microenvironment of the thymic cortex plays a major
role in positive selection. This microenvironment expresses MHC class
I and class II products and shows close interactions with lymphocytes
morphologically (at the electron microscopic level). This close
interaction is reflected in the complete inclusion of lymphocytes
inside the epithelial cytoplasm ('thymic nurse cells') (Van Ewijk,
1988). Negative selection has been ascribed to either the epithelial
compartment or the medullary dendritic cells. The different processes
occurring in early (cortical) and late (medullary) maturation are
associated with differences in the microenvironment. Epithelial cells
in the cortex and medulla differ in antigen expression,
ultrastructural characteristics, and their capacity to synthesize
thymic hormones such as thymulin, thymic humoral factor, thymosin, and
thymopoietin. These hormones have a major function late in intrathymic
T-cell maturation, and the major site of thymic hormone synthesis is
the medullary epithelium (Dabrowski & Dabrowski-Bernstein, 1990).
The cortex can be considered a primary lymphoid organ because it
is an antigen-free microenvironment with a blood-thymus barrier. In
contrast, antigens can move relatively freely into the medulla and
encounter antigen-presenting dendritic cells as well as antigen-
reactive T cells. Thus the medulla has the properties of a secondary
lymphoid organ (Van Ewijk, 1988; Kendall, 1991).
Ontogeny, growth, and involution: The thymus in rodents reaches
full development at around day 17 of gestation, that is about five
days before birth. In humans, a fully developed thymus is first seen
at the 16th to 17th week of gestation (Von Gaudecker, 1986), which is
relatively earlier in the gestation period than in rodents, since the
human immune system is more mature at birth than that of rodents.
Nevertheless, a thymus that appears histologically to be fully
developed may be functionally somewhat immature. For instance, fetal
thymus from humans (McCune et al., 1988; Namikawa et al., 1990) or
rats (De Heer et al., 1993) can be transplanted into mice with the
severe combined immunodeficiency (scid) mutation and grow for longer
than tissue obtained postnatally (see also section 4.5.3). After
birth, when the individual first comes into contact with exogenous
antigens, the thymus is called upon to provide large numbers of T
cells to the periphery, and the organ grows in a relatively short time
-- in humans within three to four weeks, from about 15 to 50 g. In
rats, the organ reaches it largest relative size about one week after
birth; the absolute weight is greatest at about six to eight weeks of
age. After adulthood is reached, the thymus starts to involute
(Steinmann, 1986; Kuper et al., 1990a; Schuurman et al., 1991a; Kuper
et al., 1992a), a process that may be related to changes in the
hormonal status of the individual; circulating thymic hormone is
reduced to very low levels in adults. The underlying mechanisms are
not fully understood. The consequences of age-associated involution
are obvious: emigration of lymphocytes from the thymus decreases
dramatically, from, for instance, 1.6 × 106/day in one-month-old
mice to 4 × 104/day in one-year-old mice (Stutman, 1986; Shortman et
al., 1990). Apparently, the persistent generation of a new antigen-
recognition repertoire in the T-cell population of adults is not
needed. Instead, the body can defend itself using the established
repertoire and extrathymic self-renewal of the T cells. Similar
processes may occur after artificial involution of the thymus caused
by toxic compounds or acute stress (including acute disease); this
aspect is further discussed below.
It should be emphasized that the basic architecture of the thymus
is not a fixed histological entity; its features depend on the age and
the stress hormone status of the individual. A 'normal' architecture
can be expected only between the late gestational period and young
adulthood, before the start of age-associated involution. This
phenomenon has important implications for the selection of rodents
according to age for studies of immune function and in the
interpretation of studies of immunotoxicity.
In mice, severe but reversible changes occur in the thymus during
pregnancy (Clarke, 1984; Clarke & Kendall, 1989; see also Figure 9).
The weight of the thymus shows an initial small rise in early
pregnancy, from about 35 to 40 mg, and dramatically decreases to 15 mg
or less at day 17 of pregnancy (Clarke, 1984). This involution is
associated with severe lymphodepletion of the cortex. Cell death is
seen by the presence of apoptotic figures and phagocytosis in
macrophages and epithelial cells. Remarkably, the large lymphoblasts
in the outer cortex remain relatively unattached, and the same applies
for thymocytes in thymic nurse cells. In birds, changes with breeding
sessions have been found (Kendall & Ward, 1974; Ward & Kendall, 1975).
In a study of a wild population of adult red-billed queleas, cyclical
enlargement and regression of the thymus were documented; at the time
of mating and laying, most birds, irrespective of sex, showed an
involuted thymus; on subsequent egg incubation the thymus size
increased, with a decline in the latter half of the rearing period.
1.2.2.4 Lymph nodes
A finely branched lymph vessel system (lymphatics) is involved in
the return of interstitial fluid in tissue to the blood circulation,
with lymph nodes spaced at regular intervals (Dunn, 1954; Tilney,
1971). The major sites of lymph nodes (or groups of lymph nodes) are
shown in Figure 3; a scheme of the areas drained by distinct lymph
nodes or lymph node groups is given in Figure 10, a schematic
presentation of individual lymph node architecture is presented in
Figure 11, and the histology of a lymph node is shown in Figure 12.
The main functions of lymph nodes are to filter pathogens from the
afferent lymph and then to initialize immune reactions. The afferent
lymphatics penetrate the lymph node capsule and connect with the
subcapsular sinus, which in turn connects with the cortical and
medullary sinuses. On the basis of the lymph flow through the node,
basic units can be recognized, each of which is supplied by its own
afferent lymph vessel and which comprise part of the paracortex
(Bélisle & Sainte-Marie, 1981; Sainte-Marie et al., 1990). Afferent
and efferent blood vessels are connected to the organ at the hilus,
where the lymph leaves the node via the efferent lymphatic(s). The
efferent lymphatics drain into other lymph nodes or directly into the
thoracic duct, which enters the bloodstream, in the rat via the left
subclavian vein.
Lymph vessels and lymph nodes occur only in mammals. In some
birds and monotremes (primitive mammals), primitive lymph nodes
directly interposed in the lymph circulation have been described.
Ectothermic vertebrates do not have lymph nodes. Some small lymphoid
organs such as lymph glands and jugular bodies have been described in
some species of frogs but not in others. These organs presumably
filter antigens from both blood and lymph and have been claimed to be
phylogenetic precursors of mammalian lymph nodes. Likewise, small
lymphoid aggregates associated with the cardinal veins occur in some
reptiles.
Lymph nodes are surrounded by a connective tissue capsule. The
nodes comprise various compartments or microenvironments: (i) the
outer cortex, with follicles and interfollicular areas; (ii) the inner
cortex or paracortex; and (iii) the medulla, with medullary cords and
medullary sinuses. These compartments are easily differentiated into
sections after conventional histological staining. In the cortex,
interfollicular areas and the paracortex (T-lymphocyte area) are
differentiated from follicles by the presence of blood vessels lined
by high endothelium (high endothelial postcapillary venules, discussed
below). Follicles (B lymphocyte area) are rounded structures, located
absence of a functionally active T-cell system is causally related to
the aplasia of the organ. The process of T-cell maturation includes a
number of steps located in different microenvironments (Schuurman et
al., 1993): The least mature cells, which enter the lobules from the
bloodstream at the cortico-medullary junction, first move to the outer
subcapsular cortex, where they appear as large lymphoblasts. They then
pass through the cortex, where they become small lymphocytes with a
scanty cytoplasm. Finally, they move to the medulla, where they appear
as medium-sized lymphocytes. These translocational stages in
development are monitored on the basis of the immunological phenotype.
For the CD4-CD8 phenotype, the cells change from CD-CD8- (so-called
double-negative) at a very immature stage, then change to a CD4-CD8+
stage and into a CD4+CD8+ (double-positive) phenotype, which is
found on almost all lymphocytes in the cortex. In the medulla, T cells
have the phenotype of mature cells, with distinct CD4+CD8- (about
two-thirds) and CD4-CD8+ (about one-third) populations.
This phenotypic change is accompanied by a crucial aspect of
intrathymic T-cell maturation: genesis of the TCR, which consists of
the alpha-ß heterodimer. Initially, the DNA genomic organization that
encodes these chains is in germline configuration, with a variety of
gene segments encoding the variable part of the receptor molecule.
Before transcription and translation into TCR becomes possible,
combinations have to be made of the gene segments that encode the
variable and constant parts of the TCR. This process of gene
rearrangement requires the thymic microenvironment, and only after it
is completed can the cell synthesize the receptor. The receptor is
then expressed on the cell membrane together with the CD3 molecule,
which may act as the transmembrane signal transducing molecule after
TCR stimulation; the CD3 molecule is already present in the cytoplasm
of the cell even before the TCR has been synthesized. T Cells at this
stage of maturation can be recognized by cytoplasmic staining with CD3
reagents.
The TCR gene rearrangement is similar to the rearrangement of
genes that encode immunoglobulin heavy and light chains, which takes
place in the bone-marrow microenvironment. Once TCR has been expressed
at the surface, however, the cell undergoes a process unique to T
cells, namely, specific selection on the basis of recognition
specificity (Blackmann et al., 1990; Sprent et al., 1990; Von Boehmer,
1990). First, the cell is examined for its capacity to recognize an
antigen in the context of its own MHC (self-restriction); then it is
allowed to expand (positive selection). Second, the cell is examined
for its capacity to recognize a self-antigen (autoreactivity). If it
recognizes a self-antigen, it is blocked from further differentiation
(negative selection). In this way, the random pool of antigen
recognition specificities of T cells is adapted to the host's
situation; the total repertoire of the alpha-ß T-cell population
(estimated at 1012 different epitopes) changes into the potentially
available repertoire (estimated at recognition of about 106
epitopes). Current theories of negative selection state that this step
is not feasible for all putative autoantigens in the body. Rather, it
applies to a selection of potentially harmful specificities (in
particular MHC antigens). If a cell is not selected during positive or
negative selection, it dies, possibly by suicide or apoptosis
(McDonald & Lees, 1990). A hallmark of apoptosis is endonuclease-
induced chromosomal fragmentation into 200 base-pair fragments
(McConkey et al., 1990). Histologically, apoptosis is recognized by
the presence of condensed, sometimes fragmented nuclei, which can be
found in phagocytic macrophages ('tingible body' or 'starry-sky'
macrophages) (Kendall, 1991).
Function of the microenvironment: It is generally accepted that
the epithelial microenvironment of the thymic cortex plays a major
role in positive selection. This microenvironment expresses MHC class
I and class II products and shows close interactions with lymphocytes
morphologically (at the electron microscopic level). This close
interaction is reflected in the complete inclusion of lymphocytes
inside the epithelial cytoplasm ('thymic nurse cells') (Van Ewijk,
1988). Negative selection has been ascribed to either the epithelial
compartment or the medullary dendritic cells. The different processes
occurring in early (cortical) and late (medullary) maturation are
associated with differences in the microenvironment. Epithelial cells
in the cortex and medulla differ in antigen expression,
ultrastructural characteristics, and their capacity to synthesize
thymic hormones such as thymulin, thymic humoral factor, thymosin, and
thymopoietin. These hormones have a major function late in intrathymic
T-cell maturation, and the major site of thymic hormone synthesis is
the medullary epithelium (Dabrowski & Dabrowski-Bernstein, 1990).
The cortex can be considered a primary lymphoid organ because it
is an antigen-free microenvironment with a blood-thymus barrier. In
contrast, antigens can move relatively freely into the medulla and
encounter antigen-presenting dendritic cells as well as antigen-
reactive T cells. Thus the medulla has the properties of a secondary
lymphoid organ (Van Ewijk, 1988; Kendall, 1991).
Ontogeny, growth, and involution: The thymus in rodents reaches
full development at around day 17 of gestation, that is about five
days before birth. In humans, a fully developed thymus is first seen
at the 16th to 17th week of gestation (Von Gaudecker, 1986), which is
relatively earlier in the gestation period than in rodents, since the
human immune system is more mature at birth than that of rodents.
Nevertheless, a thymus that appears histologically to be fully
developed may be functionally somewhat immature. For instance, fetal
thymus from humans (McCune et al., 1988; Namikawa et al., 1990) or
rats (De Heer et al., 1993) can be transplanted into mice with the
severe combined immunodeficiency (scid) mutation and grow for longer
than tissue obtained postnatally (see also section 4.5.3). After
birth, when the individual first comes into contact with exogenous
antigens, the thymus is called upon to provide large numbers of T
cells to the periphery, and the organ grows in a relatively short time
-- in humans within three to four weeks, from about 15 to 50 g. In
rats, the organ reaches it largest relative size about one week after
birth; the absolute weight is greatest at about six to eight weeks of
age. After adulthood is reached, the thymus starts to involute
(Steinmann, 1986; Kuper et al., 1990a; Schuurman et al., 1991a; Kuper
et al., 1992a), a process that may be related to changes in the
hormonal status of the individual; circulating thymic hormone is
reduced to very low levels in adults. The underlying mechanisms are
not fully understood. The consequences of age-associated involution
are obvious: emigration of lymphocytes from the thymus decreases
dramatically, from, for instance, 1.6 × 106/day in one-month-old
mice to 4 × 104/day in one-year-old mice (Stutman, 1986; Shortman et
al., 1990). Apparently, the persistent generation of a new antigen-
recognition repertoire in the T-cell population of adults is not
needed. Instead, the body can defend itself using the established
repertoire and extrathymic self-renewal of the T cells. Similar
processes may occur after artificial involution of the thymus caused
by toxic compounds or acute stress (including acute disease); this
aspect is further discussed below.
It should be emphasized that the basic architecture of the thymus
is not a fixed histological entity; its features depend on the age and
the stress hormone status of the individual. A 'normal' architecture
can be expected only between the late gestational period and young
adulthood, before the start of age-associated involution. This
phenomenon has important implications for the selection of rodents
according to age for studies of immune function and in the
interpretation of studies of immunotoxicity.
In mice, severe but reversible changes occur in the thymus during
pregnancy (Clarke, 1984; Clarke & Kendall, 1989; see also Figure 9).
The weight of the thymus shows an initial small rise in early
pregnancy, from about 35 to 40 mg, and dramatically decreases to 15 mg
or less at day 17 of pregnancy (Clarke, 1984). This involution is
associated with severe lymphodepletion of the cortex. Cell death is
seen by the presence of apoptotic figures and phagocytosis in
macrophages and epithelial cells. Remarkably, the large lymphoblasts
in the outer cortex remain relatively unattached, and the same applies
for thymocytes in thymic nurse cells. In birds, changes with breeding
sessions have been found (Kendall & Ward, 1974; Ward & Kendall, 1975).
In a study of a wild population of adult red-billed queleas, cyclical
enlargement and regression of the thymus were documented; at the time
of mating and laying, most birds, irrespective of sex, showed an
involuted thymus; on subsequent egg incubation the thymus size
increased, with a decline in the latter half of the rearing period.
1.2.2.4 Lymph nodes
A finely branched lymph vessel system (lymphatics) is involved in
the return of interstitial fluid in tissue to the blood circulation,
with lymph nodes spaced at regular intervals (Dunn, 1954; Tilney,
1971). The major sites of lymph nodes (or groups of lymph nodes) are
shown in Figure 3; a scheme of the areas drained by distinct lymph
nodes or lymph node groups is given in Figure 10, a schematic
presentation of individual lymph node architecture is presented in
Figure 11, and the histology of a lymph node is shown in Figure 12.
The main functions of lymph nodes are to filter pathogens from the
afferent lymph and then to initialize immune reactions. The afferent
lymphatics penetrate the lymph node capsule and connect with the
subcapsular sinus, which in turn connects with the cortical and
medullary sinuses. On the basis of the lymph flow through the node,
basic units can be recognized, each of which is supplied by its own
afferent lymph vessel and which comprise part of the paracortex
(Bélisle & Sainte-Marie, 1981; Sainte-Marie et al., 1990). Afferent
and efferent blood vessels are connected to the organ at the hilus,
where the lymph leaves the node via the efferent lymphatic(s). The
efferent lymphatics drain into other lymph nodes or directly into the
thoracic duct, which enters the bloodstream, in the rat via the left
subclavian vein.
Lymph vessels and lymph nodes occur only in mammals. In some
birds and monotremes (primitive mammals), primitive lymph nodes
directly interposed in the lymph circulation have been described.
Ectothermic vertebrates do not have lymph nodes. Some small lymphoid
organs such as lymph glands and jugular bodies have been described in
some species of frogs but not in others. These organs presumably
filter antigens from both blood and lymph and have been claimed to be
phylogenetic precursors of mammalian lymph nodes. Likewise, small
lymphoid aggregates associated with the cardinal veins occur in some
reptiles.
Lymph nodes are surrounded by a connective tissue capsule. The
nodes comprise various compartments or microenvironments: (i) the
outer cortex, with follicles and interfollicular areas; (ii) the inner
cortex or paracortex; and (iii) the medulla, with medullary cords and
medullary sinuses. These compartments are easily differentiated into
sections after conventional histological staining. In the cortex,
interfollicular areas and the paracortex (T-lymphocyte area) are
differentiated from follicles by the presence of blood vessels lined
by high endothelium (high endothelial postcapillary venules, discussed
below). Follicles (B lymphocyte area) are rounded structures, located







 mainly immediately underneath the capsule; they present as
accumulations of small lymphocytes (primary follicle) or a pale-
stained centre with large lymphoid cells (centrocytes, centroblasts)
and tingible-body macro-phages surrounded by a mantle with small
lymphocytes (secondary follicles). The interfollicular areas are
continuous with the paracortex, and the latter is continuous with the
medullary cords.
The arterial blood supply enters the node at the medulla and ends
in the paracortex as arteriolar capillaries, with branches in the
follicles. The capillaries feed venules that are lined with high
endothelial cells. The high endothelial venules run from the
paracortex into the medullary cords and then leave the node via the
vein in the hilus. Lymphocytes migrate through the high endothelial
venules after adhering to the endothelium by specific receptor-ligand
interactions (Picker & Butcher, 1992). The adhesion molecules on
lymphocytes and endothelium that are involved in this binding and
subsequent passage thought the endothelial layer are the addressins,
reflecting the difference in receptor-ligand interactions that exists
between lymph nodes of the internal and external lymphoid systems.
Subsequently, lymphocytes can specifically reach the various nodes,
using the same route (the blood). After the lymphocytes have migrated
into the parenchyma, they move into their microenvironment or
compartment.
Antigen encounter and immune reactivity: The main route of
access for antigens and pathogens is the afferent lymph flow; antigens
can also come into contact with the lymph node tissues via the blood.
Antigens in the lymph, either free or processed by veiled macrophages,
enter the node through the subcapsular sinus, which is rich in
macrophages that can phagocytose free antigen. From there, antigens
move to the paracortex, where they are presented to CD4+ Th cells by
the antigen-presenting cells for initiation of the immune response.
The main antigen-presenting cells in the lymph node are the
interdigitating dendritic cells, the tissue equivalents of veiled
macrophages, which can arise from Langerhans cells in the skin (for
e.g. lymph nodes draining the skin). The antigen-presenting cells
express MHC class II antigens in high density, enabling the alpha-ß
TCR of the Th cells to recognize the antigenic determinant complexed
with the polymorphic ('self') MHC class II molecule. The CD4+
molecule on the Th cell surface binds to a non-polymorphic determinant
of MHC class II molecules and strengthens the binding between Th and
antigen-presenting cells (Janeway, 1992). The cellular interaction
triggers the synthesis of cytokines like IL-1 and IL-2. This process
is down-regulated by Ts cells in a way that is not yet completely
understood.
Follicles are involved in antigen-driven B-cell activation,
somatic mutation, positive and negative selection, and memory and
plasma cell development (Szakal et al., 1989; Kroese et al., 1990; Liu
et al., 1992) and are known as primary follicles in the resting state.
They contain small, IgM+IgD+ virgin B cells in a framework of
follicular dendritic cells. During stimulation by antigens, the
follicles change into secondary follicles consisting of a germinal
centre surrounded by a mantle. Antigen may be transported into the
follicle by immune (T) cells, but this route is not yet fully
established. Antigen is presented to B cells in immune complexes, with
complement split products like C3b, which are trapped in cytoplasmic
extensions of the follicular dendritic cells. The interaction between
complement and complement receptors on these cells has a pivotal role
in the adherence of antigen to them. Fc receptors also play a role,
but only in rodents. The complement split products in the trapped
immune complexes have an accessory function in antigen presentation.
Local CD4+ Th cells assist in B-cell activation. Antigen-driven
B-cell activation and proliferation in the germinal centre are
accompanied by an isotype switch of the immunoglobulin class
synthesized by the B cell. In addition, the affinity of the antibody
increases as a result of somatic mutation (Kocks & Rajewski, 1989). A
kind of selection mechanism has been proposed in this antigen-driven
process, in which cells that produce antibody of higher affinity are
selected preferentially, and cells that produce antibody of lower
affinity are not selected and subsequently die, perhaps by apoptosis
(programmed cell death) (Liu et al., 1989). This selection resembles
that of developing T lymphocytes in the thymus; it differs from T-cell
selection by the absence of negative selection and the occurrence of
somatic mutation. Antigen may remain in the follicular compartment for
quite some time, thereby causing persistent activation of B cells,
related to the state of immunological memory within the B-cell
population. After the antigen disappears, the immunological memory in
the B cells is short-lived and disappears. B-Cell activation in
germinal centres leads to activated cells with a specific morphology,
the so-called centrocytes and centroblasts. Finally, B-cell activation
leads to the formation of plasma cells, both in the periphery of
germinal centres but more predominantly in the medullary cords of the
lymph nodes.
The main site of effector immune reactions is the medulla. The
medullary cords house macrophages, granulocytes, activated effector T
cells, and plasma cells. The effector T cells include CD4+ delayed-
type hypersensitivity T cells (mediator-producing cells) and CD8+ Tc
cells. The Tc cells bear an alpha-ß TCR that recognizes antigen in the
context of the polymorphic determinant of MHC class I molecules. The
CD8 molecule has an accessory function in this process, since it binds
a non-polymorphic class I determinant (Janeway, 1992). Antigen
recognition by Tc cells is thus different from that by Th cells. The
reaction products of the effector cells, such as lymphokines, and
effector cells like plasma cell precursors leave the lymph nodes via
the efferent lymph or blood circulation to go to other sites of the
body.
Development and aging: Lymph node morphology is dynamic: its
appearance throughout life is directly related to the type and amount
of antigenic stimulation. After antigenic contact, the organ increases
in size within a relatively short time, with high proliferative
activity of lymphocytes and germinal centre formation, depending on
the type of reaction and the choice of immunological reaction. In the
case of B-lymphocyte reactions, hyperplasia of follicles is seen (e.g.
after bacterial infection); in the case of T-cell reactions, the
interfollicular areas or paracortex become enlarged (e.g. in viral
infection). After the reaction is terminated or is transferred to the
next draining lymph node, it regains its normal small size. Germinal
centres with an interfollicular microenvironment can develop
extranodally, especially at sites of chronic inflammation.
The dynamics of the lymph node can be illustrated by several
examples. After immunization of the footpad with antigen mixed with an
adjuvant, such as Freund's complete adjuvant, containing killed
mycobacteria, the draining popliteal lymph node becomes enlarged (in
rats, from about 5 mg to more than 100 mg), and granulomatous
reactions (epitheloid-cell granuloma) can be seen histologically. The
swelling of lymph nodes is used to assess reactivity towards chemicals
and in the evaluation of immunomodulatory drugs (Gleichmann et al.,
1989). In the popliteal lymph node assay, a test substance is injected
subcutaneously into one footpad and the contralateral side is left
untreated or injected with vehicle only. The effect of the substance
is subsequently estimated from the difference in weight between the
popliteal lymph nodes. Further evidence for immunostimulatory activity
in vivo is obtained by histological appearance, often manifested as
follicular hyperplasia.
Lymph nodes develop relatively late in fetal life: At birth, the
anlage of unstimulated lymph nodes is present, containing few lymph
cells. The lymph nodes develop quickly after exposure to many new
(exogenous) antigens. In adults, they may become relatively quiescent
and small, with virgin T and B cells and primary follicles. The lymph
nodes in aged rats are capable of the same degree of activity as those
in young individuals upon antigenic stimuli. The state and type of
activation in the various nodes of adult and aged animals differ under
normal housing conditions and are a reflection of the absence or
existence of continuous local stimulation with antigens or disease
processes, like inflammation and the presence of a tumour in the
drained area (Ward, 1990). The central nodes of the mandibular and
superficial cervical group, which are continuously exposed to
(aero)antigens via the oronasopharynx, may contain a considerable
number of plasma cells and precursors in the medullary cords and
relatively well-developed germinal centres. Sinal histiocytosis (that
is, considerable numbers of macrophages in the sinuses) and
accumulations of pigmented macrophages are often present in the
mesenteric lymph nodes, which are continuously exposed to antigens via
the digestive tract. An extensive review of lymph node development and
aging is given by Losco & Harleman (1992).
1.2.2.5 Spleen
The spleen consists of two main compartments: the red and white
pulp (Van Rooijen et al., 1989; Dijkstra & Sminia, 1990; Laman et al.,
1992; Van den Eertwegh et al., 1992). A schematic drawing of the
spleen is presented in Figure 13 and histological views in Figure 14.
The red pulp consists of blood-filled sinusoids and Bilroth's cords
containing macrophages, lymphocytes, plasma cells, and NK cells.
Macrophages perform major functions in clearing blood cells (for
instance, old red blood cells) and in phagocytosis, especially of non-
opsonized particles. This high-volume filter function is made possible
by two factors: the direct contact, unobstructed by blood-vessel
walls, between phagocytic cells and blood-borne particles; and the
large blood supply, estimated at about 5% of the total blood volume
per minute. There are no lymphatics in the spleen.
The phagocytic function is especially important in the case of
intravascular pathogenic microorganisms, before antibody formation and
subsequent opsonization occur (early bacterial septicaemia). The
mononuclear phagocyte system of the liver (Kupffer cells) plays a
major role in the removal of opsonized particles. Together with the
hepatic phagocytic system, splenic macrophages synthesize complement
components, although this is done mainly by hepatocytes. In rats and
mice, the red pulp contains nests of (extramedullary) haematopoiesis,
characterized histologically by megakaryocytes and normoblasts. In the
case of systemic septicaemia, when pathogenic microorganisms have
reached the blood either directly or after inadequate filtering
through lymph nodes, the red pulp increases and contains large
proportions of (immature) granulocytes. Differentiation between
septicaemia and extramedullary haematopoiesis is not always easy; the
decreased or absent white pulp in septicaemia can be helpful in making
this differentiation.
Phylogenetically, cartilaginous fish are the first species that
have a spleen, which consists of lymphoid follicles and a red pulp
that generally houses developing erythroid cells (Zapata & Cooper,
1990). The lympho-haematopoietic masses of the intestine that are seen
in some lower vertebrates (e.g. lampreys) are not primitive spleens
but rather primitive lymphohaematopoietic organs equivalent to
mammalian bone marrow. In most bony fishes, the white pulp is poorly
developed, probably reflecting the existence of other peripheral
lymphoid organs, e.g. the kidney, which participate actively in the
mainly immediately underneath the capsule; they present as
accumulations of small lymphocytes (primary follicle) or a pale-
stained centre with large lymphoid cells (centrocytes, centroblasts)
and tingible-body macro-phages surrounded by a mantle with small
lymphocytes (secondary follicles). The interfollicular areas are
continuous with the paracortex, and the latter is continuous with the
medullary cords.
The arterial blood supply enters the node at the medulla and ends
in the paracortex as arteriolar capillaries, with branches in the
follicles. The capillaries feed venules that are lined with high
endothelial cells. The high endothelial venules run from the
paracortex into the medullary cords and then leave the node via the
vein in the hilus. Lymphocytes migrate through the high endothelial
venules after adhering to the endothelium by specific receptor-ligand
interactions (Picker & Butcher, 1992). The adhesion molecules on
lymphocytes and endothelium that are involved in this binding and
subsequent passage thought the endothelial layer are the addressins,
reflecting the difference in receptor-ligand interactions that exists
between lymph nodes of the internal and external lymphoid systems.
Subsequently, lymphocytes can specifically reach the various nodes,
using the same route (the blood). After the lymphocytes have migrated
into the parenchyma, they move into their microenvironment or
compartment.
Antigen encounter and immune reactivity: The main route of
access for antigens and pathogens is the afferent lymph flow; antigens
can also come into contact with the lymph node tissues via the blood.
Antigens in the lymph, either free or processed by veiled macrophages,
enter the node through the subcapsular sinus, which is rich in
macrophages that can phagocytose free antigen. From there, antigens
move to the paracortex, where they are presented to CD4+ Th cells by
the antigen-presenting cells for initiation of the immune response.
The main antigen-presenting cells in the lymph node are the
interdigitating dendritic cells, the tissue equivalents of veiled
macrophages, which can arise from Langerhans cells in the skin (for
e.g. lymph nodes draining the skin). The antigen-presenting cells
express MHC class II antigens in high density, enabling the alpha-ß
TCR of the Th cells to recognize the antigenic determinant complexed
with the polymorphic ('self') MHC class II molecule. The CD4+
molecule on the Th cell surface binds to a non-polymorphic determinant
of MHC class II molecules and strengthens the binding between Th and
antigen-presenting cells (Janeway, 1992). The cellular interaction
triggers the synthesis of cytokines like IL-1 and IL-2. This process
is down-regulated by Ts cells in a way that is not yet completely
understood.
Follicles are involved in antigen-driven B-cell activation,
somatic mutation, positive and negative selection, and memory and
plasma cell development (Szakal et al., 1989; Kroese et al., 1990; Liu
et al., 1992) and are known as primary follicles in the resting state.
They contain small, IgM+IgD+ virgin B cells in a framework of
follicular dendritic cells. During stimulation by antigens, the
follicles change into secondary follicles consisting of a germinal
centre surrounded by a mantle. Antigen may be transported into the
follicle by immune (T) cells, but this route is not yet fully
established. Antigen is presented to B cells in immune complexes, with
complement split products like C3b, which are trapped in cytoplasmic
extensions of the follicular dendritic cells. The interaction between
complement and complement receptors on these cells has a pivotal role
in the adherence of antigen to them. Fc receptors also play a role,
but only in rodents. The complement split products in the trapped
immune complexes have an accessory function in antigen presentation.
Local CD4+ Th cells assist in B-cell activation. Antigen-driven
B-cell activation and proliferation in the germinal centre are
accompanied by an isotype switch of the immunoglobulin class
synthesized by the B cell. In addition, the affinity of the antibody
increases as a result of somatic mutation (Kocks & Rajewski, 1989). A
kind of selection mechanism has been proposed in this antigen-driven
process, in which cells that produce antibody of higher affinity are
selected preferentially, and cells that produce antibody of lower
affinity are not selected and subsequently die, perhaps by apoptosis
(programmed cell death) (Liu et al., 1989). This selection resembles
that of developing T lymphocytes in the thymus; it differs from T-cell
selection by the absence of negative selection and the occurrence of
somatic mutation. Antigen may remain in the follicular compartment for
quite some time, thereby causing persistent activation of B cells,
related to the state of immunological memory within the B-cell
population. After the antigen disappears, the immunological memory in
the B cells is short-lived and disappears. B-Cell activation in
germinal centres leads to activated cells with a specific morphology,
the so-called centrocytes and centroblasts. Finally, B-cell activation
leads to the formation of plasma cells, both in the periphery of
germinal centres but more predominantly in the medullary cords of the
lymph nodes.
The main site of effector immune reactions is the medulla. The
medullary cords house macrophages, granulocytes, activated effector T
cells, and plasma cells. The effector T cells include CD4+ delayed-
type hypersensitivity T cells (mediator-producing cells) and CD8+ Tc
cells. The Tc cells bear an alpha-ß TCR that recognizes antigen in the
context of the polymorphic determinant of MHC class I molecules. The
CD8 molecule has an accessory function in this process, since it binds
a non-polymorphic class I determinant (Janeway, 1992). Antigen
recognition by Tc cells is thus different from that by Th cells. The
reaction products of the effector cells, such as lymphokines, and
effector cells like plasma cell precursors leave the lymph nodes via
the efferent lymph or blood circulation to go to other sites of the
body.
Development and aging: Lymph node morphology is dynamic: its
appearance throughout life is directly related to the type and amount
of antigenic stimulation. After antigenic contact, the organ increases
in size within a relatively short time, with high proliferative
activity of lymphocytes and germinal centre formation, depending on
the type of reaction and the choice of immunological reaction. In the
case of B-lymphocyte reactions, hyperplasia of follicles is seen (e.g.
after bacterial infection); in the case of T-cell reactions, the
interfollicular areas or paracortex become enlarged (e.g. in viral
infection). After the reaction is terminated or is transferred to the
next draining lymph node, it regains its normal small size. Germinal
centres with an interfollicular microenvironment can develop
extranodally, especially at sites of chronic inflammation.
The dynamics of the lymph node can be illustrated by several
examples. After immunization of the footpad with antigen mixed with an
adjuvant, such as Freund's complete adjuvant, containing killed
mycobacteria, the draining popliteal lymph node becomes enlarged (in
rats, from about 5 mg to more than 100 mg), and granulomatous
reactions (epitheloid-cell granuloma) can be seen histologically. The
swelling of lymph nodes is used to assess reactivity towards chemicals
and in the evaluation of immunomodulatory drugs (Gleichmann et al.,
1989). In the popliteal lymph node assay, a test substance is injected
subcutaneously into one footpad and the contralateral side is left
untreated or injected with vehicle only. The effect of the substance
is subsequently estimated from the difference in weight between the
popliteal lymph nodes. Further evidence for immunostimulatory activity
in vivo is obtained by histological appearance, often manifested as
follicular hyperplasia.
Lymph nodes develop relatively late in fetal life: At birth, the
anlage of unstimulated lymph nodes is present, containing few lymph
cells. The lymph nodes develop quickly after exposure to many new
(exogenous) antigens. In adults, they may become relatively quiescent
and small, with virgin T and B cells and primary follicles. The lymph
nodes in aged rats are capable of the same degree of activity as those
in young individuals upon antigenic stimuli. The state and type of
activation in the various nodes of adult and aged animals differ under
normal housing conditions and are a reflection of the absence or
existence of continuous local stimulation with antigens or disease
processes, like inflammation and the presence of a tumour in the
drained area (Ward, 1990). The central nodes of the mandibular and
superficial cervical group, which are continuously exposed to
(aero)antigens via the oronasopharynx, may contain a considerable
number of plasma cells and precursors in the medullary cords and
relatively well-developed germinal centres. Sinal histiocytosis (that
is, considerable numbers of macrophages in the sinuses) and
accumulations of pigmented macrophages are often present in the
mesenteric lymph nodes, which are continuously exposed to antigens via
the digestive tract. An extensive review of lymph node development and
aging is given by Losco & Harleman (1992).
1.2.2.5 Spleen
The spleen consists of two main compartments: the red and white
pulp (Van Rooijen et al., 1989; Dijkstra & Sminia, 1990; Laman et al.,
1992; Van den Eertwegh et al., 1992). A schematic drawing of the
spleen is presented in Figure 13 and histological views in Figure 14.
The red pulp consists of blood-filled sinusoids and Bilroth's cords
containing macrophages, lymphocytes, plasma cells, and NK cells.
Macrophages perform major functions in clearing blood cells (for
instance, old red blood cells) and in phagocytosis, especially of non-
opsonized particles. This high-volume filter function is made possible
by two factors: the direct contact, unobstructed by blood-vessel
walls, between phagocytic cells and blood-borne particles; and the
large blood supply, estimated at about 5% of the total blood volume
per minute. There are no lymphatics in the spleen.
The phagocytic function is especially important in the case of
intravascular pathogenic microorganisms, before antibody formation and
subsequent opsonization occur (early bacterial septicaemia). The
mononuclear phagocyte system of the liver (Kupffer cells) plays a
major role in the removal of opsonized particles. Together with the
hepatic phagocytic system, splenic macrophages synthesize complement
components, although this is done mainly by hepatocytes. In rats and
mice, the red pulp contains nests of (extramedullary) haematopoiesis,
characterized histologically by megakaryocytes and normoblasts. In the
case of systemic septicaemia, when pathogenic microorganisms have
reached the blood either directly or after inadequate filtering
through lymph nodes, the red pulp increases and contains large
proportions of (immature) granulocytes. Differentiation between
septicaemia and extramedullary haematopoiesis is not always easy; the
decreased or absent white pulp in septicaemia can be helpful in making
this differentiation.
Phylogenetically, cartilaginous fish are the first species that
have a spleen, which consists of lymphoid follicles and a red pulp
that generally houses developing erythroid cells (Zapata & Cooper,
1990). The lympho-haematopoietic masses of the intestine that are seen
in some lower vertebrates (e.g. lampreys) are not primitive spleens
but rather primitive lymphohaematopoietic organs equivalent to
mammalian bone marrow. In most bony fishes, the white pulp is poorly
developed, probably reflecting the existence of other peripheral
lymphoid organs, e.g. the kidney, which participate actively in the


 immune response. After antigenic stimulation, the amount of splenic
lymphoid tissue increases considerably in all lower vertebrates,
although germinal centres do not occur. At the cellular level,
however, antigen-presenting cells and cells retaining immune complexes
on their surface have been described in the spleen of some bony
fishes, anurous species, and reptiles.
White pulp: The spleen contains about one-quarter of the body's
total lymphocyte population; during lymphocyte recirculation, more
cells pass through the spleen than through all the lymph nodes.
Lymphocytes in the spleen reside in the white pulp, which consists of
a central arteriole surrounded by the periarteriolar lymphocyte
sheath, a T-lymphocyte area. The outer sheath contains B lymphocytes
and, after antigenic stimulation, plasma cells. Adjacent follicles
contain B cells. Around the periarteriolar lymphocyte sheath and
follicles is a corona containing B cells, called the marginal zone;
this region is easily distinguished, especially in rats. The
periarteriolar lymphocyte sheath has a microenvironment and a
passenger leukocyte content similar to that of the lymph node
paracortex. Some sources claim that the spleen is a rich source of Ts
cell activity, exceeding that of lymph nodes. The follicles are not
essentially different in structure and function from those of lymph
nodes. The spleen performs a major function in humoral immunity by
synthesizing IgM class antibodies, especially to blood-borne antigens.
The microenvironment of the marginal zone is unique to the
spleen. Histologically, the B cells at this site are of medium size;
on histological staining, they are larger and paler than B cells in
primary follicles and in the follicular mantle of secondary follicles
(Figure 14). In addition, they do not show the morphology of the
centrocytes or centroblasts found in germinal centres, and the
phenotypic expression (surface IgM+IgD-) indicates that the B
cells in the marginal zone are a separate population. Special
macrophage types are present, which are known as marginal zone
macrophages and marginal metallophilic macrophages. The latter are
located at the periphery of the white pulp, along the inner border of
the marginal sinus, and can be stained by silver impregnation.
The physiological function of the marginal zone has been
characterized recently. First, the site retains B-lymphocyte memory;
second, it mediates humoral responses that do not directly involve
T cells. These T-independent responses are elicited by polysaccharide
antigens of encapsulated bacteria, which are present in repeating
units on the microorganism and are presented to the B cells by
marginal zone macrophages. The response may not be completely T cell-
independent in all cases, as T cell-derived factors enhance the
response to some of these antigens. The antibodies generated are
mainly of the IgM class, as T-cell help is required for an isotype
switch.
In conclusion, the main immunological function of the spleen is
to defend the body's vascular compartment by generating T cell-
independent IgM antibody responses to bacterial polysaccharides and by
exerting an enormous phagocytic power. This function is lost after
splenectomy, when reduced nonspecific phagocytosis of non-opsonized
particles, lowered serum IgM levels, and increased susceptibility to
infections by encapsulated bacteria have been described.
1.2.2.6 Mucosa-associated lymphoid tissue
The secretory epithelial surfaces of the body form a major route
of entry for potentially pathogenic substances. These surfaces include
the epithelia of the gastrointestinal, upper and lower respiratory,
and urogenital tracts (Miller & Nicklin, 1987; Sminia et al., 1989).
The host response at these locations ranges from nonspecific
constituents, such as a physical or mechanical component (epithelial
barrier, motility of the gastrointestinal tract, and the mucociliary
escalator in the respiratory tract), to a chemical component (low
gastric pH, mucus, lysosomal and digestive enzymes), and antigen-
specific components of the immune system.
Nonspecific killer cells are found in significant numbers in the
lungs and along the epithelium of the gastrointestinal tract, where
lymphocyte-like cells have been found to kill pathogens, presumably
without prior sensitization (Hanglow et al., 1990). In mice, these
cells have been characterized as T cells with a gamma-delta TCR,
which, in contrast to Tc cells that express alpha-ß TCR, kill targets
in an MHC-nonrestricted manner (Raulet, 1989). The cells have antigen
specificity that is encoded at the DNA level by variable gene
segments, but the repertoire appears to be smaller than that which
encodes TCR alpha or ß chains. These gamma-delta T killer cells are
not generated under the strict influence of the thymus, as are their
alpha-ß T-cell counterparts (Bell, 1989; Haas et al., 1993). Apart
from their killer activity, these cells may serve as inducing elements
for the response mediated by alpha-ß TCR-expressing T subsets. In this
initiating activity, gamma-delta TCR molecules shed from the
lymphocyte surface may act as antigen-specific factors (De Weger et
al., 1989).
Lymphoid tissue: Lymphoid tissue occurs just underneath the
secretory epithelium, in the duodenum and jejunum as Peyer's patches
(Figure 15), in the appendix of the large intestine, along the bronchi
(Sminia et al., 1989), and in the oro- and nasopharyngeal regions
(Kuper et al., 1992b). These mucosal lymphoid tissues share structural
and functional characteristics and are strongly interrelated. The
common designation 'mucosa-associated lymphoid tissue' (MALT) is
therefore used to refer to bronchus-associated, gut-associated, and
nasal-associated (nasal cavity and nasopharynx) lymphoid tissue.
Nasal-associated lymphoid tissue has been identified in horses,
monkeys, and rats (Figure 16) (Kuper et al., 1990b). In humans and
domestic animals, the larger lymphoid nodules in the pharyngeal region
are called tonsils. Together with the intermediate lymphoid tissue,
the tonsils form Waldeyer's tonsillar ring.
The organization of MALT is similar to that of lymph nodes, with
B cell-containing follicles and T cell-containing interfollicular
areas. Afferent lymph vessels are lacking, because pathogens can enter
the tissue through the covering epithelial layer. The epithelial cells
at this location (the 'M' or microfold cells) are often thinner than
those at other secretory sites, in order to enable efficient passage
of antigens. Stimulated gut-associated lymphoid tissue and human
tonsils often have prominent follicles with germinal centres. In
contrast, germinal centres are scarce in stimulated bronchus-
associated and nasal-associated lymphoid tissue in rodents, due to the
fact that immunological reactions occur mainly in the draining
cervical lymph node. Th medulla-like areas seen in lymph nodes are
absent in MALT. Lymphocytes and NK cells are found in the lymphoid
tissue, in interstitial tissue in the lung, and in the lamina propria
along the gastrointestinal tract.
The homing specificity of lymphocytes into lymphoid tissue by
migration through high endothelial venules is described above. The
specificity of the homing phenomenon to MALT has the advantage that
the same circulation pathway (i.e. the blood) is used by the secretory
and internal immune system (with the intrinsic possibility of mutual
contact). In addition, the antigen message received at one secretory
site is followed by effects at all secretory surfaces. Thus, after
antigen presentation in the gastrointestinal tract, effector cells
(e.g. IgA antibody-synthesizing plasma cells, see below) are found at
the site of stimulation and at other secretory sites (e.g. the
respiratory tract). Thus, the major function of MALT is to initiate
immune responses, which are then passed on to draining lymph nodes,
such as mesenteric lymph nodes in the gastrointestinal tract.
The secretory IgA antibody response: The immune response in
MALT differs from that at other sites of the body in that it is
devoted to the generation of an IgA antibody response. Thus, MALT
contains precursors of IgA antibody plasma cells and populations of T
cells capable of promoting a B-cell immunoglobulin class switch into
IgA-producing B cells or plasma cells. B-Cell differentiation into
IgA-producing plasma cells after local antigen presentation is
accompanied by lymphocyte migration and specific homing. Precursors
move through draining lymph nodes into the blood and from there to the
secretory surface, where they lodge as IgA plasma cells in the lamina
propria. Specific homing mechanisms exist by which these cells are
able to select the secretory surface of their final mucosal
destination.
immune response. After antigenic stimulation, the amount of splenic
lymphoid tissue increases considerably in all lower vertebrates,
although germinal centres do not occur. At the cellular level,
however, antigen-presenting cells and cells retaining immune complexes
on their surface have been described in the spleen of some bony
fishes, anurous species, and reptiles.
White pulp: The spleen contains about one-quarter of the body's
total lymphocyte population; during lymphocyte recirculation, more
cells pass through the spleen than through all the lymph nodes.
Lymphocytes in the spleen reside in the white pulp, which consists of
a central arteriole surrounded by the periarteriolar lymphocyte
sheath, a T-lymphocyte area. The outer sheath contains B lymphocytes
and, after antigenic stimulation, plasma cells. Adjacent follicles
contain B cells. Around the periarteriolar lymphocyte sheath and
follicles is a corona containing B cells, called the marginal zone;
this region is easily distinguished, especially in rats. The
periarteriolar lymphocyte sheath has a microenvironment and a
passenger leukocyte content similar to that of the lymph node
paracortex. Some sources claim that the spleen is a rich source of Ts
cell activity, exceeding that of lymph nodes. The follicles are not
essentially different in structure and function from those of lymph
nodes. The spleen performs a major function in humoral immunity by
synthesizing IgM class antibodies, especially to blood-borne antigens.
The microenvironment of the marginal zone is unique to the
spleen. Histologically, the B cells at this site are of medium size;
on histological staining, they are larger and paler than B cells in
primary follicles and in the follicular mantle of secondary follicles
(Figure 14). In addition, they do not show the morphology of the
centrocytes or centroblasts found in germinal centres, and the
phenotypic expression (surface IgM+IgD-) indicates that the B
cells in the marginal zone are a separate population. Special
macrophage types are present, which are known as marginal zone
macrophages and marginal metallophilic macrophages. The latter are
located at the periphery of the white pulp, along the inner border of
the marginal sinus, and can be stained by silver impregnation.
The physiological function of the marginal zone has been
characterized recently. First, the site retains B-lymphocyte memory;
second, it mediates humoral responses that do not directly involve
T cells. These T-independent responses are elicited by polysaccharide
antigens of encapsulated bacteria, which are present in repeating
units on the microorganism and are presented to the B cells by
marginal zone macrophages. The response may not be completely T cell-
independent in all cases, as T cell-derived factors enhance the
response to some of these antigens. The antibodies generated are
mainly of the IgM class, as T-cell help is required for an isotype
switch.
In conclusion, the main immunological function of the spleen is
to defend the body's vascular compartment by generating T cell-
independent IgM antibody responses to bacterial polysaccharides and by
exerting an enormous phagocytic power. This function is lost after
splenectomy, when reduced nonspecific phagocytosis of non-opsonized
particles, lowered serum IgM levels, and increased susceptibility to
infections by encapsulated bacteria have been described.
1.2.2.6 Mucosa-associated lymphoid tissue
The secretory epithelial surfaces of the body form a major route
of entry for potentially pathogenic substances. These surfaces include
the epithelia of the gastrointestinal, upper and lower respiratory,
and urogenital tracts (Miller & Nicklin, 1987; Sminia et al., 1989).
The host response at these locations ranges from nonspecific
constituents, such as a physical or mechanical component (epithelial
barrier, motility of the gastrointestinal tract, and the mucociliary
escalator in the respiratory tract), to a chemical component (low
gastric pH, mucus, lysosomal and digestive enzymes), and antigen-
specific components of the immune system.
Nonspecific killer cells are found in significant numbers in the
lungs and along the epithelium of the gastrointestinal tract, where
lymphocyte-like cells have been found to kill pathogens, presumably
without prior sensitization (Hanglow et al., 1990). In mice, these
cells have been characterized as T cells with a gamma-delta TCR,
which, in contrast to Tc cells that express alpha-ß TCR, kill targets
in an MHC-nonrestricted manner (Raulet, 1989). The cells have antigen
specificity that is encoded at the DNA level by variable gene
segments, but the repertoire appears to be smaller than that which
encodes TCR alpha or ß chains. These gamma-delta T killer cells are
not generated under the strict influence of the thymus, as are their
alpha-ß T-cell counterparts (Bell, 1989; Haas et al., 1993). Apart
from their killer activity, these cells may serve as inducing elements
for the response mediated by alpha-ß TCR-expressing T subsets. In this
initiating activity, gamma-delta TCR molecules shed from the
lymphocyte surface may act as antigen-specific factors (De Weger et
al., 1989).
Lymphoid tissue: Lymphoid tissue occurs just underneath the
secretory epithelium, in the duodenum and jejunum as Peyer's patches
(Figure 15), in the appendix of the large intestine, along the bronchi
(Sminia et al., 1989), and in the oro- and nasopharyngeal regions
(Kuper et al., 1992b). These mucosal lymphoid tissues share structural
and functional characteristics and are strongly interrelated. The
common designation 'mucosa-associated lymphoid tissue' (MALT) is
therefore used to refer to bronchus-associated, gut-associated, and
nasal-associated (nasal cavity and nasopharynx) lymphoid tissue.
Nasal-associated lymphoid tissue has been identified in horses,
monkeys, and rats (Figure 16) (Kuper et al., 1990b). In humans and
domestic animals, the larger lymphoid nodules in the pharyngeal region
are called tonsils. Together with the intermediate lymphoid tissue,
the tonsils form Waldeyer's tonsillar ring.
The organization of MALT is similar to that of lymph nodes, with
B cell-containing follicles and T cell-containing interfollicular
areas. Afferent lymph vessels are lacking, because pathogens can enter
the tissue through the covering epithelial layer. The epithelial cells
at this location (the 'M' or microfold cells) are often thinner than
those at other secretory sites, in order to enable efficient passage
of antigens. Stimulated gut-associated lymphoid tissue and human
tonsils often have prominent follicles with germinal centres. In
contrast, germinal centres are scarce in stimulated bronchus-
associated and nasal-associated lymphoid tissue in rodents, due to the
fact that immunological reactions occur mainly in the draining
cervical lymph node. Th medulla-like areas seen in lymph nodes are
absent in MALT. Lymphocytes and NK cells are found in the lymphoid
tissue, in interstitial tissue in the lung, and in the lamina propria
along the gastrointestinal tract.
The homing specificity of lymphocytes into lymphoid tissue by
migration through high endothelial venules is described above. The
specificity of the homing phenomenon to MALT has the advantage that
the same circulation pathway (i.e. the blood) is used by the secretory
and internal immune system (with the intrinsic possibility of mutual
contact). In addition, the antigen message received at one secretory
site is followed by effects at all secretory surfaces. Thus, after
antigen presentation in the gastrointestinal tract, effector cells
(e.g. IgA antibody-synthesizing plasma cells, see below) are found at
the site of stimulation and at other secretory sites (e.g. the
respiratory tract). Thus, the major function of MALT is to initiate
immune responses, which are then passed on to draining lymph nodes,
such as mesenteric lymph nodes in the gastrointestinal tract.
The secretory IgA antibody response: The immune response in
MALT differs from that at other sites of the body in that it is
devoted to the generation of an IgA antibody response. Thus, MALT
contains precursors of IgA antibody plasma cells and populations of T
cells capable of promoting a B-cell immunoglobulin class switch into
IgA-producing B cells or plasma cells. B-Cell differentiation into
IgA-producing plasma cells after local antigen presentation is
accompanied by lymphocyte migration and specific homing. Precursors
move through draining lymph nodes into the blood and from there to the
secretory surface, where they lodge as IgA plasma cells in the lamina
propria. Specific homing mechanisms exist by which these cells are
able to select the secretory surface of their final mucosal
destination.

 In contrast to IgA produced by the bone marrow and circulating in
blood, IgA synthesized by plasma cells of MALT consists of a dimeric
immunoglobulin subunit. The two monomers are linked by a polypeptide
called the J chain (about 15 kDa). These IgA antibodies have their
main effect outside the body itself, for example in salivary and
gastrointestinal secretions. The transport from the site of synthesis
across the epithelial barrier is specifically adapted for dimeric IgA,
and to a lesser extent for pentameric IgM. Epithelial cells express a
receptor for these immunoglobulins, called secretory component
(a polypeptide of about 70 kDa). After binding to this receptor, the
molecule is transported through the epithelium, possibly through its
cytoplasm, and excreted on the luminal surface. During this process,
secretory component attaches to the immunoglobulin molecule (coiled
around the Fc fragments); the composite molecule, comprising dimeric
IgA, the J chain, and secretory component, is called secretory IgA. In
rodents, a similar secretory component-mediated transport occurs in
the liver (Brown & Kloppel, 1989). Here, a secretory component on the
hepatocyte surface mediates the passage of dimeric IgA from the
sinusoids to the bile canaliculi. In this way, dimeric IgA entering
the liver by the portal vein efficiently recirculates to the bile and
from there into the gastrointestinal lumen. Secretory IgA is more
resistant to luminal conditions (especially proteolytic enzymes) than
dimeric IgA and is thus better able to function there.
IgA lacks the effector reactivity of IgM and IgG in complement
activation by the classical cascade, opsonization and phagocytosis, or
antibody-mediated cellular cytotoxicity. This lack appears to be
related to the absence of effector systems (complement, phagocytes) in
secretory fluid. The main function of IgA is to prevent the entry of
potentially pathogenic substances into the body, by a specific antigen
exclusion function in which the epithelium is coated with 'antiseptic
paint'.
Induction of immunological tolerance: A final feature of MALT
is its capacity to generate immunological tolerance. After antigenic
priming at secretory surfaces, subsequent systemic antigenic challenge
often results in nonresponsiveness (Strobel & Ferguson, 1984;
Challacombe, 1987; Holt & Sedgwick, 1987; Mowat, 1987). Suppressor
cells have been found in the spleen and suppressor factors in the
circulation after local immunization. The induction of tolerance
pertains primarily to dead microorganisms and inactivated proteins
which come into contact with the MALT. The mechanism of tolerance
induction and different responses to live and dead microorganisms is
not completely defined but is important in tolerance to food antigens
and the development of food allergies.
In summary, the host's defence in MALT is different from the
response of the internal immune system. The responses at this first
line of defence range from NK cell activity in the epithelium to
specific IgA antibody-mediated exclusion in the secretory fluid.
Immune responses to antigens entering the body at secretory sites are
initiated by lymphocytes in the epithelium, in lymphoid tissue
immediately underneath the epithelium, and in the lymph nodes that
drain the site (e.g. the mesenteric node). The liver may also
contribute to the response, as antigens passing directly into the
portal vein are efficiently removed and processed by the hepatic
mononuclear phagocyte system (Kupffer cells). Because MALT can
function independently of the internal immune system, blood analysis
alone may not provide complete information on MALT. Instead, analysis
of secretory fluids, such as saliva (for IgA antibody), bronchoalveolar
lavage fluid, and jejunal fluid, or direct investigation of the tissue
itself, are more appropriate approaches.
1.2.2.7 Skin immune system or skin-associated lymphoid tissue
As the skin is the largest organ of the body, its principal
physical function is to act as a barrier to water-soluble compounds,
to mechanical trauma, and to trauma caused by potentially pathogenic
microorganisms and the photons of sunlight. The physicochemical
characteristics of the outermost layer, the corneal or horny substance
of the epidermis, underlie the resistance to exogenous pathogenic
substances. The skin also has a host defence function that can be
designated as immunological. Some studies have suggested that the skin
might function as a primary organ (Fichtelius et al., 1970; Bos &
Kapsenberg, 1986), but most of the relevant immune reactions in the
skin appear to be antigen driven. The components of the skin immune
system, or skin-associated lymphoid tissue, are the following
(Streilein, 1983, 1990) (Figure 17): (i) Langerhans cells in the
epidermis, which are adapted for processing antigen and transporting
it to the draining lymph node, where they are called interdigitating
cells and present the antigen to lymphocytes; (ii) epider-motrophic
recirculating T lymphocyte subpopulations (homing T lymphocytes);
(iii) keratinocytes, which can synthesize cytokines after activation,
thereby influencing T-cell differentiation and haematopoiesis; they
can have an antigen-presenting function, especially after activation
resulting in MHC class II expression; (iv) Thy-1+ dendritic epidermal
cells, described in rodent skin epidermis: a special T cell that bears
the gamma-delta TCR and has an antigen-presenting function; and (v)
skin-draining lymph nodes comprising high endothelial venules through
which lymphocytes enter from the blood circulation.
In contrast to IgA produced by the bone marrow and circulating in
blood, IgA synthesized by plasma cells of MALT consists of a dimeric
immunoglobulin subunit. The two monomers are linked by a polypeptide
called the J chain (about 15 kDa). These IgA antibodies have their
main effect outside the body itself, for example in salivary and
gastrointestinal secretions. The transport from the site of synthesis
across the epithelial barrier is specifically adapted for dimeric IgA,
and to a lesser extent for pentameric IgM. Epithelial cells express a
receptor for these immunoglobulins, called secretory component
(a polypeptide of about 70 kDa). After binding to this receptor, the
molecule is transported through the epithelium, possibly through its
cytoplasm, and excreted on the luminal surface. During this process,
secretory component attaches to the immunoglobulin molecule (coiled
around the Fc fragments); the composite molecule, comprising dimeric
IgA, the J chain, and secretory component, is called secretory IgA. In
rodents, a similar secretory component-mediated transport occurs in
the liver (Brown & Kloppel, 1989). Here, a secretory component on the
hepatocyte surface mediates the passage of dimeric IgA from the
sinusoids to the bile canaliculi. In this way, dimeric IgA entering
the liver by the portal vein efficiently recirculates to the bile and
from there into the gastrointestinal lumen. Secretory IgA is more
resistant to luminal conditions (especially proteolytic enzymes) than
dimeric IgA and is thus better able to function there.
IgA lacks the effector reactivity of IgM and IgG in complement
activation by the classical cascade, opsonization and phagocytosis, or
antibody-mediated cellular cytotoxicity. This lack appears to be
related to the absence of effector systems (complement, phagocytes) in
secretory fluid. The main function of IgA is to prevent the entry of
potentially pathogenic substances into the body, by a specific antigen
exclusion function in which the epithelium is coated with 'antiseptic
paint'.
Induction of immunological tolerance: A final feature of MALT
is its capacity to generate immunological tolerance. After antigenic
priming at secretory surfaces, subsequent systemic antigenic challenge
often results in nonresponsiveness (Strobel & Ferguson, 1984;
Challacombe, 1987; Holt & Sedgwick, 1987; Mowat, 1987). Suppressor
cells have been found in the spleen and suppressor factors in the
circulation after local immunization. The induction of tolerance
pertains primarily to dead microorganisms and inactivated proteins
which come into contact with the MALT. The mechanism of tolerance
induction and different responses to live and dead microorganisms is
not completely defined but is important in tolerance to food antigens
and the development of food allergies.
In summary, the host's defence in MALT is different from the
response of the internal immune system. The responses at this first
line of defence range from NK cell activity in the epithelium to
specific IgA antibody-mediated exclusion in the secretory fluid.
Immune responses to antigens entering the body at secretory sites are
initiated by lymphocytes in the epithelium, in lymphoid tissue
immediately underneath the epithelium, and in the lymph nodes that
drain the site (e.g. the mesenteric node). The liver may also
contribute to the response, as antigens passing directly into the
portal vein are efficiently removed and processed by the hepatic
mononuclear phagocyte system (Kupffer cells). Because MALT can
function independently of the internal immune system, blood analysis
alone may not provide complete information on MALT. Instead, analysis
of secretory fluids, such as saliva (for IgA antibody), bronchoalveolar
lavage fluid, and jejunal fluid, or direct investigation of the tissue
itself, are more appropriate approaches.
1.2.2.7 Skin immune system or skin-associated lymphoid tissue
As the skin is the largest organ of the body, its principal
physical function is to act as a barrier to water-soluble compounds,
to mechanical trauma, and to trauma caused by potentially pathogenic
microorganisms and the photons of sunlight. The physicochemical
characteristics of the outermost layer, the corneal or horny substance
of the epidermis, underlie the resistance to exogenous pathogenic
substances. The skin also has a host defence function that can be
designated as immunological. Some studies have suggested that the skin
might function as a primary organ (Fichtelius et al., 1970; Bos &
Kapsenberg, 1986), but most of the relevant immune reactions in the
skin appear to be antigen driven. The components of the skin immune
system, or skin-associated lymphoid tissue, are the following
(Streilein, 1983, 1990) (Figure 17): (i) Langerhans cells in the
epidermis, which are adapted for processing antigen and transporting
it to the draining lymph node, where they are called interdigitating
cells and present the antigen to lymphocytes; (ii) epider-motrophic
recirculating T lymphocyte subpopulations (homing T lymphocytes);
(iii) keratinocytes, which can synthesize cytokines after activation,
thereby influencing T-cell differentiation and haematopoiesis; they
can have an antigen-presenting function, especially after activation
resulting in MHC class II expression; (iv) Thy-1+ dendritic epidermal
cells, described in rodent skin epidermis: a special T cell that bears
the gamma-delta TCR and has an antigen-presenting function; and (v)
skin-draining lymph nodes comprising high endothelial venules through
which lymphocytes enter from the blood circulation.
 Immune components exist not only in the epidermis but also in the
dermis. At this location, T cells and macrophages have preferential
distributions, especially in the papillary region. T Cells,
macrophages, mast cells, endothelial cells, and dendritic cells are
found in the connective tissue of the dermis, as in connective tissue
at other locations in the body. The reactivity of these cells in the
dermis may differ, however, from those at other locations. For
instance, skin mast cells (Van Loveren et al., 1990a) behave
differently from mast cells at other places. These immunological
components cannot always be recognized in conventional histological
preparations of skin. For instance, Langerhans cells and dendritic
epidermal cells require special immunohistological staining (Figure
17).
Various inflammatory and immune mediators are also present in
skin. These include antimicrobial peptides, complement components,
immunoglobulins, cytokines, fibrinolysins, eicosanoids, and
neuropeptides. They are partly derived from the blood circulation and
are partly of local origin (Bos, 1990). Streilein (1990) described the
function of the skin-associated lymphoid tissue as follows: induction
of primary immune responses to new cutaneous antigens, expression of
immunity to previously encountered antigens, and avoidance of
deleterious immune responses to non-threatening cutaneous antigens.
1.3 Pathophysiology
1.3.1 Susceptibility to toxic action
The dynamic nature of the immune system renders it especially
vulnerable to toxic influences. The major target sites of the immune
system for toxicity are presented in Figure 18. The reactions of
lymphoid cells are associated with gene amplification, transcription,
and translation, and compounds that affect these processes of cell
proliferation and differentiation are especially immunotoxic,
particularly to the rapidly dividing thymocytes and haematopoietic
cells of the bone marrow. Thus, the disappearance of lymphoid cells
from bone marrow, blood, and tissue, and thymus weight may be the
first and most obvious signs of toxicity, as was seen in application
of the tiered approach to assessing the immunotoxicity of pesticides,
mentioned previously (Vos & Krajnc, 1983; Vos et al., 1983a). Effects
on the constituents of the framework (stationary stroma), which
support and steer the activation, proliferation, and differentiation
of lymphoid cells, are observed less often (Krajnc-Franken et al.,
1990; Schuurman et al., 1991b). Such effects result mostly in
degeneration, ending in atrophy and fibrosis. Alternatively, framework
cells and passenger leukocytes may persist but are rendered unable to
function by the toxic insult, and the delicate interactions between
these cells may be affected. This result of toxicity is not always
observed by conventional histology; it may be visualized by changes in
immunolabelling for markers that have functional significance.
Otherwise, functional assessment in vivo or in vitro is required.
As shown in Figure 18, the effects on specific cells in lymphoid
tissues are generally reflected in altered histology of lymphoid
organs, but this is not always the case for effects on responses.
The skin, respiratory tract, and gastrointestinal tract together
form an enormous surface that is in close contact with the outside
world and is potentially exposed to a vast magnitude of microbial
agents and potential toxicants. The toxic effects on these components
of the immune system can differ in (histo)pathological manifestation
from those on internal lymphoid organs. In the human respiratory
tract, these effects include asthma, fibrosis, and pulmonary
infections. Examples of inhaled pollutants that may induce these
effects are oxidant gases and particulates such as silica, asbestos,
and coal dust. The cellular and biochemical profiles of
bronchoalveolar lavage constituents after exposure of experimental
animals and humans by inhalation (Koren et al., 1989) are valuable for
screening immune-mediated lung injury. The products of pulmonary
epithelial cells and alveolar macrophages appear to be key factors. A
number of studies have indicated that lung disease progresses with
postactivational release of cytokines, such as IL-1, tumour necrosis
factor, platelet-derived growth factor, and transforming growth
factors. Alveolar macrophages secrete not only cytokines but also a
variety of short-lived products that may contribute to altered
resistance to pulmonary infections and inflammation; these include
reactive oxygen species, such as superoxide, nitric oxide, and
hydrogen peroxide, and arachidonic acid metabolites. The overall
suppression of these humoral systems, in combination with effects on
e.g. NK cells, may predispose individuals to infectious agents or
tumour development or may alter the inflammatory and degenerative
response (Van Loveren et al., 1990b; Khan & Gupta, 1991; Denis, 1992;
Denis et al., 1993).
The skin is an important target in immunotoxicology, for instance
for chemical allergens (Kimber & Cumberbatch, 1992) and ultraviolet B
(UVB) radiation (Goettsch et al., 1993). The skin can respond to many
xenobiotics by a specific immune response (contact hypersensitivity)
or by a nonspecific inflammatory response (contact irritancy); both
responses are associated with the induction of pro-inflammatory
cytokines. The cells of the immune system are readily recruited from
the circulation to the skin in response to dermal stimulation by
xenobiotics. In addition, various resident immune cells can be
activated, for instance Langerhans cells during the induction of the
Immune components exist not only in the epidermis but also in the
dermis. At this location, T cells and macrophages have preferential
distributions, especially in the papillary region. T Cells,
macrophages, mast cells, endothelial cells, and dendritic cells are
found in the connective tissue of the dermis, as in connective tissue
at other locations in the body. The reactivity of these cells in the
dermis may differ, however, from those at other locations. For
instance, skin mast cells (Van Loveren et al., 1990a) behave
differently from mast cells at other places. These immunological
components cannot always be recognized in conventional histological
preparations of skin. For instance, Langerhans cells and dendritic
epidermal cells require special immunohistological staining (Figure
17).
Various inflammatory and immune mediators are also present in
skin. These include antimicrobial peptides, complement components,
immunoglobulins, cytokines, fibrinolysins, eicosanoids, and
neuropeptides. They are partly derived from the blood circulation and
are partly of local origin (Bos, 1990). Streilein (1990) described the
function of the skin-associated lymphoid tissue as follows: induction
of primary immune responses to new cutaneous antigens, expression of
immunity to previously encountered antigens, and avoidance of
deleterious immune responses to non-threatening cutaneous antigens.
1.3 Pathophysiology
1.3.1 Susceptibility to toxic action
The dynamic nature of the immune system renders it especially
vulnerable to toxic influences. The major target sites of the immune
system for toxicity are presented in Figure 18. The reactions of
lymphoid cells are associated with gene amplification, transcription,
and translation, and compounds that affect these processes of cell
proliferation and differentiation are especially immunotoxic,
particularly to the rapidly dividing thymocytes and haematopoietic
cells of the bone marrow. Thus, the disappearance of lymphoid cells
from bone marrow, blood, and tissue, and thymus weight may be the
first and most obvious signs of toxicity, as was seen in application
of the tiered approach to assessing the immunotoxicity of pesticides,
mentioned previously (Vos & Krajnc, 1983; Vos et al., 1983a). Effects
on the constituents of the framework (stationary stroma), which
support and steer the activation, proliferation, and differentiation
of lymphoid cells, are observed less often (Krajnc-Franken et al.,
1990; Schuurman et al., 1991b). Such effects result mostly in
degeneration, ending in atrophy and fibrosis. Alternatively, framework
cells and passenger leukocytes may persist but are rendered unable to
function by the toxic insult, and the delicate interactions between
these cells may be affected. This result of toxicity is not always
observed by conventional histology; it may be visualized by changes in
immunolabelling for markers that have functional significance.
Otherwise, functional assessment in vivo or in vitro is required.
As shown in Figure 18, the effects on specific cells in lymphoid
tissues are generally reflected in altered histology of lymphoid
organs, but this is not always the case for effects on responses.
The skin, respiratory tract, and gastrointestinal tract together
form an enormous surface that is in close contact with the outside
world and is potentially exposed to a vast magnitude of microbial
agents and potential toxicants. The toxic effects on these components
of the immune system can differ in (histo)pathological manifestation
from those on internal lymphoid organs. In the human respiratory
tract, these effects include asthma, fibrosis, and pulmonary
infections. Examples of inhaled pollutants that may induce these
effects are oxidant gases and particulates such as silica, asbestos,
and coal dust. The cellular and biochemical profiles of
bronchoalveolar lavage constituents after exposure of experimental
animals and humans by inhalation (Koren et al., 1989) are valuable for
screening immune-mediated lung injury. The products of pulmonary
epithelial cells and alveolar macrophages appear to be key factors. A
number of studies have indicated that lung disease progresses with
postactivational release of cytokines, such as IL-1, tumour necrosis
factor, platelet-derived growth factor, and transforming growth
factors. Alveolar macrophages secrete not only cytokines but also a
variety of short-lived products that may contribute to altered
resistance to pulmonary infections and inflammation; these include
reactive oxygen species, such as superoxide, nitric oxide, and
hydrogen peroxide, and arachidonic acid metabolites. The overall
suppression of these humoral systems, in combination with effects on
e.g. NK cells, may predispose individuals to infectious agents or
tumour development or may alter the inflammatory and degenerative
response (Van Loveren et al., 1990b; Khan & Gupta, 1991; Denis, 1992;
Denis et al., 1993).
The skin is an important target in immunotoxicology, for instance
for chemical allergens (Kimber & Cumberbatch, 1992) and ultraviolet B
(UVB) radiation (Goettsch et al., 1993). The skin can respond to many
xenobiotics by a specific immune response (contact hypersensitivity)
or by a nonspecific inflammatory response (contact irritancy); both
responses are associated with the induction of pro-inflammatory
cytokines. The cells of the immune system are readily recruited from
the circulation to the skin in response to dermal stimulation by
xenobiotics. In addition, various resident immune cells can be
activated, for instance Langerhans cells during the induction of the
 contact hypersensitivity response. Soluble mediators can be produced
locally, and antigen-antibody complexes can be formed at the site of
inflammation. Exogenous factors such as ultraviolet light (Applegate
et al., 1989) and 7,12-dimethylbenz[ a]anthracene (DMBA) (Halliday et
al., 1988) can cause the disappearance of Langerhans cells from the
skin (or the loss of their function), with consequent disturbance or
dysregulation of the skin's immune function. Keratinocytes, which
comprise the vast majority of cells in the epidermis, have an
important role in immune and inflammatory responses, serving as a
significant source of cytokines, which contribute either
quantitatively or qualitatively to the nature of the response of the
skin to exogenous stimulation.
Reactions to drugs illustrate the skin's susceptibility to toxic
influences. Cytostatic drugs used in cancer therapy often induce bone-
marrow depression as a major side-effect, resulting in an increased
risk for infections, so that blood leukocyte counts, which reflect
bone-marrow depression, must be monitored during administration.
Conversely, a number of cytotostatic drugs are immunosuppressive. A
well-known example is azathioprine (see section 2.2.1.1). A number of
new immunosuppressive drugs inhibit DNA synthesis, including
Mizoribine (Bredinin, an imidazole nucleoside), Brequinar sodium (a
quinoline carboxylic acid derivative), and RS61443 (morpholino-
ethylester of mycophenolic acid) (Thomson, 1992). Their specificity to
cells of the immune system may be related to the distinct pathways in
purine and pyrimidine metabolism that are preferentially used by
lymphocytes, such as guanine nucleotide synthesis promoted by inosine
monophosphate dehydrogenase in the case of Mizoribine and RS61443.
Another example of the particular sensitivity of the immune system to
toxic damage is its response to irradiation. Ionizing radiation is
commonly used in cancer therapy. Of the body's constituents, the
haematopoietic system is particularly sensitive to irradiation; when
pluripotent stem cells are affected, regenerative activity is lost.
Other systems destroyed by this treatment, like the intestinal
epithelium, have an intrinsic self-renewal capacity and do not need
replacement therapy. The lymphoid constituents of the immune system
differ in radiosensitivity. The dose of radiation that causes a
reduction in the cell population by a factor of 1/e (or 0.37), the
D0, has been estimated at about 0.9 Gy for bone-marrow lymphoid stem
cells, 0.4-0.9 Gy for pre-B cells, and 0.7 Gy for peripheral
lymphocytes (Anderson & Williams, 1977; Anderson et al., 1977). The
thymus is very sensitive to irradiation (Sharp & Watkins, 1981;
Anderson et al., 1986; Adkins et al., 1988). Cortical lymphocytes
manifest a D0 value of about 0.6 Gy. The intrathymic T-cell
precursor has a D0 value of about 1.4 Gy. A refractory population
(about 20-30%) is present within the medulla, but most cells are
radiosensitive (D0, about 0.7 Gy). The capacity of thymic stroma to
support precursor T-cell processing is radioresistant (Huiskamp et
al., 1988). In addition to cell depletion, peripheral lymphoid tissues
manifest decreased lymphocyte recirculation at a dose of only 0.5 Gy
(Anderson et al., 1977), suggesting that high endothelial venules, or
cell surface molecules involved in lymphocyte migration through these
venules, are radiosensitive. The sensitivity of lymphocytes to
ionizing radiation cannot be ascribed solely to their susceptibility
to death during proliferation. Cells in the resting state disappear
after irradiation, at a time that does not correspond to their
physiological half-life. Apparently, the death of lymphocytes occurs
at phases between cell division. This phenomenon, known as interphase
death, appears to be a distinct characteristic of lymphocytes and
sensitizes the immune system to radiation. Thus, only peripheral blood
lymphocytes need be assessed as dosimeters after accidental
irradiation (such as at Chernobyl in 1986).
Toxicity to the thymus: Of the lymphoid cells of the body, the
lymphocytes of the thymus (thymocytes) are especially susceptible to
the action of toxic compounds (Schuurman et al., 1992d). Table 4
presents data on the susceptibility of various thymic components to
toxic damage, illustrating the particular vulnerability of the
passenger lymphocyte population. The microenvironment appears to be
more resistant, mainly on the basis of histopathological assessment.
Thymocyte depletion, suggestive of toxicity towards this population,
may actually be an indirect effect, in that the microenvironment is
damaged and unable to support thymocyte growth. This situation may
exist in the thymus after exposure to 2,3,7,8-tetrachlorodibenzo-
para-dioxin (TCDD), which preferentially attacks the thymic
reticular epithelium, resulting in lymphocyte depletion histologically
(see section 2.2.2.1). Similarly, the reduction in the cellularity of
the medulla of the thymus after treatment with cyclosporin A is due to
the absence of the medullary lymphocyte population, but the basis of
the reduction is the disappearance of dendritic cells (as seen by
immunohistochemistry; see section 2.2.1.2).
The susceptibility of thymocytes to toxicity is also related to
their fragile composition, especially cortical thymocytes, and to the
delicate interactions between them and their microenvironment. For
instance, they are programmed to enter apoptosis when activated during
the physiological process of selection. A decrease in size or
involution of the organ may thus be the first manifestation of
toxicity. It should be noted that stress itself can induce thymic
involution; furthermore, thymic status is dependent on nutritional
status and age. The main function of the thymus is to generate the
T-cell repertoire during fetal and early postnatal life. Its
susceptibility to toxic compounds and the subsequent effects on the
cell-mediated immune system are most evident during this period of
life.
Table 4. Sensitivity of cell populations in the thymus to toxic chemicals
Cells Location Compound
Lymphocytes
Immature lymphoblasts Outer cortex Some organotin compounds,
2,3,7,8-tetrachlorodibenzo-para-dioxin
Small cells Cortex Glucocorticosteroids, cytostatic drugs
(e.g. azathioprine)
Intermediate-sized cells Medulla Ammonia caramel (THI)
Epithelial cells Cortex 2,3,7,8-Tetrachlorodibenzo-para-dioxin
Medulla Cyclosporin, FK-506
Dendritic cells Medulla Cyclosporin, FK-506
Macrophages Cortex Ammonia caramel (THI)
THI, 2-acetyl-4(5)-tetrahydroxybutylimidazole; caramel colour III
An ever-increasing number of toxic compounds has been shown to
affect the immune system. Induced and spontaneous immunopathology in
rodents have been described in reviews and textbooks (Dean et al.,
1982, 1985; Irons, 1985; Descotes, 1986; Lebish et al., 1986; Gopinath
et al., 1987; Luster et al., 1989; Vos & Luster, 1989; Jones et al.,
1990; Krajnc-Franken et al., 1990; Vos & Krajnc-Franken, 1990;
Schuurman et al., 1991b; Dean et al., 1994; Frith et al., in press).
The weight and histology of lymphoid organs are the main parameters in
evaluating toxicity. The examples given in section 2.2 illustrate the
various ways in which the immune system is injured.
1.3.2 Regeneration
The dynamic nature of the immune system provides it with a strong
regenerative capacity. In principle, the leukocyte population is
generated by a single pluripotent progenitor cell in the bone marrow.
In the thymus, single thymocyte precursors have an enormous capacity
for expansion (Ezine et al., 1984). Thus, after exposure to xenobiotic
compounds that destroy the immune system, regeneration can occur
within a relatively short time: The original architecture is restored
within three to four weeks after involution caused by radiation or
treatment with glucocorticosteroid or organotin compounds. The
restoration process can occur in waves, depending on the sensitivity
of the precursor T cells in the thymus and bone marrow (Huiskamp et
al., 1983; Penit & Ezine, 1989). Regeneration does not occur after
destruction of the white blood cell system, e.g. by sublethal
irradiation, when the stem cells in the bone marrow are affected. In
such cases, bone-marrow transplantation is required, and the anlage of
the lymphoid organs then supports generation of the newly built immune
system. After bone-marrow transplantation, polymorphonuclear
granulocytes are the first cells to appear in the circulation, within
two to three weeks; lymphocytes appear after three to four weeks;
however, establishment of a fully developed T-cell system takes about
six months (De Gast et al., 1985). These examples illustrate the
vulnerability of the bone marrow-derived component to toxic action;
regeneration requires substitution of this component and not the
relatively more resistant stromal component in lymphoid tissue.
1.3.3 Changes in lymphoid organs
The weight and gross morphology of lymphoid organs are the first
parameters studied in assessing toxicity, as the response to injury is
often expressed as changes in tissue or organ weight, size, colour,
and gross appearance. These observations are combined with leukocyte
counts, studies of differentiation, and the results of
histopathological evaluations of lymphoid organs and tissues.
Conventional histopathology allows evaluation of the effects of
xenobiotics on the main cell subsets on the basis of their distinct
morphology, tissue location, and density. In this way, the effects on
lymphocytes, lymphoblasts, and stromal cells can be evaluated
(Schuurman et al., 1994; Kuper et al., 1995). The disappearance of
lymphocytes from blood and tissues and the decrease in size and weight
are often first seen in the thymus, as this organ is very sensitive to
toxicity (see above). Evaluations of effects on distinct
subpopulations must be confirmed by immunohistochemical
characterization (Schuurman et al., 1992a). The sensitivity of
histopathology can be increased by quantitative microscopy and
morphometry and organ cell counts.
During microscopic examination, important aspects to be
considered are the cell density and the size of the various
compartments of the lymphoid organs, as well as qualitative changes
like germinal centre development; however, microscopic examination of
lymphoid organs reveals these highly dynamic and complex processes
only at a static stage. Changes in the number of cells are best
reported by descriptive terms like 'increases' or 'reductions' in
cellularity rather than by interpretive terms like 'involution',
'atrophy', or 'hyperplasia'. The pathology working group of the IPCS-
European Union international collaborative immunotoxicity study has
started to develop such a descriptive approach. A window of control
values and gross and microscopic appearance must be established for
recognition of deviations from normal; however, normal and control
values and histological features are influenced by various endogenous
and exogenous factors, including the age and hormonal (especially sex
hormone) status of the animal (Kammüller et al., 1989; Goonewardene &
Murasko, 1992; Kuper et al., 1992a; Losco, 1992; Losco & Harleman,
1992). The influence of sex hormonal status on the histology and
histophysiology of lymphoid organs is illustrated by the following
examples. Considerable changes are seen in the lymphoid organs of mice
during pregnancy (Clarke, 1984), in birds during hatching (see
Section 2), and in ectothermic vertebrates during different seasons of
the year (see section 1.2.1.5). Castration results in an increase in
thymic size in old animals (Kendall et al., 1990), as has been
observed in old rats with an involuted thymus after treatment with an
analogue of luteinizing hormone-releasing hormone (Greenstein et al.,
1987). Figure 19 shows the wet thymic weight of groups of male rats in
order to illustrate these phenomena: the mean values were about 500 mg
in young male rats at 2.5 months of age, about 125 mg in rats at 12-15
months of age, about 250 mg in aged rats after surgical castration,
and about 300 mg in aged rats after treatment with the hormone
(Kendall et al., 1990).
The neuroendocrine system and the sex steroid hormone balance may
also underlie the changes in lymphoid organs of ectothermic
vertebrates with seasonal changes like temperature and photoperiod
(mentioned above). The thymic status is also dependent on nutritional
status (Van Logten et al., 1981; Corman, 1985; Mittal et al., 1988;
Good & Lorenz, 1992), and stressful conditions influence the
appearance of lymphoid organs, especially the thymus. Finally, housing
conditions, diet, and microbiological status are important. For
instance, the presence of gamma-delta T cells in the rodent intestinal
epithelium is dependent on the presence or absence of microbiological
stimulation (that is, whether the animals are bred and maintained
under specified pathogen-free conditions) (Bandeira et al., 1990;
Dobber et al., 1992; Umesaki et al., 1993). The role of genetic
factors is reflected in strain differences in lymphoid organ
histology, e.g. in rats (Losco & Harleman, 1992; Schuurman et al.,
1992e), seen on examination of data on concurrent and historical
controls.
Genetic factors may also contribute to the individual variability
in response to a given compound. This variability can be relatively
high, even among random-bred and inbred rats, as illustrated in the
following example (Figure 20), derived from a study of the toxicity of
the immunosuppressive drug azathioprine in random-bred Wistar rats.
The data on haematological and histopathological parameters were
subjected to factor analysis in order to facilitate examination of
individual and group responses to the drug. Factor analysis involves
clustering of parameters into composite groups, or 'factors', by
arithmetic manipulation of the data, so that the parameters within a
cluster are related mathematically. It is up to the investigator to
decide whether the clustering is meaningful biologically. Figure 20
shows that not all of the 10 animals that received the highest dose of
azathioprine had the same response: three were considered to be high
responders, six low or medium responders, and one a non-responder. The
high individual variability in response illustrates the significance
of outliers in studies with a limited number of animals.
The relevance of changes observed in a study of exposure in vivo
must be based on knowledge of the nature of the response. Current
understanding of the relationship between the structure and function
of the lymphoid organs and their components often allows only a
provisional hypothesis to be made about the mechanisms of toxicity.
Moreover, different mechanisms of tissue injury can yield similar
histopathological features. Therefore, in-depth, specific histological
examination of tissue response and immune function are indispensable
for interpreting changes and for risk assessment. In interpreting
quantitative changes, like changes in cellularity, it should be noted
that particular components of the immune system may be decreased in
number or size (suppressed or involuted) or increased (stimulated or
expanded), but this does not necessarily reflect the overall effect on
the immune system or lymphoid organs.
The presence of tissue damage, protein complex deposits, and
inflammatory cell infiltrates may indicate the induction of
autoimmunity or the presence of allergy or hypersensitivity. The site
at which such responses are seen is often not a lymphoid organ, but
blood vessels, renal glomeruli, synovial membranes, thyroid, skin,
liver, or lung, which are well-known sites of autoimmunity and
hypersensitivity. Non-lymphoid organs should also be examined in order
to determine whether the effects on the lymphoid system are secondary,
for instance mediated by acute stress. This aspect is not considered
in detail in this monograph, which concerns mainly the direct toxic
action of xenobiotics on the immune system.
contact hypersensitivity response. Soluble mediators can be produced
locally, and antigen-antibody complexes can be formed at the site of
inflammation. Exogenous factors such as ultraviolet light (Applegate
et al., 1989) and 7,12-dimethylbenz[ a]anthracene (DMBA) (Halliday et
al., 1988) can cause the disappearance of Langerhans cells from the
skin (or the loss of their function), with consequent disturbance or
dysregulation of the skin's immune function. Keratinocytes, which
comprise the vast majority of cells in the epidermis, have an
important role in immune and inflammatory responses, serving as a
significant source of cytokines, which contribute either
quantitatively or qualitatively to the nature of the response of the
skin to exogenous stimulation.
Reactions to drugs illustrate the skin's susceptibility to toxic
influences. Cytostatic drugs used in cancer therapy often induce bone-
marrow depression as a major side-effect, resulting in an increased
risk for infections, so that blood leukocyte counts, which reflect
bone-marrow depression, must be monitored during administration.
Conversely, a number of cytotostatic drugs are immunosuppressive. A
well-known example is azathioprine (see section 2.2.1.1). A number of
new immunosuppressive drugs inhibit DNA synthesis, including
Mizoribine (Bredinin, an imidazole nucleoside), Brequinar sodium (a
quinoline carboxylic acid derivative), and RS61443 (morpholino-
ethylester of mycophenolic acid) (Thomson, 1992). Their specificity to
cells of the immune system may be related to the distinct pathways in
purine and pyrimidine metabolism that are preferentially used by
lymphocytes, such as guanine nucleotide synthesis promoted by inosine
monophosphate dehydrogenase in the case of Mizoribine and RS61443.
Another example of the particular sensitivity of the immune system to
toxic damage is its response to irradiation. Ionizing radiation is
commonly used in cancer therapy. Of the body's constituents, the
haematopoietic system is particularly sensitive to irradiation; when
pluripotent stem cells are affected, regenerative activity is lost.
Other systems destroyed by this treatment, like the intestinal
epithelium, have an intrinsic self-renewal capacity and do not need
replacement therapy. The lymphoid constituents of the immune system
differ in radiosensitivity. The dose of radiation that causes a
reduction in the cell population by a factor of 1/e (or 0.37), the
D0, has been estimated at about 0.9 Gy for bone-marrow lymphoid stem
cells, 0.4-0.9 Gy for pre-B cells, and 0.7 Gy for peripheral
lymphocytes (Anderson & Williams, 1977; Anderson et al., 1977). The
thymus is very sensitive to irradiation (Sharp & Watkins, 1981;
Anderson et al., 1986; Adkins et al., 1988). Cortical lymphocytes
manifest a D0 value of about 0.6 Gy. The intrathymic T-cell
precursor has a D0 value of about 1.4 Gy. A refractory population
(about 20-30%) is present within the medulla, but most cells are
radiosensitive (D0, about 0.7 Gy). The capacity of thymic stroma to
support precursor T-cell processing is radioresistant (Huiskamp et
al., 1988). In addition to cell depletion, peripheral lymphoid tissues
manifest decreased lymphocyte recirculation at a dose of only 0.5 Gy
(Anderson et al., 1977), suggesting that high endothelial venules, or
cell surface molecules involved in lymphocyte migration through these
venules, are radiosensitive. The sensitivity of lymphocytes to
ionizing radiation cannot be ascribed solely to their susceptibility
to death during proliferation. Cells in the resting state disappear
after irradiation, at a time that does not correspond to their
physiological half-life. Apparently, the death of lymphocytes occurs
at phases between cell division. This phenomenon, known as interphase
death, appears to be a distinct characteristic of lymphocytes and
sensitizes the immune system to radiation. Thus, only peripheral blood
lymphocytes need be assessed as dosimeters after accidental
irradiation (such as at Chernobyl in 1986).
Toxicity to the thymus: Of the lymphoid cells of the body, the
lymphocytes of the thymus (thymocytes) are especially susceptible to
the action of toxic compounds (Schuurman et al., 1992d). Table 4
presents data on the susceptibility of various thymic components to
toxic damage, illustrating the particular vulnerability of the
passenger lymphocyte population. The microenvironment appears to be
more resistant, mainly on the basis of histopathological assessment.
Thymocyte depletion, suggestive of toxicity towards this population,
may actually be an indirect effect, in that the microenvironment is
damaged and unable to support thymocyte growth. This situation may
exist in the thymus after exposure to 2,3,7,8-tetrachlorodibenzo-
para-dioxin (TCDD), which preferentially attacks the thymic
reticular epithelium, resulting in lymphocyte depletion histologically
(see section 2.2.2.1). Similarly, the reduction in the cellularity of
the medulla of the thymus after treatment with cyclosporin A is due to
the absence of the medullary lymphocyte population, but the basis of
the reduction is the disappearance of dendritic cells (as seen by
immunohistochemistry; see section 2.2.1.2).
The susceptibility of thymocytes to toxicity is also related to
their fragile composition, especially cortical thymocytes, and to the
delicate interactions between them and their microenvironment. For
instance, they are programmed to enter apoptosis when activated during
the physiological process of selection. A decrease in size or
involution of the organ may thus be the first manifestation of
toxicity. It should be noted that stress itself can induce thymic
involution; furthermore, thymic status is dependent on nutritional
status and age. The main function of the thymus is to generate the
T-cell repertoire during fetal and early postnatal life. Its
susceptibility to toxic compounds and the subsequent effects on the
cell-mediated immune system are most evident during this period of
life.
Table 4. Sensitivity of cell populations in the thymus to toxic chemicals
Cells Location Compound
Lymphocytes
Immature lymphoblasts Outer cortex Some organotin compounds,
2,3,7,8-tetrachlorodibenzo-para-dioxin
Small cells Cortex Glucocorticosteroids, cytostatic drugs
(e.g. azathioprine)
Intermediate-sized cells Medulla Ammonia caramel (THI)
Epithelial cells Cortex 2,3,7,8-Tetrachlorodibenzo-para-dioxin
Medulla Cyclosporin, FK-506
Dendritic cells Medulla Cyclosporin, FK-506
Macrophages Cortex Ammonia caramel (THI)
THI, 2-acetyl-4(5)-tetrahydroxybutylimidazole; caramel colour III
An ever-increasing number of toxic compounds has been shown to
affect the immune system. Induced and spontaneous immunopathology in
rodents have been described in reviews and textbooks (Dean et al.,
1982, 1985; Irons, 1985; Descotes, 1986; Lebish et al., 1986; Gopinath
et al., 1987; Luster et al., 1989; Vos & Luster, 1989; Jones et al.,
1990; Krajnc-Franken et al., 1990; Vos & Krajnc-Franken, 1990;
Schuurman et al., 1991b; Dean et al., 1994; Frith et al., in press).
The weight and histology of lymphoid organs are the main parameters in
evaluating toxicity. The examples given in section 2.2 illustrate the
various ways in which the immune system is injured.
1.3.2 Regeneration
The dynamic nature of the immune system provides it with a strong
regenerative capacity. In principle, the leukocyte population is
generated by a single pluripotent progenitor cell in the bone marrow.
In the thymus, single thymocyte precursors have an enormous capacity
for expansion (Ezine et al., 1984). Thus, after exposure to xenobiotic
compounds that destroy the immune system, regeneration can occur
within a relatively short time: The original architecture is restored
within three to four weeks after involution caused by radiation or
treatment with glucocorticosteroid or organotin compounds. The
restoration process can occur in waves, depending on the sensitivity
of the precursor T cells in the thymus and bone marrow (Huiskamp et
al., 1983; Penit & Ezine, 1989). Regeneration does not occur after
destruction of the white blood cell system, e.g. by sublethal
irradiation, when the stem cells in the bone marrow are affected. In
such cases, bone-marrow transplantation is required, and the anlage of
the lymphoid organs then supports generation of the newly built immune
system. After bone-marrow transplantation, polymorphonuclear
granulocytes are the first cells to appear in the circulation, within
two to three weeks; lymphocytes appear after three to four weeks;
however, establishment of a fully developed T-cell system takes about
six months (De Gast et al., 1985). These examples illustrate the
vulnerability of the bone marrow-derived component to toxic action;
regeneration requires substitution of this component and not the
relatively more resistant stromal component in lymphoid tissue.
1.3.3 Changes in lymphoid organs
The weight and gross morphology of lymphoid organs are the first
parameters studied in assessing toxicity, as the response to injury is
often expressed as changes in tissue or organ weight, size, colour,
and gross appearance. These observations are combined with leukocyte
counts, studies of differentiation, and the results of
histopathological evaluations of lymphoid organs and tissues.
Conventional histopathology allows evaluation of the effects of
xenobiotics on the main cell subsets on the basis of their distinct
morphology, tissue location, and density. In this way, the effects on
lymphocytes, lymphoblasts, and stromal cells can be evaluated
(Schuurman et al., 1994; Kuper et al., 1995). The disappearance of
lymphocytes from blood and tissues and the decrease in size and weight
are often first seen in the thymus, as this organ is very sensitive to
toxicity (see above). Evaluations of effects on distinct
subpopulations must be confirmed by immunohistochemical
characterization (Schuurman et al., 1992a). The sensitivity of
histopathology can be increased by quantitative microscopy and
morphometry and organ cell counts.
During microscopic examination, important aspects to be
considered are the cell density and the size of the various
compartments of the lymphoid organs, as well as qualitative changes
like germinal centre development; however, microscopic examination of
lymphoid organs reveals these highly dynamic and complex processes
only at a static stage. Changes in the number of cells are best
reported by descriptive terms like 'increases' or 'reductions' in
cellularity rather than by interpretive terms like 'involution',
'atrophy', or 'hyperplasia'. The pathology working group of the IPCS-
European Union international collaborative immunotoxicity study has
started to develop such a descriptive approach. A window of control
values and gross and microscopic appearance must be established for
recognition of deviations from normal; however, normal and control
values and histological features are influenced by various endogenous
and exogenous factors, including the age and hormonal (especially sex
hormone) status of the animal (Kammüller et al., 1989; Goonewardene &
Murasko, 1992; Kuper et al., 1992a; Losco, 1992; Losco & Harleman,
1992). The influence of sex hormonal status on the histology and
histophysiology of lymphoid organs is illustrated by the following
examples. Considerable changes are seen in the lymphoid organs of mice
during pregnancy (Clarke, 1984), in birds during hatching (see
Section 2), and in ectothermic vertebrates during different seasons of
the year (see section 1.2.1.5). Castration results in an increase in
thymic size in old animals (Kendall et al., 1990), as has been
observed in old rats with an involuted thymus after treatment with an
analogue of luteinizing hormone-releasing hormone (Greenstein et al.,
1987). Figure 19 shows the wet thymic weight of groups of male rats in
order to illustrate these phenomena: the mean values were about 500 mg
in young male rats at 2.5 months of age, about 125 mg in rats at 12-15
months of age, about 250 mg in aged rats after surgical castration,
and about 300 mg in aged rats after treatment with the hormone
(Kendall et al., 1990).
The neuroendocrine system and the sex steroid hormone balance may
also underlie the changes in lymphoid organs of ectothermic
vertebrates with seasonal changes like temperature and photoperiod
(mentioned above). The thymic status is also dependent on nutritional
status (Van Logten et al., 1981; Corman, 1985; Mittal et al., 1988;
Good & Lorenz, 1992), and stressful conditions influence the
appearance of lymphoid organs, especially the thymus. Finally, housing
conditions, diet, and microbiological status are important. For
instance, the presence of gamma-delta T cells in the rodent intestinal
epithelium is dependent on the presence or absence of microbiological
stimulation (that is, whether the animals are bred and maintained
under specified pathogen-free conditions) (Bandeira et al., 1990;
Dobber et al., 1992; Umesaki et al., 1993). The role of genetic
factors is reflected in strain differences in lymphoid organ
histology, e.g. in rats (Losco & Harleman, 1992; Schuurman et al.,
1992e), seen on examination of data on concurrent and historical
controls.
Genetic factors may also contribute to the individual variability
in response to a given compound. This variability can be relatively
high, even among random-bred and inbred rats, as illustrated in the
following example (Figure 20), derived from a study of the toxicity of
the immunosuppressive drug azathioprine in random-bred Wistar rats.
The data on haematological and histopathological parameters were
subjected to factor analysis in order to facilitate examination of
individual and group responses to the drug. Factor analysis involves
clustering of parameters into composite groups, or 'factors', by
arithmetic manipulation of the data, so that the parameters within a
cluster are related mathematically. It is up to the investigator to
decide whether the clustering is meaningful biologically. Figure 20
shows that not all of the 10 animals that received the highest dose of
azathioprine had the same response: three were considered to be high
responders, six low or medium responders, and one a non-responder. The
high individual variability in response illustrates the significance
of outliers in studies with a limited number of animals.
The relevance of changes observed in a study of exposure in vivo
must be based on knowledge of the nature of the response. Current
understanding of the relationship between the structure and function
of the lymphoid organs and their components often allows only a
provisional hypothesis to be made about the mechanisms of toxicity.
Moreover, different mechanisms of tissue injury can yield similar
histopathological features. Therefore, in-depth, specific histological
examination of tissue response and immune function are indispensable
for interpreting changes and for risk assessment. In interpreting
quantitative changes, like changes in cellularity, it should be noted
that particular components of the immune system may be decreased in
number or size (suppressed or involuted) or increased (stimulated or
expanded), but this does not necessarily reflect the overall effect on
the immune system or lymphoid organs.
The presence of tissue damage, protein complex deposits, and
inflammatory cell infiltrates may indicate the induction of
autoimmunity or the presence of allergy or hypersensitivity. The site
at which such responses are seen is often not a lymphoid organ, but
blood vessels, renal glomeruli, synovial membranes, thyroid, skin,
liver, or lung, which are well-known sites of autoimmunity and
hypersensitivity. Non-lymphoid organs should also be examined in order
to determine whether the effects on the lymphoid system are secondary,
for instance mediated by acute stress. This aspect is not considered
in detail in this monograph, which concerns mainly the direct toxic
action of xenobiotics on the immune system.

 2. HEALTH IMPACT OF SELECTED IMMUNOTOXIC AGENTS
2.1 Description of consequences on human health
2.1.1 Consequences of immunosuppression
Since immunosuppressive drugs were introduced clinically to
prevent allograft rejection more than 25 years ago, the human
consequences of immunosuppression are well known. Infections and
cancers are the main consequences of immunosuppressive therapy, as
exemplified by a number of isolated case reports and epidemiological
studies (IARC, 1987; Descotes, 1990; IARC, 1990). The cancers are
often lymphomas and carcinomas, which are likely to be of viral
origin, especially in immunosuppressed patients. Recurrent respiratory
viral infections should also be considered as sentinel conditions for
immunotoxicity, both in individuals and in community-based epidemics,
including, but not confined to, opportunistic infections. Additional
evidence that immunosuppression can enhance the risk of cancer is the
increased incidence of an atypical form of Kaposi's sarcoma and of
lymphomas frequently located in the brains of patients with AIDS. It
is important to distinguish between profound immunodepression (mainly
seen clinically, e.g. after renal transplantation or cytotoxic therapy
for neoplasia) and the less severe suppression of immune function that
is more likely to be associated with exposure to an environmental
immunotoxic agent.
2.1.1.1 Cancer
Evidence from three sources, namely cancer patients on
chemotherapy, organ transplant patients, and patients with autoimmune
disorders undergoing long-term immunosuppressive therapy, demonstrates
that immunosuppressed patients are at a higher risk than others of
developing malignancies (Boyle et al., 1984; Penn, 1988; IARC, 1990;
Barrett et al., 1993; Bouwes Bavinck et al., 1993; Penn, 1993a,b;
Descotes & Vial, 1994), although not all immunosuppressive drugs have
been shown to be carcinogenic, e.g. prednisone and methotrexate (IARC,
1987). Immunotoxic effects might result in tumour formation through
reduced immune surveillance, i.e. tumours might escape the guard of
the immune system. Reduced immune surveillance can thus be regarded as
tumour promotion.
The risk for second malignancies after prolonged cancer
chemotherapy has been shown in numerous case reports and
epidemiological studies (Henne & Schmähl, 1985; Boivin, 1990; Blatt et
al., 1992). Acute leukaemia is the most frequently reported second
cancer (Kyle, 1984); overall, iatrogenic leukaemias account for 10% of
all leukaemias, with an incidence 5-100 times higher than in the
general population. Non-Hodgkin's lymphoma develops in 0.5-4.5% of
patients within 10 years after cytotoxic therapy. The risk for solid
tumours, e.g. carcinomas of the lung, skin, breast, colon, and
pancreas, is also increased after cytotoxic therapy but with a
different trend: the increase in risk is more prolonged and slower
(Swerdlow et al., 1992).
Although most cytotoxic drugs are genotoxic, their
immunosuppressive effects may also account for the increased risk of
second cancers, as indicated by results obtained in organ transplant
recipients. The Cincinnati Transplant Tumour Registry collected data
on more than 3600 cases of cancer in transplant patients up to June
1988 (Penn, 1988). Large numbers of cancers were also included in the
Australian and New Zealand Combined Dialysis and Transplant Registry
(Sheil et al., 1991). Overall, 1-15% of organ transplant patients
developed cancer within the first five years after transplantation;
whatever the therapeutic regimen, the incidence of cancer was at least
three times that of the general population and increased
logarithmically with the length of follow-up to reach more than 50%
after 20 years in some series. Cancers of the skin and lips were
reported in 18% of patients after 10 years of immunosuppressive
therapy. Squamous-cell carcinoma was the most frequent skin cancer and
was about 250 times more frequent in transplant patients than in the
general population (Hartevelt et al., 1990). Lymphomas accounted for
14-18% of neoplasms in transplant patients, and high-grade non-
Hodgkin's lymphomas accounted for 95% of these lymphomas. The
incidence of Kaposi's sarcoma was 400-500 times more frequent in
transplant patients than in the general population.
A similar pattern of neoplasias was observed in patients on
immunosuppressive therapy for autoimmune diseases (Sela & Shoenfeld,
1988). Non-Hodgkin's lymphomas were 11 times more frequent than in the
general population, and other cancers, namely leukaemias, primary
liver cancers, and squamous-cell carcinomas, were also found to be
more frequent (Penn, 1988; Descotes & Vial, 1994). The respective
roles of immunosuppressive therapy and of the underlying disease
remain to be established, however.
Cancers have been reported to occur after immunosuppressive
therapy with both cytotoxic and noncytotoxic drugs. Even though the
respective roles of genotoxicity and immunosuppression are difficult
to ascertain, cancers have been described in patients on azathioprine
after organ transplantation (Wessel et al., 1988; Singh et al., 1989)
or on low-dose methotrexate for autoimmune disorders (Ellman et al.,
1991; Shiroky et al., 1991; Kingsmore et al., 1992; Kamel et al.,
1993); immunosuppressive drugs increased the risk of malignancies
(especially lymphomas) in the treated patients. Interestingly, all of
the noncytotoxic immunosuppressive drugs were reportedly associated
with a variety of malignancies presumably related to immuno-
suppression. Whatever the type of tumour, the time to tumour
development after treatment with cyclosporin A was shorter (26 months
on average; 14 months for lymphomas) than after conventional
immunosuppressive therapy (60 months on average) (Penn, 1988). The
murine monoclonal antibody OKT3 was also shown to increase the risk
for lymphoproliferative disorders (Swinnen et al., 1990): Lymphomas
developed 1-18 months after starting OKT3, and a correlation was found
between the dose and time to neoplasm development. Lymphoproliferative
disorders were also shown to occur following treatment with FK 506
(Reyes et al., 1991). There are insufficient data to conclude a direct
causal relationship between immunosuppression induced by environmental
chemicals and the development of cancer; however, there is
epidemiological evidence that exposure to various potentially
immunotoxic chemicals (e.g. pesticides, benzene) is associated with
increased risks for cancers that also occur in immunosuppressed
patients (e.g. non-Hodgkin's lymphoma and leukaemia).
No marked difference was found in the relative risks for
lymphoproliferative disorders associated with the various
immunosuppressive drugs currently used, suggesting that
immunosuppression is the causative factor, particularly when account
is taken of the different carcinogenic potentials of azathioprine and
cyclosporin A, both clinical reference immunosuppressive agents
(Ryffel, 1992). Reactivation of latent viruses, e.g. Epstein-Barr
virus, due to immunosuppression was suggested to be involved. Indeed,
most lymphoproliferative disorders induced with cyclosporin A or
methotrexate were B-cell malignant lymphomas associated with this
viral infection (Starzl et al., 1984; Kamel et al., 1993). Uninhibited
proliferation of Epstein-Barr virus in B lymphocytes caused by the
efficient immunosuppression of one or more kinds of controls by
T lymphocytes is the commonly accepted mechanism. Interestingly,
Epstein-Barr virus-associated lymphomas in patients on
immunosuppressive therapy are usually reversible upon cessation of
treatment (Starzl et al., 1984).
2.1.1.2 Infectious diseases
Whatever the primary cause of the immune deficiency, patients
develop more frequent, more severe, recurring, and often atypical
infections, depending on the type and severity of the deficiency. The
complications associated with severe immunosuppression include
bacterial, viral, fungal, and parasitic infections (Waldman, 1988;
Barr et al., 1989; Mandell, 1990; Tieben et al., 1994). The pathogens
most frequently encountered in immunodeficient patients include the
bacterial agents Staphylococcus aureus, Streptococci, Escherichia
coli, Pseudomonas aeruginosa, Listeria monocytogenes, Mycobacterium
tuberculosis, and atypical mycobacteria. Herpes virus,
cytomegalovirus, Epstein-Barr virus, and human papillomavirus are the
leading causes of viral infections in immunosuppressed patients.
Fungal opportunistic infections include those induced by Candida,
Aspergillus, and Cryptococcus species. The immunotoxicity induced
by environmental chemicals often results in subtle changes in the
immune system, which have been suggested to result in increased
incidences of common infections like influenza and the common cold
(see section 2.4).
The respiratory tract is a primary target for infectious
pathogens, especially in immunosuppressed patients. Pulmonary
infections and infections of the upper respiratory tract are the most
common (Frattini & Trulock, 1993). Cytomegalovirus infections, often
asymptomatic, are particularly frequent in renal transplant patients
(Rubin, 1990). In addition, Pneumocystis carinii can cause a
particular form of pneumonia in immunosuppressed patients. Even though
respiratory diseases usually predominate, gastrointestinal infectious
diseases may constitute the leading consequences of immunosuppression
(Bodey et al., 1986). Infections of the central nervous system and
isolated fever are also extremely frequent. Interestingly, the type of
infection that develops in immunosuppressed patients is largely
dependent on the type of immune defect, as illustrated in Table 5.
Infectious complications have been commonly described in patients
treated with various cytotoxic drugs for cancer and with
immunosuppressants, such as cyclosporin A, for the prevention of
allograft rejection or the treatment of autoimmune disorders (Kim,
1989; Descotes & Vial, 1994).
Table 5. Pathogens frequently associated with immune defects
Humoral Cellular Neutrophil Complement
immunity immunity functions system
Campylobacter Candida Aspergillus Neisseria
Echovirus Coccidioides Bacteroides Staphylococci
Gardia Cryptococci Escherichia coli Streptococci
Haemophilus Cytomegalovirus Klebsiella
Pneumococci Herpes virus Pseudomonas
Salmonella
Legionella
Mycobacteria
Staphylococci
Histoplasma
Toxoplasma
Pneumocystis
Human papillomavirus
2.1.2 Consequences of immunostimulation
The health consequences of immunostimulation are less well
established than those associated with immunosuppression. A number of
adverse effects have, however, been reported after treatment with
immunostimulating drugs, including influenza-like reactions,
facilitation or exacerbation of underlying diseases, and inhibition of
hepatic drug metabolism (Descotes, 1992).
Patients with influenza-like reactions present with mild to
moderate fever associated with chills, malaise, and hypotension. The
reaction usually develops within hours after taking an
immunostimulating drug, and the patient recovers uneventfully within a
few hours. Such reactions are relatively uncommon with most
immunomodulating agents but have been shown to limit treatment with
several recombinant cytokines, e.g. IL-1 and tumour necrosis factor.
Facilitation and/or exacerbation of underlying diseases have been
ascribed to most immunostimulating drugs, but the incidence of this
adverse event differs markedly from one drug to another. Exacerbation
of chronic infections, psoriasis, and Crohn's disease have been
reported. More interestingly, several autoimmune diseases are more
frequent in patients treated with various (recombinant) cytokines,
e.g. autoimmunity treated with IFN gamma (Jacob et al., 1987),
thyroiditis with IL-2 (Vial & Descotes, 1993), and lupus erythematosus
with IFN alpha (Vial & Descotes, 1993). Exacerbation or facilitation
of allergic reactions to unrelated allergens has also been reported:
starting an immunostimulating treatment has been associated with
exacerbation of underlying eczema, asthma, or rhinitis. Allergic
reactions to radiological contrast media have been shown to be more
frequent in IL-2-treated patients than controls (Vial & Descotes,
1992).
Oxidative drug metabolism by the hepatic cytochrome P450 system
has been shown to be inhibited by immunostimulating drugs (Descotes,
1985) and by administration of bacillus Calmette-Guérin (BCG) vaccine
or interferon (Vial & Descotes, 1993). Although the mechanism of this
inhibition is still unknown, activation of macrophages resulting in
the release of IL-1 and IL-6 has been suggested to be involved.
Likewise, vaccination has been shown to compromise drug metabolism to
a sufficient extent that normally therapeutic doses of theophylline
caused acute toxicity in humans (Renton et al., 1980). Stimulation of
the immune system has also been shown to alter drug metabolism in
humans (Renton, 1986). A similar effect of infection has been reported
in laboratory animals (Selgrade et al., 1984); infection with mouse
cytomegalovirus before exposure to the insecticide parathion reduced
the total P450 concentration and dramatically increased the toxicity
of parathion.
So far, only a few environmental chemicals have been shown to
exert immunostimulating properties, e.g. hexachlorobenzene and
selenium. There have been no reports of clinical reactions to such
chemicals that are similar to the adverse effects seen with
immunostimulating drugs.
2.2 Direct immunotoxicity in laboratory animals
The following are some illustrative examples of immunotoxic
chemicals.
2.2.1 Azathioprine and cyclosporin A
The immunosuppressive effects of azathioprine and cyclosporin A
are considered because they can shed light on the direct
immunotoxicity of environmental chemicals.
2.2.1.1 Azathioprine
Azathioprine is a thiopurine that is used as cytostatic drug in
the treatment of leukaemias and as an immunosuppressant in patients
who have received allogeneic organ transplants or who have autoimmune
diseases. When used as an immunosuppressant, its main side-effect is
bone-marrow depression, reflected in blood leukocytopenia; its
administration must therefore be monitored through blood leukocyte
counts. Another side-effect, especially after long-term
administration, is tumour formation (IARC, 1987).
In rats, azathioprine is cytotoxic for all cell lineages in the
bone marrow, and strong cellular depletion is observed histologically.
It decreases the cellularity in thymus, blood, and peripheral lymphoid
organs, but it is mainly in the thymus that the immature lymphocyte
population of the cortex is affected. This effect is a general feature
of most cytostatic drugs. A similar effect is seen in the thymus after
treatment with glucocorticosteroids, but the molecular mechanism
resulting in lymphocyte depletion is obviously different: interference
with DNA synthesis resulting in lymphocyte proliferation in contrast
to binding to glucocorticosteroid receptors and cell down-modulation.
Azathioprine affects a number of indicators of immune function, like
macrophage cytotoxicity (Spreafico et al., 1987), lymphocyte
proliferation in vitro after mitogen stimulation (Weissgarten et
al., 1989) and in the mixed leukocyte reaction (Mellert et al., 1989),
and cytotoxicity by NK cells (Pedersen & Beyer, 1986; Spreafico et
al., 1987; Versluis et al., 1989). Both stimulation and suppression of
these functions have been found in experimental animals, depending on
the dosage and the time of testing after exposure. These findings are
in accordance with those in azathioprine-treated patients, who showed
no change in primary antibody response, a decrease in secondary
antibody response, and some or no effect on lymphocyte proliferation
in vitro after mitogen stimulation. The time of testing after the
start of exposure to azathioprine was a crucial factor in the
detection of effects. Azathioprine was tested in the IPCS-European
Union international collaborative immunotoxicity study (see section
1.1) and showed a significant strain-dependent sensitivity.
2.2.1.2 Cyclosporin A
Cyclosporin A is one of the most powerful immunosuppressive drugs
(Kahan, 1989). It is a neutral lipophilic cyclic peptide consisting of
11 amino acids (relative molecular mass, 1203 Da) isolated from the
fungus Tolypocladium inflatum. Its main use is in bone-marrow
transplantation to prevent transplant rejection and graft-versus-host
reactions. It is also used in the therapy of various autoimmune
diseases.
A complication of cyclosporin A treatment is nephrotoxicity.
Another side-effect, especially after long-term administration, is
tumour formation (IARC, 1987). In its immunosuppressive action,
cyclosporin A does not affect resting lymphocytes but blocks the
events occurring after stimulation, particularly the synthesis of
lymphokines, including IL-1 and IL-2, and IL-2 receptors. The
synthesis of IL-1 by antigen-presenting cells and of IL-2 by Th cells
is inhibited, and the synthesis of IFN gamma and tumour necrosis
factor is blocked. These events occur inside the cell at the
transcriptional level. Cyclosporin A binds to an intracellular
receptor, cyclophilin, forming a complex with calcineurin; this
complex in turn interferes with the activation of genes, resulting in
inhibition of lymphokine gene transcription (Baumann et al., 1992;
Sigal & Dumont, 1992).
An interesting feature of cyclosporin A is its specific action on
the thymus and the induction of autoimmune phenomena. Rats treated
with total body irradiation and syngeneic or autologous bone-marrow
transplantation, followed by treatment with cyclosporin A at a dose of
about 10 mg/kg body weight per day subcutaneously for four weeks,
developed signs of acute graft-versus-host reactions, with lymphocytic
infiltration at multiple epithelial sites (Glazier et al., 1983). A
similar pseudo-graft-versus-host reaction has also been evoked in
mice. It is associated with thymic changes, because it can be
transferred in whole thymus or thymocytes (Sakaguchi & Sakaguchi,
1988). Histologically, the medullary area is diminished (Beschorner et
al., 1987a; Schuurman et al., 1990; see also Figure 21). The medullary
stroma shows a decrease in MHC class II expression, indicating a loss
of dendritic cells, which has been confirmed by electron microscopy
(De Waal et al., 1992a). As these cells normally contribute to the
negative selection process, their depletion (or reduced MHC class II
expression) may be related to an absence of negative selection. The
autoreactive T cells may even attack the medullary epithelium.
2. HEALTH IMPACT OF SELECTED IMMUNOTOXIC AGENTS
2.1 Description of consequences on human health
2.1.1 Consequences of immunosuppression
Since immunosuppressive drugs were introduced clinically to
prevent allograft rejection more than 25 years ago, the human
consequences of immunosuppression are well known. Infections and
cancers are the main consequences of immunosuppressive therapy, as
exemplified by a number of isolated case reports and epidemiological
studies (IARC, 1987; Descotes, 1990; IARC, 1990). The cancers are
often lymphomas and carcinomas, which are likely to be of viral
origin, especially in immunosuppressed patients. Recurrent respiratory
viral infections should also be considered as sentinel conditions for
immunotoxicity, both in individuals and in community-based epidemics,
including, but not confined to, opportunistic infections. Additional
evidence that immunosuppression can enhance the risk of cancer is the
increased incidence of an atypical form of Kaposi's sarcoma and of
lymphomas frequently located in the brains of patients with AIDS. It
is important to distinguish between profound immunodepression (mainly
seen clinically, e.g. after renal transplantation or cytotoxic therapy
for neoplasia) and the less severe suppression of immune function that
is more likely to be associated with exposure to an environmental
immunotoxic agent.
2.1.1.1 Cancer
Evidence from three sources, namely cancer patients on
chemotherapy, organ transplant patients, and patients with autoimmune
disorders undergoing long-term immunosuppressive therapy, demonstrates
that immunosuppressed patients are at a higher risk than others of
developing malignancies (Boyle et al., 1984; Penn, 1988; IARC, 1990;
Barrett et al., 1993; Bouwes Bavinck et al., 1993; Penn, 1993a,b;
Descotes & Vial, 1994), although not all immunosuppressive drugs have
been shown to be carcinogenic, e.g. prednisone and methotrexate (IARC,
1987). Immunotoxic effects might result in tumour formation through
reduced immune surveillance, i.e. tumours might escape the guard of
the immune system. Reduced immune surveillance can thus be regarded as
tumour promotion.
The risk for second malignancies after prolonged cancer
chemotherapy has been shown in numerous case reports and
epidemiological studies (Henne & Schmähl, 1985; Boivin, 1990; Blatt et
al., 1992). Acute leukaemia is the most frequently reported second
cancer (Kyle, 1984); overall, iatrogenic leukaemias account for 10% of
all leukaemias, with an incidence 5-100 times higher than in the
general population. Non-Hodgkin's lymphoma develops in 0.5-4.5% of
patients within 10 years after cytotoxic therapy. The risk for solid
tumours, e.g. carcinomas of the lung, skin, breast, colon, and
pancreas, is also increased after cytotoxic therapy but with a
different trend: the increase in risk is more prolonged and slower
(Swerdlow et al., 1992).
Although most cytotoxic drugs are genotoxic, their
immunosuppressive effects may also account for the increased risk of
second cancers, as indicated by results obtained in organ transplant
recipients. The Cincinnati Transplant Tumour Registry collected data
on more than 3600 cases of cancer in transplant patients up to June
1988 (Penn, 1988). Large numbers of cancers were also included in the
Australian and New Zealand Combined Dialysis and Transplant Registry
(Sheil et al., 1991). Overall, 1-15% of organ transplant patients
developed cancer within the first five years after transplantation;
whatever the therapeutic regimen, the incidence of cancer was at least
three times that of the general population and increased
logarithmically with the length of follow-up to reach more than 50%
after 20 years in some series. Cancers of the skin and lips were
reported in 18% of patients after 10 years of immunosuppressive
therapy. Squamous-cell carcinoma was the most frequent skin cancer and
was about 250 times more frequent in transplant patients than in the
general population (Hartevelt et al., 1990). Lymphomas accounted for
14-18% of neoplasms in transplant patients, and high-grade non-
Hodgkin's lymphomas accounted for 95% of these lymphomas. The
incidence of Kaposi's sarcoma was 400-500 times more frequent in
transplant patients than in the general population.
A similar pattern of neoplasias was observed in patients on
immunosuppressive therapy for autoimmune diseases (Sela & Shoenfeld,
1988). Non-Hodgkin's lymphomas were 11 times more frequent than in the
general population, and other cancers, namely leukaemias, primary
liver cancers, and squamous-cell carcinomas, were also found to be
more frequent (Penn, 1988; Descotes & Vial, 1994). The respective
roles of immunosuppressive therapy and of the underlying disease
remain to be established, however.
Cancers have been reported to occur after immunosuppressive
therapy with both cytotoxic and noncytotoxic drugs. Even though the
respective roles of genotoxicity and immunosuppression are difficult
to ascertain, cancers have been described in patients on azathioprine
after organ transplantation (Wessel et al., 1988; Singh et al., 1989)
or on low-dose methotrexate for autoimmune disorders (Ellman et al.,
1991; Shiroky et al., 1991; Kingsmore et al., 1992; Kamel et al.,
1993); immunosuppressive drugs increased the risk of malignancies
(especially lymphomas) in the treated patients. Interestingly, all of
the noncytotoxic immunosuppressive drugs were reportedly associated
with a variety of malignancies presumably related to immuno-
suppression. Whatever the type of tumour, the time to tumour
development after treatment with cyclosporin A was shorter (26 months
on average; 14 months for lymphomas) than after conventional
immunosuppressive therapy (60 months on average) (Penn, 1988). The
murine monoclonal antibody OKT3 was also shown to increase the risk
for lymphoproliferative disorders (Swinnen et al., 1990): Lymphomas
developed 1-18 months after starting OKT3, and a correlation was found
between the dose and time to neoplasm development. Lymphoproliferative
disorders were also shown to occur following treatment with FK 506
(Reyes et al., 1991). There are insufficient data to conclude a direct
causal relationship between immunosuppression induced by environmental
chemicals and the development of cancer; however, there is
epidemiological evidence that exposure to various potentially
immunotoxic chemicals (e.g. pesticides, benzene) is associated with
increased risks for cancers that also occur in immunosuppressed
patients (e.g. non-Hodgkin's lymphoma and leukaemia).
No marked difference was found in the relative risks for
lymphoproliferative disorders associated with the various
immunosuppressive drugs currently used, suggesting that
immunosuppression is the causative factor, particularly when account
is taken of the different carcinogenic potentials of azathioprine and
cyclosporin A, both clinical reference immunosuppressive agents
(Ryffel, 1992). Reactivation of latent viruses, e.g. Epstein-Barr
virus, due to immunosuppression was suggested to be involved. Indeed,
most lymphoproliferative disorders induced with cyclosporin A or
methotrexate were B-cell malignant lymphomas associated with this
viral infection (Starzl et al., 1984; Kamel et al., 1993). Uninhibited
proliferation of Epstein-Barr virus in B lymphocytes caused by the
efficient immunosuppression of one or more kinds of controls by
T lymphocytes is the commonly accepted mechanism. Interestingly,
Epstein-Barr virus-associated lymphomas in patients on
immunosuppressive therapy are usually reversible upon cessation of
treatment (Starzl et al., 1984).
2.1.1.2 Infectious diseases
Whatever the primary cause of the immune deficiency, patients
develop more frequent, more severe, recurring, and often atypical
infections, depending on the type and severity of the deficiency. The
complications associated with severe immunosuppression include
bacterial, viral, fungal, and parasitic infections (Waldman, 1988;
Barr et al., 1989; Mandell, 1990; Tieben et al., 1994). The pathogens
most frequently encountered in immunodeficient patients include the
bacterial agents Staphylococcus aureus, Streptococci, Escherichia
coli, Pseudomonas aeruginosa, Listeria monocytogenes, Mycobacterium
tuberculosis, and atypical mycobacteria. Herpes virus,
cytomegalovirus, Epstein-Barr virus, and human papillomavirus are the
leading causes of viral infections in immunosuppressed patients.
Fungal opportunistic infections include those induced by Candida,
Aspergillus, and Cryptococcus species. The immunotoxicity induced
by environmental chemicals often results in subtle changes in the
immune system, which have been suggested to result in increased
incidences of common infections like influenza and the common cold
(see section 2.4).
The respiratory tract is a primary target for infectious
pathogens, especially in immunosuppressed patients. Pulmonary
infections and infections of the upper respiratory tract are the most
common (Frattini & Trulock, 1993). Cytomegalovirus infections, often
asymptomatic, are particularly frequent in renal transplant patients
(Rubin, 1990). In addition, Pneumocystis carinii can cause a
particular form of pneumonia in immunosuppressed patients. Even though
respiratory diseases usually predominate, gastrointestinal infectious
diseases may constitute the leading consequences of immunosuppression
(Bodey et al., 1986). Infections of the central nervous system and
isolated fever are also extremely frequent. Interestingly, the type of
infection that develops in immunosuppressed patients is largely
dependent on the type of immune defect, as illustrated in Table 5.
Infectious complications have been commonly described in patients
treated with various cytotoxic drugs for cancer and with
immunosuppressants, such as cyclosporin A, for the prevention of
allograft rejection or the treatment of autoimmune disorders (Kim,
1989; Descotes & Vial, 1994).
Table 5. Pathogens frequently associated with immune defects
Humoral Cellular Neutrophil Complement
immunity immunity functions system
Campylobacter Candida Aspergillus Neisseria
Echovirus Coccidioides Bacteroides Staphylococci
Gardia Cryptococci Escherichia coli Streptococci
Haemophilus Cytomegalovirus Klebsiella
Pneumococci Herpes virus Pseudomonas
Salmonella
Legionella
Mycobacteria
Staphylococci
Histoplasma
Toxoplasma
Pneumocystis
Human papillomavirus
2.1.2 Consequences of immunostimulation
The health consequences of immunostimulation are less well
established than those associated with immunosuppression. A number of
adverse effects have, however, been reported after treatment with
immunostimulating drugs, including influenza-like reactions,
facilitation or exacerbation of underlying diseases, and inhibition of
hepatic drug metabolism (Descotes, 1992).
Patients with influenza-like reactions present with mild to
moderate fever associated with chills, malaise, and hypotension. The
reaction usually develops within hours after taking an
immunostimulating drug, and the patient recovers uneventfully within a
few hours. Such reactions are relatively uncommon with most
immunomodulating agents but have been shown to limit treatment with
several recombinant cytokines, e.g. IL-1 and tumour necrosis factor.
Facilitation and/or exacerbation of underlying diseases have been
ascribed to most immunostimulating drugs, but the incidence of this
adverse event differs markedly from one drug to another. Exacerbation
of chronic infections, psoriasis, and Crohn's disease have been
reported. More interestingly, several autoimmune diseases are more
frequent in patients treated with various (recombinant) cytokines,
e.g. autoimmunity treated with IFN gamma (Jacob et al., 1987),
thyroiditis with IL-2 (Vial & Descotes, 1993), and lupus erythematosus
with IFN alpha (Vial & Descotes, 1993). Exacerbation or facilitation
of allergic reactions to unrelated allergens has also been reported:
starting an immunostimulating treatment has been associated with
exacerbation of underlying eczema, asthma, or rhinitis. Allergic
reactions to radiological contrast media have been shown to be more
frequent in IL-2-treated patients than controls (Vial & Descotes,
1992).
Oxidative drug metabolism by the hepatic cytochrome P450 system
has been shown to be inhibited by immunostimulating drugs (Descotes,
1985) and by administration of bacillus Calmette-Guérin (BCG) vaccine
or interferon (Vial & Descotes, 1993). Although the mechanism of this
inhibition is still unknown, activation of macrophages resulting in
the release of IL-1 and IL-6 has been suggested to be involved.
Likewise, vaccination has been shown to compromise drug metabolism to
a sufficient extent that normally therapeutic doses of theophylline
caused acute toxicity in humans (Renton et al., 1980). Stimulation of
the immune system has also been shown to alter drug metabolism in
humans (Renton, 1986). A similar effect of infection has been reported
in laboratory animals (Selgrade et al., 1984); infection with mouse
cytomegalovirus before exposure to the insecticide parathion reduced
the total P450 concentration and dramatically increased the toxicity
of parathion.
So far, only a few environmental chemicals have been shown to
exert immunostimulating properties, e.g. hexachlorobenzene and
selenium. There have been no reports of clinical reactions to such
chemicals that are similar to the adverse effects seen with
immunostimulating drugs.
2.2 Direct immunotoxicity in laboratory animals
The following are some illustrative examples of immunotoxic
chemicals.
2.2.1 Azathioprine and cyclosporin A
The immunosuppressive effects of azathioprine and cyclosporin A
are considered because they can shed light on the direct
immunotoxicity of environmental chemicals.
2.2.1.1 Azathioprine
Azathioprine is a thiopurine that is used as cytostatic drug in
the treatment of leukaemias and as an immunosuppressant in patients
who have received allogeneic organ transplants or who have autoimmune
diseases. When used as an immunosuppressant, its main side-effect is
bone-marrow depression, reflected in blood leukocytopenia; its
administration must therefore be monitored through blood leukocyte
counts. Another side-effect, especially after long-term
administration, is tumour formation (IARC, 1987).
In rats, azathioprine is cytotoxic for all cell lineages in the
bone marrow, and strong cellular depletion is observed histologically.
It decreases the cellularity in thymus, blood, and peripheral lymphoid
organs, but it is mainly in the thymus that the immature lymphocyte
population of the cortex is affected. This effect is a general feature
of most cytostatic drugs. A similar effect is seen in the thymus after
treatment with glucocorticosteroids, but the molecular mechanism
resulting in lymphocyte depletion is obviously different: interference
with DNA synthesis resulting in lymphocyte proliferation in contrast
to binding to glucocorticosteroid receptors and cell down-modulation.
Azathioprine affects a number of indicators of immune function, like
macrophage cytotoxicity (Spreafico et al., 1987), lymphocyte
proliferation in vitro after mitogen stimulation (Weissgarten et
al., 1989) and in the mixed leukocyte reaction (Mellert et al., 1989),
and cytotoxicity by NK cells (Pedersen & Beyer, 1986; Spreafico et
al., 1987; Versluis et al., 1989). Both stimulation and suppression of
these functions have been found in experimental animals, depending on
the dosage and the time of testing after exposure. These findings are
in accordance with those in azathioprine-treated patients, who showed
no change in primary antibody response, a decrease in secondary
antibody response, and some or no effect on lymphocyte proliferation
in vitro after mitogen stimulation. The time of testing after the
start of exposure to azathioprine was a crucial factor in the
detection of effects. Azathioprine was tested in the IPCS-European
Union international collaborative immunotoxicity study (see section
1.1) and showed a significant strain-dependent sensitivity.
2.2.1.2 Cyclosporin A
Cyclosporin A is one of the most powerful immunosuppressive drugs
(Kahan, 1989). It is a neutral lipophilic cyclic peptide consisting of
11 amino acids (relative molecular mass, 1203 Da) isolated from the
fungus Tolypocladium inflatum. Its main use is in bone-marrow
transplantation to prevent transplant rejection and graft-versus-host
reactions. It is also used in the therapy of various autoimmune
diseases.
A complication of cyclosporin A treatment is nephrotoxicity.
Another side-effect, especially after long-term administration, is
tumour formation (IARC, 1987). In its immunosuppressive action,
cyclosporin A does not affect resting lymphocytes but blocks the
events occurring after stimulation, particularly the synthesis of
lymphokines, including IL-1 and IL-2, and IL-2 receptors. The
synthesis of IL-1 by antigen-presenting cells and of IL-2 by Th cells
is inhibited, and the synthesis of IFN gamma and tumour necrosis
factor is blocked. These events occur inside the cell at the
transcriptional level. Cyclosporin A binds to an intracellular
receptor, cyclophilin, forming a complex with calcineurin; this
complex in turn interferes with the activation of genes, resulting in
inhibition of lymphokine gene transcription (Baumann et al., 1992;
Sigal & Dumont, 1992).
An interesting feature of cyclosporin A is its specific action on
the thymus and the induction of autoimmune phenomena. Rats treated
with total body irradiation and syngeneic or autologous bone-marrow
transplantation, followed by treatment with cyclosporin A at a dose of
about 10 mg/kg body weight per day subcutaneously for four weeks,
developed signs of acute graft-versus-host reactions, with lymphocytic
infiltration at multiple epithelial sites (Glazier et al., 1983). A
similar pseudo-graft-versus-host reaction has also been evoked in
mice. It is associated with thymic changes, because it can be
transferred in whole thymus or thymocytes (Sakaguchi & Sakaguchi,
1988). Histologically, the medullary area is diminished (Beschorner et
al., 1987a; Schuurman et al., 1990; see also Figure 21). The medullary
stroma shows a decrease in MHC class II expression, indicating a loss
of dendritic cells, which has been confirmed by electron microscopy
(De Waal et al., 1992a). As these cells normally contribute to the
negative selection process, their depletion (or reduced MHC class II
expression) may be related to an absence of negative selection. The
autoreactive T cells may even attack the medullary epithelium.

 The effect of cyclosporin A on thymic functions, i.e. the
induction of 'leakiness', with export of T cells that have not been
negatively selected, has not yet been studied for other drugs, but may
not be specific to cyclosporin A. It represents a distinct mechanism
of autoimmunity induced by the action of toxic compounds on the immune
system, mediated via thymic selection. Although the medullary area is
reduced in young rats after treatment with cyclosporin A, this is not
the case in one-year-old rats, which presumably have a lesser output
of mature T cells because of thymic involution (Beschorner et al.,
1987b).
The effect of cyclosporin A in inducing syngeneic graft-versus-
host disease in rodents has an application in clinical medicine:
Patients treated for cancer with high-dose chemotherapy and/or total
body irradiation, followed by autologous bone-marrow transplantation,
develop a recurrence of the original tumour at a higher incidence than
patients who receive an allogeneic bone-marrow transplant. This
difference has been ascribed to the addition of a graft-versus-tumour
effect to the graft-versus-host reaction. Trials have now been
initiated to induce a graft-versus-host reaction with cyclosporin A
treatment after autologous bone-marrow transplantation, in order to
reduce tumour recurrence. The initial results are promising (Hess et
al., 1992; Yeager et al., 1993; Kennedy et al., 1994).
Interestingly, two other immunosuppressive drugs, FK-506 and
rapamycin, which also interfere with gene activation in T lymphocytes,
do not bind to cyclophilin but to another intracellular receptor, the
FK-binding protein. The effect of FK-506 on the thymus is similar to
that of cyclosporin A, i.e. a decrease in the medulla (Pugh-Humphreys
et al., 1990), and rapamycin causes severe acute involution with
disappearance of lymphocytes from the cortex (Zheng et al., 1991).
These findings indicate that the two compounds have different
molecular mechanisms of action on the thymus from those of cyclosporin
A, which have not yet been elucidated.
2.2.2 Halogenated hydrocarbons
2.2.2.1 2,3,7,8-Tetrachlorodibenzo-para-dioxin
The halogenated hydrocarbon most closely studied for its
immunotoxic effects is TCDD. It has a variety of toxic effects, with a
remarkable interspecies variation; however, it causes atrophy of the
thymus and immunotoxicity in all species investigated (Vos & Luster,
1989; Holsapple et al., 1991; Neubert, 1992; Kerkvliet & Burleson,
1994). Atrophy of the thymus is reflected histologically by lymphocyte
depletion of the cortex (Figure 22). Functionally, cell-mediated
immunity appears to be suppressed in a dose-dependent fashion, as
manifested in delayed-type hypersensitivity responses, rejection of
allogeneic skin transplants, graft-versus-host reactivity, and
lymphocyte proliferation in vitro after mitogen stimulation. This
immune suppression is age-related: more severe immunotoxic effects are
observed after perinatal administration than after administration in
adulthood (Vos & Moore, 1974; Thomas & Hinsdill, 1979). TCDD can also
impair antibody-mediated immunity after primary or secondary
immunization. A sensitive parameter of the immunotoxicity of TCDD and
TCDD congeners in mice is suppression of the T cell-dependent antibody
response to sheep red blood cells in mice (Vecchi et al., 1980; Davis
& Safe, 1988; Kerkvliet et al., 1990). No effects have been observed
on classical macrophage functions.
In mice, susceptibility to TCDD is genetically determined and is
segregated at the locus that encodes a cytosolic protein which
mediates aryl hydrocarbon hydroxylase activity (Poland & Knutson,
1982). This Ah (aromatic hydrocarbon) receptor has a high affinity for
TCDD and is strongly active in mouse and rat thymus (Gasiewicz &
Rucci, 1984), particularly in epithelial cells (Greenlee et al., 1985;
Cook et al., 1987). Ah receptor-dependent immunotoxicity has been
demonstrated in mice for thymic atrophy and the antibody response to
sheep red blood cells (Tucker et al., 1986; Kerkvliet & Burleson,
1994); however, the importance of Ah receptor-mediated events in
chronic, low-level TCDD immunotoxicity is controversial (Morris et
al., 1992).
Immunosuppression in adult mice manifests almost exclusively as
suppressed antibody responses and does not appear to be related to
thymic atrophy in experiments in thymectomized (Tucker et al., 1986)
and nude (Kerkvliet & Brauner, 1987) mice. Both T and B lymphocytes
involved in antibody responses can, however, be affected by TCDD. For
example, exposure to TCDD in vivo alters regulatory lymphocyte
function (Kerkvliet & Brauner, 1987) and antigen-specific T lymphocyte
activation (Lundberg et al., 1992). TCDD also inhibits T-independent
antigen responses (Vecchi et al., 1983) and T-dependent responses when
only B cells are treated (Dooley & Holsapple, 1988). Studies of the
effects of TCDD on enriched B-cell populations in vitro have shown
that it selectively inhibits late stages of the cell cycle and the
development of B cells into plasma cells after antigen-specific
activation (Luster et al., 1988). The molecular events responsible for
TCDD immunosuppression have not been examined in detail. While early
events in B-cell maturation, such as inositol phosphate accumulation,
are not affected (Luster et al. 1988), activation of protein kinase
(Kramer et al., 1987) and tyrosine kinase (Clark et al., 1991) have
been observed.
A consequence of TCDD-induced immunosuppression is impaired
resistance to infection by bacterial, viral, and protozoan
microorganisms (Vos et al., 1991). In various mouse strains with
different treatment schedules, TCDD suppressed resistance to models
of infectious diseases with Salmonella bern, S. typhimurium,
Streptococcus pneumoniae, herpes II, Plasmodium yoelli and influenza
viruses. Various effects have been reported on resistance to
The effect of cyclosporin A on thymic functions, i.e. the
induction of 'leakiness', with export of T cells that have not been
negatively selected, has not yet been studied for other drugs, but may
not be specific to cyclosporin A. It represents a distinct mechanism
of autoimmunity induced by the action of toxic compounds on the immune
system, mediated via thymic selection. Although the medullary area is
reduced in young rats after treatment with cyclosporin A, this is not
the case in one-year-old rats, which presumably have a lesser output
of mature T cells because of thymic involution (Beschorner et al.,
1987b).
The effect of cyclosporin A in inducing syngeneic graft-versus-
host disease in rodents has an application in clinical medicine:
Patients treated for cancer with high-dose chemotherapy and/or total
body irradiation, followed by autologous bone-marrow transplantation,
develop a recurrence of the original tumour at a higher incidence than
patients who receive an allogeneic bone-marrow transplant. This
difference has been ascribed to the addition of a graft-versus-tumour
effect to the graft-versus-host reaction. Trials have now been
initiated to induce a graft-versus-host reaction with cyclosporin A
treatment after autologous bone-marrow transplantation, in order to
reduce tumour recurrence. The initial results are promising (Hess et
al., 1992; Yeager et al., 1993; Kennedy et al., 1994).
Interestingly, two other immunosuppressive drugs, FK-506 and
rapamycin, which also interfere with gene activation in T lymphocytes,
do not bind to cyclophilin but to another intracellular receptor, the
FK-binding protein. The effect of FK-506 on the thymus is similar to
that of cyclosporin A, i.e. a decrease in the medulla (Pugh-Humphreys
et al., 1990), and rapamycin causes severe acute involution with
disappearance of lymphocytes from the cortex (Zheng et al., 1991).
These findings indicate that the two compounds have different
molecular mechanisms of action on the thymus from those of cyclosporin
A, which have not yet been elucidated.
2.2.2 Halogenated hydrocarbons
2.2.2.1 2,3,7,8-Tetrachlorodibenzo-para-dioxin
The halogenated hydrocarbon most closely studied for its
immunotoxic effects is TCDD. It has a variety of toxic effects, with a
remarkable interspecies variation; however, it causes atrophy of the
thymus and immunotoxicity in all species investigated (Vos & Luster,
1989; Holsapple et al., 1991; Neubert, 1992; Kerkvliet & Burleson,
1994). Atrophy of the thymus is reflected histologically by lymphocyte
depletion of the cortex (Figure 22). Functionally, cell-mediated
immunity appears to be suppressed in a dose-dependent fashion, as
manifested in delayed-type hypersensitivity responses, rejection of
allogeneic skin transplants, graft-versus-host reactivity, and
lymphocyte proliferation in vitro after mitogen stimulation. This
immune suppression is age-related: more severe immunotoxic effects are
observed after perinatal administration than after administration in
adulthood (Vos & Moore, 1974; Thomas & Hinsdill, 1979). TCDD can also
impair antibody-mediated immunity after primary or secondary
immunization. A sensitive parameter of the immunotoxicity of TCDD and
TCDD congeners in mice is suppression of the T cell-dependent antibody
response to sheep red blood cells in mice (Vecchi et al., 1980; Davis
& Safe, 1988; Kerkvliet et al., 1990). No effects have been observed
on classical macrophage functions.
In mice, susceptibility to TCDD is genetically determined and is
segregated at the locus that encodes a cytosolic protein which
mediates aryl hydrocarbon hydroxylase activity (Poland & Knutson,
1982). This Ah (aromatic hydrocarbon) receptor has a high affinity for
TCDD and is strongly active in mouse and rat thymus (Gasiewicz &
Rucci, 1984), particularly in epithelial cells (Greenlee et al., 1985;
Cook et al., 1987). Ah receptor-dependent immunotoxicity has been
demonstrated in mice for thymic atrophy and the antibody response to
sheep red blood cells (Tucker et al., 1986; Kerkvliet & Burleson,
1994); however, the importance of Ah receptor-mediated events in
chronic, low-level TCDD immunotoxicity is controversial (Morris et
al., 1992).
Immunosuppression in adult mice manifests almost exclusively as
suppressed antibody responses and does not appear to be related to
thymic atrophy in experiments in thymectomized (Tucker et al., 1986)
and nude (Kerkvliet & Brauner, 1987) mice. Both T and B lymphocytes
involved in antibody responses can, however, be affected by TCDD. For
example, exposure to TCDD in vivo alters regulatory lymphocyte
function (Kerkvliet & Brauner, 1987) and antigen-specific T lymphocyte
activation (Lundberg et al., 1992). TCDD also inhibits T-independent
antigen responses (Vecchi et al., 1983) and T-dependent responses when
only B cells are treated (Dooley & Holsapple, 1988). Studies of the
effects of TCDD on enriched B-cell populations in vitro have shown
that it selectively inhibits late stages of the cell cycle and the
development of B cells into plasma cells after antigen-specific
activation (Luster et al., 1988). The molecular events responsible for
TCDD immunosuppression have not been examined in detail. While early
events in B-cell maturation, such as inositol phosphate accumulation,
are not affected (Luster et al. 1988), activation of protein kinase
(Kramer et al., 1987) and tyrosine kinase (Clark et al., 1991) have
been observed.
A consequence of TCDD-induced immunosuppression is impaired
resistance to infection by bacterial, viral, and protozoan
microorganisms (Vos et al., 1991). In various mouse strains with
different treatment schedules, TCDD suppressed resistance to models
of infectious diseases with Salmonella bern, S. typhimurium,
Streptococcus pneumoniae, herpes II, Plasmodium yoelli and influenza
viruses. Various effects have been reported on resistance to

 L. monocytogenes. TCDD had no effect on the mortality of mice
infected with Herpes suis (pseudorabies), whereas the mortality of
mice infected with influenza virus was enhanced by a single oral dose
of TCDD as low as 10 ng/kg body weight (Burleson et al., in press).
Many studies have been performed to investigate the mechanisms of
TCDD-induced thymic atrophy, and a number have presented evidence that
the effect may occur through an action on epithelial cells:
1. The enhanced lymphoproliferation of thymocytes after coculture
with cultured mouse and human epithelial cells was reduced when
the epithelial cells were pretreated with TCDD (Greenlee et al.,
1985; Cook et al., 1987).
2. In mouse radiation chimaeras, TCDD-induced suppression of Tc
lymphocytes is determined by the host (epithelium) and not the
donor (bone marrow, subsequently thymocytes) (Nagarkatti et al.,
1984).
3. Histological and electron microscopy studies of TCDD-exposed rats
reveal formation of epithelial aggregates and a more
differentiated state of cortical epithelium, indicating that TCDD
acts on the thymic epithelium (De Waal et al., 1992b, 1993).
A direct action of TCDD on rat thymocytes has also been
documented in vitro as cell death due to apoptosis (McConkey et al.,
1988), but this effect requires higher concentrations than those that
affect epithelial cell function in vitro. In bone marrow, TCDD
affects myelopoiesis (Luster et al., 1985a) but may be more selective
for prothymocytes (Fine et al., 1989, 1990; Holladay et al., 1991;
Blaylock et al., 1992), thus indirectly affecting thymic function.
2.2.2.2 Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are important environmental
chemicals shown in numerous studies to have immunotoxic properties.
PCB mixtures alter several morphological and functional aspects of the
immune system in rodents, guinea-pigs, rabbits, and chickens (Vos &
Luster, 1989). The first suggestion that PCBs might affect the immune
system came from observations on the weight and histology of lymphoid
organs. Oral exposure of chickens to PCBs resulted in small spleens
(Flick et al., 1965) and atrophy of lymphoid tissue (Vos & Koeman,
1970). Similar effects were noted in rabbits and guinea-pigs
(Figure 23). Dermal application of PCBs to rabbits caused lymphopenia,
atrophy of the thymic cortex, and a reduced number of germinal centres
in spleen and lymph nodes (Vos & Beems, 1971). Oral exposure of guinea-
pigs significantly reduced the number of circulating lymphocytes
and the relative thymus weight (Vos & Van Driel-Grootenhuis, 1972).
L. monocytogenes. TCDD had no effect on the mortality of mice
infected with Herpes suis (pseudorabies), whereas the mortality of
mice infected with influenza virus was enhanced by a single oral dose
of TCDD as low as 10 ng/kg body weight (Burleson et al., in press).
Many studies have been performed to investigate the mechanisms of
TCDD-induced thymic atrophy, and a number have presented evidence that
the effect may occur through an action on epithelial cells:
1. The enhanced lymphoproliferation of thymocytes after coculture
with cultured mouse and human epithelial cells was reduced when
the epithelial cells were pretreated with TCDD (Greenlee et al.,
1985; Cook et al., 1987).
2. In mouse radiation chimaeras, TCDD-induced suppression of Tc
lymphocytes is determined by the host (epithelium) and not the
donor (bone marrow, subsequently thymocytes) (Nagarkatti et al.,
1984).
3. Histological and electron microscopy studies of TCDD-exposed rats
reveal formation of epithelial aggregates and a more
differentiated state of cortical epithelium, indicating that TCDD
acts on the thymic epithelium (De Waal et al., 1992b, 1993).
A direct action of TCDD on rat thymocytes has also been
documented in vitro as cell death due to apoptosis (McConkey et al.,
1988), but this effect requires higher concentrations than those that
affect epithelial cell function in vitro. In bone marrow, TCDD
affects myelopoiesis (Luster et al., 1985a) but may be more selective
for prothymocytes (Fine et al., 1989, 1990; Holladay et al., 1991;
Blaylock et al., 1992), thus indirectly affecting thymic function.
2.2.2.2 Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are important environmental
chemicals shown in numerous studies to have immunotoxic properties.
PCB mixtures alter several morphological and functional aspects of the
immune system in rodents, guinea-pigs, rabbits, and chickens (Vos &
Luster, 1989). The first suggestion that PCBs might affect the immune
system came from observations on the weight and histology of lymphoid
organs. Oral exposure of chickens to PCBs resulted in small spleens
(Flick et al., 1965) and atrophy of lymphoid tissue (Vos & Koeman,
1970). Similar effects were noted in rabbits and guinea-pigs
(Figure 23). Dermal application of PCBs to rabbits caused lymphopenia,
atrophy of the thymic cortex, and a reduced number of germinal centres
in spleen and lymph nodes (Vos & Beems, 1971). Oral exposure of guinea-
pigs significantly reduced the number of circulating lymphocytes
and the relative thymus weight (Vos & Van Driel-Grootenhuis, 1972).
 Functional tests have been focused on humoral immune responses.
Exposure of guinea-pigs, rabbits, mice, and rats to PCBs at different
regimens reduced antibody production to foreign antigens, including
tetanus toxoid, pseudorabies virus, sheep red blood cells, and keyhole
limpet haemocyanin (Vos & Van Driel-Grootenhuis, 1972; Koller &
Thigpen, 1973; Loose et al., 1977; Wierda et al., 1981; Exon, 1985;
Kunita et al., 1985). These data are in line with the observations of
Loose et al. (1977) and Thomas & Hinsdill (1978) that exposure to PCBs
lowered circulating immunoglobulin levels in mice. No reduction was
reported in antibody responses to bovine serum albumin (Talcott &
Koller, 1983).
The response to sheep red blood cells in the plaque-forming cell
assay has been used to establish dose-response relationships for
several potentially immunotoxic Aroclors in mice given a single
intraperitoneal injection of PCB mixtures (Davis & Safe, 1989). These
studies indicate that the higher chlorinated PCB mixtures are more
immunotoxic than the lower chlorinated Aroclors (Allen & Abrahamson,
1973; Loose et al., 1978; Tryphonas, in press). Data on the effects of
PCBs on total serum immunoglobulin levels have not been reported in
non-immunized animals.
While the suppressive effects of PCBs on humoral immunity are
well documented, the effects on cell-mediated immune parameters are
less clear. The delayed-type hypersensitivity reaction to tuberculin
was suppressed in guinea-pigs (Vos & Van Driel-Grootenhuis, 1972) but
not in rabbits treated with PCBs (Street & Sharma, 1975). Decreased
delayed-type hypersensitivity reactions were reported in mice by Smith
et al. (1978) but not by others (Talcott & Koller, 1983). Kerkvliet &
Baecher-Steppan (1988) reported that 3,4,5,3',4',5'-hexachlorobiphenyl
reduced Tc lymphocyte activity in the spleens of mice. In contrast,
the graft-versus-host reaction was increased following PCB treatment
(Carter & Clancy, 1980). Studies on the mitogen-induced responses of
splenic mononuclear leukocytes from PCB-treated mice in vitro
resulted in either enhanced or unaltered responses (Bonnyns &
Bastomsky, 1976; Wierda et al., 1981; Davis & Safe, 1989; Smialowicz
et al., 1989).
Functional impairment of the non-specific resistance of macro-
phages has been reported, including reduced phagocytic activity and
clearance of pathogenic bacteria by the spleens and livers of PCB-
exposed animals (Smith et al., 1978) and decreased NK cell activity
(Talcott et al., 1985; Smialowicz et al., 1989). Exposure of mice to
PCBs also enhanced their sensitivity to endotoxin shock (Loose et al.,
1978; Thomas & Hinsdill, 1978).
PCB treatment was shown to protect mice and rats against
Ehrlich's tumour (Keck, 1981) and Walker 256 tumours (Kerkvliet &
Kimeldorf, 1977), shown as reduced tumour growth and metastasis after
transplantation; in other studies, however, no influence of PCB on
tumour-cell implants was reported (Koller, 1977; Loose et al., 1981).
PCBs also affect the resistance of animals to infectious diseases.
Thus, ducklings exposed to low levels of PCBs were more susceptible to
challenge with duck hepatitis virus (Friend & Trainer, 1970), and mice
were more susceptible to challenge with Moloney leukaemia virus
(Koller, 1977), Plasmodium berghei (Loose et al., 1978),
S. typhimurium (Loose et al., 1978), L. monocytogenes (Thomas &
Hinsdill, 1978), and Herpes simplex and Ectromelia viruses
(Imanishi et al., 1980).
The immunotoxic effects of PCBs have also been investigated in a
number of studies with non-human primates. Decreased titres of anti-
sheep red blood cells have been observed in PCB-exposed rhesus (Thomas
& Hinsdill, 1978) and cynomolgus monkeys (Hori et al., 1982; Truelove
et al., 1982; Kunita et al., 1985). Immunotoxic effects were also
reported in adult female rhesus monkeys and their infants (exposed
in utero and through lactation) after low-level exposure (Tryphonas
et al., 1989, 1991a,b). In this study, five groups of female rhesus
monkeys were administered PCB (Aroclor 1254) at 0, 5.0, 20.0, 40.0, or
80.0 µg/kg body weight per day orally. Immunological effects were
reported after both 23 and 55 months of exposure and comprised
significantly decreased IgM and IgG responses to sheep red blood cells
at the lowest dose. Alterations in T-cell subsets were reported in the
group receiving the high dose in comparison with the controls, which
were characterized by an increase in Ts/Tc (CD8) cells and a reduction
in the relative numbers of Th/inducer cells (CD4) and in the CD4:CD8
ratio. No effects were seen on total lymphocytes or on B cells or on
total serum IgG, IgM, and IgA levels. A further study indicated that
Aroclor 1254 had no effect on B lymphocytes, since antibody responses
to T-independent pneumoccocal antigen were not significantly affected.
A trend for reduced incorporation of 3H-thymidine by mitogen-induced
lymphocyte proliferation was noted only for the T mitogens
phytohaemagglutinin and concanavalin A and not for the B pokeweed
mitogen. A significant augmentation of NK cell activity was noted at
the highest dose. Total serum complement activity (CH50) was also
increased. The serum levels of corticosteroids (hydrocortisone), which
were measured throughout the study, were not affected by treatment
(Loo et al., 1989), clearly indicating that the changes in several of
the immune parameters were direct effects of Aroclor 1254 on the
immune system.
2.2.2.3 Hexachlorobenzene
Hexachlorobenzene (HCB) is a highly persistent chemical which was
used in the past as a fungicide. Emissions to the environment now
occur owing to its use as a chemical intermediate and its presence as
a by-product in several chemical processes. It is an immunotoxic
compound (Vos, 1986), with different effects in rats and mice. In
rats, the main changes seen after subacute exposure are increased
weights of spleen and lymph nodes; the serum levels of IgM are also
increased. Histologically, the spleen shows hyperplasia of follicles
and the marginal zone (Figure 24); the lymph nodes have more follicles
with germinal centres and greater proportions of high endothelial
venules, indicating activation (Figures 25 and 26). High endothelial-
type venules are also induced in the lung (Figure 27), and macrophages
accumulate in lung alveoli (Kitchin et al., 1982; Vos, 1986; see also
Figure 28).
Functional assessment showed an increase in cell-mediated
immunity (delayed-type hypersensitivity) and an even greater increase
in antibody-mediated immunity (primary and secondary antibody response
to tetanus toxoid). Macrophage functions were unaltered. Stimulation
of immune reactivity occurs at dietary levels as low as 4 mg/kg after
combined pre- and postnatal exposure for six weeks, whereas the
conventional parameters of hepatotoxicity are not altered at this dose
(Vos et al., 1979a; Vos, 1986). The developing immune system of the
rat therefore seems to be particularly vulnerable to the immunotoxic
action of HCB. Reduced NK cell activity has also been found in the
lung after oral exposure to 150-450 mg/kg HCB in the diet (Van Loveren
et al., 1990c).
Studies on the mechanism of action of HCB indicate a role for
T cells: congenitally athymic rnu/rnu rats, which lack T cells, do
not manifest the hyperplasia of B lymphocytes in splenic follicles and
the marginal zone after administration of the compound; but
endothelial cell proliferation and macrophage accumulation in the lung
are apparently T cell-independent, as these effects were seen in
athymic animals (Vos et al., 1990b). In contrast to the
immunostimulatory effect in rats, HCB suppresses cell-mediated and
antibody-mediated immunity in mice, as well as their resistance to
protozoan infections ( Leishmania and Plasmodium berghei) and to
inoculated tumours (Loose et al., 1977, 1978, 1981). The
susceptibility of mice to HCB is also higher after pre-or perinatal
administration (Barnett et al., 1987). Recent studies indicate that
the immunostimulatory effect of HCB in rats may be related to
autoimmunity:
Functional tests have been focused on humoral immune responses.
Exposure of guinea-pigs, rabbits, mice, and rats to PCBs at different
regimens reduced antibody production to foreign antigens, including
tetanus toxoid, pseudorabies virus, sheep red blood cells, and keyhole
limpet haemocyanin (Vos & Van Driel-Grootenhuis, 1972; Koller &
Thigpen, 1973; Loose et al., 1977; Wierda et al., 1981; Exon, 1985;
Kunita et al., 1985). These data are in line with the observations of
Loose et al. (1977) and Thomas & Hinsdill (1978) that exposure to PCBs
lowered circulating immunoglobulin levels in mice. No reduction was
reported in antibody responses to bovine serum albumin (Talcott &
Koller, 1983).
The response to sheep red blood cells in the plaque-forming cell
assay has been used to establish dose-response relationships for
several potentially immunotoxic Aroclors in mice given a single
intraperitoneal injection of PCB mixtures (Davis & Safe, 1989). These
studies indicate that the higher chlorinated PCB mixtures are more
immunotoxic than the lower chlorinated Aroclors (Allen & Abrahamson,
1973; Loose et al., 1978; Tryphonas, in press). Data on the effects of
PCBs on total serum immunoglobulin levels have not been reported in
non-immunized animals.
While the suppressive effects of PCBs on humoral immunity are
well documented, the effects on cell-mediated immune parameters are
less clear. The delayed-type hypersensitivity reaction to tuberculin
was suppressed in guinea-pigs (Vos & Van Driel-Grootenhuis, 1972) but
not in rabbits treated with PCBs (Street & Sharma, 1975). Decreased
delayed-type hypersensitivity reactions were reported in mice by Smith
et al. (1978) but not by others (Talcott & Koller, 1983). Kerkvliet &
Baecher-Steppan (1988) reported that 3,4,5,3',4',5'-hexachlorobiphenyl
reduced Tc lymphocyte activity in the spleens of mice. In contrast,
the graft-versus-host reaction was increased following PCB treatment
(Carter & Clancy, 1980). Studies on the mitogen-induced responses of
splenic mononuclear leukocytes from PCB-treated mice in vitro
resulted in either enhanced or unaltered responses (Bonnyns &
Bastomsky, 1976; Wierda et al., 1981; Davis & Safe, 1989; Smialowicz
et al., 1989).
Functional impairment of the non-specific resistance of macro-
phages has been reported, including reduced phagocytic activity and
clearance of pathogenic bacteria by the spleens and livers of PCB-
exposed animals (Smith et al., 1978) and decreased NK cell activity
(Talcott et al., 1985; Smialowicz et al., 1989). Exposure of mice to
PCBs also enhanced their sensitivity to endotoxin shock (Loose et al.,
1978; Thomas & Hinsdill, 1978).
PCB treatment was shown to protect mice and rats against
Ehrlich's tumour (Keck, 1981) and Walker 256 tumours (Kerkvliet &
Kimeldorf, 1977), shown as reduced tumour growth and metastasis after
transplantation; in other studies, however, no influence of PCB on
tumour-cell implants was reported (Koller, 1977; Loose et al., 1981).
PCBs also affect the resistance of animals to infectious diseases.
Thus, ducklings exposed to low levels of PCBs were more susceptible to
challenge with duck hepatitis virus (Friend & Trainer, 1970), and mice
were more susceptible to challenge with Moloney leukaemia virus
(Koller, 1977), Plasmodium berghei (Loose et al., 1978),
S. typhimurium (Loose et al., 1978), L. monocytogenes (Thomas &
Hinsdill, 1978), and Herpes simplex and Ectromelia viruses
(Imanishi et al., 1980).
The immunotoxic effects of PCBs have also been investigated in a
number of studies with non-human primates. Decreased titres of anti-
sheep red blood cells have been observed in PCB-exposed rhesus (Thomas
& Hinsdill, 1978) and cynomolgus monkeys (Hori et al., 1982; Truelove
et al., 1982; Kunita et al., 1985). Immunotoxic effects were also
reported in adult female rhesus monkeys and their infants (exposed
in utero and through lactation) after low-level exposure (Tryphonas
et al., 1989, 1991a,b). In this study, five groups of female rhesus
monkeys were administered PCB (Aroclor 1254) at 0, 5.0, 20.0, 40.0, or
80.0 µg/kg body weight per day orally. Immunological effects were
reported after both 23 and 55 months of exposure and comprised
significantly decreased IgM and IgG responses to sheep red blood cells
at the lowest dose. Alterations in T-cell subsets were reported in the
group receiving the high dose in comparison with the controls, which
were characterized by an increase in Ts/Tc (CD8) cells and a reduction
in the relative numbers of Th/inducer cells (CD4) and in the CD4:CD8
ratio. No effects were seen on total lymphocytes or on B cells or on
total serum IgG, IgM, and IgA levels. A further study indicated that
Aroclor 1254 had no effect on B lymphocytes, since antibody responses
to T-independent pneumoccocal antigen were not significantly affected.
A trend for reduced incorporation of 3H-thymidine by mitogen-induced
lymphocyte proliferation was noted only for the T mitogens
phytohaemagglutinin and concanavalin A and not for the B pokeweed
mitogen. A significant augmentation of NK cell activity was noted at
the highest dose. Total serum complement activity (CH50) was also
increased. The serum levels of corticosteroids (hydrocortisone), which
were measured throughout the study, were not affected by treatment
(Loo et al., 1989), clearly indicating that the changes in several of
the immune parameters were direct effects of Aroclor 1254 on the
immune system.
2.2.2.3 Hexachlorobenzene
Hexachlorobenzene (HCB) is a highly persistent chemical which was
used in the past as a fungicide. Emissions to the environment now
occur owing to its use as a chemical intermediate and its presence as
a by-product in several chemical processes. It is an immunotoxic
compound (Vos, 1986), with different effects in rats and mice. In
rats, the main changes seen after subacute exposure are increased
weights of spleen and lymph nodes; the serum levels of IgM are also
increased. Histologically, the spleen shows hyperplasia of follicles
and the marginal zone (Figure 24); the lymph nodes have more follicles
with germinal centres and greater proportions of high endothelial
venules, indicating activation (Figures 25 and 26). High endothelial-
type venules are also induced in the lung (Figure 27), and macrophages
accumulate in lung alveoli (Kitchin et al., 1982; Vos, 1986; see also
Figure 28).
Functional assessment showed an increase in cell-mediated
immunity (delayed-type hypersensitivity) and an even greater increase
in antibody-mediated immunity (primary and secondary antibody response
to tetanus toxoid). Macrophage functions were unaltered. Stimulation
of immune reactivity occurs at dietary levels as low as 4 mg/kg after
combined pre- and postnatal exposure for six weeks, whereas the
conventional parameters of hepatotoxicity are not altered at this dose
(Vos et al., 1979a; Vos, 1986). The developing immune system of the
rat therefore seems to be particularly vulnerable to the immunotoxic
action of HCB. Reduced NK cell activity has also been found in the
lung after oral exposure to 150-450 mg/kg HCB in the diet (Van Loveren
et al., 1990c).
Studies on the mechanism of action of HCB indicate a role for
T cells: congenitally athymic rnu/rnu rats, which lack T cells, do
not manifest the hyperplasia of B lymphocytes in splenic follicles and
the marginal zone after administration of the compound; but
endothelial cell proliferation and macrophage accumulation in the lung
are apparently T cell-independent, as these effects were seen in
athymic animals (Vos et al., 1990b). In contrast to the
immunostimulatory effect in rats, HCB suppresses cell-mediated and
antibody-mediated immunity in mice, as well as their resistance to
protozoan infections ( Leishmania and Plasmodium berghei) and to
inoculated tumours (Loose et al., 1977, 1978, 1981). The
susceptibility of mice to HCB is also higher after pre-or perinatal
administration (Barnett et al., 1987). Recent studies indicate that
the immunostimulatory effect of HCB in rats may be related to
autoimmunity:





 1. Exposure to HCB of Lewis rats, which develop autoimmune disease
after sensitization with complete Freund's adjuvant (adjuvant
arthritis) or with guinea-pig myelin (experimental allergic
encephalomyelitis), had clear effects (Van Loveren et al., 1990c):
Whereas the allergic encephalomyelitis response was severely enhanced,
the arthritic lesions were strongly suppressed.
2. Wistar rats treated with HCB produce antibodies to autoantigens;
thus, IgM, but not IgG, levels against single-stranded DNA, native
DNA, rat IgG (representing rheumatoid factor), and bromelain-treated
mouse erythrocytes (indicating that phosphatidylcholine is a major
autoantigen) were elevated. It has been suggested that HCB activates a
B-cell subset committed to the production of these autoantibodies and
associated with various systemic autoimmune diseases (Schielen et al.,
1993).
2.2.3 Pesticides
A large number of studies have focused on the immunotoxicity of
pesticides. Because of the chemical heterogeneity of these compounds
as a class, the reported effects vary widely (Barnett & Rodgers,
1994).
2.2.3.1 Organochlorine pesticides
The evidence for the immunotoxicity of organochlorine pesticides
as a class is inconclusive.
DDT: Wistar rats treated with 40 mg/kg body weight per day DDT
orally for 60 days showed increased anti-bovine serum albumin titres
(Lukic et al., 1973). Studies by Vos et al. (Vos & Krajnc, 1983, Vos
et al., 1983a), however, showed no changes in thymus or spleen
weights, leukocyte counts, or total serum IgG and IgM levels at doses
up to 800 mg/kg body weight per day.
Chlordane: Prenatal exposure of mice to chlordane was reported
to reduce contact and delayed hypersensitivity responses, suggesting
an effect on T-cell responses. Attempts to elucidate the mechanism
have, however, been unsuccessful. Johnson et al. (1986) observed
increased lymphocyte proliferation only at a dose of 8 mg/kg body
weight in B6C3F1 mice and concluded that chlordane has no significant
immunotoxicity in this model.
Chlordecone: Chlordecone reduced thymus and spleen weights by
40% in Fischer rats at at a dose of 10 mg/kg body weight per day but
had no significant effect at or below 5 mg/kg body weight per day.
T-Lymphocyte proliferation was unaffected at all doses (Smialowicz et
al., 1985a).
Lindane: Lindane had various effects on the anti-sheep red
blood cell response, depending on the immunization protocol. Specific
IgM levels were unchanged by parenteral immunization after four weeks'
treatment with 150 mg/kg in the diet, but specific IgG2b levels were
raised after intragastric immunization (André et al., 1983); however,
the duration of Giardia muris infection was significantly prolonged.
Five weeks' oral treatment with up to 12 mg/kg of diet decreased the
antibody titre to TY3 vaccine in rabbits in a dose-dependent manner
(Desi et al., 1978).
Toxaphene: Toxaphene given at 100 or 200 mg/kg of diet
decreased anti-bovine serum albumin antibody titres in Swiss mice
treated for eight weeks and in the offspring of dams given the same
diet. Macrophage activity was also reduced in these offspring, but
there were no changes in the delayed-type hypersensitivity response to
purified protein derivative (Allen et al., 1983).
Endosulfan: Endosulfan had no immunotoxic effect in Wistar rats
(Vos & Krajnc, 1983; Vos et al., 1983a).
2.2.3.2 Organophosphorus compounds
Single doses of the insecticides parathion, malathion, and
dichlorvos cause significant reductions in anti-sheep red blood cell
plaque-forming cell responses (Casale et al., 1983, 1984). The
relevance of these findings is questionable, however, as they occurred
only if cholinergic or parathion symptoms were also induced.
Administration of multiple doses of malathion resulted in conflicting
findings: C57Bl6 mice given four doses of 240 mg/kg body weight over
eight days had unchanged plaque-forming cell responses to sheep red
blood cells. In contrast, rabbits treated with 5-10 mg/kg body weight
per day over 5-6 weeks had reduced antibody titres after vaccination
with S. typhimurium. Parathion also failed to suppress anti-sheep
red blood cell plaque-forming cell formation when given as four doses
of 4 mg/kg body weight (Casale et al., 1983). Parathion-methyl given
to rabbits for four weeks did not affect immune responses (Desi et
al., 1978). In mice, however, both cellular and humoral responses were
reported to be suppressed by subacute administration of parathion
(Wiltrout et al., 1978).
The immunotoxicity of MPT-IP (the industrial compound for the
production of Wofatox EC50, containing 60% parathion-methyl) was
studied in mice given single oral doses of 8.9 mg/kg body weight or
repeated doses of 0.890 or 0.445 mg/kg body weight for four weeks.
Depending on the day of administration, the single dose increased the
IgM plaque-forming cell content of the spleen and the serum anti-sheep
red blood cell antibody titre. In the subacute system, the smaller
dose (0.445 mg/kg) increased the splenic plaque-forming cell content
and serum antibody titre (Institoris et al., 1992).
Dimethoate was administered by gavage to three generations of
Wistar rats at doses of 14.1, 9.39, and 7.04 mg/kg body weight
(equivalent to 1/50, 1/75, and 1/100 of the LD50), and parathion-
methyl was administered at doses of 0.436, 0.291, a,d 0.218 mg/kg body
weight. The highest dose of dimethoate significantly decreased the
plaque-forming capacity of spleen cells in the first generation and
increased thymic weight in the second and third generations. All three
doses of parathion-methyl decreased the number of red blood cells and
the haematocrit value, and the two highest doses decreased the
leukocyte count. The nucleated cell content of the bone marrow was
increased in the second and third generations, and decreased relative
thymic weight was seen at all three doses in the third generation
(Institoris et al., 1995). In a similar experiment, dichlorvos was
administered at doses of 1.85, 1.24, or 0.972 mg/kg body weight. A
significant decrease in leukocyte count, lowered spleen cellularity,
and decreased plaque-forming capacity were seen with the highest dose
in the second generation. In the third generation, there was a dose-
dependent decrease in femoral bone-marrow cellularity (Institoris et
al., in press). In vitro, 250 µmol/litre of paraoxon, a parathion
metabolite, suppressed mitogenic lymphocyte proliferation in spleen
cells from Sprague-Dawley rats (Pruett & Chambers, 1988).
Reduction of antibody titre against Ty3 vaccine was observed by
the end of six weeks' oral treatment of rabbits with 5-100 mg/kg body
weight of malathion or with 1.25 or 2.5 mg/kg body weight of
dichlorphos (Desi et al., 1978). In the same system, a dose-dependent
decrease was observed in the tuberculin skin reaction after
administration of 0.31, 0.62, or 1.25 mg/kg body weight of
dichlorphos. The cholinesterase activity of red blood cells was
decreased only by the two higher doses.
Convincing evidence for immunotoxicity has been obtained only for
O,O,S-trimethylphosphorothiate ( O,O,S-TMP), a contaminant of
various commercial organophosphorus formulations, such as malathion,
fenitrothion, and acephate. This compound was shown to suppress
humoral and cellular immunity in mice exposed to 10 mg/kg body weight
orally (Devens et al., 1985). Several organophosphorus derivatives can
alter some immune functions in vitro, including mitogen-induced
lymphocyte proliferation (Pruett & Chambers, 1988), T-lymphocyte
cytotoxicity, and production of hydrogen peroxide by macrophages
(Pruett, 1992), at concentrations that can theoretically be attained
in vivo.
Several mechanisms have been proposed to explain organo-
phosphorus-induced immunosuppression (Pruett, 1992). A direct
cholinergic mechanism is unlikely to be involved, as the addition of
various cholinergic agonists does not suppress immune responses in
vitro. In addition, O,O,S-trimethylphosphorodithioate, a structural
analogue of O,O,S-TMP, modulates cholinesterase activity but does
not alter immune competence. An indirect mechanism involving
stress caused by neurotoxicity has also been proposed. Finally, a
direct action on cells of the immune system, and particularly
macrophages, has been suggested to be involved. Mice treated with
O,O,S-TMP, which is not neurotoxic, generate a population of
macrophages, contraindicating lymphocyte proliferation. Antigen
processing and presentation by these highly activated (inflammatory)
macrophages are severely impaired; however, the changes in macrophage
function are not correlated with suppression of humoral or cellular
immunity. While there is no direct evidence that B and T lymphocytes
are the predominant targets of organophosphorus compounds, their
mechanisms of action on macrophages are largely unknown.
2.2.3.3 Pyrethroids
Dose-dependent decreases in the serum anti- S. typhimurium
antibody titre and in the tuberculin skin reaction were observed in
rabbits fed 25, 12.5, or 6.25 mg/kg body weight of technical-grade
cypermethrin (93.5%) for seven weeks (Desi et al., 1985). Single oral
doses (23.5, 20.7, or 18.7 mg/kg body weight) of supermethrin, the
active substance of the pyrethroid pesticide Neramethrin EC 50,
decreased the number of IgM plaque-forming cells in the spleens of
mice but had no effect on the delayed-type hypersensitivity reaction.
Repeated doses of 2.97, 1.49, and 0.743 mg/kg body weight caused only
slight changes in the leukocyte count and in the nucleated cell
content of femoral bone marrow (Siroki et al., 1994).
2.2.3.4 Carbamates
Carbaryl induced marked increases in serum IgG1 and IgG2, but not
IgA, IgG3, or IgM, levels of mice exposed to 150 mg/kg of diet for one
month (André et al., 1983). Rabbits given carbaryl at 4-150 mg/kg of
diet for four weeks had no changes in anti-sheep red blood cell
haemolysin or haemagglutinin titres or in the delayed-type
hypersensitivity response to tuberculin, whereas oral treatment with
carbofuran at 0.5-20 mg/kg of diet for four weeks induced a 60-75%
decrease in the delayed-type hypersensitivity response (Street &
Sharma, 1975). Aldicarb induced no changes in a large battery of
assays for immune function and host resistance in B6C3F1 mice exposed
to 0.1-1000 mg/litre of drinking-water daily for 34 days (Thomas et
al., 1987).
2.2.3.5 Dinocap
Dinocap is a dinitrophenol compound used as a fungicide. Female
C57Bl/6J mice were given doses of 12.5-50 mg/kg body weight per day by
gavage for 7 or 12 days. All mice given the highest dose died after
four days. Mice given 25 mg/kg for 12 days had decreased thymus
weights and cellularity and increased spleen weights but no changes in
body weight, leukocyte count, lymphoproliferative response to B- or
T-cell mitogens, mixed lymphocyte reaction, or NK cell activity of
spleen cells; lymphoproliferative responses to concanavalin A and
phytohaemagglutinin in thymocytes were reduced. In mice exposed for
seven days to 25 mg/kg body weight per day, the cytotoxic T lymphocyte
response to P815 mastocytoma cells was enhanced, and there was a
significant reduction in the IgM and IgC plaque-forming cell response
to sheep red blood cells. In vitro in murine thymocytes, a
concentration of 10 µg/ml dinocap for 72h suppressed the proliferative
response to concanavalin A and phytohaemagglutinin; exposure for as
little as 30 min suppressed the mitogen-stimulated response with no
direct cytotoxicity (Smialowicz et al., 1992a).
2.2.4 Polycyclic aromatic hydrocarbons
A major concern for human health is the carcinogenic potential of
most polycyclic aromatic hydrocarbons (PAHs). Interestingly, those
which are carcinogenic also have potent immunosuppressive properties,
whereas those which are not carcinogenic lack marked immunotoxic
effects (Ward et al., 1985; White, 1986). Suppression of humoral
immunity has been observed frequently after exposure to a number of
PAHs, including benzo[ a]pyrene, DMBA, and 3-methylcholanthrene (Ward
et al., 1985). Structure-activity studies by White et al. (1985), in
which the antibody-forming cell response was used to evaluate 10 PAHs
in B6C3F1 and DBA/2 mice, demonstrated a wide spectrum of activity:
compounds like benzo[ e]pyrene and perylene were not immunotoxic,
whereas dibenz[ a,h)anthracene and DMBA were potent immunosuppressors
of the plaque-forming cell response. Interestingly, the DBA/2 mice
were more susceptible to the immunosuppressive effects than the B6C3F1
mice.
PAHs also suppress cell-mediated immunity. T-Lymphocyte
cytotoxicity and mixed lymphocyte responsiveness were found to be
impaired by most PAHs. Differences between PAHs are seen, however, in
that benzo[ a]pyrene may be less suppressive of cell-mediated
immunity than DMBA, accounting for the greater host susceptibility to
L. monocytogenes and PYB6 sarcoma challenges in DMBA- than in
benzo[ a]pyrene-treated rodents (Ward et al., 1985). Thurmond et al.
(1987) evaluated immunosuppression in B6C3F1 ( Ah-responsive) and
DBA/2 ( Ah-nonresponsive) mice and in Ah-congenic C57Bl/6J
(responsive B6-AhbAhd and nonresponsive B6-AhdAhd) mice after
exposure to DMBA in a battery of immunological assays, including
evaluation of organ weights, plaque-forming cell response, mitogen
responses, and mixed lymphocyte responses. The authors concluded that
the immunosuppressive action of DMBA was independent of the Ah locus
and associated induction of cytochrome P1-450 metabolizing enzymes.
The mechanisms of PAH-mediated immunosuppression remain to be
elucidated. PAHs may exert their immunotoxic effects as the parent
compound or as metabolites. In vitro many of the metabolites of
benzo[ a]pyrene and DMBA are immunosuppressive, the diol metabolites
being the most potent (Kawabata & White, 1987; Ladics et al., 1991).
Several possible mechanisms of action have been proposed, including
altered interleukin levels (Lyte & Bick, 1986; Pallardy et al., 1989),
a direct effect on transmembrane signalling (Pallardy et al., 1992),
and alterations in intracellular calcium mobilization (Burchiel et
al., 1991; Davis & Burchiel, 1992).
Earlier studies suggested that Th cells or faulty antigen
recognition by T cells were possible mechanisms of DMBA-induced
immunosuppression (House et al., 1987, 1989). Myers et al. (1987) also
reported that benzo[ a]pyrene alters macrophage antigen presentation.
Studies by Ladics et al. (1992) demonstrated that the only splenic
cell type capable of metabolizing benzo[ a]pyrene was the macrophage
and that the predominant immunosuppressive metabolite formed was the
benzo[ a]pyrene-7,8 diol epoxide, which is also believed to be the
ultimate carcinogenic metabolite of benzo[ a]pyrene.
2.2.5 Solvents
2.2.5.1 Benzene
Exposure to benzene is associated with myelotoxicity, and a
strong correlation was noted between lymphocytopenia and abnormal
immunological parameters. The myelotoxicity may be due, in part, to
altered differentiation of marrow lymphoid cells, as suggested by the
finding that acute exposure of IgM+ cell-depleted marrow cultures to
hydroquinone, an oxidative metabolite of benzene, blocked the final
maturation stages of B-cell differentiation (King et al., 1987). In
addition, it was shown that the hydroquinone metabolite inhibits
lectin-stimulated lymphocyte agglutination and mitogenesis by reacting
with intracellular sulfhydryl groups (Pfeifer & Irons, 1981).
Immunosuppression associated with exposure to benzene was found
in rabbits to be an impaired antibody response together with an
increased susceptibility to tuberculosis and pneumonia. Similarly,
C57Bl/6 mice exposed to benzene had a lower antibody response and
reduced mitogen-induced lymphocyte proliferation (Wierda et al.,
1981). Chronic inhalation of concentrations as low as 30 ppm impaired
resistance to L. monocytogenes (Rosenthal & Snyder, 1985).
Similarly, increased susceptibility to PYB6 tumour cell challenge was
seen at concentrations that also impaired Tc lymphocyte function.
The mechanism of benzene-induced immunosuppression is unclear.
Cellular depletion may be the major effect, although B- and T-cell
dysfunction may also be involved. The antiproliferative effects of
benzene may be related to its ability to alter cytoskeletal
development through inhibition of microtubule assembly. Polyhydroxy
metabolites of benzene ( para-benzoquinone and hydroquinone) have
been shown to bind to sulfhydryl groups on the proteins necessary for
the integrity and polymerization of microtubules. This effect may
alter cell membrane fluidity and may explain the sublethal effect of
benzene on lymphocyte function.
2.2.5.2 Other solvents
Hexanediol (1.2 mg/kg per day for seven days) decreased thymus
and spleen weights, antibody production, and delayed-type
hypersensitivity in mice (Kannan et al., 1985). Humoral immunity was
suppressed to a greater extent in female than in male mice after a
four-month exposure to trichloroethylene in the drinking-water at
doses of 0.1, 1.0, 2.5, or 5.0 mg/ml; cell-mediated immunity and bone-
marrow stem-cell colonization were inhibited only in females (Sanders
et al., 1982). The immunotoxicity of glycol ethers and some of their
metabolites has been studied in rats by measuring the plaque-forming
cell response to trinitrophenyl lipopolysaccaride. The glycol ethers
2-methoxyethanol and 2-methoxyethylacetate were immunosuppressive, as
was the principal metabolite of the latter, 2-methoxyacetic acid. The
glycol ethers 2-(2-methoxyethoxy)ethanol, bis(2-methoxyethyl) ether,
2-ethoxyethanol and its principal metabolite 2-ethoxyacetic acid,
2-ethoxyethyl acetate, and 2-butoxyethanol were not immunosuppressive
(Smialowicz et al., 1991, 1992b, 1993)
Dichloroethylene did not induce immunotoxic changes in mice given
up to 2 mg/litre per day for 90 days (Shopp et al., 1985). Similarly
negative findings were obtained with trichloroethane (Sanders et al.,
1985).
2.2.6 Metals
Heavy metals have been shown to alter immune responsiveness in
laboratory animals (Koller, 1980). Alterations in B lymphocyte
function have been observed most frequently after exposure to lead and
cadmium, but T-cell and macrophage changes have also been described.
In addition, exposure to metals is correlated better with impaired
resistance to experimental infections than with changes in B-
or T-cell functions. Interestingly, immunostimulation has been
shown to occur at levels of exposure lower than those associated with
immunosuppression. Metals have also been shown to induce immuno-
potentiation, at lower doses than those that cause immunosuppression.
2.2.6.1 Cadmium
Conflicting results have been obtained with regard to the effect
of cadmium on humoral immunity in animals (Descotes et al., 1990).
Cell-mediated immunity, however, is consistently depressed after both
short- and long-term exposure, and phagocytosis and NK cell activity
are found to be depressed. Susceptibility to L. monocytogenes, Herpes
simplex 1 and 2, and influenza virus was increased in B6C3F1 mice
exposed for long periods (Thomas et al., 1985a).
2.2.6.2 Lead
Experimental studies suggest that lead has immunosuppressive
effects in rodents (Lawrence, 1985; Descotes et al., 1990; Koller,
1990). Early studies demonstrated that lead can suppress the humoral
immune response of mice exposed as adults (Koller & Kovacic, 1974) and
of rats exposed pre- and postnatally (Luster et al., 1978). In
contrast, no change in humoral immunity was found in mice exposed to
0.08-10 mmol/litre in drinking-water (Lawrence, 1981) or after a
10-week oral treatment with 13, 130, or 1300 mg/kg of diet (as lead
acetate) (Koller & Roan, 1980). Delayed-type hypersensitivity was
found to be depressed by lead acetate and lead chloride but not in
mice treated with lead oxide, nitrate, or carbonate. The most
consistent finding in experimental studies of the effects of lead on
host resistance, however, is increased susceptibility to infectious
agents (McCabe, 1994).
With respect to nonspecific host defence mechanisms, mice treated
with lead at doses of 5, 10, or 20 µg/kg body weight given intra-
peritoneally once or at doses of 25, 50, or 100 µg/kg body weight
given orally once showed increased clearance of colloidal carbon
(Schlick & Friedberg, 1981). Furthermore, treatment of mice with 130
or 1300 ppm of lead orally for 10 weeks impaired the phagocytosis of
sheep red blood cells (Koller & Roan, 1977). Lead also has consistent
overall effects on host resistance to infection. Thus, treatment
resulted in significantly decreased resistance of mice to Klebsiella
pneumoniae (Hemphill et al., 1971) and S. typhimurium, and decreased
resistance of rats to a bacterial endotoxin and to a challenge with
E. coli, S. epidermidis, or S. enteritidis. The increased
susceptibility of rodents to Gram-negative bacteria after exposure
to lead is likely to be due to hypersensitivity to an endotoxin of
bacterial origin (Cook et al., 1974, 1975)
Organolead compounds, such as tetrethyllead, can also be
immunotoxic (Luster et al., 1992).
2.2.6.3 Mercury
Mercuric salts have been shown repeatedly to depress both humoral
and cellular immunity and nonspecific host defences in animals. For
instance, B6C3F1 mice given mercuric chloride orally for seven weeks
had decreased thymus and spleen weights, an impaired plaque-forming
cell response, and inhibition of lymphocyte proliferation at a daily
dose of 75 mg/litre of drinking-water (Dieter et al., 1983).
Methylmercury was reported to decrease humoral immunity in mice
treated orally for three weeks with 0.5, 2, or 10 mg/litre drinking-
water (Blackley et al., 1980).
2.2.6.4 Organotins
Several organotins have been shown to be markedly immunotoxic and
are considered as prototype immunotoxicants (Penninks et al., 1990),
even though no human data are available.
Di- n-octyltin dichloride at 50 or 150 mg/kg of diet for six
weeks induced a dramatic, dose-related decrease in the weight of the
thymus in rats, associated with a less severe decrease in spleen and
lymph node weights (Seinen & Willems, 1976). The numbers of cells in
the thymus and spleen, but not the bone marrow, were decreased.
Histologically, lymphocyte depletion was seen in the thymus and in
thymus-dependent areas of the spleen. Interestingly, thymic atrophy
recovered quickly after cessation of exposure (Seinen et al., 1977).
It was later shown to be associated with a 25% decrease in peripheral
blood lymphocytes, with a preferential loss of Th lymphocytes. As
expected, T-cell functions, such as the delayed-type hypersensitivity
response and T-lymphocyte proliferation, were depressed. Inhibition of
humoral immunity was also seen, with reduced numbers of plaque-forming
cells and decreased circulating antibody titres. NK cell activity was
not affected, whereas susceptibility to L. monocytogenes infection
was markedly increased.
Immune function is not impaired in guinea-pigs or mice fed
di- n-octyltin dichloride, which correlates with the absence of
thymic atrophy (Seinen & Penninks, 1979). Mice treated intravenously
or intraperitoneally develop thymic atrophy, however, suggesting
interspecies variability in the disposition of dialkyltins after oral
intake, although other, poorly understood mechanisms may account for
this variability (Penninks et al., 1990). No interspecies differences
in lymphocyte functions were noted after exposure in vitro.
Generally similar findings were made with the trialkylorganotin,
tri- n-butyltin oxide (TBTO). As trisubstituted organotins are
rapidly metabolized to disubstituted derivatives, the latter are
considered to be involved in the reported thymic effects (Snoeij et
al., 1988). In a short-term study in rats, pronounced effects were
found on the lymphoid organs: thymus (Figure 29), spleen, and lymph
nodes. These effects were most pronounced in thymus-dependent areas
(Figure 30) (Krajnc et al., 1984). Interestingly, thymus atrophy also
occurred in fish, as guppies exposed to organotin compounds showed
severe thymic atrophy (Figure 31). In function tests (Vos et al.,
1984), rats that were exposed to TBTO for six weeks after weaning had
suppressed delayed-type hypersensitivity responses to ovalbumin and
tuberculin and suppressed IgG responses to sheep erythrocytes. In
vitro mitogen responses to concanavalin A in thymus and spleen and
NK cell activity in both the spleen and the lungs were decreased (Van
Loveren et al., 1990b). Exposure to TBTO at 20 or 80 mg/kg of diet for
six weeks led to decreased resistance to infection with
L. monocytogenes or Trichinella spiralis. The latter effect was
evidenced by increased numbers of adult worms in the gut as a result
of impaired worm expulsion, increased numbers of muscle larvae in the
striated tissue, decreased inflammatory responses around these larvae,
and decreased antibody responses to T. spiralis, especially in the
IgE class (Vos et al., 1984). After long-term exposure (15-17 months)
to 5 or 50 mg/kg of diet, delayed-type hypersensitivity was not
suppressed, but assays for NK cell activity and resistance to
infection indicated suppression.
As the immune responsiveness of older animals can be expected to
be less strong than that of younger rats, the effects of exposure to
immunotoxic chemicals may become evident less easily; however, tests
for function still indicated immunotoxicity. In experiments in which
exposure to TBTO was begun only at 12 months of age, both infection
models showed immunotoxicity to TBTO. Very few studies have focused on
the immunotoxic effects of chemicals on the gut immune system, but the
studies of TBTO showed both a decreased capacity of the host to expel
adult T. spiralis worms from the gut and increased production of
serum IgA specific for this parasite (Vos et al., 1990a).
The mechanism of the immunotoxicity of organotin compounds has
been investigated extensively (Penninks et al., 1990). A direct
influence on the synthesis of thymic hormones is uncertain, as
conflicting results have been obtained in different experiments.
Interference with the influx of prothymocytes can be ruled out, as
thymic atrophy develops too rapidly. Interestingly, organotins reduced
the proliferative activity of thymocytes and the number of
proliferating thymoblasts within 24h after exposure was begun, at a
time when thymic atrophy was not evident. This selective effect on
thymoblasts and the physiological destruction of most cortical
thymocytes would result in marked depletion and, finally, in thymic
atrophy.
2.2.6.5 Gallium arsenide
Gallium arsenide is an intermetallic compound used widely in the
electronics industry, primarily in the manufacture of transistors and
light-emitting diodes. A single intratracheal instillation of 50, 100,
or 200 mg/kg body weight into female B6C3F1 mice resulted in a dose-
related decrease in the IgM and IgG antibody response to sheep
erythrocytes. Similarly, cell-mediated immunity, as evaluated by the
delayed-type hypersensitivity reaction to keyhole limpet haemocyanin
and the mixed lymphocyte response, was also decreased in a dose-
dependent way. Increases were observed in complement C3 levels,
mitogen response to lipopolysaccharide, and NK cell activity. No
effects were observed on response to T-cell mitogens, total complement
CH50 activity, or host resistance to Plasmodium yoelii or
Streptococcus pneumoniae; however, a significant decrease in host
resistance was observed to L. monocytogenes and B16F10 tumour
challenge (Sikorski et al., 1989).
1. Exposure to HCB of Lewis rats, which develop autoimmune disease
after sensitization with complete Freund's adjuvant (adjuvant
arthritis) or with guinea-pig myelin (experimental allergic
encephalomyelitis), had clear effects (Van Loveren et al., 1990c):
Whereas the allergic encephalomyelitis response was severely enhanced,
the arthritic lesions were strongly suppressed.
2. Wistar rats treated with HCB produce antibodies to autoantigens;
thus, IgM, but not IgG, levels against single-stranded DNA, native
DNA, rat IgG (representing rheumatoid factor), and bromelain-treated
mouse erythrocytes (indicating that phosphatidylcholine is a major
autoantigen) were elevated. It has been suggested that HCB activates a
B-cell subset committed to the production of these autoantibodies and
associated with various systemic autoimmune diseases (Schielen et al.,
1993).
2.2.3 Pesticides
A large number of studies have focused on the immunotoxicity of
pesticides. Because of the chemical heterogeneity of these compounds
as a class, the reported effects vary widely (Barnett & Rodgers,
1994).
2.2.3.1 Organochlorine pesticides
The evidence for the immunotoxicity of organochlorine pesticides
as a class is inconclusive.
DDT: Wistar rats treated with 40 mg/kg body weight per day DDT
orally for 60 days showed increased anti-bovine serum albumin titres
(Lukic et al., 1973). Studies by Vos et al. (Vos & Krajnc, 1983, Vos
et al., 1983a), however, showed no changes in thymus or spleen
weights, leukocyte counts, or total serum IgG and IgM levels at doses
up to 800 mg/kg body weight per day.
Chlordane: Prenatal exposure of mice to chlordane was reported
to reduce contact and delayed hypersensitivity responses, suggesting
an effect on T-cell responses. Attempts to elucidate the mechanism
have, however, been unsuccessful. Johnson et al. (1986) observed
increased lymphocyte proliferation only at a dose of 8 mg/kg body
weight in B6C3F1 mice and concluded that chlordane has no significant
immunotoxicity in this model.
Chlordecone: Chlordecone reduced thymus and spleen weights by
40% in Fischer rats at at a dose of 10 mg/kg body weight per day but
had no significant effect at or below 5 mg/kg body weight per day.
T-Lymphocyte proliferation was unaffected at all doses (Smialowicz et
al., 1985a).
Lindane: Lindane had various effects on the anti-sheep red
blood cell response, depending on the immunization protocol. Specific
IgM levels were unchanged by parenteral immunization after four weeks'
treatment with 150 mg/kg in the diet, but specific IgG2b levels were
raised after intragastric immunization (André et al., 1983); however,
the duration of Giardia muris infection was significantly prolonged.
Five weeks' oral treatment with up to 12 mg/kg of diet decreased the
antibody titre to TY3 vaccine in rabbits in a dose-dependent manner
(Desi et al., 1978).
Toxaphene: Toxaphene given at 100 or 200 mg/kg of diet
decreased anti-bovine serum albumin antibody titres in Swiss mice
treated for eight weeks and in the offspring of dams given the same
diet. Macrophage activity was also reduced in these offspring, but
there were no changes in the delayed-type hypersensitivity response to
purified protein derivative (Allen et al., 1983).
Endosulfan: Endosulfan had no immunotoxic effect in Wistar rats
(Vos & Krajnc, 1983; Vos et al., 1983a).
2.2.3.2 Organophosphorus compounds
Single doses of the insecticides parathion, malathion, and
dichlorvos cause significant reductions in anti-sheep red blood cell
plaque-forming cell responses (Casale et al., 1983, 1984). The
relevance of these findings is questionable, however, as they occurred
only if cholinergic or parathion symptoms were also induced.
Administration of multiple doses of malathion resulted in conflicting
findings: C57Bl6 mice given four doses of 240 mg/kg body weight over
eight days had unchanged plaque-forming cell responses to sheep red
blood cells. In contrast, rabbits treated with 5-10 mg/kg body weight
per day over 5-6 weeks had reduced antibody titres after vaccination
with S. typhimurium. Parathion also failed to suppress anti-sheep
red blood cell plaque-forming cell formation when given as four doses
of 4 mg/kg body weight (Casale et al., 1983). Parathion-methyl given
to rabbits for four weeks did not affect immune responses (Desi et
al., 1978). In mice, however, both cellular and humoral responses were
reported to be suppressed by subacute administration of parathion
(Wiltrout et al., 1978).
The immunotoxicity of MPT-IP (the industrial compound for the
production of Wofatox EC50, containing 60% parathion-methyl) was
studied in mice given single oral doses of 8.9 mg/kg body weight or
repeated doses of 0.890 or 0.445 mg/kg body weight for four weeks.
Depending on the day of administration, the single dose increased the
IgM plaque-forming cell content of the spleen and the serum anti-sheep
red blood cell antibody titre. In the subacute system, the smaller
dose (0.445 mg/kg) increased the splenic plaque-forming cell content
and serum antibody titre (Institoris et al., 1992).
Dimethoate was administered by gavage to three generations of
Wistar rats at doses of 14.1, 9.39, and 7.04 mg/kg body weight
(equivalent to 1/50, 1/75, and 1/100 of the LD50), and parathion-
methyl was administered at doses of 0.436, 0.291, a,d 0.218 mg/kg body
weight. The highest dose of dimethoate significantly decreased the
plaque-forming capacity of spleen cells in the first generation and
increased thymic weight in the second and third generations. All three
doses of parathion-methyl decreased the number of red blood cells and
the haematocrit value, and the two highest doses decreased the
leukocyte count. The nucleated cell content of the bone marrow was
increased in the second and third generations, and decreased relative
thymic weight was seen at all three doses in the third generation
(Institoris et al., 1995). In a similar experiment, dichlorvos was
administered at doses of 1.85, 1.24, or 0.972 mg/kg body weight. A
significant decrease in leukocyte count, lowered spleen cellularity,
and decreased plaque-forming capacity were seen with the highest dose
in the second generation. In the third generation, there was a dose-
dependent decrease in femoral bone-marrow cellularity (Institoris et
al., in press). In vitro, 250 µmol/litre of paraoxon, a parathion
metabolite, suppressed mitogenic lymphocyte proliferation in spleen
cells from Sprague-Dawley rats (Pruett & Chambers, 1988).
Reduction of antibody titre against Ty3 vaccine was observed by
the end of six weeks' oral treatment of rabbits with 5-100 mg/kg body
weight of malathion or with 1.25 or 2.5 mg/kg body weight of
dichlorphos (Desi et al., 1978). In the same system, a dose-dependent
decrease was observed in the tuberculin skin reaction after
administration of 0.31, 0.62, or 1.25 mg/kg body weight of
dichlorphos. The cholinesterase activity of red blood cells was
decreased only by the two higher doses.
Convincing evidence for immunotoxicity has been obtained only for
O,O,S-trimethylphosphorothiate ( O,O,S-TMP), a contaminant of
various commercial organophosphorus formulations, such as malathion,
fenitrothion, and acephate. This compound was shown to suppress
humoral and cellular immunity in mice exposed to 10 mg/kg body weight
orally (Devens et al., 1985). Several organophosphorus derivatives can
alter some immune functions in vitro, including mitogen-induced
lymphocyte proliferation (Pruett & Chambers, 1988), T-lymphocyte
cytotoxicity, and production of hydrogen peroxide by macrophages
(Pruett, 1992), at concentrations that can theoretically be attained
in vivo.
Several mechanisms have been proposed to explain organo-
phosphorus-induced immunosuppression (Pruett, 1992). A direct
cholinergic mechanism is unlikely to be involved, as the addition of
various cholinergic agonists does not suppress immune responses in
vitro. In addition, O,O,S-trimethylphosphorodithioate, a structural
analogue of O,O,S-TMP, modulates cholinesterase activity but does
not alter immune competence. An indirect mechanism involving
stress caused by neurotoxicity has also been proposed. Finally, a
direct action on cells of the immune system, and particularly
macrophages, has been suggested to be involved. Mice treated with
O,O,S-TMP, which is not neurotoxic, generate a population of
macrophages, contraindicating lymphocyte proliferation. Antigen
processing and presentation by these highly activated (inflammatory)
macrophages are severely impaired; however, the changes in macrophage
function are not correlated with suppression of humoral or cellular
immunity. While there is no direct evidence that B and T lymphocytes
are the predominant targets of organophosphorus compounds, their
mechanisms of action on macrophages are largely unknown.
2.2.3.3 Pyrethroids
Dose-dependent decreases in the serum anti- S. typhimurium
antibody titre and in the tuberculin skin reaction were observed in
rabbits fed 25, 12.5, or 6.25 mg/kg body weight of technical-grade
cypermethrin (93.5%) for seven weeks (Desi et al., 1985). Single oral
doses (23.5, 20.7, or 18.7 mg/kg body weight) of supermethrin, the
active substance of the pyrethroid pesticide Neramethrin EC 50,
decreased the number of IgM plaque-forming cells in the spleens of
mice but had no effect on the delayed-type hypersensitivity reaction.
Repeated doses of 2.97, 1.49, and 0.743 mg/kg body weight caused only
slight changes in the leukocyte count and in the nucleated cell
content of femoral bone marrow (Siroki et al., 1994).
2.2.3.4 Carbamates
Carbaryl induced marked increases in serum IgG1 and IgG2, but not
IgA, IgG3, or IgM, levels of mice exposed to 150 mg/kg of diet for one
month (André et al., 1983). Rabbits given carbaryl at 4-150 mg/kg of
diet for four weeks had no changes in anti-sheep red blood cell
haemolysin or haemagglutinin titres or in the delayed-type
hypersensitivity response to tuberculin, whereas oral treatment with
carbofuran at 0.5-20 mg/kg of diet for four weeks induced a 60-75%
decrease in the delayed-type hypersensitivity response (Street &
Sharma, 1975). Aldicarb induced no changes in a large battery of
assays for immune function and host resistance in B6C3F1 mice exposed
to 0.1-1000 mg/litre of drinking-water daily for 34 days (Thomas et
al., 1987).
2.2.3.5 Dinocap
Dinocap is a dinitrophenol compound used as a fungicide. Female
C57Bl/6J mice were given doses of 12.5-50 mg/kg body weight per day by
gavage for 7 or 12 days. All mice given the highest dose died after
four days. Mice given 25 mg/kg for 12 days had decreased thymus
weights and cellularity and increased spleen weights but no changes in
body weight, leukocyte count, lymphoproliferative response to B- or
T-cell mitogens, mixed lymphocyte reaction, or NK cell activity of
spleen cells; lymphoproliferative responses to concanavalin A and
phytohaemagglutinin in thymocytes were reduced. In mice exposed for
seven days to 25 mg/kg body weight per day, the cytotoxic T lymphocyte
response to P815 mastocytoma cells was enhanced, and there was a
significant reduction in the IgM and IgC plaque-forming cell response
to sheep red blood cells. In vitro in murine thymocytes, a
concentration of 10 µg/ml dinocap for 72h suppressed the proliferative
response to concanavalin A and phytohaemagglutinin; exposure for as
little as 30 min suppressed the mitogen-stimulated response with no
direct cytotoxicity (Smialowicz et al., 1992a).
2.2.4 Polycyclic aromatic hydrocarbons
A major concern for human health is the carcinogenic potential of
most polycyclic aromatic hydrocarbons (PAHs). Interestingly, those
which are carcinogenic also have potent immunosuppressive properties,
whereas those which are not carcinogenic lack marked immunotoxic
effects (Ward et al., 1985; White, 1986). Suppression of humoral
immunity has been observed frequently after exposure to a number of
PAHs, including benzo[ a]pyrene, DMBA, and 3-methylcholanthrene (Ward
et al., 1985). Structure-activity studies by White et al. (1985), in
which the antibody-forming cell response was used to evaluate 10 PAHs
in B6C3F1 and DBA/2 mice, demonstrated a wide spectrum of activity:
compounds like benzo[ e]pyrene and perylene were not immunotoxic,
whereas dibenz[ a,h)anthracene and DMBA were potent immunosuppressors
of the plaque-forming cell response. Interestingly, the DBA/2 mice
were more susceptible to the immunosuppressive effects than the B6C3F1
mice.
PAHs also suppress cell-mediated immunity. T-Lymphocyte
cytotoxicity and mixed lymphocyte responsiveness were found to be
impaired by most PAHs. Differences between PAHs are seen, however, in
that benzo[ a]pyrene may be less suppressive of cell-mediated
immunity than DMBA, accounting for the greater host susceptibility to
L. monocytogenes and PYB6 sarcoma challenges in DMBA- than in
benzo[ a]pyrene-treated rodents (Ward et al., 1985). Thurmond et al.
(1987) evaluated immunosuppression in B6C3F1 ( Ah-responsive) and
DBA/2 ( Ah-nonresponsive) mice and in Ah-congenic C57Bl/6J
(responsive B6-AhbAhd and nonresponsive B6-AhdAhd) mice after
exposure to DMBA in a battery of immunological assays, including
evaluation of organ weights, plaque-forming cell response, mitogen
responses, and mixed lymphocyte responses. The authors concluded that
the immunosuppressive action of DMBA was independent of the Ah locus
and associated induction of cytochrome P1-450 metabolizing enzymes.
The mechanisms of PAH-mediated immunosuppression remain to be
elucidated. PAHs may exert their immunotoxic effects as the parent
compound or as metabolites. In vitro many of the metabolites of
benzo[ a]pyrene and DMBA are immunosuppressive, the diol metabolites
being the most potent (Kawabata & White, 1987; Ladics et al., 1991).
Several possible mechanisms of action have been proposed, including
altered interleukin levels (Lyte & Bick, 1986; Pallardy et al., 1989),
a direct effect on transmembrane signalling (Pallardy et al., 1992),
and alterations in intracellular calcium mobilization (Burchiel et
al., 1991; Davis & Burchiel, 1992).
Earlier studies suggested that Th cells or faulty antigen
recognition by T cells were possible mechanisms of DMBA-induced
immunosuppression (House et al., 1987, 1989). Myers et al. (1987) also
reported that benzo[ a]pyrene alters macrophage antigen presentation.
Studies by Ladics et al. (1992) demonstrated that the only splenic
cell type capable of metabolizing benzo[ a]pyrene was the macrophage
and that the predominant immunosuppressive metabolite formed was the
benzo[ a]pyrene-7,8 diol epoxide, which is also believed to be the
ultimate carcinogenic metabolite of benzo[ a]pyrene.
2.2.5 Solvents
2.2.5.1 Benzene
Exposure to benzene is associated with myelotoxicity, and a
strong correlation was noted between lymphocytopenia and abnormal
immunological parameters. The myelotoxicity may be due, in part, to
altered differentiation of marrow lymphoid cells, as suggested by the
finding that acute exposure of IgM+ cell-depleted marrow cultures to
hydroquinone, an oxidative metabolite of benzene, blocked the final
maturation stages of B-cell differentiation (King et al., 1987). In
addition, it was shown that the hydroquinone metabolite inhibits
lectin-stimulated lymphocyte agglutination and mitogenesis by reacting
with intracellular sulfhydryl groups (Pfeifer & Irons, 1981).
Immunosuppression associated with exposure to benzene was found
in rabbits to be an impaired antibody response together with an
increased susceptibility to tuberculosis and pneumonia. Similarly,
C57Bl/6 mice exposed to benzene had a lower antibody response and
reduced mitogen-induced lymphocyte proliferation (Wierda et al.,
1981). Chronic inhalation of concentrations as low as 30 ppm impaired
resistance to L. monocytogenes (Rosenthal & Snyder, 1985).
Similarly, increased susceptibility to PYB6 tumour cell challenge was
seen at concentrations that also impaired Tc lymphocyte function.
The mechanism of benzene-induced immunosuppression is unclear.
Cellular depletion may be the major effect, although B- and T-cell
dysfunction may also be involved. The antiproliferative effects of
benzene may be related to its ability to alter cytoskeletal
development through inhibition of microtubule assembly. Polyhydroxy
metabolites of benzene ( para-benzoquinone and hydroquinone) have
been shown to bind to sulfhydryl groups on the proteins necessary for
the integrity and polymerization of microtubules. This effect may
alter cell membrane fluidity and may explain the sublethal effect of
benzene on lymphocyte function.
2.2.5.2 Other solvents
Hexanediol (1.2 mg/kg per day for seven days) decreased thymus
and spleen weights, antibody production, and delayed-type
hypersensitivity in mice (Kannan et al., 1985). Humoral immunity was
suppressed to a greater extent in female than in male mice after a
four-month exposure to trichloroethylene in the drinking-water at
doses of 0.1, 1.0, 2.5, or 5.0 mg/ml; cell-mediated immunity and bone-
marrow stem-cell colonization were inhibited only in females (Sanders
et al., 1982). The immunotoxicity of glycol ethers and some of their
metabolites has been studied in rats by measuring the plaque-forming
cell response to trinitrophenyl lipopolysaccaride. The glycol ethers
2-methoxyethanol and 2-methoxyethylacetate were immunosuppressive, as
was the principal metabolite of the latter, 2-methoxyacetic acid. The
glycol ethers 2-(2-methoxyethoxy)ethanol, bis(2-methoxyethyl) ether,
2-ethoxyethanol and its principal metabolite 2-ethoxyacetic acid,
2-ethoxyethyl acetate, and 2-butoxyethanol were not immunosuppressive
(Smialowicz et al., 1991, 1992b, 1993)
Dichloroethylene did not induce immunotoxic changes in mice given
up to 2 mg/litre per day for 90 days (Shopp et al., 1985). Similarly
negative findings were obtained with trichloroethane (Sanders et al.,
1985).
2.2.6 Metals
Heavy metals have been shown to alter immune responsiveness in
laboratory animals (Koller, 1980). Alterations in B lymphocyte
function have been observed most frequently after exposure to lead and
cadmium, but T-cell and macrophage changes have also been described.
In addition, exposure to metals is correlated better with impaired
resistance to experimental infections than with changes in B-
or T-cell functions. Interestingly, immunostimulation has been
shown to occur at levels of exposure lower than those associated with
immunosuppression. Metals have also been shown to induce immuno-
potentiation, at lower doses than those that cause immunosuppression.
2.2.6.1 Cadmium
Conflicting results have been obtained with regard to the effect
of cadmium on humoral immunity in animals (Descotes et al., 1990).
Cell-mediated immunity, however, is consistently depressed after both
short- and long-term exposure, and phagocytosis and NK cell activity
are found to be depressed. Susceptibility to L. monocytogenes, Herpes
simplex 1 and 2, and influenza virus was increased in B6C3F1 mice
exposed for long periods (Thomas et al., 1985a).
2.2.6.2 Lead
Experimental studies suggest that lead has immunosuppressive
effects in rodents (Lawrence, 1985; Descotes et al., 1990; Koller,
1990). Early studies demonstrated that lead can suppress the humoral
immune response of mice exposed as adults (Koller & Kovacic, 1974) and
of rats exposed pre- and postnatally (Luster et al., 1978). In
contrast, no change in humoral immunity was found in mice exposed to
0.08-10 mmol/litre in drinking-water (Lawrence, 1981) or after a
10-week oral treatment with 13, 130, or 1300 mg/kg of diet (as lead
acetate) (Koller & Roan, 1980). Delayed-type hypersensitivity was
found to be depressed by lead acetate and lead chloride but not in
mice treated with lead oxide, nitrate, or carbonate. The most
consistent finding in experimental studies of the effects of lead on
host resistance, however, is increased susceptibility to infectious
agents (McCabe, 1994).
With respect to nonspecific host defence mechanisms, mice treated
with lead at doses of 5, 10, or 20 µg/kg body weight given intra-
peritoneally once or at doses of 25, 50, or 100 µg/kg body weight
given orally once showed increased clearance of colloidal carbon
(Schlick & Friedberg, 1981). Furthermore, treatment of mice with 130
or 1300 ppm of lead orally for 10 weeks impaired the phagocytosis of
sheep red blood cells (Koller & Roan, 1977). Lead also has consistent
overall effects on host resistance to infection. Thus, treatment
resulted in significantly decreased resistance of mice to Klebsiella
pneumoniae (Hemphill et al., 1971) and S. typhimurium, and decreased
resistance of rats to a bacterial endotoxin and to a challenge with
E. coli, S. epidermidis, or S. enteritidis. The increased
susceptibility of rodents to Gram-negative bacteria after exposure
to lead is likely to be due to hypersensitivity to an endotoxin of
bacterial origin (Cook et al., 1974, 1975)
Organolead compounds, such as tetrethyllead, can also be
immunotoxic (Luster et al., 1992).
2.2.6.3 Mercury
Mercuric salts have been shown repeatedly to depress both humoral
and cellular immunity and nonspecific host defences in animals. For
instance, B6C3F1 mice given mercuric chloride orally for seven weeks
had decreased thymus and spleen weights, an impaired plaque-forming
cell response, and inhibition of lymphocyte proliferation at a daily
dose of 75 mg/litre of drinking-water (Dieter et al., 1983).
Methylmercury was reported to decrease humoral immunity in mice
treated orally for three weeks with 0.5, 2, or 10 mg/litre drinking-
water (Blackley et al., 1980).
2.2.6.4 Organotins
Several organotins have been shown to be markedly immunotoxic and
are considered as prototype immunotoxicants (Penninks et al., 1990),
even though no human data are available.
Di- n-octyltin dichloride at 50 or 150 mg/kg of diet for six
weeks induced a dramatic, dose-related decrease in the weight of the
thymus in rats, associated with a less severe decrease in spleen and
lymph node weights (Seinen & Willems, 1976). The numbers of cells in
the thymus and spleen, but not the bone marrow, were decreased.
Histologically, lymphocyte depletion was seen in the thymus and in
thymus-dependent areas of the spleen. Interestingly, thymic atrophy
recovered quickly after cessation of exposure (Seinen et al., 1977).
It was later shown to be associated with a 25% decrease in peripheral
blood lymphocytes, with a preferential loss of Th lymphocytes. As
expected, T-cell functions, such as the delayed-type hypersensitivity
response and T-lymphocyte proliferation, were depressed. Inhibition of
humoral immunity was also seen, with reduced numbers of plaque-forming
cells and decreased circulating antibody titres. NK cell activity was
not affected, whereas susceptibility to L. monocytogenes infection
was markedly increased.
Immune function is not impaired in guinea-pigs or mice fed
di- n-octyltin dichloride, which correlates with the absence of
thymic atrophy (Seinen & Penninks, 1979). Mice treated intravenously
or intraperitoneally develop thymic atrophy, however, suggesting
interspecies variability in the disposition of dialkyltins after oral
intake, although other, poorly understood mechanisms may account for
this variability (Penninks et al., 1990). No interspecies differences
in lymphocyte functions were noted after exposure in vitro.
Generally similar findings were made with the trialkylorganotin,
tri- n-butyltin oxide (TBTO). As trisubstituted organotins are
rapidly metabolized to disubstituted derivatives, the latter are
considered to be involved in the reported thymic effects (Snoeij et
al., 1988). In a short-term study in rats, pronounced effects were
found on the lymphoid organs: thymus (Figure 29), spleen, and lymph
nodes. These effects were most pronounced in thymus-dependent areas
(Figure 30) (Krajnc et al., 1984). Interestingly, thymus atrophy also
occurred in fish, as guppies exposed to organotin compounds showed
severe thymic atrophy (Figure 31). In function tests (Vos et al.,
1984), rats that were exposed to TBTO for six weeks after weaning had
suppressed delayed-type hypersensitivity responses to ovalbumin and
tuberculin and suppressed IgG responses to sheep erythrocytes. In
vitro mitogen responses to concanavalin A in thymus and spleen and
NK cell activity in both the spleen and the lungs were decreased (Van
Loveren et al., 1990b). Exposure to TBTO at 20 or 80 mg/kg of diet for
six weeks led to decreased resistance to infection with
L. monocytogenes or Trichinella spiralis. The latter effect was
evidenced by increased numbers of adult worms in the gut as a result
of impaired worm expulsion, increased numbers of muscle larvae in the
striated tissue, decreased inflammatory responses around these larvae,
and decreased antibody responses to T. spiralis, especially in the
IgE class (Vos et al., 1984). After long-term exposure (15-17 months)
to 5 or 50 mg/kg of diet, delayed-type hypersensitivity was not
suppressed, but assays for NK cell activity and resistance to
infection indicated suppression.
As the immune responsiveness of older animals can be expected to
be less strong than that of younger rats, the effects of exposure to
immunotoxic chemicals may become evident less easily; however, tests
for function still indicated immunotoxicity. In experiments in which
exposure to TBTO was begun only at 12 months of age, both infection
models showed immunotoxicity to TBTO. Very few studies have focused on
the immunotoxic effects of chemicals on the gut immune system, but the
studies of TBTO showed both a decreased capacity of the host to expel
adult T. spiralis worms from the gut and increased production of
serum IgA specific for this parasite (Vos et al., 1990a).
The mechanism of the immunotoxicity of organotin compounds has
been investigated extensively (Penninks et al., 1990). A direct
influence on the synthesis of thymic hormones is uncertain, as
conflicting results have been obtained in different experiments.
Interference with the influx of prothymocytes can be ruled out, as
thymic atrophy develops too rapidly. Interestingly, organotins reduced
the proliferative activity of thymocytes and the number of
proliferating thymoblasts within 24h after exposure was begun, at a
time when thymic atrophy was not evident. This selective effect on
thymoblasts and the physiological destruction of most cortical
thymocytes would result in marked depletion and, finally, in thymic
atrophy.
2.2.6.5 Gallium arsenide
Gallium arsenide is an intermetallic compound used widely in the
electronics industry, primarily in the manufacture of transistors and
light-emitting diodes. A single intratracheal instillation of 50, 100,
or 200 mg/kg body weight into female B6C3F1 mice resulted in a dose-
related decrease in the IgM and IgG antibody response to sheep
erythrocytes. Similarly, cell-mediated immunity, as evaluated by the
delayed-type hypersensitivity reaction to keyhole limpet haemocyanin
and the mixed lymphocyte response, was also decreased in a dose-
dependent way. Increases were observed in complement C3 levels,
mitogen response to lipopolysaccharide, and NK cell activity. No
effects were observed on response to T-cell mitogens, total complement
CH50 activity, or host resistance to Plasmodium yoelii or
Streptococcus pneumoniae; however, a significant decrease in host
resistance was observed to L. monocytogenes and B16F10 tumour
challenge (Sikorski et al., 1989).


 2.2.6.6 Beryllium
Beryllium induces a variety of diseases, including granulomatous
lung (chronic beryllium disease) and skin conditions. These
granulomatous reactions involve a lymphocyte response to beryllium
salts. The major lymphocyte population consists of Th cells (CD4). The
T-cell response to beryllium is IL2-dependent (Saltini et al., 1989).
The antigen has not been identified, but may be a beryllium-protein
complex. There appears to be a genetic predisposition, as the majority
of patients with beryllium lung disease share a particular HLA-Dp
allele (HLA-DpB1) (Richeldi et al., 1992). The development and
maintenance of lung and skin granulomas depend on the presence of
antigen, antigen-presenting cells, and memory T lymphocytes and the
release of proinflammatory cytokines by macrophages and lymphocytes
(Boros, 1988; Kunkel et al., 1989).
2.2.7 Air pollutants
Pollutants characteristic of occupational and urban environments
may cause or aggravate pulmonary diseases. Pulmonary defence
mechanisms to pathogens comprise mechanical defences, nonspecific
defences (ingestion by phagocytic cells, lysis of virus-infected
cells), and specific immunity. A number of studies in experimental
animals have shown that exposure to air pollutants, including ozone,
nitrogen dioxide, sulfur dioxide, some volatile organic compounds, and
metal particulates, adversely affects pulmonary defences, and
primarily nonspecific defences important in clearing certain Gram-
positive bacteria from the lung (Graham & Gardner, 1985; Jakab &
Hmieleski, 1988; Selgrade & Gilmour, 1994).
In dogs, exposure to ozone at 3 ppm for 2 h per day for three
days markedly increased the number of epithelial neutrophils, whereas
the number of circulating neutrophils was decreased (O'Byrne et al.,
1984). A significant decrease in absolute thymocyte numbers was also
observed in mice continuously exposed to 0.7ppm ozone for three to
seven days (Li & Richters, 1991). Decreased spleen and thymus weights
were reported in mice exposed to ozone alone or in combination with
nitrogen dioxide (Fujimaki, 1989; Goodman et al., 1989). The numbers
of neutrophils and alveolar macrophages in bronchoalveolar lavage
fluid were found to be increased in rats, and T-lymphocyte
infiltrations were seen in ozone-induced lesions of mice.
Accumulations of macrophages are located mainly at the bronchoalveolar
junction and in alveoli (Figures 32 and 33).
2.2.6.6 Beryllium
Beryllium induces a variety of diseases, including granulomatous
lung (chronic beryllium disease) and skin conditions. These
granulomatous reactions involve a lymphocyte response to beryllium
salts. The major lymphocyte population consists of Th cells (CD4). The
T-cell response to beryllium is IL2-dependent (Saltini et al., 1989).
The antigen has not been identified, but may be a beryllium-protein
complex. There appears to be a genetic predisposition, as the majority
of patients with beryllium lung disease share a particular HLA-Dp
allele (HLA-DpB1) (Richeldi et al., 1992). The development and
maintenance of lung and skin granulomas depend on the presence of
antigen, antigen-presenting cells, and memory T lymphocytes and the
release of proinflammatory cytokines by macrophages and lymphocytes
(Boros, 1988; Kunkel et al., 1989).
2.2.7 Air pollutants
Pollutants characteristic of occupational and urban environments
may cause or aggravate pulmonary diseases. Pulmonary defence
mechanisms to pathogens comprise mechanical defences, nonspecific
defences (ingestion by phagocytic cells, lysis of virus-infected
cells), and specific immunity. A number of studies in experimental
animals have shown that exposure to air pollutants, including ozone,
nitrogen dioxide, sulfur dioxide, some volatile organic compounds, and
metal particulates, adversely affects pulmonary defences, and
primarily nonspecific defences important in clearing certain Gram-
positive bacteria from the lung (Graham & Gardner, 1985; Jakab &
Hmieleski, 1988; Selgrade & Gilmour, 1994).
In dogs, exposure to ozone at 3 ppm for 2 h per day for three
days markedly increased the number of epithelial neutrophils, whereas
the number of circulating neutrophils was decreased (O'Byrne et al.,
1984). A significant decrease in absolute thymocyte numbers was also
observed in mice continuously exposed to 0.7ppm ozone for three to
seven days (Li & Richters, 1991). Decreased spleen and thymus weights
were reported in mice exposed to ozone alone or in combination with
nitrogen dioxide (Fujimaki, 1989; Goodman et al., 1989). The numbers
of neutrophils and alveolar macrophages in bronchoalveolar lavage
fluid were found to be increased in rats, and T-lymphocyte
infiltrations were seen in ozone-induced lesions of mice.
Accumulations of macrophages are located mainly at the bronchoalveolar
junction and in alveoli (Figures 32 and 33).

 Modulation of nonspecific defence mechanisms by ozone has also
been described (Goldstein et al., 1971; Holt & Keast, 1977; Van
Loveren et al., 1988a, 1990b). Thus, phagocytic activity in alveolar
macrophages is suppressed, but this depends on the concentration and
duration of exposure; enhanced phagocytic activity was also observed.
Alterations in the macrophage production of arachidonic acid
metabolites, resulting in increased prostaglandin 2 production, have
been suggested to be involved (Gilmour et al., 1993). NK cell activity
is either unaffected or stimulated by low ozone concentrations,
whereas high concentrations decreased both the number and the activity
of splenic and pulmonary NK cells (Burleson et al., 1989; Van Loveren
et al., 1990b). Ozone also affects T cells (Dziedzic & White, 1986;
Van Loveren et al., 1988a; Bleavins & Dziedzic, 1990; Dziedzic et al.,
1990). Ozone-induced systemic dysfunction has been reported in animals
and probably contributes to impaired host defences (Aranyi et al.,
1983). Humoral immunity, e.g. circulating antibody titres to a variety
of antigens and the plaque-forming cell response to sheep
erythrocytes, is depressed after exposure to ozone; cellular immunity
is also inhibited. The numbers of all major T lymphocyte subsets,
mitogen-induced T lymphocyte proliferation, and delayed-type
hypersensitivity responses were all shown to be decreased. Numerous
studies with infectivity models show that exposure to ozone has an
adverse influence on the host defences to respiratory infections (Van
Loveren et al., 1994), and most of the studies demonstrate that the
primary targets are the alveolar macrophages (Selgrade & Gilmour,
1994).
Although the influence of other air pollutants such as nitrogen
dioxide and sulfuric acid on host defences has been the subject of
fewer studies, the available data suggest that they have similar
adverse effects (Graham & Gardner, 1985). In view of the numerous
possible targets of air pollutants on respiratory defences and because
of the intricate mechanisms involved, infectivity models in animals
are particularly relevant for ascertaining the likely consequences of
air pollution for exposed human populations.
2.2.8 Mycotoxins
Mycotoxins are structurally diverse secondary metabolites of
fungi that grow on feed. Mycotoxin-induced immunosuppression may be
manifested as depressed T- or B-lymphocyte function, decreased
antibody production, or impaired macrophage activity. Immuno-
stimulation may also be observed with the tricothecenes under
some experimental conditions. Similar effects have been found on the
proliferative responses of human and rodent lymphocytes in vitro
(Lang et al., 1993). Most of the data have been obtained in vivo or
in vitro in animal systems, and there is only limited evidence that
mycotoxins are immunosuppressive in humans (Lea et al., 1989).
Dietary exposure to various mycotoxins resulted in decreased
antibody production, T-lymphocyte proliferative response, delayed-type
hypersensitivity, and NK cell activity (Pestka & Bondy, 1990).
Interestingly, dietary intake was associated with increased
susceptibility to experimental infections.
Aflatoxin is markedly immunosuppressive in cattle and poultry
(see below). Thymic atrophy, suppression of mitogen-induced T- and
B-lymphocyte proliferation, and decreased antibody responses to
various microbial antigens and sheep erythrocytes have been observed
(Corrier, 1992). Cell-mediated immune responses appear to be affected
at lower concentrations than antibody responses. The mechanism of
action seems to be related to impaired protein synthesis.
Ochratoxin, a mycotoxin produced by several species of
Aspergillus and Penicillium, causes depletion of lymphoid cells in
the spleen and lymph nodes of dogs, swine, and mice (Corrier, 1992).
The dose, the route of administration, and the animal species appear
to be critical factors, however; for instance, administration of 13 mg
of ochratoxin to mice in six intraperitoneal injections did not impair
T-lymphocyte proliferation (Luster et al., 1987), whereas
intraperitoneal injections of 5 mg/kg body weight for 50 days did
(Prior & Sisodia, 1982). Ochratoxin also impairs NK cell activity and
increases tumour cell growth in mice (Luster et al., 1987).
The trichothecenes, including T-2 toxin and deoxynivalenol
(vomitoxin), are a structurally related group of mycotoxins produced
by Fusarium. T-2 toxin has been studied extensively and has been
shown to induce lymphoid depletion in the thymus, spleen, and lymph
nodes of numerous laboratory animals (Pestka & Bondy, 1990; Corrier,
1992). In addition, mitogen-induced T- or B-lymphocyte proliferation,
antibody production, and macrophage activation have been found to be
depressed after exposure to either T-2 toxin or vomitoxin. The
impaired immune responsiveness is associated with increased
susceptibility to a variety of experimental infections. As the
tricothecenes are currently considered to be the most potent small-
molecule inhibitors of protein synthesis in eukaryotic cells, the
immunosuppression associated with exposure to these mycotoxins is
likely to be directly or indirectly linked to inhibition of protein
synthesis.
2.2.9 Particles
2.2.9.1 Asbestos
Exposure to asbestos is associated with the development of
inflammatory, fibrotic, and malignant (i.e. pleural mesothelioma and
bronchogenic carcinoma) diseases in humans. Although the pathogenesis
of asbestos-induced lung diseases is complex, a number of observations
indicate that immune processes influence the development and
resolution of both the inflammatory response and fibrotic lesions. For
example, exposure to asbestos is associated with alterations in
cellular and humoral-mediated immunity, including reduction of
lymphocyte mitogenesis, delayed hypersensitivity responses, and
primary antibody responses (Hartmann et al., 1984; Hartmann, 1985;
Bissonette et al., 1989; Miller, 1992). In addition, immunodeficient
mice resolve asbestos-induced inflammatory and fibrotic responses only
with difficulty (Corsini et al., 1994), suggesting that immune
mediators with anti-inflammatory or anti-fibrotic activity (e.g. IL-4
or INF gamma) are involved. Furthermore, it is well established that
alveolar macrophages and type II epithelial cells secrete inflammatory
cytokines, chemokines, and growth factors in response to asbestos
(Driscoll et al., 1990; Rosenthal et al., 1994), and these mediators
are directly involved in the inflammatory responses (e.g. inflammatory
cell recruitment) and fibrogenesis (e.g. fibroblast proliferation).
2.2.9.2 Silica
Experimental animals have been used extensively to define the
pathogenesis of silicosis (Uber & McReynolds, 1982). Several immune
changes have been demonstrated in guinea-pigs, including depression of
humoral and cellular immunity and increased susceptibility to
infectious agents. Similarly, mice exposed to silica showed decreased
lipopolysaccharide-induced proliferation of B lymphocytes and
depressed plaque-forming cell responses (Scheuchenzuber et al., 1985).
Antibody responses to T-independent antigens, however, were less
markedly depressed than responses to T-dependent antigens, suggesting
an additional effect on T-cell control of humoral immunity. The
effects of silica on cellular immunity depend on the dose and route of
entry of antigens. The concanavalin A-induced proliferation response
of spleen T lymphocytes was increased, whereas that of mesenteric
lymph node T lymphocytes was depressed. The aberrations of humoral and
cellular immunity induced by silica are thus complex, and it remains
to be established how these immune changes correlate with the
induction of lung fibrosis or autoantibodies, the major adverse
consequences of exposure to silica. In addition, silica is markedly
toxic to macrophages and activates alveolar macrophages, granulocytes,
and monocytes (Gusev et al., 1993). Infectivity models consistently
show an increased susceptibility of silica-exposed rodents to
infectious pathogens.
2.2.10 Substances of abuse
The immunotoxic consequences of exposure to substances of abuse
are difficult to ascertain in most instances as confounding factors,
such as intercurrent infections secondary to intravenous injection,
may contribute to the observed changes. Recent research has provided
evidence, however, that substances of abuse can directly affect the
immune system (Descotes, 1986; Watson, 1990; Friedman et al., 1991a;
Watson, 1993).
In rodent lymphocytes in vitro, D9-tetrahydrocannabinol
depressed the proliferative responses of T lymphocytes in a dose-
dependent manner (Friedman et al., 1991b). Further to the early
findings that opiates adversely affect immune competence (Cantacuzene,
1898), an increasing body of evidence shows that exogenous opioids
have a variety of effects on cells of the immune system (Rouveix,
1993). At pharmacological concentrations, opiates suppress antibody
production, lymphocyte proliferation, and delayed-type
hypersensitivity and decrease NK cell activity in various animal
models. In addition, phagocytosis is impaired. Opioid peptides can,
however, also have a stimulatory effect on the immune system,
depending on the experimental conditions. ß-Endorphin affects cytokine
production in rat and mouse T-cell cultures in vitro; e.g. it
stimulates the synthesis of IL-2, IL-4, and INF gamma, thereby
inducing MHC class II expression on B cells (van den Bergh et al.,
1993a,b, 1994).
In general, short-term exposure of mice, rats, and guinea-pigs to
mainstream tobacco smoke either produces no significant immuno-
modulatory effect or a slight immunostimulation, which returns
to normal shortly after cessation of exposure (Johnson et al., 1990).
In contrast, subchronic or chronic (more than one year) exposure is
generally immunosuppressive: cellular immunity, e.g. mitogen-induced
lymphocyte proliferative response, and NK cell activity are impaired
after long-term exposure to tobacco smoke. The humoral immune response
is also depressed, as shown by decreased antibody titres, and animals
exposed to cigarette smoke for extended periods are more susceptible
to tumour and infectious challenge than naive animals.
2.2.11 Ultraviolet radiation
The earliest indication that ultraviolet radiation (UVR) affects
the immune system came from studies of host resistance to UVR-induced
tumours in mice (Kripke, 1974). Subsequent studies showed that low
doses of UVR suppress contact hypersensitivity responses to chemical
sensitizers (Toews et al., 1980) and that systemic immunosuppression
(depressed contact hypersensitivity in unirradiated skin) occurs after
exposure to higher doses (Jessup et al., 1978). Irradiated mice were
also found to be less resistant to infection (Giannini, 1990). Other
studies (Noonan & De Fabo, 1990) have determined that systemic
suppression of immunoreactivity is not a function of the dose of UVR
but rather of the interval between irradiation and immunization of the
mice. Thus, induction of contact hypersensitivity responses in mice
exposed to low doses of UVR was not affected when the animals were
immunized through unirradiated skin immediately after exposure to UVR;
however, sensitization was suppressed if three days were allowed to
elapse between irradiation and immunization. It has also been shown
that the dose of UVR required to induce 50% suppression of the immune
response depends on the strain of mouse and the type of antigen used
(Noonan & De Fabo, 1990; Noonan & Hoffman, 1994).
The mechanism of UVR-induced suppression of cellular immunity has
not been elucidated, nor has a single initial event been identified
that leads to suppression of immunoreactivity. Currently, induction of
pyrimidine dimers in DNA (Kripke et al., 1992) and isomerization of
urocanic acid (Noonan & De Fabo, 1992) are the leading contenders.
Increased suppressor cell activity (Brodie & Halliday, 1991) and
efferent lymphatic blockade, which inhibits lymphocyte homing, may be
responsible for the UVR-associated accumulation of lymphocytes in
lymph nodes in UVR-exposed areas (Spangrude et al., 1983) and have
been proposed as possible causes of immunosuppression. Exposure to UVR
has also been shown to alter the pattern of cytokine production by
T cells, from a response dominated by Th1 (i.e. favouring delayed
hypersensitivity responses) to one dominated by Th2 (i.e. favouring
antibody production) (Araneo et al., 1989; Simon et al., 1990).
Exposure to UVR has been reported to affect Langerhans cells directly,
such that their interaction with T cells induces specific antigen
tolerance in the Th1 subpopulation (Simon et al., 1990) and
preferential activation of the Th2 population (Simon et al., 1991).
This may be the reason that mice exposed to UVR are more susceptible
to infection with the protozoan Leishmania major (Giannini, 1992),
since resistance to infection with this intracellular parasite is
dependent on the magnitude of the Th1 response of the host (Reed &
Scott, 1993). In addition, reduced resistance to T. spiralis was
found in rats exposed to UVR on days 5-10 of infection (Goettsch et
al., 1993). Altered cytokine production profiles may also be
responsible for increased sensitivity to Mycobacterium lepraemurium,
an intracellular pathogen that induces a chronic and eventually fatal
infection in susceptible mice. In a comparison of susceptible (BALB/c)
and resistant (C57Bl/6J) mice, Brett & Butler (1986) determined that
resistance to infection is correlated with the ability of mouse
lymphocytes to elaborate cytokines that activate macrophages, rather
than with the actual development of a delayed hypersensitivity
response to bacterial antigens. Jeevan & Kripke (1990) and Jeevan et
al. (1992) reported that irradiation of BALB/c mice resulted in
decreased resistance to infection, as measured by bacterial counts and
length of survival after infection. Elevated bacterial counts were
seen in animals exposed to doses of UVR that did not suppress the
delayed hypersensitivity response to bacterial antigens, suggesting
that the underlying mechanism of UVR-induced suppression of resistance
to infection is independent of suppressed delayed hypersensitivity.
2.2.12 Food additives
There is little information about the effects of food additives
on the immune system. An early study showed that the preservative
methylparaben and the antioxidants butylated hydroxyanisole, butylated
hydroxytoluene, and propylgallate suppress the in-vitro T-dependent
antibody response, whereas vanillin and vanillic acid stimulate it
(Archer et al., 1978). The significance of these findings in vivo
has yet to be established.
The immunotoxicity of 'caramel colour', which covers a large
number of complex products used as food colorants, has been
investigated. One of the compounds in this group, 2-acetyl-4(5)-
tetrahydroxybutylimidazole (THI) (caramel colour III), has been found
to be immunotoxic in rodents (Kroplien et al., 1985). THI induces a
rapid reduction in the number of B and T cells in blood, spleen, and
lymph nodes and morphological changes in the thymus of rats, with an
increased number of mature medullary thymocytes and a decreased number
of cortical macrophages. THI might reduce the migration of mature
thymocytes into the periphery, as a decrease in the number of recent
ER4+ thymic emigrants was found in the spleens of exposed rats
(Houben et al., 1992). Functional studies indicate changes in Th cell
function, an increased capacity to clear the Gram-positive bacterium
L. monocytogenes, and modulation of the activity of adherent splenic
cells (Houben et al., 1993). It has been hypothesized that THI exerts
an antivitamin B6 action by competing with pyridoxal 5'-phosphate for
binding to the cofactor site of one or more pyridoxal 5'-phosphate-
dependent enzymes.
2.3 Immunotoxicity of environmental chemicals in wildlife and
domesticated species
Most of the concern about chemical contamination of wildlife
populations has been focused on the aquatic ecosystem, and a growing
body of literature has appeared on the effects of pollution on the
health status of aquatic life. These studies deal mainly with the
occurrence of tumours and infectious diseases in fish and marine
mammals. These are multifactorial diseases in which perturbations of
the immune system may play a part.
2.3.1 Fish and other marine species
2.3.1.1 Fish
Fish diseases are being monitored on a routine basis at various
sites in North America and Europe. In Europe, most of the programmes
are carried out under the auspices of the International Council for
Exploration of the Sea. National and local studies have been directed
to estuarine, marine, and brackish waters suspected of being polluted,
such as in the vicinity of industrial areas and after major oil
spills. The common diseases that are discussed in relation to
pollution are certain skin diseases, such as lymphocystis, papillomas,
fin rotting, and skin ulcers (Vethaak & ap Rheinallt, 1992), as they
are easily identified grossly and are therefore potentially useful for
biomonitoring. Since most diseases of fish have a viral or bacterial
etiology, and elevated incidences have been correlated with chemical
pollution, immunotoxicity may play a role. A causal relationship
between chemical pollution and a disease state induced by
immunosuppression cannot be finally established, however, since many
confounding factors exist in the natural environment. Liver neoplasia
and precursor lesions have been used to biomonitor environmental
pollution in flatfish (Malins et al., 1988; Vethaak & ap Rheinallt,
1992; Vethaak, 1993); however, the role of the immune system is not
evident.
Effects observed in field studies are modified or confounded by
numerous factors, in particular for feral fish, for which there are
deficient case histories and limited knowledge of their migratory
patterns and biology (Vethaak, 1993). Extensive epidemiological
surveys are required that include specific parameters that have been
validated in experiments under (more) controlled conditions (in
mesocosms or the laboratory). Changes in disease patterns may suggest
immune alterations, but this should be demonstrated. Since most
diseases have a complex etiology, it will be difficult to establish
the role of immunotoxic effects under field conditions. Circumstantial
evidence can be obtained in these instances, although in the case of
feral animals mesocosm or laboratory experiments must carried out in
order to reach a final conclusion (Secombes et al., 1992; Vethaak,
1993).
The etiological components and their role in the pathogenesis of
many diseases in fish in the field are, as yet, poorly understood, and
laboratory experiments are often indispensable for background
knowledge. Even when such scientific deficiencies are resolved,
laboratory studies will still be needed, since function tests under
controlled conditions yield the most reliable and sensitive methods of
assessing immunological stress and must often accompany field studies,
as mentioned above. Findings from laboratory situations do not
necessarily imply effects in the field, however; in particular, when
results from the laboratory are extrapolated to field situations,
there is often a discrepancy between the levels of exposure.
2.3.1.2 Marine mammals
Marine mammals are of special interest to the discipline of
immunotoxicology. As the highest predators in highly contaminated
marine environments, these animals are exposed to a large number of
environmental chemicals, some of which have been identified as
potentially immunotoxic. Persistent lipophilic halogenated compounds
such as PCBs, polychlorinted dibenzodioxins, polychlorinated
dibenzofurans, and pesticides accumulate in the marine food chain and
are thus biomagnified in marine mammals. The concentrations of PCBs in
the blubber layer of marine mammals are higher than in any other
wildlife species measured (Table 6). In times of food shortage and
other stressful circumstances, these lipids are mobilized, thereby
releasing their toxic burden.
Table 6. Concentrations of polychlorinated biphenyls (PCBs) in herring and the blubber
layer of marine mammals
Species Source Total PCBs Reference
(µg/g lipid)
Herring Atlantic Ocean 0.0003-0.001 De Swart et al.
(United Kingdom) (1994)
Herring Baltic Sea 0.0035-0.0045 De Swart et
(Sweden) al. (1993)
Weddell seal Weddell Sea 0.07-0.09 Luckas et al.
(Leptonychotes wedelli) (Antarctic) (1990)
Harbour seal Atlantic Ocean 1-13 Luckas et al.
(Phoca vitulina) (Iceland) (1990)
Harbour seal Baltic Sea 21-140 Luckas et al.
(Phoca vitulina) (Sweden) (1990)
Beluga whale St Lawrence 15-700 Muir et. al.
(Delphinapterus leucas) River (Canada) (1990);
Martineau et al.
(1987)
Striped dolphin Mediterranean 100-2600 Kannan et al.
(Stenella coeruleoalba) Sea (Spain) (1993)
Because of the high level of exposure of marine mammals, they may
be expected to be the first wild animals to suffer from
immunosuppression due to chronic exposure to environmental
contaminants. Toxicological research over the last 20 years has
identified environmental chemicals as the source of many disorders in
marine mammals. In both field studies and controlled experiments, PCBs
have been linked to reproductive problems. As early as 1976, a high
incidence of premature parturition was seen in California sea-lions
(Zalophus californianus), which was caused by a viral infection and
was suggested to be linked to higher levels of pollutants in animals
that aborted as compared with mothers that gave birth to healthy pups
(Gilmartin et al., 1976). Pathological changes in the uteri of seals
in the highly polluted Baltic Sea, in some cases leading to sterility,
could be correlated with increased levels of PCBs (Helle et al.,
1976). In addition, several studies have linked skeletal deformities
in grey seals (Halichoerus grypus) and harbour seals (Phoca
vitulina) in the Baltic Sea to high levels of organochlorines in
their environment (Bergman et al., 1992; Mortensen et al., 1992). In
porpoises (Phocoenoides dalli) living in the north-western part of
the North Pacific, a negative correlation was found between serum
testosterone levels and DDE concentrations in blubber (Subramanian et
al., 1987). In an experimental situation, two groups of harbour seals
were fed fish containing different levels of pollutants. Seals that
were fed fish from the heavily polluted western part of the Dutch
Wadden Sea had significantly lower pup production than seals feeding
on less polluted fish (Reijnders, 1986). In the same study, it was
shown that the seals fed polluted fish also had significantly reduced
levels of vitamin A and thyroid hormone in their serum (Brouwer et
al., 1989). No parameters of immune function were studied.
Such correlative observations in the natural environment, in
combination with the results of semi-field studies, suggest that the
current levels of contaminants may be adversely affecting certain
marine mammal populations. The occurrence of a large number of
epizootics among seals and dolphins inhabiting polluted coastal areas
-- among bottlenose dolphins (Tursiops truncatus) on the Atlantic
coast of the United States in 1987 and in the Gulf of Mexico in 1990,
striped dolphins (Stenella coeruleoalba) on the coast of France in
1989 and in the Mediterranean Sea in 1990-92, Baikal seals (Phoca
sibirica) in Lake Baikal in 1987, and harbour seals in north-west
Europe in 1988 -- led to both public and scientific discussions about
the possible contribution of environmental pollutants to these disease
outbreaks. Owing to the complexities of the relationships between
toxicants and the immune system and the difficulties in obtaining
samples fit for use in immunotoxicological studies, it has been
impossible thus far to conclusively demonstrate immunosuppression in
free-ranging marine mammals.
Another immunotoxicological experiment was carried out in which
two groups of juvenile harbour seals (Phoca vitulina) were fed fish
from the Baltic Sea or from the relatively unpolluted Atlantic Ocean.
The diets were analysed for concentrations of potential immunotoxic
chemicals: the estimated daily intakes of TCDD-like organochlorines
were 270 and 35 ng/day for the two groups, respectively. Immunological
function in the two groups was examined by measuring mitogen- and
antigen-induced proliferative responses of lymphocytes, NK cell
activity, serum antibody levels after immunization with primary
antigens, and delayed-type hypersensitivity reactions. These
techniques had to be validated for application to seals, as virtually
nothing was known about the cellular immune system of marine mammals
(De Swart et al., 1993, 1994). Seals fed herring from the contaminated
Baltic Sea had significantly depressed immune function, as measured by
decreased NK cell activity (Ross et al., in press) and T-cell mitogen-
induced lymphocyte proliferation (De Swart et al., 1994), and
significantly lower delayed-type hypersensitivity and antibody
responses to immunization with ovalbumin (Ross et al., 1995). The
functional changes were accompanied by increased numbers of
neutrophils in the peripheral blood (De Swart et al., 1994). Since NK
cells are an important line of defence against viruses, and
lymphocytes (especially T cells) play a major role in the clearance of
viral infections, functional suppression of these leukocytes may
contribute to the severity of epizootic episodes among marine mammals.
These experiments not only provide the first demonstration of
pollution-induced impairment of immune function in marine mammals but
indicate that mammals in general can undergo such impairment as a
consequence of chronic exposure to the levels of pollution found in
their natural habitats. It is still difficult, however, to link the
disease outbreaks among marine mammals directly to pollution-induced
impairment of immune function.
2.3.2 Cattle and swine
The effects of antimicrobial, corticosteroid, and hormonal
compounds have been investigated in cattle, mainly as lymphocyte
proliferative responses and neutrophil functions in vitro. The
results are in keeping with those obtained in humans (Black et al.,
1992).
Few studies have dealt specifically with the direct immunotoxic
effects of pesticides and environmental pollutants. No statistical
difference was found between control and polybrominated biphenyl-
exposed animals in the numbers of circulating total, T, and B
lymphocytes, serum immunoglobulin levels, mitogen-induced
proliferative responses of lymphocytes, antibody response to keyhole
limpet mitogen, or cell-mediated response to purified protein
derivative (Kateley & Bazzell, 1978). In contrast, peripheral blood
lymphocytes from sows fed polybrominated biphenyls for 12weeks had
significantly decreased responses to mitogens (Howard et al., 1980).
The mycotoxin tricothecene produced by Fusarium and several
other fungi was shown to reduce lymphoid tissue cellularity and serum
IgA, IgG, and IgM concentrations and to impair neutrophil migration,
chemotaxis, and phagocytosis in cattle exposed to high doses (Buening
et al., 1982; Mann et al., 1983). Similarly, aflatoxin was reported to
suppress the mitogen-induced proliferative response of bovine
lymphocytes (Paul et al., 1977). Other mycotoxins, e.g. ochratoxin A
and zearalenone, have been suggested to be immunosuppressive in cattle
(Black et al., 1992).
2.3.3 Chickens
Chickens have been used in a number of immunological studies, as
the unique bursa of Fabricius, the avian organ for B-cell development,
underlies the need for a separate avian model in immunotoxicology.
Exposure of adolescent chickens to cyclophosphamide decreased the
levels of antibodies to various antigens without decreasing graft-
versus-host reactivity (Lerman & Weidanz, 1970). Nutritional
deficiencies in selenium or vitamin E have also been shown to impair
the humoral immune responses of adolescent chickens (Marsh et al.,
1981). Exposure to cyclophosphamide in ovo results in decreased
antibody forming capacity, decreased responsiveness to
phytohaemagglutinin and concanavalin A, and decreased weights and
altered morphology of the thymus, spleen, and bursa of Fabricius
(Eskola & Toivanen, 1974). Peritoneal macrophages were not affected
after exposure to cyclophosphamide in ovo, as judged by their
number, superoxide anion production, and surface expression of Ia
antigen and transferrin receptor (Golemboski et al., 1992). Dietert et
al. (1985) showed that exposure to aflatoxin B1 in ovo did not alter
humoral immunity; however, two parameters of cell-mediated, graft-
versus-host, and cutaneous basophil hypersensitivity reactions were
depressed. Methyl methanesulfonate decreased the bactericidal action
of peritoneal macrophages for E. coli after exposure in vitro
(Qureshi et al., 1989). TCDD impairs B-cell development in the bursa
of Fabricius in chicken embryos (Nikolaidos et al., 1990).
2.4 Immunotoxicity of environmental chemicals in humans
Although limited, various lines of evidence derived from case
reports, clinical studies, and well-designed longitudinal studies
imply that environmental agents can affect the human immune system.
While these data raise concern about potential health effects, they
rarely refer to clinical disease, for a number of reasons. For
example, there may be sufficient redundancy or reserve in the immune
system that 'moderate' levels of immunosuppression do not result in
disease. Alternatively, the clinical changes most likely to be
associated with moderate immunosuppression, e.g. increased severity or
frequency of pulmonary infections, do not occur. Well-designed
clinical studies with adequate populations and appropriate monitoring,
follow-up, and documentation of exposure have rarely been conducted.
Examples of published reports that attribute immune changes in human
populations exposed to environmental agents are summarized below. As
the reader will note, these studies range from poorly defined to
relatively large longitudinal studies.
2.4.1 Case reports
In an unsubstantiated study, a cluster of cases of Hodgkin's
disease reported in a small town in Michigan (United States) was
ascribed to chronic immune stimulation by mitogenic substances in the
environment (Schwartz et al., 1978). Immunological studies of family
members revealed a large number of individuals with altered ratios of
T-lymphocyte subpopulations, autoantibodies, infections, recurrent
rashes, and NK cell function. A report of a four-year study of workers
engaged in the manufacture of benzidine, a human bladder carcinogen,
suggested that individuals with depressed cell-mediated immunity (as
judged by skin testing) had precancerous conditions and subsequent
neoplasms (Gorodilova & Mandrik, 1978); no cases of neoplastic disease
were registered in workers with normal immunological responses.
2.4.2 Air pollutants
The association betweeen changes in immunological parameters and
host resistance and inhalation of particulate materials and oxidant
gases is well established (Folinsbee, 1992). For example, decreases in
delayed-type hypersensitivity response, circulating T-cell numbers,
and T-cell proliferation have been observed with, and sometimes
preceding, asbestos-related diseases, i.e. fibrosis, asbestosis, and
mesothelioma (Kagan et al., 1977a; Gaumer et al., 1981; Lew et al.,
1986; Tsang et al., 1988). B-Cell responses are increased, however, as
evidenced by increased serum and secretory (primarily IgA)
immunoglobulins (Kagan et al., 1977b). Kagan et al. (1979) also
reported an association between exposure to asbestos and B-cell
lymphoproliferative disorders. Several studies have shown altered NK
cell activity after exposure to asbestos (Kubota et al., 1985; Tsang
et al., 1988). In studies of NK cell responses in asbestos workers,
Lew et al. (1986) found that immune changes may occur independently of
any early neoplastic process. Similarly, there have been multiple
observations of abnormal antibody production, decreased cell-mediated
immune responses, and decreased resistance to disease in people
occupationally exposed to silica (Uber & McReynolds, 1982).
Oxidant gases have been associated with an increased prevalence
of respiratory infections, particularly bacterial, and potential
immune effects, but the data are less convincing than those from
studies of rodents. The association between air pollution in
industrialized areas and altered health status has been well
established in epidemiological studies (French et al., 1973). A number
of studies have linked exposure to air pollutants (ozone, nitrogen
dioxide, sulfur dioxide, environmental tobacco smoke) with an
increased incidence, severity, or duration of symptoms associated with
respiratory infections (Lunn et al., 1967; French et al., 1973;
Durham, 1974; Harrington & Krupnick, 1985; Neas et al., 1991; Schwartz
et al., 1991; Schwartz, 1992; US Environmental Protection Agency,
1990), although several studies of nitrogen dioxide failed to show
such an association (Speizer et al., 1980; Ware et al., 1984; Samet et
al., 1993). In Ontario, Canada, increased air pollution from sulfur
dioxide and ozone during the summer was directly related to hospital
admissions for acute respiratory symptoms (Bates & Sizto, 1983).
Goings et al. (1989) subjected young adult volunteers to 1, 2, or
3 ppm nitrogen dioxide for 2h per day on two consecutive days and then
administered influenza virus intranasally. Although no statistical
differences were observed, the frequencies of infections in exposed
volunteers (91%) were higher than the 56-73% in healthy individuals,
suggesting that nitrogen dioxide may play a role in increasing
susceptibility to infection. In assessments of air pollution at home,
young children in households with gas stoves had a higher incidence of
respiratory disease and decreased pulmonary function than children in
households with electric stoves. This difference was related to
increased levels of nitrogen dioxide in homes with gas stoves, which
reached peak values of > 1100 mg/m3 (Melia et al., 1977; Speizer et
al., 1980). In contrast, Samet (1994) found no association between
indoor levels of nitrogen dioxide and respiratory infections in
children. A study of schoolchildren in Chattanooga (United States)
showed an increased incidence of respiratory illness associated with
atmospheric nitrogen dioxide levels (Shy et al., 1970).
Examination of hospital admissions in Massachusetts (United
States) in 1980 and 1982 revealed a positive association between 1-h
maximum ozone levels in the summer months and daily admissions for
pneumonia and influenza (Ozkaynak et al., 1990). An effect of
atmospheric pollution, including oxidant gases, was also seen on the
number of influenza cases in Sofia, Bulgaria (Kalpazanov et al.,
1976); however, no demonstrable adverse effect on the course of a
rhinoviral infection was seen in young adult male volunteers after
exposure to moderate levels of ozone (0.3ppm for 6 h/day over a period
of five days) (Henderson et al., 1988), and children living in areas
with high ozone concentrations had lowered CD4:CD8 ratios of
peripheral lymphocytes but no higher incidence of chest colds (Zwick
et al., 1991). The phagocytic activity of alveolar macrophages
(obtained by bronchoalveolar lavage) and other functions were impaired
in human volunteers exposed to ozone (Devlin et al., 1991). The
sensitivity of human and mouse macrophages to the effects of ozone is
similar (Selgrade et al., 1995).
Not all epidemiological studies have showed a correlation between
air pollution and respiratory disease. Some of the discrepancies from
experimental studies may be due to the parameters used to assess
enhancement of infection. In experimental studies, increases in viral
titres in the respiratory tract tend to be taken as an indication that
the exposure enhances infection, whereas epidemiological studies rely
on symptoms, many of which could be related to enhanced inflammatory
or even allergic responses to the virus in the absence of viral
replication.
Controlled studies (in an environmental chamber) showed that
acute exposure to ozone causes an inflammatory response in the lower
airways of human subjects (Koren et al., 1989; Devlin et al., 1991).
The inflammatory response was manifested by increases in various
inflammatory indicators including polymorphonuclear neutrophils
(Figure 34), proteins, fibronectin, IL-6, and tryptase (Koren et al.,
1989, 1994).
Modulation of nonspecific defence mechanisms by ozone has also
been described (Goldstein et al., 1971; Holt & Keast, 1977; Van
Loveren et al., 1988a, 1990b). Thus, phagocytic activity in alveolar
macrophages is suppressed, but this depends on the concentration and
duration of exposure; enhanced phagocytic activity was also observed.
Alterations in the macrophage production of arachidonic acid
metabolites, resulting in increased prostaglandin 2 production, have
been suggested to be involved (Gilmour et al., 1993). NK cell activity
is either unaffected or stimulated by low ozone concentrations,
whereas high concentrations decreased both the number and the activity
of splenic and pulmonary NK cells (Burleson et al., 1989; Van Loveren
et al., 1990b). Ozone also affects T cells (Dziedzic & White, 1986;
Van Loveren et al., 1988a; Bleavins & Dziedzic, 1990; Dziedzic et al.,
1990). Ozone-induced systemic dysfunction has been reported in animals
and probably contributes to impaired host defences (Aranyi et al.,
1983). Humoral immunity, e.g. circulating antibody titres to a variety
of antigens and the plaque-forming cell response to sheep
erythrocytes, is depressed after exposure to ozone; cellular immunity
is also inhibited. The numbers of all major T lymphocyte subsets,
mitogen-induced T lymphocyte proliferation, and delayed-type
hypersensitivity responses were all shown to be decreased. Numerous
studies with infectivity models show that exposure to ozone has an
adverse influence on the host defences to respiratory infections (Van
Loveren et al., 1994), and most of the studies demonstrate that the
primary targets are the alveolar macrophages (Selgrade & Gilmour,
1994).
Although the influence of other air pollutants such as nitrogen
dioxide and sulfuric acid on host defences has been the subject of
fewer studies, the available data suggest that they have similar
adverse effects (Graham & Gardner, 1985). In view of the numerous
possible targets of air pollutants on respiratory defences and because
of the intricate mechanisms involved, infectivity models in animals
are particularly relevant for ascertaining the likely consequences of
air pollution for exposed human populations.
2.2.8 Mycotoxins
Mycotoxins are structurally diverse secondary metabolites of
fungi that grow on feed. Mycotoxin-induced immunosuppression may be
manifested as depressed T- or B-lymphocyte function, decreased
antibody production, or impaired macrophage activity. Immuno-
stimulation may also be observed with the tricothecenes under
some experimental conditions. Similar effects have been found on the
proliferative responses of human and rodent lymphocytes in vitro
(Lang et al., 1993). Most of the data have been obtained in vivo or
in vitro in animal systems, and there is only limited evidence that
mycotoxins are immunosuppressive in humans (Lea et al., 1989).
Dietary exposure to various mycotoxins resulted in decreased
antibody production, T-lymphocyte proliferative response, delayed-type
hypersensitivity, and NK cell activity (Pestka & Bondy, 1990).
Interestingly, dietary intake was associated with increased
susceptibility to experimental infections.
Aflatoxin is markedly immunosuppressive in cattle and poultry
(see below). Thymic atrophy, suppression of mitogen-induced T- and
B-lymphocyte proliferation, and decreased antibody responses to
various microbial antigens and sheep erythrocytes have been observed
(Corrier, 1992). Cell-mediated immune responses appear to be affected
at lower concentrations than antibody responses. The mechanism of
action seems to be related to impaired protein synthesis.
Ochratoxin, a mycotoxin produced by several species of
Aspergillus and Penicillium, causes depletion of lymphoid cells in
the spleen and lymph nodes of dogs, swine, and mice (Corrier, 1992).
The dose, the route of administration, and the animal species appear
to be critical factors, however; for instance, administration of 13 mg
of ochratoxin to mice in six intraperitoneal injections did not impair
T-lymphocyte proliferation (Luster et al., 1987), whereas
intraperitoneal injections of 5 mg/kg body weight for 50 days did
(Prior & Sisodia, 1982). Ochratoxin also impairs NK cell activity and
increases tumour cell growth in mice (Luster et al., 1987).
The trichothecenes, including T-2 toxin and deoxynivalenol
(vomitoxin), are a structurally related group of mycotoxins produced
by Fusarium. T-2 toxin has been studied extensively and has been
shown to induce lymphoid depletion in the thymus, spleen, and lymph
nodes of numerous laboratory animals (Pestka & Bondy, 1990; Corrier,
1992). In addition, mitogen-induced T- or B-lymphocyte proliferation,
antibody production, and macrophage activation have been found to be
depressed after exposure to either T-2 toxin or vomitoxin. The
impaired immune responsiveness is associated with increased
susceptibility to a variety of experimental infections. As the
tricothecenes are currently considered to be the most potent small-
molecule inhibitors of protein synthesis in eukaryotic cells, the
immunosuppression associated with exposure to these mycotoxins is
likely to be directly or indirectly linked to inhibition of protein
synthesis.
2.2.9 Particles
2.2.9.1 Asbestos
Exposure to asbestos is associated with the development of
inflammatory, fibrotic, and malignant (i.e. pleural mesothelioma and
bronchogenic carcinoma) diseases in humans. Although the pathogenesis
of asbestos-induced lung diseases is complex, a number of observations
indicate that immune processes influence the development and
resolution of both the inflammatory response and fibrotic lesions. For
example, exposure to asbestos is associated with alterations in
cellular and humoral-mediated immunity, including reduction of
lymphocyte mitogenesis, delayed hypersensitivity responses, and
primary antibody responses (Hartmann et al., 1984; Hartmann, 1985;
Bissonette et al., 1989; Miller, 1992). In addition, immunodeficient
mice resolve asbestos-induced inflammatory and fibrotic responses only
with difficulty (Corsini et al., 1994), suggesting that immune
mediators with anti-inflammatory or anti-fibrotic activity (e.g. IL-4
or INF gamma) are involved. Furthermore, it is well established that
alveolar macrophages and type II epithelial cells secrete inflammatory
cytokines, chemokines, and growth factors in response to asbestos
(Driscoll et al., 1990; Rosenthal et al., 1994), and these mediators
are directly involved in the inflammatory responses (e.g. inflammatory
cell recruitment) and fibrogenesis (e.g. fibroblast proliferation).
2.2.9.2 Silica
Experimental animals have been used extensively to define the
pathogenesis of silicosis (Uber & McReynolds, 1982). Several immune
changes have been demonstrated in guinea-pigs, including depression of
humoral and cellular immunity and increased susceptibility to
infectious agents. Similarly, mice exposed to silica showed decreased
lipopolysaccharide-induced proliferation of B lymphocytes and
depressed plaque-forming cell responses (Scheuchenzuber et al., 1985).
Antibody responses to T-independent antigens, however, were less
markedly depressed than responses to T-dependent antigens, suggesting
an additional effect on T-cell control of humoral immunity. The
effects of silica on cellular immunity depend on the dose and route of
entry of antigens. The concanavalin A-induced proliferation response
of spleen T lymphocytes was increased, whereas that of mesenteric
lymph node T lymphocytes was depressed. The aberrations of humoral and
cellular immunity induced by silica are thus complex, and it remains
to be established how these immune changes correlate with the
induction of lung fibrosis or autoantibodies, the major adverse
consequences of exposure to silica. In addition, silica is markedly
toxic to macrophages and activates alveolar macrophages, granulocytes,
and monocytes (Gusev et al., 1993). Infectivity models consistently
show an increased susceptibility of silica-exposed rodents to
infectious pathogens.
2.2.10 Substances of abuse
The immunotoxic consequences of exposure to substances of abuse
are difficult to ascertain in most instances as confounding factors,
such as intercurrent infections secondary to intravenous injection,
may contribute to the observed changes. Recent research has provided
evidence, however, that substances of abuse can directly affect the
immune system (Descotes, 1986; Watson, 1990; Friedman et al., 1991a;
Watson, 1993).
In rodent lymphocytes in vitro, D9-tetrahydrocannabinol
depressed the proliferative responses of T lymphocytes in a dose-
dependent manner (Friedman et al., 1991b). Further to the early
findings that opiates adversely affect immune competence (Cantacuzene,
1898), an increasing body of evidence shows that exogenous opioids
have a variety of effects on cells of the immune system (Rouveix,
1993). At pharmacological concentrations, opiates suppress antibody
production, lymphocyte proliferation, and delayed-type
hypersensitivity and decrease NK cell activity in various animal
models. In addition, phagocytosis is impaired. Opioid peptides can,
however, also have a stimulatory effect on the immune system,
depending on the experimental conditions. ß-Endorphin affects cytokine
production in rat and mouse T-cell cultures in vitro; e.g. it
stimulates the synthesis of IL-2, IL-4, and INF gamma, thereby
inducing MHC class II expression on B cells (van den Bergh et al.,
1993a,b, 1994).
In general, short-term exposure of mice, rats, and guinea-pigs to
mainstream tobacco smoke either produces no significant immuno-
modulatory effect or a slight immunostimulation, which returns
to normal shortly after cessation of exposure (Johnson et al., 1990).
In contrast, subchronic or chronic (more than one year) exposure is
generally immunosuppressive: cellular immunity, e.g. mitogen-induced
lymphocyte proliferative response, and NK cell activity are impaired
after long-term exposure to tobacco smoke. The humoral immune response
is also depressed, as shown by decreased antibody titres, and animals
exposed to cigarette smoke for extended periods are more susceptible
to tumour and infectious challenge than naive animals.
2.2.11 Ultraviolet radiation
The earliest indication that ultraviolet radiation (UVR) affects
the immune system came from studies of host resistance to UVR-induced
tumours in mice (Kripke, 1974). Subsequent studies showed that low
doses of UVR suppress contact hypersensitivity responses to chemical
sensitizers (Toews et al., 1980) and that systemic immunosuppression
(depressed contact hypersensitivity in unirradiated skin) occurs after
exposure to higher doses (Jessup et al., 1978). Irradiated mice were
also found to be less resistant to infection (Giannini, 1990). Other
studies (Noonan & De Fabo, 1990) have determined that systemic
suppression of immunoreactivity is not a function of the dose of UVR
but rather of the interval between irradiation and immunization of the
mice. Thus, induction of contact hypersensitivity responses in mice
exposed to low doses of UVR was not affected when the animals were
immunized through unirradiated skin immediately after exposure to UVR;
however, sensitization was suppressed if three days were allowed to
elapse between irradiation and immunization. It has also been shown
that the dose of UVR required to induce 50% suppression of the immune
response depends on the strain of mouse and the type of antigen used
(Noonan & De Fabo, 1990; Noonan & Hoffman, 1994).
The mechanism of UVR-induced suppression of cellular immunity has
not been elucidated, nor has a single initial event been identified
that leads to suppression of immunoreactivity. Currently, induction of
pyrimidine dimers in DNA (Kripke et al., 1992) and isomerization of
urocanic acid (Noonan & De Fabo, 1992) are the leading contenders.
Increased suppressor cell activity (Brodie & Halliday, 1991) and
efferent lymphatic blockade, which inhibits lymphocyte homing, may be
responsible for the UVR-associated accumulation of lymphocytes in
lymph nodes in UVR-exposed areas (Spangrude et al., 1983) and have
been proposed as possible causes of immunosuppression. Exposure to UVR
has also been shown to alter the pattern of cytokine production by
T cells, from a response dominated by Th1 (i.e. favouring delayed
hypersensitivity responses) to one dominated by Th2 (i.e. favouring
antibody production) (Araneo et al., 1989; Simon et al., 1990).
Exposure to UVR has been reported to affect Langerhans cells directly,
such that their interaction with T cells induces specific antigen
tolerance in the Th1 subpopulation (Simon et al., 1990) and
preferential activation of the Th2 population (Simon et al., 1991).
This may be the reason that mice exposed to UVR are more susceptible
to infection with the protozoan Leishmania major (Giannini, 1992),
since resistance to infection with this intracellular parasite is
dependent on the magnitude of the Th1 response of the host (Reed &
Scott, 1993). In addition, reduced resistance to T. spiralis was
found in rats exposed to UVR on days 5-10 of infection (Goettsch et
al., 1993). Altered cytokine production profiles may also be
responsible for increased sensitivity to Mycobacterium lepraemurium,
an intracellular pathogen that induces a chronic and eventually fatal
infection in susceptible mice. In a comparison of susceptible (BALB/c)
and resistant (C57Bl/6J) mice, Brett & Butler (1986) determined that
resistance to infection is correlated with the ability of mouse
lymphocytes to elaborate cytokines that activate macrophages, rather
than with the actual development of a delayed hypersensitivity
response to bacterial antigens. Jeevan & Kripke (1990) and Jeevan et
al. (1992) reported that irradiation of BALB/c mice resulted in
decreased resistance to infection, as measured by bacterial counts and
length of survival after infection. Elevated bacterial counts were
seen in animals exposed to doses of UVR that did not suppress the
delayed hypersensitivity response to bacterial antigens, suggesting
that the underlying mechanism of UVR-induced suppression of resistance
to infection is independent of suppressed delayed hypersensitivity.
2.2.12 Food additives
There is little information about the effects of food additives
on the immune system. An early study showed that the preservative
methylparaben and the antioxidants butylated hydroxyanisole, butylated
hydroxytoluene, and propylgallate suppress the in-vitro T-dependent
antibody response, whereas vanillin and vanillic acid stimulate it
(Archer et al., 1978). The significance of these findings in vivo
has yet to be established.
The immunotoxicity of 'caramel colour', which covers a large
number of complex products used as food colorants, has been
investigated. One of the compounds in this group, 2-acetyl-4(5)-
tetrahydroxybutylimidazole (THI) (caramel colour III), has been found
to be immunotoxic in rodents (Kroplien et al., 1985). THI induces a
rapid reduction in the number of B and T cells in blood, spleen, and
lymph nodes and morphological changes in the thymus of rats, with an
increased number of mature medullary thymocytes and a decreased number
of cortical macrophages. THI might reduce the migration of mature
thymocytes into the periphery, as a decrease in the number of recent
ER4+ thymic emigrants was found in the spleens of exposed rats
(Houben et al., 1992). Functional studies indicate changes in Th cell
function, an increased capacity to clear the Gram-positive bacterium
L. monocytogenes, and modulation of the activity of adherent splenic
cells (Houben et al., 1993). It has been hypothesized that THI exerts
an antivitamin B6 action by competing with pyridoxal 5'-phosphate for
binding to the cofactor site of one or more pyridoxal 5'-phosphate-
dependent enzymes.
2.3 Immunotoxicity of environmental chemicals in wildlife and
domesticated species
Most of the concern about chemical contamination of wildlife
populations has been focused on the aquatic ecosystem, and a growing
body of literature has appeared on the effects of pollution on the
health status of aquatic life. These studies deal mainly with the
occurrence of tumours and infectious diseases in fish and marine
mammals. These are multifactorial diseases in which perturbations of
the immune system may play a part.
2.3.1 Fish and other marine species
2.3.1.1 Fish
Fish diseases are being monitored on a routine basis at various
sites in North America and Europe. In Europe, most of the programmes
are carried out under the auspices of the International Council for
Exploration of the Sea. National and local studies have been directed
to estuarine, marine, and brackish waters suspected of being polluted,
such as in the vicinity of industrial areas and after major oil
spills. The common diseases that are discussed in relation to
pollution are certain skin diseases, such as lymphocystis, papillomas,
fin rotting, and skin ulcers (Vethaak & ap Rheinallt, 1992), as they
are easily identified grossly and are therefore potentially useful for
biomonitoring. Since most diseases of fish have a viral or bacterial
etiology, and elevated incidences have been correlated with chemical
pollution, immunotoxicity may play a role. A causal relationship
between chemical pollution and a disease state induced by
immunosuppression cannot be finally established, however, since many
confounding factors exist in the natural environment. Liver neoplasia
and precursor lesions have been used to biomonitor environmental
pollution in flatfish (Malins et al., 1988; Vethaak & ap Rheinallt,
1992; Vethaak, 1993); however, the role of the immune system is not
evident.
Effects observed in field studies are modified or confounded by
numerous factors, in particular for feral fish, for which there are
deficient case histories and limited knowledge of their migratory
patterns and biology (Vethaak, 1993). Extensive epidemiological
surveys are required that include specific parameters that have been
validated in experiments under (more) controlled conditions (in
mesocosms or the laboratory). Changes in disease patterns may suggest
immune alterations, but this should be demonstrated. Since most
diseases have a complex etiology, it will be difficult to establish
the role of immunotoxic effects under field conditions. Circumstantial
evidence can be obtained in these instances, although in the case of
feral animals mesocosm or laboratory experiments must carried out in
order to reach a final conclusion (Secombes et al., 1992; Vethaak,
1993).
The etiological components and their role in the pathogenesis of
many diseases in fish in the field are, as yet, poorly understood, and
laboratory experiments are often indispensable for background
knowledge. Even when such scientific deficiencies are resolved,
laboratory studies will still be needed, since function tests under
controlled conditions yield the most reliable and sensitive methods of
assessing immunological stress and must often accompany field studies,
as mentioned above. Findings from laboratory situations do not
necessarily imply effects in the field, however; in particular, when
results from the laboratory are extrapolated to field situations,
there is often a discrepancy between the levels of exposure.
2.3.1.2 Marine mammals
Marine mammals are of special interest to the discipline of
immunotoxicology. As the highest predators in highly contaminated
marine environments, these animals are exposed to a large number of
environmental chemicals, some of which have been identified as
potentially immunotoxic. Persistent lipophilic halogenated compounds
such as PCBs, polychlorinted dibenzodioxins, polychlorinated
dibenzofurans, and pesticides accumulate in the marine food chain and
are thus biomagnified in marine mammals. The concentrations of PCBs in
the blubber layer of marine mammals are higher than in any other
wildlife species measured (Table 6). In times of food shortage and
other stressful circumstances, these lipids are mobilized, thereby
releasing their toxic burden.
Table 6. Concentrations of polychlorinated biphenyls (PCBs) in herring and the blubber
layer of marine mammals
Species Source Total PCBs Reference
(µg/g lipid)
Herring Atlantic Ocean 0.0003-0.001 De Swart et al.
(United Kingdom) (1994)
Herring Baltic Sea 0.0035-0.0045 De Swart et
(Sweden) al. (1993)
Weddell seal Weddell Sea 0.07-0.09 Luckas et al.
(Leptonychotes wedelli) (Antarctic) (1990)
Harbour seal Atlantic Ocean 1-13 Luckas et al.
(Phoca vitulina) (Iceland) (1990)
Harbour seal Baltic Sea 21-140 Luckas et al.
(Phoca vitulina) (Sweden) (1990)
Beluga whale St Lawrence 15-700 Muir et. al.
(Delphinapterus leucas) River (Canada) (1990);
Martineau et al.
(1987)
Striped dolphin Mediterranean 100-2600 Kannan et al.
(Stenella coeruleoalba) Sea (Spain) (1993)
Because of the high level of exposure of marine mammals, they may
be expected to be the first wild animals to suffer from
immunosuppression due to chronic exposure to environmental
contaminants. Toxicological research over the last 20 years has
identified environmental chemicals as the source of many disorders in
marine mammals. In both field studies and controlled experiments, PCBs
have been linked to reproductive problems. As early as 1976, a high
incidence of premature parturition was seen in California sea-lions
(Zalophus californianus), which was caused by a viral infection and
was suggested to be linked to higher levels of pollutants in animals
that aborted as compared with mothers that gave birth to healthy pups
(Gilmartin et al., 1976). Pathological changes in the uteri of seals
in the highly polluted Baltic Sea, in some cases leading to sterility,
could be correlated with increased levels of PCBs (Helle et al.,
1976). In addition, several studies have linked skeletal deformities
in grey seals (Halichoerus grypus) and harbour seals (Phoca
vitulina) in the Baltic Sea to high levels of organochlorines in
their environment (Bergman et al., 1992; Mortensen et al., 1992). In
porpoises (Phocoenoides dalli) living in the north-western part of
the North Pacific, a negative correlation was found between serum
testosterone levels and DDE concentrations in blubber (Subramanian et
al., 1987). In an experimental situation, two groups of harbour seals
were fed fish containing different levels of pollutants. Seals that
were fed fish from the heavily polluted western part of the Dutch
Wadden Sea had significantly lower pup production than seals feeding
on less polluted fish (Reijnders, 1986). In the same study, it was
shown that the seals fed polluted fish also had significantly reduced
levels of vitamin A and thyroid hormone in their serum (Brouwer et
al., 1989). No parameters of immune function were studied.
Such correlative observations in the natural environment, in
combination with the results of semi-field studies, suggest that the
current levels of contaminants may be adversely affecting certain
marine mammal populations. The occurrence of a large number of
epizootics among seals and dolphins inhabiting polluted coastal areas
-- among bottlenose dolphins (Tursiops truncatus) on the Atlantic
coast of the United States in 1987 and in the Gulf of Mexico in 1990,
striped dolphins (Stenella coeruleoalba) on the coast of France in
1989 and in the Mediterranean Sea in 1990-92, Baikal seals (Phoca
sibirica) in Lake Baikal in 1987, and harbour seals in north-west
Europe in 1988 -- led to both public and scientific discussions about
the possible contribution of environmental pollutants to these disease
outbreaks. Owing to the complexities of the relationships between
toxicants and the immune system and the difficulties in obtaining
samples fit for use in immunotoxicological studies, it has been
impossible thus far to conclusively demonstrate immunosuppression in
free-ranging marine mammals.
Another immunotoxicological experiment was carried out in which
two groups of juvenile harbour seals (Phoca vitulina) were fed fish
from the Baltic Sea or from the relatively unpolluted Atlantic Ocean.
The diets were analysed for concentrations of potential immunotoxic
chemicals: the estimated daily intakes of TCDD-like organochlorines
were 270 and 35 ng/day for the two groups, respectively. Immunological
function in the two groups was examined by measuring mitogen- and
antigen-induced proliferative responses of lymphocytes, NK cell
activity, serum antibody levels after immunization with primary
antigens, and delayed-type hypersensitivity reactions. These
techniques had to be validated for application to seals, as virtually
nothing was known about the cellular immune system of marine mammals
(De Swart et al., 1993, 1994). Seals fed herring from the contaminated
Baltic Sea had significantly depressed immune function, as measured by
decreased NK cell activity (Ross et al., in press) and T-cell mitogen-
induced lymphocyte proliferation (De Swart et al., 1994), and
significantly lower delayed-type hypersensitivity and antibody
responses to immunization with ovalbumin (Ross et al., 1995). The
functional changes were accompanied by increased numbers of
neutrophils in the peripheral blood (De Swart et al., 1994). Since NK
cells are an important line of defence against viruses, and
lymphocytes (especially T cells) play a major role in the clearance of
viral infections, functional suppression of these leukocytes may
contribute to the severity of epizootic episodes among marine mammals.
These experiments not only provide the first demonstration of
pollution-induced impairment of immune function in marine mammals but
indicate that mammals in general can undergo such impairment as a
consequence of chronic exposure to the levels of pollution found in
their natural habitats. It is still difficult, however, to link the
disease outbreaks among marine mammals directly to pollution-induced
impairment of immune function.
2.3.2 Cattle and swine
The effects of antimicrobial, corticosteroid, and hormonal
compounds have been investigated in cattle, mainly as lymphocyte
proliferative responses and neutrophil functions in vitro. The
results are in keeping with those obtained in humans (Black et al.,
1992).
Few studies have dealt specifically with the direct immunotoxic
effects of pesticides and environmental pollutants. No statistical
difference was found between control and polybrominated biphenyl-
exposed animals in the numbers of circulating total, T, and B
lymphocytes, serum immunoglobulin levels, mitogen-induced
proliferative responses of lymphocytes, antibody response to keyhole
limpet mitogen, or cell-mediated response to purified protein
derivative (Kateley & Bazzell, 1978). In contrast, peripheral blood
lymphocytes from sows fed polybrominated biphenyls for 12weeks had
significantly decreased responses to mitogens (Howard et al., 1980).
The mycotoxin tricothecene produced by Fusarium and several
other fungi was shown to reduce lymphoid tissue cellularity and serum
IgA, IgG, and IgM concentrations and to impair neutrophil migration,
chemotaxis, and phagocytosis in cattle exposed to high doses (Buening
et al., 1982; Mann et al., 1983). Similarly, aflatoxin was reported to
suppress the mitogen-induced proliferative response of bovine
lymphocytes (Paul et al., 1977). Other mycotoxins, e.g. ochratoxin A
and zearalenone, have been suggested to be immunosuppressive in cattle
(Black et al., 1992).
2.3.3 Chickens
Chickens have been used in a number of immunological studies, as
the unique bursa of Fabricius, the avian organ for B-cell development,
underlies the need for a separate avian model in immunotoxicology.
Exposure of adolescent chickens to cyclophosphamide decreased the
levels of antibodies to various antigens without decreasing graft-
versus-host reactivity (Lerman & Weidanz, 1970). Nutritional
deficiencies in selenium or vitamin E have also been shown to impair
the humoral immune responses of adolescent chickens (Marsh et al.,
1981). Exposure to cyclophosphamide in ovo results in decreased
antibody forming capacity, decreased responsiveness to
phytohaemagglutinin and concanavalin A, and decreased weights and
altered morphology of the thymus, spleen, and bursa of Fabricius
(Eskola & Toivanen, 1974). Peritoneal macrophages were not affected
after exposure to cyclophosphamide in ovo, as judged by their
number, superoxide anion production, and surface expression of Ia
antigen and transferrin receptor (Golemboski et al., 1992). Dietert et
al. (1985) showed that exposure to aflatoxin B1 in ovo did not alter
humoral immunity; however, two parameters of cell-mediated, graft-
versus-host, and cutaneous basophil hypersensitivity reactions were
depressed. Methyl methanesulfonate decreased the bactericidal action
of peritoneal macrophages for E. coli after exposure in vitro
(Qureshi et al., 1989). TCDD impairs B-cell development in the bursa
of Fabricius in chicken embryos (Nikolaidos et al., 1990).
2.4 Immunotoxicity of environmental chemicals in humans
Although limited, various lines of evidence derived from case
reports, clinical studies, and well-designed longitudinal studies
imply that environmental agents can affect the human immune system.
While these data raise concern about potential health effects, they
rarely refer to clinical disease, for a number of reasons. For
example, there may be sufficient redundancy or reserve in the immune
system that 'moderate' levels of immunosuppression do not result in
disease. Alternatively, the clinical changes most likely to be
associated with moderate immunosuppression, e.g. increased severity or
frequency of pulmonary infections, do not occur. Well-designed
clinical studies with adequate populations and appropriate monitoring,
follow-up, and documentation of exposure have rarely been conducted.
Examples of published reports that attribute immune changes in human
populations exposed to environmental agents are summarized below. As
the reader will note, these studies range from poorly defined to
relatively large longitudinal studies.
2.4.1 Case reports
In an unsubstantiated study, a cluster of cases of Hodgkin's
disease reported in a small town in Michigan (United States) was
ascribed to chronic immune stimulation by mitogenic substances in the
environment (Schwartz et al., 1978). Immunological studies of family
members revealed a large number of individuals with altered ratios of
T-lymphocyte subpopulations, autoantibodies, infections, recurrent
rashes, and NK cell function. A report of a four-year study of workers
engaged in the manufacture of benzidine, a human bladder carcinogen,
suggested that individuals with depressed cell-mediated immunity (as
judged by skin testing) had precancerous conditions and subsequent
neoplasms (Gorodilova & Mandrik, 1978); no cases of neoplastic disease
were registered in workers with normal immunological responses.
2.4.2 Air pollutants
The association betweeen changes in immunological parameters and
host resistance and inhalation of particulate materials and oxidant
gases is well established (Folinsbee, 1992). For example, decreases in
delayed-type hypersensitivity response, circulating T-cell numbers,
and T-cell proliferation have been observed with, and sometimes
preceding, asbestos-related diseases, i.e. fibrosis, asbestosis, and
mesothelioma (Kagan et al., 1977a; Gaumer et al., 1981; Lew et al.,
1986; Tsang et al., 1988). B-Cell responses are increased, however, as
evidenced by increased serum and secretory (primarily IgA)
immunoglobulins (Kagan et al., 1977b). Kagan et al. (1979) also
reported an association between exposure to asbestos and B-cell
lymphoproliferative disorders. Several studies have shown altered NK
cell activity after exposure to asbestos (Kubota et al., 1985; Tsang
et al., 1988). In studies of NK cell responses in asbestos workers,
Lew et al. (1986) found that immune changes may occur independently of
any early neoplastic process. Similarly, there have been multiple
observations of abnormal antibody production, decreased cell-mediated
immune responses, and decreased resistance to disease in people
occupationally exposed to silica (Uber & McReynolds, 1982).
Oxidant gases have been associated with an increased prevalence
of respiratory infections, particularly bacterial, and potential
immune effects, but the data are less convincing than those from
studies of rodents. The association between air pollution in
industrialized areas and altered health status has been well
established in epidemiological studies (French et al., 1973). A number
of studies have linked exposure to air pollutants (ozone, nitrogen
dioxide, sulfur dioxide, environmental tobacco smoke) with an
increased incidence, severity, or duration of symptoms associated with
respiratory infections (Lunn et al., 1967; French et al., 1973;
Durham, 1974; Harrington & Krupnick, 1985; Neas et al., 1991; Schwartz
et al., 1991; Schwartz, 1992; US Environmental Protection Agency,
1990), although several studies of nitrogen dioxide failed to show
such an association (Speizer et al., 1980; Ware et al., 1984; Samet et
al., 1993). In Ontario, Canada, increased air pollution from sulfur
dioxide and ozone during the summer was directly related to hospital
admissions for acute respiratory symptoms (Bates & Sizto, 1983).
Goings et al. (1989) subjected young adult volunteers to 1, 2, or
3 ppm nitrogen dioxide for 2h per day on two consecutive days and then
administered influenza virus intranasally. Although no statistical
differences were observed, the frequencies of infections in exposed
volunteers (91%) were higher than the 56-73% in healthy individuals,
suggesting that nitrogen dioxide may play a role in increasing
susceptibility to infection. In assessments of air pollution at home,
young children in households with gas stoves had a higher incidence of
respiratory disease and decreased pulmonary function than children in
households with electric stoves. This difference was related to
increased levels of nitrogen dioxide in homes with gas stoves, which
reached peak values of > 1100 mg/m3 (Melia et al., 1977; Speizer et
al., 1980). In contrast, Samet (1994) found no association between
indoor levels of nitrogen dioxide and respiratory infections in
children. A study of schoolchildren in Chattanooga (United States)
showed an increased incidence of respiratory illness associated with
atmospheric nitrogen dioxide levels (Shy et al., 1970).
Examination of hospital admissions in Massachusetts (United
States) in 1980 and 1982 revealed a positive association between 1-h
maximum ozone levels in the summer months and daily admissions for
pneumonia and influenza (Ozkaynak et al., 1990). An effect of
atmospheric pollution, including oxidant gases, was also seen on the
number of influenza cases in Sofia, Bulgaria (Kalpazanov et al.,
1976); however, no demonstrable adverse effect on the course of a
rhinoviral infection was seen in young adult male volunteers after
exposure to moderate levels of ozone (0.3ppm for 6 h/day over a period
of five days) (Henderson et al., 1988), and children living in areas
with high ozone concentrations had lowered CD4:CD8 ratios of
peripheral lymphocytes but no higher incidence of chest colds (Zwick
et al., 1991). The phagocytic activity of alveolar macrophages
(obtained by bronchoalveolar lavage) and other functions were impaired
in human volunteers exposed to ozone (Devlin et al., 1991). The
sensitivity of human and mouse macrophages to the effects of ozone is
similar (Selgrade et al., 1995).
Not all epidemiological studies have showed a correlation between
air pollution and respiratory disease. Some of the discrepancies from
experimental studies may be due to the parameters used to assess
enhancement of infection. In experimental studies, increases in viral
titres in the respiratory tract tend to be taken as an indication that
the exposure enhances infection, whereas epidemiological studies rely
on symptoms, many of which could be related to enhanced inflammatory
or even allergic responses to the virus in the absence of viral
replication.
Controlled studies (in an environmental chamber) showed that
acute exposure to ozone causes an inflammatory response in the lower
airways of human subjects (Koren et al., 1989; Devlin et al., 1991).
The inflammatory response was manifested by increases in various
inflammatory indicators including polymorphonuclear neutrophils
(Figure 34), proteins, fibronectin, IL-6, and tryptase (Koren et al.,
1989, 1994).
 The proinflammatory effects of ozone raise the possibility that
it can increase the sensitivity of people with atopic asthma to
challenge with a specific allergen. Several studies have investigated
the effect of exposure to pollutants on subsequent reactivity to
antigen in atopic human volunteers. Molfino et al. (1991) reported
that exposure to 0.12 ppm ozone significantly increased bronchial
responsiveness to antigen in some individuals. Although they
acknowledged weaknesses in the design of the experiment and
recommended that the findings be confirmed, their observations are in
accordance with those of the majority of epidemiological studies. In
contrast, Bascom et al. (1990) found no alteration in the acute
response to nasal antigen challenge in allergic patients pre-exposed
to ozone in comparison with exposure to air; however, they did report
increased upper respiratory tract inflammation after exposure to ozone
in these patients in the absence of antigen challenge. Similarly, the
bronchial response to inhaled grass pollen was unaffected by prior
exposure to 0.1 ppm nitrogen dioxide (Orehek et al., 1981). The topic
of sensitization and the role of ozone in exacerbating asthma has been
reviewed (Koren & Blomberg, in press).
2.4.3 Pesticides
Pesticides can alter the human immune system. For example, women
chronically exposed to low levels of aldicarb-contaminated groundwater
had altered numbers of T cells and decreased CD4:CD8 ratios (Fiore et
al., 1986). While this finding was not confirmed in studies in animals
(see section 2.2.3.4), follow-up studies by Mirkin et al. (1990)
confirmed the immune changes in those individuals still available for
study, although the population was considerably smaller.
2.4.4 Halogenated aromatic hydrocarbons
A number of chemical accidents have resulted in human exposure to
halogenated aromatic hydrocarbons. A feed supplement for lactating
cows, inadvertently contaminated with polybrominated biphenyls, was
used in more than 500 dairy herds and poultry farms in Michigan
(United States) in 1973. Diary farm residents had reduced proportions
of circulating T Iymphocytes and reducedlymphoproliferative
responsiveness in vitro (Bekesi et al., 1978); these changes
persisted during follow-up (Bekesi et al., 1987). Silva et al. (1979),
however, were unable to detect any immune abnormalities in a similarly
exposed cohort.
In Taiwan, more than 2000 people were exposed in 1979 to rice oil
contaminated with PCBs and polychlorinated dibenzofurans. The clinical
features in many of the exposed individuals included chloracne,
pigmentation of skin, liver disease, and respiratory infections. When
the immune status of the exposed individuals was examined one year
after exposure, decreased concentrations of serum IgM and IgA, but not
IgG, were reported, in addition to decreased numbers of circulating Th
cells. The proportions of Ts/Tc cells and B lymphocytes were within
the control values. Suppression of delayed-type hypersensitivity to
recall antigens (streptokinase, streptodornase, tuberculin), enhanced
mitogen-induced lymphocyte proliferation, and increases in
sinopulmonary infections have been reported in this population (Chang
et al., 1981, 1982; Lu & Wu, 1985). Many of the effects were
transient, since two years after exposure most of the clinical
abnormalities and laboratory parameters had returned to normal. A
similar incident occurred in Japan in 1978, resulting in the 'yusho'
syndrome. The immune system was assumed to be affected because of an
increased frequency of respiratory infections and lowered serum IgM
and IgA concentrations (Shigematsu et al., 1978). Another incident of
intoxication with PCBs and polychlorinated dibenzofurans occurred
after exposure to contaminated soot of fires in electrical equipment
(Elo et al., 1985). The exposed people had serum PCB concentrations up
to 30 mg/litre. The number of blood T cells was lower five weeks after
exposure but had returned to normal values seven weeks later. Lowered
CD4:CD8 ratios and lymphocyte proliferation after mitogen stimulation
were also seen. Nine of the 15 most heavily exposed persons suffered
from at least one infection of the upper respiratory tract. No overt
long-term effects or chloracne were observed.
The existing data also suggest that neonates are particularly
sensitive to the immunotoxic effects of PCBs. Thus, higher incidences
of colds and gastrointestinal (vomiting, abdominal pain) and
dermatological (eczema, itchy skin) manifestations were observed in
breast-fed infants of women occupationally exposed to the PCBs
Kanechlor 500 and 300 than in infants born to unexposed women. The
incidence of these symptoms increased with increasing length of
breast-feeding (Hara, 1985). Epidemiological studies of women who
consumed contaminated fish from the Great Lakes indicated that the
maternal serum PCB level during pregnancy was positively associated
with the number and type of infectious illnesses suffered by their
breast-fed infants, especially during the first four months of life.
The incidence of infections in the infant was correlated strongly with
the highest rate of maternal fish consumption (Swain, 1991).
A number of studies have been conducted of the immune status of
people exposed to TCDD. In 1976, an accident occurred at a chemical
plant in Seveso, Italy, in which high concentrations of TCDD were
released into the local environment. An evaluation of 44 exposed
children (20 with chloracne) showed no overt changes in immune status
(Reggiani, 1980), although the adequacy of the control populations
used has been questioned. In a study of residents of an area of
Missouri (United States) with long-term exposure (average, three
years) to low levels of TCDD in contaminated soil, no clinical
symptoms were recorded, although a number of individuals showed
changes in cell-mediated immunity, manifested as altered delayed-type
hypersensitivity (Hoffman et al., 1986). In the follow-up
investigation, however, the skin anergy was not confirmed (Evans et
al., 1988). The serum concentration of the thymic hormone thymosin-a1,
which has been related to the toxic action of this compound on the
thymus, was reduced (Stehr-Green et al., 1989; Hoffman, 1992).
Jennings et al. (1988) found an increased frequency of circulating
antinuclear antibodies and immune complexes in TCDD-exposed workers.
2.4.5 Metals
Exposure to metals may also affect the immune system. Workers
with elevated blood lead levels (30-90 µg/100 ml) had increased
suppressor cell activity (Cohen et al., 1989), lowered lymphocyte
proliferation after mitogen stimulation in vitro (Jaremin, 1983),
decreased IgA concentrations in saliva, and lowered complement C3
levels (Ewers et al., 1982). These individuals also had an enhanced
prevalence of respiratory infection (Ewers et al., 1982). The
immunotoxic effects of lead may be dose-dependent, since neither
humoral nor cellular parameters were affected after long-term, low-
level exposure (Reigart & Graber, 1976; Kimber et al., 1986). In
unrelated studies, the cationic heavy metal mercury was associated
with immune complex disease in humans (Makker & Aikawa, 1979).
In contrast to the database on the immunotoxic effects of
cadmium, lead, and mercury in experimental animals in vivo and the
results of mechanistic studies in vitro, the data on the effects of
heavy metals on the human immune system are scanty and refer mainly to
occupational exposure. These studies nevertheless provide evidence
that at least mercury and lead affect the immune system (Moszczynski
et al., 1990a; Bernier et al., in press).
Significantly decreased levels of serum IgG and IgA, but not IgM,
IgD, or IgE, were reported in workers occupationally exposed to
metallic mercury vapours for 20 years in comparison with unexposed
controls (Moszczynski et al., 1990b). These workers had blood mercury
levels of < 50 µg/litre. Similarly, significantly decreased IgG and
IgA levels were observed in workers with urinary mercury levels of
0.029-0.545 mg/litre (Bencko et al., 1990). Studies of a small number
of people exposed to mercury in dental amalgams have shown increased
levels of IgE (Anneroth et al., 1992), an increased incidence of
asthma (Drouet et al., 1990), and development of contact dermatitis
(Gonçalo et al., 1992). Total lymphocyte, CD4, and CD8 levels were
higher in exposed people than in controls (Eedy et al., 1990).
Epidemiological data indicate that the main effects of
occupational exposure to lead are on cellular aspects of the immune
system and that humoral parameters remain relatively insensitive to
such exposure. Thus, serum IgG, IgM, and IgA levels remained within
the normal range in workers exposed for 4-30years, with a mean blood
lead level of 38.4 µg/dl, in comparison with a mean control level of
11.8 µg/dl (Kimber et al., 1986). Similarly, no effects were noted on
serum IgG or IgA levels in a cohort exposed to lead oxides at a
reported concentration within the plant of 266 µg/m3, who had an
estimated blood lead level of 64 µg/dl; however, the response of
lymphocytes from the exposed group to stimulation with
phytohaemagglutinin and concanavalin A in vitro was significantly
lower than that of controls (Alomran & Shleamoon, 1988). Decreased
serum Ig levels were reported in occupationally exposed workers with a
mean blood lead level of 46.9 µg/dl, but the duration of exposure was
not reported (Castillo-Mendez et al., 1991). In another study, no
significant effects were noted on serum immunoglobulin levels after
exposure to lead, but the levels of secretory IgA, which plays a major
role in the defence against respiratory and gastrointestinal
infections, was significantly decreased in workers with blood lead
levels of 21-90 µg/dl. The incidence of influenza infections per year
was significantly higher in these workers than in the control group
(Ewers et al., 1982).
Studies on the effects of lead on lymphocyte levels in
occupationally exposed workers have produced inconclusive results. One
study found an increase in absolute B lymphocyte counts and CD8 cells
(Coscia et al., 1987), while a decrease in total T lymphocytes and the
CD4 subset was reported in another set of workers (Fischbein et al.,
1993).
2.4.6 Solvents
Certain organic solvents may induce immune changes in humans.
Benzene-induced pancytopenia with associated bone-marrow hypoplasia, a
classical sign of chronic exposure to benzene, results in an
immunodeficiency state due to the reduced numbers of immunocompetent
cells (Goldstein, 1977). Alterations in the numbers of certain
lymphocyte subsets, e.g. CD3 and CD4 lymphocytes, have also been
reported in workers exposed to solvents (Denkhaus et al., 1986),
suggesting that the effects may be somewhat specific.
2.4.7 Ultraviolet radiation
Numerous reports have shown that UVR inhibits contact
hypersensitivity of the skin to sensitizers such as
dinitrochlorobenzene (DNCB) (O'Dell et al., 1980; Halprin et al.,
1981; Hersey et al., 1983a,b; Kalimo et al., 1983; Sjovall et al.,
1985; Friedmann et al., 1989; Yoshikawa et al., 1990; Vermeer et al.,
1991; Cooper et al., 1992). The dose required to produce
immunosuppressive effects in humans is similar to that in C57Bl/6
mice, the strain phenotypically most sensitive to UVR-induced immune
suppression (Noonan & Hoffman, 1994).
Human buttock skin was exposed to four daily doses of
144 mJ/cm2 UVR, and the irradiated site was sensitized with DNCB
immediately after the last exposure; the inner surface of the forearm
was challenged 30days later with DNCB and contact hypersensitivity
assessed. Forty percent of the volunteers failed to develop contact
hypersensitivity and were designated sensitive, suggesting that, as in
mice, susceptibility to UVR is genetically controlled. The sensitive
phenotype also appeared to be a risk factor for the development of
skin cancer (Yoshikawa et al., 1990). Suppression of contact
hypersensitivity is seen in a similar percentage of black-skinned
individuals, indicating that melanin cannot protect against this
phenomenon (Vermeer et al., 1991). In another study, human buttock
skin was exposed to 0.75 or two minimal erythemal doses (MED) of UVB
(1 MED = 29.1-32.5mJ/cm2, depending on the individual) for four days
and sensitized through irradiated skin immediately after the last
exposure to DNCB; subjects were challenged with diluted DNCB at a
distal site three weeks later. Some subjects were also exposed to four
MED (moderate sunburn) and sensitized three days later with DNCB.
Analysis of overall individual responses revealed decreased
frequencies of fully successful immunizations in all UVB-exposed
groups. The rate of immunological tolerance to DNCB (lasting up to
four months) in the groups that were initially sensitized on skin that
had received erythemagenic doses of UVB was 31%, whereas it was 7% in
unirradiated controls (Cooper et al., 1992).
A dose-response relationship was established in the studies of
Cooper et al. (1992) in a comparison of subjects with types I-III skin
(fair to moderately fair) who received various doses or schedules of
UVR from a bank of FS20 fluorescent sun lamps (rich in UVB) with
respect to their ability to mount an immune response to DNCB. A linear
inhibition of immune responsiveness was seen, the first detectable
decrease occurring at 0.75 of the individual's MED, reaching complete
inhibition of responsiveness for 95% of subjects when two MED were
administered every day for four days before immunization. Similar
inhibition occurred when DNCB was administered through skin that had
been exposed to a single dose of fourMED three days earlier. A dose-
response curve for fair-skinned subjects was constructed by plotting
the dose in total MED administered against the degree of immune
response to DNCB (Figure 35). The 50% immune suppressive dose was
calculated to be about 100mJ/cm2 of UVB.
Unresponsiveness to a contact sensitizer applied to UV-irradiated
skin can thus be induced in a proportion of individuals after exposure
to moderate levels of UVR, and at least some individuals become
immunologically tolerant in a manner similar to experimental animals.
Taken together, these data suggest that the systemic immunosuppression
induced in mice by UVR also occurs in humans, possibly through a
similar mechanism. UVR appears to alter antigen presentation and the
expression of Langerhans (CDla+DR+) cells, which is followed by an
influx of CDla+DR+ monocytes that preferentially activate CD4+
(suppressor-inducer) cells, which induce maturation of CD8+ Tc
Iymphocytes (Cooper et al., 1986; Baadsgaard et al., 1990). The UVR
wavelengths responsible for induction of CDla-DR+ cells are
predominantly within the UVB band and to a lesser extent in the C band
(Baadsgaard et al., 1987, 1989).
UVR from solarium lamps also suppressed NK cell activity in the
blood of subjects exposed for 1h per day for 12 days and tested one
and seven days after exposure; the activity returned to normal by 21
days after exposure (Hersey et al., 1983a). The effects of UVR on NK
cell activity are attributed to A radiation (Hersey et al., 1983b).
The depressed immune function observed in irradiated rodents
reflects anecdotal observations in humans, i.e. that exposure to
sunlight exacerbates certain infectious diseases, particularly those
involving the skin. For example, it was noted at the turn of the
century that smallpox lesions were worsened by exposure to sunlight
(Finsen, 1901), and herpetic lesions and viral warts may be
reactivated or exacerbated by sunlight (Giannini, 1990). It has also
been hypothesized that sunlight affects susceptibility to infection
with the bacteria that cause leprosy (Patki, 1991). Lesions of Herpes
simplex virus type I and type II can be reactivated by exposure to
UVR (Spruanoe, 1985; Klein, 1986). Using the criteria established by
The proinflammatory effects of ozone raise the possibility that
it can increase the sensitivity of people with atopic asthma to
challenge with a specific allergen. Several studies have investigated
the effect of exposure to pollutants on subsequent reactivity to
antigen in atopic human volunteers. Molfino et al. (1991) reported
that exposure to 0.12 ppm ozone significantly increased bronchial
responsiveness to antigen in some individuals. Although they
acknowledged weaknesses in the design of the experiment and
recommended that the findings be confirmed, their observations are in
accordance with those of the majority of epidemiological studies. In
contrast, Bascom et al. (1990) found no alteration in the acute
response to nasal antigen challenge in allergic patients pre-exposed
to ozone in comparison with exposure to air; however, they did report
increased upper respiratory tract inflammation after exposure to ozone
in these patients in the absence of antigen challenge. Similarly, the
bronchial response to inhaled grass pollen was unaffected by prior
exposure to 0.1 ppm nitrogen dioxide (Orehek et al., 1981). The topic
of sensitization and the role of ozone in exacerbating asthma has been
reviewed (Koren & Blomberg, in press).
2.4.3 Pesticides
Pesticides can alter the human immune system. For example, women
chronically exposed to low levels of aldicarb-contaminated groundwater
had altered numbers of T cells and decreased CD4:CD8 ratios (Fiore et
al., 1986). While this finding was not confirmed in studies in animals
(see section 2.2.3.4), follow-up studies by Mirkin et al. (1990)
confirmed the immune changes in those individuals still available for
study, although the population was considerably smaller.
2.4.4 Halogenated aromatic hydrocarbons
A number of chemical accidents have resulted in human exposure to
halogenated aromatic hydrocarbons. A feed supplement for lactating
cows, inadvertently contaminated with polybrominated biphenyls, was
used in more than 500 dairy herds and poultry farms in Michigan
(United States) in 1973. Diary farm residents had reduced proportions
of circulating T Iymphocytes and reducedlymphoproliferative
responsiveness in vitro (Bekesi et al., 1978); these changes
persisted during follow-up (Bekesi et al., 1987). Silva et al. (1979),
however, were unable to detect any immune abnormalities in a similarly
exposed cohort.
In Taiwan, more than 2000 people were exposed in 1979 to rice oil
contaminated with PCBs and polychlorinated dibenzofurans. The clinical
features in many of the exposed individuals included chloracne,
pigmentation of skin, liver disease, and respiratory infections. When
the immune status of the exposed individuals was examined one year
after exposure, decreased concentrations of serum IgM and IgA, but not
IgG, were reported, in addition to decreased numbers of circulating Th
cells. The proportions of Ts/Tc cells and B lymphocytes were within
the control values. Suppression of delayed-type hypersensitivity to
recall antigens (streptokinase, streptodornase, tuberculin), enhanced
mitogen-induced lymphocyte proliferation, and increases in
sinopulmonary infections have been reported in this population (Chang
et al., 1981, 1982; Lu & Wu, 1985). Many of the effects were
transient, since two years after exposure most of the clinical
abnormalities and laboratory parameters had returned to normal. A
similar incident occurred in Japan in 1978, resulting in the 'yusho'
syndrome. The immune system was assumed to be affected because of an
increased frequency of respiratory infections and lowered serum IgM
and IgA concentrations (Shigematsu et al., 1978). Another incident of
intoxication with PCBs and polychlorinated dibenzofurans occurred
after exposure to contaminated soot of fires in electrical equipment
(Elo et al., 1985). The exposed people had serum PCB concentrations up
to 30 mg/litre. The number of blood T cells was lower five weeks after
exposure but had returned to normal values seven weeks later. Lowered
CD4:CD8 ratios and lymphocyte proliferation after mitogen stimulation
were also seen. Nine of the 15 most heavily exposed persons suffered
from at least one infection of the upper respiratory tract. No overt
long-term effects or chloracne were observed.
The existing data also suggest that neonates are particularly
sensitive to the immunotoxic effects of PCBs. Thus, higher incidences
of colds and gastrointestinal (vomiting, abdominal pain) and
dermatological (eczema, itchy skin) manifestations were observed in
breast-fed infants of women occupationally exposed to the PCBs
Kanechlor 500 and 300 than in infants born to unexposed women. The
incidence of these symptoms increased with increasing length of
breast-feeding (Hara, 1985). Epidemiological studies of women who
consumed contaminated fish from the Great Lakes indicated that the
maternal serum PCB level during pregnancy was positively associated
with the number and type of infectious illnesses suffered by their
breast-fed infants, especially during the first four months of life.
The incidence of infections in the infant was correlated strongly with
the highest rate of maternal fish consumption (Swain, 1991).
A number of studies have been conducted of the immune status of
people exposed to TCDD. In 1976, an accident occurred at a chemical
plant in Seveso, Italy, in which high concentrations of TCDD were
released into the local environment. An evaluation of 44 exposed
children (20 with chloracne) showed no overt changes in immune status
(Reggiani, 1980), although the adequacy of the control populations
used has been questioned. In a study of residents of an area of
Missouri (United States) with long-term exposure (average, three
years) to low levels of TCDD in contaminated soil, no clinical
symptoms were recorded, although a number of individuals showed
changes in cell-mediated immunity, manifested as altered delayed-type
hypersensitivity (Hoffman et al., 1986). In the follow-up
investigation, however, the skin anergy was not confirmed (Evans et
al., 1988). The serum concentration of the thymic hormone thymosin-a1,
which has been related to the toxic action of this compound on the
thymus, was reduced (Stehr-Green et al., 1989; Hoffman, 1992).
Jennings et al. (1988) found an increased frequency of circulating
antinuclear antibodies and immune complexes in TCDD-exposed workers.
2.4.5 Metals
Exposure to metals may also affect the immune system. Workers
with elevated blood lead levels (30-90 µg/100 ml) had increased
suppressor cell activity (Cohen et al., 1989), lowered lymphocyte
proliferation after mitogen stimulation in vitro (Jaremin, 1983),
decreased IgA concentrations in saliva, and lowered complement C3
levels (Ewers et al., 1982). These individuals also had an enhanced
prevalence of respiratory infection (Ewers et al., 1982). The
immunotoxic effects of lead may be dose-dependent, since neither
humoral nor cellular parameters were affected after long-term, low-
level exposure (Reigart & Graber, 1976; Kimber et al., 1986). In
unrelated studies, the cationic heavy metal mercury was associated
with immune complex disease in humans (Makker & Aikawa, 1979).
In contrast to the database on the immunotoxic effects of
cadmium, lead, and mercury in experimental animals in vivo and the
results of mechanistic studies in vitro, the data on the effects of
heavy metals on the human immune system are scanty and refer mainly to
occupational exposure. These studies nevertheless provide evidence
that at least mercury and lead affect the immune system (Moszczynski
et al., 1990a; Bernier et al., in press).
Significantly decreased levels of serum IgG and IgA, but not IgM,
IgD, or IgE, were reported in workers occupationally exposed to
metallic mercury vapours for 20 years in comparison with unexposed
controls (Moszczynski et al., 1990b). These workers had blood mercury
levels of < 50 µg/litre. Similarly, significantly decreased IgG and
IgA levels were observed in workers with urinary mercury levels of
0.029-0.545 mg/litre (Bencko et al., 1990). Studies of a small number
of people exposed to mercury in dental amalgams have shown increased
levels of IgE (Anneroth et al., 1992), an increased incidence of
asthma (Drouet et al., 1990), and development of contact dermatitis
(Gonçalo et al., 1992). Total lymphocyte, CD4, and CD8 levels were
higher in exposed people than in controls (Eedy et al., 1990).
Epidemiological data indicate that the main effects of
occupational exposure to lead are on cellular aspects of the immune
system and that humoral parameters remain relatively insensitive to
such exposure. Thus, serum IgG, IgM, and IgA levels remained within
the normal range in workers exposed for 4-30years, with a mean blood
lead level of 38.4 µg/dl, in comparison with a mean control level of
11.8 µg/dl (Kimber et al., 1986). Similarly, no effects were noted on
serum IgG or IgA levels in a cohort exposed to lead oxides at a
reported concentration within the plant of 266 µg/m3, who had an
estimated blood lead level of 64 µg/dl; however, the response of
lymphocytes from the exposed group to stimulation with
phytohaemagglutinin and concanavalin A in vitro was significantly
lower than that of controls (Alomran & Shleamoon, 1988). Decreased
serum Ig levels were reported in occupationally exposed workers with a
mean blood lead level of 46.9 µg/dl, but the duration of exposure was
not reported (Castillo-Mendez et al., 1991). In another study, no
significant effects were noted on serum immunoglobulin levels after
exposure to lead, but the levels of secretory IgA, which plays a major
role in the defence against respiratory and gastrointestinal
infections, was significantly decreased in workers with blood lead
levels of 21-90 µg/dl. The incidence of influenza infections per year
was significantly higher in these workers than in the control group
(Ewers et al., 1982).
Studies on the effects of lead on lymphocyte levels in
occupationally exposed workers have produced inconclusive results. One
study found an increase in absolute B lymphocyte counts and CD8 cells
(Coscia et al., 1987), while a decrease in total T lymphocytes and the
CD4 subset was reported in another set of workers (Fischbein et al.,
1993).
2.4.6 Solvents
Certain organic solvents may induce immune changes in humans.
Benzene-induced pancytopenia with associated bone-marrow hypoplasia, a
classical sign of chronic exposure to benzene, results in an
immunodeficiency state due to the reduced numbers of immunocompetent
cells (Goldstein, 1977). Alterations in the numbers of certain
lymphocyte subsets, e.g. CD3 and CD4 lymphocytes, have also been
reported in workers exposed to solvents (Denkhaus et al., 1986),
suggesting that the effects may be somewhat specific.
2.4.7 Ultraviolet radiation
Numerous reports have shown that UVR inhibits contact
hypersensitivity of the skin to sensitizers such as
dinitrochlorobenzene (DNCB) (O'Dell et al., 1980; Halprin et al.,
1981; Hersey et al., 1983a,b; Kalimo et al., 1983; Sjovall et al.,
1985; Friedmann et al., 1989; Yoshikawa et al., 1990; Vermeer et al.,
1991; Cooper et al., 1992). The dose required to produce
immunosuppressive effects in humans is similar to that in C57Bl/6
mice, the strain phenotypically most sensitive to UVR-induced immune
suppression (Noonan & Hoffman, 1994).
Human buttock skin was exposed to four daily doses of
144 mJ/cm2 UVR, and the irradiated site was sensitized with DNCB
immediately after the last exposure; the inner surface of the forearm
was challenged 30days later with DNCB and contact hypersensitivity
assessed. Forty percent of the volunteers failed to develop contact
hypersensitivity and were designated sensitive, suggesting that, as in
mice, susceptibility to UVR is genetically controlled. The sensitive
phenotype also appeared to be a risk factor for the development of
skin cancer (Yoshikawa et al., 1990). Suppression of contact
hypersensitivity is seen in a similar percentage of black-skinned
individuals, indicating that melanin cannot protect against this
phenomenon (Vermeer et al., 1991). In another study, human buttock
skin was exposed to 0.75 or two minimal erythemal doses (MED) of UVB
(1 MED = 29.1-32.5mJ/cm2, depending on the individual) for four days
and sensitized through irradiated skin immediately after the last
exposure to DNCB; subjects were challenged with diluted DNCB at a
distal site three weeks later. Some subjects were also exposed to four
MED (moderate sunburn) and sensitized three days later with DNCB.
Analysis of overall individual responses revealed decreased
frequencies of fully successful immunizations in all UVB-exposed
groups. The rate of immunological tolerance to DNCB (lasting up to
four months) in the groups that were initially sensitized on skin that
had received erythemagenic doses of UVB was 31%, whereas it was 7% in
unirradiated controls (Cooper et al., 1992).
A dose-response relationship was established in the studies of
Cooper et al. (1992) in a comparison of subjects with types I-III skin
(fair to moderately fair) who received various doses or schedules of
UVR from a bank of FS20 fluorescent sun lamps (rich in UVB) with
respect to their ability to mount an immune response to DNCB. A linear
inhibition of immune responsiveness was seen, the first detectable
decrease occurring at 0.75 of the individual's MED, reaching complete
inhibition of responsiveness for 95% of subjects when two MED were
administered every day for four days before immunization. Similar
inhibition occurred when DNCB was administered through skin that had
been exposed to a single dose of fourMED three days earlier. A dose-
response curve for fair-skinned subjects was constructed by plotting
the dose in total MED administered against the degree of immune
response to DNCB (Figure 35). The 50% immune suppressive dose was
calculated to be about 100mJ/cm2 of UVB.
Unresponsiveness to a contact sensitizer applied to UV-irradiated
skin can thus be induced in a proportion of individuals after exposure
to moderate levels of UVR, and at least some individuals become
immunologically tolerant in a manner similar to experimental animals.
Taken together, these data suggest that the systemic immunosuppression
induced in mice by UVR also occurs in humans, possibly through a
similar mechanism. UVR appears to alter antigen presentation and the
expression of Langerhans (CDla+DR+) cells, which is followed by an
influx of CDla+DR+ monocytes that preferentially activate CD4+
(suppressor-inducer) cells, which induce maturation of CD8+ Tc
Iymphocytes (Cooper et al., 1986; Baadsgaard et al., 1990). The UVR
wavelengths responsible for induction of CDla-DR+ cells are
predominantly within the UVB band and to a lesser extent in the C band
(Baadsgaard et al., 1987, 1989).
UVR from solarium lamps also suppressed NK cell activity in the
blood of subjects exposed for 1h per day for 12 days and tested one
and seven days after exposure; the activity returned to normal by 21
days after exposure (Hersey et al., 1983a). The effects of UVR on NK
cell activity are attributed to A radiation (Hersey et al., 1983b).
The depressed immune function observed in irradiated rodents
reflects anecdotal observations in humans, i.e. that exposure to
sunlight exacerbates certain infectious diseases, particularly those
involving the skin. For example, it was noted at the turn of the
century that smallpox lesions were worsened by exposure to sunlight
(Finsen, 1901), and herpetic lesions and viral warts may be
reactivated or exacerbated by sunlight (Giannini, 1990). It has also
been hypothesized that sunlight affects susceptibility to infection
with the bacteria that cause leprosy (Patki, 1991). Lesions of Herpes
simplex virus type I and type II can be reactivated by exposure to
UVR (Spruanoe, 1985; Klein, 1986). Using the criteria established by
 Yoshida & Streilin (1990) for the UVB-sensitive phenotype, Taylor et
al. (1994) reported that 66% of individuals who have a history of
herpes lip lesions provoked by exposure to sunlight were sensitive to
UVB, in comparison with 40-45% of the general population and 92% of
skin cancer patients. Exposure of immunosuppressed patients to
sunlight can increase the incidence of viral warts caused by
papillomavirus (Boyle et al., 1984; Dyall-Smith & Varigos, 1985). It
is also known that UVR exacerbates the clinical course of systemic
lupus erythematosus, an autoimmune disease (Epstein et al., 1965). The
effects of UVR on the risk of infectious disease have been reviewed by
Koren et al. (1994). In contrast, certain infectious diseases appear
to be cured by sunlight; the most notable are erysipelas (Giannini,
1990), a skin disease caused by Streptococcus, and skin lesions
caused by Herpes zoster virus.
2.4.8 Others
A large number of therapeutic drugs and drugs of abuse may also
alter human immune function in humans. These include:
Therapeutic agents
Alkylating agents
Nitrogen mustards: cyclophosphamide, L-phenylalanine mustard,
chlorambucil
Alkyl sulfonates: busulfan
Nitrosoureas: carmustine (BCNU), lomustine (CCNU)
Triazenes: dimethyltriazenoimidazolecarboxamide (DTIC)
Anti-inflammatory agents
Aspirin, indomethacin, penicillamine, gold salts
Adrenocorticosteroids: prednisone
Antimetabolites
Purine antagonists: 6-mercaptopurine, azathioprine, 6-thioguanine
Pyrimidine antagonists: 5-fluorouracil, cytosine arabinoside,
bromodeoxyuridine
Folic acid antagonists: methotrexate (amethopterine)
Natural products
Vinca alkaloids: vinblastine, vincristine, procarbazine
Antibiotics: actinomycin D, adriablastine, bleomycin, daunomycin,
puromycin, mitomycin C, mithramycin
Antifungal agents: griseofulvin
Enzymes: L-asparaginase
Cyclosporin A
Estrogens: diethylstilbestrol, ethinylestradiol
Substances of abuse
Ethanol
Cannabinoids
Cocaine
Opiates
Adapted from Dean & Murray (1990)
3. STRATEGIES FOR TESTING THE IMMUNOTOXICITY OF CHEMICALS IN ANIMALS
3.1 General testing of the toxicity of chemicals
The fact that substances used in various aspects of modern life
can be simultaneously beneficial and harmful to human life creates a
legislative and regulatory dilemma. In order to balance the desire to
use the many new substances that enter the market every year and the
economic benefit that is associated with their use on the one hand
with the health and safety of the population on the other is an
important challenge to governmental authorities. Legislative and
regulatory efforts to minimize and control the risk of adverse effects
on human health has resulted in a system for assessing and classifying
the potential risk of exposure to chemicals. Potential adverse effects
can be assessed in studies with experimental animals. In conducting
such studies, attention must be paid to ethical and regulatory
requirements for animal welfare and to good laboratory practice.
In assessing and evaluating the toxic characteristics of a
chemical, its oral toxicity may be determined once initial information
has been obtained by acute testing. Toxicity is routinely tested
according to guidelines, one of which is guideline No. 407 of the
Organisation for Economic Co-operation and Development (OECD) for
testing of chemicals, the 'Repeated Dose Oral Toxicity - Rodent:
28-day or 14-day Study' (Organisation for Economic Co-operation and
Development, 1981). This guideline has undergone three revisions, the
most recent of which (January 1994) includes parameters of
immunotoxicological relevance (see Table 7). Depending on the amount
of a chemical to be produced and the expected exposure to the
chemical, testing according to this guideline may in many countries
provide the only information on its safety, including potential
toxicity to the immune system. The information yielded by this type of
testing is therefore decisive in determining how chemicals are used in
society. Subsequent guidelines have been defined for use in follow-up
studies if more exposure is expected or if there is a suspicion of
toxicity on the basis of structural analogy with other known
compounds. These include 90-day studies of oral toxicity, long-term
studies, and studies of reproductive effects. Although such guidelines
include more parameters of the immune system than OECD test guideline
No. 407, detection of potential immunotoxicity may still not be
adequately addressed. In practice, the best procedure is to carry out
appropriate tests on a case-by-case basis, at increasing levels of
complexity, when concerns are raised in more general toxicological
studies.
Table 7. Parameters of OECD Test Guideline 407 that relate to the immune system
Current Guideline 407 Proposal for updating Proposal for updating Proposal for updating
(adopted 21 May 1981) Guideline 406 Guideline 407 (revision of Guideline 407 (revision of
(February 1991) January 1993) January 1994)
Total and differential Total and differential Total and differential Total and differential
leukocyte count leukocyte count leukocyte count leukocyte count
Weight of spleen and thymus Weight of spleen and thymus Weight of spleen and thymus
Histopathology of spleen Histopathology of spleen, Histopathology of spleen, Histopathology of spleen, thymus,
thymus, lymph node, and thymus, lymph nodes (one lymph nodes (one relevant to the
bone marrow relevant to route of route of administration and a
administration and a distant distant one to cover systemic
one to cover systemic effects), effects), small intestine (including
and bone marrow Peyer's patches), and bone marrow
Histopathology of target Histopathology of target Histopathology of target Histopathology of target
organs organs organs organs
OECD, Organisation for Economic Co-operation and Development
An insight into the type of information that the OECD test
guideline No. 407 yields is given below. In this test, the substance
is administered orally in daily graduated doses to groups of
experimental animals, one dose per group for 28 or 14 days. The
preferred rodent species for this test is the rat, although others may
be used. At least three doses and a control should be used. The
highest dose should result in toxic effects but not produce an
incidence of fatalities which would prevent a meaningful evaluation;
the lowest dose should not produce any evidence of toxicity and should
exceed a usable estimate of human exposure, when available. Ideally,
the intermediate dose level(s) should produce minimal observable toxic
effects.
In compliance with the guideline, the following examinations are
carried out:
(a) haematology, including haematocrit, haemoglobin concentration,
erythrocyte count, total and differential leukocyte count, and a
measure of clotting potential such as clotting time, prothrombin
time, thromboplastin time, or platelet count;
(b) clinical biochemistry of blood, including blood parameters of
liver and kidney function. The selection of specific tests is
influenced by observations on the mode of action of the
substance. Suggested determinations are: calcium, phosphorus,
chloride, sodium, potassium, fasting glucose, serum alanine
aminotransferase, serum aspartate aminotransferase, ornithine
decarboxylase, gamma-glutamyl transpeptidase, urea nitrogen,
albumin, blood creatinine, total bilirubin, and total serum
protein. Other determinations that may be necessary for an
adequate toxicological evaluation include analyses of lipids,
hormones, acid-base balance, methaemoglobin, and cholinesterase
activity. Additional clinical biochemistry may be used when
necessary, to extend the investigation of any observed effects.
(c) pathology, including gross necropsy, with examination of the
external surface of the body, all orifices, and the cranial,
thoracic, and abdominal cavities and their contents. The liver,
kidneys, adrenal glands, and testes are weighed wet as soon as
possible after dissection to avoid drying. Liver, kidney, spleen,
adrenal glands, heart, and target organs showing gross lesions or
changes in size are preserved in a suitable medium for possible
future histopathological examination. Histopathological
examination is performed on the preserved organs or tissues of
the group given the high dose and the control group. These
examinations may be extended to animals in other dosage groups,
if considered necessary to further investigate changes observed
in the high-dose group.
A properly conducted 28- or 14-day study will provide information
on the effects of repeated doses and can indicate the need for
further, longer-term studies. It can also provide information on the
selection of doses for longer-term studies.
It is clear that the 1994 guideline is not suitable for adequate
assessment of the potential adverse effects of exposure to a test
chemical on the immune system, since the immunological parameters are
restricted to total and differential leukocyte counts and the
histopathology of the spleen. An evaluation of this test (Van Loveren
& Vos, 1992) indicated that over 50% of the immunotoxic chemicals in a
series of about 20 chemicals would not have been identified as such if
the tests had strictly adhered to the guideline. In fact, it is even
doubtful if chemicals indicated as immunotoxic only on the basis of
guideline No. 407 would in practice have been picked up: For instance,
in a toxicological experiment, a small but significant change in the
percentage of basophilic leukocytes would by itself probably not be
considered to be biologically relevant in the absence of any other
parameter to suggest that an effect on the immune system might have
been present.
These data indicate that extension of OECD test guideline No. 407
is necessary in order adequately to assess potential immunotoxicity.
It is recommended that additional immunological parameters be included
in this guideline in order to increase its power (Vos & Van Loveren,
1987; Basketter et al., 1994).
Guidelines also exist for follow-up studies if greater exposure
is expected or if there is a suspicion of toxicity on the basis of
structural analogy with other compounds. In these studies, potential
toxicity to the immune system is generally addressed somewhat more
extensively than in guideline No. 407. For instance, in a 90-day study
of oral toxicity, the OECD guidelines prescribe that histopathological
examination be done on the thymus, a representative lymph node, and
the sternum with bone marrow, in addition to the spleen. Even with
these additional parameters, it is highly questionable whether
potential immunotoxicity is adequately assessed. For this purpose, a
variety of tests is available, which are described in section 4.
Depending on what is already known about the toxicity of the test
compound, different panels of tests (also referred to as tiers) are
selected for immunotoxicological evaluation. Usually, if little or no
information is available, a dose range including high doses is used;
lower, overtly nontoxic doses are chosen if some knowledge is
available about the physical and chemical properties, toxicokinetics,
structure-activity relationships, and intended use.
3.2 Organization of tests in tiers
Immunotoxicity can be assessed in a tiered approach (Luster et
al., 1988; Van Loveren & Vos, 1989). Generally, the objective of the
first tier is to identify potentially hazardous compounds (hazard
identification). If potential immunotoxicity is identified, a second
tier of tests is carried out to confirm and further characterize the
immunotoxicity.
Various approaches have been suggested for evaluating the
potential immunotoxicity of compounds. Most are similar in design, in
that the first tier is usually a screen for immunotoxicity and the
second tier consists of a more specific confirmatory set of studies or
in-depth mechanistic studies. Since the use of the tiers is usually
tailored to the goals or objectives of the organization that proposes
them, they differ in respect of the specific assays recommended and
the organization of the assays into tiers.
3.2.1 United States National Toxicology Program panel
The tiers and assays originally adopted by the NTP, based on the
proposed guidelines for immunotoxicity evaluation in mice reported by
Luster et al. (1988), are shown in Table 8.
Table 8. Panel adopted by the US National Toxicology Program for detecting immune
alterations after exposure of rodentsa to chemicals or drugs
Parameter Procedures
Screen (Tier I)
Immunopathology Haematology: complete and differential blood count
Weights: body, spleen, thymus, kidney, liver
Cellularity: spleen
Histology: spleen, thymus, lymph node
Humoral immunity Enumerate IgM antibody plaque-forming cells to
T-dependent antigen (sheep red blood cells)
Lipopolysaccharide mitogen response
Cell-medicated Lymphocyte blastogenesis to mitogens
immunity (concanavalin A)
Mixed leukocyte response to allogeneic leukocytes
Nonspecific immunity Natural killer cell activity
Comprehensive (Tier II)
Immunopathology Quantification of splenic B and T cell numbers
Humoral-mediated Enumeration of IgG antibody response to sheep red
immunity blood cells
Cell-medicated Cytotoxic T lymphocyte cytolysis; delayed
immunity hypersensitivity response
Nonspecific immunity Macrophage function: quantification of resident
peritoneal cells, and phagocytic ability (basal and
activated by macrophage activating factor)
Host resistance Syngeneic tumour cells
challenge models PYB6 sarcoma (tumour incidence)
(end-points)b B16F10 melanoma (lung burden)
Bacterial models: Listeria monocytogenes (mortality);
Streptococcus species (mortality)
Viral models: influenza (mortality)
Parasite models: Plasmodium yoelii (parasitaemia)
a The testing panel was developed using B6C3F1 female mice.
b For any particular chemical tested, only two or three host resistance models
are selected for examination.
In this approach, the tier 1 assay is limited; it includes assays
for both cell-mediated and humoral-mediated immunity and for innate
(nonspecific) immunity with the inclusion of NK cell assays. It also
includes immunohistopathology, which is part of the standard protocol
for studies of subchronic toxicity and carcinogenicity conducted by
the NTP. Tier II represents a more extensive evaluation and includes
additional assays for assessing effects on cell-mediated, humoral, and
innate immunity, in addition to host resistance. In this approach,
animals are usually evaluated at only one time, so that the
possibility for recovery or reversibility of immunological changes is
not evaluated. A 14-day exposure period is employed routinely;
however, 30- and 90-day exposures have been used, depending on the
pharmacokinetic properties of the chemical being tested. The dose used
in this tier system tends to be lower than those in several of the
other approaches followed. In the NTP approach, dose levels are
selected whenever possible that have no effect on body weight or other
toxicological end-points. The approach has therefore focused on
compounds for which the immune system is the most sensitive target.
This is in marked contrast to other approaches, in which the highest
dose is usually the maximum tolerated dose.
The assays that make up the NTP tier approach have undergone
various revisions, partly on the basis of an immunotoxicological
review of compounds evaluated in this tier structure (Luster et al.,
1992). The mitogen assays were first moved from tier I to tier II and
have now been dropped altogether: They were found to be insensitive
and to add little when run in conjunction with other assays that have
a proliferative component, such as the mixed leukocyte response and
the plaque assay. Furthermore, the only macrophage phagocytic assay
routinely carried out in immunotoxicological studies conducted for the
NTP is evaluation of the functional activity of the mononuclear
phagocyte system, which is an in-vivo assay for phagocytosis.
Studies by Luster et al. (1992) show that the potential
immunotoxicity of a compound can be reasonably predicted with a few
selected assays. As additional data become available, further changes
to the NTP tiers will most likely be forthcoming.
3.2.2 Dutch National Institute of Public Health and Environmental
Protection panel
The tier approach for immunotoxicological evaluation followed at
the National Institute of Public Health and Environmental Protection
(RIVM) in the Netherlands (Vos & Van Loveren, 1987) is shown in
Table 9. This approach is based essentially on OECD test guideline
No. 407, which suggests that the maximum tolerated dose be used as the
high dose in the study. As a result, significantly higher doses are
used than in the NTP approach in evaluating compounds for
immunotoxicity. Additionally, the standard exposure period is 28 days,
and the animal species routinely used is the rat instead of the mouse.
This type of testing can therefore be performed in the context of
studies in rats to determine the toxicological profile of a compound.
At least three doses should be used, the highest having a toxic effect
(but not mortality) and the lowest producing no evidence of toxicity.
Moreover, immunotoxicity tests carried out in the context of such
testing should not in any way influence the toxicity of the chemical
(e.g. immunization or challenge with an infectious agent). In the NTP
panel, the highest dose to which mice are exposed is chosen so that no
overt toxicity, i.e. changes in body weight or gross pathological
effects, is observed. As tests for immunotoxicity must be fairly
sensitive in order to preclude false negatives, the NTP tier I
includes functional assays. With a broader dose range that includes
overt toxicity, potential immunotoxicity is more likely to be
observed, without the inclusion of functional tests. If functional
assays are to be included in the first tier, those tests that require
sensitization of animals would require inclusion of satellite groups.
In OECD test guideline No. 407 for testing chemicals, none of the
other systems is approached functionally.
It has been suggested that the NK cell assay be added to tier 1 (Van
Loveren & Vos, 1992). Since the assay does not require animals to be
sensitized or challenged, the same animals can be used without
affecting other toxicological parameters, and thus an additional
satellite group of animals is unnecessary.
3.2.3 United States Environmental Protection Agency, Office of
Pesticides panel
The United States Environmental Protection Agency has proposed a
tiered approach to the evaluation of biochemical pest control agents,
which fall under the subdivision M guidelines for pesticides (Sjoblad,
1988). The proposed tiers are shown below. Tier 1 of this approach
includes functional assays for evaluating humoral immunity, cell-
mediated immunity, and innate immunity. Thus, while Tier 1 is
considered by the Agency to be an immunotoxicity screen, it is much
more encompassing than the first tier of the other approaches. By
providing options in the selection of assays for tier 1, the approach
can easily accommodate both the rat and the mouse as the laboratory
animal species used. In this approach, the tier 2 studies provide
information sufficient for risk evaluation, including information on
the time course of recovery from immunotoxic effects and host
resistance to infectious agents and tumour models. Additional
functional tests would be required if a dysfunction were observed in
tier 1 tests or if data from other sources indicated the compound
could produce an adverse effect on the immune response.
Table 9. Methods for detecting immunotoxic alterations in the rat evaluated by the
Dutch National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Parameters Procedures
Tier 1
Nonfunctional Routine haematology, including differential cell counts
Serum IgM, IgG, IgA, IgE determination; lymphoid organ
weights (spleen, thymus, local and distant lymph nodes)
Histopathology of lymphoid tissues, including mucosa-
associated lymphoid tissue
Bone-marrow cellularity
Analysis of lymphocyte subpopulations in spleen by flow
cytometry
Tier 2
Cell-medicated Sensitization to T-cell dependent antigens (e.g. ovalbumin,
immunity tuberculin, Listeria), and skin test challenge
Lymphoproliferative response to specific antigens (Listeria)
Mitogen responses (concanavalin A, phytohaemagglutinin)
Humoral Serum titration of IgM, IgG, IgA, IgE responses to
immunity T-dependent antigens (ovalbumin, tetanus toxoid,
Trichinella spiralis, sheep red blood cells) by ELISA
Serum titration of T-cell-independent IgM response to
lipopolysaccharide by ELISA
Mitogen response to lipopolysaccharide
Macrophaand Phagocytosis and killing of Listeria by adherent spleen
function and peritoneal cells in vitro
Cytolysis of YAC-1 lymphoma cells by adherent spleen
and peritoneal cells
Natural killer Cytolysis of YAC-1 lymphoma cells by non-adherent
function spleen and peritoneal cells.
Host resistance Trichinella spiralis challenge (muscle larvae counts and
worm expulsion)
Listeria challenge (spleen and lung clearance)
Cytomegalovirus challenge (clearance from salivary gland)
Endotoxin hypersensitivity;
Autoimmune models (adjuvant arthritis, experimental
allergic encephalomyelitis)
Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay
Subdivision M guidelines: proposed revised requirements by the US
Environmental Protection Agency for testing the immunotoxicity of
biochemical pest control agents
AI. Tier 1
A. Spleen, thymus, and bone-marrow cellularity
B. Humoral immunity (one of the following)
1. Primary and secondary IgG and IgM responses to antigen; or,
2. Antibody plaque-forming cell assay
C. Specific cell-mediated immunity (one of the following)
1. One-way mixed lymphocyte reaction assay; or,
2. Effect of agent on normal delayed-type hypersensitivity
response; or,
3. Effect of agent on generation of cytotoxic T-lymphocyte
response
D. Nonspecific cell-mediated immunity
1. Natural killer cell activity and
2. Macrophage function
II. Tier 2
A. Required if:
1. Dysfunction is observed in tier 1 tests
2. Tier 1 test results cannot be definitively interpreted
3. Data from other sources indicate immunotoxicity
B. General testing features:
1. Evaluate time course for recovery from immunotoxic effects.
2. Determine whether observed effects impair host resistance to
infectious agents or to tumour cell challenge.
3. Perform additional specific, but appropriate, testing
essential for evaluation of potential risks.
This Agency has also suggested that immunotoxicological screening
be conducted in evaluating conventional chemical pesticides
(subdivision F guidelines; see below); however, unlike those of
subdivision M, these guidelines are not designed as a tiered testing
scheme. If the immunotoxicity screen listed in subheading I were added
to subchronic and/or chronic studies in subdivision F, it would be a
more effective screen for immunotoxicity than is currently available.
If this proposed screen indicates that the immune system is a
sensitive target, the Agency considers that it may be necessary to
evaluate the risk for immunotoxic effects as under subheading
II. Currently, these suggestions have not been promulgated as official
guidelines or regulations.
Evaluations suggested by the US Environmental Protection Agency as
appropriate additions to Subdivision F guidelines for immunotoxicity
screening (subheading I) and possible additional data appropriate for
risk evaluation of chemical pesticides (subheading II)
I. Immunotoxicity screen
A. Serum immunoglobulin levels (e.g. IgG, IgM, and IgA)
B. Spleen, thymus, and lymph node weights
C. Spleen, thymus, and bone-marrow cellularity and cell
viability
D. Special histopathology (e.g. enzyme histochemistry,
immunohistochemistry)
E. More complete evaluation of 'premature' mortality of test
animals, as possibly related to immunosuppressive effects
II. Immunotoxicity risk evaluation
A. Host resistance to challenge with infectious agent and/or
tumour cells
B. Specific cell-mediated immune responses (e.g. mixed
leukocyte response, delayed-type hypersensitivity
response,cytotoxic T lymphocyte assays)a
C. Nonspecific cell-mediated immune responses (i.e. natural
killer cell activity, macrophage function)a
D. Time course for recovery from adverse immunological effects
a Measures of specific and nonspecific cell-mediated immune
responses that also may be considered useful in an immunotoxicity
screen
3.2.4 United States Food and Drug Administration, Center for Food
Safety and Applied Nutrition panel
The United States Food and Drug Administration is considering
testing guidelines for evaluating the immunotoxic potential of direct
food additives (Hinton, 1992). The multifaceted approach is included
in the draft revision of the Toxicological Principles for the Safety
Assessment of Direct Food Additives and Color Additives Used in Food
is (US Food and Drug Administration, 1993). The testing requirements
are based on the 'concern level' of the substance, assigned on the
basis of the available toxicological information or the substance's
structural similarity to known toxicants and on estimated human
exposure from its proposed use. A compound with high toxic potential
and high exposure would be assigned a high initial 'concern level'
(3), and one with low toxic potential and low exposure would be
assigned a low initial level (1).
In general, substances will be evaluated for immunotoxic
potential on a case-by-case basis. Two types of immunotoxicity tests
and procedures are defined in this approach: Type 1 tests are those
that do not involve perturbation of the test animal (i.e.
sensitization or challenge). These are further divided into 'basic'
tests, which include haematology and serum chemistry, routine
histopathological examination, and determination of organ and body
weights, and 'expanded' tests, which are logical extensions of the
'basic' tests and include those that can be performed retrospectively.
Type 2 tests include injection of or exposure to antigens, infectious
agents, vaccines, or tumour cells. In general, type 2 tests require a
satellite group of animals for immunological evaluation. The sets of
'basic' and 'expanded' type 1 tests are defined as level-I
immunotoxicity tests, and the sets of type 2 tests are defined as
level-II tests. Some level-I tests can be used to screen for
immunotoxic effects, while others focus on the mechanism of action or
the cell types affected by the test substance. Level-II tests are
conducted to define the immunotoxic effects of food and colour
additives more specifically. The recommended testing scheme is shown
below.
The functional tests generally require sensitization of exposed
rats and controls and subsequent analysis of the responses to the
sensitizing antigens. For this reason, functional tests are not
readily conducted in the first tier of immunotoxicity testing. As
guidelines for routine toxicology experiments preclude compromising
the experiment by any agent other than the test chemical, the second
tier of immunotoxicity testing, with immune function tests, requires a
separate set of experiments. The antigens that are used to sensitize
the exposed and control rats may be relatively simple antigens, such
as ovalbumin or tetanus toxoid, or more complex antigens, such as
sheep red blood cells, bacteria, or parasites. The responses can occur
in various arms of the immune system, which consequently must be
measured with different assays. For instance, humoral responses can be
measured by determination of specific antibodies in serum; the
appropriate tests for cellular responses are proliferative responses
of lymphocytes to the specific antigens ex vivo/in vitro or delayed-
type hypersensitivity responses to injection with antigen in vivo.
Recommendations of the United States Food and Drug Administration for
testing the immunotoxicity of direct food additives
Basic testing (rat model)
Complete blood count, differential white blood cell count;
Total serum protein, albumin:globulin ratio;
Histopathology, gross and microscopic (spleen, thymus, lymph
nodes, Peyer's patches, and bone marrow);
Lymphoid organ and body weights
Retrospective level-I testing (possible in a standard toxicology
study)
Electrophoretic analysis of serum proteinsa (when positive or
marginal effect is noted in basic testing);
Immunostaining of spleen and lymph nodes for B and T cellsa
(quantification of total immunoglobulins);
Serum autoantibody screen and deposition of immunoglobulins
(micrometry for semiquantification of the proliferative
response)
Enhanced level-I testing (possible for more complete screening in the
standard toxicology study core group, with a satellite animal group,
or in a follow-up study)
Cellularity of spleen (lymph nodes and thymus when indicated);
Quantification of total B and T cells (blood and/or spleen);
Mitogen stimulation assays for B and T cells (spleen);
Natural killer cell functional analysis (spleen);
Macrophage quantification and functional analysis (spleen);
Interleukin-2 functional analysis (spleen);
When indicated or for more complete analysis, other end-points
such as total haemolytic complement activity assay in serum
Level-II testing with a satellite group or follow-up study for
screening of functional immune effects
Kinetic evaluation of humoral response to T-dependent antigen
(primary and secondary responses with sheep red blood cells,
tetanus toxoid, or other);
Kinetic evaluation of primary humoral response to a
T-independent antigen such as pneumococcal polysaccharides,
trinitrophenyl-lipopolysaccharide, or other recognized
antigens;
Delayed-type hypersensitivity response to known sensitizer of
known T effector cell;
Reversibility evaluation
Recommendations (cont'd)
Enhanced level-II testing with a satellite group or follow-up study
for evaluation of potential immunotoxic risk
Tumour challenge (MADB106 or other in rat);
PYB6 sarcoma (in mouse);
Infectivity challenge (Trichinella, Candida or other in rat;
Listeria or other in mouse) a Recommended for inclusion in
basic testing
a Recommended for inclusion in basic testing
Not all functional assays require prior sensitization of the test
animals, e.g. proliferative responses of lymphocytes ex vivo/in vitro
to mitogens which are either specific for T cells, giving information
on cellular immunity, or for B cells, providing data on humoral
immunity. The phagocytic and lytic activity of macrophages and the
nonspecific cytotoxic activity of NK cells can also be measured
ex vivo/in vitro, without prior sensitization of the test animals.
Both types of activity are examples of nonspecific defence mechanisms,
directed to bacteria and certain tumour cells and to tumour cells and
virally infected cells, respectively. Since measurement of these types
of activity does not require prior sensitization of the host, such
functional tests can be considered for inclusion in the first tier of
testing for immunotoxicity in routine toxicology.
3.3 Considerations in evaluating systemic and local immunotoxicity
3.3.1 Species selection
Selection of the most appropriate animal model for
immunotoxicology studies has been a matter of great concern. Ideally,
toxicity testing should be performed with a species that responds to a
test chemical in a pharmacologically and toxicological manner similar
to that anticipated in humans, i.e. the test animals and humans
metabolize the chemical similarly and have identical target organs and
toxic responses. Toxicological studies are often conducted in several
animal species, since it is assumed that the more species that show a
specific toxic response, the more likely it is that the response will
occur in humans. Data from studies in rodents on target organ toxicity
at immunosuppressive doses for most immunosuppressive therapeutic
agents have generally been predictive of later clinical observations.
Exceptions to the predictive value of rodent toxicological data are
infrequent but occurred in studies of glucocorticoids, which are
lympholytic in rodents but not in primates (Haynes & Murad, 1985).
Although certain compounds exhibit different pharmacokinetic
properties in rodents and in humans, rodents still appear to be the
most appropriate animal model for examining the non-species-specific
immunotoxicity of compounds, because of established toxicological
knowledge, including similarities of toxicological profiles, and the
relative ease of generating data on host resistance and immune
function in rodents. Comparative toxicological studies should be
continued and expanded, however, as novel recombinant biological
compounds and natural products that enter safety testing will probably
have species-specific host interactions and toxicological profiles.
The quantitative and possibly the qualitative susceptibility of
an individual animal to the immunotoxicity of a selected agent can be
influenced by its genetic characteristics, indicating not only a need
to consider species but also strain. Rao et al. (1988) described two
approaches for selecting appropriate genotypes for toxicity studies.
The first is to select genotypes that are representative of an animal
species, which by virtue of similar metabolic profiles may also
exhibit a sensitivity similar to that of man, such as random-bred
mice. A second approach is to attempt to identify genotypes that are
uniquely suitable for evaluating a specific class of chemicals, such
as the use of Ah-responsive rodent strains in studies with
polyhalogenated aromatic hydrocarbons. In many cases, however, this
approach requires considerable knowledge of the mechanisms of toxicity
of the compound, which may not be available. One compromise has been
to use Fl hybrids which have the stability, phenotypic uniformity, and
known genetic traits of an inbred animal, yet have the vigour
associated with heterozygosity. The description of the genetic
relationships between inbred mouse strains on the basis of the
distribution of alleles at 16 loci (Taylor, 1972) has made possible
rational selection of appropriate Fl hybrids, such as the B6C3Fl
mouse.
3.3.2 Systemic immunosuppression
Because of this complexity, the initial strategies devised by
immunologists working in toxicology and safety assessment were to
select and apply a tiered panel of assays in order to identify
immunosuppressive or, in rare instances, immunostimulatory agents in
laboratory animals (US National Research Council, 1992). Although the
configuration of these testing panels varies according to the
laboratory conducting the test and the animal species used, they
include measurements of one or more of the following: (i) altered
lymphoid organ weights and histology, including immunohistology; (ii)
quantitative changes in the cellularity of lymphoid tissue, peripheral
blood leukocytes, and/or bone marrow; (iii) impairment of cell
function at the effector or regulatory level; and/or (iv) increased
susceptibility to infectious agents or transplantable tumours.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to adversely influence the
immune system. These include appropriate selection of animal models
and exposure variables, consideration of general toxicological
parameters and mechanisms of action, as well as an understanding of
the biological relevance of the end-points to be measured. Treatment
conditions should be based on the potential route, level, and duration
of human exposure, the biophysical properties of the agent, and any
available information on the mechanism of action. Moreover,
toxicokinetic parameters, such as bioavailability, distribution
volume, clearance, and half-life, should be measured. Doses should be
selected that will allow establishment of a clear dose-response curve
and a no-observed-effect level NOEL). Although, for reasons explained
earlier, it is beneficial to include a dose that induces overt
toxicity, any immune change observed at that dose should not
necessarily be considered to be biologically significant, since severe
stress and malnutrition are known to impair immune responsiveness.
Many laboratories routinely use three doses but generally conduct
studies to define the range of doses before a full-scale
immunotoxicological evaluation. If studies are being designed
specifically to establish reference doses for toxic chemicals,
additional exposure levels are advisable. In addition, inclusion of a
'positive control' group, treated with an agent that shares some of
the characteristics of the test compound, may be advantageous when
experimental and fiscal constraints permit.
The selection of the exposure route should reflect the most
probable route of human exposure, which is most often oral,
respiratory, or dermal. If it is necessary to deliver an accurate
dose, a parenteral exposure route may be required; however, this may
significantly change the metabolism or distribution of the agent from
that which would occur following natural exposure.
3.3.3 Local suppression
Local immune suppression has received less attention than
systemic immune suppression, and this is noteworthy, since the surface
that is exposed to the environment, i.e. the skin, the respiratory
tract, and the gastrointestinal tract, are the major ports of entry of
antigens and pathogens. While a variety of validated methods are
currently available to detect chemical skin sensitizers in humans and
experimental animals, there is no standard method to assess the
potential of chemicals to induce local immunosuppression in the skin.
Furthermore, although increasing evidence suggests that the
consequence of skin immunosuppression would be an increase in
neoplastic and infectious diseases of the skin, definitive data are
still lacking. In contrast, considerable efforts are being deployed to
develop sensitive models for monitoring skin irritants. For example,
human keratinocyte cultures and keratinocyte-fibroblast co-cultures
have been examined for end-points ranging from changes in cell
viability to production and loss of various bioactive products. Few
test systems are available for the gut and the respiratory tract.
4. METHODS OF IMMUNOTOXICOLOGY IN EXPERIMENTAL ANIMALS
This section comprises general descriptions of methods used for
evaluating immunotoxicity.
4.1 Nonfunctional tests
4.1.1 Organ weights
It is routine practice in toxicology to weigh organs that are
potentially affected by the compound that is being investigated. The
immunological organs that are suitable for weighing in screening for
potential immunotoxicity are: the thymus, which plays a decisive role
in the development of the immune system and which is affected by many
immunotoxicants; the spleen, which is the repository for many
recirculating lymphocytes; and the lymph nodes, which are important
for the induction of immune processes. Determination of the weight of
draining lymph nodes (depending on the route of exposure, i.e.
mesenteric nodes for oral exposure and bronchial nodes for inhalation)
in addition to distant lymph nodes (such as popliteal lymph nodes for
determining systemic effects) is the best. Mesenteric nodes, in
particular, occur in a string within non-lymphoid fatty tissue, and
care must be taken to remove this non-lymphoid tissue so that the
weight can be adequately determined. The cellularity of these organs
is another indication of the effects of chemicals on the immune
system. Furthermore, cell suspensions can be prepared from lymphoid
organs in order to assess the distribution of subpopulations of
lymphoid cells and to test their functionality within the organs.
Under OECD guideline No. 407, histopathological examination of
lymphoid organs and tissues is crucial for detecting the effects of
chemicals on the immune system. Therefore, upon termination of
exposure to a compound in a toxicological experiment, organs such as
the spleen should first be weighed; subsequently, they are divided
into parts which are also weighed, and one or more parts are used for
histopathological examination and the remainder to prepare cell
suspensions that can be evaluated for distribution of lymphocyte
subpopulations or can be assessed functionally.
4.1.2 Pathology
The histopathology of the thymus, spleen, and draining and
distant lymph nodes, of the mucosal immune system (Peyer's patches in
the gut or bronchus and nose-associated lymphoid tissue in the
respiratory tract), and of the skin immune system should be evaluated,
depending on the route of exposure. The first level of evaluation
should be of haematoxylin-eosin stained, paraffin-embedded slides. A
more sophisticated level of evaluation is immunoperoxidase staining of
special cell types.
Many monoclonal antibodies are available for mice, rats, and
humans to detect differentiation antigens, cell adhesion molecules,
and activation markers on haematolymphoid and stromal cells involved
in immune responses. A list of some monoclonal antibodies that can be
used in the identification of leukocytes and stromal cells in (frozen)
sections of lymphoid tissue is presented in Table 10; a selection of
these is reviewed below.
For a further description of these markers, and the cells that
express them, reference may be made to the introductory section and to
descriptions in the literature (Brideau et al., 1980; Bazin et al.,
1984; Dallman et al., 1984; Dijkstra et al., 1985; Joling et al.,
1985; Vaessen et al., 1985; Joling, 1987; Hünig et al., 1989; Kampinga
et al., 1989; Portoles et al., 1989; Schuurman et al., 1991a).
These markers are usually stained in frozen tissue sections of
6-8 µm, fixed in acetone. A three-step immunoperoxidase procedure is
most suitable: the first step includes the monoclonal antibody
specific for the determinants to be studied (see above), the second
step, rabbit anti-mouse immunoglobulin, and the third step, swine
anti-rabbit immunoglobulin, the latter two antibodies conjugated to
horseradish peroxidase. The peroxidase activity can be developed by
3,3-diaminobenzidine tetrahydrochloride with hydrogen peroxide as
substrate, and the sections can be counterstained with Mayer's
haematoxylin to facilitate evaluation. Negative controls are prepared
by omitting the antibody in the first step or replacing it with an
irrelevant one. Under these conditions, only the peroxidase activity
of polymorphonuclear cells, when present, is visualized, and no
immunolabelling is found.
In general, histopathological evaluation provides a semi-
quantitative estimation of effects. The experienced pathologist can do
this adequately in studies carried out 'blind', especially if the
effects are clear. For more subtle effects, morphometric analysis is a
valuable addition, especially when supported by software for assessing
the values of parameters such as size, surface, and intensity of
staining. The compartments of the immune system, i.e. specific T and B
lymphocyte areas in spleen and lymph nodes and cortical and medullary
areas of immature and differentiated thymocytes within the thymus, and
the numbers of specialized cells per surface unit are parameters that
are well suited for morphometric analysis.
4.1.3 Basal immunoglobulin level
Serum immunoglobulin levels are often altered after exposure of
rats to immunotoxic chemicals (Vos, 1980; Vos et al., 1982, 1984,
1990a; Van Loveren et al., 1993a). This is not surprising, as the
total levels measured are a function of the humoral aspects of the
immune system, which react to the antigens that the host encounters.
For this reason, measurement of antibody levels is potentially
valuable in screening for immunotoxicity. Since the amount of antibody
Table 10. Some monoclonal antibodies to leukocytes and stromal cells used in immunohistochemical studies of tissue sections and flow
cytofluorography on cell suspensions
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells
CD1 gp43,45, Ly-38 OKT6, Lymphocytes in thymic cortex, Langerhans cells in skin,
49,12 a-Leu-6 interdigitating cells
CD2 gp50 Ly-37, MRC OX-34, a-Leu-5, All T cells in thymus and peripheral lymphoid organs, subset
NSM46.7, MRC OX-54, OKT11 of macrophages (rat). Sheep erythrocyte receptor, leukocyte
RM2-5 MRC OX-55 function antigen:-2 (LFA-2). Ligand f or LFA-3 (CD58)
CD3 gp19-29 CD3-1,KT3, IF4, G4.18 a-Leu-4, T Cells in thymic medulla and peripheral lymphoid organs
145-2C11 OKT3 (T-cell receptor-associated, cytoplasmic in precursor T cells
in thymus)
CD4 gp65 Ly-4, L3T4, MRC OX-35, a-Leu-3, Lymphocytes in thymic cortex, about two-thirds of T cells in
YTS 177.9 MRC OX-38, OKT4 peripheral lymphoid organs, subset macrophages, microglia
(ER2), W3/25 T helper/inducer and delayed-hypersensitivity phenotype.
MHC class II binding, receptor for human immunodeficiency
virus
CD5 gp65-62 Ly-1,Lyt-1 MRC OX-19, a-Leu-1 Lymphocytes in thymic cortex (faint). All T cells in thymic
HIS47 medulla and peripheral lymphoid tissue, subset of B cells
CD6 gp120 Tü 33 T Cells in thymic medulla and peripheral lymphoid organs
CD Relative Mouse Rat Human Reactivity
mol. mass
CD7 gp41 WT1,B-F12, Prethymic T-cell precursors, all T cells in thymus and fewer
a-Leu-9 in peripheral lymphoid organs
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
CD8 gp32-33 Ly-2,3,Lyt-2,3, MRC OX-8 a-Leu-2, Lymphocytes in thymic cortex, about one-third of T cells in
YTS 105.8 OKT8 peripheral lymphoid organs, splenic sinusoids (T cytotoxic/
suppressor phenotype, NK cells). MHC class I binding
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells,
thymic cortex cells (rodents). Heat-stable antigen (HSA)
CD43 gp115 Ly-48 W3/13,HIS17 DFT-1, (Pro)thymocytes, T cells, plasma cells, cells in bone
WR-14 marrow, polymorphonuclear granulocytes, brain cells.
Leukosialin, sialophorin
CDw p25-30 Thy-1 (ER4), 5F10 Thymocytes, T lymphocytes, connective tissue structures,
90 MRC OX-7, epithelial cells, fibroblasts, neurons, subset of bone-marrow
HIS51 cells, plasma cells, stem cells (T-activation molecule)
Thy-2 Thymocytes
p40-55 H57-597 R73,HIS42 WT31, T-Cell receptor a-b chain. Mature T cells in thymic medulla
TalphaF1, and peripheral lymphoid tissue
TßF1
p40-55 GL3,GL4, V65 CgammaM1, T-Cell receptor gamma-delta chain
UC7-13D5 11F2,
TCR delta1,
deltaTCS1
p41-55 MRC OX-44 Prothymocytes, lymphocytes in thymic medulla, T and B
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
p41,47 MRC OX-2 Thymocytes, dendritic cells, B cells, brain cells
ER3,ER7, Subset of thymocytes and peripheral T cells, subset of
ER9,ER10 myeloid cells
HIS44 Most lymphocytes in thymic cortex, small subset of
medullary lymphocytes, erythroid cells, cells in germinal
centre
HIS45 Some lymphocytes in thymic cortex, most medullary
thymocytes and peripheral T cells, subset of B cells.
Quiescent cell antigen (QCA-1)
MHC class I (Various antibodies to polymorphic and All nucleated cells, including leukocytes and stromal
non-polymorphic epitopes) cells; for T cells absent on thymic cortex cells (human)
B Cells
CD9 gp24 BA-2 Germinal centres (faint); some cells in thymic cortex. Late
pre-B cells
CD10 gp100 BA-3, Germinal centres (faint); some cells in thymic cortex
W8E7 Common acute lymphoblastic leukaemia antigen (CALLA)
CD19 gp95 a-Leu-12, B Cells in germinal centres and mantles, follicular dendritic
B4, FMC63 cells
CD20 p35 Ly-44 B1, a-Leu-16 B Cells in germinal centres and mantles, follicular dendritic
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
CD21 gp140 B2,BL13, B Cells in germinal centres and mantles (faint), follicular
HB-5 dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus)
CD22 gp135 Lyb-8.2, a-Leu-14,To B Cells in germinal centres and mantles, cytoplasmic in
Cy34.1 To 15,RFB4, precursor B cells
SHCL-1
CD23 p45 Ly-42 a-Leu-20, Some B cells in marginal centres and mantles, activated B
Tü 1 cells, subset of follicular dendritic cells (IgE Fc receptor)
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells, thymic
cortex cells (rodents). Heat-stable antigen (HSA)
CD37 gp40-45 BL14 B Cells in germinal centres and mantles
CD38 gp45 a-Leu-17, Lymphocytes in thymic cortex, cells in germinal centres,
OKT10 plasma cells (immature lymphoid cells, plasma cells)
CDw75 p53? LN1, OKB4 B Cells in germinal centre, in corona (faint), macrophages,
epithelium
CD79a p33,40 mb-1 B Cells, Ig alpha chain
CD79b p33,40 B29 B Cells, Igß chain
p200 (HIS14) All B cells, including TdT+ precursors
p200 (HIS22) All B cells in corona, pre-B cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
MHC class II (Various antibodies to polymorphic and B Lymphocytes, activated T cells, monocytes/macrophages,
non-polymorphic epitopes) interdigitating cells, Langerhans cells, epithelia, endothelia
B Cells (surface); in germinal center IgM+IgG+IgA+ and in
anti-immunoglobulin corona IgM+IgD+, plasma cells
(cytoplasmic)
Monocytes/macrophages, myeloid cells
CD13 p130-150 ER-BMDM-1 a-Leu-M7, Monocytes, granulocytes, dendritic reticulum cells
My7 (aminopeptidase N)
CD14 p55 ED9 UCH-M1, B-A8, Monocytes, some granulocytes and macrophages
a-Leu-M3
CD15 p170-190 a-Leu-M1 Granulocytes, some monocytes (lacto-N-fucose pentaosyl)
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes,
activated macrophages. IgG-FcRIII, low affinity, complexed IgG
CD33 p67 a-Leu-M9, (Precursor) granulocytes, macrophages, Langerhans cells.
My9 Myelin-associated protein
CD68 p110 Ki-M6,Ki-M7 Macrophages (specific)
p160 F4/80 Monocytes-macrophages
p32 Mac-2 Thioglycollate-elicited peritoneal macrophages
p92-110 Mac-3 Peritoneal macrophages
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Monocytes/macrophages, myeloid cells (contd)
4F7 Dendritic cells in skin, bone marrow
ED1 Monocytes/macrophages
ED2,HIS36 Subset of macrophages (F4/80-like)
ED3 Subset of macrophages, restricted, negative in thymus
MRC OX-41 Granulocytes, macrophages, dendritic cells
MRC OX-62 Dendritic cells (integrin-like)
(IF119) Dendritic cells
HIS48 Granulocytes
Mac-387 Macrophages
Natural killer cells
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages. IgG-FcRIII, low affinity, complexed IgG
CD56 p220/135 a-Leu-19, NK cells, monocytes, neuroectodermal cells NKH-1, isoform of
B-A19 neural cell adhesion molecule (NCAM)
CD57 p110 a-Leu-7, NK cells, subset of T cells, some B cells, some epithelial cells,
VC1.1 monocytes, neuroendocrine cells, NKH-1
a-asialo-GM1 NK cells, stromal components
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Natural killer cells (contd)
NK-1.1,2B4, 3.2.3 NK cells (NKR-P1 gene family)
3A4, 5E6
Follicular dendritic cells
ED5,ED6, Ki-M4,DRC-1 Follicular dendritic cells
MRC OX-2
Epithelial cells (thymus)
(Various anti-keratin antibodies) Epithelium
(ER-TR4),4F1 HIS38 TE-3,(MR3, Thymic cortex epithelium
MR6)
(ER-TR5),IVC4 (HIS39) TE-4,(MR19), Thymic subcapsular or medullary epithelium
RFD4
Complement receptors
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells, C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD21 gp140 B2,BL13 B Cells in germinal centres and mantles (faint), follicular
dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus
CD35 p220 To 5 Follicular dendritic cells, macrophages, B cells in corona
(faint), renal glomerular epithelium. C3bR, CR1
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
IgG-Fc receptors
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages; IgG-FcRIII, low affinity, complexed IgG
CD32 gp140 Ly-17 3E1,CIKM5 B Cells, myeloid cells, macrophages; IgGFcRII, low affinity,
complexed IgG
CD64 p75 32.2 Monocytes; IgG-FcRI, high affinity, monomeric IgG
ß1-Integrin (CD29-CD49) family
CD29 p130 9EG7 B-D15 Ubiquitous, not on erythrocytes; ß1 chain of all CD49 antigens
CD49a p200 TS2/7 Activated T cells, monocytes, smooth muscle cells. Very late
antigen-1 (VLA-1), ligand of collagen, laminin
CD49b p155 31H4,AK7, T Cells, B cells, thrombocytes, fibroblasts, endothelium.
P1E6 Very late antigen-2 (VLA-2), ligand of collagen I, II, III,
and IV, laminin
CD49c p145 11G5,P1B5 B Cells, renal glomeruli, basal membranes. Very late antigen-3
(VLA-3), ligand of collagen, laminin, fibronectin, and invasin
CD49d p150 R1-2, P12520, HP2/1,44H6, Thymocytes, lymphocytes, monocytes, NK cells, eosinophilic
MFR4.B MR?4 L25.3 granulocytes, erythroblasts. Very late antigen-4 (VLA-4),
ligand of VCAM-1, fibronectin
CD49e p160 MFR5, SAM-1 Monocytes, leukocytes, memory T cells, fibroblasts,
P12750 thrombocytes and muscle cells. Very late antigen-5 (VLA-5),
ligand of fibronectin
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
ß1-Integrin (CD29-CD49) family (contd)
CD49f p150 GoH3 Go-H3,4F10 T Cells, thymocytes, monocytes, thrombocytes. Very late
antigen-6 (VLA-6), ligand of laminin and invasin
ß2-Integrin (CD11-CD18) family
CD11a p180 Ly-15, WT.1 YTH-81.5, T and B cells, NK cells, erythroid and myeloid stem cells.
2D7, B-B15, Leukocyte function-associated antigen-1 (LFA-1) involved in cell
M17/4 G-25.2 adhesion, ligand for intercellular adhesion molecule (ICAM)-1
(CD54), ICAM-2 (CD102), ICAM-3 (CD50)
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells. C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD11c p150 a-Leu-M5, Monocytes, macrophages, granulocytes (faint), activated
S-HCL-3 lymphocytes. CR4
CD18 p95 YTS213.1, WT.3 BL5 All lymphocytes. ß-Chain of CD11 antigens
C71/16,
M18/2
p160-95 ED7,ED8 CD11-CD18 molecule
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others
Terminal deoxynucleotidyl transferase (TdT) Immature (lymphoid) cells in bone marrow and thymic cortex
(nuclear staining)
CD25 p55 Ly-43, AMT13, MRC OX-39 Tac,a-IL2-R Activated lymphocytes at scattered locations in thymus and
7D4,3C7 T-cell areas in peripheral lymphoid organs. Interleukin-2
receptor alpha chain
CD122 p75 5H4, CF1,Mik-ß2, NK cells, T cells, B cells, monocytes. Interleukin-2 receptor
TM-ß1 Mik-ß3 ß chain
CD26 p120 H194-112 MRC OX-61 134-2C2 (Activated) T cells. Dipeptidyl peptidase IV, in mouse T-cell
activation molecule (THAM)
CD30 p105 Ki-1,Ber-H2 Sporadic cells in thymic (cortex) and T cell areas in
peripheral lymphoid organs, some plasma cells. Activated
lymphocytes,Hodgkin cells
CD34 MY 10,8G12 Haematopoietic progenitor cells, capillary endothelium
QBEND/10 Human progenitor cell antigen (HPCA)
CD44 p65-85 Ly-24 MRC OX-49, a-Leu-44, Prothymocytes, T cells, small B cells. Lymphocyte homing
IM7 MRC OX-50 F10-44-2 receptor. Phagocytic glycoprotein-1 (PgP-1), HCAM
CD45 p180-210 Ly-5 MRC OX-1, T29/33 All leukocytes. Common leukocyte antigen
HIS41
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others (contd)
CD45R p190-220 B220 MRC OX-22, a-Leu-18, All B cells, subset of T cells. Common leukocyte antigen.
MRC OX-32, MB1,MT2 HIS24 restricted to strains of the RT7.2 allotype and labels
HIS24 all peripheral B cells except cells in marginal zone, pre-B
cells
CD45RA p205-220 14.8 MRC OX-33 HI100 B Cells, T cytotoxic-suppressor cells (faint), subset of
thymocytes.In humans, also CD4+ subset (naive-virgin
T-cells)
CD45RO p190-220 UCH-L1 T Cells in immature and memory stage. Common leukocyte
antigen
CD54 p90 KAT-1 1A29 84H10,B-C14 Endothelial cells, many activated cell types. Intercellular
3E2 HA58 adhesion molecule-1 (ICAM-1)
CD71 p95 YTA74.4,C2 MRC OX-26 B3/25 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs, stromal
cells. Transferrin receptor
Ki-67 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs. Proliferation
antigen present in late G1, S, G2, and M phases
PCNA PCNA PCNA Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs
MEL14 Recirculating T and B cells. Lymphocyte homing receptor
MHC, major histocompatibility complex; NK, natural killer; Ig, immunoglobulin
CD nomenclature from: Clark & Lanier (1989); Knapp et al. (1989); Schlossman et al. (1994, 1995)
Antibodies within parentheses are not commercially available.
in serum is a function of the antibody's half-life, the longer the
study the more likely it is that an effect will be observed.
Immunoglobulin levels can be influenced by the cleanness of the
facility: studies conducted in facilities with excellent husbandry
will have lower basal levels than those conducted in 'dirty'
facilities where animals are constantly exposed to foreign antigens,
including pathogens.
Measurement of basal immunoglobulin levels is useful only after
subchronic or chronic exposure, i.e. with sufficient time for normal
metabolic elimination. Basal levels of immunoglobulin decrease only
when synthesis is reduced or prevented such that metabolized
immunoglobulins are not replaced. The parameter therefore yields
little information about possible mechanisms of immunotoxicity, and
should rather be regarded as a screening parameter; this is in fact
true for most non-functional tests. The IgM and G classes have usually
been measured; however, since the two other classes (A and E) are
biologically very important (for instance in mucosal immunity and
allergic manifestations), they should also be measured.
Total IgM and IgG concentrations in serum can be analysed by
means of a 'sandwich' enzyme linked immunosorbent assay (ELISA), as
described by Vos et al. (1982). Total IgA and IgE concentrations can
be analysed in an essentially similar way, except that the microtitre
plates are coated with monoclonal anti-rat IgA (Van Loveren et al.,
1988b) or monoclonal anti-rat IgE antibodies (MARE-1), respectively,
and immunoglobulins bound to these antibodies in serum samples are
detected by sheep anti-rat IgA or monoclonal anti-kappa chains of rat
immunoglobulins (MARK-1), conjugated with peroxidase.
Data from the ELISA are usually reported as percentages of
control values, and a titration curve based on pooled sera is
prepared; optimal dilutions of exposed and unexposed groups are then
plotted from this curve. The deviation of the dilution of the test
groups from the control groups is expressed, with the dilution in the
control group set at 100%. While studies in rats indicate that
measurement of basal immunoglobulin levels is useful in predicting the
immunotoxic effects of compounds, studies conducted in mice at the NTP
do not, and measurement of basal immunoglobulins is not included in
either tier of their testing panel (Luster et al., 1988). There are
several possible reasons for the difference in the usefulness of basal
immunoglobulin levels in rats and mice. First, in the studies of Vos
and colleagues, cited above, the exposure period was routinely longer
than the 14-day studies conducted within the NTP; since serum antibody
level is a function of the antibody half-life, longer studies are more
likely to detect an effect. Furthermore, the doses used at the NTP
were often lower, by design, than those used in rats. A final possible
explanation, which remains to be confirmed, is that immunoglobulin
synthesis in rats is more sensitive than that in mice.
4.1.4 Bone marrow
Bone marrow is an important haematopoietic organ and a source of
precursors for lymphocytes and other leukocytes. Changes in the bone
marrow are therefore likely to result in alterations of
immunocompetent cell populations, which may be long lasting or
permanent and thus serve as an indicator of potential immunotoxicity.
In a study to validate immunotoxicological parameters, bone-marrow
cellularity was shown to be an indicator of the immunotoxicity of
cyclosporin A, used as the model compound (Van Loveren et al., 1993a).
Determination of cellularity in stained slides of bone marrow,
evaluation of smears, and actual counts of the numbers of cells within
bone marrow are practical. For this purpose, both ends of a femur are
cut off, and bone-marrow cells are collected by flushing balanced salt
solution through the femur with a 21-gauge needle. The concentration
of nucleated cells is determined in a Coulter counter; a differential
count of cells can be done visually in May-Grunvald Giemsa-stained
cytospin preparations.
4.1.5 Enumeration of leukocytes in bronchoalveolar lavage fluid,
peritoneal cavity, and skin
Mononuclear phagocytes in the alveoli of the lung play an
important role in clearing inhaled particles, including
microorganisms, from the lung. The numbers of cells and alterations in
their function can be end-points of the toxicity of inhaled chemicals.
In order to study these parameters, methods for harvesting the cells
from the lungs should be easy to perform, guarantee the sterility of
the cell harvest, and be standardized. Methods involve use of either a
syringe (Blusse Van Oud Alblas & Van Furth, 1979) or a complex system
of syringes, tubes, and valves (Moolenbeek, 1982). These methods often
result in contamination of the harvested cell population; moreover,
they are laborious and cannot easily be standardized since the syringe
is operated manually. In a more recently developed method (Van
Soolingen et al., 1990), an excised lung is placed in a pressure
chamber and connected to a cannula through which lavage fluid can be
introduced into the lung and transferred from the lung into a test
tube. This procedure is repeated several times to obtain an optimal
yield.
Enumeration of mononuclear cells in the peritoneal cavity can
also best be performed by harvesting these cells by lavage. Because
of the architecture of this organ, three or four cycles of
intraperitoneal injections of lavage fluid, followed by gentle
massaging of the abdomen, and aspiration of the fluid with the syringe
that was also used for injection suffice.
Langerhans cells in the skin can be enumerated with
histopathological techniques. Frozen tissue sections are used, stained
with immunoperoxidase techniques including markers for MHC class II
antigens or specific markers, as indicated above. Morphometric
analysis may provide a quantitative basis for this type of evaluation.
4.1.6 Flow cytometric analysis
Evaluation of phenotypic markers has proved to be one of the most
sensitive indicators of immunotoxic compounds. The availability of
fluorescent activated cell sorter (FACS) analysis units and
fluorescent cell counter units in immunotoxicology laboratories has
made analysis of cell populations routine. Determination of the
phenotype of lymphoid cells is a non-functional assay, although it has
often been inappropriately grouped with functional tests. The presence
or absence of a particular marker on the surface of a cell does not
reveal the functional capability of the cell. The usefulness of
surface marker analysis for predicting potential immunotoxicity has
been demonstrated. In studies conducted by Luster et al. (1992), a 91%
concordance was found for correct identification of immunotoxic
compounds on the basis of studies of surface markers alone.
As indicated above (section 4.1.2), numerous markers are
expressed on the cells of the immune system. Essentially, the same
reagents as used on tissue sections are applied on cells that have
been isolated from tissues, body fluids, or lavage fluids in
suspension (see above). Furthermore, both polyclonal and monoclonal
antibodies are available for detecting these surface markers. While
many of the markers have been used in immunological investigations,
very few have been evaluated with a large number of immunosuppressive
compounds. The markers that have been routinely used in studies of
immunotoxicity conducted for the NTP in mice and the cell types they
identify are shown in Table 11. The CD4:CD8 ratio in spleen has been
shown to concord best with the immunotoxicity of these surface markers
(Luster et al., 1992).
Table 11. Phenotypic markers on lymphocyte subpopulations used in
studies of immunotoxicity by the United States National
Toxicology Program
Surface marker Cell type
sIg+ Pan B cells
Thy 1.2+ or CD3+ Pan T cells
CD4+CD8- T Helper/delayed-type
hypersensitivity cells
CD8+CD4- T Suppressor-cytotoxic cells
CD8+CD4+ Immature T cells
The identification of phenotypic markers in rats has not
developed as rapidly as in mice; however, antibodies to rat cell
surface markers are now becoming available commercially and are being
used in immunotoxicological assessments (Smialowicz et al., 1990). The
monoclonal antibodies currently used for this purpose are: OX4 or
MARK-1 for B cells, W3/13 or OX 19 for T cells, R79 for the T cell
receptor, W3/25 for CD4 cells, and OX 8 for CD8 cells.
In enumerating the cell types in lymphoid tissue, both
percentages and absolute cell numbers should be reported. Of the two,
absolute cell numbers are by far the most meaningful. Compounds that
affect all populations equally and thus do not change the relative
percentages of the various cell types may be missed if only
percentages are evaluated. In addition, significant differences in the
magnitude of an effect on one or more of the populations can be
observed when the data are evaluated as absolute numbers and not as
percentages. As indicated above, the absolute changes more closely
reflect the events occurring in the animal and should thus be given
priority in interpreting data.
FACS analysis is also being used to determine the activation
state of various cell types, on the basis of changes in detectable
activation markers. Some of the activation markers that have been
studied are F4/80 (Austyn & Gordon, 1981), Mac-1 (Springer et al.,
1979), Mac-2 (Ho & Springer, 1984), transferrin receptor (Neckers &
Cossman, 1983), and IL-2 receptor (Cantrell et al., 1988). While
activation markers are of value in studying the mechanism of action of
compounds, their usefulness as predictors of immunotoxicity has yet to
be firmly established.
4.2 Functional tests
4.2.1 Macrophage activity
Phagocytic activity is the first line of defence against many
pathogens. Macrophages can phagocytose many particles, including
bacteria, and can lyse and inactivate them. Alterations in phagocytic
activity are therefore important potentially adverse effects of
chemicals on the immune system. The capacity to ingest particles
in vitro can be measured, and activity in vivo can be measured by
determining the clearance of bacteria, such as L. monocytogenes.
This test is dealt with in section 4.2.10.1.
Several assays have been developed for evaluating various types
of phagocytosis in mice and can also be used in rats, with slight
modifications. Innate and non-immune-mediated phagocytosis by
macrophages can be evaluated by determining the uptake of fluorescent
latex covaspheres (Duke et al., 1985). Macrophages and peritoneal
exudate cells are placed on a tissue culture slide and incubated with
the covaspheres for 24h on a rocking platform. The slides are then
fixed with methanol. The slide chambers are evaluated under a
fluorescent microscope, and macrophages with more than five latex
covaspheres are counted as positive for phagocytosis. The results are
expressed as percentage of phagocytosis, which is calculated as the
ratio of macrophages positive for phagocytosis to total macrophages
counted. In order to distinguish phagocytosed latex covaspheres from
those that are merely associated with the macrophage surface, the
cells are exposed for 30-60s to methylene chloride vapour. By
immersing the slides in this manner, the covaspheres that have not
been phagocytosed are dissolved, while those inside the macrophage
remain intact (Burleson et al., 1987). Phagocytosed fluorescent latex
particles can easily be quantified under the fluorescence microscope.
While this assay is straightforward, it is labour intensive, and
reading the slides, shifting back and forth from the fluorescent
field, and counting the macrophages is time-consuming.
A radioisotopic procedure, the chicken erythrocyte assay, can be
used to evaluate both adherence to and phagocytosis of particles by
macrophages. The phagocytic capacity is measured as an immunologically
mediated (Fc receptor) response. Macrophages are added to each well of
a 24-well tissue dish and allowed to adhere for a 2-3-h incubation
period. Nonadherent cells are washed, and chicken erythrocytes
labelled with 51Cr are added to each well; then a subagglutinating
dilution of antisera to chicken erythrocytes is added to each well and
the plate incubated for 1h. The plates are then washed to remove
unbound erythrocytes; an ammonium chloride solution is added to lyse
adhered erythrocytes, and the supernatant is collected and counted to
determine adherence of the erythrocytes to the macrophages. Next, both
the macrophages and the phagocytosed chicken erythrocytes are lysed by
addition of 0.1 N sodium hydroxide, and the solution is counted to
determine the amount of phagocytosis. Three to six wells in each group
do not receive 51Cr and are used to evaluate the DNA content
(Labarca & Paigen, 1980). The data are expressed as adherence counts
per minute, phagocytosed counts per minute, and specific activity for
adherence and phagocytosis. Specific activity is determined by
dividing the number of adhered or phagocytosed counts per minute by
the DNA content per well. The data must be expressed in terms of
specific activity, since compounds that affect the macrophages'
ability to adhere to the 24-well culture dish will significantly alter
the results obtained.
While both the nonspecific and immune-mediated phagocytosis
assays are useful for understanding the potential mechanisms of action
of compounds, changes in phagocytic activity in these in-vitro assays
have not been found to be predictive of immunotoxicity. For example, a
single intratracheal exposure to gallium arsenide resulted in
increased adherence and phagocytosis by chicken erythrocytes but
decreased phagocytosis of latex covaspheres (Sikorski et al., 1989).
The phagocytosis assay that is most predictive of altered
macrophage function is evaluation of the functional ability of the
mononuclear phagocyte system. This is a holistic assay for measuring
the capacity of the fixed macrophages of the mononuclear phagocyte
system, where macrophages provide the first line of defence against
both pathogenic and non-pathogenic blood-borne particles. The fixed
macrophages of the mononuclear phagocyte system line the liver
endothelium (Kupffer cells), the spleen, the lymph nodes (reticular
cells), the lung (interstitial macrophages), and other organs such as
the thymus and bone marrow. When the assay is conducted in mice, the
animals are injected intravenously with 51Cr-labelled sheep
erythrocytes, and a 5-µl blood sample is taken from the clipped tail
at 3-min intervals over a 15-min period. A final 30-min blood sample
is taken, and 1h after injection the animals are sacrificed and the
liver, spleen, lungs, thymus, and kidneys are removed, weighed, and
counted in a gamma counter. The 60-min time interval after injection
of sheep erythrocytes was selected as the time of sacrifice since it
represents the plateau for particle uptake by the selected organs
(White et al., 1985). Blood clearance of the radiolabelled cells is
expressed as vascular half-life and as a phagocytic index, which is
determined by the slope of the clearance curve. Organ distribution is
expressed as percent organ uptake and counts per minute per milligram
of tissue (specific activity). The assay can detect both stimulation
and inhibition of the mononuclear phagocyte system. Bick et al. (1984)
reported marked stimulation of the mononuclear phagocyte system after
treatment with diethylstilbestrol; more recently, morphine sulfate was
shown to decrease vascular clearance and hepatic and splenic
phagocytosis significantly (LeVier et al., 1993).
4.2.2 Natural killer activity
NK activity against neoplastic and virus-infected targets has
been clearly demonstrated in vitro and is thought to play an
important role in vivo in providing surveillance against neoplastic
cells and as a first line of defence against viruses (Herberman &
Ortaldo, 1981). In humans, rats, and mice, most cells with NK activity
can be identified by morphological (although the definition is not
morphological) and functional characteristics (Timonen et al., 1981).
Most of the cells that show NK activity are nonadherent, non-
phagocytic lymphocytes and are morphologically associated with large
granular lymphocytes (Timonen et al., 1982). Although cells with NK
activity do not strictly belong to the T-cell lineage, they can
express T cell-associated markers and express surface receptors, such
as those for the Fc portion of IgG and the ganglioside asialo GM1.
Some of these markers are also expressed by monocytes, macrophages,
and polymorphonuclear leukocytes (Herberman & Ortaldo, 1981). Within
4h, the cells can show nonantigen-specific cytotoxic activity
in vitro and in vivo against certain (NK-sensitive) tumour cell
lines and virus-infected cells.
The cells have enhanced cytolytic function after activation with
a variety of stimuli, including viral infection (Stein-Streinlein et
al., 1983), BCG (Tracey et al., 1977), IL-2 (Henney et al., 1981;
Domzig et al., 1983; Lanier et al., 1985; Malkovsky et al., 1987),
interferon, and interferon inducers (polyI:C) (Tracey et al., 1977;
Oehler & Herberman, 1978; Djeu et al., 1979a,b). NK activity in vitro
can be stimulated with IL-2 and interferon (Tracey et al., 1977; Djeu
et al., 1979b). Anti-asialo GM1 antibody can strongly inhibit
cytotoxic NK activity both in vitro and in vivo (Kasai et al.,
1980, 1981; Yosioka et al., 1986). This antibody binds to the cell
surface glycolipid GM1 and suppresses the lytic activity of effector
cells. Large granular lymphocytes are found in several lymphoid
organs. Many cells with high NK activity are found in spleen and
peripheral blood (Rolstad et al., 1986); lymph nodes have less NK
activity, and thymus and bone marrow show only marginal activity. NK
activity can also be demonstrated in the bronchus-associated lymphoid
tissue in the lungs. Moreover, large granular lymphocytes can migrate
from the circulation into the extravascular tissue and can even be in
contact with the lumen of the alveoli (Timonen et al., 1982; Reynolds
et al., 1984; Rolstad et al., 1986; Prichard et al., 1987). The
presence of large granular lymphocytes associated with NK activity in
the lungs is probably of great importance, because the lungs
constitute a major site for neoplastic disease (metastatic spread) and
viral infections. NK cells may also operate in certain types of
bacterial infections. In experimental animals, suppression of NK cell
activity increased the numbers of metastases after transplantation of
tumours.
The clinical significance of altered NK cell activity in humans
has not clearly been established. Asymptomatic individuals with low NK
cell responses may be at some risk for developing upper respiratory
infections and for increased morbidity (Levy et al., 1991); and
extreme susceptibility to severe and repeated herpes virus infection
was reported in an individual without NK cells (Biron et al., 1989).
It is obvious therefore that exposure to toxic substances that alter
NK activity can have biological consequences, and testing this
activity is important in assessing potential immunotoxicity.
The procedure for determining NK activity is as follows: cell
populations that exert NK activity (usually enriched peripheral blood
mononuclear cells or spleen cells) are cultured with NK-sensitive
target cells. A cell type frequently used for this purpose is the YAC
lymphoma cell line, which has been applied to mice, rats, humans, and
even seals. YAC lymphoma target cells are radiolabelled with 51 Cr,
and lysis of the cells, resulting in release of chromium, within 4h is
used to estimate the cytolytic activity of the NK cells within the
cell population. This assay has been used to demonstrate the effects
of numerous compounds on NK activity in rats (e.g. TBTO, ozone, and
HCB: Vos et al., 1984; Van Loveren et al., 1990c), mice (Luster et
al., 1992), and harbour seals (Ross et al., in press).
4.2.3 Antigen-specific antibody responses
Most antibody responses require not only B cells, which, after
maturation into plasma cells, produce antibodies, but also the help of
T lymphocytes. A variety of T cell-dependent antigens can be used for
this purpose, and an excellent one is tetanus toxoid. A typical
immunization schedule in rats comprises intravenous immunization on
day 0 followed by a booster on day 10. Primary and secondary IgG and
IgM responses can then be measured in serum, taken on day 10 (just
before the booster) and day 21, respectively. The primary IgM response
is the immunoglobulin response that is least under the control of T
cells. As tetanus toxoid is also used for human immunization, the
responses to this antigen may be useful in extrapolating experimental
data to humans. The responses can be determined in an ELISA (Vos et
al., 1979b).
Another widely used T cell-dependent antigen is ovalbumin. This
antigen can be and has been used to induce all classes of antibody
responses, i.e. IgM, IgG, IgA, and IgE, that can be measured with the
ELISA (Vos et al., 1980; Van Loveren et al., 1988b). The classical
assay of specific IgE responses is the passive cutaneous anaphylaxis
reaction. Serial dilutions are injected into the skin of rats,
sensitizing local mast cells; the specific antigen is then injected
intravenously, simultaneously with Evans blue. Mast cell products are
released where IgE meets the antigen, and IgE is cross-linked on the
membranes of the mast cells, leading to extravasation of Evans blue.
The titre can be determined from the magnitude of the reaction at each
dilution of IgE. ELISA techniques and the specific reagents to detect
IgE in an ELISA that are now available make this test preferable.
Ovalbumin induces not only humoral responses but also delayed-
type hypersensitivity. Sensitization to ovalbumin in Freund's complete
adjuvant enhances responses and makes it possibile to assay both
responses in one animal. Delayed-type hypersensitivity can also be
directed to purified protein derivative, with responses induced by the
adjuvant (Vos et al., 1980). At least in mice, however, immunization
in complete adjuvant skews responses in the direction of Th1
responses, i.e. delayed hypersensitivity, and hence suppresses Th2-,
IgE-, and IgA-dependent immune responses.
A few antigens can induce humoral immune responses without
involvement of T lymphocytes. One example is trinitrophenol-Ficoll
(lipopolysaccharide). Sensitization of animals to this antigen yields
immunoglobulin responses that can be measured in an ELISA. This is a
useful test for use in mechanistic studies to separate the effects of
compounds on B and T cells.
4.2.4 Antibody responses to sheep red blood cells
4.2.4.1 Spleen immunoglobulin M and immunoglobulin G plaque-forming
cell assay to the T-dependent antigen, sheep red blood cells
A widely used particulate T cell-dependent antigen is sheep red
blood cells. Antibody titres induced after sensitization can be
assayed with various techniques; one that is widely used is the
plaque-forming cell assay, or antibody-forming cell response. This
assay is relatively simple and can be conducted with inexpensive
equipment found in most laboratories, but the optimal concentration of
sheep red blood cells must be injected. As the antigenicity of red
blood cells varies significantly from sheep to sheep, time must be
invested to obtain cells from a sheep that repeatedly gives a high
response (>= 1500 plaque-forming cells/106 spleen cells). The
number of cells administered (about 2 × 108) should also be
optimized for both mice and rats in the laboratory conducting the
assay. The intravenous route is that preferred for sensitization;
intraperitoneal injections can be used but significantly increase the
potential for nonresponding animals as a result of a poor injection.
Animals are sacrificed on day 4 after injection, and spleen cells are
prepared by mincing the spleen between two frosted microscope slides,
teasing it apart with forceps, or passing it through a small mesh
screen; all of these methods are satisfactory, and that used to
prepare single splenocyte cultures varies from laboratory to
laboratory. An aliquot of cells is added to sheep erythrocytes and
guinea-pig complement; these are placed in a microscope slide chamber
when the Cunningham assay method is used (Cunningham & Szenberg,
1968), or, in the Jerne method, cells and guinea-pig complement are
added to a test tube containing warm agar and after thorough mixing
the test tube mixture is plated in a petri dish and covered with a
microscope cover slip (Jerne et al., 1974). In either case, the
preparations are then incubated at 37°C for 3-4h to allow plaques to
develop. The plaques are counted under a Bellco plaque viewer. A
plaque results from the lysis of sheep erythrocytes and is elicited as
a result of the interaction of complement and antibodies directed
against sheep erythrocytes, which are produced in response to the
intravenous sensitization. As each plaque is generated from a single
IgM antibody-producing plasma cell, the number of IgM plaque-forming
cells present in the whole spleen can be calculated. The data are
expressed as specific activity (IgM plaque-forming cells/106 spleen
cells) and IgM plaque-forming cells per spleen.
By incorporating rabbit anti-mouse or anti-rat IgG antibody into
the preparation of spleen cells, complement, and sheep red blood
cells, the number of IgG antibody-forming cells present in the spleen
can also be determined. This number is calculated by subtracting the
number of IgM plaque-forming cells from the total number of both IgM
and IgG plaque-forming cells. The optimal IgG primary response is
observed five days after sensitization (Sikorski et al., 1989).
The T-dependent IgM response to sheep red blood cells is one of
the most sensitive immunotoxicological assays currently in use. Luster
et al. (1992) reported that the individual concordance of the plaque-
forming cell assay for predicting immunotoxicity was the highest of
all the functional assays (78%). Furthermore, use of this assay in
combination with either NK cell activity or surface marker analysis
resulted in pairwise concordances for predictability of more than 90%.
While the plaque-forming cell assay has been shown to be
sensitive and predictive, the procedure does have its limitations. As
indicated earlier, the effect of the test compound on the immune
system is evaluated only in spleen cells, and effects on other
antibody-producing organs and tissues are not determined. The assay is
somewhat laborious, and it is preferable that several people
participate, to help in removing spleens, preparing cell preparations,
counting cells, and adding preparations to either microscope slide
chambers or agar dishes. An additional drawback is that the assay must
be conducted on the same day as the animals are sacrificed. This is in
marked contrast to the ELISA, in which sera can be frozen and
evaluated at a later date. While the slides and petri dishes can be
placed in a cold room or refrigerator and counted the next day, this
procedure is not recommended, as they tend to dry out to some extent,
making viewing and discerning plaques more difficult.
4.2.4.2 Enzyme-linked immunosorbent assay of anti-sheep red blood
cell antibodies of classes M, G, and A in rats
An alternative to the plaque-forming cell assay is ELISA of anti-
sheep red blood cell antibody titres in serum. Antigen preparations
made from ghosts of sheep erythrocytes by extraction with potassium
chloride are used to coat the bottoms of the wells of 96-well
microtitre plates. Serum samples from rats immunized with sheep
erythrocytes are titrated onto these plates using specific polyclonal
antibodies to rat IgM or IgG, to which peroxidase is conjugated. IgA
has also been assayed, using monoclonal anti-rat IgA antibodies and
polyclonal rat anti-mouse IgG conjugated with peroxidase. The ELISA of
serum titres of IgM, IgG, and IgA to sheep erythrocytes is an easy,
reliable method that can be used to detect the effects of chemicals on
the immune system of the rat (Van Loveren et al., 1991; Ladics et al.,
1995).
The assay measures titres of specific antibodies, in contrast to
the plaque-forming cell assay which determines the number of cells
that are actually responsible for production. The ELISA assesses the
production of antibodies, either per cell or in terms of the total
capacity of the host to produce these antibodies in vivo. In
interpreting the effects of exposure to chemicals, account must be
taken of the fact that the cells used in the assay are derived from
specialized parts of the body, such as the spleen, and alterations in
the numbers of antibody-producing cells in such an organ in rats
immunized with sheep red blood cells cannot give information on other,
inaccessible pools of antibody-producing cells. In the ELISA,
alterations in titres due to exposure to chemicals indicate changes in
the immune potential of the exposed animals. In screening for the
effects of chemicals on the immune system, therefore, ELISAs may be
preferable, but for studies on specific immunosuppressive mechanisms,
the plaque-forming cell assay, although labour- and time-intensive, is
a powerful tool for obtaining information complementary to the data
provided by the ELISA. Unfortunately, it is not always possible to
perform the two assays with material from the same animal. The peak
response in the plaque-forming cell assay in both rats (Fischer 344)
and mice (B6C3F1) occurs on day 4 after sensitization, while the peak
response in the ELISA occurs on day 6 for rats and day 4-5 for mice
(Temple et al., 1993). In order to detect the effects of chemicals on
the immune response to sheep red blood cells, it is preferable to
choose the optimal conditions, or to follow the kinetics, of the
response.
4.2.5 Responsiveness to B-cell mitogens
Responsiveness to lipopolysaccharide is another estimate of
humoral immune response, as solely B cells respond to this mitogen.
Although the responses of rats to this mitogen are less pronounced
than those of mice, good results can be obtained, and the
immunosuppressive effects of chemicals can be detected (Vos et al.,
1984).
An alternative B-cell mitogen is S. typhimurium mitogen (STM),
a water-soluble, proteinaceous extract derived from the cell walls of
S. typhimurium; it is a more potent mitogen for rat B lymphocytes
than lipopolysaccharide (Minchin et al., 1990). In both mice and rats,
the polyclonal activation of B lymphocytes is a multistep process. In
mice, mitogens alone can provide all the signals necessary for
proliferation and differentiation; in the rat, STM stimulation induces
B lymphocytes to proliferate without differentiating. The addition of
lymphokines to STM-stimulated B cells also failed to stimulate them to
differentiate (Stunz & Feldbush, 1986). Nevertheless, this mitogen is
useful for evaluating effects on the proliferative ability of rat B
lymphocytes. Smialowicz et al. (1991) showed a decrease in the STM
response in Fischer 344 rats after oral exposure to 2-methoxyethanol.
Unlike the bell-shaped mitogen dose-response curves observed with
T-cell mitogens, the proliferative response of B lymphocytes to both
lipopolysaccharide and STM rises quickly at low concentrations of the
mitogens and plateaus at higher concentrations. As a result, a single
concentration on the plateau phase of the mitogen response curve is
sufficient to evaluate the effects of a test compound on B-cell
mitogen-driven proliferation. One of the reasons that the mitogen
assays appear to be insensitive is that the cells must remain in
culture for several days in order to obtain a peak response. As a
result, they may recover from the immunomodulatory effects of the test
compounds during this in-vitro phase. This is a common problem with
many ex-vivo/in-vitro assays, including the cytotoxic T lymphocyte and
mixed leukocyte response assays; because of the short, 4-h period of
the NK cell assay, this is less of a concern.
4.2.6 Responsiveness to T-cell mitogens
The proliferative ability of T lymphocytes after stimulation with
mitogens can be measured by the uptake of 3H-thymidine in a manner
similar to that used to measure B-cell proliferation (Anderson et al.,
1972). Concanavalin A and phytohaemagglutinin are T-cell mitogens in
both rats and mice; pokeweed mitogen stimulates the proliferation of
both T and B cells and thus lacks specificity. Although both
concanavalin A and phytohaemagglutinin stimulate T lymphocytes,
T cells responsive to concanavalin A have been reported to be less
mature than those responsive to phytohaemagglutinin (Stobo & Paul,
1973). Multiple concentrations of these mitogens should be used to
ensure that a peak response is obtained: both produce a bell-shaped
dose-response curve, and too high a concentration can result in a
suboptimal response.
Historically, mitogens have been included in the battery of tests
for evaluating potential immunotoxicity, because the assay is one that
can also be carried out in humans. Human studies, however, are
conducted on peripheral blood, while most studies of rodent lymphocyte
transformation are conducted using spleen or lymph node cells. Thus,
the argument that the assay has clinical relevance is not well
founded. Furthermore, as the response of lymphocytes is extremely
robust, the assay lacks sensitivity. After a significant number of
compounds were evaluated for potential immunotoxicity in mitogen
assays, use of this assay was shifted from the tier 1 screen
originally described by Luster et al. (1988) to the tier 2
comprehensive evaluation. Use of the mitogen assay has now been
removed completely from studies conducted for the NTP, since other
assays in which cellular proliferation is required (e.g. plaque-
forming cell assay, mixed leukocyte reaction) were considered to be
more sensitive, and the data obtained from the mitogen assays add
little if any to an evaluation of the potential immunotoxicity of test
compounds.
4.2.7 Mixed lymphocyte reaction
In the mixed lymphocyte reaction (also known as mixed lymphocyte
culture), suspensions of responder T lymphocytes from spleen or lymph
nodes are co-cultured with allogeneic stimulator cells. The foreign
histocompatibility antigen (MHC class I or class II molecules)
expressed on the allogeneic stimulator cells serves as the activating
stimulus for inbred populations. In noninbred populations, a pool of
allogeneic cells can be used as stimulators. The assay analyses the
ability of T cells to recognize allogenic cells as 'non-self' as a
result of the presence of different MHC class II antigens on their
surface. In response to the class II antigens, the spleen or node
cells proliferate. Because a sufficiently large number of T cells in
the mixed lymphocyte population respond to the stimulator population,
the responder T cells need not be primed. Proliferation of the
responder cells is one of the parameters for T-cell responsiveness to
cellular antigens. If the allogeneic stimulator cell suspension
contains T cells, their uptake of 3H-thymidine must be prevented by
gamma-irradiation or mitomycin C, in order to preclude background
thymidine uptake.
4.2.8 Cytotoxic T lymphocyte assay
The Tc lymphocyte assay is a continuation of the mixed lymphocyte
reaction response in which the T lymphocytes further differentiate
into cytotoxic effector cells under the influence of various
cytokines. In mice, the assay is usually conducted using P815
mastocytoma cells as the sensitizer and target cell (Murray et al.,
1985). Mice are exposed in vivo to the test agent, and spleen cells
are then removed and placed in culture flasks with the P815
mastocytoma cells. After a five-day co-culture period, the spleen
cells are harvested and added to fresh P815 mastocytoma cells which
have been radiolabelled with 51Cr as sodium chromate. After a 4-h
incubation, the percentage cytotoxicity is determined by measuring the
specific release of 51Cr into the supernatant. The five days of
culture are necessary for the T lymphocytes to differentiate into
cytotoxic effector cells. Unfortunately, this extended period in
culture may give the spleen cells sufficient time to recover from any
adverse effects of the test compound, although such effects may have
been present at the time the spleen cells were removed from the
animal. This inherent limitation of the assay detracts from its
usefulness in assessing the immunotoxicity of test compounds.
A holistic Tc lymphocyte assay has been described, in which the
animal is sensitized after injection of the irradiated target cells
(Devens et al., 1985). Inhibiting the ability of the sensitizing cells
to proliferate either through irradiation or mitomycin C treatment
before injection prevents development of Tc lymphocytes in the animal.
Smialowicz et al. (1989) developed an assay in rats in which effector
cells are generated in culture by incubating cells with lymph node
cells from Wistar/Furth rats, and 51Cr-labelled W/Fu-G1 tumour cells
are used as the target cells. The assay requires four days in culture
and can be run simultaneously with the rat mixed lymphocyte reaction,
thus providing information on the test compound's ability to affect
proliferation and differentiation into effector cells.
4.2.9 Delayed-type hypersensitivity responses
Delayed-type hypersensitivity responsiveness is a reflection of
the capacity of the cellular immune system to execute immune responses
and especially those dependent on IL-2 and INF gamma, which include
attraction and activation of nonspecific mononuclear leukocytes
(macrophages-monocytes). Many systems can be used, depending on the
antigen. One is sensitization to BCG, followed by challenge with
purified protein derivative, to which sensitivity is induced. Another
example is ovalbumin, to which sensitization is most efficient if the
ovalbumin is emulsified in complete Freund's adjuvant. In this system,
delayed hypersensitivity can be measured to both purified protein
derivative and ovalbumin (Vos et al., 1980). Another antigen is
L. monocytogenes: This system is particularly interesting since it
can be used in the context of experiments in which host resistance to
this pathogen is also measured (Van Loveren et al., 1988a).
Delayed hypersensitivity responses can be measured after
sensitization to Listeria by subcutaneous injection of the test
antigen into the ears. Prior to and 24 and/or 48h after challenge, the
increment in ear thickness can be measured with a micrometer by a
person unaware of the experimental group. The background ear swelling
responses of similar, unimmunized control animals are subtracted from
the swelling responses found in immunized animals.
Several delayed-type hypersensitivity assays have been developed
and used for evaluating immunotoxicity in the mouse. Most have
involved measuring swelling in either the footpad or the ear after
sensitization and challenge with a protein antigen. Studies by
LaGrange et al. (1974) demonstrated that sheep erythrocytes could
elicit a delayed-type hypersensitivity response after a single
injection into the foot pad; however, more sheep erythrocytes were
needed to elicit the delayed-type hypersensitivity response than to
produce the optimal humoral immune response. Foot pad swelling can be
measured with a micrometer, as described for rats or by a more
objective, isotopic procedure, as described by Paranjpe & Boone (1972)
and Munson et al. (1982). The delayed-type hypersensitivity response
to sheep erythrocytes was previously considered to be a good assay for
detecting effects on cell-mediated immunity; however, the lack of
persistence of the response (LaGrange et al., 1974; Askenase et al.,
1977) and the possible contribution of antibody to the response raised
concern about the specificity of the assay when sheep erythrocytes are
used as the eliciting antigen. Benzo[ a]pyrene, a compound that
selectively affects humoral but not cell-mediated immunity in adult
mice, appears to decrease cell-mediated immunity when measured in the
sheep erythrocyte assay but has no effect on delayed-type
hypersensitivity when evaluated in the keyhole limpet haemocyanin
assay. The effect in the sheep erythrocyte assay is observed at doses
of benzo[ a]pyrene that decrease antibody production, suggesting a
significant antibody component of the swelling observed (White, 1992).
Keyhole limpet haemocyanin is another protein antigen used in
evaluating delayed-type hypersensitivity responses. Holsapple et al.
(1984) characterized the response to this antigen in the mouse,
showing that it produced the classical delayed-type hypersensitivity
response both with and without adjuvant. Two immunizations with
keyhole limpet haemocyanin were required, however, to produce a
response equivalent to one obtained with complete Freund's adjuvant.
In these studies, animals were sensitized with subcutaneous injections
of keyhole limpet haemocyanin in the shoulder area, with seven days
between the sensitizations. They were then challenged with the same
antigen injected intradermally into the central portion of the pinna
of one of the ears. Increases in ear thickness were evaluated by both
micrometer readings and radioisotopically. The unchallenged ear was
used as an individual control for each animal, and a group of
unsensitized but challenged animals was used to control for
nonspecific and background effects. The results indicated that,
whenever possible, the use of adjuvant in delayed-type
hypersensitivity studies should be avoided. Despite the fact that
complete Freund's adjuvant boosted the responses to keyhole limpet
haemocyanin, it partially masked the dexamethasone-induced suppression
of the response. In some cases, however, delayed-type hypersensitivity
responses are difficult to induce without adjuvant.
The studies currently conducted in mice and rats with this assay
are holistic assays for evaluating cell-mediated immunity. Since
sensitization and challenge occur in the intact animal, all components
of the immune system are present to respond in a physiologically
relevant manner. This type of assay is much more valuable for
evaluating the effects of compounds on cell-mediated immunity than are
in-vitro assays such as the mixed leukocyte response or Tc cell assay.
Luster et al. (1992) reported that the delayed-type hypersensitivity
response assay in mice was highly predictive (100% concordance) of
immunotoxicity when used in combination with the NK cell assay and the
plaque-forming cell assay.
4.2.10 Host resistance models
4.2.10.1 Listeria monocytogenes
Relevant mechanisms of defence against L. monocytogenes include
phagocytosis by macrophages and T cell-dependent lymphokine production
which enhances phagocytosis (Mackaness, 1969; McGregor et al., 1973;
Takeya et al., 1977; Pennington, 1985; Van Loveren et al., 1987).
Humoral immunity is not relevant in protection against infection in
this model. Clearance of Listeria after infection by, for instance,
the intravenous or the intratracheal route can be assessed at various
times after infection by determining the numbers of colony forming
units in the spleen or lungs, respectively. This can be done by
classical methods (Reynolds & Thomson, 1973) that involve the
following steps: Serial dilutions of homogenates of the organs,
prepared in mortars with sterile sea sand, are plated onto sheep blood
agar plates; after a 24-h incubation at 37°C, the colonies are counted
to determine the number of viable bacteria in the organ. Differences
in the numbers of bacteria retrieved from the organs are an indication
of the clearance of the bacteria, i.e. the rate at which the host
disposes of the bacteria after infection.
Histopathology after a Listeria infection can also be valuable.
For instance, exposure to ozone before an intratracheal infection with
Listeria affects pathological lesions due to the infection (Van
Loveren et al., 1988a): Pulmonary infection with Listeria induces
histopathological lesions characterized by foci of inflammatory cells,
such as lymphoid and histiocytic cells, accompanied by local cell
degeneration and influx of granulocytes. If rats are exposed to ozone
for one week before infection, the lesions are much more severe than
in unexposed animals and persist at times when either ozone-associated
or infection-associated effects alone would have resolved. The quality
of the lesions is also influenced by prior exposure to ozone: mature
granulomas were found in Listeria-infected rats that were also
exposed to ozone.
When mice are challenged with Listeria, mortality is the usual
end-point monitored; however, clearance and organ bacterial colony
counts can also be determined. L. monocytogenes is a Gram-positive
bacterium. The resistance of mice to the organism is genetically
regulated, and the susceptibility of the B6C3F1 strain, the strain
designated by the NTP for immunotoxicity studies, comes from the C3H
parent, since the C57Bl/6 mouse is resistant (Kongshavn et al., 1980).
Listeria can easily be stored at -70°C at a stock concentration of
approximately 108 colony forming units per ml. In studies in mice,
three challenge levels are routinely selected to produce 20, 50, and
80% mortality in the vehicle control animals. Mortality is recorded
daily for 14 days. Treatment groups consisting of 12 mice per group
have been found to be useful for obtaining statistically meaningful
data on host resistance. This assay is extremely reproducible when the
organism is administered intravenously.
The Listeria assay can detect both protection from and
increased susceptibility to chemicals and drugs. Morahan et al. (1979)
used the model to demonstrate a dose-dependent decrease in host
resistance after exposure to delta-9-tetrahydrocannabinol, the major
psychoactive constituent of marijuana. The Listeria host resistance
assay is that most often used in immunotoxicological assessment of
compounds, and numerous examples of its use can be found in the
literature. It is one of the primary models used by the NTP for
evaluating immunosuppression. Since Listeria is a human pathogen,
appropriate precautions are needed in conducting the assay.
4.2.10.2 Streptococcus infectivity models
Two species of Streptococcus have been used widely in bacterial
host resistance models for immunotoxicological assessment.
S. pneumoniae has been used primarily for evaluating systemic
immunity. S. zooepidemicus has also been used to evaluate systemic
immunity but is used extensively to evaluate the effects of drugs and
chemicals on the local immunity of the pulmonary system.
S. pneumoniae is a Gram-positive coccus to which host
resistance is multifaceted (Winkelstein, 1981). The first line of
defence against this organism is the complement system. Activation of
the complement system can result in direct lysis of certain strains of
S. pneumoniae; however, owing to the nature of their cell wall, some
strains are resistant to lysis by complement. Complement can still
participate directly in the removal of these bacteria as a result of
deposition of complement component C3 on their surface, which
facilitates phagocytosis by polymorphonuclear leukocytes and
macrophages. In the later stages of the infection, antigen-specific
antibody plays a major role in controlling the infection. Thus,
compounds that affect complement, polymorphonuclear leukocytes, B-cell
maturation and proliferation, or the production of antibody can be
evaluated in this system. S. pneumoniae is an excellent model for
evaluating immunotoxicity, since it elicits multiple immune components
which participate in host resistance, each of which can be a potential
target for an adverse effect of a xenobiotic. To date, this model has
had limited success in rats.
Preparation of S. pneumoniaefor challenge is slightly more
complicated than the procedures used for Listeria; however, the
potential of the model for detecting immunotoxic compounds makes the
additional steps worthwhile. Stock preparations of S. pneumoniae
(ATCC 6314) are easily maintained at -70°C in defibrinated rabbit
blood, and aliquots of the stock preparation can be removed and grown
in culture at various dilutions to obtain the desired challenge
concentration. An alternative approach is to grow the organism in
culture and to monitor the bacterial concentrations by measuring the
turbidity of the culture. A 5-µl aliquot of the stock preparation is
used to inoculate 50ml of brain-heart infusion broth, which is
incubated at 37°C, and the turbidity of the overnight culture is
determined with an Abbott Biochromatic analyser system or another
instrument that can sensitively measure changes in culture turbidity.
The overnight culture is diluted with fresh brain-heart infusion broth
to yield an absorbence difference of 0.020-0.025. The turbidity of the
subculture is monitored periodically, and when the optimal density
reaches an absorbence difference of 0.080, the subculture is rapidly
cooled in an ice bath and diluted to the desired inoculum level. The
turbidity of each inoculum is checked in the analyser, and adjustments
are made to obtain the preselected differences in absorbence.
Routinely, one day after the last exposure, female mice are challenged
intraperitoneally with 0.2ml of the S. pneumoniae inoculum. If the
inoculum is administered intravenously, extremely high challenge
levels must be used, which may reflect the efficiency of the
mononuclear phagocyte system to clear and kill the organism. Three
innocula, each at a different concentration, are prepared to give a
range of lethality (e.g. 20, 50, and 80%), and a sample of each is
serially diluted and placed on blood agar plates to determine the
number of colony-forming units administered to the animals. Owing to
the rapid onset of infection, mortality is recorded twice daily for
seven days. In studies by White et al. (1986), when female B6C3F1 mice
were exposed daily for 14 days to 1,2,3,6,7,8-hexachlorodibenzo-
para-dioxin or TCDD by gavage, they were found to have decreased
host resistance to S. pneumoniae, which is consistent with the
decrease in complement activity caused by these compounds.
Another species of Streptococcus that has been used as a host
resistance model is S. zooepidemicus, a group C streptococcus
(Fugmann et al., 1983). Exposure to N-nitrosodimethylamine was shown
to decrease host resistance to this strain significantly. Infection
with S. zooepidemicus may be dependent on an antibody-mediated
response, since the time to death after challenge is considerably
longer than with S. pneumoniae. Numerous studies have demonstrated
that aerosolized S. zooepidemicus is one of the most sensitive
indicators of the toxicity of air pollution: Mice exposed for short
periods to single or mixed pollutants before infection with an aerosol
of S. zooepidemicus and then assessed for mortality over 20 days had
increased mortality with increasing concentrations of ozone (Coffin &
Gardner, 1972; Ehrlich et al., 1977), nitrogen dioxide (Ehrlich &
Henry, 1968; Sherwood et al., 1981), sulfur dioxide (Selgrade et al.,
1989), metal particulates (Gardner et al., 1977; Adkins et al., 1979,
1980; Aranyi et al., 1985), phosgene (Selgrade et al., 1989), and
other volatile organic compounds (Aranyi et al., 1986). With many of
these compounds, enhanced susceptibility to infection has been
demonstrated at concentrations at or below the United States national
ambient air quality standards or threshold limit values. With all of
these compounds, enhanced mortality has been associated with failure
to clear bacteria from the lung and suppression of alveolar macrophage
phagocytic function. This model has recently been adapted to rats. In
this species, both ozone (Gilmour & Selgrade, 1993) and phosgene (Yang
et al., 1995) delayed clearance of bacteria from the lungs and
enhanced the inflammatory response (polymorphonuclear leukocytes in
lavage fluid) at concentrations that do not themselves produce
inflammation; however, mortality does not occur in this species. While
the bacteria have generally been administered as aerosols, Sherwood et
al. (1988) showed that similar results could be obtained when they
were administered intratracheally or intranasally. Since some strains
of Streptococcus are pathogenic to humans, appropriate precautions
must be taken when using this host resistance model.
4.2.10.3 Viral infection model with mouse and rat cytomegalovirus
Cytomegalovirus infections are widely distributed in humans, with
about 60-90% of the population infected. Human cytomegalovirus
infections occur in several forms, the most serious being congenital
and perinatal infection and infection of immunosuppressed individuals.
Less serious forms include post-perfusion syndrome and some cases of
infectious mononucleosis; however, the vast majority of postnatal
infections in immunocompetent individuals are clinically asymptomatic.
More severe disease may occur in immunodeficient hosts, such as
transplant patients (Naraqi et al., 1977; Pass et al., 1978; Marker et
al., 1981; Rubin et al., 1981). Primarily on the basis of
morphological considerations, cytomegaloviruses are classified as
members of the family Herpesviridae. Because these viruses have a
relatively protracted replication cycle, a slowly developing
cytopathology characterized by cytomegaly, and a relatively restricted
host range, they are grouped into the beta Herpesviridae subfamily
(Roizman et al., 1981; Roizman, 1982). The roles of several arms of
the immune system in resistance to cytomegalovirus have been studied
extensively in mice. The role of humoral immunity is not well
understood. It was suggested initially that neutralizing antibodies do
not play a pivotal role in recovery from cytomegalovirus infection in
mice (Osborn et al., 1968; Tonari & Minamishima, 1983); however, the
role of antibodies in neutralization of murine cytomegalovirus and in
antibody-dependent cell-mediated cytotoxicity is now recognized
(Manischewitz & Quinnan, 1980; Quinnan et al., 1980; Farrell &
Shellam, 1991). Cytomegalovirus-specific Tc cells can be detected in
cytomegalovirus-infected mice (Ho, 1980; Quinnan et al., 1980). NK
cell activity appeared to be the most effective, especially during the
initial stages of infection (Bancroft et al., 1981; Selgrade et al.,
1982; Bukowski et al., 1984). Enhanced susceptibility to infection has
been demonstrated in mice when macrophage function was blocked by
silica, and transfer of syngeneic adult macrophages to suckling mice
significantly increased their resistance to mouse cytomegalovirus
infection (Selgrade & Osborn, 1974). An inverse correlation is seen
between the virulence of mouse cytomegalovirus and its infectivity for
peritoneal macrophages (Inada & Mims, 1985), suggesting that
attenuated virus may be controlled, in part, by macrophages. Since the
rat virus acts very much like the attenuated mouse virus, macrophages
may be even more important in rats. Macrophages may facilitate the
generation of latent infection (Booss, 1980; Yamaguchi et al., 1988).
Enhanced susceptibility to mouse cytomegalovirus has been
demonstrated after treatment of mice with cyclophosphamide,
cyclosporin A, nickel chloride, or DMBA. Treatment with benzo[ a]-
pyrene or TCDD did not affect susceptibility to this infection
(Selgrade et al., 1982). Enhanced susceptibility was correlated with
chemical suppression of virus-augmented NK cell activity during the
first week of infection. In rats, exposure to immunotoxic agents such
as organotin compounds led to altered resistance to rat
cytomegalovirus (Garssen et al., 1995).
Experimentally, rodents can be inoculated intraperitoneally with
a species-specific cytomegalovirus, and the concentration of the virus
in tissue can be determined in a plaque-forming assay, which is a
modification of the method described by Bruggeman et al. (1983, 1985).
Rat embryo-cell monolayers are prepared in 24-well plates. Different
organs (salivary gland, lung, kidney, liver, spleen), obtained at
various times after infection, are homogenized in a tissue grinder and
stored as 10% weight/volume samples at -135°C until use. The confluent
monolayers are then infected with 10-fold serial dilutions of the
organ suspensions. After centrifugation, the suspension is removed,
and 1 or 0.6% agarose is added. After incubation at 37°C in 5% carbon
dioxide for seven days, the cells are fixed in 3.7% formaldehyde
solution, the agarose layer is removed, and the monolayer is stained
with 1% aqueous methylene blue. Plaques are counted under a
stereoscopic microscope.
In PVG rats, cytomegalovirus is detectable eight days after
infection, although the virus load is much higher on days 15-20. The
viral load in the salivary gland is higher than that in other organs,
i.e. spleen, lung, kidney, and liver. In contrast, in Lewis rats and
BN rats the viral load in e.g. the kidney was higher than that in the
salivary gland during the first week after infection (Bruning, 1985),
perhaps due to a strain difference (Bruggeman et al., 1983, 1985).
Total body irradiation of PVG rats with 60Co one day before
infection with cytomegalovirus increased the viral load in the
salivary gland, lung, kidney, spleen, and liver over that in
unirradiated PVG rats; histological analysis also indicated a higher
viral load in the salivary gland of infected rats. The mucosal
epithelium of the salivary gland contains enlarged cells with nuclear
inclusion bodies; these could be detected in irradiated, infected rats
only if the salivary gland was dissected and fixed 15 days after
infection. These results are in agreement with those of Bruggeman et
al. (1983), who found that gamma irradiation also induced higher viral
loads in the salivary gland of BN rats. Taken together these results
indicate a role for cellular immunity in resistance to this virus in
rats.
4.2.10.4 Influenza virus model
Influenza virus A2/Taiwan H2N2 has been used as a viral
challenge in evaluating alterations in host resistance of mice after
exposure to various compounds. Compounds that decrease host resistance
to the virus are N-nitrosodimethylamine (Thomas et al., 1985b) and
TCDD (House et al., 1990a). Compounds that do not to alter host
resistance to this pathogen include ozone (Selgrade et al., 1988),
benzo[ a]pyrene, benzo[ e]pyrene (Munson & White, 1990), methyl
isocyanate (Luster et al., 1986), and Pyrexol (House et al., 1990b).
Mortality is the end-point routinely measured in evaluating decreased
host resistance to influenza virus, which is usually instilled
intranasally (Fenters et al., 1979). Host resistance to this virus has
been reported to be mediated by cell-mediated immunity (Ada et al.,
1981), interferon (Hoshino et al., 1983), and antibody (Vireligier,
1975). This model had been suggested for use in evaluating compounds
that affect humoral immunity; however, its inability to detect such
compounds indicates that it is not suitable. A possible explanation
for the discrepancy is that administration of the virus by intranasal
instillation may invoke local immune mechanisms in the lung and may
not adequately reflect systemic immunocompetence. In several cases,
enhanced mortality has been demonstrated in the absence of effects on
viral titres in the lung (Selgrade et al., 1988; Burleson et al., in
press), indicating that enhanced mortality does not always reflect
effects on virus-specific immune defences.
Influenza virus has been used in evaluating immunotoxicity in
rats, after adaptation. Studies by Ehrlich & Burleson (1991) showed
that rats exposed to phosgene had significantly decreased host
resistance. TCDD was shown to affect the resistance of rats to the
adapted influenza virus RAIV (Yang et al., 1994).
As influenza virus is a human pathogen, appropriate precautions
must be taken.
4.2.10.5 Parasitic infection model with Trichinella spiralis
Resistance to infection with the helminth T. spiralis has been
evaluated in both mice and rats after exposure to a variety of
chemicals. In humans, as in other carnivores, infection occurs by
eating meat containing infectious larvae. The life cycle of the worm
is as follows: Infectious larvae excyst in the acid-pepsin environment
of the stomach, rapidly migrate to the jejunum, and penetrate host
intestinal epithelial cells. Sexually mature parasites are present
within three to four days after infection. The viviparous females
produce larvae that migrate via the lymphatic and blood vessels to
host muscle, where they encyst and are encapsulated within a host-
derived structure. Encapsulated muscle larvae can survive for years
within this structure.
An intense inflammatory response, comprised mainly of mast cells
and eosinophils, accompanies intracellular infection in the intestine.
T Cell-dependent immunity plays a crucial role in this inflammatory
response (Manson-Smith et al., 1979; Vos et al., 1983b; Wakelin,
1993), which is responsible for the expulsion of adult parasites.
Antibodies damage the reproductive structures of the female parasite
(Love et al., 1976), have a major role in the rapid elimination of
subsequent infections in rats (Appleton & McGregor, 1984), and
sensitize migrating newborn larvae for destruction by granulocytes
(Ruitenberg et al., 1983).
The number of encysted muscle larvae is typically much higher in
immunosuppressed animals than in immunocompetent animals, due perhaps
to delayed expulsion of adult worms from the intestine, decreased host
control of parasite fecundity, decreased destruction of migrating
larvae, or a combination of resistance defects. These end-points of
host resistance to T. spiralis infection and class-specific antibody
titres can be measured by standard techniques (Van Loveren et al.,
1994). Histological evaluation of the inflammatory infiltrate
surrounding encysted muscle larvae has also been described (Van
Loveren et al., 1993b).
It should be noted that direct effects of the chemical under
study can affect the outcome of infection. For example Bolas-Fernandez
et al. (1988) determined that cyclosporin A delays expulsion of adult
parasites from the intestines of rats but does not increase the number
of larvae encysted in host muscle. This was determined to be a direct
effect of cyclosporin A on the fecundity of female parasites rather
than on immunity to infection. Animals are infected by oral gavage
with known numbers of larvae, isolated from infected donor muscle.
Because infection is spread only by consumption of infected meat or
freshly isolated larvae, there is little danger of the infection
spreading to other animals housed in the same room. T. spiralis is a
human pathogen and must be handled as such; normal laboratory
practices are sufficient to prevent accidental infection.
T. spiralis infection has been used as a host resistance model
in both rats and mice. In general, chemicals that suppress T-cell
function suppress resistance to T. spiralis infection. Thus, TBTO
(Vos et al., 1990b), diethylstilbestrol (Luebke et al., 1984), TCDD
(Luebke et al., 1994, 1995), and the virustatic agent acyclovir
(Stahlmann et al., 1992) had deleterious effects on resistance.
4.2.10.6 Plasmodium model
Two strains of Plasmodium have been used to evaluate the
potential immunotoxicity of compounds. P. yoelii (17XNL) is a
nonlethal strain that produces a self-limiting parasitaemia in mice.
Resistance to this organism is multifaceted and includes specific
antibody, macrophage involvement, and T cell-mediated functions
(Luster et al., 1986). In this assay, animals are injected with 106
parasitized erythrocytes, and the degree of parasitaemia is monitored
over the course of the infection by taking blood samples. In control
animals, the peak response usually occurs 10-14 days after injection.
The degree of parasitaemia can be evaluated by a variety of methods,
e.g. manually, by counting parasitized erythrocytes in blood smears
(Luebke et al., 1991). Host resistance to P. yoelii has been used to
assess the immunotoxicity of benzidine (Luster et al., 1985b),
diphenylhydantoin (Tucker et al., 1985), TCDD (Tucker et al., 1986),
pyran copolymer (Krishna et al., 1989), gallium arsenide (Sikorski et
al., 1989), and 2'-deoxycoformycin (Luebke et al., 1991).
P. berghei is lethal to mice and certain strains of rats and
has been used in assessing immunotoxicity (Loose et al., 1978). Host
resistance depends on specific antibody production and ingestion and
destruction of antibody-coated Plasmodium by phagocytic cells such
as macrophages. T Lymphocytes may also be involved in host resistance
to the organism (Bradley & Morahan, 1982). Mortality has been
evaluated after injection of 106 Plasmodium-infected erythrocytes.
Compounds that have been evaluated for immunotoxicity in this model
system include 4,4'-thiobis(6- tert-butyl- meta-cresol) (Holsapple
et al., 1988), dietary fish-oil supplement (Blok et al., 1992), and
styrene (Dogra et al., 1992). Neither P. yoelii nor P. berghei is
infectious in humans; infection of animals can occur only through
parenteral injection of contaminated blood.
4.2.10.7 B16F10 Melanoma model
The B16F10 tumour cell line is a malignant melanoma that is
syngeneic with the C57Bl/6 mouse, which is one of the parents of the
B6C3F1 mouse. This tumour line was selected for its propensity to
metastasize to the lung. The assay is an outgrowth of the work of
Fidler and colleagues (Fidler, 1973; Fidler et al., 1978). NK cells
and macrophages have been proposed to be involved in host resistance
to this metastasizing tumour; however, T lymphocytes have also been
shown to play a role (Parhar & Lala, 1987). This host resistance assay
is referred to as an artificial metastasis model, since the tumour
cells are administered by intravenous injection, usually into the tail
vein, and lodge in the lung, which is the first capillary bed they
encounter. The B16F10 tumour cells can be stored frozen and can easily
be grown in culture before use. Routinely, 1-5 × 105 cells are
injected intravenously into sentinel mice (i.e. untreated mice
injected with the highest challenge level of tumour cells), and the
tumour burden is monitored in order to select the optimal day of
assay.
Two parameters are routinely used to assess tumour burden. One is
DNA synthesis in the lungs of mice bearing tumours. Since background
DNA synthesis in the lungs of mice without tumours is extremely low,
any detectable rate is a result of the presence of a tumour. In order
to measure synthesis, mice are pulsed intraperitoneally one day before
sacrifice with 0.2 ml of 10-6 mol/litre of 5-fluorodeoxyuridine,
followed 30 min later by 2 µCi of 125I-iododeoxyuridine administered
by the intravenous route. After sacrifice, the lungs are removed,
placed in Bouin's fixative solution, and counted with a gamma counter.
A second indicator of tumour burden is visual enumeration of tumour
nodules after fixation in Bouin's solution. The visibility of the
black nodules of the melanin-producing B16F10 tumour cells on the
yellow background of the fixed lung tissue allows enumeration of up to
200-250 nodules on the surface of the lungs. A good correlation has
been shown between number of tumour nodules and radioactivity present
in the lungs (White, 1992). Thus, if the tumour nodules become too
numerous to count, the results of the study can still be determined
from the radioassay. This system has been useful in demonstrating
decreased host resistance after systemic exposure to the tumour
promoter phorbol myristate acetate (Murray et al., 1985),
intratracheal exposure to gallium arsenide (Sikorski et al., 1989),
and exposure to nickel chloride (Smialowicz et al., 1985b); it has
also been used to show enhanced host resistance after exposure to
manganese chloride (Smialowicz et al., 1984) and 4,4'-thiobis(6- tert-
butyl- meta-cresol) (Holsapple et al., 1988).
4.2.10.8 PYB6 Carcinoma model
The PYB6 tumour cell line is a fibrosarcoma originally induced
with a polyoma virus in C57Bl/6 mice. Host resistance to the tumour
includes NK cell activity and T cell-mediated killing (Urban et al.,
1982). While PYB6 cells can easily be grown in culture, they should be
passed through an animal before use in challenge studies for
immunotoxicity (Luster et al., 1988). In studies with the PYB6 line,
mice are injected in the thigh with 1-5 × 103 viable tumour cells
and are then palpated weekly to detect the development of tumours at
the injection site. The end-points evaluated include the incidence of
tumours and time to tumour appearance; tumour size can also be
measured. This assay has been useful in detecting decreased host
resistance to many compounds, including Aroclor 1254 (Lubet et al.,
1986), DMBA (Dean et al., 1986), and benzene (Rosenthal & Snyder,
1987).
4.2.10.9 MADB106 Adenocarcinoma model
A tumour model used to evaluate host resistance in rats is the
MADB106 rat mammary adenocarcinoma, which is syngeneic with the
Fischer 344 rat. NK cells appear to play a major role in host defence
to this tumour (Barlozzari et al., 1985). In this model, survival time
after injection of the cells is the usual end-point monitored.
Compounds that decrease host resistance to the tumour can decrease
both the percentage survival and the survival time of treated animals.
Control rats begin to succumb to the adenocarcinoma two to three weeks
after an intravenous injection of 2 × 106 tumour cells. Smialowicz
et al. (1985b) showed a significant decrease in the survival of
animals treated with a single intramuscular dose of nickel chloride,
which was correlated with a decrease in NK cell activity.
4.2.11 Autoimmune models
Autoimmune models can also be used to investigate whether a
compound exacerbates induced or genetically predisposed auto-immunity.
These models are used mainly to elucidate the pathogenesis of
autoimmunity and the effect of immunosuppression in
immunopharmacology. Few studies have been reported, although the
relevance of the model for extrapolation to humans may be good. A
number of autoimmune models are available in rats and mice. Autoimmune
phenomena can be either induced or occur spontaneously. In induced
models, an autoantigen is isolated from a target organ obtained from
another species (generally bovine), and the animal is immunized with
this purified antigen in adjuvant. Examples in the rat (Calder &
Lightman, 1992) are experimental encephalomyelitis elicited by bovine
spinal cord antigen (Stanley & Pender, 1991), experimental uveitis
elicited by bovine retinal S-antigen (Fox et al., 1987), and adjuvant
arthritis elicited by Mycobacterium containing H37RA adjuvant
(adjuvant arthritis) or collagen (Holmdahl et al., 1990; Klareskog &
Olsson, 1990; Wooley, 1991). Autoimmune phenomena and associated organ
pathology normally emerge in almost all immunized animals within two
to three weeks. Depending on the effector reaction and the
reversibility of the damage, the disease either stops when the damage
is complete (e.g. uveitis, resulting in blindness of the animal) or
the autoimmune reaction is transient and animals recover (adjuvant
arthritis). In some models, animals subsequently experience a relapse
around day 30 (experimental encephalomyelitis). The induction and
development of autoimmunity in these animals are mediated by T cells
that show the cytokine expression pattern (IL-2, INF gamma) of the Th1
subset. The effector phase of disease symptoms is also mainly a T
cell-mediated process, to which CD4+ cells, CD8+ cells, and
macrophages contribute. Lewis (RT11) rats are particularly
susceptible. These autoimmune models can be induced in other species,
such as mice (Baker et al., 1990) and rhesus monkeys (Rose et al.,
1991). They are generally accepted as models of human (organ-specific)
autoimmune diseases, e.g. experimental allergic encephalomyelitis as a
model of multiple sclerosis, experimental allergic uveitis as a model
of idiopathic posterior uveitis, and adjuvant arthritis as a model of
rheumatoid arthritis.
Autoimmunity can also be induced by metals (Druet et al., 1989;
Bigazzi, 1992). A well-known example is glomerulopathy induced by
mercuric chloride in BN (RT1n) rats. The process is initiated by T
cells with a cytokine synthesis pattern (IL-4) of the Th2 subset. The
relative incidence of these cells is much higher in BB rats than in
other strains. After the cells of the Th2 subset have been stimulated,
there is polyclonal stimulation of B lymphocytes, leading to synthesis
of antibodies (including pathogenic antibodies) to the glomerular
basement membrane. These antibodies subsequently mediate autoimmune
destruction of renal glomeruli. This model is the best studied model
of 'drug'-induced autoimmunity. Mercuric chloride elicits
glomerulonephritis in other rat strains, in which glomerular
destruction is not due to anti-glomerular autoantibodies but is
mediated by immune complexes deposited in the glomerulus (Druet et
al., 1989).
In spontaneous models, predisposition to the development of
autoimmune phenomena and disease is determined by the genetic
composition of the animal strain. Well-known examples are BB rats
(Like et al., 1982; Guberski, 1994) and NOD mice (Lampeter et al.,
1989; Leiter, 1993), which develop autoimmune pancreatitis and
subsequently diabetes. Within the pancreas, the islets of Langerhans
are infiltrated by T lymphocytes and macrophages; subsequent
destruction of the islets results in diabetes. These spontaneous
models are considered to be animal models of human diabetes (Dotta &
Eisenbarth, 1989; Lampeter et al., 1989; Riley, 1989). Other examples
are the systemic autoimmunity that emerges in certain mouse strains
(Guttierez-Ramos et al., 1990) like (NZB × NZW)F1 mice (Theofilopoulos
& Dixon, 1985) and the mixed lymphocyte reaction in lpr (Matsuzawa
et al., 1990) and gld mice (Roths et al., 1984). The spontaneous
pathology in these animals resembles various disease manifestations in
human systemic lupus erythematosus and is similarly mediated by immune
complexes that deposit in tissue. In (NZB × NZW)F1 mice, mainly lupus
nephritis is induced by immune complexes; in the mixed lymphocyte
reaction in lpr mice, both joint manifestations and
glomerulonephritis are seen. The genes associated with autoimmune and
immune complex disease are not known. In comparison with induced
models, these models have the advantage of spontaneous, gradual
development of autoimmune disease symptoms; however, this is a
disadvantage in experimental design, as not all animals develop
disease, and emerging disease develops at different times or ages.
In general, treatment of animals with immunosuppressive drugs
that interfere with signal transduction (cyclosporin, FK-506,
rapamycin) or cell proliferation (cytostatics like azathioprine,
mizoribine, and brequinar), and anti-inflammatory agents
(corticosteroids) inhibit the development of symptoms in these models.
Exposure to immunotoxic chemicals may also lead to alterations in the
course of disease emergence. For instance, HCB, which leads to
immunoenhancement in rats, markedly enhanced the severity of allergic
encephalomyelitis (Van Loveren at al., 1990c). In contrast, arthritic
lesions were strongly suppressed in HCB-exposed Lewis rats, indicating
that HCB has biologically significant immunotoxic effects. Although
the contrasting effects in the two autoimmune models are not yet
understood, and clear dose-effect relationships have yet to be
established, this type of information should be obtained for risk
assessment.
4.3 Assessment of immunotoxicity in non-rodent species
While most immunotoxicological evaluations are conducted in mice
or rats, use of other species is increasing.
4.3.1 Non-human primates
Various non-human primates, including Macaca mulatta (rhesus
macaques), M. nemestrina (pig-tailed macaques), Cercocebus atys
(sooty mangabeys), M. fasicularis (cynomolgus monkeys), and
marmosets have been used in immunotoxicological studies. Many of the
assays carried out in mice or rats can be adapted for use with non-
human primates. Strategies and methods used in studies of humans have
also been introduced in studies on non-human primates. Monoclonal
antibodies generated to human leukocyte subsets can be used in
phenotyping blood mononuclear cells of e.g. marmoset monkeys
(Callithrix jacchus) (Neubert et al., 1990, 1991), although the
possibility of such use differs, depending on the evolutionary
distance of the non-human primate from humans. While most of the
assays conducted in non-human primates involve serum or peripheral
blood, some assays, such as those used to measure delayed-type
hypersensitivity, are holistic, in that the animals are sensitized
in vivo and then evaluated in vivo at the challenge site (Bugelski
et al., 1990; Bleavins & Alvey, 1991).
The effects of chronic exposure to the PCB Arochlor 1254 on the
immune response of rhesus monkeys have been evaluated by Tryphonas et
al. (1989). In these studies, the lymphocyte response to concanavalin
A and phytohaemagglutinin was evaluated, as were total serum
immunoglobulin levels, antibodies to sheep red blood cells, and
numbers of T and B cells in peripheral blood. In later studies
(Tryphonas et al., 1991a,b), one-way mixed lymphocyte cultures,
antibodies to pneumococcal antigens, phagocytic mononuclear cell
function, NK function, haemolytic complement activity, and production
of IL-1, tumour necrosis factor, thymosins, and interferon were
evaluated in monkeys exposed to Arocolor 1254. Ahmed-Ansari et al.
(1989) evaluated phenotypic markers and function in three species of
non-human primate. The functional assays included NK cell activity,
lymphocyte transformation, and antigen presentation. Extensive studies
included the evaluation of more than 20 phenotypic markers or
combinations of markers for each of the three monkey species. Use of
the monkey as a test species is likely to increase as more and more
biotechnology and recombinant products are produced.
4.3.2 Dogs
While dogs are not the species of choice for immunotoxicological
studies, they are used predominantly in assessing toxicological
safety, and virtually all of the assays used for assessing immunotoxic
potential have been adapted for use in dogs. These include evaluation
of basal levels of IgA, IgG, and IgM (Glickman et al., 1988),
allergen-specific serum IgE (Kleinbeck et al., 1989), mononuclear
phagocyte function (Thiem et al., 1988), NK cell activity (Raskin et
al., 1989), Tc cell activity (Holmes et al., 1989), and mitogen-and
cell-mediated immune responses (Nimmo Wilkie et al., 1992).
4.3.3 Non-mammalian species
Non-mammalian species are also used extensively for evaluating
the potential adverse effects of compounds and agents on the immune
system.
4.3.3.1 Fish
Because of their environment, fish are an excellent model for
studying the effects of water-and sediment-borne pollutants. There are
several other good reasons for studying immunotoxicity in fish: many
of their diseases are related to environmental quality, various
environmental pollutants have immunotoxic potential, and many of the
diseases have an immune component. Moreover, there is concern about
the health status of aquatic ecosystems in relation to pollution, and
fish will be useful target species for developing biomarkers (see
box). Fish are easy to obtain, there is an extensive body of
knowledge, and their economic interest (aquaculture) facilitates the
finding of research resources. At present, immunotoxicology in fish is
not as sophisticated as that in mammals. Screening and functional
tests are being developed in the laboratory but cannot yet be applied
in the field.
A wide range of species is used for field and laboratory studies.
The choice of species depends on its biology (migratory or local,
marine or freshwater; sediment-dwelling or pelagic) and on experience
in the laboratory. A lack of consistency, e.g. becuse of a limited
number of species (as in mammalian immunotoxicology) makes this field
of research diffuse and extended and may result in limited progress;
nevertheless, a variable or consistent effect over a variety of
species is certainly a valuable observation. Some species seem to be
preferred, such as trout, salmon, and carp, which are practical, owing
to their size, for sampling blood and tissues for laboratory studies.
Smaller species, such as guppies (Poecilia reticulata) and medaka
(Oryzias latipes), have secured a niche in aquatic toxicology owing
to the ease of husbandry and relatively low cost; moreover, because of
their small size, whole animals can be used for histopathological
examination (Wester & Canton, 1991), but their application in
immunotoxicology may be limited because of difficulty in obtaining
adequate blood and tissue samples. For studies of saltwater species,
bottom-dwelling flatfish are commonly used in field studies and, to a
certain extent, in studies of mesocosms and in the laboratory. In
Europe, the flounder (Platichthys flesus) and dab (Limanda limanda)
are popular target species since they are susceptible to certain
recognizable diseases and are commonly available.
A compehensive variety of parameters is listed by Anderson (1990)
and Weeks et al. (1992). A modified set based on those lists and
assays used in rodent immunotoxicology is presented in the box above
and classified as tier 1 (screening tests) and tier 2 (functional
assays). Parameters commonly mentioned in the literature are discussed
below.
Blood cell counts and differential counts: As leukocytes play a
major role in specific and nonspecific humoral and cellular immune
responses, this parameter is used as a measure of the status of the
defence system, in particular in tier 1 testing. It is relatively easy
to test blood samples drawn from live animals, but many environmental
factors unrelated to defence may modify leukocyte status (Anderson,
1990). The use of monoclonal antibodies directed against individual
cell types may improve their identification (Bly et al., 1990; Van
Diepen et al., 1991). Another possible parameter is the haematocrit;
however, it has no known specificity for any immune function, although
it may be considered as a general indicator of stress.
Candidate biomarkers for immunotoxicity in fish
Tier 1: Screening tests
* Conventional haematology, including total and differential blood
cell counts, surface markers (flow cytometry), and macrophage
density and morphology: easy, nonspecific
* Serum immunoglobulin concentrations in naive (unstimulated) fish:
easy, limited specificity
* Lymphoid organ weight (mainly spleen, occasionally thymus):
impracticable
* Histopathology of the thymus, spleen, and kidney: possible, can
be specific
Tier 2: Functional assays
* Humoral immune response (agglutination, enzyme-linked
immunosorbent assay): possible, can be specific
* Cellular immune response (allograft rejection in scale, skin, or
eye): possible, can be specific
* Macrophage functions (phagocytosis, bacterial killing, migration,
chemiluminescence): limited specificity
* Host resistance (bacterial infections): possible, can be
specific, relevant
Nonspecific defence: Other indicators of nonspecific defence
have been proposed as indicators of immunological stress. These
include acute-phase proteins (Fletcher, 1986), the levels of which
appear to be stress hormone-dependent; and lysozyme and ceruloplasmin
activity, which are reduced in carp exposed to trichlorphon in vivo
(Siwicki et al., 1990).
Morphology: The spleen is easy to excise and weigh in animals
of adequate size and could thus serve as a biomarker, although it is
not commonly reported in the literature. One reason may be that a
major and variable portion of the spleen consists of storage blood or
erythropoietic tissue (Fänge & Nilsson, 1985); the lymphoid tissue is
poorly developed and is mainly associated with melanomacrophage
centres (Zapata, 1982, 1983; Fänge & Nilsson, 1985; Van Muiswinkel et
al., 1991); and, after immunization, only a small proportion of the
plaque-forming cells is found in the spleen, in contrast to the kidney
pronephros (Van Muiswinkel et al., 1991). The role of the spleen in
most fish species thus seems to be limited, although melanomacrophage
centres are abundant.
Experimental immunotoxicology in mammals has demonstrated that
the weight of the thymus, a primary and exclusively immunological
organ, is a sensitive indicator of thymic effects. This parameter is
not commonly used in fish. One reason is that the thymus has a complex
location in some species, which makes clean dissection nearly
impossible. Other reasons are inconsistencies in thymic morphology,
histopathology, and morphometry (Ghoneum et al., 1986; Wester &
Canton, 1987). The latter paper described studies in which guppies
exposed to TBTO showed dose-dependent atrophy of the thymus, as seen
in rats (Krajnc et al., 1984). Both species also showed a concomitant
increase in 'neutrophils', which suggests functional compensation.
This response could not be reproduced in medaka (Wester et al., 1990),
stickleback, or flounder (P. Wester, unpublished results), probably
indicating species specificity. Thymic lymphocyte function can also be
tested in vitro.
Macrophage function tests: Macrophages are an important cell
population for both specific (antigen processing and presentation) and
nonspecific (phagocytosis and destruction) defence. They are
considered to be a relatively primitive defence mechanism and are
therefore of major importance to lower animals (Ratcliffe & Rowly,
1981). Much effort has been devoted to establishing macrophage
parameters as biomarkers for immune effects in fish; a possible reason
for this preference is the fact that these cells are fairly easy to
obtain, e.g. by peritoneal washing or removal of kidney pronephros,
and many function tests do not require sophisticated techniques or
species-specific reagents or markers (Mathews et al., 1990). The tests
include determination of chemotaxis, phagocytosis, pinocytosis, and
chemiluminescence. Zelikoff et al. (1991) studied the applicability of
trout peritoneal macrophages for immunotoxicology, stressing the need
for systematic baseline information. In addition to the tests listed
above, they studied the morphology and spread of resident and
stimulated peritoneal macrophages and concluded that these cells share
many morphological and functional properties with their mammalian
counterparts and may thus be useful indicators in immunotoxicology.
Many case studies in fish species have been published, demonstrating
the sensitivity of one or more parameters to chemicals, including PAHs
(Weeks & Warinner, 1986; Zelikoff et al., 1991) and pentachlorophenol,
in vitro (Anderson & Brubacher, 1993).
Melanomacrophage centres: Melanomacrophage centres, or
macrophage aggregates, are widely distributed throughout the fish
body, in particular in spleen, liver, and kidney. They are composed of
clusters of swollen, rounded cells (macrophages) that stain pale-tan
to black. This parameter must be determined histopathologically. Their
occurrence and morphology have been described (Agius, 1985), but their
function is not yet fully understood. The presence of pigments
(haemosiderin, lipofuscin, and ceroid) indicates storage of effete
biological material (erythrocytes, biomembranes) (Wolke, 1992). The
melanin present may be a generator of the bactericidal hydrogen
peroxide (Roberts, 1975), and the presence of antigens indicates a
role in immune reactions, e.g. antigen presentation. An increase in
melanomacrophage centres can be found with age and after stress
(Blazer et al., 1987), as confirmed in field studies (Vethaak &
Wester, 1993). Moreover, a large number of relatively small, pale
centres was seen in animals caught in late winter, when conditions are
more stressful, including spawning with associated migration and
starvation (Vethaak & Wester, 1993). The presumption that the small
size and pale appearance are indicators of recent development is
supported by the observation that in liver tumours composed of
relatively young, fast-growing tissue melanomacrophage centres are
usually absent or definitely smaller. As a consequence, when these
centres are used as general parameters of stress, the study groups
must be matched for age.
Because they are characteristic for fish and because of the
multiplicity of their functions, these structures deserve special
attention in the context of defining biomarkers for immunotoxicity. In
addition, they are easy to monitor, since they do not require special
preparation other than routine histological procedures, including
morphometry. Since melanomacrophage centres can be considered
primitive analogues of the mammalian lymph follicle (Payne & Fancey,
1989), it has been suggested that their presence indicates immune
capacity or function, although their role in this context has not yet
been established and the implications of a change in this parameter
for the integrity of the defence systems remains unclear. The density
of melanomacrophage centres in liver or spleen has been successfully
correlated with environmental sediment (Payne & Fancey, 1989) and
along a gradient of pollution in the North Sea (Bucke et al., 1992).
Other studies have reported an increase in melanomacrophage centre
density after contact with chemical contaminants (Blazer et al., 1987;
Secombes et al., 1992), which may indicate accumulation of cytotoxic
waste or immune stimulation.
At present, macrophage function and melanomacrophage centres are
the most widely used and promising indicators of the effects of
environmental stress (Blazer et al., 1987). Their relationship to
other components of the immune system remains to be clarified,
however, in tests with immunotoxicants.
Humoral immune response: Determination of circulating
immunoglobulin levels in serum is useful for testing the net result on
an immunological pathway in vivo. The response can be measured in
'naive' animals (total immunoglobulin) or after exposure to an
antigen, e.g. to verify the efficacy of vaccination in aquaculture.
Sheep red blood cells can be used as a standard antigen, and the
immune response can be measured by agglutination tests. ELISA tests,
which are sensitive and specific, can also be used (Arkoosh &
Kaattari, 1990). A related test is the haemolytic plaque assay which
identifies antibody-producing cells (splenic lymphocytes) (Anderson,
1990), but which has been used to only a limited extent in fish.
Specific lymphocyte stimulation tests: Functional tests widely
used in mammalian immunotoxicology, in which lymphocytes are
stimulated in vitro by exposure to mitogens such as
lipopolysaccharide, phytohaemagglutinin, and concanavalin A, can also
be used in fish. Proliferation is monitored by measuring the
incorporation of 3H-thymidine into DNA. The test is not antigen-
specific but provides information on the capacity of the entire B
(lipopolysaccharide) or T (phytohaemagglutinin, concanavalin A) cell
population. It is used to only a limited extent in fish
immunotoxicology, although Faisal & Hugget (1993) gave an elegant
demonstration of significant suppression of this parameter in spot
(Leiostomus xanthurus) under field conditions; this was shown to be
related to site and pollution in controlled laboratory experiments.
Specific cellular immune responses: Tests described in the
literature to measure cellular immune responses are scale or skin
allograft rejection, a relatively simple test (Zeeman & Brindley,
1981), and eye allograft rejection (Khangarot & Tripathi, 1991);
delayed rejection was seen in carp after exposure to copper. These
tests are applied to only a limited extent.
The tests described above are mainly tier 2 tests. The tests most
often used in immunotoxicology, however, are those for host resistance
(challenge by infections or tumours). The results of such tests are
rarely reported in the literature and have not been validated. For
ultimate proof of immunotoxicity, all phases of a test (maintainence,
exposure, and infection) must be conducted under strictly controlled
laboratory conditions. When suitable (often species-specific)
pathogens are standardized, such tests are valuable and necessary for
estimating the practical consequences of suspected immunotoxicity.
Although the incentive for undertaking immunotoxicological studies in
fish is usually epidemiological observation of a suspected toxic
component, ultimate challenge experiments must be carried out before a
final conclusion about immunotoxic mechanisms can be drawn.
More emphasis has been given to the development of biomarkers
than to their application in the field, for several reasons, including
the lack of specificity and the lack of association between effects at
the level of the biomarker and the population (Mayer et al., 1992).
Some comments on and some needs in this field are as follows:
* Immunological biomarkers in fish have great potential: many have
not yet been fully explored, probably owing to practical
limitations of lack of specificity and predictivity.
* The number of animal species should be limited in order to
concentrate research, which often requires species-specific
knowledge and reagents. Standardization could be achieved by
choosing well-defined inbred strains of fish (e.g. carp or
trout).
* A tiered approach is highly recommended for obtaining knowledge
on the specificity of the biomarker.
* More knowledge is needed on the epidemiology, mechanisms, and
etiology of diseases in fish, and particularly the predictive
value of immune parameters and the influence of hormesis.
* In terms of relevance for the organism, a test that monitors the
net result of a cascade of reactions (e.g. specific antibody
production, host resistance) is more predictive than a single,
nonspecific cell parameter (e.g. macrophage activity in
vitro).
* In identifying potential biomarkers for immunotoxicity, evidence
should be available that the levels tested in the laboratory are
relevant for field conditions and that the effect is directly
related to the immune system.
4.3.3.2 Chickens
Another non-mammalian species that has been studied extensively
with regard to the structure and function of its immune response is
the chicken. It is therefore not surprising that the chicken has
emerged as the predominant avian model for assessing compounds for
potential immunotoxicity. Humoral responses to different antigens have
been assessed routinely (Lerman & Weidanz, 1970; Marsh et al., 1981).
The weights of the thymus, spleen, and bursa of Fabricius have been
used, in combination with decreased antibody responses in vivo and
lymphocyte responses to phytohaemagglutinin and concanavalin A
in vitro (Eskola & Toivanen, 1974). Graft-versus-host and
cutaneous basophil hypersensitivity have also been used to detect
immunosuppression in chickens (Dietert et al., 1985). The availability
of chicken cell lines (Sung et al., 1992) will facilitate studies of
the mechanisms of action of compounds on the immune responses of this
avian model.
4.4 Approaches to assessing immunosuppression in vitro
The complexity of the immune system and the requirement of many
agents for metabolism and distribution to produce an immunotoxic
response has resulted in the almost exclusive use of animal models
in vivo for immunotoxicity assessment. Culture systems have been
used extensively, however, to study the mechanisms by which agents
produce immunosuppression.
Since most of the assays used for assessing immunotoxicity are
ex-vivo/in-vitro tests, they are easily adapted to completely in-vitro
assays for assessing immunosuppression. The direct addition of
compounds in various assays, including those involving NK cells,
lymphocyte proliferation, mixed leukocytes, and Tc lymphocytes, has
been used to determine the mechanisms by which compounds alter the
immune response at the cellular and subcellular level. Similarly, the
action of benzene and its metabolites on bone marrow has been studied
extensively in vitro (Gaido & Wierda, 1987), and the effects of TCDD
on thymocytes have been well studied in thymic epithelium co-cultures
(Greenlee et al, 1985). One of the most useful in-vitro assays for
studying immunosuppression is the T-dependent antibody response to
sheep erythrocytes. This assay, also known as the Mishell-Dutton assay
(Mishell & Dutton, 1967), has been used extensively in studying the
cellular target of immunotoxicants. It is the in-vitro counterpart of
the in-vivo plaque-forming cell assay, but sensitization with sheep
erythrocytes takes place in splenic cell culture and the plaque
response is measured on day 5 after addition of the erythrocytes. The
Mishell-Dutton assay has been used to study the structure - activity
relationships of various immunosuppressive compounds (Kawabata &
White, 1987; Davis & Safe, 1991). Since T cells, B cells, and
macrophages are needed for the response and an adverse effect on any
of these cell types can produce immunomodulation, it has proved
to be a sensitive assay for evaluating compounds in vitro for
immunosuppressive activity. Furthermore, since the various cell types
that participate in the response can easily be separated, individually
treated, and then reconstituted in the culture system, it is an
excellent assay for determining which cell type is adversely affected
by the compound. Using this approach, White & Munson (1986)
demonstrated that asbestos suppresses the response by affecting
macrophages; Shopp & Munson (1985) showed that the primary action of
phorbol ester on the antibody response occurs through an effect on B
cells; and Johnson et al. (1987) found that N-nitrosodimethylamine
affects primarily B cells.
As indicated above, one of the limitations of in-vitro systems is
that exogenous metabolic activation systems are often required. While
lymphocytes can metabolize some compounds, such as benzo[ a]pyrene,
to active metabolites (Ladics et al., 1992), other potent
immunosuppressive compounds such as cyclophosphamide require a
metabolic activation system. Such preparations usually consist of a
9000 x g supernatant of liver (S9). Using S9 preparations, Tucker &
Munson (1981) showed that cyclophosphamide could be activated to an
immunosuppressive form in vitro. Similarly, naphthalene could be
metabolized to an immunosuppressive metabolite (Kawabata & White,
1990). An alternative approach to the S9 activation system is a
hepatocyte co-culture system, which has been shown to be capable of
activating several parent compounds to their immunosuppressive
metabolites (Yang et al., 1986).
Predictive in-vitro systems based on immune cells of human origin
are particularly attractive, given the uncertainties of extrapolating
the results of experimental studies to humans and the accessibility of
immune cells in human peripheral blood. Although many of the immune
cells obtained from human blood are immature forms, the large numbers
and diverse populations (i.e. polymorphonuclear leukocytes, monocytes,
NK cells, T cells, and B cells) that can be obtained provide an
attractive alternative or adjunct to conventional studies in
experimental animals. As a consequence, a number of studies have been
conducted to compare the functional response of human and rodent
lymphocytes to putative immunosuppressive agents in vitro (Cornacoff
et al., 1988; Luo et al., 1992; Wood et al., 1992; Lang et al., 1993).
Although these studies were hampered by the lack of assays to assess
primary antigen-specific immune responses in human lymphocytes, a
relatively good interspecies correlation has been observed in the
limited responses available. Furthermore, several of these assays have
been successfully modified to include co-culture with primary
hepatocytes (Kim et al., 1987) to allow for chemical metabolism.
4.5 Future directions
4.5.1 Molecular approaches in immunotoxicology
A promising avenue for early detection of immunotoxicity may be
measurement of the expression of various interleukins. Cytokines are
involved both in regulation of the immune system and in pathological
phenomena, hence alterations in their pattern of expression may be
early indicators of immunotoxicity. Such testing can be done at the
level of mRNA expression, on mRNA extracted from lymphoid tissue taken
from exposed animals, or in tissue sections, so that the alterations
can be evaluated in the context of morphological indications of the
toxic effects. The signal of the cytokine that is being tested must
therefore be strong enough to be picked up in material from exposed
animals whose immune system has not received other stimuli, i.e.
sensitization or infection. This may not be true for all cytokines;
ex-vivo stimulation of cells that are part of the immune system may be
necessary, although the tests then become more laborious and must in
fact be considered functional assays, like tests for mitogen
responsiveness.
Very sensitive analysis can be done with the semiquantitative
polymerase chain reaction, which is a powerful technique for
elucidating early kinetic changes of cytokine expression, before
translation and secretion (Saiki et al., 1985). In addition, since
immunosuppressive agents can enhance or inhibit the ultimate
production and secretion of cytokines at various stages such as
transcription, the splicing of mRNA, translation of mRNA into
polypeptides on ribosomes, post-translational processing, and
secretion, potential molecular targets can be dissected by such
techniques. Several other molecular approaches may be used, including
northern blotting, dot-slot blotting, in-situ hybridization, and
antisense oligonucleotides for inhibiting the translation of specific
mRNAs.
4.5.2 Transgenic mice
The development of molecular genetic techniques has allowed not
only the isolation and analysis of specific genes but also the
manipulation of embryonic genes. Transgenic technology can be used in
immunology to generate mice that lack virtually any genetic control
mechanism or specific cell subpopulations. As a consequence, complex
systemic responses can be dissected into individual components, and
the mechanism by which immunosuppressive agents exert their affects
can be better understood. Two strategies are used to induce genetic
aberrations in transgenic mice (Bernstein & Breitman, 1989). One
involves the introduction of genes that produce toxins, such as
diphtheria toxin or the A subunit of ricin, into targeted cell
subpopulations. The second strategy involves the thymidine kinase
(tk) gene from Herpes simplex virus: When certain nucleotide
analogues are administered and are metabolized exclusively by viral
thymidine kinase, the metabolites are lethal only to cell
subpopulations that express the tk gene. Both approaches are
inducible systems for killing cells in vivo. Although gene ablation
techniques can be used to generate mutant animals that lack specific
cells in vivo, a small proportion of cells appeared to escape from
targeted cell death in virtually every study using bacterial toxins or
viral tk genes. While this may cause problems in determining the
qualitative roles of ablated cell populations, these techniques hold
promise for understanding the selective toxicity of drugs and
environmental agents on the immune system.
Other promising avenues are the use of animals transgenic with
respect to certain specificities of the TCR. If a gene that encodes
for a certain antigen specificity is introduced into the genome, that
specificity may be the only one that is expressed by the T cells. The
effects of immunotoxicants that affect the (positive and/or negative)
selection process that takes place in the thymus could be studied
elegantly with such models, when either undesired specificities (which
should be negatively selected) or desired specificities (which should
be positively selected) are introduced.
4.5.3 Severe combined immunodeficient mice
Another approach that may warrant further exploration is the use
of severe combined immunodeficient CB-17 scid/scid (SCID) mice
grafted with human immune cells. Xenogeneic lymphoid cells and/or
tissues can be successfully transferred to SCID mice (McCune et al.,
1988; Namikawa et al., 1990; Barry et al., 1991; Greiner et al., 1991;
Surhe & Sprent, 1991). SCID mice have been grafted with human fetal
lymphoid tissue in order to study human haematopoiesis (McCune et al.,
1988) or with human peripheral blood lymphocytes to allow production
of human immunoglobulins, including secondary antibody responses
(Mosier, 1990). SCID mice have also been used to study autoimmunity
and potential antiviral therapeutics. While these animal models still
have limitations (Pollock et al., 1994), they may ultimately provide
predictive models for examining potential immunosuppressive agents.
In particular, SCID mice co-implanted with human fetal thymus and
liver tissue fragments (SCID/hu mice) offer the possibility of
studying the human thymus in vivo in an isolated xenogeneic
environment (McCune et al., 1988; Namikawa et al., 1990) and the
effects of immunotoxicants on these grafts. This system is
particularly interesting with regard to those immunotoxicants for
which the thymus is one locus of action. The placement of human fetal
thymus under the SCID mouse renal capsule, followed by an intravenous
injection of fetal liver cells (McCune et al., 1988), and
co-implantation of human fetal liver and fetal thymus under the renal
capsule of SCID mice (Namikawa et al., 1990) have resulted in
reconstitution of SCID/hu mice; the fetal thymic implants increased in
size, and were found to be vascularized. The architecture and
antigenic distribution of these thymic grafts were virtually
indistinguishable from those of normal, age-matched human thymus.
Human stem cells were found to home to and differentiate within the
grafted human thymus, and phenotypically mature and functional human
T cells were found in the peripheral circulation of these mice (McCune
et al., 1988; Krowka et al., 1991; Vandekerckhove et al., 1991). As
such, the SCID/hu model can be helpful in immunotoxicological research
on the human thymus. When data obtained in experimental animals are
extrapolated to the human situation, a 'control' model, between the
SCID/hu mouse model and the intact laboratory animal (rat), is
desirable in order to test for possible differences in thymic
behaviour, because of its location under the kidney capsule: Thymic
blood flow and therefore the toxicokinetic behaviour of the thymus may
differ. For this reason the SCID/ra model was developed, by implanting
rat fetal thymus and liver tissue fragments under the SCID mouse renal
capsule. The outcomes of exposure of rats and SCID/ra mice can be
compared and the influence of thymus location and mouse metabolism on
extrapolation from SCID/hu to humans can then be determined.
Implantation of fetal rat thymus and liver tissue yields thymic
grafts that are virtually indistinguishable from normal, age-matched
rat thymus (De Heer et al., 1993). After implantation of rat fetal
thymus and liver tissue, the thymic grafts increase considerably in
size. Histologically, the SCID/ra thymic graft bears a close
resemblance to normal rat thymus, and the (immuno)histology of the
SCID/hu and SCID/ra mouse thymic grafts is comparable. Differences are
found, however, in peripheral reconstitutions of SCID/hu and SCID/ra
mice: Whereas large numbers of circulating donor rat T cells are found
in the blood and peripheral lymphoid organs of SCID/ra mice, only a
small number of donor T cells are found in the SCID/hu. This implies
that the data for extrapolating immunotoxic data from rats to humans
must be confined to thymic effects. With this restriction in mind, the
outcome of experiments with SCID/hu and SCID/ra mice can be used to
compare the sensitivity of the human and rat thymus and can thus yield
important information for the process of human risk assessment.
4.6 Biomarkers in epidemiological studies and monitoring
There is a difference between assays of the immune system and
biomarkers. Many validated tests can indicate alterations to the
immune system, including its function, so that most assays can be
helpful for hazard identification. Not every validated assay of the
immune system is a biomarker, however. The IPCS (1994a) definition of
a biomarker is one that indicates exposure (and is specific for
exposure), indicates susceptibility to adverse effects, and/or is
predictive of disease associated with exposure. Biomarkers should be
used to characterize risk due to exposure, on the basis of
identification of the hazard.
Within this strict definition, it is clear that not many
biomarkers are available for immunotoxicity (as is true for other
systems), especially for assessing immunotoxicity or individual
susceptibility to immunotoxicity. Some assays may be useful in
epidemiological studies. In any event, more epidemiological studies
are needed to obtain a better view of the usefulness of biomarkers for
detecting immunotoxic events and hence the possible health risks that
may be associated with exposure to immunotoxicants.
4.7 Quality assurance for immunotoxicology studies
In many countries, studies to support the safety of a compound or
drug must be conducted in accordance with the requirements for 'good
laboratory practice' of the agency that is evaluating the material.
Immunotoxicological studies conducted to support the safety of a new
drug or chemical should follow at least the 'spirit' of good
laboratory practice. The OECD has published their principles of good
laboratory practice, with supporting publications on their
application, and these have been adopted into legislation in a number
of countries. IPCS (1992) has published a monograph, Quality
Management for Chemical Safety Testing, covering the important
aspects of good laboratory practice in a nonregulatory context and
quality control of chemical analyses.
In the United States, good laboratory practice for conducting
nonclinical laboratory studies for submission to the Food and Drug
Administration has been detailed. The standards for studies on
pesticides submitted to the Environmental Protection Agency were also
published, as were the procedures to be followed in conducting studies
submitted on compounds covered by the Toxic Substance Control Act.
Each of these sets of standards is periodically updated by the
respective agencies, and studies must be conducted in accordance with
the most recent updates. While there are some differences in the
wording of the standards, they are generally similar.
Good laboratory practice includes written protocols for
evaluating potential immunotoxicants and the establishment of standard
operating procedures for assays. Each laboratory must run the assays
frequently enough to establish historical control values, and
the results of any study conducted to evaluate a compound for
immunotoxicity must be judged in the context of the historical control
values for the laboratory and appropriate controls. Incorporation of
positive control compounds in the study design provides additional
confidence that the assays are being conducted correctly, particularly
when the tested compound shows no effect.
The selection of assays to be used in evaluating compounds for
immunotoxicity remains a subject of active discussion. Other parts of
this document address this issue in detail. Regardless of which assays
are used, however, they must be standardized and be recognized as
validated and meaningful. Significant advances have been made in the
standardization and harmonization of assay procedures for assessing
immunotoxicity, mainly as a result of the willingness of leading
laboratories in the field to share their standard operating procedures
openly with other laboratories. Published papers and books on
immunotoxicity test methods also contribute to the standardization
process. As a result of studies by Luster et al. (1988), the assays
used by the NTP for evaluating potential immunotoxicants have been
accepted as validated assays in mice.
4.8 Validation
An important requirement of tests for evaluating immunotoxicity
is that they be validated. While there is no agreed definition of
validation, tests must meet certain requirements. In toxicology,
validation is the process by which the reliability and relevance of a
test to identify human health risk is established (Balls et al.,
1990). A flow diagram of a proposed validation process and its end-
point, the acceptance of a method by regulatory authorities for
submission of toxicological data, is presented in Figure 36.
Four parameters must be considered in determining the validity of
a testing method: specificity, sensitivity, accuracy, and precision.
Specificity is based on the rate of false-positive results generated.
Sensitivity is determined by the ability to identify true-positive
results. These two parameters determine the level of predictability or
relevance. In order to determine specificity and sensitivity, the
method is evaluated with a set of compounds of known positive and
negative immunosuppressiveness. This approach was used by the NTP in
Yoshida & Streilin (1990) for the UVB-sensitive phenotype, Taylor et
al. (1994) reported that 66% of individuals who have a history of
herpes lip lesions provoked by exposure to sunlight were sensitive to
UVB, in comparison with 40-45% of the general population and 92% of
skin cancer patients. Exposure of immunosuppressed patients to
sunlight can increase the incidence of viral warts caused by
papillomavirus (Boyle et al., 1984; Dyall-Smith & Varigos, 1985). It
is also known that UVR exacerbates the clinical course of systemic
lupus erythematosus, an autoimmune disease (Epstein et al., 1965). The
effects of UVR on the risk of infectious disease have been reviewed by
Koren et al. (1994). In contrast, certain infectious diseases appear
to be cured by sunlight; the most notable are erysipelas (Giannini,
1990), a skin disease caused by Streptococcus, and skin lesions
caused by Herpes zoster virus.
2.4.8 Others
A large number of therapeutic drugs and drugs of abuse may also
alter human immune function in humans. These include:
Therapeutic agents
Alkylating agents
Nitrogen mustards: cyclophosphamide, L-phenylalanine mustard,
chlorambucil
Alkyl sulfonates: busulfan
Nitrosoureas: carmustine (BCNU), lomustine (CCNU)
Triazenes: dimethyltriazenoimidazolecarboxamide (DTIC)
Anti-inflammatory agents
Aspirin, indomethacin, penicillamine, gold salts
Adrenocorticosteroids: prednisone
Antimetabolites
Purine antagonists: 6-mercaptopurine, azathioprine, 6-thioguanine
Pyrimidine antagonists: 5-fluorouracil, cytosine arabinoside,
bromodeoxyuridine
Folic acid antagonists: methotrexate (amethopterine)
Natural products
Vinca alkaloids: vinblastine, vincristine, procarbazine
Antibiotics: actinomycin D, adriablastine, bleomycin, daunomycin,
puromycin, mitomycin C, mithramycin
Antifungal agents: griseofulvin
Enzymes: L-asparaginase
Cyclosporin A
Estrogens: diethylstilbestrol, ethinylestradiol
Substances of abuse
Ethanol
Cannabinoids
Cocaine
Opiates
Adapted from Dean & Murray (1990)
3. STRATEGIES FOR TESTING THE IMMUNOTOXICITY OF CHEMICALS IN ANIMALS
3.1 General testing of the toxicity of chemicals
The fact that substances used in various aspects of modern life
can be simultaneously beneficial and harmful to human life creates a
legislative and regulatory dilemma. In order to balance the desire to
use the many new substances that enter the market every year and the
economic benefit that is associated with their use on the one hand
with the health and safety of the population on the other is an
important challenge to governmental authorities. Legislative and
regulatory efforts to minimize and control the risk of adverse effects
on human health has resulted in a system for assessing and classifying
the potential risk of exposure to chemicals. Potential adverse effects
can be assessed in studies with experimental animals. In conducting
such studies, attention must be paid to ethical and regulatory
requirements for animal welfare and to good laboratory practice.
In assessing and evaluating the toxic characteristics of a
chemical, its oral toxicity may be determined once initial information
has been obtained by acute testing. Toxicity is routinely tested
according to guidelines, one of which is guideline No. 407 of the
Organisation for Economic Co-operation and Development (OECD) for
testing of chemicals, the 'Repeated Dose Oral Toxicity - Rodent:
28-day or 14-day Study' (Organisation for Economic Co-operation and
Development, 1981). This guideline has undergone three revisions, the
most recent of which (January 1994) includes parameters of
immunotoxicological relevance (see Table 7). Depending on the amount
of a chemical to be produced and the expected exposure to the
chemical, testing according to this guideline may in many countries
provide the only information on its safety, including potential
toxicity to the immune system. The information yielded by this type of
testing is therefore decisive in determining how chemicals are used in
society. Subsequent guidelines have been defined for use in follow-up
studies if more exposure is expected or if there is a suspicion of
toxicity on the basis of structural analogy with other known
compounds. These include 90-day studies of oral toxicity, long-term
studies, and studies of reproductive effects. Although such guidelines
include more parameters of the immune system than OECD test guideline
No. 407, detection of potential immunotoxicity may still not be
adequately addressed. In practice, the best procedure is to carry out
appropriate tests on a case-by-case basis, at increasing levels of
complexity, when concerns are raised in more general toxicological
studies.
Table 7. Parameters of OECD Test Guideline 407 that relate to the immune system
Current Guideline 407 Proposal for updating Proposal for updating Proposal for updating
(adopted 21 May 1981) Guideline 406 Guideline 407 (revision of Guideline 407 (revision of
(February 1991) January 1993) January 1994)
Total and differential Total and differential Total and differential Total and differential
leukocyte count leukocyte count leukocyte count leukocyte count
Weight of spleen and thymus Weight of spleen and thymus Weight of spleen and thymus
Histopathology of spleen Histopathology of spleen, Histopathology of spleen, Histopathology of spleen, thymus,
thymus, lymph node, and thymus, lymph nodes (one lymph nodes (one relevant to the
bone marrow relevant to route of route of administration and a
administration and a distant distant one to cover systemic
one to cover systemic effects), effects), small intestine (including
and bone marrow Peyer's patches), and bone marrow
Histopathology of target Histopathology of target Histopathology of target Histopathology of target
organs organs organs organs
OECD, Organisation for Economic Co-operation and Development
An insight into the type of information that the OECD test
guideline No. 407 yields is given below. In this test, the substance
is administered orally in daily graduated doses to groups of
experimental animals, one dose per group for 28 or 14 days. The
preferred rodent species for this test is the rat, although others may
be used. At least three doses and a control should be used. The
highest dose should result in toxic effects but not produce an
incidence of fatalities which would prevent a meaningful evaluation;
the lowest dose should not produce any evidence of toxicity and should
exceed a usable estimate of human exposure, when available. Ideally,
the intermediate dose level(s) should produce minimal observable toxic
effects.
In compliance with the guideline, the following examinations are
carried out:
(a) haematology, including haematocrit, haemoglobin concentration,
erythrocyte count, total and differential leukocyte count, and a
measure of clotting potential such as clotting time, prothrombin
time, thromboplastin time, or platelet count;
(b) clinical biochemistry of blood, including blood parameters of
liver and kidney function. The selection of specific tests is
influenced by observations on the mode of action of the
substance. Suggested determinations are: calcium, phosphorus,
chloride, sodium, potassium, fasting glucose, serum alanine
aminotransferase, serum aspartate aminotransferase, ornithine
decarboxylase, gamma-glutamyl transpeptidase, urea nitrogen,
albumin, blood creatinine, total bilirubin, and total serum
protein. Other determinations that may be necessary for an
adequate toxicological evaluation include analyses of lipids,
hormones, acid-base balance, methaemoglobin, and cholinesterase
activity. Additional clinical biochemistry may be used when
necessary, to extend the investigation of any observed effects.
(c) pathology, including gross necropsy, with examination of the
external surface of the body, all orifices, and the cranial,
thoracic, and abdominal cavities and their contents. The liver,
kidneys, adrenal glands, and testes are weighed wet as soon as
possible after dissection to avoid drying. Liver, kidney, spleen,
adrenal glands, heart, and target organs showing gross lesions or
changes in size are preserved in a suitable medium for possible
future histopathological examination. Histopathological
examination is performed on the preserved organs or tissues of
the group given the high dose and the control group. These
examinations may be extended to animals in other dosage groups,
if considered necessary to further investigate changes observed
in the high-dose group.
A properly conducted 28- or 14-day study will provide information
on the effects of repeated doses and can indicate the need for
further, longer-term studies. It can also provide information on the
selection of doses for longer-term studies.
It is clear that the 1994 guideline is not suitable for adequate
assessment of the potential adverse effects of exposure to a test
chemical on the immune system, since the immunological parameters are
restricted to total and differential leukocyte counts and the
histopathology of the spleen. An evaluation of this test (Van Loveren
& Vos, 1992) indicated that over 50% of the immunotoxic chemicals in a
series of about 20 chemicals would not have been identified as such if
the tests had strictly adhered to the guideline. In fact, it is even
doubtful if chemicals indicated as immunotoxic only on the basis of
guideline No. 407 would in practice have been picked up: For instance,
in a toxicological experiment, a small but significant change in the
percentage of basophilic leukocytes would by itself probably not be
considered to be biologically relevant in the absence of any other
parameter to suggest that an effect on the immune system might have
been present.
These data indicate that extension of OECD test guideline No. 407
is necessary in order adequately to assess potential immunotoxicity.
It is recommended that additional immunological parameters be included
in this guideline in order to increase its power (Vos & Van Loveren,
1987; Basketter et al., 1994).
Guidelines also exist for follow-up studies if greater exposure
is expected or if there is a suspicion of toxicity on the basis of
structural analogy with other compounds. In these studies, potential
toxicity to the immune system is generally addressed somewhat more
extensively than in guideline No. 407. For instance, in a 90-day study
of oral toxicity, the OECD guidelines prescribe that histopathological
examination be done on the thymus, a representative lymph node, and
the sternum with bone marrow, in addition to the spleen. Even with
these additional parameters, it is highly questionable whether
potential immunotoxicity is adequately assessed. For this purpose, a
variety of tests is available, which are described in section 4.
Depending on what is already known about the toxicity of the test
compound, different panels of tests (also referred to as tiers) are
selected for immunotoxicological evaluation. Usually, if little or no
information is available, a dose range including high doses is used;
lower, overtly nontoxic doses are chosen if some knowledge is
available about the physical and chemical properties, toxicokinetics,
structure-activity relationships, and intended use.
3.2 Organization of tests in tiers
Immunotoxicity can be assessed in a tiered approach (Luster et
al., 1988; Van Loveren & Vos, 1989). Generally, the objective of the
first tier is to identify potentially hazardous compounds (hazard
identification). If potential immunotoxicity is identified, a second
tier of tests is carried out to confirm and further characterize the
immunotoxicity.
Various approaches have been suggested for evaluating the
potential immunotoxicity of compounds. Most are similar in design, in
that the first tier is usually a screen for immunotoxicity and the
second tier consists of a more specific confirmatory set of studies or
in-depth mechanistic studies. Since the use of the tiers is usually
tailored to the goals or objectives of the organization that proposes
them, they differ in respect of the specific assays recommended and
the organization of the assays into tiers.
3.2.1 United States National Toxicology Program panel
The tiers and assays originally adopted by the NTP, based on the
proposed guidelines for immunotoxicity evaluation in mice reported by
Luster et al. (1988), are shown in Table 8.
Table 8. Panel adopted by the US National Toxicology Program for detecting immune
alterations after exposure of rodentsa to chemicals or drugs
Parameter Procedures
Screen (Tier I)
Immunopathology Haematology: complete and differential blood count
Weights: body, spleen, thymus, kidney, liver
Cellularity: spleen
Histology: spleen, thymus, lymph node
Humoral immunity Enumerate IgM antibody plaque-forming cells to
T-dependent antigen (sheep red blood cells)
Lipopolysaccharide mitogen response
Cell-medicated Lymphocyte blastogenesis to mitogens
immunity (concanavalin A)
Mixed leukocyte response to allogeneic leukocytes
Nonspecific immunity Natural killer cell activity
Comprehensive (Tier II)
Immunopathology Quantification of splenic B and T cell numbers
Humoral-mediated Enumeration of IgG antibody response to sheep red
immunity blood cells
Cell-medicated Cytotoxic T lymphocyte cytolysis; delayed
immunity hypersensitivity response
Nonspecific immunity Macrophage function: quantification of resident
peritoneal cells, and phagocytic ability (basal and
activated by macrophage activating factor)
Host resistance Syngeneic tumour cells
challenge models PYB6 sarcoma (tumour incidence)
(end-points)b B16F10 melanoma (lung burden)
Bacterial models: Listeria monocytogenes (mortality);
Streptococcus species (mortality)
Viral models: influenza (mortality)
Parasite models: Plasmodium yoelii (parasitaemia)
a The testing panel was developed using B6C3F1 female mice.
b For any particular chemical tested, only two or three host resistance models
are selected for examination.
In this approach, the tier 1 assay is limited; it includes assays
for both cell-mediated and humoral-mediated immunity and for innate
(nonspecific) immunity with the inclusion of NK cell assays. It also
includes immunohistopathology, which is part of the standard protocol
for studies of subchronic toxicity and carcinogenicity conducted by
the NTP. Tier II represents a more extensive evaluation and includes
additional assays for assessing effects on cell-mediated, humoral, and
innate immunity, in addition to host resistance. In this approach,
animals are usually evaluated at only one time, so that the
possibility for recovery or reversibility of immunological changes is
not evaluated. A 14-day exposure period is employed routinely;
however, 30- and 90-day exposures have been used, depending on the
pharmacokinetic properties of the chemical being tested. The dose used
in this tier system tends to be lower than those in several of the
other approaches followed. In the NTP approach, dose levels are
selected whenever possible that have no effect on body weight or other
toxicological end-points. The approach has therefore focused on
compounds for which the immune system is the most sensitive target.
This is in marked contrast to other approaches, in which the highest
dose is usually the maximum tolerated dose.
The assays that make up the NTP tier approach have undergone
various revisions, partly on the basis of an immunotoxicological
review of compounds evaluated in this tier structure (Luster et al.,
1992). The mitogen assays were first moved from tier I to tier II and
have now been dropped altogether: They were found to be insensitive
and to add little when run in conjunction with other assays that have
a proliferative component, such as the mixed leukocyte response and
the plaque assay. Furthermore, the only macrophage phagocytic assay
routinely carried out in immunotoxicological studies conducted for the
NTP is evaluation of the functional activity of the mononuclear
phagocyte system, which is an in-vivo assay for phagocytosis.
Studies by Luster et al. (1992) show that the potential
immunotoxicity of a compound can be reasonably predicted with a few
selected assays. As additional data become available, further changes
to the NTP tiers will most likely be forthcoming.
3.2.2 Dutch National Institute of Public Health and Environmental
Protection panel
The tier approach for immunotoxicological evaluation followed at
the National Institute of Public Health and Environmental Protection
(RIVM) in the Netherlands (Vos & Van Loveren, 1987) is shown in
Table 9. This approach is based essentially on OECD test guideline
No. 407, which suggests that the maximum tolerated dose be used as the
high dose in the study. As a result, significantly higher doses are
used than in the NTP approach in evaluating compounds for
immunotoxicity. Additionally, the standard exposure period is 28 days,
and the animal species routinely used is the rat instead of the mouse.
This type of testing can therefore be performed in the context of
studies in rats to determine the toxicological profile of a compound.
At least three doses should be used, the highest having a toxic effect
(but not mortality) and the lowest producing no evidence of toxicity.
Moreover, immunotoxicity tests carried out in the context of such
testing should not in any way influence the toxicity of the chemical
(e.g. immunization or challenge with an infectious agent). In the NTP
panel, the highest dose to which mice are exposed is chosen so that no
overt toxicity, i.e. changes in body weight or gross pathological
effects, is observed. As tests for immunotoxicity must be fairly
sensitive in order to preclude false negatives, the NTP tier I
includes functional assays. With a broader dose range that includes
overt toxicity, potential immunotoxicity is more likely to be
observed, without the inclusion of functional tests. If functional
assays are to be included in the first tier, those tests that require
sensitization of animals would require inclusion of satellite groups.
In OECD test guideline No. 407 for testing chemicals, none of the
other systems is approached functionally.
It has been suggested that the NK cell assay be added to tier 1 (Van
Loveren & Vos, 1992). Since the assay does not require animals to be
sensitized or challenged, the same animals can be used without
affecting other toxicological parameters, and thus an additional
satellite group of animals is unnecessary.
3.2.3 United States Environmental Protection Agency, Office of
Pesticides panel
The United States Environmental Protection Agency has proposed a
tiered approach to the evaluation of biochemical pest control agents,
which fall under the subdivision M guidelines for pesticides (Sjoblad,
1988). The proposed tiers are shown below. Tier 1 of this approach
includes functional assays for evaluating humoral immunity, cell-
mediated immunity, and innate immunity. Thus, while Tier 1 is
considered by the Agency to be an immunotoxicity screen, it is much
more encompassing than the first tier of the other approaches. By
providing options in the selection of assays for tier 1, the approach
can easily accommodate both the rat and the mouse as the laboratory
animal species used. In this approach, the tier 2 studies provide
information sufficient for risk evaluation, including information on
the time course of recovery from immunotoxic effects and host
resistance to infectious agents and tumour models. Additional
functional tests would be required if a dysfunction were observed in
tier 1 tests or if data from other sources indicated the compound
could produce an adverse effect on the immune response.
Table 9. Methods for detecting immunotoxic alterations in the rat evaluated by the
Dutch National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Parameters Procedures
Tier 1
Nonfunctional Routine haematology, including differential cell counts
Serum IgM, IgG, IgA, IgE determination; lymphoid organ
weights (spleen, thymus, local and distant lymph nodes)
Histopathology of lymphoid tissues, including mucosa-
associated lymphoid tissue
Bone-marrow cellularity
Analysis of lymphocyte subpopulations in spleen by flow
cytometry
Tier 2
Cell-medicated Sensitization to T-cell dependent antigens (e.g. ovalbumin,
immunity tuberculin, Listeria), and skin test challenge
Lymphoproliferative response to specific antigens (Listeria)
Mitogen responses (concanavalin A, phytohaemagglutinin)
Humoral Serum titration of IgM, IgG, IgA, IgE responses to
immunity T-dependent antigens (ovalbumin, tetanus toxoid,
Trichinella spiralis, sheep red blood cells) by ELISA
Serum titration of T-cell-independent IgM response to
lipopolysaccharide by ELISA
Mitogen response to lipopolysaccharide
Macrophaand Phagocytosis and killing of Listeria by adherent spleen
function and peritoneal cells in vitro
Cytolysis of YAC-1 lymphoma cells by adherent spleen
and peritoneal cells
Natural killer Cytolysis of YAC-1 lymphoma cells by non-adherent
function spleen and peritoneal cells.
Host resistance Trichinella spiralis challenge (muscle larvae counts and
worm expulsion)
Listeria challenge (spleen and lung clearance)
Cytomegalovirus challenge (clearance from salivary gland)
Endotoxin hypersensitivity;
Autoimmune models (adjuvant arthritis, experimental
allergic encephalomyelitis)
Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay
Subdivision M guidelines: proposed revised requirements by the US
Environmental Protection Agency for testing the immunotoxicity of
biochemical pest control agents
AI. Tier 1
A. Spleen, thymus, and bone-marrow cellularity
B. Humoral immunity (one of the following)
1. Primary and secondary IgG and IgM responses to antigen; or,
2. Antibody plaque-forming cell assay
C. Specific cell-mediated immunity (one of the following)
1. One-way mixed lymphocyte reaction assay; or,
2. Effect of agent on normal delayed-type hypersensitivity
response; or,
3. Effect of agent on generation of cytotoxic T-lymphocyte
response
D. Nonspecific cell-mediated immunity
1. Natural killer cell activity and
2. Macrophage function
II. Tier 2
A. Required if:
1. Dysfunction is observed in tier 1 tests
2. Tier 1 test results cannot be definitively interpreted
3. Data from other sources indicate immunotoxicity
B. General testing features:
1. Evaluate time course for recovery from immunotoxic effects.
2. Determine whether observed effects impair host resistance to
infectious agents or to tumour cell challenge.
3. Perform additional specific, but appropriate, testing
essential for evaluation of potential risks.
This Agency has also suggested that immunotoxicological screening
be conducted in evaluating conventional chemical pesticides
(subdivision F guidelines; see below); however, unlike those of
subdivision M, these guidelines are not designed as a tiered testing
scheme. If the immunotoxicity screen listed in subheading I were added
to subchronic and/or chronic studies in subdivision F, it would be a
more effective screen for immunotoxicity than is currently available.
If this proposed screen indicates that the immune system is a
sensitive target, the Agency considers that it may be necessary to
evaluate the risk for immunotoxic effects as under subheading
II. Currently, these suggestions have not been promulgated as official
guidelines or regulations.
Evaluations suggested by the US Environmental Protection Agency as
appropriate additions to Subdivision F guidelines for immunotoxicity
screening (subheading I) and possible additional data appropriate for
risk evaluation of chemical pesticides (subheading II)
I. Immunotoxicity screen
A. Serum immunoglobulin levels (e.g. IgG, IgM, and IgA)
B. Spleen, thymus, and lymph node weights
C. Spleen, thymus, and bone-marrow cellularity and cell
viability
D. Special histopathology (e.g. enzyme histochemistry,
immunohistochemistry)
E. More complete evaluation of 'premature' mortality of test
animals, as possibly related to immunosuppressive effects
II. Immunotoxicity risk evaluation
A. Host resistance to challenge with infectious agent and/or
tumour cells
B. Specific cell-mediated immune responses (e.g. mixed
leukocyte response, delayed-type hypersensitivity
response,cytotoxic T lymphocyte assays)a
C. Nonspecific cell-mediated immune responses (i.e. natural
killer cell activity, macrophage function)a
D. Time course for recovery from adverse immunological effects
a Measures of specific and nonspecific cell-mediated immune
responses that also may be considered useful in an immunotoxicity
screen
3.2.4 United States Food and Drug Administration, Center for Food
Safety and Applied Nutrition panel
The United States Food and Drug Administration is considering
testing guidelines for evaluating the immunotoxic potential of direct
food additives (Hinton, 1992). The multifaceted approach is included
in the draft revision of the Toxicological Principles for the Safety
Assessment of Direct Food Additives and Color Additives Used in Food
is (US Food and Drug Administration, 1993). The testing requirements
are based on the 'concern level' of the substance, assigned on the
basis of the available toxicological information or the substance's
structural similarity to known toxicants and on estimated human
exposure from its proposed use. A compound with high toxic potential
and high exposure would be assigned a high initial 'concern level'
(3), and one with low toxic potential and low exposure would be
assigned a low initial level (1).
In general, substances will be evaluated for immunotoxic
potential on a case-by-case basis. Two types of immunotoxicity tests
and procedures are defined in this approach: Type 1 tests are those
that do not involve perturbation of the test animal (i.e.
sensitization or challenge). These are further divided into 'basic'
tests, which include haematology and serum chemistry, routine
histopathological examination, and determination of organ and body
weights, and 'expanded' tests, which are logical extensions of the
'basic' tests and include those that can be performed retrospectively.
Type 2 tests include injection of or exposure to antigens, infectious
agents, vaccines, or tumour cells. In general, type 2 tests require a
satellite group of animals for immunological evaluation. The sets of
'basic' and 'expanded' type 1 tests are defined as level-I
immunotoxicity tests, and the sets of type 2 tests are defined as
level-II tests. Some level-I tests can be used to screen for
immunotoxic effects, while others focus on the mechanism of action or
the cell types affected by the test substance. Level-II tests are
conducted to define the immunotoxic effects of food and colour
additives more specifically. The recommended testing scheme is shown
below.
The functional tests generally require sensitization of exposed
rats and controls and subsequent analysis of the responses to the
sensitizing antigens. For this reason, functional tests are not
readily conducted in the first tier of immunotoxicity testing. As
guidelines for routine toxicology experiments preclude compromising
the experiment by any agent other than the test chemical, the second
tier of immunotoxicity testing, with immune function tests, requires a
separate set of experiments. The antigens that are used to sensitize
the exposed and control rats may be relatively simple antigens, such
as ovalbumin or tetanus toxoid, or more complex antigens, such as
sheep red blood cells, bacteria, or parasites. The responses can occur
in various arms of the immune system, which consequently must be
measured with different assays. For instance, humoral responses can be
measured by determination of specific antibodies in serum; the
appropriate tests for cellular responses are proliferative responses
of lymphocytes to the specific antigens ex vivo/in vitro or delayed-
type hypersensitivity responses to injection with antigen in vivo.
Recommendations of the United States Food and Drug Administration for
testing the immunotoxicity of direct food additives
Basic testing (rat model)
Complete blood count, differential white blood cell count;
Total serum protein, albumin:globulin ratio;
Histopathology, gross and microscopic (spleen, thymus, lymph
nodes, Peyer's patches, and bone marrow);
Lymphoid organ and body weights
Retrospective level-I testing (possible in a standard toxicology
study)
Electrophoretic analysis of serum proteinsa (when positive or
marginal effect is noted in basic testing);
Immunostaining of spleen and lymph nodes for B and T cellsa
(quantification of total immunoglobulins);
Serum autoantibody screen and deposition of immunoglobulins
(micrometry for semiquantification of the proliferative
response)
Enhanced level-I testing (possible for more complete screening in the
standard toxicology study core group, with a satellite animal group,
or in a follow-up study)
Cellularity of spleen (lymph nodes and thymus when indicated);
Quantification of total B and T cells (blood and/or spleen);
Mitogen stimulation assays for B and T cells (spleen);
Natural killer cell functional analysis (spleen);
Macrophage quantification and functional analysis (spleen);
Interleukin-2 functional analysis (spleen);
When indicated or for more complete analysis, other end-points
such as total haemolytic complement activity assay in serum
Level-II testing with a satellite group or follow-up study for
screening of functional immune effects
Kinetic evaluation of humoral response to T-dependent antigen
(primary and secondary responses with sheep red blood cells,
tetanus toxoid, or other);
Kinetic evaluation of primary humoral response to a
T-independent antigen such as pneumococcal polysaccharides,
trinitrophenyl-lipopolysaccharide, or other recognized
antigens;
Delayed-type hypersensitivity response to known sensitizer of
known T effector cell;
Reversibility evaluation
Recommendations (cont'd)
Enhanced level-II testing with a satellite group or follow-up study
for evaluation of potential immunotoxic risk
Tumour challenge (MADB106 or other in rat);
PYB6 sarcoma (in mouse);
Infectivity challenge (Trichinella, Candida or other in rat;
Listeria or other in mouse) a Recommended for inclusion in
basic testing
a Recommended for inclusion in basic testing
Not all functional assays require prior sensitization of the test
animals, e.g. proliferative responses of lymphocytes ex vivo/in vitro
to mitogens which are either specific for T cells, giving information
on cellular immunity, or for B cells, providing data on humoral
immunity. The phagocytic and lytic activity of macrophages and the
nonspecific cytotoxic activity of NK cells can also be measured
ex vivo/in vitro, without prior sensitization of the test animals.
Both types of activity are examples of nonspecific defence mechanisms,
directed to bacteria and certain tumour cells and to tumour cells and
virally infected cells, respectively. Since measurement of these types
of activity does not require prior sensitization of the host, such
functional tests can be considered for inclusion in the first tier of
testing for immunotoxicity in routine toxicology.
3.3 Considerations in evaluating systemic and local immunotoxicity
3.3.1 Species selection
Selection of the most appropriate animal model for
immunotoxicology studies has been a matter of great concern. Ideally,
toxicity testing should be performed with a species that responds to a
test chemical in a pharmacologically and toxicological manner similar
to that anticipated in humans, i.e. the test animals and humans
metabolize the chemical similarly and have identical target organs and
toxic responses. Toxicological studies are often conducted in several
animal species, since it is assumed that the more species that show a
specific toxic response, the more likely it is that the response will
occur in humans. Data from studies in rodents on target organ toxicity
at immunosuppressive doses for most immunosuppressive therapeutic
agents have generally been predictive of later clinical observations.
Exceptions to the predictive value of rodent toxicological data are
infrequent but occurred in studies of glucocorticoids, which are
lympholytic in rodents but not in primates (Haynes & Murad, 1985).
Although certain compounds exhibit different pharmacokinetic
properties in rodents and in humans, rodents still appear to be the
most appropriate animal model for examining the non-species-specific
immunotoxicity of compounds, because of established toxicological
knowledge, including similarities of toxicological profiles, and the
relative ease of generating data on host resistance and immune
function in rodents. Comparative toxicological studies should be
continued and expanded, however, as novel recombinant biological
compounds and natural products that enter safety testing will probably
have species-specific host interactions and toxicological profiles.
The quantitative and possibly the qualitative susceptibility of
an individual animal to the immunotoxicity of a selected agent can be
influenced by its genetic characteristics, indicating not only a need
to consider species but also strain. Rao et al. (1988) described two
approaches for selecting appropriate genotypes for toxicity studies.
The first is to select genotypes that are representative of an animal
species, which by virtue of similar metabolic profiles may also
exhibit a sensitivity similar to that of man, such as random-bred
mice. A second approach is to attempt to identify genotypes that are
uniquely suitable for evaluating a specific class of chemicals, such
as the use of Ah-responsive rodent strains in studies with
polyhalogenated aromatic hydrocarbons. In many cases, however, this
approach requires considerable knowledge of the mechanisms of toxicity
of the compound, which may not be available. One compromise has been
to use Fl hybrids which have the stability, phenotypic uniformity, and
known genetic traits of an inbred animal, yet have the vigour
associated with heterozygosity. The description of the genetic
relationships between inbred mouse strains on the basis of the
distribution of alleles at 16 loci (Taylor, 1972) has made possible
rational selection of appropriate Fl hybrids, such as the B6C3Fl
mouse.
3.3.2 Systemic immunosuppression
Because of this complexity, the initial strategies devised by
immunologists working in toxicology and safety assessment were to
select and apply a tiered panel of assays in order to identify
immunosuppressive or, in rare instances, immunostimulatory agents in
laboratory animals (US National Research Council, 1992). Although the
configuration of these testing panels varies according to the
laboratory conducting the test and the animal species used, they
include measurements of one or more of the following: (i) altered
lymphoid organ weights and histology, including immunohistology; (ii)
quantitative changes in the cellularity of lymphoid tissue, peripheral
blood leukocytes, and/or bone marrow; (iii) impairment of cell
function at the effector or regulatory level; and/or (iv) increased
susceptibility to infectious agents or transplantable tumours.
A variety of factors must be considered in evaluating the
potential of an environmental agent or drug to adversely influence the
immune system. These include appropriate selection of animal models
and exposure variables, consideration of general toxicological
parameters and mechanisms of action, as well as an understanding of
the biological relevance of the end-points to be measured. Treatment
conditions should be based on the potential route, level, and duration
of human exposure, the biophysical properties of the agent, and any
available information on the mechanism of action. Moreover,
toxicokinetic parameters, such as bioavailability, distribution
volume, clearance, and half-life, should be measured. Doses should be
selected that will allow establishment of a clear dose-response curve
and a no-observed-effect level NOEL). Although, for reasons explained
earlier, it is beneficial to include a dose that induces overt
toxicity, any immune change observed at that dose should not
necessarily be considered to be biologically significant, since severe
stress and malnutrition are known to impair immune responsiveness.
Many laboratories routinely use three doses but generally conduct
studies to define the range of doses before a full-scale
immunotoxicological evaluation. If studies are being designed
specifically to establish reference doses for toxic chemicals,
additional exposure levels are advisable. In addition, inclusion of a
'positive control' group, treated with an agent that shares some of
the characteristics of the test compound, may be advantageous when
experimental and fiscal constraints permit.
The selection of the exposure route should reflect the most
probable route of human exposure, which is most often oral,
respiratory, or dermal. If it is necessary to deliver an accurate
dose, a parenteral exposure route may be required; however, this may
significantly change the metabolism or distribution of the agent from
that which would occur following natural exposure.
3.3.3 Local suppression
Local immune suppression has received less attention than
systemic immune suppression, and this is noteworthy, since the surface
that is exposed to the environment, i.e. the skin, the respiratory
tract, and the gastrointestinal tract, are the major ports of entry of
antigens and pathogens. While a variety of validated methods are
currently available to detect chemical skin sensitizers in humans and
experimental animals, there is no standard method to assess the
potential of chemicals to induce local immunosuppression in the skin.
Furthermore, although increasing evidence suggests that the
consequence of skin immunosuppression would be an increase in
neoplastic and infectious diseases of the skin, definitive data are
still lacking. In contrast, considerable efforts are being deployed to
develop sensitive models for monitoring skin irritants. For example,
human keratinocyte cultures and keratinocyte-fibroblast co-cultures
have been examined for end-points ranging from changes in cell
viability to production and loss of various bioactive products. Few
test systems are available for the gut and the respiratory tract.
4. METHODS OF IMMUNOTOXICOLOGY IN EXPERIMENTAL ANIMALS
This section comprises general descriptions of methods used for
evaluating immunotoxicity.
4.1 Nonfunctional tests
4.1.1 Organ weights
It is routine practice in toxicology to weigh organs that are
potentially affected by the compound that is being investigated. The
immunological organs that are suitable for weighing in screening for
potential immunotoxicity are: the thymus, which plays a decisive role
in the development of the immune system and which is affected by many
immunotoxicants; the spleen, which is the repository for many
recirculating lymphocytes; and the lymph nodes, which are important
for the induction of immune processes. Determination of the weight of
draining lymph nodes (depending on the route of exposure, i.e.
mesenteric nodes for oral exposure and bronchial nodes for inhalation)
in addition to distant lymph nodes (such as popliteal lymph nodes for
determining systemic effects) is the best. Mesenteric nodes, in
particular, occur in a string within non-lymphoid fatty tissue, and
care must be taken to remove this non-lymphoid tissue so that the
weight can be adequately determined. The cellularity of these organs
is another indication of the effects of chemicals on the immune
system. Furthermore, cell suspensions can be prepared from lymphoid
organs in order to assess the distribution of subpopulations of
lymphoid cells and to test their functionality within the organs.
Under OECD guideline No. 407, histopathological examination of
lymphoid organs and tissues is crucial for detecting the effects of
chemicals on the immune system. Therefore, upon termination of
exposure to a compound in a toxicological experiment, organs such as
the spleen should first be weighed; subsequently, they are divided
into parts which are also weighed, and one or more parts are used for
histopathological examination and the remainder to prepare cell
suspensions that can be evaluated for distribution of lymphocyte
subpopulations or can be assessed functionally.
4.1.2 Pathology
The histopathology of the thymus, spleen, and draining and
distant lymph nodes, of the mucosal immune system (Peyer's patches in
the gut or bronchus and nose-associated lymphoid tissue in the
respiratory tract), and of the skin immune system should be evaluated,
depending on the route of exposure. The first level of evaluation
should be of haematoxylin-eosin stained, paraffin-embedded slides. A
more sophisticated level of evaluation is immunoperoxidase staining of
special cell types.
Many monoclonal antibodies are available for mice, rats, and
humans to detect differentiation antigens, cell adhesion molecules,
and activation markers on haematolymphoid and stromal cells involved
in immune responses. A list of some monoclonal antibodies that can be
used in the identification of leukocytes and stromal cells in (frozen)
sections of lymphoid tissue is presented in Table 10; a selection of
these is reviewed below.
For a further description of these markers, and the cells that
express them, reference may be made to the introductory section and to
descriptions in the literature (Brideau et al., 1980; Bazin et al.,
1984; Dallman et al., 1984; Dijkstra et al., 1985; Joling et al.,
1985; Vaessen et al., 1985; Joling, 1987; Hünig et al., 1989; Kampinga
et al., 1989; Portoles et al., 1989; Schuurman et al., 1991a).
These markers are usually stained in frozen tissue sections of
6-8 µm, fixed in acetone. A three-step immunoperoxidase procedure is
most suitable: the first step includes the monoclonal antibody
specific for the determinants to be studied (see above), the second
step, rabbit anti-mouse immunoglobulin, and the third step, swine
anti-rabbit immunoglobulin, the latter two antibodies conjugated to
horseradish peroxidase. The peroxidase activity can be developed by
3,3-diaminobenzidine tetrahydrochloride with hydrogen peroxide as
substrate, and the sections can be counterstained with Mayer's
haematoxylin to facilitate evaluation. Negative controls are prepared
by omitting the antibody in the first step or replacing it with an
irrelevant one. Under these conditions, only the peroxidase activity
of polymorphonuclear cells, when present, is visualized, and no
immunolabelling is found.
In general, histopathological evaluation provides a semi-
quantitative estimation of effects. The experienced pathologist can do
this adequately in studies carried out 'blind', especially if the
effects are clear. For more subtle effects, morphometric analysis is a
valuable addition, especially when supported by software for assessing
the values of parameters such as size, surface, and intensity of
staining. The compartments of the immune system, i.e. specific T and B
lymphocyte areas in spleen and lymph nodes and cortical and medullary
areas of immature and differentiated thymocytes within the thymus, and
the numbers of specialized cells per surface unit are parameters that
are well suited for morphometric analysis.
4.1.3 Basal immunoglobulin level
Serum immunoglobulin levels are often altered after exposure of
rats to immunotoxic chemicals (Vos, 1980; Vos et al., 1982, 1984,
1990a; Van Loveren et al., 1993a). This is not surprising, as the
total levels measured are a function of the humoral aspects of the
immune system, which react to the antigens that the host encounters.
For this reason, measurement of antibody levels is potentially
valuable in screening for immunotoxicity. Since the amount of antibody
Table 10. Some monoclonal antibodies to leukocytes and stromal cells used in immunohistochemical studies of tissue sections and flow
cytofluorography on cell suspensions
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells
CD1 gp43,45, Ly-38 OKT6, Lymphocytes in thymic cortex, Langerhans cells in skin,
49,12 a-Leu-6 interdigitating cells
CD2 gp50 Ly-37, MRC OX-34, a-Leu-5, All T cells in thymus and peripheral lymphoid organs, subset
NSM46.7, MRC OX-54, OKT11 of macrophages (rat). Sheep erythrocyte receptor, leukocyte
RM2-5 MRC OX-55 function antigen:-2 (LFA-2). Ligand f or LFA-3 (CD58)
CD3 gp19-29 CD3-1,KT3, IF4, G4.18 a-Leu-4, T Cells in thymic medulla and peripheral lymphoid organs
145-2C11 OKT3 (T-cell receptor-associated, cytoplasmic in precursor T cells
in thymus)
CD4 gp65 Ly-4, L3T4, MRC OX-35, a-Leu-3, Lymphocytes in thymic cortex, about two-thirds of T cells in
YTS 177.9 MRC OX-38, OKT4 peripheral lymphoid organs, subset macrophages, microglia
(ER2), W3/25 T helper/inducer and delayed-hypersensitivity phenotype.
MHC class II binding, receptor for human immunodeficiency
virus
CD5 gp65-62 Ly-1,Lyt-1 MRC OX-19, a-Leu-1 Lymphocytes in thymic cortex (faint). All T cells in thymic
HIS47 medulla and peripheral lymphoid tissue, subset of B cells
CD6 gp120 Tü 33 T Cells in thymic medulla and peripheral lymphoid organs
CD Relative Mouse Rat Human Reactivity
mol. mass
CD7 gp41 WT1,B-F12, Prethymic T-cell precursors, all T cells in thymus and fewer
a-Leu-9 in peripheral lymphoid organs
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
CD8 gp32-33 Ly-2,3,Lyt-2,3, MRC OX-8 a-Leu-2, Lymphocytes in thymic cortex, about one-third of T cells in
YTS 105.8 OKT8 peripheral lymphoid organs, splenic sinusoids (T cytotoxic/
suppressor phenotype, NK cells). MHC class I binding
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells,
thymic cortex cells (rodents). Heat-stable antigen (HSA)
CD43 gp115 Ly-48 W3/13,HIS17 DFT-1, (Pro)thymocytes, T cells, plasma cells, cells in bone
WR-14 marrow, polymorphonuclear granulocytes, brain cells.
Leukosialin, sialophorin
CDw p25-30 Thy-1 (ER4), 5F10 Thymocytes, T lymphocytes, connective tissue structures,
90 MRC OX-7, epithelial cells, fibroblasts, neurons, subset of bone-marrow
HIS51 cells, plasma cells, stem cells (T-activation molecule)
Thy-2 Thymocytes
p40-55 H57-597 R73,HIS42 WT31, T-Cell receptor a-b chain. Mature T cells in thymic medulla
TalphaF1, and peripheral lymphoid tissue
TßF1
p40-55 GL3,GL4, V65 CgammaM1, T-Cell receptor gamma-delta chain
UC7-13D5 11F2,
TCR delta1,
deltaTCS1
p41-55 MRC OX-44 Prothymocytes, lymphocytes in thymic medulla, T and B
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
T Cells (contd)
p41,47 MRC OX-2 Thymocytes, dendritic cells, B cells, brain cells
ER3,ER7, Subset of thymocytes and peripheral T cells, subset of
ER9,ER10 myeloid cells
HIS44 Most lymphocytes in thymic cortex, small subset of
medullary lymphocytes, erythroid cells, cells in germinal
centre
HIS45 Some lymphocytes in thymic cortex, most medullary
thymocytes and peripheral T cells, subset of B cells.
Quiescent cell antigen (QCA-1)
MHC class I (Various antibodies to polymorphic and All nucleated cells, including leukocytes and stromal
non-polymorphic epitopes) cells; for T cells absent on thymic cortex cells (human)
B Cells
CD9 gp24 BA-2 Germinal centres (faint); some cells in thymic cortex. Late
pre-B cells
CD10 gp100 BA-3, Germinal centres (faint); some cells in thymic cortex
W8E7 Common acute lymphoblastic leukaemia antigen (CALLA)
CD19 gp95 a-Leu-12, B Cells in germinal centres and mantles, follicular dendritic
B4, FMC63 cells
CD20 p35 Ly-44 B1, a-Leu-16 B Cells in germinal centres and mantles, follicular dendritic
cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
CD21 gp140 B2,BL13, B Cells in germinal centres and mantles (faint), follicular
HB-5 dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus)
CD22 gp135 Lyb-8.2, a-Leu-14,To B Cells in germinal centres and mantles, cytoplasmic in
Cy34.1 To 15,RFB4, precursor B cells
SHCL-1
CD23 p45 Ly-42 a-Leu-20, Some B cells in marginal centres and mantles, activated B
Tü 1 cells, subset of follicular dendritic cells (IgE Fc receptor)
CD24 p45,55,65 J11d,M1/69 SRT1 BA-1 B Cells in germinal centres and corona, myeloid cells, thymic
cortex cells (rodents). Heat-stable antigen (HSA)
CD37 gp40-45 BL14 B Cells in germinal centres and mantles
CD38 gp45 a-Leu-17, Lymphocytes in thymic cortex, cells in germinal centres,
OKT10 plasma cells (immature lymphoid cells, plasma cells)
CDw75 p53? LN1, OKB4 B Cells in germinal centre, in corona (faint), macrophages,
epithelium
CD79a p33,40 mb-1 B Cells, Ig alpha chain
CD79b p33,40 B29 B Cells, Igß chain
p200 (HIS14) All B cells, including TdT+ precursors
p200 (HIS22) All B cells in corona, pre-B cells
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
B Cells (contd)
MHC class II (Various antibodies to polymorphic and B Lymphocytes, activated T cells, monocytes/macrophages,
non-polymorphic epitopes) interdigitating cells, Langerhans cells, epithelia, endothelia
B Cells (surface); in germinal center IgM+IgG+IgA+ and in
anti-immunoglobulin corona IgM+IgD+, plasma cells
(cytoplasmic)
Monocytes/macrophages, myeloid cells
CD13 p130-150 ER-BMDM-1 a-Leu-M7, Monocytes, granulocytes, dendritic reticulum cells
My7 (aminopeptidase N)
CD14 p55 ED9 UCH-M1, B-A8, Monocytes, some granulocytes and macrophages
a-Leu-M3
CD15 p170-190 a-Leu-M1 Granulocytes, some monocytes (lacto-N-fucose pentaosyl)
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes,
activated macrophages. IgG-FcRIII, low affinity, complexed IgG
CD33 p67 a-Leu-M9, (Precursor) granulocytes, macrophages, Langerhans cells.
My9 Myelin-associated protein
CD68 p110 Ki-M6,Ki-M7 Macrophages (specific)
p160 F4/80 Monocytes-macrophages
p32 Mac-2 Thioglycollate-elicited peritoneal macrophages
p92-110 Mac-3 Peritoneal macrophages
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Monocytes/macrophages, myeloid cells (contd)
4F7 Dendritic cells in skin, bone marrow
ED1 Monocytes/macrophages
ED2,HIS36 Subset of macrophages (F4/80-like)
ED3 Subset of macrophages, restricted, negative in thymus
MRC OX-41 Granulocytes, macrophages, dendritic cells
MRC OX-62 Dendritic cells (integrin-like)
(IF119) Dendritic cells
HIS48 Granulocytes
Mac-387 Macrophages
Natural killer cells
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages. IgG-FcRIII, low affinity, complexed IgG
CD56 p220/135 a-Leu-19, NK cells, monocytes, neuroectodermal cells NKH-1, isoform of
B-A19 neural cell adhesion molecule (NCAM)
CD57 p110 a-Leu-7, NK cells, subset of T cells, some B cells, some epithelial cells,
VC1.1 monocytes, neuroendocrine cells, NKH-1
a-asialo-GM1 NK cells, stromal components
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Natural killer cells (contd)
NK-1.1,2B4, 3.2.3 NK cells (NKR-P1 gene family)
3A4, 5E6
Follicular dendritic cells
ED5,ED6, Ki-M4,DRC-1 Follicular dendritic cells
MRC OX-2
Epithelial cells (thymus)
(Various anti-keratin antibodies) Epithelium
(ER-TR4),4F1 HIS38 TE-3,(MR3, Thymic cortex epithelium
MR6)
(ER-TR5),IVC4 (HIS39) TE-4,(MR19), Thymic subcapsular or medullary epithelium
RFD4
Complement receptors
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells, C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD21 gp140 B2,BL13 B Cells in germinal centres and mantles (faint), follicular
dendritic cells (C3d receptor, CR2, receptor for Epstein-Barr
virus
CD35 p220 To 5 Follicular dendritic cells, macrophages, B cells in corona
(faint), renal glomerular epithelium. C3bR, CR1
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
IgG-Fc receptors
CD16 p50-70 a-Leu-11 NK cells, subset of T cells, neutrophilic granulocytes, activated
macrophages; IgG-FcRIII, low affinity, complexed IgG
CD32 gp140 Ly-17 3E1,CIKM5 B Cells, myeloid cells, macrophages; IgGFcRII, low affinity,
complexed IgG
CD64 p75 32.2 Monocytes; IgG-FcRI, high affinity, monomeric IgG
ß1-Integrin (CD29-CD49) family
CD29 p130 9EG7 B-D15 Ubiquitous, not on erythrocytes; ß1 chain of all CD49 antigens
CD49a p200 TS2/7 Activated T cells, monocytes, smooth muscle cells. Very late
antigen-1 (VLA-1), ligand of collagen, laminin
CD49b p155 31H4,AK7, T Cells, B cells, thrombocytes, fibroblasts, endothelium.
P1E6 Very late antigen-2 (VLA-2), ligand of collagen I, II, III,
and IV, laminin
CD49c p145 11G5,P1B5 B Cells, renal glomeruli, basal membranes. Very late antigen-3
(VLA-3), ligand of collagen, laminin, fibronectin, and invasin
CD49d p150 R1-2, P12520, HP2/1,44H6, Thymocytes, lymphocytes, monocytes, NK cells, eosinophilic
MFR4.B MR?4 L25.3 granulocytes, erythroblasts. Very late antigen-4 (VLA-4),
ligand of VCAM-1, fibronectin
CD49e p160 MFR5, SAM-1 Monocytes, leukocytes, memory T cells, fibroblasts,
P12750 thrombocytes and muscle cells. Very late antigen-5 (VLA-5),
ligand of fibronectin
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
ß1-Integrin (CD29-CD49) family (contd)
CD49f p150 GoH3 Go-H3,4F10 T Cells, thymocytes, monocytes, thrombocytes. Very late
antigen-6 (VLA-6), ligand of laminin and invasin
ß2-Integrin (CD11-CD18) family
CD11a p180 Ly-15, WT.1 YTH-81.5, T and B cells, NK cells, erythroid and myeloid stem cells.
2D7, B-B15, Leukocyte function-associated antigen-1 (LFA-1) involved in cell
M17/4 G-25.2 adhesion, ligand for intercellular adhesion molecule (ICAM)-1
(CD54), ICAM-2 (CD102), ICAM-3 (CD50)
CD11b p160 Ly-40, MRC OX-41, Mac-1, Granulocytes, macrophages, CD5+ B cells. C3b1R, CR3
M1/70 MRC OX-42, a-Leu-15
WT.5
CD11c p150 a-Leu-M5, Monocytes, macrophages, granulocytes (faint), activated
S-HCL-3 lymphocytes. CR4
CD18 p95 YTS213.1, WT.3 BL5 All lymphocytes. ß-Chain of CD11 antigens
C71/16,
M18/2
p160-95 ED7,ED8 CD11-CD18 molecule
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others
Terminal deoxynucleotidyl transferase (TdT) Immature (lymphoid) cells in bone marrow and thymic cortex
(nuclear staining)
CD25 p55 Ly-43, AMT13, MRC OX-39 Tac,a-IL2-R Activated lymphocytes at scattered locations in thymus and
7D4,3C7 T-cell areas in peripheral lymphoid organs. Interleukin-2
receptor alpha chain
CD122 p75 5H4, CF1,Mik-ß2, NK cells, T cells, B cells, monocytes. Interleukin-2 receptor
TM-ß1 Mik-ß3 ß chain
CD26 p120 H194-112 MRC OX-61 134-2C2 (Activated) T cells. Dipeptidyl peptidase IV, in mouse T-cell
activation molecule (THAM)
CD30 p105 Ki-1,Ber-H2 Sporadic cells in thymic (cortex) and T cell areas in
peripheral lymphoid organs, some plasma cells. Activated
lymphocytes,Hodgkin cells
CD34 MY 10,8G12 Haematopoietic progenitor cells, capillary endothelium
QBEND/10 Human progenitor cell antigen (HPCA)
CD44 p65-85 Ly-24 MRC OX-49, a-Leu-44, Prothymocytes, T cells, small B cells. Lymphocyte homing
IM7 MRC OX-50 F10-44-2 receptor. Phagocytic glycoprotein-1 (PgP-1), HCAM
CD45 p180-210 Ly-5 MRC OX-1, T29/33 All leukocytes. Common leukocyte antigen
HIS41
Table 10 (cont'd)
CD Relative Mouse Rat Human Reactivity
mol. mass
Others (contd)
CD45R p190-220 B220 MRC OX-22, a-Leu-18, All B cells, subset of T cells. Common leukocyte antigen.
MRC OX-32, MB1,MT2 HIS24 restricted to strains of the RT7.2 allotype and labels
HIS24 all peripheral B cells except cells in marginal zone, pre-B
cells
CD45RA p205-220 14.8 MRC OX-33 HI100 B Cells, T cytotoxic-suppressor cells (faint), subset of
thymocytes.In humans, also CD4+ subset (naive-virgin
T-cells)
CD45RO p190-220 UCH-L1 T Cells in immature and memory stage. Common leukocyte
antigen
CD54 p90 KAT-1 1A29 84H10,B-C14 Endothelial cells, many activated cell types. Intercellular
3E2 HA58 adhesion molecule-1 (ICAM-1)
CD71 p95 YTA74.4,C2 MRC OX-26 B3/25 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs, stromal
cells. Transferrin receptor
Ki-67 Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs. Proliferation
antigen present in late G1, S, G2, and M phases
PCNA PCNA PCNA Proliferating cells in germinal centres, some cells in thymus
and T-cell areas in peripheral lymphoid organs
MEL14 Recirculating T and B cells. Lymphocyte homing receptor
MHC, major histocompatibility complex; NK, natural killer; Ig, immunoglobulin
CD nomenclature from: Clark & Lanier (1989); Knapp et al. (1989); Schlossman et al. (1994, 1995)
Antibodies within parentheses are not commercially available.
in serum is a function of the antibody's half-life, the longer the
study the more likely it is that an effect will be observed.
Immunoglobulin levels can be influenced by the cleanness of the
facility: studies conducted in facilities with excellent husbandry
will have lower basal levels than those conducted in 'dirty'
facilities where animals are constantly exposed to foreign antigens,
including pathogens.
Measurement of basal immunoglobulin levels is useful only after
subchronic or chronic exposure, i.e. with sufficient time for normal
metabolic elimination. Basal levels of immunoglobulin decrease only
when synthesis is reduced or prevented such that metabolized
immunoglobulins are not replaced. The parameter therefore yields
little information about possible mechanisms of immunotoxicity, and
should rather be regarded as a screening parameter; this is in fact
true for most non-functional tests. The IgM and G classes have usually
been measured; however, since the two other classes (A and E) are
biologically very important (for instance in mucosal immunity and
allergic manifestations), they should also be measured.
Total IgM and IgG concentrations in serum can be analysed by
means of a 'sandwich' enzyme linked immunosorbent assay (ELISA), as
described by Vos et al. (1982). Total IgA and IgE concentrations can
be analysed in an essentially similar way, except that the microtitre
plates are coated with monoclonal anti-rat IgA (Van Loveren et al.,
1988b) or monoclonal anti-rat IgE antibodies (MARE-1), respectively,
and immunoglobulins bound to these antibodies in serum samples are
detected by sheep anti-rat IgA or monoclonal anti-kappa chains of rat
immunoglobulins (MARK-1), conjugated with peroxidase.
Data from the ELISA are usually reported as percentages of
control values, and a titration curve based on pooled sera is
prepared; optimal dilutions of exposed and unexposed groups are then
plotted from this curve. The deviation of the dilution of the test
groups from the control groups is expressed, with the dilution in the
control group set at 100%. While studies in rats indicate that
measurement of basal immunoglobulin levels is useful in predicting the
immunotoxic effects of compounds, studies conducted in mice at the NTP
do not, and measurement of basal immunoglobulins is not included in
either tier of their testing panel (Luster et al., 1988). There are
several possible reasons for the difference in the usefulness of basal
immunoglobulin levels in rats and mice. First, in the studies of Vos
and colleagues, cited above, the exposure period was routinely longer
than the 14-day studies conducted within the NTP; since serum antibody
level is a function of the antibody half-life, longer studies are more
likely to detect an effect. Furthermore, the doses used at the NTP
were often lower, by design, than those used in rats. A final possible
explanation, which remains to be confirmed, is that immunoglobulin
synthesis in rats is more sensitive than that in mice.
4.1.4 Bone marrow
Bone marrow is an important haematopoietic organ and a source of
precursors for lymphocytes and other leukocytes. Changes in the bone
marrow are therefore likely to result in alterations of
immunocompetent cell populations, which may be long lasting or
permanent and thus serve as an indicator of potential immunotoxicity.
In a study to validate immunotoxicological parameters, bone-marrow
cellularity was shown to be an indicator of the immunotoxicity of
cyclosporin A, used as the model compound (Van Loveren et al., 1993a).
Determination of cellularity in stained slides of bone marrow,
evaluation of smears, and actual counts of the numbers of cells within
bone marrow are practical. For this purpose, both ends of a femur are
cut off, and bone-marrow cells are collected by flushing balanced salt
solution through the femur with a 21-gauge needle. The concentration
of nucleated cells is determined in a Coulter counter; a differential
count of cells can be done visually in May-Grunvald Giemsa-stained
cytospin preparations.
4.1.5 Enumeration of leukocytes in bronchoalveolar lavage fluid,
peritoneal cavity, and skin
Mononuclear phagocytes in the alveoli of the lung play an
important role in clearing inhaled particles, including
microorganisms, from the lung. The numbers of cells and alterations in
their function can be end-points of the toxicity of inhaled chemicals.
In order to study these parameters, methods for harvesting the cells
from the lungs should be easy to perform, guarantee the sterility of
the cell harvest, and be standardized. Methods involve use of either a
syringe (Blusse Van Oud Alblas & Van Furth, 1979) or a complex system
of syringes, tubes, and valves (Moolenbeek, 1982). These methods often
result in contamination of the harvested cell population; moreover,
they are laborious and cannot easily be standardized since the syringe
is operated manually. In a more recently developed method (Van
Soolingen et al., 1990), an excised lung is placed in a pressure
chamber and connected to a cannula through which lavage fluid can be
introduced into the lung and transferred from the lung into a test
tube. This procedure is repeated several times to obtain an optimal
yield.
Enumeration of mononuclear cells in the peritoneal cavity can
also best be performed by harvesting these cells by lavage. Because
of the architecture of this organ, three or four cycles of
intraperitoneal injections of lavage fluid, followed by gentle
massaging of the abdomen, and aspiration of the fluid with the syringe
that was also used for injection suffice.
Langerhans cells in the skin can be enumerated with
histopathological techniques. Frozen tissue sections are used, stained
with immunoperoxidase techniques including markers for MHC class II
antigens or specific markers, as indicated above. Morphometric
analysis may provide a quantitative basis for this type of evaluation.
4.1.6 Flow cytometric analysis
Evaluation of phenotypic markers has proved to be one of the most
sensitive indicators of immunotoxic compounds. The availability of
fluorescent activated cell sorter (FACS) analysis units and
fluorescent cell counter units in immunotoxicology laboratories has
made analysis of cell populations routine. Determination of the
phenotype of lymphoid cells is a non-functional assay, although it has
often been inappropriately grouped with functional tests. The presence
or absence of a particular marker on the surface of a cell does not
reveal the functional capability of the cell. The usefulness of
surface marker analysis for predicting potential immunotoxicity has
been demonstrated. In studies conducted by Luster et al. (1992), a 91%
concordance was found for correct identification of immunotoxic
compounds on the basis of studies of surface markers alone.
As indicated above (section 4.1.2), numerous markers are
expressed on the cells of the immune system. Essentially, the same
reagents as used on tissue sections are applied on cells that have
been isolated from tissues, body fluids, or lavage fluids in
suspension (see above). Furthermore, both polyclonal and monoclonal
antibodies are available for detecting these surface markers. While
many of the markers have been used in immunological investigations,
very few have been evaluated with a large number of immunosuppressive
compounds. The markers that have been routinely used in studies of
immunotoxicity conducted for the NTP in mice and the cell types they
identify are shown in Table 11. The CD4:CD8 ratio in spleen has been
shown to concord best with the immunotoxicity of these surface markers
(Luster et al., 1992).
Table 11. Phenotypic markers on lymphocyte subpopulations used in
studies of immunotoxicity by the United States National
Toxicology Program
Surface marker Cell type
sIg+ Pan B cells
Thy 1.2+ or CD3+ Pan T cells
CD4+CD8- T Helper/delayed-type
hypersensitivity cells
CD8+CD4- T Suppressor-cytotoxic cells
CD8+CD4+ Immature T cells
The identification of phenotypic markers in rats has not
developed as rapidly as in mice; however, antibodies to rat cell
surface markers are now becoming available commercially and are being
used in immunotoxicological assessments (Smialowicz et al., 1990). The
monoclonal antibodies currently used for this purpose are: OX4 or
MARK-1 for B cells, W3/13 or OX 19 for T cells, R79 for the T cell
receptor, W3/25 for CD4 cells, and OX 8 for CD8 cells.
In enumerating the cell types in lymphoid tissue, both
percentages and absolute cell numbers should be reported. Of the two,
absolute cell numbers are by far the most meaningful. Compounds that
affect all populations equally and thus do not change the relative
percentages of the various cell types may be missed if only
percentages are evaluated. In addition, significant differences in the
magnitude of an effect on one or more of the populations can be
observed when the data are evaluated as absolute numbers and not as
percentages. As indicated above, the absolute changes more closely
reflect the events occurring in the animal and should thus be given
priority in interpreting data.
FACS analysis is also being used to determine the activation
state of various cell types, on the basis of changes in detectable
activation markers. Some of the activation markers that have been
studied are F4/80 (Austyn & Gordon, 1981), Mac-1 (Springer et al.,
1979), Mac-2 (Ho & Springer, 1984), transferrin receptor (Neckers &
Cossman, 1983), and IL-2 receptor (Cantrell et al., 1988). While
activation markers are of value in studying the mechanism of action of
compounds, their usefulness as predictors of immunotoxicity has yet to
be firmly established.
4.2 Functional tests
4.2.1 Macrophage activity
Phagocytic activity is the first line of defence against many
pathogens. Macrophages can phagocytose many particles, including
bacteria, and can lyse and inactivate them. Alterations in phagocytic
activity are therefore important potentially adverse effects of
chemicals on the immune system. The capacity to ingest particles
in vitro can be measured, and activity in vivo can be measured by
determining the clearance of bacteria, such as L. monocytogenes.
This test is dealt with in section 4.2.10.1.
Several assays have been developed for evaluating various types
of phagocytosis in mice and can also be used in rats, with slight
modifications. Innate and non-immune-mediated phagocytosis by
macrophages can be evaluated by determining the uptake of fluorescent
latex covaspheres (Duke et al., 1985). Macrophages and peritoneal
exudate cells are placed on a tissue culture slide and incubated with
the covaspheres for 24h on a rocking platform. The slides are then
fixed with methanol. The slide chambers are evaluated under a
fluorescent microscope, and macrophages with more than five latex
covaspheres are counted as positive for phagocytosis. The results are
expressed as percentage of phagocytosis, which is calculated as the
ratio of macrophages positive for phagocytosis to total macrophages
counted. In order to distinguish phagocytosed latex covaspheres from
those that are merely associated with the macrophage surface, the
cells are exposed for 30-60s to methylene chloride vapour. By
immersing the slides in this manner, the covaspheres that have not
been phagocytosed are dissolved, while those inside the macrophage
remain intact (Burleson et al., 1987). Phagocytosed fluorescent latex
particles can easily be quantified under the fluorescence microscope.
While this assay is straightforward, it is labour intensive, and
reading the slides, shifting back and forth from the fluorescent
field, and counting the macrophages is time-consuming.
A radioisotopic procedure, the chicken erythrocyte assay, can be
used to evaluate both adherence to and phagocytosis of particles by
macrophages. The phagocytic capacity is measured as an immunologically
mediated (Fc receptor) response. Macrophages are added to each well of
a 24-well tissue dish and allowed to adhere for a 2-3-h incubation
period. Nonadherent cells are washed, and chicken erythrocytes
labelled with 51Cr are added to each well; then a subagglutinating
dilution of antisera to chicken erythrocytes is added to each well and
the plate incubated for 1h. The plates are then washed to remove
unbound erythrocytes; an ammonium chloride solution is added to lyse
adhered erythrocytes, and the supernatant is collected and counted to
determine adherence of the erythrocytes to the macrophages. Next, both
the macrophages and the phagocytosed chicken erythrocytes are lysed by
addition of 0.1 N sodium hydroxide, and the solution is counted to
determine the amount of phagocytosis. Three to six wells in each group
do not receive 51Cr and are used to evaluate the DNA content
(Labarca & Paigen, 1980). The data are expressed as adherence counts
per minute, phagocytosed counts per minute, and specific activity for
adherence and phagocytosis. Specific activity is determined by
dividing the number of adhered or phagocytosed counts per minute by
the DNA content per well. The data must be expressed in terms of
specific activity, since compounds that affect the macrophages'
ability to adhere to the 24-well culture dish will significantly alter
the results obtained.
While both the nonspecific and immune-mediated phagocytosis
assays are useful for understanding the potential mechanisms of action
of compounds, changes in phagocytic activity in these in-vitro assays
have not been found to be predictive of immunotoxicity. For example, a
single intratracheal exposure to gallium arsenide resulted in
increased adherence and phagocytosis by chicken erythrocytes but
decreased phagocytosis of latex covaspheres (Sikorski et al., 1989).
The phagocytosis assay that is most predictive of altered
macrophage function is evaluation of the functional ability of the
mononuclear phagocyte system. This is a holistic assay for measuring
the capacity of the fixed macrophages of the mononuclear phagocyte
system, where macrophages provide the first line of defence against
both pathogenic and non-pathogenic blood-borne particles. The fixed
macrophages of the mononuclear phagocyte system line the liver
endothelium (Kupffer cells), the spleen, the lymph nodes (reticular
cells), the lung (interstitial macrophages), and other organs such as
the thymus and bone marrow. When the assay is conducted in mice, the
animals are injected intravenously with 51Cr-labelled sheep
erythrocytes, and a 5-µl blood sample is taken from the clipped tail
at 3-min intervals over a 15-min period. A final 30-min blood sample
is taken, and 1h after injection the animals are sacrificed and the
liver, spleen, lungs, thymus, and kidneys are removed, weighed, and
counted in a gamma counter. The 60-min time interval after injection
of sheep erythrocytes was selected as the time of sacrifice since it
represents the plateau for particle uptake by the selected organs
(White et al., 1985). Blood clearance of the radiolabelled cells is
expressed as vascular half-life and as a phagocytic index, which is
determined by the slope of the clearance curve. Organ distribution is
expressed as percent organ uptake and counts per minute per milligram
of tissue (specific activity). The assay can detect both stimulation
and inhibition of the mononuclear phagocyte system. Bick et al. (1984)
reported marked stimulation of the mononuclear phagocyte system after
treatment with diethylstilbestrol; more recently, morphine sulfate was
shown to decrease vascular clearance and hepatic and splenic
phagocytosis significantly (LeVier et al., 1993).
4.2.2 Natural killer activity
NK activity against neoplastic and virus-infected targets has
been clearly demonstrated in vitro and is thought to play an
important role in vivo in providing surveillance against neoplastic
cells and as a first line of defence against viruses (Herberman &
Ortaldo, 1981). In humans, rats, and mice, most cells with NK activity
can be identified by morphological (although the definition is not
morphological) and functional characteristics (Timonen et al., 1981).
Most of the cells that show NK activity are nonadherent, non-
phagocytic lymphocytes and are morphologically associated with large
granular lymphocytes (Timonen et al., 1982). Although cells with NK
activity do not strictly belong to the T-cell lineage, they can
express T cell-associated markers and express surface receptors, such
as those for the Fc portion of IgG and the ganglioside asialo GM1.
Some of these markers are also expressed by monocytes, macrophages,
and polymorphonuclear leukocytes (Herberman & Ortaldo, 1981). Within
4h, the cells can show nonantigen-specific cytotoxic activity
in vitro and in vivo against certain (NK-sensitive) tumour cell
lines and virus-infected cells.
The cells have enhanced cytolytic function after activation with
a variety of stimuli, including viral infection (Stein-Streinlein et
al., 1983), BCG (Tracey et al., 1977), IL-2 (Henney et al., 1981;
Domzig et al., 1983; Lanier et al., 1985; Malkovsky et al., 1987),
interferon, and interferon inducers (polyI:C) (Tracey et al., 1977;
Oehler & Herberman, 1978; Djeu et al., 1979a,b). NK activity in vitro
can be stimulated with IL-2 and interferon (Tracey et al., 1977; Djeu
et al., 1979b). Anti-asialo GM1 antibody can strongly inhibit
cytotoxic NK activity both in vitro and in vivo (Kasai et al.,
1980, 1981; Yosioka et al., 1986). This antibody binds to the cell
surface glycolipid GM1 and suppresses the lytic activity of effector
cells. Large granular lymphocytes are found in several lymphoid
organs. Many cells with high NK activity are found in spleen and
peripheral blood (Rolstad et al., 1986); lymph nodes have less NK
activity, and thymus and bone marrow show only marginal activity. NK
activity can also be demonstrated in the bronchus-associated lymphoid
tissue in the lungs. Moreover, large granular lymphocytes can migrate
from the circulation into the extravascular tissue and can even be in
contact with the lumen of the alveoli (Timonen et al., 1982; Reynolds
et al., 1984; Rolstad et al., 1986; Prichard et al., 1987). The
presence of large granular lymphocytes associated with NK activity in
the lungs is probably of great importance, because the lungs
constitute a major site for neoplastic disease (metastatic spread) and
viral infections. NK cells may also operate in certain types of
bacterial infections. In experimental animals, suppression of NK cell
activity increased the numbers of metastases after transplantation of
tumours.
The clinical significance of altered NK cell activity in humans
has not clearly been established. Asymptomatic individuals with low NK
cell responses may be at some risk for developing upper respiratory
infections and for increased morbidity (Levy et al., 1991); and
extreme susceptibility to severe and repeated herpes virus infection
was reported in an individual without NK cells (Biron et al., 1989).
It is obvious therefore that exposure to toxic substances that alter
NK activity can have biological consequences, and testing this
activity is important in assessing potential immunotoxicity.
The procedure for determining NK activity is as follows: cell
populations that exert NK activity (usually enriched peripheral blood
mononuclear cells or spleen cells) are cultured with NK-sensitive
target cells. A cell type frequently used for this purpose is the YAC
lymphoma cell line, which has been applied to mice, rats, humans, and
even seals. YAC lymphoma target cells are radiolabelled with 51 Cr,
and lysis of the cells, resulting in release of chromium, within 4h is
used to estimate the cytolytic activity of the NK cells within the
cell population. This assay has been used to demonstrate the effects
of numerous compounds on NK activity in rats (e.g. TBTO, ozone, and
HCB: Vos et al., 1984; Van Loveren et al., 1990c), mice (Luster et
al., 1992), and harbour seals (Ross et al., in press).
4.2.3 Antigen-specific antibody responses
Most antibody responses require not only B cells, which, after
maturation into plasma cells, produce antibodies, but also the help of
T lymphocytes. A variety of T cell-dependent antigens can be used for
this purpose, and an excellent one is tetanus toxoid. A typical
immunization schedule in rats comprises intravenous immunization on
day 0 followed by a booster on day 10. Primary and secondary IgG and
IgM responses can then be measured in serum, taken on day 10 (just
before the booster) and day 21, respectively. The primary IgM response
is the immunoglobulin response that is least under the control of T
cells. As tetanus toxoid is also used for human immunization, the
responses to this antigen may be useful in extrapolating experimental
data to humans. The responses can be determined in an ELISA (Vos et
al., 1979b).
Another widely used T cell-dependent antigen is ovalbumin. This
antigen can be and has been used to induce all classes of antibody
responses, i.e. IgM, IgG, IgA, and IgE, that can be measured with the
ELISA (Vos et al., 1980; Van Loveren et al., 1988b). The classical
assay of specific IgE responses is the passive cutaneous anaphylaxis
reaction. Serial dilutions are injected into the skin of rats,
sensitizing local mast cells; the specific antigen is then injected
intravenously, simultaneously with Evans blue. Mast cell products are
released where IgE meets the antigen, and IgE is cross-linked on the
membranes of the mast cells, leading to extravasation of Evans blue.
The titre can be determined from the magnitude of the reaction at each
dilution of IgE. ELISA techniques and the specific reagents to detect
IgE in an ELISA that are now available make this test preferable.
Ovalbumin induces not only humoral responses but also delayed-
type hypersensitivity. Sensitization to ovalbumin in Freund's complete
adjuvant enhances responses and makes it possibile to assay both
responses in one animal. Delayed-type hypersensitivity can also be
directed to purified protein derivative, with responses induced by the
adjuvant (Vos et al., 1980). At least in mice, however, immunization
in complete adjuvant skews responses in the direction of Th1
responses, i.e. delayed hypersensitivity, and hence suppresses Th2-,
IgE-, and IgA-dependent immune responses.
A few antigens can induce humoral immune responses without
involvement of T lymphocytes. One example is trinitrophenol-Ficoll
(lipopolysaccharide). Sensitization of animals to this antigen yields
immunoglobulin responses that can be measured in an ELISA. This is a
useful test for use in mechanistic studies to separate the effects of
compounds on B and T cells.
4.2.4 Antibody responses to sheep red blood cells
4.2.4.1 Spleen immunoglobulin M and immunoglobulin G plaque-forming
cell assay to the T-dependent antigen, sheep red blood cells
A widely used particulate T cell-dependent antigen is sheep red
blood cells. Antibody titres induced after sensitization can be
assayed with various techniques; one that is widely used is the
plaque-forming cell assay, or antibody-forming cell response. This
assay is relatively simple and can be conducted with inexpensive
equipment found in most laboratories, but the optimal concentration of
sheep red blood cells must be injected. As the antigenicity of red
blood cells varies significantly from sheep to sheep, time must be
invested to obtain cells from a sheep that repeatedly gives a high
response (>= 1500 plaque-forming cells/106 spleen cells). The
number of cells administered (about 2 × 108) should also be
optimized for both mice and rats in the laboratory conducting the
assay. The intravenous route is that preferred for sensitization;
intraperitoneal injections can be used but significantly increase the
potential for nonresponding animals as a result of a poor injection.
Animals are sacrificed on day 4 after injection, and spleen cells are
prepared by mincing the spleen between two frosted microscope slides,
teasing it apart with forceps, or passing it through a small mesh
screen; all of these methods are satisfactory, and that used to
prepare single splenocyte cultures varies from laboratory to
laboratory. An aliquot of cells is added to sheep erythrocytes and
guinea-pig complement; these are placed in a microscope slide chamber
when the Cunningham assay method is used (Cunningham & Szenberg,
1968), or, in the Jerne method, cells and guinea-pig complement are
added to a test tube containing warm agar and after thorough mixing
the test tube mixture is plated in a petri dish and covered with a
microscope cover slip (Jerne et al., 1974). In either case, the
preparations are then incubated at 37°C for 3-4h to allow plaques to
develop. The plaques are counted under a Bellco plaque viewer. A
plaque results from the lysis of sheep erythrocytes and is elicited as
a result of the interaction of complement and antibodies directed
against sheep erythrocytes, which are produced in response to the
intravenous sensitization. As each plaque is generated from a single
IgM antibody-producing plasma cell, the number of IgM plaque-forming
cells present in the whole spleen can be calculated. The data are
expressed as specific activity (IgM plaque-forming cells/106 spleen
cells) and IgM plaque-forming cells per spleen.
By incorporating rabbit anti-mouse or anti-rat IgG antibody into
the preparation of spleen cells, complement, and sheep red blood
cells, the number of IgG antibody-forming cells present in the spleen
can also be determined. This number is calculated by subtracting the
number of IgM plaque-forming cells from the total number of both IgM
and IgG plaque-forming cells. The optimal IgG primary response is
observed five days after sensitization (Sikorski et al., 1989).
The T-dependent IgM response to sheep red blood cells is one of
the most sensitive immunotoxicological assays currently in use. Luster
et al. (1992) reported that the individual concordance of the plaque-
forming cell assay for predicting immunotoxicity was the highest of
all the functional assays (78%). Furthermore, use of this assay in
combination with either NK cell activity or surface marker analysis
resulted in pairwise concordances for predictability of more than 90%.
While the plaque-forming cell assay has been shown to be
sensitive and predictive, the procedure does have its limitations. As
indicated earlier, the effect of the test compound on the immune
system is evaluated only in spleen cells, and effects on other
antibody-producing organs and tissues are not determined. The assay is
somewhat laborious, and it is preferable that several people
participate, to help in removing spleens, preparing cell preparations,
counting cells, and adding preparations to either microscope slide
chambers or agar dishes. An additional drawback is that the assay must
be conducted on the same day as the animals are sacrificed. This is in
marked contrast to the ELISA, in which sera can be frozen and
evaluated at a later date. While the slides and petri dishes can be
placed in a cold room or refrigerator and counted the next day, this
procedure is not recommended, as they tend to dry out to some extent,
making viewing and discerning plaques more difficult.
4.2.4.2 Enzyme-linked immunosorbent assay of anti-sheep red blood
cell antibodies of classes M, G, and A in rats
An alternative to the plaque-forming cell assay is ELISA of anti-
sheep red blood cell antibody titres in serum. Antigen preparations
made from ghosts of sheep erythrocytes by extraction with potassium
chloride are used to coat the bottoms of the wells of 96-well
microtitre plates. Serum samples from rats immunized with sheep
erythrocytes are titrated onto these plates using specific polyclonal
antibodies to rat IgM or IgG, to which peroxidase is conjugated. IgA
has also been assayed, using monoclonal anti-rat IgA antibodies and
polyclonal rat anti-mouse IgG conjugated with peroxidase. The ELISA of
serum titres of IgM, IgG, and IgA to sheep erythrocytes is an easy,
reliable method that can be used to detect the effects of chemicals on
the immune system of the rat (Van Loveren et al., 1991; Ladics et al.,
1995).
The assay measures titres of specific antibodies, in contrast to
the plaque-forming cell assay which determines the number of cells
that are actually responsible for production. The ELISA assesses the
production of antibodies, either per cell or in terms of the total
capacity of the host to produce these antibodies in vivo. In
interpreting the effects of exposure to chemicals, account must be
taken of the fact that the cells used in the assay are derived from
specialized parts of the body, such as the spleen, and alterations in
the numbers of antibody-producing cells in such an organ in rats
immunized with sheep red blood cells cannot give information on other,
inaccessible pools of antibody-producing cells. In the ELISA,
alterations in titres due to exposure to chemicals indicate changes in
the immune potential of the exposed animals. In screening for the
effects of chemicals on the immune system, therefore, ELISAs may be
preferable, but for studies on specific immunosuppressive mechanisms,
the plaque-forming cell assay, although labour- and time-intensive, is
a powerful tool for obtaining information complementary to the data
provided by the ELISA. Unfortunately, it is not always possible to
perform the two assays with material from the same animal. The peak
response in the plaque-forming cell assay in both rats (Fischer 344)
and mice (B6C3F1) occurs on day 4 after sensitization, while the peak
response in the ELISA occurs on day 6 for rats and day 4-5 for mice
(Temple et al., 1993). In order to detect the effects of chemicals on
the immune response to sheep red blood cells, it is preferable to
choose the optimal conditions, or to follow the kinetics, of the
response.
4.2.5 Responsiveness to B-cell mitogens
Responsiveness to lipopolysaccharide is another estimate of
humoral immune response, as solely B cells respond to this mitogen.
Although the responses of rats to this mitogen are less pronounced
than those of mice, good results can be obtained, and the
immunosuppressive effects of chemicals can be detected (Vos et al.,
1984).
An alternative B-cell mitogen is S. typhimurium mitogen (STM),
a water-soluble, proteinaceous extract derived from the cell walls of
S. typhimurium; it is a more potent mitogen for rat B lymphocytes
than lipopolysaccharide (Minchin et al., 1990). In both mice and rats,
the polyclonal activation of B lymphocytes is a multistep process. In
mice, mitogens alone can provide all the signals necessary for
proliferation and differentiation; in the rat, STM stimulation induces
B lymphocytes to proliferate without differentiating. The addition of
lymphokines to STM-stimulated B cells also failed to stimulate them to
differentiate (Stunz & Feldbush, 1986). Nevertheless, this mitogen is
useful for evaluating effects on the proliferative ability of rat B
lymphocytes. Smialowicz et al. (1991) showed a decrease in the STM
response in Fischer 344 rats after oral exposure to 2-methoxyethanol.
Unlike the bell-shaped mitogen dose-response curves observed with
T-cell mitogens, the proliferative response of B lymphocytes to both
lipopolysaccharide and STM rises quickly at low concentrations of the
mitogens and plateaus at higher concentrations. As a result, a single
concentration on the plateau phase of the mitogen response curve is
sufficient to evaluate the effects of a test compound on B-cell
mitogen-driven proliferation. One of the reasons that the mitogen
assays appear to be insensitive is that the cells must remain in
culture for several days in order to obtain a peak response. As a
result, they may recover from the immunomodulatory effects of the test
compounds during this in-vitro phase. This is a common problem with
many ex-vivo/in-vitro assays, including the cytotoxic T lymphocyte and
mixed leukocyte response assays; because of the short, 4-h period of
the NK cell assay, this is less of a concern.
4.2.6 Responsiveness to T-cell mitogens
The proliferative ability of T lymphocytes after stimulation with
mitogens can be measured by the uptake of 3H-thymidine in a manner
similar to that used to measure B-cell proliferation (Anderson et al.,
1972). Concanavalin A and phytohaemagglutinin are T-cell mitogens in
both rats and mice; pokeweed mitogen stimulates the proliferation of
both T and B cells and thus lacks specificity. Although both
concanavalin A and phytohaemagglutinin stimulate T lymphocytes,
T cells responsive to concanavalin A have been reported to be less
mature than those responsive to phytohaemagglutinin (Stobo & Paul,
1973). Multiple concentrations of these mitogens should be used to
ensure that a peak response is obtained: both produce a bell-shaped
dose-response curve, and too high a concentration can result in a
suboptimal response.
Historically, mitogens have been included in the battery of tests
for evaluating potential immunotoxicity, because the assay is one that
can also be carried out in humans. Human studies, however, are
conducted on peripheral blood, while most studies of rodent lymphocyte
transformation are conducted using spleen or lymph node cells. Thus,
the argument that the assay has clinical relevance is not well
founded. Furthermore, as the response of lymphocytes is extremely
robust, the assay lacks sensitivity. After a significant number of
compounds were evaluated for potential immunotoxicity in mitogen
assays, use of this assay was shifted from the tier 1 screen
originally described by Luster et al. (1988) to the tier 2
comprehensive evaluation. Use of the mitogen assay has now been
removed completely from studies conducted for the NTP, since other
assays in which cellular proliferation is required (e.g. plaque-
forming cell assay, mixed leukocyte reaction) were considered to be
more sensitive, and the data obtained from the mitogen assays add
little if any to an evaluation of the potential immunotoxicity of test
compounds.
4.2.7 Mixed lymphocyte reaction
In the mixed lymphocyte reaction (also known as mixed lymphocyte
culture), suspensions of responder T lymphocytes from spleen or lymph
nodes are co-cultured with allogeneic stimulator cells. The foreign
histocompatibility antigen (MHC class I or class II molecules)
expressed on the allogeneic stimulator cells serves as the activating
stimulus for inbred populations. In noninbred populations, a pool of
allogeneic cells can be used as stimulators. The assay analyses the
ability of T cells to recognize allogenic cells as 'non-self' as a
result of the presence of different MHC class II antigens on their
surface. In response to the class II antigens, the spleen or node
cells proliferate. Because a sufficiently large number of T cells in
the mixed lymphocyte population respond to the stimulator population,
the responder T cells need not be primed. Proliferation of the
responder cells is one of the parameters for T-cell responsiveness to
cellular antigens. If the allogeneic stimulator cell suspension
contains T cells, their uptake of 3H-thymidine must be prevented by
gamma-irradiation or mitomycin C, in order to preclude background
thymidine uptake.
4.2.8 Cytotoxic T lymphocyte assay
The Tc lymphocyte assay is a continuation of the mixed lymphocyte
reaction response in which the T lymphocytes further differentiate
into cytotoxic effector cells under the influence of various
cytokines. In mice, the assay is usually conducted using P815
mastocytoma cells as the sensitizer and target cell (Murray et al.,
1985). Mice are exposed in vivo to the test agent, and spleen cells
are then removed and placed in culture flasks with the P815
mastocytoma cells. After a five-day co-culture period, the spleen
cells are harvested and added to fresh P815 mastocytoma cells which
have been radiolabelled with 51Cr as sodium chromate. After a 4-h
incubation, the percentage cytotoxicity is determined by measuring the
specific release of 51Cr into the supernatant. The five days of
culture are necessary for the T lymphocytes to differentiate into
cytotoxic effector cells. Unfortunately, this extended period in
culture may give the spleen cells sufficient time to recover from any
adverse effects of the test compound, although such effects may have
been present at the time the spleen cells were removed from the
animal. This inherent limitation of the assay detracts from its
usefulness in assessing the immunotoxicity of test compounds.
A holistic Tc lymphocyte assay has been described, in which the
animal is sensitized after injection of the irradiated target cells
(Devens et al., 1985). Inhibiting the ability of the sensitizing cells
to proliferate either through irradiation or mitomycin C treatment
before injection prevents development of Tc lymphocytes in the animal.
Smialowicz et al. (1989) developed an assay in rats in which effector
cells are generated in culture by incubating cells with lymph node
cells from Wistar/Furth rats, and 51Cr-labelled W/Fu-G1 tumour cells
are used as the target cells. The assay requires four days in culture
and can be run simultaneously with the rat mixed lymphocyte reaction,
thus providing information on the test compound's ability to affect
proliferation and differentiation into effector cells.
4.2.9 Delayed-type hypersensitivity responses
Delayed-type hypersensitivity responsiveness is a reflection of
the capacity of the cellular immune system to execute immune responses
and especially those dependent on IL-2 and INF gamma, which include
attraction and activation of nonspecific mononuclear leukocytes
(macrophages-monocytes). Many systems can be used, depending on the
antigen. One is sensitization to BCG, followed by challenge with
purified protein derivative, to which sensitivity is induced. Another
example is ovalbumin, to which sensitization is most efficient if the
ovalbumin is emulsified in complete Freund's adjuvant. In this system,
delayed hypersensitivity can be measured to both purified protein
derivative and ovalbumin (Vos et al., 1980). Another antigen is
L. monocytogenes: This system is particularly interesting since it
can be used in the context of experiments in which host resistance to
this pathogen is also measured (Van Loveren et al., 1988a).
Delayed hypersensitivity responses can be measured after
sensitization to Listeria by subcutaneous injection of the test
antigen into the ears. Prior to and 24 and/or 48h after challenge, the
increment in ear thickness can be measured with a micrometer by a
person unaware of the experimental group. The background ear swelling
responses of similar, unimmunized control animals are subtracted from
the swelling responses found in immunized animals.
Several delayed-type hypersensitivity assays have been developed
and used for evaluating immunotoxicity in the mouse. Most have
involved measuring swelling in either the footpad or the ear after
sensitization and challenge with a protein antigen. Studies by
LaGrange et al. (1974) demonstrated that sheep erythrocytes could
elicit a delayed-type hypersensitivity response after a single
injection into the foot pad; however, more sheep erythrocytes were
needed to elicit the delayed-type hypersensitivity response than to
produce the optimal humoral immune response. Foot pad swelling can be
measured with a micrometer, as described for rats or by a more
objective, isotopic procedure, as described by Paranjpe & Boone (1972)
and Munson et al. (1982). The delayed-type hypersensitivity response
to sheep erythrocytes was previously considered to be a good assay for
detecting effects on cell-mediated immunity; however, the lack of
persistence of the response (LaGrange et al., 1974; Askenase et al.,
1977) and the possible contribution of antibody to the response raised
concern about the specificity of the assay when sheep erythrocytes are
used as the eliciting antigen. Benzo[ a]pyrene, a compound that
selectively affects humoral but not cell-mediated immunity in adult
mice, appears to decrease cell-mediated immunity when measured in the
sheep erythrocyte assay but has no effect on delayed-type
hypersensitivity when evaluated in the keyhole limpet haemocyanin
assay. The effect in the sheep erythrocyte assay is observed at doses
of benzo[ a]pyrene that decrease antibody production, suggesting a
significant antibody component of the swelling observed (White, 1992).
Keyhole limpet haemocyanin is another protein antigen used in
evaluating delayed-type hypersensitivity responses. Holsapple et al.
(1984) characterized the response to this antigen in the mouse,
showing that it produced the classical delayed-type hypersensitivity
response both with and without adjuvant. Two immunizations with
keyhole limpet haemocyanin were required, however, to produce a
response equivalent to one obtained with complete Freund's adjuvant.
In these studies, animals were sensitized with subcutaneous injections
of keyhole limpet haemocyanin in the shoulder area, with seven days
between the sensitizations. They were then challenged with the same
antigen injected intradermally into the central portion of the pinna
of one of the ears. Increases in ear thickness were evaluated by both
micrometer readings and radioisotopically. The unchallenged ear was
used as an individual control for each animal, and a group of
unsensitized but challenged animals was used to control for
nonspecific and background effects. The results indicated that,
whenever possible, the use of adjuvant in delayed-type
hypersensitivity studies should be avoided. Despite the fact that
complete Freund's adjuvant boosted the responses to keyhole limpet
haemocyanin, it partially masked the dexamethasone-induced suppression
of the response. In some cases, however, delayed-type hypersensitivity
responses are difficult to induce without adjuvant.
The studies currently conducted in mice and rats with this assay
are holistic assays for evaluating cell-mediated immunity. Since
sensitization and challenge occur in the intact animal, all components
of the immune system are present to respond in a physiologically
relevant manner. This type of assay is much more valuable for
evaluating the effects of compounds on cell-mediated immunity than are
in-vitro assays such as the mixed leukocyte response or Tc cell assay.
Luster et al. (1992) reported that the delayed-type hypersensitivity
response assay in mice was highly predictive (100% concordance) of
immunotoxicity when used in combination with the NK cell assay and the
plaque-forming cell assay.
4.2.10 Host resistance models
4.2.10.1 Listeria monocytogenes
Relevant mechanisms of defence against L. monocytogenes include
phagocytosis by macrophages and T cell-dependent lymphokine production
which enhances phagocytosis (Mackaness, 1969; McGregor et al., 1973;
Takeya et al., 1977; Pennington, 1985; Van Loveren et al., 1987).
Humoral immunity is not relevant in protection against infection in
this model. Clearance of Listeria after infection by, for instance,
the intravenous or the intratracheal route can be assessed at various
times after infection by determining the numbers of colony forming
units in the spleen or lungs, respectively. This can be done by
classical methods (Reynolds & Thomson, 1973) that involve the
following steps: Serial dilutions of homogenates of the organs,
prepared in mortars with sterile sea sand, are plated onto sheep blood
agar plates; after a 24-h incubation at 37°C, the colonies are counted
to determine the number of viable bacteria in the organ. Differences
in the numbers of bacteria retrieved from the organs are an indication
of the clearance of the bacteria, i.e. the rate at which the host
disposes of the bacteria after infection.
Histopathology after a Listeria infection can also be valuable.
For instance, exposure to ozone before an intratracheal infection with
Listeria affects pathological lesions due to the infection (Van
Loveren et al., 1988a): Pulmonary infection with Listeria induces
histopathological lesions characterized by foci of inflammatory cells,
such as lymphoid and histiocytic cells, accompanied by local cell
degeneration and influx of granulocytes. If rats are exposed to ozone
for one week before infection, the lesions are much more severe than
in unexposed animals and persist at times when either ozone-associated
or infection-associated effects alone would have resolved. The quality
of the lesions is also influenced by prior exposure to ozone: mature
granulomas were found in Listeria-infected rats that were also
exposed to ozone.
When mice are challenged with Listeria, mortality is the usual
end-point monitored; however, clearance and organ bacterial colony
counts can also be determined. L. monocytogenes is a Gram-positive
bacterium. The resistance of mice to the organism is genetically
regulated, and the susceptibility of the B6C3F1 strain, the strain
designated by the NTP for immunotoxicity studies, comes from the C3H
parent, since the C57Bl/6 mouse is resistant (Kongshavn et al., 1980).
Listeria can easily be stored at -70°C at a stock concentration of
approximately 108 colony forming units per ml. In studies in mice,
three challenge levels are routinely selected to produce 20, 50, and
80% mortality in the vehicle control animals. Mortality is recorded
daily for 14 days. Treatment groups consisting of 12 mice per group
have been found to be useful for obtaining statistically meaningful
data on host resistance. This assay is extremely reproducible when the
organism is administered intravenously.
The Listeria assay can detect both protection from and
increased susceptibility to chemicals and drugs. Morahan et al. (1979)
used the model to demonstrate a dose-dependent decrease in host
resistance after exposure to delta-9-tetrahydrocannabinol, the major
psychoactive constituent of marijuana. The Listeria host resistance
assay is that most often used in immunotoxicological assessment of
compounds, and numerous examples of its use can be found in the
literature. It is one of the primary models used by the NTP for
evaluating immunosuppression. Since Listeria is a human pathogen,
appropriate precautions are needed in conducting the assay.
4.2.10.2 Streptococcus infectivity models
Two species of Streptococcus have been used widely in bacterial
host resistance models for immunotoxicological assessment.
S. pneumoniae has been used primarily for evaluating systemic
immunity. S. zooepidemicus has also been used to evaluate systemic
immunity but is used extensively to evaluate the effects of drugs and
chemicals on the local immunity of the pulmonary system.
S. pneumoniae is a Gram-positive coccus to which host
resistance is multifaceted (Winkelstein, 1981). The first line of
defence against this organism is the complement system. Activation of
the complement system can result in direct lysis of certain strains of
S. pneumoniae; however, owing to the nature of their cell wall, some
strains are resistant to lysis by complement. Complement can still
participate directly in the removal of these bacteria as a result of
deposition of complement component C3 on their surface, which
facilitates phagocytosis by polymorphonuclear leukocytes and
macrophages. In the later stages of the infection, antigen-specific
antibody plays a major role in controlling the infection. Thus,
compounds that affect complement, polymorphonuclear leukocytes, B-cell
maturation and proliferation, or the production of antibody can be
evaluated in this system. S. pneumoniae is an excellent model for
evaluating immunotoxicity, since it elicits multiple immune components
which participate in host resistance, each of which can be a potential
target for an adverse effect of a xenobiotic. To date, this model has
had limited success in rats.
Preparation of S. pneumoniaefor challenge is slightly more
complicated than the procedures used for Listeria; however, the
potential of the model for detecting immunotoxic compounds makes the
additional steps worthwhile. Stock preparations of S. pneumoniae
(ATCC 6314) are easily maintained at -70°C in defibrinated rabbit
blood, and aliquots of the stock preparation can be removed and grown
in culture at various dilutions to obtain the desired challenge
concentration. An alternative approach is to grow the organism in
culture and to monitor the bacterial concentrations by measuring the
turbidity of the culture. A 5-µl aliquot of the stock preparation is
used to inoculate 50ml of brain-heart infusion broth, which is
incubated at 37°C, and the turbidity of the overnight culture is
determined with an Abbott Biochromatic analyser system or another
instrument that can sensitively measure changes in culture turbidity.
The overnight culture is diluted with fresh brain-heart infusion broth
to yield an absorbence difference of 0.020-0.025. The turbidity of the
subculture is monitored periodically, and when the optimal density
reaches an absorbence difference of 0.080, the subculture is rapidly
cooled in an ice bath and diluted to the desired inoculum level. The
turbidity of each inoculum is checked in the analyser, and adjustments
are made to obtain the preselected differences in absorbence.
Routinely, one day after the last exposure, female mice are challenged
intraperitoneally with 0.2ml of the S. pneumoniae inoculum. If the
inoculum is administered intravenously, extremely high challenge
levels must be used, which may reflect the efficiency of the
mononuclear phagocyte system to clear and kill the organism. Three
innocula, each at a different concentration, are prepared to give a
range of lethality (e.g. 20, 50, and 80%), and a sample of each is
serially diluted and placed on blood agar plates to determine the
number of colony-forming units administered to the animals. Owing to
the rapid onset of infection, mortality is recorded twice daily for
seven days. In studies by White et al. (1986), when female B6C3F1 mice
were exposed daily for 14 days to 1,2,3,6,7,8-hexachlorodibenzo-
para-dioxin or TCDD by gavage, they were found to have decreased
host resistance to S. pneumoniae, which is consistent with the
decrease in complement activity caused by these compounds.
Another species of Streptococcus that has been used as a host
resistance model is S. zooepidemicus, a group C streptococcus
(Fugmann et al., 1983). Exposure to N-nitrosodimethylamine was shown
to decrease host resistance to this strain significantly. Infection
with S. zooepidemicus may be dependent on an antibody-mediated
response, since the time to death after challenge is considerably
longer than with S. pneumoniae. Numerous studies have demonstrated
that aerosolized S. zooepidemicus is one of the most sensitive
indicators of the toxicity of air pollution: Mice exposed for short
periods to single or mixed pollutants before infection with an aerosol
of S. zooepidemicus and then assessed for mortality over 20 days had
increased mortality with increasing concentrations of ozone (Coffin &
Gardner, 1972; Ehrlich et al., 1977), nitrogen dioxide (Ehrlich &
Henry, 1968; Sherwood et al., 1981), sulfur dioxide (Selgrade et al.,
1989), metal particulates (Gardner et al., 1977; Adkins et al., 1979,
1980; Aranyi et al., 1985), phosgene (Selgrade et al., 1989), and
other volatile organic compounds (Aranyi et al., 1986). With many of
these compounds, enhanced susceptibility to infection has been
demonstrated at concentrations at or below the United States national
ambient air quality standards or threshold limit values. With all of
these compounds, enhanced mortality has been associated with failure
to clear bacteria from the lung and suppression of alveolar macrophage
phagocytic function. This model has recently been adapted to rats. In
this species, both ozone (Gilmour & Selgrade, 1993) and phosgene (Yang
et al., 1995) delayed clearance of bacteria from the lungs and
enhanced the inflammatory response (polymorphonuclear leukocytes in
lavage fluid) at concentrations that do not themselves produce
inflammation; however, mortality does not occur in this species. While
the bacteria have generally been administered as aerosols, Sherwood et
al. (1988) showed that similar results could be obtained when they
were administered intratracheally or intranasally. Since some strains
of Streptococcus are pathogenic to humans, appropriate precautions
must be taken when using this host resistance model.
4.2.10.3 Viral infection model with mouse and rat cytomegalovirus
Cytomegalovirus infections are widely distributed in humans, with
about 60-90% of the population infected. Human cytomegalovirus
infections occur in several forms, the most serious being congenital
and perinatal infection and infection of immunosuppressed individuals.
Less serious forms include post-perfusion syndrome and some cases of
infectious mononucleosis; however, the vast majority of postnatal
infections in immunocompetent individuals are clinically asymptomatic.
More severe disease may occur in immunodeficient hosts, such as
transplant patients (Naraqi et al., 1977; Pass et al., 1978; Marker et
al., 1981; Rubin et al., 1981). Primarily on the basis of
morphological considerations, cytomegaloviruses are classified as
members of the family Herpesviridae. Because these viruses have a
relatively protracted replication cycle, a slowly developing
cytopathology characterized by cytomegaly, and a relatively restricted
host range, they are grouped into the beta Herpesviridae subfamily
(Roizman et al., 1981; Roizman, 1982). The roles of several arms of
the immune system in resistance to cytomegalovirus have been studied
extensively in mice. The role of humoral immunity is not well
understood. It was suggested initially that neutralizing antibodies do
not play a pivotal role in recovery from cytomegalovirus infection in
mice (Osborn et al., 1968; Tonari & Minamishima, 1983); however, the
role of antibodies in neutralization of murine cytomegalovirus and in
antibody-dependent cell-mediated cytotoxicity is now recognized
(Manischewitz & Quinnan, 1980; Quinnan et al., 1980; Farrell &
Shellam, 1991). Cytomegalovirus-specific Tc cells can be detected in
cytomegalovirus-infected mice (Ho, 1980; Quinnan et al., 1980). NK
cell activity appeared to be the most effective, especially during the
initial stages of infection (Bancroft et al., 1981; Selgrade et al.,
1982; Bukowski et al., 1984). Enhanced susceptibility to infection has
been demonstrated in mice when macrophage function was blocked by
silica, and transfer of syngeneic adult macrophages to suckling mice
significantly increased their resistance to mouse cytomegalovirus
infection (Selgrade & Osborn, 1974). An inverse correlation is seen
between the virulence of mouse cytomegalovirus and its infectivity for
peritoneal macrophages (Inada & Mims, 1985), suggesting that
attenuated virus may be controlled, in part, by macrophages. Since the
rat virus acts very much like the attenuated mouse virus, macrophages
may be even more important in rats. Macrophages may facilitate the
generation of latent infection (Booss, 1980; Yamaguchi et al., 1988).
Enhanced susceptibility to mouse cytomegalovirus has been
demonstrated after treatment of mice with cyclophosphamide,
cyclosporin A, nickel chloride, or DMBA. Treatment with benzo[ a]-
pyrene or TCDD did not affect susceptibility to this infection
(Selgrade et al., 1982). Enhanced susceptibility was correlated with
chemical suppression of virus-augmented NK cell activity during the
first week of infection. In rats, exposure to immunotoxic agents such
as organotin compounds led to altered resistance to rat
cytomegalovirus (Garssen et al., 1995).
Experimentally, rodents can be inoculated intraperitoneally with
a species-specific cytomegalovirus, and the concentration of the virus
in tissue can be determined in a plaque-forming assay, which is a
modification of the method described by Bruggeman et al. (1983, 1985).
Rat embryo-cell monolayers are prepared in 24-well plates. Different
organs (salivary gland, lung, kidney, liver, spleen), obtained at
various times after infection, are homogenized in a tissue grinder and
stored as 10% weight/volume samples at -135°C until use. The confluent
monolayers are then infected with 10-fold serial dilutions of the
organ suspensions. After centrifugation, the suspension is removed,
and 1 or 0.6% agarose is added. After incubation at 37°C in 5% carbon
dioxide for seven days, the cells are fixed in 3.7% formaldehyde
solution, the agarose layer is removed, and the monolayer is stained
with 1% aqueous methylene blue. Plaques are counted under a
stereoscopic microscope.
In PVG rats, cytomegalovirus is detectable eight days after
infection, although the virus load is much higher on days 15-20. The
viral load in the salivary gland is higher than that in other organs,
i.e. spleen, lung, kidney, and liver. In contrast, in Lewis rats and
BN rats the viral load in e.g. the kidney was higher than that in the
salivary gland during the first week after infection (Bruning, 1985),
perhaps due to a strain difference (Bruggeman et al., 1983, 1985).
Total body irradiation of PVG rats with 60Co one day before
infection with cytomegalovirus increased the viral load in the
salivary gland, lung, kidney, spleen, and liver over that in
unirradiated PVG rats; histological analysis also indicated a higher
viral load in the salivary gland of infected rats. The mucosal
epithelium of the salivary gland contains enlarged cells with nuclear
inclusion bodies; these could be detected in irradiated, infected rats
only if the salivary gland was dissected and fixed 15 days after
infection. These results are in agreement with those of Bruggeman et
al. (1983), who found that gamma irradiation also induced higher viral
loads in the salivary gland of BN rats. Taken together these results
indicate a role for cellular immunity in resistance to this virus in
rats.
4.2.10.4 Influenza virus model
Influenza virus A2/Taiwan H2N2 has been used as a viral
challenge in evaluating alterations in host resistance of mice after
exposure to various compounds. Compounds that decrease host resistance
to the virus are N-nitrosodimethylamine (Thomas et al., 1985b) and
TCDD (House et al., 1990a). Compounds that do not to alter host
resistance to this pathogen include ozone (Selgrade et al., 1988),
benzo[ a]pyrene, benzo[ e]pyrene (Munson & White, 1990), methyl
isocyanate (Luster et al., 1986), and Pyrexol (House et al., 1990b).
Mortality is the end-point routinely measured in evaluating decreased
host resistance to influenza virus, which is usually instilled
intranasally (Fenters et al., 1979). Host resistance to this virus has
been reported to be mediated by cell-mediated immunity (Ada et al.,
1981), interferon (Hoshino et al., 1983), and antibody (Vireligier,
1975). This model had been suggested for use in evaluating compounds
that affect humoral immunity; however, its inability to detect such
compounds indicates that it is not suitable. A possible explanation
for the discrepancy is that administration of the virus by intranasal
instillation may invoke local immune mechanisms in the lung and may
not adequately reflect systemic immunocompetence. In several cases,
enhanced mortality has been demonstrated in the absence of effects on
viral titres in the lung (Selgrade et al., 1988; Burleson et al., in
press), indicating that enhanced mortality does not always reflect
effects on virus-specific immune defences.
Influenza virus has been used in evaluating immunotoxicity in
rats, after adaptation. Studies by Ehrlich & Burleson (1991) showed
that rats exposed to phosgene had significantly decreased host
resistance. TCDD was shown to affect the resistance of rats to the
adapted influenza virus RAIV (Yang et al., 1994).
As influenza virus is a human pathogen, appropriate precautions
must be taken.
4.2.10.5 Parasitic infection model with Trichinella spiralis
Resistance to infection with the helminth T. spiralis has been
evaluated in both mice and rats after exposure to a variety of
chemicals. In humans, as in other carnivores, infection occurs by
eating meat containing infectious larvae. The life cycle of the worm
is as follows: Infectious larvae excyst in the acid-pepsin environment
of the stomach, rapidly migrate to the jejunum, and penetrate host
intestinal epithelial cells. Sexually mature parasites are present
within three to four days after infection. The viviparous females
produce larvae that migrate via the lymphatic and blood vessels to
host muscle, where they encyst and are encapsulated within a host-
derived structure. Encapsulated muscle larvae can survive for years
within this structure.
An intense inflammatory response, comprised mainly of mast cells
and eosinophils, accompanies intracellular infection in the intestine.
T Cell-dependent immunity plays a crucial role in this inflammatory
response (Manson-Smith et al., 1979; Vos et al., 1983b; Wakelin,
1993), which is responsible for the expulsion of adult parasites.
Antibodies damage the reproductive structures of the female parasite
(Love et al., 1976), have a major role in the rapid elimination of
subsequent infections in rats (Appleton & McGregor, 1984), and
sensitize migrating newborn larvae for destruction by granulocytes
(Ruitenberg et al., 1983).
The number of encysted muscle larvae is typically much higher in
immunosuppressed animals than in immunocompetent animals, due perhaps
to delayed expulsion of adult worms from the intestine, decreased host
control of parasite fecundity, decreased destruction of migrating
larvae, or a combination of resistance defects. These end-points of
host resistance to T. spiralis infection and class-specific antibody
titres can be measured by standard techniques (Van Loveren et al.,
1994). Histological evaluation of the inflammatory infiltrate
surrounding encysted muscle larvae has also been described (Van
Loveren et al., 1993b).
It should be noted that direct effects of the chemical under
study can affect the outcome of infection. For example Bolas-Fernandez
et al. (1988) determined that cyclosporin A delays expulsion of adult
parasites from the intestines of rats but does not increase the number
of larvae encysted in host muscle. This was determined to be a direct
effect of cyclosporin A on the fecundity of female parasites rather
than on immunity to infection. Animals are infected by oral gavage
with known numbers of larvae, isolated from infected donor muscle.
Because infection is spread only by consumption of infected meat or
freshly isolated larvae, there is little danger of the infection
spreading to other animals housed in the same room. T. spiralis is a
human pathogen and must be handled as such; normal laboratory
practices are sufficient to prevent accidental infection.
T. spiralis infection has been used as a host resistance model
in both rats and mice. In general, chemicals that suppress T-cell
function suppress resistance to T. spiralis infection. Thus, TBTO
(Vos et al., 1990b), diethylstilbestrol (Luebke et al., 1984), TCDD
(Luebke et al., 1994, 1995), and the virustatic agent acyclovir
(Stahlmann et al., 1992) had deleterious effects on resistance.
4.2.10.6 Plasmodium model
Two strains of Plasmodium have been used to evaluate the
potential immunotoxicity of compounds. P. yoelii (17XNL) is a
nonlethal strain that produces a self-limiting parasitaemia in mice.
Resistance to this organism is multifaceted and includes specific
antibody, macrophage involvement, and T cell-mediated functions
(Luster et al., 1986). In this assay, animals are injected with 106
parasitized erythrocytes, and the degree of parasitaemia is monitored
over the course of the infection by taking blood samples. In control
animals, the peak response usually occurs 10-14 days after injection.
The degree of parasitaemia can be evaluated by a variety of methods,
e.g. manually, by counting parasitized erythrocytes in blood smears
(Luebke et al., 1991). Host resistance to P. yoelii has been used to
assess the immunotoxicity of benzidine (Luster et al., 1985b),
diphenylhydantoin (Tucker et al., 1985), TCDD (Tucker et al., 1986),
pyran copolymer (Krishna et al., 1989), gallium arsenide (Sikorski et
al., 1989), and 2'-deoxycoformycin (Luebke et al., 1991).
P. berghei is lethal to mice and certain strains of rats and
has been used in assessing immunotoxicity (Loose et al., 1978). Host
resistance depends on specific antibody production and ingestion and
destruction of antibody-coated Plasmodium by phagocytic cells such
as macrophages. T Lymphocytes may also be involved in host resistance
to the organism (Bradley & Morahan, 1982). Mortality has been
evaluated after injection of 106 Plasmodium-infected erythrocytes.
Compounds that have been evaluated for immunotoxicity in this model
system include 4,4'-thiobis(6- tert-butyl- meta-cresol) (Holsapple
et al., 1988), dietary fish-oil supplement (Blok et al., 1992), and
styrene (Dogra et al., 1992). Neither P. yoelii nor P. berghei is
infectious in humans; infection of animals can occur only through
parenteral injection of contaminated blood.
4.2.10.7 B16F10 Melanoma model
The B16F10 tumour cell line is a malignant melanoma that is
syngeneic with the C57Bl/6 mouse, which is one of the parents of the
B6C3F1 mouse. This tumour line was selected for its propensity to
metastasize to the lung. The assay is an outgrowth of the work of
Fidler and colleagues (Fidler, 1973; Fidler et al., 1978). NK cells
and macrophages have been proposed to be involved in host resistance
to this metastasizing tumour; however, T lymphocytes have also been
shown to play a role (Parhar & Lala, 1987). This host resistance assay
is referred to as an artificial metastasis model, since the tumour
cells are administered by intravenous injection, usually into the tail
vein, and lodge in the lung, which is the first capillary bed they
encounter. The B16F10 tumour cells can be stored frozen and can easily
be grown in culture before use. Routinely, 1-5 × 105 cells are
injected intravenously into sentinel mice (i.e. untreated mice
injected with the highest challenge level of tumour cells), and the
tumour burden is monitored in order to select the optimal day of
assay.
Two parameters are routinely used to assess tumour burden. One is
DNA synthesis in the lungs of mice bearing tumours. Since background
DNA synthesis in the lungs of mice without tumours is extremely low,
any detectable rate is a result of the presence of a tumour. In order
to measure synthesis, mice are pulsed intraperitoneally one day before
sacrifice with 0.2 ml of 10-6 mol/litre of 5-fluorodeoxyuridine,
followed 30 min later by 2 µCi of 125I-iododeoxyuridine administered
by the intravenous route. After sacrifice, the lungs are removed,
placed in Bouin's fixative solution, and counted with a gamma counter.
A second indicator of tumour burden is visual enumeration of tumour
nodules after fixation in Bouin's solution. The visibility of the
black nodules of the melanin-producing B16F10 tumour cells on the
yellow background of the fixed lung tissue allows enumeration of up to
200-250 nodules on the surface of the lungs. A good correlation has
been shown between number of tumour nodules and radioactivity present
in the lungs (White, 1992). Thus, if the tumour nodules become too
numerous to count, the results of the study can still be determined
from the radioassay. This system has been useful in demonstrating
decreased host resistance after systemic exposure to the tumour
promoter phorbol myristate acetate (Murray et al., 1985),
intratracheal exposure to gallium arsenide (Sikorski et al., 1989),
and exposure to nickel chloride (Smialowicz et al., 1985b); it has
also been used to show enhanced host resistance after exposure to
manganese chloride (Smialowicz et al., 1984) and 4,4'-thiobis(6- tert-
butyl- meta-cresol) (Holsapple et al., 1988).
4.2.10.8 PYB6 Carcinoma model
The PYB6 tumour cell line is a fibrosarcoma originally induced
with a polyoma virus in C57Bl/6 mice. Host resistance to the tumour
includes NK cell activity and T cell-mediated killing (Urban et al.,
1982). While PYB6 cells can easily be grown in culture, they should be
passed through an animal before use in challenge studies for
immunotoxicity (Luster et al., 1988). In studies with the PYB6 line,
mice are injected in the thigh with 1-5 × 103 viable tumour cells
and are then palpated weekly to detect the development of tumours at
the injection site. The end-points evaluated include the incidence of
tumours and time to tumour appearance; tumour size can also be
measured. This assay has been useful in detecting decreased host
resistance to many compounds, including Aroclor 1254 (Lubet et al.,
1986), DMBA (Dean et al., 1986), and benzene (Rosenthal & Snyder,
1987).
4.2.10.9 MADB106 Adenocarcinoma model
A tumour model used to evaluate host resistance in rats is the
MADB106 rat mammary adenocarcinoma, which is syngeneic with the
Fischer 344 rat. NK cells appear to play a major role in host defence
to this tumour (Barlozzari et al., 1985). In this model, survival time
after injection of the cells is the usual end-point monitored.
Compounds that decrease host resistance to the tumour can decrease
both the percentage survival and the survival time of treated animals.
Control rats begin to succumb to the adenocarcinoma two to three weeks
after an intravenous injection of 2 × 106 tumour cells. Smialowicz
et al. (1985b) showed a significant decrease in the survival of
animals treated with a single intramuscular dose of nickel chloride,
which was correlated with a decrease in NK cell activity.
4.2.11 Autoimmune models
Autoimmune models can also be used to investigate whether a
compound exacerbates induced or genetically predisposed auto-immunity.
These models are used mainly to elucidate the pathogenesis of
autoimmunity and the effect of immunosuppression in
immunopharmacology. Few studies have been reported, although the
relevance of the model for extrapolation to humans may be good. A
number of autoimmune models are available in rats and mice. Autoimmune
phenomena can be either induced or occur spontaneously. In induced
models, an autoantigen is isolated from a target organ obtained from
another species (generally bovine), and the animal is immunized with
this purified antigen in adjuvant. Examples in the rat (Calder &
Lightman, 1992) are experimental encephalomyelitis elicited by bovine
spinal cord antigen (Stanley & Pender, 1991), experimental uveitis
elicited by bovine retinal S-antigen (Fox et al., 1987), and adjuvant
arthritis elicited by Mycobacterium containing H37RA adjuvant
(adjuvant arthritis) or collagen (Holmdahl et al., 1990; Klareskog &
Olsson, 1990; Wooley, 1991). Autoimmune phenomena and associated organ
pathology normally emerge in almost all immunized animals within two
to three weeks. Depending on the effector reaction and the
reversibility of the damage, the disease either stops when the damage
is complete (e.g. uveitis, resulting in blindness of the animal) or
the autoimmune reaction is transient and animals recover (adjuvant
arthritis). In some models, animals subsequently experience a relapse
around day 30 (experimental encephalomyelitis). The induction and
development of autoimmunity in these animals are mediated by T cells
that show the cytokine expression pattern (IL-2, INF gamma) of the Th1
subset. The effector phase of disease symptoms is also mainly a T
cell-mediated process, to which CD4+ cells, CD8+ cells, and
macrophages contribute. Lewis (RT11) rats are particularly
susceptible. These autoimmune models can be induced in other species,
such as mice (Baker et al., 1990) and rhesus monkeys (Rose et al.,
1991). They are generally accepted as models of human (organ-specific)
autoimmune diseases, e.g. experimental allergic encephalomyelitis as a
model of multiple sclerosis, experimental allergic uveitis as a model
of idiopathic posterior uveitis, and adjuvant arthritis as a model of
rheumatoid arthritis.
Autoimmunity can also be induced by metals (Druet et al., 1989;
Bigazzi, 1992). A well-known example is glomerulopathy induced by
mercuric chloride in BN (RT1n) rats. The process is initiated by T
cells with a cytokine synthesis pattern (IL-4) of the Th2 subset. The
relative incidence of these cells is much higher in BB rats than in
other strains. After the cells of the Th2 subset have been stimulated,
there is polyclonal stimulation of B lymphocytes, leading to synthesis
of antibodies (including pathogenic antibodies) to the glomerular
basement membrane. These antibodies subsequently mediate autoimmune
destruction of renal glomeruli. This model is the best studied model
of 'drug'-induced autoimmunity. Mercuric chloride elicits
glomerulonephritis in other rat strains, in which glomerular
destruction is not due to anti-glomerular autoantibodies but is
mediated by immune complexes deposited in the glomerulus (Druet et
al., 1989).
In spontaneous models, predisposition to the development of
autoimmune phenomena and disease is determined by the genetic
composition of the animal strain. Well-known examples are BB rats
(Like et al., 1982; Guberski, 1994) and NOD mice (Lampeter et al.,
1989; Leiter, 1993), which develop autoimmune pancreatitis and
subsequently diabetes. Within the pancreas, the islets of Langerhans
are infiltrated by T lymphocytes and macrophages; subsequent
destruction of the islets results in diabetes. These spontaneous
models are considered to be animal models of human diabetes (Dotta &
Eisenbarth, 1989; Lampeter et al., 1989; Riley, 1989). Other examples
are the systemic autoimmunity that emerges in certain mouse strains
(Guttierez-Ramos et al., 1990) like (NZB × NZW)F1 mice (Theofilopoulos
& Dixon, 1985) and the mixed lymphocyte reaction in lpr (Matsuzawa
et al., 1990) and gld mice (Roths et al., 1984). The spontaneous
pathology in these animals resembles various disease manifestations in
human systemic lupus erythematosus and is similarly mediated by immune
complexes that deposit in tissue. In (NZB × NZW)F1 mice, mainly lupus
nephritis is induced by immune complexes; in the mixed lymphocyte
reaction in lpr mice, both joint manifestations and
glomerulonephritis are seen. The genes associated with autoimmune and
immune complex disease are not known. In comparison with induced
models, these models have the advantage of spontaneous, gradual
development of autoimmune disease symptoms; however, this is a
disadvantage in experimental design, as not all animals develop
disease, and emerging disease develops at different times or ages.
In general, treatment of animals with immunosuppressive drugs
that interfere with signal transduction (cyclosporin, FK-506,
rapamycin) or cell proliferation (cytostatics like azathioprine,
mizoribine, and brequinar), and anti-inflammatory agents
(corticosteroids) inhibit the development of symptoms in these models.
Exposure to immunotoxic chemicals may also lead to alterations in the
course of disease emergence. For instance, HCB, which leads to
immunoenhancement in rats, markedly enhanced the severity of allergic
encephalomyelitis (Van Loveren at al., 1990c). In contrast, arthritic
lesions were strongly suppressed in HCB-exposed Lewis rats, indicating
that HCB has biologically significant immunotoxic effects. Although
the contrasting effects in the two autoimmune models are not yet
understood, and clear dose-effect relationships have yet to be
established, this type of information should be obtained for risk
assessment.
4.3 Assessment of immunotoxicity in non-rodent species
While most immunotoxicological evaluations are conducted in mice
or rats, use of other species is increasing.
4.3.1 Non-human primates
Various non-human primates, including Macaca mulatta (rhesus
macaques), M. nemestrina (pig-tailed macaques), Cercocebus atys
(sooty mangabeys), M. fasicularis (cynomolgus monkeys), and
marmosets have been used in immunotoxicological studies. Many of the
assays carried out in mice or rats can be adapted for use with non-
human primates. Strategies and methods used in studies of humans have
also been introduced in studies on non-human primates. Monoclonal
antibodies generated to human leukocyte subsets can be used in
phenotyping blood mononuclear cells of e.g. marmoset monkeys
(Callithrix jacchus) (Neubert et al., 1990, 1991), although the
possibility of such use differs, depending on the evolutionary
distance of the non-human primate from humans. While most of the
assays conducted in non-human primates involve serum or peripheral
blood, some assays, such as those used to measure delayed-type
hypersensitivity, are holistic, in that the animals are sensitized
in vivo and then evaluated in vivo at the challenge site (Bugelski
et al., 1990; Bleavins & Alvey, 1991).
The effects of chronic exposure to the PCB Arochlor 1254 on the
immune response of rhesus monkeys have been evaluated by Tryphonas et
al. (1989). In these studies, the lymphocyte response to concanavalin
A and phytohaemagglutinin was evaluated, as were total serum
immunoglobulin levels, antibodies to sheep red blood cells, and
numbers of T and B cells in peripheral blood. In later studies
(Tryphonas et al., 1991a,b), one-way mixed lymphocyte cultures,
antibodies to pneumococcal antigens, phagocytic mononuclear cell
function, NK function, haemolytic complement activity, and production
of IL-1, tumour necrosis factor, thymosins, and interferon were
evaluated in monkeys exposed to Arocolor 1254. Ahmed-Ansari et al.
(1989) evaluated phenotypic markers and function in three species of
non-human primate. The functional assays included NK cell activity,
lymphocyte transformation, and antigen presentation. Extensive studies
included the evaluation of more than 20 phenotypic markers or
combinations of markers for each of the three monkey species. Use of
the monkey as a test species is likely to increase as more and more
biotechnology and recombinant products are produced.
4.3.2 Dogs
While dogs are not the species of choice for immunotoxicological
studies, they are used predominantly in assessing toxicological
safety, and virtually all of the assays used for assessing immunotoxic
potential have been adapted for use in dogs. These include evaluation
of basal levels of IgA, IgG, and IgM (Glickman et al., 1988),
allergen-specific serum IgE (Kleinbeck et al., 1989), mononuclear
phagocyte function (Thiem et al., 1988), NK cell activity (Raskin et
al., 1989), Tc cell activity (Holmes et al., 1989), and mitogen-and
cell-mediated immune responses (Nimmo Wilkie et al., 1992).
4.3.3 Non-mammalian species
Non-mammalian species are also used extensively for evaluating
the potential adverse effects of compounds and agents on the immune
system.
4.3.3.1 Fish
Because of their environment, fish are an excellent model for
studying the effects of water-and sediment-borne pollutants. There are
several other good reasons for studying immunotoxicity in fish: many
of their diseases are related to environmental quality, various
environmental pollutants have immunotoxic potential, and many of the
diseases have an immune component. Moreover, there is concern about
the health status of aquatic ecosystems in relation to pollution, and
fish will be useful target species for developing biomarkers (see
box). Fish are easy to obtain, there is an extensive body of
knowledge, and their economic interest (aquaculture) facilitates the
finding of research resources. At present, immunotoxicology in fish is
not as sophisticated as that in mammals. Screening and functional
tests are being developed in the laboratory but cannot yet be applied
in the field.
A wide range of species is used for field and laboratory studies.
The choice of species depends on its biology (migratory or local,
marine or freshwater; sediment-dwelling or pelagic) and on experience
in the laboratory. A lack of consistency, e.g. becuse of a limited
number of species (as in mammalian immunotoxicology) makes this field
of research diffuse and extended and may result in limited progress;
nevertheless, a variable or consistent effect over a variety of
species is certainly a valuable observation. Some species seem to be
preferred, such as trout, salmon, and carp, which are practical, owing
to their size, for sampling blood and tissues for laboratory studies.
Smaller species, such as guppies (Poecilia reticulata) and medaka
(Oryzias latipes), have secured a niche in aquatic toxicology owing
to the ease of husbandry and relatively low cost; moreover, because of
their small size, whole animals can be used for histopathological
examination (Wester & Canton, 1991), but their application in
immunotoxicology may be limited because of difficulty in obtaining
adequate blood and tissue samples. For studies of saltwater species,
bottom-dwelling flatfish are commonly used in field studies and, to a
certain extent, in studies of mesocosms and in the laboratory. In
Europe, the flounder (Platichthys flesus) and dab (Limanda limanda)
are popular target species since they are susceptible to certain
recognizable diseases and are commonly available.
A compehensive variety of parameters is listed by Anderson (1990)
and Weeks et al. (1992). A modified set based on those lists and
assays used in rodent immunotoxicology is presented in the box above
and classified as tier 1 (screening tests) and tier 2 (functional
assays). Parameters commonly mentioned in the literature are discussed
below.
Blood cell counts and differential counts: As leukocytes play a
major role in specific and nonspecific humoral and cellular immune
responses, this parameter is used as a measure of the status of the
defence system, in particular in tier 1 testing. It is relatively easy
to test blood samples drawn from live animals, but many environmental
factors unrelated to defence may modify leukocyte status (Anderson,
1990). The use of monoclonal antibodies directed against individual
cell types may improve their identification (Bly et al., 1990; Van
Diepen et al., 1991). Another possible parameter is the haematocrit;
however, it has no known specificity for any immune function, although
it may be considered as a general indicator of stress.
Candidate biomarkers for immunotoxicity in fish
Tier 1: Screening tests
* Conventional haematology, including total and differential blood
cell counts, surface markers (flow cytometry), and macrophage
density and morphology: easy, nonspecific
* Serum immunoglobulin concentrations in naive (unstimulated) fish:
easy, limited specificity
* Lymphoid organ weight (mainly spleen, occasionally thymus):
impracticable
* Histopathology of the thymus, spleen, and kidney: possible, can
be specific
Tier 2: Functional assays
* Humoral immune response (agglutination, enzyme-linked
immunosorbent assay): possible, can be specific
* Cellular immune response (allograft rejection in scale, skin, or
eye): possible, can be specific
* Macrophage functions (phagocytosis, bacterial killing, migration,
chemiluminescence): limited specificity
* Host resistance (bacterial infections): possible, can be
specific, relevant
Nonspecific defence: Other indicators of nonspecific defence
have been proposed as indicators of immunological stress. These
include acute-phase proteins (Fletcher, 1986), the levels of which
appear to be stress hormone-dependent; and lysozyme and ceruloplasmin
activity, which are reduced in carp exposed to trichlorphon in vivo
(Siwicki et al., 1990).
Morphology: The spleen is easy to excise and weigh in animals
of adequate size and could thus serve as a biomarker, although it is
not commonly reported in the literature. One reason may be that a
major and variable portion of the spleen consists of storage blood or
erythropoietic tissue (Fänge & Nilsson, 1985); the lymphoid tissue is
poorly developed and is mainly associated with melanomacrophage
centres (Zapata, 1982, 1983; Fänge & Nilsson, 1985; Van Muiswinkel et
al., 1991); and, after immunization, only a small proportion of the
plaque-forming cells is found in the spleen, in contrast to the kidney
pronephros (Van Muiswinkel et al., 1991). The role of the spleen in
most fish species thus seems to be limited, although melanomacrophage
centres are abundant.
Experimental immunotoxicology in mammals has demonstrated that
the weight of the thymus, a primary and exclusively immunological
organ, is a sensitive indicator of thymic effects. This parameter is
not commonly used in fish. One reason is that the thymus has a complex
location in some species, which makes clean dissection nearly
impossible. Other reasons are inconsistencies in thymic morphology,
histopathology, and morphometry (Ghoneum et al., 1986; Wester &
Canton, 1987). The latter paper described studies in which guppies
exposed to TBTO showed dose-dependent atrophy of the thymus, as seen
in rats (Krajnc et al., 1984). Both species also showed a concomitant
increase in 'neutrophils', which suggests functional compensation.
This response could not be reproduced in medaka (Wester et al., 1990),
stickleback, or flounder (P. Wester, unpublished results), probably
indicating species specificity. Thymic lymphocyte function can also be
tested in vitro.
Macrophage function tests: Macrophages are an important cell
population for both specific (antigen processing and presentation) and
nonspecific (phagocytosis and destruction) defence. They are
considered to be a relatively primitive defence mechanism and are
therefore of major importance to lower animals (Ratcliffe & Rowly,
1981). Much effort has been devoted to establishing macrophage
parameters as biomarkers for immune effects in fish; a possible reason
for this preference is the fact that these cells are fairly easy to
obtain, e.g. by peritoneal washing or removal of kidney pronephros,
and many function tests do not require sophisticated techniques or
species-specific reagents or markers (Mathews et al., 1990). The tests
include determination of chemotaxis, phagocytosis, pinocytosis, and
chemiluminescence. Zelikoff et al. (1991) studied the applicability of
trout peritoneal macrophages for immunotoxicology, stressing the need
for systematic baseline information. In addition to the tests listed
above, they studied the morphology and spread of resident and
stimulated peritoneal macrophages and concluded that these cells share
many morphological and functional properties with their mammalian
counterparts and may thus be useful indicators in immunotoxicology.
Many case studies in fish species have been published, demonstrating
the sensitivity of one or more parameters to chemicals, including PAHs
(Weeks & Warinner, 1986; Zelikoff et al., 1991) and pentachlorophenol,
in vitro (Anderson & Brubacher, 1993).
Melanomacrophage centres: Melanomacrophage centres, or
macrophage aggregates, are widely distributed throughout the fish
body, in particular in spleen, liver, and kidney. They are composed of
clusters of swollen, rounded cells (macrophages) that stain pale-tan
to black. This parameter must be determined histopathologically. Their
occurrence and morphology have been described (Agius, 1985), but their
function is not yet fully understood. The presence of pigments
(haemosiderin, lipofuscin, and ceroid) indicates storage of effete
biological material (erythrocytes, biomembranes) (Wolke, 1992). The
melanin present may be a generator of the bactericidal hydrogen
peroxide (Roberts, 1975), and the presence of antigens indicates a
role in immune reactions, e.g. antigen presentation. An increase in
melanomacrophage centres can be found with age and after stress
(Blazer et al., 1987), as confirmed in field studies (Vethaak &
Wester, 1993). Moreover, a large number of relatively small, pale
centres was seen in animals caught in late winter, when conditions are
more stressful, including spawning with associated migration and
starvation (Vethaak & Wester, 1993). The presumption that the small
size and pale appearance are indicators of recent development is
supported by the observation that in liver tumours composed of
relatively young, fast-growing tissue melanomacrophage centres are
usually absent or definitely smaller. As a consequence, when these
centres are used as general parameters of stress, the study groups
must be matched for age.
Because they are characteristic for fish and because of the
multiplicity of their functions, these structures deserve special
attention in the context of defining biomarkers for immunotoxicity. In
addition, they are easy to monitor, since they do not require special
preparation other than routine histological procedures, including
morphometry. Since melanomacrophage centres can be considered
primitive analogues of the mammalian lymph follicle (Payne & Fancey,
1989), it has been suggested that their presence indicates immune
capacity or function, although their role in this context has not yet
been established and the implications of a change in this parameter
for the integrity of the defence systems remains unclear. The density
of melanomacrophage centres in liver or spleen has been successfully
correlated with environmental sediment (Payne & Fancey, 1989) and
along a gradient of pollution in the North Sea (Bucke et al., 1992).
Other studies have reported an increase in melanomacrophage centre
density after contact with chemical contaminants (Blazer et al., 1987;
Secombes et al., 1992), which may indicate accumulation of cytotoxic
waste or immune stimulation.
At present, macrophage function and melanomacrophage centres are
the most widely used and promising indicators of the effects of
environmental stress (Blazer et al., 1987). Their relationship to
other components of the immune system remains to be clarified,
however, in tests with immunotoxicants.
Humoral immune response: Determination of circulating
immunoglobulin levels in serum is useful for testing the net result on
an immunological pathway in vivo. The response can be measured in
'naive' animals (total immunoglobulin) or after exposure to an
antigen, e.g. to verify the efficacy of vaccination in aquaculture.
Sheep red blood cells can be used as a standard antigen, and the
immune response can be measured by agglutination tests. ELISA tests,
which are sensitive and specific, can also be used (Arkoosh &
Kaattari, 1990). A related test is the haemolytic plaque assay which
identifies antibody-producing cells (splenic lymphocytes) (Anderson,
1990), but which has been used to only a limited extent in fish.
Specific lymphocyte stimulation tests: Functional tests widely
used in mammalian immunotoxicology, in which lymphocytes are
stimulated in vitro by exposure to mitogens such as
lipopolysaccharide, phytohaemagglutinin, and concanavalin A, can also
be used in fish. Proliferation is monitored by measuring the
incorporation of 3H-thymidine into DNA. The test is not antigen-
specific but provides information on the capacity of the entire B
(lipopolysaccharide) or T (phytohaemagglutinin, concanavalin A) cell
population. It is used to only a limited extent in fish
immunotoxicology, although Faisal & Hugget (1993) gave an elegant
demonstration of significant suppression of this parameter in spot
(Leiostomus xanthurus) under field conditions; this was shown to be
related to site and pollution in controlled laboratory experiments.
Specific cellular immune responses: Tests described in the
literature to measure cellular immune responses are scale or skin
allograft rejection, a relatively simple test (Zeeman & Brindley,
1981), and eye allograft rejection (Khangarot & Tripathi, 1991);
delayed rejection was seen in carp after exposure to copper. These
tests are applied to only a limited extent.
The tests described above are mainly tier 2 tests. The tests most
often used in immunotoxicology, however, are those for host resistance
(challenge by infections or tumours). The results of such tests are
rarely reported in the literature and have not been validated. For
ultimate proof of immunotoxicity, all phases of a test (maintainence,
exposure, and infection) must be conducted under strictly controlled
laboratory conditions. When suitable (often species-specific)
pathogens are standardized, such tests are valuable and necessary for
estimating the practical consequences of suspected immunotoxicity.
Although the incentive for undertaking immunotoxicological studies in
fish is usually epidemiological observation of a suspected toxic
component, ultimate challenge experiments must be carried out before a
final conclusion about immunotoxic mechanisms can be drawn.
More emphasis has been given to the development of biomarkers
than to their application in the field, for several reasons, including
the lack of specificity and the lack of association between effects at
the level of the biomarker and the population (Mayer et al., 1992).
Some comments on and some needs in this field are as follows:
* Immunological biomarkers in fish have great potential: many have
not yet been fully explored, probably owing to practical
limitations of lack of specificity and predictivity.
* The number of animal species should be limited in order to
concentrate research, which often requires species-specific
knowledge and reagents. Standardization could be achieved by
choosing well-defined inbred strains of fish (e.g. carp or
trout).
* A tiered approach is highly recommended for obtaining knowledge
on the specificity of the biomarker.
* More knowledge is needed on the epidemiology, mechanisms, and
etiology of diseases in fish, and particularly the predictive
value of immune parameters and the influence of hormesis.
* In terms of relevance for the organism, a test that monitors the
net result of a cascade of reactions (e.g. specific antibody
production, host resistance) is more predictive than a single,
nonspecific cell parameter (e.g. macrophage activity in
vitro).
* In identifying potential biomarkers for immunotoxicity, evidence
should be available that the levels tested in the laboratory are
relevant for field conditions and that the effect is directly
related to the immune system.
4.3.3.2 Chickens
Another non-mammalian species that has been studied extensively
with regard to the structure and function of its immune response is
the chicken. It is therefore not surprising that the chicken has
emerged as the predominant avian model for assessing compounds for
potential immunotoxicity. Humoral responses to different antigens have
been assessed routinely (Lerman & Weidanz, 1970; Marsh et al., 1981).
The weights of the thymus, spleen, and bursa of Fabricius have been
used, in combination with decreased antibody responses in vivo and
lymphocyte responses to phytohaemagglutinin and concanavalin A
in vitro (Eskola & Toivanen, 1974). Graft-versus-host and
cutaneous basophil hypersensitivity have also been used to detect
immunosuppression in chickens (Dietert et al., 1985). The availability
of chicken cell lines (Sung et al., 1992) will facilitate studies of
the mechanisms of action of compounds on the immune responses of this
avian model.
4.4 Approaches to assessing immunosuppression in vitro
The complexity of the immune system and the requirement of many
agents for metabolism and distribution to produce an immunotoxic
response has resulted in the almost exclusive use of animal models
in vivo for immunotoxicity assessment. Culture systems have been
used extensively, however, to study the mechanisms by which agents
produce immunosuppression.
Since most of the assays used for assessing immunotoxicity are
ex-vivo/in-vitro tests, they are easily adapted to completely in-vitro
assays for assessing immunosuppression. The direct addition of
compounds in various assays, including those involving NK cells,
lymphocyte proliferation, mixed leukocytes, and Tc lymphocytes, has
been used to determine the mechanisms by which compounds alter the
immune response at the cellular and subcellular level. Similarly, the
action of benzene and its metabolites on bone marrow has been studied
extensively in vitro (Gaido & Wierda, 1987), and the effects of TCDD
on thymocytes have been well studied in thymic epithelium co-cultures
(Greenlee et al, 1985). One of the most useful in-vitro assays for
studying immunosuppression is the T-dependent antibody response to
sheep erythrocytes. This assay, also known as the Mishell-Dutton assay
(Mishell & Dutton, 1967), has been used extensively in studying the
cellular target of immunotoxicants. It is the in-vitro counterpart of
the in-vivo plaque-forming cell assay, but sensitization with sheep
erythrocytes takes place in splenic cell culture and the plaque
response is measured on day 5 after addition of the erythrocytes. The
Mishell-Dutton assay has been used to study the structure - activity
relationships of various immunosuppressive compounds (Kawabata &
White, 1987; Davis & Safe, 1991). Since T cells, B cells, and
macrophages are needed for the response and an adverse effect on any
of these cell types can produce immunomodulation, it has proved
to be a sensitive assay for evaluating compounds in vitro for
immunosuppressive activity. Furthermore, since the various cell types
that participate in the response can easily be separated, individually
treated, and then reconstituted in the culture system, it is an
excellent assay for determining which cell type is adversely affected
by the compound. Using this approach, White & Munson (1986)
demonstrated that asbestos suppresses the response by affecting
macrophages; Shopp & Munson (1985) showed that the primary action of
phorbol ester on the antibody response occurs through an effect on B
cells; and Johnson et al. (1987) found that N-nitrosodimethylamine
affects primarily B cells.
As indicated above, one of the limitations of in-vitro systems is
that exogenous metabolic activation systems are often required. While
lymphocytes can metabolize some compounds, such as benzo[ a]pyrene,
to active metabolites (Ladics et al., 1992), other potent
immunosuppressive compounds such as cyclophosphamide require a
metabolic activation system. Such preparations usually consist of a
9000 x g supernatant of liver (S9). Using S9 preparations, Tucker &
Munson (1981) showed that cyclophosphamide could be activated to an
immunosuppressive form in vitro. Similarly, naphthalene could be
metabolized to an immunosuppressive metabolite (Kawabata & White,
1990). An alternative approach to the S9 activation system is a
hepatocyte co-culture system, which has been shown to be capable of
activating several parent compounds to their immunosuppressive
metabolites (Yang et al., 1986).
Predictive in-vitro systems based on immune cells of human origin
are particularly attractive, given the uncertainties of extrapolating
the results of experimental studies to humans and the accessibility of
immune cells in human peripheral blood. Although many of the immune
cells obtained from human blood are immature forms, the large numbers
and diverse populations (i.e. polymorphonuclear leukocytes, monocytes,
NK cells, T cells, and B cells) that can be obtained provide an
attractive alternative or adjunct to conventional studies in
experimental animals. As a consequence, a number of studies have been
conducted to compare the functional response of human and rodent
lymphocytes to putative immunosuppressive agents in vitro (Cornacoff
et al., 1988; Luo et al., 1992; Wood et al., 1992; Lang et al., 1993).
Although these studies were hampered by the lack of assays to assess
primary antigen-specific immune responses in human lymphocytes, a
relatively good interspecies correlation has been observed in the
limited responses available. Furthermore, several of these assays have
been successfully modified to include co-culture with primary
hepatocytes (Kim et al., 1987) to allow for chemical metabolism.
4.5 Future directions
4.5.1 Molecular approaches in immunotoxicology
A promising avenue for early detection of immunotoxicity may be
measurement of the expression of various interleukins. Cytokines are
involved both in regulation of the immune system and in pathological
phenomena, hence alterations in their pattern of expression may be
early indicators of immunotoxicity. Such testing can be done at the
level of mRNA expression, on mRNA extracted from lymphoid tissue taken
from exposed animals, or in tissue sections, so that the alterations
can be evaluated in the context of morphological indications of the
toxic effects. The signal of the cytokine that is being tested must
therefore be strong enough to be picked up in material from exposed
animals whose immune system has not received other stimuli, i.e.
sensitization or infection. This may not be true for all cytokines;
ex-vivo stimulation of cells that are part of the immune system may be
necessary, although the tests then become more laborious and must in
fact be considered functional assays, like tests for mitogen
responsiveness.
Very sensitive analysis can be done with the semiquantitative
polymerase chain reaction, which is a powerful technique for
elucidating early kinetic changes of cytokine expression, before
translation and secretion (Saiki et al., 1985). In addition, since
immunosuppressive agents can enhance or inhibit the ultimate
production and secretion of cytokines at various stages such as
transcription, the splicing of mRNA, translation of mRNA into
polypeptides on ribosomes, post-translational processing, and
secretion, potential molecular targets can be dissected by such
techniques. Several other molecular approaches may be used, including
northern blotting, dot-slot blotting, in-situ hybridization, and
antisense oligonucleotides for inhibiting the translation of specific
mRNAs.
4.5.2 Transgenic mice
The development of molecular genetic techniques has allowed not
only the isolation and analysis of specific genes but also the
manipulation of embryonic genes. Transgenic technology can be used in
immunology to generate mice that lack virtually any genetic control
mechanism or specific cell subpopulations. As a consequence, complex
systemic responses can be dissected into individual components, and
the mechanism by which immunosuppressive agents exert their affects
can be better understood. Two strategies are used to induce genetic
aberrations in transgenic mice (Bernstein & Breitman, 1989). One
involves the introduction of genes that produce toxins, such as
diphtheria toxin or the A subunit of ricin, into targeted cell
subpopulations. The second strategy involves the thymidine kinase
(tk) gene from Herpes simplex virus: When certain nucleotide
analogues are administered and are metabolized exclusively by viral
thymidine kinase, the metabolites are lethal only to cell
subpopulations that express the tk gene. Both approaches are
inducible systems for killing cells in vivo. Although gene ablation
techniques can be used to generate mutant animals that lack specific
cells in vivo, a small proportion of cells appeared to escape from
targeted cell death in virtually every study using bacterial toxins or
viral tk genes. While this may cause problems in determining the
qualitative roles of ablated cell populations, these techniques hold
promise for understanding the selective toxicity of drugs and
environmental agents on the immune system.
Other promising avenues are the use of animals transgenic with
respect to certain specificities of the TCR. If a gene that encodes
for a certain antigen specificity is introduced into the genome, that
specificity may be the only one that is expressed by the T cells. The
effects of immunotoxicants that affect the (positive and/or negative)
selection process that takes place in the thymus could be studied
elegantly with such models, when either undesired specificities (which
should be negatively selected) or desired specificities (which should
be positively selected) are introduced.
4.5.3 Severe combined immunodeficient mice
Another approach that may warrant further exploration is the use
of severe combined immunodeficient CB-17 scid/scid (SCID) mice
grafted with human immune cells. Xenogeneic lymphoid cells and/or
tissues can be successfully transferred to SCID mice (McCune et al.,
1988; Namikawa et al., 1990; Barry et al., 1991; Greiner et al., 1991;
Surhe & Sprent, 1991). SCID mice have been grafted with human fetal
lymphoid tissue in order to study human haematopoiesis (McCune et al.,
1988) or with human peripheral blood lymphocytes to allow production
of human immunoglobulins, including secondary antibody responses
(Mosier, 1990). SCID mice have also been used to study autoimmunity
and potential antiviral therapeutics. While these animal models still
have limitations (Pollock et al., 1994), they may ultimately provide
predictive models for examining potential immunosuppressive agents.
In particular, SCID mice co-implanted with human fetal thymus and
liver tissue fragments (SCID/hu mice) offer the possibility of
studying the human thymus in vivo in an isolated xenogeneic
environment (McCune et al., 1988; Namikawa et al., 1990) and the
effects of immunotoxicants on these grafts. This system is
particularly interesting with regard to those immunotoxicants for
which the thymus is one locus of action. The placement of human fetal
thymus under the SCID mouse renal capsule, followed by an intravenous
injection of fetal liver cells (McCune et al., 1988), and
co-implantation of human fetal liver and fetal thymus under the renal
capsule of SCID mice (Namikawa et al., 1990) have resulted in
reconstitution of SCID/hu mice; the fetal thymic implants increased in
size, and were found to be vascularized. The architecture and
antigenic distribution of these thymic grafts were virtually
indistinguishable from those of normal, age-matched human thymus.
Human stem cells were found to home to and differentiate within the
grafted human thymus, and phenotypically mature and functional human
T cells were found in the peripheral circulation of these mice (McCune
et al., 1988; Krowka et al., 1991; Vandekerckhove et al., 1991). As
such, the SCID/hu model can be helpful in immunotoxicological research
on the human thymus. When data obtained in experimental animals are
extrapolated to the human situation, a 'control' model, between the
SCID/hu mouse model and the intact laboratory animal (rat), is
desirable in order to test for possible differences in thymic
behaviour, because of its location under the kidney capsule: Thymic
blood flow and therefore the toxicokinetic behaviour of the thymus may
differ. For this reason the SCID/ra model was developed, by implanting
rat fetal thymus and liver tissue fragments under the SCID mouse renal
capsule. The outcomes of exposure of rats and SCID/ra mice can be
compared and the influence of thymus location and mouse metabolism on
extrapolation from SCID/hu to humans can then be determined.
Implantation of fetal rat thymus and liver tissue yields thymic
grafts that are virtually indistinguishable from normal, age-matched
rat thymus (De Heer et al., 1993). After implantation of rat fetal
thymus and liver tissue, the thymic grafts increase considerably in
size. Histologically, the SCID/ra thymic graft bears a close
resemblance to normal rat thymus, and the (immuno)histology of the
SCID/hu and SCID/ra mouse thymic grafts is comparable. Differences are
found, however, in peripheral reconstitutions of SCID/hu and SCID/ra
mice: Whereas large numbers of circulating donor rat T cells are found
in the blood and peripheral lymphoid organs of SCID/ra mice, only a
small number of donor T cells are found in the SCID/hu. This implies
that the data for extrapolating immunotoxic data from rats to humans
must be confined to thymic effects. With this restriction in mind, the
outcome of experiments with SCID/hu and SCID/ra mice can be used to
compare the sensitivity of the human and rat thymus and can thus yield
important information for the process of human risk assessment.
4.6 Biomarkers in epidemiological studies and monitoring
There is a difference between assays of the immune system and
biomarkers. Many validated tests can indicate alterations to the
immune system, including its function, so that most assays can be
helpful for hazard identification. Not every validated assay of the
immune system is a biomarker, however. The IPCS (1994a) definition of
a biomarker is one that indicates exposure (and is specific for
exposure), indicates susceptibility to adverse effects, and/or is
predictive of disease associated with exposure. Biomarkers should be
used to characterize risk due to exposure, on the basis of
identification of the hazard.
Within this strict definition, it is clear that not many
biomarkers are available for immunotoxicity (as is true for other
systems), especially for assessing immunotoxicity or individual
susceptibility to immunotoxicity. Some assays may be useful in
epidemiological studies. In any event, more epidemiological studies
are needed to obtain a better view of the usefulness of biomarkers for
detecting immunotoxic events and hence the possible health risks that
may be associated with exposure to immunotoxicants.
4.7 Quality assurance for immunotoxicology studies
In many countries, studies to support the safety of a compound or
drug must be conducted in accordance with the requirements for 'good
laboratory practice' of the agency that is evaluating the material.
Immunotoxicological studies conducted to support the safety of a new
drug or chemical should follow at least the 'spirit' of good
laboratory practice. The OECD has published their principles of good
laboratory practice, with supporting publications on their
application, and these have been adopted into legislation in a number
of countries. IPCS (1992) has published a monograph, Quality
Management for Chemical Safety Testing, covering the important
aspects of good laboratory practice in a nonregulatory context and
quality control of chemical analyses.
In the United States, good laboratory practice for conducting
nonclinical laboratory studies for submission to the Food and Drug
Administration has been detailed. The standards for studies on
pesticides submitted to the Environmental Protection Agency were also
published, as were the procedures to be followed in conducting studies
submitted on compounds covered by the Toxic Substance Control Act.
Each of these sets of standards is periodically updated by the
respective agencies, and studies must be conducted in accordance with
the most recent updates. While there are some differences in the
wording of the standards, they are generally similar.
Good laboratory practice includes written protocols for
evaluating potential immunotoxicants and the establishment of standard
operating procedures for assays. Each laboratory must run the assays
frequently enough to establish historical control values, and
the results of any study conducted to evaluate a compound for
immunotoxicity must be judged in the context of the historical control
values for the laboratory and appropriate controls. Incorporation of
positive control compounds in the study design provides additional
confidence that the assays are being conducted correctly, particularly
when the tested compound shows no effect.
The selection of assays to be used in evaluating compounds for
immunotoxicity remains a subject of active discussion. Other parts of
this document address this issue in detail. Regardless of which assays
are used, however, they must be standardized and be recognized as
validated and meaningful. Significant advances have been made in the
standardization and harmonization of assay procedures for assessing
immunotoxicity, mainly as a result of the willingness of leading
laboratories in the field to share their standard operating procedures
openly with other laboratories. Published papers and books on
immunotoxicity test methods also contribute to the standardization
process. As a result of studies by Luster et al. (1988), the assays
used by the NTP for evaluating potential immunotoxicants have been
accepted as validated assays in mice.
4.8 Validation
An important requirement of tests for evaluating immunotoxicity
is that they be validated. While there is no agreed definition of
validation, tests must meet certain requirements. In toxicology,
validation is the process by which the reliability and relevance of a
test to identify human health risk is established (Balls et al.,
1990). A flow diagram of a proposed validation process and its end-
point, the acceptance of a method by regulatory authorities for
submission of toxicological data, is presented in Figure 36.
Four parameters must be considered in determining the validity of
a testing method: specificity, sensitivity, accuracy, and precision.
Specificity is based on the rate of false-positive results generated.
Sensitivity is determined by the ability to identify true-positive
results. These two parameters determine the level of predictability or
relevance. In order to determine specificity and sensitivity, the
method is evaluated with a set of compounds of known positive and
negative immunosuppressiveness. This approach was used by the NTP in
 evaluating the predictability of various assays (Luster et al., 1988).
In a subsequent study, the potential immunotoxicity (defined as a
dose-related effect on any of two immunotoxicological parameters with
no effect on body weight) was determined for 51 chemicals in mice,
using a variety of general and functional immunological parameters
(Luster et al., 1992).
Accuracy is determined by the ability to measure the intended
end-point truly. Precision is the ability to reproduce results from
experiment to experiment or between laboratories. In the NTP studies
in mice (Luster et al., 1988), four laboratories participated in the
inter-laboratory validation process. A number of international studies
are in progress on the precision of several assays, using the rat as a
model for immunotoxicological evaluations, in an attempt to bring the
level of acceptance of immunotoxicological studies in rats to the
level that has been achieved for mice. Most of these studies are
multinational and represent an interaction of industry, government,
and academia to achieve this common goal.
A comparative study in Fischer 344 rats with cyclosporin A (White
et al., 1994) encompasses nine laboratories in Canada, Europe, and the
United States. The primary focus of the study is on the use of
functional assays for detecting immunosuppression; lymphoid organs and
tissue are weighed and examined histopathologically in several of the
laboratories. The functional assays used in this protocol include the
plaque-forming cell assay or ELISA to sheep erythrocytes, splenocyte
proliferative assays to concanavalin A and STM, the NK cell assay, and
the mixed leukocyte response. Splenocyte surface markers were also
analysed. The study design was similar to that used by the NTP, with a
14-day exposure and administration by oral gavage. The preliminary
results demonstrated excellent reproducibility of the results for the
plaque-forming cell assay, splenocyte proliferative assays to
concanavalin A and STM, and the mixed leukocyte response. Differences
were observed between the laboratories in the results of the test for
NK cell activity.
The IPCS-European Union international collaborative
immunotoxicity study in rats is also in progress. The study involves
20 laboratories in Canada, Europe, Japan, and the United States; its
design is based on the OECD test guideline No. 407 for a 28-day
toxicity study. The study focuses primarily on the ability to detect
immunotoxic compounds on the basis of organ weights, pathological
findings, and 'enhanced pathology', which includes additional
evaluation of lymphoid tissues not currently required by test
guideline No. 407. Functional assays were also conducted; the core
assays included the plaque-forming cell assay or ELISA to sheep
erythrocytes, splenocyte proliferative assays (concanavalin A and
STM), and the NK cell assay. The study was conducted in two phases. In
the first phase, azathioprine was used as the test compound, various
strains of rat were used, and each laboratory established its own
doses on the basis of a predetermined maximum tolerated dose. In the
second phase, cyclosporin A was the test compound, only three strains
of rat were used, and a more structured protocol was followed. A
report on phase I of the study has been drafted, and the data from
phase II are currently being analysed. All of the laboratories found
that azathioprine is immunosuppressive, even though several strains of
rats were used, different dose levels were administered, and no
standard protocol was followed. The preliminary results with
cyclosporin A show good agreement between the laboratories for the
plaque-forming cell assay but some differences for the splenocyte
proliferative assays (concanavalin A and STM) and the NK cell assay.
A third interlaboratory study in rats has been organized by the
German Bundesgesundheitsamt, in Berlin, with German and French
participants. The design is also based on OECD test guideline No. 407
for a 28-day repeated-dose study in Wistar rats. Cyclosporin A was
selected as the test substance. The live phase of this study has been
completed, the data are being analysed, and the final report is being
prepared.
Information obtained from studies of predictability, accuracy,
and precision, such as those described above, must undergo peer review
before publication. A major goal of the validation process is to
determine which methods should be recommended in the testing
guidelines of regulatory agencies. Figure 36 shows each input and
output of information and the action steps. The process of developing
and obtaining acceptance of testing guidelines is based on three major
inputs: (1) publication of reports in peer-reviewed journals;
(2) guidelines for deciding whether a method is valid; and
(3) implementation of test methods that are interpretable by
scientists involved in assessing biologically relevant risks and the
results of which can be incorporated into quantitative dose-response
analyses. The proposed process is built around the generation of these
major inputs. Two of the issues that will arise in the development of
guidelines are: 'How many and what type of compounds should be
included in the validation process?' and 'Should the compounds be
shown to be immunosuppressive in both humans and mice?'
5. ESSENTIALS OF IMMUNOTOXICITY ASSESSMENT IN HUMANS
5.1 Introduction: immunocompetence and immunosuppression
An immune response in the fully mature, immunologically competent
individual provides protection against a myriad of infectious agents
and environmental hazards. The immune system acts as a self-restoring
(homeostatic) system which can quickly return to normal levels of
function after periods of marked stimulation and response. This self-
regulation allows the individual to recover from or circumvent the
toxic effects of many potentially damaging environmental hazards.
There are many well-known clinical conditions of inherited deficiency
in immunological function; some result in specific defects in antibody
formation, others consist of T-cell and/or metabolic defects, while
others include impairment of both B- and T-cell function. These
conditions, known as primary immunodeficiency disorders, are due to
definable, inheritable genetic defects. Clinical studies of these
disorders have demonstrated the importance of the immune system to
host defence and individual survival, showing that individuals with
partial or absolute defects in T-cell function rarely survive beyond
infancy or early childhood. In contrast, individuals with defects in
B-cell function, resulting in a deficiency in antibody formation, may
suffer from a variety of chronic, recurrent infectious diseases and
diminished health but can survive with appropriate therapy when the
underlying disorder is recognized. Study of these genetic
immunodeficiency states has also provided considerable information
about the functions of human B and T cells which would otherwise not
have been determined.
Impairment of the function of a key component of the immune
system results in a diminished immune response (immunosuppression) or
immunodeficiency. Acquired immunodeficiency states were recognized
only sporadically until the late 1970s, when a syndrome appeared that
spread rapidly through certain groups and produced a generalized type
of immunosuppression known as acquired immunodeficiency syndrome
(AIDS). AIDS was found to be due to retroviruses that infect and
destroy Th (CD4+) cells in humans (Fauci et al., 1991). CD+
lymphocytes have been identified in experimental studies as the key
cells in the recognition and secondary processing of antigens. Thus,
progression of AIDS is associated with progressive loss of Th cells
and increased frequencies of infection by bacterial, fungal, viral,
and parasitic agents and of certain types of neoplasms.
5.2 Considerations in assessing human immune status related to
immunotoxicity
The assessment of immunotoxicity in humans exposed to potentially
immunotoxic compounds is much more complicated than in experimental
animals. Issues such as logistics, appropriate controls, magnitude and
pattern of exposure, and confounding parameters such as medication,
drug abuse, and illness must be considered. Other considerations that
should be taken into account in comparing human immune status with
that in laboratory animals in relation to immunotoxicity are as
follows:
(1) The human population is heterogeneous and genetically
disparate; it can be considered as 'wildlife'. Inbred laboratory
animals are, by definition, genetically identical; outbred laboratory
animals typically have a larger genetic variability than inbred
animals but a variability that is much smaller than that in wildlife
populations. Genetic constitution, which accounts for the variability,
has consequences for the antigen recognition capacity of the immune
system, especially for the T-lymphocyte population. Antigen
recognition by T cells is restricted to the MHC haplotype of the
individual and therefore differs between (allogeneic) individuals.
Inbred, and most outbred, laboratory animals are much more alike in
antigen recognition capacity than wildlife populations. For instance,
the repertoire of certain inbred mouse strains lacks part of the
spectrum of T-cell specificity, as seen by the absence of T cells that
express distinct 'variable gene families' in the repertoire of TCR
specificities. Such 'gaps' in the repertoire have thus far not been
detected in the outbred human population by similar methods of
detection (Hu et al., 1993), perhaps because each individual in an
outbred population expresses the MHC products of both parents and can
in principle multiply the repertoire of MHC-restricted reactions by a
factor of 2 (including MHC I and MHC II).
Interindividual variability has obvious consequences for
immunotoxicity, in which the response to the chemical or drug
underlies the mechanism of toxicity. Its effect on direct toxicity is
presumably less, but the manifestation of toxicity is often reduced
immune reactivity (e.g. increased incidence of infections) and hence
determined by individual reactions to antigens.
(2) The human population, like populations of 'wildlife' animals,
is continuously exposed to environmental stimuli. It is well known
that it is not necessary that each member of a population be protected
('immune') but that a certain proportion of protected individuals must
be reached in order to achieve 'herd immunity'. That is to say, the
whole population is protected when a certain percentage of individuals
is immune. In contrast, when this percentage falls below the required
incidence (which differs for different infectious agents), the
population as a whole loses its protected state, and an infectious
epidemic can result. This situation easily arises in small groups in a
country where vaccination is minimal. Well-known examples are the
outbreaks of poliomyelitis in the Netherlands and the hepatitis A
virus outbreaks in China. This phenomenon should be taken into account
in epidemiological studies and associated laboratory investigations
(e.g. antibody levels to microorganisms) in assessing immunotoxicity
in human populations.
(3) Most of the human population is continuously exposed to
environmental stimuli and maintains its ability to respond to foreign
material from the pool of immunological memory. From the first
postnatal period through to adulthood, the T-cell repertoire is
generated by the thymus; later, this generation of cells is reduced to
a low level. Strict MHC restriction implies that the T-cell population
cannot easily change specificity, e.g. by somatic mutation of
the genes that encode the TCR. There is some evidence from
immunophenotyping, both in mice and humans, that the T-cell population
shifts gradually during life, from naive (committed) T cells to memory
T cells. Within the B-cell population, the situation is different.
Here, the repertoire changes continuously, due to somatic mutation
presumably associated with 'affinity maturation' in lymph nodes. This
phenomenon may result in the emergence of B cells with a strong
affinity for stimuli and the disappearance of low-affinity cells. It
is not known whether neoantigens or pathogens are recognized by
'affinity matured' or memory B cells or by naive B cells. In the
absence of information on this aspect, it can be suggested that most
of the immune capacity of adults is deployed for memory reactions,
whereas in young people the contribution from the naive pool is
higher. This is reflected in infectious epidemics, when microorganisms
like influenza change to phenotypes that cannot be recognized by the
memory pool, an aspect to be kept in mind when choosing immune tests
to be used in evaluating immunotoxicity. Primary responses, like those
to keyhole limpet haemocyanin antigen, are considered to be more
sensitive than secondary responses, like those to tetanus toxoid.
Another example of this effect is the composition of the recall
antigens used in testing delayed-type hypersensitivity (Borleffs et
al., 1993). Both primary and secondary antibody responses, however,
are valuable for evaluating the intrinsic naive and memory immune
capacity of individuals, although secondary responses are less
sensitive to immunological insults.
(4) For a number of infectious microorganisms, the immune
response does not result in complete elimination of the invader but
rather in its 'silent' integration into the genome. Certain viruses,
like herpes viruses, cytomegalovirus, and Epstein-Barr virus, are
dealt with in this way. An individual is considered to be a carrier of
the virus (postinfection status) on the basis of the presence of
antibodies. In other words, individual postinfection is a continuous
defence against these viruses, often with sufficient capacity to keep
the virus in a silent form. In diminished immune capacity, this
natural protection can be lost, and infections can re-occur after
viral reactivation. Primary infection and reactivation therefore have
different pathogeneses, although the subsequent disease may be
characterized by the same symptoms. This situation is well known
clinically, when high doses of immunosuppressive drugs are given for
long periods. The relevance of this observation in immunotoxicity
testing must be established.
(5) Ex-vivo diagnosis in humans is often restricted to
haematological investigations, so that only information on the
circulatory pool of cells and plasma factors is obtained. For example,
the distribution of immunoglobulins differs in the intravascular and
extravascular spaces. Only about 1% of the total lymphocyte pool is
present in blood (1010 cells out of the total of about 1012), and
this population represents only the recirculating pool of cells and
not the tissue-bound cells that participate actively in immunological
responses. Investigations of peripheral blood cells can be somewhat
misleading: for instance, patients infected with the human
immunodeficiency virus (HIV)-1 may show severe depression of CD+
cells in blood but less reduction in CD cells in lymphoid tissue
(Schuurman et al., 1985).
It is considerably more complex to establish immune changes in
humans than in animals, since noninvasive tests are limited, the
levels of exposure to an agent (i.e. dose) are difficult to establish,
and the responses in the population are extremely heterogeneous. With
respect to the latter, the variation in immune responses (genetic or
environmental) can exceed a coefficient of variation greater than
20-30%. Because many of the immune changes in humans that follow
exposure to chemicals may be sporadic and subtle, recently exposed
populations must be studied and sensitive tests for assessing the
immune system be performed. Since many of the immune tests used in
humans have a certain degree of overlap (redundancy), it is also
important that a positive diagnosis not be based on a change in one
test but on a profile (pattern) of changes, similar to that observed
in primary or secondary immunodeficiency diseases. For example, low
CD:CD8 ratios are often accompanied by changes in skin reactions to
recall antigens. The Clinical Immunology Subcommittee of WHO and the
International Union of Immunological Societies published methods for
examining changes in the human immune system and described their
pitfalls (Bentwich et al., 1982, 1988); however, most of the tests
were selected on the basis of observations in patients with primary
immunodeficiency diseases. Such individuals suffer from severe
recurring infections, and their degree of immunosuppression is
probably considerably greater than that induced by chemicals. Thus,
some of the methods may be of limited value for examining potential
chemical-induced immunosuppression, and further evaluation of methods
is needed.
In view of the difficulties in identifying chemical-induced
immunosuppression in humans, establishment of exposure levels (e.g. in
blood or tissue) would not only be useful but would in many instances
be essential for determining a cause-effect relationship. Clinical
disease may not necessarily have to be present in order for
immunosuppression to be meaningful, for several reasons. Firstly,
there are uncertainties about the extent of the reserve capacity of
the immune system and whether the relationship between immune function
and clinical disease shows a linear or a threshold response. In a
linear relationship, even minor changes in immune function would be
related to an increase in disease, if the population examined is large
enough. While the relationship at the low end of the dose-response
curve is unclear, at the high end of the curve (i.e. severe
immunosuppression), clinical disease is readily apparent. This is
exemplified by increased incidences of the opportunistic infections
that occur in AIDS patients. Secondly, clinical disease may be
difficult to establish, as neoplastic diseases may involve a
10-20-year latency before tumour appearance, and increased infection
rates are difficult to ascertain in epidemiological surveys (e.g.
increased numbers of cases of severe common cold).
5.3 Confounding variables
The normal population has a wide range of immunological
responses, with no apparent health impact. In addition to the
underlying population variability, certain host characteristics and
common exposures may be associated with significant, predictable
alterations in immunological parameters. If not recognized and
effectively addressed in study design or statistical analysis, these
confounding factors may severely alter the results of population
studies.
Examples of factors associated with measurable alterations in
immunological parameters are age, race, sex, pregnancy status, acute
stress and the ability to cope with stress, coexistent disease or
infection, nutritional status, lifestyle, tobacco smoking, and some
medications. The effect of acute stress on the immune system is
mentioned in section 1.2.1.5. Protein calorie restriction and
deficiencies of trace elements such as zinc have also been associated
with immune deficiency (Chandra, 1992; Good & Lorenz, 1992; Chandra,
1993). Periodic influences, ranging from daily to seasonal, also
exist; some are relatively minor, but others are of a magnitude that
may rival the expected effect of a low-level exposure to a toxic
agent. They are therefore of primary concern in large epidemiological
studies. For example, African Americans have, on average, serum IgG
levels that are 15-20% higher, neutrophil counts that are 10-15%
lower, and a proportion of circulating B cells that is significantly
higher than those of Caucasians, with no discernible health
implications. Cigarette smoking is associated with a significant
decrease in IgG level and an increase in leukocyte count, independent
of ethnic differences. Therefore, it is imperative that population
norms and reference ranges be supplemented by detailed analysis of
potential confounding factors. Study designs should include
considerations of matching, stratification, and subgroup analysis to
control for these potential effects. As new immunological assays are
developed, normative data will be required, particularly for ethnic
minorities, children, the aged, and certain groups potentially at high
risk, such as pregnant and lactating women.
Certain endocrine diseases and conditions may be associated with
significant alterations in immune function (e.g. adrenal dysfunction).
Some medications, such as corticosteroids, phenytoin, and nonsteroidal
anti-inflammatory agents, may depress a variety of immune functions.
Questionnaires and population surveys should allow the collection of
sufficient information to make it possible to consider these factors
in data analysis and interpretation.
An increasingly important consideration in any analysis of immune
function, particularly in relation to immune deficiency, is the
potential presence of HIV infection, which causes widespread
alterations in virtually all elements of the immune system. Even a
small proportion of unrecognized HIV-infected individuals in a study
population may significantly affect the results and the interpretation
of data. When immunological studies indicate decreased immune
parameters consistent with HIV infection, testing for the virus should
be considered; otherwise, interpretation of the results of
immunological tests of immune dysfunction, particularly among
populations with potentially high rates of HIV infection, may be
severely limited.
In assessing human immunotoxicity, it is useful to establish the
presence of infectious, allergic, or autoimmune diseases in order to
ensure completeness and to rule out additional confounding variables.
A clue to the type of immunological defect is often provided by the
kind of infection observed. For example, patients with impaired
humoral immunity have an increased incidence of recurrent infections
with encapsulated bacterial pathogens (e.g. Pneumococcus and
Haemophilus influenzae), which can induce chronic sinopulmonary
infection, bacteraemia, and meningitis. In contrast, if cellular
immunity is intact, the patients will have less severe infections with
fungal and viral agents. Abnormalities of T cells and impairment of
cell-mediated immunity predispose individuals to infections with a
wide variety of agents, including viruses that cause disseminated
infections (e.g. Herpes simplex virus, varicella-zoster virus, and
cytomegalovirus), fungi that cause mucocutaneous candidiasis, and
parasitic organisms including the protozoan Pneumocystis carinii.
5.4 Considerations in the design of epidemiological studies
An important factor in assessing the usefulness of an
epidemiological study for risk assessment is its design. The commonest
design used in immunotoxicology is the cross-sectional study, in which
exposure and disease status (in this case, changes in immunological
function) are measured at one time or over a short period. The immune
function of 'exposed' subjects is compared with that of a comparable
group of 'unexposed' individuals. Important considerations in using
the data provided by such studies in risk assessment have been
discussed (E. Ward, unpublished manuscript):
(1) What is the relationship between changes in immune function and
human health risk?
(2) Are the selection procedures for study subjects adequately
documented?
(3) Is there evidence that the exposed group was actually exposed to
the substance of interest?
(4) Has the possibility that other exposures, of either the entire
population or individuals, been accounted for?
(5) Are the 'exposed' and 'unexposed' populations comparable with
respect to factors other than the exposure of interest?
(6) Have major individual aspects (such as illness and use of
medications) that may influence the outcome of tests for immune
function been accounted for?
(7) Has inter- or intralaboratory variability been controlled for?
Was the laboratory that ran the tests for immune function able to
distinguish between samples from 'exposed' and 'unexposed'
individuals?
5.5 Proposed testing regimen
Biological research involving human subjects must be conducted in
accordance with ethical standards and involve scientific procedures
designed to ensure the safety of the subjects (Council for
International Organizations of Medical Sciences, 1993). Below are
shown a testing scheme proposed by WHO for preliminary evaluation of
individuals exposed to immunotoxicants, an approach developed by a
working group organized by the United States Centers for Disease
Control and Agency for Toxic Substances and Disease Registry, and that
proposed by a panel of the United States National Academy of Science
(US National Research Council, 1992). The three approaches have many
similarities.
5.6 Assays for assessing immune status
A plethora of tests has been developed to assess immunity in
humans (Bentwich et al., 1982, 1988), which are described in
laboratory manuals (Lawlor & Fischer, 1988; Miller et al., 1991;
Coligan et al., 1994). Many of these tests are now commercially
available in kits. A systematic approach to the evaluation of immune
function, which is based on simple screening procedures, followed by
appropriate specialized tests of immune function, usually permits the
definition of an immune alteration. The tests should include
evaluation of the B-cell system, of the T-cell system, and of
nonspecific resistance (polymorphonuclear leukocytes, monocytes and
macrophages, NK cells, the complement system). Although some exogenous
Assays suggested by WHO for assessing immunotoxicity in all persons
exposed to immunotoxicants
1. Complete blood count with differential counts
2. Antibody-mediated immunity (one or more of following):
* Primary antibody response to protein antigen (e.g. epitope-
labelled influenza vaccine)
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
* Secondary antibody response to protein antigen (diphtheria,
tetanus, or poliomyelitis)
* Natural immunity to blood-group antigens (e.g. anti-A and
anti-B)
3. Phenotypic analysis of lymphocytes by flow cytometry
* Surface analysis of CD3, CD4, CD8, CD20
4. Cellular immunity
* Delayed-type hypersensitivity skin testing using Multitest
Biomerieux(R)
* Primary delayed-type hypersensitivity reaction to protein
(keyhole limpet haemocyanin)
* Proliferation to recall antigens
5. Autoantibodies and inflammation
* C-Reactive protein
* Autoantibody titres to nuclei, DNA, mitochondria and IgE
(rheumatoid factor)
* IgE to allergens
6. Measure of nonspecific immunity
* Numbers of natural killer cells (CD56 or CD60) or cytolytic
activity against K562
* Phagocytosis (nitroblue tetrazolium or chemiluminescence)
7. Clinical chemistry
Screening panel recommended for human studies by the United States Centers for
Disease Control and Agency for Toxic Substances and Disease Registry
* Complete blood count with differential counts
- absolute lymphocyte count
- granulocyte count
- platelet count
- absolute eosinophil count
- examination of peripheral smear
* Immunoglobulins
- IgG
- IgA (optional)
- IgM (optional)
* C-Reactive protein
* Autoantibody screening panel
- Antinuclear antibody
- Rheumatoid factor
- Anti-thyroglobulin antibody
* Peripheral blood leukocyte surface markers
- CD2/CD3
- CD4/CD8
- CD8/CD3
- CD19/CD20
* Clinical chemistry in serum
- Blood urea nitrogen
- Cholesterol
- Creatinine
- Total bilirubin
- Alkaline transaminase
- Alkaline phosphatase
- Total protein (albumin:globulin ratio)
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
Tier 1 (all persons exposed to immunotoxicants)
I. Humoral immunity
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
and immunofixation electrophoresis
* Natural immunity: Antibody levels to ubiquitous antigens
(e.g. anti-A and anti-B group substances in individuals of
non-AB blood type)
* Secondary antibody responses to proteins (e.g. diphtheria,
tetanus, poliomyelitis) and polysaccharides (e.g.
pneumococcal, meningococcal)
Note: In immunization studies, live microorganisms should not be
given to persons suspected of being severely immunocompromised.
II. Lymphocytes
* Enumeration of B and T cells in blood
* Surface analysis of CD3, CD4, CD8, CD20
* Secondary delayed-type hypersensitivity reaction (e.g.
candida, diphtheria, tetanus)
* Alternative: Multiple antigen skin test kit
III. Autoantibody titres (to red blood cells, nuclei, DNA,
mitochondria, IgE (rheumatoid factor)
Tier 2 (all persons with abnormal Tier 1 test results and
a fraction of the total exposed population to be determined
by a statistician)
I. Humoral immunity
* Primary antibody response to protein and polysaccharide
antigens
Note: A panel of antigens should be developed that can be used
in sequential studies on a given individual, since a particular
antigen can be used only once to assess a primary response.
II. Cellular immunity
* Proliferative response to mitogens (phytohaemagglutinin,
concanavalin A) and possible antigens such as tetanus;
primary delayed-type hypersensitivity reaction to keyhole
limpet haemocyanin
Note: Here, too, a panel of standard antigens is needed for
sequential testing; these could be the same as those used to
assess primary antibody responses.
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
III. Natural killer cells, monocytes, and other T- and B-cell markers
* CD5, CD11, CD16, CD19, CD23, CD64; class II MHC on T cells
by two-colour flow cytometry for co-expression of class II
and a T-cell marker such as CD3
IV. Serum levels of cytokines (e.g. IL-1, IL-2, IL-6) and of shed or
secreted cellular activation markers and receptors (e.g. CD25).
V. Class I and II MHC antigen typing
Tier 3 (to be considered for persons with abnormalities
in Tier 2 tests or for a random fraction of the entire
population in Tier 2)
* If a proportion of CD16 cells of Tier 2, III, is abnormal:
nonspecific killing of a tumour cell line to test for natural
killer function
* If primary delayed-type hypersensitivity reaction in Tier 2, II,
is abnormal:cell proliferation in response to phorbol ester and
calcium ionophore,anti-CD3 antibody, and a staphylococcal
enterotoxin B (experimental)
* Generation of secondary cell-mediated immune reactions
(proliferation and MHC-restricted cytotoxicity) in vivo, e.g.
with influenza virus (experimental)
* Immunoglobulin subclass levels in serum (IgA1, IgA2, IgG1-4)
* Antiviral titres (e.g. influenza, parainfluenza,
cytomegalovirus, human immunodeficiency virus) in serum (no
deliberate immunization)
agents can alter several elements of the human immune system, others
have a primary effect on a single element. For example, low doses of
cyclosporin A selectively affect T cells by acting on the production
of IL2 and IL2 receptors. Conversely, the anticonvulsive drug
phenytoin acts primarily on the humoral immune system, leading to a
selective deficiency of IgA.
A number of immune function assays recommended for inclusion in
a simple screening panel for assessing human immune function after
potential exposure to xenobiotics believed to affect the immune system
are described below. It should be noted that there are many indicators
of altered immune function in humans which may not be specific markers
for exposure, immune disease or susceptibility (IPCS, 1994a;
IPCS/Department of Health, 1995).
5.6.1 Total blood count and differential
A complete blood count, with differential absolute counts of
lymphocytes, granulocytes, eosinophils, and platelets, are basic
components of immunotoxicology. These tests are useful in defining the
general health status of a population, since they are relatively well
standardized over most age, sex, and race groups. Such counts are also
essential for interpreting the results of ex-vivo/in-vitro functional
tests, described below, since functional tests reflect a combination
of numbers of cell types and activity per cell.
The absolute lymphocyte count is critical: Higher absolute
counts are found in children than in adults, but lymphocyte counts
consistently below 1500/mm3 are indicative of lymphocytopenia, and a
higher count signals a defect in the T-cell system or effects on the
bone marrow. Lymphocytopenia can occur not only in primary immune
deficiency but also as a result of viral infections, malnutrition,
stress, autoimmune diseases, and haematopoietic malignancies.
Examination of bone marrow may be indicated to exclude some other
factors once lymphocytopenia has been confirmed. Additional assessment
of cell mediated immunity and direct measurement of T-cell parameters,
such as lymphocyte phenotypic markers, may also be indicated.
Review of peripheral smears for morphological abnormalities of
the white and red cells adds useful information for interpreting raw
cell counts, such as the atypical lymphocytosis of many acute viral
infections. The absolute eosinophil count can be very helpful in
delineating allergic disorders, vascular collagen diseases, and
parasitic manifestations.
5.6.2 Tests of the antibody-mediated immune system
Evaluation of antibody-mediated immunity involves measurement of
serum concentrations of immunoglobulins and assessment of antibody
formation after immunization or measurement of 'natural antibodies'.
5.6.2.1 Immunoglobulin concentration
Several methods are available for measuring the concentrations
of the five major classes of immunoglobulin, IgM, IgG, IgA, IgD, and
IgE, in serum, including single radial diffusion, double diffusion in
agar gel, immunoelectrodiffusion, radioimmunoassay, ELISA, and
automated laser nephelometry. Single radial diffusion is widely used.
Gel diffusion methods are very sensitive to differences in diffusion
constants and thus to differences in molecular size.
The serum concentration of each of the major immunoglobulins
should be determined, with the exception of IgD (which occurs
predominantly on the cell surface). The determinations must be well
standardized because antisera vary in quality. Since serum
immunoglobulin concentrations vary with age and environment,
appropriate norms must be used. Patients can manifest a deficiency in
all immunoglobulin classes (common variable hypogammaglobulinaemia),
or they may have a deficiency in only a single class (IgA deficiency
as a primary defect or after phenytoin therapy).
The concentration of immunoglobulins cannot be used as the
sole criterion for a diagnosis of immunodeficiency. Diminished
immunoglobulin concentrations can result from loss into the
gastrointestinal tract as well as from decreased synthesis. An
indication of loss can be obtained by measuring serum albumin, which
is usually lost concomitantly.
5.6.2.2 Specific antibodies
Antibody-mediated immunity can be assessed from antibody
responses to common specific antigens (basal levels). Humoral immunity
after immunization can be assessed in the same way. The response to
antigenic stimulation with both protein and polysaccharide antigens
must be defined if immunodeficiency is strongly suspected. Failure to
respond to one or more classes of antigen has been observed in
patients with normal or high levels of immunoglobulins, regardless of
whether they have an isolated immunoglobulin class or subclass
deficiency. Specifically, patients with the Wiskott-Aldrich syndrome
may have normal or even elevated immunoglobulin concentrations, yet
have multiple infections because of their failure to mount a specific
immune response, especially when they are exposed predominantly to
polysaccharide antigens.
Natural antibodies: Isohaemagglutinins are naturally occurring
antibodies to blood group A and B antigens found in all normal
individuals except those with type AB red cells. By three years of
age, 98% of normal persons with type A, B, or O blood have
isohaemagglutinin titres of at least 1:16. Patients with the Wiskott-
Aldrich syndrome may have normal immunoglobulin levels yet lack
isohaemagglutinins as an indicator of their antibody-deficient state.
Other natural antibodies that can be assayed include heterolysins
(e.g. against sheep or rabbit red blood cells) and antistreptolysin.
Antibody response to immunization: In order to test for
T cell-dependent antibody responses, commercially available
diphtheria-tetanus vaccine can be given in recommended doses. Blood is
taken two weeks after each injection and tetanus and diphtheria
antibodies are determined. In patients who have been immunized with
diphtheria-tetanus or diphtheria-pertussis-tetanus vaccine, one
booster injection is given before determination of antibodies. In
testing for T cell-independent antibody responses, commercially
available pneumococcal vaccine can be given in recommended doses.
Three doses of killed poliomyelitis vaccine (1.0 ml intramuscularly,
at intervals of two weeks) can also be used as the immunogen. Blood is
taken two weeks after the last injection, and antibody is usually
determined by virus neutralization. There is strong consensus that
quantification of a primary immune response (antibody and/or cellular)
after immunization is not only a very relevant test but also very
sensitive. Although such tests are not routine in clinical immunology,
they have been used successfully for determining immune integrity.
While keyhole limpet haemocyanin has been used as the antigen,
beneficial immunizations such as influenza vaccine linked to a marker
epitope can also be used.
5.6.3 Tests for inflammation and autoantibodies
5.6.3.1 C-Reactive protein
Inclusion of an acute-phase reactant marker is helpful for
clinical interpretation of other laboratory biomarkers such as
autoantibodies. The concentration of C-reactive protein rises and
falls to baseline values in direct proportion to tissue damage and is
thus a sensitive indicator of the presence of a generalized
inflammatory state. It offers the best example of an accepted,
standardized procedure that can be used in large population studies,
because it is less subject to transport variables than other
procedures.
5.6.3.2 Antinuclear antibody
Antinuclear antibody is a common autoantibody that may be
associated with various rheumatic disorders and, classically, systemic
lupus erythematosus. Several commercial kits are available to detect
the presence of autoantibodies. Progressively greater titres increase
the specificity for a disease. Positive sera should be titred at
dilutions of > 1:40 to 1:640.
5.6.3.3 Rheumatoid factor
Rheumatoid factor is an autoantibody to immunoglobulin (usually
IgM) that occurs in a high percentage (50-70%) of individuals with
classical rheumatoid arthritis but may also develop in a variety of
other disorders, including infections and immunological diseases.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.3.4 Thyroglobulin antibody
This antibody occurs in association with a variety of thyroid
disorders but may be found without detectable thyroid dysfunction.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.4 Tests for cellular immunity
A variety of tests are commonly used to assess cell-mediated
immunity, including enumeration of T cells and T-cell subsets,
identification of delayed skin reactions, and measurement in vitro
of stimulation of lymphocytes to proliferate and form blast cells.
Other tests are available to measure T-cell effector or regulatory
function in vitro. As for humoral immunity, a series of simple tests
is available to screen for defects in cell-mediated immunity. The
proportion of circulating T cells in a mononuclear cell preparation
can be determined by immunofluorescence with CD2 or CD3 monoclonal
antibodies. Normally, T cells constitute 55-80% of peripheral blood
lymphocytes. The normal values reported for absolute numbers of
circulating T cells are 1620-4320/mm3 during the first week up to
18 months of life and 590-3090/mm3 after 18 months of age (Fleisher
et al., 1975).
5.6.4.1 Flow cytometry
Antibodies that may be used in immunological phenotyping are
listed in Table 10 in section 4.1.2. Subsets of T cells have been
defined with monoclonal antibodies specific to cell-surface antigens.
The association of a particular T-cell subset with a given function
has caused some confusion in the analysis of immunological data for
patients with immunodeficiency states, as discussed in more detail in
section 1.2.2.1. For example, CD-positive cells have commonly been
associated with helper functions, and CD8 cells have been associated
with cytotoxic functions. This approach is an oversimplification,
which became evident with the finding that CD and CD8 cells recognize
foreign antigens in the context of MHC class II and class I antigens,
respectively. Thus, the CD population contains helper cells, memory
cells, and cytotoxic cells for targets bearing MHC class II molecules.
The CD8 population contains cytotoxic cells and also cells that can
recognize antigens presented by macrophages and cells that can augment
the interaction of CD-positive cells with B cells. Abnormalities in
the number of CD or CD8 cells, or their ratio, can be associated with
abnormalities in the ability to recognize antigens and regulate T-cell
function that can lead to immune incompetence or autoimmunity.
5.6.4.2 Delayed-type hypersensitivity
The ability of patients to manifest pre-existing T-cell immunity
has been evaluated in vivo with a series of skin antigens that
normally produce a delayed-type cutaneous hypersensitivity response.
Because delayed cutaneous hypersensitivity, a localized immunological
skin response, depends on functional thymus-derived lymphocytes, it is
used in detecting T cell-mediated immunodeficiency. A device
(Multitest(R), Institut Merieux, Lyon, France) is available that
enables the simultaneous intradermal injection of seven standardized
antigens and so overcomes the inconvenience of giving seven separate
injections of antigens and the technical difficulty of ensuring their
intradermal rather than subcutaneous injection. The Multitest(R),
consisting of eight tined, preloaded heads, delivers a glycerol
control and the seven test antigens dissolved in glycerol. The size of
the induration produced by each antigen should be measured at 48h in
two diameters: reactions of less than 2 mm are scored as negative. No
reaction should be seen with the glycerol control. The test includes
as antigens: tetanus, diphtheria, streptococcus, tuberculin, Candida,
Trichophyton, and Proteus.
Recall of delayed-type hypersensitivity as a test for cell-
mediated immune competence was assessed with the Multitest(R) device
in 254 subjects. When 77 subjects were tested concurrently with the
Multitest(R) and a conventional panel of six antigens (Frazer et
al., 1985), similar results ( r = 0.65) were obtained with the two
systems. The reproducibility of the Multitest(R) among three
observers, who assessed the aggregate size of reactions in 45 subjects
independently, was high ( r = 0.89); the correlation for the reaction
score in 24 subjects tested twice, three months apart, was also high
( r = 0.88), demonstrating the suitability of the test for serial
studies of immune function.
5.6.4.3 Proliferation of mononuclear cells in vitro
A common test of lymphocyte function is measurement of the
capacity of lymphocyte subsets to enlarge and convert into blast-like
cells that synthesize DNA and incorporate thymidine after stimulation
in vitro. In this test, lymphocytes can be activated by antigens
(e.g. purified protein derivative, Candida, streptokinase, tetanus,
or diphtheria); allogeneic cells are also used in the one-way mixed
lymphocyte culture to stimulate T-cell proliferation. T-Cell blast
transformation can be assessed directly by measuring blastogenesis and
proliferation of cells, expression of activation antigens (e.g. CD69
or CD25 and HLA DR), and release of mediators. The blastogenic
response is assessed as incorporation of 3H-thymidine, usually for
16-24h, followed by cell precipitation on filter paper and liquid
scintillation counting. Non-isotope assay alternatives are also
available. The responses to various antigenic stimuli by different
types of responding cells must be interpreted with caution. The mixed
leukocyte reaction is the result of T-cell reactivity to MHC-encoded
peptides displayed on the surface of B cells and monocytes. The
T cells in the population of normal irradiated or mitomycin C-treated
lymphocytes used as the stimulators can secrete factors that induce
blastogenesis in the patient's lymphocytes. As this can be misleading,
it is preferable to use B-cell lines or T cell-depleted normal cells
as the stimulators.
5.6.5 Tests for nonspecific immunity
5.6.5.1 Natural killer cells
The differences between NK cell function, phenotyping for NK
cells, and the cytology of large granular lymphocytes are described in
section 1.2.2.1. The identification of NK cells remains problematic
owing to this apparent heterogeneity. The cells can be evaluated in
ex-vivo/in-vivo tests with enriched peripheral blood mononuclear
cells. The proportion of NK cells can be identified with appropriate
monoclonal antibodies (see Table 10). Functional assays of NK activity
involve the ability of the appropriate mononuclear cells to kill
specific NK targets, such as the K562 cell, in which cell-mediated
cytolysis in vitro is quantified by release of 51Cr from the
target cells.
5.6.5.2 Polymorphonuclear granulocytes
Some defects of phagocytic function affect polymorphonuclear
phagocytes. Neutrophil function depends on movement in response to
chemotactic stimulus, adherence, endocytosis, and destruction of the
ingested particles. Phagocyte mobility depends on the integrity of the
cytoskeleton and contractile system. Defects in intracellular killing
of ingested microorganisms usually result from failure of the
'respiratory burst' that is critical to production of superoxide
radicals and hydrogen peroxide. The organisms cultured from lesions of
patients with this type of defect generally contain catalase and
include staphylococci, E. coli, Serratia marcescens, fungi, and
Nocardia. Patients with defects in mobility, adherence, and
endocytosis usually have infections of the skin, periodontitis, and
intestinal or perianal fistulae; patients with normal endocytosis and
defective killing tend to have chronic granulomas. Measurement of
nitroblue tetrazolium dye reduction or chemiluminescence by actively
phagocytosing leukocytes has been accepted as a standard measure for
the adequacy of the respiratory burst. Recently, methods have been
developed to measure the production of reactive oxygen intermediates
by flow cytometry (Emmendorfer et al., 1994). Kits are commercially
available for assessing phagocytic capacities and the production of
reactive oxygen intermediates by phagocytes. Assays for bacterial
killing yield highly variable results, depending on the bacterial
species used in the assay, and are not recommended for routine use.
The activation state of neutrophilic granulocytes can be assessed by
flow cytometry with the antibodies CD11b, CD14, CD16, CD54, and CD64
(Spiekermann et al., 1994). Activation of platelets can also be
assessed by flow cytometry (Tschöpe et al., 1990)
5.6.5.3 Complement
The classic complement system consists of nine components (C1-9)
and a series of regulatory proteins (C1 inhibitor, C4 binding protein,
and properdin factors). Many biological activities important in the
inflammatory response and in host resistance to infection occur at
various points in the classical and alternative pathways of complement
activation. Three clinical states should raise a suspicion of
deficiency in a complement component: systemic lupus erythematosus,
recurrent infections of the type seen in hypogammaglobulinaemia in
patients with normal immunoglobulin levels, and severe Neisseria
infection. Laboratory measurement of serum haemolytic complement
(CH50) is a useful test. Serum haemolytic complement is usually absent
and rarely above 10% of the normal value in inherited complement
deficiencies, with the exception of hypercatabolism of C3. More
detailed analysis of the complement system requires functional and
antigenic measurements of the individual components, usually best
performed in laboratories that specialize in the complement system.
5.6.6 Clinical chemistry
Assessment of clinical abnormalities in standard serum
chemistry, such as liver function, renal function, glucose, and
albumin, is indicated to facilitate reasonable interpretation of
specific changes in the immune system as secondary to another
condition.
5.6.7 Additional confirmatory tests
After activation, mononuclear cells from peripheral blood
express the genes that encode a series of interleukins and colony-
stimulating factors. Activated T cells and monocytes synthesize and
secrete IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, interferon, other
cytokines such as tumour necrosis factor, and cytokine receptors.
These cytokines are involved in the growth and differentiation of
T and B cells, eosinophils, and basophils. The supernatants of
mononuclear cells from peripheral blood stimulated by soluble
phytohaemagglutinin can be assessed for IL-2 by determining their
capacity to stimulate 3H-thymidine uptake by mouse IL-2-dependent,
cultured T-cell lines. Other biological assays, radioimmunoassays, and
ELISAs have been developed to quantify the production of lymphokines
and colony-stimulating factors. With molecular cloning techniques,
messenger RNA transcription by each of the lymphokines can be
quantified after appropriate lymphocyte activation. In the assessment
of lymphokines, T cells or peripheral blood mononuclear cells are
usually activated with concanavalin A, pokeweed mitogen, or
insolubilized CD3 antibodies; then, the appropriate assays are
performed to quantify the specific lymphokine(s) produced in the
culture media. Different patterns of lymphokine secretion have been
observed with different subsets of T cells: INF gamma and IL-2 are
produced by the Th1 subset, while IL-4, IL-5, and IL-10 are produced
by the Th2 subset, as originally documented for cloned CD T-cell lines
from mice (Mosmann & Coffman, 1989). Thus, an assay of the pattern of
lymphokine production could be used in pinpointing the action of an
immunotoxicant on a particular subset of immune cells.
6. RISK ASSESSMENT
6.1 Introduction
Publications on immunotoxicology published by IPCS and the
European Union (Berlin et al., 1987; Dayan et al., 1990), the United
States Office of Technology Assessment (1991), and the United States
National Research Council (1992) demonstrate the growing interest and
concern within scientific and public communities on the capacity of
environmental agents to perturb normal immune processes. The
incorporation of experimental data on toxicant-induced alterations in
the immune system into evaluations of drugs, chemicals, and biological
agents for human risk assessment has thus become increasingly common.
For example, in the United States, the Environmental Protection Agency
(Sjoblad, 1988) and Food and Drug Administration (Hoyle & Cooper,
1990; Hinton, 1992) indicate the benefits of testing the
immunosuppressive potential of biochemical pest control agents,
antiviral drugs, and food additives. Furthermore, the Environmental
Protection Agency has established reference doses (an estimate of the
daily exposure of the human population that is likely to have no
appreciable risk of deleterious effects during a lifetime), on the
basis of data on the immunotoxicity of several compounds, including
1,1,2-trichloroethane, 2,4-dichlorophenol, and dibutyltin oxide. The
United States Agency for Toxic Substances and Disease Registry has
derived 'minimal risk levels' for arsenic, dieldrin, nickel,
1,2-dichloroethane, and 2,4-dichlorophenol on the basis of immune
end-points.
Risk assessment is a process whereby relevant biological, dose-
response, and exposure data for a particular agent are analysed in an
attempt to establish qualitative and quantitative estimates of adverse
outcomes (Scala, 1991). Such data are sometimes used in the
development of standards for regulating the manufacture, use, and
release of chemicals into the environment (Kimmel, 1990). As defined
by the United States National Academy of Science (US National Research
Council, 1983), risk assessment comprises four steps: hazard
identification, dose-response assessment, exposure assessment, and
risk characterization. The process of assessing the risk of both
cancer and non-cancer end-points, including immunotoxicity, may be
adapted to this form.
The first step, hazard identification, involves a qualitative
evaluation of available human and animal data to determine whether a
chemical agent poses a potential hazard. Consideration is given to
dose, route, and duration of exposure. The precise quantitative
relationships between changes in immune function or in the
histological appearance of lymphoid organs and host resistance to
infectious agents or neoplastic diseases are unclear. It can be
assumed that any significant difference from appropriate controls in
the ability of the immune system to respond to a challenge may evolve
into an adverse effect and thus present a potential hazard. This
applies to adaptive as well as nonadaptive responses.
After hazard identification, 'dose-response' is assessed. For
non-cancer toxicity, a no-observed-adverse-effect-level (NOAEL) is
established for an adverse response of interest. This process is no
different in immunotoxicology and is the same as for the other target
organ systems. The NOAEL value is either obtained from the dose-
response curve or is estimated from the lowest-observed-adverse-
effect-level (LOAEL). Once the NOAEL has been determined, safety and
uncertainty factors can be applied to allow for various uncertainties,
such as species or interspecies variability, irreversible effects, and
chronic exposure. The use of safety factors, however, should be
flexible and should allow incorporation of any relevant information on
the mechanism of action of the chemical under review. Ideally,
however, dose-response relationships should be established from human
epidemiological data that include the exposure levels expected on the
basis of human contact with the agent in the environment. As
illustrated in an assessment of developmental toxicity (Kimmel &
Gaylor, 1988), use of the risk assessment and management paradigm of
the United States National Academy of Sciences (US National Research
Council, 1983) to non-cancer end-points such as immunotoxicity, offers
some serious challenges. For example, since the presence or absence of
an effect is based upon whether a statistically and/or biologically
significant response is observed at a certain dose or doses, and since
multiple assays are routinely conducted, the NOAEL will depend heavily
on the sensitivity of the assay. The differences may be particularly
exaggerated when continuous responses (i.e. the results of most immune
function tests) are compared with categorical data, the latter being
routinely expressed as proportions. For example, in experimental
animals, changes in many immune functions may be statistically
significant when they vary by as little as 15-25% from the control
values. In contrast, host resistance, when expressed categorically
(e.g. tumour frequency), must change by 80% to reach a comparable
degree of significance, assuming group sizes of about 15 animals and
effective doses of 20% in the control group. Furthermore, immune tests
are often, but not always, interdependent (Luster et al., 1992), and
individual or combinations of tests might have to be ranked in order
of sensitivity and degree of interdependence before dose-response is
assessed. This has not been done in the past.
Exposure assessment (step 3) is done in parallel with hazard
identification and dose-response assessment (Scala, 1991). It often
involves field measurements and other estimates of human exposure,
such as the composition and size of the population, biological or
clinical effects and types, and the magnitude, frequency, and duration
of exposure to the agent. Many of these parameters are difficult to
determine accurately in a longitudinal study; even measurements of
body burden can be misleading since the concentrations at the target
organ (e.g. lymphoid tissue) are not determined. The problems in
immune testing in humans are similar to those in testing other organs
and systems and include differences in individual responses due to
unique sensitivities (e.g. age, pregnancy, genetics) and confounding
factors (e.g. smoking, stress, drugs).
Risk characterization (step 4) is the aggregation of the three
previous processes. It provides an estimate of the incidence of
adverse effects in a population and the potential health problems. As
part of risk characterization, the strengths and weaknesses of each
component of the assessment are considered, with assumptions,
scientific judgements, and, to the extent possible, estimated
uncertainties. Most assessments of the risks presented by chemical
agents have focused on the estimated incidence of cancer after
lifetime exposure to a chemical at some unit dose, assuming that there
is essentially no threshold for carcinogenicity. The assessment of
non-cancer end-points, such as disorders of the neurological,
developmental, and reproductive systems, is somewhat similar to that
of cancer, in that it involves calculations that include both
assumptions and uncertainties. For example, considerations in risk
assessment include ranking the value of epidemiological against
experimental data, extrapolations from high to low doses, from
subchronic to chronic exposure, and from animals to humans, and
appropriate use of mechanistic and pharmacokinetic data. Data from
immunotoxicology, like those from developmental toxicology (Schwetz &
Tyl, 1987), do not easily lend themselves to the mathematical models
used in cancer risk assessment, which usually involve non-threshold
models for genotoxic carcinogens. For more accurate assessment,
mechanistic models will be required which include the concept of
'threshold'. It is assumed that threshold levels exist below which no
adverse immunological effect can be demonstrated. Complex mixtures of
chemicals, in which each chemical occurs at a subthreshold
concentration, may reach or exceed a threshold for immunotoxicity
(Germolec et al., 1989), although problems associated with mixtures of
compounds are not unique to the field of immunotoxicology.
The approaches currently used by the US Environmental Protection
Agency (1986) in extrapolating the risk for developmental toxicity
have been outlined, and similar guidelines have been developed by IPCS
(1994b). One method is to apply uncertainty factors to the NOEL or
NOAEL for the most sensitive animal species tested. The uncertainty
factor is usually composed of a 10-fold factor to account for
interspecies differences and a 10-fold factor for intraspecies
variability. If no NOEL is available, an additional 10-fold factor may
be applied to the lowest-observed-effect level (LOEL). Another
approach is to calculate a margin of safety, which is the NOEL divided
by the estimated level of human exposure from all potential sources.
The margin of safety can then be evaluated for adequacy to protect
human health. There are several drawbacks to both of these approaches,
the primary one being that they use only one point on the dose-
response curve (NOEL or LOEL) and ignore the rest of the data. Also,
since the variability around the NOEL and LOEL is usually not taken
into account, these approaches may rely on poor studies, i.e. studies
that result in a higher NOEL because of their limited ability to
detect small changes over the background.
Since the purpose of risk assessment is to make inferences about
potential risk to human health, the most appropriate data are those
derived from studies of humans; however, adequate data are seldom
available, and most risk assessments are based on results obtained in
experimental animals. In order to use these results, a number of
assumptions must be made about their relevance to potential human
health risk. Firstly, it is assumed that experimental animals respond
to the agent of interest in a pharmacological and toxicological manner
similar to that anticipated in humans (i.e. the test animals and
humans metabolize the chemical similarly and have identical responses
and toxicity at the target organ). Secondly, the immune system of the
experimental species must be very similar to that of humans: the
vertebrate immune system is highly conserved among higher vertebrate
species, and the immune components and their interactions in mice,
rats, and humans are closely similar. Thus, if toxicokinetic
properties are similar, it is reasonable to test for potential adverse
effects in humans using laboratory rodents.
As immunosuppressive agents cannot ethically be administered to
humans, quantitative comparisons of dose-responses in humans and
experimental animals are limited (although it is possible to do so in
hypersensitivity tests). Nonetheless, controlled human exposures have
been studied and the results compared with the immune effects observed
in animals. As an example, the immune effects of cyclosporin A in
various species are compiled in Table 12, which shows that the mouse
is much less sensitive to cyclosporin A than other species, in
particular the rat, and that humans are slightly more sensitive than
other species (Dean & Thurmond, 1987); however, for the most part,
there was good qualitative and quantitative agreement between the
species examined. Selgrade et al. (1995) compared phagocytosis by
human and murine alveolar macrophages after exposure to ozone
in vitro and in vivo (Table 13): The effects of ozone on alveolar
macrophage function in murine species are predictive of effects that
occur in humans, and the effects on macrophage phagocytosis seen
in vitro are predictive of those that occur in vivo. Quantitative
comparisons have also been made in mice and humans for the ability of
UVR to inhibit the contact hypersensitivity response (Table 14). As
described in section 2.2.11, exposure to UVB inhibits delayed-type
hypersensitivity (Kripke et al., 1979). Noonan & Hoffman (1994)
described three strains of mice with low, intermediate, and high
susceptibility to UVR-induced immunosuppression, and Oberhelman et al.
(1992) reported that suppression of the hypersensitivity response also
occurs in humans and that the dose of radiation required to induce 50%
suppression in fair-and dark-skinned individuals is similar to that
required to inhibit the response in mice with high and intermediate
susceptibility, respectively.
Table 12. Comparison of doses of cyclosporin A that suppress
the immune response in various species
Species Response Cyclosporin A
(mg/kg body
weight)
Mouse Antibody production 50-300
Cell-mediated immunity 100-300
(delayed-type hypersensitivity)
Graft-versus-host reaction 50-250
Rat Antibody production 20-50
Graft-versus-host reaction 10-60
Guinea-pig Cell-mediated immunity 10-100
(delayed-type hypersensitivity)
Dog Cell-mediated immunity 15-30
(delayed-type hypersensitivity)
Rhesus monkey Antibody production 50-250
Human Cell-mediated immunity 10-20
Adapted from Dean & Thurmond (1987); White et al. (1994)
Data from immunotoxicology also differ from those for
carcinogenicity and possibly other non-cancer end-points because the
immune system contains components with overlapping functions. For
example, when an individual is exposed to an infectious agent,
multiple immune components may work either independently or in concert
to help defend the host; i.e. there is redundancy between immune
functions. Furthermore, while a significant change in any immune
function can be considered potentially deleterious, in that it may
increase the risk of developing clinical disease, a change in immune
function does not necessarily precipitate a disease or clinical health
affect. That is, immunocompromised individuals function normally in
the absence of infectious agents. Thus, immune function reserve and
redundancy are relative terms, depending on the dose of infectious
agent. This complicates dose-response assessment, and models should be
developed that incorporate available information on the quantitative
relationship between immune function and clinical disease and
potential redundancy.
Table 13. Effect of exposure to ozone on phagocytosis by alveolar
macrophages
Treatment Phagocytic index (no. fluorescent
particles ingested/100 macrophages)
--------------------------------------
Mice Humans
---------------- -------------
Mean SE Mean SE
Air in vitro 369.2 26.4 386.7 50.5
(n = 6) (n = 6)
Ozone in vitro 291.7 17.4* 275.0 45.1*
(n = 6) (n = 6)
Suppression 21% 29%
Air in vivo 330.6 10.4 714.9 46.1
(n = 4) (n = 10)
Ozone in vivoa 194.0 19.7* 539.2 22.3*
(n = 4) (n = 10)
Suppression 42% 25%
Suppression corrected 28% 25%
for dosimetric differenceb
Adapted from Selgrade et al. (1995)
* Significantly different from air control (P < 0.05; Student's
t test)
a Mice were exposed to 0.8 ppm for 3 h; humans were exposed to
0.08 ppm for 6.6 h while undergoing intermittent exercise.
b On the basis of studies using 18O, alveolar macrophages of
mice exposed to 0.8 ppm ozone for 3 h receive roughly 1.5 times
more ozone than those of humans exposed to 0.08 ppm ozone for 6.6 h
while exercising moderately.
Table 14. Comparison of doses of ultraviolet radiation that cause
50% suppression of contact sensitivity in mice and humans
Mousea Humanb
--------------------------------------------- --------------
Sensitivity kJ/m2 mJ/cm2 Skin type
mJ/cm2
(phenotype)
High (C57Bl) 0.7-2.3 70-230 Fair 100
Intermediate (C3H) 4.7-6.9 470-690 Dark 225
Low (BALB/c) 9.6-12.3 960-1230
Adapted from Selgrade et al. (1995)
a Data from Noonan & Hoffman (1994)
b Data from Oberhelman et al. (1992)
The increasing evidence that environmental contaminants affect
wildlife populations has led to risk assessment at the level of the
ecosystem; however, the limited evidence for immunotoxic effects in
wildlife precludes an adequate understanding of the risks posed by
current levels of environmental pollution. The demonstration of
immunosuppression in harbour seals fed herring from the contaminated
Baltic Sea in a semi-field experiment (De Swart et al., 1994; Ross et
al., 1995) provided a first indication that ambient levels of
contaminants in certain areas present an immunotoxic risk to mammalian
wildlife occupying a high trophic level. These results may partially
explain the severity of a series of unrelated epizootic viral episodes
in various marine mammalian populations in coastal areas of Europe and
North America (Dietz et al., 1989; Van Bressem et al., 1991). In
similar semi-field experiments in a bottom-dwelling fish species,
flounder exposed to contaminated sediments had more viral lymphocytic
infection and liver tumours than controls (Vethaak & Wester, 1993).
While the difficulties in conducting adequate immunological studies
with wildlife may preclude an approach as comprehensive as that in
humans, such semi-field strategies may provide the best available
direction.
Should the application of field studies be possible, correlative
approaches to immunotoxicology may substantiate an effect on the
ecosystem. Such an approach was used to establish a correlation
between the induction of mixed-function oxidases and pollutant burden
(as measured by the toxic equivalence of organochlorine chemicals) in
cormorant (Phalocrocorax carbo) chicks collected from various sites
in the Netherlands (van den Berg et al., 1994). A combination of
laboratory experiments under controlled conditions, semi-field
experiments under controlled conditions with exposure to environmental
mixtures of pollutants, and correlative field studies is necessary to
understand immunotoxicity in wildlife populations.
6.2 Complements to extrapolating experimental data
6.2.1 In-vitro approaches
The complexities of the immune system and the requirement of
many agents for metabolism and distribution in order to produce an
immunotoxic response have resulted in the almost exclusive use of
animal models in vivo for assessing immunotoxicity. Culture systems
have been used extensively, however, to study the mechanisms by which
agents induce immunosuppression. In-vitro test systems with immune
cells of human origin are particularly attractive, given the
uncertainties in extrapolating the results of studies in experimental
animals to humans and the accessibility of human peripheral blood
cells. Although many of the immune cells obtained from human blood are
immature forms, the large numbers and diverse populations (i.e.
polymorphonuclear granulocytes, monocytes, NK cells, T cells, and
B cells) that can be obtained and the ease of conducting challenge
assays in vitro provide an attractive alternative (or, preferably,
adjunct) to more conventional studies in animals. Surprisingly few
laboratories have conducted studies with immunosuppressive agents in
which immune function responses in human immune cells are compared
with thosein rodents in vivo (Cornacoff et al., 1988; Luo et al.,
1992; Wood et al., 1992; Lang et al., 1993). Althoughstudies in human
cells in vitro have been hampered by a lack of assays to assess
primary antigen-specific immune responses, a relatively good
interspecies correlation has been observed in the limited responses
examined. Furthermore, some of these assays have been successfully
modified to include metabolic fractions of liver homogenates (Shand,
1975) or co-culture with primary hepatocytes (Kim et al., 1987) to
allow for chemical metabolism.
6.2.2 Parallelograms
Interpretation of studies in experimental animals or in vitro
can be improved when even limited data on human exposure in vivo are
available, using a 'parallelogram' approach. In general, if a
parallelogram can be constructed in which data are available for five
of the six angles (Figure 37), it may be easier to predict the outcome
at the remaining angle, at least qualitatively. For example, cytokine
and phagocytic responses of alveolar macrophages or pulmonary
epithelial cells after exposure to ozone in vitro can be compared
with the responses to ozone after exposure of humans and animals
evaluating the predictability of various assays (Luster et al., 1988).
In a subsequent study, the potential immunotoxicity (defined as a
dose-related effect on any of two immunotoxicological parameters with
no effect on body weight) was determined for 51 chemicals in mice,
using a variety of general and functional immunological parameters
(Luster et al., 1992).
Accuracy is determined by the ability to measure the intended
end-point truly. Precision is the ability to reproduce results from
experiment to experiment or between laboratories. In the NTP studies
in mice (Luster et al., 1988), four laboratories participated in the
inter-laboratory validation process. A number of international studies
are in progress on the precision of several assays, using the rat as a
model for immunotoxicological evaluations, in an attempt to bring the
level of acceptance of immunotoxicological studies in rats to the
level that has been achieved for mice. Most of these studies are
multinational and represent an interaction of industry, government,
and academia to achieve this common goal.
A comparative study in Fischer 344 rats with cyclosporin A (White
et al., 1994) encompasses nine laboratories in Canada, Europe, and the
United States. The primary focus of the study is on the use of
functional assays for detecting immunosuppression; lymphoid organs and
tissue are weighed and examined histopathologically in several of the
laboratories. The functional assays used in this protocol include the
plaque-forming cell assay or ELISA to sheep erythrocytes, splenocyte
proliferative assays to concanavalin A and STM, the NK cell assay, and
the mixed leukocyte response. Splenocyte surface markers were also
analysed. The study design was similar to that used by the NTP, with a
14-day exposure and administration by oral gavage. The preliminary
results demonstrated excellent reproducibility of the results for the
plaque-forming cell assay, splenocyte proliferative assays to
concanavalin A and STM, and the mixed leukocyte response. Differences
were observed between the laboratories in the results of the test for
NK cell activity.
The IPCS-European Union international collaborative
immunotoxicity study in rats is also in progress. The study involves
20 laboratories in Canada, Europe, Japan, and the United States; its
design is based on the OECD test guideline No. 407 for a 28-day
toxicity study. The study focuses primarily on the ability to detect
immunotoxic compounds on the basis of organ weights, pathological
findings, and 'enhanced pathology', which includes additional
evaluation of lymphoid tissues not currently required by test
guideline No. 407. Functional assays were also conducted; the core
assays included the plaque-forming cell assay or ELISA to sheep
erythrocytes, splenocyte proliferative assays (concanavalin A and
STM), and the NK cell assay. The study was conducted in two phases. In
the first phase, azathioprine was used as the test compound, various
strains of rat were used, and each laboratory established its own
doses on the basis of a predetermined maximum tolerated dose. In the
second phase, cyclosporin A was the test compound, only three strains
of rat were used, and a more structured protocol was followed. A
report on phase I of the study has been drafted, and the data from
phase II are currently being analysed. All of the laboratories found
that azathioprine is immunosuppressive, even though several strains of
rats were used, different dose levels were administered, and no
standard protocol was followed. The preliminary results with
cyclosporin A show good agreement between the laboratories for the
plaque-forming cell assay but some differences for the splenocyte
proliferative assays (concanavalin A and STM) and the NK cell assay.
A third interlaboratory study in rats has been organized by the
German Bundesgesundheitsamt, in Berlin, with German and French
participants. The design is also based on OECD test guideline No. 407
for a 28-day repeated-dose study in Wistar rats. Cyclosporin A was
selected as the test substance. The live phase of this study has been
completed, the data are being analysed, and the final report is being
prepared.
Information obtained from studies of predictability, accuracy,
and precision, such as those described above, must undergo peer review
before publication. A major goal of the validation process is to
determine which methods should be recommended in the testing
guidelines of regulatory agencies. Figure 36 shows each input and
output of information and the action steps. The process of developing
and obtaining acceptance of testing guidelines is based on three major
inputs: (1) publication of reports in peer-reviewed journals;
(2) guidelines for deciding whether a method is valid; and
(3) implementation of test methods that are interpretable by
scientists involved in assessing biologically relevant risks and the
results of which can be incorporated into quantitative dose-response
analyses. The proposed process is built around the generation of these
major inputs. Two of the issues that will arise in the development of
guidelines are: 'How many and what type of compounds should be
included in the validation process?' and 'Should the compounds be
shown to be immunosuppressive in both humans and mice?'
5. ESSENTIALS OF IMMUNOTOXICITY ASSESSMENT IN HUMANS
5.1 Introduction: immunocompetence and immunosuppression
An immune response in the fully mature, immunologically competent
individual provides protection against a myriad of infectious agents
and environmental hazards. The immune system acts as a self-restoring
(homeostatic) system which can quickly return to normal levels of
function after periods of marked stimulation and response. This self-
regulation allows the individual to recover from or circumvent the
toxic effects of many potentially damaging environmental hazards.
There are many well-known clinical conditions of inherited deficiency
in immunological function; some result in specific defects in antibody
formation, others consist of T-cell and/or metabolic defects, while
others include impairment of both B- and T-cell function. These
conditions, known as primary immunodeficiency disorders, are due to
definable, inheritable genetic defects. Clinical studies of these
disorders have demonstrated the importance of the immune system to
host defence and individual survival, showing that individuals with
partial or absolute defects in T-cell function rarely survive beyond
infancy or early childhood. In contrast, individuals with defects in
B-cell function, resulting in a deficiency in antibody formation, may
suffer from a variety of chronic, recurrent infectious diseases and
diminished health but can survive with appropriate therapy when the
underlying disorder is recognized. Study of these genetic
immunodeficiency states has also provided considerable information
about the functions of human B and T cells which would otherwise not
have been determined.
Impairment of the function of a key component of the immune
system results in a diminished immune response (immunosuppression) or
immunodeficiency. Acquired immunodeficiency states were recognized
only sporadically until the late 1970s, when a syndrome appeared that
spread rapidly through certain groups and produced a generalized type
of immunosuppression known as acquired immunodeficiency syndrome
(AIDS). AIDS was found to be due to retroviruses that infect and
destroy Th (CD4+) cells in humans (Fauci et al., 1991). CD+
lymphocytes have been identified in experimental studies as the key
cells in the recognition and secondary processing of antigens. Thus,
progression of AIDS is associated with progressive loss of Th cells
and increased frequencies of infection by bacterial, fungal, viral,
and parasitic agents and of certain types of neoplasms.
5.2 Considerations in assessing human immune status related to
immunotoxicity
The assessment of immunotoxicity in humans exposed to potentially
immunotoxic compounds is much more complicated than in experimental
animals. Issues such as logistics, appropriate controls, magnitude and
pattern of exposure, and confounding parameters such as medication,
drug abuse, and illness must be considered. Other considerations that
should be taken into account in comparing human immune status with
that in laboratory animals in relation to immunotoxicity are as
follows:
(1) The human population is heterogeneous and genetically
disparate; it can be considered as 'wildlife'. Inbred laboratory
animals are, by definition, genetically identical; outbred laboratory
animals typically have a larger genetic variability than inbred
animals but a variability that is much smaller than that in wildlife
populations. Genetic constitution, which accounts for the variability,
has consequences for the antigen recognition capacity of the immune
system, especially for the T-lymphocyte population. Antigen
recognition by T cells is restricted to the MHC haplotype of the
individual and therefore differs between (allogeneic) individuals.
Inbred, and most outbred, laboratory animals are much more alike in
antigen recognition capacity than wildlife populations. For instance,
the repertoire of certain inbred mouse strains lacks part of the
spectrum of T-cell specificity, as seen by the absence of T cells that
express distinct 'variable gene families' in the repertoire of TCR
specificities. Such 'gaps' in the repertoire have thus far not been
detected in the outbred human population by similar methods of
detection (Hu et al., 1993), perhaps because each individual in an
outbred population expresses the MHC products of both parents and can
in principle multiply the repertoire of MHC-restricted reactions by a
factor of 2 (including MHC I and MHC II).
Interindividual variability has obvious consequences for
immunotoxicity, in which the response to the chemical or drug
underlies the mechanism of toxicity. Its effect on direct toxicity is
presumably less, but the manifestation of toxicity is often reduced
immune reactivity (e.g. increased incidence of infections) and hence
determined by individual reactions to antigens.
(2) The human population, like populations of 'wildlife' animals,
is continuously exposed to environmental stimuli. It is well known
that it is not necessary that each member of a population be protected
('immune') but that a certain proportion of protected individuals must
be reached in order to achieve 'herd immunity'. That is to say, the
whole population is protected when a certain percentage of individuals
is immune. In contrast, when this percentage falls below the required
incidence (which differs for different infectious agents), the
population as a whole loses its protected state, and an infectious
epidemic can result. This situation easily arises in small groups in a
country where vaccination is minimal. Well-known examples are the
outbreaks of poliomyelitis in the Netherlands and the hepatitis A
virus outbreaks in China. This phenomenon should be taken into account
in epidemiological studies and associated laboratory investigations
(e.g. antibody levels to microorganisms) in assessing immunotoxicity
in human populations.
(3) Most of the human population is continuously exposed to
environmental stimuli and maintains its ability to respond to foreign
material from the pool of immunological memory. From the first
postnatal period through to adulthood, the T-cell repertoire is
generated by the thymus; later, this generation of cells is reduced to
a low level. Strict MHC restriction implies that the T-cell population
cannot easily change specificity, e.g. by somatic mutation of
the genes that encode the TCR. There is some evidence from
immunophenotyping, both in mice and humans, that the T-cell population
shifts gradually during life, from naive (committed) T cells to memory
T cells. Within the B-cell population, the situation is different.
Here, the repertoire changes continuously, due to somatic mutation
presumably associated with 'affinity maturation' in lymph nodes. This
phenomenon may result in the emergence of B cells with a strong
affinity for stimuli and the disappearance of low-affinity cells. It
is not known whether neoantigens or pathogens are recognized by
'affinity matured' or memory B cells or by naive B cells. In the
absence of information on this aspect, it can be suggested that most
of the immune capacity of adults is deployed for memory reactions,
whereas in young people the contribution from the naive pool is
higher. This is reflected in infectious epidemics, when microorganisms
like influenza change to phenotypes that cannot be recognized by the
memory pool, an aspect to be kept in mind when choosing immune tests
to be used in evaluating immunotoxicity. Primary responses, like those
to keyhole limpet haemocyanin antigen, are considered to be more
sensitive than secondary responses, like those to tetanus toxoid.
Another example of this effect is the composition of the recall
antigens used in testing delayed-type hypersensitivity (Borleffs et
al., 1993). Both primary and secondary antibody responses, however,
are valuable for evaluating the intrinsic naive and memory immune
capacity of individuals, although secondary responses are less
sensitive to immunological insults.
(4) For a number of infectious microorganisms, the immune
response does not result in complete elimination of the invader but
rather in its 'silent' integration into the genome. Certain viruses,
like herpes viruses, cytomegalovirus, and Epstein-Barr virus, are
dealt with in this way. An individual is considered to be a carrier of
the virus (postinfection status) on the basis of the presence of
antibodies. In other words, individual postinfection is a continuous
defence against these viruses, often with sufficient capacity to keep
the virus in a silent form. In diminished immune capacity, this
natural protection can be lost, and infections can re-occur after
viral reactivation. Primary infection and reactivation therefore have
different pathogeneses, although the subsequent disease may be
characterized by the same symptoms. This situation is well known
clinically, when high doses of immunosuppressive drugs are given for
long periods. The relevance of this observation in immunotoxicity
testing must be established.
(5) Ex-vivo diagnosis in humans is often restricted to
haematological investigations, so that only information on the
circulatory pool of cells and plasma factors is obtained. For example,
the distribution of immunoglobulins differs in the intravascular and
extravascular spaces. Only about 1% of the total lymphocyte pool is
present in blood (1010 cells out of the total of about 1012), and
this population represents only the recirculating pool of cells and
not the tissue-bound cells that participate actively in immunological
responses. Investigations of peripheral blood cells can be somewhat
misleading: for instance, patients infected with the human
immunodeficiency virus (HIV)-1 may show severe depression of CD+
cells in blood but less reduction in CD cells in lymphoid tissue
(Schuurman et al., 1985).
It is considerably more complex to establish immune changes in
humans than in animals, since noninvasive tests are limited, the
levels of exposure to an agent (i.e. dose) are difficult to establish,
and the responses in the population are extremely heterogeneous. With
respect to the latter, the variation in immune responses (genetic or
environmental) can exceed a coefficient of variation greater than
20-30%. Because many of the immune changes in humans that follow
exposure to chemicals may be sporadic and subtle, recently exposed
populations must be studied and sensitive tests for assessing the
immune system be performed. Since many of the immune tests used in
humans have a certain degree of overlap (redundancy), it is also
important that a positive diagnosis not be based on a change in one
test but on a profile (pattern) of changes, similar to that observed
in primary or secondary immunodeficiency diseases. For example, low
CD:CD8 ratios are often accompanied by changes in skin reactions to
recall antigens. The Clinical Immunology Subcommittee of WHO and the
International Union of Immunological Societies published methods for
examining changes in the human immune system and described their
pitfalls (Bentwich et al., 1982, 1988); however, most of the tests
were selected on the basis of observations in patients with primary
immunodeficiency diseases. Such individuals suffer from severe
recurring infections, and their degree of immunosuppression is
probably considerably greater than that induced by chemicals. Thus,
some of the methods may be of limited value for examining potential
chemical-induced immunosuppression, and further evaluation of methods
is needed.
In view of the difficulties in identifying chemical-induced
immunosuppression in humans, establishment of exposure levels (e.g. in
blood or tissue) would not only be useful but would in many instances
be essential for determining a cause-effect relationship. Clinical
disease may not necessarily have to be present in order for
immunosuppression to be meaningful, for several reasons. Firstly,
there are uncertainties about the extent of the reserve capacity of
the immune system and whether the relationship between immune function
and clinical disease shows a linear or a threshold response. In a
linear relationship, even minor changes in immune function would be
related to an increase in disease, if the population examined is large
enough. While the relationship at the low end of the dose-response
curve is unclear, at the high end of the curve (i.e. severe
immunosuppression), clinical disease is readily apparent. This is
exemplified by increased incidences of the opportunistic infections
that occur in AIDS patients. Secondly, clinical disease may be
difficult to establish, as neoplastic diseases may involve a
10-20-year latency before tumour appearance, and increased infection
rates are difficult to ascertain in epidemiological surveys (e.g.
increased numbers of cases of severe common cold).
5.3 Confounding variables
The normal population has a wide range of immunological
responses, with no apparent health impact. In addition to the
underlying population variability, certain host characteristics and
common exposures may be associated with significant, predictable
alterations in immunological parameters. If not recognized and
effectively addressed in study design or statistical analysis, these
confounding factors may severely alter the results of population
studies.
Examples of factors associated with measurable alterations in
immunological parameters are age, race, sex, pregnancy status, acute
stress and the ability to cope with stress, coexistent disease or
infection, nutritional status, lifestyle, tobacco smoking, and some
medications. The effect of acute stress on the immune system is
mentioned in section 1.2.1.5. Protein calorie restriction and
deficiencies of trace elements such as zinc have also been associated
with immune deficiency (Chandra, 1992; Good & Lorenz, 1992; Chandra,
1993). Periodic influences, ranging from daily to seasonal, also
exist; some are relatively minor, but others are of a magnitude that
may rival the expected effect of a low-level exposure to a toxic
agent. They are therefore of primary concern in large epidemiological
studies. For example, African Americans have, on average, serum IgG
levels that are 15-20% higher, neutrophil counts that are 10-15%
lower, and a proportion of circulating B cells that is significantly
higher than those of Caucasians, with no discernible health
implications. Cigarette smoking is associated with a significant
decrease in IgG level and an increase in leukocyte count, independent
of ethnic differences. Therefore, it is imperative that population
norms and reference ranges be supplemented by detailed analysis of
potential confounding factors. Study designs should include
considerations of matching, stratification, and subgroup analysis to
control for these potential effects. As new immunological assays are
developed, normative data will be required, particularly for ethnic
minorities, children, the aged, and certain groups potentially at high
risk, such as pregnant and lactating women.
Certain endocrine diseases and conditions may be associated with
significant alterations in immune function (e.g. adrenal dysfunction).
Some medications, such as corticosteroids, phenytoin, and nonsteroidal
anti-inflammatory agents, may depress a variety of immune functions.
Questionnaires and population surveys should allow the collection of
sufficient information to make it possible to consider these factors
in data analysis and interpretation.
An increasingly important consideration in any analysis of immune
function, particularly in relation to immune deficiency, is the
potential presence of HIV infection, which causes widespread
alterations in virtually all elements of the immune system. Even a
small proportion of unrecognized HIV-infected individuals in a study
population may significantly affect the results and the interpretation
of data. When immunological studies indicate decreased immune
parameters consistent with HIV infection, testing for the virus should
be considered; otherwise, interpretation of the results of
immunological tests of immune dysfunction, particularly among
populations with potentially high rates of HIV infection, may be
severely limited.
In assessing human immunotoxicity, it is useful to establish the
presence of infectious, allergic, or autoimmune diseases in order to
ensure completeness and to rule out additional confounding variables.
A clue to the type of immunological defect is often provided by the
kind of infection observed. For example, patients with impaired
humoral immunity have an increased incidence of recurrent infections
with encapsulated bacterial pathogens (e.g. Pneumococcus and
Haemophilus influenzae), which can induce chronic sinopulmonary
infection, bacteraemia, and meningitis. In contrast, if cellular
immunity is intact, the patients will have less severe infections with
fungal and viral agents. Abnormalities of T cells and impairment of
cell-mediated immunity predispose individuals to infections with a
wide variety of agents, including viruses that cause disseminated
infections (e.g. Herpes simplex virus, varicella-zoster virus, and
cytomegalovirus), fungi that cause mucocutaneous candidiasis, and
parasitic organisms including the protozoan Pneumocystis carinii.
5.4 Considerations in the design of epidemiological studies
An important factor in assessing the usefulness of an
epidemiological study for risk assessment is its design. The commonest
design used in immunotoxicology is the cross-sectional study, in which
exposure and disease status (in this case, changes in immunological
function) are measured at one time or over a short period. The immune
function of 'exposed' subjects is compared with that of a comparable
group of 'unexposed' individuals. Important considerations in using
the data provided by such studies in risk assessment have been
discussed (E. Ward, unpublished manuscript):
(1) What is the relationship between changes in immune function and
human health risk?
(2) Are the selection procedures for study subjects adequately
documented?
(3) Is there evidence that the exposed group was actually exposed to
the substance of interest?
(4) Has the possibility that other exposures, of either the entire
population or individuals, been accounted for?
(5) Are the 'exposed' and 'unexposed' populations comparable with
respect to factors other than the exposure of interest?
(6) Have major individual aspects (such as illness and use of
medications) that may influence the outcome of tests for immune
function been accounted for?
(7) Has inter- or intralaboratory variability been controlled for?
Was the laboratory that ran the tests for immune function able to
distinguish between samples from 'exposed' and 'unexposed'
individuals?
5.5 Proposed testing regimen
Biological research involving human subjects must be conducted in
accordance with ethical standards and involve scientific procedures
designed to ensure the safety of the subjects (Council for
International Organizations of Medical Sciences, 1993). Below are
shown a testing scheme proposed by WHO for preliminary evaluation of
individuals exposed to immunotoxicants, an approach developed by a
working group organized by the United States Centers for Disease
Control and Agency for Toxic Substances and Disease Registry, and that
proposed by a panel of the United States National Academy of Science
(US National Research Council, 1992). The three approaches have many
similarities.
5.6 Assays for assessing immune status
A plethora of tests has been developed to assess immunity in
humans (Bentwich et al., 1982, 1988), which are described in
laboratory manuals (Lawlor & Fischer, 1988; Miller et al., 1991;
Coligan et al., 1994). Many of these tests are now commercially
available in kits. A systematic approach to the evaluation of immune
function, which is based on simple screening procedures, followed by
appropriate specialized tests of immune function, usually permits the
definition of an immune alteration. The tests should include
evaluation of the B-cell system, of the T-cell system, and of
nonspecific resistance (polymorphonuclear leukocytes, monocytes and
macrophages, NK cells, the complement system). Although some exogenous
Assays suggested by WHO for assessing immunotoxicity in all persons
exposed to immunotoxicants
1. Complete blood count with differential counts
2. Antibody-mediated immunity (one or more of following):
* Primary antibody response to protein antigen (e.g. epitope-
labelled influenza vaccine)
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
* Secondary antibody response to protein antigen (diphtheria,
tetanus, or poliomyelitis)
* Natural immunity to blood-group antigens (e.g. anti-A and
anti-B)
3. Phenotypic analysis of lymphocytes by flow cytometry
* Surface analysis of CD3, CD4, CD8, CD20
4. Cellular immunity
* Delayed-type hypersensitivity skin testing using Multitest
Biomerieux(R)
* Primary delayed-type hypersensitivity reaction to protein
(keyhole limpet haemocyanin)
* Proliferation to recall antigens
5. Autoantibodies and inflammation
* C-Reactive protein
* Autoantibody titres to nuclei, DNA, mitochondria and IgE
(rheumatoid factor)
* IgE to allergens
6. Measure of nonspecific immunity
* Numbers of natural killer cells (CD56 or CD60) or cytolytic
activity against K562
* Phagocytosis (nitroblue tetrazolium or chemiluminescence)
7. Clinical chemistry
Screening panel recommended for human studies by the United States Centers for
Disease Control and Agency for Toxic Substances and Disease Registry
* Complete blood count with differential counts
- absolute lymphocyte count
- granulocyte count
- platelet count
- absolute eosinophil count
- examination of peripheral smear
* Immunoglobulins
- IgG
- IgA (optional)
- IgM (optional)
* C-Reactive protein
* Autoantibody screening panel
- Antinuclear antibody
- Rheumatoid factor
- Anti-thyroglobulin antibody
* Peripheral blood leukocyte surface markers
- CD2/CD3
- CD4/CD8
- CD8/CD3
- CD19/CD20
* Clinical chemistry in serum
- Blood urea nitrogen
- Cholesterol
- Creatinine
- Total bilirubin
- Alkaline transaminase
- Alkaline phosphatase
- Total protein (albumin:globulin ratio)
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
Tier 1 (all persons exposed to immunotoxicants)
I. Humoral immunity
* Immunoglobulin concentrations in serum (IgM, IgG, IgA, IgE)
and immunofixation electrophoresis
* Natural immunity: Antibody levels to ubiquitous antigens
(e.g. anti-A and anti-B group substances in individuals of
non-AB blood type)
* Secondary antibody responses to proteins (e.g. diphtheria,
tetanus, poliomyelitis) and polysaccharides (e.g.
pneumococcal, meningococcal)
Note: In immunization studies, live microorganisms should not be
given to persons suspected of being severely immunocompromised.
II. Lymphocytes
* Enumeration of B and T cells in blood
* Surface analysis of CD3, CD4, CD8, CD20
* Secondary delayed-type hypersensitivity reaction (e.g.
candida, diphtheria, tetanus)
* Alternative: Multiple antigen skin test kit
III. Autoantibody titres (to red blood cells, nuclei, DNA,
mitochondria, IgE (rheumatoid factor)
Tier 2 (all persons with abnormal Tier 1 test results and
a fraction of the total exposed population to be determined
by a statistician)
I. Humoral immunity
* Primary antibody response to protein and polysaccharide
antigens
Note: A panel of antigens should be developed that can be used
in sequential studies on a given individual, since a particular
antigen can be used only once to assess a primary response.
II. Cellular immunity
* Proliferative response to mitogens (phytohaemagglutinin,
concanavalin A) and possible antigens such as tetanus;
primary delayed-type hypersensitivity reaction to keyhole
limpet haemocyanin
Note: Here, too, a panel of standard antigens is needed for
sequential testing; these could be the same as those used to
assess primary antibody responses.
Tests recommended by the panel of the United States National Academy of Sciences
for studies of persons exposed to immunotoxicants
III. Natural killer cells, monocytes, and other T- and B-cell markers
* CD5, CD11, CD16, CD19, CD23, CD64; class II MHC on T cells
by two-colour flow cytometry for co-expression of class II
and a T-cell marker such as CD3
IV. Serum levels of cytokines (e.g. IL-1, IL-2, IL-6) and of shed or
secreted cellular activation markers and receptors (e.g. CD25).
V. Class I and II MHC antigen typing
Tier 3 (to be considered for persons with abnormalities
in Tier 2 tests or for a random fraction of the entire
population in Tier 2)
* If a proportion of CD16 cells of Tier 2, III, is abnormal:
nonspecific killing of a tumour cell line to test for natural
killer function
* If primary delayed-type hypersensitivity reaction in Tier 2, II,
is abnormal:cell proliferation in response to phorbol ester and
calcium ionophore,anti-CD3 antibody, and a staphylococcal
enterotoxin B (experimental)
* Generation of secondary cell-mediated immune reactions
(proliferation and MHC-restricted cytotoxicity) in vivo, e.g.
with influenza virus (experimental)
* Immunoglobulin subclass levels in serum (IgA1, IgA2, IgG1-4)
* Antiviral titres (e.g. influenza, parainfluenza,
cytomegalovirus, human immunodeficiency virus) in serum (no
deliberate immunization)
agents can alter several elements of the human immune system, others
have a primary effect on a single element. For example, low doses of
cyclosporin A selectively affect T cells by acting on the production
of IL2 and IL2 receptors. Conversely, the anticonvulsive drug
phenytoin acts primarily on the humoral immune system, leading to a
selective deficiency of IgA.
A number of immune function assays recommended for inclusion in
a simple screening panel for assessing human immune function after
potential exposure to xenobiotics believed to affect the immune system
are described below. It should be noted that there are many indicators
of altered immune function in humans which may not be specific markers
for exposure, immune disease or susceptibility (IPCS, 1994a;
IPCS/Department of Health, 1995).
5.6.1 Total blood count and differential
A complete blood count, with differential absolute counts of
lymphocytes, granulocytes, eosinophils, and platelets, are basic
components of immunotoxicology. These tests are useful in defining the
general health status of a population, since they are relatively well
standardized over most age, sex, and race groups. Such counts are also
essential for interpreting the results of ex-vivo/in-vitro functional
tests, described below, since functional tests reflect a combination
of numbers of cell types and activity per cell.
The absolute lymphocyte count is critical: Higher absolute
counts are found in children than in adults, but lymphocyte counts
consistently below 1500/mm3 are indicative of lymphocytopenia, and a
higher count signals a defect in the T-cell system or effects on the
bone marrow. Lymphocytopenia can occur not only in primary immune
deficiency but also as a result of viral infections, malnutrition,
stress, autoimmune diseases, and haematopoietic malignancies.
Examination of bone marrow may be indicated to exclude some other
factors once lymphocytopenia has been confirmed. Additional assessment
of cell mediated immunity and direct measurement of T-cell parameters,
such as lymphocyte phenotypic markers, may also be indicated.
Review of peripheral smears for morphological abnormalities of
the white and red cells adds useful information for interpreting raw
cell counts, such as the atypical lymphocytosis of many acute viral
infections. The absolute eosinophil count can be very helpful in
delineating allergic disorders, vascular collagen diseases, and
parasitic manifestations.
5.6.2 Tests of the antibody-mediated immune system
Evaluation of antibody-mediated immunity involves measurement of
serum concentrations of immunoglobulins and assessment of antibody
formation after immunization or measurement of 'natural antibodies'.
5.6.2.1 Immunoglobulin concentration
Several methods are available for measuring the concentrations
of the five major classes of immunoglobulin, IgM, IgG, IgA, IgD, and
IgE, in serum, including single radial diffusion, double diffusion in
agar gel, immunoelectrodiffusion, radioimmunoassay, ELISA, and
automated laser nephelometry. Single radial diffusion is widely used.
Gel diffusion methods are very sensitive to differences in diffusion
constants and thus to differences in molecular size.
The serum concentration of each of the major immunoglobulins
should be determined, with the exception of IgD (which occurs
predominantly on the cell surface). The determinations must be well
standardized because antisera vary in quality. Since serum
immunoglobulin concentrations vary with age and environment,
appropriate norms must be used. Patients can manifest a deficiency in
all immunoglobulin classes (common variable hypogammaglobulinaemia),
or they may have a deficiency in only a single class (IgA deficiency
as a primary defect or after phenytoin therapy).
The concentration of immunoglobulins cannot be used as the
sole criterion for a diagnosis of immunodeficiency. Diminished
immunoglobulin concentrations can result from loss into the
gastrointestinal tract as well as from decreased synthesis. An
indication of loss can be obtained by measuring serum albumin, which
is usually lost concomitantly.
5.6.2.2 Specific antibodies
Antibody-mediated immunity can be assessed from antibody
responses to common specific antigens (basal levels). Humoral immunity
after immunization can be assessed in the same way. The response to
antigenic stimulation with both protein and polysaccharide antigens
must be defined if immunodeficiency is strongly suspected. Failure to
respond to one or more classes of antigen has been observed in
patients with normal or high levels of immunoglobulins, regardless of
whether they have an isolated immunoglobulin class or subclass
deficiency. Specifically, patients with the Wiskott-Aldrich syndrome
may have normal or even elevated immunoglobulin concentrations, yet
have multiple infections because of their failure to mount a specific
immune response, especially when they are exposed predominantly to
polysaccharide antigens.
Natural antibodies: Isohaemagglutinins are naturally occurring
antibodies to blood group A and B antigens found in all normal
individuals except those with type AB red cells. By three years of
age, 98% of normal persons with type A, B, or O blood have
isohaemagglutinin titres of at least 1:16. Patients with the Wiskott-
Aldrich syndrome may have normal immunoglobulin levels yet lack
isohaemagglutinins as an indicator of their antibody-deficient state.
Other natural antibodies that can be assayed include heterolysins
(e.g. against sheep or rabbit red blood cells) and antistreptolysin.
Antibody response to immunization: In order to test for
T cell-dependent antibody responses, commercially available
diphtheria-tetanus vaccine can be given in recommended doses. Blood is
taken two weeks after each injection and tetanus and diphtheria
antibodies are determined. In patients who have been immunized with
diphtheria-tetanus or diphtheria-pertussis-tetanus vaccine, one
booster injection is given before determination of antibodies. In
testing for T cell-independent antibody responses, commercially
available pneumococcal vaccine can be given in recommended doses.
Three doses of killed poliomyelitis vaccine (1.0 ml intramuscularly,
at intervals of two weeks) can also be used as the immunogen. Blood is
taken two weeks after the last injection, and antibody is usually
determined by virus neutralization. There is strong consensus that
quantification of a primary immune response (antibody and/or cellular)
after immunization is not only a very relevant test but also very
sensitive. Although such tests are not routine in clinical immunology,
they have been used successfully for determining immune integrity.
While keyhole limpet haemocyanin has been used as the antigen,
beneficial immunizations such as influenza vaccine linked to a marker
epitope can also be used.
5.6.3 Tests for inflammation and autoantibodies
5.6.3.1 C-Reactive protein
Inclusion of an acute-phase reactant marker is helpful for
clinical interpretation of other laboratory biomarkers such as
autoantibodies. The concentration of C-reactive protein rises and
falls to baseline values in direct proportion to tissue damage and is
thus a sensitive indicator of the presence of a generalized
inflammatory state. It offers the best example of an accepted,
standardized procedure that can be used in large population studies,
because it is less subject to transport variables than other
procedures.
5.6.3.2 Antinuclear antibody
Antinuclear antibody is a common autoantibody that may be
associated with various rheumatic disorders and, classically, systemic
lupus erythematosus. Several commercial kits are available to detect
the presence of autoantibodies. Progressively greater titres increase
the specificity for a disease. Positive sera should be titred at
dilutions of > 1:40 to 1:640.
5.6.3.3 Rheumatoid factor
Rheumatoid factor is an autoantibody to immunoglobulin (usually
IgM) that occurs in a high percentage (50-70%) of individuals with
classical rheumatoid arthritis but may also develop in a variety of
other disorders, including infections and immunological diseases.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.3.4 Thyroglobulin antibody
This antibody occurs in association with a variety of thyroid
disorders but may be found without detectable thyroid dysfunction.
Positive sera should be titred at dilutions of > 1:40 to 1:640.
5.6.4 Tests for cellular immunity
A variety of tests are commonly used to assess cell-mediated
immunity, including enumeration of T cells and T-cell subsets,
identification of delayed skin reactions, and measurement in vitro
of stimulation of lymphocytes to proliferate and form blast cells.
Other tests are available to measure T-cell effector or regulatory
function in vitro. As for humoral immunity, a series of simple tests
is available to screen for defects in cell-mediated immunity. The
proportion of circulating T cells in a mononuclear cell preparation
can be determined by immunofluorescence with CD2 or CD3 monoclonal
antibodies. Normally, T cells constitute 55-80% of peripheral blood
lymphocytes. The normal values reported for absolute numbers of
circulating T cells are 1620-4320/mm3 during the first week up to
18 months of life and 590-3090/mm3 after 18 months of age (Fleisher
et al., 1975).
5.6.4.1 Flow cytometry
Antibodies that may be used in immunological phenotyping are
listed in Table 10 in section 4.1.2. Subsets of T cells have been
defined with monoclonal antibodies specific to cell-surface antigens.
The association of a particular T-cell subset with a given function
has caused some confusion in the analysis of immunological data for
patients with immunodeficiency states, as discussed in more detail in
section 1.2.2.1. For example, CD-positive cells have commonly been
associated with helper functions, and CD8 cells have been associated
with cytotoxic functions. This approach is an oversimplification,
which became evident with the finding that CD and CD8 cells recognize
foreign antigens in the context of MHC class II and class I antigens,
respectively. Thus, the CD population contains helper cells, memory
cells, and cytotoxic cells for targets bearing MHC class II molecules.
The CD8 population contains cytotoxic cells and also cells that can
recognize antigens presented by macrophages and cells that can augment
the interaction of CD-positive cells with B cells. Abnormalities in
the number of CD or CD8 cells, or their ratio, can be associated with
abnormalities in the ability to recognize antigens and regulate T-cell
function that can lead to immune incompetence or autoimmunity.
5.6.4.2 Delayed-type hypersensitivity
The ability of patients to manifest pre-existing T-cell immunity
has been evaluated in vivo with a series of skin antigens that
normally produce a delayed-type cutaneous hypersensitivity response.
Because delayed cutaneous hypersensitivity, a localized immunological
skin response, depends on functional thymus-derived lymphocytes, it is
used in detecting T cell-mediated immunodeficiency. A device
(Multitest(R), Institut Merieux, Lyon, France) is available that
enables the simultaneous intradermal injection of seven standardized
antigens and so overcomes the inconvenience of giving seven separate
injections of antigens and the technical difficulty of ensuring their
intradermal rather than subcutaneous injection. The Multitest(R),
consisting of eight tined, preloaded heads, delivers a glycerol
control and the seven test antigens dissolved in glycerol. The size of
the induration produced by each antigen should be measured at 48h in
two diameters: reactions of less than 2 mm are scored as negative. No
reaction should be seen with the glycerol control. The test includes
as antigens: tetanus, diphtheria, streptococcus, tuberculin, Candida,
Trichophyton, and Proteus.
Recall of delayed-type hypersensitivity as a test for cell-
mediated immune competence was assessed with the Multitest(R) device
in 254 subjects. When 77 subjects were tested concurrently with the
Multitest(R) and a conventional panel of six antigens (Frazer et
al., 1985), similar results ( r = 0.65) were obtained with the two
systems. The reproducibility of the Multitest(R) among three
observers, who assessed the aggregate size of reactions in 45 subjects
independently, was high ( r = 0.89); the correlation for the reaction
score in 24 subjects tested twice, three months apart, was also high
( r = 0.88), demonstrating the suitability of the test for serial
studies of immune function.
5.6.4.3 Proliferation of mononuclear cells in vitro
A common test of lymphocyte function is measurement of the
capacity of lymphocyte subsets to enlarge and convert into blast-like
cells that synthesize DNA and incorporate thymidine after stimulation
in vitro. In this test, lymphocytes can be activated by antigens
(e.g. purified protein derivative, Candida, streptokinase, tetanus,
or diphtheria); allogeneic cells are also used in the one-way mixed
lymphocyte culture to stimulate T-cell proliferation. T-Cell blast
transformation can be assessed directly by measuring blastogenesis and
proliferation of cells, expression of activation antigens (e.g. CD69
or CD25 and HLA DR), and release of mediators. The blastogenic
response is assessed as incorporation of 3H-thymidine, usually for
16-24h, followed by cell precipitation on filter paper and liquid
scintillation counting. Non-isotope assay alternatives are also
available. The responses to various antigenic stimuli by different
types of responding cells must be interpreted with caution. The mixed
leukocyte reaction is the result of T-cell reactivity to MHC-encoded
peptides displayed on the surface of B cells and monocytes. The
T cells in the population of normal irradiated or mitomycin C-treated
lymphocytes used as the stimulators can secrete factors that induce
blastogenesis in the patient's lymphocytes. As this can be misleading,
it is preferable to use B-cell lines or T cell-depleted normal cells
as the stimulators.
5.6.5 Tests for nonspecific immunity
5.6.5.1 Natural killer cells
The differences between NK cell function, phenotyping for NK
cells, and the cytology of large granular lymphocytes are described in
section 1.2.2.1. The identification of NK cells remains problematic
owing to this apparent heterogeneity. The cells can be evaluated in
ex-vivo/in-vivo tests with enriched peripheral blood mononuclear
cells. The proportion of NK cells can be identified with appropriate
monoclonal antibodies (see Table 10). Functional assays of NK activity
involve the ability of the appropriate mononuclear cells to kill
specific NK targets, such as the K562 cell, in which cell-mediated
cytolysis in vitro is quantified by release of 51Cr from the
target cells.
5.6.5.2 Polymorphonuclear granulocytes
Some defects of phagocytic function affect polymorphonuclear
phagocytes. Neutrophil function depends on movement in response to
chemotactic stimulus, adherence, endocytosis, and destruction of the
ingested particles. Phagocyte mobility depends on the integrity of the
cytoskeleton and contractile system. Defects in intracellular killing
of ingested microorganisms usually result from failure of the
'respiratory burst' that is critical to production of superoxide
radicals and hydrogen peroxide. The organisms cultured from lesions of
patients with this type of defect generally contain catalase and
include staphylococci, E. coli, Serratia marcescens, fungi, and
Nocardia. Patients with defects in mobility, adherence, and
endocytosis usually have infections of the skin, periodontitis, and
intestinal or perianal fistulae; patients with normal endocytosis and
defective killing tend to have chronic granulomas. Measurement of
nitroblue tetrazolium dye reduction or chemiluminescence by actively
phagocytosing leukocytes has been accepted as a standard measure for
the adequacy of the respiratory burst. Recently, methods have been
developed to measure the production of reactive oxygen intermediates
by flow cytometry (Emmendorfer et al., 1994). Kits are commercially
available for assessing phagocytic capacities and the production of
reactive oxygen intermediates by phagocytes. Assays for bacterial
killing yield highly variable results, depending on the bacterial
species used in the assay, and are not recommended for routine use.
The activation state of neutrophilic granulocytes can be assessed by
flow cytometry with the antibodies CD11b, CD14, CD16, CD54, and CD64
(Spiekermann et al., 1994). Activation of platelets can also be
assessed by flow cytometry (Tschöpe et al., 1990)
5.6.5.3 Complement
The classic complement system consists of nine components (C1-9)
and a series of regulatory proteins (C1 inhibitor, C4 binding protein,
and properdin factors). Many biological activities important in the
inflammatory response and in host resistance to infection occur at
various points in the classical and alternative pathways of complement
activation. Three clinical states should raise a suspicion of
deficiency in a complement component: systemic lupus erythematosus,
recurrent infections of the type seen in hypogammaglobulinaemia in
patients with normal immunoglobulin levels, and severe Neisseria
infection. Laboratory measurement of serum haemolytic complement
(CH50) is a useful test. Serum haemolytic complement is usually absent
and rarely above 10% of the normal value in inherited complement
deficiencies, with the exception of hypercatabolism of C3. More
detailed analysis of the complement system requires functional and
antigenic measurements of the individual components, usually best
performed in laboratories that specialize in the complement system.
5.6.6 Clinical chemistry
Assessment of clinical abnormalities in standard serum
chemistry, such as liver function, renal function, glucose, and
albumin, is indicated to facilitate reasonable interpretation of
specific changes in the immune system as secondary to another
condition.
5.6.7 Additional confirmatory tests
After activation, mononuclear cells from peripheral blood
express the genes that encode a series of interleukins and colony-
stimulating factors. Activated T cells and monocytes synthesize and
secrete IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, interferon, other
cytokines such as tumour necrosis factor, and cytokine receptors.
These cytokines are involved in the growth and differentiation of
T and B cells, eosinophils, and basophils. The supernatants of
mononuclear cells from peripheral blood stimulated by soluble
phytohaemagglutinin can be assessed for IL-2 by determining their
capacity to stimulate 3H-thymidine uptake by mouse IL-2-dependent,
cultured T-cell lines. Other biological assays, radioimmunoassays, and
ELISAs have been developed to quantify the production of lymphokines
and colony-stimulating factors. With molecular cloning techniques,
messenger RNA transcription by each of the lymphokines can be
quantified after appropriate lymphocyte activation. In the assessment
of lymphokines, T cells or peripheral blood mononuclear cells are
usually activated with concanavalin A, pokeweed mitogen, or
insolubilized CD3 antibodies; then, the appropriate assays are
performed to quantify the specific lymphokine(s) produced in the
culture media. Different patterns of lymphokine secretion have been
observed with different subsets of T cells: INF gamma and IL-2 are
produced by the Th1 subset, while IL-4, IL-5, and IL-10 are produced
by the Th2 subset, as originally documented for cloned CD T-cell lines
from mice (Mosmann & Coffman, 1989). Thus, an assay of the pattern of
lymphokine production could be used in pinpointing the action of an
immunotoxicant on a particular subset of immune cells.
6. RISK ASSESSMENT
6.1 Introduction
Publications on immunotoxicology published by IPCS and the
European Union (Berlin et al., 1987; Dayan et al., 1990), the United
States Office of Technology Assessment (1991), and the United States
National Research Council (1992) demonstrate the growing interest and
concern within scientific and public communities on the capacity of
environmental agents to perturb normal immune processes. The
incorporation of experimental data on toxicant-induced alterations in
the immune system into evaluations of drugs, chemicals, and biological
agents for human risk assessment has thus become increasingly common.
For example, in the United States, the Environmental Protection Agency
(Sjoblad, 1988) and Food and Drug Administration (Hoyle & Cooper,
1990; Hinton, 1992) indicate the benefits of testing the
immunosuppressive potential of biochemical pest control agents,
antiviral drugs, and food additives. Furthermore, the Environmental
Protection Agency has established reference doses (an estimate of the
daily exposure of the human population that is likely to have no
appreciable risk of deleterious effects during a lifetime), on the
basis of data on the immunotoxicity of several compounds, including
1,1,2-trichloroethane, 2,4-dichlorophenol, and dibutyltin oxide. The
United States Agency for Toxic Substances and Disease Registry has
derived 'minimal risk levels' for arsenic, dieldrin, nickel,
1,2-dichloroethane, and 2,4-dichlorophenol on the basis of immune
end-points.
Risk assessment is a process whereby relevant biological, dose-
response, and exposure data for a particular agent are analysed in an
attempt to establish qualitative and quantitative estimates of adverse
outcomes (Scala, 1991). Such data are sometimes used in the
development of standards for regulating the manufacture, use, and
release of chemicals into the environment (Kimmel, 1990). As defined
by the United States National Academy of Science (US National Research
Council, 1983), risk assessment comprises four steps: hazard
identification, dose-response assessment, exposure assessment, and
risk characterization. The process of assessing the risk of both
cancer and non-cancer end-points, including immunotoxicity, may be
adapted to this form.
The first step, hazard identification, involves a qualitative
evaluation of available human and animal data to determine whether a
chemical agent poses a potential hazard. Consideration is given to
dose, route, and duration of exposure. The precise quantitative
relationships between changes in immune function or in the
histological appearance of lymphoid organs and host resistance to
infectious agents or neoplastic diseases are unclear. It can be
assumed that any significant difference from appropriate controls in
the ability of the immune system to respond to a challenge may evolve
into an adverse effect and thus present a potential hazard. This
applies to adaptive as well as nonadaptive responses.
After hazard identification, 'dose-response' is assessed. For
non-cancer toxicity, a no-observed-adverse-effect-level (NOAEL) is
established for an adverse response of interest. This process is no
different in immunotoxicology and is the same as for the other target
organ systems. The NOAEL value is either obtained from the dose-
response curve or is estimated from the lowest-observed-adverse-
effect-level (LOAEL). Once the NOAEL has been determined, safety and
uncertainty factors can be applied to allow for various uncertainties,
such as species or interspecies variability, irreversible effects, and
chronic exposure. The use of safety factors, however, should be
flexible and should allow incorporation of any relevant information on
the mechanism of action of the chemical under review. Ideally,
however, dose-response relationships should be established from human
epidemiological data that include the exposure levels expected on the
basis of human contact with the agent in the environment. As
illustrated in an assessment of developmental toxicity (Kimmel &
Gaylor, 1988), use of the risk assessment and management paradigm of
the United States National Academy of Sciences (US National Research
Council, 1983) to non-cancer end-points such as immunotoxicity, offers
some serious challenges. For example, since the presence or absence of
an effect is based upon whether a statistically and/or biologically
significant response is observed at a certain dose or doses, and since
multiple assays are routinely conducted, the NOAEL will depend heavily
on the sensitivity of the assay. The differences may be particularly
exaggerated when continuous responses (i.e. the results of most immune
function tests) are compared with categorical data, the latter being
routinely expressed as proportions. For example, in experimental
animals, changes in many immune functions may be statistically
significant when they vary by as little as 15-25% from the control
values. In contrast, host resistance, when expressed categorically
(e.g. tumour frequency), must change by 80% to reach a comparable
degree of significance, assuming group sizes of about 15 animals and
effective doses of 20% in the control group. Furthermore, immune tests
are often, but not always, interdependent (Luster et al., 1992), and
individual or combinations of tests might have to be ranked in order
of sensitivity and degree of interdependence before dose-response is
assessed. This has not been done in the past.
Exposure assessment (step 3) is done in parallel with hazard
identification and dose-response assessment (Scala, 1991). It often
involves field measurements and other estimates of human exposure,
such as the composition and size of the population, biological or
clinical effects and types, and the magnitude, frequency, and duration
of exposure to the agent. Many of these parameters are difficult to
determine accurately in a longitudinal study; even measurements of
body burden can be misleading since the concentrations at the target
organ (e.g. lymphoid tissue) are not determined. The problems in
immune testing in humans are similar to those in testing other organs
and systems and include differences in individual responses due to
unique sensitivities (e.g. age, pregnancy, genetics) and confounding
factors (e.g. smoking, stress, drugs).
Risk characterization (step 4) is the aggregation of the three
previous processes. It provides an estimate of the incidence of
adverse effects in a population and the potential health problems. As
part of risk characterization, the strengths and weaknesses of each
component of the assessment are considered, with assumptions,
scientific judgements, and, to the extent possible, estimated
uncertainties. Most assessments of the risks presented by chemical
agents have focused on the estimated incidence of cancer after
lifetime exposure to a chemical at some unit dose, assuming that there
is essentially no threshold for carcinogenicity. The assessment of
non-cancer end-points, such as disorders of the neurological,
developmental, and reproductive systems, is somewhat similar to that
of cancer, in that it involves calculations that include both
assumptions and uncertainties. For example, considerations in risk
assessment include ranking the value of epidemiological against
experimental data, extrapolations from high to low doses, from
subchronic to chronic exposure, and from animals to humans, and
appropriate use of mechanistic and pharmacokinetic data. Data from
immunotoxicology, like those from developmental toxicology (Schwetz &
Tyl, 1987), do not easily lend themselves to the mathematical models
used in cancer risk assessment, which usually involve non-threshold
models for genotoxic carcinogens. For more accurate assessment,
mechanistic models will be required which include the concept of
'threshold'. It is assumed that threshold levels exist below which no
adverse immunological effect can be demonstrated. Complex mixtures of
chemicals, in which each chemical occurs at a subthreshold
concentration, may reach or exceed a threshold for immunotoxicity
(Germolec et al., 1989), although problems associated with mixtures of
compounds are not unique to the field of immunotoxicology.
The approaches currently used by the US Environmental Protection
Agency (1986) in extrapolating the risk for developmental toxicity
have been outlined, and similar guidelines have been developed by IPCS
(1994b). One method is to apply uncertainty factors to the NOEL or
NOAEL for the most sensitive animal species tested. The uncertainty
factor is usually composed of a 10-fold factor to account for
interspecies differences and a 10-fold factor for intraspecies
variability. If no NOEL is available, an additional 10-fold factor may
be applied to the lowest-observed-effect level (LOEL). Another
approach is to calculate a margin of safety, which is the NOEL divided
by the estimated level of human exposure from all potential sources.
The margin of safety can then be evaluated for adequacy to protect
human health. There are several drawbacks to both of these approaches,
the primary one being that they use only one point on the dose-
response curve (NOEL or LOEL) and ignore the rest of the data. Also,
since the variability around the NOEL and LOEL is usually not taken
into account, these approaches may rely on poor studies, i.e. studies
that result in a higher NOEL because of their limited ability to
detect small changes over the background.
Since the purpose of risk assessment is to make inferences about
potential risk to human health, the most appropriate data are those
derived from studies of humans; however, adequate data are seldom
available, and most risk assessments are based on results obtained in
experimental animals. In order to use these results, a number of
assumptions must be made about their relevance to potential human
health risk. Firstly, it is assumed that experimental animals respond
to the agent of interest in a pharmacological and toxicological manner
similar to that anticipated in humans (i.e. the test animals and
humans metabolize the chemical similarly and have identical responses
and toxicity at the target organ). Secondly, the immune system of the
experimental species must be very similar to that of humans: the
vertebrate immune system is highly conserved among higher vertebrate
species, and the immune components and their interactions in mice,
rats, and humans are closely similar. Thus, if toxicokinetic
properties are similar, it is reasonable to test for potential adverse
effects in humans using laboratory rodents.
As immunosuppressive agents cannot ethically be administered to
humans, quantitative comparisons of dose-responses in humans and
experimental animals are limited (although it is possible to do so in
hypersensitivity tests). Nonetheless, controlled human exposures have
been studied and the results compared with the immune effects observed
in animals. As an example, the immune effects of cyclosporin A in
various species are compiled in Table 12, which shows that the mouse
is much less sensitive to cyclosporin A than other species, in
particular the rat, and that humans are slightly more sensitive than
other species (Dean & Thurmond, 1987); however, for the most part,
there was good qualitative and quantitative agreement between the
species examined. Selgrade et al. (1995) compared phagocytosis by
human and murine alveolar macrophages after exposure to ozone
in vitro and in vivo (Table 13): The effects of ozone on alveolar
macrophage function in murine species are predictive of effects that
occur in humans, and the effects on macrophage phagocytosis seen
in vitro are predictive of those that occur in vivo. Quantitative
comparisons have also been made in mice and humans for the ability of
UVR to inhibit the contact hypersensitivity response (Table 14). As
described in section 2.2.11, exposure to UVB inhibits delayed-type
hypersensitivity (Kripke et al., 1979). Noonan & Hoffman (1994)
described three strains of mice with low, intermediate, and high
susceptibility to UVR-induced immunosuppression, and Oberhelman et al.
(1992) reported that suppression of the hypersensitivity response also
occurs in humans and that the dose of radiation required to induce 50%
suppression in fair-and dark-skinned individuals is similar to that
required to inhibit the response in mice with high and intermediate
susceptibility, respectively.
Table 12. Comparison of doses of cyclosporin A that suppress
the immune response in various species
Species Response Cyclosporin A
(mg/kg body
weight)
Mouse Antibody production 50-300
Cell-mediated immunity 100-300
(delayed-type hypersensitivity)
Graft-versus-host reaction 50-250
Rat Antibody production 20-50
Graft-versus-host reaction 10-60
Guinea-pig Cell-mediated immunity 10-100
(delayed-type hypersensitivity)
Dog Cell-mediated immunity 15-30
(delayed-type hypersensitivity)
Rhesus monkey Antibody production 50-250
Human Cell-mediated immunity 10-20
Adapted from Dean & Thurmond (1987); White et al. (1994)
Data from immunotoxicology also differ from those for
carcinogenicity and possibly other non-cancer end-points because the
immune system contains components with overlapping functions. For
example, when an individual is exposed to an infectious agent,
multiple immune components may work either independently or in concert
to help defend the host; i.e. there is redundancy between immune
functions. Furthermore, while a significant change in any immune
function can be considered potentially deleterious, in that it may
increase the risk of developing clinical disease, a change in immune
function does not necessarily precipitate a disease or clinical health
affect. That is, immunocompromised individuals function normally in
the absence of infectious agents. Thus, immune function reserve and
redundancy are relative terms, depending on the dose of infectious
agent. This complicates dose-response assessment, and models should be
developed that incorporate available information on the quantitative
relationship between immune function and clinical disease and
potential redundancy.
Table 13. Effect of exposure to ozone on phagocytosis by alveolar
macrophages
Treatment Phagocytic index (no. fluorescent
particles ingested/100 macrophages)
--------------------------------------
Mice Humans
---------------- -------------
Mean SE Mean SE
Air in vitro 369.2 26.4 386.7 50.5
(n = 6) (n = 6)
Ozone in vitro 291.7 17.4* 275.0 45.1*
(n = 6) (n = 6)
Suppression 21% 29%
Air in vivo 330.6 10.4 714.9 46.1
(n = 4) (n = 10)
Ozone in vivoa 194.0 19.7* 539.2 22.3*
(n = 4) (n = 10)
Suppression 42% 25%
Suppression corrected 28% 25%
for dosimetric differenceb
Adapted from Selgrade et al. (1995)
* Significantly different from air control (P < 0.05; Student's
t test)
a Mice were exposed to 0.8 ppm for 3 h; humans were exposed to
0.08 ppm for 6.6 h while undergoing intermittent exercise.
b On the basis of studies using 18O, alveolar macrophages of
mice exposed to 0.8 ppm ozone for 3 h receive roughly 1.5 times
more ozone than those of humans exposed to 0.08 ppm ozone for 6.6 h
while exercising moderately.
Table 14. Comparison of doses of ultraviolet radiation that cause
50% suppression of contact sensitivity in mice and humans
Mousea Humanb
--------------------------------------------- --------------
Sensitivity kJ/m2 mJ/cm2 Skin type
mJ/cm2
(phenotype)
High (C57Bl) 0.7-2.3 70-230 Fair 100
Intermediate (C3H) 4.7-6.9 470-690 Dark 225
Low (BALB/c) 9.6-12.3 960-1230
Adapted from Selgrade et al. (1995)
a Data from Noonan & Hoffman (1994)
b Data from Oberhelman et al. (1992)
The increasing evidence that environmental contaminants affect
wildlife populations has led to risk assessment at the level of the
ecosystem; however, the limited evidence for immunotoxic effects in
wildlife precludes an adequate understanding of the risks posed by
current levels of environmental pollution. The demonstration of
immunosuppression in harbour seals fed herring from the contaminated
Baltic Sea in a semi-field experiment (De Swart et al., 1994; Ross et
al., 1995) provided a first indication that ambient levels of
contaminants in certain areas present an immunotoxic risk to mammalian
wildlife occupying a high trophic level. These results may partially
explain the severity of a series of unrelated epizootic viral episodes
in various marine mammalian populations in coastal areas of Europe and
North America (Dietz et al., 1989; Van Bressem et al., 1991). In
similar semi-field experiments in a bottom-dwelling fish species,
flounder exposed to contaminated sediments had more viral lymphocytic
infection and liver tumours than controls (Vethaak & Wester, 1993).
While the difficulties in conducting adequate immunological studies
with wildlife may preclude an approach as comprehensive as that in
humans, such semi-field strategies may provide the best available
direction.
Should the application of field studies be possible, correlative
approaches to immunotoxicology may substantiate an effect on the
ecosystem. Such an approach was used to establish a correlation
between the induction of mixed-function oxidases and pollutant burden
(as measured by the toxic equivalence of organochlorine chemicals) in
cormorant (Phalocrocorax carbo) chicks collected from various sites
in the Netherlands (van den Berg et al., 1994). A combination of
laboratory experiments under controlled conditions, semi-field
experiments under controlled conditions with exposure to environmental
mixtures of pollutants, and correlative field studies is necessary to
understand immunotoxicity in wildlife populations.
6.2 Complements to extrapolating experimental data
6.2.1 In-vitro approaches
The complexities of the immune system and the requirement of
many agents for metabolism and distribution in order to produce an
immunotoxic response have resulted in the almost exclusive use of
animal models in vivo for assessing immunotoxicity. Culture systems
have been used extensively, however, to study the mechanisms by which
agents induce immunosuppression. In-vitro test systems with immune
cells of human origin are particularly attractive, given the
uncertainties in extrapolating the results of studies in experimental
animals to humans and the accessibility of human peripheral blood
cells. Although many of the immune cells obtained from human blood are
immature forms, the large numbers and diverse populations (i.e.
polymorphonuclear granulocytes, monocytes, NK cells, T cells, and
B cells) that can be obtained and the ease of conducting challenge
assays in vitro provide an attractive alternative (or, preferably,
adjunct) to more conventional studies in animals. Surprisingly few
laboratories have conducted studies with immunosuppressive agents in
which immune function responses in human immune cells are compared
with thosein rodents in vivo (Cornacoff et al., 1988; Luo et al.,
1992; Wood et al., 1992; Lang et al., 1993). Althoughstudies in human
cells in vitro have been hampered by a lack of assays to assess
primary antigen-specific immune responses, a relatively good
interspecies correlation has been observed in the limited responses
examined. Furthermore, some of these assays have been successfully
modified to include metabolic fractions of liver homogenates (Shand,
1975) or co-culture with primary hepatocytes (Kim et al., 1987) to
allow for chemical metabolism.
6.2.2 Parallelograms
Interpretation of studies in experimental animals or in vitro
can be improved when even limited data on human exposure in vivo are
available, using a 'parallelogram' approach. In general, if a
parallelogram can be constructed in which data are available for five
of the six angles (Figure 37), it may be easier to predict the outcome
at the remaining angle, at least qualitatively. For example, cytokine
and phagocytic responses of alveolar macrophages or pulmonary
epithelial cells after exposure to ozone in vitro can be compared
with the responses to ozone after exposure of humans and animals
 in vivo. If the in-vitro data prove to be predictive indicators of
the in-vivo effects in humans, more weight can be given to in-vitro
studies with similar agents or with compounds that are too toxic to be
assessed in clinical studies but for which data are available on both
animals in vivo and animals and humans in vitro. A similar
approach can be used to establish the relationship between acute and
subchronic effects as a means of extrapolating from acute effects in
humans to chronic effects, for which few data are usually available.
Another situation in which this approach may be applicable is in
extrapolating deficits in immune function to increased susceptibility
to disease in animal models, as a means of interpreting the risk of
disease in humans, for whom data on immune function but not infectious
disease may be available.
6.2.3 Severe combined immunodeficient mice
Another approach, which warrants further exploration, is the use
of SCID mice grafted with human immune cells. This model is described
in section 4.5.3. In short, SCID mice have been successfully
grafted with human fetal lymphoid tissue in order to study human
haematopoiesis (McCune et al., 1988) or with human peripheral blood
lymphocytes, which allow production of human immunoglobulins, to study
secondary antibody responses (Mosier, 1990). Reconstituted mice have
been used to study autoimmunity and the efficacy of antiviral
therapeutic agents. There are still limitations to the use of these
animals for immunotoxicology (Pollock et al., 1994).
6.3 Host resistance and clinical disease
A major limitation in assessing the risk of immunotoxicity is
the difficulty in establishing quantitative relationships between
immunosuppression and clinical diseases. The diseases are usually
manifested as increases in the frequency, duration, or severity of
infections, increased incidences of certain cancers, such as Kaposi's
sarcoma and non-Hodgkin's lymphoma (malignancies often observed in
immunosuppressed individuals), or an increased incidence of autoimmune
diseases.
Despite overwhelming experimental and clinical evidence that
increases in the incidences of neoplastic and/or infectious diseases
occur in animals and individuals with secondary immunodeficiency
(Austin et al., 1989; Ehrke et al., 1989), neither the most
appropriate immune end-points for predicting clinical disease nor the
quantitative relationship between changes in immune function and
impairment of host defence are clearly defined. For example, it would
be useful to determine whether certain immune end-points (or quantity
of changes) predict certain outcomes (e.g. increased susceptibility to
influenza and decreased antibody responses). A better understanding of
these relationships would be particularly beneficial for risk
assessment, since changes in immune function are more readily
quantifiable in populations at risk than are changes in the frequency
or severity of infections. A particularly relevant question for risk
estimation is whether increases in host susceptibility to challenge
agents follow linear or 'threshold-like' models as a function of
increased immunosuppression. While terms such as 'immune reserve' and
'immunological redundancy' are applicable for individual responses, it
is unclear how they would be applied to large populations. Since the
potential outcomes of immunosuppression are increases in infections or
neoplastic diseases and there is already a background incidence of
these diseases in the population (Centers for Disease Control, 1991),
it would be helpful to determine the additional frequency of disease
that is associated with increased loss of immune function. Qualitative
relationships are well established, but the quantitative relationship
between immune function and clinical disease in humans has proved
difficult to explore, owing, in part, to the complexity of the immune
system, overlapping (i.e. redundant) immune responses, and variability
in the virulence of infectious agents. Nonetheless, several studies
have shown quantitative relationships in humans. For example, in
longitudinal studies of a relatively large population, asymptomatic
individuals with low NK cell activity were found to be at risk for
developing upper respiratory infections (Levy et al., 1991). Larger
population studies have been conducted in AIDS patients, as the
depletion of CD4+ cells following HIV-1 infection is a clinical
hallmark of the disease. The normal human range of CD4+ cells is
800-1200 cells/µl, but this level generally declines to less than 500
cells/µl within three to four years after HIV-1 infection and to 200
cells/µl before overt opportunistic infections are seen (Masur et al.,
1989; Phair et al., 1990). It has also been shown in seropositive
individuals that a drop in CD4+ cells by 7% or more in a year
increases the relative risk of developing AIDS (Burcham et al., 1991).
Because of the uncertainties about the quantitative relationship
between immune function and disease, there has been continuing
interest in developing sensitive, reproducible experimental models of
host resistance to define altered immune function after exposure to
environmental agents. Most of these models were developed in mice and,
to a lesser extent, in rats; they include bacterial, viral, protozoan,
fungal, and syngeneic or semisyngeneic transplantable tumour cell
models. Although the target organs and general host defence activities
have been defined for most of these models, multiple immune and
nonimmune, mechanisms are involved in resistance, making it difficult
to determine the exact defect without assessing immune function
responses to the challenge agent. For example, defence against
extracellular organisms involves the interactions of T lymphocytes,
B lymphocytes, macrophages, and polymorphonuclear granulocytes, in
addition to a variety of cell-secreted products, whereas resistance to
generalized infection from intracellular pathogens and neoplastic
diseases is likely to involve macrophages, NK cells, and T cell-
mediated immune processes.
Although many host resistance assays are relatively simple to
perform, they normally require large numbers of animals and appear to
be less sensitive than immune function tests (Luster et al., 1993).
Other studies have shown that host resistance assays are more
sensitive than immune function tests (Vos et al., 1991; Burleson et
al., in press). The dose of challenge agent used in experimental
studies is important, since too low or too high a dose will not allow
detection of changes in immunocompromised groups in comparison with
controls (Selgrade et al., 1982; Luster et al., 1993). The sensitivity
of a host resistance assay also depends on the end-point measured. For
example, tests involving survival or tumour models (i.e. dichotomous)
are by nature less sensitive than those with end-points that provide
continuous data, such as enumeration of tumours, bacteria, or soluble
immune activation markers, and several end-points in one model of
infection, such as T. spiralis. This is attributable partly to
differences in the types of statistical analyses used to establish
group differences. With dichotomous data, two approaches can be used.
Some laboratories use a challenge dose that produces a response in
10-30% of animals in the control group. An alternative is to use a
dose slightly below that which would induce the desired response in
any of the animals in the control group. The latter design increases
the statistical power of data analysis. In most cases, extreme
accuracy is needed in the delivery of the agent, to ensure that the
administered dose of agent is only slightly smaller than that which
will give the desired response. In either approach, statistical
significance is heavily dependent on the dose of challenge agent and
the number of animals in each treatment group: Table 15 shows that
doubling the number of animals in a study greatly increases the
ability to detect a significant change. Even more obvious is the
increased ability to detect significant differences when the dose of
the agent in the control group is lowered to a subclinical
concentration. These hypothetical values demonstrate how statistically
significant changes can be obtained in susceptibility assays by
modifying the experimental design. In the first design, an effective
dose of 30% is used in the control group (i.e. a concentration of
agent that produces a response in 30% of normal animals) with 15
animals per treatment group. In the second design, increasing the
group size to 30 allows for even greater statistical significance. In
the third design, a challenge inoculation is given which produces no
effect in the control group (effective dose, 0), allowing for greater
statistical significance.
Two variables that influence the quantitative relationship
between immune function and disease are the virulence and amount of
the infectious agent. These remain a constant in most experimental
studies but may vary between experiments as well as in the human
population. For example, in the general population one can assume that
an infectious disease such as influenza can develop in any individual,
independently of their immune capacity or prior immunization, provided
that the virulence or quantity of the challenging agent is sufficient
to overwhelm the individual's defensive capacities. In Figure 38,
in vivo. If the in-vitro data prove to be predictive indicators of
the in-vivo effects in humans, more weight can be given to in-vitro
studies with similar agents or with compounds that are too toxic to be
assessed in clinical studies but for which data are available on both
animals in vivo and animals and humans in vitro. A similar
approach can be used to establish the relationship between acute and
subchronic effects as a means of extrapolating from acute effects in
humans to chronic effects, for which few data are usually available.
Another situation in which this approach may be applicable is in
extrapolating deficits in immune function to increased susceptibility
to disease in animal models, as a means of interpreting the risk of
disease in humans, for whom data on immune function but not infectious
disease may be available.
6.2.3 Severe combined immunodeficient mice
Another approach, which warrants further exploration, is the use
of SCID mice grafted with human immune cells. This model is described
in section 4.5.3. In short, SCID mice have been successfully
grafted with human fetal lymphoid tissue in order to study human
haematopoiesis (McCune et al., 1988) or with human peripheral blood
lymphocytes, which allow production of human immunoglobulins, to study
secondary antibody responses (Mosier, 1990). Reconstituted mice have
been used to study autoimmunity and the efficacy of antiviral
therapeutic agents. There are still limitations to the use of these
animals for immunotoxicology (Pollock et al., 1994).
6.3 Host resistance and clinical disease
A major limitation in assessing the risk of immunotoxicity is
the difficulty in establishing quantitative relationships between
immunosuppression and clinical diseases. The diseases are usually
manifested as increases in the frequency, duration, or severity of
infections, increased incidences of certain cancers, such as Kaposi's
sarcoma and non-Hodgkin's lymphoma (malignancies often observed in
immunosuppressed individuals), or an increased incidence of autoimmune
diseases.
Despite overwhelming experimental and clinical evidence that
increases in the incidences of neoplastic and/or infectious diseases
occur in animals and individuals with secondary immunodeficiency
(Austin et al., 1989; Ehrke et al., 1989), neither the most
appropriate immune end-points for predicting clinical disease nor the
quantitative relationship between changes in immune function and
impairment of host defence are clearly defined. For example, it would
be useful to determine whether certain immune end-points (or quantity
of changes) predict certain outcomes (e.g. increased susceptibility to
influenza and decreased antibody responses). A better understanding of
these relationships would be particularly beneficial for risk
assessment, since changes in immune function are more readily
quantifiable in populations at risk than are changes in the frequency
or severity of infections. A particularly relevant question for risk
estimation is whether increases in host susceptibility to challenge
agents follow linear or 'threshold-like' models as a function of
increased immunosuppression. While terms such as 'immune reserve' and
'immunological redundancy' are applicable for individual responses, it
is unclear how they would be applied to large populations. Since the
potential outcomes of immunosuppression are increases in infections or
neoplastic diseases and there is already a background incidence of
these diseases in the population (Centers for Disease Control, 1991),
it would be helpful to determine the additional frequency of disease
that is associated with increased loss of immune function. Qualitative
relationships are well established, but the quantitative relationship
between immune function and clinical disease in humans has proved
difficult to explore, owing, in part, to the complexity of the immune
system, overlapping (i.e. redundant) immune responses, and variability
in the virulence of infectious agents. Nonetheless, several studies
have shown quantitative relationships in humans. For example, in
longitudinal studies of a relatively large population, asymptomatic
individuals with low NK cell activity were found to be at risk for
developing upper respiratory infections (Levy et al., 1991). Larger
population studies have been conducted in AIDS patients, as the
depletion of CD4+ cells following HIV-1 infection is a clinical
hallmark of the disease. The normal human range of CD4+ cells is
800-1200 cells/µl, but this level generally declines to less than 500
cells/µl within three to four years after HIV-1 infection and to 200
cells/µl before overt opportunistic infections are seen (Masur et al.,
1989; Phair et al., 1990). It has also been shown in seropositive
individuals that a drop in CD4+ cells by 7% or more in a year
increases the relative risk of developing AIDS (Burcham et al., 1991).
Because of the uncertainties about the quantitative relationship
between immune function and disease, there has been continuing
interest in developing sensitive, reproducible experimental models of
host resistance to define altered immune function after exposure to
environmental agents. Most of these models were developed in mice and,
to a lesser extent, in rats; they include bacterial, viral, protozoan,
fungal, and syngeneic or semisyngeneic transplantable tumour cell
models. Although the target organs and general host defence activities
have been defined for most of these models, multiple immune and
nonimmune, mechanisms are involved in resistance, making it difficult
to determine the exact defect without assessing immune function
responses to the challenge agent. For example, defence against
extracellular organisms involves the interactions of T lymphocytes,
B lymphocytes, macrophages, and polymorphonuclear granulocytes, in
addition to a variety of cell-secreted products, whereas resistance to
generalized infection from intracellular pathogens and neoplastic
diseases is likely to involve macrophages, NK cells, and T cell-
mediated immune processes.
Although many host resistance assays are relatively simple to
perform, they normally require large numbers of animals and appear to
be less sensitive than immune function tests (Luster et al., 1993).
Other studies have shown that host resistance assays are more
sensitive than immune function tests (Vos et al., 1991; Burleson et
al., in press). The dose of challenge agent used in experimental
studies is important, since too low or too high a dose will not allow
detection of changes in immunocompromised groups in comparison with
controls (Selgrade et al., 1982; Luster et al., 1993). The sensitivity
of a host resistance assay also depends on the end-point measured. For
example, tests involving survival or tumour models (i.e. dichotomous)
are by nature less sensitive than those with end-points that provide
continuous data, such as enumeration of tumours, bacteria, or soluble
immune activation markers, and several end-points in one model of
infection, such as T. spiralis. This is attributable partly to
differences in the types of statistical analyses used to establish
group differences. With dichotomous data, two approaches can be used.
Some laboratories use a challenge dose that produces a response in
10-30% of animals in the control group. An alternative is to use a
dose slightly below that which would induce the desired response in
any of the animals in the control group. The latter design increases
the statistical power of data analysis. In most cases, extreme
accuracy is needed in the delivery of the agent, to ensure that the
administered dose of agent is only slightly smaller than that which
will give the desired response. In either approach, statistical
significance is heavily dependent on the dose of challenge agent and
the number of animals in each treatment group: Table 15 shows that
doubling the number of animals in a study greatly increases the
ability to detect a significant change. Even more obvious is the
increased ability to detect significant differences when the dose of
the agent in the control group is lowered to a subclinical
concentration. These hypothetical values demonstrate how statistically
significant changes can be obtained in susceptibility assays by
modifying the experimental design. In the first design, an effective
dose of 30% is used in the control group (i.e. a concentration of
agent that produces a response in 30% of normal animals) with 15
animals per treatment group. In the second design, increasing the
group size to 30 allows for even greater statistical significance. In
the third design, a challenge inoculation is given which produces no
effect in the control group (effective dose, 0), allowing for greater
statistical significance.
Two variables that influence the quantitative relationship
between immune function and disease are the virulence and amount of
the infectious agent. These remain a constant in most experimental
studies but may vary between experiments as well as in the human
population. For example, in the general population one can assume that
an infectious disease such as influenza can develop in any individual,
independently of their immune capacity or prior immunization, provided
that the virulence or quantity of the challenging agent is sufficient
to overwhelm the individual's defensive capacities. In Figure 38,
 Table 15. Chi-squared values (hypothetical)
Treatment Design 1 Design 2 Design 3
------------------------------- ------------------------------- --------------------------------
No. affected/ One-tailed No. affected/ One-tailed No. affected/ One-tailed
no. tested P value no. tested P value no. tested P value
Control 3/15 - 6/30 - 0/15 -
Dose 1 4//15 0.532 8/30 0.428 1/15 0.516
2 5/15 0.411 10/30 0.273 2/15 0.274
3 6/15 0.312 12/30 0.165 3/15 0.150
4 7/15 0.233 14/30 0.096 4/15 0.084
5 8/15 0.173 16/30 0.055 5/15 0.048*
6 9/15 0.128 18/30 0.031* 6/15 0.028*
7 10/15 0.094 20/30 0.017* 7/15 0.017*
8 11/15 0.069 22/30 0.009* 8/15 0.010*
9 12/15 0.051 24/30 0.005* 9/15 0.006*
10 13/15 0.038* 26/30 0.003* 10/15 0.004*
11 14/15 0.028* 28/30 0.002* 11/15 0.002*
12 15/15 0.021* 30/30 0.001* 12/15 0.002*
groups of mice pretreated with either vehicle (saline), 50 mg/kg
cyclophosphamide (causing minimal immunosuppression), or 200 mg/kg
cyclophosphamide (causing severe immunosuppression) were administered
various numbers of PYB6 tumour cells. Even vehicle-treated mice
developed a high frequency of tumours, provided that the challenge was
sufficiently high (i.e. 8 × 104 tumour cells). In contrast, severely
immunosuppressed mice (high dose of cyclophosphamide) developed an
increased tumour frequency at all challenge levels of PYB6 tumour
cells. The groups treated with the low dose of cyclophosphamide were
of particular interest, since evidence of increased susceptibility
appeared but only as a function of the tumour cell concentration.
Assuming that these observations are applicable to human populations,
even small changes in immune function could increase the likelihood of
disease.
As indicated earlier, while experimental data have been used
occasionally in risk assessment, most immunotoxicological data have
focused on hazard identification. Although comparative data on the
effects of specific immunotoxic agents in humans and animals are
limited, other factors contribute to the minimal use of these data in
risk assessment, including the concern that immunotoxicity testing has
often been conducted without full knowledge of its predictive value in
humans or its quantitative relationship to immune-mediated diseases.
Luster et al. (1988) reported on the design and content of a
screening battery involving a tier approach for detecting potential
immunosuppressive compounds in mice. This battery has been used to
examine a variety of compounds, and the database, generated on over 50
compounds, has been analysed in an attempt to improve the accuracy and
efficiency of tests for screening chemicals for immunosuppression and
to identify better those tests that predict experimentally induced
immune-mediated diseases (Luster et al., 1992, 1993). Specifically,
attempts have been made to develop a 'streamlined' test configuration
for accurately predicting immunotoxic agents and to establish models
that could be used to provide insight into the qualitative and
quantitative relationships between the immune and host resistance
assays commonly used to examine potential immunotoxic chemicals in
experimental animals. While the analyses had a number of limitations,
several conclusions can be drawn from the results:
(1) With this particular testing configuration, examination of
only two or three immune parameters was needed in order to identify
potential immunotoxicants. Lymphocyte enumeration and quantification
of the T cell-dependent antibody response appeared to be particularly
useful. Furthermore, some commonly employed measures (e.g. leukocyte
counts, lymphoid organ weights), while probably good predictors of
immunotoxicity, are apparently not as sensitive as the other tests.
Obviously, inclusion of additional tests that are not part of the
original battery may improve the prediction of immunotoxicity.
(2) A good correlation was found between changes in immune tests
and altered host resistance, in that there was no instance in which
host resistance was altered without a significant change in the immune
test(s). In many instances, however, immune changes were observed in
the absence of detectable changes in host resistance (Table 16),
indicating that immune tests are generally more sensitive than host
resistance assays.
(3) No single immune test could be considered highly predictive
for altered host resistance; however, many of the tests were good
indicators, while others, such as leukocyte counts and proliferative
response to lipopolysaccharide, were relatively poor indicators of a
change in host resistance. Some of the tests that showed the highest
association with host resistance were those described previously as
the best indicators of immunotoxicity, such as the plaque-forming
assay and surface markers, but also included tests such as delayed-
type hypersensitivity and thymic weight.
(4) Regression modelling, using a large data set on one chemical
agent, indicated that most, but not all, of the immune function-host
resistance relationships follow a linear model. It was not possible,
however, to establish linear or threshold models for most of the
chemicals studied when the data from all 50 chemicals were combined;
thus, a more mechanistically based mathematical model will have to be
developed. A similar conclusion was drawn on the basis of a limited
data set collected by the Environmental Protection Agency (Selgrade et
al., 1992), in which changes in NK cell activity were correlated with
changes in susceptibility to cytomegalovirus in a murine model. It is
impossible, at present, to determine how applicable these analyses
will be for immunotoxic compounds with different immune profiles;
however, as more analyses become available, the ability to estimate
potential clinical effects accurately from the results of
immunological tests should increase.
Table 16. Association between the results of host resistance models and immune tests
Challenge No. of Frequencya
agent tests
Specificity Sensitivity Concordance
(-/-) (+/+) (Total)
Listeria monocytogenes 34 100 52 65**
PYB6 tumour 24 100 39 54
Streptococcus pneumoniae 19 100 38 58
B16F10 melanoma 19 100 40 68
Plasmodium yoelii 11 100 38 55
Influenza 9 100 17 44
Any of the aboveb 46 100 68 78*
From Luster et al. (1993)
* Agreement statistically significant at P < 0.05
a Frequency is defined as: specificity, the percentage of non-immunotoxic
chemicals with no effect on the host resistance models; sensitivity,
the percentage of potentially immunotoxic chemicals causing a change
in a host resistance model; concordance, percentage of qualitative agreement
b Frequency calculated on the basis of all of the host resistance models used to
study an agent
7. SOME TERMS USED IN IMMUNOTOXICOLOGY
Accessory cell. Passenger cell (leukocyte, mainly monocytes) or
stationary cell (reticulum cell, epithelial cell, endothelial cell)
that aids T or B lymphocytes in inducing immunological reactions,
either by direct contact or by releasing factors; normally expresses
MHC class II molecules
Acquired immunity. A state of protection against pathogen-induced
injury, with rapid immune elimination of pathogenic invaders; due to
previous immunization or vaccination
Activation. The process of going from a resting or inactive state to
a functionally active state, of leukocytes (lymphocytes, monocytes) or
proteins (complement, coagulation)
Acute-phase protein. Non-antibody humoral factor that emerges in
increasing amounts in the circulation shortly after induction of an
inflammatory response; e.g. alpha 2-macroglobulin, C-reactive protein,
fibrinogen, alpha 1-antitrypsin, and complement components
Adaptive immunity. A state of specific acquired protection against
pathogenic invaders, induced by immunization or vaccination
Addressin. Receptor for lymphocytes on endothelial cells of venules,
involved in homing of cells in lymphoid tissue; belongs to the
immunoglobulin gene superfamily, integrins, and selectins
Adenoid. See Tonsil
Adhesion receptors Molecule involved in cellular adhesion between
passenger cells and the extracellular stationary matrix (endothelium,
connective tissue); comprises three main families; member of the
immunoglobulin gene superfamily, integrins, and selectins
Adjuvant. Material that enhances an immune response
Adoptive immunity or tolerance. Transfer of a state of immunity or
tolerance via cells or serum from an immune or tolerant individual to
a naive individual
Affinity. Binding strength of an antibody-combining site to an
antigenic determinant (epitope); expressed as an association constant
(Ka)
Agglutination. Process of aggregation of visible antigenic particles
(e.g. erythrocytes) mediated by antibodies directed towards the
particles
Allele. One or more genes at the same chromosomal locus which
control alternative forms (phenotypes) of a particular inherited
characteristic
Allergen. Antigen that induces an allergic or hypersensitivity
reaction, resulting in immune-mediated or nonimmune-mediated tissue
damage; restricted mainly to immediate hypersensitivity or
anaphylactic reactions
Allergy. State of altered immunity, resulting in hypersensitivity
reaction on contact with antigen or allergen; often restricted to
immediate hypersensitivity or anaphylaxis
Alloantigen. Antigen that differs between different (not inbred)
individuals within one species
Allogeneic. Genetically different phenotypes in different (not
inbred) individuals within one species; opposite of isogeneic, or
xenogeneic
Allotype. Genetically different antigenic determinants on protein of
(not inbred) individuals within one species
alpha Chain. First chain of a multimeric receptor molecule: in
immunoglobulin molecules, the a heavy chain forming the IgA class; in
T-cell receptor molecules, one of the chains forming the dimeric
alpha-ß receptor molecule; in MHC class I molecules, the main
polypeptide chain associated with the ß2-microglobulin molecule; in
MHC class II molecules, one of the chains in the dimeric molecule
Alternative pathway of complement activation. Activation of
complement pathway by substances other than antigen-antibody
complexes; involves factor B, properdin, and complement component C3
Anaphylatoxin. Activated components of complement components C3 and
C5 (C3a and C5a, respectively), which induce anaphylactic reactions by
activating mast cells and basophilic granulocytes
Anaphylaxis or anaphylactic reaction. Local or systemic immediate
hypersensitivity reaction initiated by mediators released after
immunological stimulation; symptoms can be a drop in blood pressure
related to vascular permeability and vascular dilatation, and
obstruction of airways related to smooth muscle contraction or
bronchoconstriction
Anergy. State of unresponsiveness to antigenic stimulation, due to
the absence of responding elements or the loss of capacity of existing
elements to mount a reaction; synonym for tolerance
Antibody. Immunoglobulin molecule produced in response to immunization
or sensitization, which specifically reacts with antigen Antibody-dependent
cell-mediated cytotoxicity. Cytotoxic reaction in which an antibody forms
the bridge between the cytotoxic cell (lymphocyte, macrophage) and the
target cell
Antigen. Any substance that induces a specific immunological
response
Antigen-binding site (paratope). Part on an antibody molecule that
binds antigen (antigenic determinant, or epitope); part of the T-cell
receptor that binds the complex of antigen and MHC molecule
Antigenic determinant (epitope). Part of an antigen that binds to
antibody or T-cell receptor (the latter in combination with MHC
molecule)
Antigenicity. Capacity to react with components of the specific
immune system (antibody, receptors on lymphocytes)
Antigen presentation. Process of enabling lymphocytes to recognize
antigen on a specific receptor on the cell surface. For presentation
to T lymphocytes, includes intracellular processing and complexing of
processed peptides with MHC molecule on the cell membrane of the
antigen-presenting cell. For presentation to B lymphocytes, can
include formation of immune complexes (in germinal centres)
Antigen-presenting cell. Cell that presents antigen to lymphocytes,
making possible specific recognition by receptors on the cell surface.
In a more restricted way, used to describe MHC class II-positive
(accessory) cells which can present (processed) antigenic peptides
complexed with MHC class II molecules to T helper-inducer lymphocytes.
Includes macrophage populations (in particular, Langerhans cells and
dendritic or interdigitating cells), B lymphocytes, activated T
lymphocytes, certain epithelia (after MHC class II antigen induction
by e.g. interferon gamma); others are follicular dendritic cells, not
of bone-marrow origin, which present antigen in the form of immune
complexes to B cells in germinal centres of peripheral lymphoid
tissue; marginal zone macrophages in splenic marginal zone, which
present antigen, without contact with T helper cells, to B cells at
this location (T cell-independent response, e.g. to bacterial
polysaccharide). In rodent skin epidermis, a dendritic epidermal cell,
of T-cell origin, has an antigen-presenting function.
Antigen receptor. Multichain molecule on lymphocytes, to which
antigens bind. For B lymphocytes, an immunoglobulin molecule that
recognizes nominal antigen; for T lymphocytes, a T-cell receptor
molecule that recognizes antigenic peptide in combination with the
polymorphic determinant of an MHC molecule
Antinuclear antibody. Antibody directed to nuclear antigen; can have
various specificities (e.g. to single- or double-stranded DNA or
histone proteins); frequently observed in patients with rheumatoid
arthritis, scleroderma, Sjögren's syndrome, systemic lupus
erythematosus, and mixed connective tissue disease. Also called
antinuclear factor
Antiserum. Serum from an individual that contains antibodies to a
given antigen
Aplasia. Absence of tissue structure or cellular component, either
congenital or acquired
Apoptosis (programmed cell death). Process whereby the cell kills
itself after activation, by Ca2+-dependent endonuclease-induced
chromosomal fragmentation into fragments of about 200 base-pairs
Appendix. Lymphoid organ in the gastrointestinal tract, at the
junction of ileum and caecum; forms part of gut-associated lymphoid
tissue
Arthus reaction. Inflammatory response, generally in skin, induced
by immune complexes formed after injection of antigen into an
individual that contains antibodies
Asthma. Chronic inflammatory disease characterized by bronchial
hyperresponsiveness to various stimuli
Atopy. In general terms, 'unwanted reactivity'; used mostly to
describe the state of general systemic or local hypersensitivity
reactions related to genetic predisposition
Auto-antibody. Antibody to component in the individual itself
Auto-antigen. Antigen to which an autoimmune reaction is directed
Autoimmunity. A state of immune reactivity towards self
Autologous. Derived from self; components of an immunological
reaction (e.g. antibody, lymphocytes, grafted tissue) from the same
individual; opposite of heterologous
Avidity. Binding strength between antibody and antigen, or receptor
and ligand; for antibody, represents the product of more than one
interaction between antigen-binding site and antigenic determinant
Bacteraemia. Presence of bacteria in blood
Basophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with acid glycoproteins stained by basic (blue) dyes; after
release, glycoproteins induce anaphylactic reactions
B-Cell growth and differentiation factor, B-cell growth factor, and
B-cell stimulating factor. See Interleukin-4 and -5
ß Chain. In T-cell receptor molecules, one of the chains forming the
dimeric alpha-ß receptor molecule; in MHC class II molecules, one of
the chains in the dimeric molecule
ß2-Microglobulin. A peptide of 12 kDa, which forms part of MHC class
I molecule
B Lymphocyte or cell. Lymphocytes that recognize nominal antigen by
immunoglobulin (antibody) surface receptor (on virgin B cell, IgM and
IgD) and, after activation, proliferate and differentiate into
antibody-producing plasma cells. During a T-dependent process, there
is immunoglobulin class switch (IgM into IgG, IgA, IgD, or IgE), with
maintenance of the antigen-combining structure. For T-independent
antigens, cells differentiate only in IgM-producing plasma cells. B
Lymphocytes originate from precursor cells in bone marrow; in avian
species, they undergo maturation in the bursa of Fabricius (B, bursa-
dependent); in mammals, in the bone marrow
B Lymphocyte area. That part of an lymphoid organ or tissue that is
occupied by B lymphocytes, e.g. follicles in peripheral lymphoid
tissue, marginal zone in spleen
Birbeck granule. Rod-shaped structure with rounded end,
approximately 6 nm thick, found in the cytoplasm of Langerhans cells
in the epidermis, and interdigitating dendritic cells in T-lymphocyte
area of lymphoid tissues
Blast cell. Large cell (about 15 µm or more) with dispersed nuclear
chromatin and cytoplasm rich in ribosomes; in an active stage of the
cell cycle before mitosis
Blast transformation. Process of activation of lymphocytes into cell
cycle and to form blastoid cells before mitosis
Blocking antibody. Antibody that can interfere with another antibody
or with reactive cells in binding antigen, thereby preventing effector
reactions (often used in association with an allergic reaction or
tissue damage)
Bone marrow. Soft tissue in hollow bones, containing haematopoietic
stem cells and precursor cells of all blood cell subpopulations
(primary lymphoid organ); major site of plasma cell and antibody
production (secondary lymphoid organ)
Booster. Dose of antigen given after immunization or sensitization
to evoke a secondary response
Bradykinin. Peptide of nine amino acids split from alpha 2-
macroglobulin by the enzyme kallikrein; causes contraction of smooth
muscle
Bronchus-associated lymphoid tissue. Lymphoid tissue located along
the bronchi, considered to represent the location of presentation of
antigens entering the airways; contributes to mucosa-associated
lymphoid tissue
Bursa equivalent. Site where B-cell precursors undergo maturation
into immunocompetent cells in non-avian species; bone marrow in adult
mammals
Bursa of Fabricius. Primary lymphoid organ in avian species, located
in cloaca, with an epithelial reticulum, where precursors of B
lymphocytes from the bone marrow undergo maturation into
immunocompetent cells and then move to peripheral lymphoid organs
C (constant) gene. Gene that encodes the constant part of
immunoglobulin chains or T-cell receptor chains (e.g. Cµ, Cdelta
for immunoglobulin heavy chains, Ckappa for immunoglobulin kappa
light chain, Calpha for T-cell receptor alpha chain)
C (constant) region. Region at carboxy terminal of immunoglobulin
chains or T-cell receptor chains, identical for a given immunoglobulin
class or subclass or for a given T-cell receptor chain; encoded by C
genes in DNA
Cachectin. See Tumour necrosis factor
CD (cluster of differentiation). Group of (monoclonal mouse)
antibodies that react to identical leukocyte surface molecules in
humans (but not necessarily to identical epitopes), on the basis of
comparative evaluations during international workshops and transferred
to other species by analogy. Does not include MHC or immunoglobulin
molecules
CD3 molecule. Molecule consisting of at least four invariant
polypeptide chains, present on the surface of T lymphocytes associated
with the T-cell receptor; mediates transmembrane signalling (tyrosine
phosphorylation) after antigen binding to T-cell receptor
CD4 molecule. Glycoprotein of 55 kDa on the surface of T lymphocytes
and part of monocytes and macrophages. On mature T cells, restricted
to T helper (inducer) cells; has an accessory function to antigen
binding by T-cell receptors, by binding to a non-polymorphic
determinant of MHC class II molecule
CD8 molecule. Complex of dimers or higher multimers of 32-34 kDa
glycosated polypeptides linked by disulfide bridges, on the surface of
T lymphocytes. On mature T cells, the presence is restricted to
T cytotoxic-suppressor cells; has an accessory function to antigen
binding by the T-cell receptor, by binding to a non-polymorphic
determinant of MHC class I molecule
Cell-mediated immunity. Immunological reactivity mediated by
T lymphocytes
Central (primary) lymphoid organ. Lymphoid organ in which precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment, to form immunocompetent cells; not antigen-driven
but can be influenced by mediators produced as a result of antigen
stimulation
Centroblast. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; a medium to large,
12-18-µm cell, with a round to ovoid nucleus that has moderately
condensed heterochromatin and medium-sized nucleoli close to the
nuclear membrane, medium-sized to broad cytoplasm containing many
polyribosomes and a variable amount of rough endoplasmic reticulum
Centrocyte. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; medium-sized,
8-12-µm lymphoid cell with irregular nucleus with condensed
heterochromatin; small cytoplasm containing a few organelles
CH50. The amount of serum (or dilution of serum) that is required to
lyse 50% of erythrocytes in a standard haemolytic complement assay
Chemiluminescence. Luminescence produced by direct transformation of
chemical energy into light energy
Chemotactic factor. Substance that attracts cells to inflammatory
lesions
Chemotaxis. Process of attracting cells to a given location, where
they contribute to an inflammatory lesion
Class I MHC molecule. Molecule coded by the A, B, or C locus in the
HLA complex, the K and D locus in the mouse H2 complex, and less well
defined MHC I gene loci in other species, in association with the
ß2-microglobulin molecule. Two-chain molecule occurring on all
nucleated cells, without allelic exclusion
Class II MHC molecule. Molecule coded by the D (DR, DP, DQ) locus in
the HLA complex, the I-A and I-E locus in the mouse H2 complex, and
less well defined MHC II gene loci in other species, comprising an
alpha and a ß chain (intracellular, associated with an 'invariant'
chain). Two-chain molecule occurring, without allelic exclusion, on B
lymphocytes, activated T lymphocytes, monocytes-macrophages,
interdigitating dendritic cells, some epithelial and endothelial cells
(variable, dependent on species and state of activation); antigen-
presenting cells
Class switch. See Immunoglobulin class switch
Classical pathway of complement activation. Activation of complement
pathway by antigen-antibody complexes, starting with complement
component C1 and ending with complement component C3
Clone. Population of cells that emerge from a single precursor cell;
within T or B lymphocytes, cells with a fixed rearrangement of genes
coding for T-cell receptor or immunoglobulin
Clonal expansion. Proliferation of cells that have a genetically
identical constitution; when uncontrolled, may result in tumour
formation
Colony-stimulating factor. Substance that supports clonal cell
growth of haematopoietic cells
Complement. Group of about 20 proteinase precursors that activate
and split each other, in sequential order. The various components are
present in inactive (precursor) form, except for C3, which in a normal
state shows a low turnover (major split products C3a and C3b). The
split products are either bound to the activating substance (immune
complex or antibody-coated cell) or are released as active mediators.
The classical cascade starts by activation of component C1,
subsequently C4, C2, and C3, and is initiated by (IgG/IgM) immune
complexes. The alternative cascade starts by activation of C3b and
factor B, subsequently factors D and C3, and is initiated by nonimmune
specific activators like microbial polysaccharides and some
'allergens'. C3 split products (C3b) activate the amplification loop,
in which factors D and B are also used, activate C5, and thereafter
the terminal cascade C6, C7, C8, and C9, which attack the cell
membrane and kill the cell (microorganism). The cascade is under the
control of various inhibitors. Major effects of complement split
products are adherence to receptors on phagocytes (C3b, C3d); mediator
activity, like chemoattraction of inflammatory cells, vasodilatation,
increased vascular permeability, and smooth muscle contraction
(C3a, C5a); cell lysis by membrane lesions (C6-C9)
Complement fixation. Binding and consumption of complement by
antigen-antibody complexes; often used in association with assays for
complement activity
Complement receptor (CR). Cell surface molecule that can bind
activated complement components in e.g. antigen-antibody complexes;
CR1 (CD35) is a receptor for C3b, present on B lymphocytes, monocytes
and macrophages, granulocytes, and erythrocytes; CR2 (CD21) is a
receptor for C3d, present on mature B lymphocytes; CR3 (CD11b/CD18) is
a receptor for C3b present on macrophages, granulocytes, natural
killer cells, and a subset of CD5+ B lymphocytes; CR4 (CD11c/CD18)
is a receptor for C3b present on monocytes, macrophages, granulocytes,
and natural killer cells
Contact sensitivity. Hypersensitivity reaction evoked in skin by
placing sensitizing agents or substances on skin
Cords of Billroth. Medullary cords in spleen
Corona. See Mantle
Cortex. Outer parenchymal layer of organs
Cross-reactivity. Reactivity of antigen-specific elements (T lymphocytes
sensitized by T-cell receptor, B lymphocytes by antibody; antibody
molecules) towards antigens other than those used in original
sensitization, owing to shared antigenic epitopes on different
antigenic molecules; also used to describe reactions towards antigenic
determinants other than those originally used in sensitization, due to
similarities in structure
Cytokine. Biologically active peptide, synthesized mainly by
lymphocytes (lymphokines) or monocytes and macrophages (monokines);
modulates the function of cells in immunological reactions; include
interleukins. Some (pleotrophic cytokines) have a broad spectrum of
biological action, including neuromodulation, growth factor activity,
and proinflammatory activity
Cytokine receptor. Ligand for cytokine on target cell, acts in
signal transduction through the cell membrane; many are multichain
molecules belonging to different receptor families
Cytolytic antibody. Antibody that can mediate lysis of the target to
which it is directed, either in combination with complement or as
bridge between cytotoxic cell and target
Cytotoxic cell (killer cell). Effector T cell, natural killer cell,
or activated macrophage; kills target cells and tissue extracellularly
after binding; mediated by release of substances from cytolytic
granules (including serine esterase, cytolysin, and perforins)
Cytotoxic reaction. Effector reaction of antibody or cells, resulting
in lysis of target cell or tissue
Cytotoxic T lymphocyte. Subpopulation of T lymphocytes with CD8
phenotype; after recognition of antigen in an MHC class I-restricted
manner, differentiates from precursor to effector cytotoxic cell and
subsequently kills target cells
Degranulation. Process of fusion of cytoplasmic granules with cell
membrane, whereby the content of the granules is released into the
extracellular space; mainly used in association with immediate
hypersensitivity reactions
Delayed-type hypersensitivity. Inflammatory lesion mediated by
effector T lymphocytes or their products, with attraction mainly of
macrophages towards the inflammatory lesion. Term originates from the
classical skin reaction after challenge of a sensitized individual;
maximal (wheal and flare) response reached within 24-72 h
Delayed-type hypersensitivity T cell. Subpopulation of T lymphocytes
with CD4 phenotype; after recognition of antigen in an MHC class
II-restricted manner, secretes mediators involved in inflammatory
responses, e.g. INF gamma and tumour necrosis factor
delta Chain. In immunoglobulin molecules, delta heavy chain forming
the IgD immunoglobulin class; in T-cell receptor molecules, one of the
chains forming the dimeric gamma-delta receptor molecule; one of the
chains in the CD3 molecule associated with the T-cell receptor
Dendritic cell. Cell in tissue that shows elongations or protrusions
of cytoplasm into the parenchyma. Often used in a restricted manner to
designate a type of antigen-presenting cell, of which two categories
exist: one of bone-marrow origin belonging to the macrophage lineage,
including Langerhans cells in skin and interdigitating dendritic cells
in T-cell areas of lymphoid tissue and a very small leukocyte
population in blood; the second of tissue parenchymal origin
(presumably pericytes around blood vessels), the follicular dendritic
cells in B-cell areas (follicles) of lymphoid tissue
Dendritic epidermal cell. T Cell in the epidermis that has dendritic
morphology; has antigen-presenting function but is not a (macrophage-
related) Langerhans cell. Occurs in rodents but not in humans;
contributes to skin immune system
Dermal immune system. See Skin immune system
Desensitization. Induction of anergy or tolerance to allergic
substances by active intervention in immune reactivity (exhaustion of
reactive elements or induction of blocking phenomena)
Diapedesis. Passage of cells through blood vessel walls into tissue
parenchyma, mediated by constriction of endothelial cells
D (diversity) gene. Gene that encodes the variable part of
immunoglobulin heavy chains, or T-cell receptor alpha, ß, or gamma
chain (e.g. DH1-DHn for immunoglobulin heavy chains, Ddelta
1-Vdelta n for T-cell receptor alpha or delta chain)
Domain. Part of polypeptide chain folded to a relatively rigid
globular tertiary structure fixed by disulfide bonds. Molecules of the
immunoglobulin gene superfamily have a tertiary domain-like structure:
each domain is about 110 amino acids long and arranged in a sandwich
of two sheets of anti-parallel ß strands. See also Homologous,
Homology
Ectoderm. Outermost of the three cellular layers of the embryo;
produces the epidermis and neuronal tissue
Eczema. Superficial inflammation in skin, involving primarily the
epidermis; characterized by redness, itching, minute papules and
vesicles, weeping, oozing, and crusting. Histological changes include
microvesiculation and oedema of the epidermis and an infiltrate of
lymphocytes and macrophages in the dermis
Effector cell. General term to describe a cell that mediates a
function after a stage of activation, differentiation, and
proliferation
Endocytosis. Process of uptake of material by a cell; special forms
are phagocytosis and pinocytosis
Endoderm. Innermost of the three cellular layers of the embryo;
produces the gastrointestinal lining and some internal organs, such as
liver and pancreas
Endoplasmic reticulum. Membrane-like structure in cell cytoplasm;
site of protein synthesis
Endothelium. Cells that line blood vessels; exert a major function
in traffic of leukocytes from blood into tissue, by altered expression
of adhesion molecules (modulation of numbers of receptors; maturation
and activation resulting in altered glycosylation, expression of new
ligands or altered ligand binding affinity; change in cytoskeleton
organization). A special endothelial cell type occurs in T-lymphocyte
areas of lymphoid tissue in the high endothelial (postcapillary)
venule.
Endotoxin. Lipopolysaccharide from the cell wall of Gram-negative
bacteria; has toxic, pyrogenic, and immunoactivating effects
Enzyme-linked immunosorbent assay. Immunoenzymetric assay based on
the use of antigens or antibodies labelled with a specific enzyme;
combines the virtues of solid-phase technology and enzyme-labelled
immunoreagents. The antigen-antibody complex is determined by an
enzyme assay involving the incubation of the complex with an
appropriate substrate of the enzyme.
Eosinophil chemotactic factor. Acidic tetrapeptide of 0.5 kDa
produced by mast cells (preformed mediator); attracts eosinophils to
the site of inflammation
Eosinophilia. State of increased proportions of eosinophilic
granulocytes in blood
Eosinophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with basic proteins stained by acidophilic (red) dyes; after
release, the proteins modulate inflammatory reactions
Epithelium. Cells covering the surface of the body and forming the
first line of defence against pathogenic invaders. Reticular
epithelium forming the stroma of tissue occurs in thymus (in avian
species in the bursa of Fabricius); these cells have a major function
in processing precursor cells to immunocompetent lymphocytes
Epithelioid cell. Cell of macrophage origin in chronic inflammatory
lesions, which resembles an epithelial cell morphologically
Epitope (antigenic determinant). Part of antigen that binds to
antibody or T-cell receptors (the latter in combination with MHC
molecule)
epsilon Chain (see also alpha Chain). In immunoglobulin molecules,
the epsilon heavy chain forming the IgE immunoglobulin class; one of
the chains in the CD3 molecule associated with the T-cell receptor
Erythema. Redness of skin produced by congestion of blood
capillaries due to dermal arterial vasodilatation
Erythrocyte. Red blood cell; a bone marrow-derived blood cell
component involved in oxygen transport to tissue; contains a nucleus
in distinct avian species like chickens but does not have a nucleus in
mammals
Exudation. Inflammation in tissue; contains blood cells and fluid
comprising serum proteins of high relative molecular mass
Ex vivo/in vitro. An assay method in which the effects of a
xenobiotic are evaluated in vitro in cells isolated from an animal
or human exposed to the compound of interest
Fab fragment. Part of an antibody molecule in which monovalent
binding of an antigenic determinant occurs; formed by the three-
dimensional structure of variable parts (domain) of one heavy and one
light chain and the adjacent part of the constant part (constant
domain); ab, antigen binding
F(ab')2 fragment. Part of an antibody molecule in which divalent
binding of antigenic determinants occurs; formed by the
three-dimensional structure of both Fab fragments
Fc fragment. Part of an antibody molecule formed by the three-
dimensional structure of the constant part (constant domains) of the
heavy chains (except that adjacent to the variable domain), involved
in antibody effector functions; c, crystallizable
Fc receptor. Structure on leukocytes (lymphocytes, monocytes,
macrophages, granulocytes) that mediates binding of immunoglobulin or
antibody, alone or after forming aggregates in antigen-antibody
complexes. Receptors for IgE (Fc epsilon) occur on mast cells and
basophilic granulocytes and are involved in immediate hypersensitivity
reactions; receptors for IgG are of three classes: low-affinity FcR
III, CD16, on natural killer cells, monocytes, macrophages, and
granulocytes; low-affinity FcR II, CD32, on B cells, myeloid cells,
Langerhans cells, and interdigitating dendritic cells; high-affinity
FcR III, CD64, on monocytes and macrophages
Follicle. Round to oval structure in lymphoid tissue, where B cells
are lodged. Primary follicles contain only small resting B cells;
secondary follicles comprise a pale-stained germinal centre, with
centrocytes and centroblast, and contain B lymphocytes in a state of
activation or proliferation and macrophages, the stroma consisting of
follicular dendritic cells. The germinal centre is surrounded by a
mantle with small B lymphocytes
Follicular dendritic cell. Cell forming the stationary micro-
environment of germinal centres of follicles in lymphoid tissue;
elongated, often binucleated cell with long branches extending between
germinal centre cells and forming a labyrinth-like structure; linked
by desmosomes. Of local parenchymal origin, presumably from pericytes
surrounding blood vessels. Its main function is presentation of
antigen, trapped as immune complex in the labyrinth, to B lymphocytes.
gamma Chain (see also alpha Chain). In immunoglobulin molecules,
the gamma heavy chain forming the IgG immunoglobulin class; in T-cell
receptor molecules, one of the chains forming the dimeric gamma-delta
receptor molecule; one of the chains in the CD3 molecule associated
with the T-cell receptor
gamma-delta T cell. T Lymphocyte with an antigen receptor composed
of a gamma and a delta chain associated with CD3 transmembrane
molecule; develops in part inside the thymus (including intrathymic
selection), in part outside the thymus. Has a major role as a
cytotoxic cell in the first phase of the (innate) immune response,
e.g. in rodents in the mucosal epithelium
Gammaglobulin. Part of serum proteins that move towards the negative
electrode (gamma fraction) upon electrophoresis; contains
immunoglobulins
Gene rearrangement. For immunoglobulin and T-cell receptor, the
process whereby the germline chromosomal genomic structure of variable
(diversity), joining, and constant segments recombine to form a
specific V-(D-)J-C combination, enabling transcription into mRNA and
translation into protein. The V-(D-)J combination of different chains
determines the specificity of the receptor (immunoglobulin or T-cell
receptor).
Germinal centre. The pale-staining centre in follicles of lymphoid
tissue, where B lymphocytes are activated by antigen in a
T lymphocyte-dependent manner and subsequently go into proliferation
and differentiation, acquiring the morphology of centroblasts,
centrocytes, and plasma cells. Has a special microenvironment made up
of follicular dendritic cells and large macrophages (tingible body or
starry-sky macrophages)
Glomerulonephritis. Inflammation of glomeruli in kidney, often
associated with deposition of immune complexes along the glomerular
basement membrane or in the mesangium, and influx of polymorpho-
nuclear granulocytes
Golgi apparatus. Tubular structures in cytoplasm, involved in
secretion of synthesized proteins
Granulocyte colony-stimulating factor. Synthesized by T lymphocytes
and macrophages, epithelial cells, fibroblasts, and endothelial cells;
supports growth of granulocyte progenitors, in synergism with IL-3 and
granulocyte-macrophage colony-stimulating factor of monocyte-
macrophage progenitors
Granulocyte-macrophage colony-stimulating factor. Produced by T
lymphocytes, endothelial cells, macrophages, and lung cells; supports
growth and differentiation of macrophages and granulocyte progenitors;
activates macrophages and polymorphonuclear macrophages to become
tumoricidal and produce superoxide anion
Granuloma. Chronic inflammatory reaction in tissue comprising macro-
phages (epitheloid cells), lymphocytes, and fibroblasts; formed in a
cell-mediated response towards poorly degradable material, in
immunological reactions as part of a delayed-type hypersensitivity
reaction
Gut-associated lymphoid tissue. Lymphoid organs and tissue located
along the gastrointestinal tract, presumed to be a first location of
presentation of antigens entering through the digestive tract;
comprises Peyer's patches, appendix, in part mesenteric lymph nodes,
adenoids, and tonsils; contributes to the mucosa-associated lymphoid
tissue
H-2. Major histocompatibility complex of mouse
Haemagglutinin. Antibody or substance that induces agglutination of
erythrocytes
Haematopoiesis. Production of cells of blood; subdivided into
erythropoiesis, lymphopoiesis, and myelopoiesis
Haematopoietic malignancy. Malignancy of blood-forming cells
Haemolysis. Process of lysis of erythrocytes, with release of
haemoglobin
Haemolytic agent (haemolysin). Antibody or substance that induces
lysis of erythrocytes
Haemopoietin. Growth factor that induces production of distinct
types of blood cells; also enhances the function of the mature cells
Haplotype. Phenotype of inherited characteristic on closely linked
genes on one chromosome
Hapten. Structure around one antigenic determinant, which itself
does not evoke an immune response unless coupled to a carrier
substance but can react with the products (antibodies, cells) of an
immune response
Hassall's corpuscle. Epithelial aggregate in onion-like structure,
often with debris of other cells; in the medulla of the thymus,
surrounded by large epithelial cells secreting thymic hormones; does
not occur in rodent thymus
Heat-shock protein. Family of proteins (60-90 kDa) with conserved
sequence in evolution; play a prime role in regulation and transport
of intracellular proteins. Expression is upregulated when cells are
under 'stress' (originally induced by heating), such as inflammatory
conditions, and may act as autoantigen in triggering and perpetuating
an auto-immune response
H (heavy) chain. One of the 45-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and three
constant domains (four constant domains in the 55-kDa µ chain). The
combination of the constant part of two heavy chains (alpha, ß, delta,
gamma, or µ) forms the immunoglobulin class-associated part of the
molecule (IgA, IgD, IgE, IgG, or IgM class)
Helper (inducer) T cell. Cell in a subpopulation of T lymphocytes,
with CD4 phenotype; after recognizing antigen in an MHC class
II-restricted manner, induces immunological reactions, secretes
interleukins, and cooperates (supports) B lymphocytes, cytotoxic
T-cell precursors, and macrophages
Helper T cell subpopulations. Th1 and Th2: Th1 cells produce
interleukin-2 and interleukin-3, interferon gamma, tumour necrosis
factor alpha and ß, and granulocyte-macrophage colony stimulating
factor, and function in induction of delayed-type hypersensitivity,
macrophage activation, and IgG2a synthesis. Th2 cells produce
interleukin-3, interleukin-4, and interleukin-5, tumour necrosis
factor alpha and granulocyte-macrophage colony stimulating factor, and
function in induction of IgG1, IgA, and IgE synthesis and induction of
eosinophilic granulocytes
Heterologous. Derived from foreign source or species; components of
an immunological reaction (e.g. antibody, lymphocytes, grafted tissue)
derived from another individual of the same species or another
species; opposite of autologous
Heterophilic antigen. Antigen in unrelated species; can be directed
towards xenogeneic immune reactivity; often has carbohydrate
structure; opposite of homocytotropic antibody
High endothelial (postcapillary) venule. Specialized blood vessels
in T-lymphocyte area of lymphoid tissue, through which circulating
lymphocytes pass into the parenchyma
Hinge region. Stretch in immunoglobulin molecule between Fab and Fc
fragments (first constant domain and other constant domains of the
heavy chain), where the quaternary structure of the molecule is not
rigid but flexible; bending of the hinge region after antigen binding
serves as a signal transduction, resulting in effector reactions
Histamine. ß-Imidazolylethylamine; component of granules in mast
cells and basophilic granulocytes that is released upon activation and
induces immediate hypersensitivity reaction, e.g. vasodilatation,
vascular permeability, smooth muscle contraction, and broncho-
constriction
Histiocyte (histiocytic reticulum cell). Monocyte in tissue. See
Macrophage
Histiocytosis. Increase in proportion of macrophages in tissue
HLA, human leukocyte antigen. Major histocompatibility complex of
humans
Homocytotropic antibody. Antibody that binds preferentially to cells
from the same species rather than to cells from other species;
opposite of heterologous antibody
Homologous, Homology. Similarity in primary structure between
substances; homology region is a synonym for domain
Host defence. Ability of an individual or species to protect itself
against opportunistic agents and to eliminate certain tumours and
exogenous agents such as (micro)organisms, viruses, and particles that
can cause disease
Hot spot. See Hypervariable region
Humoral immunity. Immunological reactivity mediated by antibody
Hybridoma. Transformed cell line or cell clone formed by fusion of
two different parental cell lines or clones
Hyperplasia. Reversible increase in cell number, usually as the
result of a physiological stimulus or persistent cell injury due to
irritating compounds
Hypersensitivity. Increased reactivity or sensitivity; in
immunological reactions, often associated with tissue destruction
Hypervariable region. Amino acid sequences in the variable regions
of antibody molecules or T-cell receptor chains where variability is
highest and which together form the antigen-binding site. Synonym for
hot spot
Hypoplasia. Reversible decrease in cell number, usually as a result
of a physiological stimulus
Ia antigen. MHC class II cell surface molecule
Idiotype. Antigenic determinant of variable domain of immunoglobulin
molecules or T-cell receptor
Immediate hypersensitivity. Inflammatory response that occurs within
minutes after exposure to allergen; caused by physical or
immunological stimulus, with vascular dilatation, increased vascular
permeability, and oedema as the main effects. Term originates from the
classical skin reaction after challenge of a sensitized individual in
skin, which takes 20-30 min to reach maximal (wheal and flare)
response and is mimicked by injection of mediator only (histamine)
Immune adherence. Binding of antigen-antibody complexes (antibody-
coated particles) to erythrocytes, platelets, or leukocytes; mediated
by activation of complement C3
Immune complex. Complex between antigen and antibody
Immune elimination. Rapid clearance of pathogen from the circulation
by components of the immune system; often used in association with
antibody molecules (removal by immune complex formation and
phagocytosis)
Immune exclusion. Process whereby entry of pathogens at mucosal
surfaces is prevented by the action of specific (secretory IgA)
antibody
Immune interferon. Former name for interferon gamma
Immune surveillance. Function (still hypothetical) of the immune
system in preventing or eliminating cells after malignant
transformation to a neoplastic process
Immunity. State of protection against pathogen-induced injury, with
fast immune elimination of pathogenic invaders due to previous antigen
contact or a special acquired state of responsiveness
Immunization (vaccination). Active intervention resulting in
immunity; used mainly in the context of presentation of (inactivated
or attenuated, nonpathogenic) substance to induce immunological
memory. Passive immunization is the adoptive transfer of immune system
components after previous contact with the pathogen and is performed
mainly with antibodies
Immunoblast. Intermediately differentiated B lymphocyte in lymphoid
tissue; a large, 15-20 µm, round-to-spherical cell with a rounded
euchromatic nucleus. The abundant cytoplasm contains many ribosomes,
well-developed rough endoplasmic reticulum and Golgi complex
Immunocompetence. Capacity of B or T lymphocytes to specifically
recognize antigen, resulting in a specific immunological reaction
Immunodeficiency. Defects in the immune system resulting in
decreased or absent reactivity to pathogens. Primary immunodeficiency
is mainly intrinsic defects in the differentiation of T or B
lymphocytes and can be congenital or acquired. Secondary
immunodeficiency is defects of which the cause is outside the immune
system (malnutrition; stress; protein loss after burns, nephrotic
syndrome, or intestinal bleeding; viral infection; therapy with
immunosuppressive or cytostatic drugs; irradiation).
Immunogen. A substance that can induce an immunological reaction
Immunogenicity. Capacity to evoke an immune response
Immunoglobulin. Formerly the electrophoretically-defined
gammaglobulin (in blood) but is also present in the ß fraction;
synthesized by plasma cells. The basic subunit consists of two
identical heavy chains (about 500 amino acid residues, organized into
four homologous domains; for µ chain in IgM, about 600 amino acid
residues, organized into five homologous domains) and two identical
light chains (about 250 amino acid residues organized into two
homologous domains), each consisting of a variable domain and one to
three constant domains (in the µ chain, four constant domains). The
antigen-binding fragment (Fab) consists of variable domains of heavy
and light chains (two per basic subunit). Five classes of
immunoglobulins exist, which differ according to heavy chain type
(constant domains): IgG (major immunoglobulin in blood), IgM
(pentamer, consisting of five basic units), IgA (major immunoglobulin
in secretions; present mainly as a dimeric molecule), IgD (major
function, receptor on B lymphocytes), and IgE. Effector functions
after antigen binding are mediated by constant domains of the heavy
chain (Fc part of the molecule) and include complement activation
(IgG, IgM), binding to phagocytic cells (IgG), sensitization and
antibody-dependent cell-mediated cytotoxicity (IgG), adherence to
platelets (IgG), sensitization and degranulation of mast cells and
basophils (IgE). IgA lacks these effector functions and acts mainly in
immune exclusion (prevention of entry in the body) at secretory
surfaces ('antiseptic paint').
I mmunoglobulin class. Subfamily of immunoglobulins, based on
difference in heavy chain. Five classes exist: IgA, secretory
immunoglobulin, dimeric; IgD, immunoglobulin on B cells that acts as
antigen receptor; IgE, immunoglobulin fixed to mast cells and
basophilic granulocytes, involved in immediate hypersensitivity
reactions; IgG, main immunoglobulin in circulation; IgM, pentameric
immunoglobulin with optimal agglutinating capacity, produced on first
antigen contact
Immunoglobulin class switch. Process whereby synthesis of IgM
antibody changes into synthesis of antibody of another immunoglobulin
class, with maintenance of the same variable part of the
immunoglobulin molecule. At the genomic level, this includes gene
rearrangement, with an exchange of a constant gene segment to a fixed
V-D-J gene segment combination. This switch is thought to occur in
germinal centres of follicles in lymphoid tissue, during the change of
a primary into a secondary response, and is under the control of
cytokines (switch factors)
Immunoglobulin gene superfamily. Group of molecules including
immunoglobulins, T-cell receptors, MHC molecules, and others, like the
lymphocyte function-related antigens LFA-2 (CD2) and LFA-3 (CD58), the
intercellular adhesion molecules ICAM-1 (CD54) and ICAM-2, the
vascular cell adhesion molecule VCAM-1, the neural cell adhesion
molecule NCAM (CD56), and the CD4 and CD8 molecules, which have a
similar tertiary basic domain-like structure, in which each domain is
about 110 amino acids long and stabilized by a disulfide bridge. These
molecules are known to be important for specific recognition and
adhesion functions
Immunoglobulin light chain type. Defines the light chain in the
immunoglobulin unit, either kappa or lamda, each defined at the
germline DNA level by individual constant (C), joining (J), and
variable (V) gene segments
Immunoglobulin subclass. Subfamily within a distinct immunoglobulin
class, based on subtle differences in heavy chain. For instance, in
humans there are two IgA subclasses, IgA1 (alpha1 heavy chain) and
IgA2 (alpha 2 heavy chain), and four IgG subclasses, IgG1-IgG4
(gamma 1-gamma 4 heavy chain). In rodents, these are designated IgG1
(gamma 1), IgG2a (gamma 2a), IgG2b (gamma 2b), and IgG3 (gamma 3)
Immunological memory. Acquired state of the immune system after
first contact with antigen, whereby the reaction upon subsequent
contact is faster, more intense, and of higher affinity. For antibody
response, associated with an immunoglobulin class switch and 'affinity
maturation' (by somatic mutation)
Immunosuppression. Prevention or diminution of the immune response
by administration of antineoplastic or antimetabolic drugs,
antilymphocyte serum, or exposure to e.g. environmental chemicals or
microorganisms (viruses)
Immunotoxicant. Drug, chemical, or other agent that is toxic to
cells or other components of the immune system
Inducer (helper) T cell. See Helper (inducer) T cell
Inflammation. Process whereby blood proteins or leukocytes enter
tissue in response to or in association with infection or tissue
injury
Inflammatory cell. General description of cells in an inflammatory
infiltrate; in acute inflammation, mainly polymorphonuclear
leukocytes; in chronic inflammation, mainly lymphocytes and
macrophages
Innate immunity. State of protection against pathogen-induced
injury; does not require previous immunization or vaccination
Innocent bystander. Cell or tissue component that is destroyed by an
immunological reaction specifically directed against a unrelated
antigen
Integrin. Family of heterodimeric molecules sharing a ß chain (ß1,
ß2, ß3, about 750 amino acids long), each with a different alpha chain
(about 1100 amino acids long), with a major function in cell adhesion
and migration. Form a protein family rather than a superfamily on the
basis of strong structural and functional similarities. Examples:
leukocyte function-related antigen LFA-1 (alpha L/ß1, CD11a/CD18;
receptor for intercellular adhesion molecules ICAM-1, ICAM-2, and
ICAM-3); Mac-1 (alpha M/ß2, CD11b/CD18; complement C3 receptor, CR3);
p150,95 (alpha X/ß2, CD11c/CD18); very late antigens VLA-1
(alpha 1/ß1, CD49a/CD29; laminin, collagen receptor), VLA-2
(alpha 2/ß1, CD49b/CD29; laminin, collagen receptor), VLA-3
(alpha 3/ß1, CD49c/CD29; laminin, collagen, fibronectin receptor),
VLA-4/LPAM-1 (alpha 4/ß1, CD49d/CD29; receptor for fibronectin and
VCAM-1), VLA-5 (alpha 5/ß1, CD49e/CD29, fibronectin receptor), and
VLA-6 (alpha 6/ß1, CD49f/CD29; laminin receptor, and alpha V/ß1,
CD51/CD29; vitronectin receptor); LPAM-2 (alpha 4/ßp, CD49d/.., or
alpha 4/ß7)
Interdigitating dendritic cell. Leukocyte belonging to the monocyte-
macrophage cell lineage, present in T-cell areas in lymphoid organs;
has a major function in presentation of antigen (MHC class
II-restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures called Birbeck granules. Its
equivalent in epidermis is the Langerhans cell, and that in lymph, the
veiled macrophage.
Interferon. Low-relative-molecular-mass substance produced mainly
during viral infection by leukocytes (IFN alpha), fibroblasts (IFNß),
and lymphocytes (IFN gamma); has a major function in interfering with
viral replication
Interferon-alpha. Produced by leukocytes; stimulates B cells to
proliferate and differentiate; stimulates natural killer cells and
increases cytotoxic T cell generation, but blocks T-cell proliferation
and lymphokine-activated killer activity; stimulates macrophage
accessory activity and enhances Fc receptor expression and MHC class I
and II expression on various cell types; induces antiviral state in
cells and is cytostatic for tumour cells, inhibits fibroblast and
adipocyte differentiation, and enhances promyelocytic and monoblastic
cell differentiation
Interferon-ß. Produced by fibroblasts and epithelia; activity
similar to that of IFN alpha
Interferon-gamma. Produced by T cells; induces antiviral state and
is cytostatic for tumour cells; enhances MHC class I and II expression
on various cell types, is antagonistic to interleukin-4 in IgE/IgG1
synthesis, and stimulates IgG2a synthesis; activates macrophages to
become cytolytic and enhances natural killer and lymphokine-activated
killer activity.
Interfollicular area. Area between follicles in lymphoid tissue,
where mainly small T lymphocytes are lodged; recognized by presence of
high endothelial venules. In lymph nodes, located in the outer cortex
and continuous with the paracortex
Interleukin. Immunoregulatory protein, also known as lymphokine,
monokine, interferon, or cytokine. Generally, low relative molecular
mass (< 80 kDa) and frequent glycosylation; regulates immune cell
function and inflammation by binding to specific cell surface
receptors; transient and local production; acts in paracrine or
autocrine manner, with stimulatory or blocking effect on growth and
differentiation; very potent, functions at picomolar concentrations.
Represents an extensive series of mediators (interleukins 1-12), with
a wide range of overlapping functions. Other mediators in this series
are c-kit ligand, interferon, tumour necrosis factor, and transforming
growth factor
Interleukin 1. Comprises two forms, IL-1 alpha and IL-1ß; produced
mainly by cells of the mononuclear phagocytic system (macrophages),
astrocytes, endothelium, and some epithelia, following stimulation by
e.g. microorganisms, immune complexes, or particulate compounds.
IL-1 alpha is mainly cell-associated; IL-1ß is released. IL-1 has
(together with IL-6 and tumour necrosis factor) multiple effects in
the systemic acute-phase response and in local acute and chronic
inflammation: it stimulates T (helper) cells to synthesize IL-2 and
IL-2 receptors, interferon gamma, and other lymphokines, B cells
(proliferation and differentiation), neutrophils, and natural killer
cells; stimulates monocytes and macrophages to produce IL-1, IL-6, and
tumour necrosis factor; acts in the acute-phase response by inducing
synthesis of acute-phase proteins in liver and reducing cytochrome
P450 synthesis; induces natriuresis in kidney, insulin production in
pancreas ß cells, muscular proteolysis ('easy' energy generation) in
muscle cells, slow-wave sleep in cerebral cortex; raises the
temperature set-point (fever) in hypothalamus; stimulates
haematopoiesis and prostaglandin synthesis by various cell types
(fibroblasts, macrophages, endothelium); inhibits gastric motility
in vitro ; induces collagenase production by synovial cells and
osteoclasts, and antiviral state; inhibits gastric smooth muscle
in vitro ; is cytostatic for tumour cells and activates endothelium
Interleukin 2. Synthesized by T helper cells after activation;
stimulates (autocrine) T cells to divide and release mediators,
B cells to proliferate and differentiate; activates monocytes and
natural killer cells; stimulates lymphokine-activated killer cells;
promotes generation of T helper 1 cells.
Interleukin 3. Formerly called multi-colony-stimulating factor;
synthesized by T helper cells; promotes growth of pluripotent
haematopoietic progenitor cells to granulocytes (eosinophilic,
basophilic, neutrophilic), mast cells, macrophages, megakaryocytes,
and, together with erythropoietin, to normoblasts and erythrocytes;
activates eosinophils and mast cells; stimulates haematopoiesis and
B-cell differentiation; blocks lymphokine-activated killer cells
Interleukin 4. Formerly called B-cell growth factor or B-cell
stimulating factor; synthesized by T helper and B cells; stimulates
IgE and IgG1 production by B cells and enhances MHC class II and IgE
receptor expression on B cells; acts in synergism with IL-2 in killer
cell generation, is mitogenic for T cells, and activates macrophages.
It is the dominant interleukin in generating T helper 2 cells, with a
negative feedback on the generation of T helper 1 cells.
Interleukin 5. Formerly called T-cell replacing factor or B-cell
growth and differentiation factor II; synthesized by T helper cells;
activates B cells and eosinophils, and stimulates IgA production by
B cells
Interleukin 6. Formerly called interferon ß2; synthesized by T
cells, monocytes, endothelial cells, fibroblasts, and smooth muscle
cells, among others, during inflammatory reactions; stimulates T and B
cells to proliferate and differentiate; has properties similar to IL-1
and acts synergistically with it in the acute-phase response (fever,
synthesis of acute-phase proteins); synergizes with IL-3 in promoting
haematopoietic progenitor cell proliferation; inhibits production of
IL-1 and tumour necrosis factor by monocytes
Interleukin 7. Formerly called lymphopoietin; synthesized by bone-
marrow stroma; induces growth of immature T and B lymphocytes
Interleukin 8. Formerly called neutrophil-activating protein;
synthesized by monocytes and various tissue cells in response to
inflammatory stimuli; performs chemotaxis of neutrophilic granulocytes
and subsequent granule exocytosis and respiratory burst; induces
increased expression of adhesion molecules CD11b/CD18 (complement C3
receptor CR3) and promotes vascular leakage. Endothelium-derived IL-8
inhibits adhesion of neutrophilic granulocytes induced by IL-1
Interleukin 9. Synthesized by T lymphocytes; stimulates growth of
erythroid and megakaryocyte precursors and promotes (mucosal) mast-
cell growth; acts synergistically with IL-4 in modulating IgE and IgG
production
Interleukin 10. Synthesized by T and B lymphocytes; inhibits
mediator synthesis (IL-2, IL-3, tumour necrosis factor, interferon
gamma, granulocyte-macrophage colony-stimulating factor) by T helper
1 cells, inhibits mediator synthesis (IL-1 alpha, IL-1ß, IL-6, IL-8,
and tumour necrosis factor alpha) by monocytes; stimulates IL-2-
dependent growth and cytotoxicity of cytotoxic T cells and stimulates
mast cell growth together with IL-2 or IL-3 and IL-4; induces MHC
class II antigen expression on B cells, but down-regulates MHC class
II on monocytes; promotes generation of T helper 2 cells
Interleukin 11. Synthesized by fibroblasts and bone-marrow stromal
cells; resembles IL-6 in function: stimulates haematopoietic cell
growth and differentiation (myeloid, erythroid, megakaryocyte
lineage); enhances T-cell-dependent antibody response; and suppresses
adipocyte differentiation and lipoprotein lipase production
Interleukin 12. Also called natural killer cell stimulatory factor;
synthesized by monocytes-macrophages, B cells, and accessory cells, in
response to bacteria or parasites; stimulates T-lymphocyte
proliferation, activates natural killer cells, and stimulates
lymphokine-activated killer activity; synergizes with IL-2 in
activation of cytotoxic lymphocytes; induces production of interferon
gamma and other cytokines by lymphocytes. It is the dominant
interleukin in generating T helper 1 cells and has a negative feedback
on the generation of T helper 2 cells.
in vitro. In the context of this book, exposure of cells or cell
systems to the immunotoxic agent in vitro. If the donors of cells or
cell systems are exposed but these are analysed in vitro, the term
ex vivo/in vitro is used
Isohaemagglutinin. Antibodies mainly of the IgM class that react
with (carbohydrate) antigens on erythrocytes from individuals of the
same species, resulting in agglutination in vitro
Isologous. Synonym for isogeneic
Isotype. Antigenic determinant that defines class or subclass of
immunoglobulin molecules
J (joining) chain. A 15-kDa polypeptide chain that acts
intracellularly to combine (identical) IgA or IgM immunoglobulin
units, consisting of two heavy and two light chains, to form a dimeric
IgA or a pentameric IgM molecule
J (joining) genes. Genes that encode the variable part of
immunoglobulin or T-cell receptor chains (e.g. JH1-JHn for
immunoglobulin heavy chains, Jkappa 1-Jkappa n for immunoglobulin
kappa light chain, Jalpha 1-Jalpha n for T-cell receptor alpha
chain)
Kallikrein (kininogenase). Arises in tissue fluids after cleavage of
prekallikrein; acts on kininogen to produce kinins, resulting in
immediate hypersensitivity reaction, e.g. vasodilatation and oedema.
It is a preformed mediator present in mast cell granules
kappa Chain. In immunoglobulin molecules, the kappa light chain
forms the light chain type of the molecule
Keratinocyte. Epithelial cell in the epidermis; in some
circumstances, can manifest antigen presentation and produce
immunoregulatory cytokines; hence belongs to the skin immune system
Killer cell (K cell). See Cytotoxic cell
Kinin system. Humoral amplification system involved in inflammation,
whereby substrate proteins become active after enzymatic cleavage;
cause vasodilatation, increased vascular permeability, hypotension,
and contraction of smooth muscle
c-Kit ligand. Also called stem cell growth factor or mast cell
growth factor; synthesized by various stromal cells, fibroblasts, and
liver cells; stimulates growth of early pluripotent progenitor cells
and that of myeloid, erythroid, and lymphoid progenitors in synergy
with interleukin-1, -3, -6, -7, and granulocyte-macrophage colony-
stimulating factor; promotes growth of mast cells
Kupffer cells. Macrophages on or between endothelial cells lining
the sinusoids of the liver
lamba Chain. In immunoglobulin molecules, the lamba light chain
forms the light chain type of the molecule
Lamina propria. Thin layer of connective tissue under the villous
epithelium of the gastrointestinal tract; site of plasma cells,
producing mainly dimeric IgA, including J chain
Langerhans cell. Leukocyte belonging to the monocyte-macrophage cell
lineage, present in skin epidermis; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II
restricted) to T helper lymphocytes. Cytoplasm contains characteristic
rod-like structures, Birbeck granules. Its equivalent in lymphoid
tissue is the interdigitating dendritic cell, and that in lymph,
veiled macrophage; forms part of the skin immune system
Large granular lymphocyte. Intermediate-sized, 10-12-µm lymphocyte
with a kidney-shaped nucleus and prominent, large, azurophilic
granules in the cytoplasm; occurs in the circulation and in tissue and
has a major function as a natural killer cell; forms a heterogeneous
population with either T markers or monocyte-macrophage markers.
Lectin. Plant-derived substance that binds to lymphocytes and can
induce cell proliferation; some also bind to other haematopoietic
cells
Leukaemia. Neoplasia of lymphoid cells in blood or bone marrow
Leukocyte. Bone marrow-derived white blood cell, including cells in
the lymphoid, myeloid, and monocyte lineages; sometimes used to
describe only granulocytes
Leukocytosis. Increased proportion of leukocytes in blood
Leukopenia, leukocytopenia. Reduced proportion of leukocytes in
blood
Leukotriene. Formerly called slow-reacting substance of anaphylaxis;
products of arachidonic acid metabolism following the lipoxygenase
pathway, which act as mediators in the immediate hypersensitivity
reaction, mainly as chemoattractants for granulocytes and monocytes,
and in smooth muscle contraction; newly synthesized by mast cells upon
activation
L (light) chain. One of the 22-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and a
constant domain. The light chain, either kappa or lamba, determines
the light chain type of the immunoglobulin molecule
Ly antigen. T Lymphocyte antigen in mice
Lymph. Fluid in lymphatic vessels
Lymph node. Secondary (peripheral) lymphoid organ, the main function
of which is to filter lymphatic vessels. Present throughout the body,
at connecting places of lymphatics and blood vessels; forms a major
site of encounter between pathogenic substances in the lymph and
lymphocytes entering from blood vessels, and subsequent initiation of
antigen-specific immunological reactions
Lymphatic. Vessel that collects fluid from interstitial spaces and
goes via lymph nodes (filtering) to the thoracic duct and blood
Lymphocyte. Cell belonging to the lymphoid lineage of bone marrow-
derived haematopoietic cells. In a restricted way, the designation of
a small, resting or recirculating mononuclear cell in blood or
lymphoid tissue, which measures about 7-8 µm, has a round nucleus
containing densely aggregated chromatin, and little cytoplasm. Plays a
key role in immune reactions by specific recognition of antigens
Lymphocytosis. Increased proportions of lymphocytes in blood
Lymphoid organ. Tissue in the body where cells of the immune system,
mainly lymphocytes, are lodged in an organized microenvironment,
either in a resting state or in a state of activation,
differentiation, or proliferation. Includes bone marrow, thymus, lymph
nodes, spleen, and mucosa-associated lymphoid tissue
Lymphokine. Hormonal substance synthesized by lymphocytes, which
modulates the function of cells in immunological reactions
Lymphoma. Neoplasia of lymphoid cells in tissue
Lymphopenia, lymphocytopenia. Reduced proportions of lymphocytes in
blood
Lymphotoxin. Former name for tumour necrosis factor ß; lymphokine
synthesized by T lymphocytes, which kills selected target cells
Lysosome. Granule present in many cell types that contain hydrolytic
enzymes; also performs intracellular degradation of pathogens after
phagocytosis
Lysozyme (muramidase). A low-relative-molecular-mass, cationic
enzyme present in tissue fluids and secretions, which degrades
mucopeptides of bacterial cell walls
Macroglobulin. Glycoprotein of relative molecular mass > 200 kDa
Macrophage. Large, 12-20-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in tissue, and forming the
mononuclear phagocytic system. Its reniform nucleus usually has
pronounced peripheral condensation of nuclear chromatin; its cytoplasm
contains a great variety of cell organelles, including rough
endoplasmic reticulum, mitochondria, ribosomes, lysosomes, and Golgi
complex. Has a major function in (chronic) inflammatory reactions, by
virtue of its phagocytic capacity, with immunoglobulin Fc and
complement C3 receptors which bind to immune complexes. Macrophages
develop into killer cells after activation by e.g. T-cell factors and
can mediate antibody-dependent cell-mediated cytotoxicity; also
functions as an accessory cell in induction of immune responses
(antigen presentation, mediator secretion). Macrophages in blood are
called monocytes. Subtypes with special functions are interdigitating
dendritic cells: T-cell area of lymphoid tissue, Langerhans cell
(skin), Kupffer cells (liver), metallophilic macrophages (spleen),
microglia (brain), osteoclasts (bone), tingible body macrophage
(starry-sky macrophage), veiled macrophage (lymph).
Macrophage colony-stimulating factor. Synthesized mainly by
endothelial cells and fibroblasts, and possibly macrophages; supports
growth of monocyte-macrophage progenitors.
Major histocompatibility complex (MHC). Set of genes that codes for
tissue compatibility markers, which are targets in allograft rejection
and hence determine the fate of allografts; plays a central role in
control of cellular interactions during immunological reactions.
Tissue compatibility is coded by classes I and II loci (see Class I
and Class II MHC molecule). Genes within or closely linked to MHC
control certain complement components (MHC class III genes). The MHC
complex of humans is HLA, that of mice H-2, and that of rats, RT-1.
Mantle (corona). Zone in secondary follicles surrounding the central
germinal centre, densely packed with small resting B lymphocytes
Marginal zone. Outer layer of white pulp in spleen, surrounding
follicles, and periarteriolar lymphocyte sheath; separated from these
by the marginal sinus; populated by intermediate-sized, slightly
pyroninophilic B cells which have a major function in the T cell-
independent antibody response. The microenvironment manifests a
special type of macrophage, the marginal metallophilic macrophage
Margination. Adherence of blood leukocytes to endothelium during
inflammatory reactions
Mast cell. A bone marrow-derived polymorphonuclear leukocyte present
in tissue; has a major function in immediate hypersensitivity
reactions; has a round or oval nucleus and abundant cytoplasm with
basophilic (blue) granules stained by metachromatic dyes; granules
contain mediators of immediate hypersensitivity reactions, e.g.
heparin, histamine, serotonin, tryptase, kallikrein, and
chemoattractants for neutrophilic and eosinophilic granulocytes; has a
high-affinity receptor for IgE. After activation (physical stimuli,
cross-linking via allergen-IgE-IgE receptor), there is immediate
granule release and synthesis of other mediators, including
prostaglandins, thromboxanes, leukotrienes, and platelet-activating
factor. The cell can exert modulatory activity by secreting cytokines
such as IL-3, IL-6, and tumour necrosis factor. Two subtypes exist, in
the mucosa and in connective tissue; the equivalent in the circulation
is the basophilic granulocyte
Mast cell growth factor. See Interleukin 9
Medulla. Inner parenchymal layer of organs
Medullary cord. Parenchyma in medulla of lymph nodes separating
lymphatic sinusoids
Megakaryocyte. Large, multinucleated giant cell, precursor of blood
platelets, formed by separation of portions of membrane-bound
cytoplasm; occurs in haematopoietic tissue, including bone marrow
Memory. See Immunological memory
Mesoderm. Middle of the three cellular layers of the embryo;
produces connective tissue and blood cells
Metallophilic macrophage. Subtype of macrophage identified by silver
impregnation, present at the inner border of the marginal zone in
spleen
MHC restriction. Immunological reactions can occur only in
associated recognition with the polymorphic determinant of a given MHC
molecule and not with that of another MHC molecule. Applies to T
lymphocytes with an alpha-ß T-cell receptor, which recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules, and part of the T cell population with the gamma-delta
T-cell receptor.
Microglia. Macrophages in central nervous system
Migration inhibitory factor. A lymphokine that inhibits the movement
of macrophages
Milky spot. Aggregate of lymphoid cells in omentum, macroscopically
visible as a small white spot; not organized tissue but rather the
product of immune stimulation in that area of the body
Minor histocompatibility antigen. Ill-defined histocompatibility
marker not encoded by the MHC, which is a target in allograft
reactions (apart from products of the MHC)
Mitogen. Substance that activates resting cells to transform and
proliferate
Monoclonal. Derived from a single clone. For T and B lymphocytes, a
cell population in which all cells have a distinct V-D-J gene
rearrangement product (as seen in lymphoma and leukaemia). Monoclonal
antibodies are products of hybridomas prepared after fusion of
antibody-producing cells and a transformed (non-producing)
plasmacytoid cell line.
Monocyte. Large, 10-15-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in the blood and in lymphatic
vessels
Monokine. Hormonal substance synthesized by monocytes-macrophages;
modulates the function of cells in immunological reactions
Mononuclear cell. Leukocyte with a single rounded nucleus, e.g.
lymphocytes and monocytes-macrophages
Mononuclear phagocytic system. Formerly called reticuloendothelial
system; composite of phagocytic cells in the body, including monocytes
and tissue macrophages. Main populations are Kupffer cells in liver,
microglia in the central nervous system, macrophages in red pulp of
spleen, alveolar macrophages in lung, and, after induction, peritoneal
macrophages in the peritoneal cavity; others are mesangial macrophages
in kidney and osteoclasts in bone
µ Chain. In immunoglobulin molecules, the µ heavy chain forming the
IgM immunoglobulin class
Mucosa-associated lymphoid tissue. Lymphoid tissue or organs in
immediate contact with the mucous-secreting mucosal layer in nasal
cavity and nasopharynx (nasal-associated lymphoid tissue), airways
(bronchus-associated lymphoid tissue), and intestinal tract (gut-
associated lymphoid tissue). Serves as the immunological defence at
secretory surfaces, to some extent independent of the systemic
(internal) response; includes IgA synthesis by plasma cells in the
lamina propria and excretion into the lumen
Multiple myeloma. Tumour of plasma cells in bone marrow
Muramidase. See Lysozyme
Myeloblast. Immature precursor cell in the lineage of polymorpho-
nuclear cells (granulocytes, mast cells)
Nasal-associated lymphoid tissue. Lymphoid organs or tissue located
in the nasal cavity and nasopharynx, presumed to be a first location
for presentation of antigens entering through the nose; contributes to
the mucosa-associated lymphoid tissue
Natural antibody. Antibody in serum of individuals with no previous
exposure to the corresponding antigen; often generated by contact with
cross-reacting agents, e.g. bacterial products; often restricted to
antibodies that react to xenogeneic antigens
Natural killer cell. Leukocyte with a limited repertoire to
recognize antigen; can kill target cells without prior sensitization;
can be of lymphoid or monocyte-macrophage origin. Large granular
lymphocytes are the main population
Necrosis. Death of tissue and cells
Neoantigen. New antigen appearing on cells or tissue during
malignant transformation or (viral) infection
Neoplasia. Uncontrolled malignant transformation of cells resulting
in tumour formation
Neutralization. Process whereby a pathogenic substance becomes
inactivated by effector components (antibodies) of the immunological
reaction
Neutropenia. Reduced proportions of neutrophilic granulocytes in
blood
Neutrophil chemotactic factor. Preformed mediator with a relative
molecular mass > 750 kDa, present in granules of mast cells and
basophilic granulocytes; released after activation and attracts
neutrophilic granulocytes to the site of inflammation or
hypersensitivity
Neutrophilic granulocyte. Polymorphonuclear leukocyte that contains
granules stained by neither acidophilic nor basophilic dyes; can
phagocytose immune complexes by receptor-mediated endocytosis,
followed by intracellular degradation in lysosomes. Degranulation
releases catepsins and lysosomal enzymes, resulting in tissue damage.
Nonspecific immunity. Immunity induced by non-immunological
mechanisms, for instance by action of complement, lysozyme,
phagocytosis, or interferon
Oedema. Swelling of tissue due to extravasation of fluid from the
intravascular space following increase in vascular permeability
Ontogeny. Life cycle of an organism; in immunological terms, often
used to describe the process whereby the immune system develops
immunocompetence
Opsonization. Adherence of pathogen to phagocytic cell due to action
of antibody or activated complement
Osteoclast. Macrophage in bony tissue involved in bone resorption
Paracortex. Area in the inner cortex of lymph node where T
lymphocytes are lodged; recognized by the presence of high endothelial
venules; continuous with interfollicular areas in the outer cortex on
one side and with the medullary cords on the other
Paratope (antigen-binding site). Part of antibody that binds antigen
(antigenic determinant, or epitope); part of T-cell receptor that
binds the complex of antigen and MHC molecule
Periarteriolar lymphocyte sheath. Area in white pulp of spleen
surrounding the central artery, populated mainly by small
T lymphocytes
Peripheral (secondary) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of these
reactions
Peyer's patch. Lymphoid tissue in wall of small intestine,
particularly ileum, separated from the gut lumen by a domed area and
an epithelial layer ('dome' epithelium); forms part of gut-associated
lymphoid tissue; main function is initiation of immunological
reactions towards pathogens entering through dome epithelium
Phagocytosis. Uptake of material >1 µm by cells, by receptor-
mediated endocytosis, by cells of the mononuclear phagocytic system;
requires Fc receptors, with accessory help of complement receptors; is
blocked by cytochalasins. Occurs via a 'zipper' mechanism, in which
the opsonized particle (coated with antibody or complement) becomes
enclosed by the cell membrane of the phagocyte; a second mechanism
involves oxidative burst, with formation of superoxide anion, peroxide
anion, and hydroxyl radicals, which kill or degrade the phagocytosed
particle
Phagolysosome. Membrane-bound cytoplasmic vesicle formed by fusion
of a phagosome and a lysosome
Phagosome. Membrane-bound vesicle in phagocytic cells containing
phagocytosed material
Pharmacokinetics. Fate of drugs or chemicals in the body over time,
including the processes of absorption and distribution in tissues,
biotransformation, and excretion
Phenotype. Characteristic of a distinct cell or individual,
reflecting the expression of a genetically determined property
Phenotypic marker. Expressed characteristic(s), for instance an
antigenic determinant, of a given cell or molecule, associated with
function or specificity
Phylogeny. Evolutionary history of a particular species
Pinocytosis. Uptake of material < 1 µm by cells; often restricted
to a receptor-mediated process in leukocytes of the monocyte-
macrophage series, e.g. uptake of lipoproteins and viruses into
clathrin-coated vesicles
Plasma. Fluid of uncoagulated blood after removal of cells
Plasma cell. Terminally differentiated B lymphocyte that synthesizes
and secretes immunoglobulin; these medium-sized, 10-15-µm cells have a
small excentric nucleus, with heterochromatin organized in a
'cartwheel'-like structure, and abundant cytoplasm filled with rough
endoplasmic reticulum.
Platelet. Small bone marrow-derived cytoplasmic fragment in blood
responsible for coagulation; main role is to block damaged vessel
walls and prevent haemorrhage, by clumping and aggregation; contains
heparin and serotonin, which contribute after release to the acute
vascular response in hypersensitivity reactions and produce oxygen
radicals
Platelet-activating factor. Low-relative-molecular-mass phospholipid
generated from alkyl phospholipids in mast cells, basophilic and
neutrophilic granulocytes, and monocytes-macrophages, which mediates
microthrombus formation of platelets in hypersensitivity reactions
Polyclonal. Derived from many different clones; for T and B
lymphocytes, cell populations in which the cells have different V-D-J
gene rearrangement products. Polyclonal activation is stimulation of
multiple lymphocyte clones, resulting in a heterogeneous response
Polymorphonuclear granulocyte. Leukocyte of bone-marrow origin, with
a lobulated nucleus, involved in acute inflammatory reactions. Main
subsets are basophilic, eosinophilic, and neutrophilic granulocytes
(different cytoplasmic granule colours after haematological staining).
Contributes to (acute) inflammatory reactions after attraction by
specific (immune complex-mediated) or nonspecific stimuli (including
complement components); after activation, releases granules containing
various hydrolytic enzymes
Postcapillary venule. Small blood vessel though which blood flows
after leaving the capillaries before reaching veins; often the site
where inflammatory cells leave the circulation and enter the tissue
Primary (central) lymphoid organ. Lymphoid organ where precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment to form immunocompetent cells; not antigen-driven but
can be influenced by mediators produced as a result of antigen
stimulation
Primary follicle. See Follicle
Primary response. Immunological reaction after first contact with
antigen, resulting in generation of immunological memory
Programmed cell death. See Apoptosis
Prostaglandin. Aliphatic acid produced by arachidonic acid
metabolism following the cyclo-oxygenase pathway; synthesized by mast
cells after activation; mediates immediate hypersensitivity reactions,
mainly smooth muscle contraction or bronchoconstriction; also
decreases the threshold for pain
Prothymocyte. Precursor of T lymphocytes in bone marrow before
moving to the thymus, or present in the thymus just before intrathymic
processing
Pyrogen. Substance that increases the temperature in the central
nervous system, resulting in fever; examples are bacterial endotoxin
and IL-1
Reactive oxygen intermediate. Reactive species of oxygen produced
e.g. by phagocytes (granulocytes and monocyte-macrophages) in response
to phagocytic stimuli like bacteria
Reagin. Former designation of IgE class antibody
Recall antigen. Antigen used to elicit a response from an individual
already sensitized to that antigen; may be one that the host has
knowingly been sensitized to or, in humans, one that it is assumed
that most individuals have been sensitized to
Red pulp. Area in spleen comprising venous sinuses filled with blood
and splenic cords; venous sinuses mainly contain erythrocytes
surrounded by endothelial cells; cords comprise macrophages,
lymphocytes, and occasionally megakaryocytes, but other types of blood
cells can also be present. Main function is phagocytosis of
particulate material and removal of old erythrocytes from blood. In
rodents, the red pulp can also be a site of haematopoiesis.
Reticuloendothelial system. See Mononuclear phagocytic system
Repertoire. All specific antigen-recognizing capacities (diversity)
within a population of T or B lymphocytes
RT-1. The major histocompatibility complex of rats
Secretory immunoglobulin. Immunoglobulin encountered in secretions
like tears, saliva, and jejunal juice; concerns mainly secretory IgA,
a dimer of the basic four-chain immunoglobulin structure, linked by a
J chain and surrounded by a secretory piece molecule.
Secretory piece. A 70-kDa molecule produced by epithelial cells
covering mucosa-associated lymphoid tissue; functions as a receptor
for IgA or IgM, thereby facilitating intercellular transport of these
molecules into the lumen. During this process, the secretory piece
becomes associated with the immunoglobulin, thereby enhancing its
stability in nonphysiological conditions of secretory fluid
Secondary follicle. See Follicle
Secondary (peripheral) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of those
reactions
Secondary response. Response after first contact (immunization,
primary response) with an antigen, based on the presence of
immunological memory; characterized as faster, more intense, and of
higher affinity; for the antibody response, associated with an
immunoglobulin class switch
Selectin. Cell surface glycoprotein that has a prominent function in
the interaction between lymphocytes, monocytes, neutrophilic
granulocytes, and endothelium. They share an N-terminal domain of
approximately 120 amino acids that is homologous to many Ca2+-
dependent animal lectins and binds to carbohydrates. Examples are
L-selectin (MEL-14, LAM-1, present on leukocytes; adherence of
endothelial cells, role in lymphocyte recirculation and neutrophil and
leukocyte inflammation); E-selectin (ELAM-1, present on endothelium;
adherence of monocytes, neutrophils, and T cells; role in
inflammation); P-selectin (PADGEM, GMP-140, CD62, present on platelets
and endothelium; adherence of monocytes, neutrophils, and T cells;
role in inflammation).
Self-MHC restriction. See MHC restriction; applies to MHC
molecules of the individual
Sensitization. Induction of specialized immunological memory in an
individual by exposure to antigen
Serotonin. 5-Hydroxytryptamine; catecholamine with relative molecular
mass of 176 Da; preformed mediator of immediate hypersensitivity
reactions, present in granules of mast cells and in platelets. After
activation, is released and mediates vasodilatation and increased
vascular permeability
Serum. Fluid of blood after coagulation (removal of fibrinogen) and
removal of cells
Serum sickness. Systemic vasculitis, glomerulonephritis, or arthritis
due to immune complex formation after the reaction between antibody and
injected foreign antigen (serum)
Skin-associated lymphoid tissue. See Skin immune system
Skin immune system. Combination of immune system components and
their function, present in skin; antigen presentation by Langerhans
cells, by dendritic epidermal cells, and in some conditions by
keratinocytes; immunoregulation by e.g. keratinocyte-derived
cytokines, and distinct dermatotropic T-cell populations
Slow-reacting substance of anaphylaxis. See Leukotriene
Somatic mutation. Small changes in genes resulting in alterations in
amino acids built into protein chains. For immunoglobulin molecules,
changes in diversity of antigen-binding site (variable region)
Spleen. Lymphoid organ in the left abdominal cavity, for filtering
blood; main function is phagocytosis of particles from blood, removal
of old erythrocytes in red pulp, and initiation of immunological
reactions in white pulp. The marginal zone of the white pulp serves as
the main site of T cell-independent antibody formation.
Starry-sky macrophage. See Tingible body macrophage
Stem cell. Multipotential, self-renewing precursor cell of all
haematopoietic cell lineages, present in bone marrow
Stem-cell growth factor (synonym for c-Kit ligand). An interleukin
that supports continuous growth of mast cells and augments the
response of progenitor cells to stem growth factors; interacts via
c-kit proto-oncogene
Subcapsular sinus. Area in lymph node just under the capsule and
surrounding the cortex, which is connected with afferent lymphatics,
and through cortical (peritrabecular) sinuses with medullary sinuses;
contains dendritic macrophages
Superantigen. Antigenic moiety that, in MHC-restricted presentation
to T lymphocytes, is not present in the 'groove' made by the
quaternary structure of the MHC molecule but is complexed with the MHC
molecule. Examples are the endogenous viral-encoded Mlsa (minor
lymphocyte stimulatory) antigen, which is present in certain mouse
strains, and Staphylococcus enterotoxin A
Suppressor T cell. Subpopulation of T lymphocytes with CD8 phenotype;
after recognition of antigen in an MHC class I-restricted manner,
suppresses immunological reactions, in part by cytotoxic activity
Systemic lupus erythematosus. Chronic, remitting, relapsing,
inflammatory, and often febrile multisystemic disorder of connective
tissues, with possible involvement of the central nervous system,
skin, joints, kidneys, and serosal membranes; can be acute or
insidious in onset. The etiology is unknown but is thought to follow a
failure of the regulatory mechanisms of the immune system that sustain
self-tolerance. Many drugs and chemicals can induce lupus-like
symptoms (drug-induced lupus erythematosus)
T-Cell receptor. Heterodimeric molecule on the surface of the T
lymphocyte that recognizes antigen. The polypeptide chains have a
variable and a constant part, and can be alpha, ß, gamma, or delta.
The alpha-ß T-cell receptor occurs on most T cells and recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules (self-MHC restricted). The gamma-delta T-cell receptor
occurs on a small subpopulation, e.g. in mucosal epithelium, and can
recognize antigen in a non-MHC restricted manner. The T-cell receptor
occurs exclusively with the CD3 molecule, which is thought to mediate
transmembrane signalling.
T-Dependent antigen. Antigen for which antibody formation requires
T cells.
Terminal pathway of complement activation. Activation of complement
components C6-C9, with formation of the membrane attack complex and
subsequent lysis of the cell
Tingible body macrophage (starry-sky macrophage). Large macrophage
in cortex of thymus and germinal centres of follicles in lymphoid
tissue, filled with condensed nuclear material with high affinity for
dyes; has a major function in phagocytosis, presumably of apoptotic
cells
T Lymphocyte or cell. Lymphocyte that induces, regulates, and
effects specific immunological reactions after stimulation by antigen,
mostly in the form of processed antigen complexed with MHC product on
an antigen-presenting cell. They originate from precursors in the bone
marrow and undergo maturation in the thymus (T, thymus-dependent).
Most T lymphocytes recognize antigen by a heterodimeric alpha-ß
surface receptor molecule associated with the CD3 molecule, which
mediates transmembrane signalling. Subsets include helper-inducer and
suppressor-cytotoxic cells.
T-Lymphocyte area. That part of a lymphoid organ or tissue that is
occupied by T lymphocytes, e.g. paracortex or interfollicular area in
lymph node, periarteriolar lymphocyte sheath in spleen
Thrombocyte. See Platelet
Thrombocytopenia. Reduced proportion of platelets in blood
Thromboxane. Product of arachidonic acid following the cyclo-
oxygenase pathway; synthesized by mast cells after activation and
mediates immediate hypersensitivity reactions, mainly smooth muscle
contraction, bronchoconstriction, and platelet aggregation
Thymocyte. Lymphocyte in the thymus
Thymoma. Tumour of the thymus; neoplastic cell is an epithelial cell
Thymus. Central lymphoid organ located dorsal to the cranial part of
the sternum in the thorax, comprising two lobes, each consisting of
many lobules. Its main function is generation of immunocompetent
T lymphocytes from prothymocytes from the bone marrow
Tolerance. State of unresponsiveness to antigenic stimulation, due
to the absence of responding elements or loss of capacity of existing
elements to mount a reaction. Synonym for anergy
Tolerogen. Antigen that evokes tolerance
Tonsil. Organized mucosa-associated lymphoid tissue in
oronasopharynx. Adenoids strictu sensu are also tonsils. The main
function is initiation of immunological reactions towards pathogens
entering through the mouth. Contributes in part to the gut-associated
lymphoid tissue. Together with lymphoid aggregates in oronasopharynx,
these tissues form the ring of Waldeyer
Transforming growth factor ß. Mediator synthesized by lymphocytes or
macrophages, with a function in down-regulation of immune reactions;
suppresses T- and B-lymphocyte growth, IgM and IgG production, and
down-regulates MHC class II expression; interferes with production of
tumour necrosis factor and adhesion of granulocytes to endothelial
cells; is chemotactic for monocytes and induces interleukin-1 and
interleukin-6 expression
Transudation. Transfer of fluid and low-relative-molecular-mass
proteins from intravascular to extravascular tissue during
inflammatory processes
Tryptase. Proteolytic enzyme present in granules of mast cells;
released after activation and activates complement component C3, with
formation of the anaphylatoxin C3a
Tumour necrosis factor. General mediator of inflammation and septic
shock; formerly named cachectin and lymphotoxin. Two forms: alpha and
ß, both produced by monocytes-macrophages, TNF-ß also by T lymphocytes
and natural killer cells. Has activity similar to interleukin-1 and
acts synergistically with it; promotes antiviral state and is
cytotoxic for tumour cells; stimulates granulocytes and eosinophils,
activates macrophages to interleukin-1 synthesis, stimulates B cells
to proliferate and differentiate, T cells to proliferate,interleukin-2
receptor synthesis, and interferon gamma synthesis; induces
fibroblasts to synthesize prostaglandin and proliferate; induces fever
and synthesis of acute-phase proteins; reduces cytochrome P450
synthesis; activates endothelium and promotes adherence of
neutrophilic granulocytes to endothelium; induces cell adhesion
molecules like lymphocyte function-associated antigens LFA-1 and
LFA-3, ICAM-1, and ELAM-1; inhibits gastric motility in vitro;
reduces lipoprotein lipase synthesis by adipocytes; and activates
osteoclasts to bone resorption
Urticaria. Transient eruption of skin characterized by erythematous
or oedematous swelling (wheal) of the dermis or subcutaneous tissue
Vaccination. See Immunization
Valency. Number of antigenic determinants or ligands that can bind
to one antibody molecule or receptor
V (variable) gene. Gene that encodes the variable part of
immunoglobulin or T-cell receptor chains (e.g. VH1-VHn for
immunoglobulin heavy chains, Vkappa 1-Vkappa n for immunoglobulin
kappa light chain, Valpha 1-Valpha n for T-cell receptor alpha
chain)
Variable gene family. Groups of germline V genes (which encode
immunoglobulin chains or T-cell receptor genes) that have more than
about 80% nucleotide sequence identity
V (variable) region. Region at the amino terminal of immunoglobulin
or T-cell receptor chains, which contributes to the antigen-binding
site of the molecule. Encoded by V (variable), D (diversity), and J
(joining) genes in DNA
Vasoconstriction. Contraction of capillary venules, resulting in
decreased blood flow
Vasodilatation. Dilatation of capillary venules, resulting in
increased blood flow through capillaries and lowering of local blood
pressure
Veiled macrophage. Leukocyte belonging to the monocyte-macrophage
lineage, present in lymph; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II-
restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures, Birbeck granules. Its equivalent
in lymphoid tissue is the interdigitating dendritic cell, and that in
skin is the Langerhans cell
Waldeyer's ring. Lymphoid tissue of tonsils and adenoids located
around the junction of the pharynx and oral cavity in humans and
domestic animals. Main function is initiation of immunological
reactions towards pathogens entering through the mouth. Contributes to
the gut-associated lymphoid tissue
White blood cell. Polymorphonuclear leukocyte, lymphocyte, or
monocyte in peripheral blood
White pulp. Area in spleen around central arterioles where lymphoid
cells reside. Comprises three major compartments: the periarteriolar
lymphocyte sheath, follicles, and marginal zone
Xenobiotic. Chemical or substance that is foreign to the biological
system
Xenogeneic. Genetically different phenotypes in individuals of
different species; opposite of allogeneic, or isogeneic
zeta Chain (see epsilon Chain). One of the chains in the CD3
molecule associated with the T-cell receptor
REFERENCES
Ada GL, Leung KN, & Ertl H (1981) An analysis of effector T-cell
generation and function in mice exposed to influenza A or Sendai
virus. Immunol Rev, 50: 5-24.
Ader, R, Felten D, & Cohen, N (1990) Interactions between the brain
and the immune system. Ann Rev Pharmacol Toxicol, 30: 561-602.
Adkins B, Richards JH, & Gardner DE (1979) Enhancement of experimental
respiratory infection following nickel inhalation. Environ Res,
20: 33-42.
Adkins B, Luginbuhl GH, Miller, FJ, & Gardner DE (1980) Increased
pulmonary susceptibility to Streptococcal infection following
inhalation of manganese oxide. Environ Res, 23: 110-120.
Adkins B, Gandour D, Strober S & Weissman I (1988) Total lymphoid
irradiation leads to transient depletion of the mouse thymic medulla
and persistent abnormalities among medullary stromal cells. J Immunol,
140: 3373-3379.
Adorini L ed. (1990) The molecular basis of antigen presentation to
T lymphocytes: Novel possibilities for immunointervention. Int Rev
Immunol, 6: 1-88.
Agius C (1985) The melanomacrophage centers of fish; a review.
In: Manning MJ & Tatner MF, eds, Fish Immunology. London, Academic
Press, pp 85-105.
Ahmed-Ansari A, Brodie AR, Fultz PN, Anderson DC, Sell KW, & McClure
HM (1989) Flow microfluorometric analysis of peripheral blood
mononuclear cells from nonhuman primates: Correlation of phenotype
with immune function. Am J Primatol, 17: 107-131.
Allen JR, & Abrahamson LJ (1973) Morphological and biochemical changes
in the liver of rats fed polychlorinated biphenyls. Arch Environ
Contam Toxicol, 1: 265-280.
Allen AL, Koller LD, & Pollock GA (1983) Effect of toxaphen exposure
on immune responses in mice. J Toxicol Environ Health, 11: 61-69.
Alomran, AH & Shleamoon, MN (1988) The influence of chronic lead
exposure on lymphocyte proliferative response and immunoglobulin
levels in storage battery workers. J Biol Sci Res, 19: 575-585.
Anderson DP (1990) Immunological indicators: Effects of environmental
stress on immune protection and disease outbreaks. Am Fish Soc Symp
8: 38-50.
Anderson RS & Brubacher LL (1993) Inhibition by pentachlorophenol of
production of reactive-oxygen intermediates by medaka phagocytic blood
cells. Marine Environ Res, 35: 125-129.
Anderson RE & Williams WL (1977) Radiosensitivity of T and B
lymphocytes. V. Effects of whole-body irradiation on numbers of
recirculating T cells and sensitization to primary skin grafts in
mice. Am J Pathol, 89: 367-378.
Anderson J, Moller G & Sjorberg O (1972) Selective induction of DNA
synthesis in T and B lymphocytes. Cell Immunol, 4: 381-393.
Anderson RE, Olson GB, Autry JR, Howarth JL, Troup GM ,& Bartels PH
(1977) Radiosensitivity of T and B lymphocytes. IV. Effect of whole
body irradiation upon various lymphoid tissues and numbers of
recirculating lymphocytes. J Immunol, 118: 1191-1200.
Anderson RE, Standefer JC, & Tokuda S (1986) The structural and
functional assessment of cytotoxic injury of the immune system with
particular reference to the effects of ionizing radiation and
cyclophosphamide. Br J Cancer, 53 (Suppl 7): 140-160.
André F, Gillon F, André C, Lafont S, & Jourdan G (1983) Pesticide-
containing diet augments anti-sheep red blood cell non reagenic
antibody responses in mice but may prolong murine infection with
Giardia muris. Environ Res 32: 145-150.
Anneroth G, Ericson T, Johannson I, Mornstad H, Ryberg M, Skoglund A,
& Stegmayr B (1992) Comprehensive medical examination of a group of
patients with alleged adverse effects from dental amalgam. Acta
Odontol Scand, 50: 101-107.
Applegate LA, Ley RD, Alcalay J, & Kripke ML (1989) Identification of
the molecular target for the suppression of contact hypersensitivity
by UV radiation. J Exp Med, 170: 1117-1131.
Appleton JA & McGregor DD (1984) Rapid expulsion of Trichinella
spiralisin suckling rats. Science, 226: 70-72.
Araneo BAT, Dowell T, Moon HB; & Daynes RA (1989) Regulation of murine
lymphokine production in vivo: Ultraviolet radiation exposure
depresses IL-2 and enhances IL-4 production by T cells through an IL-1
independent mechanism. J Immunol, 143: 1737-1745.
Aranyi C, Vana SC, Thomas PT, Bradof JN, Fenters JD, Graham JA, &
Miller FJ (1983) Effects of subchronic exposure to a mixture of O3,
SO2 and (NH4)SO4 on host defenses in mice. Environ Res,
12: 55-71.
Aranyi C, Bradof JN, O'Shea WJ, Graham JA, & Miller FJ (1985) Effects
of arsenic trioxide inhalation exposure on pulmonary antibacterial
defenses in mice. J Toxicol Environ Health, 15: 163-172.
Aranyi C, O'Shea WJ, Graham JA & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6: 713-720.
Archer DL, Smith BG, & Bukovic-Wess JA (1978) Use of an in vitro
antibody producing system for recognizing potentially
immunosuppressive compounds. Int Arch Allergy Appl Immunol, 56: 90-93.
Arkoosh MR & Kaattari SL (1990) Quantitation of fish antibody to a
specific antigen by an enzyme-linked immunosorbent assay (ELISA).
In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS & van Muiswinkel
WB ed. Techniques in Fish Immunology. Fair Haven, NJ, SOS
Publications, Vol 1, pp 15-24.
Askenase PW, Hayden B, & Gershon RK (1977) Evanescent delayed
hypersensitivity: Mediation by effector cells with a short life span.
J Immunol, 119: 1830-1835.
Austin JH, Schulman LL, & Mastrobattista JD (1989) Pulmonary infection
after cardiac transplantation: Clinical and radiologic correlations.
Radiology, 172: 259-265.
Austyn JM & Gordon S (1981) F4/80, a monoclonal antibody directed
specifically against the mouse macrophage. Eur J Immunol, 11: 805-815.
Baadsgaard O, Wulf HC, Wantzin GL, & Cooper KD (1987) UVB and UVC, but
not UVA, potently induce the appearance of T6-DR+ antigen-presenting
cells in human epidermis. J Invest Dermatol, 89: 113-118.
Baadsgaard O, Lisby S, Wulf HC, Wantzin GL, & Cooper KD (1989) Rapid
recovery of Langerhans cell alloreactivity, without induction of
autoreactivity, after in vivo ultraviolet A, but not ultraviolet
B exposure of human skin. J Immunol, 142: 4213-4218.
Baadsgaard O, Salvo B, Mannie A, Dass B, Fox DA, & Cooper KD (1990)
In vivo ultraviolet-exposed human epidermal cells activate T
suppressor cell pathways that involve CD4+CD45RA+ suppressor-inducer
T cells. J Immunol, 145: 2854-2861.
Baker D, O'Neill JK, Gschmeissner SE, Wilcox CE, Butter C, & Turk JL
(1990) Induction of chronic relapsing experimental allergic
encephalomyelitis in Biozzi mice. J Neuroimmunol, 28: 261-270.
Balls M, Blaauboer B, Brusick D, Frazier J, Lamb D, Pemberton M,
Reinhardt C, Roberfroid M, Rosenkranz H, Schmid B, Spielmann H,
Stammati A-L, & Walum E (1990) Report and recommendations of the
CAAT/ERGATT workshop on the validation of toxicity test procedures.
ATLA, 18: 313-337.
Bancroft GJ, Shellam GR, & Chalmer JE (1981) Genetic influences on the
augmentation of natural killer (NK) cells during murine
cytomegalovirus infection: Correlation with patterns of resistance. J
Immunol, 126: 988-994.
Bandeira A, Mota-Santos T, Otohara S, Degermann S, Heusser C, Tonegawa
S, & Coutinho A (1990) Localization of gamma/delta cells to the
intestinal epithelium is independent of normal microbial colonization.
J Exp Med, 172: 239-244. 306
Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, & Reynolds CW
(1985) Direct evidence for the role of LGL in the inhibition of
experimental tumor metastases. J Immunol, 134: 2783-2789.
Barnett JB & Rodgers KE (1994) Pesticides. In: Dean JH, Luster MI,
Munson AE, & Kimber I ed. Immunotoxicity and immunopharmacology, 2nd
Ed. New York, Raven Press, pp 191-212.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LS
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39: 263-274.
Barr BBB, Benton EC, McLaren K, Bunney MH, Smith IW, Blessing K,
& Hunter JAA (1989) Human papilloma virus infection and skin cancer in
renal allograft recipients. Lancet, i: 124-129.
Barrett WL, First MR, Aron BS, & Penn I (1993) Clinical course of
malignancies in renal transplant recipients. Cancer, 72: 2186-2189.
Barry TS, Jones DM, Richter CB, & Haynes BF (1991) Successful
engraftment of human postnatal thymus in severe combined immune
deficient (SCID) mice: Differential engraftment of thymic components
with irradiation versus anti-asialo GM-1 immunosuppressive regimens.
J Exp Med, 173: 167-180.
Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, & Penny R (1977)
Depressed lymphocyte function after bereavement. Lancet, i: 834-836.
Bascom R, Naclerio RM, Fitzgerald TK, Kagey-Sobotka A, & Proud D
(1990) Effect of ozone inhalation on the response to nasal challenge
of allergic subjects. Am Rev Respir Dis, 142: 594-601.
Basketter DA, Bremmer JN, Kammuller ME, Kawabata T, Kimber I, Loveless
SE, Magda S, Pal THM, Stringer DA, & Vohr HW (1994) The identification
of chemicals with sensitizing or immunosuppressive properties in
routine toxicology. Food Chem Toxicol, 32: 289-296.
Bates DV & Sizto R (1983) Relationship between air pollutant levels
and hospital admissions in southern Ontario. Can J Public Health,
74: 117-122.
Baumann G, Zenke G, Wenger R, Hiestand P, Quesniaux V, Andersen E, &
Schreier MH (1992) Molecular mechanisms of immunosuppression. J
Autoimmunol, 5 (Suppl A): 67-72.
Bazin H, Xhurdebise LM, Burtonboy G, Lebacq AM, de Clerq L, & Cormont
F (1984) Rat monoclonal antibodies. I. Rapid purification from
in vitro culture supernatants. J Immunol Meth 66: 261-269.
Behrendt H, Krämer U, Dolgner R, Hinrinchs J, Willer H, Hagenbeck H, &
Schlipköter HW (1993) Elevated levels of total serum IgE in East
German children: Atopy, parasites and pollutants. J Allergol, 2:31.
Behrendt H, Friedrichs K-H, Krämer U, Hitzfeld B, Becker W-M, & Ring J
(1994) The role of indoor and outdoor air pollution in allergic
diseases. In: Vos JG, Younes M, & Smith E ed. Allergic
hypersensitivities induced by chemicals: Recommendations for
prevention. Boca Raton, Florida, CRC Press, pp 173-182.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Roboz JP, Fischbein A, & Mason P (1987) Immunotoxicology,
environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the State of Michigan. Cancer Detect
Prev, Suppl 1: 29-37.
Bélisle C & Sainte-Marie G (1981) Tridimensional study of the deep
cortex of the rat lymph node. II. Relation of deep cortex units to
afferent lymphatic vessels. Anat Rec, 199: 61-72.
Bell EB (1989) Thymus-derived and non-thymus-derived T-like cells. The
origin and function of cells bearing gd receptors. Thymus, 14: 3-17.
Bencko V, Wagner V, Wagnerova M, & Ondrejcak K (1990) Immunological
profiles in workers occupationally exposed to inorganic mercury. J Hyg
Epidemiol Microbiol Immunol, 34: 9-14.
Bentwich Z, Bianco N, Jager L, Houba V, Lambert PH, Knaap W, Rose N,
Seligman M, Thompson R, Torrigiani G, & de Weck A (1982) Use and abuse
of laboratory tests in clinical immunology: Critical considerations of
eight widely used diagnostic procedures. report of a Joint IUIS/WHO
Meeting on Assessment of Tests Used in Clinical Immunology. Clin
Immunol Immunopathol, 24: 122-138.
Bentwich Z, Beverley PCL, Hammarstrom L, Kalden JR, Lambert PH, Rose
NR, & Thompson RA (1988) Laboratory investigations in clinical
immunology: Methods, pitfalls, and clinical indications. Clin Immunol
Immunopathol 49: 478-497.
van den Bergh P, Rozing J, & Nagelkerken L (1991) Opioid peptides as
cytokines in T cell activation. Adv Neuroimmunol, 1: 189-203.
van den Bergh P, Rozing J, & Nagelkerken L (1993a) Identification of
two moieties of ß-endorphin with opposing effects on rat T-cell
proliferation. Immunology, 79: 18-23.
van den Bergh P, Rozing J, & Nagelkerken L (1993b) b-Endorphin
stimulates Ia expression on mouse B cells by inducing interleukin-4
secretion by CD4+ cells. Cell Immunol, 149: 180-192
van den Bergh P, Dobber R, Ramlal S, Rozing J, & Nagelkerken L (1994)
Role of opioid peptides in the regulation of cytokine production by
murine CD4+ T cells. Cell Immunol, 154: 109-122.
Bergman A, Olsson M, & Reiland S (1992) Skull-bone lesions in the
Baltic grey seal (Halichoerus grypus). Ambio, 21: 517-519.
Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F ed. (1987)
Immunotoxicology. Dordrecht, Martinus Nijhoff Publishers, pp xi-xxvii.
Bernier J, Brousseau P, Krzystyniak K, Tryphonas H, & Fournier M (in
press) Immunotoxicity of heavy metals in relation to Great Lakes.
Environ Health Perspectives, Suppl 9.
Bernstein A & Breitman M (1989) Genetic ablation in transgenic mice.
Mol Biol Med, 6: 523-530.
Beschorner WE, Namnoun JD, Hess AD, Shinn CA, & Santos GW (1987a)
Cyclosporin A and the thymus immunopathology. Am J Pathol,
126: 487-496.
Beschorner, WE, Di Gennaro KA, Hess AD, & Santos GW (1987b)
Cyclosporine and the thymus: Influence of irradiation and age on
thymic immunopathology and recovery. Cell Immunol, 110: 350-364.
Bevilacqua MP (1993) Endothelial-leukocyte adhesion molecules. Ann Rev
Immunol, 11: 767-804.
Bick PH, Tucker AN, White KL, & Holsapple MP (1984) Effects of
subchronic exposure to diethylstilbestrol on humoral immune function
in adult female (C3B6)F1 mice. Immunopharmacology, 7: 27-39.
Bigazzi PE (1992) Lessons from animal models: The scope of mercury-
induced autoimmunity. Clin Immunol Immunopathol 65: 81-84.
Biron CA, Byron KS, & Sullivan JL (1989) Severe herpesvirus infection
in an adolescent without natural killer cells. New Engl J Med,
320: 1731-1735.
Bissonette E, Dubois C, & Rola-Pleszczynski M (1989) Changes in
lymphocyte function and lung histology during the development of
asbestosis and silicosis in the mouse. Res Commun Chem Pathol
Pharmacol, 65: 211-227.
Black RD, McVey DS, & Oehme FW (1992) Immunotoxicity in the bovine
animal: A review. Vet Hum Toxicol, 34: 438-442.
Blackley BR, Sisodia CS, & Mukkur TK (1980) The effects of
methylmercury, tetraethyllead and sodium arsenite on the humoral
immune response in mice. Toxicol Appl Pharmacol, 52: 245-254.
Blackmann M, Kappler J, & Marrack P (1990) The role of the T cell
receptor in positive and negative selection of developing T cells.
Science, 248: 1335-1341.
Blatt J, Olshan A, Gula MJ, Dickman PS, & Zarenek B (1992) Second
malignancies in very long-term survivors of childhood cancer. Am J
Med, 93: 57-60.
Blaylock BL, Holladay SD, Comment CE, Heindel JJ, & Luster MI (1992)
Exposure to tetrachlorodibenzo- p-dioxin (TCDD) alters fetal
thymocyte maturation. Toxicol Appl Pharmacol, 112: 207-213.
Blazer VS, Wolke RE, Brown J, & Powell CA (1987) Piscine macrophage
aggregate parameters as health monitors: Effects of age, sex, relative
weight, season and site quality in largemouth bass (Micropterus
salmoides). Aquat Toxicol, 10: 199-215.
Bleavins MR & Alvey JD (1991) Immunotoxicologic evaluation of a purine
nucleoside phosphorylase inhibitor in the cynomolgus monkey.
Toxicologist, 11: 205.
Bleavins MR & Dziedzic D (1990) An immunofluorescence study of T and B
lymphocytes in ozone-induced pulmonary lesions in the mouse. Toxicol
Appl Pharmacol, 105: 93-102.
Blok WL, Vogels MT, Curfs JH, Eling WM, Buurman WA, & Van Der Meer JW
(1992) Dietary fish-oil supplementation in experimental Gram negative
infection and in cerebral malaria in mice. J Infect Dis, 165: 898-903.
Bloom BR, Salgame P, & Diamond B (1992) Revisiting and revising
suppressor T cells. Immunol Today, 131: 131-136.
Blusse Van Oud Alblas A, & Van Furth R (1979) Origin, kinetics and
characteristics of pulmonary macrophages in the normal steady state.
J Exp Med, 149: 1504-1518.
Bly JE, Miller NW, & Clem LW (1990) A monoclonal antibody specific for
neutrophils in normal and stressed channel catfish. Dev Comp Immunol,
14: 211-221.
Bodey GP, Fainstein V, & Guerrant R (1986) Infections of the
gastrointestinal tract in the immunocompromised patient Ann Rev Med,
37: 271-281.
Bohus B, Koolhaas JM, De Ruiter AJH, & Heijnen CJ (1991) Stress and
differential alterations in immune system functions: Conclusions from
social stress studies in animals. Neth J Med, 39: 306-315.
Boivin JF (1990) Second cancers and other late side-effects of cancer
treatment: A review. Cancer, 65: 770-775.
Bolas-Fernandez F, Grencis RK & Wakelin D (1988) Cyclosporin A and
Trichinella spiralis: Anthelminthic effects in immunosuppressed
mice. Parasite Immunol, 10: 111-116.
Bonnyns M & Bastomsky CH (1976) Polychlorinated biphenyl-induced
modifica-tion of lymphocyte response to plant mitogens in rats.
Experientia, 15: 522-523.
Booss J (1980) Establishment of cytomegalovirus infection in mice:
Role of a macrophage-enriched subpopulation. J Infect Dis,
141: 466-472.
Borleffs JCC, Schuurman H-J, Vrehen HM, Van Schaik M, & Bast EJEG
(1993) in vitro and in vivo measurement of cell-mediated immunity
in patients with HIV-1-infection. Scand J Immunol, 37: 634-636.
Boros DL (1988) Mechanisms of granuloma formation in the lung.
In: Daniele RP ed. Immunology and immunologic diseases of the lung.
Boston, Blackwell Scientific Publications, pp 263-287.
Bos J ed. (1990) The skin immune system. Boca Raton, Florida, CRC
Press.
Bos JD & Kapsenberg ML (1986) The skin immune system (SIS): Its
cellular constituents and their interactions. Immunol Today,
7: 235-240.
Bouwes Bavinck JN, de Boer A, Vermeer BJ, Hartevelt MM, van der Woude
FJ, Claas FHJ, Wolterbeek R, & Vandenbroucke JP (1993) Sunlight,
keratotic skin lesions and skin cancer in renal transplant recipients.
Br J Dermatol, 129: 242-249.
Boyce WT, Jensen EW, Cassel JC, Collier AM, Smith AH, & Ramey CT
(1977) Influence of life events and family routines on childhood
respiratory tract illness. Pediatrics, 60: 609-615.
Boyd RL, Wilson TJ, Bean AG, Ward HA, & Gershwin ME (1992) Phenotypic
characterization of chicken thymic stromal elements. Dev Immunol,
2: 51-66.
Boyle J, Mackie RM, Briggs JD, Junor BJR, & Aitchison TC (1984)
Cancer, warts, and sunshine in renal transplant patients: A case-
control study. Lancet, i: 702-705.
Bradley SG & Morahan PS (1982) Approaches to assessing host
resistance. Environ Health Perspectives, 43: 61-69.
Brekelmans P & Van Ewijk W (1990) Phenotypic characterization of
murine thymic microenvironments. Sem Immunol, 2: 13-24.
Brett SJ & Butler R (1986) Resistance to Mycobacterium lepraemurium
is correlated with the capacity to generate macrophage activating
factor(s) in response to mycobacterial antigens in vitro.
Immunology, 59: 339-345.
Brideau RJ, Carter PB, McMaster WR, Mason DW, & Williams AF (1980) Two
subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J
Immunol, 10: 609-615.
Brigham KL (1989) Mediators in inflammation. In: Henderson PM & Murphy
RC ed. Handbook of inflammation, Vol. 6: Mediators of the inflammatory
process. Amsterdam, Elsevier Science Publishers, pp 1-14.
Brodie AM & Halliday WJ (1991) Suppressor cells and soluble factors
from the spleens of UV-irradiated mice. Immunol Cell Biol,
69: 199-204.
Brostoff J, Scadding GK, Male D, & Roitt IM (1991) Clinical
immunology. London, Gower Medical Publishers.
Brouwer A, Reijnders PJH, & Koeman JH (1989) Polychlorinated biphenyl
(PCB)-contaminated fish induces vitamin A and thyroid hormone
deficiency in the common seal (Phoca vitulina). Aquat Toxicol,
15: 99-106.
Brown WR & Kloppel TM (1989) The liver and IgA: Immunological, cell
biological and clinical implications. Hepatology, 9: 763-778.
Bruggeman CA, Debie WMH, Grauls G, Majoor G, & Boven CPA (1983)
Infection of laboratory rats with a new cytomegalo-like virus. Arch
Virol, 76: 189-199.
Bruggeman CA, Meijer H, Bosman F, & Boven CPA (1985) Biology of rat
cytomegalovirus. Intervirology, 24: 1-9.
Bruning JH (1985) Cytomegalovirus infections in renal transplantation;
study in a rat model. PhD thesis, Utrecht, Utrecht University.
Bucke D, Vethaak AD, & Lang T (1992) Quantitative assessment of
melanomacrophage centers (MMC's) in dab (Limanda limanda) along a
pollution transect in the German Bight. Mar Ecol Prog Ser,
91: 193-196.
Buckingham JC, Safieh B, Singh S, Arduino LA, Cover PO, & Kendall MD
(1992) Interactions between hypothalamo-pituitary adrenal axis and the
thymus in the rat: A role for corticotropin in the control of thymulin
release. J Neuroendocrinol, 4: 295-301.
Buening GM, Mann DD, Hook B, & Osweiler BP (1982) The effect of T-2
toxin on the bovine immunity system: Cellular factors. Vet Immunol
Immunopathol, 3: 411-417.
Bugelski PJ, Thiem PA, Solleveld HA, & Morgan DG (1990) Effects of
sensitization to dinitrochlorobenzene (DNCB) on clinical pathology
parameters and mitogen-mediated blastogenesis in cynomolgus monkeys
(Macaca fascicularis). Toxicol Pathol, 18: 643-650.
Bukowski JF, Woda BA, & Welsh RM (1984) Pathogenesis of murine
cytomegalovirus infection in natural killer cell-depleted mice.
J Virol, 52: 119-128.
Bulloch K (1987) The innervation of immune system tissues and organs.
In: Cotman CW, Brinton RE, Galaburda A, McEwen B, & Schneider DM ed.
The neuro-immune-endocrine connection. New York, Raven Press,
pp 33-47.
Burcham J, Marmor M, Dubin N, Tindall B, Cooper DA, Berry G, & Penny R
(1991) CD4+ is the best predictor of development of AIDS in a cohort
of HIV-infected homosexual men. AIDS, 5: 365-372.
Burchiel SW, Thompson TA, & Davis AP (1991) Alterations in mitogen-
induced calcium mobilization and intracellular free calcium produced
by 7,12-dimethylbenz (a)anthracene in the Jurkat human T cell line.
Int J Immunopharmacol, 13: 109-115.
Burleson GR, Fuller LB, Menache MG, & Graham JA (1987) Poly(I):
poly(C)-enhanced alveolar and peritoneal macrophage phagocytosis:
Quantification by a new method utilizing fluorescent beads. Proc Soc
Exp Biol Med, 184: 468-476.
Burleson GR, Keyes LL, & Stutzman JD (1989) Immunosuppression of
pulmonary natural killer activity by exposure to ozone.
Immunopharmacol Immunotoxicol, 11: 715-735.
Burleson GR, Lebrec H, Yang YG, lbanes JD, Pennington KN, & Birnbaum
LS (in press) Effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)
on influenza virus host resistance in mice. Fundam Appl Toxicol.
Calder VL & Lightman SL (1992) Experimental autoimmune uveoretinitis
(EAU) versus experimental allergic encephalomyelitis (EAE): A
comparison of T cell-mediated mechanisms. Clin Exp Immunol,
89: 165-169.
Cantacuzene MJ (1898) Nouvelles recherches sur le mode de destruction
des virions dans l'organisme. Ann Inst Pasteur, 12: 274-300.
Cantrell DA, Collins MKL, & Crumpton DA (1988) Autocrine regulation of
T-lymphocyte proliferation: Differential induction of IL-2 and IL-2
receptor. Immunology, 65: 343-349.
Capurro PU (1980) The effects of solvents and pesticides on health.
Clin Toxicol, 16: 549-553.
Carter JW & Clancy J (1980) Acutely administered polychlorinated
biphenyls (PCBs) decrease splenic cellularity but increase its
ability to cause graft-versus-host reactions in BALB/c mice.
Immunopharmacology, 2: 341-347.
Casale GP, Cohen SD, & DiCapua RA (1983) The effects of
organophosphate-induced cholinergic stimulation on the antibody
response to sheep erythrocytes in inbred mice. Toxicol Appl
Pharmacol, 68: 198-205.
Casale GP, Cohen SD, & DiCapua RA (1984) Parathion-induced suppression
of humoral immunity in inbred mice. Toxicol Lett, 23: 239-247.
Castillo-Mendez A, Rodriguez-Diaz T, Leon-Lobeck A, & Gravalosa-Cruz
AJ (1991) Influence of occupational lead exposure on the concentration
of immunoglobins and immune cellular functions in human. Rev Allergol
Mex, 38: 69.
Centers for Disease Control (1991) Summary of notifiable diseases,
United States, 1990. Morb Mortal Weekly Rep, 39 (53).
Challacombe SJ (1987) The investigation of secretory and systemic
immune responses to ingested material in animal models. In: Miller K
& Nicklin S ed. Immunology of the gastrointestinal tract, Vol 2. Boca
Raton, Florida, CRC Press, pp 99-124.
Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, &
Hiseroth JC (1989) Monoclonal antibody to a triggering structure
expressed on rat natural killer cells and adherent lymphokine-
activated killer cells. J Exp Med 169: 1373-1389.
Chan AC, Irving BA, & Weiss A (1992) New insights into T-cell antigen
receptor structure and signal transduction. Curr Opin Immunol,
4: 246-251.
Chanarin I (1985) Structure and function of the bone marrow. In: Irons
RD ed. Toxicology of the blood and bone marrow. New York, Raven Press,
pp 1-16.
Chandra RK (1992) Nutrition and immunoregulation. Significance of host
resistance to tumors and infectious diseases in humans and rodents.
J Nutr, 122 (Suppl): 754-757.
Chandra RK (1993) Nutrition and the immune system. Proc Nutr Soc,
52: 77-84.
Chang KH, Hsieh KH, Lee TP, Tang SY, & Tung TC (1981) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Determination of lymphocyte subpopulations. Toxicol Appl Pharmacol,
61: 58-63.
Chang KH, Hsieh KH, Tang SY, Tung TC, & Lee TP (1982) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Evaluation of delayed-type skin hypersensitivity response and its
relation to clinical studies. J Toxicol Environ Health, 9: 217-223.
Clark EA & Lanier LL (1989) Report from Vienna: In search of all
surface molecules expressed on human leukocytes. J Clin Immunol,
9: 265-272.
Clark GC, Blank JA, Germolec DR & Luster MI (1991)
2,3,7,8-Tetrachlorodibenzo- p-dioxin stimulation of tyrosine
phosphorylation in B lymphocytes: Potential role in
immunosuppression. Mol Pharmacol, 39: 495-501.
Clarke AG (1984) Immunological studies on pregnancy in the mouse.
In: Crighton DB ed. Immunological aspects of reproduction in mammals.
London, Butterworths, pp 153-182.
Clarke AG & Kendall MD (1989) Histological changes in the thymus
during mouse pregnancy. Thymus, 14: 65-78.
Clevers H, Alarcon B, Wileman T, & Terhorst C (1988) The T cell
receptor/CD3 complex: A dynamic protein ensemble. Ann Rev Immunol,
6: 629-662.
Clover RD, Abell T, Becker LA, Crawford S, & Ramsey CN (1989) Family
functioning and stress as predictors of influenza B infection.
J Family Practice, 28: 535-539.
Coffin DL & Gardner DE (1972) Interaction of biological agents and
chemical air pollutants. Ann Occup Hyg, 15: 219-234.
Cohen N, Modai D, Golik A, Weissgarten J, Peller S, Katz A, Averbuhk
Z, & Shaked U (l989) Increased concanavalin A-induced suppressor cell
activity in humans with occupational lead exposure. Environ Res,
48: 1-6.
Coligan JE, Kruisbeek, AM, Margulies DH, Shevach EM, & Strober W ed.
(1994) Current protocols in immunology, Vols 1 and 2. New York, John
Wiley & Sons.
Cook JA, Marconi EA, & DiLuzio NR (1974) Lead, cadmium, endotoxin
interaction: Effect on mortality and hepatic function. Toxicol Appl
Pharmacol, 28: 292.
Cook JA, Hoffman EO, & DiLuzio NR (1975) Influence of lead and cadmium
on the susceptibility of rats to bacterial challenge (39117). Proc Soc
Exp Biol Med, 150: 741.
Cook JC, Gaido KW, receptor: & Greenlee WF Relevance of (1987) Ah
mechanistic studies to human risk assessment. Environ Health
Perspectives, 76: 71-77.
Cooper EL (1982) How the immune system evolved. In: General
immunology. Oxford, Pergamon Press, pp 279-323.
Cooper KD, Neises GR, & Katz SI (1986) Antigen-presenting OKM5+
melanophages appear in human epidermis after ultraviolet radiation.
J Invest Dermatol, 86: 363-370.
Cooper KD, Oberhelman BS, Hamilton MS, Baadsgaard O, Terhune M, Levee
G, Anderson T, & Koren H (1992) UV exposure reduces immunization rates
and promotes tolerance to epicutaneous antigens in humans;
relationship to dose, CDla DR+ epidermal macrophage inductlon and
Langerhans cell depletion. Proc Natl Acad Sci USA, 89: 8497-8501.
Corman LC (1985) Effects of specific nutrients on the immune response.
Selected clinical applications. Med Clin North Am, 69: 759-791.
Cornacoff JB, Tucker AN, & Dean JH (1988) Development of a human
peripheral blood mononuclear leucocyte model for immunotoxicity
evaluation. In Vitro Toxicol, 2: 81-89.
Corrier DE (1992) Mycotoxicosis: Mechanisms of immunosuppression. Vet
Immunol Immunopathol, 30: 73-87.
Corsini E, Luster MI, Mahler J, Craig WA, Blazka M, & Rosenthal GJ
(1994) A protective role for T-lymphocytes in asbestos-induced
pulmonary inflammation and collagen deposition. Am J Respir Cell Mol
Biol, 7: 60-82.
Coscia GC, Discalzi G, & Ponzetti C (1987) Immunological aspects of
occupational lead exposure. Med Lav, 78: 360-364.
Council for International Organizations of Medical Sciences (1993)
International ethical guidelines for biomedical research involving
human subjects. Geneva, 63 pp.
Cunningham AJ & Szenberg A (1968) Further improvements in the plaque
tech-nique for detecting single antibody-forming cells. Immunology,
14: 559-600.
Dabrowski MP & Dabrowski-Bernstein BK (1990) Immunoregulatory role of
the thymus. Boca Raton, Florida, CRC Press.
Dallman MJ, Thomas ML, & Green JR (1984) MRC OX-19: A monoclonal
antibody that labels rat T lymphocytes and augments in
vitroproliferative responses. Eur J Immunol, 14: 260-267.
Davis AP & Burchiel SW (1992) Inhibition of calcium-dependent pathways
of B-cell activation by DMBA. Toxicol Appl Pharmacol, 116: 202-208.
Davis D & Safe S (1988) Immunosuppressive activities of
polychlorinated dibenzofuran congeners: Quantitative structure-
activity relationships and interactive effects. Toxicol Appl
Pharmacol, 94: 141-149.
Davis D & Safe S (1989) Dose-response immunotoxicities of commercial
polychlorinated biphenyls (PCBs) and their interaction with
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol Lett, 48: 35-43.
Davis D & Safe S (1991) Halogenated aryl hydrocarbon-induced
suppression of the in vitro plaque-forming cell response to
sheep red blood cells is not dependent on the Ah receptor.
Immunopharmacology, 21: 183-190.
Dayan AD, Hertel RF, Heseltine E, Kazantis G, Smith EM, & Van der
Venne MT ed. (1990) Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press.
Dean JH & Murray MJ (1990) Toxic responses of the immune system.
In: Klaassen CD, Amdur MO, & Doull J ed. Toxicology: The basic science
of poisons, Vol 4. New York, MacMillan Press, pp 282-333.
Dean JH & Thurmond LM (1987) Immunotoxicology: An overview. Toxicol
Pathol, 15: 265-271.
Dean JH, Luster MI, & Boorman GA (1982) Immunotoxicology. In: Sirois
P, & Pleszczynski MR ed. Immunopharmacology. Amsterdam, Elsevier
Science Publishers, pp 349-397.
Dean JH, Luster MI, Munson AE, & Amos HA (1985) Immunotoxicology and
immunopharmacology. New York, Raven Press.
Dean JH, Ward EC, Murray MJ, Lauer LD, & House RV (1986)
Immunosuppression following 7,12-dimethylbenz-a-anthracene exposure
in B6C3F1 mice. II. Altered cell-mediated immunity and tumor
resistance. Int J Immunopharmacol, 8: 189-198.
Dean JH, Luster MI, Munson AE, & Kimber I (1994) Immunotoxicology and
immunopharmacology, 2nd Ed. New York, Raven Press.
De Gast GC, Verdonck LF, Middeldorp JM, The TH, Hekker A, Van der
Linden JA, Kreeft HA, & Bast BJ (1985) Recovery of T cell subsets
after autologous bone marrow transplantation is mainly due to
proliferation of mature T cells in the graft. Blood, 66: 428-431.
De Heer C, Verlaan APJ, Penninks AH, Schuurman HJ, & Van Loveren H
(1993) The SCID-RA mouse: Rat T cell differentiation in the severe
combined immunodeficient mouse. APMIS, 101: 467-479.
Denis M (1992) Mouse hypersensitivity pneumonitis: Depletion of NK
cells abrogates the spontaneous regression phase and leads to massive
fibrosis. Exp Lung Res, 18: 761-773.
Denis M, Bisson D, & Ghardirian E (1993) Cellular and cytokine
profiles in spontaneous regression phase of hypersensitivity
pneumonitis. Exp Lung Res, 19: 257-271.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
Deo MG, Gangal S, Bhisey AN, Somasundaram R, Balsara Gulwani B,
Darbaru BS, Bhiide S, & Maru B (1987) Immunological, mutagenic and
genotoxic investigations in gas exposed population of Bhopal. Indian
J Med Res, 86 (Suppl): 63-76.
Descotes J (1985) Immunomodulating agents and hepatic drug metabolism.
Drug Metab Rev, 16: 175-183.
Descotes J (1986) Immunotoxicology of drugs and chemicals. Amsterdam,
Elsevier Science Publishers.
Descotes J (1990) Drug-induced immune diseases. Amsterdam, Elsevier
Science Publishers.
Descotes J (1992) Immunomodulating agents. In: Dukes MNG ed. Meyler's
side-effects of drugs, 12th Ed. Amsterdam, Elsevier Science
Publishers, pp 954-958.
Descotes J & Vial T (1994) Cytoreductive drugs. In: Dean JH, Luster
MI, Munson AE, & White K ed. Immunotoxicology and immunopharmacology,
2nd Ed. New York, Raven Press, pp 293-301.
Descotes J, Verdier F, Brouland JP, & Pulce C (1990) Immunotoxicity of
lead, cadmium and arsenic: Experimental data and their relevance to
man. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM, &
Van der Venne, M-T ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, pp 209-213.
Desi L, Varga L, & Farkas I (1978) Studies on the immunosuppressive
effect of organochlorine and organophosporic pesticides in subacute
experiments. J Hyg Epidemiol Microbiol Immunol, 22: 115-122.
Desi L, Varga L, Dobronyi L, & Szklenarik G (1985) Immunotoxicological
investigation of the effects of a pesticide; cypermethrin. Arch
Toxicol, Suppl 8: 305-309.
De Swart RL, Kluten RMG, Huizing CJ, Vedder LJ, Reijnders PJH, Visser
IKG, UytdeHaag FGCM, & Osterhaus ADME (1993) Mitogen and antigen
induced B and T cell responses of peripheral blood mononuclear cells
from the harbour seal (Phoca vitulina). Vet Immunol Immunopathol,
37: 217-230.
De Swart RL, Ross PS, Vedder EJ, Timmerman HH, Heisterkamp SH, Van
Loveren H, Vos JG, Reijnders PJH, & Osterhaus ADME (1994) Impairment
of immune function in harbour seals (Phoca vitulina) feeding on fish
from polluted waters. Ambio, 23: 155-159.
Devens BH, Grayson MH, Imamura IK, & Rodgers KE (1985)
O,O,S-Trimethyl-phosphorothioate effect on immunocompetence.
Pestic Biochem Physiol, 24: 251-259.
Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers
D, & Koren H (1991) Exposure of humans to ambient levels of ozone for
6.6 hours causes cellular and biochemical changes in the lung.
Am J Resp Cell Mol Biol, 4: 72-81.
De Waal EJ, Rademakers LHPM, Schuurman H-J, & Van Loveren H (1992a)
Interdigitating cells in the rat thymus during cyclosporin A
treatment: Ultrastructural observations. Thymus, 20: 163-170.
De Waal EJ, Schuurman H-J, Loeber JG, Van Loveren H, & Vos JG (1992b)
Alterations in the cortical thymic epithelium of rats after
in vivo exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD):
An (immuno)histological study. Toxicol Appl Pharmacol, 115: 80-88.
De Waal EJ, Rademakers LHPM, Schuurman H-J, Van Loveren H, & Vos JG
(1993) The cortical epithelium of the rat thymus after in vivo
exposure to bis(tri-n-butyltin)oxide (TBTO). Arch Toxicol,
67: 186-192.
DeWailly E, Nantel A, Brunean S, Laliberte C, Ferron L, & Gingras S
(1992) Breast milk contamination by PCDDs, PCDFs, and PCBs in Arctic
Quebec: A preliminary assessment. Chemosphere, 25: 1245-1249.
De Weger RA, Van Loveren H, Vandebriel RJ, & Parmentier HK (1989).
Sequential activity of gamma-delta T-cell receptor bearing
lymphocytes, specific T-cell factors, and alpha-ß T-cell receptor
bearing T-cells: A hypothesis. Thymus, 14: 19-30.
Dieter MP, Luster MI, Boorman GA, Jameson CW, Dean JH, & Cox JW (1983)
Immunological and biochemical responses in mice treated with mercuric
chloride Toxicol Appl Pharmacol, 68: 218-228.
Dietert RR, Qureshi MA, Nanna UC, & Bloom SE (1985) Embryonic exposure
to aflatoxin-B1: Mutagenicity and influence on development and
immunity. Environ Mutag, 7: 715-725.
Dietz R, Heide-Jorgensen MP, & Härkönen T (1989) Mass deaths of harbor
seals (Phoca vitulina) in Europe. Ambio, 18: 258-264.
Dijkstra CD & Sminia T (1990) Normal anatomy, histology,
immunohistology, ultrastructure, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI monograph). Berlin, Springer
Verlag, pp 185-193.
Dijkstra CD, Döpp EA, Joling P, & Kraal G (1985) The heterogeneity of
mononuclear phagocytes in lymphoid organs: Distinct macrophage
subpopu-lations in rat recognized by monoclonal antibodies ED1, ED2
and ED3. Immunology, 54: 589-599.
Dinarello CA (1992) Role of interleukin-1 in infectious diseases.
Immunol Rev, 127: 119-146.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979a) Role of
macrophages in the augmentation of mouse natural killer activity by
polyI:C and interferon. J Immunol, 122: 182-188.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979b) Augmentation
of mouse natural killer cell activity by interferon and interferon
inducers. J Immunol, 122: 175-181.
Dobber R, Hertog-Huijbregts A, Rozing J, Bottomly K, & Nagelkerken L
(1992) The involvement of the intestinal microflora in the expansion
of CD4+ T cells with a naive phenotype in the periphery. Dev
Immunol, 2: 141-150.
Dogra RK, Chandra K, Chandra S, Gupta S, Khanna S, Srivastava SN,
Shukla LJ, Katiyar JC, & Shanker R (1992) Host resistance assays as
predictive models in styrene immunomodulation. Int J Immunopharmacol,
14: 1003-1009.
Domzig W, Stadler BM, & Herberman RB (1983) Interleukin-2 dependence
of human natural killer (NK) cell activity. J Immunol, 130: 1970-1973.
Dooley RK & Holsapple MP (1988) Elucidation of cellular targets
responsible for tetrachlorodibenzo- p-dioxin (TCDD)-induced
suppression of antibody responses: I. The role of the B lymphocyte.
Immunopharmacology, 16: 167-180.
Dotta F & Eisenbarth GS (1989) Type I diabetes mellitus: A predictable
autoimmune disease with interindividual variation in the rate of
ß cell destruction. Clin Immunol Immunopathol, 50: S85-S95.
Driscoll KE, Higgins JM, Leytart MJ, & Corsby LL (1990) Differential
effects of mineral dusts on the in vitro activation of alveolar
macrophage eicosanoid and cytokine release. Toxicity in vitro,
4, 284-288.
Drouet M, Le-Sellin J, Bonneau JC, & Sabbah A(1990) Is mercury an
allergen of the respiratory tract. Allerg Immunol (Paris),
22: 81, 84-85, 87.
Druet P, Pelletier L, Rossert J, Druet E, Hirsch F, & Sapin C (1989)
Autoimmune reactions induced by metals. In: Kammüller ME, Bloksma N,
& Seinen W ed. Autoimmunity and toxicology. Immune disregulation
induced by drugs and chemicals. Amsterdam, Elsevier Science
Publishers, pp 347-361.
Duijvestijn A & Hamann A (1989) Mechanisms and regulation of
lymphocyte migration. Immunol Today, 10: 23-28.
Duke SS, Schook LB, & Holsapple MP (1985) Effects of
N-nitrosodimethylamine on tumor susceptibility. J Leukocyte
Biol, 37: 383-394.
Dunn TB (1954) Normal and pathologic anatomy of the reticular tissue
in laboratory mice, with a classification and discussion of neoplasms.
J Natl Cancer Inst, 14: 1281-1433.
Du Pasquier L (1989) Evolution of the immune system. In: Paul WE ed.
Fundamental immunology, 2nd Ed. New York, Raven Press, pp 139-165.
Durham WH (1974) Air pollution and student health. Arch Environ
Health, 28: 241-254.
Dutch Health Council, Committee for Immunotoxicology (1991)
Immunotoxicity of compounds. The Hague.
Dyall-Smith C & Varigos G (1985) The malignant potential of
papillomavirus. Aust J Dermatol, 26: 102-107.
Dziedzic D & White HJ (1986) T-Cell activation in pulmonary lymph
nodes of mice exposed to ozone. Environ Res, 41: 610-622.
Dziedzic D, Wright ES, & Sargent HE (1990) Pulmonary response to
ozone. Reaction of bronchus-associated lymphoid tissue and lymph node
lymphocytes in the rat. Environ Res, 51: 194-208.
Eedy DJ, Burrows D, Clifford T, & Fay A (1990) Elevated T cell
subpopulations in dental students. J Prosthet Dent, 63: 593.
Ehrke MJ, Mihich E, Berd D, & Mastrangelo MJ (1989) Effects of
anticancer drugs on the immune system in humans. Semin Oncol,
16: 230-253.
Ehrlich JP & Burleson GR (1991) Enhanced and prolonged pulmonary
influenza virus infection following phosgene inhalation. J Toxicol
Environ Health, 34: 259-273.
Ehrlich R & Henry MC (1968) Chronic toxicity of nitrogen dioxide.
I. Effect on resistance to bacterial pneumonia. Arch Environ Health,
17: 860-865.
Ehrlich R, Findlay JC, Fonters JD, & Gardner DE (1977) Health effects
of short-term inhalation of nitrogen dioxide and ozone mixtures.
Environ Res, 4: 223-231.
Ellman MH, Hurwitz H, Thomas C, & Kozloff M (1991) Lymphoma developing
in a patient with rheumatoid arthritis taking low dose weekly
methotrexate. J Rheumatol, 18: 1741-1743.
Elo O, Vuojolahti P, Janhunen R, & Rantanen J (1985) Recent PCB
accidents in Finland. Environ Health Perspectives, 60: 315-319.
Emmendorfer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML,
& Roesler J (1994) Evaluation of flow cytometric methods for diagnosis
of chronic granulomatous disease variants under routine laboratory
conditions. Cytometry, 18: 147-155.
Engelhard VH (1994) Structure of peptides associated with class I and
class II MHC molecules. Ann Rev Immunol, 12: 181-207.
Engleman EG, Benike CJ, Grumet FC, & Evans RL (1981) Activation of
human T lymphocyte subsets: Helper and suppressor/cytotoxic T cells
recognize and respond to distinct histocompatibility antigens.
J Immunol, 127: 2124-2129.
Epstein JH, Tuffanelli DL, & Dubois EL (1965) Light sensitivity and
lupus erythematosus. Arch Dermatol, 91: 483-485.
Eskola J & Toivanen P (1974) Effect of in ovo treatment with
cyclophosphamide on lymphoid system in chicken. Cell Immunol,
13: 459-471.
Evans RG, Webb KB, Knutsen AP, Roodman ST, Roberts DW, Bagby JR,
Garrett WA, & Andrews JS (1988) A medical follow-up of the health
effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. Arch Environ Health, 43: 273-278.
Ewers U, Stiller-Winkler R, & Idel H (1982) Serum immunoglobulin,
complement C3, and salivary IgA levels in lead workers. Environ Res,
29: 351-357.
Exon JR (1985) Effect of lead, polychlorinated biphenyls and
cyclophosphamide on rat natural killer cells, interleukin 2, and
antibody synthesis. Fundam Appl Toxicol, 5: 158-164.
Ezine S, Weissman IL, & Rouse RV (1984) Bone marrow cells give rise to
distinct cell clones within the thymus. Nature, 309: 629-631.
Fabry Z, Raine CS, & Hart MN (1994) Nervous tissue as an immune
compartment: Dialect of the immune response in the CNS. Immunol Today,
15: 218-224.
Faisal M & Hugget RJ (1993) Effects of polycyclic aromatic
hydrocarbons on the lymphocyte mitogenic response in spot, Leiostomus
xanthurus. Mar Environ Res, 35: 121-124.
Faisal M, Chiapelli F, Ahmed II, Cooper EL, & Weiner H (1989) Social
confrontation stress in aggressive fish is associated with an
endogenous opioid-mediated suppression of proliferative response to
mitogens and nonspecific cytotoxicity. Brain Behav Immunol,
3: 223-233.
Fänge R & Nilsson S (1985) The fish spleen: Structure and function.
Experientia, 41: 152-158.
Farrell HE & Shellam GR (1991) Protection against murine
cytomegalovirus infection by passive transfer of neutralizing and non-
neutralizing monoclonal antibodies. Infect Immunol, 72: 149-156.
Fauci A, Schnittman SM, & Poli G (1991) Immunopathologenic mechanisms
in human immunodeficiency virus (HIV) infection. Ann Intern Med.,
114: 678-693.
Fellah JS, Wiles MV, Charlemagne J, & Scwager J (1992) Evolution of
vertebrate IgM: Complete amino acid sequence of the constant region of
Ambystoma mexicanum µ chain deduced from cDNA sequence. Eur J
Immunol, 22: 2595-2601.
Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden
KS, Olschowka JA, & Livnat S (1987) Noradrenergic sympathetic neural
interaction with the immune system: Structure and function. Immunol
Rev, 100: 225-260.
Fenters J, Bradof J, Aranyi C, Ketels K, Ehrlich R, & Gardner D (1979)
Health effects of long-term inhalation of sulfuric acid mist-carbon
particle mixtures. Environ Res, 19: 244-257.
Fichtelius KE, Groth O, & Liden S (1970) The skin, a first level
lymphoid organ? Int Arch Allergy, 37: 607-620.
Fidler IJ (1973) Selection of successive tumor lines for metastasis.
Nature New Biol, 242: 148-149.
Fidler IJ, Gersten DM, & Hart IR (1978) The biology of cancer invasion
and metastases. Adv Cancer Res, 28: 149-250.
Fine JS, Gasiewicz TA, & Silverstone AE (1989) Lymphocyte
stem cell alterations following perinatal exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol Pharmacol, 35: 18-25.
Fine JS, Gasiewicz TA, Fiore NC, & Silverstone AE (1990) Prothymocyte
activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo- p-dioxin
exposure. J Exp Pharmacol Ther, 255: 1-5.
Finsen NR (1901) The treatment of lupus vulgaris by concentrated rays.
In: Phototherapy. London, Edward Arnold, pp 73-75.
Fiore MC, Anderson HA, Hong R, Golubjatnokov R, Seiser JE, Nordstrom
D, Hanrahan L, & Belluck D (1986) Chronic exposure to aldicarb-
contaminated groundwater and human immune function. Environ Res,
41: 633-645.
Fischbein A, Tsang P, Luo JC, Roboz JP, Jiang JD, & Bekesi JG (1993)
Phenotypic aberrations of CD3+ and CD4+ cells and functional
impairments of lymphocytes at low-level occupational exposure to lead.
Clin Immunol Immunopathol, 66: 163-171.
Flajnik MF, Canel C, Kramer J, & Kasahara M (1991) Evolution of the
major histocompatibility complex class I from the amphibian Xenopus.
Proc Natl Acad Sci USA, 88: 537-541.
Fleisher TA, Luckasen JR, Sabad A, Gehrtz RC, & Kersey JH (1975) T and
B lymphocyte subpopulations in children. Pediatrics, 55: 162-165.
Fletcher TC (1986) Modulation of non specific host defences in fish.
Vet Immunol Immunopathol, 12: 59-67.
Fletcher FA & Williams DE (1992) Recent progress in the discovery and
invention of novel hematopoietic cytokines. Crit Rev Oncol Hematol,
13: 1-15.
Flick DF, O'Dell RG, & Childs VA (1965) Studies on the chick edema
disease. 3. Similarity of symptoms produced by feeding chlorinated
biphenyls. Poultry Sci, 44: 1460-1465.
Folinsbee LJ (1992) Human health effects of air pollution. Environ
Health Perspectives, 100: 45-56.
Fontana A, Constamm DB, Frei K, Malipiero U, & Pfister HW (1992)
Modulation of the immune response by transforming growth factor beta.
Int Arch Allergy Immunol, 99: 1-7.
Food Safety Council (1978) Proposed system for food safety assessment.
Food Cosmet Toxicol, 16 (Suppl 1): 1-136.
Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, & Gery
I (1987) Experimental autoimmune uveoretinitis (EAU) induced by
retinal interphotoreceptor retinoid-binding protein (IRBP):
Differences between EAU induced by IRBP and by S-antigen. Clin Immunol
Immunopathol, 43: 256-264.
Foxwell BM, Barrett K, & Feldmann M (1992) Cytokine receptors:
Structure and signal transduction. Clin Exp Immunol, 90: 161-169.
Frasca D, Adorini L, & Doria G (1987) Enhanced frequency of mitogen-
responsive T cell precursors in old mice injected with thymosin alpha
1. Eur J Immunol, 17: 727-730.
Frattini JE & Trulock EP (1993) Respiratory infections in
immunocompromised patients. Immunol Allergy Clin North Am,
13: 193-204.
Frazer IH, Collins EJ, Fox JS, Jones B, Oliphant RC, & Mackay IR
(1985) Assessment of delayed-type hypersensitivity in man: A
comparison of the 'Multitest' and conventional intradermal injection
of six antigens. Clin Immunol Immunopathol, 35: 182-190.
Freier S, ed. (1990) The neuroendocrine-immune network. Boca Raton,
Florida, CRC Press.
French JG, Lowrimore G, Nelson WC, Finklea JF, English T, & Hertz M
(1973) The effect of sulfur dioxide and suspended sulfates on acute
respiratory disease. Arch Environ Health, 27: 129-133.
Friedman H, Klein T, & Specter S (1991a) Immunosuppression by
marijuana and its components. In: Ader R, Felten DL, & Cohen N ed.
Psychoneuroimmunology, 2nd Ed. San Diego, Academic Press, pp 931-953.
Friedman H, Specter S, & Klein TW (1991b) Drugs of abuse, immunity and
immunodeficiency (Advances in Experimental Biology and Medicine,
Volume 288). New York, Plenum Press.
Friedmann PS, White SI, Parker S, Moss C, & Mattews JNS (1989)
Antigenic stimulation during ultraviolet therapy in man does not
result in immunological tolerance. Clin Exp Immunol, 76: 68-72.
Friend M & Trainer DO (1970) Polychlorinated biphenyl: Interaction
with duck hepatitis virus. Science, 170: 1314-1316.
Frith CH, Ward JM, Brown RH, Tyler RD, Chandra M, & Stromberg PC (in
press) Proliferative lesions of the hematopoietic system in rats. In:
Streett CS, Burek JD, Hardisty JF, Garner FM, Leiniger JR, Pletcher
JM, & Moch RW ed. Standardized system of nomenclature and diagnostic
criteria. Guides for toxicologic pathology. Washington DC, Society of
Toxicologic Pathologists, Armed Forces Institute of Pathology,
American Registry of Pathology.
Fugmann RA, Aranyi C, Barbera PW, Bradof JN, Gibbons RD, & Fenters JD
(1983) The effects of DES as measured by host resistance and tumor
susceptibility assays in mice. J Toxicol Environ Health, 11: 827-841.
Fujimaki H (1989) Impairment of humoral responses in mice exposed to
nitrogen dioxide and ozone mixtures. Environ Res, 48: 211-217.
Gahmberg CG, Nortamo P, Li R, & Valmu L (1992) Leukocyte cell adhesion
proteins: From molecular dissection to clinical applications. Ann Med,
24: 329-335.
Gaido KW & Wierda D (1987) Suppression of bone marrow stromal cell
function by benzene and hydroquinone is ameliorated by indomethacin.
Toxicol Appl Pharmacol, 89: 378-390.
Gardner DE, Miller FJ, Illing JW, & Kirtz JM (1977) Alterations in
bacterial defense mechanisms of the lung induced by inhalation of
cadmium. Bull Eur Physiopathol Resp, 13: 157-174.
Garssen J, Van der Vliet H, De Klerk A, Dormans JAMA, & Bruggeman CA
(1995) A rat cytomegalovirus infection model as a tool for
immunotoxicity testing. Eur J Pharmacol, 292: 233-281.
Gasiewicz TA & Rucci G (1984) Cytosolic receptor for
2,3,7,8-tetrachloro-dibenzo- p-dioxin Evidence for a homologous
nature among various mammalian species. Mol Pharmacol, 26: 90-98.
Gaumer HS, Doll NJ, Kaimal J, Schuyler M, & Salvaggio JE (1981)
Diminished suppressor cell function in patients with asbestosis.
Clin Exp Immunol, 44: 108.
Germain RN & Margulies DH (1993) The biochemistry and cell biology of
antigen processing and presentation. Ann Rev Immunol, 11: 403-450.
Germolec DR, Yang RSH, Ackermann MF, Rosenthal GJ, Boorman GA, Blair
P, & Luster MI (1989) Toxicology studies of a chemical mixture of 25
groundwater contaminants. II. Immunosuppression in B6C3F1 mice.
Fundam Appl Toxicol, 13: 377-387.
Ghoneum MH, Egami N, Ljiri K & Cooper EL (1986) Effect of
corticosteroids on the thymus of the fish Oryzias latipes. Dev Comp
Immunol, 10: 35-44.
Giannini SH (1990) Effects of UVB on infectious diseases. In: White JC
ed. Global atmospheric change and public health. Amsterdam, Elsevier
Science Publishers, pp 33-45.
Giannini SH (1992) Effects of UV radiation on cutaneous leishmaniasis.
Parasitol Today, 8: 44-48.
Gibson GG, Hubbard R, & Parke DV (1983) Immunotoxicology. London,
Academic Press.
Gilmartin WG, Delong RL, Smith AW, Sweeney JC, De Lappe BW, Risebrough
RW, Griner LA, Dailey MD, & Peakall DB (1976) Premature parturition in
the California sea lion. J Wildl Dis, 12: 104-115.
Gilmour MI & Selgrade MJK (1993) A comparison of the pulmonary
defenses against streptococcal infection in rats and mice following
O3exposures: A possible disease resistance in rats. Toxicol Appl
Pharmacol, 1233: 211-218.
Gilmour MI, Park P, Doefler D, & Selgrade MJK (1993) Factors which
influence the suppression of pulmonary anti-bacterial defenses in mice
exposed to ozone. Exp Lung Res, 9: 229-314.
Glaser R, Kiecolt-Glaser JK, Speicher CE, & Holliday JE (1985) Stress,
loneliness, and changes in herpesvirus latency. J Behav Med,
8: 249-250.
Glazier A, Tutschka PJ, Farmer ER, & Santos GW (1983) Graft-versus-
host disease in cyclosporin A-treated rats after syngeneic and
autologous bone marrow reconstitution J Exp Med, 158: 1-8.
Gleichmann E, Vohr, H-W, Stringer C, Nuyens J, & Gleichmann H (1989)
Testing the sensitization of T cell to chemicals. From murine graft-
versus-host (GVH) reactions to chemical-induced GVH-like immunological
diseases. In: Kammüller ME, Bloksma N, & Seinen W ed. Autoimmunity and
toxicology: Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers, pp 364-390.
Glickman LT, Shofer FS, Payton AJ, Laster LL, & Felsburg PJ (1988)
Survey of serum IgA, IgG, and IgM concentrations in a large beagle
population in which IgA deficiency had been identified. Am J Vet Res,
49: 1240-1245.
Goettsch W, Garssen J, De Gruijl FR, & Van Loveren H (1993) UV-B and
the immune system: A review with special emphasis on T cell-mediated
immunity. Thymus, 21: 93-114.
Goings SAJ, Kulle TJ, Bascom R, Sauder LR, Green DJ, Hebel R, &
Clements ML (1989) Effects of nitrogen dioxide exposure on
susceptibility to influenza A virus infection in healthy adults.
Am Rev Respir Dis, 139: 1075-1081.
Goldstein BD (1977) Hematotoxicity in humans. J Toxicol Environ
Health, 2(Suppl): 69-106.
Goldstein A, Lowney LI, & Pal BK (1971) Stereospecific and nonspecific
interactions of the morphine congener levorphanol in subcellular
fractions of mouse brain. Proc Natl Acad Sci USA, 68: 1742-1752.
Golemboski KA, Bloom SE, & Dietert RR (1992) Assessment of neonatal
avian inflammatory macrophage function following embryonic
cyclophospha-mide exposure. Int J Immunopharmacol, 14: 19-26.
Gonçalo S, Gonçalo M, Azenha A, Barros MA, Bastos AS, Brandao FM,
Faria A, Marques MSJ, Pecegueiro M, Rodrigues JB, Salgueiro E, &
Torres V (1992) Allergic contact dermatitis in children. Contact Derm,
26: 112.
Good RA & Lorenz E (1992) Nutrition and cellular immunity. Int J
Immunopharmacol, 14: 361-366.
Goodman JW, Peter-Fizaine FE, Shinpock SG, Hall EA, & Famie DJ (1989)
Immunologic and hematologic consequences in mice of exposure to ozone.
JEPTO, 9: 243-252.
Goonewardene IM & Murasko DM (1992) Changes in the immune response.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat. Washington DC, ILSI Press, pp 3-7.
Gopinath C, Prentice DE, & Lewis DJ (1987) The lymphoid system.
In: Atlas of experimental toxicologic pathology. Boston, MTP Press,
pp 122-137.
Gorodilova VV & Mandrik EV (1978) The use of some immunological
reactions for studying the immune response in persons presenting a
high oncological risk. Sov Med, 8: 50-53.
Graham JA & Gardner DE (1985) Immunotoxicity of air pollutants.
In: Dean JH, Luster MI, & Munson AE ed. Immunotoxicology and
Immunopharmacology. New York, Raven Press, pp 367-380.
Gray, D (1993) Immunological memory. Ann Rev Immunol, 11: 47-77.
Greenlee WF, Dold KM, Irons RD, & Osborne R (1985) Evidence for direct
action of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) on thymic
epithelium. Toxicol Appl Pharmacol, 79: 112--120.
Greenstein BD, Fitzpatrick FTA, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 112: 345-350.
Greiner DL, Shultz LD, Rossini AA, Mordes JP, Handler ES, & Rajan TV
(1991) Recapitulation of normal and abnormal BB rat immune system
development in SCID mouse/rat lymphohemopoietic chimeras. J Clin
Invest, 88: 717-719.
Guberski DL (1994) Diabetes-prone and diabetes-resistant BB rats:
Animal models of spontaneous and virally induced diabetes mellitus,
lymphocytic thyroiditis, and collagen-induced arthritis. ILAR News,
35: 29-37.
Gumbiner BM & Yamada KM ed. (1992) Cell-to-cell contact and
extracellular matrix. Curr Opin Cell Biol, 4: 757-874.
Gusev VA, Danilovskaya YV, Vatolkina OY, Lomonosova OS, & Velichkowsky
BT (1993) Effect of quartz and alumina dust on generation of
superoxide radicals and hydrogen peroxide by alveolar macrophages,
granulocytes, and monocytes. Br J Ind Med, 50: 732-735.
Gutierrez-Ramos JC, Andreu JL, Moreno de Alboran I, Rodriguez J,
Leonardo E, Kroemer G, Marcos MAR, & Martinez AC (1990) Insights into
autoimmunity: From classical models to current perspectives. Immunol
Rev, 118: 73-101.
Haas W, Pereira P, & Tonegawa S (1993) Gamma/delta cells. Ann Rev
Immunol, 11: 637-685.
Halliday GM, Cavanaugh LL, & Muller HK (1988) Antigen present in local
lymph node by cells from dimethylbenzanthracene-treated murine
epidermis activate suppressor cells. Cell Immunol, 109: 206-221.
Halprin KM, Comerford M, Presser SE, & Taylor JR (1981) Ultraviolet
light treatment delays contact sensitization to nitrogen mustard.
Br J Dermatol, 105: 71-76.
Hanglow AC, Ernst PB, & Bienenstock J (1990) Intraepithelial
leukocytes, murine. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed.
Hemopoietic system (ILSI Monograph). Berlin, Springer Verlag,
pp 315-322.
Hanley PJ, Hook JW, Raftos DA, Gooley AA, Trent R, & Raison RL (1992)
Hagfish humoral defense protein exhibits structural and functional
homology with mammalian complement components. Proc Natl Acad Sci USA,
89: 7910-7914.
Hansch GM (1992) The complement attack phase: Control of lysis and
non-lethal effects of C5b-9. Immunopharmacology, 24: 107-117.
Hara I (1985) Health status and PCBs in blood of workers exposed to
PCBs and of their children. Environ Health Perspectives, 59: 85-90.
Harriman W, Völk H, Defranoux N, & Wabl M (1993) Immunoglobulin class
switch recombination. Ann Rev Immunol, 11: 361-384.
Harrington W & Krupnick AJ (1985) Short-term nitrogen dioxide exposure
and acute respiratory disease in children. J Air Pollut Control Assoc,
35: 1061-1067.
Hartevelt MM, Bouwes Bavinck JN, Kootte AMM, Vermeer BJ, &
Vandenbroucke JP (1990) Incidence of skin cancer after renal
transplantation in the Netherlands. Transplantation, 49: 506-509.
Hartmann DP (1985) Immunological consequences of asbestos exposure.
Surv Immunol Res, 4: 65-68.
Hartmann DP, Georgian MM, Oghiso Y, & Kagan E (1984) Enhanced
interleukin activity following asbestos inhalation. Clin Exp Immunol,
55: 643-650.
Hasemann CA & Capra JD (1989) Immunoglobulins: Structure and function.
In: Paul WE ed. Fundamental immunology, 2nd Ed. New York, Raven Press,
pp 209-233.
Hashimoto K, Nakanishi T, & Kurosawa Y (1990) Isolation of carp genes
encoding major histocompatibility complex antigens. Proc Natl Acad Sci
USA, 87: 6863-6867.
Haynes RC & Murad F (1985) Adrenocortical steroids and their synthetic
analogs: Inhibitors of adrenocortical steroid biosynthesis. In: Gilman
AG, Goodman LS, Rall TW, & Murad F ed. Goodman and Gilman's
pharmacological basis of therapeutics. New York, Macmillan, p 1459.
Hedrick SM (1989) T Lymphocyte receptors. In: Paul WE ed. Fundamental
immunology, 2nd Ed. New York, Raven Press, pp 291-313.
Heijnen CJ & Kavelaars A (1991) The contribution of neuroendocrine
substances to the immune response. Neth J Med, 39: 281-294.
Heijnen CJ, Kavelaars A, & Ballieux RE (1991) ß-Endorphin: Cytokine
and neuropeptide. Immunol Rev, 119: 41-63.
Helle E, Olsson M, & Jensen S (1976) PCB levels correlated with
pathological changes in seal uteri. Ambio, 5: 261-263.
Hemphill FE, Kaeberle ML, & Buck WB (1971) Lead suppression of mouse
resistance to Salmonella typhimurium. Science, 172: 1031.
Henderson FW, Dubovy EJ, Harder S, Seal E, & Graham D (1988)
Experimental rhinovirus infection in human volunteers exposed to
ozone. Am Rev Respir Dis, 137: 124-127.
Henne T & Schmähl D (1985) Occurrence of second primary malignancies
in man: A second look. Cancer Treat Rev, 12: 77-94.
Henney CS, Kuriayashi K, Kern BE, & Gillis S (1981) Interleukin-2
augments natural killer cell activity. Nature, 291: 335-338.
Herberman RB & Ortaldo JR (1981) Natural killer cells: Their role in
defenses against disease. Science, 214: 24-30.
Herman A, Kappler JW, Marrack P, & Pullen AM (1991) Superantigens:
Mechanism of T-cell stimulation and role in immune responses. Ann Rev
Immunol, 9: 745-772.
Hersey P, Hasic E, Edwards A, Bradley M, Haran G, & McCarthy WH
(1983a) Immunological effects of solarium exposure. Lancet,
i: 545-548.
Hersey P, Haran G, Hasic E, & Edwards A (1983b) Alteration of T cell
subsets and induction of suppressor T cell activity in normal subjects
after exposure to sunlight. J Immunol, 31: 171-174.
Hess AD, Jones RJ, Morris LE, Noga SJ, Vogelsang GB, & Santos GW
(1992) Autologous graft-versus-host disease: A novel approach for
antitumor immunotherapy. Hum Immunol 34: 219-224.
Hinton DM (1992) Testing guidelines for evaluation of the immunotoxic
potential of direct food additives. Crit Rev Food Sci Nutr,
32: 173-190.
Ho M (1980) Role of specific cytotoxic lymphocytes in cellular
immunity against murine cytomegalovirus. Infect Immunol, 27: 767-776.
Ho MK & Springer TA (1984) Preparation and use of monoclonal
antimacrophage antibodies. Methods Enzymol, 108: 313-324.
Hoffman RE (1992) Recent studies of the human immunotoxic effects of
2,3,7,8-tetrachlorodibenzo- p-dioxin. In: Newcombe DS, Rose NR, &
Bloom JC ed. Clinical immunotoxicology. New York, Raven Press,
pp 339-347.
Hoffman RE, Stehr-Green PA, Webb KB, Evans G, Knutsen AP, Schramm WF,
Staake JL, Gibson BG, & Steinberg KK (1986) Health effects of long-
term exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin. J Am Med
Assoc, 255: 2031-2038.
Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR,
Heindell JJ, & Luster MI (1991) Perinatal thymocyte antigen expression
and postnatal immune development altered by gestational exposure to
tetrachlorodibenzo- p-dioxin (TCDD). Teratology, 44: 385-393.
Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, & Mo
JA (1990) Type II collagen autoimmunity in animals and provocations
leading to arthritis. Immunol Rev, 118: 193-232.
Holmes KL & Morse HC (1988) Murine hematopoetic cell surface antigen
expression. Immunol Today, 9: 344-350.
Holmes MA, Duffus WP, & Gorman NT (1989) Natural cytotoxicity in the
dog: Description of two new allogeneic tumour targets. Vet Immunol
Immunopathol, 23: 161-170.
Holsapple MP, Page DG, Shopp GM, & Bick PH (1984) Characterization of
the delayed hypersensitivity response to a protein antigen in the
mouse. Int J Immunopharmacol, 6: 399-405.
Holsapple MP, White KL, McCay JA, Bradley SG, & Munson AE (1988) An
immunotoxicological evaluation of 4,4'-thiobis-(6- t-butyl- m-cresol)
in female B6C3F1 mice. Fundam Appl Toxicol, 10: 701-716.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo- p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-251.
Holt PG & Keast D (1977) Environmentally induced changes in
immunological function: Acute and chronic effects of inhalation of
tobacco smoke and other atmospheric contaminants in man and
experimental animals. Bacteriol Rev, 41: 205-216.
Holt PG & Sedgwick JD (1987) Suppression of IgE responses following
inhalation of antigen. A natural homeostatic mechanism which limits
sensitization to aeroallergens. Immunol Today, 8: 14-15.
Hordvik I, Grimholt U, Fosse VM, Lie O, & Endresen C (1993) Cloning
and sequence analysis of cDNAs encoding the MHC class II ß chain in
Atlantic salmon (Salmo salar). Immunogenetics, 37: 437-441.
Hori S, Obana H, Kashimoto T, Otake T, Nishimura H, Ikegami N, Kunita
N, & Uda H (1982) Effect of polychlorinated biphenyls and
polychlorinated quaterphenyls in cynomolgus monkey (Macaca
fascicularis). Toxicology, 24: 123-139.
Hoshino A, Takenaka H, Mizukoshi O, Imanishi J, Kishida T, & Tovey M
(1983) Effect of anti-interferon serum of influenza virus infection in
mice. Antiviral Res, 3: 59-65.
Houben GF, Van Dokkum W, Van Loveren H, Penninks AH, Seinen W,
Spanhaak S, & Ockhuizen T (1992) Effects of caramel colour III on the
number of blood lymphocytes: A human study on caramel colour III
immunotoxicity and a comparison of the results with data from rat
studies. Food Chem Toxicol, 5: 427-430.
Houben GF, Penninks AH, Seinen W, Vos JG, & Van Loveren H (1993)
Immunotoxic effects of the color additive caramel color III: Immune
function studies in rats. Fundam Appl Toxicol, 20: 30-37.
House RV, Lauer LD, Murray MJ, & Dean JH (1987) Suppression of
T-helper cell function in mice following exposure to the carcinogen
7,12-dimethylbenz[ a]-anthracene and its restoration by
interleukin-2. Int J Immunopharmacol, 9: 89-97.
House RV, Pallardy MJ & Dean JH (1989) Suppression of murine
cytotoxic T-lymphocyte induction following exposure to
7,12-dimethylbenz[ a]anthracene: Dysfunction of antigen recognition.
Int J Immunopharmacol, 11: 207-215.
House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR,
& Dean JH (1990a) Examination of immune parameters and host
resistance mechanisms in B6C3F1 mice following adult exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. J Toxicol Environ Health,
31: 203-215.
House RV, Pearson FC, & Thomas PT (1990b) Selective potentiation of
host resistance in mice following treatment with pyrexol. Mol Biother,
2: 175-178.
Howard SK, Werner PR, & Sleigh SD (1980) Polybrominated biphenyl
toxicosis in swine: Effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol,
55: 146-153.
Hoyle PC & Cooper EC (1990) Nonclinical toxicity studies of antiviral
drugs indicated for the treatment of non-life threatening diseases:
Evaluation of drug toxicity prior to phase I clinical studies. Regul
Toxicol Pharmacol, 11: 81-89.
Hu HZ, Queirò MR, Tilanus MGJ, De Weger RA, & Schuurman H-J (1993)
Expression of T-cell receptor aand b variable genes in normal and
malignant human T cells. Br J Haematol, 84: 39-48.
Hughes & Nei (1993) Evolutionary relationships of the classes of major
histocompatibility complex genes. Immunogenetics, 93: 337-346.
Huiskamp R, Davids JAG, & Vos O (1983) Short- and long-term effects of
whole-body irradiation with fission neutrons or X rays on the thymus
in CBA mice. Radiat Res, 95: 370-381.
Huiskamp R, Davids JAG, & Van Ewijk W (1988) The effect of graded
doses of fission neutrons or X rays on the stromal compartment of the
thymus in mice. Radiat Res, 113: 25-39.
Hünig T, Wallny HJ, Hastley JK, Lawetzky A, & Tiefenthaler G (1989) A
monoclonal antibody to a constant determinant of the rat T cell
antigen receptor that induces T cell activation. Differential
reactivity with subsets of immature and mature T lymphocytes. J Exp
Med, 169: 73-86.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42 (IARC monographs on the evaluation
of carcinogenic risks to humans, Suppl 7). Lyon.
IARC (1990) Ciclosporin. In: Pharmaceutical drugs (IARC monographs on
the avaluation of carcinogenic risks to humans, Vol. 50). Lyon,
pp 77-114.
Imanishi J, Nomura H, Matsubara M, Kita M, Won S-J, Mizutani T, &
Kishida T (1980) Effect of polychlorinated biphenyl on viral
infections in mice. Infect Immunol, 29: 275-277.
Inada T & Mims CA (1985) Association of virulence of murine
cytomegalovirus with macrophage susceptibility and with virion-bound
non-neutralizing antibody. J Gen Virol, 66: 879-882.
Institoris L, Siroki O, Toth S, & Desi I (1992) Immunotoxic effects of
MTP-IP containing 60% methylparathion in mice. Hum Exp Toxicol,
11: 11-16.
Institoris L, Siroki O, & Desi I (1995) Immunotoxicity study of
repeated small doses of dimethoate and methylparathion administered to
rats over three generations. Hum Exp Toxicol, 14: 879-883.
Institoris L, Siroki O, Fekete K, & Desi I (in press)
Immunotoxicological investigation of repeated small doses of
dichlorvos (DDPV) in three generations of rats. Int J Environ Health
Res.
IPCS (1978) Environmental health criteria 6: Principles and methods
for evaluating the toxicity of chemicals. Part I. Geneva, WHO.
IPCS (1986) Immunotoxicology. Development of predictive testing for
determining the immunotoxic potential of chemicals. Report of a
technical review meeting. London, November 3-5, 1986. Geneva, WHO.
IPCS (1992) Environmental health criteria 141: Quality management for
chemical safety testing. Geneva, WHO.
IPCS (1994a) Environmental health criteria 155: Biomarkers and risk
assessment: Concepts and principles. Geneva, WHO.
IPCS (1994b) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO.
IPCS/Department of Health (1995) Proceedings of International
Programme on Chemical Safety and UK Department of Health International
Workshop on Environmental Immunotoxicology and Human Health, Oxford,
22-25 March, 1994. Hum Exp Toxicol, 14: 77-154.
Irons RD (1985) Histology of the immune system: Structure and
function. In: Dean J, Luster MI, Munson AE, & Amos H ed.
Immunotoxicology and immunopharmacology. New York, Raven Press,
pp 11-22.
Ishiguro H, Kobayashi K Suzuki M, Titani K, Tomonaga S, & Kurosawa Y
(1992) Isolation of a hagfish gene that encodes a complement
component. EMBO J, 11: 829-837.
Jacob CO, Van Der Meide PH, & McDevitt HO (1987) In vivo treatment of
(NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma-
interferon. J Exp Med, 166: 798-803.
Jakab GJ & Hmieleski RR (1988) Reduction of influenza virus
pathogenesis by exposure to 0.5 ppm ozone. J Toxicol Environ Health,
23: 455-472.
Janeway CA (1992) The T-cell receptor as a multicomponent signalling
machine: CD4/CD8 coreceptors and CD45 in T-cell activation. Ann Rev
Immunol, 10: 645-674.
Jaremin B (1983) Blast lymphocyte transformation (LTT), rosette
(E-RFC) and leukocyte migration inhibition (MIF) tests in persons
exposed to the action of lead during work. Report II. Bull Inst Mar
Trop Med (Gdynia), 34: 187-197.
Jeevan A & Kripke ML (1990) Alteration of the immune response to
Mycobacterium bovis BCG in mice exposed chronically to low doses of
UV radiation. Cell Immunol, 130: 32-41.
Jeevan A, Gilliam K, Heard H, & Kripke ML (1992) Effects of
ultraviolet radiation on the pathogenesis of Mycobacterium
lepraemurium infection in mice. Exp Dermatol, 1: 152-160
Jefferies WA (1988) Hemopoietic and T-lymphocyte marker antigens of
the rat characterized with monoclonal antibodies. In: Miyasaka M &
Trnka Z ed. Differentiation antigens in lymphohemopoietic tissues. New
York, Marcel Dekker, pp 173-247.
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunological
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachloro- p-dioxin. Br J Ind Med, 45: 701-704.
Jerne NK, Henry C, Nordin AA, Fun H, Koros MC, & Lefkovits I (1974)
Plaque forming cells: Methodology and theory. Transplant Rev,
18: 130-191.
Jessup JM, Hanna N, Palaszynski E, & Kripke ML (1978) Mechanisms of
depressed reactivity to dinitrochlorobenzene and ultraviolet-induced
tumours during ultraviolet carcinogenesis in BALB/c mice. Cell
Immunol, 38: 105-115.
Johnson KW, Holsapple MP, & Munson AE (1986) An immunotoxicological
evaluation of g-chlordane. Fundam Appl Toxicol, 6: 317.
Johnson KW, Munson AE, Kim DH, & Holsapple MP (1987) Role of
reactive metabolites in suppression of humoral immunity by
N-nitrosodimethylamine. J Pharmacol Exp Ther, 240: 847-855.
Johnson JD, Houchens DP, De Kluwe WM, Craig DK, & Fisher GL (1990)
Effects of mainstream and environmental tobacco smoke on the immune
system of animals and humans: A review. CRC Crit Rev Toxicol,
20: 369-395.
Joling P, Tielen FJ, Vaessen LMB, Hesse CJ, & Rozing J (1985)
Intrathymic differentiation in the rat. Adv Exp Med Biol,
186: 235-244.
Joling P (1987) Allografting and the T cell system. A multiparameter
analysis of rejection in the rat. PhD Thesis, Rotterdam, Erasmus
University.
Jones TC, Ward JM, Mohr U, & Hunt RD (1990) Hemopoietic system (ILSI
monograph). Berlin, Springer Verlag.
Kafafi SA, Afeefy HY, Ali AH, Said HK, & Kafafi AG (1993) Binding of
polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ
Health Perspectives, 101: 422-425.
Kagan E, Solomon A, Cochrane JC, Beissner EI, Gluckman J, Rocks PH, &
Webster I (1977a) Immunological studies of patients with asbestosis.
I. Studies of cell-mediated immunity. Clin Exp Immunol, 28: 261-267.
Kagan E, Solomon A, Cochrane JC, Kuba P, Rocks PH, & Webster I (1977b)
Immunological studies of patients with asbestosis. II. Studies of
circulating lymphoid numbers and humoral immunity. Clin Exp Immunol,
28: 268-275.
Kagan E, Jacobson RJ, Yeung KY, Haidak DJ, & Nachnani GH (1979)
Asbestos-associated neoplasms of B cell lineage. Am J Med,
67: 325-330.
Kahan BD (1989) Cyclosporine. New Engl J Med, 321: 1725-1738.
Kalimo K, Koulu L, & Jansen CT (1983) UV exposure and
dinitrochlorobenzene (DNCB). Arch Dermatol Res, 275: 374-378.
Kalpazanov Y, Stamenova M, & Kurchatova G (1976) Air pollution and the
1974 - 1975 influenza epidemic in Sofia. Environ Res, 12: 1-8.
Kamel OW, Van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA,
Montgomery PG, Warnke RA, & Dorfman RF (1993) Reversible lymphoma
associated with Epstein-Barr virus occurring during methotrexate
therapy for rheumatoid arthritis and dermatomyositis. New Engl J Med,
328: 1317-1321.
Kammüller ME & Bloksma N (1994) Drug-induced autoimmunity. In: Dean J,
Luster MI, Munson AE, & Kimber I ed. Immunotoxicology and
immunopharmacology. New York, Raven Press, pp 573-588.
Kammüller ME, Bloksma N, & Seinen W (1989) Autoimmunity and
toxicology. Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers.
Kampinga J, Berges S, Boyd RL, Brekelmans P, Colic M, Van Ewijk W,
Kendall M, Ladyman H, Nieuwenhuis P, Ritter MA, Schuurman HJ, &
Tournefier A (1989) Thymic epithelial antibodies: Immunohistological
analysis and introduction of nomenclature. Thymus, 13: 165-173.
Kannan K, Singh KP, Goel SK, & Shanker R (1985) Effect of
2,5-hexanediol on immunocompetence of mice. Environ Res, 36: 14.
Kannan K, Tanabe S, Borrell A, Aguilar A, Focardi S, & Tatsukawa R
(1993) Isomer-specific analysis and toxic evaluation of
polychlorinated biphenyls in striped dolphins affected by an epizootic
in the western Mediterranean Sea. Arch Environ Contam Toxicol,
25: 227-233.
Kasahara M, Vazquez M, Sato K, McKinney EC, & Flajnik MF (1992)
Evolution of the major histocompatibility complex: Isolation of class
II A cDNA clones from the cartilagenous fish. Proc Natl Acad Sci USA,
89: 6688-6692.
Kasai M, Iwamari M, Nagai T, Okumura K, & Tada T (1980) A glycolipid
on the surface of mouse natural killer cells. Eur J Immunol,
10: 175-180.
Kasai M, Yoneda T, Habu S, Maruyana Y, Okumura K, & Tokunaga T (1981)
In vivo effect of anti-asialo GM-1 antibody on natural killer cell
activity. Nature, 291: 334-335.
Kasl SV, Evans AS, & Neiderman JC (1979) Psychosocial risk factors
in the development of infectious mononucleosis. Psychosom Med,
41: 445-466.
Kateley JR & Bazzell SJ (1978) Immunological studies in cattle exposed
to polybrominated biphenyls. Environ Health Perspectives, 23: 75-82.
Kawabata TT & White KL (1987) Suppression of the in vitro humoral
immune response of mouse splenocytes by benzo( a)pyrene metabolites
and inhibition of benzo( a)pyrene-induced immunosuppression by
a-naphthoflavone. Cancer Res, 47: 2317-2322.
Kawabata TT & White KL (1990) Effects of naphthalene and naphthalene
metabolites on the in vitro humoral immune response. J Toxicol
Environ Health, 30: 53-67.
Keck G (1981) Effets de la contamination par les polychlorobiphenyles
(PCB) sur le developpement de la tumeur d'Ehrlich chez la souris
Swiss. Toxicol Eur Res, 3: 229-236.
Kendall MD (1980) The occurrence of erythropoiesis in the thymus of
the bank vole (Clethrionomys glareolus). Cell Tissue Res,
212: 307-314.
Kendall MD (1991) Functional anatomy of the thymic microenvironment.
J Anat, 177: 1-29.
Kendall MD & Al-Shawaf AA (1991) Innervation of the thymus gland.
Brain Behav Immunol, 5: 9-28.
Kendall MD & Frazier JA (1979) Ultrastructural studies on
erythropoiesis in the avian thymus. I. Description of cell types. Cell
Tissue Res, 199: 37-61.
Kendall MD & Singh J (1980) The presence of erythroid cells in the
thymus gland of man. J Anat, 130: 183-189.
Kendall MD & Ward P (1974) Erythropoiesis in an avian thymus. Nature,
249: 3 66-367.
Kendall MD, Fitzpatrick FTA, Greenstein BD, Khoylou F, Safieh B, &
Hamblin A (1990) Reversal of ageing changes in the thymus of rats by
chemical or surgical castration. Cell Tissue Res, 261: 555-564.
Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V,
Huelskamp AM, & Davidson NE (1994) Phase I trial of interferon gamma
to potentiate cyclosporine-induced graft-versus-host disease in women
undergoing autologous bone marrow transplantation for breast cancer.
J Clin Oncol, 12: 249-257.
Kerkvliet NI & Baecher-Steppan L (1988) Suppression of allograft
immunity by 3,4,5,3 ',4 ',5 '-hexachlorobiphenyl. I. Effects of
exposure on tumor rejection and cytotoxic T cell activity.
Immunopharmacology, 16: 1-12.
Kerkvliet NI & Brauner JA (1987) Mechanisms of 1,2,3,4,6,7,8-
heptachlorodi-benzo- p-dioxin (HpCDD)-induced humoral suppression:
Evidence of primary defect in T-cell regulation. Toxicol Appl
Pharmacol, 87: 18-31.
Kerkvliet NI & Burleson GR (1994) Immunotoxicity of TCDD and related
halogenated aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE,
& Kimber I ed. Immunotoxicity and immunopharmacology, 2nd Ed. New
York, Raven Press, pp 97-121.
Kerkvliet NI & Kimeldorf DJ (1977) Antitumor activity of a
polychlorinated biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J Natl Cancer Inst, 59: 951-955.
Kerkvliet NI, Steppan LB, Smith BB, Youngberg JA, Henderson MC, &
Buhler DR (1990) Role of the Ah locus in suppression of cytotoxic T
lymphocyte (CTL) activity by halogenated aromatic hydrocarbons (PCBs
and TCDD): Structure-activity relationships and effects in C57Bl/6
mice. Fundam Appl Toxicol, 14: 532-541.
Khan MF & Gupta GS (1991) Cellular and biochemical indices of
bronchoalveolar lavage for detection of lung injury following insult
by airborne toxicants. Toxicol Lett, 58: 239-255
Khangarot BS & Tripathi DM (1991) Changes in humoral and cell-mediated
immune responses and in skin and respiratory surfaces of catfish,
Saccobranchus fossilis, following copper exposure. Ecotox Environ
Saf, 22: 291-308.
Khansari DN, Murgo AJ, & Faith RE (1990) Effects of stress on the
immune system. Immunol Today, 11: 170-175.
Kiecolt-Glaser JK & Glaser R (1986) Psychological influences on
immunity. Psychonomatics, 27: 621-624.
Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday
JE, & Speicher CE (1986) Modulation of cellular immunity in medical
students. J Behav Med, 9: 5-21.
Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, &
Glaser R (1987) Marital quality, marital disruption, and immune
function. Psychosom Med, 49: 13-33.
Kim JH (1989) Infection and cyclosporin. Rev Infect Dis, 11: 677-690.
Kim DH, Yang KH, Johnson KW, & Holsapple MP (1987) Suppression of
in vitro antibody production by dimethylnitrosamine in mixed
cultures of mouse primary hepatocytes and mouse splenocytes. Toxicol
Appl Pharmacol, 87: 32-42.
Kimber I & Cumberbatch M (1992) Dendritic cells and cutaneous immune
responses to chemical allergens. Toxicol Appl Pharmacol, 117: 137-146.
Kimber I, Stonard MD, Gidlow DA, & Niewola Z (1986) Influence of
chronic low-level exposure to lead on plasma immunoglobulin
concentration and cellular immune function in man. Int Arch Occup
Environ Health, 57: 117-125.
Kimbrough RD (1987) Human health effects of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls (PBBs). Ann Rev Pharmacol Toxicol,
27: 87-11.
Kimchi A (1992) Cytokine triggered molecular pathways that control
cell cycle arrest. J Cell Biochem, 50: 1-9.
Kimmel CA (1990) Quantitative approaches to human risk assessment for
noncancer health effects. Neurotoxicology, 11: 189-198.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Kincade PW (1992) Cell interaction molecules and cytokines which
participate in B lymphopoiesis. Baillieres Clin Haematol, 5: 575-598.
King AG, Landbreth KS, & Wierda D (1987) Hydroquinone inhibits bone
marrow pre-B cell maturation in vitro. Mol Pharmacol, 32: 807-812.
Kingsmore SF, Hall BD, Alen NB, Rice JR, & Caldwell DS (1992)
Association of methotrexate, rheumatoid arthritis and lymphoma: Report
of 2 cases and literature review. J Rheumatol, 19: 1462-1465.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Klareskog L & Olsson T (1990) Autoimmunity to collagen II and myelin
basic protein: Comparative studies in humans and rodents. Immunol Rev,
118: 285-310.
Klein J (1986) Natural history of the major histocompatibility
complex. New York, John Wiley and Sons.
Klein J (1990) Immunology. Oxford, Blackwell Scientific Publications.
Kleinbeck ML, Hites MJ, Loker JL, Halliwell RE,& Lee KW (1989) Enzyme-
linked immunosorbent assay for measurement of allergen-specific IgE
antibodies in canine serum. Am J Vet Res, 50: 1831-1839.
Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, & Von dem
Borne AEGK ed. (1989) Leucocyte typing IV: White cell differentiation
antigens. Oxford, Oxford University Press.
Knight R, Sarlis N, & Stephanou A (1992) Interleukins and
neurohormones: A common language. Postgrad Med J, 68: 603-605.
Kocks C & Rajewski K (1989) Stable expression and somatic
hypermutation of antibody V regions in B cell developmental pathways.
Ann Rev Immunol, 7: 537-559.
Koller LD (1977) Enhanced polychlorinated biphenyl lesions in Moloney
leukemia virus-infected mice. Clin Toxicol, 11: 107-116.
Koller LD (1980) Immunotoxicology of heavy metals. Int J
Immunopharmacol, 2: 269-279.
Koller LD (1990) The immunotoxic effects of lead in lead-exposed
laboratory animals. Ann NY Acad Sci, 587: 160-167.
Koller LD & Kovacic S (1974) Decreased antibody formation in mice
exposed to lead. Nature, 250: 148-150.
Koller LD & Roan JG (1977) Effects of lead and cadmium on mouse
peritoneal macrophages. J Reticuloendothel Soc, 21: 7.
Koller LD & Roan JG (1980) Effects of lead, cadmium and methylmercury
on immunological memory. J Reticuloendothel Soc, 21: 7-12.
Koller LD & Thigpen JE (1973) Biphenyl-exposed rabbits. Am J Vet Res,
34: 1605-1606.
Kongshavn PAL, Sadarangani C, & Skamene E (1980) Cellular mechanisms
of genetically-determined resistance to Listeria monocytogenes.
In: Skamene E ed. Genetic control of natural resistance. New York,
Academic Press, pp 149-163.
Koren HS & Blomberg PA (in press) Respiratory responses of asthmatics
to ozone. Int Arch Allergy.
Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo
WJ, Becker S, House DE, McDonnel WF, & Bromberg PA (1989) Ozone-
induced inflammation in the lower airways of human subjects. Am Rev
Respir Dis, 139: 407-415.
Koren HS, Devlin RB, & Becker S (1994) Ozone-induced inflammatory
response in pulmonary cells. In: Schook LB & Laskin DL ed. Xenobiotics
and inflammation: Roles of cytokines and growth factors. New York,
Academic Press, pp 249-281.
Krajnc EI, Wester PW, Loeber JG, Van Leeuwen FXR, Vos JG, Vaessen
HAMG, & Van der Heijden CA (1984) Toxicity of bis(tri-n-butyltin)oxide
in the rat. I. Short-term effects on general parameters and on the
endocrine and lymphoid systems. Toxicol Appl Pharmacol, 75: 363-386.
Krajnc-Franken MAM, Van Loveren H, Schuurman HJ, & Vos JG (1990) The
immune system as a target for toxicity. A tiered approach of testing,
with special emphasis on histopathology. In: Dayan AD, Hertel RF,
Heseltine E, Kazantis G, Smith EM, & Van der Venne MT ed.
Immunotoxicity of metals and immunotoxicology. New York, Plenum Press,
pp 241-264.
Kramer CM, Johnson KW, Dooley RK, & Holsapple MP (1987) 2,3,7,8-
Tetrachlorodibenzo- p-dioxin (TCDD) enhances antibody production and
protein kinase activity in murine B cells. Biochem Biophys Res Commun,
145: 25-33.
Kripke ML (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J Natl Cancer Inst, 53: 1333-1336.
Kripke ML, Thorn RM, Lill PH, Civin CI, Pazmino NH, & Fisher MS (1979)
Further characterization of immunological unresponsiveness induced in
mice by ultraviolet radiation. Transplantation, 28: 212-217.
Kripke ML, Cox PA, Alas LG, & Yarosh DB (1992) Pyrimidine dimers in
DNA initiate systemic immunosuppression in UV-irradiated mice. Proc
Natl Acad Sci USA, 89: 7516-7520.
Krishna VR, Fouant MM, & Bradley SG (1989) Effects of pyran copolymer
on host resistance of mice to Plasmodium yoelii. J Parasitol,
75: 405-410.
Kroese FGM, Timens W, & Nieuwenhuis P (1990) Germinal center reaction
and B lymphocytes: Morphology and function. In: Grundmann E & Vollmer
E ed. Reaction patterns of the lymph node. Part 1. Cell types and
functions. Berlin, Springer Verlag, pp 103-148.
Kroplien U, Rosdorfer J, van der Greef J, Long RC, & Goldstein JH
(1985) 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)imidazole: Detection
in commercial caramel color III and preparation by a model browning
reaction. J Org Chem, 50: 1131-1133.
Krowka JF, Sarin S, Namikawa R, McCune JM, & Kaneshima H (1991) Human
T cells in the SCID-hu mouse are phenotypically normal and
functionally competent. J Immunol, 146: 3751-3756.
Kubota M, Kagamimori S, Yokoyama K, & Okada A (1985) Reduced killer
cell activity of lymphocytes from patients with asbestosis. Br J Ind
Med, 42: 276-280.
Kunita N, Hori S, Obana H, Otake T, Nishimura H, Kashimoto T, &
Ikegami N (1985) Biological effect of PCBS, PCQs and PCDFs present in
the oil causing yusho and yu-cheng. Environ Health Perspectives,
59: 79-84.
Kunkel SL, Chensue SW, Strieter RM, Lynch JP, & Remick DG (1989)
Cellular and molecular aspects of granulomatous inflammation. Am J
Respir Cell Mol Biol, 1: 439-447.
Kuper CF, Beems RB, & Hollanders VMH (1990a) Development and aging;
thymus, rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic
system (ILSI Monograph). Berlin, Springer Verlag, pp 257-263.
Kuper CF, Hameleers DMH, Bruijntjes JP, Van der Ven I, Biewenga J, &
Sminia T (1990b) Lymphoid and non-lymphoid cells in nasal-associated
lymphoid tissue (NALT) in the rat. An immuno- and enzymehistochemical
study. Cell Tissue Res, 259: 371-377.
Kuper CF, Beems RB, Bruijntjes JP, Schuurman H-J, & Vos JG (1992a)
Normal development, growth and aging of the thymus. In: Mohr U,
Dungworth DL, & Capen CC ed. Pathobiology of the aging rat, Vol. 1.
Washington DC, ILSI Press, pp 25-49.
Kuper CF, Koornstra PJ, Hameleers DMH, Biewenga J, Spit BJ,
Duijvestijn AM, Van Breda Vriesman PJC, & Sminia T (1992b) The role of
nasopharyngeal lymphoid tissue. Immunol Today, 13: 219-224.
Kuper CF, Schuurman H-J, & Vos JG (1995) Pathology in immunotoxicology.
In: Burleson G, Munson A, & Dean J ed. Modern methods in immuno-
toxicology. New York, John Wiley & Sons, pp 397-436.
Kyle RA (1984) Second malignancies associated with chemotherapy. In:
Perry MC & Yarbro JW ed. Toxicity of chemotherapy. New York, Grune &
Stratton, pp 479-506.
Labarca C & Paigen KA (1980) A simple, rapid, and sensitive DNA assay
procedure. Anal Biochem, 102: 344-352.
Ladics GS, Kawabata TT, & White KL (1991) Suppression of the
in vitro humoral immune response of mouse splenocytes by
7,12-dimethylbenz[ a]anthracene metabolites and inhibition of
immunosuppression by a-naphthflavone. Toxicol Appl Pharmacol,
110: 31-44.
Ladics GS, Kawabata TT, Munson AE, & White KL (1992) Metabolism of
benzo(a)pyrene by murine splenic cell types. Toxicol Appl Pharmacol,
116: 248-257.
Ladics GS, Smith C, Heaps K, Elliott GS, Slone TW, & Loveless SE
(1995) Possible incorporation of an immunotoxicological functional
assay for assessing humoral immunity for hazard identification
purposes in rats on standard toxicology study. Toxicology,
96: 225-238.
LaGrange PH, Mackaness GB, & Miller TE (1974) Influence of dose and
route of antigen injection on the immunological induction of T cells.
J Exp Med, 139: 528-542.
Laman JD, Van den Eertwegh AJM, Claassen E, & Van Rooijen N (1992)
Cell-cell interactions: In situ studies of splenic humoral immune
responses. In: Fornusek L & Vetvicka V ed. Immune system accessory
cells. Boca Raton, Florida, CRC Press, pp 201-231.
Lambris JD (1993) The chemistry, biology and phylogeny of C3.
In: Cruse JM & Lewis RE ed. Complement today, Vol. 1. Basel, Karger,
pp 16-45.
Lampeter EF, Signore A, Gale EAM, & Pozzilli P (1989) Lesson from the
NOD mouse for the pathogenesis and immunotherapy of human type 1
(insulin-dependent) diabetes mellitus. Diabetologia, 32: 703-708.
Lang D, Meier KL, & Luster MI (1993) Comparative effects of
immunotoxic chemicals on in vitro proliferative responses of human
and rodent lymphocytes. Fundam Appl Toxicol, 21: 535-545.
Lanier LL, Benike CJ, Phillips JH, & Engleman EG (1985) Recombinant
interleukin-2 enhanced natural killer cell-mediated cytotoxicity in
human lymphocyte subpopulations expressing the leu-7 and leu-11
antigens. J Immunol, 2: 794-801.
Larrick JW & Wright SC (1992) Native cytokine antagonists. Baillieres
Clin Haematol, 5: 681-702.
Lasky LA (1992) Selectins: Interpreters of cell-specific carbohydrate
information during inflammation. Science, 258: 964-969.
Lawlor GJ Jr & Fischer TJ ed. (1988) Manual of allergy and immunology.
Diagnosis and therapy, 2nd Ed. Boston, Little, Brown & Co.
Lawrence DH (1981) In vivo and in vitro effects of lead on humoral
and cell-mediated immunity. Infect Immun, 31: 136-143.
Lawrence DA (1985) Immunotoxicity of heavy metals. In: Dean JH, Luster
MI, Munson AE, & Amos H ed. Immunotoxicology and immunopharmacology.
New York, Raven Press, p 341.
Lawrence D, Mudzinski S, Rudofsky U, & Warner A (1987) Mechanism of
metal-induced immunotoxicity. In: Berlin A, Dean J, Draper MH, Smith
EMB, & Spreafico F ed. Immunotoxicology. Dordrecht, Martinus Nijhoff,
pp 293-307.
Lea T, Stelen K, & Stormer FC (1989) Mechanism of ochratoxin A-induced
immunosuppression. Mycopatholgia, 107: 153-159.
Lebish IJ, Hurvitz A, Lewis RM, Cramer DV, & Krakowka S (1986)
Immunopathology of laboratory animals. Toxicol Pathol, 14: 129-134.
Leiter EH (1993) The NOD mouse: A model for analyzing the interplay
between heredity and environment in development of autoimmune disease.
ILAR News, 35: 4-14.
Lerman SP & Weidanz WP (1970) The effect of cyclophosphamide on the
ontogeny of the humoral immune response in chickens. J Immunol,
105: 614-619.
LeVier DG, Brown RD, McCay JA, Fuchs BA, Harris LS, & Munson AE (1993)
Hepatic and splenic phagocytosis in female B6C3F1 mice implanted with
morphine sulfate pellets. J Pharmacol Exp Ther, 267: 357-363.
Levy SM, Herberman RB, Lee J, Whiteside T, Beadle M, Heiden L ,&
Simons A (1991) Persistently low natural killer cell activity, age,
and environmental stress as predictors of infectious morbidity. Nat
Immun Cell Growth Regul, 10: 289-307.
Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, & Bekesi JG (1986)
High frequency of immune dysfunctions in asbestos workers and in
patients with malignant mesothelioma. J Clin Immunol, 6: 225-233.
Li FY & Richters A (1991) Effects of 0.7 ppm ozone exposure on
thymocytes: In vivo and in vitro studies. Inhal Toxicol, 3: 61-71.
Lichtman MA (1981) The ultrastructure of the hemopoietic environment
of the marrow: A review. Exp Hematol, 9: 391-410.
Like AA, Butler L, Williams M, Appel MC, Weringer EJ, & Rossini AA
(1982) Spontaneous autoimmune diabetes mellitus in the BB rat.
Diabetes, 31(Suppl 1): 7-13.
Litman GW, Rast JP, Shamblott MJ, Haire RN, Hulst M, Roess W, Litman
RT, Hind-Frey KR, Zilch A, & Amemiya CT (1993) Phylogenetic
diversification of immunoglobulin genes and the antibody repertoire.
Mol Biol Evol, 10: 60-72.
Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, & MacLennan ICM
(1989) Mechanism of antigen-driven selection in germinal centres.
Nature, 342: 929-931.
Liu Y-J, Johnson GD, Gordon J, & MacLennan ICM (1992) Germinal centres
in T-cell-dependent antibody responses. Immunol Today, 13: 17-21.
Loo JCK, Tryphonas H, Jordan N, Brien R, Karpinski KF, & Arnold DL
(1989) Effects of Aroclor 1254 on hydrocortisone levels in adult
rhesus monkeys (Macaca mulatta). Bull Environ Contam Toxicol,
43: 667-669.
Loose LD, Pittman KA, Benitz K-F, & Silkworth JB (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J Reticuloendothel Soc, 22: 253-271.
Loose LD, Silkworth JB, Pittman KA, Benitz K-F, & Mueller W (1978)
Impaired host resistance to endotoxin and malaria in polychlorinated
biphenyl- and hexachlorobenzene-treated mice. Infect Immun, 20: 30-35.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspectives, 39: 79-91.
Losco P (1992) Normal development, growth and aging of the spleen.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat, Vol. 1. Washington DC, ILSI Press, pp 75-95.
Losco P & Harleman H (1992) Normal development, growth, and aging of
the lymph node. In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology
of the aging rat, Vol. 1. Washington DC, ILSI Press, pp 49-75.
Love RJ, Ogilvie BM, & McLaren DJ (1976) The immune mechanism which
expels the intestinal stage of Trichinella spiralis from rats.
Immunology, 30: 7-13.
Lu YC & Wu YC (1985) Clinical findings and immunological abnormalities
in yu-cheng patients. Environ Health Perspectives, 59: 17-29.
Lubet RA, Lemaire BN, Avery D, & Kouri RE (1986) Induction of
immunotoxicity in mice by polyhalogenated biphenyls. Arch Toxicol,
59: 71-77.
Luckas B, Vetter W, Fischer P, Heidemann G, & Plötz J (1990)
Characteristic chlorinated hydrocarbon patterns in the blubber of
seals from different marine regions. Chemosphere, 21: 13-19.
Luebke RW, Luster MI, Dean JH, & Hayes HT (1984) Altered host
resistance to Trichinella spiralis infection following subchronic
exposure to diethylstilbestrol. Int J Immunopharmacol, 6: 609-617.
Luebke RW, Andrews DL, Copeland CB, Riddle MM, Rogers RR, & Smialowicz
RJ (1991) Host resistance to murine malaria in mice exposed to the
adenosine deaminase inhibitor, 2'-deoxycoformycin. Int J
Immunopharmacol, 13: 987-997.
Luebke RW, Copeland CB, Diliberto JJ, Akubue PI, Andrews DL,
Riddle MM, Williams WC, & Birnbaum LS (1994) Assessment of host
resistance to Trichinella spiralis in mice following preinfection
exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol, 125: 7-16.
Luebke RW, Copeland CB, & Andrews DL (1995) Host resistance
to Trichinella spiralis infection in rats exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Fundam Appl Toxicol,
24: 285-289.
Lukic ML, Popeskovic L, & Jankovic BD (1973) Potentiation of immune
responsiveness in rats treated with DDT. Fed Proc, 32: 1037.
Lundberg K, Dencker L, & Gronvik KO (1992) 2,3,7,8-Tetrachlorodibenzo-
p-dioxin (TCDD) inhibits the activation of antigen-specific T cells
in mice. Int J Immunopharmacol, 14: 699-705.
Lunn JE Knowelden J, & Handyside AJ (1967) Patterns of respiratory
illness in Sheffield infant schoolchildren. Br J Prev Soc Med,
21: 7-16.
Luo YD, Patel MK, Wiederholt MD, & Ou DW (1992) Effects of
cannabinoids and cocaine on the mitogen-induced transformations of
lymphocytes of human and mouse origins. Int J Immunopharmacol,
14: 49-56.
Luster MI, Faith RE, & Moore JA (1978) Depression of humoral immunity
in rats following chronic developmental lead exposure. J Environ
Pathol Toxicol, 1: 397-402.
Luster MI, Hong LH, Boorman GH, Clark G, Hayes HT, Greenlee WF, Dold
K, & Tucker AN (1985a) Acute myelotoxic responses in mice exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Toxicol Appl Pharmacol,
81: 151-165.
Luster MI, Tucker AN, Hayes HT, Pung OJ, Burka T, McMillan R, & Eling
T (1985b) Immunosuppressive effects of benzidine in mice: Evidence of
alterations in arachidonic acid metabolism. J Immunol, 135: 2754-2761.
Luster MI, Tucker AN, Germolec DR, Silver MT, Thomas PT, Vore SJ, &
Bucher JR (1986) Immunotoxicity studies in mice exposed to methyl
isocyanate. Toxicol Appl Pharmacol, 86: 140-144.
Luster MI, Germolec DR, Burleson GR, Jameson CW, Ackerman MF, Lamm KR,
& Hayes HT (1987) Selective immunosuppression in mice of natural
killer cell activity by ochratoxin A. Cancer Res, 47: 2259-2263.
Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL,
Lauer LD, Germolec DR, Rosenthal GJ, & Dean JH (1988) Development of a
testing battery to assess chemical-induced immunotoxicity: National
Toxicology Program's guidelines for immunotoxicity evaluation in mice.
Fundam Appl Toxicol, 10: 2-19.
Luster MI, Ackermann MF, Germolec DR, & Rosenthal GJ (1989)
Perturbations of the immune system by xenobiotics. Environ Health
Perspectives, 81: 157-162.
Luster MI, Portier C, Pait DG, White KL, Gennings C, Munson AE, &
Rosenthal GJ (1992) Risk assessment in immunotoxicology I. Sensitivity
and predictability of immune tests. Fundam Appl Toxicol., 18: 200-210.
Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E,
Blaylock BL, Pollock P, Kouchi Y, Craig W, White KL, Munson AE, &
Comment CE (1993) Risk assessment in immunotoxicology. II.
Relationship between immune and host resistance tests. Fundam Appl
Toxicol, 21: 71-82.
Lyte M & Bick PH (1986) Modulation of interleukin-1 production by
macrophages following benzo( a)pyrene exposure. Int J
Immunopharmacol, 8: 377-381.
Mackaness GB (1969) The influence of immunologically committed
lymphoid cells on macrophage activity in vivo. J Exp Med,
129: 973-992.
Makker SP & Aikawa M (1979) Mesangial glomerulonephropathy with
deposition of IgG, IgM, and C3 induced by mercuric chloride. Lab
Invest, 41: 45-49.
Malins DC, McClain BB, Landahl JT, Meyers MS, Krahn MM, Brow DW, Chan
SL, & Roubal WT (1988) Neoplasia and other diseases in fish in
relation to toxic chemicals: An overview. Aquat Toxicol, 11: 43-67.
Malkovsky M, Loveland B, North M, Asherson GL, Gao L, Word P, & Fiers
W (1987) Recombinant Interleukin-2 directly augments the cytotoxicity
of human monocytes. Nature, 325: 262-265.
Mandell LA (1990) Infections in the immunocompromised host. J Intern
Med Res, 18: 177-190.
Manischewitz JE & Quinnan GV (1980) Antivirus antibody-dependent cell-
mediated immunity during murine cytomegalovirus infection. Infect
Immun, 29: 1050-1054.
Mann DD, Buening GM, Hook B, & Osweiler GD (1983) Effects of T-2 toxin
on bovine serum proteins. Am J Vet Res, 44: 1751-1759.
Manson-Smith DF, Bruce RG, & Parrott DMV (1979) Villous atrophy and
expul-sion of intestinal Trichinella spiralis are mediated by T
cells. Cell Immunol, 47: 285-292.
Marchalonis JJ, Hohman VS, Thomas C, & Schluter SF (1993) Antibody
production in sharks and humans: A role for natural antibodies. Dev
Comp Immunol, 17: 41-53.
Marker SC, Howard RJ, Simmons RL, Kalis JM, Conelly DP, Najarian JS, &
Balfour HH (1981) Cytomegalovirus infection: A quantitative
prospective study of 320 consecutive renal transplants. Surgery,
89: 660-671.
Marsh JA, Dietert RR, & Combs GF (1981) Influence of dietary selenium
and vitamin E on the humoral immune response of the chick. Proc Soc
Exp Biol Med, 166: 228-236.
Martineau D, Béland P, Desjardins C, & Lagacé A (1987) Levels of
organochlorine chemicals in tissues of beluga whales (Delphinapterus
leucas) from the St Lawrence Estuary, Québec, Canada. Arch Environ
Contam Toxicol, 16: 137-147.
Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird FF, Travis W,
Suffredini AF, Deyton l, Kovacs JA, Falloon J, Davey R, Polis M,
Metcalf J, Baseler M, Wesley R, Gill VJ, Fauci AS, & Lane HC (1989)
CD4 counts as predictors of opportunistic pneumonias in human
immunodeficiency virus (HIV) infection. Ann Intern Med, 111: 223-231.
Mathews SE, Warinner JE, & Weeks BA (1990) Assays of immune function
in fish macrophages. In: Stolen JS, Fletcher TC, Anderson DP, Roberson
BS, & van Muiswinkel WB ed. Techniques in fish immunology, Vol. 1.
Fair Haven, New Jersey, SOS Publications, pp 155-163.
Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, &
Katagiri T (1990) A new allele of the lpr locus, lprcg, that
complements the gld gene in induction of lymphadenopathy in the mouse.
J Exp Med, 171: 519-531.
Mayani H, Guilbert LJ, & Janowska-Wieczorek A (1992) Biology of the
hemopoietic microenvironment. Eur J Haematol, 49: 225-233.
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, &
Rattner BA (1992) Physiological and nonspecific biomarkers. In:
Biomarkers, biochemical, physiological and histological markers of
anthropogenic stress. London, Lewis Publishers, pp 5-85.
McCabe MJ (1994) Mechanisms and consequences of immunomodulation by
lead. In: Dean JH, Luster MI, Munson AE, & Kimber I ed. Immunotoxicity
and immunopharmacology, 2nd Ed. New York, Raven Press, pp 143-162.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo- p-dioxin kills immature thymocytes by
Ca2+-mediated endonuclease activation. Science, 242: 256-259.
McConkey DJ, Orrenius S, & Jondal M (1990) Cellular signalling in
programmed cell death (apoptosis). Immunol Today, 11: 120-121.
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, & Weissman
IL (1988) The SCID-hu mouse: Murine model for the analysis of human
hematolymphoid differentiation and function. Science, 241: 1632-1639.
McDonald HR & Lees RK (1990) Programmed death of autoreactive
thymocytes. Nature, 343: 642-644.
McGregor DD, Hahn HH, & Mackaness GB (1973) The mediator of cellular
immunity. V. Development of cellular resistance to infection in
thymectomized irradiated rats. Cell Immunol, 6: 186-199.
Melia RJW, Du V, Florey C, Altman DG, & Swan AV (1977) Association
between gas cooking and respiratory disease in children. Br Med J,
ii: 149-152.
Mellert J, Hopt UT, Erath F, & Holzer H (1989) Differential effects of
azathioprine (aza), cyclosporin A (CSA) and dexamethasone (DEXA) on
lymphokine mediated inflammation (LMI) in rejecting allografts.
Transplant Proc, 21: 98-99.
Meuer SC, Fitzgerald KA, Hussey RE, Hodgdon JC, Schlossman SF, &
Reinherz EL (1983) Clonotypic structures involved in antigen-specific
human T cell function. Relationship to the T3 molecular complex. J Exp
Med, 157: 705-719.
Miller K (1992) Mineral dusts: Asbestos, silica and others.
In: Miller K, Turk JL ,& Nicklin S ed. Principles and practice of
immunotoxicology. Oxford, Blackwell Scientific Publications,
pp 217-227.
Miller MD & Krangel MS (1992) Biology and biochemistry of the
chemokines: A family of chemotactic and inflammatory cytokines. Crit
Rev Immunol, 12: 17-46.
Miller K, & Nicklin S, eds (1987) Immunology of the gastrointestinal
tract. Boca Raton, Florida, CRC Press.
Miller LE, Ludke HR, Peacock JE, & Tomar RH (1991) Manual of
laboratory immunology, 2nd Ed. Philadelphia, Lea & Febiger.
Minchin SA, Leitenberg D, Stunz LL, & Feldbush TL (1990) Polyclonal
activation of rat B cells. II. Dextran sulfate as a cofactor in
mitogen-induced and antigen-induced differentiation of rat B
lymphocytes. J Immunol, 145: 2427-2433.
Mirkin IR, Anderson HA, Handaran L, Hong R, Gilubjatnikov R, & Belluck
D (1990) Changes in T-lymphocyte distribution associated with
ingestion of aldi-carb-contaminated drinking water: A follow-up study.
Environ Res, 51: 35-50.
Mishell RI & Dutton RW (1967) Immunization of dissociated spleen cell
cultures from normal mice. J Exp Med, 126: 423-442.
Mittal A, Woodward B, & Chandra RK (1988) Involution of thymic
epithelium and low serum thymulin bioactivity in weanling mice
subjected to severe food intake restriction or severe protein
deficiency. Exp Mol Pathol, 48: 226-235.
Molfino NA, Wright SC, Katz I, Tarlo S, Silverman F, McClean PA,
Szalai JP, Raizenne M, Slutski AS, & Zamel N (1991) Effect of low
concentrations of ozone on inhaled allergen responses in asthmatic
subjects. Lancet, 338: 199-203.
Moolenbeek C (1982) Obtaining alveolar macrophages from small
laboratory animals. Lab Anim, 16: 56-58.
Morahan PS, Klykken PC, Smith SH, Harris LS, & Munson AE (1979)
Effects of cannabinoids on host resistance to Listeria
monocytogenesand herpes simplex virus. Infect Immun, 23: 670-674.
Morris DL, Snyder NK, Gokani V, Blair RE, & Holsapple MP (1992)
Enhanced suppression of humoral immunity in DBA/2 mice following
subchronic exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD).
Toxicol Appl Pharmacol, 112: 128-132.
Mortensen P, Bergman A, Bignert A, Hansen H-J, Harkonen T, & Olsson
M (1992) Prevalence of skull lesions in harbor seals (Phoca vitulina)
in Swedish and Danish museum collections: 1835-1988. Ambio,
21: 520-524.
Mosier DE (1990) Immunodeficient mice xenografted with human lymphoid
cells: New models for in vivo studies of human immunobiology and
infectious diseases. J Clin Immunol, 10: 185-191.
Mosmann TR & Coffman RL (1989) Heterogeneity and cytokine secretion
patterns and functions of helper T cells. Adv Immunol, 46: 111-147.
Moszczynski P, Bem S, Moszczynski P, & Bartus R (1990a) Indicators of
immunity and acute phase reaction in men in relation to the duration
of exposure to mercury vapors. Med Pract, 41: 169.
Moszczynski P, Lisiewicz J, Bartus R, & Bem S (1990b) The serum
immunoglobulins in workers after prolonged occupational exposure to
the mercury vapors. Rev Roum Méd Intern, 28: 25-30.
Mowat AM (1987) The regulation of immune responses to dietary protein
antigens. Immunol Today, 8: 93-98.
Muir DCG, Ford CA, Stewart REA, Smith TG, Addison RF, Zinck ME, &
Béland P (1990) Organochlorine contaminants in belugas
(Delphinapterus leucas), from Canadian waters. Can Bull Fish Aquat
Sci, 224: 165-190.
Munson AE & White KL (1990) Immunotoxicology. In: Clayson DB, Munro
IC, Shubik P, & Swenberg JA ed. Progress in predictive toxicology.
Amsterdam, Elsevier Scientific Publishers, pp 285-313.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspectives, 43: 41-52.
Murray MJ, Lauer LD, Luster MI, Luebke RW, Adams DO, & Dean JH (1985)
Correlation of murine susceptibility to tumor, parasite and bacterial
challenge with altered cell-mediated immunity following systemic
exposure to the tumor promoter phorbol myristate acetate. Int J
Immunopharmacol, 7: 491-500.
Myers MJ, Schook LB, & Bick PH (1987) Mechanisms of benzo( a)pyrene-
induced modulation of antigen presentation. J Pharmacol Exp Ther,
242: 399-404.
Nagarkatti PS, Sweeney GC, Gauldie J, & Clark DA (1984) Sensitivity to
suppression of cytotoxic T-cell generation by 2,3,7,8-tetra-
chlorodibenzo- p-dioxin (TCDD) is dependent on the Ah genotype of the
murine host. Toxicol Appl Pharmacol, 72: 169-176.
Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, & McCune JM (1990)
Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med,
172: 1055-1063.
Naraqi S, Jackson GG, Jonasson O, & Yamashiroya HJ (1977) Prospective
study of prevalence, incidence and source of herpesvirus infections in
patients with renal allografts. J Infect Dis, 136: 531.
Neas LM, Dockery DD, Ware JH, Spengler JD, Speizer FE, & Ferris BG
(1991) Association of indoor nitrogen dioxide with respiratory
symptoms and pulmonary function in children. Am J Epidemiol,
134: 204-218
Neckers LM & Cossman J (1983) Transferrin receptor induction in
mitogen-stimulated human T lymphocytes is required for DNA synthesis
and cell division and is regulated by interleukin 2. Proc Natl Acad
Sci USA, 80: 3494-3498.
Neefjes JJ & Momburg F (1993) Cell biology of antigen processing. Curr
Opin Immunol, 5: 27-34.
Neubert D (1992) Evaluation of toxicity of TCDD in animals as a basis
for human risk assessment. Toxic Subst J, 12: 237-276.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1990)
Polyhalo-genated dibenzo- p-dioxins and dibenzofurans and the immune
system. 1. Effects on peripheral lymphocyte subpopulations of a
non-human primate (Callitrix jacchus) after treatment with
2,3,7,8-tetrachlorodibenzo-p -dioxin (TCDD). Arch Toxicol,
64: 345-359.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1991)
Polyhalogenated dibenzo- p-dioxins and dibenzofurans and the immune
system. 2. In vitro effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) on lymphocytes of venous blood from man and a non-human primate
(Callitrix jacchus). Arch Toxicol, 65: 213-219.
Newcombe DS, Rose NR, & Bloom JC (1992) Clinical immunotoxicology. New
York, Raven Press.
Nickoloff BJ ed. (1993) Heterogeneity among CD36+ cells in normal
diseased human skin. In: Dermal immune system. Boca Raton, Florida,
CRC Press, pp 245-275.
Nigam SK, Karnik AB, Chattopadhyay P, Lakkad K, Venkaiah K,
& Kashyap SK (1993) Clinical and biochemical investigations to
evolve early diagnosis in workers involved in the manufacture of
hexachlorocyclohexane. Int Arch Occup Environ Health, 65: S193-S196.
Nikolaidos E, Brunström B, Dencker L, & Veromaa T (1990) TCDD inhibits
the support of B-cell development by the bursa of Fabricius. Pharmacol
Toxicol, 67: 22-26.
Nimmo Wilkie JS, Yager JA, Wilkie BN, & Pascoe PJ (1992) Changes in
cell-mediated immune responses after experimentally-induced
anaphylaxis in dogs. Vet Immunol Immunopathol, 32: 325-338.
Noonan FP & De Fabo EC (1990) Ultraviolet-B-dose-response curves for
local and systemic immunosuppression are identical. Photochem
Photobiol, 52: 801-810.
Noonan FP & De Fabo EC (1992) Immunosuppression by ultraviolet-B
radiation: Initiation by urocanic acid. Immunol Today, 13: 250-254.
Noonan FP & Hoffman HA (1994) Susceptibility to immunosuppression by
ultraviolet B radiation in the mouse. Immunogenetics, 39: 29-39.
Nossal GJV (1987) Immunology: The basic components of the immune
system. New Engl J Med, 316: 1320-1325.
Oberhelman L, Koren H, & Cooper KD (1992) Depressed capacity to
contact sensitize women at the onset of menses; elevated contact
sensitization capacity during mid cycle can be inhibited by UV.
Clin Res, 40: 542A.
O'Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE,
Nadel JA, & Holtzman (1984) Neutrophil depletion inhibits airway
hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis,
130: 214-219.
O'Dell BL, Jessen RT, Becker LE, Jackson RT, & Smith EB (1980)
Diminished immune response in sun-damaged skin. Arch Dermatol,
116: 559-561.
Oehler R & Herberman RB (1978) Natural cell-mediated cytotoxicity in
rats. III. Effects of immunopharmacologic treatments on natural
reactivity and reactivity augmented by plyinosinic-polycytidylicacid.
Int J Cancer, 21: 221-229.
Ono H, Klein D, Vincek V, Figueroa F, O'Huigin C, Tichy H, & Klein J
(1992) Major histocompatibility complex class II genes of zebra fish.
Proc Natl Acad Sci USA, 89: 11886-11890.
Orehek J, Grimaldi F, Muls E, Durand JP, Viala A, & Charpin J (1981)
Bronchial response to allergens after controlled NO2exposure. Bull Eur
Physiopathol Respir, 17: 911-915.
Organisation for Economic Co-operation and Development (1981) OECD
guidelines for testing chemicals. Repeated dose oral toxicity -
Rodent: 28-day or 14-day study (Guideline 407). Paris.
Osborn JE, Blazkovec AA, & Walker DL (1968) Immunosuppression during
acute cytomegalovirus infection. J Immunol, 100: 835-844.
Ozkaynak H, Kinney PL, & Burbank B (1990) Recent epidemiological
findings on morbidity and mortality effects of ozone. Air Waste Manage
Assoc Rep, 6: 90-150.
Pallardy MJ, House RV, & Dean JH (1989) Molecular mechanism of
7,12-dimethylbenz[ a]anthracene-induced immunosuppression: Evidence
for action via the interleukin-2 pathway. Mol Pharmacol, 36: 128-133.
Pallardy MJ, Mishal Z, Lebrec H, & Bohuon C (1992) Immune
modification due to chemical interference with transmembrane
signalling: Applications to polycyclic aromatic hydrocarbons.
Int J Immunopharmacol, 14: 377-382.
Paranjpe MS & Boone CW (1972) Delayed hypersensitivity to simian virus
40 tumor cells in BALB/c mice demonstrated by a radioisotopic foot-pad
assay. J Natl Cancer Inst, 48: 563-566.
Parhar RS & Lala PK (1987) Amelioration of B16F10 melanoma lung
metastasis in mice by a combination therapy with indomethacin and
interleukin 2. J Exp Med, 165: 14-28.
Pass RF, Long WK, Whitley RJ, Soong S, Diethelm A, Reynolds DW, &
Alford CA (1978) Productive infection with CMV and HSV in renal
transplant recipients: Role of source of kidney. J Infect Dis,
137: 556.
Patarroyo M (1991) Leukocyte adhesion in host defence and tissue
injury. Clin Immunol Immunopathol, 60: 333-348.
Patki AH (1991) Hypothesis: Solar ultraviolet radiation and the
initial skin lesion of leprosy. Int J Leprosy, 59: 492-493.
Paul WE (1993) Fundamental immunology, 3rd Ed. New York, Raven Press.
Paul PS, Johnson DW, Mirocha CJ, Soper FF, Thoen CO, Muscoplat CC, &
Weber AF (1977) In vitro stimulation of bovine peripheral blood
lymphocytes: Suppression of phytomitogen and specific antigen
lymphocyte responses by aflatoxin. Am J Vet Res, 38: 2033-2035.
Payne JF & Fancey LF (1989) Effect of polycyclic aromatic hydrocarbons
on immune response in fish: Change in melanomacrophage centers in
flounder (Pseudopleuronectes americanus) exposed to hydrocarbon-
contaminated sediments. Mar Environ Res, 28: 431-435.
Pedersen BK & Beyer JM (1986) A longitudinal study of the influence of
azathioprine on natural killer cell activity. Allergy, 41: 286-289.
Penit C & Ezine S (1989) Cell proliferation and thymocyte subset
reconstitution in sublethally irradiated mice: Compared kinetics of
endogenous and intrathymically transferred progenitors. Proc Natl Acad
Sci USA, 86: 5547-5551.
Penn I (1988) Cancer is a long-term hazard of immunosuppressive
therapy. J Autoimmun, 1: 545-558.
Penn I (1993a) Incidence and treatment of neoplasia after
transplantation. J Heart Lung Transplant, 12: 328-336.
Penn I (1993b) Tumors after renal and cardiac transplantation. Hematol
Oncol Clin North Am, 7: 431-445.
Pennington JE (1985) Immunosuppression of pulmonary host defense. Adv
Host Def Mech, 4: 141-164.
Penninks AH, Snoeij NJ, Peiters RHH, & Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: An
overview. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM,
& Van der Venne MT ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, p 191.
Pestka JJ & Bondy GS (1990) Alteration of immune function following
dietary mycotoxin exposure. Can J Physiol Pharmacol, 68: 1009-1016.
Pfeifer R & Irons R (1981) Inhibition of lecithin-stimulated
lymphocyte agglutination and mitogenesis by hydroquinone; reactivity
with sulfhydyl groups. Exp Mol Pathol, 35: 189-198.
Phair J, Munoz A, Detels RI, Kaslow R, Rinaldo C, & Saah A (1990) The
risk of Pneumocystis carinii pneumonia among men infected with human
immunodeficiency virus type 1. New Engl J Med, 322: 161-165.
Picker LJ & Butcher EC (1992) Physiological and molecular mechanisms
of lymphocyte homing. Ann Rev Immunol, 10: 561-591.
Pleiman CM, D'Ambrosio D, & Cambier JC (1994) The B-cell antigen
receptor complex: Structure and signal transduction. Immunol Today,
15: 393-399.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p -dioxin and
related halogenated aromatic hydrocarbons: Examination of the
mechanism of toxicity. Ann Rev Pharmacol Toxicol, 22: 517-554.
Pollock PL, Germolec DR, Comment CE, Rosenthal GJ, & Luster MI (1994)
Development of human lymphocyte engrafted SCID mice as a model for
immunotoxicity assessment. Fundam Appl Toxicol., 22: 130-138.
Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L,
Janeway CA, Murphy DB, & Bottomly K (1989) Monoclonal antibodies to
murine CD3e defined distinct epitopes, one of which may interact with
CD4 during T cell activation. J Immunol, 142: 4169-4175.
Prichard MG, Boerth LW, & Pennington JE (1987) Compartmental analysis
of resting and activated pulmonary natural killer cells. Exp Lung Res,
12: 239-251.
Prior AC & Sisodia CS (1982) The effects of ochratoxin A on the immune
responses of Swiss mice. Can J Comp Med, 46: 91-96.
Pruett SB (1992) Immunotoxicity of organophosphorus compounds.
In: Chambers JE & Levi PE ed. Organophosphates. Chemistry, fate and
effects. New York, Academic Press, pp 367-385.
Pruett SB & Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin
2 system. Toxicol Lett, 40: 11-20.
Pugh-Humphreys RGP, Ross CSK, & Thomson AW (1990) The influence of
FK-506 on the thymus. An immunophenotypic and structural analysis.
Immunology, 70: 398-404.
Quesniaux VFJ (1992) Interleukins 9, 10, 11 and 12 and kit ligand:
A brief overview. Res Immunol, 143: 385-400.
Quinnan GV, Manischewitz JE, & Ennis FA (1980) Role of cytotoxic
T-lymphocytes in murine cytomegalovirus infection. J Gen Virol,
47: 503-508.
Qureshi MA, Bloom SE, Hamilton JW, & Dietert RR (1989) Toxic effects
of methyl methanesulfonate (MMS) on activated macrophages from
chickens. Environ Mol Mutag, 13: 253-262
Rao GN, Bimbaurn LS, Collins JJ, Tennant RW, & Skow LC (1988) Mouse
strains for chemical carcinogenicity studies: Overview of a workshop.
Fundam Appl Toxicol, 10: 385-394.
Raskin RE, Tvedten HW, Bull RW, Crow SE, Dunstan RW, & Krehbiel JD
(1989) Natural killer cell activity in untreated and treated dogs with
lymphoma. Am J Vet Res, 4: 483-487.
Ratajczak HV, Thomas PT, Sothern RB, Vollmuth T, & Heck D (1993)
Evidence for genetic basis of seasonal differences in antibody
formation between two mouse strains. Chronobiol Int, 10: 383-394.
Ratcliffe NA & Rowly AF (1981) Intervertebrate blood cells. London,
Academic Press.
Raulet DH (1989) The structure, function, and molecular genetics of
the gamma/delta T cell receptor. Ann Rev Immunol, 7: 175-207.
Reed SG & Scott P (1993) T-Cell and cytokine responses in
leishmaniasis. Curr Opin Immunol, 5: 524-531.
Reggiani G (1980) Acute human exposure to TCDD in Seveso, Italy.
J Toxicol Environ Health, 6: 27-43.
Reigart JR & Graber CD (1976) Evaluation of the humoral immune
response of children with low lead exposure. Bull Environ Contam
Toxicol, 16: 112-117.
Reijnders PJH (1986) Reproductive failure in common seals feeding on
fish from polluted coastal waters. Nature, 324: 456-457.
Renton KW (1986) Factors affecting drug biotransformation. Clin
Biochem, 19: 72-75.
Renton KW, Gray JD, & Hall RI (1980) Decreased elimination of
theophylline after influenza vaccination. Can Med Assoc J,
123: 288-290.
Reyes J, Tzakis A, Green M, Nour B, Nalesnik M, Van Thiel D, Martin M,
Breinig MK, Fung LL, Cooper M, & Starzl TE (1991) Post-transplant
lymphoproliferative disorders under primary FK506 immunosuppression.
Transplant Proc, 23: 3044-3046
Reynolds HY & Thomson RE (1973) Pulmonary host defenses.
II. Interaction of respiratory antibodies with Pseudomonas aeruginosa
and alveolar macrophages. J Immunol, 111: 369-380.
Reynolds CW, Wenn AC, Barlozzari T, Wiltrout T, Reichardt WA, &
Herberman RB (1984) Natural killer activity in the rat. IV.
Distribution of large granular lymohocytes (LGL) following intravenous
and intraperitoneal transfer. Cell Immunol, 86: 371-380.
Richeldi L, Persichini T, Ferrara GB, Crystal RG, Markham T, Luisetti
M, Bertrorelli G, Guerritore D, & Saltini C (1992) Chronic beryllium
disease association with the HLA-DP 2.1 allele. Am Rev Respir Dis,
145 (Suppl): A324.
Riley WJ (1989) Insulin dependent diabetes mellitus, an autoimmune
disorder? Clin Immunol Immunopathol, 53: S92-S98.
Roberts RJ (1975) Melanin-containing cells of the teleost fish and
their relation to disease. In: Ribelin WE & Migaki G ed. The pathology
of fishes. Madison, Wisconsin, University of Wisconsin Press,
pp 399-428.
Roitt IM (1991) Essential immunology, 7th Ed. Oxford, Blackwell
Scientific Publications.
Roitt IM, Brostoff J & Male DK (1993) Immunology, 3rd Ed. London,
Gower Medical Publications.
Roizman B (1982) The family Herpesviridae. General description,
taxonomy, and classification. In: Roizman B ed. The viruses, Vol 1:
Herpesviruses. New York, Plenum Press, pp 1-23.
Roizman B, Carmichael LE, Deinhardt F, de The G, Nahmias AJ, Plowright
W, Rapp F, Sheldrick P, Takahashi M, & Wolf K (1981) Herpesviridae.
Definition, provisional nomenclature and taxonomy. Intervirology,
16: 201-217.
Rolstad B, Herberman RB, & Reynolds GW (1986) Natural killer activity
in the rat: V. The circulation patterns and tissue localization of
peripheral blood lymphocytes. J Immunol, 136: 2600-2808.
Rose LM, Richards TL, Petersen R, Peterson J, Hruby S, & Alvord EC Jr
(1991) Remitting-relapsing EAE in nonhuman primates: A valid model of
multiple sclerosis. Clin Immunol Immunopathol, 59: 1-15.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502-510.
Rosenthal GJ & Snyder CA (1987) Inhaled benzene reduces aspects of
cell-mediated tumor surveillance in mice. Toxicol Appl Pharmacol,
88: 35-43.
Rosenthal GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock
P, Ling-Yuan K, Kwon J, & Luster MI (1994) Asbestos stimulates
IL-8 production from human lung epithelial cells. J Immunol,
153: 3237-3244.
Ross GD (1992) Function of neutrophils in host defence and immunity.
In: Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca
Raton, Florida, CRC Press, pp 263-286.
Ross PS, De Swart RL, Reijnders PJH, Van Loveren H, Vos JG, &
Osterhaus ADME (1995) Contaminant-related suppression of delayed-type
hypersensitivity and antibody responses in harbor seals fed herring
from the Baltic Sea. Environ Health Perspectives, 103: 162-167.
Ross PS, De Swart RL, Timmerman HH, Reijnders PJH, Vos JG, Van Loveren
H, & Osterhaus ADME (in press) Suppression of natural killer activity
in harbour seals (Phoca virulina) fed Baltic Sea herring. Aquat
Toxicol.
Rothbard JB & Gefter ML (1991) Interactions between immunogenic
peptides and MHC molecules. Ann Rev Immunol, 9: 527-565.
Roths JB, Murphy ED, & Eicher EM (1984) A new mutation, gld, that
produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp
Med, 159: 1-20.
Rouveix B(1993) Opiates and immune function. Thérapie, 47: 503-512.
Rubin RH (1990) Impact of cytomegalovirus infection in organ
transplant recipients. Rev Infect Dis, 12: S754-S766.
Rubin RH, Cosimi AB, Hirsch MS, Herrin JT, Russell PS, & Tolkoff Rubin
NE (1981) Effects of antithymocyte globulin on CMV infection in renal
transplant recipients. Transplantation, 31: 143-145.
Ruitenberg EJ, Buys J, Teppema JS, & Elgersma A (1983) Rat mononuclear
cells and neutrophils are more effective than eosinophils in antibody-
mediated stage-specific killing of Trichinella spiralis in vitro.
Parasitenkunde, 69: 807-815.
Ryffel B (1992) The carcinogenicity of ciclosporin. Toxicology,
73: 1-22.
Saiki RK, Scharf SJ, Faloona FA, Mullis KB, Hom GT, Erlich HA, &
Arnheim N (1985) Enzymatic amplification of ß-globulin genomic
sequences and restriction site analysis for diagnosis of sickle cell
anemia. Science, 230: 1350-1354.
Sainte-Marie G, Bélisle C, & Peng FS (1990) The deep cortex of the
lymph node: Morphological variations and functional aspects. In:
Grundmann E & Vollmer E ed. Reaction patterns of the lymph node, Part
1. Cell types and functions. Berlin, Springer Verlag, pp 33-63.
Saito M (1992) Recent trends in research on differentiation of
hematopoietic cells and lymphokines (hemopoietins). Hum Cell,
5: 54-69.
Sakaguchi S & Sakaguchi N (1988) Thymus and autoimmunity. Transplanta-
tion of the thymus from cyclosporin A-treated mice causes organ-
specific autoimmune disease in athymic nude mice. J Exp Med,
167: 1479-1485.
Saltini C, Winestock K, Kirby M, Pinkston P, & Crystal RB (1989)
Maintenance of alveolitis in patients with chronic beryllium disease
by beryllium-specific helper T cells. New Engl J Med, 320: 1103-1109.
Samet JM (1994) Nitrogen dioxide and respiratory infection. Am Rev
Respir Dis, 139: 1073-1074.
Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA,
McLaren LC, Schwab M, & Spengler JD (1993) Nitrogen dioxide and
respiratory illnesses in infants. Am Rev Respir Dis, 148: 1258-1265.
Sanders VM, Tucker AN, White KL, Kauffmann BM, Hallett P, Carchman RA,
Borzelleca JF, & Munson AE (1982) Humoral and cell-mediated immune
status in mice exposed to trichloroethylene in the drinking water.
Toxicol Appl Pharmacol, 62: 358-368.
Sanders VM, White KL, Shopp GM, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to 1,1,2-trichloroethane. Drug
Chem Toxicol, 8: 357.
Sato K, Flajnik MF, Du Pasquier L, Katagiri M, & Kasahara M (1993)
Evolution of the MHC: Isolation of class II ß-chain cDNA clones from
the amphibian Xenopus laevis. J Immunol, 150: 2831-2843.
Scala R (1991) Risk assessment. In: Amdur M, Doull J, & Klaasen C ed.
Casarett and Doull's Toxicology, 3rd Ed. Elmsford, New York, Pergamon
Press, pp 985-996.
Scheuchenzuber WJ, Eskew ML, & Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environ Res, 38: 389-399.
Schielen P, Schoo W, Tekstra J, Oostermeijer HH, Seinen W, & Bloksma N
(1993) Autoimmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schlick E & Friedberg KD (1981) The influence of low lead doses on the
reticulo-endothelial system and leukocytes of mice. Arch Toxicol,
47: 197.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
C, Ritz J, Shaw S, Silverstein RL, Springer TA, Tedder TF, & Todd RF
(1994) CD antigens 1993. Immunol Today, 15: 98-99.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
T, Ritz J, Shaw S, Springer TA, Tedder TF, & Todd RF (1995) Leucocyte
typing, V: White cell differentiation antigens. Oxford, Oxford
University Press.
Schouk LB & Laskin DL (1994) Xenobiotics and inflammation. San Diego,
California, Academic Press.
Schuurman H-J, Kluin PM, Gmelig-Meyling FHJ, Van Unnik JAM, & Kater L
(1985) Lymphocyte status of lymph node and blood in acquired
immunodeficien-cy syndrome (AIDS) and AIDS-related complex disease.
J Pathol, 147: 269-280.
Schuurman H-J, Van Loveren H, Rozing J, Van Dijk A, Loeber JG, & Vos
JG (1990) Cyclosporin and the rat thymus. An immunohistochemical
study. Thymus, 16: 235-254.
Schuurman H-J, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1991a) Immune system. In: Haschek WM & Rousseaux CG ed. Handbook of
toxicologic pathology. San Diego, Academic Press, pp 421-487.
Schuurman H-J, Nagelkerken L, De Weger RA, & Rozing J (1991b) Age-
associated involution: Significance of a physiologic process. Thymus,
18: 1-6.
Schuurman H-J, De Weger RA, Van Loveren H, Krajnc-Franken MAM, & Vos
JG (1992a) Histopathological approaches. In: Miller K, Turk JL, &
Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 279-303.
Schuurman H-J, Hougen HP, & Van Loveren H (1992b) The rnu (Rowett
nude) and rnuN ( nznu, New Zealand nude) rat: An update. ILAR
News, 34: 3-12.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijt BC,
De Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Pass D, Vankerkom
J, Boot R, Broekhuizen R, Dormans J, van Herck H, Hermans PGC, Hoekman
J, van der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992c)
Comparative evaluation of the immune status of congenitally athymic
and euthymic rat strains bred and maintained at different institutes:
2. Athymic rats. J Exp Anim Sci, 35: 33-48.
Schuurman H-J, Van Loveren H, Rozing J, & Vos JG (1992d) Chemicals
trophic for the thymus: Risk for immunodeficiency and autoimmunity.
Int J Immunopharmacol, 14: 369-375.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijht BC,
de Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Vankerkom J, Boot
R, Broekhuizen R, Dormans J, van Herck H, Hermans GC, Hoekman J, van
der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992e) Comparative
evaluation of the immune status of congenitally athymic and euthymic
rat strains bred and maintained at different institutes: 1. Euthymic
rats. J Exp Anim Sci, 35: 16-32.
Schuurman H-J, Hu HZ, De Weger RA, & Clevers HC (1993) Thoughts on
thymus and T-lymphocyte repertoire. Relevance for tolerance of the
immune response. Neth J Med, 43: 38-54.
Schuurman H-J, Kuper CF, & Vos JG (1994) Histopathology of the immune
system as a tool to assess immunotoxicity. Toxicology, 86: 197-212.
Schwartz J (1992) Air pollution and the duration of acute respiratory
symptoms. Arch Environ Health, 47: 116-122.
Schwartz RS, Callen JP, & Silva JR (1978) A cluster of Hodgkin's
disease in a small community: Evidence for environmental factors.
Am J Epidemiol, 108: 19-26.
Schwartz J, Spix C, Wichmann HE, & Malin E (1991) Air pollution and
acute respiratory illness in five German communities. Environ Res,
56: 1-14.
Schwetz BA & Tyl RW (1987) Consensus workshop on the evaluation of
maternal and developmental toxicity. Workgroup III report. Low dose
extrapolation and other considerations for risk assessment - models
and applications. Teratog Carcinog Mutag, 1: 1-7.
Secombes CJ, Fletcher TC, White A, Costello MJ, Stagg R, & Houlihan DF
(1992) Effects of sewage sludge on immune response in the dab,
Limanda limanda. Aquat Toxicol, 23: 217-230.
Seinen W & Penninks AH (1979) Immune suppression as a consequence of a
selective cytotoxic activity of certain organometallic compounds on
thymus and thymus-dependent lymphocytes. Ann NY Acad Sci,
320: 499-517.
Seinen W & Willems MI (1976) Toxicity of organotin compounds.
I. Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di- n-octyltin dichloride. Toxicol Appl Pharmacol, 35: 63-75.
Seinen W, Vos JG, Van Krieken R, Penninks AH, Brands R, & Hooykaas H
(1977) Toxicity of organotin compounds. II. Comparative in vivo and
in vitro studies with various organotin and organolead compounds in
different animal species with special emphasis on lymphocyte
cytotoxicity. Toxicol Appl Pharmacol, 42: 197-212.
Sela O & Shoenfeld Y (1988) Cancer in autoimmune diseases. Semin Arthr
Rheum, 18: 78-87.
Selgrade MJK & Gilmour MI (1994) Effects of gaseous air pollutants on
immune responses and susceptibility to infectious and allergic
disease. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicity and immunopharmacology, 2nd Ed. New York, Raven Press,
pp 395-411.
Selgrade MJK & Osborn TE (1974) Role of macrophages in resistance to
murine cytomegalovirus. Infect Immun, 10: 1383-1390.
Selgrade MJK, Daniels MJ, Hu PC, Miller FJ, & Graham JA (1982) Effects
of immunosuppression with cyclophosphamide on acute murine
cytomegalovirus infection and virus-augmented natural killer cell
activity. Infect Immun, 38: 1046-1055.
Selgrade MK, Daniels MJ, Illing JW, Ralston AL, Grady MA, Chartlet E,
& Graham JA (1984) Increased susceptibility to parathion poisoning
following murine cytomegalovirus infection. Toxicol Appl Pharmacol,
76: 356-364.
Selgrade MJK, Illing JW, Starnes DM, Stead AG, Menache MG, & Stevens
MA (1988) Evaluation of effects of ozone exposure on influenza
infection in mice using several indicators of susceptibility. Fundam
Appl Toxicol, 11: 169-180.
Selgrade MJK, Starnes DM, Illing JW, Daniels MJ, & Graham JA (1989)
Effects of phosgene exposure on bacterial, viral, and neoplastic lung
disease susceptibility in mice. Inhal Toxicol, 1: 243-259.
Selgrade MJK, Daniels MJ, & Dean JH (1992) Correlation between
chemical suppression of natural killer cell activity in mice and
susceptibility to cytomegalovirus: Rationale for applying murine
cytomegalovirus as a host resistance model and for interpreting
immunotoxicity testing in terms of risk of disease. J Toxicol Environ
Health, 37: 123-137.
Selgrade MJK, Cooper KD, Devlin RB, Van Loveren H, Biagini RE, &
Luster MI (1995) Immunotoxicity - Bridging the gap between animal
research and human health effects. Fundam Appl Toxicol, 24: 13-21.
Sell S (1987) Immunology, immunopathology and immunity, 4th Ed.
Norwalk, Connecticut, Appleton & Lange.
Shabtai M, Malinowski K, Waltzer WC, Pullis C, Raisbeck AP, & Rapaport
FT (1991) Quantitative analysis of surface marker densities after
exposure of T cells to concanavalin A (ConA): A sensitive early index
of cellular activation. Cell Immunol, 133: 519-525.
Shand FL (1975) Review/commentary: The immunopharmacology of
cyclophosphamide. Int J Immunopharmacol, 1: 165-171.
Sharp JG & Watkins EB (1981) Cellular and immunological consequences
of thymic irradiation. In: Dubois JB, Serrou B, & Rosenfeld C ed.
Immunopharmacologic effects of radiation therapy. New York, Raven
Press, pp 137-179.
Sheil AG, Disney AP, Mathew TH, Amiss N, & Excell L (1991) Cancer
development in cadaveric donor renal allograft recipients treated with
azathioprine (AZA) or cyclosporine (CyA) or AZA/CyA. Transplant Proc,
23: 1111-1112.
Sherwood RL, Tarkington B, Lippert WE, & Goldstein E (1981) Effect of
ferrous sulphate aerosols and nitrogen dioxide aerosols on murine
pulmonary defense. Arch Environ Health, 36: 130-135.
Sherwood RL, Thomas PT, Kawanishi CY, & Fenters JD (1988) Comparison
of Streptococcus zooepidemicus and influenza virus pathogenicity in
mice by three pulmonary exposure routes. Appl Environ Microbiol,
54: 1744-1751.
Shigematsu N, Ishimaru S, Saito R, Ikeda T, Matsuba K, Sigiyama K, &
Masuda Y (1978) Respiratory involvement in polychlorinated biphenyls
poisoning. Environ Res, 16: 92-100.
Shiroky JB, Frosp A, Skelton JD, Haegert DG, Newkirk MM, & Neville
C (1991) Complications of immunosuppression associated with weekly low
dose methrotrexate. J Rheum, 18: 1172-1175.
Shopp GM & Munson AE (1985) Suppression of the antibody response by
phorbol esters in the mouse is due to an effect on the nylon wool
adherent cell population. Agents Actions, 6: 535-541.
Shopp GM, Sanders VM, White KL, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to trans-12-dichlorethylene.
Drug Chem Toxicol, 8: 393.
Shortman K, Egerton M, Spangrude GJ, & Scollay R (1990) The generation
and fate of thymocytes. Sem Immunol, 2: 3-13.
Shy CM, Creason JP, Pearlman ME, McClain KE, & Benson FB (1970) The
Chattanooga study: Effects of community exposure to nitrogen dioxide.
II. Incidence of acute respiratory illness. J Air Pollut Control
Assoc, 20: 582-588.
Sibinga NES & Goldstein A (1988) Opioid peptides and opioid receptors
in cells of the immune system. Ann Rev Immunol, 6: 219-249.
Sigal NH & Dumont FJ (1992) Cyclosporin A, FK-506, and Rapamycin:
Pharmacologic probes of lymphocyte signal transduction. Ann Rev
Immunol, 10: 519-560.
Sikorski EE, McCay JA, White KL, Bradley SG, & Munson AE (1989)
Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1
mice. Fundam Appl Toxicol, 13: 843-858.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW,
Wilcox KR, VanAmburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls.
J Reticuloendothel Soc, 26: 341.
Sima P & Vetvicka V (1992) Evolution of immune accessory function. In:
Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca Raton,
Florida, CRC Press, pp 1-56.
Simon JC, Cruz PD, Bergstresser PR, & Tigelaar RE (1990) Low dose
ultraviolet B-irradiated Langerhans cells preferentially activate
CD4+ cells of the T helper 2 subset. J Immunol, 145: 2087-2094.
Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, & Cruz PD (1991)
Ultraviolet B radiation converts Langerhans cells from immunogenic to
tolerogenic antigen-presenting cells. Induction of specific clonal
anergy in CD4+ T helper 1 cells. J Immunol, 146: 485-491.
Singh G, Fries JF, Spitz P, & Williams CA (1989) Toxic effects of
azathioprine in rheumatoid arthritis patients. A national post-
marketing perspective. Arthr Rheum, 32: 837-843.
Siroki O, Institoris L, Tatar F, & Desi I (1994) Immunotoxicological
investigation of SCMF, a new pyrethroid pesticide in mice. Hum Exp
Toxicol, 13: 337-343.
Siwicki AK, Cossarini-Dunier M, Studnicka M, & Demael A (1990)
In vivo effect of the organophosphorus insecticide trichlorphon on
immune response of carp (Cyprinus carpio). Ecotoxicol Environ Saf,
19: 99-105.
Sjoblad RD (1988) Potential future requirements for immunotoxicity
testing of pesticides. Toxicol Ind Health, 4: 391-394.
Sjovall P, Christensen OB, & Moller H (1985) Single exposure to
ultraviolet irradiation and elicitation of human allergic contact
dermatitis. Acta Dermatol Venereol, 65: 93-96.
Smialowicz RJ, Rogers RR, Riddle MM, Luebke RW, Rowe DG, & Garner RJ
(1984) Manganese chloride enhances murine cell-mediated cytotoxicity:
Effects on natural killer cells. J Immunopharmacol, 6: 1-23.
Smialowicz R, Luebke RW, Riddle MM, Rogers RR, & Rowe DG (1985a)
Evaluation of the immunotoxic potential of chlordecone with comparison
to cyclophosphamide. J Toxicol Environ Health, 15: 561-574.
Smialowicz RJ, Luebke RW, Rogers RR, Riddle MM, & Rowe DG (1985b)
Evaluation of immune function in mice exposed to ordram. Toxicology,
37: 307-314.
Smialowicz RJ, Rogers RR, Riddle MM, Garner RJ, Rowe DG, & Luebke RW
(1985c) Immunologic effects of nickel. II. Suppression of natural
killer cell activity. Environ Res, 36: 56-66.
Smialowicz RJ, Andrews JE, Riddle MM, Rogers RR, Luebke RW, & Copeland
CB (1989) Evaluation of the immunotoxicity of low level PCB exposure
in the rat. Toxicology, 56: 197-211.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB, & Ernest
GG (1990) Immune alterations in rats following subacute exposure to
tributyltin oxide. Toxicology, 64: 169-178.
Smialowicz RJ, Riddle MM, Luebke RW, Copeland CB, Andrews D, Rogers
RR, Gray LE, & Laskey JW (1991) Immunotoxicity of 2-methoxyethanol
following oral administration in Fischer 344 rats. Toxicol Appl
Pharmacol, 109: 494-506.
Smialowicz RJ, Luebke RW, & Riddle MM (1992a) Assessment of the
immunotoxic potential of the fungicide dinocap in mice. Toxicology,
75: 235-247.
Smialowicz RJ, Williams WC, Riddle MM, Andrews DL, Luebke RW, &
Copeland CB (1992b) Comparative immunosuppression of various glycol
ethers orally administered to Fischer 344 rats. Fundam Appl Toxicol,
18: 621-627.
Smialowicz RJ, Riddle MM, & Williams WC (1993) Methoxyacetaldehyde, an
intermediate metabolite of 2-methoxyethanol, is immunosuppressive in
the rat. Fundam Appl Toxicol, 21: 1-7.
Sminia T, Van der Brugge-Gamelkoorn GJ, & Jeurissen SHM (1989)
Structure and function of bronchus-associated lymphoid tissue (BALT).
Crit Rev Immunol, 9: 119-150.
Smith SH, Sanders VM, Barrett BA, Borzellaca JF, & Munson AE (1978)
Immunotoxicological evaluation on mice exposed to polychlorinated
biphenyls. Toxicol Appl Pharmacol, 45: 330.
Snoeij NJ, Penninks AH, & Seinen W (1988) Dibutyltin and tributyltin
compounds induce thymic atrophy in rats due to a selective action on
thymic lymphoblasts. Int J Immunopharmacol, 10: 889-891.
Snyder CA (1989) The neuroendocrine system, an opportunity for
immunotoxicologists. Environ Health Perspectives, 81: 165-166.
Spangrude GJ, Berhard EJ, Ajoika RS, & Daynes RA (1983) Alterations in
lymphocyte homing patterns within mice exposed to ultraviolet
radiation. J Immunol, 130: 2974-2981.
Speizer FE, Ferris B, Bishop YMM, & Spengler J (1980) Respiratory
disease rates and pulmonary function in children associated with NO2
exposure. Am Rev Respir Dis, 121: 3-10.
Spiekermann K, Emmendoerfer A, Elsner J, Raeder E, Lohmann-Matthes ML,
Prahst A, Link H, Freund M, Welte K, & Roesler J (1994) Altered
surface marker expression and function of G-CSF induced neutrophils
from test subjects and patients under chemotherapy. Br J Haematol,
87: 31-38.
Spreafico F, Merendino A, Braceschi L, & Sozanni S (1987)
Immunodepressive drugs as prototype immunotoxicants. In: Berlin A,
Dean J, Draper MH, Smith EMB, & Van Der Venne, M-T ed.
Immunotoxicology. Dordrecht, Martinus Nijhoff, pp 192-207.
Sprent J, Gao EK, & Webb SR (1990) T Cell reactivity to MHC molecules:
Immunity versus tolerance. Science, 248: 1357-1363.
Springer TA (1990) Adhesion receptors of the immune system. Nature,
346: 425-434.
Springer T, Galfré G, Secher DS, & Milstein C (1979) Mac-1: A
macrophage differentiation antigen identified by monclonal antibody.
Eur J Immunol, 9: 301-306.
Spruanoe SL (1985) Pathogenesis of herpes simplex labialis:
Experimental induction of lesions with UV light. J Clin Microbiol,
22: 366-368.
Stahlmann R, Korte M, Van Loveren H, Vos JG, Thiel R, & Neubert D
(1992) Abnormal thymus development and impaired function of the immune
system in rats after prenatal exposure to aciclovir. Arch Toxicol,
66: 551-559.
Stanley GP & Pender MP (1991) The pathophysiology of chronic relapsing
experimental allergic encephalomyelitis in the Lewis rat. Brain,
114: 1827-1853.
Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP,
Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL, Atchison RW, Jaffe R, &
Bahnson HT (1984) Reversibility of lymphomas and lymphoproliferative
lesions developing under cyclosporin-steroid therapy. Lancet,
i: 583-587.
Stehr-Green PA, Naylor PH, & Hoffman RE (1989) Diminished thymosin
alpha-1 levels in persons exposed to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. J Toxicol Environ Health, 28: 285-295.
Steinmann GG (1986) Changes in the human thymus during aging. Curr
Topics Pathol, 75: 43-88.
Stein-Streinlein J, Bennett M, Mann D, & Kumar V (1983) NK cells in
mouse lungs: Surface phenotype, target preference and response to
local influenza virus infection. J Immunol, 131: 2699-2704.
Stet RJM & Egberts E (1991) The histocompatibility system in
teleostean fishes: From multiple histocompatibility loci to a major
histocompatibility complex. Fish Shellfish Immunol, 1: 1-16.
Stingl G & Steiner G (1989) Immunological host defence of the skin.
Curr Prob Dermatol, 18: 22-30.
Stobo JD & Paul WE (1973) Functional heterogeneity of murine
lymphoid cells. III. Differential responsiveness of T cells to
phytohemagglutinin and concanavalin A for T cell subsets. J Immunol,
110: 362-369.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral responses by pesticides and chemicals of environmental
concern: Quantitative studies of immunosuppression by DDT, Aroclor,
carbaryl, carbofuran and methylparathion. Toxicol Appl Pharmacol,
32: 587-602.
Streilein JW (1983) Skin associated lymphoid tissues (SALT): Origins
and functions. J Invest Dermatol, 80 (Suppl): 12s-16s.
Streilein JW (1990) Skin-associated lymphoid tissues (SALT): The next
generation. In: Bos J ed. The skin immune system. Boca Raton, Florida,
CRC Press, pp 25-48.
Strobel S & Ferguson A (1984) Immune responses to fed protein antigens
in mice. 3. Systemic tolerance or priming is related to age at which
antigen is first encountered. Ped Res, 18: 588-594.
Stunz LL & Feldbush TL (1986) Polyclonal activation of rat B
lymphocytes. I. A single mitogenic signal can stimulate proliferation,
but three signals are required for differentiation. J Immunol,
136: 4006-4012.
Stutman O (1986) Postthymic T-cell development. Immunol Rev,
91: 160-194.
Subramanian A, Tanabe S, Tatsukawa R, Saito S, & Miyazaki N (1987)
Reduction in the testosterone levels by PCBs and DDE in Dall's
porpoises of northwestern North Pacific. Mar Pollut Bull, 18: 643-646.
Sung YJ, Hotchkiss JH, Austic RE, & Dietert RR (1992) Direct
measurement of nitric oxide in headspace gas produced by a chicken
macrophage cell line in a closed culture system. Biochem Biophys Res
Commun, 184: 36-42.
Surhe CD & Sprent J (1991) Long-term xenogeneic chimeras. Full
differentiation of rat T and B cells in SCID mice. J Immunol,
147: 2148-2154.
Svensson BG, Nilsson A, Hansson M, Rappe C, Åkesson B, & Skerving S
(1991) Exposure to dioxins and dibenzofurans through the consumption
of fish. New Engl J Med, 324: 8-12.
Swain WR (1991) Effects of organochlorine chemicals on the
reproductive outcome of humans who consumed contaminated Great Lakes
fish: An epidemiologic consideration. J Toxicol Environ Health,
33: 587-639.
Swerdlow AJ, Douglas AJ, Vaughan Hudson G, Vaughan Hudson B, Bennett
MH, & MacLennan KA (1992) Risk of second primary cancers after
Hodgkin's disease by type of treatment: Analysis of 2846 patients in
the British National Lymphoma Investigation. Br Med J, 304: 1137-1143.
Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR,
Heroux AL, Dizikes GJ, Pifarre R, & Fisher RI (1990) Increased
incidence of lymphoproliferative disorder after immunosuppression with
the monoclonal antibody OKT3 in cardiac-transplant recipient. New Engl
J Med, 323: 1723-1728.
Szakal AK, Kosco MH, & Tew JG (1989) Microanatomy of lymphoid tissue
during humoral immune responses: Structure function relationships. Ann
Rev Immunol, 7: 91-109.
Taga T, & Kishimoto T (1992) Cytokine receptors and signal
transduction. FASEB J, 6: 3387-3396.
Takeya U, Shimotori S, Tanaguchi T, & Nomoto K (1977) Cellular
mechanisms in the protection against infection by Listeria
monocytogenesin mice. J Gen Microbiol, 100: 373-379.
Talcott PA & Koller LD (1983) The effect of inorganic lead and/or a
polychlorinated biphenyl on the developing immune system of mice. J
Toxicol Environ Health, 12: 337-352.
Talcott PA, Koller LD, & Exon JH (1985) The effect of lead and
polychlorinated biphenyl exposure on rat natural killer cell
cytotoxicity. Int J Immunopharmacol, 7: 255-261.
Taylor BA (1972) Genetic relationships between inbred strains of mice.
J Hered, 63: 83-86.
Taylor JR, Schmieder GF, Shimizu T, Tie C, & Streilin JW (1994)
Interrelationship between ultraviolet light and recurrent herpes
simplex infections in man. J Dermatol Sci, 8: 224-232.
Temple L, Kawabata TT, Munson AE, & White KL (1993) Comparison of
ELISA and plaque-forming cell assays for measuring the humoral immune
response to SRBC in rats and mice treated with benzo[ a]pyrene or
cyclophosphamide. Fundam Appl Toxicol, 21: 412-419.
Theofilopoulos AN & Dixon FJ (1985) Murine models of systemic lupus
erythematosus. Adv Immunol, 37: 269-390.
Thiem PA, Halper LK, & Bloom JC (1988) Techniques for assessing canine
mononuclear phagocytic function as part of an immunotoxicologic
evaluation. Int J Immunopharmacol, 10: 765-771.
Thomas PT & Hinsdill RD (1978) Effect of polychlorinated biphenyls on
the immune responses of rhesus monkeys and mice. Toxicol Appl
Pharmacol, 44: 41-51.
Thomas PT & Hinsdill RD (1979) The effect of perinatal exposure to
tetrachlorodibenzo- p-dioxin on the immune response of young mice.
Drug Chem Toxicol, 2: 77-98.
Thomas PT, Ratajczak HV, Aranyi C, Gibbons R, & Fenters JD (1985a)
Evaluation of host resistance and immune function in cadmium-exposed
mice. Toxicol Appl Pharmacol, 80: 446-456.
Thomas PT, Fugmann R, Aranyi C, Barbera P, Gibbons R, & Fenters J
(1985b) Effect of dimethylnitrosamine on host resistance and immunity.
Toxicol Appl Pharmacol, 77: 219-229.
Thomas PT, Ratajczak HV, Eisenberg WC, Furedi-Machacek M, Ketels K, &
Barbera PW (1987) Evaluation of host resistance and immunity in mice
exposed to the carbamate pesticide aldicarb. Fundam Appl Toxicol,
9: 82-89.
Thomson AW (1992) The spectrum of action of new immunosuppressive
drugs. Clin Exp Immunol, 89: 170-173.
Thurmond LM, Lauer LD, House RV, Cook JC, & Dean JH (1987) Immuno-
suppression following exposure to 7,12-dimethylbenz[ a]anthracene
(DMBA) in Ah-responsive and Ah-nonresponsive mice. Toxicol
Appl Pharmacol, 91: 450-460.
Tieben LM, Berkhout RJM, Smits HL, Bouwes Bavinck JN, Vermeer BJ,
Bruijn JA, van der Woude FJ, & ter Schegget J (1994) Detection of
epidermodysplasia verruciformis-like human papillomavirus types in
renal allograft recipients. Br J Dermatol, 131: 226-230.
Tilney NL (1971) Patterns of lymphatic drainage in the adult
laboratory rat. J Anat, 109: 369-383.
Timonen T, Ortaldo JR, & Herberman RB (1981) Characteristics of human
large granular lymphocytes and relationship to natural killer and K
cells. J Exp Med, 153: 569-582.
Timonen T, Ortaldo JR, & Herberman RB (1982) Isolation of human and
rat natural killer cells. J Immunol Meth, 51: 269-277.
Toews GB, Bergstresser PR, Streilein JW, & Sullivan S (1980) Epidermal
Langerhans cell density determines whether contact hypersensitivity or
unresponsiveness follows skin painting with DNFB. J Immunol,
124: 445-453.
Tomlinson S (1993) Complement defense mechanisms. Curr Opin Immunol,
5: 83-89.
Tonari Y, & Minamishima Y (1983) Pathogenicity and immunogenicity of
temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol,
64: 1983-1990.
Tracey DE, Walfe SA, Durdik JM ,& Henney CS (1977) BCG-induced murine
effector cells; cytolytic activity in peritoneal exudates: An early
response to BCG. J Immunol, 119: 1145-1151.
Truelove J, Grant D, Mes J, Tryphonas H, Tryphonas L, & Zawidzka Z
(1982) Polychlorinated biphenyl toxicity in the pregnant cynomolgus
monkey: A pilot study. Arch Environ Contam Toxicol, 11: 583-588.
Tryphonas H (in press) Immunotoxicity of PCBs (Aroclors) in relation
to Great Lakes. Environ Health Perspectives, Suppl 9.
Tryphonas H, Hayward S, O'Grady L, Loo JCK, Arnold DL, Bryce F, &
Zawidzka ZZ (1989) Immunotoxicity studies of PCB (Aroclor 1254) in the
adult rhesus (Macaca mulatta) monkey--Preliminary report. Int J
Immunopharmacol, 11: 199-206.
Tryphonas H, Luster MI, White KL, Naylor PH, Erdos MR, Burleson GR,
Germolec D, Hodgen M, Hayward S, & Arnold DL (1991a) Effects of PCB
(Aroclor 1254) on non-specific immune parameters in rhesus (Macaca
mulatta) monkeys. Int J Immunopharmacol, 13: 639-648.
Tryphonas H, Luster MI, Schiffman G, Dawson L-L, Hodgen M, Germolec D,
Hayward S, Bryce, F, Loo JCK, Mandy F, & Arnold DL (1991b) Effects of
chronic exposure of PCB (Aroclor 1254) on specific and non-specific
immune parameters in the rhesus (Macaca mulatta) monkey. Fundam Appl
Toxicol, 16: 773-786.
Tsang PH, Chu FN, Fischbein A, & Bekesi JG (1988) Impairments in
functional subsets of T-suppressor (CD8) lymphocytes, monocytes, and
natural killer cells among asbestos-exposed workers. Clin Immunol
Immunopathol, 47: 323-332.
Tschöpe D, Roesen B, Scwippert B, Kehrel B, Scauseil S, Esser J, &
Gries FA (1990) Platelet analysis using flow cytometry procedures.
Platelets, 1: 127-133.
Tucker AN & Munson AE (1981) In vitro inhibition of the primary
antibody response to sheep erythrocytes by cyclophosphamide. Toxicol
Appl Pharmacol, 59: 617-619.
Tucker AN, Hong L, Boorman GA, Pung O, & Luster MI (1985) Alteration
of bone marrow cell cycle kinetics by diphenylhydantoin: Relationship
to folate utilization and immune function. J Pharmacol Exp Ther,
234: 57-62.
Tucker AN, Vore SJ, & Luster MI (1986) Suppression of B cell
differentiation by 2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol
Pharmacol, 29: 372-377.
Uber CL & McReynolds RA (1982) Immunotoxicology of silica. CRC Crit
Rev Toxicol, 10: 303-319.
Umesaki Y, Setoyama H, Matsumoto S, & Okada Y (1993) Expansion of
alpha ß T-cell receptor-bearing intestinal intraepithelial lymphocytes
after microbial colonization in germ-free mice and its independence
from thymus. Immunology, 79: 32-37.
Urban JL, Burton RC, Holland JM, Kripke ML, & Schreiber H (1982)
Mechanisms of syngeneic tumor rejection, susceptibility of host-
selected progressor variants to various immunological effector cells.
J Exp Med, 155: 557-573.
US Environmental Protection Agency (1986) Guidelines for the health
assessment of suspect developmental toxicants. Fed Regist,
51: 34027-34040.
US Environmental Protection Agency (1990) Respiratory health effects
of passive smoking: Lung cancer and other disorders
(EPA/600/6-90/006F). Cincinnati, Ohio, Center for Environmental
Research Information.
US Environmental Protection Agency (1991) Workshop report on toxicity
equivalency factors for polychlorinated biphenyl congeners. Washington
DC, Risk Assessment Forum.
US Food and Drug Administration (1993) Draft toxicological principles
for the safety assessment of direct food additives and color additives
used in food ('Redbook II'). Washington DC, Center for Food Safety and
Applied Nutrition.
US National Academy of Sciences (1977) Committee for the revision of
NAS publication No. 1138: Principles and procedures for evaluating the
toxicity of household substances. Washington DC.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Washington DC, National Academy
Press, pp 1-50.
US National Research Council (1989) Biologic markers in pulmonary
toxicology. Washington DC, National Academy Press.
US National Research Council (1992) Biologic markers in
immunotoxicology. Washington DC, National Academy Press.
US Office of Technology Assessment (1991) Identifying and controlling
immunotoxic substances: Background paper (OTA-BP-BA-75). Washington
DC, Congress of the United States, US Government Printing Office.
Vaessen LMB, Joling P, Nieuwenhuis P, Van Haperen MJ, & Rozing J
(1985) T Cell markers on pre-B cells in the rat. Adv Exp Med Biol,
186: 17-26.
Vallejo AN, Miller NW, Jorgensen T, & Clem LW (1990) Phylogeny of
immune recognition: Antigen processing/presentation in channel
catfish; immune responses to hemocyanins. Cell Immunol, 130: 364-377.
Vallejo AN, Miller NW, & Clem LW (1992) Antigen processing and
presentation in teleost immune responses. Ann Rev Fish Dis,
2: 167-183.
Valli VE, Villeneuve DC, Reed B, Barsoum N, & Smith G (1990)
Evaluation of blood and bone marrow, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI Monograph). Berlin, Springer
Verlag, pp 9-26.
Van Bressem MF, Visser IKG, Van de Bildt MWG, Teppema JS, Raga JA, &
Osterhaus ADME (1991) Morbillivirus infection in Mediterranean striped
dolphins (Stenella coeruleoalba). Vet Rec, 129: 471-472.
Vandekerckhove BAE, Krowka JF, McCune JM, De Vries JE, Spits H, &
Roncarolo MG (1991) Clonal analysis of the peripheral T cell
compartment of the SCID-hu mouse. J Immunol, 146: 4173-4179.
Van den Eertwegh AJM, Boersma WJA, & Claassen E (1992) Immunological
functions and in vivo cell-cell interactions of T cells in the
spleen. Crit Rev Immunol, 11: 337-380.
Van Deuren M, Dofferhoff AS, & Van der Meer JW (1992) Cytokines and
the response to infection. J Pathol, 168: 349-356.
Van Diepen JCE, Wagenaar GTM, & Rombout JHWM (1991) Immunocytochemical
detection of membrane antigens of carp leukocytes using light and
electron microscopy. Fish Shellfish Immunol, 1: 47-57.
Van Ewijk W (1988) Cell surface topography of thymic microenvironments.
Lab Invest, 59: 579-590.
Van Ewijk W (1991) T-Cell differentiation is influenced by thymic
microenvironments. Ann Rev Immunol, 9: 591-615.
Van Logten MJ, Gupta BN, McConnell EE, & Moore JA (1981) The influence
of malnutrition on the toxicity of tetrachlorodibenzo- p-dioxin
(TCDD) in rats. Toxicology, 21: 77-88.
Van Loveren H & Vos JG (1989) Immunotoxicological considerations: A
practical approach to immunotoxicity testing in the rat. In: Dayan AD
& Paine AJ ed. Advances in applied toxicology. London, Taylor &
Francis, pp 143-165.
Van Loveren H & Vos JG (1992) Evaluation of OECD guideline # 407 for
assessment of toxicity of chemicals with respect to potential adverse
effects to the immune system (RIVM report no. 158801001). Bilthoven,
National Institute of Public Health and Environmental Protection.
Van Loveren H, Postma GW, Van Soolingen D, Kruizinga W, Groothuis D, &
Vos JG (1987) Enhanced macrophage activity and deficient acquired
resistance to Listeria monocytogenes in nude rats. In: Rygaard J,
Brunner N, Graem N, & Spang-Thomson M ed. Immune-deficient animals in
biomedical research. Basel, Karger.
Van Loveren H, Rombout PJA, Wagenaar SS, Walvoort HC, & Vos JG (1988a)
Effects of ozone on the defence to a respiratory Listeria
monocytogenes infection in the rat. Suppression of macrophage
function and cellular immunity and aggravation of histopathology in
lung and liver during infection. Toxicol Appl Pharmacol, 94: 374-393.
Van Loveren H, Osterhaus ADME, Nagel J, Schurrman HJ, & Vos JG (1988b)
Quantification of IgA responses in the rat. Detection of IgA
antibodies and enumeration of IgA antibody producing cells specific
for ovalbumin or Trichinella spiralis. Scand J Immunol, 28: 377-381.
Van Loveren H, Teppema JS, & Askenase PW (1990a) Skin mast cells.
In: Bos J ed. The skin immune system. Boca Raton, Florida, CRC Press,
pp 171-193.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, & Vos JG (1990b)
Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Loveren H, Van Eden W, Krajnc EI, Krajnc-Franken MAM, De Kort W, &
Vos JG (1990c) Hexachlorobenzene interferes with the development of
experimental autoimmune disease in the Lewis rat. Toxicologist,
10: 221.
Van Loveren H, Verlaan APJ, & Vos JG (1991) An enzyme-linked
immunosorbent assay of anti-sheep red blood cell antibodies of the
classes M, G and A in the rat. Int J Immunopharmacol, 13: 689-695.
Van Loveren H, Timmerman H, Dortant P, & De Waal E (1993a) Validation
of a screening battery for immunotoxicity of drugs within a 28 day
oral toxicity study, using cyclosporin as a model compound (RIVM
report no. 312620001). Bilthoven, National Institute of Public Health
and Environmental Protection.
Van Loveren H Rombout PJA, Fischer P, Lebret E, & Van Bree L (1993b)
Effects of oxidant air pollution on resistance to respiratory
infection, a review (RIVM report no. 619002003). Bilthoven, National
Institute of Public Health and Environmental Protection.
Van Loveren H, Luebke RL, & Vos JG (1994) Assessment of immunotoxicity
using the host infectivity model Trichinella spiralis. In: Burleson
G, Munson A, & Dean J ed. Methods in immunotoxicology. Chicago,
Wiley-Liss.
Van Muiswinkel WB, Lamers CHJ, & Rombout JHWM (1991) Structural and
functional aspects of the spleen in bony fish. Res Immunol,
142: 362-366.
Van Rooijen N, Claassen E, Kraal G, & Dijkstra CD (1989) Cytological
basis of immune functions of the spleen. Immunocytochemical
characterization of lymphoid and non-lymphoid cells involved in the
'in situ' immune response. Progr Histochem Cytochem, 19: 1-71.
Van Soolingen D, Moolenbeek C, & Van Loveren H (1990) An improved
method of bronchoalveolar lavage of lungs of small laboratory animals:
Short report. Lab Anim, 24: 197-199.
Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, & Garattini S
(1980) Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin on humoral antibody production. Chem-Biol Interactions,
30: 337-342.
Vecchi A, Sironi M, Canegrail MA, Recchia M, & Garattini S (1983)
Immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo- p-dioxin in
strains of mice with different susceptibility to induction of aryl
hydrocarbon hydroxylase. Toxicol Appl Pharmacol, 68: 434-441.
Vercelli D & Geha RS (1992) Regulation of isotype switching. Curr Opin
Immunol, 4: 794-797.
Vermeer M, Schmieder GJ, Yoshikawa T, Van Den Berg J-W, Metzman MS,
Taylor JR, & Streilin JW (1991) Effects of ultraviolet B light on
cutaneous immune responses of humans with deeply pigmented skin. J
Invest Dermatol, 97: 729-734.
Versluis DJ, Bijma AM, Vaessen LMB, & Weimar W (1989) Changes in
immunological parameters after conversion from cyclosporin A to
azathioprine in renal transplant recipients. Int J Immunopharmacol,
11: 157-164.
Vethaak D (1993) A large-scale mesocosm study of the effects of marine
pollution on disease development in flounder (Platichthys flesus).
In: Fish disease and marine pollution: A case study of the flounder
(Platichthys flesus) in Dutch coastal and estuarine waters. Thesis
(Meetkundige Dienst RWS, ISBN 90-369-0413-7), Amsterdam, University of
Amsterdam, pp 107-127.
Vethaak AD & ap Rheinallt T (1992) Fish disease as a monitor for
marine pollution: The case of the North Sea. Rev Fish Biol Fish,
2: 1-32.
Vethaak AD & Wester PW (1993) Diseases of flounder (Platichthys
flesus) in Dutch coastal waters, with particular reference to
environmental stress factors. Part II. Liver histopathology. In: Fish
disease and marine pollution, Thesis (Meetkundige Dienst RWS, ISBN
90-369-0413-7). Amsterdam, University of Amsterdam, pp 60-78.
Vetvicka V & Fornusek L (1992) Macrophages. In: Fornusek L & Vetvicka
V ed. Immune system accessory cells. Boca Raton, Florida, CRC Press,
pp 95-108.
Vial T & Descotes J (1992) Clinical toxicity of interleukin-2. Drug
Saf, 7: 417-433.
Vial T & Descotes J (1994) Clinical toxicity of the interferons. Drug
Saf, 10: 115-150.
Vireligier J (1975) Host defenses against influenza virus: The role of
anti-hemagglutinin antibody. J Immunol, 115: 434-439.
Von Boehmer H (1990) Developmental biology of T cells in T cell-
receptor transgenic mice. Ann Rev Immunol, 8: 531-536.
Von Gaudecker B (1986) The development of the human thymus
microenvironment. Curr Topics Pathol, 75: 1-41.
Von Gaudecker B (1991) Functional histology of the thymus. Anat
Embryol, 183: 1-15.
Von Mutius E, Fritzsch C, Weiland SK, Roll G, & Magnussen H (1992)
Prevalence of asthma and allergic disorders among children in united
Germany. Br Med J, 305: 1395-1399.
Vos JG (1977) Immune suppression as related to toxicology. CRC Crit
Rev Toxicol, 5: 67-101.
Vos JG (1980) Immunotoxicity assessment: Screening and function
studies. Arch Toxicol, 4 (Suppl): 95-108.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an international
symposium (IARC Scientific Publications No 77). Lyon, International
Agency for Research on Cancer, pp 347-356.
Vos JG & Beems RB (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits. Toxicol
Appl Pharmacol, 19: 617-633.
Vos JG & Koeman TH (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis, and tissue residues.
Toxicol Appl Pharmacol, 17: 656-668.
Vos JG & Krajnc EI (1983) Immunotoxicology of pesticides. In: Hayes
AW, Schnell RC, & Miya TS ed. Developments in the science and practice
of toxicology. Amsterdam, Elsevier Science Publishers, pp 229-240.
Vos JG & Krajnc-Franken MAM (1990) Toxic effects on the immune system:
Rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system.
Berlin, Springer Verlag, pp 168-181.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Elsevier Science
Publishers, pp 295-322.
Vos JG & Moore JA (1974) Suppression of cellular immunity in rats and
mice by maternal treatment with 2,3,7,8-tetrachlorobenzo- p-dioxin.
Int Arch Allergy Appl Immunol, 47: 777-794.
Vos JG & Van Driel-Grootenhuis, L (1972) PCB-induced suppression of
the humoral and cell-mediated immunity in guinea pigs. Sci Total
Environ, 1: 289-302.
Vos JG & Van Genderen H (1973) Toxicological aspects of
immunosuppression. In: Pesticides and the environment: A continuing
controversy. North Miami, Florida, Symposia Specialists, pp 527-545.
Vos JG & Van Loveren H (1987) Immunotoxicity testing in the rat. In:
Burger EJ, Tardiff RG, & Bellanti JA ed. Advances in modern
environmental toxicology, Vol 13: Environmental chemical exposures and
immune system integrity. Princeton, New Jersey, Princeton Scientific
Publishing, pp 167-180.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979a) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Buys J, Hanstede JG, & Hagenaars AM (1979b) Comparison of
enzyme-linked immunosorbent assay and passive haemagglutination method
for quantification of antibodies to lipopolysaccharide and tetanus
toxoid in rats. Infect Immun, 24: 798-803.
Vos JG, Boerkamp J, Buys J, & Steerenberg PA (1980) Ovalbumin immunity
in the rat: Simultaneous testing of IgM, IgG and IgE response measured
by ELISA and delayed-type hypersensitivity. Scand J Immunol,
12: 289-295.
Vos JG, Krajnc EI, & Beekhof P (1982) Use of the enzyme-linked
immunosorbent assy (elisa) in immunotoxicity testing. Environ Health
Perspectives, 43: 115-121.
Vos JG, Krajnc EI, Beekhof PK, & Van Logten MJ (1983a) Methods for
testing immune effects of toxic chemicals: Evaluation of the
immunotoxicity of various pesticides in the rat. In: Miyamoto T ed.
IUPAC pesticide chemistry: Human welfare and the environment. Oxford,
Pergamon Press, pp 497-504.
Vos JG, Ruitenberg EJ, Van Basten N, Buys J, Elgersma A, & Kruizinga W
(1983b) The athymic nude rat. IV. Immunocytochemical study to detect
T cells, and immunological and histopathological reactions against
Trichinella spiralis. Parasite Immunol, 5: 195-215.
Vos JG, De Klerk A, Krajnc EI, Kruizinga W, Van Ommen B, & Rozing J
(1984) Toxicity of bis(tri-n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicol Appl
Pharmacol, 75: 387-408.
Vos JG, Van Loveren H, Wester P, & Vethaak D (1989) Toxic effects of
environmental chemicals on the immune system. TiPs, 10: 289-292.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rozing J (1990a)
Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on
thymus-dependent-immunity and non-specific resistance following long-
term exposure in young versus aged rats. Toxicol Appl Pharmacol,
105: 144-155.
Vos JG, Dormans JAMA, Kraal G, Krajnc EI, Krajnc-Franken MAM, & Van
Loveren H (1990b) Immunotoxicity of hexachlorobenzene in the rat is
thymus-dependent. Toxicologist, 10: 221.
Vos JG, Van Loveren H, & Schuurman H-J (1991) Immunotoxicity of
dioxin: Immune function and host resistance in laboratory animals and
humans. In: Biological basis for risk assessment of dioxins and
related compounds (Banbury report 35). Cold Spring Harbor, New York,
CSH Press, pp 79-93.
Wakelin D (1993) Allergic inflammation as a hypothesis for the
expulsion of worms from tissues. Parasitol Today, 9: 115-116.
Waldman TA (1988) Immunodeficiency diseases: Primary and acquired.
In: Immunological diseases. Boston, Little Brown Co, pp 441-466.
Ward JM (1990) Classification of reactive lesions of lymph nodes.
In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system (ILSI
Monograph). Berlin, Springer Verlag, pp 155-162.
Ward P & Kendall MD (1975) Morphological changes in the thymus of
young and adult red-billed queleas Quelea quelea (aves). Phil Trans
R Soc Lond B, 273: 55-64.
Ward EC, Murray MJ, & Dean JH (1985) Immunotoxicity of nonhalogenated
polycyclic aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE, &
Amos H ed. Immunotoxicology and immunopharmacology. New York, Raven
Press, pp 291-304.
Ware JH, Dockery DW, Spiro A, Speizer FE, & Ferris BG (1984) Passive
smoking, gas cooking, and respiratory health of children living in six
cities. Am Rev Respir Dis, 129: 366-374.
Watson RR (1990) Drugs of abuse and immune function. Boca Raton,
Florida, CRC Press.
Watson RR (1993) Alcohol, drugs of abuse and immunomodulation
(Advances in the Biosciences, Vol 86). Oxford, Pergamon Press.
Weeks BA & Warinner JE (1986) Functional evaluation of macrophages in
fish from a polluted estuary. Vet Immunol Immunopathol, 12: 313-320.
Weeks BA, Anderson DP, Dufour AP, Fairbrother A, Goven AJ, Lahvis GP,
& Peters G (1992) Immunological biomarkers to assess environmental
stress. In: Biomarkers, biochemical, physiological and histological
markers of anthropo-genic stress. London, Lewis Publishers, pp 5-85.
Weigent DA & Blalock JE (1987) Interactions between the neuroendocrine
and immune systems: Common hormones and receptors. Immunol Rev,
100: 79-108.
Weiss L & Geduldig U (1991) Barrier cells: Stromal regulation of
hematopoiesis and blood release in normal and stressed murine bone
marrow. Blood, 78: 975-990.
Weiss L & Sakai H (1984) The hematopoietic stroma. Am J Anat,
170: 447-463.
Weissgarten J, Freidman CNGE, & Cardella CJ (1989) B Lymphocytes from
stable renal transplant patients do not respond to exogenous
lymphokines: Comparison of azathioprine and cyclosporin treated
patients. Transplant Proc, 21: 393-394.
Weissler JC, Nicod LP, & Toews GB (1987) Pulmonary natural killer cell
activity is reduced in patients with bronchogenic carcinoma. Am Rev
Respir Dis, 135: 1353-1357.
Wessel G, Abenroth K, & Wisheit M (1988) Malignant transformation
during immunosuppressive therapy (azathioprine) of rheumatoid
arthritis and systemic lupus erythematosus. Scand J Rheumatol, 67
(Suppl): 73-75.
Wester PW, & Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to bis(tri- n-
butyltin)oxide (TBTO) and di- n-butyltindichloride (DBTC). Aquat
Toxicol 10: 143-165.
Wester PW & Canton JH (1991) The usefulness of histopathology in
aquatic toxicity studies. Comp Biochem Physiol, 100C: 115-117.
Wester PW, Canton JH, Van Iersel AAJ, Krajnc EI ,& Vaessen HAMG
(1990) The toxicity of bis(tri- n-butyltin)oxide (TBTO) and
di- n-butyltindichloride (DBTC) in the small fish species Oryzias
latipes (medaka) and Poecilia reticulata (guppy). Aquat Toxicol,
16: 53-72.
Wester PW, Vethaak AD, & Van Muiswinkel WB (1994) Fish as biomarkers
in immunotoxicology. Toxicology, 86: 213-232.
White KL (1986) An overview of immunotoxicology and carcinogenic
polycyclic aromatic hydrocarbons. J Environ Health Sci, C4: 163-202.
White KL (1992) Specific immune function assays. In: Miller K, Turk J,
& Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 304-323.
White KL & Munson AE (1986) Suppression of the in vitro humoral
immune response by chrysotile asbestos. Toxicol Appl Pharmacol,
82: 493-504.
White KL, Sander VM, Barnes DW, Shopp GM, & Munson AE (1985)
Immunotoxicological investigations in the mouse: General approach and
methods. Drug Chem Toxicol, 8: 299-331.
White KL, Lysy HH, McCay JA, & Anderson AC (1986) Modulation of serum
complement levels following exposure to polychlorinated dibenzo- p-
dioxins. Toxicol Appl Pharmacol, 84: 209-219.
White KL, Gennings C, Murray MJ, & Dean JH (1994) Summary of
international methods: Validation study, carried out in nine
laboratories, on immunological assessment of cyclosporin A in the rat.
In Vitro Toxicity, 8: 957-961.
WHO (1990) Revised guidelines for preparation of Environmental Health
Criteria monographs (PCS/90.69), Geneva
Wierda D, Irons RD, & Greenlee WF (1981) Immunotoxicity in C57Bl/6
mice exposed to benzene and Aroclor 1254. Toxicol Appl Pharmacol,
60: 410-417.
Williams ME & Quesenberry PJ (1992) Hematopoietic growth factors.
Hematol Pathol, 6: 105-124.
Wilson MR & Warr GW (1992) Fish immunoglobulins and the genes that
encode them. Ann Rev Fish Dis, 201-221.
Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, & Steinberg C
(1992) What limits affinity maturation of antibodies in Xenopus: The
rate of somatic mutation or the ability to select mutants? EMBO J,
11: 4337-4347.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity in
mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20: 423.
Winkelstein JA (1981) The role of complement in the host defense
against Streptococcus pneumoniae. Rev Infect Dis, 3: 289-298.
Wolke RE (1992) Piscine macrophage aggregates: A review. Ann Rev Fish
Dis, 91-108.
Wood SC, Karras JG, & Holsapple MP (1992) Integration of the human
lymphocyte into immunotoxicological investigations. Fundam Appl
Toxicol, 18: 450-459.
Wooley PH (1991) Animal models of rheumatoid arthritis. Curr Opin
Rheumatol, 3: 407-420.
Wright EG & Pragnell IB (1992) Stem cell proliferation inhibitors.
Bailliere's Clin Haematol, 5: 723-739.
Yamaguchi T, Shinagawal Y, & Pollard RB (1988) Relationship between
the production of murine cytomegalovirus and interferon in
macrophages. J Gen Virol, 69: 2961-2971.
Yang KH, Kim BS, Munson AE, & Holsapple MP (1986) Immunosuppression
induced by chemicals requiring metabolic activation in mixed cultures
of rat hepatocytes and murine splenocytes. Toxicol Appl Pharmacol,
83: 420-429.
Yang YG, Lebrec H, & Burleson GR (1994) Effect of 2,3,7,8
tetrachlorodibenzo- p-dioxin (TCDD) on viral replication and natural
killer (NK) activity in rats. Fundam Appl Toxicol, 23: 125-131.
Yang YG, Gilmour MI, Lange R, Burleson GR, & Selgrade MJK (1995)
Effects of acute exposure to phosgene on pulmonary host defenses and
resistance to infection. Inhal Toxicol, 7: 393-404.
Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, & Santos GW
(1993) Cyclosporine-induced graft-versus-host disease after autologous
bone marrow transplantation for acute myeloid leukemia. Leukemia
Lymphoma, 11: 215-220.
Yoshida T & Streilin JW (1990) On the genetic basis of the effects of
ultraviolet B on cutaneous immunity. Evidence that polymorphisms at
the TNFa and Lps loci govern susceptibility. Immunogenetics,
32: 398-405.
Yoshikawa T, Rae V, Bruins-Slot W, Van Den Berg J-W, Taylor JR, &
Streilin JW (1990) Susceptibility to effects of UVB radiation on
induction of contact hypersensitivity as a risk factor for skin cancer
in humans. J Invest Dermatol, 95: 530-536.
Yosioka T, Sato S, Fujiwana H, & Hamacka T (1986) The role of GM-1
antibody-sensitive cells in the implementation of tumor-specific
T-cell mediated immunity in vivo. Jpn J Cancer Res, 77: 825-832.
Zapata A (1982) Lymphoid organs of teleost fish III. Splenic lymphoid
tissue of Rutilus rutilus and Gobio gobio. Dev Comp Immunol,
6: 87-94.
Zapata A (1983) Phylogeny of the fish immune system. Bull Inst
Pasteur, 81: 185-186.
Zapata AG & Cooper EL (1990) The immune system: Comparative
histophysiology. New York, John Wiley & Sons.
Zapata AG, Varas A, & Torroba M (1992) Seasonal variations in the
immune system of lower vertebrates. Immunol Today, 13: 142-147.
Zeeman MG & Brindley WA (1981) Effects of toxic agents upon fish
immune systems: A review. In: Sharma RP, ed. Immunologic
considerations in toxicology, Vol II. Boca Raton, Florida, CRC Press,
pp 1-60.
Zelikoff JT, Enane NA, Bowser D, Squibb KS, & Frenkel K (1991)
Development of fish peritoneal macrophages as a model for higher
vertebrates in immunotoxicological studies. Fundam Appl Toxicol,
16: 576-589.
Zheng B, Shorthouse R, Masek MA, Berry G, Billingham ME, & Morris RE
(1991) Effect of the new and highly active immunosuppressant,
Rapamycin, on lymphoid tissues and cells in vivo. Transplant Proc,
23: 851-855.
Zwick H, Popp W, Wagner C, Reiser K, Schmoger J, Bock A, Herkner K, &
Radunsky K (1991) Effects of ozone on the respiratory health allergic
sensitization, and cellular immune system in children. Am Rev Respir
Dis, 144: 1075-1079.
1. RESUME
1. Le système immunitaire a évolué de manière à contrer les
atteintes que peuvent porter à l'intégrité du soi des microorganismes
ou des cellules ayant échappé au contrôle de l'organisme. Une
intrusion xénobiotique peut perturber le fonctionnement du système
immunitaire et c'est la reconnaissance de cet état de choses qui a
permis les progrès de ces vingt dernières années. Toute une
méthodologie expérimentale a été développée et validée
(essentiellement sur des rongeurs) par des études impliquant une
multitude de laboratoires. La présente monographie examine les
fonctions et l'histophysiologie du système immunitaire et présente les
données nécessaires à la compréhension et à l'interprétation des
modifications pathologiques provoquées par les agressions
immmunotoxiques. L'accent est mis sur le système immunitaire de
l'homme et des rongeurs, mais sans négliger d'autres espèces, les
poissons en particulier, auxquelles des études immunotoxicologiques
ont été consacrées. Il importe, pour comprendre l'impact des effets
immunotoxiques, de connaître la pathophysiologie du système
immunitaire, et notamment la sensibilité variable de ses constituants,
les modifications subies par les organes lymphoïdes et la réversibilité
de ces modifications.
2. L'immunosuppression et l'immunostimulation ont toutes deux des
conséquences sur le plan clinique. On a établi une corrélation entre
les états d'immunodéficience ou d'immunodépression grave que l'on
observe par exemple chez des patients greffés ou sous traitement
cytostatique, et l'accroissement de l'incidence des maladies
infectieuses (opportunistes en particulier) et du cancer. L'exposition
à des substances chimiques immunotoxiques présentes dans l'environnement
peut toutefois donner lieu à des formes plus subtiles d'immunodépression,
difficiles à déceler, qui entraînent une augmentation de l'incidence de
maladies infectieuses comme la grippe ou le rhume. Des travaux effectués
sur des animaux de laboratoire et sur l'homme montrent que de nombreux
produits chimiques présents dans l'environnement dépriment la réponse
immunitaire. Ces substances xénobiotiques immunotoxiques ne se limitent
pas à untype particulier de composé chimique ou d'agent physique. Il y
a, parmi les médicaments, les pesticides, les solvants, les hydro-
carbures halogénés, les hydrocarbures aromatiques et les métaux, des
substances susceptibles d'altérer le système immunitaire et le
rayonnement ultra-violet a également cette propriété. L'administration,
à des fins thérapeutiques, d'agents immunostimulants, peut avoir des
effets nocifs et certaines substances (béryllium, silice, hexa-
chlorobenzène) présentes dans l'environnement, ont des propriétés
immunostimulantes qui peuvent se traduire par des effets cliniques.
3. Du fait de sa complexité, le système immunitaire offre une
multiplicité de cibles potentielles à l'action de ces agents avec
toutes sortes de séquelles pathologiques. Les premières stratégies
élaborées par les immunotoxicologues ont consisté à choisir et à
mettre en oeuvre une batterie de tests multiphasiques sur animaux de
laboratoire afin d'identifier les agents immunosuppresseurs ou
immunostimulants. Ces batteries de tests peuvent varier selon
l'organisme ou le laboratoire qui les mettent en oeuvre ou encore
selon l'espèce animale utilisée, mais elles comportent toutes un ou
plusieurs des éléments suivants: modification du poids ou de
l'histologie des organes lymphoïdes; examen des leucocytes du sang
périphérique, des cellules du tissu lymphoïde ou de la moelle osseuse
à la recherche de modifications; intégrité de la fonction des cellules
effectrices et régulatrices et modification éventuelle de la
sensibilité à une exposition à des agents infectieux ou à des cellules
tumorales.
La directive expérimentale originale No 407 de l'Organisation de
coopération et de développement économiques, publiée en 1981, n'avait
pas pour but la mise en évidence d'une immunotoxicité potentielle,
aussi a-t-elle été modifiée pour la rendre plus adaptée à
l'identification des substances immunotoxiques. Des systèmes d'épreuve
multiphasiques permettant une investigation plus large de
l'immunotoxicité ont été conçus par l'US National Toxicology Program,
l'Institut Néerlandais de la Santé Publique et de la Protection de
l'Environnement, l'US Environmental Protection Agency (Office of
Pesticides), ainsi que par le Center for Food Safety andApplied
Nutrition de l'US Food and Drug Administration. Des études ont été
effectuées sur des souris et, à un moindre degré, sur des rats afin
d'évaluer la spécificité, la précision (reproductibilité), la
sensibilité, l'exactitude et la pertinence, pour l'appréciation du
risque sanitaire, de diverses mesures de l'état immunitaire. On a
entrepris la validation interlaboratoires, au niveau international, de
toutes ces méthodes, dans le cadre de l'Etude collective
internationale sur l'immunotoxicité organisée par le PISC, l'Union
européenne et le Bundesinstitut für Gesundheitllichen
Verbraucherschutz und Veterinärmedizin. Des études analogues ont été
menées sur des rats Fischer 344 à propos de la cyclosporine A.
4. Les épreuves utilisées dans le système multiphasique sont
décrites à la section 3 avec les raisons qui ont guidé ce choix et un
exposé des difficultés que l'on peut rencontrer dans leur mise en
oeuvre. Bien que conçus à l'origine pour des études sur des rats et
des souris, certains de ces protocoles ont pu être utilisés avec fruit
pour des travaux d'immunotoxicologie portant sur d'autres espèces,
notamment des primates non humains, des mammifères marins, des chiens,
des oiseaux et des poissons.
Lorsqu'on se propose de déterminer dans quelle mesure un agent
environnemental ou un médicament est susceptible d'avoir une influence
nocive sur le système immunitaire, il faut prendre en considération un
certain nombre de facteurs. Il s'agit notamment du choix d'un modèle
animal et de variables d'exposition appropriés, de la prise en compte
des paramètres toxicologiques généraux, de la pertinence biologique
des points d'aboutissement retenus, de la mesure de grandeurs dûment
validées et de la mise en oeuvre d'un système d'assurance de la
qualité. Les conditions expérimentales doivent tenir compte des
modalités de l'exposition humaine (voie de pénétration et
concentration ou intensité) et de toute information disponible d'ordre
toxicodynamique ou toxicocinétique. Les doses et la taille des
échantillons doivent être choisies de manière à permettre l'obtention
de bonnes courbes dose-réponse et la détermination de la dose sans
effet (nocif ou non) observable. Cesstratégies sont améliorées en
permanence afin de permettre une meilleure prévision des situations
susceptibles d'entraîner une pathologie. En outre, on devrait pouvoir
disposer de techniques qui faciliteraient l'étude du mode d'action des
agents en cause. Il pourrait s'agir de méthodes in vitro, de l'étude
des réponses immunitaires locales (par exemple au niveau de la peau,
des poumons et de l'intestin), de techniques de biologie moléculaire
et de l'utilisation d'animaux génétiquement modifiés.
5. Mettre en évidence des modifications d'ordre immunologique après
une exposition à des composés potentiellement immunotoxiques est plus
complexe chez l'homme que chez l'animal de laboratoire. Les
possibilités d'expérimentation sont limitées, il est difficile
d'établir le niveau d'exposition à l'agent en cause (c'est-à-dire la
dose) et de plus, l'état immunitaire des populations est extrêmement
hétérogène. Cette hétérogénéité trouve son origine dans un certain
nombre de facteurs: âge, sexe, race, gravidité, stress et aptitude à y
faire face, pathologies et états infectieux cocomitants, état
nutritionnel, tabagisme et prise de certains médicaments. L'intérêt de
telle ou telle étude pour l'évaluation du risque est conditionné par
un élément important, à savoir le concept épidémiologique qui la sous-
tend. La plupart du temps on procède à une étude transversale, qui
consiste à déterminer l'exposition et la morbidité à un moment donné
ou sur une courte période. On compare ensuite la fonction immunitaire
des sujets exposés à celle d'un groupe analogue de sujets non exposés.
Ce genre d'étude peut receler un certain nombre de pièges.
Nombre des altérations que produit l'exposition à une substance
chimique ne se manifestent chez l'homme que de manière subtile et
sporadique, aussi faut-il étudier des populations récemment exposées
et utiliser des épreuves sensibles.
La plupart des épreuves concernant l'immunité spécifique (à
médiation cellulaire ou humorale), l'immunité non spécifique et les
processus inflammatoires ont été conçues pour rechercher des anomalies
chez des patients atteints d'une déficience immunitaire et ne sont pas
forcément capables de déceler les modifications subtiles provoquées
par les substances chimiques. Le PISC, les Centers for Disease Control
et l'Académie des Sciences des Etats-Unis ont, chacun de leur côté,
défini une méthodologie pour évaluer les modifications du système
immunitaire quipourraient résulter d'une exposition à des produits
immunotoxiques mais les épreuves proposées demandent encore à être
validées.
6. L'évaluation du risque consiste à analyser les données
pertinentes sur un agent donné (effets biologiques, relations dose-
réponse et exposition) pour tenter d'obtenir une estimation
qualitative et quantitative des diverses conséquences nocives de la
présence de cet agent. Elle se caractérise par quatre phases
principales: reconnaître le danger, établir la relation dose-réponse,
évaluer l'exposition et caractériser le risque. Jusqu'ici,
l'immunotoxicologie s'est essentiellement attachée à reconnaître le
danger et, dans une certaine mesure, à établir des relations dose-
réponse, mais très peu d'études ont été consacrées à l'évaluation du
risque ou à sa caractérisation.
Comme dans d'autres domaines de la toxicologie, des incertitudes
demeurent qui sont susceptibles de gêner l'interprétation des données
immunotoxicologiques dans l'optique du risque pour la santé humaine.
Les deux questions qui sont actuellement les plus problématiques -
l'extrapolation à l'organe dans son ensemble des effets constatés sur
la cellule et l'extrapolation à l'homme des données obtenues sur
l'animal - valent pour la plupart des points d'aboutissement des
effets, cancer excepté. Le premier problème tient aux incertitudes que
comporte l'établissement d'une relation quatitative entre les
modifications subies par la fonction immunitaire d'un individu et
l'altération de sa résistance aux maladies infectieuses et
néoplasiques. Le deuxième est lié à l'incertitude qui entache
l'évaluation du risque pour la santé humaine à partir des résultats
obtenus sur des animaux de laboratoire.
L'évaluation du risque a pour finalité de protéger la santé
humaine et l'environnement. Il faut donc que le choix des modéles
expérimentaux soit judicieux. La toxicocinétique de la substance
étudiée et la réponse immunitaire suscitée dans le modèle doivent
pouvoir être comparées à celles qu'on observerait chez l'homme.
Pour tirer une limite d'exposition des résultats expérimentaux,
il est convenu d'appliquer un facteur d'incertitude aux données
d'évaluation du risque. Cette convention ne tient pas compte de la
réserve fonctionnelle ou de la redondance du système immunitaire. Une
méthode plus récente d'évaluation du risque consiste à ultiliser des
modèles in vitro en complément aux études sur animaux de laboratoire.
Cette méthode a l'avantage de permettre une extrapolation plus fidèle
de l'animal à l'homme et de ne nécessiter qu'un minimum d'animaux.
Elle permet également de pallier l'absence de données dans les cas où
des considérations d'éthique limitent l'expérimentation sur l'homme.
Le Chapitre 6 donne deux exemples de situation où les données obtenues
in vitro permettent de lever en partie les incertitudes dans
l'évaluation du risque dû à l'exposition à l'ozone et au rayonnement
ultra-violet. L'utilisation des données immunotoxicologiques dans
l'évaluation du risque reste limitée par la difficulté d'établir des
relations quantitatives entre l'immunodépression et les manifestations
cliniques d'une pathologie donnée.
RESUMEN
1. El sistema inmunitario ha evolucionado para hacer frente a las
amenazas a la integridad del organismo vivo provenientes de
microorganismos o de células que han escapado a los mecanismos de
control del organismo. El reconocimiento de que las sustancias
xenobióticas pueden trastornar el funcionamiento del sistema
inmunitario ha llevado a avances en el campo de la inmunotoxicología
durante los dos últimos decenios. Se han formulado métodos
experimentales (empleando principalmente especies de roedores), que
han sido validados en estudios multilaboratorio. En esta monografía se
examinan la función y la histofisiología del sistema inmunitario,
presentándose la información necesaria para la comprensión e
interpretación de los cambios patológicos causados por las agresiones
inmunotóxicas. Si bien se hace hincapié en los sistemas inmunitarios
del ser humano y de las especies de roedores, se hace referencia a
otras especies, incluidos los peces, que han sido objeto de estudios
inmunotoxicológicos. La fisiopatología del sistema inmunitario,
incluidas la sensibilidad variable de sus componentes, las
alteraciones de los órganos linfoides y la reversibilidad de los
cambios, es importante para comprender las repercusiones de la
inmunotoxicidad.
2. Tanto la inmunosupresión como la inmunoestimulación tienen
consecuencias clínicas. Se ha observado que los estados de
inmunodeficiencia y de inmunosupresión grave, como los que se pueden
presentar en casos de trasplante y de terapia citostática, van
acompañados de mayor incidencia de enfermedades infecciosas
(especialmente las oportunistas) y de cáncer. Con todo, cabe prever
que la exposición a los productos químicos inmunotóxicos enel medio
ambiente dará origen a formas más sutiles de inmunosupresión cuya
detección podría resultar difícil, lo que se traduciría en una mayor
incidencia de infecciones tales como la gripe y el resfriado común.
Estudios realizados con animales de laboratorio y seres humanos han
mostrado que muchos de los productos químicos presentes en el medio
ambiente provocan la supresión de la respuesta inmunitaria. Las
sustancias xenobióticas inmunotóxicas no se limitan a una clase
determinada de productos químicos. Entre los compuestos que tienen
efectos nocivos para el sistema inmunitario se cuentan fármacos,
plaguicidas, disolventes, hidrocarburos halogenados y aromáticos y
metales; la radiación ultravioleta puede resultar también
inmunotóxica. La administración terapéutica de agentes
inmunoestimulantes puede provocar reacciones adversas; asimismo,
algunos de los productos químicos presentes en el medio ambiente que
poseen propiedades inmunoestimulantes (berilio, sílice,
hexaclorobenceno) pueden tener consecuencias clínicas.
3. La complejidad del sistema inmunitario lleva aparejadas
multiplicidad de posibles puntos vulnerables y secuelas patológicas.
Los métodos iniciales ideados por los inmunotoxicólogos que realizan
investigaciones sobre toxicología y evaluación de la inocuidad
consistían en seleccionar y aplicar una serie de valoraciones
escalonadas para identificar los agentes inmunosupresores e
inmunoestimulantes en animales de laboratorio. Si bien la
configuración de esas series de análisis puede variar según la
institución o el laboratorio en que se llevan a cabo, así como según
las especies de animales empleadas, en todas se mide unoo más de los
siguientes parámetros: alteraciones del peso y de la histología de los
órganos linfoides; cambios en la celularidad del tejido linfoide, de
los leucocitos en la sangre periférica y/o de la médula ósea;
trastornos de la función celular a nivel de los efectores o de la
regulación, y alteración de la sensibilidad a la amenaza que presentan
los agentes infecciosos o las células tumorales.
La Directriz de pruebas inicial No 407 de la Organización de
Cooperación y Desarrollo Económicos, publicada en 1981, no preveía la
detección de los riesgos de inmunotoxicidad, y se han propuesto
modificaciones destinadas a aumentar la utilidad de esa Directriz para
la identificación de las sustancias inmunotóxicas. El Programa
Nacional de Toxicología de los Estados Unidos, el Instituto Nacional
de Salud Pública y Protección del Medio Ambiente de los Países Bajos,
la Oficina de Plaguicidas de la Agencia para la Protección del Medio
Ambiente de los Estados Unidos y el Centro de Seguridad de los
Alimentos y Nutrición Aplicada de la Administración de Alimentos y
Medicamentos de los Estados Unidos han elaborado sistemas de pruebas
escalonadas para la investigación en mayor escala de los riesgos de
inmunotoxicidad. Se han realizado estudios con ratones, y en menor
medida con ratas, de diferentes indicadores del estado inmunológico
con el fin de determinar su especificidad, precisión (reproducibilidad),
sensibilidad, exactitud y pertinencia para la evaluación de los riesgos
para la salud humana. Los métodos han sido objeto de validaciones
internacionales interlaboratorios en el marco del Estudio Internacional
en Colaboración sobre Inmunotoxicidad del IPCS, la Unión Europea y el
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinärmedizin, yen estudios sobre la ciclosporina A en ratas
Fisher 344.
4. Las pruebas empleadas en los programas de verificaciones
escalonadas se describen en la Sección 3, en la que se indican la
razón de ser de su selección y las complejidades que entraña su
realización. Si bien esos protocolos fueron diseñados para estudios
realizados con ratas y ratones, algunos de ellos se han aplicado con
buenos resultados al estudio de la inmunotoxicidad en otras especies
animales, incluidos primates no humanos, mamíferos marinos, perros,
aves y peces.
A la hora de evaluar las posibles repercusiones negativas de un
agente ambiental o fármaco sobre el sistema inmunitario de los
animales experimentales deberán considerarse diversos factores. Entre
ellos cabe señalar: la selección de los modelos y las variables de
exposición apropiados para los animales, la inclusión de parámetros
toxicológicos generales, la comprensión de la importancia de los
parámetros objeto de medición, el empleo de medidas validadas y el
control de la calidad. En las condiciones experimentales deberán
tenerse en cuenta las vías y el nivel posibles de exposición del ser
humano, así como toda la información disponible sobre toxicodinámica y
toxicocinética. Las dosis y el tamaño de las muestras deberán
seleccionarse de manera que se puedan obtener curvas de dosis-
respuesta bien definidas, además del nivel sin efectos adversos
observados y del nivel sin efectos observados. Los métodos se
perfeccionan continuamente para poder predecir mejor las condiciones
que podrían ser causantes de enfermedades. Además, deberán elaborarse
técnicas que contribuyana la identificación de mecanismos de acción;
éstos podrían incluir los métodos in vitro, el examen de las
respuestas inmunitarias locales (por ejemplo, en la piel, los pulmones
y los intestinos), y el empleo de las técnicas de la biología
molecular y de animales modificados genéticamente.
5. La detección de los cambios inmunológicos ocurridos tras la
exposición a compuestos que podrían ser inmunotóxicos resulta más
complicada en el ser humano que en los animales de laboratorio. Las
posibilidades de realizar pruebas son limitadas; los niveles de
exposición al agente (es decir, la dosis) son difíciles de establecer
y el estado inmunitario de las poblaciones es sumamente heterogéneo.
La edad, la raza, el sexo, la gestación, el estrés agudo y la
capacidad para hacerle frente, las enfermedades e infecciones
coexistentes, el estado nutricional, el humo del tabaco y algunos
medicamentos se cuentan entre los factores que contribuyen a esa
heterogeneidad.
El diseño de los estudios epidemiológicos es un factor importante
para determinar la utilidad de un estudio determinado para la
evaluación de riesgos. El tipo de diseño empleado más frecuentemente
en materia de inmunotoxicidad son los estudios transversales, en los
que se miden las condiciones de exposición y el estado de la
enfermedad en un momento dado, o durante un periodo breve. A
continuación se compara la función inmunitaria de los sujetos
«expuestos» con la de un grupo comparable de individuos
«no expuestos». Ese tipo de diseño lleva aparejados posibles escollos.
Como muchos de los cambios observados en la respuesta inmunitaria
de los seres humanos tras su exposición a un productoquímico podrían
ser esporádicos y sutiles, habrá que estudiar las poblaciones que han
estado expuestas recientemente empleando pruebas de gran sensibilidad
para la evaluación del sistema inmunitario. Las conclusiones sobre los
efectos inmunotóxicos deberán estar basadas en las variaciones
detectadas no en un parámetro aislado sino en el perfil inmunológico
del individuo o de la población.
La mayoría de las pruebas existentes para determinar la inmunidad
específica (de base celular y humoral), la inmunidad no específica y
la inflamación han sido concebidas para detectar las alteraciones
inmunitarias en pacientes que padecen de inmunodeficiencia y no
siempre resultan adecuadas para detectar las alteraciones sutiles
provocadas por los productos químicos presentes en el medio ambiente.
El IPCS, los Centros de Control de Enfermedades y la Academia de
Ciencias de los Estados Unidos han descrito procedimientos para
evaluar los cambios que ocurren en el sistema inmunitario del ser
humano como consecuencia de la exposición a sustancias inmunotóxicas;
con todo, las pruebas descritas deberán ser evaluadas con este fin.
6. La evaluación de riesgos es un proceso en el que se analizan la
información pertinente sobre los efectos biológicos, las relaciones
dosis-respuesta y la exposición a un agente determinado con miras a
establecer estimaciones cualitativas y cuantitativas de los resultados
adversos. Por regla general, la evaluación de los riesgos supone
cuatro pasos fundamentales: identificación de los riesgos; evaluación
de la relación dosis-respuesta; evaluación de la exposición y
caracterización de los riesgos. Hasta ahora, lainmunotoxicología se ha
centrado principalmente en la identificación de los riesgos y, en
cierta medida, en la evaluación de las relaciones dosis-respuesta; muy
contados han sido los estudios que han incluido la evaluación de la
exposición o la caracterización de los riesgos.
Al igual que ocurre en otros campos de la toxicología, existen
incertidumbres que podrían afectar a la interpretación de los datos
sobre inmunotoxicidad en cuanto a los riesgos para la salud humana.
Las dos cuestiones más problemáticas - la extrapolación de los efectos
observados en células individuales a todo un órgano, o a niveles
superiores, y la extrapolación al ser humano de los resultados
obtenidos en experimentos con animales - son comunes a la mayoría de
los parámetros de valoración no relacionados con el cáncer. La primera
obedece a las incertidumbres vinculadas al establecimiento de una
relación cuantitativa entre los cambios observados en la función
inmunitaria del individuo y la perturbación de la resistencia a las
infecciones y enfermedades neoplásicas. La segunda cuestión es
consecuencia de las incertidumbres que lleva aparejadas la evaluación
de los riesgos para la salud humana basándose en los estudios
realizados con animales de laboratorio.
El objetivo fundamental de la evaluación de los riesgos es la
protección de la salud de los seres humanos y del medio ambiente. Por
lo tanto, deberán seleccionarse sistemas modelo idóneos. La
toxicocinética del material de prueba y la índole y magnitud de la
respuesta inmunitaria generada en el modelo deberán ser comparables a
la de los seres humanos.
Habitualmente, en la evaluación de los riesgos se emplean
factores empíricos de incertidumbre para determinar el límite de
exposición aceptable a partir de los resultados experimentales. Ese
procedimiento no toma en cuenta la reserva funcional ni la redundancia
del sistema inmunitario. Un adelanto más reciente en materia de
evaluación de riesgos es el empleo de modelos in vitro como
complemento de los estudios realizados con animales de laboratorio.
Ese procedimiento tiene la ventaja de que permite aumentar la
exactitud de la extrapolación al ser humano de los resultados
obtenidos en los experimentos realizados con animales, reduciendo al
mínimo el número de animales necesarios; asimismo, permite colmar la
brecha entre ambos tipos de información, sobre todo en los casos en
que los experimentos con seres humanos se ven limitados por
consideraciones de índole ética. En el capítulo 6 se presentan dos
ejemplos de cómo la información in vitro permite reducir las
incertidumbres en materia de evaluación de riesgos relacionadas con la
exposición al ozono y a la radiación ultravioleta. La dificultad para
establecer relaciones cuantitativas entre la inmunosupresión y las
enfermedades clínicas ha limitado el empleo de los datos
inmunotoxicológicos en la evaluación de los riesgos.
Table 15. Chi-squared values (hypothetical)
Treatment Design 1 Design 2 Design 3
------------------------------- ------------------------------- --------------------------------
No. affected/ One-tailed No. affected/ One-tailed No. affected/ One-tailed
no. tested P value no. tested P value no. tested P value
Control 3/15 - 6/30 - 0/15 -
Dose 1 4//15 0.532 8/30 0.428 1/15 0.516
2 5/15 0.411 10/30 0.273 2/15 0.274
3 6/15 0.312 12/30 0.165 3/15 0.150
4 7/15 0.233 14/30 0.096 4/15 0.084
5 8/15 0.173 16/30 0.055 5/15 0.048*
6 9/15 0.128 18/30 0.031* 6/15 0.028*
7 10/15 0.094 20/30 0.017* 7/15 0.017*
8 11/15 0.069 22/30 0.009* 8/15 0.010*
9 12/15 0.051 24/30 0.005* 9/15 0.006*
10 13/15 0.038* 26/30 0.003* 10/15 0.004*
11 14/15 0.028* 28/30 0.002* 11/15 0.002*
12 15/15 0.021* 30/30 0.001* 12/15 0.002*
groups of mice pretreated with either vehicle (saline), 50 mg/kg
cyclophosphamide (causing minimal immunosuppression), or 200 mg/kg
cyclophosphamide (causing severe immunosuppression) were administered
various numbers of PYB6 tumour cells. Even vehicle-treated mice
developed a high frequency of tumours, provided that the challenge was
sufficiently high (i.e. 8 × 104 tumour cells). In contrast, severely
immunosuppressed mice (high dose of cyclophosphamide) developed an
increased tumour frequency at all challenge levels of PYB6 tumour
cells. The groups treated with the low dose of cyclophosphamide were
of particular interest, since evidence of increased susceptibility
appeared but only as a function of the tumour cell concentration.
Assuming that these observations are applicable to human populations,
even small changes in immune function could increase the likelihood of
disease.
As indicated earlier, while experimental data have been used
occasionally in risk assessment, most immunotoxicological data have
focused on hazard identification. Although comparative data on the
effects of specific immunotoxic agents in humans and animals are
limited, other factors contribute to the minimal use of these data in
risk assessment, including the concern that immunotoxicity testing has
often been conducted without full knowledge of its predictive value in
humans or its quantitative relationship to immune-mediated diseases.
Luster et al. (1988) reported on the design and content of a
screening battery involving a tier approach for detecting potential
immunosuppressive compounds in mice. This battery has been used to
examine a variety of compounds, and the database, generated on over 50
compounds, has been analysed in an attempt to improve the accuracy and
efficiency of tests for screening chemicals for immunosuppression and
to identify better those tests that predict experimentally induced
immune-mediated diseases (Luster et al., 1992, 1993). Specifically,
attempts have been made to develop a 'streamlined' test configuration
for accurately predicting immunotoxic agents and to establish models
that could be used to provide insight into the qualitative and
quantitative relationships between the immune and host resistance
assays commonly used to examine potential immunotoxic chemicals in
experimental animals. While the analyses had a number of limitations,
several conclusions can be drawn from the results:
(1) With this particular testing configuration, examination of
only two or three immune parameters was needed in order to identify
potential immunotoxicants. Lymphocyte enumeration and quantification
of the T cell-dependent antibody response appeared to be particularly
useful. Furthermore, some commonly employed measures (e.g. leukocyte
counts, lymphoid organ weights), while probably good predictors of
immunotoxicity, are apparently not as sensitive as the other tests.
Obviously, inclusion of additional tests that are not part of the
original battery may improve the prediction of immunotoxicity.
(2) A good correlation was found between changes in immune tests
and altered host resistance, in that there was no instance in which
host resistance was altered without a significant change in the immune
test(s). In many instances, however, immune changes were observed in
the absence of detectable changes in host resistance (Table 16),
indicating that immune tests are generally more sensitive than host
resistance assays.
(3) No single immune test could be considered highly predictive
for altered host resistance; however, many of the tests were good
indicators, while others, such as leukocyte counts and proliferative
response to lipopolysaccharide, were relatively poor indicators of a
change in host resistance. Some of the tests that showed the highest
association with host resistance were those described previously as
the best indicators of immunotoxicity, such as the plaque-forming
assay and surface markers, but also included tests such as delayed-
type hypersensitivity and thymic weight.
(4) Regression modelling, using a large data set on one chemical
agent, indicated that most, but not all, of the immune function-host
resistance relationships follow a linear model. It was not possible,
however, to establish linear or threshold models for most of the
chemicals studied when the data from all 50 chemicals were combined;
thus, a more mechanistically based mathematical model will have to be
developed. A similar conclusion was drawn on the basis of a limited
data set collected by the Environmental Protection Agency (Selgrade et
al., 1992), in which changes in NK cell activity were correlated with
changes in susceptibility to cytomegalovirus in a murine model. It is
impossible, at present, to determine how applicable these analyses
will be for immunotoxic compounds with different immune profiles;
however, as more analyses become available, the ability to estimate
potential clinical effects accurately from the results of
immunological tests should increase.
Table 16. Association between the results of host resistance models and immune tests
Challenge No. of Frequencya
agent tests
Specificity Sensitivity Concordance
(-/-) (+/+) (Total)
Listeria monocytogenes 34 100 52 65**
PYB6 tumour 24 100 39 54
Streptococcus pneumoniae 19 100 38 58
B16F10 melanoma 19 100 40 68
Plasmodium yoelii 11 100 38 55
Influenza 9 100 17 44
Any of the aboveb 46 100 68 78*
From Luster et al. (1993)
* Agreement statistically significant at P < 0.05
a Frequency is defined as: specificity, the percentage of non-immunotoxic
chemicals with no effect on the host resistance models; sensitivity,
the percentage of potentially immunotoxic chemicals causing a change
in a host resistance model; concordance, percentage of qualitative agreement
b Frequency calculated on the basis of all of the host resistance models used to
study an agent
7. SOME TERMS USED IN IMMUNOTOXICOLOGY
Accessory cell. Passenger cell (leukocyte, mainly monocytes) or
stationary cell (reticulum cell, epithelial cell, endothelial cell)
that aids T or B lymphocytes in inducing immunological reactions,
either by direct contact or by releasing factors; normally expresses
MHC class II molecules
Acquired immunity. A state of protection against pathogen-induced
injury, with rapid immune elimination of pathogenic invaders; due to
previous immunization or vaccination
Activation. The process of going from a resting or inactive state to
a functionally active state, of leukocytes (lymphocytes, monocytes) or
proteins (complement, coagulation)
Acute-phase protein. Non-antibody humoral factor that emerges in
increasing amounts in the circulation shortly after induction of an
inflammatory response; e.g. alpha 2-macroglobulin, C-reactive protein,
fibrinogen, alpha 1-antitrypsin, and complement components
Adaptive immunity. A state of specific acquired protection against
pathogenic invaders, induced by immunization or vaccination
Addressin. Receptor for lymphocytes on endothelial cells of venules,
involved in homing of cells in lymphoid tissue; belongs to the
immunoglobulin gene superfamily, integrins, and selectins
Adenoid. See Tonsil
Adhesion receptors Molecule involved in cellular adhesion between
passenger cells and the extracellular stationary matrix (endothelium,
connective tissue); comprises three main families; member of the
immunoglobulin gene superfamily, integrins, and selectins
Adjuvant. Material that enhances an immune response
Adoptive immunity or tolerance. Transfer of a state of immunity or
tolerance via cells or serum from an immune or tolerant individual to
a naive individual
Affinity. Binding strength of an antibody-combining site to an
antigenic determinant (epitope); expressed as an association constant
(Ka)
Agglutination. Process of aggregation of visible antigenic particles
(e.g. erythrocytes) mediated by antibodies directed towards the
particles
Allele. One or more genes at the same chromosomal locus which
control alternative forms (phenotypes) of a particular inherited
characteristic
Allergen. Antigen that induces an allergic or hypersensitivity
reaction, resulting in immune-mediated or nonimmune-mediated tissue
damage; restricted mainly to immediate hypersensitivity or
anaphylactic reactions
Allergy. State of altered immunity, resulting in hypersensitivity
reaction on contact with antigen or allergen; often restricted to
immediate hypersensitivity or anaphylaxis
Alloantigen. Antigen that differs between different (not inbred)
individuals within one species
Allogeneic. Genetically different phenotypes in different (not
inbred) individuals within one species; opposite of isogeneic, or
xenogeneic
Allotype. Genetically different antigenic determinants on protein of
(not inbred) individuals within one species
alpha Chain. First chain of a multimeric receptor molecule: in
immunoglobulin molecules, the a heavy chain forming the IgA class; in
T-cell receptor molecules, one of the chains forming the dimeric
alpha-ß receptor molecule; in MHC class I molecules, the main
polypeptide chain associated with the ß2-microglobulin molecule; in
MHC class II molecules, one of the chains in the dimeric molecule
Alternative pathway of complement activation. Activation of
complement pathway by substances other than antigen-antibody
complexes; involves factor B, properdin, and complement component C3
Anaphylatoxin. Activated components of complement components C3 and
C5 (C3a and C5a, respectively), which induce anaphylactic reactions by
activating mast cells and basophilic granulocytes
Anaphylaxis or anaphylactic reaction. Local or systemic immediate
hypersensitivity reaction initiated by mediators released after
immunological stimulation; symptoms can be a drop in blood pressure
related to vascular permeability and vascular dilatation, and
obstruction of airways related to smooth muscle contraction or
bronchoconstriction
Anergy. State of unresponsiveness to antigenic stimulation, due to
the absence of responding elements or the loss of capacity of existing
elements to mount a reaction; synonym for tolerance
Antibody. Immunoglobulin molecule produced in response to immunization
or sensitization, which specifically reacts with antigen Antibody-dependent
cell-mediated cytotoxicity. Cytotoxic reaction in which an antibody forms
the bridge between the cytotoxic cell (lymphocyte, macrophage) and the
target cell
Antigen. Any substance that induces a specific immunological
response
Antigen-binding site (paratope). Part on an antibody molecule that
binds antigen (antigenic determinant, or epitope); part of the T-cell
receptor that binds the complex of antigen and MHC molecule
Antigenic determinant (epitope). Part of an antigen that binds to
antibody or T-cell receptor (the latter in combination with MHC
molecule)
Antigenicity. Capacity to react with components of the specific
immune system (antibody, receptors on lymphocytes)
Antigen presentation. Process of enabling lymphocytes to recognize
antigen on a specific receptor on the cell surface. For presentation
to T lymphocytes, includes intracellular processing and complexing of
processed peptides with MHC molecule on the cell membrane of the
antigen-presenting cell. For presentation to B lymphocytes, can
include formation of immune complexes (in germinal centres)
Antigen-presenting cell. Cell that presents antigen to lymphocytes,
making possible specific recognition by receptors on the cell surface.
In a more restricted way, used to describe MHC class II-positive
(accessory) cells which can present (processed) antigenic peptides
complexed with MHC class II molecules to T helper-inducer lymphocytes.
Includes macrophage populations (in particular, Langerhans cells and
dendritic or interdigitating cells), B lymphocytes, activated T
lymphocytes, certain epithelia (after MHC class II antigen induction
by e.g. interferon gamma); others are follicular dendritic cells, not
of bone-marrow origin, which present antigen in the form of immune
complexes to B cells in germinal centres of peripheral lymphoid
tissue; marginal zone macrophages in splenic marginal zone, which
present antigen, without contact with T helper cells, to B cells at
this location (T cell-independent response, e.g. to bacterial
polysaccharide). In rodent skin epidermis, a dendritic epidermal cell,
of T-cell origin, has an antigen-presenting function.
Antigen receptor. Multichain molecule on lymphocytes, to which
antigens bind. For B lymphocytes, an immunoglobulin molecule that
recognizes nominal antigen; for T lymphocytes, a T-cell receptor
molecule that recognizes antigenic peptide in combination with the
polymorphic determinant of an MHC molecule
Antinuclear antibody. Antibody directed to nuclear antigen; can have
various specificities (e.g. to single- or double-stranded DNA or
histone proteins); frequently observed in patients with rheumatoid
arthritis, scleroderma, Sjögren's syndrome, systemic lupus
erythematosus, and mixed connective tissue disease. Also called
antinuclear factor
Antiserum. Serum from an individual that contains antibodies to a
given antigen
Aplasia. Absence of tissue structure or cellular component, either
congenital or acquired
Apoptosis (programmed cell death). Process whereby the cell kills
itself after activation, by Ca2+-dependent endonuclease-induced
chromosomal fragmentation into fragments of about 200 base-pairs
Appendix. Lymphoid organ in the gastrointestinal tract, at the
junction of ileum and caecum; forms part of gut-associated lymphoid
tissue
Arthus reaction. Inflammatory response, generally in skin, induced
by immune complexes formed after injection of antigen into an
individual that contains antibodies
Asthma. Chronic inflammatory disease characterized by bronchial
hyperresponsiveness to various stimuli
Atopy. In general terms, 'unwanted reactivity'; used mostly to
describe the state of general systemic or local hypersensitivity
reactions related to genetic predisposition
Auto-antibody. Antibody to component in the individual itself
Auto-antigen. Antigen to which an autoimmune reaction is directed
Autoimmunity. A state of immune reactivity towards self
Autologous. Derived from self; components of an immunological
reaction (e.g. antibody, lymphocytes, grafted tissue) from the same
individual; opposite of heterologous
Avidity. Binding strength between antibody and antigen, or receptor
and ligand; for antibody, represents the product of more than one
interaction between antigen-binding site and antigenic determinant
Bacteraemia. Presence of bacteria in blood
Basophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with acid glycoproteins stained by basic (blue) dyes; after
release, glycoproteins induce anaphylactic reactions
B-Cell growth and differentiation factor, B-cell growth factor, and
B-cell stimulating factor. See Interleukin-4 and -5
ß Chain. In T-cell receptor molecules, one of the chains forming the
dimeric alpha-ß receptor molecule; in MHC class II molecules, one of
the chains in the dimeric molecule
ß2-Microglobulin. A peptide of 12 kDa, which forms part of MHC class
I molecule
B Lymphocyte or cell. Lymphocytes that recognize nominal antigen by
immunoglobulin (antibody) surface receptor (on virgin B cell, IgM and
IgD) and, after activation, proliferate and differentiate into
antibody-producing plasma cells. During a T-dependent process, there
is immunoglobulin class switch (IgM into IgG, IgA, IgD, or IgE), with
maintenance of the antigen-combining structure. For T-independent
antigens, cells differentiate only in IgM-producing plasma cells. B
Lymphocytes originate from precursor cells in bone marrow; in avian
species, they undergo maturation in the bursa of Fabricius (B, bursa-
dependent); in mammals, in the bone marrow
B Lymphocyte area. That part of an lymphoid organ or tissue that is
occupied by B lymphocytes, e.g. follicles in peripheral lymphoid
tissue, marginal zone in spleen
Birbeck granule. Rod-shaped structure with rounded end,
approximately 6 nm thick, found in the cytoplasm of Langerhans cells
in the epidermis, and interdigitating dendritic cells in T-lymphocyte
area of lymphoid tissues
Blast cell. Large cell (about 15 µm or more) with dispersed nuclear
chromatin and cytoplasm rich in ribosomes; in an active stage of the
cell cycle before mitosis
Blast transformation. Process of activation of lymphocytes into cell
cycle and to form blastoid cells before mitosis
Blocking antibody. Antibody that can interfere with another antibody
or with reactive cells in binding antigen, thereby preventing effector
reactions (often used in association with an allergic reaction or
tissue damage)
Bone marrow. Soft tissue in hollow bones, containing haematopoietic
stem cells and precursor cells of all blood cell subpopulations
(primary lymphoid organ); major site of plasma cell and antibody
production (secondary lymphoid organ)
Booster. Dose of antigen given after immunization or sensitization
to evoke a secondary response
Bradykinin. Peptide of nine amino acids split from alpha 2-
macroglobulin by the enzyme kallikrein; causes contraction of smooth
muscle
Bronchus-associated lymphoid tissue. Lymphoid tissue located along
the bronchi, considered to represent the location of presentation of
antigens entering the airways; contributes to mucosa-associated
lymphoid tissue
Bursa equivalent. Site where B-cell precursors undergo maturation
into immunocompetent cells in non-avian species; bone marrow in adult
mammals
Bursa of Fabricius. Primary lymphoid organ in avian species, located
in cloaca, with an epithelial reticulum, where precursors of B
lymphocytes from the bone marrow undergo maturation into
immunocompetent cells and then move to peripheral lymphoid organs
C (constant) gene. Gene that encodes the constant part of
immunoglobulin chains or T-cell receptor chains (e.g. Cµ, Cdelta
for immunoglobulin heavy chains, Ckappa for immunoglobulin kappa
light chain, Calpha for T-cell receptor alpha chain)
C (constant) region. Region at carboxy terminal of immunoglobulin
chains or T-cell receptor chains, identical for a given immunoglobulin
class or subclass or for a given T-cell receptor chain; encoded by C
genes in DNA
Cachectin. See Tumour necrosis factor
CD (cluster of differentiation). Group of (monoclonal mouse)
antibodies that react to identical leukocyte surface molecules in
humans (but not necessarily to identical epitopes), on the basis of
comparative evaluations during international workshops and transferred
to other species by analogy. Does not include MHC or immunoglobulin
molecules
CD3 molecule. Molecule consisting of at least four invariant
polypeptide chains, present on the surface of T lymphocytes associated
with the T-cell receptor; mediates transmembrane signalling (tyrosine
phosphorylation) after antigen binding to T-cell receptor
CD4 molecule. Glycoprotein of 55 kDa on the surface of T lymphocytes
and part of monocytes and macrophages. On mature T cells, restricted
to T helper (inducer) cells; has an accessory function to antigen
binding by T-cell receptors, by binding to a non-polymorphic
determinant of MHC class II molecule
CD8 molecule. Complex of dimers or higher multimers of 32-34 kDa
glycosated polypeptides linked by disulfide bridges, on the surface of
T lymphocytes. On mature T cells, the presence is restricted to
T cytotoxic-suppressor cells; has an accessory function to antigen
binding by the T-cell receptor, by binding to a non-polymorphic
determinant of MHC class I molecule
Cell-mediated immunity. Immunological reactivity mediated by
T lymphocytes
Central (primary) lymphoid organ. Lymphoid organ in which precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment, to form immunocompetent cells; not antigen-driven
but can be influenced by mediators produced as a result of antigen
stimulation
Centroblast. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; a medium to large,
12-18-µm cell, with a round to ovoid nucleus that has moderately
condensed heterochromatin and medium-sized nucleoli close to the
nuclear membrane, medium-sized to broad cytoplasm containing many
polyribosomes and a variable amount of rough endoplasmic reticulum
Centrocyte. Intermediately differentiated B lymphocyte present in
germinal centres of follicles in lymphoid tissue; medium-sized,
8-12-µm lymphoid cell with irregular nucleus with condensed
heterochromatin; small cytoplasm containing a few organelles
CH50. The amount of serum (or dilution of serum) that is required to
lyse 50% of erythrocytes in a standard haemolytic complement assay
Chemiluminescence. Luminescence produced by direct transformation of
chemical energy into light energy
Chemotactic factor. Substance that attracts cells to inflammatory
lesions
Chemotaxis. Process of attracting cells to a given location, where
they contribute to an inflammatory lesion
Class I MHC molecule. Molecule coded by the A, B, or C locus in the
HLA complex, the K and D locus in the mouse H2 complex, and less well
defined MHC I gene loci in other species, in association with the
ß2-microglobulin molecule. Two-chain molecule occurring on all
nucleated cells, without allelic exclusion
Class II MHC molecule. Molecule coded by the D (DR, DP, DQ) locus in
the HLA complex, the I-A and I-E locus in the mouse H2 complex, and
less well defined MHC II gene loci in other species, comprising an
alpha and a ß chain (intracellular, associated with an 'invariant'
chain). Two-chain molecule occurring, without allelic exclusion, on B
lymphocytes, activated T lymphocytes, monocytes-macrophages,
interdigitating dendritic cells, some epithelial and endothelial cells
(variable, dependent on species and state of activation); antigen-
presenting cells
Class switch. See Immunoglobulin class switch
Classical pathway of complement activation. Activation of complement
pathway by antigen-antibody complexes, starting with complement
component C1 and ending with complement component C3
Clone. Population of cells that emerge from a single precursor cell;
within T or B lymphocytes, cells with a fixed rearrangement of genes
coding for T-cell receptor or immunoglobulin
Clonal expansion. Proliferation of cells that have a genetically
identical constitution; when uncontrolled, may result in tumour
formation
Colony-stimulating factor. Substance that supports clonal cell
growth of haematopoietic cells
Complement. Group of about 20 proteinase precursors that activate
and split each other, in sequential order. The various components are
present in inactive (precursor) form, except for C3, which in a normal
state shows a low turnover (major split products C3a and C3b). The
split products are either bound to the activating substance (immune
complex or antibody-coated cell) or are released as active mediators.
The classical cascade starts by activation of component C1,
subsequently C4, C2, and C3, and is initiated by (IgG/IgM) immune
complexes. The alternative cascade starts by activation of C3b and
factor B, subsequently factors D and C3, and is initiated by nonimmune
specific activators like microbial polysaccharides and some
'allergens'. C3 split products (C3b) activate the amplification loop,
in which factors D and B are also used, activate C5, and thereafter
the terminal cascade C6, C7, C8, and C9, which attack the cell
membrane and kill the cell (microorganism). The cascade is under the
control of various inhibitors. Major effects of complement split
products are adherence to receptors on phagocytes (C3b, C3d); mediator
activity, like chemoattraction of inflammatory cells, vasodilatation,
increased vascular permeability, and smooth muscle contraction
(C3a, C5a); cell lysis by membrane lesions (C6-C9)
Complement fixation. Binding and consumption of complement by
antigen-antibody complexes; often used in association with assays for
complement activity
Complement receptor (CR). Cell surface molecule that can bind
activated complement components in e.g. antigen-antibody complexes;
CR1 (CD35) is a receptor for C3b, present on B lymphocytes, monocytes
and macrophages, granulocytes, and erythrocytes; CR2 (CD21) is a
receptor for C3d, present on mature B lymphocytes; CR3 (CD11b/CD18) is
a receptor for C3b present on macrophages, granulocytes, natural
killer cells, and a subset of CD5+ B lymphocytes; CR4 (CD11c/CD18)
is a receptor for C3b present on monocytes, macrophages, granulocytes,
and natural killer cells
Contact sensitivity. Hypersensitivity reaction evoked in skin by
placing sensitizing agents or substances on skin
Cords of Billroth. Medullary cords in spleen
Corona. See Mantle
Cortex. Outer parenchymal layer of organs
Cross-reactivity. Reactivity of antigen-specific elements (T lymphocytes
sensitized by T-cell receptor, B lymphocytes by antibody; antibody
molecules) towards antigens other than those used in original
sensitization, owing to shared antigenic epitopes on different
antigenic molecules; also used to describe reactions towards antigenic
determinants other than those originally used in sensitization, due to
similarities in structure
Cytokine. Biologically active peptide, synthesized mainly by
lymphocytes (lymphokines) or monocytes and macrophages (monokines);
modulates the function of cells in immunological reactions; include
interleukins. Some (pleotrophic cytokines) have a broad spectrum of
biological action, including neuromodulation, growth factor activity,
and proinflammatory activity
Cytokine receptor. Ligand for cytokine on target cell, acts in
signal transduction through the cell membrane; many are multichain
molecules belonging to different receptor families
Cytolytic antibody. Antibody that can mediate lysis of the target to
which it is directed, either in combination with complement or as
bridge between cytotoxic cell and target
Cytotoxic cell (killer cell). Effector T cell, natural killer cell,
or activated macrophage; kills target cells and tissue extracellularly
after binding; mediated by release of substances from cytolytic
granules (including serine esterase, cytolysin, and perforins)
Cytotoxic reaction. Effector reaction of antibody or cells, resulting
in lysis of target cell or tissue
Cytotoxic T lymphocyte. Subpopulation of T lymphocytes with CD8
phenotype; after recognition of antigen in an MHC class I-restricted
manner, differentiates from precursor to effector cytotoxic cell and
subsequently kills target cells
Degranulation. Process of fusion of cytoplasmic granules with cell
membrane, whereby the content of the granules is released into the
extracellular space; mainly used in association with immediate
hypersensitivity reactions
Delayed-type hypersensitivity. Inflammatory lesion mediated by
effector T lymphocytes or their products, with attraction mainly of
macrophages towards the inflammatory lesion. Term originates from the
classical skin reaction after challenge of a sensitized individual;
maximal (wheal and flare) response reached within 24-72 h
Delayed-type hypersensitivity T cell. Subpopulation of T lymphocytes
with CD4 phenotype; after recognition of antigen in an MHC class
II-restricted manner, secretes mediators involved in inflammatory
responses, e.g. INF gamma and tumour necrosis factor
delta Chain. In immunoglobulin molecules, delta heavy chain forming
the IgD immunoglobulin class; in T-cell receptor molecules, one of the
chains forming the dimeric gamma-delta receptor molecule; one of the
chains in the CD3 molecule associated with the T-cell receptor
Dendritic cell. Cell in tissue that shows elongations or protrusions
of cytoplasm into the parenchyma. Often used in a restricted manner to
designate a type of antigen-presenting cell, of which two categories
exist: one of bone-marrow origin belonging to the macrophage lineage,
including Langerhans cells in skin and interdigitating dendritic cells
in T-cell areas of lymphoid tissue and a very small leukocyte
population in blood; the second of tissue parenchymal origin
(presumably pericytes around blood vessels), the follicular dendritic
cells in B-cell areas (follicles) of lymphoid tissue
Dendritic epidermal cell. T Cell in the epidermis that has dendritic
morphology; has antigen-presenting function but is not a (macrophage-
related) Langerhans cell. Occurs in rodents but not in humans;
contributes to skin immune system
Dermal immune system. See Skin immune system
Desensitization. Induction of anergy or tolerance to allergic
substances by active intervention in immune reactivity (exhaustion of
reactive elements or induction of blocking phenomena)
Diapedesis. Passage of cells through blood vessel walls into tissue
parenchyma, mediated by constriction of endothelial cells
D (diversity) gene. Gene that encodes the variable part of
immunoglobulin heavy chains, or T-cell receptor alpha, ß, or gamma
chain (e.g. DH1-DHn for immunoglobulin heavy chains, Ddelta
1-Vdelta n for T-cell receptor alpha or delta chain)
Domain. Part of polypeptide chain folded to a relatively rigid
globular tertiary structure fixed by disulfide bonds. Molecules of the
immunoglobulin gene superfamily have a tertiary domain-like structure:
each domain is about 110 amino acids long and arranged in a sandwich
of two sheets of anti-parallel ß strands. See also Homologous,
Homology
Ectoderm. Outermost of the three cellular layers of the embryo;
produces the epidermis and neuronal tissue
Eczema. Superficial inflammation in skin, involving primarily the
epidermis; characterized by redness, itching, minute papules and
vesicles, weeping, oozing, and crusting. Histological changes include
microvesiculation and oedema of the epidermis and an infiltrate of
lymphocytes and macrophages in the dermis
Effector cell. General term to describe a cell that mediates a
function after a stage of activation, differentiation, and
proliferation
Endocytosis. Process of uptake of material by a cell; special forms
are phagocytosis and pinocytosis
Endoderm. Innermost of the three cellular layers of the embryo;
produces the gastrointestinal lining and some internal organs, such as
liver and pancreas
Endoplasmic reticulum. Membrane-like structure in cell cytoplasm;
site of protein synthesis
Endothelium. Cells that line blood vessels; exert a major function
in traffic of leukocytes from blood into tissue, by altered expression
of adhesion molecules (modulation of numbers of receptors; maturation
and activation resulting in altered glycosylation, expression of new
ligands or altered ligand binding affinity; change in cytoskeleton
organization). A special endothelial cell type occurs in T-lymphocyte
areas of lymphoid tissue in the high endothelial (postcapillary)
venule.
Endotoxin. Lipopolysaccharide from the cell wall of Gram-negative
bacteria; has toxic, pyrogenic, and immunoactivating effects
Enzyme-linked immunosorbent assay. Immunoenzymetric assay based on
the use of antigens or antibodies labelled with a specific enzyme;
combines the virtues of solid-phase technology and enzyme-labelled
immunoreagents. The antigen-antibody complex is determined by an
enzyme assay involving the incubation of the complex with an
appropriate substrate of the enzyme.
Eosinophil chemotactic factor. Acidic tetrapeptide of 0.5 kDa
produced by mast cells (preformed mediator); attracts eosinophils to
the site of inflammation
Eosinophilia. State of increased proportions of eosinophilic
granulocytes in blood
Eosinophilic granulocyte. Polymorphonuclear leukocyte that contains
granules with basic proteins stained by acidophilic (red) dyes; after
release, the proteins modulate inflammatory reactions
Epithelium. Cells covering the surface of the body and forming the
first line of defence against pathogenic invaders. Reticular
epithelium forming the stroma of tissue occurs in thymus (in avian
species in the bursa of Fabricius); these cells have a major function
in processing precursor cells to immunocompetent lymphocytes
Epithelioid cell. Cell of macrophage origin in chronic inflammatory
lesions, which resembles an epithelial cell morphologically
Epitope (antigenic determinant). Part of antigen that binds to
antibody or T-cell receptors (the latter in combination with MHC
molecule)
epsilon Chain (see also alpha Chain). In immunoglobulin molecules,
the epsilon heavy chain forming the IgE immunoglobulin class; one of
the chains in the CD3 molecule associated with the T-cell receptor
Erythema. Redness of skin produced by congestion of blood
capillaries due to dermal arterial vasodilatation
Erythrocyte. Red blood cell; a bone marrow-derived blood cell
component involved in oxygen transport to tissue; contains a nucleus
in distinct avian species like chickens but does not have a nucleus in
mammals
Exudation. Inflammation in tissue; contains blood cells and fluid
comprising serum proteins of high relative molecular mass
Ex vivo/in vitro. An assay method in which the effects of a
xenobiotic are evaluated in vitro in cells isolated from an animal
or human exposed to the compound of interest
Fab fragment. Part of an antibody molecule in which monovalent
binding of an antigenic determinant occurs; formed by the three-
dimensional structure of variable parts (domain) of one heavy and one
light chain and the adjacent part of the constant part (constant
domain); ab, antigen binding
F(ab')2 fragment. Part of an antibody molecule in which divalent
binding of antigenic determinants occurs; formed by the
three-dimensional structure of both Fab fragments
Fc fragment. Part of an antibody molecule formed by the three-
dimensional structure of the constant part (constant domains) of the
heavy chains (except that adjacent to the variable domain), involved
in antibody effector functions; c, crystallizable
Fc receptor. Structure on leukocytes (lymphocytes, monocytes,
macrophages, granulocytes) that mediates binding of immunoglobulin or
antibody, alone or after forming aggregates in antigen-antibody
complexes. Receptors for IgE (Fc epsilon) occur on mast cells and
basophilic granulocytes and are involved in immediate hypersensitivity
reactions; receptors for IgG are of three classes: low-affinity FcR
III, CD16, on natural killer cells, monocytes, macrophages, and
granulocytes; low-affinity FcR II, CD32, on B cells, myeloid cells,
Langerhans cells, and interdigitating dendritic cells; high-affinity
FcR III, CD64, on monocytes and macrophages
Follicle. Round to oval structure in lymphoid tissue, where B cells
are lodged. Primary follicles contain only small resting B cells;
secondary follicles comprise a pale-stained germinal centre, with
centrocytes and centroblast, and contain B lymphocytes in a state of
activation or proliferation and macrophages, the stroma consisting of
follicular dendritic cells. The germinal centre is surrounded by a
mantle with small B lymphocytes
Follicular dendritic cell. Cell forming the stationary micro-
environment of germinal centres of follicles in lymphoid tissue;
elongated, often binucleated cell with long branches extending between
germinal centre cells and forming a labyrinth-like structure; linked
by desmosomes. Of local parenchymal origin, presumably from pericytes
surrounding blood vessels. Its main function is presentation of
antigen, trapped as immune complex in the labyrinth, to B lymphocytes.
gamma Chain (see also alpha Chain). In immunoglobulin molecules,
the gamma heavy chain forming the IgG immunoglobulin class; in T-cell
receptor molecules, one of the chains forming the dimeric gamma-delta
receptor molecule; one of the chains in the CD3 molecule associated
with the T-cell receptor
gamma-delta T cell. T Lymphocyte with an antigen receptor composed
of a gamma and a delta chain associated with CD3 transmembrane
molecule; develops in part inside the thymus (including intrathymic
selection), in part outside the thymus. Has a major role as a
cytotoxic cell in the first phase of the (innate) immune response,
e.g. in rodents in the mucosal epithelium
Gammaglobulin. Part of serum proteins that move towards the negative
electrode (gamma fraction) upon electrophoresis; contains
immunoglobulins
Gene rearrangement. For immunoglobulin and T-cell receptor, the
process whereby the germline chromosomal genomic structure of variable
(diversity), joining, and constant segments recombine to form a
specific V-(D-)J-C combination, enabling transcription into mRNA and
translation into protein. The V-(D-)J combination of different chains
determines the specificity of the receptor (immunoglobulin or T-cell
receptor).
Germinal centre. The pale-staining centre in follicles of lymphoid
tissue, where B lymphocytes are activated by antigen in a
T lymphocyte-dependent manner and subsequently go into proliferation
and differentiation, acquiring the morphology of centroblasts,
centrocytes, and plasma cells. Has a special microenvironment made up
of follicular dendritic cells and large macrophages (tingible body or
starry-sky macrophages)
Glomerulonephritis. Inflammation of glomeruli in kidney, often
associated with deposition of immune complexes along the glomerular
basement membrane or in the mesangium, and influx of polymorpho-
nuclear granulocytes
Golgi apparatus. Tubular structures in cytoplasm, involved in
secretion of synthesized proteins
Granulocyte colony-stimulating factor. Synthesized by T lymphocytes
and macrophages, epithelial cells, fibroblasts, and endothelial cells;
supports growth of granulocyte progenitors, in synergism with IL-3 and
granulocyte-macrophage colony-stimulating factor of monocyte-
macrophage progenitors
Granulocyte-macrophage colony-stimulating factor. Produced by T
lymphocytes, endothelial cells, macrophages, and lung cells; supports
growth and differentiation of macrophages and granulocyte progenitors;
activates macrophages and polymorphonuclear macrophages to become
tumoricidal and produce superoxide anion
Granuloma. Chronic inflammatory reaction in tissue comprising macro-
phages (epitheloid cells), lymphocytes, and fibroblasts; formed in a
cell-mediated response towards poorly degradable material, in
immunological reactions as part of a delayed-type hypersensitivity
reaction
Gut-associated lymphoid tissue. Lymphoid organs and tissue located
along the gastrointestinal tract, presumed to be a first location of
presentation of antigens entering through the digestive tract;
comprises Peyer's patches, appendix, in part mesenteric lymph nodes,
adenoids, and tonsils; contributes to the mucosa-associated lymphoid
tissue
H-2. Major histocompatibility complex of mouse
Haemagglutinin. Antibody or substance that induces agglutination of
erythrocytes
Haematopoiesis. Production of cells of blood; subdivided into
erythropoiesis, lymphopoiesis, and myelopoiesis
Haematopoietic malignancy. Malignancy of blood-forming cells
Haemolysis. Process of lysis of erythrocytes, with release of
haemoglobin
Haemolytic agent (haemolysin). Antibody or substance that induces
lysis of erythrocytes
Haemopoietin. Growth factor that induces production of distinct
types of blood cells; also enhances the function of the mature cells
Haplotype. Phenotype of inherited characteristic on closely linked
genes on one chromosome
Hapten. Structure around one antigenic determinant, which itself
does not evoke an immune response unless coupled to a carrier
substance but can react with the products (antibodies, cells) of an
immune response
Hassall's corpuscle. Epithelial aggregate in onion-like structure,
often with debris of other cells; in the medulla of the thymus,
surrounded by large epithelial cells secreting thymic hormones; does
not occur in rodent thymus
Heat-shock protein. Family of proteins (60-90 kDa) with conserved
sequence in evolution; play a prime role in regulation and transport
of intracellular proteins. Expression is upregulated when cells are
under 'stress' (originally induced by heating), such as inflammatory
conditions, and may act as autoantigen in triggering and perpetuating
an auto-immune response
H (heavy) chain. One of the 45-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and three
constant domains (four constant domains in the 55-kDa µ chain). The
combination of the constant part of two heavy chains (alpha, ß, delta,
gamma, or µ) forms the immunoglobulin class-associated part of the
molecule (IgA, IgD, IgE, IgG, or IgM class)
Helper (inducer) T cell. Cell in a subpopulation of T lymphocytes,
with CD4 phenotype; after recognizing antigen in an MHC class
II-restricted manner, induces immunological reactions, secretes
interleukins, and cooperates (supports) B lymphocytes, cytotoxic
T-cell precursors, and macrophages
Helper T cell subpopulations. Th1 and Th2: Th1 cells produce
interleukin-2 and interleukin-3, interferon gamma, tumour necrosis
factor alpha and ß, and granulocyte-macrophage colony stimulating
factor, and function in induction of delayed-type hypersensitivity,
macrophage activation, and IgG2a synthesis. Th2 cells produce
interleukin-3, interleukin-4, and interleukin-5, tumour necrosis
factor alpha and granulocyte-macrophage colony stimulating factor, and
function in induction of IgG1, IgA, and IgE synthesis and induction of
eosinophilic granulocytes
Heterologous. Derived from foreign source or species; components of
an immunological reaction (e.g. antibody, lymphocytes, grafted tissue)
derived from another individual of the same species or another
species; opposite of autologous
Heterophilic antigen. Antigen in unrelated species; can be directed
towards xenogeneic immune reactivity; often has carbohydrate
structure; opposite of homocytotropic antibody
High endothelial (postcapillary) venule. Specialized blood vessels
in T-lymphocyte area of lymphoid tissue, through which circulating
lymphocytes pass into the parenchyma
Hinge region. Stretch in immunoglobulin molecule between Fab and Fc
fragments (first constant domain and other constant domains of the
heavy chain), where the quaternary structure of the molecule is not
rigid but flexible; bending of the hinge region after antigen binding
serves as a signal transduction, resulting in effector reactions
Histamine. ß-Imidazolylethylamine; component of granules in mast
cells and basophilic granulocytes that is released upon activation and
induces immediate hypersensitivity reaction, e.g. vasodilatation,
vascular permeability, smooth muscle contraction, and broncho-
constriction
Histiocyte (histiocytic reticulum cell). Monocyte in tissue. See
Macrophage
Histiocytosis. Increase in proportion of macrophages in tissue
HLA, human leukocyte antigen. Major histocompatibility complex of
humans
Homocytotropic antibody. Antibody that binds preferentially to cells
from the same species rather than to cells from other species;
opposite of heterologous antibody
Homologous, Homology. Similarity in primary structure between
substances; homology region is a synonym for domain
Host defence. Ability of an individual or species to protect itself
against opportunistic agents and to eliminate certain tumours and
exogenous agents such as (micro)organisms, viruses, and particles that
can cause disease
Hot spot. See Hypervariable region
Humoral immunity. Immunological reactivity mediated by antibody
Hybridoma. Transformed cell line or cell clone formed by fusion of
two different parental cell lines or clones
Hyperplasia. Reversible increase in cell number, usually as the
result of a physiological stimulus or persistent cell injury due to
irritating compounds
Hypersensitivity. Increased reactivity or sensitivity; in
immunological reactions, often associated with tissue destruction
Hypervariable region. Amino acid sequences in the variable regions
of antibody molecules or T-cell receptor chains where variability is
highest and which together form the antigen-binding site. Synonym for
hot spot
Hypoplasia. Reversible decrease in cell number, usually as a result
of a physiological stimulus
Ia antigen. MHC class II cell surface molecule
Idiotype. Antigenic determinant of variable domain of immunoglobulin
molecules or T-cell receptor
Immediate hypersensitivity. Inflammatory response that occurs within
minutes after exposure to allergen; caused by physical or
immunological stimulus, with vascular dilatation, increased vascular
permeability, and oedema as the main effects. Term originates from the
classical skin reaction after challenge of a sensitized individual in
skin, which takes 20-30 min to reach maximal (wheal and flare)
response and is mimicked by injection of mediator only (histamine)
Immune adherence. Binding of antigen-antibody complexes (antibody-
coated particles) to erythrocytes, platelets, or leukocytes; mediated
by activation of complement C3
Immune complex. Complex between antigen and antibody
Immune elimination. Rapid clearance of pathogen from the circulation
by components of the immune system; often used in association with
antibody molecules (removal by immune complex formation and
phagocytosis)
Immune exclusion. Process whereby entry of pathogens at mucosal
surfaces is prevented by the action of specific (secretory IgA)
antibody
Immune interferon. Former name for interferon gamma
Immune surveillance. Function (still hypothetical) of the immune
system in preventing or eliminating cells after malignant
transformation to a neoplastic process
Immunity. State of protection against pathogen-induced injury, with
fast immune elimination of pathogenic invaders due to previous antigen
contact or a special acquired state of responsiveness
Immunization (vaccination). Active intervention resulting in
immunity; used mainly in the context of presentation of (inactivated
or attenuated, nonpathogenic) substance to induce immunological
memory. Passive immunization is the adoptive transfer of immune system
components after previous contact with the pathogen and is performed
mainly with antibodies
Immunoblast. Intermediately differentiated B lymphocyte in lymphoid
tissue; a large, 15-20 µm, round-to-spherical cell with a rounded
euchromatic nucleus. The abundant cytoplasm contains many ribosomes,
well-developed rough endoplasmic reticulum and Golgi complex
Immunocompetence. Capacity of B or T lymphocytes to specifically
recognize antigen, resulting in a specific immunological reaction
Immunodeficiency. Defects in the immune system resulting in
decreased or absent reactivity to pathogens. Primary immunodeficiency
is mainly intrinsic defects in the differentiation of T or B
lymphocytes and can be congenital or acquired. Secondary
immunodeficiency is defects of which the cause is outside the immune
system (malnutrition; stress; protein loss after burns, nephrotic
syndrome, or intestinal bleeding; viral infection; therapy with
immunosuppressive or cytostatic drugs; irradiation).
Immunogen. A substance that can induce an immunological reaction
Immunogenicity. Capacity to evoke an immune response
Immunoglobulin. Formerly the electrophoretically-defined
gammaglobulin (in blood) but is also present in the ß fraction;
synthesized by plasma cells. The basic subunit consists of two
identical heavy chains (about 500 amino acid residues, organized into
four homologous domains; for µ chain in IgM, about 600 amino acid
residues, organized into five homologous domains) and two identical
light chains (about 250 amino acid residues organized into two
homologous domains), each consisting of a variable domain and one to
three constant domains (in the µ chain, four constant domains). The
antigen-binding fragment (Fab) consists of variable domains of heavy
and light chains (two per basic subunit). Five classes of
immunoglobulins exist, which differ according to heavy chain type
(constant domains): IgG (major immunoglobulin in blood), IgM
(pentamer, consisting of five basic units), IgA (major immunoglobulin
in secretions; present mainly as a dimeric molecule), IgD (major
function, receptor on B lymphocytes), and IgE. Effector functions
after antigen binding are mediated by constant domains of the heavy
chain (Fc part of the molecule) and include complement activation
(IgG, IgM), binding to phagocytic cells (IgG), sensitization and
antibody-dependent cell-mediated cytotoxicity (IgG), adherence to
platelets (IgG), sensitization and degranulation of mast cells and
basophils (IgE). IgA lacks these effector functions and acts mainly in
immune exclusion (prevention of entry in the body) at secretory
surfaces ('antiseptic paint').
I mmunoglobulin class. Subfamily of immunoglobulins, based on
difference in heavy chain. Five classes exist: IgA, secretory
immunoglobulin, dimeric; IgD, immunoglobulin on B cells that acts as
antigen receptor; IgE, immunoglobulin fixed to mast cells and
basophilic granulocytes, involved in immediate hypersensitivity
reactions; IgG, main immunoglobulin in circulation; IgM, pentameric
immunoglobulin with optimal agglutinating capacity, produced on first
antigen contact
Immunoglobulin class switch. Process whereby synthesis of IgM
antibody changes into synthesis of antibody of another immunoglobulin
class, with maintenance of the same variable part of the
immunoglobulin molecule. At the genomic level, this includes gene
rearrangement, with an exchange of a constant gene segment to a fixed
V-D-J gene segment combination. This switch is thought to occur in
germinal centres of follicles in lymphoid tissue, during the change of
a primary into a secondary response, and is under the control of
cytokines (switch factors)
Immunoglobulin gene superfamily. Group of molecules including
immunoglobulins, T-cell receptors, MHC molecules, and others, like the
lymphocyte function-related antigens LFA-2 (CD2) and LFA-3 (CD58), the
intercellular adhesion molecules ICAM-1 (CD54) and ICAM-2, the
vascular cell adhesion molecule VCAM-1, the neural cell adhesion
molecule NCAM (CD56), and the CD4 and CD8 molecules, which have a
similar tertiary basic domain-like structure, in which each domain is
about 110 amino acids long and stabilized by a disulfide bridge. These
molecules are known to be important for specific recognition and
adhesion functions
Immunoglobulin light chain type. Defines the light chain in the
immunoglobulin unit, either kappa or lamda, each defined at the
germline DNA level by individual constant (C), joining (J), and
variable (V) gene segments
Immunoglobulin subclass. Subfamily within a distinct immunoglobulin
class, based on subtle differences in heavy chain. For instance, in
humans there are two IgA subclasses, IgA1 (alpha1 heavy chain) and
IgA2 (alpha 2 heavy chain), and four IgG subclasses, IgG1-IgG4
(gamma 1-gamma 4 heavy chain). In rodents, these are designated IgG1
(gamma 1), IgG2a (gamma 2a), IgG2b (gamma 2b), and IgG3 (gamma 3)
Immunological memory. Acquired state of the immune system after
first contact with antigen, whereby the reaction upon subsequent
contact is faster, more intense, and of higher affinity. For antibody
response, associated with an immunoglobulin class switch and 'affinity
maturation' (by somatic mutation)
Immunosuppression. Prevention or diminution of the immune response
by administration of antineoplastic or antimetabolic drugs,
antilymphocyte serum, or exposure to e.g. environmental chemicals or
microorganisms (viruses)
Immunotoxicant. Drug, chemical, or other agent that is toxic to
cells or other components of the immune system
Inducer (helper) T cell. See Helper (inducer) T cell
Inflammation. Process whereby blood proteins or leukocytes enter
tissue in response to or in association with infection or tissue
injury
Inflammatory cell. General description of cells in an inflammatory
infiltrate; in acute inflammation, mainly polymorphonuclear
leukocytes; in chronic inflammation, mainly lymphocytes and
macrophages
Innate immunity. State of protection against pathogen-induced
injury; does not require previous immunization or vaccination
Innocent bystander. Cell or tissue component that is destroyed by an
immunological reaction specifically directed against a unrelated
antigen
Integrin. Family of heterodimeric molecules sharing a ß chain (ß1,
ß2, ß3, about 750 amino acids long), each with a different alpha chain
(about 1100 amino acids long), with a major function in cell adhesion
and migration. Form a protein family rather than a superfamily on the
basis of strong structural and functional similarities. Examples:
leukocyte function-related antigen LFA-1 (alpha L/ß1, CD11a/CD18;
receptor for intercellular adhesion molecules ICAM-1, ICAM-2, and
ICAM-3); Mac-1 (alpha M/ß2, CD11b/CD18; complement C3 receptor, CR3);
p150,95 (alpha X/ß2, CD11c/CD18); very late antigens VLA-1
(alpha 1/ß1, CD49a/CD29; laminin, collagen receptor), VLA-2
(alpha 2/ß1, CD49b/CD29; laminin, collagen receptor), VLA-3
(alpha 3/ß1, CD49c/CD29; laminin, collagen, fibronectin receptor),
VLA-4/LPAM-1 (alpha 4/ß1, CD49d/CD29; receptor for fibronectin and
VCAM-1), VLA-5 (alpha 5/ß1, CD49e/CD29, fibronectin receptor), and
VLA-6 (alpha 6/ß1, CD49f/CD29; laminin receptor, and alpha V/ß1,
CD51/CD29; vitronectin receptor); LPAM-2 (alpha 4/ßp, CD49d/.., or
alpha 4/ß7)
Interdigitating dendritic cell. Leukocyte belonging to the monocyte-
macrophage cell lineage, present in T-cell areas in lymphoid organs;
has a major function in presentation of antigen (MHC class
II-restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures called Birbeck granules. Its
equivalent in epidermis is the Langerhans cell, and that in lymph, the
veiled macrophage.
Interferon. Low-relative-molecular-mass substance produced mainly
during viral infection by leukocytes (IFN alpha), fibroblasts (IFNß),
and lymphocytes (IFN gamma); has a major function in interfering with
viral replication
Interferon-alpha. Produced by leukocytes; stimulates B cells to
proliferate and differentiate; stimulates natural killer cells and
increases cytotoxic T cell generation, but blocks T-cell proliferation
and lymphokine-activated killer activity; stimulates macrophage
accessory activity and enhances Fc receptor expression and MHC class I
and II expression on various cell types; induces antiviral state in
cells and is cytostatic for tumour cells, inhibits fibroblast and
adipocyte differentiation, and enhances promyelocytic and monoblastic
cell differentiation
Interferon-ß. Produced by fibroblasts and epithelia; activity
similar to that of IFN alpha
Interferon-gamma. Produced by T cells; induces antiviral state and
is cytostatic for tumour cells; enhances MHC class I and II expression
on various cell types, is antagonistic to interleukin-4 in IgE/IgG1
synthesis, and stimulates IgG2a synthesis; activates macrophages to
become cytolytic and enhances natural killer and lymphokine-activated
killer activity.
Interfollicular area. Area between follicles in lymphoid tissue,
where mainly small T lymphocytes are lodged; recognized by presence of
high endothelial venules. In lymph nodes, located in the outer cortex
and continuous with the paracortex
Interleukin. Immunoregulatory protein, also known as lymphokine,
monokine, interferon, or cytokine. Generally, low relative molecular
mass (< 80 kDa) and frequent glycosylation; regulates immune cell
function and inflammation by binding to specific cell surface
receptors; transient and local production; acts in paracrine or
autocrine manner, with stimulatory or blocking effect on growth and
differentiation; very potent, functions at picomolar concentrations.
Represents an extensive series of mediators (interleukins 1-12), with
a wide range of overlapping functions. Other mediators in this series
are c-kit ligand, interferon, tumour necrosis factor, and transforming
growth factor
Interleukin 1. Comprises two forms, IL-1 alpha and IL-1ß; produced
mainly by cells of the mononuclear phagocytic system (macrophages),
astrocytes, endothelium, and some epithelia, following stimulation by
e.g. microorganisms, immune complexes, or particulate compounds.
IL-1 alpha is mainly cell-associated; IL-1ß is released. IL-1 has
(together with IL-6 and tumour necrosis factor) multiple effects in
the systemic acute-phase response and in local acute and chronic
inflammation: it stimulates T (helper) cells to synthesize IL-2 and
IL-2 receptors, interferon gamma, and other lymphokines, B cells
(proliferation and differentiation), neutrophils, and natural killer
cells; stimulates monocytes and macrophages to produce IL-1, IL-6, and
tumour necrosis factor; acts in the acute-phase response by inducing
synthesis of acute-phase proteins in liver and reducing cytochrome
P450 synthesis; induces natriuresis in kidney, insulin production in
pancreas ß cells, muscular proteolysis ('easy' energy generation) in
muscle cells, slow-wave sleep in cerebral cortex; raises the
temperature set-point (fever) in hypothalamus; stimulates
haematopoiesis and prostaglandin synthesis by various cell types
(fibroblasts, macrophages, endothelium); inhibits gastric motility
in vitro ; induces collagenase production by synovial cells and
osteoclasts, and antiviral state; inhibits gastric smooth muscle
in vitro ; is cytostatic for tumour cells and activates endothelium
Interleukin 2. Synthesized by T helper cells after activation;
stimulates (autocrine) T cells to divide and release mediators,
B cells to proliferate and differentiate; activates monocytes and
natural killer cells; stimulates lymphokine-activated killer cells;
promotes generation of T helper 1 cells.
Interleukin 3. Formerly called multi-colony-stimulating factor;
synthesized by T helper cells; promotes growth of pluripotent
haematopoietic progenitor cells to granulocytes (eosinophilic,
basophilic, neutrophilic), mast cells, macrophages, megakaryocytes,
and, together with erythropoietin, to normoblasts and erythrocytes;
activates eosinophils and mast cells; stimulates haematopoiesis and
B-cell differentiation; blocks lymphokine-activated killer cells
Interleukin 4. Formerly called B-cell growth factor or B-cell
stimulating factor; synthesized by T helper and B cells; stimulates
IgE and IgG1 production by B cells and enhances MHC class II and IgE
receptor expression on B cells; acts in synergism with IL-2 in killer
cell generation, is mitogenic for T cells, and activates macrophages.
It is the dominant interleukin in generating T helper 2 cells, with a
negative feedback on the generation of T helper 1 cells.
Interleukin 5. Formerly called T-cell replacing factor or B-cell
growth and differentiation factor II; synthesized by T helper cells;
activates B cells and eosinophils, and stimulates IgA production by
B cells
Interleukin 6. Formerly called interferon ß2; synthesized by T
cells, monocytes, endothelial cells, fibroblasts, and smooth muscle
cells, among others, during inflammatory reactions; stimulates T and B
cells to proliferate and differentiate; has properties similar to IL-1
and acts synergistically with it in the acute-phase response (fever,
synthesis of acute-phase proteins); synergizes with IL-3 in promoting
haematopoietic progenitor cell proliferation; inhibits production of
IL-1 and tumour necrosis factor by monocytes
Interleukin 7. Formerly called lymphopoietin; synthesized by bone-
marrow stroma; induces growth of immature T and B lymphocytes
Interleukin 8. Formerly called neutrophil-activating protein;
synthesized by monocytes and various tissue cells in response to
inflammatory stimuli; performs chemotaxis of neutrophilic granulocytes
and subsequent granule exocytosis and respiratory burst; induces
increased expression of adhesion molecules CD11b/CD18 (complement C3
receptor CR3) and promotes vascular leakage. Endothelium-derived IL-8
inhibits adhesion of neutrophilic granulocytes induced by IL-1
Interleukin 9. Synthesized by T lymphocytes; stimulates growth of
erythroid and megakaryocyte precursors and promotes (mucosal) mast-
cell growth; acts synergistically with IL-4 in modulating IgE and IgG
production
Interleukin 10. Synthesized by T and B lymphocytes; inhibits
mediator synthesis (IL-2, IL-3, tumour necrosis factor, interferon
gamma, granulocyte-macrophage colony-stimulating factor) by T helper
1 cells, inhibits mediator synthesis (IL-1 alpha, IL-1ß, IL-6, IL-8,
and tumour necrosis factor alpha) by monocytes; stimulates IL-2-
dependent growth and cytotoxicity of cytotoxic T cells and stimulates
mast cell growth together with IL-2 or IL-3 and IL-4; induces MHC
class II antigen expression on B cells, but down-regulates MHC class
II on monocytes; promotes generation of T helper 2 cells
Interleukin 11. Synthesized by fibroblasts and bone-marrow stromal
cells; resembles IL-6 in function: stimulates haematopoietic cell
growth and differentiation (myeloid, erythroid, megakaryocyte
lineage); enhances T-cell-dependent antibody response; and suppresses
adipocyte differentiation and lipoprotein lipase production
Interleukin 12. Also called natural killer cell stimulatory factor;
synthesized by monocytes-macrophages, B cells, and accessory cells, in
response to bacteria or parasites; stimulates T-lymphocyte
proliferation, activates natural killer cells, and stimulates
lymphokine-activated killer activity; synergizes with IL-2 in
activation of cytotoxic lymphocytes; induces production of interferon
gamma and other cytokines by lymphocytes. It is the dominant
interleukin in generating T helper 1 cells and has a negative feedback
on the generation of T helper 2 cells.
in vitro. In the context of this book, exposure of cells or cell
systems to the immunotoxic agent in vitro. If the donors of cells or
cell systems are exposed but these are analysed in vitro, the term
ex vivo/in vitro is used
Isohaemagglutinin. Antibodies mainly of the IgM class that react
with (carbohydrate) antigens on erythrocytes from individuals of the
same species, resulting in agglutination in vitro
Isologous. Synonym for isogeneic
Isotype. Antigenic determinant that defines class or subclass of
immunoglobulin molecules
J (joining) chain. A 15-kDa polypeptide chain that acts
intracellularly to combine (identical) IgA or IgM immunoglobulin
units, consisting of two heavy and two light chains, to form a dimeric
IgA or a pentameric IgM molecule
J (joining) genes. Genes that encode the variable part of
immunoglobulin or T-cell receptor chains (e.g. JH1-JHn for
immunoglobulin heavy chains, Jkappa 1-Jkappa n for immunoglobulin
kappa light chain, Jalpha 1-Jalpha n for T-cell receptor alpha
chain)
Kallikrein (kininogenase). Arises in tissue fluids after cleavage of
prekallikrein; acts on kininogen to produce kinins, resulting in
immediate hypersensitivity reaction, e.g. vasodilatation and oedema.
It is a preformed mediator present in mast cell granules
kappa Chain. In immunoglobulin molecules, the kappa light chain
forms the light chain type of the molecule
Keratinocyte. Epithelial cell in the epidermis; in some
circumstances, can manifest antigen presentation and produce
immunoregulatory cytokines; hence belongs to the skin immune system
Killer cell (K cell). See Cytotoxic cell
Kinin system. Humoral amplification system involved in inflammation,
whereby substrate proteins become active after enzymatic cleavage;
cause vasodilatation, increased vascular permeability, hypotension,
and contraction of smooth muscle
c-Kit ligand. Also called stem cell growth factor or mast cell
growth factor; synthesized by various stromal cells, fibroblasts, and
liver cells; stimulates growth of early pluripotent progenitor cells
and that of myeloid, erythroid, and lymphoid progenitors in synergy
with interleukin-1, -3, -6, -7, and granulocyte-macrophage colony-
stimulating factor; promotes growth of mast cells
Kupffer cells. Macrophages on or between endothelial cells lining
the sinusoids of the liver
lamba Chain. In immunoglobulin molecules, the lamba light chain
forms the light chain type of the molecule
Lamina propria. Thin layer of connective tissue under the villous
epithelium of the gastrointestinal tract; site of plasma cells,
producing mainly dimeric IgA, including J chain
Langerhans cell. Leukocyte belonging to the monocyte-macrophage cell
lineage, present in skin epidermis; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II
restricted) to T helper lymphocytes. Cytoplasm contains characteristic
rod-like structures, Birbeck granules. Its equivalent in lymphoid
tissue is the interdigitating dendritic cell, and that in lymph,
veiled macrophage; forms part of the skin immune system
Large granular lymphocyte. Intermediate-sized, 10-12-µm lymphocyte
with a kidney-shaped nucleus and prominent, large, azurophilic
granules in the cytoplasm; occurs in the circulation and in tissue and
has a major function as a natural killer cell; forms a heterogeneous
population with either T markers or monocyte-macrophage markers.
Lectin. Plant-derived substance that binds to lymphocytes and can
induce cell proliferation; some also bind to other haematopoietic
cells
Leukaemia. Neoplasia of lymphoid cells in blood or bone marrow
Leukocyte. Bone marrow-derived white blood cell, including cells in
the lymphoid, myeloid, and monocyte lineages; sometimes used to
describe only granulocytes
Leukocytosis. Increased proportion of leukocytes in blood
Leukopenia, leukocytopenia. Reduced proportion of leukocytes in
blood
Leukotriene. Formerly called slow-reacting substance of anaphylaxis;
products of arachidonic acid metabolism following the lipoxygenase
pathway, which act as mediators in the immediate hypersensitivity
reaction, mainly as chemoattractants for granulocytes and monocytes,
and in smooth muscle contraction; newly synthesized by mast cells upon
activation
L (light) chain. One of the 22-kDa polypeptide chains in
immunoglobulin molecules, consisting of a variable domain and a
constant domain. The light chain, either kappa or lamba, determines
the light chain type of the immunoglobulin molecule
Ly antigen. T Lymphocyte antigen in mice
Lymph. Fluid in lymphatic vessels
Lymph node. Secondary (peripheral) lymphoid organ, the main function
of which is to filter lymphatic vessels. Present throughout the body,
at connecting places of lymphatics and blood vessels; forms a major
site of encounter between pathogenic substances in the lymph and
lymphocytes entering from blood vessels, and subsequent initiation of
antigen-specific immunological reactions
Lymphatic. Vessel that collects fluid from interstitial spaces and
goes via lymph nodes (filtering) to the thoracic duct and blood
Lymphocyte. Cell belonging to the lymphoid lineage of bone marrow-
derived haematopoietic cells. In a restricted way, the designation of
a small, resting or recirculating mononuclear cell in blood or
lymphoid tissue, which measures about 7-8 µm, has a round nucleus
containing densely aggregated chromatin, and little cytoplasm. Plays a
key role in immune reactions by specific recognition of antigens
Lymphocytosis. Increased proportions of lymphocytes in blood
Lymphoid organ. Tissue in the body where cells of the immune system,
mainly lymphocytes, are lodged in an organized microenvironment,
either in a resting state or in a state of activation,
differentiation, or proliferation. Includes bone marrow, thymus, lymph
nodes, spleen, and mucosa-associated lymphoid tissue
Lymphokine. Hormonal substance synthesized by lymphocytes, which
modulates the function of cells in immunological reactions
Lymphoma. Neoplasia of lymphoid cells in tissue
Lymphopenia, lymphocytopenia. Reduced proportions of lymphocytes in
blood
Lymphotoxin. Former name for tumour necrosis factor ß; lymphokine
synthesized by T lymphocytes, which kills selected target cells
Lysosome. Granule present in many cell types that contain hydrolytic
enzymes; also performs intracellular degradation of pathogens after
phagocytosis
Lysozyme (muramidase). A low-relative-molecular-mass, cationic
enzyme present in tissue fluids and secretions, which degrades
mucopeptides of bacterial cell walls
Macroglobulin. Glycoprotein of relative molecular mass > 200 kDa
Macrophage. Large, 12-20-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in tissue, and forming the
mononuclear phagocytic system. Its reniform nucleus usually has
pronounced peripheral condensation of nuclear chromatin; its cytoplasm
contains a great variety of cell organelles, including rough
endoplasmic reticulum, mitochondria, ribosomes, lysosomes, and Golgi
complex. Has a major function in (chronic) inflammatory reactions, by
virtue of its phagocytic capacity, with immunoglobulin Fc and
complement C3 receptors which bind to immune complexes. Macrophages
develop into killer cells after activation by e.g. T-cell factors and
can mediate antibody-dependent cell-mediated cytotoxicity; also
functions as an accessory cell in induction of immune responses
(antigen presentation, mediator secretion). Macrophages in blood are
called monocytes. Subtypes with special functions are interdigitating
dendritic cells: T-cell area of lymphoid tissue, Langerhans cell
(skin), Kupffer cells (liver), metallophilic macrophages (spleen),
microglia (brain), osteoclasts (bone), tingible body macrophage
(starry-sky macrophage), veiled macrophage (lymph).
Macrophage colony-stimulating factor. Synthesized mainly by
endothelial cells and fibroblasts, and possibly macrophages; supports
growth of monocyte-macrophage progenitors.
Major histocompatibility complex (MHC). Set of genes that codes for
tissue compatibility markers, which are targets in allograft rejection
and hence determine the fate of allografts; plays a central role in
control of cellular interactions during immunological reactions.
Tissue compatibility is coded by classes I and II loci (see Class I
and Class II MHC molecule). Genes within or closely linked to MHC
control certain complement components (MHC class III genes). The MHC
complex of humans is HLA, that of mice H-2, and that of rats, RT-1.
Mantle (corona). Zone in secondary follicles surrounding the central
germinal centre, densely packed with small resting B lymphocytes
Marginal zone. Outer layer of white pulp in spleen, surrounding
follicles, and periarteriolar lymphocyte sheath; separated from these
by the marginal sinus; populated by intermediate-sized, slightly
pyroninophilic B cells which have a major function in the T cell-
independent antibody response. The microenvironment manifests a
special type of macrophage, the marginal metallophilic macrophage
Margination. Adherence of blood leukocytes to endothelium during
inflammatory reactions
Mast cell. A bone marrow-derived polymorphonuclear leukocyte present
in tissue; has a major function in immediate hypersensitivity
reactions; has a round or oval nucleus and abundant cytoplasm with
basophilic (blue) granules stained by metachromatic dyes; granules
contain mediators of immediate hypersensitivity reactions, e.g.
heparin, histamine, serotonin, tryptase, kallikrein, and
chemoattractants for neutrophilic and eosinophilic granulocytes; has a
high-affinity receptor for IgE. After activation (physical stimuli,
cross-linking via allergen-IgE-IgE receptor), there is immediate
granule release and synthesis of other mediators, including
prostaglandins, thromboxanes, leukotrienes, and platelet-activating
factor. The cell can exert modulatory activity by secreting cytokines
such as IL-3, IL-6, and tumour necrosis factor. Two subtypes exist, in
the mucosa and in connective tissue; the equivalent in the circulation
is the basophilic granulocyte
Mast cell growth factor. See Interleukin 9
Medulla. Inner parenchymal layer of organs
Medullary cord. Parenchyma in medulla of lymph nodes separating
lymphatic sinusoids
Megakaryocyte. Large, multinucleated giant cell, precursor of blood
platelets, formed by separation of portions of membrane-bound
cytoplasm; occurs in haematopoietic tissue, including bone marrow
Memory. See Immunological memory
Mesoderm. Middle of the three cellular layers of the embryo;
produces connective tissue and blood cells
Metallophilic macrophage. Subtype of macrophage identified by silver
impregnation, present at the inner border of the marginal zone in
spleen
MHC restriction. Immunological reactions can occur only in
associated recognition with the polymorphic determinant of a given MHC
molecule and not with that of another MHC molecule. Applies to T
lymphocytes with an alpha-ß T-cell receptor, which recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules, and part of the T cell population with the gamma-delta
T-cell receptor.
Microglia. Macrophages in central nervous system
Migration inhibitory factor. A lymphokine that inhibits the movement
of macrophages
Milky spot. Aggregate of lymphoid cells in omentum, macroscopically
visible as a small white spot; not organized tissue but rather the
product of immune stimulation in that area of the body
Minor histocompatibility antigen. Ill-defined histocompatibility
marker not encoded by the MHC, which is a target in allograft
reactions (apart from products of the MHC)
Mitogen. Substance that activates resting cells to transform and
proliferate
Monoclonal. Derived from a single clone. For T and B lymphocytes, a
cell population in which all cells have a distinct V-D-J gene
rearrangement product (as seen in lymphoma and leukaemia). Monoclonal
antibodies are products of hybridomas prepared after fusion of
antibody-producing cells and a transformed (non-producing)
plasmacytoid cell line.
Monocyte. Large, 10-15-µm bone marrow-derived mononuclear cell in
the monocyte-macrophage lineage, present in the blood and in lymphatic
vessels
Monokine. Hormonal substance synthesized by monocytes-macrophages;
modulates the function of cells in immunological reactions
Mononuclear cell. Leukocyte with a single rounded nucleus, e.g.
lymphocytes and monocytes-macrophages
Mononuclear phagocytic system. Formerly called reticuloendothelial
system; composite of phagocytic cells in the body, including monocytes
and tissue macrophages. Main populations are Kupffer cells in liver,
microglia in the central nervous system, macrophages in red pulp of
spleen, alveolar macrophages in lung, and, after induction, peritoneal
macrophages in the peritoneal cavity; others are mesangial macrophages
in kidney and osteoclasts in bone
µ Chain. In immunoglobulin molecules, the µ heavy chain forming the
IgM immunoglobulin class
Mucosa-associated lymphoid tissue. Lymphoid tissue or organs in
immediate contact with the mucous-secreting mucosal layer in nasal
cavity and nasopharynx (nasal-associated lymphoid tissue), airways
(bronchus-associated lymphoid tissue), and intestinal tract (gut-
associated lymphoid tissue). Serves as the immunological defence at
secretory surfaces, to some extent independent of the systemic
(internal) response; includes IgA synthesis by plasma cells in the
lamina propria and excretion into the lumen
Multiple myeloma. Tumour of plasma cells in bone marrow
Muramidase. See Lysozyme
Myeloblast. Immature precursor cell in the lineage of polymorpho-
nuclear cells (granulocytes, mast cells)
Nasal-associated lymphoid tissue. Lymphoid organs or tissue located
in the nasal cavity and nasopharynx, presumed to be a first location
for presentation of antigens entering through the nose; contributes to
the mucosa-associated lymphoid tissue
Natural antibody. Antibody in serum of individuals with no previous
exposure to the corresponding antigen; often generated by contact with
cross-reacting agents, e.g. bacterial products; often restricted to
antibodies that react to xenogeneic antigens
Natural killer cell. Leukocyte with a limited repertoire to
recognize antigen; can kill target cells without prior sensitization;
can be of lymphoid or monocyte-macrophage origin. Large granular
lymphocytes are the main population
Necrosis. Death of tissue and cells
Neoantigen. New antigen appearing on cells or tissue during
malignant transformation or (viral) infection
Neoplasia. Uncontrolled malignant transformation of cells resulting
in tumour formation
Neutralization. Process whereby a pathogenic substance becomes
inactivated by effector components (antibodies) of the immunological
reaction
Neutropenia. Reduced proportions of neutrophilic granulocytes in
blood
Neutrophil chemotactic factor. Preformed mediator with a relative
molecular mass > 750 kDa, present in granules of mast cells and
basophilic granulocytes; released after activation and attracts
neutrophilic granulocytes to the site of inflammation or
hypersensitivity
Neutrophilic granulocyte. Polymorphonuclear leukocyte that contains
granules stained by neither acidophilic nor basophilic dyes; can
phagocytose immune complexes by receptor-mediated endocytosis,
followed by intracellular degradation in lysosomes. Degranulation
releases catepsins and lysosomal enzymes, resulting in tissue damage.
Nonspecific immunity. Immunity induced by non-immunological
mechanisms, for instance by action of complement, lysozyme,
phagocytosis, or interferon
Oedema. Swelling of tissue due to extravasation of fluid from the
intravascular space following increase in vascular permeability
Ontogeny. Life cycle of an organism; in immunological terms, often
used to describe the process whereby the immune system develops
immunocompetence
Opsonization. Adherence of pathogen to phagocytic cell due to action
of antibody or activated complement
Osteoclast. Macrophage in bony tissue involved in bone resorption
Paracortex. Area in the inner cortex of lymph node where T
lymphocytes are lodged; recognized by the presence of high endothelial
venules; continuous with interfollicular areas in the outer cortex on
one side and with the medullary cords on the other
Paratope (antigen-binding site). Part of antibody that binds antigen
(antigenic determinant, or epitope); part of T-cell receptor that
binds the complex of antigen and MHC molecule
Periarteriolar lymphocyte sheath. Area in white pulp of spleen
surrounding the central artery, populated mainly by small
T lymphocytes
Peripheral (secondary) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of these
reactions
Peyer's patch. Lymphoid tissue in wall of small intestine,
particularly ileum, separated from the gut lumen by a domed area and
an epithelial layer ('dome' epithelium); forms part of gut-associated
lymphoid tissue; main function is initiation of immunological
reactions towards pathogens entering through dome epithelium
Phagocytosis. Uptake of material >1 µm by cells, by receptor-
mediated endocytosis, by cells of the mononuclear phagocytic system;
requires Fc receptors, with accessory help of complement receptors; is
blocked by cytochalasins. Occurs via a 'zipper' mechanism, in which
the opsonized particle (coated with antibody or complement) becomes
enclosed by the cell membrane of the phagocyte; a second mechanism
involves oxidative burst, with formation of superoxide anion, peroxide
anion, and hydroxyl radicals, which kill or degrade the phagocytosed
particle
Phagolysosome. Membrane-bound cytoplasmic vesicle formed by fusion
of a phagosome and a lysosome
Phagosome. Membrane-bound vesicle in phagocytic cells containing
phagocytosed material
Pharmacokinetics. Fate of drugs or chemicals in the body over time,
including the processes of absorption and distribution in tissues,
biotransformation, and excretion
Phenotype. Characteristic of a distinct cell or individual,
reflecting the expression of a genetically determined property
Phenotypic marker. Expressed characteristic(s), for instance an
antigenic determinant, of a given cell or molecule, associated with
function or specificity
Phylogeny. Evolutionary history of a particular species
Pinocytosis. Uptake of material < 1 µm by cells; often restricted
to a receptor-mediated process in leukocytes of the monocyte-
macrophage series, e.g. uptake of lipoproteins and viruses into
clathrin-coated vesicles
Plasma. Fluid of uncoagulated blood after removal of cells
Plasma cell. Terminally differentiated B lymphocyte that synthesizes
and secretes immunoglobulin; these medium-sized, 10-15-µm cells have a
small excentric nucleus, with heterochromatin organized in a
'cartwheel'-like structure, and abundant cytoplasm filled with rough
endoplasmic reticulum.
Platelet. Small bone marrow-derived cytoplasmic fragment in blood
responsible for coagulation; main role is to block damaged vessel
walls and prevent haemorrhage, by clumping and aggregation; contains
heparin and serotonin, which contribute after release to the acute
vascular response in hypersensitivity reactions and produce oxygen
radicals
Platelet-activating factor. Low-relative-molecular-mass phospholipid
generated from alkyl phospholipids in mast cells, basophilic and
neutrophilic granulocytes, and monocytes-macrophages, which mediates
microthrombus formation of platelets in hypersensitivity reactions
Polyclonal. Derived from many different clones; for T and B
lymphocytes, cell populations in which the cells have different V-D-J
gene rearrangement products. Polyclonal activation is stimulation of
multiple lymphocyte clones, resulting in a heterogeneous response
Polymorphonuclear granulocyte. Leukocyte of bone-marrow origin, with
a lobulated nucleus, involved in acute inflammatory reactions. Main
subsets are basophilic, eosinophilic, and neutrophilic granulocytes
(different cytoplasmic granule colours after haematological staining).
Contributes to (acute) inflammatory reactions after attraction by
specific (immune complex-mediated) or nonspecific stimuli (including
complement components); after activation, releases granules containing
various hydrolytic enzymes
Postcapillary venule. Small blood vessel though which blood flows
after leaving the capillaries before reaching veins; often the site
where inflammatory cells leave the circulation and enter the tissue
Primary (central) lymphoid organ. Lymphoid organ where precursor
lymphocytes differentiate and proliferate in close contact with the
microenvironment to form immunocompetent cells; not antigen-driven but
can be influenced by mediators produced as a result of antigen
stimulation
Primary follicle. See Follicle
Primary response. Immunological reaction after first contact with
antigen, resulting in generation of immunological memory
Programmed cell death. See Apoptosis
Prostaglandin. Aliphatic acid produced by arachidonic acid
metabolism following the cyclo-oxygenase pathway; synthesized by mast
cells after activation; mediates immediate hypersensitivity reactions,
mainly smooth muscle contraction or bronchoconstriction; also
decreases the threshold for pain
Prothymocyte. Precursor of T lymphocytes in bone marrow before
moving to the thymus, or present in the thymus just before intrathymic
processing
Pyrogen. Substance that increases the temperature in the central
nervous system, resulting in fever; examples are bacterial endotoxin
and IL-1
Reactive oxygen intermediate. Reactive species of oxygen produced
e.g. by phagocytes (granulocytes and monocyte-macrophages) in response
to phagocytic stimuli like bacteria
Reagin. Former designation of IgE class antibody
Recall antigen. Antigen used to elicit a response from an individual
already sensitized to that antigen; may be one that the host has
knowingly been sensitized to or, in humans, one that it is assumed
that most individuals have been sensitized to
Red pulp. Area in spleen comprising venous sinuses filled with blood
and splenic cords; venous sinuses mainly contain erythrocytes
surrounded by endothelial cells; cords comprise macrophages,
lymphocytes, and occasionally megakaryocytes, but other types of blood
cells can also be present. Main function is phagocytosis of
particulate material and removal of old erythrocytes from blood. In
rodents, the red pulp can also be a site of haematopoiesis.
Reticuloendothelial system. See Mononuclear phagocytic system
Repertoire. All specific antigen-recognizing capacities (diversity)
within a population of T or B lymphocytes
RT-1. The major histocompatibility complex of rats
Secretory immunoglobulin. Immunoglobulin encountered in secretions
like tears, saliva, and jejunal juice; concerns mainly secretory IgA,
a dimer of the basic four-chain immunoglobulin structure, linked by a
J chain and surrounded by a secretory piece molecule.
Secretory piece. A 70-kDa molecule produced by epithelial cells
covering mucosa-associated lymphoid tissue; functions as a receptor
for IgA or IgM, thereby facilitating intercellular transport of these
molecules into the lumen. During this process, the secretory piece
becomes associated with the immunoglobulin, thereby enhancing its
stability in nonphysiological conditions of secretory fluid
Secondary follicle. See Follicle
Secondary (peripheral) lymphoid organ. Lymphoid organ in which
immunocompetent lymphocytes recognize antigen, subsequently initiate
immunological reactions, and produce effector elements of those
reactions
Secondary response. Response after first contact (immunization,
primary response) with an antigen, based on the presence of
immunological memory; characterized as faster, more intense, and of
higher affinity; for the antibody response, associated with an
immunoglobulin class switch
Selectin. Cell surface glycoprotein that has a prominent function in
the interaction between lymphocytes, monocytes, neutrophilic
granulocytes, and endothelium. They share an N-terminal domain of
approximately 120 amino acids that is homologous to many Ca2+-
dependent animal lectins and binds to carbohydrates. Examples are
L-selectin (MEL-14, LAM-1, present on leukocytes; adherence of
endothelial cells, role in lymphocyte recirculation and neutrophil and
leukocyte inflammation); E-selectin (ELAM-1, present on endothelium;
adherence of monocytes, neutrophils, and T cells; role in
inflammation); P-selectin (PADGEM, GMP-140, CD62, present on platelets
and endothelium; adherence of monocytes, neutrophils, and T cells;
role in inflammation).
Self-MHC restriction. See MHC restriction; applies to MHC
molecules of the individual
Sensitization. Induction of specialized immunological memory in an
individual by exposure to antigen
Serotonin. 5-Hydroxytryptamine; catecholamine with relative molecular
mass of 176 Da; preformed mediator of immediate hypersensitivity
reactions, present in granules of mast cells and in platelets. After
activation, is released and mediates vasodilatation and increased
vascular permeability
Serum. Fluid of blood after coagulation (removal of fibrinogen) and
removal of cells
Serum sickness. Systemic vasculitis, glomerulonephritis, or arthritis
due to immune complex formation after the reaction between antibody and
injected foreign antigen (serum)
Skin-associated lymphoid tissue. See Skin immune system
Skin immune system. Combination of immune system components and
their function, present in skin; antigen presentation by Langerhans
cells, by dendritic epidermal cells, and in some conditions by
keratinocytes; immunoregulation by e.g. keratinocyte-derived
cytokines, and distinct dermatotropic T-cell populations
Slow-reacting substance of anaphylaxis. See Leukotriene
Somatic mutation. Small changes in genes resulting in alterations in
amino acids built into protein chains. For immunoglobulin molecules,
changes in diversity of antigen-binding site (variable region)
Spleen. Lymphoid organ in the left abdominal cavity, for filtering
blood; main function is phagocytosis of particles from blood, removal
of old erythrocytes in red pulp, and initiation of immunological
reactions in white pulp. The marginal zone of the white pulp serves as
the main site of T cell-independent antibody formation.
Starry-sky macrophage. See Tingible body macrophage
Stem cell. Multipotential, self-renewing precursor cell of all
haematopoietic cell lineages, present in bone marrow
Stem-cell growth factor (synonym for c-Kit ligand). An interleukin
that supports continuous growth of mast cells and augments the
response of progenitor cells to stem growth factors; interacts via
c-kit proto-oncogene
Subcapsular sinus. Area in lymph node just under the capsule and
surrounding the cortex, which is connected with afferent lymphatics,
and through cortical (peritrabecular) sinuses with medullary sinuses;
contains dendritic macrophages
Superantigen. Antigenic moiety that, in MHC-restricted presentation
to T lymphocytes, is not present in the 'groove' made by the
quaternary structure of the MHC molecule but is complexed with the MHC
molecule. Examples are the endogenous viral-encoded Mlsa (minor
lymphocyte stimulatory) antigen, which is present in certain mouse
strains, and Staphylococcus enterotoxin A
Suppressor T cell. Subpopulation of T lymphocytes with CD8 phenotype;
after recognition of antigen in an MHC class I-restricted manner,
suppresses immunological reactions, in part by cytotoxic activity
Systemic lupus erythematosus. Chronic, remitting, relapsing,
inflammatory, and often febrile multisystemic disorder of connective
tissues, with possible involvement of the central nervous system,
skin, joints, kidneys, and serosal membranes; can be acute or
insidious in onset. The etiology is unknown but is thought to follow a
failure of the regulatory mechanisms of the immune system that sustain
self-tolerance. Many drugs and chemicals can induce lupus-like
symptoms (drug-induced lupus erythematosus)
T-Cell receptor. Heterodimeric molecule on the surface of the T
lymphocyte that recognizes antigen. The polypeptide chains have a
variable and a constant part, and can be alpha, ß, gamma, or delta.
The alpha-ß T-cell receptor occurs on most T cells and recognizes
antigenic peptides in combination with the polymorphic determinant of
MHC molecules (self-MHC restricted). The gamma-delta T-cell receptor
occurs on a small subpopulation, e.g. in mucosal epithelium, and can
recognize antigen in a non-MHC restricted manner. The T-cell receptor
occurs exclusively with the CD3 molecule, which is thought to mediate
transmembrane signalling.
T-Dependent antigen. Antigen for which antibody formation requires
T cells.
Terminal pathway of complement activation. Activation of complement
components C6-C9, with formation of the membrane attack complex and
subsequent lysis of the cell
Tingible body macrophage (starry-sky macrophage). Large macrophage
in cortex of thymus and germinal centres of follicles in lymphoid
tissue, filled with condensed nuclear material with high affinity for
dyes; has a major function in phagocytosis, presumably of apoptotic
cells
T Lymphocyte or cell. Lymphocyte that induces, regulates, and
effects specific immunological reactions after stimulation by antigen,
mostly in the form of processed antigen complexed with MHC product on
an antigen-presenting cell. They originate from precursors in the bone
marrow and undergo maturation in the thymus (T, thymus-dependent).
Most T lymphocytes recognize antigen by a heterodimeric alpha-ß
surface receptor molecule associated with the CD3 molecule, which
mediates transmembrane signalling. Subsets include helper-inducer and
suppressor-cytotoxic cells.
T-Lymphocyte area. That part of a lymphoid organ or tissue that is
occupied by T lymphocytes, e.g. paracortex or interfollicular area in
lymph node, periarteriolar lymphocyte sheath in spleen
Thrombocyte. See Platelet
Thrombocytopenia. Reduced proportion of platelets in blood
Thromboxane. Product of arachidonic acid following the cyclo-
oxygenase pathway; synthesized by mast cells after activation and
mediates immediate hypersensitivity reactions, mainly smooth muscle
contraction, bronchoconstriction, and platelet aggregation
Thymocyte. Lymphocyte in the thymus
Thymoma. Tumour of the thymus; neoplastic cell is an epithelial cell
Thymus. Central lymphoid organ located dorsal to the cranial part of
the sternum in the thorax, comprising two lobes, each consisting of
many lobules. Its main function is generation of immunocompetent
T lymphocytes from prothymocytes from the bone marrow
Tolerance. State of unresponsiveness to antigenic stimulation, due
to the absence of responding elements or loss of capacity of existing
elements to mount a reaction. Synonym for anergy
Tolerogen. Antigen that evokes tolerance
Tonsil. Organized mucosa-associated lymphoid tissue in
oronasopharynx. Adenoids strictu sensu are also tonsils. The main
function is initiation of immunological reactions towards pathogens
entering through the mouth. Contributes in part to the gut-associated
lymphoid tissue. Together with lymphoid aggregates in oronasopharynx,
these tissues form the ring of Waldeyer
Transforming growth factor ß. Mediator synthesized by lymphocytes or
macrophages, with a function in down-regulation of immune reactions;
suppresses T- and B-lymphocyte growth, IgM and IgG production, and
down-regulates MHC class II expression; interferes with production of
tumour necrosis factor and adhesion of granulocytes to endothelial
cells; is chemotactic for monocytes and induces interleukin-1 and
interleukin-6 expression
Transudation. Transfer of fluid and low-relative-molecular-mass
proteins from intravascular to extravascular tissue during
inflammatory processes
Tryptase. Proteolytic enzyme present in granules of mast cells;
released after activation and activates complement component C3, with
formation of the anaphylatoxin C3a
Tumour necrosis factor. General mediator of inflammation and septic
shock; formerly named cachectin and lymphotoxin. Two forms: alpha and
ß, both produced by monocytes-macrophages, TNF-ß also by T lymphocytes
and natural killer cells. Has activity similar to interleukin-1 and
acts synergistically with it; promotes antiviral state and is
cytotoxic for tumour cells; stimulates granulocytes and eosinophils,
activates macrophages to interleukin-1 synthesis, stimulates B cells
to proliferate and differentiate, T cells to proliferate,interleukin-2
receptor synthesis, and interferon gamma synthesis; induces
fibroblasts to synthesize prostaglandin and proliferate; induces fever
and synthesis of acute-phase proteins; reduces cytochrome P450
synthesis; activates endothelium and promotes adherence of
neutrophilic granulocytes to endothelium; induces cell adhesion
molecules like lymphocyte function-associated antigens LFA-1 and
LFA-3, ICAM-1, and ELAM-1; inhibits gastric motility in vitro;
reduces lipoprotein lipase synthesis by adipocytes; and activates
osteoclasts to bone resorption
Urticaria. Transient eruption of skin characterized by erythematous
or oedematous swelling (wheal) of the dermis or subcutaneous tissue
Vaccination. See Immunization
Valency. Number of antigenic determinants or ligands that can bind
to one antibody molecule or receptor
V (variable) gene. Gene that encodes the variable part of
immunoglobulin or T-cell receptor chains (e.g. VH1-VHn for
immunoglobulin heavy chains, Vkappa 1-Vkappa n for immunoglobulin
kappa light chain, Valpha 1-Valpha n for T-cell receptor alpha
chain)
Variable gene family. Groups of germline V genes (which encode
immunoglobulin chains or T-cell receptor genes) that have more than
about 80% nucleotide sequence identity
V (variable) region. Region at the amino terminal of immunoglobulin
or T-cell receptor chains, which contributes to the antigen-binding
site of the molecule. Encoded by V (variable), D (diversity), and J
(joining) genes in DNA
Vasoconstriction. Contraction of capillary venules, resulting in
decreased blood flow
Vasodilatation. Dilatation of capillary venules, resulting in
increased blood flow through capillaries and lowering of local blood
pressure
Veiled macrophage. Leukocyte belonging to the monocyte-macrophage
lineage, present in lymph; has a major function in uptake and
processing of antigen, followed by presentation (MHC class II-
restricted) to helper-inducer T lymphocytes. Cytoplasm contains
characteristic rod-like structures, Birbeck granules. Its equivalent
in lymphoid tissue is the interdigitating dendritic cell, and that in
skin is the Langerhans cell
Waldeyer's ring. Lymphoid tissue of tonsils and adenoids located
around the junction of the pharynx and oral cavity in humans and
domestic animals. Main function is initiation of immunological
reactions towards pathogens entering through the mouth. Contributes to
the gut-associated lymphoid tissue
White blood cell. Polymorphonuclear leukocyte, lymphocyte, or
monocyte in peripheral blood
White pulp. Area in spleen around central arterioles where lymphoid
cells reside. Comprises three major compartments: the periarteriolar
lymphocyte sheath, follicles, and marginal zone
Xenobiotic. Chemical or substance that is foreign to the biological
system
Xenogeneic. Genetically different phenotypes in individuals of
different species; opposite of allogeneic, or isogeneic
zeta Chain (see epsilon Chain). One of the chains in the CD3
molecule associated with the T-cell receptor
REFERENCES
Ada GL, Leung KN, & Ertl H (1981) An analysis of effector T-cell
generation and function in mice exposed to influenza A or Sendai
virus. Immunol Rev, 50: 5-24.
Ader, R, Felten D, & Cohen, N (1990) Interactions between the brain
and the immune system. Ann Rev Pharmacol Toxicol, 30: 561-602.
Adkins B, Richards JH, & Gardner DE (1979) Enhancement of experimental
respiratory infection following nickel inhalation. Environ Res,
20: 33-42.
Adkins B, Luginbuhl GH, Miller, FJ, & Gardner DE (1980) Increased
pulmonary susceptibility to Streptococcal infection following
inhalation of manganese oxide. Environ Res, 23: 110-120.
Adkins B, Gandour D, Strober S & Weissman I (1988) Total lymphoid
irradiation leads to transient depletion of the mouse thymic medulla
and persistent abnormalities among medullary stromal cells. J Immunol,
140: 3373-3379.
Adorini L ed. (1990) The molecular basis of antigen presentation to
T lymphocytes: Novel possibilities for immunointervention. Int Rev
Immunol, 6: 1-88.
Agius C (1985) The melanomacrophage centers of fish; a review.
In: Manning MJ & Tatner MF, eds, Fish Immunology. London, Academic
Press, pp 85-105.
Ahmed-Ansari A, Brodie AR, Fultz PN, Anderson DC, Sell KW, & McClure
HM (1989) Flow microfluorometric analysis of peripheral blood
mononuclear cells from nonhuman primates: Correlation of phenotype
with immune function. Am J Primatol, 17: 107-131.
Allen JR, & Abrahamson LJ (1973) Morphological and biochemical changes
in the liver of rats fed polychlorinated biphenyls. Arch Environ
Contam Toxicol, 1: 265-280.
Allen AL, Koller LD, & Pollock GA (1983) Effect of toxaphen exposure
on immune responses in mice. J Toxicol Environ Health, 11: 61-69.
Alomran, AH & Shleamoon, MN (1988) The influence of chronic lead
exposure on lymphocyte proliferative response and immunoglobulin
levels in storage battery workers. J Biol Sci Res, 19: 575-585.
Anderson DP (1990) Immunological indicators: Effects of environmental
stress on immune protection and disease outbreaks. Am Fish Soc Symp
8: 38-50.
Anderson RS & Brubacher LL (1993) Inhibition by pentachlorophenol of
production of reactive-oxygen intermediates by medaka phagocytic blood
cells. Marine Environ Res, 35: 125-129.
Anderson RE & Williams WL (1977) Radiosensitivity of T and B
lymphocytes. V. Effects of whole-body irradiation on numbers of
recirculating T cells and sensitization to primary skin grafts in
mice. Am J Pathol, 89: 367-378.
Anderson J, Moller G & Sjorberg O (1972) Selective induction of DNA
synthesis in T and B lymphocytes. Cell Immunol, 4: 381-393.
Anderson RE, Olson GB, Autry JR, Howarth JL, Troup GM ,& Bartels PH
(1977) Radiosensitivity of T and B lymphocytes. IV. Effect of whole
body irradiation upon various lymphoid tissues and numbers of
recirculating lymphocytes. J Immunol, 118: 1191-1200.
Anderson RE, Standefer JC, & Tokuda S (1986) The structural and
functional assessment of cytotoxic injury of the immune system with
particular reference to the effects of ionizing radiation and
cyclophosphamide. Br J Cancer, 53 (Suppl 7): 140-160.
André F, Gillon F, André C, Lafont S, & Jourdan G (1983) Pesticide-
containing diet augments anti-sheep red blood cell non reagenic
antibody responses in mice but may prolong murine infection with
Giardia muris. Environ Res 32: 145-150.
Anneroth G, Ericson T, Johannson I, Mornstad H, Ryberg M, Skoglund A,
& Stegmayr B (1992) Comprehensive medical examination of a group of
patients with alleged adverse effects from dental amalgam. Acta
Odontol Scand, 50: 101-107.
Applegate LA, Ley RD, Alcalay J, & Kripke ML (1989) Identification of
the molecular target for the suppression of contact hypersensitivity
by UV radiation. J Exp Med, 170: 1117-1131.
Appleton JA & McGregor DD (1984) Rapid expulsion of Trichinella
spiralisin suckling rats. Science, 226: 70-72.
Araneo BAT, Dowell T, Moon HB; & Daynes RA (1989) Regulation of murine
lymphokine production in vivo: Ultraviolet radiation exposure
depresses IL-2 and enhances IL-4 production by T cells through an IL-1
independent mechanism. J Immunol, 143: 1737-1745.
Aranyi C, Vana SC, Thomas PT, Bradof JN, Fenters JD, Graham JA, &
Miller FJ (1983) Effects of subchronic exposure to a mixture of O3,
SO2 and (NH4)SO4 on host defenses in mice. Environ Res,
12: 55-71.
Aranyi C, Bradof JN, O'Shea WJ, Graham JA, & Miller FJ (1985) Effects
of arsenic trioxide inhalation exposure on pulmonary antibacterial
defenses in mice. J Toxicol Environ Health, 15: 163-172.
Aranyi C, O'Shea WJ, Graham JA & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6: 713-720.
Archer DL, Smith BG, & Bukovic-Wess JA (1978) Use of an in vitro
antibody producing system for recognizing potentially
immunosuppressive compounds. Int Arch Allergy Appl Immunol, 56: 90-93.
Arkoosh MR & Kaattari SL (1990) Quantitation of fish antibody to a
specific antigen by an enzyme-linked immunosorbent assay (ELISA).
In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS & van Muiswinkel
WB ed. Techniques in Fish Immunology. Fair Haven, NJ, SOS
Publications, Vol 1, pp 15-24.
Askenase PW, Hayden B, & Gershon RK (1977) Evanescent delayed
hypersensitivity: Mediation by effector cells with a short life span.
J Immunol, 119: 1830-1835.
Austin JH, Schulman LL, & Mastrobattista JD (1989) Pulmonary infection
after cardiac transplantation: Clinical and radiologic correlations.
Radiology, 172: 259-265.
Austyn JM & Gordon S (1981) F4/80, a monoclonal antibody directed
specifically against the mouse macrophage. Eur J Immunol, 11: 805-815.
Baadsgaard O, Wulf HC, Wantzin GL, & Cooper KD (1987) UVB and UVC, but
not UVA, potently induce the appearance of T6-DR+ antigen-presenting
cells in human epidermis. J Invest Dermatol, 89: 113-118.
Baadsgaard O, Lisby S, Wulf HC, Wantzin GL, & Cooper KD (1989) Rapid
recovery of Langerhans cell alloreactivity, without induction of
autoreactivity, after in vivo ultraviolet A, but not ultraviolet
B exposure of human skin. J Immunol, 142: 4213-4218.
Baadsgaard O, Salvo B, Mannie A, Dass B, Fox DA, & Cooper KD (1990)
In vivo ultraviolet-exposed human epidermal cells activate T
suppressor cell pathways that involve CD4+CD45RA+ suppressor-inducer
T cells. J Immunol, 145: 2854-2861.
Baker D, O'Neill JK, Gschmeissner SE, Wilcox CE, Butter C, & Turk JL
(1990) Induction of chronic relapsing experimental allergic
encephalomyelitis in Biozzi mice. J Neuroimmunol, 28: 261-270.
Balls M, Blaauboer B, Brusick D, Frazier J, Lamb D, Pemberton M,
Reinhardt C, Roberfroid M, Rosenkranz H, Schmid B, Spielmann H,
Stammati A-L, & Walum E (1990) Report and recommendations of the
CAAT/ERGATT workshop on the validation of toxicity test procedures.
ATLA, 18: 313-337.
Bancroft GJ, Shellam GR, & Chalmer JE (1981) Genetic influences on the
augmentation of natural killer (NK) cells during murine
cytomegalovirus infection: Correlation with patterns of resistance. J
Immunol, 126: 988-994.
Bandeira A, Mota-Santos T, Otohara S, Degermann S, Heusser C, Tonegawa
S, & Coutinho A (1990) Localization of gamma/delta cells to the
intestinal epithelium is independent of normal microbial colonization.
J Exp Med, 172: 239-244. 306
Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, & Reynolds CW
(1985) Direct evidence for the role of LGL in the inhibition of
experimental tumor metastases. J Immunol, 134: 2783-2789.
Barnett JB & Rodgers KE (1994) Pesticides. In: Dean JH, Luster MI,
Munson AE, & Kimber I ed. Immunotoxicity and immunopharmacology, 2nd
Ed. New York, Raven Press, pp 191-212.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LS
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39: 263-274.
Barr BBB, Benton EC, McLaren K, Bunney MH, Smith IW, Blessing K,
& Hunter JAA (1989) Human papilloma virus infection and skin cancer in
renal allograft recipients. Lancet, i: 124-129.
Barrett WL, First MR, Aron BS, & Penn I (1993) Clinical course of
malignancies in renal transplant recipients. Cancer, 72: 2186-2189.
Barry TS, Jones DM, Richter CB, & Haynes BF (1991) Successful
engraftment of human postnatal thymus in severe combined immune
deficient (SCID) mice: Differential engraftment of thymic components
with irradiation versus anti-asialo GM-1 immunosuppressive regimens.
J Exp Med, 173: 167-180.
Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, & Penny R (1977)
Depressed lymphocyte function after bereavement. Lancet, i: 834-836.
Bascom R, Naclerio RM, Fitzgerald TK, Kagey-Sobotka A, & Proud D
(1990) Effect of ozone inhalation on the response to nasal challenge
of allergic subjects. Am Rev Respir Dis, 142: 594-601.
Basketter DA, Bremmer JN, Kammuller ME, Kawabata T, Kimber I, Loveless
SE, Magda S, Pal THM, Stringer DA, & Vohr HW (1994) The identification
of chemicals with sensitizing or immunosuppressive properties in
routine toxicology. Food Chem Toxicol, 32: 289-296.
Bates DV & Sizto R (1983) Relationship between air pollutant levels
and hospital admissions in southern Ontario. Can J Public Health,
74: 117-122.
Baumann G, Zenke G, Wenger R, Hiestand P, Quesniaux V, Andersen E, &
Schreier MH (1992) Molecular mechanisms of immunosuppression. J
Autoimmunol, 5 (Suppl A): 67-72.
Bazin H, Xhurdebise LM, Burtonboy G, Lebacq AM, de Clerq L, & Cormont
F (1984) Rat monoclonal antibodies. I. Rapid purification from
in vitro culture supernatants. J Immunol Meth 66: 261-269.
Behrendt H, Krämer U, Dolgner R, Hinrinchs J, Willer H, Hagenbeck H, &
Schlipköter HW (1993) Elevated levels of total serum IgE in East
German children: Atopy, parasites and pollutants. J Allergol, 2:31.
Behrendt H, Friedrichs K-H, Krämer U, Hitzfeld B, Becker W-M, & Ring J
(1994) The role of indoor and outdoor air pollution in allergic
diseases. In: Vos JG, Younes M, & Smith E ed. Allergic
hypersensitivities induced by chemicals: Recommendations for
prevention. Boca Raton, Florida, CRC Press, pp 173-182.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Roboz JP, Fischbein A, & Mason P (1987) Immunotoxicology,
environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the State of Michigan. Cancer Detect
Prev, Suppl 1: 29-37.
Bélisle C & Sainte-Marie G (1981) Tridimensional study of the deep
cortex of the rat lymph node. II. Relation of deep cortex units to
afferent lymphatic vessels. Anat Rec, 199: 61-72.
Bell EB (1989) Thymus-derived and non-thymus-derived T-like cells. The
origin and function of cells bearing gd receptors. Thymus, 14: 3-17.
Bencko V, Wagner V, Wagnerova M, & Ondrejcak K (1990) Immunological
profiles in workers occupationally exposed to inorganic mercury. J Hyg
Epidemiol Microbiol Immunol, 34: 9-14.
Bentwich Z, Bianco N, Jager L, Houba V, Lambert PH, Knaap W, Rose N,
Seligman M, Thompson R, Torrigiani G, & de Weck A (1982) Use and abuse
of laboratory tests in clinical immunology: Critical considerations of
eight widely used diagnostic procedures. report of a Joint IUIS/WHO
Meeting on Assessment of Tests Used in Clinical Immunology. Clin
Immunol Immunopathol, 24: 122-138.
Bentwich Z, Beverley PCL, Hammarstrom L, Kalden JR, Lambert PH, Rose
NR, & Thompson RA (1988) Laboratory investigations in clinical
immunology: Methods, pitfalls, and clinical indications. Clin Immunol
Immunopathol 49: 478-497.
van den Bergh P, Rozing J, & Nagelkerken L (1991) Opioid peptides as
cytokines in T cell activation. Adv Neuroimmunol, 1: 189-203.
van den Bergh P, Rozing J, & Nagelkerken L (1993a) Identification of
two moieties of ß-endorphin with opposing effects on rat T-cell
proliferation. Immunology, 79: 18-23.
van den Bergh P, Rozing J, & Nagelkerken L (1993b) b-Endorphin
stimulates Ia expression on mouse B cells by inducing interleukin-4
secretion by CD4+ cells. Cell Immunol, 149: 180-192
van den Bergh P, Dobber R, Ramlal S, Rozing J, & Nagelkerken L (1994)
Role of opioid peptides in the regulation of cytokine production by
murine CD4+ T cells. Cell Immunol, 154: 109-122.
Bergman A, Olsson M, & Reiland S (1992) Skull-bone lesions in the
Baltic grey seal (Halichoerus grypus). Ambio, 21: 517-519.
Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F ed. (1987)
Immunotoxicology. Dordrecht, Martinus Nijhoff Publishers, pp xi-xxvii.
Bernier J, Brousseau P, Krzystyniak K, Tryphonas H, & Fournier M (in
press) Immunotoxicity of heavy metals in relation to Great Lakes.
Environ Health Perspectives, Suppl 9.
Bernstein A & Breitman M (1989) Genetic ablation in transgenic mice.
Mol Biol Med, 6: 523-530.
Beschorner WE, Namnoun JD, Hess AD, Shinn CA, & Santos GW (1987a)
Cyclosporin A and the thymus immunopathology. Am J Pathol,
126: 487-496.
Beschorner, WE, Di Gennaro KA, Hess AD, & Santos GW (1987b)
Cyclosporine and the thymus: Influence of irradiation and age on
thymic immunopathology and recovery. Cell Immunol, 110: 350-364.
Bevilacqua MP (1993) Endothelial-leukocyte adhesion molecules. Ann Rev
Immunol, 11: 767-804.
Bick PH, Tucker AN, White KL, & Holsapple MP (1984) Effects of
subchronic exposure to diethylstilbestrol on humoral immune function
in adult female (C3B6)F1 mice. Immunopharmacology, 7: 27-39.
Bigazzi PE (1992) Lessons from animal models: The scope of mercury-
induced autoimmunity. Clin Immunol Immunopathol 65: 81-84.
Biron CA, Byron KS, & Sullivan JL (1989) Severe herpesvirus infection
in an adolescent without natural killer cells. New Engl J Med,
320: 1731-1735.
Bissonette E, Dubois C, & Rola-Pleszczynski M (1989) Changes in
lymphocyte function and lung histology during the development of
asbestosis and silicosis in the mouse. Res Commun Chem Pathol
Pharmacol, 65: 211-227.
Black RD, McVey DS, & Oehme FW (1992) Immunotoxicity in the bovine
animal: A review. Vet Hum Toxicol, 34: 438-442.
Blackley BR, Sisodia CS, & Mukkur TK (1980) The effects of
methylmercury, tetraethyllead and sodium arsenite on the humoral
immune response in mice. Toxicol Appl Pharmacol, 52: 245-254.
Blackmann M, Kappler J, & Marrack P (1990) The role of the T cell
receptor in positive and negative selection of developing T cells.
Science, 248: 1335-1341.
Blatt J, Olshan A, Gula MJ, Dickman PS, & Zarenek B (1992) Second
malignancies in very long-term survivors of childhood cancer. Am J
Med, 93: 57-60.
Blaylock BL, Holladay SD, Comment CE, Heindel JJ, & Luster MI (1992)
Exposure to tetrachlorodibenzo- p-dioxin (TCDD) alters fetal
thymocyte maturation. Toxicol Appl Pharmacol, 112: 207-213.
Blazer VS, Wolke RE, Brown J, & Powell CA (1987) Piscine macrophage
aggregate parameters as health monitors: Effects of age, sex, relative
weight, season and site quality in largemouth bass (Micropterus
salmoides). Aquat Toxicol, 10: 199-215.
Bleavins MR & Alvey JD (1991) Immunotoxicologic evaluation of a purine
nucleoside phosphorylase inhibitor in the cynomolgus monkey.
Toxicologist, 11: 205.
Bleavins MR & Dziedzic D (1990) An immunofluorescence study of T and B
lymphocytes in ozone-induced pulmonary lesions in the mouse. Toxicol
Appl Pharmacol, 105: 93-102.
Blok WL, Vogels MT, Curfs JH, Eling WM, Buurman WA, & Van Der Meer JW
(1992) Dietary fish-oil supplementation in experimental Gram negative
infection and in cerebral malaria in mice. J Infect Dis, 165: 898-903.
Bloom BR, Salgame P, & Diamond B (1992) Revisiting and revising
suppressor T cells. Immunol Today, 131: 131-136.
Blusse Van Oud Alblas A, & Van Furth R (1979) Origin, kinetics and
characteristics of pulmonary macrophages in the normal steady state.
J Exp Med, 149: 1504-1518.
Bly JE, Miller NW, & Clem LW (1990) A monoclonal antibody specific for
neutrophils in normal and stressed channel catfish. Dev Comp Immunol,
14: 211-221.
Bodey GP, Fainstein V, & Guerrant R (1986) Infections of the
gastrointestinal tract in the immunocompromised patient Ann Rev Med,
37: 271-281.
Bohus B, Koolhaas JM, De Ruiter AJH, & Heijnen CJ (1991) Stress and
differential alterations in immune system functions: Conclusions from
social stress studies in animals. Neth J Med, 39: 306-315.
Boivin JF (1990) Second cancers and other late side-effects of cancer
treatment: A review. Cancer, 65: 770-775.
Bolas-Fernandez F, Grencis RK & Wakelin D (1988) Cyclosporin A and
Trichinella spiralis: Anthelminthic effects in immunosuppressed
mice. Parasite Immunol, 10: 111-116.
Bonnyns M & Bastomsky CH (1976) Polychlorinated biphenyl-induced
modifica-tion of lymphocyte response to plant mitogens in rats.
Experientia, 15: 522-523.
Booss J (1980) Establishment of cytomegalovirus infection in mice:
Role of a macrophage-enriched subpopulation. J Infect Dis,
141: 466-472.
Borleffs JCC, Schuurman H-J, Vrehen HM, Van Schaik M, & Bast EJEG
(1993) in vitro and in vivo measurement of cell-mediated immunity
in patients with HIV-1-infection. Scand J Immunol, 37: 634-636.
Boros DL (1988) Mechanisms of granuloma formation in the lung.
In: Daniele RP ed. Immunology and immunologic diseases of the lung.
Boston, Blackwell Scientific Publications, pp 263-287.
Bos J ed. (1990) The skin immune system. Boca Raton, Florida, CRC
Press.
Bos JD & Kapsenberg ML (1986) The skin immune system (SIS): Its
cellular constituents and their interactions. Immunol Today,
7: 235-240.
Bouwes Bavinck JN, de Boer A, Vermeer BJ, Hartevelt MM, van der Woude
FJ, Claas FHJ, Wolterbeek R, & Vandenbroucke JP (1993) Sunlight,
keratotic skin lesions and skin cancer in renal transplant recipients.
Br J Dermatol, 129: 242-249.
Boyce WT, Jensen EW, Cassel JC, Collier AM, Smith AH, & Ramey CT
(1977) Influence of life events and family routines on childhood
respiratory tract illness. Pediatrics, 60: 609-615.
Boyd RL, Wilson TJ, Bean AG, Ward HA, & Gershwin ME (1992) Phenotypic
characterization of chicken thymic stromal elements. Dev Immunol,
2: 51-66.
Boyle J, Mackie RM, Briggs JD, Junor BJR, & Aitchison TC (1984)
Cancer, warts, and sunshine in renal transplant patients: A case-
control study. Lancet, i: 702-705.
Bradley SG & Morahan PS (1982) Approaches to assessing host
resistance. Environ Health Perspectives, 43: 61-69.
Brekelmans P & Van Ewijk W (1990) Phenotypic characterization of
murine thymic microenvironments. Sem Immunol, 2: 13-24.
Brett SJ & Butler R (1986) Resistance to Mycobacterium lepraemurium
is correlated with the capacity to generate macrophage activating
factor(s) in response to mycobacterial antigens in vitro.
Immunology, 59: 339-345.
Brideau RJ, Carter PB, McMaster WR, Mason DW, & Williams AF (1980) Two
subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J
Immunol, 10: 609-615.
Brigham KL (1989) Mediators in inflammation. In: Henderson PM & Murphy
RC ed. Handbook of inflammation, Vol. 6: Mediators of the inflammatory
process. Amsterdam, Elsevier Science Publishers, pp 1-14.
Brodie AM & Halliday WJ (1991) Suppressor cells and soluble factors
from the spleens of UV-irradiated mice. Immunol Cell Biol,
69: 199-204.
Brostoff J, Scadding GK, Male D, & Roitt IM (1991) Clinical
immunology. London, Gower Medical Publishers.
Brouwer A, Reijnders PJH, & Koeman JH (1989) Polychlorinated biphenyl
(PCB)-contaminated fish induces vitamin A and thyroid hormone
deficiency in the common seal (Phoca vitulina). Aquat Toxicol,
15: 99-106.
Brown WR & Kloppel TM (1989) The liver and IgA: Immunological, cell
biological and clinical implications. Hepatology, 9: 763-778.
Bruggeman CA, Debie WMH, Grauls G, Majoor G, & Boven CPA (1983)
Infection of laboratory rats with a new cytomegalo-like virus. Arch
Virol, 76: 189-199.
Bruggeman CA, Meijer H, Bosman F, & Boven CPA (1985) Biology of rat
cytomegalovirus. Intervirology, 24: 1-9.
Bruning JH (1985) Cytomegalovirus infections in renal transplantation;
study in a rat model. PhD thesis, Utrecht, Utrecht University.
Bucke D, Vethaak AD, & Lang T (1992) Quantitative assessment of
melanomacrophage centers (MMC's) in dab (Limanda limanda) along a
pollution transect in the German Bight. Mar Ecol Prog Ser,
91: 193-196.
Buckingham JC, Safieh B, Singh S, Arduino LA, Cover PO, & Kendall MD
(1992) Interactions between hypothalamo-pituitary adrenal axis and the
thymus in the rat: A role for corticotropin in the control of thymulin
release. J Neuroendocrinol, 4: 295-301.
Buening GM, Mann DD, Hook B, & Osweiler BP (1982) The effect of T-2
toxin on the bovine immunity system: Cellular factors. Vet Immunol
Immunopathol, 3: 411-417.
Bugelski PJ, Thiem PA, Solleveld HA, & Morgan DG (1990) Effects of
sensitization to dinitrochlorobenzene (DNCB) on clinical pathology
parameters and mitogen-mediated blastogenesis in cynomolgus monkeys
(Macaca fascicularis). Toxicol Pathol, 18: 643-650.
Bukowski JF, Woda BA, & Welsh RM (1984) Pathogenesis of murine
cytomegalovirus infection in natural killer cell-depleted mice.
J Virol, 52: 119-128.
Bulloch K (1987) The innervation of immune system tissues and organs.
In: Cotman CW, Brinton RE, Galaburda A, McEwen B, & Schneider DM ed.
The neuro-immune-endocrine connection. New York, Raven Press,
pp 33-47.
Burcham J, Marmor M, Dubin N, Tindall B, Cooper DA, Berry G, & Penny R
(1991) CD4+ is the best predictor of development of AIDS in a cohort
of HIV-infected homosexual men. AIDS, 5: 365-372.
Burchiel SW, Thompson TA, & Davis AP (1991) Alterations in mitogen-
induced calcium mobilization and intracellular free calcium produced
by 7,12-dimethylbenz (a)anthracene in the Jurkat human T cell line.
Int J Immunopharmacol, 13: 109-115.
Burleson GR, Fuller LB, Menache MG, & Graham JA (1987) Poly(I):
poly(C)-enhanced alveolar and peritoneal macrophage phagocytosis:
Quantification by a new method utilizing fluorescent beads. Proc Soc
Exp Biol Med, 184: 468-476.
Burleson GR, Keyes LL, & Stutzman JD (1989) Immunosuppression of
pulmonary natural killer activity by exposure to ozone.
Immunopharmacol Immunotoxicol, 11: 715-735.
Burleson GR, Lebrec H, Yang YG, lbanes JD, Pennington KN, & Birnbaum
LS (in press) Effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)
on influenza virus host resistance in mice. Fundam Appl Toxicol.
Calder VL & Lightman SL (1992) Experimental autoimmune uveoretinitis
(EAU) versus experimental allergic encephalomyelitis (EAE): A
comparison of T cell-mediated mechanisms. Clin Exp Immunol,
89: 165-169.
Cantacuzene MJ (1898) Nouvelles recherches sur le mode de destruction
des virions dans l'organisme. Ann Inst Pasteur, 12: 274-300.
Cantrell DA, Collins MKL, & Crumpton DA (1988) Autocrine regulation of
T-lymphocyte proliferation: Differential induction of IL-2 and IL-2
receptor. Immunology, 65: 343-349.
Capurro PU (1980) The effects of solvents and pesticides on health.
Clin Toxicol, 16: 549-553.
Carter JW & Clancy J (1980) Acutely administered polychlorinated
biphenyls (PCBs) decrease splenic cellularity but increase its
ability to cause graft-versus-host reactions in BALB/c mice.
Immunopharmacology, 2: 341-347.
Casale GP, Cohen SD, & DiCapua RA (1983) The effects of
organophosphate-induced cholinergic stimulation on the antibody
response to sheep erythrocytes in inbred mice. Toxicol Appl
Pharmacol, 68: 198-205.
Casale GP, Cohen SD, & DiCapua RA (1984) Parathion-induced suppression
of humoral immunity in inbred mice. Toxicol Lett, 23: 239-247.
Castillo-Mendez A, Rodriguez-Diaz T, Leon-Lobeck A, & Gravalosa-Cruz
AJ (1991) Influence of occupational lead exposure on the concentration
of immunoglobins and immune cellular functions in human. Rev Allergol
Mex, 38: 69.
Centers for Disease Control (1991) Summary of notifiable diseases,
United States, 1990. Morb Mortal Weekly Rep, 39 (53).
Challacombe SJ (1987) The investigation of secretory and systemic
immune responses to ingested material in animal models. In: Miller K
& Nicklin S ed. Immunology of the gastrointestinal tract, Vol 2. Boca
Raton, Florida, CRC Press, pp 99-124.
Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, &
Hiseroth JC (1989) Monoclonal antibody to a triggering structure
expressed on rat natural killer cells and adherent lymphokine-
activated killer cells. J Exp Med 169: 1373-1389.
Chan AC, Irving BA, & Weiss A (1992) New insights into T-cell antigen
receptor structure and signal transduction. Curr Opin Immunol,
4: 246-251.
Chanarin I (1985) Structure and function of the bone marrow. In: Irons
RD ed. Toxicology of the blood and bone marrow. New York, Raven Press,
pp 1-16.
Chandra RK (1992) Nutrition and immunoregulation. Significance of host
resistance to tumors and infectious diseases in humans and rodents.
J Nutr, 122 (Suppl): 754-757.
Chandra RK (1993) Nutrition and the immune system. Proc Nutr Soc,
52: 77-84.
Chang KH, Hsieh KH, Lee TP, Tang SY, & Tung TC (1981) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Determination of lymphocyte subpopulations. Toxicol Appl Pharmacol,
61: 58-63.
Chang KH, Hsieh KH, Tang SY, Tung TC, & Lee TP (1982) Immunologic
evaluation of patients with polychlorinated biphenyl poisoning:
Evaluation of delayed-type skin hypersensitivity response and its
relation to clinical studies. J Toxicol Environ Health, 9: 217-223.
Clark EA & Lanier LL (1989) Report from Vienna: In search of all
surface molecules expressed on human leukocytes. J Clin Immunol,
9: 265-272.
Clark GC, Blank JA, Germolec DR & Luster MI (1991)
2,3,7,8-Tetrachlorodibenzo- p-dioxin stimulation of tyrosine
phosphorylation in B lymphocytes: Potential role in
immunosuppression. Mol Pharmacol, 39: 495-501.
Clarke AG (1984) Immunological studies on pregnancy in the mouse.
In: Crighton DB ed. Immunological aspects of reproduction in mammals.
London, Butterworths, pp 153-182.
Clarke AG & Kendall MD (1989) Histological changes in the thymus
during mouse pregnancy. Thymus, 14: 65-78.
Clevers H, Alarcon B, Wileman T, & Terhorst C (1988) The T cell
receptor/CD3 complex: A dynamic protein ensemble. Ann Rev Immunol,
6: 629-662.
Clover RD, Abell T, Becker LA, Crawford S, & Ramsey CN (1989) Family
functioning and stress as predictors of influenza B infection.
J Family Practice, 28: 535-539.
Coffin DL & Gardner DE (1972) Interaction of biological agents and
chemical air pollutants. Ann Occup Hyg, 15: 219-234.
Cohen N, Modai D, Golik A, Weissgarten J, Peller S, Katz A, Averbuhk
Z, & Shaked U (l989) Increased concanavalin A-induced suppressor cell
activity in humans with occupational lead exposure. Environ Res,
48: 1-6.
Coligan JE, Kruisbeek, AM, Margulies DH, Shevach EM, & Strober W ed.
(1994) Current protocols in immunology, Vols 1 and 2. New York, John
Wiley & Sons.
Cook JA, Marconi EA, & DiLuzio NR (1974) Lead, cadmium, endotoxin
interaction: Effect on mortality and hepatic function. Toxicol Appl
Pharmacol, 28: 292.
Cook JA, Hoffman EO, & DiLuzio NR (1975) Influence of lead and cadmium
on the susceptibility of rats to bacterial challenge (39117). Proc Soc
Exp Biol Med, 150: 741.
Cook JC, Gaido KW, receptor: & Greenlee WF Relevance of (1987) Ah
mechanistic studies to human risk assessment. Environ Health
Perspectives, 76: 71-77.
Cooper EL (1982) How the immune system evolved. In: General
immunology. Oxford, Pergamon Press, pp 279-323.
Cooper KD, Neises GR, & Katz SI (1986) Antigen-presenting OKM5+
melanophages appear in human epidermis after ultraviolet radiation.
J Invest Dermatol, 86: 363-370.
Cooper KD, Oberhelman BS, Hamilton MS, Baadsgaard O, Terhune M, Levee
G, Anderson T, & Koren H (1992) UV exposure reduces immunization rates
and promotes tolerance to epicutaneous antigens in humans;
relationship to dose, CDla DR+ epidermal macrophage inductlon and
Langerhans cell depletion. Proc Natl Acad Sci USA, 89: 8497-8501.
Corman LC (1985) Effects of specific nutrients on the immune response.
Selected clinical applications. Med Clin North Am, 69: 759-791.
Cornacoff JB, Tucker AN, & Dean JH (1988) Development of a human
peripheral blood mononuclear leucocyte model for immunotoxicity
evaluation. In Vitro Toxicol, 2: 81-89.
Corrier DE (1992) Mycotoxicosis: Mechanisms of immunosuppression. Vet
Immunol Immunopathol, 30: 73-87.
Corsini E, Luster MI, Mahler J, Craig WA, Blazka M, & Rosenthal GJ
(1994) A protective role for T-lymphocytes in asbestos-induced
pulmonary inflammation and collagen deposition. Am J Respir Cell Mol
Biol, 7: 60-82.
Coscia GC, Discalzi G, & Ponzetti C (1987) Immunological aspects of
occupational lead exposure. Med Lav, 78: 360-364.
Council for International Organizations of Medical Sciences (1993)
International ethical guidelines for biomedical research involving
human subjects. Geneva, 63 pp.
Cunningham AJ & Szenberg A (1968) Further improvements in the plaque
tech-nique for detecting single antibody-forming cells. Immunology,
14: 559-600.
Dabrowski MP & Dabrowski-Bernstein BK (1990) Immunoregulatory role of
the thymus. Boca Raton, Florida, CRC Press.
Dallman MJ, Thomas ML, & Green JR (1984) MRC OX-19: A monoclonal
antibody that labels rat T lymphocytes and augments in
vitroproliferative responses. Eur J Immunol, 14: 260-267.
Davis AP & Burchiel SW (1992) Inhibition of calcium-dependent pathways
of B-cell activation by DMBA. Toxicol Appl Pharmacol, 116: 202-208.
Davis D & Safe S (1988) Immunosuppressive activities of
polychlorinated dibenzofuran congeners: Quantitative structure-
activity relationships and interactive effects. Toxicol Appl
Pharmacol, 94: 141-149.
Davis D & Safe S (1989) Dose-response immunotoxicities of commercial
polychlorinated biphenyls (PCBs) and their interaction with
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol Lett, 48: 35-43.
Davis D & Safe S (1991) Halogenated aryl hydrocarbon-induced
suppression of the in vitro plaque-forming cell response to
sheep red blood cells is not dependent on the Ah receptor.
Immunopharmacology, 21: 183-190.
Dayan AD, Hertel RF, Heseltine E, Kazantis G, Smith EM, & Van der
Venne MT ed. (1990) Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press.
Dean JH & Murray MJ (1990) Toxic responses of the immune system.
In: Klaassen CD, Amdur MO, & Doull J ed. Toxicology: The basic science
of poisons, Vol 4. New York, MacMillan Press, pp 282-333.
Dean JH & Thurmond LM (1987) Immunotoxicology: An overview. Toxicol
Pathol, 15: 265-271.
Dean JH, Luster MI, & Boorman GA (1982) Immunotoxicology. In: Sirois
P, & Pleszczynski MR ed. Immunopharmacology. Amsterdam, Elsevier
Science Publishers, pp 349-397.
Dean JH, Luster MI, Munson AE, & Amos HA (1985) Immunotoxicology and
immunopharmacology. New York, Raven Press.
Dean JH, Ward EC, Murray MJ, Lauer LD, & House RV (1986)
Immunosuppression following 7,12-dimethylbenz-a-anthracene exposure
in B6C3F1 mice. II. Altered cell-mediated immunity and tumor
resistance. Int J Immunopharmacol, 8: 189-198.
Dean JH, Luster MI, Munson AE, & Kimber I (1994) Immunotoxicology and
immunopharmacology, 2nd Ed. New York, Raven Press.
De Gast GC, Verdonck LF, Middeldorp JM, The TH, Hekker A, Van der
Linden JA, Kreeft HA, & Bast BJ (1985) Recovery of T cell subsets
after autologous bone marrow transplantation is mainly due to
proliferation of mature T cells in the graft. Blood, 66: 428-431.
De Heer C, Verlaan APJ, Penninks AH, Schuurman HJ, & Van Loveren H
(1993) The SCID-RA mouse: Rat T cell differentiation in the severe
combined immunodeficient mouse. APMIS, 101: 467-479.
Denis M (1992) Mouse hypersensitivity pneumonitis: Depletion of NK
cells abrogates the spontaneous regression phase and leads to massive
fibrosis. Exp Lung Res, 18: 761-773.
Denis M, Bisson D, & Ghardirian E (1993) Cellular and cytokine
profiles in spontaneous regression phase of hypersensitivity
pneumonitis. Exp Lung Res, 19: 257-271.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
Deo MG, Gangal S, Bhisey AN, Somasundaram R, Balsara Gulwani B,
Darbaru BS, Bhiide S, & Maru B (1987) Immunological, mutagenic and
genotoxic investigations in gas exposed population of Bhopal. Indian
J Med Res, 86 (Suppl): 63-76.
Descotes J (1985) Immunomodulating agents and hepatic drug metabolism.
Drug Metab Rev, 16: 175-183.
Descotes J (1986) Immunotoxicology of drugs and chemicals. Amsterdam,
Elsevier Science Publishers.
Descotes J (1990) Drug-induced immune diseases. Amsterdam, Elsevier
Science Publishers.
Descotes J (1992) Immunomodulating agents. In: Dukes MNG ed. Meyler's
side-effects of drugs, 12th Ed. Amsterdam, Elsevier Science
Publishers, pp 954-958.
Descotes J & Vial T (1994) Cytoreductive drugs. In: Dean JH, Luster
MI, Munson AE, & White K ed. Immunotoxicology and immunopharmacology,
2nd Ed. New York, Raven Press, pp 293-301.
Descotes J, Verdier F, Brouland JP, & Pulce C (1990) Immunotoxicity of
lead, cadmium and arsenic: Experimental data and their relevance to
man. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM, &
Van der Venne, M-T ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, pp 209-213.
Desi L, Varga L, & Farkas I (1978) Studies on the immunosuppressive
effect of organochlorine and organophosporic pesticides in subacute
experiments. J Hyg Epidemiol Microbiol Immunol, 22: 115-122.
Desi L, Varga L, Dobronyi L, & Szklenarik G (1985) Immunotoxicological
investigation of the effects of a pesticide; cypermethrin. Arch
Toxicol, Suppl 8: 305-309.
De Swart RL, Kluten RMG, Huizing CJ, Vedder LJ, Reijnders PJH, Visser
IKG, UytdeHaag FGCM, & Osterhaus ADME (1993) Mitogen and antigen
induced B and T cell responses of peripheral blood mononuclear cells
from the harbour seal (Phoca vitulina). Vet Immunol Immunopathol,
37: 217-230.
De Swart RL, Ross PS, Vedder EJ, Timmerman HH, Heisterkamp SH, Van
Loveren H, Vos JG, Reijnders PJH, & Osterhaus ADME (1994) Impairment
of immune function in harbour seals (Phoca vitulina) feeding on fish
from polluted waters. Ambio, 23: 155-159.
Devens BH, Grayson MH, Imamura IK, & Rodgers KE (1985)
O,O,S-Trimethyl-phosphorothioate effect on immunocompetence.
Pestic Biochem Physiol, 24: 251-259.
Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers
D, & Koren H (1991) Exposure of humans to ambient levels of ozone for
6.6 hours causes cellular and biochemical changes in the lung.
Am J Resp Cell Mol Biol, 4: 72-81.
De Waal EJ, Rademakers LHPM, Schuurman H-J, & Van Loveren H (1992a)
Interdigitating cells in the rat thymus during cyclosporin A
treatment: Ultrastructural observations. Thymus, 20: 163-170.
De Waal EJ, Schuurman H-J, Loeber JG, Van Loveren H, & Vos JG (1992b)
Alterations in the cortical thymic epithelium of rats after
in vivo exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD):
An (immuno)histological study. Toxicol Appl Pharmacol, 115: 80-88.
De Waal EJ, Rademakers LHPM, Schuurman H-J, Van Loveren H, & Vos JG
(1993) The cortical epithelium of the rat thymus after in vivo
exposure to bis(tri-n-butyltin)oxide (TBTO). Arch Toxicol,
67: 186-192.
DeWailly E, Nantel A, Brunean S, Laliberte C, Ferron L, & Gingras S
(1992) Breast milk contamination by PCDDs, PCDFs, and PCBs in Arctic
Quebec: A preliminary assessment. Chemosphere, 25: 1245-1249.
De Weger RA, Van Loveren H, Vandebriel RJ, & Parmentier HK (1989).
Sequential activity of gamma-delta T-cell receptor bearing
lymphocytes, specific T-cell factors, and alpha-ß T-cell receptor
bearing T-cells: A hypothesis. Thymus, 14: 19-30.
Dieter MP, Luster MI, Boorman GA, Jameson CW, Dean JH, & Cox JW (1983)
Immunological and biochemical responses in mice treated with mercuric
chloride Toxicol Appl Pharmacol, 68: 218-228.
Dietert RR, Qureshi MA, Nanna UC, & Bloom SE (1985) Embryonic exposure
to aflatoxin-B1: Mutagenicity and influence on development and
immunity. Environ Mutag, 7: 715-725.
Dietz R, Heide-Jorgensen MP, & Härkönen T (1989) Mass deaths of harbor
seals (Phoca vitulina) in Europe. Ambio, 18: 258-264.
Dijkstra CD & Sminia T (1990) Normal anatomy, histology,
immunohistology, ultrastructure, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI monograph). Berlin, Springer
Verlag, pp 185-193.
Dijkstra CD, Döpp EA, Joling P, & Kraal G (1985) The heterogeneity of
mononuclear phagocytes in lymphoid organs: Distinct macrophage
subpopu-lations in rat recognized by monoclonal antibodies ED1, ED2
and ED3. Immunology, 54: 589-599.
Dinarello CA (1992) Role of interleukin-1 in infectious diseases.
Immunol Rev, 127: 119-146.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979a) Role of
macrophages in the augmentation of mouse natural killer activity by
polyI:C and interferon. J Immunol, 122: 182-188.
Djeu JY, Heinbaugh JA, Holden HT, & Herberman RB (1979b) Augmentation
of mouse natural killer cell activity by interferon and interferon
inducers. J Immunol, 122: 175-181.
Dobber R, Hertog-Huijbregts A, Rozing J, Bottomly K, & Nagelkerken L
(1992) The involvement of the intestinal microflora in the expansion
of CD4+ T cells with a naive phenotype in the periphery. Dev
Immunol, 2: 141-150.
Dogra RK, Chandra K, Chandra S, Gupta S, Khanna S, Srivastava SN,
Shukla LJ, Katiyar JC, & Shanker R (1992) Host resistance assays as
predictive models in styrene immunomodulation. Int J Immunopharmacol,
14: 1003-1009.
Domzig W, Stadler BM, & Herberman RB (1983) Interleukin-2 dependence
of human natural killer (NK) cell activity. J Immunol, 130: 1970-1973.
Dooley RK & Holsapple MP (1988) Elucidation of cellular targets
responsible for tetrachlorodibenzo- p-dioxin (TCDD)-induced
suppression of antibody responses: I. The role of the B lymphocyte.
Immunopharmacology, 16: 167-180.
Dotta F & Eisenbarth GS (1989) Type I diabetes mellitus: A predictable
autoimmune disease with interindividual variation in the rate of
ß cell destruction. Clin Immunol Immunopathol, 50: S85-S95.
Driscoll KE, Higgins JM, Leytart MJ, & Corsby LL (1990) Differential
effects of mineral dusts on the in vitro activation of alveolar
macrophage eicosanoid and cytokine release. Toxicity in vitro,
4, 284-288.
Drouet M, Le-Sellin J, Bonneau JC, & Sabbah A(1990) Is mercury an
allergen of the respiratory tract. Allerg Immunol (Paris),
22: 81, 84-85, 87.
Druet P, Pelletier L, Rossert J, Druet E, Hirsch F, & Sapin C (1989)
Autoimmune reactions induced by metals. In: Kammüller ME, Bloksma N,
& Seinen W ed. Autoimmunity and toxicology. Immune disregulation
induced by drugs and chemicals. Amsterdam, Elsevier Science
Publishers, pp 347-361.
Duijvestijn A & Hamann A (1989) Mechanisms and regulation of
lymphocyte migration. Immunol Today, 10: 23-28.
Duke SS, Schook LB, & Holsapple MP (1985) Effects of
N-nitrosodimethylamine on tumor susceptibility. J Leukocyte
Biol, 37: 383-394.
Dunn TB (1954) Normal and pathologic anatomy of the reticular tissue
in laboratory mice, with a classification and discussion of neoplasms.
J Natl Cancer Inst, 14: 1281-1433.
Du Pasquier L (1989) Evolution of the immune system. In: Paul WE ed.
Fundamental immunology, 2nd Ed. New York, Raven Press, pp 139-165.
Durham WH (1974) Air pollution and student health. Arch Environ
Health, 28: 241-254.
Dutch Health Council, Committee for Immunotoxicology (1991)
Immunotoxicity of compounds. The Hague.
Dyall-Smith C & Varigos G (1985) The malignant potential of
papillomavirus. Aust J Dermatol, 26: 102-107.
Dziedzic D & White HJ (1986) T-Cell activation in pulmonary lymph
nodes of mice exposed to ozone. Environ Res, 41: 610-622.
Dziedzic D, Wright ES, & Sargent HE (1990) Pulmonary response to
ozone. Reaction of bronchus-associated lymphoid tissue and lymph node
lymphocytes in the rat. Environ Res, 51: 194-208.
Eedy DJ, Burrows D, Clifford T, & Fay A (1990) Elevated T cell
subpopulations in dental students. J Prosthet Dent, 63: 593.
Ehrke MJ, Mihich E, Berd D, & Mastrangelo MJ (1989) Effects of
anticancer drugs on the immune system in humans. Semin Oncol,
16: 230-253.
Ehrlich JP & Burleson GR (1991) Enhanced and prolonged pulmonary
influenza virus infection following phosgene inhalation. J Toxicol
Environ Health, 34: 259-273.
Ehrlich R & Henry MC (1968) Chronic toxicity of nitrogen dioxide.
I. Effect on resistance to bacterial pneumonia. Arch Environ Health,
17: 860-865.
Ehrlich R, Findlay JC, Fonters JD, & Gardner DE (1977) Health effects
of short-term inhalation of nitrogen dioxide and ozone mixtures.
Environ Res, 4: 223-231.
Ellman MH, Hurwitz H, Thomas C, & Kozloff M (1991) Lymphoma developing
in a patient with rheumatoid arthritis taking low dose weekly
methotrexate. J Rheumatol, 18: 1741-1743.
Elo O, Vuojolahti P, Janhunen R, & Rantanen J (1985) Recent PCB
accidents in Finland. Environ Health Perspectives, 60: 315-319.
Emmendorfer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML,
& Roesler J (1994) Evaluation of flow cytometric methods for diagnosis
of chronic granulomatous disease variants under routine laboratory
conditions. Cytometry, 18: 147-155.
Engelhard VH (1994) Structure of peptides associated with class I and
class II MHC molecules. Ann Rev Immunol, 12: 181-207.
Engleman EG, Benike CJ, Grumet FC, & Evans RL (1981) Activation of
human T lymphocyte subsets: Helper and suppressor/cytotoxic T cells
recognize and respond to distinct histocompatibility antigens.
J Immunol, 127: 2124-2129.
Epstein JH, Tuffanelli DL, & Dubois EL (1965) Light sensitivity and
lupus erythematosus. Arch Dermatol, 91: 483-485.
Eskola J & Toivanen P (1974) Effect of in ovo treatment with
cyclophosphamide on lymphoid system in chicken. Cell Immunol,
13: 459-471.
Evans RG, Webb KB, Knutsen AP, Roodman ST, Roberts DW, Bagby JR,
Garrett WA, & Andrews JS (1988) A medical follow-up of the health
effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. Arch Environ Health, 43: 273-278.
Ewers U, Stiller-Winkler R, & Idel H (1982) Serum immunoglobulin,
complement C3, and salivary IgA levels in lead workers. Environ Res,
29: 351-357.
Exon JR (1985) Effect of lead, polychlorinated biphenyls and
cyclophosphamide on rat natural killer cells, interleukin 2, and
antibody synthesis. Fundam Appl Toxicol, 5: 158-164.
Ezine S, Weissman IL, & Rouse RV (1984) Bone marrow cells give rise to
distinct cell clones within the thymus. Nature, 309: 629-631.
Fabry Z, Raine CS, & Hart MN (1994) Nervous tissue as an immune
compartment: Dialect of the immune response in the CNS. Immunol Today,
15: 218-224.
Faisal M & Hugget RJ (1993) Effects of polycyclic aromatic
hydrocarbons on the lymphocyte mitogenic response in spot, Leiostomus
xanthurus. Mar Environ Res, 35: 121-124.
Faisal M, Chiapelli F, Ahmed II, Cooper EL, & Weiner H (1989) Social
confrontation stress in aggressive fish is associated with an
endogenous opioid-mediated suppression of proliferative response to
mitogens and nonspecific cytotoxicity. Brain Behav Immunol,
3: 223-233.
Fänge R & Nilsson S (1985) The fish spleen: Structure and function.
Experientia, 41: 152-158.
Farrell HE & Shellam GR (1991) Protection against murine
cytomegalovirus infection by passive transfer of neutralizing and non-
neutralizing monoclonal antibodies. Infect Immunol, 72: 149-156.
Fauci A, Schnittman SM, & Poli G (1991) Immunopathologenic mechanisms
in human immunodeficiency virus (HIV) infection. Ann Intern Med.,
114: 678-693.
Fellah JS, Wiles MV, Charlemagne J, & Scwager J (1992) Evolution of
vertebrate IgM: Complete amino acid sequence of the constant region of
Ambystoma mexicanum µ chain deduced from cDNA sequence. Eur J
Immunol, 22: 2595-2601.
Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden
KS, Olschowka JA, & Livnat S (1987) Noradrenergic sympathetic neural
interaction with the immune system: Structure and function. Immunol
Rev, 100: 225-260.
Fenters J, Bradof J, Aranyi C, Ketels K, Ehrlich R, & Gardner D (1979)
Health effects of long-term inhalation of sulfuric acid mist-carbon
particle mixtures. Environ Res, 19: 244-257.
Fichtelius KE, Groth O, & Liden S (1970) The skin, a first level
lymphoid organ? Int Arch Allergy, 37: 607-620.
Fidler IJ (1973) Selection of successive tumor lines for metastasis.
Nature New Biol, 242: 148-149.
Fidler IJ, Gersten DM, & Hart IR (1978) The biology of cancer invasion
and metastases. Adv Cancer Res, 28: 149-250.
Fine JS, Gasiewicz TA, & Silverstone AE (1989) Lymphocyte
stem cell alterations following perinatal exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol Pharmacol, 35: 18-25.
Fine JS, Gasiewicz TA, Fiore NC, & Silverstone AE (1990) Prothymocyte
activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo- p-dioxin
exposure. J Exp Pharmacol Ther, 255: 1-5.
Finsen NR (1901) The treatment of lupus vulgaris by concentrated rays.
In: Phototherapy. London, Edward Arnold, pp 73-75.
Fiore MC, Anderson HA, Hong R, Golubjatnokov R, Seiser JE, Nordstrom
D, Hanrahan L, & Belluck D (1986) Chronic exposure to aldicarb-
contaminated groundwater and human immune function. Environ Res,
41: 633-645.
Fischbein A, Tsang P, Luo JC, Roboz JP, Jiang JD, & Bekesi JG (1993)
Phenotypic aberrations of CD3+ and CD4+ cells and functional
impairments of lymphocytes at low-level occupational exposure to lead.
Clin Immunol Immunopathol, 66: 163-171.
Flajnik MF, Canel C, Kramer J, & Kasahara M (1991) Evolution of the
major histocompatibility complex class I from the amphibian Xenopus.
Proc Natl Acad Sci USA, 88: 537-541.
Fleisher TA, Luckasen JR, Sabad A, Gehrtz RC, & Kersey JH (1975) T and
B lymphocyte subpopulations in children. Pediatrics, 55: 162-165.
Fletcher TC (1986) Modulation of non specific host defences in fish.
Vet Immunol Immunopathol, 12: 59-67.
Fletcher FA & Williams DE (1992) Recent progress in the discovery and
invention of novel hematopoietic cytokines. Crit Rev Oncol Hematol,
13: 1-15.
Flick DF, O'Dell RG, & Childs VA (1965) Studies on the chick edema
disease. 3. Similarity of symptoms produced by feeding chlorinated
biphenyls. Poultry Sci, 44: 1460-1465.
Folinsbee LJ (1992) Human health effects of air pollution. Environ
Health Perspectives, 100: 45-56.
Fontana A, Constamm DB, Frei K, Malipiero U, & Pfister HW (1992)
Modulation of the immune response by transforming growth factor beta.
Int Arch Allergy Immunol, 99: 1-7.
Food Safety Council (1978) Proposed system for food safety assessment.
Food Cosmet Toxicol, 16 (Suppl 1): 1-136.
Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, & Gery
I (1987) Experimental autoimmune uveoretinitis (EAU) induced by
retinal interphotoreceptor retinoid-binding protein (IRBP):
Differences between EAU induced by IRBP and by S-antigen. Clin Immunol
Immunopathol, 43: 256-264.
Foxwell BM, Barrett K, & Feldmann M (1992) Cytokine receptors:
Structure and signal transduction. Clin Exp Immunol, 90: 161-169.
Frasca D, Adorini L, & Doria G (1987) Enhanced frequency of mitogen-
responsive T cell precursors in old mice injected with thymosin alpha
1. Eur J Immunol, 17: 727-730.
Frattini JE & Trulock EP (1993) Respiratory infections in
immunocompromised patients. Immunol Allergy Clin North Am,
13: 193-204.
Frazer IH, Collins EJ, Fox JS, Jones B, Oliphant RC, & Mackay IR
(1985) Assessment of delayed-type hypersensitivity in man: A
comparison of the 'Multitest' and conventional intradermal injection
of six antigens. Clin Immunol Immunopathol, 35: 182-190.
Freier S, ed. (1990) The neuroendocrine-immune network. Boca Raton,
Florida, CRC Press.
French JG, Lowrimore G, Nelson WC, Finklea JF, English T, & Hertz M
(1973) The effect of sulfur dioxide and suspended sulfates on acute
respiratory disease. Arch Environ Health, 27: 129-133.
Friedman H, Klein T, & Specter S (1991a) Immunosuppression by
marijuana and its components. In: Ader R, Felten DL, & Cohen N ed.
Psychoneuroimmunology, 2nd Ed. San Diego, Academic Press, pp 931-953.
Friedman H, Specter S, & Klein TW (1991b) Drugs of abuse, immunity and
immunodeficiency (Advances in Experimental Biology and Medicine,
Volume 288). New York, Plenum Press.
Friedmann PS, White SI, Parker S, Moss C, & Mattews JNS (1989)
Antigenic stimulation during ultraviolet therapy in man does not
result in immunological tolerance. Clin Exp Immunol, 76: 68-72.
Friend M & Trainer DO (1970) Polychlorinated biphenyl: Interaction
with duck hepatitis virus. Science, 170: 1314-1316.
Frith CH, Ward JM, Brown RH, Tyler RD, Chandra M, & Stromberg PC (in
press) Proliferative lesions of the hematopoietic system in rats. In:
Streett CS, Burek JD, Hardisty JF, Garner FM, Leiniger JR, Pletcher
JM, & Moch RW ed. Standardized system of nomenclature and diagnostic
criteria. Guides for toxicologic pathology. Washington DC, Society of
Toxicologic Pathologists, Armed Forces Institute of Pathology,
American Registry of Pathology.
Fugmann RA, Aranyi C, Barbera PW, Bradof JN, Gibbons RD, & Fenters JD
(1983) The effects of DES as measured by host resistance and tumor
susceptibility assays in mice. J Toxicol Environ Health, 11: 827-841.
Fujimaki H (1989) Impairment of humoral responses in mice exposed to
nitrogen dioxide and ozone mixtures. Environ Res, 48: 211-217.
Gahmberg CG, Nortamo P, Li R, & Valmu L (1992) Leukocyte cell adhesion
proteins: From molecular dissection to clinical applications. Ann Med,
24: 329-335.
Gaido KW & Wierda D (1987) Suppression of bone marrow stromal cell
function by benzene and hydroquinone is ameliorated by indomethacin.
Toxicol Appl Pharmacol, 89: 378-390.
Gardner DE, Miller FJ, Illing JW, & Kirtz JM (1977) Alterations in
bacterial defense mechanisms of the lung induced by inhalation of
cadmium. Bull Eur Physiopathol Resp, 13: 157-174.
Garssen J, Van der Vliet H, De Klerk A, Dormans JAMA, & Bruggeman CA
(1995) A rat cytomegalovirus infection model as a tool for
immunotoxicity testing. Eur J Pharmacol, 292: 233-281.
Gasiewicz TA & Rucci G (1984) Cytosolic receptor for
2,3,7,8-tetrachloro-dibenzo- p-dioxin Evidence for a homologous
nature among various mammalian species. Mol Pharmacol, 26: 90-98.
Gaumer HS, Doll NJ, Kaimal J, Schuyler M, & Salvaggio JE (1981)
Diminished suppressor cell function in patients with asbestosis.
Clin Exp Immunol, 44: 108.
Germain RN & Margulies DH (1993) The biochemistry and cell biology of
antigen processing and presentation. Ann Rev Immunol, 11: 403-450.
Germolec DR, Yang RSH, Ackermann MF, Rosenthal GJ, Boorman GA, Blair
P, & Luster MI (1989) Toxicology studies of a chemical mixture of 25
groundwater contaminants. II. Immunosuppression in B6C3F1 mice.
Fundam Appl Toxicol, 13: 377-387.
Ghoneum MH, Egami N, Ljiri K & Cooper EL (1986) Effect of
corticosteroids on the thymus of the fish Oryzias latipes. Dev Comp
Immunol, 10: 35-44.
Giannini SH (1990) Effects of UVB on infectious diseases. In: White JC
ed. Global atmospheric change and public health. Amsterdam, Elsevier
Science Publishers, pp 33-45.
Giannini SH (1992) Effects of UV radiation on cutaneous leishmaniasis.
Parasitol Today, 8: 44-48.
Gibson GG, Hubbard R, & Parke DV (1983) Immunotoxicology. London,
Academic Press.
Gilmartin WG, Delong RL, Smith AW, Sweeney JC, De Lappe BW, Risebrough
RW, Griner LA, Dailey MD, & Peakall DB (1976) Premature parturition in
the California sea lion. J Wildl Dis, 12: 104-115.
Gilmour MI & Selgrade MJK (1993) A comparison of the pulmonary
defenses against streptococcal infection in rats and mice following
O3exposures: A possible disease resistance in rats. Toxicol Appl
Pharmacol, 1233: 211-218.
Gilmour MI, Park P, Doefler D, & Selgrade MJK (1993) Factors which
influence the suppression of pulmonary anti-bacterial defenses in mice
exposed to ozone. Exp Lung Res, 9: 229-314.
Glaser R, Kiecolt-Glaser JK, Speicher CE, & Holliday JE (1985) Stress,
loneliness, and changes in herpesvirus latency. J Behav Med,
8: 249-250.
Glazier A, Tutschka PJ, Farmer ER, & Santos GW (1983) Graft-versus-
host disease in cyclosporin A-treated rats after syngeneic and
autologous bone marrow reconstitution J Exp Med, 158: 1-8.
Gleichmann E, Vohr, H-W, Stringer C, Nuyens J, & Gleichmann H (1989)
Testing the sensitization of T cell to chemicals. From murine graft-
versus-host (GVH) reactions to chemical-induced GVH-like immunological
diseases. In: Kammüller ME, Bloksma N, & Seinen W ed. Autoimmunity and
toxicology: Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers, pp 364-390.
Glickman LT, Shofer FS, Payton AJ, Laster LL, & Felsburg PJ (1988)
Survey of serum IgA, IgG, and IgM concentrations in a large beagle
population in which IgA deficiency had been identified. Am J Vet Res,
49: 1240-1245.
Goettsch W, Garssen J, De Gruijl FR, & Van Loveren H (1993) UV-B and
the immune system: A review with special emphasis on T cell-mediated
immunity. Thymus, 21: 93-114.
Goings SAJ, Kulle TJ, Bascom R, Sauder LR, Green DJ, Hebel R, &
Clements ML (1989) Effects of nitrogen dioxide exposure on
susceptibility to influenza A virus infection in healthy adults.
Am Rev Respir Dis, 139: 1075-1081.
Goldstein BD (1977) Hematotoxicity in humans. J Toxicol Environ
Health, 2(Suppl): 69-106.
Goldstein A, Lowney LI, & Pal BK (1971) Stereospecific and nonspecific
interactions of the morphine congener levorphanol in subcellular
fractions of mouse brain. Proc Natl Acad Sci USA, 68: 1742-1752.
Golemboski KA, Bloom SE, & Dietert RR (1992) Assessment of neonatal
avian inflammatory macrophage function following embryonic
cyclophospha-mide exposure. Int J Immunopharmacol, 14: 19-26.
Gonçalo S, Gonçalo M, Azenha A, Barros MA, Bastos AS, Brandao FM,
Faria A, Marques MSJ, Pecegueiro M, Rodrigues JB, Salgueiro E, &
Torres V (1992) Allergic contact dermatitis in children. Contact Derm,
26: 112.
Good RA & Lorenz E (1992) Nutrition and cellular immunity. Int J
Immunopharmacol, 14: 361-366.
Goodman JW, Peter-Fizaine FE, Shinpock SG, Hall EA, & Famie DJ (1989)
Immunologic and hematologic consequences in mice of exposure to ozone.
JEPTO, 9: 243-252.
Goonewardene IM & Murasko DM (1992) Changes in the immune response.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat. Washington DC, ILSI Press, pp 3-7.
Gopinath C, Prentice DE, & Lewis DJ (1987) The lymphoid system.
In: Atlas of experimental toxicologic pathology. Boston, MTP Press,
pp 122-137.
Gorodilova VV & Mandrik EV (1978) The use of some immunological
reactions for studying the immune response in persons presenting a
high oncological risk. Sov Med, 8: 50-53.
Graham JA & Gardner DE (1985) Immunotoxicity of air pollutants.
In: Dean JH, Luster MI, & Munson AE ed. Immunotoxicology and
Immunopharmacology. New York, Raven Press, pp 367-380.
Gray, D (1993) Immunological memory. Ann Rev Immunol, 11: 47-77.
Greenlee WF, Dold KM, Irons RD, & Osborne R (1985) Evidence for direct
action of 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) on thymic
epithelium. Toxicol Appl Pharmacol, 79: 112--120.
Greenstein BD, Fitzpatrick FTA, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 112: 345-350.
Greiner DL, Shultz LD, Rossini AA, Mordes JP, Handler ES, & Rajan TV
(1991) Recapitulation of normal and abnormal BB rat immune system
development in SCID mouse/rat lymphohemopoietic chimeras. J Clin
Invest, 88: 717-719.
Guberski DL (1994) Diabetes-prone and diabetes-resistant BB rats:
Animal models of spontaneous and virally induced diabetes mellitus,
lymphocytic thyroiditis, and collagen-induced arthritis. ILAR News,
35: 29-37.
Gumbiner BM & Yamada KM ed. (1992) Cell-to-cell contact and
extracellular matrix. Curr Opin Cell Biol, 4: 757-874.
Gusev VA, Danilovskaya YV, Vatolkina OY, Lomonosova OS, & Velichkowsky
BT (1993) Effect of quartz and alumina dust on generation of
superoxide radicals and hydrogen peroxide by alveolar macrophages,
granulocytes, and monocytes. Br J Ind Med, 50: 732-735.
Gutierrez-Ramos JC, Andreu JL, Moreno de Alboran I, Rodriguez J,
Leonardo E, Kroemer G, Marcos MAR, & Martinez AC (1990) Insights into
autoimmunity: From classical models to current perspectives. Immunol
Rev, 118: 73-101.
Haas W, Pereira P, & Tonegawa S (1993) Gamma/delta cells. Ann Rev
Immunol, 11: 637-685.
Halliday GM, Cavanaugh LL, & Muller HK (1988) Antigen present in local
lymph node by cells from dimethylbenzanthracene-treated murine
epidermis activate suppressor cells. Cell Immunol, 109: 206-221.
Halprin KM, Comerford M, Presser SE, & Taylor JR (1981) Ultraviolet
light treatment delays contact sensitization to nitrogen mustard.
Br J Dermatol, 105: 71-76.
Hanglow AC, Ernst PB, & Bienenstock J (1990) Intraepithelial
leukocytes, murine. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed.
Hemopoietic system (ILSI Monograph). Berlin, Springer Verlag,
pp 315-322.
Hanley PJ, Hook JW, Raftos DA, Gooley AA, Trent R, & Raison RL (1992)
Hagfish humoral defense protein exhibits structural and functional
homology with mammalian complement components. Proc Natl Acad Sci USA,
89: 7910-7914.
Hansch GM (1992) The complement attack phase: Control of lysis and
non-lethal effects of C5b-9. Immunopharmacology, 24: 107-117.
Hara I (1985) Health status and PCBs in blood of workers exposed to
PCBs and of their children. Environ Health Perspectives, 59: 85-90.
Harriman W, Völk H, Defranoux N, & Wabl M (1993) Immunoglobulin class
switch recombination. Ann Rev Immunol, 11: 361-384.
Harrington W & Krupnick AJ (1985) Short-term nitrogen dioxide exposure
and acute respiratory disease in children. J Air Pollut Control Assoc,
35: 1061-1067.
Hartevelt MM, Bouwes Bavinck JN, Kootte AMM, Vermeer BJ, &
Vandenbroucke JP (1990) Incidence of skin cancer after renal
transplantation in the Netherlands. Transplantation, 49: 506-509.
Hartmann DP (1985) Immunological consequences of asbestos exposure.
Surv Immunol Res, 4: 65-68.
Hartmann DP, Georgian MM, Oghiso Y, & Kagan E (1984) Enhanced
interleukin activity following asbestos inhalation. Clin Exp Immunol,
55: 643-650.
Hasemann CA & Capra JD (1989) Immunoglobulins: Structure and function.
In: Paul WE ed. Fundamental immunology, 2nd Ed. New York, Raven Press,
pp 209-233.
Hashimoto K, Nakanishi T, & Kurosawa Y (1990) Isolation of carp genes
encoding major histocompatibility complex antigens. Proc Natl Acad Sci
USA, 87: 6863-6867.
Haynes RC & Murad F (1985) Adrenocortical steroids and their synthetic
analogs: Inhibitors of adrenocortical steroid biosynthesis. In: Gilman
AG, Goodman LS, Rall TW, & Murad F ed. Goodman and Gilman's
pharmacological basis of therapeutics. New York, Macmillan, p 1459.
Hedrick SM (1989) T Lymphocyte receptors. In: Paul WE ed. Fundamental
immunology, 2nd Ed. New York, Raven Press, pp 291-313.
Heijnen CJ & Kavelaars A (1991) The contribution of neuroendocrine
substances to the immune response. Neth J Med, 39: 281-294.
Heijnen CJ, Kavelaars A, & Ballieux RE (1991) ß-Endorphin: Cytokine
and neuropeptide. Immunol Rev, 119: 41-63.
Helle E, Olsson M, & Jensen S (1976) PCB levels correlated with
pathological changes in seal uteri. Ambio, 5: 261-263.
Hemphill FE, Kaeberle ML, & Buck WB (1971) Lead suppression of mouse
resistance to Salmonella typhimurium. Science, 172: 1031.
Henderson FW, Dubovy EJ, Harder S, Seal E, & Graham D (1988)
Experimental rhinovirus infection in human volunteers exposed to
ozone. Am Rev Respir Dis, 137: 124-127.
Henne T & Schmähl D (1985) Occurrence of second primary malignancies
in man: A second look. Cancer Treat Rev, 12: 77-94.
Henney CS, Kuriayashi K, Kern BE, & Gillis S (1981) Interleukin-2
augments natural killer cell activity. Nature, 291: 335-338.
Herberman RB & Ortaldo JR (1981) Natural killer cells: Their role in
defenses against disease. Science, 214: 24-30.
Herman A, Kappler JW, Marrack P, & Pullen AM (1991) Superantigens:
Mechanism of T-cell stimulation and role in immune responses. Ann Rev
Immunol, 9: 745-772.
Hersey P, Hasic E, Edwards A, Bradley M, Haran G, & McCarthy WH
(1983a) Immunological effects of solarium exposure. Lancet,
i: 545-548.
Hersey P, Haran G, Hasic E, & Edwards A (1983b) Alteration of T cell
subsets and induction of suppressor T cell activity in normal subjects
after exposure to sunlight. J Immunol, 31: 171-174.
Hess AD, Jones RJ, Morris LE, Noga SJ, Vogelsang GB, & Santos GW
(1992) Autologous graft-versus-host disease: A novel approach for
antitumor immunotherapy. Hum Immunol 34: 219-224.
Hinton DM (1992) Testing guidelines for evaluation of the immunotoxic
potential of direct food additives. Crit Rev Food Sci Nutr,
32: 173-190.
Ho M (1980) Role of specific cytotoxic lymphocytes in cellular
immunity against murine cytomegalovirus. Infect Immunol, 27: 767-776.
Ho MK & Springer TA (1984) Preparation and use of monoclonal
antimacrophage antibodies. Methods Enzymol, 108: 313-324.
Hoffman RE (1992) Recent studies of the human immunotoxic effects of
2,3,7,8-tetrachlorodibenzo- p-dioxin. In: Newcombe DS, Rose NR, &
Bloom JC ed. Clinical immunotoxicology. New York, Raven Press,
pp 339-347.
Hoffman RE, Stehr-Green PA, Webb KB, Evans G, Knutsen AP, Schramm WF,
Staake JL, Gibson BG, & Steinberg KK (1986) Health effects of long-
term exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin. J Am Med
Assoc, 255: 2031-2038.
Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR,
Heindell JJ, & Luster MI (1991) Perinatal thymocyte antigen expression
and postnatal immune development altered by gestational exposure to
tetrachlorodibenzo- p-dioxin (TCDD). Teratology, 44: 385-393.
Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, & Mo
JA (1990) Type II collagen autoimmunity in animals and provocations
leading to arthritis. Immunol Rev, 118: 193-232.
Holmes KL & Morse HC (1988) Murine hematopoetic cell surface antigen
expression. Immunol Today, 9: 344-350.
Holmes MA, Duffus WP, & Gorman NT (1989) Natural cytotoxicity in the
dog: Description of two new allogeneic tumour targets. Vet Immunol
Immunopathol, 23: 161-170.
Holsapple MP, Page DG, Shopp GM, & Bick PH (1984) Characterization of
the delayed hypersensitivity response to a protein antigen in the
mouse. Int J Immunopharmacol, 6: 399-405.
Holsapple MP, White KL, McCay JA, Bradley SG, & Munson AE (1988) An
immunotoxicological evaluation of 4,4'-thiobis-(6- t-butyl- m-cresol)
in female B6C3F1 mice. Fundam Appl Toxicol, 10: 701-716.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo- p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-251.
Holt PG & Keast D (1977) Environmentally induced changes in
immunological function: Acute and chronic effects of inhalation of
tobacco smoke and other atmospheric contaminants in man and
experimental animals. Bacteriol Rev, 41: 205-216.
Holt PG & Sedgwick JD (1987) Suppression of IgE responses following
inhalation of antigen. A natural homeostatic mechanism which limits
sensitization to aeroallergens. Immunol Today, 8: 14-15.
Hordvik I, Grimholt U, Fosse VM, Lie O, & Endresen C (1993) Cloning
and sequence analysis of cDNAs encoding the MHC class II ß chain in
Atlantic salmon (Salmo salar). Immunogenetics, 37: 437-441.
Hori S, Obana H, Kashimoto T, Otake T, Nishimura H, Ikegami N, Kunita
N, & Uda H (1982) Effect of polychlorinated biphenyls and
polychlorinated quaterphenyls in cynomolgus monkey (Macaca
fascicularis). Toxicology, 24: 123-139.
Hoshino A, Takenaka H, Mizukoshi O, Imanishi J, Kishida T, & Tovey M
(1983) Effect of anti-interferon serum of influenza virus infection in
mice. Antiviral Res, 3: 59-65.
Houben GF, Van Dokkum W, Van Loveren H, Penninks AH, Seinen W,
Spanhaak S, & Ockhuizen T (1992) Effects of caramel colour III on the
number of blood lymphocytes: A human study on caramel colour III
immunotoxicity and a comparison of the results with data from rat
studies. Food Chem Toxicol, 5: 427-430.
Houben GF, Penninks AH, Seinen W, Vos JG, & Van Loveren H (1993)
Immunotoxic effects of the color additive caramel color III: Immune
function studies in rats. Fundam Appl Toxicol, 20: 30-37.
House RV, Lauer LD, Murray MJ, & Dean JH (1987) Suppression of
T-helper cell function in mice following exposure to the carcinogen
7,12-dimethylbenz[ a]-anthracene and its restoration by
interleukin-2. Int J Immunopharmacol, 9: 89-97.
House RV, Pallardy MJ & Dean JH (1989) Suppression of murine
cytotoxic T-lymphocyte induction following exposure to
7,12-dimethylbenz[ a]anthracene: Dysfunction of antigen recognition.
Int J Immunopharmacol, 11: 207-215.
House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR,
& Dean JH (1990a) Examination of immune parameters and host
resistance mechanisms in B6C3F1 mice following adult exposure to
2,3,7,8-tetrachlorodibenzo- p-dioxin. J Toxicol Environ Health,
31: 203-215.
House RV, Pearson FC, & Thomas PT (1990b) Selective potentiation of
host resistance in mice following treatment with pyrexol. Mol Biother,
2: 175-178.
Howard SK, Werner PR, & Sleigh SD (1980) Polybrominated biphenyl
toxicosis in swine: Effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol,
55: 146-153.
Hoyle PC & Cooper EC (1990) Nonclinical toxicity studies of antiviral
drugs indicated for the treatment of non-life threatening diseases:
Evaluation of drug toxicity prior to phase I clinical studies. Regul
Toxicol Pharmacol, 11: 81-89.
Hu HZ, Queirò MR, Tilanus MGJ, De Weger RA, & Schuurman H-J (1993)
Expression of T-cell receptor aand b variable genes in normal and
malignant human T cells. Br J Haematol, 84: 39-48.
Hughes & Nei (1993) Evolutionary relationships of the classes of major
histocompatibility complex genes. Immunogenetics, 93: 337-346.
Huiskamp R, Davids JAG, & Vos O (1983) Short- and long-term effects of
whole-body irradiation with fission neutrons or X rays on the thymus
in CBA mice. Radiat Res, 95: 370-381.
Huiskamp R, Davids JAG, & Van Ewijk W (1988) The effect of graded
doses of fission neutrons or X rays on the stromal compartment of the
thymus in mice. Radiat Res, 113: 25-39.
Hünig T, Wallny HJ, Hastley JK, Lawetzky A, & Tiefenthaler G (1989) A
monoclonal antibody to a constant determinant of the rat T cell
antigen receptor that induces T cell activation. Differential
reactivity with subsets of immature and mature T lymphocytes. J Exp
Med, 169: 73-86.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42 (IARC monographs on the evaluation
of carcinogenic risks to humans, Suppl 7). Lyon.
IARC (1990) Ciclosporin. In: Pharmaceutical drugs (IARC monographs on
the avaluation of carcinogenic risks to humans, Vol. 50). Lyon,
pp 77-114.
Imanishi J, Nomura H, Matsubara M, Kita M, Won S-J, Mizutani T, &
Kishida T (1980) Effect of polychlorinated biphenyl on viral
infections in mice. Infect Immunol, 29: 275-277.
Inada T & Mims CA (1985) Association of virulence of murine
cytomegalovirus with macrophage susceptibility and with virion-bound
non-neutralizing antibody. J Gen Virol, 66: 879-882.
Institoris L, Siroki O, Toth S, & Desi I (1992) Immunotoxic effects of
MTP-IP containing 60% methylparathion in mice. Hum Exp Toxicol,
11: 11-16.
Institoris L, Siroki O, & Desi I (1995) Immunotoxicity study of
repeated small doses of dimethoate and methylparathion administered to
rats over three generations. Hum Exp Toxicol, 14: 879-883.
Institoris L, Siroki O, Fekete K, & Desi I (in press)
Immunotoxicological investigation of repeated small doses of
dichlorvos (DDPV) in three generations of rats. Int J Environ Health
Res.
IPCS (1978) Environmental health criteria 6: Principles and methods
for evaluating the toxicity of chemicals. Part I. Geneva, WHO.
IPCS (1986) Immunotoxicology. Development of predictive testing for
determining the immunotoxic potential of chemicals. Report of a
technical review meeting. London, November 3-5, 1986. Geneva, WHO.
IPCS (1992) Environmental health criteria 141: Quality management for
chemical safety testing. Geneva, WHO.
IPCS (1994a) Environmental health criteria 155: Biomarkers and risk
assessment: Concepts and principles. Geneva, WHO.
IPCS (1994b) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO.
IPCS/Department of Health (1995) Proceedings of International
Programme on Chemical Safety and UK Department of Health International
Workshop on Environmental Immunotoxicology and Human Health, Oxford,
22-25 March, 1994. Hum Exp Toxicol, 14: 77-154.
Irons RD (1985) Histology of the immune system: Structure and
function. In: Dean J, Luster MI, Munson AE, & Amos H ed.
Immunotoxicology and immunopharmacology. New York, Raven Press,
pp 11-22.
Ishiguro H, Kobayashi K Suzuki M, Titani K, Tomonaga S, & Kurosawa Y
(1992) Isolation of a hagfish gene that encodes a complement
component. EMBO J, 11: 829-837.
Jacob CO, Van Der Meide PH, & McDevitt HO (1987) In vivo treatment of
(NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma-
interferon. J Exp Med, 166: 798-803.
Jakab GJ & Hmieleski RR (1988) Reduction of influenza virus
pathogenesis by exposure to 0.5 ppm ozone. J Toxicol Environ Health,
23: 455-472.
Janeway CA (1992) The T-cell receptor as a multicomponent signalling
machine: CD4/CD8 coreceptors and CD45 in T-cell activation. Ann Rev
Immunol, 10: 645-674.
Jaremin B (1983) Blast lymphocyte transformation (LTT), rosette
(E-RFC) and leukocyte migration inhibition (MIF) tests in persons
exposed to the action of lead during work. Report II. Bull Inst Mar
Trop Med (Gdynia), 34: 187-197.
Jeevan A & Kripke ML (1990) Alteration of the immune response to
Mycobacterium bovis BCG in mice exposed chronically to low doses of
UV radiation. Cell Immunol, 130: 32-41.
Jeevan A, Gilliam K, Heard H, & Kripke ML (1992) Effects of
ultraviolet radiation on the pathogenesis of Mycobacterium
lepraemurium infection in mice. Exp Dermatol, 1: 152-160
Jefferies WA (1988) Hemopoietic and T-lymphocyte marker antigens of
the rat characterized with monoclonal antibodies. In: Miyasaka M &
Trnka Z ed. Differentiation antigens in lymphohemopoietic tissues. New
York, Marcel Dekker, pp 173-247.
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunological
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachloro- p-dioxin. Br J Ind Med, 45: 701-704.
Jerne NK, Henry C, Nordin AA, Fun H, Koros MC, & Lefkovits I (1974)
Plaque forming cells: Methodology and theory. Transplant Rev,
18: 130-191.
Jessup JM, Hanna N, Palaszynski E, & Kripke ML (1978) Mechanisms of
depressed reactivity to dinitrochlorobenzene and ultraviolet-induced
tumours during ultraviolet carcinogenesis in BALB/c mice. Cell
Immunol, 38: 105-115.
Johnson KW, Holsapple MP, & Munson AE (1986) An immunotoxicological
evaluation of g-chlordane. Fundam Appl Toxicol, 6: 317.
Johnson KW, Munson AE, Kim DH, & Holsapple MP (1987) Role of
reactive metabolites in suppression of humoral immunity by
N-nitrosodimethylamine. J Pharmacol Exp Ther, 240: 847-855.
Johnson JD, Houchens DP, De Kluwe WM, Craig DK, & Fisher GL (1990)
Effects of mainstream and environmental tobacco smoke on the immune
system of animals and humans: A review. CRC Crit Rev Toxicol,
20: 369-395.
Joling P, Tielen FJ, Vaessen LMB, Hesse CJ, & Rozing J (1985)
Intrathymic differentiation in the rat. Adv Exp Med Biol,
186: 235-244.
Joling P (1987) Allografting and the T cell system. A multiparameter
analysis of rejection in the rat. PhD Thesis, Rotterdam, Erasmus
University.
Jones TC, Ward JM, Mohr U, & Hunt RD (1990) Hemopoietic system (ILSI
monograph). Berlin, Springer Verlag.
Kafafi SA, Afeefy HY, Ali AH, Said HK, & Kafafi AG (1993) Binding of
polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ
Health Perspectives, 101: 422-425.
Kagan E, Solomon A, Cochrane JC, Beissner EI, Gluckman J, Rocks PH, &
Webster I (1977a) Immunological studies of patients with asbestosis.
I. Studies of cell-mediated immunity. Clin Exp Immunol, 28: 261-267.
Kagan E, Solomon A, Cochrane JC, Kuba P, Rocks PH, & Webster I (1977b)
Immunological studies of patients with asbestosis. II. Studies of
circulating lymphoid numbers and humoral immunity. Clin Exp Immunol,
28: 268-275.
Kagan E, Jacobson RJ, Yeung KY, Haidak DJ, & Nachnani GH (1979)
Asbestos-associated neoplasms of B cell lineage. Am J Med,
67: 325-330.
Kahan BD (1989) Cyclosporine. New Engl J Med, 321: 1725-1738.
Kalimo K, Koulu L, & Jansen CT (1983) UV exposure and
dinitrochlorobenzene (DNCB). Arch Dermatol Res, 275: 374-378.
Kalpazanov Y, Stamenova M, & Kurchatova G (1976) Air pollution and the
1974 - 1975 influenza epidemic in Sofia. Environ Res, 12: 1-8.
Kamel OW, Van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA,
Montgomery PG, Warnke RA, & Dorfman RF (1993) Reversible lymphoma
associated with Epstein-Barr virus occurring during methotrexate
therapy for rheumatoid arthritis and dermatomyositis. New Engl J Med,
328: 1317-1321.
Kammüller ME & Bloksma N (1994) Drug-induced autoimmunity. In: Dean J,
Luster MI, Munson AE, & Kimber I ed. Immunotoxicology and
immunopharmacology. New York, Raven Press, pp 573-588.
Kammüller ME, Bloksma N, & Seinen W (1989) Autoimmunity and
toxicology. Immune dysregulation induced by drugs and chemicals.
Amsterdam, Elsevier Science Publishers.
Kampinga J, Berges S, Boyd RL, Brekelmans P, Colic M, Van Ewijk W,
Kendall M, Ladyman H, Nieuwenhuis P, Ritter MA, Schuurman HJ, &
Tournefier A (1989) Thymic epithelial antibodies: Immunohistological
analysis and introduction of nomenclature. Thymus, 13: 165-173.
Kannan K, Singh KP, Goel SK, & Shanker R (1985) Effect of
2,5-hexanediol on immunocompetence of mice. Environ Res, 36: 14.
Kannan K, Tanabe S, Borrell A, Aguilar A, Focardi S, & Tatsukawa R
(1993) Isomer-specific analysis and toxic evaluation of
polychlorinated biphenyls in striped dolphins affected by an epizootic
in the western Mediterranean Sea. Arch Environ Contam Toxicol,
25: 227-233.
Kasahara M, Vazquez M, Sato K, McKinney EC, & Flajnik MF (1992)
Evolution of the major histocompatibility complex: Isolation of class
II A cDNA clones from the cartilagenous fish. Proc Natl Acad Sci USA,
89: 6688-6692.
Kasai M, Iwamari M, Nagai T, Okumura K, & Tada T (1980) A glycolipid
on the surface of mouse natural killer cells. Eur J Immunol,
10: 175-180.
Kasai M, Yoneda T, Habu S, Maruyana Y, Okumura K, & Tokunaga T (1981)
In vivo effect of anti-asialo GM-1 antibody on natural killer cell
activity. Nature, 291: 334-335.
Kasl SV, Evans AS, & Neiderman JC (1979) Psychosocial risk factors
in the development of infectious mononucleosis. Psychosom Med,
41: 445-466.
Kateley JR & Bazzell SJ (1978) Immunological studies in cattle exposed
to polybrominated biphenyls. Environ Health Perspectives, 23: 75-82.
Kawabata TT & White KL (1987) Suppression of the in vitro humoral
immune response of mouse splenocytes by benzo( a)pyrene metabolites
and inhibition of benzo( a)pyrene-induced immunosuppression by
a-naphthoflavone. Cancer Res, 47: 2317-2322.
Kawabata TT & White KL (1990) Effects of naphthalene and naphthalene
metabolites on the in vitro humoral immune response. J Toxicol
Environ Health, 30: 53-67.
Keck G (1981) Effets de la contamination par les polychlorobiphenyles
(PCB) sur le developpement de la tumeur d'Ehrlich chez la souris
Swiss. Toxicol Eur Res, 3: 229-236.
Kendall MD (1980) The occurrence of erythropoiesis in the thymus of
the bank vole (Clethrionomys glareolus). Cell Tissue Res,
212: 307-314.
Kendall MD (1991) Functional anatomy of the thymic microenvironment.
J Anat, 177: 1-29.
Kendall MD & Al-Shawaf AA (1991) Innervation of the thymus gland.
Brain Behav Immunol, 5: 9-28.
Kendall MD & Frazier JA (1979) Ultrastructural studies on
erythropoiesis in the avian thymus. I. Description of cell types. Cell
Tissue Res, 199: 37-61.
Kendall MD & Singh J (1980) The presence of erythroid cells in the
thymus gland of man. J Anat, 130: 183-189.
Kendall MD & Ward P (1974) Erythropoiesis in an avian thymus. Nature,
249: 3 66-367.
Kendall MD, Fitzpatrick FTA, Greenstein BD, Khoylou F, Safieh B, &
Hamblin A (1990) Reversal of ageing changes in the thymus of rats by
chemical or surgical castration. Cell Tissue Res, 261: 555-564.
Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V,
Huelskamp AM, & Davidson NE (1994) Phase I trial of interferon gamma
to potentiate cyclosporine-induced graft-versus-host disease in women
undergoing autologous bone marrow transplantation for breast cancer.
J Clin Oncol, 12: 249-257.
Kerkvliet NI & Baecher-Steppan L (1988) Suppression of allograft
immunity by 3,4,5,3 ',4 ',5 '-hexachlorobiphenyl. I. Effects of
exposure on tumor rejection and cytotoxic T cell activity.
Immunopharmacology, 16: 1-12.
Kerkvliet NI & Brauner JA (1987) Mechanisms of 1,2,3,4,6,7,8-
heptachlorodi-benzo- p-dioxin (HpCDD)-induced humoral suppression:
Evidence of primary defect in T-cell regulation. Toxicol Appl
Pharmacol, 87: 18-31.
Kerkvliet NI & Burleson GR (1994) Immunotoxicity of TCDD and related
halogenated aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE,
& Kimber I ed. Immunotoxicity and immunopharmacology, 2nd Ed. New
York, Raven Press, pp 97-121.
Kerkvliet NI & Kimeldorf DJ (1977) Antitumor activity of a
polychlorinated biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J Natl Cancer Inst, 59: 951-955.
Kerkvliet NI, Steppan LB, Smith BB, Youngberg JA, Henderson MC, &
Buhler DR (1990) Role of the Ah locus in suppression of cytotoxic T
lymphocyte (CTL) activity by halogenated aromatic hydrocarbons (PCBs
and TCDD): Structure-activity relationships and effects in C57Bl/6
mice. Fundam Appl Toxicol, 14: 532-541.
Khan MF & Gupta GS (1991) Cellular and biochemical indices of
bronchoalveolar lavage for detection of lung injury following insult
by airborne toxicants. Toxicol Lett, 58: 239-255
Khangarot BS & Tripathi DM (1991) Changes in humoral and cell-mediated
immune responses and in skin and respiratory surfaces of catfish,
Saccobranchus fossilis, following copper exposure. Ecotox Environ
Saf, 22: 291-308.
Khansari DN, Murgo AJ, & Faith RE (1990) Effects of stress on the
immune system. Immunol Today, 11: 170-175.
Kiecolt-Glaser JK & Glaser R (1986) Psychological influences on
immunity. Psychonomatics, 27: 621-624.
Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday
JE, & Speicher CE (1986) Modulation of cellular immunity in medical
students. J Behav Med, 9: 5-21.
Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, &
Glaser R (1987) Marital quality, marital disruption, and immune
function. Psychosom Med, 49: 13-33.
Kim JH (1989) Infection and cyclosporin. Rev Infect Dis, 11: 677-690.
Kim DH, Yang KH, Johnson KW, & Holsapple MP (1987) Suppression of
in vitro antibody production by dimethylnitrosamine in mixed
cultures of mouse primary hepatocytes and mouse splenocytes. Toxicol
Appl Pharmacol, 87: 32-42.
Kimber I & Cumberbatch M (1992) Dendritic cells and cutaneous immune
responses to chemical allergens. Toxicol Appl Pharmacol, 117: 137-146.
Kimber I, Stonard MD, Gidlow DA, & Niewola Z (1986) Influence of
chronic low-level exposure to lead on plasma immunoglobulin
concentration and cellular immune function in man. Int Arch Occup
Environ Health, 57: 117-125.
Kimbrough RD (1987) Human health effects of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls (PBBs). Ann Rev Pharmacol Toxicol,
27: 87-11.
Kimchi A (1992) Cytokine triggered molecular pathways that control
cell cycle arrest. J Cell Biochem, 50: 1-9.
Kimmel CA (1990) Quantitative approaches to human risk assessment for
noncancer health effects. Neurotoxicology, 11: 189-198.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Kincade PW (1992) Cell interaction molecules and cytokines which
participate in B lymphopoiesis. Baillieres Clin Haematol, 5: 575-598.
King AG, Landbreth KS, & Wierda D (1987) Hydroquinone inhibits bone
marrow pre-B cell maturation in vitro. Mol Pharmacol, 32: 807-812.
Kingsmore SF, Hall BD, Alen NB, Rice JR, & Caldwell DS (1992)
Association of methotrexate, rheumatoid arthritis and lymphoma: Report
of 2 cases and literature review. J Rheumatol, 19: 1462-1465.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Klareskog L & Olsson T (1990) Autoimmunity to collagen II and myelin
basic protein: Comparative studies in humans and rodents. Immunol Rev,
118: 285-310.
Klein J (1986) Natural history of the major histocompatibility
complex. New York, John Wiley and Sons.
Klein J (1990) Immunology. Oxford, Blackwell Scientific Publications.
Kleinbeck ML, Hites MJ, Loker JL, Halliwell RE,& Lee KW (1989) Enzyme-
linked immunosorbent assay for measurement of allergen-specific IgE
antibodies in canine serum. Am J Vet Res, 50: 1831-1839.
Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, & Von dem
Borne AEGK ed. (1989) Leucocyte typing IV: White cell differentiation
antigens. Oxford, Oxford University Press.
Knight R, Sarlis N, & Stephanou A (1992) Interleukins and
neurohormones: A common language. Postgrad Med J, 68: 603-605.
Kocks C & Rajewski K (1989) Stable expression and somatic
hypermutation of antibody V regions in B cell developmental pathways.
Ann Rev Immunol, 7: 537-559.
Koller LD (1977) Enhanced polychlorinated biphenyl lesions in Moloney
leukemia virus-infected mice. Clin Toxicol, 11: 107-116.
Koller LD (1980) Immunotoxicology of heavy metals. Int J
Immunopharmacol, 2: 269-279.
Koller LD (1990) The immunotoxic effects of lead in lead-exposed
laboratory animals. Ann NY Acad Sci, 587: 160-167.
Koller LD & Kovacic S (1974) Decreased antibody formation in mice
exposed to lead. Nature, 250: 148-150.
Koller LD & Roan JG (1977) Effects of lead and cadmium on mouse
peritoneal macrophages. J Reticuloendothel Soc, 21: 7.
Koller LD & Roan JG (1980) Effects of lead, cadmium and methylmercury
on immunological memory. J Reticuloendothel Soc, 21: 7-12.
Koller LD & Thigpen JE (1973) Biphenyl-exposed rabbits. Am J Vet Res,
34: 1605-1606.
Kongshavn PAL, Sadarangani C, & Skamene E (1980) Cellular mechanisms
of genetically-determined resistance to Listeria monocytogenes.
In: Skamene E ed. Genetic control of natural resistance. New York,
Academic Press, pp 149-163.
Koren HS & Blomberg PA (in press) Respiratory responses of asthmatics
to ozone. Int Arch Allergy.
Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo
WJ, Becker S, House DE, McDonnel WF, & Bromberg PA (1989) Ozone-
induced inflammation in the lower airways of human subjects. Am Rev
Respir Dis, 139: 407-415.
Koren HS, Devlin RB, & Becker S (1994) Ozone-induced inflammatory
response in pulmonary cells. In: Schook LB & Laskin DL ed. Xenobiotics
and inflammation: Roles of cytokines and growth factors. New York,
Academic Press, pp 249-281.
Krajnc EI, Wester PW, Loeber JG, Van Leeuwen FXR, Vos JG, Vaessen
HAMG, & Van der Heijden CA (1984) Toxicity of bis(tri-n-butyltin)oxide
in the rat. I. Short-term effects on general parameters and on the
endocrine and lymphoid systems. Toxicol Appl Pharmacol, 75: 363-386.
Krajnc-Franken MAM, Van Loveren H, Schuurman HJ, & Vos JG (1990) The
immune system as a target for toxicity. A tiered approach of testing,
with special emphasis on histopathology. In: Dayan AD, Hertel RF,
Heseltine E, Kazantis G, Smith EM, & Van der Venne MT ed.
Immunotoxicity of metals and immunotoxicology. New York, Plenum Press,
pp 241-264.
Kramer CM, Johnson KW, Dooley RK, & Holsapple MP (1987) 2,3,7,8-
Tetrachlorodibenzo- p-dioxin (TCDD) enhances antibody production and
protein kinase activity in murine B cells. Biochem Biophys Res Commun,
145: 25-33.
Kripke ML (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J Natl Cancer Inst, 53: 1333-1336.
Kripke ML, Thorn RM, Lill PH, Civin CI, Pazmino NH, & Fisher MS (1979)
Further characterization of immunological unresponsiveness induced in
mice by ultraviolet radiation. Transplantation, 28: 212-217.
Kripke ML, Cox PA, Alas LG, & Yarosh DB (1992) Pyrimidine dimers in
DNA initiate systemic immunosuppression in UV-irradiated mice. Proc
Natl Acad Sci USA, 89: 7516-7520.
Krishna VR, Fouant MM, & Bradley SG (1989) Effects of pyran copolymer
on host resistance of mice to Plasmodium yoelii. J Parasitol,
75: 405-410.
Kroese FGM, Timens W, & Nieuwenhuis P (1990) Germinal center reaction
and B lymphocytes: Morphology and function. In: Grundmann E & Vollmer
E ed. Reaction patterns of the lymph node. Part 1. Cell types and
functions. Berlin, Springer Verlag, pp 103-148.
Kroplien U, Rosdorfer J, van der Greef J, Long RC, & Goldstein JH
(1985) 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)imidazole: Detection
in commercial caramel color III and preparation by a model browning
reaction. J Org Chem, 50: 1131-1133.
Krowka JF, Sarin S, Namikawa R, McCune JM, & Kaneshima H (1991) Human
T cells in the SCID-hu mouse are phenotypically normal and
functionally competent. J Immunol, 146: 3751-3756.
Kubota M, Kagamimori S, Yokoyama K, & Okada A (1985) Reduced killer
cell activity of lymphocytes from patients with asbestosis. Br J Ind
Med, 42: 276-280.
Kunita N, Hori S, Obana H, Otake T, Nishimura H, Kashimoto T, &
Ikegami N (1985) Biological effect of PCBS, PCQs and PCDFs present in
the oil causing yusho and yu-cheng. Environ Health Perspectives,
59: 79-84.
Kunkel SL, Chensue SW, Strieter RM, Lynch JP, & Remick DG (1989)
Cellular and molecular aspects of granulomatous inflammation. Am J
Respir Cell Mol Biol, 1: 439-447.
Kuper CF, Beems RB, & Hollanders VMH (1990a) Development and aging;
thymus, rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic
system (ILSI Monograph). Berlin, Springer Verlag, pp 257-263.
Kuper CF, Hameleers DMH, Bruijntjes JP, Van der Ven I, Biewenga J, &
Sminia T (1990b) Lymphoid and non-lymphoid cells in nasal-associated
lymphoid tissue (NALT) in the rat. An immuno- and enzymehistochemical
study. Cell Tissue Res, 259: 371-377.
Kuper CF, Beems RB, Bruijntjes JP, Schuurman H-J, & Vos JG (1992a)
Normal development, growth and aging of the thymus. In: Mohr U,
Dungworth DL, & Capen CC ed. Pathobiology of the aging rat, Vol. 1.
Washington DC, ILSI Press, pp 25-49.
Kuper CF, Koornstra PJ, Hameleers DMH, Biewenga J, Spit BJ,
Duijvestijn AM, Van Breda Vriesman PJC, & Sminia T (1992b) The role of
nasopharyngeal lymphoid tissue. Immunol Today, 13: 219-224.
Kuper CF, Schuurman H-J, & Vos JG (1995) Pathology in immunotoxicology.
In: Burleson G, Munson A, & Dean J ed. Modern methods in immuno-
toxicology. New York, John Wiley & Sons, pp 397-436.
Kyle RA (1984) Second malignancies associated with chemotherapy. In:
Perry MC & Yarbro JW ed. Toxicity of chemotherapy. New York, Grune &
Stratton, pp 479-506.
Labarca C & Paigen KA (1980) A simple, rapid, and sensitive DNA assay
procedure. Anal Biochem, 102: 344-352.
Ladics GS, Kawabata TT, & White KL (1991) Suppression of the
in vitro humoral immune response of mouse splenocytes by
7,12-dimethylbenz[ a]anthracene metabolites and inhibition of
immunosuppression by a-naphthflavone. Toxicol Appl Pharmacol,
110: 31-44.
Ladics GS, Kawabata TT, Munson AE, & White KL (1992) Metabolism of
benzo(a)pyrene by murine splenic cell types. Toxicol Appl Pharmacol,
116: 248-257.
Ladics GS, Smith C, Heaps K, Elliott GS, Slone TW, & Loveless SE
(1995) Possible incorporation of an immunotoxicological functional
assay for assessing humoral immunity for hazard identification
purposes in rats on standard toxicology study. Toxicology,
96: 225-238.
LaGrange PH, Mackaness GB, & Miller TE (1974) Influence of dose and
route of antigen injection on the immunological induction of T cells.
J Exp Med, 139: 528-542.
Laman JD, Van den Eertwegh AJM, Claassen E, & Van Rooijen N (1992)
Cell-cell interactions: In situ studies of splenic humoral immune
responses. In: Fornusek L & Vetvicka V ed. Immune system accessory
cells. Boca Raton, Florida, CRC Press, pp 201-231.
Lambris JD (1993) The chemistry, biology and phylogeny of C3.
In: Cruse JM & Lewis RE ed. Complement today, Vol. 1. Basel, Karger,
pp 16-45.
Lampeter EF, Signore A, Gale EAM, & Pozzilli P (1989) Lesson from the
NOD mouse for the pathogenesis and immunotherapy of human type 1
(insulin-dependent) diabetes mellitus. Diabetologia, 32: 703-708.
Lang D, Meier KL, & Luster MI (1993) Comparative effects of
immunotoxic chemicals on in vitro proliferative responses of human
and rodent lymphocytes. Fundam Appl Toxicol, 21: 535-545.
Lanier LL, Benike CJ, Phillips JH, & Engleman EG (1985) Recombinant
interleukin-2 enhanced natural killer cell-mediated cytotoxicity in
human lymphocyte subpopulations expressing the leu-7 and leu-11
antigens. J Immunol, 2: 794-801.
Larrick JW & Wright SC (1992) Native cytokine antagonists. Baillieres
Clin Haematol, 5: 681-702.
Lasky LA (1992) Selectins: Interpreters of cell-specific carbohydrate
information during inflammation. Science, 258: 964-969.
Lawlor GJ Jr & Fischer TJ ed. (1988) Manual of allergy and immunology.
Diagnosis and therapy, 2nd Ed. Boston, Little, Brown & Co.
Lawrence DH (1981) In vivo and in vitro effects of lead on humoral
and cell-mediated immunity. Infect Immun, 31: 136-143.
Lawrence DA (1985) Immunotoxicity of heavy metals. In: Dean JH, Luster
MI, Munson AE, & Amos H ed. Immunotoxicology and immunopharmacology.
New York, Raven Press, p 341.
Lawrence D, Mudzinski S, Rudofsky U, & Warner A (1987) Mechanism of
metal-induced immunotoxicity. In: Berlin A, Dean J, Draper MH, Smith
EMB, & Spreafico F ed. Immunotoxicology. Dordrecht, Martinus Nijhoff,
pp 293-307.
Lea T, Stelen K, & Stormer FC (1989) Mechanism of ochratoxin A-induced
immunosuppression. Mycopatholgia, 107: 153-159.
Lebish IJ, Hurvitz A, Lewis RM, Cramer DV, & Krakowka S (1986)
Immunopathology of laboratory animals. Toxicol Pathol, 14: 129-134.
Leiter EH (1993) The NOD mouse: A model for analyzing the interplay
between heredity and environment in development of autoimmune disease.
ILAR News, 35: 4-14.
Lerman SP & Weidanz WP (1970) The effect of cyclophosphamide on the
ontogeny of the humoral immune response in chickens. J Immunol,
105: 614-619.
LeVier DG, Brown RD, McCay JA, Fuchs BA, Harris LS, & Munson AE (1993)
Hepatic and splenic phagocytosis in female B6C3F1 mice implanted with
morphine sulfate pellets. J Pharmacol Exp Ther, 267: 357-363.
Levy SM, Herberman RB, Lee J, Whiteside T, Beadle M, Heiden L ,&
Simons A (1991) Persistently low natural killer cell activity, age,
and environmental stress as predictors of infectious morbidity. Nat
Immun Cell Growth Regul, 10: 289-307.
Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, & Bekesi JG (1986)
High frequency of immune dysfunctions in asbestos workers and in
patients with malignant mesothelioma. J Clin Immunol, 6: 225-233.
Li FY & Richters A (1991) Effects of 0.7 ppm ozone exposure on
thymocytes: In vivo and in vitro studies. Inhal Toxicol, 3: 61-71.
Lichtman MA (1981) The ultrastructure of the hemopoietic environment
of the marrow: A review. Exp Hematol, 9: 391-410.
Like AA, Butler L, Williams M, Appel MC, Weringer EJ, & Rossini AA
(1982) Spontaneous autoimmune diabetes mellitus in the BB rat.
Diabetes, 31(Suppl 1): 7-13.
Litman GW, Rast JP, Shamblott MJ, Haire RN, Hulst M, Roess W, Litman
RT, Hind-Frey KR, Zilch A, & Amemiya CT (1993) Phylogenetic
diversification of immunoglobulin genes and the antibody repertoire.
Mol Biol Evol, 10: 60-72.
Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, & MacLennan ICM
(1989) Mechanism of antigen-driven selection in germinal centres.
Nature, 342: 929-931.
Liu Y-J, Johnson GD, Gordon J, & MacLennan ICM (1992) Germinal centres
in T-cell-dependent antibody responses. Immunol Today, 13: 17-21.
Loo JCK, Tryphonas H, Jordan N, Brien R, Karpinski KF, & Arnold DL
(1989) Effects of Aroclor 1254 on hydrocortisone levels in adult
rhesus monkeys (Macaca mulatta). Bull Environ Contam Toxicol,
43: 667-669.
Loose LD, Pittman KA, Benitz K-F, & Silkworth JB (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J Reticuloendothel Soc, 22: 253-271.
Loose LD, Silkworth JB, Pittman KA, Benitz K-F, & Mueller W (1978)
Impaired host resistance to endotoxin and malaria in polychlorinated
biphenyl- and hexachlorobenzene-treated mice. Infect Immun, 20: 30-35.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspectives, 39: 79-91.
Losco P (1992) Normal development, growth and aging of the spleen.
In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology of the aging
rat, Vol. 1. Washington DC, ILSI Press, pp 75-95.
Losco P & Harleman H (1992) Normal development, growth, and aging of
the lymph node. In: Mohr U, Dungworth DL, & Capen CC ed. Pathobiology
of the aging rat, Vol. 1. Washington DC, ILSI Press, pp 49-75.
Love RJ, Ogilvie BM, & McLaren DJ (1976) The immune mechanism which
expels the intestinal stage of Trichinella spiralis from rats.
Immunology, 30: 7-13.
Lu YC & Wu YC (1985) Clinical findings and immunological abnormalities
in yu-cheng patients. Environ Health Perspectives, 59: 17-29.
Lubet RA, Lemaire BN, Avery D, & Kouri RE (1986) Induction of
immunotoxicity in mice by polyhalogenated biphenyls. Arch Toxicol,
59: 71-77.
Luckas B, Vetter W, Fischer P, Heidemann G, & Plötz J (1990)
Characteristic chlorinated hydrocarbon patterns in the blubber of
seals from different marine regions. Chemosphere, 21: 13-19.
Luebke RW, Luster MI, Dean JH, & Hayes HT (1984) Altered host
resistance to Trichinella spiralis infection following subchronic
exposure to diethylstilbestrol. Int J Immunopharmacol, 6: 609-617.
Luebke RW, Andrews DL, Copeland CB, Riddle MM, Rogers RR, & Smialowicz
RJ (1991) Host resistance to murine malaria in mice exposed to the
adenosine deaminase inhibitor, 2'-deoxycoformycin. Int J
Immunopharmacol, 13: 987-997.
Luebke RW, Copeland CB, Diliberto JJ, Akubue PI, Andrews DL,
Riddle MM, Williams WC, & Birnbaum LS (1994) Assessment of host
resistance to Trichinella spiralis in mice following preinfection
exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol, 125: 7-16.
Luebke RW, Copeland CB, & Andrews DL (1995) Host resistance
to Trichinella spiralis infection in rats exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Fundam Appl Toxicol,
24: 285-289.
Lukic ML, Popeskovic L, & Jankovic BD (1973) Potentiation of immune
responsiveness in rats treated with DDT. Fed Proc, 32: 1037.
Lundberg K, Dencker L, & Gronvik KO (1992) 2,3,7,8-Tetrachlorodibenzo-
p-dioxin (TCDD) inhibits the activation of antigen-specific T cells
in mice. Int J Immunopharmacol, 14: 699-705.
Lunn JE Knowelden J, & Handyside AJ (1967) Patterns of respiratory
illness in Sheffield infant schoolchildren. Br J Prev Soc Med,
21: 7-16.
Luo YD, Patel MK, Wiederholt MD, & Ou DW (1992) Effects of
cannabinoids and cocaine on the mitogen-induced transformations of
lymphocytes of human and mouse origins. Int J Immunopharmacol,
14: 49-56.
Luster MI, Faith RE, & Moore JA (1978) Depression of humoral immunity
in rats following chronic developmental lead exposure. J Environ
Pathol Toxicol, 1: 397-402.
Luster MI, Hong LH, Boorman GH, Clark G, Hayes HT, Greenlee WF, Dold
K, & Tucker AN (1985a) Acute myelotoxic responses in mice exposed to
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD). Toxicol Appl Pharmacol,
81: 151-165.
Luster MI, Tucker AN, Hayes HT, Pung OJ, Burka T, McMillan R, & Eling
T (1985b) Immunosuppressive effects of benzidine in mice: Evidence of
alterations in arachidonic acid metabolism. J Immunol, 135: 2754-2761.
Luster MI, Tucker AN, Germolec DR, Silver MT, Thomas PT, Vore SJ, &
Bucher JR (1986) Immunotoxicity studies in mice exposed to methyl
isocyanate. Toxicol Appl Pharmacol, 86: 140-144.
Luster MI, Germolec DR, Burleson GR, Jameson CW, Ackerman MF, Lamm KR,
& Hayes HT (1987) Selective immunosuppression in mice of natural
killer cell activity by ochratoxin A. Cancer Res, 47: 2259-2263.
Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL,
Lauer LD, Germolec DR, Rosenthal GJ, & Dean JH (1988) Development of a
testing battery to assess chemical-induced immunotoxicity: National
Toxicology Program's guidelines for immunotoxicity evaluation in mice.
Fundam Appl Toxicol, 10: 2-19.
Luster MI, Ackermann MF, Germolec DR, & Rosenthal GJ (1989)
Perturbations of the immune system by xenobiotics. Environ Health
Perspectives, 81: 157-162.
Luster MI, Portier C, Pait DG, White KL, Gennings C, Munson AE, &
Rosenthal GJ (1992) Risk assessment in immunotoxicology I. Sensitivity
and predictability of immune tests. Fundam Appl Toxicol., 18: 200-210.
Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E,
Blaylock BL, Pollock P, Kouchi Y, Craig W, White KL, Munson AE, &
Comment CE (1993) Risk assessment in immunotoxicology. II.
Relationship between immune and host resistance tests. Fundam Appl
Toxicol, 21: 71-82.
Lyte M & Bick PH (1986) Modulation of interleukin-1 production by
macrophages following benzo( a)pyrene exposure. Int J
Immunopharmacol, 8: 377-381.
Mackaness GB (1969) The influence of immunologically committed
lymphoid cells on macrophage activity in vivo. J Exp Med,
129: 973-992.
Makker SP & Aikawa M (1979) Mesangial glomerulonephropathy with
deposition of IgG, IgM, and C3 induced by mercuric chloride. Lab
Invest, 41: 45-49.
Malins DC, McClain BB, Landahl JT, Meyers MS, Krahn MM, Brow DW, Chan
SL, & Roubal WT (1988) Neoplasia and other diseases in fish in
relation to toxic chemicals: An overview. Aquat Toxicol, 11: 43-67.
Malkovsky M, Loveland B, North M, Asherson GL, Gao L, Word P, & Fiers
W (1987) Recombinant Interleukin-2 directly augments the cytotoxicity
of human monocytes. Nature, 325: 262-265.
Mandell LA (1990) Infections in the immunocompromised host. J Intern
Med Res, 18: 177-190.
Manischewitz JE & Quinnan GV (1980) Antivirus antibody-dependent cell-
mediated immunity during murine cytomegalovirus infection. Infect
Immun, 29: 1050-1054.
Mann DD, Buening GM, Hook B, & Osweiler GD (1983) Effects of T-2 toxin
on bovine serum proteins. Am J Vet Res, 44: 1751-1759.
Manson-Smith DF, Bruce RG, & Parrott DMV (1979) Villous atrophy and
expul-sion of intestinal Trichinella spiralis are mediated by T
cells. Cell Immunol, 47: 285-292.
Marchalonis JJ, Hohman VS, Thomas C, & Schluter SF (1993) Antibody
production in sharks and humans: A role for natural antibodies. Dev
Comp Immunol, 17: 41-53.
Marker SC, Howard RJ, Simmons RL, Kalis JM, Conelly DP, Najarian JS, &
Balfour HH (1981) Cytomegalovirus infection: A quantitative
prospective study of 320 consecutive renal transplants. Surgery,
89: 660-671.
Marsh JA, Dietert RR, & Combs GF (1981) Influence of dietary selenium
and vitamin E on the humoral immune response of the chick. Proc Soc
Exp Biol Med, 166: 228-236.
Martineau D, Béland P, Desjardins C, & Lagacé A (1987) Levels of
organochlorine chemicals in tissues of beluga whales (Delphinapterus
leucas) from the St Lawrence Estuary, Québec, Canada. Arch Environ
Contam Toxicol, 16: 137-147.
Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird FF, Travis W,
Suffredini AF, Deyton l, Kovacs JA, Falloon J, Davey R, Polis M,
Metcalf J, Baseler M, Wesley R, Gill VJ, Fauci AS, & Lane HC (1989)
CD4 counts as predictors of opportunistic pneumonias in human
immunodeficiency virus (HIV) infection. Ann Intern Med, 111: 223-231.
Mathews SE, Warinner JE, & Weeks BA (1990) Assays of immune function
in fish macrophages. In: Stolen JS, Fletcher TC, Anderson DP, Roberson
BS, & van Muiswinkel WB ed. Techniques in fish immunology, Vol. 1.
Fair Haven, New Jersey, SOS Publications, pp 155-163.
Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, &
Katagiri T (1990) A new allele of the lpr locus, lprcg, that
complements the gld gene in induction of lymphadenopathy in the mouse.
J Exp Med, 171: 519-531.
Mayani H, Guilbert LJ, & Janowska-Wieczorek A (1992) Biology of the
hemopoietic microenvironment. Eur J Haematol, 49: 225-233.
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, &
Rattner BA (1992) Physiological and nonspecific biomarkers. In:
Biomarkers, biochemical, physiological and histological markers of
anthropogenic stress. London, Lewis Publishers, pp 5-85.
McCabe MJ (1994) Mechanisms and consequences of immunomodulation by
lead. In: Dean JH, Luster MI, Munson AE, & Kimber I ed. Immunotoxicity
and immunopharmacology, 2nd Ed. New York, Raven Press, pp 143-162.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo- p-dioxin kills immature thymocytes by
Ca2+-mediated endonuclease activation. Science, 242: 256-259.
McConkey DJ, Orrenius S, & Jondal M (1990) Cellular signalling in
programmed cell death (apoptosis). Immunol Today, 11: 120-121.
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, & Weissman
IL (1988) The SCID-hu mouse: Murine model for the analysis of human
hematolymphoid differentiation and function. Science, 241: 1632-1639.
McDonald HR & Lees RK (1990) Programmed death of autoreactive
thymocytes. Nature, 343: 642-644.
McGregor DD, Hahn HH, & Mackaness GB (1973) The mediator of cellular
immunity. V. Development of cellular resistance to infection in
thymectomized irradiated rats. Cell Immunol, 6: 186-199.
Melia RJW, Du V, Florey C, Altman DG, & Swan AV (1977) Association
between gas cooking and respiratory disease in children. Br Med J,
ii: 149-152.
Mellert J, Hopt UT, Erath F, & Holzer H (1989) Differential effects of
azathioprine (aza), cyclosporin A (CSA) and dexamethasone (DEXA) on
lymphokine mediated inflammation (LMI) in rejecting allografts.
Transplant Proc, 21: 98-99.
Meuer SC, Fitzgerald KA, Hussey RE, Hodgdon JC, Schlossman SF, &
Reinherz EL (1983) Clonotypic structures involved in antigen-specific
human T cell function. Relationship to the T3 molecular complex. J Exp
Med, 157: 705-719.
Miller K (1992) Mineral dusts: Asbestos, silica and others.
In: Miller K, Turk JL ,& Nicklin S ed. Principles and practice of
immunotoxicology. Oxford, Blackwell Scientific Publications,
pp 217-227.
Miller MD & Krangel MS (1992) Biology and biochemistry of the
chemokines: A family of chemotactic and inflammatory cytokines. Crit
Rev Immunol, 12: 17-46.
Miller K, & Nicklin S, eds (1987) Immunology of the gastrointestinal
tract. Boca Raton, Florida, CRC Press.
Miller LE, Ludke HR, Peacock JE, & Tomar RH (1991) Manual of
laboratory immunology, 2nd Ed. Philadelphia, Lea & Febiger.
Minchin SA, Leitenberg D, Stunz LL, & Feldbush TL (1990) Polyclonal
activation of rat B cells. II. Dextran sulfate as a cofactor in
mitogen-induced and antigen-induced differentiation of rat B
lymphocytes. J Immunol, 145: 2427-2433.
Mirkin IR, Anderson HA, Handaran L, Hong R, Gilubjatnikov R, & Belluck
D (1990) Changes in T-lymphocyte distribution associated with
ingestion of aldi-carb-contaminated drinking water: A follow-up study.
Environ Res, 51: 35-50.
Mishell RI & Dutton RW (1967) Immunization of dissociated spleen cell
cultures from normal mice. J Exp Med, 126: 423-442.
Mittal A, Woodward B, & Chandra RK (1988) Involution of thymic
epithelium and low serum thymulin bioactivity in weanling mice
subjected to severe food intake restriction or severe protein
deficiency. Exp Mol Pathol, 48: 226-235.
Molfino NA, Wright SC, Katz I, Tarlo S, Silverman F, McClean PA,
Szalai JP, Raizenne M, Slutski AS, & Zamel N (1991) Effect of low
concentrations of ozone on inhaled allergen responses in asthmatic
subjects. Lancet, 338: 199-203.
Moolenbeek C (1982) Obtaining alveolar macrophages from small
laboratory animals. Lab Anim, 16: 56-58.
Morahan PS, Klykken PC, Smith SH, Harris LS, & Munson AE (1979)
Effects of cannabinoids on host resistance to Listeria
monocytogenesand herpes simplex virus. Infect Immun, 23: 670-674.
Morris DL, Snyder NK, Gokani V, Blair RE, & Holsapple MP (1992)
Enhanced suppression of humoral immunity in DBA/2 mice following
subchronic exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD).
Toxicol Appl Pharmacol, 112: 128-132.
Mortensen P, Bergman A, Bignert A, Hansen H-J, Harkonen T, & Olsson
M (1992) Prevalence of skull lesions in harbor seals (Phoca vitulina)
in Swedish and Danish museum collections: 1835-1988. Ambio,
21: 520-524.
Mosier DE (1990) Immunodeficient mice xenografted with human lymphoid
cells: New models for in vivo studies of human immunobiology and
infectious diseases. J Clin Immunol, 10: 185-191.
Mosmann TR & Coffman RL (1989) Heterogeneity and cytokine secretion
patterns and functions of helper T cells. Adv Immunol, 46: 111-147.
Moszczynski P, Bem S, Moszczynski P, & Bartus R (1990a) Indicators of
immunity and acute phase reaction in men in relation to the duration
of exposure to mercury vapors. Med Pract, 41: 169.
Moszczynski P, Lisiewicz J, Bartus R, & Bem S (1990b) The serum
immunoglobulins in workers after prolonged occupational exposure to
the mercury vapors. Rev Roum Méd Intern, 28: 25-30.
Mowat AM (1987) The regulation of immune responses to dietary protein
antigens. Immunol Today, 8: 93-98.
Muir DCG, Ford CA, Stewart REA, Smith TG, Addison RF, Zinck ME, &
Béland P (1990) Organochlorine contaminants in belugas
(Delphinapterus leucas), from Canadian waters. Can Bull Fish Aquat
Sci, 224: 165-190.
Munson AE & White KL (1990) Immunotoxicology. In: Clayson DB, Munro
IC, Shubik P, & Swenberg JA ed. Progress in predictive toxicology.
Amsterdam, Elsevier Scientific Publishers, pp 285-313.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspectives, 43: 41-52.
Murray MJ, Lauer LD, Luster MI, Luebke RW, Adams DO, & Dean JH (1985)
Correlation of murine susceptibility to tumor, parasite and bacterial
challenge with altered cell-mediated immunity following systemic
exposure to the tumor promoter phorbol myristate acetate. Int J
Immunopharmacol, 7: 491-500.
Myers MJ, Schook LB, & Bick PH (1987) Mechanisms of benzo( a)pyrene-
induced modulation of antigen presentation. J Pharmacol Exp Ther,
242: 399-404.
Nagarkatti PS, Sweeney GC, Gauldie J, & Clark DA (1984) Sensitivity to
suppression of cytotoxic T-cell generation by 2,3,7,8-tetra-
chlorodibenzo- p-dioxin (TCDD) is dependent on the Ah genotype of the
murine host. Toxicol Appl Pharmacol, 72: 169-176.
Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, & McCune JM (1990)
Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med,
172: 1055-1063.
Naraqi S, Jackson GG, Jonasson O, & Yamashiroya HJ (1977) Prospective
study of prevalence, incidence and source of herpesvirus infections in
patients with renal allografts. J Infect Dis, 136: 531.
Neas LM, Dockery DD, Ware JH, Spengler JD, Speizer FE, & Ferris BG
(1991) Association of indoor nitrogen dioxide with respiratory
symptoms and pulmonary function in children. Am J Epidemiol,
134: 204-218
Neckers LM & Cossman J (1983) Transferrin receptor induction in
mitogen-stimulated human T lymphocytes is required for DNA synthesis
and cell division and is regulated by interleukin 2. Proc Natl Acad
Sci USA, 80: 3494-3498.
Neefjes JJ & Momburg F (1993) Cell biology of antigen processing. Curr
Opin Immunol, 5: 27-34.
Neubert D (1992) Evaluation of toxicity of TCDD in animals as a basis
for human risk assessment. Toxic Subst J, 12: 237-276.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1990)
Polyhalo-genated dibenzo- p-dioxins and dibenzofurans and the immune
system. 1. Effects on peripheral lymphocyte subpopulations of a
non-human primate (Callitrix jacchus) after treatment with
2,3,7,8-tetrachlorodibenzo-p -dioxin (TCDD). Arch Toxicol,
64: 345-359.
Neubert R, Jacob-Miller U, Stahlman R, Helge H, & Neubert D (1991)
Polyhalogenated dibenzo- p-dioxins and dibenzofurans and the immune
system. 2. In vitro effect of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) on lymphocytes of venous blood from man and a non-human primate
(Callitrix jacchus). Arch Toxicol, 65: 213-219.
Newcombe DS, Rose NR, & Bloom JC (1992) Clinical immunotoxicology. New
York, Raven Press.
Nickoloff BJ ed. (1993) Heterogeneity among CD36+ cells in normal
diseased human skin. In: Dermal immune system. Boca Raton, Florida,
CRC Press, pp 245-275.
Nigam SK, Karnik AB, Chattopadhyay P, Lakkad K, Venkaiah K,
& Kashyap SK (1993) Clinical and biochemical investigations to
evolve early diagnosis in workers involved in the manufacture of
hexachlorocyclohexane. Int Arch Occup Environ Health, 65: S193-S196.
Nikolaidos E, Brunström B, Dencker L, & Veromaa T (1990) TCDD inhibits
the support of B-cell development by the bursa of Fabricius. Pharmacol
Toxicol, 67: 22-26.
Nimmo Wilkie JS, Yager JA, Wilkie BN, & Pascoe PJ (1992) Changes in
cell-mediated immune responses after experimentally-induced
anaphylaxis in dogs. Vet Immunol Immunopathol, 32: 325-338.
Noonan FP & De Fabo EC (1990) Ultraviolet-B-dose-response curves for
local and systemic immunosuppression are identical. Photochem
Photobiol, 52: 801-810.
Noonan FP & De Fabo EC (1992) Immunosuppression by ultraviolet-B
radiation: Initiation by urocanic acid. Immunol Today, 13: 250-254.
Noonan FP & Hoffman HA (1994) Susceptibility to immunosuppression by
ultraviolet B radiation in the mouse. Immunogenetics, 39: 29-39.
Nossal GJV (1987) Immunology: The basic components of the immune
system. New Engl J Med, 316: 1320-1325.
Oberhelman L, Koren H, & Cooper KD (1992) Depressed capacity to
contact sensitize women at the onset of menses; elevated contact
sensitization capacity during mid cycle can be inhibited by UV.
Clin Res, 40: 542A.
O'Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE,
Nadel JA, & Holtzman (1984) Neutrophil depletion inhibits airway
hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis,
130: 214-219.
O'Dell BL, Jessen RT, Becker LE, Jackson RT, & Smith EB (1980)
Diminished immune response in sun-damaged skin. Arch Dermatol,
116: 559-561.
Oehler R & Herberman RB (1978) Natural cell-mediated cytotoxicity in
rats. III. Effects of immunopharmacologic treatments on natural
reactivity and reactivity augmented by plyinosinic-polycytidylicacid.
Int J Cancer, 21: 221-229.
Ono H, Klein D, Vincek V, Figueroa F, O'Huigin C, Tichy H, & Klein J
(1992) Major histocompatibility complex class II genes of zebra fish.
Proc Natl Acad Sci USA, 89: 11886-11890.
Orehek J, Grimaldi F, Muls E, Durand JP, Viala A, & Charpin J (1981)
Bronchial response to allergens after controlled NO2exposure. Bull Eur
Physiopathol Respir, 17: 911-915.
Organisation for Economic Co-operation and Development (1981) OECD
guidelines for testing chemicals. Repeated dose oral toxicity -
Rodent: 28-day or 14-day study (Guideline 407). Paris.
Osborn JE, Blazkovec AA, & Walker DL (1968) Immunosuppression during
acute cytomegalovirus infection. J Immunol, 100: 835-844.
Ozkaynak H, Kinney PL, & Burbank B (1990) Recent epidemiological
findings on morbidity and mortality effects of ozone. Air Waste Manage
Assoc Rep, 6: 90-150.
Pallardy MJ, House RV, & Dean JH (1989) Molecular mechanism of
7,12-dimethylbenz[ a]anthracene-induced immunosuppression: Evidence
for action via the interleukin-2 pathway. Mol Pharmacol, 36: 128-133.
Pallardy MJ, Mishal Z, Lebrec H, & Bohuon C (1992) Immune
modification due to chemical interference with transmembrane
signalling: Applications to polycyclic aromatic hydrocarbons.
Int J Immunopharmacol, 14: 377-382.
Paranjpe MS & Boone CW (1972) Delayed hypersensitivity to simian virus
40 tumor cells in BALB/c mice demonstrated by a radioisotopic foot-pad
assay. J Natl Cancer Inst, 48: 563-566.
Parhar RS & Lala PK (1987) Amelioration of B16F10 melanoma lung
metastasis in mice by a combination therapy with indomethacin and
interleukin 2. J Exp Med, 165: 14-28.
Pass RF, Long WK, Whitley RJ, Soong S, Diethelm A, Reynolds DW, &
Alford CA (1978) Productive infection with CMV and HSV in renal
transplant recipients: Role of source of kidney. J Infect Dis,
137: 556.
Patarroyo M (1991) Leukocyte adhesion in host defence and tissue
injury. Clin Immunol Immunopathol, 60: 333-348.
Patki AH (1991) Hypothesis: Solar ultraviolet radiation and the
initial skin lesion of leprosy. Int J Leprosy, 59: 492-493.
Paul WE (1993) Fundamental immunology, 3rd Ed. New York, Raven Press.
Paul PS, Johnson DW, Mirocha CJ, Soper FF, Thoen CO, Muscoplat CC, &
Weber AF (1977) In vitro stimulation of bovine peripheral blood
lymphocytes: Suppression of phytomitogen and specific antigen
lymphocyte responses by aflatoxin. Am J Vet Res, 38: 2033-2035.
Payne JF & Fancey LF (1989) Effect of polycyclic aromatic hydrocarbons
on immune response in fish: Change in melanomacrophage centers in
flounder (Pseudopleuronectes americanus) exposed to hydrocarbon-
contaminated sediments. Mar Environ Res, 28: 431-435.
Pedersen BK & Beyer JM (1986) A longitudinal study of the influence of
azathioprine on natural killer cell activity. Allergy, 41: 286-289.
Penit C & Ezine S (1989) Cell proliferation and thymocyte subset
reconstitution in sublethally irradiated mice: Compared kinetics of
endogenous and intrathymically transferred progenitors. Proc Natl Acad
Sci USA, 86: 5547-5551.
Penn I (1988) Cancer is a long-term hazard of immunosuppressive
therapy. J Autoimmun, 1: 545-558.
Penn I (1993a) Incidence and treatment of neoplasia after
transplantation. J Heart Lung Transplant, 12: 328-336.
Penn I (1993b) Tumors after renal and cardiac transplantation. Hematol
Oncol Clin North Am, 7: 431-445.
Pennington JE (1985) Immunosuppression of pulmonary host defense. Adv
Host Def Mech, 4: 141-164.
Penninks AH, Snoeij NJ, Peiters RHH, & Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: An
overview. In: Dayan AD, Hertel RF, Heseltine E, Kazantzis G, Smith EM,
& Van der Venne MT ed. Immunotoxicity of metals and immunotoxicology.
New York, Plenum Press, p 191.
Pestka JJ & Bondy GS (1990) Alteration of immune function following
dietary mycotoxin exposure. Can J Physiol Pharmacol, 68: 1009-1016.
Pfeifer R & Irons R (1981) Inhibition of lecithin-stimulated
lymphocyte agglutination and mitogenesis by hydroquinone; reactivity
with sulfhydyl groups. Exp Mol Pathol, 35: 189-198.
Phair J, Munoz A, Detels RI, Kaslow R, Rinaldo C, & Saah A (1990) The
risk of Pneumocystis carinii pneumonia among men infected with human
immunodeficiency virus type 1. New Engl J Med, 322: 161-165.
Picker LJ & Butcher EC (1992) Physiological and molecular mechanisms
of lymphocyte homing. Ann Rev Immunol, 10: 561-591.
Pleiman CM, D'Ambrosio D, & Cambier JC (1994) The B-cell antigen
receptor complex: Structure and signal transduction. Immunol Today,
15: 393-399.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p -dioxin and
related halogenated aromatic hydrocarbons: Examination of the
mechanism of toxicity. Ann Rev Pharmacol Toxicol, 22: 517-554.
Pollock PL, Germolec DR, Comment CE, Rosenthal GJ, & Luster MI (1994)
Development of human lymphocyte engrafted SCID mice as a model for
immunotoxicity assessment. Fundam Appl Toxicol., 22: 130-138.
Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L,
Janeway CA, Murphy DB, & Bottomly K (1989) Monoclonal antibodies to
murine CD3e defined distinct epitopes, one of which may interact with
CD4 during T cell activation. J Immunol, 142: 4169-4175.
Prichard MG, Boerth LW, & Pennington JE (1987) Compartmental analysis
of resting and activated pulmonary natural killer cells. Exp Lung Res,
12: 239-251.
Prior AC & Sisodia CS (1982) The effects of ochratoxin A on the immune
responses of Swiss mice. Can J Comp Med, 46: 91-96.
Pruett SB (1992) Immunotoxicity of organophosphorus compounds.
In: Chambers JE & Levi PE ed. Organophosphates. Chemistry, fate and
effects. New York, Academic Press, pp 367-385.
Pruett SB & Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin
2 system. Toxicol Lett, 40: 11-20.
Pugh-Humphreys RGP, Ross CSK, & Thomson AW (1990) The influence of
FK-506 on the thymus. An immunophenotypic and structural analysis.
Immunology, 70: 398-404.
Quesniaux VFJ (1992) Interleukins 9, 10, 11 and 12 and kit ligand:
A brief overview. Res Immunol, 143: 385-400.
Quinnan GV, Manischewitz JE, & Ennis FA (1980) Role of cytotoxic
T-lymphocytes in murine cytomegalovirus infection. J Gen Virol,
47: 503-508.
Qureshi MA, Bloom SE, Hamilton JW, & Dietert RR (1989) Toxic effects
of methyl methanesulfonate (MMS) on activated macrophages from
chickens. Environ Mol Mutag, 13: 253-262
Rao GN, Bimbaurn LS, Collins JJ, Tennant RW, & Skow LC (1988) Mouse
strains for chemical carcinogenicity studies: Overview of a workshop.
Fundam Appl Toxicol, 10: 385-394.
Raskin RE, Tvedten HW, Bull RW, Crow SE, Dunstan RW, & Krehbiel JD
(1989) Natural killer cell activity in untreated and treated dogs with
lymphoma. Am J Vet Res, 4: 483-487.
Ratajczak HV, Thomas PT, Sothern RB, Vollmuth T, & Heck D (1993)
Evidence for genetic basis of seasonal differences in antibody
formation between two mouse strains. Chronobiol Int, 10: 383-394.
Ratcliffe NA & Rowly AF (1981) Intervertebrate blood cells. London,
Academic Press.
Raulet DH (1989) The structure, function, and molecular genetics of
the gamma/delta T cell receptor. Ann Rev Immunol, 7: 175-207.
Reed SG & Scott P (1993) T-Cell and cytokine responses in
leishmaniasis. Curr Opin Immunol, 5: 524-531.
Reggiani G (1980) Acute human exposure to TCDD in Seveso, Italy.
J Toxicol Environ Health, 6: 27-43.
Reigart JR & Graber CD (1976) Evaluation of the humoral immune
response of children with low lead exposure. Bull Environ Contam
Toxicol, 16: 112-117.
Reijnders PJH (1986) Reproductive failure in common seals feeding on
fish from polluted coastal waters. Nature, 324: 456-457.
Renton KW (1986) Factors affecting drug biotransformation. Clin
Biochem, 19: 72-75.
Renton KW, Gray JD, & Hall RI (1980) Decreased elimination of
theophylline after influenza vaccination. Can Med Assoc J,
123: 288-290.
Reyes J, Tzakis A, Green M, Nour B, Nalesnik M, Van Thiel D, Martin M,
Breinig MK, Fung LL, Cooper M, & Starzl TE (1991) Post-transplant
lymphoproliferative disorders under primary FK506 immunosuppression.
Transplant Proc, 23: 3044-3046
Reynolds HY & Thomson RE (1973) Pulmonary host defenses.
II. Interaction of respiratory antibodies with Pseudomonas aeruginosa
and alveolar macrophages. J Immunol, 111: 369-380.
Reynolds CW, Wenn AC, Barlozzari T, Wiltrout T, Reichardt WA, &
Herberman RB (1984) Natural killer activity in the rat. IV.
Distribution of large granular lymohocytes (LGL) following intravenous
and intraperitoneal transfer. Cell Immunol, 86: 371-380.
Richeldi L, Persichini T, Ferrara GB, Crystal RG, Markham T, Luisetti
M, Bertrorelli G, Guerritore D, & Saltini C (1992) Chronic beryllium
disease association with the HLA-DP 2.1 allele. Am Rev Respir Dis,
145 (Suppl): A324.
Riley WJ (1989) Insulin dependent diabetes mellitus, an autoimmune
disorder? Clin Immunol Immunopathol, 53: S92-S98.
Roberts RJ (1975) Melanin-containing cells of the teleost fish and
their relation to disease. In: Ribelin WE & Migaki G ed. The pathology
of fishes. Madison, Wisconsin, University of Wisconsin Press,
pp 399-428.
Roitt IM (1991) Essential immunology, 7th Ed. Oxford, Blackwell
Scientific Publications.
Roitt IM, Brostoff J & Male DK (1993) Immunology, 3rd Ed. London,
Gower Medical Publications.
Roizman B (1982) The family Herpesviridae. General description,
taxonomy, and classification. In: Roizman B ed. The viruses, Vol 1:
Herpesviruses. New York, Plenum Press, pp 1-23.
Roizman B, Carmichael LE, Deinhardt F, de The G, Nahmias AJ, Plowright
W, Rapp F, Sheldrick P, Takahashi M, & Wolf K (1981) Herpesviridae.
Definition, provisional nomenclature and taxonomy. Intervirology,
16: 201-217.
Rolstad B, Herberman RB, & Reynolds GW (1986) Natural killer activity
in the rat: V. The circulation patterns and tissue localization of
peripheral blood lymphocytes. J Immunol, 136: 2600-2808.
Rose LM, Richards TL, Petersen R, Peterson J, Hruby S, & Alvord EC Jr
(1991) Remitting-relapsing EAE in nonhuman primates: A valid model of
multiple sclerosis. Clin Immunol Immunopathol, 59: 1-15.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502-510.
Rosenthal GJ & Snyder CA (1987) Inhaled benzene reduces aspects of
cell-mediated tumor surveillance in mice. Toxicol Appl Pharmacol,
88: 35-43.
Rosenthal GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock
P, Ling-Yuan K, Kwon J, & Luster MI (1994) Asbestos stimulates
IL-8 production from human lung epithelial cells. J Immunol,
153: 3237-3244.
Ross GD (1992) Function of neutrophils in host defence and immunity.
In: Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca
Raton, Florida, CRC Press, pp 263-286.
Ross PS, De Swart RL, Reijnders PJH, Van Loveren H, Vos JG, &
Osterhaus ADME (1995) Contaminant-related suppression of delayed-type
hypersensitivity and antibody responses in harbor seals fed herring
from the Baltic Sea. Environ Health Perspectives, 103: 162-167.
Ross PS, De Swart RL, Timmerman HH, Reijnders PJH, Vos JG, Van Loveren
H, & Osterhaus ADME (in press) Suppression of natural killer activity
in harbour seals (Phoca virulina) fed Baltic Sea herring. Aquat
Toxicol.
Rothbard JB & Gefter ML (1991) Interactions between immunogenic
peptides and MHC molecules. Ann Rev Immunol, 9: 527-565.
Roths JB, Murphy ED, & Eicher EM (1984) A new mutation, gld, that
produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp
Med, 159: 1-20.
Rouveix B(1993) Opiates and immune function. Thérapie, 47: 503-512.
Rubin RH (1990) Impact of cytomegalovirus infection in organ
transplant recipients. Rev Infect Dis, 12: S754-S766.
Rubin RH, Cosimi AB, Hirsch MS, Herrin JT, Russell PS, & Tolkoff Rubin
NE (1981) Effects of antithymocyte globulin on CMV infection in renal
transplant recipients. Transplantation, 31: 143-145.
Ruitenberg EJ, Buys J, Teppema JS, & Elgersma A (1983) Rat mononuclear
cells and neutrophils are more effective than eosinophils in antibody-
mediated stage-specific killing of Trichinella spiralis in vitro.
Parasitenkunde, 69: 807-815.
Ryffel B (1992) The carcinogenicity of ciclosporin. Toxicology,
73: 1-22.
Saiki RK, Scharf SJ, Faloona FA, Mullis KB, Hom GT, Erlich HA, &
Arnheim N (1985) Enzymatic amplification of ß-globulin genomic
sequences and restriction site analysis for diagnosis of sickle cell
anemia. Science, 230: 1350-1354.
Sainte-Marie G, Bélisle C, & Peng FS (1990) The deep cortex of the
lymph node: Morphological variations and functional aspects. In:
Grundmann E & Vollmer E ed. Reaction patterns of the lymph node, Part
1. Cell types and functions. Berlin, Springer Verlag, pp 33-63.
Saito M (1992) Recent trends in research on differentiation of
hematopoietic cells and lymphokines (hemopoietins). Hum Cell,
5: 54-69.
Sakaguchi S & Sakaguchi N (1988) Thymus and autoimmunity. Transplanta-
tion of the thymus from cyclosporin A-treated mice causes organ-
specific autoimmune disease in athymic nude mice. J Exp Med,
167: 1479-1485.
Saltini C, Winestock K, Kirby M, Pinkston P, & Crystal RB (1989)
Maintenance of alveolitis in patients with chronic beryllium disease
by beryllium-specific helper T cells. New Engl J Med, 320: 1103-1109.
Samet JM (1994) Nitrogen dioxide and respiratory infection. Am Rev
Respir Dis, 139: 1073-1074.
Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA,
McLaren LC, Schwab M, & Spengler JD (1993) Nitrogen dioxide and
respiratory illnesses in infants. Am Rev Respir Dis, 148: 1258-1265.
Sanders VM, Tucker AN, White KL, Kauffmann BM, Hallett P, Carchman RA,
Borzelleca JF, & Munson AE (1982) Humoral and cell-mediated immune
status in mice exposed to trichloroethylene in the drinking water.
Toxicol Appl Pharmacol, 62: 358-368.
Sanders VM, White KL, Shopp GM, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to 1,1,2-trichloroethane. Drug
Chem Toxicol, 8: 357.
Sato K, Flajnik MF, Du Pasquier L, Katagiri M, & Kasahara M (1993)
Evolution of the MHC: Isolation of class II ß-chain cDNA clones from
the amphibian Xenopus laevis. J Immunol, 150: 2831-2843.
Scala R (1991) Risk assessment. In: Amdur M, Doull J, & Klaasen C ed.
Casarett and Doull's Toxicology, 3rd Ed. Elmsford, New York, Pergamon
Press, pp 985-996.
Scheuchenzuber WJ, Eskew ML, & Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environ Res, 38: 389-399.
Schielen P, Schoo W, Tekstra J, Oostermeijer HH, Seinen W, & Bloksma N
(1993) Autoimmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schlick E & Friedberg KD (1981) The influence of low lead doses on the
reticulo-endothelial system and leukocytes of mice. Arch Toxicol,
47: 197.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
C, Ritz J, Shaw S, Silverstein RL, Springer TA, Tedder TF, & Todd RF
(1994) CD antigens 1993. Immunol Today, 15: 98-99.
Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto
T, Ritz J, Shaw S, Springer TA, Tedder TF, & Todd RF (1995) Leucocyte
typing, V: White cell differentiation antigens. Oxford, Oxford
University Press.
Schouk LB & Laskin DL (1994) Xenobiotics and inflammation. San Diego,
California, Academic Press.
Schuurman H-J, Kluin PM, Gmelig-Meyling FHJ, Van Unnik JAM, & Kater L
(1985) Lymphocyte status of lymph node and blood in acquired
immunodeficien-cy syndrome (AIDS) and AIDS-related complex disease.
J Pathol, 147: 269-280.
Schuurman H-J, Van Loveren H, Rozing J, Van Dijk A, Loeber JG, & Vos
JG (1990) Cyclosporin and the rat thymus. An immunohistochemical
study. Thymus, 16: 235-254.
Schuurman H-J, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1991a) Immune system. In: Haschek WM & Rousseaux CG ed. Handbook of
toxicologic pathology. San Diego, Academic Press, pp 421-487.
Schuurman H-J, Nagelkerken L, De Weger RA, & Rozing J (1991b) Age-
associated involution: Significance of a physiologic process. Thymus,
18: 1-6.
Schuurman H-J, De Weger RA, Van Loveren H, Krajnc-Franken MAM, & Vos
JG (1992a) Histopathological approaches. In: Miller K, Turk JL, &
Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 279-303.
Schuurman H-J, Hougen HP, & Van Loveren H (1992b) The rnu (Rowett
nude) and rnuN ( nznu, New Zealand nude) rat: An update. ILAR
News, 34: 3-12.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijt BC,
De Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Pass D, Vankerkom
J, Boot R, Broekhuizen R, Dormans J, van Herck H, Hermans PGC, Hoekman
J, van der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992c)
Comparative evaluation of the immune status of congenitally athymic
and euthymic rat strains bred and maintained at different institutes:
2. Athymic rats. J Exp Anim Sci, 35: 33-48.
Schuurman H-J, Van Loveren H, Rozing J, & Vos JG (1992d) Chemicals
trophic for the thymus: Risk for immunodeficiency and autoimmunity.
Int J Immunopharmacol, 14: 369-375.
Schuurman H-J, Bell EB, Gärtner K, Hedrich HJ, Hansen AK, Kruijht BC,
de Vrey P, Leyten R, Maeder SJ, Moutier R, Mohnle U, Vankerkom J, Boot
R, Broekhuizen R, Dormans J, van Herck H, Hermans GC, Hoekman J, van
der Logt J, van Wijk M, Vos JG, & van Zutphen LFM (1992e) Comparative
evaluation of the immune status of congenitally athymic and euthymic
rat strains bred and maintained at different institutes: 1. Euthymic
rats. J Exp Anim Sci, 35: 16-32.
Schuurman H-J, Hu HZ, De Weger RA, & Clevers HC (1993) Thoughts on
thymus and T-lymphocyte repertoire. Relevance for tolerance of the
immune response. Neth J Med, 43: 38-54.
Schuurman H-J, Kuper CF, & Vos JG (1994) Histopathology of the immune
system as a tool to assess immunotoxicity. Toxicology, 86: 197-212.
Schwartz J (1992) Air pollution and the duration of acute respiratory
symptoms. Arch Environ Health, 47: 116-122.
Schwartz RS, Callen JP, & Silva JR (1978) A cluster of Hodgkin's
disease in a small community: Evidence for environmental factors.
Am J Epidemiol, 108: 19-26.
Schwartz J, Spix C, Wichmann HE, & Malin E (1991) Air pollution and
acute respiratory illness in five German communities. Environ Res,
56: 1-14.
Schwetz BA & Tyl RW (1987) Consensus workshop on the evaluation of
maternal and developmental toxicity. Workgroup III report. Low dose
extrapolation and other considerations for risk assessment - models
and applications. Teratog Carcinog Mutag, 1: 1-7.
Secombes CJ, Fletcher TC, White A, Costello MJ, Stagg R, & Houlihan DF
(1992) Effects of sewage sludge on immune response in the dab,
Limanda limanda. Aquat Toxicol, 23: 217-230.
Seinen W & Penninks AH (1979) Immune suppression as a consequence of a
selective cytotoxic activity of certain organometallic compounds on
thymus and thymus-dependent lymphocytes. Ann NY Acad Sci,
320: 499-517.
Seinen W & Willems MI (1976) Toxicity of organotin compounds.
I. Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di- n-octyltin dichloride. Toxicol Appl Pharmacol, 35: 63-75.
Seinen W, Vos JG, Van Krieken R, Penninks AH, Brands R, & Hooykaas H
(1977) Toxicity of organotin compounds. II. Comparative in vivo and
in vitro studies with various organotin and organolead compounds in
different animal species with special emphasis on lymphocyte
cytotoxicity. Toxicol Appl Pharmacol, 42: 197-212.
Sela O & Shoenfeld Y (1988) Cancer in autoimmune diseases. Semin Arthr
Rheum, 18: 78-87.
Selgrade MJK & Gilmour MI (1994) Effects of gaseous air pollutants on
immune responses and susceptibility to infectious and allergic
disease. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicity and immunopharmacology, 2nd Ed. New York, Raven Press,
pp 395-411.
Selgrade MJK & Osborn TE (1974) Role of macrophages in resistance to
murine cytomegalovirus. Infect Immun, 10: 1383-1390.
Selgrade MJK, Daniels MJ, Hu PC, Miller FJ, & Graham JA (1982) Effects
of immunosuppression with cyclophosphamide on acute murine
cytomegalovirus infection and virus-augmented natural killer cell
activity. Infect Immun, 38: 1046-1055.
Selgrade MK, Daniels MJ, Illing JW, Ralston AL, Grady MA, Chartlet E,
& Graham JA (1984) Increased susceptibility to parathion poisoning
following murine cytomegalovirus infection. Toxicol Appl Pharmacol,
76: 356-364.
Selgrade MJK, Illing JW, Starnes DM, Stead AG, Menache MG, & Stevens
MA (1988) Evaluation of effects of ozone exposure on influenza
infection in mice using several indicators of susceptibility. Fundam
Appl Toxicol, 11: 169-180.
Selgrade MJK, Starnes DM, Illing JW, Daniels MJ, & Graham JA (1989)
Effects of phosgene exposure on bacterial, viral, and neoplastic lung
disease susceptibility in mice. Inhal Toxicol, 1: 243-259.
Selgrade MJK, Daniels MJ, & Dean JH (1992) Correlation between
chemical suppression of natural killer cell activity in mice and
susceptibility to cytomegalovirus: Rationale for applying murine
cytomegalovirus as a host resistance model and for interpreting
immunotoxicity testing in terms of risk of disease. J Toxicol Environ
Health, 37: 123-137.
Selgrade MJK, Cooper KD, Devlin RB, Van Loveren H, Biagini RE, &
Luster MI (1995) Immunotoxicity - Bridging the gap between animal
research and human health effects. Fundam Appl Toxicol, 24: 13-21.
Sell S (1987) Immunology, immunopathology and immunity, 4th Ed.
Norwalk, Connecticut, Appleton & Lange.
Shabtai M, Malinowski K, Waltzer WC, Pullis C, Raisbeck AP, & Rapaport
FT (1991) Quantitative analysis of surface marker densities after
exposure of T cells to concanavalin A (ConA): A sensitive early index
of cellular activation. Cell Immunol, 133: 519-525.
Shand FL (1975) Review/commentary: The immunopharmacology of
cyclophosphamide. Int J Immunopharmacol, 1: 165-171.
Sharp JG & Watkins EB (1981) Cellular and immunological consequences
of thymic irradiation. In: Dubois JB, Serrou B, & Rosenfeld C ed.
Immunopharmacologic effects of radiation therapy. New York, Raven
Press, pp 137-179.
Sheil AG, Disney AP, Mathew TH, Amiss N, & Excell L (1991) Cancer
development in cadaveric donor renal allograft recipients treated with
azathioprine (AZA) or cyclosporine (CyA) or AZA/CyA. Transplant Proc,
23: 1111-1112.
Sherwood RL, Tarkington B, Lippert WE, & Goldstein E (1981) Effect of
ferrous sulphate aerosols and nitrogen dioxide aerosols on murine
pulmonary defense. Arch Environ Health, 36: 130-135.
Sherwood RL, Thomas PT, Kawanishi CY, & Fenters JD (1988) Comparison
of Streptococcus zooepidemicus and influenza virus pathogenicity in
mice by three pulmonary exposure routes. Appl Environ Microbiol,
54: 1744-1751.
Shigematsu N, Ishimaru S, Saito R, Ikeda T, Matsuba K, Sigiyama K, &
Masuda Y (1978) Respiratory involvement in polychlorinated biphenyls
poisoning. Environ Res, 16: 92-100.
Shiroky JB, Frosp A, Skelton JD, Haegert DG, Newkirk MM, & Neville
C (1991) Complications of immunosuppression associated with weekly low
dose methrotrexate. J Rheum, 18: 1172-1175.
Shopp GM & Munson AE (1985) Suppression of the antibody response by
phorbol esters in the mouse is due to an effect on the nylon wool
adherent cell population. Agents Actions, 6: 535-541.
Shopp GM, Sanders VM, White KL, & Munson AE (1985) Humoral and cell-
mediated immune status of mice exposed to trans-12-dichlorethylene.
Drug Chem Toxicol, 8: 393.
Shortman K, Egerton M, Spangrude GJ, & Scollay R (1990) The generation
and fate of thymocytes. Sem Immunol, 2: 3-13.
Shy CM, Creason JP, Pearlman ME, McClain KE, & Benson FB (1970) The
Chattanooga study: Effects of community exposure to nitrogen dioxide.
II. Incidence of acute respiratory illness. J Air Pollut Control
Assoc, 20: 582-588.
Sibinga NES & Goldstein A (1988) Opioid peptides and opioid receptors
in cells of the immune system. Ann Rev Immunol, 6: 219-249.
Sigal NH & Dumont FJ (1992) Cyclosporin A, FK-506, and Rapamycin:
Pharmacologic probes of lymphocyte signal transduction. Ann Rev
Immunol, 10: 519-560.
Sikorski EE, McCay JA, White KL, Bradley SG, & Munson AE (1989)
Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1
mice. Fundam Appl Toxicol, 13: 843-858.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW,
Wilcox KR, VanAmburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls.
J Reticuloendothel Soc, 26: 341.
Sima P & Vetvicka V (1992) Evolution of immune accessory function. In:
Fornusek L & Vetvicka V ed. Immune system accessory cells. Boca Raton,
Florida, CRC Press, pp 1-56.
Simon JC, Cruz PD, Bergstresser PR, & Tigelaar RE (1990) Low dose
ultraviolet B-irradiated Langerhans cells preferentially activate
CD4+ cells of the T helper 2 subset. J Immunol, 145: 2087-2094.
Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, & Cruz PD (1991)
Ultraviolet B radiation converts Langerhans cells from immunogenic to
tolerogenic antigen-presenting cells. Induction of specific clonal
anergy in CD4+ T helper 1 cells. J Immunol, 146: 485-491.
Singh G, Fries JF, Spitz P, & Williams CA (1989) Toxic effects of
azathioprine in rheumatoid arthritis patients. A national post-
marketing perspective. Arthr Rheum, 32: 837-843.
Siroki O, Institoris L, Tatar F, & Desi I (1994) Immunotoxicological
investigation of SCMF, a new pyrethroid pesticide in mice. Hum Exp
Toxicol, 13: 337-343.
Siwicki AK, Cossarini-Dunier M, Studnicka M, & Demael A (1990)
In vivo effect of the organophosphorus insecticide trichlorphon on
immune response of carp (Cyprinus carpio). Ecotoxicol Environ Saf,
19: 99-105.
Sjoblad RD (1988) Potential future requirements for immunotoxicity
testing of pesticides. Toxicol Ind Health, 4: 391-394.
Sjovall P, Christensen OB, & Moller H (1985) Single exposure to
ultraviolet irradiation and elicitation of human allergic contact
dermatitis. Acta Dermatol Venereol, 65: 93-96.
Smialowicz RJ, Rogers RR, Riddle MM, Luebke RW, Rowe DG, & Garner RJ
(1984) Manganese chloride enhances murine cell-mediated cytotoxicity:
Effects on natural killer cells. J Immunopharmacol, 6: 1-23.
Smialowicz R, Luebke RW, Riddle MM, Rogers RR, & Rowe DG (1985a)
Evaluation of the immunotoxic potential of chlordecone with comparison
to cyclophosphamide. J Toxicol Environ Health, 15: 561-574.
Smialowicz RJ, Luebke RW, Rogers RR, Riddle MM, & Rowe DG (1985b)
Evaluation of immune function in mice exposed to ordram. Toxicology,
37: 307-314.
Smialowicz RJ, Rogers RR, Riddle MM, Garner RJ, Rowe DG, & Luebke RW
(1985c) Immunologic effects of nickel. II. Suppression of natural
killer cell activity. Environ Res, 36: 56-66.
Smialowicz RJ, Andrews JE, Riddle MM, Rogers RR, Luebke RW, & Copeland
CB (1989) Evaluation of the immunotoxicity of low level PCB exposure
in the rat. Toxicology, 56: 197-211.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB, & Ernest
GG (1990) Immune alterations in rats following subacute exposure to
tributyltin oxide. Toxicology, 64: 169-178.
Smialowicz RJ, Riddle MM, Luebke RW, Copeland CB, Andrews D, Rogers
RR, Gray LE, & Laskey JW (1991) Immunotoxicity of 2-methoxyethanol
following oral administration in Fischer 344 rats. Toxicol Appl
Pharmacol, 109: 494-506.
Smialowicz RJ, Luebke RW, & Riddle MM (1992a) Assessment of the
immunotoxic potential of the fungicide dinocap in mice. Toxicology,
75: 235-247.
Smialowicz RJ, Williams WC, Riddle MM, Andrews DL, Luebke RW, &
Copeland CB (1992b) Comparative immunosuppression of various glycol
ethers orally administered to Fischer 344 rats. Fundam Appl Toxicol,
18: 621-627.
Smialowicz RJ, Riddle MM, & Williams WC (1993) Methoxyacetaldehyde, an
intermediate metabolite of 2-methoxyethanol, is immunosuppressive in
the rat. Fundam Appl Toxicol, 21: 1-7.
Sminia T, Van der Brugge-Gamelkoorn GJ, & Jeurissen SHM (1989)
Structure and function of bronchus-associated lymphoid tissue (BALT).
Crit Rev Immunol, 9: 119-150.
Smith SH, Sanders VM, Barrett BA, Borzellaca JF, & Munson AE (1978)
Immunotoxicological evaluation on mice exposed to polychlorinated
biphenyls. Toxicol Appl Pharmacol, 45: 330.
Snoeij NJ, Penninks AH, & Seinen W (1988) Dibutyltin and tributyltin
compounds induce thymic atrophy in rats due to a selective action on
thymic lymphoblasts. Int J Immunopharmacol, 10: 889-891.
Snyder CA (1989) The neuroendocrine system, an opportunity for
immunotoxicologists. Environ Health Perspectives, 81: 165-166.
Spangrude GJ, Berhard EJ, Ajoika RS, & Daynes RA (1983) Alterations in
lymphocyte homing patterns within mice exposed to ultraviolet
radiation. J Immunol, 130: 2974-2981.
Speizer FE, Ferris B, Bishop YMM, & Spengler J (1980) Respiratory
disease rates and pulmonary function in children associated with NO2
exposure. Am Rev Respir Dis, 121: 3-10.
Spiekermann K, Emmendoerfer A, Elsner J, Raeder E, Lohmann-Matthes ML,
Prahst A, Link H, Freund M, Welte K, & Roesler J (1994) Altered
surface marker expression and function of G-CSF induced neutrophils
from test subjects and patients under chemotherapy. Br J Haematol,
87: 31-38.
Spreafico F, Merendino A, Braceschi L, & Sozanni S (1987)
Immunodepressive drugs as prototype immunotoxicants. In: Berlin A,
Dean J, Draper MH, Smith EMB, & Van Der Venne, M-T ed.
Immunotoxicology. Dordrecht, Martinus Nijhoff, pp 192-207.
Sprent J, Gao EK, & Webb SR (1990) T Cell reactivity to MHC molecules:
Immunity versus tolerance. Science, 248: 1357-1363.
Springer TA (1990) Adhesion receptors of the immune system. Nature,
346: 425-434.
Springer T, Galfré G, Secher DS, & Milstein C (1979) Mac-1: A
macrophage differentiation antigen identified by monclonal antibody.
Eur J Immunol, 9: 301-306.
Spruanoe SL (1985) Pathogenesis of herpes simplex labialis:
Experimental induction of lesions with UV light. J Clin Microbiol,
22: 366-368.
Stahlmann R, Korte M, Van Loveren H, Vos JG, Thiel R, & Neubert D
(1992) Abnormal thymus development and impaired function of the immune
system in rats after prenatal exposure to aciclovir. Arch Toxicol,
66: 551-559.
Stanley GP & Pender MP (1991) The pathophysiology of chronic relapsing
experimental allergic encephalomyelitis in the Lewis rat. Brain,
114: 1827-1853.
Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP,
Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL, Atchison RW, Jaffe R, &
Bahnson HT (1984) Reversibility of lymphomas and lymphoproliferative
lesions developing under cyclosporin-steroid therapy. Lancet,
i: 583-587.
Stehr-Green PA, Naylor PH, & Hoffman RE (1989) Diminished thymosin
alpha-1 levels in persons exposed to 2,3,7,8-tetrachlorodibenzo-
p-dioxin. J Toxicol Environ Health, 28: 285-295.
Steinmann GG (1986) Changes in the human thymus during aging. Curr
Topics Pathol, 75: 43-88.
Stein-Streinlein J, Bennett M, Mann D, & Kumar V (1983) NK cells in
mouse lungs: Surface phenotype, target preference and response to
local influenza virus infection. J Immunol, 131: 2699-2704.
Stet RJM & Egberts E (1991) The histocompatibility system in
teleostean fishes: From multiple histocompatibility loci to a major
histocompatibility complex. Fish Shellfish Immunol, 1: 1-16.
Stingl G & Steiner G (1989) Immunological host defence of the skin.
Curr Prob Dermatol, 18: 22-30.
Stobo JD & Paul WE (1973) Functional heterogeneity of murine
lymphoid cells. III. Differential responsiveness of T cells to
phytohemagglutinin and concanavalin A for T cell subsets. J Immunol,
110: 362-369.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral responses by pesticides and chemicals of environmental
concern: Quantitative studies of immunosuppression by DDT, Aroclor,
carbaryl, carbofuran and methylparathion. Toxicol Appl Pharmacol,
32: 587-602.
Streilein JW (1983) Skin associated lymphoid tissues (SALT): Origins
and functions. J Invest Dermatol, 80 (Suppl): 12s-16s.
Streilein JW (1990) Skin-associated lymphoid tissues (SALT): The next
generation. In: Bos J ed. The skin immune system. Boca Raton, Florida,
CRC Press, pp 25-48.
Strobel S & Ferguson A (1984) Immune responses to fed protein antigens
in mice. 3. Systemic tolerance or priming is related to age at which
antigen is first encountered. Ped Res, 18: 588-594.
Stunz LL & Feldbush TL (1986) Polyclonal activation of rat B
lymphocytes. I. A single mitogenic signal can stimulate proliferation,
but three signals are required for differentiation. J Immunol,
136: 4006-4012.
Stutman O (1986) Postthymic T-cell development. Immunol Rev,
91: 160-194.
Subramanian A, Tanabe S, Tatsukawa R, Saito S, & Miyazaki N (1987)
Reduction in the testosterone levels by PCBs and DDE in Dall's
porpoises of northwestern North Pacific. Mar Pollut Bull, 18: 643-646.
Sung YJ, Hotchkiss JH, Austic RE, & Dietert RR (1992) Direct
measurement of nitric oxide in headspace gas produced by a chicken
macrophage cell line in a closed culture system. Biochem Biophys Res
Commun, 184: 36-42.
Surhe CD & Sprent J (1991) Long-term xenogeneic chimeras. Full
differentiation of rat T and B cells in SCID mice. J Immunol,
147: 2148-2154.
Svensson BG, Nilsson A, Hansson M, Rappe C, Åkesson B, & Skerving S
(1991) Exposure to dioxins and dibenzofurans through the consumption
of fish. New Engl J Med, 324: 8-12.
Swain WR (1991) Effects of organochlorine chemicals on the
reproductive outcome of humans who consumed contaminated Great Lakes
fish: An epidemiologic consideration. J Toxicol Environ Health,
33: 587-639.
Swerdlow AJ, Douglas AJ, Vaughan Hudson G, Vaughan Hudson B, Bennett
MH, & MacLennan KA (1992) Risk of second primary cancers after
Hodgkin's disease by type of treatment: Analysis of 2846 patients in
the British National Lymphoma Investigation. Br Med J, 304: 1137-1143.
Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR,
Heroux AL, Dizikes GJ, Pifarre R, & Fisher RI (1990) Increased
incidence of lymphoproliferative disorder after immunosuppression with
the monoclonal antibody OKT3 in cardiac-transplant recipient. New Engl
J Med, 323: 1723-1728.
Szakal AK, Kosco MH, & Tew JG (1989) Microanatomy of lymphoid tissue
during humoral immune responses: Structure function relationships. Ann
Rev Immunol, 7: 91-109.
Taga T, & Kishimoto T (1992) Cytokine receptors and signal
transduction. FASEB J, 6: 3387-3396.
Takeya U, Shimotori S, Tanaguchi T, & Nomoto K (1977) Cellular
mechanisms in the protection against infection by Listeria
monocytogenesin mice. J Gen Microbiol, 100: 373-379.
Talcott PA & Koller LD (1983) The effect of inorganic lead and/or a
polychlorinated biphenyl on the developing immune system of mice. J
Toxicol Environ Health, 12: 337-352.
Talcott PA, Koller LD, & Exon JH (1985) The effect of lead and
polychlorinated biphenyl exposure on rat natural killer cell
cytotoxicity. Int J Immunopharmacol, 7: 255-261.
Taylor BA (1972) Genetic relationships between inbred strains of mice.
J Hered, 63: 83-86.
Taylor JR, Schmieder GF, Shimizu T, Tie C, & Streilin JW (1994)
Interrelationship between ultraviolet light and recurrent herpes
simplex infections in man. J Dermatol Sci, 8: 224-232.
Temple L, Kawabata TT, Munson AE, & White KL (1993) Comparison of
ELISA and plaque-forming cell assays for measuring the humoral immune
response to SRBC in rats and mice treated with benzo[ a]pyrene or
cyclophosphamide. Fundam Appl Toxicol, 21: 412-419.
Theofilopoulos AN & Dixon FJ (1985) Murine models of systemic lupus
erythematosus. Adv Immunol, 37: 269-390.
Thiem PA, Halper LK, & Bloom JC (1988) Techniques for assessing canine
mononuclear phagocytic function as part of an immunotoxicologic
evaluation. Int J Immunopharmacol, 10: 765-771.
Thomas PT & Hinsdill RD (1978) Effect of polychlorinated biphenyls on
the immune responses of rhesus monkeys and mice. Toxicol Appl
Pharmacol, 44: 41-51.
Thomas PT & Hinsdill RD (1979) The effect of perinatal exposure to
tetrachlorodibenzo- p-dioxin on the immune response of young mice.
Drug Chem Toxicol, 2: 77-98.
Thomas PT, Ratajczak HV, Aranyi C, Gibbons R, & Fenters JD (1985a)
Evaluation of host resistance and immune function in cadmium-exposed
mice. Toxicol Appl Pharmacol, 80: 446-456.
Thomas PT, Fugmann R, Aranyi C, Barbera P, Gibbons R, & Fenters J
(1985b) Effect of dimethylnitrosamine on host resistance and immunity.
Toxicol Appl Pharmacol, 77: 219-229.
Thomas PT, Ratajczak HV, Eisenberg WC, Furedi-Machacek M, Ketels K, &
Barbera PW (1987) Evaluation of host resistance and immunity in mice
exposed to the carbamate pesticide aldicarb. Fundam Appl Toxicol,
9: 82-89.
Thomson AW (1992) The spectrum of action of new immunosuppressive
drugs. Clin Exp Immunol, 89: 170-173.
Thurmond LM, Lauer LD, House RV, Cook JC, & Dean JH (1987) Immuno-
suppression following exposure to 7,12-dimethylbenz[ a]anthracene
(DMBA) in Ah-responsive and Ah-nonresponsive mice. Toxicol
Appl Pharmacol, 91: 450-460.
Tieben LM, Berkhout RJM, Smits HL, Bouwes Bavinck JN, Vermeer BJ,
Bruijn JA, van der Woude FJ, & ter Schegget J (1994) Detection of
epidermodysplasia verruciformis-like human papillomavirus types in
renal allograft recipients. Br J Dermatol, 131: 226-230.
Tilney NL (1971) Patterns of lymphatic drainage in the adult
laboratory rat. J Anat, 109: 369-383.
Timonen T, Ortaldo JR, & Herberman RB (1981) Characteristics of human
large granular lymphocytes and relationship to natural killer and K
cells. J Exp Med, 153: 569-582.
Timonen T, Ortaldo JR, & Herberman RB (1982) Isolation of human and
rat natural killer cells. J Immunol Meth, 51: 269-277.
Toews GB, Bergstresser PR, Streilein JW, & Sullivan S (1980) Epidermal
Langerhans cell density determines whether contact hypersensitivity or
unresponsiveness follows skin painting with DNFB. J Immunol,
124: 445-453.
Tomlinson S (1993) Complement defense mechanisms. Curr Opin Immunol,
5: 83-89.
Tonari Y, & Minamishima Y (1983) Pathogenicity and immunogenicity of
temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol,
64: 1983-1990.
Tracey DE, Walfe SA, Durdik JM ,& Henney CS (1977) BCG-induced murine
effector cells; cytolytic activity in peritoneal exudates: An early
response to BCG. J Immunol, 119: 1145-1151.
Truelove J, Grant D, Mes J, Tryphonas H, Tryphonas L, & Zawidzka Z
(1982) Polychlorinated biphenyl toxicity in the pregnant cynomolgus
monkey: A pilot study. Arch Environ Contam Toxicol, 11: 583-588.
Tryphonas H (in press) Immunotoxicity of PCBs (Aroclors) in relation
to Great Lakes. Environ Health Perspectives, Suppl 9.
Tryphonas H, Hayward S, O'Grady L, Loo JCK, Arnold DL, Bryce F, &
Zawidzka ZZ (1989) Immunotoxicity studies of PCB (Aroclor 1254) in the
adult rhesus (Macaca mulatta) monkey--Preliminary report. Int J
Immunopharmacol, 11: 199-206.
Tryphonas H, Luster MI, White KL, Naylor PH, Erdos MR, Burleson GR,
Germolec D, Hodgen M, Hayward S, & Arnold DL (1991a) Effects of PCB
(Aroclor 1254) on non-specific immune parameters in rhesus (Macaca
mulatta) monkeys. Int J Immunopharmacol, 13: 639-648.
Tryphonas H, Luster MI, Schiffman G, Dawson L-L, Hodgen M, Germolec D,
Hayward S, Bryce, F, Loo JCK, Mandy F, & Arnold DL (1991b) Effects of
chronic exposure of PCB (Aroclor 1254) on specific and non-specific
immune parameters in the rhesus (Macaca mulatta) monkey. Fundam Appl
Toxicol, 16: 773-786.
Tsang PH, Chu FN, Fischbein A, & Bekesi JG (1988) Impairments in
functional subsets of T-suppressor (CD8) lymphocytes, monocytes, and
natural killer cells among asbestos-exposed workers. Clin Immunol
Immunopathol, 47: 323-332.
Tschöpe D, Roesen B, Scwippert B, Kehrel B, Scauseil S, Esser J, &
Gries FA (1990) Platelet analysis using flow cytometry procedures.
Platelets, 1: 127-133.
Tucker AN & Munson AE (1981) In vitro inhibition of the primary
antibody response to sheep erythrocytes by cyclophosphamide. Toxicol
Appl Pharmacol, 59: 617-619.
Tucker AN, Hong L, Boorman GA, Pung O, & Luster MI (1985) Alteration
of bone marrow cell cycle kinetics by diphenylhydantoin: Relationship
to folate utilization and immune function. J Pharmacol Exp Ther,
234: 57-62.
Tucker AN, Vore SJ, & Luster MI (1986) Suppression of B cell
differentiation by 2,3,7,8-tetrachlorodibenzo- p-dioxin. Mol
Pharmacol, 29: 372-377.
Uber CL & McReynolds RA (1982) Immunotoxicology of silica. CRC Crit
Rev Toxicol, 10: 303-319.
Umesaki Y, Setoyama H, Matsumoto S, & Okada Y (1993) Expansion of
alpha ß T-cell receptor-bearing intestinal intraepithelial lymphocytes
after microbial colonization in germ-free mice and its independence
from thymus. Immunology, 79: 32-37.
Urban JL, Burton RC, Holland JM, Kripke ML, & Schreiber H (1982)
Mechanisms of syngeneic tumor rejection, susceptibility of host-
selected progressor variants to various immunological effector cells.
J Exp Med, 155: 557-573.
US Environmental Protection Agency (1986) Guidelines for the health
assessment of suspect developmental toxicants. Fed Regist,
51: 34027-34040.
US Environmental Protection Agency (1990) Respiratory health effects
of passive smoking: Lung cancer and other disorders
(EPA/600/6-90/006F). Cincinnati, Ohio, Center for Environmental
Research Information.
US Environmental Protection Agency (1991) Workshop report on toxicity
equivalency factors for polychlorinated biphenyl congeners. Washington
DC, Risk Assessment Forum.
US Food and Drug Administration (1993) Draft toxicological principles
for the safety assessment of direct food additives and color additives
used in food ('Redbook II'). Washington DC, Center for Food Safety and
Applied Nutrition.
US National Academy of Sciences (1977) Committee for the revision of
NAS publication No. 1138: Principles and procedures for evaluating the
toxicity of household substances. Washington DC.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Washington DC, National Academy
Press, pp 1-50.
US National Research Council (1989) Biologic markers in pulmonary
toxicology. Washington DC, National Academy Press.
US National Research Council (1992) Biologic markers in
immunotoxicology. Washington DC, National Academy Press.
US Office of Technology Assessment (1991) Identifying and controlling
immunotoxic substances: Background paper (OTA-BP-BA-75). Washington
DC, Congress of the United States, US Government Printing Office.
Vaessen LMB, Joling P, Nieuwenhuis P, Van Haperen MJ, & Rozing J
(1985) T Cell markers on pre-B cells in the rat. Adv Exp Med Biol,
186: 17-26.
Vallejo AN, Miller NW, Jorgensen T, & Clem LW (1990) Phylogeny of
immune recognition: Antigen processing/presentation in channel
catfish; immune responses to hemocyanins. Cell Immunol, 130: 364-377.
Vallejo AN, Miller NW, & Clem LW (1992) Antigen processing and
presentation in teleost immune responses. Ann Rev Fish Dis,
2: 167-183.
Valli VE, Villeneuve DC, Reed B, Barsoum N, & Smith G (1990)
Evaluation of blood and bone marrow, rat. In: Jones TC, Ward JM, Mohr
U, & Hunt RD ed. Hemopoietic system (ILSI Monograph). Berlin, Springer
Verlag, pp 9-26.
Van Bressem MF, Visser IKG, Van de Bildt MWG, Teppema JS, Raga JA, &
Osterhaus ADME (1991) Morbillivirus infection in Mediterranean striped
dolphins (Stenella coeruleoalba). Vet Rec, 129: 471-472.
Vandekerckhove BAE, Krowka JF, McCune JM, De Vries JE, Spits H, &
Roncarolo MG (1991) Clonal analysis of the peripheral T cell
compartment of the SCID-hu mouse. J Immunol, 146: 4173-4179.
Van den Eertwegh AJM, Boersma WJA, & Claassen E (1992) Immunological
functions and in vivo cell-cell interactions of T cells in the
spleen. Crit Rev Immunol, 11: 337-380.
Van Deuren M, Dofferhoff AS, & Van der Meer JW (1992) Cytokines and
the response to infection. J Pathol, 168: 349-356.
Van Diepen JCE, Wagenaar GTM, & Rombout JHWM (1991) Immunocytochemical
detection of membrane antigens of carp leukocytes using light and
electron microscopy. Fish Shellfish Immunol, 1: 47-57.
Van Ewijk W (1988) Cell surface topography of thymic microenvironments.
Lab Invest, 59: 579-590.
Van Ewijk W (1991) T-Cell differentiation is influenced by thymic
microenvironments. Ann Rev Immunol, 9: 591-615.
Van Logten MJ, Gupta BN, McConnell EE, & Moore JA (1981) The influence
of malnutrition on the toxicity of tetrachlorodibenzo- p-dioxin
(TCDD) in rats. Toxicology, 21: 77-88.
Van Loveren H & Vos JG (1989) Immunotoxicological considerations: A
practical approach to immunotoxicity testing in the rat. In: Dayan AD
& Paine AJ ed. Advances in applied toxicology. London, Taylor &
Francis, pp 143-165.
Van Loveren H & Vos JG (1992) Evaluation of OECD guideline # 407 for
assessment of toxicity of chemicals with respect to potential adverse
effects to the immune system (RIVM report no. 158801001). Bilthoven,
National Institute of Public Health and Environmental Protection.
Van Loveren H, Postma GW, Van Soolingen D, Kruizinga W, Groothuis D, &
Vos JG (1987) Enhanced macrophage activity and deficient acquired
resistance to Listeria monocytogenes in nude rats. In: Rygaard J,
Brunner N, Graem N, & Spang-Thomson M ed. Immune-deficient animals in
biomedical research. Basel, Karger.
Van Loveren H, Rombout PJA, Wagenaar SS, Walvoort HC, & Vos JG (1988a)
Effects of ozone on the defence to a respiratory Listeria
monocytogenes infection in the rat. Suppression of macrophage
function and cellular immunity and aggravation of histopathology in
lung and liver during infection. Toxicol Appl Pharmacol, 94: 374-393.
Van Loveren H, Osterhaus ADME, Nagel J, Schurrman HJ, & Vos JG (1988b)
Quantification of IgA responses in the rat. Detection of IgA
antibodies and enumeration of IgA antibody producing cells specific
for ovalbumin or Trichinella spiralis. Scand J Immunol, 28: 377-381.
Van Loveren H, Teppema JS, & Askenase PW (1990a) Skin mast cells.
In: Bos J ed. The skin immune system. Boca Raton, Florida, CRC Press,
pp 171-193.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, & Vos JG (1990b)
Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Loveren H, Van Eden W, Krajnc EI, Krajnc-Franken MAM, De Kort W, &
Vos JG (1990c) Hexachlorobenzene interferes with the development of
experimental autoimmune disease in the Lewis rat. Toxicologist,
10: 221.
Van Loveren H, Verlaan APJ, & Vos JG (1991) An enzyme-linked
immunosorbent assay of anti-sheep red blood cell antibodies of the
classes M, G and A in the rat. Int J Immunopharmacol, 13: 689-695.
Van Loveren H, Timmerman H, Dortant P, & De Waal E (1993a) Validation
of a screening battery for immunotoxicity of drugs within a 28 day
oral toxicity study, using cyclosporin as a model compound (RIVM
report no. 312620001). Bilthoven, National Institute of Public Health
and Environmental Protection.
Van Loveren H Rombout PJA, Fischer P, Lebret E, & Van Bree L (1993b)
Effects of oxidant air pollution on resistance to respiratory
infection, a review (RIVM report no. 619002003). Bilthoven, National
Institute of Public Health and Environmental Protection.
Van Loveren H, Luebke RL, & Vos JG (1994) Assessment of immunotoxicity
using the host infectivity model Trichinella spiralis. In: Burleson
G, Munson A, & Dean J ed. Methods in immunotoxicology. Chicago,
Wiley-Liss.
Van Muiswinkel WB, Lamers CHJ, & Rombout JHWM (1991) Structural and
functional aspects of the spleen in bony fish. Res Immunol,
142: 362-366.
Van Rooijen N, Claassen E, Kraal G, & Dijkstra CD (1989) Cytological
basis of immune functions of the spleen. Immunocytochemical
characterization of lymphoid and non-lymphoid cells involved in the
'in situ' immune response. Progr Histochem Cytochem, 19: 1-71.
Van Soolingen D, Moolenbeek C, & Van Loveren H (1990) An improved
method of bronchoalveolar lavage of lungs of small laboratory animals:
Short report. Lab Anim, 24: 197-199.
Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, & Garattini S
(1980) Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo-
p-dioxin on humoral antibody production. Chem-Biol Interactions,
30: 337-342.
Vecchi A, Sironi M, Canegrail MA, Recchia M, & Garattini S (1983)
Immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo- p-dioxin in
strains of mice with different susceptibility to induction of aryl
hydrocarbon hydroxylase. Toxicol Appl Pharmacol, 68: 434-441.
Vercelli D & Geha RS (1992) Regulation of isotype switching. Curr Opin
Immunol, 4: 794-797.
Vermeer M, Schmieder GJ, Yoshikawa T, Van Den Berg J-W, Metzman MS,
Taylor JR, & Streilin JW (1991) Effects of ultraviolet B light on
cutaneous immune responses of humans with deeply pigmented skin. J
Invest Dermatol, 97: 729-734.
Versluis DJ, Bijma AM, Vaessen LMB, & Weimar W (1989) Changes in
immunological parameters after conversion from cyclosporin A to
azathioprine in renal transplant recipients. Int J Immunopharmacol,
11: 157-164.
Vethaak D (1993) A large-scale mesocosm study of the effects of marine
pollution on disease development in flounder (Platichthys flesus).
In: Fish disease and marine pollution: A case study of the flounder
(Platichthys flesus) in Dutch coastal and estuarine waters. Thesis
(Meetkundige Dienst RWS, ISBN 90-369-0413-7), Amsterdam, University of
Amsterdam, pp 107-127.
Vethaak AD & ap Rheinallt T (1992) Fish disease as a monitor for
marine pollution: The case of the North Sea. Rev Fish Biol Fish,
2: 1-32.
Vethaak AD & Wester PW (1993) Diseases of flounder (Platichthys
flesus) in Dutch coastal waters, with particular reference to
environmental stress factors. Part II. Liver histopathology. In: Fish
disease and marine pollution, Thesis (Meetkundige Dienst RWS, ISBN
90-369-0413-7). Amsterdam, University of Amsterdam, pp 60-78.
Vetvicka V & Fornusek L (1992) Macrophages. In: Fornusek L & Vetvicka
V ed. Immune system accessory cells. Boca Raton, Florida, CRC Press,
pp 95-108.
Vial T & Descotes J (1992) Clinical toxicity of interleukin-2. Drug
Saf, 7: 417-433.
Vial T & Descotes J (1994) Clinical toxicity of the interferons. Drug
Saf, 10: 115-150.
Vireligier J (1975) Host defenses against influenza virus: The role of
anti-hemagglutinin antibody. J Immunol, 115: 434-439.
Von Boehmer H (1990) Developmental biology of T cells in T cell-
receptor transgenic mice. Ann Rev Immunol, 8: 531-536.
Von Gaudecker B (1986) The development of the human thymus
microenvironment. Curr Topics Pathol, 75: 1-41.
Von Gaudecker B (1991) Functional histology of the thymus. Anat
Embryol, 183: 1-15.
Von Mutius E, Fritzsch C, Weiland SK, Roll G, & Magnussen H (1992)
Prevalence of asthma and allergic disorders among children in united
Germany. Br Med J, 305: 1395-1399.
Vos JG (1977) Immune suppression as related to toxicology. CRC Crit
Rev Toxicol, 5: 67-101.
Vos JG (1980) Immunotoxicity assessment: Screening and function
studies. Arch Toxicol, 4 (Suppl): 95-108.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an international
symposium (IARC Scientific Publications No 77). Lyon, International
Agency for Research on Cancer, pp 347-356.
Vos JG & Beems RB (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits. Toxicol
Appl Pharmacol, 19: 617-633.
Vos JG & Koeman TH (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis, and tissue residues.
Toxicol Appl Pharmacol, 17: 656-668.
Vos JG & Krajnc EI (1983) Immunotoxicology of pesticides. In: Hayes
AW, Schnell RC, & Miya TS ed. Developments in the science and practice
of toxicology. Amsterdam, Elsevier Science Publishers, pp 229-240.
Vos JG & Krajnc-Franken MAM (1990) Toxic effects on the immune system:
Rat. In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system.
Berlin, Springer Verlag, pp 168-181.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Elsevier Science
Publishers, pp 295-322.
Vos JG & Moore JA (1974) Suppression of cellular immunity in rats and
mice by maternal treatment with 2,3,7,8-tetrachlorobenzo- p-dioxin.
Int Arch Allergy Appl Immunol, 47: 777-794.
Vos JG & Van Driel-Grootenhuis, L (1972) PCB-induced suppression of
the humoral and cell-mediated immunity in guinea pigs. Sci Total
Environ, 1: 289-302.
Vos JG & Van Genderen H (1973) Toxicological aspects of
immunosuppression. In: Pesticides and the environment: A continuing
controversy. North Miami, Florida, Symposia Specialists, pp 527-545.
Vos JG & Van Loveren H (1987) Immunotoxicity testing in the rat. In:
Burger EJ, Tardiff RG, & Bellanti JA ed. Advances in modern
environmental toxicology, Vol 13: Environmental chemical exposures and
immune system integrity. Princeton, New Jersey, Princeton Scientific
Publishing, pp 167-180.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979a) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Buys J, Hanstede JG, & Hagenaars AM (1979b) Comparison of
enzyme-linked immunosorbent assay and passive haemagglutination method
for quantification of antibodies to lipopolysaccharide and tetanus
toxoid in rats. Infect Immun, 24: 798-803.
Vos JG, Boerkamp J, Buys J, & Steerenberg PA (1980) Ovalbumin immunity
in the rat: Simultaneous testing of IgM, IgG and IgE response measured
by ELISA and delayed-type hypersensitivity. Scand J Immunol,
12: 289-295.
Vos JG, Krajnc EI, & Beekhof P (1982) Use of the enzyme-linked
immunosorbent assy (elisa) in immunotoxicity testing. Environ Health
Perspectives, 43: 115-121.
Vos JG, Krajnc EI, Beekhof PK, & Van Logten MJ (1983a) Methods for
testing immune effects of toxic chemicals: Evaluation of the
immunotoxicity of various pesticides in the rat. In: Miyamoto T ed.
IUPAC pesticide chemistry: Human welfare and the environment. Oxford,
Pergamon Press, pp 497-504.
Vos JG, Ruitenberg EJ, Van Basten N, Buys J, Elgersma A, & Kruizinga W
(1983b) The athymic nude rat. IV. Immunocytochemical study to detect
T cells, and immunological and histopathological reactions against
Trichinella spiralis. Parasite Immunol, 5: 195-215.
Vos JG, De Klerk A, Krajnc EI, Kruizinga W, Van Ommen B, & Rozing J
(1984) Toxicity of bis(tri-n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicol Appl
Pharmacol, 75: 387-408.
Vos JG, Van Loveren H, Wester P, & Vethaak D (1989) Toxic effects of
environmental chemicals on the immune system. TiPs, 10: 289-292.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rozing J (1990a)
Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on
thymus-dependent-immunity and non-specific resistance following long-
term exposure in young versus aged rats. Toxicol Appl Pharmacol,
105: 144-155.
Vos JG, Dormans JAMA, Kraal G, Krajnc EI, Krajnc-Franken MAM, & Van
Loveren H (1990b) Immunotoxicity of hexachlorobenzene in the rat is
thymus-dependent. Toxicologist, 10: 221.
Vos JG, Van Loveren H, & Schuurman H-J (1991) Immunotoxicity of
dioxin: Immune function and host resistance in laboratory animals and
humans. In: Biological basis for risk assessment of dioxins and
related compounds (Banbury report 35). Cold Spring Harbor, New York,
CSH Press, pp 79-93.
Wakelin D (1993) Allergic inflammation as a hypothesis for the
expulsion of worms from tissues. Parasitol Today, 9: 115-116.
Waldman TA (1988) Immunodeficiency diseases: Primary and acquired.
In: Immunological diseases. Boston, Little Brown Co, pp 441-466.
Ward JM (1990) Classification of reactive lesions of lymph nodes.
In: Jones TC, Ward JM, Mohr U, & Hunt RD ed. Hemopoietic system (ILSI
Monograph). Berlin, Springer Verlag, pp 155-162.
Ward P & Kendall MD (1975) Morphological changes in the thymus of
young and adult red-billed queleas Quelea quelea (aves). Phil Trans
R Soc Lond B, 273: 55-64.
Ward EC, Murray MJ, & Dean JH (1985) Immunotoxicity of nonhalogenated
polycyclic aromatic hydrocarbons. In: Dean JH, Luster MI, Munson AE, &
Amos H ed. Immunotoxicology and immunopharmacology. New York, Raven
Press, pp 291-304.
Ware JH, Dockery DW, Spiro A, Speizer FE, & Ferris BG (1984) Passive
smoking, gas cooking, and respiratory health of children living in six
cities. Am Rev Respir Dis, 129: 366-374.
Watson RR (1990) Drugs of abuse and immune function. Boca Raton,
Florida, CRC Press.
Watson RR (1993) Alcohol, drugs of abuse and immunomodulation
(Advances in the Biosciences, Vol 86). Oxford, Pergamon Press.
Weeks BA & Warinner JE (1986) Functional evaluation of macrophages in
fish from a polluted estuary. Vet Immunol Immunopathol, 12: 313-320.
Weeks BA, Anderson DP, Dufour AP, Fairbrother A, Goven AJ, Lahvis GP,
& Peters G (1992) Immunological biomarkers to assess environmental
stress. In: Biomarkers, biochemical, physiological and histological
markers of anthropo-genic stress. London, Lewis Publishers, pp 5-85.
Weigent DA & Blalock JE (1987) Interactions between the neuroendocrine
and immune systems: Common hormones and receptors. Immunol Rev,
100: 79-108.
Weiss L & Geduldig U (1991) Barrier cells: Stromal regulation of
hematopoiesis and blood release in normal and stressed murine bone
marrow. Blood, 78: 975-990.
Weiss L & Sakai H (1984) The hematopoietic stroma. Am J Anat,
170: 447-463.
Weissgarten J, Freidman CNGE, & Cardella CJ (1989) B Lymphocytes from
stable renal transplant patients do not respond to exogenous
lymphokines: Comparison of azathioprine and cyclosporin treated
patients. Transplant Proc, 21: 393-394.
Weissler JC, Nicod LP, & Toews GB (1987) Pulmonary natural killer cell
activity is reduced in patients with bronchogenic carcinoma. Am Rev
Respir Dis, 135: 1353-1357.
Wessel G, Abenroth K, & Wisheit M (1988) Malignant transformation
during immunosuppressive therapy (azathioprine) of rheumatoid
arthritis and systemic lupus erythematosus. Scand J Rheumatol, 67
(Suppl): 73-75.
Wester PW, & Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to bis(tri- n-
butyltin)oxide (TBTO) and di- n-butyltindichloride (DBTC). Aquat
Toxicol 10: 143-165.
Wester PW & Canton JH (1991) The usefulness of histopathology in
aquatic toxicity studies. Comp Biochem Physiol, 100C: 115-117.
Wester PW, Canton JH, Van Iersel AAJ, Krajnc EI ,& Vaessen HAMG
(1990) The toxicity of bis(tri- n-butyltin)oxide (TBTO) and
di- n-butyltindichloride (DBTC) in the small fish species Oryzias
latipes (medaka) and Poecilia reticulata (guppy). Aquat Toxicol,
16: 53-72.
Wester PW, Vethaak AD, & Van Muiswinkel WB (1994) Fish as biomarkers
in immunotoxicology. Toxicology, 86: 213-232.
White KL (1986) An overview of immunotoxicology and carcinogenic
polycyclic aromatic hydrocarbons. J Environ Health Sci, C4: 163-202.
White KL (1992) Specific immune function assays. In: Miller K, Turk J,
& Nicklin S ed. Principles and practice of immunotoxicology. Oxford,
Blackwell Scientific Publications, pp 304-323.
White KL & Munson AE (1986) Suppression of the in vitro humoral
immune response by chrysotile asbestos. Toxicol Appl Pharmacol,
82: 493-504.
White KL, Sander VM, Barnes DW, Shopp GM, & Munson AE (1985)
Immunotoxicological investigations in the mouse: General approach and
methods. Drug Chem Toxicol, 8: 299-331.
White KL, Lysy HH, McCay JA, & Anderson AC (1986) Modulation of serum
complement levels following exposure to polychlorinated dibenzo- p-
dioxins. Toxicol Appl Pharmacol, 84: 209-219.
White KL, Gennings C, Murray MJ, & Dean JH (1994) Summary of
international methods: Validation study, carried out in nine
laboratories, on immunological assessment of cyclosporin A in the rat.
In Vitro Toxicity, 8: 957-961.
WHO (1990) Revised guidelines for preparation of Environmental Health
Criteria monographs (PCS/90.69), Geneva
Wierda D, Irons RD, & Greenlee WF (1981) Immunotoxicity in C57Bl/6
mice exposed to benzene and Aroclor 1254. Toxicol Appl Pharmacol,
60: 410-417.
Williams ME & Quesenberry PJ (1992) Hematopoietic growth factors.
Hematol Pathol, 6: 105-124.
Wilson MR & Warr GW (1992) Fish immunoglobulins and the genes that
encode them. Ann Rev Fish Dis, 201-221.
Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, & Steinberg C
(1992) What limits affinity maturation of antibodies in Xenopus: The
rate of somatic mutation or the ability to select mutants? EMBO J,
11: 4337-4347.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity in
mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20: 423.
Winkelstein JA (1981) The role of complement in the host defense
against Streptococcus pneumoniae. Rev Infect Dis, 3: 289-298.
Wolke RE (1992) Piscine macrophage aggregates: A review. Ann Rev Fish
Dis, 91-108.
Wood SC, Karras JG, & Holsapple MP (1992) Integration of the human
lymphocyte into immunotoxicological investigations. Fundam Appl
Toxicol, 18: 450-459.
Wooley PH (1991) Animal models of rheumatoid arthritis. Curr Opin
Rheumatol, 3: 407-420.
Wright EG & Pragnell IB (1992) Stem cell proliferation inhibitors.
Bailliere's Clin Haematol, 5: 723-739.
Yamaguchi T, Shinagawal Y, & Pollard RB (1988) Relationship between
the production of murine cytomegalovirus and interferon in
macrophages. J Gen Virol, 69: 2961-2971.
Yang KH, Kim BS, Munson AE, & Holsapple MP (1986) Immunosuppression
induced by chemicals requiring metabolic activation in mixed cultures
of rat hepatocytes and murine splenocytes. Toxicol Appl Pharmacol,
83: 420-429.
Yang YG, Lebrec H, & Burleson GR (1994) Effect of 2,3,7,8
tetrachlorodibenzo- p-dioxin (TCDD) on viral replication and natural
killer (NK) activity in rats. Fundam Appl Toxicol, 23: 125-131.
Yang YG, Gilmour MI, Lange R, Burleson GR, & Selgrade MJK (1995)
Effects of acute exposure to phosgene on pulmonary host defenses and
resistance to infection. Inhal Toxicol, 7: 393-404.
Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, & Santos GW
(1993) Cyclosporine-induced graft-versus-host disease after autologous
bone marrow transplantation for acute myeloid leukemia. Leukemia
Lymphoma, 11: 215-220.
Yoshida T & Streilin JW (1990) On the genetic basis of the effects of
ultraviolet B on cutaneous immunity. Evidence that polymorphisms at
the TNFa and Lps loci govern susceptibility. Immunogenetics,
32: 398-405.
Yoshikawa T, Rae V, Bruins-Slot W, Van Den Berg J-W, Taylor JR, &
Streilin JW (1990) Susceptibility to effects of UVB radiation on
induction of contact hypersensitivity as a risk factor for skin cancer
in humans. J Invest Dermatol, 95: 530-536.
Yosioka T, Sato S, Fujiwana H, & Hamacka T (1986) The role of GM-1
antibody-sensitive cells in the implementation of tumor-specific
T-cell mediated immunity in vivo. Jpn J Cancer Res, 77: 825-832.
Zapata A (1982) Lymphoid organs of teleost fish III. Splenic lymphoid
tissue of Rutilus rutilus and Gobio gobio. Dev Comp Immunol,
6: 87-94.
Zapata A (1983) Phylogeny of the fish immune system. Bull Inst
Pasteur, 81: 185-186.
Zapata AG & Cooper EL (1990) The immune system: Comparative
histophysiology. New York, John Wiley & Sons.
Zapata AG, Varas A, & Torroba M (1992) Seasonal variations in the
immune system of lower vertebrates. Immunol Today, 13: 142-147.
Zeeman MG & Brindley WA (1981) Effects of toxic agents upon fish
immune systems: A review. In: Sharma RP, ed. Immunologic
considerations in toxicology, Vol II. Boca Raton, Florida, CRC Press,
pp 1-60.
Zelikoff JT, Enane NA, Bowser D, Squibb KS, & Frenkel K (1991)
Development of fish peritoneal macrophages as a model for higher
vertebrates in immunotoxicological studies. Fundam Appl Toxicol,
16: 576-589.
Zheng B, Shorthouse R, Masek MA, Berry G, Billingham ME, & Morris RE
(1991) Effect of the new and highly active immunosuppressant,
Rapamycin, on lymphoid tissues and cells in vivo. Transplant Proc,
23: 851-855.
Zwick H, Popp W, Wagner C, Reiser K, Schmoger J, Bock A, Herkner K, &
Radunsky K (1991) Effects of ozone on the respiratory health allergic
sensitization, and cellular immune system in children. Am Rev Respir
Dis, 144: 1075-1079.
1. RESUME
1. Le système immunitaire a évolué de manière à contrer les
atteintes que peuvent porter à l'intégrité du soi des microorganismes
ou des cellules ayant échappé au contrôle de l'organisme. Une
intrusion xénobiotique peut perturber le fonctionnement du système
immunitaire et c'est la reconnaissance de cet état de choses qui a
permis les progrès de ces vingt dernières années. Toute une
méthodologie expérimentale a été développée et validée
(essentiellement sur des rongeurs) par des études impliquant une
multitude de laboratoires. La présente monographie examine les
fonctions et l'histophysiologie du système immunitaire et présente les
données nécessaires à la compréhension et à l'interprétation des
modifications pathologiques provoquées par les agressions
immmunotoxiques. L'accent est mis sur le système immunitaire de
l'homme et des rongeurs, mais sans négliger d'autres espèces, les
poissons en particulier, auxquelles des études immunotoxicologiques
ont été consacrées. Il importe, pour comprendre l'impact des effets
immunotoxiques, de connaître la pathophysiologie du système
immunitaire, et notamment la sensibilité variable de ses constituants,
les modifications subies par les organes lymphoïdes et la réversibilité
de ces modifications.
2. L'immunosuppression et l'immunostimulation ont toutes deux des
conséquences sur le plan clinique. On a établi une corrélation entre
les états d'immunodéficience ou d'immunodépression grave que l'on
observe par exemple chez des patients greffés ou sous traitement
cytostatique, et l'accroissement de l'incidence des maladies
infectieuses (opportunistes en particulier) et du cancer. L'exposition
à des substances chimiques immunotoxiques présentes dans l'environnement
peut toutefois donner lieu à des formes plus subtiles d'immunodépression,
difficiles à déceler, qui entraînent une augmentation de l'incidence de
maladies infectieuses comme la grippe ou le rhume. Des travaux effectués
sur des animaux de laboratoire et sur l'homme montrent que de nombreux
produits chimiques présents dans l'environnement dépriment la réponse
immunitaire. Ces substances xénobiotiques immunotoxiques ne se limitent
pas à untype particulier de composé chimique ou d'agent physique. Il y
a, parmi les médicaments, les pesticides, les solvants, les hydro-
carbures halogénés, les hydrocarbures aromatiques et les métaux, des
substances susceptibles d'altérer le système immunitaire et le
rayonnement ultra-violet a également cette propriété. L'administration,
à des fins thérapeutiques, d'agents immunostimulants, peut avoir des
effets nocifs et certaines substances (béryllium, silice, hexa-
chlorobenzène) présentes dans l'environnement, ont des propriétés
immunostimulantes qui peuvent se traduire par des effets cliniques.
3. Du fait de sa complexité, le système immunitaire offre une
multiplicité de cibles potentielles à l'action de ces agents avec
toutes sortes de séquelles pathologiques. Les premières stratégies
élaborées par les immunotoxicologues ont consisté à choisir et à
mettre en oeuvre une batterie de tests multiphasiques sur animaux de
laboratoire afin d'identifier les agents immunosuppresseurs ou
immunostimulants. Ces batteries de tests peuvent varier selon
l'organisme ou le laboratoire qui les mettent en oeuvre ou encore
selon l'espèce animale utilisée, mais elles comportent toutes un ou
plusieurs des éléments suivants: modification du poids ou de
l'histologie des organes lymphoïdes; examen des leucocytes du sang
périphérique, des cellules du tissu lymphoïde ou de la moelle osseuse
à la recherche de modifications; intégrité de la fonction des cellules
effectrices et régulatrices et modification éventuelle de la
sensibilité à une exposition à des agents infectieux ou à des cellules
tumorales.
La directive expérimentale originale No 407 de l'Organisation de
coopération et de développement économiques, publiée en 1981, n'avait
pas pour but la mise en évidence d'une immunotoxicité potentielle,
aussi a-t-elle été modifiée pour la rendre plus adaptée à
l'identification des substances immunotoxiques. Des systèmes d'épreuve
multiphasiques permettant une investigation plus large de
l'immunotoxicité ont été conçus par l'US National Toxicology Program,
l'Institut Néerlandais de la Santé Publique et de la Protection de
l'Environnement, l'US Environmental Protection Agency (Office of
Pesticides), ainsi que par le Center for Food Safety andApplied
Nutrition de l'US Food and Drug Administration. Des études ont été
effectuées sur des souris et, à un moindre degré, sur des rats afin
d'évaluer la spécificité, la précision (reproductibilité), la
sensibilité, l'exactitude et la pertinence, pour l'appréciation du
risque sanitaire, de diverses mesures de l'état immunitaire. On a
entrepris la validation interlaboratoires, au niveau international, de
toutes ces méthodes, dans le cadre de l'Etude collective
internationale sur l'immunotoxicité organisée par le PISC, l'Union
européenne et le Bundesinstitut für Gesundheitllichen
Verbraucherschutz und Veterinärmedizin. Des études analogues ont été
menées sur des rats Fischer 344 à propos de la cyclosporine A.
4. Les épreuves utilisées dans le système multiphasique sont
décrites à la section 3 avec les raisons qui ont guidé ce choix et un
exposé des difficultés que l'on peut rencontrer dans leur mise en
oeuvre. Bien que conçus à l'origine pour des études sur des rats et
des souris, certains de ces protocoles ont pu être utilisés avec fruit
pour des travaux d'immunotoxicologie portant sur d'autres espèces,
notamment des primates non humains, des mammifères marins, des chiens,
des oiseaux et des poissons.
Lorsqu'on se propose de déterminer dans quelle mesure un agent
environnemental ou un médicament est susceptible d'avoir une influence
nocive sur le système immunitaire, il faut prendre en considération un
certain nombre de facteurs. Il s'agit notamment du choix d'un modèle
animal et de variables d'exposition appropriés, de la prise en compte
des paramètres toxicologiques généraux, de la pertinence biologique
des points d'aboutissement retenus, de la mesure de grandeurs dûment
validées et de la mise en oeuvre d'un système d'assurance de la
qualité. Les conditions expérimentales doivent tenir compte des
modalités de l'exposition humaine (voie de pénétration et
concentration ou intensité) et de toute information disponible d'ordre
toxicodynamique ou toxicocinétique. Les doses et la taille des
échantillons doivent être choisies de manière à permettre l'obtention
de bonnes courbes dose-réponse et la détermination de la dose sans
effet (nocif ou non) observable. Cesstratégies sont améliorées en
permanence afin de permettre une meilleure prévision des situations
susceptibles d'entraîner une pathologie. En outre, on devrait pouvoir
disposer de techniques qui faciliteraient l'étude du mode d'action des
agents en cause. Il pourrait s'agir de méthodes in vitro, de l'étude
des réponses immunitaires locales (par exemple au niveau de la peau,
des poumons et de l'intestin), de techniques de biologie moléculaire
et de l'utilisation d'animaux génétiquement modifiés.
5. Mettre en évidence des modifications d'ordre immunologique après
une exposition à des composés potentiellement immunotoxiques est plus
complexe chez l'homme que chez l'animal de laboratoire. Les
possibilités d'expérimentation sont limitées, il est difficile
d'établir le niveau d'exposition à l'agent en cause (c'est-à-dire la
dose) et de plus, l'état immunitaire des populations est extrêmement
hétérogène. Cette hétérogénéité trouve son origine dans un certain
nombre de facteurs: âge, sexe, race, gravidité, stress et aptitude à y
faire face, pathologies et états infectieux cocomitants, état
nutritionnel, tabagisme et prise de certains médicaments. L'intérêt de
telle ou telle étude pour l'évaluation du risque est conditionné par
un élément important, à savoir le concept épidémiologique qui la sous-
tend. La plupart du temps on procède à une étude transversale, qui
consiste à déterminer l'exposition et la morbidité à un moment donné
ou sur une courte période. On compare ensuite la fonction immunitaire
des sujets exposés à celle d'un groupe analogue de sujets non exposés.
Ce genre d'étude peut receler un certain nombre de pièges.
Nombre des altérations que produit l'exposition à une substance
chimique ne se manifestent chez l'homme que de manière subtile et
sporadique, aussi faut-il étudier des populations récemment exposées
et utiliser des épreuves sensibles.
La plupart des épreuves concernant l'immunité spécifique (à
médiation cellulaire ou humorale), l'immunité non spécifique et les
processus inflammatoires ont été conçues pour rechercher des anomalies
chez des patients atteints d'une déficience immunitaire et ne sont pas
forcément capables de déceler les modifications subtiles provoquées
par les substances chimiques. Le PISC, les Centers for Disease Control
et l'Académie des Sciences des Etats-Unis ont, chacun de leur côté,
défini une méthodologie pour évaluer les modifications du système
immunitaire quipourraient résulter d'une exposition à des produits
immunotoxiques mais les épreuves proposées demandent encore à être
validées.
6. L'évaluation du risque consiste à analyser les données
pertinentes sur un agent donné (effets biologiques, relations dose-
réponse et exposition) pour tenter d'obtenir une estimation
qualitative et quantitative des diverses conséquences nocives de la
présence de cet agent. Elle se caractérise par quatre phases
principales: reconnaître le danger, établir la relation dose-réponse,
évaluer l'exposition et caractériser le risque. Jusqu'ici,
l'immunotoxicologie s'est essentiellement attachée à reconnaître le
danger et, dans une certaine mesure, à établir des relations dose-
réponse, mais très peu d'études ont été consacrées à l'évaluation du
risque ou à sa caractérisation.
Comme dans d'autres domaines de la toxicologie, des incertitudes
demeurent qui sont susceptibles de gêner l'interprétation des données
immunotoxicologiques dans l'optique du risque pour la santé humaine.
Les deux questions qui sont actuellement les plus problématiques -
l'extrapolation à l'organe dans son ensemble des effets constatés sur
la cellule et l'extrapolation à l'homme des données obtenues sur
l'animal - valent pour la plupart des points d'aboutissement des
effets, cancer excepté. Le premier problème tient aux incertitudes que
comporte l'établissement d'une relation quatitative entre les
modifications subies par la fonction immunitaire d'un individu et
l'altération de sa résistance aux maladies infectieuses et
néoplasiques. Le deuxième est lié à l'incertitude qui entache
l'évaluation du risque pour la santé humaine à partir des résultats
obtenus sur des animaux de laboratoire.
L'évaluation du risque a pour finalité de protéger la santé
humaine et l'environnement. Il faut donc que le choix des modéles
expérimentaux soit judicieux. La toxicocinétique de la substance
étudiée et la réponse immunitaire suscitée dans le modèle doivent
pouvoir être comparées à celles qu'on observerait chez l'homme.
Pour tirer une limite d'exposition des résultats expérimentaux,
il est convenu d'appliquer un facteur d'incertitude aux données
d'évaluation du risque. Cette convention ne tient pas compte de la
réserve fonctionnelle ou de la redondance du système immunitaire. Une
méthode plus récente d'évaluation du risque consiste à ultiliser des
modèles in vitro en complément aux études sur animaux de laboratoire.
Cette méthode a l'avantage de permettre une extrapolation plus fidèle
de l'animal à l'homme et de ne nécessiter qu'un minimum d'animaux.
Elle permet également de pallier l'absence de données dans les cas où
des considérations d'éthique limitent l'expérimentation sur l'homme.
Le Chapitre 6 donne deux exemples de situation où les données obtenues
in vitro permettent de lever en partie les incertitudes dans
l'évaluation du risque dû à l'exposition à l'ozone et au rayonnement
ultra-violet. L'utilisation des données immunotoxicologiques dans
l'évaluation du risque reste limitée par la difficulté d'établir des
relations quantitatives entre l'immunodépression et les manifestations
cliniques d'une pathologie donnée.
RESUMEN
1. El sistema inmunitario ha evolucionado para hacer frente a las
amenazas a la integridad del organismo vivo provenientes de
microorganismos o de células que han escapado a los mecanismos de
control del organismo. El reconocimiento de que las sustancias
xenobióticas pueden trastornar el funcionamiento del sistema
inmunitario ha llevado a avances en el campo de la inmunotoxicología
durante los dos últimos decenios. Se han formulado métodos
experimentales (empleando principalmente especies de roedores), que
han sido validados en estudios multilaboratorio. En esta monografía se
examinan la función y la histofisiología del sistema inmunitario,
presentándose la información necesaria para la comprensión e
interpretación de los cambios patológicos causados por las agresiones
inmunotóxicas. Si bien se hace hincapié en los sistemas inmunitarios
del ser humano y de las especies de roedores, se hace referencia a
otras especies, incluidos los peces, que han sido objeto de estudios
inmunotoxicológicos. La fisiopatología del sistema inmunitario,
incluidas la sensibilidad variable de sus componentes, las
alteraciones de los órganos linfoides y la reversibilidad de los
cambios, es importante para comprender las repercusiones de la
inmunotoxicidad.
2. Tanto la inmunosupresión como la inmunoestimulación tienen
consecuencias clínicas. Se ha observado que los estados de
inmunodeficiencia y de inmunosupresión grave, como los que se pueden
presentar en casos de trasplante y de terapia citostática, van
acompañados de mayor incidencia de enfermedades infecciosas
(especialmente las oportunistas) y de cáncer. Con todo, cabe prever
que la exposición a los productos químicos inmunotóxicos enel medio
ambiente dará origen a formas más sutiles de inmunosupresión cuya
detección podría resultar difícil, lo que se traduciría en una mayor
incidencia de infecciones tales como la gripe y el resfriado común.
Estudios realizados con animales de laboratorio y seres humanos han
mostrado que muchos de los productos químicos presentes en el medio
ambiente provocan la supresión de la respuesta inmunitaria. Las
sustancias xenobióticas inmunotóxicas no se limitan a una clase
determinada de productos químicos. Entre los compuestos que tienen
efectos nocivos para el sistema inmunitario se cuentan fármacos,
plaguicidas, disolventes, hidrocarburos halogenados y aromáticos y
metales; la radiación ultravioleta puede resultar también
inmunotóxica. La administración terapéutica de agentes
inmunoestimulantes puede provocar reacciones adversas; asimismo,
algunos de los productos químicos presentes en el medio ambiente que
poseen propiedades inmunoestimulantes (berilio, sílice,
hexaclorobenceno) pueden tener consecuencias clínicas.
3. La complejidad del sistema inmunitario lleva aparejadas
multiplicidad de posibles puntos vulnerables y secuelas patológicas.
Los métodos iniciales ideados por los inmunotoxicólogos que realizan
investigaciones sobre toxicología y evaluación de la inocuidad
consistían en seleccionar y aplicar una serie de valoraciones
escalonadas para identificar los agentes inmunosupresores e
inmunoestimulantes en animales de laboratorio. Si bien la
configuración de esas series de análisis puede variar según la
institución o el laboratorio en que se llevan a cabo, así como según
las especies de animales empleadas, en todas se mide unoo más de los
siguientes parámetros: alteraciones del peso y de la histología de los
órganos linfoides; cambios en la celularidad del tejido linfoide, de
los leucocitos en la sangre periférica y/o de la médula ósea;
trastornos de la función celular a nivel de los efectores o de la
regulación, y alteración de la sensibilidad a la amenaza que presentan
los agentes infecciosos o las células tumorales.
La Directriz de pruebas inicial No 407 de la Organización de
Cooperación y Desarrollo Económicos, publicada en 1981, no preveía la
detección de los riesgos de inmunotoxicidad, y se han propuesto
modificaciones destinadas a aumentar la utilidad de esa Directriz para
la identificación de las sustancias inmunotóxicas. El Programa
Nacional de Toxicología de los Estados Unidos, el Instituto Nacional
de Salud Pública y Protección del Medio Ambiente de los Países Bajos,
la Oficina de Plaguicidas de la Agencia para la Protección del Medio
Ambiente de los Estados Unidos y el Centro de Seguridad de los
Alimentos y Nutrición Aplicada de la Administración de Alimentos y
Medicamentos de los Estados Unidos han elaborado sistemas de pruebas
escalonadas para la investigación en mayor escala de los riesgos de
inmunotoxicidad. Se han realizado estudios con ratones, y en menor
medida con ratas, de diferentes indicadores del estado inmunológico
con el fin de determinar su especificidad, precisión (reproducibilidad),
sensibilidad, exactitud y pertinencia para la evaluación de los riesgos
para la salud humana. Los métodos han sido objeto de validaciones
internacionales interlaboratorios en el marco del Estudio Internacional
en Colaboración sobre Inmunotoxicidad del IPCS, la Unión Europea y el
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinärmedizin, yen estudios sobre la ciclosporina A en ratas
Fisher 344.
4. Las pruebas empleadas en los programas de verificaciones
escalonadas se describen en la Sección 3, en la que se indican la
razón de ser de su selección y las complejidades que entraña su
realización. Si bien esos protocolos fueron diseñados para estudios
realizados con ratas y ratones, algunos de ellos se han aplicado con
buenos resultados al estudio de la inmunotoxicidad en otras especies
animales, incluidos primates no humanos, mamíferos marinos, perros,
aves y peces.
A la hora de evaluar las posibles repercusiones negativas de un
agente ambiental o fármaco sobre el sistema inmunitario de los
animales experimentales deberán considerarse diversos factores. Entre
ellos cabe señalar: la selección de los modelos y las variables de
exposición apropiados para los animales, la inclusión de parámetros
toxicológicos generales, la comprensión de la importancia de los
parámetros objeto de medición, el empleo de medidas validadas y el
control de la calidad. En las condiciones experimentales deberán
tenerse en cuenta las vías y el nivel posibles de exposición del ser
humano, así como toda la información disponible sobre toxicodinámica y
toxicocinética. Las dosis y el tamaño de las muestras deberán
seleccionarse de manera que se puedan obtener curvas de dosis-
respuesta bien definidas, además del nivel sin efectos adversos
observados y del nivel sin efectos observados. Los métodos se
perfeccionan continuamente para poder predecir mejor las condiciones
que podrían ser causantes de enfermedades. Además, deberán elaborarse
técnicas que contribuyana la identificación de mecanismos de acción;
éstos podrían incluir los métodos in vitro, el examen de las
respuestas inmunitarias locales (por ejemplo, en la piel, los pulmones
y los intestinos), y el empleo de las técnicas de la biología
molecular y de animales modificados genéticamente.
5. La detección de los cambios inmunológicos ocurridos tras la
exposición a compuestos que podrían ser inmunotóxicos resulta más
complicada en el ser humano que en los animales de laboratorio. Las
posibilidades de realizar pruebas son limitadas; los niveles de
exposición al agente (es decir, la dosis) son difíciles de establecer
y el estado inmunitario de las poblaciones es sumamente heterogéneo.
La edad, la raza, el sexo, la gestación, el estrés agudo y la
capacidad para hacerle frente, las enfermedades e infecciones
coexistentes, el estado nutricional, el humo del tabaco y algunos
medicamentos se cuentan entre los factores que contribuyen a esa
heterogeneidad.
El diseño de los estudios epidemiológicos es un factor importante
para determinar la utilidad de un estudio determinado para la
evaluación de riesgos. El tipo de diseño empleado más frecuentemente
en materia de inmunotoxicidad son los estudios transversales, en los
que se miden las condiciones de exposición y el estado de la
enfermedad en un momento dado, o durante un periodo breve. A
continuación se compara la función inmunitaria de los sujetos
«expuestos» con la de un grupo comparable de individuos
«no expuestos». Ese tipo de diseño lleva aparejados posibles escollos.
Como muchos de los cambios observados en la respuesta inmunitaria
de los seres humanos tras su exposición a un productoquímico podrían
ser esporádicos y sutiles, habrá que estudiar las poblaciones que han
estado expuestas recientemente empleando pruebas de gran sensibilidad
para la evaluación del sistema inmunitario. Las conclusiones sobre los
efectos inmunotóxicos deberán estar basadas en las variaciones
detectadas no en un parámetro aislado sino en el perfil inmunológico
del individuo o de la población.
La mayoría de las pruebas existentes para determinar la inmunidad
específica (de base celular y humoral), la inmunidad no específica y
la inflamación han sido concebidas para detectar las alteraciones
inmunitarias en pacientes que padecen de inmunodeficiencia y no
siempre resultan adecuadas para detectar las alteraciones sutiles
provocadas por los productos químicos presentes en el medio ambiente.
El IPCS, los Centros de Control de Enfermedades y la Academia de
Ciencias de los Estados Unidos han descrito procedimientos para
evaluar los cambios que ocurren en el sistema inmunitario del ser
humano como consecuencia de la exposición a sustancias inmunotóxicas;
con todo, las pruebas descritas deberán ser evaluadas con este fin.
6. La evaluación de riesgos es un proceso en el que se analizan la
información pertinente sobre los efectos biológicos, las relaciones
dosis-respuesta y la exposición a un agente determinado con miras a
establecer estimaciones cualitativas y cuantitativas de los resultados
adversos. Por regla general, la evaluación de los riesgos supone
cuatro pasos fundamentales: identificación de los riesgos; evaluación
de la relación dosis-respuesta; evaluación de la exposición y
caracterización de los riesgos. Hasta ahora, lainmunotoxicología se ha
centrado principalmente en la identificación de los riesgos y, en
cierta medida, en la evaluación de las relaciones dosis-respuesta; muy
contados han sido los estudios que han incluido la evaluación de la
exposición o la caracterización de los riesgos.
Al igual que ocurre en otros campos de la toxicología, existen
incertidumbres que podrían afectar a la interpretación de los datos
sobre inmunotoxicidad en cuanto a los riesgos para la salud humana.
Las dos cuestiones más problemáticas - la extrapolación de los efectos
observados en células individuales a todo un órgano, o a niveles
superiores, y la extrapolación al ser humano de los resultados
obtenidos en experimentos con animales - son comunes a la mayoría de
los parámetros de valoración no relacionados con el cáncer. La primera
obedece a las incertidumbres vinculadas al establecimiento de una
relación cuantitativa entre los cambios observados en la función
inmunitaria del individuo y la perturbación de la resistencia a las
infecciones y enfermedades neoplásicas. La segunda cuestión es
consecuencia de las incertidumbres que lleva aparejadas la evaluación
de los riesgos para la salud humana basándose en los estudios
realizados con animales de laboratorio.
El objetivo fundamental de la evaluación de los riesgos es la
protección de la salud de los seres humanos y del medio ambiente. Por
lo tanto, deberán seleccionarse sistemas modelo idóneos. La
toxicocinética del material de prueba y la índole y magnitud de la
respuesta inmunitaria generada en el modelo deberán ser comparables a
la de los seres humanos.
Habitualmente, en la evaluación de los riesgos se emplean
factores empíricos de incertidumbre para determinar el límite de
exposición aceptable a partir de los resultados experimentales. Ese
procedimiento no toma en cuenta la reserva funcional ni la redundancia
del sistema inmunitario. Un adelanto más reciente en materia de
evaluación de riesgos es el empleo de modelos in vitro como
complemento de los estudios realizados con animales de laboratorio.
Ese procedimiento tiene la ventaja de que permite aumentar la
exactitud de la extrapolación al ser humano de los resultados
obtenidos en los experimentos realizados con animales, reduciendo al
mínimo el número de animales necesarios; asimismo, permite colmar la
brecha entre ambos tipos de información, sobre todo en los casos en
que los experimentos con seres humanos se ven limitados por
consideraciones de índole ética. En el capítulo 6 se presentan dos
ejemplos de cómo la información in vitro permite reducir las
incertidumbres en materia de evaluación de riesgos relacionadas con la
exposición al ozono y a la radiación ultravioleta. La dificultad para
establecer relaciones cuantitativas entre la inmunosupresión y las
enfermedades clínicas ha limitado el empleo de los datos
inmunotoxicológicos en la evaluación de los riesgos.
