
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 179
Morpholine
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. J. Kielhorn and Dr. G. Rosner, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Morpholine.
(Environmental health criteria ; 179)
1.Morpholine 2.Solvents 3.Chemical industry
4.Environmental exposure I.Series
ISBN 92 4 157179 9 (NLM Classification: TP 247.5)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Physical and chemical properties
1.2. Analytical methods
1.3. Sources of human and environmental exposure
1.4. Environmental transport, distribution and transformation
1.5. Environmental levels and human exposure
1.6. Kinetics and metabolism in laboratory animals and humans
1.7. Effects on laboratory mammals and in vitro test systems
1.8. Effects on humans
1.9. Effects on other organisms in the laboratory and field
1.10. Evaluation of human health risks and effects on the
environment
1.10.1. Evaluation of effects on human health
1.10.2. Evaluation of effects on the environment
1.11. Conclusions and recommendations
1.11.1. Recommendations for protection of human health
1.11.2. Recommendations for protection of the environment
1.11.3. Recommendations for further research
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Technical product
2.1.2. Impurities
2.2. Physical and chemical properties
2.2.1. Physical properties of morpholine
2.2.1.1 Storage of morpholine
2.2.2. Chemical properties of morpholine
2.3. Conversion factors for morpholine
2.4. Analytical methods
2.4.1. Determination of morpholine in air
2.4.2. Determination of morpholine in water
2.4.3. Determination of morpholine in soil and sediments
2.4.4. Determination in biological and other material
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.1.1 World producers
3.2.1.2 Production figures
3.2.1.3 Production processes
3.2.1.4 Losses to the environment during normal
production
3.2.1.5 Methods of transport
3.2.1.6 Accidental release
3.2.2. Uses
3.2.2.1 Rubber chemicals
3.2.2.2 Anticorrosion agent
3.2.2.3 Waxes and polishes
3.2.2.4 Optical brighteners
3.2.2.5 Catalysts
3.2.2.6 Pharmaceuticals
3.2.2.7 Bactericides, fungicides and herbicides
3.2.2.8 Food additive applications
3.2.2.9 Cosmetics
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Volatilization
4.2. Transformation
4.2.1. Biodegradation
4.2.1.1 Batch biodegradation tests
4.2.1.2 Biodegradation in laboratory-scale
wastewater treatment plants
4.2.2. Abiotic degradation
4.2.2.1 Hydrolytic degradation
4.2.2.2 Photochemical degradation
4.2.2.3 Degradation by physico-chemical
processes
4.2.3. Bioaccumulation
4.3. Interaction with other physical, chemical or biological
factors
4.4. Ultimate fate following use
4.4.1. Fate of morpholine in various products
4.4.2. Waste disposal
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Ambient air
5.1.2. Water
5.1.2.1 River water
5.1.2.2 Wastewater
5.1.3. Sediment
5.1.4. Soil
5.1.5. Terrestrial and aquatic organisms
5.2. General population exposure
5.2.1. Indoor air
5.2.2. Drinking-water and food
5.2.3. Tobacco
5.2.4. Cosmetics and toiletry articles
5.2.5. Rubber articles
5.3. Occupational exposure during manufacture, formulation or
use
5.3.1. Exposure to morpholine
5.3.2. Exposure to N-nitrosomorpholine
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.4.1. Expired air
6.4.2. Urine
6.4.3. Faeces
6.5. Retention and turnover
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral
7.1.2. Inhalation
7.1.3. Dermal
7.1.4. Intraperitoneal
7.2. Short-term exposure
7.2.1. Oral
7.2.2. Inhalation
7.2.3. Dermal
7.3. Long-term exposure
7.3.1. Oral
7.3.2. Inhalation
7.3.3. Dermal
7.4. Skin and eye irritation; sensitization
7.4.1. Eye irritation
7.4.2. Skin irritation
7.4.3. Sensitization
7.5. Reproductive toxicity, embryotoxicity and teratogenicity
7.6. Mutagenicity and related end-points
7.6.1. Mutagenicity of morpholine
7.6.1.1 Bacteria
7.6.1.2 Yeast
7.6.1.3 Mammalian cells in vitro
7.6.1.4 In vivo studies in mammals
7.6.2. Mutagenicity of morpholine in the presence of
nitrite and nitrate
7.6.3. Mutagenicity of N-Nitrosomorpholine
7.7. Carcinogenicity
7.7.1. Morpholine
7.7.1.1 Oral studies
7.7.1.2 Inhalation studies
7.7.2. Morpholine and nitrite
7.7.2.1 Oral studies
7.7.3. Carcinogenicity of N-nitrosomorpholine
7.8. Factors modifying toxicity; toxicity of metabolites
7.8.1. Factors modifying toxicity
7.8.2. Morpholine metabolites
7.9. Mechanisms of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Controlled human studies
8.1.1.1 Organoleptic effects
8.1.2. Epidemiological studies
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Microorganisms in water
9.1.1.2 Microorganisms in soil
9.1.1.3 Pathogenic microorganisms
9.1.2. Other aquatic organisms
9.1.2.1 Monocellular green algae
9.1.2.2 Invertebrates
9.1.2.3 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Animals
9.2. Field observations
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the
environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
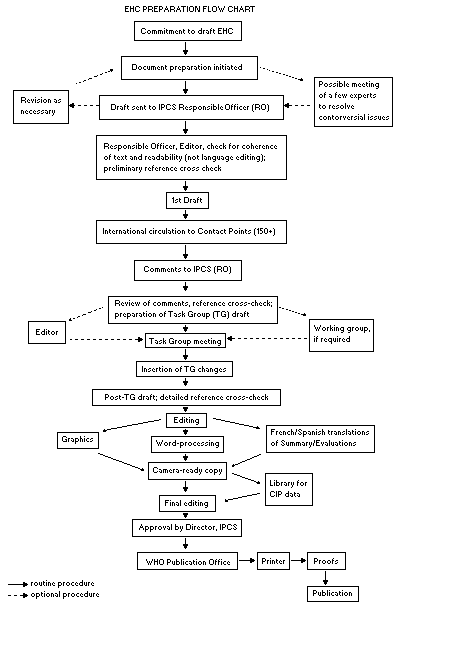
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
 health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
Members
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany (Joint Rapporteur)
Dr J.S. Knapp, Department of Microbiology, University of Leeds, Leeds,
United Kingdom (Joint Rapporteur)
Dr I. Linhart, Centre of Industrial Hygiene and Occupational Diseases,
National Institute of Public Health, Prague, Czech Republic
Dr U. Schiecke, Federal Environmental Agency, Berlin, Germany
Dr J.A. Sokal, Institute of Occupational Medicine and Environmental
Health, Sosnowiec, Poland (Chairman)
Representatives of other organizations
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International
Union of Toxicology (Vice-Chairman)
Secretariat
Mrs C. Partensky, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
A WHO Task Group on Environmental Health Criteria for Morpholine
met at the World Health Organization, Geneva, from 8 to 11 November
1994. Dr E.M. Smith, IPCS, welcomed the participants on behalf of Dr
M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to
morpholine.
The first draft of this monograph was prepared by Dr J. Kielhorn
and Dr G. Rosner, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany. The second revised draft was prepared by
Dr J. Kielhorn. Dr E.M. Smith and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
CHO Chinese hamster ovary
DOC dissolved organic carbon
FID flame ionization detector
FPD flame photometric detector
GC gas chromatography
HPLC high-performance liquid chromatography
IC ion chromatography
MLSS mixed liquor suspended solids
MS mass spectrometry
NMOR N-nitrosomorpholine
NO nitrogen oxide
NOAEL no-observed-adverse-effect level
NSD nitrogen selective detector
OECD Organisation for Economic Co-operation and Development
TEA thermal energy analyser
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Physical and chemical properties
Morpholine (1-oxa-4-azacyclohexane) is a colourless, oily,
hygroscopic, volatile liquid with a characteristic amine ("fishy")
odour. It is completely miscible with water, as well as with many
organic solvents, but has limited solubility in alkaline aqueous
solutions. It is a base, the pKa of the conjugated acid being 8.33.
Correspondingly, the octanol-water partition coefficient is pH-
dependent (log Pow -2.55 at pH 7 and -0.84 at pH 10; 35°C). The
vapour pressure of aqueous solutions of morpholine is very close to
that of water.
Morpholine can undergo a variety of reactions. It behaves
chemically as a secondary amine. Under environmental and
physiological conditions, the proven animal carcinogen
N-nitrosomorpholine (NMOR) is formed by reaction of solutions of
nitrite or gaseous nitrogen oxides with dilute solutions of
morpholine. Nitrogen oxide (NO) levels may be of importance in
nitrosation. The conditions of nitrosation, in particular pH, play a
significant role.
1.2 Analytical methods
Morpholine can be determined by gas chromatography (GC) with
packed as well as capillary columns, high-performance liquid
chromatography (HPLC) and ion chromatography (IC). Detectors used
include flame ionization detector (FID), flame photometric detector
(FPD), nitrogen selective detector (NSD), and mass spectrometry (MS)
and thermal energy analyser (TEA) for GC, and UV-detector and TEA for
HPLC. For the determination of trace amounts, derivatization is
required. The method of choice for sensitivity seems to be GC with
TEA following the derivatization to NMOR (the detection limit is
2-3 µg/kg in various matrices). Low concentrations of morpholine in
air can be determined by GC with NSD.
1.3 Sources of human and environmental exposure
It is estimated that around 25 000 tonnes of morpholine per year
are produced industrially world-wide, but details of production from
some countries are lacking.
The main production process used appears to be the reaction of
diethylene glycol with ammonia in the presence of hydrogen and
catalysts.
Morpholine is an extremely versatile chemical but knowledge of
its uses is incomplete. It is important as a chemical intermediate in
the rubber industry, as a corrosion inhibitor, and in the synthesis of
optical brighteners, crop protection agents, dyes and drugs.
Morpholine is used as a solvent for a large variety of organic
materials, including resins, dyes and waxes. It can be used as a
catalyst. Morpholine is still used in some countries in toiletry and
cosmetic products. It is used in some countries in several direct and
indirect food additive applications.
Human and environmental exposure arises from both gaseous and
aqueous emissions and directly from some of its uses, including, for
example, its use in cosmetic formulations and waxes. The main
emissions probably result from its manufacture and its use in the
chemical industry (notably in production and use of rubber chemicals)
and as an anti-corrosion agent. Morpholine has been detected in a
wide variety of foods and tobacco. It could be that this morpholine
arises from the wax coatings on fruit or on packaging, but in some
cases its origin is unknown.
1.4 Environmental transport, distribution and transformation
Morpholine is chemically stable in the biosphere although it is
subject to chemical and biological nitrosation to NMOR.
Morpholine is inherently biodegradable. Under the conditions of
model activated sludge plants, morpholine is biodegradable. However,
under non-adapted conditions there is probably no significant
degradation of morpholine. The mean solid retention time in activated
sludge plants is of crucial importance and must be over 8 days if
reliable morpholine degradation is to be achieved.
There are inadequate data on the bioaccumulation of morpholine in
aquatic and terrestrial organisms. From the n-octanol/water
partition coefficient for morpholine (log Pow = -2.55 at pH7), no
bioaccumulation would be expected.
As morpholine is an important industrial chemical with a wide
range of applications, the presence of the compound or its derivatives
is to be expected in many industrial effluents. Its use as a
corrosion inhibitor in boiler water means that it will be found in
boiler wastewater, including that from power plants using morpholine.
Its use in the manufacture of rubber additives results in an
undefinable amount of morpholine being released into the hydrosphere
or geosphere through tyre abrasion and disposal of used tyres.
As a result of its use in waxes and polishes, morpholine is
released into the environment through volatilization. It is quickly
adsorbed by moisture. The main compartment for accumulation of
morpholine is therefore the hydrosphere. The limited data suggest that
morpholine does not accumulate in the hydrosphere.
Incineration is the preferred method of disposal for undiluted
morpholine, but nitrogen oxide emission controls may be required to
meet environmental regulations. For aqueous effluents, activated
sludge treatment is adequate, but only if the plant is carefully
controlled (see above).
1.5 Environmental levels and human exposure
There are no data available on levels of morpholine in ambient
and residential indoor air and in drinking-water. There are limited
data on its occurrence in natural waters and no information on its
occurrence in soil.
Based on the available data, the main source of general
population exposure to morpholine is food, which can be contaminated
with morpholine through direct treatment of fruit with waxes
containing morpholine for conservation purposes, through steam
treatment during food processing, and by the use of packaging material
containing morpholine. However, quantitative data on food
contamination by morpholine and NMOR are limited. For example, in
prepacked milk products, values ranged from 5 to 77 µg/kg morpholine
and up to 3.3 µg/kg NMOR. Morpholine content in various food samples
(fish, meat, plant products, beverages) usually did not exceed
1 mg/kg. Higher levels (up to 71.1 mg/kg) were detected in citrus
fruits in Japan. A survey in Italy did not identify NMOR in a variety
of foods at a detection limit of 0.3 µg/kg. Existing data do not
permit an estimation of the intake of morpholine and NMOR from food.
Morpholine has been found in cigarette tobacco at a concentration
of 0.3 mg/kg, and in snuff and chewing tobacco at concentrations up to
4.0 mg/kg. Levels of NMOR up to 0.7 mg/kg have been reported in the
past in snuff. These were probably associated with the use of
morpholine-containing waxes in packaging.
NMOR has been detected in some toiletry and cosmetic products,
e.g., shampoos and eye make-up, and in rubber articles, e.g. baby
pacifiers and feeding bottle teats, at levels up to 3.5 mg/kg.
Occupational exposure to morpholine may occur in several
industries. There are few data on exposure of workers to morpholine.
All reported values are below 3 mg/m3. Occupational exposure to
NMOR has been found in the rubber industry, where concentrations up to
250 µg/m3 have been measured.
The data currently available provide an indication of the
potential for human exposure but do not allow a precise estimation of
the levels of exposure of the general and occupational populations to
morpholine and NMOR.
1.6 Kinetics and metabolism in laboratory animals and humans
Morpholine is absorbed after oral, dermal and inhalation
exposure. In the rat following oral and intravenous administration,
morpholine is rapidly distributed, the highest concentrations being
found in the intestine and muscle.
In the rabbit, following intravenous and inhalation exposure,
morpholine is preferentially distributed to the kidneys, lower
concentrations reaching the lung, liver and blood.
Morpholine does not bind significantly to plasma proteins.
Plasma half-lives have been reported to be 115 (rat), 120 (hamster),
and 300 min (guinea-pig).
Morpholine is excreted mainly via the renal route, as the
unchanged compound, in a variety of species. One day after
administration, 70-90% of morpholine was found in urine.
Neutralization of morpholine enhances the rate of excretion of the
compound. A small percentage of morpholine is excreted in expired air
and faeces.
Studies in rats, mice, hamster and rabbit indicate that
morpholine is eliminated almost completely as the unmetabolized
compound. In the guinea-pig, N-methylation followed by Noxidation
can occur, with up to 20% of the administered dose being metabolized.
In the presence of nitrite, morpholine can be converted to NMOR both
in vitro and in vivo. Depending on the dose, 0-12% of morpholine
administered to rats with nitrites may be nitrosated.
Immunostimulation, involving macrophage activation, may increase
the extent of nitrosation.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of morpholine after oral administration shows
LD50 values of 1-1.9 g/kg body weight and 0.9 g/kg body weight in
the rat and guinea-pig, respectively. Rats receiving neutralized
morpholine (1 g/kg body weight) survived. After intraperitoneal
administration, the LD50 was 0.4 g/kg body weight in the mouse and
between 0.1 and 0.4 g/kg body weight in the rat. After inhalation
exposure, the LD50 was about 8 g/m3 in the rat and between 5 and
7 g/m3 in the mouse. The dermal LD50 was 0.5 ml/kg of undiluted
morpholine in the rabbit. The acute toxicity of morpholine is
characterized by gastrointestinal haemorrhage and diarrhoea after oral
exposure, and irritation and haemorrhage of the nose, mouth, eyes and
lung after inhalation. In a 30-day gavage study on rats at doses of
0.16 - 0.8 g/kg body weight, there were severe toxic effects and
mortality at all dose levels. In the guinea-pig at doses of
0.09 - 0.45 g/kg body weight there was also severe toxicity and
mortality at all dose levels.
After short-term inhalation exposure to morpholine (7.2 g/m3,
4 h/day, 4 days and 1.63 g/m3, 4 h/day, 5 days/week, 30 days),
alterations in lung function have been reported in rats. Mortality
rate in the rat ranged from 0 to 100% depending on exposure level
(0.36-18.1 g/m3, 6 h/day, 9 days). Inhalation toxicity was dose-
related with various degrees of local irritation (eyes, mouth, nose,
lung) and haemorrhage at the higher exposure levels. One study
reported increased function of thyroid gland and another necrosis of
liver and renal tubules after inhalation exposure.
A 90-day study showed that morpholine administered orally
(0.2-0.7 g/kg body weight per day) for 90 days may reduce body weight
gain and renal function in the mouse. After 672 days of oral exposure
to morpholine (0.28-0.5 g/kg body weight per day), forestomach
epithelium hyperplasia was reported (mouse).
In a 13-week inhalation study, morpholine (0.09-0.9 g/m3,
6 h/day, 5 days/week) has been reported to cause dose-related lesions
of nasal mucosa and pneumonia at the higher exposure levels (0.36 and
0.9 mg/m3). No treatment-related changes to a number of parameters
were observed at 0.09 g/m3; this concentration may be considered a
no-observed-adverse-effect level (NOAEL) under the conditions of
sub-chronic inhalation exposure.
Morpholine in the undiluted and unneutralized form is highly
irritant for the eye and skin, probably due to its alkaline
properties. Dilution and neutralization of its pH may significantly
reduce its topical toxicity. Morpholine (2%) did not induce
sensitivity in the guinea-pig using the modified Buehler method.
Morpholine did not induce mutations in bacteria or yeasts with
and without metabolic activation (with one exception at a very high
concentration). It was negative in the host-mediated assay.
Morpholine did not induce DNA-repair in primary rat hepatocytes
and did not induce a significant increase in sister chromatid exchange
in Chinese hamster ovary cells. Morpholine was considered to be weakly
mutagenic in the L5178Y mouse lymphoma assay. It increased type III
foci in the BALB/3T3 malignant cell transformation assay, although
neutralized morpholine did not.
Morpholine caused neither point mutation nor chromosomal
aberration in hamster embryos exposed in utero.
No increase in the incidence of tumours was seen in rats given up
to 0.5 g/m3 morpholine by inhalation for 104 weeks nor in mice given
1% morpholine oleate in their drinking-water for 96 weeks. In a long-
term study on a group of 104 rats given 1000 mg morpholine/kg diet,
there were three liver cell carcinoma, two lung and another
angiosarcoma (unspecified) and two malignant glioma, whereas in a
control group of 156 rats there were no tumours. With hamsters under
the same conditions, no tumours were found.
Morpholine given simultaneously with nitrite yields positive
results in the host-mediated assay, probably due to the formation of
NMOR. Morpholine fed simultaneously with nitrite induced liver and
lung tumours in rats and liver tumours in hamsters probably due to the
endogenous formation of NMOR. NMOR is mutagenic in bacteria and
yeasts; weakly positive results were reported for sister chromatid
exchange in CHO cells and for mutations in mouse lymphoma L5178Y
cells. NMOR is carcinogenic in mice, rats, hamsters and various
fishes, producing liver and lung tumours in mice, liver, kidney and
blood vessel tumours in rats, liver, upper digestive and respiratory
tract tumours in hamsters, and liver tumours in fish.
1.8 Effects on humans
There have been no reports on incidents of acute poisoning or on
the effects of short- or long-term exposure to morpholine by the
general population.
The phenomenon known as blue vision or glaucopsia, as well as
some instances of skin and respiratory tract irritation, have been
described in reports of occupational exposure to morpholine; however,
no atmospheric concentrations of morpholine were given. It was
reported that the number of chromosomal aberrations in the lymphocytes
of peripheral blood of workers exposed for 3-10 years to morpholine at
concentrations of 0.54-0.93 mg/m3 did not differ significantly from
controls.
Undiluted morpholine is strongly irritant to skin; a dilute
solution (1 to 40) was mildly irritant.
The potential carcinogenicity of morpholine in exposed human
populations has not been investigated.
1.9 Effects on other organisms in the laboratory and field
Among the aquatic organisms tested, certain cyanobacteria
(Microcystis) and unicellular green algae (Scenedesmus) appear to
be the most sensitive taxa as toxicity threshold values (criterion:
inhibition of population growth) of 1.7 mg/litre for Microcystis and
4.1 mg/litre for Scenedesmus have been reported (duration of
exposure: 8 days).
Aerobic bacteria like Pseudomonas proved to be much more
resistant: the 16-h toxicity threshold and the NOEC for population
growth have been cited as 310 and 8700 mg/litre, respectively.
However, 1000 mg/litre inhibited respiration and dehydrogenase
activity (up to 20%) in activated sludge from industrial treatment
plants.
Among aquatic protozoans tested so far, representatives of the
genera Entosiphon and Chilomonas (with threshold values of 12 and
18 mg/litre, respectively, for the inhibition of population growth)
turned out to be the most sensitive. The 24-h EC values
(E=immobilisation) for Daphnia were in the range of
100-120 mg/litre. The 48- to 96-h LC50 values reported for fish
tested in fresh, brackish or seawater were > 180 mg/litre, rainbow
trout being the most sensitive species.
No data on long-term effects in aquatic invertebrates and
vertebrates are available. Information about the toxicity of
morpholine in free-living soil organisms is almost entirely lacking,
being restricted to a 3-day EC value of about 400 mg/litre given for
germination inhibition in lettuce.
1.10 Evaluation of human health risks and effects on the environment
1.10.1 Evaluation of effects on human health
The general population is primarily exposed to morpholine by
consumption of contaminated food. Contamination of tobacco and
tobacco products, and cosmetic and toiletry articles and rubber
products may also contribute to overall exposure. Occupational
exposure to morpholine occurs in many industries; the compound is
absorbed by inhalation and skin absorption. Data are inadequate to
determine the degree of exposure of the general population. Data on
occupational exposure to morpholine are also limited.
Morpholine is not highly toxic under conditions of acute
exposure. The LD50 after oral administration is 1-1.9 g/kg body
weight in rats and 0.9 g/kg body weight in guinea-pigs. LC50 values
of 7.8 mg/m3 (rats) and 4.9-6.9 g/m3 (mice) have been reported.
In the conditions of short-term and long-term inhalation
exposure, the critical effects appear to be irritation of the eyes and
respiratory tract. A concentration of 90 mg/m3 may be considered the
NOAEL in the conditions of the 13-week experiment in rats (6 h/day,
5 days/week). In a long-term inhalation study (104 weeks), increased
incidences of inflammation of the cornea, and inflammation and
necrosis of the nasal cavity were observed in rats at 540 mg/m3.
Increased incidence of irritation of eyes and nose was also observed
at 36 and 180 mg/m3.
High exposures to morpholine causes severe damage to the liver
and kidneys of rats and guinea-pigs. Fatty degeneration of the liver
was reported in rats after feeding morpholine (0.5 g/kg body weight)
daily for 56 days. When administered morpholine oleic acid salt in
the drinking-water at a dose of about 0.7 g/kg body weight per day for
13 weeks, mice showed cloudy swelling of the kidney proximal tubules.
Decreased body weight gain was observed in female mice in the long-
term (672 days) feeding experiment at dose levels between 0.05 and
0.4 g morpholine (as oleic acid salt).
At the reported levels of the present occupational and
environmental exposures, morpholine does not seem to create any
significant risk of systemic toxic effects. Local effects (irritation)
of the eyes and respiratory tract may occur in non-controlled
occupational and incidental exposures to high concentrations of
airborne morpholine, and skin irritation may result from contact with
liquid (even diluted) morpholine.
Morpholine does not appear to be mutagenic or carcinogenic in
animals. However, it can be easily nitrosated to form NMOR, which is
mutagenic and carcinogenic in several species of experimental animals.
Morpholine fed to rats sequentially with nitrite caused an increase in
tumours, mostly hepatocellular carcinoma and sarcomas of the liver and
lungs. It is therefore prudent to consider exposure to morpholine as
increasing the carcinogenic risk in exposed populations.
1.10.2 Evaluation of effects on the environment
In view of the very restricted knowledge regarding environmental
exposure, the lack of effect data relating to long-term exposure in
water and to short- and long-term exposure in the terrestrial
environment, a sound risk assessment cannot be carried out at present.
On the basis of the reported properties of morpholine, the available
ecotoxicological information and the few data on environmental
concentrations, certain conclusions can be drawn.
The high water solubility of morpholine and its low volatility
(under environmental conditions) make the hydrosphere the pre-dominant
environmental sink.
Morpholine is inherently biodegradable and, although degradation
is slow, there are no data to suggest accumulation in the hydrosphere.
Bioaccumulation is unlikely.
There are relatively few data on toxicity of morpholine to free-
living organisms. However, it seems unlikely that current levels of
morpholine emission cause any significant damage to the wider
environment. Local effects, due for example to factory emissions or
to morpholine release due to wear of tyres, remain to be evaluated.
Contamination of some foods, e.g., fish, with morpholine may be
due to environmental contamination, but this is uncertain.
Conversion of morpholine to NMOR is the main cause of concern,
especially with respect to vertebrate populations. NMOR has been
reported in industrial wastewater and in soil near a factory. The
presence of morpholine in water destined for processing to drinking-
water is a cause for concern.
1.11 Conclusions and recommendations
Morpholine does not present a toxic risk to humans at the usual
levels of exposure, but its conversion to the carcinogenic NMOR should
be noted.
There is no evidence at present levels of exposure that
morpholine poses a substantial risk to biota in the environment.
1.11.1 Recommendations for protection of human health
a) Human exposure to morpholine should be avoided as far as
possible.
b) Contamination of food through food packaging and food processing
should be avoided.
c) Morpholine should not be used in rubber products intended for
direct contact with humans.
d) Morpholine should not be used in toiletry or cosmetic
preparations.
e) Industrial effluents should be rigorously treated to avoid entry
of morpholine into drinking-water.
f) In the light of the formation of carcinogenic NMOR the present
occupational exposure limits should be reconsidered.
1.11.2 Recommendations for protection of the environment
Spills and shock loads to effluent treatment plants should be
avoided.
1.11.3 Recommendations for further research
Studies should be undertaken on the following topics:
a) reproductive toxicity in mammals;
b) long-term toxicity in mammals;
c) effect of exposure of mammals to low levels of morpholine with
and without nitrite and nitrate;
d) transnitrosation by NMOR under in vivo and in vitro
conditions;
e) biodegradation under anaerobic conditions, especially under
nitrate-reducing conditions;
f) microbial catalysis of N-nitrosation under realistic
conditions;
g) environmental levels of morpholine in groundwater, soil and
rivers used for drinking-water;
h) environmental levels of morpholine around morpholine-producing
and -processing factories;
i) metabolism and toxicokinetics in humans as a part of the
development of methods for biological monitoring of morpholine;
j) monitoring of morpholine and NMOR levels in food, drinking-water
and indoor air;
k) data on occupational exposure should be collected and made
available.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
CAS/IUPAC name: Morpholine
Chemical formula: C4H9NO
Chemical structure:
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
Members
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany (Joint Rapporteur)
Dr J.S. Knapp, Department of Microbiology, University of Leeds, Leeds,
United Kingdom (Joint Rapporteur)
Dr I. Linhart, Centre of Industrial Hygiene and Occupational Diseases,
National Institute of Public Health, Prague, Czech Republic
Dr U. Schiecke, Federal Environmental Agency, Berlin, Germany
Dr J.A. Sokal, Institute of Occupational Medicine and Environmental
Health, Sosnowiec, Poland (Chairman)
Representatives of other organizations
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International
Union of Toxicology (Vice-Chairman)
Secretariat
Mrs C. Partensky, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
A WHO Task Group on Environmental Health Criteria for Morpholine
met at the World Health Organization, Geneva, from 8 to 11 November
1994. Dr E.M. Smith, IPCS, welcomed the participants on behalf of Dr
M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to
morpholine.
The first draft of this monograph was prepared by Dr J. Kielhorn
and Dr G. Rosner, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany. The second revised draft was prepared by
Dr J. Kielhorn. Dr E.M. Smith and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
CHO Chinese hamster ovary
DOC dissolved organic carbon
FID flame ionization detector
FPD flame photometric detector
GC gas chromatography
HPLC high-performance liquid chromatography
IC ion chromatography
MLSS mixed liquor suspended solids
MS mass spectrometry
NMOR N-nitrosomorpholine
NO nitrogen oxide
NOAEL no-observed-adverse-effect level
NSD nitrogen selective detector
OECD Organisation for Economic Co-operation and Development
TEA thermal energy analyser
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Physical and chemical properties
Morpholine (1-oxa-4-azacyclohexane) is a colourless, oily,
hygroscopic, volatile liquid with a characteristic amine ("fishy")
odour. It is completely miscible with water, as well as with many
organic solvents, but has limited solubility in alkaline aqueous
solutions. It is a base, the pKa of the conjugated acid being 8.33.
Correspondingly, the octanol-water partition coefficient is pH-
dependent (log Pow -2.55 at pH 7 and -0.84 at pH 10; 35°C). The
vapour pressure of aqueous solutions of morpholine is very close to
that of water.
Morpholine can undergo a variety of reactions. It behaves
chemically as a secondary amine. Under environmental and
physiological conditions, the proven animal carcinogen
N-nitrosomorpholine (NMOR) is formed by reaction of solutions of
nitrite or gaseous nitrogen oxides with dilute solutions of
morpholine. Nitrogen oxide (NO) levels may be of importance in
nitrosation. The conditions of nitrosation, in particular pH, play a
significant role.
1.2 Analytical methods
Morpholine can be determined by gas chromatography (GC) with
packed as well as capillary columns, high-performance liquid
chromatography (HPLC) and ion chromatography (IC). Detectors used
include flame ionization detector (FID), flame photometric detector
(FPD), nitrogen selective detector (NSD), and mass spectrometry (MS)
and thermal energy analyser (TEA) for GC, and UV-detector and TEA for
HPLC. For the determination of trace amounts, derivatization is
required. The method of choice for sensitivity seems to be GC with
TEA following the derivatization to NMOR (the detection limit is
2-3 µg/kg in various matrices). Low concentrations of morpholine in
air can be determined by GC with NSD.
1.3 Sources of human and environmental exposure
It is estimated that around 25 000 tonnes of morpholine per year
are produced industrially world-wide, but details of production from
some countries are lacking.
The main production process used appears to be the reaction of
diethylene glycol with ammonia in the presence of hydrogen and
catalysts.
Morpholine is an extremely versatile chemical but knowledge of
its uses is incomplete. It is important as a chemical intermediate in
the rubber industry, as a corrosion inhibitor, and in the synthesis of
optical brighteners, crop protection agents, dyes and drugs.
Morpholine is used as a solvent for a large variety of organic
materials, including resins, dyes and waxes. It can be used as a
catalyst. Morpholine is still used in some countries in toiletry and
cosmetic products. It is used in some countries in several direct and
indirect food additive applications.
Human and environmental exposure arises from both gaseous and
aqueous emissions and directly from some of its uses, including, for
example, its use in cosmetic formulations and waxes. The main
emissions probably result from its manufacture and its use in the
chemical industry (notably in production and use of rubber chemicals)
and as an anti-corrosion agent. Morpholine has been detected in a
wide variety of foods and tobacco. It could be that this morpholine
arises from the wax coatings on fruit or on packaging, but in some
cases its origin is unknown.
1.4 Environmental transport, distribution and transformation
Morpholine is chemically stable in the biosphere although it is
subject to chemical and biological nitrosation to NMOR.
Morpholine is inherently biodegradable. Under the conditions of
model activated sludge plants, morpholine is biodegradable. However,
under non-adapted conditions there is probably no significant
degradation of morpholine. The mean solid retention time in activated
sludge plants is of crucial importance and must be over 8 days if
reliable morpholine degradation is to be achieved.
There are inadequate data on the bioaccumulation of morpholine in
aquatic and terrestrial organisms. From the n-octanol/water
partition coefficient for morpholine (log Pow = -2.55 at pH7), no
bioaccumulation would be expected.
As morpholine is an important industrial chemical with a wide
range of applications, the presence of the compound or its derivatives
is to be expected in many industrial effluents. Its use as a
corrosion inhibitor in boiler water means that it will be found in
boiler wastewater, including that from power plants using morpholine.
Its use in the manufacture of rubber additives results in an
undefinable amount of morpholine being released into the hydrosphere
or geosphere through tyre abrasion and disposal of used tyres.
As a result of its use in waxes and polishes, morpholine is
released into the environment through volatilization. It is quickly
adsorbed by moisture. The main compartment for accumulation of
morpholine is therefore the hydrosphere. The limited data suggest that
morpholine does not accumulate in the hydrosphere.
Incineration is the preferred method of disposal for undiluted
morpholine, but nitrogen oxide emission controls may be required to
meet environmental regulations. For aqueous effluents, activated
sludge treatment is adequate, but only if the plant is carefully
controlled (see above).
1.5 Environmental levels and human exposure
There are no data available on levels of morpholine in ambient
and residential indoor air and in drinking-water. There are limited
data on its occurrence in natural waters and no information on its
occurrence in soil.
Based on the available data, the main source of general
population exposure to morpholine is food, which can be contaminated
with morpholine through direct treatment of fruit with waxes
containing morpholine for conservation purposes, through steam
treatment during food processing, and by the use of packaging material
containing morpholine. However, quantitative data on food
contamination by morpholine and NMOR are limited. For example, in
prepacked milk products, values ranged from 5 to 77 µg/kg morpholine
and up to 3.3 µg/kg NMOR. Morpholine content in various food samples
(fish, meat, plant products, beverages) usually did not exceed
1 mg/kg. Higher levels (up to 71.1 mg/kg) were detected in citrus
fruits in Japan. A survey in Italy did not identify NMOR in a variety
of foods at a detection limit of 0.3 µg/kg. Existing data do not
permit an estimation of the intake of morpholine and NMOR from food.
Morpholine has been found in cigarette tobacco at a concentration
of 0.3 mg/kg, and in snuff and chewing tobacco at concentrations up to
4.0 mg/kg. Levels of NMOR up to 0.7 mg/kg have been reported in the
past in snuff. These were probably associated with the use of
morpholine-containing waxes in packaging.
NMOR has been detected in some toiletry and cosmetic products,
e.g., shampoos and eye make-up, and in rubber articles, e.g. baby
pacifiers and feeding bottle teats, at levels up to 3.5 mg/kg.
Occupational exposure to morpholine may occur in several
industries. There are few data on exposure of workers to morpholine.
All reported values are below 3 mg/m3. Occupational exposure to
NMOR has been found in the rubber industry, where concentrations up to
250 µg/m3 have been measured.
The data currently available provide an indication of the
potential for human exposure but do not allow a precise estimation of
the levels of exposure of the general and occupational populations to
morpholine and NMOR.
1.6 Kinetics and metabolism in laboratory animals and humans
Morpholine is absorbed after oral, dermal and inhalation
exposure. In the rat following oral and intravenous administration,
morpholine is rapidly distributed, the highest concentrations being
found in the intestine and muscle.
In the rabbit, following intravenous and inhalation exposure,
morpholine is preferentially distributed to the kidneys, lower
concentrations reaching the lung, liver and blood.
Morpholine does not bind significantly to plasma proteins.
Plasma half-lives have been reported to be 115 (rat), 120 (hamster),
and 300 min (guinea-pig).
Morpholine is excreted mainly via the renal route, as the
unchanged compound, in a variety of species. One day after
administration, 70-90% of morpholine was found in urine.
Neutralization of morpholine enhances the rate of excretion of the
compound. A small percentage of morpholine is excreted in expired air
and faeces.
Studies in rats, mice, hamster and rabbit indicate that
morpholine is eliminated almost completely as the unmetabolized
compound. In the guinea-pig, N-methylation followed by Noxidation
can occur, with up to 20% of the administered dose being metabolized.
In the presence of nitrite, morpholine can be converted to NMOR both
in vitro and in vivo. Depending on the dose, 0-12% of morpholine
administered to rats with nitrites may be nitrosated.
Immunostimulation, involving macrophage activation, may increase
the extent of nitrosation.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of morpholine after oral administration shows
LD50 values of 1-1.9 g/kg body weight and 0.9 g/kg body weight in
the rat and guinea-pig, respectively. Rats receiving neutralized
morpholine (1 g/kg body weight) survived. After intraperitoneal
administration, the LD50 was 0.4 g/kg body weight in the mouse and
between 0.1 and 0.4 g/kg body weight in the rat. After inhalation
exposure, the LD50 was about 8 g/m3 in the rat and between 5 and
7 g/m3 in the mouse. The dermal LD50 was 0.5 ml/kg of undiluted
morpholine in the rabbit. The acute toxicity of morpholine is
characterized by gastrointestinal haemorrhage and diarrhoea after oral
exposure, and irritation and haemorrhage of the nose, mouth, eyes and
lung after inhalation. In a 30-day gavage study on rats at doses of
0.16 - 0.8 g/kg body weight, there were severe toxic effects and
mortality at all dose levels. In the guinea-pig at doses of
0.09 - 0.45 g/kg body weight there was also severe toxicity and
mortality at all dose levels.
After short-term inhalation exposure to morpholine (7.2 g/m3,
4 h/day, 4 days and 1.63 g/m3, 4 h/day, 5 days/week, 30 days),
alterations in lung function have been reported in rats. Mortality
rate in the rat ranged from 0 to 100% depending on exposure level
(0.36-18.1 g/m3, 6 h/day, 9 days). Inhalation toxicity was dose-
related with various degrees of local irritation (eyes, mouth, nose,
lung) and haemorrhage at the higher exposure levels. One study
reported increased function of thyroid gland and another necrosis of
liver and renal tubules after inhalation exposure.
A 90-day study showed that morpholine administered orally
(0.2-0.7 g/kg body weight per day) for 90 days may reduce body weight
gain and renal function in the mouse. After 672 days of oral exposure
to morpholine (0.28-0.5 g/kg body weight per day), forestomach
epithelium hyperplasia was reported (mouse).
In a 13-week inhalation study, morpholine (0.09-0.9 g/m3,
6 h/day, 5 days/week) has been reported to cause dose-related lesions
of nasal mucosa and pneumonia at the higher exposure levels (0.36 and
0.9 mg/m3). No treatment-related changes to a number of parameters
were observed at 0.09 g/m3; this concentration may be considered a
no-observed-adverse-effect level (NOAEL) under the conditions of
sub-chronic inhalation exposure.
Morpholine in the undiluted and unneutralized form is highly
irritant for the eye and skin, probably due to its alkaline
properties. Dilution and neutralization of its pH may significantly
reduce its topical toxicity. Morpholine (2%) did not induce
sensitivity in the guinea-pig using the modified Buehler method.
Morpholine did not induce mutations in bacteria or yeasts with
and without metabolic activation (with one exception at a very high
concentration). It was negative in the host-mediated assay.
Morpholine did not induce DNA-repair in primary rat hepatocytes
and did not induce a significant increase in sister chromatid exchange
in Chinese hamster ovary cells. Morpholine was considered to be weakly
mutagenic in the L5178Y mouse lymphoma assay. It increased type III
foci in the BALB/3T3 malignant cell transformation assay, although
neutralized morpholine did not.
Morpholine caused neither point mutation nor chromosomal
aberration in hamster embryos exposed in utero.
No increase in the incidence of tumours was seen in rats given up
to 0.5 g/m3 morpholine by inhalation for 104 weeks nor in mice given
1% morpholine oleate in their drinking-water for 96 weeks. In a long-
term study on a group of 104 rats given 1000 mg morpholine/kg diet,
there were three liver cell carcinoma, two lung and another
angiosarcoma (unspecified) and two malignant glioma, whereas in a
control group of 156 rats there were no tumours. With hamsters under
the same conditions, no tumours were found.
Morpholine given simultaneously with nitrite yields positive
results in the host-mediated assay, probably due to the formation of
NMOR. Morpholine fed simultaneously with nitrite induced liver and
lung tumours in rats and liver tumours in hamsters probably due to the
endogenous formation of NMOR. NMOR is mutagenic in bacteria and
yeasts; weakly positive results were reported for sister chromatid
exchange in CHO cells and for mutations in mouse lymphoma L5178Y
cells. NMOR is carcinogenic in mice, rats, hamsters and various
fishes, producing liver and lung tumours in mice, liver, kidney and
blood vessel tumours in rats, liver, upper digestive and respiratory
tract tumours in hamsters, and liver tumours in fish.
1.8 Effects on humans
There have been no reports on incidents of acute poisoning or on
the effects of short- or long-term exposure to morpholine by the
general population.
The phenomenon known as blue vision or glaucopsia, as well as
some instances of skin and respiratory tract irritation, have been
described in reports of occupational exposure to morpholine; however,
no atmospheric concentrations of morpholine were given. It was
reported that the number of chromosomal aberrations in the lymphocytes
of peripheral blood of workers exposed for 3-10 years to morpholine at
concentrations of 0.54-0.93 mg/m3 did not differ significantly from
controls.
Undiluted morpholine is strongly irritant to skin; a dilute
solution (1 to 40) was mildly irritant.
The potential carcinogenicity of morpholine in exposed human
populations has not been investigated.
1.9 Effects on other organisms in the laboratory and field
Among the aquatic organisms tested, certain cyanobacteria
(Microcystis) and unicellular green algae (Scenedesmus) appear to
be the most sensitive taxa as toxicity threshold values (criterion:
inhibition of population growth) of 1.7 mg/litre for Microcystis and
4.1 mg/litre for Scenedesmus have been reported (duration of
exposure: 8 days).
Aerobic bacteria like Pseudomonas proved to be much more
resistant: the 16-h toxicity threshold and the NOEC for population
growth have been cited as 310 and 8700 mg/litre, respectively.
However, 1000 mg/litre inhibited respiration and dehydrogenase
activity (up to 20%) in activated sludge from industrial treatment
plants.
Among aquatic protozoans tested so far, representatives of the
genera Entosiphon and Chilomonas (with threshold values of 12 and
18 mg/litre, respectively, for the inhibition of population growth)
turned out to be the most sensitive. The 24-h EC values
(E=immobilisation) for Daphnia were in the range of
100-120 mg/litre. The 48- to 96-h LC50 values reported for fish
tested in fresh, brackish or seawater were > 180 mg/litre, rainbow
trout being the most sensitive species.
No data on long-term effects in aquatic invertebrates and
vertebrates are available. Information about the toxicity of
morpholine in free-living soil organisms is almost entirely lacking,
being restricted to a 3-day EC value of about 400 mg/litre given for
germination inhibition in lettuce.
1.10 Evaluation of human health risks and effects on the environment
1.10.1 Evaluation of effects on human health
The general population is primarily exposed to morpholine by
consumption of contaminated food. Contamination of tobacco and
tobacco products, and cosmetic and toiletry articles and rubber
products may also contribute to overall exposure. Occupational
exposure to morpholine occurs in many industries; the compound is
absorbed by inhalation and skin absorption. Data are inadequate to
determine the degree of exposure of the general population. Data on
occupational exposure to morpholine are also limited.
Morpholine is not highly toxic under conditions of acute
exposure. The LD50 after oral administration is 1-1.9 g/kg body
weight in rats and 0.9 g/kg body weight in guinea-pigs. LC50 values
of 7.8 mg/m3 (rats) and 4.9-6.9 g/m3 (mice) have been reported.
In the conditions of short-term and long-term inhalation
exposure, the critical effects appear to be irritation of the eyes and
respiratory tract. A concentration of 90 mg/m3 may be considered the
NOAEL in the conditions of the 13-week experiment in rats (6 h/day,
5 days/week). In a long-term inhalation study (104 weeks), increased
incidences of inflammation of the cornea, and inflammation and
necrosis of the nasal cavity were observed in rats at 540 mg/m3.
Increased incidence of irritation of eyes and nose was also observed
at 36 and 180 mg/m3.
High exposures to morpholine causes severe damage to the liver
and kidneys of rats and guinea-pigs. Fatty degeneration of the liver
was reported in rats after feeding morpholine (0.5 g/kg body weight)
daily for 56 days. When administered morpholine oleic acid salt in
the drinking-water at a dose of about 0.7 g/kg body weight per day for
13 weeks, mice showed cloudy swelling of the kidney proximal tubules.
Decreased body weight gain was observed in female mice in the long-
term (672 days) feeding experiment at dose levels between 0.05 and
0.4 g morpholine (as oleic acid salt).
At the reported levels of the present occupational and
environmental exposures, morpholine does not seem to create any
significant risk of systemic toxic effects. Local effects (irritation)
of the eyes and respiratory tract may occur in non-controlled
occupational and incidental exposures to high concentrations of
airborne morpholine, and skin irritation may result from contact with
liquid (even diluted) morpholine.
Morpholine does not appear to be mutagenic or carcinogenic in
animals. However, it can be easily nitrosated to form NMOR, which is
mutagenic and carcinogenic in several species of experimental animals.
Morpholine fed to rats sequentially with nitrite caused an increase in
tumours, mostly hepatocellular carcinoma and sarcomas of the liver and
lungs. It is therefore prudent to consider exposure to morpholine as
increasing the carcinogenic risk in exposed populations.
1.10.2 Evaluation of effects on the environment
In view of the very restricted knowledge regarding environmental
exposure, the lack of effect data relating to long-term exposure in
water and to short- and long-term exposure in the terrestrial
environment, a sound risk assessment cannot be carried out at present.
On the basis of the reported properties of morpholine, the available
ecotoxicological information and the few data on environmental
concentrations, certain conclusions can be drawn.
The high water solubility of morpholine and its low volatility
(under environmental conditions) make the hydrosphere the pre-dominant
environmental sink.
Morpholine is inherently biodegradable and, although degradation
is slow, there are no data to suggest accumulation in the hydrosphere.
Bioaccumulation is unlikely.
There are relatively few data on toxicity of morpholine to free-
living organisms. However, it seems unlikely that current levels of
morpholine emission cause any significant damage to the wider
environment. Local effects, due for example to factory emissions or
to morpholine release due to wear of tyres, remain to be evaluated.
Contamination of some foods, e.g., fish, with morpholine may be
due to environmental contamination, but this is uncertain.
Conversion of morpholine to NMOR is the main cause of concern,
especially with respect to vertebrate populations. NMOR has been
reported in industrial wastewater and in soil near a factory. The
presence of morpholine in water destined for processing to drinking-
water is a cause for concern.
1.11 Conclusions and recommendations
Morpholine does not present a toxic risk to humans at the usual
levels of exposure, but its conversion to the carcinogenic NMOR should
be noted.
There is no evidence at present levels of exposure that
morpholine poses a substantial risk to biota in the environment.
1.11.1 Recommendations for protection of human health
a) Human exposure to morpholine should be avoided as far as
possible.
b) Contamination of food through food packaging and food processing
should be avoided.
c) Morpholine should not be used in rubber products intended for
direct contact with humans.
d) Morpholine should not be used in toiletry or cosmetic
preparations.
e) Industrial effluents should be rigorously treated to avoid entry
of morpholine into drinking-water.
f) In the light of the formation of carcinogenic NMOR the present
occupational exposure limits should be reconsidered.
1.11.2 Recommendations for protection of the environment
Spills and shock loads to effluent treatment plants should be
avoided.
1.11.3 Recommendations for further research
Studies should be undertaken on the following topics:
a) reproductive toxicity in mammals;
b) long-term toxicity in mammals;
c) effect of exposure of mammals to low levels of morpholine with
and without nitrite and nitrate;
d) transnitrosation by NMOR under in vivo and in vitro
conditions;
e) biodegradation under anaerobic conditions, especially under
nitrate-reducing conditions;
f) microbial catalysis of N-nitrosation under realistic
conditions;
g) environmental levels of morpholine in groundwater, soil and
rivers used for drinking-water;
h) environmental levels of morpholine around morpholine-producing
and -processing factories;
i) metabolism and toxicokinetics in humans as a part of the
development of methods for biological monitoring of morpholine;
j) monitoring of morpholine and NMOR levels in food, drinking-water
and indoor air;
k) data on occupational exposure should be collected and made
available.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
CAS/IUPAC name: Morpholine
Chemical formula: C4H9NO
Chemical structure: 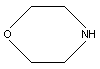 CAS registry number: 110-91-8
EEC number: 613-028-00-9
EINECS number: 2038151
UN number: 2054
Synonyms: 1-oxa-4-azacyclohexane
tetrahydro-2H-1,4-oxazine
tetrahydro-1,4-oxazine
tetrahydro-1,4-isoxazine
diethylene oximide
diethyleneimide oxide
diethylene imidoxide
Relative molecular mass: 87.12
2.1.1 Technical product
The compound is marketed under the name of "Morpholine". It is
distributed as an anhydrous liquid and as 40% and 88% solutions with
water (Air Products and Chemicals, 1989).
2.1.2 Impurities
Morpholine is marketed as a product with approximately 99% purity
(Cosmetic Ingredient Review, 1989; BUA, 1991). The exact chemical
nature of the impurities depends on the production process (see
section 3.2.1.3). When produced from diethylene glycol,
2-(2-aminoethoxy)ethanol is a by-product, which can be isolated and
recycled (Heilen et al., 1989). Reported impurities are
N-ethylmorpholine and ethylenediamine (Heilen et al., 1989) and
2-methoxy ethanol (BUA, 1991).
During the production of morpholine from diethanolamine, it is
possible that N-hydroxyethylmorpholine may be formed (Cosmetic
Ingredient Review, 1989).
Impurities in cosmetic grade morpholine have been reported to
include arsenic (up to 3 mg/kg) and lead (up to 20 mg/kg) (Estrin et
al., 1982). The Cosmetic Ingredient Review (1991a) lists morpholine
as having insufficient data on impurities.
Fajen et al. (1979) found 0.8 mg/kg N-nitrosomorpholine (NMOR)
in a morpholine charge used for the production of a vulcanization
accelerator in a chemical factory in Ohio. NMOR could not be detected
(detection limit: 50 µg/kg) in morpholine stored under nitrogen in
Germany (BUA, 1991).
2.2 Physical and chemical properties
2.2.1 Physical properties of morpholine
Morpholine is a colourless, oily, hygroscopic, volatile liquid
with a characteristic amine smell (Reinhardt & Brittelli, 1981).
Morpholine vapour is heavier than air and as a result, the vapour can
travel a significant distance to a source of ignition and "flash
back".
It is completely miscible with water, soluble in the usual
solvents and can itself be used as a solvent (Heilen et al., 1989).
It has a low solubility in alkaline aqueous solutions.
Morpholine is a strong base, the 0.01% (w/w) mixture having a
pH of 9.4, and the 10% (w/w) mixture having a pH of 11.2 (Texaco,
1986).
Some physical and chemical properties are presented in Table 1.
2.2.1.1 Storage of morpholine
Morpholine can be stored for an unlimited time in iron or steel
containers if protected from atmospheric moisture and carbon dioxide.
However, it is unstable in the presence of copper, zinc and their
alloys and these metals should not be used in storage containers for
morpholine (Heilen et al., 1989; Air Products and Chemicals, 1989).
2.2.2 Chemical properties of morpholine
Morpholine can undergo a diversity of reactions. It is an amino
ether; the ether function of the molecule is typically inert and most
of the reactions involve the secondary amine group.
Table 1. Some physical and chemical properties of morpholine
Melting point (°C) -3.1a; -4.9b,c; -5d
Boiling point (°C at 1013 hPa) 128.2a; 128.3c; 128.9b; 128-130d
Flash point (°C) - Open cup 38b;
- Closed cup 35c; 31d
Autoignition temperature (°C) 275a,d; 310c;
Explosion limits in air 1.4-13.1 vol% d; 1.8-11 vol% e;
1.8-15.2 vol% a
Decomposition temperature > 330°Cd; > 550°C
(in steam cycles)a
pKa (conjugated acid) 8.33 (25°C)f;
8.36c (temperature not given)
Vapour pressure (°C) 10 20 40 60 80 100 120
(kPa) 0.6 1.1 3.2 8.3 10.5 40.9 81.8
Density g/cm3 (20°C) 0.994b; 0.999c; 1.00d; 1.007a
log n-octanol/water partition -0.723 (free base; calculated)g
coefficient (log Pow) -1.08 (free base; calculated)h
-0.66 (free base; calculated)i
Solubility in water completely miscible with watera
Solubility in organic solvents completely miscible with, for
instance, methanol, ethanol, acetone,
diethylether, benzene, toluene,
xylolc,e
Refractive index 1.4537-1.4545 at 20°Ce
Human olfactory threshold 0.036 (mg/m3)j
a Heilen et al. (1989); b Brown (1966); c Texaco (1986);
d BASF (1987); e Cosmetic Ingredient Review (1989);
f Lide (1990); g UBA (1990); h Leo et al. (1971);
i Le Therizien et al. (1980); j Hellman & Small (1974)
It reacts with inorganic acids and acid gases such as CO2,
H2S, or HCN to form morpholine salts. This property is of use in
the addition of morpholine as an anticorrosive in boiler systems
(Brown, 1966). Morpholine can react with oxidizing agents, undergo
direct chlorination, and form complexes with metallic halides. It
reacts with carboxylic acids, anhydrides, chlorides and esters to form
morpholides (Brown, 1966). Alkyl morpholides are formed by reaction
of morpholine with alkyl halides, dialkyl sulfates or trialkyl
phosphates. The N-alkylmorpholides, particularly
N-methylmorpholides, and N-ethylmorpholides, are widely used as
catalysts in the preparation of polyurethanes (Brown, 1966).
Morpholine reacts with formaldehyde to form N-formyl-morpholine,
which is used industrially as a selective solvent for the extraction
of very pure aromatic compounds (Heilen et al., 1989).
Morpholine reacts with fatty acids to form soaps which are used
in household and automotive waxes and polishes. Their principal
advantage is that the morpholine evaporates at the same rate as water,
leaving a water-resistant wax base (Mjos, 1978; Texaco, 1986).
Vulcanizing agents for the rubber industry are formed by the reaction
of morpholine with sulfur and sulfur-containing compounds (Taylor &
Son, 1982).
Morpholine is flammable. Violent reaction and fire may result
when the product is mixed with oxidizing agents (Air Products and
Chemicals, 1989).
N-nitrosomorpholine (NMOR) can be formed by reaction of aqueous
solutions of nitrite with morpholine or by reaction of gaseous
nitrogen oxides in aqueous solutions of morpholine (see section 4.3).
2.3 Conversion factors for morpholine
1 mg/m3 = 0.276 ppm at 20°C and 1013 hPa
1 ppm = 3.62 mg/m3
2.4 Analytical methods
Methods suitable for measuring trace levels of morpholine include
ion chromatography (IC), gas chromatography (GC) with packed as well
as capillary columns, and high-performance liquid chromatography
(HPLC), usually using reverse phase (RP) columns.
The poor UV absorptivity of morpholine necessitates chemical
derivatization to detect trace amounts.
Detection methods include UV detectors (for HPLC) and flame
ionisation detectors (FID, following GC), as well as thermal energy
analysers (TEA). Photochemical methods are used but are not specific
for morpholine.
An overview of the analytical methods for determining morpholine
in various matrices is given in Table 2.
For the detection of trace amounts of NMOR, GC or HPLC together
with TEA has proved to be the method of choice. The use of internal
standards helps to distinguish NMOR in the sample from artifacts
caused by nitrosation or transnitrosation during the work-up procedure
(BUA,1991; ECETOC, 1991).
2.4.1 Determination of morpholine in air
Table 2 summarizes the available methods.
Air samples can be collected and concentrated by passing through
silica gel or an impinger containing dilute acid. A 20-litre sample
is recommended to reach concentrations between 7 and 210 mg/m3
(NIOSH, 1977). Bianchi & Muccioli (1978) collected air samples
without absorption on a solvent and rapidly performed the GC.
Sollenberg & Hansen (1987) described an isotachophoretic
determination of morpholine using 10 mM potassium cacodylate (pH 6.5)
as leading electrolyte, and 10 mM creatinine with 5 mM HCl as
terminating electrolyte. This method has been used primarily to
measure N-methylmorpholine in air samples from a polyurethane foam
factory (Hansen et al., 1986). Aarts et al. (1990) also used an
isotachophoretic method for determining morpholine in rubber samples
(see Table 2).
2.4.2 Determination of morpholine in water
The methods given in Table 2 (water) are suitable for the
determination of morpholine in steam condensates or non-aqueous
solvents.
2.4.3 Determination of morpholine in soil and sediments
A GC/MS method has been used to detect morpholine in sediment and
soil (Spies et al., 1987).
2.4.4 Determination in biological and other materials
Morpholine has been determined in biological tissues and fluids
using GC/FID (Tombropoulos, 1979). Morpholine and some of its
metabolites ( N-hydroxymorpholine, N-methylmorpholine and
N-methylmorpholine- N-oxide) could be separated using two
complementary HPLCs, one using reversed-phase and the other
ion-exchange chromatography (Sohn et al., 1982a).
Morpholine has been determined in a number of foods and beverages
as well as in tobacco, snuff and packaging material (see section
5.2.2). The methods used are summarized in Table 2. Generally,
morpholine is extracted from the samples using steam distillation
followed by purification and derivatization.
Table 2. Methods for the analysis of morpholinea
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Air adsorption on silica gel, GC FID 7 mg/m3c 100 NIOSH (1977)
desorption with H2SO4,
neutralization with NaOH
Air collected directly GC FID 36 mg/m3 not given Bianchi &
Muccioli (1978)
Air absorption in 1 N KOH (impinger); GC TEA not given not given Fajen et al.
extraction with dichloromethane (1979)
Air adsorption on silica gel; HPLC UV not given 90-96 Simon & Lemacon
derivatization to m-toluamides (235-255 nm) (1987)
Air absorption on silica gel, GC NSD 0.03 mg/m3 93 ± 5% BIA (1989)
extraction with methanol
+ 2% KOH
Water derivatization to p-tosylamide, GC FID 70 ng/litre 45-67 Singer &
acidification with HCl (pH 1), Lijinsky (1976a)
extraction with diethylether
Water addition of Cu(II), CS2 in UV/VIS VIS 10 µg/litre 89 Karweik &
chloroform and NH3/NH4Cl-buffer (434 nm) Meyers (1979)
Water, with Ni(II); phosphate buffer HPLC UV not given 95-100 Moriyasu et al.
solutions (325 nm)d (1984)
Water Cu(II); remainder; titrated titration Cu-ion- lower 98 Hassan et al.
with EDTA selective mg range (1985)
electrode
Table 2 (cont'd)
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Water derivatization with HPLC VIS < 10 µg/litrec 9-97 Koga &
1,2-naphthoquinone-4-sulfonate, (436 nm) Akiyama (1985)
extraction with dichloromethane
Water derivatization to benzene- GC FPD < 2 ng approx.100 Hamano et al.
sulfonamide; extraction with (1980)
n-hexane
Steam addition of KOH to pH > 10 GC FID 1 mg/litre > 90 Malaiyandi et
condensate al. (1979)
Steam none IC CD 100 µg/litre 91-97 Gilbert et al.
condensate (1984)
Steam acidification with HCl; derivatization HPLC VIS 30 µg/litre 96 Lamarre et al.
condensate to dabsyl amide, addition of NaHCO3 (456 nm) (1989)
Blood, extraction with methanol; purificaction GC FID < 4 mg/kgc 55-70 Tombropoulos
tissue, over picrate; neutralisation (tissue) (1979)
urine with CaCO3 < 21 mg/litrec
(blood/urine)
Urine, extraction with methanol homogenized HPLCe UV (196 nm) not given not given Sohn et al.
tissues in KCl, phosphate buffer, a) RP (1982a)
extraction with methane b) IC
Food, steam distillation; derivatization GC/GC-MS FID 200 µg/kg 45-67 Singer &
drinks to p-tosylamide (food) Lijinsky (1976a)
4 µg/litre
(drinks)
Table 2 (cont'd)
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Food homogenization with HCl and GC FPD 10 µg/kg 89-100 Hamano et al.
methanol; derivatization to benzene (1981)
sulfonamide; extraction with n-hexane
Food, addition of alkali; injection GC TEA 87 µg/litre not given Rounbehler &
drinks of the liquid sample Fine (1982)
Citrus steam distillation GC/GC-MS FID 200 µg/kg 95; Ohnishi et al.
fruits 24-87f (1983)
Tobacco, steam distillation; derivatization GC/ FID < 0.3 mg/kgc 50 Singer &
smoke to p-tosylamide GC-MS Lijinsky (1976b)
condensate
Snuff, extraction with water; filtration; GC/ TEA 2 µg/kg 70-80 Brunnemann
tobacco, acidification; extraction with GC-MS et al. (1982)
packing diethylether; nitrosation;
material extraction with dichloromethane
Paper, extraction with HCl; nitrosation with GC TEA 3 µg/kg 90 Hotchkiss &
cardboard NaNO2; extraction with dichloromethane Vecchio (1983)
Rubber extraction/reextraction with GC PND 2 mg/kgc not given Lakritz &
articles dichloromethane/HCl HPLC Kimoto (1980)
Rubber air passed through powdered sample; isotachophoresis not given not given not given Aarts et al.
articles trapped in dil. HCl (1990)
a adapted from BUA (1991); b HPLC = high-performance liquid chromatography, UV = ultraviolet, GC = gas chromatography,
FID = flame ionisation detector, IC = ion chromatography, CD = conductivity detector, TEA = thermal energy analyser,
VIS = visible, FPD = flame photometric detection, PND = phosphorus nitrogen detector, NSD = nitrogen selective detector,
RP = reversed phase; c smallest measurable value (detection limit not given); d measured as diethyldithiocarbamate;
e method used primarily for the separation of morpholine metabolites; f removal efficiency of morpholine from peel
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Morpholine is not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 World producers
a) Producers in USA (Chemical Marketing Reporter, 1989; 1990)
- Air Products and Chemicals
- BASF Co.
- Dow Chemical Co. (up to the end of 1990)
- Texaco Chemical Co.
b) Producers in western Europe (SRI, 1990)
- BASF AG, Ludwigshafen, Germany
- Chemische Werke Hüls AG, Marl, Germany (up to mid-1990)
- Texaco Ltd., Dyfed, Wales, United Kingdom
c) Producers in Japan (Japan Chemical Week, 1991)
- Koei Chemical
- Nippon Nyukazai
- Osaka Organic Chemical Ind.
d) Producers in other countries
Morpholine is manufactured in India and in the Common-wealth of
Independent States (CIS).
3.2.1.2 Production figures
Between 1974 and 1981, USA production was stable at about
11 000 tonnes/year (NRC, 1981). Two new plants were planned in the USA
in the 1980s, namely BASF (with an estimated capacity of
8200 tonnes/year) and Air Products and Chemicals (no capacity given).
BASF reported that in 1988 it manufactured morpholine at Geismar,
Louisiana, USA, as well as importing it from the parent plant in
Germany. The combined import/production volumes were about 30% of a
9000 tonnes/year market, i.e. 2700 tonnes per year (Dynamac
Corporation, 1988). In Germany, about 12 000 tonnes were produced
in 1988, around 75% being exported (BUA, 1991). Production figures
from other European countries are not available. In Japan,
1500-1600 tonnes/year is produced (Japan Chemical Week, 1991). In
India, 200-500 kg/day (60-150 tonnes per year) is manufactured
(Subrahmanyam et al., 1983). Production data from other countries are
not available.
It is estimated that elsewhere in the world around 1000 tonnes of
morpholine are produced annually.
3.2.1.3 Production processes
Three methods of producing morpholine have been described:
a) Reductive ammonation of diethylene glycol and hydrogen at a
pressure of 30-400 × 105 Pa and temperature of 150-400°C.
Possible catalysts include copper, nickel, cobalt, chromium,
molybdenum, manganese, platinum, palladium, rhodium and
ruthenium. Morpholine is recovered by fractional distillation
(Mjos, 1978).
b) Dehydration of diethanolamine with a strong acid such as oleum,
concentrated sulfuric acid or concentrated hydrochloric acid.
The acid is neutralized by the addition of an alkali to give the
free base of morpholine. Morpholine is recovered by extraction
using an organic solvent or concentrated aqueous alkali followed
by distillation (Mjos, 1978).
c) Heating bis(chloroethyl)ether and anhydrous ammonia in a closed
vessel to 50°C for 24 h. After venting the excess ammonia, the
product is filtered from ammonium chloride, and purified
morpholine obtained by distillation (Mjos, 1978).
BASF (Germany) uses method a in a continuous process in a closed
system, and the Texaco Chemical Company also uses method a. Hüls
(Germany) produced morpholine up to 1990 using method b (BUA, 1991).
Air Products and Chemicals use a low-pressure process in their plant
at Pace, Florida, USA (NRC, 1981).
3.2.1.4 Losses to the environment during normal production
A USA study on atmospheric morpholine releases was conducted by
Anderson (1983). No direct measurements were taken, and estimates of
morpholine emissions were based on analogy with emissions from
ethylene oxide production. Total annual emissions (process, storage
and fugitive emissions) from the processing to rubber accelerators (at
96 USA sites) and optical brighteners (at 128 USA sites) were
estimated at 5100 kg/year. Morpholine emission from miscellaneous
uses were estimated at an additional 900 kg/year (Anderson, 1983).
3.2.1.5 Methods of transport
Morpholine should be stored and transported in iron or steel
containers (Air Products and Chemicals, 1989).
3.2.1.6 Accidental release
There are no reports available on accidental releases of
morpholine.
3.2.2 Uses
Morpholine is an extremely versatile chemical. It is most
important as a chemical intermediate in the rubber industry, in
corrosion control, and in the synthesis of a large number of drugs,
crop protection agents, dyes and optical brighteners (Texaco, 1986;
Heilen et al., 1989). Morpholine is a solvent for a large variety of
organic materials, including resins, dyes and waxes (Texaco, 1986).
It can be used as a catalyst. Morpholine is still used in the USA in
toiletry and cosmetic products at concentrations up to 5% (Cosmetic
Ingredient Review, 1989). It is permitted for use in the USA in
several direct and indirect food additive applications.
The use pattern, which varies from country to country, is shown
in Table 3.
Approximately 33% of USA-produced morpholine is used as
intermediates for rubber accelerators and 25% as corrosion inhibitor
in steam boiler systems (Mjos, 1978). A high proportion (25-50%) of
the morpholine produced in Germany is used for optical brighteners in
detergent formulations. In Germany, morpholine-based vulcanization
auxiliaries are either imported or have been replaced by other
products. The use of about half of the morpholine produced in Germany
could not be identified (BUA, 1991).
3.2.2.1 Rubber chemicals
Morpholine derivatives are used in rubber vulcanization,
stabilization and the manufacture of special high-speed tyres.
Morpholine may be released during rubber processing (Mjos, 1978;
Heilen et al., 1989; BUA, 1991).
3.2.2.2 Anticorrosion agent
Morpholine has a volatility similar to water. It is therefore
widely used as a neutralizing amine in combating carbonic acid
corrosion in condensate return lines in steam boiler systems as well
as in aqueous hydraulic liquids and similar systems.
Table 3. Use pattern for morpholine (tonnes/year)
USA (1981)a Germany
(1988-90)b
Rubber chemicals 4920 (40%)
Corrosion inhibitors 3690 (30%) small amounts
Optical brighteners 615 (5%) 750-1500 (25-50%)
Alkyl morpholines 300-400 (10-13%)
Waxes and polishes 615 (5%) < 100 (< 3%)
Diazotype/blueprints 100 (3%)
Miscellaneous/no information 2460 (20%) < 900-1750 (30-60%)
a From: Mannsville Chemical Products (1981)
b From: BUA (1991)
Morpholine vapours protect silver and other metals against
corrosion and tarnish by acid fumes such as SO2 and H2S.
Corrosion of metal aerosol containers and valves can also be prevented
by the use of low levels of morpholine (Texaco, 1986). Morpholine is
effective in hydraulic system fluids based on glycols, where various
metals are in contact with the fluid at the same time (Brown, 1966).
Morpholine derivatives have been used as corrosion inhibitors in
mineral lubricating oil, turbine oils, for protecting storage tanks,
pipes and other devices used in handling petroleum distillates, and
for inhibiting the corrosive action of grease-proof paper on steel and
other metals (Texaco, 1986).
3.2.2.3 Waxes and polishes
Salts of morpholine with long-chain fatty acids, such as oleic or
stearic acid, have wax-like properties and are used as emulsifying
agents in the formulation of water-resistant waxes and polishes for
automobiles, floors, leather and furniture. When the loosely-bound
fatty acid-morpholine compound breaks down, the morpholine component
evaporates at approximately the same rate as water, leaving a film
highly resistant to water spotting and deterioration. Morpholine is
typically present in concentrations up to 2% (Texaco, 1986).
Morpholine is no longer employed in the production of waxes and
polishes in Germany (BUA,1991).
3.2.2.4 Optical brighteners
Optical brighteners are used in detergent formulations in the
soap and detergent industry. The diaminostilbene triazine type
brightener with morpholine as a substituent on one of the triazine
rings is particularly effective on cellulosics and is used in home
laundry detergents because it is stable to chlorine bleaches (Texaco,
1986).
3.2.2.5 Catalysts
Morpholine derivatives such as N-methylmorpholine and
N-ethylmorpholine are used as catalysts for the production of
polyurethane foams.
3.2.2.6 Pharmaceuticals
Morpholine derivatives are used as analgesics and local
anaesthetics (Texaco, 1986; Fisher, 1986; Rekka et al. 1990; Cusano &
Luciano, 1993), antibiotics (Kleemann & Engel, 1982; Schröder et al.
1982; BUA, 1991), antimycotics (Lauharanta, 1992; Reinel & Clarke,
1992) and for plaque control in dentistry (Collaert et al., 1992a,b).
3.2.2.7 Bactericides, fungicides and herbicides
Several morpholine derivatives, e.g., morpholinium salts of
certain acylated sulfonamides, possess bactericidal activity.
Morpholine hydroperiodide has been used as a water disinfectant
(Texaco, 1986).
Morpholine fungicides are used for agricultural purposes (Mercer,
1991), as foliar fungicides with protective and curative properties
for the control of powdery mildew and rust (Brouwers et al., 1992;
Leenheers et al. 1992), and as foliar fungicides for cereals
(Ackermann et al., 1989). Morpholine is also used in the preparation
of herbicides that can be applied either to the soil before the weeds
emerge or to the growing plants (Texaco, 1986).
3.2.2.8 Food additive applications
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (Cosmetic Ingredient
Review, 1989). Certain fatty acid salts of morpholine can be used as
components of protective coatings applied to fruits and vegetables
with the concentrations not allowed to exceed the level required to
produce the intended effect (US FDA, 1988). Indirect food additive
possibilities include the use of morpholine as a corrosion inhibitor
for steel and or tinplate used in food containers (US FDA, 1984a), as
a defoaming agent used in the manufacture of paper and paperboard for
food-packaging materials (US FDA, 1984b), as a component of adhesives
(US FDA, 1984c), and as a defoaming agent in animal glue used for
packaging materials (US FDA, 1984d). Morpholine is only allowed as a
boiler-water additive in concentrations up to 36 mg/m3 (10 ppm), but
is not permitted when the steam comes into contact with food, milk or
milk products (US FDA, 1984e).
In Germany, the use of morpholine in water-repellent food
packaging material is forbidden (BUA, 1991).
3.2.2.9 Cosmetics
Morpholine is used in the USA by the cosmetic industry. Data
submitted to the US Food and Drug Administration (US FDA) in 1981 and
1986 (Cosmetic Ingredient Review, 1989) and in 1991 (Cosmetic
Ingredient Review, 1991a) show that at least in the USA, morpholine is
still used in cosmetic products. In 1981, morpholine was used in 38
cosmetic preparations, the majority (32) being mascara. It is also
used in eyeliner, eye shadow and skin care preparations. Morpholine
is listed by the Cosmetic Ingredient Review as an ingredient used in
cosmetics, although there are insufficient data to substantiate safety
(Cosmetic Ingredient Review, 1989,1991a).
Morpholine is listed in Annex II of the EEC Cosmetics Directive.
Annex II lists compounds that must not be used in cosmetic
formulations. In Germany, the use of morpholine in cosmetic
preparations has been forbidden since 1985 (BUA, 1991) and in the EU
since 1986 (EEC, 1990).
Hydroxybenzomorpholine (HBM) is used as a colour additive for
hair dyes or colorants. In the FDA voluntary cosmetic registration
programme, it is listed as a component of 46 products.
Isostearamidopropyl morpholine lactate (IML), an antistatic agent
primarily used in hair conditioners and products, is present in five
reported cosmetic items. Quaternary morpholinium salts are given as
possible ingredients in hair conditioners and deodorants in wave
formulations (Mjos, 1978). The presence of morpholine as an
ingredient in shampoos has been reported (Spiegelhalder & Preussmann,
1984). However, a German survey in 1990 showed that morpholine was
not present in shampoos in Germany (BUA, 1991).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Volatilization
As morpholine is freely miscible with water, a Henry's constant
cannot be reliably calculated. However, estimates for this constant
(BUA, 1991) have been published. Donath et al. (1977) measured the
distribution coefficient (between vapour and liquid phase) for
morpholine as a function of temperature (50 to 130°C). They found that
the rate of volatilization was dependent on the concentration of
morpholine in the liquid phase. Extrapolation of their curve to 20°C
for morpholine concentrations of 10-15 mg per litre gives a value of
0.02, corresponding to a Henry constant value of 49 Pa.m3.mol-1.
Calculations from Bosholm (1983) give a value corresponding to
244 Pa.m3.mol-1.
According to the classification of Smith et al. (1980),
morpholine belongs to the group of "moderately volatile" substances.
4.2 Transformation
4.2.1 Biodegradation
Morpholine seems to be degraded only by a restricted range of
microbes, mostly Mycobacterium spp., which have specially adapted
(acclimated) themselves to this substrate under specific conditions
(Knapp et al., 1982; Cech et al., 1988; Knapp & Brown, 1988; Brown &
Knapp, 1990). Dmitrenko et al. (1985, 1987) identified an
Arthobacter sp. capable of doing this. Dmitrenko & Gvozdyak (1988)
reported the isolation of morpholine-degrading mycobacteria and found
that these organisms could utilize morpholine anaerobically with
nitrate as a terminal electron acceptor. Calamari et al. (1980) and
Tölgyessy et al. (1986) both reported the resistance of morpholine to
biodegradation. In addition, Tanaka et al. (1968) and Subrahmanyam et
al. (1983) both reported the failure of effluent treatment systems to
degrade morpholine.
Knapp & Brown (1988) isolated 13 morpholine-degrading bacterial
strains of Mycobacterium spp. in pure culture from their laboratory
activated sludge plant (ASP). They also found morpholine-degrading
bacteria in samples from a number of other habitats, including
activated sludge (from two sewage works), water from two rivers,
compost, soil and leaf litter. In all cases, there was a lag period of
10 to 20 days before degradation could be detected. The growth rate of
these morpholine-degrading strains is slow not only on morpholine but
also on other substrates.
Swain et al. (1991) studied the catabolic pathway for morpholine
when Mycobacterium strain MorG was grown with morpholine as sole
source of carbon and nitrogen. The results indicated that morpholine
is initially catabolized to 2-(2-aminoethoxy)acetate which can be
oxidatively cleaved to give rise to glycolate and indirectly to
ethanolamine. Mazure (1993) showed that morpholine can be degraded by
mixed cultures of gram-negative bacteria. Two mixed cultures were
studied, one containing 9 and the other 10 bacterial strains, mostly
pseudomonads. Interestingly, none of the individual strains was
capable of sustained growth with morpholine as a sole carbon and
nitrogen source. The rate at which the two mixed cultures degraded
morpholine was similar to that shown by Mycobacterium aurum in a
study by Cech et al. (1988).
Emtiazi (1993) reported that several Gram-negative bacteria
isolated as degraders of pyrrolidine and piperidine could oxidize
morpholine but could not grow on it as the sole source of carbon and
nitrogen. However, at least one strain could utilize morpholine as a
source of nitrogen if given succinate as a carbon source; the
degradation of morpholine was slower than that shown by mycobacteria.
4.2.1.1 Batch biodegradation tests
In several early studies all employing some form of biological
oxygen demand (BOD) test with unadapted inocula, morpholine was found
to be resistant to biodegradation (Swope & Kenna, 1950; Lamb &
Jenkins, 1952; Mills & Stack, 1953). However, Mills & Stack (1955) in
a later study utilized an inoculum adapted (for 116 days) to the
presence of morpholine and found that morpholine was degraded in a BOD
test after 4 days.
Strotmann et al. (1993) assessed the biodegradability of
morpholine using a test similar to that of the modified OECD Screening
Test (die-away test in an open system with low bacterial density)
(OECD guideline 301 E; OECD, 1981a,b). The inoculum used was taken
from an industrial sewage plant. As morpholine was regularly
discharged into this treatment plant, the inoculum was regarded as
adapted. An unadapted inoculum was obtained from a laboratory-scale
wastewater treatment plant operated with municipal wastewater. The
extent of degradation during the 28-day test (20°C incubation) was
determined by following the decrease in dissolved organic carbon
(DOC). The results showed that morpholine was degraded by both
adapted and unadapted inoculum. The lag period before start of
degradation was about 15 days for the adapted inoculum and 16 days for
the unadapted. The lag period given for the adapted cultures in this
study was rather long, especially considering the result of the
Zahn-Wellens test (below) carried out using the same inoculum. The
degradation period was 5 to 7 days for both unadapted and adapted
cultures. Under the conditions in this test, morpholine showed ready
biodegradability. The activated sludge concentration was about 30 mg
mixed liquor suspended solids (MLSS) per litre. Initial morpholine
concentration was 36 mg/litre.
A Zahn-Wellens Test (a test to estimate inherent
biodegradability) according to OECD guideline 302 B (OECD, 1981a,b)
was also performed by Strotmann et al. (1993). The adapted and
unadapted sludges were obtained as above, but the activated sludge
concentration was higher (1 g MLSS/litre). The concentration of
morpholine was about 725 mg/litre resulting in an initial DOC of
400 mg/litre; test duration was 31 days. Results showed that the lag
period with unadapted and adapted cultures was about 16-20 days and
7 days, respectively. In both cultures the extent of DOC removal was
more than 90% (morpholine was therefore rated as "inherently
biodegradable"). After the lag period, the maximum biodegradation
rates for adapted and unadapted activated sludges were
6 g morpholine/kg MLSS per h and 3 g morpholine/kg MLSS per h,
respectively. In this test the use of an adapted inoculum
significantly shortened the lag time. The authors suggested that this
effect, which was not observed in the modified OECD screening test,
might be due to the higher inoculum concentration used in the
Zahn-Wellens test.
4.2.1.2 Biodegradation in laboratory-scale wastewater treatment
plants
A laboratory-scale wastewater treatment plant operating with
municipal wastewater was supplemented with 4.5 to
5.0 mg morpholine/litre. More than 99% of the ammonia could be
eliminated by nitrification. The total organic carbon (TOC)
degradation ranged between 80 and 94%. The time taken for the sludge
to adapt to morpholine was 10 to 12 days. The adapted sludge of this
treatment plant was reported to be able to degrade morpholine for a
period of more than one month to more than 90% (Strotmann et al.,
1993)
In a die-away test (EEC, 1983), the kinetics of morpholine
biodegradation in the above treatment plant were determined (Strotmann
et al., 1993). At 20 h after adding 40 mg morpholine per litre, 65%
of the morpholine was degraded; after 25 h less than 10% of the added
morpholine was still present. In this adapted treatment plant, the
degradation occurred without any lag period, the maximum degradation
rate (3 g morpholine/kg MLSS per h) being reached after 18 h.
According to the authors, morpholine concentrations of 5 mg/litre in
wastewater can be well degraded in an adapted wastewater treatment
plant. However, shock loading with high concentrations (35 mg/litre)
can result in high concentrations of undegraded morpholine in the
effluent.
A model activated sludge plant capable of treating a simple
industrial waste influent (pH 5.4-5.6) containing morpholine, acetate
and salicylate and mineral salts was set up (Brown & Knapp, 1990).
The activated sludge was taken from the treatment plant of a
morpholine-containing effluent from a large chemical factory. It was
found that when morpholine was absent from the influent, the ability
of the activated sludge to degrade this compound was subsequently
reduced. This was shown by an increase in the lag period before
morpholine degradation could be detected in a die-away test from over
40 days, and was accounted for by a decline in the specific population
of morpholine-degrading microorganisms. The morpholine degradative
phenotype was shown to be genetically unstable in several pure
cultures of mycobacteria (Brown et al., 1990).
Since morpholine-degraders have a low growth rate, they can only
establish themselves in activated sludge if the Mean Solids Retention
Time (sludge age) is relatively long. Under semi-continuous
conditions (800 mg morpholine/litre), a sludge age of 8 days was
needed to achieve complete morpholine degradation (Cech & Chudoba,
1988).
In their investigations into morpholine-degrading bacteria in
river water from several different sites in Yorkshire, United Kingdom,
over a 3-month period, Knapp & Whytell (1990) found, as a general
trend, that the numbers of morpholine-degraders increased and die-away
lag times decreased as water passed downstream. This was probably
related to the cumulative polluting effects of discharges of effluent
to the rivers. The number of morpholine-degraders found in this
investigation agreed with similar studies from rivers in eastern
England. Of the 58 die-away tests carried out on 29 water samples,
only 3 (all from water classed as very clean) failed to reveal
morpholine biodegradation, although in several sites the numbers were
near the limits of detection (Knapp & Whytell, 1990).
4.2.2 Abiotic degradation
4.2.2.1 Hydrolytic degradation
Morpholine can thermally decompose at temperatures used in boiler
steam cycles. Agarwala (1982) found that, at 316°C, morpholine
decomposed in 12 h by 2-5% only, when used in boilers at 95 kg/cm2
and 108 kg/cm2, the decomposition products being ammonia and
carbonic acid products. Under the conditions found in steam-water
cycles in nuclear power plants (260°C and 4.55 MPa), ammonia,
methylamine, ethylamine, ethanolamine and 2-(2-aminoethoxy)ethanol
were identified as morpholine degradation products (Gilbert & Saheb,
1987; Lamarre et al., 1989).
Under normal field conditions, it is assumed that morpholine is
stable. However, no experimental data are available to confirm this.
4.2.2.2 Photochemical degradation
Amines react rapidly with hydroxyl radicals, and the irradiation
of amine-NOx mixtures in air results in the rapid conversion of NO
to NO2 and in the formation of ozone, carbonyls and other products
(Grosjean, 1991). The rate constant for the degradation of morpholine
in the atmosphere by hydroxyl radicals has not yet been measured
experimentally. Grosjean (1991) postulated a rate constant of
2-10 × 10-11 cm3.mol-1.sec-1 and gave a tentative reaction
scheme based on experimental data for dialkylamines.
Using the method of Atkinson (1988), a half-life (for morpholine)
of less than one day has been calculated (BUA, 1991).
As morpholine shows no absorption in the UV spectrum
(lambda > 260 nm), direct photochemical degradation in the atmosphere
or in the hydrosphere is unlikely (BUA, 1991).
4.2.2.3 Degradation by physico-chemical processes
Upon combustion in the presence of sufficient oxygen, carbon
monoxide, carbon dioxide and nitrogen gases are produced.
Combustion under oxygen-starved conditions can result in the
production of carbon monoxide, hydrogen cyanide, nitriles, cyanic
acid, isocyanates, cyanogens, nitrosamines, amides and carbamates
(Air Products and Chemicals, 1989).
4.2.3 Bioaccumulation
There are no data on the bioaccumulation of morpholine in aquatic
and terrestrial organisms. However, as the n-octanol/water
partition coefficient for morpholine is log Pow = -2.55 (at pH 7),
bioaccumulation is not expected (BUA, 1991).
4.3 Interaction with other physical, chemical or biological factors
Due to its carcinogenic properties the formation of NMOR from
morpholine has to be taken into account when assessing health and
environmental aspects of morpholine. NMOR can be formed by reaction
of aqueous solutions of nitrite with morpholine (Mirvish, 1975) or by
reaction of gaseous nitrogen oxides, e.g., N2O3, N2O4, NOx
in aqueous solutions of morpholine, even under normal environmental
conditions (Challis & Kyrtopoulos, 1979; Mirvish et al., 1988;
Schuster et al., 1990). Nitrogen oxide (NO) levels may be higher than
was previously thought (Cooney et al. 1992; Hibbs, 1992). The
conditions of nitrosation, in particular the pH, plays a significant
role.
In aqueous solutions, the reaction is as follows:
CAS registry number: 110-91-8
EEC number: 613-028-00-9
EINECS number: 2038151
UN number: 2054
Synonyms: 1-oxa-4-azacyclohexane
tetrahydro-2H-1,4-oxazine
tetrahydro-1,4-oxazine
tetrahydro-1,4-isoxazine
diethylene oximide
diethyleneimide oxide
diethylene imidoxide
Relative molecular mass: 87.12
2.1.1 Technical product
The compound is marketed under the name of "Morpholine". It is
distributed as an anhydrous liquid and as 40% and 88% solutions with
water (Air Products and Chemicals, 1989).
2.1.2 Impurities
Morpholine is marketed as a product with approximately 99% purity
(Cosmetic Ingredient Review, 1989; BUA, 1991). The exact chemical
nature of the impurities depends on the production process (see
section 3.2.1.3). When produced from diethylene glycol,
2-(2-aminoethoxy)ethanol is a by-product, which can be isolated and
recycled (Heilen et al., 1989). Reported impurities are
N-ethylmorpholine and ethylenediamine (Heilen et al., 1989) and
2-methoxy ethanol (BUA, 1991).
During the production of morpholine from diethanolamine, it is
possible that N-hydroxyethylmorpholine may be formed (Cosmetic
Ingredient Review, 1989).
Impurities in cosmetic grade morpholine have been reported to
include arsenic (up to 3 mg/kg) and lead (up to 20 mg/kg) (Estrin et
al., 1982). The Cosmetic Ingredient Review (1991a) lists morpholine
as having insufficient data on impurities.
Fajen et al. (1979) found 0.8 mg/kg N-nitrosomorpholine (NMOR)
in a morpholine charge used for the production of a vulcanization
accelerator in a chemical factory in Ohio. NMOR could not be detected
(detection limit: 50 µg/kg) in morpholine stored under nitrogen in
Germany (BUA, 1991).
2.2 Physical and chemical properties
2.2.1 Physical properties of morpholine
Morpholine is a colourless, oily, hygroscopic, volatile liquid
with a characteristic amine smell (Reinhardt & Brittelli, 1981).
Morpholine vapour is heavier than air and as a result, the vapour can
travel a significant distance to a source of ignition and "flash
back".
It is completely miscible with water, soluble in the usual
solvents and can itself be used as a solvent (Heilen et al., 1989).
It has a low solubility in alkaline aqueous solutions.
Morpholine is a strong base, the 0.01% (w/w) mixture having a
pH of 9.4, and the 10% (w/w) mixture having a pH of 11.2 (Texaco,
1986).
Some physical and chemical properties are presented in Table 1.
2.2.1.1 Storage of morpholine
Morpholine can be stored for an unlimited time in iron or steel
containers if protected from atmospheric moisture and carbon dioxide.
However, it is unstable in the presence of copper, zinc and their
alloys and these metals should not be used in storage containers for
morpholine (Heilen et al., 1989; Air Products and Chemicals, 1989).
2.2.2 Chemical properties of morpholine
Morpholine can undergo a diversity of reactions. It is an amino
ether; the ether function of the molecule is typically inert and most
of the reactions involve the secondary amine group.
Table 1. Some physical and chemical properties of morpholine
Melting point (°C) -3.1a; -4.9b,c; -5d
Boiling point (°C at 1013 hPa) 128.2a; 128.3c; 128.9b; 128-130d
Flash point (°C) - Open cup 38b;
- Closed cup 35c; 31d
Autoignition temperature (°C) 275a,d; 310c;
Explosion limits in air 1.4-13.1 vol% d; 1.8-11 vol% e;
1.8-15.2 vol% a
Decomposition temperature > 330°Cd; > 550°C
(in steam cycles)a
pKa (conjugated acid) 8.33 (25°C)f;
8.36c (temperature not given)
Vapour pressure (°C) 10 20 40 60 80 100 120
(kPa) 0.6 1.1 3.2 8.3 10.5 40.9 81.8
Density g/cm3 (20°C) 0.994b; 0.999c; 1.00d; 1.007a
log n-octanol/water partition -0.723 (free base; calculated)g
coefficient (log Pow) -1.08 (free base; calculated)h
-0.66 (free base; calculated)i
Solubility in water completely miscible with watera
Solubility in organic solvents completely miscible with, for
instance, methanol, ethanol, acetone,
diethylether, benzene, toluene,
xylolc,e
Refractive index 1.4537-1.4545 at 20°Ce
Human olfactory threshold 0.036 (mg/m3)j
a Heilen et al. (1989); b Brown (1966); c Texaco (1986);
d BASF (1987); e Cosmetic Ingredient Review (1989);
f Lide (1990); g UBA (1990); h Leo et al. (1971);
i Le Therizien et al. (1980); j Hellman & Small (1974)
It reacts with inorganic acids and acid gases such as CO2,
H2S, or HCN to form morpholine salts. This property is of use in
the addition of morpholine as an anticorrosive in boiler systems
(Brown, 1966). Morpholine can react with oxidizing agents, undergo
direct chlorination, and form complexes with metallic halides. It
reacts with carboxylic acids, anhydrides, chlorides and esters to form
morpholides (Brown, 1966). Alkyl morpholides are formed by reaction
of morpholine with alkyl halides, dialkyl sulfates or trialkyl
phosphates. The N-alkylmorpholides, particularly
N-methylmorpholides, and N-ethylmorpholides, are widely used as
catalysts in the preparation of polyurethanes (Brown, 1966).
Morpholine reacts with formaldehyde to form N-formyl-morpholine,
which is used industrially as a selective solvent for the extraction
of very pure aromatic compounds (Heilen et al., 1989).
Morpholine reacts with fatty acids to form soaps which are used
in household and automotive waxes and polishes. Their principal
advantage is that the morpholine evaporates at the same rate as water,
leaving a water-resistant wax base (Mjos, 1978; Texaco, 1986).
Vulcanizing agents for the rubber industry are formed by the reaction
of morpholine with sulfur and sulfur-containing compounds (Taylor &
Son, 1982).
Morpholine is flammable. Violent reaction and fire may result
when the product is mixed with oxidizing agents (Air Products and
Chemicals, 1989).
N-nitrosomorpholine (NMOR) can be formed by reaction of aqueous
solutions of nitrite with morpholine or by reaction of gaseous
nitrogen oxides in aqueous solutions of morpholine (see section 4.3).
2.3 Conversion factors for morpholine
1 mg/m3 = 0.276 ppm at 20°C and 1013 hPa
1 ppm = 3.62 mg/m3
2.4 Analytical methods
Methods suitable for measuring trace levels of morpholine include
ion chromatography (IC), gas chromatography (GC) with packed as well
as capillary columns, and high-performance liquid chromatography
(HPLC), usually using reverse phase (RP) columns.
The poor UV absorptivity of morpholine necessitates chemical
derivatization to detect trace amounts.
Detection methods include UV detectors (for HPLC) and flame
ionisation detectors (FID, following GC), as well as thermal energy
analysers (TEA). Photochemical methods are used but are not specific
for morpholine.
An overview of the analytical methods for determining morpholine
in various matrices is given in Table 2.
For the detection of trace amounts of NMOR, GC or HPLC together
with TEA has proved to be the method of choice. The use of internal
standards helps to distinguish NMOR in the sample from artifacts
caused by nitrosation or transnitrosation during the work-up procedure
(BUA,1991; ECETOC, 1991).
2.4.1 Determination of morpholine in air
Table 2 summarizes the available methods.
Air samples can be collected and concentrated by passing through
silica gel or an impinger containing dilute acid. A 20-litre sample
is recommended to reach concentrations between 7 and 210 mg/m3
(NIOSH, 1977). Bianchi & Muccioli (1978) collected air samples
without absorption on a solvent and rapidly performed the GC.
Sollenberg & Hansen (1987) described an isotachophoretic
determination of morpholine using 10 mM potassium cacodylate (pH 6.5)
as leading electrolyte, and 10 mM creatinine with 5 mM HCl as
terminating electrolyte. This method has been used primarily to
measure N-methylmorpholine in air samples from a polyurethane foam
factory (Hansen et al., 1986). Aarts et al. (1990) also used an
isotachophoretic method for determining morpholine in rubber samples
(see Table 2).
2.4.2 Determination of morpholine in water
The methods given in Table 2 (water) are suitable for the
determination of morpholine in steam condensates or non-aqueous
solvents.
2.4.3 Determination of morpholine in soil and sediments
A GC/MS method has been used to detect morpholine in sediment and
soil (Spies et al., 1987).
2.4.4 Determination in biological and other materials
Morpholine has been determined in biological tissues and fluids
using GC/FID (Tombropoulos, 1979). Morpholine and some of its
metabolites ( N-hydroxymorpholine, N-methylmorpholine and
N-methylmorpholine- N-oxide) could be separated using two
complementary HPLCs, one using reversed-phase and the other
ion-exchange chromatography (Sohn et al., 1982a).
Morpholine has been determined in a number of foods and beverages
as well as in tobacco, snuff and packaging material (see section
5.2.2). The methods used are summarized in Table 2. Generally,
morpholine is extracted from the samples using steam distillation
followed by purification and derivatization.
Table 2. Methods for the analysis of morpholinea
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Air adsorption on silica gel, GC FID 7 mg/m3c 100 NIOSH (1977)
desorption with H2SO4,
neutralization with NaOH
Air collected directly GC FID 36 mg/m3 not given Bianchi &
Muccioli (1978)
Air absorption in 1 N KOH (impinger); GC TEA not given not given Fajen et al.
extraction with dichloromethane (1979)
Air adsorption on silica gel; HPLC UV not given 90-96 Simon & Lemacon
derivatization to m-toluamides (235-255 nm) (1987)
Air absorption on silica gel, GC NSD 0.03 mg/m3 93 ± 5% BIA (1989)
extraction with methanol
+ 2% KOH
Water derivatization to p-tosylamide, GC FID 70 ng/litre 45-67 Singer &
acidification with HCl (pH 1), Lijinsky (1976a)
extraction with diethylether
Water addition of Cu(II), CS2 in UV/VIS VIS 10 µg/litre 89 Karweik &
chloroform and NH3/NH4Cl-buffer (434 nm) Meyers (1979)
Water, with Ni(II); phosphate buffer HPLC UV not given 95-100 Moriyasu et al.
solutions (325 nm)d (1984)
Water Cu(II); remainder; titrated titration Cu-ion- lower 98 Hassan et al.
with EDTA selective mg range (1985)
electrode
Table 2 (cont'd)
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Water derivatization with HPLC VIS < 10 µg/litrec 9-97 Koga &
1,2-naphthoquinone-4-sulfonate, (436 nm) Akiyama (1985)
extraction with dichloromethane
Water derivatization to benzene- GC FPD < 2 ng approx.100 Hamano et al.
sulfonamide; extraction with (1980)
n-hexane
Steam addition of KOH to pH > 10 GC FID 1 mg/litre > 90 Malaiyandi et
condensate al. (1979)
Steam none IC CD 100 µg/litre 91-97 Gilbert et al.
condensate (1984)
Steam acidification with HCl; derivatization HPLC VIS 30 µg/litre 96 Lamarre et al.
condensate to dabsyl amide, addition of NaHCO3 (456 nm) (1989)
Blood, extraction with methanol; purificaction GC FID < 4 mg/kgc 55-70 Tombropoulos
tissue, over picrate; neutralisation (tissue) (1979)
urine with CaCO3 < 21 mg/litrec
(blood/urine)
Urine, extraction with methanol homogenized HPLCe UV (196 nm) not given not given Sohn et al.
tissues in KCl, phosphate buffer, a) RP (1982a)
extraction with methane b) IC
Food, steam distillation; derivatization GC/GC-MS FID 200 µg/kg 45-67 Singer &
drinks to p-tosylamide (food) Lijinsky (1976a)
4 µg/litre
(drinks)
Table 2 (cont'd)
Matrix Sample preparation Methodb Detectorb Detection Recovery References
limit (%)
Food homogenization with HCl and GC FPD 10 µg/kg 89-100 Hamano et al.
methanol; derivatization to benzene (1981)
sulfonamide; extraction with n-hexane
Food, addition of alkali; injection GC TEA 87 µg/litre not given Rounbehler &
drinks of the liquid sample Fine (1982)
Citrus steam distillation GC/GC-MS FID 200 µg/kg 95; Ohnishi et al.
fruits 24-87f (1983)
Tobacco, steam distillation; derivatization GC/ FID < 0.3 mg/kgc 50 Singer &
smoke to p-tosylamide GC-MS Lijinsky (1976b)
condensate
Snuff, extraction with water; filtration; GC/ TEA 2 µg/kg 70-80 Brunnemann
tobacco, acidification; extraction with GC-MS et al. (1982)
packing diethylether; nitrosation;
material extraction with dichloromethane
Paper, extraction with HCl; nitrosation with GC TEA 3 µg/kg 90 Hotchkiss &
cardboard NaNO2; extraction with dichloromethane Vecchio (1983)
Rubber extraction/reextraction with GC PND 2 mg/kgc not given Lakritz &
articles dichloromethane/HCl HPLC Kimoto (1980)
Rubber air passed through powdered sample; isotachophoresis not given not given not given Aarts et al.
articles trapped in dil. HCl (1990)
a adapted from BUA (1991); b HPLC = high-performance liquid chromatography, UV = ultraviolet, GC = gas chromatography,
FID = flame ionisation detector, IC = ion chromatography, CD = conductivity detector, TEA = thermal energy analyser,
VIS = visible, FPD = flame photometric detection, PND = phosphorus nitrogen detector, NSD = nitrogen selective detector,
RP = reversed phase; c smallest measurable value (detection limit not given); d measured as diethyldithiocarbamate;
e method used primarily for the separation of morpholine metabolites; f removal efficiency of morpholine from peel
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Morpholine is not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 World producers
a) Producers in USA (Chemical Marketing Reporter, 1989; 1990)
- Air Products and Chemicals
- BASF Co.
- Dow Chemical Co. (up to the end of 1990)
- Texaco Chemical Co.
b) Producers in western Europe (SRI, 1990)
- BASF AG, Ludwigshafen, Germany
- Chemische Werke Hüls AG, Marl, Germany (up to mid-1990)
- Texaco Ltd., Dyfed, Wales, United Kingdom
c) Producers in Japan (Japan Chemical Week, 1991)
- Koei Chemical
- Nippon Nyukazai
- Osaka Organic Chemical Ind.
d) Producers in other countries
Morpholine is manufactured in India and in the Common-wealth of
Independent States (CIS).
3.2.1.2 Production figures
Between 1974 and 1981, USA production was stable at about
11 000 tonnes/year (NRC, 1981). Two new plants were planned in the USA
in the 1980s, namely BASF (with an estimated capacity of
8200 tonnes/year) and Air Products and Chemicals (no capacity given).
BASF reported that in 1988 it manufactured morpholine at Geismar,
Louisiana, USA, as well as importing it from the parent plant in
Germany. The combined import/production volumes were about 30% of a
9000 tonnes/year market, i.e. 2700 tonnes per year (Dynamac
Corporation, 1988). In Germany, about 12 000 tonnes were produced
in 1988, around 75% being exported (BUA, 1991). Production figures
from other European countries are not available. In Japan,
1500-1600 tonnes/year is produced (Japan Chemical Week, 1991). In
India, 200-500 kg/day (60-150 tonnes per year) is manufactured
(Subrahmanyam et al., 1983). Production data from other countries are
not available.
It is estimated that elsewhere in the world around 1000 tonnes of
morpholine are produced annually.
3.2.1.3 Production processes
Three methods of producing morpholine have been described:
a) Reductive ammonation of diethylene glycol and hydrogen at a
pressure of 30-400 × 105 Pa and temperature of 150-400°C.
Possible catalysts include copper, nickel, cobalt, chromium,
molybdenum, manganese, platinum, palladium, rhodium and
ruthenium. Morpholine is recovered by fractional distillation
(Mjos, 1978).
b) Dehydration of diethanolamine with a strong acid such as oleum,
concentrated sulfuric acid or concentrated hydrochloric acid.
The acid is neutralized by the addition of an alkali to give the
free base of morpholine. Morpholine is recovered by extraction
using an organic solvent or concentrated aqueous alkali followed
by distillation (Mjos, 1978).
c) Heating bis(chloroethyl)ether and anhydrous ammonia in a closed
vessel to 50°C for 24 h. After venting the excess ammonia, the
product is filtered from ammonium chloride, and purified
morpholine obtained by distillation (Mjos, 1978).
BASF (Germany) uses method a in a continuous process in a closed
system, and the Texaco Chemical Company also uses method a. Hüls
(Germany) produced morpholine up to 1990 using method b (BUA, 1991).
Air Products and Chemicals use a low-pressure process in their plant
at Pace, Florida, USA (NRC, 1981).
3.2.1.4 Losses to the environment during normal production
A USA study on atmospheric morpholine releases was conducted by
Anderson (1983). No direct measurements were taken, and estimates of
morpholine emissions were based on analogy with emissions from
ethylene oxide production. Total annual emissions (process, storage
and fugitive emissions) from the processing to rubber accelerators (at
96 USA sites) and optical brighteners (at 128 USA sites) were
estimated at 5100 kg/year. Morpholine emission from miscellaneous
uses were estimated at an additional 900 kg/year (Anderson, 1983).
3.2.1.5 Methods of transport
Morpholine should be stored and transported in iron or steel
containers (Air Products and Chemicals, 1989).
3.2.1.6 Accidental release
There are no reports available on accidental releases of
morpholine.
3.2.2 Uses
Morpholine is an extremely versatile chemical. It is most
important as a chemical intermediate in the rubber industry, in
corrosion control, and in the synthesis of a large number of drugs,
crop protection agents, dyes and optical brighteners (Texaco, 1986;
Heilen et al., 1989). Morpholine is a solvent for a large variety of
organic materials, including resins, dyes and waxes (Texaco, 1986).
It can be used as a catalyst. Morpholine is still used in the USA in
toiletry and cosmetic products at concentrations up to 5% (Cosmetic
Ingredient Review, 1989). It is permitted for use in the USA in
several direct and indirect food additive applications.
The use pattern, which varies from country to country, is shown
in Table 3.
Approximately 33% of USA-produced morpholine is used as
intermediates for rubber accelerators and 25% as corrosion inhibitor
in steam boiler systems (Mjos, 1978). A high proportion (25-50%) of
the morpholine produced in Germany is used for optical brighteners in
detergent formulations. In Germany, morpholine-based vulcanization
auxiliaries are either imported or have been replaced by other
products. The use of about half of the morpholine produced in Germany
could not be identified (BUA, 1991).
3.2.2.1 Rubber chemicals
Morpholine derivatives are used in rubber vulcanization,
stabilization and the manufacture of special high-speed tyres.
Morpholine may be released during rubber processing (Mjos, 1978;
Heilen et al., 1989; BUA, 1991).
3.2.2.2 Anticorrosion agent
Morpholine has a volatility similar to water. It is therefore
widely used as a neutralizing amine in combating carbonic acid
corrosion in condensate return lines in steam boiler systems as well
as in aqueous hydraulic liquids and similar systems.
Table 3. Use pattern for morpholine (tonnes/year)
USA (1981)a Germany
(1988-90)b
Rubber chemicals 4920 (40%)
Corrosion inhibitors 3690 (30%) small amounts
Optical brighteners 615 (5%) 750-1500 (25-50%)
Alkyl morpholines 300-400 (10-13%)
Waxes and polishes 615 (5%) < 100 (< 3%)
Diazotype/blueprints 100 (3%)
Miscellaneous/no information 2460 (20%) < 900-1750 (30-60%)
a From: Mannsville Chemical Products (1981)
b From: BUA (1991)
Morpholine vapours protect silver and other metals against
corrosion and tarnish by acid fumes such as SO2 and H2S.
Corrosion of metal aerosol containers and valves can also be prevented
by the use of low levels of morpholine (Texaco, 1986). Morpholine is
effective in hydraulic system fluids based on glycols, where various
metals are in contact with the fluid at the same time (Brown, 1966).
Morpholine derivatives have been used as corrosion inhibitors in
mineral lubricating oil, turbine oils, for protecting storage tanks,
pipes and other devices used in handling petroleum distillates, and
for inhibiting the corrosive action of grease-proof paper on steel and
other metals (Texaco, 1986).
3.2.2.3 Waxes and polishes
Salts of morpholine with long-chain fatty acids, such as oleic or
stearic acid, have wax-like properties and are used as emulsifying
agents in the formulation of water-resistant waxes and polishes for
automobiles, floors, leather and furniture. When the loosely-bound
fatty acid-morpholine compound breaks down, the morpholine component
evaporates at approximately the same rate as water, leaving a film
highly resistant to water spotting and deterioration. Morpholine is
typically present in concentrations up to 2% (Texaco, 1986).
Morpholine is no longer employed in the production of waxes and
polishes in Germany (BUA,1991).
3.2.2.4 Optical brighteners
Optical brighteners are used in detergent formulations in the
soap and detergent industry. The diaminostilbene triazine type
brightener with morpholine as a substituent on one of the triazine
rings is particularly effective on cellulosics and is used in home
laundry detergents because it is stable to chlorine bleaches (Texaco,
1986).
3.2.2.5 Catalysts
Morpholine derivatives such as N-methylmorpholine and
N-ethylmorpholine are used as catalysts for the production of
polyurethane foams.
3.2.2.6 Pharmaceuticals
Morpholine derivatives are used as analgesics and local
anaesthetics (Texaco, 1986; Fisher, 1986; Rekka et al. 1990; Cusano &
Luciano, 1993), antibiotics (Kleemann & Engel, 1982; Schröder et al.
1982; BUA, 1991), antimycotics (Lauharanta, 1992; Reinel & Clarke,
1992) and for plaque control in dentistry (Collaert et al., 1992a,b).
3.2.2.7 Bactericides, fungicides and herbicides
Several morpholine derivatives, e.g., morpholinium salts of
certain acylated sulfonamides, possess bactericidal activity.
Morpholine hydroperiodide has been used as a water disinfectant
(Texaco, 1986).
Morpholine fungicides are used for agricultural purposes (Mercer,
1991), as foliar fungicides with protective and curative properties
for the control of powdery mildew and rust (Brouwers et al., 1992;
Leenheers et al. 1992), and as foliar fungicides for cereals
(Ackermann et al., 1989). Morpholine is also used in the preparation
of herbicides that can be applied either to the soil before the weeds
emerge or to the growing plants (Texaco, 1986).
3.2.2.8 Food additive applications
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (Cosmetic Ingredient
Review, 1989). Certain fatty acid salts of morpholine can be used as
components of protective coatings applied to fruits and vegetables
with the concentrations not allowed to exceed the level required to
produce the intended effect (US FDA, 1988). Indirect food additive
possibilities include the use of morpholine as a corrosion inhibitor
for steel and or tinplate used in food containers (US FDA, 1984a), as
a defoaming agent used in the manufacture of paper and paperboard for
food-packaging materials (US FDA, 1984b), as a component of adhesives
(US FDA, 1984c), and as a defoaming agent in animal glue used for
packaging materials (US FDA, 1984d). Morpholine is only allowed as a
boiler-water additive in concentrations up to 36 mg/m3 (10 ppm), but
is not permitted when the steam comes into contact with food, milk or
milk products (US FDA, 1984e).
In Germany, the use of morpholine in water-repellent food
packaging material is forbidden (BUA, 1991).
3.2.2.9 Cosmetics
Morpholine is used in the USA by the cosmetic industry. Data
submitted to the US Food and Drug Administration (US FDA) in 1981 and
1986 (Cosmetic Ingredient Review, 1989) and in 1991 (Cosmetic
Ingredient Review, 1991a) show that at least in the USA, morpholine is
still used in cosmetic products. In 1981, morpholine was used in 38
cosmetic preparations, the majority (32) being mascara. It is also
used in eyeliner, eye shadow and skin care preparations. Morpholine
is listed by the Cosmetic Ingredient Review as an ingredient used in
cosmetics, although there are insufficient data to substantiate safety
(Cosmetic Ingredient Review, 1989,1991a).
Morpholine is listed in Annex II of the EEC Cosmetics Directive.
Annex II lists compounds that must not be used in cosmetic
formulations. In Germany, the use of morpholine in cosmetic
preparations has been forbidden since 1985 (BUA, 1991) and in the EU
since 1986 (EEC, 1990).
Hydroxybenzomorpholine (HBM) is used as a colour additive for
hair dyes or colorants. In the FDA voluntary cosmetic registration
programme, it is listed as a component of 46 products.
Isostearamidopropyl morpholine lactate (IML), an antistatic agent
primarily used in hair conditioners and products, is present in five
reported cosmetic items. Quaternary morpholinium salts are given as
possible ingredients in hair conditioners and deodorants in wave
formulations (Mjos, 1978). The presence of morpholine as an
ingredient in shampoos has been reported (Spiegelhalder & Preussmann,
1984). However, a German survey in 1990 showed that morpholine was
not present in shampoos in Germany (BUA, 1991).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Volatilization
As morpholine is freely miscible with water, a Henry's constant
cannot be reliably calculated. However, estimates for this constant
(BUA, 1991) have been published. Donath et al. (1977) measured the
distribution coefficient (between vapour and liquid phase) for
morpholine as a function of temperature (50 to 130°C). They found that
the rate of volatilization was dependent on the concentration of
morpholine in the liquid phase. Extrapolation of their curve to 20°C
for morpholine concentrations of 10-15 mg per litre gives a value of
0.02, corresponding to a Henry constant value of 49 Pa.m3.mol-1.
Calculations from Bosholm (1983) give a value corresponding to
244 Pa.m3.mol-1.
According to the classification of Smith et al. (1980),
morpholine belongs to the group of "moderately volatile" substances.
4.2 Transformation
4.2.1 Biodegradation
Morpholine seems to be degraded only by a restricted range of
microbes, mostly Mycobacterium spp., which have specially adapted
(acclimated) themselves to this substrate under specific conditions
(Knapp et al., 1982; Cech et al., 1988; Knapp & Brown, 1988; Brown &
Knapp, 1990). Dmitrenko et al. (1985, 1987) identified an
Arthobacter sp. capable of doing this. Dmitrenko & Gvozdyak (1988)
reported the isolation of morpholine-degrading mycobacteria and found
that these organisms could utilize morpholine anaerobically with
nitrate as a terminal electron acceptor. Calamari et al. (1980) and
Tölgyessy et al. (1986) both reported the resistance of morpholine to
biodegradation. In addition, Tanaka et al. (1968) and Subrahmanyam et
al. (1983) both reported the failure of effluent treatment systems to
degrade morpholine.
Knapp & Brown (1988) isolated 13 morpholine-degrading bacterial
strains of Mycobacterium spp. in pure culture from their laboratory
activated sludge plant (ASP). They also found morpholine-degrading
bacteria in samples from a number of other habitats, including
activated sludge (from two sewage works), water from two rivers,
compost, soil and leaf litter. In all cases, there was a lag period of
10 to 20 days before degradation could be detected. The growth rate of
these morpholine-degrading strains is slow not only on morpholine but
also on other substrates.
Swain et al. (1991) studied the catabolic pathway for morpholine
when Mycobacterium strain MorG was grown with morpholine as sole
source of carbon and nitrogen. The results indicated that morpholine
is initially catabolized to 2-(2-aminoethoxy)acetate which can be
oxidatively cleaved to give rise to glycolate and indirectly to
ethanolamine. Mazure (1993) showed that morpholine can be degraded by
mixed cultures of gram-negative bacteria. Two mixed cultures were
studied, one containing 9 and the other 10 bacterial strains, mostly
pseudomonads. Interestingly, none of the individual strains was
capable of sustained growth with morpholine as a sole carbon and
nitrogen source. The rate at which the two mixed cultures degraded
morpholine was similar to that shown by Mycobacterium aurum in a
study by Cech et al. (1988).
Emtiazi (1993) reported that several Gram-negative bacteria
isolated as degraders of pyrrolidine and piperidine could oxidize
morpholine but could not grow on it as the sole source of carbon and
nitrogen. However, at least one strain could utilize morpholine as a
source of nitrogen if given succinate as a carbon source; the
degradation of morpholine was slower than that shown by mycobacteria.
4.2.1.1 Batch biodegradation tests
In several early studies all employing some form of biological
oxygen demand (BOD) test with unadapted inocula, morpholine was found
to be resistant to biodegradation (Swope & Kenna, 1950; Lamb &
Jenkins, 1952; Mills & Stack, 1953). However, Mills & Stack (1955) in
a later study utilized an inoculum adapted (for 116 days) to the
presence of morpholine and found that morpholine was degraded in a BOD
test after 4 days.
Strotmann et al. (1993) assessed the biodegradability of
morpholine using a test similar to that of the modified OECD Screening
Test (die-away test in an open system with low bacterial density)
(OECD guideline 301 E; OECD, 1981a,b). The inoculum used was taken
from an industrial sewage plant. As morpholine was regularly
discharged into this treatment plant, the inoculum was regarded as
adapted. An unadapted inoculum was obtained from a laboratory-scale
wastewater treatment plant operated with municipal wastewater. The
extent of degradation during the 28-day test (20°C incubation) was
determined by following the decrease in dissolved organic carbon
(DOC). The results showed that morpholine was degraded by both
adapted and unadapted inoculum. The lag period before start of
degradation was about 15 days for the adapted inoculum and 16 days for
the unadapted. The lag period given for the adapted cultures in this
study was rather long, especially considering the result of the
Zahn-Wellens test (below) carried out using the same inoculum. The
degradation period was 5 to 7 days for both unadapted and adapted
cultures. Under the conditions in this test, morpholine showed ready
biodegradability. The activated sludge concentration was about 30 mg
mixed liquor suspended solids (MLSS) per litre. Initial morpholine
concentration was 36 mg/litre.
A Zahn-Wellens Test (a test to estimate inherent
biodegradability) according to OECD guideline 302 B (OECD, 1981a,b)
was also performed by Strotmann et al. (1993). The adapted and
unadapted sludges were obtained as above, but the activated sludge
concentration was higher (1 g MLSS/litre). The concentration of
morpholine was about 725 mg/litre resulting in an initial DOC of
400 mg/litre; test duration was 31 days. Results showed that the lag
period with unadapted and adapted cultures was about 16-20 days and
7 days, respectively. In both cultures the extent of DOC removal was
more than 90% (morpholine was therefore rated as "inherently
biodegradable"). After the lag period, the maximum biodegradation
rates for adapted and unadapted activated sludges were
6 g morpholine/kg MLSS per h and 3 g morpholine/kg MLSS per h,
respectively. In this test the use of an adapted inoculum
significantly shortened the lag time. The authors suggested that this
effect, which was not observed in the modified OECD screening test,
might be due to the higher inoculum concentration used in the
Zahn-Wellens test.
4.2.1.2 Biodegradation in laboratory-scale wastewater treatment
plants
A laboratory-scale wastewater treatment plant operating with
municipal wastewater was supplemented with 4.5 to
5.0 mg morpholine/litre. More than 99% of the ammonia could be
eliminated by nitrification. The total organic carbon (TOC)
degradation ranged between 80 and 94%. The time taken for the sludge
to adapt to morpholine was 10 to 12 days. The adapted sludge of this
treatment plant was reported to be able to degrade morpholine for a
period of more than one month to more than 90% (Strotmann et al.,
1993)
In a die-away test (EEC, 1983), the kinetics of morpholine
biodegradation in the above treatment plant were determined (Strotmann
et al., 1993). At 20 h after adding 40 mg morpholine per litre, 65%
of the morpholine was degraded; after 25 h less than 10% of the added
morpholine was still present. In this adapted treatment plant, the
degradation occurred without any lag period, the maximum degradation
rate (3 g morpholine/kg MLSS per h) being reached after 18 h.
According to the authors, morpholine concentrations of 5 mg/litre in
wastewater can be well degraded in an adapted wastewater treatment
plant. However, shock loading with high concentrations (35 mg/litre)
can result in high concentrations of undegraded morpholine in the
effluent.
A model activated sludge plant capable of treating a simple
industrial waste influent (pH 5.4-5.6) containing morpholine, acetate
and salicylate and mineral salts was set up (Brown & Knapp, 1990).
The activated sludge was taken from the treatment plant of a
morpholine-containing effluent from a large chemical factory. It was
found that when morpholine was absent from the influent, the ability
of the activated sludge to degrade this compound was subsequently
reduced. This was shown by an increase in the lag period before
morpholine degradation could be detected in a die-away test from over
40 days, and was accounted for by a decline in the specific population
of morpholine-degrading microorganisms. The morpholine degradative
phenotype was shown to be genetically unstable in several pure
cultures of mycobacteria (Brown et al., 1990).
Since morpholine-degraders have a low growth rate, they can only
establish themselves in activated sludge if the Mean Solids Retention
Time (sludge age) is relatively long. Under semi-continuous
conditions (800 mg morpholine/litre), a sludge age of 8 days was
needed to achieve complete morpholine degradation (Cech & Chudoba,
1988).
In their investigations into morpholine-degrading bacteria in
river water from several different sites in Yorkshire, United Kingdom,
over a 3-month period, Knapp & Whytell (1990) found, as a general
trend, that the numbers of morpholine-degraders increased and die-away
lag times decreased as water passed downstream. This was probably
related to the cumulative polluting effects of discharges of effluent
to the rivers. The number of morpholine-degraders found in this
investigation agreed with similar studies from rivers in eastern
England. Of the 58 die-away tests carried out on 29 water samples,
only 3 (all from water classed as very clean) failed to reveal
morpholine biodegradation, although in several sites the numbers were
near the limits of detection (Knapp & Whytell, 1990).
4.2.2 Abiotic degradation
4.2.2.1 Hydrolytic degradation
Morpholine can thermally decompose at temperatures used in boiler
steam cycles. Agarwala (1982) found that, at 316°C, morpholine
decomposed in 12 h by 2-5% only, when used in boilers at 95 kg/cm2
and 108 kg/cm2, the decomposition products being ammonia and
carbonic acid products. Under the conditions found in steam-water
cycles in nuclear power plants (260°C and 4.55 MPa), ammonia,
methylamine, ethylamine, ethanolamine and 2-(2-aminoethoxy)ethanol
were identified as morpholine degradation products (Gilbert & Saheb,
1987; Lamarre et al., 1989).
Under normal field conditions, it is assumed that morpholine is
stable. However, no experimental data are available to confirm this.
4.2.2.2 Photochemical degradation
Amines react rapidly with hydroxyl radicals, and the irradiation
of amine-NOx mixtures in air results in the rapid conversion of NO
to NO2 and in the formation of ozone, carbonyls and other products
(Grosjean, 1991). The rate constant for the degradation of morpholine
in the atmosphere by hydroxyl radicals has not yet been measured
experimentally. Grosjean (1991) postulated a rate constant of
2-10 × 10-11 cm3.mol-1.sec-1 and gave a tentative reaction
scheme based on experimental data for dialkylamines.
Using the method of Atkinson (1988), a half-life (for morpholine)
of less than one day has been calculated (BUA, 1991).
As morpholine shows no absorption in the UV spectrum
(lambda > 260 nm), direct photochemical degradation in the atmosphere
or in the hydrosphere is unlikely (BUA, 1991).
4.2.2.3 Degradation by physico-chemical processes
Upon combustion in the presence of sufficient oxygen, carbon
monoxide, carbon dioxide and nitrogen gases are produced.
Combustion under oxygen-starved conditions can result in the
production of carbon monoxide, hydrogen cyanide, nitriles, cyanic
acid, isocyanates, cyanogens, nitrosamines, amides and carbamates
(Air Products and Chemicals, 1989).
4.2.3 Bioaccumulation
There are no data on the bioaccumulation of morpholine in aquatic
and terrestrial organisms. However, as the n-octanol/water
partition coefficient for morpholine is log Pow = -2.55 (at pH 7),
bioaccumulation is not expected (BUA, 1991).
4.3 Interaction with other physical, chemical or biological factors
Due to its carcinogenic properties the formation of NMOR from
morpholine has to be taken into account when assessing health and
environmental aspects of morpholine. NMOR can be formed by reaction
of aqueous solutions of nitrite with morpholine (Mirvish, 1975) or by
reaction of gaseous nitrogen oxides, e.g., N2O3, N2O4, NOx
in aqueous solutions of morpholine, even under normal environmental
conditions (Challis & Kyrtopoulos, 1979; Mirvish et al., 1988;
Schuster et al., 1990). Nitrogen oxide (NO) levels may be higher than
was previously thought (Cooney et al. 1992; Hibbs, 1992). The
conditions of nitrosation, in particular the pH, plays a significant
role.
In aqueous solutions, the reaction is as follows:
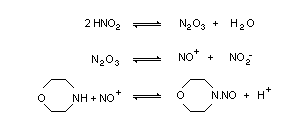 The rate of reaction of the nitrosation of morpholine by nitrite
is greatest at a pH value of 3.4, where the rate constant is
0.42 mol-2.s-1. An increase in the pH value has been shown to
result in a decrease in the rate of nitrosation with nitrite (Mirvish,
1975; Archer et al., 1977), and the rate was almost zero at pH > 7
(Archer et al., 1977).
In contrast, nitrosation with gaseous nitrogen oxides (N2O3,
N2O4, NOx) can take place over the whole pH range (Challis &
Kyrtopoulos, 1979; Meiners et al., 1980). Cooney et al. (1987) found
that, under certain conditions, the yield of NMOR at pH 7 was ten
times higher than at pH 2, but there was no further increase beyond
this pH.
Some nitrosamines, particularly alpha-nitrosamine aldehydes, are
potent transnitrosation reagents and are capable of nitrosating
morpholine at pH 7.9 (Loeppky et al., 1987).
Numerous reaction accelerators are known, e.g., thiocyanate
(Boyland et al., 1971), halides (Mirvish, 1975), formaldehyde (Archer
et al., 1977) and nitrosophenols, e.g., p-nitroso- o-cresol (Davies
et al., 1980). Enhancement of the nitrosation of morpholine by
nitrogen dioxide was reported in the presence of iodine (Challis &
Outram, 1979), vanillin and related phenols (Cooney & Ross, 1987) and
halides, particularly bromide (Cooney et al., 1987).
In contrast, the following compounds have been reported to
inhibit the nitrosation of morpholine almost completely: ascorbic acid
(Lathia & Schellhöh, 1981; Leach et al., 1991); urea or ammonium
sulfamate (Mirvish et al., 1972); gallic acid and sulfite (Mirvish,
1975); L-cysteine and DL-methionine ( in vitro study under
physiological conditions, Lathia & Edeler, 1989), catechol and
4-hydroxychavicol (Shenoy & Choughuley, 1989); alpha-tocopherol
(Norkus et al., 1986; Cooney et al., 1987; Schuster et al., 1990);
sulfhydryl compounds such as cysteine, cysteamine, glutathione and
thioglycolic acid, as well as extracts of onion and garlic juice
(Shenoy & Choughuley, 1992). Vitamin C, glucose, mannitol, cabbage
juice, orange juice, shiitake mushroom extract and saliva inhibited
the nitrosation of morpholine in vitro, but catechin, epicatechin
and tea extract enhanced the same reaction (Ohnishi, 1984). The
inhibitory effect of Chinese tea on the formation of NMOR in vitro
and in vivo has also been described (Wang & Wu, 1991).
Several C-nitro compounds, in particular tetranitromethane, have
been demonstrated to transnitrosate morpholine to form NMOR (Fan et
al., 1978). C-nitro compounds are widely used in industry as
pesticides, bactericides, colouring agents, drugs and perfumes.
Singer (1980) described the transnitrosation of morpholine with
nitrosamines and nitrosureas under acid conditions in the presence of
thiocyanate. These reactions are dependent on the pH value and steric
and electronic factors, as well as on the basicity of the amines. In a
model study, the nitrosation of morpholine by nitro-nitroso compounds,
such as those found in fried bacon, was observed (Liu et al., 1988).
NMOR can be formed in vivo in humans and has been found in
various tissues and fluids such as human saliva (Boyland et al., 1971;
Wishnok & Tannenbaum, 1977) and human gastric juice (Ziebarth, 1973;
1974; Sen & Baddoo, 1989; Yurchenko et al., 1990). NMOR formation has
been reported in rat lungs (Postlethwait & Mustafa, 1983), whole mice
(Iqbal et al. 1980; Norkus et al. 1984), stomach (Furman & Rubenchik,
1991), hepatocytes isolated from woodchucks (Marmota monax) (Liu et
al., 1992) and microorganisms (Archer et al., 1979; O'Donnell et al.,
1988; Calmels et al., 1991a,b). Bacterial catalysis of
N-nitrosation of morpholine has been reported in a range of bacteria
often isolated from the human gut or urinary tract infections (Suzuki
& Mitsuoka, 1984; Calmels et al., 1987, 1988; Mackerness et al.,
1989), including the ubiquitous gut bacterium Escherichia coli and
Pseudomonas aeruginosa, which is also widespread in the aquatic
environment. Bacterial catalysis of N-nitrosation of morpholine is
heat labile and is optimal at neutral to slightly alkaline pH (Calmels
et al., 1985; Leach et al., 1987). N-nitrosation by bacteria is
generally associated with the ability to reduce nitrate. It appears
that those that reduce nitrate to nitrogen or nitrogen oxides (e.g.,
P. aeruginosa) can nitrosate at much greater rates than those (e.g.,
E. coli) that only reduce nitrate to nitrite (Leach et al.,1987;
Calmels et al., 1988). There is considerable variation between
strains of the same species. Bacterial N-nitrosation of morpholine
has been shown to follow Michaelis-Menten kinetics (Calmels et al.,
1985; Leach et al., 1987). E. coli A10, for example, displays Km
values of 7.4 mmol/litre for morpholine and 11.4 mmol/litre for sodium
nitrite. It has been shown that the rate of bacterial N-nitrosation
of secondary amines is inversely related to the pKa of the amine
(Calmels et al., 1985; Leach et al., 1987, 1991), with a linear
relationship between log10 of the rate of nitrosation and pKa.
Morpholine, having a relatively low pKa, is thus relatively
susceptible to nitrosation compared, for example, to alkyl amines.
It has been shown that ascorbate is capable of inhibiting
nitrosation of morpholine by P. aeruginosa (Leach et al., 1991).
Although most nitrosation studies have used whole bacteria, an enzyme
catalyzing N-nitrosation of morpholine has been isolated and
purified from two denitrifying bacteria (Calmels at al., 1990).
4.4 Ultimate fate following use
4.4.1 Fate of morpholine in various products
Morpholine is an important industrial chemical with a wide range
of applications (see section 3.2.2) and therefore may be present in
many industrial emissions.
Its use as a corrosion inhibitor in boiler water means that
morpholine and its decomposition products will be found in boiler
wastewater, including water from power plants using morpholine. In a
study by McCain & Peck (1976), morpholine concentrations in the
discharge streams of three Hawaiian power plants ranged from not
detectable to 0.008 mg/litre, suggesting that the potential for human
exposure is small.
Its use in the manufacture of rubber additives results in an
indefinable amount of morpholine being released into the hydrosphere
or geosphere not only during manufacturing processes but also through
tyre abrasion and disposal of used tyres.
Morpholine is released during vulcanization processes using
morpholine-containing accelerators such as 2-( N-morpholino-
thio)benzothiazole (MBS) (Badura et al., 1989). Some of the amine is
released into the atmosphere and some is bound to the rubber. Even
the accelerator itself can contain free amine. The morpholine content
of MBS is < 0.4% by weight. This level can be higher if the
accelerator is not stored properly and is exposed to heat or moisture
(BUA, 1991).
Aarts et al. (1990) detected free volatile morpholine at
concentrations of between 70 (new) and 230 mg/kg (old) in samples of
dithio-bis-morpholine (DTBM). After extraction in water for one hour
in an ultrasonic bath, ten times this amount was detected, i.e.
960 mg/kg in newly made and 2750 mg/kg in stored DTBM. These
quantities of amine could be released during vulcanization.
Optical brighteners adhere to clothes during the first wash but
tend to be released into the wastewater in subsequent washings.
Although these substances are not themselves biologically degradable,
they have been found to disappear from wastewater after a two-step
biological treatment presumably due to the high rate of adsorption to
the sludge particles (Jakobi et al., 1983).
As mentioned in section 3.2.2, morpholine is released into the
environment by volatilization through its use in waxes and polishes
(Texaco, 1986).
4.4.2 Waste disposal
Controlled incineration is the preferred method of disposal
(Sittig, 1985; Air Products and Chemicals, 1989). The incinerator
should be equipped with a scrubber or thermal unit. Nitrogen oxide
emissions should meet environmental regulations.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
No data are available on levels of morpholine in ambient air.
5.1.2 Water
5.1.2.1 River water
Since mid-1990, the levels of morpholine in some rivers in North
Rhine-Westphalia, Germany, have been monitored (BUA, 1991). No
morpholine could be detected at three different points in the River
Rhine or in five of its tributaries (detection limit, 5 µg/litre). No
morpholine was found in samples of Tennessee freshwater (detection
limit, 0.07 µg/litre) (Singer & Lijinsky, 1976a). In 1979, 33 water
samples were collected at 11 sites in Japan, but no morpholine could
be detected (detection limit, 1-5 µg/litre) in any of the samples
(Environment Agency Japan, 1980).
5.1.2.2 Wastewater
There are no data on morpholine levels in wastewater.
A single sample of wastewater from a tyre chemical factory in
Ohio, USA was found to contain 3 µg NMOR/litre (Fajen et al., 1979).
In England, samples were taken from the inlets and outlets of
four sewage treatment plants (Richardson et al., 1980). NMOR
(100 µg/litre) was found only in the outlet of a cutting-fluid
recovery plant.
5.1.3 Sediment
Spies et al. (1987) examined contaminated sediments in San
Francisco Bay, USA and found several benzothiazoles, including
2-(4-morpholinyl)-benzothiazole, which is present as an impurity in
commercial 2-(morpholinothio)-benzothiazole used in motor tyres. The
authors carried out weathering tests on this latter commercial
substance and found that the morpholine impurity was environmentally
stable. They suggested that the 2-(4-morpholinyl)-benzothiazole found
in the sediments (up to 0.36 mg/kg dry weight) was a result of
accumulated street run-off. Morpholine itself could not be detected.
In 1979, 33 bottom sediment samples were collected at 11 sites in
Japan, but no morpholine could be detected (detection limit,
0.01-0.5 mg/kg) in any of the samples (Environment Agency Japan,
1980).
5.1.4 Soil
There are no data on the presence of morpholine in soil.
2-(4-Morpholinyl)-benzothiaozole (273 µg/kg dry weight) was detected
1.6 km from a motorway in California, USA (Spies et al., 1987) (see
also section 5.1.2).
NMOR (4.4 mg/kg) was detected in a single sample of soil near to
a tyre chemical factory in Ohio, USA (Fajen et al., 1979).
5.1.5 Terrestrial and aquatic organisms
Levels of morpholine found in single or small samples of fish are
given in Table 6, but the sample numbers are too low to make an
evaluation. No other data are available.
5.2 General population exposure
5.2.1 Indoor air
No data on indoor air exposure to morpholine are available.
Analysis for NMOR in the air inside new cars showed levels of up
to 2.5 µg/m3. Levels were 4 to 10 times lower when the air-venting
system was working, indicating that NMOR exposure is limited to the
first few minutes of each trip (Rounbehler et al., 1980). During a
simulation of conditions inside cars on a hot day, concentrations of
up to 0.4 µg NMOR/m3 were measured at 60°C (Dropkin, 1985).
5.2.2 Drinking-water and food
There are no data on the morpholine content of drinking-water.
Food can become contaminated with morpholine in several ways:
(a) through direct treatment of fruit with waxes containing morpholine
for conservation purposes; (b) by use of packaging material
containing morpholine, and (c) through steam treatment during
processing.
Ohnishi et al. (1983) found morpholine at concentrations of
< 71.1 mg/kg in the peel of retail citrus fruits in Japan. In the
pulp (flesh) of the fruits the level was much lower, being less than
0.7 mg/kg (Table 4). Marmalade made from whole fruits contained
concentrations of morpholine between 0.3 to 0.7 mg/kg. If the fruits
were previously washed in washing-up liquid, morpholine concentrations
were reduced, but only by 25%. Even if the fruit was boiled for
20 minutes, a third to a quarter of the morpholine still remained. The
morpholine removed by these processes could be detected quantitatively
in the washing and boiling water (Ohnishi et al., 1983).
Table 4. Morpholine content of citrus fruits and marmalade from
citrus fruitsa
Sample Number Morpholine
(mg/kg)b
Orange (variety a)c peel 12
n.d.-57.0
fruit pulp 3 0.2-0.7
Orange (variety b)c peel 6 5.0-71.1
fruit pulp 1 0.3
Mandarine peel 2 16.1-18.0
fruit pulp 1 n.d.
Lemon peel 2 n.d.-5.2
fruit pulp 1 n.d.
Grapefruit peel 2 2.8-7.0
fruit pulp 1 n.d.
Marmalade from citrus fruits 4 0.3-0.7
a adapted from Ohnishi et al. (1983)
b n.d. = not detectable (detection level 0.2 mg/kg),
presumably fresh weight
c variety not specified
Sen & Baddoo (1989) reported the morpholine and NMOR content of
waxed and unwaxed apples of Canadian origin, obtained either direct
from the packers or from retail sources. Liquid wax spray is used as
a protective coating on fruit and vegetables to reduce moisture loss
and thereby extend the shelf-life of the product. Apple homogenates
and liquid waxes were analysed for their morpholine contents
(Table 5). Although the concentrations of morpholine found in waxed
apples were high, NMOR could not be found in any of the waxed or
unwaxed samples. Low levels of morpholine in the unwaxed apples could
be due to contamination during packing or transport.
Singer & Lijinski (1976a) analysed a variety of foodstuffs for
the presence of morpholine but the sample size was too small to draw
any conclusions. The results are given in Table 6. The sources of
contamination with morpholine are in these cases not clear. The
possibility of artifacts is unlikely according to the authors.
Table 5. Concentration of morpholine and NMOR (mg/kg) in samples of
liquid waxes and waxed and unwaxed applesa
Liquid wax Unwaxed apples Waxed apples
NMOR morpholine NMOR morpholine NMOR morpholine
0.286 27 300 n.d. n.d. n.d. 4.3
0.668 31 500 n.d. 0.118 n.d. 4.9
0.138 24 400 n.d. 0.016 n.d. 6.3
0.277 38 500 n.d. 0.041 n.d. 7.1
0.152 22 500 n.d. n.d. n.d. 4.0
0.585 33 300 n.d. 0.018 n.d. 7.7
a adapted from Sen & Baddoo (1989); n.d. = not detected (detection
limit: 0.005 mg/kg for morpholine, 0.0005 mg/kg for NMOR)
Table 7 summarizes the results of investigations into the
concentrations of morpholine and NMOR in prepacked milk products
(Hoffmann et al., 1982). The values range from 5-77 µg/kg for
morpholine and "not detectable" to 3.3 µg/kg for NMOR. Contamination
of prepacked foodstuffs with morpholine might be explained by the use
of morpholine in steam boiler systems for paper and cardboard
production.
Hotchkiss & Vecchio (1983) found morpholine concentrations of
between 0.098 and 8.4 mg/kg (mean 0.38 mg/kg) in food packaging. A
sample of flour nearest the wall of the paper bag contained 1.1 µg
NMOR/kg. The bag itself contained 33.0 µg NMOR/kg. In an experimental
72-h incubation at 100°C, between 1 and 2.3 µg NMOR/kg migrated from
packaging material to various dry foods.
Sen & Baddoo (1986) investigated the migration of NMOR out of
waxed packaging material into margarine. Waxed wrappings and margarine
samples taken 1 cm from the outer layer of the block contained NMOR
(5-73 µg/kg and 0.5-1.4 µg/kg, respectively). NMOR was not detectable
in those samples taken from the inside of the block. Samples taken of
margarine packed in aluminium backed wrappers, specially coated waxed
papers or plastic containers were negative for NMOR (detection level,
0.5 µg/kg).
Aitzetmüller & Thiele (1982) found no NMOR in 20 margarine
samples from different countries (detection limit, 0.5 µg/kg).
Table 6. Morpholine content in various food samplesa
Food No. of Concentration References
samples (mg/kg)b
Fish
Fish sausage 5 n.d. Hamano et al. (1981)
Cod roe 3 n.d. Hamano et al. (1981)
Codc -d tracese Singer & Lijinsky (1976a)
Spotted troutc 1 6 Singer & Lijinsky (1976a)
Smallmouth bassc 1 < 0.7 Singer & Lijinsky (1976a)
Salmonc 1 1 Singer & Lijinsky (1976a)
Ocean perchc -d 9.0 Singer & Lijinsky (1976a)
Tuna (in tins) -d < 0.6 Singer & Lijinsky (1976a)
Meat
Baked ham 5 0.2 Hamano et al. (1981)
Baked ham -d 0.5 Singer & Lijinsky (1976a)
Frankfurter sausages -d 0.4 Singer & Lijinsky (1976a)
Plant products
Spinach 2 n.d. Hamano et al. (1981)
Miso (from soja) 2 n.d. Hamano et al. (1981)
Beverages
Evaporated milk -d 0.2 Singer & Lijinsky (1976a)
Coffee -d 1.2 Singer & Lijinsky (1976a)
Tea -d tracesf Singer & Lijinsky (1976a)
Beer (in tins) -d 0.4 Singer & Lijinsky (1976a)
Beer (in bottles) -d < 0.2 Singer & Lijinsky (1976a)
Wine -d < 0.7 Singer & Lijinsky (1976a)
a adapted from BUA (1991)
b fresh weight except for tea and coffee (dry weight);
n.d. = not detected (detection limit 0.01 mg/kg)
c frozen from Tennesee & Columbia rivers
d average from several samples; exact number not given
e < 0.3 mg/kg
f < 0.1 mg/kg dry weight
Table 7. Morpholine and NMOR content (mg/kg) in food products
and their waxed containersa
Sample Food Container
NMOR morpholine NMOR morpholine
Butter 0.0032 0.058 0.0019 0.22
Cream cheese 0.0009 0.077 n.d. 0.68
Yoghurt n.d. 0.038 n.d. 3.06
Cottage cheese 0.0004 0.044 0.0054 17.2
"Cheese" (semi-soft)
Country of origin:
Germany 0.0033 0.009 n.d. 0.026
Denmark 0.0031 0.01 0.0016 0.025
Austria 0.0007 0.005 0.0012 0.022
USA 0.0014 0.008 n.d. 0.132
Gouda 0.0016 0.035 n.d. 0.035
Frozen peas n.d. 0.026 0.0031 0.057
and carrots
a adapted from Hoffmann et al. (1982); n.d. = not detected
(detection limit for NMOR, 0.0002 mg/kg)
Gavinelli et al. (1988), in a survey in Italy, found no NMOR in a
variety of foodstuffs including canned beef, pork, poultry, cured
meat, milk products, malt products, domestic Italian canned wines and
beers. The limit of detection was 0.3 µg/kg. Similarly, Pensabene &
Fiddler (1988) found no NMOR in samples of different USA fish-meat
frankfurter sausages (limit of detection, 0.2 µg/kg). These authors
assumed that the positive results in their earlier work were due to
artifacts.
Groenen et al. (1987) found NMOR in cheese (2.8 µg/kg) and cured
meat products (34 µg/kg) after addition of morpholine (10 mg/kg) in
the alkaline pH range.
Liu et al. (1988) suggested that the NMOR in fried bacon was
formed by transnitrosation.
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (US FDA, 1984a,b,c,d,e;
1988; see section 3.2.2). Although the US Food and Drug Administration
allows morpholine to be used as a direct or indirect food additive, in
1980 one of the biggest USA producers recommended its customers not to
use morpholine in this way (Litton Bionetics, 1980).
5.2.3 Tobacco
Morpholine was found in unburned cigarette tobacco at a
concentration of 0.3 mg/kg (Singer & Lijinsky, 1976b). In cigarette
smoke condensate < 5 mg/kg was detected, equivalent to
0.08 µg/cigarette. It has been established that an appreciable portion
of the inhaled smoke is swallowed and retained in the stomach where
nitrosation of ingested amines is known to occur (Sander & Bürkle,
1969).
Brunnemann et al. (1982) analysed 10 popular snuff brands from
the USA and Sweden for morpholine and volatile N-nitrosamines (see
Table 8). In the five USA brands, morpholine concentrations of between
1.5 and 4.0 mg/kg were found; in the Swedish products, the
concentrations were between 0.2 and 2.5 mg/kg. An analysis of the
snuff containers that were made of waxed cardboard gave morpholine
values of 0.17 to 4.74 mg/kg (USA) and 0.46 to 4.83 mg/kg (Swedish).
The morpholine content in the snuff could have come through diffusion
from the container made of waxed cardboard. Another possibility was
contamination during the tobacco processing as suggested by the very
high morpholine content (19.4 mg/kg) of a single sample of chewing
tobacco, packaged in aluminium. NMOR formed by nitrosation from
morpholine was found in 5/5 of the USA and 2/5 of the Swedish snuff
samples (Brunnemann et al., 1982).
Brunnemann & Hoffmann (1991) reported that since 1981 they had
routinely determined the concentrations of NMOR in a leading snuff
brand in the USA with the aim of creating awareness of the fact that
morpholine is a precursor of NMOR and must be removed. In eight
commercial snuff brands analysed in 1986 (Hoffmann et al., 1987), only
three were found with traces of NMOR (9-39 µg/kg). Brunnemann &
Hoffmann (1991) noted that the decrease in the NMOR content (from
about 700 µg/kg in 1981 to an undetectable level in 1990) is most
probably due to modifications in packaging, since snuff products in
the USA are now sold in plastic containers that have no wax coatings
(Table 9).
Österdahl & Slorach (1983) analysed snuff and chewing tobacco on
the Swedish market for volatile N-nitrosamines. NMOR was found in
9/30 samples in concentrations between 0.4 and 4.0 µg/kg (Swedish
brands) but in none of the three USA brands. One of three chewing
tobacco brands contained 0.8 µg morpholine/kg.
Table 8. Nitrosomorpholine and morpholine in snuff containersa
Snuff brand Snuff tobacco (µg/kg)b Snuff container
(µg/kg)c
NMOR morpholine NMOR morpholine
USA I 24 2800 34 845
II 690 1500 10 170
III 690 4000 230 4740
IV 630 3200 4 90
V 31 2200 3 140
Sweden I 44 820 4 1750
II < 2d 200 3 460
III < 2d 780 13 4830
IV 10 940 23 4290
V < 2d 2500 n.d.e n.d.
a from Brunnemann et al. (1982)
b based on dry weight
c uncorrected for moisture; had previously contained snuff.
Containers of USA I-III and Sweden I-IV were cardboard boxes
with a metal lid, USA IV were plastic containers with individual
snuff portions in porous paper bags; USA V was a plastic container;
Sweden V were individual snuff portions in Al-bags.
d detection limit = 2 µg/kg
e n.d. = not determined
Table 9. NMOR in fine-cut snuff (on the basis of dry tobacco weight)a
Year Yield (µg/kg)
1981 690
1984 29.4
1984/85 238
1985 29
1986/87 < 2b
1987 43
1990 < 2
a from Brunnemann & Hoffmann (1991)
b detection limit = 2 µg/kg
A survey of smokeless tobacco products commercially available in
1987-1988 showed that trace levels of NMOR were still found in some
United Kingdom (mean 0.5 µg/kg) and Swedish (mean 1 µg/kg) oral
tobacco products packed in waxed containers, but no NMOR was detected
in Indian zarda or European nasal snuff (Tricker & Preussmann, 1991).
5.2.4 Cosmetics and toiletry articles
Morpholine is used in some countries in cosmetic products as a
surfactant and emulsifier at concentrations up to 5% (Cosmetic
Ingredient Review, 1989).
Data submitted to the Food and Drug Administration (FDA) in 1981
by cosmetic firms participating in the voluntary cosmetic registration
programme indicated that morpholine was used in a total of 38 cosmetic
products including eyeliner, eye shadow, mascara and skin care
preparations (Cosmetic Ingredient Review, 1989). The greatest use of
morpholine was in mascara (32 products). The reported concentration
ranges of morpholine in these products were < 0.1% (4 products),
> 0.1-1% (17 products) and > 1-5% (17 products). Table 10
summarizes data submitted to the FDA in 1986 showing that the greatest
use of morpholine was in eye make-up removers, at concentrations of
> 0.1-1% and > 1-5% (Cosmetic Ingredient Review, 1989). These data
show that the ocular region and the skin around are the areas directly
exposed to cosmetic products containing morpholine. The potential also
exists for morpholine-containing products to come in contact with the
conjunctiva and cornea. Additionally, it must be emphasized that these
morpholine-containing products may be applied several times daily over
the course of several years. Due to lack of appropriate safety data,
the Cosmetic Ingredient Review Expert Panel could not conclude that
morpholine was safe for use in cosmetic products (Cosmetic Ingredient
Review, 1989).
Morpholine is listed in Annex II of the European Economic
Community (EEC) Cosmetics Directive and therefore must not be used in
cosmetic formulations (EEC, 1986). Investigations in 1988 and 1989 in
the Federal Republic of Germany showed that no morpholine could be
detected in toiletry articles (BUA, 1991).
Data from a 1985 US Food and Drug Administration report given in
Table 11 show that NMOR was found at concentrations between 48 and
1240 µg/kg in seven mascara products (Cosmetic Ingredient Review,
1989). It is not known whether the NMOR detected was formed in the
cosmetics. Table 11 also shows the results of an analysis of various
toiletry articles in the Federal Republic of Germany prior to the EEC
Directive in 1986. NMOR was detected in 18% of the products
(Spiegelhalder & Preussman, 1984).
Table 10. Product formulation data for morpholinea
Product Total no. of Total no. No. of product formulations
category formulations containing within estimated
in category ingredient concentration ranges
> 0-1% > 1 > 1-5%
Eye make-up 77 29 11 18
remover
Eye, face, or body 1264 2 2
preparations other
than eye make-up
removers
1986 totals 1341 31 11 2 18
a US FDA (1986)
5.2.5 Rubber articles
NMOR was found in commercial samples of rubber chemicals at
concentrations of 60-3500 µg/kg by Spiegelhalder & Preussmann (1982),
who noted that the occurrence of nitrosamines was directly related to
the use of corresponding vulcanization accelerators. The nitrosamines
could be leached out by water, buffer solutions or milk but could not
be completely removed even after boiling or treating with acid or
alkaline solutions (Spiegelhalder & Preussmann, 1982).
Several rubber articles which come into contact with skin or
food, in particular baby bottle teats and pacifiers (dummies), were
assayed for NMOR; 10 µg/kg migrated from a silicone rubber pacifier
(1 out of 11 different samples; detection level, 1 µg/kg) after
incubation for 24 h at 40°C (Spiegelhalder & Preussmann, 1982). Sen et
al. (1984) found NMOR in 2 out of 10 brands of nipples and in one
pacifier at concentrations of between 0.1 µg/kg (detection level) and
86 µg/kg. The same authors found NMOR (5.7 µg/kg) in one plastic
nipple shield, no NMOR being found in the other 41 samples of nipples
and pacifiers (detection limit, 1 µg/kg) (Sen et al., 1985).
Westin et al. (1987) tested 16 types of children's pacifiers and
baby-bottle nipples on sale in Israel from nine different countries
and found NMOR at levels of up to 2 µg/kg.
Table 11. N-nitrosomorpholine (NMOR) in toiletry articles and
cosmetics
Concentration
Productsa No. of No. (µg/kg)
samples positive
maximum average
Shampoos 45 13 640 133
Colour toners 7 - - -
Hair conditioners 16 - - -
Foam baths 7 - - -
Shower gels 9 4 380 145
Cream and oil baths 8 2 440 -
Cosmetic bath additives 5 - - -
Children's shampoos 5 1 230 -
Children's bath and
skin care products 8 6 360 80
Body lotions and rubs 6 - - -
Face tonics, cleaners, 29 - - -
and masks
Mascarab 7 7 1240 306
a from Spiegelhalder & Preussmann (1984), with the exception of
mascara
b from CIR (1986)
In a survey in Germany in 1990, no morpholine or NMOR was found
in the rubber articles tested; these included balloons, baby
dummies/teats, rubber gloves, condoms and rubber rings for bottling
(BUA, 1991).
5.3 Occupational exposure during manufacture, formulation or use
A USA National Occupational Hazard Survey, conducted by NIOSH,
detected worker exposure to morpholine in 283 different industries
(NRC, 1981). In 1970, 501 283 workers were potentially exposed to the
actual product, 22% were exposed to a product known to contain
morpholine, and 74% were exposed to a generic product suspected to
contain morpholine.
Data from the USA National Occupational Exposure Survey (NOES) in
1988 indicated that in a total of 30 industries and 8711 plants
surveyed, 146 511 workers, including 44 839 women, were potentially
exposed to morpholine in the workplace (NIOSH, 1988).
Many countries recommend for morpholine an 8-h time-weighted
average exposure limit of 70 mg/m3 (skin notation) and a 15-min
short-term exposure limit (STEL) of 105 mg/m3 (ILO, 1991).
5.3.1 Exposure to morpholine
Only a few studies have been reported concerning the exposure of
workers to morpholine.
An analysis in Ontario, Canada of condensed steam samples in four
hospitals and one food processing plant using corrosion inhibitors in
the steam-generating plants gave mean values for morpholine of
2.41 mg/litre in the hospitals (9 samples; range 2.1-2.9 mg/litre) and
1.1 mg/litre in the factory (9 samples; range 0.6-1.8 mg/litre)
(Malaiyandi et al., 1979).
Fajen et al. (1979) found morpholine levels of 230 and
42 µg/m3, respectively, in air samples from two out of four rubber
industry factories; these two were a tyre factory and its chemical
factory.
Taft & Stroman (1979) described an investigation into workers
exposed to a number of chemicals in the drying and bagging departments
of a tyre and rubber company manufacturing the rubber accelerator,
4-morpholino-2-benzothiazolyl [(4-morphinyl-2-benzothiazole)]
disulfide. This rubber accelerator is manu-factured by reacting
morpholine with 2-mercaptobenzothiazole (MBT). With the exception of
one personal sample showing 0.05 mg of morpholine/sample (0.4 ppm) at
the level of detection, all other samples showed negative results.
Occupational exposure to morpholine was measured in 15 factories
of the chemical, plastic and rubber industries in Germany from 1980 to
1990, and in no case was the German workplace exposure limit of
70 mg/m3 reached. Of 35 measurements, 90% were below 0.26 mg/m3
(BUA, 1991).
Katosova et al. (1991) reported average levels of 0.54 to 0.93 mg
morpholine/m3 with maximum concentrations of 0.73 to 2.14 mg/m3 in
a morpholine production factory in the former-USSR.
5.3.2 Exposure to N-nitrosomorpholine
Occupational exposure to NMOR has been found in the rubber
industry. Table 12 gives a summary of the data published.
Fajen et al. (1979) found NMOR in air samples from a tyre
chemical factory and an aircraft tyre factory. In the chemical
factory, NMOR was also found as an impurity in morpholine (0.8 mg/kg)
and in bismorpholine carbamylsulfenamide (BMCS), a vulcanisation
accelerator (0.4 to 0.7 mg/kg), as well as in wastewater
(0.003 mg/kg), utility steam condensate (0.002 mg/kg), and dirt
scrapings on a staircase (730 mg/kg). Outside the chemical plant, a
soil sample contained 4.4 mg/kg (Fajen et al., 1979). It is possible
that the NMOR in these samples was formed by the transnitrosation from
N-nitrosodiphenylamine, also produced in the tyre chemical factory,
together with morpholine present in the steam condensate (BUA, 1991).
A survey was carried out by NIOSH and a tyre manufacturing
company in 1979 into the levels of NMOR in four different work areas
in the factory as well as personal exposure levels (Ringenburg &
Fajen, 1980; London & Lee, 1987). Personal breathing-zone air samples
ranged from 0.6 to 1.8 µg/m3 (mean 0.8 µg/m3), and workplace
levels from 0.8 to 3.7 µg/m3. In a survey at another tyre
manufacturing company during the same year, nitrosamine levels were
highest (1.62 µg/m3) during the extrusion process of racing tyre
components (McGlothlin, 1980; McGlothlin & Wilcox, 1984). Exposure to
NMOR in rubber press operator fumes was investigated by NIOSH in
another company. The highest personal exposure to NMOR was
0.063 µg/m3, and the highest area sample 0.038 µg/m3.
In a factory producing rubber parts for automobile interiors, the
highest level of NMOR (19 µg/m3) was found in the process sample
taken directly above one of the extruder ovens. Other personal and
process levels were in the range 0.1 to 1.4 µg NMOR/m3.
Between 1980 and 1990, 775 samples of workplace air from 124
factories in Germany involved in the production and fabrication of
rubber articles were analysed for NMOR (BUA, 1991). Of the samples,
71% showed concentrations less than the guidance value of 1 µg/m3
(BUA, 1991).
According to Spiegelhalder (1983) and Schuster et al. (1990),
higher concentrations of NMOR are to be found in work areas where
higher temperatures are required for fabrication/vulcanization. High
levels of NMOR from storage areas probably result from continued
emission of volatile NMOR.
NMOR was not detected in the environment of a metal factory using
metal-working fluids (Fadlallah et al., 1990).
Table 12. Occupational exposure to NMORa
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Tyre chemical factory USA 1978 10 area 0.07-5.1 Fajen et al. (1979)
Tyre manufacturer USA 1978 20 area 0.6-27 Rounbehler & Fajen
12 area 0.08-8.5 (1983)
7 area 0.02-3.9
6 process 0.41-2.9
6 area < 0.01-0.60
7 process < 0.1-22.0
7 area < 0.002-250
8 process 0.1-25
Tyre manufacturer USA 1979/ 13 area 0.1-1.62 McGlothlin (1980),
1980 McGlothlin & Wilcox (1984)
Tyre manufacturer USA 1979/ 4 area 0.85-3.7 Ringenburg & Fajen (1980);
1980 6 personal 0.6-1.8 London & Lee (1987)
Industrial rubber articles USA 1978 4 area 0 Fajen et al. (1979)
Industrial rubber articles USA 1980/ 11 area < 0.005-0.0376 Hollett et al. (1982)
1981 7 personal < 0.0096-0.0629
Rubber articles for USA 1982 4 area 0.8-19 Lee (1982)
car outfitting 13 personal 0.4-1.2
Rubber industry Germany 1979/ 20 monitoring area and 0.1-17 Spiegelhalder &
1981 points in 17 personal Preussmann (1982)
factories
Table 12 (cont'd)
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Rubber industry Germany 1987 545 personalc < 2.5 (73%), TRGS (1989)
> 2.5 (27%)
max. 41
Chemical industry Germany 1987 22 not given < 1 (91%)d TRGS (1989)
Leather industry Germany 1987 50 not given < 1 (100%) TRGS (1989)
Foundries Germany not given 658 not given < 1 (95%), TRGS (1989)
> 1 (5%)
max. 2.1
a adapted from BUA (1991)
b the percentage of samples which had this given concentration is given in parentheses
c sum of all measured N-nitrosamines
d in 82% below the detection level (0.2 µg/m3)
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Toxicity experiments on rodents have shown that morpholine is
absorbed after oral, dermal and inhalation exposure (see Table 13).
6.2 Distribution
Tanaka et al. (1978) determined the distribution of
[14C]-labelled morpholine in male Wistar rats (3 animals/group,
250-300 g) after oral (200 mg/kg) and intravenous injections
(150 mg/kg). The radioactivity was determined in the dried, powdered
organs. Large amounts were only found in muscle and intestine. In
rats sacrificed 2 h after oral administration of morpholine-HCl, 29%
of the radioactivity was found in the intestine and 26% in the muscle.
Similarly, 2 h after intravenous injections, 19 and 27% of the dose
was found in the intestine and muscle, respectively.
Female New Zealand rabbits (number not given) were exposed to
morpholine (905 mg/m3) for 5 h by nose-only inhalation
(Tombropoulos, 1979). At the end of the exposure, the animals were
sacrificed and the tissue and body fluids analysed. Concentrations of
morpholine were highest in urine (324 mg/litre) and kidney
(118 mg/kg), the other tissues having concentrations below 40 mg/kg.
Van Stee et al. (1981) injected six male New Zealand rabbits
intravenously with 5 mmol [14C]-labelled morpholine/kg body weight
(435 mg/kg). The distribution of radioactivity after 30 min showed
the highest concentrations in the renal medulla (36 mmol/kg) and
cortex (15.4 mmol/kg), followed by lung (5.1 mmol/kg), liver
(4.7 mmol/kg) and blood (2.3 mmol/litre). Morpholine was not bound to
serum proteins.
6.3 Metabolic transformation
Morpholine is eliminated mainly in a non-metabolized form in the
urine of the rat, mouse, hamster and rabbit (Griffiths, 1968; Tanaka
et al., 1978; Van Stee et al., 1981; Sohn et al., 1982b). However,
Sohn et al. (1982b) reported that morpholine is metabolized by
N-methylation followed by N-oxidation in the guinea-pig. After an
intraperitoneal injection of 125 mg/kg [14C]-labelled morpholine in
guinea-pigs, 20% of the radioactivity was found in the urine as
N-methylmorpholine- N-oxide. However, the morpholine ring can be
cleaved in mammalian systems. In several studies on the metabolism of
morpholine derivatives in the rat, ring cleavage products have been
noted (Tatsumi et al., 1975; Hecht & Young, 1981; Kamimura et al.,
1987).
Table 13. Single exposure toxicity data for morpholinea
Species No./sex Dosage Results Cause of death/symptoms Reference
Oral
Rat 5 1.05 g/kg body weight LD50 n.g. Smyth et al. (1954)
Rat 57 1.6 g/kg body weightb LD50 gastrointestinal haemorrhage Shea (1939)
Rat 5 m + 5 f 1.9 g/kg body weight LD50 dyspnoea, haemorrhagic BASF (1967)
enteritis
Rat 7 m 1.0 g/kg body weight; pH 7 no deaths n.g. Börzsönyi et al. (1981)
Guinea-pig 33 0.9 g/kg body weightb LD50 gastrointestinal Shea (1939)
haemorrhage, diarrhoea
Intraperitoneal
Rat (Wistar) 4 m 0.4 g/kg body weight all died n.g. Stewart & Farber (1973)
Rat (Wistar) 4 m 0.1 g/kg body weight lethal n.g. Stewart & Farber (1973)
for 1/4
Mouse 5 m + 5 f 0.4 g/kg body weight LD50 irritation around BASF (1967)
injection area, dyspnoea
Inhalation
Rat 5 m + 5 f 18.1 g/m3; 6 h lethal haemorrhage of nose, mouth Hazleton (1981)
for 9/10 and eyes; spasms; tremors
Rat 6 saturated atmosphere;c all died spasms; caustic burns BASF (1967)
5´ h on nose and extremities
Table 13 (cont'd)
Species No./sex Dosage Results Cause of death/symptoms Reference
Inhalation (contd)
Rat n.g./m 8.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g./f 7.8 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g. 65.2 g/m3; 8 h lethal irritation of nose, Shea (1939)
eyes; lung haemorrhage
Rat n.g. 43.4 g/m3; 8 h lethal n.g. Shea (1939)
Mouse n.g./f 6.9 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g./m 5.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g. 4.9 g/m3; LC50 n.g. Zaeva et al. (1968)
Dermal
Rabbit (New n.g. 0.5 ml/kg body weight LC50 n.g. Smyth et al. (1954)
Zealand)
a adapted from BUA (1991); n.g. = no details given
b morpholine diluted with 4 parts water; deaths within a week
c inhalation of a saturated atmosphere at 20°C formed by bubbling air through a 5-cm layer of morpholine
In the presence of nitrite, morpholine can be converted to NMOR
(see section 4.3). NMOR was found in the stomachs of rats that had
been fed on diets containing morpholine and nitrite (Sander et al.,
1968; Inui et al., 1979).
Immunostimulation of rats by intraperitoneal treatment with
E. coli lipopolysaccharide (LPS; 1 mg/kg) led to a large increase in
urinary nitrate and urinary metabolites of NMOR when morpholine
(80 µmol/kg) and L-arginine (400 µmol/kg) were injected
intraperitoneally. The replacement of LPS with nitrate (330 µmol/kg
intraperitoneal) did not increase urinary metabolites of NMOR (Leaf et
al., 1991). This result is consistent with endogenous nitrosation of
morpholine by nitrogen oxide (NO) from oxidation of the guanido group
of arginine by induced NO synthase (Hibbs, 1992).
Hecht & Morrison (1984) developed a method to monitor the
in vivo formation of NMOR by measuring N-nitroso
(2-hydroxyethyl)glycine, its major urinary metabolite. The formation
of NMOR was measured in F-344 rats over wide range of doses of
morpholine (38.3-0.92 µmol) and sodium nitrite (191-4.8 µmol).
According to estimates by the authors, 0.5 to 12% of the morpholine,
depending on the dose, was nitrosated.
In vitro nitrosation of morpholine has been reported. NMOR was
formed when morpholine was added to human saliva (Tannenbaum et al.,
1978). Additionally, a new type of metabolite N-cyanomorpholine was
identified in human saliva (Wishnok & Tannenbaum, 1976).
6.4 Elimination and excretion
6.4.1 Expired air
Elimination of 14C from labelled morpholine (intraperitoneal
injection) through expired air is minimal. In rats, experiments have
shown that only about 0.5% of the dose of radioactively labelled
morpholine is exhaled as 14CO2 (Sohn et al., 1982b). In rabbits
0.0008% of the administered dose was 14CO2 (Van Stee et al.,
1981).
6.4.2 Urine
Elimination studies on male Wistar rats (200-350 g) were carried
out by administering morpholine-HCl (500 mg/kg) or [14C]-labelled
morpholine-HCl (200 mg/kg) orally and morpholine-HCl (250 mg/kg)
intravenously. In all cases, over 85% of the dose was excreted in
urine within 24 h. A further portion, up to 5%, was excreted during
the next three days. [14C]-morpholine palmitate was eliminated
slightly more slowly, but the urinary excretion within 3 days
following oral administration amounted to 90% of the dose (Tanaka et
al., 1978). Of the radioactive morpholine administered to rats,
62-77.5% was excreted in the urine after 24 h (Griffiths, 1968;
Ohnishi, 1984). Following intraperitoneal administration to rats,
urinary excretion within 24 h was 87.8% of the dose (Maller &
Heidelberger, 1957). In the dog, 70-80% of the radioactive morpholine
was excreted in the urine (Rhodes & Case, 1977).
The time-course of urinary excretion of 14C by Sprague-Dawley
rats, Syrian golden hamsters, and strain II guinea-pigs treated with
[14C]-morpholine was compared by Sohn et al. (1982b). Although in
all three species over 80% was excreted in 3 days, the rate of urinary
excretion within the first 6 h was greatest in the hamster and least
in the guinea-pig.
Van Stee et al. (1981) infused rabbits intravenously with
[14C]-morpholine (5 mmol/kg) which had been neutralized with HCl.
After 4 h, 18.5% of the dose was excreted in the urine. When the pH
of the urine was lowered from 7.8-7.9 to 7.1-7.2 by administration of
ammonium chloride (10 g/litre) in drinking-water prior to the
injection, the urinary excretion more than doubled (to 43%).
These data suggest that the urinary excretion of morpholine is
enhanced by its neutralization with acid.
6.4.3 Faeces
Rats dosed orally or intravenously with morpholine hydrochloride
excreted not more than 1.7% of the dose in the faeces (Griffiths,
1968; Tanaka et al., 1978). However, when dosed orally with
morpholine palmitate (Tanaka et al., 1978, Ohnishi, 1984), up to 7%
was excreted in faeces.
6.5 Retention and turnover
Plasma concentration-time curves of 14C after intraperitoneal
injections of [14C]-morpholine (125 mg/kg in 0.9% NaCl) in Sprague-
Dawley rats, Syrian golden hamsters, and strain II guinea-pigs
declined biexponentially. Whereas rates of first phase of elimination
from plasma in rats and hamsters were similar (half-lives of 115 and
120 min, respectively), the half-life in guinea-pigs was significantly
longer (300 min) (Sohn et al., 1982b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Table 13 summarizes the toxicity data on single exposure to
morpholine.
7.1.1 Oral
Oral administration of morpholine to rats resulted in LD50
values of 1-2 g/kg body weight (Shea, 1939; Smyth et al., 1954; BASF,
1967). Gastrointestinal and nasal haemorrhage were reported. In
contrast, when morpholine was administered to seven male rats at a
neutral pH, no deaths occurred with concentrations of 1 g/kg body
weight (Börzsönyi et al., 1981).
A study on guinea-pigs resulted in a LD50 of 0.9 g morpholine
per kg body weight. Gastrointestinal and nasal haemorrhage were
reported (Shea, 1939).
7.1.2 Inhalation
There have been reports of inhalation studies with morpholine on
rats (Shea, 1939; BASF, 1967; International Labour Office, 1972;
Lam & Van Stee, 1978; Hazleton, 1981) and mice (Zaeva et al., 1968;
Lam & Van Stee, 1978).
Exposure to morpholine at vapour saturation concentrations
resulted in almost 100% lethality after 5.5 h (BASF, 1967). It had
irritating and corrosive properties.
In studies with lower concentrations, Lam & Van Stee (1978)
obtained LC50 values of 7.8 and 8.2 g/m3 for female and male rats,
respectively, whereas other authors reported no deaths at three times
this concentration (see Table 13).
Reported LC50 values for mice are consistently in the range of
5-7 g/m3 (see Table 13).
7.1.3 Dermal
Smyth et al. (1954) noted necrosis on the clipped skin of albino
rabbits within 24 h of application of undiluted morpholine. Mortality
within 14 days in New Zealand rabbits after penetration of morpholine
into the skin gave an LD50 of 0.5 ml/kg body weight.
Other reports are discussed in section 7.4.2.
7.1.4 Intraperitoneal
When morpholine was administered intraperitoneally to rats
(Stewart & Farber, 1973) and mice (BASF, 1967), the LD50 was in the
range 0.1-0.4 g/kg body weight for rats and 0.4 g/kg body weight for
mice (see Table 13).
7.2 Short-term exposure
As with single-exposure, the effects of morpholine after short-
term exposure depend on the method of exposure. The available data on
short-term exposure are summarized in Table 14.
7.2.1 Oral
Rats and guinea-pigs were fed morpholine at concentrations of
0.16-0.8 g/kg body weight and 0.09-0.45 g/kg body weight,
respectively, by gavage for 30 days (Shea, 1939). At concentrations of
half of the LD50, nearly all the animals died within 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver and necrosis of the stomach
glandular epithelium.
Fatty degeneration (lipidosis) of the liver in rats was noted
after feeding morpholine (0.5 g/kg body weight) daily for 56 days
(Sander & Bürkle, 1969).
Shibata et al. (1987a) carried out a 13-week toxicity study in
B6C3F1 mice using morpholine as the fatty acid salt, morpholine
oleic acid salt (MOAS), at dosage levels of 0%, 0.15%, 0.3%, 0.6%,
1.25% and 2.5% MOAS in the drinking-water. At the highest MOAS level,
body weight gains were slightly reduced. Histopathological analysis
showed cloudy swelling of the proximal tubules, but no other
alterations were observed in the organs of either sex. Urinalysis
showed increases in both specific gravity and plasma urea nitrogen in
some dosage groups, suggesting a possible malfunctioning of the kidney
(see Table 14).
7.2.2 Inhalation
In rats that died after 5 days repeated exposure to 65.2 g/m3,
lung haemorrhage, severe damage to the secreting tubules of the
kidney, and fatty degeneration of the liver were observed (Shea,
1939).
Table 14. Short-term exposure to morpholinea
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
Oral
Rat 20 0.16 g/kg body weight per 8/20 beginning necrosis of liver, kidney Shea (1939)
day; 30 days; in 2 ml H2O and stomach mucous membrane
(gavage)
20 0.32 g/kg body weight per 8/20 necrosis of liver, kidney, stomach
day; 30 days; in 2 ml H2O
(gavage)
20 0.8 g/kg body weight per 19/20 weight loss, lethargy, severe necrosis
day; 30 days; in 2 ml H2O of liver, kidney, stomach
(gavage)
Rat 7 f 0.5 g/kg body weight per sacrifice after 270 days, moderate Sander & Bürkle
(Sprague- day; 56 days liver adipose degeneration (1969)
Dawley)
Mouse 10 m + 10 f 0.15 and 0.3% MOAS; 91 days no significant changes Shibata et al.
(B6C3F1) (0.07 or 0.14 g morpholine/ (1987a)
kg body weight per day)
10 m + 10 f 0.6% MOAS; 91 days m: increase in specific gravity of
(ca. 0.2 g morpholine/kg urine; f: increase in blood urea
body weight) per day
10 m + 10 f 1.25% MOAS; 91 days increase in specific gravity of
(ca. 0.4 g morpholine/kg urine and blood urea
body weight per day)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
10 m + 10 f 2.5% MOAS; 91 days reduced weight gain; increase in specific Shibata et al.
(ca. 0.7 g morpholine/kg gravity of urine and blood urea; (1987a)
body weight per day) increased relative kidney weight with
swelling of proximal tubules
Guinea-pig 20 0.09 g/kg body weight 3/20 slight alterations in liver, kidney Shea (1939)
per day; 30 days (syringe)
20 0.18 g/kg body weight 12/20 necrosis of liver, kidney, stomach
per day; 30 days (syringe)
20 0.45 g/kg body weight 16/20 severe degeneration of liver, kidney,
per day; 30 days (syringe) stomach
Inhalation
Rat n.g. 7.2 g/m3; 4 h/day; 4 days n.g. increased residual volume and Takezawa &
total lung capacity Lam (1978)
Rat n.g. 65.2 g/m3; 34 h over 5 days some deathsb necrosis of liver and kidney tubules; Shea (1939)
lung irritation
Rat 6-8 m 0.08 g/m3; 4 h/day; 8 days no deaths hypersecretion of thyroid gland, Grodeckaja
higher accumulation of 131I in the & Karamzina
thyroid gland (1973)
Rat 5 m + 5 f 0.36 g/m3; 6 h/day; 9 days 0/10 red stains around nose and mouth; Hazleton
weight loss in females (1981)
5 m + 5 f 1.81 g/m3; 6 h/day; 9 days 2/10 irritation to nose and eyes; weight loss; Hazleton
decrease in spleen/brain weight ratios (1981)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
5 m + 5 f 3.62 and 18.1 g/m3; 10/10 bleeding from eyes, nose and Hazleton
6 h/day; 9 days mouth (1981)
Rat n.g. 1.63 g/m3; 4 h/day; n.g. reduced weight gain; increased residual Takezawa &
5 days/week; 30 days volume and total lung capacity Lam (1978)
Rat 20 m + 20 f 0.09 g/m3; 6 h/day; no significant differences in body Conaway et al.
(Sprague- 5 days/week; 13 weeks weight, clinical chemistry, haematology (1984b)
Dawley) or organ weight data; no nasal lesions
20 m + 20 f 0.36 g/m3; 6 h/day; in 2/20 females focal necrosis in
5 days/week; 13 weeks nasal cavity
20 m + 20 f 0.9 g/m3; 6 h/day; lesions of nose and mouth; after 7
5 days/week; 13 weeks weeks in 8 animals focal metaplasia
and necrosis in nasal turbinates; after
13 weeks, increased incidence and
severity; pneumonia
Rabbit 4 m 0.9 g/m3; 6 h/day; increased hydrolytic enzyme content Tombropoulos
5 days/week; 33 days in alveolar macrophages et al. (1983)
a adapted from BUA (1991); b.w. = body weight; n.g. = not given
b exact numbers not clear
Rats inhaling morpholine at 3.62 or 18.1 g/m3 for 9 days, 6 h
per day, died within the exposure period (Hazleton, 1981). At lower
concentrations (1.81 g/m3), weight loss and irritation to nose and
eyes, as well as two deaths, were reported. The report concluded that
the maximal tolerated dose for rats is about or just below 0.3 g/m3.
Increased thyroid activity, shown as increased uptake of injected
131I, was observed in male rats after exposure to 0.08 g
morpholine/m3, 4 h/day, for 4 days (Grodeckaja & Karamzina, 1973).
Takezawa & Lam (1978) reported an increase in lung weight,
residual volume and total lung capacity in rats exposed to morpholine
(7.2 g/m3, 4 h/day for 4 days or 1.63 g/m3 for 30 days).
Tombropoulos et al. (1983) examined the induction of lysosomal
enzymes by morpholine in rabbits. Two acid hydrolases,
alpha-mannosidase and acid phosphatase, were induced in the lung
alveolar macrophages during the course of inhalation exposure
(905 mg/m3, 250 ppm, 6 h/day, 5 days/week for a total of 33
exposures). The induction was also observed when macrophages were
cultured in the presence of morpholine.
Conaway et al. (1984b) exposed groups of 40 rats to morpholine
(0, 0.09, 0.36 and 0.9 g/m3) for 7 and 13 weeks. Shallow, rapid
breathing was noted in all groups except the controls. Lesions of the
nasal septum, anterior cavities, nasoturbinates and maxilloturbinates
were observed in the 0.36 and 0.9 g/m3 groups but not in the lowest
exposure group.
Lung sections of all rats killed after 7 weeks contained early
lesions of chronic murine pneumonia. In the upper dosage group, these
lesions had developed in severity by 13 weeks. There were no apparent
treatment-related effects in any of the haematology, clinical
chemistry or urinalysis data at weeks 7 or 13.
7.2.3 Dermal
Shea (1939) investigated the effects of single and repeated
dermal application of morpholine on rabbits and guinea-pigs. He found
that whereas unneutralized, undiluted morpholine caused 100% lethality
and even the diluted compound caused mortality and severe necrotic
burns and inflammation, application of undiluted morpholine
neutralized to pH 7 with sulfuric acid caused neither gross nor
microscopic pathology of the skin with the exception that the dermis
was thickened at the site of application.
Undiluted morpholine applied for 5-15 min to rabbit skin led to
severe necrosis (BASF, 1967).
Data on short-term dermal exposure to morpholine are also
discussed in section 7.4.2.
7.3 Long-term exposure
Table 15 summarizes the data on long-term exposure to morpholine.
7.3.1 Oral
Mice (10 males and 10 females/group) were given 0%, 0.25% or 1.0%
MOAS in their drinking-water for 96 weeks and then given normal tap
water for a further 8 weeks (Shibata et al., 1987b). During the study,
the physical appearance and general behaviour did not appear to be
affected by treatment. Decreased body weight gain was noted at 1% MOAS
(both sexes) and at 0.25% MOAS (females). Gross pathological and
extensive biochemical examinations did not reveal treatment-related
effects. The incidence of hyperplasia in forestomach epithelium of
males in the 1% group was statistically higher than in the controls,
but otherwise no significant increase in the incidence of non-
neoplastic and neoplastic lesions could be found.
7.3.2 Inhalation
Migukina (1973) reported increased nervous system activity and
increases in haemoglobin and peripheral red blood cell counts in rats
and guinea-pigs exposed to morpholine (0.008 and 0.07 g/m3) for 4
months. An increase in the chromosomal aberrations of the bone marrow
cells was also noted (see section 7.6). This study was deficient with
respect to the description of study methods.
Harbison et al. (1989) carried out an extensive long-term
exposure inhalation study. Groups of 70 rats of each sex were exposed
to morpholine (0, 0.036, 0.181, 0.543 g/m3) 6 h per day, 5 days per
week, for 104 weeks, with an interim sacrifice at week 53. Levels of
nitrates and nitrites in the drinking-water were reported to be
< 0.1 mg/litre and 0.01 mg/litre, respectively. Survival, body weight
gain, organ weight and haematology and clinical chemistry data were
normal in exposed groups, compared to the controls. In-life clinical
examinations revealed increased incidences of inflammation of the
cornea, inflammation and squamous metaplasia of the turbinate
epithelium, and necrosis of the turbinate bones in the nasal cavity of
both male and female rats. No increase in the incidence of neoplasms
was found. Only tissues from the respiratory tract and eyes were
examined histologically in the case of the mid- and low-dose groups.
7.3.3 Dermal
No data are available on long-term dermal exposure to morpholine.
Table 15. Long-term exposure to morpholinea
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Oral
Mouse 50 m 0.25% MOAS; 672 days (ca. 0.05-0.14 g no significant changes Shibata et al.
(B6C3F1)b morpholine/kg body weight per day) (1987b)
50 f 0.25% MOAS; 672 days (ca. 0.07-0.17 g decreased body weight gain
morpholine/kg body weight per day)
50 m 1% MOAS; 672 days (ca. 0.28-0.5 g decreased body weight gain; increase in blood
morpholine/kg body weight per day) urea nitrogen; hyperplasia in forestomach
epithelium
50 f 1% MOAS; 672 days (ca. 0.21-0.57 g decreased body weight gain
morpholine/kg body weight per day)
Inhalation
Rat 84 m 0.008 g/m3; 34 h within 5 days; reversible changes in haemogram, kidney, Migukina (1973)
16 weeks liver, lung, spleen, myocardium
84 m 0.07 g/m3; 4 h/day; changes in haemogram, liver, kidney
5 days/week; 16 weeks lung, spleen, myocardium
Rat 20 1.09 g/m3; 6 h/day; no significant increase in spinal cord Savolainen &
5 days/week; 15 weeks axonal succinate dehydrogenase activity; no Rosenberg (1983)
significant alteration of enzyme activity in
muscle; morpholine concentration in brain
increased with length of exposure
Table 15 (cont'd)
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Rat 70 m + 0.036 and 0.18 g/m3; 6 h/day; increased incidence of irritation around Harbison et al.
(Sprague- 70 f 5 days/week; 104 weeksc eyes and nose (1989)
Dawley)
70 m + 0.54 g/m3; 6 h/day; local necrosis around eyes and nose;
70 f 5 days/week; 104 weeksc kerativitis
Guinea-pig 24 0.008 g/m3; 4 h/day; swollen lymph nodes of the spleen Migukina (1973)
5 days/week; 16 weeks
24 0.07 g/m3; 4 h/day; swollen alveoli; atrophy of lymph vessels in
5 days/week; 16 weeks lung and spleen; increase in haemoglobin and
erythrocyte number, decrease in leucocytes
a adapted from BUA (1991); b.w. = body weight
b MOAS: morpholine administered as oleic acid salt in drinking-water; in brackets, the daily intake of morpholine
calculated from MOAS intake
c 10 m + 10 f killed at 53 weeks
7.4 Skin and eye irritation; sensitization
7.4.1. Eye irritation
Eye injury in rabbits was tested by Smyth et al. (1954), who
reported severe eye burns with 0.5 ml of a 1% solution. BASF (1967)
reported that 1 drop of undiluted morpholine in rabbit eyes, repeated
once after 5 min, caused oedema, opacity, staphyloma and corrosion of
the eye mucous membranes within 24 h.
A solution of morpholine (0.02 mol/litre) neutralized with HCl
had no injurious effect on the eyes of rabbits when applied
continuously for 10 min after removal of the corneal epithelium to
facilitate penetration. Similarly, the irritative reaction of 10-20%
aqueous morpholine was reduced on neutralization (Grant, 1974).
In-life clinical examinations in rats exposed to up to
0.54 g/m3 (150 ppm) morpholine revealed increased incidences in
inflammation of the cornea at week 103 of the study (Harbison et al.,
1989). Findings included keratitis, oedema, abrasion, scarring, and
ulceration with or without neovascularization and corneal epithelial
hyperplasia. A high incidence of retinal degeneration was observed,
primarily in female animals, which was probably an age-related light-
induced retinal degeneration.
Albino rabbits, three of each sex, were treated with a mascara
composite containing 1% morpholine; 0.1 ml of the cosmetic was
instilled into one eye of each rabbit daily for 14 days. Before the
application each day, the eye was evaluated for ocular irritation. A
slight redness of the conjunctiva was noted throughout the duration of
the study but this cleared within 24 h of the last treatment. A sodium
fluorescein dye test performed at the conclusion of the study
indicated no abnormalities of the cornea or its iris membranes
(Cosmetic Ingredient Review, 1989).
7.4.2 Skin irritation
In rabbits treated with aqueous solutions of 2, 20, 40 and 60%
morpholine, the skin reactions were evaluated after 0.5, 24, 48 and
72 h (Lodén et al., 1985). A 2% solution of morpholine caused skin
irritation after 72 h, whereas 40% and 60% solutions immediately
caused reddening of the skin.
Wang & Suskind (1988) measured the irritant potential of
morpholine by applying 0.1 g mixture (0.1, 0.5, 2, 5 and 10%
morpholine in petrolatum) to guinea-pig skin for 24 h. Observations
were made 1, 24 and 48 h after removal of the test materials, and no
noticeable effects were found.
A mascara composite containing 1% morpholine was applied to
normal and abraded skin of six albino rabbits (Cosmetic Ingredient
Review, 1989). At the end of the 14-day treatment period, no dermal
toxicity or irritation was observed.
7.4.3 Sensitization
Sensitization studies (modified Buehler) on guinea-pig skin using
2% morpholine in petrolatum gave negative results (Wang & Suskind,
1988). In an in vitro study into the biochemical causes of
sensitization, it was found that morpholine as a hapten did not react
with the amino acids glycine, lysine and cystine, as did related
compounds which showed sensitizing reactions (Wang & Tabor, 1988).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
No adequate studies on reproductive toxicity, embryotoxicity or
teratogenicity have been reported.
7.6 Mutagenicity and related end-points
7.6.1 Mutagenicity of morpholine
Table 16 summarizes the short-term mutagenicity tests on
morpholine.
7.6.1.1 Bacteria
No mutagenic response was observed in plate tests with strains of
Salmonella typhimurium, either with or without metabolic activator,
exposed to morpholine at up to 10 µl/plate (approximately 10 mg/plate)
(Texaco, 1979a; Haworth et al., 1983), whereas a 99.8% purity sample
did induce weak mutagenic responses in S. typhimurium TA100 and
Escherichia coli WP2 uvr A at the unusually high dose level of
50 mg/plate (Glatt & Oesch, 1981). The activity in strain TA100 did
not require S9 mix and is, therefore, unlikely to have been due to
0.2% contamination by NMOR (see below).
In mouse peritoneal cavity host-mediated assays with
S. typhimurium TA1530 and TA1930, no increase in the proportion of
mutants was observed following the oral administration of morpholine
(Braun et al., 1977; Edwards et al., 1979). These groups served as
controls in a study of nitrosation (see below).
7.6.1.2 Yeast
No gene conversion was induced in Saccharomyces cerevisiae D4
by morpholine concentrations up to 10 µg/litre per plate. Toxicity
was observed at the highest concentration (Texaco, 1979a).
Table 16. Short-term in vitro microbial mutagenicity assays with morpholinea
Test organisms Strain Analyzed Metabolic Dosage range Result with/ Reference
purity (%) activityb without S9-mix
Salmonella typhimurium TA98/100/1535/1537 not given RA 0.109-10.9 mg/plate -/- Haworth et al. (1983)
S. typhimurium TA98/100/1535/1537 not given RA 0.005-10 µl/plate -/- Texaco (1979a)
1538c
Saccharomyces D4 not given RA 0.005-10 µl/platec -/- Texaco (1979a)
cerevisiae
S. typhimurium TA98/1535/1537 99.8 RA 0.0158-50 mg/plate -/- Glatt & Oesch (1981)
S. typhimurium TA100 99.8 RA 0.0158-50 mg/plate (+)/(+)d Glatt & Oesch (1981)
Escherichia coli WP2 uvrA 99.8 RA 0.0158-50 mg/plate (+)d/- Glatt & Oesch (1981)
S. typhimurium TA1950 99 HM1 1450-2900 µmol/kg - Braun et al. (1977)
S. typhimurium TA1530 not given HM2 4 mg/kg body weight - Edwards et al. (1979)
a adapted from BUA (1991)
b RA = Arochlor-induced rat liver-S9; HM1 = Host-mediated assay NMRI-mice; HM2 = Host-mediated assay CD-1 mice
c toxic at 10 µl/plate for strain Salmonella typhimurium TA1538 and Saccharomyces cerevisiae D4
d (+) = positive only at the 50 mg/plate dose
7.6.1.3 Mammalian cells in vitro
When tested over the range 0.625-1.25 µl/ml (approximately
0.625-1.25 mg/ml) morpholine induced small increases in the fraction
of tk mutants in mouse lymphoma L5178Y cells. No exogenous metabolic
activation system was required for this weak activity (Texaco, 1979b;
Conaway et al., 1982a,b).
Morpholine induced no DNA-repair in the primary cultures of rat
hepatocytes (0.1-100 µg/ml) (Conaway et al., 1984a).
Morpholine induced small increases in the frequency of SCEs in
Chinese hamster ovary (CHO) cells. No exogenous metabolic activation
system was required for this activity, which was observed at
concentrations of 50 and 100 nl/ml (approximately 50 and 100 µg/ml) in
the absence of S9 mix (Litton Bionetics, 1980).
Morpholine increased the numbers of type III foci in the
Balb/C3T3 cell transformation assay tested at different dosages
(0.001-0.3 µl/ml), corresponding to 78-52% survival in the
cytotoxicity test (Texaco, 1979b, Litton Bionetics, 1979a,b; Conaway
et al., 1982a,b).
In another study of in vitro transformation of Balb/3T3 cells
with and without metabolic activation, the morpholine in the culture
medium was neutralized before testing (Litton Bionetics, 1982). No
significant increases were induced in transformed foci over the tested
concentration ranges (0.015 to 1.400 µl/ml in the non-activation
assay; 0.0175-0.7 µl/ml in the activation assay). Morpholine was
therefore considered as being inactive in this test.
7.6.1.4 In vivo studies in mammals
Inui et al. (1979) administered a dosage of 500 mg morpholine
per kg body weight to female Syrian hamsters on the 11th or 12th day
of pregnancy. The embryos were removed 24 h later and embryo cells
examined. For detection of induced mutations, embryo cells were
cultured in normal medium for 72 h and then transferred to a medium
containing 8-azaguanine (10 or 20 mg per litre) or ouabain (1 mM). No
chromosomal aberrations, micro-nucleus formation, or 8-azaguanine- or
ouabain-resistant mutations were found.
Migukina (1973) reported an increase in the number of chromosomal
aberrations, particularly "fragmentations", in bone marrow cells of
rats and guinea-pigs exposed for 4 months to 8 or 70 mg
morpholine/m3 (see also section 7.3.2). However, there were
deficiencies in the study report (BUA, 1991).
7.6.2 Mutagenicity of morpholine in the presence of nitrite and
nitrate
Although morpholine has given negative responses in a number of
mutagenicity assays, there is concern for its ability to be nitrosated
readily to form NMOR (see section 4.3).
Two mouse peritoneal cavity host-mediated assays with
S. typhimurium have been performed. In one, with strain TA1530,
different morpholine doses were administered orally to mice
simultaneously with a standard sodium nitrite dose of 120 mg/kg. The
mixtures were adjusted to pH 3.4. Significant increases in the mutant
fraction were observed with morpholine doses of 4-40 mg/kg, the full
range tested (Edwards et al., 1979). In the other assay, with strain
TA1950, equimolar mixtures of morpholine and sodium nitrite (1450 or
2900 µmol/kg, approximately 125 or 250 mg/kg) were administered orally
to mice (pH 7.0). Significant increases in the mutant fraction were
observed at both dose levels. When the nitrite was administered to the
mice 10 min before the morpholine treatment, no mutagenic response was
induced (Braun et al., 1977).
Mutations were found in S. typhimurium TA1535 used to test the
urine of OF1 mice after morpholine (250 mg/kg body weight) had been
administered in the presence of nitrate (2000 mg/kg). Lower levels of
nitrate (333 and 666 mg/kg) caused no mutations (Perez et al., 1990).
7.6.3 Mutagenicity of N-nitrosomorpholine
NMOR is mutagenic to S. typhimurium TA100 in the presence of S9
mix at dose levels above 2000 µg/plate. In yeast, it induced forward
mutation and aneuploidy, but not mitotic recombination. In cultured
mammalian cells, conflicting results were obtained in unscheduled DNA
synthesis (UDS) assays in fibroblasts, while weakly positive results
were reported for sister-chromatid exchange (SCE) in CHO cells, for tk
locus mutation in mouse lymphoma L5178Y cells, and for enhanced colony
growth of BHK cells in soft agar. NMOR was negative in an in vitro
cytogenetic assay using RL1 (rat liver cell line) cells (IARC, 1978;
NRC, 1981).
7.7 Carcinogenicity
Carcinogenicity studies have been carried out with morpholine
alone, as well as in the presence of nitrite, to investigate the
possible formation and effect of NMOR.
7.7.1 Morpholine
7.7.1.1 Oral studies
Greenblatt et al. (1971) treated 40 Swiss mice (20 male and
20 female) with 6.33 g morpholine/kg food (estimated dosage, 0.9 g/kg
body weight per day for 28 weeks. The control group consisted of
80 mice of each sex. After a further 12 weeks of observation the
surviving animals were sacrificed. No increase in the lung tumour rate
(0.1 adenoma/mouse) was found compared to the controls
(0.18 adenoma/mouse). It should be noted that the duration of exposure
in this study was shorter than that normally used in a well-designed
long-term carcinogenicity study.
Multi-generation oral studies were performed on Sprague-Dawley
rats fed 5, 50 or 1000 mg/kg morpholine, together with various dietary
concentrations of sodium nitrite (0, 5, 50 or 1000 mg/kg diet)
(Newberne & Shank, 1973; Shank & Newberne, 1976). From the day of
conception, the pregnant animals were given 0 or 1000 mg morpholine/kg
feed. The F1 and F2 generations were fed likewise for the length
of the experiment. The estimated dosage for the young animals was
10 mg/day and for mature animals 20 mg/day. The average life-span was
117 weeks for the treated animals and 109 weeks for the controls. F1
and F2 generations were studied, the survivors being sacrificed in
the 125th week. Table 17 summarizes the results. Three liver cell
carcinomas, two lung angiosarcomas and one other, and two malignant
gliomas were found in the group of 104 rats (F1 and F2) treated
with morpholine.
In a similar study using Syrian golden hamsters, only the F1
generation was studied, the survivors being sacrificed in the 110th
week (Shank & Newberne. 1976). With morpholine alone (1000 mg per kg),
no liver tumours were found but the number in the group (22) was
small.
Morpholine oleic acid salt (MOAS), at dosage levels of 0%, 0.25%
or 1.0%, was added to the drinking-water of B6C3F1 mice for 96
weeks, and this was followed by normal tap water for a further 8 weeks
(Shibata et al., 1987b). Details are given in Table 15 and section
7.3.1. Extensive biochemical, gross pathological and histological
studies were performed. Only the incidence of hyperplasia in
forestomach epithelium in the males of the 1% MOAS group was
statistically higher than in the controls; otherwise, no significant
increases in the incidence of non-neoplastic or neoplastic lesions
were found.
7.7.1.2 Inhalation studies
In a long-term inhalation study over 2 years, Sprague-Dawley rats
(70 of each sex per group) received morpholine at mean exposure
concentrations of 0, 0.036, 0.181 or 0.543 g/m3 (6 h/day,
5 days/week) for up to 104 weeks (Harbison et al., 1989). Further
details are given in section 7.3.2 and Table 15. Tissues from the
high-dose and control groups were subjected to extensive
Table 17. Tumour incidence (liver and lung) in rats and hamsters after feeding morpholine, sodium nitrite or N-nitrosomorpholinea
Dietary levels (mg/kg) Tumour incidence in rats (%) Tumour incidence in hamsters (%)
Morpholine Sodium N-Nitrosomorpholine No. of Liver cell Liver angio Lung angio No. of Liver cell
nitrite animalsb carcinoma sarcoma sarcoma animals carcinoma
0 0 0 156 0 0 0 23 4
0 1000 0 96 1 0 0 30 0
1000 0 0 104 3 0 2 22 0
1000 1000 0 159 97 14 23 16 31
50 1000 0 117 59 5 6 32 0
5 1000 0 154 28 12 8 400
1000 50 0 109 3 2 1 22 0
1000 5 0 172 1 2 1 19 0
50 50 0 152 2 1 1 30 0
5 5 0 125 1 2 2 40 0
0 0 5 128 58 15 9 35 0
0 0 50 94 93 21 20 18 6
a adapted from Shank & Newberne (1976)
b F1 and F2 generations together
histopathological examination; in the middle- and low-dose groups,
this examination was limited to the eye and the respiratory tract.
Ten males and 10 females per group were sacrificed at week 53.
Survival at termination in the control, low-, middle- and high-dose
groups, respectively, was 40, 44, 33 and 32 in males and 35, 27, 32
and 35 in females. No significant increase in the incidence of
tumours was seen in rats of either sex.
7.7.2 Morpholine and nitrite
7.7.2.1 Oral studies
Sander & Bürkle (1969) fed a group of seven female Sprague-Dawley
rats 5 g morpholine together with 5 g nitrite/kg diet for 12 weeks.
After 39 weeks, all of the animals developed hepato-cellular adenomas,
six out of the seven hepatocellular carcinomas, two
hemangioendotheliomas of the liver, one a cyst-adenocarcinoma of the
liver and one a renal adenoma. Rats fed morpholine or nitrite alone
did not develop tumours.
As described in section 7.7.1.1, Shank & Newberne (1976) fed
pregnant rats on a diet containing various concentrations of
morpholine, sodium nitrite and NMOR. Table 17 summarizes the dietary
concentrations and the tumour incidences for the experimental groups.
Hepatocellular carcinoma and haemangio-sarcomas of the liver and
angiosarcoma of the lungs were the most common tumours observed in the
rats. The neoplasms induced by nitrite and morpholine were
morphologically similar to those induced by NMOR. High concentrations
(1000 mg/kg) of morpholine and nitrite together were carcinogenic to
rats. When the morpholine concentration was reduced and the nitrite
concentration remained high, the incidence of hepatic cell carcinomas
decreased with a linear dose-response relationship. When morpholine
concentration remained high, with decreasing nitrite concentration,
the number of hepatic tumours was sharply reduced. This agrees with
the observation in vitro that the nitrosation of morpholine depends
on the square of the nitrite concentration (Mirvish et al., 1975).
Groups of 40 male MRC Wistar rats were treated for 2 years with
either 10 g morpholine/kg diet and drinking-water containing 3 g
sodium nitrite/litre, or with drinking-water containing 0.15 g
NMOR/litre. In both cases, one group of rats was also given sodium
ascorbate (22.7 g/kg diet). The results of treatment with morpholine
plus nitrite or with NMOR were similar to those reported above. When
ascorbate was present, the liver tumours induced by morpholine plus
nitrite had a longer induction period (93 versus 54 weeks) and a
slightly lower incidence (49% versus 65%). However, ascorbate did not
affect liver tumour induction by preformed NMOR. Of those treated
with morpholine, nitrite and ascorbate, 21/39 animals developed
forestomach tumours (Mirvish et al. 1976).
In a similar study using Syrian hamsters (see section 7.7.1.1 and
Table 17), a high morpholine, high nitrite diet (1000 mg/kg) induced
liver cancer in some animals. In contrast, hamsters fed NMOR in the
diet seemed to have greater resistance to tumour induction (Shank &
Newberne, 1976).
7.7.3 Carcinogenicity of N-nitrosomorpholine
NMOR has been shown to be carcinogenic in mice, rats, hamsters
and various fish. Benign and malignant tumours of the liver and lung
in mice, of the liver, kidney and blood vessels in rats, and of the
liver in hamsters have been reported following oral administration of
NMOR. After its subcutaneous injection, it produces tumours of the
upper digestive and respiratory tract in hamsters. NMOR produces liver
tumours following its intravenous injection in rats and liver tumours
in various fish following its administration in tank water (IARC,
1978).
7.8 Factors modifying toxicity; toxicity of metabolites
7.8.1 Factors modifying toxicity
Leaf et al. (1991) reported that rats (Sprague-Dawley, male)
challenged with an E. coli lipopolysaccharide (LPS; 1 mg/kg body
weight), followed 6 and 10 h later by morpholine (80 or 100 µmol/kg
intraperitoneally) and arginine (400 µmol/kg) treatment, showed
significantly increased NMOR formation compared with unchallenged
control rats. The NMOR formation was measured by monitoring the NMOR
metabolite N-nitroso-(2-hydroxyethyl) glycine in the urine. Furman
& Rubenchik (1991) showed that endogenous synthesis of NMOR in the
stomach of adult mice administered nitrite (oral) in combination with
morpholine (intraperitoneal or subcutaneous) was increased after
activation of peritoneal macrophages induced by intraperitoneal
injection of E. coli LPS.
7.8.2 Morpholine metabolites
Morpholine is eliminated almost entirely in a non-metabolized
form in the urine of the rat, mouse, hamster and rabbit (section 6.3).
N-cyanomorpholine ( N-morpholinocarbonitrile) was formed when
morpholine was incubated in vitro with whole human saliva (Wishnok &
Tannenbaum, 1976).
Sohn et al. (1982a,b) identified a metabolite
N-methylmorpholine- N-oxide (NMMO) in guinea-pig urine.
Conaway et al. (1984a) reported that NMMO (0.0001-10 mg/ml) and
other putative metabolites, N-hydroxymorpholine (0.0001-1 mg/ml) and
3-morpholinone (0.001-0.1 mg/ml), did not induce DNA repair at the
non-toxic concentrations tested. The polyurethane foam catalyst,
N-butylmorpholine (0.0001-0.1 mg/ml) was also inactive in the
primary rat hepatocyte/DNA repair assay (Conaway et al., 1984a).
The chemical intermediate N-hydroxyethylmorpholine induced DNA
repair within the dose range 1-5 mg/ml (Conaway et al., 1984a).
The genotoxicity of substituted morpholines might be a function
of the substituent moiety rather than morpholine itself (Conaway et
al., 1984a).
7.9 Mechanisms of toxicity - mode of action
The irritating and corrosive properties of morpholine are due to
its basicity. The mechanism of action of its systemic effects is not
known.
Morpholine fungicides have been shown to act by inhibiting
several enzymes of sterol biosynthesis (Hesselink et al. 1990; Mercer,
1991).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available on the effects of short- and long-term
exposure to morpholine on the general population. There are no
reports of poisonings by morpholine.
8.1.1 Controlled human studies
The Cosmetic Ingredient Review (1989, 1991b) reported unpublished
studies of occlusive patch testing and in-use testing of two mascara
products containing 1% morpholine by 320 women between the ages of 18
and 65. All 320 volunteer subjects underwent occlusive patch testing
(using the Shelanski/Jordan repeat insult procedure). After 24 h, the
patches were removed and the sites graded. This procedure was repeated
several times for a total of 10 applications (3´ weeks). Following the
10th application, there was a 10-day to 2-week rest period, after
which the subjects were again patch tested, this time for 48 h, and
then scored. After another 10-day to 2-week rest period, the subjects
were again patch-tested for 48 h and scored. A final reading took
place 24 h after this. Of the 320 subjects patch-tested with charcoal
mascara, 314 showed no reaction, the remaining six showed varying
reactions. The researchers explained this irritation as being either
non-specific or due to the occlusive patching procedure. The same
results were found with the blue mascara. It was concluded from this
study that neither of the mascara products containing 1% morpholine
was a primary irritant, nor were they contact sensitizers (Cosmetic
Ingredient Review, 1989, 1991b).
In the in-use testing, 50 women used charcoal mascara once daily
for 4 weeks, and 50 women used blue mascara likewise. A further 100
women used once daily lash conditioner, eye make-up remover and
mascara (50 for each colour). In another group, 100 subjects used
lash conditioner and eye make-up remover but no mascara. All underwent
the above-described patch testing simultaneously.
There were a few complaints associated with the in-use testing.
The charcoal mascara caused a mild burning sensation in three
subjects, some itching in a further three, and minor irritation in
three. These findings were put down to improper use of the product,
which resulted in the product entering the eye and causing mild
irritation (Cosmetic Ingredient Review, 1989, 1991b).
Shea (1939) exposed himself to 43 g morpholine/m3 (12 000 ppm)
for 90 seconds. He experienced irritation of the nose followed by
coughing. Pipetting of pure morpholine from the stock solution led to
inhalation of vapour, a severe sore throat, and violently reddened
mucous membrane.
Shea (1939) reported that when applied to human finger tips
undiluted morpholine caused a cracking of the eponychium and
hyponychium about the nail and an intense stinging sensation. Diluted
morpholine (1 to 40) was a mild irritant.
8.1.1.1 Organoleptic effects
Hellman & Small (1974) tested the absolute and recognition
threshold odour index of 101 petrochemicals using a trained panel.
During a 20-min exposure, 0.036 mg morpholine/m3 was recognized by
50% of the panel and 0.5 mg morpholine/m3 by all of the panel
(giving an odour index of 65.9). It was described as an unpleasant
fishy smell.
The phenomenon known as blue vision, gray vision or haloes
"glaucopsia" is a well-documented effect of amines, including
morpholine and its derivatives, on the eyes of workers, particularly
in the foam plastic industry (Mastromatteo, 1965; Jones & Kipling,
1972). The vision becomes misty and haloes appear several hours after
the subjects have been exposed to these vapours at concentrations too
low to cause discomfort or disability during several hours of
exposure. The disturbances lasted for 4-6 h after leaving work. In a
minority of the workers examined, mild conjunctival infection was
observed; no corneal oedema or alteration in visual acuity was
detected by inspection or by ophthalmoscopy. The atmospheric
concentrations of morpholine and other similar compounds were not
reported.
8.1.2 Epidemiological studies
No data from epidemiological studies for morpholine have been
reported.
8.2 Occupational exposure
Studies into the levels of morpholine and NMOR in workplace air
are described in section 5.3.
Katosova et al. (1991) reported a cytogenetic analysis of the
lymphocytes in the peripheral blood of 24 workers (16 men, 8 women)
with 3 to 10 years of exposure to morpholine during production. These
were compared to a control group of the same size from the same town
having no contact with chemicals at work. The morpholine workers were
exposed to concentrations of morpholine in air of 0.54-0.93 mg/m3,
the maximum single concentration being 0.74-2.14 mg/m3. The
lymphocytes were cultivated for 56 h and all chromatid and chromosome
types as well as chromosome gaps were registered. From 2561 cells
analysed from the test group, only 2.08% had aberrations, compared to
1.61% out of 2050 cells in the control group, showing no significant
increase in the number of cells with chromosome aberrations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The data reported in this section refer to nominal
concentrations.
9.1.1 Microorganisms
9.1.1.1 Microorganisms in water
a) Bacterial and cyanobacterial cultures
In a 16-h cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 25°C), the toxicity threshold (3% reduction in
population growth) of morpholine for Pseudomonas putida was
310 mg/litre (Bringmann & Kühn, 1977a).
The effect of morpholine at neutral pH on the growth of
Pseudomonas strains in succinate mineral salt medium has been
reported by Knapp et al. (1982) and Emtiazi & Knapp (1994). Both
studies used two strains of Pseudomonas (all four were different)
which had not previously been exposed to morpholine and found that
morpholine at 8.7 g/litre had no effect on the rate or extent of
growth.
The mixed bacterial cultures studied by Mazure (1993) were able
to degrade morpholine rapidly at 5, 10 and 20 g/litre. The
degradation rate was highest at 10 g/litre but was only a little less
at 20 g/litre. At 40 g/litre degradation was markedly inhibited.
As reported in section 4.2.1, morpholine at up to 870 mg/litre
does not inhibit certain strains of Mycobacterium spp. that have
been found to biodegrade the compound.
In a cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 192 h) under continual lighting conditions using
Microcystis aeruginosa, a cyanobacterium, the onset of inhibition,
defined variously as a 1% (Bringmann, 1975) or 3% (Bringmann & Kühn,
1978) reduction in population growth, was found to occur at a
morpholine concentration of 1.7 mg/litre.
b) Activated sludge
The effect of morpholine on the respiratory, dehydrogenase and
nitrification activities of activated sludge was determined by
Strotmann et al. (1993). Short-term respiration assays (pH 7.0;
incubation time 30 min) were performed according to the OECD guideline
209 (OECD, 1984). The dehydrogenase activity was determined by
measuring the reduction of resazurin to resorufin. At concentrations
of 1000 mg morpholine/litre, both respiratory and dehydrogenase
activities were inhibited by up to 20%. A pH-dependent inhibitory
effect on the dehydrogenase activity was not found (pH 6.0-7.5).
Nitrification activity was determined using a specially adapted
culture of nitrifying bacteria. Suspended biomass, previously
inoculated with activated sludge and acclimated to a high-strength
ammonium feed, was incubated for 30 min at pH 7.2 with different
amounts of morpholine. The extent of inhibition was based on the
reduction in oxygen uptake rate, compared to a control containing no
morpholine. Nitrification activity was inhibited by 5% at a
morpholine concentration of 5%.
c) Protozoa
The toxicity thresholds (defined as a 5% reduction in population
growth) of morpholine for the protozoa Entosiphon sulcatum
(Bringmann, 1978), Uronema parduczi and Chilomonas paramaecium
(Bringmann & Kühn (1980), as measured in cell multiplication
inhibition tests under given conditions, are summarized in Table 18.
9.1.1.2 Microorganisms in soil
No data are available concerning soil bacteria or fungi.
9.1.1.3 Pathogenic microorganisms
Morpholine, like other amines, shows antibacterial and anti-
mycotic action. Kubis et al. (1983) demonstrated that 0.5% morpholine
inhibits the growth of a variety of pathogenic bacteria on agar plates
and in liquid medium. Application of 10% morpholine was shown to cure
an experimental mycosis in guinea-pigs caused by Trychophyton
mentagrophytes var. granulosum (Kubis et al., 1981). The pH was not
specified.
9.1.2 Other aquatic organisms
9.1.2.1 Monocellular green algae
Table 19 summarizes the tests carried out concerning the effect
of morpholine on algae.
In a 192-h test, the onset of inhibition by morpholine
(in distilled water, pH 7.0) of cell multiplication (here defined as
3% in population growth) in the green alga Scenedesmus quadricauda
in a turbidity test was noted at a concentration of 4.1 mg/litre
(Bringmann & Kühn, 1977a, 1978; see parallel results test with
Microcystis aeruginosa, section 9.1.1.1a).
Table 18. Toxicity threshold (TT) of morpholine for protozoa in the cell multiplication
inhibition test (growth factor: cell number)
Species Test duration pH Temperature TT Reference
(h) (°C) (mg/litre)
Entosiphon sulcatuma 72 6.9 25 12 Bringmann (1978)
Uronema parduczia 20 6.9 25 815 Bringmann & Kühn (1980)
Chilomonas paramaeciumb 48 6.9 20 18 Bringmann et al. (1980)
a bacteriovorous ciliate
b saprozoic flagellate
The results of Adams et al. (1985) showed that Selenastrum
capricornutum grows exponentially without a lag phase in the
presence as well as in the absence of morpholine, the growth being
inhibited first at a concentration of 100 mg/litre. The inhibition was
most marked after 6 days, shortly before reaching the stationary
phase; the no-observed-effect level (NOEL) was 10 mg/litre. Using the
same species, Calamari et al. (1980) evaluated algal growth by
measuring in vivo the fluorometric units at 48, 72, 96 h and 7 days.
A 96-h EC50 of 28 mg/litre was determined.
Millington et al. (1988) investigated the effects of varying
growth medium composition on the toxicity of morpholine to three
freshwater green algae: Selenastrum capricornutum, Scenedesmus
subspicatus and Chlorella vulgaris. They reported that the toxic
effect of morpholine on algae depended upon the species as well as
upon the test medium used (see Table 19).
9.1.2.2 Invertebrates
Table 20 summarizes the results of three investigations into the
toxicity of morpholine, as measured by the immobilization of Daphnia
magna (Bringmann & Kühn, 1977b, 1982; Calamari et al., 1980). The
test conditions were almost identical (pH 7.6-8.0; 18-22°C) and the
resulting EC50 values (100-119 mg/litre) were in good agreement.
Kramer et al. (1983) investigated the relative toxicity of
organic solvents including morpholine to mosquito (Aedes aegypti)
larvae (2 to 3 instar) by exposing 10-20 of them to 50 ml of test
solution in a glass container for 4 h at 22-24°C. The test was run in
triplicate. An LC50 of 1000 mg/litre was reported, together with
the observation that death was accompanied by convulsive larval
twitching.
9.1.2.3 Vertebrates
The acute toxicity of morpholine for fish has been tested on a
number of fresh, sea and brackish water species (see Table 21). The
lowest LC50 (180 mg/litre) was found for rainbow trout
(Oncorhynchus mykiss; formerly Salmo gairdneri) in very soft water
(20 mg CaCO3/litre). In hard water (320 mg CaCO3/litre), the
poisoning effect, with reference to the LC50 value, was less than
half (Calamari et al., 1980). Brachydanio rerio proved to be
relatively insensitive (LC0 value > 1000 mg/litre) (Wellens, 1982).
LC50 values for the freshwater fish Leuciscus idus melanotus and
Lepomis macrochirus were found to be 240-285 mg/litre (48 h) and
350 mg/litre (96 h), respectively (Juhnke & Lüdemann, 1978; Dawson et
al., 1977). The 96-h LC50 for the marine fish Menidia beryllina
was reported to be 400 mg/litre (Dawson et al., 1977). McCain & Peck
(1976) investigated the effect of morpholine on common Hawaiian fish.
Table 19. Toxicity of morpholine for algaea
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Scenedesmus inhibition of cell 192 neutral 27 TT: 4.1 Bringmann & Kühn (1978)
quadricauda multiplication (turbidity)
Selenastrum cell number after: 24 not given 22 NOEC: 100 Adams et al. (1985)
capricornutum 48/72 NOEC: 80
96/120 NOEC: 50
144 NOEC: 10
area under 24-72 NOEC: 80
growth curve 24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 50
growth rate 24-72 NOEC: 80
24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 80
S. capricornutum growth rate (in vivo 96 not given 24 EC0: 10c Calamari et al. (1980)d
fluorescence) EC50: 28c
EC100: 80c
S. capricornutum area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 50f
ECL0: 50g
Scenedesmus area under growth 24-120 not given 22 ECL0: 50e Millington et al. (1988)
subspicatus curve (cell number) ECL0: 5f
ECL0: 10g
Table 19 (cont'd)
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Chlorella vulgaris area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 80f
ECL0: 5g
a Adapted from BUA (1991)
b TT = toxicity threshold; NOEC = no-observed-effect concentration; EC0, EC50, EC100 = effective concentration inhibiting for the growth
of 0, 50 and 100% of the population; ECL0 = lowest-tested concentration with significant growth inhibition
(from 5, 10, 50, 80, 100 mg/litre)
c equivalent to GC-FID measured concentration ± 10%
d method according to Chiaudini & Vighi (1978)
e Bolds basal medium (BBM): rich medium; N/P-ratio 14:1
f OECD medium: less N than BBM; N/P-ratio 24:1
g EPA medium: less N than BBM; N/P-ratio 50:1
Table 20. Acute toxicity of morpholine for the water flea Daphnia magna
(test duration: 24 h; effect: immobilization)a
Test systemb pH Temperature Effect/concentration Reference
(°C) (mg/litre)
Static 7.6-7.7 20-22 EC0: 16 Bringmann &
EC50: 100 Kühn (1977b)
EC100: 500
Static 8 ± 0.2 20 EC0: 68 Bringmann &
EC50: 101 (83-122)c Kühn (1982)
EC100: 260
Static 7.9 ± 0.3 18-22 EC50: 119 (112-127)c Calamari et
al. (1980)
a adapted from BUA (1991)
b in accordance with OECD-guideline 202 part I
c 95% confidence limits
The 96-h LC50 (TLm) was between 100 and 180 mg/litre for white
mullet (Vala mugil engeli; former designation: Chelon engeli),
between 320 and 560 mg/litre for Gambusia affinis, and more than
1000 mg/litre for Tilapia sp.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Reynolds (1989) found that 4.4±0.9 mol morpholine/m3
(383 mg/litre) lowers the percentage germination of lettuce seeds
(Lactuca sativa) by 50% of the control value. The seeds were kept on
agar for 3 days in sealed plastic containers at a temperature of 30°C.
9.1.3.2 Animals
No data are available concerning the effects of morpholine on
terrestrial animals.
9.2 Field observations
No data have been reported concerning field observations
involving morpholine.
Table 21. Acute toxicity of morpholine in fish static test systemsa
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
Rainbow trout freshwater hardness: 320 mg CaCO3/litre; pH 7.4; 96 LC50 380 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (375-460)b (1980)
Rainbow trout freshwater hardness: 20 mg CaCO3/litre; pH 7.4; 96 LC50 180 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (1980)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 285 Juhnke &
(Leuciscus idus continual aeration (Juhnke laboratory) (250/340)d Lüdemann
melanotus) (1978)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 240 Juhnke &
(L. idus melanotus) continual aeration (Lüdemann laboratory) (100/301)d Lüdemann (1978)
Zebra fish freshwater hardness: 250 mg CaCO3/litre; pH 7.5; 22°C; 96 LC50> 1000 Wellens (1982)
(Brachydanio rerio) no aeration (> 1000/> 1000)d
Bluegill sunfish freshwater hardness: 55 mg CaCO3/litre; pH 7.6-7.9; 23°C; 96 LC50 350 Dawson et
(Lepomis macrochirus) intermittent aeration 24 h after test; from al. (1977)
commercial hatcheries; 14 days acclimatization
Tidewater silverside marine artificial seawater; 20°C; continual aeration; 96 LC50 400 Dawson et
(Menidia beryllina) caught wild Horseshoe Bay (New Jersey); al. (1977)
40-100 mm length; 14 days acclimatization
Mosquito fish marinee,f natural seawater (32% salinity); continual 96 LC0 320 McCain &
(Gambusia affinis) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 560 Peck (1976)
Hawaii); 38-64 mm length; 7 days
acclimatization
Table 21 (cont'd)
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
"Coloured perch" marinee,f natural seawater (32% salinity); continual 96 LC0 1000 McCain &
(Tilapia sp.) aeration; 25.5-27.8°C; caught wild (Oahu, Peck (1976)
Hawaii); 30-65 mm length; 7 days
acclimatization
White mullet marinee natural seawater (32% salinity); continual 96 LC0 100 McCain &
(Chelon engeli) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 320 Peck (1976)
Hawaii); 91-128 mm length; 7 days
acclimatization
a adapted from BUA (1991)
b 95% confidence limits
c parallel tests in two different laboratories
d LC0/LC100
e the fish were caught in rivers, presumably in the estuary area
f fresh or brackish water fish that were exposed to the marine system.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks were evaluated by an International Agency
for Research on Cancer ad hoc Expert Group in 1988. It was
concluded that there is inadequate evidence for the carcinogenicity
of morpholine in experimental animals. No data were available from
studies in humans on the carcinogenicity of morpholine. The overall
evaluation was that morpholine was not classifiable for its
carcinogenicity to humans (IARC, 1989).
IARC also evaluated N-nitrosomorpholine (NMOR). It concluded
that there is sufficient evidence for a carcinogenic effect of NMOR in
several experimental animal species. No human data, case reports or
epidemiological studies were available, nor was it possible to
identify exposed groups. In spite of the absence of epidemiological
data, NMOR should be regarded for practical purposes as if it were
carcinogenic to humans (IARC, 1978).
REFERENCES
Aarts AJ, Benson GB, Duchateau NL, & Davies KM (1990) Modern
analytical techniques for the detection of rubber chemicals and their
decomposition products in the environment. Int J Environ Anal Chem,
38: 85-95.
Ackermann P, Margot P, & Klotzsche, C (1989) Agricultural fungicides.
In: Ullmann's encyclopedia of industrial chemistry, 5th ed. Weinheim,
VCH Verlagsgesellschaft, vol A12, p 107.
Adams N, Goulding KH, & Dobbs AJ (1985) Toxicity of eight water-
soluble organic chemicals to Selenastrum capricornutum: a study of
methods for calculating toxic values using different growth
parameters. Arch Environ Contam Toxicol, 14: 333-345.
Agarwala KK (1982) Study on stability of morpholine in high pressure
boiler system. Fertil Technol, 19: 73-75.
Air Products and Chemicals (1989) Material safety data sheet.
Allentown, Pennsylvania, Air Products and Chemicals, Inc.
Aitzetmüller K & Thiele E (1982) Absence of volatile N-nitrosamines in
margarines. J Am Oil Chem Soc, 59: 387-389.
Anderson GE (1983) Human exposure to atmospheric concentrations of
selected chemicals. Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Quality Planning and
Standards, pp 455-473 (Report submitted by Systems Applications, Inc.,
San Rafael, California) (EPA No 83-265249).
Archer MC, Tannenbaum SR, & Wishnok JS (1977) Nitrosamine formation in
the presence of carbonyl compounds. In: Walker EA, Bogovski P, &
Griciute L ed. Environmental N-nitroso compounds analysis and
formation. Lyon, International Agency for Research on Cancer,
pp 141-145 (IARC Scientific Publications No. 14).
Archer MC, Yang HS, & Okun JD (1979) Acceleration of nitrosamine
formation at pH 3.5 by microorganisms. In: Walker EA, Castegnaro M,
Griciute L, & Lyle RE ed. Environmental aspects of N-nitroso
compounds. Lyon, International Agency for Research on Cancer,
pp 239-246 (IARC Scientific Publications No. 19).
Atkinson R (1988) Estimation of gas-phase hydroxyl radical rate
constants for organic chemicals. Environ Toxicol Chem, 7: 435-442.
Badura R, Linde H, & Schuster RH (1989) Analysis of volatile products
in vulcanisation fumes. In: Proceedings of the International Rubber
Conference, Prague, 8-11 May. London, International Rubber
Association, pp 19-38.
BASF (1967) [Morpholine: Toxicological data.] Ludwigshafen, BASF AG,
2 pp (Internal report) (in German).
BASF (1987) [Data sheets: Morpholine.] Ludwigshafen, BASF AG, 8 pp
(in German).
BASF (1990) [Test report: Zahn-Wellens-Test.] Ludwigshafen, BASF AG,
12 pp (Unpublished report) (in German).
BIA (Industries Association Institute for Work Safety) (1989)
[Measurement of dangerous substances - BIA data sheet.] Bielefeld,
Erich Schmidt Verlag (in German).
Bianchi A & Muccioli G (1978) [Determination of morpholine together
with isopropyl alcohol, toluene and xylenes (o-m-p) in the atmosphere
by means of gas-chromatography.] Ann Ist Super Sanita, 14: 441-445
(in Italian with English summary).
Börzsönyi M, Török G, Surján A, Challis BC, & Bär V (1981) Protective
effect of a new antioxidant on acute hepatotoxicity caused by
morpholine plus nitrite in rats. Toxicol Lett, 7: 285-288.
Bosholm J (1983) [Description of the behaviour of gaseous compounds in
water/steam systems.] Kernenergie, 26: 198-201 (in German).
Boyland E, Nice E, & Williams K (1971) The catalysis of nitrosation by
thiocyanate from saliva. Food Cosmet Toxicol, 9: 639-643.
Braun R, Schöneich J, & Ziebarth D (1977) In vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Bringmann G (1975) [Determination of the biological effect of water
pollutants on blue algae Microcystis using the cell multiplication
inhibition test.] Gesund-Ing, 96: 238-241 (in German).
Bringmann G (1978) [Determination of the biological effect of water
pollutants in protozoa I Bacteriovorous flagellate protozoa (Model
organism: Entosiphon sulcatum Stein).] Z Wasser Abwasser Forsch,
11: 210-215 (in German).
Bringmann G & Kühn R (1977a) [Toxicity thresholds of water pollutants
for bacteria (Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1977b) [Results of toxic action of water
pollutants on Daphia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1978) [Toxicity thresholds of water pollutants
for blue algae (Microcystis aeruginosa) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) [Determination of the biological effect of
water pollutants in protozoa. II. Bacteriovorous ciliates.] Z Wasser
Abwasser Forsch, 1: 26-31 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic action of water
pollutants on Daphnia magna tested by an improved standardized
procedure system.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa III Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German).
Brouwer R, Marquart H, de Mik G, & van Hemmen J (1992) Risk assessment
of dermal exposure of greenhouse workers to pesticides after re-entry.
Arch Environ Contam Toxicol, 23: 273-280
Brown AR (1966) Morpholine: its properties and uses. Manuf Chem
Aerosol News, Dec 1966: 50-52.
Brown VR & Knapp JS (1990) The effect of withdrawal of morpholine from
the influent and its reinstatement on the performance and microbial
ecology of a model activated sludge plant treating a morpholine-
containing influent. J Appl Bacteriol, 69: 43-53.
Brown VR, Knapp JS, & Heritage J (1990) Instability of the morpholine-
degradative phenotype in mycobacteria isolated from activated sludge.
J Appl Bacteriol, 69: 54-62.
Brunnemann KD & Hoffmann D (1991) Decreased concentrations of
N-nitrosodiethanolamine and N-nitrosomorpholine in commercial tobacco
products. J Agric Food Chem, 39: 207-208.
Brunnemann KD, Scott JC, & Hoffmann D (1982) N-nitroso-morpholine
and other volatile N-nitrosamines in snuff tobacco. Carcinogenesis,
3: 693-696.
BUA (Society of German Chemists, Advisory Committee on Existing
Chemicals of Environmental Relevance) (1991) [Morpholine.] Weinheim,
VCH Verlagsgesellschaft, 181 pp (BUA Report No. 56) (in German).
Calamari D, Da Gasso R, Galassi S, Provini A, & Vighi M (1980)
Biodegradation and toxicity of selected amines on aquatic organisms.
Chemosphere, 9: 753-762.
Calmels S, Ohshima H, Vincent P, Gounot A-M, & Bartsch H (1985)
Screening of micro-organisms for nitrosation catalysis at Ph 7 and
kinetics studies on nitrosamines formation from secondary amines by
E. coli strains. Carcinogenesis, 6: 911-915.
Calmels S, Ohshima H, Crespi M, Leclerc H, Cattoen C, & Bartsch H
(1987) N-nitrosoamine formation by microorganisms isolated from
human gastric juice and urine: biochemical studies on bacteria-
catalysed nitrosation. In: Bartsch H, O`Neill IK, & Schulte-Hermann R
ed. The relevance of N-nitroso compounds to human cancer: Exposures
and mechanisms. Lyon, International Agency for Research on Cancer,
pp 391-395 (IARC Scientific Publications No. 84).
Calmels S, Ohshima H, & Bartsch H (1988) Nitrosamine formation by
denitrifying and non-denitrifying bacteria: Implication of nitrite
reductase and nitrate reductase in nitrosation catalysis. J Gen
Microbiol, 134: 221-226.
Calmels S, Dalla Venezia N, & Bartsch H (1990) Isolation of an enzyme
catalysing nitrosamine formation in Pseudomonas aeruginosa and
Neisseria mucosae. Biochem Biophys Res Commun, 171(2): 655-660.
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991a) Bacterial
formation of N-nitroso compounds in the rat stomach after omeprazole-
induced achlorhydria. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 187-191 (IARC Scientific Publications No. 105).
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991b) Bacterial
formation of N-nitroso compounds in the rat stomach after
omeprazole-induced achlorhydria. Carcinogenesis, 12: 435-439.
Cech JS & Chudoba J (1988) Effect of the solids retention time on the
rate of biodegradation of organic compounds. Acta Hydrochim Hydrobiol,
16: 313-323.
Cech JS, Hartman P, Slosárek M, & Chudoba J (1988) Isolation and
identification of a morpholine-degrading bacterium. Appl Environ
Microbiol, 54: 619-621.
Challis BC & Kyrtopoulos SA (1979) The chemistry of nitroso-compounds.
Part 11. Nitrosation of amines by the two-phase interaction of amines
in solution with gaseous oxides of nitrogen. J Chem Soc Perkin Trans
I, 1979: 299-304.
Challis BC & Outram JR (1979) The chemistry of nitroso-compounds. Part
15. Formation of N-nitrosoamines in solution from gaseous nitric oxide
in the presence of iodine. J Chem Soc Perkin Trans I, 1979: 2768-2775.
Chemical Marketing Reporter (1989) Aromatics. Morpholine. Chem Mark
Rep, July 17: 13.
Chemical Marketing Reporter (1990) Dow to exit morpholine. Chem Mark
Rep, May 28: 7.
Chiaudani G & Vighi M (1978) The use of Selenastrum capricornutum
batch cultures in toxicity studies. Mitt Int Ver Limnol, 21: 316-329.
Collaert B, Attström R, De Bruyn H, & Movert R (1992a) The effect of
delmopinol rinsing on dental plaque formation and gingivitis healing.
J Clin Periodontol, 19: 274-280.
Collaert B, Edwardsson S, Attström R, Hase J, Aström M, & Movert R
(1992b) Rinsing with delmopinol 0.2% and chlorhexidine 0.2%: Short-
term effect on salivary microbiology, plaque, and gingivitis.
J Periodontol, 63: 618-625.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982a) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Presented at the 13th Annual
Meeting of the Environmental Mutagen Society, Boston, 22-28 February
1982. New York, John Wiley and Sons, pp 1-15.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982b) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Environ Mutagen, 4: 390
(Abstract).
Conaway CC, Tong C, & Williams GM (1984a) Evaluation of morpholine,
3-morpholine, and N-substituted morpholines in the rat hepatocyte
primary culture/DNA repair test. Mutat Res, 136: 153-157.
Conaway CC, Coate WB, & Voelker RW (1984b) Subchronic inhalation
toxicity of morpholine in rats. Fundam Appl Toxicol, 4: 465-472.
Cooney RV & Ross PD (1987) N-nitrosation and N-nitration of morpholine
by nitrogen dioxide in aqueous solution: effects of vanillin and
related phenols. J Agric Food Chem, 35: 789-793.
Cooney RV, Ross PD, Bartolini GL, & Ramseyer J (1987) N-nitrosoamine
and N-nitroamine formation: Factors influencing the aqueous reactions
of nitrogen dioxide with morpholine. Environ Sci Technol, 21: 77-83.
Cooney RV, Ross PD, Hatch-Pigott V, & Ramseyer J (1992) Carcinogenic
N-nitrosamine formation: A requirement for nitric oxide. J Environ Sci
Health, 27(3): 789-801.
Cosmetic Ingredient Review (1986) Scientific literature review on
morpholine. Washington, DC, The Cosmetic, Toiletry and Fragrance
Association, 71 pp.
Cosmetic Ingredient Review (1989) Final report on the safety
assessment of morpholine. J Am Colloq Toxicol, 8: 707-748.
Cosmetic Ingredient Review (1991a) CIR annual report 1991. Washington,
DC, The Cosmetic, Toiletry & Fragrance Association, 67 pp.
Cosmetic Ingredient Review (1991b) Protocol: Repeated insult patch
testing and in-use testing. Washington, DC, The Cosmetic, Toiletry &
Fragrance Association, 16 pp (Unpublished report).
Cusano F & Luciano S (1993) Contact dermatitis from pramoxine. Contact
Dermatitis, 28: 39:
Davies R, Massey RC, & McWeeny DJ (1980) The catalysis of the
N-nitrosation of secondary amines by nitrosophenols. Food Chem,
6: 115-122.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J Hazard Mater, 1: 303-318.
Dmitrenko GN & Gvozdyak P (1988) Detection of morpholine by
mycobacteria. In: Proceedings of a Conference on Microbiological
Methods for Protecting the Environment, Puschino, USSR, 5-7 April
1988. Puschino, Scientific Centre for Biological Research of the
Academy of Sciences, 141 pp.
Dmitrenko GN, Udod VM, & Gvozdyak PI (1985) Destruction of morpholine
by fixed bacteria. Khim Tekhnol Vody, 7: 71-73.
Dmitrenko GN, Gvodzdyak PI, & Udod VM (1987) Selection of destructor
microorganisms for heterocyclic xenobiotics. Khim Tekhnol Vody,
9(5): 442-445.
Dodson JJ & Bitterman ME (1989) Compound uniqueness and the
interactive role of morpholine in fish chemoreception. Biol Behav,
14: 13-27.
Donath G, Heitmann HG, Messer J, & Schott M (1977) [Determination of
the distribution coefficients of volatile alkalising agents used in
power plants between steam and water phases and their importance for
the corrosive behaviour of materials.] Vom Wasser, 49: 221-243
(in German).
Dropkin D (1985) Sampling of automobile interiors for organic
emissions. Research Triangle Park, North Carolina, US Environmental
Protection Agency, 20 pp (PB85-172567).
Dynamac Corporation (1988) Information review: Morpholine (EPA
contract No. 68-02-4251). Rockville, Maryland, Dynamac Corporation,
pp 1-52 (IR-514) (Report prepared for TSCA Interagency Testing
Committee).
ECETOC (1991) Critical evaluation of methods for the determination of
N-nitrosamines in personal care and household products. Brussels,
European Chemical Industry Ecology and Toxicology Centre (Technical
Report No. 42).
EEC (1983) Council directive 79831 - Annex V, Part C: Methods for
determination of ecotoxicity. 5.2 Degradation - Biotic degradation
Manometric Respirometry. Brussels, Commission of the European Union.
EEC (1990) Proposal for a council directive on the approximation of
the laws of the Member States relating to cosmetic products (SEC(90)
1985 final) (90/C 322/06). Off J Eur Communities, C/322: 29-43.
Edwards G, Whong W-Z, & Speciner N (1979) Intrahepatic mutagenesis
assay: a sensitive method for detecting N-nitrosomorpholine and
in vivo nitrosation of morpholine. Mutat Res, 64: 415-423.
Emtiazi G (1993) Microbial degradation of linear and cyclic amines.
Leeds, University of Leeds, Department of Microbiology (Thesis).
Emtiazi G & Knapp JS (1994) The biodegradation of piperazine and
structurally-related linear anc cyclic amines. Biodegradation,
5: 83-92.
Environment Agency, Japan (1980) Annual report on chemicals in the
environment. Tokyo, Environment Agency, pp 72-73.
Estrin NF, Haynes CR, & Whelan JM (1982) Specifications/spectra. CTFA
compendium of cosmetic ingredient composition. Washington, DC, The
Cosmetic, Toiletry and Fragrance Association, Inc.
Fadlallah S, Cooper SF, Fournier M, Drolet D, & Perrault G (1990)
Determination of N-nitroso compounds in the environment of a metal
factory using metalworking fluids. Int J Environ Anal Chem,
39: 281-288.
Fajen JM, Carson GA, Rounbehler DP, Fan TY, Vita R, Goff UE, Wolf MH,
Edwards GS, Fine DH, Reinhold V, & Biemann K (1979) N-nitrosamines in
the rubber and tire industry. Science, 205: 1262-1264.
Fan TY, Vita R, & Fine DH (1978) C-nitro compounds: a new class of
nitrosating agents. Toxicol Lett, 2: 5-10.
Fisher AA (1986) Contact Dermatitis. Philadelphia, Pennsylvania, Lea &
Febiger.
Furman MA & Rubenchik BL (1991) Formation of carcinogenic N-nitro
compounds after intraperitoneal administration of nitrosating
precursors in C57BLl/6 line mice. Eksp Onkol, 13(2): 14-22.
Gavinelli M, Fanelli R, Bonfanti M, Davoli E, & Airoldi L (1988)
Volatile nitrosamines in foods and beverages: preliminary survey of
the Italian market. Bull Environ Contam Toxicol, 40: 41-46.
Gilbert R & Saheb SE (1987) Field measurement of the distribution
coefficients of chemical additives used for corrosion control in
steam-water cycles. Mater Perform, 26: 30-36.
Gilbert R, Rioux R, & Saheb SE (1984) Ion chromatographic
determination of morpholine and cyclohexylamine in aqueous solutions
containing ammonia and hydrazine. Anal Chem, 56: 106-109.
Glatt HR & Oesch F (1981) [Ames test for morpholine.] Ludwigshafen,
BASF AG, 11 pp (Unpublished report submitted by the Pharmacological
Institute, University of Mainz, Germany).
Grant WM (1974) Morpholine. In: Toxicology of the eye, 2nd ed.
Sringfield, Illinois, Charles C. Thomas,vol 2, pp 722-723.
Greenblatt M, Mirvish S, & So BT (1971) Nitrosamine studies: induction
of lung adenomas by concurrent administration of sodium nitrite and
secondary amines in Swiss mice. J Natl Cancer Inst, 46: 1029-1034
(Abstract).
Griffiths MH (1968) The metabolism of N-triphenylmethylmorpholine in
the dog and rat. Biochem J, 108: 731-740.
Grodeckaja NS & Karamzina NM (1973) [Initial reactions by the organism
to the effects of industrial substances in concentrations of minimal
effect (Limac, Limch).] Toksikol Nov Prom Chim Veshchestv,
13: 12-23 (in Russian).
Groenen PJ, Busink E, & van Wandelen M (1987) Determination of
volatile nitrosamines in cheese and cured meat products. Model study
of a temperature- and pH-dependent artefact formation phenomenon in
alkaline medium. Z Lebensm.Unters Forsch, 185: 24-30.
Grosjean D (1991) Atmospheric chemistry of toxic contaminants.
6. Nitrosamines: Dialkyl nitrosamines and nitrosomorpholine. J Air
Waste Manage Assoc, 41: 306-311.
Hamano T, Mitsuhashi Y, & Matsuki Y (1980) Improved gas
chromatographic method for the quantitative determination of secondary
amines as sulphonamides formed by reaction with benzene-sulphonyl
chloride. J Chromatogr, 190: 462-465.
Hamano T, Mitsuhashi Y, & Matsuki Y (1981) Glass capillary gas
chromatography of secondary amines in foods with flame photometric
detection after derivatization with benzenesulfonyl chloride. Agric
Biol Chem, 45: 2237-2243.
Hansen L, Akesson B, Sollenberg J, & Lundh T (1986) Determination of
N-methylmorpholine in air samples from a polyurethane foam factory.
Scand J Work Environ Health, 12: 66-69.
Harbison RD, Marino DJ, Conaway CC, Rubin LF, & Gandy J (1989) Chronic
morpholine exposure of rats. Fundam Appl Toxicol, 12: 491-507.
Hassan SSM, Tadros FS, & Selig W (1985) Microdetermination of
secondary aliphatic amines using a copper ion-selective electrode.
Microchem J, 31: 1-6.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hazleton (1981) Final report: 9-day acute inhalation toxicity study in
rats. Vienna, Virginia, Hazleton Laboratories America, Inc., 26 pp
(Submitted to Texaco Chemical Company).
Hecht SS & Morrison JB (1984) A sensitive method for detecting
in vivo formation of N-nitrosomorpholine and its application to
rats given low doses of morpholine and sodium nitrite. Cancer Res,
7: 2873-2877.
Hecht SS & Young R (1981) Metabolic alpha-hydroxylation of
N-nitrosomorpholine and 3,3,5,5-tetradeutero-N-nitrosomorpholine in
the F344 rat. Cancer Res, 41: 5039-5043.
Heilen G, Mercker HJ, Frank D, Reck RA, & Jäckh R (1989) [Amines,
aliphatic.] In: [Ullmann's encyclopedia of industrial chemistry], 5th
ed. Weinheim, VCH Verlagsgesellschaft, vol A2, pp 1-36 (in German).
Hellman TM & Small FH (1974) Characterization of the odor properties
of 101 petrochemicals using sensory methods. J Air Pollut Control
Assoc, 24: 979-982.
Hesselink PGM, Kerkenaar A, & Witholt B (1990) Inhibition of microbial
cholesterol oxidases by dimethylmorpholines. J Steroid Biochem,
35: 107-113.
Hibbs JB Jr (1992) Immunology: Overview of cytotoxic mechanisms and
defence of the intracellular environment against microbes. In: Moncada
S, Marletta MA, Hibbs JB Jr, Higgs EA ed. The biology of nitric oxide.
London,d Chapel Hill, Portland Press, pp 201-206.
Hoffmann D, Brunnemann KD, Adams JD, Rivenson A, & Hecht SS (1982)
N-nitrosamines in tobacco carcinogenesis. In: Magee PN ed.
Nitrosamines and human cancer. Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp 211-225 (Banbury Report No. 12).
Hoffmann D, Adams JD, Lisk D, Fisenne I, & Brunnemann KD (1987) Toxic
and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst,
79: 1281-1286.
Hollett BA, Klemme JC, & Andjelkovich D (1982) Health hazard
evaluation report HETA 81-045B-1216: Uniroyal, Inc., Mishawaka,
Indiana. Cincinnati Ohio, National Institute for Occupational Safety
and Health, 20 pp (PB84-183615).
Hotchkiss JH & Vecchio AJ (1983) Analysis of direct contact paper and
paperboard food packaging for N-nitrosomorpholine and morpholine. J
Food Sci, 48: 240-242.
IARC (1978) N-nitrosomorpholine. In: Some N-nitroso compounds. Lyon,
International Agency for Research on Cancer, pp 263-280 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 17).
IARC (1989) Morpholine. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 199-213 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office, pp 282-283 (Occupational
Safety and Health Series No. 37).
Inui N, Nishi Y, Taketomi M, Mori M, Yamamoto M, Yamada T, & Tanimura
A (1979) Transplacental mutagenesis of products formed in the stomach
of golden hamsters given sodium nitrite and morpholine. Int J Cancer,
24: 365-372.
Iqbal ZM, Dahl K, & Epstein SE (1980) Role of nitrogen dioxide in the
biosynthesis of nitrosamines in mice. Science, 207(4432): 1475-1476.
Jakobi G, Löhr A, Schwuger MJ, Jung D, Fischer W, & Gloxhuber C (1983)
[Washing agents.] In: [Ullmann's encyclopedia of industrial
chemistry], 4th ed. Weinheim, VCH Verlagsgesellschaft, vol 24, pp 105,
138-139 (in German).
Japan Chemical Week (1991) Speciality chemicals handbook, 4th ed.
Tokyo, The Chemical Daily Co., Ltd, 8 pp.
Jones WT & Kipling MD (1972) Glaucopsia - blue-grey vision. Br J Ind
Med, 29: 460-461.
Juhnke I & Lüdemann D (1978) [Results of the testing of 200 selected
chemical compounds for acute fish toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Kamimura H, Enjoji Y, Sasaki H, Kawai R, Kaniwa H, Niigata K, &
Kageyama S (1987) Disposition and metabolism of indeloxazine
hydrochloride, a cerebral activator, in rats. Xenobiotica,
17(6): 645-658.
Karweik DH & Meyers CH (1979) Spectrophotometric determination of
secondary amines. Anal Chem, 51: 319-320.
Katosova LD, Fomenko VN, & Davydenko LN (1991) Air pollution and
industrial hygiene. Gig Tr Prof Zabol, 6: 35-36.
Kleemann A & Engel J (1982) [Midodrin, Minaprin, Minocyclin;
Trimethobenzamid, Trimethoprin, Trimetozin, Trimipramin,
Tripelennamin.] In: [Pharmaceutical substances.] Stuttgart, New York,
Georg Thieme Verlag, pp 602-603, 923-928 (in German).
Knapp JS & Brown VR (1988) Morpholine biodegradation. Int Biodeterior,
24: 299-306.
Knapp JS & Whytell AJ (1990) The biodegradation of morpholine in river
water and activated sludge. Environ Pollut, 68: 67-79.
Knapp JS, Callely AG, & Mainprize J (1982) The microbial degradation
of morpholine. J Appl Bacteriol, 52: 5-13.
Koga M & Akiyama T (1985) Determination of trace heterocyclic amines
in water as their 1,2,-naphthoquinone derivatives by high performance
liquid chromatography. Anal Sci, 1: 285-288.
Kramer VC, Schnell DJ, & Nickerson KW (1983) Relative toxicity of
organic solvents to Aedes aegypti larvae. J Invertebr Pathol,
42: 285-287.
Kubis A, Witek R, Baran E, Jadach W, Matecka K, & Zaba A (1981)
[ In vivo antimycotic effect of topically applied morpholine.]
Pharmazie, 36: 429-431 (in German).
Kubis A, Witek R, & Krutul H (1983) [Investigation on antibacterial
action of some amines.] Pharmazie, 38: 488-489 (in German).
Lakritz L & Kimoto W (1980) N-nitrosamines - contaminants in blood-
collection tubes. Food Cosmet Toxicol, 18: 31-34.
Lam HF & Van Stee EW (1978) A re-evaluation of the toxicity of
morpholine. Fed Proc, 37: 679 (Abstract).
Lamarre C, Gilbert R, & Gendron A (1989) Liquid chromatographic
determination of morpholine and its thermal breakdown products in
steam-water cycles at nuclear power plants. J Chromatogr,
467: 249-258.
Lamb CB & Jenkins GF (1952) B.O.D. of synthetic organic chemicals. In:
Proceedings of the 7th Industrial Waste Conference, Purdue University,
West Lafayette, Indiana, 7-9 May 1952. Ann Arbor, Michigan, Ann Arbor
Science Publishers, pp 326-339.
Lathia D & Edeler A (1989) [Influence of sulfur-containing amino acids
on in vitro nitrosamine formation under conditions similar to those
found in the stomach.] Ernährungsphysiologie, 36: 21-24 (in German).
Lathia D & Schellhöh B (1981) Inhibition of nitrosamine formation
in vitro in presence of both ascorbic acid and sorbic acid. Recent
Adv Clin Nutr, 1: 189-190.
Lauharanta J (1992) Comparative efficacy and safety of amorolfine nail
lacquer 2% versus 5% once weekly. Clin Exp Dermatol, 705: 41-43.
Leach SA, Cook AR, Challis BC, Hill MJ, & Thompson MH (1987)
Bacterially mediated N-nitrosation reactions and endogenous
formation of N-nitroso compounds. In: Bartsch H, O`Neill IK, &
Schulte-Hermann R ed. Relevance of N-nitroso compounds to human
cancer: Exposures and mechanisms. Lyon, International Agency for
Research on Cancer, pp 396-403 (IARC Scientific Publications No. 84).
Leach SA, Mackerness CW, Hill MJ, & Thompson MH (1991) Inhibition of
bacterially mediated N-nitrosation by ascorbate: Therapeutic and
mechanistic considerations. In: O`Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 571-578 (IARC Scientific Publications No. 105).
Leaf CD, Wishnok JS, & Tannenbaum SR (1991) Endogenous incorporation
of nitric oxide from L-arginine into N-nitrosomorpholine stimulated by
Escherichia coli lipopolysaccharide in the rat. Carcinogenesis,
224: 537-539.
Lee SA (1982) Health hazard evaluation report HETA 82-156-1231:
Sheller-Globe Corporation, Keokuk, Iowa. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 8 pp (PB84-172915).
Leenheers LH, Ravensberg JC, Kerstens HJ, & Jongen MJM (1992) Gas
chromatographic determination of the pesticide dodemorph for
assessment of occupational exposure. J Chromatogr Sci, 132: 228-232.
Leo A, Hansch C, & Elkins D (1971) Partition coefficients and their
uses. Chem Rev, 71: 525, 564.
Le Therizien L, Heymans F, Redeuilh C, Godfroid J-J, & Busch N (1980)
Partition coefficient additivity. 1. Morpholine and
N-(N',N'-disubstituted amino acetyl)-arylamine series. Eur J Med Chem
Chim Ther, 15: 311-316.
Lide DR ed. (1990) CRC handbook of chemistry and physics, 71st ed.
Boca Raton, Florida, CRC Press, pp 8-34.
Litton Bionetics (1979a) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp (Report submitted to Texaco Petrochemicals,
Bellaire, Texas).
Litton Bionetics (1979b) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp Report submitted to Texaco, Inc., Beacon, New
York).
Litton Bionetics (1980) Mutagenicity evaluation of morpholine in the
sister chromatid exchange assay with chinese hamster ovary (CHO)
cells. Kensingtn, Maryland, Litton Bionetics, Inc., 10 pp (Report
submitted to Texaco, Inc., Beacon, New York).
Litton Bionetics (1982) Evaluation of morpholine in the in vitro
transformation of Balb/3T3 cells with and without metabolic activation
assay 80/490. Kensington, Maryland, Liotton Bionetics, Inc. 21 pp
(Unpublished report submitted to BASF AG, Ludwigshafen).
Liu RH, Conboy JJ, & Hotchkiss JH (1988) Nitrosation by nitro-nitroso
derivatives of olefins: a potential mechanism for N-nitrosamine
formation in fried bacon. J Agric Food Chem, 36: 984-987.
Liu RH, Jacob JR, Tennant BC, & Hotchkiss JH (1992) Nitrite and
nitrosamine synthesis by hepatocytes isolated from normal woodchucks.
Cancer Res, 52: 4139-4143.
Lodén M, Larsson R, Häggqvist I, & Karlsson N (1985) [The dermal
irritancy/corrosion of 20 compounds in aqueous solutions.] Umea,
Sweden, Försvarets Forskningsanstalt, 76 pp (FOA Report No. E/40023)
(in Swedish with English summary).
Loeppky RN, Tomasik W, & Kerrick BE (1987) Nitroso transfer from
alpha-nitrosamino aldehydes: implications for carcinogenesis.
Carcinogenesis, 8(7): 941-946.
London MA & Lee S (1987) Health hazard evaluation report HETA
85-003-1834: BF Goodrich, Woodburn, Indiana. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 27 pp
(PB88-162672/XAD).
McCain JC & Peck JM Jr (1976) The toxicity of selected chemicals used
in power generating stations to Hawaiian fishes NOAA. Washington, DC,
US Department of Commerce, National Technical Information Service,
23 pp (NTIS No PB262437).
McGlothlin JD (1980) Health hazard evaluation report HETA 80-67-749:
Firestone Fire and Rubber Company, Akron, Ohio. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 18 pp
(PB82-103144).
McGlothlin J & Wilcox T (1984) Health hazard evaluation report HETA
79-109-1538: Kelly Springfield Tire Company, Cumberland, Maryland.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 50 pp (PB85-244424).
Mackerness CW, Leach SA, Thompson MH, & Hill MJ (1989) The inhibition
of bacterially mediated N-nitrosation by vitamin C: relevance to the
inhibition of endogenous N-nitrosation in the achlorhydric stomach.
Carcinogenesis, 10(2): 397-399.
Malaiyandi M, Thomas GH, & Meek ME (1979) Sampling and analysis of
some corrosion inhibiting amines in steam condensates. J Environ Sci
Health, A14: 609-627.
Maller RK & Heidelberger C (1957) Studies on OPSPA. IV. Metabolism of
OPSPA in the rat and human. Cancer Res, 17: 296-301.
Mannsville Chemical Products (1981) Chemical products synopsis:
Morpholine. Cortland, New York, Mannsville Chemical Products
Corporation, 2 pp.
Mastromatteo E (1965) Recent occupational health experiences in
Ontario. J Occup Med, 702: 502-511.
Mazure N (1993) Etude de la biodégradation de la morpholine par une
culture pure, Mycobacterium aurum, et des cultures mixtes
Pseudomonas. Compiègne, France, University of Technology (Thesis).
Meiners AF, Gadberry H, Carson BL, Owens HP, & Lapp TW (1980) Volatile
corrosion inhibitors and boiler water additives: potential for
nitrosamine formation. Washington, DC, US Environmental Protection
Agency, Office of Pesticides and Toxic Substances, 99 pp (Report
submitted by the Midwest Research Institute, Kansas City, Missouri)
(NTIS/PB80-221105).
Mercer EI (1991) Morpholine antifungals and their mode of action.
Biochem Soc Trans, 19: 788-793.
Migukina NV (1973) [Evaluation of the danger [toxicity] of morpholine
by chronic exposure.] Toksikol Nov Prom Chim Veshchestv, 13: 92-100
(in Russian).
Millington LA, Goulding KH, & Adams N (1988) The influence of growth
medium composition on the toxicity of chemicals to algae. Water Res,
22: 1593-1597.
Mills EJ & Stack VT (1953) Biological oxidation of synthetic organic
chemicals. In: Proceedings of the 8th Industrial Waste Conference,
Purdue University, West Lafayette, Indiana, 4-6 May 1953. Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp 492-517.
Mills EJ & Stack VT (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage Ind Wastes,
27: 1061-1064.
Mirvish SS (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol Appl Pharmacol,
31: 325-351.
Mirvish SS, Wallcave L, Eagen M, & Shubik P (1972) Ascorbate-nitrite
reaction: possible means of blocking the formation of carcinogenic
N-nitroso compounds. Science, 177: 65-68.
Mirvish SS, Cardesa A, Wallcave L, & Shubik P (1975) Induction of
mouse lung adenomas by amines or ureas plus nitrite and by N-nitroso
compounds: effect of ascorbate, gallic acid, thiocyanate, and
caffeine. J Natl Cancer Inst, 55: 633-636.
Mirvish SS, Pelfrene AF, Garcia H, & Shubik P (1976) Effect of sodium
ascorbate on tumor induction in rats treated with morpholine and
sodium nitrate, and with nitrosomorpholine. Cancer Lett, 2: 101-108.
Mirvish SS, Ramm MD, Sams JP, & Babcock DM (1988) Nitrosamine
formation from amines applied to the skin of mice after and before
exposure to nitrogen dioxide. Cancer Res, 48: 1095-1099.
Mjos K (1978) Cyclic amines. In: Kirk-Othmer encyclopedia of chemical
technology, 3rd ed. New York, John Wiley and Sons, vol 2, pp 295-308.
Moriyasu M, Endo M, Hashimoto Y, & Koeda T (1984) High-performance
liquid chromatographic determination of organic substances by metal
chelate derivatization. II. Microdetermination of methamphetamine and
amphetamine. Chem Pharm Bull, 32: 600-608.
Newberne PM & Shank RC (1973) Induction of liver and lung tumours in
rats by the simultaneous administration of sodium nitrite and
morpholine. Food Cosmet Toxicol, 11: 819-825.
NIOSH (1977) Manual of analytical methods, 2nd ed. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 3, pp
S150/1-S150/9.
NIOSH (1988) National occupational exposure survey as of 16.12.1988:
Morpholine. Cincinnati, Ohio, National Institute of Occupational
Safety and Health, 1 p.
Norkus EP, Boyle S, Kuenzig WA, & Mergens WJ (1984) Formation of
N-nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen oxide. Carcinogenesis, 5: 549-554.
Norkus EP, Kuenzig WA, Chau J, Mergens WJ, & Conney AH (1986)
Inhibitory effect of alpha-tocopherol on the formation of
nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen dioxide. Carcinogenesis, 7: 357-360.
NRC (1981) Selected aliphatic amines and related compounds: an
assessment of the biological and environmental effects. Washington,
DC, National Research Council, Board on Toxicology and Environmental
Health Hazards (Report prepared for the US Environmental Protection
Agency, Washington) (NTIS/PB83-133066).
OECD (1981a) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Ready biodegradability. 301 E - Modified
OECD screening test. Paris, Organisation for Economic Co-operation and
Development.
OECD (1981b) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Inherent biodegradability. 302 B -
Modified Zahn-Wellens test. Paris, Organisation for Economic
Co-operation and Development.
OECD (1984) Guidelines for testing of chemicals No 202, Part I:
Daphnia sp, acute immobilisation test. Paris, Organisation for
Economic Co-operation and Development.
O'Donnell CM, Edwards C, & Ware J (1988) Nitrosamine formation by
clinical isolates of enteric bacteria. FEMS Microbiol Lett,
51: 193-197.
Ohnishi T (1984) [Morpholine. Studies on mutagenicity of the food
additive morpholine (fatty acid salt).] Nippon Eiseigaku Zasski,
39: 729-745 (in Japanese with English summary).
Ohnishi T, Kubota M, Okada A, & Tonami K (1983) [Residual survey
investigation and removal efficiency by washing with kitchen detergent
of food additive morpholine.] Hokuriku Koshu Eisei Gakkaishi,
10: 63-67 (in Japanese with English summary).
Österdahl B-G & Slorach SA (1983) Volatile N-nitrosamines in snuff and
chewing tobacco on the Swedish market. Food Chem Toxicol, 21: 759-762.
Pensabene JW & Fiddler W (1988) Food additives. Determination of
volatile N-nitrosamines in Frankfurters containing minced fish and
surimi. J Assoc Off Anal Chem, 71: 839-843.
Perez A, Fernandez SI, Garcia-Roche MO, De Las Cagigas A, Castillo A,
Fonseca G, & Herrera M (1990) Mutagenicity of N-nitrosomorpholine
biosynthesized from morpholine in the presence of nitrate and its
inhibition by ascorbic acid. Nahrung, 34: 661-664.
Postlethwait EM & Mustafa MG (1983) Formation of N-nitrosamine in
isolated rat lungs during nitrogen dioxide ventilation.
Carcinogenesis, 4: 777-778.
Reinel D & Clarke C (1992) Comparative efficacy and safety of
amorolfine nail lacquer 5% in onychomycosis, once-weekly versus
twice-weekly. Clin Exp Dermatol, 705: 44-49.
Reinhardt CF & Brittelli MR (1981) Heterocyclic and miscellaneous
nitrogen compounds. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology: Vol 2A. Toxicology. New York, John Wiley and
Sons, pp 2671-2822.
Rekka E, Retsas S, Demopoulos VJ, & Kourounakis PN (1990)
Lipophilicity of some substituted morpholine derivatives synthesized
as potential antinociceptive agents. Arch Pharmacol, 323: 53-56.
Reynolds T (1989) Comparative effects of heterocyclic compounds on
inhibition of lettuce fruit germination. J Exp Bot, 40: 391-404.
Rhodes C & Case DE (1977) Non-metabolite residues of ICI 58,834
(viloxazine). Studies with (14C)morpholine, (14C)ethanolamine and
(14C)glyoxylate. Xenobiotica, 7: 112.
Richardson ML, Webb KS, & Gough TA (1980) The detection of some
N-nitrosamines in the water cycle. Ecotoxicol Environ Saf, 4: 207-212.
Ringenburg V & Fajen JM (1980) Survey for N-nitroso compounds at B.F.
Goodrich, Woodburn, Indiana, December 12, 1979. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 11 pp
(PB88-250477/XAD).
Rounbehler DP & Fine DH (1982) Specific detection of amines and other
nitrogen-containing compounds with a modified TEA analyzer. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 209-219 (IARC Scientific
Publications No. 41).
Rounbehler DP & Fajen JM (1983) N-nitroso compounds in the factory
environment. National Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 228 pp (PB84-145770).
Rounbehler DP, Reisch J, & Fine DH (1980) Nitrosamines in new motor-
cars. Food Cosmet Toxicol, 18: 147-151.
Sander J & Bürkle G (1969) [Induction of malignant tumours in rats by
simultaneous feeding of nitrite and secondary amines.] Z Krebsforsch,
73: 54-66 (in German).
Sander J, Schweinsberg F, & Menz H-P (1968) [Studies on the formation
of carcinogenic nitrosamines in the stomach.] Hoppe-Seyler's Z Physiol
Chem, 349: 1691-1697 (in German).
Savolainen H & Rosenberg C (1983) Morpholine vapour inhalation and
interactions of simultaneous nitrite intake. Biochemical effects on
rat spinal cord axons and skeletal muscle. Arch Toxicol, 53: 143-150.
Schröder E, Rufer C, & Schmiechen R (1982) [Nitrofuran.] In:
[Pharmaceutical Chemistry.] Stuttgart, New York, Georg Thieme Verlag,
pp 858-863 (in German).
Schuster RH, Nabholz F, & Gmünder M (1990) [The inhibition of the
formation of N-nitrosamines: Part 1. The situation and the effect of
alpha-tocopherol.] Kautschuk Gummi Kunstst, 43: 95-106 (in German).
Sen NP & Baddoo PA (1986) Origin of N-nitrosomorpholine contamination
in margarine. J Food Sci, 51: 216-217.
Sen NP & Baddoo PA (1989) An investigation on the possible presence of
morpholine and N-nitrosomorpholine in wax-coated apples. J Food Saf,
9: 183-191.
Sen NP, Seaman S, Clarkson S, Garrod F, & Lalonde P (1984) Volatile
N-nitrosamines in baby bottle rubber nipples and pacifiers. Analysis,
occurrence and migration. In: O'Neill IK, vn Borstel RC, Miller CT,
Long J, & Bartsch H ed. N-nitroso compounds: Occurrence, biological
effects and relevance to human cancer. Lyon, International Agency for
Research on Cancer, pp 51-57 (IARC Scientific Publications No. 57).
Sen NP, Kushwaha SC, Seaman SW, & Clarkson SG (1985) Nitrosamines in
baby bottle nipples and pacifiers: occurrence, migration, and effect
of infant formulas and fruit juices on in vitro formation of
nitrosamines under simulated gastric conditions. J Agric Food Chem,
33: 428-433.
Shank RC & Newberne PM (1976) Dose-response study of the
carcinogenicity of dietary sodium nitrite and morpholine in rats and
hamsters. Food Cosmet Toxicol, 14: 1-8.
Shea TE Jr (1939) The acute and sub-acute toxicity of morpholine. J
Ind Hyg Toxicol, 21: 236-245.
Shenoy NR & Choughuley ASU (1989) Effect of certain plant phenolics on
nitrosamine formation. J Agric Food Chem, 37: 721-725.
Shenoy NR & Choughuley ASU (1992) Inhibitory effect of diet related
sulphydryl compounds on the formation of carcinogenic nitrosamines.
Cancer Lett, 67: 227-232.
Shibata M-A, Kurata Y, Tamano S, Ogiso T, Fukushima S, & Ito N (1987a)
13-week subchronic toxicity study with morpholine oleic acid salt
administered to B6C3F1 mice. J Toxicol Environ Health, 22: 187-194.
Shibata M-A, Kurata Y, Ogiso T, Tamano S, Fukushima S, & Ito N (1987b)
Combined chronic toxicity and carcinogenicity studies of morpholine
oleic acid salt in B6C3F1 mice. Food Chem Toxicol, 25: 569-574.
Simon P & Lemacon C (1987) Determination of aliphatic primary and
secondary amines and polyamines in air by high-performance liquid
chromatography. Anal Chem, 59: 480-484.
Singer GM & Lijinsky W (1976a) Naturally occurring nitrosatable
compounds. I. Secondary amines in foodstuffs. J Agric Food Chem,
24: 550-553.
Singer GM & Lijinsky W (1976b) Naturally occurring nitrosatable
amines. II Secondary amines in tobacco and cigarette smoke condensate.
J Agric Food Chem, 24: 553-555.
Singer SS (1980) Transnitrosation by nitrosamines and nitrosoureas.
In: Walker EA, Griciute L, Castegnaro M, & Börzsönyi M ed. N-nitroso
compounds: Analysis, formation and occurrence. Lyon, International
Agency for Research on Cancer, pp 111-117 (IARC Scientific
Publications No. 31).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, Noyes Publications, pp 626-627.
Smith JH, Bomberger DC Jr, & Haynes DL (1980) Prediction of the
volatilization rates of high-volatility chemicals from natural water
bodies. Environ Sci Technol, 14: 1332-1337.
Smyth HF Jr, Carpenter CP, Weil CS, & Pozzani UC (1954) Range-finding
toxicity data. Arch Ind Hyg Occup Med, 10: 61-68.
Sohn OS, Fiala E, Conaway CC, & Weisburger JH (1982a) Separation of
morpholine and some of its metabolites by high-performance liquid
chromatography. J Chromatogr, 242: 374-380.
Sohn OS, Fiala ES, Conaway CC, & Weisburger JH (1982b) Metabolism and
disposition of morpholine in the rat, hamster and guinea pig. Toxicol
Appl Pharmacol, 64: 486-491.
Sollenberg J & Hansen L (1987) Isotachophoretic determination of
amines from workroom air. J Chromatogr, 390: 133-140.
Spiegelhalder B (1983) [Nitrosamines and rubber.] In: Preussmann R ed.
[The nitrosamine problem.] Weinheim, VCH Verlagsgesellschaft,
pp 235-244 (in German).
Spiegelhalder B & Preussmann R (1982) Nitrosamines and rubber. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 231-2439 (IARC Scientific
Publications No. 41).
Spiegelhalder B & Preussmann R (1984) Contamination of toiletries and
cosmetic products with volatile and nonvolatile N-nitroso carcinogens.
J Cancer Res Clin Oncol, 108: 160-163.
Spies RB, Andresen BD, & Rice DW Jr (1987) Benzthiazoles in estuarine
sediments as indicators of street runoff. Nature (Lond), 327: 697-699.
SRI (1990) Directory of chemical producers, Western Europe:
Morpholine. Menlo Park, California, SRI International, vol 2, p 1563.
Stewart BW & Farber E (1973) Strand breakage in rat liver DNA and its
repair following administration of cyclic nitrosamines. Cancer Res,
33: 3209-3215.
Strotmann UJ, Weberruss U, & Bias WR (1993) Degradation of morpholine
in several biodegradation tests and in wastewater treatment plants.
Chemosphere, 75: 1729-1742.
Subrahmanyam PVR, Khadakkar SN, Chakrabarti T, & Sundaresan BB (1983)
Wastewater-treatment of a phthalate plasticizer, ethanolamine and
morpholine manufacturing plant: a case study. Proc Ind Waste Conf,
37: 13-20.
Suzuki K & Mitsuoka T (1984) N-nitrosamine formation by intestinal
bacteria. In: O'Neill IK, vn Borstel RC, Miller CT, Long J, & Bartsch
H ed. N-nitroso compounds: Occurrence, biological effects and
relevance to human cancer. Lyon, International Agency for Research on
Cancer, pp 275-281 (IARC Scientific Publications No. 57).
Swain A, Waterhouse KV, Venables WA, Callely AG, & Lowe SE (1991)
Biochemical studies of morpholine catabolism by an environmental
mycobacterium. Appl Microbiol Biotechnol, 29: 110-114.
Swope HG & Kenna M (1950) Effect of organic compounds on biochemical
oxygen demand. Sew Ind Wastes Eng, 21: 467-468.
Taft RM & Stroman RE (1979) Health hazard evaluation report HETA
78-131-586: Goodyear Tire and Rubber Company, Niagara Falls, New York.
Cincinnati Ohio, National Institute of Occupational Safety and Health,
Hazard Evaluations and Technical Assistance Branch, 13 pp
(NTIS/PB80-196736).
Takezawa J & Lam HF (1978) Toxic effect of morpholine on rat lungs.
Fed Proc, 37: 247 (Abstract).
Tanaka M, Okada Z, Mihashi K, & Seiko Y (1968) Industrial waste
treatment by activated sludge. XV. Treatment of waste from an organic
vulcanization accelerator producing plant. Kogyo Gijutsuin Hakko
Kenkyusho Kenkyu Hokoku, 33: 19-29.
Tanaka A, Tokieda T, Nambaru S, Osawa M, & Yamaha T (1978) Excretion
and distribution of morpholine salts in rats. J Food Hyg Soc,
19: 329-334.
Tannenbaum SR, Archer MC, Wishnok JS, & Bishop WW (1978) Nitrosamine
formation in human saliva. J Natl Cancer Inst, 60(2): 251-253.
Tatsumi K, Kitamura S, Yoshimura Y, Tanaka S, Hashimoto K, & Igarashi
T (1975) The metabolism of phenyl o-(2-N-morpholinoethoxy)-phenyl
ether hydrochloride in the rabbit and rat. Xenobiotica, 5(6): 377-388.
Taylor R & Son PN (1982) Rubber chemicals. In: Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 20, pp 337-364.
Texaco (1979a) Mutagenicity evaluation of morpholine in the Ames
salmonella/microsome plate test. Bellaire, Texas, Texaco
Petrochemicals, 8 pp.
Texaco (1979b) Mutagenicity evaluation of morpholine in the mouse
lymphoma forward mutation assay. Bellaire, Texas, Texaco
Petrochemicals, 15 pp.
Texaco (1986) Texaco product brochure - Morpholine. Austin, Texas,
Texaco Chemical Company, Research and Technical Services, 29 pp.
Tölgyessy P, Kollár M, Vanco D, & Piatrik M (1986) Bio-degradability
of morpholine. J Radioanal Nucl Chem Lett, 107: 291-295.
Tombropoulos EG (1979) Micromethod for the gas chromatographic
determination of morpholine in biological tissues and fluids. J
Chromatogr, 164: 95-99.
Tombropoulos EG, Koo JO, Gibson W, & Hook GER (1983) Induction by
morpholine of lysosomal alpha-mannosidase and acid phosphatase in
rabbit alveolar macrophages in vivo and in vitro. Toxicol Appl
Pharmacol, 70: 1-6.
TRGS (1989) [Technical regulations for dangerous chemicals:
Nitrosamine.] Cologne, Carl Heymanns Verlag KG, 13 pp (TRGS 552)
(in German).
Tricker AR & Preussmann R (1991) Occurrence of and exposure to
N-nitroso compounds in tobacco. In: O'Neill IK, Chen J, & Bartsch H
ed. Relevance to human cancer of N-nitroso compounds, tobacco smoke
and mycotoxins. Lyon, International Agency for Research on Cancer,
pp 493-495 (IARC Scientific Publications No. 105).
UBA (1990) Calculation of log POW with the Program CLOGP (data
sheet). Berlin, Environment Office, 2 pp.
US FDA (1984a) Code of federal regulations (April 1, 1984) - Title 21,
Part 178.3300: Corrosion inhibitors used for steel or tinplate.
Washington, DC, US Food and Drug Administration, p 312.
US FDA (1984b) Code of federal regulations (April 1, 1984) - Title 21,
Part 176.210: Defoaming agents used in the manufacture of paper and
paperboard. Washington, DC, US Food and Drug Administration,
pp 192-193.
US FDA (1984c) Code of federal regulations (April 1, 1984) - Title 21,
Part 175.105: Substances for use only as components of adhesives.
Washington, DC, US Food and Drug Administration, pp 124-137.
US FDA (1984d) Code of federal regulations - Title 21, Part 178.310:
Animal glue. Washington, DC, US Food and Drug Administration, p 308.
US FDA (1984e) Code of federal regulations - Title 21, Part 173.310:
Boiler water additives. Washington, DC, US Food and Drug
Administration, pp 115-118.
US FDA (1986) Cosmetic product formulation data: Ingredients used in
each product category. Washington, DC, US Food and Drug
Administration.
US FDA (1988) Code of federal regulations - Title 21, Part 172.235:
Morpholine. Washington, DC, US Food and Drug Administration, pp 33-35.
Van Stee EW, Wynns PC, & Moorman MP (1981) Distribution and
disposition of morpholine in the rabbit. Toxicology, 20: 53-60.
Wang X & Suskind RR (1988) Comparative studies of the sensitization
potential of morpholine, 2-mercaptobenzothiazole and 2 of their
derivatives in guinea pigs. Contact Dermatitis, 19: 11-15.
Wang X & Tabor MW (1988) Studies of the reactivity of morpholine,
2-mercaptobenzothiazole and 2 of their derivatives with selected amino
acids. Contact Dermatitis, 19: 16-21.
Wang H & Wu Y (1991) Inhibitory effect of Chinese tea on N-nitrosation
in vitro and in vivo. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 546-548 (IARC Scientific Publications No. 105).
Wellens H (1982) [Comparison of the sensitivity of Brachydanio rerio
und Leuciscus idus by testing the fish toxicity of chemicals and
wastewaters.] Z Wasser Abwasser Forsch, 2: 49-52 (in German).
Westin JB, Castegnaro MJ-J, & Friesen MD (1987) N-nitrosamines and
nitrosatable amines, potential precursors of N-nitramines, in
children's pacifiers and baby-bottle nipples. Environ Res,
43: 126-134.
Wishnok JS & Tannenbaum SE (1976) Formation of cyanamides from
secondary amines in human saliva. Science, 191: 1179-1180.
Wishnok JS & Tannenbaum SR (1977) An unknown salivary morpholine
metabolite. Anal Chem, 49: 715a-716a, 718a.
Yurchenko VA, Ilnitskii AP, Ermilow VB, Mistakopulo GM, & Nechipai AM
(1990) [Investigation of mutagenic action of natural zeolite and
chrysotile-asbest dusts.] Eksp Onkol, 12: 24-26 (in Russian with
English summary).
Zaeva GN, Timofievskaya LA, Bazarova LA, & Migukina NV (1968)
[Comparative toxicity of a group of cyclic imino-compounds.] Toksikol
Nov Prom Chim Veshchestv, 10: 25-35 (in Russian).
Ziebarth D (1973) N-nitrosation of secondary amines and particularly
of drugs, in buffer solutions and human gastric juice. In: Bogovski P
& Walker EA ed. N-nitroso compounds in the environment. Lyon,
International Agency for Research on Cancer, pp 137-141 (IARC
Scientific Publications No. 9).
Ziebarth D. (1974) [Investigations into the nitrosation of secondary
amines in buffer solutions and in human gastric juice.] Arch
Geschwulstforsch, 43: 42-41 (in German).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Propriétés physiques et chimiques
La morpholine (1-oxa-4-azacyclohexane) est un liquide incolore,
huileux, hygroscopique et volatil qui possède l'odeur de poisson
caractéristique des amines. Elle est entièrement miscible à l'eau,
ainsi qu'à de nombreux solvants organiques, mais sa solubilité est
limitée dans les solutions aqueuses alcalines. C'est une base, dont
le pKa de l'acide conjugué est de 8,33. Il s'ensuit que son
coefficient de partage entre l'octanol et l'eau dépend du pH
(log Pow = -2,55 à pH 7 et -0.84 à pH 10 et à 35°C). La tension de
vapeur des solutions aqueuses de morpholine est très proche de celle
de l'eau.
La morpholine peut entrer en réaction de diverses manières.
Chimiquement, elle se comporte comme une amine secondaire. Dans les
conditions environnementales et physiologiques, la
N-nitrosomorpholine (NMOR), dont le pouvoir cancérogène chez
l'animal est prouvé, se forme par réaction entre des solutions de
nitrite ou des oxydes d'azote gazeux et des solutions diluées de
morpholine. La concentration en oxyde d'azote (NO) peut être
importante pour la nitrosation. Les conditions de nitrosation, en
particulier la valeur du pH, jouent un rôle important.
2. Méthodes d'analyse
On peut doser la morpholine par chromatographie en phase gazeuse
avec des colonnes à remplissage ou des colonnes capillaires, par
chromatographie liquide à haute performance (HPLC) et par
chromatographie ionique. Les détecteurs utilisés sont les détecteurs
à ionisation de flamme, les détecteurs à photométrie de flamme, les
détecteurs sélectifs d'azote, les détecteurs thermiques pour la
chromatographie en phase gazeuse et, pour la chromatographie en phase
liquide à haute performance, les détecteurs à UV et les détecteurs
thermiques. Pour le dosage des traces, il faut passer par un dérivé.
La méthode de choix sur le plan de la sensibilité semble être la
chromatographie en phase gazeuse avec détection thermique après
transformation de la morpholine en NMOR (limite de détection: 2 à
3 µg/kg dans diverses matrices). Dans l'air, de faibles
concentrations de morpholine peuvent être mesurées par chromatographie
en phase gazeuse avec un détecteur sélectif d'azote.
3. Sources d'exposition humaine et environnementale
On estime qu'environ 25 000 tonnes de morpholine sont produites
chaque année, toutefois on ne connaît pas dans le détail la production
de certains pays.
Le principal procédé de production est, semble-t-il, la réaction
du diéthylène-glycol sur l'ammoniaque en présence d'hydrogène et de
catalyseurs.
La morpholine est un produit chimique à tout faire mais on n'en
connaît pas tous les usages. Il joue un rôle important comme
intermédiaire dans l'industrie du caoutchouc, comme inhibiteur de la
corrosion ainsi que pour la synthèse d'éclaircissants optiques,
d'agents pour la protection des récoltes, de colorants et de
médicaments. La morpholine est utilisée comme solvant pour les
dérivés organiques les plus divers, comme par exemple les résines, les
colorants et les cires. On peut également l'utiliser comme
catalyseur. On utilise encore de la morpholine dans certains pays
pour la confection de produits de toilette et de cosmétiques. Dans
d'autres, elle entre dans la composition d'additifs alimentaires, soit
directement, soit indirectement.
Lorsqu'il y a exposition humaine ou environnementale, elle peut
être due soit à des émissions de gaz, soit à des décharges de
solutions aqueuses, à moins qu'elle ne se produise directement lors de
l'utilisation de certains produits contenant de la morpholine, comme
par exemple des produits cosmétiques ou des cires. Le gros des
émissions et des décharges trouve probablement son origine dans la
fabrication et l'utilisation de la morpholine dans l'industrie
chimique (notamment lors de la production et de l'utilisation des
produits destinés à l'industrie du caoutchouc) ainsi que dans
l'application de la morpholine comme agent anti-corrosion. On a
trouvé de la morpholine dans des denrées alimentaires très variées
ainsi que dans le tabac. Il est possible que cette présence
s'explique par la migration, dans la denrée alimentaire, de la
morpholine incorporée à l'enduit qui recouvre les fruits ou
l'emballage, mais dans un certain nombre de cas, on ignore d'où la
morpholine peut provenir.
4. Transport, distribution et transformation dans l'environnement
La morpholine est chimiquement stable dans la biosphère bien
qu'elle puisse être soumise à une nitrosation chimique ou biologique
qui la transforme en NMOR.
La morpholine est intrinsèquement biodégradable. On a vérifié
cette propriété dans des conditions reproduisant celles qui règnent
dans les usines de traitement des boues activées. Cependant, dans des
conditions de non adaptation, la morpholine n'est probablement pas
décomposée en proportion importante. Le temps de rétention moyen sur
les matières solides présentes dans les usines de traitement des boues
activées est d'une importance cruciale et il doit dépasser les 8 jours
pour que l'on puisse obtenir une bonne dégradation de la morpholine.
Les données relatives à la bioaccumulation de la morpholine par
les organismes aquatiques et terrestres sont insuffisantes. D'après
la valeur du coefficient de partage entre le n-octanol et l'eau
(log Pow = -2,55 à pH 7), on peut s'attendre à ce qu'il n'y ait
aucune bioaccumulation.
Comme la morpholine est un produit chimique industriel important
dont les applications sont variées, il faut s'attendre à retrouver ce
composé ou ses dérivés dans un grand nombre d'effluents industriels.
De plus, étant donné qu'on l'utilise comme inhibiteur de la corrosion
dans l'eau des chaudières, on va le retrouver dans les eaux usées de
ces chaudières, et en particulier les eaux usées provenant de
certaines centrales thermiques. Comme la morpholine entre également
dans la composition des additifs du caoutchouc, on va en retrouver
également, en quantité mal définie, dans l'hydrosphère et la
géosphère, par suite de l'usure des pneus et du rejet des pneus usés.
La morpholine peut pénétrer dans l'environnement en se
volatilisant à partir des encaustiques et des cirages dont elle est un
constituant. Elle est rapidement captée par l'humidité. C'est donc
principalement dans l'hydrosphère qu'elle devrait s'accumuler, mais
les données limitées dont on dispose incitent à penser que la
morpholine ne s'accumule pas dans ce compartiment.
La meilleure méthode pour éliminer la morpholine concentrée est
l'incinération, toutefois il peut être nécessaire de veiller aux
émissions d'oxydes d'azote afin de respecter les normes de protection
de l'environnement. En ce qui concerne les effluents aqueux, le
traitement des boues activées est suffisant, à la condition toutefois
que l'usine fasse l'objet d'un contrôle rigoureux (voir ci-dessus).
5. Concentrations dans l'environnement et exposition humaine
On ne dispose d'aucune donnée sur la concentration de la
morpholine dans l'air ambiant ainsi que dans l'air intérieur des
immeubles résidentiels ou encore dans l'eau de boisson. Si l'on
possède quelques données sur sa présence dans les eaux naturelles, on
n'en a en revanche aucune sur sa présence dans le sol.
D'après les données disponibles, c'est les denrées alimentaires
qui constituent la principale source d'exposition à la morpholine de
la population générale, les produits alimentaires pouvant être
contaminés par suite d'un traitement conservateur direct des fruits à
l'aide de cires contenant de la morpholine, lors du traitement à la
vapeur au cours de la préparation et enfin par l'utilisation de
matériaux d'emballage dans la composition desquels entre la
morpholine. Cependant on ne dispose que de données quantitatives
limitées sur la contamination des denrées alimentaires par la
morpholine et la NMOR. Par exemple, dans les produits laitiers pré-
emballés, les valeurs mesurées vont de 5 à 77 µg/kg pour la morpholine
et jusqu'à 3,3 µg/kg pour la NMOR. En général, les mesures effectuées
ont montré que la teneur en morpholine de divers échantillons de
produits alimentaires (poisson, viande, produits d'origine végétale,
boissons) ne dépassait généralement pas 1 mg/kg. Des valeurs plus
élevées (jusqu'à 71 mg/kg) ont été relevées au Japon dans des agrumes.
Une enquête effectuée en Italie n'a pas permis de repérer la présence
de NMOR dans diverses denrées alimentaires au seuil de détection de
0,3 µg/kg. Les données existantes ne permettent pas d'évaluer
l'apport de morpholine et de NMOR par la voie alimentaire.
On a trouvé de la morpholine dans le tabac de cigarette à la
concentration de 0,3 mg/kg ainsi que dans le tabac à priser et à
chiquer à des concentrations allant jusqu'à 4,0 mg/kg. Il est arrivé
que l'on trouve dans du tabac à priser des concentrations de
morpholine allant jusqu'à 0,7 mg/kg. La présence de ce produit était
probablement la conséquence de l'utilisation de cires à base de
morpholine pour le conditionnement de ce tabac.
De la NMOR a été mise en évidence dans certains produits de
toilette et dans des cosmétiques, par exemple des shampoings, du
rimmel ainsi que dans des articles en caoutchouc comme les sucettes
pour bébés et les tétines pour biberon, à des concentrations allant
jusqu'à 3,5 mg/kg.
Il peut y avoir exposition professionnelle à la morpholine dans
diverses industries. On ne possède guère de données sur l'exposition
des travailleurs à la morpholine. Toutes les valeurs signalées sont
inférieures à 3 mg/m3. On a signalé des cas d'exposition
professionnelle à la NMOR dans l'industrie du caoutchouc où des
concentrations allant jusqu'à 250 µg/m3 ont été mesurées.
Les données actuellement disponibles permettent de se faire une
idée du risque d'exposition humaine mais elles ne permettent pas une
estimation précise de l'intensité de l'exposition à la morpholine et
la NMOR de la population générale et des diverses catégories
professionnelles.
6. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après exposition par voie orale, cutanée ou respiratoire, il y a
absorption de la morpholine. Chez le rat, après administration par
voie orale ou intraveineuse, la morpholine se répartit rapidement dans
l'organisme, atteignant sa concentration la plus élevée dans
l'intestin et les muscles.
Chez le lapin, après exposition par voie intraveineuse ou
respiratoire, la morpholine se répartit de préférence dans les reins,
les concentrations étant plus faibles dans les poumons, le foie et le
sang.
La morpholine ne se fixe pas de manière importante aux protéines
plasmatiques. Sa demi-vie dans le plasma est de 115 minutes chez le
rat, de 120 minutes chez le hamster et de 300 minutes chez le cobaye.
La morpholine est principalement excrétée sans modification par
les reins chez un certain nombre d'espèces. Un jour après
l'administration, on a constaté que 70 à 90% de la morpholine se
retrouvaient dans les urines. La neutralisation en augmente la
vitesse d'excrétion. Une faible proportion de la morpholine est
excrétée dans l'air expiré et dans les matières fécales.
Les études effectuées sur des rats, des souris, des hamsters et
des lapins indiquent que la morpholine s'élimine presque complètement
sans métabolisation. Chez le cobaye, il peut y avoir une
N-méthylation suivie d'une N-oxydation, la dose administrée
pouvant être métabolisée jusqu'à hauteur de 20%. En présence de
nitrites, la morpholine peut être transformée en NMOR, tant in vitro
qu' in vivo. On a constaté, en administrant de la morpholine à des
rats avec des nitrites, que celle-ci pouvait être nitrosée dans la
proportion de 0 à 12%, selon la dose.
Le taux de nitrosation peut augmenter par le fait d'une immuno-
stimulation comportant l'activation des macrophages.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Après administration de morpholine par voie orale à des rats et à
des cobayes, on a obtenu pour la DL50 des valeurs respectives de
1-1,9 g/kg de poids corporel et 0,9 g/kg de poids corporel, ce qui
permet d'avoir une idée de la toxicité aiguë du produit. Des rats qui
avaient reçu de la morpholine neutralisée à raison de 1 g/kg de poids
corporel ont survécu. Après administration par voie intrapéritonéale,
on a obtenu une DL50 de 0,4 g/kg de poids corporel chez la souris et
de 0,1-0,4 g/kg de poids corporel chez le rat. Après exposition par
la voie respiratoire, la DL50 était d'environ 8 g/m3 chez le rat
et de 5-7 g/m3 chez la souris. La DL50 de la morpholine
concentrée par voie percutanée était de 0,5 ml/kg chez le lapin. La
toxicité aiguë de la morpholine se caractérise par des hémorragies
gastrointestinales et des diarrhées lorsqu'elle est administrée par
voie orale et par une irritation et des hémorragies nasales, buccales,
oculaires et pulmonaires lorsqu'elle est administrée par voie
respiratoire. Lors d'une étude de 30 jours au cours de laquelle des
rats ont reçu de la morpholine par gavage à des doses de 0,16 à
0,8 g/kg de poids corporel, on a observé des effets toxiques graves et
une mortalité à toutes les doses. Les mêmes observations ont été
effectuées sur des cobayes à des doses comprises entre 0,09 et
0,045 g/kg de poids corporel.
Chez des rats soumis pendant une brève période à des inhalations
de morpholine (7,2 g/m3, 4 heures par jour pendant 4 jours ou bien
1,63 g/m3, 4 heures par jour, 5 jours par semaine pendant 30 jours),
on a observé une altération de la fonction pulmonaire. Le taux de
mortalité chez d'autres rats allait de 0 à 100% selon le niveau
d'exposition (0,36-18,1 g/m3, 6 heures par jour pendant 9 jours).
Par la voie respiratoire, on a constaté que la toxicité dépendait de
la dose avec une irritation locale (yeux, bouche, nez et poumons) et
des hémorragies de gravité variables aux niveaux d'exposition les plus
élevés. Dans une étude, on a observé un hyperfonctionnement de la
glande thyroïde et dans une autre, une nécrose du foie et des tubules
rénaux après exposition par la voie respiratoire.
Une étude de 90 jours a montré que la morpholine administrée par
voie orale (0.2-0,7 g quotidiennement par kg de poids corporel)
pouvait réduire la gain de poids et entraîner une insuffisance rénale
chez la souris. Après 672 jours d'administration de morpholine par
voie orale (0,28-0,5 g/kg de poids corporel quotidiennement), on a
observé chez la souris une hyperplasie au niveau de l'épithélium de la
portion cardiaque de l'estomac.
Selon une étude d'inhalation de 13 semaines, la morpholine
(administrée à la dose de 0,09-0,9 g/m3 6 heures par jour, 5 jours
par semaine) provoque des lésions liées à la dose au niveau de la
muqueuse nasale ainsi qu'une pneumopathie aux doses les plus élevées
(0,36 et 0,9 mg/m3). A la dose de 0,09 g/m3 on n'a observé aucune
modification d'un certain nombre de paramètres qui puisse être
imputable à ce traitement; cette concentration peut être considérée
comme la dose sans effets nocifs observables dans les conditions d'une
exposition subchronique par la voie respiratoire.
Sous sa forme concentrée et non neutralisée, la morpholine est
extrêmement irritante pour les yeux et la peau, probablement en raison
de sa basicité. En la diluant et en la ramenant à un pH neutre, on
peut sensiblement en réduire la toxicité topique. Selon une variante
de la méthode de Buehler, la morpholine à 2% n'a pas produit de
sensibilisation chez le cobaye.
La morpholine ne produit pas de mutation chez des bactéries ou
des levures en présence ou en l'absence d'activation métabolique
(à l'exception d'un essai effectué à très forte concentration). Le
passage sur hôte a également donné des résultats négatifs.
On n'a pas non plus observé de réparation de l'ADN sous
l'influence de la morpholine dans des cultures primaires d'hépatocytes
de rats et ce composé n'a pas augmenté de façon sensible les échanges
entre chromatides soeurs dans des cellules ovariennes de hamsters
chinois. Les résultats d'une épreuve sur cellules lymphomateuses de
souris L5178Y ont conduit à considérer la morpholine comme faiblement
mutagène. Ce composé a entraîné une augmentation des foyers de type
III lors d'une épreuve de transformation cellulaire maligne effectuée
sur des cellules BALB/3T3, phénomène qui n'a pas été constaté avec la
morpholine neutralisée.
On n'a pas non plus constaté de mutation ponctuelle ni
d'aberration chromosomique chez des embryons de hamsters exposés
in utero à de la morpholine.
On n'a pas constaté d'augmentation dans l'incidence des tumeurs
chez des rats qui avaient été exposés par voie respiratoire pendant
104 semaines à des concentrations de morpholine allant jusqu'à
0,5 g/m3, ni chez des souris qui avaient reçu pendant 96 semaines
dans leur eau de boisson de l'oléate de morpholine à 1%. Une étude à
long terme sur un groupe de 104 rats qui recevaient dans leur
alimentation 1000 mg de morpholine par kg de nourriture, a permis de
mettre en évidence trois carcinomes hépatocellulaires, deux
angiosarcomes pulmonaires ainsi qu'un troisième angiosarcome de
localisation non précisée et enfin deux gliomes malins, alors
qu'aucune tumeur n'a été observée dans le groupe témoin comportant
156 animaux. Chez des hamsters exposés dans les mêmes conditions,
aucune tumeur n'a été observée.
Administrée en même temps qu'un nitrite, la morpholine donne un
résultat positif à l'épreuve de passage sur hôte, qui s'explique
probablement par la formation de NMOR. De la morpholine administrée
en même temps qu'un nitrite par mélange à la nourriture, a provoqué
chez des rats des tumeurs hépatiques et pulmonaires et chez des
hamsters des tumeurs hépatiques qui s'expliquent probablement par la
formation endogène de NMOR. La NMOR est mutagène pour les bactéries
et les levures; on a également observé avec ce composé des résultats
faiblement positifs dans l'épreuve d'échange de chromatides soeurs sur
des cellules CHO et lors de la recherche de mutations dans des
cultures de cellules lymphomateuses de souris L5178Y. La NMOR est
cancérogène pour la souris, le rat, le hamster et divers poissons;
elle provoque chez la souris des tumeurs hépatiques et pulmonaires,
chez le rats des tumeurs du foie, du rein et des vaisseaux sanguins,
chez le hamster des tumeurs des voies digestives et respiratoires
supérieures et enfin chez les poissons, des tumeurs hépatiques.
8. Effets sur l'homme
On ne dispose d'aucun rapport faisant état de cas d'intoxication
aiguë ou décrivant les effets d'une exposition à court ou à long terme
à la morpholine dans la population générale.
Un phénomène connu sous le nom de glaucopsie (vision bleutée)
ainsi que dans certains cas une irritation de la peau et des voies
respiratoires, ont été décrits dans des rapports d'exposition
professionnelle à la morpholine; toutefois, ces rapports ne précisent
pas la concentration de la morpholine dans l'air. On a indiqué que
chez des ouvriers exposés pendant 3 à 10 ans à de la morpholine à des
concentrations de 0,54-0,93 mg/m3, le nombre d'aberrations
chromosomiques dans les lymphocytes du sang périphérique ne présentait
pas de différence notable par rapport aux témoins.
La morpholine concentrée est fortement irritante pour la peau;
en solution (à 1/40) elle se révèle encore légèrement irritante.
On n'a pas étudié la cancérogénicité potentielle de la morpholine
chez les populations humaines exposées.
9. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Parmi les organismes aquatiques étudiés, certaines cyanobactéries
(Microcystis) et certaines algues bleues unicellulaires
(Scenedesmus) se révèlent être les taxa les plus sensibles puisque
le seuil de toxicité (critère: inhibition de la croissance des
populations) se situe à 1,7 mg/litre pour Microcystis et à
4,1 mg/litre pour Scenedesmus (durée de l'exposition: 8 jours).
Des bactéries aérobies telles que Pseudomonas se sont révélées
beaucoup plus résistantes; le seuil de toxicité à 16 heures ainsi que
la concentration sans effets observables sur la croissance des
populations atteindraient respectivement 310 et 8700 mg/litre.
Cependant une concentration de 1000 mg/litre a inhibé la respiration
et l'activité de la déshydrogénase (dans une proportion allant jusqu'à
20%) chez les bactéries de boues activées provenant d'usines de
traitement des effluents industriels.
Chez les protozoaires aquatiques étudiés jusqu'ici, des spécimens
des genres Entosiphon et Chilomonas (avec des valeursseuil
respectivement égales à 12 et 18 mg/litre pour ce qui est de
l'inhibition de la croissance des populations) se sont révélés être
les plus sensibles. Les valeurs de la CE50 à 24 heures
(E=immobilisation) pour la daphnie se situaient dans les limites de
100 à 120 mg/litre. Chez les poissons, les valeurs de la CL50 à
48 et 96 heures se sont révélées > 180 mg/litre lors d'épreuves
effectuées en eau douce, en eau saumâtre ou en eau de mer, la truite
arc-en-ciel étant l'espèce la plus sensible.
On ne dispose d'aucune donnée relative aux effets à long terme de
la morpholine sur les invertébrés et les vertébrés aquatiques.
L'absence d'information est également presque totale à propos de la
toxicité de ce composé pour les organismes terricoles, puisque les
seules données dont on dispose se limitent à la valeur de la CE à
3 jours, égale à 400 mg/litre, en ce qui concerne l'inhibition de la
germination des laitues.
10. Evaluation des risques pour la santé humaine et effets sur
l'environnement
10.1 Evaluation des effets sur la santé humaine
Les cas d'exposition de la population générale à la morpholine
résultent principalement de la consommation de denrées alimentaires
contaminées. La contamination du tabac et des produits du tabac, des
articles de toilette et des cosmétiques, de même que des articles en
caoutchouc, peuvent contribuer à l'exposition globale. Il peut y
avoir exposition professionnelle à la morpholine dans de nombreuses
industries; ce composé peut être absorbé soit par inhalation, soit
par la voie percutanée. On ne dispose pas de données suffisantes pour
déterminer le degré d'exposition de la population générale.
Les données relatives à l'exposition professionnelle sont
également limitées.
La morpholine n'est pas extrêmement toxique dans les conditions
d'une exposition aiguë. Après administration par voie orale, la
DL50 était de 1-1,9 g/kg de poids corporel chez le rat et de
0,9 g/kg de poids corporel chez le cobaye. Par ailleurs, on a trouvé,
pour la CL50, des valeurs de 7,8 mg/m3 chez le rat et de 4,9 à
6,9 g/m3 chez la souris.
Dans les conditions d'une exposition à court ou à long terme par
la voie respiratoire, c'est l'irritation des yeux et des voies
respiratoires qui constitue l'effet critique. Lors d'une étude de
13 semaines au cours de laquelle des rats ont été exposés 6 heures par
jour et 5 jours par semaine à de la morpholine, on a pu fixer à
90 mg/m3 la concentration sans effets nocifs observables. Lors
d'une autre étude d'inhalation, à long terme cette fois
(104 semaines), on a observé une augmentation de l'incidence des cas
d'inflammation de la cornée ou d'inflammation et de nécrose des fosses
nasales, à la dose de 540 mg/m3 chez le rat. L'irritation des yeux
et du nez présentait également une incidence accrue aux doses de 36 et
180 mg/m3.
Une forte exposition à la morpholine entraîne de graves lésions
hépatiques et rénales chez le rat et le cobaye. Après administration
de morpholine à des rats, par mélange à leur nourriture (0,5 g/kg de
poids corporel) tous les jours pendant 56 jours, on a observé une
dégénérescence graisseuse du foie. Des souris qui avaient reçu tous
les jours, pendant 13 semaines, de l'oléate de morpholine mêlé à leur
eau de boisson à la dose d'environ 0,7 g/kg de poids corporel, ont
présenté une dégénérescence albuminoïde au niveau des tubules
proximaux du rein (désignée également par l'expression "tuméfaction
trouble" par certains auteurs). Chez des souris femelles qui avaient
reçu pendant 672 jours de la morpholine dans leur alimentation, on a
observé aux doses comprises entre 0,05 et 0,4 g de morpholine
administrée sous forme d'oléate, une réduction du gain de poids.
Aux concentrations que l'on observe actuellement dans les cas
d'exposition professionnelle ou environnementale, il ne semble pas que
la morpholine risque véritablement d'entraîner des effets toxiques
généraux. Cependant lors d'une exposition accidentelle ou
professionnelle non contrôlée à de fortes concentrations de morpholine
présentes dans l'air, il peut y avoir des effets locaux qui prennent
la forme d'une irritation de la muqueuse oculaire et des voies
respiratoires; en outre, un contact avec de la morpholine liquide
(même diluée) peut entraîner une irritation cutanée.
Il ne semble pas que la morpholine soit mutagène ou cancérogène
chez l'animal. Toutefois elle peut facilement être nitrosée pour
donner naissance à de la NMOR qui s'est révélée, elle, mutagène et
cancérogène chez plusieurs espèces d'animaux de laboratoire.
L'administration de morpholine, puis d'un nitrite, mêlés à la
nourriture d'animaux de laboratoire a provoqué un accroissement de
l'incidence des tumeurs, pour la plupart des carcinomes
hépatocellulaires et des sarcomes du foie et du poumon. Il est donc
prudent de considérer l'exposition à la morpholine comme un facteur
supplémentaire de risque cancérogène chez les populations exposées.
10.2 Evaluation des effets sur l'environnement
Comme on sait très peu de choses sur l'exposition
environnementale et que les données relatives à l'exposition à long
terme dans l'hydrosphère ou à court et à long terme dans le milieu
terrestre font défaut, il est impossible, pour l'instant, de procéder
valablement à une appréciation du risque. On peut toutefois tirer un
certain nombre de conclusions sur la base des propriétés observées de
la morpholine, des données écotoxicologiques disponibles et des
quelques renseignements dont on dispose sur les concentrations dans
l'environnement.
La forte solubilité dans l'eau de la morpholine et sa faible
volatilité (dans les conditions du milieu) font que l'hydrosphère en
constitue le principal milieu récepteur.
La morpholine est intrinsèquement biodégradable et, bien que
cette biodégradation soit lente, rien n'indique qu'elle s'accumule
dans l'hydrosphère. En outre, sa bioaccumulation est également peu
probable.
On ne possède que relativement peu de données sur la toxicité de
la morpholine pour les organismes vagiles. Toutefois, il paraît
improbable qu'au niveau actuel des émissions de morpholine, des
dommages importants puissent être causés à l'environnement dans son
ensemble. Les effets locaux, dus par exemple aux émissions
industrielles ou à la libération de morpholine dans l'environnement
par suite de l'usure des pneumatiques, restent à évaluer.
Il peut y avoir contamination de certains produits alimentaires
comme le poisson, par de la morpholine, contamination qui viendrait de
l'environnement, mais cela n'est pas certain.
La transformation de la morpholine en NMOR est la principale
cause d'inquiétude, en particulier en ce qui concerne les populations
de vertébrés. On a signalé la présence de NMOR dans des eaux
résiduaires industrielles et dans le sol aux alentours d'une usine.
On peut s'inquiéter de la présence de morpholine dans de l'eau
destinée à être traitée pour la rendre potable.
11. Conclusions et recommandations
La morpholine ne présente aucun risque toxique pour l'homme au
niveau habituel d'exposition mais il faut prendre garde à sa
transformation en NMOR qui est cancérogène.
Rien n'indique qu'aux nivaux actuels d'exposition, la morpholine
constitue un risque important pour les biotes présents dans
l'environnement.
11.1 Recommandations en vue de la protection de la santé humaine
a) Il faut éviter dans la mesure du possible toute exposition
humaine à la morpholine.
b) Il faut éviter la contamination des denrées alimentaires par leur
emballage ou lors de leur transformation.
c) La morpholine ne doit pas entrer dans la composition d'articles
en caoutchouc destinés en entrer en contact direct avec l'homme.
d) La morpholine ne doit pas entrer dans la composition de
préparations destinées à la toilette ou à un usage cosmétique.
e) Les effluents industriels doivent être traités avec soin afin
d'éviter que la morpholine ne pénètre dans l'eau destinée à la
boisson.
f) Compte tenu de la possibilité de formation de NMOR cancérogène,
les limites d'exposition professionnelle actuelles devront être
réexaminées.
11.2 Recommandations en vue de la protection de l'environnement
Il faut éviter les déversements et les décharges massives dans
les usines de traitement des effluents.
11.3 Recommandations en vue de recherches ultérieures
Des travaux doivent être entrepris dans les domaines suivants:
a) toxicité pour la fonction de reproduction des mammifères;
b) toxicité à long terme pour les mammifères;
c) effets sur les mammifères d'une exposition à de faibles
concentrations de morpholine en présence ou non de nitrites et de
nitrates;
d) transnitrosation par la NMOR in vivo et in vitro;
e) biodégradation en anaérobiose, en particulier dans des conditions
entraînant la réduction des nitrates;
f) catalyse microbienne de la N-nitrosation en situation réelle;
g) concentrations de la morpholine dans les eaux souterraines, le
sol et les rivières dont l'eau est utilisée pour la boisson;
h) concentration de morpholine aux alentours d'usines qui produisent
ou transforment cette substance;
i) métabolisme et toxicocinétique chez l'homme en vue de la mise au
point de méthodes pour la surveillance biologique de la
morpholine;
j) surveillance de la concentration de morpholine et de NMOR dans
les denrées alimentaires, dans l'eau de boisson et l'air
intérieur aux habitations;
k) collecte et diffusion de données sur l'exposition
professionnelle.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Propiedades físicas y químicas
La morfolina (1-oxa-4-azaciclohexano) es un líquido incoloro,
oleoso, higroscópico y volátil que desprende un característico olor a
amina ("a pescado"). Es totalmente miscible en agua, así como en
numerosos disolventes orgánicos, y parcialmente soluble en soluciones
acuosas alcalinas. Se trata de una base, y el pKa del ácido conjugado
es de 8,33. En consecuencia, el coeficiente de reparto octanol/agua
depende del pH (log Pow -2,55 a pH 7, y - 0,84 a pH 10; 35°C). La
presión de vapor de las soluciones acuosas de morfolina es casi como
la del agua.
La morfolina puede sufrir diversas reacciones. Se comporta
químicamente como una amina secundaria. En condiciones ambientales y
fisiológicas, como resultado de la reacción de las soluciones de
nitrito o de óxidos de nitrógeno gaseosos con las soluciones diluidas
de morfolina, se forma N-nitrosomorfolina (NMOR), conocido
carcinógeno para los animales. Los niveles de óxido de nitrógeno (NO)
pueden ser importantes en la nitrosación. Las condiciones de
nitrosación, en particular el pH, tienen una considerable influencia.
2. Métodos analíticos
La morfolina se puede determinar mediante cromatografía de gases
(GC) en columnas empacadas o capilares, cromatografía líquida de alta
resolución (HPLC) y cromatografía iónica. Entre los detectores
utilizados cabe citar el detector de ionización por conductor, el
detector de fotometría de llama, el detector selectivo de nitrógeno
(NSD), y la espectrometría de masas y el analizador de energía térmica
(TEA) para la GC, y el detector de UV y el TEA para la HPLC. Para
determinar cantidades ínfimas hay que recurrir a la derivatización.
El método de elección en lo que respecta a sensibilidad es al parecer
la GC combinada con el TEA, previa transformación por derivatización
en NMOR (límite de detección de 2-3 µg/kg en diversas matrices). Las
bajas concentraciones de morfolina en el aire se pueden determinar
mediante GC y NSD.
3. Fuentes de exposición humana y ambiental
Se estima que cada año se producen industrialmente en todo el
mundo unas 25 000 toneladas de morfolina, pero no se conoce con
detalle la producción de algunos países.
El principal proceso de producción utilizado para su obtención es
al parecer la reacción de dietilenglicol con amoniáco en presencia de
hidrógeno y de catalizadores.
La morfolina es una sustancia química que puede utilizarse con
muy diversos fines, pero no se conocen todos sus posibles usos. Es
importante como producto intermedio en la industria del caucho, como
inhibidor de la corrosión, y en la síntesis de abrillantadores
ópticos, protectores de cultivos, colorantes y medicamentos. La
morfolina se utiliza como disolvente de una amplia variedad de
productos orgánicos, entre ellos resinas, colorantes y ceras. Se
puede utilizar como catalizador. La morfolina se usa aún en algunos
países para elaborar productos cosméticos y de tocador. En algunos
países se usa también en varias aplicaciones relacionadas directa o
indirectamente con los aditivos alimentarios.
La exposición humana y ambiental se debe a emisiones tanto
gaseosas como acuosas, y es también el resultado directo de alguno de
sus usos, por ejemplo como componente de productos cosméticos y de
ceras. Las emisiones más importantes son resultado probablemente de
su fabricación y de su uso en la industria química (sobre todo en la
producción y el uso de productos químicos derivados del caucho) y como
agente anticorrosión. Se ha detectado morfolina en muchos tipos de
alimento y de tabaco. En estos casos el origen del producto podría
ser la parafina empleada para proteger la fruta o en determinados
envases, pero a veces no se puede establecer su procedencia.
4. Transporte, distribución y transformación en el medio ambiente
La morfolina es químicamente estable en la biosfera, aunque sufre
nitrosación química y biológica, transformándose así en NMOR.
La morfolina es por naturaleza biodegradable. Así es en las
condiciones reinantes en las plantas de fangos activados que funcionan
correctamente. No obstante, en condiciones no idóneas probablemente
no se produce una degradación importante. El tiempo medio de
retención del sólido en las plantas de fangos activados tiene una
importancia crucial y debe ser superior a ocho días para que se
produzca una degradación fiable de la morfolina.
No se dispone de datos suficientes sobre la bioacumulación de
morfolina en organismos acuáticos y terrestres. Según el coeficiente
de reparto n-octanol/agua del producto (log Pow = -2,55 a pH 7),
no debería producirse bioacumulación.
Como es un importante producto químico industrial con una amplia
gama de aplicaciones, es previsible la presencia de morfolina o de sus
derivados en numerosos efluentes industriales. Usada como inhibidor
de la corrosión en el agua de calderas, aparece en los efluentes de
éstas, incluidos los de las centrales eléctricas en que se utiliza
morfolina. Su uso en la fabricación de aditivos del caucho da lugar a
la liberación de una cantidad indeterminada de morfolina a la
hidrosfera y la geosfera como consecuencia del desgaste de los
neumáticos y de la eliminación de neumáticos usados.
Componente de parafinas y abrillantadores, la morfolina se libera
al medio ambiente por volatilización. Es adsorbida rápidamente por la
humedad, y el principal compartimiento de posible acumulación de la
morfolina es, por tanto, la hidrosfera. No obstante, los datos
limitados disponibles parecen indicar que el producto no se acumula en
la hidrosfera.
La incineración es el método preferido de eliminación de la
morfolina no diluida, pero a veces es necesario controlar las
emisiones de óxido de nitrógeno para respetar las reglamentaciones en
materia de medio ambiente. Por lo que se refiere a los efluentes
acuosos, el tratamiento de fangos activados es suficiente, a condición
de que la planta sea cuidadosamente controlada (véase más arriba).
5. Niveles ambientales y exposición humana
No hay datos disponibles sobre los niveles de morfolina en el
aire ambiental y de locales cerrados y en el agua de bebida. Hay
datos limitados sobre su presencia en aguas naturales, y se carece de
información sobre su presencia en el suelo.
A tenor de los datos disponibles, la fuente principal de
exposición de la población general a la morfolina son los alimentos,
que pueden estar contaminados como consecuencia del tratamiento
directo de la fruta con parafinas contenedoras de morfolina a efectos
de conservación, de los tratamientos a base de vapor empleados durante
la elaboración de los alimentos, y del uso de material de envasado con
morfolina. No obstante, los datos cuantitativos disponibles acerca de
la contaminación de los alimentos por morfolina y NMOR son limitados.
Por ejemplo, en productos lácteos preenvasados se han hallado valores
comprendidos entre 5 y 77 µg/kg de morfolina y de hasta 3,3 µg/kg de
NMOR. La concentración de morfolina en diversas muestras de alimentos
(pescado, carne, productos vegetales, bebidas) no rebasaba por lo
general el valor de 1 mg/kg. Se han detectado niveles más altos
(hasta 71,1 mg/kg) en frutos cítricos en el Japón. En un estudio
realizado en Italia trabajando con un límite de detección de 0,3 µg/kg
no se halló NMOR en una serie de alimentos. Los datos disponibles no
permiten hacer una estimación de la ingesta de morfolina y NMOR a
través de los alimentos.
Se ha detectado morfolina en tabaco de cigarrillos a una
concentración de 0,3 mg/kg, y en tabaco en polvo y tabaco mascable a
concentraciones de hasta 4,0 mg/kg. En otras ocasiones se ha
notificado el hallazgo de nitrosomorfolina a niveles de hasta
0,7 mg/kg en el tabaco en polvo. En estos casos el producto provenía
probablemente de la parafina de los envases utilizados.
Se ha detectado NMOR en algunos productos cosméticos y de
tocador, como por ejemplo champús y maquillaje de ojos, así como en
artículos de goma, tales como chupetes y tetillas de biberón, a
niveles de hasta 3,5 mg/kg.
En varias industrias puede darse una exposición ocupacional a la
morfolina, pero se dispone de pocos datos sobre la exposición de
trabajadores al producto. Todos los valores notificados son
inferiores a 3 mg/m3. Se ha detectado la exposición ocupacional a
NMOR en la industria del caucho, donde se han hallado concentraciones
de hasta 250 µg/m3.
Los datos actualmente disponibles permiten hacerse una idea del
riesgo potencial de exposición humana, pero no estimar con exactitud
los niveles de exposición de las poblaciones general y laboral a la
morfolina y la NMOR.
6. Cinética y metabolismo en animales de laboratorio y en el hombre
La morfolina se absorbe por vía oral, por vía cutánea y por
inhalación. En la rata, tras su administración oral o intravenosa, la
morfolina se distribuye rápidamente y se concentra sobre todo en el
intestino y el músculo.
En el conejo, la morfolina administrada por vía intravenosa o por
inhalación se concentra preferentemente en los riñones, y en menor
medida en los pulmones, el hígado y la sangre.
La morfolina no se une de forma importante a las proteínas del
plasma. Se han notificado semividas plasmáticas de 115 (rata),
120 (hámster) y 300 minutos (cobayo).
La morfolina se excreta principalmente inalterada por vía renal
en diversas especies. Al cabo de un día de su administración, se
halló en la orina el 70%-90% de la morfolina. La neutralización de la
morfolina acelera su excreción. Un pequeño porcentaje se excreta a
través del aire espirado y de las heces.
Estudios realizados en la rata, el ratón, el hámster y el conejo
muestran que la morfolina se elimina casi enteramente en su forma no
metabolizada. En el cobayo, puede darse una reacción de
N-metilación seguida de N-oxidación, metabolizándose así hasta un
20% de la dosis administrada. En presencia de nitrito, la morfolina
se puede transformar en NMOR tanto in vitro como in vivo. En
función de la dosis, entre el 0% y el 12% de la morfolina administrada
a ratas junto con nitritos puede sufrir nitrosación.
La inmunoestimulación, que entraña la activación de macrófagos,
puede aumentar el grado de nitrosación.
7. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
En lo que respecta a la toxicidad aguda, la DL50 de la
morfolina administrada oralmente es de 1-1,9 g/kg de peso corporal y
0,9 g/kg de peso corporal en la rata y el cobayo, respectivamente.
Las ratas que recibieron morfolina neutralizada (1 g/kg de peso
corporal) sobrevivieron. Tras administración interperitoneal, la
DL50 fue de 0,4 g/kg de peso corporal en el ratón y de 0,1 -
0,4 g/kg de peso corporal en la rata. En los experimentos de
exposición por inhalación, la DL50 fue de aproximadamente 8 g/m3
en la rata y de entre 5 y 7 g/m3 en el ratón. Por vía cutánea la
DL50 en el conejo fue de 0,5 ml/kg de morfolina no diluida. La
intoxicación aguda por morfolina se caracteriza por la aparición de
hemorragia gastrointestinal y diarrea tras la exposición oral, y de
irritación y hemorragias en la nariz, la boca, los ojos y los pulmones
tras la inhalación. En un estudio realizado durante 30 días con ratas
a las que se administraron con sonda dosis de 0,16 - 0,8 g/kg de peso
corporal, se observaron efectos tóxicos graves y mortalidad a todas
las dosis empleadas. En el cobayo se observó también toxicidad grave
y mortalidad a todas las dosis en el margen de 0,09 a 0,45 g/kg de
peso corporal.
Se ha notificado la aparición de alteraciones de la función
pulmonar en la rata tras la exposición a morfolina por inhalación
durante cortos periodos (7,2 g/m3, 4 h/día, 4 días y 1,63 g/m3,
4 h/día, 5 días/semana, 30 días). La mortalidad en la rata osciló
entre 0% y 100% según el nivel de exposición (0,36 - 18,1 g/m3,
6 h/día, 9 días). La toxicidad por inhalación dependía de la dosis,
observándose diversos grados de irritación local (ojos, boca, nariz,
pulmones) y hemorragias a los niveles más altos de exposición. En un
estudio se detectó un aumento de la función de la glándula tiroides, y
en otro necrosis del hígado y de los túbulos renales, como resultado
de la exposición por inhalación.
Un estudio de 90 días de duración reveló que la morfolina
administrada por vía oral (0,2 - 0,7 g/kg de peso corporal al día)
durante ese espacio de tiempo puede reducir el aumento del peso
corporal y la función renal en el ratón. Se ha notificado la
aparición de hiperplasia del epitelio del estómago anterior en el
ratón como resultado de la exposición oral a morfolina
(0,28 - 0,5 g/kg de peso corporal al día) durante 672 días.
En un estudio de 13 semanas sobre los efectos de su inhalación,
la morfolina (0,09 - 0,9 g/m3, 6 h/día, 5 días/semana) causó
lesiones dosis-dependientes de la mucosa nasal y neumonía a los
niveles de exposición más altos (0,36 y 0,9 mg/m3). Un cierto
número de parámetros no variaron en respuesta al tratamiento cuando se
utilizaron 0,09 g/m3; esta concentración puede considerarse el nivel
sin efectos adversos observados (NOAEL) en las condiciones de
exposición por inhalación subcrónica.
La morfolina no diluida y no neutralizada es altamente irritante
para los ojos y la piel, probablemente a causa de sus propiedades
alcalinas. La dilución y la neutralización de su pH pueden reducir
considerablemente su toxicidad tópica. La morfolina (2%) no indujo
sensibilidad en el cobayo al aplicar el método modificado de Buehler.
La morfolina no indujo la aparición de mutaciones en bacterias o
levaduras, con o sin activación metabólica (salvo en un caso a una
concentración muy alta). Se obtuvieron resultados negativos en el
ensayo realizado por mediación de un huésped.
La morfolina no indujo reparación del ADN en hepatocitos
primarios de rata, así como tampoco un aumento importante del
intercambio de cromátides hermanas en células ováricas de hámster
chino. Se consideró que la morfolina tenía efectos ligeramente
mutagénicos en el ensayo con células L5178Y de linfoma de ratón. El
producto aumentó los focos de tipo III en el ensayo de transformación
de células malignas BALB/3T3, lo que no ocurrió con la morfolina
neutralizada.
La morfolina no causó ni mutaciones puntuales ni aberraciones
cromosómicas en embriones de hámster expuestos in utero.
No se observó ningún aumento de la incidencia de tumores en ratas
sometidas a niveles de hasta 0,5 g/m3 de morfolina por inhalación
durante 104 semanas, así como tampoco en ratones que ingirieron oleato
de morfolina al 1% con el agua de bebida durante 96 semanas. En un
estudio de larga duración realizado con un grupo de 104 ratas que
recibieron 1000 mg de morfolina/kg dieta, se observaron tres casos de
carcinoma de células hepáticas, dos de pulmón y uno de angiosarcoma
(no especificado), así como dos gliomas malignos, mientras que en un
grupo testigo de 156 ratas no se observaron tumores. No se detectaron
tumores en hámsters sometidos a idénticas condiciones.
La morfolina administrada al mismo tiempo que nitrito da lugar a
resultados positivos en el ensayo realizado por mediación de un
huésped, probablemente debido a la formación de NMOR. La ingestión
simultánea de morfolina y nitrito indujo la aparición de tumores
hepáticos y pulmonares en la rata y de tumores hepáticos en el
hámster, probablemente a causa de la formación endógena de NMOR. La
NMOR es mutágena en bacterias y levaduras; se notificaron resultados
ligeramente positivos en lo que respecta al intercambio de cromátides
hermanas en células CHO, así como a la aparición de mutaciones en
células L5178Y de linfoma de ratón. La NMOR es carcinógena en el
ratón, la rata, el hámster y diversos peces, y produce tumores de
hígado y de pulmón en el ratón; de hígado, riñón y vasos sanguíneos en
la rata; de hígado y de las vías digestivas y respiratorias superiores
en el hámster, y de hígado en peces.
8. Efectos en el hombre
No se han descrito casos de intoxicación aguda o de efectos a
corto o largo plazo de la exposición a morfolina en la población
general.
En informes sobre la exposición ocupacional a la morfolina se ha
descrito el fenómeno conocido como visión azul o glaucopsia, así como
algunos casos de irritación de la piel y del tracto respiratorio, pero
sin hacer mención de las concentraciones atmosféricas de morfolina.
Se señaló que el número de aberraciones cromosómicas en los linfocitos
de sangre periférica de trabajadores expuestos durante 3 a 10 años a
concentraciones de morfolina de 0,54 - 0,93 mg/m3 no diferían
significativamente del número hallado en los testigos.
La morfolina no diluida es muy irritante para la piel; una
solución diluida (1/40) tuvo efectos moderadamente irritantes.
No se ha investigado el potencial carcinógeno de la morfolina en
poblaciones humanas expuestas.
9. Efectos sobre otros organismos en el laboratorio y en el terreno
Entre los microorganismos acuáticos estudiados, determinadas
cianobacterias (Microcystis) y algas verdes unicelulares
(Scenedesmus) son al parecer las especies más sensibles según se
desprende de los valores de la toxicidad liminal (empleando como
criterio la inhibición del crecimiento de la población) notificados
(duración de la exposición: 8 días): 1,7 mg/litro para Microcystis,
y 4,1 mg/litro para Scenedesmus.
Bacterias aerobias tales como Pseudomonas resultaron ser mucho
más resistentes: la toxicidad liminal a las 16 horas y la NOEC para
el crecimiento de la población se han cifrado, respectivamente, en
310 y 8700 mg/litro. No obstante, una concentración de 1000 mg/litro
inhibía la respiración y la actividad deshidrogenasa (hasta un 20%) en
fangos activados de plantas de tratamiento industrial.
Entre los protozoos acuáticos analizados hasta ahora, la mayor
sensibilidad corresponde a ejemplares de los géneros Entosiphon y
Chilomonas (con toxicidades liminales de 12 y 18 mg/litro,
respectivamente, para la inhibición del crecimiento de la población).
En Daphnia, la CE a las 24 horas (E = inmovilización) se ha
establecido en valores comprendidos entre 100 y 120 mg/litro. Los
valores notificados para la CL50 a las 48 - 96 horas en peces
estudiados en agua dulce, salobre o marina fueron > 180 mg/litro, y
la especial más sensible en este sentido es la trucha arco iris.
No se dispone de datos sobre los efectos a largo plazo en
invertebrados y vertebrados acuáticos. La información sobre la
toxicidad de la morfolina en organismos silvestres del suelo es casi
inexistente, pues se limita a una CE a los 3 días de aproximadamente
400 mg/litro para la inhibición de la germinación en la lechuga.
10. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
10.1 Evaluación de los efectos en la salud humana
La principal vía de exposición de la población general a la
morfolina es el consumo de alimentos contaminados. También puede
contribuir a la exposición general la contaminación del tabaco y de
los productos derivados del tabaco, así como de los artículos
cosméticos y de tocador y de los productos de goma. En numerosas
industrias se da una exposición ocupacional a la morfolina; el
compuesto es absorbido por inhalación y por vía cutánea. No hay datos
suficientes para determinar el grado de exposición de la población
general. Los datos disponibles sobre la exposición ocupacional al
producto también son limitados.
La morfolina no es altamente tóxica en condiciones de exposición
aguda. La DL50 tras administración oral es de 1 a 1,9 g/kg de peso
corporal en la rata y de 0,9 g/kg de peso corporal en el cobayo. Se
han notificado CL50 de 7,8 mg/m3 (rata) y 4,9 - 6,9 g/m3
(ratón).
En las condiciones de exposición por inhalación de corta y larga
duración, los efectos críticos son al parecer la irritación de los
ojos y las vías respiratorias. Puede considerarse que el NOAEL
corresponde a una concentración de 90 mg/m3 en las condiciones en
que se llevó a cabo el experimento de 13 semanas en la rata (6 h/día,
5 días/semana). En un estudio de larga duración (104 semanas) sobre
los efectos de la inhalación del producto se observó una mayor
incidencia de inflamación de la córnea y de inflamación y necrosis de
la cavidad nasal en ratas sometidas a 540 mg/m3. A concentraciones
de 36 y 180 mg/m3 se observó también una mayor incidencia de
irritación de los ojos y de la cavidad nasal.
Las exposiciones altas a la morfolina causan lesiones graves del
hígado y los riñones en la rata y el cobayo. Se ha notificado la
aparición de degeneración grasa del hígado en la rata como resultado
de la ingestión diaria de morfolina (0,5 g/kg de peso corporal)
durante 56 días. En el ratón la administración de una sal de
morfolina y ácido oleico en el agua de bebida a una dosis de
aproximadamente 0,7 g/kg de peso corporal al día durante 13 semanas
dio lugar a una hinchazón turbia de los túbulos proximales renales.
Se observó una disminución del aumento del peso corporal en los
ratones hembra del experimento de administración prolongada (672 días)
de una dieta con dosis comprendidas entre 0,05 y 0,4 g de morfolina
(en forma de sal del ácido oleico).
A los niveles que según se ha notificado alcanza actualmente la
exposición ocupacional y ambiental, no parece que la morfolina entrañe
ningún riesgo importante de efectos tóxicos sistémicos. Pueden
aparecer efectos locales (irritación) en los ojos y las vías
respiratorias en las exposiciones ocupacionales y accidentales no
controladas a altas concentraciones de morfolina transmitida por el
aire, y el contacto con morfolina líquida (incluso diluida) puede
causar irritación cutánea.
La morfolina no parece tener efectos mutágenos o carcinógenos en
los animales. No obstante, fácilmente puede nitrosarse y convertirse
en NMOR producto mutágeno y carcinógeno en varias especies de animales
de experimentación. En la rata, la morfolina ingerida secuencialmente
con nitrito dio lugar a un aumento de los tumores, sobre todo de
carcinoma hepatocelular y de sarcomas del hígado y los pulmones. Así
pues, por prudencia, conviene considerar que la exposición a la
morfolina aumenta el riesgo de carcinogénesis en las poblaciones
expuestas.
10.2 Evaluación de los efectos en el medio ambiente
Habida cuenta de los muy limitados conocimientos respecto a la
exposición ambiental, así como de la carencia de datos sobre los
efectos de la exposición de larga duración en el agua y la exposición
de corta y larga duración en el medio terrestre, por el momento no es
posible hacer una evaluación rigurosa de los riesgos. Sobre la base
de las propiedades conocidas de la morfolina, de la información
ecotoxicológica disponible y de los escasos datos sobre su
concentración en el medio ambiente, es posible extraer algunas
conclusiones.
Debido a la alta hidrosolubilidad de la morfolina y a su baja
volatilidad (en condiciones ambientales), su principal sumidero
ambiental es la hidrosfera.
La morfolina es por naturaleza biodegradable y, aunque la
degradación es lenta, no hay datos que lleven a pensar que se acumula
en la hidrosfera. Su bioacumulación es improbable.
Hay relativamente pocos datos sobre la toxicidad de la morfolina
en organismos silvestres. No obstante, parece improbable que los
niveles actuales de emisión de morfolina puedan causar daños
importantes en un radio de acción amplio. Restan por evaluar los
efectos locales, como por ejemplo los debidos a emisiones industriales
o a la morfolina liberada por el desgaste de los neumáticos.
La contaminación de algunos alimentos, como el pescado, por
morfolina puede deberse a contaminación ambiental, pero ello no es
seguro.
La conversión de la morfolina en NMOR es la principal causa de
inquietud, sobre todo en relación con las poblaciones de vertebrados.
Se ha notificado la presencia de NMOR en aguas residuales industriales
y en el suelo próximo a una fábrica. La presencia de morfolina en
agua destinada a transformarse por elaboración en agua de bebida
suscita preocupación.
11. Conclusiones y recomendaciones
La morfolina no entraña riesgos de toxicidad para el hombre a los
niveles habituales de exposición, pero hay que tener en cuenta que
puede transformarse en el carcinógeno NMOR.
No hay ningún indicio de que a los niveles actuales de exposición
la morfolina entrañe un riesgo sustancial para la biota en el medio
ambiente.
11.1 Recomendaciones para la protección de la salud humana
a) En la medida de lo posible debe evitarse la exposición humana a
la morfolina.
b) Debe evitarse que los alimentos se contaminen durante su envasado
y elaboración.
c) Se evitará que contengan morfolina los productos de goma con los
que el hombre haya de tener contacto directo.
d) No debe emplearse morfolina en la preparación de productos
cosméticos o de tocador.
e) Los efluentes industriales deben ser objeto de un riguroso
tratamiento para evitar la contaminación por morfolina del agua
de bebida.
f) Habida cuenta de la formación del carcinógeno NMOR es necesario
replantearse los actuales límites de exposición ocupacional.
11.2 Recomendaciones para la protección del medio ambiente
Debe evitarse que las plantas de tratamiento de efluentes sufran
derrames y sobrecargas.
11.3 Recomendaciones para ulteriores investigaciones
Deben emprenderse estudios sobre los siguientes temas:
a) toxicidad reproductiva en mamíferos;
b) toxicidad a largo plazo en mamíferos;
c) efecto de la exposición de mamíferos a niveles bajos de
morfolina, con y sin nitrito y nitrato;
d) transnitrosación por NMOR in vivo e in vitro;
e) biodegradación en condiciones anaerobias, sobre todo en
condiciones favorecedoras de la reducción de nitratos;
f) catálisis microbiana de la N-nitrosación en condiciones
realistas;
g) niveles ambientales de morfolina en las aguas subterráneas, el
suelo y los ríos a partir de los cuales se obtenga agua de
bebida;
h) niveles ambientales de morfolina en torno a las fábricas
productoras y procesadoras de morfolina;
i) metabolismo y toxicocinética en el hombre, como parte del
desarrollo de métodos para la vigilancia biológica de la
morfolina;
j) vigilancia de los niveles de morfolina y de NMOR en los
alimentos, el agua de bebida y el aire de locales cerrados;
k) se deben reunir y poner a disposición datos sobre la exposición
ocupacional.
The rate of reaction of the nitrosation of morpholine by nitrite
is greatest at a pH value of 3.4, where the rate constant is
0.42 mol-2.s-1. An increase in the pH value has been shown to
result in a decrease in the rate of nitrosation with nitrite (Mirvish,
1975; Archer et al., 1977), and the rate was almost zero at pH > 7
(Archer et al., 1977).
In contrast, nitrosation with gaseous nitrogen oxides (N2O3,
N2O4, NOx) can take place over the whole pH range (Challis &
Kyrtopoulos, 1979; Meiners et al., 1980). Cooney et al. (1987) found
that, under certain conditions, the yield of NMOR at pH 7 was ten
times higher than at pH 2, but there was no further increase beyond
this pH.
Some nitrosamines, particularly alpha-nitrosamine aldehydes, are
potent transnitrosation reagents and are capable of nitrosating
morpholine at pH 7.9 (Loeppky et al., 1987).
Numerous reaction accelerators are known, e.g., thiocyanate
(Boyland et al., 1971), halides (Mirvish, 1975), formaldehyde (Archer
et al., 1977) and nitrosophenols, e.g., p-nitroso- o-cresol (Davies
et al., 1980). Enhancement of the nitrosation of morpholine by
nitrogen dioxide was reported in the presence of iodine (Challis &
Outram, 1979), vanillin and related phenols (Cooney & Ross, 1987) and
halides, particularly bromide (Cooney et al., 1987).
In contrast, the following compounds have been reported to
inhibit the nitrosation of morpholine almost completely: ascorbic acid
(Lathia & Schellhöh, 1981; Leach et al., 1991); urea or ammonium
sulfamate (Mirvish et al., 1972); gallic acid and sulfite (Mirvish,
1975); L-cysteine and DL-methionine ( in vitro study under
physiological conditions, Lathia & Edeler, 1989), catechol and
4-hydroxychavicol (Shenoy & Choughuley, 1989); alpha-tocopherol
(Norkus et al., 1986; Cooney et al., 1987; Schuster et al., 1990);
sulfhydryl compounds such as cysteine, cysteamine, glutathione and
thioglycolic acid, as well as extracts of onion and garlic juice
(Shenoy & Choughuley, 1992). Vitamin C, glucose, mannitol, cabbage
juice, orange juice, shiitake mushroom extract and saliva inhibited
the nitrosation of morpholine in vitro, but catechin, epicatechin
and tea extract enhanced the same reaction (Ohnishi, 1984). The
inhibitory effect of Chinese tea on the formation of NMOR in vitro
and in vivo has also been described (Wang & Wu, 1991).
Several C-nitro compounds, in particular tetranitromethane, have
been demonstrated to transnitrosate morpholine to form NMOR (Fan et
al., 1978). C-nitro compounds are widely used in industry as
pesticides, bactericides, colouring agents, drugs and perfumes.
Singer (1980) described the transnitrosation of morpholine with
nitrosamines and nitrosureas under acid conditions in the presence of
thiocyanate. These reactions are dependent on the pH value and steric
and electronic factors, as well as on the basicity of the amines. In a
model study, the nitrosation of morpholine by nitro-nitroso compounds,
such as those found in fried bacon, was observed (Liu et al., 1988).
NMOR can be formed in vivo in humans and has been found in
various tissues and fluids such as human saliva (Boyland et al., 1971;
Wishnok & Tannenbaum, 1977) and human gastric juice (Ziebarth, 1973;
1974; Sen & Baddoo, 1989; Yurchenko et al., 1990). NMOR formation has
been reported in rat lungs (Postlethwait & Mustafa, 1983), whole mice
(Iqbal et al. 1980; Norkus et al. 1984), stomach (Furman & Rubenchik,
1991), hepatocytes isolated from woodchucks (Marmota monax) (Liu et
al., 1992) and microorganisms (Archer et al., 1979; O'Donnell et al.,
1988; Calmels et al., 1991a,b). Bacterial catalysis of
N-nitrosation of morpholine has been reported in a range of bacteria
often isolated from the human gut or urinary tract infections (Suzuki
& Mitsuoka, 1984; Calmels et al., 1987, 1988; Mackerness et al.,
1989), including the ubiquitous gut bacterium Escherichia coli and
Pseudomonas aeruginosa, which is also widespread in the aquatic
environment. Bacterial catalysis of N-nitrosation of morpholine is
heat labile and is optimal at neutral to slightly alkaline pH (Calmels
et al., 1985; Leach et al., 1987). N-nitrosation by bacteria is
generally associated with the ability to reduce nitrate. It appears
that those that reduce nitrate to nitrogen or nitrogen oxides (e.g.,
P. aeruginosa) can nitrosate at much greater rates than those (e.g.,
E. coli) that only reduce nitrate to nitrite (Leach et al.,1987;
Calmels et al., 1988). There is considerable variation between
strains of the same species. Bacterial N-nitrosation of morpholine
has been shown to follow Michaelis-Menten kinetics (Calmels et al.,
1985; Leach et al., 1987). E. coli A10, for example, displays Km
values of 7.4 mmol/litre for morpholine and 11.4 mmol/litre for sodium
nitrite. It has been shown that the rate of bacterial N-nitrosation
of secondary amines is inversely related to the pKa of the amine
(Calmels et al., 1985; Leach et al., 1987, 1991), with a linear
relationship between log10 of the rate of nitrosation and pKa.
Morpholine, having a relatively low pKa, is thus relatively
susceptible to nitrosation compared, for example, to alkyl amines.
It has been shown that ascorbate is capable of inhibiting
nitrosation of morpholine by P. aeruginosa (Leach et al., 1991).
Although most nitrosation studies have used whole bacteria, an enzyme
catalyzing N-nitrosation of morpholine has been isolated and
purified from two denitrifying bacteria (Calmels at al., 1990).
4.4 Ultimate fate following use
4.4.1 Fate of morpholine in various products
Morpholine is an important industrial chemical with a wide range
of applications (see section 3.2.2) and therefore may be present in
many industrial emissions.
Its use as a corrosion inhibitor in boiler water means that
morpholine and its decomposition products will be found in boiler
wastewater, including water from power plants using morpholine. In a
study by McCain & Peck (1976), morpholine concentrations in the
discharge streams of three Hawaiian power plants ranged from not
detectable to 0.008 mg/litre, suggesting that the potential for human
exposure is small.
Its use in the manufacture of rubber additives results in an
indefinable amount of morpholine being released into the hydrosphere
or geosphere not only during manufacturing processes but also through
tyre abrasion and disposal of used tyres.
Morpholine is released during vulcanization processes using
morpholine-containing accelerators such as 2-( N-morpholino-
thio)benzothiazole (MBS) (Badura et al., 1989). Some of the amine is
released into the atmosphere and some is bound to the rubber. Even
the accelerator itself can contain free amine. The morpholine content
of MBS is < 0.4% by weight. This level can be higher if the
accelerator is not stored properly and is exposed to heat or moisture
(BUA, 1991).
Aarts et al. (1990) detected free volatile morpholine at
concentrations of between 70 (new) and 230 mg/kg (old) in samples of
dithio-bis-morpholine (DTBM). After extraction in water for one hour
in an ultrasonic bath, ten times this amount was detected, i.e.
960 mg/kg in newly made and 2750 mg/kg in stored DTBM. These
quantities of amine could be released during vulcanization.
Optical brighteners adhere to clothes during the first wash but
tend to be released into the wastewater in subsequent washings.
Although these substances are not themselves biologically degradable,
they have been found to disappear from wastewater after a two-step
biological treatment presumably due to the high rate of adsorption to
the sludge particles (Jakobi et al., 1983).
As mentioned in section 3.2.2, morpholine is released into the
environment by volatilization through its use in waxes and polishes
(Texaco, 1986).
4.4.2 Waste disposal
Controlled incineration is the preferred method of disposal
(Sittig, 1985; Air Products and Chemicals, 1989). The incinerator
should be equipped with a scrubber or thermal unit. Nitrogen oxide
emissions should meet environmental regulations.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
No data are available on levels of morpholine in ambient air.
5.1.2 Water
5.1.2.1 River water
Since mid-1990, the levels of morpholine in some rivers in North
Rhine-Westphalia, Germany, have been monitored (BUA, 1991). No
morpholine could be detected at three different points in the River
Rhine or in five of its tributaries (detection limit, 5 µg/litre). No
morpholine was found in samples of Tennessee freshwater (detection
limit, 0.07 µg/litre) (Singer & Lijinsky, 1976a). In 1979, 33 water
samples were collected at 11 sites in Japan, but no morpholine could
be detected (detection limit, 1-5 µg/litre) in any of the samples
(Environment Agency Japan, 1980).
5.1.2.2 Wastewater
There are no data on morpholine levels in wastewater.
A single sample of wastewater from a tyre chemical factory in
Ohio, USA was found to contain 3 µg NMOR/litre (Fajen et al., 1979).
In England, samples were taken from the inlets and outlets of
four sewage treatment plants (Richardson et al., 1980). NMOR
(100 µg/litre) was found only in the outlet of a cutting-fluid
recovery plant.
5.1.3 Sediment
Spies et al. (1987) examined contaminated sediments in San
Francisco Bay, USA and found several benzothiazoles, including
2-(4-morpholinyl)-benzothiazole, which is present as an impurity in
commercial 2-(morpholinothio)-benzothiazole used in motor tyres. The
authors carried out weathering tests on this latter commercial
substance and found that the morpholine impurity was environmentally
stable. They suggested that the 2-(4-morpholinyl)-benzothiazole found
in the sediments (up to 0.36 mg/kg dry weight) was a result of
accumulated street run-off. Morpholine itself could not be detected.
In 1979, 33 bottom sediment samples were collected at 11 sites in
Japan, but no morpholine could be detected (detection limit,
0.01-0.5 mg/kg) in any of the samples (Environment Agency Japan,
1980).
5.1.4 Soil
There are no data on the presence of morpholine in soil.
2-(4-Morpholinyl)-benzothiaozole (273 µg/kg dry weight) was detected
1.6 km from a motorway in California, USA (Spies et al., 1987) (see
also section 5.1.2).
NMOR (4.4 mg/kg) was detected in a single sample of soil near to
a tyre chemical factory in Ohio, USA (Fajen et al., 1979).
5.1.5 Terrestrial and aquatic organisms
Levels of morpholine found in single or small samples of fish are
given in Table 6, but the sample numbers are too low to make an
evaluation. No other data are available.
5.2 General population exposure
5.2.1 Indoor air
No data on indoor air exposure to morpholine are available.
Analysis for NMOR in the air inside new cars showed levels of up
to 2.5 µg/m3. Levels were 4 to 10 times lower when the air-venting
system was working, indicating that NMOR exposure is limited to the
first few minutes of each trip (Rounbehler et al., 1980). During a
simulation of conditions inside cars on a hot day, concentrations of
up to 0.4 µg NMOR/m3 were measured at 60°C (Dropkin, 1985).
5.2.2 Drinking-water and food
There are no data on the morpholine content of drinking-water.
Food can become contaminated with morpholine in several ways:
(a) through direct treatment of fruit with waxes containing morpholine
for conservation purposes; (b) by use of packaging material
containing morpholine, and (c) through steam treatment during
processing.
Ohnishi et al. (1983) found morpholine at concentrations of
< 71.1 mg/kg in the peel of retail citrus fruits in Japan. In the
pulp (flesh) of the fruits the level was much lower, being less than
0.7 mg/kg (Table 4). Marmalade made from whole fruits contained
concentrations of morpholine between 0.3 to 0.7 mg/kg. If the fruits
were previously washed in washing-up liquid, morpholine concentrations
were reduced, but only by 25%. Even if the fruit was boiled for
20 minutes, a third to a quarter of the morpholine still remained. The
morpholine removed by these processes could be detected quantitatively
in the washing and boiling water (Ohnishi et al., 1983).
Table 4. Morpholine content of citrus fruits and marmalade from
citrus fruitsa
Sample Number Morpholine
(mg/kg)b
Orange (variety a)c peel 12
n.d.-57.0
fruit pulp 3 0.2-0.7
Orange (variety b)c peel 6 5.0-71.1
fruit pulp 1 0.3
Mandarine peel 2 16.1-18.0
fruit pulp 1 n.d.
Lemon peel 2 n.d.-5.2
fruit pulp 1 n.d.
Grapefruit peel 2 2.8-7.0
fruit pulp 1 n.d.
Marmalade from citrus fruits 4 0.3-0.7
a adapted from Ohnishi et al. (1983)
b n.d. = not detectable (detection level 0.2 mg/kg),
presumably fresh weight
c variety not specified
Sen & Baddoo (1989) reported the morpholine and NMOR content of
waxed and unwaxed apples of Canadian origin, obtained either direct
from the packers or from retail sources. Liquid wax spray is used as
a protective coating on fruit and vegetables to reduce moisture loss
and thereby extend the shelf-life of the product. Apple homogenates
and liquid waxes were analysed for their morpholine contents
(Table 5). Although the concentrations of morpholine found in waxed
apples were high, NMOR could not be found in any of the waxed or
unwaxed samples. Low levels of morpholine in the unwaxed apples could
be due to contamination during packing or transport.
Singer & Lijinski (1976a) analysed a variety of foodstuffs for
the presence of morpholine but the sample size was too small to draw
any conclusions. The results are given in Table 6. The sources of
contamination with morpholine are in these cases not clear. The
possibility of artifacts is unlikely according to the authors.
Table 5. Concentration of morpholine and NMOR (mg/kg) in samples of
liquid waxes and waxed and unwaxed applesa
Liquid wax Unwaxed apples Waxed apples
NMOR morpholine NMOR morpholine NMOR morpholine
0.286 27 300 n.d. n.d. n.d. 4.3
0.668 31 500 n.d. 0.118 n.d. 4.9
0.138 24 400 n.d. 0.016 n.d. 6.3
0.277 38 500 n.d. 0.041 n.d. 7.1
0.152 22 500 n.d. n.d. n.d. 4.0
0.585 33 300 n.d. 0.018 n.d. 7.7
a adapted from Sen & Baddoo (1989); n.d. = not detected (detection
limit: 0.005 mg/kg for morpholine, 0.0005 mg/kg for NMOR)
Table 7 summarizes the results of investigations into the
concentrations of morpholine and NMOR in prepacked milk products
(Hoffmann et al., 1982). The values range from 5-77 µg/kg for
morpholine and "not detectable" to 3.3 µg/kg for NMOR. Contamination
of prepacked foodstuffs with morpholine might be explained by the use
of morpholine in steam boiler systems for paper and cardboard
production.
Hotchkiss & Vecchio (1983) found morpholine concentrations of
between 0.098 and 8.4 mg/kg (mean 0.38 mg/kg) in food packaging. A
sample of flour nearest the wall of the paper bag contained 1.1 µg
NMOR/kg. The bag itself contained 33.0 µg NMOR/kg. In an experimental
72-h incubation at 100°C, between 1 and 2.3 µg NMOR/kg migrated from
packaging material to various dry foods.
Sen & Baddoo (1986) investigated the migration of NMOR out of
waxed packaging material into margarine. Waxed wrappings and margarine
samples taken 1 cm from the outer layer of the block contained NMOR
(5-73 µg/kg and 0.5-1.4 µg/kg, respectively). NMOR was not detectable
in those samples taken from the inside of the block. Samples taken of
margarine packed in aluminium backed wrappers, specially coated waxed
papers or plastic containers were negative for NMOR (detection level,
0.5 µg/kg).
Aitzetmüller & Thiele (1982) found no NMOR in 20 margarine
samples from different countries (detection limit, 0.5 µg/kg).
Table 6. Morpholine content in various food samplesa
Food No. of Concentration References
samples (mg/kg)b
Fish
Fish sausage 5 n.d. Hamano et al. (1981)
Cod roe 3 n.d. Hamano et al. (1981)
Codc -d tracese Singer & Lijinsky (1976a)
Spotted troutc 1 6 Singer & Lijinsky (1976a)
Smallmouth bassc 1 < 0.7 Singer & Lijinsky (1976a)
Salmonc 1 1 Singer & Lijinsky (1976a)
Ocean perchc -d 9.0 Singer & Lijinsky (1976a)
Tuna (in tins) -d < 0.6 Singer & Lijinsky (1976a)
Meat
Baked ham 5 0.2 Hamano et al. (1981)
Baked ham -d 0.5 Singer & Lijinsky (1976a)
Frankfurter sausages -d 0.4 Singer & Lijinsky (1976a)
Plant products
Spinach 2 n.d. Hamano et al. (1981)
Miso (from soja) 2 n.d. Hamano et al. (1981)
Beverages
Evaporated milk -d 0.2 Singer & Lijinsky (1976a)
Coffee -d 1.2 Singer & Lijinsky (1976a)
Tea -d tracesf Singer & Lijinsky (1976a)
Beer (in tins) -d 0.4 Singer & Lijinsky (1976a)
Beer (in bottles) -d < 0.2 Singer & Lijinsky (1976a)
Wine -d < 0.7 Singer & Lijinsky (1976a)
a adapted from BUA (1991)
b fresh weight except for tea and coffee (dry weight);
n.d. = not detected (detection limit 0.01 mg/kg)
c frozen from Tennesee & Columbia rivers
d average from several samples; exact number not given
e < 0.3 mg/kg
f < 0.1 mg/kg dry weight
Table 7. Morpholine and NMOR content (mg/kg) in food products
and their waxed containersa
Sample Food Container
NMOR morpholine NMOR morpholine
Butter 0.0032 0.058 0.0019 0.22
Cream cheese 0.0009 0.077 n.d. 0.68
Yoghurt n.d. 0.038 n.d. 3.06
Cottage cheese 0.0004 0.044 0.0054 17.2
"Cheese" (semi-soft)
Country of origin:
Germany 0.0033 0.009 n.d. 0.026
Denmark 0.0031 0.01 0.0016 0.025
Austria 0.0007 0.005 0.0012 0.022
USA 0.0014 0.008 n.d. 0.132
Gouda 0.0016 0.035 n.d. 0.035
Frozen peas n.d. 0.026 0.0031 0.057
and carrots
a adapted from Hoffmann et al. (1982); n.d. = not detected
(detection limit for NMOR, 0.0002 mg/kg)
Gavinelli et al. (1988), in a survey in Italy, found no NMOR in a
variety of foodstuffs including canned beef, pork, poultry, cured
meat, milk products, malt products, domestic Italian canned wines and
beers. The limit of detection was 0.3 µg/kg. Similarly, Pensabene &
Fiddler (1988) found no NMOR in samples of different USA fish-meat
frankfurter sausages (limit of detection, 0.2 µg/kg). These authors
assumed that the positive results in their earlier work were due to
artifacts.
Groenen et al. (1987) found NMOR in cheese (2.8 µg/kg) and cured
meat products (34 µg/kg) after addition of morpholine (10 mg/kg) in
the alkaline pH range.
Liu et al. (1988) suggested that the NMOR in fried bacon was
formed by transnitrosation.
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (US FDA, 1984a,b,c,d,e;
1988; see section 3.2.2). Although the US Food and Drug Administration
allows morpholine to be used as a direct or indirect food additive, in
1980 one of the biggest USA producers recommended its customers not to
use morpholine in this way (Litton Bionetics, 1980).
5.2.3 Tobacco
Morpholine was found in unburned cigarette tobacco at a
concentration of 0.3 mg/kg (Singer & Lijinsky, 1976b). In cigarette
smoke condensate < 5 mg/kg was detected, equivalent to
0.08 µg/cigarette. It has been established that an appreciable portion
of the inhaled smoke is swallowed and retained in the stomach where
nitrosation of ingested amines is known to occur (Sander & Bürkle,
1969).
Brunnemann et al. (1982) analysed 10 popular snuff brands from
the USA and Sweden for morpholine and volatile N-nitrosamines (see
Table 8). In the five USA brands, morpholine concentrations of between
1.5 and 4.0 mg/kg were found; in the Swedish products, the
concentrations were between 0.2 and 2.5 mg/kg. An analysis of the
snuff containers that were made of waxed cardboard gave morpholine
values of 0.17 to 4.74 mg/kg (USA) and 0.46 to 4.83 mg/kg (Swedish).
The morpholine content in the snuff could have come through diffusion
from the container made of waxed cardboard. Another possibility was
contamination during the tobacco processing as suggested by the very
high morpholine content (19.4 mg/kg) of a single sample of chewing
tobacco, packaged in aluminium. NMOR formed by nitrosation from
morpholine was found in 5/5 of the USA and 2/5 of the Swedish snuff
samples (Brunnemann et al., 1982).
Brunnemann & Hoffmann (1991) reported that since 1981 they had
routinely determined the concentrations of NMOR in a leading snuff
brand in the USA with the aim of creating awareness of the fact that
morpholine is a precursor of NMOR and must be removed. In eight
commercial snuff brands analysed in 1986 (Hoffmann et al., 1987), only
three were found with traces of NMOR (9-39 µg/kg). Brunnemann &
Hoffmann (1991) noted that the decrease in the NMOR content (from
about 700 µg/kg in 1981 to an undetectable level in 1990) is most
probably due to modifications in packaging, since snuff products in
the USA are now sold in plastic containers that have no wax coatings
(Table 9).
Österdahl & Slorach (1983) analysed snuff and chewing tobacco on
the Swedish market for volatile N-nitrosamines. NMOR was found in
9/30 samples in concentrations between 0.4 and 4.0 µg/kg (Swedish
brands) but in none of the three USA brands. One of three chewing
tobacco brands contained 0.8 µg morpholine/kg.
Table 8. Nitrosomorpholine and morpholine in snuff containersa
Snuff brand Snuff tobacco (µg/kg)b Snuff container
(µg/kg)c
NMOR morpholine NMOR morpholine
USA I 24 2800 34 845
II 690 1500 10 170
III 690 4000 230 4740
IV 630 3200 4 90
V 31 2200 3 140
Sweden I 44 820 4 1750
II < 2d 200 3 460
III < 2d 780 13 4830
IV 10 940 23 4290
V < 2d 2500 n.d.e n.d.
a from Brunnemann et al. (1982)
b based on dry weight
c uncorrected for moisture; had previously contained snuff.
Containers of USA I-III and Sweden I-IV were cardboard boxes
with a metal lid, USA IV were plastic containers with individual
snuff portions in porous paper bags; USA V was a plastic container;
Sweden V were individual snuff portions in Al-bags.
d detection limit = 2 µg/kg
e n.d. = not determined
Table 9. NMOR in fine-cut snuff (on the basis of dry tobacco weight)a
Year Yield (µg/kg)
1981 690
1984 29.4
1984/85 238
1985 29
1986/87 < 2b
1987 43
1990 < 2
a from Brunnemann & Hoffmann (1991)
b detection limit = 2 µg/kg
A survey of smokeless tobacco products commercially available in
1987-1988 showed that trace levels of NMOR were still found in some
United Kingdom (mean 0.5 µg/kg) and Swedish (mean 1 µg/kg) oral
tobacco products packed in waxed containers, but no NMOR was detected
in Indian zarda or European nasal snuff (Tricker & Preussmann, 1991).
5.2.4 Cosmetics and toiletry articles
Morpholine is used in some countries in cosmetic products as a
surfactant and emulsifier at concentrations up to 5% (Cosmetic
Ingredient Review, 1989).
Data submitted to the Food and Drug Administration (FDA) in 1981
by cosmetic firms participating in the voluntary cosmetic registration
programme indicated that morpholine was used in a total of 38 cosmetic
products including eyeliner, eye shadow, mascara and skin care
preparations (Cosmetic Ingredient Review, 1989). The greatest use of
morpholine was in mascara (32 products). The reported concentration
ranges of morpholine in these products were < 0.1% (4 products),
> 0.1-1% (17 products) and > 1-5% (17 products). Table 10
summarizes data submitted to the FDA in 1986 showing that the greatest
use of morpholine was in eye make-up removers, at concentrations of
> 0.1-1% and > 1-5% (Cosmetic Ingredient Review, 1989). These data
show that the ocular region and the skin around are the areas directly
exposed to cosmetic products containing morpholine. The potential also
exists for morpholine-containing products to come in contact with the
conjunctiva and cornea. Additionally, it must be emphasized that these
morpholine-containing products may be applied several times daily over
the course of several years. Due to lack of appropriate safety data,
the Cosmetic Ingredient Review Expert Panel could not conclude that
morpholine was safe for use in cosmetic products (Cosmetic Ingredient
Review, 1989).
Morpholine is listed in Annex II of the European Economic
Community (EEC) Cosmetics Directive and therefore must not be used in
cosmetic formulations (EEC, 1986). Investigations in 1988 and 1989 in
the Federal Republic of Germany showed that no morpholine could be
detected in toiletry articles (BUA, 1991).
Data from a 1985 US Food and Drug Administration report given in
Table 11 show that NMOR was found at concentrations between 48 and
1240 µg/kg in seven mascara products (Cosmetic Ingredient Review,
1989). It is not known whether the NMOR detected was formed in the
cosmetics. Table 11 also shows the results of an analysis of various
toiletry articles in the Federal Republic of Germany prior to the EEC
Directive in 1986. NMOR was detected in 18% of the products
(Spiegelhalder & Preussman, 1984).
Table 10. Product formulation data for morpholinea
Product Total no. of Total no. No. of product formulations
category formulations containing within estimated
in category ingredient concentration ranges
> 0-1% > 1 > 1-5%
Eye make-up 77 29 11 18
remover
Eye, face, or body 1264 2 2
preparations other
than eye make-up
removers
1986 totals 1341 31 11 2 18
a US FDA (1986)
5.2.5 Rubber articles
NMOR was found in commercial samples of rubber chemicals at
concentrations of 60-3500 µg/kg by Spiegelhalder & Preussmann (1982),
who noted that the occurrence of nitrosamines was directly related to
the use of corresponding vulcanization accelerators. The nitrosamines
could be leached out by water, buffer solutions or milk but could not
be completely removed even after boiling or treating with acid or
alkaline solutions (Spiegelhalder & Preussmann, 1982).
Several rubber articles which come into contact with skin or
food, in particular baby bottle teats and pacifiers (dummies), were
assayed for NMOR; 10 µg/kg migrated from a silicone rubber pacifier
(1 out of 11 different samples; detection level, 1 µg/kg) after
incubation for 24 h at 40°C (Spiegelhalder & Preussmann, 1982). Sen et
al. (1984) found NMOR in 2 out of 10 brands of nipples and in one
pacifier at concentrations of between 0.1 µg/kg (detection level) and
86 µg/kg. The same authors found NMOR (5.7 µg/kg) in one plastic
nipple shield, no NMOR being found in the other 41 samples of nipples
and pacifiers (detection limit, 1 µg/kg) (Sen et al., 1985).
Westin et al. (1987) tested 16 types of children's pacifiers and
baby-bottle nipples on sale in Israel from nine different countries
and found NMOR at levels of up to 2 µg/kg.
Table 11. N-nitrosomorpholine (NMOR) in toiletry articles and
cosmetics
Concentration
Productsa No. of No. (µg/kg)
samples positive
maximum average
Shampoos 45 13 640 133
Colour toners 7 - - -
Hair conditioners 16 - - -
Foam baths 7 - - -
Shower gels 9 4 380 145
Cream and oil baths 8 2 440 -
Cosmetic bath additives 5 - - -
Children's shampoos 5 1 230 -
Children's bath and
skin care products 8 6 360 80
Body lotions and rubs 6 - - -
Face tonics, cleaners, 29 - - -
and masks
Mascarab 7 7 1240 306
a from Spiegelhalder & Preussmann (1984), with the exception of
mascara
b from CIR (1986)
In a survey in Germany in 1990, no morpholine or NMOR was found
in the rubber articles tested; these included balloons, baby
dummies/teats, rubber gloves, condoms and rubber rings for bottling
(BUA, 1991).
5.3 Occupational exposure during manufacture, formulation or use
A USA National Occupational Hazard Survey, conducted by NIOSH,
detected worker exposure to morpholine in 283 different industries
(NRC, 1981). In 1970, 501 283 workers were potentially exposed to the
actual product, 22% were exposed to a product known to contain
morpholine, and 74% were exposed to a generic product suspected to
contain morpholine.
Data from the USA National Occupational Exposure Survey (NOES) in
1988 indicated that in a total of 30 industries and 8711 plants
surveyed, 146 511 workers, including 44 839 women, were potentially
exposed to morpholine in the workplace (NIOSH, 1988).
Many countries recommend for morpholine an 8-h time-weighted
average exposure limit of 70 mg/m3 (skin notation) and a 15-min
short-term exposure limit (STEL) of 105 mg/m3 (ILO, 1991).
5.3.1 Exposure to morpholine
Only a few studies have been reported concerning the exposure of
workers to morpholine.
An analysis in Ontario, Canada of condensed steam samples in four
hospitals and one food processing plant using corrosion inhibitors in
the steam-generating plants gave mean values for morpholine of
2.41 mg/litre in the hospitals (9 samples; range 2.1-2.9 mg/litre) and
1.1 mg/litre in the factory (9 samples; range 0.6-1.8 mg/litre)
(Malaiyandi et al., 1979).
Fajen et al. (1979) found morpholine levels of 230 and
42 µg/m3, respectively, in air samples from two out of four rubber
industry factories; these two were a tyre factory and its chemical
factory.
Taft & Stroman (1979) described an investigation into workers
exposed to a number of chemicals in the drying and bagging departments
of a tyre and rubber company manufacturing the rubber accelerator,
4-morpholino-2-benzothiazolyl [(4-morphinyl-2-benzothiazole)]
disulfide. This rubber accelerator is manu-factured by reacting
morpholine with 2-mercaptobenzothiazole (MBT). With the exception of
one personal sample showing 0.05 mg of morpholine/sample (0.4 ppm) at
the level of detection, all other samples showed negative results.
Occupational exposure to morpholine was measured in 15 factories
of the chemical, plastic and rubber industries in Germany from 1980 to
1990, and in no case was the German workplace exposure limit of
70 mg/m3 reached. Of 35 measurements, 90% were below 0.26 mg/m3
(BUA, 1991).
Katosova et al. (1991) reported average levels of 0.54 to 0.93 mg
morpholine/m3 with maximum concentrations of 0.73 to 2.14 mg/m3 in
a morpholine production factory in the former-USSR.
5.3.2 Exposure to N-nitrosomorpholine
Occupational exposure to NMOR has been found in the rubber
industry. Table 12 gives a summary of the data published.
Fajen et al. (1979) found NMOR in air samples from a tyre
chemical factory and an aircraft tyre factory. In the chemical
factory, NMOR was also found as an impurity in morpholine (0.8 mg/kg)
and in bismorpholine carbamylsulfenamide (BMCS), a vulcanisation
accelerator (0.4 to 0.7 mg/kg), as well as in wastewater
(0.003 mg/kg), utility steam condensate (0.002 mg/kg), and dirt
scrapings on a staircase (730 mg/kg). Outside the chemical plant, a
soil sample contained 4.4 mg/kg (Fajen et al., 1979). It is possible
that the NMOR in these samples was formed by the transnitrosation from
N-nitrosodiphenylamine, also produced in the tyre chemical factory,
together with morpholine present in the steam condensate (BUA, 1991).
A survey was carried out by NIOSH and a tyre manufacturing
company in 1979 into the levels of NMOR in four different work areas
in the factory as well as personal exposure levels (Ringenburg &
Fajen, 1980; London & Lee, 1987). Personal breathing-zone air samples
ranged from 0.6 to 1.8 µg/m3 (mean 0.8 µg/m3), and workplace
levels from 0.8 to 3.7 µg/m3. In a survey at another tyre
manufacturing company during the same year, nitrosamine levels were
highest (1.62 µg/m3) during the extrusion process of racing tyre
components (McGlothlin, 1980; McGlothlin & Wilcox, 1984). Exposure to
NMOR in rubber press operator fumes was investigated by NIOSH in
another company. The highest personal exposure to NMOR was
0.063 µg/m3, and the highest area sample 0.038 µg/m3.
In a factory producing rubber parts for automobile interiors, the
highest level of NMOR (19 µg/m3) was found in the process sample
taken directly above one of the extruder ovens. Other personal and
process levels were in the range 0.1 to 1.4 µg NMOR/m3.
Between 1980 and 1990, 775 samples of workplace air from 124
factories in Germany involved in the production and fabrication of
rubber articles were analysed for NMOR (BUA, 1991). Of the samples,
71% showed concentrations less than the guidance value of 1 µg/m3
(BUA, 1991).
According to Spiegelhalder (1983) and Schuster et al. (1990),
higher concentrations of NMOR are to be found in work areas where
higher temperatures are required for fabrication/vulcanization. High
levels of NMOR from storage areas probably result from continued
emission of volatile NMOR.
NMOR was not detected in the environment of a metal factory using
metal-working fluids (Fadlallah et al., 1990).
Table 12. Occupational exposure to NMORa
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Tyre chemical factory USA 1978 10 area 0.07-5.1 Fajen et al. (1979)
Tyre manufacturer USA 1978 20 area 0.6-27 Rounbehler & Fajen
12 area 0.08-8.5 (1983)
7 area 0.02-3.9
6 process 0.41-2.9
6 area < 0.01-0.60
7 process < 0.1-22.0
7 area < 0.002-250
8 process 0.1-25
Tyre manufacturer USA 1979/ 13 area 0.1-1.62 McGlothlin (1980),
1980 McGlothlin & Wilcox (1984)
Tyre manufacturer USA 1979/ 4 area 0.85-3.7 Ringenburg & Fajen (1980);
1980 6 personal 0.6-1.8 London & Lee (1987)
Industrial rubber articles USA 1978 4 area 0 Fajen et al. (1979)
Industrial rubber articles USA 1980/ 11 area < 0.005-0.0376 Hollett et al. (1982)
1981 7 personal < 0.0096-0.0629
Rubber articles for USA 1982 4 area 0.8-19 Lee (1982)
car outfitting 13 personal 0.4-1.2
Rubber industry Germany 1979/ 20 monitoring area and 0.1-17 Spiegelhalder &
1981 points in 17 personal Preussmann (1982)
factories
Table 12 (cont'd)
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Rubber industry Germany 1987 545 personalc < 2.5 (73%), TRGS (1989)
> 2.5 (27%)
max. 41
Chemical industry Germany 1987 22 not given < 1 (91%)d TRGS (1989)
Leather industry Germany 1987 50 not given < 1 (100%) TRGS (1989)
Foundries Germany not given 658 not given < 1 (95%), TRGS (1989)
> 1 (5%)
max. 2.1
a adapted from BUA (1991)
b the percentage of samples which had this given concentration is given in parentheses
c sum of all measured N-nitrosamines
d in 82% below the detection level (0.2 µg/m3)
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Toxicity experiments on rodents have shown that morpholine is
absorbed after oral, dermal and inhalation exposure (see Table 13).
6.2 Distribution
Tanaka et al. (1978) determined the distribution of
[14C]-labelled morpholine in male Wistar rats (3 animals/group,
250-300 g) after oral (200 mg/kg) and intravenous injections
(150 mg/kg). The radioactivity was determined in the dried, powdered
organs. Large amounts were only found in muscle and intestine. In
rats sacrificed 2 h after oral administration of morpholine-HCl, 29%
of the radioactivity was found in the intestine and 26% in the muscle.
Similarly, 2 h after intravenous injections, 19 and 27% of the dose
was found in the intestine and muscle, respectively.
Female New Zealand rabbits (number not given) were exposed to
morpholine (905 mg/m3) for 5 h by nose-only inhalation
(Tombropoulos, 1979). At the end of the exposure, the animals were
sacrificed and the tissue and body fluids analysed. Concentrations of
morpholine were highest in urine (324 mg/litre) and kidney
(118 mg/kg), the other tissues having concentrations below 40 mg/kg.
Van Stee et al. (1981) injected six male New Zealand rabbits
intravenously with 5 mmol [14C]-labelled morpholine/kg body weight
(435 mg/kg). The distribution of radioactivity after 30 min showed
the highest concentrations in the renal medulla (36 mmol/kg) and
cortex (15.4 mmol/kg), followed by lung (5.1 mmol/kg), liver
(4.7 mmol/kg) and blood (2.3 mmol/litre). Morpholine was not bound to
serum proteins.
6.3 Metabolic transformation
Morpholine is eliminated mainly in a non-metabolized form in the
urine of the rat, mouse, hamster and rabbit (Griffiths, 1968; Tanaka
et al., 1978; Van Stee et al., 1981; Sohn et al., 1982b). However,
Sohn et al. (1982b) reported that morpholine is metabolized by
N-methylation followed by N-oxidation in the guinea-pig. After an
intraperitoneal injection of 125 mg/kg [14C]-labelled morpholine in
guinea-pigs, 20% of the radioactivity was found in the urine as
N-methylmorpholine- N-oxide. However, the morpholine ring can be
cleaved in mammalian systems. In several studies on the metabolism of
morpholine derivatives in the rat, ring cleavage products have been
noted (Tatsumi et al., 1975; Hecht & Young, 1981; Kamimura et al.,
1987).
Table 13. Single exposure toxicity data for morpholinea
Species No./sex Dosage Results Cause of death/symptoms Reference
Oral
Rat 5 1.05 g/kg body weight LD50 n.g. Smyth et al. (1954)
Rat 57 1.6 g/kg body weightb LD50 gastrointestinal haemorrhage Shea (1939)
Rat 5 m + 5 f 1.9 g/kg body weight LD50 dyspnoea, haemorrhagic BASF (1967)
enteritis
Rat 7 m 1.0 g/kg body weight; pH 7 no deaths n.g. Börzsönyi et al. (1981)
Guinea-pig 33 0.9 g/kg body weightb LD50 gastrointestinal Shea (1939)
haemorrhage, diarrhoea
Intraperitoneal
Rat (Wistar) 4 m 0.4 g/kg body weight all died n.g. Stewart & Farber (1973)
Rat (Wistar) 4 m 0.1 g/kg body weight lethal n.g. Stewart & Farber (1973)
for 1/4
Mouse 5 m + 5 f 0.4 g/kg body weight LD50 irritation around BASF (1967)
injection area, dyspnoea
Inhalation
Rat 5 m + 5 f 18.1 g/m3; 6 h lethal haemorrhage of nose, mouth Hazleton (1981)
for 9/10 and eyes; spasms; tremors
Rat 6 saturated atmosphere;c all died spasms; caustic burns BASF (1967)
5´ h on nose and extremities
Table 13 (cont'd)
Species No./sex Dosage Results Cause of death/symptoms Reference
Inhalation (contd)
Rat n.g./m 8.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g./f 7.8 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g. 65.2 g/m3; 8 h lethal irritation of nose, Shea (1939)
eyes; lung haemorrhage
Rat n.g. 43.4 g/m3; 8 h lethal n.g. Shea (1939)
Mouse n.g./f 6.9 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g./m 5.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g. 4.9 g/m3; LC50 n.g. Zaeva et al. (1968)
Dermal
Rabbit (New n.g. 0.5 ml/kg body weight LC50 n.g. Smyth et al. (1954)
Zealand)
a adapted from BUA (1991); n.g. = no details given
b morpholine diluted with 4 parts water; deaths within a week
c inhalation of a saturated atmosphere at 20°C formed by bubbling air through a 5-cm layer of morpholine
In the presence of nitrite, morpholine can be converted to NMOR
(see section 4.3). NMOR was found in the stomachs of rats that had
been fed on diets containing morpholine and nitrite (Sander et al.,
1968; Inui et al., 1979).
Immunostimulation of rats by intraperitoneal treatment with
E. coli lipopolysaccharide (LPS; 1 mg/kg) led to a large increase in
urinary nitrate and urinary metabolites of NMOR when morpholine
(80 µmol/kg) and L-arginine (400 µmol/kg) were injected
intraperitoneally. The replacement of LPS with nitrate (330 µmol/kg
intraperitoneal) did not increase urinary metabolites of NMOR (Leaf et
al., 1991). This result is consistent with endogenous nitrosation of
morpholine by nitrogen oxide (NO) from oxidation of the guanido group
of arginine by induced NO synthase (Hibbs, 1992).
Hecht & Morrison (1984) developed a method to monitor the
in vivo formation of NMOR by measuring N-nitroso
(2-hydroxyethyl)glycine, its major urinary metabolite. The formation
of NMOR was measured in F-344 rats over wide range of doses of
morpholine (38.3-0.92 µmol) and sodium nitrite (191-4.8 µmol).
According to estimates by the authors, 0.5 to 12% of the morpholine,
depending on the dose, was nitrosated.
In vitro nitrosation of morpholine has been reported. NMOR was
formed when morpholine was added to human saliva (Tannenbaum et al.,
1978). Additionally, a new type of metabolite N-cyanomorpholine was
identified in human saliva (Wishnok & Tannenbaum, 1976).
6.4 Elimination and excretion
6.4.1 Expired air
Elimination of 14C from labelled morpholine (intraperitoneal
injection) through expired air is minimal. In rats, experiments have
shown that only about 0.5% of the dose of radioactively labelled
morpholine is exhaled as 14CO2 (Sohn et al., 1982b). In rabbits
0.0008% of the administered dose was 14CO2 (Van Stee et al.,
1981).
6.4.2 Urine
Elimination studies on male Wistar rats (200-350 g) were carried
out by administering morpholine-HCl (500 mg/kg) or [14C]-labelled
morpholine-HCl (200 mg/kg) orally and morpholine-HCl (250 mg/kg)
intravenously. In all cases, over 85% of the dose was excreted in
urine within 24 h. A further portion, up to 5%, was excreted during
the next three days. [14C]-morpholine palmitate was eliminated
slightly more slowly, but the urinary excretion within 3 days
following oral administration amounted to 90% of the dose (Tanaka et
al., 1978). Of the radioactive morpholine administered to rats,
62-77.5% was excreted in the urine after 24 h (Griffiths, 1968;
Ohnishi, 1984). Following intraperitoneal administration to rats,
urinary excretion within 24 h was 87.8% of the dose (Maller &
Heidelberger, 1957). In the dog, 70-80% of the radioactive morpholine
was excreted in the urine (Rhodes & Case, 1977).
The time-course of urinary excretion of 14C by Sprague-Dawley
rats, Syrian golden hamsters, and strain II guinea-pigs treated with
[14C]-morpholine was compared by Sohn et al. (1982b). Although in
all three species over 80% was excreted in 3 days, the rate of urinary
excretion within the first 6 h was greatest in the hamster and least
in the guinea-pig.
Van Stee et al. (1981) infused rabbits intravenously with
[14C]-morpholine (5 mmol/kg) which had been neutralized with HCl.
After 4 h, 18.5% of the dose was excreted in the urine. When the pH
of the urine was lowered from 7.8-7.9 to 7.1-7.2 by administration of
ammonium chloride (10 g/litre) in drinking-water prior to the
injection, the urinary excretion more than doubled (to 43%).
These data suggest that the urinary excretion of morpholine is
enhanced by its neutralization with acid.
6.4.3 Faeces
Rats dosed orally or intravenously with morpholine hydrochloride
excreted not more than 1.7% of the dose in the faeces (Griffiths,
1968; Tanaka et al., 1978). However, when dosed orally with
morpholine palmitate (Tanaka et al., 1978, Ohnishi, 1984), up to 7%
was excreted in faeces.
6.5 Retention and turnover
Plasma concentration-time curves of 14C after intraperitoneal
injections of [14C]-morpholine (125 mg/kg in 0.9% NaCl) in Sprague-
Dawley rats, Syrian golden hamsters, and strain II guinea-pigs
declined biexponentially. Whereas rates of first phase of elimination
from plasma in rats and hamsters were similar (half-lives of 115 and
120 min, respectively), the half-life in guinea-pigs was significantly
longer (300 min) (Sohn et al., 1982b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Table 13 summarizes the toxicity data on single exposure to
morpholine.
7.1.1 Oral
Oral administration of morpholine to rats resulted in LD50
values of 1-2 g/kg body weight (Shea, 1939; Smyth et al., 1954; BASF,
1967). Gastrointestinal and nasal haemorrhage were reported. In
contrast, when morpholine was administered to seven male rats at a
neutral pH, no deaths occurred with concentrations of 1 g/kg body
weight (Börzsönyi et al., 1981).
A study on guinea-pigs resulted in a LD50 of 0.9 g morpholine
per kg body weight. Gastrointestinal and nasal haemorrhage were
reported (Shea, 1939).
7.1.2 Inhalation
There have been reports of inhalation studies with morpholine on
rats (Shea, 1939; BASF, 1967; International Labour Office, 1972;
Lam & Van Stee, 1978; Hazleton, 1981) and mice (Zaeva et al., 1968;
Lam & Van Stee, 1978).
Exposure to morpholine at vapour saturation concentrations
resulted in almost 100% lethality after 5.5 h (BASF, 1967). It had
irritating and corrosive properties.
In studies with lower concentrations, Lam & Van Stee (1978)
obtained LC50 values of 7.8 and 8.2 g/m3 for female and male rats,
respectively, whereas other authors reported no deaths at three times
this concentration (see Table 13).
Reported LC50 values for mice are consistently in the range of
5-7 g/m3 (see Table 13).
7.1.3 Dermal
Smyth et al. (1954) noted necrosis on the clipped skin of albino
rabbits within 24 h of application of undiluted morpholine. Mortality
within 14 days in New Zealand rabbits after penetration of morpholine
into the skin gave an LD50 of 0.5 ml/kg body weight.
Other reports are discussed in section 7.4.2.
7.1.4 Intraperitoneal
When morpholine was administered intraperitoneally to rats
(Stewart & Farber, 1973) and mice (BASF, 1967), the LD50 was in the
range 0.1-0.4 g/kg body weight for rats and 0.4 g/kg body weight for
mice (see Table 13).
7.2 Short-term exposure
As with single-exposure, the effects of morpholine after short-
term exposure depend on the method of exposure. The available data on
short-term exposure are summarized in Table 14.
7.2.1 Oral
Rats and guinea-pigs were fed morpholine at concentrations of
0.16-0.8 g/kg body weight and 0.09-0.45 g/kg body weight,
respectively, by gavage for 30 days (Shea, 1939). At concentrations of
half of the LD50, nearly all the animals died within 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver and necrosis of the stomach
glandular epithelium.
Fatty degeneration (lipidosis) of the liver in rats was noted
after feeding morpholine (0.5 g/kg body weight) daily for 56 days
(Sander & Bürkle, 1969).
Shibata et al. (1987a) carried out a 13-week toxicity study in
B6C3F1 mice using morpholine as the fatty acid salt, morpholine
oleic acid salt (MOAS), at dosage levels of 0%, 0.15%, 0.3%, 0.6%,
1.25% and 2.5% MOAS in the drinking-water. At the highest MOAS level,
body weight gains were slightly reduced. Histopathological analysis
showed cloudy swelling of the proximal tubules, but no other
alterations were observed in the organs of either sex. Urinalysis
showed increases in both specific gravity and plasma urea nitrogen in
some dosage groups, suggesting a possible malfunctioning of the kidney
(see Table 14).
7.2.2 Inhalation
In rats that died after 5 days repeated exposure to 65.2 g/m3,
lung haemorrhage, severe damage to the secreting tubules of the
kidney, and fatty degeneration of the liver were observed (Shea,
1939).
Table 14. Short-term exposure to morpholinea
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
Oral
Rat 20 0.16 g/kg body weight per 8/20 beginning necrosis of liver, kidney Shea (1939)
day; 30 days; in 2 ml H2O and stomach mucous membrane
(gavage)
20 0.32 g/kg body weight per 8/20 necrosis of liver, kidney, stomach
day; 30 days; in 2 ml H2O
(gavage)
20 0.8 g/kg body weight per 19/20 weight loss, lethargy, severe necrosis
day; 30 days; in 2 ml H2O of liver, kidney, stomach
(gavage)
Rat 7 f 0.5 g/kg body weight per sacrifice after 270 days, moderate Sander & Bürkle
(Sprague- day; 56 days liver adipose degeneration (1969)
Dawley)
Mouse 10 m + 10 f 0.15 and 0.3% MOAS; 91 days no significant changes Shibata et al.
(B6C3F1) (0.07 or 0.14 g morpholine/ (1987a)
kg body weight per day)
10 m + 10 f 0.6% MOAS; 91 days m: increase in specific gravity of
(ca. 0.2 g morpholine/kg urine; f: increase in blood urea
body weight) per day
10 m + 10 f 1.25% MOAS; 91 days increase in specific gravity of
(ca. 0.4 g morpholine/kg urine and blood urea
body weight per day)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
10 m + 10 f 2.5% MOAS; 91 days reduced weight gain; increase in specific Shibata et al.
(ca. 0.7 g morpholine/kg gravity of urine and blood urea; (1987a)
body weight per day) increased relative kidney weight with
swelling of proximal tubules
Guinea-pig 20 0.09 g/kg body weight 3/20 slight alterations in liver, kidney Shea (1939)
per day; 30 days (syringe)
20 0.18 g/kg body weight 12/20 necrosis of liver, kidney, stomach
per day; 30 days (syringe)
20 0.45 g/kg body weight 16/20 severe degeneration of liver, kidney,
per day; 30 days (syringe) stomach
Inhalation
Rat n.g. 7.2 g/m3; 4 h/day; 4 days n.g. increased residual volume and Takezawa &
total lung capacity Lam (1978)
Rat n.g. 65.2 g/m3; 34 h over 5 days some deathsb necrosis of liver and kidney tubules; Shea (1939)
lung irritation
Rat 6-8 m 0.08 g/m3; 4 h/day; 8 days no deaths hypersecretion of thyroid gland, Grodeckaja
higher accumulation of 131I in the & Karamzina
thyroid gland (1973)
Rat 5 m + 5 f 0.36 g/m3; 6 h/day; 9 days 0/10 red stains around nose and mouth; Hazleton
weight loss in females (1981)
5 m + 5 f 1.81 g/m3; 6 h/day; 9 days 2/10 irritation to nose and eyes; weight loss; Hazleton
decrease in spleen/brain weight ratios (1981)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
5 m + 5 f 3.62 and 18.1 g/m3; 10/10 bleeding from eyes, nose and Hazleton
6 h/day; 9 days mouth (1981)
Rat n.g. 1.63 g/m3; 4 h/day; n.g. reduced weight gain; increased residual Takezawa &
5 days/week; 30 days volume and total lung capacity Lam (1978)
Rat 20 m + 20 f 0.09 g/m3; 6 h/day; no significant differences in body Conaway et al.
(Sprague- 5 days/week; 13 weeks weight, clinical chemistry, haematology (1984b)
Dawley) or organ weight data; no nasal lesions
20 m + 20 f 0.36 g/m3; 6 h/day; in 2/20 females focal necrosis in
5 days/week; 13 weeks nasal cavity
20 m + 20 f 0.9 g/m3; 6 h/day; lesions of nose and mouth; after 7
5 days/week; 13 weeks weeks in 8 animals focal metaplasia
and necrosis in nasal turbinates; after
13 weeks, increased incidence and
severity; pneumonia
Rabbit 4 m 0.9 g/m3; 6 h/day; increased hydrolytic enzyme content Tombropoulos
5 days/week; 33 days in alveolar macrophages et al. (1983)
a adapted from BUA (1991); b.w. = body weight; n.g. = not given
b exact numbers not clear
Rats inhaling morpholine at 3.62 or 18.1 g/m3 for 9 days, 6 h
per day, died within the exposure period (Hazleton, 1981). At lower
concentrations (1.81 g/m3), weight loss and irritation to nose and
eyes, as well as two deaths, were reported. The report concluded that
the maximal tolerated dose for rats is about or just below 0.3 g/m3.
Increased thyroid activity, shown as increased uptake of injected
131I, was observed in male rats after exposure to 0.08 g
morpholine/m3, 4 h/day, for 4 days (Grodeckaja & Karamzina, 1973).
Takezawa & Lam (1978) reported an increase in lung weight,
residual volume and total lung capacity in rats exposed to morpholine
(7.2 g/m3, 4 h/day for 4 days or 1.63 g/m3 for 30 days).
Tombropoulos et al. (1983) examined the induction of lysosomal
enzymes by morpholine in rabbits. Two acid hydrolases,
alpha-mannosidase and acid phosphatase, were induced in the lung
alveolar macrophages during the course of inhalation exposure
(905 mg/m3, 250 ppm, 6 h/day, 5 days/week for a total of 33
exposures). The induction was also observed when macrophages were
cultured in the presence of morpholine.
Conaway et al. (1984b) exposed groups of 40 rats to morpholine
(0, 0.09, 0.36 and 0.9 g/m3) for 7 and 13 weeks. Shallow, rapid
breathing was noted in all groups except the controls. Lesions of the
nasal septum, anterior cavities, nasoturbinates and maxilloturbinates
were observed in the 0.36 and 0.9 g/m3 groups but not in the lowest
exposure group.
Lung sections of all rats killed after 7 weeks contained early
lesions of chronic murine pneumonia. In the upper dosage group, these
lesions had developed in severity by 13 weeks. There were no apparent
treatment-related effects in any of the haematology, clinical
chemistry or urinalysis data at weeks 7 or 13.
7.2.3 Dermal
Shea (1939) investigated the effects of single and repeated
dermal application of morpholine on rabbits and guinea-pigs. He found
that whereas unneutralized, undiluted morpholine caused 100% lethality
and even the diluted compound caused mortality and severe necrotic
burns and inflammation, application of undiluted morpholine
neutralized to pH 7 with sulfuric acid caused neither gross nor
microscopic pathology of the skin with the exception that the dermis
was thickened at the site of application.
Undiluted morpholine applied for 5-15 min to rabbit skin led to
severe necrosis (BASF, 1967).
Data on short-term dermal exposure to morpholine are also
discussed in section 7.4.2.
7.3 Long-term exposure
Table 15 summarizes the data on long-term exposure to morpholine.
7.3.1 Oral
Mice (10 males and 10 females/group) were given 0%, 0.25% or 1.0%
MOAS in their drinking-water for 96 weeks and then given normal tap
water for a further 8 weeks (Shibata et al., 1987b). During the study,
the physical appearance and general behaviour did not appear to be
affected by treatment. Decreased body weight gain was noted at 1% MOAS
(both sexes) and at 0.25% MOAS (females). Gross pathological and
extensive biochemical examinations did not reveal treatment-related
effects. The incidence of hyperplasia in forestomach epithelium of
males in the 1% group was statistically higher than in the controls,
but otherwise no significant increase in the incidence of non-
neoplastic and neoplastic lesions could be found.
7.3.2 Inhalation
Migukina (1973) reported increased nervous system activity and
increases in haemoglobin and peripheral red blood cell counts in rats
and guinea-pigs exposed to morpholine (0.008 and 0.07 g/m3) for 4
months. An increase in the chromosomal aberrations of the bone marrow
cells was also noted (see section 7.6). This study was deficient with
respect to the description of study methods.
Harbison et al. (1989) carried out an extensive long-term
exposure inhalation study. Groups of 70 rats of each sex were exposed
to morpholine (0, 0.036, 0.181, 0.543 g/m3) 6 h per day, 5 days per
week, for 104 weeks, with an interim sacrifice at week 53. Levels of
nitrates and nitrites in the drinking-water were reported to be
< 0.1 mg/litre and 0.01 mg/litre, respectively. Survival, body weight
gain, organ weight and haematology and clinical chemistry data were
normal in exposed groups, compared to the controls. In-life clinical
examinations revealed increased incidences of inflammation of the
cornea, inflammation and squamous metaplasia of the turbinate
epithelium, and necrosis of the turbinate bones in the nasal cavity of
both male and female rats. No increase in the incidence of neoplasms
was found. Only tissues from the respiratory tract and eyes were
examined histologically in the case of the mid- and low-dose groups.
7.3.3 Dermal
No data are available on long-term dermal exposure to morpholine.
Table 15. Long-term exposure to morpholinea
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Oral
Mouse 50 m 0.25% MOAS; 672 days (ca. 0.05-0.14 g no significant changes Shibata et al.
(B6C3F1)b morpholine/kg body weight per day) (1987b)
50 f 0.25% MOAS; 672 days (ca. 0.07-0.17 g decreased body weight gain
morpholine/kg body weight per day)
50 m 1% MOAS; 672 days (ca. 0.28-0.5 g decreased body weight gain; increase in blood
morpholine/kg body weight per day) urea nitrogen; hyperplasia in forestomach
epithelium
50 f 1% MOAS; 672 days (ca. 0.21-0.57 g decreased body weight gain
morpholine/kg body weight per day)
Inhalation
Rat 84 m 0.008 g/m3; 34 h within 5 days; reversible changes in haemogram, kidney, Migukina (1973)
16 weeks liver, lung, spleen, myocardium
84 m 0.07 g/m3; 4 h/day; changes in haemogram, liver, kidney
5 days/week; 16 weeks lung, spleen, myocardium
Rat 20 1.09 g/m3; 6 h/day; no significant increase in spinal cord Savolainen &
5 days/week; 15 weeks axonal succinate dehydrogenase activity; no Rosenberg (1983)
significant alteration of enzyme activity in
muscle; morpholine concentration in brain
increased with length of exposure
Table 15 (cont'd)
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Rat 70 m + 0.036 and 0.18 g/m3; 6 h/day; increased incidence of irritation around Harbison et al.
(Sprague- 70 f 5 days/week; 104 weeksc eyes and nose (1989)
Dawley)
70 m + 0.54 g/m3; 6 h/day; local necrosis around eyes and nose;
70 f 5 days/week; 104 weeksc kerativitis
Guinea-pig 24 0.008 g/m3; 4 h/day; swollen lymph nodes of the spleen Migukina (1973)
5 days/week; 16 weeks
24 0.07 g/m3; 4 h/day; swollen alveoli; atrophy of lymph vessels in
5 days/week; 16 weeks lung and spleen; increase in haemoglobin and
erythrocyte number, decrease in leucocytes
a adapted from BUA (1991); b.w. = body weight
b MOAS: morpholine administered as oleic acid salt in drinking-water; in brackets, the daily intake of morpholine
calculated from MOAS intake
c 10 m + 10 f killed at 53 weeks
7.4 Skin and eye irritation; sensitization
7.4.1. Eye irritation
Eye injury in rabbits was tested by Smyth et al. (1954), who
reported severe eye burns with 0.5 ml of a 1% solution. BASF (1967)
reported that 1 drop of undiluted morpholine in rabbit eyes, repeated
once after 5 min, caused oedema, opacity, staphyloma and corrosion of
the eye mucous membranes within 24 h.
A solution of morpholine (0.02 mol/litre) neutralized with HCl
had no injurious effect on the eyes of rabbits when applied
continuously for 10 min after removal of the corneal epithelium to
facilitate penetration. Similarly, the irritative reaction of 10-20%
aqueous morpholine was reduced on neutralization (Grant, 1974).
In-life clinical examinations in rats exposed to up to
0.54 g/m3 (150 ppm) morpholine revealed increased incidences in
inflammation of the cornea at week 103 of the study (Harbison et al.,
1989). Findings included keratitis, oedema, abrasion, scarring, and
ulceration with or without neovascularization and corneal epithelial
hyperplasia. A high incidence of retinal degeneration was observed,
primarily in female animals, which was probably an age-related light-
induced retinal degeneration.
Albino rabbits, three of each sex, were treated with a mascara
composite containing 1% morpholine; 0.1 ml of the cosmetic was
instilled into one eye of each rabbit daily for 14 days. Before the
application each day, the eye was evaluated for ocular irritation. A
slight redness of the conjunctiva was noted throughout the duration of
the study but this cleared within 24 h of the last treatment. A sodium
fluorescein dye test performed at the conclusion of the study
indicated no abnormalities of the cornea or its iris membranes
(Cosmetic Ingredient Review, 1989).
7.4.2 Skin irritation
In rabbits treated with aqueous solutions of 2, 20, 40 and 60%
morpholine, the skin reactions were evaluated after 0.5, 24, 48 and
72 h (Lodén et al., 1985). A 2% solution of morpholine caused skin
irritation after 72 h, whereas 40% and 60% solutions immediately
caused reddening of the skin.
Wang & Suskind (1988) measured the irritant potential of
morpholine by applying 0.1 g mixture (0.1, 0.5, 2, 5 and 10%
morpholine in petrolatum) to guinea-pig skin for 24 h. Observations
were made 1, 24 and 48 h after removal of the test materials, and no
noticeable effects were found.
A mascara composite containing 1% morpholine was applied to
normal and abraded skin of six albino rabbits (Cosmetic Ingredient
Review, 1989). At the end of the 14-day treatment period, no dermal
toxicity or irritation was observed.
7.4.3 Sensitization
Sensitization studies (modified Buehler) on guinea-pig skin using
2% morpholine in petrolatum gave negative results (Wang & Suskind,
1988). In an in vitro study into the biochemical causes of
sensitization, it was found that morpholine as a hapten did not react
with the amino acids glycine, lysine and cystine, as did related
compounds which showed sensitizing reactions (Wang & Tabor, 1988).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
No adequate studies on reproductive toxicity, embryotoxicity or
teratogenicity have been reported.
7.6 Mutagenicity and related end-points
7.6.1 Mutagenicity of morpholine
Table 16 summarizes the short-term mutagenicity tests on
morpholine.
7.6.1.1 Bacteria
No mutagenic response was observed in plate tests with strains of
Salmonella typhimurium, either with or without metabolic activator,
exposed to morpholine at up to 10 µl/plate (approximately 10 mg/plate)
(Texaco, 1979a; Haworth et al., 1983), whereas a 99.8% purity sample
did induce weak mutagenic responses in S. typhimurium TA100 and
Escherichia coli WP2 uvr A at the unusually high dose level of
50 mg/plate (Glatt & Oesch, 1981). The activity in strain TA100 did
not require S9 mix and is, therefore, unlikely to have been due to
0.2% contamination by NMOR (see below).
In mouse peritoneal cavity host-mediated assays with
S. typhimurium TA1530 and TA1930, no increase in the proportion of
mutants was observed following the oral administration of morpholine
(Braun et al., 1977; Edwards et al., 1979). These groups served as
controls in a study of nitrosation (see below).
7.6.1.2 Yeast
No gene conversion was induced in Saccharomyces cerevisiae D4
by morpholine concentrations up to 10 µg/litre per plate. Toxicity
was observed at the highest concentration (Texaco, 1979a).
Table 16. Short-term in vitro microbial mutagenicity assays with morpholinea
Test organisms Strain Analyzed Metabolic Dosage range Result with/ Reference
purity (%) activityb without S9-mix
Salmonella typhimurium TA98/100/1535/1537 not given RA 0.109-10.9 mg/plate -/- Haworth et al. (1983)
S. typhimurium TA98/100/1535/1537 not given RA 0.005-10 µl/plate -/- Texaco (1979a)
1538c
Saccharomyces D4 not given RA 0.005-10 µl/platec -/- Texaco (1979a)
cerevisiae
S. typhimurium TA98/1535/1537 99.8 RA 0.0158-50 mg/plate -/- Glatt & Oesch (1981)
S. typhimurium TA100 99.8 RA 0.0158-50 mg/plate (+)/(+)d Glatt & Oesch (1981)
Escherichia coli WP2 uvrA 99.8 RA 0.0158-50 mg/plate (+)d/- Glatt & Oesch (1981)
S. typhimurium TA1950 99 HM1 1450-2900 µmol/kg - Braun et al. (1977)
S. typhimurium TA1530 not given HM2 4 mg/kg body weight - Edwards et al. (1979)
a adapted from BUA (1991)
b RA = Arochlor-induced rat liver-S9; HM1 = Host-mediated assay NMRI-mice; HM2 = Host-mediated assay CD-1 mice
c toxic at 10 µl/plate for strain Salmonella typhimurium TA1538 and Saccharomyces cerevisiae D4
d (+) = positive only at the 50 mg/plate dose
7.6.1.3 Mammalian cells in vitro
When tested over the range 0.625-1.25 µl/ml (approximately
0.625-1.25 mg/ml) morpholine induced small increases in the fraction
of tk mutants in mouse lymphoma L5178Y cells. No exogenous metabolic
activation system was required for this weak activity (Texaco, 1979b;
Conaway et al., 1982a,b).
Morpholine induced no DNA-repair in the primary cultures of rat
hepatocytes (0.1-100 µg/ml) (Conaway et al., 1984a).
Morpholine induced small increases in the frequency of SCEs in
Chinese hamster ovary (CHO) cells. No exogenous metabolic activation
system was required for this activity, which was observed at
concentrations of 50 and 100 nl/ml (approximately 50 and 100 µg/ml) in
the absence of S9 mix (Litton Bionetics, 1980).
Morpholine increased the numbers of type III foci in the
Balb/C3T3 cell transformation assay tested at different dosages
(0.001-0.3 µl/ml), corresponding to 78-52% survival in the
cytotoxicity test (Texaco, 1979b, Litton Bionetics, 1979a,b; Conaway
et al., 1982a,b).
In another study of in vitro transformation of Balb/3T3 cells
with and without metabolic activation, the morpholine in the culture
medium was neutralized before testing (Litton Bionetics, 1982). No
significant increases were induced in transformed foci over the tested
concentration ranges (0.015 to 1.400 µl/ml in the non-activation
assay; 0.0175-0.7 µl/ml in the activation assay). Morpholine was
therefore considered as being inactive in this test.
7.6.1.4 In vivo studies in mammals
Inui et al. (1979) administered a dosage of 500 mg morpholine
per kg body weight to female Syrian hamsters on the 11th or 12th day
of pregnancy. The embryos were removed 24 h later and embryo cells
examined. For detection of induced mutations, embryo cells were
cultured in normal medium for 72 h and then transferred to a medium
containing 8-azaguanine (10 or 20 mg per litre) or ouabain (1 mM). No
chromosomal aberrations, micro-nucleus formation, or 8-azaguanine- or
ouabain-resistant mutations were found.
Migukina (1973) reported an increase in the number of chromosomal
aberrations, particularly "fragmentations", in bone marrow cells of
rats and guinea-pigs exposed for 4 months to 8 or 70 mg
morpholine/m3 (see also section 7.3.2). However, there were
deficiencies in the study report (BUA, 1991).
7.6.2 Mutagenicity of morpholine in the presence of nitrite and
nitrate
Although morpholine has given negative responses in a number of
mutagenicity assays, there is concern for its ability to be nitrosated
readily to form NMOR (see section 4.3).
Two mouse peritoneal cavity host-mediated assays with
S. typhimurium have been performed. In one, with strain TA1530,
different morpholine doses were administered orally to mice
simultaneously with a standard sodium nitrite dose of 120 mg/kg. The
mixtures were adjusted to pH 3.4. Significant increases in the mutant
fraction were observed with morpholine doses of 4-40 mg/kg, the full
range tested (Edwards et al., 1979). In the other assay, with strain
TA1950, equimolar mixtures of morpholine and sodium nitrite (1450 or
2900 µmol/kg, approximately 125 or 250 mg/kg) were administered orally
to mice (pH 7.0). Significant increases in the mutant fraction were
observed at both dose levels. When the nitrite was administered to the
mice 10 min before the morpholine treatment, no mutagenic response was
induced (Braun et al., 1977).
Mutations were found in S. typhimurium TA1535 used to test the
urine of OF1 mice after morpholine (250 mg/kg body weight) had been
administered in the presence of nitrate (2000 mg/kg). Lower levels of
nitrate (333 and 666 mg/kg) caused no mutations (Perez et al., 1990).
7.6.3 Mutagenicity of N-nitrosomorpholine
NMOR is mutagenic to S. typhimurium TA100 in the presence of S9
mix at dose levels above 2000 µg/plate. In yeast, it induced forward
mutation and aneuploidy, but not mitotic recombination. In cultured
mammalian cells, conflicting results were obtained in unscheduled DNA
synthesis (UDS) assays in fibroblasts, while weakly positive results
were reported for sister-chromatid exchange (SCE) in CHO cells, for tk
locus mutation in mouse lymphoma L5178Y cells, and for enhanced colony
growth of BHK cells in soft agar. NMOR was negative in an in vitro
cytogenetic assay using RL1 (rat liver cell line) cells (IARC, 1978;
NRC, 1981).
7.7 Carcinogenicity
Carcinogenicity studies have been carried out with morpholine
alone, as well as in the presence of nitrite, to investigate the
possible formation and effect of NMOR.
7.7.1 Morpholine
7.7.1.1 Oral studies
Greenblatt et al. (1971) treated 40 Swiss mice (20 male and
20 female) with 6.33 g morpholine/kg food (estimated dosage, 0.9 g/kg
body weight per day for 28 weeks. The control group consisted of
80 mice of each sex. After a further 12 weeks of observation the
surviving animals were sacrificed. No increase in the lung tumour rate
(0.1 adenoma/mouse) was found compared to the controls
(0.18 adenoma/mouse). It should be noted that the duration of exposure
in this study was shorter than that normally used in a well-designed
long-term carcinogenicity study.
Multi-generation oral studies were performed on Sprague-Dawley
rats fed 5, 50 or 1000 mg/kg morpholine, together with various dietary
concentrations of sodium nitrite (0, 5, 50 or 1000 mg/kg diet)
(Newberne & Shank, 1973; Shank & Newberne, 1976). From the day of
conception, the pregnant animals were given 0 or 1000 mg morpholine/kg
feed. The F1 and F2 generations were fed likewise for the length
of the experiment. The estimated dosage for the young animals was
10 mg/day and for mature animals 20 mg/day. The average life-span was
117 weeks for the treated animals and 109 weeks for the controls. F1
and F2 generations were studied, the survivors being sacrificed in
the 125th week. Table 17 summarizes the results. Three liver cell
carcinomas, two lung angiosarcomas and one other, and two malignant
gliomas were found in the group of 104 rats (F1 and F2) treated
with morpholine.
In a similar study using Syrian golden hamsters, only the F1
generation was studied, the survivors being sacrificed in the 110th
week (Shank & Newberne. 1976). With morpholine alone (1000 mg per kg),
no liver tumours were found but the number in the group (22) was
small.
Morpholine oleic acid salt (MOAS), at dosage levels of 0%, 0.25%
or 1.0%, was added to the drinking-water of B6C3F1 mice for 96
weeks, and this was followed by normal tap water for a further 8 weeks
(Shibata et al., 1987b). Details are given in Table 15 and section
7.3.1. Extensive biochemical, gross pathological and histological
studies were performed. Only the incidence of hyperplasia in
forestomach epithelium in the males of the 1% MOAS group was
statistically higher than in the controls; otherwise, no significant
increases in the incidence of non-neoplastic or neoplastic lesions
were found.
7.7.1.2 Inhalation studies
In a long-term inhalation study over 2 years, Sprague-Dawley rats
(70 of each sex per group) received morpholine at mean exposure
concentrations of 0, 0.036, 0.181 or 0.543 g/m3 (6 h/day,
5 days/week) for up to 104 weeks (Harbison et al., 1989). Further
details are given in section 7.3.2 and Table 15. Tissues from the
high-dose and control groups were subjected to extensive
Table 17. Tumour incidence (liver and lung) in rats and hamsters after feeding morpholine, sodium nitrite or N-nitrosomorpholinea
Dietary levels (mg/kg) Tumour incidence in rats (%) Tumour incidence in hamsters (%)
Morpholine Sodium N-Nitrosomorpholine No. of Liver cell Liver angio Lung angio No. of Liver cell
nitrite animalsb carcinoma sarcoma sarcoma animals carcinoma
0 0 0 156 0 0 0 23 4
0 1000 0 96 1 0 0 30 0
1000 0 0 104 3 0 2 22 0
1000 1000 0 159 97 14 23 16 31
50 1000 0 117 59 5 6 32 0
5 1000 0 154 28 12 8 400
1000 50 0 109 3 2 1 22 0
1000 5 0 172 1 2 1 19 0
50 50 0 152 2 1 1 30 0
5 5 0 125 1 2 2 40 0
0 0 5 128 58 15 9 35 0
0 0 50 94 93 21 20 18 6
a adapted from Shank & Newberne (1976)
b F1 and F2 generations together
histopathological examination; in the middle- and low-dose groups,
this examination was limited to the eye and the respiratory tract.
Ten males and 10 females per group were sacrificed at week 53.
Survival at termination in the control, low-, middle- and high-dose
groups, respectively, was 40, 44, 33 and 32 in males and 35, 27, 32
and 35 in females. No significant increase in the incidence of
tumours was seen in rats of either sex.
7.7.2 Morpholine and nitrite
7.7.2.1 Oral studies
Sander & Bürkle (1969) fed a group of seven female Sprague-Dawley
rats 5 g morpholine together with 5 g nitrite/kg diet for 12 weeks.
After 39 weeks, all of the animals developed hepato-cellular adenomas,
six out of the seven hepatocellular carcinomas, two
hemangioendotheliomas of the liver, one a cyst-adenocarcinoma of the
liver and one a renal adenoma. Rats fed morpholine or nitrite alone
did not develop tumours.
As described in section 7.7.1.1, Shank & Newberne (1976) fed
pregnant rats on a diet containing various concentrations of
morpholine, sodium nitrite and NMOR. Table 17 summarizes the dietary
concentrations and the tumour incidences for the experimental groups.
Hepatocellular carcinoma and haemangio-sarcomas of the liver and
angiosarcoma of the lungs were the most common tumours observed in the
rats. The neoplasms induced by nitrite and morpholine were
morphologically similar to those induced by NMOR. High concentrations
(1000 mg/kg) of morpholine and nitrite together were carcinogenic to
rats. When the morpholine concentration was reduced and the nitrite
concentration remained high, the incidence of hepatic cell carcinomas
decreased with a linear dose-response relationship. When morpholine
concentration remained high, with decreasing nitrite concentration,
the number of hepatic tumours was sharply reduced. This agrees with
the observation in vitro that the nitrosation of morpholine depends
on the square of the nitrite concentration (Mirvish et al., 1975).
Groups of 40 male MRC Wistar rats were treated for 2 years with
either 10 g morpholine/kg diet and drinking-water containing 3 g
sodium nitrite/litre, or with drinking-water containing 0.15 g
NMOR/litre. In both cases, one group of rats was also given sodium
ascorbate (22.7 g/kg diet). The results of treatment with morpholine
plus nitrite or with NMOR were similar to those reported above. When
ascorbate was present, the liver tumours induced by morpholine plus
nitrite had a longer induction period (93 versus 54 weeks) and a
slightly lower incidence (49% versus 65%). However, ascorbate did not
affect liver tumour induction by preformed NMOR. Of those treated
with morpholine, nitrite and ascorbate, 21/39 animals developed
forestomach tumours (Mirvish et al. 1976).
In a similar study using Syrian hamsters (see section 7.7.1.1 and
Table 17), a high morpholine, high nitrite diet (1000 mg/kg) induced
liver cancer in some animals. In contrast, hamsters fed NMOR in the
diet seemed to have greater resistance to tumour induction (Shank &
Newberne, 1976).
7.7.3 Carcinogenicity of N-nitrosomorpholine
NMOR has been shown to be carcinogenic in mice, rats, hamsters
and various fish. Benign and malignant tumours of the liver and lung
in mice, of the liver, kidney and blood vessels in rats, and of the
liver in hamsters have been reported following oral administration of
NMOR. After its subcutaneous injection, it produces tumours of the
upper digestive and respiratory tract in hamsters. NMOR produces liver
tumours following its intravenous injection in rats and liver tumours
in various fish following its administration in tank water (IARC,
1978).
7.8 Factors modifying toxicity; toxicity of metabolites
7.8.1 Factors modifying toxicity
Leaf et al. (1991) reported that rats (Sprague-Dawley, male)
challenged with an E. coli lipopolysaccharide (LPS; 1 mg/kg body
weight), followed 6 and 10 h later by morpholine (80 or 100 µmol/kg
intraperitoneally) and arginine (400 µmol/kg) treatment, showed
significantly increased NMOR formation compared with unchallenged
control rats. The NMOR formation was measured by monitoring the NMOR
metabolite N-nitroso-(2-hydroxyethyl) glycine in the urine. Furman
& Rubenchik (1991) showed that endogenous synthesis of NMOR in the
stomach of adult mice administered nitrite (oral) in combination with
morpholine (intraperitoneal or subcutaneous) was increased after
activation of peritoneal macrophages induced by intraperitoneal
injection of E. coli LPS.
7.8.2 Morpholine metabolites
Morpholine is eliminated almost entirely in a non-metabolized
form in the urine of the rat, mouse, hamster and rabbit (section 6.3).
N-cyanomorpholine ( N-morpholinocarbonitrile) was formed when
morpholine was incubated in vitro with whole human saliva (Wishnok &
Tannenbaum, 1976).
Sohn et al. (1982a,b) identified a metabolite
N-methylmorpholine- N-oxide (NMMO) in guinea-pig urine.
Conaway et al. (1984a) reported that NMMO (0.0001-10 mg/ml) and
other putative metabolites, N-hydroxymorpholine (0.0001-1 mg/ml) and
3-morpholinone (0.001-0.1 mg/ml), did not induce DNA repair at the
non-toxic concentrations tested. The polyurethane foam catalyst,
N-butylmorpholine (0.0001-0.1 mg/ml) was also inactive in the
primary rat hepatocyte/DNA repair assay (Conaway et al., 1984a).
The chemical intermediate N-hydroxyethylmorpholine induced DNA
repair within the dose range 1-5 mg/ml (Conaway et al., 1984a).
The genotoxicity of substituted morpholines might be a function
of the substituent moiety rather than morpholine itself (Conaway et
al., 1984a).
7.9 Mechanisms of toxicity - mode of action
The irritating and corrosive properties of morpholine are due to
its basicity. The mechanism of action of its systemic effects is not
known.
Morpholine fungicides have been shown to act by inhibiting
several enzymes of sterol biosynthesis (Hesselink et al. 1990; Mercer,
1991).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available on the effects of short- and long-term
exposure to morpholine on the general population. There are no
reports of poisonings by morpholine.
8.1.1 Controlled human studies
The Cosmetic Ingredient Review (1989, 1991b) reported unpublished
studies of occlusive patch testing and in-use testing of two mascara
products containing 1% morpholine by 320 women between the ages of 18
and 65. All 320 volunteer subjects underwent occlusive patch testing
(using the Shelanski/Jordan repeat insult procedure). After 24 h, the
patches were removed and the sites graded. This procedure was repeated
several times for a total of 10 applications (3´ weeks). Following the
10th application, there was a 10-day to 2-week rest period, after
which the subjects were again patch tested, this time for 48 h, and
then scored. After another 10-day to 2-week rest period, the subjects
were again patch-tested for 48 h and scored. A final reading took
place 24 h after this. Of the 320 subjects patch-tested with charcoal
mascara, 314 showed no reaction, the remaining six showed varying
reactions. The researchers explained this irritation as being either
non-specific or due to the occlusive patching procedure. The same
results were found with the blue mascara. It was concluded from this
study that neither of the mascara products containing 1% morpholine
was a primary irritant, nor were they contact sensitizers (Cosmetic
Ingredient Review, 1989, 1991b).
In the in-use testing, 50 women used charcoal mascara once daily
for 4 weeks, and 50 women used blue mascara likewise. A further 100
women used once daily lash conditioner, eye make-up remover and
mascara (50 for each colour). In another group, 100 subjects used
lash conditioner and eye make-up remover but no mascara. All underwent
the above-described patch testing simultaneously.
There were a few complaints associated with the in-use testing.
The charcoal mascara caused a mild burning sensation in three
subjects, some itching in a further three, and minor irritation in
three. These findings were put down to improper use of the product,
which resulted in the product entering the eye and causing mild
irritation (Cosmetic Ingredient Review, 1989, 1991b).
Shea (1939) exposed himself to 43 g morpholine/m3 (12 000 ppm)
for 90 seconds. He experienced irritation of the nose followed by
coughing. Pipetting of pure morpholine from the stock solution led to
inhalation of vapour, a severe sore throat, and violently reddened
mucous membrane.
Shea (1939) reported that when applied to human finger tips
undiluted morpholine caused a cracking of the eponychium and
hyponychium about the nail and an intense stinging sensation. Diluted
morpholine (1 to 40) was a mild irritant.
8.1.1.1 Organoleptic effects
Hellman & Small (1974) tested the absolute and recognition
threshold odour index of 101 petrochemicals using a trained panel.
During a 20-min exposure, 0.036 mg morpholine/m3 was recognized by
50% of the panel and 0.5 mg morpholine/m3 by all of the panel
(giving an odour index of 65.9). It was described as an unpleasant
fishy smell.
The phenomenon known as blue vision, gray vision or haloes
"glaucopsia" is a well-documented effect of amines, including
morpholine and its derivatives, on the eyes of workers, particularly
in the foam plastic industry (Mastromatteo, 1965; Jones & Kipling,
1972). The vision becomes misty and haloes appear several hours after
the subjects have been exposed to these vapours at concentrations too
low to cause discomfort or disability during several hours of
exposure. The disturbances lasted for 4-6 h after leaving work. In a
minority of the workers examined, mild conjunctival infection was
observed; no corneal oedema or alteration in visual acuity was
detected by inspection or by ophthalmoscopy. The atmospheric
concentrations of morpholine and other similar compounds were not
reported.
8.1.2 Epidemiological studies
No data from epidemiological studies for morpholine have been
reported.
8.2 Occupational exposure
Studies into the levels of morpholine and NMOR in workplace air
are described in section 5.3.
Katosova et al. (1991) reported a cytogenetic analysis of the
lymphocytes in the peripheral blood of 24 workers (16 men, 8 women)
with 3 to 10 years of exposure to morpholine during production. These
were compared to a control group of the same size from the same town
having no contact with chemicals at work. The morpholine workers were
exposed to concentrations of morpholine in air of 0.54-0.93 mg/m3,
the maximum single concentration being 0.74-2.14 mg/m3. The
lymphocytes were cultivated for 56 h and all chromatid and chromosome
types as well as chromosome gaps were registered. From 2561 cells
analysed from the test group, only 2.08% had aberrations, compared to
1.61% out of 2050 cells in the control group, showing no significant
increase in the number of cells with chromosome aberrations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The data reported in this section refer to nominal
concentrations.
9.1.1 Microorganisms
9.1.1.1 Microorganisms in water
a) Bacterial and cyanobacterial cultures
In a 16-h cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 25°C), the toxicity threshold (3% reduction in
population growth) of morpholine for Pseudomonas putida was
310 mg/litre (Bringmann & Kühn, 1977a).
The effect of morpholine at neutral pH on the growth of
Pseudomonas strains in succinate mineral salt medium has been
reported by Knapp et al. (1982) and Emtiazi & Knapp (1994). Both
studies used two strains of Pseudomonas (all four were different)
which had not previously been exposed to morpholine and found that
morpholine at 8.7 g/litre had no effect on the rate or extent of
growth.
The mixed bacterial cultures studied by Mazure (1993) were able
to degrade morpholine rapidly at 5, 10 and 20 g/litre. The
degradation rate was highest at 10 g/litre but was only a little less
at 20 g/litre. At 40 g/litre degradation was markedly inhibited.
As reported in section 4.2.1, morpholine at up to 870 mg/litre
does not inhibit certain strains of Mycobacterium spp. that have
been found to biodegrade the compound.
In a cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 192 h) under continual lighting conditions using
Microcystis aeruginosa, a cyanobacterium, the onset of inhibition,
defined variously as a 1% (Bringmann, 1975) or 3% (Bringmann & Kühn,
1978) reduction in population growth, was found to occur at a
morpholine concentration of 1.7 mg/litre.
b) Activated sludge
The effect of morpholine on the respiratory, dehydrogenase and
nitrification activities of activated sludge was determined by
Strotmann et al. (1993). Short-term respiration assays (pH 7.0;
incubation time 30 min) were performed according to the OECD guideline
209 (OECD, 1984). The dehydrogenase activity was determined by
measuring the reduction of resazurin to resorufin. At concentrations
of 1000 mg morpholine/litre, both respiratory and dehydrogenase
activities were inhibited by up to 20%. A pH-dependent inhibitory
effect on the dehydrogenase activity was not found (pH 6.0-7.5).
Nitrification activity was determined using a specially adapted
culture of nitrifying bacteria. Suspended biomass, previously
inoculated with activated sludge and acclimated to a high-strength
ammonium feed, was incubated for 30 min at pH 7.2 with different
amounts of morpholine. The extent of inhibition was based on the
reduction in oxygen uptake rate, compared to a control containing no
morpholine. Nitrification activity was inhibited by 5% at a
morpholine concentration of 5%.
c) Protozoa
The toxicity thresholds (defined as a 5% reduction in population
growth) of morpholine for the protozoa Entosiphon sulcatum
(Bringmann, 1978), Uronema parduczi and Chilomonas paramaecium
(Bringmann & Kühn (1980), as measured in cell multiplication
inhibition tests under given conditions, are summarized in Table 18.
9.1.1.2 Microorganisms in soil
No data are available concerning soil bacteria or fungi.
9.1.1.3 Pathogenic microorganisms
Morpholine, like other amines, shows antibacterial and anti-
mycotic action. Kubis et al. (1983) demonstrated that 0.5% morpholine
inhibits the growth of a variety of pathogenic bacteria on agar plates
and in liquid medium. Application of 10% morpholine was shown to cure
an experimental mycosis in guinea-pigs caused by Trychophyton
mentagrophytes var. granulosum (Kubis et al., 1981). The pH was not
specified.
9.1.2 Other aquatic organisms
9.1.2.1 Monocellular green algae
Table 19 summarizes the tests carried out concerning the effect
of morpholine on algae.
In a 192-h test, the onset of inhibition by morpholine
(in distilled water, pH 7.0) of cell multiplication (here defined as
3% in population growth) in the green alga Scenedesmus quadricauda
in a turbidity test was noted at a concentration of 4.1 mg/litre
(Bringmann & Kühn, 1977a, 1978; see parallel results test with
Microcystis aeruginosa, section 9.1.1.1a).
Table 18. Toxicity threshold (TT) of morpholine for protozoa in the cell multiplication
inhibition test (growth factor: cell number)
Species Test duration pH Temperature TT Reference
(h) (°C) (mg/litre)
Entosiphon sulcatuma 72 6.9 25 12 Bringmann (1978)
Uronema parduczia 20 6.9 25 815 Bringmann & Kühn (1980)
Chilomonas paramaeciumb 48 6.9 20 18 Bringmann et al. (1980)
a bacteriovorous ciliate
b saprozoic flagellate
The results of Adams et al. (1985) showed that Selenastrum
capricornutum grows exponentially without a lag phase in the
presence as well as in the absence of morpholine, the growth being
inhibited first at a concentration of 100 mg/litre. The inhibition was
most marked after 6 days, shortly before reaching the stationary
phase; the no-observed-effect level (NOEL) was 10 mg/litre. Using the
same species, Calamari et al. (1980) evaluated algal growth by
measuring in vivo the fluorometric units at 48, 72, 96 h and 7 days.
A 96-h EC50 of 28 mg/litre was determined.
Millington et al. (1988) investigated the effects of varying
growth medium composition on the toxicity of morpholine to three
freshwater green algae: Selenastrum capricornutum, Scenedesmus
subspicatus and Chlorella vulgaris. They reported that the toxic
effect of morpholine on algae depended upon the species as well as
upon the test medium used (see Table 19).
9.1.2.2 Invertebrates
Table 20 summarizes the results of three investigations into the
toxicity of morpholine, as measured by the immobilization of Daphnia
magna (Bringmann & Kühn, 1977b, 1982; Calamari et al., 1980). The
test conditions were almost identical (pH 7.6-8.0; 18-22°C) and the
resulting EC50 values (100-119 mg/litre) were in good agreement.
Kramer et al. (1983) investigated the relative toxicity of
organic solvents including morpholine to mosquito (Aedes aegypti)
larvae (2 to 3 instar) by exposing 10-20 of them to 50 ml of test
solution in a glass container for 4 h at 22-24°C. The test was run in
triplicate. An LC50 of 1000 mg/litre was reported, together with
the observation that death was accompanied by convulsive larval
twitching.
9.1.2.3 Vertebrates
The acute toxicity of morpholine for fish has been tested on a
number of fresh, sea and brackish water species (see Table 21). The
lowest LC50 (180 mg/litre) was found for rainbow trout
(Oncorhynchus mykiss; formerly Salmo gairdneri) in very soft water
(20 mg CaCO3/litre). In hard water (320 mg CaCO3/litre), the
poisoning effect, with reference to the LC50 value, was less than
half (Calamari et al., 1980). Brachydanio rerio proved to be
relatively insensitive (LC0 value > 1000 mg/litre) (Wellens, 1982).
LC50 values for the freshwater fish Leuciscus idus melanotus and
Lepomis macrochirus were found to be 240-285 mg/litre (48 h) and
350 mg/litre (96 h), respectively (Juhnke & Lüdemann, 1978; Dawson et
al., 1977). The 96-h LC50 for the marine fish Menidia beryllina
was reported to be 400 mg/litre (Dawson et al., 1977). McCain & Peck
(1976) investigated the effect of morpholine on common Hawaiian fish.
Table 19. Toxicity of morpholine for algaea
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Scenedesmus inhibition of cell 192 neutral 27 TT: 4.1 Bringmann & Kühn (1978)
quadricauda multiplication (turbidity)
Selenastrum cell number after: 24 not given 22 NOEC: 100 Adams et al. (1985)
capricornutum 48/72 NOEC: 80
96/120 NOEC: 50
144 NOEC: 10
area under 24-72 NOEC: 80
growth curve 24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 50
growth rate 24-72 NOEC: 80
24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 80
S. capricornutum growth rate (in vivo 96 not given 24 EC0: 10c Calamari et al. (1980)d
fluorescence) EC50: 28c
EC100: 80c
S. capricornutum area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 50f
ECL0: 50g
Scenedesmus area under growth 24-120 not given 22 ECL0: 50e Millington et al. (1988)
subspicatus curve (cell number) ECL0: 5f
ECL0: 10g
Table 19 (cont'd)
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Chlorella vulgaris area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 80f
ECL0: 5g
a Adapted from BUA (1991)
b TT = toxicity threshold; NOEC = no-observed-effect concentration; EC0, EC50, EC100 = effective concentration inhibiting for the growth
of 0, 50 and 100% of the population; ECL0 = lowest-tested concentration with significant growth inhibition
(from 5, 10, 50, 80, 100 mg/litre)
c equivalent to GC-FID measured concentration ± 10%
d method according to Chiaudini & Vighi (1978)
e Bolds basal medium (BBM): rich medium; N/P-ratio 14:1
f OECD medium: less N than BBM; N/P-ratio 24:1
g EPA medium: less N than BBM; N/P-ratio 50:1
Table 20. Acute toxicity of morpholine for the water flea Daphnia magna
(test duration: 24 h; effect: immobilization)a
Test systemb pH Temperature Effect/concentration Reference
(°C) (mg/litre)
Static 7.6-7.7 20-22 EC0: 16 Bringmann &
EC50: 100 Kühn (1977b)
EC100: 500
Static 8 ± 0.2 20 EC0: 68 Bringmann &
EC50: 101 (83-122)c Kühn (1982)
EC100: 260
Static 7.9 ± 0.3 18-22 EC50: 119 (112-127)c Calamari et
al. (1980)
a adapted from BUA (1991)
b in accordance with OECD-guideline 202 part I
c 95% confidence limits
The 96-h LC50 (TLm) was between 100 and 180 mg/litre for white
mullet (Vala mugil engeli; former designation: Chelon engeli),
between 320 and 560 mg/litre for Gambusia affinis, and more than
1000 mg/litre for Tilapia sp.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Reynolds (1989) found that 4.4±0.9 mol morpholine/m3
(383 mg/litre) lowers the percentage germination of lettuce seeds
(Lactuca sativa) by 50% of the control value. The seeds were kept on
agar for 3 days in sealed plastic containers at a temperature of 30°C.
9.1.3.2 Animals
No data are available concerning the effects of morpholine on
terrestrial animals.
9.2 Field observations
No data have been reported concerning field observations
involving morpholine.
Table 21. Acute toxicity of morpholine in fish static test systemsa
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
Rainbow trout freshwater hardness: 320 mg CaCO3/litre; pH 7.4; 96 LC50 380 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (375-460)b (1980)
Rainbow trout freshwater hardness: 20 mg CaCO3/litre; pH 7.4; 96 LC50 180 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (1980)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 285 Juhnke &
(Leuciscus idus continual aeration (Juhnke laboratory) (250/340)d Lüdemann
melanotus) (1978)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 240 Juhnke &
(L. idus melanotus) continual aeration (Lüdemann laboratory) (100/301)d Lüdemann (1978)
Zebra fish freshwater hardness: 250 mg CaCO3/litre; pH 7.5; 22°C; 96 LC50> 1000 Wellens (1982)
(Brachydanio rerio) no aeration (> 1000/> 1000)d
Bluegill sunfish freshwater hardness: 55 mg CaCO3/litre; pH 7.6-7.9; 23°C; 96 LC50 350 Dawson et
(Lepomis macrochirus) intermittent aeration 24 h after test; from al. (1977)
commercial hatcheries; 14 days acclimatization
Tidewater silverside marine artificial seawater; 20°C; continual aeration; 96 LC50 400 Dawson et
(Menidia beryllina) caught wild Horseshoe Bay (New Jersey); al. (1977)
40-100 mm length; 14 days acclimatization
Mosquito fish marinee,f natural seawater (32% salinity); continual 96 LC0 320 McCain &
(Gambusia affinis) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 560 Peck (1976)
Hawaii); 38-64 mm length; 7 days
acclimatization
Table 21 (cont'd)
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
"Coloured perch" marinee,f natural seawater (32% salinity); continual 96 LC0 1000 McCain &
(Tilapia sp.) aeration; 25.5-27.8°C; caught wild (Oahu, Peck (1976)
Hawaii); 30-65 mm length; 7 days
acclimatization
White mullet marinee natural seawater (32% salinity); continual 96 LC0 100 McCain &
(Chelon engeli) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 320 Peck (1976)
Hawaii); 91-128 mm length; 7 days
acclimatization
a adapted from BUA (1991)
b 95% confidence limits
c parallel tests in two different laboratories
d LC0/LC100
e the fish were caught in rivers, presumably in the estuary area
f fresh or brackish water fish that were exposed to the marine system.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks were evaluated by an International Agency
for Research on Cancer ad hoc Expert Group in 1988. It was
concluded that there is inadequate evidence for the carcinogenicity
of morpholine in experimental animals. No data were available from
studies in humans on the carcinogenicity of morpholine. The overall
evaluation was that morpholine was not classifiable for its
carcinogenicity to humans (IARC, 1989).
IARC also evaluated N-nitrosomorpholine (NMOR). It concluded
that there is sufficient evidence for a carcinogenic effect of NMOR in
several experimental animal species. No human data, case reports or
epidemiological studies were available, nor was it possible to
identify exposed groups. In spite of the absence of epidemiological
data, NMOR should be regarded for practical purposes as if it were
carcinogenic to humans (IARC, 1978).
REFERENCES
Aarts AJ, Benson GB, Duchateau NL, & Davies KM (1990) Modern
analytical techniques for the detection of rubber chemicals and their
decomposition products in the environment. Int J Environ Anal Chem,
38: 85-95.
Ackermann P, Margot P, & Klotzsche, C (1989) Agricultural fungicides.
In: Ullmann's encyclopedia of industrial chemistry, 5th ed. Weinheim,
VCH Verlagsgesellschaft, vol A12, p 107.
Adams N, Goulding KH, & Dobbs AJ (1985) Toxicity of eight water-
soluble organic chemicals to Selenastrum capricornutum: a study of
methods for calculating toxic values using different growth
parameters. Arch Environ Contam Toxicol, 14: 333-345.
Agarwala KK (1982) Study on stability of morpholine in high pressure
boiler system. Fertil Technol, 19: 73-75.
Air Products and Chemicals (1989) Material safety data sheet.
Allentown, Pennsylvania, Air Products and Chemicals, Inc.
Aitzetmüller K & Thiele E (1982) Absence of volatile N-nitrosamines in
margarines. J Am Oil Chem Soc, 59: 387-389.
Anderson GE (1983) Human exposure to atmospheric concentrations of
selected chemicals. Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Quality Planning and
Standards, pp 455-473 (Report submitted by Systems Applications, Inc.,
San Rafael, California) (EPA No 83-265249).
Archer MC, Tannenbaum SR, & Wishnok JS (1977) Nitrosamine formation in
the presence of carbonyl compounds. In: Walker EA, Bogovski P, &
Griciute L ed. Environmental N-nitroso compounds analysis and
formation. Lyon, International Agency for Research on Cancer,
pp 141-145 (IARC Scientific Publications No. 14).
Archer MC, Yang HS, & Okun JD (1979) Acceleration of nitrosamine
formation at pH 3.5 by microorganisms. In: Walker EA, Castegnaro M,
Griciute L, & Lyle RE ed. Environmental aspects of N-nitroso
compounds. Lyon, International Agency for Research on Cancer,
pp 239-246 (IARC Scientific Publications No. 19).
Atkinson R (1988) Estimation of gas-phase hydroxyl radical rate
constants for organic chemicals. Environ Toxicol Chem, 7: 435-442.
Badura R, Linde H, & Schuster RH (1989) Analysis of volatile products
in vulcanisation fumes. In: Proceedings of the International Rubber
Conference, Prague, 8-11 May. London, International Rubber
Association, pp 19-38.
BASF (1967) [Morpholine: Toxicological data.] Ludwigshafen, BASF AG,
2 pp (Internal report) (in German).
BASF (1987) [Data sheets: Morpholine.] Ludwigshafen, BASF AG, 8 pp
(in German).
BASF (1990) [Test report: Zahn-Wellens-Test.] Ludwigshafen, BASF AG,
12 pp (Unpublished report) (in German).
BIA (Industries Association Institute for Work Safety) (1989)
[Measurement of dangerous substances - BIA data sheet.] Bielefeld,
Erich Schmidt Verlag (in German).
Bianchi A & Muccioli G (1978) [Determination of morpholine together
with isopropyl alcohol, toluene and xylenes (o-m-p) in the atmosphere
by means of gas-chromatography.] Ann Ist Super Sanita, 14: 441-445
(in Italian with English summary).
Börzsönyi M, Török G, Surján A, Challis BC, & Bär V (1981) Protective
effect of a new antioxidant on acute hepatotoxicity caused by
morpholine plus nitrite in rats. Toxicol Lett, 7: 285-288.
Bosholm J (1983) [Description of the behaviour of gaseous compounds in
water/steam systems.] Kernenergie, 26: 198-201 (in German).
Boyland E, Nice E, & Williams K (1971) The catalysis of nitrosation by
thiocyanate from saliva. Food Cosmet Toxicol, 9: 639-643.
Braun R, Schöneich J, & Ziebarth D (1977) In vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Bringmann G (1975) [Determination of the biological effect of water
pollutants on blue algae Microcystis using the cell multiplication
inhibition test.] Gesund-Ing, 96: 238-241 (in German).
Bringmann G (1978) [Determination of the biological effect of water
pollutants in protozoa I Bacteriovorous flagellate protozoa (Model
organism: Entosiphon sulcatum Stein).] Z Wasser Abwasser Forsch,
11: 210-215 (in German).
Bringmann G & Kühn R (1977a) [Toxicity thresholds of water pollutants
for bacteria (Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1977b) [Results of toxic action of water
pollutants on Daphia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1978) [Toxicity thresholds of water pollutants
for blue algae (Microcystis aeruginosa) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) [Determination of the biological effect of
water pollutants in protozoa. II. Bacteriovorous ciliates.] Z Wasser
Abwasser Forsch, 1: 26-31 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic action of water
pollutants on Daphnia magna tested by an improved standardized
procedure system.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa III Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German).
Brouwer R, Marquart H, de Mik G, & van Hemmen J (1992) Risk assessment
of dermal exposure of greenhouse workers to pesticides after re-entry.
Arch Environ Contam Toxicol, 23: 273-280
Brown AR (1966) Morpholine: its properties and uses. Manuf Chem
Aerosol News, Dec 1966: 50-52.
Brown VR & Knapp JS (1990) The effect of withdrawal of morpholine from
the influent and its reinstatement on the performance and microbial
ecology of a model activated sludge plant treating a morpholine-
containing influent. J Appl Bacteriol, 69: 43-53.
Brown VR, Knapp JS, & Heritage J (1990) Instability of the morpholine-
degradative phenotype in mycobacteria isolated from activated sludge.
J Appl Bacteriol, 69: 54-62.
Brunnemann KD & Hoffmann D (1991) Decreased concentrations of
N-nitrosodiethanolamine and N-nitrosomorpholine in commercial tobacco
products. J Agric Food Chem, 39: 207-208.
Brunnemann KD, Scott JC, & Hoffmann D (1982) N-nitroso-morpholine
and other volatile N-nitrosamines in snuff tobacco. Carcinogenesis,
3: 693-696.
BUA (Society of German Chemists, Advisory Committee on Existing
Chemicals of Environmental Relevance) (1991) [Morpholine.] Weinheim,
VCH Verlagsgesellschaft, 181 pp (BUA Report No. 56) (in German).
Calamari D, Da Gasso R, Galassi S, Provini A, & Vighi M (1980)
Biodegradation and toxicity of selected amines on aquatic organisms.
Chemosphere, 9: 753-762.
Calmels S, Ohshima H, Vincent P, Gounot A-M, & Bartsch H (1985)
Screening of micro-organisms for nitrosation catalysis at Ph 7 and
kinetics studies on nitrosamines formation from secondary amines by
E. coli strains. Carcinogenesis, 6: 911-915.
Calmels S, Ohshima H, Crespi M, Leclerc H, Cattoen C, & Bartsch H
(1987) N-nitrosoamine formation by microorganisms isolated from
human gastric juice and urine: biochemical studies on bacteria-
catalysed nitrosation. In: Bartsch H, O`Neill IK, & Schulte-Hermann R
ed. The relevance of N-nitroso compounds to human cancer: Exposures
and mechanisms. Lyon, International Agency for Research on Cancer,
pp 391-395 (IARC Scientific Publications No. 84).
Calmels S, Ohshima H, & Bartsch H (1988) Nitrosamine formation by
denitrifying and non-denitrifying bacteria: Implication of nitrite
reductase and nitrate reductase in nitrosation catalysis. J Gen
Microbiol, 134: 221-226.
Calmels S, Dalla Venezia N, & Bartsch H (1990) Isolation of an enzyme
catalysing nitrosamine formation in Pseudomonas aeruginosa and
Neisseria mucosae. Biochem Biophys Res Commun, 171(2): 655-660.
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991a) Bacterial
formation of N-nitroso compounds in the rat stomach after omeprazole-
induced achlorhydria. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 187-191 (IARC Scientific Publications No. 105).
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991b) Bacterial
formation of N-nitroso compounds in the rat stomach after
omeprazole-induced achlorhydria. Carcinogenesis, 12: 435-439.
Cech JS & Chudoba J (1988) Effect of the solids retention time on the
rate of biodegradation of organic compounds. Acta Hydrochim Hydrobiol,
16: 313-323.
Cech JS, Hartman P, Slosárek M, & Chudoba J (1988) Isolation and
identification of a morpholine-degrading bacterium. Appl Environ
Microbiol, 54: 619-621.
Challis BC & Kyrtopoulos SA (1979) The chemistry of nitroso-compounds.
Part 11. Nitrosation of amines by the two-phase interaction of amines
in solution with gaseous oxides of nitrogen. J Chem Soc Perkin Trans
I, 1979: 299-304.
Challis BC & Outram JR (1979) The chemistry of nitroso-compounds. Part
15. Formation of N-nitrosoamines in solution from gaseous nitric oxide
in the presence of iodine. J Chem Soc Perkin Trans I, 1979: 2768-2775.
Chemical Marketing Reporter (1989) Aromatics. Morpholine. Chem Mark
Rep, July 17: 13.
Chemical Marketing Reporter (1990) Dow to exit morpholine. Chem Mark
Rep, May 28: 7.
Chiaudani G & Vighi M (1978) The use of Selenastrum capricornutum
batch cultures in toxicity studies. Mitt Int Ver Limnol, 21: 316-329.
Collaert B, Attström R, De Bruyn H, & Movert R (1992a) The effect of
delmopinol rinsing on dental plaque formation and gingivitis healing.
J Clin Periodontol, 19: 274-280.
Collaert B, Edwardsson S, Attström R, Hase J, Aström M, & Movert R
(1992b) Rinsing with delmopinol 0.2% and chlorhexidine 0.2%: Short-
term effect on salivary microbiology, plaque, and gingivitis.
J Periodontol, 63: 618-625.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982a) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Presented at the 13th Annual
Meeting of the Environmental Mutagen Society, Boston, 22-28 February
1982. New York, John Wiley and Sons, pp 1-15.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982b) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Environ Mutagen, 4: 390
(Abstract).
Conaway CC, Tong C, & Williams GM (1984a) Evaluation of morpholine,
3-morpholine, and N-substituted morpholines in the rat hepatocyte
primary culture/DNA repair test. Mutat Res, 136: 153-157.
Conaway CC, Coate WB, & Voelker RW (1984b) Subchronic inhalation
toxicity of morpholine in rats. Fundam Appl Toxicol, 4: 465-472.
Cooney RV & Ross PD (1987) N-nitrosation and N-nitration of morpholine
by nitrogen dioxide in aqueous solution: effects of vanillin and
related phenols. J Agric Food Chem, 35: 789-793.
Cooney RV, Ross PD, Bartolini GL, & Ramseyer J (1987) N-nitrosoamine
and N-nitroamine formation: Factors influencing the aqueous reactions
of nitrogen dioxide with morpholine. Environ Sci Technol, 21: 77-83.
Cooney RV, Ross PD, Hatch-Pigott V, & Ramseyer J (1992) Carcinogenic
N-nitrosamine formation: A requirement for nitric oxide. J Environ Sci
Health, 27(3): 789-801.
Cosmetic Ingredient Review (1986) Scientific literature review on
morpholine. Washington, DC, The Cosmetic, Toiletry and Fragrance
Association, 71 pp.
Cosmetic Ingredient Review (1989) Final report on the safety
assessment of morpholine. J Am Colloq Toxicol, 8: 707-748.
Cosmetic Ingredient Review (1991a) CIR annual report 1991. Washington,
DC, The Cosmetic, Toiletry & Fragrance Association, 67 pp.
Cosmetic Ingredient Review (1991b) Protocol: Repeated insult patch
testing and in-use testing. Washington, DC, The Cosmetic, Toiletry &
Fragrance Association, 16 pp (Unpublished report).
Cusano F & Luciano S (1993) Contact dermatitis from pramoxine. Contact
Dermatitis, 28: 39:
Davies R, Massey RC, & McWeeny DJ (1980) The catalysis of the
N-nitrosation of secondary amines by nitrosophenols. Food Chem,
6: 115-122.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J Hazard Mater, 1: 303-318.
Dmitrenko GN & Gvozdyak P (1988) Detection of morpholine by
mycobacteria. In: Proceedings of a Conference on Microbiological
Methods for Protecting the Environment, Puschino, USSR, 5-7 April
1988. Puschino, Scientific Centre for Biological Research of the
Academy of Sciences, 141 pp.
Dmitrenko GN, Udod VM, & Gvozdyak PI (1985) Destruction of morpholine
by fixed bacteria. Khim Tekhnol Vody, 7: 71-73.
Dmitrenko GN, Gvodzdyak PI, & Udod VM (1987) Selection of destructor
microorganisms for heterocyclic xenobiotics. Khim Tekhnol Vody,
9(5): 442-445.
Dodson JJ & Bitterman ME (1989) Compound uniqueness and the
interactive role of morpholine in fish chemoreception. Biol Behav,
14: 13-27.
Donath G, Heitmann HG, Messer J, & Schott M (1977) [Determination of
the distribution coefficients of volatile alkalising agents used in
power plants between steam and water phases and their importance for
the corrosive behaviour of materials.] Vom Wasser, 49: 221-243
(in German).
Dropkin D (1985) Sampling of automobile interiors for organic
emissions. Research Triangle Park, North Carolina, US Environmental
Protection Agency, 20 pp (PB85-172567).
Dynamac Corporation (1988) Information review: Morpholine (EPA
contract No. 68-02-4251). Rockville, Maryland, Dynamac Corporation,
pp 1-52 (IR-514) (Report prepared for TSCA Interagency Testing
Committee).
ECETOC (1991) Critical evaluation of methods for the determination of
N-nitrosamines in personal care and household products. Brussels,
European Chemical Industry Ecology and Toxicology Centre (Technical
Report No. 42).
EEC (1983) Council directive 79831 - Annex V, Part C: Methods for
determination of ecotoxicity. 5.2 Degradation - Biotic degradation
Manometric Respirometry. Brussels, Commission of the European Union.
EEC (1990) Proposal for a council directive on the approximation of
the laws of the Member States relating to cosmetic products (SEC(90)
1985 final) (90/C 322/06). Off J Eur Communities, C/322: 29-43.
Edwards G, Whong W-Z, & Speciner N (1979) Intrahepatic mutagenesis
assay: a sensitive method for detecting N-nitrosomorpholine and
in vivo nitrosation of morpholine. Mutat Res, 64: 415-423.
Emtiazi G (1993) Microbial degradation of linear and cyclic amines.
Leeds, University of Leeds, Department of Microbiology (Thesis).
Emtiazi G & Knapp JS (1994) The biodegradation of piperazine and
structurally-related linear anc cyclic amines. Biodegradation,
5: 83-92.
Environment Agency, Japan (1980) Annual report on chemicals in the
environment. Tokyo, Environment Agency, pp 72-73.
Estrin NF, Haynes CR, & Whelan JM (1982) Specifications/spectra. CTFA
compendium of cosmetic ingredient composition. Washington, DC, The
Cosmetic, Toiletry and Fragrance Association, Inc.
Fadlallah S, Cooper SF, Fournier M, Drolet D, & Perrault G (1990)
Determination of N-nitroso compounds in the environment of a metal
factory using metalworking fluids. Int J Environ Anal Chem,
39: 281-288.
Fajen JM, Carson GA, Rounbehler DP, Fan TY, Vita R, Goff UE, Wolf MH,
Edwards GS, Fine DH, Reinhold V, & Biemann K (1979) N-nitrosamines in
the rubber and tire industry. Science, 205: 1262-1264.
Fan TY, Vita R, & Fine DH (1978) C-nitro compounds: a new class of
nitrosating agents. Toxicol Lett, 2: 5-10.
Fisher AA (1986) Contact Dermatitis. Philadelphia, Pennsylvania, Lea &
Febiger.
Furman MA & Rubenchik BL (1991) Formation of carcinogenic N-nitro
compounds after intraperitoneal administration of nitrosating
precursors in C57BLl/6 line mice. Eksp Onkol, 13(2): 14-22.
Gavinelli M, Fanelli R, Bonfanti M, Davoli E, & Airoldi L (1988)
Volatile nitrosamines in foods and beverages: preliminary survey of
the Italian market. Bull Environ Contam Toxicol, 40: 41-46.
Gilbert R & Saheb SE (1987) Field measurement of the distribution
coefficients of chemical additives used for corrosion control in
steam-water cycles. Mater Perform, 26: 30-36.
Gilbert R, Rioux R, & Saheb SE (1984) Ion chromatographic
determination of morpholine and cyclohexylamine in aqueous solutions
containing ammonia and hydrazine. Anal Chem, 56: 106-109.
Glatt HR & Oesch F (1981) [Ames test for morpholine.] Ludwigshafen,
BASF AG, 11 pp (Unpublished report submitted by the Pharmacological
Institute, University of Mainz, Germany).
Grant WM (1974) Morpholine. In: Toxicology of the eye, 2nd ed.
Sringfield, Illinois, Charles C. Thomas,vol 2, pp 722-723.
Greenblatt M, Mirvish S, & So BT (1971) Nitrosamine studies: induction
of lung adenomas by concurrent administration of sodium nitrite and
secondary amines in Swiss mice. J Natl Cancer Inst, 46: 1029-1034
(Abstract).
Griffiths MH (1968) The metabolism of N-triphenylmethylmorpholine in
the dog and rat. Biochem J, 108: 731-740.
Grodeckaja NS & Karamzina NM (1973) [Initial reactions by the organism
to the effects of industrial substances in concentrations of minimal
effect (Limac, Limch).] Toksikol Nov Prom Chim Veshchestv,
13: 12-23 (in Russian).
Groenen PJ, Busink E, & van Wandelen M (1987) Determination of
volatile nitrosamines in cheese and cured meat products. Model study
of a temperature- and pH-dependent artefact formation phenomenon in
alkaline medium. Z Lebensm.Unters Forsch, 185: 24-30.
Grosjean D (1991) Atmospheric chemistry of toxic contaminants.
6. Nitrosamines: Dialkyl nitrosamines and nitrosomorpholine. J Air
Waste Manage Assoc, 41: 306-311.
Hamano T, Mitsuhashi Y, & Matsuki Y (1980) Improved gas
chromatographic method for the quantitative determination of secondary
amines as sulphonamides formed by reaction with benzene-sulphonyl
chloride. J Chromatogr, 190: 462-465.
Hamano T, Mitsuhashi Y, & Matsuki Y (1981) Glass capillary gas
chromatography of secondary amines in foods with flame photometric
detection after derivatization with benzenesulfonyl chloride. Agric
Biol Chem, 45: 2237-2243.
Hansen L, Akesson B, Sollenberg J, & Lundh T (1986) Determination of
N-methylmorpholine in air samples from a polyurethane foam factory.
Scand J Work Environ Health, 12: 66-69.
Harbison RD, Marino DJ, Conaway CC, Rubin LF, & Gandy J (1989) Chronic
morpholine exposure of rats. Fundam Appl Toxicol, 12: 491-507.
Hassan SSM, Tadros FS, & Selig W (1985) Microdetermination of
secondary aliphatic amines using a copper ion-selective electrode.
Microchem J, 31: 1-6.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hazleton (1981) Final report: 9-day acute inhalation toxicity study in
rats. Vienna, Virginia, Hazleton Laboratories America, Inc., 26 pp
(Submitted to Texaco Chemical Company).
Hecht SS & Morrison JB (1984) A sensitive method for detecting
in vivo formation of N-nitrosomorpholine and its application to
rats given low doses of morpholine and sodium nitrite. Cancer Res,
7: 2873-2877.
Hecht SS & Young R (1981) Metabolic alpha-hydroxylation of
N-nitrosomorpholine and 3,3,5,5-tetradeutero-N-nitrosomorpholine in
the F344 rat. Cancer Res, 41: 5039-5043.
Heilen G, Mercker HJ, Frank D, Reck RA, & Jäckh R (1989) [Amines,
aliphatic.] In: [Ullmann's encyclopedia of industrial chemistry], 5th
ed. Weinheim, VCH Verlagsgesellschaft, vol A2, pp 1-36 (in German).
Hellman TM & Small FH (1974) Characterization of the odor properties
of 101 petrochemicals using sensory methods. J Air Pollut Control
Assoc, 24: 979-982.
Hesselink PGM, Kerkenaar A, & Witholt B (1990) Inhibition of microbial
cholesterol oxidases by dimethylmorpholines. J Steroid Biochem,
35: 107-113.
Hibbs JB Jr (1992) Immunology: Overview of cytotoxic mechanisms and
defence of the intracellular environment against microbes. In: Moncada
S, Marletta MA, Hibbs JB Jr, Higgs EA ed. The biology of nitric oxide.
London,d Chapel Hill, Portland Press, pp 201-206.
Hoffmann D, Brunnemann KD, Adams JD, Rivenson A, & Hecht SS (1982)
N-nitrosamines in tobacco carcinogenesis. In: Magee PN ed.
Nitrosamines and human cancer. Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp 211-225 (Banbury Report No. 12).
Hoffmann D, Adams JD, Lisk D, Fisenne I, & Brunnemann KD (1987) Toxic
and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst,
79: 1281-1286.
Hollett BA, Klemme JC, & Andjelkovich D (1982) Health hazard
evaluation report HETA 81-045B-1216: Uniroyal, Inc., Mishawaka,
Indiana. Cincinnati Ohio, National Institute for Occupational Safety
and Health, 20 pp (PB84-183615).
Hotchkiss JH & Vecchio AJ (1983) Analysis of direct contact paper and
paperboard food packaging for N-nitrosomorpholine and morpholine. J
Food Sci, 48: 240-242.
IARC (1978) N-nitrosomorpholine. In: Some N-nitroso compounds. Lyon,
International Agency for Research on Cancer, pp 263-280 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 17).
IARC (1989) Morpholine. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 199-213 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office, pp 282-283 (Occupational
Safety and Health Series No. 37).
Inui N, Nishi Y, Taketomi M, Mori M, Yamamoto M, Yamada T, & Tanimura
A (1979) Transplacental mutagenesis of products formed in the stomach
of golden hamsters given sodium nitrite and morpholine. Int J Cancer,
24: 365-372.
Iqbal ZM, Dahl K, & Epstein SE (1980) Role of nitrogen dioxide in the
biosynthesis of nitrosamines in mice. Science, 207(4432): 1475-1476.
Jakobi G, Löhr A, Schwuger MJ, Jung D, Fischer W, & Gloxhuber C (1983)
[Washing agents.] In: [Ullmann's encyclopedia of industrial
chemistry], 4th ed. Weinheim, VCH Verlagsgesellschaft, vol 24, pp 105,
138-139 (in German).
Japan Chemical Week (1991) Speciality chemicals handbook, 4th ed.
Tokyo, The Chemical Daily Co., Ltd, 8 pp.
Jones WT & Kipling MD (1972) Glaucopsia - blue-grey vision. Br J Ind
Med, 29: 460-461.
Juhnke I & Lüdemann D (1978) [Results of the testing of 200 selected
chemical compounds for acute fish toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Kamimura H, Enjoji Y, Sasaki H, Kawai R, Kaniwa H, Niigata K, &
Kageyama S (1987) Disposition and metabolism of indeloxazine
hydrochloride, a cerebral activator, in rats. Xenobiotica,
17(6): 645-658.
Karweik DH & Meyers CH (1979) Spectrophotometric determination of
secondary amines. Anal Chem, 51: 319-320.
Katosova LD, Fomenko VN, & Davydenko LN (1991) Air pollution and
industrial hygiene. Gig Tr Prof Zabol, 6: 35-36.
Kleemann A & Engel J (1982) [Midodrin, Minaprin, Minocyclin;
Trimethobenzamid, Trimethoprin, Trimetozin, Trimipramin,
Tripelennamin.] In: [Pharmaceutical substances.] Stuttgart, New York,
Georg Thieme Verlag, pp 602-603, 923-928 (in German).
Knapp JS & Brown VR (1988) Morpholine biodegradation. Int Biodeterior,
24: 299-306.
Knapp JS & Whytell AJ (1990) The biodegradation of morpholine in river
water and activated sludge. Environ Pollut, 68: 67-79.
Knapp JS, Callely AG, & Mainprize J (1982) The microbial degradation
of morpholine. J Appl Bacteriol, 52: 5-13.
Koga M & Akiyama T (1985) Determination of trace heterocyclic amines
in water as their 1,2,-naphthoquinone derivatives by high performance
liquid chromatography. Anal Sci, 1: 285-288.
Kramer VC, Schnell DJ, & Nickerson KW (1983) Relative toxicity of
organic solvents to Aedes aegypti larvae. J Invertebr Pathol,
42: 285-287.
Kubis A, Witek R, Baran E, Jadach W, Matecka K, & Zaba A (1981)
[ In vivo antimycotic effect of topically applied morpholine.]
Pharmazie, 36: 429-431 (in German).
Kubis A, Witek R, & Krutul H (1983) [Investigation on antibacterial
action of some amines.] Pharmazie, 38: 488-489 (in German).
Lakritz L & Kimoto W (1980) N-nitrosamines - contaminants in blood-
collection tubes. Food Cosmet Toxicol, 18: 31-34.
Lam HF & Van Stee EW (1978) A re-evaluation of the toxicity of
morpholine. Fed Proc, 37: 679 (Abstract).
Lamarre C, Gilbert R, & Gendron A (1989) Liquid chromatographic
determination of morpholine and its thermal breakdown products in
steam-water cycles at nuclear power plants. J Chromatogr,
467: 249-258.
Lamb CB & Jenkins GF (1952) B.O.D. of synthetic organic chemicals. In:
Proceedings of the 7th Industrial Waste Conference, Purdue University,
West Lafayette, Indiana, 7-9 May 1952. Ann Arbor, Michigan, Ann Arbor
Science Publishers, pp 326-339.
Lathia D & Edeler A (1989) [Influence of sulfur-containing amino acids
on in vitro nitrosamine formation under conditions similar to those
found in the stomach.] Ernährungsphysiologie, 36: 21-24 (in German).
Lathia D & Schellhöh B (1981) Inhibition of nitrosamine formation
in vitro in presence of both ascorbic acid and sorbic acid. Recent
Adv Clin Nutr, 1: 189-190.
Lauharanta J (1992) Comparative efficacy and safety of amorolfine nail
lacquer 2% versus 5% once weekly. Clin Exp Dermatol, 705: 41-43.
Leach SA, Cook AR, Challis BC, Hill MJ, & Thompson MH (1987)
Bacterially mediated N-nitrosation reactions and endogenous
formation of N-nitroso compounds. In: Bartsch H, O`Neill IK, &
Schulte-Hermann R ed. Relevance of N-nitroso compounds to human
cancer: Exposures and mechanisms. Lyon, International Agency for
Research on Cancer, pp 396-403 (IARC Scientific Publications No. 84).
Leach SA, Mackerness CW, Hill MJ, & Thompson MH (1991) Inhibition of
bacterially mediated N-nitrosation by ascorbate: Therapeutic and
mechanistic considerations. In: O`Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 571-578 (IARC Scientific Publications No. 105).
Leaf CD, Wishnok JS, & Tannenbaum SR (1991) Endogenous incorporation
of nitric oxide from L-arginine into N-nitrosomorpholine stimulated by
Escherichia coli lipopolysaccharide in the rat. Carcinogenesis,
224: 537-539.
Lee SA (1982) Health hazard evaluation report HETA 82-156-1231:
Sheller-Globe Corporation, Keokuk, Iowa. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 8 pp (PB84-172915).
Leenheers LH, Ravensberg JC, Kerstens HJ, & Jongen MJM (1992) Gas
chromatographic determination of the pesticide dodemorph for
assessment of occupational exposure. J Chromatogr Sci, 132: 228-232.
Leo A, Hansch C, & Elkins D (1971) Partition coefficients and their
uses. Chem Rev, 71: 525, 564.
Le Therizien L, Heymans F, Redeuilh C, Godfroid J-J, & Busch N (1980)
Partition coefficient additivity. 1. Morpholine and
N-(N',N'-disubstituted amino acetyl)-arylamine series. Eur J Med Chem
Chim Ther, 15: 311-316.
Lide DR ed. (1990) CRC handbook of chemistry and physics, 71st ed.
Boca Raton, Florida, CRC Press, pp 8-34.
Litton Bionetics (1979a) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp (Report submitted to Texaco Petrochemicals,
Bellaire, Texas).
Litton Bionetics (1979b) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp Report submitted to Texaco, Inc., Beacon, New
York).
Litton Bionetics (1980) Mutagenicity evaluation of morpholine in the
sister chromatid exchange assay with chinese hamster ovary (CHO)
cells. Kensingtn, Maryland, Litton Bionetics, Inc., 10 pp (Report
submitted to Texaco, Inc., Beacon, New York).
Litton Bionetics (1982) Evaluation of morpholine in the in vitro
transformation of Balb/3T3 cells with and without metabolic activation
assay 80/490. Kensington, Maryland, Liotton Bionetics, Inc. 21 pp
(Unpublished report submitted to BASF AG, Ludwigshafen).
Liu RH, Conboy JJ, & Hotchkiss JH (1988) Nitrosation by nitro-nitroso
derivatives of olefins: a potential mechanism for N-nitrosamine
formation in fried bacon. J Agric Food Chem, 36: 984-987.
Liu RH, Jacob JR, Tennant BC, & Hotchkiss JH (1992) Nitrite and
nitrosamine synthesis by hepatocytes isolated from normal woodchucks.
Cancer Res, 52: 4139-4143.
Lodén M, Larsson R, Häggqvist I, & Karlsson N (1985) [The dermal
irritancy/corrosion of 20 compounds in aqueous solutions.] Umea,
Sweden, Försvarets Forskningsanstalt, 76 pp (FOA Report No. E/40023)
(in Swedish with English summary).
Loeppky RN, Tomasik W, & Kerrick BE (1987) Nitroso transfer from
alpha-nitrosamino aldehydes: implications for carcinogenesis.
Carcinogenesis, 8(7): 941-946.
London MA & Lee S (1987) Health hazard evaluation report HETA
85-003-1834: BF Goodrich, Woodburn, Indiana. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 27 pp
(PB88-162672/XAD).
McCain JC & Peck JM Jr (1976) The toxicity of selected chemicals used
in power generating stations to Hawaiian fishes NOAA. Washington, DC,
US Department of Commerce, National Technical Information Service,
23 pp (NTIS No PB262437).
McGlothlin JD (1980) Health hazard evaluation report HETA 80-67-749:
Firestone Fire and Rubber Company, Akron, Ohio. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 18 pp
(PB82-103144).
McGlothlin J & Wilcox T (1984) Health hazard evaluation report HETA
79-109-1538: Kelly Springfield Tire Company, Cumberland, Maryland.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 50 pp (PB85-244424).
Mackerness CW, Leach SA, Thompson MH, & Hill MJ (1989) The inhibition
of bacterially mediated N-nitrosation by vitamin C: relevance to the
inhibition of endogenous N-nitrosation in the achlorhydric stomach.
Carcinogenesis, 10(2): 397-399.
Malaiyandi M, Thomas GH, & Meek ME (1979) Sampling and analysis of
some corrosion inhibiting amines in steam condensates. J Environ Sci
Health, A14: 609-627.
Maller RK & Heidelberger C (1957) Studies on OPSPA. IV. Metabolism of
OPSPA in the rat and human. Cancer Res, 17: 296-301.
Mannsville Chemical Products (1981) Chemical products synopsis:
Morpholine. Cortland, New York, Mannsville Chemical Products
Corporation, 2 pp.
Mastromatteo E (1965) Recent occupational health experiences in
Ontario. J Occup Med, 702: 502-511.
Mazure N (1993) Etude de la biodégradation de la morpholine par une
culture pure, Mycobacterium aurum, et des cultures mixtes
Pseudomonas. Compiègne, France, University of Technology (Thesis).
Meiners AF, Gadberry H, Carson BL, Owens HP, & Lapp TW (1980) Volatile
corrosion inhibitors and boiler water additives: potential for
nitrosamine formation. Washington, DC, US Environmental Protection
Agency, Office of Pesticides and Toxic Substances, 99 pp (Report
submitted by the Midwest Research Institute, Kansas City, Missouri)
(NTIS/PB80-221105).
Mercer EI (1991) Morpholine antifungals and their mode of action.
Biochem Soc Trans, 19: 788-793.
Migukina NV (1973) [Evaluation of the danger [toxicity] of morpholine
by chronic exposure.] Toksikol Nov Prom Chim Veshchestv, 13: 92-100
(in Russian).
Millington LA, Goulding KH, & Adams N (1988) The influence of growth
medium composition on the toxicity of chemicals to algae. Water Res,
22: 1593-1597.
Mills EJ & Stack VT (1953) Biological oxidation of synthetic organic
chemicals. In: Proceedings of the 8th Industrial Waste Conference,
Purdue University, West Lafayette, Indiana, 4-6 May 1953. Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp 492-517.
Mills EJ & Stack VT (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage Ind Wastes,
27: 1061-1064.
Mirvish SS (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol Appl Pharmacol,
31: 325-351.
Mirvish SS, Wallcave L, Eagen M, & Shubik P (1972) Ascorbate-nitrite
reaction: possible means of blocking the formation of carcinogenic
N-nitroso compounds. Science, 177: 65-68.
Mirvish SS, Cardesa A, Wallcave L, & Shubik P (1975) Induction of
mouse lung adenomas by amines or ureas plus nitrite and by N-nitroso
compounds: effect of ascorbate, gallic acid, thiocyanate, and
caffeine. J Natl Cancer Inst, 55: 633-636.
Mirvish SS, Pelfrene AF, Garcia H, & Shubik P (1976) Effect of sodium
ascorbate on tumor induction in rats treated with morpholine and
sodium nitrate, and with nitrosomorpholine. Cancer Lett, 2: 101-108.
Mirvish SS, Ramm MD, Sams JP, & Babcock DM (1988) Nitrosamine
formation from amines applied to the skin of mice after and before
exposure to nitrogen dioxide. Cancer Res, 48: 1095-1099.
Mjos K (1978) Cyclic amines. In: Kirk-Othmer encyclopedia of chemical
technology, 3rd ed. New York, John Wiley and Sons, vol 2, pp 295-308.
Moriyasu M, Endo M, Hashimoto Y, & Koeda T (1984) High-performance
liquid chromatographic determination of organic substances by metal
chelate derivatization. II. Microdetermination of methamphetamine and
amphetamine. Chem Pharm Bull, 32: 600-608.
Newberne PM & Shank RC (1973) Induction of liver and lung tumours in
rats by the simultaneous administration of sodium nitrite and
morpholine. Food Cosmet Toxicol, 11: 819-825.
NIOSH (1977) Manual of analytical methods, 2nd ed. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 3, pp
S150/1-S150/9.
NIOSH (1988) National occupational exposure survey as of 16.12.1988:
Morpholine. Cincinnati, Ohio, National Institute of Occupational
Safety and Health, 1 p.
Norkus EP, Boyle S, Kuenzig WA, & Mergens WJ (1984) Formation of
N-nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen oxide. Carcinogenesis, 5: 549-554.
Norkus EP, Kuenzig WA, Chau J, Mergens WJ, & Conney AH (1986)
Inhibitory effect of alpha-tocopherol on the formation of
nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen dioxide. Carcinogenesis, 7: 357-360.
NRC (1981) Selected aliphatic amines and related compounds: an
assessment of the biological and environmental effects. Washington,
DC, National Research Council, Board on Toxicology and Environmental
Health Hazards (Report prepared for the US Environmental Protection
Agency, Washington) (NTIS/PB83-133066).
OECD (1981a) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Ready biodegradability. 301 E - Modified
OECD screening test. Paris, Organisation for Economic Co-operation and
Development.
OECD (1981b) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Inherent biodegradability. 302 B -
Modified Zahn-Wellens test. Paris, Organisation for Economic
Co-operation and Development.
OECD (1984) Guidelines for testing of chemicals No 202, Part I:
Daphnia sp, acute immobilisation test. Paris, Organisation for
Economic Co-operation and Development.
O'Donnell CM, Edwards C, & Ware J (1988) Nitrosamine formation by
clinical isolates of enteric bacteria. FEMS Microbiol Lett,
51: 193-197.
Ohnishi T (1984) [Morpholine. Studies on mutagenicity of the food
additive morpholine (fatty acid salt).] Nippon Eiseigaku Zasski,
39: 729-745 (in Japanese with English summary).
Ohnishi T, Kubota M, Okada A, & Tonami K (1983) [Residual survey
investigation and removal efficiency by washing with kitchen detergent
of food additive morpholine.] Hokuriku Koshu Eisei Gakkaishi,
10: 63-67 (in Japanese with English summary).
Österdahl B-G & Slorach SA (1983) Volatile N-nitrosamines in snuff and
chewing tobacco on the Swedish market. Food Chem Toxicol, 21: 759-762.
Pensabene JW & Fiddler W (1988) Food additives. Determination of
volatile N-nitrosamines in Frankfurters containing minced fish and
surimi. J Assoc Off Anal Chem, 71: 839-843.
Perez A, Fernandez SI, Garcia-Roche MO, De Las Cagigas A, Castillo A,
Fonseca G, & Herrera M (1990) Mutagenicity of N-nitrosomorpholine
biosynthesized from morpholine in the presence of nitrate and its
inhibition by ascorbic acid. Nahrung, 34: 661-664.
Postlethwait EM & Mustafa MG (1983) Formation of N-nitrosamine in
isolated rat lungs during nitrogen dioxide ventilation.
Carcinogenesis, 4: 777-778.
Reinel D & Clarke C (1992) Comparative efficacy and safety of
amorolfine nail lacquer 5% in onychomycosis, once-weekly versus
twice-weekly. Clin Exp Dermatol, 705: 44-49.
Reinhardt CF & Brittelli MR (1981) Heterocyclic and miscellaneous
nitrogen compounds. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology: Vol 2A. Toxicology. New York, John Wiley and
Sons, pp 2671-2822.
Rekka E, Retsas S, Demopoulos VJ, & Kourounakis PN (1990)
Lipophilicity of some substituted morpholine derivatives synthesized
as potential antinociceptive agents. Arch Pharmacol, 323: 53-56.
Reynolds T (1989) Comparative effects of heterocyclic compounds on
inhibition of lettuce fruit germination. J Exp Bot, 40: 391-404.
Rhodes C & Case DE (1977) Non-metabolite residues of ICI 58,834
(viloxazine). Studies with (14C)morpholine, (14C)ethanolamine and
(14C)glyoxylate. Xenobiotica, 7: 112.
Richardson ML, Webb KS, & Gough TA (1980) The detection of some
N-nitrosamines in the water cycle. Ecotoxicol Environ Saf, 4: 207-212.
Ringenburg V & Fajen JM (1980) Survey for N-nitroso compounds at B.F.
Goodrich, Woodburn, Indiana, December 12, 1979. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 11 pp
(PB88-250477/XAD).
Rounbehler DP & Fine DH (1982) Specific detection of amines and other
nitrogen-containing compounds with a modified TEA analyzer. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 209-219 (IARC Scientific
Publications No. 41).
Rounbehler DP & Fajen JM (1983) N-nitroso compounds in the factory
environment. National Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 228 pp (PB84-145770).
Rounbehler DP, Reisch J, & Fine DH (1980) Nitrosamines in new motor-
cars. Food Cosmet Toxicol, 18: 147-151.
Sander J & Bürkle G (1969) [Induction of malignant tumours in rats by
simultaneous feeding of nitrite and secondary amines.] Z Krebsforsch,
73: 54-66 (in German).
Sander J, Schweinsberg F, & Menz H-P (1968) [Studies on the formation
of carcinogenic nitrosamines in the stomach.] Hoppe-Seyler's Z Physiol
Chem, 349: 1691-1697 (in German).
Savolainen H & Rosenberg C (1983) Morpholine vapour inhalation and
interactions of simultaneous nitrite intake. Biochemical effects on
rat spinal cord axons and skeletal muscle. Arch Toxicol, 53: 143-150.
Schröder E, Rufer C, & Schmiechen R (1982) [Nitrofuran.] In:
[Pharmaceutical Chemistry.] Stuttgart, New York, Georg Thieme Verlag,
pp 858-863 (in German).
Schuster RH, Nabholz F, & Gmünder M (1990) [The inhibition of the
formation of N-nitrosamines: Part 1. The situation and the effect of
alpha-tocopherol.] Kautschuk Gummi Kunstst, 43: 95-106 (in German).
Sen NP & Baddoo PA (1986) Origin of N-nitrosomorpholine contamination
in margarine. J Food Sci, 51: 216-217.
Sen NP & Baddoo PA (1989) An investigation on the possible presence of
morpholine and N-nitrosomorpholine in wax-coated apples. J Food Saf,
9: 183-191.
Sen NP, Seaman S, Clarkson S, Garrod F, & Lalonde P (1984) Volatile
N-nitrosamines in baby bottle rubber nipples and pacifiers. Analysis,
occurrence and migration. In: O'Neill IK, vn Borstel RC, Miller CT,
Long J, & Bartsch H ed. N-nitroso compounds: Occurrence, biological
effects and relevance to human cancer. Lyon, International Agency for
Research on Cancer, pp 51-57 (IARC Scientific Publications No. 57).
Sen NP, Kushwaha SC, Seaman SW, & Clarkson SG (1985) Nitrosamines in
baby bottle nipples and pacifiers: occurrence, migration, and effect
of infant formulas and fruit juices on in vitro formation of
nitrosamines under simulated gastric conditions. J Agric Food Chem,
33: 428-433.
Shank RC & Newberne PM (1976) Dose-response study of the
carcinogenicity of dietary sodium nitrite and morpholine in rats and
hamsters. Food Cosmet Toxicol, 14: 1-8.
Shea TE Jr (1939) The acute and sub-acute toxicity of morpholine. J
Ind Hyg Toxicol, 21: 236-245.
Shenoy NR & Choughuley ASU (1989) Effect of certain plant phenolics on
nitrosamine formation. J Agric Food Chem, 37: 721-725.
Shenoy NR & Choughuley ASU (1992) Inhibitory effect of diet related
sulphydryl compounds on the formation of carcinogenic nitrosamines.
Cancer Lett, 67: 227-232.
Shibata M-A, Kurata Y, Tamano S, Ogiso T, Fukushima S, & Ito N (1987a)
13-week subchronic toxicity study with morpholine oleic acid salt
administered to B6C3F1 mice. J Toxicol Environ Health, 22: 187-194.
Shibata M-A, Kurata Y, Ogiso T, Tamano S, Fukushima S, & Ito N (1987b)
Combined chronic toxicity and carcinogenicity studies of morpholine
oleic acid salt in B6C3F1 mice. Food Chem Toxicol, 25: 569-574.
Simon P & Lemacon C (1987) Determination of aliphatic primary and
secondary amines and polyamines in air by high-performance liquid
chromatography. Anal Chem, 59: 480-484.
Singer GM & Lijinsky W (1976a) Naturally occurring nitrosatable
compounds. I. Secondary amines in foodstuffs. J Agric Food Chem,
24: 550-553.
Singer GM & Lijinsky W (1976b) Naturally occurring nitrosatable
amines. II Secondary amines in tobacco and cigarette smoke condensate.
J Agric Food Chem, 24: 553-555.
Singer SS (1980) Transnitrosation by nitrosamines and nitrosoureas.
In: Walker EA, Griciute L, Castegnaro M, & Börzsönyi M ed. N-nitroso
compounds: Analysis, formation and occurrence. Lyon, International
Agency for Research on Cancer, pp 111-117 (IARC Scientific
Publications No. 31).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, Noyes Publications, pp 626-627.
Smith JH, Bomberger DC Jr, & Haynes DL (1980) Prediction of the
volatilization rates of high-volatility chemicals from natural water
bodies. Environ Sci Technol, 14: 1332-1337.
Smyth HF Jr, Carpenter CP, Weil CS, & Pozzani UC (1954) Range-finding
toxicity data. Arch Ind Hyg Occup Med, 10: 61-68.
Sohn OS, Fiala E, Conaway CC, & Weisburger JH (1982a) Separation of
morpholine and some of its metabolites by high-performance liquid
chromatography. J Chromatogr, 242: 374-380.
Sohn OS, Fiala ES, Conaway CC, & Weisburger JH (1982b) Metabolism and
disposition of morpholine in the rat, hamster and guinea pig. Toxicol
Appl Pharmacol, 64: 486-491.
Sollenberg J & Hansen L (1987) Isotachophoretic determination of
amines from workroom air. J Chromatogr, 390: 133-140.
Spiegelhalder B (1983) [Nitrosamines and rubber.] In: Preussmann R ed.
[The nitrosamine problem.] Weinheim, VCH Verlagsgesellschaft,
pp 235-244 (in German).
Spiegelhalder B & Preussmann R (1982) Nitrosamines and rubber. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 231-2439 (IARC Scientific
Publications No. 41).
Spiegelhalder B & Preussmann R (1984) Contamination of toiletries and
cosmetic products with volatile and nonvolatile N-nitroso carcinogens.
J Cancer Res Clin Oncol, 108: 160-163.
Spies RB, Andresen BD, & Rice DW Jr (1987) Benzthiazoles in estuarine
sediments as indicators of street runoff. Nature (Lond), 327: 697-699.
SRI (1990) Directory of chemical producers, Western Europe:
Morpholine. Menlo Park, California, SRI International, vol 2, p 1563.
Stewart BW & Farber E (1973) Strand breakage in rat liver DNA and its
repair following administration of cyclic nitrosamines. Cancer Res,
33: 3209-3215.
Strotmann UJ, Weberruss U, & Bias WR (1993) Degradation of morpholine
in several biodegradation tests and in wastewater treatment plants.
Chemosphere, 75: 1729-1742.
Subrahmanyam PVR, Khadakkar SN, Chakrabarti T, & Sundaresan BB (1983)
Wastewater-treatment of a phthalate plasticizer, ethanolamine and
morpholine manufacturing plant: a case study. Proc Ind Waste Conf,
37: 13-20.
Suzuki K & Mitsuoka T (1984) N-nitrosamine formation by intestinal
bacteria. In: O'Neill IK, vn Borstel RC, Miller CT, Long J, & Bartsch
H ed. N-nitroso compounds: Occurrence, biological effects and
relevance to human cancer. Lyon, International Agency for Research on
Cancer, pp 275-281 (IARC Scientific Publications No. 57).
Swain A, Waterhouse KV, Venables WA, Callely AG, & Lowe SE (1991)
Biochemical studies of morpholine catabolism by an environmental
mycobacterium. Appl Microbiol Biotechnol, 29: 110-114.
Swope HG & Kenna M (1950) Effect of organic compounds on biochemical
oxygen demand. Sew Ind Wastes Eng, 21: 467-468.
Taft RM & Stroman RE (1979) Health hazard evaluation report HETA
78-131-586: Goodyear Tire and Rubber Company, Niagara Falls, New York.
Cincinnati Ohio, National Institute of Occupational Safety and Health,
Hazard Evaluations and Technical Assistance Branch, 13 pp
(NTIS/PB80-196736).
Takezawa J & Lam HF (1978) Toxic effect of morpholine on rat lungs.
Fed Proc, 37: 247 (Abstract).
Tanaka M, Okada Z, Mihashi K, & Seiko Y (1968) Industrial waste
treatment by activated sludge. XV. Treatment of waste from an organic
vulcanization accelerator producing plant. Kogyo Gijutsuin Hakko
Kenkyusho Kenkyu Hokoku, 33: 19-29.
Tanaka A, Tokieda T, Nambaru S, Osawa M, & Yamaha T (1978) Excretion
and distribution of morpholine salts in rats. J Food Hyg Soc,
19: 329-334.
Tannenbaum SR, Archer MC, Wishnok JS, & Bishop WW (1978) Nitrosamine
formation in human saliva. J Natl Cancer Inst, 60(2): 251-253.
Tatsumi K, Kitamura S, Yoshimura Y, Tanaka S, Hashimoto K, & Igarashi
T (1975) The metabolism of phenyl o-(2-N-morpholinoethoxy)-phenyl
ether hydrochloride in the rabbit and rat. Xenobiotica, 5(6): 377-388.
Taylor R & Son PN (1982) Rubber chemicals. In: Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 20, pp 337-364.
Texaco (1979a) Mutagenicity evaluation of morpholine in the Ames
salmonella/microsome plate test. Bellaire, Texas, Texaco
Petrochemicals, 8 pp.
Texaco (1979b) Mutagenicity evaluation of morpholine in the mouse
lymphoma forward mutation assay. Bellaire, Texas, Texaco
Petrochemicals, 15 pp.
Texaco (1986) Texaco product brochure - Morpholine. Austin, Texas,
Texaco Chemical Company, Research and Technical Services, 29 pp.
Tölgyessy P, Kollár M, Vanco D, & Piatrik M (1986) Bio-degradability
of morpholine. J Radioanal Nucl Chem Lett, 107: 291-295.
Tombropoulos EG (1979) Micromethod for the gas chromatographic
determination of morpholine in biological tissues and fluids. J
Chromatogr, 164: 95-99.
Tombropoulos EG, Koo JO, Gibson W, & Hook GER (1983) Induction by
morpholine of lysosomal alpha-mannosidase and acid phosphatase in
rabbit alveolar macrophages in vivo and in vitro. Toxicol Appl
Pharmacol, 70: 1-6.
TRGS (1989) [Technical regulations for dangerous chemicals:
Nitrosamine.] Cologne, Carl Heymanns Verlag KG, 13 pp (TRGS 552)
(in German).
Tricker AR & Preussmann R (1991) Occurrence of and exposure to
N-nitroso compounds in tobacco. In: O'Neill IK, Chen J, & Bartsch H
ed. Relevance to human cancer of N-nitroso compounds, tobacco smoke
and mycotoxins. Lyon, International Agency for Research on Cancer,
pp 493-495 (IARC Scientific Publications No. 105).
UBA (1990) Calculation of log POW with the Program CLOGP (data
sheet). Berlin, Environment Office, 2 pp.
US FDA (1984a) Code of federal regulations (April 1, 1984) - Title 21,
Part 178.3300: Corrosion inhibitors used for steel or tinplate.
Washington, DC, US Food and Drug Administration, p 312.
US FDA (1984b) Code of federal regulations (April 1, 1984) - Title 21,
Part 176.210: Defoaming agents used in the manufacture of paper and
paperboard. Washington, DC, US Food and Drug Administration,
pp 192-193.
US FDA (1984c) Code of federal regulations (April 1, 1984) - Title 21,
Part 175.105: Substances for use only as components of adhesives.
Washington, DC, US Food and Drug Administration, pp 124-137.
US FDA (1984d) Code of federal regulations - Title 21, Part 178.310:
Animal glue. Washington, DC, US Food and Drug Administration, p 308.
US FDA (1984e) Code of federal regulations - Title 21, Part 173.310:
Boiler water additives. Washington, DC, US Food and Drug
Administration, pp 115-118.
US FDA (1986) Cosmetic product formulation data: Ingredients used in
each product category. Washington, DC, US Food and Drug
Administration.
US FDA (1988) Code of federal regulations - Title 21, Part 172.235:
Morpholine. Washington, DC, US Food and Drug Administration, pp 33-35.
Van Stee EW, Wynns PC, & Moorman MP (1981) Distribution and
disposition of morpholine in the rabbit. Toxicology, 20: 53-60.
Wang X & Suskind RR (1988) Comparative studies of the sensitization
potential of morpholine, 2-mercaptobenzothiazole and 2 of their
derivatives in guinea pigs. Contact Dermatitis, 19: 11-15.
Wang X & Tabor MW (1988) Studies of the reactivity of morpholine,
2-mercaptobenzothiazole and 2 of their derivatives with selected amino
acids. Contact Dermatitis, 19: 16-21.
Wang H & Wu Y (1991) Inhibitory effect of Chinese tea on N-nitrosation
in vitro and in vivo. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 546-548 (IARC Scientific Publications No. 105).
Wellens H (1982) [Comparison of the sensitivity of Brachydanio rerio
und Leuciscus idus by testing the fish toxicity of chemicals and
wastewaters.] Z Wasser Abwasser Forsch, 2: 49-52 (in German).
Westin JB, Castegnaro MJ-J, & Friesen MD (1987) N-nitrosamines and
nitrosatable amines, potential precursors of N-nitramines, in
children's pacifiers and baby-bottle nipples. Environ Res,
43: 126-134.
Wishnok JS & Tannenbaum SE (1976) Formation of cyanamides from
secondary amines in human saliva. Science, 191: 1179-1180.
Wishnok JS & Tannenbaum SR (1977) An unknown salivary morpholine
metabolite. Anal Chem, 49: 715a-716a, 718a.
Yurchenko VA, Ilnitskii AP, Ermilow VB, Mistakopulo GM, & Nechipai AM
(1990) [Investigation of mutagenic action of natural zeolite and
chrysotile-asbest dusts.] Eksp Onkol, 12: 24-26 (in Russian with
English summary).
Zaeva GN, Timofievskaya LA, Bazarova LA, & Migukina NV (1968)
[Comparative toxicity of a group of cyclic imino-compounds.] Toksikol
Nov Prom Chim Veshchestv, 10: 25-35 (in Russian).
Ziebarth D (1973) N-nitrosation of secondary amines and particularly
of drugs, in buffer solutions and human gastric juice. In: Bogovski P
& Walker EA ed. N-nitroso compounds in the environment. Lyon,
International Agency for Research on Cancer, pp 137-141 (IARC
Scientific Publications No. 9).
Ziebarth D. (1974) [Investigations into the nitrosation of secondary
amines in buffer solutions and in human gastric juice.] Arch
Geschwulstforsch, 43: 42-41 (in German).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Propriétés physiques et chimiques
La morpholine (1-oxa-4-azacyclohexane) est un liquide incolore,
huileux, hygroscopique et volatil qui possède l'odeur de poisson
caractéristique des amines. Elle est entièrement miscible à l'eau,
ainsi qu'à de nombreux solvants organiques, mais sa solubilité est
limitée dans les solutions aqueuses alcalines. C'est une base, dont
le pKa de l'acide conjugué est de 8,33. Il s'ensuit que son
coefficient de partage entre l'octanol et l'eau dépend du pH
(log Pow = -2,55 à pH 7 et -0.84 à pH 10 et à 35°C). La tension de
vapeur des solutions aqueuses de morpholine est très proche de celle
de l'eau.
La morpholine peut entrer en réaction de diverses manières.
Chimiquement, elle se comporte comme une amine secondaire. Dans les
conditions environnementales et physiologiques, la
N-nitrosomorpholine (NMOR), dont le pouvoir cancérogène chez
l'animal est prouvé, se forme par réaction entre des solutions de
nitrite ou des oxydes d'azote gazeux et des solutions diluées de
morpholine. La concentration en oxyde d'azote (NO) peut être
importante pour la nitrosation. Les conditions de nitrosation, en
particulier la valeur du pH, jouent un rôle important.
2. Méthodes d'analyse
On peut doser la morpholine par chromatographie en phase gazeuse
avec des colonnes à remplissage ou des colonnes capillaires, par
chromatographie liquide à haute performance (HPLC) et par
chromatographie ionique. Les détecteurs utilisés sont les détecteurs
à ionisation de flamme, les détecteurs à photométrie de flamme, les
détecteurs sélectifs d'azote, les détecteurs thermiques pour la
chromatographie en phase gazeuse et, pour la chromatographie en phase
liquide à haute performance, les détecteurs à UV et les détecteurs
thermiques. Pour le dosage des traces, il faut passer par un dérivé.
La méthode de choix sur le plan de la sensibilité semble être la
chromatographie en phase gazeuse avec détection thermique après
transformation de la morpholine en NMOR (limite de détection: 2 à
3 µg/kg dans diverses matrices). Dans l'air, de faibles
concentrations de morpholine peuvent être mesurées par chromatographie
en phase gazeuse avec un détecteur sélectif d'azote.
3. Sources d'exposition humaine et environnementale
On estime qu'environ 25 000 tonnes de morpholine sont produites
chaque année, toutefois on ne connaît pas dans le détail la production
de certains pays.
Le principal procédé de production est, semble-t-il, la réaction
du diéthylène-glycol sur l'ammoniaque en présence d'hydrogène et de
catalyseurs.
La morpholine est un produit chimique à tout faire mais on n'en
connaît pas tous les usages. Il joue un rôle important comme
intermédiaire dans l'industrie du caoutchouc, comme inhibiteur de la
corrosion ainsi que pour la synthèse d'éclaircissants optiques,
d'agents pour la protection des récoltes, de colorants et de
médicaments. La morpholine est utilisée comme solvant pour les
dérivés organiques les plus divers, comme par exemple les résines, les
colorants et les cires. On peut également l'utiliser comme
catalyseur. On utilise encore de la morpholine dans certains pays
pour la confection de produits de toilette et de cosmétiques. Dans
d'autres, elle entre dans la composition d'additifs alimentaires, soit
directement, soit indirectement.
Lorsqu'il y a exposition humaine ou environnementale, elle peut
être due soit à des émissions de gaz, soit à des décharges de
solutions aqueuses, à moins qu'elle ne se produise directement lors de
l'utilisation de certains produits contenant de la morpholine, comme
par exemple des produits cosmétiques ou des cires. Le gros des
émissions et des décharges trouve probablement son origine dans la
fabrication et l'utilisation de la morpholine dans l'industrie
chimique (notamment lors de la production et de l'utilisation des
produits destinés à l'industrie du caoutchouc) ainsi que dans
l'application de la morpholine comme agent anti-corrosion. On a
trouvé de la morpholine dans des denrées alimentaires très variées
ainsi que dans le tabac. Il est possible que cette présence
s'explique par la migration, dans la denrée alimentaire, de la
morpholine incorporée à l'enduit qui recouvre les fruits ou
l'emballage, mais dans un certain nombre de cas, on ignore d'où la
morpholine peut provenir.
4. Transport, distribution et transformation dans l'environnement
La morpholine est chimiquement stable dans la biosphère bien
qu'elle puisse être soumise à une nitrosation chimique ou biologique
qui la transforme en NMOR.
La morpholine est intrinsèquement biodégradable. On a vérifié
cette propriété dans des conditions reproduisant celles qui règnent
dans les usines de traitement des boues activées. Cependant, dans des
conditions de non adaptation, la morpholine n'est probablement pas
décomposée en proportion importante. Le temps de rétention moyen sur
les matières solides présentes dans les usines de traitement des boues
activées est d'une importance cruciale et il doit dépasser les 8 jours
pour que l'on puisse obtenir une bonne dégradation de la morpholine.
Les données relatives à la bioaccumulation de la morpholine par
les organismes aquatiques et terrestres sont insuffisantes. D'après
la valeur du coefficient de partage entre le n-octanol et l'eau
(log Pow = -2,55 à pH 7), on peut s'attendre à ce qu'il n'y ait
aucune bioaccumulation.
Comme la morpholine est un produit chimique industriel important
dont les applications sont variées, il faut s'attendre à retrouver ce
composé ou ses dérivés dans un grand nombre d'effluents industriels.
De plus, étant donné qu'on l'utilise comme inhibiteur de la corrosion
dans l'eau des chaudières, on va le retrouver dans les eaux usées de
ces chaudières, et en particulier les eaux usées provenant de
certaines centrales thermiques. Comme la morpholine entre également
dans la composition des additifs du caoutchouc, on va en retrouver
également, en quantité mal définie, dans l'hydrosphère et la
géosphère, par suite de l'usure des pneus et du rejet des pneus usés.
La morpholine peut pénétrer dans l'environnement en se
volatilisant à partir des encaustiques et des cirages dont elle est un
constituant. Elle est rapidement captée par l'humidité. C'est donc
principalement dans l'hydrosphère qu'elle devrait s'accumuler, mais
les données limitées dont on dispose incitent à penser que la
morpholine ne s'accumule pas dans ce compartiment.
La meilleure méthode pour éliminer la morpholine concentrée est
l'incinération, toutefois il peut être nécessaire de veiller aux
émissions d'oxydes d'azote afin de respecter les normes de protection
de l'environnement. En ce qui concerne les effluents aqueux, le
traitement des boues activées est suffisant, à la condition toutefois
que l'usine fasse l'objet d'un contrôle rigoureux (voir ci-dessus).
5. Concentrations dans l'environnement et exposition humaine
On ne dispose d'aucune donnée sur la concentration de la
morpholine dans l'air ambiant ainsi que dans l'air intérieur des
immeubles résidentiels ou encore dans l'eau de boisson. Si l'on
possède quelques données sur sa présence dans les eaux naturelles, on
n'en a en revanche aucune sur sa présence dans le sol.
D'après les données disponibles, c'est les denrées alimentaires
qui constituent la principale source d'exposition à la morpholine de
la population générale, les produits alimentaires pouvant être
contaminés par suite d'un traitement conservateur direct des fruits à
l'aide de cires contenant de la morpholine, lors du traitement à la
vapeur au cours de la préparation et enfin par l'utilisation de
matériaux d'emballage dans la composition desquels entre la
morpholine. Cependant on ne dispose que de données quantitatives
limitées sur la contamination des denrées alimentaires par la
morpholine et la NMOR. Par exemple, dans les produits laitiers pré-
emballés, les valeurs mesurées vont de 5 à 77 µg/kg pour la morpholine
et jusqu'à 3,3 µg/kg pour la NMOR. En général, les mesures effectuées
ont montré que la teneur en morpholine de divers échantillons de
produits alimentaires (poisson, viande, produits d'origine végétale,
boissons) ne dépassait généralement pas 1 mg/kg. Des valeurs plus
élevées (jusqu'à 71 mg/kg) ont été relevées au Japon dans des agrumes.
Une enquête effectuée en Italie n'a pas permis de repérer la présence
de NMOR dans diverses denrées alimentaires au seuil de détection de
0,3 µg/kg. Les données existantes ne permettent pas d'évaluer
l'apport de morpholine et de NMOR par la voie alimentaire.
On a trouvé de la morpholine dans le tabac de cigarette à la
concentration de 0,3 mg/kg ainsi que dans le tabac à priser et à
chiquer à des concentrations allant jusqu'à 4,0 mg/kg. Il est arrivé
que l'on trouve dans du tabac à priser des concentrations de
morpholine allant jusqu'à 0,7 mg/kg. La présence de ce produit était
probablement la conséquence de l'utilisation de cires à base de
morpholine pour le conditionnement de ce tabac.
De la NMOR a été mise en évidence dans certains produits de
toilette et dans des cosmétiques, par exemple des shampoings, du
rimmel ainsi que dans des articles en caoutchouc comme les sucettes
pour bébés et les tétines pour biberon, à des concentrations allant
jusqu'à 3,5 mg/kg.
Il peut y avoir exposition professionnelle à la morpholine dans
diverses industries. On ne possède guère de données sur l'exposition
des travailleurs à la morpholine. Toutes les valeurs signalées sont
inférieures à 3 mg/m3. On a signalé des cas d'exposition
professionnelle à la NMOR dans l'industrie du caoutchouc où des
concentrations allant jusqu'à 250 µg/m3 ont été mesurées.
Les données actuellement disponibles permettent de se faire une
idée du risque d'exposition humaine mais elles ne permettent pas une
estimation précise de l'intensité de l'exposition à la morpholine et
la NMOR de la population générale et des diverses catégories
professionnelles.
6. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après exposition par voie orale, cutanée ou respiratoire, il y a
absorption de la morpholine. Chez le rat, après administration par
voie orale ou intraveineuse, la morpholine se répartit rapidement dans
l'organisme, atteignant sa concentration la plus élevée dans
l'intestin et les muscles.
Chez le lapin, après exposition par voie intraveineuse ou
respiratoire, la morpholine se répartit de préférence dans les reins,
les concentrations étant plus faibles dans les poumons, le foie et le
sang.
La morpholine ne se fixe pas de manière importante aux protéines
plasmatiques. Sa demi-vie dans le plasma est de 115 minutes chez le
rat, de 120 minutes chez le hamster et de 300 minutes chez le cobaye.
La morpholine est principalement excrétée sans modification par
les reins chez un certain nombre d'espèces. Un jour après
l'administration, on a constaté que 70 à 90% de la morpholine se
retrouvaient dans les urines. La neutralisation en augmente la
vitesse d'excrétion. Une faible proportion de la morpholine est
excrétée dans l'air expiré et dans les matières fécales.
Les études effectuées sur des rats, des souris, des hamsters et
des lapins indiquent que la morpholine s'élimine presque complètement
sans métabolisation. Chez le cobaye, il peut y avoir une
N-méthylation suivie d'une N-oxydation, la dose administrée
pouvant être métabolisée jusqu'à hauteur de 20%. En présence de
nitrites, la morpholine peut être transformée en NMOR, tant in vitro
qu' in vivo. On a constaté, en administrant de la morpholine à des
rats avec des nitrites, que celle-ci pouvait être nitrosée dans la
proportion de 0 à 12%, selon la dose.
Le taux de nitrosation peut augmenter par le fait d'une immuno-
stimulation comportant l'activation des macrophages.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Après administration de morpholine par voie orale à des rats et à
des cobayes, on a obtenu pour la DL50 des valeurs respectives de
1-1,9 g/kg de poids corporel et 0,9 g/kg de poids corporel, ce qui
permet d'avoir une idée de la toxicité aiguë du produit. Des rats qui
avaient reçu de la morpholine neutralisée à raison de 1 g/kg de poids
corporel ont survécu. Après administration par voie intrapéritonéale,
on a obtenu une DL50 de 0,4 g/kg de poids corporel chez la souris et
de 0,1-0,4 g/kg de poids corporel chez le rat. Après exposition par
la voie respiratoire, la DL50 était d'environ 8 g/m3 chez le rat
et de 5-7 g/m3 chez la souris. La DL50 de la morpholine
concentrée par voie percutanée était de 0,5 ml/kg chez le lapin. La
toxicité aiguë de la morpholine se caractérise par des hémorragies
gastrointestinales et des diarrhées lorsqu'elle est administrée par
voie orale et par une irritation et des hémorragies nasales, buccales,
oculaires et pulmonaires lorsqu'elle est administrée par voie
respiratoire. Lors d'une étude de 30 jours au cours de laquelle des
rats ont reçu de la morpholine par gavage à des doses de 0,16 à
0,8 g/kg de poids corporel, on a observé des effets toxiques graves et
une mortalité à toutes les doses. Les mêmes observations ont été
effectuées sur des cobayes à des doses comprises entre 0,09 et
0,045 g/kg de poids corporel.
Chez des rats soumis pendant une brève période à des inhalations
de morpholine (7,2 g/m3, 4 heures par jour pendant 4 jours ou bien
1,63 g/m3, 4 heures par jour, 5 jours par semaine pendant 30 jours),
on a observé une altération de la fonction pulmonaire. Le taux de
mortalité chez d'autres rats allait de 0 à 100% selon le niveau
d'exposition (0,36-18,1 g/m3, 6 heures par jour pendant 9 jours).
Par la voie respiratoire, on a constaté que la toxicité dépendait de
la dose avec une irritation locale (yeux, bouche, nez et poumons) et
des hémorragies de gravité variables aux niveaux d'exposition les plus
élevés. Dans une étude, on a observé un hyperfonctionnement de la
glande thyroïde et dans une autre, une nécrose du foie et des tubules
rénaux après exposition par la voie respiratoire.
Une étude de 90 jours a montré que la morpholine administrée par
voie orale (0.2-0,7 g quotidiennement par kg de poids corporel)
pouvait réduire la gain de poids et entraîner une insuffisance rénale
chez la souris. Après 672 jours d'administration de morpholine par
voie orale (0,28-0,5 g/kg de poids corporel quotidiennement), on a
observé chez la souris une hyperplasie au niveau de l'épithélium de la
portion cardiaque de l'estomac.
Selon une étude d'inhalation de 13 semaines, la morpholine
(administrée à la dose de 0,09-0,9 g/m3 6 heures par jour, 5 jours
par semaine) provoque des lésions liées à la dose au niveau de la
muqueuse nasale ainsi qu'une pneumopathie aux doses les plus élevées
(0,36 et 0,9 mg/m3). A la dose de 0,09 g/m3 on n'a observé aucune
modification d'un certain nombre de paramètres qui puisse être
imputable à ce traitement; cette concentration peut être considérée
comme la dose sans effets nocifs observables dans les conditions d'une
exposition subchronique par la voie respiratoire.
Sous sa forme concentrée et non neutralisée, la morpholine est
extrêmement irritante pour les yeux et la peau, probablement en raison
de sa basicité. En la diluant et en la ramenant à un pH neutre, on
peut sensiblement en réduire la toxicité topique. Selon une variante
de la méthode de Buehler, la morpholine à 2% n'a pas produit de
sensibilisation chez le cobaye.
La morpholine ne produit pas de mutation chez des bactéries ou
des levures en présence ou en l'absence d'activation métabolique
(à l'exception d'un essai effectué à très forte concentration). Le
passage sur hôte a également donné des résultats négatifs.
On n'a pas non plus observé de réparation de l'ADN sous
l'influence de la morpholine dans des cultures primaires d'hépatocytes
de rats et ce composé n'a pas augmenté de façon sensible les échanges
entre chromatides soeurs dans des cellules ovariennes de hamsters
chinois. Les résultats d'une épreuve sur cellules lymphomateuses de
souris L5178Y ont conduit à considérer la morpholine comme faiblement
mutagène. Ce composé a entraîné une augmentation des foyers de type
III lors d'une épreuve de transformation cellulaire maligne effectuée
sur des cellules BALB/3T3, phénomène qui n'a pas été constaté avec la
morpholine neutralisée.
On n'a pas non plus constaté de mutation ponctuelle ni
d'aberration chromosomique chez des embryons de hamsters exposés
in utero à de la morpholine.
On n'a pas constaté d'augmentation dans l'incidence des tumeurs
chez des rats qui avaient été exposés par voie respiratoire pendant
104 semaines à des concentrations de morpholine allant jusqu'à
0,5 g/m3, ni chez des souris qui avaient reçu pendant 96 semaines
dans leur eau de boisson de l'oléate de morpholine à 1%. Une étude à
long terme sur un groupe de 104 rats qui recevaient dans leur
alimentation 1000 mg de morpholine par kg de nourriture, a permis de
mettre en évidence trois carcinomes hépatocellulaires, deux
angiosarcomes pulmonaires ainsi qu'un troisième angiosarcome de
localisation non précisée et enfin deux gliomes malins, alors
qu'aucune tumeur n'a été observée dans le groupe témoin comportant
156 animaux. Chez des hamsters exposés dans les mêmes conditions,
aucune tumeur n'a été observée.
Administrée en même temps qu'un nitrite, la morpholine donne un
résultat positif à l'épreuve de passage sur hôte, qui s'explique
probablement par la formation de NMOR. De la morpholine administrée
en même temps qu'un nitrite par mélange à la nourriture, a provoqué
chez des rats des tumeurs hépatiques et pulmonaires et chez des
hamsters des tumeurs hépatiques qui s'expliquent probablement par la
formation endogène de NMOR. La NMOR est mutagène pour les bactéries
et les levures; on a également observé avec ce composé des résultats
faiblement positifs dans l'épreuve d'échange de chromatides soeurs sur
des cellules CHO et lors de la recherche de mutations dans des
cultures de cellules lymphomateuses de souris L5178Y. La NMOR est
cancérogène pour la souris, le rat, le hamster et divers poissons;
elle provoque chez la souris des tumeurs hépatiques et pulmonaires,
chez le rats des tumeurs du foie, du rein et des vaisseaux sanguins,
chez le hamster des tumeurs des voies digestives et respiratoires
supérieures et enfin chez les poissons, des tumeurs hépatiques.
8. Effets sur l'homme
On ne dispose d'aucun rapport faisant état de cas d'intoxication
aiguë ou décrivant les effets d'une exposition à court ou à long terme
à la morpholine dans la population générale.
Un phénomène connu sous le nom de glaucopsie (vision bleutée)
ainsi que dans certains cas une irritation de la peau et des voies
respiratoires, ont été décrits dans des rapports d'exposition
professionnelle à la morpholine; toutefois, ces rapports ne précisent
pas la concentration de la morpholine dans l'air. On a indiqué que
chez des ouvriers exposés pendant 3 à 10 ans à de la morpholine à des
concentrations de 0,54-0,93 mg/m3, le nombre d'aberrations
chromosomiques dans les lymphocytes du sang périphérique ne présentait
pas de différence notable par rapport aux témoins.
La morpholine concentrée est fortement irritante pour la peau;
en solution (à 1/40) elle se révèle encore légèrement irritante.
On n'a pas étudié la cancérogénicité potentielle de la morpholine
chez les populations humaines exposées.
9. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Parmi les organismes aquatiques étudiés, certaines cyanobactéries
(Microcystis) et certaines algues bleues unicellulaires
(Scenedesmus) se révèlent être les taxa les plus sensibles puisque
le seuil de toxicité (critère: inhibition de la croissance des
populations) se situe à 1,7 mg/litre pour Microcystis et à
4,1 mg/litre pour Scenedesmus (durée de l'exposition: 8 jours).
Des bactéries aérobies telles que Pseudomonas se sont révélées
beaucoup plus résistantes; le seuil de toxicité à 16 heures ainsi que
la concentration sans effets observables sur la croissance des
populations atteindraient respectivement 310 et 8700 mg/litre.
Cependant une concentration de 1000 mg/litre a inhibé la respiration
et l'activité de la déshydrogénase (dans une proportion allant jusqu'à
20%) chez les bactéries de boues activées provenant d'usines de
traitement des effluents industriels.
Chez les protozoaires aquatiques étudiés jusqu'ici, des spécimens
des genres Entosiphon et Chilomonas (avec des valeursseuil
respectivement égales à 12 et 18 mg/litre pour ce qui est de
l'inhibition de la croissance des populations) se sont révélés être
les plus sensibles. Les valeurs de la CE50 à 24 heures
(E=immobilisation) pour la daphnie se situaient dans les limites de
100 à 120 mg/litre. Chez les poissons, les valeurs de la CL50 à
48 et 96 heures se sont révélées > 180 mg/litre lors d'épreuves
effectuées en eau douce, en eau saumâtre ou en eau de mer, la truite
arc-en-ciel étant l'espèce la plus sensible.
On ne dispose d'aucune donnée relative aux effets à long terme de
la morpholine sur les invertébrés et les vertébrés aquatiques.
L'absence d'information est également presque totale à propos de la
toxicité de ce composé pour les organismes terricoles, puisque les
seules données dont on dispose se limitent à la valeur de la CE à
3 jours, égale à 400 mg/litre, en ce qui concerne l'inhibition de la
germination des laitues.
10. Evaluation des risques pour la santé humaine et effets sur
l'environnement
10.1 Evaluation des effets sur la santé humaine
Les cas d'exposition de la population générale à la morpholine
résultent principalement de la consommation de denrées alimentaires
contaminées. La contamination du tabac et des produits du tabac, des
articles de toilette et des cosmétiques, de même que des articles en
caoutchouc, peuvent contribuer à l'exposition globale. Il peut y
avoir exposition professionnelle à la morpholine dans de nombreuses
industries; ce composé peut être absorbé soit par inhalation, soit
par la voie percutanée. On ne dispose pas de données suffisantes pour
déterminer le degré d'exposition de la population générale.
Les données relatives à l'exposition professionnelle sont
également limitées.
La morpholine n'est pas extrêmement toxique dans les conditions
d'une exposition aiguë. Après administration par voie orale, la
DL50 était de 1-1,9 g/kg de poids corporel chez le rat et de
0,9 g/kg de poids corporel chez le cobaye. Par ailleurs, on a trouvé,
pour la CL50, des valeurs de 7,8 mg/m3 chez le rat et de 4,9 à
6,9 g/m3 chez la souris.
Dans les conditions d'une exposition à court ou à long terme par
la voie respiratoire, c'est l'irritation des yeux et des voies
respiratoires qui constitue l'effet critique. Lors d'une étude de
13 semaines au cours de laquelle des rats ont été exposés 6 heures par
jour et 5 jours par semaine à de la morpholine, on a pu fixer à
90 mg/m3 la concentration sans effets nocifs observables. Lors
d'une autre étude d'inhalation, à long terme cette fois
(104 semaines), on a observé une augmentation de l'incidence des cas
d'inflammation de la cornée ou d'inflammation et de nécrose des fosses
nasales, à la dose de 540 mg/m3 chez le rat. L'irritation des yeux
et du nez présentait également une incidence accrue aux doses de 36 et
180 mg/m3.
Une forte exposition à la morpholine entraîne de graves lésions
hépatiques et rénales chez le rat et le cobaye. Après administration
de morpholine à des rats, par mélange à leur nourriture (0,5 g/kg de
poids corporel) tous les jours pendant 56 jours, on a observé une
dégénérescence graisseuse du foie. Des souris qui avaient reçu tous
les jours, pendant 13 semaines, de l'oléate de morpholine mêlé à leur
eau de boisson à la dose d'environ 0,7 g/kg de poids corporel, ont
présenté une dégénérescence albuminoïde au niveau des tubules
proximaux du rein (désignée également par l'expression "tuméfaction
trouble" par certains auteurs). Chez des souris femelles qui avaient
reçu pendant 672 jours de la morpholine dans leur alimentation, on a
observé aux doses comprises entre 0,05 et 0,4 g de morpholine
administrée sous forme d'oléate, une réduction du gain de poids.
Aux concentrations que l'on observe actuellement dans les cas
d'exposition professionnelle ou environnementale, il ne semble pas que
la morpholine risque véritablement d'entraîner des effets toxiques
généraux. Cependant lors d'une exposition accidentelle ou
professionnelle non contrôlée à de fortes concentrations de morpholine
présentes dans l'air, il peut y avoir des effets locaux qui prennent
la forme d'une irritation de la muqueuse oculaire et des voies
respiratoires; en outre, un contact avec de la morpholine liquide
(même diluée) peut entraîner une irritation cutanée.
Il ne semble pas que la morpholine soit mutagène ou cancérogène
chez l'animal. Toutefois elle peut facilement être nitrosée pour
donner naissance à de la NMOR qui s'est révélée, elle, mutagène et
cancérogène chez plusieurs espèces d'animaux de laboratoire.
L'administration de morpholine, puis d'un nitrite, mêlés à la
nourriture d'animaux de laboratoire a provoqué un accroissement de
l'incidence des tumeurs, pour la plupart des carcinomes
hépatocellulaires et des sarcomes du foie et du poumon. Il est donc
prudent de considérer l'exposition à la morpholine comme un facteur
supplémentaire de risque cancérogène chez les populations exposées.
10.2 Evaluation des effets sur l'environnement
Comme on sait très peu de choses sur l'exposition
environnementale et que les données relatives à l'exposition à long
terme dans l'hydrosphère ou à court et à long terme dans le milieu
terrestre font défaut, il est impossible, pour l'instant, de procéder
valablement à une appréciation du risque. On peut toutefois tirer un
certain nombre de conclusions sur la base des propriétés observées de
la morpholine, des données écotoxicologiques disponibles et des
quelques renseignements dont on dispose sur les concentrations dans
l'environnement.
La forte solubilité dans l'eau de la morpholine et sa faible
volatilité (dans les conditions du milieu) font que l'hydrosphère en
constitue le principal milieu récepteur.
La morpholine est intrinsèquement biodégradable et, bien que
cette biodégradation soit lente, rien n'indique qu'elle s'accumule
dans l'hydrosphère. En outre, sa bioaccumulation est également peu
probable.
On ne possède que relativement peu de données sur la toxicité de
la morpholine pour les organismes vagiles. Toutefois, il paraît
improbable qu'au niveau actuel des émissions de morpholine, des
dommages importants puissent être causés à l'environnement dans son
ensemble. Les effets locaux, dus par exemple aux émissions
industrielles ou à la libération de morpholine dans l'environnement
par suite de l'usure des pneumatiques, restent à évaluer.
Il peut y avoir contamination de certains produits alimentaires
comme le poisson, par de la morpholine, contamination qui viendrait de
l'environnement, mais cela n'est pas certain.
La transformation de la morpholine en NMOR est la principale
cause d'inquiétude, en particulier en ce qui concerne les populations
de vertébrés. On a signalé la présence de NMOR dans des eaux
résiduaires industrielles et dans le sol aux alentours d'une usine.
On peut s'inquiéter de la présence de morpholine dans de l'eau
destinée à être traitée pour la rendre potable.
11. Conclusions et recommandations
La morpholine ne présente aucun risque toxique pour l'homme au
niveau habituel d'exposition mais il faut prendre garde à sa
transformation en NMOR qui est cancérogène.
Rien n'indique qu'aux nivaux actuels d'exposition, la morpholine
constitue un risque important pour les biotes présents dans
l'environnement.
11.1 Recommandations en vue de la protection de la santé humaine
a) Il faut éviter dans la mesure du possible toute exposition
humaine à la morpholine.
b) Il faut éviter la contamination des denrées alimentaires par leur
emballage ou lors de leur transformation.
c) La morpholine ne doit pas entrer dans la composition d'articles
en caoutchouc destinés en entrer en contact direct avec l'homme.
d) La morpholine ne doit pas entrer dans la composition de
préparations destinées à la toilette ou à un usage cosmétique.
e) Les effluents industriels doivent être traités avec soin afin
d'éviter que la morpholine ne pénètre dans l'eau destinée à la
boisson.
f) Compte tenu de la possibilité de formation de NMOR cancérogène,
les limites d'exposition professionnelle actuelles devront être
réexaminées.
11.2 Recommandations en vue de la protection de l'environnement
Il faut éviter les déversements et les décharges massives dans
les usines de traitement des effluents.
11.3 Recommandations en vue de recherches ultérieures
Des travaux doivent être entrepris dans les domaines suivants:
a) toxicité pour la fonction de reproduction des mammifères;
b) toxicité à long terme pour les mammifères;
c) effets sur les mammifères d'une exposition à de faibles
concentrations de morpholine en présence ou non de nitrites et de
nitrates;
d) transnitrosation par la NMOR in vivo et in vitro;
e) biodégradation en anaérobiose, en particulier dans des conditions
entraînant la réduction des nitrates;
f) catalyse microbienne de la N-nitrosation en situation réelle;
g) concentrations de la morpholine dans les eaux souterraines, le
sol et les rivières dont l'eau est utilisée pour la boisson;
h) concentration de morpholine aux alentours d'usines qui produisent
ou transforment cette substance;
i) métabolisme et toxicocinétique chez l'homme en vue de la mise au
point de méthodes pour la surveillance biologique de la
morpholine;
j) surveillance de la concentration de morpholine et de NMOR dans
les denrées alimentaires, dans l'eau de boisson et l'air
intérieur aux habitations;
k) collecte et diffusion de données sur l'exposition
professionnelle.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Propiedades físicas y químicas
La morfolina (1-oxa-4-azaciclohexano) es un líquido incoloro,
oleoso, higroscópico y volátil que desprende un característico olor a
amina ("a pescado"). Es totalmente miscible en agua, así como en
numerosos disolventes orgánicos, y parcialmente soluble en soluciones
acuosas alcalinas. Se trata de una base, y el pKa del ácido conjugado
es de 8,33. En consecuencia, el coeficiente de reparto octanol/agua
depende del pH (log Pow -2,55 a pH 7, y - 0,84 a pH 10; 35°C). La
presión de vapor de las soluciones acuosas de morfolina es casi como
la del agua.
La morfolina puede sufrir diversas reacciones. Se comporta
químicamente como una amina secundaria. En condiciones ambientales y
fisiológicas, como resultado de la reacción de las soluciones de
nitrito o de óxidos de nitrógeno gaseosos con las soluciones diluidas
de morfolina, se forma N-nitrosomorfolina (NMOR), conocido
carcinógeno para los animales. Los niveles de óxido de nitrógeno (NO)
pueden ser importantes en la nitrosación. Las condiciones de
nitrosación, en particular el pH, tienen una considerable influencia.
2. Métodos analíticos
La morfolina se puede determinar mediante cromatografía de gases
(GC) en columnas empacadas o capilares, cromatografía líquida de alta
resolución (HPLC) y cromatografía iónica. Entre los detectores
utilizados cabe citar el detector de ionización por conductor, el
detector de fotometría de llama, el detector selectivo de nitrógeno
(NSD), y la espectrometría de masas y el analizador de energía térmica
(TEA) para la GC, y el detector de UV y el TEA para la HPLC. Para
determinar cantidades ínfimas hay que recurrir a la derivatización.
El método de elección en lo que respecta a sensibilidad es al parecer
la GC combinada con el TEA, previa transformación por derivatización
en NMOR (límite de detección de 2-3 µg/kg en diversas matrices). Las
bajas concentraciones de morfolina en el aire se pueden determinar
mediante GC y NSD.
3. Fuentes de exposición humana y ambiental
Se estima que cada año se producen industrialmente en todo el
mundo unas 25 000 toneladas de morfolina, pero no se conoce con
detalle la producción de algunos países.
El principal proceso de producción utilizado para su obtención es
al parecer la reacción de dietilenglicol con amoniáco en presencia de
hidrógeno y de catalizadores.
La morfolina es una sustancia química que puede utilizarse con
muy diversos fines, pero no se conocen todos sus posibles usos. Es
importante como producto intermedio en la industria del caucho, como
inhibidor de la corrosión, y en la síntesis de abrillantadores
ópticos, protectores de cultivos, colorantes y medicamentos. La
morfolina se utiliza como disolvente de una amplia variedad de
productos orgánicos, entre ellos resinas, colorantes y ceras. Se
puede utilizar como catalizador. La morfolina se usa aún en algunos
países para elaborar productos cosméticos y de tocador. En algunos
países se usa también en varias aplicaciones relacionadas directa o
indirectamente con los aditivos alimentarios.
La exposición humana y ambiental se debe a emisiones tanto
gaseosas como acuosas, y es también el resultado directo de alguno de
sus usos, por ejemplo como componente de productos cosméticos y de
ceras. Las emisiones más importantes son resultado probablemente de
su fabricación y de su uso en la industria química (sobre todo en la
producción y el uso de productos químicos derivados del caucho) y como
agente anticorrosión. Se ha detectado morfolina en muchos tipos de
alimento y de tabaco. En estos casos el origen del producto podría
ser la parafina empleada para proteger la fruta o en determinados
envases, pero a veces no se puede establecer su procedencia.
4. Transporte, distribución y transformación en el medio ambiente
La morfolina es químicamente estable en la biosfera, aunque sufre
nitrosación química y biológica, transformándose así en NMOR.
La morfolina es por naturaleza biodegradable. Así es en las
condiciones reinantes en las plantas de fangos activados que funcionan
correctamente. No obstante, en condiciones no idóneas probablemente
no se produce una degradación importante. El tiempo medio de
retención del sólido en las plantas de fangos activados tiene una
importancia crucial y debe ser superior a ocho días para que se
produzca una degradación fiable de la morfolina.
No se dispone de datos suficientes sobre la bioacumulación de
morfolina en organismos acuáticos y terrestres. Según el coeficiente
de reparto n-octanol/agua del producto (log Pow = -2,55 a pH 7),
no debería producirse bioacumulación.
Como es un importante producto químico industrial con una amplia
gama de aplicaciones, es previsible la presencia de morfolina o de sus
derivados en numerosos efluentes industriales. Usada como inhibidor
de la corrosión en el agua de calderas, aparece en los efluentes de
éstas, incluidos los de las centrales eléctricas en que se utiliza
morfolina. Su uso en la fabricación de aditivos del caucho da lugar a
la liberación de una cantidad indeterminada de morfolina a la
hidrosfera y la geosfera como consecuencia del desgaste de los
neumáticos y de la eliminación de neumáticos usados.
Componente de parafinas y abrillantadores, la morfolina se libera
al medio ambiente por volatilización. Es adsorbida rápidamente por la
humedad, y el principal compartimiento de posible acumulación de la
morfolina es, por tanto, la hidrosfera. No obstante, los datos
limitados disponibles parecen indicar que el producto no se acumula en
la hidrosfera.
La incineración es el método preferido de eliminación de la
morfolina no diluida, pero a veces es necesario controlar las
emisiones de óxido de nitrógeno para respetar las reglamentaciones en
materia de medio ambiente. Por lo que se refiere a los efluentes
acuosos, el tratamiento de fangos activados es suficiente, a condición
de que la planta sea cuidadosamente controlada (véase más arriba).
5. Niveles ambientales y exposición humana
No hay datos disponibles sobre los niveles de morfolina en el
aire ambiental y de locales cerrados y en el agua de bebida. Hay
datos limitados sobre su presencia en aguas naturales, y se carece de
información sobre su presencia en el suelo.
A tenor de los datos disponibles, la fuente principal de
exposición de la población general a la morfolina son los alimentos,
que pueden estar contaminados como consecuencia del tratamiento
directo de la fruta con parafinas contenedoras de morfolina a efectos
de conservación, de los tratamientos a base de vapor empleados durante
la elaboración de los alimentos, y del uso de material de envasado con
morfolina. No obstante, los datos cuantitativos disponibles acerca de
la contaminación de los alimentos por morfolina y NMOR son limitados.
Por ejemplo, en productos lácteos preenvasados se han hallado valores
comprendidos entre 5 y 77 µg/kg de morfolina y de hasta 3,3 µg/kg de
NMOR. La concentración de morfolina en diversas muestras de alimentos
(pescado, carne, productos vegetales, bebidas) no rebasaba por lo
general el valor de 1 mg/kg. Se han detectado niveles más altos
(hasta 71,1 mg/kg) en frutos cítricos en el Japón. En un estudio
realizado en Italia trabajando con un límite de detección de 0,3 µg/kg
no se halló NMOR en una serie de alimentos. Los datos disponibles no
permiten hacer una estimación de la ingesta de morfolina y NMOR a
través de los alimentos.
Se ha detectado morfolina en tabaco de cigarrillos a una
concentración de 0,3 mg/kg, y en tabaco en polvo y tabaco mascable a
concentraciones de hasta 4,0 mg/kg. En otras ocasiones se ha
notificado el hallazgo de nitrosomorfolina a niveles de hasta
0,7 mg/kg en el tabaco en polvo. En estos casos el producto provenía
probablemente de la parafina de los envases utilizados.
Se ha detectado NMOR en algunos productos cosméticos y de
tocador, como por ejemplo champús y maquillaje de ojos, así como en
artículos de goma, tales como chupetes y tetillas de biberón, a
niveles de hasta 3,5 mg/kg.
En varias industrias puede darse una exposición ocupacional a la
morfolina, pero se dispone de pocos datos sobre la exposición de
trabajadores al producto. Todos los valores notificados son
inferiores a 3 mg/m3. Se ha detectado la exposición ocupacional a
NMOR en la industria del caucho, donde se han hallado concentraciones
de hasta 250 µg/m3.
Los datos actualmente disponibles permiten hacerse una idea del
riesgo potencial de exposición humana, pero no estimar con exactitud
los niveles de exposición de las poblaciones general y laboral a la
morfolina y la NMOR.
6. Cinética y metabolismo en animales de laboratorio y en el hombre
La morfolina se absorbe por vía oral, por vía cutánea y por
inhalación. En la rata, tras su administración oral o intravenosa, la
morfolina se distribuye rápidamente y se concentra sobre todo en el
intestino y el músculo.
En el conejo, la morfolina administrada por vía intravenosa o por
inhalación se concentra preferentemente en los riñones, y en menor
medida en los pulmones, el hígado y la sangre.
La morfolina no se une de forma importante a las proteínas del
plasma. Se han notificado semividas plasmáticas de 115 (rata),
120 (hámster) y 300 minutos (cobayo).
La morfolina se excreta principalmente inalterada por vía renal
en diversas especies. Al cabo de un día de su administración, se
halló en la orina el 70%-90% de la morfolina. La neutralización de la
morfolina acelera su excreción. Un pequeño porcentaje se excreta a
través del aire espirado y de las heces.
Estudios realizados en la rata, el ratón, el hámster y el conejo
muestran que la morfolina se elimina casi enteramente en su forma no
metabolizada. En el cobayo, puede darse una reacción de
N-metilación seguida de N-oxidación, metabolizándose así hasta un
20% de la dosis administrada. En presencia de nitrito, la morfolina
se puede transformar en NMOR tanto in vitro como in vivo. En
función de la dosis, entre el 0% y el 12% de la morfolina administrada
a ratas junto con nitritos puede sufrir nitrosación.
La inmunoestimulación, que entraña la activación de macrófagos,
puede aumentar el grado de nitrosación.
7. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
En lo que respecta a la toxicidad aguda, la DL50 de la
morfolina administrada oralmente es de 1-1,9 g/kg de peso corporal y
0,9 g/kg de peso corporal en la rata y el cobayo, respectivamente.
Las ratas que recibieron morfolina neutralizada (1 g/kg de peso
corporal) sobrevivieron. Tras administración interperitoneal, la
DL50 fue de 0,4 g/kg de peso corporal en el ratón y de 0,1 -
0,4 g/kg de peso corporal en la rata. En los experimentos de
exposición por inhalación, la DL50 fue de aproximadamente 8 g/m3
en la rata y de entre 5 y 7 g/m3 en el ratón. Por vía cutánea la
DL50 en el conejo fue de 0,5 ml/kg de morfolina no diluida. La
intoxicación aguda por morfolina se caracteriza por la aparición de
hemorragia gastrointestinal y diarrea tras la exposición oral, y de
irritación y hemorragias en la nariz, la boca, los ojos y los pulmones
tras la inhalación. En un estudio realizado durante 30 días con ratas
a las que se administraron con sonda dosis de 0,16 - 0,8 g/kg de peso
corporal, se observaron efectos tóxicos graves y mortalidad a todas
las dosis empleadas. En el cobayo se observó también toxicidad grave
y mortalidad a todas las dosis en el margen de 0,09 a 0,45 g/kg de
peso corporal.
Se ha notificado la aparición de alteraciones de la función
pulmonar en la rata tras la exposición a morfolina por inhalación
durante cortos periodos (7,2 g/m3, 4 h/día, 4 días y 1,63 g/m3,
4 h/día, 5 días/semana, 30 días). La mortalidad en la rata osciló
entre 0% y 100% según el nivel de exposición (0,36 - 18,1 g/m3,
6 h/día, 9 días). La toxicidad por inhalación dependía de la dosis,
observándose diversos grados de irritación local (ojos, boca, nariz,
pulmones) y hemorragias a los niveles más altos de exposición. En un
estudio se detectó un aumento de la función de la glándula tiroides, y
en otro necrosis del hígado y de los túbulos renales, como resultado
de la exposición por inhalación.
Un estudio de 90 días de duración reveló que la morfolina
administrada por vía oral (0,2 - 0,7 g/kg de peso corporal al día)
durante ese espacio de tiempo puede reducir el aumento del peso
corporal y la función renal en el ratón. Se ha notificado la
aparición de hiperplasia del epitelio del estómago anterior en el
ratón como resultado de la exposición oral a morfolina
(0,28 - 0,5 g/kg de peso corporal al día) durante 672 días.
En un estudio de 13 semanas sobre los efectos de su inhalación,
la morfolina (0,09 - 0,9 g/m3, 6 h/día, 5 días/semana) causó
lesiones dosis-dependientes de la mucosa nasal y neumonía a los
niveles de exposición más altos (0,36 y 0,9 mg/m3). Un cierto
número de parámetros no variaron en respuesta al tratamiento cuando se
utilizaron 0,09 g/m3; esta concentración puede considerarse el nivel
sin efectos adversos observados (NOAEL) en las condiciones de
exposición por inhalación subcrónica.
La morfolina no diluida y no neutralizada es altamente irritante
para los ojos y la piel, probablemente a causa de sus propiedades
alcalinas. La dilución y la neutralización de su pH pueden reducir
considerablemente su toxicidad tópica. La morfolina (2%) no indujo
sensibilidad en el cobayo al aplicar el método modificado de Buehler.
La morfolina no indujo la aparición de mutaciones en bacterias o
levaduras, con o sin activación metabólica (salvo en un caso a una
concentración muy alta). Se obtuvieron resultados negativos en el
ensayo realizado por mediación de un huésped.
La morfolina no indujo reparación del ADN en hepatocitos
primarios de rata, así como tampoco un aumento importante del
intercambio de cromátides hermanas en células ováricas de hámster
chino. Se consideró que la morfolina tenía efectos ligeramente
mutagénicos en el ensayo con células L5178Y de linfoma de ratón. El
producto aumentó los focos de tipo III en el ensayo de transformación
de células malignas BALB/3T3, lo que no ocurrió con la morfolina
neutralizada.
La morfolina no causó ni mutaciones puntuales ni aberraciones
cromosómicas en embriones de hámster expuestos in utero.
No se observó ningún aumento de la incidencia de tumores en ratas
sometidas a niveles de hasta 0,5 g/m3 de morfolina por inhalación
durante 104 semanas, así como tampoco en ratones que ingirieron oleato
de morfolina al 1% con el agua de bebida durante 96 semanas. En un
estudio de larga duración realizado con un grupo de 104 ratas que
recibieron 1000 mg de morfolina/kg dieta, se observaron tres casos de
carcinoma de células hepáticas, dos de pulmón y uno de angiosarcoma
(no especificado), así como dos gliomas malignos, mientras que en un
grupo testigo de 156 ratas no se observaron tumores. No se detectaron
tumores en hámsters sometidos a idénticas condiciones.
La morfolina administrada al mismo tiempo que nitrito da lugar a
resultados positivos en el ensayo realizado por mediación de un
huésped, probablemente debido a la formación de NMOR. La ingestión
simultánea de morfolina y nitrito indujo la aparición de tumores
hepáticos y pulmonares en la rata y de tumores hepáticos en el
hámster, probablemente a causa de la formación endógena de NMOR. La
NMOR es mutágena en bacterias y levaduras; se notificaron resultados
ligeramente positivos en lo que respecta al intercambio de cromátides
hermanas en células CHO, así como a la aparición de mutaciones en
células L5178Y de linfoma de ratón. La NMOR es carcinógena en el
ratón, la rata, el hámster y diversos peces, y produce tumores de
hígado y de pulmón en el ratón; de hígado, riñón y vasos sanguíneos en
la rata; de hígado y de las vías digestivas y respiratorias superiores
en el hámster, y de hígado en peces.
8. Efectos en el hombre
No se han descrito casos de intoxicación aguda o de efectos a
corto o largo plazo de la exposición a morfolina en la población
general.
En informes sobre la exposición ocupacional a la morfolina se ha
descrito el fenómeno conocido como visión azul o glaucopsia, así como
algunos casos de irritación de la piel y del tracto respiratorio, pero
sin hacer mención de las concentraciones atmosféricas de morfolina.
Se señaló que el número de aberraciones cromosómicas en los linfocitos
de sangre periférica de trabajadores expuestos durante 3 a 10 años a
concentraciones de morfolina de 0,54 - 0,93 mg/m3 no diferían
significativamente del número hallado en los testigos.
La morfolina no diluida es muy irritante para la piel; una
solución diluida (1/40) tuvo efectos moderadamente irritantes.
No se ha investigado el potencial carcinógeno de la morfolina en
poblaciones humanas expuestas.
9. Efectos sobre otros organismos en el laboratorio y en el terreno
Entre los microorganismos acuáticos estudiados, determinadas
cianobacterias (Microcystis) y algas verdes unicelulares
(Scenedesmus) son al parecer las especies más sensibles según se
desprende de los valores de la toxicidad liminal (empleando como
criterio la inhibición del crecimiento de la población) notificados
(duración de la exposición: 8 días): 1,7 mg/litro para Microcystis,
y 4,1 mg/litro para Scenedesmus.
Bacterias aerobias tales como Pseudomonas resultaron ser mucho
más resistentes: la toxicidad liminal a las 16 horas y la NOEC para
el crecimiento de la población se han cifrado, respectivamente, en
310 y 8700 mg/litro. No obstante, una concentración de 1000 mg/litro
inhibía la respiración y la actividad deshidrogenasa (hasta un 20%) en
fangos activados de plantas de tratamiento industrial.
Entre los protozoos acuáticos analizados hasta ahora, la mayor
sensibilidad corresponde a ejemplares de los géneros Entosiphon y
Chilomonas (con toxicidades liminales de 12 y 18 mg/litro,
respectivamente, para la inhibición del crecimiento de la población).
En Daphnia, la CE a las 24 horas (E = inmovilización) se ha
establecido en valores comprendidos entre 100 y 120 mg/litro. Los
valores notificados para la CL50 a las 48 - 96 horas en peces
estudiados en agua dulce, salobre o marina fueron > 180 mg/litro, y
la especial más sensible en este sentido es la trucha arco iris.
No se dispone de datos sobre los efectos a largo plazo en
invertebrados y vertebrados acuáticos. La información sobre la
toxicidad de la morfolina en organismos silvestres del suelo es casi
inexistente, pues se limita a una CE a los 3 días de aproximadamente
400 mg/litro para la inhibición de la germinación en la lechuga.
10. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
10.1 Evaluación de los efectos en la salud humana
La principal vía de exposición de la población general a la
morfolina es el consumo de alimentos contaminados. También puede
contribuir a la exposición general la contaminación del tabaco y de
los productos derivados del tabaco, así como de los artículos
cosméticos y de tocador y de los productos de goma. En numerosas
industrias se da una exposición ocupacional a la morfolina; el
compuesto es absorbido por inhalación y por vía cutánea. No hay datos
suficientes para determinar el grado de exposición de la población
general. Los datos disponibles sobre la exposición ocupacional al
producto también son limitados.
La morfolina no es altamente tóxica en condiciones de exposición
aguda. La DL50 tras administración oral es de 1 a 1,9 g/kg de peso
corporal en la rata y de 0,9 g/kg de peso corporal en el cobayo. Se
han notificado CL50 de 7,8 mg/m3 (rata) y 4,9 - 6,9 g/m3
(ratón).
En las condiciones de exposición por inhalación de corta y larga
duración, los efectos críticos son al parecer la irritación de los
ojos y las vías respiratorias. Puede considerarse que el NOAEL
corresponde a una concentración de 90 mg/m3 en las condiciones en
que se llevó a cabo el experimento de 13 semanas en la rata (6 h/día,
5 días/semana). En un estudio de larga duración (104 semanas) sobre
los efectos de la inhalación del producto se observó una mayor
incidencia de inflamación de la córnea y de inflamación y necrosis de
la cavidad nasal en ratas sometidas a 540 mg/m3. A concentraciones
de 36 y 180 mg/m3 se observó también una mayor incidencia de
irritación de los ojos y de la cavidad nasal.
Las exposiciones altas a la morfolina causan lesiones graves del
hígado y los riñones en la rata y el cobayo. Se ha notificado la
aparición de degeneración grasa del hígado en la rata como resultado
de la ingestión diaria de morfolina (0,5 g/kg de peso corporal)
durante 56 días. En el ratón la administración de una sal de
morfolina y ácido oleico en el agua de bebida a una dosis de
aproximadamente 0,7 g/kg de peso corporal al día durante 13 semanas
dio lugar a una hinchazón turbia de los túbulos proximales renales.
Se observó una disminución del aumento del peso corporal en los
ratones hembra del experimento de administración prolongada (672 días)
de una dieta con dosis comprendidas entre 0,05 y 0,4 g de morfolina
(en forma de sal del ácido oleico).
A los niveles que según se ha notificado alcanza actualmente la
exposición ocupacional y ambiental, no parece que la morfolina entrañe
ningún riesgo importante de efectos tóxicos sistémicos. Pueden
aparecer efectos locales (irritación) en los ojos y las vías
respiratorias en las exposiciones ocupacionales y accidentales no
controladas a altas concentraciones de morfolina transmitida por el
aire, y el contacto con morfolina líquida (incluso diluida) puede
causar irritación cutánea.
La morfolina no parece tener efectos mutágenos o carcinógenos en
los animales. No obstante, fácilmente puede nitrosarse y convertirse
en NMOR producto mutágeno y carcinógeno en varias especies de animales
de experimentación. En la rata, la morfolina ingerida secuencialmente
con nitrito dio lugar a un aumento de los tumores, sobre todo de
carcinoma hepatocelular y de sarcomas del hígado y los pulmones. Así
pues, por prudencia, conviene considerar que la exposición a la
morfolina aumenta el riesgo de carcinogénesis en las poblaciones
expuestas.
10.2 Evaluación de los efectos en el medio ambiente
Habida cuenta de los muy limitados conocimientos respecto a la
exposición ambiental, así como de la carencia de datos sobre los
efectos de la exposición de larga duración en el agua y la exposición
de corta y larga duración en el medio terrestre, por el momento no es
posible hacer una evaluación rigurosa de los riesgos. Sobre la base
de las propiedades conocidas de la morfolina, de la información
ecotoxicológica disponible y de los escasos datos sobre su
concentración en el medio ambiente, es posible extraer algunas
conclusiones.
Debido a la alta hidrosolubilidad de la morfolina y a su baja
volatilidad (en condiciones ambientales), su principal sumidero
ambiental es la hidrosfera.
La morfolina es por naturaleza biodegradable y, aunque la
degradación es lenta, no hay datos que lleven a pensar que se acumula
en la hidrosfera. Su bioacumulación es improbable.
Hay relativamente pocos datos sobre la toxicidad de la morfolina
en organismos silvestres. No obstante, parece improbable que los
niveles actuales de emisión de morfolina puedan causar daños
importantes en un radio de acción amplio. Restan por evaluar los
efectos locales, como por ejemplo los debidos a emisiones industriales
o a la morfolina liberada por el desgaste de los neumáticos.
La contaminación de algunos alimentos, como el pescado, por
morfolina puede deberse a contaminación ambiental, pero ello no es
seguro.
La conversión de la morfolina en NMOR es la principal causa de
inquietud, sobre todo en relación con las poblaciones de vertebrados.
Se ha notificado la presencia de NMOR en aguas residuales industriales
y en el suelo próximo a una fábrica. La presencia de morfolina en
agua destinada a transformarse por elaboración en agua de bebida
suscita preocupación.
11. Conclusiones y recomendaciones
La morfolina no entraña riesgos de toxicidad para el hombre a los
niveles habituales de exposición, pero hay que tener en cuenta que
puede transformarse en el carcinógeno NMOR.
No hay ningún indicio de que a los niveles actuales de exposición
la morfolina entrañe un riesgo sustancial para la biota en el medio
ambiente.
11.1 Recomendaciones para la protección de la salud humana
a) En la medida de lo posible debe evitarse la exposición humana a
la morfolina.
b) Debe evitarse que los alimentos se contaminen durante su envasado
y elaboración.
c) Se evitará que contengan morfolina los productos de goma con los
que el hombre haya de tener contacto directo.
d) No debe emplearse morfolina en la preparación de productos
cosméticos o de tocador.
e) Los efluentes industriales deben ser objeto de un riguroso
tratamiento para evitar la contaminación por morfolina del agua
de bebida.
f) Habida cuenta de la formación del carcinógeno NMOR es necesario
replantearse los actuales límites de exposición ocupacional.
11.2 Recomendaciones para la protección del medio ambiente
Debe evitarse que las plantas de tratamiento de efluentes sufran
derrames y sobrecargas.
11.3 Recomendaciones para ulteriores investigaciones
Deben emprenderse estudios sobre los siguientes temas:
a) toxicidad reproductiva en mamíferos;
b) toxicidad a largo plazo en mamíferos;
c) efecto de la exposición de mamíferos a niveles bajos de
morfolina, con y sin nitrito y nitrato;
d) transnitrosación por NMOR in vivo e in vitro;
e) biodegradación en condiciones anaerobias, sobre todo en
condiciones favorecedoras de la reducción de nitratos;
f) catálisis microbiana de la N-nitrosación en condiciones
realistas;
g) niveles ambientales de morfolina en las aguas subterráneas, el
suelo y los ríos a partir de los cuales se obtenga agua de
bebida;
h) niveles ambientales de morfolina en torno a las fábricas
productoras y procesadoras de morfolina;
i) metabolismo y toxicocinética en el hombre, como parte del
desarrollo de métodos para la vigilancia biológica de la
morfolina;
j) vigilancia de los niveles de morfolina y de NMOR en los
alimentos, el agua de bebida y el aire de locales cerrados;
k) se deben reunir y poner a disposición datos sobre la exposición
ocupacional.
 health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
Members
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany (Joint Rapporteur)
Dr J.S. Knapp, Department of Microbiology, University of Leeds, Leeds,
United Kingdom (Joint Rapporteur)
Dr I. Linhart, Centre of Industrial Hygiene and Occupational Diseases,
National Institute of Public Health, Prague, Czech Republic
Dr U. Schiecke, Federal Environmental Agency, Berlin, Germany
Dr J.A. Sokal, Institute of Occupational Medicine and Environmental
Health, Sosnowiec, Poland (Chairman)
Representatives of other organizations
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International
Union of Toxicology (Vice-Chairman)
Secretariat
Mrs C. Partensky, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
A WHO Task Group on Environmental Health Criteria for Morpholine
met at the World Health Organization, Geneva, from 8 to 11 November
1994. Dr E.M. Smith, IPCS, welcomed the participants on behalf of Dr
M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to
morpholine.
The first draft of this monograph was prepared by Dr J. Kielhorn
and Dr G. Rosner, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany. The second revised draft was prepared by
Dr J. Kielhorn. Dr E.M. Smith and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
CHO Chinese hamster ovary
DOC dissolved organic carbon
FID flame ionization detector
FPD flame photometric detector
GC gas chromatography
HPLC high-performance liquid chromatography
IC ion chromatography
MLSS mixed liquor suspended solids
MS mass spectrometry
NMOR N-nitrosomorpholine
NO nitrogen oxide
NOAEL no-observed-adverse-effect level
NSD nitrogen selective detector
OECD Organisation for Economic Co-operation and Development
TEA thermal energy analyser
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Physical and chemical properties
Morpholine (1-oxa-4-azacyclohexane) is a colourless, oily,
hygroscopic, volatile liquid with a characteristic amine ("fishy")
odour. It is completely miscible with water, as well as with many
organic solvents, but has limited solubility in alkaline aqueous
solutions. It is a base, the pKa of the conjugated acid being 8.33.
Correspondingly, the octanol-water partition coefficient is pH-
dependent (log Pow -2.55 at pH 7 and -0.84 at pH 10; 35°C). The
vapour pressure of aqueous solutions of morpholine is very close to
that of water.
Morpholine can undergo a variety of reactions. It behaves
chemically as a secondary amine. Under environmental and
physiological conditions, the proven animal carcinogen
N-nitrosomorpholine (NMOR) is formed by reaction of solutions of
nitrite or gaseous nitrogen oxides with dilute solutions of
morpholine. Nitrogen oxide (NO) levels may be of importance in
nitrosation. The conditions of nitrosation, in particular pH, play a
significant role.
1.2 Analytical methods
Morpholine can be determined by gas chromatography (GC) with
packed as well as capillary columns, high-performance liquid
chromatography (HPLC) and ion chromatography (IC). Detectors used
include flame ionization detector (FID), flame photometric detector
(FPD), nitrogen selective detector (NSD), and mass spectrometry (MS)
and thermal energy analyser (TEA) for GC, and UV-detector and TEA for
HPLC. For the determination of trace amounts, derivatization is
required. The method of choice for sensitivity seems to be GC with
TEA following the derivatization to NMOR (the detection limit is
2-3 µg/kg in various matrices). Low concentrations of morpholine in
air can be determined by GC with NSD.
1.3 Sources of human and environmental exposure
It is estimated that around 25 000 tonnes of morpholine per year
are produced industrially world-wide, but details of production from
some countries are lacking.
The main production process used appears to be the reaction of
diethylene glycol with ammonia in the presence of hydrogen and
catalysts.
Morpholine is an extremely versatile chemical but knowledge of
its uses is incomplete. It is important as a chemical intermediate in
the rubber industry, as a corrosion inhibitor, and in the synthesis of
optical brighteners, crop protection agents, dyes and drugs.
Morpholine is used as a solvent for a large variety of organic
materials, including resins, dyes and waxes. It can be used as a
catalyst. Morpholine is still used in some countries in toiletry and
cosmetic products. It is used in some countries in several direct and
indirect food additive applications.
Human and environmental exposure arises from both gaseous and
aqueous emissions and directly from some of its uses, including, for
example, its use in cosmetic formulations and waxes. The main
emissions probably result from its manufacture and its use in the
chemical industry (notably in production and use of rubber chemicals)
and as an anti-corrosion agent. Morpholine has been detected in a
wide variety of foods and tobacco. It could be that this morpholine
arises from the wax coatings on fruit or on packaging, but in some
cases its origin is unknown.
1.4 Environmental transport, distribution and transformation
Morpholine is chemically stable in the biosphere although it is
subject to chemical and biological nitrosation to NMOR.
Morpholine is inherently biodegradable. Under the conditions of
model activated sludge plants, morpholine is biodegradable. However,
under non-adapted conditions there is probably no significant
degradation of morpholine. The mean solid retention time in activated
sludge plants is of crucial importance and must be over 8 days if
reliable morpholine degradation is to be achieved.
There are inadequate data on the bioaccumulation of morpholine in
aquatic and terrestrial organisms. From the n-octanol/water
partition coefficient for morpholine (log Pow = -2.55 at pH7), no
bioaccumulation would be expected.
As morpholine is an important industrial chemical with a wide
range of applications, the presence of the compound or its derivatives
is to be expected in many industrial effluents. Its use as a
corrosion inhibitor in boiler water means that it will be found in
boiler wastewater, including that from power plants using morpholine.
Its use in the manufacture of rubber additives results in an
undefinable amount of morpholine being released into the hydrosphere
or geosphere through tyre abrasion and disposal of used tyres.
As a result of its use in waxes and polishes, morpholine is
released into the environment through volatilization. It is quickly
adsorbed by moisture. The main compartment for accumulation of
morpholine is therefore the hydrosphere. The limited data suggest that
morpholine does not accumulate in the hydrosphere.
Incineration is the preferred method of disposal for undiluted
morpholine, but nitrogen oxide emission controls may be required to
meet environmental regulations. For aqueous effluents, activated
sludge treatment is adequate, but only if the plant is carefully
controlled (see above).
1.5 Environmental levels and human exposure
There are no data available on levels of morpholine in ambient
and residential indoor air and in drinking-water. There are limited
data on its occurrence in natural waters and no information on its
occurrence in soil.
Based on the available data, the main source of general
population exposure to morpholine is food, which can be contaminated
with morpholine through direct treatment of fruit with waxes
containing morpholine for conservation purposes, through steam
treatment during food processing, and by the use of packaging material
containing morpholine. However, quantitative data on food
contamination by morpholine and NMOR are limited. For example, in
prepacked milk products, values ranged from 5 to 77 µg/kg morpholine
and up to 3.3 µg/kg NMOR. Morpholine content in various food samples
(fish, meat, plant products, beverages) usually did not exceed
1 mg/kg. Higher levels (up to 71.1 mg/kg) were detected in citrus
fruits in Japan. A survey in Italy did not identify NMOR in a variety
of foods at a detection limit of 0.3 µg/kg. Existing data do not
permit an estimation of the intake of morpholine and NMOR from food.
Morpholine has been found in cigarette tobacco at a concentration
of 0.3 mg/kg, and in snuff and chewing tobacco at concentrations up to
4.0 mg/kg. Levels of NMOR up to 0.7 mg/kg have been reported in the
past in snuff. These were probably associated with the use of
morpholine-containing waxes in packaging.
NMOR has been detected in some toiletry and cosmetic products,
e.g., shampoos and eye make-up, and in rubber articles, e.g. baby
pacifiers and feeding bottle teats, at levels up to 3.5 mg/kg.
Occupational exposure to morpholine may occur in several
industries. There are few data on exposure of workers to morpholine.
All reported values are below 3 mg/m3. Occupational exposure to
NMOR has been found in the rubber industry, where concentrations up to
250 µg/m3 have been measured.
The data currently available provide an indication of the
potential for human exposure but do not allow a precise estimation of
the levels of exposure of the general and occupational populations to
morpholine and NMOR.
1.6 Kinetics and metabolism in laboratory animals and humans
Morpholine is absorbed after oral, dermal and inhalation
exposure. In the rat following oral and intravenous administration,
morpholine is rapidly distributed, the highest concentrations being
found in the intestine and muscle.
In the rabbit, following intravenous and inhalation exposure,
morpholine is preferentially distributed to the kidneys, lower
concentrations reaching the lung, liver and blood.
Morpholine does not bind significantly to plasma proteins.
Plasma half-lives have been reported to be 115 (rat), 120 (hamster),
and 300 min (guinea-pig).
Morpholine is excreted mainly via the renal route, as the
unchanged compound, in a variety of species. One day after
administration, 70-90% of morpholine was found in urine.
Neutralization of morpholine enhances the rate of excretion of the
compound. A small percentage of morpholine is excreted in expired air
and faeces.
Studies in rats, mice, hamster and rabbit indicate that
morpholine is eliminated almost completely as the unmetabolized
compound. In the guinea-pig, N-methylation followed by Noxidation
can occur, with up to 20% of the administered dose being metabolized.
In the presence of nitrite, morpholine can be converted to NMOR both
in vitro and in vivo. Depending on the dose, 0-12% of morpholine
administered to rats with nitrites may be nitrosated.
Immunostimulation, involving macrophage activation, may increase
the extent of nitrosation.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of morpholine after oral administration shows
LD50 values of 1-1.9 g/kg body weight and 0.9 g/kg body weight in
the rat and guinea-pig, respectively. Rats receiving neutralized
morpholine (1 g/kg body weight) survived. After intraperitoneal
administration, the LD50 was 0.4 g/kg body weight in the mouse and
between 0.1 and 0.4 g/kg body weight in the rat. After inhalation
exposure, the LD50 was about 8 g/m3 in the rat and between 5 and
7 g/m3 in the mouse. The dermal LD50 was 0.5 ml/kg of undiluted
morpholine in the rabbit. The acute toxicity of morpholine is
characterized by gastrointestinal haemorrhage and diarrhoea after oral
exposure, and irritation and haemorrhage of the nose, mouth, eyes and
lung after inhalation. In a 30-day gavage study on rats at doses of
0.16 - 0.8 g/kg body weight, there were severe toxic effects and
mortality at all dose levels. In the guinea-pig at doses of
0.09 - 0.45 g/kg body weight there was also severe toxicity and
mortality at all dose levels.
After short-term inhalation exposure to morpholine (7.2 g/m3,
4 h/day, 4 days and 1.63 g/m3, 4 h/day, 5 days/week, 30 days),
alterations in lung function have been reported in rats. Mortality
rate in the rat ranged from 0 to 100% depending on exposure level
(0.36-18.1 g/m3, 6 h/day, 9 days). Inhalation toxicity was dose-
related with various degrees of local irritation (eyes, mouth, nose,
lung) and haemorrhage at the higher exposure levels. One study
reported increased function of thyroid gland and another necrosis of
liver and renal tubules after inhalation exposure.
A 90-day study showed that morpholine administered orally
(0.2-0.7 g/kg body weight per day) for 90 days may reduce body weight
gain and renal function in the mouse. After 672 days of oral exposure
to morpholine (0.28-0.5 g/kg body weight per day), forestomach
epithelium hyperplasia was reported (mouse).
In a 13-week inhalation study, morpholine (0.09-0.9 g/m3,
6 h/day, 5 days/week) has been reported to cause dose-related lesions
of nasal mucosa and pneumonia at the higher exposure levels (0.36 and
0.9 mg/m3). No treatment-related changes to a number of parameters
were observed at 0.09 g/m3; this concentration may be considered a
no-observed-adverse-effect level (NOAEL) under the conditions of
sub-chronic inhalation exposure.
Morpholine in the undiluted and unneutralized form is highly
irritant for the eye and skin, probably due to its alkaline
properties. Dilution and neutralization of its pH may significantly
reduce its topical toxicity. Morpholine (2%) did not induce
sensitivity in the guinea-pig using the modified Buehler method.
Morpholine did not induce mutations in bacteria or yeasts with
and without metabolic activation (with one exception at a very high
concentration). It was negative in the host-mediated assay.
Morpholine did not induce DNA-repair in primary rat hepatocytes
and did not induce a significant increase in sister chromatid exchange
in Chinese hamster ovary cells. Morpholine was considered to be weakly
mutagenic in the L5178Y mouse lymphoma assay. It increased type III
foci in the BALB/3T3 malignant cell transformation assay, although
neutralized morpholine did not.
Morpholine caused neither point mutation nor chromosomal
aberration in hamster embryos exposed in utero.
No increase in the incidence of tumours was seen in rats given up
to 0.5 g/m3 morpholine by inhalation for 104 weeks nor in mice given
1% morpholine oleate in their drinking-water for 96 weeks. In a long-
term study on a group of 104 rats given 1000 mg morpholine/kg diet,
there were three liver cell carcinoma, two lung and another
angiosarcoma (unspecified) and two malignant glioma, whereas in a
control group of 156 rats there were no tumours. With hamsters under
the same conditions, no tumours were found.
Morpholine given simultaneously with nitrite yields positive
results in the host-mediated assay, probably due to the formation of
NMOR. Morpholine fed simultaneously with nitrite induced liver and
lung tumours in rats and liver tumours in hamsters probably due to the
endogenous formation of NMOR. NMOR is mutagenic in bacteria and
yeasts; weakly positive results were reported for sister chromatid
exchange in CHO cells and for mutations in mouse lymphoma L5178Y
cells. NMOR is carcinogenic in mice, rats, hamsters and various
fishes, producing liver and lung tumours in mice, liver, kidney and
blood vessel tumours in rats, liver, upper digestive and respiratory
tract tumours in hamsters, and liver tumours in fish.
1.8 Effects on humans
There have been no reports on incidents of acute poisoning or on
the effects of short- or long-term exposure to morpholine by the
general population.
The phenomenon known as blue vision or glaucopsia, as well as
some instances of skin and respiratory tract irritation, have been
described in reports of occupational exposure to morpholine; however,
no atmospheric concentrations of morpholine were given. It was
reported that the number of chromosomal aberrations in the lymphocytes
of peripheral blood of workers exposed for 3-10 years to morpholine at
concentrations of 0.54-0.93 mg/m3 did not differ significantly from
controls.
Undiluted morpholine is strongly irritant to skin; a dilute
solution (1 to 40) was mildly irritant.
The potential carcinogenicity of morpholine in exposed human
populations has not been investigated.
1.9 Effects on other organisms in the laboratory and field
Among the aquatic organisms tested, certain cyanobacteria
(Microcystis) and unicellular green algae (Scenedesmus) appear to
be the most sensitive taxa as toxicity threshold values (criterion:
inhibition of population growth) of 1.7 mg/litre for Microcystis and
4.1 mg/litre for Scenedesmus have been reported (duration of
exposure: 8 days).
Aerobic bacteria like Pseudomonas proved to be much more
resistant: the 16-h toxicity threshold and the NOEC for population
growth have been cited as 310 and 8700 mg/litre, respectively.
However, 1000 mg/litre inhibited respiration and dehydrogenase
activity (up to 20%) in activated sludge from industrial treatment
plants.
Among aquatic protozoans tested so far, representatives of the
genera Entosiphon and Chilomonas (with threshold values of 12 and
18 mg/litre, respectively, for the inhibition of population growth)
turned out to be the most sensitive. The 24-h EC values
(E=immobilisation) for Daphnia were in the range of
100-120 mg/litre. The 48- to 96-h LC50 values reported for fish
tested in fresh, brackish or seawater were > 180 mg/litre, rainbow
trout being the most sensitive species.
No data on long-term effects in aquatic invertebrates and
vertebrates are available. Information about the toxicity of
morpholine in free-living soil organisms is almost entirely lacking,
being restricted to a 3-day EC value of about 400 mg/litre given for
germination inhibition in lettuce.
1.10 Evaluation of human health risks and effects on the environment
1.10.1 Evaluation of effects on human health
The general population is primarily exposed to morpholine by
consumption of contaminated food. Contamination of tobacco and
tobacco products, and cosmetic and toiletry articles and rubber
products may also contribute to overall exposure. Occupational
exposure to morpholine occurs in many industries; the compound is
absorbed by inhalation and skin absorption. Data are inadequate to
determine the degree of exposure of the general population. Data on
occupational exposure to morpholine are also limited.
Morpholine is not highly toxic under conditions of acute
exposure. The LD50 after oral administration is 1-1.9 g/kg body
weight in rats and 0.9 g/kg body weight in guinea-pigs. LC50 values
of 7.8 mg/m3 (rats) and 4.9-6.9 g/m3 (mice) have been reported.
In the conditions of short-term and long-term inhalation
exposure, the critical effects appear to be irritation of the eyes and
respiratory tract. A concentration of 90 mg/m3 may be considered the
NOAEL in the conditions of the 13-week experiment in rats (6 h/day,
5 days/week). In a long-term inhalation study (104 weeks), increased
incidences of inflammation of the cornea, and inflammation and
necrosis of the nasal cavity were observed in rats at 540 mg/m3.
Increased incidence of irritation of eyes and nose was also observed
at 36 and 180 mg/m3.
High exposures to morpholine causes severe damage to the liver
and kidneys of rats and guinea-pigs. Fatty degeneration of the liver
was reported in rats after feeding morpholine (0.5 g/kg body weight)
daily for 56 days. When administered morpholine oleic acid salt in
the drinking-water at a dose of about 0.7 g/kg body weight per day for
13 weeks, mice showed cloudy swelling of the kidney proximal tubules.
Decreased body weight gain was observed in female mice in the long-
term (672 days) feeding experiment at dose levels between 0.05 and
0.4 g morpholine (as oleic acid salt).
At the reported levels of the present occupational and
environmental exposures, morpholine does not seem to create any
significant risk of systemic toxic effects. Local effects (irritation)
of the eyes and respiratory tract may occur in non-controlled
occupational and incidental exposures to high concentrations of
airborne morpholine, and skin irritation may result from contact with
liquid (even diluted) morpholine.
Morpholine does not appear to be mutagenic or carcinogenic in
animals. However, it can be easily nitrosated to form NMOR, which is
mutagenic and carcinogenic in several species of experimental animals.
Morpholine fed to rats sequentially with nitrite caused an increase in
tumours, mostly hepatocellular carcinoma and sarcomas of the liver and
lungs. It is therefore prudent to consider exposure to morpholine as
increasing the carcinogenic risk in exposed populations.
1.10.2 Evaluation of effects on the environment
In view of the very restricted knowledge regarding environmental
exposure, the lack of effect data relating to long-term exposure in
water and to short- and long-term exposure in the terrestrial
environment, a sound risk assessment cannot be carried out at present.
On the basis of the reported properties of morpholine, the available
ecotoxicological information and the few data on environmental
concentrations, certain conclusions can be drawn.
The high water solubility of morpholine and its low volatility
(under environmental conditions) make the hydrosphere the pre-dominant
environmental sink.
Morpholine is inherently biodegradable and, although degradation
is slow, there are no data to suggest accumulation in the hydrosphere.
Bioaccumulation is unlikely.
There are relatively few data on toxicity of morpholine to free-
living organisms. However, it seems unlikely that current levels of
morpholine emission cause any significant damage to the wider
environment. Local effects, due for example to factory emissions or
to morpholine release due to wear of tyres, remain to be evaluated.
Contamination of some foods, e.g., fish, with morpholine may be
due to environmental contamination, but this is uncertain.
Conversion of morpholine to NMOR is the main cause of concern,
especially with respect to vertebrate populations. NMOR has been
reported in industrial wastewater and in soil near a factory. The
presence of morpholine in water destined for processing to drinking-
water is a cause for concern.
1.11 Conclusions and recommendations
Morpholine does not present a toxic risk to humans at the usual
levels of exposure, but its conversion to the carcinogenic NMOR should
be noted.
There is no evidence at present levels of exposure that
morpholine poses a substantial risk to biota in the environment.
1.11.1 Recommendations for protection of human health
a) Human exposure to morpholine should be avoided as far as
possible.
b) Contamination of food through food packaging and food processing
should be avoided.
c) Morpholine should not be used in rubber products intended for
direct contact with humans.
d) Morpholine should not be used in toiletry or cosmetic
preparations.
e) Industrial effluents should be rigorously treated to avoid entry
of morpholine into drinking-water.
f) In the light of the formation of carcinogenic NMOR the present
occupational exposure limits should be reconsidered.
1.11.2 Recommendations for protection of the environment
Spills and shock loads to effluent treatment plants should be
avoided.
1.11.3 Recommendations for further research
Studies should be undertaken on the following topics:
a) reproductive toxicity in mammals;
b) long-term toxicity in mammals;
c) effect of exposure of mammals to low levels of morpholine with
and without nitrite and nitrate;
d) transnitrosation by NMOR under in vivo and in vitro
conditions;
e) biodegradation under anaerobic conditions, especially under
nitrate-reducing conditions;
f) microbial catalysis of N-nitrosation under realistic
conditions;
g) environmental levels of morpholine in groundwater, soil and
rivers used for drinking-water;
h) environmental levels of morpholine around morpholine-producing
and -processing factories;
i) metabolism and toxicokinetics in humans as a part of the
development of methods for biological monitoring of morpholine;
j) monitoring of morpholine and NMOR levels in food, drinking-water
and indoor air;
k) data on occupational exposure should be collected and made
available.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
CAS/IUPAC name: Morpholine
Chemical formula: C4H9NO
Chemical structure:
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
Members
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany (Joint Rapporteur)
Dr J.S. Knapp, Department of Microbiology, University of Leeds, Leeds,
United Kingdom (Joint Rapporteur)
Dr I. Linhart, Centre of Industrial Hygiene and Occupational Diseases,
National Institute of Public Health, Prague, Czech Republic
Dr U. Schiecke, Federal Environmental Agency, Berlin, Germany
Dr J.A. Sokal, Institute of Occupational Medicine and Environmental
Health, Sosnowiec, Poland (Chairman)
Representatives of other organizations
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International
Union of Toxicology (Vice-Chairman)
Secretariat
Mrs C. Partensky, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR MORPHOLINE
A WHO Task Group on Environmental Health Criteria for Morpholine
met at the World Health Organization, Geneva, from 8 to 11 November
1994. Dr E.M. Smith, IPCS, welcomed the participants on behalf of Dr
M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to
morpholine.
The first draft of this monograph was prepared by Dr J. Kielhorn
and Dr G. Rosner, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany. The second revised draft was prepared by
Dr J. Kielhorn. Dr E.M. Smith and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
CHO Chinese hamster ovary
DOC dissolved organic carbon
FID flame ionization detector
FPD flame photometric detector
GC gas chromatography
HPLC high-performance liquid chromatography
IC ion chromatography
MLSS mixed liquor suspended solids
MS mass spectrometry
NMOR N-nitrosomorpholine
NO nitrogen oxide
NOAEL no-observed-adverse-effect level
NSD nitrogen selective detector
OECD Organisation for Economic Co-operation and Development
TEA thermal energy analyser
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Physical and chemical properties
Morpholine (1-oxa-4-azacyclohexane) is a colourless, oily,
hygroscopic, volatile liquid with a characteristic amine ("fishy")
odour. It is completely miscible with water, as well as with many
organic solvents, but has limited solubility in alkaline aqueous
solutions. It is a base, the pKa of the conjugated acid being 8.33.
Correspondingly, the octanol-water partition coefficient is pH-
dependent (log Pow -2.55 at pH 7 and -0.84 at pH 10; 35°C). The
vapour pressure of aqueous solutions of morpholine is very close to
that of water.
Morpholine can undergo a variety of reactions. It behaves
chemically as a secondary amine. Under environmental and
physiological conditions, the proven animal carcinogen
N-nitrosomorpholine (NMOR) is formed by reaction of solutions of
nitrite or gaseous nitrogen oxides with dilute solutions of
morpholine. Nitrogen oxide (NO) levels may be of importance in
nitrosation. The conditions of nitrosation, in particular pH, play a
significant role.
1.2 Analytical methods
Morpholine can be determined by gas chromatography (GC) with
packed as well as capillary columns, high-performance liquid
chromatography (HPLC) and ion chromatography (IC). Detectors used
include flame ionization detector (FID), flame photometric detector
(FPD), nitrogen selective detector (NSD), and mass spectrometry (MS)
and thermal energy analyser (TEA) for GC, and UV-detector and TEA for
HPLC. For the determination of trace amounts, derivatization is
required. The method of choice for sensitivity seems to be GC with
TEA following the derivatization to NMOR (the detection limit is
2-3 µg/kg in various matrices). Low concentrations of morpholine in
air can be determined by GC with NSD.
1.3 Sources of human and environmental exposure
It is estimated that around 25 000 tonnes of morpholine per year
are produced industrially world-wide, but details of production from
some countries are lacking.
The main production process used appears to be the reaction of
diethylene glycol with ammonia in the presence of hydrogen and
catalysts.
Morpholine is an extremely versatile chemical but knowledge of
its uses is incomplete. It is important as a chemical intermediate in
the rubber industry, as a corrosion inhibitor, and in the synthesis of
optical brighteners, crop protection agents, dyes and drugs.
Morpholine is used as a solvent for a large variety of organic
materials, including resins, dyes and waxes. It can be used as a
catalyst. Morpholine is still used in some countries in toiletry and
cosmetic products. It is used in some countries in several direct and
indirect food additive applications.
Human and environmental exposure arises from both gaseous and
aqueous emissions and directly from some of its uses, including, for
example, its use in cosmetic formulations and waxes. The main
emissions probably result from its manufacture and its use in the
chemical industry (notably in production and use of rubber chemicals)
and as an anti-corrosion agent. Morpholine has been detected in a
wide variety of foods and tobacco. It could be that this morpholine
arises from the wax coatings on fruit or on packaging, but in some
cases its origin is unknown.
1.4 Environmental transport, distribution and transformation
Morpholine is chemically stable in the biosphere although it is
subject to chemical and biological nitrosation to NMOR.
Morpholine is inherently biodegradable. Under the conditions of
model activated sludge plants, morpholine is biodegradable. However,
under non-adapted conditions there is probably no significant
degradation of morpholine. The mean solid retention time in activated
sludge plants is of crucial importance and must be over 8 days if
reliable morpholine degradation is to be achieved.
There are inadequate data on the bioaccumulation of morpholine in
aquatic and terrestrial organisms. From the n-octanol/water
partition coefficient for morpholine (log Pow = -2.55 at pH7), no
bioaccumulation would be expected.
As morpholine is an important industrial chemical with a wide
range of applications, the presence of the compound or its derivatives
is to be expected in many industrial effluents. Its use as a
corrosion inhibitor in boiler water means that it will be found in
boiler wastewater, including that from power plants using morpholine.
Its use in the manufacture of rubber additives results in an
undefinable amount of morpholine being released into the hydrosphere
or geosphere through tyre abrasion and disposal of used tyres.
As a result of its use in waxes and polishes, morpholine is
released into the environment through volatilization. It is quickly
adsorbed by moisture. The main compartment for accumulation of
morpholine is therefore the hydrosphere. The limited data suggest that
morpholine does not accumulate in the hydrosphere.
Incineration is the preferred method of disposal for undiluted
morpholine, but nitrogen oxide emission controls may be required to
meet environmental regulations. For aqueous effluents, activated
sludge treatment is adequate, but only if the plant is carefully
controlled (see above).
1.5 Environmental levels and human exposure
There are no data available on levels of morpholine in ambient
and residential indoor air and in drinking-water. There are limited
data on its occurrence in natural waters and no information on its
occurrence in soil.
Based on the available data, the main source of general
population exposure to morpholine is food, which can be contaminated
with morpholine through direct treatment of fruit with waxes
containing morpholine for conservation purposes, through steam
treatment during food processing, and by the use of packaging material
containing morpholine. However, quantitative data on food
contamination by morpholine and NMOR are limited. For example, in
prepacked milk products, values ranged from 5 to 77 µg/kg morpholine
and up to 3.3 µg/kg NMOR. Morpholine content in various food samples
(fish, meat, plant products, beverages) usually did not exceed
1 mg/kg. Higher levels (up to 71.1 mg/kg) were detected in citrus
fruits in Japan. A survey in Italy did not identify NMOR in a variety
of foods at a detection limit of 0.3 µg/kg. Existing data do not
permit an estimation of the intake of morpholine and NMOR from food.
Morpholine has been found in cigarette tobacco at a concentration
of 0.3 mg/kg, and in snuff and chewing tobacco at concentrations up to
4.0 mg/kg. Levels of NMOR up to 0.7 mg/kg have been reported in the
past in snuff. These were probably associated with the use of
morpholine-containing waxes in packaging.
NMOR has been detected in some toiletry and cosmetic products,
e.g., shampoos and eye make-up, and in rubber articles, e.g. baby
pacifiers and feeding bottle teats, at levels up to 3.5 mg/kg.
Occupational exposure to morpholine may occur in several
industries. There are few data on exposure of workers to morpholine.
All reported values are below 3 mg/m3. Occupational exposure to
NMOR has been found in the rubber industry, where concentrations up to
250 µg/m3 have been measured.
The data currently available provide an indication of the
potential for human exposure but do not allow a precise estimation of
the levels of exposure of the general and occupational populations to
morpholine and NMOR.
1.6 Kinetics and metabolism in laboratory animals and humans
Morpholine is absorbed after oral, dermal and inhalation
exposure. In the rat following oral and intravenous administration,
morpholine is rapidly distributed, the highest concentrations being
found in the intestine and muscle.
In the rabbit, following intravenous and inhalation exposure,
morpholine is preferentially distributed to the kidneys, lower
concentrations reaching the lung, liver and blood.
Morpholine does not bind significantly to plasma proteins.
Plasma half-lives have been reported to be 115 (rat), 120 (hamster),
and 300 min (guinea-pig).
Morpholine is excreted mainly via the renal route, as the
unchanged compound, in a variety of species. One day after
administration, 70-90% of morpholine was found in urine.
Neutralization of morpholine enhances the rate of excretion of the
compound. A small percentage of morpholine is excreted in expired air
and faeces.
Studies in rats, mice, hamster and rabbit indicate that
morpholine is eliminated almost completely as the unmetabolized
compound. In the guinea-pig, N-methylation followed by Noxidation
can occur, with up to 20% of the administered dose being metabolized.
In the presence of nitrite, morpholine can be converted to NMOR both
in vitro and in vivo. Depending on the dose, 0-12% of morpholine
administered to rats with nitrites may be nitrosated.
Immunostimulation, involving macrophage activation, may increase
the extent of nitrosation.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of morpholine after oral administration shows
LD50 values of 1-1.9 g/kg body weight and 0.9 g/kg body weight in
the rat and guinea-pig, respectively. Rats receiving neutralized
morpholine (1 g/kg body weight) survived. After intraperitoneal
administration, the LD50 was 0.4 g/kg body weight in the mouse and
between 0.1 and 0.4 g/kg body weight in the rat. After inhalation
exposure, the LD50 was about 8 g/m3 in the rat and between 5 and
7 g/m3 in the mouse. The dermal LD50 was 0.5 ml/kg of undiluted
morpholine in the rabbit. The acute toxicity of morpholine is
characterized by gastrointestinal haemorrhage and diarrhoea after oral
exposure, and irritation and haemorrhage of the nose, mouth, eyes and
lung after inhalation. In a 30-day gavage study on rats at doses of
0.16 - 0.8 g/kg body weight, there were severe toxic effects and
mortality at all dose levels. In the guinea-pig at doses of
0.09 - 0.45 g/kg body weight there was also severe toxicity and
mortality at all dose levels.
After short-term inhalation exposure to morpholine (7.2 g/m3,
4 h/day, 4 days and 1.63 g/m3, 4 h/day, 5 days/week, 30 days),
alterations in lung function have been reported in rats. Mortality
rate in the rat ranged from 0 to 100% depending on exposure level
(0.36-18.1 g/m3, 6 h/day, 9 days). Inhalation toxicity was dose-
related with various degrees of local irritation (eyes, mouth, nose,
lung) and haemorrhage at the higher exposure levels. One study
reported increased function of thyroid gland and another necrosis of
liver and renal tubules after inhalation exposure.
A 90-day study showed that morpholine administered orally
(0.2-0.7 g/kg body weight per day) for 90 days may reduce body weight
gain and renal function in the mouse. After 672 days of oral exposure
to morpholine (0.28-0.5 g/kg body weight per day), forestomach
epithelium hyperplasia was reported (mouse).
In a 13-week inhalation study, morpholine (0.09-0.9 g/m3,
6 h/day, 5 days/week) has been reported to cause dose-related lesions
of nasal mucosa and pneumonia at the higher exposure levels (0.36 and
0.9 mg/m3). No treatment-related changes to a number of parameters
were observed at 0.09 g/m3; this concentration may be considered a
no-observed-adverse-effect level (NOAEL) under the conditions of
sub-chronic inhalation exposure.
Morpholine in the undiluted and unneutralized form is highly
irritant for the eye and skin, probably due to its alkaline
properties. Dilution and neutralization of its pH may significantly
reduce its topical toxicity. Morpholine (2%) did not induce
sensitivity in the guinea-pig using the modified Buehler method.
Morpholine did not induce mutations in bacteria or yeasts with
and without metabolic activation (with one exception at a very high
concentration). It was negative in the host-mediated assay.
Morpholine did not induce DNA-repair in primary rat hepatocytes
and did not induce a significant increase in sister chromatid exchange
in Chinese hamster ovary cells. Morpholine was considered to be weakly
mutagenic in the L5178Y mouse lymphoma assay. It increased type III
foci in the BALB/3T3 malignant cell transformation assay, although
neutralized morpholine did not.
Morpholine caused neither point mutation nor chromosomal
aberration in hamster embryos exposed in utero.
No increase in the incidence of tumours was seen in rats given up
to 0.5 g/m3 morpholine by inhalation for 104 weeks nor in mice given
1% morpholine oleate in their drinking-water for 96 weeks. In a long-
term study on a group of 104 rats given 1000 mg morpholine/kg diet,
there were three liver cell carcinoma, two lung and another
angiosarcoma (unspecified) and two malignant glioma, whereas in a
control group of 156 rats there were no tumours. With hamsters under
the same conditions, no tumours were found.
Morpholine given simultaneously with nitrite yields positive
results in the host-mediated assay, probably due to the formation of
NMOR. Morpholine fed simultaneously with nitrite induced liver and
lung tumours in rats and liver tumours in hamsters probably due to the
endogenous formation of NMOR. NMOR is mutagenic in bacteria and
yeasts; weakly positive results were reported for sister chromatid
exchange in CHO cells and for mutations in mouse lymphoma L5178Y
cells. NMOR is carcinogenic in mice, rats, hamsters and various
fishes, producing liver and lung tumours in mice, liver, kidney and
blood vessel tumours in rats, liver, upper digestive and respiratory
tract tumours in hamsters, and liver tumours in fish.
1.8 Effects on humans
There have been no reports on incidents of acute poisoning or on
the effects of short- or long-term exposure to morpholine by the
general population.
The phenomenon known as blue vision or glaucopsia, as well as
some instances of skin and respiratory tract irritation, have been
described in reports of occupational exposure to morpholine; however,
no atmospheric concentrations of morpholine were given. It was
reported that the number of chromosomal aberrations in the lymphocytes
of peripheral blood of workers exposed for 3-10 years to morpholine at
concentrations of 0.54-0.93 mg/m3 did not differ significantly from
controls.
Undiluted morpholine is strongly irritant to skin; a dilute
solution (1 to 40) was mildly irritant.
The potential carcinogenicity of morpholine in exposed human
populations has not been investigated.
1.9 Effects on other organisms in the laboratory and field
Among the aquatic organisms tested, certain cyanobacteria
(Microcystis) and unicellular green algae (Scenedesmus) appear to
be the most sensitive taxa as toxicity threshold values (criterion:
inhibition of population growth) of 1.7 mg/litre for Microcystis and
4.1 mg/litre for Scenedesmus have been reported (duration of
exposure: 8 days).
Aerobic bacteria like Pseudomonas proved to be much more
resistant: the 16-h toxicity threshold and the NOEC for population
growth have been cited as 310 and 8700 mg/litre, respectively.
However, 1000 mg/litre inhibited respiration and dehydrogenase
activity (up to 20%) in activated sludge from industrial treatment
plants.
Among aquatic protozoans tested so far, representatives of the
genera Entosiphon and Chilomonas (with threshold values of 12 and
18 mg/litre, respectively, for the inhibition of population growth)
turned out to be the most sensitive. The 24-h EC values
(E=immobilisation) for Daphnia were in the range of
100-120 mg/litre. The 48- to 96-h LC50 values reported for fish
tested in fresh, brackish or seawater were > 180 mg/litre, rainbow
trout being the most sensitive species.
No data on long-term effects in aquatic invertebrates and
vertebrates are available. Information about the toxicity of
morpholine in free-living soil organisms is almost entirely lacking,
being restricted to a 3-day EC value of about 400 mg/litre given for
germination inhibition in lettuce.
1.10 Evaluation of human health risks and effects on the environment
1.10.1 Evaluation of effects on human health
The general population is primarily exposed to morpholine by
consumption of contaminated food. Contamination of tobacco and
tobacco products, and cosmetic and toiletry articles and rubber
products may also contribute to overall exposure. Occupational
exposure to morpholine occurs in many industries; the compound is
absorbed by inhalation and skin absorption. Data are inadequate to
determine the degree of exposure of the general population. Data on
occupational exposure to morpholine are also limited.
Morpholine is not highly toxic under conditions of acute
exposure. The LD50 after oral administration is 1-1.9 g/kg body
weight in rats and 0.9 g/kg body weight in guinea-pigs. LC50 values
of 7.8 mg/m3 (rats) and 4.9-6.9 g/m3 (mice) have been reported.
In the conditions of short-term and long-term inhalation
exposure, the critical effects appear to be irritation of the eyes and
respiratory tract. A concentration of 90 mg/m3 may be considered the
NOAEL in the conditions of the 13-week experiment in rats (6 h/day,
5 days/week). In a long-term inhalation study (104 weeks), increased
incidences of inflammation of the cornea, and inflammation and
necrosis of the nasal cavity were observed in rats at 540 mg/m3.
Increased incidence of irritation of eyes and nose was also observed
at 36 and 180 mg/m3.
High exposures to morpholine causes severe damage to the liver
and kidneys of rats and guinea-pigs. Fatty degeneration of the liver
was reported in rats after feeding morpholine (0.5 g/kg body weight)
daily for 56 days. When administered morpholine oleic acid salt in
the drinking-water at a dose of about 0.7 g/kg body weight per day for
13 weeks, mice showed cloudy swelling of the kidney proximal tubules.
Decreased body weight gain was observed in female mice in the long-
term (672 days) feeding experiment at dose levels between 0.05 and
0.4 g morpholine (as oleic acid salt).
At the reported levels of the present occupational and
environmental exposures, morpholine does not seem to create any
significant risk of systemic toxic effects. Local effects (irritation)
of the eyes and respiratory tract may occur in non-controlled
occupational and incidental exposures to high concentrations of
airborne morpholine, and skin irritation may result from contact with
liquid (even diluted) morpholine.
Morpholine does not appear to be mutagenic or carcinogenic in
animals. However, it can be easily nitrosated to form NMOR, which is
mutagenic and carcinogenic in several species of experimental animals.
Morpholine fed to rats sequentially with nitrite caused an increase in
tumours, mostly hepatocellular carcinoma and sarcomas of the liver and
lungs. It is therefore prudent to consider exposure to morpholine as
increasing the carcinogenic risk in exposed populations.
1.10.2 Evaluation of effects on the environment
In view of the very restricted knowledge regarding environmental
exposure, the lack of effect data relating to long-term exposure in
water and to short- and long-term exposure in the terrestrial
environment, a sound risk assessment cannot be carried out at present.
On the basis of the reported properties of morpholine, the available
ecotoxicological information and the few data on environmental
concentrations, certain conclusions can be drawn.
The high water solubility of morpholine and its low volatility
(under environmental conditions) make the hydrosphere the pre-dominant
environmental sink.
Morpholine is inherently biodegradable and, although degradation
is slow, there are no data to suggest accumulation in the hydrosphere.
Bioaccumulation is unlikely.
There are relatively few data on toxicity of morpholine to free-
living organisms. However, it seems unlikely that current levels of
morpholine emission cause any significant damage to the wider
environment. Local effects, due for example to factory emissions or
to morpholine release due to wear of tyres, remain to be evaluated.
Contamination of some foods, e.g., fish, with morpholine may be
due to environmental contamination, but this is uncertain.
Conversion of morpholine to NMOR is the main cause of concern,
especially with respect to vertebrate populations. NMOR has been
reported in industrial wastewater and in soil near a factory. The
presence of morpholine in water destined for processing to drinking-
water is a cause for concern.
1.11 Conclusions and recommendations
Morpholine does not present a toxic risk to humans at the usual
levels of exposure, but its conversion to the carcinogenic NMOR should
be noted.
There is no evidence at present levels of exposure that
morpholine poses a substantial risk to biota in the environment.
1.11.1 Recommendations for protection of human health
a) Human exposure to morpholine should be avoided as far as
possible.
b) Contamination of food through food packaging and food processing
should be avoided.
c) Morpholine should not be used in rubber products intended for
direct contact with humans.
d) Morpholine should not be used in toiletry or cosmetic
preparations.
e) Industrial effluents should be rigorously treated to avoid entry
of morpholine into drinking-water.
f) In the light of the formation of carcinogenic NMOR the present
occupational exposure limits should be reconsidered.
1.11.2 Recommendations for protection of the environment
Spills and shock loads to effluent treatment plants should be
avoided.
1.11.3 Recommendations for further research
Studies should be undertaken on the following topics:
a) reproductive toxicity in mammals;
b) long-term toxicity in mammals;
c) effect of exposure of mammals to low levels of morpholine with
and without nitrite and nitrate;
d) transnitrosation by NMOR under in vivo and in vitro
conditions;
e) biodegradation under anaerobic conditions, especially under
nitrate-reducing conditions;
f) microbial catalysis of N-nitrosation under realistic
conditions;
g) environmental levels of morpholine in groundwater, soil and
rivers used for drinking-water;
h) environmental levels of morpholine around morpholine-producing
and -processing factories;
i) metabolism and toxicokinetics in humans as a part of the
development of methods for biological monitoring of morpholine;
j) monitoring of morpholine and NMOR levels in food, drinking-water
and indoor air;
k) data on occupational exposure should be collected and made
available.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
CAS/IUPAC name: Morpholine
Chemical formula: C4H9NO
Chemical structure:  CAS registry number:
CAS registry number: 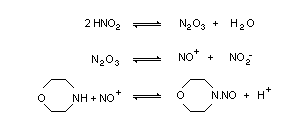 The rate of reaction of the nitrosation of morpholine by nitrite
is greatest at a pH value of 3.4, where the rate constant is
0.42 mol-2.s-1. An increase in the pH value has been shown to
result in a decrease in the rate of nitrosation with nitrite (Mirvish,
1975; Archer et al., 1977), and the rate was almost zero at pH > 7
(Archer et al., 1977).
In contrast, nitrosation with gaseous nitrogen oxides (N2O3,
N2O4, NOx) can take place over the whole pH range (Challis &
Kyrtopoulos, 1979; Meiners et al., 1980). Cooney et al. (1987) found
that, under certain conditions, the yield of NMOR at pH 7 was ten
times higher than at pH 2, but there was no further increase beyond
this pH.
Some nitrosamines, particularly alpha-nitrosamine aldehydes, are
potent transnitrosation reagents and are capable of nitrosating
morpholine at pH 7.9 (Loeppky et al., 1987).
Numerous reaction accelerators are known, e.g., thiocyanate
(Boyland et al., 1971), halides (Mirvish, 1975), formaldehyde (Archer
et al., 1977) and nitrosophenols, e.g., p-nitroso- o-cresol (Davies
et al., 1980). Enhancement of the nitrosation of morpholine by
nitrogen dioxide was reported in the presence of iodine (Challis &
Outram, 1979), vanillin and related phenols (Cooney & Ross, 1987) and
halides, particularly bromide (Cooney et al., 1987).
In contrast, the following compounds have been reported to
inhibit the nitrosation of morpholine almost completely: ascorbic acid
(Lathia & Schellhöh, 1981; Leach et al., 1991); urea or ammonium
sulfamate (Mirvish et al., 1972); gallic acid and sulfite (Mirvish,
1975); L-cysteine and DL-methionine ( in vitro study under
physiological conditions, Lathia & Edeler, 1989), catechol and
4-hydroxychavicol (Shenoy & Choughuley, 1989); alpha-tocopherol
(Norkus et al., 1986; Cooney et al., 1987; Schuster et al., 1990);
sulfhydryl compounds such as cysteine, cysteamine, glutathione and
thioglycolic acid, as well as extracts of onion and garlic juice
(Shenoy & Choughuley, 1992). Vitamin C, glucose, mannitol, cabbage
juice, orange juice, shiitake mushroom extract and saliva inhibited
the nitrosation of morpholine in vitro, but catechin, epicatechin
and tea extract enhanced the same reaction (Ohnishi, 1984). The
inhibitory effect of Chinese tea on the formation of NMOR in vitro
and in vivo has also been described (Wang & Wu, 1991).
Several C-nitro compounds, in particular tetranitromethane, have
been demonstrated to transnitrosate morpholine to form NMOR (Fan et
al., 1978). C-nitro compounds are widely used in industry as
pesticides, bactericides, colouring agents, drugs and perfumes.
Singer (1980) described the transnitrosation of morpholine with
nitrosamines and nitrosureas under acid conditions in the presence of
thiocyanate. These reactions are dependent on the pH value and steric
and electronic factors, as well as on the basicity of the amines. In a
model study, the nitrosation of morpholine by nitro-nitroso compounds,
such as those found in fried bacon, was observed (Liu et al., 1988).
NMOR can be formed in vivo in humans and has been found in
various tissues and fluids such as human saliva (Boyland et al., 1971;
Wishnok & Tannenbaum, 1977) and human gastric juice (Ziebarth, 1973;
1974; Sen & Baddoo, 1989; Yurchenko et al., 1990). NMOR formation has
been reported in rat lungs (Postlethwait & Mustafa, 1983), whole mice
(Iqbal et al. 1980; Norkus et al. 1984), stomach (Furman & Rubenchik,
1991), hepatocytes isolated from woodchucks (Marmota monax) (Liu et
al., 1992) and microorganisms (Archer et al., 1979; O'Donnell et al.,
1988; Calmels et al., 1991a,b). Bacterial catalysis of
N-nitrosation of morpholine has been reported in a range of bacteria
often isolated from the human gut or urinary tract infections (Suzuki
& Mitsuoka, 1984; Calmels et al., 1987, 1988; Mackerness et al.,
1989), including the ubiquitous gut bacterium Escherichia coli and
Pseudomonas aeruginosa, which is also widespread in the aquatic
environment. Bacterial catalysis of N-nitrosation of morpholine is
heat labile and is optimal at neutral to slightly alkaline pH (Calmels
et al., 1985; Leach et al., 1987). N-nitrosation by bacteria is
generally associated with the ability to reduce nitrate. It appears
that those that reduce nitrate to nitrogen or nitrogen oxides (e.g.,
P. aeruginosa) can nitrosate at much greater rates than those (e.g.,
E. coli) that only reduce nitrate to nitrite (Leach et al.,1987;
Calmels et al., 1988). There is considerable variation between
strains of the same species. Bacterial N-nitrosation of morpholine
has been shown to follow Michaelis-Menten kinetics (Calmels et al.,
1985; Leach et al., 1987). E. coli A10, for example, displays Km
values of 7.4 mmol/litre for morpholine and 11.4 mmol/litre for sodium
nitrite. It has been shown that the rate of bacterial N-nitrosation
of secondary amines is inversely related to the pKa of the amine
(Calmels et al., 1985; Leach et al., 1987, 1991), with a linear
relationship between log10 of the rate of nitrosation and pKa.
Morpholine, having a relatively low pKa, is thus relatively
susceptible to nitrosation compared, for example, to alkyl amines.
It has been shown that ascorbate is capable of inhibiting
nitrosation of morpholine by P. aeruginosa (Leach et al., 1991).
Although most nitrosation studies have used whole bacteria, an enzyme
catalyzing N-nitrosation of morpholine has been isolated and
purified from two denitrifying bacteria (Calmels at al., 1990).
4.4 Ultimate fate following use
4.4.1 Fate of morpholine in various products
Morpholine is an important industrial chemical with a wide range
of applications (see section 3.2.2) and therefore may be present in
many industrial emissions.
Its use as a corrosion inhibitor in boiler water means that
morpholine and its decomposition products will be found in boiler
wastewater, including water from power plants using morpholine. In a
study by McCain & Peck (1976), morpholine concentrations in the
discharge streams of three Hawaiian power plants ranged from not
detectable to 0.008 mg/litre, suggesting that the potential for human
exposure is small.
Its use in the manufacture of rubber additives results in an
indefinable amount of morpholine being released into the hydrosphere
or geosphere not only during manufacturing processes but also through
tyre abrasion and disposal of used tyres.
Morpholine is released during vulcanization processes using
morpholine-containing accelerators such as 2-( N-morpholino-
thio)benzothiazole (MBS) (Badura et al., 1989). Some of the amine is
released into the atmosphere and some is bound to the rubber. Even
the accelerator itself can contain free amine. The morpholine content
of MBS is < 0.4% by weight. This level can be higher if the
accelerator is not stored properly and is exposed to heat or moisture
(BUA, 1991).
Aarts et al. (1990) detected free volatile morpholine at
concentrations of between 70 (new) and 230 mg/kg (old) in samples of
dithio-bis-morpholine (DTBM). After extraction in water for one hour
in an ultrasonic bath, ten times this amount was detected, i.e.
960 mg/kg in newly made and 2750 mg/kg in stored DTBM. These
quantities of amine could be released during vulcanization.
Optical brighteners adhere to clothes during the first wash but
tend to be released into the wastewater in subsequent washings.
Although these substances are not themselves biologically degradable,
they have been found to disappear from wastewater after a two-step
biological treatment presumably due to the high rate of adsorption to
the sludge particles (Jakobi et al., 1983).
As mentioned in section 3.2.2, morpholine is released into the
environment by volatilization through its use in waxes and polishes
(Texaco, 1986).
4.4.2 Waste disposal
Controlled incineration is the preferred method of disposal
(Sittig, 1985; Air Products and Chemicals, 1989). The incinerator
should be equipped with a scrubber or thermal unit. Nitrogen oxide
emissions should meet environmental regulations.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
No data are available on levels of morpholine in ambient air.
5.1.2 Water
5.1.2.1 River water
Since mid-1990, the levels of morpholine in some rivers in North
Rhine-Westphalia, Germany, have been monitored (BUA, 1991). No
morpholine could be detected at three different points in the River
Rhine or in five of its tributaries (detection limit, 5 µg/litre). No
morpholine was found in samples of Tennessee freshwater (detection
limit, 0.07 µg/litre) (Singer & Lijinsky, 1976a). In 1979, 33 water
samples were collected at 11 sites in Japan, but no morpholine could
be detected (detection limit, 1-5 µg/litre) in any of the samples
(Environment Agency Japan, 1980).
5.1.2.2 Wastewater
There are no data on morpholine levels in wastewater.
A single sample of wastewater from a tyre chemical factory in
Ohio, USA was found to contain 3 µg NMOR/litre (Fajen et al., 1979).
In England, samples were taken from the inlets and outlets of
four sewage treatment plants (Richardson et al., 1980). NMOR
(100 µg/litre) was found only in the outlet of a cutting-fluid
recovery plant.
5.1.3 Sediment
Spies et al. (1987) examined contaminated sediments in San
Francisco Bay, USA and found several benzothiazoles, including
2-(4-morpholinyl)-benzothiazole, which is present as an impurity in
commercial 2-(morpholinothio)-benzothiazole used in motor tyres. The
authors carried out weathering tests on this latter commercial
substance and found that the morpholine impurity was environmentally
stable. They suggested that the 2-(4-morpholinyl)-benzothiazole found
in the sediments (up to 0.36 mg/kg dry weight) was a result of
accumulated street run-off. Morpholine itself could not be detected.
In 1979, 33 bottom sediment samples were collected at 11 sites in
Japan, but no morpholine could be detected (detection limit,
0.01-0.5 mg/kg) in any of the samples (Environment Agency Japan,
1980).
5.1.4 Soil
There are no data on the presence of morpholine in soil.
2-(4-Morpholinyl)-benzothiaozole (273 µg/kg dry weight) was detected
1.6 km from a motorway in California, USA (Spies et al., 1987) (see
also section 5.1.2).
NMOR (4.4 mg/kg) was detected in a single sample of soil near to
a tyre chemical factory in Ohio, USA (Fajen et al., 1979).
5.1.5 Terrestrial and aquatic organisms
Levels of morpholine found in single or small samples of fish are
given in Table 6, but the sample numbers are too low to make an
evaluation. No other data are available.
5.2 General population exposure
5.2.1 Indoor air
No data on indoor air exposure to morpholine are available.
Analysis for NMOR in the air inside new cars showed levels of up
to 2.5 µg/m3. Levels were 4 to 10 times lower when the air-venting
system was working, indicating that NMOR exposure is limited to the
first few minutes of each trip (Rounbehler et al., 1980). During a
simulation of conditions inside cars on a hot day, concentrations of
up to 0.4 µg NMOR/m3 were measured at 60°C (Dropkin, 1985).
5.2.2 Drinking-water and food
There are no data on the morpholine content of drinking-water.
Food can become contaminated with morpholine in several ways:
(a) through direct treatment of fruit with waxes containing morpholine
for conservation purposes; (b) by use of packaging material
containing morpholine, and (c) through steam treatment during
processing.
Ohnishi et al. (1983) found morpholine at concentrations of
< 71.1 mg/kg in the peel of retail citrus fruits in Japan. In the
pulp (flesh) of the fruits the level was much lower, being less than
0.7 mg/kg (Table 4). Marmalade made from whole fruits contained
concentrations of morpholine between 0.3 to 0.7 mg/kg. If the fruits
were previously washed in washing-up liquid, morpholine concentrations
were reduced, but only by 25%. Even if the fruit was boiled for
20 minutes, a third to a quarter of the morpholine still remained. The
morpholine removed by these processes could be detected quantitatively
in the washing and boiling water (Ohnishi et al., 1983).
Table 4. Morpholine content of citrus fruits and marmalade from
citrus fruitsa
Sample Number Morpholine
(mg/kg)b
Orange (variety a)c peel 12
n.d.-57.0
fruit pulp 3 0.2-0.7
Orange (variety b)c peel 6 5.0-71.1
fruit pulp 1 0.3
Mandarine peel 2 16.1-18.0
fruit pulp 1 n.d.
Lemon peel 2 n.d.-5.2
fruit pulp 1 n.d.
Grapefruit peel 2 2.8-7.0
fruit pulp 1 n.d.
Marmalade from citrus fruits 4 0.3-0.7
a adapted from Ohnishi et al. (1983)
b n.d. = not detectable (detection level 0.2 mg/kg),
presumably fresh weight
c variety not specified
Sen & Baddoo (1989) reported the morpholine and NMOR content of
waxed and unwaxed apples of Canadian origin, obtained either direct
from the packers or from retail sources. Liquid wax spray is used as
a protective coating on fruit and vegetables to reduce moisture loss
and thereby extend the shelf-life of the product. Apple homogenates
and liquid waxes were analysed for their morpholine contents
(Table 5). Although the concentrations of morpholine found in waxed
apples were high, NMOR could not be found in any of the waxed or
unwaxed samples. Low levels of morpholine in the unwaxed apples could
be due to contamination during packing or transport.
Singer & Lijinski (1976a) analysed a variety of foodstuffs for
the presence of morpholine but the sample size was too small to draw
any conclusions. The results are given in Table 6. The sources of
contamination with morpholine are in these cases not clear. The
possibility of artifacts is unlikely according to the authors.
Table 5. Concentration of morpholine and NMOR (mg/kg) in samples of
liquid waxes and waxed and unwaxed applesa
Liquid wax Unwaxed apples Waxed apples
NMOR morpholine NMOR morpholine NMOR morpholine
0.286 27 300 n.d. n.d. n.d. 4.3
0.668 31 500 n.d. 0.118 n.d. 4.9
0.138 24 400 n.d. 0.016 n.d. 6.3
0.277 38 500 n.d. 0.041 n.d. 7.1
0.152 22 500 n.d. n.d. n.d. 4.0
0.585 33 300 n.d. 0.018 n.d. 7.7
a adapted from Sen & Baddoo (1989); n.d. = not detected (detection
limit: 0.005 mg/kg for morpholine, 0.0005 mg/kg for NMOR)
Table 7 summarizes the results of investigations into the
concentrations of morpholine and NMOR in prepacked milk products
(Hoffmann et al., 1982). The values range from 5-77 µg/kg for
morpholine and "not detectable" to 3.3 µg/kg for NMOR. Contamination
of prepacked foodstuffs with morpholine might be explained by the use
of morpholine in steam boiler systems for paper and cardboard
production.
Hotchkiss & Vecchio (1983) found morpholine concentrations of
between 0.098 and 8.4 mg/kg (mean 0.38 mg/kg) in food packaging. A
sample of flour nearest the wall of the paper bag contained 1.1 µg
NMOR/kg. The bag itself contained 33.0 µg NMOR/kg. In an experimental
72-h incubation at 100°C, between 1 and 2.3 µg NMOR/kg migrated from
packaging material to various dry foods.
Sen & Baddoo (1986) investigated the migration of NMOR out of
waxed packaging material into margarine. Waxed wrappings and margarine
samples taken 1 cm from the outer layer of the block contained NMOR
(5-73 µg/kg and 0.5-1.4 µg/kg, respectively). NMOR was not detectable
in those samples taken from the inside of the block. Samples taken of
margarine packed in aluminium backed wrappers, specially coated waxed
papers or plastic containers were negative for NMOR (detection level,
0.5 µg/kg).
Aitzetmüller & Thiele (1982) found no NMOR in 20 margarine
samples from different countries (detection limit, 0.5 µg/kg).
Table 6. Morpholine content in various food samplesa
Food No. of Concentration References
samples (mg/kg)b
Fish
Fish sausage 5 n.d. Hamano et al. (1981)
Cod roe 3 n.d. Hamano et al. (1981)
Codc -d tracese Singer & Lijinsky (1976a)
Spotted troutc 1 6 Singer & Lijinsky (1976a)
Smallmouth bassc 1 < 0.7 Singer & Lijinsky (1976a)
Salmonc 1 1 Singer & Lijinsky (1976a)
Ocean perchc -d 9.0 Singer & Lijinsky (1976a)
Tuna (in tins) -d < 0.6 Singer & Lijinsky (1976a)
Meat
Baked ham 5 0.2 Hamano et al. (1981)
Baked ham -d 0.5 Singer & Lijinsky (1976a)
Frankfurter sausages -d 0.4 Singer & Lijinsky (1976a)
Plant products
Spinach 2 n.d. Hamano et al. (1981)
Miso (from soja) 2 n.d. Hamano et al. (1981)
Beverages
Evaporated milk -d 0.2 Singer & Lijinsky (1976a)
Coffee -d 1.2 Singer & Lijinsky (1976a)
Tea -d tracesf Singer & Lijinsky (1976a)
Beer (in tins) -d 0.4 Singer & Lijinsky (1976a)
Beer (in bottles) -d < 0.2 Singer & Lijinsky (1976a)
Wine -d < 0.7 Singer & Lijinsky (1976a)
a adapted from BUA (1991)
b fresh weight except for tea and coffee (dry weight);
n.d. = not detected (detection limit 0.01 mg/kg)
c frozen from Tennesee & Columbia rivers
d average from several samples; exact number not given
e < 0.3 mg/kg
f < 0.1 mg/kg dry weight
Table 7. Morpholine and NMOR content (mg/kg) in food products
and their waxed containersa
Sample Food Container
NMOR morpholine NMOR morpholine
Butter 0.0032 0.058 0.0019 0.22
Cream cheese 0.0009 0.077 n.d. 0.68
Yoghurt n.d. 0.038 n.d. 3.06
Cottage cheese 0.0004 0.044 0.0054 17.2
"Cheese" (semi-soft)
Country of origin:
Germany 0.0033 0.009 n.d. 0.026
Denmark 0.0031 0.01 0.0016 0.025
Austria 0.0007 0.005 0.0012 0.022
USA 0.0014 0.008 n.d. 0.132
Gouda 0.0016 0.035 n.d. 0.035
Frozen peas n.d. 0.026 0.0031 0.057
and carrots
a adapted from Hoffmann et al. (1982); n.d. = not detected
(detection limit for NMOR, 0.0002 mg/kg)
Gavinelli et al. (1988), in a survey in Italy, found no NMOR in a
variety of foodstuffs including canned beef, pork, poultry, cured
meat, milk products, malt products, domestic Italian canned wines and
beers. The limit of detection was 0.3 µg/kg. Similarly, Pensabene &
Fiddler (1988) found no NMOR in samples of different USA fish-meat
frankfurter sausages (limit of detection, 0.2 µg/kg). These authors
assumed that the positive results in their earlier work were due to
artifacts.
Groenen et al. (1987) found NMOR in cheese (2.8 µg/kg) and cured
meat products (34 µg/kg) after addition of morpholine (10 mg/kg) in
the alkaline pH range.
Liu et al. (1988) suggested that the NMOR in fried bacon was
formed by transnitrosation.
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (US FDA, 1984a,b,c,d,e;
1988; see section 3.2.2). Although the US Food and Drug Administration
allows morpholine to be used as a direct or indirect food additive, in
1980 one of the biggest USA producers recommended its customers not to
use morpholine in this way (Litton Bionetics, 1980).
5.2.3 Tobacco
Morpholine was found in unburned cigarette tobacco at a
concentration of 0.3 mg/kg (Singer & Lijinsky, 1976b). In cigarette
smoke condensate < 5 mg/kg was detected, equivalent to
0.08 µg/cigarette. It has been established that an appreciable portion
of the inhaled smoke is swallowed and retained in the stomach where
nitrosation of ingested amines is known to occur (Sander & Bürkle,
1969).
Brunnemann et al. (1982) analysed 10 popular snuff brands from
the USA and Sweden for morpholine and volatile N-nitrosamines (see
Table 8). In the five USA brands, morpholine concentrations of between
1.5 and 4.0 mg/kg were found; in the Swedish products, the
concentrations were between 0.2 and 2.5 mg/kg. An analysis of the
snuff containers that were made of waxed cardboard gave morpholine
values of 0.17 to 4.74 mg/kg (USA) and 0.46 to 4.83 mg/kg (Swedish).
The morpholine content in the snuff could have come through diffusion
from the container made of waxed cardboard. Another possibility was
contamination during the tobacco processing as suggested by the very
high morpholine content (19.4 mg/kg) of a single sample of chewing
tobacco, packaged in aluminium. NMOR formed by nitrosation from
morpholine was found in 5/5 of the USA and 2/5 of the Swedish snuff
samples (Brunnemann et al., 1982).
Brunnemann & Hoffmann (1991) reported that since 1981 they had
routinely determined the concentrations of NMOR in a leading snuff
brand in the USA with the aim of creating awareness of the fact that
morpholine is a precursor of NMOR and must be removed. In eight
commercial snuff brands analysed in 1986 (Hoffmann et al., 1987), only
three were found with traces of NMOR (9-39 µg/kg). Brunnemann &
Hoffmann (1991) noted that the decrease in the NMOR content (from
about 700 µg/kg in 1981 to an undetectable level in 1990) is most
probably due to modifications in packaging, since snuff products in
the USA are now sold in plastic containers that have no wax coatings
(Table 9).
Österdahl & Slorach (1983) analysed snuff and chewing tobacco on
the Swedish market for volatile N-nitrosamines. NMOR was found in
9/30 samples in concentrations between 0.4 and 4.0 µg/kg (Swedish
brands) but in none of the three USA brands. One of three chewing
tobacco brands contained 0.8 µg morpholine/kg.
Table 8. Nitrosomorpholine and morpholine in snuff containersa
Snuff brand Snuff tobacco (µg/kg)b Snuff container
(µg/kg)c
NMOR morpholine NMOR morpholine
USA I 24 2800 34 845
II 690 1500 10 170
III 690 4000 230 4740
IV 630 3200 4 90
V 31 2200 3 140
Sweden I 44 820 4 1750
II < 2d 200 3 460
III < 2d 780 13 4830
IV 10 940 23 4290
V < 2d 2500 n.d.e n.d.
a from Brunnemann et al. (1982)
b based on dry weight
c uncorrected for moisture; had previously contained snuff.
Containers of USA I-III and Sweden I-IV were cardboard boxes
with a metal lid, USA IV were plastic containers with individual
snuff portions in porous paper bags; USA V was a plastic container;
Sweden V were individual snuff portions in Al-bags.
d detection limit = 2 µg/kg
e n.d. = not determined
Table 9. NMOR in fine-cut snuff (on the basis of dry tobacco weight)a
Year Yield (µg/kg)
1981 690
1984 29.4
1984/85 238
1985 29
1986/87 < 2b
1987 43
1990 < 2
a from Brunnemann & Hoffmann (1991)
b detection limit = 2 µg/kg
A survey of smokeless tobacco products commercially available in
1987-1988 showed that trace levels of NMOR were still found in some
United Kingdom (mean 0.5 µg/kg) and Swedish (mean 1 µg/kg) oral
tobacco products packed in waxed containers, but no NMOR was detected
in Indian zarda or European nasal snuff (Tricker & Preussmann, 1991).
5.2.4 Cosmetics and toiletry articles
Morpholine is used in some countries in cosmetic products as a
surfactant and emulsifier at concentrations up to 5% (Cosmetic
Ingredient Review, 1989).
Data submitted to the Food and Drug Administration (FDA) in 1981
by cosmetic firms participating in the voluntary cosmetic registration
programme indicated that morpholine was used in a total of 38 cosmetic
products including eyeliner, eye shadow, mascara and skin care
preparations (Cosmetic Ingredient Review, 1989). The greatest use of
morpholine was in mascara (32 products). The reported concentration
ranges of morpholine in these products were < 0.1% (4 products),
> 0.1-1% (17 products) and > 1-5% (17 products). Table 10
summarizes data submitted to the FDA in 1986 showing that the greatest
use of morpholine was in eye make-up removers, at concentrations of
> 0.1-1% and > 1-5% (Cosmetic Ingredient Review, 1989). These data
show that the ocular region and the skin around are the areas directly
exposed to cosmetic products containing morpholine. The potential also
exists for morpholine-containing products to come in contact with the
conjunctiva and cornea. Additionally, it must be emphasized that these
morpholine-containing products may be applied several times daily over
the course of several years. Due to lack of appropriate safety data,
the Cosmetic Ingredient Review Expert Panel could not conclude that
morpholine was safe for use in cosmetic products (Cosmetic Ingredient
Review, 1989).
Morpholine is listed in Annex II of the European Economic
Community (EEC) Cosmetics Directive and therefore must not be used in
cosmetic formulations (EEC, 1986). Investigations in 1988 and 1989 in
the Federal Republic of Germany showed that no morpholine could be
detected in toiletry articles (BUA, 1991).
Data from a 1985 US Food and Drug Administration report given in
Table 11 show that NMOR was found at concentrations between 48 and
1240 µg/kg in seven mascara products (Cosmetic Ingredient Review,
1989). It is not known whether the NMOR detected was formed in the
cosmetics. Table 11 also shows the results of an analysis of various
toiletry articles in the Federal Republic of Germany prior to the EEC
Directive in 1986. NMOR was detected in 18% of the products
(Spiegelhalder & Preussman, 1984).
Table 10. Product formulation data for morpholinea
Product Total no. of Total no. No. of product formulations
category formulations containing within estimated
in category ingredient concentration ranges
> 0-1% > 1 > 1-5%
Eye make-up 77 29 11 18
remover
Eye, face, or body 1264 2 2
preparations other
than eye make-up
removers
1986 totals 1341 31 11 2 18
a US FDA (1986)
5.2.5 Rubber articles
NMOR was found in commercial samples of rubber chemicals at
concentrations of 60-3500 µg/kg by Spiegelhalder & Preussmann (1982),
who noted that the occurrence of nitrosamines was directly related to
the use of corresponding vulcanization accelerators. The nitrosamines
could be leached out by water, buffer solutions or milk but could not
be completely removed even after boiling or treating with acid or
alkaline solutions (Spiegelhalder & Preussmann, 1982).
Several rubber articles which come into contact with skin or
food, in particular baby bottle teats and pacifiers (dummies), were
assayed for NMOR; 10 µg/kg migrated from a silicone rubber pacifier
(1 out of 11 different samples; detection level, 1 µg/kg) after
incubation for 24 h at 40°C (Spiegelhalder & Preussmann, 1982). Sen et
al. (1984) found NMOR in 2 out of 10 brands of nipples and in one
pacifier at concentrations of between 0.1 µg/kg (detection level) and
86 µg/kg. The same authors found NMOR (5.7 µg/kg) in one plastic
nipple shield, no NMOR being found in the other 41 samples of nipples
and pacifiers (detection limit, 1 µg/kg) (Sen et al., 1985).
Westin et al. (1987) tested 16 types of children's pacifiers and
baby-bottle nipples on sale in Israel from nine different countries
and found NMOR at levels of up to 2 µg/kg.
Table 11. N-nitrosomorpholine (NMOR) in toiletry articles and
cosmetics
Concentration
Productsa No. of No. (µg/kg)
samples positive
maximum average
Shampoos 45 13 640 133
Colour toners 7 - - -
Hair conditioners 16 - - -
Foam baths 7 - - -
Shower gels 9 4 380 145
Cream and oil baths 8 2 440 -
Cosmetic bath additives 5 - - -
Children's shampoos 5 1 230 -
Children's bath and
skin care products 8 6 360 80
Body lotions and rubs 6 - - -
Face tonics, cleaners, 29 - - -
and masks
Mascarab 7 7 1240 306
a from Spiegelhalder & Preussmann (1984), with the exception of
mascara
b from CIR (1986)
In a survey in Germany in 1990, no morpholine or NMOR was found
in the rubber articles tested; these included balloons, baby
dummies/teats, rubber gloves, condoms and rubber rings for bottling
(BUA, 1991).
5.3 Occupational exposure during manufacture, formulation or use
A USA National Occupational Hazard Survey, conducted by NIOSH,
detected worker exposure to morpholine in 283 different industries
(NRC, 1981). In 1970, 501 283 workers were potentially exposed to the
actual product, 22% were exposed to a product known to contain
morpholine, and 74% were exposed to a generic product suspected to
contain morpholine.
Data from the USA National Occupational Exposure Survey (NOES) in
1988 indicated that in a total of 30 industries and 8711 plants
surveyed, 146 511 workers, including 44 839 women, were potentially
exposed to morpholine in the workplace (NIOSH, 1988).
Many countries recommend for morpholine an 8-h time-weighted
average exposure limit of 70 mg/m3 (skin notation) and a 15-min
short-term exposure limit (STEL) of 105 mg/m3 (ILO, 1991).
5.3.1 Exposure to morpholine
Only a few studies have been reported concerning the exposure of
workers to morpholine.
An analysis in Ontario, Canada of condensed steam samples in four
hospitals and one food processing plant using corrosion inhibitors in
the steam-generating plants gave mean values for morpholine of
2.41 mg/litre in the hospitals (9 samples; range 2.1-2.9 mg/litre) and
1.1 mg/litre in the factory (9 samples; range 0.6-1.8 mg/litre)
(Malaiyandi et al., 1979).
Fajen et al. (1979) found morpholine levels of 230 and
42 µg/m3, respectively, in air samples from two out of four rubber
industry factories; these two were a tyre factory and its chemical
factory.
Taft & Stroman (1979) described an investigation into workers
exposed to a number of chemicals in the drying and bagging departments
of a tyre and rubber company manufacturing the rubber accelerator,
4-morpholino-2-benzothiazolyl [(4-morphinyl-2-benzothiazole)]
disulfide. This rubber accelerator is manu-factured by reacting
morpholine with 2-mercaptobenzothiazole (MBT). With the exception of
one personal sample showing 0.05 mg of morpholine/sample (0.4 ppm) at
the level of detection, all other samples showed negative results.
Occupational exposure to morpholine was measured in 15 factories
of the chemical, plastic and rubber industries in Germany from 1980 to
1990, and in no case was the German workplace exposure limit of
70 mg/m3 reached. Of 35 measurements, 90% were below 0.26 mg/m3
(BUA, 1991).
Katosova et al. (1991) reported average levels of 0.54 to 0.93 mg
morpholine/m3 with maximum concentrations of 0.73 to 2.14 mg/m3 in
a morpholine production factory in the former-USSR.
5.3.2 Exposure to N-nitrosomorpholine
Occupational exposure to NMOR has been found in the rubber
industry. Table 12 gives a summary of the data published.
Fajen et al. (1979) found NMOR in air samples from a tyre
chemical factory and an aircraft tyre factory. In the chemical
factory, NMOR was also found as an impurity in morpholine (0.8 mg/kg)
and in bismorpholine carbamylsulfenamide (BMCS), a vulcanisation
accelerator (0.4 to 0.7 mg/kg), as well as in wastewater
(0.003 mg/kg), utility steam condensate (0.002 mg/kg), and dirt
scrapings on a staircase (730 mg/kg). Outside the chemical plant, a
soil sample contained 4.4 mg/kg (Fajen et al., 1979). It is possible
that the NMOR in these samples was formed by the transnitrosation from
N-nitrosodiphenylamine, also produced in the tyre chemical factory,
together with morpholine present in the steam condensate (BUA, 1991).
A survey was carried out by NIOSH and a tyre manufacturing
company in 1979 into the levels of NMOR in four different work areas
in the factory as well as personal exposure levels (Ringenburg &
Fajen, 1980; London & Lee, 1987). Personal breathing-zone air samples
ranged from 0.6 to 1.8 µg/m3 (mean 0.8 µg/m3), and workplace
levels from 0.8 to 3.7 µg/m3. In a survey at another tyre
manufacturing company during the same year, nitrosamine levels were
highest (1.62 µg/m3) during the extrusion process of racing tyre
components (McGlothlin, 1980; McGlothlin & Wilcox, 1984). Exposure to
NMOR in rubber press operator fumes was investigated by NIOSH in
another company. The highest personal exposure to NMOR was
0.063 µg/m3, and the highest area sample 0.038 µg/m3.
In a factory producing rubber parts for automobile interiors, the
highest level of NMOR (19 µg/m3) was found in the process sample
taken directly above one of the extruder ovens. Other personal and
process levels were in the range 0.1 to 1.4 µg NMOR/m3.
Between 1980 and 1990, 775 samples of workplace air from 124
factories in Germany involved in the production and fabrication of
rubber articles were analysed for NMOR (BUA, 1991). Of the samples,
71% showed concentrations less than the guidance value of 1 µg/m3
(BUA, 1991).
According to Spiegelhalder (1983) and Schuster et al. (1990),
higher concentrations of NMOR are to be found in work areas where
higher temperatures are required for fabrication/vulcanization. High
levels of NMOR from storage areas probably result from continued
emission of volatile NMOR.
NMOR was not detected in the environment of a metal factory using
metal-working fluids (Fadlallah et al., 1990).
Table 12. Occupational exposure to NMORa
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Tyre chemical factory USA 1978 10 area 0.07-5.1 Fajen et al. (1979)
Tyre manufacturer USA 1978 20 area 0.6-27 Rounbehler & Fajen
12 area 0.08-8.5 (1983)
7 area 0.02-3.9
6 process 0.41-2.9
6 area < 0.01-0.60
7 process < 0.1-22.0
7 area < 0.002-250
8 process 0.1-25
Tyre manufacturer USA 1979/ 13 area 0.1-1.62 McGlothlin (1980),
1980 McGlothlin & Wilcox (1984)
Tyre manufacturer USA 1979/ 4 area 0.85-3.7 Ringenburg & Fajen (1980);
1980 6 personal 0.6-1.8 London & Lee (1987)
Industrial rubber articles USA 1978 4 area 0 Fajen et al. (1979)
Industrial rubber articles USA 1980/ 11 area < 0.005-0.0376 Hollett et al. (1982)
1981 7 personal < 0.0096-0.0629
Rubber articles for USA 1982 4 area 0.8-19 Lee (1982)
car outfitting 13 personal 0.4-1.2
Rubber industry Germany 1979/ 20 monitoring area and 0.1-17 Spiegelhalder &
1981 points in 17 personal Preussmann (1982)
factories
Table 12 (cont'd)
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Rubber industry Germany 1987 545 personalc < 2.5 (73%), TRGS (1989)
> 2.5 (27%)
max. 41
Chemical industry Germany 1987 22 not given < 1 (91%)d TRGS (1989)
Leather industry Germany 1987 50 not given < 1 (100%) TRGS (1989)
Foundries Germany not given 658 not given < 1 (95%), TRGS (1989)
> 1 (5%)
max. 2.1
a adapted from BUA (1991)
b the percentage of samples which had this given concentration is given in parentheses
c sum of all measured N-nitrosamines
d in 82% below the detection level (0.2 µg/m3)
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Toxicity experiments on rodents have shown that morpholine is
absorbed after oral, dermal and inhalation exposure (see Table 13).
6.2 Distribution
Tanaka et al. (1978) determined the distribution of
[14C]-labelled morpholine in male Wistar rats (3 animals/group,
250-300 g) after oral (200 mg/kg) and intravenous injections
(150 mg/kg). The radioactivity was determined in the dried, powdered
organs. Large amounts were only found in muscle and intestine. In
rats sacrificed 2 h after oral administration of morpholine-HCl, 29%
of the radioactivity was found in the intestine and 26% in the muscle.
Similarly, 2 h after intravenous injections, 19 and 27% of the dose
was found in the intestine and muscle, respectively.
Female New Zealand rabbits (number not given) were exposed to
morpholine (905 mg/m3) for 5 h by nose-only inhalation
(Tombropoulos, 1979). At the end of the exposure, the animals were
sacrificed and the tissue and body fluids analysed. Concentrations of
morpholine were highest in urine (324 mg/litre) and kidney
(118 mg/kg), the other tissues having concentrations below 40 mg/kg.
Van Stee et al. (1981) injected six male New Zealand rabbits
intravenously with 5 mmol [14C]-labelled morpholine/kg body weight
(435 mg/kg). The distribution of radioactivity after 30 min showed
the highest concentrations in the renal medulla (36 mmol/kg) and
cortex (15.4 mmol/kg), followed by lung (5.1 mmol/kg), liver
(4.7 mmol/kg) and blood (2.3 mmol/litre). Morpholine was not bound to
serum proteins.
6.3 Metabolic transformation
Morpholine is eliminated mainly in a non-metabolized form in the
urine of the rat, mouse, hamster and rabbit (Griffiths, 1968; Tanaka
et al., 1978; Van Stee et al., 1981; Sohn et al., 1982b). However,
Sohn et al. (1982b) reported that morpholine is metabolized by
N-methylation followed by N-oxidation in the guinea-pig. After an
intraperitoneal injection of 125 mg/kg [14C]-labelled morpholine in
guinea-pigs, 20% of the radioactivity was found in the urine as
N-methylmorpholine- N-oxide. However, the morpholine ring can be
cleaved in mammalian systems. In several studies on the metabolism of
morpholine derivatives in the rat, ring cleavage products have been
noted (Tatsumi et al., 1975; Hecht & Young, 1981; Kamimura et al.,
1987).
Table 13. Single exposure toxicity data for morpholinea
Species No./sex Dosage Results Cause of death/symptoms Reference
Oral
Rat 5 1.05 g/kg body weight LD50 n.g. Smyth et al. (1954)
Rat 57 1.6 g/kg body weightb LD50 gastrointestinal haemorrhage Shea (1939)
Rat 5 m + 5 f 1.9 g/kg body weight LD50 dyspnoea, haemorrhagic BASF (1967)
enteritis
Rat 7 m 1.0 g/kg body weight; pH 7 no deaths n.g. Börzsönyi et al. (1981)
Guinea-pig 33 0.9 g/kg body weightb LD50 gastrointestinal Shea (1939)
haemorrhage, diarrhoea
Intraperitoneal
Rat (Wistar) 4 m 0.4 g/kg body weight all died n.g. Stewart & Farber (1973)
Rat (Wistar) 4 m 0.1 g/kg body weight lethal n.g. Stewart & Farber (1973)
for 1/4
Mouse 5 m + 5 f 0.4 g/kg body weight LD50 irritation around BASF (1967)
injection area, dyspnoea
Inhalation
Rat 5 m + 5 f 18.1 g/m3; 6 h lethal haemorrhage of nose, mouth Hazleton (1981)
for 9/10 and eyes; spasms; tremors
Rat 6 saturated atmosphere;c all died spasms; caustic burns BASF (1967)
5´ h on nose and extremities
Table 13 (cont'd)
Species No./sex Dosage Results Cause of death/symptoms Reference
Inhalation (contd)
Rat n.g./m 8.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g./f 7.8 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g. 65.2 g/m3; 8 h lethal irritation of nose, Shea (1939)
eyes; lung haemorrhage
Rat n.g. 43.4 g/m3; 8 h lethal n.g. Shea (1939)
Mouse n.g./f 6.9 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g./m 5.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g. 4.9 g/m3; LC50 n.g. Zaeva et al. (1968)
Dermal
Rabbit (New n.g. 0.5 ml/kg body weight LC50 n.g. Smyth et al. (1954)
Zealand)
a adapted from BUA (1991); n.g. = no details given
b morpholine diluted with 4 parts water; deaths within a week
c inhalation of a saturated atmosphere at 20°C formed by bubbling air through a 5-cm layer of morpholine
In the presence of nitrite, morpholine can be converted to NMOR
(see section 4.3). NMOR was found in the stomachs of rats that had
been fed on diets containing morpholine and nitrite (Sander et al.,
1968; Inui et al., 1979).
Immunostimulation of rats by intraperitoneal treatment with
E. coli lipopolysaccharide (LPS; 1 mg/kg) led to a large increase in
urinary nitrate and urinary metabolites of NMOR when morpholine
(80 µmol/kg) and L-arginine (400 µmol/kg) were injected
intraperitoneally. The replacement of LPS with nitrate (330 µmol/kg
intraperitoneal) did not increase urinary metabolites of NMOR (Leaf et
al., 1991). This result is consistent with endogenous nitrosation of
morpholine by nitrogen oxide (NO) from oxidation of the guanido group
of arginine by induced NO synthase (Hibbs, 1992).
Hecht & Morrison (1984) developed a method to monitor the
in vivo formation of NMOR by measuring N-nitroso
(2-hydroxyethyl)glycine, its major urinary metabolite. The formation
of NMOR was measured in F-344 rats over wide range of doses of
morpholine (38.3-0.92 µmol) and sodium nitrite (191-4.8 µmol).
According to estimates by the authors, 0.5 to 12% of the morpholine,
depending on the dose, was nitrosated.
In vitro nitrosation of morpholine has been reported. NMOR was
formed when morpholine was added to human saliva (Tannenbaum et al.,
1978). Additionally, a new type of metabolite N-cyanomorpholine was
identified in human saliva (Wishnok & Tannenbaum, 1976).
6.4 Elimination and excretion
6.4.1 Expired air
Elimination of 14C from labelled morpholine (intraperitoneal
injection) through expired air is minimal. In rats, experiments have
shown that only about 0.5% of the dose of radioactively labelled
morpholine is exhaled as 14CO2 (Sohn et al., 1982b). In rabbits
0.0008% of the administered dose was 14CO2 (Van Stee et al.,
1981).
6.4.2 Urine
Elimination studies on male Wistar rats (200-350 g) were carried
out by administering morpholine-HCl (500 mg/kg) or [14C]-labelled
morpholine-HCl (200 mg/kg) orally and morpholine-HCl (250 mg/kg)
intravenously. In all cases, over 85% of the dose was excreted in
urine within 24 h. A further portion, up to 5%, was excreted during
the next three days. [14C]-morpholine palmitate was eliminated
slightly more slowly, but the urinary excretion within 3 days
following oral administration amounted to 90% of the dose (Tanaka et
al., 1978). Of the radioactive morpholine administered to rats,
62-77.5% was excreted in the urine after 24 h (Griffiths, 1968;
Ohnishi, 1984). Following intraperitoneal administration to rats,
urinary excretion within 24 h was 87.8% of the dose (Maller &
Heidelberger, 1957). In the dog, 70-80% of the radioactive morpholine
was excreted in the urine (Rhodes & Case, 1977).
The time-course of urinary excretion of 14C by Sprague-Dawley
rats, Syrian golden hamsters, and strain II guinea-pigs treated with
[14C]-morpholine was compared by Sohn et al. (1982b). Although in
all three species over 80% was excreted in 3 days, the rate of urinary
excretion within the first 6 h was greatest in the hamster and least
in the guinea-pig.
Van Stee et al. (1981) infused rabbits intravenously with
[14C]-morpholine (5 mmol/kg) which had been neutralized with HCl.
After 4 h, 18.5% of the dose was excreted in the urine. When the pH
of the urine was lowered from 7.8-7.9 to 7.1-7.2 by administration of
ammonium chloride (10 g/litre) in drinking-water prior to the
injection, the urinary excretion more than doubled (to 43%).
These data suggest that the urinary excretion of morpholine is
enhanced by its neutralization with acid.
6.4.3 Faeces
Rats dosed orally or intravenously with morpholine hydrochloride
excreted not more than 1.7% of the dose in the faeces (Griffiths,
1968; Tanaka et al., 1978). However, when dosed orally with
morpholine palmitate (Tanaka et al., 1978, Ohnishi, 1984), up to 7%
was excreted in faeces.
6.5 Retention and turnover
Plasma concentration-time curves of 14C after intraperitoneal
injections of [14C]-morpholine (125 mg/kg in 0.9% NaCl) in Sprague-
Dawley rats, Syrian golden hamsters, and strain II guinea-pigs
declined biexponentially. Whereas rates of first phase of elimination
from plasma in rats and hamsters were similar (half-lives of 115 and
120 min, respectively), the half-life in guinea-pigs was significantly
longer (300 min) (Sohn et al., 1982b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Table 13 summarizes the toxicity data on single exposure to
morpholine.
7.1.1 Oral
Oral administration of morpholine to rats resulted in LD50
values of 1-2 g/kg body weight (Shea, 1939; Smyth et al., 1954; BASF,
1967). Gastrointestinal and nasal haemorrhage were reported. In
contrast, when morpholine was administered to seven male rats at a
neutral pH, no deaths occurred with concentrations of 1 g/kg body
weight (Börzsönyi et al., 1981).
A study on guinea-pigs resulted in a LD50 of 0.9 g morpholine
per kg body weight. Gastrointestinal and nasal haemorrhage were
reported (Shea, 1939).
7.1.2 Inhalation
There have been reports of inhalation studies with morpholine on
rats (Shea, 1939; BASF, 1967; International Labour Office, 1972;
Lam & Van Stee, 1978; Hazleton, 1981) and mice (Zaeva et al., 1968;
Lam & Van Stee, 1978).
Exposure to morpholine at vapour saturation concentrations
resulted in almost 100% lethality after 5.5 h (BASF, 1967). It had
irritating and corrosive properties.
In studies with lower concentrations, Lam & Van Stee (1978)
obtained LC50 values of 7.8 and 8.2 g/m3 for female and male rats,
respectively, whereas other authors reported no deaths at three times
this concentration (see Table 13).
Reported LC50 values for mice are consistently in the range of
5-7 g/m3 (see Table 13).
7.1.3 Dermal
Smyth et al. (1954) noted necrosis on the clipped skin of albino
rabbits within 24 h of application of undiluted morpholine. Mortality
within 14 days in New Zealand rabbits after penetration of morpholine
into the skin gave an LD50 of 0.5 ml/kg body weight.
Other reports are discussed in section 7.4.2.
7.1.4 Intraperitoneal
When morpholine was administered intraperitoneally to rats
(Stewart & Farber, 1973) and mice (BASF, 1967), the LD50 was in the
range 0.1-0.4 g/kg body weight for rats and 0.4 g/kg body weight for
mice (see Table 13).
7.2 Short-term exposure
As with single-exposure, the effects of morpholine after short-
term exposure depend on the method of exposure. The available data on
short-term exposure are summarized in Table 14.
7.2.1 Oral
Rats and guinea-pigs were fed morpholine at concentrations of
0.16-0.8 g/kg body weight and 0.09-0.45 g/kg body weight,
respectively, by gavage for 30 days (Shea, 1939). At concentrations of
half of the LD50, nearly all the animals died within 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver and necrosis of the stomach
glandular epithelium.
Fatty degeneration (lipidosis) of the liver in rats was noted
after feeding morpholine (0.5 g/kg body weight) daily for 56 days
(Sander & Bürkle, 1969).
Shibata et al. (1987a) carried out a 13-week toxicity study in
B6C3F1 mice using morpholine as the fatty acid salt, morpholine
oleic acid salt (MOAS), at dosage levels of 0%, 0.15%, 0.3%, 0.6%,
1.25% and 2.5% MOAS in the drinking-water. At the highest MOAS level,
body weight gains were slightly reduced. Histopathological analysis
showed cloudy swelling of the proximal tubules, but no other
alterations were observed in the organs of either sex. Urinalysis
showed increases in both specific gravity and plasma urea nitrogen in
some dosage groups, suggesting a possible malfunctioning of the kidney
(see Table 14).
7.2.2 Inhalation
In rats that died after 5 days repeated exposure to 65.2 g/m3,
lung haemorrhage, severe damage to the secreting tubules of the
kidney, and fatty degeneration of the liver were observed (Shea,
1939).
Table 14. Short-term exposure to morpholinea
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
Oral
Rat 20 0.16 g/kg body weight per 8/20 beginning necrosis of liver, kidney Shea (1939)
day; 30 days; in 2 ml H2O and stomach mucous membrane
(gavage)
20 0.32 g/kg body weight per 8/20 necrosis of liver, kidney, stomach
day; 30 days; in 2 ml H2O
(gavage)
20 0.8 g/kg body weight per 19/20 weight loss, lethargy, severe necrosis
day; 30 days; in 2 ml H2O of liver, kidney, stomach
(gavage)
Rat 7 f 0.5 g/kg body weight per sacrifice after 270 days, moderate Sander & Bürkle
(Sprague- day; 56 days liver adipose degeneration (1969)
Dawley)
Mouse 10 m + 10 f 0.15 and 0.3% MOAS; 91 days no significant changes Shibata et al.
(B6C3F1) (0.07 or 0.14 g morpholine/ (1987a)
kg body weight per day)
10 m + 10 f 0.6% MOAS; 91 days m: increase in specific gravity of
(ca. 0.2 g morpholine/kg urine; f: increase in blood urea
body weight) per day
10 m + 10 f 1.25% MOAS; 91 days increase in specific gravity of
(ca. 0.4 g morpholine/kg urine and blood urea
body weight per day)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
10 m + 10 f 2.5% MOAS; 91 days reduced weight gain; increase in specific Shibata et al.
(ca. 0.7 g morpholine/kg gravity of urine and blood urea; (1987a)
body weight per day) increased relative kidney weight with
swelling of proximal tubules
Guinea-pig 20 0.09 g/kg body weight 3/20 slight alterations in liver, kidney Shea (1939)
per day; 30 days (syringe)
20 0.18 g/kg body weight 12/20 necrosis of liver, kidney, stomach
per day; 30 days (syringe)
20 0.45 g/kg body weight 16/20 severe degeneration of liver, kidney,
per day; 30 days (syringe) stomach
Inhalation
Rat n.g. 7.2 g/m3; 4 h/day; 4 days n.g. increased residual volume and Takezawa &
total lung capacity Lam (1978)
Rat n.g. 65.2 g/m3; 34 h over 5 days some deathsb necrosis of liver and kidney tubules; Shea (1939)
lung irritation
Rat 6-8 m 0.08 g/m3; 4 h/day; 8 days no deaths hypersecretion of thyroid gland, Grodeckaja
higher accumulation of 131I in the & Karamzina
thyroid gland (1973)
Rat 5 m + 5 f 0.36 g/m3; 6 h/day; 9 days 0/10 red stains around nose and mouth; Hazleton
weight loss in females (1981)
5 m + 5 f 1.81 g/m3; 6 h/day; 9 days 2/10 irritation to nose and eyes; weight loss; Hazleton
decrease in spleen/brain weight ratios (1981)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
5 m + 5 f 3.62 and 18.1 g/m3; 10/10 bleeding from eyes, nose and Hazleton
6 h/day; 9 days mouth (1981)
Rat n.g. 1.63 g/m3; 4 h/day; n.g. reduced weight gain; increased residual Takezawa &
5 days/week; 30 days volume and total lung capacity Lam (1978)
Rat 20 m + 20 f 0.09 g/m3; 6 h/day; no significant differences in body Conaway et al.
(Sprague- 5 days/week; 13 weeks weight, clinical chemistry, haematology (1984b)
Dawley) or organ weight data; no nasal lesions
20 m + 20 f 0.36 g/m3; 6 h/day; in 2/20 females focal necrosis in
5 days/week; 13 weeks nasal cavity
20 m + 20 f 0.9 g/m3; 6 h/day; lesions of nose and mouth; after 7
5 days/week; 13 weeks weeks in 8 animals focal metaplasia
and necrosis in nasal turbinates; after
13 weeks, increased incidence and
severity; pneumonia
Rabbit 4 m 0.9 g/m3; 6 h/day; increased hydrolytic enzyme content Tombropoulos
5 days/week; 33 days in alveolar macrophages et al. (1983)
a adapted from BUA (1991); b.w. = body weight; n.g. = not given
b exact numbers not clear
Rats inhaling morpholine at 3.62 or 18.1 g/m3 for 9 days, 6 h
per day, died within the exposure period (Hazleton, 1981). At lower
concentrations (1.81 g/m3), weight loss and irritation to nose and
eyes, as well as two deaths, were reported. The report concluded that
the maximal tolerated dose for rats is about or just below 0.3 g/m3.
Increased thyroid activity, shown as increased uptake of injected
131I, was observed in male rats after exposure to 0.08 g
morpholine/m3, 4 h/day, for 4 days (Grodeckaja & Karamzina, 1973).
Takezawa & Lam (1978) reported an increase in lung weight,
residual volume and total lung capacity in rats exposed to morpholine
(7.2 g/m3, 4 h/day for 4 days or 1.63 g/m3 for 30 days).
Tombropoulos et al. (1983) examined the induction of lysosomal
enzymes by morpholine in rabbits. Two acid hydrolases,
alpha-mannosidase and acid phosphatase, were induced in the lung
alveolar macrophages during the course of inhalation exposure
(905 mg/m3, 250 ppm, 6 h/day, 5 days/week for a total of 33
exposures). The induction was also observed when macrophages were
cultured in the presence of morpholine.
Conaway et al. (1984b) exposed groups of 40 rats to morpholine
(0, 0.09, 0.36 and 0.9 g/m3) for 7 and 13 weeks. Shallow, rapid
breathing was noted in all groups except the controls. Lesions of the
nasal septum, anterior cavities, nasoturbinates and maxilloturbinates
were observed in the 0.36 and 0.9 g/m3 groups but not in the lowest
exposure group.
Lung sections of all rats killed after 7 weeks contained early
lesions of chronic murine pneumonia. In the upper dosage group, these
lesions had developed in severity by 13 weeks. There were no apparent
treatment-related effects in any of the haematology, clinical
chemistry or urinalysis data at weeks 7 or 13.
7.2.3 Dermal
Shea (1939) investigated the effects of single and repeated
dermal application of morpholine on rabbits and guinea-pigs. He found
that whereas unneutralized, undiluted morpholine caused 100% lethality
and even the diluted compound caused mortality and severe necrotic
burns and inflammation, application of undiluted morpholine
neutralized to pH 7 with sulfuric acid caused neither gross nor
microscopic pathology of the skin with the exception that the dermis
was thickened at the site of application.
Undiluted morpholine applied for 5-15 min to rabbit skin led to
severe necrosis (BASF, 1967).
Data on short-term dermal exposure to morpholine are also
discussed in section 7.4.2.
7.3 Long-term exposure
Table 15 summarizes the data on long-term exposure to morpholine.
7.3.1 Oral
Mice (10 males and 10 females/group) were given 0%, 0.25% or 1.0%
MOAS in their drinking-water for 96 weeks and then given normal tap
water for a further 8 weeks (Shibata et al., 1987b). During the study,
the physical appearance and general behaviour did not appear to be
affected by treatment. Decreased body weight gain was noted at 1% MOAS
(both sexes) and at 0.25% MOAS (females). Gross pathological and
extensive biochemical examinations did not reveal treatment-related
effects. The incidence of hyperplasia in forestomach epithelium of
males in the 1% group was statistically higher than in the controls,
but otherwise no significant increase in the incidence of non-
neoplastic and neoplastic lesions could be found.
7.3.2 Inhalation
Migukina (1973) reported increased nervous system activity and
increases in haemoglobin and peripheral red blood cell counts in rats
and guinea-pigs exposed to morpholine (0.008 and 0.07 g/m3) for 4
months. An increase in the chromosomal aberrations of the bone marrow
cells was also noted (see section 7.6). This study was deficient with
respect to the description of study methods.
Harbison et al. (1989) carried out an extensive long-term
exposure inhalation study. Groups of 70 rats of each sex were exposed
to morpholine (0, 0.036, 0.181, 0.543 g/m3) 6 h per day, 5 days per
week, for 104 weeks, with an interim sacrifice at week 53. Levels of
nitrates and nitrites in the drinking-water were reported to be
< 0.1 mg/litre and 0.01 mg/litre, respectively. Survival, body weight
gain, organ weight and haematology and clinical chemistry data were
normal in exposed groups, compared to the controls. In-life clinical
examinations revealed increased incidences of inflammation of the
cornea, inflammation and squamous metaplasia of the turbinate
epithelium, and necrosis of the turbinate bones in the nasal cavity of
both male and female rats. No increase in the incidence of neoplasms
was found. Only tissues from the respiratory tract and eyes were
examined histologically in the case of the mid- and low-dose groups.
7.3.3 Dermal
No data are available on long-term dermal exposure to morpholine.
Table 15. Long-term exposure to morpholinea
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Oral
Mouse 50 m 0.25% MOAS; 672 days (ca. 0.05-0.14 g no significant changes Shibata et al.
(B6C3F1)b morpholine/kg body weight per day) (1987b)
50 f 0.25% MOAS; 672 days (ca. 0.07-0.17 g decreased body weight gain
morpholine/kg body weight per day)
50 m 1% MOAS; 672 days (ca. 0.28-0.5 g decreased body weight gain; increase in blood
morpholine/kg body weight per day) urea nitrogen; hyperplasia in forestomach
epithelium
50 f 1% MOAS; 672 days (ca. 0.21-0.57 g decreased body weight gain
morpholine/kg body weight per day)
Inhalation
Rat 84 m 0.008 g/m3; 34 h within 5 days; reversible changes in haemogram, kidney, Migukina (1973)
16 weeks liver, lung, spleen, myocardium
84 m 0.07 g/m3; 4 h/day; changes in haemogram, liver, kidney
5 days/week; 16 weeks lung, spleen, myocardium
Rat 20 1.09 g/m3; 6 h/day; no significant increase in spinal cord Savolainen &
5 days/week; 15 weeks axonal succinate dehydrogenase activity; no Rosenberg (1983)
significant alteration of enzyme activity in
muscle; morpholine concentration in brain
increased with length of exposure
Table 15 (cont'd)
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Rat 70 m + 0.036 and 0.18 g/m3; 6 h/day; increased incidence of irritation around Harbison et al.
(Sprague- 70 f 5 days/week; 104 weeksc eyes and nose (1989)
Dawley)
70 m + 0.54 g/m3; 6 h/day; local necrosis around eyes and nose;
70 f 5 days/week; 104 weeksc kerativitis
Guinea-pig 24 0.008 g/m3; 4 h/day; swollen lymph nodes of the spleen Migukina (1973)
5 days/week; 16 weeks
24 0.07 g/m3; 4 h/day; swollen alveoli; atrophy of lymph vessels in
5 days/week; 16 weeks lung and spleen; increase in haemoglobin and
erythrocyte number, decrease in leucocytes
a adapted from BUA (1991); b.w. = body weight
b MOAS: morpholine administered as oleic acid salt in drinking-water; in brackets, the daily intake of morpholine
calculated from MOAS intake
c 10 m + 10 f killed at 53 weeks
7.4 Skin and eye irritation; sensitization
7.4.1. Eye irritation
Eye injury in rabbits was tested by Smyth et al. (1954), who
reported severe eye burns with 0.5 ml of a 1% solution. BASF (1967)
reported that 1 drop of undiluted morpholine in rabbit eyes, repeated
once after 5 min, caused oedema, opacity, staphyloma and corrosion of
the eye mucous membranes within 24 h.
A solution of morpholine (0.02 mol/litre) neutralized with HCl
had no injurious effect on the eyes of rabbits when applied
continuously for 10 min after removal of the corneal epithelium to
facilitate penetration. Similarly, the irritative reaction of 10-20%
aqueous morpholine was reduced on neutralization (Grant, 1974).
In-life clinical examinations in rats exposed to up to
0.54 g/m3 (150 ppm) morpholine revealed increased incidences in
inflammation of the cornea at week 103 of the study (Harbison et al.,
1989). Findings included keratitis, oedema, abrasion, scarring, and
ulceration with or without neovascularization and corneal epithelial
hyperplasia. A high incidence of retinal degeneration was observed,
primarily in female animals, which was probably an age-related light-
induced retinal degeneration.
Albino rabbits, three of each sex, were treated with a mascara
composite containing 1% morpholine; 0.1 ml of the cosmetic was
instilled into one eye of each rabbit daily for 14 days. Before the
application each day, the eye was evaluated for ocular irritation. A
slight redness of the conjunctiva was noted throughout the duration of
the study but this cleared within 24 h of the last treatment. A sodium
fluorescein dye test performed at the conclusion of the study
indicated no abnormalities of the cornea or its iris membranes
(Cosmetic Ingredient Review, 1989).
7.4.2 Skin irritation
In rabbits treated with aqueous solutions of 2, 20, 40 and 60%
morpholine, the skin reactions were evaluated after 0.5, 24, 48 and
72 h (Lodén et al., 1985). A 2% solution of morpholine caused skin
irritation after 72 h, whereas 40% and 60% solutions immediately
caused reddening of the skin.
Wang & Suskind (1988) measured the irritant potential of
morpholine by applying 0.1 g mixture (0.1, 0.5, 2, 5 and 10%
morpholine in petrolatum) to guinea-pig skin for 24 h. Observations
were made 1, 24 and 48 h after removal of the test materials, and no
noticeable effects were found.
A mascara composite containing 1% morpholine was applied to
normal and abraded skin of six albino rabbits (Cosmetic Ingredient
Review, 1989). At the end of the 14-day treatment period, no dermal
toxicity or irritation was observed.
7.4.3 Sensitization
Sensitization studies (modified Buehler) on guinea-pig skin using
2% morpholine in petrolatum gave negative results (Wang & Suskind,
1988). In an in vitro study into the biochemical causes of
sensitization, it was found that morpholine as a hapten did not react
with the amino acids glycine, lysine and cystine, as did related
compounds which showed sensitizing reactions (Wang & Tabor, 1988).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
No adequate studies on reproductive toxicity, embryotoxicity or
teratogenicity have been reported.
7.6 Mutagenicity and related end-points
7.6.1 Mutagenicity of morpholine
Table 16 summarizes the short-term mutagenicity tests on
morpholine.
7.6.1.1 Bacteria
No mutagenic response was observed in plate tests with strains of
Salmonella typhimurium, either with or without metabolic activator,
exposed to morpholine at up to 10 µl/plate (approximately 10 mg/plate)
(Texaco, 1979a; Haworth et al., 1983), whereas a 99.8% purity sample
did induce weak mutagenic responses in S. typhimurium TA100 and
Escherichia coli WP2 uvr A at the unusually high dose level of
50 mg/plate (Glatt & Oesch, 1981). The activity in strain TA100 did
not require S9 mix and is, therefore, unlikely to have been due to
0.2% contamination by NMOR (see below).
In mouse peritoneal cavity host-mediated assays with
S. typhimurium TA1530 and TA1930, no increase in the proportion of
mutants was observed following the oral administration of morpholine
(Braun et al., 1977; Edwards et al., 1979). These groups served as
controls in a study of nitrosation (see below).
7.6.1.2 Yeast
No gene conversion was induced in Saccharomyces cerevisiae D4
by morpholine concentrations up to 10 µg/litre per plate. Toxicity
was observed at the highest concentration (Texaco, 1979a).
Table 16. Short-term in vitro microbial mutagenicity assays with morpholinea
Test organisms Strain Analyzed Metabolic Dosage range Result with/ Reference
purity (%) activityb without S9-mix
Salmonella typhimurium TA98/100/1535/1537 not given RA 0.109-10.9 mg/plate -/- Haworth et al. (1983)
S. typhimurium TA98/100/1535/1537 not given RA 0.005-10 µl/plate -/- Texaco (1979a)
1538c
Saccharomyces D4 not given RA 0.005-10 µl/platec -/- Texaco (1979a)
cerevisiae
S. typhimurium TA98/1535/1537 99.8 RA 0.0158-50 mg/plate -/- Glatt & Oesch (1981)
S. typhimurium TA100 99.8 RA 0.0158-50 mg/plate (+)/(+)d Glatt & Oesch (1981)
Escherichia coli WP2 uvrA 99.8 RA 0.0158-50 mg/plate (+)d/- Glatt & Oesch (1981)
S. typhimurium TA1950 99 HM1 1450-2900 µmol/kg - Braun et al. (1977)
S. typhimurium TA1530 not given HM2 4 mg/kg body weight - Edwards et al. (1979)
a adapted from BUA (1991)
b RA = Arochlor-induced rat liver-S9; HM1 = Host-mediated assay NMRI-mice; HM2 = Host-mediated assay CD-1 mice
c toxic at 10 µl/plate for strain Salmonella typhimurium TA1538 and Saccharomyces cerevisiae D4
d (+) = positive only at the 50 mg/plate dose
7.6.1.3 Mammalian cells in vitro
When tested over the range 0.625-1.25 µl/ml (approximately
0.625-1.25 mg/ml) morpholine induced small increases in the fraction
of tk mutants in mouse lymphoma L5178Y cells. No exogenous metabolic
activation system was required for this weak activity (Texaco, 1979b;
Conaway et al., 1982a,b).
Morpholine induced no DNA-repair in the primary cultures of rat
hepatocytes (0.1-100 µg/ml) (Conaway et al., 1984a).
Morpholine induced small increases in the frequency of SCEs in
Chinese hamster ovary (CHO) cells. No exogenous metabolic activation
system was required for this activity, which was observed at
concentrations of 50 and 100 nl/ml (approximately 50 and 100 µg/ml) in
the absence of S9 mix (Litton Bionetics, 1980).
Morpholine increased the numbers of type III foci in the
Balb/C3T3 cell transformation assay tested at different dosages
(0.001-0.3 µl/ml), corresponding to 78-52% survival in the
cytotoxicity test (Texaco, 1979b, Litton Bionetics, 1979a,b; Conaway
et al., 1982a,b).
In another study of in vitro transformation of Balb/3T3 cells
with and without metabolic activation, the morpholine in the culture
medium was neutralized before testing (Litton Bionetics, 1982). No
significant increases were induced in transformed foci over the tested
concentration ranges (0.015 to 1.400 µl/ml in the non-activation
assay; 0.0175-0.7 µl/ml in the activation assay). Morpholine was
therefore considered as being inactive in this test.
7.6.1.4 In vivo studies in mammals
Inui et al. (1979) administered a dosage of 500 mg morpholine
per kg body weight to female Syrian hamsters on the 11th or 12th day
of pregnancy. The embryos were removed 24 h later and embryo cells
examined. For detection of induced mutations, embryo cells were
cultured in normal medium for 72 h and then transferred to a medium
containing 8-azaguanine (10 or 20 mg per litre) or ouabain (1 mM). No
chromosomal aberrations, micro-nucleus formation, or 8-azaguanine- or
ouabain-resistant mutations were found.
Migukina (1973) reported an increase in the number of chromosomal
aberrations, particularly "fragmentations", in bone marrow cells of
rats and guinea-pigs exposed for 4 months to 8 or 70 mg
morpholine/m3 (see also section 7.3.2). However, there were
deficiencies in the study report (BUA, 1991).
7.6.2 Mutagenicity of morpholine in the presence of nitrite and
nitrate
Although morpholine has given negative responses in a number of
mutagenicity assays, there is concern for its ability to be nitrosated
readily to form NMOR (see section 4.3).
Two mouse peritoneal cavity host-mediated assays with
S. typhimurium have been performed. In one, with strain TA1530,
different morpholine doses were administered orally to mice
simultaneously with a standard sodium nitrite dose of 120 mg/kg. The
mixtures were adjusted to pH 3.4. Significant increases in the mutant
fraction were observed with morpholine doses of 4-40 mg/kg, the full
range tested (Edwards et al., 1979). In the other assay, with strain
TA1950, equimolar mixtures of morpholine and sodium nitrite (1450 or
2900 µmol/kg, approximately 125 or 250 mg/kg) were administered orally
to mice (pH 7.0). Significant increases in the mutant fraction were
observed at both dose levels. When the nitrite was administered to the
mice 10 min before the morpholine treatment, no mutagenic response was
induced (Braun et al., 1977).
Mutations were found in S. typhimurium TA1535 used to test the
urine of OF1 mice after morpholine (250 mg/kg body weight) had been
administered in the presence of nitrate (2000 mg/kg). Lower levels of
nitrate (333 and 666 mg/kg) caused no mutations (Perez et al., 1990).
7.6.3 Mutagenicity of N-nitrosomorpholine
NMOR is mutagenic to S. typhimurium TA100 in the presence of S9
mix at dose levels above 2000 µg/plate. In yeast, it induced forward
mutation and aneuploidy, but not mitotic recombination. In cultured
mammalian cells, conflicting results were obtained in unscheduled DNA
synthesis (UDS) assays in fibroblasts, while weakly positive results
were reported for sister-chromatid exchange (SCE) in CHO cells, for tk
locus mutation in mouse lymphoma L5178Y cells, and for enhanced colony
growth of BHK cells in soft agar. NMOR was negative in an in vitro
cytogenetic assay using RL1 (rat liver cell line) cells (IARC, 1978;
NRC, 1981).
7.7 Carcinogenicity
Carcinogenicity studies have been carried out with morpholine
alone, as well as in the presence of nitrite, to investigate the
possible formation and effect of NMOR.
7.7.1 Morpholine
7.7.1.1 Oral studies
Greenblatt et al. (1971) treated 40 Swiss mice (20 male and
20 female) with 6.33 g morpholine/kg food (estimated dosage, 0.9 g/kg
body weight per day for 28 weeks. The control group consisted of
80 mice of each sex. After a further 12 weeks of observation the
surviving animals were sacrificed. No increase in the lung tumour rate
(0.1 adenoma/mouse) was found compared to the controls
(0.18 adenoma/mouse). It should be noted that the duration of exposure
in this study was shorter than that normally used in a well-designed
long-term carcinogenicity study.
Multi-generation oral studies were performed on Sprague-Dawley
rats fed 5, 50 or 1000 mg/kg morpholine, together with various dietary
concentrations of sodium nitrite (0, 5, 50 or 1000 mg/kg diet)
(Newberne & Shank, 1973; Shank & Newberne, 1976). From the day of
conception, the pregnant animals were given 0 or 1000 mg morpholine/kg
feed. The F1 and F2 generations were fed likewise for the length
of the experiment. The estimated dosage for the young animals was
10 mg/day and for mature animals 20 mg/day. The average life-span was
117 weeks for the treated animals and 109 weeks for the controls. F1
and F2 generations were studied, the survivors being sacrificed in
the 125th week. Table 17 summarizes the results. Three liver cell
carcinomas, two lung angiosarcomas and one other, and two malignant
gliomas were found in the group of 104 rats (F1 and F2) treated
with morpholine.
In a similar study using Syrian golden hamsters, only the F1
generation was studied, the survivors being sacrificed in the 110th
week (Shank & Newberne. 1976). With morpholine alone (1000 mg per kg),
no liver tumours were found but the number in the group (22) was
small.
Morpholine oleic acid salt (MOAS), at dosage levels of 0%, 0.25%
or 1.0%, was added to the drinking-water of B6C3F1 mice for 96
weeks, and this was followed by normal tap water for a further 8 weeks
(Shibata et al., 1987b). Details are given in Table 15 and section
7.3.1. Extensive biochemical, gross pathological and histological
studies were performed. Only the incidence of hyperplasia in
forestomach epithelium in the males of the 1% MOAS group was
statistically higher than in the controls; otherwise, no significant
increases in the incidence of non-neoplastic or neoplastic lesions
were found.
7.7.1.2 Inhalation studies
In a long-term inhalation study over 2 years, Sprague-Dawley rats
(70 of each sex per group) received morpholine at mean exposure
concentrations of 0, 0.036, 0.181 or 0.543 g/m3 (6 h/day,
5 days/week) for up to 104 weeks (Harbison et al., 1989). Further
details are given in section 7.3.2 and Table 15. Tissues from the
high-dose and control groups were subjected to extensive
Table 17. Tumour incidence (liver and lung) in rats and hamsters after feeding morpholine, sodium nitrite or N-nitrosomorpholinea
Dietary levels (mg/kg) Tumour incidence in rats (%) Tumour incidence in hamsters (%)
Morpholine Sodium N-Nitrosomorpholine No. of Liver cell Liver angio Lung angio No. of Liver cell
nitrite animalsb carcinoma sarcoma sarcoma animals carcinoma
0 0 0 156 0 0 0 23 4
0 1000 0 96 1 0 0 30 0
1000 0 0 104 3 0 2 22 0
1000 1000 0 159 97 14 23 16 31
50 1000 0 117 59 5 6 32 0
5 1000 0 154 28 12 8 400
1000 50 0 109 3 2 1 22 0
1000 5 0 172 1 2 1 19 0
50 50 0 152 2 1 1 30 0
5 5 0 125 1 2 2 40 0
0 0 5 128 58 15 9 35 0
0 0 50 94 93 21 20 18 6
a adapted from Shank & Newberne (1976)
b F1 and F2 generations together
histopathological examination; in the middle- and low-dose groups,
this examination was limited to the eye and the respiratory tract.
Ten males and 10 females per group were sacrificed at week 53.
Survival at termination in the control, low-, middle- and high-dose
groups, respectively, was 40, 44, 33 and 32 in males and 35, 27, 32
and 35 in females. No significant increase in the incidence of
tumours was seen in rats of either sex.
7.7.2 Morpholine and nitrite
7.7.2.1 Oral studies
Sander & Bürkle (1969) fed a group of seven female Sprague-Dawley
rats 5 g morpholine together with 5 g nitrite/kg diet for 12 weeks.
After 39 weeks, all of the animals developed hepato-cellular adenomas,
six out of the seven hepatocellular carcinomas, two
hemangioendotheliomas of the liver, one a cyst-adenocarcinoma of the
liver and one a renal adenoma. Rats fed morpholine or nitrite alone
did not develop tumours.
As described in section 7.7.1.1, Shank & Newberne (1976) fed
pregnant rats on a diet containing various concentrations of
morpholine, sodium nitrite and NMOR. Table 17 summarizes the dietary
concentrations and the tumour incidences for the experimental groups.
Hepatocellular carcinoma and haemangio-sarcomas of the liver and
angiosarcoma of the lungs were the most common tumours observed in the
rats. The neoplasms induced by nitrite and morpholine were
morphologically similar to those induced by NMOR. High concentrations
(1000 mg/kg) of morpholine and nitrite together were carcinogenic to
rats. When the morpholine concentration was reduced and the nitrite
concentration remained high, the incidence of hepatic cell carcinomas
decreased with a linear dose-response relationship. When morpholine
concentration remained high, with decreasing nitrite concentration,
the number of hepatic tumours was sharply reduced. This agrees with
the observation in vitro that the nitrosation of morpholine depends
on the square of the nitrite concentration (Mirvish et al., 1975).
Groups of 40 male MRC Wistar rats were treated for 2 years with
either 10 g morpholine/kg diet and drinking-water containing 3 g
sodium nitrite/litre, or with drinking-water containing 0.15 g
NMOR/litre. In both cases, one group of rats was also given sodium
ascorbate (22.7 g/kg diet). The results of treatment with morpholine
plus nitrite or with NMOR were similar to those reported above. When
ascorbate was present, the liver tumours induced by morpholine plus
nitrite had a longer induction period (93 versus 54 weeks) and a
slightly lower incidence (49% versus 65%). However, ascorbate did not
affect liver tumour induction by preformed NMOR. Of those treated
with morpholine, nitrite and ascorbate, 21/39 animals developed
forestomach tumours (Mirvish et al. 1976).
In a similar study using Syrian hamsters (see section 7.7.1.1 and
Table 17), a high morpholine, high nitrite diet (1000 mg/kg) induced
liver cancer in some animals. In contrast, hamsters fed NMOR in the
diet seemed to have greater resistance to tumour induction (Shank &
Newberne, 1976).
7.7.3 Carcinogenicity of N-nitrosomorpholine
NMOR has been shown to be carcinogenic in mice, rats, hamsters
and various fish. Benign and malignant tumours of the liver and lung
in mice, of the liver, kidney and blood vessels in rats, and of the
liver in hamsters have been reported following oral administration of
NMOR. After its subcutaneous injection, it produces tumours of the
upper digestive and respiratory tract in hamsters. NMOR produces liver
tumours following its intravenous injection in rats and liver tumours
in various fish following its administration in tank water (IARC,
1978).
7.8 Factors modifying toxicity; toxicity of metabolites
7.8.1 Factors modifying toxicity
Leaf et al. (1991) reported that rats (Sprague-Dawley, male)
challenged with an E. coli lipopolysaccharide (LPS; 1 mg/kg body
weight), followed 6 and 10 h later by morpholine (80 or 100 µmol/kg
intraperitoneally) and arginine (400 µmol/kg) treatment, showed
significantly increased NMOR formation compared with unchallenged
control rats. The NMOR formation was measured by monitoring the NMOR
metabolite N-nitroso-(2-hydroxyethyl) glycine in the urine. Furman
& Rubenchik (1991) showed that endogenous synthesis of NMOR in the
stomach of adult mice administered nitrite (oral) in combination with
morpholine (intraperitoneal or subcutaneous) was increased after
activation of peritoneal macrophages induced by intraperitoneal
injection of E. coli LPS.
7.8.2 Morpholine metabolites
Morpholine is eliminated almost entirely in a non-metabolized
form in the urine of the rat, mouse, hamster and rabbit (section 6.3).
N-cyanomorpholine ( N-morpholinocarbonitrile) was formed when
morpholine was incubated in vitro with whole human saliva (Wishnok &
Tannenbaum, 1976).
Sohn et al. (1982a,b) identified a metabolite
N-methylmorpholine- N-oxide (NMMO) in guinea-pig urine.
Conaway et al. (1984a) reported that NMMO (0.0001-10 mg/ml) and
other putative metabolites, N-hydroxymorpholine (0.0001-1 mg/ml) and
3-morpholinone (0.001-0.1 mg/ml), did not induce DNA repair at the
non-toxic concentrations tested. The polyurethane foam catalyst,
N-butylmorpholine (0.0001-0.1 mg/ml) was also inactive in the
primary rat hepatocyte/DNA repair assay (Conaway et al., 1984a).
The chemical intermediate N-hydroxyethylmorpholine induced DNA
repair within the dose range 1-5 mg/ml (Conaway et al., 1984a).
The genotoxicity of substituted morpholines might be a function
of the substituent moiety rather than morpholine itself (Conaway et
al., 1984a).
7.9 Mechanisms of toxicity - mode of action
The irritating and corrosive properties of morpholine are due to
its basicity. The mechanism of action of its systemic effects is not
known.
Morpholine fungicides have been shown to act by inhibiting
several enzymes of sterol biosynthesis (Hesselink et al. 1990; Mercer,
1991).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available on the effects of short- and long-term
exposure to morpholine on the general population. There are no
reports of poisonings by morpholine.
8.1.1 Controlled human studies
The Cosmetic Ingredient Review (1989, 1991b) reported unpublished
studies of occlusive patch testing and in-use testing of two mascara
products containing 1% morpholine by 320 women between the ages of 18
and 65. All 320 volunteer subjects underwent occlusive patch testing
(using the Shelanski/Jordan repeat insult procedure). After 24 h, the
patches were removed and the sites graded. This procedure was repeated
several times for a total of 10 applications (3´ weeks). Following the
10th application, there was a 10-day to 2-week rest period, after
which the subjects were again patch tested, this time for 48 h, and
then scored. After another 10-day to 2-week rest period, the subjects
were again patch-tested for 48 h and scored. A final reading took
place 24 h after this. Of the 320 subjects patch-tested with charcoal
mascara, 314 showed no reaction, the remaining six showed varying
reactions. The researchers explained this irritation as being either
non-specific or due to the occlusive patching procedure. The same
results were found with the blue mascara. It was concluded from this
study that neither of the mascara products containing 1% morpholine
was a primary irritant, nor were they contact sensitizers (Cosmetic
Ingredient Review, 1989, 1991b).
In the in-use testing, 50 women used charcoal mascara once daily
for 4 weeks, and 50 women used blue mascara likewise. A further 100
women used once daily lash conditioner, eye make-up remover and
mascara (50 for each colour). In another group, 100 subjects used
lash conditioner and eye make-up remover but no mascara. All underwent
the above-described patch testing simultaneously.
There were a few complaints associated with the in-use testing.
The charcoal mascara caused a mild burning sensation in three
subjects, some itching in a further three, and minor irritation in
three. These findings were put down to improper use of the product,
which resulted in the product entering the eye and causing mild
irritation (Cosmetic Ingredient Review, 1989, 1991b).
Shea (1939) exposed himself to 43 g morpholine/m3 (12 000 ppm)
for 90 seconds. He experienced irritation of the nose followed by
coughing. Pipetting of pure morpholine from the stock solution led to
inhalation of vapour, a severe sore throat, and violently reddened
mucous membrane.
Shea (1939) reported that when applied to human finger tips
undiluted morpholine caused a cracking of the eponychium and
hyponychium about the nail and an intense stinging sensation. Diluted
morpholine (1 to 40) was a mild irritant.
8.1.1.1 Organoleptic effects
Hellman & Small (1974) tested the absolute and recognition
threshold odour index of 101 petrochemicals using a trained panel.
During a 20-min exposure, 0.036 mg morpholine/m3 was recognized by
50% of the panel and 0.5 mg morpholine/m3 by all of the panel
(giving an odour index of 65.9). It was described as an unpleasant
fishy smell.
The phenomenon known as blue vision, gray vision or haloes
"glaucopsia" is a well-documented effect of amines, including
morpholine and its derivatives, on the eyes of workers, particularly
in the foam plastic industry (Mastromatteo, 1965; Jones & Kipling,
1972). The vision becomes misty and haloes appear several hours after
the subjects have been exposed to these vapours at concentrations too
low to cause discomfort or disability during several hours of
exposure. The disturbances lasted for 4-6 h after leaving work. In a
minority of the workers examined, mild conjunctival infection was
observed; no corneal oedema or alteration in visual acuity was
detected by inspection or by ophthalmoscopy. The atmospheric
concentrations of morpholine and other similar compounds were not
reported.
8.1.2 Epidemiological studies
No data from epidemiological studies for morpholine have been
reported.
8.2 Occupational exposure
Studies into the levels of morpholine and NMOR in workplace air
are described in section 5.3.
Katosova et al. (1991) reported a cytogenetic analysis of the
lymphocytes in the peripheral blood of 24 workers (16 men, 8 women)
with 3 to 10 years of exposure to morpholine during production. These
were compared to a control group of the same size from the same town
having no contact with chemicals at work. The morpholine workers were
exposed to concentrations of morpholine in air of 0.54-0.93 mg/m3,
the maximum single concentration being 0.74-2.14 mg/m3. The
lymphocytes were cultivated for 56 h and all chromatid and chromosome
types as well as chromosome gaps were registered. From 2561 cells
analysed from the test group, only 2.08% had aberrations, compared to
1.61% out of 2050 cells in the control group, showing no significant
increase in the number of cells with chromosome aberrations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The data reported in this section refer to nominal
concentrations.
9.1.1 Microorganisms
9.1.1.1 Microorganisms in water
a) Bacterial and cyanobacterial cultures
In a 16-h cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 25°C), the toxicity threshold (3% reduction in
population growth) of morpholine for Pseudomonas putida was
310 mg/litre (Bringmann & Kühn, 1977a).
The effect of morpholine at neutral pH on the growth of
Pseudomonas strains in succinate mineral salt medium has been
reported by Knapp et al. (1982) and Emtiazi & Knapp (1994). Both
studies used two strains of Pseudomonas (all four were different)
which had not previously been exposed to morpholine and found that
morpholine at 8.7 g/litre had no effect on the rate or extent of
growth.
The mixed bacterial cultures studied by Mazure (1993) were able
to degrade morpholine rapidly at 5, 10 and 20 g/litre. The
degradation rate was highest at 10 g/litre but was only a little less
at 20 g/litre. At 40 g/litre degradation was markedly inhibited.
As reported in section 4.2.1, morpholine at up to 870 mg/litre
does not inhibit certain strains of Mycobacterium spp. that have
been found to biodegrade the compound.
In a cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 192 h) under continual lighting conditions using
Microcystis aeruginosa, a cyanobacterium, the onset of inhibition,
defined variously as a 1% (Bringmann, 1975) or 3% (Bringmann & Kühn,
1978) reduction in population growth, was found to occur at a
morpholine concentration of 1.7 mg/litre.
b) Activated sludge
The effect of morpholine on the respiratory, dehydrogenase and
nitrification activities of activated sludge was determined by
Strotmann et al. (1993). Short-term respiration assays (pH 7.0;
incubation time 30 min) were performed according to the OECD guideline
209 (OECD, 1984). The dehydrogenase activity was determined by
measuring the reduction of resazurin to resorufin. At concentrations
of 1000 mg morpholine/litre, both respiratory and dehydrogenase
activities were inhibited by up to 20%. A pH-dependent inhibitory
effect on the dehydrogenase activity was not found (pH 6.0-7.5).
Nitrification activity was determined using a specially adapted
culture of nitrifying bacteria. Suspended biomass, previously
inoculated with activated sludge and acclimated to a high-strength
ammonium feed, was incubated for 30 min at pH 7.2 with different
amounts of morpholine. The extent of inhibition was based on the
reduction in oxygen uptake rate, compared to a control containing no
morpholine. Nitrification activity was inhibited by 5% at a
morpholine concentration of 5%.
c) Protozoa
The toxicity thresholds (defined as a 5% reduction in population
growth) of morpholine for the protozoa Entosiphon sulcatum
(Bringmann, 1978), Uronema parduczi and Chilomonas paramaecium
(Bringmann & Kühn (1980), as measured in cell multiplication
inhibition tests under given conditions, are summarized in Table 18.
9.1.1.2 Microorganisms in soil
No data are available concerning soil bacteria or fungi.
9.1.1.3 Pathogenic microorganisms
Morpholine, like other amines, shows antibacterial and anti-
mycotic action. Kubis et al. (1983) demonstrated that 0.5% morpholine
inhibits the growth of a variety of pathogenic bacteria on agar plates
and in liquid medium. Application of 10% morpholine was shown to cure
an experimental mycosis in guinea-pigs caused by Trychophyton
mentagrophytes var. granulosum (Kubis et al., 1981). The pH was not
specified.
9.1.2 Other aquatic organisms
9.1.2.1 Monocellular green algae
Table 19 summarizes the tests carried out concerning the effect
of morpholine on algae.
In a 192-h test, the onset of inhibition by morpholine
(in distilled water, pH 7.0) of cell multiplication (here defined as
3% in population growth) in the green alga Scenedesmus quadricauda
in a turbidity test was noted at a concentration of 4.1 mg/litre
(Bringmann & Kühn, 1977a, 1978; see parallel results test with
Microcystis aeruginosa, section 9.1.1.1a).
Table 18. Toxicity threshold (TT) of morpholine for protozoa in the cell multiplication
inhibition test (growth factor: cell number)
Species Test duration pH Temperature TT Reference
(h) (°C) (mg/litre)
Entosiphon sulcatuma 72 6.9 25 12 Bringmann (1978)
Uronema parduczia 20 6.9 25 815 Bringmann & Kühn (1980)
Chilomonas paramaeciumb 48 6.9 20 18 Bringmann et al. (1980)
a bacteriovorous ciliate
b saprozoic flagellate
The results of Adams et al. (1985) showed that Selenastrum
capricornutum grows exponentially without a lag phase in the
presence as well as in the absence of morpholine, the growth being
inhibited first at a concentration of 100 mg/litre. The inhibition was
most marked after 6 days, shortly before reaching the stationary
phase; the no-observed-effect level (NOEL) was 10 mg/litre. Using the
same species, Calamari et al. (1980) evaluated algal growth by
measuring in vivo the fluorometric units at 48, 72, 96 h and 7 days.
A 96-h EC50 of 28 mg/litre was determined.
Millington et al. (1988) investigated the effects of varying
growth medium composition on the toxicity of morpholine to three
freshwater green algae: Selenastrum capricornutum, Scenedesmus
subspicatus and Chlorella vulgaris. They reported that the toxic
effect of morpholine on algae depended upon the species as well as
upon the test medium used (see Table 19).
9.1.2.2 Invertebrates
Table 20 summarizes the results of three investigations into the
toxicity of morpholine, as measured by the immobilization of Daphnia
magna (Bringmann & Kühn, 1977b, 1982; Calamari et al., 1980). The
test conditions were almost identical (pH 7.6-8.0; 18-22°C) and the
resulting EC50 values (100-119 mg/litre) were in good agreement.
Kramer et al. (1983) investigated the relative toxicity of
organic solvents including morpholine to mosquito (Aedes aegypti)
larvae (2 to 3 instar) by exposing 10-20 of them to 50 ml of test
solution in a glass container for 4 h at 22-24°C. The test was run in
triplicate. An LC50 of 1000 mg/litre was reported, together with
the observation that death was accompanied by convulsive larval
twitching.
9.1.2.3 Vertebrates
The acute toxicity of morpholine for fish has been tested on a
number of fresh, sea and brackish water species (see Table 21). The
lowest LC50 (180 mg/litre) was found for rainbow trout
(Oncorhynchus mykiss; formerly Salmo gairdneri) in very soft water
(20 mg CaCO3/litre). In hard water (320 mg CaCO3/litre), the
poisoning effect, with reference to the LC50 value, was less than
half (Calamari et al., 1980). Brachydanio rerio proved to be
relatively insensitive (LC0 value > 1000 mg/litre) (Wellens, 1982).
LC50 values for the freshwater fish Leuciscus idus melanotus and
Lepomis macrochirus were found to be 240-285 mg/litre (48 h) and
350 mg/litre (96 h), respectively (Juhnke & Lüdemann, 1978; Dawson et
al., 1977). The 96-h LC50 for the marine fish Menidia beryllina
was reported to be 400 mg/litre (Dawson et al., 1977). McCain & Peck
(1976) investigated the effect of morpholine on common Hawaiian fish.
Table 19. Toxicity of morpholine for algaea
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Scenedesmus inhibition of cell 192 neutral 27 TT: 4.1 Bringmann & Kühn (1978)
quadricauda multiplication (turbidity)
Selenastrum cell number after: 24 not given 22 NOEC: 100 Adams et al. (1985)
capricornutum 48/72 NOEC: 80
96/120 NOEC: 50
144 NOEC: 10
area under 24-72 NOEC: 80
growth curve 24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 50
growth rate 24-72 NOEC: 80
24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 80
S. capricornutum growth rate (in vivo 96 not given 24 EC0: 10c Calamari et al. (1980)d
fluorescence) EC50: 28c
EC100: 80c
S. capricornutum area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 50f
ECL0: 50g
Scenedesmus area under growth 24-120 not given 22 ECL0: 50e Millington et al. (1988)
subspicatus curve (cell number) ECL0: 5f
ECL0: 10g
Table 19 (cont'd)
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Chlorella vulgaris area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 80f
ECL0: 5g
a Adapted from BUA (1991)
b TT = toxicity threshold; NOEC = no-observed-effect concentration; EC0, EC50, EC100 = effective concentration inhibiting for the growth
of 0, 50 and 100% of the population; ECL0 = lowest-tested concentration with significant growth inhibition
(from 5, 10, 50, 80, 100 mg/litre)
c equivalent to GC-FID measured concentration ± 10%
d method according to Chiaudini & Vighi (1978)
e Bolds basal medium (BBM): rich medium; N/P-ratio 14:1
f OECD medium: less N than BBM; N/P-ratio 24:1
g EPA medium: less N than BBM; N/P-ratio 50:1
Table 20. Acute toxicity of morpholine for the water flea Daphnia magna
(test duration: 24 h; effect: immobilization)a
Test systemb pH Temperature Effect/concentration Reference
(°C) (mg/litre)
Static 7.6-7.7 20-22 EC0: 16 Bringmann &
EC50: 100 Kühn (1977b)
EC100: 500
Static 8 ± 0.2 20 EC0: 68 Bringmann &
EC50: 101 (83-122)c Kühn (1982)
EC100: 260
Static 7.9 ± 0.3 18-22 EC50: 119 (112-127)c Calamari et
al. (1980)
a adapted from BUA (1991)
b in accordance with OECD-guideline 202 part I
c 95% confidence limits
The 96-h LC50 (TLm) was between 100 and 180 mg/litre for white
mullet (Vala mugil engeli; former designation: Chelon engeli),
between 320 and 560 mg/litre for Gambusia affinis, and more than
1000 mg/litre for Tilapia sp.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Reynolds (1989) found that 4.4±0.9 mol morpholine/m3
(383 mg/litre) lowers the percentage germination of lettuce seeds
(Lactuca sativa) by 50% of the control value. The seeds were kept on
agar for 3 days in sealed plastic containers at a temperature of 30°C.
9.1.3.2 Animals
No data are available concerning the effects of morpholine on
terrestrial animals.
9.2 Field observations
No data have been reported concerning field observations
involving morpholine.
Table 21. Acute toxicity of morpholine in fish static test systemsa
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
Rainbow trout freshwater hardness: 320 mg CaCO3/litre; pH 7.4; 96 LC50 380 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (375-460)b (1980)
Rainbow trout freshwater hardness: 20 mg CaCO3/litre; pH 7.4; 96 LC50 180 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (1980)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 285 Juhnke &
(Leuciscus idus continual aeration (Juhnke laboratory) (250/340)d Lüdemann
melanotus) (1978)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 240 Juhnke &
(L. idus melanotus) continual aeration (Lüdemann laboratory) (100/301)d Lüdemann (1978)
Zebra fish freshwater hardness: 250 mg CaCO3/litre; pH 7.5; 22°C; 96 LC50> 1000 Wellens (1982)
(Brachydanio rerio) no aeration (> 1000/> 1000)d
Bluegill sunfish freshwater hardness: 55 mg CaCO3/litre; pH 7.6-7.9; 23°C; 96 LC50 350 Dawson et
(Lepomis macrochirus) intermittent aeration 24 h after test; from al. (1977)
commercial hatcheries; 14 days acclimatization
Tidewater silverside marine artificial seawater; 20°C; continual aeration; 96 LC50 400 Dawson et
(Menidia beryllina) caught wild Horseshoe Bay (New Jersey); al. (1977)
40-100 mm length; 14 days acclimatization
Mosquito fish marinee,f natural seawater (32% salinity); continual 96 LC0 320 McCain &
(Gambusia affinis) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 560 Peck (1976)
Hawaii); 38-64 mm length; 7 days
acclimatization
Table 21 (cont'd)
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
"Coloured perch" marinee,f natural seawater (32% salinity); continual 96 LC0 1000 McCain &
(Tilapia sp.) aeration; 25.5-27.8°C; caught wild (Oahu, Peck (1976)
Hawaii); 30-65 mm length; 7 days
acclimatization
White mullet marinee natural seawater (32% salinity); continual 96 LC0 100 McCain &
(Chelon engeli) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 320 Peck (1976)
Hawaii); 91-128 mm length; 7 days
acclimatization
a adapted from BUA (1991)
b 95% confidence limits
c parallel tests in two different laboratories
d LC0/LC100
e the fish were caught in rivers, presumably in the estuary area
f fresh or brackish water fish that were exposed to the marine system.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks were evaluated by an International Agency
for Research on Cancer ad hoc Expert Group in 1988. It was
concluded that there is inadequate evidence for the carcinogenicity
of morpholine in experimental animals. No data were available from
studies in humans on the carcinogenicity of morpholine. The overall
evaluation was that morpholine was not classifiable for its
carcinogenicity to humans (IARC, 1989).
IARC also evaluated N-nitrosomorpholine (NMOR). It concluded
that there is sufficient evidence for a carcinogenic effect of NMOR in
several experimental animal species. No human data, case reports or
epidemiological studies were available, nor was it possible to
identify exposed groups. In spite of the absence of epidemiological
data, NMOR should be regarded for practical purposes as if it were
carcinogenic to humans (IARC, 1978).
REFERENCES
Aarts AJ, Benson GB, Duchateau NL, & Davies KM (1990) Modern
analytical techniques for the detection of rubber chemicals and their
decomposition products in the environment. Int J Environ Anal Chem,
38: 85-95.
Ackermann P, Margot P, & Klotzsche, C (1989) Agricultural fungicides.
In: Ullmann's encyclopedia of industrial chemistry, 5th ed. Weinheim,
VCH Verlagsgesellschaft, vol A12, p 107.
Adams N, Goulding KH, & Dobbs AJ (1985) Toxicity of eight water-
soluble organic chemicals to Selenastrum capricornutum: a study of
methods for calculating toxic values using different growth
parameters. Arch Environ Contam Toxicol, 14: 333-345.
Agarwala KK (1982) Study on stability of morpholine in high pressure
boiler system. Fertil Technol, 19: 73-75.
Air Products and Chemicals (1989) Material safety data sheet.
Allentown, Pennsylvania, Air Products and Chemicals, Inc.
Aitzetmüller K & Thiele E (1982) Absence of volatile N-nitrosamines in
margarines. J Am Oil Chem Soc, 59: 387-389.
Anderson GE (1983) Human exposure to atmospheric concentrations of
selected chemicals. Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Quality Planning and
Standards, pp 455-473 (Report submitted by Systems Applications, Inc.,
San Rafael, California) (EPA No 83-265249).
Archer MC, Tannenbaum SR, & Wishnok JS (1977) Nitrosamine formation in
the presence of carbonyl compounds. In: Walker EA, Bogovski P, &
Griciute L ed. Environmental N-nitroso compounds analysis and
formation. Lyon, International Agency for Research on Cancer,
pp 141-145 (IARC Scientific Publications No. 14).
Archer MC, Yang HS, & Okun JD (1979) Acceleration of nitrosamine
formation at pH 3.5 by microorganisms. In: Walker EA, Castegnaro M,
Griciute L, & Lyle RE ed. Environmental aspects of N-nitroso
compounds. Lyon, International Agency for Research on Cancer,
pp 239-246 (IARC Scientific Publications No. 19).
Atkinson R (1988) Estimation of gas-phase hydroxyl radical rate
constants for organic chemicals. Environ Toxicol Chem, 7: 435-442.
Badura R, Linde H, & Schuster RH (1989) Analysis of volatile products
in vulcanisation fumes. In: Proceedings of the International Rubber
Conference, Prague, 8-11 May. London, International Rubber
Association, pp 19-38.
BASF (1967) [Morpholine: Toxicological data.] Ludwigshafen, BASF AG,
2 pp (Internal report) (in German).
BASF (1987) [Data sheets: Morpholine.] Ludwigshafen, BASF AG, 8 pp
(in German).
BASF (1990) [Test report: Zahn-Wellens-Test.] Ludwigshafen, BASF AG,
12 pp (Unpublished report) (in German).
BIA (Industries Association Institute for Work Safety) (1989)
[Measurement of dangerous substances - BIA data sheet.] Bielefeld,
Erich Schmidt Verlag (in German).
Bianchi A & Muccioli G (1978) [Determination of morpholine together
with isopropyl alcohol, toluene and xylenes (o-m-p) in the atmosphere
by means of gas-chromatography.] Ann Ist Super Sanita, 14: 441-445
(in Italian with English summary).
Börzsönyi M, Török G, Surján A, Challis BC, & Bär V (1981) Protective
effect of a new antioxidant on acute hepatotoxicity caused by
morpholine plus nitrite in rats. Toxicol Lett, 7: 285-288.
Bosholm J (1983) [Description of the behaviour of gaseous compounds in
water/steam systems.] Kernenergie, 26: 198-201 (in German).
Boyland E, Nice E, & Williams K (1971) The catalysis of nitrosation by
thiocyanate from saliva. Food Cosmet Toxicol, 9: 639-643.
Braun R, Schöneich J, & Ziebarth D (1977) In vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Bringmann G (1975) [Determination of the biological effect of water
pollutants on blue algae Microcystis using the cell multiplication
inhibition test.] Gesund-Ing, 96: 238-241 (in German).
Bringmann G (1978) [Determination of the biological effect of water
pollutants in protozoa I Bacteriovorous flagellate protozoa (Model
organism: Entosiphon sulcatum Stein).] Z Wasser Abwasser Forsch,
11: 210-215 (in German).
Bringmann G & Kühn R (1977a) [Toxicity thresholds of water pollutants
for bacteria (Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1977b) [Results of toxic action of water
pollutants on Daphia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1978) [Toxicity thresholds of water pollutants
for blue algae (Microcystis aeruginosa) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) [Determination of the biological effect of
water pollutants in protozoa. II. Bacteriovorous ciliates.] Z Wasser
Abwasser Forsch, 1: 26-31 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic action of water
pollutants on Daphnia magna tested by an improved standardized
procedure system.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa III Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German).
Brouwer R, Marquart H, de Mik G, & van Hemmen J (1992) Risk assessment
of dermal exposure of greenhouse workers to pesticides after re-entry.
Arch Environ Contam Toxicol, 23: 273-280
Brown AR (1966) Morpholine: its properties and uses. Manuf Chem
Aerosol News, Dec 1966: 50-52.
Brown VR & Knapp JS (1990) The effect of withdrawal of morpholine from
the influent and its reinstatement on the performance and microbial
ecology of a model activated sludge plant treating a morpholine-
containing influent. J Appl Bacteriol, 69: 43-53.
Brown VR, Knapp JS, & Heritage J (1990) Instability of the morpholine-
degradative phenotype in mycobacteria isolated from activated sludge.
J Appl Bacteriol, 69: 54-62.
Brunnemann KD & Hoffmann D (1991) Decreased concentrations of
N-nitrosodiethanolamine and N-nitrosomorpholine in commercial tobacco
products. J Agric Food Chem, 39: 207-208.
Brunnemann KD, Scott JC, & Hoffmann D (1982) N-nitroso-morpholine
and other volatile N-nitrosamines in snuff tobacco. Carcinogenesis,
3: 693-696.
BUA (Society of German Chemists, Advisory Committee on Existing
Chemicals of Environmental Relevance) (1991) [Morpholine.] Weinheim,
VCH Verlagsgesellschaft, 181 pp (BUA Report No. 56) (in German).
Calamari D, Da Gasso R, Galassi S, Provini A, & Vighi M (1980)
Biodegradation and toxicity of selected amines on aquatic organisms.
Chemosphere, 9: 753-762.
Calmels S, Ohshima H, Vincent P, Gounot A-M, & Bartsch H (1985)
Screening of micro-organisms for nitrosation catalysis at Ph 7 and
kinetics studies on nitrosamines formation from secondary amines by
E. coli strains. Carcinogenesis, 6: 911-915.
Calmels S, Ohshima H, Crespi M, Leclerc H, Cattoen C, & Bartsch H
(1987) N-nitrosoamine formation by microorganisms isolated from
human gastric juice and urine: biochemical studies on bacteria-
catalysed nitrosation. In: Bartsch H, O`Neill IK, & Schulte-Hermann R
ed. The relevance of N-nitroso compounds to human cancer: Exposures
and mechanisms. Lyon, International Agency for Research on Cancer,
pp 391-395 (IARC Scientific Publications No. 84).
Calmels S, Ohshima H, & Bartsch H (1988) Nitrosamine formation by
denitrifying and non-denitrifying bacteria: Implication of nitrite
reductase and nitrate reductase in nitrosation catalysis. J Gen
Microbiol, 134: 221-226.
Calmels S, Dalla Venezia N, & Bartsch H (1990) Isolation of an enzyme
catalysing nitrosamine formation in Pseudomonas aeruginosa and
Neisseria mucosae. Biochem Biophys Res Commun, 171(2): 655-660.
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991a) Bacterial
formation of N-nitroso compounds in the rat stomach after omeprazole-
induced achlorhydria. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 187-191 (IARC Scientific Publications No. 105).
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991b) Bacterial
formation of N-nitroso compounds in the rat stomach after
omeprazole-induced achlorhydria. Carcinogenesis, 12: 435-439.
Cech JS & Chudoba J (1988) Effect of the solids retention time on the
rate of biodegradation of organic compounds. Acta Hydrochim Hydrobiol,
16: 313-323.
Cech JS, Hartman P, Slosárek M, & Chudoba J (1988) Isolation and
identification of a morpholine-degrading bacterium. Appl Environ
Microbiol, 54: 619-621.
Challis BC & Kyrtopoulos SA (1979) The chemistry of nitroso-compounds.
Part 11. Nitrosation of amines by the two-phase interaction of amines
in solution with gaseous oxides of nitrogen. J Chem Soc Perkin Trans
I, 1979: 299-304.
Challis BC & Outram JR (1979) The chemistry of nitroso-compounds. Part
15. Formation of N-nitrosoamines in solution from gaseous nitric oxide
in the presence of iodine. J Chem Soc Perkin Trans I, 1979: 2768-2775.
Chemical Marketing Reporter (1989) Aromatics. Morpholine. Chem Mark
Rep, July 17: 13.
Chemical Marketing Reporter (1990) Dow to exit morpholine. Chem Mark
Rep, May 28: 7.
Chiaudani G & Vighi M (1978) The use of Selenastrum capricornutum
batch cultures in toxicity studies. Mitt Int Ver Limnol, 21: 316-329.
Collaert B, Attström R, De Bruyn H, & Movert R (1992a) The effect of
delmopinol rinsing on dental plaque formation and gingivitis healing.
J Clin Periodontol, 19: 274-280.
Collaert B, Edwardsson S, Attström R, Hase J, Aström M, & Movert R
(1992b) Rinsing with delmopinol 0.2% and chlorhexidine 0.2%: Short-
term effect on salivary microbiology, plaque, and gingivitis.
J Periodontol, 63: 618-625.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982a) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Presented at the 13th Annual
Meeting of the Environmental Mutagen Society, Boston, 22-28 February
1982. New York, John Wiley and Sons, pp 1-15.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982b) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Environ Mutagen, 4: 390
(Abstract).
Conaway CC, Tong C, & Williams GM (1984a) Evaluation of morpholine,
3-morpholine, and N-substituted morpholines in the rat hepatocyte
primary culture/DNA repair test. Mutat Res, 136: 153-157.
Conaway CC, Coate WB, & Voelker RW (1984b) Subchronic inhalation
toxicity of morpholine in rats. Fundam Appl Toxicol, 4: 465-472.
Cooney RV & Ross PD (1987) N-nitrosation and N-nitration of morpholine
by nitrogen dioxide in aqueous solution: effects of vanillin and
related phenols. J Agric Food Chem, 35: 789-793.
Cooney RV, Ross PD, Bartolini GL, & Ramseyer J (1987) N-nitrosoamine
and N-nitroamine formation: Factors influencing the aqueous reactions
of nitrogen dioxide with morpholine. Environ Sci Technol, 21: 77-83.
Cooney RV, Ross PD, Hatch-Pigott V, & Ramseyer J (1992) Carcinogenic
N-nitrosamine formation: A requirement for nitric oxide. J Environ Sci
Health, 27(3): 789-801.
Cosmetic Ingredient Review (1986) Scientific literature review on
morpholine. Washington, DC, The Cosmetic, Toiletry and Fragrance
Association, 71 pp.
Cosmetic Ingredient Review (1989) Final report on the safety
assessment of morpholine. J Am Colloq Toxicol, 8: 707-748.
Cosmetic Ingredient Review (1991a) CIR annual report 1991. Washington,
DC, The Cosmetic, Toiletry & Fragrance Association, 67 pp.
Cosmetic Ingredient Review (1991b) Protocol: Repeated insult patch
testing and in-use testing. Washington, DC, The Cosmetic, Toiletry &
Fragrance Association, 16 pp (Unpublished report).
Cusano F & Luciano S (1993) Contact dermatitis from pramoxine. Contact
Dermatitis, 28: 39:
Davies R, Massey RC, & McWeeny DJ (1980) The catalysis of the
N-nitrosation of secondary amines by nitrosophenols. Food Chem,
6: 115-122.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J Hazard Mater, 1: 303-318.
Dmitrenko GN & Gvozdyak P (1988) Detection of morpholine by
mycobacteria. In: Proceedings of a Conference on Microbiological
Methods for Protecting the Environment, Puschino, USSR, 5-7 April
1988. Puschino, Scientific Centre for Biological Research of the
Academy of Sciences, 141 pp.
Dmitrenko GN, Udod VM, & Gvozdyak PI (1985) Destruction of morpholine
by fixed bacteria. Khim Tekhnol Vody, 7: 71-73.
Dmitrenko GN, Gvodzdyak PI, & Udod VM (1987) Selection of destructor
microorganisms for heterocyclic xenobiotics. Khim Tekhnol Vody,
9(5): 442-445.
Dodson JJ & Bitterman ME (1989) Compound uniqueness and the
interactive role of morpholine in fish chemoreception. Biol Behav,
14: 13-27.
Donath G, Heitmann HG, Messer J, & Schott M (1977) [Determination of
the distribution coefficients of volatile alkalising agents used in
power plants between steam and water phases and their importance for
the corrosive behaviour of materials.] Vom Wasser, 49: 221-243
(in German).
Dropkin D (1985) Sampling of automobile interiors for organic
emissions. Research Triangle Park, North Carolina, US Environmental
Protection Agency, 20 pp (PB85-172567).
Dynamac Corporation (1988) Information review: Morpholine (EPA
contract No. 68-02-4251). Rockville, Maryland, Dynamac Corporation,
pp 1-52 (IR-514) (Report prepared for TSCA Interagency Testing
Committee).
ECETOC (1991) Critical evaluation of methods for the determination of
N-nitrosamines in personal care and household products. Brussels,
European Chemical Industry Ecology and Toxicology Centre (Technical
Report No. 42).
EEC (1983) Council directive 79831 - Annex V, Part C: Methods for
determination of ecotoxicity. 5.2 Degradation - Biotic degradation
Manometric Respirometry. Brussels, Commission of the European Union.
EEC (1990) Proposal for a council directive on the approximation of
the laws of the Member States relating to cosmetic products (SEC(90)
1985 final) (90/C 322/06). Off J Eur Communities, C/322: 29-43.
Edwards G, Whong W-Z, & Speciner N (1979) Intrahepatic mutagenesis
assay: a sensitive method for detecting N-nitrosomorpholine and
in vivo nitrosation of morpholine. Mutat Res, 64: 415-423.
Emtiazi G (1993) Microbial degradation of linear and cyclic amines.
Leeds, University of Leeds, Department of Microbiology (Thesis).
Emtiazi G & Knapp JS (1994) The biodegradation of piperazine and
structurally-related linear anc cyclic amines. Biodegradation,
5: 83-92.
Environment Agency, Japan (1980) Annual report on chemicals in the
environment. Tokyo, Environment Agency, pp 72-73.
Estrin NF, Haynes CR, & Whelan JM (1982) Specifications/spectra. CTFA
compendium of cosmetic ingredient composition. Washington, DC, The
Cosmetic, Toiletry and Fragrance Association, Inc.
Fadlallah S, Cooper SF, Fournier M, Drolet D, & Perrault G (1990)
Determination of N-nitroso compounds in the environment of a metal
factory using metalworking fluids. Int J Environ Anal Chem,
39: 281-288.
Fajen JM, Carson GA, Rounbehler DP, Fan TY, Vita R, Goff UE, Wolf MH,
Edwards GS, Fine DH, Reinhold V, & Biemann K (1979) N-nitrosamines in
the rubber and tire industry. Science, 205: 1262-1264.
Fan TY, Vita R, & Fine DH (1978) C-nitro compounds: a new class of
nitrosating agents. Toxicol Lett, 2: 5-10.
Fisher AA (1986) Contact Dermatitis. Philadelphia, Pennsylvania, Lea &
Febiger.
Furman MA & Rubenchik BL (1991) Formation of carcinogenic N-nitro
compounds after intraperitoneal administration of nitrosating
precursors in C57BLl/6 line mice. Eksp Onkol, 13(2): 14-22.
Gavinelli M, Fanelli R, Bonfanti M, Davoli E, & Airoldi L (1988)
Volatile nitrosamines in foods and beverages: preliminary survey of
the Italian market. Bull Environ Contam Toxicol, 40: 41-46.
Gilbert R & Saheb SE (1987) Field measurement of the distribution
coefficients of chemical additives used for corrosion control in
steam-water cycles. Mater Perform, 26: 30-36.
Gilbert R, Rioux R, & Saheb SE (1984) Ion chromatographic
determination of morpholine and cyclohexylamine in aqueous solutions
containing ammonia and hydrazine. Anal Chem, 56: 106-109.
Glatt HR & Oesch F (1981) [Ames test for morpholine.] Ludwigshafen,
BASF AG, 11 pp (Unpublished report submitted by the Pharmacological
Institute, University of Mainz, Germany).
Grant WM (1974) Morpholine. In: Toxicology of the eye, 2nd ed.
Sringfield, Illinois, Charles C. Thomas,vol 2, pp 722-723.
Greenblatt M, Mirvish S, & So BT (1971) Nitrosamine studies: induction
of lung adenomas by concurrent administration of sodium nitrite and
secondary amines in Swiss mice. J Natl Cancer Inst, 46: 1029-1034
(Abstract).
Griffiths MH (1968) The metabolism of N-triphenylmethylmorpholine in
the dog and rat. Biochem J, 108: 731-740.
Grodeckaja NS & Karamzina NM (1973) [Initial reactions by the organism
to the effects of industrial substances in concentrations of minimal
effect (Limac, Limch).] Toksikol Nov Prom Chim Veshchestv,
13: 12-23 (in Russian).
Groenen PJ, Busink E, & van Wandelen M (1987) Determination of
volatile nitrosamines in cheese and cured meat products. Model study
of a temperature- and pH-dependent artefact formation phenomenon in
alkaline medium. Z Lebensm.Unters Forsch, 185: 24-30.
Grosjean D (1991) Atmospheric chemistry of toxic contaminants.
6. Nitrosamines: Dialkyl nitrosamines and nitrosomorpholine. J Air
Waste Manage Assoc, 41: 306-311.
Hamano T, Mitsuhashi Y, & Matsuki Y (1980) Improved gas
chromatographic method for the quantitative determination of secondary
amines as sulphonamides formed by reaction with benzene-sulphonyl
chloride. J Chromatogr, 190: 462-465.
Hamano T, Mitsuhashi Y, & Matsuki Y (1981) Glass capillary gas
chromatography of secondary amines in foods with flame photometric
detection after derivatization with benzenesulfonyl chloride. Agric
Biol Chem, 45: 2237-2243.
Hansen L, Akesson B, Sollenberg J, & Lundh T (1986) Determination of
N-methylmorpholine in air samples from a polyurethane foam factory.
Scand J Work Environ Health, 12: 66-69.
Harbison RD, Marino DJ, Conaway CC, Rubin LF, & Gandy J (1989) Chronic
morpholine exposure of rats. Fundam Appl Toxicol, 12: 491-507.
Hassan SSM, Tadros FS, & Selig W (1985) Microdetermination of
secondary aliphatic amines using a copper ion-selective electrode.
Microchem J, 31: 1-6.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hazleton (1981) Final report: 9-day acute inhalation toxicity study in
rats. Vienna, Virginia, Hazleton Laboratories America, Inc., 26 pp
(Submitted to Texaco Chemical Company).
Hecht SS & Morrison JB (1984) A sensitive method for detecting
in vivo formation of N-nitrosomorpholine and its application to
rats given low doses of morpholine and sodium nitrite. Cancer Res,
7: 2873-2877.
Hecht SS & Young R (1981) Metabolic alpha-hydroxylation of
N-nitrosomorpholine and 3,3,5,5-tetradeutero-N-nitrosomorpholine in
the F344 rat. Cancer Res, 41: 5039-5043.
Heilen G, Mercker HJ, Frank D, Reck RA, & Jäckh R (1989) [Amines,
aliphatic.] In: [Ullmann's encyclopedia of industrial chemistry], 5th
ed. Weinheim, VCH Verlagsgesellschaft, vol A2, pp 1-36 (in German).
Hellman TM & Small FH (1974) Characterization of the odor properties
of 101 petrochemicals using sensory methods. J Air Pollut Control
Assoc, 24: 979-982.
Hesselink PGM, Kerkenaar A, & Witholt B (1990) Inhibition of microbial
cholesterol oxidases by dimethylmorpholines. J Steroid Biochem,
35: 107-113.
Hibbs JB Jr (1992) Immunology: Overview of cytotoxic mechanisms and
defence of the intracellular environment against microbes. In: Moncada
S, Marletta MA, Hibbs JB Jr, Higgs EA ed. The biology of nitric oxide.
London,d Chapel Hill, Portland Press, pp 201-206.
Hoffmann D, Brunnemann KD, Adams JD, Rivenson A, & Hecht SS (1982)
N-nitrosamines in tobacco carcinogenesis. In: Magee PN ed.
Nitrosamines and human cancer. Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp 211-225 (Banbury Report No. 12).
Hoffmann D, Adams JD, Lisk D, Fisenne I, & Brunnemann KD (1987) Toxic
and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst,
79: 1281-1286.
Hollett BA, Klemme JC, & Andjelkovich D (1982) Health hazard
evaluation report HETA 81-045B-1216: Uniroyal, Inc., Mishawaka,
Indiana. Cincinnati Ohio, National Institute for Occupational Safety
and Health, 20 pp (PB84-183615).
Hotchkiss JH & Vecchio AJ (1983) Analysis of direct contact paper and
paperboard food packaging for N-nitrosomorpholine and morpholine. J
Food Sci, 48: 240-242.
IARC (1978) N-nitrosomorpholine. In: Some N-nitroso compounds. Lyon,
International Agency for Research on Cancer, pp 263-280 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 17).
IARC (1989) Morpholine. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 199-213 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office, pp 282-283 (Occupational
Safety and Health Series No. 37).
Inui N, Nishi Y, Taketomi M, Mori M, Yamamoto M, Yamada T, & Tanimura
A (1979) Transplacental mutagenesis of products formed in the stomach
of golden hamsters given sodium nitrite and morpholine. Int J Cancer,
24: 365-372.
Iqbal ZM, Dahl K, & Epstein SE (1980) Role of nitrogen dioxide in the
biosynthesis of nitrosamines in mice. Science, 207(4432): 1475-1476.
Jakobi G, Löhr A, Schwuger MJ, Jung D, Fischer W, & Gloxhuber C (1983)
[Washing agents.] In: [Ullmann's encyclopedia of industrial
chemistry], 4th ed. Weinheim, VCH Verlagsgesellschaft, vol 24, pp 105,
138-139 (in German).
Japan Chemical Week (1991) Speciality chemicals handbook, 4th ed.
Tokyo, The Chemical Daily Co., Ltd, 8 pp.
Jones WT & Kipling MD (1972) Glaucopsia - blue-grey vision. Br J Ind
Med, 29: 460-461.
Juhnke I & Lüdemann D (1978) [Results of the testing of 200 selected
chemical compounds for acute fish toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Kamimura H, Enjoji Y, Sasaki H, Kawai R, Kaniwa H, Niigata K, &
Kageyama S (1987) Disposition and metabolism of indeloxazine
hydrochloride, a cerebral activator, in rats. Xenobiotica,
17(6): 645-658.
Karweik DH & Meyers CH (1979) Spectrophotometric determination of
secondary amines. Anal Chem, 51: 319-320.
Katosova LD, Fomenko VN, & Davydenko LN (1991) Air pollution and
industrial hygiene. Gig Tr Prof Zabol, 6: 35-36.
Kleemann A & Engel J (1982) [Midodrin, Minaprin, Minocyclin;
Trimethobenzamid, Trimethoprin, Trimetozin, Trimipramin,
Tripelennamin.] In: [Pharmaceutical substances.] Stuttgart, New York,
Georg Thieme Verlag, pp 602-603, 923-928 (in German).
Knapp JS & Brown VR (1988) Morpholine biodegradation. Int Biodeterior,
24: 299-306.
Knapp JS & Whytell AJ (1990) The biodegradation of morpholine in river
water and activated sludge. Environ Pollut, 68: 67-79.
Knapp JS, Callely AG, & Mainprize J (1982) The microbial degradation
of morpholine. J Appl Bacteriol, 52: 5-13.
Koga M & Akiyama T (1985) Determination of trace heterocyclic amines
in water as their 1,2,-naphthoquinone derivatives by high performance
liquid chromatography. Anal Sci, 1: 285-288.
Kramer VC, Schnell DJ, & Nickerson KW (1983) Relative toxicity of
organic solvents to Aedes aegypti larvae. J Invertebr Pathol,
42: 285-287.
Kubis A, Witek R, Baran E, Jadach W, Matecka K, & Zaba A (1981)
[ In vivo antimycotic effect of topically applied morpholine.]
Pharmazie, 36: 429-431 (in German).
Kubis A, Witek R, & Krutul H (1983) [Investigation on antibacterial
action of some amines.] Pharmazie, 38: 488-489 (in German).
Lakritz L & Kimoto W (1980) N-nitrosamines - contaminants in blood-
collection tubes. Food Cosmet Toxicol, 18: 31-34.
Lam HF & Van Stee EW (1978) A re-evaluation of the toxicity of
morpholine. Fed Proc, 37: 679 (Abstract).
Lamarre C, Gilbert R, & Gendron A (1989) Liquid chromatographic
determination of morpholine and its thermal breakdown products in
steam-water cycles at nuclear power plants. J Chromatogr,
467: 249-258.
Lamb CB & Jenkins GF (1952) B.O.D. of synthetic organic chemicals. In:
Proceedings of the 7th Industrial Waste Conference, Purdue University,
West Lafayette, Indiana, 7-9 May 1952. Ann Arbor, Michigan, Ann Arbor
Science Publishers, pp 326-339.
Lathia D & Edeler A (1989) [Influence of sulfur-containing amino acids
on in vitro nitrosamine formation under conditions similar to those
found in the stomach.] Ernährungsphysiologie, 36: 21-24 (in German).
Lathia D & Schellhöh B (1981) Inhibition of nitrosamine formation
in vitro in presence of both ascorbic acid and sorbic acid. Recent
Adv Clin Nutr, 1: 189-190.
Lauharanta J (1992) Comparative efficacy and safety of amorolfine nail
lacquer 2% versus 5% once weekly. Clin Exp Dermatol, 705: 41-43.
Leach SA, Cook AR, Challis BC, Hill MJ, & Thompson MH (1987)
Bacterially mediated N-nitrosation reactions and endogenous
formation of N-nitroso compounds. In: Bartsch H, O`Neill IK, &
Schulte-Hermann R ed. Relevance of N-nitroso compounds to human
cancer: Exposures and mechanisms. Lyon, International Agency for
Research on Cancer, pp 396-403 (IARC Scientific Publications No. 84).
Leach SA, Mackerness CW, Hill MJ, & Thompson MH (1991) Inhibition of
bacterially mediated N-nitrosation by ascorbate: Therapeutic and
mechanistic considerations. In: O`Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 571-578 (IARC Scientific Publications No. 105).
Leaf CD, Wishnok JS, & Tannenbaum SR (1991) Endogenous incorporation
of nitric oxide from L-arginine into N-nitrosomorpholine stimulated by
Escherichia coli lipopolysaccharide in the rat. Carcinogenesis,
224: 537-539.
Lee SA (1982) Health hazard evaluation report HETA 82-156-1231:
Sheller-Globe Corporation, Keokuk, Iowa. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 8 pp (PB84-172915).
Leenheers LH, Ravensberg JC, Kerstens HJ, & Jongen MJM (1992) Gas
chromatographic determination of the pesticide dodemorph for
assessment of occupational exposure. J Chromatogr Sci, 132: 228-232.
Leo A, Hansch C, & Elkins D (1971) Partition coefficients and their
uses. Chem Rev, 71: 525, 564.
Le Therizien L, Heymans F, Redeuilh C, Godfroid J-J, & Busch N (1980)
Partition coefficient additivity. 1. Morpholine and
N-(N',N'-disubstituted amino acetyl)-arylamine series. Eur J Med Chem
Chim Ther, 15: 311-316.
Lide DR ed. (1990) CRC handbook of chemistry and physics, 71st ed.
Boca Raton, Florida, CRC Press, pp 8-34.
Litton Bionetics (1979a) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp (Report submitted to Texaco Petrochemicals,
Bellaire, Texas).
Litton Bionetics (1979b) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp Report submitted to Texaco, Inc., Beacon, New
York).
Litton Bionetics (1980) Mutagenicity evaluation of morpholine in the
sister chromatid exchange assay with chinese hamster ovary (CHO)
cells. Kensingtn, Maryland, Litton Bionetics, Inc., 10 pp (Report
submitted to Texaco, Inc., Beacon, New York).
Litton Bionetics (1982) Evaluation of morpholine in the in vitro
transformation of Balb/3T3 cells with and without metabolic activation
assay 80/490. Kensington, Maryland, Liotton Bionetics, Inc. 21 pp
(Unpublished report submitted to BASF AG, Ludwigshafen).
Liu RH, Conboy JJ, & Hotchkiss JH (1988) Nitrosation by nitro-nitroso
derivatives of olefins: a potential mechanism for N-nitrosamine
formation in fried bacon. J Agric Food Chem, 36: 984-987.
Liu RH, Jacob JR, Tennant BC, & Hotchkiss JH (1992) Nitrite and
nitrosamine synthesis by hepatocytes isolated from normal woodchucks.
Cancer Res, 52: 4139-4143.
Lodén M, Larsson R, Häggqvist I, & Karlsson N (1985) [The dermal
irritancy/corrosion of 20 compounds in aqueous solutions.] Umea,
Sweden, Försvarets Forskningsanstalt, 76 pp (FOA Report No. E/40023)
(in Swedish with English summary).
Loeppky RN, Tomasik W, & Kerrick BE (1987) Nitroso transfer from
alpha-nitrosamino aldehydes: implications for carcinogenesis.
Carcinogenesis, 8(7): 941-946.
London MA & Lee S (1987) Health hazard evaluation report HETA
85-003-1834: BF Goodrich, Woodburn, Indiana. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 27 pp
(PB88-162672/XAD).
McCain JC & Peck JM Jr (1976) The toxicity of selected chemicals used
in power generating stations to Hawaiian fishes NOAA. Washington, DC,
US Department of Commerce, National Technical Information Service,
23 pp (NTIS No PB262437).
McGlothlin JD (1980) Health hazard evaluation report HETA 80-67-749:
Firestone Fire and Rubber Company, Akron, Ohio. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 18 pp
(PB82-103144).
McGlothlin J & Wilcox T (1984) Health hazard evaluation report HETA
79-109-1538: Kelly Springfield Tire Company, Cumberland, Maryland.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 50 pp (PB85-244424).
Mackerness CW, Leach SA, Thompson MH, & Hill MJ (1989) The inhibition
of bacterially mediated N-nitrosation by vitamin C: relevance to the
inhibition of endogenous N-nitrosation in the achlorhydric stomach.
Carcinogenesis, 10(2): 397-399.
Malaiyandi M, Thomas GH, & Meek ME (1979) Sampling and analysis of
some corrosion inhibiting amines in steam condensates. J Environ Sci
Health, A14: 609-627.
Maller RK & Heidelberger C (1957) Studies on OPSPA. IV. Metabolism of
OPSPA in the rat and human. Cancer Res, 17: 296-301.
Mannsville Chemical Products (1981) Chemical products synopsis:
Morpholine. Cortland, New York, Mannsville Chemical Products
Corporation, 2 pp.
Mastromatteo E (1965) Recent occupational health experiences in
Ontario. J Occup Med, 702: 502-511.
Mazure N (1993) Etude de la biodégradation de la morpholine par une
culture pure, Mycobacterium aurum, et des cultures mixtes
Pseudomonas. Compiègne, France, University of Technology (Thesis).
Meiners AF, Gadberry H, Carson BL, Owens HP, & Lapp TW (1980) Volatile
corrosion inhibitors and boiler water additives: potential for
nitrosamine formation. Washington, DC, US Environmental Protection
Agency, Office of Pesticides and Toxic Substances, 99 pp (Report
submitted by the Midwest Research Institute, Kansas City, Missouri)
(NTIS/PB80-221105).
Mercer EI (1991) Morpholine antifungals and their mode of action.
Biochem Soc Trans, 19: 788-793.
Migukina NV (1973) [Evaluation of the danger [toxicity] of morpholine
by chronic exposure.] Toksikol Nov Prom Chim Veshchestv, 13: 92-100
(in Russian).
Millington LA, Goulding KH, & Adams N (1988) The influence of growth
medium composition on the toxicity of chemicals to algae. Water Res,
22: 1593-1597.
Mills EJ & Stack VT (1953) Biological oxidation of synthetic organic
chemicals. In: Proceedings of the 8th Industrial Waste Conference,
Purdue University, West Lafayette, Indiana, 4-6 May 1953. Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp 492-517.
Mills EJ & Stack VT (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage Ind Wastes,
27: 1061-1064.
Mirvish SS (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol Appl Pharmacol,
31: 325-351.
Mirvish SS, Wallcave L, Eagen M, & Shubik P (1972) Ascorbate-nitrite
reaction: possible means of blocking the formation of carcinogenic
N-nitroso compounds. Science, 177: 65-68.
Mirvish SS, Cardesa A, Wallcave L, & Shubik P (1975) Induction of
mouse lung adenomas by amines or ureas plus nitrite and by N-nitroso
compounds: effect of ascorbate, gallic acid, thiocyanate, and
caffeine. J Natl Cancer Inst, 55: 633-636.
Mirvish SS, Pelfrene AF, Garcia H, & Shubik P (1976) Effect of sodium
ascorbate on tumor induction in rats treated with morpholine and
sodium nitrate, and with nitrosomorpholine. Cancer Lett, 2: 101-108.
Mirvish SS, Ramm MD, Sams JP, & Babcock DM (1988) Nitrosamine
formation from amines applied to the skin of mice after and before
exposure to nitrogen dioxide. Cancer Res, 48: 1095-1099.
Mjos K (1978) Cyclic amines. In: Kirk-Othmer encyclopedia of chemical
technology, 3rd ed. New York, John Wiley and Sons, vol 2, pp 295-308.
Moriyasu M, Endo M, Hashimoto Y, & Koeda T (1984) High-performance
liquid chromatographic determination of organic substances by metal
chelate derivatization. II. Microdetermination of methamphetamine and
amphetamine. Chem Pharm Bull, 32: 600-608.
Newberne PM & Shank RC (1973) Induction of liver and lung tumours in
rats by the simultaneous administration of sodium nitrite and
morpholine. Food Cosmet Toxicol, 11: 819-825.
NIOSH (1977) Manual of analytical methods, 2nd ed. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 3, pp
S150/1-S150/9.
NIOSH (1988) National occupational exposure survey as of 16.12.1988:
Morpholine. Cincinnati, Ohio, National Institute of Occupational
Safety and Health, 1 p.
Norkus EP, Boyle S, Kuenzig WA, & Mergens WJ (1984) Formation of
N-nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen oxide. Carcinogenesis, 5: 549-554.
Norkus EP, Kuenzig WA, Chau J, Mergens WJ, & Conney AH (1986)
Inhibitory effect of alpha-tocopherol on the formation of
nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen dioxide. Carcinogenesis, 7: 357-360.
NRC (1981) Selected aliphatic amines and related compounds: an
assessment of the biological and environmental effects. Washington,
DC, National Research Council, Board on Toxicology and Environmental
Health Hazards (Report prepared for the US Environmental Protection
Agency, Washington) (NTIS/PB83-133066).
OECD (1981a) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Ready biodegradability. 301 E - Modified
OECD screening test. Paris, Organisation for Economic Co-operation and
Development.
OECD (1981b) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Inherent biodegradability. 302 B -
Modified Zahn-Wellens test. Paris, Organisation for Economic
Co-operation and Development.
OECD (1984) Guidelines for testing of chemicals No 202, Part I:
Daphnia sp, acute immobilisation test. Paris, Organisation for
Economic Co-operation and Development.
O'Donnell CM, Edwards C, & Ware J (1988) Nitrosamine formation by
clinical isolates of enteric bacteria. FEMS Microbiol Lett,
51: 193-197.
Ohnishi T (1984) [Morpholine. Studies on mutagenicity of the food
additive morpholine (fatty acid salt).] Nippon Eiseigaku Zasski,
39: 729-745 (in Japanese with English summary).
Ohnishi T, Kubota M, Okada A, & Tonami K (1983) [Residual survey
investigation and removal efficiency by washing with kitchen detergent
of food additive morpholine.] Hokuriku Koshu Eisei Gakkaishi,
10: 63-67 (in Japanese with English summary).
Österdahl B-G & Slorach SA (1983) Volatile N-nitrosamines in snuff and
chewing tobacco on the Swedish market. Food Chem Toxicol, 21: 759-762.
Pensabene JW & Fiddler W (1988) Food additives. Determination of
volatile N-nitrosamines in Frankfurters containing minced fish and
surimi. J Assoc Off Anal Chem, 71: 839-843.
Perez A, Fernandez SI, Garcia-Roche MO, De Las Cagigas A, Castillo A,
Fonseca G, & Herrera M (1990) Mutagenicity of N-nitrosomorpholine
biosynthesized from morpholine in the presence of nitrate and its
inhibition by ascorbic acid. Nahrung, 34: 661-664.
Postlethwait EM & Mustafa MG (1983) Formation of N-nitrosamine in
isolated rat lungs during nitrogen dioxide ventilation.
Carcinogenesis, 4: 777-778.
Reinel D & Clarke C (1992) Comparative efficacy and safety of
amorolfine nail lacquer 5% in onychomycosis, once-weekly versus
twice-weekly. Clin Exp Dermatol, 705: 44-49.
Reinhardt CF & Brittelli MR (1981) Heterocyclic and miscellaneous
nitrogen compounds. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology: Vol 2A. Toxicology. New York, John Wiley and
Sons, pp 2671-2822.
Rekka E, Retsas S, Demopoulos VJ, & Kourounakis PN (1990)
Lipophilicity of some substituted morpholine derivatives synthesized
as potential antinociceptive agents. Arch Pharmacol, 323: 53-56.
Reynolds T (1989) Comparative effects of heterocyclic compounds on
inhibition of lettuce fruit germination. J Exp Bot, 40: 391-404.
Rhodes C & Case DE (1977) Non-metabolite residues of ICI 58,834
(viloxazine). Studies with (14C)morpholine, (14C)ethanolamine and
(14C)glyoxylate. Xenobiotica, 7: 112.
Richardson ML, Webb KS, & Gough TA (1980) The detection of some
N-nitrosamines in the water cycle. Ecotoxicol Environ Saf, 4: 207-212.
Ringenburg V & Fajen JM (1980) Survey for N-nitroso compounds at B.F.
Goodrich, Woodburn, Indiana, December 12, 1979. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 11 pp
(PB88-250477/XAD).
Rounbehler DP & Fine DH (1982) Specific detection of amines and other
nitrogen-containing compounds with a modified TEA analyzer. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 209-219 (IARC Scientific
Publications No. 41).
Rounbehler DP & Fajen JM (1983) N-nitroso compounds in the factory
environment. National Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 228 pp (PB84-145770).
Rounbehler DP, Reisch J, & Fine DH (1980) Nitrosamines in new motor-
cars. Food Cosmet Toxicol, 18: 147-151.
Sander J & Bürkle G (1969) [Induction of malignant tumours in rats by
simultaneous feeding of nitrite and secondary amines.] Z Krebsforsch,
73: 54-66 (in German).
Sander J, Schweinsberg F, & Menz H-P (1968) [Studies on the formation
of carcinogenic nitrosamines in the stomach.] Hoppe-Seyler's Z Physiol
Chem, 349: 1691-1697 (in German).
Savolainen H & Rosenberg C (1983) Morpholine vapour inhalation and
interactions of simultaneous nitrite intake. Biochemical effects on
rat spinal cord axons and skeletal muscle. Arch Toxicol, 53: 143-150.
Schröder E, Rufer C, & Schmiechen R (1982) [Nitrofuran.] In:
[Pharmaceutical Chemistry.] Stuttgart, New York, Georg Thieme Verlag,
pp 858-863 (in German).
Schuster RH, Nabholz F, & Gmünder M (1990) [The inhibition of the
formation of N-nitrosamines: Part 1. The situation and the effect of
alpha-tocopherol.] Kautschuk Gummi Kunstst, 43: 95-106 (in German).
Sen NP & Baddoo PA (1986) Origin of N-nitrosomorpholine contamination
in margarine. J Food Sci, 51: 216-217.
Sen NP & Baddoo PA (1989) An investigation on the possible presence of
morpholine and N-nitrosomorpholine in wax-coated apples. J Food Saf,
9: 183-191.
Sen NP, Seaman S, Clarkson S, Garrod F, & Lalonde P (1984) Volatile
N-nitrosamines in baby bottle rubber nipples and pacifiers. Analysis,
occurrence and migration. In: O'Neill IK, vn Borstel RC, Miller CT,
Long J, & Bartsch H ed. N-nitroso compounds: Occurrence, biological
effects and relevance to human cancer. Lyon, International Agency for
Research on Cancer, pp 51-57 (IARC Scientific Publications No. 57).
Sen NP, Kushwaha SC, Seaman SW, & Clarkson SG (1985) Nitrosamines in
baby bottle nipples and pacifiers: occurrence, migration, and effect
of infant formulas and fruit juices on in vitro formation of
nitrosamines under simulated gastric conditions. J Agric Food Chem,
33: 428-433.
Shank RC & Newberne PM (1976) Dose-response study of the
carcinogenicity of dietary sodium nitrite and morpholine in rats and
hamsters. Food Cosmet Toxicol, 14: 1-8.
Shea TE Jr (1939) The acute and sub-acute toxicity of morpholine. J
Ind Hyg Toxicol, 21: 236-245.
Shenoy NR & Choughuley ASU (1989) Effect of certain plant phenolics on
nitrosamine formation. J Agric Food Chem, 37: 721-725.
Shenoy NR & Choughuley ASU (1992) Inhibitory effect of diet related
sulphydryl compounds on the formation of carcinogenic nitrosamines.
Cancer Lett, 67: 227-232.
Shibata M-A, Kurata Y, Tamano S, Ogiso T, Fukushima S, & Ito N (1987a)
13-week subchronic toxicity study with morpholine oleic acid salt
administered to B6C3F1 mice. J Toxicol Environ Health, 22: 187-194.
Shibata M-A, Kurata Y, Ogiso T, Tamano S, Fukushima S, & Ito N (1987b)
Combined chronic toxicity and carcinogenicity studies of morpholine
oleic acid salt in B6C3F1 mice. Food Chem Toxicol, 25: 569-574.
Simon P & Lemacon C (1987) Determination of aliphatic primary and
secondary amines and polyamines in air by high-performance liquid
chromatography. Anal Chem, 59: 480-484.
Singer GM & Lijinsky W (1976a) Naturally occurring nitrosatable
compounds. I. Secondary amines in foodstuffs. J Agric Food Chem,
24: 550-553.
Singer GM & Lijinsky W (1976b) Naturally occurring nitrosatable
amines. II Secondary amines in tobacco and cigarette smoke condensate.
J Agric Food Chem, 24: 553-555.
Singer SS (1980) Transnitrosation by nitrosamines and nitrosoureas.
In: Walker EA, Griciute L, Castegnaro M, & Börzsönyi M ed. N-nitroso
compounds: Analysis, formation and occurrence. Lyon, International
Agency for Research on Cancer, pp 111-117 (IARC Scientific
Publications No. 31).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, Noyes Publications, pp 626-627.
Smith JH, Bomberger DC Jr, & Haynes DL (1980) Prediction of the
volatilization rates of high-volatility chemicals from natural water
bodies. Environ Sci Technol, 14: 1332-1337.
Smyth HF Jr, Carpenter CP, Weil CS, & Pozzani UC (1954) Range-finding
toxicity data. Arch Ind Hyg Occup Med, 10: 61-68.
Sohn OS, Fiala E, Conaway CC, & Weisburger JH (1982a) Separation of
morpholine and some of its metabolites by high-performance liquid
chromatography. J Chromatogr, 242: 374-380.
Sohn OS, Fiala ES, Conaway CC, & Weisburger JH (1982b) Metabolism and
disposition of morpholine in the rat, hamster and guinea pig. Toxicol
Appl Pharmacol, 64: 486-491.
Sollenberg J & Hansen L (1987) Isotachophoretic determination of
amines from workroom air. J Chromatogr, 390: 133-140.
Spiegelhalder B (1983) [Nitrosamines and rubber.] In: Preussmann R ed.
[The nitrosamine problem.] Weinheim, VCH Verlagsgesellschaft,
pp 235-244 (in German).
Spiegelhalder B & Preussmann R (1982) Nitrosamines and rubber. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 231-2439 (IARC Scientific
Publications No. 41).
Spiegelhalder B & Preussmann R (1984) Contamination of toiletries and
cosmetic products with volatile and nonvolatile N-nitroso carcinogens.
J Cancer Res Clin Oncol, 108: 160-163.
Spies RB, Andresen BD, & Rice DW Jr (1987) Benzthiazoles in estuarine
sediments as indicators of street runoff. Nature (Lond), 327: 697-699.
SRI (1990) Directory of chemical producers, Western Europe:
Morpholine. Menlo Park, California, SRI International, vol 2, p 1563.
Stewart BW & Farber E (1973) Strand breakage in rat liver DNA and its
repair following administration of cyclic nitrosamines. Cancer Res,
33: 3209-3215.
Strotmann UJ, Weberruss U, & Bias WR (1993) Degradation of morpholine
in several biodegradation tests and in wastewater treatment plants.
Chemosphere, 75: 1729-1742.
Subrahmanyam PVR, Khadakkar SN, Chakrabarti T, & Sundaresan BB (1983)
Wastewater-treatment of a phthalate plasticizer, ethanolamine and
morpholine manufacturing plant: a case study. Proc Ind Waste Conf,
37: 13-20.
Suzuki K & Mitsuoka T (1984) N-nitrosamine formation by intestinal
bacteria. In: O'Neill IK, vn Borstel RC, Miller CT, Long J, & Bartsch
H ed. N-nitroso compounds: Occurrence, biological effects and
relevance to human cancer. Lyon, International Agency for Research on
Cancer, pp 275-281 (IARC Scientific Publications No. 57).
Swain A, Waterhouse KV, Venables WA, Callely AG, & Lowe SE (1991)
Biochemical studies of morpholine catabolism by an environmental
mycobacterium. Appl Microbiol Biotechnol, 29: 110-114.
Swope HG & Kenna M (1950) Effect of organic compounds on biochemical
oxygen demand. Sew Ind Wastes Eng, 21: 467-468.
Taft RM & Stroman RE (1979) Health hazard evaluation report HETA
78-131-586: Goodyear Tire and Rubber Company, Niagara Falls, New York.
Cincinnati Ohio, National Institute of Occupational Safety and Health,
Hazard Evaluations and Technical Assistance Branch, 13 pp
(NTIS/PB80-196736).
Takezawa J & Lam HF (1978) Toxic effect of morpholine on rat lungs.
Fed Proc, 37: 247 (Abstract).
Tanaka M, Okada Z, Mihashi K, & Seiko Y (1968) Industrial waste
treatment by activated sludge. XV. Treatment of waste from an organic
vulcanization accelerator producing plant. Kogyo Gijutsuin Hakko
Kenkyusho Kenkyu Hokoku, 33: 19-29.
Tanaka A, Tokieda T, Nambaru S, Osawa M, & Yamaha T (1978) Excretion
and distribution of morpholine salts in rats. J Food Hyg Soc,
19: 329-334.
Tannenbaum SR, Archer MC, Wishnok JS, & Bishop WW (1978) Nitrosamine
formation in human saliva. J Natl Cancer Inst, 60(2): 251-253.
Tatsumi K, Kitamura S, Yoshimura Y, Tanaka S, Hashimoto K, & Igarashi
T (1975) The metabolism of phenyl o-(2-N-morpholinoethoxy)-phenyl
ether hydrochloride in the rabbit and rat. Xenobiotica, 5(6): 377-388.
Taylor R & Son PN (1982) Rubber chemicals. In: Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 20, pp 337-364.
Texaco (1979a) Mutagenicity evaluation of morpholine in the Ames
salmonella/microsome plate test. Bellaire, Texas, Texaco
Petrochemicals, 8 pp.
Texaco (1979b) Mutagenicity evaluation of morpholine in the mouse
lymphoma forward mutation assay. Bellaire, Texas, Texaco
Petrochemicals, 15 pp.
Texaco (1986) Texaco product brochure - Morpholine. Austin, Texas,
Texaco Chemical Company, Research and Technical Services, 29 pp.
Tölgyessy P, Kollár M, Vanco D, & Piatrik M (1986) Bio-degradability
of morpholine. J Radioanal Nucl Chem Lett, 107: 291-295.
Tombropoulos EG (1979) Micromethod for the gas chromatographic
determination of morpholine in biological tissues and fluids. J
Chromatogr, 164: 95-99.
Tombropoulos EG, Koo JO, Gibson W, & Hook GER (1983) Induction by
morpholine of lysosomal alpha-mannosidase and acid phosphatase in
rabbit alveolar macrophages in vivo and in vitro. Toxicol Appl
Pharmacol, 70: 1-6.
TRGS (1989) [Technical regulations for dangerous chemicals:
Nitrosamine.] Cologne, Carl Heymanns Verlag KG, 13 pp (TRGS 552)
(in German).
Tricker AR & Preussmann R (1991) Occurrence of and exposure to
N-nitroso compounds in tobacco. In: O'Neill IK, Chen J, & Bartsch H
ed. Relevance to human cancer of N-nitroso compounds, tobacco smoke
and mycotoxins. Lyon, International Agency for Research on Cancer,
pp 493-495 (IARC Scientific Publications No. 105).
UBA (1990) Calculation of log POW with the Program CLOGP (data
sheet). Berlin, Environment Office, 2 pp.
US FDA (1984a) Code of federal regulations (April 1, 1984) - Title 21,
Part 178.3300: Corrosion inhibitors used for steel or tinplate.
Washington, DC, US Food and Drug Administration, p 312.
US FDA (1984b) Code of federal regulations (April 1, 1984) - Title 21,
Part 176.210: Defoaming agents used in the manufacture of paper and
paperboard. Washington, DC, US Food and Drug Administration,
pp 192-193.
US FDA (1984c) Code of federal regulations (April 1, 1984) - Title 21,
Part 175.105: Substances for use only as components of adhesives.
Washington, DC, US Food and Drug Administration, pp 124-137.
US FDA (1984d) Code of federal regulations - Title 21, Part 178.310:
Animal glue. Washington, DC, US Food and Drug Administration, p 308.
US FDA (1984e) Code of federal regulations - Title 21, Part 173.310:
Boiler water additives. Washington, DC, US Food and Drug
Administration, pp 115-118.
US FDA (1986) Cosmetic product formulation data: Ingredients used in
each product category. Washington, DC, US Food and Drug
Administration.
US FDA (1988) Code of federal regulations - Title 21, Part 172.235:
Morpholine. Washington, DC, US Food and Drug Administration, pp 33-35.
Van Stee EW, Wynns PC, & Moorman MP (1981) Distribution and
disposition of morpholine in the rabbit. Toxicology, 20: 53-60.
Wang X & Suskind RR (1988) Comparative studies of the sensitization
potential of morpholine, 2-mercaptobenzothiazole and 2 of their
derivatives in guinea pigs. Contact Dermatitis, 19: 11-15.
Wang X & Tabor MW (1988) Studies of the reactivity of morpholine,
2-mercaptobenzothiazole and 2 of their derivatives with selected amino
acids. Contact Dermatitis, 19: 16-21.
Wang H & Wu Y (1991) Inhibitory effect of Chinese tea on N-nitrosation
in vitro and in vivo. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 546-548 (IARC Scientific Publications No. 105).
Wellens H (1982) [Comparison of the sensitivity of Brachydanio rerio
und Leuciscus idus by testing the fish toxicity of chemicals and
wastewaters.] Z Wasser Abwasser Forsch, 2: 49-52 (in German).
Westin JB, Castegnaro MJ-J, & Friesen MD (1987) N-nitrosamines and
nitrosatable amines, potential precursors of N-nitramines, in
children's pacifiers and baby-bottle nipples. Environ Res,
43: 126-134.
Wishnok JS & Tannenbaum SE (1976) Formation of cyanamides from
secondary amines in human saliva. Science, 191: 1179-1180.
Wishnok JS & Tannenbaum SR (1977) An unknown salivary morpholine
metabolite. Anal Chem, 49: 715a-716a, 718a.
Yurchenko VA, Ilnitskii AP, Ermilow VB, Mistakopulo GM, & Nechipai AM
(1990) [Investigation of mutagenic action of natural zeolite and
chrysotile-asbest dusts.] Eksp Onkol, 12: 24-26 (in Russian with
English summary).
Zaeva GN, Timofievskaya LA, Bazarova LA, & Migukina NV (1968)
[Comparative toxicity of a group of cyclic imino-compounds.] Toksikol
Nov Prom Chim Veshchestv, 10: 25-35 (in Russian).
Ziebarth D (1973) N-nitrosation of secondary amines and particularly
of drugs, in buffer solutions and human gastric juice. In: Bogovski P
& Walker EA ed. N-nitroso compounds in the environment. Lyon,
International Agency for Research on Cancer, pp 137-141 (IARC
Scientific Publications No. 9).
Ziebarth D. (1974) [Investigations into the nitrosation of secondary
amines in buffer solutions and in human gastric juice.] Arch
Geschwulstforsch, 43: 42-41 (in German).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Propriétés physiques et chimiques
La morpholine (1-oxa-4-azacyclohexane) est un liquide incolore,
huileux, hygroscopique et volatil qui possède l'odeur de poisson
caractéristique des amines. Elle est entièrement miscible à l'eau,
ainsi qu'à de nombreux solvants organiques, mais sa solubilité est
limitée dans les solutions aqueuses alcalines. C'est une base, dont
le pKa de l'acide conjugué est de 8,33. Il s'ensuit que son
coefficient de partage entre l'octanol et l'eau dépend du pH
(log Pow = -2,55 à pH 7 et -0.84 à pH 10 et à 35°C). La tension de
vapeur des solutions aqueuses de morpholine est très proche de celle
de l'eau.
La morpholine peut entrer en réaction de diverses manières.
Chimiquement, elle se comporte comme une amine secondaire. Dans les
conditions environnementales et physiologiques, la
N-nitrosomorpholine (NMOR), dont le pouvoir cancérogène chez
l'animal est prouvé, se forme par réaction entre des solutions de
nitrite ou des oxydes d'azote gazeux et des solutions diluées de
morpholine. La concentration en oxyde d'azote (NO) peut être
importante pour la nitrosation. Les conditions de nitrosation, en
particulier la valeur du pH, jouent un rôle important.
2. Méthodes d'analyse
On peut doser la morpholine par chromatographie en phase gazeuse
avec des colonnes à remplissage ou des colonnes capillaires, par
chromatographie liquide à haute performance (HPLC) et par
chromatographie ionique. Les détecteurs utilisés sont les détecteurs
à ionisation de flamme, les détecteurs à photométrie de flamme, les
détecteurs sélectifs d'azote, les détecteurs thermiques pour la
chromatographie en phase gazeuse et, pour la chromatographie en phase
liquide à haute performance, les détecteurs à UV et les détecteurs
thermiques. Pour le dosage des traces, il faut passer par un dérivé.
La méthode de choix sur le plan de la sensibilité semble être la
chromatographie en phase gazeuse avec détection thermique après
transformation de la morpholine en NMOR (limite de détection: 2 à
3 µg/kg dans diverses matrices). Dans l'air, de faibles
concentrations de morpholine peuvent être mesurées par chromatographie
en phase gazeuse avec un détecteur sélectif d'azote.
3. Sources d'exposition humaine et environnementale
On estime qu'environ 25 000 tonnes de morpholine sont produites
chaque année, toutefois on ne connaît pas dans le détail la production
de certains pays.
Le principal procédé de production est, semble-t-il, la réaction
du diéthylène-glycol sur l'ammoniaque en présence d'hydrogène et de
catalyseurs.
La morpholine est un produit chimique à tout faire mais on n'en
connaît pas tous les usages. Il joue un rôle important comme
intermédiaire dans l'industrie du caoutchouc, comme inhibiteur de la
corrosion ainsi que pour la synthèse d'éclaircissants optiques,
d'agents pour la protection des récoltes, de colorants et de
médicaments. La morpholine est utilisée comme solvant pour les
dérivés organiques les plus divers, comme par exemple les résines, les
colorants et les cires. On peut également l'utiliser comme
catalyseur. On utilise encore de la morpholine dans certains pays
pour la confection de produits de toilette et de cosmétiques. Dans
d'autres, elle entre dans la composition d'additifs alimentaires, soit
directement, soit indirectement.
Lorsqu'il y a exposition humaine ou environnementale, elle peut
être due soit à des émissions de gaz, soit à des décharges de
solutions aqueuses, à moins qu'elle ne se produise directement lors de
l'utilisation de certains produits contenant de la morpholine, comme
par exemple des produits cosmétiques ou des cires. Le gros des
émissions et des décharges trouve probablement son origine dans la
fabrication et l'utilisation de la morpholine dans l'industrie
chimique (notamment lors de la production et de l'utilisation des
produits destinés à l'industrie du caoutchouc) ainsi que dans
l'application de la morpholine comme agent anti-corrosion. On a
trouvé de la morpholine dans des denrées alimentaires très variées
ainsi que dans le tabac. Il est possible que cette présence
s'explique par la migration, dans la denrée alimentaire, de la
morpholine incorporée à l'enduit qui recouvre les fruits ou
l'emballage, mais dans un certain nombre de cas, on ignore d'où la
morpholine peut provenir.
4. Transport, distribution et transformation dans l'environnement
La morpholine est chimiquement stable dans la biosphère bien
qu'elle puisse être soumise à une nitrosation chimique ou biologique
qui la transforme en NMOR.
La morpholine est intrinsèquement biodégradable. On a vérifié
cette propriété dans des conditions reproduisant celles qui règnent
dans les usines de traitement des boues activées. Cependant, dans des
conditions de non adaptation, la morpholine n'est probablement pas
décomposée en proportion importante. Le temps de rétention moyen sur
les matières solides présentes dans les usines de traitement des boues
activées est d'une importance cruciale et il doit dépasser les 8 jours
pour que l'on puisse obtenir une bonne dégradation de la morpholine.
Les données relatives à la bioaccumulation de la morpholine par
les organismes aquatiques et terrestres sont insuffisantes. D'après
la valeur du coefficient de partage entre le n-octanol et l'eau
(log Pow = -2,55 à pH 7), on peut s'attendre à ce qu'il n'y ait
aucune bioaccumulation.
Comme la morpholine est un produit chimique industriel important
dont les applications sont variées, il faut s'attendre à retrouver ce
composé ou ses dérivés dans un grand nombre d'effluents industriels.
De plus, étant donné qu'on l'utilise comme inhibiteur de la corrosion
dans l'eau des chaudières, on va le retrouver dans les eaux usées de
ces chaudières, et en particulier les eaux usées provenant de
certaines centrales thermiques. Comme la morpholine entre également
dans la composition des additifs du caoutchouc, on va en retrouver
également, en quantité mal définie, dans l'hydrosphère et la
géosphère, par suite de l'usure des pneus et du rejet des pneus usés.
La morpholine peut pénétrer dans l'environnement en se
volatilisant à partir des encaustiques et des cirages dont elle est un
constituant. Elle est rapidement captée par l'humidité. C'est donc
principalement dans l'hydrosphère qu'elle devrait s'accumuler, mais
les données limitées dont on dispose incitent à penser que la
morpholine ne s'accumule pas dans ce compartiment.
La meilleure méthode pour éliminer la morpholine concentrée est
l'incinération, toutefois il peut être nécessaire de veiller aux
émissions d'oxydes d'azote afin de respecter les normes de protection
de l'environnement. En ce qui concerne les effluents aqueux, le
traitement des boues activées est suffisant, à la condition toutefois
que l'usine fasse l'objet d'un contrôle rigoureux (voir ci-dessus).
5. Concentrations dans l'environnement et exposition humaine
On ne dispose d'aucune donnée sur la concentration de la
morpholine dans l'air ambiant ainsi que dans l'air intérieur des
immeubles résidentiels ou encore dans l'eau de boisson. Si l'on
possède quelques données sur sa présence dans les eaux naturelles, on
n'en a en revanche aucune sur sa présence dans le sol.
D'après les données disponibles, c'est les denrées alimentaires
qui constituent la principale source d'exposition à la morpholine de
la population générale, les produits alimentaires pouvant être
contaminés par suite d'un traitement conservateur direct des fruits à
l'aide de cires contenant de la morpholine, lors du traitement à la
vapeur au cours de la préparation et enfin par l'utilisation de
matériaux d'emballage dans la composition desquels entre la
morpholine. Cependant on ne dispose que de données quantitatives
limitées sur la contamination des denrées alimentaires par la
morpholine et la NMOR. Par exemple, dans les produits laitiers pré-
emballés, les valeurs mesurées vont de 5 à 77 µg/kg pour la morpholine
et jusqu'à 3,3 µg/kg pour la NMOR. En général, les mesures effectuées
ont montré que la teneur en morpholine de divers échantillons de
produits alimentaires (poisson, viande, produits d'origine végétale,
boissons) ne dépassait généralement pas 1 mg/kg. Des valeurs plus
élevées (jusqu'à 71 mg/kg) ont été relevées au Japon dans des agrumes.
Une enquête effectuée en Italie n'a pas permis de repérer la présence
de NMOR dans diverses denrées alimentaires au seuil de détection de
0,3 µg/kg. Les données existantes ne permettent pas d'évaluer
l'apport de morpholine et de NMOR par la voie alimentaire.
On a trouvé de la morpholine dans le tabac de cigarette à la
concentration de 0,3 mg/kg ainsi que dans le tabac à priser et à
chiquer à des concentrations allant jusqu'à 4,0 mg/kg. Il est arrivé
que l'on trouve dans du tabac à priser des concentrations de
morpholine allant jusqu'à 0,7 mg/kg. La présence de ce produit était
probablement la conséquence de l'utilisation de cires à base de
morpholine pour le conditionnement de ce tabac.
De la NMOR a été mise en évidence dans certains produits de
toilette et dans des cosmétiques, par exemple des shampoings, du
rimmel ainsi que dans des articles en caoutchouc comme les sucettes
pour bébés et les tétines pour biberon, à des concentrations allant
jusqu'à 3,5 mg/kg.
Il peut y avoir exposition professionnelle à la morpholine dans
diverses industries. On ne possède guère de données sur l'exposition
des travailleurs à la morpholine. Toutes les valeurs signalées sont
inférieures à 3 mg/m3. On a signalé des cas d'exposition
professionnelle à la NMOR dans l'industrie du caoutchouc où des
concentrations allant jusqu'à 250 µg/m3 ont été mesurées.
Les données actuellement disponibles permettent de se faire une
idée du risque d'exposition humaine mais elles ne permettent pas une
estimation précise de l'intensité de l'exposition à la morpholine et
la NMOR de la population générale et des diverses catégories
professionnelles.
6. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après exposition par voie orale, cutanée ou respiratoire, il y a
absorption de la morpholine. Chez le rat, après administration par
voie orale ou intraveineuse, la morpholine se répartit rapidement dans
l'organisme, atteignant sa concentration la plus élevée dans
l'intestin et les muscles.
Chez le lapin, après exposition par voie intraveineuse ou
respiratoire, la morpholine se répartit de préférence dans les reins,
les concentrations étant plus faibles dans les poumons, le foie et le
sang.
La morpholine ne se fixe pas de manière importante aux protéines
plasmatiques. Sa demi-vie dans le plasma est de 115 minutes chez le
rat, de 120 minutes chez le hamster et de 300 minutes chez le cobaye.
La morpholine est principalement excrétée sans modification par
les reins chez un certain nombre d'espèces. Un jour après
l'administration, on a constaté que 70 à 90% de la morpholine se
retrouvaient dans les urines. La neutralisation en augmente la
vitesse d'excrétion. Une faible proportion de la morpholine est
excrétée dans l'air expiré et dans les matières fécales.
Les études effectuées sur des rats, des souris, des hamsters et
des lapins indiquent que la morpholine s'élimine presque complètement
sans métabolisation. Chez le cobaye, il peut y avoir une
N-méthylation suivie d'une N-oxydation, la dose administrée
pouvant être métabolisée jusqu'à hauteur de 20%. En présence de
nitrites, la morpholine peut être transformée en NMOR, tant in vitro
qu' in vivo. On a constaté, en administrant de la morpholine à des
rats avec des nitrites, que celle-ci pouvait être nitrosée dans la
proportion de 0 à 12%, selon la dose.
Le taux de nitrosation peut augmenter par le fait d'une immuno-
stimulation comportant l'activation des macrophages.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Après administration de morpholine par voie orale à des rats et à
des cobayes, on a obtenu pour la DL50 des valeurs respectives de
1-1,9 g/kg de poids corporel et 0,9 g/kg de poids corporel, ce qui
permet d'avoir une idée de la toxicité aiguë du produit. Des rats qui
avaient reçu de la morpholine neutralisée à raison de 1 g/kg de poids
corporel ont survécu. Après administration par voie intrapéritonéale,
on a obtenu une DL50 de 0,4 g/kg de poids corporel chez la souris et
de 0,1-0,4 g/kg de poids corporel chez le rat. Après exposition par
la voie respiratoire, la DL50 était d'environ 8 g/m3 chez le rat
et de 5-7 g/m3 chez la souris. La DL50 de la morpholine
concentrée par voie percutanée était de 0,5 ml/kg chez le lapin. La
toxicité aiguë de la morpholine se caractérise par des hémorragies
gastrointestinales et des diarrhées lorsqu'elle est administrée par
voie orale et par une irritation et des hémorragies nasales, buccales,
oculaires et pulmonaires lorsqu'elle est administrée par voie
respiratoire. Lors d'une étude de 30 jours au cours de laquelle des
rats ont reçu de la morpholine par gavage à des doses de 0,16 à
0,8 g/kg de poids corporel, on a observé des effets toxiques graves et
une mortalité à toutes les doses. Les mêmes observations ont été
effectuées sur des cobayes à des doses comprises entre 0,09 et
0,045 g/kg de poids corporel.
Chez des rats soumis pendant une brève période à des inhalations
de morpholine (7,2 g/m3, 4 heures par jour pendant 4 jours ou bien
1,63 g/m3, 4 heures par jour, 5 jours par semaine pendant 30 jours),
on a observé une altération de la fonction pulmonaire. Le taux de
mortalité chez d'autres rats allait de 0 à 100% selon le niveau
d'exposition (0,36-18,1 g/m3, 6 heures par jour pendant 9 jours).
Par la voie respiratoire, on a constaté que la toxicité dépendait de
la dose avec une irritation locale (yeux, bouche, nez et poumons) et
des hémorragies de gravité variables aux niveaux d'exposition les plus
élevés. Dans une étude, on a observé un hyperfonctionnement de la
glande thyroïde et dans une autre, une nécrose du foie et des tubules
rénaux après exposition par la voie respiratoire.
Une étude de 90 jours a montré que la morpholine administrée par
voie orale (0.2-0,7 g quotidiennement par kg de poids corporel)
pouvait réduire la gain de poids et entraîner une insuffisance rénale
chez la souris. Après 672 jours d'administration de morpholine par
voie orale (0,28-0,5 g/kg de poids corporel quotidiennement), on a
observé chez la souris une hyperplasie au niveau de l'épithélium de la
portion cardiaque de l'estomac.
Selon une étude d'inhalation de 13 semaines, la morpholine
(administrée à la dose de 0,09-0,9 g/m3 6 heures par jour, 5 jours
par semaine) provoque des lésions liées à la dose au niveau de la
muqueuse nasale ainsi qu'une pneumopathie aux doses les plus élevées
(0,36 et 0,9 mg/m3). A la dose de 0,09 g/m3 on n'a observé aucune
modification d'un certain nombre de paramètres qui puisse être
imputable à ce traitement; cette concentration peut être considérée
comme la dose sans effets nocifs observables dans les conditions d'une
exposition subchronique par la voie respiratoire.
Sous sa forme concentrée et non neutralisée, la morpholine est
extrêmement irritante pour les yeux et la peau, probablement en raison
de sa basicité. En la diluant et en la ramenant à un pH neutre, on
peut sensiblement en réduire la toxicité topique. Selon une variante
de la méthode de Buehler, la morpholine à 2% n'a pas produit de
sensibilisation chez le cobaye.
La morpholine ne produit pas de mutation chez des bactéries ou
des levures en présence ou en l'absence d'activation métabolique
(à l'exception d'un essai effectué à très forte concentration). Le
passage sur hôte a également donné des résultats négatifs.
On n'a pas non plus observé de réparation de l'ADN sous
l'influence de la morpholine dans des cultures primaires d'hépatocytes
de rats et ce composé n'a pas augmenté de façon sensible les échanges
entre chromatides soeurs dans des cellules ovariennes de hamsters
chinois. Les résultats d'une épreuve sur cellules lymphomateuses de
souris L5178Y ont conduit à considérer la morpholine comme faiblement
mutagène. Ce composé a entraîné une augmentation des foyers de type
III lors d'une épreuve de transformation cellulaire maligne effectuée
sur des cellules BALB/3T3, phénomène qui n'a pas été constaté avec la
morpholine neutralisée.
On n'a pas non plus constaté de mutation ponctuelle ni
d'aberration chromosomique chez des embryons de hamsters exposés
in utero à de la morpholine.
On n'a pas constaté d'augmentation dans l'incidence des tumeurs
chez des rats qui avaient été exposés par voie respiratoire pendant
104 semaines à des concentrations de morpholine allant jusqu'à
0,5 g/m3, ni chez des souris qui avaient reçu pendant 96 semaines
dans leur eau de boisson de l'oléate de morpholine à 1%. Une étude à
long terme sur un groupe de 104 rats qui recevaient dans leur
alimentation 1000 mg de morpholine par kg de nourriture, a permis de
mettre en évidence trois carcinomes hépatocellulaires, deux
angiosarcomes pulmonaires ainsi qu'un troisième angiosarcome de
localisation non précisée et enfin deux gliomes malins, alors
qu'aucune tumeur n'a été observée dans le groupe témoin comportant
156 animaux. Chez des hamsters exposés dans les mêmes conditions,
aucune tumeur n'a été observée.
Administrée en même temps qu'un nitrite, la morpholine donne un
résultat positif à l'épreuve de passage sur hôte, qui s'explique
probablement par la formation de NMOR. De la morpholine administrée
en même temps qu'un nitrite par mélange à la nourriture, a provoqué
chez des rats des tumeurs hépatiques et pulmonaires et chez des
hamsters des tumeurs hépatiques qui s'expliquent probablement par la
formation endogène de NMOR. La NMOR est mutagène pour les bactéries
et les levures; on a également observé avec ce composé des résultats
faiblement positifs dans l'épreuve d'échange de chromatides soeurs sur
des cellules CHO et lors de la recherche de mutations dans des
cultures de cellules lymphomateuses de souris L5178Y. La NMOR est
cancérogène pour la souris, le rat, le hamster et divers poissons;
elle provoque chez la souris des tumeurs hépatiques et pulmonaires,
chez le rats des tumeurs du foie, du rein et des vaisseaux sanguins,
chez le hamster des tumeurs des voies digestives et respiratoires
supérieures et enfin chez les poissons, des tumeurs hépatiques.
8. Effets sur l'homme
On ne dispose d'aucun rapport faisant état de cas d'intoxication
aiguë ou décrivant les effets d'une exposition à court ou à long terme
à la morpholine dans la population générale.
Un phénomène connu sous le nom de glaucopsie (vision bleutée)
ainsi que dans certains cas une irritation de la peau et des voies
respiratoires, ont été décrits dans des rapports d'exposition
professionnelle à la morpholine; toutefois, ces rapports ne précisent
pas la concentration de la morpholine dans l'air. On a indiqué que
chez des ouvriers exposés pendant 3 à 10 ans à de la morpholine à des
concentrations de 0,54-0,93 mg/m3, le nombre d'aberrations
chromosomiques dans les lymphocytes du sang périphérique ne présentait
pas de différence notable par rapport aux témoins.
La morpholine concentrée est fortement irritante pour la peau;
en solution (à 1/40) elle se révèle encore légèrement irritante.
On n'a pas étudié la cancérogénicité potentielle de la morpholine
chez les populations humaines exposées.
9. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Parmi les organismes aquatiques étudiés, certaines cyanobactéries
(Microcystis) et certaines algues bleues unicellulaires
(Scenedesmus) se révèlent être les taxa les plus sensibles puisque
le seuil de toxicité (critère: inhibition de la croissance des
populations) se situe à 1,7 mg/litre pour Microcystis et à
4,1 mg/litre pour Scenedesmus (durée de l'exposition: 8 jours).
Des bactéries aérobies telles que Pseudomonas se sont révélées
beaucoup plus résistantes; le seuil de toxicité à 16 heures ainsi que
la concentration sans effets observables sur la croissance des
populations atteindraient respectivement 310 et 8700 mg/litre.
Cependant une concentration de 1000 mg/litre a inhibé la respiration
et l'activité de la déshydrogénase (dans une proportion allant jusqu'à
20%) chez les bactéries de boues activées provenant d'usines de
traitement des effluents industriels.
Chez les protozoaires aquatiques étudiés jusqu'ici, des spécimens
des genres Entosiphon et Chilomonas (avec des valeursseuil
respectivement égales à 12 et 18 mg/litre pour ce qui est de
l'inhibition de la croissance des populations) se sont révélés être
les plus sensibles. Les valeurs de la CE50 à 24 heures
(E=immobilisation) pour la daphnie se situaient dans les limites de
100 à 120 mg/litre. Chez les poissons, les valeurs de la CL50 à
48 et 96 heures se sont révélées > 180 mg/litre lors d'épreuves
effectuées en eau douce, en eau saumâtre ou en eau de mer, la truite
arc-en-ciel étant l'espèce la plus sensible.
On ne dispose d'aucune donnée relative aux effets à long terme de
la morpholine sur les invertébrés et les vertébrés aquatiques.
L'absence d'information est également presque totale à propos de la
toxicité de ce composé pour les organismes terricoles, puisque les
seules données dont on dispose se limitent à la valeur de la CE à
3 jours, égale à 400 mg/litre, en ce qui concerne l'inhibition de la
germination des laitues.
10. Evaluation des risques pour la santé humaine et effets sur
l'environnement
10.1 Evaluation des effets sur la santé humaine
Les cas d'exposition de la population générale à la morpholine
résultent principalement de la consommation de denrées alimentaires
contaminées. La contamination du tabac et des produits du tabac, des
articles de toilette et des cosmétiques, de même que des articles en
caoutchouc, peuvent contribuer à l'exposition globale. Il peut y
avoir exposition professionnelle à la morpholine dans de nombreuses
industries; ce composé peut être absorbé soit par inhalation, soit
par la voie percutanée. On ne dispose pas de données suffisantes pour
déterminer le degré d'exposition de la population générale.
Les données relatives à l'exposition professionnelle sont
également limitées.
La morpholine n'est pas extrêmement toxique dans les conditions
d'une exposition aiguë. Après administration par voie orale, la
DL50 était de 1-1,9 g/kg de poids corporel chez le rat et de
0,9 g/kg de poids corporel chez le cobaye. Par ailleurs, on a trouvé,
pour la CL50, des valeurs de 7,8 mg/m3 chez le rat et de 4,9 à
6,9 g/m3 chez la souris.
Dans les conditions d'une exposition à court ou à long terme par
la voie respiratoire, c'est l'irritation des yeux et des voies
respiratoires qui constitue l'effet critique. Lors d'une étude de
13 semaines au cours de laquelle des rats ont été exposés 6 heures par
jour et 5 jours par semaine à de la morpholine, on a pu fixer à
90 mg/m3 la concentration sans effets nocifs observables. Lors
d'une autre étude d'inhalation, à long terme cette fois
(104 semaines), on a observé une augmentation de l'incidence des cas
d'inflammation de la cornée ou d'inflammation et de nécrose des fosses
nasales, à la dose de 540 mg/m3 chez le rat. L'irritation des yeux
et du nez présentait également une incidence accrue aux doses de 36 et
180 mg/m3.
Une forte exposition à la morpholine entraîne de graves lésions
hépatiques et rénales chez le rat et le cobaye. Après administration
de morpholine à des rats, par mélange à leur nourriture (0,5 g/kg de
poids corporel) tous les jours pendant 56 jours, on a observé une
dégénérescence graisseuse du foie. Des souris qui avaient reçu tous
les jours, pendant 13 semaines, de l'oléate de morpholine mêlé à leur
eau de boisson à la dose d'environ 0,7 g/kg de poids corporel, ont
présenté une dégénérescence albuminoïde au niveau des tubules
proximaux du rein (désignée également par l'expression "tuméfaction
trouble" par certains auteurs). Chez des souris femelles qui avaient
reçu pendant 672 jours de la morpholine dans leur alimentation, on a
observé aux doses comprises entre 0,05 et 0,4 g de morpholine
administrée sous forme d'oléate, une réduction du gain de poids.
Aux concentrations que l'on observe actuellement dans les cas
d'exposition professionnelle ou environnementale, il ne semble pas que
la morpholine risque véritablement d'entraîner des effets toxiques
généraux. Cependant lors d'une exposition accidentelle ou
professionnelle non contrôlée à de fortes concentrations de morpholine
présentes dans l'air, il peut y avoir des effets locaux qui prennent
la forme d'une irritation de la muqueuse oculaire et des voies
respiratoires; en outre, un contact avec de la morpholine liquide
(même diluée) peut entraîner une irritation cutanée.
Il ne semble pas que la morpholine soit mutagène ou cancérogène
chez l'animal. Toutefois elle peut facilement être nitrosée pour
donner naissance à de la NMOR qui s'est révélée, elle, mutagène et
cancérogène chez plusieurs espèces d'animaux de laboratoire.
L'administration de morpholine, puis d'un nitrite, mêlés à la
nourriture d'animaux de laboratoire a provoqué un accroissement de
l'incidence des tumeurs, pour la plupart des carcinomes
hépatocellulaires et des sarcomes du foie et du poumon. Il est donc
prudent de considérer l'exposition à la morpholine comme un facteur
supplémentaire de risque cancérogène chez les populations exposées.
10.2 Evaluation des effets sur l'environnement
Comme on sait très peu de choses sur l'exposition
environnementale et que les données relatives à l'exposition à long
terme dans l'hydrosphère ou à court et à long terme dans le milieu
terrestre font défaut, il est impossible, pour l'instant, de procéder
valablement à une appréciation du risque. On peut toutefois tirer un
certain nombre de conclusions sur la base des propriétés observées de
la morpholine, des données écotoxicologiques disponibles et des
quelques renseignements dont on dispose sur les concentrations dans
l'environnement.
La forte solubilité dans l'eau de la morpholine et sa faible
volatilité (dans les conditions du milieu) font que l'hydrosphère en
constitue le principal milieu récepteur.
La morpholine est intrinsèquement biodégradable et, bien que
cette biodégradation soit lente, rien n'indique qu'elle s'accumule
dans l'hydrosphère. En outre, sa bioaccumulation est également peu
probable.
On ne possède que relativement peu de données sur la toxicité de
la morpholine pour les organismes vagiles. Toutefois, il paraît
improbable qu'au niveau actuel des émissions de morpholine, des
dommages importants puissent être causés à l'environnement dans son
ensemble. Les effets locaux, dus par exemple aux émissions
industrielles ou à la libération de morpholine dans l'environnement
par suite de l'usure des pneumatiques, restent à évaluer.
Il peut y avoir contamination de certains produits alimentaires
comme le poisson, par de la morpholine, contamination qui viendrait de
l'environnement, mais cela n'est pas certain.
La transformation de la morpholine en NMOR est la principale
cause d'inquiétude, en particulier en ce qui concerne les populations
de vertébrés. On a signalé la présence de NMOR dans des eaux
résiduaires industrielles et dans le sol aux alentours d'une usine.
On peut s'inquiéter de la présence de morpholine dans de l'eau
destinée à être traitée pour la rendre potable.
11. Conclusions et recommandations
La morpholine ne présente aucun risque toxique pour l'homme au
niveau habituel d'exposition mais il faut prendre garde à sa
transformation en NMOR qui est cancérogène.
Rien n'indique qu'aux nivaux actuels d'exposition, la morpholine
constitue un risque important pour les biotes présents dans
l'environnement.
11.1 Recommandations en vue de la protection de la santé humaine
a) Il faut éviter dans la mesure du possible toute exposition
humaine à la morpholine.
b) Il faut éviter la contamination des denrées alimentaires par leur
emballage ou lors de leur transformation.
c) La morpholine ne doit pas entrer dans la composition d'articles
en caoutchouc destinés en entrer en contact direct avec l'homme.
d) La morpholine ne doit pas entrer dans la composition de
préparations destinées à la toilette ou à un usage cosmétique.
e) Les effluents industriels doivent être traités avec soin afin
d'éviter que la morpholine ne pénètre dans l'eau destinée à la
boisson.
f) Compte tenu de la possibilité de formation de NMOR cancérogène,
les limites d'exposition professionnelle actuelles devront être
réexaminées.
11.2 Recommandations en vue de la protection de l'environnement
Il faut éviter les déversements et les décharges massives dans
les usines de traitement des effluents.
11.3 Recommandations en vue de recherches ultérieures
Des travaux doivent être entrepris dans les domaines suivants:
a) toxicité pour la fonction de reproduction des mammifères;
b) toxicité à long terme pour les mammifères;
c) effets sur les mammifères d'une exposition à de faibles
concentrations de morpholine en présence ou non de nitrites et de
nitrates;
d) transnitrosation par la NMOR in vivo et in vitro;
e) biodégradation en anaérobiose, en particulier dans des conditions
entraînant la réduction des nitrates;
f) catalyse microbienne de la N-nitrosation en situation réelle;
g) concentrations de la morpholine dans les eaux souterraines, le
sol et les rivières dont l'eau est utilisée pour la boisson;
h) concentration de morpholine aux alentours d'usines qui produisent
ou transforment cette substance;
i) métabolisme et toxicocinétique chez l'homme en vue de la mise au
point de méthodes pour la surveillance biologique de la
morpholine;
j) surveillance de la concentration de morpholine et de NMOR dans
les denrées alimentaires, dans l'eau de boisson et l'air
intérieur aux habitations;
k) collecte et diffusion de données sur l'exposition
professionnelle.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Propiedades físicas y químicas
La morfolina (1-oxa-4-azaciclohexano) es un líquido incoloro,
oleoso, higroscópico y volátil que desprende un característico olor a
amina ("a pescado"). Es totalmente miscible en agua, así como en
numerosos disolventes orgánicos, y parcialmente soluble en soluciones
acuosas alcalinas. Se trata de una base, y el pKa del ácido conjugado
es de 8,33. En consecuencia, el coeficiente de reparto octanol/agua
depende del pH (log Pow -2,55 a pH 7, y - 0,84 a pH 10; 35°C). La
presión de vapor de las soluciones acuosas de morfolina es casi como
la del agua.
La morfolina puede sufrir diversas reacciones. Se comporta
químicamente como una amina secundaria. En condiciones ambientales y
fisiológicas, como resultado de la reacción de las soluciones de
nitrito o de óxidos de nitrógeno gaseosos con las soluciones diluidas
de morfolina, se forma N-nitrosomorfolina (NMOR), conocido
carcinógeno para los animales. Los niveles de óxido de nitrógeno (NO)
pueden ser importantes en la nitrosación. Las condiciones de
nitrosación, en particular el pH, tienen una considerable influencia.
2. Métodos analíticos
La morfolina se puede determinar mediante cromatografía de gases
(GC) en columnas empacadas o capilares, cromatografía líquida de alta
resolución (HPLC) y cromatografía iónica. Entre los detectores
utilizados cabe citar el detector de ionización por conductor, el
detector de fotometría de llama, el detector selectivo de nitrógeno
(NSD), y la espectrometría de masas y el analizador de energía térmica
(TEA) para la GC, y el detector de UV y el TEA para la HPLC. Para
determinar cantidades ínfimas hay que recurrir a la derivatización.
El método de elección en lo que respecta a sensibilidad es al parecer
la GC combinada con el TEA, previa transformación por derivatización
en NMOR (límite de detección de 2-3 µg/kg en diversas matrices). Las
bajas concentraciones de morfolina en el aire se pueden determinar
mediante GC y NSD.
3. Fuentes de exposición humana y ambiental
Se estima que cada año se producen industrialmente en todo el
mundo unas 25 000 toneladas de morfolina, pero no se conoce con
detalle la producción de algunos países.
El principal proceso de producción utilizado para su obtención es
al parecer la reacción de dietilenglicol con amoniáco en presencia de
hidrógeno y de catalizadores.
La morfolina es una sustancia química que puede utilizarse con
muy diversos fines, pero no se conocen todos sus posibles usos. Es
importante como producto intermedio en la industria del caucho, como
inhibidor de la corrosión, y en la síntesis de abrillantadores
ópticos, protectores de cultivos, colorantes y medicamentos. La
morfolina se utiliza como disolvente de una amplia variedad de
productos orgánicos, entre ellos resinas, colorantes y ceras. Se
puede utilizar como catalizador. La morfolina se usa aún en algunos
países para elaborar productos cosméticos y de tocador. En algunos
países se usa también en varias aplicaciones relacionadas directa o
indirectamente con los aditivos alimentarios.
La exposición humana y ambiental se debe a emisiones tanto
gaseosas como acuosas, y es también el resultado directo de alguno de
sus usos, por ejemplo como componente de productos cosméticos y de
ceras. Las emisiones más importantes son resultado probablemente de
su fabricación y de su uso en la industria química (sobre todo en la
producción y el uso de productos químicos derivados del caucho) y como
agente anticorrosión. Se ha detectado morfolina en muchos tipos de
alimento y de tabaco. En estos casos el origen del producto podría
ser la parafina empleada para proteger la fruta o en determinados
envases, pero a veces no se puede establecer su procedencia.
4. Transporte, distribución y transformación en el medio ambiente
La morfolina es químicamente estable en la biosfera, aunque sufre
nitrosación química y biológica, transformándose así en NMOR.
La morfolina es por naturaleza biodegradable. Así es en las
condiciones reinantes en las plantas de fangos activados que funcionan
correctamente. No obstante, en condiciones no idóneas probablemente
no se produce una degradación importante. El tiempo medio de
retención del sólido en las plantas de fangos activados tiene una
importancia crucial y debe ser superior a ocho días para que se
produzca una degradación fiable de la morfolina.
No se dispone de datos suficientes sobre la bioacumulación de
morfolina en organismos acuáticos y terrestres. Según el coeficiente
de reparto n-octanol/agua del producto (log Pow = -2,55 a pH 7),
no debería producirse bioacumulación.
Como es un importante producto químico industrial con una amplia
gama de aplicaciones, es previsible la presencia de morfolina o de sus
derivados en numerosos efluentes industriales. Usada como inhibidor
de la corrosión en el agua de calderas, aparece en los efluentes de
éstas, incluidos los de las centrales eléctricas en que se utiliza
morfolina. Su uso en la fabricación de aditivos del caucho da lugar a
la liberación de una cantidad indeterminada de morfolina a la
hidrosfera y la geosfera como consecuencia del desgaste de los
neumáticos y de la eliminación de neumáticos usados.
Componente de parafinas y abrillantadores, la morfolina se libera
al medio ambiente por volatilización. Es adsorbida rápidamente por la
humedad, y el principal compartimiento de posible acumulación de la
morfolina es, por tanto, la hidrosfera. No obstante, los datos
limitados disponibles parecen indicar que el producto no se acumula en
la hidrosfera.
La incineración es el método preferido de eliminación de la
morfolina no diluida, pero a veces es necesario controlar las
emisiones de óxido de nitrógeno para respetar las reglamentaciones en
materia de medio ambiente. Por lo que se refiere a los efluentes
acuosos, el tratamiento de fangos activados es suficiente, a condición
de que la planta sea cuidadosamente controlada (véase más arriba).
5. Niveles ambientales y exposición humana
No hay datos disponibles sobre los niveles de morfolina en el
aire ambiental y de locales cerrados y en el agua de bebida. Hay
datos limitados sobre su presencia en aguas naturales, y se carece de
información sobre su presencia en el suelo.
A tenor de los datos disponibles, la fuente principal de
exposición de la población general a la morfolina son los alimentos,
que pueden estar contaminados como consecuencia del tratamiento
directo de la fruta con parafinas contenedoras de morfolina a efectos
de conservación, de los tratamientos a base de vapor empleados durante
la elaboración de los alimentos, y del uso de material de envasado con
morfolina. No obstante, los datos cuantitativos disponibles acerca de
la contaminación de los alimentos por morfolina y NMOR son limitados.
Por ejemplo, en productos lácteos preenvasados se han hallado valores
comprendidos entre 5 y 77 µg/kg de morfolina y de hasta 3,3 µg/kg de
NMOR. La concentración de morfolina en diversas muestras de alimentos
(pescado, carne, productos vegetales, bebidas) no rebasaba por lo
general el valor de 1 mg/kg. Se han detectado niveles más altos
(hasta 71,1 mg/kg) en frutos cítricos en el Japón. En un estudio
realizado en Italia trabajando con un límite de detección de 0,3 µg/kg
no se halló NMOR en una serie de alimentos. Los datos disponibles no
permiten hacer una estimación de la ingesta de morfolina y NMOR a
través de los alimentos.
Se ha detectado morfolina en tabaco de cigarrillos a una
concentración de 0,3 mg/kg, y en tabaco en polvo y tabaco mascable a
concentraciones de hasta 4,0 mg/kg. En otras ocasiones se ha
notificado el hallazgo de nitrosomorfolina a niveles de hasta
0,7 mg/kg en el tabaco en polvo. En estos casos el producto provenía
probablemente de la parafina de los envases utilizados.
Se ha detectado NMOR en algunos productos cosméticos y de
tocador, como por ejemplo champús y maquillaje de ojos, así como en
artículos de goma, tales como chupetes y tetillas de biberón, a
niveles de hasta 3,5 mg/kg.
En varias industrias puede darse una exposición ocupacional a la
morfolina, pero se dispone de pocos datos sobre la exposición de
trabajadores al producto. Todos los valores notificados son
inferiores a 3 mg/m3. Se ha detectado la exposición ocupacional a
NMOR en la industria del caucho, donde se han hallado concentraciones
de hasta 250 µg/m3.
Los datos actualmente disponibles permiten hacerse una idea del
riesgo potencial de exposición humana, pero no estimar con exactitud
los niveles de exposición de las poblaciones general y laboral a la
morfolina y la NMOR.
6. Cinética y metabolismo en animales de laboratorio y en el hombre
La morfolina se absorbe por vía oral, por vía cutánea y por
inhalación. En la rata, tras su administración oral o intravenosa, la
morfolina se distribuye rápidamente y se concentra sobre todo en el
intestino y el músculo.
En el conejo, la morfolina administrada por vía intravenosa o por
inhalación se concentra preferentemente en los riñones, y en menor
medida en los pulmones, el hígado y la sangre.
La morfolina no se une de forma importante a las proteínas del
plasma. Se han notificado semividas plasmáticas de 115 (rata),
120 (hámster) y 300 minutos (cobayo).
La morfolina se excreta principalmente inalterada por vía renal
en diversas especies. Al cabo de un día de su administración, se
halló en la orina el 70%-90% de la morfolina. La neutralización de la
morfolina acelera su excreción. Un pequeño porcentaje se excreta a
través del aire espirado y de las heces.
Estudios realizados en la rata, el ratón, el hámster y el conejo
muestran que la morfolina se elimina casi enteramente en su forma no
metabolizada. En el cobayo, puede darse una reacción de
N-metilación seguida de N-oxidación, metabolizándose así hasta un
20% de la dosis administrada. En presencia de nitrito, la morfolina
se puede transformar en NMOR tanto in vitro como in vivo. En
función de la dosis, entre el 0% y el 12% de la morfolina administrada
a ratas junto con nitritos puede sufrir nitrosación.
La inmunoestimulación, que entraña la activación de macrófagos,
puede aumentar el grado de nitrosación.
7. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
En lo que respecta a la toxicidad aguda, la DL50 de la
morfolina administrada oralmente es de 1-1,9 g/kg de peso corporal y
0,9 g/kg de peso corporal en la rata y el cobayo, respectivamente.
Las ratas que recibieron morfolina neutralizada (1 g/kg de peso
corporal) sobrevivieron. Tras administración interperitoneal, la
DL50 fue de 0,4 g/kg de peso corporal en el ratón y de 0,1 -
0,4 g/kg de peso corporal en la rata. En los experimentos de
exposición por inhalación, la DL50 fue de aproximadamente 8 g/m3
en la rata y de entre 5 y 7 g/m3 en el ratón. Por vía cutánea la
DL50 en el conejo fue de 0,5 ml/kg de morfolina no diluida. La
intoxicación aguda por morfolina se caracteriza por la aparición de
hemorragia gastrointestinal y diarrea tras la exposición oral, y de
irritación y hemorragias en la nariz, la boca, los ojos y los pulmones
tras la inhalación. En un estudio realizado durante 30 días con ratas
a las que se administraron con sonda dosis de 0,16 - 0,8 g/kg de peso
corporal, se observaron efectos tóxicos graves y mortalidad a todas
las dosis empleadas. En el cobayo se observó también toxicidad grave
y mortalidad a todas las dosis en el margen de 0,09 a 0,45 g/kg de
peso corporal.
Se ha notificado la aparición de alteraciones de la función
pulmonar en la rata tras la exposición a morfolina por inhalación
durante cortos periodos (7,2 g/m3, 4 h/día, 4 días y 1,63 g/m3,
4 h/día, 5 días/semana, 30 días). La mortalidad en la rata osciló
entre 0% y 100% según el nivel de exposición (0,36 - 18,1 g/m3,
6 h/día, 9 días). La toxicidad por inhalación dependía de la dosis,
observándose diversos grados de irritación local (ojos, boca, nariz,
pulmones) y hemorragias a los niveles más altos de exposición. En un
estudio se detectó un aumento de la función de la glándula tiroides, y
en otro necrosis del hígado y de los túbulos renales, como resultado
de la exposición por inhalación.
Un estudio de 90 días de duración reveló que la morfolina
administrada por vía oral (0,2 - 0,7 g/kg de peso corporal al día)
durante ese espacio de tiempo puede reducir el aumento del peso
corporal y la función renal en el ratón. Se ha notificado la
aparición de hiperplasia del epitelio del estómago anterior en el
ratón como resultado de la exposición oral a morfolina
(0,28 - 0,5 g/kg de peso corporal al día) durante 672 días.
En un estudio de 13 semanas sobre los efectos de su inhalación,
la morfolina (0,09 - 0,9 g/m3, 6 h/día, 5 días/semana) causó
lesiones dosis-dependientes de la mucosa nasal y neumonía a los
niveles de exposición más altos (0,36 y 0,9 mg/m3). Un cierto
número de parámetros no variaron en respuesta al tratamiento cuando se
utilizaron 0,09 g/m3; esta concentración puede considerarse el nivel
sin efectos adversos observados (NOAEL) en las condiciones de
exposición por inhalación subcrónica.
La morfolina no diluida y no neutralizada es altamente irritante
para los ojos y la piel, probablemente a causa de sus propiedades
alcalinas. La dilución y la neutralización de su pH pueden reducir
considerablemente su toxicidad tópica. La morfolina (2%) no indujo
sensibilidad en el cobayo al aplicar el método modificado de Buehler.
La morfolina no indujo la aparición de mutaciones en bacterias o
levaduras, con o sin activación metabólica (salvo en un caso a una
concentración muy alta). Se obtuvieron resultados negativos en el
ensayo realizado por mediación de un huésped.
La morfolina no indujo reparación del ADN en hepatocitos
primarios de rata, así como tampoco un aumento importante del
intercambio de cromátides hermanas en células ováricas de hámster
chino. Se consideró que la morfolina tenía efectos ligeramente
mutagénicos en el ensayo con células L5178Y de linfoma de ratón. El
producto aumentó los focos de tipo III en el ensayo de transformación
de células malignas BALB/3T3, lo que no ocurrió con la morfolina
neutralizada.
La morfolina no causó ni mutaciones puntuales ni aberraciones
cromosómicas en embriones de hámster expuestos in utero.
No se observó ningún aumento de la incidencia de tumores en ratas
sometidas a niveles de hasta 0,5 g/m3 de morfolina por inhalación
durante 104 semanas, así como tampoco en ratones que ingirieron oleato
de morfolina al 1% con el agua de bebida durante 96 semanas. En un
estudio de larga duración realizado con un grupo de 104 ratas que
recibieron 1000 mg de morfolina/kg dieta, se observaron tres casos de
carcinoma de células hepáticas, dos de pulmón y uno de angiosarcoma
(no especificado), así como dos gliomas malignos, mientras que en un
grupo testigo de 156 ratas no se observaron tumores. No se detectaron
tumores en hámsters sometidos a idénticas condiciones.
La morfolina administrada al mismo tiempo que nitrito da lugar a
resultados positivos en el ensayo realizado por mediación de un
huésped, probablemente debido a la formación de NMOR. La ingestión
simultánea de morfolina y nitrito indujo la aparición de tumores
hepáticos y pulmonares en la rata y de tumores hepáticos en el
hámster, probablemente a causa de la formación endógena de NMOR. La
NMOR es mutágena en bacterias y levaduras; se notificaron resultados
ligeramente positivos en lo que respecta al intercambio de cromátides
hermanas en células CHO, así como a la aparición de mutaciones en
células L5178Y de linfoma de ratón. La NMOR es carcinógena en el
ratón, la rata, el hámster y diversos peces, y produce tumores de
hígado y de pulmón en el ratón; de hígado, riñón y vasos sanguíneos en
la rata; de hígado y de las vías digestivas y respiratorias superiores
en el hámster, y de hígado en peces.
8. Efectos en el hombre
No se han descrito casos de intoxicación aguda o de efectos a
corto o largo plazo de la exposición a morfolina en la población
general.
En informes sobre la exposición ocupacional a la morfolina se ha
descrito el fenómeno conocido como visión azul o glaucopsia, así como
algunos casos de irritación de la piel y del tracto respiratorio, pero
sin hacer mención de las concentraciones atmosféricas de morfolina.
Se señaló que el número de aberraciones cromosómicas en los linfocitos
de sangre periférica de trabajadores expuestos durante 3 a 10 años a
concentraciones de morfolina de 0,54 - 0,93 mg/m3 no diferían
significativamente del número hallado en los testigos.
La morfolina no diluida es muy irritante para la piel; una
solución diluida (1/40) tuvo efectos moderadamente irritantes.
No se ha investigado el potencial carcinógeno de la morfolina en
poblaciones humanas expuestas.
9. Efectos sobre otros organismos en el laboratorio y en el terreno
Entre los microorganismos acuáticos estudiados, determinadas
cianobacterias (Microcystis) y algas verdes unicelulares
(Scenedesmus) son al parecer las especies más sensibles según se
desprende de los valores de la toxicidad liminal (empleando como
criterio la inhibición del crecimiento de la población) notificados
(duración de la exposición: 8 días): 1,7 mg/litro para Microcystis,
y 4,1 mg/litro para Scenedesmus.
Bacterias aerobias tales como Pseudomonas resultaron ser mucho
más resistentes: la toxicidad liminal a las 16 horas y la NOEC para
el crecimiento de la población se han cifrado, respectivamente, en
310 y 8700 mg/litro. No obstante, una concentración de 1000 mg/litro
inhibía la respiración y la actividad deshidrogenasa (hasta un 20%) en
fangos activados de plantas de tratamiento industrial.
Entre los protozoos acuáticos analizados hasta ahora, la mayor
sensibilidad corresponde a ejemplares de los géneros Entosiphon y
Chilomonas (con toxicidades liminales de 12 y 18 mg/litro,
respectivamente, para la inhibición del crecimiento de la población).
En Daphnia, la CE a las 24 horas (E = inmovilización) se ha
establecido en valores comprendidos entre 100 y 120 mg/litro. Los
valores notificados para la CL50 a las 48 - 96 horas en peces
estudiados en agua dulce, salobre o marina fueron > 180 mg/litro, y
la especial más sensible en este sentido es la trucha arco iris.
No se dispone de datos sobre los efectos a largo plazo en
invertebrados y vertebrados acuáticos. La información sobre la
toxicidad de la morfolina en organismos silvestres del suelo es casi
inexistente, pues se limita a una CE a los 3 días de aproximadamente
400 mg/litro para la inhibición de la germinación en la lechuga.
10. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
10.1 Evaluación de los efectos en la salud humana
La principal vía de exposición de la población general a la
morfolina es el consumo de alimentos contaminados. También puede
contribuir a la exposición general la contaminación del tabaco y de
los productos derivados del tabaco, así como de los artículos
cosméticos y de tocador y de los productos de goma. En numerosas
industrias se da una exposición ocupacional a la morfolina; el
compuesto es absorbido por inhalación y por vía cutánea. No hay datos
suficientes para determinar el grado de exposición de la población
general. Los datos disponibles sobre la exposición ocupacional al
producto también son limitados.
La morfolina no es altamente tóxica en condiciones de exposición
aguda. La DL50 tras administración oral es de 1 a 1,9 g/kg de peso
corporal en la rata y de 0,9 g/kg de peso corporal en el cobayo. Se
han notificado CL50 de 7,8 mg/m3 (rata) y 4,9 - 6,9 g/m3
(ratón).
En las condiciones de exposición por inhalación de corta y larga
duración, los efectos críticos son al parecer la irritación de los
ojos y las vías respiratorias. Puede considerarse que el NOAEL
corresponde a una concentración de 90 mg/m3 en las condiciones en
que se llevó a cabo el experimento de 13 semanas en la rata (6 h/día,
5 días/semana). En un estudio de larga duración (104 semanas) sobre
los efectos de la inhalación del producto se observó una mayor
incidencia de inflamación de la córnea y de inflamación y necrosis de
la cavidad nasal en ratas sometidas a 540 mg/m3. A concentraciones
de 36 y 180 mg/m3 se observó también una mayor incidencia de
irritación de los ojos y de la cavidad nasal.
Las exposiciones altas a la morfolina causan lesiones graves del
hígado y los riñones en la rata y el cobayo. Se ha notificado la
aparición de degeneración grasa del hígado en la rata como resultado
de la ingestión diaria de morfolina (0,5 g/kg de peso corporal)
durante 56 días. En el ratón la administración de una sal de
morfolina y ácido oleico en el agua de bebida a una dosis de
aproximadamente 0,7 g/kg de peso corporal al día durante 13 semanas
dio lugar a una hinchazón turbia de los túbulos proximales renales.
Se observó una disminución del aumento del peso corporal en los
ratones hembra del experimento de administración prolongada (672 días)
de una dieta con dosis comprendidas entre 0,05 y 0,4 g de morfolina
(en forma de sal del ácido oleico).
A los niveles que según se ha notificado alcanza actualmente la
exposición ocupacional y ambiental, no parece que la morfolina entrañe
ningún riesgo importante de efectos tóxicos sistémicos. Pueden
aparecer efectos locales (irritación) en los ojos y las vías
respiratorias en las exposiciones ocupacionales y accidentales no
controladas a altas concentraciones de morfolina transmitida por el
aire, y el contacto con morfolina líquida (incluso diluida) puede
causar irritación cutánea.
La morfolina no parece tener efectos mutágenos o carcinógenos en
los animales. No obstante, fácilmente puede nitrosarse y convertirse
en NMOR producto mutágeno y carcinógeno en varias especies de animales
de experimentación. En la rata, la morfolina ingerida secuencialmente
con nitrito dio lugar a un aumento de los tumores, sobre todo de
carcinoma hepatocelular y de sarcomas del hígado y los pulmones. Así
pues, por prudencia, conviene considerar que la exposición a la
morfolina aumenta el riesgo de carcinogénesis en las poblaciones
expuestas.
10.2 Evaluación de los efectos en el medio ambiente
Habida cuenta de los muy limitados conocimientos respecto a la
exposición ambiental, así como de la carencia de datos sobre los
efectos de la exposición de larga duración en el agua y la exposición
de corta y larga duración en el medio terrestre, por el momento no es
posible hacer una evaluación rigurosa de los riesgos. Sobre la base
de las propiedades conocidas de la morfolina, de la información
ecotoxicológica disponible y de los escasos datos sobre su
concentración en el medio ambiente, es posible extraer algunas
conclusiones.
Debido a la alta hidrosolubilidad de la morfolina y a su baja
volatilidad (en condiciones ambientales), su principal sumidero
ambiental es la hidrosfera.
La morfolina es por naturaleza biodegradable y, aunque la
degradación es lenta, no hay datos que lleven a pensar que se acumula
en la hidrosfera. Su bioacumulación es improbable.
Hay relativamente pocos datos sobre la toxicidad de la morfolina
en organismos silvestres. No obstante, parece improbable que los
niveles actuales de emisión de morfolina puedan causar daños
importantes en un radio de acción amplio. Restan por evaluar los
efectos locales, como por ejemplo los debidos a emisiones industriales
o a la morfolina liberada por el desgaste de los neumáticos.
La contaminación de algunos alimentos, como el pescado, por
morfolina puede deberse a contaminación ambiental, pero ello no es
seguro.
La conversión de la morfolina en NMOR es la principal causa de
inquietud, sobre todo en relación con las poblaciones de vertebrados.
Se ha notificado la presencia de NMOR en aguas residuales industriales
y en el suelo próximo a una fábrica. La presencia de morfolina en
agua destinada a transformarse por elaboración en agua de bebida
suscita preocupación.
11. Conclusiones y recomendaciones
La morfolina no entraña riesgos de toxicidad para el hombre a los
niveles habituales de exposición, pero hay que tener en cuenta que
puede transformarse en el carcinógeno NMOR.
No hay ningún indicio de que a los niveles actuales de exposición
la morfolina entrañe un riesgo sustancial para la biota en el medio
ambiente.
11.1 Recomendaciones para la protección de la salud humana
a) En la medida de lo posible debe evitarse la exposición humana a
la morfolina.
b) Debe evitarse que los alimentos se contaminen durante su envasado
y elaboración.
c) Se evitará que contengan morfolina los productos de goma con los
que el hombre haya de tener contacto directo.
d) No debe emplearse morfolina en la preparación de productos
cosméticos o de tocador.
e) Los efluentes industriales deben ser objeto de un riguroso
tratamiento para evitar la contaminación por morfolina del agua
de bebida.
f) Habida cuenta de la formación del carcinógeno NMOR es necesario
replantearse los actuales límites de exposición ocupacional.
11.2 Recomendaciones para la protección del medio ambiente
Debe evitarse que las plantas de tratamiento de efluentes sufran
derrames y sobrecargas.
11.3 Recomendaciones para ulteriores investigaciones
Deben emprenderse estudios sobre los siguientes temas:
a) toxicidad reproductiva en mamíferos;
b) toxicidad a largo plazo en mamíferos;
c) efecto de la exposición de mamíferos a niveles bajos de
morfolina, con y sin nitrito y nitrato;
d) transnitrosación por NMOR in vivo e in vitro;
e) biodegradación en condiciones anaerobias, sobre todo en
condiciones favorecedoras de la reducción de nitratos;
f) catálisis microbiana de la N-nitrosación en condiciones
realistas;
g) niveles ambientales de morfolina en las aguas subterráneas, el
suelo y los ríos a partir de los cuales se obtenga agua de
bebida;
h) niveles ambientales de morfolina en torno a las fábricas
productoras y procesadoras de morfolina;
i) metabolismo y toxicocinética en el hombre, como parte del
desarrollo de métodos para la vigilancia biológica de la
morfolina;
j) vigilancia de los niveles de morfolina y de NMOR en los
alimentos, el agua de bebida y el aire de locales cerrados;
k) se deben reunir y poner a disposición datos sobre la exposición
ocupacional.
The rate of reaction of the nitrosation of morpholine by nitrite
is greatest at a pH value of 3.4, where the rate constant is
0.42 mol-2.s-1. An increase in the pH value has been shown to
result in a decrease in the rate of nitrosation with nitrite (Mirvish,
1975; Archer et al., 1977), and the rate was almost zero at pH > 7
(Archer et al., 1977).
In contrast, nitrosation with gaseous nitrogen oxides (N2O3,
N2O4, NOx) can take place over the whole pH range (Challis &
Kyrtopoulos, 1979; Meiners et al., 1980). Cooney et al. (1987) found
that, under certain conditions, the yield of NMOR at pH 7 was ten
times higher than at pH 2, but there was no further increase beyond
this pH.
Some nitrosamines, particularly alpha-nitrosamine aldehydes, are
potent transnitrosation reagents and are capable of nitrosating
morpholine at pH 7.9 (Loeppky et al., 1987).
Numerous reaction accelerators are known, e.g., thiocyanate
(Boyland et al., 1971), halides (Mirvish, 1975), formaldehyde (Archer
et al., 1977) and nitrosophenols, e.g., p-nitroso- o-cresol (Davies
et al., 1980). Enhancement of the nitrosation of morpholine by
nitrogen dioxide was reported in the presence of iodine (Challis &
Outram, 1979), vanillin and related phenols (Cooney & Ross, 1987) and
halides, particularly bromide (Cooney et al., 1987).
In contrast, the following compounds have been reported to
inhibit the nitrosation of morpholine almost completely: ascorbic acid
(Lathia & Schellhöh, 1981; Leach et al., 1991); urea or ammonium
sulfamate (Mirvish et al., 1972); gallic acid and sulfite (Mirvish,
1975); L-cysteine and DL-methionine ( in vitro study under
physiological conditions, Lathia & Edeler, 1989), catechol and
4-hydroxychavicol (Shenoy & Choughuley, 1989); alpha-tocopherol
(Norkus et al., 1986; Cooney et al., 1987; Schuster et al., 1990);
sulfhydryl compounds such as cysteine, cysteamine, glutathione and
thioglycolic acid, as well as extracts of onion and garlic juice
(Shenoy & Choughuley, 1992). Vitamin C, glucose, mannitol, cabbage
juice, orange juice, shiitake mushroom extract and saliva inhibited
the nitrosation of morpholine in vitro, but catechin, epicatechin
and tea extract enhanced the same reaction (Ohnishi, 1984). The
inhibitory effect of Chinese tea on the formation of NMOR in vitro
and in vivo has also been described (Wang & Wu, 1991).
Several C-nitro compounds, in particular tetranitromethane, have
been demonstrated to transnitrosate morpholine to form NMOR (Fan et
al., 1978). C-nitro compounds are widely used in industry as
pesticides, bactericides, colouring agents, drugs and perfumes.
Singer (1980) described the transnitrosation of morpholine with
nitrosamines and nitrosureas under acid conditions in the presence of
thiocyanate. These reactions are dependent on the pH value and steric
and electronic factors, as well as on the basicity of the amines. In a
model study, the nitrosation of morpholine by nitro-nitroso compounds,
such as those found in fried bacon, was observed (Liu et al., 1988).
NMOR can be formed in vivo in humans and has been found in
various tissues and fluids such as human saliva (Boyland et al., 1971;
Wishnok & Tannenbaum, 1977) and human gastric juice (Ziebarth, 1973;
1974; Sen & Baddoo, 1989; Yurchenko et al., 1990). NMOR formation has
been reported in rat lungs (Postlethwait & Mustafa, 1983), whole mice
(Iqbal et al. 1980; Norkus et al. 1984), stomach (Furman & Rubenchik,
1991), hepatocytes isolated from woodchucks (Marmota monax) (Liu et
al., 1992) and microorganisms (Archer et al., 1979; O'Donnell et al.,
1988; Calmels et al., 1991a,b). Bacterial catalysis of
N-nitrosation of morpholine has been reported in a range of bacteria
often isolated from the human gut or urinary tract infections (Suzuki
& Mitsuoka, 1984; Calmels et al., 1987, 1988; Mackerness et al.,
1989), including the ubiquitous gut bacterium Escherichia coli and
Pseudomonas aeruginosa, which is also widespread in the aquatic
environment. Bacterial catalysis of N-nitrosation of morpholine is
heat labile and is optimal at neutral to slightly alkaline pH (Calmels
et al., 1985; Leach et al., 1987). N-nitrosation by bacteria is
generally associated with the ability to reduce nitrate. It appears
that those that reduce nitrate to nitrogen or nitrogen oxides (e.g.,
P. aeruginosa) can nitrosate at much greater rates than those (e.g.,
E. coli) that only reduce nitrate to nitrite (Leach et al.,1987;
Calmels et al., 1988). There is considerable variation between
strains of the same species. Bacterial N-nitrosation of morpholine
has been shown to follow Michaelis-Menten kinetics (Calmels et al.,
1985; Leach et al., 1987). E. coli A10, for example, displays Km
values of 7.4 mmol/litre for morpholine and 11.4 mmol/litre for sodium
nitrite. It has been shown that the rate of bacterial N-nitrosation
of secondary amines is inversely related to the pKa of the amine
(Calmels et al., 1985; Leach et al., 1987, 1991), with a linear
relationship between log10 of the rate of nitrosation and pKa.
Morpholine, having a relatively low pKa, is thus relatively
susceptible to nitrosation compared, for example, to alkyl amines.
It has been shown that ascorbate is capable of inhibiting
nitrosation of morpholine by P. aeruginosa (Leach et al., 1991).
Although most nitrosation studies have used whole bacteria, an enzyme
catalyzing N-nitrosation of morpholine has been isolated and
purified from two denitrifying bacteria (Calmels at al., 1990).
4.4 Ultimate fate following use
4.4.1 Fate of morpholine in various products
Morpholine is an important industrial chemical with a wide range
of applications (see section 3.2.2) and therefore may be present in
many industrial emissions.
Its use as a corrosion inhibitor in boiler water means that
morpholine and its decomposition products will be found in boiler
wastewater, including water from power plants using morpholine. In a
study by McCain & Peck (1976), morpholine concentrations in the
discharge streams of three Hawaiian power plants ranged from not
detectable to 0.008 mg/litre, suggesting that the potential for human
exposure is small.
Its use in the manufacture of rubber additives results in an
indefinable amount of morpholine being released into the hydrosphere
or geosphere not only during manufacturing processes but also through
tyre abrasion and disposal of used tyres.
Morpholine is released during vulcanization processes using
morpholine-containing accelerators such as 2-( N-morpholino-
thio)benzothiazole (MBS) (Badura et al., 1989). Some of the amine is
released into the atmosphere and some is bound to the rubber. Even
the accelerator itself can contain free amine. The morpholine content
of MBS is < 0.4% by weight. This level can be higher if the
accelerator is not stored properly and is exposed to heat or moisture
(BUA, 1991).
Aarts et al. (1990) detected free volatile morpholine at
concentrations of between 70 (new) and 230 mg/kg (old) in samples of
dithio-bis-morpholine (DTBM). After extraction in water for one hour
in an ultrasonic bath, ten times this amount was detected, i.e.
960 mg/kg in newly made and 2750 mg/kg in stored DTBM. These
quantities of amine could be released during vulcanization.
Optical brighteners adhere to clothes during the first wash but
tend to be released into the wastewater in subsequent washings.
Although these substances are not themselves biologically degradable,
they have been found to disappear from wastewater after a two-step
biological treatment presumably due to the high rate of adsorption to
the sludge particles (Jakobi et al., 1983).
As mentioned in section 3.2.2, morpholine is released into the
environment by volatilization through its use in waxes and polishes
(Texaco, 1986).
4.4.2 Waste disposal
Controlled incineration is the preferred method of disposal
(Sittig, 1985; Air Products and Chemicals, 1989). The incinerator
should be equipped with a scrubber or thermal unit. Nitrogen oxide
emissions should meet environmental regulations.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
No data are available on levels of morpholine in ambient air.
5.1.2 Water
5.1.2.1 River water
Since mid-1990, the levels of morpholine in some rivers in North
Rhine-Westphalia, Germany, have been monitored (BUA, 1991). No
morpholine could be detected at three different points in the River
Rhine or in five of its tributaries (detection limit, 5 µg/litre). No
morpholine was found in samples of Tennessee freshwater (detection
limit, 0.07 µg/litre) (Singer & Lijinsky, 1976a). In 1979, 33 water
samples were collected at 11 sites in Japan, but no morpholine could
be detected (detection limit, 1-5 µg/litre) in any of the samples
(Environment Agency Japan, 1980).
5.1.2.2 Wastewater
There are no data on morpholine levels in wastewater.
A single sample of wastewater from a tyre chemical factory in
Ohio, USA was found to contain 3 µg NMOR/litre (Fajen et al., 1979).
In England, samples were taken from the inlets and outlets of
four sewage treatment plants (Richardson et al., 1980). NMOR
(100 µg/litre) was found only in the outlet of a cutting-fluid
recovery plant.
5.1.3 Sediment
Spies et al. (1987) examined contaminated sediments in San
Francisco Bay, USA and found several benzothiazoles, including
2-(4-morpholinyl)-benzothiazole, which is present as an impurity in
commercial 2-(morpholinothio)-benzothiazole used in motor tyres. The
authors carried out weathering tests on this latter commercial
substance and found that the morpholine impurity was environmentally
stable. They suggested that the 2-(4-morpholinyl)-benzothiazole found
in the sediments (up to 0.36 mg/kg dry weight) was a result of
accumulated street run-off. Morpholine itself could not be detected.
In 1979, 33 bottom sediment samples were collected at 11 sites in
Japan, but no morpholine could be detected (detection limit,
0.01-0.5 mg/kg) in any of the samples (Environment Agency Japan,
1980).
5.1.4 Soil
There are no data on the presence of morpholine in soil.
2-(4-Morpholinyl)-benzothiaozole (273 µg/kg dry weight) was detected
1.6 km from a motorway in California, USA (Spies et al., 1987) (see
also section 5.1.2).
NMOR (4.4 mg/kg) was detected in a single sample of soil near to
a tyre chemical factory in Ohio, USA (Fajen et al., 1979).
5.1.5 Terrestrial and aquatic organisms
Levels of morpholine found in single or small samples of fish are
given in Table 6, but the sample numbers are too low to make an
evaluation. No other data are available.
5.2 General population exposure
5.2.1 Indoor air
No data on indoor air exposure to morpholine are available.
Analysis for NMOR in the air inside new cars showed levels of up
to 2.5 µg/m3. Levels were 4 to 10 times lower when the air-venting
system was working, indicating that NMOR exposure is limited to the
first few minutes of each trip (Rounbehler et al., 1980). During a
simulation of conditions inside cars on a hot day, concentrations of
up to 0.4 µg NMOR/m3 were measured at 60°C (Dropkin, 1985).
5.2.2 Drinking-water and food
There are no data on the morpholine content of drinking-water.
Food can become contaminated with morpholine in several ways:
(a) through direct treatment of fruit with waxes containing morpholine
for conservation purposes; (b) by use of packaging material
containing morpholine, and (c) through steam treatment during
processing.
Ohnishi et al. (1983) found morpholine at concentrations of
< 71.1 mg/kg in the peel of retail citrus fruits in Japan. In the
pulp (flesh) of the fruits the level was much lower, being less than
0.7 mg/kg (Table 4). Marmalade made from whole fruits contained
concentrations of morpholine between 0.3 to 0.7 mg/kg. If the fruits
were previously washed in washing-up liquid, morpholine concentrations
were reduced, but only by 25%. Even if the fruit was boiled for
20 minutes, a third to a quarter of the morpholine still remained. The
morpholine removed by these processes could be detected quantitatively
in the washing and boiling water (Ohnishi et al., 1983).
Table 4. Morpholine content of citrus fruits and marmalade from
citrus fruitsa
Sample Number Morpholine
(mg/kg)b
Orange (variety a)c peel 12
n.d.-57.0
fruit pulp 3 0.2-0.7
Orange (variety b)c peel 6 5.0-71.1
fruit pulp 1 0.3
Mandarine peel 2 16.1-18.0
fruit pulp 1 n.d.
Lemon peel 2 n.d.-5.2
fruit pulp 1 n.d.
Grapefruit peel 2 2.8-7.0
fruit pulp 1 n.d.
Marmalade from citrus fruits 4 0.3-0.7
a adapted from Ohnishi et al. (1983)
b n.d. = not detectable (detection level 0.2 mg/kg),
presumably fresh weight
c variety not specified
Sen & Baddoo (1989) reported the morpholine and NMOR content of
waxed and unwaxed apples of Canadian origin, obtained either direct
from the packers or from retail sources. Liquid wax spray is used as
a protective coating on fruit and vegetables to reduce moisture loss
and thereby extend the shelf-life of the product. Apple homogenates
and liquid waxes were analysed for their morpholine contents
(Table 5). Although the concentrations of morpholine found in waxed
apples were high, NMOR could not be found in any of the waxed or
unwaxed samples. Low levels of morpholine in the unwaxed apples could
be due to contamination during packing or transport.
Singer & Lijinski (1976a) analysed a variety of foodstuffs for
the presence of morpholine but the sample size was too small to draw
any conclusions. The results are given in Table 6. The sources of
contamination with morpholine are in these cases not clear. The
possibility of artifacts is unlikely according to the authors.
Table 5. Concentration of morpholine and NMOR (mg/kg) in samples of
liquid waxes and waxed and unwaxed applesa
Liquid wax Unwaxed apples Waxed apples
NMOR morpholine NMOR morpholine NMOR morpholine
0.286 27 300 n.d. n.d. n.d. 4.3
0.668 31 500 n.d. 0.118 n.d. 4.9
0.138 24 400 n.d. 0.016 n.d. 6.3
0.277 38 500 n.d. 0.041 n.d. 7.1
0.152 22 500 n.d. n.d. n.d. 4.0
0.585 33 300 n.d. 0.018 n.d. 7.7
a adapted from Sen & Baddoo (1989); n.d. = not detected (detection
limit: 0.005 mg/kg for morpholine, 0.0005 mg/kg for NMOR)
Table 7 summarizes the results of investigations into the
concentrations of morpholine and NMOR in prepacked milk products
(Hoffmann et al., 1982). The values range from 5-77 µg/kg for
morpholine and "not detectable" to 3.3 µg/kg for NMOR. Contamination
of prepacked foodstuffs with morpholine might be explained by the use
of morpholine in steam boiler systems for paper and cardboard
production.
Hotchkiss & Vecchio (1983) found morpholine concentrations of
between 0.098 and 8.4 mg/kg (mean 0.38 mg/kg) in food packaging. A
sample of flour nearest the wall of the paper bag contained 1.1 µg
NMOR/kg. The bag itself contained 33.0 µg NMOR/kg. In an experimental
72-h incubation at 100°C, between 1 and 2.3 µg NMOR/kg migrated from
packaging material to various dry foods.
Sen & Baddoo (1986) investigated the migration of NMOR out of
waxed packaging material into margarine. Waxed wrappings and margarine
samples taken 1 cm from the outer layer of the block contained NMOR
(5-73 µg/kg and 0.5-1.4 µg/kg, respectively). NMOR was not detectable
in those samples taken from the inside of the block. Samples taken of
margarine packed in aluminium backed wrappers, specially coated waxed
papers or plastic containers were negative for NMOR (detection level,
0.5 µg/kg).
Aitzetmüller & Thiele (1982) found no NMOR in 20 margarine
samples from different countries (detection limit, 0.5 µg/kg).
Table 6. Morpholine content in various food samplesa
Food No. of Concentration References
samples (mg/kg)b
Fish
Fish sausage 5 n.d. Hamano et al. (1981)
Cod roe 3 n.d. Hamano et al. (1981)
Codc -d tracese Singer & Lijinsky (1976a)
Spotted troutc 1 6 Singer & Lijinsky (1976a)
Smallmouth bassc 1 < 0.7 Singer & Lijinsky (1976a)
Salmonc 1 1 Singer & Lijinsky (1976a)
Ocean perchc -d 9.0 Singer & Lijinsky (1976a)
Tuna (in tins) -d < 0.6 Singer & Lijinsky (1976a)
Meat
Baked ham 5 0.2 Hamano et al. (1981)
Baked ham -d 0.5 Singer & Lijinsky (1976a)
Frankfurter sausages -d 0.4 Singer & Lijinsky (1976a)
Plant products
Spinach 2 n.d. Hamano et al. (1981)
Miso (from soja) 2 n.d. Hamano et al. (1981)
Beverages
Evaporated milk -d 0.2 Singer & Lijinsky (1976a)
Coffee -d 1.2 Singer & Lijinsky (1976a)
Tea -d tracesf Singer & Lijinsky (1976a)
Beer (in tins) -d 0.4 Singer & Lijinsky (1976a)
Beer (in bottles) -d < 0.2 Singer & Lijinsky (1976a)
Wine -d < 0.7 Singer & Lijinsky (1976a)
a adapted from BUA (1991)
b fresh weight except for tea and coffee (dry weight);
n.d. = not detected (detection limit 0.01 mg/kg)
c frozen from Tennesee & Columbia rivers
d average from several samples; exact number not given
e < 0.3 mg/kg
f < 0.1 mg/kg dry weight
Table 7. Morpholine and NMOR content (mg/kg) in food products
and their waxed containersa
Sample Food Container
NMOR morpholine NMOR morpholine
Butter 0.0032 0.058 0.0019 0.22
Cream cheese 0.0009 0.077 n.d. 0.68
Yoghurt n.d. 0.038 n.d. 3.06
Cottage cheese 0.0004 0.044 0.0054 17.2
"Cheese" (semi-soft)
Country of origin:
Germany 0.0033 0.009 n.d. 0.026
Denmark 0.0031 0.01 0.0016 0.025
Austria 0.0007 0.005 0.0012 0.022
USA 0.0014 0.008 n.d. 0.132
Gouda 0.0016 0.035 n.d. 0.035
Frozen peas n.d. 0.026 0.0031 0.057
and carrots
a adapted from Hoffmann et al. (1982); n.d. = not detected
(detection limit for NMOR, 0.0002 mg/kg)
Gavinelli et al. (1988), in a survey in Italy, found no NMOR in a
variety of foodstuffs including canned beef, pork, poultry, cured
meat, milk products, malt products, domestic Italian canned wines and
beers. The limit of detection was 0.3 µg/kg. Similarly, Pensabene &
Fiddler (1988) found no NMOR in samples of different USA fish-meat
frankfurter sausages (limit of detection, 0.2 µg/kg). These authors
assumed that the positive results in their earlier work were due to
artifacts.
Groenen et al. (1987) found NMOR in cheese (2.8 µg/kg) and cured
meat products (34 µg/kg) after addition of morpholine (10 mg/kg) in
the alkaline pH range.
Liu et al. (1988) suggested that the NMOR in fried bacon was
formed by transnitrosation.
USA Federal regulations permit the use of morpholine in several
direct and indirect food additive applications (US FDA, 1984a,b,c,d,e;
1988; see section 3.2.2). Although the US Food and Drug Administration
allows morpholine to be used as a direct or indirect food additive, in
1980 one of the biggest USA producers recommended its customers not to
use morpholine in this way (Litton Bionetics, 1980).
5.2.3 Tobacco
Morpholine was found in unburned cigarette tobacco at a
concentration of 0.3 mg/kg (Singer & Lijinsky, 1976b). In cigarette
smoke condensate < 5 mg/kg was detected, equivalent to
0.08 µg/cigarette. It has been established that an appreciable portion
of the inhaled smoke is swallowed and retained in the stomach where
nitrosation of ingested amines is known to occur (Sander & Bürkle,
1969).
Brunnemann et al. (1982) analysed 10 popular snuff brands from
the USA and Sweden for morpholine and volatile N-nitrosamines (see
Table 8). In the five USA brands, morpholine concentrations of between
1.5 and 4.0 mg/kg were found; in the Swedish products, the
concentrations were between 0.2 and 2.5 mg/kg. An analysis of the
snuff containers that were made of waxed cardboard gave morpholine
values of 0.17 to 4.74 mg/kg (USA) and 0.46 to 4.83 mg/kg (Swedish).
The morpholine content in the snuff could have come through diffusion
from the container made of waxed cardboard. Another possibility was
contamination during the tobacco processing as suggested by the very
high morpholine content (19.4 mg/kg) of a single sample of chewing
tobacco, packaged in aluminium. NMOR formed by nitrosation from
morpholine was found in 5/5 of the USA and 2/5 of the Swedish snuff
samples (Brunnemann et al., 1982).
Brunnemann & Hoffmann (1991) reported that since 1981 they had
routinely determined the concentrations of NMOR in a leading snuff
brand in the USA with the aim of creating awareness of the fact that
morpholine is a precursor of NMOR and must be removed. In eight
commercial snuff brands analysed in 1986 (Hoffmann et al., 1987), only
three were found with traces of NMOR (9-39 µg/kg). Brunnemann &
Hoffmann (1991) noted that the decrease in the NMOR content (from
about 700 µg/kg in 1981 to an undetectable level in 1990) is most
probably due to modifications in packaging, since snuff products in
the USA are now sold in plastic containers that have no wax coatings
(Table 9).
Österdahl & Slorach (1983) analysed snuff and chewing tobacco on
the Swedish market for volatile N-nitrosamines. NMOR was found in
9/30 samples in concentrations between 0.4 and 4.0 µg/kg (Swedish
brands) but in none of the three USA brands. One of three chewing
tobacco brands contained 0.8 µg morpholine/kg.
Table 8. Nitrosomorpholine and morpholine in snuff containersa
Snuff brand Snuff tobacco (µg/kg)b Snuff container
(µg/kg)c
NMOR morpholine NMOR morpholine
USA I 24 2800 34 845
II 690 1500 10 170
III 690 4000 230 4740
IV 630 3200 4 90
V 31 2200 3 140
Sweden I 44 820 4 1750
II < 2d 200 3 460
III < 2d 780 13 4830
IV 10 940 23 4290
V < 2d 2500 n.d.e n.d.
a from Brunnemann et al. (1982)
b based on dry weight
c uncorrected for moisture; had previously contained snuff.
Containers of USA I-III and Sweden I-IV were cardboard boxes
with a metal lid, USA IV were plastic containers with individual
snuff portions in porous paper bags; USA V was a plastic container;
Sweden V were individual snuff portions in Al-bags.
d detection limit = 2 µg/kg
e n.d. = not determined
Table 9. NMOR in fine-cut snuff (on the basis of dry tobacco weight)a
Year Yield (µg/kg)
1981 690
1984 29.4
1984/85 238
1985 29
1986/87 < 2b
1987 43
1990 < 2
a from Brunnemann & Hoffmann (1991)
b detection limit = 2 µg/kg
A survey of smokeless tobacco products commercially available in
1987-1988 showed that trace levels of NMOR were still found in some
United Kingdom (mean 0.5 µg/kg) and Swedish (mean 1 µg/kg) oral
tobacco products packed in waxed containers, but no NMOR was detected
in Indian zarda or European nasal snuff (Tricker & Preussmann, 1991).
5.2.4 Cosmetics and toiletry articles
Morpholine is used in some countries in cosmetic products as a
surfactant and emulsifier at concentrations up to 5% (Cosmetic
Ingredient Review, 1989).
Data submitted to the Food and Drug Administration (FDA) in 1981
by cosmetic firms participating in the voluntary cosmetic registration
programme indicated that morpholine was used in a total of 38 cosmetic
products including eyeliner, eye shadow, mascara and skin care
preparations (Cosmetic Ingredient Review, 1989). The greatest use of
morpholine was in mascara (32 products). The reported concentration
ranges of morpholine in these products were < 0.1% (4 products),
> 0.1-1% (17 products) and > 1-5% (17 products). Table 10
summarizes data submitted to the FDA in 1986 showing that the greatest
use of morpholine was in eye make-up removers, at concentrations of
> 0.1-1% and > 1-5% (Cosmetic Ingredient Review, 1989). These data
show that the ocular region and the skin around are the areas directly
exposed to cosmetic products containing morpholine. The potential also
exists for morpholine-containing products to come in contact with the
conjunctiva and cornea. Additionally, it must be emphasized that these
morpholine-containing products may be applied several times daily over
the course of several years. Due to lack of appropriate safety data,
the Cosmetic Ingredient Review Expert Panel could not conclude that
morpholine was safe for use in cosmetic products (Cosmetic Ingredient
Review, 1989).
Morpholine is listed in Annex II of the European Economic
Community (EEC) Cosmetics Directive and therefore must not be used in
cosmetic formulations (EEC, 1986). Investigations in 1988 and 1989 in
the Federal Republic of Germany showed that no morpholine could be
detected in toiletry articles (BUA, 1991).
Data from a 1985 US Food and Drug Administration report given in
Table 11 show that NMOR was found at concentrations between 48 and
1240 µg/kg in seven mascara products (Cosmetic Ingredient Review,
1989). It is not known whether the NMOR detected was formed in the
cosmetics. Table 11 also shows the results of an analysis of various
toiletry articles in the Federal Republic of Germany prior to the EEC
Directive in 1986. NMOR was detected in 18% of the products
(Spiegelhalder & Preussman, 1984).
Table 10. Product formulation data for morpholinea
Product Total no. of Total no. No. of product formulations
category formulations containing within estimated
in category ingredient concentration ranges
> 0-1% > 1 > 1-5%
Eye make-up 77 29 11 18
remover
Eye, face, or body 1264 2 2
preparations other
than eye make-up
removers
1986 totals 1341 31 11 2 18
a US FDA (1986)
5.2.5 Rubber articles
NMOR was found in commercial samples of rubber chemicals at
concentrations of 60-3500 µg/kg by Spiegelhalder & Preussmann (1982),
who noted that the occurrence of nitrosamines was directly related to
the use of corresponding vulcanization accelerators. The nitrosamines
could be leached out by water, buffer solutions or milk but could not
be completely removed even after boiling or treating with acid or
alkaline solutions (Spiegelhalder & Preussmann, 1982).
Several rubber articles which come into contact with skin or
food, in particular baby bottle teats and pacifiers (dummies), were
assayed for NMOR; 10 µg/kg migrated from a silicone rubber pacifier
(1 out of 11 different samples; detection level, 1 µg/kg) after
incubation for 24 h at 40°C (Spiegelhalder & Preussmann, 1982). Sen et
al. (1984) found NMOR in 2 out of 10 brands of nipples and in one
pacifier at concentrations of between 0.1 µg/kg (detection level) and
86 µg/kg. The same authors found NMOR (5.7 µg/kg) in one plastic
nipple shield, no NMOR being found in the other 41 samples of nipples
and pacifiers (detection limit, 1 µg/kg) (Sen et al., 1985).
Westin et al. (1987) tested 16 types of children's pacifiers and
baby-bottle nipples on sale in Israel from nine different countries
and found NMOR at levels of up to 2 µg/kg.
Table 11. N-nitrosomorpholine (NMOR) in toiletry articles and
cosmetics
Concentration
Productsa No. of No. (µg/kg)
samples positive
maximum average
Shampoos 45 13 640 133
Colour toners 7 - - -
Hair conditioners 16 - - -
Foam baths 7 - - -
Shower gels 9 4 380 145
Cream and oil baths 8 2 440 -
Cosmetic bath additives 5 - - -
Children's shampoos 5 1 230 -
Children's bath and
skin care products 8 6 360 80
Body lotions and rubs 6 - - -
Face tonics, cleaners, 29 - - -
and masks
Mascarab 7 7 1240 306
a from Spiegelhalder & Preussmann (1984), with the exception of
mascara
b from CIR (1986)
In a survey in Germany in 1990, no morpholine or NMOR was found
in the rubber articles tested; these included balloons, baby
dummies/teats, rubber gloves, condoms and rubber rings for bottling
(BUA, 1991).
5.3 Occupational exposure during manufacture, formulation or use
A USA National Occupational Hazard Survey, conducted by NIOSH,
detected worker exposure to morpholine in 283 different industries
(NRC, 1981). In 1970, 501 283 workers were potentially exposed to the
actual product, 22% were exposed to a product known to contain
morpholine, and 74% were exposed to a generic product suspected to
contain morpholine.
Data from the USA National Occupational Exposure Survey (NOES) in
1988 indicated that in a total of 30 industries and 8711 plants
surveyed, 146 511 workers, including 44 839 women, were potentially
exposed to morpholine in the workplace (NIOSH, 1988).
Many countries recommend for morpholine an 8-h time-weighted
average exposure limit of 70 mg/m3 (skin notation) and a 15-min
short-term exposure limit (STEL) of 105 mg/m3 (ILO, 1991).
5.3.1 Exposure to morpholine
Only a few studies have been reported concerning the exposure of
workers to morpholine.
An analysis in Ontario, Canada of condensed steam samples in four
hospitals and one food processing plant using corrosion inhibitors in
the steam-generating plants gave mean values for morpholine of
2.41 mg/litre in the hospitals (9 samples; range 2.1-2.9 mg/litre) and
1.1 mg/litre in the factory (9 samples; range 0.6-1.8 mg/litre)
(Malaiyandi et al., 1979).
Fajen et al. (1979) found morpholine levels of 230 and
42 µg/m3, respectively, in air samples from two out of four rubber
industry factories; these two were a tyre factory and its chemical
factory.
Taft & Stroman (1979) described an investigation into workers
exposed to a number of chemicals in the drying and bagging departments
of a tyre and rubber company manufacturing the rubber accelerator,
4-morpholino-2-benzothiazolyl [(4-morphinyl-2-benzothiazole)]
disulfide. This rubber accelerator is manu-factured by reacting
morpholine with 2-mercaptobenzothiazole (MBT). With the exception of
one personal sample showing 0.05 mg of morpholine/sample (0.4 ppm) at
the level of detection, all other samples showed negative results.
Occupational exposure to morpholine was measured in 15 factories
of the chemical, plastic and rubber industries in Germany from 1980 to
1990, and in no case was the German workplace exposure limit of
70 mg/m3 reached. Of 35 measurements, 90% were below 0.26 mg/m3
(BUA, 1991).
Katosova et al. (1991) reported average levels of 0.54 to 0.93 mg
morpholine/m3 with maximum concentrations of 0.73 to 2.14 mg/m3 in
a morpholine production factory in the former-USSR.
5.3.2 Exposure to N-nitrosomorpholine
Occupational exposure to NMOR has been found in the rubber
industry. Table 12 gives a summary of the data published.
Fajen et al. (1979) found NMOR in air samples from a tyre
chemical factory and an aircraft tyre factory. In the chemical
factory, NMOR was also found as an impurity in morpholine (0.8 mg/kg)
and in bismorpholine carbamylsulfenamide (BMCS), a vulcanisation
accelerator (0.4 to 0.7 mg/kg), as well as in wastewater
(0.003 mg/kg), utility steam condensate (0.002 mg/kg), and dirt
scrapings on a staircase (730 mg/kg). Outside the chemical plant, a
soil sample contained 4.4 mg/kg (Fajen et al., 1979). It is possible
that the NMOR in these samples was formed by the transnitrosation from
N-nitrosodiphenylamine, also produced in the tyre chemical factory,
together with morpholine present in the steam condensate (BUA, 1991).
A survey was carried out by NIOSH and a tyre manufacturing
company in 1979 into the levels of NMOR in four different work areas
in the factory as well as personal exposure levels (Ringenburg &
Fajen, 1980; London & Lee, 1987). Personal breathing-zone air samples
ranged from 0.6 to 1.8 µg/m3 (mean 0.8 µg/m3), and workplace
levels from 0.8 to 3.7 µg/m3. In a survey at another tyre
manufacturing company during the same year, nitrosamine levels were
highest (1.62 µg/m3) during the extrusion process of racing tyre
components (McGlothlin, 1980; McGlothlin & Wilcox, 1984). Exposure to
NMOR in rubber press operator fumes was investigated by NIOSH in
another company. The highest personal exposure to NMOR was
0.063 µg/m3, and the highest area sample 0.038 µg/m3.
In a factory producing rubber parts for automobile interiors, the
highest level of NMOR (19 µg/m3) was found in the process sample
taken directly above one of the extruder ovens. Other personal and
process levels were in the range 0.1 to 1.4 µg NMOR/m3.
Between 1980 and 1990, 775 samples of workplace air from 124
factories in Germany involved in the production and fabrication of
rubber articles were analysed for NMOR (BUA, 1991). Of the samples,
71% showed concentrations less than the guidance value of 1 µg/m3
(BUA, 1991).
According to Spiegelhalder (1983) and Schuster et al. (1990),
higher concentrations of NMOR are to be found in work areas where
higher temperatures are required for fabrication/vulcanization. High
levels of NMOR from storage areas probably result from continued
emission of volatile NMOR.
NMOR was not detected in the environment of a metal factory using
metal-working fluids (Fadlallah et al., 1990).
Table 12. Occupational exposure to NMORa
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Tyre chemical factory USA 1978 10 area 0.07-5.1 Fajen et al. (1979)
Tyre manufacturer USA 1978 20 area 0.6-27 Rounbehler & Fajen
12 area 0.08-8.5 (1983)
7 area 0.02-3.9
6 process 0.41-2.9
6 area < 0.01-0.60
7 process < 0.1-22.0
7 area < 0.002-250
8 process 0.1-25
Tyre manufacturer USA 1979/ 13 area 0.1-1.62 McGlothlin (1980),
1980 McGlothlin & Wilcox (1984)
Tyre manufacturer USA 1979/ 4 area 0.85-3.7 Ringenburg & Fajen (1980);
1980 6 personal 0.6-1.8 London & Lee (1987)
Industrial rubber articles USA 1978 4 area 0 Fajen et al. (1979)
Industrial rubber articles USA 1980/ 11 area < 0.005-0.0376 Hollett et al. (1982)
1981 7 personal < 0.0096-0.0629
Rubber articles for USA 1982 4 area 0.8-19 Lee (1982)
car outfitting 13 personal 0.4-1.2
Rubber industry Germany 1979/ 20 monitoring area and 0.1-17 Spiegelhalder &
1981 points in 17 personal Preussmann (1982)
factories
Table 12 (cont'd)
Production area Country Year No. of monitoring Type of Concentration Reference
places monitoring (µg/m3)b
Rubber industry Germany 1987 545 personalc < 2.5 (73%), TRGS (1989)
> 2.5 (27%)
max. 41
Chemical industry Germany 1987 22 not given < 1 (91%)d TRGS (1989)
Leather industry Germany 1987 50 not given < 1 (100%) TRGS (1989)
Foundries Germany not given 658 not given < 1 (95%), TRGS (1989)
> 1 (5%)
max. 2.1
a adapted from BUA (1991)
b the percentage of samples which had this given concentration is given in parentheses
c sum of all measured N-nitrosamines
d in 82% below the detection level (0.2 µg/m3)
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Toxicity experiments on rodents have shown that morpholine is
absorbed after oral, dermal and inhalation exposure (see Table 13).
6.2 Distribution
Tanaka et al. (1978) determined the distribution of
[14C]-labelled morpholine in male Wistar rats (3 animals/group,
250-300 g) after oral (200 mg/kg) and intravenous injections
(150 mg/kg). The radioactivity was determined in the dried, powdered
organs. Large amounts were only found in muscle and intestine. In
rats sacrificed 2 h after oral administration of morpholine-HCl, 29%
of the radioactivity was found in the intestine and 26% in the muscle.
Similarly, 2 h after intravenous injections, 19 and 27% of the dose
was found in the intestine and muscle, respectively.
Female New Zealand rabbits (number not given) were exposed to
morpholine (905 mg/m3) for 5 h by nose-only inhalation
(Tombropoulos, 1979). At the end of the exposure, the animals were
sacrificed and the tissue and body fluids analysed. Concentrations of
morpholine were highest in urine (324 mg/litre) and kidney
(118 mg/kg), the other tissues having concentrations below 40 mg/kg.
Van Stee et al. (1981) injected six male New Zealand rabbits
intravenously with 5 mmol [14C]-labelled morpholine/kg body weight
(435 mg/kg). The distribution of radioactivity after 30 min showed
the highest concentrations in the renal medulla (36 mmol/kg) and
cortex (15.4 mmol/kg), followed by lung (5.1 mmol/kg), liver
(4.7 mmol/kg) and blood (2.3 mmol/litre). Morpholine was not bound to
serum proteins.
6.3 Metabolic transformation
Morpholine is eliminated mainly in a non-metabolized form in the
urine of the rat, mouse, hamster and rabbit (Griffiths, 1968; Tanaka
et al., 1978; Van Stee et al., 1981; Sohn et al., 1982b). However,
Sohn et al. (1982b) reported that morpholine is metabolized by
N-methylation followed by N-oxidation in the guinea-pig. After an
intraperitoneal injection of 125 mg/kg [14C]-labelled morpholine in
guinea-pigs, 20% of the radioactivity was found in the urine as
N-methylmorpholine- N-oxide. However, the morpholine ring can be
cleaved in mammalian systems. In several studies on the metabolism of
morpholine derivatives in the rat, ring cleavage products have been
noted (Tatsumi et al., 1975; Hecht & Young, 1981; Kamimura et al.,
1987).
Table 13. Single exposure toxicity data for morpholinea
Species No./sex Dosage Results Cause of death/symptoms Reference
Oral
Rat 5 1.05 g/kg body weight LD50 n.g. Smyth et al. (1954)
Rat 57 1.6 g/kg body weightb LD50 gastrointestinal haemorrhage Shea (1939)
Rat 5 m + 5 f 1.9 g/kg body weight LD50 dyspnoea, haemorrhagic BASF (1967)
enteritis
Rat 7 m 1.0 g/kg body weight; pH 7 no deaths n.g. Börzsönyi et al. (1981)
Guinea-pig 33 0.9 g/kg body weightb LD50 gastrointestinal Shea (1939)
haemorrhage, diarrhoea
Intraperitoneal
Rat (Wistar) 4 m 0.4 g/kg body weight all died n.g. Stewart & Farber (1973)
Rat (Wistar) 4 m 0.1 g/kg body weight lethal n.g. Stewart & Farber (1973)
for 1/4
Mouse 5 m + 5 f 0.4 g/kg body weight LD50 irritation around BASF (1967)
injection area, dyspnoea
Inhalation
Rat 5 m + 5 f 18.1 g/m3; 6 h lethal haemorrhage of nose, mouth Hazleton (1981)
for 9/10 and eyes; spasms; tremors
Rat 6 saturated atmosphere;c all died spasms; caustic burns BASF (1967)
5´ h on nose and extremities
Table 13 (cont'd)
Species No./sex Dosage Results Cause of death/symptoms Reference
Inhalation (contd)
Rat n.g./m 8.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g./f 7.8 g/m3; LC50 n.g. Lam & Van Stee (1978)
Rat n.g. 65.2 g/m3; 8 h lethal irritation of nose, Shea (1939)
eyes; lung haemorrhage
Rat n.g. 43.4 g/m3; 8 h lethal n.g. Shea (1939)
Mouse n.g./f 6.9 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g./m 5.2 g/m3; LC50 n.g. Lam & Van Stee (1978)
Mouse n.g. 4.9 g/m3; LC50 n.g. Zaeva et al. (1968)
Dermal
Rabbit (New n.g. 0.5 ml/kg body weight LC50 n.g. Smyth et al. (1954)
Zealand)
a adapted from BUA (1991); n.g. = no details given
b morpholine diluted with 4 parts water; deaths within a week
c inhalation of a saturated atmosphere at 20°C formed by bubbling air through a 5-cm layer of morpholine
In the presence of nitrite, morpholine can be converted to NMOR
(see section 4.3). NMOR was found in the stomachs of rats that had
been fed on diets containing morpholine and nitrite (Sander et al.,
1968; Inui et al., 1979).
Immunostimulation of rats by intraperitoneal treatment with
E. coli lipopolysaccharide (LPS; 1 mg/kg) led to a large increase in
urinary nitrate and urinary metabolites of NMOR when morpholine
(80 µmol/kg) and L-arginine (400 µmol/kg) were injected
intraperitoneally. The replacement of LPS with nitrate (330 µmol/kg
intraperitoneal) did not increase urinary metabolites of NMOR (Leaf et
al., 1991). This result is consistent with endogenous nitrosation of
morpholine by nitrogen oxide (NO) from oxidation of the guanido group
of arginine by induced NO synthase (Hibbs, 1992).
Hecht & Morrison (1984) developed a method to monitor the
in vivo formation of NMOR by measuring N-nitroso
(2-hydroxyethyl)glycine, its major urinary metabolite. The formation
of NMOR was measured in F-344 rats over wide range of doses of
morpholine (38.3-0.92 µmol) and sodium nitrite (191-4.8 µmol).
According to estimates by the authors, 0.5 to 12% of the morpholine,
depending on the dose, was nitrosated.
In vitro nitrosation of morpholine has been reported. NMOR was
formed when morpholine was added to human saliva (Tannenbaum et al.,
1978). Additionally, a new type of metabolite N-cyanomorpholine was
identified in human saliva (Wishnok & Tannenbaum, 1976).
6.4 Elimination and excretion
6.4.1 Expired air
Elimination of 14C from labelled morpholine (intraperitoneal
injection) through expired air is minimal. In rats, experiments have
shown that only about 0.5% of the dose of radioactively labelled
morpholine is exhaled as 14CO2 (Sohn et al., 1982b). In rabbits
0.0008% of the administered dose was 14CO2 (Van Stee et al.,
1981).
6.4.2 Urine
Elimination studies on male Wistar rats (200-350 g) were carried
out by administering morpholine-HCl (500 mg/kg) or [14C]-labelled
morpholine-HCl (200 mg/kg) orally and morpholine-HCl (250 mg/kg)
intravenously. In all cases, over 85% of the dose was excreted in
urine within 24 h. A further portion, up to 5%, was excreted during
the next three days. [14C]-morpholine palmitate was eliminated
slightly more slowly, but the urinary excretion within 3 days
following oral administration amounted to 90% of the dose (Tanaka et
al., 1978). Of the radioactive morpholine administered to rats,
62-77.5% was excreted in the urine after 24 h (Griffiths, 1968;
Ohnishi, 1984). Following intraperitoneal administration to rats,
urinary excretion within 24 h was 87.8% of the dose (Maller &
Heidelberger, 1957). In the dog, 70-80% of the radioactive morpholine
was excreted in the urine (Rhodes & Case, 1977).
The time-course of urinary excretion of 14C by Sprague-Dawley
rats, Syrian golden hamsters, and strain II guinea-pigs treated with
[14C]-morpholine was compared by Sohn et al. (1982b). Although in
all three species over 80% was excreted in 3 days, the rate of urinary
excretion within the first 6 h was greatest in the hamster and least
in the guinea-pig.
Van Stee et al. (1981) infused rabbits intravenously with
[14C]-morpholine (5 mmol/kg) which had been neutralized with HCl.
After 4 h, 18.5% of the dose was excreted in the urine. When the pH
of the urine was lowered from 7.8-7.9 to 7.1-7.2 by administration of
ammonium chloride (10 g/litre) in drinking-water prior to the
injection, the urinary excretion more than doubled (to 43%).
These data suggest that the urinary excretion of morpholine is
enhanced by its neutralization with acid.
6.4.3 Faeces
Rats dosed orally or intravenously with morpholine hydrochloride
excreted not more than 1.7% of the dose in the faeces (Griffiths,
1968; Tanaka et al., 1978). However, when dosed orally with
morpholine palmitate (Tanaka et al., 1978, Ohnishi, 1984), up to 7%
was excreted in faeces.
6.5 Retention and turnover
Plasma concentration-time curves of 14C after intraperitoneal
injections of [14C]-morpholine (125 mg/kg in 0.9% NaCl) in Sprague-
Dawley rats, Syrian golden hamsters, and strain II guinea-pigs
declined biexponentially. Whereas rates of first phase of elimination
from plasma in rats and hamsters were similar (half-lives of 115 and
120 min, respectively), the half-life in guinea-pigs was significantly
longer (300 min) (Sohn et al., 1982b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Table 13 summarizes the toxicity data on single exposure to
morpholine.
7.1.1 Oral
Oral administration of morpholine to rats resulted in LD50
values of 1-2 g/kg body weight (Shea, 1939; Smyth et al., 1954; BASF,
1967). Gastrointestinal and nasal haemorrhage were reported. In
contrast, when morpholine was administered to seven male rats at a
neutral pH, no deaths occurred with concentrations of 1 g/kg body
weight (Börzsönyi et al., 1981).
A study on guinea-pigs resulted in a LD50 of 0.9 g morpholine
per kg body weight. Gastrointestinal and nasal haemorrhage were
reported (Shea, 1939).
7.1.2 Inhalation
There have been reports of inhalation studies with morpholine on
rats (Shea, 1939; BASF, 1967; International Labour Office, 1972;
Lam & Van Stee, 1978; Hazleton, 1981) and mice (Zaeva et al., 1968;
Lam & Van Stee, 1978).
Exposure to morpholine at vapour saturation concentrations
resulted in almost 100% lethality after 5.5 h (BASF, 1967). It had
irritating and corrosive properties.
In studies with lower concentrations, Lam & Van Stee (1978)
obtained LC50 values of 7.8 and 8.2 g/m3 for female and male rats,
respectively, whereas other authors reported no deaths at three times
this concentration (see Table 13).
Reported LC50 values for mice are consistently in the range of
5-7 g/m3 (see Table 13).
7.1.3 Dermal
Smyth et al. (1954) noted necrosis on the clipped skin of albino
rabbits within 24 h of application of undiluted morpholine. Mortality
within 14 days in New Zealand rabbits after penetration of morpholine
into the skin gave an LD50 of 0.5 ml/kg body weight.
Other reports are discussed in section 7.4.2.
7.1.4 Intraperitoneal
When morpholine was administered intraperitoneally to rats
(Stewart & Farber, 1973) and mice (BASF, 1967), the LD50 was in the
range 0.1-0.4 g/kg body weight for rats and 0.4 g/kg body weight for
mice (see Table 13).
7.2 Short-term exposure
As with single-exposure, the effects of morpholine after short-
term exposure depend on the method of exposure. The available data on
short-term exposure are summarized in Table 14.
7.2.1 Oral
Rats and guinea-pigs were fed morpholine at concentrations of
0.16-0.8 g/kg body weight and 0.09-0.45 g/kg body weight,
respectively, by gavage for 30 days (Shea, 1939). At concentrations of
half of the LD50, nearly all the animals died within 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver and necrosis of the stomach
glandular epithelium.
Fatty degeneration (lipidosis) of the liver in rats was noted
after feeding morpholine (0.5 g/kg body weight) daily for 56 days
(Sander & Bürkle, 1969).
Shibata et al. (1987a) carried out a 13-week toxicity study in
B6C3F1 mice using morpholine as the fatty acid salt, morpholine
oleic acid salt (MOAS), at dosage levels of 0%, 0.15%, 0.3%, 0.6%,
1.25% and 2.5% MOAS in the drinking-water. At the highest MOAS level,
body weight gains were slightly reduced. Histopathological analysis
showed cloudy swelling of the proximal tubules, but no other
alterations were observed in the organs of either sex. Urinalysis
showed increases in both specific gravity and plasma urea nitrogen in
some dosage groups, suggesting a possible malfunctioning of the kidney
(see Table 14).
7.2.2 Inhalation
In rats that died after 5 days repeated exposure to 65.2 g/m3,
lung haemorrhage, severe damage to the secreting tubules of the
kidney, and fatty degeneration of the liver were observed (Shea,
1939).
Table 14. Short-term exposure to morpholinea
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
Oral
Rat 20 0.16 g/kg body weight per 8/20 beginning necrosis of liver, kidney Shea (1939)
day; 30 days; in 2 ml H2O and stomach mucous membrane
(gavage)
20 0.32 g/kg body weight per 8/20 necrosis of liver, kidney, stomach
day; 30 days; in 2 ml H2O
(gavage)
20 0.8 g/kg body weight per 19/20 weight loss, lethargy, severe necrosis
day; 30 days; in 2 ml H2O of liver, kidney, stomach
(gavage)
Rat 7 f 0.5 g/kg body weight per sacrifice after 270 days, moderate Sander & Bürkle
(Sprague- day; 56 days liver adipose degeneration (1969)
Dawley)
Mouse 10 m + 10 f 0.15 and 0.3% MOAS; 91 days no significant changes Shibata et al.
(B6C3F1) (0.07 or 0.14 g morpholine/ (1987a)
kg body weight per day)
10 m + 10 f 0.6% MOAS; 91 days m: increase in specific gravity of
(ca. 0.2 g morpholine/kg urine; f: increase in blood urea
body weight) per day
10 m + 10 f 1.25% MOAS; 91 days increase in specific gravity of
(ca. 0.4 g morpholine/kg urine and blood urea
body weight per day)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
10 m + 10 f 2.5% MOAS; 91 days reduced weight gain; increase in specific Shibata et al.
(ca. 0.7 g morpholine/kg gravity of urine and blood urea; (1987a)
body weight per day) increased relative kidney weight with
swelling of proximal tubules
Guinea-pig 20 0.09 g/kg body weight 3/20 slight alterations in liver, kidney Shea (1939)
per day; 30 days (syringe)
20 0.18 g/kg body weight 12/20 necrosis of liver, kidney, stomach
per day; 30 days (syringe)
20 0.45 g/kg body weight 16/20 severe degeneration of liver, kidney,
per day; 30 days (syringe) stomach
Inhalation
Rat n.g. 7.2 g/m3; 4 h/day; 4 days n.g. increased residual volume and Takezawa &
total lung capacity Lam (1978)
Rat n.g. 65.2 g/m3; 34 h over 5 days some deathsb necrosis of liver and kidney tubules; Shea (1939)
lung irritation
Rat 6-8 m 0.08 g/m3; 4 h/day; 8 days no deaths hypersecretion of thyroid gland, Grodeckaja
higher accumulation of 131I in the & Karamzina
thyroid gland (1973)
Rat 5 m + 5 f 0.36 g/m3; 6 h/day; 9 days 0/10 red stains around nose and mouth; Hazleton
weight loss in females (1981)
5 m + 5 f 1.81 g/m3; 6 h/day; 9 days 2/10 irritation to nose and eyes; weight loss; Hazleton
decrease in spleen/brain weight ratios (1981)
Table 14 (cont'd)
Species No./sex Dosage/concentration/ Mortality Pathological, histopathological Reference
length of exposure or biochemical changes
5 m + 5 f 3.62 and 18.1 g/m3; 10/10 bleeding from eyes, nose and Hazleton
6 h/day; 9 days mouth (1981)
Rat n.g. 1.63 g/m3; 4 h/day; n.g. reduced weight gain; increased residual Takezawa &
5 days/week; 30 days volume and total lung capacity Lam (1978)
Rat 20 m + 20 f 0.09 g/m3; 6 h/day; no significant differences in body Conaway et al.
(Sprague- 5 days/week; 13 weeks weight, clinical chemistry, haematology (1984b)
Dawley) or organ weight data; no nasal lesions
20 m + 20 f 0.36 g/m3; 6 h/day; in 2/20 females focal necrosis in
5 days/week; 13 weeks nasal cavity
20 m + 20 f 0.9 g/m3; 6 h/day; lesions of nose and mouth; after 7
5 days/week; 13 weeks weeks in 8 animals focal metaplasia
and necrosis in nasal turbinates; after
13 weeks, increased incidence and
severity; pneumonia
Rabbit 4 m 0.9 g/m3; 6 h/day; increased hydrolytic enzyme content Tombropoulos
5 days/week; 33 days in alveolar macrophages et al. (1983)
a adapted from BUA (1991); b.w. = body weight; n.g. = not given
b exact numbers not clear
Rats inhaling morpholine at 3.62 or 18.1 g/m3 for 9 days, 6 h
per day, died within the exposure period (Hazleton, 1981). At lower
concentrations (1.81 g/m3), weight loss and irritation to nose and
eyes, as well as two deaths, were reported. The report concluded that
the maximal tolerated dose for rats is about or just below 0.3 g/m3.
Increased thyroid activity, shown as increased uptake of injected
131I, was observed in male rats after exposure to 0.08 g
morpholine/m3, 4 h/day, for 4 days (Grodeckaja & Karamzina, 1973).
Takezawa & Lam (1978) reported an increase in lung weight,
residual volume and total lung capacity in rats exposed to morpholine
(7.2 g/m3, 4 h/day for 4 days or 1.63 g/m3 for 30 days).
Tombropoulos et al. (1983) examined the induction of lysosomal
enzymes by morpholine in rabbits. Two acid hydrolases,
alpha-mannosidase and acid phosphatase, were induced in the lung
alveolar macrophages during the course of inhalation exposure
(905 mg/m3, 250 ppm, 6 h/day, 5 days/week for a total of 33
exposures). The induction was also observed when macrophages were
cultured in the presence of morpholine.
Conaway et al. (1984b) exposed groups of 40 rats to morpholine
(0, 0.09, 0.36 and 0.9 g/m3) for 7 and 13 weeks. Shallow, rapid
breathing was noted in all groups except the controls. Lesions of the
nasal septum, anterior cavities, nasoturbinates and maxilloturbinates
were observed in the 0.36 and 0.9 g/m3 groups but not in the lowest
exposure group.
Lung sections of all rats killed after 7 weeks contained early
lesions of chronic murine pneumonia. In the upper dosage group, these
lesions had developed in severity by 13 weeks. There were no apparent
treatment-related effects in any of the haematology, clinical
chemistry or urinalysis data at weeks 7 or 13.
7.2.3 Dermal
Shea (1939) investigated the effects of single and repeated
dermal application of morpholine on rabbits and guinea-pigs. He found
that whereas unneutralized, undiluted morpholine caused 100% lethality
and even the diluted compound caused mortality and severe necrotic
burns and inflammation, application of undiluted morpholine
neutralized to pH 7 with sulfuric acid caused neither gross nor
microscopic pathology of the skin with the exception that the dermis
was thickened at the site of application.
Undiluted morpholine applied for 5-15 min to rabbit skin led to
severe necrosis (BASF, 1967).
Data on short-term dermal exposure to morpholine are also
discussed in section 7.4.2.
7.3 Long-term exposure
Table 15 summarizes the data on long-term exposure to morpholine.
7.3.1 Oral
Mice (10 males and 10 females/group) were given 0%, 0.25% or 1.0%
MOAS in their drinking-water for 96 weeks and then given normal tap
water for a further 8 weeks (Shibata et al., 1987b). During the study,
the physical appearance and general behaviour did not appear to be
affected by treatment. Decreased body weight gain was noted at 1% MOAS
(both sexes) and at 0.25% MOAS (females). Gross pathological and
extensive biochemical examinations did not reveal treatment-related
effects. The incidence of hyperplasia in forestomach epithelium of
males in the 1% group was statistically higher than in the controls,
but otherwise no significant increase in the incidence of non-
neoplastic and neoplastic lesions could be found.
7.3.2 Inhalation
Migukina (1973) reported increased nervous system activity and
increases in haemoglobin and peripheral red blood cell counts in rats
and guinea-pigs exposed to morpholine (0.008 and 0.07 g/m3) for 4
months. An increase in the chromosomal aberrations of the bone marrow
cells was also noted (see section 7.6). This study was deficient with
respect to the description of study methods.
Harbison et al. (1989) carried out an extensive long-term
exposure inhalation study. Groups of 70 rats of each sex were exposed
to morpholine (0, 0.036, 0.181, 0.543 g/m3) 6 h per day, 5 days per
week, for 104 weeks, with an interim sacrifice at week 53. Levels of
nitrates and nitrites in the drinking-water were reported to be
< 0.1 mg/litre and 0.01 mg/litre, respectively. Survival, body weight
gain, organ weight and haematology and clinical chemistry data were
normal in exposed groups, compared to the controls. In-life clinical
examinations revealed increased incidences of inflammation of the
cornea, inflammation and squamous metaplasia of the turbinate
epithelium, and necrosis of the turbinate bones in the nasal cavity of
both male and female rats. No increase in the incidence of neoplasms
was found. Only tissues from the respiratory tract and eyes were
examined histologically in the case of the mid- and low-dose groups.
7.3.3 Dermal
No data are available on long-term dermal exposure to morpholine.
Table 15. Long-term exposure to morpholinea
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Oral
Mouse 50 m 0.25% MOAS; 672 days (ca. 0.05-0.14 g no significant changes Shibata et al.
(B6C3F1)b morpholine/kg body weight per day) (1987b)
50 f 0.25% MOAS; 672 days (ca. 0.07-0.17 g decreased body weight gain
morpholine/kg body weight per day)
50 m 1% MOAS; 672 days (ca. 0.28-0.5 g decreased body weight gain; increase in blood
morpholine/kg body weight per day) urea nitrogen; hyperplasia in forestomach
epithelium
50 f 1% MOAS; 672 days (ca. 0.21-0.57 g decreased body weight gain
morpholine/kg body weight per day)
Inhalation
Rat 84 m 0.008 g/m3; 34 h within 5 days; reversible changes in haemogram, kidney, Migukina (1973)
16 weeks liver, lung, spleen, myocardium
84 m 0.07 g/m3; 4 h/day; changes in haemogram, liver, kidney
5 days/week; 16 weeks lung, spleen, myocardium
Rat 20 1.09 g/m3; 6 h/day; no significant increase in spinal cord Savolainen &
5 days/week; 15 weeks axonal succinate dehydrogenase activity; no Rosenberg (1983)
significant alteration of enzyme activity in
muscle; morpholine concentration in brain
increased with length of exposure
Table 15 (cont'd)
Species No./sex Dosage/concentration, Effect Reference
length of exposure
Rat 70 m + 0.036 and 0.18 g/m3; 6 h/day; increased incidence of irritation around Harbison et al.
(Sprague- 70 f 5 days/week; 104 weeksc eyes and nose (1989)
Dawley)
70 m + 0.54 g/m3; 6 h/day; local necrosis around eyes and nose;
70 f 5 days/week; 104 weeksc kerativitis
Guinea-pig 24 0.008 g/m3; 4 h/day; swollen lymph nodes of the spleen Migukina (1973)
5 days/week; 16 weeks
24 0.07 g/m3; 4 h/day; swollen alveoli; atrophy of lymph vessels in
5 days/week; 16 weeks lung and spleen; increase in haemoglobin and
erythrocyte number, decrease in leucocytes
a adapted from BUA (1991); b.w. = body weight
b MOAS: morpholine administered as oleic acid salt in drinking-water; in brackets, the daily intake of morpholine
calculated from MOAS intake
c 10 m + 10 f killed at 53 weeks
7.4 Skin and eye irritation; sensitization
7.4.1. Eye irritation
Eye injury in rabbits was tested by Smyth et al. (1954), who
reported severe eye burns with 0.5 ml of a 1% solution. BASF (1967)
reported that 1 drop of undiluted morpholine in rabbit eyes, repeated
once after 5 min, caused oedema, opacity, staphyloma and corrosion of
the eye mucous membranes within 24 h.
A solution of morpholine (0.02 mol/litre) neutralized with HCl
had no injurious effect on the eyes of rabbits when applied
continuously for 10 min after removal of the corneal epithelium to
facilitate penetration. Similarly, the irritative reaction of 10-20%
aqueous morpholine was reduced on neutralization (Grant, 1974).
In-life clinical examinations in rats exposed to up to
0.54 g/m3 (150 ppm) morpholine revealed increased incidences in
inflammation of the cornea at week 103 of the study (Harbison et al.,
1989). Findings included keratitis, oedema, abrasion, scarring, and
ulceration with or without neovascularization and corneal epithelial
hyperplasia. A high incidence of retinal degeneration was observed,
primarily in female animals, which was probably an age-related light-
induced retinal degeneration.
Albino rabbits, three of each sex, were treated with a mascara
composite containing 1% morpholine; 0.1 ml of the cosmetic was
instilled into one eye of each rabbit daily for 14 days. Before the
application each day, the eye was evaluated for ocular irritation. A
slight redness of the conjunctiva was noted throughout the duration of
the study but this cleared within 24 h of the last treatment. A sodium
fluorescein dye test performed at the conclusion of the study
indicated no abnormalities of the cornea or its iris membranes
(Cosmetic Ingredient Review, 1989).
7.4.2 Skin irritation
In rabbits treated with aqueous solutions of 2, 20, 40 and 60%
morpholine, the skin reactions were evaluated after 0.5, 24, 48 and
72 h (Lodén et al., 1985). A 2% solution of morpholine caused skin
irritation after 72 h, whereas 40% and 60% solutions immediately
caused reddening of the skin.
Wang & Suskind (1988) measured the irritant potential of
morpholine by applying 0.1 g mixture (0.1, 0.5, 2, 5 and 10%
morpholine in petrolatum) to guinea-pig skin for 24 h. Observations
were made 1, 24 and 48 h after removal of the test materials, and no
noticeable effects were found.
A mascara composite containing 1% morpholine was applied to
normal and abraded skin of six albino rabbits (Cosmetic Ingredient
Review, 1989). At the end of the 14-day treatment period, no dermal
toxicity or irritation was observed.
7.4.3 Sensitization
Sensitization studies (modified Buehler) on guinea-pig skin using
2% morpholine in petrolatum gave negative results (Wang & Suskind,
1988). In an in vitro study into the biochemical causes of
sensitization, it was found that morpholine as a hapten did not react
with the amino acids glycine, lysine and cystine, as did related
compounds which showed sensitizing reactions (Wang & Tabor, 1988).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
No adequate studies on reproductive toxicity, embryotoxicity or
teratogenicity have been reported.
7.6 Mutagenicity and related end-points
7.6.1 Mutagenicity of morpholine
Table 16 summarizes the short-term mutagenicity tests on
morpholine.
7.6.1.1 Bacteria
No mutagenic response was observed in plate tests with strains of
Salmonella typhimurium, either with or without metabolic activator,
exposed to morpholine at up to 10 µl/plate (approximately 10 mg/plate)
(Texaco, 1979a; Haworth et al., 1983), whereas a 99.8% purity sample
did induce weak mutagenic responses in S. typhimurium TA100 and
Escherichia coli WP2 uvr A at the unusually high dose level of
50 mg/plate (Glatt & Oesch, 1981). The activity in strain TA100 did
not require S9 mix and is, therefore, unlikely to have been due to
0.2% contamination by NMOR (see below).
In mouse peritoneal cavity host-mediated assays with
S. typhimurium TA1530 and TA1930, no increase in the proportion of
mutants was observed following the oral administration of morpholine
(Braun et al., 1977; Edwards et al., 1979). These groups served as
controls in a study of nitrosation (see below).
7.6.1.2 Yeast
No gene conversion was induced in Saccharomyces cerevisiae D4
by morpholine concentrations up to 10 µg/litre per plate. Toxicity
was observed at the highest concentration (Texaco, 1979a).
Table 16. Short-term in vitro microbial mutagenicity assays with morpholinea
Test organisms Strain Analyzed Metabolic Dosage range Result with/ Reference
purity (%) activityb without S9-mix
Salmonella typhimurium TA98/100/1535/1537 not given RA 0.109-10.9 mg/plate -/- Haworth et al. (1983)
S. typhimurium TA98/100/1535/1537 not given RA 0.005-10 µl/plate -/- Texaco (1979a)
1538c
Saccharomyces D4 not given RA 0.005-10 µl/platec -/- Texaco (1979a)
cerevisiae
S. typhimurium TA98/1535/1537 99.8 RA 0.0158-50 mg/plate -/- Glatt & Oesch (1981)
S. typhimurium TA100 99.8 RA 0.0158-50 mg/plate (+)/(+)d Glatt & Oesch (1981)
Escherichia coli WP2 uvrA 99.8 RA 0.0158-50 mg/plate (+)d/- Glatt & Oesch (1981)
S. typhimurium TA1950 99 HM1 1450-2900 µmol/kg - Braun et al. (1977)
S. typhimurium TA1530 not given HM2 4 mg/kg body weight - Edwards et al. (1979)
a adapted from BUA (1991)
b RA = Arochlor-induced rat liver-S9; HM1 = Host-mediated assay NMRI-mice; HM2 = Host-mediated assay CD-1 mice
c toxic at 10 µl/plate for strain Salmonella typhimurium TA1538 and Saccharomyces cerevisiae D4
d (+) = positive only at the 50 mg/plate dose
7.6.1.3 Mammalian cells in vitro
When tested over the range 0.625-1.25 µl/ml (approximately
0.625-1.25 mg/ml) morpholine induced small increases in the fraction
of tk mutants in mouse lymphoma L5178Y cells. No exogenous metabolic
activation system was required for this weak activity (Texaco, 1979b;
Conaway et al., 1982a,b).
Morpholine induced no DNA-repair in the primary cultures of rat
hepatocytes (0.1-100 µg/ml) (Conaway et al., 1984a).
Morpholine induced small increases in the frequency of SCEs in
Chinese hamster ovary (CHO) cells. No exogenous metabolic activation
system was required for this activity, which was observed at
concentrations of 50 and 100 nl/ml (approximately 50 and 100 µg/ml) in
the absence of S9 mix (Litton Bionetics, 1980).
Morpholine increased the numbers of type III foci in the
Balb/C3T3 cell transformation assay tested at different dosages
(0.001-0.3 µl/ml), corresponding to 78-52% survival in the
cytotoxicity test (Texaco, 1979b, Litton Bionetics, 1979a,b; Conaway
et al., 1982a,b).
In another study of in vitro transformation of Balb/3T3 cells
with and without metabolic activation, the morpholine in the culture
medium was neutralized before testing (Litton Bionetics, 1982). No
significant increases were induced in transformed foci over the tested
concentration ranges (0.015 to 1.400 µl/ml in the non-activation
assay; 0.0175-0.7 µl/ml in the activation assay). Morpholine was
therefore considered as being inactive in this test.
7.6.1.4 In vivo studies in mammals
Inui et al. (1979) administered a dosage of 500 mg morpholine
per kg body weight to female Syrian hamsters on the 11th or 12th day
of pregnancy. The embryos were removed 24 h later and embryo cells
examined. For detection of induced mutations, embryo cells were
cultured in normal medium for 72 h and then transferred to a medium
containing 8-azaguanine (10 or 20 mg per litre) or ouabain (1 mM). No
chromosomal aberrations, micro-nucleus formation, or 8-azaguanine- or
ouabain-resistant mutations were found.
Migukina (1973) reported an increase in the number of chromosomal
aberrations, particularly "fragmentations", in bone marrow cells of
rats and guinea-pigs exposed for 4 months to 8 or 70 mg
morpholine/m3 (see also section 7.3.2). However, there were
deficiencies in the study report (BUA, 1991).
7.6.2 Mutagenicity of morpholine in the presence of nitrite and
nitrate
Although morpholine has given negative responses in a number of
mutagenicity assays, there is concern for its ability to be nitrosated
readily to form NMOR (see section 4.3).
Two mouse peritoneal cavity host-mediated assays with
S. typhimurium have been performed. In one, with strain TA1530,
different morpholine doses were administered orally to mice
simultaneously with a standard sodium nitrite dose of 120 mg/kg. The
mixtures were adjusted to pH 3.4. Significant increases in the mutant
fraction were observed with morpholine doses of 4-40 mg/kg, the full
range tested (Edwards et al., 1979). In the other assay, with strain
TA1950, equimolar mixtures of morpholine and sodium nitrite (1450 or
2900 µmol/kg, approximately 125 or 250 mg/kg) were administered orally
to mice (pH 7.0). Significant increases in the mutant fraction were
observed at both dose levels. When the nitrite was administered to the
mice 10 min before the morpholine treatment, no mutagenic response was
induced (Braun et al., 1977).
Mutations were found in S. typhimurium TA1535 used to test the
urine of OF1 mice after morpholine (250 mg/kg body weight) had been
administered in the presence of nitrate (2000 mg/kg). Lower levels of
nitrate (333 and 666 mg/kg) caused no mutations (Perez et al., 1990).
7.6.3 Mutagenicity of N-nitrosomorpholine
NMOR is mutagenic to S. typhimurium TA100 in the presence of S9
mix at dose levels above 2000 µg/plate. In yeast, it induced forward
mutation and aneuploidy, but not mitotic recombination. In cultured
mammalian cells, conflicting results were obtained in unscheduled DNA
synthesis (UDS) assays in fibroblasts, while weakly positive results
were reported for sister-chromatid exchange (SCE) in CHO cells, for tk
locus mutation in mouse lymphoma L5178Y cells, and for enhanced colony
growth of BHK cells in soft agar. NMOR was negative in an in vitro
cytogenetic assay using RL1 (rat liver cell line) cells (IARC, 1978;
NRC, 1981).
7.7 Carcinogenicity
Carcinogenicity studies have been carried out with morpholine
alone, as well as in the presence of nitrite, to investigate the
possible formation and effect of NMOR.
7.7.1 Morpholine
7.7.1.1 Oral studies
Greenblatt et al. (1971) treated 40 Swiss mice (20 male and
20 female) with 6.33 g morpholine/kg food (estimated dosage, 0.9 g/kg
body weight per day for 28 weeks. The control group consisted of
80 mice of each sex. After a further 12 weeks of observation the
surviving animals were sacrificed. No increase in the lung tumour rate
(0.1 adenoma/mouse) was found compared to the controls
(0.18 adenoma/mouse). It should be noted that the duration of exposure
in this study was shorter than that normally used in a well-designed
long-term carcinogenicity study.
Multi-generation oral studies were performed on Sprague-Dawley
rats fed 5, 50 or 1000 mg/kg morpholine, together with various dietary
concentrations of sodium nitrite (0, 5, 50 or 1000 mg/kg diet)
(Newberne & Shank, 1973; Shank & Newberne, 1976). From the day of
conception, the pregnant animals were given 0 or 1000 mg morpholine/kg
feed. The F1 and F2 generations were fed likewise for the length
of the experiment. The estimated dosage for the young animals was
10 mg/day and for mature animals 20 mg/day. The average life-span was
117 weeks for the treated animals and 109 weeks for the controls. F1
and F2 generations were studied, the survivors being sacrificed in
the 125th week. Table 17 summarizes the results. Three liver cell
carcinomas, two lung angiosarcomas and one other, and two malignant
gliomas were found in the group of 104 rats (F1 and F2) treated
with morpholine.
In a similar study using Syrian golden hamsters, only the F1
generation was studied, the survivors being sacrificed in the 110th
week (Shank & Newberne. 1976). With morpholine alone (1000 mg per kg),
no liver tumours were found but the number in the group (22) was
small.
Morpholine oleic acid salt (MOAS), at dosage levels of 0%, 0.25%
or 1.0%, was added to the drinking-water of B6C3F1 mice for 96
weeks, and this was followed by normal tap water for a further 8 weeks
(Shibata et al., 1987b). Details are given in Table 15 and section
7.3.1. Extensive biochemical, gross pathological and histological
studies were performed. Only the incidence of hyperplasia in
forestomach epithelium in the males of the 1% MOAS group was
statistically higher than in the controls; otherwise, no significant
increases in the incidence of non-neoplastic or neoplastic lesions
were found.
7.7.1.2 Inhalation studies
In a long-term inhalation study over 2 years, Sprague-Dawley rats
(70 of each sex per group) received morpholine at mean exposure
concentrations of 0, 0.036, 0.181 or 0.543 g/m3 (6 h/day,
5 days/week) for up to 104 weeks (Harbison et al., 1989). Further
details are given in section 7.3.2 and Table 15. Tissues from the
high-dose and control groups were subjected to extensive
Table 17. Tumour incidence (liver and lung) in rats and hamsters after feeding morpholine, sodium nitrite or N-nitrosomorpholinea
Dietary levels (mg/kg) Tumour incidence in rats (%) Tumour incidence in hamsters (%)
Morpholine Sodium N-Nitrosomorpholine No. of Liver cell Liver angio Lung angio No. of Liver cell
nitrite animalsb carcinoma sarcoma sarcoma animals carcinoma
0 0 0 156 0 0 0 23 4
0 1000 0 96 1 0 0 30 0
1000 0 0 104 3 0 2 22 0
1000 1000 0 159 97 14 23 16 31
50 1000 0 117 59 5 6 32 0
5 1000 0 154 28 12 8 400
1000 50 0 109 3 2 1 22 0
1000 5 0 172 1 2 1 19 0
50 50 0 152 2 1 1 30 0
5 5 0 125 1 2 2 40 0
0 0 5 128 58 15 9 35 0
0 0 50 94 93 21 20 18 6
a adapted from Shank & Newberne (1976)
b F1 and F2 generations together
histopathological examination; in the middle- and low-dose groups,
this examination was limited to the eye and the respiratory tract.
Ten males and 10 females per group were sacrificed at week 53.
Survival at termination in the control, low-, middle- and high-dose
groups, respectively, was 40, 44, 33 and 32 in males and 35, 27, 32
and 35 in females. No significant increase in the incidence of
tumours was seen in rats of either sex.
7.7.2 Morpholine and nitrite
7.7.2.1 Oral studies
Sander & Bürkle (1969) fed a group of seven female Sprague-Dawley
rats 5 g morpholine together with 5 g nitrite/kg diet for 12 weeks.
After 39 weeks, all of the animals developed hepato-cellular adenomas,
six out of the seven hepatocellular carcinomas, two
hemangioendotheliomas of the liver, one a cyst-adenocarcinoma of the
liver and one a renal adenoma. Rats fed morpholine or nitrite alone
did not develop tumours.
As described in section 7.7.1.1, Shank & Newberne (1976) fed
pregnant rats on a diet containing various concentrations of
morpholine, sodium nitrite and NMOR. Table 17 summarizes the dietary
concentrations and the tumour incidences for the experimental groups.
Hepatocellular carcinoma and haemangio-sarcomas of the liver and
angiosarcoma of the lungs were the most common tumours observed in the
rats. The neoplasms induced by nitrite and morpholine were
morphologically similar to those induced by NMOR. High concentrations
(1000 mg/kg) of morpholine and nitrite together were carcinogenic to
rats. When the morpholine concentration was reduced and the nitrite
concentration remained high, the incidence of hepatic cell carcinomas
decreased with a linear dose-response relationship. When morpholine
concentration remained high, with decreasing nitrite concentration,
the number of hepatic tumours was sharply reduced. This agrees with
the observation in vitro that the nitrosation of morpholine depends
on the square of the nitrite concentration (Mirvish et al., 1975).
Groups of 40 male MRC Wistar rats were treated for 2 years with
either 10 g morpholine/kg diet and drinking-water containing 3 g
sodium nitrite/litre, or with drinking-water containing 0.15 g
NMOR/litre. In both cases, one group of rats was also given sodium
ascorbate (22.7 g/kg diet). The results of treatment with morpholine
plus nitrite or with NMOR were similar to those reported above. When
ascorbate was present, the liver tumours induced by morpholine plus
nitrite had a longer induction period (93 versus 54 weeks) and a
slightly lower incidence (49% versus 65%). However, ascorbate did not
affect liver tumour induction by preformed NMOR. Of those treated
with morpholine, nitrite and ascorbate, 21/39 animals developed
forestomach tumours (Mirvish et al. 1976).
In a similar study using Syrian hamsters (see section 7.7.1.1 and
Table 17), a high morpholine, high nitrite diet (1000 mg/kg) induced
liver cancer in some animals. In contrast, hamsters fed NMOR in the
diet seemed to have greater resistance to tumour induction (Shank &
Newberne, 1976).
7.7.3 Carcinogenicity of N-nitrosomorpholine
NMOR has been shown to be carcinogenic in mice, rats, hamsters
and various fish. Benign and malignant tumours of the liver and lung
in mice, of the liver, kidney and blood vessels in rats, and of the
liver in hamsters have been reported following oral administration of
NMOR. After its subcutaneous injection, it produces tumours of the
upper digestive and respiratory tract in hamsters. NMOR produces liver
tumours following its intravenous injection in rats and liver tumours
in various fish following its administration in tank water (IARC,
1978).
7.8 Factors modifying toxicity; toxicity of metabolites
7.8.1 Factors modifying toxicity
Leaf et al. (1991) reported that rats (Sprague-Dawley, male)
challenged with an E. coli lipopolysaccharide (LPS; 1 mg/kg body
weight), followed 6 and 10 h later by morpholine (80 or 100 µmol/kg
intraperitoneally) and arginine (400 µmol/kg) treatment, showed
significantly increased NMOR formation compared with unchallenged
control rats. The NMOR formation was measured by monitoring the NMOR
metabolite N-nitroso-(2-hydroxyethyl) glycine in the urine. Furman
& Rubenchik (1991) showed that endogenous synthesis of NMOR in the
stomach of adult mice administered nitrite (oral) in combination with
morpholine (intraperitoneal or subcutaneous) was increased after
activation of peritoneal macrophages induced by intraperitoneal
injection of E. coli LPS.
7.8.2 Morpholine metabolites
Morpholine is eliminated almost entirely in a non-metabolized
form in the urine of the rat, mouse, hamster and rabbit (section 6.3).
N-cyanomorpholine ( N-morpholinocarbonitrile) was formed when
morpholine was incubated in vitro with whole human saliva (Wishnok &
Tannenbaum, 1976).
Sohn et al. (1982a,b) identified a metabolite
N-methylmorpholine- N-oxide (NMMO) in guinea-pig urine.
Conaway et al. (1984a) reported that NMMO (0.0001-10 mg/ml) and
other putative metabolites, N-hydroxymorpholine (0.0001-1 mg/ml) and
3-morpholinone (0.001-0.1 mg/ml), did not induce DNA repair at the
non-toxic concentrations tested. The polyurethane foam catalyst,
N-butylmorpholine (0.0001-0.1 mg/ml) was also inactive in the
primary rat hepatocyte/DNA repair assay (Conaway et al., 1984a).
The chemical intermediate N-hydroxyethylmorpholine induced DNA
repair within the dose range 1-5 mg/ml (Conaway et al., 1984a).
The genotoxicity of substituted morpholines might be a function
of the substituent moiety rather than morpholine itself (Conaway et
al., 1984a).
7.9 Mechanisms of toxicity - mode of action
The irritating and corrosive properties of morpholine are due to
its basicity. The mechanism of action of its systemic effects is not
known.
Morpholine fungicides have been shown to act by inhibiting
several enzymes of sterol biosynthesis (Hesselink et al. 1990; Mercer,
1991).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available on the effects of short- and long-term
exposure to morpholine on the general population. There are no
reports of poisonings by morpholine.
8.1.1 Controlled human studies
The Cosmetic Ingredient Review (1989, 1991b) reported unpublished
studies of occlusive patch testing and in-use testing of two mascara
products containing 1% morpholine by 320 women between the ages of 18
and 65. All 320 volunteer subjects underwent occlusive patch testing
(using the Shelanski/Jordan repeat insult procedure). After 24 h, the
patches were removed and the sites graded. This procedure was repeated
several times for a total of 10 applications (3´ weeks). Following the
10th application, there was a 10-day to 2-week rest period, after
which the subjects were again patch tested, this time for 48 h, and
then scored. After another 10-day to 2-week rest period, the subjects
were again patch-tested for 48 h and scored. A final reading took
place 24 h after this. Of the 320 subjects patch-tested with charcoal
mascara, 314 showed no reaction, the remaining six showed varying
reactions. The researchers explained this irritation as being either
non-specific or due to the occlusive patching procedure. The same
results were found with the blue mascara. It was concluded from this
study that neither of the mascara products containing 1% morpholine
was a primary irritant, nor were they contact sensitizers (Cosmetic
Ingredient Review, 1989, 1991b).
In the in-use testing, 50 women used charcoal mascara once daily
for 4 weeks, and 50 women used blue mascara likewise. A further 100
women used once daily lash conditioner, eye make-up remover and
mascara (50 for each colour). In another group, 100 subjects used
lash conditioner and eye make-up remover but no mascara. All underwent
the above-described patch testing simultaneously.
There were a few complaints associated with the in-use testing.
The charcoal mascara caused a mild burning sensation in three
subjects, some itching in a further three, and minor irritation in
three. These findings were put down to improper use of the product,
which resulted in the product entering the eye and causing mild
irritation (Cosmetic Ingredient Review, 1989, 1991b).
Shea (1939) exposed himself to 43 g morpholine/m3 (12 000 ppm)
for 90 seconds. He experienced irritation of the nose followed by
coughing. Pipetting of pure morpholine from the stock solution led to
inhalation of vapour, a severe sore throat, and violently reddened
mucous membrane.
Shea (1939) reported that when applied to human finger tips
undiluted morpholine caused a cracking of the eponychium and
hyponychium about the nail and an intense stinging sensation. Diluted
morpholine (1 to 40) was a mild irritant.
8.1.1.1 Organoleptic effects
Hellman & Small (1974) tested the absolute and recognition
threshold odour index of 101 petrochemicals using a trained panel.
During a 20-min exposure, 0.036 mg morpholine/m3 was recognized by
50% of the panel and 0.5 mg morpholine/m3 by all of the panel
(giving an odour index of 65.9). It was described as an unpleasant
fishy smell.
The phenomenon known as blue vision, gray vision or haloes
"glaucopsia" is a well-documented effect of amines, including
morpholine and its derivatives, on the eyes of workers, particularly
in the foam plastic industry (Mastromatteo, 1965; Jones & Kipling,
1972). The vision becomes misty and haloes appear several hours after
the subjects have been exposed to these vapours at concentrations too
low to cause discomfort or disability during several hours of
exposure. The disturbances lasted for 4-6 h after leaving work. In a
minority of the workers examined, mild conjunctival infection was
observed; no corneal oedema or alteration in visual acuity was
detected by inspection or by ophthalmoscopy. The atmospheric
concentrations of morpholine and other similar compounds were not
reported.
8.1.2 Epidemiological studies
No data from epidemiological studies for morpholine have been
reported.
8.2 Occupational exposure
Studies into the levels of morpholine and NMOR in workplace air
are described in section 5.3.
Katosova et al. (1991) reported a cytogenetic analysis of the
lymphocytes in the peripheral blood of 24 workers (16 men, 8 women)
with 3 to 10 years of exposure to morpholine during production. These
were compared to a control group of the same size from the same town
having no contact with chemicals at work. The morpholine workers were
exposed to concentrations of morpholine in air of 0.54-0.93 mg/m3,
the maximum single concentration being 0.74-2.14 mg/m3. The
lymphocytes were cultivated for 56 h and all chromatid and chromosome
types as well as chromosome gaps were registered. From 2561 cells
analysed from the test group, only 2.08% had aberrations, compared to
1.61% out of 2050 cells in the control group, showing no significant
increase in the number of cells with chromosome aberrations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The data reported in this section refer to nominal
concentrations.
9.1.1 Microorganisms
9.1.1.1 Microorganisms in water
a) Bacterial and cyanobacterial cultures
In a 16-h cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 25°C), the toxicity threshold (3% reduction in
population growth) of morpholine for Pseudomonas putida was
310 mg/litre (Bringmann & Kühn, 1977a).
The effect of morpholine at neutral pH on the growth of
Pseudomonas strains in succinate mineral salt medium has been
reported by Knapp et al. (1982) and Emtiazi & Knapp (1994). Both
studies used two strains of Pseudomonas (all four were different)
which had not previously been exposed to morpholine and found that
morpholine at 8.7 g/litre had no effect on the rate or extent of
growth.
The mixed bacterial cultures studied by Mazure (1993) were able
to degrade morpholine rapidly at 5, 10 and 20 g/litre. The
degradation rate was highest at 10 g/litre but was only a little less
at 20 g/litre. At 40 g/litre degradation was markedly inhibited.
As reported in section 4.2.1, morpholine at up to 870 mg/litre
does not inhibit certain strains of Mycobacterium spp. that have
been found to biodegrade the compound.
In a cell multiplication inhibition test (growth parameter:
turbidity; pH 7; 192 h) under continual lighting conditions using
Microcystis aeruginosa, a cyanobacterium, the onset of inhibition,
defined variously as a 1% (Bringmann, 1975) or 3% (Bringmann & Kühn,
1978) reduction in population growth, was found to occur at a
morpholine concentration of 1.7 mg/litre.
b) Activated sludge
The effect of morpholine on the respiratory, dehydrogenase and
nitrification activities of activated sludge was determined by
Strotmann et al. (1993). Short-term respiration assays (pH 7.0;
incubation time 30 min) were performed according to the OECD guideline
209 (OECD, 1984). The dehydrogenase activity was determined by
measuring the reduction of resazurin to resorufin. At concentrations
of 1000 mg morpholine/litre, both respiratory and dehydrogenase
activities were inhibited by up to 20%. A pH-dependent inhibitory
effect on the dehydrogenase activity was not found (pH 6.0-7.5).
Nitrification activity was determined using a specially adapted
culture of nitrifying bacteria. Suspended biomass, previously
inoculated with activated sludge and acclimated to a high-strength
ammonium feed, was incubated for 30 min at pH 7.2 with different
amounts of morpholine. The extent of inhibition was based on the
reduction in oxygen uptake rate, compared to a control containing no
morpholine. Nitrification activity was inhibited by 5% at a
morpholine concentration of 5%.
c) Protozoa
The toxicity thresholds (defined as a 5% reduction in population
growth) of morpholine for the protozoa Entosiphon sulcatum
(Bringmann, 1978), Uronema parduczi and Chilomonas paramaecium
(Bringmann & Kühn (1980), as measured in cell multiplication
inhibition tests under given conditions, are summarized in Table 18.
9.1.1.2 Microorganisms in soil
No data are available concerning soil bacteria or fungi.
9.1.1.3 Pathogenic microorganisms
Morpholine, like other amines, shows antibacterial and anti-
mycotic action. Kubis et al. (1983) demonstrated that 0.5% morpholine
inhibits the growth of a variety of pathogenic bacteria on agar plates
and in liquid medium. Application of 10% morpholine was shown to cure
an experimental mycosis in guinea-pigs caused by Trychophyton
mentagrophytes var. granulosum (Kubis et al., 1981). The pH was not
specified.
9.1.2 Other aquatic organisms
9.1.2.1 Monocellular green algae
Table 19 summarizes the tests carried out concerning the effect
of morpholine on algae.
In a 192-h test, the onset of inhibition by morpholine
(in distilled water, pH 7.0) of cell multiplication (here defined as
3% in population growth) in the green alga Scenedesmus quadricauda
in a turbidity test was noted at a concentration of 4.1 mg/litre
(Bringmann & Kühn, 1977a, 1978; see parallel results test with
Microcystis aeruginosa, section 9.1.1.1a).
Table 18. Toxicity threshold (TT) of morpholine for protozoa in the cell multiplication
inhibition test (growth factor: cell number)
Species Test duration pH Temperature TT Reference
(h) (°C) (mg/litre)
Entosiphon sulcatuma 72 6.9 25 12 Bringmann (1978)
Uronema parduczia 20 6.9 25 815 Bringmann & Kühn (1980)
Chilomonas paramaeciumb 48 6.9 20 18 Bringmann et al. (1980)
a bacteriovorous ciliate
b saprozoic flagellate
The results of Adams et al. (1985) showed that Selenastrum
capricornutum grows exponentially without a lag phase in the
presence as well as in the absence of morpholine, the growth being
inhibited first at a concentration of 100 mg/litre. The inhibition was
most marked after 6 days, shortly before reaching the stationary
phase; the no-observed-effect level (NOEL) was 10 mg/litre. Using the
same species, Calamari et al. (1980) evaluated algal growth by
measuring in vivo the fluorometric units at 48, 72, 96 h and 7 days.
A 96-h EC50 of 28 mg/litre was determined.
Millington et al. (1988) investigated the effects of varying
growth medium composition on the toxicity of morpholine to three
freshwater green algae: Selenastrum capricornutum, Scenedesmus
subspicatus and Chlorella vulgaris. They reported that the toxic
effect of morpholine on algae depended upon the species as well as
upon the test medium used (see Table 19).
9.1.2.2 Invertebrates
Table 20 summarizes the results of three investigations into the
toxicity of morpholine, as measured by the immobilization of Daphnia
magna (Bringmann & Kühn, 1977b, 1982; Calamari et al., 1980). The
test conditions were almost identical (pH 7.6-8.0; 18-22°C) and the
resulting EC50 values (100-119 mg/litre) were in good agreement.
Kramer et al. (1983) investigated the relative toxicity of
organic solvents including morpholine to mosquito (Aedes aegypti)
larvae (2 to 3 instar) by exposing 10-20 of them to 50 ml of test
solution in a glass container for 4 h at 22-24°C. The test was run in
triplicate. An LC50 of 1000 mg/litre was reported, together with
the observation that death was accompanied by convulsive larval
twitching.
9.1.2.3 Vertebrates
The acute toxicity of morpholine for fish has been tested on a
number of fresh, sea and brackish water species (see Table 21). The
lowest LC50 (180 mg/litre) was found for rainbow trout
(Oncorhynchus mykiss; formerly Salmo gairdneri) in very soft water
(20 mg CaCO3/litre). In hard water (320 mg CaCO3/litre), the
poisoning effect, with reference to the LC50 value, was less than
half (Calamari et al., 1980). Brachydanio rerio proved to be
relatively insensitive (LC0 value > 1000 mg/litre) (Wellens, 1982).
LC50 values for the freshwater fish Leuciscus idus melanotus and
Lepomis macrochirus were found to be 240-285 mg/litre (48 h) and
350 mg/litre (96 h), respectively (Juhnke & Lüdemann, 1978; Dawson et
al., 1977). The 96-h LC50 for the marine fish Menidia beryllina
was reported to be 400 mg/litre (Dawson et al., 1977). McCain & Peck
(1976) investigated the effect of morpholine on common Hawaiian fish.
Table 19. Toxicity of morpholine for algaea
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Scenedesmus inhibition of cell 192 neutral 27 TT: 4.1 Bringmann & Kühn (1978)
quadricauda multiplication (turbidity)
Selenastrum cell number after: 24 not given 22 NOEC: 100 Adams et al. (1985)
capricornutum 48/72 NOEC: 80
96/120 NOEC: 50
144 NOEC: 10
area under 24-72 NOEC: 80
growth curve 24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 50
growth rate 24-72 NOEC: 80
24-96 NOEC: 80
24-120 NOEC: 80
24-144 NOEC: 80
S. capricornutum growth rate (in vivo 96 not given 24 EC0: 10c Calamari et al. (1980)d
fluorescence) EC50: 28c
EC100: 80c
S. capricornutum area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 50f
ECL0: 50g
Scenedesmus area under growth 24-120 not given 22 ECL0: 50e Millington et al. (1988)
subspicatus curve (cell number) ECL0: 5f
ECL0: 10g
Table 19 (cont'd)
Species Growth parameters Test duration pH Temperature Effectb/concentration Reference
(h) (°C) (mg/litre)
Chlorella vulgaris area under growth 24-120 not given 22 ECL0: 100e Millington et al. (1988)
curve (cell number) ECL0: 80f
ECL0: 5g
a Adapted from BUA (1991)
b TT = toxicity threshold; NOEC = no-observed-effect concentration; EC0, EC50, EC100 = effective concentration inhibiting for the growth
of 0, 50 and 100% of the population; ECL0 = lowest-tested concentration with significant growth inhibition
(from 5, 10, 50, 80, 100 mg/litre)
c equivalent to GC-FID measured concentration ± 10%
d method according to Chiaudini & Vighi (1978)
e Bolds basal medium (BBM): rich medium; N/P-ratio 14:1
f OECD medium: less N than BBM; N/P-ratio 24:1
g EPA medium: less N than BBM; N/P-ratio 50:1
Table 20. Acute toxicity of morpholine for the water flea Daphnia magna
(test duration: 24 h; effect: immobilization)a
Test systemb pH Temperature Effect/concentration Reference
(°C) (mg/litre)
Static 7.6-7.7 20-22 EC0: 16 Bringmann &
EC50: 100 Kühn (1977b)
EC100: 500
Static 8 ± 0.2 20 EC0: 68 Bringmann &
EC50: 101 (83-122)c Kühn (1982)
EC100: 260
Static 7.9 ± 0.3 18-22 EC50: 119 (112-127)c Calamari et
al. (1980)
a adapted from BUA (1991)
b in accordance with OECD-guideline 202 part I
c 95% confidence limits
The 96-h LC50 (TLm) was between 100 and 180 mg/litre for white
mullet (Vala mugil engeli; former designation: Chelon engeli),
between 320 and 560 mg/litre for Gambusia affinis, and more than
1000 mg/litre for Tilapia sp.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Reynolds (1989) found that 4.4±0.9 mol morpholine/m3
(383 mg/litre) lowers the percentage germination of lettuce seeds
(Lactuca sativa) by 50% of the control value. The seeds were kept on
agar for 3 days in sealed plastic containers at a temperature of 30°C.
9.1.3.2 Animals
No data are available concerning the effects of morpholine on
terrestrial animals.
9.2 Field observations
No data have been reported concerning field observations
involving morpholine.
Table 21. Acute toxicity of morpholine in fish static test systemsa
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
Rainbow trout freshwater hardness: 320 mg CaCO3/litre; pH 7.4; 96 LC50 380 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (375-460)b (1980)
Rainbow trout freshwater hardness: 20 mg CaCO3/litre; pH 7.4; 96 LC50 180 Calamari et al.
(Salmo gairdneri) 15°C; > 95% O2 saturation (1980)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 285 Juhnke &
(Leuciscus idus continual aeration (Juhnke laboratory) (250/340)d Lüdemann
melanotus) (1978)
Golden orfe freshwaterc hardness: 220-320 mg CaCO3/litre; pH 8; 20°C; 48 LC50 240 Juhnke &
(L. idus melanotus) continual aeration (Lüdemann laboratory) (100/301)d Lüdemann (1978)
Zebra fish freshwater hardness: 250 mg CaCO3/litre; pH 7.5; 22°C; 96 LC50> 1000 Wellens (1982)
(Brachydanio rerio) no aeration (> 1000/> 1000)d
Bluegill sunfish freshwater hardness: 55 mg CaCO3/litre; pH 7.6-7.9; 23°C; 96 LC50 350 Dawson et
(Lepomis macrochirus) intermittent aeration 24 h after test; from al. (1977)
commercial hatcheries; 14 days acclimatization
Tidewater silverside marine artificial seawater; 20°C; continual aeration; 96 LC50 400 Dawson et
(Menidia beryllina) caught wild Horseshoe Bay (New Jersey); al. (1977)
40-100 mm length; 14 days acclimatization
Mosquito fish marinee,f natural seawater (32% salinity); continual 96 LC0 320 McCain &
(Gambusia affinis) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 560 Peck (1976)
Hawaii); 38-64 mm length; 7 days
acclimatization
Table 21 (cont'd)
Species Aquatic system Test conditions Test Effect/ Reference
duration concentration
(h) (mg/litre)
"Coloured perch" marinee,f natural seawater (32% salinity); continual 96 LC0 1000 McCain &
(Tilapia sp.) aeration; 25.5-27.8°C; caught wild (Oahu, Peck (1976)
Hawaii); 30-65 mm length; 7 days
acclimatization
White mullet marinee natural seawater (32% salinity); continual 96 LC0 100 McCain &
(Chelon engeli) aeration; 25.5-27.8°C; caught wild (Oahu, LC100 320 Peck (1976)
Hawaii); 91-128 mm length; 7 days
acclimatization
a adapted from BUA (1991)
b 95% confidence limits
c parallel tests in two different laboratories
d LC0/LC100
e the fish were caught in rivers, presumably in the estuary area
f fresh or brackish water fish that were exposed to the marine system.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks were evaluated by an International Agency
for Research on Cancer ad hoc Expert Group in 1988. It was
concluded that there is inadequate evidence for the carcinogenicity
of morpholine in experimental animals. No data were available from
studies in humans on the carcinogenicity of morpholine. The overall
evaluation was that morpholine was not classifiable for its
carcinogenicity to humans (IARC, 1989).
IARC also evaluated N-nitrosomorpholine (NMOR). It concluded
that there is sufficient evidence for a carcinogenic effect of NMOR in
several experimental animal species. No human data, case reports or
epidemiological studies were available, nor was it possible to
identify exposed groups. In spite of the absence of epidemiological
data, NMOR should be regarded for practical purposes as if it were
carcinogenic to humans (IARC, 1978).
REFERENCES
Aarts AJ, Benson GB, Duchateau NL, & Davies KM (1990) Modern
analytical techniques for the detection of rubber chemicals and their
decomposition products in the environment. Int J Environ Anal Chem,
38: 85-95.
Ackermann P, Margot P, & Klotzsche, C (1989) Agricultural fungicides.
In: Ullmann's encyclopedia of industrial chemistry, 5th ed. Weinheim,
VCH Verlagsgesellschaft, vol A12, p 107.
Adams N, Goulding KH, & Dobbs AJ (1985) Toxicity of eight water-
soluble organic chemicals to Selenastrum capricornutum: a study of
methods for calculating toxic values using different growth
parameters. Arch Environ Contam Toxicol, 14: 333-345.
Agarwala KK (1982) Study on stability of morpholine in high pressure
boiler system. Fertil Technol, 19: 73-75.
Air Products and Chemicals (1989) Material safety data sheet.
Allentown, Pennsylvania, Air Products and Chemicals, Inc.
Aitzetmüller K & Thiele E (1982) Absence of volatile N-nitrosamines in
margarines. J Am Oil Chem Soc, 59: 387-389.
Anderson GE (1983) Human exposure to atmospheric concentrations of
selected chemicals. Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Quality Planning and
Standards, pp 455-473 (Report submitted by Systems Applications, Inc.,
San Rafael, California) (EPA No 83-265249).
Archer MC, Tannenbaum SR, & Wishnok JS (1977) Nitrosamine formation in
the presence of carbonyl compounds. In: Walker EA, Bogovski P, &
Griciute L ed. Environmental N-nitroso compounds analysis and
formation. Lyon, International Agency for Research on Cancer,
pp 141-145 (IARC Scientific Publications No. 14).
Archer MC, Yang HS, & Okun JD (1979) Acceleration of nitrosamine
formation at pH 3.5 by microorganisms. In: Walker EA, Castegnaro M,
Griciute L, & Lyle RE ed. Environmental aspects of N-nitroso
compounds. Lyon, International Agency for Research on Cancer,
pp 239-246 (IARC Scientific Publications No. 19).
Atkinson R (1988) Estimation of gas-phase hydroxyl radical rate
constants for organic chemicals. Environ Toxicol Chem, 7: 435-442.
Badura R, Linde H, & Schuster RH (1989) Analysis of volatile products
in vulcanisation fumes. In: Proceedings of the International Rubber
Conference, Prague, 8-11 May. London, International Rubber
Association, pp 19-38.
BASF (1967) [Morpholine: Toxicological data.] Ludwigshafen, BASF AG,
2 pp (Internal report) (in German).
BASF (1987) [Data sheets: Morpholine.] Ludwigshafen, BASF AG, 8 pp
(in German).
BASF (1990) [Test report: Zahn-Wellens-Test.] Ludwigshafen, BASF AG,
12 pp (Unpublished report) (in German).
BIA (Industries Association Institute for Work Safety) (1989)
[Measurement of dangerous substances - BIA data sheet.] Bielefeld,
Erich Schmidt Verlag (in German).
Bianchi A & Muccioli G (1978) [Determination of morpholine together
with isopropyl alcohol, toluene and xylenes (o-m-p) in the atmosphere
by means of gas-chromatography.] Ann Ist Super Sanita, 14: 441-445
(in Italian with English summary).
Börzsönyi M, Török G, Surján A, Challis BC, & Bär V (1981) Protective
effect of a new antioxidant on acute hepatotoxicity caused by
morpholine plus nitrite in rats. Toxicol Lett, 7: 285-288.
Bosholm J (1983) [Description of the behaviour of gaseous compounds in
water/steam systems.] Kernenergie, 26: 198-201 (in German).
Boyland E, Nice E, & Williams K (1971) The catalysis of nitrosation by
thiocyanate from saliva. Food Cosmet Toxicol, 9: 639-643.
Braun R, Schöneich J, & Ziebarth D (1977) In vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Bringmann G (1975) [Determination of the biological effect of water
pollutants on blue algae Microcystis using the cell multiplication
inhibition test.] Gesund-Ing, 96: 238-241 (in German).
Bringmann G (1978) [Determination of the biological effect of water
pollutants in protozoa I Bacteriovorous flagellate protozoa (Model
organism: Entosiphon sulcatum Stein).] Z Wasser Abwasser Forsch,
11: 210-215 (in German).
Bringmann G & Kühn R (1977a) [Toxicity thresholds of water pollutants
for bacteria (Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1977b) [Results of toxic action of water
pollutants on Daphia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1978) [Toxicity thresholds of water pollutants
for blue algae (Microcystis aeruginosa) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) [Determination of the biological effect of
water pollutants in protozoa. II. Bacteriovorous ciliates.] Z Wasser
Abwasser Forsch, 1: 26-31 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic action of water
pollutants on Daphnia magna tested by an improved standardized
procedure system.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa III Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German).
Brouwer R, Marquart H, de Mik G, & van Hemmen J (1992) Risk assessment
of dermal exposure of greenhouse workers to pesticides after re-entry.
Arch Environ Contam Toxicol, 23: 273-280
Brown AR (1966) Morpholine: its properties and uses. Manuf Chem
Aerosol News, Dec 1966: 50-52.
Brown VR & Knapp JS (1990) The effect of withdrawal of morpholine from
the influent and its reinstatement on the performance and microbial
ecology of a model activated sludge plant treating a morpholine-
containing influent. J Appl Bacteriol, 69: 43-53.
Brown VR, Knapp JS, & Heritage J (1990) Instability of the morpholine-
degradative phenotype in mycobacteria isolated from activated sludge.
J Appl Bacteriol, 69: 54-62.
Brunnemann KD & Hoffmann D (1991) Decreased concentrations of
N-nitrosodiethanolamine and N-nitrosomorpholine in commercial tobacco
products. J Agric Food Chem, 39: 207-208.
Brunnemann KD, Scott JC, & Hoffmann D (1982) N-nitroso-morpholine
and other volatile N-nitrosamines in snuff tobacco. Carcinogenesis,
3: 693-696.
BUA (Society of German Chemists, Advisory Committee on Existing
Chemicals of Environmental Relevance) (1991) [Morpholine.] Weinheim,
VCH Verlagsgesellschaft, 181 pp (BUA Report No. 56) (in German).
Calamari D, Da Gasso R, Galassi S, Provini A, & Vighi M (1980)
Biodegradation and toxicity of selected amines on aquatic organisms.
Chemosphere, 9: 753-762.
Calmels S, Ohshima H, Vincent P, Gounot A-M, & Bartsch H (1985)
Screening of micro-organisms for nitrosation catalysis at Ph 7 and
kinetics studies on nitrosamines formation from secondary amines by
E. coli strains. Carcinogenesis, 6: 911-915.
Calmels S, Ohshima H, Crespi M, Leclerc H, Cattoen C, & Bartsch H
(1987) N-nitrosoamine formation by microorganisms isolated from
human gastric juice and urine: biochemical studies on bacteria-
catalysed nitrosation. In: Bartsch H, O`Neill IK, & Schulte-Hermann R
ed. The relevance of N-nitroso compounds to human cancer: Exposures
and mechanisms. Lyon, International Agency for Research on Cancer,
pp 391-395 (IARC Scientific Publications No. 84).
Calmels S, Ohshima H, & Bartsch H (1988) Nitrosamine formation by
denitrifying and non-denitrifying bacteria: Implication of nitrite
reductase and nitrate reductase in nitrosation catalysis. J Gen
Microbiol, 134: 221-226.
Calmels S, Dalla Venezia N, & Bartsch H (1990) Isolation of an enzyme
catalysing nitrosamine formation in Pseudomonas aeruginosa and
Neisseria mucosae. Biochem Biophys Res Commun, 171(2): 655-660.
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991a) Bacterial
formation of N-nitroso compounds in the rat stomach after omeprazole-
induced achlorhydria. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 187-191 (IARC Scientific Publications No. 105).
Calmels S, Béréziat J-C, Ohshima H, & Bartsch H (1991b) Bacterial
formation of N-nitroso compounds in the rat stomach after
omeprazole-induced achlorhydria. Carcinogenesis, 12: 435-439.
Cech JS & Chudoba J (1988) Effect of the solids retention time on the
rate of biodegradation of organic compounds. Acta Hydrochim Hydrobiol,
16: 313-323.
Cech JS, Hartman P, Slosárek M, & Chudoba J (1988) Isolation and
identification of a morpholine-degrading bacterium. Appl Environ
Microbiol, 54: 619-621.
Challis BC & Kyrtopoulos SA (1979) The chemistry of nitroso-compounds.
Part 11. Nitrosation of amines by the two-phase interaction of amines
in solution with gaseous oxides of nitrogen. J Chem Soc Perkin Trans
I, 1979: 299-304.
Challis BC & Outram JR (1979) The chemistry of nitroso-compounds. Part
15. Formation of N-nitrosoamines in solution from gaseous nitric oxide
in the presence of iodine. J Chem Soc Perkin Trans I, 1979: 2768-2775.
Chemical Marketing Reporter (1989) Aromatics. Morpholine. Chem Mark
Rep, July 17: 13.
Chemical Marketing Reporter (1990) Dow to exit morpholine. Chem Mark
Rep, May 28: 7.
Chiaudani G & Vighi M (1978) The use of Selenastrum capricornutum
batch cultures in toxicity studies. Mitt Int Ver Limnol, 21: 316-329.
Collaert B, Attström R, De Bruyn H, & Movert R (1992a) The effect of
delmopinol rinsing on dental plaque formation and gingivitis healing.
J Clin Periodontol, 19: 274-280.
Collaert B, Edwardsson S, Attström R, Hase J, Aström M, & Movert R
(1992b) Rinsing with delmopinol 0.2% and chlorhexidine 0.2%: Short-
term effect on salivary microbiology, plaque, and gingivitis.
J Periodontol, 63: 618-625.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982a) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Presented at the 13th Annual
Meeting of the Environmental Mutagen Society, Boston, 22-28 February
1982. New York, John Wiley and Sons, pp 1-15.
Conaway CC, Myhr BC, Rundell JO, & Brusick DJ (1982b) Evaluation of
morpholine, piperazine and analogues in the L5178Y mouse lymphoma
assay and Balb/3T3 transformation assay. Environ Mutagen, 4: 390
(Abstract).
Conaway CC, Tong C, & Williams GM (1984a) Evaluation of morpholine,
3-morpholine, and N-substituted morpholines in the rat hepatocyte
primary culture/DNA repair test. Mutat Res, 136: 153-157.
Conaway CC, Coate WB, & Voelker RW (1984b) Subchronic inhalation
toxicity of morpholine in rats. Fundam Appl Toxicol, 4: 465-472.
Cooney RV & Ross PD (1987) N-nitrosation and N-nitration of morpholine
by nitrogen dioxide in aqueous solution: effects of vanillin and
related phenols. J Agric Food Chem, 35: 789-793.
Cooney RV, Ross PD, Bartolini GL, & Ramseyer J (1987) N-nitrosoamine
and N-nitroamine formation: Factors influencing the aqueous reactions
of nitrogen dioxide with morpholine. Environ Sci Technol, 21: 77-83.
Cooney RV, Ross PD, Hatch-Pigott V, & Ramseyer J (1992) Carcinogenic
N-nitrosamine formation: A requirement for nitric oxide. J Environ Sci
Health, 27(3): 789-801.
Cosmetic Ingredient Review (1986) Scientific literature review on
morpholine. Washington, DC, The Cosmetic, Toiletry and Fragrance
Association, 71 pp.
Cosmetic Ingredient Review (1989) Final report on the safety
assessment of morpholine. J Am Colloq Toxicol, 8: 707-748.
Cosmetic Ingredient Review (1991a) CIR annual report 1991. Washington,
DC, The Cosmetic, Toiletry & Fragrance Association, 67 pp.
Cosmetic Ingredient Review (1991b) Protocol: Repeated insult patch
testing and in-use testing. Washington, DC, The Cosmetic, Toiletry &
Fragrance Association, 16 pp (Unpublished report).
Cusano F & Luciano S (1993) Contact dermatitis from pramoxine. Contact
Dermatitis, 28: 39:
Davies R, Massey RC, & McWeeny DJ (1980) The catalysis of the
N-nitrosation of secondary amines by nitrosophenols. Food Chem,
6: 115-122.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J Hazard Mater, 1: 303-318.
Dmitrenko GN & Gvozdyak P (1988) Detection of morpholine by
mycobacteria. In: Proceedings of a Conference on Microbiological
Methods for Protecting the Environment, Puschino, USSR, 5-7 April
1988. Puschino, Scientific Centre for Biological Research of the
Academy of Sciences, 141 pp.
Dmitrenko GN, Udod VM, & Gvozdyak PI (1985) Destruction of morpholine
by fixed bacteria. Khim Tekhnol Vody, 7: 71-73.
Dmitrenko GN, Gvodzdyak PI, & Udod VM (1987) Selection of destructor
microorganisms for heterocyclic xenobiotics. Khim Tekhnol Vody,
9(5): 442-445.
Dodson JJ & Bitterman ME (1989) Compound uniqueness and the
interactive role of morpholine in fish chemoreception. Biol Behav,
14: 13-27.
Donath G, Heitmann HG, Messer J, & Schott M (1977) [Determination of
the distribution coefficients of volatile alkalising agents used in
power plants between steam and water phases and their importance for
the corrosive behaviour of materials.] Vom Wasser, 49: 221-243
(in German).
Dropkin D (1985) Sampling of automobile interiors for organic
emissions. Research Triangle Park, North Carolina, US Environmental
Protection Agency, 20 pp (PB85-172567).
Dynamac Corporation (1988) Information review: Morpholine (EPA
contract No. 68-02-4251). Rockville, Maryland, Dynamac Corporation,
pp 1-52 (IR-514) (Report prepared for TSCA Interagency Testing
Committee).
ECETOC (1991) Critical evaluation of methods for the determination of
N-nitrosamines in personal care and household products. Brussels,
European Chemical Industry Ecology and Toxicology Centre (Technical
Report No. 42).
EEC (1983) Council directive 79831 - Annex V, Part C: Methods for
determination of ecotoxicity. 5.2 Degradation - Biotic degradation
Manometric Respirometry. Brussels, Commission of the European Union.
EEC (1990) Proposal for a council directive on the approximation of
the laws of the Member States relating to cosmetic products (SEC(90)
1985 final) (90/C 322/06). Off J Eur Communities, C/322: 29-43.
Edwards G, Whong W-Z, & Speciner N (1979) Intrahepatic mutagenesis
assay: a sensitive method for detecting N-nitrosomorpholine and
in vivo nitrosation of morpholine. Mutat Res, 64: 415-423.
Emtiazi G (1993) Microbial degradation of linear and cyclic amines.
Leeds, University of Leeds, Department of Microbiology (Thesis).
Emtiazi G & Knapp JS (1994) The biodegradation of piperazine and
structurally-related linear anc cyclic amines. Biodegradation,
5: 83-92.
Environment Agency, Japan (1980) Annual report on chemicals in the
environment. Tokyo, Environment Agency, pp 72-73.
Estrin NF, Haynes CR, & Whelan JM (1982) Specifications/spectra. CTFA
compendium of cosmetic ingredient composition. Washington, DC, The
Cosmetic, Toiletry and Fragrance Association, Inc.
Fadlallah S, Cooper SF, Fournier M, Drolet D, & Perrault G (1990)
Determination of N-nitroso compounds in the environment of a metal
factory using metalworking fluids. Int J Environ Anal Chem,
39: 281-288.
Fajen JM, Carson GA, Rounbehler DP, Fan TY, Vita R, Goff UE, Wolf MH,
Edwards GS, Fine DH, Reinhold V, & Biemann K (1979) N-nitrosamines in
the rubber and tire industry. Science, 205: 1262-1264.
Fan TY, Vita R, & Fine DH (1978) C-nitro compounds: a new class of
nitrosating agents. Toxicol Lett, 2: 5-10.
Fisher AA (1986) Contact Dermatitis. Philadelphia, Pennsylvania, Lea &
Febiger.
Furman MA & Rubenchik BL (1991) Formation of carcinogenic N-nitro
compounds after intraperitoneal administration of nitrosating
precursors in C57BLl/6 line mice. Eksp Onkol, 13(2): 14-22.
Gavinelli M, Fanelli R, Bonfanti M, Davoli E, & Airoldi L (1988)
Volatile nitrosamines in foods and beverages: preliminary survey of
the Italian market. Bull Environ Contam Toxicol, 40: 41-46.
Gilbert R & Saheb SE (1987) Field measurement of the distribution
coefficients of chemical additives used for corrosion control in
steam-water cycles. Mater Perform, 26: 30-36.
Gilbert R, Rioux R, & Saheb SE (1984) Ion chromatographic
determination of morpholine and cyclohexylamine in aqueous solutions
containing ammonia and hydrazine. Anal Chem, 56: 106-109.
Glatt HR & Oesch F (1981) [Ames test for morpholine.] Ludwigshafen,
BASF AG, 11 pp (Unpublished report submitted by the Pharmacological
Institute, University of Mainz, Germany).
Grant WM (1974) Morpholine. In: Toxicology of the eye, 2nd ed.
Sringfield, Illinois, Charles C. Thomas,vol 2, pp 722-723.
Greenblatt M, Mirvish S, & So BT (1971) Nitrosamine studies: induction
of lung adenomas by concurrent administration of sodium nitrite and
secondary amines in Swiss mice. J Natl Cancer Inst, 46: 1029-1034
(Abstract).
Griffiths MH (1968) The metabolism of N-triphenylmethylmorpholine in
the dog and rat. Biochem J, 108: 731-740.
Grodeckaja NS & Karamzina NM (1973) [Initial reactions by the organism
to the effects of industrial substances in concentrations of minimal
effect (Limac, Limch).] Toksikol Nov Prom Chim Veshchestv,
13: 12-23 (in Russian).
Groenen PJ, Busink E, & van Wandelen M (1987) Determination of
volatile nitrosamines in cheese and cured meat products. Model study
of a temperature- and pH-dependent artefact formation phenomenon in
alkaline medium. Z Lebensm.Unters Forsch, 185: 24-30.
Grosjean D (1991) Atmospheric chemistry of toxic contaminants.
6. Nitrosamines: Dialkyl nitrosamines and nitrosomorpholine. J Air
Waste Manage Assoc, 41: 306-311.
Hamano T, Mitsuhashi Y, & Matsuki Y (1980) Improved gas
chromatographic method for the quantitative determination of secondary
amines as sulphonamides formed by reaction with benzene-sulphonyl
chloride. J Chromatogr, 190: 462-465.
Hamano T, Mitsuhashi Y, & Matsuki Y (1981) Glass capillary gas
chromatography of secondary amines in foods with flame photometric
detection after derivatization with benzenesulfonyl chloride. Agric
Biol Chem, 45: 2237-2243.
Hansen L, Akesson B, Sollenberg J, & Lundh T (1986) Determination of
N-methylmorpholine in air samples from a polyurethane foam factory.
Scand J Work Environ Health, 12: 66-69.
Harbison RD, Marino DJ, Conaway CC, Rubin LF, & Gandy J (1989) Chronic
morpholine exposure of rats. Fundam Appl Toxicol, 12: 491-507.
Hassan SSM, Tadros FS, & Selig W (1985) Microdetermination of
secondary aliphatic amines using a copper ion-selective electrode.
Microchem J, 31: 1-6.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hazleton (1981) Final report: 9-day acute inhalation toxicity study in
rats. Vienna, Virginia, Hazleton Laboratories America, Inc., 26 pp
(Submitted to Texaco Chemical Company).
Hecht SS & Morrison JB (1984) A sensitive method for detecting
in vivo formation of N-nitrosomorpholine and its application to
rats given low doses of morpholine and sodium nitrite. Cancer Res,
7: 2873-2877.
Hecht SS & Young R (1981) Metabolic alpha-hydroxylation of
N-nitrosomorpholine and 3,3,5,5-tetradeutero-N-nitrosomorpholine in
the F344 rat. Cancer Res, 41: 5039-5043.
Heilen G, Mercker HJ, Frank D, Reck RA, & Jäckh R (1989) [Amines,
aliphatic.] In: [Ullmann's encyclopedia of industrial chemistry], 5th
ed. Weinheim, VCH Verlagsgesellschaft, vol A2, pp 1-36 (in German).
Hellman TM & Small FH (1974) Characterization of the odor properties
of 101 petrochemicals using sensory methods. J Air Pollut Control
Assoc, 24: 979-982.
Hesselink PGM, Kerkenaar A, & Witholt B (1990) Inhibition of microbial
cholesterol oxidases by dimethylmorpholines. J Steroid Biochem,
35: 107-113.
Hibbs JB Jr (1992) Immunology: Overview of cytotoxic mechanisms and
defence of the intracellular environment against microbes. In: Moncada
S, Marletta MA, Hibbs JB Jr, Higgs EA ed. The biology of nitric oxide.
London,d Chapel Hill, Portland Press, pp 201-206.
Hoffmann D, Brunnemann KD, Adams JD, Rivenson A, & Hecht SS (1982)
N-nitrosamines in tobacco carcinogenesis. In: Magee PN ed.
Nitrosamines and human cancer. Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp 211-225 (Banbury Report No. 12).
Hoffmann D, Adams JD, Lisk D, Fisenne I, & Brunnemann KD (1987) Toxic
and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst,
79: 1281-1286.
Hollett BA, Klemme JC, & Andjelkovich D (1982) Health hazard
evaluation report HETA 81-045B-1216: Uniroyal, Inc., Mishawaka,
Indiana. Cincinnati Ohio, National Institute for Occupational Safety
and Health, 20 pp (PB84-183615).
Hotchkiss JH & Vecchio AJ (1983) Analysis of direct contact paper and
paperboard food packaging for N-nitrosomorpholine and morpholine. J
Food Sci, 48: 240-242.
IARC (1978) N-nitrosomorpholine. In: Some N-nitroso compounds. Lyon,
International Agency for Research on Cancer, pp 263-280 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 17).
IARC (1989) Morpholine. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 199-213 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office, pp 282-283 (Occupational
Safety and Health Series No. 37).
Inui N, Nishi Y, Taketomi M, Mori M, Yamamoto M, Yamada T, & Tanimura
A (1979) Transplacental mutagenesis of products formed in the stomach
of golden hamsters given sodium nitrite and morpholine. Int J Cancer,
24: 365-372.
Iqbal ZM, Dahl K, & Epstein SE (1980) Role of nitrogen dioxide in the
biosynthesis of nitrosamines in mice. Science, 207(4432): 1475-1476.
Jakobi G, Löhr A, Schwuger MJ, Jung D, Fischer W, & Gloxhuber C (1983)
[Washing agents.] In: [Ullmann's encyclopedia of industrial
chemistry], 4th ed. Weinheim, VCH Verlagsgesellschaft, vol 24, pp 105,
138-139 (in German).
Japan Chemical Week (1991) Speciality chemicals handbook, 4th ed.
Tokyo, The Chemical Daily Co., Ltd, 8 pp.
Jones WT & Kipling MD (1972) Glaucopsia - blue-grey vision. Br J Ind
Med, 29: 460-461.
Juhnke I & Lüdemann D (1978) [Results of the testing of 200 selected
chemical compounds for acute fish toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Kamimura H, Enjoji Y, Sasaki H, Kawai R, Kaniwa H, Niigata K, &
Kageyama S (1987) Disposition and metabolism of indeloxazine
hydrochloride, a cerebral activator, in rats. Xenobiotica,
17(6): 645-658.
Karweik DH & Meyers CH (1979) Spectrophotometric determination of
secondary amines. Anal Chem, 51: 319-320.
Katosova LD, Fomenko VN, & Davydenko LN (1991) Air pollution and
industrial hygiene. Gig Tr Prof Zabol, 6: 35-36.
Kleemann A & Engel J (1982) [Midodrin, Minaprin, Minocyclin;
Trimethobenzamid, Trimethoprin, Trimetozin, Trimipramin,
Tripelennamin.] In: [Pharmaceutical substances.] Stuttgart, New York,
Georg Thieme Verlag, pp 602-603, 923-928 (in German).
Knapp JS & Brown VR (1988) Morpholine biodegradation. Int Biodeterior,
24: 299-306.
Knapp JS & Whytell AJ (1990) The biodegradation of morpholine in river
water and activated sludge. Environ Pollut, 68: 67-79.
Knapp JS, Callely AG, & Mainprize J (1982) The microbial degradation
of morpholine. J Appl Bacteriol, 52: 5-13.
Koga M & Akiyama T (1985) Determination of trace heterocyclic amines
in water as their 1,2,-naphthoquinone derivatives by high performance
liquid chromatography. Anal Sci, 1: 285-288.
Kramer VC, Schnell DJ, & Nickerson KW (1983) Relative toxicity of
organic solvents to Aedes aegypti larvae. J Invertebr Pathol,
42: 285-287.
Kubis A, Witek R, Baran E, Jadach W, Matecka K, & Zaba A (1981)
[ In vivo antimycotic effect of topically applied morpholine.]
Pharmazie, 36: 429-431 (in German).
Kubis A, Witek R, & Krutul H (1983) [Investigation on antibacterial
action of some amines.] Pharmazie, 38: 488-489 (in German).
Lakritz L & Kimoto W (1980) N-nitrosamines - contaminants in blood-
collection tubes. Food Cosmet Toxicol, 18: 31-34.
Lam HF & Van Stee EW (1978) A re-evaluation of the toxicity of
morpholine. Fed Proc, 37: 679 (Abstract).
Lamarre C, Gilbert R, & Gendron A (1989) Liquid chromatographic
determination of morpholine and its thermal breakdown products in
steam-water cycles at nuclear power plants. J Chromatogr,
467: 249-258.
Lamb CB & Jenkins GF (1952) B.O.D. of synthetic organic chemicals. In:
Proceedings of the 7th Industrial Waste Conference, Purdue University,
West Lafayette, Indiana, 7-9 May 1952. Ann Arbor, Michigan, Ann Arbor
Science Publishers, pp 326-339.
Lathia D & Edeler A (1989) [Influence of sulfur-containing amino acids
on in vitro nitrosamine formation under conditions similar to those
found in the stomach.] Ernährungsphysiologie, 36: 21-24 (in German).
Lathia D & Schellhöh B (1981) Inhibition of nitrosamine formation
in vitro in presence of both ascorbic acid and sorbic acid. Recent
Adv Clin Nutr, 1: 189-190.
Lauharanta J (1992) Comparative efficacy and safety of amorolfine nail
lacquer 2% versus 5% once weekly. Clin Exp Dermatol, 705: 41-43.
Leach SA, Cook AR, Challis BC, Hill MJ, & Thompson MH (1987)
Bacterially mediated N-nitrosation reactions and endogenous
formation of N-nitroso compounds. In: Bartsch H, O`Neill IK, &
Schulte-Hermann R ed. Relevance of N-nitroso compounds to human
cancer: Exposures and mechanisms. Lyon, International Agency for
Research on Cancer, pp 396-403 (IARC Scientific Publications No. 84).
Leach SA, Mackerness CW, Hill MJ, & Thompson MH (1991) Inhibition of
bacterially mediated N-nitrosation by ascorbate: Therapeutic and
mechanistic considerations. In: O`Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 571-578 (IARC Scientific Publications No. 105).
Leaf CD, Wishnok JS, & Tannenbaum SR (1991) Endogenous incorporation
of nitric oxide from L-arginine into N-nitrosomorpholine stimulated by
Escherichia coli lipopolysaccharide in the rat. Carcinogenesis,
224: 537-539.
Lee SA (1982) Health hazard evaluation report HETA 82-156-1231:
Sheller-Globe Corporation, Keokuk, Iowa. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 8 pp (PB84-172915).
Leenheers LH, Ravensberg JC, Kerstens HJ, & Jongen MJM (1992) Gas
chromatographic determination of the pesticide dodemorph for
assessment of occupational exposure. J Chromatogr Sci, 132: 228-232.
Leo A, Hansch C, & Elkins D (1971) Partition coefficients and their
uses. Chem Rev, 71: 525, 564.
Le Therizien L, Heymans F, Redeuilh C, Godfroid J-J, & Busch N (1980)
Partition coefficient additivity. 1. Morpholine and
N-(N',N'-disubstituted amino acetyl)-arylamine series. Eur J Med Chem
Chim Ther, 15: 311-316.
Lide DR ed. (1990) CRC handbook of chemistry and physics, 71st ed.
Boca Raton, Florida, CRC Press, pp 8-34.
Litton Bionetics (1979a) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp (Report submitted to Texaco Petrochemicals,
Bellaire, Texas).
Litton Bionetics (1979b) Evaluation of morpholine in the in vitro
transformation of BALB/3T3 cells assay. Kensington, Maryland, Litton
Bionetics, Inc., 13 pp Report submitted to Texaco, Inc., Beacon, New
York).
Litton Bionetics (1980) Mutagenicity evaluation of morpholine in the
sister chromatid exchange assay with chinese hamster ovary (CHO)
cells. Kensingtn, Maryland, Litton Bionetics, Inc., 10 pp (Report
submitted to Texaco, Inc., Beacon, New York).
Litton Bionetics (1982) Evaluation of morpholine in the in vitro
transformation of Balb/3T3 cells with and without metabolic activation
assay 80/490. Kensington, Maryland, Liotton Bionetics, Inc. 21 pp
(Unpublished report submitted to BASF AG, Ludwigshafen).
Liu RH, Conboy JJ, & Hotchkiss JH (1988) Nitrosation by nitro-nitroso
derivatives of olefins: a potential mechanism for N-nitrosamine
formation in fried bacon. J Agric Food Chem, 36: 984-987.
Liu RH, Jacob JR, Tennant BC, & Hotchkiss JH (1992) Nitrite and
nitrosamine synthesis by hepatocytes isolated from normal woodchucks.
Cancer Res, 52: 4139-4143.
Lodén M, Larsson R, Häggqvist I, & Karlsson N (1985) [The dermal
irritancy/corrosion of 20 compounds in aqueous solutions.] Umea,
Sweden, Försvarets Forskningsanstalt, 76 pp (FOA Report No. E/40023)
(in Swedish with English summary).
Loeppky RN, Tomasik W, & Kerrick BE (1987) Nitroso transfer from
alpha-nitrosamino aldehydes: implications for carcinogenesis.
Carcinogenesis, 8(7): 941-946.
London MA & Lee S (1987) Health hazard evaluation report HETA
85-003-1834: BF Goodrich, Woodburn, Indiana. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 27 pp
(PB88-162672/XAD).
McCain JC & Peck JM Jr (1976) The toxicity of selected chemicals used
in power generating stations to Hawaiian fishes NOAA. Washington, DC,
US Department of Commerce, National Technical Information Service,
23 pp (NTIS No PB262437).
McGlothlin JD (1980) Health hazard evaluation report HETA 80-67-749:
Firestone Fire and Rubber Company, Akron, Ohio. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 18 pp
(PB82-103144).
McGlothlin J & Wilcox T (1984) Health hazard evaluation report HETA
79-109-1538: Kelly Springfield Tire Company, Cumberland, Maryland.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 50 pp (PB85-244424).
Mackerness CW, Leach SA, Thompson MH, & Hill MJ (1989) The inhibition
of bacterially mediated N-nitrosation by vitamin C: relevance to the
inhibition of endogenous N-nitrosation in the achlorhydric stomach.
Carcinogenesis, 10(2): 397-399.
Malaiyandi M, Thomas GH, & Meek ME (1979) Sampling and analysis of
some corrosion inhibiting amines in steam condensates. J Environ Sci
Health, A14: 609-627.
Maller RK & Heidelberger C (1957) Studies on OPSPA. IV. Metabolism of
OPSPA in the rat and human. Cancer Res, 17: 296-301.
Mannsville Chemical Products (1981) Chemical products synopsis:
Morpholine. Cortland, New York, Mannsville Chemical Products
Corporation, 2 pp.
Mastromatteo E (1965) Recent occupational health experiences in
Ontario. J Occup Med, 702: 502-511.
Mazure N (1993) Etude de la biodégradation de la morpholine par une
culture pure, Mycobacterium aurum, et des cultures mixtes
Pseudomonas. Compiègne, France, University of Technology (Thesis).
Meiners AF, Gadberry H, Carson BL, Owens HP, & Lapp TW (1980) Volatile
corrosion inhibitors and boiler water additives: potential for
nitrosamine formation. Washington, DC, US Environmental Protection
Agency, Office of Pesticides and Toxic Substances, 99 pp (Report
submitted by the Midwest Research Institute, Kansas City, Missouri)
(NTIS/PB80-221105).
Mercer EI (1991) Morpholine antifungals and their mode of action.
Biochem Soc Trans, 19: 788-793.
Migukina NV (1973) [Evaluation of the danger [toxicity] of morpholine
by chronic exposure.] Toksikol Nov Prom Chim Veshchestv, 13: 92-100
(in Russian).
Millington LA, Goulding KH, & Adams N (1988) The influence of growth
medium composition on the toxicity of chemicals to algae. Water Res,
22: 1593-1597.
Mills EJ & Stack VT (1953) Biological oxidation of synthetic organic
chemicals. In: Proceedings of the 8th Industrial Waste Conference,
Purdue University, West Lafayette, Indiana, 4-6 May 1953. Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp 492-517.
Mills EJ & Stack VT (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage Ind Wastes,
27: 1061-1064.
Mirvish SS (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol Appl Pharmacol,
31: 325-351.
Mirvish SS, Wallcave L, Eagen M, & Shubik P (1972) Ascorbate-nitrite
reaction: possible means of blocking the formation of carcinogenic
N-nitroso compounds. Science, 177: 65-68.
Mirvish SS, Cardesa A, Wallcave L, & Shubik P (1975) Induction of
mouse lung adenomas by amines or ureas plus nitrite and by N-nitroso
compounds: effect of ascorbate, gallic acid, thiocyanate, and
caffeine. J Natl Cancer Inst, 55: 633-636.
Mirvish SS, Pelfrene AF, Garcia H, & Shubik P (1976) Effect of sodium
ascorbate on tumor induction in rats treated with morpholine and
sodium nitrate, and with nitrosomorpholine. Cancer Lett, 2: 101-108.
Mirvish SS, Ramm MD, Sams JP, & Babcock DM (1988) Nitrosamine
formation from amines applied to the skin of mice after and before
exposure to nitrogen dioxide. Cancer Res, 48: 1095-1099.
Mjos K (1978) Cyclic amines. In: Kirk-Othmer encyclopedia of chemical
technology, 3rd ed. New York, John Wiley and Sons, vol 2, pp 295-308.
Moriyasu M, Endo M, Hashimoto Y, & Koeda T (1984) High-performance
liquid chromatographic determination of organic substances by metal
chelate derivatization. II. Microdetermination of methamphetamine and
amphetamine. Chem Pharm Bull, 32: 600-608.
Newberne PM & Shank RC (1973) Induction of liver and lung tumours in
rats by the simultaneous administration of sodium nitrite and
morpholine. Food Cosmet Toxicol, 11: 819-825.
NIOSH (1977) Manual of analytical methods, 2nd ed. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 3, pp
S150/1-S150/9.
NIOSH (1988) National occupational exposure survey as of 16.12.1988:
Morpholine. Cincinnati, Ohio, National Institute of Occupational
Safety and Health, 1 p.
Norkus EP, Boyle S, Kuenzig WA, & Mergens WJ (1984) Formation of
N-nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen oxide. Carcinogenesis, 5: 549-554.
Norkus EP, Kuenzig WA, Chau J, Mergens WJ, & Conney AH (1986)
Inhibitory effect of alpha-tocopherol on the formation of
nitrosomorpholine in mice treated with morpholine and exposed to
nitrogen dioxide. Carcinogenesis, 7: 357-360.
NRC (1981) Selected aliphatic amines and related compounds: an
assessment of the biological and environmental effects. Washington,
DC, National Research Council, Board on Toxicology and Environmental
Health Hazards (Report prepared for the US Environmental Protection
Agency, Washington) (NTIS/PB83-133066).
OECD (1981a) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Ready biodegradability. 301 E - Modified
OECD screening test. Paris, Organisation for Economic Co-operation and
Development.
OECD (1981b) OECD guidelines for testing of chemicals. Section 3.
Degradation and accumulation: Inherent biodegradability. 302 B -
Modified Zahn-Wellens test. Paris, Organisation for Economic
Co-operation and Development.
OECD (1984) Guidelines for testing of chemicals No 202, Part I:
Daphnia sp, acute immobilisation test. Paris, Organisation for
Economic Co-operation and Development.
O'Donnell CM, Edwards C, & Ware J (1988) Nitrosamine formation by
clinical isolates of enteric bacteria. FEMS Microbiol Lett,
51: 193-197.
Ohnishi T (1984) [Morpholine. Studies on mutagenicity of the food
additive morpholine (fatty acid salt).] Nippon Eiseigaku Zasski,
39: 729-745 (in Japanese with English summary).
Ohnishi T, Kubota M, Okada A, & Tonami K (1983) [Residual survey
investigation and removal efficiency by washing with kitchen detergent
of food additive morpholine.] Hokuriku Koshu Eisei Gakkaishi,
10: 63-67 (in Japanese with English summary).
Österdahl B-G & Slorach SA (1983) Volatile N-nitrosamines in snuff and
chewing tobacco on the Swedish market. Food Chem Toxicol, 21: 759-762.
Pensabene JW & Fiddler W (1988) Food additives. Determination of
volatile N-nitrosamines in Frankfurters containing minced fish and
surimi. J Assoc Off Anal Chem, 71: 839-843.
Perez A, Fernandez SI, Garcia-Roche MO, De Las Cagigas A, Castillo A,
Fonseca G, & Herrera M (1990) Mutagenicity of N-nitrosomorpholine
biosynthesized from morpholine in the presence of nitrate and its
inhibition by ascorbic acid. Nahrung, 34: 661-664.
Postlethwait EM & Mustafa MG (1983) Formation of N-nitrosamine in
isolated rat lungs during nitrogen dioxide ventilation.
Carcinogenesis, 4: 777-778.
Reinel D & Clarke C (1992) Comparative efficacy and safety of
amorolfine nail lacquer 5% in onychomycosis, once-weekly versus
twice-weekly. Clin Exp Dermatol, 705: 44-49.
Reinhardt CF & Brittelli MR (1981) Heterocyclic and miscellaneous
nitrogen compounds. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology: Vol 2A. Toxicology. New York, John Wiley and
Sons, pp 2671-2822.
Rekka E, Retsas S, Demopoulos VJ, & Kourounakis PN (1990)
Lipophilicity of some substituted morpholine derivatives synthesized
as potential antinociceptive agents. Arch Pharmacol, 323: 53-56.
Reynolds T (1989) Comparative effects of heterocyclic compounds on
inhibition of lettuce fruit germination. J Exp Bot, 40: 391-404.
Rhodes C & Case DE (1977) Non-metabolite residues of ICI 58,834
(viloxazine). Studies with (14C)morpholine, (14C)ethanolamine and
(14C)glyoxylate. Xenobiotica, 7: 112.
Richardson ML, Webb KS, & Gough TA (1980) The detection of some
N-nitrosamines in the water cycle. Ecotoxicol Environ Saf, 4: 207-212.
Ringenburg V & Fajen JM (1980) Survey for N-nitroso compounds at B.F.
Goodrich, Woodburn, Indiana, December 12, 1979. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 11 pp
(PB88-250477/XAD).
Rounbehler DP & Fine DH (1982) Specific detection of amines and other
nitrogen-containing compounds with a modified TEA analyzer. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 209-219 (IARC Scientific
Publications No. 41).
Rounbehler DP & Fajen JM (1983) N-nitroso compounds in the factory
environment. National Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 228 pp (PB84-145770).
Rounbehler DP, Reisch J, & Fine DH (1980) Nitrosamines in new motor-
cars. Food Cosmet Toxicol, 18: 147-151.
Sander J & Bürkle G (1969) [Induction of malignant tumours in rats by
simultaneous feeding of nitrite and secondary amines.] Z Krebsforsch,
73: 54-66 (in German).
Sander J, Schweinsberg F, & Menz H-P (1968) [Studies on the formation
of carcinogenic nitrosamines in the stomach.] Hoppe-Seyler's Z Physiol
Chem, 349: 1691-1697 (in German).
Savolainen H & Rosenberg C (1983) Morpholine vapour inhalation and
interactions of simultaneous nitrite intake. Biochemical effects on
rat spinal cord axons and skeletal muscle. Arch Toxicol, 53: 143-150.
Schröder E, Rufer C, & Schmiechen R (1982) [Nitrofuran.] In:
[Pharmaceutical Chemistry.] Stuttgart, New York, Georg Thieme Verlag,
pp 858-863 (in German).
Schuster RH, Nabholz F, & Gmünder M (1990) [The inhibition of the
formation of N-nitrosamines: Part 1. The situation and the effect of
alpha-tocopherol.] Kautschuk Gummi Kunstst, 43: 95-106 (in German).
Sen NP & Baddoo PA (1986) Origin of N-nitrosomorpholine contamination
in margarine. J Food Sci, 51: 216-217.
Sen NP & Baddoo PA (1989) An investigation on the possible presence of
morpholine and N-nitrosomorpholine in wax-coated apples. J Food Saf,
9: 183-191.
Sen NP, Seaman S, Clarkson S, Garrod F, & Lalonde P (1984) Volatile
N-nitrosamines in baby bottle rubber nipples and pacifiers. Analysis,
occurrence and migration. In: O'Neill IK, vn Borstel RC, Miller CT,
Long J, & Bartsch H ed. N-nitroso compounds: Occurrence, biological
effects and relevance to human cancer. Lyon, International Agency for
Research on Cancer, pp 51-57 (IARC Scientific Publications No. 57).
Sen NP, Kushwaha SC, Seaman SW, & Clarkson SG (1985) Nitrosamines in
baby bottle nipples and pacifiers: occurrence, migration, and effect
of infant formulas and fruit juices on in vitro formation of
nitrosamines under simulated gastric conditions. J Agric Food Chem,
33: 428-433.
Shank RC & Newberne PM (1976) Dose-response study of the
carcinogenicity of dietary sodium nitrite and morpholine in rats and
hamsters. Food Cosmet Toxicol, 14: 1-8.
Shea TE Jr (1939) The acute and sub-acute toxicity of morpholine. J
Ind Hyg Toxicol, 21: 236-245.
Shenoy NR & Choughuley ASU (1989) Effect of certain plant phenolics on
nitrosamine formation. J Agric Food Chem, 37: 721-725.
Shenoy NR & Choughuley ASU (1992) Inhibitory effect of diet related
sulphydryl compounds on the formation of carcinogenic nitrosamines.
Cancer Lett, 67: 227-232.
Shibata M-A, Kurata Y, Tamano S, Ogiso T, Fukushima S, & Ito N (1987a)
13-week subchronic toxicity study with morpholine oleic acid salt
administered to B6C3F1 mice. J Toxicol Environ Health, 22: 187-194.
Shibata M-A, Kurata Y, Ogiso T, Tamano S, Fukushima S, & Ito N (1987b)
Combined chronic toxicity and carcinogenicity studies of morpholine
oleic acid salt in B6C3F1 mice. Food Chem Toxicol, 25: 569-574.
Simon P & Lemacon C (1987) Determination of aliphatic primary and
secondary amines and polyamines in air by high-performance liquid
chromatography. Anal Chem, 59: 480-484.
Singer GM & Lijinsky W (1976a) Naturally occurring nitrosatable
compounds. I. Secondary amines in foodstuffs. J Agric Food Chem,
24: 550-553.
Singer GM & Lijinsky W (1976b) Naturally occurring nitrosatable
amines. II Secondary amines in tobacco and cigarette smoke condensate.
J Agric Food Chem, 24: 553-555.
Singer SS (1980) Transnitrosation by nitrosamines and nitrosoureas.
In: Walker EA, Griciute L, Castegnaro M, & Börzsönyi M ed. N-nitroso
compounds: Analysis, formation and occurrence. Lyon, International
Agency for Research on Cancer, pp 111-117 (IARC Scientific
Publications No. 31).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, Noyes Publications, pp 626-627.
Smith JH, Bomberger DC Jr, & Haynes DL (1980) Prediction of the
volatilization rates of high-volatility chemicals from natural water
bodies. Environ Sci Technol, 14: 1332-1337.
Smyth HF Jr, Carpenter CP, Weil CS, & Pozzani UC (1954) Range-finding
toxicity data. Arch Ind Hyg Occup Med, 10: 61-68.
Sohn OS, Fiala E, Conaway CC, & Weisburger JH (1982a) Separation of
morpholine and some of its metabolites by high-performance liquid
chromatography. J Chromatogr, 242: 374-380.
Sohn OS, Fiala ES, Conaway CC, & Weisburger JH (1982b) Metabolism and
disposition of morpholine in the rat, hamster and guinea pig. Toxicol
Appl Pharmacol, 64: 486-491.
Sollenberg J & Hansen L (1987) Isotachophoretic determination of
amines from workroom air. J Chromatogr, 390: 133-140.
Spiegelhalder B (1983) [Nitrosamines and rubber.] In: Preussmann R ed.
[The nitrosamine problem.] Weinheim, VCH Verlagsgesellschaft,
pp 235-244 (in German).
Spiegelhalder B & Preussmann R (1982) Nitrosamines and rubber. In:
Bartsch H, O'Neill IK, Castegnaro M, & Okada M ed. N-nitroso
compounds: Occurrence and biological effects. Lyon, International
Agency for Research on Cancer, pp 231-2439 (IARC Scientific
Publications No. 41).
Spiegelhalder B & Preussmann R (1984) Contamination of toiletries and
cosmetic products with volatile and nonvolatile N-nitroso carcinogens.
J Cancer Res Clin Oncol, 108: 160-163.
Spies RB, Andresen BD, & Rice DW Jr (1987) Benzthiazoles in estuarine
sediments as indicators of street runoff. Nature (Lond), 327: 697-699.
SRI (1990) Directory of chemical producers, Western Europe:
Morpholine. Menlo Park, California, SRI International, vol 2, p 1563.
Stewart BW & Farber E (1973) Strand breakage in rat liver DNA and its
repair following administration of cyclic nitrosamines. Cancer Res,
33: 3209-3215.
Strotmann UJ, Weberruss U, & Bias WR (1993) Degradation of morpholine
in several biodegradation tests and in wastewater treatment plants.
Chemosphere, 75: 1729-1742.
Subrahmanyam PVR, Khadakkar SN, Chakrabarti T, & Sundaresan BB (1983)
Wastewater-treatment of a phthalate plasticizer, ethanolamine and
morpholine manufacturing plant: a case study. Proc Ind Waste Conf,
37: 13-20.
Suzuki K & Mitsuoka T (1984) N-nitrosamine formation by intestinal
bacteria. In: O'Neill IK, vn Borstel RC, Miller CT, Long J, & Bartsch
H ed. N-nitroso compounds: Occurrence, biological effects and
relevance to human cancer. Lyon, International Agency for Research on
Cancer, pp 275-281 (IARC Scientific Publications No. 57).
Swain A, Waterhouse KV, Venables WA, Callely AG, & Lowe SE (1991)
Biochemical studies of morpholine catabolism by an environmental
mycobacterium. Appl Microbiol Biotechnol, 29: 110-114.
Swope HG & Kenna M (1950) Effect of organic compounds on biochemical
oxygen demand. Sew Ind Wastes Eng, 21: 467-468.
Taft RM & Stroman RE (1979) Health hazard evaluation report HETA
78-131-586: Goodyear Tire and Rubber Company, Niagara Falls, New York.
Cincinnati Ohio, National Institute of Occupational Safety and Health,
Hazard Evaluations and Technical Assistance Branch, 13 pp
(NTIS/PB80-196736).
Takezawa J & Lam HF (1978) Toxic effect of morpholine on rat lungs.
Fed Proc, 37: 247 (Abstract).
Tanaka M, Okada Z, Mihashi K, & Seiko Y (1968) Industrial waste
treatment by activated sludge. XV. Treatment of waste from an organic
vulcanization accelerator producing plant. Kogyo Gijutsuin Hakko
Kenkyusho Kenkyu Hokoku, 33: 19-29.
Tanaka A, Tokieda T, Nambaru S, Osawa M, & Yamaha T (1978) Excretion
and distribution of morpholine salts in rats. J Food Hyg Soc,
19: 329-334.
Tannenbaum SR, Archer MC, Wishnok JS, & Bishop WW (1978) Nitrosamine
formation in human saliva. J Natl Cancer Inst, 60(2): 251-253.
Tatsumi K, Kitamura S, Yoshimura Y, Tanaka S, Hashimoto K, & Igarashi
T (1975) The metabolism of phenyl o-(2-N-morpholinoethoxy)-phenyl
ether hydrochloride in the rabbit and rat. Xenobiotica, 5(6): 377-388.
Taylor R & Son PN (1982) Rubber chemicals. In: Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 20, pp 337-364.
Texaco (1979a) Mutagenicity evaluation of morpholine in the Ames
salmonella/microsome plate test. Bellaire, Texas, Texaco
Petrochemicals, 8 pp.
Texaco (1979b) Mutagenicity evaluation of morpholine in the mouse
lymphoma forward mutation assay. Bellaire, Texas, Texaco
Petrochemicals, 15 pp.
Texaco (1986) Texaco product brochure - Morpholine. Austin, Texas,
Texaco Chemical Company, Research and Technical Services, 29 pp.
Tölgyessy P, Kollár M, Vanco D, & Piatrik M (1986) Bio-degradability
of morpholine. J Radioanal Nucl Chem Lett, 107: 291-295.
Tombropoulos EG (1979) Micromethod for the gas chromatographic
determination of morpholine in biological tissues and fluids. J
Chromatogr, 164: 95-99.
Tombropoulos EG, Koo JO, Gibson W, & Hook GER (1983) Induction by
morpholine of lysosomal alpha-mannosidase and acid phosphatase in
rabbit alveolar macrophages in vivo and in vitro. Toxicol Appl
Pharmacol, 70: 1-6.
TRGS (1989) [Technical regulations for dangerous chemicals:
Nitrosamine.] Cologne, Carl Heymanns Verlag KG, 13 pp (TRGS 552)
(in German).
Tricker AR & Preussmann R (1991) Occurrence of and exposure to
N-nitroso compounds in tobacco. In: O'Neill IK, Chen J, & Bartsch H
ed. Relevance to human cancer of N-nitroso compounds, tobacco smoke
and mycotoxins. Lyon, International Agency for Research on Cancer,
pp 493-495 (IARC Scientific Publications No. 105).
UBA (1990) Calculation of log POW with the Program CLOGP (data
sheet). Berlin, Environment Office, 2 pp.
US FDA (1984a) Code of federal regulations (April 1, 1984) - Title 21,
Part 178.3300: Corrosion inhibitors used for steel or tinplate.
Washington, DC, US Food and Drug Administration, p 312.
US FDA (1984b) Code of federal regulations (April 1, 1984) - Title 21,
Part 176.210: Defoaming agents used in the manufacture of paper and
paperboard. Washington, DC, US Food and Drug Administration,
pp 192-193.
US FDA (1984c) Code of federal regulations (April 1, 1984) - Title 21,
Part 175.105: Substances for use only as components of adhesives.
Washington, DC, US Food and Drug Administration, pp 124-137.
US FDA (1984d) Code of federal regulations - Title 21, Part 178.310:
Animal glue. Washington, DC, US Food and Drug Administration, p 308.
US FDA (1984e) Code of federal regulations - Title 21, Part 173.310:
Boiler water additives. Washington, DC, US Food and Drug
Administration, pp 115-118.
US FDA (1986) Cosmetic product formulation data: Ingredients used in
each product category. Washington, DC, US Food and Drug
Administration.
US FDA (1988) Code of federal regulations - Title 21, Part 172.235:
Morpholine. Washington, DC, US Food and Drug Administration, pp 33-35.
Van Stee EW, Wynns PC, & Moorman MP (1981) Distribution and
disposition of morpholine in the rabbit. Toxicology, 20: 53-60.
Wang X & Suskind RR (1988) Comparative studies of the sensitization
potential of morpholine, 2-mercaptobenzothiazole and 2 of their
derivatives in guinea pigs. Contact Dermatitis, 19: 11-15.
Wang X & Tabor MW (1988) Studies of the reactivity of morpholine,
2-mercaptobenzothiazole and 2 of their derivatives with selected amino
acids. Contact Dermatitis, 19: 16-21.
Wang H & Wu Y (1991) Inhibitory effect of Chinese tea on N-nitrosation
in vitro and in vivo. In: O'Neill IK, Chen J, & Bartsch H ed.
Relevance to human cancer of N-nitroso compounds, tobacco smoke and
mycotoxins. Lyon, International Agency for Research on Cancer,
pp 546-548 (IARC Scientific Publications No. 105).
Wellens H (1982) [Comparison of the sensitivity of Brachydanio rerio
und Leuciscus idus by testing the fish toxicity of chemicals and
wastewaters.] Z Wasser Abwasser Forsch, 2: 49-52 (in German).
Westin JB, Castegnaro MJ-J, & Friesen MD (1987) N-nitrosamines and
nitrosatable amines, potential precursors of N-nitramines, in
children's pacifiers and baby-bottle nipples. Environ Res,
43: 126-134.
Wishnok JS & Tannenbaum SE (1976) Formation of cyanamides from
secondary amines in human saliva. Science, 191: 1179-1180.
Wishnok JS & Tannenbaum SR (1977) An unknown salivary morpholine
metabolite. Anal Chem, 49: 715a-716a, 718a.
Yurchenko VA, Ilnitskii AP, Ermilow VB, Mistakopulo GM, & Nechipai AM
(1990) [Investigation of mutagenic action of natural zeolite and
chrysotile-asbest dusts.] Eksp Onkol, 12: 24-26 (in Russian with
English summary).
Zaeva GN, Timofievskaya LA, Bazarova LA, & Migukina NV (1968)
[Comparative toxicity of a group of cyclic imino-compounds.] Toksikol
Nov Prom Chim Veshchestv, 10: 25-35 (in Russian).
Ziebarth D (1973) N-nitrosation of secondary amines and particularly
of drugs, in buffer solutions and human gastric juice. In: Bogovski P
& Walker EA ed. N-nitroso compounds in the environment. Lyon,
International Agency for Research on Cancer, pp 137-141 (IARC
Scientific Publications No. 9).
Ziebarth D. (1974) [Investigations into the nitrosation of secondary
amines in buffer solutions and in human gastric juice.] Arch
Geschwulstforsch, 43: 42-41 (in German).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Propriétés physiques et chimiques
La morpholine (1-oxa-4-azacyclohexane) est un liquide incolore,
huileux, hygroscopique et volatil qui possède l'odeur de poisson
caractéristique des amines. Elle est entièrement miscible à l'eau,
ainsi qu'à de nombreux solvants organiques, mais sa solubilité est
limitée dans les solutions aqueuses alcalines. C'est une base, dont
le pKa de l'acide conjugué est de 8,33. Il s'ensuit que son
coefficient de partage entre l'octanol et l'eau dépend du pH
(log Pow = -2,55 à pH 7 et -0.84 à pH 10 et à 35°C). La tension de
vapeur des solutions aqueuses de morpholine est très proche de celle
de l'eau.
La morpholine peut entrer en réaction de diverses manières.
Chimiquement, elle se comporte comme une amine secondaire. Dans les
conditions environnementales et physiologiques, la
N-nitrosomorpholine (NMOR), dont le pouvoir cancérogène chez
l'animal est prouvé, se forme par réaction entre des solutions de
nitrite ou des oxydes d'azote gazeux et des solutions diluées de
morpholine. La concentration en oxyde d'azote (NO) peut être
importante pour la nitrosation. Les conditions de nitrosation, en
particulier la valeur du pH, jouent un rôle important.
2. Méthodes d'analyse
On peut doser la morpholine par chromatographie en phase gazeuse
avec des colonnes à remplissage ou des colonnes capillaires, par
chromatographie liquide à haute performance (HPLC) et par
chromatographie ionique. Les détecteurs utilisés sont les détecteurs
à ionisation de flamme, les détecteurs à photométrie de flamme, les
détecteurs sélectifs d'azote, les détecteurs thermiques pour la
chromatographie en phase gazeuse et, pour la chromatographie en phase
liquide à haute performance, les détecteurs à UV et les détecteurs
thermiques. Pour le dosage des traces, il faut passer par un dérivé.
La méthode de choix sur le plan de la sensibilité semble être la
chromatographie en phase gazeuse avec détection thermique après
transformation de la morpholine en NMOR (limite de détection: 2 à
3 µg/kg dans diverses matrices). Dans l'air, de faibles
concentrations de morpholine peuvent être mesurées par chromatographie
en phase gazeuse avec un détecteur sélectif d'azote.
3. Sources d'exposition humaine et environnementale
On estime qu'environ 25 000 tonnes de morpholine sont produites
chaque année, toutefois on ne connaît pas dans le détail la production
de certains pays.
Le principal procédé de production est, semble-t-il, la réaction
du diéthylène-glycol sur l'ammoniaque en présence d'hydrogène et de
catalyseurs.
La morpholine est un produit chimique à tout faire mais on n'en
connaît pas tous les usages. Il joue un rôle important comme
intermédiaire dans l'industrie du caoutchouc, comme inhibiteur de la
corrosion ainsi que pour la synthèse d'éclaircissants optiques,
d'agents pour la protection des récoltes, de colorants et de
médicaments. La morpholine est utilisée comme solvant pour les
dérivés organiques les plus divers, comme par exemple les résines, les
colorants et les cires. On peut également l'utiliser comme
catalyseur. On utilise encore de la morpholine dans certains pays
pour la confection de produits de toilette et de cosmétiques. Dans
d'autres, elle entre dans la composition d'additifs alimentaires, soit
directement, soit indirectement.
Lorsqu'il y a exposition humaine ou environnementale, elle peut
être due soit à des émissions de gaz, soit à des décharges de
solutions aqueuses, à moins qu'elle ne se produise directement lors de
l'utilisation de certains produits contenant de la morpholine, comme
par exemple des produits cosmétiques ou des cires. Le gros des
émissions et des décharges trouve probablement son origine dans la
fabrication et l'utilisation de la morpholine dans l'industrie
chimique (notamment lors de la production et de l'utilisation des
produits destinés à l'industrie du caoutchouc) ainsi que dans
l'application de la morpholine comme agent anti-corrosion. On a
trouvé de la morpholine dans des denrées alimentaires très variées
ainsi que dans le tabac. Il est possible que cette présence
s'explique par la migration, dans la denrée alimentaire, de la
morpholine incorporée à l'enduit qui recouvre les fruits ou
l'emballage, mais dans un certain nombre de cas, on ignore d'où la
morpholine peut provenir.
4. Transport, distribution et transformation dans l'environnement
La morpholine est chimiquement stable dans la biosphère bien
qu'elle puisse être soumise à une nitrosation chimique ou biologique
qui la transforme en NMOR.
La morpholine est intrinsèquement biodégradable. On a vérifié
cette propriété dans des conditions reproduisant celles qui règnent
dans les usines de traitement des boues activées. Cependant, dans des
conditions de non adaptation, la morpholine n'est probablement pas
décomposée en proportion importante. Le temps de rétention moyen sur
les matières solides présentes dans les usines de traitement des boues
activées est d'une importance cruciale et il doit dépasser les 8 jours
pour que l'on puisse obtenir une bonne dégradation de la morpholine.
Les données relatives à la bioaccumulation de la morpholine par
les organismes aquatiques et terrestres sont insuffisantes. D'après
la valeur du coefficient de partage entre le n-octanol et l'eau
(log Pow = -2,55 à pH 7), on peut s'attendre à ce qu'il n'y ait
aucune bioaccumulation.
Comme la morpholine est un produit chimique industriel important
dont les applications sont variées, il faut s'attendre à retrouver ce
composé ou ses dérivés dans un grand nombre d'effluents industriels.
De plus, étant donné qu'on l'utilise comme inhibiteur de la corrosion
dans l'eau des chaudières, on va le retrouver dans les eaux usées de
ces chaudières, et en particulier les eaux usées provenant de
certaines centrales thermiques. Comme la morpholine entre également
dans la composition des additifs du caoutchouc, on va en retrouver
également, en quantité mal définie, dans l'hydrosphère et la
géosphère, par suite de l'usure des pneus et du rejet des pneus usés.
La morpholine peut pénétrer dans l'environnement en se
volatilisant à partir des encaustiques et des cirages dont elle est un
constituant. Elle est rapidement captée par l'humidité. C'est donc
principalement dans l'hydrosphère qu'elle devrait s'accumuler, mais
les données limitées dont on dispose incitent à penser que la
morpholine ne s'accumule pas dans ce compartiment.
La meilleure méthode pour éliminer la morpholine concentrée est
l'incinération, toutefois il peut être nécessaire de veiller aux
émissions d'oxydes d'azote afin de respecter les normes de protection
de l'environnement. En ce qui concerne les effluents aqueux, le
traitement des boues activées est suffisant, à la condition toutefois
que l'usine fasse l'objet d'un contrôle rigoureux (voir ci-dessus).
5. Concentrations dans l'environnement et exposition humaine
On ne dispose d'aucune donnée sur la concentration de la
morpholine dans l'air ambiant ainsi que dans l'air intérieur des
immeubles résidentiels ou encore dans l'eau de boisson. Si l'on
possède quelques données sur sa présence dans les eaux naturelles, on
n'en a en revanche aucune sur sa présence dans le sol.
D'après les données disponibles, c'est les denrées alimentaires
qui constituent la principale source d'exposition à la morpholine de
la population générale, les produits alimentaires pouvant être
contaminés par suite d'un traitement conservateur direct des fruits à
l'aide de cires contenant de la morpholine, lors du traitement à la
vapeur au cours de la préparation et enfin par l'utilisation de
matériaux d'emballage dans la composition desquels entre la
morpholine. Cependant on ne dispose que de données quantitatives
limitées sur la contamination des denrées alimentaires par la
morpholine et la NMOR. Par exemple, dans les produits laitiers pré-
emballés, les valeurs mesurées vont de 5 à 77 µg/kg pour la morpholine
et jusqu'à 3,3 µg/kg pour la NMOR. En général, les mesures effectuées
ont montré que la teneur en morpholine de divers échantillons de
produits alimentaires (poisson, viande, produits d'origine végétale,
boissons) ne dépassait généralement pas 1 mg/kg. Des valeurs plus
élevées (jusqu'à 71 mg/kg) ont été relevées au Japon dans des agrumes.
Une enquête effectuée en Italie n'a pas permis de repérer la présence
de NMOR dans diverses denrées alimentaires au seuil de détection de
0,3 µg/kg. Les données existantes ne permettent pas d'évaluer
l'apport de morpholine et de NMOR par la voie alimentaire.
On a trouvé de la morpholine dans le tabac de cigarette à la
concentration de 0,3 mg/kg ainsi que dans le tabac à priser et à
chiquer à des concentrations allant jusqu'à 4,0 mg/kg. Il est arrivé
que l'on trouve dans du tabac à priser des concentrations de
morpholine allant jusqu'à 0,7 mg/kg. La présence de ce produit était
probablement la conséquence de l'utilisation de cires à base de
morpholine pour le conditionnement de ce tabac.
De la NMOR a été mise en évidence dans certains produits de
toilette et dans des cosmétiques, par exemple des shampoings, du
rimmel ainsi que dans des articles en caoutchouc comme les sucettes
pour bébés et les tétines pour biberon, à des concentrations allant
jusqu'à 3,5 mg/kg.
Il peut y avoir exposition professionnelle à la morpholine dans
diverses industries. On ne possède guère de données sur l'exposition
des travailleurs à la morpholine. Toutes les valeurs signalées sont
inférieures à 3 mg/m3. On a signalé des cas d'exposition
professionnelle à la NMOR dans l'industrie du caoutchouc où des
concentrations allant jusqu'à 250 µg/m3 ont été mesurées.
Les données actuellement disponibles permettent de se faire une
idée du risque d'exposition humaine mais elles ne permettent pas une
estimation précise de l'intensité de l'exposition à la morpholine et
la NMOR de la population générale et des diverses catégories
professionnelles.
6. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après exposition par voie orale, cutanée ou respiratoire, il y a
absorption de la morpholine. Chez le rat, après administration par
voie orale ou intraveineuse, la morpholine se répartit rapidement dans
l'organisme, atteignant sa concentration la plus élevée dans
l'intestin et les muscles.
Chez le lapin, après exposition par voie intraveineuse ou
respiratoire, la morpholine se répartit de préférence dans les reins,
les concentrations étant plus faibles dans les poumons, le foie et le
sang.
La morpholine ne se fixe pas de manière importante aux protéines
plasmatiques. Sa demi-vie dans le plasma est de 115 minutes chez le
rat, de 120 minutes chez le hamster et de 300 minutes chez le cobaye.
La morpholine est principalement excrétée sans modification par
les reins chez un certain nombre d'espèces. Un jour après
l'administration, on a constaté que 70 à 90% de la morpholine se
retrouvaient dans les urines. La neutralisation en augmente la
vitesse d'excrétion. Une faible proportion de la morpholine est
excrétée dans l'air expiré et dans les matières fécales.
Les études effectuées sur des rats, des souris, des hamsters et
des lapins indiquent que la morpholine s'élimine presque complètement
sans métabolisation. Chez le cobaye, il peut y avoir une
N-méthylation suivie d'une N-oxydation, la dose administrée
pouvant être métabolisée jusqu'à hauteur de 20%. En présence de
nitrites, la morpholine peut être transformée en NMOR, tant in vitro
qu' in vivo. On a constaté, en administrant de la morpholine à des
rats avec des nitrites, que celle-ci pouvait être nitrosée dans la
proportion de 0 à 12%, selon la dose.
Le taux de nitrosation peut augmenter par le fait d'une immuno-
stimulation comportant l'activation des macrophages.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Après administration de morpholine par voie orale à des rats et à
des cobayes, on a obtenu pour la DL50 des valeurs respectives de
1-1,9 g/kg de poids corporel et 0,9 g/kg de poids corporel, ce qui
permet d'avoir une idée de la toxicité aiguë du produit. Des rats qui
avaient reçu de la morpholine neutralisée à raison de 1 g/kg de poids
corporel ont survécu. Après administration par voie intrapéritonéale,
on a obtenu une DL50 de 0,4 g/kg de poids corporel chez la souris et
de 0,1-0,4 g/kg de poids corporel chez le rat. Après exposition par
la voie respiratoire, la DL50 était d'environ 8 g/m3 chez le rat
et de 5-7 g/m3 chez la souris. La DL50 de la morpholine
concentrée par voie percutanée était de 0,5 ml/kg chez le lapin. La
toxicité aiguë de la morpholine se caractérise par des hémorragies
gastrointestinales et des diarrhées lorsqu'elle est administrée par
voie orale et par une irritation et des hémorragies nasales, buccales,
oculaires et pulmonaires lorsqu'elle est administrée par voie
respiratoire. Lors d'une étude de 30 jours au cours de laquelle des
rats ont reçu de la morpholine par gavage à des doses de 0,16 à
0,8 g/kg de poids corporel, on a observé des effets toxiques graves et
une mortalité à toutes les doses. Les mêmes observations ont été
effectuées sur des cobayes à des doses comprises entre 0,09 et
0,045 g/kg de poids corporel.
Chez des rats soumis pendant une brève période à des inhalations
de morpholine (7,2 g/m3, 4 heures par jour pendant 4 jours ou bien
1,63 g/m3, 4 heures par jour, 5 jours par semaine pendant 30 jours),
on a observé une altération de la fonction pulmonaire. Le taux de
mortalité chez d'autres rats allait de 0 à 100% selon le niveau
d'exposition (0,36-18,1 g/m3, 6 heures par jour pendant 9 jours).
Par la voie respiratoire, on a constaté que la toxicité dépendait de
la dose avec une irritation locale (yeux, bouche, nez et poumons) et
des hémorragies de gravité variables aux niveaux d'exposition les plus
élevés. Dans une étude, on a observé un hyperfonctionnement de la
glande thyroïde et dans une autre, une nécrose du foie et des tubules
rénaux après exposition par la voie respiratoire.
Une étude de 90 jours a montré que la morpholine administrée par
voie orale (0.2-0,7 g quotidiennement par kg de poids corporel)
pouvait réduire la gain de poids et entraîner une insuffisance rénale
chez la souris. Après 672 jours d'administration de morpholine par
voie orale (0,28-0,5 g/kg de poids corporel quotidiennement), on a
observé chez la souris une hyperplasie au niveau de l'épithélium de la
portion cardiaque de l'estomac.
Selon une étude d'inhalation de 13 semaines, la morpholine
(administrée à la dose de 0,09-0,9 g/m3 6 heures par jour, 5 jours
par semaine) provoque des lésions liées à la dose au niveau de la
muqueuse nasale ainsi qu'une pneumopathie aux doses les plus élevées
(0,36 et 0,9 mg/m3). A la dose de 0,09 g/m3 on n'a observé aucune
modification d'un certain nombre de paramètres qui puisse être
imputable à ce traitement; cette concentration peut être considérée
comme la dose sans effets nocifs observables dans les conditions d'une
exposition subchronique par la voie respiratoire.
Sous sa forme concentrée et non neutralisée, la morpholine est
extrêmement irritante pour les yeux et la peau, probablement en raison
de sa basicité. En la diluant et en la ramenant à un pH neutre, on
peut sensiblement en réduire la toxicité topique. Selon une variante
de la méthode de Buehler, la morpholine à 2% n'a pas produit de
sensibilisation chez le cobaye.
La morpholine ne produit pas de mutation chez des bactéries ou
des levures en présence ou en l'absence d'activation métabolique
(à l'exception d'un essai effectué à très forte concentration). Le
passage sur hôte a également donné des résultats négatifs.
On n'a pas non plus observé de réparation de l'ADN sous
l'influence de la morpholine dans des cultures primaires d'hépatocytes
de rats et ce composé n'a pas augmenté de façon sensible les échanges
entre chromatides soeurs dans des cellules ovariennes de hamsters
chinois. Les résultats d'une épreuve sur cellules lymphomateuses de
souris L5178Y ont conduit à considérer la morpholine comme faiblement
mutagène. Ce composé a entraîné une augmentation des foyers de type
III lors d'une épreuve de transformation cellulaire maligne effectuée
sur des cellules BALB/3T3, phénomène qui n'a pas été constaté avec la
morpholine neutralisée.
On n'a pas non plus constaté de mutation ponctuelle ni
d'aberration chromosomique chez des embryons de hamsters exposés
in utero à de la morpholine.
On n'a pas constaté d'augmentation dans l'incidence des tumeurs
chez des rats qui avaient été exposés par voie respiratoire pendant
104 semaines à des concentrations de morpholine allant jusqu'à
0,5 g/m3, ni chez des souris qui avaient reçu pendant 96 semaines
dans leur eau de boisson de l'oléate de morpholine à 1%. Une étude à
long terme sur un groupe de 104 rats qui recevaient dans leur
alimentation 1000 mg de morpholine par kg de nourriture, a permis de
mettre en évidence trois carcinomes hépatocellulaires, deux
angiosarcomes pulmonaires ainsi qu'un troisième angiosarcome de
localisation non précisée et enfin deux gliomes malins, alors
qu'aucune tumeur n'a été observée dans le groupe témoin comportant
156 animaux. Chez des hamsters exposés dans les mêmes conditions,
aucune tumeur n'a été observée.
Administrée en même temps qu'un nitrite, la morpholine donne un
résultat positif à l'épreuve de passage sur hôte, qui s'explique
probablement par la formation de NMOR. De la morpholine administrée
en même temps qu'un nitrite par mélange à la nourriture, a provoqué
chez des rats des tumeurs hépatiques et pulmonaires et chez des
hamsters des tumeurs hépatiques qui s'expliquent probablement par la
formation endogène de NMOR. La NMOR est mutagène pour les bactéries
et les levures; on a également observé avec ce composé des résultats
faiblement positifs dans l'épreuve d'échange de chromatides soeurs sur
des cellules CHO et lors de la recherche de mutations dans des
cultures de cellules lymphomateuses de souris L5178Y. La NMOR est
cancérogène pour la souris, le rat, le hamster et divers poissons;
elle provoque chez la souris des tumeurs hépatiques et pulmonaires,
chez le rats des tumeurs du foie, du rein et des vaisseaux sanguins,
chez le hamster des tumeurs des voies digestives et respiratoires
supérieures et enfin chez les poissons, des tumeurs hépatiques.
8. Effets sur l'homme
On ne dispose d'aucun rapport faisant état de cas d'intoxication
aiguë ou décrivant les effets d'une exposition à court ou à long terme
à la morpholine dans la population générale.
Un phénomène connu sous le nom de glaucopsie (vision bleutée)
ainsi que dans certains cas une irritation de la peau et des voies
respiratoires, ont été décrits dans des rapports d'exposition
professionnelle à la morpholine; toutefois, ces rapports ne précisent
pas la concentration de la morpholine dans l'air. On a indiqué que
chez des ouvriers exposés pendant 3 à 10 ans à de la morpholine à des
concentrations de 0,54-0,93 mg/m3, le nombre d'aberrations
chromosomiques dans les lymphocytes du sang périphérique ne présentait
pas de différence notable par rapport aux témoins.
La morpholine concentrée est fortement irritante pour la peau;
en solution (à 1/40) elle se révèle encore légèrement irritante.
On n'a pas étudié la cancérogénicité potentielle de la morpholine
chez les populations humaines exposées.
9. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Parmi les organismes aquatiques étudiés, certaines cyanobactéries
(Microcystis) et certaines algues bleues unicellulaires
(Scenedesmus) se révèlent être les taxa les plus sensibles puisque
le seuil de toxicité (critère: inhibition de la croissance des
populations) se situe à 1,7 mg/litre pour Microcystis et à
4,1 mg/litre pour Scenedesmus (durée de l'exposition: 8 jours).
Des bactéries aérobies telles que Pseudomonas se sont révélées
beaucoup plus résistantes; le seuil de toxicité à 16 heures ainsi que
la concentration sans effets observables sur la croissance des
populations atteindraient respectivement 310 et 8700 mg/litre.
Cependant une concentration de 1000 mg/litre a inhibé la respiration
et l'activité de la déshydrogénase (dans une proportion allant jusqu'à
20%) chez les bactéries de boues activées provenant d'usines de
traitement des effluents industriels.
Chez les protozoaires aquatiques étudiés jusqu'ici, des spécimens
des genres Entosiphon et Chilomonas (avec des valeursseuil
respectivement égales à 12 et 18 mg/litre pour ce qui est de
l'inhibition de la croissance des populations) se sont révélés être
les plus sensibles. Les valeurs de la CE50 à 24 heures
(E=immobilisation) pour la daphnie se situaient dans les limites de
100 à 120 mg/litre. Chez les poissons, les valeurs de la CL50 à
48 et 96 heures se sont révélées > 180 mg/litre lors d'épreuves
effectuées en eau douce, en eau saumâtre ou en eau de mer, la truite
arc-en-ciel étant l'espèce la plus sensible.
On ne dispose d'aucune donnée relative aux effets à long terme de
la morpholine sur les invertébrés et les vertébrés aquatiques.
L'absence d'information est également presque totale à propos de la
toxicité de ce composé pour les organismes terricoles, puisque les
seules données dont on dispose se limitent à la valeur de la CE à
3 jours, égale à 400 mg/litre, en ce qui concerne l'inhibition de la
germination des laitues.
10. Evaluation des risques pour la santé humaine et effets sur
l'environnement
10.1 Evaluation des effets sur la santé humaine
Les cas d'exposition de la population générale à la morpholine
résultent principalement de la consommation de denrées alimentaires
contaminées. La contamination du tabac et des produits du tabac, des
articles de toilette et des cosmétiques, de même que des articles en
caoutchouc, peuvent contribuer à l'exposition globale. Il peut y
avoir exposition professionnelle à la morpholine dans de nombreuses
industries; ce composé peut être absorbé soit par inhalation, soit
par la voie percutanée. On ne dispose pas de données suffisantes pour
déterminer le degré d'exposition de la population générale.
Les données relatives à l'exposition professionnelle sont
également limitées.
La morpholine n'est pas extrêmement toxique dans les conditions
d'une exposition aiguë. Après administration par voie orale, la
DL50 était de 1-1,9 g/kg de poids corporel chez le rat et de
0,9 g/kg de poids corporel chez le cobaye. Par ailleurs, on a trouvé,
pour la CL50, des valeurs de 7,8 mg/m3 chez le rat et de 4,9 à
6,9 g/m3 chez la souris.
Dans les conditions d'une exposition à court ou à long terme par
la voie respiratoire, c'est l'irritation des yeux et des voies
respiratoires qui constitue l'effet critique. Lors d'une étude de
13 semaines au cours de laquelle des rats ont été exposés 6 heures par
jour et 5 jours par semaine à de la morpholine, on a pu fixer à
90 mg/m3 la concentration sans effets nocifs observables. Lors
d'une autre étude d'inhalation, à long terme cette fois
(104 semaines), on a observé une augmentation de l'incidence des cas
d'inflammation de la cornée ou d'inflammation et de nécrose des fosses
nasales, à la dose de 540 mg/m3 chez le rat. L'irritation des yeux
et du nez présentait également une incidence accrue aux doses de 36 et
180 mg/m3.
Une forte exposition à la morpholine entraîne de graves lésions
hépatiques et rénales chez le rat et le cobaye. Après administration
de morpholine à des rats, par mélange à leur nourriture (0,5 g/kg de
poids corporel) tous les jours pendant 56 jours, on a observé une
dégénérescence graisseuse du foie. Des souris qui avaient reçu tous
les jours, pendant 13 semaines, de l'oléate de morpholine mêlé à leur
eau de boisson à la dose d'environ 0,7 g/kg de poids corporel, ont
présenté une dégénérescence albuminoïde au niveau des tubules
proximaux du rein (désignée également par l'expression "tuméfaction
trouble" par certains auteurs). Chez des souris femelles qui avaient
reçu pendant 672 jours de la morpholine dans leur alimentation, on a
observé aux doses comprises entre 0,05 et 0,4 g de morpholine
administrée sous forme d'oléate, une réduction du gain de poids.
Aux concentrations que l'on observe actuellement dans les cas
d'exposition professionnelle ou environnementale, il ne semble pas que
la morpholine risque véritablement d'entraîner des effets toxiques
généraux. Cependant lors d'une exposition accidentelle ou
professionnelle non contrôlée à de fortes concentrations de morpholine
présentes dans l'air, il peut y avoir des effets locaux qui prennent
la forme d'une irritation de la muqueuse oculaire et des voies
respiratoires; en outre, un contact avec de la morpholine liquide
(même diluée) peut entraîner une irritation cutanée.
Il ne semble pas que la morpholine soit mutagène ou cancérogène
chez l'animal. Toutefois elle peut facilement être nitrosée pour
donner naissance à de la NMOR qui s'est révélée, elle, mutagène et
cancérogène chez plusieurs espèces d'animaux de laboratoire.
L'administration de morpholine, puis d'un nitrite, mêlés à la
nourriture d'animaux de laboratoire a provoqué un accroissement de
l'incidence des tumeurs, pour la plupart des carcinomes
hépatocellulaires et des sarcomes du foie et du poumon. Il est donc
prudent de considérer l'exposition à la morpholine comme un facteur
supplémentaire de risque cancérogène chez les populations exposées.
10.2 Evaluation des effets sur l'environnement
Comme on sait très peu de choses sur l'exposition
environnementale et que les données relatives à l'exposition à long
terme dans l'hydrosphère ou à court et à long terme dans le milieu
terrestre font défaut, il est impossible, pour l'instant, de procéder
valablement à une appréciation du risque. On peut toutefois tirer un
certain nombre de conclusions sur la base des propriétés observées de
la morpholine, des données écotoxicologiques disponibles et des
quelques renseignements dont on dispose sur les concentrations dans
l'environnement.
La forte solubilité dans l'eau de la morpholine et sa faible
volatilité (dans les conditions du milieu) font que l'hydrosphère en
constitue le principal milieu récepteur.
La morpholine est intrinsèquement biodégradable et, bien que
cette biodégradation soit lente, rien n'indique qu'elle s'accumule
dans l'hydrosphère. En outre, sa bioaccumulation est également peu
probable.
On ne possède que relativement peu de données sur la toxicité de
la morpholine pour les organismes vagiles. Toutefois, il paraît
improbable qu'au niveau actuel des émissions de morpholine, des
dommages importants puissent être causés à l'environnement dans son
ensemble. Les effets locaux, dus par exemple aux émissions
industrielles ou à la libération de morpholine dans l'environnement
par suite de l'usure des pneumatiques, restent à évaluer.
Il peut y avoir contamination de certains produits alimentaires
comme le poisson, par de la morpholine, contamination qui viendrait de
l'environnement, mais cela n'est pas certain.
La transformation de la morpholine en NMOR est la principale
cause d'inquiétude, en particulier en ce qui concerne les populations
de vertébrés. On a signalé la présence de NMOR dans des eaux
résiduaires industrielles et dans le sol aux alentours d'une usine.
On peut s'inquiéter de la présence de morpholine dans de l'eau
destinée à être traitée pour la rendre potable.
11. Conclusions et recommandations
La morpholine ne présente aucun risque toxique pour l'homme au
niveau habituel d'exposition mais il faut prendre garde à sa
transformation en NMOR qui est cancérogène.
Rien n'indique qu'aux nivaux actuels d'exposition, la morpholine
constitue un risque important pour les biotes présents dans
l'environnement.
11.1 Recommandations en vue de la protection de la santé humaine
a) Il faut éviter dans la mesure du possible toute exposition
humaine à la morpholine.
b) Il faut éviter la contamination des denrées alimentaires par leur
emballage ou lors de leur transformation.
c) La morpholine ne doit pas entrer dans la composition d'articles
en caoutchouc destinés en entrer en contact direct avec l'homme.
d) La morpholine ne doit pas entrer dans la composition de
préparations destinées à la toilette ou à un usage cosmétique.
e) Les effluents industriels doivent être traités avec soin afin
d'éviter que la morpholine ne pénètre dans l'eau destinée à la
boisson.
f) Compte tenu de la possibilité de formation de NMOR cancérogène,
les limites d'exposition professionnelle actuelles devront être
réexaminées.
11.2 Recommandations en vue de la protection de l'environnement
Il faut éviter les déversements et les décharges massives dans
les usines de traitement des effluents.
11.3 Recommandations en vue de recherches ultérieures
Des travaux doivent être entrepris dans les domaines suivants:
a) toxicité pour la fonction de reproduction des mammifères;
b) toxicité à long terme pour les mammifères;
c) effets sur les mammifères d'une exposition à de faibles
concentrations de morpholine en présence ou non de nitrites et de
nitrates;
d) transnitrosation par la NMOR in vivo et in vitro;
e) biodégradation en anaérobiose, en particulier dans des conditions
entraînant la réduction des nitrates;
f) catalyse microbienne de la N-nitrosation en situation réelle;
g) concentrations de la morpholine dans les eaux souterraines, le
sol et les rivières dont l'eau est utilisée pour la boisson;
h) concentration de morpholine aux alentours d'usines qui produisent
ou transforment cette substance;
i) métabolisme et toxicocinétique chez l'homme en vue de la mise au
point de méthodes pour la surveillance biologique de la
morpholine;
j) surveillance de la concentration de morpholine et de NMOR dans
les denrées alimentaires, dans l'eau de boisson et l'air
intérieur aux habitations;
k) collecte et diffusion de données sur l'exposition
professionnelle.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Propiedades físicas y químicas
La morfolina (1-oxa-4-azaciclohexano) es un líquido incoloro,
oleoso, higroscópico y volátil que desprende un característico olor a
amina ("a pescado"). Es totalmente miscible en agua, así como en
numerosos disolventes orgánicos, y parcialmente soluble en soluciones
acuosas alcalinas. Se trata de una base, y el pKa del ácido conjugado
es de 8,33. En consecuencia, el coeficiente de reparto octanol/agua
depende del pH (log Pow -2,55 a pH 7, y - 0,84 a pH 10; 35°C). La
presión de vapor de las soluciones acuosas de morfolina es casi como
la del agua.
La morfolina puede sufrir diversas reacciones. Se comporta
químicamente como una amina secundaria. En condiciones ambientales y
fisiológicas, como resultado de la reacción de las soluciones de
nitrito o de óxidos de nitrógeno gaseosos con las soluciones diluidas
de morfolina, se forma N-nitrosomorfolina (NMOR), conocido
carcinógeno para los animales. Los niveles de óxido de nitrógeno (NO)
pueden ser importantes en la nitrosación. Las condiciones de
nitrosación, en particular el pH, tienen una considerable influencia.
2. Métodos analíticos
La morfolina se puede determinar mediante cromatografía de gases
(GC) en columnas empacadas o capilares, cromatografía líquida de alta
resolución (HPLC) y cromatografía iónica. Entre los detectores
utilizados cabe citar el detector de ionización por conductor, el
detector de fotometría de llama, el detector selectivo de nitrógeno
(NSD), y la espectrometría de masas y el analizador de energía térmica
(TEA) para la GC, y el detector de UV y el TEA para la HPLC. Para
determinar cantidades ínfimas hay que recurrir a la derivatización.
El método de elección en lo que respecta a sensibilidad es al parecer
la GC combinada con el TEA, previa transformación por derivatización
en NMOR (límite de detección de 2-3 µg/kg en diversas matrices). Las
bajas concentraciones de morfolina en el aire se pueden determinar
mediante GC y NSD.
3. Fuentes de exposición humana y ambiental
Se estima que cada año se producen industrialmente en todo el
mundo unas 25 000 toneladas de morfolina, pero no se conoce con
detalle la producción de algunos países.
El principal proceso de producción utilizado para su obtención es
al parecer la reacción de dietilenglicol con amoniáco en presencia de
hidrógeno y de catalizadores.
La morfolina es una sustancia química que puede utilizarse con
muy diversos fines, pero no se conocen todos sus posibles usos. Es
importante como producto intermedio en la industria del caucho, como
inhibidor de la corrosión, y en la síntesis de abrillantadores
ópticos, protectores de cultivos, colorantes y medicamentos. La
morfolina se utiliza como disolvente de una amplia variedad de
productos orgánicos, entre ellos resinas, colorantes y ceras. Se
puede utilizar como catalizador. La morfolina se usa aún en algunos
países para elaborar productos cosméticos y de tocador. En algunos
países se usa también en varias aplicaciones relacionadas directa o
indirectamente con los aditivos alimentarios.
La exposición humana y ambiental se debe a emisiones tanto
gaseosas como acuosas, y es también el resultado directo de alguno de
sus usos, por ejemplo como componente de productos cosméticos y de
ceras. Las emisiones más importantes son resultado probablemente de
su fabricación y de su uso en la industria química (sobre todo en la
producción y el uso de productos químicos derivados del caucho) y como
agente anticorrosión. Se ha detectado morfolina en muchos tipos de
alimento y de tabaco. En estos casos el origen del producto podría
ser la parafina empleada para proteger la fruta o en determinados
envases, pero a veces no se puede establecer su procedencia.
4. Transporte, distribución y transformación en el medio ambiente
La morfolina es químicamente estable en la biosfera, aunque sufre
nitrosación química y biológica, transformándose así en NMOR.
La morfolina es por naturaleza biodegradable. Así es en las
condiciones reinantes en las plantas de fangos activados que funcionan
correctamente. No obstante, en condiciones no idóneas probablemente
no se produce una degradación importante. El tiempo medio de
retención del sólido en las plantas de fangos activados tiene una
importancia crucial y debe ser superior a ocho días para que se
produzca una degradación fiable de la morfolina.
No se dispone de datos suficientes sobre la bioacumulación de
morfolina en organismos acuáticos y terrestres. Según el coeficiente
de reparto n-octanol/agua del producto (log Pow = -2,55 a pH 7),
no debería producirse bioacumulación.
Como es un importante producto químico industrial con una amplia
gama de aplicaciones, es previsible la presencia de morfolina o de sus
derivados en numerosos efluentes industriales. Usada como inhibidor
de la corrosión en el agua de calderas, aparece en los efluentes de
éstas, incluidos los de las centrales eléctricas en que se utiliza
morfolina. Su uso en la fabricación de aditivos del caucho da lugar a
la liberación de una cantidad indeterminada de morfolina a la
hidrosfera y la geosfera como consecuencia del desgaste de los
neumáticos y de la eliminación de neumáticos usados.
Componente de parafinas y abrillantadores, la morfolina se libera
al medio ambiente por volatilización. Es adsorbida rápidamente por la
humedad, y el principal compartimiento de posible acumulación de la
morfolina es, por tanto, la hidrosfera. No obstante, los datos
limitados disponibles parecen indicar que el producto no se acumula en
la hidrosfera.
La incineración es el método preferido de eliminación de la
morfolina no diluida, pero a veces es necesario controlar las
emisiones de óxido de nitrógeno para respetar las reglamentaciones en
materia de medio ambiente. Por lo que se refiere a los efluentes
acuosos, el tratamiento de fangos activados es suficiente, a condición
de que la planta sea cuidadosamente controlada (véase más arriba).
5. Niveles ambientales y exposición humana
No hay datos disponibles sobre los niveles de morfolina en el
aire ambiental y de locales cerrados y en el agua de bebida. Hay
datos limitados sobre su presencia en aguas naturales, y se carece de
información sobre su presencia en el suelo.
A tenor de los datos disponibles, la fuente principal de
exposición de la población general a la morfolina son los alimentos,
que pueden estar contaminados como consecuencia del tratamiento
directo de la fruta con parafinas contenedoras de morfolina a efectos
de conservación, de los tratamientos a base de vapor empleados durante
la elaboración de los alimentos, y del uso de material de envasado con
morfolina. No obstante, los datos cuantitativos disponibles acerca de
la contaminación de los alimentos por morfolina y NMOR son limitados.
Por ejemplo, en productos lácteos preenvasados se han hallado valores
comprendidos entre 5 y 77 µg/kg de morfolina y de hasta 3,3 µg/kg de
NMOR. La concentración de morfolina en diversas muestras de alimentos
(pescado, carne, productos vegetales, bebidas) no rebasaba por lo
general el valor de 1 mg/kg. Se han detectado niveles más altos
(hasta 71,1 mg/kg) en frutos cítricos en el Japón. En un estudio
realizado en Italia trabajando con un límite de detección de 0,3 µg/kg
no se halló NMOR en una serie de alimentos. Los datos disponibles no
permiten hacer una estimación de la ingesta de morfolina y NMOR a
través de los alimentos.
Se ha detectado morfolina en tabaco de cigarrillos a una
concentración de 0,3 mg/kg, y en tabaco en polvo y tabaco mascable a
concentraciones de hasta 4,0 mg/kg. En otras ocasiones se ha
notificado el hallazgo de nitrosomorfolina a niveles de hasta
0,7 mg/kg en el tabaco en polvo. En estos casos el producto provenía
probablemente de la parafina de los envases utilizados.
Se ha detectado NMOR en algunos productos cosméticos y de
tocador, como por ejemplo champús y maquillaje de ojos, así como en
artículos de goma, tales como chupetes y tetillas de biberón, a
niveles de hasta 3,5 mg/kg.
En varias industrias puede darse una exposición ocupacional a la
morfolina, pero se dispone de pocos datos sobre la exposición de
trabajadores al producto. Todos los valores notificados son
inferiores a 3 mg/m3. Se ha detectado la exposición ocupacional a
NMOR en la industria del caucho, donde se han hallado concentraciones
de hasta 250 µg/m3.
Los datos actualmente disponibles permiten hacerse una idea del
riesgo potencial de exposición humana, pero no estimar con exactitud
los niveles de exposición de las poblaciones general y laboral a la
morfolina y la NMOR.
6. Cinética y metabolismo en animales de laboratorio y en el hombre
La morfolina se absorbe por vía oral, por vía cutánea y por
inhalación. En la rata, tras su administración oral o intravenosa, la
morfolina se distribuye rápidamente y se concentra sobre todo en el
intestino y el músculo.
En el conejo, la morfolina administrada por vía intravenosa o por
inhalación se concentra preferentemente en los riñones, y en menor
medida en los pulmones, el hígado y la sangre.
La morfolina no se une de forma importante a las proteínas del
plasma. Se han notificado semividas plasmáticas de 115 (rata),
120 (hámster) y 300 minutos (cobayo).
La morfolina se excreta principalmente inalterada por vía renal
en diversas especies. Al cabo de un día de su administración, se
halló en la orina el 70%-90% de la morfolina. La neutralización de la
morfolina acelera su excreción. Un pequeño porcentaje se excreta a
través del aire espirado y de las heces.
Estudios realizados en la rata, el ratón, el hámster y el conejo
muestran que la morfolina se elimina casi enteramente en su forma no
metabolizada. En el cobayo, puede darse una reacción de
N-metilación seguida de N-oxidación, metabolizándose así hasta un
20% de la dosis administrada. En presencia de nitrito, la morfolina
se puede transformar en NMOR tanto in vitro como in vivo. En
función de la dosis, entre el 0% y el 12% de la morfolina administrada
a ratas junto con nitritos puede sufrir nitrosación.
La inmunoestimulación, que entraña la activación de macrófagos,
puede aumentar el grado de nitrosación.
7. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
En lo que respecta a la toxicidad aguda, la DL50 de la
morfolina administrada oralmente es de 1-1,9 g/kg de peso corporal y
0,9 g/kg de peso corporal en la rata y el cobayo, respectivamente.
Las ratas que recibieron morfolina neutralizada (1 g/kg de peso
corporal) sobrevivieron. Tras administración interperitoneal, la
DL50 fue de 0,4 g/kg de peso corporal en el ratón y de 0,1 -
0,4 g/kg de peso corporal en la rata. En los experimentos de
exposición por inhalación, la DL50 fue de aproximadamente 8 g/m3
en la rata y de entre 5 y 7 g/m3 en el ratón. Por vía cutánea la
DL50 en el conejo fue de 0,5 ml/kg de morfolina no diluida. La
intoxicación aguda por morfolina se caracteriza por la aparición de
hemorragia gastrointestinal y diarrea tras la exposición oral, y de
irritación y hemorragias en la nariz, la boca, los ojos y los pulmones
tras la inhalación. En un estudio realizado durante 30 días con ratas
a las que se administraron con sonda dosis de 0,16 - 0,8 g/kg de peso
corporal, se observaron efectos tóxicos graves y mortalidad a todas
las dosis empleadas. En el cobayo se observó también toxicidad grave
y mortalidad a todas las dosis en el margen de 0,09 a 0,45 g/kg de
peso corporal.
Se ha notificado la aparición de alteraciones de la función
pulmonar en la rata tras la exposición a morfolina por inhalación
durante cortos periodos (7,2 g/m3, 4 h/día, 4 días y 1,63 g/m3,
4 h/día, 5 días/semana, 30 días). La mortalidad en la rata osciló
entre 0% y 100% según el nivel de exposición (0,36 - 18,1 g/m3,
6 h/día, 9 días). La toxicidad por inhalación dependía de la dosis,
observándose diversos grados de irritación local (ojos, boca, nariz,
pulmones) y hemorragias a los niveles más altos de exposición. En un
estudio se detectó un aumento de la función de la glándula tiroides, y
en otro necrosis del hígado y de los túbulos renales, como resultado
de la exposición por inhalación.
Un estudio de 90 días de duración reveló que la morfolina
administrada por vía oral (0,2 - 0,7 g/kg de peso corporal al día)
durante ese espacio de tiempo puede reducir el aumento del peso
corporal y la función renal en el ratón. Se ha notificado la
aparición de hiperplasia del epitelio del estómago anterior en el
ratón como resultado de la exposición oral a morfolina
(0,28 - 0,5 g/kg de peso corporal al día) durante 672 días.
En un estudio de 13 semanas sobre los efectos de su inhalación,
la morfolina (0,09 - 0,9 g/m3, 6 h/día, 5 días/semana) causó
lesiones dosis-dependientes de la mucosa nasal y neumonía a los
niveles de exposición más altos (0,36 y 0,9 mg/m3). Un cierto
número de parámetros no variaron en respuesta al tratamiento cuando se
utilizaron 0,09 g/m3; esta concentración puede considerarse el nivel
sin efectos adversos observados (NOAEL) en las condiciones de
exposición por inhalación subcrónica.
La morfolina no diluida y no neutralizada es altamente irritante
para los ojos y la piel, probablemente a causa de sus propiedades
alcalinas. La dilución y la neutralización de su pH pueden reducir
considerablemente su toxicidad tópica. La morfolina (2%) no indujo
sensibilidad en el cobayo al aplicar el método modificado de Buehler.
La morfolina no indujo la aparición de mutaciones en bacterias o
levaduras, con o sin activación metabólica (salvo en un caso a una
concentración muy alta). Se obtuvieron resultados negativos en el
ensayo realizado por mediación de un huésped.
La morfolina no indujo reparación del ADN en hepatocitos
primarios de rata, así como tampoco un aumento importante del
intercambio de cromátides hermanas en células ováricas de hámster
chino. Se consideró que la morfolina tenía efectos ligeramente
mutagénicos en el ensayo con células L5178Y de linfoma de ratón. El
producto aumentó los focos de tipo III en el ensayo de transformación
de células malignas BALB/3T3, lo que no ocurrió con la morfolina
neutralizada.
La morfolina no causó ni mutaciones puntuales ni aberraciones
cromosómicas en embriones de hámster expuestos in utero.
No se observó ningún aumento de la incidencia de tumores en ratas
sometidas a niveles de hasta 0,5 g/m3 de morfolina por inhalación
durante 104 semanas, así como tampoco en ratones que ingirieron oleato
de morfolina al 1% con el agua de bebida durante 96 semanas. En un
estudio de larga duración realizado con un grupo de 104 ratas que
recibieron 1000 mg de morfolina/kg dieta, se observaron tres casos de
carcinoma de células hepáticas, dos de pulmón y uno de angiosarcoma
(no especificado), así como dos gliomas malignos, mientras que en un
grupo testigo de 156 ratas no se observaron tumores. No se detectaron
tumores en hámsters sometidos a idénticas condiciones.
La morfolina administrada al mismo tiempo que nitrito da lugar a
resultados positivos en el ensayo realizado por mediación de un
huésped, probablemente debido a la formación de NMOR. La ingestión
simultánea de morfolina y nitrito indujo la aparición de tumores
hepáticos y pulmonares en la rata y de tumores hepáticos en el
hámster, probablemente a causa de la formación endógena de NMOR. La
NMOR es mutágena en bacterias y levaduras; se notificaron resultados
ligeramente positivos en lo que respecta al intercambio de cromátides
hermanas en células CHO, así como a la aparición de mutaciones en
células L5178Y de linfoma de ratón. La NMOR es carcinógena en el
ratón, la rata, el hámster y diversos peces, y produce tumores de
hígado y de pulmón en el ratón; de hígado, riñón y vasos sanguíneos en
la rata; de hígado y de las vías digestivas y respiratorias superiores
en el hámster, y de hígado en peces.
8. Efectos en el hombre
No se han descrito casos de intoxicación aguda o de efectos a
corto o largo plazo de la exposición a morfolina en la población
general.
En informes sobre la exposición ocupacional a la morfolina se ha
descrito el fenómeno conocido como visión azul o glaucopsia, así como
algunos casos de irritación de la piel y del tracto respiratorio, pero
sin hacer mención de las concentraciones atmosféricas de morfolina.
Se señaló que el número de aberraciones cromosómicas en los linfocitos
de sangre periférica de trabajadores expuestos durante 3 a 10 años a
concentraciones de morfolina de 0,54 - 0,93 mg/m3 no diferían
significativamente del número hallado en los testigos.
La morfolina no diluida es muy irritante para la piel; una
solución diluida (1/40) tuvo efectos moderadamente irritantes.
No se ha investigado el potencial carcinógeno de la morfolina en
poblaciones humanas expuestas.
9. Efectos sobre otros organismos en el laboratorio y en el terreno
Entre los microorganismos acuáticos estudiados, determinadas
cianobacterias (Microcystis) y algas verdes unicelulares
(Scenedesmus) son al parecer las especies más sensibles según se
desprende de los valores de la toxicidad liminal (empleando como
criterio la inhibición del crecimiento de la población) notificados
(duración de la exposición: 8 días): 1,7 mg/litro para Microcystis,
y 4,1 mg/litro para Scenedesmus.
Bacterias aerobias tales como Pseudomonas resultaron ser mucho
más resistentes: la toxicidad liminal a las 16 horas y la NOEC para
el crecimiento de la población se han cifrado, respectivamente, en
310 y 8700 mg/litro. No obstante, una concentración de 1000 mg/litro
inhibía la respiración y la actividad deshidrogenasa (hasta un 20%) en
fangos activados de plantas de tratamiento industrial.
Entre los protozoos acuáticos analizados hasta ahora, la mayor
sensibilidad corresponde a ejemplares de los géneros Entosiphon y
Chilomonas (con toxicidades liminales de 12 y 18 mg/litro,
respectivamente, para la inhibición del crecimiento de la población).
En Daphnia, la CE a las 24 horas (E = inmovilización) se ha
establecido en valores comprendidos entre 100 y 120 mg/litro. Los
valores notificados para la CL50 a las 48 - 96 horas en peces
estudiados en agua dulce, salobre o marina fueron > 180 mg/litro, y
la especial más sensible en este sentido es la trucha arco iris.
No se dispone de datos sobre los efectos a largo plazo en
invertebrados y vertebrados acuáticos. La información sobre la
toxicidad de la morfolina en organismos silvestres del suelo es casi
inexistente, pues se limita a una CE a los 3 días de aproximadamente
400 mg/litro para la inhibición de la germinación en la lechuga.
10. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
10.1 Evaluación de los efectos en la salud humana
La principal vía de exposición de la población general a la
morfolina es el consumo de alimentos contaminados. También puede
contribuir a la exposición general la contaminación del tabaco y de
los productos derivados del tabaco, así como de los artículos
cosméticos y de tocador y de los productos de goma. En numerosas
industrias se da una exposición ocupacional a la morfolina; el
compuesto es absorbido por inhalación y por vía cutánea. No hay datos
suficientes para determinar el grado de exposición de la población
general. Los datos disponibles sobre la exposición ocupacional al
producto también son limitados.
La morfolina no es altamente tóxica en condiciones de exposición
aguda. La DL50 tras administración oral es de 1 a 1,9 g/kg de peso
corporal en la rata y de 0,9 g/kg de peso corporal en el cobayo. Se
han notificado CL50 de 7,8 mg/m3 (rata) y 4,9 - 6,9 g/m3
(ratón).
En las condiciones de exposición por inhalación de corta y larga
duración, los efectos críticos son al parecer la irritación de los
ojos y las vías respiratorias. Puede considerarse que el NOAEL
corresponde a una concentración de 90 mg/m3 en las condiciones en
que se llevó a cabo el experimento de 13 semanas en la rata (6 h/día,
5 días/semana). En un estudio de larga duración (104 semanas) sobre
los efectos de la inhalación del producto se observó una mayor
incidencia de inflamación de la córnea y de inflamación y necrosis de
la cavidad nasal en ratas sometidas a 540 mg/m3. A concentraciones
de 36 y 180 mg/m3 se observó también una mayor incidencia de
irritación de los ojos y de la cavidad nasal.
Las exposiciones altas a la morfolina causan lesiones graves del
hígado y los riñones en la rata y el cobayo. Se ha notificado la
aparición de degeneración grasa del hígado en la rata como resultado
de la ingestión diaria de morfolina (0,5 g/kg de peso corporal)
durante 56 días. En el ratón la administración de una sal de
morfolina y ácido oleico en el agua de bebida a una dosis de
aproximadamente 0,7 g/kg de peso corporal al día durante 13 semanas
dio lugar a una hinchazón turbia de los túbulos proximales renales.
Se observó una disminución del aumento del peso corporal en los
ratones hembra del experimento de administración prolongada (672 días)
de una dieta con dosis comprendidas entre 0,05 y 0,4 g de morfolina
(en forma de sal del ácido oleico).
A los niveles que según se ha notificado alcanza actualmente la
exposición ocupacional y ambiental, no parece que la morfolina entrañe
ningún riesgo importante de efectos tóxicos sistémicos. Pueden
aparecer efectos locales (irritación) en los ojos y las vías
respiratorias en las exposiciones ocupacionales y accidentales no
controladas a altas concentraciones de morfolina transmitida por el
aire, y el contacto con morfolina líquida (incluso diluida) puede
causar irritación cutánea.
La morfolina no parece tener efectos mutágenos o carcinógenos en
los animales. No obstante, fácilmente puede nitrosarse y convertirse
en NMOR producto mutágeno y carcinógeno en varias especies de animales
de experimentación. En la rata, la morfolina ingerida secuencialmente
con nitrito dio lugar a un aumento de los tumores, sobre todo de
carcinoma hepatocelular y de sarcomas del hígado y los pulmones. Así
pues, por prudencia, conviene considerar que la exposición a la
morfolina aumenta el riesgo de carcinogénesis en las poblaciones
expuestas.
10.2 Evaluación de los efectos en el medio ambiente
Habida cuenta de los muy limitados conocimientos respecto a la
exposición ambiental, así como de la carencia de datos sobre los
efectos de la exposición de larga duración en el agua y la exposición
de corta y larga duración en el medio terrestre, por el momento no es
posible hacer una evaluación rigurosa de los riesgos. Sobre la base
de las propiedades conocidas de la morfolina, de la información
ecotoxicológica disponible y de los escasos datos sobre su
concentración en el medio ambiente, es posible extraer algunas
conclusiones.
Debido a la alta hidrosolubilidad de la morfolina y a su baja
volatilidad (en condiciones ambientales), su principal sumidero
ambiental es la hidrosfera.
La morfolina es por naturaleza biodegradable y, aunque la
degradación es lenta, no hay datos que lleven a pensar que se acumula
en la hidrosfera. Su bioacumulación es improbable.
Hay relativamente pocos datos sobre la toxicidad de la morfolina
en organismos silvestres. No obstante, parece improbable que los
niveles actuales de emisión de morfolina puedan causar daños
importantes en un radio de acción amplio. Restan por evaluar los
efectos locales, como por ejemplo los debidos a emisiones industriales
o a la morfolina liberada por el desgaste de los neumáticos.
La contaminación de algunos alimentos, como el pescado, por
morfolina puede deberse a contaminación ambiental, pero ello no es
seguro.
La conversión de la morfolina en NMOR es la principal causa de
inquietud, sobre todo en relación con las poblaciones de vertebrados.
Se ha notificado la presencia de NMOR en aguas residuales industriales
y en el suelo próximo a una fábrica. La presencia de morfolina en
agua destinada a transformarse por elaboración en agua de bebida
suscita preocupación.
11. Conclusiones y recomendaciones
La morfolina no entraña riesgos de toxicidad para el hombre a los
niveles habituales de exposición, pero hay que tener en cuenta que
puede transformarse en el carcinógeno NMOR.
No hay ningún indicio de que a los niveles actuales de exposición
la morfolina entrañe un riesgo sustancial para la biota en el medio
ambiente.
11.1 Recomendaciones para la protección de la salud humana
a) En la medida de lo posible debe evitarse la exposición humana a
la morfolina.
b) Debe evitarse que los alimentos se contaminen durante su envasado
y elaboración.
c) Se evitará que contengan morfolina los productos de goma con los
que el hombre haya de tener contacto directo.
d) No debe emplearse morfolina en la preparación de productos
cosméticos o de tocador.
e) Los efluentes industriales deben ser objeto de un riguroso
tratamiento para evitar la contaminación por morfolina del agua
de bebida.
f) Habida cuenta de la formación del carcinógeno NMOR es necesario
replantearse los actuales límites de exposición ocupacional.
11.2 Recomendaciones para la protección del medio ambiente
Debe evitarse que las plantas de tratamiento de efluentes sufran
derrames y sobrecargas.
11.3 Recomendaciones para ulteriores investigaciones
Deben emprenderse estudios sobre los siguientes temas:
a) toxicidad reproductiva en mamíferos;
b) toxicidad a largo plazo en mamíferos;
c) efecto de la exposición de mamíferos a niveles bajos de
morfolina, con y sin nitrito y nitrato;
d) transnitrosación por NMOR in vivo e in vitro;
e) biodegradación en condiciones anaerobias, sobre todo en
condiciones favorecedoras de la reducción de nitratos;
f) catálisis microbiana de la N-nitrosación en condiciones
realistas;
g) niveles ambientales de morfolina en las aguas subterráneas, el
suelo y los ríos a partir de los cuales se obtenga agua de
bebida;
h) niveles ambientales de morfolina en torno a las fábricas
productoras y procesadoras de morfolina;
i) metabolismo y toxicocinética en el hombre, como parte del
desarrollo de métodos para la vigilancia biológica de la
morfolina;
j) vigilancia de los niveles de morfolina y de NMOR en los
alimentos, el agua de bebida y el aire de locales cerrados;
k) se deben reunir y poner a disposición datos sobre la exposición
ocupacional.
