
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 175
ANTICOAGULANT RODENTICIDES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr M. Tasheva, National Centre of Hygiene,
Medical Ecology and Nutrition, Sofia, Bulgaria
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Anticoagulant rodenticides.
(Environmental health criteria ; 175)
1.Rodenticides 2.Anticoagulants
3.Occupational exposure I.Series
ISBN 92 4 157175 1 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT RODENTICIDES
Preamble
Introduction
1. SUMMARY
1.1. General
1.2. Properties and analytical methods
1.3. Sources of human and environmental exposure
1.4. Environmental distribution, levels and exposures
1.5. Mode of action and metabolism
1.6. Effects on mammals and in vitro test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
1.9. Evaluation and conclusion
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air, water and soil
4.1.2. Vegetation and wildlife
4.2. Transformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.2.1 Photolysis
4.2.2.2 Hydrolysis
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure
6. MODE OF ACTION AND METABOLISM
6.1. Vitamin K and its antagonists
6.2. Metabolism
6.2.1. Absorption, distribution and elimination
6.2.2. Metabolic transformation
6.2.3. Retention and turnover
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute effects
7.1.1. Rodent species
7.1.2. Non-target species
7.2. Short-term exposure
7.2.1. Rodent species
7.2.2. Non-target species
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.5. Reproductive toxicity and teratogenicity
7.6. Mutagenicity
7.7. Factors modifying toxicity
7.8. Adverse effects in domestic and farm animals
7.8.1. Domestic animals
7.8.1.1 Poisoning incidents
7.8.1.2 Diagnosis and treatment of poisoning
7.8.2. Farm animals
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Acute poisoning
8.1.2. Poisoning incidents
8.1.3. Controlled human studies
8.2. Monitoring of biological effects
8.2.1. Effects of short- and long-term exposure
8.2.2. Epidemiological studies
8.3. Developmental effects
8.4. Other adverse effects
8.5. Methods for assessing absorption and effects of
anticoagulant rodenticides
8.6. Treatment of anticoagulant rodenticide poisoning
8.6.1. Minimizing the absorption
8.6.2. Specific pharmacological treatment
8.6.2.1 Vitamin K1 (phytomenadione)
8.6.2.2 Blood components
8.6.2.3 Phenobarbital
8.6.3. Response to therapy
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.3. Terrestrial organisms
9.1.3.1 Acute toxicity
9.1.3.2 Primary toxicity
9.1.3.3 Secondary toxicity
9.2. Field observations
9.2.1. Primary poisonings
9.2.2. Secondary poisonings
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations for protection of human health and the
environment
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-15
from the National Institute of Environmental Health Sciences, National
Institutes of Health, USA, and by financial support from the European
Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
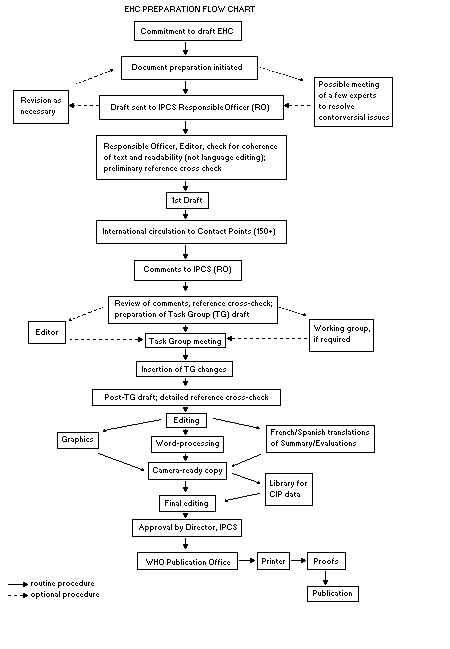 When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT
RODENTICIDES
Members
Dr N. Gratz, Commugny, Switzerland
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr W. Jacobs, Office of Pesticide Programs, US Environmental
Protection Agency, Washington, USA
Mrs M. Palmborg, Swedish Poison Information Centre, Stockholm, Sweden
Dr A.F. Pelfrène, Technology Sciences Group (TSG) International Inc.,
Brussels, Belgium (Chairman)
Mr D. Renshaw, Health Aspects of Environment and Food (Medical),
Department of Health, London, United Kingdom
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (Rapporteur)
Dr C. Vermeer, University of Limburg, Maastricht, Netherlands
Observers
Dr A. Buckle, ZENECA Public Health, Haslemere, Surrey, United Kingdom
(Representative of GIFAP)
Dr Y. Cohet, Lipha SA, Lyon, France (Representative of GIFAP)
Secretariat
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT RODENTICIDES
A WHO Task Group on Environmental Health Criteria for
Anticoagulant Rodenticides met in Geneva from 14 to 18 November 1994.
Dr R. Plestina, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO).
The first draft was prepared by Dr M. Tasheva of the National
Centre of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria.
The second draft was prepared by Dr R. Plestina, incorporating
comments received following the circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs. The
Task Group reviewed and revised the draft document and made an
evaluation of risks for human health and the environment from exposure
to anticoagulant rodenticides. Dr R. Plestina and Dr P.G. Jenkins,
both members of the IPCS Central Unit, were responsible for the
overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AAPCC American Association of Poison Control Centers
DT50 degradation time for 50% of a compound
EC50 median effect concentration
FD fluorescence detection
GC gas chromatography
HPLC high-performance liquid chromatography
I50 concentration of an inhibitor causing 50% inhibition of an
enzyme under given conditions
IUPAC International Union of Pure and Applied Chemistry Kal
adsorption coefficient
LD50 median lethal dose
MS mass spectrometry
MTD maximum tolerated dose
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PT prothrombin time
PTT partial thromboplastin time
WISN warfarin-induced skin necrosis
INTRODUCTION
The anticoagulants included in this review are those that are
used as rodenticides. The development of coumarin anticoagulants
occurred during the Second World War and they were introduced as
effective antithrombotic agents for treatment of thromboembolic
disease in humans. Warfarin has been used both as a drug and a
rodenticide, and has been extensively evaluated. Several
hydroxycoumarin and indandione derivatives have been synthesized and
introduced as effective rodenticides. They act by interfering with
the blood coagulation mechanism.
The appearance of rat strains resistant to warfarin and some
other anticoagulants has stimulated the development of more potent,
second-generation anticoagulants, some of which are also "single dose"
anticoagulants or "superwarfarins".
Many anticoagulant rodenticides are known, but it is not the aim
of this monograph to include all available information on each
compound. The purpose is to describe the general characteristics of
anticoagulants, using suitable illustrations to indicate their impact
on humans and the environment.
A distinction needs to be made between the characteristics of the
technical compounds and those of their formulated products concerning
the risks that their use poses to human health and the environment.
1. SUMMARY
1.1 General
The anticoagulants described in this monograph are those used
mainly in agriculture and urban rodent control. Warfarin, the first
widely used anticoagulant rodenticide, was introduced as an effective
agent for treatment of thromboembolic disease in humans.
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories, hydroxycoumarins and indandiones,
although their mechanisms of action are similar.
1.2 Properties and analytical methods
Anticoagulant rodenticides come in a solid crystalline or powder
form, and are slightly soluble in water. Most of them are stable
under normal storage conditions.
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography.
1.3 Sources of human and environmental exposure
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
first-generation anticoagulants led to the development of more potent,
second-generation anticoagulants. The concentrations of active
ingredients in baits vary according to the efficacy of the
rodenticides.
1.4 Environmental distribution, levels and exposures
Anticoagulant rodenticides are used mainly as bait formulations.
Since their volatility is low, concentrations in the air will be
negligible. As they are only slightly soluble in water, their use is
unlikely to be a source of water contamination.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant foodstuffs are
expected.
Non-target vertebrates are exposed to rodenticides primarily
through consumption of bait and secondarily from consumption of
poisoned rodents. Small pellets and whole grain baits are highly
attractive to birds.
Warfarin is used as a therapeutic agent for thromboembolic
disease.
There is a potential for occupational exposure to anticoagulant
rodenticides during manufacture, formulation and bait application, but
data on the levels of exposure are not available.
1.5 Mode of action and metabolism
Anticoagulant rodenticides are vitamin K antagonists. The main
site of their action is the liver, where several of the blood
coagulation precursors undergo vitamin-K-dependent posttranslation
processing before they are converted into the respective procoagulant
zymogens. The point of action appears to be the inhibition of K1
epoxide reductase.
Anticoagulant rodenticides are easily absorbed from the
gastrointestinal tract, and may also be absorbed through the skin and
respiratory system. After oral administration, the major route of
elimination in various species is through the faeces.
The metabolic degradation of warfarin and indandiones in rats
mainly involves hydroxylation. However, the second-generation
anticoagulants are mainly eliminated as unchanged compounds. The low
urinary excretion precludes isolation of metabolites from the urine.
The liver is the main organ for accumulation and storage of
rodenticide anticoagulants. Accumulation also occurs in the fat.
1.6 Effects on mammals and in vitro test systems
Signs of poisoning in rats and mice are those associated with
increased bleeding tendency.
There is wide variation in the LD50 of anticoagulant
rodenticides, toxicity being greatest by the oral route. Dermal and
inhalation toxicities of anticoagulants are also high.
Some anticoagulants show a similar range of acute toxicity for
non-target mammals as for target rodents, but toxicity spectra for
anticoagulants may vary between species.
Following repeated oral administration in rats, the main effects
seen are those associated with the anticoagulant action.
There are few data available on repeated exposure of non-rodent
species.
One study on warfarin in rats has indicated developmental
effects. Otherwise, there is no convincing evidence that
anticoagulants are teratogenic in experimental animals.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on individual compounds to demonstrate an absence of mutagenicity.
Strain, sex and diet are important factors modifying the toxicity of
anticoagulants in rodents.
Poisoning incidents in domestic animals after consumption of
anticoagulant baits have been reported. Fatalities and severe
clinical syndromes are generally due to the second-generation
anticoagulants. The major difference between warfarin and the other
anticoagulants (both indandiones and second-generation
hydroxycoumarins) is that they have a longer retention time in the
body and consequently a more prolonged effect than warfarin.
Therefore in cases of poisoning, antidote treatment with vitamin K1
needs to be continued for a longer period.
1.7 Effects on humans
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxications from occupational
exposure to anticoagulants have also occurred. Symptoms of acute
intoxication by anticoagulant rodenticides range from increased
bleeding tendency in minor or moderate poisoning to massive
haemorrhage in more severe cases. The signs of poisoning develop with
a delay of one to several days after absorption.
Warfarin is associated in humans with the induction of
developmental malformations when taken as a therapeutic agent during
pregnancy. No cases of developmental defects following the use of
anticoagulants as rodenticides have been reported.
The plasma prothrombin concentration is one guide to the severity
of intoxication. This is a more sensitive indication than overall
tests such as prothrombin time. In repeated occupational exposure,
direct measurement of either trace amounts of circulating
descarboxyprothrombin or circulating vitamin K 2,3-epoxide may provide
a more sensitive assessment.
Treatment of anticoagulant poisoning is graded according to the
severity of intoxication. Specific pharmacological treatment consists
of parenteral administration of vitamin K1 with, in serious cases,
co-administration of blood components. Measurement of prothrombin
time helps to determine the effectiveness and required duration of
treatment.
1.8 Effects on other organisms in the laboratory and field
The possible effects of anticoagulant rodenticides on non-target
organisms can be considered to fall into two categories: primary
(direct poisoning through consumption of bait) and secondary (through
consumption of poisoned rodents).
In the form of the technical product, anticoagulants are highly
toxic to fish. As bait formulations they are unlikely to present any
hazard because of their low water solubility. For this reason, they
will not be available to fish unless misused.
Bird species vary in their susceptibility to anticoagulant
rodenticides. It is difficult to assess the risks to birds resulting
from direct consumption because most published studies consist of
toxicity trials in laboratory conditions. The attractiveness of whole
grain bait to small birds increases the risk in field conditions.
Secondary toxicity laboratory studies with wildlife have shown
that captive predators can be intoxicated by no-choice feeding with
anticoagulant-poisoned or -dosed prey. Some deaths of predators in
the field have been reported.
1.9 Evaluation and conclusion
Anticoagulant rodenticides disrupt the normal blood-clotting
mechanisms, resulting in increased bleeding tendency and,
eventually, profuse haemorrhage.
Unintentional exposure of the general population to anticoagulant
rodenticides is unlikely.
Occupational contact is a potential source of significant
exposure. It may occur during manufacture and formulation as well as
during bait preparation and application.
Anticoagulant rodenticide compounds are readily absorbed from the
gastrointestinal tract, and through the skin and respiratory system.
The liver is the major organ for accumulation and storage. The plasma
prothrombin concentration is a suitable guide to the severity of acute
intoxication and to the effectiveness and required duration of the
therapy.
The specific antidote is vitamin K1.
The major difference between first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention and therefore tend to lead to a longer period of bleeding.
Most anticoagulants are stable under conditions of normal use.
Their low water solubility and low concentration in baits make them
unlikely to be a source of water contamination. They also appear to
bind quickly to soil particles, with very slow desorption and no
leaching properties.
Non-target organisms are potentially at risk from direct
consumption of baits (primary hazard) and from eating poisoned rodents
(secondary hazard).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories:
* hydroxycoumarins:
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT
RODENTICIDES
Members
Dr N. Gratz, Commugny, Switzerland
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr W. Jacobs, Office of Pesticide Programs, US Environmental
Protection Agency, Washington, USA
Mrs M. Palmborg, Swedish Poison Information Centre, Stockholm, Sweden
Dr A.F. Pelfrène, Technology Sciences Group (TSG) International Inc.,
Brussels, Belgium (Chairman)
Mr D. Renshaw, Health Aspects of Environment and Food (Medical),
Department of Health, London, United Kingdom
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (Rapporteur)
Dr C. Vermeer, University of Limburg, Maastricht, Netherlands
Observers
Dr A. Buckle, ZENECA Public Health, Haslemere, Surrey, United Kingdom
(Representative of GIFAP)
Dr Y. Cohet, Lipha SA, Lyon, France (Representative of GIFAP)
Secretariat
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT RODENTICIDES
A WHO Task Group on Environmental Health Criteria for
Anticoagulant Rodenticides met in Geneva from 14 to 18 November 1994.
Dr R. Plestina, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO).
The first draft was prepared by Dr M. Tasheva of the National
Centre of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria.
The second draft was prepared by Dr R. Plestina, incorporating
comments received following the circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs. The
Task Group reviewed and revised the draft document and made an
evaluation of risks for human health and the environment from exposure
to anticoagulant rodenticides. Dr R. Plestina and Dr P.G. Jenkins,
both members of the IPCS Central Unit, were responsible for the
overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AAPCC American Association of Poison Control Centers
DT50 degradation time for 50% of a compound
EC50 median effect concentration
FD fluorescence detection
GC gas chromatography
HPLC high-performance liquid chromatography
I50 concentration of an inhibitor causing 50% inhibition of an
enzyme under given conditions
IUPAC International Union of Pure and Applied Chemistry Kal
adsorption coefficient
LD50 median lethal dose
MS mass spectrometry
MTD maximum tolerated dose
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PT prothrombin time
PTT partial thromboplastin time
WISN warfarin-induced skin necrosis
INTRODUCTION
The anticoagulants included in this review are those that are
used as rodenticides. The development of coumarin anticoagulants
occurred during the Second World War and they were introduced as
effective antithrombotic agents for treatment of thromboembolic
disease in humans. Warfarin has been used both as a drug and a
rodenticide, and has been extensively evaluated. Several
hydroxycoumarin and indandione derivatives have been synthesized and
introduced as effective rodenticides. They act by interfering with
the blood coagulation mechanism.
The appearance of rat strains resistant to warfarin and some
other anticoagulants has stimulated the development of more potent,
second-generation anticoagulants, some of which are also "single dose"
anticoagulants or "superwarfarins".
Many anticoagulant rodenticides are known, but it is not the aim
of this monograph to include all available information on each
compound. The purpose is to describe the general characteristics of
anticoagulants, using suitable illustrations to indicate their impact
on humans and the environment.
A distinction needs to be made between the characteristics of the
technical compounds and those of their formulated products concerning
the risks that their use poses to human health and the environment.
1. SUMMARY
1.1 General
The anticoagulants described in this monograph are those used
mainly in agriculture and urban rodent control. Warfarin, the first
widely used anticoagulant rodenticide, was introduced as an effective
agent for treatment of thromboembolic disease in humans.
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories, hydroxycoumarins and indandiones,
although their mechanisms of action are similar.
1.2 Properties and analytical methods
Anticoagulant rodenticides come in a solid crystalline or powder
form, and are slightly soluble in water. Most of them are stable
under normal storage conditions.
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography.
1.3 Sources of human and environmental exposure
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
first-generation anticoagulants led to the development of more potent,
second-generation anticoagulants. The concentrations of active
ingredients in baits vary according to the efficacy of the
rodenticides.
1.4 Environmental distribution, levels and exposures
Anticoagulant rodenticides are used mainly as bait formulations.
Since their volatility is low, concentrations in the air will be
negligible. As they are only slightly soluble in water, their use is
unlikely to be a source of water contamination.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant foodstuffs are
expected.
Non-target vertebrates are exposed to rodenticides primarily
through consumption of bait and secondarily from consumption of
poisoned rodents. Small pellets and whole grain baits are highly
attractive to birds.
Warfarin is used as a therapeutic agent for thromboembolic
disease.
There is a potential for occupational exposure to anticoagulant
rodenticides during manufacture, formulation and bait application, but
data on the levels of exposure are not available.
1.5 Mode of action and metabolism
Anticoagulant rodenticides are vitamin K antagonists. The main
site of their action is the liver, where several of the blood
coagulation precursors undergo vitamin-K-dependent posttranslation
processing before they are converted into the respective procoagulant
zymogens. The point of action appears to be the inhibition of K1
epoxide reductase.
Anticoagulant rodenticides are easily absorbed from the
gastrointestinal tract, and may also be absorbed through the skin and
respiratory system. After oral administration, the major route of
elimination in various species is through the faeces.
The metabolic degradation of warfarin and indandiones in rats
mainly involves hydroxylation. However, the second-generation
anticoagulants are mainly eliminated as unchanged compounds. The low
urinary excretion precludes isolation of metabolites from the urine.
The liver is the main organ for accumulation and storage of
rodenticide anticoagulants. Accumulation also occurs in the fat.
1.6 Effects on mammals and in vitro test systems
Signs of poisoning in rats and mice are those associated with
increased bleeding tendency.
There is wide variation in the LD50 of anticoagulant
rodenticides, toxicity being greatest by the oral route. Dermal and
inhalation toxicities of anticoagulants are also high.
Some anticoagulants show a similar range of acute toxicity for
non-target mammals as for target rodents, but toxicity spectra for
anticoagulants may vary between species.
Following repeated oral administration in rats, the main effects
seen are those associated with the anticoagulant action.
There are few data available on repeated exposure of non-rodent
species.
One study on warfarin in rats has indicated developmental
effects. Otherwise, there is no convincing evidence that
anticoagulants are teratogenic in experimental animals.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on individual compounds to demonstrate an absence of mutagenicity.
Strain, sex and diet are important factors modifying the toxicity of
anticoagulants in rodents.
Poisoning incidents in domestic animals after consumption of
anticoagulant baits have been reported. Fatalities and severe
clinical syndromes are generally due to the second-generation
anticoagulants. The major difference between warfarin and the other
anticoagulants (both indandiones and second-generation
hydroxycoumarins) is that they have a longer retention time in the
body and consequently a more prolonged effect than warfarin.
Therefore in cases of poisoning, antidote treatment with vitamin K1
needs to be continued for a longer period.
1.7 Effects on humans
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxications from occupational
exposure to anticoagulants have also occurred. Symptoms of acute
intoxication by anticoagulant rodenticides range from increased
bleeding tendency in minor or moderate poisoning to massive
haemorrhage in more severe cases. The signs of poisoning develop with
a delay of one to several days after absorption.
Warfarin is associated in humans with the induction of
developmental malformations when taken as a therapeutic agent during
pregnancy. No cases of developmental defects following the use of
anticoagulants as rodenticides have been reported.
The plasma prothrombin concentration is one guide to the severity
of intoxication. This is a more sensitive indication than overall
tests such as prothrombin time. In repeated occupational exposure,
direct measurement of either trace amounts of circulating
descarboxyprothrombin or circulating vitamin K 2,3-epoxide may provide
a more sensitive assessment.
Treatment of anticoagulant poisoning is graded according to the
severity of intoxication. Specific pharmacological treatment consists
of parenteral administration of vitamin K1 with, in serious cases,
co-administration of blood components. Measurement of prothrombin
time helps to determine the effectiveness and required duration of
treatment.
1.8 Effects on other organisms in the laboratory and field
The possible effects of anticoagulant rodenticides on non-target
organisms can be considered to fall into two categories: primary
(direct poisoning through consumption of bait) and secondary (through
consumption of poisoned rodents).
In the form of the technical product, anticoagulants are highly
toxic to fish. As bait formulations they are unlikely to present any
hazard because of their low water solubility. For this reason, they
will not be available to fish unless misused.
Bird species vary in their susceptibility to anticoagulant
rodenticides. It is difficult to assess the risks to birds resulting
from direct consumption because most published studies consist of
toxicity trials in laboratory conditions. The attractiveness of whole
grain bait to small birds increases the risk in field conditions.
Secondary toxicity laboratory studies with wildlife have shown
that captive predators can be intoxicated by no-choice feeding with
anticoagulant-poisoned or -dosed prey. Some deaths of predators in
the field have been reported.
1.9 Evaluation and conclusion
Anticoagulant rodenticides disrupt the normal blood-clotting
mechanisms, resulting in increased bleeding tendency and,
eventually, profuse haemorrhage.
Unintentional exposure of the general population to anticoagulant
rodenticides is unlikely.
Occupational contact is a potential source of significant
exposure. It may occur during manufacture and formulation as well as
during bait preparation and application.
Anticoagulant rodenticide compounds are readily absorbed from the
gastrointestinal tract, and through the skin and respiratory system.
The liver is the major organ for accumulation and storage. The plasma
prothrombin concentration is a suitable guide to the severity of acute
intoxication and to the effectiveness and required duration of the
therapy.
The specific antidote is vitamin K1.
The major difference between first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention and therefore tend to lead to a longer period of bleeding.
Most anticoagulants are stable under conditions of normal use.
Their low water solubility and low concentration in baits make them
unlikely to be a source of water contamination. They also appear to
bind quickly to soil particles, with very slow desorption and no
leaching properties.
Non-target organisms are potentially at risk from direct
consumption of baits (primary hazard) and from eating poisoned rodents
(secondary hazard).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories:
* hydroxycoumarins:
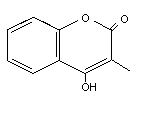 * indandiones:
* indandiones:
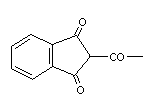 The common and chemical names of the rodenticides are given in
Table 1. Trade names, chemical structures, RTECS and CAS numbers,
molecular formulae and relative molecular masses are listed in
Table 2.
2.2 Physical and chemical properties
Anticoagulant rodenticides are solids (crystalline or powders),
slightly soluble in water (Table 3) and readily soluble in acetone.
Most of them are stable under normal storage conditions.
Table 1. Identity of anticoagulant rodenticides
Common name CAS name IUPAC name
First generation hydroxycoumarins
Coumachlor 3-[1-(4-chlorophenyl)-3 oxobutyl]-4-hydroxy- 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumafuryl 3-[1-(2-furanyl)-3 oxobutyl]-4-hydroxy- 3-[1-(2-furyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumatetralyl 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthalenyl)- 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthyl) coumarin
2H-1-benzopyran-2-one
Warfarin 4-hydroxy-3-(3-oxo-1-phenylbutyl-2H-1-benzopyran-2-one (RS)4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin
Second generation hydroxycoumarins
Brodifacoum 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[3-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-
1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 1-naphthyl]-4-hydroxycoumarin
Bromadiolone 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1- 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-
phenylpropyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difenacoum 3-[3-(1,1'-biphenyl)-4-yl-1,2,3,4-tetrahydro-1- 3-(3-biphenyl-4-yl-1,2,3,4-tetrahydro-1-naphthyl)-
naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difethialone 3-[3-(4-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[1RS,3RS;1RS,3SR)-3-(4'-bromobiphenyl-4-yl)-1,2,3,4-
1-naphthalenyl]-4-hydroxy-2H-1-benzothiopyran-2-one tetrahydro-1-naphthyl]-4-hydroxy-1-benzothi-in-2-one
Flocoumafen 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-[-(trifluoromethyl) 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-
phenyl]methoxy]phenyl-1-naphthalenyl]-2H-1-benzopyran-2-one (4-trifluoromethylbenzyloxy)phenyl]-1-naphthyl] coumarin
Table 1 (contd).
Common name CAS name IUPAC name
Indandione derivatives
Chlorophacinone 2-[(4-chlorophenyl)phenylacetyl]-1H-indene-1,3 (2H)-dione 2-[2-(4-chlorophenyl)-2-phenylacetyl]indan-1,3-dione
Diphacinone 2-(diphenylacetyl)-1H-indene-1,3 (2H)-dione 2-(diphenylacetyl)indan-1,3-dione
Pindone 2-(2,2-dimethyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-pivaloylindan-1,3-dione
Valone 2-(3-methyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-isovaleryl-1,3-indandione
Table 2. Names, structures and identification details
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Brodifacoum Finale GN4934750 56073-10-0 C31H23BrO3 523.4
Folgorat
Havoc
Klerat
Matikus
Mouser
Ratak +
Rodend
Talon
Volak
Volid
The common and chemical names of the rodenticides are given in
Table 1. Trade names, chemical structures, RTECS and CAS numbers,
molecular formulae and relative molecular masses are listed in
Table 2.
2.2 Physical and chemical properties
Anticoagulant rodenticides are solids (crystalline or powders),
slightly soluble in water (Table 3) and readily soluble in acetone.
Most of them are stable under normal storage conditions.
Table 1. Identity of anticoagulant rodenticides
Common name CAS name IUPAC name
First generation hydroxycoumarins
Coumachlor 3-[1-(4-chlorophenyl)-3 oxobutyl]-4-hydroxy- 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumafuryl 3-[1-(2-furanyl)-3 oxobutyl]-4-hydroxy- 3-[1-(2-furyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumatetralyl 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthalenyl)- 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthyl) coumarin
2H-1-benzopyran-2-one
Warfarin 4-hydroxy-3-(3-oxo-1-phenylbutyl-2H-1-benzopyran-2-one (RS)4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin
Second generation hydroxycoumarins
Brodifacoum 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[3-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-
1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 1-naphthyl]-4-hydroxycoumarin
Bromadiolone 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1- 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-
phenylpropyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difenacoum 3-[3-(1,1'-biphenyl)-4-yl-1,2,3,4-tetrahydro-1- 3-(3-biphenyl-4-yl-1,2,3,4-tetrahydro-1-naphthyl)-
naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difethialone 3-[3-(4-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[1RS,3RS;1RS,3SR)-3-(4'-bromobiphenyl-4-yl)-1,2,3,4-
1-naphthalenyl]-4-hydroxy-2H-1-benzothiopyran-2-one tetrahydro-1-naphthyl]-4-hydroxy-1-benzothi-in-2-one
Flocoumafen 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-[-(trifluoromethyl) 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-
phenyl]methoxy]phenyl-1-naphthalenyl]-2H-1-benzopyran-2-one (4-trifluoromethylbenzyloxy)phenyl]-1-naphthyl] coumarin
Table 1 (contd).
Common name CAS name IUPAC name
Indandione derivatives
Chlorophacinone 2-[(4-chlorophenyl)phenylacetyl]-1H-indene-1,3 (2H)-dione 2-[2-(4-chlorophenyl)-2-phenylacetyl]indan-1,3-dione
Diphacinone 2-(diphenylacetyl)-1H-indene-1,3 (2H)-dione 2-(diphenylacetyl)indan-1,3-dione
Pindone 2-(2,2-dimethyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-pivaloylindan-1,3-dione
Valone 2-(3-methyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-isovaleryl-1,3-indandione
Table 2. Names, structures and identification details
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Brodifacoum Finale GN4934750 56073-10-0 C31H23BrO3 523.4
Folgorat
Havoc
Klerat
Matikus
Mouser
Ratak +
Rodend
Talon
Volak
Volid
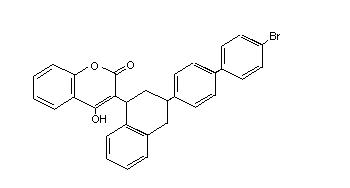 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Bromadiolone Apobas, Bromard, GN4934700 28772-56-7 C30H23BrO4 527.4
Bromorat, Bromatrol,
Contrac, Deadline,
Hurex, Lanirat,
Maki, Morfaron,
Musal, Ramortal,
Ratimon, Rodine-c,
Slaymor, Super-caid,
Topidon
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Bromadiolone Apobas, Bromard, GN4934700 28772-56-7 C30H23BrO4 527.4
Bromorat, Bromatrol,
Contrac, Deadline,
Hurex, Lanirat,
Maki, Morfaron,
Musal, Ramortal,
Ratimon, Rodine-c,
Slaymor, Super-caid,
Topidon
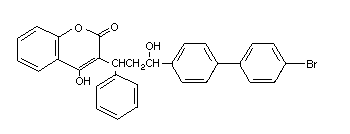 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Chlorophacinone Caid NK5335000 3691-35-8 C23H15ClO3 374.8
Delta
Drat
Lepit
Liphadione
Microzul
Muriol
Patrol
Quick
Raviac
Redentin OC
Rozol
Saviac
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Chlorophacinone Caid NK5335000 3691-35-8 C23H15ClO3 374.8
Delta
Drat
Lepit
Liphadione
Microzul
Muriol
Patrol
Quick
Raviac
Redentin OC
Rozol
Saviac
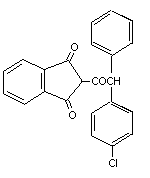 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumachlor Ratilan GN4830000 81-82-3 C19H15ClO4 342.8
Tomorin
(Discontinued by
Ciba-Geigy in 1984)
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumachlor Ratilan GN4830000 81-82-3 C19H15ClO4 342.8
Tomorin
(Discontinued by
Ciba-Geigy in 1984)
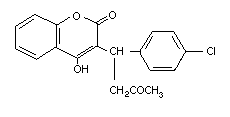 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumafuryl Fumarin GN4850000 117-52-2 C17H14O5 298.3
(Discontinued by
Rhône-Poulenc)
Fumasol
Kill-ko rat
Krumkil
Kumatox
Lurat
Mouse blues
Ratafin
Rat-a-way
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumafuryl Fumarin GN4850000 117-52-2 C17H14O5 298.3
(Discontinued by
Rhône-Poulenc)
Fumasol
Kill-ko rat
Krumkil
Kumatox
Lurat
Mouse blues
Ratafin
Rat-a-way
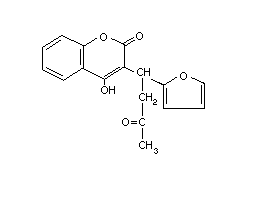 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumatetralyl Racumin GN7630000 5836-29-3 C19H16O3 292.4
Raukumin 57
Rodentin
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumatetralyl Racumin GN7630000 5836-29-3 C19H16O3 292.4
Raukumin 57
Rodentin
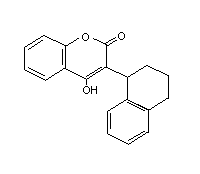 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difenacoum Compo GN4934500 56073-07-5 C31H24O3 444.5
Diphenacoum
Matrak
Neosorexa
Rastop
Ratak
Ratrick
Silo
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difenacoum Compo GN4934500 56073-07-5 C31H24O3 444.5
Diphenacoum
Matrak
Neosorexa
Rastop
Ratak
Ratrick
Silo
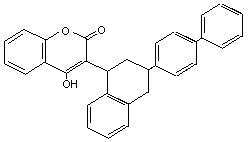 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difethialone Baraki DM0013800 104653-34-1 C31H23BrO2S 539.5
Frap
Quell
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difethialone Baraki DM0013800 104653-34-1 C31H23BrO2S 539.5
Frap
Quell
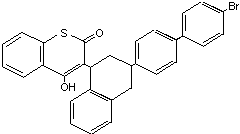 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Diphacinone Diphacine NK5600000 82-66-6 C23H16O3 340.4
Gold Crest
Kill-ko rat killer
Pid
Promar
Ramik
Ratindan 1
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Diphacinone Diphacine NK5600000 82-66-6 C23H16O3 340.4
Gold Crest
Kill-ko rat killer
Pid
Promar
Ramik
Ratindan 1
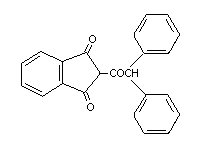 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Flocoumafen Stratagem DJ3100300 90035-08-8 C33H25F3O4 542.6
Storm
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Flocoumafen Stratagem DJ3100300 90035-08-8 C33H25F3O4 542.6
Storm
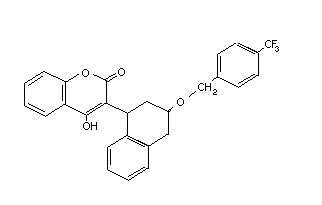 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Pindone Pivaldione NK6300000 83-26-1 C14H14O3 230.3
Pival
Pivalyn
Tri-ban
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Pindone Pivaldione NK6300000 83-26-1 C14H14O3 230.3
Pival
Pivalyn
Tri-ban
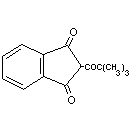 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Valone Motomco trading NK5775000 83-28-3 C14H14O3 230.3
powder
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Valone Motomco trading NK5775000 83-28-3 C14H14O3 230.3
powder
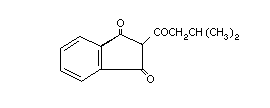 Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Warfarin Arthrombine-K GN4550000 81-81-2 C19H16O4 308.4
Dethmore
Panwarfin
Warfarat
Warfarin +
Warficide
Zoocoumarin
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Warfarin Arthrombine-K GN4550000 81-81-2 C19H16O4 308.4
Dethmore
Panwarfin
Warfarat
Warfarin +
Warficide
Zoocoumarin
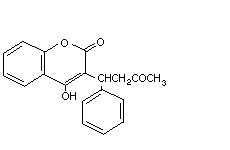 Table 3. Water solubility and vapour pressure of various anticoagulant rodenticides
Rodenticide Solubility Vapour pressure
in water (mg/litre) at temperature (°C) at pH mPa at temperature (°C)
Brodifacoum < 10 20 7 < 0.13 25
Bromadiolone 19 20 0.002 20
Chlorophacinone 100 20 negligible 20
Coumachlor 0.5 20 4.5 < 10 20
Coumatetralyl 4 20 4.2 8.5 × 10-6 20
20 20 5
425 20 7
Difenacoum < 10 20 7 0.16 45
Difethialone 0.39 25 0.074 25
Diphacinone 0.3 13.7 × 10-6 25
Flocoumafen 1.1 22 0.133 × 10-6 25
Pindone 18 25 very low 25
Warfarin practically insoluble
2.3 Analytical methods
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography
(Hunter, 1983; Hoogenboom & Rammell, 1983; Murphy et al., 1989;
O'Bryan & Constable, 1991; Chalermchaikit et al., 1993; Kelly et al.,
1993).
Warfarin is an acid which, in its hydrogenated form, is
practically insoluble in distilled water. At neutral or higher pH,
however, it is ionized and as such it readily dissolves in water. In
addition, compounds contaminating the water (such as proteins or
detergents) may substantially increase the solubility of warfarin.
Hunter (1983) developed a multi-residue method for the
determination of warfarin, coumatetralyl, bromadiolone, difenacoum and
brodifacoum in animal tissues by high-performance liquid
chromatography with fluorescence detection. A chloroformacetone (1:1)
mixture was significantly better than chloroform for the extraction of
residues of these rodenticides from liver tissues. Detection limits
in animal tissues of 2 µg/kg for coumatetralyl, difenacoum and
brodifacoum, 10 µg/kg for bromadiolone, and 20 µg/kg for warfarin
could be routinely achieved.
Felice et al. (1991) developed a reversed-phase liquid
chromatographic method with fluorescence detection for multicomponent
determination of the above-mentioned five rodenticides in blood serum
with detection limits of 10 to 20 ng/ml. Acetonitrile was used for
the extraction.
Braselton et al. (1992) developed a special method for confirming
the presence of indandione rodenticides (diphacinone and
chlorophacinone) in intoxicated domestic animals by using mass
spectrometry/mass spectrometry with collision-activated dissociation.
More details of analytical methods for individual rodenticides are
given in Table 4.
Table 4. Methods for the determination of anticoagulant rodenticides
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Animal tissues Chloroform-acetone (1:1) HPLC/FD 2 µg/kg coumatetralyl, difenacoum, Hunter (1983)
brodifacoum
10 µg/kg bromadiolone Hunter (1983)
20 µg/kg warfarin Hunter (1983)
Animal tissues Chloroform-acetone (1:1) HPLC/FDa 10 µg/kg warfarin Hunter (1985)
2 µg/kg other rodenticides Hunter (1985)
Serum Acetonitrile and diethyl ether HPLC 10 µg/litre brodifacoum, coumatetralyl, Felice et al. (1991)
difenacoum
20 µg/litre bromadiolone, warfarin Felice et al. (1981)
Serum Acetonitrile and diethyl ether HPLC 1 µg/litre brodifacoum Felice & Murphy (1989)
Serum twice with diethyl ether and HPLC/FD 3 µg/litre brodifacoum Murphy et al. (1989)
twice with acetonitrile-ether
(1:1)
Table 4 (contd).
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Plasma Acetonitrile-ethyl ether (9:1) HPLC/FD 2 µg/litre brodifacoum; no interference O'Bryan & Constable
with bromadiolone, (1991)
Liver tissue 5 µg/kg coumarin, difenacoum,
diphacinone, warfarin
and vitamin K1
Liver tissue Chloroform and acetone GC/MS 60 µg/kg protocol did not differentiate Ray et al. (1989)
between brodifacoum and
bromadiolone
a Post-column pH-switching fluorescence detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Anticoagulant rodenticides do not occur naturally in the
environment, although some plants do contain coumarinic derivatives.
Huebner & Link (1941), Overman et al. (1944) and Alstad et al. (1985)
described the anticoagulant properties of dicumarol found in spoiled
sweet clover and in connection with haemorrhagic disease in cattle.
3.2 Anthropogenic sources
Anticoagulant rodenticides are used worldwide, but figures for
the total world production are not available.
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
early anticoagulant rodenticides stimulated the development of
second-generation anticoagulants. About 95% of all commensal rodent
control in the USA is carried out with anticoagulants (Marsh, 1985a).
More than 50% of rodenticides used by professional pest controllers in
the USA contain brodifacoum (Dubock, 1986).
Depending on the toxicity of the rodenticide, the concentration
of the active ingredient varies from 0.005 to 0.05% for indandiones
and second-generation hydroxycoumarins and from 0.025 to 0.05% for
first-generation anticoagulants.
Anticoagulant rodenticides are available in a variety of
different formulations, including paraffin wax blocks, whole grain
baits, pelleted baits and tracking powder (FAO, 1979). Baits are the
most widely used formulations for rodent control.
Some manufacturers have added bittering agents, such as Bitrex
(denatonium benzoate), to anticoagulant baits. According to Kaukeinen
& Buckle (1992), adult humans found wax-block and pelleted placebo
baits containing denatonium benzoate (10 mg/kg) to be unpalatable.
However, the concentration of Bitrex cannot be increased to levels
that would make baits unpalatable to target rodents, and there is no
evidence that concentrations of Bitrex that target rodents readily
accept will deter bait-eating by non-target animals or by children
under 14 months of age.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air, water and soil
Since anticoagulant rodenticides are generally used as bait
formulations and have low volatility, increased levels in the air are
unlikely. As mentioned in section 2.2, most anticoagulants are
slightly soluble in water and therefore their use is unlikely to be a
source of water pollution.
Newby & White (1978) studied the adsorption and desorption of
14C-brodifacoum in soil under laboratory conditions. Adsorption
coefficients (kd) for course sand (pH 6.6), sandy clay loam (pH 7.1)
and calcareous sandy loam (pH 7.6) were 625, 1320 and 1180,
respectively, indicating strong adsorption to soil particles.
Adsorption equilibria were established fairly rapidly with the large
water:soil ratios used and despite very low brodifacoum water
solubility. Desorption was reported to be very slow and much less
than that required for a reversible interaction.
Lewis (1992b) applied 14C-difenacoum at 0.2 mg/kg (dry weight)
to a sandy soil with low humous content. After 142 days of incubation
(the approximate half-life of difenacoum in this soil type), two soil
samples were transferred to the top of soil columns. The columns were
eluted with deionized water at a rate and amount equivalent to
approximately 200 mm of rain falling onto the soil surface area
(91.6 cm2) for 50 h. The percentages of applied radioactivity
present in the leachates were 0.41 and 0.47%, representing only a very
small amount of leaching under these test conditions.
The leaching characteristics of aged soil residues of 14C-
brodifacoum in four soil types were investigated. 14C-
Brodifacoum was applied to soil at a nominal application rate of
0.4 mg/kg and incubated under aerobic conditions for 30 days. Samples
were taken and transferred to soil columns. After leaching, most of
the radioactivity applied to the soil was recovered in the top segment
of each column. No detectable levels of 14C residues were found in
the leachates. The results indicated that 14C-brodifacoum was
effectively immobile in all the soils tested (Jackson & Hall, 1992).
A study was carried out with 14C-bromadiolone in four types of
soil. With a soil rich in clay and organic compounds, bromadiolone
stayed in the superficial layer and scarcely moved. However, in soil
poor in clay and organic compounds, 67% of the added bromadiolone was
eluted (Spare et al., 1980).
4.1.2 Vegetation and wildlife
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant food stuffs are
expected. Unlike conventional crop protection products, which must be
applied over relatively large crop areas, rodenticides are applied to
discrete sites in the form of low concentration baits. Even if the
bait is spilled, it will not be taken up by plants.
Small pellets and whole grain baits are highly attractive to
birds and other non-target vertebrates. The formulation in wax blocks
consequently decreases the risk of primary poisoning of non-target
species.
Rodenticides may present a risk not only of primary poisoning
(from direct consumption of the bait) but also of secondary poisoning
(from consumption of poisoned rodents), in spite of the fact that many
of the target rodents die below ground in their burrows (Gorenzel et
al., 1982). Commensal and wild rodents poisoned by anticoagulants may
lead to the death of cats, pigs, foxes and birds of prey. The risk of
secondary poisoning depends mainly on the extent to which predators
feed on the target animals (Dubock, 1986).
4.2 Transformation
4.2.1 Biodegradation
Coveney & Forbes (1987) studied the degradation of flocoumafen in
rat carcasses, rat faeces, loose grain and wax block baits placed on
small soil plots. Overall losses of flocoumafen ranged from 85% to
95% over the 12-month study. The majority of the rodenticide present
in samples collected after 4 months was found in the upper 15 cm of
the soil. Only very small quantities were found in the lower soil
layers.
The degradation of 14C-difenacoum was studied in two standard
soils under controlled conditions for a period of 108 days.
Degradation time (DT50) values for the two soils were 146 and 439
days, indicating that difenacoum is a relatively long-lived compound
in soils (Lewis, 1992a).
Hall & Priestley (1992) monitored the metabolism of 14C-
brodifacoum in soil under aerobic conditions after applying it
at a nominal rate of 0.4 mg/kg and incubating for up to 52 weeks. A
mean total of 35.8% of the applied radioactivity was recovered as
14CO2 within the test period. 14C-Brodifacoum was the major
radiolabelled component in the soil extracts throughout the 52 weeks.
Under the conditions of the study the half-life of brodifacoum was
calculated to be 157 days.
A study was carried out with 14C-bromadiolone in four types of
soil. The rodenticide was degraded significantly with half-lives
ranging from 1.8 to 7.4 days (Wölkl & Galicia, 1992).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
A photolysis study was carried out with 14C-bromadiolone
(1 mg/litre) in a solution at pH 7.3 (Spare, 1982). The rodenticide
was very quickly degraded by exposure to artificial sunlight with a
half-life of 2.1 h.
The photolytic stability of 14C-difenacoum was investigated in
sterile buffered aqueous solutions of pH 5, 7 and 9 over a 24-h
irradiation period. The photolytic half-lives for total difenacoum
were calculated to be 3.26, 8.05 and 7.32 h at pH 5, 7 and 9,
respectively (Hall et al., 1992).
4.2.2.2 Hydrolysis
Lewis (1992c) studied the stability of 14C-difenacoum in
sterile buffered aqueous solutions of pH 5, 7 and 9. No hydrolysis
was observed at pH 5, at pH 7 there was very slow hydrolysis
(half-life estimated to be 847-1332 days), and at pH 9 the half-life
was estimated to be 77-85 days.
Jackson et al. (1991) studied the hydrolytic stability of
14C-brodifacoum (0.04 mg/kg) in sterile buffered aqueous solutions
at pH 5, 7 and 9 over a 30-day period. The hydrolytic half-life of
brodifacoum at pH 7 and 9 was found to be much greater than 30 days,
but precise calculation was not possible because the degradation seen
after one day did not continue.
Spare (1992) demonstrated that 14C-bromadiolone was slowly
hydrolysed in pH 5 buffer, with an estimated half-life of 392 days.
No degradation was observed at pH 7 and 9.
In the absence of a co-solvent, bromadiolone has a half-life of
67 days at pH 7 and 20°C (Morin, 1988). Degradation is more
significant in the presence of H3O+ ions, in saline water and at
increased temperatures.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
There is no information available on concentrations of
anticoagulant rodenticides in air, water and soil.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plants are expected.
Residues of difenacoum and brodifacoum were detected in the
bodies of 15 out of a total of 145 dead barn owls (Tylo alba) received
from various parts of the United Kingdom during the period 1983-1989.
Levels of difenacoum were in the range of 0.005-0.106 mg/kg body
weight, whilst levels of brodifacoum were in the range
0.019-0.515 mg/kg body weight (Newton et al., 1990).
Merson & Byers (1984) analysed eastern screech owl (Otus asio)
pellets following the application of 0.001% brodifacoum to an orchard
for rodent control. The brodifacoum residues in pellet samples ranged
from 0.06 to 0.09 mg/kg, indicating some exposure of the birds.
Hegdal & Colvin (1988) analysed screech owl tissues up to 52 days
after application of brodifacoum in an orchard. Brodifacoum was
detected in livers (detection limit = 0.3 mg/kg) from 9 out of 16
birds, the concentrations ranging from 0.3 to 0.8 mg/kg. No
detectable residues were found in the remainder of the carcasses
(detection limit = 0.1 mg/kg).
Hegdal & Blaskiewicz (1984) sampled six barn owls of different
ages in the vicinity of farm buildings treated with brodifacoum.
Analysis of carcasses revealed only one with trace (< 0.05 mg/kg)
levels of brodifacoum; the other carcasses did not contain detectable
concentrations.
Brodifacoum residues in the liver, muscle and fatty tissue of
rabbits poisoned during field trials with bait containing 0.005%
active ingredient were 4.4, 0.26 and 0.86 mg/kg, respectively. During
the same field trials, brodifacoum residues in seven poisoned birds of
various species ranged from 0.12 to 8.1 mg/kg in the liver, < 0.05 to
0.14 mg/kg in muscle and < 0.05 to 0.25 mg/kg in fatty tissue (Rammel
et al., 1984).
5.2 General population exposure
As mentioned in the previous section, residues are unlikely to be
found in plant foods. The use of dry baits to protect grain stores
can result in contamination of the stored food. Although on average
the concentration of residues would be expected to be low, occasional
areas of high concentration can occur.
With respect to residues in animals used for human food (pigs,
sheep and birds), there are no residue data concerning animals that
have survived anticoagulant poisoning. It should be emphasized,
however, that in some countries rodents are used as food.
Warfarin is widely used as a therapeutic agent.
5.3 Occupational exposure
Exposure may occur during manufacture, formulation and bait
application. The available information is discussed in section 8.2.
6. MODE OF ACTION AND METABOLISM
6.1 Vitamin K and its antagonists
Vitamin K is a collective name for a number of related compounds,
which all may function as co-enzymes for the enzyme gamma-glutamate
carboxylase. They all contain the functional naphthoquinone ring
structure, but differ in their aliphatic side chains. Vitamin K1
(phytomenadione) contains a side chain composed of four isoprenoid
residues, one of which is unsaturated. The vitamin K2 compounds
(menaquinones) have side chains which vary from 1 to 13 isoprenoid
residues, all of which are unsaturated. They are generally referred
to as MK-n, where n is the number of isoprenoid residues. Vitamin
K3 (menadione) has no side chain, but upon ingestion it is converted
into MK-4 by a liver enzyme. The two products commercially available
for human use are K1 and MK-4. Both are equally active, but for some
reason K1 is almost exclusively used in Europe and North America,
whereas MK-4 (also known as menatetrenone) is used in Asia, notably
Japan. K3 is not used any more for humans because of its adverse
side effect, haemolysis, but is frequently added to animal food.
Both 4-hydroxycoumarin derivatives and indandiones (also known as
oral anticoagulants) are antagonists of vitamin K. Their use as
rodenticides is based on the inhibition of the vitamin K-dependent
step in the synthesis of a number of blood coagulation factors. The
vitamin K-dependent proteins involved in the coagulation cascade
(Fig. 1) are the procoagulant factors II (prothrombin), VII
(proconvertin), IX (Christmas factor) and X (Stuart-Prower factor),
and the coagulation-inhibiting proteins C and S. All these proteins
are synthesized in the liver. Before they are released into the
circulation the various precursor proteins undergo substantial
(intracellular) post-translational modification. Vitamin K functions
as a co-enzyme in one of these modifications, namely the carboxylation
at well-defined positions of 10-12 glutamate residues into
gamma-carboxyglutamate (Gla). The presence of these Gla residues is
essential for the procoagulant activity of the various coagulations
factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its
oxidation to vitamin K 2,3-epoxide (KO) provides the energy required
for the carboxylation reaction. The epoxide is than recycled in two
reduction steps mediated by the enzyme KO reductase (Fig. 2). The
latter enzyme is the target enzyme for coumarin anticoagulants. Their
blocking of the KO reductase leads to a rapid exhaustion of the supply
of KH2, and thus to an effective prevention of the formation of Gla
residues. This leads to an accumulation of non-carboxylated
coagulation factor precursors in the liver. In some cases these
precursors are processed further without being carboxylated, and
(depending on the species) may appear in the circulation. At that
stage the under-carboxylated proteins are designated as descarboxy
coagulation factors (Stenflo et al., 1974; Nelsestuen et al., 1974).
Normal coagulation factors circulate in the form of zymogens, which
can only participate in the coagulation cascade after being activated
by limited proteolytic degradation (see Fig. 1). Descarboxy
coagulation factors have no procoagulant activity (i.e. they cannot be
activated) and neither they can be converted into the active zymogens
by vitamin K action. Whereas in anticoagulated humans high levels of
circulating descarboxy coagulation factors are detectable, these
levels are negligible in warfarin-treated rats and mice. Reviews by
Vermeer (1990) and Furie & Furie (1990) give further details.
Leck & Park (1981) compared the effects of warfarin and
brodifacoum on vitamin K metabolism and blood-clotting factor activity
in warfarin-susceptible and warfarin-resistant rats. In
warfarin-susceptible rats both brodifacoum and warfarin induced a
significant increase in the circulating KO (measured as the KO/K ratio
using 3H-vitamin K1), indicating that KO reductase is the target
enzyme for both drugs. However, whereas warfarin (1 mg/kg) only
inhibited the KO reductase in the susceptible strain, brodifacoum
(1 mg/kg) produced the same decrease of plasma prothrombin
concentration in both warfarin-susceptible and warfarin-resistant
animals.
The KO/K ratio in warfarin-resistant rats is five times higher
than in warfarin-susceptible animals. This is explained by the fact
that the hepatic KO reductase in the resistant animals has not only a
reduced affinity for warfarin, but also for KO. Hence the vitamin K
requirement of warfarin-resistant animals is 5-10 times higher than
that of warfarin-susceptible ones. Second-generation anticoagulants,
if given in doses which cause anticoagulation, further increase the
KO/K ratio (Leck & Park, 1981).
The much stronger potency of difenacoum and brodifacoum, as
vitamin K-antagonists, was reported by Park & Leck (1982), who
concluded that in the case of poisoning with these second-generation
anticoagulants it will be necessary to give repeated and frequent
doses of vitamin K to maintain clotting factor synthesis. The potency
of second-generation anticoagulants can be partly explained by their
highly lipophilic nature, which enables them to bind strongly to
membranes. Their target enzyme KO reductase is an integral membrane
protein with, in addition, a highly lipophilic nature. It is to be
expected that the dissociation of enzyme/inhibitor complexes will be
extremely slow. Moreover, their effectiveness in warfarin-resistant
rats demonstrates that the mutation leading to warfarin resistance
does not significantly affect their interaction with the KO reductase.
Table 3. Water solubility and vapour pressure of various anticoagulant rodenticides
Rodenticide Solubility Vapour pressure
in water (mg/litre) at temperature (°C) at pH mPa at temperature (°C)
Brodifacoum < 10 20 7 < 0.13 25
Bromadiolone 19 20 0.002 20
Chlorophacinone 100 20 negligible 20
Coumachlor 0.5 20 4.5 < 10 20
Coumatetralyl 4 20 4.2 8.5 × 10-6 20
20 20 5
425 20 7
Difenacoum < 10 20 7 0.16 45
Difethialone 0.39 25 0.074 25
Diphacinone 0.3 13.7 × 10-6 25
Flocoumafen 1.1 22 0.133 × 10-6 25
Pindone 18 25 very low 25
Warfarin practically insoluble
2.3 Analytical methods
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography
(Hunter, 1983; Hoogenboom & Rammell, 1983; Murphy et al., 1989;
O'Bryan & Constable, 1991; Chalermchaikit et al., 1993; Kelly et al.,
1993).
Warfarin is an acid which, in its hydrogenated form, is
practically insoluble in distilled water. At neutral or higher pH,
however, it is ionized and as such it readily dissolves in water. In
addition, compounds contaminating the water (such as proteins or
detergents) may substantially increase the solubility of warfarin.
Hunter (1983) developed a multi-residue method for the
determination of warfarin, coumatetralyl, bromadiolone, difenacoum and
brodifacoum in animal tissues by high-performance liquid
chromatography with fluorescence detection. A chloroformacetone (1:1)
mixture was significantly better than chloroform for the extraction of
residues of these rodenticides from liver tissues. Detection limits
in animal tissues of 2 µg/kg for coumatetralyl, difenacoum and
brodifacoum, 10 µg/kg for bromadiolone, and 20 µg/kg for warfarin
could be routinely achieved.
Felice et al. (1991) developed a reversed-phase liquid
chromatographic method with fluorescence detection for multicomponent
determination of the above-mentioned five rodenticides in blood serum
with detection limits of 10 to 20 ng/ml. Acetonitrile was used for
the extraction.
Braselton et al. (1992) developed a special method for confirming
the presence of indandione rodenticides (diphacinone and
chlorophacinone) in intoxicated domestic animals by using mass
spectrometry/mass spectrometry with collision-activated dissociation.
More details of analytical methods for individual rodenticides are
given in Table 4.
Table 4. Methods for the determination of anticoagulant rodenticides
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Animal tissues Chloroform-acetone (1:1) HPLC/FD 2 µg/kg coumatetralyl, difenacoum, Hunter (1983)
brodifacoum
10 µg/kg bromadiolone Hunter (1983)
20 µg/kg warfarin Hunter (1983)
Animal tissues Chloroform-acetone (1:1) HPLC/FDa 10 µg/kg warfarin Hunter (1985)
2 µg/kg other rodenticides Hunter (1985)
Serum Acetonitrile and diethyl ether HPLC 10 µg/litre brodifacoum, coumatetralyl, Felice et al. (1991)
difenacoum
20 µg/litre bromadiolone, warfarin Felice et al. (1981)
Serum Acetonitrile and diethyl ether HPLC 1 µg/litre brodifacoum Felice & Murphy (1989)
Serum twice with diethyl ether and HPLC/FD 3 µg/litre brodifacoum Murphy et al. (1989)
twice with acetonitrile-ether
(1:1)
Table 4 (contd).
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Plasma Acetonitrile-ethyl ether (9:1) HPLC/FD 2 µg/litre brodifacoum; no interference O'Bryan & Constable
with bromadiolone, (1991)
Liver tissue 5 µg/kg coumarin, difenacoum,
diphacinone, warfarin
and vitamin K1
Liver tissue Chloroform and acetone GC/MS 60 µg/kg protocol did not differentiate Ray et al. (1989)
between brodifacoum and
bromadiolone
a Post-column pH-switching fluorescence detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Anticoagulant rodenticides do not occur naturally in the
environment, although some plants do contain coumarinic derivatives.
Huebner & Link (1941), Overman et al. (1944) and Alstad et al. (1985)
described the anticoagulant properties of dicumarol found in spoiled
sweet clover and in connection with haemorrhagic disease in cattle.
3.2 Anthropogenic sources
Anticoagulant rodenticides are used worldwide, but figures for
the total world production are not available.
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
early anticoagulant rodenticides stimulated the development of
second-generation anticoagulants. About 95% of all commensal rodent
control in the USA is carried out with anticoagulants (Marsh, 1985a).
More than 50% of rodenticides used by professional pest controllers in
the USA contain brodifacoum (Dubock, 1986).
Depending on the toxicity of the rodenticide, the concentration
of the active ingredient varies from 0.005 to 0.05% for indandiones
and second-generation hydroxycoumarins and from 0.025 to 0.05% for
first-generation anticoagulants.
Anticoagulant rodenticides are available in a variety of
different formulations, including paraffin wax blocks, whole grain
baits, pelleted baits and tracking powder (FAO, 1979). Baits are the
most widely used formulations for rodent control.
Some manufacturers have added bittering agents, such as Bitrex
(denatonium benzoate), to anticoagulant baits. According to Kaukeinen
& Buckle (1992), adult humans found wax-block and pelleted placebo
baits containing denatonium benzoate (10 mg/kg) to be unpalatable.
However, the concentration of Bitrex cannot be increased to levels
that would make baits unpalatable to target rodents, and there is no
evidence that concentrations of Bitrex that target rodents readily
accept will deter bait-eating by non-target animals or by children
under 14 months of age.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air, water and soil
Since anticoagulant rodenticides are generally used as bait
formulations and have low volatility, increased levels in the air are
unlikely. As mentioned in section 2.2, most anticoagulants are
slightly soluble in water and therefore their use is unlikely to be a
source of water pollution.
Newby & White (1978) studied the adsorption and desorption of
14C-brodifacoum in soil under laboratory conditions. Adsorption
coefficients (kd) for course sand (pH 6.6), sandy clay loam (pH 7.1)
and calcareous sandy loam (pH 7.6) were 625, 1320 and 1180,
respectively, indicating strong adsorption to soil particles.
Adsorption equilibria were established fairly rapidly with the large
water:soil ratios used and despite very low brodifacoum water
solubility. Desorption was reported to be very slow and much less
than that required for a reversible interaction.
Lewis (1992b) applied 14C-difenacoum at 0.2 mg/kg (dry weight)
to a sandy soil with low humous content. After 142 days of incubation
(the approximate half-life of difenacoum in this soil type), two soil
samples were transferred to the top of soil columns. The columns were
eluted with deionized water at a rate and amount equivalent to
approximately 200 mm of rain falling onto the soil surface area
(91.6 cm2) for 50 h. The percentages of applied radioactivity
present in the leachates were 0.41 and 0.47%, representing only a very
small amount of leaching under these test conditions.
The leaching characteristics of aged soil residues of 14C-
brodifacoum in four soil types were investigated. 14C-
Brodifacoum was applied to soil at a nominal application rate of
0.4 mg/kg and incubated under aerobic conditions for 30 days. Samples
were taken and transferred to soil columns. After leaching, most of
the radioactivity applied to the soil was recovered in the top segment
of each column. No detectable levels of 14C residues were found in
the leachates. The results indicated that 14C-brodifacoum was
effectively immobile in all the soils tested (Jackson & Hall, 1992).
A study was carried out with 14C-bromadiolone in four types of
soil. With a soil rich in clay and organic compounds, bromadiolone
stayed in the superficial layer and scarcely moved. However, in soil
poor in clay and organic compounds, 67% of the added bromadiolone was
eluted (Spare et al., 1980).
4.1.2 Vegetation and wildlife
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant food stuffs are
expected. Unlike conventional crop protection products, which must be
applied over relatively large crop areas, rodenticides are applied to
discrete sites in the form of low concentration baits. Even if the
bait is spilled, it will not be taken up by plants.
Small pellets and whole grain baits are highly attractive to
birds and other non-target vertebrates. The formulation in wax blocks
consequently decreases the risk of primary poisoning of non-target
species.
Rodenticides may present a risk not only of primary poisoning
(from direct consumption of the bait) but also of secondary poisoning
(from consumption of poisoned rodents), in spite of the fact that many
of the target rodents die below ground in their burrows (Gorenzel et
al., 1982). Commensal and wild rodents poisoned by anticoagulants may
lead to the death of cats, pigs, foxes and birds of prey. The risk of
secondary poisoning depends mainly on the extent to which predators
feed on the target animals (Dubock, 1986).
4.2 Transformation
4.2.1 Biodegradation
Coveney & Forbes (1987) studied the degradation of flocoumafen in
rat carcasses, rat faeces, loose grain and wax block baits placed on
small soil plots. Overall losses of flocoumafen ranged from 85% to
95% over the 12-month study. The majority of the rodenticide present
in samples collected after 4 months was found in the upper 15 cm of
the soil. Only very small quantities were found in the lower soil
layers.
The degradation of 14C-difenacoum was studied in two standard
soils under controlled conditions for a period of 108 days.
Degradation time (DT50) values for the two soils were 146 and 439
days, indicating that difenacoum is a relatively long-lived compound
in soils (Lewis, 1992a).
Hall & Priestley (1992) monitored the metabolism of 14C-
brodifacoum in soil under aerobic conditions after applying it
at a nominal rate of 0.4 mg/kg and incubating for up to 52 weeks. A
mean total of 35.8% of the applied radioactivity was recovered as
14CO2 within the test period. 14C-Brodifacoum was the major
radiolabelled component in the soil extracts throughout the 52 weeks.
Under the conditions of the study the half-life of brodifacoum was
calculated to be 157 days.
A study was carried out with 14C-bromadiolone in four types of
soil. The rodenticide was degraded significantly with half-lives
ranging from 1.8 to 7.4 days (Wölkl & Galicia, 1992).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
A photolysis study was carried out with 14C-bromadiolone
(1 mg/litre) in a solution at pH 7.3 (Spare, 1982). The rodenticide
was very quickly degraded by exposure to artificial sunlight with a
half-life of 2.1 h.
The photolytic stability of 14C-difenacoum was investigated in
sterile buffered aqueous solutions of pH 5, 7 and 9 over a 24-h
irradiation period. The photolytic half-lives for total difenacoum
were calculated to be 3.26, 8.05 and 7.32 h at pH 5, 7 and 9,
respectively (Hall et al., 1992).
4.2.2.2 Hydrolysis
Lewis (1992c) studied the stability of 14C-difenacoum in
sterile buffered aqueous solutions of pH 5, 7 and 9. No hydrolysis
was observed at pH 5, at pH 7 there was very slow hydrolysis
(half-life estimated to be 847-1332 days), and at pH 9 the half-life
was estimated to be 77-85 days.
Jackson et al. (1991) studied the hydrolytic stability of
14C-brodifacoum (0.04 mg/kg) in sterile buffered aqueous solutions
at pH 5, 7 and 9 over a 30-day period. The hydrolytic half-life of
brodifacoum at pH 7 and 9 was found to be much greater than 30 days,
but precise calculation was not possible because the degradation seen
after one day did not continue.
Spare (1992) demonstrated that 14C-bromadiolone was slowly
hydrolysed in pH 5 buffer, with an estimated half-life of 392 days.
No degradation was observed at pH 7 and 9.
In the absence of a co-solvent, bromadiolone has a half-life of
67 days at pH 7 and 20°C (Morin, 1988). Degradation is more
significant in the presence of H3O+ ions, in saline water and at
increased temperatures.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
There is no information available on concentrations of
anticoagulant rodenticides in air, water and soil.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plants are expected.
Residues of difenacoum and brodifacoum were detected in the
bodies of 15 out of a total of 145 dead barn owls (Tylo alba) received
from various parts of the United Kingdom during the period 1983-1989.
Levels of difenacoum were in the range of 0.005-0.106 mg/kg body
weight, whilst levels of brodifacoum were in the range
0.019-0.515 mg/kg body weight (Newton et al., 1990).
Merson & Byers (1984) analysed eastern screech owl (Otus asio)
pellets following the application of 0.001% brodifacoum to an orchard
for rodent control. The brodifacoum residues in pellet samples ranged
from 0.06 to 0.09 mg/kg, indicating some exposure of the birds.
Hegdal & Colvin (1988) analysed screech owl tissues up to 52 days
after application of brodifacoum in an orchard. Brodifacoum was
detected in livers (detection limit = 0.3 mg/kg) from 9 out of 16
birds, the concentrations ranging from 0.3 to 0.8 mg/kg. No
detectable residues were found in the remainder of the carcasses
(detection limit = 0.1 mg/kg).
Hegdal & Blaskiewicz (1984) sampled six barn owls of different
ages in the vicinity of farm buildings treated with brodifacoum.
Analysis of carcasses revealed only one with trace (< 0.05 mg/kg)
levels of brodifacoum; the other carcasses did not contain detectable
concentrations.
Brodifacoum residues in the liver, muscle and fatty tissue of
rabbits poisoned during field trials with bait containing 0.005%
active ingredient were 4.4, 0.26 and 0.86 mg/kg, respectively. During
the same field trials, brodifacoum residues in seven poisoned birds of
various species ranged from 0.12 to 8.1 mg/kg in the liver, < 0.05 to
0.14 mg/kg in muscle and < 0.05 to 0.25 mg/kg in fatty tissue (Rammel
et al., 1984).
5.2 General population exposure
As mentioned in the previous section, residues are unlikely to be
found in plant foods. The use of dry baits to protect grain stores
can result in contamination of the stored food. Although on average
the concentration of residues would be expected to be low, occasional
areas of high concentration can occur.
With respect to residues in animals used for human food (pigs,
sheep and birds), there are no residue data concerning animals that
have survived anticoagulant poisoning. It should be emphasized,
however, that in some countries rodents are used as food.
Warfarin is widely used as a therapeutic agent.
5.3 Occupational exposure
Exposure may occur during manufacture, formulation and bait
application. The available information is discussed in section 8.2.
6. MODE OF ACTION AND METABOLISM
6.1 Vitamin K and its antagonists
Vitamin K is a collective name for a number of related compounds,
which all may function as co-enzymes for the enzyme gamma-glutamate
carboxylase. They all contain the functional naphthoquinone ring
structure, but differ in their aliphatic side chains. Vitamin K1
(phytomenadione) contains a side chain composed of four isoprenoid
residues, one of which is unsaturated. The vitamin K2 compounds
(menaquinones) have side chains which vary from 1 to 13 isoprenoid
residues, all of which are unsaturated. They are generally referred
to as MK-n, where n is the number of isoprenoid residues. Vitamin
K3 (menadione) has no side chain, but upon ingestion it is converted
into MK-4 by a liver enzyme. The two products commercially available
for human use are K1 and MK-4. Both are equally active, but for some
reason K1 is almost exclusively used in Europe and North America,
whereas MK-4 (also known as menatetrenone) is used in Asia, notably
Japan. K3 is not used any more for humans because of its adverse
side effect, haemolysis, but is frequently added to animal food.
Both 4-hydroxycoumarin derivatives and indandiones (also known as
oral anticoagulants) are antagonists of vitamin K. Their use as
rodenticides is based on the inhibition of the vitamin K-dependent
step in the synthesis of a number of blood coagulation factors. The
vitamin K-dependent proteins involved in the coagulation cascade
(Fig. 1) are the procoagulant factors II (prothrombin), VII
(proconvertin), IX (Christmas factor) and X (Stuart-Prower factor),
and the coagulation-inhibiting proteins C and S. All these proteins
are synthesized in the liver. Before they are released into the
circulation the various precursor proteins undergo substantial
(intracellular) post-translational modification. Vitamin K functions
as a co-enzyme in one of these modifications, namely the carboxylation
at well-defined positions of 10-12 glutamate residues into
gamma-carboxyglutamate (Gla). The presence of these Gla residues is
essential for the procoagulant activity of the various coagulations
factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its
oxidation to vitamin K 2,3-epoxide (KO) provides the energy required
for the carboxylation reaction. The epoxide is than recycled in two
reduction steps mediated by the enzyme KO reductase (Fig. 2). The
latter enzyme is the target enzyme for coumarin anticoagulants. Their
blocking of the KO reductase leads to a rapid exhaustion of the supply
of KH2, and thus to an effective prevention of the formation of Gla
residues. This leads to an accumulation of non-carboxylated
coagulation factor precursors in the liver. In some cases these
precursors are processed further without being carboxylated, and
(depending on the species) may appear in the circulation. At that
stage the under-carboxylated proteins are designated as descarboxy
coagulation factors (Stenflo et al., 1974; Nelsestuen et al., 1974).
Normal coagulation factors circulate in the form of zymogens, which
can only participate in the coagulation cascade after being activated
by limited proteolytic degradation (see Fig. 1). Descarboxy
coagulation factors have no procoagulant activity (i.e. they cannot be
activated) and neither they can be converted into the active zymogens
by vitamin K action. Whereas in anticoagulated humans high levels of
circulating descarboxy coagulation factors are detectable, these
levels are negligible in warfarin-treated rats and mice. Reviews by
Vermeer (1990) and Furie & Furie (1990) give further details.
Leck & Park (1981) compared the effects of warfarin and
brodifacoum on vitamin K metabolism and blood-clotting factor activity
in warfarin-susceptible and warfarin-resistant rats. In
warfarin-susceptible rats both brodifacoum and warfarin induced a
significant increase in the circulating KO (measured as the KO/K ratio
using 3H-vitamin K1), indicating that KO reductase is the target
enzyme for both drugs. However, whereas warfarin (1 mg/kg) only
inhibited the KO reductase in the susceptible strain, brodifacoum
(1 mg/kg) produced the same decrease of plasma prothrombin
concentration in both warfarin-susceptible and warfarin-resistant
animals.
The KO/K ratio in warfarin-resistant rats is five times higher
than in warfarin-susceptible animals. This is explained by the fact
that the hepatic KO reductase in the resistant animals has not only a
reduced affinity for warfarin, but also for KO. Hence the vitamin K
requirement of warfarin-resistant animals is 5-10 times higher than
that of warfarin-susceptible ones. Second-generation anticoagulants,
if given in doses which cause anticoagulation, further increase the
KO/K ratio (Leck & Park, 1981).
The much stronger potency of difenacoum and brodifacoum, as
vitamin K-antagonists, was reported by Park & Leck (1982), who
concluded that in the case of poisoning with these second-generation
anticoagulants it will be necessary to give repeated and frequent
doses of vitamin K to maintain clotting factor synthesis. The potency
of second-generation anticoagulants can be partly explained by their
highly lipophilic nature, which enables them to bind strongly to
membranes. Their target enzyme KO reductase is an integral membrane
protein with, in addition, a highly lipophilic nature. It is to be
expected that the dissociation of enzyme/inhibitor complexes will be
extremely slow. Moreover, their effectiveness in warfarin-resistant
rats demonstrates that the mutation leading to warfarin resistance
does not significantly affect their interaction with the KO reductase.
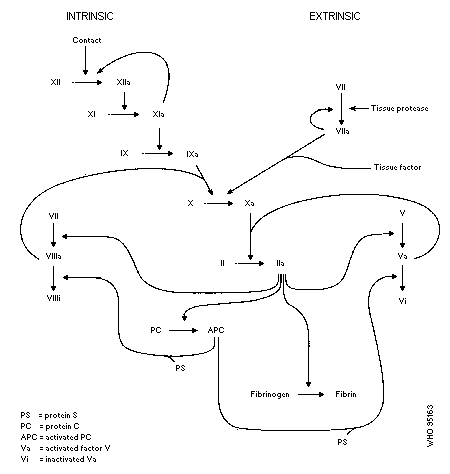
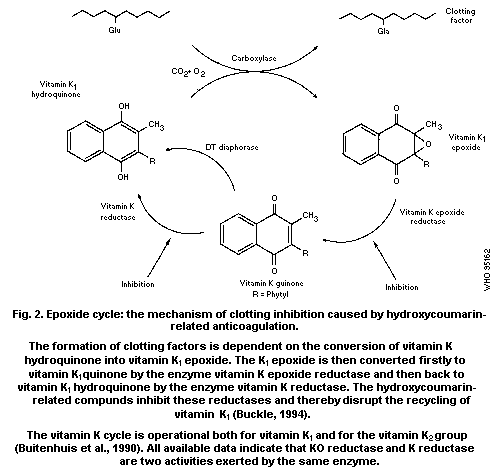 Vermeer & Soute (1992) compared the inhibition of each of the
three enzymes from the vitamin K cycle by four anticoagulants
(warfarin, flocoumafen, difenacoum and brodifacoum). The studies were
performed using in vitro enzyme systems prepared from rat, cow and
human liver. It was shown that in all three species the inhibitor
concentration required for 50% inhibition (I50) was comparable for
the KO reductase and K reductase activity, but that the I50 for
gamma-glutamylcarboxylase was 2-3 orders of magnitude higher. It was
concluded that for all four anticoagulants the reductions of KO and K
are the target reactions for inhibition. Moreover, it was found that
there is no species specificity of the inhibitors, which means that
they are equally active in cell-free systems derived from rat, cow and
human liver. Any species-dependent differences which might be found
in vivo will presumably be brought about by a different
pharmacokinetic or pharmacodynamic behaviour in these species.
6.2 Metabolism
6.2.1 Absorption, distribution and elimination
Anticoagulant rodenticides are easily absorbed through the
gastrointestinal tract, skin and respiratory system.
After a single oral dose of 14C-flocoumafen (0.14 mg/kg body
weight) to rats, the absorption into blood was rapid, reaching maximum
concentrations (0.03-0.05 µg/ml) in plasma within 4 h (Huckle et al.,
1989).
The major route of elimination in rats and sheep after oral
administration of anticoagulants is through the faeces. The
intestinal levels of brodifacoum in rats began increasing 24 to 72 h
after an oral dose of 0.2 mg/kg body weight (Bachmann & Sullivan,
1983). Faecal elimination of radiolabelled flocoumafen following an
oral dose of 0.14 mg/kg body weight accounted for 23-26% of the dose
over the 7-day period; approximately half of this was recovered within
the first 24 h. Less than 0.5% of the dose appeared in the urine
within 7 days (Huckle et al., 1989).
After single oral administration of brodifacoum (0.2 and 2 mg/kg
body weight) to sheep, about 20% and 30%, respectively, was excreted
in the faeces within 8 days (Laas et al., 1985).
A larger proportion of a percutaneous dose of 14C-flocoumafen
(0.17 mg/kg body weight) dissolved in acetone was found in the urine
of rats (10%) than in the case of an equivalent oral dose (less than
0.5%) over a 7-day period. Faecal elimination accounted for 31% of
the percutaneous dose (Huckle & Warburton, 1986b).
After oral 14C-flocoumafen doses of 0.02 mg/kg body weight or
0.1 mg/kg body weight were given to rats, once weekly for up to 14
weeks, approximately one-third of each weekly low dose was eliminated
through the faeces within 3 days, mostly within the first 24 h. At
the higher dose the elimination ranged from 18% after the first dose
to 59% after the tenth dose (Huckle et al., 1988).
Following repeated oral administration of 14C-flocoumafen to
rats at 0.02 mg/kg body weight per week for 14 weeks or 0.1 mg/kg body
weight per week for 10 weeks, appreciable accumulation was seen in the
liver. At both dose levels tissue concentrations were highest in the
liver, followed by the kidney > skin > muscle > fat > blood. The
hepatic residue in the low-dose group ranged from 0.1 mg/kg tissue
after one week to 2.1 mg/kg by week 14 (Huckle & Warburton, 1986a).
Brodifacoum could not be detected in the omental fat of sheep 8
days after the oral administration of 0.2 and 2 mg/kg body weight
(Laas et al., 1985).
6.2.2 Metabolic transformation
Warfarin is readily hydroxylated in vitro and in vivo by rat
liver microsomal enzymes to form 6-, 8- and, especially
7-hydroxy-warfarin (Ullrich & Staundinger, 1968; Ikeda et al.,
1986a,b). These inactive metabolites are to some extent conjugated
with glucuronic acid, undergo enterohepatic recirculation, and are
excreted in the urine and faeces (Ellenhorn & Barceloux, 1988).
The metabolic pattern of indandiones in rats also mainly involves
hydroxylation (Yu et al., 1982).
The second-generation anticoagulants have mainly been found as
unchanged compounds (Bachmann & Sullivan, 1983; Huckle et al., 1988).
The low urinary elimination following oral dosing has precluded
accurate isolation of metabolites in urine (Warburton & Hutson, 1985;
Waburton & Huckle, 1986; Huckle & Warburton, 1986a).
Following administration of flocoumafen, liver residues in rats
consisted mainly of unchanged flocoumafen, although in a repeat dose
study a polar metabolite was detected. Eight urinary metabolites were
detected after percutaneous exposure to 14C-flocoumafen (Huckle &
Warburton, 1986b).
Studies in male Japanese quail have shown more rapid metabolism
and elimination than in the rat following an oral dose of
14C-flocoumafen. Up to 12 radioactive components were detected in
the excreta (Huckle & Warburton, 1986c).
Bromadiolone, brodifacoum and coumatetralyl were also found in
rats as unchanged parent compounds, whereas in the case of difenacoum
metabolites predominated (Parmar et al., 1987). The metabolism and
elimination of the difenacoum trans isomer was more rapid than for the
cis isomer (Bratt, 1987).
The suggestion that the anticoagulant effect in rats is mediated
by the unchanged compound itself rather than by its metabolites has
been confirmed by the effects of phenobarbital and SKF525A
pretreatments on the general pattern of responses to warfarin and
brodifacoum (Bachmann & Sullivan, 1983).
6.2.3 Retention and turnover
Metabolic studies of anticoagulant rodenticides show that the
liver is the main organ of accumulation and storage. Liver
concentrations of brodifacoum after a single oral dose of 0.2 mg/kg
body weight to rats remained high and relatively constant for 96 h,
with a maximum of 5.0 mg/kg after 50 h (Bachmann & Sullivan, 1983).
A high degree of body retention was found 7 days after a single
oral dose of 0.14 mg/kg body weight 14C-flocoumafen (74-76% of the
administered dose); approximately half the dose was found in the liver
(Huckle et al., 1989).
Brodifacoum was detected in the liver of sheep 128 days after
oral administration (0.2 and 2 mg/kg body weight) in concentrations of
0.64 and 1.07 mg/kg dry weight (equivalent to 0.22 and 0.36 mg/kg wet
weight), respectively. The peak levels occurred at 2 days in the
high-dose group and at 8 days in the low-dose group, being 6.50 and
1.87 mg/kg dry weight (2.21 and 0.64 mg/kg wet weight), respectively
(Laas et al., 1985). Woody et al. (1992) observed an elimination
half-life for brodifacoum in serum of 6 ± 4 days in four dogs.
The largest proportion of a percutaneous flocoumafen dose of
0.17 mg/kg body weight was located in the liver (25% of the dose at a
concentration of 0.8 mg/kg), although this was 10 times lower than
that following an oral dose (Huckle & Warburton, 1986b).
Parmar et al. (1987) found that elimination of radiolabelled
brodifacoum, bromadiolone and difenacoum from the liver was biphasic,
consisting of an rapid initial phase lasting from days 2 to 8 after
dosing and a slower terminal phase when the elimination half-lives
were 130, 170 and 120 days, respectively. Elimination of
coumatetralyl was more rapid, with a half-life of 55 days.
Similar results for difenacoum were found by Bratt (1987). After
a single oral 14C-difenacoum dose of 1.2 mg/kg body weight, the
highest concentration of radioactivity (41.5% of the dose) was found
in the rat liver 24 h after dosing. The elimination from the liver
was biphasic. The half-life of elimination of the radioactivity
during the first rapid phase was three days, and for the slower phase
was 118 days. A similar biphasic elimination was also apparent in the
kidney. In the pancreas the concentration declined more slowly than
in any of the other tissues (182 days). The parent compound was the
major component in the liver 24 h after dosing (42%).
Unchanged flocoumafen comprised the major proportion of the
hepatic radioactivity in rats and was eliminated with a half-life of
220 days (Huckle et al., 1989). Veenstra et al. (1991) found
retention of about 8% of an administered flocoumafen dose of 0.4 mg/kg
in the liver of beagle dogs 43 weeks after dosing.
Despite the more rapid metabolism of flocoumafen in Japanese
quail, a proportion of the administered dose is retained in the liver,
with an elimination half-life of 115 days after oral dosing (Huckle &
Warburton, 1986c).
Six Hereford heifers weighing approximately 230 kg each were
dosed with diphacinone (1 mg/kg body weight) by injecting it into the
rumen. The highest residue level of parent compound found in the
liver was 0.15 mg/kg at days 30 and 90 after treatment. No detectable
levels (> 0.01 mg/kg) could be found in any of the other tissues
analysed (kidney, plasma, brain, heart, muscle and fat). The residues
in the liver were almost constant from 30 to 90 days post-treatment
(Bullard et al., 1976).
The plasma half-life of brodifacoum determined in three patients
with severe bleeding disorders was found to be approximately 16 to 36
days (Weitzel et al., 1990).
The half-life for disappearance from the plasma of human
volunteers given a single oral or intravenous warfarin dose of
1.5 mg/kg body weight varied from 15 to 58 h, with a mean of 42 h
(O'Reilly et al., 1963).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute effects
7.1.1 Rodent species
Wide variations exist in the literature for LD50 values of
anticoagulant rodenticides. A particular reason for these variables
is the use of single or repeated (5-day) doses. LD50 values also
vary according to the animal strain and sex, but both have not always
been indicated in reported data (Ashton et al., 1987)
(Tables 5 and 6).
Table 5. Acute oral LD50 values for various rodenticides in albino
Norway rats
Rodenticide LD50 (mg/kg) Reference
Brodifacoum 0.26 Redfern et al. (1976)
Bromadiolone 1.125 Grand (1976)
Chlorophacinone 20.5 Thomson (1988)
Coumachlor 900.0 Thomson (1988)
Coumafuril 0.4 Wiswesser (1976)
Coumatetralyl 16.5 Thomson (1988)
Difenacoum 1.8 Bull (1976)
Difethialone 0.56 Lechevin & Grand (1987)
Diphacinone 3.0 Thomson (1988)
Flocoumafen 0.46 Sharples (1983a)
Pindone 50.0 Tomlin (1994)
Warfarin 58.0 Thomson (1988)
Reported oral LD50 values for warfarin in rats vary by a
considerable magnitude. Values of 11 mg/kg body weight (Lund, 1982),
58 mg/kg body weight (Thomson, 1988) and 58 mg/kg body weight and
323 mg/kg body weight for female and male, respectively (Hagan &
Radomski, 1953), have been reported.
The second-generation anticoagulants are more toxic than the
first-generation ones in the sense that a single feeding may be
lethal. Apparent discrepancies between the single oral LD50 values
for Norway rats, as shown in Table 5, and the multiple dose oral
LD50 values, shown in Table 6, can be explained by the cumulative
effects resulting from multiple dose (5 day) administration (Table 6)
and characteristics of this family of compounds.
Table 6. Five-day oral LD50 (mg/kg body weight per day) of various anticoagulants
for the Norway rat (Rattus norvegicus)a
Anticoagulant Strainb Male Female Both sexes Number of
animalsc
Pindone SD 1.21 (0.70-2.11) 1.60 (0.83-3.08) 1.34 (0.87-2.06) 40
wild 7.60 (2.61-22.2) 25.60 (5.34-123) 12.80 (1.73-84.8) 40
Warfarin SD 0.29 (0.14-0.57) 0.38 (0.22-0.66) 0.33 (0.22-0.50) 40
wild 0.39 (0.16-0.91) 0.60 (0.23-1.09) 0.44 (0.25-0.76) 40
Diphacinone SD 0.19 (0.11-0.33) 0.23 (0.12-0.43) 0.21 (0.14-0.31) 40
wild 0.39 (0.15-0.84) 0.60 (0.22-0.57) 0.44 (0.23-0.54) 40
Chlorophacinone SD 0.18 (0.18-0.18) 0.20 (0.15-0.27) 0.19 (0.16-0.22) 40
wild 0.13 (0.10-0.19) 0.23 (0.14-0.36) 0.16 (0.12-0.22) 40
Bromadiolone SD 0.13 (0.10-0.19) 0.10 (0.08-0.13) 0.12 (0.10-0.15) 40
wild 0.06 (0.03-0.12) 0.09 (0.09-0.09) 0.07 (0.05-0.10) 16
a Modified from: Ashton et al. (1987); figures in parentheses represent 95% confidence limits
b SD = Sprague-Dawley
c 50% males and 50% females in all tests
Reported oral LD50 values for mice show similar variations,
from less than 1 mg/kg body weight for second-generation rodenticides
to 374 mg/kg body weight for warfarin (Hagan & Radomski, 1953; Redfern
et al., 1976; Bull, 1976; Sharples, 1983a).
Signs of anticoagulant poisoning in rats and mice include
lethargy, hunched posture and vein clearing in the ears. Blood around
the eyes, mouth and anus, indicating internal haemorrhaging, appears
prior to death (Sharples, 1984).
The percutaneous toxicity of anticoagulants to rats varied for
different compounds from an LD50 of 0.54 mg/kg body weight for
flocoumafen (master mix in corn oil) to > 50 mg/kg body weight for
brodifacoum and difenacoum (Price, 1985b; Tomlin, 1994). The signs of
intoxication were identical to those observed after an oral dose.
Second-generation anticoagulants appeared to be highly toxic by
inhalation. An acute inhalation study in Wistar rats exposed (nose
only) for 4 h was conducted using the 0.5% manufacturing master mix
specifically prepared to give a mass median aerodynamic diameter of
less than 5 µm. The acute LC50 values were between 0.16 and
1.4 mg/litre. Signs of intoxication, characteristic of an
anticoagulant action, were observed within 3 days after exposure with
deaths occurring between 4 and 9 days (Blair, 1984).
7.1.2 Non-target species
The acute toxicity of various anticoagulant rodenticides to
non-target mammalian species is presented in Table 7.
From the data presented it appears that some anticoagulants show
a similar range of acute toxicity for both non-target mammals and
target rodents.
7.2 Short-term exposure
7.2.1 Rodent species
In a study by Hadler (1974), groups of Wistar rats (male and
female) were given brodifacoum by gavage (0.01, 0.02, 0.05, 0.1 and
0.2 mg/kg body weight) for 5 consecutive days. All rats receiving the
two lowest doses survived the 21-day experimental period, but all rats
given the two highest doses died within 11 days of cessation of
dosing. No abnormalities were detected in surviving animals
sacrificed at the end of the observation period. In animals which
died during the study, only massive internal haemorrhages, mainly in
the peritoneum, were observed. The no-observed-effect level of
brodifacoum for Wistar rats was 0.02 mg/kg body weight per day.
Table 7. Acute oral toxicity (LD50, mg/kg) of various anticoagulant rodenticides for non-target mammalian speciesa
Rodenticide Guinea-pig Rabbit Dog Cat Sheep Pig References
Brodifacoum 2.78 (F) 0.29 (M) 0.25-1 (F) approx. 25 (F) > 25 (M) 0.5-2 Hadler (1975a,b); Parkinson
3.56 11-33 (1975, 1976); Godfrey (1984);
Godfrey et al. (1985)
Bromadiolone 2.8 1.0 10 (F) MTD > 25 MTD 3 Grand (1976)
Chlorophacinone 50 Pelfrène (1991)
Difenacoum 50 (F) 2 (M) approx. 50 100 100 80-100 Bull (1976); Tomlin (1994)
Diphacinone 35 3-7.5 14.7 150 Kosmin & Barlow (1976)
Difethialone 0.75 5 MTD > 16 MTD 2-3 Lechevin & Grand (1987)
Flocoumafen > 10 (M) 0.7 (M/F) 0.075-0.25 > 10 (M/F) > 5 approx. 60 (M,F) Sharples (1983b); Price (1985a);
(M/F) Chesterman et al. (1984);
Roberts et al. (1985a, 1986)
Warfarin 800 20-50 6-40 1-5 Anonymous (1976)
a F = female; M = male; MTD = maximum tolerated dose
Wistar rats (male and female) were continuously given a diet
containing brodifacoum at 0.1 mg/kg body weight, with no choice of
other feed, for 12 weeks. Prothrombin time was increased and
mortality occurred in 9 out of 20 males and 5 out of 20 females.
Macroscopic examination of the major organs of surviving rats revealed
no abnormalities other than those expected of anticoagulant action
(Hadler, 1976).
Feeding Fisher-344 rats with diets containing flocoumafen at
concentrations of 0.2 or 0.4 mg/kg feed for 5 days produced no
toxicologically significant effects during the 15-day observation
period (Price, 1985c).
In a 28-day feeding study in rats, fed on a diet containing 0,
0.01, 0.05, 0.1 or 0.2 mg-flocoumafen/kg, there were no overt signs of
toxicity. Highest-dose females showed a slight but significant
increase in mean prothrombin time (PT) and activated partial
thromboplastin time (PTT), and a slight decrease in mean plasma total
protein. No toxicological or pathological changes were observed in
rats fed diets containing 0.01 or 0.05 mg/kg of diet (Price, 1985d).
No effect on prothrombin time was observed in a 12-week study
with Wistar rats administered oral flocoumafen doses (by gavage) of
0.0125, 0.0625 and 0.125 mg/kg body weight once a week (Forsey, 1985).
Groups of six male and six female Sprague-Dawley rats were fed
diphacinone in their diets at concentrations of 0 (control), 0.0313,
0.0625, 0.125, 0.25 and 0.5 mg/kg diet (equivalent to 0, 1.7, 3.3,
6.4, 13 and 27 µg/kg body weight per day) for 90 days (Elias & Johns,
1981). Additional satellite groups of one rat of each sex and each
dose group were killed for gross pathological examination at 30 and
60 days. Mortality was unaffected by the treatment. In the survivors
of the main groups and satellite groups, there were no gross
pathological changes, but in the two males that died prematurely (in
the 0.0625 and 0.25 mg/kg groups) there was subdural haemorrhage.
Prothrombin time was unaffected by treatment. Routine haematological
and clinical chemistry tests were performed on only two rats of each
sex from each group, and no effects were observed apart from a reduced
blood fibrinogen concentration in both sexes at the highest dose
level.
As no clear NOAEL was indicated by the results of the 90-day
study, a 21-day study was performed using two rats of each sex at
diphacinone levels of 0 (control), 0.125, 0.25, 0.5, 1, 2 and 4 mg/kg
diet. All the rats in the 2 and 4 mg/kg groups died within the first
14 days, but all others survived until the end of the study. The mean
dosages received by the survivors were equivalent to 0, 9, 17, 34 and
67 µg/kg body weight per day. At 21 days, there was no effect on
prothrombin time. Gross necropsy revealed haemorrhage in the thymus
of one rat in the 0.5 mg/kg group, but no effects were seen in any
other survivors. All the animals in the 2 and 4 mg/kg groups had
massive and extensive internal haemorrhage (Elias & Johns, 1981).
7.2.2 Non-target species
There are few data available on the repeated exposure of
non-rodent species to anticoagulant rodenticides.
Death followed five daily warfarin doses of 3 mg/kg body weight
in cats and 1 mg/kg body weight in pigs (Tomlin, 1994). The route of
administration was not specified.
The dermal administration of warfarin (188 mg/kg per day) to
female baboons caused profuse bleeding in 5 days followed by death
(Dreyfus et al., 1983).
When it became known that vitamin K was not an antagonist for
warfarin action in bone, it became possible to study the long-term
effects of this anticoagulant in bones without the risk of inducing
haemorrhages and bleeding. By feeding lambs with high doses of
warfarin (up to 150 mg/kg body weight per day) under the protection of
4 mg/kg body weight per day of vitamin K, Pastoureau et al. (1993)
showed that within 3 months the lambs had developed a marked
osteopenia that resulted from a decrease in resorption and a much more
pronounced decrease in bone formation. As a result the bone density in
the warfarin-treated animals was substantially lower than that in
control animals. This is in agreement with earlier studies in rats
(Price et al., 1982) where it was found that, under the protection of
vitamin K, warfarin induced excessive mineralization and growth plate
closure, due to which bone growth came to a halt.
The maximum tolerated 5-day oral dose of bromadiolone was
considered to be 25 mg in pigs (Large White strain) weighing 25 kg.
After 45 oral daily doses of 0.5 mg no change in the prothrombin time
was observed (Grand, 1976).
Woody et al. (1992) studied the effect of a cumulative dose of
1.1 mg brodifacoum/kg body weight administered orally to dogs over a
3-day period. Signs of coagulopathic effects appeared within 24 h
(greater PT and PTT) and increased over a 10-day period. Treatment
with Vitamin K1 at 10 days post-exposure reduced the effects.
Six horses were treated by gavage with brodifacoum containing
bait at a dosage of 0.125 mg/kg body weight (Boermans et al., 1991).
Four of the horses became anorexic and depressed, one requiring K1
therapy. Peak plasma concentration occurred 2-3 h after
administration. Pharmacokinetic evaluation indicated that brodifacoum
has a plasma half-life of 1.22 days. An increase in clotting time was
observed as early as 24 h after dosing, returning to the pre-treatment
level by day 12.
Male pigs (five per group) were fed difenacoum in the diet for 14
days at dose levels of 0.01, 0.05, 0.1, 0.5 and 1.0 mg/kg diet. With
the exception of the lowest-dose group, all groups showed a marked
increase of prothrombin time values. Extensive subcutaneous, inter-
and intra-muscular haemorrhage and oedema was observed in animals
dosed at levels of 0.5 mg/kg or more (Ross et al., 1979).
7.3 Long-term exposure
No data on long-term exposure are available.
Studies with second-generation anticoagulants are difficult to
carry out for more than a few weeks due to the rapid acute effects,
and NOEL values for second-generation rodenticides and longer exposure
periods have not been established. In any chronic study approaching
two years in length, the dose level would have to be less than the
analytical limit of detection.
7.4 Skin and eye irritation; sensitization
Brodifacoum is a slight skin irritant and a mild eye irritant in
the rabbit (Hadler, 1975c). No skin or eye irritation was observed in
New Zealand white rabbits treated with flocoumafen (Forsey, 1983a,b).
Neither of these rodenticides was a skin sensitizer when tested in the
guinea-pig maximization test (Parkinson, 1979; Price, 1986).
7.5 Reproductive toxicity and teratogenicity
Brodifacoum was given by oral gavage to female rats at daily dose
levels of 0.001, 0.01 or 0.02 mg/kg body weight during days 6-15 of
pregnancy. There was no evidence of adverse effects on the fetus at
termination. Higher daily doses (above 0.05 mg/kg) caused an
anticoagulant effect in the dams which resulted in a high incidence of
abortion (Hodge et al., 1980a).
Pregnant female rabbits were given oral gavage doses of 0.001,
0.002 or 0.005 mg brodifacoum/kg body weight per day from days 6-18 of
pregnancy. A the highest dose level a high proportion of maternal
deaths occurred as a result of haemorrhage. Although the survivors
showed signs of haemorrhage, there were no effects on the developing
fetus. No effects were observed at either of the other dose levels
used (Hodge et al., 1980b,c).
Bromadiolone was given orally to four groups of 25 female rats
from day 6 to 15 of pregnancy at doses of 0, 17.5, 35 and 70 µg/kg
body weight per day. Maternal toxicity occurred at the higher dose
levels. There was no evidence of embryotoxicity or teratogenic
effects at any dose level (Monnot et al., 1981). A similar absence of
effects was reported in a study on rabbits treated orally with daily
doses of either 2, 4 or 8 µg/kg body weight per day on days 6-18 of
pregnancy, although there was maternal toxicity at the highest dose
level (Virat, 1981).
Groups of 18 pregnant F-344 rats were given daily oral doses of
0, 0.01 or 0.04 mg flocoumafen/kg body weight from day 8 to 17 of
gestation. Eight animals in the 0.04 mg/kg body weight group either
died or were killed with signs of anticoagulant poisoning.
In contrast, Mirkova & Antov (1983) found warfarin to be
embryotoxic and teratogenic to Wistar rats when administered by gavage
in single doses or repeatedly throughout the periods of
pre-implantation (1-7 days of gestation) and organogenesis (8-16
days), and also throughout the whole gestation (1-21 days) at a wide
range of dose levels (0.04-8 mg/kg body weight). At these dose levels
and treatment regimens, warfarin induced substantially increased rates
of embryolethality, subcutaneous and internal haemorrhage and gross
structural malformations (pes varus, internal hydrocephalus and
anomalies of skeletal ossification).
7.6 Mutagenicity
Various in vitro and in vivo studies have been undertaken to
assess the genotoxic potential of brodifacoum. No mutagenic activity
was detected in the Salmonella reverse mutation assay in any of the
five tester strains employed (TA98, TA100., TA1535, TA1537 and TA1538)
either in the presence or absence of Arochlor 1254-induced rat liver
S9 fraction at brodifacoum concentrations ranging from 1.6 to
5000 µg/plate (Callander, 1984). Brodifacoum showed no activity in a
forward mutation assay using L5178 mouse lymphoma cells, either with
or without metabolic activation, at concentrations of 47.5, 63.3 and
84.4 mg/litre (Cross & Clay, 1984).
Brodifacoum caused no significant chromosomal aberrations in
cultured human lymphocytes (concentrations 1, 10, 100 and
1000 mg/litre), either with or without metabolic activation, and did
not induce unscheduled DNA synthesis in cultured HeLa cells at the
same range of concentrations (Mellano, 1984a,b). Difenacoum did not
induce unscheduled DNA synthesis in rat hepatocytes in vivo at
either dose level or time-point (Kennelly, 1990).
An in vivo micronucleus test, in which mice were given single
brodifacoum intraperitoneal doses of 0.187 or 0.30 mg/kg body weight,
showed no induction of micronuclei in bone marrow polychromatic
erythrocytes (Sheldon et al., 1984).
Bromadiolone was tested in the Salmonella reverse mutation assay
at concentrations ranging from 10 to 3330 µg per plate on strains
TA1535, TA1537 and TA1538. No evidence of mutagenic effect was found
either with or without Aroclor metabolic activation (Lawlor, 1992).
Bromadiolone did not induce forward mutations in Chinese hamster
ovary cells either with or without metabolic activation
(Cifone, 1993).
In a mouse micronucleus test at four dose levels from 50 to
400 mg/kg, bromadiolone did not induce micronuclei in bone marrow
polychromatic erythrocytes (Murli, 1993).
Flocoumafen did not induce reverse gene mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 nor in
Escherichia coli WP2uvrA pkm 101 either with or without metabolic
activation. Flocoumafen was tested at concentrations ranging from 31
to 2000 µg/plate, beyond which precipitation from suspension occurred
(Brooks et al., 1984).
Flocoumafen did not increase the frequency of mutation to
6-thioguanine resistance in Chinese hamster V79 cells either in the
presence or absence of an Arochlor-induced rat liver S9 fraction.
Doses ranging from 5 to 150 mg/litre were used, beyond which
cytotoxicity occurred (Clare & Wiggins, 1986).
Flocoumafen did not induce in vitro cell transformation in
C3H1OT´ mouse fibroblasts, either in the presence or absence of a rat
S9 metabolizing system, at concentrations ranging from 12.5 to
100 µg/litre (Meyer & Wiggins, 1986).
Flocoumafen did not induce mitotic gene conversion in liquid
suspension cultures of Saccharomyces cerevisiae JD1, either in the
presence or absence of a rat liver S9 fraction, at concentrations
ranging from 0.01 to 2 g/litre (Brooks et al., 1984).
When incubated at concentrations ranging from 5 to 25 mg/litre
for 24 h in monolayer cultures of rat liver RL4 cells, flocoumafen did
not induce in vitro chromosomal damage (Brooks et al., 1984).
Oral administration of flocoumafen to rats at doses of 0.25 mg/kg
or 1000 mg/kg body weight (a dose 4000 times the acute oral LD50 for
rats) did not produce chromosomal damage (Allen et al., 1986).
The cytogenic effect of chlorophacinone was investigated in vivo
in metaphase bone marrow cells taken at 48 and 96 h after oral dosing
of male CFLP mice with 20 mg/kg body weight. No induction of
chromosomal aberrations was observed (Nehéz et al., 1985).
In the same study, chlorophacinone was investigated in
spermatocytes taken from male CFLP mice at 1, 2, 3 and 4 weeks after a
single oral dose of 20 mg/kg body weight. The spermatocytes were
analysed in the diakinesis phase of meiosis. There was no increased
incidence of chromosomal aberrations (Nehéz et al., 1985).
Rabbits were treated orally with 20 mg chlorophacinone/kg body
weight and chromosomal analysis was performed in bone marrow and
spermatocytes taken at 48 h post-dosing. No increase in the incidence
of chromosomal aberrations was seen (Selypes et al., 1984).
7.7 Factors modifying toxicity
Phenobarbital pretreatment of rats followed by a single
administration of brodifacoum or warfarin decreased the anticoagulant
effects of both compounds, more markedly in the case of warfarin
(Bachmann & Sullivan, 1983).
The non-steroid anti-inflammatory drugs ibuprofen and
phenylbutazone potentiated the anticoagulant effects of brodifacoum
and bromadiolone in rats (Sridhara & Krishnamurthy, 1992).
Strain and sex are important factors modifying the toxicity of
anticoagulants in rodents (see Table 5, section 7.1.1). Winn et al.
(1987) observed greater sensitivity of male rats and mice to
difenacoum, compared with female rats and mice. The possible
explanation of different responses to difenacoum was the greater
turnover of plasma proteins in male rats or the marked
inter-individual variation of vitamin K1 level in male rat liver.
Large amounts (relative to farm animals' dietary requirements) of
vitamin K3 (menadione and its salts) are sometimes added to animal
feedstuffs. This gives rodent pests ready access to a substance which
acts as an antidote to anticoagulant rodenticides, and thus can reduce
the efficacy of these rodenticides.
Differences in metabolism to inhibitors of vitamin K synthesis
among strains of mice and rats has been attributed to a number of
different factors (Misenheimer et al., 1994). Among these are: 1)
reduced sensitivity of vitamin K epoxide hydrolase to inhibition; 2)
greater reversibility of inhibition of epoxide hydrolase; and 3)
faster clearance of the rodenticide. The mechanism of resistance
differs between strains.
A single flocoumafen dose of 0.5 mg/kg body weight resulted in
clear signs of anticoagulation in five out of eight beagle dogs
(Veenstra et al., 1991). Administration of a second dose 5 weeks
later resulted in clinical evidence of anticoagulation in two out of
the three remaining dogs. Vitamin K treatment (2 to 5 mg/kg
subcutaneously) reversed the effects in all cases.
7.8 Adverse effects in domestic and farm animals
7.8.1 Domestic animals
7.8.1.1 Poisoning incidents
The main cause for accidental poisoning of domestic animals is
direct consumption of anticoagulant baits. Secondary poisoning
through the consumption of rats and mice killed with anticoagulants
may occur in dogs and cats in urban situations, but is more likely in
farm situations (Marsh, 1985b). The majority of fatalities and severe
clinical syndromes are connected with the second-generation
anticoagulants (Dodds & Frantz, 1984). Du Vall et al. (1989) studied
10 cases of second-generation anticoagulant rodenticide poisoning in
dogs and cats. The presence of anticoagulants (brodifacoum,
bromadiolone or diphacinone) in serum or liver was confirmed by HPLC
or GC/MS. Several other cases of brodifacoum poisonings in dogs have
been reported and some of them were fatal (Mc Sporran & Phillips,
1983; Stowe et al., 1983; Dodds & Frantz, 1984).
Until 1983, only first-generation anticoagulants were available
in New Zealand and, between 1977 and 1983, warfarin, coumatetralyl and
diphacinone were involved in 45 reported incidents of accidental
poisoning of domestic and farm animals. Brodifacoum and flocoumafen
were introduced after 1983. From 1983 to 1994, 40 cases of poisoning
of domestic and farm animals resulted from the use of
second-generation compounds and 17 cases were due to the ingestion of
first-generation anticoagulants (Hoogenbroom, 1994).
Schulman et al. (1986) reported five cases of coagulopathy in
dogs caused by consumption of diphacinone-containing baits. Lethargy
and respiratory distress, associated with pulmonary interstitial
haemorrhage and pleural and/or pericardial effusion, were the most
consistent signs. The prothrombin time and the activated partial
thromboplastin time were moderately to markedly prolonged.
7.8.1.2 Diagnosis and treatment of poisoning
The major difference between warfarin and the other anticoagulant
rodenticides is that the latter have longer body retention and a
tendency to induce bleeding for a longer period of time. It is
therefore necessary to continue the treatment for weeks rather than
days.
Signs of poisoning occur after a latent period of 12 h to several
days and may include:
* bruising easily with occasional nose or gum bleeds
* blood in stools or urine
* excessive bleeding from minor cuts or abrasions
* laboured breathing
* pale mouth and cold gums
* anorexia and general weakness
A reliable indication of an anticoagulant effect is the
determination of prothrombin time.
Vitamin K1 (phytomenadione) is the antidote of choice. The
recommended dosage is 2-5 mg/kg body weight in dogs. Intravenous
injection is the quickest route but it is not recommended because of
reported anaphylaxis. Vitamin K1 is much safer if given
subcutaneously for the initial 2-3 treatments. A dose of 0.25 to
2.5 mg/kg body weight is recommended for use in small animals (Mount
et al., 1982). The recommended period of treatment with vitamin K1
is 3-6 weeks (Braithwaite, 1982; Mackintosh et al., 1988).
Mount & Feldman (1983) observed that therapy effective for
warfarin poisoning was ineffective against diphacinone toxicosis due
to inadequate vitamin K1 dosage and duration of therapy. Oral
therapy was ineffective.
In a study on dogs, Mount & Kas (1989) found that the serum
concentration of vitamin K epoxide was significantly higher in
diphacinone-treated dogs than in controls, the difference being
significant as early as 1-4 h after vitamin K application. The
authors suggested that this phenomenon could be used as diagnostic
information.
In cases of severe blood loss, fresh plasma should be infused
every 6 h to the extent of 5-10% of total blood volume, assuming this
to be 90 ml/kg in the dog and 70 ml/kg in the cat (Mount et al.,
1982).
Vitamin K1 (phytomenadione) is the antidote for treating dogs
exposed to anticoagulant rodenticides. The therapeutic dosages and
duration of treatment needed vary with the compound involved. Mount &
Feldman (1983) reported that dogs poisoned with diphacinone require
treatments for at least three weeks, while treatment for 4-6 days is
sufficient for treating warfarin poisonings. Initial treatments are
by subcutaneous injection, subsequent therapy being given orally.
For animals exposed to the second-generation rodenticides
brodifacoum, bromadiolone, difenacoum and flocoumafen, the treatment
regimen that has been recommended includes one or more injections of
vitamin K1 at 2-5 mg/kg body weight until prothrombin times return
to normal levels. Once this has been achieved, daily oral doses of
vitamin K1 are recommended for a period of 3-4 weeks. Oral doses of
2-5 mg/kg body weight are recommended initially, with some reduction
being possible over time if the animal is observed closely for signs
of recurrence of symptoms. If prothrombin times increase following
withdrawal of vitamin K1, the oral dosing should be resumed for 2-3
weeks. If blood loss was severe, transfusions (10-15 ml/kg body
weight) with fresh whole blood may be needed (Anonymous, 1988).
7.8.2 Farm animals
Feinsod et al. (1986) reported an outbreak of abortions and
haemorrhages in sheep and goats in Egypt caused by brodifacoum
intoxication. Clinical signs appeared within 3-7 days after exposure
to the rodenticide and included epistaxis, haematochezia, recumbency,
subcutaneous haemorrhage, flank ecchymosis, lameness, abdominal
bloating and abortion of various stages of gestation. The signs
resembled epidemic Rift valley fever, but there was no fever, icterus
or loss of appetite in affected animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Incidents of human exposures to rodenticides are reported to
poison control centres in countries where such facilities exist. In
1988, for example, the American Association of Poison Control Centers
(AAPCC) received accounts of 10 626 cases of human exposures to
rodenticides. These incidents represented 17% of reported exposures
involving pesticides and 0.8% of the total number of cases reported in
the AAPCC system. The rodenticide incidents included 4190 cases
involving "anticoagulants" (principally warfarin) and 5133 involving
"long-acting anticoagulants" (second-generation anticoagulants plus
the indandione compounds). More than 95% of the rodenticide cases
were classified as "accidental". Most of the remainder were
classified as "intentional" and included attempted suicides. Of the
10 540 rodenticide incidents for which the ages of victims were
reported, 9406 (89%) involved children under 6 years of age (Litovitz
et al., 1994).
Victims in nearly 32% of the rodenticide exposure incidents
reported to the AAPCC in 1988 were treated in health care facilities.
However, the medical outcome "none" was reported in more than 93% of
the 5708 incidents for which information regarding outcomes was
reported. The remaining 380 cases included 333 with "minor" medical
effects, 41 with "moderate" effects, 4 with "major" effects, and two
deaths (Litovitz et al., 1994).
In 1993, the Swedish Poison Information Centre received 338
enquiries concerning exposures to anticoagulant rodenticides. This
number represented 0.6% of all enquiries to the centre and 37% of the
enquiries concerning pesticides. Of the anticoagulant rodenticide
enquiries, 202 pertained to warfarin and 136 to "superwarfarin"
compounds (Persson, 1994).
Human exposure to second-generation and indandione anticoagulants
produces symptoms consistent with anticoagulation effects (e.g.,
haematomas, haematemesis, haematuria, easy bruisability). Treatment
of cases of exposure, particularly of substantial and repeated
exposure, may require vitamin K1 therapy and monitoring of
prothrombin times for periods of many months (Rauch et al., 1994).
Suicide and/or unintentional poisonings with anticoagulant
rodenticides have occurred in many countries. Thus, Ungvary (1994)
reported 70 cases, mostly involving children, that occurred in Hungary
between 1988 and 1993.
Warfarin is widely used as a therapeutic and preventive agent in
the treatment of thromboembolic disease. Patients have been
maintained for years on this treatment with control of the prothrombin
level, which should be kept between 10 and 30% of normal.
Diphacinone has also been used as a drug because of its
long-lasting action (the half-life in humans is 15-20 days). It
ceased to be listed in the American Medical Association Drug
Evaluations, (AMA, 1980) because of its structural relation to
phenindion, which had been reported to have adverse effects.
8.1.1 Acute poisoning
Typical features of poisoning result from increased bleeding
tendency and include:
* minor poisoning: coagulation disturbance detected only by
laboratory analyses;
* moderate poisoning: coagulation disturbance resulting in
haematomata, haematuria, blood in faeces or excessive bleeding
from minor cuts or abrasions, gum bleeding;
* severe poisoning: retroperitoneal haemorrhage, severe
gastrointestinal bleeding, cerebrovascular accidents, massive
haemorrhage (internal bleeding) resulting in shock.
If anaemia or liver disease is present then the above features
may be more severe and persistent and the poisoning may be more
difficult to control (Anonymous, 1988).
The onset of the signs of poisoning may not be evident until a
few days after ingestion.
8.1.2 Poisoning incidents
Cases of human poisoning with "superwarfarins" were reviewed by
Katona & Wason (1989).
Fourteen members of a family in the Republic of Korea were
poisoned by eating warfarin-containing maize meal. The first symptoms
appeared 7-10 days after the beginning of exposure and were followed
by massive bruises or haematomata on the buttocks in all cases (Lange
& Terveer, 1954).
Pribilla (1966) reported a total dose of about 1000 mg of
warfarin to be fatal after 13 days of consumption.
Out of a total of 741 infants, 177 died after the use of
warfarin-contaminated talc in Viet Nam. The concentrations of
warfarin in the powder varied from 1.7 to 6.5% (Martin-Bouyer et al.,
1983).
A 73-year-old woman suffered from recurrent episodes of
hypoprothrombinaemia. Clotting tests and further investigation showed
that this was due to a warfarin rodenticide intentionally mixed in the
woman's cough syrup by her daughter-in-law. As the patient had as
many as seven relapses, it was possible to compare different types of
therapy. Menadione had no effect (Nilsson, 1957).
Several suicidal attempts with chlorophacinone have been
reported. Murdoch (1983) reported a case of ingestion of 625 mg
chlorophacinone (250 ml of a 0.25% concentrate formulation) by a
37-year-old woman. The prolonged anticoagulant action of
chlorophacinone persisted for at least 45 days even though treatment
was given. It was found that menadiol, the synthetic analogue of
vitamin K1, was ineffective. The natural form, phytomenadione, was
effective only when given at high dosage (20 mg daily) 30 days after
the ingestion of chlorophacinone.
In a case reported by Dusein et al. (1984), the amount of
ingested chlorophacinone was unknown. After adequate therapy, the
prothrombin level became normal within 4 weeks.
Vogel et al. (1988) reported the case of an 18-year-old woman
hospitalized 3 days after ingesting approximately 100 mg
chlorophacinone. Under high-dose vitamin K1 therapy (160 mg) the
prothrombin time was normalized, but it increased again following
withdrawal of vitamin K1. After prolonged vitamin K1 administration,
the prothrombin time finally became normal after 7 weeks.
Brodifacoum poisoning has occurred in South Sumatra, Indonesia.
Some of the villagers used a 0.005% brodifacoum rice grain bait as a
food source even though they knew it was poisonous and unfit for human
consumption. They attempted to remove the rodenticide by repeated
washing, rinsing and cooking before eating the rice. Because of the
delay in the appearance of poisoning symptoms it appeared that they
had been successful, thus encouraging further attempts to purify the
rice baits. As a result, deaths occurred before appropriate remedial
treatment could be initiated (Anonymous, 1985).
Jones et al. (1984) reported the first case of human brodifacoum
poisoning in a 17-year-old boy who attempted suicide by ingesting
approximately 7.5 mg (0.12 mg/kg) of brodifacoum in Canada. He was
initially seen with gross haematuria, followed by epistaxis and gum
bleeding. The prothrombin time and the activated partial
thromboplastin time were notably prolonged. He was treated for 56
days with either parenteral or oral vitamin K1 and either fresh or
stored plasma until coagulation values remained normal and stable.
Lipton & Klass (1984) reported a similar case in a 31-year-old
mentally disturbed woman who ingested over a 2-day period
approximately thirty 50-g packages of Talon-G (approximately 75 mg of
brodifacoum). Prothrombin time and activated partial thromboplastin
time were considerably prolonged (respectively 6-fold and 4-fold above
normal values). After 4 days of therapy with high doses of vitamin
K1 (up to 125 mg/day), partial correction in the prothrombin time
occurred. Vitamin K1 therapy continued with interruptions for 8
months until normal prothrombin time levels were found.
Chong et al. (1986) reported a case of suicidal poisoning after
ingestion of 10 mg brodifacoum (as 0.05% Klerat). The coagulation test
became normal after large doses and prolonged use of vitamin K1 over
6 months.
A case of intentional ingestion of brodifacoum (200 g of Talon G,
0.005% brodifacoum) was reported by Hoffman et al. (1988). A profound
decrease in the levels of factors II, VII, IX and X, lasting 43 days
after ingestion, was observed. Treatment with subcutaneous vitamin
K1 in doses up to 100 mg per day was effective.
Weitzel et al. (1990) described three patients with severe
bleeding disorders due to deficiency of the vitamin K-dependent blood
clotting proteins after ingestion of an anticoagulant. Although the
patients denied any ingestion, brodifacoum was detected in their serum
at concentrations of 7.6 nmol/litre, 270.7 nmol/litre and
2759 nmol/litre, respectively. The anticoagulant effect was found to
persist long after brodifacoum was no longer detectable in the serum.
A half-life of approximately 16-36 days was determined for brodifacoum
in the plasma.
Kruse & Carlson (1992) reported the case of a 25-year-old man who
attempted suicide by consuming a brodifacoum rodenticide. He
developed a severe coagulopathy that was treated with vitamin K1 and
fresh frozen plasma and he was discharged from hospital with oral
phytomenadione. Fifteen weeks later the man presented again with a
history of further brodifacoum ingestion. He suddenly became comatose
and computer tomography revealed a subarachnoid haemorrhage that led
to brain death 24 h later.
Wallace et al. (1990) described the clinical course of a patient
poisoned with brodifacoum in a suicide attempt. He developed
microhaematuria and melaena. His clotting factors were depressed and
were poorly responsive to vitamin K treatment.
Barlow et al. (1982) reported a case of attempted suicide with
25 mg of difenacoum (500 g of rat bait) followed several months later
by 1800 g of rat bait. The patient was treated with vitamin K1
(phytomenadione) for 48 and 42 days, respectively, until the
pharmacological effect of difenacoum ceased.
Nighoghossian et al. (1990) reported an unusual coagulopathy
after accidental exposure to a diphenacoum rodenticide. A 59-year-old
man developed subacute tetraparesis following severe sudden neck pain,
which on clinical examination was shown to be due to a subdural
cervical haematoma. Prothrombin complex activity was low and
diphenacoum was present in the plasma. Specific medical management
led to a complete recovery.
Greeff et al. (1987) reported accidental bromadiolone poisoning
in two children, resulting in prolonged anticoagulation.
Descarboxyprothrombin levels were increased in both cases by 27% and
29.9%, respectively (normal, non-detectable level). The first child
rapidly recovered after treatment with high-dose intravenous factor
IX-prothrombin complex and vitamin K1. The clotting profile became
normal on the third day after admission. The second child gave a poor
response to 10 mg intravenous vitamin K1 and the dose was increased
to 20 mg.
8.1.3 Controlled human studies
Single oral doses of 60, 70, 80 or 120 mg warfarin decreased the
prothrombin concentrations in volunteers to zero by the third day.
After the administration of 50 mg vitamin K1, the prothrombin
concentrations returned by the sixth day to 60, 70, 55 and 63%,
respectively, of the normal value (Anonymous, 1965).
When a single oral dose of 20 mg chlorophacinone was given to
three volunteers, the lowest prothrombin times were 35, 34 and 38% of
the pretreatment value on days 2, 4 and 2, respectively. Eight days
after administration without any treatment the values were 80, 100 and
90%, respectively (Anonymous, 1965).
8.2 Monitoring of biological effects
8.2.1 Effects of short- and long-term exposure
Two cases of occupational exposure to brodifacoum and difenacoum
were reported by Park et al. (1986). The exposure was of a chronic
nature (2 and 4 years, respectively). Plasma analysis in the first
patient revealed the presence of both difenacoum and brodifacoum in
the range of 30-50 µg/litre. In both patients unexpectedly high
concentrations of vitamin K1 2,3-epoxide were found in the presence
of normal clotting factor activities and antigen levels suggesting the
presence of coumarin anticoagulants in the liver.
A case of poisoning in a 23-year-old man resulting from prolonged
skin contact during the process of preparing and distributing warfarin
baits has been reported (Fristedt & Sterner, 1965).
8.2.2 Epidemiological studies
During a production run preparing ready-to-use flocoumafen bait
(0.005% in baits) in a formulation plant, the effect of the
rodenticide on blood coagulation factors was monitored in 12 subjects,
using the classical prothrombin time test, a modified prothrombin time
technique and measurement of prothrombin (factor II) concentration in
blood. No adverse health effects were observed in any subject
involved in formulation operations. No changes were observed in any
of the three tests that could be ascribed to absorption of flocoumafen
into the body (Tuinman & Van Sittert, 1986).
8.3 Developmental effects
Developmental effects have been reported when anticoagulants,
particularly warfarin, have been administered as therapeutic agent
during pregnancy. Hall et al. (1980) reviewed 418 reported
pregnancies in which coumarin and indan-1,3-dione derivatives were
used, and found that one-sixth resulted in abnormal liveborn infants,
one-sixth in abortion or stillbirth, and two-thirds in apparently
normal infants.
According to Hall (1976), there are two types of defects
associated with anticoagulants, dependent upon the time of
administration during pregnancy. The first, a characteristic
embryopathy described by the terms "warfarin embryopathy" or "fetal
warfarin syndrome", occurs from early, first-trimester use. Fetal
wastage and other abnormalities, especially central nervous system
anomalies, result from treatment later during gestation (usually the
second or third trimesters). Clotting factors are not present in
first-trimester embryos and this may explain the differences in fetal
abnormalities.
The most consistent feature of warfarin embryopathy is nasal
hypoplasia. Choanal stenosis has been observed, and respiratory
difficulty is typical because of the narrowed nasal passages (Smith &
Cameron, 1979; Pauli & Hall, 1979).
The other common feature is bone abnormalities of the axial and
appendicular skeleton. Laryngeal and tracheal-thyroid calcification
has also been noted (Schardein, 1985).
Other non-skeletal abnormalities reported include
ophthalmological malformations of several types, including defects
leading to blindness, developmental delay, low birth weight (premature
birth), mental retardation, hypotonia and ear anomalies (Carson &
Reid, 1976; Schardein, 1985).
No cases of embryopathy from anticoagulants in their use as
rodenticides have been reported.
8.4 Other adverse effects
One of the more common side-effects of coumarin therapy is skin
necrosis (Brooks & Blais, 1991; Eby, 1993; Locht & Lindström, 1993).
Although this effect has been associated with a number of different
agents, it is commonly referred to as warfarin-induced skin necrosis
(WISN). The frequency of this effect has been estimated to be between
1 in 100 and 1 in 10 000, with the majority of cases occurring in
women. Symptoms of WISN typically appear 3-6 days after the
initiation of warfarin therapy, and the areas of skin involved are
most frequently areas with subcutaneous fat, particularly the breasts,
thighs, and buttocks. There is some evidence that deficiencies in
vitamin K-dependent anticoagulant proteins (protein C and protein S)
may underlie the susceptibilities of at least some individuals to WISN
(Anderson et al., 1992). The occurrence of WISN may be avoided in at
least some cases by the co-administration of heparin.
Warfarin therapy has been noted to result in increases in the
incidence of haematomas, including intraspinal epidural haematoma
(Murphy & Nye, 1992), liver haematoma (Erichsen et al., 1993), and
intramural haematoma of the small intestine (Avent et al., 1992).
Bone contains three vitamin-K-dependent proteins, i.e.
osteocalcin, matrix Gla-protein and protein S. It has been shown that
oral anticoagulants (phenprocoumon, acenocoumarol) reduce both the
plasma antigen level as well as the Gla content of osteocalcin (Van
Haarlem et al., 1988). Furthermore it was found that a poor vitamin K
status was associated with a high urinary calcium loss (Knapen et al.,
1993). These data are consistent with the observation that the bone
mass in patients on long-term anticoagulant treatment was
significantly lower than in a group of age- and sex-matched controls
(Fiore et al., 1990; Resch et al., 1991). Vitamin-K-dependent proteins
have also been identified in calcified atherosclerotic plaques, where
they were suggested to contribute to the prevention of vascular
calcification (Gijsbers et al., 1990). In this respect it is
remarkable that warfarin treatment has been shown to increase rather
than to reduce atherosclerosis in an animal model (Antov et al.,
1985). Vitamin-K-dependent proteins not related to blood coagulation
are still discovered regularly (Manfioletti et al., 1993), and it is
important to remain alert for unexpected side-effects of vitamin K
antagonists, notably after long-term exposure.
8.5 Methods for assessing absorption and effects of anticoagulant
rodenticides
The laboratory control of orally administered coumarin
derivatives has been carried out using the classical one-stage
prothrombin time test (Quick, 1935) or modified techniques such as
"Thrombotest" (Owren, 1959). However, these tests have been designed
for clinical monitoring of circulating clotting factors during
anticoagulant therapy. The monitoring of occupational exposure to
rodenticides requires the prothrombin time test to be of sufficient
sensitivity to measure changes in the normal range (Tuinman & Van
Sittert, 1985).
Repeated occupational exposure to low levels of anticoagulant
rodenticides could gradually deplete vitamin-K-dependent coagulation
factors in the blood. To detect unwanted exposure of humans in an
early state, a careful screening of those at risk is recommended. The
question is which screening method is the most suitable to monitor low
levels of rodenticide ingestion. Prothrombin time and related tests
are "overall" clotting tests, which were developed for monitoring
patients under deep anticoagulation. These tests are easy to perform
and do not require complicated equipment, but they are relatively
insensitive when used for monitoring milder anticoagulation states
(Tuinman & Van Sittert, 1985; Ross et al., 1992; Travis et al., 1993).
If possible, specific and more sensitive tests should be used. The
most sensitive test, applicable over a wide range of anticoagulation
states, is the direct detection of descarboxy-prothrombin using a
monoclonal antibody specifically recognizing the descarboxy form of
prothrombin (Widdershoven et al., 1987). Another marker for
monitoring poor vitamin K status at an early stage is
descarboxy-osteocalcin (Knapen et al., 1993), but the commercial test
kits presently available need to be substantially improved and
simplified before they can be recommended for this purpose in routine
laboratories.
Another method was suggested by Park et al. (1986), who
repeatedly injected 10 mg of vitamin K1 into factory workers who had
been exposed to brodifacoum. The authors observed that 2-4 h after
injection the circulating KO/K ratios were significantly elevated even
18 months after the prothrombin times had returned to normal values.
This suggests that very low liver concentrations of brodifacoum can be
detected from the altered KO/K ratio rather than from tests based on
blood coagulation parameters. Because this method requires vitamin K
administration shortly before blood sampling, it is only applicable in
cases of anticoagulant poisoning, and not for the routine control of
plant workers.
Methods for the direct detection of coumarin anticoagulants in
plasma and serum have been reported, all of which are based on the
extraction of plasma and pre-purification of the sample, followed by
HPLC analysis with fluorescence detection (Hunter, 1983; Murphy et
al., 1989; Felice & Murphy, 1989; Felice et al., 1991; O'Bryan &
Constable, 1991). However, such facilities will not be available in
most routine laboratories. Moreover, the blood sampling should be
performed within a reasonably short period after ingestion of the
coumarins, because these drugs are rapidly cleared by the liver. This
places severe restrictions on the applicability of these techniques,
particularly for the second-generation anticoagulants.
Plasma chlorophacinone determinations were performed in three
cases of intoxication. The risk of bleeding was minimal when the
plasma level was below 1 mg/litre (Burcuoa et al., 1989).
Brodifacoum was detected in a case of self-ingestion by a
25-year-old woman who denied any intake of anticoagulants but was in
need of vitamin K1 treatment for 8 months. Factor assays revealed a
marked reduction in the levels of the vitamin K-dependent factors II,
VII, IX and X and normal levels of factor V and VIII:C (Exner et al.,
1992).
The diagnosis in a case of difenacoum intoxication was confirmed
by analysis of difenacoum in serum (0.6 mg/litre) (Butcher et al.,
1992).
Bromadiolone was analysed in the serum of a 27-year-old female
with a history of bleeding. She denied any contact with a rodenticide
but the bromadiolone concentration in serum was 40 mg/litre (Chow et
al., 1992).
Hollinger & Pastoor (1993) reported results of comparison of
plasma brodifacoum concentrations and prothrombin levels over time in
a case of brodifacoum poisoning. Brodifacoum was eliminated according
to a two-compartment mode, with an initial half-life of 18 h and a
terminal half-life of 24.2 days. On admission, the brodifacoum level
was 731 µg/litre. Treatment with large doses of phytonadione lasted
for 4 months.
8.6 Treatment of anticoagulant rodenticide poisoning
All suspected poisoned patients should receive medical attention
immediately. Rapid determination of prothrombin time and search for
evidence of bleeding is essential and may have to be maintained for
several weeks.
8.6.1 Minimizing the absorption
Gastric lavage or induction of emesis are indicated in all cases
of superwarfarin rodenticide ingestion if it was recent and the amount
is possibly lethal or uncertain. Repeated administration of activated
charcoal is useful. Cathartics could also be administered (Sullivan
et al., 1989; Smolinske et al., 1989; Donovan et al., 1990).
8.6.2 Specific pharmacological treatment
8.6.2.1 Vitamin K1 (phytomenadione)
Vitamin K1 is the specific antidote of choice. Depending on
whether the poisoning is due to warfarin or superwarfarin, the dosage
may differ as well as the duration of treatment. Dosage is dependent
on coagulation parameters, mainly prothrombin time.
If the patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection.
Prothrombin time should be checked at 3-hourly intervals in severe
cases and after 8-10 h in less severe cases. If no improvement
occurs, vitamin K1 injection should be repeated. Doses of up to
125-200 mg/day have been given without adverse effects (Lipton &
Klass, 1984; Sheen et al., 1994).
In moderate to minor cases of poisoning, vitamin K1 may be
given in lower doses.
After initial parenteral vitamin K1 administration, oral
treatment can be continued for a prolonged period of time. Oral
treatment can also be sufficient in minor cases.
The major difference between warfarin and second-generation
rodenticides is that the latter can cause increased bleeding for a
longer period of time than warfarin, as they have a much longer
half-life in the body. Therefore vitamin K1 should be given for
months rather than weeks. It is also prudent to monitor prothrombin
time for some time after cessation of this treatment to ensure that
there is no regression.
In warfarin-resistant individuals, 10 times the normal dose of
warfarin is required to achieve a reduction in the plasma prothrombin
level. However, these individuals also respond more strongly to the
effect of vitamin K (O'Reilly et al., 1963; O'Reilly et al., 1964).
8.6.2.2 Blood components
The following products could be considered, subject to
availability:
* whole blood
* fresh frozen plasma and fresh blood should be used in cases of
acute severe bleeding in order to rapidly restore the blood
clotting factors
* factor concentrate may be considered in severe cases, especially
if the amount of plasma would be too great (volume overload)
8.6.2.3 Phenobarbital
In the cases reported by Lipton & Klass (1984) and Jones et al.
(1984), phenobarbital therapy was included. It was presumed that
anticoagulants had been metabolized by the mixed function oxidase
system based on animal experiments (Bachmann & Sullivan, 1983) and
that, by inducing this enzyme system, phenobarbital might increase
their metabolism.
Robinson & Mac Donald (1966) found that the pharmacological
activity of warfarin in humans decreased when it was combined with
phenobarbital.
8.6.3 Response to therapy
Patient should be kept in hospital until the prothrombin time has
remained normal for 3 days. It is suggested that oral treatment with
10 mg vitamin K1 twice daily may be necessary for up to 60 days with
close monitoring of prothrombin time.
According to Hoffman et al. (1988), factor analysis allows for a
detailed evaluation of the course of toxicity, and the response to
therapy. Monitoring the prothrombin time alone could offer a false
sense of confidence and delay effective treatment.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
No data are available for the effects on microorganisms in water
and in soil.
9.1.2 Aquatic organisms
The acute toxicity of technical flocoumafen to planktonic algae
(Selenastrum capricornutum) was determined in a 4-day growth test.
The 96-h EC50, based on cell counts on day 4, was calculated to be
1.1 mg/litre (Pearson & Wallace, 1984).
Pearson (1984) reported static acute toxicity tests for
flocoumafen on Daphnia magna. The 48-h EC50 values, based on
immobilization, were 1.4 mg/litre for technical grade flocoumafen and
280 mg/litre for 0.5% flocoumafen. Pearson & Wallace (1984) found a
48-h EC50 for Daphnia magna of 0.66 mg/litre for technical grade
flocoumafen.
Anticoagulants are highly toxic to fish when tested as technical
formulations (Hill et al., 1976; Pearson & Wallace, 1984) (Table 8).
9.1.3 Terrestrial organisms
9.1.3.1 Acute toxicity
Bird species vary in their susceptibility to anticoagulant
rodenticides. The acute oral LD50 of brodifacoum for the mallard
duck (Anas platyrhynchos) is 2.0 mg/kg (Ross et al., 1978).
Symptoms of poisoning became apparent approximately 7 days after
dosing and included lethargy, weakness and lack of muscular
coordination. Prolonged bleeding occurred from any small wounds and
extensive bruising and subcutaneous haemorrhage were noted. Blood was
observed in the faeces. Deaths occurred principally during the period
of 7-14 days after administration. No deaths occurred more than 4
weeks after administration.
The acute toxicity of flocoumafen to the mallard duck appears to
be dependent on the age of birds. The LD50 values for 12- and
18-week-old birds are 24 mg/kg and 94 mg/kg, respectively (Roberts et
al., 1985b,c).
Table 8. Acute toxicity of anticoagulant rodenticides for aquatic organisms (96-h LC50)
Rodenticide Species Stat/flowa LC50 (mg/litre) Reference
Brodifacoum Rainbow trout flow 0.051 Hill et al. (1976)
Diphacinone (95%) Bluegill sunfish 7.6 Kosmin & Barlow (1976)
Channel catfish 2.09 Kosmin & Barlow (1976)
Rainbow trout 2.82 Kosmin & Barlow (1976)
Pink shrimp > 10 Kosmin & Barlow (1976)
Fiddler crab > 10 Kosmin & Barlow (1976)
Diphacinone (0.005%) Bluegill sunfish 288 Kosmin & Barlow (1976)
Channel catfish 285 Kosmin & Barlow (1976)
Rainbow trout 425 Kosmin & Barlow (1976)
Pink shrimp 33 Kosmin & Barlow (1976)
Fiddler crab 37 Kosmin & Barlow (1976)
Warfarin Bluegill sunfish 88 Brorson et al. (1994)
Flocoumafen (0.5%) Rainbow trout static 190 Pearson (1984)
Flocoumafen (technical grade) Rainbow trout static 0.95 Pearson (1984)
Rainbow trout static 0.32 Pearson & Wallace (1984)
Rainbow trout static 0.091 Pearson & Wallace (1984)
Common carp static 0.22 Pearson & Wallace (1984)
Bromadiolone Rainbow trout 1.4 Tomlin (1994)
a stat = static conditions (water unchanged for duration of test)
Sex differences were observed in the acute toxicity for bobwhite
quail (Colinus virginianus) of difenacoum. LD50 values for male
and female birds were 140 mg/kg body weight and 56 mg/kg body weight,
respectively (Ross et al., 1980c).
Birds seem to be relatively tolerant to diphacinone, the LD50
for mallard duck being 3158 mg/kg (Kosmin & Barlow, 1976).
The acute toxicity of brodifacoum for various species of birds is
presented in Table 9.
Table 9. The acute toxicity of brodifacoum to birds (Godfrey, 1985)
Species Acute oral LD50
(mg/kg)
Black-backed gull (Larus dominicans) < 0.75
Black-billed gull (L. bulleri) < 5.0
Canada goose (Branta canadensis canadensis) < 0.75
Pukeko (Porphyris porphyris melanotus) 0.95
Mallard duck (Anas platyrhynchus) 4.6
California quail (Lophortyx californica) 3.3
Blackbird (Turdus merula) > 3
House sparrow (Passer domesticus) > 6
Hedge sparrow (Prunella modularis occidentalis) > 3
Wax-eye (Zosterops lateralis) > 6
Harrier hawk (Circus approximans) 10.0
Ring-necked pheasant (Phasianus colchicus) 10.0
Paradise duck (Tadorna variegata) > 20
9.1.3.2 Primary toxicity
The subacute (5-day) dietary toxicity (LC50) of difenacoum for
the bobwhite quail was found to be between 0.25 and 7.00 mg/kg diet
(Ross et al., 1980a). The 5-day LC50 of difenacoum for the mallard
duck was calculated to be 18.9 mg/kg diet (Ross et al., 1980b).
Similarly to the acute toxicity studies, diphacinone was found to
be less toxic in 8-day dietary studies. The LC50 for the bobwhite
quail was 4485 mg/kg diet and for the mallard duck was > 10 000 mg/kg
diet (Kosmin & Barlow, 1976).
White Leghorn hens (4 per group) were fed warfarin,
coumatetralyl, bromadiolone, difenacoum or brodifacoum in baits as a
choice to non-poisonous chicken food for 15 days. No symptoms
appeared at a total warfarin intake of up to 171 mg/kg body weight.
Coumatetralyl and brodifacoum killed all hens after intakes of
79-137 mg/kg body weight of active ingredient and 7.1-15 mg/kg body
weight of active ingredient, respectively. Both bromadiolone and
difenacoum killed two of the hens, at 5.9 and 15.9 mg/kg body weight
of active ingredient for bromadiolone, and at 18.9 and 26.3 mg/kg
body weight active ingredient for difenacoum within 10-15 days (Lund,
1981).
Christopher et al. (1984) dosed male hybrid leghorn chickens with
anticoagulant rodenticides. No signs of poisoning were observed in
chickens fed 183.7 mg warfarin/kg (mean a.i. ingested) for 3 days. No
mortality occurred after the ingestion of a total dose of 36.9 mg
bromadiolone/kg over a 3-day period. Birds fed a diet containing
brodifacoum for 3 days ingested a mean of 28.9 mg/kg (active
ingredient). One bird died on day 4 while the other five birds
exposed had died by day 16.
Two-day no-choice tests in which chukar partridges ( Alectoris
graeca cypriotes Hartert) were fed 0.005% bromadiolone or difenacoum
resulted in no mortality. Ten-day no-choice feeding tests resulted in
6 out of 12 and 8 out of 12 dead partridges for bromadiolone and
difenacoum, respectively. The highest quantity of bait consumed by an
individual bird without any lethal effects was 411 g of bait with
bromadiolone (34.8 mg a.i./kg body weight) and 326.2 g of bait with
difenacoum (29.1 mg a.i./kg body weight) (Krambias & Hoppe, 1987).
9.1.3.3 Secondary toxicity
Barn owls (Tyto alba) were fed rats poisoned with diphacinone,
chlorophacinone, coumafuryl, difenacoum, bromadiolone or brodifacoum.
Five out of six owls died of haemorrhaging after feeding on rats
killed with brodifacoum after 8 to 11 days. Sublethal haemorrhaging,
but no mortality, occurred in owls fed rats killed with difenacoum.
One owl died following 10 days of treatment with bromadiolone-poisoned
rats, while five showed no symptoms. No abnormalities were observed
in two owls fed rats killed with diphacinone, coumafuryl or
chlorophacinone. Owls that died behaved normally until 24 h or less
before death, when they became lethargic and stopped eating
(Mendenhall & Pank, 1980).
Mice (weighing 35 g) were fed for 1 day (no choice) on a mixture
containing either 0.005% difenacoum or 0.002% brodifacoum. The mean
mass of residue on the day of death in a whole mouse was estimated to
be 10.17 µg for difenacoum and 15.36 µg for brodifacoum. Difenacoum-
and brodifacoumpoisoned mice were fed to captive barn owls for
successive periods of 1, 3 and 6 days. All of the owls fed on
difenacoum-poisoned mice survived the treatments and none showed
external bleeding. In contrast, four of the six owls fed
brodifacoum-poisoned mice died within 6-17 days after a 1-day dose.
The estimated lethal dose of brodifacoum was 0.15-0.18 mg/kg. After
death these owls had 0.63-1.25 mg brodifacoum/kg in their livers
(Newton et al., 1990).
Newton et al. (1994) fed barn owls flocoumafen-dosed mice for
consecutive periods of 1, 3 and 6 days. Four of the birds survived;
the fifth bird died from haemorrhaging 5 days after the final dose.
During the feeding trial birds received cumulative flocoumafen doses
ranging from 0.78 to 1.25 mg/kg.
Gray et al. (1992) investigated the toxicity of brodifacoum,
difenacoum and flocoumafen for barn owls fed poisoned mice. For each
rodenticide, the owls survived a cumulative dose of at least 1.9 mg/kg
owl weight over 15 days of treatment. All owls with cumulative doses
in excess of 1.9 mg/kg body weight showed multiple treatment-related
effects. The three rodenticides had approximately the same order of
magnitude of toxicity to barn owls. Gray et al. (1994) developed a
non-invasive method for monitoring the exposure of these birds to
second-generation rodenticides by measuring compounds in regurgitated
owl feed.
Radvanyi et al. (1988) fed American kestrels (Falco sparverius)
on meadow voles that had been maintained on 2% chlorophacinone. Voles
consumed approximately 53 mg of 2% chlorophacinone (1.14 mg a.i.)
before dying within 6 days. No kestrels fed poisoned mice, for up to
21 consecutive days, died. Haematomas were observed on the pectoral
muscles, lungs, liver and heart of exposed birds.
In a study by Townsend et al. (1981), captive-bred tawny owls
(Strix aluco) were given warfarin-treated mice for 3 months. No
behavioural changes were observed. Prothrombin levels decreased to
less than 10-12% of normal but returned to normal after 9 days.
Townsend et al. (1984) found that daily warfarin intakes
approaching 0.3 mg/kg body weight or 30 µg of warfarin/day can cause
the death of list weasels (Mustela nivalis) by secondary poisoning.
Lethal exposure occurred if mice were contaminated with 1.0-1.5 mg
warfarin/kg, an amount reached following exposure to 0.005 or 0.02%
sodium warfarin for about 3 days.
9.2 Field observations
9.2.1 Primary poisonings
There are few data available on the non-target hazard of
anticoagulant rodenticides in field conditions.
Greig-Smith et al. (1988) reported incidents in England and Wales
with barn owls and foxes. There were three incidents in which barn
owls were found to have residues of the anticoagulant rodenticide
brodifacoum in their livers. In two birds, residues were thought to
represent lethal poisoning (0.61 and 0.29 mg/kg) but in the third the
level was only 0.099 mg/kg. There were six separate incidents in
which dead foxes were found to contain residues of brodifacoum,
bromadiolone, coumatetralyl, difenacoum or warfarin. Residue levels
showed a wide range from 0.009 to 0.46 mg/kg tissue. Three of the
foxes contained more than one rodenticide, one animal revealing
residues of difenacoum, bromadiolone and warfarin.
Poisonings of pheasants and partridges by chlorophacinone used
against Microtus arvalis have been reported (Giban, 1974).
Reece et al. (1985) reported that, a few days after bromadiolone
was placed out for control of rodents, three pea fowl
(Pavo cristatus), a little raven (Corvus mellori) and an eastern
swamp hen (Porphyrio porphyrio melanotus) died. Necropsy showed
that the birds had been in good condition. One of the pea fowl and
the swamp hen had massive intra-abdominal haemorrhage without evidence
of external trauma. All had severe congestion of the liver and lungs.
The birds had negligible gut contents, so analysis for bromadiolone
could not be performed.
9.2.2 Secondary poisonings
Difenacoum and brodifacoum were detected in 15 of a total of 145
dead barn owls in Britain, and on postmortem the cause of death for
one owl was diagnosed as rodenticide poisoning (Newton et al., 1990,
see section 5.1).
A study of 35 active nests and the radio-telemetry of 34 barn
owls in an area where brodifacoum baits (0.005%) were used in and
around farm buildings in New Jersey, USA, showed no adverse effects of
the rodenticide on the owls studied. In this study, rats and house
mice were the target organisms and barn owls were found not to feed on
these animals to any extent. Chicks were fledged from at least eight
nests where poisoned rodents had been available during part of the
nesting and feeding period, but no rodenticide-induced mortality was
observed. Traces of brodifacoum (less than 0.05 mg/kg) were found in
a barn owl that had been accidentally electrocuted (Hegdal &
Blaskiewicz, 1984).
Merson & Byers (1984) monitored eastern screech owls (Otus asio)
using radio-transmitters following the use of 0.005% brodifacoum for
rodent control in a commercial orchard. Brodifacoum was detected in
screech owl pellets (see section 5.1). There were no owl deaths which
could be attributed to brodifacoum poisoning. Two owls were analysed
at the end of the study; one showed signs of haemorrhage and
contained 0.21 mg brodifacoum/kg and the other owl contained no
brodifacoum. In a larger study, Hegdal & Colvin (1988) monitored 38
eastern screech owls and several other species of owl following
brodifacoum application in an orchard. Minimum mortality was 58%
among screech owls for which more than 20% of the home range was
treated, as compared with 17% among those for which less than 10% of
the home range was treated. Secondary brodifacoum poisoning was the
most probable cause of death in six screech owls. Six radio-equipped
owls were collected 1-2 months post-treatment, and four contained
detectable concentrations of brodifacoum.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Anticoagulants, both hydroxycoumarins and indandiones, known also
as vitamin K antagonists, are widely used in urban rodent control and
against rodent pests in agriculture. They act by inhibiting the
vitamin K1 epoxide cycle, thereby depleting the active form of
vitamin K1 necessary for producing blood-clotting factors.
Since anticoagulant rodenticides are used in general as
low-concentration bait formulations and have low volatility, increased
levels in the air are unlikely. Being only slightly soluble in water
their use could not be a major source of water contamination.
Anticoagulant rodenticides are not intended for direct application to
growing crops. Hence no residues in plant foodstuffs are expected.
The controlled medicinal use of warfarin exposes more people to
higher concentrations over a longer period than would be expected to
occur as a result of accidental human exposure due to its use as a
rodenticide.
Occupational exposure may occur during manufacture, formulation
and bait application, but figures indicating the levels of exposure
are not available.
Anticoagulant rodenticides are readily absorbed through the
gastrointestinal tract, skin and respiratory system. The major route
of elimination in various species after oral administration is through
the faeces. The urine is a very minor route of elimination. The liver
is the major organ for accumulation and storage of anticoagulants.
The metabolism pattern of warfarin and indandiones mainly
involves hydroxylation. The second-generation hydroxycoumarins are
found principally as unchanged parent compounds. Their elimination
from the liver is slow with a biphasic rate, where a rapid initial
phase is followed by a prolonged second phase.
Most of the anticoagulants have high acute toxicity by oral,
percutaneous and inhalation routes of exposure. The second-generation
anticoagulants are more toxic than the first-generation one in the
sense that a single feeding may be lethal. Signs of poisoning in all
species, including humans, are associated with increased bleeding
tendency.
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxication from occupational
exposure to anticoagulants have also been observed.
The level of prothrombin concentration is a satisfactory guide to
the severity of acute intoxication and the effectiveness and duration
of the therapy. The specific antidote is vitamin K1. The high
retention of the second-generation anticoagulants in the liver means
that any treatment of intoxication should be prolonged to ensure that
anticoagulant activity does not recur.
The addition of bittering agents to anticoagulant rodenticides is
aimed at discouraging human consumption.
Warfarin has been found to be teratogenic in both rats and
humans. Second-generation anticoagulant rodenticides do not
demonstrate teratogenicity in laboratory animals. Developmental
effects in humans are observed when warfarin has been taken as a
therapeutic agent during pregnancy. No cases of embryopathy from
anticoagulants in their use as rodenticides have been reported.
Second-generation anticoagulants are not intended to be used as
therapeutic agents, and the risks associated with, for example,
warfarin will not apply.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on some compounds to demonstrate an absence of mutagenicity.
There is experimental evidence that coumarin anticoagulants not
only inhibit the vitamin K cycle in the liver, but also in other
tissues such as bone. In humans it has been demonstrated that even
low levels of vitamin K deficiency form a risk factor for developing
osteoporosis (Hart et al., 1985; Szulc et al., 1993). Therefore, it
appears that long-term exposure to low levels of anticoagulant may
have an adverse effect on bone metabolism.
10.2 Evaluation of effects on the environment
Unlike conventional crop protection products, which must be
applied over relatively large crop areas, anticoagulant rodenticides
are applied to discrete sites in the form of low concentration baits.
Most of the anticoagulants are stable under normal conditions.
They are slightly soluble in water, and as bait formulations their use
is unlikely to be a source of water contamination. They appear to
bind rapidly in the soil, with very slow desorption and no leaching.
In general, anticoagulants are highly toxic to aquatic organisms when
tested as technical material.
Non-target organisms are potentially at risk in two ways: from
direct consumption of baits (primary hazard) and through eating
poisoned rodents (secondary hazard).
Bird species vary in their susceptibility to anticoagulant
rodenticides. Small pellets and whole grain baits are highly
attractive to birds. Wax block formulation appear to decrease the
attractiveness to the birds and so reduce the possibility of poisoning
incidents. It is difficult to assess the risks to birds due to direct
consumption of baits because most of the published studies are
toxicity trials in laboratory conditions.
The main cause for poisoning of domestic animals is through
direct consumption of anticoagulant baits.
Some of the anticoagulants show a similar range of acute toxicity
for non-target non-rodent mammals as for target rodents. The primary
hazard is usually expressed by the amount of finished bait which must
be consumed to approach the lethal dose. The bait concentration of
second-generation rodenticides is usually around 0.005%, whereas the
concentration of the first-generation rodenticides in the bait may be
5 to 10 times higher. Thus, to reach the toxic or lethal dose,
non-target animals must consume comparatively large amounts of bait
with low concentrations of active ingredient.
Secondary poisoning through the consumption of rats and mice
killed with anticoagulants may occur in dogs and cats in urban
situations but is more likely in farm situations.
Some secondary toxicity laboratory studies with wildlife have
shown that captive predators could be intoxicated by no-choice feeding
of anticoagulant-poisoned or dosed prey. The significance of these
results in terms of hazard under field conditions is difficult to
assess because the predators would not be expected to eat only
poisoned animals. However, where they occur, predators may take
poisoned, but not dead, small mammals preferentially. In areas close
to baiting, poisoned rodents may represent a high proportion of the
diet for individual birds. However, only a few individuals will be
affected except in situations of very widespread and constant use of
baits. Therefore, some kills of owls will be expected but no severe
population effects. This agrees with observed field effects with small
numbers of poisoned owls.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
a) Exposure of the general population to anticoagulant rodenticides
via food and drinking-water is unlikely and does not constitute a
significant health hazard.
b) Poisoning incidents may occur in cases of massive intentional or
unintentional ingestion or prolonged skin contact during
manufacture and formulation.
c) Anticoagulant rodenticides are relatively persistent in the
environment, but their specific use as low-concentration bait
formulations limits the potential for environmental
contamination.
d) Direct and secondary poisoning of birds, domestic and farm
animals, and wildlife may occur.
e) The major difference between the first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention, resulting in an increased tendency for bleeding over a
longer period of time.
f) The mode of action of anticoagulant rodenticides is known and an
effective antidote is available, i.e. vitamin K1.
g) There is no available evidence that this class of compound is
mutagenic or carcinogenic.
h) Only warfarin has been shown to possess some teratogenic
potential in both rats and humans.
11.2 Recommendations for protection of human health and the
environment
a) Exposed workers should receive appropriate biomonitoring and
health evaluation.
b) The inclusion of a bittering agent in formulations at appropriate
concentrations may reduce accidental ingestion.
c) For preventing primary poisoning, baits less attractive to birds
and domestic animals, as well as pulsating baiting, should be
used.
d) The location of bait placement should be carefully selected.
e) Killed rodents should be burned or buried to reduce the risk of
secondary poisoning of predators.
f) Training in the safe handling of rodenticides is essential.
12. FURTHER RESEARCH
a) Studies of exposed humans are required, particularly with regard
to possible teratogenic and embryotoxic effects.
b) More information is needed in order to evaluate the risks of
occupational exposure to anticoagulant rodenticides.
c) Methods more sensitive than prothrombin time determination need
to be developed for routinely assessing the absorption and
effects of anticoagulants.
d) More data are required to assess secondary effects on non-target
organism populations.
e) The extent to which second-generation anticoagulant rodenticides
are transferred across the placenta should be evaluated.
f) More information should be collected concerning the tissue
distribution of anticoagulant rodenticides after their ingestion
and their effects on physiological processes other than blood
coagulation, notably calcium and bone metabolism. This is
particularly necessary for long-term, low-level exposure to these
compounds.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by Hazard
(WHO, 1994), anticoagulant rodenticides were classified, according to
their acute oral LD50, as follows:
Class Ia (Extremely hazardous) Oral LD50 (mg/kg)
Brodifacoum 0.3
Bromadiolone 1.12
Chlorophacinone 3.1
Difenacoum 1.8
Difethialone 0.56
Diphacinone 2.3
Flocoumafen 0.25
Class Ib (Highly hazardous)
Coumachlor 33
Coumatetralyl 16
Warfarin 10
Class II (Moderately hazardous)
Pindone 50
A Poison Information Monograph for brodifacoum has been issued
(IPCS, 1992), and one on warfarin is in preparation.
REFERENCES
Allen JA, Proudlock RJ, & McCaffrey KJ (1986) Genotoxicity studies
with WL 108366 (rodenticide): in vivo chromosome studies with rat
bone marrow cells. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL 85/8610).
Alstad AD, Casper HH, & Johnson LJ (1985) Vitamin K treatment of sweet
clover poisoning in calves. J Am Med Assoc, 187: 729-731.
Anderson DR, Brill-Edwards P, & Walker I (1992) Warfarin-induced skin
necrosis in 2 patients with protein S deficiency: Successful
reinstatement of warfarin therapy. Haemostasis, 22: 124-128.
AMA (1980) AMA drug evaluations, 4th ed. Chicago, Illinois, American
Medical Association.
Anonymous (1965) Technical report on chlorophacinone. Lyon, France,
Lipha S.A.
Anonymous (1976) 'Ratak' containing difenacoum: A new rodenticide.
Haslemere, Surrey, Imperial Chemical Industries Ltd, Plant Protection
Division, 18 pp.
Anonymous (1985) Brodifacoum: safety in use. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division, 8 pp
(ICI Agrochemical Information Bulletin).
Anonymous (1988) The treatment of anticoagulant rodenticide poisoning.
Imperial Chemical Industries Ltd, Lipha S.A., Shell International
Chemical Company Ltd, and Sorex Ltd.
Antov G, Kasakova B, & Ajanova A (1985) Effect of warfarin on
experimentally induced sclerotic processes. In: Pathological models in
toxicological studies. Bucharest, Romania, Ministry of Chemical
Industry, Industrial Head-Office for Medicinal Drugs and Cosmetics,
pp 153-157.
Ashton AD, Jackson WB, & Peters H (1987) Comparative evaluation of
LD50 values for various anticoagulant rodenticides. In: Richards CGL
& Ku TY ed. Control of mammal pests. London, New York, Philadelphia,
Taylor & Francis, pp 187-197.
Avent ML, Canady BR, & Sawyer WT (1992) Warfarin-induced intramural
hematoma of the small intestine. Clin Pharm, 11: 632-635.
Bachmann KA & Sullivan TJ (1983) Dispositional and pharmacodynamic
characteristics of brodifacoum in warfarin-sensitive rats.
Pharmacology, 27: 281-288.
Barlow AM, Gay AL, & Park BK (1982) Difenacoum (Neosorexa) poisoning.
Br Med J, 285: 541.
Blair D (1984) Toxicology of a candidate rodenticide: The acute 4-hour
inhalation toxicity of manufacturing master mix (bait concentrate)
containing 0.5% m/m WL 108366. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 71 pp (Report No. SBGR.84.151).
Boermans HJ, Johnstone J, Black WD, & Murphy M (1991) Clinical signs,
laboratory changes and toxicokinetics of brodifacoum in horses. Can J
Vet Res, 55: 21-27.
Braithwaite GB (1982) Vitamin K and brodifacoum. J Am Vet Med Assoc,
181(6): 531, 534.
Bratt H (1987) Difenacoum: Elimination from tissues of rats following
administration of a single oral dose. Macclesfield, Surrey, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report
No. CTL/P/1592).
Braselton WE Jr, Neiger RD, & Poppenga RH (1992) Confirmation of
indandione rodenticide toxicoses by mass spectrometry/mass
spectrometry. J Vet Diagn Invest, 4, 441-446.
Brooks LW & Blais FX (1991) Coumarin-induced skin necrosis. J Am
Osteopath Assoc, 91: 601-605.
Brooks TM, Clare MG, & Wiggins DE (1984) Genotoxicity studies with
WL 108366 (candidate rodenticide). Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 64 pp (Report No. SBGR.84.160).
Brorson T, Björklund I, Svenstam G, & Lantz R (1994) Comparison of two
strategies for assessing ecotoxicological aspects of complex
wastewater from a chemical-pharmaceutical plant. Environ Toxicol Chem,
14(4): 543-552.
Buckle AP (1994) Rodent control methods in rodent pests and their
control. Wallingford, Oxon, Commonwealth Agriculture Bureau (CAB)
International.
Buitenhuis HC, Soute BAM, & Vermeer C (1990) Comparison of the
vitamins K1, K2 and K3 as cofactors for the hepatic vitamin
K-dependent carboxylase. Biochim Biophys Acta, 1034: 170-175.
Bull JO (1976) Laboratory and field investigations with difenacoum, a
promising new rodenticide. In: Proceedings of the 77th Vertebrate Pest
Conference, Monterey, California, March 1976. Davis, California,
University of California, pp 72-84.
Bullard RW, Thompson RD, & Holguin G (1976) Diphenadione residues in
tissues of cattle. J Agric Food Chem, 24(2): 261-263.
Burucoa Ch, Mura P, Robert R, Boinot C, Bouquet S, & Piriou A (1989)
Chlorophacinone intoxication: A biological and toxicological study.
Clin Toxicol, 27: 79-89.
Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, & Ind PW (1992)
Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol,
11: 553-554.
Callander RD (1984) Product registration. Brodifacoum: An evaluation
in the salmonella mutagenicity assay. Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V. (ICI CTL Report No. CTL/P/949).
Carson M & Reid M (1976) Warfarin and fetal abnormality. Lancet,
1: 1127.
Chalermchaikit T, Felice LJ, & Murphy MJ (1993) Simultaneous
determination of eight anticoagulant rodenticides in blood serum and
liver. J Anal Toxicol, 17: 56-61.
Chesterman H, Burford P, Harling RJ, & Heywood R (1984) WL 108366
rodenticide: Acute oral toxicity in Beagle dogs. Huntingdon, United
Kingdom, Huntingdon Research Centre (HRC Report No. SLL/72/84757).
Chong LL, Chan WK, & Ho CH (1986) A case of 'superwarfarin' poisoning.
Scand J Haematol, 36: 314-315.
Chow EY, Haley LP, Vickars LM, & Murphy MJ (1992) A case of
bromadiolone (superwarfarin) ingestion. Can Med Assoc J, 147: 60-62.
Christopher MJ, Balasubramanyam M, & Purushotham KR (1984) Toxicity of
three anticoagulant rodenticides to male hybrid Leghorns. Z Angew
Zool, 71(3): 275-281.
Cifone MA (1993) Mutagenicity test on bromadiolone technical in the
CHO/HGPRT forward mutation assay (HWA Study No. 15310-0-435). Vienna,
Virginia, Hazleton Washington Inc., 48 pp.
Clare MG & Wiggins DE (1986) In vitro mutagenicity studies with
WL 108366 (rodenticide) using cultured Chinese hamster V 79 cells.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research
Centre, 33 pp (Report No. SBGR.86.014).
Coveney PC & Forbes S (1987) Degradation of WL 108366 (Storm) in rat
baits, carcasses and faeces in field situations. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.87.171).
Cross M & Clay P (1984) Product registration. Brodifacoum: Assessment
of mutagenic potential using L5178Y mouse lymphoma cells. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/975).
Dodds WJ & Frantz SC (1984) Dog and cat poisonings. Pest Control
Technol, 12(3): 14.
Donovan JW, Ballard JO, & Murphy MJ (1990) Brodifacoum therapy with
activated charcoal: Effect on elimination kinetics. Vet Hum Toxicol,
32: 350.
Dreyfus M, Dubouch P, Pierson K, Neveu Y, Martin E, & Tchernia G
(1983) Warfarin-contaminated talc. Lancet, 1: 1110.
Dubock AC (1986) The evaluation of potential effects on non-target
vertebrate populations as a result of pesticide use. In: Proceedings
of the Second Symposium on Recent Advances in Rodent Control, Kuwait,
1986, pp 257-269.
Dusein P, Manigand G, & Taillandier J (1984) Hypoprothrombinémie
sévère et prolongée après intoxication par chlorphacinone. Presse Méd,
13(30): 1845.
DuVall MD, Murphy MJ, Ray AC, & Reagor JC (1989) Case studies on
second-generation anticoagulant rodenticide toxicities in non-target
species. J Vet Diagn Invest, 1: 66-68.
Eby CS (1993) Warfarin-induced skin necrosis. Hematol/Oncol Clin North
Am, 7: 1291-1300.
Elias DJ & Johns BE (1981) Response of rats to chronic ingestion of
diphacinone. Bull Environ Contam Toxicol, 27: 559-567.
Ellenhorn MJ & Barceloux DG (1988) Anticoagulant poisoning. In:
Medical toxicology, diagnosis and treatment of human poisoning.
Amsterdam, New York, Elsevier Science Publishing Company Inc.,
pp 211-221.
Erichsen C, Sudenaa K, Sreide JA, Andersen E, & Tysvoer A (1993)
Spontaneous liver hematomas induced by anti-coagulation therapy.
Hepato-Gastroenterology, 40: 402-406.
Exner DV, Brien WF, & Murphy MJ (1992) Superwarfarin ingestion. Can
Med Assoc J, 146(1): 34-35.
FAO (1979) Rodenticides: Analyses specifications, formulations. Rome,
Food and Agriculture Organization of the United Nations, pp 1-79 (FAO
Plant Production and Protection Paper 16).
Feinsod FM, Allam I, Ragai A, & Saah AJ (1986) Rodenticide-induced
signs simulating Rift Valley fever in sheep and goats in Egypt. Vet
Rec, 118: 270-272.
Felice LJ & Murphy MH (1989) The determination of the anticoagulant
rodenticide brodifacoum in blood serum by liquid chromatography with
fluorescence detection. J Anal Toxicol, 13: 229-231.
Felice LJ, Chalermchaikit T, & Murphy MJ (1991) Multicomponent
determination of 4-hydroxycoumarin anticoagulant rodenticides in blood
serum by liquid chromatography with fluorescence detection. J Anal
Toxicol, 15: 126-129.
Fiore CE, Tamburino C, Foti R, & Grimaldi D (1990) Reduced bone
mineral content in patients taking an oral anticoagulant. South Med J,
83: 538-542.
Forsey JD (1983a) The acute skin irritation of novel anticoagulants in
male rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 3 pp.
Forsey JD (1983b) Acute eye irritation of novel anticoagulants in male
rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 4 pp.
Forsey JD (1985) An evaluation of the long term sub-acute oral
toxicity of WL 108366 in Wistar rats. Widnes, United Kingdom, Sorex
Ltd, Agricultural Research Department, 10 pp.
Fristedt B & Sterner N (1965) Warfarin intoxication from percutaneous
absorption. Arch Environ Health, 11: 205-208.
Furie B & Furie BC (1990) Molecular basis of vitamin K-dependent
gamma-carboxylation. Blood, 75: 1753-1762.
Giban J (1974) Use of chlorophacinone in the struggle against the
common vole (Microtus arvalis Pallas) and against the musk rat
(Ondatra zibethicus). In: Proceedings of the 6th Vertebrate Pest
Conference, Sacramento, California. Davis, California, University of
California, pp 263-271.
Gijsbers BLMG, van Haarlem LJM, Soute BAM, Ebberink RHM, & Vermeer C
(1990) Characterization of a gla-containing protein from calcified
human atherosclerotic plaques. Arteriosclerosis, 10: 991-995.
Godfrey MER (1984) The toxicology of brodifacoum to target and
non-target species in New Zealand. Proceedings of a Conference on the
Organization and Practice of Vertebrate Pest Control, Elvetham Hall,
Hampshire, England, 30 August-3 September 1982. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division,
pp 631-637.
Godfrey MER (1985) Non-target and secondary poisoning hazards of
"second generation" anticoagulants. Acta Zool Fennica, 173: 209-212.
Godfrey MER, Laas FJ, & Rammell CG (1985) Acute toxicity of
brodifacoum to sheep. N Z J Exp Agric, 13: 23-25.
Gorenzel WP, Marsh RE, & Salmon TR (1982) Single-feeding
anticoagulants. Pest Control, 50(2): 32.
Grand M (1976) Données expérimentales sur un nouveau raticide
anticoagulant: le bromadiolone. Phytiatrie-Phytopharmacie, 25: 69-88.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1992) Toxicity of
second generation rodenticides to barn owls. In: Proceedings of the
Brighton Crop Protection Conference - Pests and Diseases, 1992,
vol 2, pp 781-786.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1994) Non-invasive
method for monitoring the exposure of barn owls to second-generation
rodenticides. Pestic Sci, 41: 339-343.
Greeff MC, Mashile O, & MacDougall LG (1987) "Superwarfarin"
(bromadiolone) poisoning in two children resulting in prolonged
anticoagulation. Lancet, 1: 1269.
Greig-Smith PW, Fletcher MR, Hunter K, Quick MP, Ruthven AD, & Shaw IC
(1988) Pesticide poisoning of animals 1988: Investigations of
suspected incidents in Great Britain. A Report of the Environmental
Panel of the Advisory Committee on Pesticides.
Hadler MR (1974) Sub-acute five day oral toxicity of WBA 8119 to male
rats. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research.
Hadler MR (1975a) Acute oral toxicity of WBA 8119 to male rabbit.
Appendix 5. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research, 3 pp.
Hadler MR (1975b) Acute oral toxicity of WBA 8119 to female Guinea
pig. Appendix 6. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research, 4 pp.
Hadler MR (1975c) Acute eye irritation of WBA 8119 to the rabbit.
Appendix 31. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research.
Hadler MR (1976) WBA 8119-12 week feeding study in rats. Widnes,
Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural Research, 7 pp
(Laboratory report).
Hagan EC & Radomski JL (1953) The toxicity of 3-(acetonylbenzyl)-
4-hydroxycoumarin (warfarin) to laboratory animals. J Am Pharm Assoc,
42(6): 379-382.
Hall JG (1976) Warfarin and fetal abnormality. Lancet, 1: 1127.
Hall BE & Priestley I (1992) Brodifacoum: Metabolism in soil under
aerobic conditions (IRI Project No. 381441). Haslemere, Surrey, Zeneca
Agrochemicals, 63 pp (Report No. 8795).
Hall JG, Pauli RM, & Wilson KM (1980) Maternal and fetal sequelae of
anticoagulants during pregnancy. Am J Med, 68: 122-140.
Hall BE, Jackson R, & Priestley I (1992) Difenacoum: Photolysis in
buffered aqueous solutions (IRI Project No. 381614). Haslemere,
Surrey, Zeneca Agrochemicals, 123 pp (Report No. 8704).
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN,
Dodds RA, Bitensky L, & Chayen J (1985) Electrochemical detection of
depressed circulating levels of vitamin K1 in osteoporosis. J Clin
Endocrinol Metab, 60(6): 1268-1269.
Hegdal PL & Blaskiewicz RW (1984) Evaluation of the potential hazard
to barn owls of Talon (brodifacoum bait) used to control rats and
house mice. Environ Toxicol Chem, 3: 167-179.
Hegdal PL & Colvin BA (1988) Potential hazard to eastern screech-owls
and other raptors of brodifacoum bait used for vole control in
orchards. Environ Toxicol Chem, 7: 245-260.
Hill RW, Maddock BG, Hart B, & Bowles PF (1976) Determination of the
acute toxicity of PP 581 to Rainbow trout (Salmo gairdneri). Brixham,
Devon, Imperial Chemical Industries Ltd, Brixham Laboratory (Report
No. BL/B/1758).
Hodge MCE, Banham PB, Richards D, Weight TM, & Wilson J (1980a)
Product registration. Brodifacoum: Teratogenicity study in the rat.
Geneva, Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.
(ICI CTL Report No. CTL/P/437).
Hodge MCE, Banham PB, Richards D, & Weight TM (1980b) Product
registration. Brodifacoum: Teratogenicity study in the rabbit. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/459).
Hodge MCE, Banham PB, Richards D & Weight TM (1980c) Product
registration. Brodifacoum: Teratogenicity study in the rabbit
(Appendices A-E). Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V. (ICI CTL Report No. CTL/P/459 S).
Hoffman RS, Smilkstein MJ, & Goldfrank LR (1988) Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose. Clin Toxicol, 26(3/4): 233-248.
Hollinger BR & Pastoor TP (1993) Case management and plasma half-life
in a case of brodifacoum poisoning. Arch Intern Med, 153: 1925-1928.
Hoogenboom JJL (1994) Anticoagulant poisoning cases 1977-1994.
Wellington, New Zealand Central Animal Health Laboratory.
Hoogenboom JJL & Rammell CG (1983) Improved HPLC method for
determining brodifacoum in animal tissues. J Environ Contam Toxicol,
31: 239-243.
Huckle KR & Warburton PA (1986a) Elimination, metabolism and
disposition of 14C-WL 108366 in the Fischer 344 rat following
repeated oral administration. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre (Report No. SBGR.86.084).
Huckle KR & Warburton PA (1986b) Percutaneous absorption metabolism
and elimination of WL 108366 in the rat. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.218).
Huckle KR & Warburton PA (1986c) WL 108366: Absorption, metabolism and
disposition in Japanese quail (Coturnix coturnix Japonica) following a
single dose by intraperitoneal or oral administration. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.192).
Huckle KR, Hutson DH, & Warburton PA (1988) Elimination and
accumulation of the rodenticide flocoumafen in rats following repeated
oral administration. Xenobiotica, 18(12): 1465-1479.
Huckle KR, Hutson DH, Logan CJ, Morrison BJ, & Warburton PA (1989) The
fate of the rodenticide flocoumafen in the rat: Retention and
elimination of a single oral dose. Pestic Sci, 25: 297-312.
Huebner CF & Link KP (1941) Studies on the hemorrhagic sweet clover
disease. VI. The synthesis of the delta-diketone derived from the
hemorrhagic agent through alkaline degradation. J Biol Chem,
138: 529-534.
Hunter K (1983) Determination of coumarin anticoagulant rodenticide
residues in animal tissues by high-performance liquid chromatography:
Fluorescence detection using post-column techniques. J Chromatogr,
270: 267-276.
Hunter K (1985) High-performance liquid chromatographic strategies for
the determination and confirmation of anticoagulant rodenticides in
animal tissues. J Chromatogr, 321: 255-272.
Ikeda M, Conney AH, & Burns JJ (1968a) Stimulatory effect of
phenobarbital and insecticides on warfarin metabolism in the rat.
J Pharmacol Exp Ther, 162: 338-343.
Ikeda M, Ullrich V, & Staudinger H (1968b) Metabolism in vitro of
warfarin by enzymic and nonenzymic systems. Biochem Pharmacol,
17: 1663-1669.
IPCS (1992) IPCS/INTOX project. Poison Information Monograph:
Brodifacoum. Geneva, World Health Organization, 16 pp
(IPCS/INTOX/PIM.077).
Jackson R & Hall BE (1992) Aged soil leaching of [14C]-brodifacoum
(IRI Project No. 381986). Haslemere, Surrey, Zeneca Agrochemicals,
72 pp (Report No. 8879).
Jackson R, Priestley I, & Hall BE (1991) The determination of the
hydrolytic stability of [14C]-brodifacoum (IRI Project No. 381420).
Haslemere, Surrey, Zeneca Agrochemicals. 77 pp (Report No. 8330).
Jones EC, Growe GH, & Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. J Am Med Assoc, 252(21): 3005-3007.
Katona B & Wason S (1989) Superwarfarin poisoning. J Emerg Med,
7: 627-631.
Kaukeinen DE & Buckle AP (1992) Evaluations of aversive agents to
increase the selectivity of rodenticides, with emphasis on denatonium
benzoate (Bitrex(R)) bittering agent. In: Borrecco JE & Marsh RE ed.
Proceedings of the 15th Vertebrate Pest Conference. Davis, California,
University of California, pp 192-198.
Kelly MJ, Chambers J, & MacNicoll AD (1993) Simple and rapid method
for the determination of the diastereomers of difenacoum in blood and
liver using high-performance liquid chromatography with fluorescence
detection. J Chromatogr, 620: 105-112.
Kennelly JC (1990) Difenacoum: Assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 42 pp (Report No. CTL/P/3017.
Knapen MHJ, Jie K-SG, Hamulyák K, & Vermeer C (1993) Vitamin K-induced
changes in markers for osteoblast activity and urinary calcium loss.
Calcif Tissue Int, 53: 81-85.
Kosmin M & Barlow JN (1976) Rodent control using a novel formulation
of diphacinone, Ramik. Presented at the First Afro-Asian Vertebrate
Pest Conference, Cairo. Rosemont, Illinois, Velsicol Chemical
Corporation, 7 pp.
Krambias A & Hoppe AH (1987) The response of captive partridges to
dosing with anticoagulant rodenticides. In: Richards CGL & Ku TY ed.
Control of mammal pests. London, New York, Philadelphia, Taylor &
Francis, pp 181-186.
Kruse JA & Carlson RW (1992) Fatal rodenticide poisoning with
brodifacoum. Ann Emerg Med, 21: 331-336.
Laas FJ, Forss DA, & Goodfrey MER (1985) Retention of brodifacoum in
sheep tissues and excretion in faeces. N Z J Agric Res, 28: 357-359.
Lange PF & Terveer J (1954) Warfarin poisoning. US Armed Forces Med J,
5: 872-877.
Lawlor TE (1992) Mutagenicity test on bromadiolone in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test)
(HWA Study No. 15310-0-401). Vienna, Virginia, Hazleton Washington
Inc., 34 pp.
Lechevin JC & Grand M (1987) LM 2219 (proposed common name:
difethialone). Summary of the file submitted in the request for
approval (struggle against commensal rodents). Lyon, France, Lipha
S.A., Research and Development Centre, 15 pp (Report No. L-JCL/SP).
Leck JB & Park BK (1981) A comparative study of the effects of
warfarin and brodifacoum on the relationship between vitamin K1
metabolism and clotting factor activity in warfarin-susceptible and
warfarin-resistant rats. Biochem Pharmacol, 30: 123-128.
Lewis CJ (1992a) (14-C)-Difenacoum: A study of the degradation in two
soils. Haslemere, Surrey, Zeneca Agrochemicals (Report No. 6927).
Lewis CJ (1992b) (14C)-Difenacoum: Aged soil leaching. Haslemere,
Surrey, Zeneca Agrochemicals.
Lewis CJ (1992c) Difenacoum: Hydrolysis study. Haslemere, Surrey,
Zeneca Agrochemicals (Report No. 7031).
Lipton RA & Klass EM (1984) Human ingestion of a 'superwafarin'
rodenticide resulting in a prolonged anticoagulant effect. J Am Med
Assoc, 252: 3004-3005.
Litovitz TL, Clark LR, & Soloway RA (1994) 1993 Annual report of the
American Association of Poison Control Centers, Toxic Exposure
Surveillance System. Am J Emerg Med, 12: 546-584.
Locht H & Lindström FD (1993) Severe skin necrosis following warfarin
therapy in a patient with protein C deficiency. J Intern Med,
233: 287-289.
Lund M (1981) Hens, eggs and anticoagulants. Int Pest Control,
5: 126-128.
Lund M (1982) Annual report 1981. Lyngby, Danish Pest Infestation
Laboratory, pp 58-75.
Lund M (1984) Resistance to the second generation anticoagulant
rodenticides. In: Clark DO ed. Proceedings of the 11th Vertebrate Pest
Conference. Davis, California, University of California, pp 89-94.
Mackintosh CG, Laas FJ, Godfrey MER, & Turner K (1988) Vitamin K1
treatment of brodifacoum poisoning in dogs. In: Crabb AC & Marsh RE
ed. Proceedings of the 13th Vertebrate Pest Conference. Davis,
California, University of California, pp 86-90.
McSporran KD & Phillips CA (1983) Brodifacoum poisoning in a dog.
N Z Vet J, 31: 185-186.
Manfioletti G, Brancolini C, Avanzi G, & Schneider C (1993) The
protein encoded by a growth arrest-specific gene (gas6) is a new
member of the vitamin K-dependent proteins related to protein S, a
negative coregulator in the blood coagulation cascade. Mol Cell Biol,
13(5): 4976-4985.
Marsh RE (1985a) Are anticoagulant rodenticides a problem for
household pets? Pest Control, 53(8): 20-22.
Marsh RE (1985b) Anticoagulant rodenticides provide pet problems.
Part II. Pest Control, 53(9): 25-28.
Martin-Bouyer G, Linh PD, Tuan LC, Barin C, Khahn MB, Hoa DQ, Tourneau
J, & Guerbois H (1983) Epidemic of haemorrhagic disease in Vietnamese
infants caused by warfarin-contaminated talc. Lancet, 1: 230-232.
Mellano D (1984a) Product registration. In vitro study of chromosome
aberration induced by the article brodifacoum in cultured human
lymphocytes. Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V., 17 pp (Report No. CTL/C/1258).
Mellano D (1984b) Product registration. Study of the capacity of test
article brodifacoum to induce unscheduled DNA synthesis in cultured
Hela cells (autoradiographic method). Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V., 14 pp (Report No. CTL/C/1257).
Mendenhall VM & Pank LF (1980) Secondary poisoning of owls by
anticoagulant rodenticides. Wildl Soc Bull, 8(4): 311-315.
Merson MH & Byers RE (1984) Residues of the rodenticide brodifacoum in
voles and raptors after orchard treatment. J Wildl Manage,
48: 212-216.
Meyer AL & Wiggins DE (1986) Genotoxicity studies with WL 108366
(rodenticide): In vitro cell transformation studies. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.289).
Mirkova E & Antov G (1983) Experimental evaluation of the risk of
prenatal pathology under the effect of warfarin coumarine rodenticide.
Hig Zdrav, 25(6): 476-482.
Misenheimer TM, Lund ML, Baker EM, & Suttie JW (1994) Biochemical
basis of warfarin and bromadiolone resistance in the house mouse,
Mus musculus domesticus. Biochem Pharmacol, 47: 673-678.
Monnot G, Fave A, Illat T, & Briet Ph (1981) Phase 3 reformat of
MRID 0007-9375: Teratology study in the rat with LM 637 bromadiolone.
L'Arborasle, France, Institut Français de Recherches et Essais
Biologiques, 124 pp (Report No. MRID 0007-9375).
Morin M-F (1988) Study of the impact on the natural environment of
bromadiolone, an anticoagulant rodenticide: Evolution in aqueous
medium and bioaccumulation by terrestrial and aquatic organisms.
University of Poitiers (Thesis) ((Order No. 176).
Mosterd JJ & Thijssen HHW (1991) The long-term effects of the
rodenticide, brodifacoum, on blood coagulation and vitamin K
metabolism in rats. Br J Pharmacol, 104: 531-534.
Mount ME & Feldman BF (1983) Mechanism of diphacinone rodenticide
toxicosis in the dog and its therapeutic implications. Am J Vet Res,
44(11): 2009-2017.
Mount ME & Kass PH (1989) Diagnostic importance of vitamin K1 and
its epoxide measured in serum of dogs exposed to an anticoagulant
rodenticide. Am J Vet Res, 50: 1704-1709.
Mount ME, Feldman BF, & Buffington T (1982) Vitamin K and its
therapeutic importance. J Am Vet Med Assoc, 180(11): 1354-1356.
Murdoch DA (1983) Prolonged anticoagulation in chlorphacinone
poisoning. Lancet, 1: 355-356.
Murli H (1993) Mutagenicity test on bromadiolone, technical in an
in vivo mouse micronucleus assay (HWA Study No. 15310-0-455).
Washington, DC, Hazleton Laboratories.
Murphy MA & Nye DH (1991) Thoracic intramedullary haematoma as a
complication of warfarin: Case report and literature review. Aust N Z
J Surg, 61: 789-792.
Murphy MJ, Ray AC, & Bailey EM (1989) A high performance liquid
chromatography method for the detection of Brodifacoum in serum. Vet
Hum Toxicol, 31(3): 228-231.
Nehéz M, Kékes-Szabo A, Mazzag E, Selypes A, & Jarmay K (1985)
Histologic and cytogenetic effects of redentin on the spermiogenesis
and bone marrow cells of the mouse, an in vivo experiment. Regul
Toxicol Pharmacol, 5: 204-206.
Nelsestuen GL, Zytkovicz TH, & Howard JB (1974) The mode of action of
vitamin K: Identification of gamma-carboxyglutamic acid as a component
of prothrombin. J Biol Chem, 249: 6347-6350.
Newby S & White BG (1978) Brodifacoum: Adsorption and desorption in
soils measured under laboratory conditions. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division (Report
No TMJ 1764B).
Newton I, Wyllie I, & Freestone P (1990) Rodenticides in British barn
owls. Environ Pollut, 68: 101-117.
Newton I, Wyllie I, Gray A, Eadsforth CV (1994) The toxicity of the
rodenticide flocoumafen to barn owls and its elimination via pellets.
Pestic Sci, 41: 187-193.
Nighoghossian N, Ruel JH, French P, Froment JC, & Trouillas P (1990)
Hématome sous-dural cervico-dorsal par intoxication aux raticides
coumariniques. Rev Neurol (Paris), 146(3): 221-223.
Nilsson IM (1957) Recurrent hypoprothrombinaemia due to poisoning with
dicumarol-containing rat-killer. Acta Haematol, 17: 176-182.
O'Bryan SM & Constable DJC (1991) Quantification of brodifacoum in
plasma and liver tissue by HPLC. J Anal Toxicol, 15: 144-147.
O'Reilly RA, Aggeler PM, & Leong LS (1963) Studies on the coumarin
anticoagulant drugs: The pharmacodynamics of warfarin in man. J Clin
Invest, 4: 1542-1551.
O'Reilly RA, Aggeler PM, Haag MS, Leong LS, & Kropatkin ML (1964)
Hereditary transmission of exceptional resistance to coumarin
anticoagulant drugs. N Engl J Med, 271: 809-815.
Overman RS, Stahmann MA, Huebner CF, Sullivan WR, Spero L, Doherty DG,
Ikawa M, Graf L, Roseman S, & Link KP (1944) Studies on the
hemorrhagic sweet clover disease. XIII. Anticoagulant activity and
structure in the 4-hydroxycoumarin group. J Biol Chem, 153: 5-24.
Owren PA (1959) Thrombotest: A new method for controlling
anticoagulant therapy. Lancet, 2: 754-758.
Park BK & Leck JB (1982) A comparison of vitamin K antagonism by
warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol,
31(22): 3635-3639.
Park BK, Choonara IA, Haynes BP, Breckenridge AM, Malia RG, & Preston
FE (1986) Abnormal vitamin K metabolism in the presence of normal
clotting factor activity in factory workers exposed to
4-hydroxycoumarins. Br J Clin Pharmacol, 21: 289-294.
Parkinson GR (1975) WBA 8119: Acute oral toxicity. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 6 pp (Report No. CTL/P/216 (revised)).
Parkinson GR (1976) PP581: Acute oral toxicity to sheep. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 3 pp (Report No. CTL/P/259 (revised)).
Parkinson GR (1979) PP 581: Acute oral toxicity and skin
sensitization. Macclesfield, Cheshire, Imperial Chemical Industries
Ltd, Central Toxicology Laboratory, 3 pp (Report No. CTL/P/260
(revised)).
Parmar G, Bratt H, Moore R, & Batten PL (1987) Evidence for common
binding site in vivo for the retention of anticoagulants in rat
liver. Hum Toxicol, 6: 431-432.
Pastoureau P, Vergnaud P, Meunier PJ, & Delmas PD (1993) Ostopenia and
bone-remodeling abnormalities in warfarin-treated lambs. J Bone Miner
Res, 8: 1417-1426.
Pauli RM & Hall JG (1979) Warfarin embryopathy. Lancet, 2: 144.
Pearson N (1984) WL 108366 manufacturing master mix: Acute toxicity to
Salmo gairdneri and Daphnia magna. Sittingbourne, Shell Research Ltd
(Report No. SBGR.84.274).
Pearson N & Wallace BG (1984) Technical WL 108366: Acute toxicity to
Salmo gairdneri, Cyprinus carpio, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Kent, Shell Research Ltd, Sittingbourne
Research Centre (Report No. SBGR.84.211).
Pelfrène AF (1991) Synthetic organic rodenticides. In: Hayes WJ Jr &
Laws ER Jr ed. Handbook of pesticide toxicology. New York, Academic
Press, vol 3, pp 1271-1316.
Persson H (1994) [Annual report 1993.] Stockholm, Swedish Poison
Information Centre, 24 pp (in Swedish).
Pribilla O (1966) Murder caused by warfarin. Arch Toxicol,
21: 235-249.
Price JB (1985a) Toxicology of rodenticides: The acute oral toxicity
of WL 108366 in rabbits and hamsters. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.189).
Price JB (1985b) Toxicology of rodenticides: The percutaneous toxicity
of WL 108366 corn oil manufacturing master mix. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.84.227).
Price JB (1985c) Toxicology of rodenticides - WL 108366: A five day
range finding feeding study in rats. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.84.279).
Price JB (1985d) WL 108366: A 28-day feeding study in rats.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.84.235).
Price JB (1986) Toxicology of rodenticides ("Storm"): The skin
sensitizing potential of WL 108366. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.86.091).
Price PA, Williamson MK, Haba T, Dell RB, & Jee WS (1982) Excessive
mineralization with growth plate closure in rats on chronic warfarin
treatment. Proc Natl Acad Sci (USA), 79: 7734-7738.
Quick AJ (1935) The prothrombin in haemophilia and in obstructive
jaundice. J Biol Chem, 109: 73.
Radvanyi A, Weaver P, Massari C, Bid D, & Broughton E (1988) Effects
of chlorophacinone on captive kestrels. Bull Environ Contam Toxicol,
41: 441-448.
Rammell CG, Hoogenboom JJL, & Cotter M (1984) Brodifacoum residues in
target and non-target animals following rabbit poisoning trials. N Z J
Exp Agric, 12: 107-111.
Rauch AE, Weininger R, Pasquale D, Burkart PT, Dunn HG, Weissman C, &
Rydzak E (1994) Superwarfarin poisoning: A significant public health
problem. J Community Health, 19: 55-65.
Ray AC, Murphy MJ, DuVall MD, & Reagor JC (1989) Determination of
brodifacoum and bromadiolone residues in rodent and canine liver. Am J
Vet Res, 50(4): 546-550.
Redfern R, Gill JE, & Hadler MR (1976) Laboratory evaluation of WBA
8119 as a rodenticide for use against warfarin resistant and
non-resistant rats and mice. J Hyg Camb, 77: 419-426.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 16 November: 525-527.
Resch H, Pietschmann P, Krexner E, & Willvonseder R (1991) Decreased
peripheral bone mineral content in patients under anticoagulant
therapy with phenprocoumon. Eur Heart J, 12: 439-441.
Roberts NL, Cameron DM, & Street AE (1985a) WL 108366 - Acute oral
toxicity to pigs. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL/76/851108).
Roberts NL, Fairley C, & Baldwin MK (1985b) The acute oral toxicity
(LD50) of WL 108366 to the Mallard duck. Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Report No. SLL 67/BT/84925).
Roberts NL, Fairley C, & Baldwin MK (1985c) The acute oral toxicity of
WL 108366 to the Mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Centre (HRC Report No. SLL/73/BT/8572).
Robinson DS & MacDonald MG (1966) The effect of phenobarbital
administration on the control of coagulation achieved during warfarin
therapy in man. J Pharmacol Exp Ther, 153: 250-253.
Ross DB, Roberts NL, & Cameron DM (1978) The acute oral toxicity
(LD50) of PP581 to the Mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No ICI 122WL/78507).
Ross DB, Roberts NL, & Phillips C (1979) The subacute toxicity of
difenacoum (Ratak) to pigs. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No.
ICI 262/79697).
Ross DB, Roberts NL, & Fairley C (1980a) The subacute dietary toxicity
(LC50) of difenacoum to Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 305/791197).
Ross DB, Roberts NL, & Fairley C (1980b) The subacute dietary toxicity
(LC50) of difenacoum to the mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 304/791198).
Ross DB, Roberts NL & Fairley C (1980c) The acute oral toxicity
(LD50) of difenacoum to the Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 309/WL/8076).
Ross GS, Zacharski LR, Robert D, & Rabin DL (1992) An acquired
hemorrhagic disorder from long-acting rodenticide ingestion. Arch
Intern Med, 152: 410-412.
Schardein JL (1985) Anticoagulants. In: Chemically induced birth
defects. New York, Basel, Marcel Dekker Inc., pp 89-100.
Schulman A, Lusk R, Lippincott CL, & Ettinger SJ (1986)
Diphacinone-induced coagulopathy in the dog. J Am Vet Med Assoc,
188(4): 403-405.
Selypes A, Kékes-Szabo A, & Nehéz M (1984) Study of the mutagenic
effect of redentin on various species of animals. Acta Vet Hung,
32(3-4): 213-217.
Sharples R (1983a) The acute oral toxicity of a series of novel
anticoagulants in Wistar rats and C57BL/10 mice. Widnes, United
Kingdom, Sorex Ltd, Agricultural Research Department, 7 pp.
Sharples R (1983b) The acute oral toxicity of WL 108366 in
Dunkin-Hartley guinea pigs. Widnes, United Kingdom, Sorex Ltd,
Agricultural Research Department, 3 pp.
Sharples R (1984) The acute oral toxicity of WL 108366 in C57BL/10
mice. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 6 pp.
Sheen SR, Spiller HA, & Grossman D (1994) Symptomatic brodifacoum
ingestion requiring high-dose phytonadione therapy. Vet Hum Toxicol,
36(3): 216-217.
Sheldon T, Richardson CR, & Shaw J (1984) Product registration. An
evaluation of brodifacoum in the mouse micronucleus test. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.,
19 pp (Report No. CTL/P/1006).
Smith MF & Cameron MD (1979) Warfarin as teratogen. Lancet, 1: 727.
Smolinske SC, Scherger DL, Kearns PS, Wruk KM, Kulig KW, & Rumack BH
(1989) Superwarfarin poisoning in children: A prospective study.
Pediatrics, 84: 490-494.
Spare WC (1982) Aqueous photodegradation of 14C-bromadiolone.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Spare WC (1992) Hydrolysis of bromadiolone. Beltsville, Maryland,
Biospherics Inc. (Unpublished report submitted to WHO by Lipha S.A.).
Spare WC & Olson SB (1980) 14C-Bromadiolone soil leaching.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Sridhara S & Krishnamurthy TR (1992) Potentiation of anticoagulant
toxicity to Rattus rattus by two non-steroid anti-inflammatory drugs.
In: Borrecco JE & Marsh RE ed. Proceedings of the 15th Vertebrate Pest
Conference. Davis, California, University of California, pp 212-217.
Stenflo J, Fernlund P, Egan W, & Roepstorff P (1974) Vitamin K
dependent modifications of glutamic acid residues in prothrombin. Proc
Natl Acad Sci (USA), 71(7): 2730-2733.
Stowe CM, Metz AL, Arendt TD, & Schulman J (1983) Apparent brodifacoum
poisoning in a dog. J Am Vet Med Assoc, 182(8): 817-818.
Sullivan MP, Dean BS, & Krenzelok EO (1989) Long-acting anticoagulant
rodenticides: An evaluation of 88 cases. Vet Hum Toxicol, 31: 361.
Szulc P, Chapuy M-C, Meunier PJ, & Delmas PD (1993) Serum
undercarboxylated osteocalcin is a marker of the risk of hip fracture
in elderly women. J Clin Invest, 91: 1769-1774.
Thomson WT (1988) Agricultural chemicals. Book III - Miscellaneous
chemicals: Fumigants, growth regulators, repellents, and rodenticides
(1988/89 Revision). Fresno, California, Thomson Publications.
Tomlin C ed. (1994) The pesticide manual incorporating the
agrochemical handbook, 10th ed. Farnham, Surrey, The British Crop
Protection Council.
Townsend MG, Fletcher MR, Odam EM, & Stanley PI (1981) An assessment
of the secondary poisoning hazard of warfarin to tawny owls. J Wildl
Manage, 45(1): 242-248.
Townsend MG, Bunyan PJ, Odam EM, Stanley PI, & Wardall HP (1984)
Assessment of secondary poisoning hazard of warfarin to least weasels.
J Wildl Manage, 48(2): 628-632.
Travis SF, Warfield W, Greenbaum BH, Mobkisher M, & Siegel JE (1993)
Spontaneous hemorrhage associated with accidental brodifacoum
poisoning in a child. J Pediatr, 122: 982-984.
Tuinman CP & Van Sittert NJ (1985) Biomedical monitoring of personnel
in Sorex Ltd (Widnes, UK) involved in a formulation run with the
rodenticide WL 108366. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report No. HSE 85.006).
Ullrich V & Staundinger H (1968) Metabolism in vitro of warfarin by
enzymic and nonenzymic systems. Biochem Pharmacol, 17: 1663-1669.
Ungvary G (1994) Anticoagulant poisoning incidents in Hungary.
Budapest, National Institute of Occupational Health (Unpublished
report).
Van Haarlem LJM, Knapen MHJ, Hamulyák K, & Vermeer C (1988)
Circulating osteocalcin during oral anticoagulant therapy. Thromb
Haemost, 60: 79-82.
Veenstra GE, Owen DE, & Huckle KR (1991) Metabolic and toxicological
studies on the anticoagulant rodenticide, flocoumafen. Arch Toxicol,
14(Suppl): 160-165.
Vermeer C (1990) gamma-Carboxyglutamate-containing proteins and the
vitamin K-dependent carboxylase. Biochem J, 266: 625-636.
Vermeer C & Soute BAM (1992) Comparison of rodenticide anticoagulant
action in vitro and in vivo. Sittingbourne, Kent, Shell Research
Ltd (Report No. HSE/50).
Virat M (1981) LM-637-Bromadiolone: Teratology study in the rabbit by
oral route. L'Arborasle, France, Institut Français de Recherches et
Essais Biologiques (Report No. 106206).
Vogel JJ, de Moerloose Ph, Bouvier CA, Gaspoz J, & Riant P (1988)
Anticoagulation prolongée lors d'une intoxication à la chlorphacinone.
Schweiz Med Wochenschr, 118: 1915-1917.
Völkl S & Galicia H (1992) 14C-Bromadiolone: Degradation and
metabolism in soils incubated under aerobic conditions. Itingen,
Switzerland, Umweltchemie AG (Unpublished report submitted to WHO by
Lipha S.A., Lyon).
Wallace S, Paull P, Worsnop C, & Mashford ML (1990) Covert self
poisoning with brodifacoum, a "superwarfarin". Aust N Z J Med,
20: 713-715.
Warburton PA & Huckle KR (1986) WL 108366 - Fate of a single oral dose
of 14C WL 108366 in rats. Part III: Metabolism. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.294).
Warburton PA & Hutson DH (1985) WL 108366 - Fate of a single oral dose
of 14C-WL 108366 in rats. Part I: Elimination and retention of
radioactivity and effect of WL 108366 on prothrombin time.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.85.053).
Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, Murphy
MJ, & Furie B (1990) Surreptitious ingestion of a long-acting vitamin
K antagonist/rodenticide, brodifacoum: Clinical and metabolic studies
of three cases. Blood, 76(12): 2555-2559.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, 64 pp (WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II). Clin Chem,
33(11): 2074-2078.
Winn MJ, Clegg JAD, & Park BK (1987) An investigation of sex-linked
differences to the toxic and to the pharmacological action of
difenacoum: Studies in mice and rats. J Pharm Pharmacol, 39: 219-222.
Wiswesser WJ (1976) Pesticide index, 5th ed. College Park, Maryland,
Entomological Society of America.
Woody BJ, Murphy MJ, Ray AC, & Green RA (1992) Coagulopathic effects
and therapy of brodifacoum toxicosis in dogs. J Vet Intern Med,
6: 23-28.
Yu CC, Atallah YH, & Whitacre DM (1982) Metabolism and disposition of
diphacinone in rats and mice. Drug Metab Dispos, 10: 645-648.
RESUME
1. Généralités
Les anticoagulants qui sont décrits dans la présente monographie
sont ceux que l'on utilise principalement en agriculture et pour la
destruction des rongeurs en milieu urbain. La warfarine, premier
rodenticide anticoagulant à connaître une large utilisation, était à
l'origine un médicament efficace pour le traitement des
thromboembolies chez l'homme.
Selon leur structure chimique, les rodenticides anticoagulants
peuvent se répartir en deux catégories, les hydroxycoumarines et les
indanediones, mais leur mode d'action est analogue.
2. Propriétés et méthodes d'analyse
Les rodenticides anticoagulants se présentent sous la forme de
solides cristallins ou de poudres et sont légèrement solubles dans
l'eau. La plupart d'entre eux sont stables dans les conditions
normales de conservation.
La plupart des méthodes de dosage des rodenticides anticoagulants
reposent sur la chromatographie en phase liquide à haute performance.
3. Sources d'exposition humaine et environnementale
Les hydroxycoumarines de la première génération ont commencé à
être utilisées comme rodenticides vers la fin des années 1940. Par la
suite, l'apparition d'une résistance à la warfarine et aux autres
anticoagulants de la première génération a conduit à mettre au point
des produits de deuxième génération, plus actifs. La concentration du
principe actif dans les appâts varie selon l'efficacité du
rodenticide.
4. Distribution, concentration et exposition dans l'environnement
Les rodenticides anticoagulants sont principalement utilisés sous
la forme d'appâts. Comme ils sont peu volatils, leur concentration
dans l'air est négligeable. De même n'étant que légèrement solubles
dans l'eau, il est peu probable que leur utilisation conduise à la
contamination des eaux.
Etant donné que les rodenticides anticoagulants ne sont pas
destinés à être appliqués directement sur les cultures, il n'y a pas
lieu de s'attendre à en trouver sous forme de résidus dans les
aliments d'origine végétale.
L'exposition aux rodenticides des vertébrés non visés peut se
produire directement par la consommation d'appâts empoisonnés et
indirectement par celle de rongeurs contaminés. Les petits granulés
et les appâts constitués de grains entiers attirent fortement les
oiseaux.
La warfarine est utilisé dans le traitement des thromboembolies.
Il existe une possibilité d'exposition professionnelle aux
rodenticides anticoagulants lors de leur fabrication ou formulation ou
encore lors de la pose d'appâts empoisonnés, mais on ne dispose pas de
données sur l'ampleur de cette exposition.
5. Mode d'action et métabolisme
Les rodenticides anticoagulants sont des antagonistes de la
vitamine K. Leur action est principalement localisée dans le foie où
plusieurs précurseurs de l'hémostase subissent un processus
post-traductionnel dépendant de la vitamine K avant d'être convertis
en zymogènes procoagulants. L'action proprement dite consiste dans
l'inhibition de la K1-époxyde-réductase.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives et pourraient l'être également à travers
la peau et dans les vois respiratoires. Après administration par voie
orale, c'est principalement dans les matières fécales que ces composés
sont éliminés chez diverses espèces.
Il est possible que la décomposition métabolique de la warfarine
et des indanediones chez le rat s'effectue principalement par
l'intermédiaire d'une hydroxylation. En revanche, les anti-coagulants
de la deuxième génération s'éliminent essentiellement tels quels. Le
faible taux d'excrétion urinaire empêche d'isoler les métabolites de
l'urine.
Le foie est le principal organe où s'accumulent les rodenticides
anticoagulants. Cette accumulation a lieu également dans les
graisses.
6. Effets sur les mammifères et les systèmes d'épreuve in vitro
Chez le rat et la souris, les signes d'intoxication consistent en
une tendance accrue au saignement.
La DL50 est très variable, et c'est par la voie orale que les
composés sont les plus toxiques. La toxicité est également élevée par
la voie percutanée et par la voie respiratoire.
Certains anticoagulants présentent une toxicité aiguë du même
ordre pour les mammifères non visés que pour les rongeurs à détruire,
toutefois leur spectre toxique peut varier d'une espèce à l'autre.
Après administration répétée par voie orale à des rats, les
principaux effets observés sont ceux qui résultent de l'activité
anticoagulante.
On ne possède que peu de données sur les expositions répétées aux
anticoagulants chez les espèces n'appartenant pas à l'ordre des
rongeurs.
Une étude relative aux effets de la warfarine sur le rat a fait
ressortir une action sur le développement de ces animaux. En dehors
de cela, rien n'indique que les anticoagulants aient une action
tératogène sur les animaux d'expérience.
Rien n'indique non plus que les rodenticides anticoagulants
soient mutagènes, toutefois les données relatives aux différents
composés sont insuffisantes pour qu'on puisse en conclure à l'absence
de mutagénicité. La souche, le sexe et le régime alimentaire sont des
facteurs importants qui influent sur la toxicité des anticoagulants
chez les rongeurs. On a fait état d'épisodes d'intoxication chez des
animaux domestiques qui avaient consommé des appâts additionnés
d'anticoagulants. Lorsqu'il y a issue fatale ou du moins un tableau
clinique grave, c'est généralement qu'il y a eu consommation
d'anticoagulants de la deuxième génération. La principale différence
entre la warfarine et les autres anticoagulants (qu'il s'agisse des
indanediones ou des hydroxycoumarines de la deuxième génération),
c'est que ceux-ci persistent plus longtemps dans l'organisme et ont
par conséquent un effet plus durable que la warfarine. Dans ces
conditions, devant une intoxication, il faut poursuivre plus longtemps
l'administration de l'antidote, c'est-à-dire de la vitamine K1.
7. Effets sur l'homme
De nombreuses intoxications (qu'elles soient intentionnelles on
non) ont été signalées. Il y a eu également quelques cas
d'intoxication imputables à une exposition professionnelle. En cas
d'intoxication aiguë par des rodenticides anticoagulants, les
symptômes vont de l'accroissement de la tendance au saignement en cas
d'intoxication minime ou modérée, à une hémorragie massive dans les
cas plus graves. Les signes cliniques se manifestent un à plusieurs
jours après l'absorption.
Chez l'homme, on attribue certaines malformations congénitales à
des traitements par la warfarine pendant la période de grossesse.
Aucune malformation de ce type n'a été observée par suite de
l'utilisation d'anticoagulants comme rodenticides.
La concentration de la prothrombine plasmatique donne une
indication de la gravité de l'intoxication. Elle est plus sensible
que des épreuves globales telles que le temps de Quick. En cas
d'exposition professionnelle répétée, le dosage direct des traces de
carboxyprothrombine circulante ou du 2,3-époxyde de la vitamine K peut
permettre un bilan plus sensible.
Le traitement d'une intoxication par des anticoagulants doit être
adapté à la gravité de l'intoxication. Il est basé sur l'action
pharmacologique spécifique de la vitamine K1 que l'on administre par
voie parentérale avec, dans les cas graves, administration simultanée
de constituants sanguins. La mesure du temps de Quick permet
d'apprécier l'efficacité du traitement et de déterminer sa durée.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On peut répartir en deux catégories les effets possibles de
rodenticides anticoagulants sur les organismes non visés: les effets
directs (par consommation d'appâts) et les effets indirects (par
consommation de rongeurs empoisonnés).
Sous forme de produit technique, les anticoagulants sont
extrêmement toxiques pour les poissons. Incorporés à des appâts, il
est peu probable qu'ils présentent un danger, du fait de leur faible
solubilité dans l'eau. C'est pourquoi, à moins d'une erreur de
manipulation, ils ne devraient pas parvenir jusqu'aux poissons.
Les différentes espèces d'oiseaux sont d'une sensibilité variable
aux rodenticides anticoagulants. Il est difficile d'apprécier les
risques que représente, pour les oiseaux, la consommation directe
d'appâts car la plupart des travaux publiés sont des études
toxicologiques effectuées au laboratoire. Le caractère attractif des
appâts constitués de grains entiers pour les petits oiseaux en accroît
le danger dans la nature.
Des études de toxicité indirecte effectuées en laboratoire sur
des animaux sauvages ont montré que des prédateurs captifs pouvaient
s'intoxiquer si on leur donnait de la nourriture empoisonnée sans
autre choix ou des proies empoisonnées. On a également constaté la
mort de prédateurs dans le milieu naturel.
9. Evaluation et conclusion
Les rodenticides anticoagulants bloquent le mécanisme normal de
l'hémostase, d'où une tendance accrue au saignement qui peut déboucher
sur une forte hémorragie.
Il est peu probable que la population générale puisse être
exposée involontairement à des rodenticides anticoagulants.
Il peut y avoir une exposition non négligeable par suite de
contacts lors de l'activité professionnelle. Ces contacts peuvent se
produire au cours des opérations de production et de formulation aussi
bien que lors de la préparation et de la pose des appâts.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives ainsi qu'à travers la peau et les voies
respiratoires. C'est principalement dans le foie qu'ils sont retenus
et s'accumulent. La concentration de prothrombine plasmatique est un
bon indicateur de la gravité d'une intoxication aiguë et permet
d'avoir une idée de l'efficacité et de la durée nécessaire du
traitement.
L'antidote spécifique est la vitamine K1.
La principale différence entre les rodenticides anticoagulants de
première et de deuxième génération tient au fait que ces derniers
séjournent plus longtemps dans l'organisme et ont donc tendance à
prolonger l'effet hémorragique.
La plupart des anticoagulants sont stables dans les conditions
normales d'utilisation. Comme ils sont peu solubles dans l'eau et que
les appâts n'en contiennent qu'une faible quantité, il est peu
probable qu'ils puissent contaminer les étendues d'eau. Par ailleurs,
ils se fixent rapidement aux particules du sol, ne s'en désorbent que
très lentement et ne sont pas lessivés.
Aucun organisme non visé ne court de risque d'intoxication
directe par consommation d'appâts, ni de risque d'intoxication
indirecte par consommation de rongeurs contaminés.
RESUMEN
1. Generalidades
Los anticoagulantes descritos en esta monografía son los
utilizados principalmente en agricultura y en la lucha contra los
roedores urbanos. La warfarina, el primer rodenticida anticoagulante
de uso generalizado, se introdujo como agente eficaz para el
tratamiento de la tromboembolia en el ser humano.
De acuerdo con su estructura química, los rodenticidas
anticoagulantes pueden agruparse en dos categorías, las
hidroxicumarinas y las indandonas, aunque su mecanismo de acción es
similar.
2. Propiedades y métodos analíticos
Los rodenticidas anticoagulantes se presentan en forma cristalina
sólida o en polvo, y son ligeramente solubles en agua. La mayoría de
ellos son estables en condiciones de almacenamiento normales.
La mayoría de los procedimientos para la determinación de los
rodenticidas anticoagulantes se basan en cromatografía líquida de alta
resolución.
3. Fuentes de exposición humana y ambiental
Las hidroxicumarinas de primera generación se introdujeron como
rodenticidas a finales de los años cuarenta. La aparición de
resistencia a la warfarina y a otros anticoagulantes de primera
generación dio lugar a la elaboración de anticoagulantes más potentes,
de segunda generación. Las concentraciones de componentes activos en
los cebos varían en función de la eficacia de los rodenticidas.
4. Distribución, niveles y exposición ambientales
Los rodenticidas anticoagulantes se utilizan principalmente como
formulaciones para cebo. Dada su baja volatilidad, las
concentraciones en el aire son insignificantes. Como son muy poco
solubles en agua, es improbable que su uso sea fuente de contaminación
del agua.
Como los rodenticidas anticoagulantes no están pensados para su
aplicación directa a cosechas en pie, no son de prever residuos en
alimentos vegetales.
Los vertebrados no destinatarios están expuestos a los
rodenticidas principalmente por medio del consumo del cebo y de forma
secundaria por el consumo de roedores envenenados. Los cebos en
bolitas y de grano entero son muy atractivos para las aves.
La warfarina se utiliza como agente terapéutico para la
tromboembolia.
Hay un potencial de exposición ocupacional a los rodenticidas
anticoagulantes durante la fabricación, formulación y aplicación del
cebo, pero no se dispone de datos sobre los niveles de exposición.
5. Modo de acción y metabolismo
Los rodenticidas anticoagulantes son antagonistas de la vitamina
K. Su lugar principal de acción es el hígado, donde varios de los
precursores de la coagulación de la sangre sufren un procesamiento
post-traslación dependiente de la vitamina K antes de convertirse en
los zimógenos procoagulantes respectivos. Parece que el mecanismo de
acción es la inhibición de la reductasa epoxídica K1.
Los rodenticidas anticoagulantes se absorben fácilmente por el
tracto intestinal, y también pueden absorberse por la piel y el
sistema respiratorio. Tras la administración oral, la principal vía
de eliminación en diversas especies son las heces.
La degradación metabólica de la warfarina y las indandonas en
ratas es principalmente la hidroxilación. Sin embargo, los
anticoagulantes de segunda generación se eliminan principalmente como
compuestos inalterados. El bajo nivel de excreción urinaria impide
aislar los metabolitos a partir de la orina.
El hígado es el órgano principal para la acumulación y
almacenamiento de anticoagulantes rodenticidas. La acumulación
también tiene lugar en la grasa.
6. Efectos en los mamíferos y en los sistemas de prueba in vitro
Los signos de envenenamiento en ratas y ratones son los asociados
a una mayor tendencia a la hemorragia.
Hay una gran variación en la DL50 de los rodenticidas
anticoagulantes, siendo máxima la toxicidad por vía oral. También es
alta la toxicidad cutánea y por inhalación.
Los márgenes de toxicidad aguda de algunos anticoagulantes son
similares en el caso de los mamíferos no destinatarios y de los
roedores de destino, pero los espectros de toxicidad para los
anticoagulantes pueden variar entre las especies.
Tras una administración oral repetida en ratas, los principales
efectos observados son los asociados a la acción anticoagulante.
Se dispone de pocos datos sobre la exposición repetida de
especies distintas de los roedores.
Un estudio sobre la warfarina en ratas ha indicado efectos sobre
el desarrollo. Por lo demás, no hay pruebas convincentes de que los
anticoagulantes sean teratogénicos en animales de experimentación.
No hay prueba que sugiera que los rodenticidas anticoagulantes
son mutagénicos, pero se dispone de datos insuficientes sobre los
compuestos individuales para demostrar la inexistencia de
mutagenicidad. La sobrecarga, el sexo y la alimentación son
importantes factores modificadores de la toxicidad de los
anticoagulantes en los roedores.
Se han dado casos de envenenamiento de animales domésticos tras
la ingestión de cebos anticoagulantes. Las muertes y los síndromes
clínicos graves se deben por lo general a anticoagulantes de segunda
generación. La diferencia principal entre la warfarina y los demás
anticoagulantes (tanto las indandonas como las hidroxicumarinas de
segunda generación) es que éstos tienen mayor tiempo de retención en
el organismo y por consiguiente un efecto más prolongado que la
warfarina. Por ello, en los casos de envenenamiento, el tratamiento
antídoto con vitamina K1 debe proseguir durante un periodo más
largo.
7. Efectos en el ser humano
Se han notificado muchos casos de envenenamiento (tanto
intencionados como no intencionados). También se han producido unos
pocos casos de intoxicación por exposición ocupacional a los
anticoagulantes. Los síntomas de intoxicación aguda por rodenticidas
anticoagulantes van desde una mayor tendencia a la hemorragia en el
envenenamiento leve o moderado a una hemorragia masiva en casos más
graves. Los signos de envenenamiento aparecen con un retraso de uno a
varios días después de la absorción.
La warfarina va asociada en el ser humano a la producción de
malformaciones del desarrollo cuando se toma como agente terapéutico
durante el embarazo. No se han notificado casos de defectos de
desarrollo tras el uso de anticoagulantes como rodenticidas.
La concentración de protrombina plasmática orienta sobre la
gravedad de la intoxicación. Es una indicación más sensible que
pruebas generales como el tiempo de protrombina. En la exposición
ocupacional repetida, la medición directa de cantidades ínfimas de
descarboxiprotrombina en circulación o de 2,3-epóxido de vitamina K en
circulación pueden constituir una evaluación más sensible.
El tratamiento del envenenamiento con anticoagulantes se gradúa
de acuerdo con la gravedad de la intoxicación. El tratamiento
farmacológico específico consiste en la administración parenteral de
vitamina K1 con administración simultánea, en los casos graves, de
componentes sanguíneos. La medición del tiempo de protrombina ayuda a
determinar la eficacia y la duración de tratamiento necesaria.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los posibles efectos de los rodenticidas en organismos no
destinatarios pueden dividirse en dos categorías: primarios
(envenenamiento directo por consumo de cebo) y secundario (por consumo
de roedores envenenados).
En la forma del producto técnico, los anticoagulantes son muy
tóxicos para los peces. Como formulaciones para cebo es improbable
que planteen riesgos en razón de su baja solubilidad en agua. Por
esta razón, a menos que se utilicen de forma indebida, no están al
alcance de los peces.
La susceptibilidad de las aves a los rodenticidas anticoagulantes
es variable. Es difícil evaluar los riesgos para las aves que entraña
el consumo directo porque la mayoría de los estudios publicados
consisten en ensayos de toxicidad en condiciones de laboratorio. El
atractivo del cebo de grano integral para las aves pequeñas aumenta el
riesgo en las condiciones de campo.
Los estudios en laboratorio de la toxicidad secundaria con la
fauna silvestre han mostrado que los predadores cautivos pueden
intoxicarse mediante alimentación sin otra elección con presas que se
han envenenado con anticoagulante o a las que se ha administrado este
producto. Se tiene noticias de algunas muertes de predadores en su
medio natural.
9. Evaluación y conclusión
Los rodenticidas anticoagulantes perturban los mecanismos
normales de coagulación de la sangre, determinando una mayor tendencia
a la hemorragia y, por último, una hemorragia abundante.
La exposición no intencionada de la población general a los
rodenticidas anticoagulantes es improbable.
El contacto ocupacional es una fuente potencial de exposición
significativa. Puede tener lugar durante la elaboración y
formulación, así como durante la preparación y aplicación del cebo.
Los compuestos de rodenticida anticoagulante se absorben
fácilmente por el tracto intestinal, y por la piel y el sistema
respiratorio. El hígado es el órgano principal de acumulación y
almacenamiento. La concentración de protrombina plasmática es una
buena orientación de la gravedad de la intoxicación aguda y de la
eficacia y la duración necesaria de la terapia.
El antídoto específico es la vitamina K1.
La principal diferencia entre los rodenticidas anticoagulantes de
la primera generación y los de la segunda es que éstos últimos tienen
una mayor retención en el organismo y por ello suelen dar lugar a un
periodo de hemorragia más prolongado.
La mayoría de los anticoagulantes son estables en condiciones de
uso normal. Su baja solubilidad en agua y baja concentración en los
cebos hace improbable que sean una fuente de contaminación del agua.
Parece que se asocian rápidamente a las partículas del suelo, con
desorción muy lenta y nula propiedad de lixiviación.
Los organismos no destinatarios pueden correr al riesgo de
consumir directamente cebos (riesgo primario) y de ingerir roedores
envenenados (riesgo secundario).
Vermeer & Soute (1992) compared the inhibition of each of the
three enzymes from the vitamin K cycle by four anticoagulants
(warfarin, flocoumafen, difenacoum and brodifacoum). The studies were
performed using in vitro enzyme systems prepared from rat, cow and
human liver. It was shown that in all three species the inhibitor
concentration required for 50% inhibition (I50) was comparable for
the KO reductase and K reductase activity, but that the I50 for
gamma-glutamylcarboxylase was 2-3 orders of magnitude higher. It was
concluded that for all four anticoagulants the reductions of KO and K
are the target reactions for inhibition. Moreover, it was found that
there is no species specificity of the inhibitors, which means that
they are equally active in cell-free systems derived from rat, cow and
human liver. Any species-dependent differences which might be found
in vivo will presumably be brought about by a different
pharmacokinetic or pharmacodynamic behaviour in these species.
6.2 Metabolism
6.2.1 Absorption, distribution and elimination
Anticoagulant rodenticides are easily absorbed through the
gastrointestinal tract, skin and respiratory system.
After a single oral dose of 14C-flocoumafen (0.14 mg/kg body
weight) to rats, the absorption into blood was rapid, reaching maximum
concentrations (0.03-0.05 µg/ml) in plasma within 4 h (Huckle et al.,
1989).
The major route of elimination in rats and sheep after oral
administration of anticoagulants is through the faeces. The
intestinal levels of brodifacoum in rats began increasing 24 to 72 h
after an oral dose of 0.2 mg/kg body weight (Bachmann & Sullivan,
1983). Faecal elimination of radiolabelled flocoumafen following an
oral dose of 0.14 mg/kg body weight accounted for 23-26% of the dose
over the 7-day period; approximately half of this was recovered within
the first 24 h. Less than 0.5% of the dose appeared in the urine
within 7 days (Huckle et al., 1989).
After single oral administration of brodifacoum (0.2 and 2 mg/kg
body weight) to sheep, about 20% and 30%, respectively, was excreted
in the faeces within 8 days (Laas et al., 1985).
A larger proportion of a percutaneous dose of 14C-flocoumafen
(0.17 mg/kg body weight) dissolved in acetone was found in the urine
of rats (10%) than in the case of an equivalent oral dose (less than
0.5%) over a 7-day period. Faecal elimination accounted for 31% of
the percutaneous dose (Huckle & Warburton, 1986b).
After oral 14C-flocoumafen doses of 0.02 mg/kg body weight or
0.1 mg/kg body weight were given to rats, once weekly for up to 14
weeks, approximately one-third of each weekly low dose was eliminated
through the faeces within 3 days, mostly within the first 24 h. At
the higher dose the elimination ranged from 18% after the first dose
to 59% after the tenth dose (Huckle et al., 1988).
Following repeated oral administration of 14C-flocoumafen to
rats at 0.02 mg/kg body weight per week for 14 weeks or 0.1 mg/kg body
weight per week for 10 weeks, appreciable accumulation was seen in the
liver. At both dose levels tissue concentrations were highest in the
liver, followed by the kidney > skin > muscle > fat > blood. The
hepatic residue in the low-dose group ranged from 0.1 mg/kg tissue
after one week to 2.1 mg/kg by week 14 (Huckle & Warburton, 1986a).
Brodifacoum could not be detected in the omental fat of sheep 8
days after the oral administration of 0.2 and 2 mg/kg body weight
(Laas et al., 1985).
6.2.2 Metabolic transformation
Warfarin is readily hydroxylated in vitro and in vivo by rat
liver microsomal enzymes to form 6-, 8- and, especially
7-hydroxy-warfarin (Ullrich & Staundinger, 1968; Ikeda et al.,
1986a,b). These inactive metabolites are to some extent conjugated
with glucuronic acid, undergo enterohepatic recirculation, and are
excreted in the urine and faeces (Ellenhorn & Barceloux, 1988).
The metabolic pattern of indandiones in rats also mainly involves
hydroxylation (Yu et al., 1982).
The second-generation anticoagulants have mainly been found as
unchanged compounds (Bachmann & Sullivan, 1983; Huckle et al., 1988).
The low urinary elimination following oral dosing has precluded
accurate isolation of metabolites in urine (Warburton & Hutson, 1985;
Waburton & Huckle, 1986; Huckle & Warburton, 1986a).
Following administration of flocoumafen, liver residues in rats
consisted mainly of unchanged flocoumafen, although in a repeat dose
study a polar metabolite was detected. Eight urinary metabolites were
detected after percutaneous exposure to 14C-flocoumafen (Huckle &
Warburton, 1986b).
Studies in male Japanese quail have shown more rapid metabolism
and elimination than in the rat following an oral dose of
14C-flocoumafen. Up to 12 radioactive components were detected in
the excreta (Huckle & Warburton, 1986c).
Bromadiolone, brodifacoum and coumatetralyl were also found in
rats as unchanged parent compounds, whereas in the case of difenacoum
metabolites predominated (Parmar et al., 1987). The metabolism and
elimination of the difenacoum trans isomer was more rapid than for the
cis isomer (Bratt, 1987).
The suggestion that the anticoagulant effect in rats is mediated
by the unchanged compound itself rather than by its metabolites has
been confirmed by the effects of phenobarbital and SKF525A
pretreatments on the general pattern of responses to warfarin and
brodifacoum (Bachmann & Sullivan, 1983).
6.2.3 Retention and turnover
Metabolic studies of anticoagulant rodenticides show that the
liver is the main organ of accumulation and storage. Liver
concentrations of brodifacoum after a single oral dose of 0.2 mg/kg
body weight to rats remained high and relatively constant for 96 h,
with a maximum of 5.0 mg/kg after 50 h (Bachmann & Sullivan, 1983).
A high degree of body retention was found 7 days after a single
oral dose of 0.14 mg/kg body weight 14C-flocoumafen (74-76% of the
administered dose); approximately half the dose was found in the liver
(Huckle et al., 1989).
Brodifacoum was detected in the liver of sheep 128 days after
oral administration (0.2 and 2 mg/kg body weight) in concentrations of
0.64 and 1.07 mg/kg dry weight (equivalent to 0.22 and 0.36 mg/kg wet
weight), respectively. The peak levels occurred at 2 days in the
high-dose group and at 8 days in the low-dose group, being 6.50 and
1.87 mg/kg dry weight (2.21 and 0.64 mg/kg wet weight), respectively
(Laas et al., 1985). Woody et al. (1992) observed an elimination
half-life for brodifacoum in serum of 6 ± 4 days in four dogs.
The largest proportion of a percutaneous flocoumafen dose of
0.17 mg/kg body weight was located in the liver (25% of the dose at a
concentration of 0.8 mg/kg), although this was 10 times lower than
that following an oral dose (Huckle & Warburton, 1986b).
Parmar et al. (1987) found that elimination of radiolabelled
brodifacoum, bromadiolone and difenacoum from the liver was biphasic,
consisting of an rapid initial phase lasting from days 2 to 8 after
dosing and a slower terminal phase when the elimination half-lives
were 130, 170 and 120 days, respectively. Elimination of
coumatetralyl was more rapid, with a half-life of 55 days.
Similar results for difenacoum were found by Bratt (1987). After
a single oral 14C-difenacoum dose of 1.2 mg/kg body weight, the
highest concentration of radioactivity (41.5% of the dose) was found
in the rat liver 24 h after dosing. The elimination from the liver
was biphasic. The half-life of elimination of the radioactivity
during the first rapid phase was three days, and for the slower phase
was 118 days. A similar biphasic elimination was also apparent in the
kidney. In the pancreas the concentration declined more slowly than
in any of the other tissues (182 days). The parent compound was the
major component in the liver 24 h after dosing (42%).
Unchanged flocoumafen comprised the major proportion of the
hepatic radioactivity in rats and was eliminated with a half-life of
220 days (Huckle et al., 1989). Veenstra et al. (1991) found
retention of about 8% of an administered flocoumafen dose of 0.4 mg/kg
in the liver of beagle dogs 43 weeks after dosing.
Despite the more rapid metabolism of flocoumafen in Japanese
quail, a proportion of the administered dose is retained in the liver,
with an elimination half-life of 115 days after oral dosing (Huckle &
Warburton, 1986c).
Six Hereford heifers weighing approximately 230 kg each were
dosed with diphacinone (1 mg/kg body weight) by injecting it into the
rumen. The highest residue level of parent compound found in the
liver was 0.15 mg/kg at days 30 and 90 after treatment. No detectable
levels (> 0.01 mg/kg) could be found in any of the other tissues
analysed (kidney, plasma, brain, heart, muscle and fat). The residues
in the liver were almost constant from 30 to 90 days post-treatment
(Bullard et al., 1976).
The plasma half-life of brodifacoum determined in three patients
with severe bleeding disorders was found to be approximately 16 to 36
days (Weitzel et al., 1990).
The half-life for disappearance from the plasma of human
volunteers given a single oral or intravenous warfarin dose of
1.5 mg/kg body weight varied from 15 to 58 h, with a mean of 42 h
(O'Reilly et al., 1963).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute effects
7.1.1 Rodent species
Wide variations exist in the literature for LD50 values of
anticoagulant rodenticides. A particular reason for these variables
is the use of single or repeated (5-day) doses. LD50 values also
vary according to the animal strain and sex, but both have not always
been indicated in reported data (Ashton et al., 1987)
(Tables 5 and 6).
Table 5. Acute oral LD50 values for various rodenticides in albino
Norway rats
Rodenticide LD50 (mg/kg) Reference
Brodifacoum 0.26 Redfern et al. (1976)
Bromadiolone 1.125 Grand (1976)
Chlorophacinone 20.5 Thomson (1988)
Coumachlor 900.0 Thomson (1988)
Coumafuril 0.4 Wiswesser (1976)
Coumatetralyl 16.5 Thomson (1988)
Difenacoum 1.8 Bull (1976)
Difethialone 0.56 Lechevin & Grand (1987)
Diphacinone 3.0 Thomson (1988)
Flocoumafen 0.46 Sharples (1983a)
Pindone 50.0 Tomlin (1994)
Warfarin 58.0 Thomson (1988)
Reported oral LD50 values for warfarin in rats vary by a
considerable magnitude. Values of 11 mg/kg body weight (Lund, 1982),
58 mg/kg body weight (Thomson, 1988) and 58 mg/kg body weight and
323 mg/kg body weight for female and male, respectively (Hagan &
Radomski, 1953), have been reported.
The second-generation anticoagulants are more toxic than the
first-generation ones in the sense that a single feeding may be
lethal. Apparent discrepancies between the single oral LD50 values
for Norway rats, as shown in Table 5, and the multiple dose oral
LD50 values, shown in Table 6, can be explained by the cumulative
effects resulting from multiple dose (5 day) administration (Table 6)
and characteristics of this family of compounds.
Table 6. Five-day oral LD50 (mg/kg body weight per day) of various anticoagulants
for the Norway rat (Rattus norvegicus)a
Anticoagulant Strainb Male Female Both sexes Number of
animalsc
Pindone SD 1.21 (0.70-2.11) 1.60 (0.83-3.08) 1.34 (0.87-2.06) 40
wild 7.60 (2.61-22.2) 25.60 (5.34-123) 12.80 (1.73-84.8) 40
Warfarin SD 0.29 (0.14-0.57) 0.38 (0.22-0.66) 0.33 (0.22-0.50) 40
wild 0.39 (0.16-0.91) 0.60 (0.23-1.09) 0.44 (0.25-0.76) 40
Diphacinone SD 0.19 (0.11-0.33) 0.23 (0.12-0.43) 0.21 (0.14-0.31) 40
wild 0.39 (0.15-0.84) 0.60 (0.22-0.57) 0.44 (0.23-0.54) 40
Chlorophacinone SD 0.18 (0.18-0.18) 0.20 (0.15-0.27) 0.19 (0.16-0.22) 40
wild 0.13 (0.10-0.19) 0.23 (0.14-0.36) 0.16 (0.12-0.22) 40
Bromadiolone SD 0.13 (0.10-0.19) 0.10 (0.08-0.13) 0.12 (0.10-0.15) 40
wild 0.06 (0.03-0.12) 0.09 (0.09-0.09) 0.07 (0.05-0.10) 16
a Modified from: Ashton et al. (1987); figures in parentheses represent 95% confidence limits
b SD = Sprague-Dawley
c 50% males and 50% females in all tests
Reported oral LD50 values for mice show similar variations,
from less than 1 mg/kg body weight for second-generation rodenticides
to 374 mg/kg body weight for warfarin (Hagan & Radomski, 1953; Redfern
et al., 1976; Bull, 1976; Sharples, 1983a).
Signs of anticoagulant poisoning in rats and mice include
lethargy, hunched posture and vein clearing in the ears. Blood around
the eyes, mouth and anus, indicating internal haemorrhaging, appears
prior to death (Sharples, 1984).
The percutaneous toxicity of anticoagulants to rats varied for
different compounds from an LD50 of 0.54 mg/kg body weight for
flocoumafen (master mix in corn oil) to > 50 mg/kg body weight for
brodifacoum and difenacoum (Price, 1985b; Tomlin, 1994). The signs of
intoxication were identical to those observed after an oral dose.
Second-generation anticoagulants appeared to be highly toxic by
inhalation. An acute inhalation study in Wistar rats exposed (nose
only) for 4 h was conducted using the 0.5% manufacturing master mix
specifically prepared to give a mass median aerodynamic diameter of
less than 5 µm. The acute LC50 values were between 0.16 and
1.4 mg/litre. Signs of intoxication, characteristic of an
anticoagulant action, were observed within 3 days after exposure with
deaths occurring between 4 and 9 days (Blair, 1984).
7.1.2 Non-target species
The acute toxicity of various anticoagulant rodenticides to
non-target mammalian species is presented in Table 7.
From the data presented it appears that some anticoagulants show
a similar range of acute toxicity for both non-target mammals and
target rodents.
7.2 Short-term exposure
7.2.1 Rodent species
In a study by Hadler (1974), groups of Wistar rats (male and
female) were given brodifacoum by gavage (0.01, 0.02, 0.05, 0.1 and
0.2 mg/kg body weight) for 5 consecutive days. All rats receiving the
two lowest doses survived the 21-day experimental period, but all rats
given the two highest doses died within 11 days of cessation of
dosing. No abnormalities were detected in surviving animals
sacrificed at the end of the observation period. In animals which
died during the study, only massive internal haemorrhages, mainly in
the peritoneum, were observed. The no-observed-effect level of
brodifacoum for Wistar rats was 0.02 mg/kg body weight per day.
Table 7. Acute oral toxicity (LD50, mg/kg) of various anticoagulant rodenticides for non-target mammalian speciesa
Rodenticide Guinea-pig Rabbit Dog Cat Sheep Pig References
Brodifacoum 2.78 (F) 0.29 (M) 0.25-1 (F) approx. 25 (F) > 25 (M) 0.5-2 Hadler (1975a,b); Parkinson
3.56 11-33 (1975, 1976); Godfrey (1984);
Godfrey et al. (1985)
Bromadiolone 2.8 1.0 10 (F) MTD > 25 MTD 3 Grand (1976)
Chlorophacinone 50 Pelfrène (1991)
Difenacoum 50 (F) 2 (M) approx. 50 100 100 80-100 Bull (1976); Tomlin (1994)
Diphacinone 35 3-7.5 14.7 150 Kosmin & Barlow (1976)
Difethialone 0.75 5 MTD > 16 MTD 2-3 Lechevin & Grand (1987)
Flocoumafen > 10 (M) 0.7 (M/F) 0.075-0.25 > 10 (M/F) > 5 approx. 60 (M,F) Sharples (1983b); Price (1985a);
(M/F) Chesterman et al. (1984);
Roberts et al. (1985a, 1986)
Warfarin 800 20-50 6-40 1-5 Anonymous (1976)
a F = female; M = male; MTD = maximum tolerated dose
Wistar rats (male and female) were continuously given a diet
containing brodifacoum at 0.1 mg/kg body weight, with no choice of
other feed, for 12 weeks. Prothrombin time was increased and
mortality occurred in 9 out of 20 males and 5 out of 20 females.
Macroscopic examination of the major organs of surviving rats revealed
no abnormalities other than those expected of anticoagulant action
(Hadler, 1976).
Feeding Fisher-344 rats with diets containing flocoumafen at
concentrations of 0.2 or 0.4 mg/kg feed for 5 days produced no
toxicologically significant effects during the 15-day observation
period (Price, 1985c).
In a 28-day feeding study in rats, fed on a diet containing 0,
0.01, 0.05, 0.1 or 0.2 mg-flocoumafen/kg, there were no overt signs of
toxicity. Highest-dose females showed a slight but significant
increase in mean prothrombin time (PT) and activated partial
thromboplastin time (PTT), and a slight decrease in mean plasma total
protein. No toxicological or pathological changes were observed in
rats fed diets containing 0.01 or 0.05 mg/kg of diet (Price, 1985d).
No effect on prothrombin time was observed in a 12-week study
with Wistar rats administered oral flocoumafen doses (by gavage) of
0.0125, 0.0625 and 0.125 mg/kg body weight once a week (Forsey, 1985).
Groups of six male and six female Sprague-Dawley rats were fed
diphacinone in their diets at concentrations of 0 (control), 0.0313,
0.0625, 0.125, 0.25 and 0.5 mg/kg diet (equivalent to 0, 1.7, 3.3,
6.4, 13 and 27 µg/kg body weight per day) for 90 days (Elias & Johns,
1981). Additional satellite groups of one rat of each sex and each
dose group were killed for gross pathological examination at 30 and
60 days. Mortality was unaffected by the treatment. In the survivors
of the main groups and satellite groups, there were no gross
pathological changes, but in the two males that died prematurely (in
the 0.0625 and 0.25 mg/kg groups) there was subdural haemorrhage.
Prothrombin time was unaffected by treatment. Routine haematological
and clinical chemistry tests were performed on only two rats of each
sex from each group, and no effects were observed apart from a reduced
blood fibrinogen concentration in both sexes at the highest dose
level.
As no clear NOAEL was indicated by the results of the 90-day
study, a 21-day study was performed using two rats of each sex at
diphacinone levels of 0 (control), 0.125, 0.25, 0.5, 1, 2 and 4 mg/kg
diet. All the rats in the 2 and 4 mg/kg groups died within the first
14 days, but all others survived until the end of the study. The mean
dosages received by the survivors were equivalent to 0, 9, 17, 34 and
67 µg/kg body weight per day. At 21 days, there was no effect on
prothrombin time. Gross necropsy revealed haemorrhage in the thymus
of one rat in the 0.5 mg/kg group, but no effects were seen in any
other survivors. All the animals in the 2 and 4 mg/kg groups had
massive and extensive internal haemorrhage (Elias & Johns, 1981).
7.2.2 Non-target species
There are few data available on the repeated exposure of
non-rodent species to anticoagulant rodenticides.
Death followed five daily warfarin doses of 3 mg/kg body weight
in cats and 1 mg/kg body weight in pigs (Tomlin, 1994). The route of
administration was not specified.
The dermal administration of warfarin (188 mg/kg per day) to
female baboons caused profuse bleeding in 5 days followed by death
(Dreyfus et al., 1983).
When it became known that vitamin K was not an antagonist for
warfarin action in bone, it became possible to study the long-term
effects of this anticoagulant in bones without the risk of inducing
haemorrhages and bleeding. By feeding lambs with high doses of
warfarin (up to 150 mg/kg body weight per day) under the protection of
4 mg/kg body weight per day of vitamin K, Pastoureau et al. (1993)
showed that within 3 months the lambs had developed a marked
osteopenia that resulted from a decrease in resorption and a much more
pronounced decrease in bone formation. As a result the bone density in
the warfarin-treated animals was substantially lower than that in
control animals. This is in agreement with earlier studies in rats
(Price et al., 1982) where it was found that, under the protection of
vitamin K, warfarin induced excessive mineralization and growth plate
closure, due to which bone growth came to a halt.
The maximum tolerated 5-day oral dose of bromadiolone was
considered to be 25 mg in pigs (Large White strain) weighing 25 kg.
After 45 oral daily doses of 0.5 mg no change in the prothrombin time
was observed (Grand, 1976).
Woody et al. (1992) studied the effect of a cumulative dose of
1.1 mg brodifacoum/kg body weight administered orally to dogs over a
3-day period. Signs of coagulopathic effects appeared within 24 h
(greater PT and PTT) and increased over a 10-day period. Treatment
with Vitamin K1 at 10 days post-exposure reduced the effects.
Six horses were treated by gavage with brodifacoum containing
bait at a dosage of 0.125 mg/kg body weight (Boermans et al., 1991).
Four of the horses became anorexic and depressed, one requiring K1
therapy. Peak plasma concentration occurred 2-3 h after
administration. Pharmacokinetic evaluation indicated that brodifacoum
has a plasma half-life of 1.22 days. An increase in clotting time was
observed as early as 24 h after dosing, returning to the pre-treatment
level by day 12.
Male pigs (five per group) were fed difenacoum in the diet for 14
days at dose levels of 0.01, 0.05, 0.1, 0.5 and 1.0 mg/kg diet. With
the exception of the lowest-dose group, all groups showed a marked
increase of prothrombin time values. Extensive subcutaneous, inter-
and intra-muscular haemorrhage and oedema was observed in animals
dosed at levels of 0.5 mg/kg or more (Ross et al., 1979).
7.3 Long-term exposure
No data on long-term exposure are available.
Studies with second-generation anticoagulants are difficult to
carry out for more than a few weeks due to the rapid acute effects,
and NOEL values for second-generation rodenticides and longer exposure
periods have not been established. In any chronic study approaching
two years in length, the dose level would have to be less than the
analytical limit of detection.
7.4 Skin and eye irritation; sensitization
Brodifacoum is a slight skin irritant and a mild eye irritant in
the rabbit (Hadler, 1975c). No skin or eye irritation was observed in
New Zealand white rabbits treated with flocoumafen (Forsey, 1983a,b).
Neither of these rodenticides was a skin sensitizer when tested in the
guinea-pig maximization test (Parkinson, 1979; Price, 1986).
7.5 Reproductive toxicity and teratogenicity
Brodifacoum was given by oral gavage to female rats at daily dose
levels of 0.001, 0.01 or 0.02 mg/kg body weight during days 6-15 of
pregnancy. There was no evidence of adverse effects on the fetus at
termination. Higher daily doses (above 0.05 mg/kg) caused an
anticoagulant effect in the dams which resulted in a high incidence of
abortion (Hodge et al., 1980a).
Pregnant female rabbits were given oral gavage doses of 0.001,
0.002 or 0.005 mg brodifacoum/kg body weight per day from days 6-18 of
pregnancy. A the highest dose level a high proportion of maternal
deaths occurred as a result of haemorrhage. Although the survivors
showed signs of haemorrhage, there were no effects on the developing
fetus. No effects were observed at either of the other dose levels
used (Hodge et al., 1980b,c).
Bromadiolone was given orally to four groups of 25 female rats
from day 6 to 15 of pregnancy at doses of 0, 17.5, 35 and 70 µg/kg
body weight per day. Maternal toxicity occurred at the higher dose
levels. There was no evidence of embryotoxicity or teratogenic
effects at any dose level (Monnot et al., 1981). A similar absence of
effects was reported in a study on rabbits treated orally with daily
doses of either 2, 4 or 8 µg/kg body weight per day on days 6-18 of
pregnancy, although there was maternal toxicity at the highest dose
level (Virat, 1981).
Groups of 18 pregnant F-344 rats were given daily oral doses of
0, 0.01 or 0.04 mg flocoumafen/kg body weight from day 8 to 17 of
gestation. Eight animals in the 0.04 mg/kg body weight group either
died or were killed with signs of anticoagulant poisoning.
In contrast, Mirkova & Antov (1983) found warfarin to be
embryotoxic and teratogenic to Wistar rats when administered by gavage
in single doses or repeatedly throughout the periods of
pre-implantation (1-7 days of gestation) and organogenesis (8-16
days), and also throughout the whole gestation (1-21 days) at a wide
range of dose levels (0.04-8 mg/kg body weight). At these dose levels
and treatment regimens, warfarin induced substantially increased rates
of embryolethality, subcutaneous and internal haemorrhage and gross
structural malformations (pes varus, internal hydrocephalus and
anomalies of skeletal ossification).
7.6 Mutagenicity
Various in vitro and in vivo studies have been undertaken to
assess the genotoxic potential of brodifacoum. No mutagenic activity
was detected in the Salmonella reverse mutation assay in any of the
five tester strains employed (TA98, TA100., TA1535, TA1537 and TA1538)
either in the presence or absence of Arochlor 1254-induced rat liver
S9 fraction at brodifacoum concentrations ranging from 1.6 to
5000 µg/plate (Callander, 1984). Brodifacoum showed no activity in a
forward mutation assay using L5178 mouse lymphoma cells, either with
or without metabolic activation, at concentrations of 47.5, 63.3 and
84.4 mg/litre (Cross & Clay, 1984).
Brodifacoum caused no significant chromosomal aberrations in
cultured human lymphocytes (concentrations 1, 10, 100 and
1000 mg/litre), either with or without metabolic activation, and did
not induce unscheduled DNA synthesis in cultured HeLa cells at the
same range of concentrations (Mellano, 1984a,b). Difenacoum did not
induce unscheduled DNA synthesis in rat hepatocytes in vivo at
either dose level or time-point (Kennelly, 1990).
An in vivo micronucleus test, in which mice were given single
brodifacoum intraperitoneal doses of 0.187 or 0.30 mg/kg body weight,
showed no induction of micronuclei in bone marrow polychromatic
erythrocytes (Sheldon et al., 1984).
Bromadiolone was tested in the Salmonella reverse mutation assay
at concentrations ranging from 10 to 3330 µg per plate on strains
TA1535, TA1537 and TA1538. No evidence of mutagenic effect was found
either with or without Aroclor metabolic activation (Lawlor, 1992).
Bromadiolone did not induce forward mutations in Chinese hamster
ovary cells either with or without metabolic activation
(Cifone, 1993).
In a mouse micronucleus test at four dose levels from 50 to
400 mg/kg, bromadiolone did not induce micronuclei in bone marrow
polychromatic erythrocytes (Murli, 1993).
Flocoumafen did not induce reverse gene mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 nor in
Escherichia coli WP2uvrA pkm 101 either with or without metabolic
activation. Flocoumafen was tested at concentrations ranging from 31
to 2000 µg/plate, beyond which precipitation from suspension occurred
(Brooks et al., 1984).
Flocoumafen did not increase the frequency of mutation to
6-thioguanine resistance in Chinese hamster V79 cells either in the
presence or absence of an Arochlor-induced rat liver S9 fraction.
Doses ranging from 5 to 150 mg/litre were used, beyond which
cytotoxicity occurred (Clare & Wiggins, 1986).
Flocoumafen did not induce in vitro cell transformation in
C3H1OT´ mouse fibroblasts, either in the presence or absence of a rat
S9 metabolizing system, at concentrations ranging from 12.5 to
100 µg/litre (Meyer & Wiggins, 1986).
Flocoumafen did not induce mitotic gene conversion in liquid
suspension cultures of Saccharomyces cerevisiae JD1, either in the
presence or absence of a rat liver S9 fraction, at concentrations
ranging from 0.01 to 2 g/litre (Brooks et al., 1984).
When incubated at concentrations ranging from 5 to 25 mg/litre
for 24 h in monolayer cultures of rat liver RL4 cells, flocoumafen did
not induce in vitro chromosomal damage (Brooks et al., 1984).
Oral administration of flocoumafen to rats at doses of 0.25 mg/kg
or 1000 mg/kg body weight (a dose 4000 times the acute oral LD50 for
rats) did not produce chromosomal damage (Allen et al., 1986).
The cytogenic effect of chlorophacinone was investigated in vivo
in metaphase bone marrow cells taken at 48 and 96 h after oral dosing
of male CFLP mice with 20 mg/kg body weight. No induction of
chromosomal aberrations was observed (Nehéz et al., 1985).
In the same study, chlorophacinone was investigated in
spermatocytes taken from male CFLP mice at 1, 2, 3 and 4 weeks after a
single oral dose of 20 mg/kg body weight. The spermatocytes were
analysed in the diakinesis phase of meiosis. There was no increased
incidence of chromosomal aberrations (Nehéz et al., 1985).
Rabbits were treated orally with 20 mg chlorophacinone/kg body
weight and chromosomal analysis was performed in bone marrow and
spermatocytes taken at 48 h post-dosing. No increase in the incidence
of chromosomal aberrations was seen (Selypes et al., 1984).
7.7 Factors modifying toxicity
Phenobarbital pretreatment of rats followed by a single
administration of brodifacoum or warfarin decreased the anticoagulant
effects of both compounds, more markedly in the case of warfarin
(Bachmann & Sullivan, 1983).
The non-steroid anti-inflammatory drugs ibuprofen and
phenylbutazone potentiated the anticoagulant effects of brodifacoum
and bromadiolone in rats (Sridhara & Krishnamurthy, 1992).
Strain and sex are important factors modifying the toxicity of
anticoagulants in rodents (see Table 5, section 7.1.1). Winn et al.
(1987) observed greater sensitivity of male rats and mice to
difenacoum, compared with female rats and mice. The possible
explanation of different responses to difenacoum was the greater
turnover of plasma proteins in male rats or the marked
inter-individual variation of vitamin K1 level in male rat liver.
Large amounts (relative to farm animals' dietary requirements) of
vitamin K3 (menadione and its salts) are sometimes added to animal
feedstuffs. This gives rodent pests ready access to a substance which
acts as an antidote to anticoagulant rodenticides, and thus can reduce
the efficacy of these rodenticides.
Differences in metabolism to inhibitors of vitamin K synthesis
among strains of mice and rats has been attributed to a number of
different factors (Misenheimer et al., 1994). Among these are: 1)
reduced sensitivity of vitamin K epoxide hydrolase to inhibition; 2)
greater reversibility of inhibition of epoxide hydrolase; and 3)
faster clearance of the rodenticide. The mechanism of resistance
differs between strains.
A single flocoumafen dose of 0.5 mg/kg body weight resulted in
clear signs of anticoagulation in five out of eight beagle dogs
(Veenstra et al., 1991). Administration of a second dose 5 weeks
later resulted in clinical evidence of anticoagulation in two out of
the three remaining dogs. Vitamin K treatment (2 to 5 mg/kg
subcutaneously) reversed the effects in all cases.
7.8 Adverse effects in domestic and farm animals
7.8.1 Domestic animals
7.8.1.1 Poisoning incidents
The main cause for accidental poisoning of domestic animals is
direct consumption of anticoagulant baits. Secondary poisoning
through the consumption of rats and mice killed with anticoagulants
may occur in dogs and cats in urban situations, but is more likely in
farm situations (Marsh, 1985b). The majority of fatalities and severe
clinical syndromes are connected with the second-generation
anticoagulants (Dodds & Frantz, 1984). Du Vall et al. (1989) studied
10 cases of second-generation anticoagulant rodenticide poisoning in
dogs and cats. The presence of anticoagulants (brodifacoum,
bromadiolone or diphacinone) in serum or liver was confirmed by HPLC
or GC/MS. Several other cases of brodifacoum poisonings in dogs have
been reported and some of them were fatal (Mc Sporran & Phillips,
1983; Stowe et al., 1983; Dodds & Frantz, 1984).
Until 1983, only first-generation anticoagulants were available
in New Zealand and, between 1977 and 1983, warfarin, coumatetralyl and
diphacinone were involved in 45 reported incidents of accidental
poisoning of domestic and farm animals. Brodifacoum and flocoumafen
were introduced after 1983. From 1983 to 1994, 40 cases of poisoning
of domestic and farm animals resulted from the use of
second-generation compounds and 17 cases were due to the ingestion of
first-generation anticoagulants (Hoogenbroom, 1994).
Schulman et al. (1986) reported five cases of coagulopathy in
dogs caused by consumption of diphacinone-containing baits. Lethargy
and respiratory distress, associated with pulmonary interstitial
haemorrhage and pleural and/or pericardial effusion, were the most
consistent signs. The prothrombin time and the activated partial
thromboplastin time were moderately to markedly prolonged.
7.8.1.2 Diagnosis and treatment of poisoning
The major difference between warfarin and the other anticoagulant
rodenticides is that the latter have longer body retention and a
tendency to induce bleeding for a longer period of time. It is
therefore necessary to continue the treatment for weeks rather than
days.
Signs of poisoning occur after a latent period of 12 h to several
days and may include:
* bruising easily with occasional nose or gum bleeds
* blood in stools or urine
* excessive bleeding from minor cuts or abrasions
* laboured breathing
* pale mouth and cold gums
* anorexia and general weakness
A reliable indication of an anticoagulant effect is the
determination of prothrombin time.
Vitamin K1 (phytomenadione) is the antidote of choice. The
recommended dosage is 2-5 mg/kg body weight in dogs. Intravenous
injection is the quickest route but it is not recommended because of
reported anaphylaxis. Vitamin K1 is much safer if given
subcutaneously for the initial 2-3 treatments. A dose of 0.25 to
2.5 mg/kg body weight is recommended for use in small animals (Mount
et al., 1982). The recommended period of treatment with vitamin K1
is 3-6 weeks (Braithwaite, 1982; Mackintosh et al., 1988).
Mount & Feldman (1983) observed that therapy effective for
warfarin poisoning was ineffective against diphacinone toxicosis due
to inadequate vitamin K1 dosage and duration of therapy. Oral
therapy was ineffective.
In a study on dogs, Mount & Kas (1989) found that the serum
concentration of vitamin K epoxide was significantly higher in
diphacinone-treated dogs than in controls, the difference being
significant as early as 1-4 h after vitamin K application. The
authors suggested that this phenomenon could be used as diagnostic
information.
In cases of severe blood loss, fresh plasma should be infused
every 6 h to the extent of 5-10% of total blood volume, assuming this
to be 90 ml/kg in the dog and 70 ml/kg in the cat (Mount et al.,
1982).
Vitamin K1 (phytomenadione) is the antidote for treating dogs
exposed to anticoagulant rodenticides. The therapeutic dosages and
duration of treatment needed vary with the compound involved. Mount &
Feldman (1983) reported that dogs poisoned with diphacinone require
treatments for at least three weeks, while treatment for 4-6 days is
sufficient for treating warfarin poisonings. Initial treatments are
by subcutaneous injection, subsequent therapy being given orally.
For animals exposed to the second-generation rodenticides
brodifacoum, bromadiolone, difenacoum and flocoumafen, the treatment
regimen that has been recommended includes one or more injections of
vitamin K1 at 2-5 mg/kg body weight until prothrombin times return
to normal levels. Once this has been achieved, daily oral doses of
vitamin K1 are recommended for a period of 3-4 weeks. Oral doses of
2-5 mg/kg body weight are recommended initially, with some reduction
being possible over time if the animal is observed closely for signs
of recurrence of symptoms. If prothrombin times increase following
withdrawal of vitamin K1, the oral dosing should be resumed for 2-3
weeks. If blood loss was severe, transfusions (10-15 ml/kg body
weight) with fresh whole blood may be needed (Anonymous, 1988).
7.8.2 Farm animals
Feinsod et al. (1986) reported an outbreak of abortions and
haemorrhages in sheep and goats in Egypt caused by brodifacoum
intoxication. Clinical signs appeared within 3-7 days after exposure
to the rodenticide and included epistaxis, haematochezia, recumbency,
subcutaneous haemorrhage, flank ecchymosis, lameness, abdominal
bloating and abortion of various stages of gestation. The signs
resembled epidemic Rift valley fever, but there was no fever, icterus
or loss of appetite in affected animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Incidents of human exposures to rodenticides are reported to
poison control centres in countries where such facilities exist. In
1988, for example, the American Association of Poison Control Centers
(AAPCC) received accounts of 10 626 cases of human exposures to
rodenticides. These incidents represented 17% of reported exposures
involving pesticides and 0.8% of the total number of cases reported in
the AAPCC system. The rodenticide incidents included 4190 cases
involving "anticoagulants" (principally warfarin) and 5133 involving
"long-acting anticoagulants" (second-generation anticoagulants plus
the indandione compounds). More than 95% of the rodenticide cases
were classified as "accidental". Most of the remainder were
classified as "intentional" and included attempted suicides. Of the
10 540 rodenticide incidents for which the ages of victims were
reported, 9406 (89%) involved children under 6 years of age (Litovitz
et al., 1994).
Victims in nearly 32% of the rodenticide exposure incidents
reported to the AAPCC in 1988 were treated in health care facilities.
However, the medical outcome "none" was reported in more than 93% of
the 5708 incidents for which information regarding outcomes was
reported. The remaining 380 cases included 333 with "minor" medical
effects, 41 with "moderate" effects, 4 with "major" effects, and two
deaths (Litovitz et al., 1994).
In 1993, the Swedish Poison Information Centre received 338
enquiries concerning exposures to anticoagulant rodenticides. This
number represented 0.6% of all enquiries to the centre and 37% of the
enquiries concerning pesticides. Of the anticoagulant rodenticide
enquiries, 202 pertained to warfarin and 136 to "superwarfarin"
compounds (Persson, 1994).
Human exposure to second-generation and indandione anticoagulants
produces symptoms consistent with anticoagulation effects (e.g.,
haematomas, haematemesis, haematuria, easy bruisability). Treatment
of cases of exposure, particularly of substantial and repeated
exposure, may require vitamin K1 therapy and monitoring of
prothrombin times for periods of many months (Rauch et al., 1994).
Suicide and/or unintentional poisonings with anticoagulant
rodenticides have occurred in many countries. Thus, Ungvary (1994)
reported 70 cases, mostly involving children, that occurred in Hungary
between 1988 and 1993.
Warfarin is widely used as a therapeutic and preventive agent in
the treatment of thromboembolic disease. Patients have been
maintained for years on this treatment with control of the prothrombin
level, which should be kept between 10 and 30% of normal.
Diphacinone has also been used as a drug because of its
long-lasting action (the half-life in humans is 15-20 days). It
ceased to be listed in the American Medical Association Drug
Evaluations, (AMA, 1980) because of its structural relation to
phenindion, which had been reported to have adverse effects.
8.1.1 Acute poisoning
Typical features of poisoning result from increased bleeding
tendency and include:
* minor poisoning: coagulation disturbance detected only by
laboratory analyses;
* moderate poisoning: coagulation disturbance resulting in
haematomata, haematuria, blood in faeces or excessive bleeding
from minor cuts or abrasions, gum bleeding;
* severe poisoning: retroperitoneal haemorrhage, severe
gastrointestinal bleeding, cerebrovascular accidents, massive
haemorrhage (internal bleeding) resulting in shock.
If anaemia or liver disease is present then the above features
may be more severe and persistent and the poisoning may be more
difficult to control (Anonymous, 1988).
The onset of the signs of poisoning may not be evident until a
few days after ingestion.
8.1.2 Poisoning incidents
Cases of human poisoning with "superwarfarins" were reviewed by
Katona & Wason (1989).
Fourteen members of a family in the Republic of Korea were
poisoned by eating warfarin-containing maize meal. The first symptoms
appeared 7-10 days after the beginning of exposure and were followed
by massive bruises or haematomata on the buttocks in all cases (Lange
& Terveer, 1954).
Pribilla (1966) reported a total dose of about 1000 mg of
warfarin to be fatal after 13 days of consumption.
Out of a total of 741 infants, 177 died after the use of
warfarin-contaminated talc in Viet Nam. The concentrations of
warfarin in the powder varied from 1.7 to 6.5% (Martin-Bouyer et al.,
1983).
A 73-year-old woman suffered from recurrent episodes of
hypoprothrombinaemia. Clotting tests and further investigation showed
that this was due to a warfarin rodenticide intentionally mixed in the
woman's cough syrup by her daughter-in-law. As the patient had as
many as seven relapses, it was possible to compare different types of
therapy. Menadione had no effect (Nilsson, 1957).
Several suicidal attempts with chlorophacinone have been
reported. Murdoch (1983) reported a case of ingestion of 625 mg
chlorophacinone (250 ml of a 0.25% concentrate formulation) by a
37-year-old woman. The prolonged anticoagulant action of
chlorophacinone persisted for at least 45 days even though treatment
was given. It was found that menadiol, the synthetic analogue of
vitamin K1, was ineffective. The natural form, phytomenadione, was
effective only when given at high dosage (20 mg daily) 30 days after
the ingestion of chlorophacinone.
In a case reported by Dusein et al. (1984), the amount of
ingested chlorophacinone was unknown. After adequate therapy, the
prothrombin level became normal within 4 weeks.
Vogel et al. (1988) reported the case of an 18-year-old woman
hospitalized 3 days after ingesting approximately 100 mg
chlorophacinone. Under high-dose vitamin K1 therapy (160 mg) the
prothrombin time was normalized, but it increased again following
withdrawal of vitamin K1. After prolonged vitamin K1 administration,
the prothrombin time finally became normal after 7 weeks.
Brodifacoum poisoning has occurred in South Sumatra, Indonesia.
Some of the villagers used a 0.005% brodifacoum rice grain bait as a
food source even though they knew it was poisonous and unfit for human
consumption. They attempted to remove the rodenticide by repeated
washing, rinsing and cooking before eating the rice. Because of the
delay in the appearance of poisoning symptoms it appeared that they
had been successful, thus encouraging further attempts to purify the
rice baits. As a result, deaths occurred before appropriate remedial
treatment could be initiated (Anonymous, 1985).
Jones et al. (1984) reported the first case of human brodifacoum
poisoning in a 17-year-old boy who attempted suicide by ingesting
approximately 7.5 mg (0.12 mg/kg) of brodifacoum in Canada. He was
initially seen with gross haematuria, followed by epistaxis and gum
bleeding. The prothrombin time and the activated partial
thromboplastin time were notably prolonged. He was treated for 56
days with either parenteral or oral vitamin K1 and either fresh or
stored plasma until coagulation values remained normal and stable.
Lipton & Klass (1984) reported a similar case in a 31-year-old
mentally disturbed woman who ingested over a 2-day period
approximately thirty 50-g packages of Talon-G (approximately 75 mg of
brodifacoum). Prothrombin time and activated partial thromboplastin
time were considerably prolonged (respectively 6-fold and 4-fold above
normal values). After 4 days of therapy with high doses of vitamin
K1 (up to 125 mg/day), partial correction in the prothrombin time
occurred. Vitamin K1 therapy continued with interruptions for 8
months until normal prothrombin time levels were found.
Chong et al. (1986) reported a case of suicidal poisoning after
ingestion of 10 mg brodifacoum (as 0.05% Klerat). The coagulation test
became normal after large doses and prolonged use of vitamin K1 over
6 months.
A case of intentional ingestion of brodifacoum (200 g of Talon G,
0.005% brodifacoum) was reported by Hoffman et al. (1988). A profound
decrease in the levels of factors II, VII, IX and X, lasting 43 days
after ingestion, was observed. Treatment with subcutaneous vitamin
K1 in doses up to 100 mg per day was effective.
Weitzel et al. (1990) described three patients with severe
bleeding disorders due to deficiency of the vitamin K-dependent blood
clotting proteins after ingestion of an anticoagulant. Although the
patients denied any ingestion, brodifacoum was detected in their serum
at concentrations of 7.6 nmol/litre, 270.7 nmol/litre and
2759 nmol/litre, respectively. The anticoagulant effect was found to
persist long after brodifacoum was no longer detectable in the serum.
A half-life of approximately 16-36 days was determined for brodifacoum
in the plasma.
Kruse & Carlson (1992) reported the case of a 25-year-old man who
attempted suicide by consuming a brodifacoum rodenticide. He
developed a severe coagulopathy that was treated with vitamin K1 and
fresh frozen plasma and he was discharged from hospital with oral
phytomenadione. Fifteen weeks later the man presented again with a
history of further brodifacoum ingestion. He suddenly became comatose
and computer tomography revealed a subarachnoid haemorrhage that led
to brain death 24 h later.
Wallace et al. (1990) described the clinical course of a patient
poisoned with brodifacoum in a suicide attempt. He developed
microhaematuria and melaena. His clotting factors were depressed and
were poorly responsive to vitamin K treatment.
Barlow et al. (1982) reported a case of attempted suicide with
25 mg of difenacoum (500 g of rat bait) followed several months later
by 1800 g of rat bait. The patient was treated with vitamin K1
(phytomenadione) for 48 and 42 days, respectively, until the
pharmacological effect of difenacoum ceased.
Nighoghossian et al. (1990) reported an unusual coagulopathy
after accidental exposure to a diphenacoum rodenticide. A 59-year-old
man developed subacute tetraparesis following severe sudden neck pain,
which on clinical examination was shown to be due to a subdural
cervical haematoma. Prothrombin complex activity was low and
diphenacoum was present in the plasma. Specific medical management
led to a complete recovery.
Greeff et al. (1987) reported accidental bromadiolone poisoning
in two children, resulting in prolonged anticoagulation.
Descarboxyprothrombin levels were increased in both cases by 27% and
29.9%, respectively (normal, non-detectable level). The first child
rapidly recovered after treatment with high-dose intravenous factor
IX-prothrombin complex and vitamin K1. The clotting profile became
normal on the third day after admission. The second child gave a poor
response to 10 mg intravenous vitamin K1 and the dose was increased
to 20 mg.
8.1.3 Controlled human studies
Single oral doses of 60, 70, 80 or 120 mg warfarin decreased the
prothrombin concentrations in volunteers to zero by the third day.
After the administration of 50 mg vitamin K1, the prothrombin
concentrations returned by the sixth day to 60, 70, 55 and 63%,
respectively, of the normal value (Anonymous, 1965).
When a single oral dose of 20 mg chlorophacinone was given to
three volunteers, the lowest prothrombin times were 35, 34 and 38% of
the pretreatment value on days 2, 4 and 2, respectively. Eight days
after administration without any treatment the values were 80, 100 and
90%, respectively (Anonymous, 1965).
8.2 Monitoring of biological effects
8.2.1 Effects of short- and long-term exposure
Two cases of occupational exposure to brodifacoum and difenacoum
were reported by Park et al. (1986). The exposure was of a chronic
nature (2 and 4 years, respectively). Plasma analysis in the first
patient revealed the presence of both difenacoum and brodifacoum in
the range of 30-50 µg/litre. In both patients unexpectedly high
concentrations of vitamin K1 2,3-epoxide were found in the presence
of normal clotting factor activities and antigen levels suggesting the
presence of coumarin anticoagulants in the liver.
A case of poisoning in a 23-year-old man resulting from prolonged
skin contact during the process of preparing and distributing warfarin
baits has been reported (Fristedt & Sterner, 1965).
8.2.2 Epidemiological studies
During a production run preparing ready-to-use flocoumafen bait
(0.005% in baits) in a formulation plant, the effect of the
rodenticide on blood coagulation factors was monitored in 12 subjects,
using the classical prothrombin time test, a modified prothrombin time
technique and measurement of prothrombin (factor II) concentration in
blood. No adverse health effects were observed in any subject
involved in formulation operations. No changes were observed in any
of the three tests that could be ascribed to absorption of flocoumafen
into the body (Tuinman & Van Sittert, 1986).
8.3 Developmental effects
Developmental effects have been reported when anticoagulants,
particularly warfarin, have been administered as therapeutic agent
during pregnancy. Hall et al. (1980) reviewed 418 reported
pregnancies in which coumarin and indan-1,3-dione derivatives were
used, and found that one-sixth resulted in abnormal liveborn infants,
one-sixth in abortion or stillbirth, and two-thirds in apparently
normal infants.
According to Hall (1976), there are two types of defects
associated with anticoagulants, dependent upon the time of
administration during pregnancy. The first, a characteristic
embryopathy described by the terms "warfarin embryopathy" or "fetal
warfarin syndrome", occurs from early, first-trimester use. Fetal
wastage and other abnormalities, especially central nervous system
anomalies, result from treatment later during gestation (usually the
second or third trimesters). Clotting factors are not present in
first-trimester embryos and this may explain the differences in fetal
abnormalities.
The most consistent feature of warfarin embryopathy is nasal
hypoplasia. Choanal stenosis has been observed, and respiratory
difficulty is typical because of the narrowed nasal passages (Smith &
Cameron, 1979; Pauli & Hall, 1979).
The other common feature is bone abnormalities of the axial and
appendicular skeleton. Laryngeal and tracheal-thyroid calcification
has also been noted (Schardein, 1985).
Other non-skeletal abnormalities reported include
ophthalmological malformations of several types, including defects
leading to blindness, developmental delay, low birth weight (premature
birth), mental retardation, hypotonia and ear anomalies (Carson &
Reid, 1976; Schardein, 1985).
No cases of embryopathy from anticoagulants in their use as
rodenticides have been reported.
8.4 Other adverse effects
One of the more common side-effects of coumarin therapy is skin
necrosis (Brooks & Blais, 1991; Eby, 1993; Locht & Lindström, 1993).
Although this effect has been associated with a number of different
agents, it is commonly referred to as warfarin-induced skin necrosis
(WISN). The frequency of this effect has been estimated to be between
1 in 100 and 1 in 10 000, with the majority of cases occurring in
women. Symptoms of WISN typically appear 3-6 days after the
initiation of warfarin therapy, and the areas of skin involved are
most frequently areas with subcutaneous fat, particularly the breasts,
thighs, and buttocks. There is some evidence that deficiencies in
vitamin K-dependent anticoagulant proteins (protein C and protein S)
may underlie the susceptibilities of at least some individuals to WISN
(Anderson et al., 1992). The occurrence of WISN may be avoided in at
least some cases by the co-administration of heparin.
Warfarin therapy has been noted to result in increases in the
incidence of haematomas, including intraspinal epidural haematoma
(Murphy & Nye, 1992), liver haematoma (Erichsen et al., 1993), and
intramural haematoma of the small intestine (Avent et al., 1992).
Bone contains three vitamin-K-dependent proteins, i.e.
osteocalcin, matrix Gla-protein and protein S. It has been shown that
oral anticoagulants (phenprocoumon, acenocoumarol) reduce both the
plasma antigen level as well as the Gla content of osteocalcin (Van
Haarlem et al., 1988). Furthermore it was found that a poor vitamin K
status was associated with a high urinary calcium loss (Knapen et al.,
1993). These data are consistent with the observation that the bone
mass in patients on long-term anticoagulant treatment was
significantly lower than in a group of age- and sex-matched controls
(Fiore et al., 1990; Resch et al., 1991). Vitamin-K-dependent proteins
have also been identified in calcified atherosclerotic plaques, where
they were suggested to contribute to the prevention of vascular
calcification (Gijsbers et al., 1990). In this respect it is
remarkable that warfarin treatment has been shown to increase rather
than to reduce atherosclerosis in an animal model (Antov et al.,
1985). Vitamin-K-dependent proteins not related to blood coagulation
are still discovered regularly (Manfioletti et al., 1993), and it is
important to remain alert for unexpected side-effects of vitamin K
antagonists, notably after long-term exposure.
8.5 Methods for assessing absorption and effects of anticoagulant
rodenticides
The laboratory control of orally administered coumarin
derivatives has been carried out using the classical one-stage
prothrombin time test (Quick, 1935) or modified techniques such as
"Thrombotest" (Owren, 1959). However, these tests have been designed
for clinical monitoring of circulating clotting factors during
anticoagulant therapy. The monitoring of occupational exposure to
rodenticides requires the prothrombin time test to be of sufficient
sensitivity to measure changes in the normal range (Tuinman & Van
Sittert, 1985).
Repeated occupational exposure to low levels of anticoagulant
rodenticides could gradually deplete vitamin-K-dependent coagulation
factors in the blood. To detect unwanted exposure of humans in an
early state, a careful screening of those at risk is recommended. The
question is which screening method is the most suitable to monitor low
levels of rodenticide ingestion. Prothrombin time and related tests
are "overall" clotting tests, which were developed for monitoring
patients under deep anticoagulation. These tests are easy to perform
and do not require complicated equipment, but they are relatively
insensitive when used for monitoring milder anticoagulation states
(Tuinman & Van Sittert, 1985; Ross et al., 1992; Travis et al., 1993).
If possible, specific and more sensitive tests should be used. The
most sensitive test, applicable over a wide range of anticoagulation
states, is the direct detection of descarboxy-prothrombin using a
monoclonal antibody specifically recognizing the descarboxy form of
prothrombin (Widdershoven et al., 1987). Another marker for
monitoring poor vitamin K status at an early stage is
descarboxy-osteocalcin (Knapen et al., 1993), but the commercial test
kits presently available need to be substantially improved and
simplified before they can be recommended for this purpose in routine
laboratories.
Another method was suggested by Park et al. (1986), who
repeatedly injected 10 mg of vitamin K1 into factory workers who had
been exposed to brodifacoum. The authors observed that 2-4 h after
injection the circulating KO/K ratios were significantly elevated even
18 months after the prothrombin times had returned to normal values.
This suggests that very low liver concentrations of brodifacoum can be
detected from the altered KO/K ratio rather than from tests based on
blood coagulation parameters. Because this method requires vitamin K
administration shortly before blood sampling, it is only applicable in
cases of anticoagulant poisoning, and not for the routine control of
plant workers.
Methods for the direct detection of coumarin anticoagulants in
plasma and serum have been reported, all of which are based on the
extraction of plasma and pre-purification of the sample, followed by
HPLC analysis with fluorescence detection (Hunter, 1983; Murphy et
al., 1989; Felice & Murphy, 1989; Felice et al., 1991; O'Bryan &
Constable, 1991). However, such facilities will not be available in
most routine laboratories. Moreover, the blood sampling should be
performed within a reasonably short period after ingestion of the
coumarins, because these drugs are rapidly cleared by the liver. This
places severe restrictions on the applicability of these techniques,
particularly for the second-generation anticoagulants.
Plasma chlorophacinone determinations were performed in three
cases of intoxication. The risk of bleeding was minimal when the
plasma level was below 1 mg/litre (Burcuoa et al., 1989).
Brodifacoum was detected in a case of self-ingestion by a
25-year-old woman who denied any intake of anticoagulants but was in
need of vitamin K1 treatment for 8 months. Factor assays revealed a
marked reduction in the levels of the vitamin K-dependent factors II,
VII, IX and X and normal levels of factor V and VIII:C (Exner et al.,
1992).
The diagnosis in a case of difenacoum intoxication was confirmed
by analysis of difenacoum in serum (0.6 mg/litre) (Butcher et al.,
1992).
Bromadiolone was analysed in the serum of a 27-year-old female
with a history of bleeding. She denied any contact with a rodenticide
but the bromadiolone concentration in serum was 40 mg/litre (Chow et
al., 1992).
Hollinger & Pastoor (1993) reported results of comparison of
plasma brodifacoum concentrations and prothrombin levels over time in
a case of brodifacoum poisoning. Brodifacoum was eliminated according
to a two-compartment mode, with an initial half-life of 18 h and a
terminal half-life of 24.2 days. On admission, the brodifacoum level
was 731 µg/litre. Treatment with large doses of phytonadione lasted
for 4 months.
8.6 Treatment of anticoagulant rodenticide poisoning
All suspected poisoned patients should receive medical attention
immediately. Rapid determination of prothrombin time and search for
evidence of bleeding is essential and may have to be maintained for
several weeks.
8.6.1 Minimizing the absorption
Gastric lavage or induction of emesis are indicated in all cases
of superwarfarin rodenticide ingestion if it was recent and the amount
is possibly lethal or uncertain. Repeated administration of activated
charcoal is useful. Cathartics could also be administered (Sullivan
et al., 1989; Smolinske et al., 1989; Donovan et al., 1990).
8.6.2 Specific pharmacological treatment
8.6.2.1 Vitamin K1 (phytomenadione)
Vitamin K1 is the specific antidote of choice. Depending on
whether the poisoning is due to warfarin or superwarfarin, the dosage
may differ as well as the duration of treatment. Dosage is dependent
on coagulation parameters, mainly prothrombin time.
If the patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection.
Prothrombin time should be checked at 3-hourly intervals in severe
cases and after 8-10 h in less severe cases. If no improvement
occurs, vitamin K1 injection should be repeated. Doses of up to
125-200 mg/day have been given without adverse effects (Lipton &
Klass, 1984; Sheen et al., 1994).
In moderate to minor cases of poisoning, vitamin K1 may be
given in lower doses.
After initial parenteral vitamin K1 administration, oral
treatment can be continued for a prolonged period of time. Oral
treatment can also be sufficient in minor cases.
The major difference between warfarin and second-generation
rodenticides is that the latter can cause increased bleeding for a
longer period of time than warfarin, as they have a much longer
half-life in the body. Therefore vitamin K1 should be given for
months rather than weeks. It is also prudent to monitor prothrombin
time for some time after cessation of this treatment to ensure that
there is no regression.
In warfarin-resistant individuals, 10 times the normal dose of
warfarin is required to achieve a reduction in the plasma prothrombin
level. However, these individuals also respond more strongly to the
effect of vitamin K (O'Reilly et al., 1963; O'Reilly et al., 1964).
8.6.2.2 Blood components
The following products could be considered, subject to
availability:
* whole blood
* fresh frozen plasma and fresh blood should be used in cases of
acute severe bleeding in order to rapidly restore the blood
clotting factors
* factor concentrate may be considered in severe cases, especially
if the amount of plasma would be too great (volume overload)
8.6.2.3 Phenobarbital
In the cases reported by Lipton & Klass (1984) and Jones et al.
(1984), phenobarbital therapy was included. It was presumed that
anticoagulants had been metabolized by the mixed function oxidase
system based on animal experiments (Bachmann & Sullivan, 1983) and
that, by inducing this enzyme system, phenobarbital might increase
their metabolism.
Robinson & Mac Donald (1966) found that the pharmacological
activity of warfarin in humans decreased when it was combined with
phenobarbital.
8.6.3 Response to therapy
Patient should be kept in hospital until the prothrombin time has
remained normal for 3 days. It is suggested that oral treatment with
10 mg vitamin K1 twice daily may be necessary for up to 60 days with
close monitoring of prothrombin time.
According to Hoffman et al. (1988), factor analysis allows for a
detailed evaluation of the course of toxicity, and the response to
therapy. Monitoring the prothrombin time alone could offer a false
sense of confidence and delay effective treatment.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
No data are available for the effects on microorganisms in water
and in soil.
9.1.2 Aquatic organisms
The acute toxicity of technical flocoumafen to planktonic algae
(Selenastrum capricornutum) was determined in a 4-day growth test.
The 96-h EC50, based on cell counts on day 4, was calculated to be
1.1 mg/litre (Pearson & Wallace, 1984).
Pearson (1984) reported static acute toxicity tests for
flocoumafen on Daphnia magna. The 48-h EC50 values, based on
immobilization, were 1.4 mg/litre for technical grade flocoumafen and
280 mg/litre for 0.5% flocoumafen. Pearson & Wallace (1984) found a
48-h EC50 for Daphnia magna of 0.66 mg/litre for technical grade
flocoumafen.
Anticoagulants are highly toxic to fish when tested as technical
formulations (Hill et al., 1976; Pearson & Wallace, 1984) (Table 8).
9.1.3 Terrestrial organisms
9.1.3.1 Acute toxicity
Bird species vary in their susceptibility to anticoagulant
rodenticides. The acute oral LD50 of brodifacoum for the mallard
duck (Anas platyrhynchos) is 2.0 mg/kg (Ross et al., 1978).
Symptoms of poisoning became apparent approximately 7 days after
dosing and included lethargy, weakness and lack of muscular
coordination. Prolonged bleeding occurred from any small wounds and
extensive bruising and subcutaneous haemorrhage were noted. Blood was
observed in the faeces. Deaths occurred principally during the period
of 7-14 days after administration. No deaths occurred more than 4
weeks after administration.
The acute toxicity of flocoumafen to the mallard duck appears to
be dependent on the age of birds. The LD50 values for 12- and
18-week-old birds are 24 mg/kg and 94 mg/kg, respectively (Roberts et
al., 1985b,c).
Table 8. Acute toxicity of anticoagulant rodenticides for aquatic organisms (96-h LC50)
Rodenticide Species Stat/flowa LC50 (mg/litre) Reference
Brodifacoum Rainbow trout flow 0.051 Hill et al. (1976)
Diphacinone (95%) Bluegill sunfish 7.6 Kosmin & Barlow (1976)
Channel catfish 2.09 Kosmin & Barlow (1976)
Rainbow trout 2.82 Kosmin & Barlow (1976)
Pink shrimp > 10 Kosmin & Barlow (1976)
Fiddler crab > 10 Kosmin & Barlow (1976)
Diphacinone (0.005%) Bluegill sunfish 288 Kosmin & Barlow (1976)
Channel catfish 285 Kosmin & Barlow (1976)
Rainbow trout 425 Kosmin & Barlow (1976)
Pink shrimp 33 Kosmin & Barlow (1976)
Fiddler crab 37 Kosmin & Barlow (1976)
Warfarin Bluegill sunfish 88 Brorson et al. (1994)
Flocoumafen (0.5%) Rainbow trout static 190 Pearson (1984)
Flocoumafen (technical grade) Rainbow trout static 0.95 Pearson (1984)
Rainbow trout static 0.32 Pearson & Wallace (1984)
Rainbow trout static 0.091 Pearson & Wallace (1984)
Common carp static 0.22 Pearson & Wallace (1984)
Bromadiolone Rainbow trout 1.4 Tomlin (1994)
a stat = static conditions (water unchanged for duration of test)
Sex differences were observed in the acute toxicity for bobwhite
quail (Colinus virginianus) of difenacoum. LD50 values for male
and female birds were 140 mg/kg body weight and 56 mg/kg body weight,
respectively (Ross et al., 1980c).
Birds seem to be relatively tolerant to diphacinone, the LD50
for mallard duck being 3158 mg/kg (Kosmin & Barlow, 1976).
The acute toxicity of brodifacoum for various species of birds is
presented in Table 9.
Table 9. The acute toxicity of brodifacoum to birds (Godfrey, 1985)
Species Acute oral LD50
(mg/kg)
Black-backed gull (Larus dominicans) < 0.75
Black-billed gull (L. bulleri) < 5.0
Canada goose (Branta canadensis canadensis) < 0.75
Pukeko (Porphyris porphyris melanotus) 0.95
Mallard duck (Anas platyrhynchus) 4.6
California quail (Lophortyx californica) 3.3
Blackbird (Turdus merula) > 3
House sparrow (Passer domesticus) > 6
Hedge sparrow (Prunella modularis occidentalis) > 3
Wax-eye (Zosterops lateralis) > 6
Harrier hawk (Circus approximans) 10.0
Ring-necked pheasant (Phasianus colchicus) 10.0
Paradise duck (Tadorna variegata) > 20
9.1.3.2 Primary toxicity
The subacute (5-day) dietary toxicity (LC50) of difenacoum for
the bobwhite quail was found to be between 0.25 and 7.00 mg/kg diet
(Ross et al., 1980a). The 5-day LC50 of difenacoum for the mallard
duck was calculated to be 18.9 mg/kg diet (Ross et al., 1980b).
Similarly to the acute toxicity studies, diphacinone was found to
be less toxic in 8-day dietary studies. The LC50 for the bobwhite
quail was 4485 mg/kg diet and for the mallard duck was > 10 000 mg/kg
diet (Kosmin & Barlow, 1976).
White Leghorn hens (4 per group) were fed warfarin,
coumatetralyl, bromadiolone, difenacoum or brodifacoum in baits as a
choice to non-poisonous chicken food for 15 days. No symptoms
appeared at a total warfarin intake of up to 171 mg/kg body weight.
Coumatetralyl and brodifacoum killed all hens after intakes of
79-137 mg/kg body weight of active ingredient and 7.1-15 mg/kg body
weight of active ingredient, respectively. Both bromadiolone and
difenacoum killed two of the hens, at 5.9 and 15.9 mg/kg body weight
of active ingredient for bromadiolone, and at 18.9 and 26.3 mg/kg
body weight active ingredient for difenacoum within 10-15 days (Lund,
1981).
Christopher et al. (1984) dosed male hybrid leghorn chickens with
anticoagulant rodenticides. No signs of poisoning were observed in
chickens fed 183.7 mg warfarin/kg (mean a.i. ingested) for 3 days. No
mortality occurred after the ingestion of a total dose of 36.9 mg
bromadiolone/kg over a 3-day period. Birds fed a diet containing
brodifacoum for 3 days ingested a mean of 28.9 mg/kg (active
ingredient). One bird died on day 4 while the other five birds
exposed had died by day 16.
Two-day no-choice tests in which chukar partridges ( Alectoris
graeca cypriotes Hartert) were fed 0.005% bromadiolone or difenacoum
resulted in no mortality. Ten-day no-choice feeding tests resulted in
6 out of 12 and 8 out of 12 dead partridges for bromadiolone and
difenacoum, respectively. The highest quantity of bait consumed by an
individual bird without any lethal effects was 411 g of bait with
bromadiolone (34.8 mg a.i./kg body weight) and 326.2 g of bait with
difenacoum (29.1 mg a.i./kg body weight) (Krambias & Hoppe, 1987).
9.1.3.3 Secondary toxicity
Barn owls (Tyto alba) were fed rats poisoned with diphacinone,
chlorophacinone, coumafuryl, difenacoum, bromadiolone or brodifacoum.
Five out of six owls died of haemorrhaging after feeding on rats
killed with brodifacoum after 8 to 11 days. Sublethal haemorrhaging,
but no mortality, occurred in owls fed rats killed with difenacoum.
One owl died following 10 days of treatment with bromadiolone-poisoned
rats, while five showed no symptoms. No abnormalities were observed
in two owls fed rats killed with diphacinone, coumafuryl or
chlorophacinone. Owls that died behaved normally until 24 h or less
before death, when they became lethargic and stopped eating
(Mendenhall & Pank, 1980).
Mice (weighing 35 g) were fed for 1 day (no choice) on a mixture
containing either 0.005% difenacoum or 0.002% brodifacoum. The mean
mass of residue on the day of death in a whole mouse was estimated to
be 10.17 µg for difenacoum and 15.36 µg for brodifacoum. Difenacoum-
and brodifacoumpoisoned mice were fed to captive barn owls for
successive periods of 1, 3 and 6 days. All of the owls fed on
difenacoum-poisoned mice survived the treatments and none showed
external bleeding. In contrast, four of the six owls fed
brodifacoum-poisoned mice died within 6-17 days after a 1-day dose.
The estimated lethal dose of brodifacoum was 0.15-0.18 mg/kg. After
death these owls had 0.63-1.25 mg brodifacoum/kg in their livers
(Newton et al., 1990).
Newton et al. (1994) fed barn owls flocoumafen-dosed mice for
consecutive periods of 1, 3 and 6 days. Four of the birds survived;
the fifth bird died from haemorrhaging 5 days after the final dose.
During the feeding trial birds received cumulative flocoumafen doses
ranging from 0.78 to 1.25 mg/kg.
Gray et al. (1992) investigated the toxicity of brodifacoum,
difenacoum and flocoumafen for barn owls fed poisoned mice. For each
rodenticide, the owls survived a cumulative dose of at least 1.9 mg/kg
owl weight over 15 days of treatment. All owls with cumulative doses
in excess of 1.9 mg/kg body weight showed multiple treatment-related
effects. The three rodenticides had approximately the same order of
magnitude of toxicity to barn owls. Gray et al. (1994) developed a
non-invasive method for monitoring the exposure of these birds to
second-generation rodenticides by measuring compounds in regurgitated
owl feed.
Radvanyi et al. (1988) fed American kestrels (Falco sparverius)
on meadow voles that had been maintained on 2% chlorophacinone. Voles
consumed approximately 53 mg of 2% chlorophacinone (1.14 mg a.i.)
before dying within 6 days. No kestrels fed poisoned mice, for up to
21 consecutive days, died. Haematomas were observed on the pectoral
muscles, lungs, liver and heart of exposed birds.
In a study by Townsend et al. (1981), captive-bred tawny owls
(Strix aluco) were given warfarin-treated mice for 3 months. No
behavioural changes were observed. Prothrombin levels decreased to
less than 10-12% of normal but returned to normal after 9 days.
Townsend et al. (1984) found that daily warfarin intakes
approaching 0.3 mg/kg body weight or 30 µg of warfarin/day can cause
the death of list weasels (Mustela nivalis) by secondary poisoning.
Lethal exposure occurred if mice were contaminated with 1.0-1.5 mg
warfarin/kg, an amount reached following exposure to 0.005 or 0.02%
sodium warfarin for about 3 days.
9.2 Field observations
9.2.1 Primary poisonings
There are few data available on the non-target hazard of
anticoagulant rodenticides in field conditions.
Greig-Smith et al. (1988) reported incidents in England and Wales
with barn owls and foxes. There were three incidents in which barn
owls were found to have residues of the anticoagulant rodenticide
brodifacoum in their livers. In two birds, residues were thought to
represent lethal poisoning (0.61 and 0.29 mg/kg) but in the third the
level was only 0.099 mg/kg. There were six separate incidents in
which dead foxes were found to contain residues of brodifacoum,
bromadiolone, coumatetralyl, difenacoum or warfarin. Residue levels
showed a wide range from 0.009 to 0.46 mg/kg tissue. Three of the
foxes contained more than one rodenticide, one animal revealing
residues of difenacoum, bromadiolone and warfarin.
Poisonings of pheasants and partridges by chlorophacinone used
against Microtus arvalis have been reported (Giban, 1974).
Reece et al. (1985) reported that, a few days after bromadiolone
was placed out for control of rodents, three pea fowl
(Pavo cristatus), a little raven (Corvus mellori) and an eastern
swamp hen (Porphyrio porphyrio melanotus) died. Necropsy showed
that the birds had been in good condition. One of the pea fowl and
the swamp hen had massive intra-abdominal haemorrhage without evidence
of external trauma. All had severe congestion of the liver and lungs.
The birds had negligible gut contents, so analysis for bromadiolone
could not be performed.
9.2.2 Secondary poisonings
Difenacoum and brodifacoum were detected in 15 of a total of 145
dead barn owls in Britain, and on postmortem the cause of death for
one owl was diagnosed as rodenticide poisoning (Newton et al., 1990,
see section 5.1).
A study of 35 active nests and the radio-telemetry of 34 barn
owls in an area where brodifacoum baits (0.005%) were used in and
around farm buildings in New Jersey, USA, showed no adverse effects of
the rodenticide on the owls studied. In this study, rats and house
mice were the target organisms and barn owls were found not to feed on
these animals to any extent. Chicks were fledged from at least eight
nests where poisoned rodents had been available during part of the
nesting and feeding period, but no rodenticide-induced mortality was
observed. Traces of brodifacoum (less than 0.05 mg/kg) were found in
a barn owl that had been accidentally electrocuted (Hegdal &
Blaskiewicz, 1984).
Merson & Byers (1984) monitored eastern screech owls (Otus asio)
using radio-transmitters following the use of 0.005% brodifacoum for
rodent control in a commercial orchard. Brodifacoum was detected in
screech owl pellets (see section 5.1). There were no owl deaths which
could be attributed to brodifacoum poisoning. Two owls were analysed
at the end of the study; one showed signs of haemorrhage and
contained 0.21 mg brodifacoum/kg and the other owl contained no
brodifacoum. In a larger study, Hegdal & Colvin (1988) monitored 38
eastern screech owls and several other species of owl following
brodifacoum application in an orchard. Minimum mortality was 58%
among screech owls for which more than 20% of the home range was
treated, as compared with 17% among those for which less than 10% of
the home range was treated. Secondary brodifacoum poisoning was the
most probable cause of death in six screech owls. Six radio-equipped
owls were collected 1-2 months post-treatment, and four contained
detectable concentrations of brodifacoum.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Anticoagulants, both hydroxycoumarins and indandiones, known also
as vitamin K antagonists, are widely used in urban rodent control and
against rodent pests in agriculture. They act by inhibiting the
vitamin K1 epoxide cycle, thereby depleting the active form of
vitamin K1 necessary for producing blood-clotting factors.
Since anticoagulant rodenticides are used in general as
low-concentration bait formulations and have low volatility, increased
levels in the air are unlikely. Being only slightly soluble in water
their use could not be a major source of water contamination.
Anticoagulant rodenticides are not intended for direct application to
growing crops. Hence no residues in plant foodstuffs are expected.
The controlled medicinal use of warfarin exposes more people to
higher concentrations over a longer period than would be expected to
occur as a result of accidental human exposure due to its use as a
rodenticide.
Occupational exposure may occur during manufacture, formulation
and bait application, but figures indicating the levels of exposure
are not available.
Anticoagulant rodenticides are readily absorbed through the
gastrointestinal tract, skin and respiratory system. The major route
of elimination in various species after oral administration is through
the faeces. The urine is a very minor route of elimination. The liver
is the major organ for accumulation and storage of anticoagulants.
The metabolism pattern of warfarin and indandiones mainly
involves hydroxylation. The second-generation hydroxycoumarins are
found principally as unchanged parent compounds. Their elimination
from the liver is slow with a biphasic rate, where a rapid initial
phase is followed by a prolonged second phase.
Most of the anticoagulants have high acute toxicity by oral,
percutaneous and inhalation routes of exposure. The second-generation
anticoagulants are more toxic than the first-generation one in the
sense that a single feeding may be lethal. Signs of poisoning in all
species, including humans, are associated with increased bleeding
tendency.
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxication from occupational
exposure to anticoagulants have also been observed.
The level of prothrombin concentration is a satisfactory guide to
the severity of acute intoxication and the effectiveness and duration
of the therapy. The specific antidote is vitamin K1. The high
retention of the second-generation anticoagulants in the liver means
that any treatment of intoxication should be prolonged to ensure that
anticoagulant activity does not recur.
The addition of bittering agents to anticoagulant rodenticides is
aimed at discouraging human consumption.
Warfarin has been found to be teratogenic in both rats and
humans. Second-generation anticoagulant rodenticides do not
demonstrate teratogenicity in laboratory animals. Developmental
effects in humans are observed when warfarin has been taken as a
therapeutic agent during pregnancy. No cases of embryopathy from
anticoagulants in their use as rodenticides have been reported.
Second-generation anticoagulants are not intended to be used as
therapeutic agents, and the risks associated with, for example,
warfarin will not apply.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on some compounds to demonstrate an absence of mutagenicity.
There is experimental evidence that coumarin anticoagulants not
only inhibit the vitamin K cycle in the liver, but also in other
tissues such as bone. In humans it has been demonstrated that even
low levels of vitamin K deficiency form a risk factor for developing
osteoporosis (Hart et al., 1985; Szulc et al., 1993). Therefore, it
appears that long-term exposure to low levels of anticoagulant may
have an adverse effect on bone metabolism.
10.2 Evaluation of effects on the environment
Unlike conventional crop protection products, which must be
applied over relatively large crop areas, anticoagulant rodenticides
are applied to discrete sites in the form of low concentration baits.
Most of the anticoagulants are stable under normal conditions.
They are slightly soluble in water, and as bait formulations their use
is unlikely to be a source of water contamination. They appear to
bind rapidly in the soil, with very slow desorption and no leaching.
In general, anticoagulants are highly toxic to aquatic organisms when
tested as technical material.
Non-target organisms are potentially at risk in two ways: from
direct consumption of baits (primary hazard) and through eating
poisoned rodents (secondary hazard).
Bird species vary in their susceptibility to anticoagulant
rodenticides. Small pellets and whole grain baits are highly
attractive to birds. Wax block formulation appear to decrease the
attractiveness to the birds and so reduce the possibility of poisoning
incidents. It is difficult to assess the risks to birds due to direct
consumption of baits because most of the published studies are
toxicity trials in laboratory conditions.
The main cause for poisoning of domestic animals is through
direct consumption of anticoagulant baits.
Some of the anticoagulants show a similar range of acute toxicity
for non-target non-rodent mammals as for target rodents. The primary
hazard is usually expressed by the amount of finished bait which must
be consumed to approach the lethal dose. The bait concentration of
second-generation rodenticides is usually around 0.005%, whereas the
concentration of the first-generation rodenticides in the bait may be
5 to 10 times higher. Thus, to reach the toxic or lethal dose,
non-target animals must consume comparatively large amounts of bait
with low concentrations of active ingredient.
Secondary poisoning through the consumption of rats and mice
killed with anticoagulants may occur in dogs and cats in urban
situations but is more likely in farm situations.
Some secondary toxicity laboratory studies with wildlife have
shown that captive predators could be intoxicated by no-choice feeding
of anticoagulant-poisoned or dosed prey. The significance of these
results in terms of hazard under field conditions is difficult to
assess because the predators would not be expected to eat only
poisoned animals. However, where they occur, predators may take
poisoned, but not dead, small mammals preferentially. In areas close
to baiting, poisoned rodents may represent a high proportion of the
diet for individual birds. However, only a few individuals will be
affected except in situations of very widespread and constant use of
baits. Therefore, some kills of owls will be expected but no severe
population effects. This agrees with observed field effects with small
numbers of poisoned owls.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
a) Exposure of the general population to anticoagulant rodenticides
via food and drinking-water is unlikely and does not constitute a
significant health hazard.
b) Poisoning incidents may occur in cases of massive intentional or
unintentional ingestion or prolonged skin contact during
manufacture and formulation.
c) Anticoagulant rodenticides are relatively persistent in the
environment, but their specific use as low-concentration bait
formulations limits the potential for environmental
contamination.
d) Direct and secondary poisoning of birds, domestic and farm
animals, and wildlife may occur.
e) The major difference between the first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention, resulting in an increased tendency for bleeding over a
longer period of time.
f) The mode of action of anticoagulant rodenticides is known and an
effective antidote is available, i.e. vitamin K1.
g) There is no available evidence that this class of compound is
mutagenic or carcinogenic.
h) Only warfarin has been shown to possess some teratogenic
potential in both rats and humans.
11.2 Recommendations for protection of human health and the
environment
a) Exposed workers should receive appropriate biomonitoring and
health evaluation.
b) The inclusion of a bittering agent in formulations at appropriate
concentrations may reduce accidental ingestion.
c) For preventing primary poisoning, baits less attractive to birds
and domestic animals, as well as pulsating baiting, should be
used.
d) The location of bait placement should be carefully selected.
e) Killed rodents should be burned or buried to reduce the risk of
secondary poisoning of predators.
f) Training in the safe handling of rodenticides is essential.
12. FURTHER RESEARCH
a) Studies of exposed humans are required, particularly with regard
to possible teratogenic and embryotoxic effects.
b) More information is needed in order to evaluate the risks of
occupational exposure to anticoagulant rodenticides.
c) Methods more sensitive than prothrombin time determination need
to be developed for routinely assessing the absorption and
effects of anticoagulants.
d) More data are required to assess secondary effects on non-target
organism populations.
e) The extent to which second-generation anticoagulant rodenticides
are transferred across the placenta should be evaluated.
f) More information should be collected concerning the tissue
distribution of anticoagulant rodenticides after their ingestion
and their effects on physiological processes other than blood
coagulation, notably calcium and bone metabolism. This is
particularly necessary for long-term, low-level exposure to these
compounds.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by Hazard
(WHO, 1994), anticoagulant rodenticides were classified, according to
their acute oral LD50, as follows:
Class Ia (Extremely hazardous) Oral LD50 (mg/kg)
Brodifacoum 0.3
Bromadiolone 1.12
Chlorophacinone 3.1
Difenacoum 1.8
Difethialone 0.56
Diphacinone 2.3
Flocoumafen 0.25
Class Ib (Highly hazardous)
Coumachlor 33
Coumatetralyl 16
Warfarin 10
Class II (Moderately hazardous)
Pindone 50
A Poison Information Monograph for brodifacoum has been issued
(IPCS, 1992), and one on warfarin is in preparation.
REFERENCES
Allen JA, Proudlock RJ, & McCaffrey KJ (1986) Genotoxicity studies
with WL 108366 (rodenticide): in vivo chromosome studies with rat
bone marrow cells. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL 85/8610).
Alstad AD, Casper HH, & Johnson LJ (1985) Vitamin K treatment of sweet
clover poisoning in calves. J Am Med Assoc, 187: 729-731.
Anderson DR, Brill-Edwards P, & Walker I (1992) Warfarin-induced skin
necrosis in 2 patients with protein S deficiency: Successful
reinstatement of warfarin therapy. Haemostasis, 22: 124-128.
AMA (1980) AMA drug evaluations, 4th ed. Chicago, Illinois, American
Medical Association.
Anonymous (1965) Technical report on chlorophacinone. Lyon, France,
Lipha S.A.
Anonymous (1976) 'Ratak' containing difenacoum: A new rodenticide.
Haslemere, Surrey, Imperial Chemical Industries Ltd, Plant Protection
Division, 18 pp.
Anonymous (1985) Brodifacoum: safety in use. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division, 8 pp
(ICI Agrochemical Information Bulletin).
Anonymous (1988) The treatment of anticoagulant rodenticide poisoning.
Imperial Chemical Industries Ltd, Lipha S.A., Shell International
Chemical Company Ltd, and Sorex Ltd.
Antov G, Kasakova B, & Ajanova A (1985) Effect of warfarin on
experimentally induced sclerotic processes. In: Pathological models in
toxicological studies. Bucharest, Romania, Ministry of Chemical
Industry, Industrial Head-Office for Medicinal Drugs and Cosmetics,
pp 153-157.
Ashton AD, Jackson WB, & Peters H (1987) Comparative evaluation of
LD50 values for various anticoagulant rodenticides. In: Richards CGL
& Ku TY ed. Control of mammal pests. London, New York, Philadelphia,
Taylor & Francis, pp 187-197.
Avent ML, Canady BR, & Sawyer WT (1992) Warfarin-induced intramural
hematoma of the small intestine. Clin Pharm, 11: 632-635.
Bachmann KA & Sullivan TJ (1983) Dispositional and pharmacodynamic
characteristics of brodifacoum in warfarin-sensitive rats.
Pharmacology, 27: 281-288.
Barlow AM, Gay AL, & Park BK (1982) Difenacoum (Neosorexa) poisoning.
Br Med J, 285: 541.
Blair D (1984) Toxicology of a candidate rodenticide: The acute 4-hour
inhalation toxicity of manufacturing master mix (bait concentrate)
containing 0.5% m/m WL 108366. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 71 pp (Report No. SBGR.84.151).
Boermans HJ, Johnstone J, Black WD, & Murphy M (1991) Clinical signs,
laboratory changes and toxicokinetics of brodifacoum in horses. Can J
Vet Res, 55: 21-27.
Braithwaite GB (1982) Vitamin K and brodifacoum. J Am Vet Med Assoc,
181(6): 531, 534.
Bratt H (1987) Difenacoum: Elimination from tissues of rats following
administration of a single oral dose. Macclesfield, Surrey, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report
No. CTL/P/1592).
Braselton WE Jr, Neiger RD, & Poppenga RH (1992) Confirmation of
indandione rodenticide toxicoses by mass spectrometry/mass
spectrometry. J Vet Diagn Invest, 4, 441-446.
Brooks LW & Blais FX (1991) Coumarin-induced skin necrosis. J Am
Osteopath Assoc, 91: 601-605.
Brooks TM, Clare MG, & Wiggins DE (1984) Genotoxicity studies with
WL 108366 (candidate rodenticide). Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 64 pp (Report No. SBGR.84.160).
Brorson T, Björklund I, Svenstam G, & Lantz R (1994) Comparison of two
strategies for assessing ecotoxicological aspects of complex
wastewater from a chemical-pharmaceutical plant. Environ Toxicol Chem,
14(4): 543-552.
Buckle AP (1994) Rodent control methods in rodent pests and their
control. Wallingford, Oxon, Commonwealth Agriculture Bureau (CAB)
International.
Buitenhuis HC, Soute BAM, & Vermeer C (1990) Comparison of the
vitamins K1, K2 and K3 as cofactors for the hepatic vitamin
K-dependent carboxylase. Biochim Biophys Acta, 1034: 170-175.
Bull JO (1976) Laboratory and field investigations with difenacoum, a
promising new rodenticide. In: Proceedings of the 77th Vertebrate Pest
Conference, Monterey, California, March 1976. Davis, California,
University of California, pp 72-84.
Bullard RW, Thompson RD, & Holguin G (1976) Diphenadione residues in
tissues of cattle. J Agric Food Chem, 24(2): 261-263.
Burucoa Ch, Mura P, Robert R, Boinot C, Bouquet S, & Piriou A (1989)
Chlorophacinone intoxication: A biological and toxicological study.
Clin Toxicol, 27: 79-89.
Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, & Ind PW (1992)
Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol,
11: 553-554.
Callander RD (1984) Product registration. Brodifacoum: An evaluation
in the salmonella mutagenicity assay. Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V. (ICI CTL Report No. CTL/P/949).
Carson M & Reid M (1976) Warfarin and fetal abnormality. Lancet,
1: 1127.
Chalermchaikit T, Felice LJ, & Murphy MJ (1993) Simultaneous
determination of eight anticoagulant rodenticides in blood serum and
liver. J Anal Toxicol, 17: 56-61.
Chesterman H, Burford P, Harling RJ, & Heywood R (1984) WL 108366
rodenticide: Acute oral toxicity in Beagle dogs. Huntingdon, United
Kingdom, Huntingdon Research Centre (HRC Report No. SLL/72/84757).
Chong LL, Chan WK, & Ho CH (1986) A case of 'superwarfarin' poisoning.
Scand J Haematol, 36: 314-315.
Chow EY, Haley LP, Vickars LM, & Murphy MJ (1992) A case of
bromadiolone (superwarfarin) ingestion. Can Med Assoc J, 147: 60-62.
Christopher MJ, Balasubramanyam M, & Purushotham KR (1984) Toxicity of
three anticoagulant rodenticides to male hybrid Leghorns. Z Angew
Zool, 71(3): 275-281.
Cifone MA (1993) Mutagenicity test on bromadiolone technical in the
CHO/HGPRT forward mutation assay (HWA Study No. 15310-0-435). Vienna,
Virginia, Hazleton Washington Inc., 48 pp.
Clare MG & Wiggins DE (1986) In vitro mutagenicity studies with
WL 108366 (rodenticide) using cultured Chinese hamster V 79 cells.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research
Centre, 33 pp (Report No. SBGR.86.014).
Coveney PC & Forbes S (1987) Degradation of WL 108366 (Storm) in rat
baits, carcasses and faeces in field situations. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.87.171).
Cross M & Clay P (1984) Product registration. Brodifacoum: Assessment
of mutagenic potential using L5178Y mouse lymphoma cells. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/975).
Dodds WJ & Frantz SC (1984) Dog and cat poisonings. Pest Control
Technol, 12(3): 14.
Donovan JW, Ballard JO, & Murphy MJ (1990) Brodifacoum therapy with
activated charcoal: Effect on elimination kinetics. Vet Hum Toxicol,
32: 350.
Dreyfus M, Dubouch P, Pierson K, Neveu Y, Martin E, & Tchernia G
(1983) Warfarin-contaminated talc. Lancet, 1: 1110.
Dubock AC (1986) The evaluation of potential effects on non-target
vertebrate populations as a result of pesticide use. In: Proceedings
of the Second Symposium on Recent Advances in Rodent Control, Kuwait,
1986, pp 257-269.
Dusein P, Manigand G, & Taillandier J (1984) Hypoprothrombinémie
sévère et prolongée après intoxication par chlorphacinone. Presse Méd,
13(30): 1845.
DuVall MD, Murphy MJ, Ray AC, & Reagor JC (1989) Case studies on
second-generation anticoagulant rodenticide toxicities in non-target
species. J Vet Diagn Invest, 1: 66-68.
Eby CS (1993) Warfarin-induced skin necrosis. Hematol/Oncol Clin North
Am, 7: 1291-1300.
Elias DJ & Johns BE (1981) Response of rats to chronic ingestion of
diphacinone. Bull Environ Contam Toxicol, 27: 559-567.
Ellenhorn MJ & Barceloux DG (1988) Anticoagulant poisoning. In:
Medical toxicology, diagnosis and treatment of human poisoning.
Amsterdam, New York, Elsevier Science Publishing Company Inc.,
pp 211-221.
Erichsen C, Sudenaa K, Sreide JA, Andersen E, & Tysvoer A (1993)
Spontaneous liver hematomas induced by anti-coagulation therapy.
Hepato-Gastroenterology, 40: 402-406.
Exner DV, Brien WF, & Murphy MJ (1992) Superwarfarin ingestion. Can
Med Assoc J, 146(1): 34-35.
FAO (1979) Rodenticides: Analyses specifications, formulations. Rome,
Food and Agriculture Organization of the United Nations, pp 1-79 (FAO
Plant Production and Protection Paper 16).
Feinsod FM, Allam I, Ragai A, & Saah AJ (1986) Rodenticide-induced
signs simulating Rift Valley fever in sheep and goats in Egypt. Vet
Rec, 118: 270-272.
Felice LJ & Murphy MH (1989) The determination of the anticoagulant
rodenticide brodifacoum in blood serum by liquid chromatography with
fluorescence detection. J Anal Toxicol, 13: 229-231.
Felice LJ, Chalermchaikit T, & Murphy MJ (1991) Multicomponent
determination of 4-hydroxycoumarin anticoagulant rodenticides in blood
serum by liquid chromatography with fluorescence detection. J Anal
Toxicol, 15: 126-129.
Fiore CE, Tamburino C, Foti R, & Grimaldi D (1990) Reduced bone
mineral content in patients taking an oral anticoagulant. South Med J,
83: 538-542.
Forsey JD (1983a) The acute skin irritation of novel anticoagulants in
male rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 3 pp.
Forsey JD (1983b) Acute eye irritation of novel anticoagulants in male
rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 4 pp.
Forsey JD (1985) An evaluation of the long term sub-acute oral
toxicity of WL 108366 in Wistar rats. Widnes, United Kingdom, Sorex
Ltd, Agricultural Research Department, 10 pp.
Fristedt B & Sterner N (1965) Warfarin intoxication from percutaneous
absorption. Arch Environ Health, 11: 205-208.
Furie B & Furie BC (1990) Molecular basis of vitamin K-dependent
gamma-carboxylation. Blood, 75: 1753-1762.
Giban J (1974) Use of chlorophacinone in the struggle against the
common vole (Microtus arvalis Pallas) and against the musk rat
(Ondatra zibethicus). In: Proceedings of the 6th Vertebrate Pest
Conference, Sacramento, California. Davis, California, University of
California, pp 263-271.
Gijsbers BLMG, van Haarlem LJM, Soute BAM, Ebberink RHM, & Vermeer C
(1990) Characterization of a gla-containing protein from calcified
human atherosclerotic plaques. Arteriosclerosis, 10: 991-995.
Godfrey MER (1984) The toxicology of brodifacoum to target and
non-target species in New Zealand. Proceedings of a Conference on the
Organization and Practice of Vertebrate Pest Control, Elvetham Hall,
Hampshire, England, 30 August-3 September 1982. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division,
pp 631-637.
Godfrey MER (1985) Non-target and secondary poisoning hazards of
"second generation" anticoagulants. Acta Zool Fennica, 173: 209-212.
Godfrey MER, Laas FJ, & Rammell CG (1985) Acute toxicity of
brodifacoum to sheep. N Z J Exp Agric, 13: 23-25.
Gorenzel WP, Marsh RE, & Salmon TR (1982) Single-feeding
anticoagulants. Pest Control, 50(2): 32.
Grand M (1976) Données expérimentales sur un nouveau raticide
anticoagulant: le bromadiolone. Phytiatrie-Phytopharmacie, 25: 69-88.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1992) Toxicity of
second generation rodenticides to barn owls. In: Proceedings of the
Brighton Crop Protection Conference - Pests and Diseases, 1992,
vol 2, pp 781-786.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1994) Non-invasive
method for monitoring the exposure of barn owls to second-generation
rodenticides. Pestic Sci, 41: 339-343.
Greeff MC, Mashile O, & MacDougall LG (1987) "Superwarfarin"
(bromadiolone) poisoning in two children resulting in prolonged
anticoagulation. Lancet, 1: 1269.
Greig-Smith PW, Fletcher MR, Hunter K, Quick MP, Ruthven AD, & Shaw IC
(1988) Pesticide poisoning of animals 1988: Investigations of
suspected incidents in Great Britain. A Report of the Environmental
Panel of the Advisory Committee on Pesticides.
Hadler MR (1974) Sub-acute five day oral toxicity of WBA 8119 to male
rats. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research.
Hadler MR (1975a) Acute oral toxicity of WBA 8119 to male rabbit.
Appendix 5. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research, 3 pp.
Hadler MR (1975b) Acute oral toxicity of WBA 8119 to female Guinea
pig. Appendix 6. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research, 4 pp.
Hadler MR (1975c) Acute eye irritation of WBA 8119 to the rabbit.
Appendix 31. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research.
Hadler MR (1976) WBA 8119-12 week feeding study in rats. Widnes,
Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural Research, 7 pp
(Laboratory report).
Hagan EC & Radomski JL (1953) The toxicity of 3-(acetonylbenzyl)-
4-hydroxycoumarin (warfarin) to laboratory animals. J Am Pharm Assoc,
42(6): 379-382.
Hall JG (1976) Warfarin and fetal abnormality. Lancet, 1: 1127.
Hall BE & Priestley I (1992) Brodifacoum: Metabolism in soil under
aerobic conditions (IRI Project No. 381441). Haslemere, Surrey, Zeneca
Agrochemicals, 63 pp (Report No. 8795).
Hall JG, Pauli RM, & Wilson KM (1980) Maternal and fetal sequelae of
anticoagulants during pregnancy. Am J Med, 68: 122-140.
Hall BE, Jackson R, & Priestley I (1992) Difenacoum: Photolysis in
buffered aqueous solutions (IRI Project No. 381614). Haslemere,
Surrey, Zeneca Agrochemicals, 123 pp (Report No. 8704).
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN,
Dodds RA, Bitensky L, & Chayen J (1985) Electrochemical detection of
depressed circulating levels of vitamin K1 in osteoporosis. J Clin
Endocrinol Metab, 60(6): 1268-1269.
Hegdal PL & Blaskiewicz RW (1984) Evaluation of the potential hazard
to barn owls of Talon (brodifacoum bait) used to control rats and
house mice. Environ Toxicol Chem, 3: 167-179.
Hegdal PL & Colvin BA (1988) Potential hazard to eastern screech-owls
and other raptors of brodifacoum bait used for vole control in
orchards. Environ Toxicol Chem, 7: 245-260.
Hill RW, Maddock BG, Hart B, & Bowles PF (1976) Determination of the
acute toxicity of PP 581 to Rainbow trout (Salmo gairdneri). Brixham,
Devon, Imperial Chemical Industries Ltd, Brixham Laboratory (Report
No. BL/B/1758).
Hodge MCE, Banham PB, Richards D, Weight TM, & Wilson J (1980a)
Product registration. Brodifacoum: Teratogenicity study in the rat.
Geneva, Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.
(ICI CTL Report No. CTL/P/437).
Hodge MCE, Banham PB, Richards D, & Weight TM (1980b) Product
registration. Brodifacoum: Teratogenicity study in the rabbit. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/459).
Hodge MCE, Banham PB, Richards D & Weight TM (1980c) Product
registration. Brodifacoum: Teratogenicity study in the rabbit
(Appendices A-E). Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V. (ICI CTL Report No. CTL/P/459 S).
Hoffman RS, Smilkstein MJ, & Goldfrank LR (1988) Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose. Clin Toxicol, 26(3/4): 233-248.
Hollinger BR & Pastoor TP (1993) Case management and plasma half-life
in a case of brodifacoum poisoning. Arch Intern Med, 153: 1925-1928.
Hoogenboom JJL (1994) Anticoagulant poisoning cases 1977-1994.
Wellington, New Zealand Central Animal Health Laboratory.
Hoogenboom JJL & Rammell CG (1983) Improved HPLC method for
determining brodifacoum in animal tissues. J Environ Contam Toxicol,
31: 239-243.
Huckle KR & Warburton PA (1986a) Elimination, metabolism and
disposition of 14C-WL 108366 in the Fischer 344 rat following
repeated oral administration. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre (Report No. SBGR.86.084).
Huckle KR & Warburton PA (1986b) Percutaneous absorption metabolism
and elimination of WL 108366 in the rat. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.218).
Huckle KR & Warburton PA (1986c) WL 108366: Absorption, metabolism and
disposition in Japanese quail (Coturnix coturnix Japonica) following a
single dose by intraperitoneal or oral administration. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.192).
Huckle KR, Hutson DH, & Warburton PA (1988) Elimination and
accumulation of the rodenticide flocoumafen in rats following repeated
oral administration. Xenobiotica, 18(12): 1465-1479.
Huckle KR, Hutson DH, Logan CJ, Morrison BJ, & Warburton PA (1989) The
fate of the rodenticide flocoumafen in the rat: Retention and
elimination of a single oral dose. Pestic Sci, 25: 297-312.
Huebner CF & Link KP (1941) Studies on the hemorrhagic sweet clover
disease. VI. The synthesis of the delta-diketone derived from the
hemorrhagic agent through alkaline degradation. J Biol Chem,
138: 529-534.
Hunter K (1983) Determination of coumarin anticoagulant rodenticide
residues in animal tissues by high-performance liquid chromatography:
Fluorescence detection using post-column techniques. J Chromatogr,
270: 267-276.
Hunter K (1985) High-performance liquid chromatographic strategies for
the determination and confirmation of anticoagulant rodenticides in
animal tissues. J Chromatogr, 321: 255-272.
Ikeda M, Conney AH, & Burns JJ (1968a) Stimulatory effect of
phenobarbital and insecticides on warfarin metabolism in the rat.
J Pharmacol Exp Ther, 162: 338-343.
Ikeda M, Ullrich V, & Staudinger H (1968b) Metabolism in vitro of
warfarin by enzymic and nonenzymic systems. Biochem Pharmacol,
17: 1663-1669.
IPCS (1992) IPCS/INTOX project. Poison Information Monograph:
Brodifacoum. Geneva, World Health Organization, 16 pp
(IPCS/INTOX/PIM.077).
Jackson R & Hall BE (1992) Aged soil leaching of [14C]-brodifacoum
(IRI Project No. 381986). Haslemere, Surrey, Zeneca Agrochemicals,
72 pp (Report No. 8879).
Jackson R, Priestley I, & Hall BE (1991) The determination of the
hydrolytic stability of [14C]-brodifacoum (IRI Project No. 381420).
Haslemere, Surrey, Zeneca Agrochemicals. 77 pp (Report No. 8330).
Jones EC, Growe GH, & Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. J Am Med Assoc, 252(21): 3005-3007.
Katona B & Wason S (1989) Superwarfarin poisoning. J Emerg Med,
7: 627-631.
Kaukeinen DE & Buckle AP (1992) Evaluations of aversive agents to
increase the selectivity of rodenticides, with emphasis on denatonium
benzoate (Bitrex(R)) bittering agent. In: Borrecco JE & Marsh RE ed.
Proceedings of the 15th Vertebrate Pest Conference. Davis, California,
University of California, pp 192-198.
Kelly MJ, Chambers J, & MacNicoll AD (1993) Simple and rapid method
for the determination of the diastereomers of difenacoum in blood and
liver using high-performance liquid chromatography with fluorescence
detection. J Chromatogr, 620: 105-112.
Kennelly JC (1990) Difenacoum: Assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 42 pp (Report No. CTL/P/3017.
Knapen MHJ, Jie K-SG, Hamulyák K, & Vermeer C (1993) Vitamin K-induced
changes in markers for osteoblast activity and urinary calcium loss.
Calcif Tissue Int, 53: 81-85.
Kosmin M & Barlow JN (1976) Rodent control using a novel formulation
of diphacinone, Ramik. Presented at the First Afro-Asian Vertebrate
Pest Conference, Cairo. Rosemont, Illinois, Velsicol Chemical
Corporation, 7 pp.
Krambias A & Hoppe AH (1987) The response of captive partridges to
dosing with anticoagulant rodenticides. In: Richards CGL & Ku TY ed.
Control of mammal pests. London, New York, Philadelphia, Taylor &
Francis, pp 181-186.
Kruse JA & Carlson RW (1992) Fatal rodenticide poisoning with
brodifacoum. Ann Emerg Med, 21: 331-336.
Laas FJ, Forss DA, & Goodfrey MER (1985) Retention of brodifacoum in
sheep tissues and excretion in faeces. N Z J Agric Res, 28: 357-359.
Lange PF & Terveer J (1954) Warfarin poisoning. US Armed Forces Med J,
5: 872-877.
Lawlor TE (1992) Mutagenicity test on bromadiolone in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test)
(HWA Study No. 15310-0-401). Vienna, Virginia, Hazleton Washington
Inc., 34 pp.
Lechevin JC & Grand M (1987) LM 2219 (proposed common name:
difethialone). Summary of the file submitted in the request for
approval (struggle against commensal rodents). Lyon, France, Lipha
S.A., Research and Development Centre, 15 pp (Report No. L-JCL/SP).
Leck JB & Park BK (1981) A comparative study of the effects of
warfarin and brodifacoum on the relationship between vitamin K1
metabolism and clotting factor activity in warfarin-susceptible and
warfarin-resistant rats. Biochem Pharmacol, 30: 123-128.
Lewis CJ (1992a) (14-C)-Difenacoum: A study of the degradation in two
soils. Haslemere, Surrey, Zeneca Agrochemicals (Report No. 6927).
Lewis CJ (1992b) (14C)-Difenacoum: Aged soil leaching. Haslemere,
Surrey, Zeneca Agrochemicals.
Lewis CJ (1992c) Difenacoum: Hydrolysis study. Haslemere, Surrey,
Zeneca Agrochemicals (Report No. 7031).
Lipton RA & Klass EM (1984) Human ingestion of a 'superwafarin'
rodenticide resulting in a prolonged anticoagulant effect. J Am Med
Assoc, 252: 3004-3005.
Litovitz TL, Clark LR, & Soloway RA (1994) 1993 Annual report of the
American Association of Poison Control Centers, Toxic Exposure
Surveillance System. Am J Emerg Med, 12: 546-584.
Locht H & Lindström FD (1993) Severe skin necrosis following warfarin
therapy in a patient with protein C deficiency. J Intern Med,
233: 287-289.
Lund M (1981) Hens, eggs and anticoagulants. Int Pest Control,
5: 126-128.
Lund M (1982) Annual report 1981. Lyngby, Danish Pest Infestation
Laboratory, pp 58-75.
Lund M (1984) Resistance to the second generation anticoagulant
rodenticides. In: Clark DO ed. Proceedings of the 11th Vertebrate Pest
Conference. Davis, California, University of California, pp 89-94.
Mackintosh CG, Laas FJ, Godfrey MER, & Turner K (1988) Vitamin K1
treatment of brodifacoum poisoning in dogs. In: Crabb AC & Marsh RE
ed. Proceedings of the 13th Vertebrate Pest Conference. Davis,
California, University of California, pp 86-90.
McSporran KD & Phillips CA (1983) Brodifacoum poisoning in a dog.
N Z Vet J, 31: 185-186.
Manfioletti G, Brancolini C, Avanzi G, & Schneider C (1993) The
protein encoded by a growth arrest-specific gene (gas6) is a new
member of the vitamin K-dependent proteins related to protein S, a
negative coregulator in the blood coagulation cascade. Mol Cell Biol,
13(5): 4976-4985.
Marsh RE (1985a) Are anticoagulant rodenticides a problem for
household pets? Pest Control, 53(8): 20-22.
Marsh RE (1985b) Anticoagulant rodenticides provide pet problems.
Part II. Pest Control, 53(9): 25-28.
Martin-Bouyer G, Linh PD, Tuan LC, Barin C, Khahn MB, Hoa DQ, Tourneau
J, & Guerbois H (1983) Epidemic of haemorrhagic disease in Vietnamese
infants caused by warfarin-contaminated talc. Lancet, 1: 230-232.
Mellano D (1984a) Product registration. In vitro study of chromosome
aberration induced by the article brodifacoum in cultured human
lymphocytes. Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V., 17 pp (Report No. CTL/C/1258).
Mellano D (1984b) Product registration. Study of the capacity of test
article brodifacoum to induce unscheduled DNA synthesis in cultured
Hela cells (autoradiographic method). Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V., 14 pp (Report No. CTL/C/1257).
Mendenhall VM & Pank LF (1980) Secondary poisoning of owls by
anticoagulant rodenticides. Wildl Soc Bull, 8(4): 311-315.
Merson MH & Byers RE (1984) Residues of the rodenticide brodifacoum in
voles and raptors after orchard treatment. J Wildl Manage,
48: 212-216.
Meyer AL & Wiggins DE (1986) Genotoxicity studies with WL 108366
(rodenticide): In vitro cell transformation studies. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.289).
Mirkova E & Antov G (1983) Experimental evaluation of the risk of
prenatal pathology under the effect of warfarin coumarine rodenticide.
Hig Zdrav, 25(6): 476-482.
Misenheimer TM, Lund ML, Baker EM, & Suttie JW (1994) Biochemical
basis of warfarin and bromadiolone resistance in the house mouse,
Mus musculus domesticus. Biochem Pharmacol, 47: 673-678.
Monnot G, Fave A, Illat T, & Briet Ph (1981) Phase 3 reformat of
MRID 0007-9375: Teratology study in the rat with LM 637 bromadiolone.
L'Arborasle, France, Institut Français de Recherches et Essais
Biologiques, 124 pp (Report No. MRID 0007-9375).
Morin M-F (1988) Study of the impact on the natural environment of
bromadiolone, an anticoagulant rodenticide: Evolution in aqueous
medium and bioaccumulation by terrestrial and aquatic organisms.
University of Poitiers (Thesis) ((Order No. 176).
Mosterd JJ & Thijssen HHW (1991) The long-term effects of the
rodenticide, brodifacoum, on blood coagulation and vitamin K
metabolism in rats. Br J Pharmacol, 104: 531-534.
Mount ME & Feldman BF (1983) Mechanism of diphacinone rodenticide
toxicosis in the dog and its therapeutic implications. Am J Vet Res,
44(11): 2009-2017.
Mount ME & Kass PH (1989) Diagnostic importance of vitamin K1 and
its epoxide measured in serum of dogs exposed to an anticoagulant
rodenticide. Am J Vet Res, 50: 1704-1709.
Mount ME, Feldman BF, & Buffington T (1982) Vitamin K and its
therapeutic importance. J Am Vet Med Assoc, 180(11): 1354-1356.
Murdoch DA (1983) Prolonged anticoagulation in chlorphacinone
poisoning. Lancet, 1: 355-356.
Murli H (1993) Mutagenicity test on bromadiolone, technical in an
in vivo mouse micronucleus assay (HWA Study No. 15310-0-455).
Washington, DC, Hazleton Laboratories.
Murphy MA & Nye DH (1991) Thoracic intramedullary haematoma as a
complication of warfarin: Case report and literature review. Aust N Z
J Surg, 61: 789-792.
Murphy MJ, Ray AC, & Bailey EM (1989) A high performance liquid
chromatography method for the detection of Brodifacoum in serum. Vet
Hum Toxicol, 31(3): 228-231.
Nehéz M, Kékes-Szabo A, Mazzag E, Selypes A, & Jarmay K (1985)
Histologic and cytogenetic effects of redentin on the spermiogenesis
and bone marrow cells of the mouse, an in vivo experiment. Regul
Toxicol Pharmacol, 5: 204-206.
Nelsestuen GL, Zytkovicz TH, & Howard JB (1974) The mode of action of
vitamin K: Identification of gamma-carboxyglutamic acid as a component
of prothrombin. J Biol Chem, 249: 6347-6350.
Newby S & White BG (1978) Brodifacoum: Adsorption and desorption in
soils measured under laboratory conditions. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division (Report
No TMJ 1764B).
Newton I, Wyllie I, & Freestone P (1990) Rodenticides in British barn
owls. Environ Pollut, 68: 101-117.
Newton I, Wyllie I, Gray A, Eadsforth CV (1994) The toxicity of the
rodenticide flocoumafen to barn owls and its elimination via pellets.
Pestic Sci, 41: 187-193.
Nighoghossian N, Ruel JH, French P, Froment JC, & Trouillas P (1990)
Hématome sous-dural cervico-dorsal par intoxication aux raticides
coumariniques. Rev Neurol (Paris), 146(3): 221-223.
Nilsson IM (1957) Recurrent hypoprothrombinaemia due to poisoning with
dicumarol-containing rat-killer. Acta Haematol, 17: 176-182.
O'Bryan SM & Constable DJC (1991) Quantification of brodifacoum in
plasma and liver tissue by HPLC. J Anal Toxicol, 15: 144-147.
O'Reilly RA, Aggeler PM, & Leong LS (1963) Studies on the coumarin
anticoagulant drugs: The pharmacodynamics of warfarin in man. J Clin
Invest, 4: 1542-1551.
O'Reilly RA, Aggeler PM, Haag MS, Leong LS, & Kropatkin ML (1964)
Hereditary transmission of exceptional resistance to coumarin
anticoagulant drugs. N Engl J Med, 271: 809-815.
Overman RS, Stahmann MA, Huebner CF, Sullivan WR, Spero L, Doherty DG,
Ikawa M, Graf L, Roseman S, & Link KP (1944) Studies on the
hemorrhagic sweet clover disease. XIII. Anticoagulant activity and
structure in the 4-hydroxycoumarin group. J Biol Chem, 153: 5-24.
Owren PA (1959) Thrombotest: A new method for controlling
anticoagulant therapy. Lancet, 2: 754-758.
Park BK & Leck JB (1982) A comparison of vitamin K antagonism by
warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol,
31(22): 3635-3639.
Park BK, Choonara IA, Haynes BP, Breckenridge AM, Malia RG, & Preston
FE (1986) Abnormal vitamin K metabolism in the presence of normal
clotting factor activity in factory workers exposed to
4-hydroxycoumarins. Br J Clin Pharmacol, 21: 289-294.
Parkinson GR (1975) WBA 8119: Acute oral toxicity. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 6 pp (Report No. CTL/P/216 (revised)).
Parkinson GR (1976) PP581: Acute oral toxicity to sheep. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 3 pp (Report No. CTL/P/259 (revised)).
Parkinson GR (1979) PP 581: Acute oral toxicity and skin
sensitization. Macclesfield, Cheshire, Imperial Chemical Industries
Ltd, Central Toxicology Laboratory, 3 pp (Report No. CTL/P/260
(revised)).
Parmar G, Bratt H, Moore R, & Batten PL (1987) Evidence for common
binding site in vivo for the retention of anticoagulants in rat
liver. Hum Toxicol, 6: 431-432.
Pastoureau P, Vergnaud P, Meunier PJ, & Delmas PD (1993) Ostopenia and
bone-remodeling abnormalities in warfarin-treated lambs. J Bone Miner
Res, 8: 1417-1426.
Pauli RM & Hall JG (1979) Warfarin embryopathy. Lancet, 2: 144.
Pearson N (1984) WL 108366 manufacturing master mix: Acute toxicity to
Salmo gairdneri and Daphnia magna. Sittingbourne, Shell Research Ltd
(Report No. SBGR.84.274).
Pearson N & Wallace BG (1984) Technical WL 108366: Acute toxicity to
Salmo gairdneri, Cyprinus carpio, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Kent, Shell Research Ltd, Sittingbourne
Research Centre (Report No. SBGR.84.211).
Pelfrène AF (1991) Synthetic organic rodenticides. In: Hayes WJ Jr &
Laws ER Jr ed. Handbook of pesticide toxicology. New York, Academic
Press, vol 3, pp 1271-1316.
Persson H (1994) [Annual report 1993.] Stockholm, Swedish Poison
Information Centre, 24 pp (in Swedish).
Pribilla O (1966) Murder caused by warfarin. Arch Toxicol,
21: 235-249.
Price JB (1985a) Toxicology of rodenticides: The acute oral toxicity
of WL 108366 in rabbits and hamsters. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.189).
Price JB (1985b) Toxicology of rodenticides: The percutaneous toxicity
of WL 108366 corn oil manufacturing master mix. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.84.227).
Price JB (1985c) Toxicology of rodenticides - WL 108366: A five day
range finding feeding study in rats. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.84.279).
Price JB (1985d) WL 108366: A 28-day feeding study in rats.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.84.235).
Price JB (1986) Toxicology of rodenticides ("Storm"): The skin
sensitizing potential of WL 108366. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.86.091).
Price PA, Williamson MK, Haba T, Dell RB, & Jee WS (1982) Excessive
mineralization with growth plate closure in rats on chronic warfarin
treatment. Proc Natl Acad Sci (USA), 79: 7734-7738.
Quick AJ (1935) The prothrombin in haemophilia and in obstructive
jaundice. J Biol Chem, 109: 73.
Radvanyi A, Weaver P, Massari C, Bid D, & Broughton E (1988) Effects
of chlorophacinone on captive kestrels. Bull Environ Contam Toxicol,
41: 441-448.
Rammell CG, Hoogenboom JJL, & Cotter M (1984) Brodifacoum residues in
target and non-target animals following rabbit poisoning trials. N Z J
Exp Agric, 12: 107-111.
Rauch AE, Weininger R, Pasquale D, Burkart PT, Dunn HG, Weissman C, &
Rydzak E (1994) Superwarfarin poisoning: A significant public health
problem. J Community Health, 19: 55-65.
Ray AC, Murphy MJ, DuVall MD, & Reagor JC (1989) Determination of
brodifacoum and bromadiolone residues in rodent and canine liver. Am J
Vet Res, 50(4): 546-550.
Redfern R, Gill JE, & Hadler MR (1976) Laboratory evaluation of WBA
8119 as a rodenticide for use against warfarin resistant and
non-resistant rats and mice. J Hyg Camb, 77: 419-426.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 16 November: 525-527.
Resch H, Pietschmann P, Krexner E, & Willvonseder R (1991) Decreased
peripheral bone mineral content in patients under anticoagulant
therapy with phenprocoumon. Eur Heart J, 12: 439-441.
Roberts NL, Cameron DM, & Street AE (1985a) WL 108366 - Acute oral
toxicity to pigs. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL/76/851108).
Roberts NL, Fairley C, & Baldwin MK (1985b) The acute oral toxicity
(LD50) of WL 108366 to the Mallard duck. Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Report No. SLL 67/BT/84925).
Roberts NL, Fairley C, & Baldwin MK (1985c) The acute oral toxicity of
WL 108366 to the Mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Centre (HRC Report No. SLL/73/BT/8572).
Robinson DS & MacDonald MG (1966) The effect of phenobarbital
administration on the control of coagulation achieved during warfarin
therapy in man. J Pharmacol Exp Ther, 153: 250-253.
Ross DB, Roberts NL, & Cameron DM (1978) The acute oral toxicity
(LD50) of PP581 to the Mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No ICI 122WL/78507).
Ross DB, Roberts NL, & Phillips C (1979) The subacute toxicity of
difenacoum (Ratak) to pigs. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No.
ICI 262/79697).
Ross DB, Roberts NL, & Fairley C (1980a) The subacute dietary toxicity
(LC50) of difenacoum to Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 305/791197).
Ross DB, Roberts NL, & Fairley C (1980b) The subacute dietary toxicity
(LC50) of difenacoum to the mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 304/791198).
Ross DB, Roberts NL & Fairley C (1980c) The acute oral toxicity
(LD50) of difenacoum to the Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 309/WL/8076).
Ross GS, Zacharski LR, Robert D, & Rabin DL (1992) An acquired
hemorrhagic disorder from long-acting rodenticide ingestion. Arch
Intern Med, 152: 410-412.
Schardein JL (1985) Anticoagulants. In: Chemically induced birth
defects. New York, Basel, Marcel Dekker Inc., pp 89-100.
Schulman A, Lusk R, Lippincott CL, & Ettinger SJ (1986)
Diphacinone-induced coagulopathy in the dog. J Am Vet Med Assoc,
188(4): 403-405.
Selypes A, Kékes-Szabo A, & Nehéz M (1984) Study of the mutagenic
effect of redentin on various species of animals. Acta Vet Hung,
32(3-4): 213-217.
Sharples R (1983a) The acute oral toxicity of a series of novel
anticoagulants in Wistar rats and C57BL/10 mice. Widnes, United
Kingdom, Sorex Ltd, Agricultural Research Department, 7 pp.
Sharples R (1983b) The acute oral toxicity of WL 108366 in
Dunkin-Hartley guinea pigs. Widnes, United Kingdom, Sorex Ltd,
Agricultural Research Department, 3 pp.
Sharples R (1984) The acute oral toxicity of WL 108366 in C57BL/10
mice. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 6 pp.
Sheen SR, Spiller HA, & Grossman D (1994) Symptomatic brodifacoum
ingestion requiring high-dose phytonadione therapy. Vet Hum Toxicol,
36(3): 216-217.
Sheldon T, Richardson CR, & Shaw J (1984) Product registration. An
evaluation of brodifacoum in the mouse micronucleus test. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.,
19 pp (Report No. CTL/P/1006).
Smith MF & Cameron MD (1979) Warfarin as teratogen. Lancet, 1: 727.
Smolinske SC, Scherger DL, Kearns PS, Wruk KM, Kulig KW, & Rumack BH
(1989) Superwarfarin poisoning in children: A prospective study.
Pediatrics, 84: 490-494.
Spare WC (1982) Aqueous photodegradation of 14C-bromadiolone.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Spare WC (1992) Hydrolysis of bromadiolone. Beltsville, Maryland,
Biospherics Inc. (Unpublished report submitted to WHO by Lipha S.A.).
Spare WC & Olson SB (1980) 14C-Bromadiolone soil leaching.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Sridhara S & Krishnamurthy TR (1992) Potentiation of anticoagulant
toxicity to Rattus rattus by two non-steroid anti-inflammatory drugs.
In: Borrecco JE & Marsh RE ed. Proceedings of the 15th Vertebrate Pest
Conference. Davis, California, University of California, pp 212-217.
Stenflo J, Fernlund P, Egan W, & Roepstorff P (1974) Vitamin K
dependent modifications of glutamic acid residues in prothrombin. Proc
Natl Acad Sci (USA), 71(7): 2730-2733.
Stowe CM, Metz AL, Arendt TD, & Schulman J (1983) Apparent brodifacoum
poisoning in a dog. J Am Vet Med Assoc, 182(8): 817-818.
Sullivan MP, Dean BS, & Krenzelok EO (1989) Long-acting anticoagulant
rodenticides: An evaluation of 88 cases. Vet Hum Toxicol, 31: 361.
Szulc P, Chapuy M-C, Meunier PJ, & Delmas PD (1993) Serum
undercarboxylated osteocalcin is a marker of the risk of hip fracture
in elderly women. J Clin Invest, 91: 1769-1774.
Thomson WT (1988) Agricultural chemicals. Book III - Miscellaneous
chemicals: Fumigants, growth regulators, repellents, and rodenticides
(1988/89 Revision). Fresno, California, Thomson Publications.
Tomlin C ed. (1994) The pesticide manual incorporating the
agrochemical handbook, 10th ed. Farnham, Surrey, The British Crop
Protection Council.
Townsend MG, Fletcher MR, Odam EM, & Stanley PI (1981) An assessment
of the secondary poisoning hazard of warfarin to tawny owls. J Wildl
Manage, 45(1): 242-248.
Townsend MG, Bunyan PJ, Odam EM, Stanley PI, & Wardall HP (1984)
Assessment of secondary poisoning hazard of warfarin to least weasels.
J Wildl Manage, 48(2): 628-632.
Travis SF, Warfield W, Greenbaum BH, Mobkisher M, & Siegel JE (1993)
Spontaneous hemorrhage associated with accidental brodifacoum
poisoning in a child. J Pediatr, 122: 982-984.
Tuinman CP & Van Sittert NJ (1985) Biomedical monitoring of personnel
in Sorex Ltd (Widnes, UK) involved in a formulation run with the
rodenticide WL 108366. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report No. HSE 85.006).
Ullrich V & Staundinger H (1968) Metabolism in vitro of warfarin by
enzymic and nonenzymic systems. Biochem Pharmacol, 17: 1663-1669.
Ungvary G (1994) Anticoagulant poisoning incidents in Hungary.
Budapest, National Institute of Occupational Health (Unpublished
report).
Van Haarlem LJM, Knapen MHJ, Hamulyák K, & Vermeer C (1988)
Circulating osteocalcin during oral anticoagulant therapy. Thromb
Haemost, 60: 79-82.
Veenstra GE, Owen DE, & Huckle KR (1991) Metabolic and toxicological
studies on the anticoagulant rodenticide, flocoumafen. Arch Toxicol,
14(Suppl): 160-165.
Vermeer C (1990) gamma-Carboxyglutamate-containing proteins and the
vitamin K-dependent carboxylase. Biochem J, 266: 625-636.
Vermeer C & Soute BAM (1992) Comparison of rodenticide anticoagulant
action in vitro and in vivo. Sittingbourne, Kent, Shell Research
Ltd (Report No. HSE/50).
Virat M (1981) LM-637-Bromadiolone: Teratology study in the rabbit by
oral route. L'Arborasle, France, Institut Français de Recherches et
Essais Biologiques (Report No. 106206).
Vogel JJ, de Moerloose Ph, Bouvier CA, Gaspoz J, & Riant P (1988)
Anticoagulation prolongée lors d'une intoxication à la chlorphacinone.
Schweiz Med Wochenschr, 118: 1915-1917.
Völkl S & Galicia H (1992) 14C-Bromadiolone: Degradation and
metabolism in soils incubated under aerobic conditions. Itingen,
Switzerland, Umweltchemie AG (Unpublished report submitted to WHO by
Lipha S.A., Lyon).
Wallace S, Paull P, Worsnop C, & Mashford ML (1990) Covert self
poisoning with brodifacoum, a "superwarfarin". Aust N Z J Med,
20: 713-715.
Warburton PA & Huckle KR (1986) WL 108366 - Fate of a single oral dose
of 14C WL 108366 in rats. Part III: Metabolism. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.294).
Warburton PA & Hutson DH (1985) WL 108366 - Fate of a single oral dose
of 14C-WL 108366 in rats. Part I: Elimination and retention of
radioactivity and effect of WL 108366 on prothrombin time.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.85.053).
Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, Murphy
MJ, & Furie B (1990) Surreptitious ingestion of a long-acting vitamin
K antagonist/rodenticide, brodifacoum: Clinical and metabolic studies
of three cases. Blood, 76(12): 2555-2559.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, 64 pp (WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II). Clin Chem,
33(11): 2074-2078.
Winn MJ, Clegg JAD, & Park BK (1987) An investigation of sex-linked
differences to the toxic and to the pharmacological action of
difenacoum: Studies in mice and rats. J Pharm Pharmacol, 39: 219-222.
Wiswesser WJ (1976) Pesticide index, 5th ed. College Park, Maryland,
Entomological Society of America.
Woody BJ, Murphy MJ, Ray AC, & Green RA (1992) Coagulopathic effects
and therapy of brodifacoum toxicosis in dogs. J Vet Intern Med,
6: 23-28.
Yu CC, Atallah YH, & Whitacre DM (1982) Metabolism and disposition of
diphacinone in rats and mice. Drug Metab Dispos, 10: 645-648.
RESUME
1. Généralités
Les anticoagulants qui sont décrits dans la présente monographie
sont ceux que l'on utilise principalement en agriculture et pour la
destruction des rongeurs en milieu urbain. La warfarine, premier
rodenticide anticoagulant à connaître une large utilisation, était à
l'origine un médicament efficace pour le traitement des
thromboembolies chez l'homme.
Selon leur structure chimique, les rodenticides anticoagulants
peuvent se répartir en deux catégories, les hydroxycoumarines et les
indanediones, mais leur mode d'action est analogue.
2. Propriétés et méthodes d'analyse
Les rodenticides anticoagulants se présentent sous la forme de
solides cristallins ou de poudres et sont légèrement solubles dans
l'eau. La plupart d'entre eux sont stables dans les conditions
normales de conservation.
La plupart des méthodes de dosage des rodenticides anticoagulants
reposent sur la chromatographie en phase liquide à haute performance.
3. Sources d'exposition humaine et environnementale
Les hydroxycoumarines de la première génération ont commencé à
être utilisées comme rodenticides vers la fin des années 1940. Par la
suite, l'apparition d'une résistance à la warfarine et aux autres
anticoagulants de la première génération a conduit à mettre au point
des produits de deuxième génération, plus actifs. La concentration du
principe actif dans les appâts varie selon l'efficacité du
rodenticide.
4. Distribution, concentration et exposition dans l'environnement
Les rodenticides anticoagulants sont principalement utilisés sous
la forme d'appâts. Comme ils sont peu volatils, leur concentration
dans l'air est négligeable. De même n'étant que légèrement solubles
dans l'eau, il est peu probable que leur utilisation conduise à la
contamination des eaux.
Etant donné que les rodenticides anticoagulants ne sont pas
destinés à être appliqués directement sur les cultures, il n'y a pas
lieu de s'attendre à en trouver sous forme de résidus dans les
aliments d'origine végétale.
L'exposition aux rodenticides des vertébrés non visés peut se
produire directement par la consommation d'appâts empoisonnés et
indirectement par celle de rongeurs contaminés. Les petits granulés
et les appâts constitués de grains entiers attirent fortement les
oiseaux.
La warfarine est utilisé dans le traitement des thromboembolies.
Il existe une possibilité d'exposition professionnelle aux
rodenticides anticoagulants lors de leur fabrication ou formulation ou
encore lors de la pose d'appâts empoisonnés, mais on ne dispose pas de
données sur l'ampleur de cette exposition.
5. Mode d'action et métabolisme
Les rodenticides anticoagulants sont des antagonistes de la
vitamine K. Leur action est principalement localisée dans le foie où
plusieurs précurseurs de l'hémostase subissent un processus
post-traductionnel dépendant de la vitamine K avant d'être convertis
en zymogènes procoagulants. L'action proprement dite consiste dans
l'inhibition de la K1-époxyde-réductase.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives et pourraient l'être également à travers
la peau et dans les vois respiratoires. Après administration par voie
orale, c'est principalement dans les matières fécales que ces composés
sont éliminés chez diverses espèces.
Il est possible que la décomposition métabolique de la warfarine
et des indanediones chez le rat s'effectue principalement par
l'intermédiaire d'une hydroxylation. En revanche, les anti-coagulants
de la deuxième génération s'éliminent essentiellement tels quels. Le
faible taux d'excrétion urinaire empêche d'isoler les métabolites de
l'urine.
Le foie est le principal organe où s'accumulent les rodenticides
anticoagulants. Cette accumulation a lieu également dans les
graisses.
6. Effets sur les mammifères et les systèmes d'épreuve in vitro
Chez le rat et la souris, les signes d'intoxication consistent en
une tendance accrue au saignement.
La DL50 est très variable, et c'est par la voie orale que les
composés sont les plus toxiques. La toxicité est également élevée par
la voie percutanée et par la voie respiratoire.
Certains anticoagulants présentent une toxicité aiguë du même
ordre pour les mammifères non visés que pour les rongeurs à détruire,
toutefois leur spectre toxique peut varier d'une espèce à l'autre.
Après administration répétée par voie orale à des rats, les
principaux effets observés sont ceux qui résultent de l'activité
anticoagulante.
On ne possède que peu de données sur les expositions répétées aux
anticoagulants chez les espèces n'appartenant pas à l'ordre des
rongeurs.
Une étude relative aux effets de la warfarine sur le rat a fait
ressortir une action sur le développement de ces animaux. En dehors
de cela, rien n'indique que les anticoagulants aient une action
tératogène sur les animaux d'expérience.
Rien n'indique non plus que les rodenticides anticoagulants
soient mutagènes, toutefois les données relatives aux différents
composés sont insuffisantes pour qu'on puisse en conclure à l'absence
de mutagénicité. La souche, le sexe et le régime alimentaire sont des
facteurs importants qui influent sur la toxicité des anticoagulants
chez les rongeurs. On a fait état d'épisodes d'intoxication chez des
animaux domestiques qui avaient consommé des appâts additionnés
d'anticoagulants. Lorsqu'il y a issue fatale ou du moins un tableau
clinique grave, c'est généralement qu'il y a eu consommation
d'anticoagulants de la deuxième génération. La principale différence
entre la warfarine et les autres anticoagulants (qu'il s'agisse des
indanediones ou des hydroxycoumarines de la deuxième génération),
c'est que ceux-ci persistent plus longtemps dans l'organisme et ont
par conséquent un effet plus durable que la warfarine. Dans ces
conditions, devant une intoxication, il faut poursuivre plus longtemps
l'administration de l'antidote, c'est-à-dire de la vitamine K1.
7. Effets sur l'homme
De nombreuses intoxications (qu'elles soient intentionnelles on
non) ont été signalées. Il y a eu également quelques cas
d'intoxication imputables à une exposition professionnelle. En cas
d'intoxication aiguë par des rodenticides anticoagulants, les
symptômes vont de l'accroissement de la tendance au saignement en cas
d'intoxication minime ou modérée, à une hémorragie massive dans les
cas plus graves. Les signes cliniques se manifestent un à plusieurs
jours après l'absorption.
Chez l'homme, on attribue certaines malformations congénitales à
des traitements par la warfarine pendant la période de grossesse.
Aucune malformation de ce type n'a été observée par suite de
l'utilisation d'anticoagulants comme rodenticides.
La concentration de la prothrombine plasmatique donne une
indication de la gravité de l'intoxication. Elle est plus sensible
que des épreuves globales telles que le temps de Quick. En cas
d'exposition professionnelle répétée, le dosage direct des traces de
carboxyprothrombine circulante ou du 2,3-époxyde de la vitamine K peut
permettre un bilan plus sensible.
Le traitement d'une intoxication par des anticoagulants doit être
adapté à la gravité de l'intoxication. Il est basé sur l'action
pharmacologique spécifique de la vitamine K1 que l'on administre par
voie parentérale avec, dans les cas graves, administration simultanée
de constituants sanguins. La mesure du temps de Quick permet
d'apprécier l'efficacité du traitement et de déterminer sa durée.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On peut répartir en deux catégories les effets possibles de
rodenticides anticoagulants sur les organismes non visés: les effets
directs (par consommation d'appâts) et les effets indirects (par
consommation de rongeurs empoisonnés).
Sous forme de produit technique, les anticoagulants sont
extrêmement toxiques pour les poissons. Incorporés à des appâts, il
est peu probable qu'ils présentent un danger, du fait de leur faible
solubilité dans l'eau. C'est pourquoi, à moins d'une erreur de
manipulation, ils ne devraient pas parvenir jusqu'aux poissons.
Les différentes espèces d'oiseaux sont d'une sensibilité variable
aux rodenticides anticoagulants. Il est difficile d'apprécier les
risques que représente, pour les oiseaux, la consommation directe
d'appâts car la plupart des travaux publiés sont des études
toxicologiques effectuées au laboratoire. Le caractère attractif des
appâts constitués de grains entiers pour les petits oiseaux en accroît
le danger dans la nature.
Des études de toxicité indirecte effectuées en laboratoire sur
des animaux sauvages ont montré que des prédateurs captifs pouvaient
s'intoxiquer si on leur donnait de la nourriture empoisonnée sans
autre choix ou des proies empoisonnées. On a également constaté la
mort de prédateurs dans le milieu naturel.
9. Evaluation et conclusion
Les rodenticides anticoagulants bloquent le mécanisme normal de
l'hémostase, d'où une tendance accrue au saignement qui peut déboucher
sur une forte hémorragie.
Il est peu probable que la population générale puisse être
exposée involontairement à des rodenticides anticoagulants.
Il peut y avoir une exposition non négligeable par suite de
contacts lors de l'activité professionnelle. Ces contacts peuvent se
produire au cours des opérations de production et de formulation aussi
bien que lors de la préparation et de la pose des appâts.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives ainsi qu'à travers la peau et les voies
respiratoires. C'est principalement dans le foie qu'ils sont retenus
et s'accumulent. La concentration de prothrombine plasmatique est un
bon indicateur de la gravité d'une intoxication aiguë et permet
d'avoir une idée de l'efficacité et de la durée nécessaire du
traitement.
L'antidote spécifique est la vitamine K1.
La principale différence entre les rodenticides anticoagulants de
première et de deuxième génération tient au fait que ces derniers
séjournent plus longtemps dans l'organisme et ont donc tendance à
prolonger l'effet hémorragique.
La plupart des anticoagulants sont stables dans les conditions
normales d'utilisation. Comme ils sont peu solubles dans l'eau et que
les appâts n'en contiennent qu'une faible quantité, il est peu
probable qu'ils puissent contaminer les étendues d'eau. Par ailleurs,
ils se fixent rapidement aux particules du sol, ne s'en désorbent que
très lentement et ne sont pas lessivés.
Aucun organisme non visé ne court de risque d'intoxication
directe par consommation d'appâts, ni de risque d'intoxication
indirecte par consommation de rongeurs contaminés.
RESUMEN
1. Generalidades
Los anticoagulantes descritos en esta monografía son los
utilizados principalmente en agricultura y en la lucha contra los
roedores urbanos. La warfarina, el primer rodenticida anticoagulante
de uso generalizado, se introdujo como agente eficaz para el
tratamiento de la tromboembolia en el ser humano.
De acuerdo con su estructura química, los rodenticidas
anticoagulantes pueden agruparse en dos categorías, las
hidroxicumarinas y las indandonas, aunque su mecanismo de acción es
similar.
2. Propiedades y métodos analíticos
Los rodenticidas anticoagulantes se presentan en forma cristalina
sólida o en polvo, y son ligeramente solubles en agua. La mayoría de
ellos son estables en condiciones de almacenamiento normales.
La mayoría de los procedimientos para la determinación de los
rodenticidas anticoagulantes se basan en cromatografía líquida de alta
resolución.
3. Fuentes de exposición humana y ambiental
Las hidroxicumarinas de primera generación se introdujeron como
rodenticidas a finales de los años cuarenta. La aparición de
resistencia a la warfarina y a otros anticoagulantes de primera
generación dio lugar a la elaboración de anticoagulantes más potentes,
de segunda generación. Las concentraciones de componentes activos en
los cebos varían en función de la eficacia de los rodenticidas.
4. Distribución, niveles y exposición ambientales
Los rodenticidas anticoagulantes se utilizan principalmente como
formulaciones para cebo. Dada su baja volatilidad, las
concentraciones en el aire son insignificantes. Como son muy poco
solubles en agua, es improbable que su uso sea fuente de contaminación
del agua.
Como los rodenticidas anticoagulantes no están pensados para su
aplicación directa a cosechas en pie, no son de prever residuos en
alimentos vegetales.
Los vertebrados no destinatarios están expuestos a los
rodenticidas principalmente por medio del consumo del cebo y de forma
secundaria por el consumo de roedores envenenados. Los cebos en
bolitas y de grano entero son muy atractivos para las aves.
La warfarina se utiliza como agente terapéutico para la
tromboembolia.
Hay un potencial de exposición ocupacional a los rodenticidas
anticoagulantes durante la fabricación, formulación y aplicación del
cebo, pero no se dispone de datos sobre los niveles de exposición.
5. Modo de acción y metabolismo
Los rodenticidas anticoagulantes son antagonistas de la vitamina
K. Su lugar principal de acción es el hígado, donde varios de los
precursores de la coagulación de la sangre sufren un procesamiento
post-traslación dependiente de la vitamina K antes de convertirse en
los zimógenos procoagulantes respectivos. Parece que el mecanismo de
acción es la inhibición de la reductasa epoxídica K1.
Los rodenticidas anticoagulantes se absorben fácilmente por el
tracto intestinal, y también pueden absorberse por la piel y el
sistema respiratorio. Tras la administración oral, la principal vía
de eliminación en diversas especies son las heces.
La degradación metabólica de la warfarina y las indandonas en
ratas es principalmente la hidroxilación. Sin embargo, los
anticoagulantes de segunda generación se eliminan principalmente como
compuestos inalterados. El bajo nivel de excreción urinaria impide
aislar los metabolitos a partir de la orina.
El hígado es el órgano principal para la acumulación y
almacenamiento de anticoagulantes rodenticidas. La acumulación
también tiene lugar en la grasa.
6. Efectos en los mamíferos y en los sistemas de prueba in vitro
Los signos de envenenamiento en ratas y ratones son los asociados
a una mayor tendencia a la hemorragia.
Hay una gran variación en la DL50 de los rodenticidas
anticoagulantes, siendo máxima la toxicidad por vía oral. También es
alta la toxicidad cutánea y por inhalación.
Los márgenes de toxicidad aguda de algunos anticoagulantes son
similares en el caso de los mamíferos no destinatarios y de los
roedores de destino, pero los espectros de toxicidad para los
anticoagulantes pueden variar entre las especies.
Tras una administración oral repetida en ratas, los principales
efectos observados son los asociados a la acción anticoagulante.
Se dispone de pocos datos sobre la exposición repetida de
especies distintas de los roedores.
Un estudio sobre la warfarina en ratas ha indicado efectos sobre
el desarrollo. Por lo demás, no hay pruebas convincentes de que los
anticoagulantes sean teratogénicos en animales de experimentación.
No hay prueba que sugiera que los rodenticidas anticoagulantes
son mutagénicos, pero se dispone de datos insuficientes sobre los
compuestos individuales para demostrar la inexistencia de
mutagenicidad. La sobrecarga, el sexo y la alimentación son
importantes factores modificadores de la toxicidad de los
anticoagulantes en los roedores.
Se han dado casos de envenenamiento de animales domésticos tras
la ingestión de cebos anticoagulantes. Las muertes y los síndromes
clínicos graves se deben por lo general a anticoagulantes de segunda
generación. La diferencia principal entre la warfarina y los demás
anticoagulantes (tanto las indandonas como las hidroxicumarinas de
segunda generación) es que éstos tienen mayor tiempo de retención en
el organismo y por consiguiente un efecto más prolongado que la
warfarina. Por ello, en los casos de envenenamiento, el tratamiento
antídoto con vitamina K1 debe proseguir durante un periodo más
largo.
7. Efectos en el ser humano
Se han notificado muchos casos de envenenamiento (tanto
intencionados como no intencionados). También se han producido unos
pocos casos de intoxicación por exposición ocupacional a los
anticoagulantes. Los síntomas de intoxicación aguda por rodenticidas
anticoagulantes van desde una mayor tendencia a la hemorragia en el
envenenamiento leve o moderado a una hemorragia masiva en casos más
graves. Los signos de envenenamiento aparecen con un retraso de uno a
varios días después de la absorción.
La warfarina va asociada en el ser humano a la producción de
malformaciones del desarrollo cuando se toma como agente terapéutico
durante el embarazo. No se han notificado casos de defectos de
desarrollo tras el uso de anticoagulantes como rodenticidas.
La concentración de protrombina plasmática orienta sobre la
gravedad de la intoxicación. Es una indicación más sensible que
pruebas generales como el tiempo de protrombina. En la exposición
ocupacional repetida, la medición directa de cantidades ínfimas de
descarboxiprotrombina en circulación o de 2,3-epóxido de vitamina K en
circulación pueden constituir una evaluación más sensible.
El tratamiento del envenenamiento con anticoagulantes se gradúa
de acuerdo con la gravedad de la intoxicación. El tratamiento
farmacológico específico consiste en la administración parenteral de
vitamina K1 con administración simultánea, en los casos graves, de
componentes sanguíneos. La medición del tiempo de protrombina ayuda a
determinar la eficacia y la duración de tratamiento necesaria.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los posibles efectos de los rodenticidas en organismos no
destinatarios pueden dividirse en dos categorías: primarios
(envenenamiento directo por consumo de cebo) y secundario (por consumo
de roedores envenenados).
En la forma del producto técnico, los anticoagulantes son muy
tóxicos para los peces. Como formulaciones para cebo es improbable
que planteen riesgos en razón de su baja solubilidad en agua. Por
esta razón, a menos que se utilicen de forma indebida, no están al
alcance de los peces.
La susceptibilidad de las aves a los rodenticidas anticoagulantes
es variable. Es difícil evaluar los riesgos para las aves que entraña
el consumo directo porque la mayoría de los estudios publicados
consisten en ensayos de toxicidad en condiciones de laboratorio. El
atractivo del cebo de grano integral para las aves pequeñas aumenta el
riesgo en las condiciones de campo.
Los estudios en laboratorio de la toxicidad secundaria con la
fauna silvestre han mostrado que los predadores cautivos pueden
intoxicarse mediante alimentación sin otra elección con presas que se
han envenenado con anticoagulante o a las que se ha administrado este
producto. Se tiene noticias de algunas muertes de predadores en su
medio natural.
9. Evaluación y conclusión
Los rodenticidas anticoagulantes perturban los mecanismos
normales de coagulación de la sangre, determinando una mayor tendencia
a la hemorragia y, por último, una hemorragia abundante.
La exposición no intencionada de la población general a los
rodenticidas anticoagulantes es improbable.
El contacto ocupacional es una fuente potencial de exposición
significativa. Puede tener lugar durante la elaboración y
formulación, así como durante la preparación y aplicación del cebo.
Los compuestos de rodenticida anticoagulante se absorben
fácilmente por el tracto intestinal, y por la piel y el sistema
respiratorio. El hígado es el órgano principal de acumulación y
almacenamiento. La concentración de protrombina plasmática es una
buena orientación de la gravedad de la intoxicación aguda y de la
eficacia y la duración necesaria de la terapia.
El antídoto específico es la vitamina K1.
La principal diferencia entre los rodenticidas anticoagulantes de
la primera generación y los de la segunda es que éstos últimos tienen
una mayor retención en el organismo y por ello suelen dar lugar a un
periodo de hemorragia más prolongado.
La mayoría de los anticoagulantes son estables en condiciones de
uso normal. Su baja solubilidad en agua y baja concentración en los
cebos hace improbable que sean una fuente de contaminación del agua.
Parece que se asocian rápidamente a las partículas del suelo, con
desorción muy lenta y nula propiedad de lixiviación.
Los organismos no destinatarios pueden correr al riesgo de
consumir directamente cebos (riesgo primario) y de ingerir roedores
envenenados (riesgo secundario).
 When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT
RODENTICIDES
Members
Dr N. Gratz, Commugny, Switzerland
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr W. Jacobs, Office of Pesticide Programs, US Environmental
Protection Agency, Washington, USA
Mrs M. Palmborg, Swedish Poison Information Centre, Stockholm, Sweden
Dr A.F. Pelfrène, Technology Sciences Group (TSG) International Inc.,
Brussels, Belgium (Chairman)
Mr D. Renshaw, Health Aspects of Environment and Food (Medical),
Department of Health, London, United Kingdom
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (Rapporteur)
Dr C. Vermeer, University of Limburg, Maastricht, Netherlands
Observers
Dr A. Buckle, ZENECA Public Health, Haslemere, Surrey, United Kingdom
(Representative of GIFAP)
Dr Y. Cohet, Lipha SA, Lyon, France (Representative of GIFAP)
Secretariat
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT RODENTICIDES
A WHO Task Group on Environmental Health Criteria for
Anticoagulant Rodenticides met in Geneva from 14 to 18 November 1994.
Dr R. Plestina, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO).
The first draft was prepared by Dr M. Tasheva of the National
Centre of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria.
The second draft was prepared by Dr R. Plestina, incorporating
comments received following the circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs. The
Task Group reviewed and revised the draft document and made an
evaluation of risks for human health and the environment from exposure
to anticoagulant rodenticides. Dr R. Plestina and Dr P.G. Jenkins,
both members of the IPCS Central Unit, were responsible for the
overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AAPCC American Association of Poison Control Centers
DT50 degradation time for 50% of a compound
EC50 median effect concentration
FD fluorescence detection
GC gas chromatography
HPLC high-performance liquid chromatography
I50 concentration of an inhibitor causing 50% inhibition of an
enzyme under given conditions
IUPAC International Union of Pure and Applied Chemistry Kal
adsorption coefficient
LD50 median lethal dose
MS mass spectrometry
MTD maximum tolerated dose
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PT prothrombin time
PTT partial thromboplastin time
WISN warfarin-induced skin necrosis
INTRODUCTION
The anticoagulants included in this review are those that are
used as rodenticides. The development of coumarin anticoagulants
occurred during the Second World War and they were introduced as
effective antithrombotic agents for treatment of thromboembolic
disease in humans. Warfarin has been used both as a drug and a
rodenticide, and has been extensively evaluated. Several
hydroxycoumarin and indandione derivatives have been synthesized and
introduced as effective rodenticides. They act by interfering with
the blood coagulation mechanism.
The appearance of rat strains resistant to warfarin and some
other anticoagulants has stimulated the development of more potent,
second-generation anticoagulants, some of which are also "single dose"
anticoagulants or "superwarfarins".
Many anticoagulant rodenticides are known, but it is not the aim
of this monograph to include all available information on each
compound. The purpose is to describe the general characteristics of
anticoagulants, using suitable illustrations to indicate their impact
on humans and the environment.
A distinction needs to be made between the characteristics of the
technical compounds and those of their formulated products concerning
the risks that their use poses to human health and the environment.
1. SUMMARY
1.1 General
The anticoagulants described in this monograph are those used
mainly in agriculture and urban rodent control. Warfarin, the first
widely used anticoagulant rodenticide, was introduced as an effective
agent for treatment of thromboembolic disease in humans.
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories, hydroxycoumarins and indandiones,
although their mechanisms of action are similar.
1.2 Properties and analytical methods
Anticoagulant rodenticides come in a solid crystalline or powder
form, and are slightly soluble in water. Most of them are stable
under normal storage conditions.
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography.
1.3 Sources of human and environmental exposure
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
first-generation anticoagulants led to the development of more potent,
second-generation anticoagulants. The concentrations of active
ingredients in baits vary according to the efficacy of the
rodenticides.
1.4 Environmental distribution, levels and exposures
Anticoagulant rodenticides are used mainly as bait formulations.
Since their volatility is low, concentrations in the air will be
negligible. As they are only slightly soluble in water, their use is
unlikely to be a source of water contamination.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant foodstuffs are
expected.
Non-target vertebrates are exposed to rodenticides primarily
through consumption of bait and secondarily from consumption of
poisoned rodents. Small pellets and whole grain baits are highly
attractive to birds.
Warfarin is used as a therapeutic agent for thromboembolic
disease.
There is a potential for occupational exposure to anticoagulant
rodenticides during manufacture, formulation and bait application, but
data on the levels of exposure are not available.
1.5 Mode of action and metabolism
Anticoagulant rodenticides are vitamin K antagonists. The main
site of their action is the liver, where several of the blood
coagulation precursors undergo vitamin-K-dependent posttranslation
processing before they are converted into the respective procoagulant
zymogens. The point of action appears to be the inhibition of K1
epoxide reductase.
Anticoagulant rodenticides are easily absorbed from the
gastrointestinal tract, and may also be absorbed through the skin and
respiratory system. After oral administration, the major route of
elimination in various species is through the faeces.
The metabolic degradation of warfarin and indandiones in rats
mainly involves hydroxylation. However, the second-generation
anticoagulants are mainly eliminated as unchanged compounds. The low
urinary excretion precludes isolation of metabolites from the urine.
The liver is the main organ for accumulation and storage of
rodenticide anticoagulants. Accumulation also occurs in the fat.
1.6 Effects on mammals and in vitro test systems
Signs of poisoning in rats and mice are those associated with
increased bleeding tendency.
There is wide variation in the LD50 of anticoagulant
rodenticides, toxicity being greatest by the oral route. Dermal and
inhalation toxicities of anticoagulants are also high.
Some anticoagulants show a similar range of acute toxicity for
non-target mammals as for target rodents, but toxicity spectra for
anticoagulants may vary between species.
Following repeated oral administration in rats, the main effects
seen are those associated with the anticoagulant action.
There are few data available on repeated exposure of non-rodent
species.
One study on warfarin in rats has indicated developmental
effects. Otherwise, there is no convincing evidence that
anticoagulants are teratogenic in experimental animals.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on individual compounds to demonstrate an absence of mutagenicity.
Strain, sex and diet are important factors modifying the toxicity of
anticoagulants in rodents.
Poisoning incidents in domestic animals after consumption of
anticoagulant baits have been reported. Fatalities and severe
clinical syndromes are generally due to the second-generation
anticoagulants. The major difference between warfarin and the other
anticoagulants (both indandiones and second-generation
hydroxycoumarins) is that they have a longer retention time in the
body and consequently a more prolonged effect than warfarin.
Therefore in cases of poisoning, antidote treatment with vitamin K1
needs to be continued for a longer period.
1.7 Effects on humans
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxications from occupational
exposure to anticoagulants have also occurred. Symptoms of acute
intoxication by anticoagulant rodenticides range from increased
bleeding tendency in minor or moderate poisoning to massive
haemorrhage in more severe cases. The signs of poisoning develop with
a delay of one to several days after absorption.
Warfarin is associated in humans with the induction of
developmental malformations when taken as a therapeutic agent during
pregnancy. No cases of developmental defects following the use of
anticoagulants as rodenticides have been reported.
The plasma prothrombin concentration is one guide to the severity
of intoxication. This is a more sensitive indication than overall
tests such as prothrombin time. In repeated occupational exposure,
direct measurement of either trace amounts of circulating
descarboxyprothrombin or circulating vitamin K 2,3-epoxide may provide
a more sensitive assessment.
Treatment of anticoagulant poisoning is graded according to the
severity of intoxication. Specific pharmacological treatment consists
of parenteral administration of vitamin K1 with, in serious cases,
co-administration of blood components. Measurement of prothrombin
time helps to determine the effectiveness and required duration of
treatment.
1.8 Effects on other organisms in the laboratory and field
The possible effects of anticoagulant rodenticides on non-target
organisms can be considered to fall into two categories: primary
(direct poisoning through consumption of bait) and secondary (through
consumption of poisoned rodents).
In the form of the technical product, anticoagulants are highly
toxic to fish. As bait formulations they are unlikely to present any
hazard because of their low water solubility. For this reason, they
will not be available to fish unless misused.
Bird species vary in their susceptibility to anticoagulant
rodenticides. It is difficult to assess the risks to birds resulting
from direct consumption because most published studies consist of
toxicity trials in laboratory conditions. The attractiveness of whole
grain bait to small birds increases the risk in field conditions.
Secondary toxicity laboratory studies with wildlife have shown
that captive predators can be intoxicated by no-choice feeding with
anticoagulant-poisoned or -dosed prey. Some deaths of predators in
the field have been reported.
1.9 Evaluation and conclusion
Anticoagulant rodenticides disrupt the normal blood-clotting
mechanisms, resulting in increased bleeding tendency and,
eventually, profuse haemorrhage.
Unintentional exposure of the general population to anticoagulant
rodenticides is unlikely.
Occupational contact is a potential source of significant
exposure. It may occur during manufacture and formulation as well as
during bait preparation and application.
Anticoagulant rodenticide compounds are readily absorbed from the
gastrointestinal tract, and through the skin and respiratory system.
The liver is the major organ for accumulation and storage. The plasma
prothrombin concentration is a suitable guide to the severity of acute
intoxication and to the effectiveness and required duration of the
therapy.
The specific antidote is vitamin K1.
The major difference between first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention and therefore tend to lead to a longer period of bleeding.
Most anticoagulants are stable under conditions of normal use.
Their low water solubility and low concentration in baits make them
unlikely to be a source of water contamination. They also appear to
bind quickly to soil particles, with very slow desorption and no
leaching properties.
Non-target organisms are potentially at risk from direct
consumption of baits (primary hazard) and from eating poisoned rodents
(secondary hazard).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories:
* hydroxycoumarins:
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT
RODENTICIDES
Members
Dr N. Gratz, Commugny, Switzerland
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr W. Jacobs, Office of Pesticide Programs, US Environmental
Protection Agency, Washington, USA
Mrs M. Palmborg, Swedish Poison Information Centre, Stockholm, Sweden
Dr A.F. Pelfrène, Technology Sciences Group (TSG) International Inc.,
Brussels, Belgium (Chairman)
Mr D. Renshaw, Health Aspects of Environment and Food (Medical),
Department of Health, London, United Kingdom
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (Rapporteur)
Dr C. Vermeer, University of Limburg, Maastricht, Netherlands
Observers
Dr A. Buckle, ZENECA Public Health, Haslemere, Surrey, United Kingdom
(Representative of GIFAP)
Dr Y. Cohet, Lipha SA, Lyon, France (Representative of GIFAP)
Secretariat
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ANTICOAGULANT RODENTICIDES
A WHO Task Group on Environmental Health Criteria for
Anticoagulant Rodenticides met in Geneva from 14 to 18 November 1994.
Dr R. Plestina, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO).
The first draft was prepared by Dr M. Tasheva of the National
Centre of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria.
The second draft was prepared by Dr R. Plestina, incorporating
comments received following the circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs. The
Task Group reviewed and revised the draft document and made an
evaluation of risks for human health and the environment from exposure
to anticoagulant rodenticides. Dr R. Plestina and Dr P.G. Jenkins,
both members of the IPCS Central Unit, were responsible for the
overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AAPCC American Association of Poison Control Centers
DT50 degradation time for 50% of a compound
EC50 median effect concentration
FD fluorescence detection
GC gas chromatography
HPLC high-performance liquid chromatography
I50 concentration of an inhibitor causing 50% inhibition of an
enzyme under given conditions
IUPAC International Union of Pure and Applied Chemistry Kal
adsorption coefficient
LD50 median lethal dose
MS mass spectrometry
MTD maximum tolerated dose
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PT prothrombin time
PTT partial thromboplastin time
WISN warfarin-induced skin necrosis
INTRODUCTION
The anticoagulants included in this review are those that are
used as rodenticides. The development of coumarin anticoagulants
occurred during the Second World War and they were introduced as
effective antithrombotic agents for treatment of thromboembolic
disease in humans. Warfarin has been used both as a drug and a
rodenticide, and has been extensively evaluated. Several
hydroxycoumarin and indandione derivatives have been synthesized and
introduced as effective rodenticides. They act by interfering with
the blood coagulation mechanism.
The appearance of rat strains resistant to warfarin and some
other anticoagulants has stimulated the development of more potent,
second-generation anticoagulants, some of which are also "single dose"
anticoagulants or "superwarfarins".
Many anticoagulant rodenticides are known, but it is not the aim
of this monograph to include all available information on each
compound. The purpose is to describe the general characteristics of
anticoagulants, using suitable illustrations to indicate their impact
on humans and the environment.
A distinction needs to be made between the characteristics of the
technical compounds and those of their formulated products concerning
the risks that their use poses to human health and the environment.
1. SUMMARY
1.1 General
The anticoagulants described in this monograph are those used
mainly in agriculture and urban rodent control. Warfarin, the first
widely used anticoagulant rodenticide, was introduced as an effective
agent for treatment of thromboembolic disease in humans.
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories, hydroxycoumarins and indandiones,
although their mechanisms of action are similar.
1.2 Properties and analytical methods
Anticoagulant rodenticides come in a solid crystalline or powder
form, and are slightly soluble in water. Most of them are stable
under normal storage conditions.
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography.
1.3 Sources of human and environmental exposure
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
first-generation anticoagulants led to the development of more potent,
second-generation anticoagulants. The concentrations of active
ingredients in baits vary according to the efficacy of the
rodenticides.
1.4 Environmental distribution, levels and exposures
Anticoagulant rodenticides are used mainly as bait formulations.
Since their volatility is low, concentrations in the air will be
negligible. As they are only slightly soluble in water, their use is
unlikely to be a source of water contamination.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant foodstuffs are
expected.
Non-target vertebrates are exposed to rodenticides primarily
through consumption of bait and secondarily from consumption of
poisoned rodents. Small pellets and whole grain baits are highly
attractive to birds.
Warfarin is used as a therapeutic agent for thromboembolic
disease.
There is a potential for occupational exposure to anticoagulant
rodenticides during manufacture, formulation and bait application, but
data on the levels of exposure are not available.
1.5 Mode of action and metabolism
Anticoagulant rodenticides are vitamin K antagonists. The main
site of their action is the liver, where several of the blood
coagulation precursors undergo vitamin-K-dependent posttranslation
processing before they are converted into the respective procoagulant
zymogens. The point of action appears to be the inhibition of K1
epoxide reductase.
Anticoagulant rodenticides are easily absorbed from the
gastrointestinal tract, and may also be absorbed through the skin and
respiratory system. After oral administration, the major route of
elimination in various species is through the faeces.
The metabolic degradation of warfarin and indandiones in rats
mainly involves hydroxylation. However, the second-generation
anticoagulants are mainly eliminated as unchanged compounds. The low
urinary excretion precludes isolation of metabolites from the urine.
The liver is the main organ for accumulation and storage of
rodenticide anticoagulants. Accumulation also occurs in the fat.
1.6 Effects on mammals and in vitro test systems
Signs of poisoning in rats and mice are those associated with
increased bleeding tendency.
There is wide variation in the LD50 of anticoagulant
rodenticides, toxicity being greatest by the oral route. Dermal and
inhalation toxicities of anticoagulants are also high.
Some anticoagulants show a similar range of acute toxicity for
non-target mammals as for target rodents, but toxicity spectra for
anticoagulants may vary between species.
Following repeated oral administration in rats, the main effects
seen are those associated with the anticoagulant action.
There are few data available on repeated exposure of non-rodent
species.
One study on warfarin in rats has indicated developmental
effects. Otherwise, there is no convincing evidence that
anticoagulants are teratogenic in experimental animals.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on individual compounds to demonstrate an absence of mutagenicity.
Strain, sex and diet are important factors modifying the toxicity of
anticoagulants in rodents.
Poisoning incidents in domestic animals after consumption of
anticoagulant baits have been reported. Fatalities and severe
clinical syndromes are generally due to the second-generation
anticoagulants. The major difference between warfarin and the other
anticoagulants (both indandiones and second-generation
hydroxycoumarins) is that they have a longer retention time in the
body and consequently a more prolonged effect than warfarin.
Therefore in cases of poisoning, antidote treatment with vitamin K1
needs to be continued for a longer period.
1.7 Effects on humans
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxications from occupational
exposure to anticoagulants have also occurred. Symptoms of acute
intoxication by anticoagulant rodenticides range from increased
bleeding tendency in minor or moderate poisoning to massive
haemorrhage in more severe cases. The signs of poisoning develop with
a delay of one to several days after absorption.
Warfarin is associated in humans with the induction of
developmental malformations when taken as a therapeutic agent during
pregnancy. No cases of developmental defects following the use of
anticoagulants as rodenticides have been reported.
The plasma prothrombin concentration is one guide to the severity
of intoxication. This is a more sensitive indication than overall
tests such as prothrombin time. In repeated occupational exposure,
direct measurement of either trace amounts of circulating
descarboxyprothrombin or circulating vitamin K 2,3-epoxide may provide
a more sensitive assessment.
Treatment of anticoagulant poisoning is graded according to the
severity of intoxication. Specific pharmacological treatment consists
of parenteral administration of vitamin K1 with, in serious cases,
co-administration of blood components. Measurement of prothrombin
time helps to determine the effectiveness and required duration of
treatment.
1.8 Effects on other organisms in the laboratory and field
The possible effects of anticoagulant rodenticides on non-target
organisms can be considered to fall into two categories: primary
(direct poisoning through consumption of bait) and secondary (through
consumption of poisoned rodents).
In the form of the technical product, anticoagulants are highly
toxic to fish. As bait formulations they are unlikely to present any
hazard because of their low water solubility. For this reason, they
will not be available to fish unless misused.
Bird species vary in their susceptibility to anticoagulant
rodenticides. It is difficult to assess the risks to birds resulting
from direct consumption because most published studies consist of
toxicity trials in laboratory conditions. The attractiveness of whole
grain bait to small birds increases the risk in field conditions.
Secondary toxicity laboratory studies with wildlife have shown
that captive predators can be intoxicated by no-choice feeding with
anticoagulant-poisoned or -dosed prey. Some deaths of predators in
the field have been reported.
1.9 Evaluation and conclusion
Anticoagulant rodenticides disrupt the normal blood-clotting
mechanisms, resulting in increased bleeding tendency and,
eventually, profuse haemorrhage.
Unintentional exposure of the general population to anticoagulant
rodenticides is unlikely.
Occupational contact is a potential source of significant
exposure. It may occur during manufacture and formulation as well as
during bait preparation and application.
Anticoagulant rodenticide compounds are readily absorbed from the
gastrointestinal tract, and through the skin and respiratory system.
The liver is the major organ for accumulation and storage. The plasma
prothrombin concentration is a suitable guide to the severity of acute
intoxication and to the effectiveness and required duration of the
therapy.
The specific antidote is vitamin K1.
The major difference between first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention and therefore tend to lead to a longer period of bleeding.
Most anticoagulants are stable under conditions of normal use.
Their low water solubility and low concentration in baits make them
unlikely to be a source of water contamination. They also appear to
bind quickly to soil particles, with very slow desorption and no
leaching properties.
Non-target organisms are potentially at risk from direct
consumption of baits (primary hazard) and from eating poisoned rodents
(secondary hazard).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Based on their chemical structure, anticoagulant rodenticides may
be grouped into two categories:
* hydroxycoumarins:
 * indandiones:
* indandiones:
 The common and chemical names of the rodenticides are given in
Table 1. Trade names, chemical structures, RTECS and CAS numbers,
molecular formulae and relative molecular masses are listed in
Table 2.
2.2 Physical and chemical properties
Anticoagulant rodenticides are solids (crystalline or powders),
slightly soluble in water (Table 3) and readily soluble in acetone.
Most of them are stable under normal storage conditions.
Table 1. Identity of anticoagulant rodenticides
Common name CAS name IUPAC name
First generation hydroxycoumarins
Coumachlor 3-[1-(4-chlorophenyl)-3 oxobutyl]-4-hydroxy- 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumafuryl 3-[1-(2-furanyl)-3 oxobutyl]-4-hydroxy- 3-[1-(2-furyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumatetralyl 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthalenyl)- 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthyl) coumarin
2H-1-benzopyran-2-one
Warfarin 4-hydroxy-3-(3-oxo-1-phenylbutyl-2H-1-benzopyran-2-one (RS)4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin
Second generation hydroxycoumarins
Brodifacoum 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[3-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-
1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 1-naphthyl]-4-hydroxycoumarin
Bromadiolone 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1- 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-
phenylpropyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difenacoum 3-[3-(1,1'-biphenyl)-4-yl-1,2,3,4-tetrahydro-1- 3-(3-biphenyl-4-yl-1,2,3,4-tetrahydro-1-naphthyl)-
naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difethialone 3-[3-(4-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[1RS,3RS;1RS,3SR)-3-(4'-bromobiphenyl-4-yl)-1,2,3,4-
1-naphthalenyl]-4-hydroxy-2H-1-benzothiopyran-2-one tetrahydro-1-naphthyl]-4-hydroxy-1-benzothi-in-2-one
Flocoumafen 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-[-(trifluoromethyl) 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-
phenyl]methoxy]phenyl-1-naphthalenyl]-2H-1-benzopyran-2-one (4-trifluoromethylbenzyloxy)phenyl]-1-naphthyl] coumarin
Table 1 (contd).
Common name CAS name IUPAC name
Indandione derivatives
Chlorophacinone 2-[(4-chlorophenyl)phenylacetyl]-1H-indene-1,3 (2H)-dione 2-[2-(4-chlorophenyl)-2-phenylacetyl]indan-1,3-dione
Diphacinone 2-(diphenylacetyl)-1H-indene-1,3 (2H)-dione 2-(diphenylacetyl)indan-1,3-dione
Pindone 2-(2,2-dimethyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-pivaloylindan-1,3-dione
Valone 2-(3-methyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-isovaleryl-1,3-indandione
Table 2. Names, structures and identification details
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Brodifacoum Finale GN4934750
The common and chemical names of the rodenticides are given in
Table 1. Trade names, chemical structures, RTECS and CAS numbers,
molecular formulae and relative molecular masses are listed in
Table 2.
2.2 Physical and chemical properties
Anticoagulant rodenticides are solids (crystalline or powders),
slightly soluble in water (Table 3) and readily soluble in acetone.
Most of them are stable under normal storage conditions.
Table 1. Identity of anticoagulant rodenticides
Common name CAS name IUPAC name
First generation hydroxycoumarins
Coumachlor 3-[1-(4-chlorophenyl)-3 oxobutyl]-4-hydroxy- 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumafuryl 3-[1-(2-furanyl)-3 oxobutyl]-4-hydroxy- 3-[1-(2-furyl)-3-oxobutyl]-4-hydroxycoumarin
2H-1-benzopyran-2-one
Coumatetralyl 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthalenyl)- 4-hydroxy-3-(1,2,3,4-tetrahydro-1-naphthyl) coumarin
2H-1-benzopyran-2-one
Warfarin 4-hydroxy-3-(3-oxo-1-phenylbutyl-2H-1-benzopyran-2-one (RS)4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin
Second generation hydroxycoumarins
Brodifacoum 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[3-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-
1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 1-naphthyl]-4-hydroxycoumarin
Bromadiolone 3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1- 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-
phenylpropyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difenacoum 3-[3-(1,1'-biphenyl)-4-yl-1,2,3,4-tetrahydro-1- 3-(3-biphenyl-4-yl-1,2,3,4-tetrahydro-1-naphthyl)-
naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one 4-hydroxycoumarin
Difethialone 3-[3-(4-bromo-[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro- 3-[1RS,3RS;1RS,3SR)-3-(4'-bromobiphenyl-4-yl)-1,2,3,4-
1-naphthalenyl]-4-hydroxy-2H-1-benzothiopyran-2-one tetrahydro-1-naphthyl]-4-hydroxy-1-benzothi-in-2-one
Flocoumafen 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-[-(trifluoromethyl) 4-hydroxy-3-[1,2,3,4-tetrahydro-3-[4-
phenyl]methoxy]phenyl-1-naphthalenyl]-2H-1-benzopyran-2-one (4-trifluoromethylbenzyloxy)phenyl]-1-naphthyl] coumarin
Table 1 (contd).
Common name CAS name IUPAC name
Indandione derivatives
Chlorophacinone 2-[(4-chlorophenyl)phenylacetyl]-1H-indene-1,3 (2H)-dione 2-[2-(4-chlorophenyl)-2-phenylacetyl]indan-1,3-dione
Diphacinone 2-(diphenylacetyl)-1H-indene-1,3 (2H)-dione 2-(diphenylacetyl)indan-1,3-dione
Pindone 2-(2,2-dimethyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-pivaloylindan-1,3-dione
Valone 2-(3-methyl-1-oxopropyl)-1H-indene-1,3 (2H)-dione 2-isovaleryl-1,3-indandione
Table 2. Names, structures and identification details
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Brodifacoum Finale GN4934750  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Bromadiolone Apobas, Bromard, GN4934700
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Bromadiolone Apobas, Bromard, GN4934700  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Chlorophacinone Caid NK5335000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Chlorophacinone Caid NK5335000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumachlor Ratilan GN4830000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumachlor Ratilan GN4830000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumafuryl Fumarin GN4850000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumafuryl Fumarin GN4850000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumatetralyl Racumin GN7630000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Coumatetralyl Racumin GN7630000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difenacoum Compo GN4934500
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difenacoum Compo GN4934500  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difethialone Baraki DM0013800
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Difethialone Baraki DM0013800  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Diphacinone Diphacine NK5600000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Diphacinone Diphacine NK5600000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Flocoumafen Stratagem DJ3100300
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Flocoumafen Stratagem DJ3100300  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Pindone Pivaldione NK6300000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Pindone Pivaldione NK6300000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Valone Motomco trading NK5775000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Valone Motomco trading NK5775000  Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Warfarin Arthrombine-K GN4550000
Table 2 (cont'd)
Common name Trade/other Chemical structure RTECS CAS Molecular Relative molecular
names number number formula mass
Warfarin Arthrombine-K GN4550000  Table 3. Water solubility and vapour pressure of various anticoagulant rodenticides
Rodenticide Solubility Vapour pressure
in water (mg/litre) at temperature (°C) at pH mPa at temperature (°C)
Brodifacoum < 10 20 7 < 0.13 25
Bromadiolone 19 20 0.002 20
Chlorophacinone 100 20 negligible 20
Coumachlor 0.5 20 4.5 < 10 20
Coumatetralyl 4 20 4.2 8.5 × 10-6 20
20 20 5
425 20 7
Difenacoum < 10 20 7 0.16 45
Difethialone 0.39 25 0.074 25
Diphacinone 0.3 13.7 × 10-6 25
Flocoumafen 1.1 22 0.133 × 10-6 25
Pindone 18 25 very low 25
Warfarin practically insoluble
2.3 Analytical methods
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography
(Hunter, 1983; Hoogenboom & Rammell, 1983; Murphy et al., 1989;
O'Bryan & Constable, 1991; Chalermchaikit et al., 1993; Kelly et al.,
1993).
Warfarin is an acid which, in its hydrogenated form, is
practically insoluble in distilled water. At neutral or higher pH,
however, it is ionized and as such it readily dissolves in water. In
addition, compounds contaminating the water (such as proteins or
detergents) may substantially increase the solubility of warfarin.
Hunter (1983) developed a multi-residue method for the
determination of warfarin, coumatetralyl, bromadiolone, difenacoum and
brodifacoum in animal tissues by high-performance liquid
chromatography with fluorescence detection. A chloroformacetone (1:1)
mixture was significantly better than chloroform for the extraction of
residues of these rodenticides from liver tissues. Detection limits
in animal tissues of 2 µg/kg for coumatetralyl, difenacoum and
brodifacoum, 10 µg/kg for bromadiolone, and 20 µg/kg for warfarin
could be routinely achieved.
Felice et al. (1991) developed a reversed-phase liquid
chromatographic method with fluorescence detection for multicomponent
determination of the above-mentioned five rodenticides in blood serum
with detection limits of 10 to 20 ng/ml. Acetonitrile was used for
the extraction.
Braselton et al. (1992) developed a special method for confirming
the presence of indandione rodenticides (diphacinone and
chlorophacinone) in intoxicated domestic animals by using mass
spectrometry/mass spectrometry with collision-activated dissociation.
More details of analytical methods for individual rodenticides are
given in Table 4.
Table 4. Methods for the determination of anticoagulant rodenticides
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Animal tissues Chloroform-acetone (1:1) HPLC/FD 2 µg/kg coumatetralyl, difenacoum, Hunter (1983)
brodifacoum
10 µg/kg bromadiolone Hunter (1983)
20 µg/kg warfarin Hunter (1983)
Animal tissues Chloroform-acetone (1:1) HPLC/FDa 10 µg/kg warfarin Hunter (1985)
2 µg/kg other rodenticides Hunter (1985)
Serum Acetonitrile and diethyl ether HPLC 10 µg/litre brodifacoum, coumatetralyl, Felice et al. (1991)
difenacoum
20 µg/litre bromadiolone, warfarin Felice et al. (1981)
Serum Acetonitrile and diethyl ether HPLC 1 µg/litre brodifacoum Felice & Murphy (1989)
Serum twice with diethyl ether and HPLC/FD 3 µg/litre brodifacoum Murphy et al. (1989)
twice with acetonitrile-ether
(1:1)
Table 4 (contd).
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Plasma Acetonitrile-ethyl ether (9:1) HPLC/FD 2 µg/litre brodifacoum; no interference O'Bryan & Constable
with bromadiolone, (1991)
Liver tissue 5 µg/kg coumarin, difenacoum,
diphacinone, warfarin
and vitamin K1
Liver tissue Chloroform and acetone GC/MS 60 µg/kg protocol did not differentiate Ray et al. (1989)
between brodifacoum and
bromadiolone
a Post-column pH-switching fluorescence detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Anticoagulant rodenticides do not occur naturally in the
environment, although some plants do contain coumarinic derivatives.
Huebner & Link (1941), Overman et al. (1944) and Alstad et al. (1985)
described the anticoagulant properties of dicumarol found in spoiled
sweet clover and in connection with haemorrhagic disease in cattle.
3.2 Anthropogenic sources
Anticoagulant rodenticides are used worldwide, but figures for
the total world production are not available.
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
early anticoagulant rodenticides stimulated the development of
second-generation anticoagulants. About 95% of all commensal rodent
control in the USA is carried out with anticoagulants (Marsh, 1985a).
More than 50% of rodenticides used by professional pest controllers in
the USA contain brodifacoum (Dubock, 1986).
Depending on the toxicity of the rodenticide, the concentration
of the active ingredient varies from 0.005 to 0.05% for indandiones
and second-generation hydroxycoumarins and from 0.025 to 0.05% for
first-generation anticoagulants.
Anticoagulant rodenticides are available in a variety of
different formulations, including paraffin wax blocks, whole grain
baits, pelleted baits and tracking powder (FAO, 1979). Baits are the
most widely used formulations for rodent control.
Some manufacturers have added bittering agents, such as Bitrex
(denatonium benzoate), to anticoagulant baits. According to Kaukeinen
& Buckle (1992), adult humans found wax-block and pelleted placebo
baits containing denatonium benzoate (10 mg/kg) to be unpalatable.
However, the concentration of Bitrex cannot be increased to levels
that would make baits unpalatable to target rodents, and there is no
evidence that concentrations of Bitrex that target rodents readily
accept will deter bait-eating by non-target animals or by children
under 14 months of age.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air, water and soil
Since anticoagulant rodenticides are generally used as bait
formulations and have low volatility, increased levels in the air are
unlikely. As mentioned in section 2.2, most anticoagulants are
slightly soluble in water and therefore their use is unlikely to be a
source of water pollution.
Newby & White (1978) studied the adsorption and desorption of
14C-brodifacoum in soil under laboratory conditions. Adsorption
coefficients (kd) for course sand (pH 6.6), sandy clay loam (pH 7.1)
and calcareous sandy loam (pH 7.6) were 625, 1320 and 1180,
respectively, indicating strong adsorption to soil particles.
Adsorption equilibria were established fairly rapidly with the large
water:soil ratios used and despite very low brodifacoum water
solubility. Desorption was reported to be very slow and much less
than that required for a reversible interaction.
Lewis (1992b) applied 14C-difenacoum at 0.2 mg/kg (dry weight)
to a sandy soil with low humous content. After 142 days of incubation
(the approximate half-life of difenacoum in this soil type), two soil
samples were transferred to the top of soil columns. The columns were
eluted with deionized water at a rate and amount equivalent to
approximately 200 mm of rain falling onto the soil surface area
(91.6 cm2) for 50 h. The percentages of applied radioactivity
present in the leachates were 0.41 and 0.47%, representing only a very
small amount of leaching under these test conditions.
The leaching characteristics of aged soil residues of 14C-
brodifacoum in four soil types were investigated. 14C-
Brodifacoum was applied to soil at a nominal application rate of
0.4 mg/kg and incubated under aerobic conditions for 30 days. Samples
were taken and transferred to soil columns. After leaching, most of
the radioactivity applied to the soil was recovered in the top segment
of each column. No detectable levels of 14C residues were found in
the leachates. The results indicated that 14C-brodifacoum was
effectively immobile in all the soils tested (Jackson & Hall, 1992).
A study was carried out with 14C-bromadiolone in four types of
soil. With a soil rich in clay and organic compounds, bromadiolone
stayed in the superficial layer and scarcely moved. However, in soil
poor in clay and organic compounds, 67% of the added bromadiolone was
eluted (Spare et al., 1980).
4.1.2 Vegetation and wildlife
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant food stuffs are
expected. Unlike conventional crop protection products, which must be
applied over relatively large crop areas, rodenticides are applied to
discrete sites in the form of low concentration baits. Even if the
bait is spilled, it will not be taken up by plants.
Small pellets and whole grain baits are highly attractive to
birds and other non-target vertebrates. The formulation in wax blocks
consequently decreases the risk of primary poisoning of non-target
species.
Rodenticides may present a risk not only of primary poisoning
(from direct consumption of the bait) but also of secondary poisoning
(from consumption of poisoned rodents), in spite of the fact that many
of the target rodents die below ground in their burrows (Gorenzel et
al., 1982). Commensal and wild rodents poisoned by anticoagulants may
lead to the death of cats, pigs, foxes and birds of prey. The risk of
secondary poisoning depends mainly on the extent to which predators
feed on the target animals (Dubock, 1986).
4.2 Transformation
4.2.1 Biodegradation
Coveney & Forbes (1987) studied the degradation of flocoumafen in
rat carcasses, rat faeces, loose grain and wax block baits placed on
small soil plots. Overall losses of flocoumafen ranged from 85% to
95% over the 12-month study. The majority of the rodenticide present
in samples collected after 4 months was found in the upper 15 cm of
the soil. Only very small quantities were found in the lower soil
layers.
The degradation of 14C-difenacoum was studied in two standard
soils under controlled conditions for a period of 108 days.
Degradation time (DT50) values for the two soils were 146 and 439
days, indicating that difenacoum is a relatively long-lived compound
in soils (Lewis, 1992a).
Hall & Priestley (1992) monitored the metabolism of 14C-
brodifacoum in soil under aerobic conditions after applying it
at a nominal rate of 0.4 mg/kg and incubating for up to 52 weeks. A
mean total of 35.8% of the applied radioactivity was recovered as
14CO2 within the test period. 14C-Brodifacoum was the major
radiolabelled component in the soil extracts throughout the 52 weeks.
Under the conditions of the study the half-life of brodifacoum was
calculated to be 157 days.
A study was carried out with 14C-bromadiolone in four types of
soil. The rodenticide was degraded significantly with half-lives
ranging from 1.8 to 7.4 days (Wölkl & Galicia, 1992).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
A photolysis study was carried out with 14C-bromadiolone
(1 mg/litre) in a solution at pH 7.3 (Spare, 1982). The rodenticide
was very quickly degraded by exposure to artificial sunlight with a
half-life of 2.1 h.
The photolytic stability of 14C-difenacoum was investigated in
sterile buffered aqueous solutions of pH 5, 7 and 9 over a 24-h
irradiation period. The photolytic half-lives for total difenacoum
were calculated to be 3.26, 8.05 and 7.32 h at pH 5, 7 and 9,
respectively (Hall et al., 1992).
4.2.2.2 Hydrolysis
Lewis (1992c) studied the stability of 14C-difenacoum in
sterile buffered aqueous solutions of pH 5, 7 and 9. No hydrolysis
was observed at pH 5, at pH 7 there was very slow hydrolysis
(half-life estimated to be 847-1332 days), and at pH 9 the half-life
was estimated to be 77-85 days.
Jackson et al. (1991) studied the hydrolytic stability of
14C-brodifacoum (0.04 mg/kg) in sterile buffered aqueous solutions
at pH 5, 7 and 9 over a 30-day period. The hydrolytic half-life of
brodifacoum at pH 7 and 9 was found to be much greater than 30 days,
but precise calculation was not possible because the degradation seen
after one day did not continue.
Spare (1992) demonstrated that 14C-bromadiolone was slowly
hydrolysed in pH 5 buffer, with an estimated half-life of 392 days.
No degradation was observed at pH 7 and 9.
In the absence of a co-solvent, bromadiolone has a half-life of
67 days at pH 7 and 20°C (Morin, 1988). Degradation is more
significant in the presence of H3O+ ions, in saline water and at
increased temperatures.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
There is no information available on concentrations of
anticoagulant rodenticides in air, water and soil.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plants are expected.
Residues of difenacoum and brodifacoum were detected in the
bodies of 15 out of a total of 145 dead barn owls (Tylo alba) received
from various parts of the United Kingdom during the period 1983-1989.
Levels of difenacoum were in the range of 0.005-0.106 mg/kg body
weight, whilst levels of brodifacoum were in the range
0.019-0.515 mg/kg body weight (Newton et al., 1990).
Merson & Byers (1984) analysed eastern screech owl (Otus asio)
pellets following the application of 0.001% brodifacoum to an orchard
for rodent control. The brodifacoum residues in pellet samples ranged
from 0.06 to 0.09 mg/kg, indicating some exposure of the birds.
Hegdal & Colvin (1988) analysed screech owl tissues up to 52 days
after application of brodifacoum in an orchard. Brodifacoum was
detected in livers (detection limit = 0.3 mg/kg) from 9 out of 16
birds, the concentrations ranging from 0.3 to 0.8 mg/kg. No
detectable residues were found in the remainder of the carcasses
(detection limit = 0.1 mg/kg).
Hegdal & Blaskiewicz (1984) sampled six barn owls of different
ages in the vicinity of farm buildings treated with brodifacoum.
Analysis of carcasses revealed only one with trace (< 0.05 mg/kg)
levels of brodifacoum; the other carcasses did not contain detectable
concentrations.
Brodifacoum residues in the liver, muscle and fatty tissue of
rabbits poisoned during field trials with bait containing 0.005%
active ingredient were 4.4, 0.26 and 0.86 mg/kg, respectively. During
the same field trials, brodifacoum residues in seven poisoned birds of
various species ranged from 0.12 to 8.1 mg/kg in the liver, < 0.05 to
0.14 mg/kg in muscle and < 0.05 to 0.25 mg/kg in fatty tissue (Rammel
et al., 1984).
5.2 General population exposure
As mentioned in the previous section, residues are unlikely to be
found in plant foods. The use of dry baits to protect grain stores
can result in contamination of the stored food. Although on average
the concentration of residues would be expected to be low, occasional
areas of high concentration can occur.
With respect to residues in animals used for human food (pigs,
sheep and birds), there are no residue data concerning animals that
have survived anticoagulant poisoning. It should be emphasized,
however, that in some countries rodents are used as food.
Warfarin is widely used as a therapeutic agent.
5.3 Occupational exposure
Exposure may occur during manufacture, formulation and bait
application. The available information is discussed in section 8.2.
6. MODE OF ACTION AND METABOLISM
6.1 Vitamin K and its antagonists
Vitamin K is a collective name for a number of related compounds,
which all may function as co-enzymes for the enzyme gamma-glutamate
carboxylase. They all contain the functional naphthoquinone ring
structure, but differ in their aliphatic side chains. Vitamin K1
(phytomenadione) contains a side chain composed of four isoprenoid
residues, one of which is unsaturated. The vitamin K2 compounds
(menaquinones) have side chains which vary from 1 to 13 isoprenoid
residues, all of which are unsaturated. They are generally referred
to as MK-n, where n is the number of isoprenoid residues. Vitamin
K3 (menadione) has no side chain, but upon ingestion it is converted
into MK-4 by a liver enzyme. The two products commercially available
for human use are K1 and MK-4. Both are equally active, but for some
reason K1 is almost exclusively used in Europe and North America,
whereas MK-4 (also known as menatetrenone) is used in Asia, notably
Japan. K3 is not used any more for humans because of its adverse
side effect, haemolysis, but is frequently added to animal food.
Both 4-hydroxycoumarin derivatives and indandiones (also known as
oral anticoagulants) are antagonists of vitamin K. Their use as
rodenticides is based on the inhibition of the vitamin K-dependent
step in the synthesis of a number of blood coagulation factors. The
vitamin K-dependent proteins involved in the coagulation cascade
(Fig. 1) are the procoagulant factors II (prothrombin), VII
(proconvertin), IX (Christmas factor) and X (Stuart-Prower factor),
and the coagulation-inhibiting proteins C and S. All these proteins
are synthesized in the liver. Before they are released into the
circulation the various precursor proteins undergo substantial
(intracellular) post-translational modification. Vitamin K functions
as a co-enzyme in one of these modifications, namely the carboxylation
at well-defined positions of 10-12 glutamate residues into
gamma-carboxyglutamate (Gla). The presence of these Gla residues is
essential for the procoagulant activity of the various coagulations
factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its
oxidation to vitamin K 2,3-epoxide (KO) provides the energy required
for the carboxylation reaction. The epoxide is than recycled in two
reduction steps mediated by the enzyme KO reductase (Fig. 2). The
latter enzyme is the target enzyme for coumarin anticoagulants. Their
blocking of the KO reductase leads to a rapid exhaustion of the supply
of KH2, and thus to an effective prevention of the formation of Gla
residues. This leads to an accumulation of non-carboxylated
coagulation factor precursors in the liver. In some cases these
precursors are processed further without being carboxylated, and
(depending on the species) may appear in the circulation. At that
stage the under-carboxylated proteins are designated as descarboxy
coagulation factors (Stenflo et al., 1974; Nelsestuen et al., 1974).
Normal coagulation factors circulate in the form of zymogens, which
can only participate in the coagulation cascade after being activated
by limited proteolytic degradation (see Fig. 1). Descarboxy
coagulation factors have no procoagulant activity (i.e. they cannot be
activated) and neither they can be converted into the active zymogens
by vitamin K action. Whereas in anticoagulated humans high levels of
circulating descarboxy coagulation factors are detectable, these
levels are negligible in warfarin-treated rats and mice. Reviews by
Vermeer (1990) and Furie & Furie (1990) give further details.
Leck & Park (1981) compared the effects of warfarin and
brodifacoum on vitamin K metabolism and blood-clotting factor activity
in warfarin-susceptible and warfarin-resistant rats. In
warfarin-susceptible rats both brodifacoum and warfarin induced a
significant increase in the circulating KO (measured as the KO/K ratio
using 3H-vitamin K1), indicating that KO reductase is the target
enzyme for both drugs. However, whereas warfarin (1 mg/kg) only
inhibited the KO reductase in the susceptible strain, brodifacoum
(1 mg/kg) produced the same decrease of plasma prothrombin
concentration in both warfarin-susceptible and warfarin-resistant
animals.
The KO/K ratio in warfarin-resistant rats is five times higher
than in warfarin-susceptible animals. This is explained by the fact
that the hepatic KO reductase in the resistant animals has not only a
reduced affinity for warfarin, but also for KO. Hence the vitamin K
requirement of warfarin-resistant animals is 5-10 times higher than
that of warfarin-susceptible ones. Second-generation anticoagulants,
if given in doses which cause anticoagulation, further increase the
KO/K ratio (Leck & Park, 1981).
The much stronger potency of difenacoum and brodifacoum, as
vitamin K-antagonists, was reported by Park & Leck (1982), who
concluded that in the case of poisoning with these second-generation
anticoagulants it will be necessary to give repeated and frequent
doses of vitamin K to maintain clotting factor synthesis. The potency
of second-generation anticoagulants can be partly explained by their
highly lipophilic nature, which enables them to bind strongly to
membranes. Their target enzyme KO reductase is an integral membrane
protein with, in addition, a highly lipophilic nature. It is to be
expected that the dissociation of enzyme/inhibitor complexes will be
extremely slow. Moreover, their effectiveness in warfarin-resistant
rats demonstrates that the mutation leading to warfarin resistance
does not significantly affect their interaction with the KO reductase.
Table 3. Water solubility and vapour pressure of various anticoagulant rodenticides
Rodenticide Solubility Vapour pressure
in water (mg/litre) at temperature (°C) at pH mPa at temperature (°C)
Brodifacoum < 10 20 7 < 0.13 25
Bromadiolone 19 20 0.002 20
Chlorophacinone 100 20 negligible 20
Coumachlor 0.5 20 4.5 < 10 20
Coumatetralyl 4 20 4.2 8.5 × 10-6 20
20 20 5
425 20 7
Difenacoum < 10 20 7 0.16 45
Difethialone 0.39 25 0.074 25
Diphacinone 0.3 13.7 × 10-6 25
Flocoumafen 1.1 22 0.133 × 10-6 25
Pindone 18 25 very low 25
Warfarin practically insoluble
2.3 Analytical methods
Most of the procedures for the determination of anticoagulant
rodenticides are based on high-performance liquid chromatography
(Hunter, 1983; Hoogenboom & Rammell, 1983; Murphy et al., 1989;
O'Bryan & Constable, 1991; Chalermchaikit et al., 1993; Kelly et al.,
1993).
Warfarin is an acid which, in its hydrogenated form, is
practically insoluble in distilled water. At neutral or higher pH,
however, it is ionized and as such it readily dissolves in water. In
addition, compounds contaminating the water (such as proteins or
detergents) may substantially increase the solubility of warfarin.
Hunter (1983) developed a multi-residue method for the
determination of warfarin, coumatetralyl, bromadiolone, difenacoum and
brodifacoum in animal tissues by high-performance liquid
chromatography with fluorescence detection. A chloroformacetone (1:1)
mixture was significantly better than chloroform for the extraction of
residues of these rodenticides from liver tissues. Detection limits
in animal tissues of 2 µg/kg for coumatetralyl, difenacoum and
brodifacoum, 10 µg/kg for bromadiolone, and 20 µg/kg for warfarin
could be routinely achieved.
Felice et al. (1991) developed a reversed-phase liquid
chromatographic method with fluorescence detection for multicomponent
determination of the above-mentioned five rodenticides in blood serum
with detection limits of 10 to 20 ng/ml. Acetonitrile was used for
the extraction.
Braselton et al. (1992) developed a special method for confirming
the presence of indandione rodenticides (diphacinone and
chlorophacinone) in intoxicated domestic animals by using mass
spectrometry/mass spectrometry with collision-activated dissociation.
More details of analytical methods for individual rodenticides are
given in Table 4.
Table 4. Methods for the determination of anticoagulant rodenticides
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Animal tissues Chloroform-acetone (1:1) HPLC/FD 2 µg/kg coumatetralyl, difenacoum, Hunter (1983)
brodifacoum
10 µg/kg bromadiolone Hunter (1983)
20 µg/kg warfarin Hunter (1983)
Animal tissues Chloroform-acetone (1:1) HPLC/FDa 10 µg/kg warfarin Hunter (1985)
2 µg/kg other rodenticides Hunter (1985)
Serum Acetonitrile and diethyl ether HPLC 10 µg/litre brodifacoum, coumatetralyl, Felice et al. (1991)
difenacoum
20 µg/litre bromadiolone, warfarin Felice et al. (1981)
Serum Acetonitrile and diethyl ether HPLC 1 µg/litre brodifacoum Felice & Murphy (1989)
Serum twice with diethyl ether and HPLC/FD 3 µg/litre brodifacoum Murphy et al. (1989)
twice with acetonitrile-ether
(1:1)
Table 4 (contd).
Sample type Extraction Analytical Limit of Rodenticide Reference
method detection
Plasma Acetonitrile-ethyl ether (9:1) HPLC/FD 2 µg/litre brodifacoum; no interference O'Bryan & Constable
with bromadiolone, (1991)
Liver tissue 5 µg/kg coumarin, difenacoum,
diphacinone, warfarin
and vitamin K1
Liver tissue Chloroform and acetone GC/MS 60 µg/kg protocol did not differentiate Ray et al. (1989)
between brodifacoum and
bromadiolone
a Post-column pH-switching fluorescence detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Anticoagulant rodenticides do not occur naturally in the
environment, although some plants do contain coumarinic derivatives.
Huebner & Link (1941), Overman et al. (1944) and Alstad et al. (1985)
described the anticoagulant properties of dicumarol found in spoiled
sweet clover and in connection with haemorrhagic disease in cattle.
3.2 Anthropogenic sources
Anticoagulant rodenticides are used worldwide, but figures for
the total world production are not available.
First-generation hydroxycoumarins were introduced as rodenticides
in the late 1940s. The appearance of resistance to warfarin and other
early anticoagulant rodenticides stimulated the development of
second-generation anticoagulants. About 95% of all commensal rodent
control in the USA is carried out with anticoagulants (Marsh, 1985a).
More than 50% of rodenticides used by professional pest controllers in
the USA contain brodifacoum (Dubock, 1986).
Depending on the toxicity of the rodenticide, the concentration
of the active ingredient varies from 0.005 to 0.05% for indandiones
and second-generation hydroxycoumarins and from 0.025 to 0.05% for
first-generation anticoagulants.
Anticoagulant rodenticides are available in a variety of
different formulations, including paraffin wax blocks, whole grain
baits, pelleted baits and tracking powder (FAO, 1979). Baits are the
most widely used formulations for rodent control.
Some manufacturers have added bittering agents, such as Bitrex
(denatonium benzoate), to anticoagulant baits. According to Kaukeinen
& Buckle (1992), adult humans found wax-block and pelleted placebo
baits containing denatonium benzoate (10 mg/kg) to be unpalatable.
However, the concentration of Bitrex cannot be increased to levels
that would make baits unpalatable to target rodents, and there is no
evidence that concentrations of Bitrex that target rodents readily
accept will deter bait-eating by non-target animals or by children
under 14 months of age.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air, water and soil
Since anticoagulant rodenticides are generally used as bait
formulations and have low volatility, increased levels in the air are
unlikely. As mentioned in section 2.2, most anticoagulants are
slightly soluble in water and therefore their use is unlikely to be a
source of water pollution.
Newby & White (1978) studied the adsorption and desorption of
14C-brodifacoum in soil under laboratory conditions. Adsorption
coefficients (kd) for course sand (pH 6.6), sandy clay loam (pH 7.1)
and calcareous sandy loam (pH 7.6) were 625, 1320 and 1180,
respectively, indicating strong adsorption to soil particles.
Adsorption equilibria were established fairly rapidly with the large
water:soil ratios used and despite very low brodifacoum water
solubility. Desorption was reported to be very slow and much less
than that required for a reversible interaction.
Lewis (1992b) applied 14C-difenacoum at 0.2 mg/kg (dry weight)
to a sandy soil with low humous content. After 142 days of incubation
(the approximate half-life of difenacoum in this soil type), two soil
samples were transferred to the top of soil columns. The columns were
eluted with deionized water at a rate and amount equivalent to
approximately 200 mm of rain falling onto the soil surface area
(91.6 cm2) for 50 h. The percentages of applied radioactivity
present in the leachates were 0.41 and 0.47%, representing only a very
small amount of leaching under these test conditions.
The leaching characteristics of aged soil residues of 14C-
brodifacoum in four soil types were investigated. 14C-
Brodifacoum was applied to soil at a nominal application rate of
0.4 mg/kg and incubated under aerobic conditions for 30 days. Samples
were taken and transferred to soil columns. After leaching, most of
the radioactivity applied to the soil was recovered in the top segment
of each column. No detectable levels of 14C residues were found in
the leachates. The results indicated that 14C-brodifacoum was
effectively immobile in all the soils tested (Jackson & Hall, 1992).
A study was carried out with 14C-bromadiolone in four types of
soil. With a soil rich in clay and organic compounds, bromadiolone
stayed in the superficial layer and scarcely moved. However, in soil
poor in clay and organic compounds, 67% of the added bromadiolone was
eluted (Spare et al., 1980).
4.1.2 Vegetation and wildlife
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plant food stuffs are
expected. Unlike conventional crop protection products, which must be
applied over relatively large crop areas, rodenticides are applied to
discrete sites in the form of low concentration baits. Even if the
bait is spilled, it will not be taken up by plants.
Small pellets and whole grain baits are highly attractive to
birds and other non-target vertebrates. The formulation in wax blocks
consequently decreases the risk of primary poisoning of non-target
species.
Rodenticides may present a risk not only of primary poisoning
(from direct consumption of the bait) but also of secondary poisoning
(from consumption of poisoned rodents), in spite of the fact that many
of the target rodents die below ground in their burrows (Gorenzel et
al., 1982). Commensal and wild rodents poisoned by anticoagulants may
lead to the death of cats, pigs, foxes and birds of prey. The risk of
secondary poisoning depends mainly on the extent to which predators
feed on the target animals (Dubock, 1986).
4.2 Transformation
4.2.1 Biodegradation
Coveney & Forbes (1987) studied the degradation of flocoumafen in
rat carcasses, rat faeces, loose grain and wax block baits placed on
small soil plots. Overall losses of flocoumafen ranged from 85% to
95% over the 12-month study. The majority of the rodenticide present
in samples collected after 4 months was found in the upper 15 cm of
the soil. Only very small quantities were found in the lower soil
layers.
The degradation of 14C-difenacoum was studied in two standard
soils under controlled conditions for a period of 108 days.
Degradation time (DT50) values for the two soils were 146 and 439
days, indicating that difenacoum is a relatively long-lived compound
in soils (Lewis, 1992a).
Hall & Priestley (1992) monitored the metabolism of 14C-
brodifacoum in soil under aerobic conditions after applying it
at a nominal rate of 0.4 mg/kg and incubating for up to 52 weeks. A
mean total of 35.8% of the applied radioactivity was recovered as
14CO2 within the test period. 14C-Brodifacoum was the major
radiolabelled component in the soil extracts throughout the 52 weeks.
Under the conditions of the study the half-life of brodifacoum was
calculated to be 157 days.
A study was carried out with 14C-bromadiolone in four types of
soil. The rodenticide was degraded significantly with half-lives
ranging from 1.8 to 7.4 days (Wölkl & Galicia, 1992).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
A photolysis study was carried out with 14C-bromadiolone
(1 mg/litre) in a solution at pH 7.3 (Spare, 1982). The rodenticide
was very quickly degraded by exposure to artificial sunlight with a
half-life of 2.1 h.
The photolytic stability of 14C-difenacoum was investigated in
sterile buffered aqueous solutions of pH 5, 7 and 9 over a 24-h
irradiation period. The photolytic half-lives for total difenacoum
were calculated to be 3.26, 8.05 and 7.32 h at pH 5, 7 and 9,
respectively (Hall et al., 1992).
4.2.2.2 Hydrolysis
Lewis (1992c) studied the stability of 14C-difenacoum in
sterile buffered aqueous solutions of pH 5, 7 and 9. No hydrolysis
was observed at pH 5, at pH 7 there was very slow hydrolysis
(half-life estimated to be 847-1332 days), and at pH 9 the half-life
was estimated to be 77-85 days.
Jackson et al. (1991) studied the hydrolytic stability of
14C-brodifacoum (0.04 mg/kg) in sterile buffered aqueous solutions
at pH 5, 7 and 9 over a 30-day period. The hydrolytic half-life of
brodifacoum at pH 7 and 9 was found to be much greater than 30 days,
but precise calculation was not possible because the degradation seen
after one day did not continue.
Spare (1992) demonstrated that 14C-bromadiolone was slowly
hydrolysed in pH 5 buffer, with an estimated half-life of 392 days.
No degradation was observed at pH 7 and 9.
In the absence of a co-solvent, bromadiolone has a half-life of
67 days at pH 7 and 20°C (Morin, 1988). Degradation is more
significant in the presence of H3O+ ions, in saline water and at
increased temperatures.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
There is no information available on concentrations of
anticoagulant rodenticides in air, water and soil.
Since anticoagulant rodenticides are not intended for direct
application to growing crops, no residues in plants are expected.
Residues of difenacoum and brodifacoum were detected in the
bodies of 15 out of a total of 145 dead barn owls (Tylo alba) received
from various parts of the United Kingdom during the period 1983-1989.
Levels of difenacoum were in the range of 0.005-0.106 mg/kg body
weight, whilst levels of brodifacoum were in the range
0.019-0.515 mg/kg body weight (Newton et al., 1990).
Merson & Byers (1984) analysed eastern screech owl (Otus asio)
pellets following the application of 0.001% brodifacoum to an orchard
for rodent control. The brodifacoum residues in pellet samples ranged
from 0.06 to 0.09 mg/kg, indicating some exposure of the birds.
Hegdal & Colvin (1988) analysed screech owl tissues up to 52 days
after application of brodifacoum in an orchard. Brodifacoum was
detected in livers (detection limit = 0.3 mg/kg) from 9 out of 16
birds, the concentrations ranging from 0.3 to 0.8 mg/kg. No
detectable residues were found in the remainder of the carcasses
(detection limit = 0.1 mg/kg).
Hegdal & Blaskiewicz (1984) sampled six barn owls of different
ages in the vicinity of farm buildings treated with brodifacoum.
Analysis of carcasses revealed only one with trace (< 0.05 mg/kg)
levels of brodifacoum; the other carcasses did not contain detectable
concentrations.
Brodifacoum residues in the liver, muscle and fatty tissue of
rabbits poisoned during field trials with bait containing 0.005%
active ingredient were 4.4, 0.26 and 0.86 mg/kg, respectively. During
the same field trials, brodifacoum residues in seven poisoned birds of
various species ranged from 0.12 to 8.1 mg/kg in the liver, < 0.05 to
0.14 mg/kg in muscle and < 0.05 to 0.25 mg/kg in fatty tissue (Rammel
et al., 1984).
5.2 General population exposure
As mentioned in the previous section, residues are unlikely to be
found in plant foods. The use of dry baits to protect grain stores
can result in contamination of the stored food. Although on average
the concentration of residues would be expected to be low, occasional
areas of high concentration can occur.
With respect to residues in animals used for human food (pigs,
sheep and birds), there are no residue data concerning animals that
have survived anticoagulant poisoning. It should be emphasized,
however, that in some countries rodents are used as food.
Warfarin is widely used as a therapeutic agent.
5.3 Occupational exposure
Exposure may occur during manufacture, formulation and bait
application. The available information is discussed in section 8.2.
6. MODE OF ACTION AND METABOLISM
6.1 Vitamin K and its antagonists
Vitamin K is a collective name for a number of related compounds,
which all may function as co-enzymes for the enzyme gamma-glutamate
carboxylase. They all contain the functional naphthoquinone ring
structure, but differ in their aliphatic side chains. Vitamin K1
(phytomenadione) contains a side chain composed of four isoprenoid
residues, one of which is unsaturated. The vitamin K2 compounds
(menaquinones) have side chains which vary from 1 to 13 isoprenoid
residues, all of which are unsaturated. They are generally referred
to as MK-n, where n is the number of isoprenoid residues. Vitamin
K3 (menadione) has no side chain, but upon ingestion it is converted
into MK-4 by a liver enzyme. The two products commercially available
for human use are K1 and MK-4. Both are equally active, but for some
reason K1 is almost exclusively used in Europe and North America,
whereas MK-4 (also known as menatetrenone) is used in Asia, notably
Japan. K3 is not used any more for humans because of its adverse
side effect, haemolysis, but is frequently added to animal food.
Both 4-hydroxycoumarin derivatives and indandiones (also known as
oral anticoagulants) are antagonists of vitamin K. Their use as
rodenticides is based on the inhibition of the vitamin K-dependent
step in the synthesis of a number of blood coagulation factors. The
vitamin K-dependent proteins involved in the coagulation cascade
(Fig. 1) are the procoagulant factors II (prothrombin), VII
(proconvertin), IX (Christmas factor) and X (Stuart-Prower factor),
and the coagulation-inhibiting proteins C and S. All these proteins
are synthesized in the liver. Before they are released into the
circulation the various precursor proteins undergo substantial
(intracellular) post-translational modification. Vitamin K functions
as a co-enzyme in one of these modifications, namely the carboxylation
at well-defined positions of 10-12 glutamate residues into
gamma-carboxyglutamate (Gla). The presence of these Gla residues is
essential for the procoagulant activity of the various coagulations
factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its
oxidation to vitamin K 2,3-epoxide (KO) provides the energy required
for the carboxylation reaction. The epoxide is than recycled in two
reduction steps mediated by the enzyme KO reductase (Fig. 2). The
latter enzyme is the target enzyme for coumarin anticoagulants. Their
blocking of the KO reductase leads to a rapid exhaustion of the supply
of KH2, and thus to an effective prevention of the formation of Gla
residues. This leads to an accumulation of non-carboxylated
coagulation factor precursors in the liver. In some cases these
precursors are processed further without being carboxylated, and
(depending on the species) may appear in the circulation. At that
stage the under-carboxylated proteins are designated as descarboxy
coagulation factors (Stenflo et al., 1974; Nelsestuen et al., 1974).
Normal coagulation factors circulate in the form of zymogens, which
can only participate in the coagulation cascade after being activated
by limited proteolytic degradation (see Fig. 1). Descarboxy
coagulation factors have no procoagulant activity (i.e. they cannot be
activated) and neither they can be converted into the active zymogens
by vitamin K action. Whereas in anticoagulated humans high levels of
circulating descarboxy coagulation factors are detectable, these
levels are negligible in warfarin-treated rats and mice. Reviews by
Vermeer (1990) and Furie & Furie (1990) give further details.
Leck & Park (1981) compared the effects of warfarin and
brodifacoum on vitamin K metabolism and blood-clotting factor activity
in warfarin-susceptible and warfarin-resistant rats. In
warfarin-susceptible rats both brodifacoum and warfarin induced a
significant increase in the circulating KO (measured as the KO/K ratio
using 3H-vitamin K1), indicating that KO reductase is the target
enzyme for both drugs. However, whereas warfarin (1 mg/kg) only
inhibited the KO reductase in the susceptible strain, brodifacoum
(1 mg/kg) produced the same decrease of plasma prothrombin
concentration in both warfarin-susceptible and warfarin-resistant
animals.
The KO/K ratio in warfarin-resistant rats is five times higher
than in warfarin-susceptible animals. This is explained by the fact
that the hepatic KO reductase in the resistant animals has not only a
reduced affinity for warfarin, but also for KO. Hence the vitamin K
requirement of warfarin-resistant animals is 5-10 times higher than
that of warfarin-susceptible ones. Second-generation anticoagulants,
if given in doses which cause anticoagulation, further increase the
KO/K ratio (Leck & Park, 1981).
The much stronger potency of difenacoum and brodifacoum, as
vitamin K-antagonists, was reported by Park & Leck (1982), who
concluded that in the case of poisoning with these second-generation
anticoagulants it will be necessary to give repeated and frequent
doses of vitamin K to maintain clotting factor synthesis. The potency
of second-generation anticoagulants can be partly explained by their
highly lipophilic nature, which enables them to bind strongly to
membranes. Their target enzyme KO reductase is an integral membrane
protein with, in addition, a highly lipophilic nature. It is to be
expected that the dissociation of enzyme/inhibitor complexes will be
extremely slow. Moreover, their effectiveness in warfarin-resistant
rats demonstrates that the mutation leading to warfarin resistance
does not significantly affect their interaction with the KO reductase.

 Vermeer & Soute (1992) compared the inhibition of each of the
three enzymes from the vitamin K cycle by four anticoagulants
(warfarin, flocoumafen, difenacoum and brodifacoum). The studies were
performed using in vitro enzyme systems prepared from rat, cow and
human liver. It was shown that in all three species the inhibitor
concentration required for 50% inhibition (I50) was comparable for
the KO reductase and K reductase activity, but that the I50 for
gamma-glutamylcarboxylase was 2-3 orders of magnitude higher. It was
concluded that for all four anticoagulants the reductions of KO and K
are the target reactions for inhibition. Moreover, it was found that
there is no species specificity of the inhibitors, which means that
they are equally active in cell-free systems derived from rat, cow and
human liver. Any species-dependent differences which might be found
in vivo will presumably be brought about by a different
pharmacokinetic or pharmacodynamic behaviour in these species.
6.2 Metabolism
6.2.1 Absorption, distribution and elimination
Anticoagulant rodenticides are easily absorbed through the
gastrointestinal tract, skin and respiratory system.
After a single oral dose of 14C-flocoumafen (0.14 mg/kg body
weight) to rats, the absorption into blood was rapid, reaching maximum
concentrations (0.03-0.05 µg/ml) in plasma within 4 h (Huckle et al.,
1989).
The major route of elimination in rats and sheep after oral
administration of anticoagulants is through the faeces. The
intestinal levels of brodifacoum in rats began increasing 24 to 72 h
after an oral dose of 0.2 mg/kg body weight (Bachmann & Sullivan,
1983). Faecal elimination of radiolabelled flocoumafen following an
oral dose of 0.14 mg/kg body weight accounted for 23-26% of the dose
over the 7-day period; approximately half of this was recovered within
the first 24 h. Less than 0.5% of the dose appeared in the urine
within 7 days (Huckle et al., 1989).
After single oral administration of brodifacoum (0.2 and 2 mg/kg
body weight) to sheep, about 20% and 30%, respectively, was excreted
in the faeces within 8 days (Laas et al., 1985).
A larger proportion of a percutaneous dose of 14C-flocoumafen
(0.17 mg/kg body weight) dissolved in acetone was found in the urine
of rats (10%) than in the case of an equivalent oral dose (less than
0.5%) over a 7-day period. Faecal elimination accounted for 31% of
the percutaneous dose (Huckle & Warburton, 1986b).
After oral 14C-flocoumafen doses of 0.02 mg/kg body weight or
0.1 mg/kg body weight were given to rats, once weekly for up to 14
weeks, approximately one-third of each weekly low dose was eliminated
through the faeces within 3 days, mostly within the first 24 h. At
the higher dose the elimination ranged from 18% after the first dose
to 59% after the tenth dose (Huckle et al., 1988).
Following repeated oral administration of 14C-flocoumafen to
rats at 0.02 mg/kg body weight per week for 14 weeks or 0.1 mg/kg body
weight per week for 10 weeks, appreciable accumulation was seen in the
liver. At both dose levels tissue concentrations were highest in the
liver, followed by the kidney > skin > muscle > fat > blood. The
hepatic residue in the low-dose group ranged from 0.1 mg/kg tissue
after one week to 2.1 mg/kg by week 14 (Huckle & Warburton, 1986a).
Brodifacoum could not be detected in the omental fat of sheep 8
days after the oral administration of 0.2 and 2 mg/kg body weight
(Laas et al., 1985).
6.2.2 Metabolic transformation
Warfarin is readily hydroxylated in vitro and in vivo by rat
liver microsomal enzymes to form 6-, 8- and, especially
7-hydroxy-warfarin (Ullrich & Staundinger, 1968; Ikeda et al.,
1986a,b). These inactive metabolites are to some extent conjugated
with glucuronic acid, undergo enterohepatic recirculation, and are
excreted in the urine and faeces (Ellenhorn & Barceloux, 1988).
The metabolic pattern of indandiones in rats also mainly involves
hydroxylation (Yu et al., 1982).
The second-generation anticoagulants have mainly been found as
unchanged compounds (Bachmann & Sullivan, 1983; Huckle et al., 1988).
The low urinary elimination following oral dosing has precluded
accurate isolation of metabolites in urine (Warburton & Hutson, 1985;
Waburton & Huckle, 1986; Huckle & Warburton, 1986a).
Following administration of flocoumafen, liver residues in rats
consisted mainly of unchanged flocoumafen, although in a repeat dose
study a polar metabolite was detected. Eight urinary metabolites were
detected after percutaneous exposure to 14C-flocoumafen (Huckle &
Warburton, 1986b).
Studies in male Japanese quail have shown more rapid metabolism
and elimination than in the rat following an oral dose of
14C-flocoumafen. Up to 12 radioactive components were detected in
the excreta (Huckle & Warburton, 1986c).
Bromadiolone, brodifacoum and coumatetralyl were also found in
rats as unchanged parent compounds, whereas in the case of difenacoum
metabolites predominated (Parmar et al., 1987). The metabolism and
elimination of the difenacoum trans isomer was more rapid than for the
cis isomer (Bratt, 1987).
The suggestion that the anticoagulant effect in rats is mediated
by the unchanged compound itself rather than by its metabolites has
been confirmed by the effects of phenobarbital and SKF525A
pretreatments on the general pattern of responses to warfarin and
brodifacoum (Bachmann & Sullivan, 1983).
6.2.3 Retention and turnover
Metabolic studies of anticoagulant rodenticides show that the
liver is the main organ of accumulation and storage. Liver
concentrations of brodifacoum after a single oral dose of 0.2 mg/kg
body weight to rats remained high and relatively constant for 96 h,
with a maximum of 5.0 mg/kg after 50 h (Bachmann & Sullivan, 1983).
A high degree of body retention was found 7 days after a single
oral dose of 0.14 mg/kg body weight 14C-flocoumafen (74-76% of the
administered dose); approximately half the dose was found in the liver
(Huckle et al., 1989).
Brodifacoum was detected in the liver of sheep 128 days after
oral administration (0.2 and 2 mg/kg body weight) in concentrations of
0.64 and 1.07 mg/kg dry weight (equivalent to 0.22 and 0.36 mg/kg wet
weight), respectively. The peak levels occurred at 2 days in the
high-dose group and at 8 days in the low-dose group, being 6.50 and
1.87 mg/kg dry weight (2.21 and 0.64 mg/kg wet weight), respectively
(Laas et al., 1985). Woody et al. (1992) observed an elimination
half-life for brodifacoum in serum of 6 ± 4 days in four dogs.
The largest proportion of a percutaneous flocoumafen dose of
0.17 mg/kg body weight was located in the liver (25% of the dose at a
concentration of 0.8 mg/kg), although this was 10 times lower than
that following an oral dose (Huckle & Warburton, 1986b).
Parmar et al. (1987) found that elimination of radiolabelled
brodifacoum, bromadiolone and difenacoum from the liver was biphasic,
consisting of an rapid initial phase lasting from days 2 to 8 after
dosing and a slower terminal phase when the elimination half-lives
were 130, 170 and 120 days, respectively. Elimination of
coumatetralyl was more rapid, with a half-life of 55 days.
Similar results for difenacoum were found by Bratt (1987). After
a single oral 14C-difenacoum dose of 1.2 mg/kg body weight, the
highest concentration of radioactivity (41.5% of the dose) was found
in the rat liver 24 h after dosing. The elimination from the liver
was biphasic. The half-life of elimination of the radioactivity
during the first rapid phase was three days, and for the slower phase
was 118 days. A similar biphasic elimination was also apparent in the
kidney. In the pancreas the concentration declined more slowly than
in any of the other tissues (182 days). The parent compound was the
major component in the liver 24 h after dosing (42%).
Unchanged flocoumafen comprised the major proportion of the
hepatic radioactivity in rats and was eliminated with a half-life of
220 days (Huckle et al., 1989). Veenstra et al. (1991) found
retention of about 8% of an administered flocoumafen dose of 0.4 mg/kg
in the liver of beagle dogs 43 weeks after dosing.
Despite the more rapid metabolism of flocoumafen in Japanese
quail, a proportion of the administered dose is retained in the liver,
with an elimination half-life of 115 days after oral dosing (Huckle &
Warburton, 1986c).
Six Hereford heifers weighing approximately 230 kg each were
dosed with diphacinone (1 mg/kg body weight) by injecting it into the
rumen. The highest residue level of parent compound found in the
liver was 0.15 mg/kg at days 30 and 90 after treatment. No detectable
levels (> 0.01 mg/kg) could be found in any of the other tissues
analysed (kidney, plasma, brain, heart, muscle and fat). The residues
in the liver were almost constant from 30 to 90 days post-treatment
(Bullard et al., 1976).
The plasma half-life of brodifacoum determined in three patients
with severe bleeding disorders was found to be approximately 16 to 36
days (Weitzel et al., 1990).
The half-life for disappearance from the plasma of human
volunteers given a single oral or intravenous warfarin dose of
1.5 mg/kg body weight varied from 15 to 58 h, with a mean of 42 h
(O'Reilly et al., 1963).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute effects
7.1.1 Rodent species
Wide variations exist in the literature for LD50 values of
anticoagulant rodenticides. A particular reason for these variables
is the use of single or repeated (5-day) doses. LD50 values also
vary according to the animal strain and sex, but both have not always
been indicated in reported data (Ashton et al., 1987)
(Tables 5 and 6).
Table 5. Acute oral LD50 values for various rodenticides in albino
Norway rats
Rodenticide LD50 (mg/kg) Reference
Brodifacoum 0.26 Redfern et al. (1976)
Bromadiolone 1.125 Grand (1976)
Chlorophacinone 20.5 Thomson (1988)
Coumachlor 900.0 Thomson (1988)
Coumafuril 0.4 Wiswesser (1976)
Coumatetralyl 16.5 Thomson (1988)
Difenacoum 1.8 Bull (1976)
Difethialone 0.56 Lechevin & Grand (1987)
Diphacinone 3.0 Thomson (1988)
Flocoumafen 0.46 Sharples (1983a)
Pindone 50.0 Tomlin (1994)
Warfarin 58.0 Thomson (1988)
Reported oral LD50 values for warfarin in rats vary by a
considerable magnitude. Values of 11 mg/kg body weight (Lund, 1982),
58 mg/kg body weight (Thomson, 1988) and 58 mg/kg body weight and
323 mg/kg body weight for female and male, respectively (Hagan &
Radomski, 1953), have been reported.
The second-generation anticoagulants are more toxic than the
first-generation ones in the sense that a single feeding may be
lethal. Apparent discrepancies between the single oral LD50 values
for Norway rats, as shown in Table 5, and the multiple dose oral
LD50 values, shown in Table 6, can be explained by the cumulative
effects resulting from multiple dose (5 day) administration (Table 6)
and characteristics of this family of compounds.
Table 6. Five-day oral LD50 (mg/kg body weight per day) of various anticoagulants
for the Norway rat (Rattus norvegicus)a
Anticoagulant Strainb Male Female Both sexes Number of
animalsc
Pindone SD 1.21 (0.70-2.11) 1.60 (0.83-3.08) 1.34 (0.87-2.06) 40
wild 7.60 (2.61-22.2) 25.60 (5.34-123) 12.80 (1.73-84.8) 40
Warfarin SD 0.29 (0.14-0.57) 0.38 (0.22-0.66) 0.33 (0.22-0.50) 40
wild 0.39 (0.16-0.91) 0.60 (0.23-1.09) 0.44 (0.25-0.76) 40
Diphacinone SD 0.19 (0.11-0.33) 0.23 (0.12-0.43) 0.21 (0.14-0.31) 40
wild 0.39 (0.15-0.84) 0.60 (0.22-0.57) 0.44 (0.23-0.54) 40
Chlorophacinone SD 0.18 (0.18-0.18) 0.20 (0.15-0.27) 0.19 (0.16-0.22) 40
wild 0.13 (0.10-0.19) 0.23 (0.14-0.36) 0.16 (0.12-0.22) 40
Bromadiolone SD 0.13 (0.10-0.19) 0.10 (0.08-0.13) 0.12 (0.10-0.15) 40
wild 0.06 (0.03-0.12) 0.09 (0.09-0.09) 0.07 (0.05-0.10) 16
a Modified from: Ashton et al. (1987); figures in parentheses represent 95% confidence limits
b SD = Sprague-Dawley
c 50% males and 50% females in all tests
Reported oral LD50 values for mice show similar variations,
from less than 1 mg/kg body weight for second-generation rodenticides
to 374 mg/kg body weight for warfarin (Hagan & Radomski, 1953; Redfern
et al., 1976; Bull, 1976; Sharples, 1983a).
Signs of anticoagulant poisoning in rats and mice include
lethargy, hunched posture and vein clearing in the ears. Blood around
the eyes, mouth and anus, indicating internal haemorrhaging, appears
prior to death (Sharples, 1984).
The percutaneous toxicity of anticoagulants to rats varied for
different compounds from an LD50 of 0.54 mg/kg body weight for
flocoumafen (master mix in corn oil) to > 50 mg/kg body weight for
brodifacoum and difenacoum (Price, 1985b; Tomlin, 1994). The signs of
intoxication were identical to those observed after an oral dose.
Second-generation anticoagulants appeared to be highly toxic by
inhalation. An acute inhalation study in Wistar rats exposed (nose
only) for 4 h was conducted using the 0.5% manufacturing master mix
specifically prepared to give a mass median aerodynamic diameter of
less than 5 µm. The acute LC50 values were between 0.16 and
1.4 mg/litre. Signs of intoxication, characteristic of an
anticoagulant action, were observed within 3 days after exposure with
deaths occurring between 4 and 9 days (Blair, 1984).
7.1.2 Non-target species
The acute toxicity of various anticoagulant rodenticides to
non-target mammalian species is presented in Table 7.
From the data presented it appears that some anticoagulants show
a similar range of acute toxicity for both non-target mammals and
target rodents.
7.2 Short-term exposure
7.2.1 Rodent species
In a study by Hadler (1974), groups of Wistar rats (male and
female) were given brodifacoum by gavage (0.01, 0.02, 0.05, 0.1 and
0.2 mg/kg body weight) for 5 consecutive days. All rats receiving the
two lowest doses survived the 21-day experimental period, but all rats
given the two highest doses died within 11 days of cessation of
dosing. No abnormalities were detected in surviving animals
sacrificed at the end of the observation period. In animals which
died during the study, only massive internal haemorrhages, mainly in
the peritoneum, were observed. The no-observed-effect level of
brodifacoum for Wistar rats was 0.02 mg/kg body weight per day.
Table 7. Acute oral toxicity (LD50, mg/kg) of various anticoagulant rodenticides for non-target mammalian speciesa
Rodenticide Guinea-pig Rabbit Dog Cat Sheep Pig References
Brodifacoum 2.78 (F) 0.29 (M) 0.25-1 (F) approx. 25 (F) > 25 (M) 0.5-2 Hadler (1975a,b); Parkinson
3.56 11-33 (1975, 1976); Godfrey (1984);
Godfrey et al. (1985)
Bromadiolone 2.8 1.0 10 (F) MTD > 25 MTD 3 Grand (1976)
Chlorophacinone 50 Pelfrène (1991)
Difenacoum 50 (F) 2 (M) approx. 50 100 100 80-100 Bull (1976); Tomlin (1994)
Diphacinone 35 3-7.5 14.7 150 Kosmin & Barlow (1976)
Difethialone 0.75 5 MTD > 16 MTD 2-3 Lechevin & Grand (1987)
Flocoumafen > 10 (M) 0.7 (M/F) 0.075-0.25 > 10 (M/F) > 5 approx. 60 (M,F) Sharples (1983b); Price (1985a);
(M/F) Chesterman et al. (1984);
Roberts et al. (1985a, 1986)
Warfarin 800 20-50 6-40 1-5 Anonymous (1976)
a F = female; M = male; MTD = maximum tolerated dose
Wistar rats (male and female) were continuously given a diet
containing brodifacoum at 0.1 mg/kg body weight, with no choice of
other feed, for 12 weeks. Prothrombin time was increased and
mortality occurred in 9 out of 20 males and 5 out of 20 females.
Macroscopic examination of the major organs of surviving rats revealed
no abnormalities other than those expected of anticoagulant action
(Hadler, 1976).
Feeding Fisher-344 rats with diets containing flocoumafen at
concentrations of 0.2 or 0.4 mg/kg feed for 5 days produced no
toxicologically significant effects during the 15-day observation
period (Price, 1985c).
In a 28-day feeding study in rats, fed on a diet containing 0,
0.01, 0.05, 0.1 or 0.2 mg-flocoumafen/kg, there were no overt signs of
toxicity. Highest-dose females showed a slight but significant
increase in mean prothrombin time (PT) and activated partial
thromboplastin time (PTT), and a slight decrease in mean plasma total
protein. No toxicological or pathological changes were observed in
rats fed diets containing 0.01 or 0.05 mg/kg of diet (Price, 1985d).
No effect on prothrombin time was observed in a 12-week study
with Wistar rats administered oral flocoumafen doses (by gavage) of
0.0125, 0.0625 and 0.125 mg/kg body weight once a week (Forsey, 1985).
Groups of six male and six female Sprague-Dawley rats were fed
diphacinone in their diets at concentrations of 0 (control), 0.0313,
0.0625, 0.125, 0.25 and 0.5 mg/kg diet (equivalent to 0, 1.7, 3.3,
6.4, 13 and 27 µg/kg body weight per day) for 90 days (Elias & Johns,
1981). Additional satellite groups of one rat of each sex and each
dose group were killed for gross pathological examination at 30 and
60 days. Mortality was unaffected by the treatment. In the survivors
of the main groups and satellite groups, there were no gross
pathological changes, but in the two males that died prematurely (in
the 0.0625 and 0.25 mg/kg groups) there was subdural haemorrhage.
Prothrombin time was unaffected by treatment. Routine haematological
and clinical chemistry tests were performed on only two rats of each
sex from each group, and no effects were observed apart from a reduced
blood fibrinogen concentration in both sexes at the highest dose
level.
As no clear NOAEL was indicated by the results of the 90-day
study, a 21-day study was performed using two rats of each sex at
diphacinone levels of 0 (control), 0.125, 0.25, 0.5, 1, 2 and 4 mg/kg
diet. All the rats in the 2 and 4 mg/kg groups died within the first
14 days, but all others survived until the end of the study. The mean
dosages received by the survivors were equivalent to 0, 9, 17, 34 and
67 µg/kg body weight per day. At 21 days, there was no effect on
prothrombin time. Gross necropsy revealed haemorrhage in the thymus
of one rat in the 0.5 mg/kg group, but no effects were seen in any
other survivors. All the animals in the 2 and 4 mg/kg groups had
massive and extensive internal haemorrhage (Elias & Johns, 1981).
7.2.2 Non-target species
There are few data available on the repeated exposure of
non-rodent species to anticoagulant rodenticides.
Death followed five daily warfarin doses of 3 mg/kg body weight
in cats and 1 mg/kg body weight in pigs (Tomlin, 1994). The route of
administration was not specified.
The dermal administration of warfarin (188 mg/kg per day) to
female baboons caused profuse bleeding in 5 days followed by death
(Dreyfus et al., 1983).
When it became known that vitamin K was not an antagonist for
warfarin action in bone, it became possible to study the long-term
effects of this anticoagulant in bones without the risk of inducing
haemorrhages and bleeding. By feeding lambs with high doses of
warfarin (up to 150 mg/kg body weight per day) under the protection of
4 mg/kg body weight per day of vitamin K, Pastoureau et al. (1993)
showed that within 3 months the lambs had developed a marked
osteopenia that resulted from a decrease in resorption and a much more
pronounced decrease in bone formation. As a result the bone density in
the warfarin-treated animals was substantially lower than that in
control animals. This is in agreement with earlier studies in rats
(Price et al., 1982) where it was found that, under the protection of
vitamin K, warfarin induced excessive mineralization and growth plate
closure, due to which bone growth came to a halt.
The maximum tolerated 5-day oral dose of bromadiolone was
considered to be 25 mg in pigs (Large White strain) weighing 25 kg.
After 45 oral daily doses of 0.5 mg no change in the prothrombin time
was observed (Grand, 1976).
Woody et al. (1992) studied the effect of a cumulative dose of
1.1 mg brodifacoum/kg body weight administered orally to dogs over a
3-day period. Signs of coagulopathic effects appeared within 24 h
(greater PT and PTT) and increased over a 10-day period. Treatment
with Vitamin K1 at 10 days post-exposure reduced the effects.
Six horses were treated by gavage with brodifacoum containing
bait at a dosage of 0.125 mg/kg body weight (Boermans et al., 1991).
Four of the horses became anorexic and depressed, one requiring K1
therapy. Peak plasma concentration occurred 2-3 h after
administration. Pharmacokinetic evaluation indicated that brodifacoum
has a plasma half-life of 1.22 days. An increase in clotting time was
observed as early as 24 h after dosing, returning to the pre-treatment
level by day 12.
Male pigs (five per group) were fed difenacoum in the diet for 14
days at dose levels of 0.01, 0.05, 0.1, 0.5 and 1.0 mg/kg diet. With
the exception of the lowest-dose group, all groups showed a marked
increase of prothrombin time values. Extensive subcutaneous, inter-
and intra-muscular haemorrhage and oedema was observed in animals
dosed at levels of 0.5 mg/kg or more (Ross et al., 1979).
7.3 Long-term exposure
No data on long-term exposure are available.
Studies with second-generation anticoagulants are difficult to
carry out for more than a few weeks due to the rapid acute effects,
and NOEL values for second-generation rodenticides and longer exposure
periods have not been established. In any chronic study approaching
two years in length, the dose level would have to be less than the
analytical limit of detection.
7.4 Skin and eye irritation; sensitization
Brodifacoum is a slight skin irritant and a mild eye irritant in
the rabbit (Hadler, 1975c). No skin or eye irritation was observed in
New Zealand white rabbits treated with flocoumafen (Forsey, 1983a,b).
Neither of these rodenticides was a skin sensitizer when tested in the
guinea-pig maximization test (Parkinson, 1979; Price, 1986).
7.5 Reproductive toxicity and teratogenicity
Brodifacoum was given by oral gavage to female rats at daily dose
levels of 0.001, 0.01 or 0.02 mg/kg body weight during days 6-15 of
pregnancy. There was no evidence of adverse effects on the fetus at
termination. Higher daily doses (above 0.05 mg/kg) caused an
anticoagulant effect in the dams which resulted in a high incidence of
abortion (Hodge et al., 1980a).
Pregnant female rabbits were given oral gavage doses of 0.001,
0.002 or 0.005 mg brodifacoum/kg body weight per day from days 6-18 of
pregnancy. A the highest dose level a high proportion of maternal
deaths occurred as a result of haemorrhage. Although the survivors
showed signs of haemorrhage, there were no effects on the developing
fetus. No effects were observed at either of the other dose levels
used (Hodge et al., 1980b,c).
Bromadiolone was given orally to four groups of 25 female rats
from day 6 to 15 of pregnancy at doses of 0, 17.5, 35 and 70 µg/kg
body weight per day. Maternal toxicity occurred at the higher dose
levels. There was no evidence of embryotoxicity or teratogenic
effects at any dose level (Monnot et al., 1981). A similar absence of
effects was reported in a study on rabbits treated orally with daily
doses of either 2, 4 or 8 µg/kg body weight per day on days 6-18 of
pregnancy, although there was maternal toxicity at the highest dose
level (Virat, 1981).
Groups of 18 pregnant F-344 rats were given daily oral doses of
0, 0.01 or 0.04 mg flocoumafen/kg body weight from day 8 to 17 of
gestation. Eight animals in the 0.04 mg/kg body weight group either
died or were killed with signs of anticoagulant poisoning.
In contrast, Mirkova & Antov (1983) found warfarin to be
embryotoxic and teratogenic to Wistar rats when administered by gavage
in single doses or repeatedly throughout the periods of
pre-implantation (1-7 days of gestation) and organogenesis (8-16
days), and also throughout the whole gestation (1-21 days) at a wide
range of dose levels (0.04-8 mg/kg body weight). At these dose levels
and treatment regimens, warfarin induced substantially increased rates
of embryolethality, subcutaneous and internal haemorrhage and gross
structural malformations (pes varus, internal hydrocephalus and
anomalies of skeletal ossification).
7.6 Mutagenicity
Various in vitro and in vivo studies have been undertaken to
assess the genotoxic potential of brodifacoum. No mutagenic activity
was detected in the Salmonella reverse mutation assay in any of the
five tester strains employed (TA98, TA100., TA1535, TA1537 and TA1538)
either in the presence or absence of Arochlor 1254-induced rat liver
S9 fraction at brodifacoum concentrations ranging from 1.6 to
5000 µg/plate (Callander, 1984). Brodifacoum showed no activity in a
forward mutation assay using L5178 mouse lymphoma cells, either with
or without metabolic activation, at concentrations of 47.5, 63.3 and
84.4 mg/litre (Cross & Clay, 1984).
Brodifacoum caused no significant chromosomal aberrations in
cultured human lymphocytes (concentrations 1, 10, 100 and
1000 mg/litre), either with or without metabolic activation, and did
not induce unscheduled DNA synthesis in cultured HeLa cells at the
same range of concentrations (Mellano, 1984a,b). Difenacoum did not
induce unscheduled DNA synthesis in rat hepatocytes in vivo at
either dose level or time-point (Kennelly, 1990).
An in vivo micronucleus test, in which mice were given single
brodifacoum intraperitoneal doses of 0.187 or 0.30 mg/kg body weight,
showed no induction of micronuclei in bone marrow polychromatic
erythrocytes (Sheldon et al., 1984).
Bromadiolone was tested in the Salmonella reverse mutation assay
at concentrations ranging from 10 to 3330 µg per plate on strains
TA1535, TA1537 and TA1538. No evidence of mutagenic effect was found
either with or without Aroclor metabolic activation (Lawlor, 1992).
Bromadiolone did not induce forward mutations in Chinese hamster
ovary cells either with or without metabolic activation
(Cifone, 1993).
In a mouse micronucleus test at four dose levels from 50 to
400 mg/kg, bromadiolone did not induce micronuclei in bone marrow
polychromatic erythrocytes (Murli, 1993).
Flocoumafen did not induce reverse gene mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 nor in
Escherichia coli WP2uvrA pkm 101 either with or without metabolic
activation. Flocoumafen was tested at concentrations ranging from 31
to 2000 µg/plate, beyond which precipitation from suspension occurred
(Brooks et al., 1984).
Flocoumafen did not increase the frequency of mutation to
6-thioguanine resistance in Chinese hamster V79 cells either in the
presence or absence of an Arochlor-induced rat liver S9 fraction.
Doses ranging from 5 to 150 mg/litre were used, beyond which
cytotoxicity occurred (Clare & Wiggins, 1986).
Flocoumafen did not induce in vitro cell transformation in
C3H1OT´ mouse fibroblasts, either in the presence or absence of a rat
S9 metabolizing system, at concentrations ranging from 12.5 to
100 µg/litre (Meyer & Wiggins, 1986).
Flocoumafen did not induce mitotic gene conversion in liquid
suspension cultures of Saccharomyces cerevisiae JD1, either in the
presence or absence of a rat liver S9 fraction, at concentrations
ranging from 0.01 to 2 g/litre (Brooks et al., 1984).
When incubated at concentrations ranging from 5 to 25 mg/litre
for 24 h in monolayer cultures of rat liver RL4 cells, flocoumafen did
not induce in vitro chromosomal damage (Brooks et al., 1984).
Oral administration of flocoumafen to rats at doses of 0.25 mg/kg
or 1000 mg/kg body weight (a dose 4000 times the acute oral LD50 for
rats) did not produce chromosomal damage (Allen et al., 1986).
The cytogenic effect of chlorophacinone was investigated in vivo
in metaphase bone marrow cells taken at 48 and 96 h after oral dosing
of male CFLP mice with 20 mg/kg body weight. No induction of
chromosomal aberrations was observed (Nehéz et al., 1985).
In the same study, chlorophacinone was investigated in
spermatocytes taken from male CFLP mice at 1, 2, 3 and 4 weeks after a
single oral dose of 20 mg/kg body weight. The spermatocytes were
analysed in the diakinesis phase of meiosis. There was no increased
incidence of chromosomal aberrations (Nehéz et al., 1985).
Rabbits were treated orally with 20 mg chlorophacinone/kg body
weight and chromosomal analysis was performed in bone marrow and
spermatocytes taken at 48 h post-dosing. No increase in the incidence
of chromosomal aberrations was seen (Selypes et al., 1984).
7.7 Factors modifying toxicity
Phenobarbital pretreatment of rats followed by a single
administration of brodifacoum or warfarin decreased the anticoagulant
effects of both compounds, more markedly in the case of warfarin
(Bachmann & Sullivan, 1983).
The non-steroid anti-inflammatory drugs ibuprofen and
phenylbutazone potentiated the anticoagulant effects of brodifacoum
and bromadiolone in rats (Sridhara & Krishnamurthy, 1992).
Strain and sex are important factors modifying the toxicity of
anticoagulants in rodents (see Table 5, section 7.1.1). Winn et al.
(1987) observed greater sensitivity of male rats and mice to
difenacoum, compared with female rats and mice. The possible
explanation of different responses to difenacoum was the greater
turnover of plasma proteins in male rats or the marked
inter-individual variation of vitamin K1 level in male rat liver.
Large amounts (relative to farm animals' dietary requirements) of
vitamin K3 (menadione and its salts) are sometimes added to animal
feedstuffs. This gives rodent pests ready access to a substance which
acts as an antidote to anticoagulant rodenticides, and thus can reduce
the efficacy of these rodenticides.
Differences in metabolism to inhibitors of vitamin K synthesis
among strains of mice and rats has been attributed to a number of
different factors (Misenheimer et al., 1994). Among these are: 1)
reduced sensitivity of vitamin K epoxide hydrolase to inhibition; 2)
greater reversibility of inhibition of epoxide hydrolase; and 3)
faster clearance of the rodenticide. The mechanism of resistance
differs between strains.
A single flocoumafen dose of 0.5 mg/kg body weight resulted in
clear signs of anticoagulation in five out of eight beagle dogs
(Veenstra et al., 1991). Administration of a second dose 5 weeks
later resulted in clinical evidence of anticoagulation in two out of
the three remaining dogs. Vitamin K treatment (2 to 5 mg/kg
subcutaneously) reversed the effects in all cases.
7.8 Adverse effects in domestic and farm animals
7.8.1 Domestic animals
7.8.1.1 Poisoning incidents
The main cause for accidental poisoning of domestic animals is
direct consumption of anticoagulant baits. Secondary poisoning
through the consumption of rats and mice killed with anticoagulants
may occur in dogs and cats in urban situations, but is more likely in
farm situations (Marsh, 1985b). The majority of fatalities and severe
clinical syndromes are connected with the second-generation
anticoagulants (Dodds & Frantz, 1984). Du Vall et al. (1989) studied
10 cases of second-generation anticoagulant rodenticide poisoning in
dogs and cats. The presence of anticoagulants (brodifacoum,
bromadiolone or diphacinone) in serum or liver was confirmed by HPLC
or GC/MS. Several other cases of brodifacoum poisonings in dogs have
been reported and some of them were fatal (Mc Sporran & Phillips,
1983; Stowe et al., 1983; Dodds & Frantz, 1984).
Until 1983, only first-generation anticoagulants were available
in New Zealand and, between 1977 and 1983, warfarin, coumatetralyl and
diphacinone were involved in 45 reported incidents of accidental
poisoning of domestic and farm animals. Brodifacoum and flocoumafen
were introduced after 1983. From 1983 to 1994, 40 cases of poisoning
of domestic and farm animals resulted from the use of
second-generation compounds and 17 cases were due to the ingestion of
first-generation anticoagulants (Hoogenbroom, 1994).
Schulman et al. (1986) reported five cases of coagulopathy in
dogs caused by consumption of diphacinone-containing baits. Lethargy
and respiratory distress, associated with pulmonary interstitial
haemorrhage and pleural and/or pericardial effusion, were the most
consistent signs. The prothrombin time and the activated partial
thromboplastin time were moderately to markedly prolonged.
7.8.1.2 Diagnosis and treatment of poisoning
The major difference between warfarin and the other anticoagulant
rodenticides is that the latter have longer body retention and a
tendency to induce bleeding for a longer period of time. It is
therefore necessary to continue the treatment for weeks rather than
days.
Signs of poisoning occur after a latent period of 12 h to several
days and may include:
* bruising easily with occasional nose or gum bleeds
* blood in stools or urine
* excessive bleeding from minor cuts or abrasions
* laboured breathing
* pale mouth and cold gums
* anorexia and general weakness
A reliable indication of an anticoagulant effect is the
determination of prothrombin time.
Vitamin K1 (phytomenadione) is the antidote of choice. The
recommended dosage is 2-5 mg/kg body weight in dogs. Intravenous
injection is the quickest route but it is not recommended because of
reported anaphylaxis. Vitamin K1 is much safer if given
subcutaneously for the initial 2-3 treatments. A dose of 0.25 to
2.5 mg/kg body weight is recommended for use in small animals (Mount
et al., 1982). The recommended period of treatment with vitamin K1
is 3-6 weeks (Braithwaite, 1982; Mackintosh et al., 1988).
Mount & Feldman (1983) observed that therapy effective for
warfarin poisoning was ineffective against diphacinone toxicosis due
to inadequate vitamin K1 dosage and duration of therapy. Oral
therapy was ineffective.
In a study on dogs, Mount & Kas (1989) found that the serum
concentration of vitamin K epoxide was significantly higher in
diphacinone-treated dogs than in controls, the difference being
significant as early as 1-4 h after vitamin K application. The
authors suggested that this phenomenon could be used as diagnostic
information.
In cases of severe blood loss, fresh plasma should be infused
every 6 h to the extent of 5-10% of total blood volume, assuming this
to be 90 ml/kg in the dog and 70 ml/kg in the cat (Mount et al.,
1982).
Vitamin K1 (phytomenadione) is the antidote for treating dogs
exposed to anticoagulant rodenticides. The therapeutic dosages and
duration of treatment needed vary with the compound involved. Mount &
Feldman (1983) reported that dogs poisoned with diphacinone require
treatments for at least three weeks, while treatment for 4-6 days is
sufficient for treating warfarin poisonings. Initial treatments are
by subcutaneous injection, subsequent therapy being given orally.
For animals exposed to the second-generation rodenticides
brodifacoum, bromadiolone, difenacoum and flocoumafen, the treatment
regimen that has been recommended includes one or more injections of
vitamin K1 at 2-5 mg/kg body weight until prothrombin times return
to normal levels. Once this has been achieved, daily oral doses of
vitamin K1 are recommended for a period of 3-4 weeks. Oral doses of
2-5 mg/kg body weight are recommended initially, with some reduction
being possible over time if the animal is observed closely for signs
of recurrence of symptoms. If prothrombin times increase following
withdrawal of vitamin K1, the oral dosing should be resumed for 2-3
weeks. If blood loss was severe, transfusions (10-15 ml/kg body
weight) with fresh whole blood may be needed (Anonymous, 1988).
7.8.2 Farm animals
Feinsod et al. (1986) reported an outbreak of abortions and
haemorrhages in sheep and goats in Egypt caused by brodifacoum
intoxication. Clinical signs appeared within 3-7 days after exposure
to the rodenticide and included epistaxis, haematochezia, recumbency,
subcutaneous haemorrhage, flank ecchymosis, lameness, abdominal
bloating and abortion of various stages of gestation. The signs
resembled epidemic Rift valley fever, but there was no fever, icterus
or loss of appetite in affected animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Incidents of human exposures to rodenticides are reported to
poison control centres in countries where such facilities exist. In
1988, for example, the American Association of Poison Control Centers
(AAPCC) received accounts of 10 626 cases of human exposures to
rodenticides. These incidents represented 17% of reported exposures
involving pesticides and 0.8% of the total number of cases reported in
the AAPCC system. The rodenticide incidents included 4190 cases
involving "anticoagulants" (principally warfarin) and 5133 involving
"long-acting anticoagulants" (second-generation anticoagulants plus
the indandione compounds). More than 95% of the rodenticide cases
were classified as "accidental". Most of the remainder were
classified as "intentional" and included attempted suicides. Of the
10 540 rodenticide incidents for which the ages of victims were
reported, 9406 (89%) involved children under 6 years of age (Litovitz
et al., 1994).
Victims in nearly 32% of the rodenticide exposure incidents
reported to the AAPCC in 1988 were treated in health care facilities.
However, the medical outcome "none" was reported in more than 93% of
the 5708 incidents for which information regarding outcomes was
reported. The remaining 380 cases included 333 with "minor" medical
effects, 41 with "moderate" effects, 4 with "major" effects, and two
deaths (Litovitz et al., 1994).
In 1993, the Swedish Poison Information Centre received 338
enquiries concerning exposures to anticoagulant rodenticides. This
number represented 0.6% of all enquiries to the centre and 37% of the
enquiries concerning pesticides. Of the anticoagulant rodenticide
enquiries, 202 pertained to warfarin and 136 to "superwarfarin"
compounds (Persson, 1994).
Human exposure to second-generation and indandione anticoagulants
produces symptoms consistent with anticoagulation effects (e.g.,
haematomas, haematemesis, haematuria, easy bruisability). Treatment
of cases of exposure, particularly of substantial and repeated
exposure, may require vitamin K1 therapy and monitoring of
prothrombin times for periods of many months (Rauch et al., 1994).
Suicide and/or unintentional poisonings with anticoagulant
rodenticides have occurred in many countries. Thus, Ungvary (1994)
reported 70 cases, mostly involving children, that occurred in Hungary
between 1988 and 1993.
Warfarin is widely used as a therapeutic and preventive agent in
the treatment of thromboembolic disease. Patients have been
maintained for years on this treatment with control of the prothrombin
level, which should be kept between 10 and 30% of normal.
Diphacinone has also been used as a drug because of its
long-lasting action (the half-life in humans is 15-20 days). It
ceased to be listed in the American Medical Association Drug
Evaluations, (AMA, 1980) because of its structural relation to
phenindion, which had been reported to have adverse effects.
8.1.1 Acute poisoning
Typical features of poisoning result from increased bleeding
tendency and include:
* minor poisoning: coagulation disturbance detected only by
laboratory analyses;
* moderate poisoning: coagulation disturbance resulting in
haematomata, haematuria, blood in faeces or excessive bleeding
from minor cuts or abrasions, gum bleeding;
* severe poisoning: retroperitoneal haemorrhage, severe
gastrointestinal bleeding, cerebrovascular accidents, massive
haemorrhage (internal bleeding) resulting in shock.
If anaemia or liver disease is present then the above features
may be more severe and persistent and the poisoning may be more
difficult to control (Anonymous, 1988).
The onset of the signs of poisoning may not be evident until a
few days after ingestion.
8.1.2 Poisoning incidents
Cases of human poisoning with "superwarfarins" were reviewed by
Katona & Wason (1989).
Fourteen members of a family in the Republic of Korea were
poisoned by eating warfarin-containing maize meal. The first symptoms
appeared 7-10 days after the beginning of exposure and were followed
by massive bruises or haematomata on the buttocks in all cases (Lange
& Terveer, 1954).
Pribilla (1966) reported a total dose of about 1000 mg of
warfarin to be fatal after 13 days of consumption.
Out of a total of 741 infants, 177 died after the use of
warfarin-contaminated talc in Viet Nam. The concentrations of
warfarin in the powder varied from 1.7 to 6.5% (Martin-Bouyer et al.,
1983).
A 73-year-old woman suffered from recurrent episodes of
hypoprothrombinaemia. Clotting tests and further investigation showed
that this was due to a warfarin rodenticide intentionally mixed in the
woman's cough syrup by her daughter-in-law. As the patient had as
many as seven relapses, it was possible to compare different types of
therapy. Menadione had no effect (Nilsson, 1957).
Several suicidal attempts with chlorophacinone have been
reported. Murdoch (1983) reported a case of ingestion of 625 mg
chlorophacinone (250 ml of a 0.25% concentrate formulation) by a
37-year-old woman. The prolonged anticoagulant action of
chlorophacinone persisted for at least 45 days even though treatment
was given. It was found that menadiol, the synthetic analogue of
vitamin K1, was ineffective. The natural form, phytomenadione, was
effective only when given at high dosage (20 mg daily) 30 days after
the ingestion of chlorophacinone.
In a case reported by Dusein et al. (1984), the amount of
ingested chlorophacinone was unknown. After adequate therapy, the
prothrombin level became normal within 4 weeks.
Vogel et al. (1988) reported the case of an 18-year-old woman
hospitalized 3 days after ingesting approximately 100 mg
chlorophacinone. Under high-dose vitamin K1 therapy (160 mg) the
prothrombin time was normalized, but it increased again following
withdrawal of vitamin K1. After prolonged vitamin K1 administration,
the prothrombin time finally became normal after 7 weeks.
Brodifacoum poisoning has occurred in South Sumatra, Indonesia.
Some of the villagers used a 0.005% brodifacoum rice grain bait as a
food source even though they knew it was poisonous and unfit for human
consumption. They attempted to remove the rodenticide by repeated
washing, rinsing and cooking before eating the rice. Because of the
delay in the appearance of poisoning symptoms it appeared that they
had been successful, thus encouraging further attempts to purify the
rice baits. As a result, deaths occurred before appropriate remedial
treatment could be initiated (Anonymous, 1985).
Jones et al. (1984) reported the first case of human brodifacoum
poisoning in a 17-year-old boy who attempted suicide by ingesting
approximately 7.5 mg (0.12 mg/kg) of brodifacoum in Canada. He was
initially seen with gross haematuria, followed by epistaxis and gum
bleeding. The prothrombin time and the activated partial
thromboplastin time were notably prolonged. He was treated for 56
days with either parenteral or oral vitamin K1 and either fresh or
stored plasma until coagulation values remained normal and stable.
Lipton & Klass (1984) reported a similar case in a 31-year-old
mentally disturbed woman who ingested over a 2-day period
approximately thirty 50-g packages of Talon-G (approximately 75 mg of
brodifacoum). Prothrombin time and activated partial thromboplastin
time were considerably prolonged (respectively 6-fold and 4-fold above
normal values). After 4 days of therapy with high doses of vitamin
K1 (up to 125 mg/day), partial correction in the prothrombin time
occurred. Vitamin K1 therapy continued with interruptions for 8
months until normal prothrombin time levels were found.
Chong et al. (1986) reported a case of suicidal poisoning after
ingestion of 10 mg brodifacoum (as 0.05% Klerat). The coagulation test
became normal after large doses and prolonged use of vitamin K1 over
6 months.
A case of intentional ingestion of brodifacoum (200 g of Talon G,
0.005% brodifacoum) was reported by Hoffman et al. (1988). A profound
decrease in the levels of factors II, VII, IX and X, lasting 43 days
after ingestion, was observed. Treatment with subcutaneous vitamin
K1 in doses up to 100 mg per day was effective.
Weitzel et al. (1990) described three patients with severe
bleeding disorders due to deficiency of the vitamin K-dependent blood
clotting proteins after ingestion of an anticoagulant. Although the
patients denied any ingestion, brodifacoum was detected in their serum
at concentrations of 7.6 nmol/litre, 270.7 nmol/litre and
2759 nmol/litre, respectively. The anticoagulant effect was found to
persist long after brodifacoum was no longer detectable in the serum.
A half-life of approximately 16-36 days was determined for brodifacoum
in the plasma.
Kruse & Carlson (1992) reported the case of a 25-year-old man who
attempted suicide by consuming a brodifacoum rodenticide. He
developed a severe coagulopathy that was treated with vitamin K1 and
fresh frozen plasma and he was discharged from hospital with oral
phytomenadione. Fifteen weeks later the man presented again with a
history of further brodifacoum ingestion. He suddenly became comatose
and computer tomography revealed a subarachnoid haemorrhage that led
to brain death 24 h later.
Wallace et al. (1990) described the clinical course of a patient
poisoned with brodifacoum in a suicide attempt. He developed
microhaematuria and melaena. His clotting factors were depressed and
were poorly responsive to vitamin K treatment.
Barlow et al. (1982) reported a case of attempted suicide with
25 mg of difenacoum (500 g of rat bait) followed several months later
by 1800 g of rat bait. The patient was treated with vitamin K1
(phytomenadione) for 48 and 42 days, respectively, until the
pharmacological effect of difenacoum ceased.
Nighoghossian et al. (1990) reported an unusual coagulopathy
after accidental exposure to a diphenacoum rodenticide. A 59-year-old
man developed subacute tetraparesis following severe sudden neck pain,
which on clinical examination was shown to be due to a subdural
cervical haematoma. Prothrombin complex activity was low and
diphenacoum was present in the plasma. Specific medical management
led to a complete recovery.
Greeff et al. (1987) reported accidental bromadiolone poisoning
in two children, resulting in prolonged anticoagulation.
Descarboxyprothrombin levels were increased in both cases by 27% and
29.9%, respectively (normal, non-detectable level). The first child
rapidly recovered after treatment with high-dose intravenous factor
IX-prothrombin complex and vitamin K1. The clotting profile became
normal on the third day after admission. The second child gave a poor
response to 10 mg intravenous vitamin K1 and the dose was increased
to 20 mg.
8.1.3 Controlled human studies
Single oral doses of 60, 70, 80 or 120 mg warfarin decreased the
prothrombin concentrations in volunteers to zero by the third day.
After the administration of 50 mg vitamin K1, the prothrombin
concentrations returned by the sixth day to 60, 70, 55 and 63%,
respectively, of the normal value (Anonymous, 1965).
When a single oral dose of 20 mg chlorophacinone was given to
three volunteers, the lowest prothrombin times were 35, 34 and 38% of
the pretreatment value on days 2, 4 and 2, respectively. Eight days
after administration without any treatment the values were 80, 100 and
90%, respectively (Anonymous, 1965).
8.2 Monitoring of biological effects
8.2.1 Effects of short- and long-term exposure
Two cases of occupational exposure to brodifacoum and difenacoum
were reported by Park et al. (1986). The exposure was of a chronic
nature (2 and 4 years, respectively). Plasma analysis in the first
patient revealed the presence of both difenacoum and brodifacoum in
the range of 30-50 µg/litre. In both patients unexpectedly high
concentrations of vitamin K1 2,3-epoxide were found in the presence
of normal clotting factor activities and antigen levels suggesting the
presence of coumarin anticoagulants in the liver.
A case of poisoning in a 23-year-old man resulting from prolonged
skin contact during the process of preparing and distributing warfarin
baits has been reported (Fristedt & Sterner, 1965).
8.2.2 Epidemiological studies
During a production run preparing ready-to-use flocoumafen bait
(0.005% in baits) in a formulation plant, the effect of the
rodenticide on blood coagulation factors was monitored in 12 subjects,
using the classical prothrombin time test, a modified prothrombin time
technique and measurement of prothrombin (factor II) concentration in
blood. No adverse health effects were observed in any subject
involved in formulation operations. No changes were observed in any
of the three tests that could be ascribed to absorption of flocoumafen
into the body (Tuinman & Van Sittert, 1986).
8.3 Developmental effects
Developmental effects have been reported when anticoagulants,
particularly warfarin, have been administered as therapeutic agent
during pregnancy. Hall et al. (1980) reviewed 418 reported
pregnancies in which coumarin and indan-1,3-dione derivatives were
used, and found that one-sixth resulted in abnormal liveborn infants,
one-sixth in abortion or stillbirth, and two-thirds in apparently
normal infants.
According to Hall (1976), there are two types of defects
associated with anticoagulants, dependent upon the time of
administration during pregnancy. The first, a characteristic
embryopathy described by the terms "warfarin embryopathy" or "fetal
warfarin syndrome", occurs from early, first-trimester use. Fetal
wastage and other abnormalities, especially central nervous system
anomalies, result from treatment later during gestation (usually the
second or third trimesters). Clotting factors are not present in
first-trimester embryos and this may explain the differences in fetal
abnormalities.
The most consistent feature of warfarin embryopathy is nasal
hypoplasia. Choanal stenosis has been observed, and respiratory
difficulty is typical because of the narrowed nasal passages (Smith &
Cameron, 1979; Pauli & Hall, 1979).
The other common feature is bone abnormalities of the axial and
appendicular skeleton. Laryngeal and tracheal-thyroid calcification
has also been noted (Schardein, 1985).
Other non-skeletal abnormalities reported include
ophthalmological malformations of several types, including defects
leading to blindness, developmental delay, low birth weight (premature
birth), mental retardation, hypotonia and ear anomalies (Carson &
Reid, 1976; Schardein, 1985).
No cases of embryopathy from anticoagulants in their use as
rodenticides have been reported.
8.4 Other adverse effects
One of the more common side-effects of coumarin therapy is skin
necrosis (Brooks & Blais, 1991; Eby, 1993; Locht & Lindström, 1993).
Although this effect has been associated with a number of different
agents, it is commonly referred to as warfarin-induced skin necrosis
(WISN). The frequency of this effect has been estimated to be between
1 in 100 and 1 in 10 000, with the majority of cases occurring in
women. Symptoms of WISN typically appear 3-6 days after the
initiation of warfarin therapy, and the areas of skin involved are
most frequently areas with subcutaneous fat, particularly the breasts,
thighs, and buttocks. There is some evidence that deficiencies in
vitamin K-dependent anticoagulant proteins (protein C and protein S)
may underlie the susceptibilities of at least some individuals to WISN
(Anderson et al., 1992). The occurrence of WISN may be avoided in at
least some cases by the co-administration of heparin.
Warfarin therapy has been noted to result in increases in the
incidence of haematomas, including intraspinal epidural haematoma
(Murphy & Nye, 1992), liver haematoma (Erichsen et al., 1993), and
intramural haematoma of the small intestine (Avent et al., 1992).
Bone contains three vitamin-K-dependent proteins, i.e.
osteocalcin, matrix Gla-protein and protein S. It has been shown that
oral anticoagulants (phenprocoumon, acenocoumarol) reduce both the
plasma antigen level as well as the Gla content of osteocalcin (Van
Haarlem et al., 1988). Furthermore it was found that a poor vitamin K
status was associated with a high urinary calcium loss (Knapen et al.,
1993). These data are consistent with the observation that the bone
mass in patients on long-term anticoagulant treatment was
significantly lower than in a group of age- and sex-matched controls
(Fiore et al., 1990; Resch et al., 1991). Vitamin-K-dependent proteins
have also been identified in calcified atherosclerotic plaques, where
they were suggested to contribute to the prevention of vascular
calcification (Gijsbers et al., 1990). In this respect it is
remarkable that warfarin treatment has been shown to increase rather
than to reduce atherosclerosis in an animal model (Antov et al.,
1985). Vitamin-K-dependent proteins not related to blood coagulation
are still discovered regularly (Manfioletti et al., 1993), and it is
important to remain alert for unexpected side-effects of vitamin K
antagonists, notably after long-term exposure.
8.5 Methods for assessing absorption and effects of anticoagulant
rodenticides
The laboratory control of orally administered coumarin
derivatives has been carried out using the classical one-stage
prothrombin time test (Quick, 1935) or modified techniques such as
"Thrombotest" (Owren, 1959). However, these tests have been designed
for clinical monitoring of circulating clotting factors during
anticoagulant therapy. The monitoring of occupational exposure to
rodenticides requires the prothrombin time test to be of sufficient
sensitivity to measure changes in the normal range (Tuinman & Van
Sittert, 1985).
Repeated occupational exposure to low levels of anticoagulant
rodenticides could gradually deplete vitamin-K-dependent coagulation
factors in the blood. To detect unwanted exposure of humans in an
early state, a careful screening of those at risk is recommended. The
question is which screening method is the most suitable to monitor low
levels of rodenticide ingestion. Prothrombin time and related tests
are "overall" clotting tests, which were developed for monitoring
patients under deep anticoagulation. These tests are easy to perform
and do not require complicated equipment, but they are relatively
insensitive when used for monitoring milder anticoagulation states
(Tuinman & Van Sittert, 1985; Ross et al., 1992; Travis et al., 1993).
If possible, specific and more sensitive tests should be used. The
most sensitive test, applicable over a wide range of anticoagulation
states, is the direct detection of descarboxy-prothrombin using a
monoclonal antibody specifically recognizing the descarboxy form of
prothrombin (Widdershoven et al., 1987). Another marker for
monitoring poor vitamin K status at an early stage is
descarboxy-osteocalcin (Knapen et al., 1993), but the commercial test
kits presently available need to be substantially improved and
simplified before they can be recommended for this purpose in routine
laboratories.
Another method was suggested by Park et al. (1986), who
repeatedly injected 10 mg of vitamin K1 into factory workers who had
been exposed to brodifacoum. The authors observed that 2-4 h after
injection the circulating KO/K ratios were significantly elevated even
18 months after the prothrombin times had returned to normal values.
This suggests that very low liver concentrations of brodifacoum can be
detected from the altered KO/K ratio rather than from tests based on
blood coagulation parameters. Because this method requires vitamin K
administration shortly before blood sampling, it is only applicable in
cases of anticoagulant poisoning, and not for the routine control of
plant workers.
Methods for the direct detection of coumarin anticoagulants in
plasma and serum have been reported, all of which are based on the
extraction of plasma and pre-purification of the sample, followed by
HPLC analysis with fluorescence detection (Hunter, 1983; Murphy et
al., 1989; Felice & Murphy, 1989; Felice et al., 1991; O'Bryan &
Constable, 1991). However, such facilities will not be available in
most routine laboratories. Moreover, the blood sampling should be
performed within a reasonably short period after ingestion of the
coumarins, because these drugs are rapidly cleared by the liver. This
places severe restrictions on the applicability of these techniques,
particularly for the second-generation anticoagulants.
Plasma chlorophacinone determinations were performed in three
cases of intoxication. The risk of bleeding was minimal when the
plasma level was below 1 mg/litre (Burcuoa et al., 1989).
Brodifacoum was detected in a case of self-ingestion by a
25-year-old woman who denied any intake of anticoagulants but was in
need of vitamin K1 treatment for 8 months. Factor assays revealed a
marked reduction in the levels of the vitamin K-dependent factors II,
VII, IX and X and normal levels of factor V and VIII:C (Exner et al.,
1992).
The diagnosis in a case of difenacoum intoxication was confirmed
by analysis of difenacoum in serum (0.6 mg/litre) (Butcher et al.,
1992).
Bromadiolone was analysed in the serum of a 27-year-old female
with a history of bleeding. She denied any contact with a rodenticide
but the bromadiolone concentration in serum was 40 mg/litre (Chow et
al., 1992).
Hollinger & Pastoor (1993) reported results of comparison of
plasma brodifacoum concentrations and prothrombin levels over time in
a case of brodifacoum poisoning. Brodifacoum was eliminated according
to a two-compartment mode, with an initial half-life of 18 h and a
terminal half-life of 24.2 days. On admission, the brodifacoum level
was 731 µg/litre. Treatment with large doses of phytonadione lasted
for 4 months.
8.6 Treatment of anticoagulant rodenticide poisoning
All suspected poisoned patients should receive medical attention
immediately. Rapid determination of prothrombin time and search for
evidence of bleeding is essential and may have to be maintained for
several weeks.
8.6.1 Minimizing the absorption
Gastric lavage or induction of emesis are indicated in all cases
of superwarfarin rodenticide ingestion if it was recent and the amount
is possibly lethal or uncertain. Repeated administration of activated
charcoal is useful. Cathartics could also be administered (Sullivan
et al., 1989; Smolinske et al., 1989; Donovan et al., 1990).
8.6.2 Specific pharmacological treatment
8.6.2.1 Vitamin K1 (phytomenadione)
Vitamin K1 is the specific antidote of choice. Depending on
whether the poisoning is due to warfarin or superwarfarin, the dosage
may differ as well as the duration of treatment. Dosage is dependent
on coagulation parameters, mainly prothrombin time.
If the patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection.
Prothrombin time should be checked at 3-hourly intervals in severe
cases and after 8-10 h in less severe cases. If no improvement
occurs, vitamin K1 injection should be repeated. Doses of up to
125-200 mg/day have been given without adverse effects (Lipton &
Klass, 1984; Sheen et al., 1994).
In moderate to minor cases of poisoning, vitamin K1 may be
given in lower doses.
After initial parenteral vitamin K1 administration, oral
treatment can be continued for a prolonged period of time. Oral
treatment can also be sufficient in minor cases.
The major difference between warfarin and second-generation
rodenticides is that the latter can cause increased bleeding for a
longer period of time than warfarin, as they have a much longer
half-life in the body. Therefore vitamin K1 should be given for
months rather than weeks. It is also prudent to monitor prothrombin
time for some time after cessation of this treatment to ensure that
there is no regression.
In warfarin-resistant individuals, 10 times the normal dose of
warfarin is required to achieve a reduction in the plasma prothrombin
level. However, these individuals also respond more strongly to the
effect of vitamin K (O'Reilly et al., 1963; O'Reilly et al., 1964).
8.6.2.2 Blood components
The following products could be considered, subject to
availability:
* whole blood
* fresh frozen plasma and fresh blood should be used in cases of
acute severe bleeding in order to rapidly restore the blood
clotting factors
* factor concentrate may be considered in severe cases, especially
if the amount of plasma would be too great (volume overload)
8.6.2.3 Phenobarbital
In the cases reported by Lipton & Klass (1984) and Jones et al.
(1984), phenobarbital therapy was included. It was presumed that
anticoagulants had been metabolized by the mixed function oxidase
system based on animal experiments (Bachmann & Sullivan, 1983) and
that, by inducing this enzyme system, phenobarbital might increase
their metabolism.
Robinson & Mac Donald (1966) found that the pharmacological
activity of warfarin in humans decreased when it was combined with
phenobarbital.
8.6.3 Response to therapy
Patient should be kept in hospital until the prothrombin time has
remained normal for 3 days. It is suggested that oral treatment with
10 mg vitamin K1 twice daily may be necessary for up to 60 days with
close monitoring of prothrombin time.
According to Hoffman et al. (1988), factor analysis allows for a
detailed evaluation of the course of toxicity, and the response to
therapy. Monitoring the prothrombin time alone could offer a false
sense of confidence and delay effective treatment.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
No data are available for the effects on microorganisms in water
and in soil.
9.1.2 Aquatic organisms
The acute toxicity of technical flocoumafen to planktonic algae
(Selenastrum capricornutum) was determined in a 4-day growth test.
The 96-h EC50, based on cell counts on day 4, was calculated to be
1.1 mg/litre (Pearson & Wallace, 1984).
Pearson (1984) reported static acute toxicity tests for
flocoumafen on Daphnia magna. The 48-h EC50 values, based on
immobilization, were 1.4 mg/litre for technical grade flocoumafen and
280 mg/litre for 0.5% flocoumafen. Pearson & Wallace (1984) found a
48-h EC50 for Daphnia magna of 0.66 mg/litre for technical grade
flocoumafen.
Anticoagulants are highly toxic to fish when tested as technical
formulations (Hill et al., 1976; Pearson & Wallace, 1984) (Table 8).
9.1.3 Terrestrial organisms
9.1.3.1 Acute toxicity
Bird species vary in their susceptibility to anticoagulant
rodenticides. The acute oral LD50 of brodifacoum for the mallard
duck (Anas platyrhynchos) is 2.0 mg/kg (Ross et al., 1978).
Symptoms of poisoning became apparent approximately 7 days after
dosing and included lethargy, weakness and lack of muscular
coordination. Prolonged bleeding occurred from any small wounds and
extensive bruising and subcutaneous haemorrhage were noted. Blood was
observed in the faeces. Deaths occurred principally during the period
of 7-14 days after administration. No deaths occurred more than 4
weeks after administration.
The acute toxicity of flocoumafen to the mallard duck appears to
be dependent on the age of birds. The LD50 values for 12- and
18-week-old birds are 24 mg/kg and 94 mg/kg, respectively (Roberts et
al., 1985b,c).
Table 8. Acute toxicity of anticoagulant rodenticides for aquatic organisms (96-h LC50)
Rodenticide Species Stat/flowa LC50 (mg/litre) Reference
Brodifacoum Rainbow trout flow 0.051 Hill et al. (1976)
Diphacinone (95%) Bluegill sunfish 7.6 Kosmin & Barlow (1976)
Channel catfish 2.09 Kosmin & Barlow (1976)
Rainbow trout 2.82 Kosmin & Barlow (1976)
Pink shrimp > 10 Kosmin & Barlow (1976)
Fiddler crab > 10 Kosmin & Barlow (1976)
Diphacinone (0.005%) Bluegill sunfish 288 Kosmin & Barlow (1976)
Channel catfish 285 Kosmin & Barlow (1976)
Rainbow trout 425 Kosmin & Barlow (1976)
Pink shrimp 33 Kosmin & Barlow (1976)
Fiddler crab 37 Kosmin & Barlow (1976)
Warfarin Bluegill sunfish 88 Brorson et al. (1994)
Flocoumafen (0.5%) Rainbow trout static 190 Pearson (1984)
Flocoumafen (technical grade) Rainbow trout static 0.95 Pearson (1984)
Rainbow trout static 0.32 Pearson & Wallace (1984)
Rainbow trout static 0.091 Pearson & Wallace (1984)
Common carp static 0.22 Pearson & Wallace (1984)
Bromadiolone Rainbow trout 1.4 Tomlin (1994)
a stat = static conditions (water unchanged for duration of test)
Sex differences were observed in the acute toxicity for bobwhite
quail (Colinus virginianus) of difenacoum. LD50 values for male
and female birds were 140 mg/kg body weight and 56 mg/kg body weight,
respectively (Ross et al., 1980c).
Birds seem to be relatively tolerant to diphacinone, the LD50
for mallard duck being 3158 mg/kg (Kosmin & Barlow, 1976).
The acute toxicity of brodifacoum for various species of birds is
presented in Table 9.
Table 9. The acute toxicity of brodifacoum to birds (Godfrey, 1985)
Species Acute oral LD50
(mg/kg)
Black-backed gull (Larus dominicans) < 0.75
Black-billed gull (L. bulleri) < 5.0
Canada goose (Branta canadensis canadensis) < 0.75
Pukeko (Porphyris porphyris melanotus) 0.95
Mallard duck (Anas platyrhynchus) 4.6
California quail (Lophortyx californica) 3.3
Blackbird (Turdus merula) > 3
House sparrow (Passer domesticus) > 6
Hedge sparrow (Prunella modularis occidentalis) > 3
Wax-eye (Zosterops lateralis) > 6
Harrier hawk (Circus approximans) 10.0
Ring-necked pheasant (Phasianus colchicus) 10.0
Paradise duck (Tadorna variegata) > 20
9.1.3.2 Primary toxicity
The subacute (5-day) dietary toxicity (LC50) of difenacoum for
the bobwhite quail was found to be between 0.25 and 7.00 mg/kg diet
(Ross et al., 1980a). The 5-day LC50 of difenacoum for the mallard
duck was calculated to be 18.9 mg/kg diet (Ross et al., 1980b).
Similarly to the acute toxicity studies, diphacinone was found to
be less toxic in 8-day dietary studies. The LC50 for the bobwhite
quail was 4485 mg/kg diet and for the mallard duck was > 10 000 mg/kg
diet (Kosmin & Barlow, 1976).
White Leghorn hens (4 per group) were fed warfarin,
coumatetralyl, bromadiolone, difenacoum or brodifacoum in baits as a
choice to non-poisonous chicken food for 15 days. No symptoms
appeared at a total warfarin intake of up to 171 mg/kg body weight.
Coumatetralyl and brodifacoum killed all hens after intakes of
79-137 mg/kg body weight of active ingredient and 7.1-15 mg/kg body
weight of active ingredient, respectively. Both bromadiolone and
difenacoum killed two of the hens, at 5.9 and 15.9 mg/kg body weight
of active ingredient for bromadiolone, and at 18.9 and 26.3 mg/kg
body weight active ingredient for difenacoum within 10-15 days (Lund,
1981).
Christopher et al. (1984) dosed male hybrid leghorn chickens with
anticoagulant rodenticides. No signs of poisoning were observed in
chickens fed 183.7 mg warfarin/kg (mean a.i. ingested) for 3 days. No
mortality occurred after the ingestion of a total dose of 36.9 mg
bromadiolone/kg over a 3-day period. Birds fed a diet containing
brodifacoum for 3 days ingested a mean of 28.9 mg/kg (active
ingredient). One bird died on day 4 while the other five birds
exposed had died by day 16.
Two-day no-choice tests in which chukar partridges ( Alectoris
graeca cypriotes Hartert) were fed 0.005% bromadiolone or difenacoum
resulted in no mortality. Ten-day no-choice feeding tests resulted in
6 out of 12 and 8 out of 12 dead partridges for bromadiolone and
difenacoum, respectively. The highest quantity of bait consumed by an
individual bird without any lethal effects was 411 g of bait with
bromadiolone (34.8 mg a.i./kg body weight) and 326.2 g of bait with
difenacoum (29.1 mg a.i./kg body weight) (Krambias & Hoppe, 1987).
9.1.3.3 Secondary toxicity
Barn owls (Tyto alba) were fed rats poisoned with diphacinone,
chlorophacinone, coumafuryl, difenacoum, bromadiolone or brodifacoum.
Five out of six owls died of haemorrhaging after feeding on rats
killed with brodifacoum after 8 to 11 days. Sublethal haemorrhaging,
but no mortality, occurred in owls fed rats killed with difenacoum.
One owl died following 10 days of treatment with bromadiolone-poisoned
rats, while five showed no symptoms. No abnormalities were observed
in two owls fed rats killed with diphacinone, coumafuryl or
chlorophacinone. Owls that died behaved normally until 24 h or less
before death, when they became lethargic and stopped eating
(Mendenhall & Pank, 1980).
Mice (weighing 35 g) were fed for 1 day (no choice) on a mixture
containing either 0.005% difenacoum or 0.002% brodifacoum. The mean
mass of residue on the day of death in a whole mouse was estimated to
be 10.17 µg for difenacoum and 15.36 µg for brodifacoum. Difenacoum-
and brodifacoumpoisoned mice were fed to captive barn owls for
successive periods of 1, 3 and 6 days. All of the owls fed on
difenacoum-poisoned mice survived the treatments and none showed
external bleeding. In contrast, four of the six owls fed
brodifacoum-poisoned mice died within 6-17 days after a 1-day dose.
The estimated lethal dose of brodifacoum was 0.15-0.18 mg/kg. After
death these owls had 0.63-1.25 mg brodifacoum/kg in their livers
(Newton et al., 1990).
Newton et al. (1994) fed barn owls flocoumafen-dosed mice for
consecutive periods of 1, 3 and 6 days. Four of the birds survived;
the fifth bird died from haemorrhaging 5 days after the final dose.
During the feeding trial birds received cumulative flocoumafen doses
ranging from 0.78 to 1.25 mg/kg.
Gray et al. (1992) investigated the toxicity of brodifacoum,
difenacoum and flocoumafen for barn owls fed poisoned mice. For each
rodenticide, the owls survived a cumulative dose of at least 1.9 mg/kg
owl weight over 15 days of treatment. All owls with cumulative doses
in excess of 1.9 mg/kg body weight showed multiple treatment-related
effects. The three rodenticides had approximately the same order of
magnitude of toxicity to barn owls. Gray et al. (1994) developed a
non-invasive method for monitoring the exposure of these birds to
second-generation rodenticides by measuring compounds in regurgitated
owl feed.
Radvanyi et al. (1988) fed American kestrels (Falco sparverius)
on meadow voles that had been maintained on 2% chlorophacinone. Voles
consumed approximately 53 mg of 2% chlorophacinone (1.14 mg a.i.)
before dying within 6 days. No kestrels fed poisoned mice, for up to
21 consecutive days, died. Haematomas were observed on the pectoral
muscles, lungs, liver and heart of exposed birds.
In a study by Townsend et al. (1981), captive-bred tawny owls
(Strix aluco) were given warfarin-treated mice for 3 months. No
behavioural changes were observed. Prothrombin levels decreased to
less than 10-12% of normal but returned to normal after 9 days.
Townsend et al. (1984) found that daily warfarin intakes
approaching 0.3 mg/kg body weight or 30 µg of warfarin/day can cause
the death of list weasels (Mustela nivalis) by secondary poisoning.
Lethal exposure occurred if mice were contaminated with 1.0-1.5 mg
warfarin/kg, an amount reached following exposure to 0.005 or 0.02%
sodium warfarin for about 3 days.
9.2 Field observations
9.2.1 Primary poisonings
There are few data available on the non-target hazard of
anticoagulant rodenticides in field conditions.
Greig-Smith et al. (1988) reported incidents in England and Wales
with barn owls and foxes. There were three incidents in which barn
owls were found to have residues of the anticoagulant rodenticide
brodifacoum in their livers. In two birds, residues were thought to
represent lethal poisoning (0.61 and 0.29 mg/kg) but in the third the
level was only 0.099 mg/kg. There were six separate incidents in
which dead foxes were found to contain residues of brodifacoum,
bromadiolone, coumatetralyl, difenacoum or warfarin. Residue levels
showed a wide range from 0.009 to 0.46 mg/kg tissue. Three of the
foxes contained more than one rodenticide, one animal revealing
residues of difenacoum, bromadiolone and warfarin.
Poisonings of pheasants and partridges by chlorophacinone used
against Microtus arvalis have been reported (Giban, 1974).
Reece et al. (1985) reported that, a few days after bromadiolone
was placed out for control of rodents, three pea fowl
(Pavo cristatus), a little raven (Corvus mellori) and an eastern
swamp hen (Porphyrio porphyrio melanotus) died. Necropsy showed
that the birds had been in good condition. One of the pea fowl and
the swamp hen had massive intra-abdominal haemorrhage without evidence
of external trauma. All had severe congestion of the liver and lungs.
The birds had negligible gut contents, so analysis for bromadiolone
could not be performed.
9.2.2 Secondary poisonings
Difenacoum and brodifacoum were detected in 15 of a total of 145
dead barn owls in Britain, and on postmortem the cause of death for
one owl was diagnosed as rodenticide poisoning (Newton et al., 1990,
see section 5.1).
A study of 35 active nests and the radio-telemetry of 34 barn
owls in an area where brodifacoum baits (0.005%) were used in and
around farm buildings in New Jersey, USA, showed no adverse effects of
the rodenticide on the owls studied. In this study, rats and house
mice were the target organisms and barn owls were found not to feed on
these animals to any extent. Chicks were fledged from at least eight
nests where poisoned rodents had been available during part of the
nesting and feeding period, but no rodenticide-induced mortality was
observed. Traces of brodifacoum (less than 0.05 mg/kg) were found in
a barn owl that had been accidentally electrocuted (Hegdal &
Blaskiewicz, 1984).
Merson & Byers (1984) monitored eastern screech owls (Otus asio)
using radio-transmitters following the use of 0.005% brodifacoum for
rodent control in a commercial orchard. Brodifacoum was detected in
screech owl pellets (see section 5.1). There were no owl deaths which
could be attributed to brodifacoum poisoning. Two owls were analysed
at the end of the study; one showed signs of haemorrhage and
contained 0.21 mg brodifacoum/kg and the other owl contained no
brodifacoum. In a larger study, Hegdal & Colvin (1988) monitored 38
eastern screech owls and several other species of owl following
brodifacoum application in an orchard. Minimum mortality was 58%
among screech owls for which more than 20% of the home range was
treated, as compared with 17% among those for which less than 10% of
the home range was treated. Secondary brodifacoum poisoning was the
most probable cause of death in six screech owls. Six radio-equipped
owls were collected 1-2 months post-treatment, and four contained
detectable concentrations of brodifacoum.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Anticoagulants, both hydroxycoumarins and indandiones, known also
as vitamin K antagonists, are widely used in urban rodent control and
against rodent pests in agriculture. They act by inhibiting the
vitamin K1 epoxide cycle, thereby depleting the active form of
vitamin K1 necessary for producing blood-clotting factors.
Since anticoagulant rodenticides are used in general as
low-concentration bait formulations and have low volatility, increased
levels in the air are unlikely. Being only slightly soluble in water
their use could not be a major source of water contamination.
Anticoagulant rodenticides are not intended for direct application to
growing crops. Hence no residues in plant foodstuffs are expected.
The controlled medicinal use of warfarin exposes more people to
higher concentrations over a longer period than would be expected to
occur as a result of accidental human exposure due to its use as a
rodenticide.
Occupational exposure may occur during manufacture, formulation
and bait application, but figures indicating the levels of exposure
are not available.
Anticoagulant rodenticides are readily absorbed through the
gastrointestinal tract, skin and respiratory system. The major route
of elimination in various species after oral administration is through
the faeces. The urine is a very minor route of elimination. The liver
is the major organ for accumulation and storage of anticoagulants.
The metabolism pattern of warfarin and indandiones mainly
involves hydroxylation. The second-generation hydroxycoumarins are
found principally as unchanged parent compounds. Their elimination
from the liver is slow with a biphasic rate, where a rapid initial
phase is followed by a prolonged second phase.
Most of the anticoagulants have high acute toxicity by oral,
percutaneous and inhalation routes of exposure. The second-generation
anticoagulants are more toxic than the first-generation one in the
sense that a single feeding may be lethal. Signs of poisoning in all
species, including humans, are associated with increased bleeding
tendency.
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxication from occupational
exposure to anticoagulants have also been observed.
The level of prothrombin concentration is a satisfactory guide to
the severity of acute intoxication and the effectiveness and duration
of the therapy. The specific antidote is vitamin K1. The high
retention of the second-generation anticoagulants in the liver means
that any treatment of intoxication should be prolonged to ensure that
anticoagulant activity does not recur.
The addition of bittering agents to anticoagulant rodenticides is
aimed at discouraging human consumption.
Warfarin has been found to be teratogenic in both rats and
humans. Second-generation anticoagulant rodenticides do not
demonstrate teratogenicity in laboratory animals. Developmental
effects in humans are observed when warfarin has been taken as a
therapeutic agent during pregnancy. No cases of embryopathy from
anticoagulants in their use as rodenticides have been reported.
Second-generation anticoagulants are not intended to be used as
therapeutic agents, and the risks associated with, for example,
warfarin will not apply.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on some compounds to demonstrate an absence of mutagenicity.
There is experimental evidence that coumarin anticoagulants not
only inhibit the vitamin K cycle in the liver, but also in other
tissues such as bone. In humans it has been demonstrated that even
low levels of vitamin K deficiency form a risk factor for developing
osteoporosis (Hart et al., 1985; Szulc et al., 1993). Therefore, it
appears that long-term exposure to low levels of anticoagulant may
have an adverse effect on bone metabolism.
10.2 Evaluation of effects on the environment
Unlike conventional crop protection products, which must be
applied over relatively large crop areas, anticoagulant rodenticides
are applied to discrete sites in the form of low concentration baits.
Most of the anticoagulants are stable under normal conditions.
They are slightly soluble in water, and as bait formulations their use
is unlikely to be a source of water contamination. They appear to
bind rapidly in the soil, with very slow desorption and no leaching.
In general, anticoagulants are highly toxic to aquatic organisms when
tested as technical material.
Non-target organisms are potentially at risk in two ways: from
direct consumption of baits (primary hazard) and through eating
poisoned rodents (secondary hazard).
Bird species vary in their susceptibility to anticoagulant
rodenticides. Small pellets and whole grain baits are highly
attractive to birds. Wax block formulation appear to decrease the
attractiveness to the birds and so reduce the possibility of poisoning
incidents. It is difficult to assess the risks to birds due to direct
consumption of baits because most of the published studies are
toxicity trials in laboratory conditions.
The main cause for poisoning of domestic animals is through
direct consumption of anticoagulant baits.
Some of the anticoagulants show a similar range of acute toxicity
for non-target non-rodent mammals as for target rodents. The primary
hazard is usually expressed by the amount of finished bait which must
be consumed to approach the lethal dose. The bait concentration of
second-generation rodenticides is usually around 0.005%, whereas the
concentration of the first-generation rodenticides in the bait may be
5 to 10 times higher. Thus, to reach the toxic or lethal dose,
non-target animals must consume comparatively large amounts of bait
with low concentrations of active ingredient.
Secondary poisoning through the consumption of rats and mice
killed with anticoagulants may occur in dogs and cats in urban
situations but is more likely in farm situations.
Some secondary toxicity laboratory studies with wildlife have
shown that captive predators could be intoxicated by no-choice feeding
of anticoagulant-poisoned or dosed prey. The significance of these
results in terms of hazard under field conditions is difficult to
assess because the predators would not be expected to eat only
poisoned animals. However, where they occur, predators may take
poisoned, but not dead, small mammals preferentially. In areas close
to baiting, poisoned rodents may represent a high proportion of the
diet for individual birds. However, only a few individuals will be
affected except in situations of very widespread and constant use of
baits. Therefore, some kills of owls will be expected but no severe
population effects. This agrees with observed field effects with small
numbers of poisoned owls.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
a) Exposure of the general population to anticoagulant rodenticides
via food and drinking-water is unlikely and does not constitute a
significant health hazard.
b) Poisoning incidents may occur in cases of massive intentional or
unintentional ingestion or prolonged skin contact during
manufacture and formulation.
c) Anticoagulant rodenticides are relatively persistent in the
environment, but their specific use as low-concentration bait
formulations limits the potential for environmental
contamination.
d) Direct and secondary poisoning of birds, domestic and farm
animals, and wildlife may occur.
e) The major difference between the first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention, resulting in an increased tendency for bleeding over a
longer period of time.
f) The mode of action of anticoagulant rodenticides is known and an
effective antidote is available, i.e. vitamin K1.
g) There is no available evidence that this class of compound is
mutagenic or carcinogenic.
h) Only warfarin has been shown to possess some teratogenic
potential in both rats and humans.
11.2 Recommendations for protection of human health and the
environment
a) Exposed workers should receive appropriate biomonitoring and
health evaluation.
b) The inclusion of a bittering agent in formulations at appropriate
concentrations may reduce accidental ingestion.
c) For preventing primary poisoning, baits less attractive to birds
and domestic animals, as well as pulsating baiting, should be
used.
d) The location of bait placement should be carefully selected.
e) Killed rodents should be burned or buried to reduce the risk of
secondary poisoning of predators.
f) Training in the safe handling of rodenticides is essential.
12. FURTHER RESEARCH
a) Studies of exposed humans are required, particularly with regard
to possible teratogenic and embryotoxic effects.
b) More information is needed in order to evaluate the risks of
occupational exposure to anticoagulant rodenticides.
c) Methods more sensitive than prothrombin time determination need
to be developed for routinely assessing the absorption and
effects of anticoagulants.
d) More data are required to assess secondary effects on non-target
organism populations.
e) The extent to which second-generation anticoagulant rodenticides
are transferred across the placenta should be evaluated.
f) More information should be collected concerning the tissue
distribution of anticoagulant rodenticides after their ingestion
and their effects on physiological processes other than blood
coagulation, notably calcium and bone metabolism. This is
particularly necessary for long-term, low-level exposure to these
compounds.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by Hazard
(WHO, 1994), anticoagulant rodenticides were classified, according to
their acute oral LD50, as follows:
Class Ia (Extremely hazardous) Oral LD50 (mg/kg)
Brodifacoum 0.3
Bromadiolone 1.12
Chlorophacinone 3.1
Difenacoum 1.8
Difethialone 0.56
Diphacinone 2.3
Flocoumafen 0.25
Class Ib (Highly hazardous)
Coumachlor 33
Coumatetralyl 16
Warfarin 10
Class II (Moderately hazardous)
Pindone 50
A Poison Information Monograph for brodifacoum has been issued
(IPCS, 1992), and one on warfarin is in preparation.
REFERENCES
Allen JA, Proudlock RJ, & McCaffrey KJ (1986) Genotoxicity studies
with WL 108366 (rodenticide): in vivo chromosome studies with rat
bone marrow cells. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL 85/8610).
Alstad AD, Casper HH, & Johnson LJ (1985) Vitamin K treatment of sweet
clover poisoning in calves. J Am Med Assoc, 187: 729-731.
Anderson DR, Brill-Edwards P, & Walker I (1992) Warfarin-induced skin
necrosis in 2 patients with protein S deficiency: Successful
reinstatement of warfarin therapy. Haemostasis, 22: 124-128.
AMA (1980) AMA drug evaluations, 4th ed. Chicago, Illinois, American
Medical Association.
Anonymous (1965) Technical report on chlorophacinone. Lyon, France,
Lipha S.A.
Anonymous (1976) 'Ratak' containing difenacoum: A new rodenticide.
Haslemere, Surrey, Imperial Chemical Industries Ltd, Plant Protection
Division, 18 pp.
Anonymous (1985) Brodifacoum: safety in use. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division, 8 pp
(ICI Agrochemical Information Bulletin).
Anonymous (1988) The treatment of anticoagulant rodenticide poisoning.
Imperial Chemical Industries Ltd, Lipha S.A., Shell International
Chemical Company Ltd, and Sorex Ltd.
Antov G, Kasakova B, & Ajanova A (1985) Effect of warfarin on
experimentally induced sclerotic processes. In: Pathological models in
toxicological studies. Bucharest, Romania, Ministry of Chemical
Industry, Industrial Head-Office for Medicinal Drugs and Cosmetics,
pp 153-157.
Ashton AD, Jackson WB, & Peters H (1987) Comparative evaluation of
LD50 values for various anticoagulant rodenticides. In: Richards CGL
& Ku TY ed. Control of mammal pests. London, New York, Philadelphia,
Taylor & Francis, pp 187-197.
Avent ML, Canady BR, & Sawyer WT (1992) Warfarin-induced intramural
hematoma of the small intestine. Clin Pharm, 11: 632-635.
Bachmann KA & Sullivan TJ (1983) Dispositional and pharmacodynamic
characteristics of brodifacoum in warfarin-sensitive rats.
Pharmacology, 27: 281-288.
Barlow AM, Gay AL, & Park BK (1982) Difenacoum (Neosorexa) poisoning.
Br Med J, 285: 541.
Blair D (1984) Toxicology of a candidate rodenticide: The acute 4-hour
inhalation toxicity of manufacturing master mix (bait concentrate)
containing 0.5% m/m WL 108366. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 71 pp (Report No. SBGR.84.151).
Boermans HJ, Johnstone J, Black WD, & Murphy M (1991) Clinical signs,
laboratory changes and toxicokinetics of brodifacoum in horses. Can J
Vet Res, 55: 21-27.
Braithwaite GB (1982) Vitamin K and brodifacoum. J Am Vet Med Assoc,
181(6): 531, 534.
Bratt H (1987) Difenacoum: Elimination from tissues of rats following
administration of a single oral dose. Macclesfield, Surrey, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report
No. CTL/P/1592).
Braselton WE Jr, Neiger RD, & Poppenga RH (1992) Confirmation of
indandione rodenticide toxicoses by mass spectrometry/mass
spectrometry. J Vet Diagn Invest, 4, 441-446.
Brooks LW & Blais FX (1991) Coumarin-induced skin necrosis. J Am
Osteopath Assoc, 91: 601-605.
Brooks TM, Clare MG, & Wiggins DE (1984) Genotoxicity studies with
WL 108366 (candidate rodenticide). Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 64 pp (Report No. SBGR.84.160).
Brorson T, Björklund I, Svenstam G, & Lantz R (1994) Comparison of two
strategies for assessing ecotoxicological aspects of complex
wastewater from a chemical-pharmaceutical plant. Environ Toxicol Chem,
14(4): 543-552.
Buckle AP (1994) Rodent control methods in rodent pests and their
control. Wallingford, Oxon, Commonwealth Agriculture Bureau (CAB)
International.
Buitenhuis HC, Soute BAM, & Vermeer C (1990) Comparison of the
vitamins K1, K2 and K3 as cofactors for the hepatic vitamin
K-dependent carboxylase. Biochim Biophys Acta, 1034: 170-175.
Bull JO (1976) Laboratory and field investigations with difenacoum, a
promising new rodenticide. In: Proceedings of the 77th Vertebrate Pest
Conference, Monterey, California, March 1976. Davis, California,
University of California, pp 72-84.
Bullard RW, Thompson RD, & Holguin G (1976) Diphenadione residues in
tissues of cattle. J Agric Food Chem, 24(2): 261-263.
Burucoa Ch, Mura P, Robert R, Boinot C, Bouquet S, & Piriou A (1989)
Chlorophacinone intoxication: A biological and toxicological study.
Clin Toxicol, 27: 79-89.
Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, & Ind PW (1992)
Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol,
11: 553-554.
Callander RD (1984) Product registration. Brodifacoum: An evaluation
in the salmonella mutagenicity assay. Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V. (ICI CTL Report No. CTL/P/949).
Carson M & Reid M (1976) Warfarin and fetal abnormality. Lancet,
1: 1127.
Chalermchaikit T, Felice LJ, & Murphy MJ (1993) Simultaneous
determination of eight anticoagulant rodenticides in blood serum and
liver. J Anal Toxicol, 17: 56-61.
Chesterman H, Burford P, Harling RJ, & Heywood R (1984) WL 108366
rodenticide: Acute oral toxicity in Beagle dogs. Huntingdon, United
Kingdom, Huntingdon Research Centre (HRC Report No. SLL/72/84757).
Chong LL, Chan WK, & Ho CH (1986) A case of 'superwarfarin' poisoning.
Scand J Haematol, 36: 314-315.
Chow EY, Haley LP, Vickars LM, & Murphy MJ (1992) A case of
bromadiolone (superwarfarin) ingestion. Can Med Assoc J, 147: 60-62.
Christopher MJ, Balasubramanyam M, & Purushotham KR (1984) Toxicity of
three anticoagulant rodenticides to male hybrid Leghorns. Z Angew
Zool, 71(3): 275-281.
Cifone MA (1993) Mutagenicity test on bromadiolone technical in the
CHO/HGPRT forward mutation assay (HWA Study No. 15310-0-435). Vienna,
Virginia, Hazleton Washington Inc., 48 pp.
Clare MG & Wiggins DE (1986) In vitro mutagenicity studies with
WL 108366 (rodenticide) using cultured Chinese hamster V 79 cells.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research
Centre, 33 pp (Report No. SBGR.86.014).
Coveney PC & Forbes S (1987) Degradation of WL 108366 (Storm) in rat
baits, carcasses and faeces in field situations. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.87.171).
Cross M & Clay P (1984) Product registration. Brodifacoum: Assessment
of mutagenic potential using L5178Y mouse lymphoma cells. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/975).
Dodds WJ & Frantz SC (1984) Dog and cat poisonings. Pest Control
Technol, 12(3): 14.
Donovan JW, Ballard JO, & Murphy MJ (1990) Brodifacoum therapy with
activated charcoal: Effect on elimination kinetics. Vet Hum Toxicol,
32: 350.
Dreyfus M, Dubouch P, Pierson K, Neveu Y, Martin E, & Tchernia G
(1983) Warfarin-contaminated talc. Lancet, 1: 1110.
Dubock AC (1986) The evaluation of potential effects on non-target
vertebrate populations as a result of pesticide use. In: Proceedings
of the Second Symposium on Recent Advances in Rodent Control, Kuwait,
1986, pp 257-269.
Dusein P, Manigand G, & Taillandier J (1984) Hypoprothrombinémie
sévère et prolongée après intoxication par chlorphacinone. Presse Méd,
13(30): 1845.
DuVall MD, Murphy MJ, Ray AC, & Reagor JC (1989) Case studies on
second-generation anticoagulant rodenticide toxicities in non-target
species. J Vet Diagn Invest, 1: 66-68.
Eby CS (1993) Warfarin-induced skin necrosis. Hematol/Oncol Clin North
Am, 7: 1291-1300.
Elias DJ & Johns BE (1981) Response of rats to chronic ingestion of
diphacinone. Bull Environ Contam Toxicol, 27: 559-567.
Ellenhorn MJ & Barceloux DG (1988) Anticoagulant poisoning. In:
Medical toxicology, diagnosis and treatment of human poisoning.
Amsterdam, New York, Elsevier Science Publishing Company Inc.,
pp 211-221.
Erichsen C, Sudenaa K, Sreide JA, Andersen E, & Tysvoer A (1993)
Spontaneous liver hematomas induced by anti-coagulation therapy.
Hepato-Gastroenterology, 40: 402-406.
Exner DV, Brien WF, & Murphy MJ (1992) Superwarfarin ingestion. Can
Med Assoc J, 146(1): 34-35.
FAO (1979) Rodenticides: Analyses specifications, formulations. Rome,
Food and Agriculture Organization of the United Nations, pp 1-79 (FAO
Plant Production and Protection Paper 16).
Feinsod FM, Allam I, Ragai A, & Saah AJ (1986) Rodenticide-induced
signs simulating Rift Valley fever in sheep and goats in Egypt. Vet
Rec, 118: 270-272.
Felice LJ & Murphy MH (1989) The determination of the anticoagulant
rodenticide brodifacoum in blood serum by liquid chromatography with
fluorescence detection. J Anal Toxicol, 13: 229-231.
Felice LJ, Chalermchaikit T, & Murphy MJ (1991) Multicomponent
determination of 4-hydroxycoumarin anticoagulant rodenticides in blood
serum by liquid chromatography with fluorescence detection. J Anal
Toxicol, 15: 126-129.
Fiore CE, Tamburino C, Foti R, & Grimaldi D (1990) Reduced bone
mineral content in patients taking an oral anticoagulant. South Med J,
83: 538-542.
Forsey JD (1983a) The acute skin irritation of novel anticoagulants in
male rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 3 pp.
Forsey JD (1983b) Acute eye irritation of novel anticoagulants in male
rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 4 pp.
Forsey JD (1985) An evaluation of the long term sub-acute oral
toxicity of WL 108366 in Wistar rats. Widnes, United Kingdom, Sorex
Ltd, Agricultural Research Department, 10 pp.
Fristedt B & Sterner N (1965) Warfarin intoxication from percutaneous
absorption. Arch Environ Health, 11: 205-208.
Furie B & Furie BC (1990) Molecular basis of vitamin K-dependent
gamma-carboxylation. Blood, 75: 1753-1762.
Giban J (1974) Use of chlorophacinone in the struggle against the
common vole (Microtus arvalis Pallas) and against the musk rat
(Ondatra zibethicus). In: Proceedings of the 6th Vertebrate Pest
Conference, Sacramento, California. Davis, California, University of
California, pp 263-271.
Gijsbers BLMG, van Haarlem LJM, Soute BAM, Ebberink RHM, & Vermeer C
(1990) Characterization of a gla-containing protein from calcified
human atherosclerotic plaques. Arteriosclerosis, 10: 991-995.
Godfrey MER (1984) The toxicology of brodifacoum to target and
non-target species in New Zealand. Proceedings of a Conference on the
Organization and Practice of Vertebrate Pest Control, Elvetham Hall,
Hampshire, England, 30 August-3 September 1982. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division,
pp 631-637.
Godfrey MER (1985) Non-target and secondary poisoning hazards of
"second generation" anticoagulants. Acta Zool Fennica, 173: 209-212.
Godfrey MER, Laas FJ, & Rammell CG (1985) Acute toxicity of
brodifacoum to sheep. N Z J Exp Agric, 13: 23-25.
Gorenzel WP, Marsh RE, & Salmon TR (1982) Single-feeding
anticoagulants. Pest Control, 50(2): 32.
Grand M (1976) Données expérimentales sur un nouveau raticide
anticoagulant: le bromadiolone. Phytiatrie-Phytopharmacie, 25: 69-88.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1992) Toxicity of
second generation rodenticides to barn owls. In: Proceedings of the
Brighton Crop Protection Conference - Pests and Diseases, 1992,
vol 2, pp 781-786.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1994) Non-invasive
method for monitoring the exposure of barn owls to second-generation
rodenticides. Pestic Sci, 41: 339-343.
Greeff MC, Mashile O, & MacDougall LG (1987) "Superwarfarin"
(bromadiolone) poisoning in two children resulting in prolonged
anticoagulation. Lancet, 1: 1269.
Greig-Smith PW, Fletcher MR, Hunter K, Quick MP, Ruthven AD, & Shaw IC
(1988) Pesticide poisoning of animals 1988: Investigations of
suspected incidents in Great Britain. A Report of the Environmental
Panel of the Advisory Committee on Pesticides.
Hadler MR (1974) Sub-acute five day oral toxicity of WBA 8119 to male
rats. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research.
Hadler MR (1975a) Acute oral toxicity of WBA 8119 to male rabbit.
Appendix 5. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research, 3 pp.
Hadler MR (1975b) Acute oral toxicity of WBA 8119 to female Guinea
pig. Appendix 6. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research, 4 pp.
Hadler MR (1975c) Acute eye irritation of WBA 8119 to the rabbit.
Appendix 31. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research.
Hadler MR (1976) WBA 8119-12 week feeding study in rats. Widnes,
Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural Research, 7 pp
(Laboratory report).
Hagan EC & Radomski JL (1953) The toxicity of 3-(acetonylbenzyl)-
4-hydroxycoumarin (warfarin) to laboratory animals. J Am Pharm Assoc,
42(6): 379-382.
Hall JG (1976) Warfarin and fetal abnormality. Lancet, 1: 1127.
Hall BE & Priestley I (1992) Brodifacoum: Metabolism in soil under
aerobic conditions (IRI Project No. 381441). Haslemere, Surrey, Zeneca
Agrochemicals, 63 pp (Report No. 8795).
Hall JG, Pauli RM, & Wilson KM (1980) Maternal and fetal sequelae of
anticoagulants during pregnancy. Am J Med, 68: 122-140.
Hall BE, Jackson R, & Priestley I (1992) Difenacoum: Photolysis in
buffered aqueous solutions (IRI Project No. 381614). Haslemere,
Surrey, Zeneca Agrochemicals, 123 pp (Report No. 8704).
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN,
Dodds RA, Bitensky L, & Chayen J (1985) Electrochemical detection of
depressed circulating levels of vitamin K1 in osteoporosis. J Clin
Endocrinol Metab, 60(6): 1268-1269.
Hegdal PL & Blaskiewicz RW (1984) Evaluation of the potential hazard
to barn owls of Talon (brodifacoum bait) used to control rats and
house mice. Environ Toxicol Chem, 3: 167-179.
Hegdal PL & Colvin BA (1988) Potential hazard to eastern screech-owls
and other raptors of brodifacoum bait used for vole control in
orchards. Environ Toxicol Chem, 7: 245-260.
Hill RW, Maddock BG, Hart B, & Bowles PF (1976) Determination of the
acute toxicity of PP 581 to Rainbow trout (Salmo gairdneri). Brixham,
Devon, Imperial Chemical Industries Ltd, Brixham Laboratory (Report
No. BL/B/1758).
Hodge MCE, Banham PB, Richards D, Weight TM, & Wilson J (1980a)
Product registration. Brodifacoum: Teratogenicity study in the rat.
Geneva, Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.
(ICI CTL Report No. CTL/P/437).
Hodge MCE, Banham PB, Richards D, & Weight TM (1980b) Product
registration. Brodifacoum: Teratogenicity study in the rabbit. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/459).
Hodge MCE, Banham PB, Richards D & Weight TM (1980c) Product
registration. Brodifacoum: Teratogenicity study in the rabbit
(Appendices A-E). Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V. (ICI CTL Report No. CTL/P/459 S).
Hoffman RS, Smilkstein MJ, & Goldfrank LR (1988) Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose. Clin Toxicol, 26(3/4): 233-248.
Hollinger BR & Pastoor TP (1993) Case management and plasma half-life
in a case of brodifacoum poisoning. Arch Intern Med, 153: 1925-1928.
Hoogenboom JJL (1994) Anticoagulant poisoning cases 1977-1994.
Wellington, New Zealand Central Animal Health Laboratory.
Hoogenboom JJL & Rammell CG (1983) Improved HPLC method for
determining brodifacoum in animal tissues. J Environ Contam Toxicol,
31: 239-243.
Huckle KR & Warburton PA (1986a) Elimination, metabolism and
disposition of 14C-WL 108366 in the Fischer 344 rat following
repeated oral administration. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre (Report No. SBGR.86.084).
Huckle KR & Warburton PA (1986b) Percutaneous absorption metabolism
and elimination of WL 108366 in the rat. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.218).
Huckle KR & Warburton PA (1986c) WL 108366: Absorption, metabolism and
disposition in Japanese quail (Coturnix coturnix Japonica) following a
single dose by intraperitoneal or oral administration. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.192).
Huckle KR, Hutson DH, & Warburton PA (1988) Elimination and
accumulation of the rodenticide flocoumafen in rats following repeated
oral administration. Xenobiotica, 18(12): 1465-1479.
Huckle KR, Hutson DH, Logan CJ, Morrison BJ, & Warburton PA (1989) The
fate of the rodenticide flocoumafen in the rat: Retention and
elimination of a single oral dose. Pestic Sci, 25: 297-312.
Huebner CF & Link KP (1941) Studies on the hemorrhagic sweet clover
disease. VI. The synthesis of the delta-diketone derived from the
hemorrhagic agent through alkaline degradation. J Biol Chem,
138: 529-534.
Hunter K (1983) Determination of coumarin anticoagulant rodenticide
residues in animal tissues by high-performance liquid chromatography:
Fluorescence detection using post-column techniques. J Chromatogr,
270: 267-276.
Hunter K (1985) High-performance liquid chromatographic strategies for
the determination and confirmation of anticoagulant rodenticides in
animal tissues. J Chromatogr, 321: 255-272.
Ikeda M, Conney AH, & Burns JJ (1968a) Stimulatory effect of
phenobarbital and insecticides on warfarin metabolism in the rat.
J Pharmacol Exp Ther, 162: 338-343.
Ikeda M, Ullrich V, & Staudinger H (1968b) Metabolism in vitro of
warfarin by enzymic and nonenzymic systems. Biochem Pharmacol,
17: 1663-1669.
IPCS (1992) IPCS/INTOX project. Poison Information Monograph:
Brodifacoum. Geneva, World Health Organization, 16 pp
(IPCS/INTOX/PIM.077).
Jackson R & Hall BE (1992) Aged soil leaching of [14C]-brodifacoum
(IRI Project No. 381986). Haslemere, Surrey, Zeneca Agrochemicals,
72 pp (Report No. 8879).
Jackson R, Priestley I, & Hall BE (1991) The determination of the
hydrolytic stability of [14C]-brodifacoum (IRI Project No. 381420).
Haslemere, Surrey, Zeneca Agrochemicals. 77 pp (Report No. 8330).
Jones EC, Growe GH, & Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. J Am Med Assoc, 252(21): 3005-3007.
Katona B & Wason S (1989) Superwarfarin poisoning. J Emerg Med,
7: 627-631.
Kaukeinen DE & Buckle AP (1992) Evaluations of aversive agents to
increase the selectivity of rodenticides, with emphasis on denatonium
benzoate (Bitrex(R)) bittering agent. In: Borrecco JE & Marsh RE ed.
Proceedings of the 15th Vertebrate Pest Conference. Davis, California,
University of California, pp 192-198.
Kelly MJ, Chambers J, & MacNicoll AD (1993) Simple and rapid method
for the determination of the diastereomers of difenacoum in blood and
liver using high-performance liquid chromatography with fluorescence
detection. J Chromatogr, 620: 105-112.
Kennelly JC (1990) Difenacoum: Assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 42 pp (Report No. CTL/P/3017.
Knapen MHJ, Jie K-SG, Hamulyák K, & Vermeer C (1993) Vitamin K-induced
changes in markers for osteoblast activity and urinary calcium loss.
Calcif Tissue Int, 53: 81-85.
Kosmin M & Barlow JN (1976) Rodent control using a novel formulation
of diphacinone, Ramik. Presented at the First Afro-Asian Vertebrate
Pest Conference, Cairo. Rosemont, Illinois, Velsicol Chemical
Corporation, 7 pp.
Krambias A & Hoppe AH (1987) The response of captive partridges to
dosing with anticoagulant rodenticides. In: Richards CGL & Ku TY ed.
Control of mammal pests. London, New York, Philadelphia, Taylor &
Francis, pp 181-186.
Kruse JA & Carlson RW (1992) Fatal rodenticide poisoning with
brodifacoum. Ann Emerg Med, 21: 331-336.
Laas FJ, Forss DA, & Goodfrey MER (1985) Retention of brodifacoum in
sheep tissues and excretion in faeces. N Z J Agric Res, 28: 357-359.
Lange PF & Terveer J (1954) Warfarin poisoning. US Armed Forces Med J,
5: 872-877.
Lawlor TE (1992) Mutagenicity test on bromadiolone in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test)
(HWA Study No. 15310-0-401). Vienna, Virginia, Hazleton Washington
Inc., 34 pp.
Lechevin JC & Grand M (1987) LM 2219 (proposed common name:
difethialone). Summary of the file submitted in the request for
approval (struggle against commensal rodents). Lyon, France, Lipha
S.A., Research and Development Centre, 15 pp (Report No. L-JCL/SP).
Leck JB & Park BK (1981) A comparative study of the effects of
warfarin and brodifacoum on the relationship between vitamin K1
metabolism and clotting factor activity in warfarin-susceptible and
warfarin-resistant rats. Biochem Pharmacol, 30: 123-128.
Lewis CJ (1992a) (14-C)-Difenacoum: A study of the degradation in two
soils. Haslemere, Surrey, Zeneca Agrochemicals (Report No. 6927).
Lewis CJ (1992b) (14C)-Difenacoum: Aged soil leaching. Haslemere,
Surrey, Zeneca Agrochemicals.
Lewis CJ (1992c) Difenacoum: Hydrolysis study. Haslemere, Surrey,
Zeneca Agrochemicals (Report No. 7031).
Lipton RA & Klass EM (1984) Human ingestion of a 'superwafarin'
rodenticide resulting in a prolonged anticoagulant effect. J Am Med
Assoc, 252: 3004-3005.
Litovitz TL, Clark LR, & Soloway RA (1994) 1993 Annual report of the
American Association of Poison Control Centers, Toxic Exposure
Surveillance System. Am J Emerg Med, 12: 546-584.
Locht H & Lindström FD (1993) Severe skin necrosis following warfarin
therapy in a patient with protein C deficiency. J Intern Med,
233: 287-289.
Lund M (1981) Hens, eggs and anticoagulants. Int Pest Control,
5: 126-128.
Lund M (1982) Annual report 1981. Lyngby, Danish Pest Infestation
Laboratory, pp 58-75.
Lund M (1984) Resistance to the second generation anticoagulant
rodenticides. In: Clark DO ed. Proceedings of the 11th Vertebrate Pest
Conference. Davis, California, University of California, pp 89-94.
Mackintosh CG, Laas FJ, Godfrey MER, & Turner K (1988) Vitamin K1
treatment of brodifacoum poisoning in dogs. In: Crabb AC & Marsh RE
ed. Proceedings of the 13th Vertebrate Pest Conference. Davis,
California, University of California, pp 86-90.
McSporran KD & Phillips CA (1983) Brodifacoum poisoning in a dog.
N Z Vet J, 31: 185-186.
Manfioletti G, Brancolini C, Avanzi G, & Schneider C (1993) The
protein encoded by a growth arrest-specific gene (gas6) is a new
member of the vitamin K-dependent proteins related to protein S, a
negative coregulator in the blood coagulation cascade. Mol Cell Biol,
13(5): 4976-4985.
Marsh RE (1985a) Are anticoagulant rodenticides a problem for
household pets? Pest Control, 53(8): 20-22.
Marsh RE (1985b) Anticoagulant rodenticides provide pet problems.
Part II. Pest Control, 53(9): 25-28.
Martin-Bouyer G, Linh PD, Tuan LC, Barin C, Khahn MB, Hoa DQ, Tourneau
J, & Guerbois H (1983) Epidemic of haemorrhagic disease in Vietnamese
infants caused by warfarin-contaminated talc. Lancet, 1: 230-232.
Mellano D (1984a) Product registration. In vitro study of chromosome
aberration induced by the article brodifacoum in cultured human
lymphocytes. Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V., 17 pp (Report No. CTL/C/1258).
Mellano D (1984b) Product registration. Study of the capacity of test
article brodifacoum to induce unscheduled DNA synthesis in cultured
Hela cells (autoradiographic method). Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V., 14 pp (Report No. CTL/C/1257).
Mendenhall VM & Pank LF (1980) Secondary poisoning of owls by
anticoagulant rodenticides. Wildl Soc Bull, 8(4): 311-315.
Merson MH & Byers RE (1984) Residues of the rodenticide brodifacoum in
voles and raptors after orchard treatment. J Wildl Manage,
48: 212-216.
Meyer AL & Wiggins DE (1986) Genotoxicity studies with WL 108366
(rodenticide): In vitro cell transformation studies. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.289).
Mirkova E & Antov G (1983) Experimental evaluation of the risk of
prenatal pathology under the effect of warfarin coumarine rodenticide.
Hig Zdrav, 25(6): 476-482.
Misenheimer TM, Lund ML, Baker EM, & Suttie JW (1994) Biochemical
basis of warfarin and bromadiolone resistance in the house mouse,
Mus musculus domesticus. Biochem Pharmacol, 47: 673-678.
Monnot G, Fave A, Illat T, & Briet Ph (1981) Phase 3 reformat of
MRID 0007-9375: Teratology study in the rat with LM 637 bromadiolone.
L'Arborasle, France, Institut Français de Recherches et Essais
Biologiques, 124 pp (Report No. MRID 0007-9375).
Morin M-F (1988) Study of the impact on the natural environment of
bromadiolone, an anticoagulant rodenticide: Evolution in aqueous
medium and bioaccumulation by terrestrial and aquatic organisms.
University of Poitiers (Thesis) ((Order No. 176).
Mosterd JJ & Thijssen HHW (1991) The long-term effects of the
rodenticide, brodifacoum, on blood coagulation and vitamin K
metabolism in rats. Br J Pharmacol, 104: 531-534.
Mount ME & Feldman BF (1983) Mechanism of diphacinone rodenticide
toxicosis in the dog and its therapeutic implications. Am J Vet Res,
44(11): 2009-2017.
Mount ME & Kass PH (1989) Diagnostic importance of vitamin K1 and
its epoxide measured in serum of dogs exposed to an anticoagulant
rodenticide. Am J Vet Res, 50: 1704-1709.
Mount ME, Feldman BF, & Buffington T (1982) Vitamin K and its
therapeutic importance. J Am Vet Med Assoc, 180(11): 1354-1356.
Murdoch DA (1983) Prolonged anticoagulation in chlorphacinone
poisoning. Lancet, 1: 355-356.
Murli H (1993) Mutagenicity test on bromadiolone, technical in an
in vivo mouse micronucleus assay (HWA Study No. 15310-0-455).
Washington, DC, Hazleton Laboratories.
Murphy MA & Nye DH (1991) Thoracic intramedullary haematoma as a
complication of warfarin: Case report and literature review. Aust N Z
J Surg, 61: 789-792.
Murphy MJ, Ray AC, & Bailey EM (1989) A high performance liquid
chromatography method for the detection of Brodifacoum in serum. Vet
Hum Toxicol, 31(3): 228-231.
Nehéz M, Kékes-Szabo A, Mazzag E, Selypes A, & Jarmay K (1985)
Histologic and cytogenetic effects of redentin on the spermiogenesis
and bone marrow cells of the mouse, an in vivo experiment. Regul
Toxicol Pharmacol, 5: 204-206.
Nelsestuen GL, Zytkovicz TH, & Howard JB (1974) The mode of action of
vitamin K: Identification of gamma-carboxyglutamic acid as a component
of prothrombin. J Biol Chem, 249: 6347-6350.
Newby S & White BG (1978) Brodifacoum: Adsorption and desorption in
soils measured under laboratory conditions. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division (Report
No TMJ 1764B).
Newton I, Wyllie I, & Freestone P (1990) Rodenticides in British barn
owls. Environ Pollut, 68: 101-117.
Newton I, Wyllie I, Gray A, Eadsforth CV (1994) The toxicity of the
rodenticide flocoumafen to barn owls and its elimination via pellets.
Pestic Sci, 41: 187-193.
Nighoghossian N, Ruel JH, French P, Froment JC, & Trouillas P (1990)
Hématome sous-dural cervico-dorsal par intoxication aux raticides
coumariniques. Rev Neurol (Paris), 146(3): 221-223.
Nilsson IM (1957) Recurrent hypoprothrombinaemia due to poisoning with
dicumarol-containing rat-killer. Acta Haematol, 17: 176-182.
O'Bryan SM & Constable DJC (1991) Quantification of brodifacoum in
plasma and liver tissue by HPLC. J Anal Toxicol, 15: 144-147.
O'Reilly RA, Aggeler PM, & Leong LS (1963) Studies on the coumarin
anticoagulant drugs: The pharmacodynamics of warfarin in man. J Clin
Invest, 4: 1542-1551.
O'Reilly RA, Aggeler PM, Haag MS, Leong LS, & Kropatkin ML (1964)
Hereditary transmission of exceptional resistance to coumarin
anticoagulant drugs. N Engl J Med, 271: 809-815.
Overman RS, Stahmann MA, Huebner CF, Sullivan WR, Spero L, Doherty DG,
Ikawa M, Graf L, Roseman S, & Link KP (1944) Studies on the
hemorrhagic sweet clover disease. XIII. Anticoagulant activity and
structure in the 4-hydroxycoumarin group. J Biol Chem, 153: 5-24.
Owren PA (1959) Thrombotest: A new method for controlling
anticoagulant therapy. Lancet, 2: 754-758.
Park BK & Leck JB (1982) A comparison of vitamin K antagonism by
warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol,
31(22): 3635-3639.
Park BK, Choonara IA, Haynes BP, Breckenridge AM, Malia RG, & Preston
FE (1986) Abnormal vitamin K metabolism in the presence of normal
clotting factor activity in factory workers exposed to
4-hydroxycoumarins. Br J Clin Pharmacol, 21: 289-294.
Parkinson GR (1975) WBA 8119: Acute oral toxicity. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 6 pp (Report No. CTL/P/216 (revised)).
Parkinson GR (1976) PP581: Acute oral toxicity to sheep. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 3 pp (Report No. CTL/P/259 (revised)).
Parkinson GR (1979) PP 581: Acute oral toxicity and skin
sensitization. Macclesfield, Cheshire, Imperial Chemical Industries
Ltd, Central Toxicology Laboratory, 3 pp (Report No. CTL/P/260
(revised)).
Parmar G, Bratt H, Moore R, & Batten PL (1987) Evidence for common
binding site in vivo for the retention of anticoagulants in rat
liver. Hum Toxicol, 6: 431-432.
Pastoureau P, Vergnaud P, Meunier PJ, & Delmas PD (1993) Ostopenia and
bone-remodeling abnormalities in warfarin-treated lambs. J Bone Miner
Res, 8: 1417-1426.
Pauli RM & Hall JG (1979) Warfarin embryopathy. Lancet, 2: 144.
Pearson N (1984) WL 108366 manufacturing master mix: Acute toxicity to
Salmo gairdneri and Daphnia magna. Sittingbourne, Shell Research Ltd
(Report No. SBGR.84.274).
Pearson N & Wallace BG (1984) Technical WL 108366: Acute toxicity to
Salmo gairdneri, Cyprinus carpio, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Kent, Shell Research Ltd, Sittingbourne
Research Centre (Report No. SBGR.84.211).
Pelfrène AF (1991) Synthetic organic rodenticides. In: Hayes WJ Jr &
Laws ER Jr ed. Handbook of pesticide toxicology. New York, Academic
Press, vol 3, pp 1271-1316.
Persson H (1994) [Annual report 1993.] Stockholm, Swedish Poison
Information Centre, 24 pp (in Swedish).
Pribilla O (1966) Murder caused by warfarin. Arch Toxicol,
21: 235-249.
Price JB (1985a) Toxicology of rodenticides: The acute oral toxicity
of WL 108366 in rabbits and hamsters. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.189).
Price JB (1985b) Toxicology of rodenticides: The percutaneous toxicity
of WL 108366 corn oil manufacturing master mix. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.84.227).
Price JB (1985c) Toxicology of rodenticides - WL 108366: A five day
range finding feeding study in rats. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.84.279).
Price JB (1985d) WL 108366: A 28-day feeding study in rats.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.84.235).
Price JB (1986) Toxicology of rodenticides ("Storm"): The skin
sensitizing potential of WL 108366. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.86.091).
Price PA, Williamson MK, Haba T, Dell RB, & Jee WS (1982) Excessive
mineralization with growth plate closure in rats on chronic warfarin
treatment. Proc Natl Acad Sci (USA), 79: 7734-7738.
Quick AJ (1935) The prothrombin in haemophilia and in obstructive
jaundice. J Biol Chem, 109: 73.
Radvanyi A, Weaver P, Massari C, Bid D, & Broughton E (1988) Effects
of chlorophacinone on captive kestrels. Bull Environ Contam Toxicol,
41: 441-448.
Rammell CG, Hoogenboom JJL, & Cotter M (1984) Brodifacoum residues in
target and non-target animals following rabbit poisoning trials. N Z J
Exp Agric, 12: 107-111.
Rauch AE, Weininger R, Pasquale D, Burkart PT, Dunn HG, Weissman C, &
Rydzak E (1994) Superwarfarin poisoning: A significant public health
problem. J Community Health, 19: 55-65.
Ray AC, Murphy MJ, DuVall MD, & Reagor JC (1989) Determination of
brodifacoum and bromadiolone residues in rodent and canine liver. Am J
Vet Res, 50(4): 546-550.
Redfern R, Gill JE, & Hadler MR (1976) Laboratory evaluation of WBA
8119 as a rodenticide for use against warfarin resistant and
non-resistant rats and mice. J Hyg Camb, 77: 419-426.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 16 November: 525-527.
Resch H, Pietschmann P, Krexner E, & Willvonseder R (1991) Decreased
peripheral bone mineral content in patients under anticoagulant
therapy with phenprocoumon. Eur Heart J, 12: 439-441.
Roberts NL, Cameron DM, & Street AE (1985a) WL 108366 - Acute oral
toxicity to pigs. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL/76/851108).
Roberts NL, Fairley C, & Baldwin MK (1985b) The acute oral toxicity
(LD50) of WL 108366 to the Mallard duck. Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Report No. SLL 67/BT/84925).
Roberts NL, Fairley C, & Baldwin MK (1985c) The acute oral toxicity of
WL 108366 to the Mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Centre (HRC Report No. SLL/73/BT/8572).
Robinson DS & MacDonald MG (1966) The effect of phenobarbital
administration on the control of coagulation achieved during warfarin
therapy in man. J Pharmacol Exp Ther, 153: 250-253.
Ross DB, Roberts NL, & Cameron DM (1978) The acute oral toxicity
(LD50) of PP581 to the Mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No ICI 122WL/78507).
Ross DB, Roberts NL, & Phillips C (1979) The subacute toxicity of
difenacoum (Ratak) to pigs. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No.
ICI 262/79697).
Ross DB, Roberts NL, & Fairley C (1980a) The subacute dietary toxicity
(LC50) of difenacoum to Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 305/791197).
Ross DB, Roberts NL, & Fairley C (1980b) The subacute dietary toxicity
(LC50) of difenacoum to the mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 304/791198).
Ross DB, Roberts NL & Fairley C (1980c) The acute oral toxicity
(LD50) of difenacoum to the Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 309/WL/8076).
Ross GS, Zacharski LR, Robert D, & Rabin DL (1992) An acquired
hemorrhagic disorder from long-acting rodenticide ingestion. Arch
Intern Med, 152: 410-412.
Schardein JL (1985) Anticoagulants. In: Chemically induced birth
defects. New York, Basel, Marcel Dekker Inc., pp 89-100.
Schulman A, Lusk R, Lippincott CL, & Ettinger SJ (1986)
Diphacinone-induced coagulopathy in the dog. J Am Vet Med Assoc,
188(4): 403-405.
Selypes A, Kékes-Szabo A, & Nehéz M (1984) Study of the mutagenic
effect of redentin on various species of animals. Acta Vet Hung,
32(3-4): 213-217.
Sharples R (1983a) The acute oral toxicity of a series of novel
anticoagulants in Wistar rats and C57BL/10 mice. Widnes, United
Kingdom, Sorex Ltd, Agricultural Research Department, 7 pp.
Sharples R (1983b) The acute oral toxicity of WL 108366 in
Dunkin-Hartley guinea pigs. Widnes, United Kingdom, Sorex Ltd,
Agricultural Research Department, 3 pp.
Sharples R (1984) The acute oral toxicity of WL 108366 in C57BL/10
mice. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 6 pp.
Sheen SR, Spiller HA, & Grossman D (1994) Symptomatic brodifacoum
ingestion requiring high-dose phytonadione therapy. Vet Hum Toxicol,
36(3): 216-217.
Sheldon T, Richardson CR, & Shaw J (1984) Product registration. An
evaluation of brodifacoum in the mouse micronucleus test. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.,
19 pp (Report No. CTL/P/1006).
Smith MF & Cameron MD (1979) Warfarin as teratogen. Lancet, 1: 727.
Smolinske SC, Scherger DL, Kearns PS, Wruk KM, Kulig KW, & Rumack BH
(1989) Superwarfarin poisoning in children: A prospective study.
Pediatrics, 84: 490-494.
Spare WC (1982) Aqueous photodegradation of 14C-bromadiolone.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Spare WC (1992) Hydrolysis of bromadiolone. Beltsville, Maryland,
Biospherics Inc. (Unpublished report submitted to WHO by Lipha S.A.).
Spare WC & Olson SB (1980) 14C-Bromadiolone soil leaching.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Sridhara S & Krishnamurthy TR (1992) Potentiation of anticoagulant
toxicity to Rattus rattus by two non-steroid anti-inflammatory drugs.
In: Borrecco JE & Marsh RE ed. Proceedings of the 15th Vertebrate Pest
Conference. Davis, California, University of California, pp 212-217.
Stenflo J, Fernlund P, Egan W, & Roepstorff P (1974) Vitamin K
dependent modifications of glutamic acid residues in prothrombin. Proc
Natl Acad Sci (USA), 71(7): 2730-2733.
Stowe CM, Metz AL, Arendt TD, & Schulman J (1983) Apparent brodifacoum
poisoning in a dog. J Am Vet Med Assoc, 182(8): 817-818.
Sullivan MP, Dean BS, & Krenzelok EO (1989) Long-acting anticoagulant
rodenticides: An evaluation of 88 cases. Vet Hum Toxicol, 31: 361.
Szulc P, Chapuy M-C, Meunier PJ, & Delmas PD (1993) Serum
undercarboxylated osteocalcin is a marker of the risk of hip fracture
in elderly women. J Clin Invest, 91: 1769-1774.
Thomson WT (1988) Agricultural chemicals. Book III - Miscellaneous
chemicals: Fumigants, growth regulators, repellents, and rodenticides
(1988/89 Revision). Fresno, California, Thomson Publications.
Tomlin C ed. (1994) The pesticide manual incorporating the
agrochemical handbook, 10th ed. Farnham, Surrey, The British Crop
Protection Council.
Townsend MG, Fletcher MR, Odam EM, & Stanley PI (1981) An assessment
of the secondary poisoning hazard of warfarin to tawny owls. J Wildl
Manage, 45(1): 242-248.
Townsend MG, Bunyan PJ, Odam EM, Stanley PI, & Wardall HP (1984)
Assessment of secondary poisoning hazard of warfarin to least weasels.
J Wildl Manage, 48(2): 628-632.
Travis SF, Warfield W, Greenbaum BH, Mobkisher M, & Siegel JE (1993)
Spontaneous hemorrhage associated with accidental brodifacoum
poisoning in a child. J Pediatr, 122: 982-984.
Tuinman CP & Van Sittert NJ (1985) Biomedical monitoring of personnel
in Sorex Ltd (Widnes, UK) involved in a formulation run with the
rodenticide WL 108366. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report No. HSE 85.006).
Ullrich V & Staundinger H (1968) Metabolism in vitro of warfarin by
enzymic and nonenzymic systems. Biochem Pharmacol, 17: 1663-1669.
Ungvary G (1994) Anticoagulant poisoning incidents in Hungary.
Budapest, National Institute of Occupational Health (Unpublished
report).
Van Haarlem LJM, Knapen MHJ, Hamulyák K, & Vermeer C (1988)
Circulating osteocalcin during oral anticoagulant therapy. Thromb
Haemost, 60: 79-82.
Veenstra GE, Owen DE, & Huckle KR (1991) Metabolic and toxicological
studies on the anticoagulant rodenticide, flocoumafen. Arch Toxicol,
14(Suppl): 160-165.
Vermeer C (1990) gamma-Carboxyglutamate-containing proteins and the
vitamin K-dependent carboxylase. Biochem J, 266: 625-636.
Vermeer C & Soute BAM (1992) Comparison of rodenticide anticoagulant
action in vitro and in vivo. Sittingbourne, Kent, Shell Research
Ltd (Report No. HSE/50).
Virat M (1981) LM-637-Bromadiolone: Teratology study in the rabbit by
oral route. L'Arborasle, France, Institut Français de Recherches et
Essais Biologiques (Report No. 106206).
Vogel JJ, de Moerloose Ph, Bouvier CA, Gaspoz J, & Riant P (1988)
Anticoagulation prolongée lors d'une intoxication à la chlorphacinone.
Schweiz Med Wochenschr, 118: 1915-1917.
Völkl S & Galicia H (1992) 14C-Bromadiolone: Degradation and
metabolism in soils incubated under aerobic conditions. Itingen,
Switzerland, Umweltchemie AG (Unpublished report submitted to WHO by
Lipha S.A., Lyon).
Wallace S, Paull P, Worsnop C, & Mashford ML (1990) Covert self
poisoning with brodifacoum, a "superwarfarin". Aust N Z J Med,
20: 713-715.
Warburton PA & Huckle KR (1986) WL 108366 - Fate of a single oral dose
of 14C WL 108366 in rats. Part III: Metabolism. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.294).
Warburton PA & Hutson DH (1985) WL 108366 - Fate of a single oral dose
of 14C-WL 108366 in rats. Part I: Elimination and retention of
radioactivity and effect of WL 108366 on prothrombin time.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.85.053).
Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, Murphy
MJ, & Furie B (1990) Surreptitious ingestion of a long-acting vitamin
K antagonist/rodenticide, brodifacoum: Clinical and metabolic studies
of three cases. Blood, 76(12): 2555-2559.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, 64 pp (WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II). Clin Chem,
33(11): 2074-2078.
Winn MJ, Clegg JAD, & Park BK (1987) An investigation of sex-linked
differences to the toxic and to the pharmacological action of
difenacoum: Studies in mice and rats. J Pharm Pharmacol, 39: 219-222.
Wiswesser WJ (1976) Pesticide index, 5th ed. College Park, Maryland,
Entomological Society of America.
Woody BJ, Murphy MJ, Ray AC, & Green RA (1992) Coagulopathic effects
and therapy of brodifacoum toxicosis in dogs. J Vet Intern Med,
6: 23-28.
Yu CC, Atallah YH, & Whitacre DM (1982) Metabolism and disposition of
diphacinone in rats and mice. Drug Metab Dispos, 10: 645-648.
RESUME
1. Généralités
Les anticoagulants qui sont décrits dans la présente monographie
sont ceux que l'on utilise principalement en agriculture et pour la
destruction des rongeurs en milieu urbain. La warfarine, premier
rodenticide anticoagulant à connaître une large utilisation, était à
l'origine un médicament efficace pour le traitement des
thromboembolies chez l'homme.
Selon leur structure chimique, les rodenticides anticoagulants
peuvent se répartir en deux catégories, les hydroxycoumarines et les
indanediones, mais leur mode d'action est analogue.
2. Propriétés et méthodes d'analyse
Les rodenticides anticoagulants se présentent sous la forme de
solides cristallins ou de poudres et sont légèrement solubles dans
l'eau. La plupart d'entre eux sont stables dans les conditions
normales de conservation.
La plupart des méthodes de dosage des rodenticides anticoagulants
reposent sur la chromatographie en phase liquide à haute performance.
3. Sources d'exposition humaine et environnementale
Les hydroxycoumarines de la première génération ont commencé à
être utilisées comme rodenticides vers la fin des années 1940. Par la
suite, l'apparition d'une résistance à la warfarine et aux autres
anticoagulants de la première génération a conduit à mettre au point
des produits de deuxième génération, plus actifs. La concentration du
principe actif dans les appâts varie selon l'efficacité du
rodenticide.
4. Distribution, concentration et exposition dans l'environnement
Les rodenticides anticoagulants sont principalement utilisés sous
la forme d'appâts. Comme ils sont peu volatils, leur concentration
dans l'air est négligeable. De même n'étant que légèrement solubles
dans l'eau, il est peu probable que leur utilisation conduise à la
contamination des eaux.
Etant donné que les rodenticides anticoagulants ne sont pas
destinés à être appliqués directement sur les cultures, il n'y a pas
lieu de s'attendre à en trouver sous forme de résidus dans les
aliments d'origine végétale.
L'exposition aux rodenticides des vertébrés non visés peut se
produire directement par la consommation d'appâts empoisonnés et
indirectement par celle de rongeurs contaminés. Les petits granulés
et les appâts constitués de grains entiers attirent fortement les
oiseaux.
La warfarine est utilisé dans le traitement des thromboembolies.
Il existe une possibilité d'exposition professionnelle aux
rodenticides anticoagulants lors de leur fabrication ou formulation ou
encore lors de la pose d'appâts empoisonnés, mais on ne dispose pas de
données sur l'ampleur de cette exposition.
5. Mode d'action et métabolisme
Les rodenticides anticoagulants sont des antagonistes de la
vitamine K. Leur action est principalement localisée dans le foie où
plusieurs précurseurs de l'hémostase subissent un processus
post-traductionnel dépendant de la vitamine K avant d'être convertis
en zymogènes procoagulants. L'action proprement dite consiste dans
l'inhibition de la K1-époxyde-réductase.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives et pourraient l'être également à travers
la peau et dans les vois respiratoires. Après administration par voie
orale, c'est principalement dans les matières fécales que ces composés
sont éliminés chez diverses espèces.
Il est possible que la décomposition métabolique de la warfarine
et des indanediones chez le rat s'effectue principalement par
l'intermédiaire d'une hydroxylation. En revanche, les anti-coagulants
de la deuxième génération s'éliminent essentiellement tels quels. Le
faible taux d'excrétion urinaire empêche d'isoler les métabolites de
l'urine.
Le foie est le principal organe où s'accumulent les rodenticides
anticoagulants. Cette accumulation a lieu également dans les
graisses.
6. Effets sur les mammifères et les systèmes d'épreuve in vitro
Chez le rat et la souris, les signes d'intoxication consistent en
une tendance accrue au saignement.
La DL50 est très variable, et c'est par la voie orale que les
composés sont les plus toxiques. La toxicité est également élevée par
la voie percutanée et par la voie respiratoire.
Certains anticoagulants présentent une toxicité aiguë du même
ordre pour les mammifères non visés que pour les rongeurs à détruire,
toutefois leur spectre toxique peut varier d'une espèce à l'autre.
Après administration répétée par voie orale à des rats, les
principaux effets observés sont ceux qui résultent de l'activité
anticoagulante.
On ne possède que peu de données sur les expositions répétées aux
anticoagulants chez les espèces n'appartenant pas à l'ordre des
rongeurs.
Une étude relative aux effets de la warfarine sur le rat a fait
ressortir une action sur le développement de ces animaux. En dehors
de cela, rien n'indique que les anticoagulants aient une action
tératogène sur les animaux d'expérience.
Rien n'indique non plus que les rodenticides anticoagulants
soient mutagènes, toutefois les données relatives aux différents
composés sont insuffisantes pour qu'on puisse en conclure à l'absence
de mutagénicité. La souche, le sexe et le régime alimentaire sont des
facteurs importants qui influent sur la toxicité des anticoagulants
chez les rongeurs. On a fait état d'épisodes d'intoxication chez des
animaux domestiques qui avaient consommé des appâts additionnés
d'anticoagulants. Lorsqu'il y a issue fatale ou du moins un tableau
clinique grave, c'est généralement qu'il y a eu consommation
d'anticoagulants de la deuxième génération. La principale différence
entre la warfarine et les autres anticoagulants (qu'il s'agisse des
indanediones ou des hydroxycoumarines de la deuxième génération),
c'est que ceux-ci persistent plus longtemps dans l'organisme et ont
par conséquent un effet plus durable que la warfarine. Dans ces
conditions, devant une intoxication, il faut poursuivre plus longtemps
l'administration de l'antidote, c'est-à-dire de la vitamine K1.
7. Effets sur l'homme
De nombreuses intoxications (qu'elles soient intentionnelles on
non) ont été signalées. Il y a eu également quelques cas
d'intoxication imputables à une exposition professionnelle. En cas
d'intoxication aiguë par des rodenticides anticoagulants, les
symptômes vont de l'accroissement de la tendance au saignement en cas
d'intoxication minime ou modérée, à une hémorragie massive dans les
cas plus graves. Les signes cliniques se manifestent un à plusieurs
jours après l'absorption.
Chez l'homme, on attribue certaines malformations congénitales à
des traitements par la warfarine pendant la période de grossesse.
Aucune malformation de ce type n'a été observée par suite de
l'utilisation d'anticoagulants comme rodenticides.
La concentration de la prothrombine plasmatique donne une
indication de la gravité de l'intoxication. Elle est plus sensible
que des épreuves globales telles que le temps de Quick. En cas
d'exposition professionnelle répétée, le dosage direct des traces de
carboxyprothrombine circulante ou du 2,3-époxyde de la vitamine K peut
permettre un bilan plus sensible.
Le traitement d'une intoxication par des anticoagulants doit être
adapté à la gravité de l'intoxication. Il est basé sur l'action
pharmacologique spécifique de la vitamine K1 que l'on administre par
voie parentérale avec, dans les cas graves, administration simultanée
de constituants sanguins. La mesure du temps de Quick permet
d'apprécier l'efficacité du traitement et de déterminer sa durée.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On peut répartir en deux catégories les effets possibles de
rodenticides anticoagulants sur les organismes non visés: les effets
directs (par consommation d'appâts) et les effets indirects (par
consommation de rongeurs empoisonnés).
Sous forme de produit technique, les anticoagulants sont
extrêmement toxiques pour les poissons. Incorporés à des appâts, il
est peu probable qu'ils présentent un danger, du fait de leur faible
solubilité dans l'eau. C'est pourquoi, à moins d'une erreur de
manipulation, ils ne devraient pas parvenir jusqu'aux poissons.
Les différentes espèces d'oiseaux sont d'une sensibilité variable
aux rodenticides anticoagulants. Il est difficile d'apprécier les
risques que représente, pour les oiseaux, la consommation directe
d'appâts car la plupart des travaux publiés sont des études
toxicologiques effectuées au laboratoire. Le caractère attractif des
appâts constitués de grains entiers pour les petits oiseaux en accroît
le danger dans la nature.
Des études de toxicité indirecte effectuées en laboratoire sur
des animaux sauvages ont montré que des prédateurs captifs pouvaient
s'intoxiquer si on leur donnait de la nourriture empoisonnée sans
autre choix ou des proies empoisonnées. On a également constaté la
mort de prédateurs dans le milieu naturel.
9. Evaluation et conclusion
Les rodenticides anticoagulants bloquent le mécanisme normal de
l'hémostase, d'où une tendance accrue au saignement qui peut déboucher
sur une forte hémorragie.
Il est peu probable que la population générale puisse être
exposée involontairement à des rodenticides anticoagulants.
Il peut y avoir une exposition non négligeable par suite de
contacts lors de l'activité professionnelle. Ces contacts peuvent se
produire au cours des opérations de production et de formulation aussi
bien que lors de la préparation et de la pose des appâts.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives ainsi qu'à travers la peau et les voies
respiratoires. C'est principalement dans le foie qu'ils sont retenus
et s'accumulent. La concentration de prothrombine plasmatique est un
bon indicateur de la gravité d'une intoxication aiguë et permet
d'avoir une idée de l'efficacité et de la durée nécessaire du
traitement.
L'antidote spécifique est la vitamine K1.
La principale différence entre les rodenticides anticoagulants de
première et de deuxième génération tient au fait que ces derniers
séjournent plus longtemps dans l'organisme et ont donc tendance à
prolonger l'effet hémorragique.
La plupart des anticoagulants sont stables dans les conditions
normales d'utilisation. Comme ils sont peu solubles dans l'eau et que
les appâts n'en contiennent qu'une faible quantité, il est peu
probable qu'ils puissent contaminer les étendues d'eau. Par ailleurs,
ils se fixent rapidement aux particules du sol, ne s'en désorbent que
très lentement et ne sont pas lessivés.
Aucun organisme non visé ne court de risque d'intoxication
directe par consommation d'appâts, ni de risque d'intoxication
indirecte par consommation de rongeurs contaminés.
RESUMEN
1. Generalidades
Los anticoagulantes descritos en esta monografía son los
utilizados principalmente en agricultura y en la lucha contra los
roedores urbanos. La warfarina, el primer rodenticida anticoagulante
de uso generalizado, se introdujo como agente eficaz para el
tratamiento de la tromboembolia en el ser humano.
De acuerdo con su estructura química, los rodenticidas
anticoagulantes pueden agruparse en dos categorías, las
hidroxicumarinas y las indandonas, aunque su mecanismo de acción es
similar.
2. Propiedades y métodos analíticos
Los rodenticidas anticoagulantes se presentan en forma cristalina
sólida o en polvo, y son ligeramente solubles en agua. La mayoría de
ellos son estables en condiciones de almacenamiento normales.
La mayoría de los procedimientos para la determinación de los
rodenticidas anticoagulantes se basan en cromatografía líquida de alta
resolución.
3. Fuentes de exposición humana y ambiental
Las hidroxicumarinas de primera generación se introdujeron como
rodenticidas a finales de los años cuarenta. La aparición de
resistencia a la warfarina y a otros anticoagulantes de primera
generación dio lugar a la elaboración de anticoagulantes más potentes,
de segunda generación. Las concentraciones de componentes activos en
los cebos varían en función de la eficacia de los rodenticidas.
4. Distribución, niveles y exposición ambientales
Los rodenticidas anticoagulantes se utilizan principalmente como
formulaciones para cebo. Dada su baja volatilidad, las
concentraciones en el aire son insignificantes. Como son muy poco
solubles en agua, es improbable que su uso sea fuente de contaminación
del agua.
Como los rodenticidas anticoagulantes no están pensados para su
aplicación directa a cosechas en pie, no son de prever residuos en
alimentos vegetales.
Los vertebrados no destinatarios están expuestos a los
rodenticidas principalmente por medio del consumo del cebo y de forma
secundaria por el consumo de roedores envenenados. Los cebos en
bolitas y de grano entero son muy atractivos para las aves.
La warfarina se utiliza como agente terapéutico para la
tromboembolia.
Hay un potencial de exposición ocupacional a los rodenticidas
anticoagulantes durante la fabricación, formulación y aplicación del
cebo, pero no se dispone de datos sobre los niveles de exposición.
5. Modo de acción y metabolismo
Los rodenticidas anticoagulantes son antagonistas de la vitamina
K. Su lugar principal de acción es el hígado, donde varios de los
precursores de la coagulación de la sangre sufren un procesamiento
post-traslación dependiente de la vitamina K antes de convertirse en
los zimógenos procoagulantes respectivos. Parece que el mecanismo de
acción es la inhibición de la reductasa epoxídica K1.
Los rodenticidas anticoagulantes se absorben fácilmente por el
tracto intestinal, y también pueden absorberse por la piel y el
sistema respiratorio. Tras la administración oral, la principal vía
de eliminación en diversas especies son las heces.
La degradación metabólica de la warfarina y las indandonas en
ratas es principalmente la hidroxilación. Sin embargo, los
anticoagulantes de segunda generación se eliminan principalmente como
compuestos inalterados. El bajo nivel de excreción urinaria impide
aislar los metabolitos a partir de la orina.
El hígado es el órgano principal para la acumulación y
almacenamiento de anticoagulantes rodenticidas. La acumulación
también tiene lugar en la grasa.
6. Efectos en los mamíferos y en los sistemas de prueba in vitro
Los signos de envenenamiento en ratas y ratones son los asociados
a una mayor tendencia a la hemorragia.
Hay una gran variación en la DL50 de los rodenticidas
anticoagulantes, siendo máxima la toxicidad por vía oral. También es
alta la toxicidad cutánea y por inhalación.
Los márgenes de toxicidad aguda de algunos anticoagulantes son
similares en el caso de los mamíferos no destinatarios y de los
roedores de destino, pero los espectros de toxicidad para los
anticoagulantes pueden variar entre las especies.
Tras una administración oral repetida en ratas, los principales
efectos observados son los asociados a la acción anticoagulante.
Se dispone de pocos datos sobre la exposición repetida de
especies distintas de los roedores.
Un estudio sobre la warfarina en ratas ha indicado efectos sobre
el desarrollo. Por lo demás, no hay pruebas convincentes de que los
anticoagulantes sean teratogénicos en animales de experimentación.
No hay prueba que sugiera que los rodenticidas anticoagulantes
son mutagénicos, pero se dispone de datos insuficientes sobre los
compuestos individuales para demostrar la inexistencia de
mutagenicidad. La sobrecarga, el sexo y la alimentación son
importantes factores modificadores de la toxicidad de los
anticoagulantes en los roedores.
Se han dado casos de envenenamiento de animales domésticos tras
la ingestión de cebos anticoagulantes. Las muertes y los síndromes
clínicos graves se deben por lo general a anticoagulantes de segunda
generación. La diferencia principal entre la warfarina y los demás
anticoagulantes (tanto las indandonas como las hidroxicumarinas de
segunda generación) es que éstos tienen mayor tiempo de retención en
el organismo y por consiguiente un efecto más prolongado que la
warfarina. Por ello, en los casos de envenenamiento, el tratamiento
antídoto con vitamina K1 debe proseguir durante un periodo más
largo.
7. Efectos en el ser humano
Se han notificado muchos casos de envenenamiento (tanto
intencionados como no intencionados). También se han producido unos
pocos casos de intoxicación por exposición ocupacional a los
anticoagulantes. Los síntomas de intoxicación aguda por rodenticidas
anticoagulantes van desde una mayor tendencia a la hemorragia en el
envenenamiento leve o moderado a una hemorragia masiva en casos más
graves. Los signos de envenenamiento aparecen con un retraso de uno a
varios días después de la absorción.
La warfarina va asociada en el ser humano a la producción de
malformaciones del desarrollo cuando se toma como agente terapéutico
durante el embarazo. No se han notificado casos de defectos de
desarrollo tras el uso de anticoagulantes como rodenticidas.
La concentración de protrombina plasmática orienta sobre la
gravedad de la intoxicación. Es una indicación más sensible que
pruebas generales como el tiempo de protrombina. En la exposición
ocupacional repetida, la medición directa de cantidades ínfimas de
descarboxiprotrombina en circulación o de 2,3-epóxido de vitamina K en
circulación pueden constituir una evaluación más sensible.
El tratamiento del envenenamiento con anticoagulantes se gradúa
de acuerdo con la gravedad de la intoxicación. El tratamiento
farmacológico específico consiste en la administración parenteral de
vitamina K1 con administración simultánea, en los casos graves, de
componentes sanguíneos. La medición del tiempo de protrombina ayuda a
determinar la eficacia y la duración de tratamiento necesaria.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los posibles efectos de los rodenticidas en organismos no
destinatarios pueden dividirse en dos categorías: primarios
(envenenamiento directo por consumo de cebo) y secundario (por consumo
de roedores envenenados).
En la forma del producto técnico, los anticoagulantes son muy
tóxicos para los peces. Como formulaciones para cebo es improbable
que planteen riesgos en razón de su baja solubilidad en agua. Por
esta razón, a menos que se utilicen de forma indebida, no están al
alcance de los peces.
La susceptibilidad de las aves a los rodenticidas anticoagulantes
es variable. Es difícil evaluar los riesgos para las aves que entraña
el consumo directo porque la mayoría de los estudios publicados
consisten en ensayos de toxicidad en condiciones de laboratorio. El
atractivo del cebo de grano integral para las aves pequeñas aumenta el
riesgo en las condiciones de campo.
Los estudios en laboratorio de la toxicidad secundaria con la
fauna silvestre han mostrado que los predadores cautivos pueden
intoxicarse mediante alimentación sin otra elección con presas que se
han envenenado con anticoagulante o a las que se ha administrado este
producto. Se tiene noticias de algunas muertes de predadores en su
medio natural.
9. Evaluación y conclusión
Los rodenticidas anticoagulantes perturban los mecanismos
normales de coagulación de la sangre, determinando una mayor tendencia
a la hemorragia y, por último, una hemorragia abundante.
La exposición no intencionada de la población general a los
rodenticidas anticoagulantes es improbable.
El contacto ocupacional es una fuente potencial de exposición
significativa. Puede tener lugar durante la elaboración y
formulación, así como durante la preparación y aplicación del cebo.
Los compuestos de rodenticida anticoagulante se absorben
fácilmente por el tracto intestinal, y por la piel y el sistema
respiratorio. El hígado es el órgano principal de acumulación y
almacenamiento. La concentración de protrombina plasmática es una
buena orientación de la gravedad de la intoxicación aguda y de la
eficacia y la duración necesaria de la terapia.
El antídoto específico es la vitamina K1.
La principal diferencia entre los rodenticidas anticoagulantes de
la primera generación y los de la segunda es que éstos últimos tienen
una mayor retención en el organismo y por ello suelen dar lugar a un
periodo de hemorragia más prolongado.
La mayoría de los anticoagulantes son estables en condiciones de
uso normal. Su baja solubilidad en agua y baja concentración en los
cebos hace improbable que sean una fuente de contaminación del agua.
Parece que se asocian rápidamente a las partículas del suelo, con
desorción muy lenta y nula propiedad de lixiviación.
Los organismos no destinatarios pueden correr al riesgo de
consumir directamente cebos (riesgo primario) y de ingerir roedores
envenenados (riesgo secundario).
Vermeer & Soute (1992) compared the inhibition of each of the
three enzymes from the vitamin K cycle by four anticoagulants
(warfarin, flocoumafen, difenacoum and brodifacoum). The studies were
performed using in vitro enzyme systems prepared from rat, cow and
human liver. It was shown that in all three species the inhibitor
concentration required for 50% inhibition (I50) was comparable for
the KO reductase and K reductase activity, but that the I50 for
gamma-glutamylcarboxylase was 2-3 orders of magnitude higher. It was
concluded that for all four anticoagulants the reductions of KO and K
are the target reactions for inhibition. Moreover, it was found that
there is no species specificity of the inhibitors, which means that
they are equally active in cell-free systems derived from rat, cow and
human liver. Any species-dependent differences which might be found
in vivo will presumably be brought about by a different
pharmacokinetic or pharmacodynamic behaviour in these species.
6.2 Metabolism
6.2.1 Absorption, distribution and elimination
Anticoagulant rodenticides are easily absorbed through the
gastrointestinal tract, skin and respiratory system.
After a single oral dose of 14C-flocoumafen (0.14 mg/kg body
weight) to rats, the absorption into blood was rapid, reaching maximum
concentrations (0.03-0.05 µg/ml) in plasma within 4 h (Huckle et al.,
1989).
The major route of elimination in rats and sheep after oral
administration of anticoagulants is through the faeces. The
intestinal levels of brodifacoum in rats began increasing 24 to 72 h
after an oral dose of 0.2 mg/kg body weight (Bachmann & Sullivan,
1983). Faecal elimination of radiolabelled flocoumafen following an
oral dose of 0.14 mg/kg body weight accounted for 23-26% of the dose
over the 7-day period; approximately half of this was recovered within
the first 24 h. Less than 0.5% of the dose appeared in the urine
within 7 days (Huckle et al., 1989).
After single oral administration of brodifacoum (0.2 and 2 mg/kg
body weight) to sheep, about 20% and 30%, respectively, was excreted
in the faeces within 8 days (Laas et al., 1985).
A larger proportion of a percutaneous dose of 14C-flocoumafen
(0.17 mg/kg body weight) dissolved in acetone was found in the urine
of rats (10%) than in the case of an equivalent oral dose (less than
0.5%) over a 7-day period. Faecal elimination accounted for 31% of
the percutaneous dose (Huckle & Warburton, 1986b).
After oral 14C-flocoumafen doses of 0.02 mg/kg body weight or
0.1 mg/kg body weight were given to rats, once weekly for up to 14
weeks, approximately one-third of each weekly low dose was eliminated
through the faeces within 3 days, mostly within the first 24 h. At
the higher dose the elimination ranged from 18% after the first dose
to 59% after the tenth dose (Huckle et al., 1988).
Following repeated oral administration of 14C-flocoumafen to
rats at 0.02 mg/kg body weight per week for 14 weeks or 0.1 mg/kg body
weight per week for 10 weeks, appreciable accumulation was seen in the
liver. At both dose levels tissue concentrations were highest in the
liver, followed by the kidney > skin > muscle > fat > blood. The
hepatic residue in the low-dose group ranged from 0.1 mg/kg tissue
after one week to 2.1 mg/kg by week 14 (Huckle & Warburton, 1986a).
Brodifacoum could not be detected in the omental fat of sheep 8
days after the oral administration of 0.2 and 2 mg/kg body weight
(Laas et al., 1985).
6.2.2 Metabolic transformation
Warfarin is readily hydroxylated in vitro and in vivo by rat
liver microsomal enzymes to form 6-, 8- and, especially
7-hydroxy-warfarin (Ullrich & Staundinger, 1968; Ikeda et al.,
1986a,b). These inactive metabolites are to some extent conjugated
with glucuronic acid, undergo enterohepatic recirculation, and are
excreted in the urine and faeces (Ellenhorn & Barceloux, 1988).
The metabolic pattern of indandiones in rats also mainly involves
hydroxylation (Yu et al., 1982).
The second-generation anticoagulants have mainly been found as
unchanged compounds (Bachmann & Sullivan, 1983; Huckle et al., 1988).
The low urinary elimination following oral dosing has precluded
accurate isolation of metabolites in urine (Warburton & Hutson, 1985;
Waburton & Huckle, 1986; Huckle & Warburton, 1986a).
Following administration of flocoumafen, liver residues in rats
consisted mainly of unchanged flocoumafen, although in a repeat dose
study a polar metabolite was detected. Eight urinary metabolites were
detected after percutaneous exposure to 14C-flocoumafen (Huckle &
Warburton, 1986b).
Studies in male Japanese quail have shown more rapid metabolism
and elimination than in the rat following an oral dose of
14C-flocoumafen. Up to 12 radioactive components were detected in
the excreta (Huckle & Warburton, 1986c).
Bromadiolone, brodifacoum and coumatetralyl were also found in
rats as unchanged parent compounds, whereas in the case of difenacoum
metabolites predominated (Parmar et al., 1987). The metabolism and
elimination of the difenacoum trans isomer was more rapid than for the
cis isomer (Bratt, 1987).
The suggestion that the anticoagulant effect in rats is mediated
by the unchanged compound itself rather than by its metabolites has
been confirmed by the effects of phenobarbital and SKF525A
pretreatments on the general pattern of responses to warfarin and
brodifacoum (Bachmann & Sullivan, 1983).
6.2.3 Retention and turnover
Metabolic studies of anticoagulant rodenticides show that the
liver is the main organ of accumulation and storage. Liver
concentrations of brodifacoum after a single oral dose of 0.2 mg/kg
body weight to rats remained high and relatively constant for 96 h,
with a maximum of 5.0 mg/kg after 50 h (Bachmann & Sullivan, 1983).
A high degree of body retention was found 7 days after a single
oral dose of 0.14 mg/kg body weight 14C-flocoumafen (74-76% of the
administered dose); approximately half the dose was found in the liver
(Huckle et al., 1989).
Brodifacoum was detected in the liver of sheep 128 days after
oral administration (0.2 and 2 mg/kg body weight) in concentrations of
0.64 and 1.07 mg/kg dry weight (equivalent to 0.22 and 0.36 mg/kg wet
weight), respectively. The peak levels occurred at 2 days in the
high-dose group and at 8 days in the low-dose group, being 6.50 and
1.87 mg/kg dry weight (2.21 and 0.64 mg/kg wet weight), respectively
(Laas et al., 1985). Woody et al. (1992) observed an elimination
half-life for brodifacoum in serum of 6 ± 4 days in four dogs.
The largest proportion of a percutaneous flocoumafen dose of
0.17 mg/kg body weight was located in the liver (25% of the dose at a
concentration of 0.8 mg/kg), although this was 10 times lower than
that following an oral dose (Huckle & Warburton, 1986b).
Parmar et al. (1987) found that elimination of radiolabelled
brodifacoum, bromadiolone and difenacoum from the liver was biphasic,
consisting of an rapid initial phase lasting from days 2 to 8 after
dosing and a slower terminal phase when the elimination half-lives
were 130, 170 and 120 days, respectively. Elimination of
coumatetralyl was more rapid, with a half-life of 55 days.
Similar results for difenacoum were found by Bratt (1987). After
a single oral 14C-difenacoum dose of 1.2 mg/kg body weight, the
highest concentration of radioactivity (41.5% of the dose) was found
in the rat liver 24 h after dosing. The elimination from the liver
was biphasic. The half-life of elimination of the radioactivity
during the first rapid phase was three days, and for the slower phase
was 118 days. A similar biphasic elimination was also apparent in the
kidney. In the pancreas the concentration declined more slowly than
in any of the other tissues (182 days). The parent compound was the
major component in the liver 24 h after dosing (42%).
Unchanged flocoumafen comprised the major proportion of the
hepatic radioactivity in rats and was eliminated with a half-life of
220 days (Huckle et al., 1989). Veenstra et al. (1991) found
retention of about 8% of an administered flocoumafen dose of 0.4 mg/kg
in the liver of beagle dogs 43 weeks after dosing.
Despite the more rapid metabolism of flocoumafen in Japanese
quail, a proportion of the administered dose is retained in the liver,
with an elimination half-life of 115 days after oral dosing (Huckle &
Warburton, 1986c).
Six Hereford heifers weighing approximately 230 kg each were
dosed with diphacinone (1 mg/kg body weight) by injecting it into the
rumen. The highest residue level of parent compound found in the
liver was 0.15 mg/kg at days 30 and 90 after treatment. No detectable
levels (> 0.01 mg/kg) could be found in any of the other tissues
analysed (kidney, plasma, brain, heart, muscle and fat). The residues
in the liver were almost constant from 30 to 90 days post-treatment
(Bullard et al., 1976).
The plasma half-life of brodifacoum determined in three patients
with severe bleeding disorders was found to be approximately 16 to 36
days (Weitzel et al., 1990).
The half-life for disappearance from the plasma of human
volunteers given a single oral or intravenous warfarin dose of
1.5 mg/kg body weight varied from 15 to 58 h, with a mean of 42 h
(O'Reilly et al., 1963).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute effects
7.1.1 Rodent species
Wide variations exist in the literature for LD50 values of
anticoagulant rodenticides. A particular reason for these variables
is the use of single or repeated (5-day) doses. LD50 values also
vary according to the animal strain and sex, but both have not always
been indicated in reported data (Ashton et al., 1987)
(Tables 5 and 6).
Table 5. Acute oral LD50 values for various rodenticides in albino
Norway rats
Rodenticide LD50 (mg/kg) Reference
Brodifacoum 0.26 Redfern et al. (1976)
Bromadiolone 1.125 Grand (1976)
Chlorophacinone 20.5 Thomson (1988)
Coumachlor 900.0 Thomson (1988)
Coumafuril 0.4 Wiswesser (1976)
Coumatetralyl 16.5 Thomson (1988)
Difenacoum 1.8 Bull (1976)
Difethialone 0.56 Lechevin & Grand (1987)
Diphacinone 3.0 Thomson (1988)
Flocoumafen 0.46 Sharples (1983a)
Pindone 50.0 Tomlin (1994)
Warfarin 58.0 Thomson (1988)
Reported oral LD50 values for warfarin in rats vary by a
considerable magnitude. Values of 11 mg/kg body weight (Lund, 1982),
58 mg/kg body weight (Thomson, 1988) and 58 mg/kg body weight and
323 mg/kg body weight for female and male, respectively (Hagan &
Radomski, 1953), have been reported.
The second-generation anticoagulants are more toxic than the
first-generation ones in the sense that a single feeding may be
lethal. Apparent discrepancies between the single oral LD50 values
for Norway rats, as shown in Table 5, and the multiple dose oral
LD50 values, shown in Table 6, can be explained by the cumulative
effects resulting from multiple dose (5 day) administration (Table 6)
and characteristics of this family of compounds.
Table 6. Five-day oral LD50 (mg/kg body weight per day) of various anticoagulants
for the Norway rat (Rattus norvegicus)a
Anticoagulant Strainb Male Female Both sexes Number of
animalsc
Pindone SD 1.21 (0.70-2.11) 1.60 (0.83-3.08) 1.34 (0.87-2.06) 40
wild 7.60 (2.61-22.2) 25.60 (5.34-123) 12.80 (1.73-84.8) 40
Warfarin SD 0.29 (0.14-0.57) 0.38 (0.22-0.66) 0.33 (0.22-0.50) 40
wild 0.39 (0.16-0.91) 0.60 (0.23-1.09) 0.44 (0.25-0.76) 40
Diphacinone SD 0.19 (0.11-0.33) 0.23 (0.12-0.43) 0.21 (0.14-0.31) 40
wild 0.39 (0.15-0.84) 0.60 (0.22-0.57) 0.44 (0.23-0.54) 40
Chlorophacinone SD 0.18 (0.18-0.18) 0.20 (0.15-0.27) 0.19 (0.16-0.22) 40
wild 0.13 (0.10-0.19) 0.23 (0.14-0.36) 0.16 (0.12-0.22) 40
Bromadiolone SD 0.13 (0.10-0.19) 0.10 (0.08-0.13) 0.12 (0.10-0.15) 40
wild 0.06 (0.03-0.12) 0.09 (0.09-0.09) 0.07 (0.05-0.10) 16
a Modified from: Ashton et al. (1987); figures in parentheses represent 95% confidence limits
b SD = Sprague-Dawley
c 50% males and 50% females in all tests
Reported oral LD50 values for mice show similar variations,
from less than 1 mg/kg body weight for second-generation rodenticides
to 374 mg/kg body weight for warfarin (Hagan & Radomski, 1953; Redfern
et al., 1976; Bull, 1976; Sharples, 1983a).
Signs of anticoagulant poisoning in rats and mice include
lethargy, hunched posture and vein clearing in the ears. Blood around
the eyes, mouth and anus, indicating internal haemorrhaging, appears
prior to death (Sharples, 1984).
The percutaneous toxicity of anticoagulants to rats varied for
different compounds from an LD50 of 0.54 mg/kg body weight for
flocoumafen (master mix in corn oil) to > 50 mg/kg body weight for
brodifacoum and difenacoum (Price, 1985b; Tomlin, 1994). The signs of
intoxication were identical to those observed after an oral dose.
Second-generation anticoagulants appeared to be highly toxic by
inhalation. An acute inhalation study in Wistar rats exposed (nose
only) for 4 h was conducted using the 0.5% manufacturing master mix
specifically prepared to give a mass median aerodynamic diameter of
less than 5 µm. The acute LC50 values were between 0.16 and
1.4 mg/litre. Signs of intoxication, characteristic of an
anticoagulant action, were observed within 3 days after exposure with
deaths occurring between 4 and 9 days (Blair, 1984).
7.1.2 Non-target species
The acute toxicity of various anticoagulant rodenticides to
non-target mammalian species is presented in Table 7.
From the data presented it appears that some anticoagulants show
a similar range of acute toxicity for both non-target mammals and
target rodents.
7.2 Short-term exposure
7.2.1 Rodent species
In a study by Hadler (1974), groups of Wistar rats (male and
female) were given brodifacoum by gavage (0.01, 0.02, 0.05, 0.1 and
0.2 mg/kg body weight) for 5 consecutive days. All rats receiving the
two lowest doses survived the 21-day experimental period, but all rats
given the two highest doses died within 11 days of cessation of
dosing. No abnormalities were detected in surviving animals
sacrificed at the end of the observation period. In animals which
died during the study, only massive internal haemorrhages, mainly in
the peritoneum, were observed. The no-observed-effect level of
brodifacoum for Wistar rats was 0.02 mg/kg body weight per day.
Table 7. Acute oral toxicity (LD50, mg/kg) of various anticoagulant rodenticides for non-target mammalian speciesa
Rodenticide Guinea-pig Rabbit Dog Cat Sheep Pig References
Brodifacoum 2.78 (F) 0.29 (M) 0.25-1 (F) approx. 25 (F) > 25 (M) 0.5-2 Hadler (1975a,b); Parkinson
3.56 11-33 (1975, 1976); Godfrey (1984);
Godfrey et al. (1985)
Bromadiolone 2.8 1.0 10 (F) MTD > 25 MTD 3 Grand (1976)
Chlorophacinone 50 Pelfrène (1991)
Difenacoum 50 (F) 2 (M) approx. 50 100 100 80-100 Bull (1976); Tomlin (1994)
Diphacinone 35 3-7.5 14.7 150 Kosmin & Barlow (1976)
Difethialone 0.75 5 MTD > 16 MTD 2-3 Lechevin & Grand (1987)
Flocoumafen > 10 (M) 0.7 (M/F) 0.075-0.25 > 10 (M/F) > 5 approx. 60 (M,F) Sharples (1983b); Price (1985a);
(M/F) Chesterman et al. (1984);
Roberts et al. (1985a, 1986)
Warfarin 800 20-50 6-40 1-5 Anonymous (1976)
a F = female; M = male; MTD = maximum tolerated dose
Wistar rats (male and female) were continuously given a diet
containing brodifacoum at 0.1 mg/kg body weight, with no choice of
other feed, for 12 weeks. Prothrombin time was increased and
mortality occurred in 9 out of 20 males and 5 out of 20 females.
Macroscopic examination of the major organs of surviving rats revealed
no abnormalities other than those expected of anticoagulant action
(Hadler, 1976).
Feeding Fisher-344 rats with diets containing flocoumafen at
concentrations of 0.2 or 0.4 mg/kg feed for 5 days produced no
toxicologically significant effects during the 15-day observation
period (Price, 1985c).
In a 28-day feeding study in rats, fed on a diet containing 0,
0.01, 0.05, 0.1 or 0.2 mg-flocoumafen/kg, there were no overt signs of
toxicity. Highest-dose females showed a slight but significant
increase in mean prothrombin time (PT) and activated partial
thromboplastin time (PTT), and a slight decrease in mean plasma total
protein. No toxicological or pathological changes were observed in
rats fed diets containing 0.01 or 0.05 mg/kg of diet (Price, 1985d).
No effect on prothrombin time was observed in a 12-week study
with Wistar rats administered oral flocoumafen doses (by gavage) of
0.0125, 0.0625 and 0.125 mg/kg body weight once a week (Forsey, 1985).
Groups of six male and six female Sprague-Dawley rats were fed
diphacinone in their diets at concentrations of 0 (control), 0.0313,
0.0625, 0.125, 0.25 and 0.5 mg/kg diet (equivalent to 0, 1.7, 3.3,
6.4, 13 and 27 µg/kg body weight per day) for 90 days (Elias & Johns,
1981). Additional satellite groups of one rat of each sex and each
dose group were killed for gross pathological examination at 30 and
60 days. Mortality was unaffected by the treatment. In the survivors
of the main groups and satellite groups, there were no gross
pathological changes, but in the two males that died prematurely (in
the 0.0625 and 0.25 mg/kg groups) there was subdural haemorrhage.
Prothrombin time was unaffected by treatment. Routine haematological
and clinical chemistry tests were performed on only two rats of each
sex from each group, and no effects were observed apart from a reduced
blood fibrinogen concentration in both sexes at the highest dose
level.
As no clear NOAEL was indicated by the results of the 90-day
study, a 21-day study was performed using two rats of each sex at
diphacinone levels of 0 (control), 0.125, 0.25, 0.5, 1, 2 and 4 mg/kg
diet. All the rats in the 2 and 4 mg/kg groups died within the first
14 days, but all others survived until the end of the study. The mean
dosages received by the survivors were equivalent to 0, 9, 17, 34 and
67 µg/kg body weight per day. At 21 days, there was no effect on
prothrombin time. Gross necropsy revealed haemorrhage in the thymus
of one rat in the 0.5 mg/kg group, but no effects were seen in any
other survivors. All the animals in the 2 and 4 mg/kg groups had
massive and extensive internal haemorrhage (Elias & Johns, 1981).
7.2.2 Non-target species
There are few data available on the repeated exposure of
non-rodent species to anticoagulant rodenticides.
Death followed five daily warfarin doses of 3 mg/kg body weight
in cats and 1 mg/kg body weight in pigs (Tomlin, 1994). The route of
administration was not specified.
The dermal administration of warfarin (188 mg/kg per day) to
female baboons caused profuse bleeding in 5 days followed by death
(Dreyfus et al., 1983).
When it became known that vitamin K was not an antagonist for
warfarin action in bone, it became possible to study the long-term
effects of this anticoagulant in bones without the risk of inducing
haemorrhages and bleeding. By feeding lambs with high doses of
warfarin (up to 150 mg/kg body weight per day) under the protection of
4 mg/kg body weight per day of vitamin K, Pastoureau et al. (1993)
showed that within 3 months the lambs had developed a marked
osteopenia that resulted from a decrease in resorption and a much more
pronounced decrease in bone formation. As a result the bone density in
the warfarin-treated animals was substantially lower than that in
control animals. This is in agreement with earlier studies in rats
(Price et al., 1982) where it was found that, under the protection of
vitamin K, warfarin induced excessive mineralization and growth plate
closure, due to which bone growth came to a halt.
The maximum tolerated 5-day oral dose of bromadiolone was
considered to be 25 mg in pigs (Large White strain) weighing 25 kg.
After 45 oral daily doses of 0.5 mg no change in the prothrombin time
was observed (Grand, 1976).
Woody et al. (1992) studied the effect of a cumulative dose of
1.1 mg brodifacoum/kg body weight administered orally to dogs over a
3-day period. Signs of coagulopathic effects appeared within 24 h
(greater PT and PTT) and increased over a 10-day period. Treatment
with Vitamin K1 at 10 days post-exposure reduced the effects.
Six horses were treated by gavage with brodifacoum containing
bait at a dosage of 0.125 mg/kg body weight (Boermans et al., 1991).
Four of the horses became anorexic and depressed, one requiring K1
therapy. Peak plasma concentration occurred 2-3 h after
administration. Pharmacokinetic evaluation indicated that brodifacoum
has a plasma half-life of 1.22 days. An increase in clotting time was
observed as early as 24 h after dosing, returning to the pre-treatment
level by day 12.
Male pigs (five per group) were fed difenacoum in the diet for 14
days at dose levels of 0.01, 0.05, 0.1, 0.5 and 1.0 mg/kg diet. With
the exception of the lowest-dose group, all groups showed a marked
increase of prothrombin time values. Extensive subcutaneous, inter-
and intra-muscular haemorrhage and oedema was observed in animals
dosed at levels of 0.5 mg/kg or more (Ross et al., 1979).
7.3 Long-term exposure
No data on long-term exposure are available.
Studies with second-generation anticoagulants are difficult to
carry out for more than a few weeks due to the rapid acute effects,
and NOEL values for second-generation rodenticides and longer exposure
periods have not been established. In any chronic study approaching
two years in length, the dose level would have to be less than the
analytical limit of detection.
7.4 Skin and eye irritation; sensitization
Brodifacoum is a slight skin irritant and a mild eye irritant in
the rabbit (Hadler, 1975c). No skin or eye irritation was observed in
New Zealand white rabbits treated with flocoumafen (Forsey, 1983a,b).
Neither of these rodenticides was a skin sensitizer when tested in the
guinea-pig maximization test (Parkinson, 1979; Price, 1986).
7.5 Reproductive toxicity and teratogenicity
Brodifacoum was given by oral gavage to female rats at daily dose
levels of 0.001, 0.01 or 0.02 mg/kg body weight during days 6-15 of
pregnancy. There was no evidence of adverse effects on the fetus at
termination. Higher daily doses (above 0.05 mg/kg) caused an
anticoagulant effect in the dams which resulted in a high incidence of
abortion (Hodge et al., 1980a).
Pregnant female rabbits were given oral gavage doses of 0.001,
0.002 or 0.005 mg brodifacoum/kg body weight per day from days 6-18 of
pregnancy. A the highest dose level a high proportion of maternal
deaths occurred as a result of haemorrhage. Although the survivors
showed signs of haemorrhage, there were no effects on the developing
fetus. No effects were observed at either of the other dose levels
used (Hodge et al., 1980b,c).
Bromadiolone was given orally to four groups of 25 female rats
from day 6 to 15 of pregnancy at doses of 0, 17.5, 35 and 70 µg/kg
body weight per day. Maternal toxicity occurred at the higher dose
levels. There was no evidence of embryotoxicity or teratogenic
effects at any dose level (Monnot et al., 1981). A similar absence of
effects was reported in a study on rabbits treated orally with daily
doses of either 2, 4 or 8 µg/kg body weight per day on days 6-18 of
pregnancy, although there was maternal toxicity at the highest dose
level (Virat, 1981).
Groups of 18 pregnant F-344 rats were given daily oral doses of
0, 0.01 or 0.04 mg flocoumafen/kg body weight from day 8 to 17 of
gestation. Eight animals in the 0.04 mg/kg body weight group either
died or were killed with signs of anticoagulant poisoning.
In contrast, Mirkova & Antov (1983) found warfarin to be
embryotoxic and teratogenic to Wistar rats when administered by gavage
in single doses or repeatedly throughout the periods of
pre-implantation (1-7 days of gestation) and organogenesis (8-16
days), and also throughout the whole gestation (1-21 days) at a wide
range of dose levels (0.04-8 mg/kg body weight). At these dose levels
and treatment regimens, warfarin induced substantially increased rates
of embryolethality, subcutaneous and internal haemorrhage and gross
structural malformations (pes varus, internal hydrocephalus and
anomalies of skeletal ossification).
7.6 Mutagenicity
Various in vitro and in vivo studies have been undertaken to
assess the genotoxic potential of brodifacoum. No mutagenic activity
was detected in the Salmonella reverse mutation assay in any of the
five tester strains employed (TA98, TA100., TA1535, TA1537 and TA1538)
either in the presence or absence of Arochlor 1254-induced rat liver
S9 fraction at brodifacoum concentrations ranging from 1.6 to
5000 µg/plate (Callander, 1984). Brodifacoum showed no activity in a
forward mutation assay using L5178 mouse lymphoma cells, either with
or without metabolic activation, at concentrations of 47.5, 63.3 and
84.4 mg/litre (Cross & Clay, 1984).
Brodifacoum caused no significant chromosomal aberrations in
cultured human lymphocytes (concentrations 1, 10, 100 and
1000 mg/litre), either with or without metabolic activation, and did
not induce unscheduled DNA synthesis in cultured HeLa cells at the
same range of concentrations (Mellano, 1984a,b). Difenacoum did not
induce unscheduled DNA synthesis in rat hepatocytes in vivo at
either dose level or time-point (Kennelly, 1990).
An in vivo micronucleus test, in which mice were given single
brodifacoum intraperitoneal doses of 0.187 or 0.30 mg/kg body weight,
showed no induction of micronuclei in bone marrow polychromatic
erythrocytes (Sheldon et al., 1984).
Bromadiolone was tested in the Salmonella reverse mutation assay
at concentrations ranging from 10 to 3330 µg per plate on strains
TA1535, TA1537 and TA1538. No evidence of mutagenic effect was found
either with or without Aroclor metabolic activation (Lawlor, 1992).
Bromadiolone did not induce forward mutations in Chinese hamster
ovary cells either with or without metabolic activation
(Cifone, 1993).
In a mouse micronucleus test at four dose levels from 50 to
400 mg/kg, bromadiolone did not induce micronuclei in bone marrow
polychromatic erythrocytes (Murli, 1993).
Flocoumafen did not induce reverse gene mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 nor in
Escherichia coli WP2uvrA pkm 101 either with or without metabolic
activation. Flocoumafen was tested at concentrations ranging from 31
to 2000 µg/plate, beyond which precipitation from suspension occurred
(Brooks et al., 1984).
Flocoumafen did not increase the frequency of mutation to
6-thioguanine resistance in Chinese hamster V79 cells either in the
presence or absence of an Arochlor-induced rat liver S9 fraction.
Doses ranging from 5 to 150 mg/litre were used, beyond which
cytotoxicity occurred (Clare & Wiggins, 1986).
Flocoumafen did not induce in vitro cell transformation in
C3H1OT´ mouse fibroblasts, either in the presence or absence of a rat
S9 metabolizing system, at concentrations ranging from 12.5 to
100 µg/litre (Meyer & Wiggins, 1986).
Flocoumafen did not induce mitotic gene conversion in liquid
suspension cultures of Saccharomyces cerevisiae JD1, either in the
presence or absence of a rat liver S9 fraction, at concentrations
ranging from 0.01 to 2 g/litre (Brooks et al., 1984).
When incubated at concentrations ranging from 5 to 25 mg/litre
for 24 h in monolayer cultures of rat liver RL4 cells, flocoumafen did
not induce in vitro chromosomal damage (Brooks et al., 1984).
Oral administration of flocoumafen to rats at doses of 0.25 mg/kg
or 1000 mg/kg body weight (a dose 4000 times the acute oral LD50 for
rats) did not produce chromosomal damage (Allen et al., 1986).
The cytogenic effect of chlorophacinone was investigated in vivo
in metaphase bone marrow cells taken at 48 and 96 h after oral dosing
of male CFLP mice with 20 mg/kg body weight. No induction of
chromosomal aberrations was observed (Nehéz et al., 1985).
In the same study, chlorophacinone was investigated in
spermatocytes taken from male CFLP mice at 1, 2, 3 and 4 weeks after a
single oral dose of 20 mg/kg body weight. The spermatocytes were
analysed in the diakinesis phase of meiosis. There was no increased
incidence of chromosomal aberrations (Nehéz et al., 1985).
Rabbits were treated orally with 20 mg chlorophacinone/kg body
weight and chromosomal analysis was performed in bone marrow and
spermatocytes taken at 48 h post-dosing. No increase in the incidence
of chromosomal aberrations was seen (Selypes et al., 1984).
7.7 Factors modifying toxicity
Phenobarbital pretreatment of rats followed by a single
administration of brodifacoum or warfarin decreased the anticoagulant
effects of both compounds, more markedly in the case of warfarin
(Bachmann & Sullivan, 1983).
The non-steroid anti-inflammatory drugs ibuprofen and
phenylbutazone potentiated the anticoagulant effects of brodifacoum
and bromadiolone in rats (Sridhara & Krishnamurthy, 1992).
Strain and sex are important factors modifying the toxicity of
anticoagulants in rodents (see Table 5, section 7.1.1). Winn et al.
(1987) observed greater sensitivity of male rats and mice to
difenacoum, compared with female rats and mice. The possible
explanation of different responses to difenacoum was the greater
turnover of plasma proteins in male rats or the marked
inter-individual variation of vitamin K1 level in male rat liver.
Large amounts (relative to farm animals' dietary requirements) of
vitamin K3 (menadione and its salts) are sometimes added to animal
feedstuffs. This gives rodent pests ready access to a substance which
acts as an antidote to anticoagulant rodenticides, and thus can reduce
the efficacy of these rodenticides.
Differences in metabolism to inhibitors of vitamin K synthesis
among strains of mice and rats has been attributed to a number of
different factors (Misenheimer et al., 1994). Among these are: 1)
reduced sensitivity of vitamin K epoxide hydrolase to inhibition; 2)
greater reversibility of inhibition of epoxide hydrolase; and 3)
faster clearance of the rodenticide. The mechanism of resistance
differs between strains.
A single flocoumafen dose of 0.5 mg/kg body weight resulted in
clear signs of anticoagulation in five out of eight beagle dogs
(Veenstra et al., 1991). Administration of a second dose 5 weeks
later resulted in clinical evidence of anticoagulation in two out of
the three remaining dogs. Vitamin K treatment (2 to 5 mg/kg
subcutaneously) reversed the effects in all cases.
7.8 Adverse effects in domestic and farm animals
7.8.1 Domestic animals
7.8.1.1 Poisoning incidents
The main cause for accidental poisoning of domestic animals is
direct consumption of anticoagulant baits. Secondary poisoning
through the consumption of rats and mice killed with anticoagulants
may occur in dogs and cats in urban situations, but is more likely in
farm situations (Marsh, 1985b). The majority of fatalities and severe
clinical syndromes are connected with the second-generation
anticoagulants (Dodds & Frantz, 1984). Du Vall et al. (1989) studied
10 cases of second-generation anticoagulant rodenticide poisoning in
dogs and cats. The presence of anticoagulants (brodifacoum,
bromadiolone or diphacinone) in serum or liver was confirmed by HPLC
or GC/MS. Several other cases of brodifacoum poisonings in dogs have
been reported and some of them were fatal (Mc Sporran & Phillips,
1983; Stowe et al., 1983; Dodds & Frantz, 1984).
Until 1983, only first-generation anticoagulants were available
in New Zealand and, between 1977 and 1983, warfarin, coumatetralyl and
diphacinone were involved in 45 reported incidents of accidental
poisoning of domestic and farm animals. Brodifacoum and flocoumafen
were introduced after 1983. From 1983 to 1994, 40 cases of poisoning
of domestic and farm animals resulted from the use of
second-generation compounds and 17 cases were due to the ingestion of
first-generation anticoagulants (Hoogenbroom, 1994).
Schulman et al. (1986) reported five cases of coagulopathy in
dogs caused by consumption of diphacinone-containing baits. Lethargy
and respiratory distress, associated with pulmonary interstitial
haemorrhage and pleural and/or pericardial effusion, were the most
consistent signs. The prothrombin time and the activated partial
thromboplastin time were moderately to markedly prolonged.
7.8.1.2 Diagnosis and treatment of poisoning
The major difference between warfarin and the other anticoagulant
rodenticides is that the latter have longer body retention and a
tendency to induce bleeding for a longer period of time. It is
therefore necessary to continue the treatment for weeks rather than
days.
Signs of poisoning occur after a latent period of 12 h to several
days and may include:
* bruising easily with occasional nose or gum bleeds
* blood in stools or urine
* excessive bleeding from minor cuts or abrasions
* laboured breathing
* pale mouth and cold gums
* anorexia and general weakness
A reliable indication of an anticoagulant effect is the
determination of prothrombin time.
Vitamin K1 (phytomenadione) is the antidote of choice. The
recommended dosage is 2-5 mg/kg body weight in dogs. Intravenous
injection is the quickest route but it is not recommended because of
reported anaphylaxis. Vitamin K1 is much safer if given
subcutaneously for the initial 2-3 treatments. A dose of 0.25 to
2.5 mg/kg body weight is recommended for use in small animals (Mount
et al., 1982). The recommended period of treatment with vitamin K1
is 3-6 weeks (Braithwaite, 1982; Mackintosh et al., 1988).
Mount & Feldman (1983) observed that therapy effective for
warfarin poisoning was ineffective against diphacinone toxicosis due
to inadequate vitamin K1 dosage and duration of therapy. Oral
therapy was ineffective.
In a study on dogs, Mount & Kas (1989) found that the serum
concentration of vitamin K epoxide was significantly higher in
diphacinone-treated dogs than in controls, the difference being
significant as early as 1-4 h after vitamin K application. The
authors suggested that this phenomenon could be used as diagnostic
information.
In cases of severe blood loss, fresh plasma should be infused
every 6 h to the extent of 5-10% of total blood volume, assuming this
to be 90 ml/kg in the dog and 70 ml/kg in the cat (Mount et al.,
1982).
Vitamin K1 (phytomenadione) is the antidote for treating dogs
exposed to anticoagulant rodenticides. The therapeutic dosages and
duration of treatment needed vary with the compound involved. Mount &
Feldman (1983) reported that dogs poisoned with diphacinone require
treatments for at least three weeks, while treatment for 4-6 days is
sufficient for treating warfarin poisonings. Initial treatments are
by subcutaneous injection, subsequent therapy being given orally.
For animals exposed to the second-generation rodenticides
brodifacoum, bromadiolone, difenacoum and flocoumafen, the treatment
regimen that has been recommended includes one or more injections of
vitamin K1 at 2-5 mg/kg body weight until prothrombin times return
to normal levels. Once this has been achieved, daily oral doses of
vitamin K1 are recommended for a period of 3-4 weeks. Oral doses of
2-5 mg/kg body weight are recommended initially, with some reduction
being possible over time if the animal is observed closely for signs
of recurrence of symptoms. If prothrombin times increase following
withdrawal of vitamin K1, the oral dosing should be resumed for 2-3
weeks. If blood loss was severe, transfusions (10-15 ml/kg body
weight) with fresh whole blood may be needed (Anonymous, 1988).
7.8.2 Farm animals
Feinsod et al. (1986) reported an outbreak of abortions and
haemorrhages in sheep and goats in Egypt caused by brodifacoum
intoxication. Clinical signs appeared within 3-7 days after exposure
to the rodenticide and included epistaxis, haematochezia, recumbency,
subcutaneous haemorrhage, flank ecchymosis, lameness, abdominal
bloating and abortion of various stages of gestation. The signs
resembled epidemic Rift valley fever, but there was no fever, icterus
or loss of appetite in affected animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Incidents of human exposures to rodenticides are reported to
poison control centres in countries where such facilities exist. In
1988, for example, the American Association of Poison Control Centers
(AAPCC) received accounts of 10 626 cases of human exposures to
rodenticides. These incidents represented 17% of reported exposures
involving pesticides and 0.8% of the total number of cases reported in
the AAPCC system. The rodenticide incidents included 4190 cases
involving "anticoagulants" (principally warfarin) and 5133 involving
"long-acting anticoagulants" (second-generation anticoagulants plus
the indandione compounds). More than 95% of the rodenticide cases
were classified as "accidental". Most of the remainder were
classified as "intentional" and included attempted suicides. Of the
10 540 rodenticide incidents for which the ages of victims were
reported, 9406 (89%) involved children under 6 years of age (Litovitz
et al., 1994).
Victims in nearly 32% of the rodenticide exposure incidents
reported to the AAPCC in 1988 were treated in health care facilities.
However, the medical outcome "none" was reported in more than 93% of
the 5708 incidents for which information regarding outcomes was
reported. The remaining 380 cases included 333 with "minor" medical
effects, 41 with "moderate" effects, 4 with "major" effects, and two
deaths (Litovitz et al., 1994).
In 1993, the Swedish Poison Information Centre received 338
enquiries concerning exposures to anticoagulant rodenticides. This
number represented 0.6% of all enquiries to the centre and 37% of the
enquiries concerning pesticides. Of the anticoagulant rodenticide
enquiries, 202 pertained to warfarin and 136 to "superwarfarin"
compounds (Persson, 1994).
Human exposure to second-generation and indandione anticoagulants
produces symptoms consistent with anticoagulation effects (e.g.,
haematomas, haematemesis, haematuria, easy bruisability). Treatment
of cases of exposure, particularly of substantial and repeated
exposure, may require vitamin K1 therapy and monitoring of
prothrombin times for periods of many months (Rauch et al., 1994).
Suicide and/or unintentional poisonings with anticoagulant
rodenticides have occurred in many countries. Thus, Ungvary (1994)
reported 70 cases, mostly involving children, that occurred in Hungary
between 1988 and 1993.
Warfarin is widely used as a therapeutic and preventive agent in
the treatment of thromboembolic disease. Patients have been
maintained for years on this treatment with control of the prothrombin
level, which should be kept between 10 and 30% of normal.
Diphacinone has also been used as a drug because of its
long-lasting action (the half-life in humans is 15-20 days). It
ceased to be listed in the American Medical Association Drug
Evaluations, (AMA, 1980) because of its structural relation to
phenindion, which had been reported to have adverse effects.
8.1.1 Acute poisoning
Typical features of poisoning result from increased bleeding
tendency and include:
* minor poisoning: coagulation disturbance detected only by
laboratory analyses;
* moderate poisoning: coagulation disturbance resulting in
haematomata, haematuria, blood in faeces or excessive bleeding
from minor cuts or abrasions, gum bleeding;
* severe poisoning: retroperitoneal haemorrhage, severe
gastrointestinal bleeding, cerebrovascular accidents, massive
haemorrhage (internal bleeding) resulting in shock.
If anaemia or liver disease is present then the above features
may be more severe and persistent and the poisoning may be more
difficult to control (Anonymous, 1988).
The onset of the signs of poisoning may not be evident until a
few days after ingestion.
8.1.2 Poisoning incidents
Cases of human poisoning with "superwarfarins" were reviewed by
Katona & Wason (1989).
Fourteen members of a family in the Republic of Korea were
poisoned by eating warfarin-containing maize meal. The first symptoms
appeared 7-10 days after the beginning of exposure and were followed
by massive bruises or haematomata on the buttocks in all cases (Lange
& Terveer, 1954).
Pribilla (1966) reported a total dose of about 1000 mg of
warfarin to be fatal after 13 days of consumption.
Out of a total of 741 infants, 177 died after the use of
warfarin-contaminated talc in Viet Nam. The concentrations of
warfarin in the powder varied from 1.7 to 6.5% (Martin-Bouyer et al.,
1983).
A 73-year-old woman suffered from recurrent episodes of
hypoprothrombinaemia. Clotting tests and further investigation showed
that this was due to a warfarin rodenticide intentionally mixed in the
woman's cough syrup by her daughter-in-law. As the patient had as
many as seven relapses, it was possible to compare different types of
therapy. Menadione had no effect (Nilsson, 1957).
Several suicidal attempts with chlorophacinone have been
reported. Murdoch (1983) reported a case of ingestion of 625 mg
chlorophacinone (250 ml of a 0.25% concentrate formulation) by a
37-year-old woman. The prolonged anticoagulant action of
chlorophacinone persisted for at least 45 days even though treatment
was given. It was found that menadiol, the synthetic analogue of
vitamin K1, was ineffective. The natural form, phytomenadione, was
effective only when given at high dosage (20 mg daily) 30 days after
the ingestion of chlorophacinone.
In a case reported by Dusein et al. (1984), the amount of
ingested chlorophacinone was unknown. After adequate therapy, the
prothrombin level became normal within 4 weeks.
Vogel et al. (1988) reported the case of an 18-year-old woman
hospitalized 3 days after ingesting approximately 100 mg
chlorophacinone. Under high-dose vitamin K1 therapy (160 mg) the
prothrombin time was normalized, but it increased again following
withdrawal of vitamin K1. After prolonged vitamin K1 administration,
the prothrombin time finally became normal after 7 weeks.
Brodifacoum poisoning has occurred in South Sumatra, Indonesia.
Some of the villagers used a 0.005% brodifacoum rice grain bait as a
food source even though they knew it was poisonous and unfit for human
consumption. They attempted to remove the rodenticide by repeated
washing, rinsing and cooking before eating the rice. Because of the
delay in the appearance of poisoning symptoms it appeared that they
had been successful, thus encouraging further attempts to purify the
rice baits. As a result, deaths occurred before appropriate remedial
treatment could be initiated (Anonymous, 1985).
Jones et al. (1984) reported the first case of human brodifacoum
poisoning in a 17-year-old boy who attempted suicide by ingesting
approximately 7.5 mg (0.12 mg/kg) of brodifacoum in Canada. He was
initially seen with gross haematuria, followed by epistaxis and gum
bleeding. The prothrombin time and the activated partial
thromboplastin time were notably prolonged. He was treated for 56
days with either parenteral or oral vitamin K1 and either fresh or
stored plasma until coagulation values remained normal and stable.
Lipton & Klass (1984) reported a similar case in a 31-year-old
mentally disturbed woman who ingested over a 2-day period
approximately thirty 50-g packages of Talon-G (approximately 75 mg of
brodifacoum). Prothrombin time and activated partial thromboplastin
time were considerably prolonged (respectively 6-fold and 4-fold above
normal values). After 4 days of therapy with high doses of vitamin
K1 (up to 125 mg/day), partial correction in the prothrombin time
occurred. Vitamin K1 therapy continued with interruptions for 8
months until normal prothrombin time levels were found.
Chong et al. (1986) reported a case of suicidal poisoning after
ingestion of 10 mg brodifacoum (as 0.05% Klerat). The coagulation test
became normal after large doses and prolonged use of vitamin K1 over
6 months.
A case of intentional ingestion of brodifacoum (200 g of Talon G,
0.005% brodifacoum) was reported by Hoffman et al. (1988). A profound
decrease in the levels of factors II, VII, IX and X, lasting 43 days
after ingestion, was observed. Treatment with subcutaneous vitamin
K1 in doses up to 100 mg per day was effective.
Weitzel et al. (1990) described three patients with severe
bleeding disorders due to deficiency of the vitamin K-dependent blood
clotting proteins after ingestion of an anticoagulant. Although the
patients denied any ingestion, brodifacoum was detected in their serum
at concentrations of 7.6 nmol/litre, 270.7 nmol/litre and
2759 nmol/litre, respectively. The anticoagulant effect was found to
persist long after brodifacoum was no longer detectable in the serum.
A half-life of approximately 16-36 days was determined for brodifacoum
in the plasma.
Kruse & Carlson (1992) reported the case of a 25-year-old man who
attempted suicide by consuming a brodifacoum rodenticide. He
developed a severe coagulopathy that was treated with vitamin K1 and
fresh frozen plasma and he was discharged from hospital with oral
phytomenadione. Fifteen weeks later the man presented again with a
history of further brodifacoum ingestion. He suddenly became comatose
and computer tomography revealed a subarachnoid haemorrhage that led
to brain death 24 h later.
Wallace et al. (1990) described the clinical course of a patient
poisoned with brodifacoum in a suicide attempt. He developed
microhaematuria and melaena. His clotting factors were depressed and
were poorly responsive to vitamin K treatment.
Barlow et al. (1982) reported a case of attempted suicide with
25 mg of difenacoum (500 g of rat bait) followed several months later
by 1800 g of rat bait. The patient was treated with vitamin K1
(phytomenadione) for 48 and 42 days, respectively, until the
pharmacological effect of difenacoum ceased.
Nighoghossian et al. (1990) reported an unusual coagulopathy
after accidental exposure to a diphenacoum rodenticide. A 59-year-old
man developed subacute tetraparesis following severe sudden neck pain,
which on clinical examination was shown to be due to a subdural
cervical haematoma. Prothrombin complex activity was low and
diphenacoum was present in the plasma. Specific medical management
led to a complete recovery.
Greeff et al. (1987) reported accidental bromadiolone poisoning
in two children, resulting in prolonged anticoagulation.
Descarboxyprothrombin levels were increased in both cases by 27% and
29.9%, respectively (normal, non-detectable level). The first child
rapidly recovered after treatment with high-dose intravenous factor
IX-prothrombin complex and vitamin K1. The clotting profile became
normal on the third day after admission. The second child gave a poor
response to 10 mg intravenous vitamin K1 and the dose was increased
to 20 mg.
8.1.3 Controlled human studies
Single oral doses of 60, 70, 80 or 120 mg warfarin decreased the
prothrombin concentrations in volunteers to zero by the third day.
After the administration of 50 mg vitamin K1, the prothrombin
concentrations returned by the sixth day to 60, 70, 55 and 63%,
respectively, of the normal value (Anonymous, 1965).
When a single oral dose of 20 mg chlorophacinone was given to
three volunteers, the lowest prothrombin times were 35, 34 and 38% of
the pretreatment value on days 2, 4 and 2, respectively. Eight days
after administration without any treatment the values were 80, 100 and
90%, respectively (Anonymous, 1965).
8.2 Monitoring of biological effects
8.2.1 Effects of short- and long-term exposure
Two cases of occupational exposure to brodifacoum and difenacoum
were reported by Park et al. (1986). The exposure was of a chronic
nature (2 and 4 years, respectively). Plasma analysis in the first
patient revealed the presence of both difenacoum and brodifacoum in
the range of 30-50 µg/litre. In both patients unexpectedly high
concentrations of vitamin K1 2,3-epoxide were found in the presence
of normal clotting factor activities and antigen levels suggesting the
presence of coumarin anticoagulants in the liver.
A case of poisoning in a 23-year-old man resulting from prolonged
skin contact during the process of preparing and distributing warfarin
baits has been reported (Fristedt & Sterner, 1965).
8.2.2 Epidemiological studies
During a production run preparing ready-to-use flocoumafen bait
(0.005% in baits) in a formulation plant, the effect of the
rodenticide on blood coagulation factors was monitored in 12 subjects,
using the classical prothrombin time test, a modified prothrombin time
technique and measurement of prothrombin (factor II) concentration in
blood. No adverse health effects were observed in any subject
involved in formulation operations. No changes were observed in any
of the three tests that could be ascribed to absorption of flocoumafen
into the body (Tuinman & Van Sittert, 1986).
8.3 Developmental effects
Developmental effects have been reported when anticoagulants,
particularly warfarin, have been administered as therapeutic agent
during pregnancy. Hall et al. (1980) reviewed 418 reported
pregnancies in which coumarin and indan-1,3-dione derivatives were
used, and found that one-sixth resulted in abnormal liveborn infants,
one-sixth in abortion or stillbirth, and two-thirds in apparently
normal infants.
According to Hall (1976), there are two types of defects
associated with anticoagulants, dependent upon the time of
administration during pregnancy. The first, a characteristic
embryopathy described by the terms "warfarin embryopathy" or "fetal
warfarin syndrome", occurs from early, first-trimester use. Fetal
wastage and other abnormalities, especially central nervous system
anomalies, result from treatment later during gestation (usually the
second or third trimesters). Clotting factors are not present in
first-trimester embryos and this may explain the differences in fetal
abnormalities.
The most consistent feature of warfarin embryopathy is nasal
hypoplasia. Choanal stenosis has been observed, and respiratory
difficulty is typical because of the narrowed nasal passages (Smith &
Cameron, 1979; Pauli & Hall, 1979).
The other common feature is bone abnormalities of the axial and
appendicular skeleton. Laryngeal and tracheal-thyroid calcification
has also been noted (Schardein, 1985).
Other non-skeletal abnormalities reported include
ophthalmological malformations of several types, including defects
leading to blindness, developmental delay, low birth weight (premature
birth), mental retardation, hypotonia and ear anomalies (Carson &
Reid, 1976; Schardein, 1985).
No cases of embryopathy from anticoagulants in their use as
rodenticides have been reported.
8.4 Other adverse effects
One of the more common side-effects of coumarin therapy is skin
necrosis (Brooks & Blais, 1991; Eby, 1993; Locht & Lindström, 1993).
Although this effect has been associated with a number of different
agents, it is commonly referred to as warfarin-induced skin necrosis
(WISN). The frequency of this effect has been estimated to be between
1 in 100 and 1 in 10 000, with the majority of cases occurring in
women. Symptoms of WISN typically appear 3-6 days after the
initiation of warfarin therapy, and the areas of skin involved are
most frequently areas with subcutaneous fat, particularly the breasts,
thighs, and buttocks. There is some evidence that deficiencies in
vitamin K-dependent anticoagulant proteins (protein C and protein S)
may underlie the susceptibilities of at least some individuals to WISN
(Anderson et al., 1992). The occurrence of WISN may be avoided in at
least some cases by the co-administration of heparin.
Warfarin therapy has been noted to result in increases in the
incidence of haematomas, including intraspinal epidural haematoma
(Murphy & Nye, 1992), liver haematoma (Erichsen et al., 1993), and
intramural haematoma of the small intestine (Avent et al., 1992).
Bone contains three vitamin-K-dependent proteins, i.e.
osteocalcin, matrix Gla-protein and protein S. It has been shown that
oral anticoagulants (phenprocoumon, acenocoumarol) reduce both the
plasma antigen level as well as the Gla content of osteocalcin (Van
Haarlem et al., 1988). Furthermore it was found that a poor vitamin K
status was associated with a high urinary calcium loss (Knapen et al.,
1993). These data are consistent with the observation that the bone
mass in patients on long-term anticoagulant treatment was
significantly lower than in a group of age- and sex-matched controls
(Fiore et al., 1990; Resch et al., 1991). Vitamin-K-dependent proteins
have also been identified in calcified atherosclerotic plaques, where
they were suggested to contribute to the prevention of vascular
calcification (Gijsbers et al., 1990). In this respect it is
remarkable that warfarin treatment has been shown to increase rather
than to reduce atherosclerosis in an animal model (Antov et al.,
1985). Vitamin-K-dependent proteins not related to blood coagulation
are still discovered regularly (Manfioletti et al., 1993), and it is
important to remain alert for unexpected side-effects of vitamin K
antagonists, notably after long-term exposure.
8.5 Methods for assessing absorption and effects of anticoagulant
rodenticides
The laboratory control of orally administered coumarin
derivatives has been carried out using the classical one-stage
prothrombin time test (Quick, 1935) or modified techniques such as
"Thrombotest" (Owren, 1959). However, these tests have been designed
for clinical monitoring of circulating clotting factors during
anticoagulant therapy. The monitoring of occupational exposure to
rodenticides requires the prothrombin time test to be of sufficient
sensitivity to measure changes in the normal range (Tuinman & Van
Sittert, 1985).
Repeated occupational exposure to low levels of anticoagulant
rodenticides could gradually deplete vitamin-K-dependent coagulation
factors in the blood. To detect unwanted exposure of humans in an
early state, a careful screening of those at risk is recommended. The
question is which screening method is the most suitable to monitor low
levels of rodenticide ingestion. Prothrombin time and related tests
are "overall" clotting tests, which were developed for monitoring
patients under deep anticoagulation. These tests are easy to perform
and do not require complicated equipment, but they are relatively
insensitive when used for monitoring milder anticoagulation states
(Tuinman & Van Sittert, 1985; Ross et al., 1992; Travis et al., 1993).
If possible, specific and more sensitive tests should be used. The
most sensitive test, applicable over a wide range of anticoagulation
states, is the direct detection of descarboxy-prothrombin using a
monoclonal antibody specifically recognizing the descarboxy form of
prothrombin (Widdershoven et al., 1987). Another marker for
monitoring poor vitamin K status at an early stage is
descarboxy-osteocalcin (Knapen et al., 1993), but the commercial test
kits presently available need to be substantially improved and
simplified before they can be recommended for this purpose in routine
laboratories.
Another method was suggested by Park et al. (1986), who
repeatedly injected 10 mg of vitamin K1 into factory workers who had
been exposed to brodifacoum. The authors observed that 2-4 h after
injection the circulating KO/K ratios were significantly elevated even
18 months after the prothrombin times had returned to normal values.
This suggests that very low liver concentrations of brodifacoum can be
detected from the altered KO/K ratio rather than from tests based on
blood coagulation parameters. Because this method requires vitamin K
administration shortly before blood sampling, it is only applicable in
cases of anticoagulant poisoning, and not for the routine control of
plant workers.
Methods for the direct detection of coumarin anticoagulants in
plasma and serum have been reported, all of which are based on the
extraction of plasma and pre-purification of the sample, followed by
HPLC analysis with fluorescence detection (Hunter, 1983; Murphy et
al., 1989; Felice & Murphy, 1989; Felice et al., 1991; O'Bryan &
Constable, 1991). However, such facilities will not be available in
most routine laboratories. Moreover, the blood sampling should be
performed within a reasonably short period after ingestion of the
coumarins, because these drugs are rapidly cleared by the liver. This
places severe restrictions on the applicability of these techniques,
particularly for the second-generation anticoagulants.
Plasma chlorophacinone determinations were performed in three
cases of intoxication. The risk of bleeding was minimal when the
plasma level was below 1 mg/litre (Burcuoa et al., 1989).
Brodifacoum was detected in a case of self-ingestion by a
25-year-old woman who denied any intake of anticoagulants but was in
need of vitamin K1 treatment for 8 months. Factor assays revealed a
marked reduction in the levels of the vitamin K-dependent factors II,
VII, IX and X and normal levels of factor V and VIII:C (Exner et al.,
1992).
The diagnosis in a case of difenacoum intoxication was confirmed
by analysis of difenacoum in serum (0.6 mg/litre) (Butcher et al.,
1992).
Bromadiolone was analysed in the serum of a 27-year-old female
with a history of bleeding. She denied any contact with a rodenticide
but the bromadiolone concentration in serum was 40 mg/litre (Chow et
al., 1992).
Hollinger & Pastoor (1993) reported results of comparison of
plasma brodifacoum concentrations and prothrombin levels over time in
a case of brodifacoum poisoning. Brodifacoum was eliminated according
to a two-compartment mode, with an initial half-life of 18 h and a
terminal half-life of 24.2 days. On admission, the brodifacoum level
was 731 µg/litre. Treatment with large doses of phytonadione lasted
for 4 months.
8.6 Treatment of anticoagulant rodenticide poisoning
All suspected poisoned patients should receive medical attention
immediately. Rapid determination of prothrombin time and search for
evidence of bleeding is essential and may have to be maintained for
several weeks.
8.6.1 Minimizing the absorption
Gastric lavage or induction of emesis are indicated in all cases
of superwarfarin rodenticide ingestion if it was recent and the amount
is possibly lethal or uncertain. Repeated administration of activated
charcoal is useful. Cathartics could also be administered (Sullivan
et al., 1989; Smolinske et al., 1989; Donovan et al., 1990).
8.6.2 Specific pharmacological treatment
8.6.2.1 Vitamin K1 (phytomenadione)
Vitamin K1 is the specific antidote of choice. Depending on
whether the poisoning is due to warfarin or superwarfarin, the dosage
may differ as well as the duration of treatment. Dosage is dependent
on coagulation parameters, mainly prothrombin time.
If the patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection.
Prothrombin time should be checked at 3-hourly intervals in severe
cases and after 8-10 h in less severe cases. If no improvement
occurs, vitamin K1 injection should be repeated. Doses of up to
125-200 mg/day have been given without adverse effects (Lipton &
Klass, 1984; Sheen et al., 1994).
In moderate to minor cases of poisoning, vitamin K1 may be
given in lower doses.
After initial parenteral vitamin K1 administration, oral
treatment can be continued for a prolonged period of time. Oral
treatment can also be sufficient in minor cases.
The major difference between warfarin and second-generation
rodenticides is that the latter can cause increased bleeding for a
longer period of time than warfarin, as they have a much longer
half-life in the body. Therefore vitamin K1 should be given for
months rather than weeks. It is also prudent to monitor prothrombin
time for some time after cessation of this treatment to ensure that
there is no regression.
In warfarin-resistant individuals, 10 times the normal dose of
warfarin is required to achieve a reduction in the plasma prothrombin
level. However, these individuals also respond more strongly to the
effect of vitamin K (O'Reilly et al., 1963; O'Reilly et al., 1964).
8.6.2.2 Blood components
The following products could be considered, subject to
availability:
* whole blood
* fresh frozen plasma and fresh blood should be used in cases of
acute severe bleeding in order to rapidly restore the blood
clotting factors
* factor concentrate may be considered in severe cases, especially
if the amount of plasma would be too great (volume overload)
8.6.2.3 Phenobarbital
In the cases reported by Lipton & Klass (1984) and Jones et al.
(1984), phenobarbital therapy was included. It was presumed that
anticoagulants had been metabolized by the mixed function oxidase
system based on animal experiments (Bachmann & Sullivan, 1983) and
that, by inducing this enzyme system, phenobarbital might increase
their metabolism.
Robinson & Mac Donald (1966) found that the pharmacological
activity of warfarin in humans decreased when it was combined with
phenobarbital.
8.6.3 Response to therapy
Patient should be kept in hospital until the prothrombin time has
remained normal for 3 days. It is suggested that oral treatment with
10 mg vitamin K1 twice daily may be necessary for up to 60 days with
close monitoring of prothrombin time.
According to Hoffman et al. (1988), factor analysis allows for a
detailed evaluation of the course of toxicity, and the response to
therapy. Monitoring the prothrombin time alone could offer a false
sense of confidence and delay effective treatment.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
No data are available for the effects on microorganisms in water
and in soil.
9.1.2 Aquatic organisms
The acute toxicity of technical flocoumafen to planktonic algae
(Selenastrum capricornutum) was determined in a 4-day growth test.
The 96-h EC50, based on cell counts on day 4, was calculated to be
1.1 mg/litre (Pearson & Wallace, 1984).
Pearson (1984) reported static acute toxicity tests for
flocoumafen on Daphnia magna. The 48-h EC50 values, based on
immobilization, were 1.4 mg/litre for technical grade flocoumafen and
280 mg/litre for 0.5% flocoumafen. Pearson & Wallace (1984) found a
48-h EC50 for Daphnia magna of 0.66 mg/litre for technical grade
flocoumafen.
Anticoagulants are highly toxic to fish when tested as technical
formulations (Hill et al., 1976; Pearson & Wallace, 1984) (Table 8).
9.1.3 Terrestrial organisms
9.1.3.1 Acute toxicity
Bird species vary in their susceptibility to anticoagulant
rodenticides. The acute oral LD50 of brodifacoum for the mallard
duck (Anas platyrhynchos) is 2.0 mg/kg (Ross et al., 1978).
Symptoms of poisoning became apparent approximately 7 days after
dosing and included lethargy, weakness and lack of muscular
coordination. Prolonged bleeding occurred from any small wounds and
extensive bruising and subcutaneous haemorrhage were noted. Blood was
observed in the faeces. Deaths occurred principally during the period
of 7-14 days after administration. No deaths occurred more than 4
weeks after administration.
The acute toxicity of flocoumafen to the mallard duck appears to
be dependent on the age of birds. The LD50 values for 12- and
18-week-old birds are 24 mg/kg and 94 mg/kg, respectively (Roberts et
al., 1985b,c).
Table 8. Acute toxicity of anticoagulant rodenticides for aquatic organisms (96-h LC50)
Rodenticide Species Stat/flowa LC50 (mg/litre) Reference
Brodifacoum Rainbow trout flow 0.051 Hill et al. (1976)
Diphacinone (95%) Bluegill sunfish 7.6 Kosmin & Barlow (1976)
Channel catfish 2.09 Kosmin & Barlow (1976)
Rainbow trout 2.82 Kosmin & Barlow (1976)
Pink shrimp > 10 Kosmin & Barlow (1976)
Fiddler crab > 10 Kosmin & Barlow (1976)
Diphacinone (0.005%) Bluegill sunfish 288 Kosmin & Barlow (1976)
Channel catfish 285 Kosmin & Barlow (1976)
Rainbow trout 425 Kosmin & Barlow (1976)
Pink shrimp 33 Kosmin & Barlow (1976)
Fiddler crab 37 Kosmin & Barlow (1976)
Warfarin Bluegill sunfish 88 Brorson et al. (1994)
Flocoumafen (0.5%) Rainbow trout static 190 Pearson (1984)
Flocoumafen (technical grade) Rainbow trout static 0.95 Pearson (1984)
Rainbow trout static 0.32 Pearson & Wallace (1984)
Rainbow trout static 0.091 Pearson & Wallace (1984)
Common carp static 0.22 Pearson & Wallace (1984)
Bromadiolone Rainbow trout 1.4 Tomlin (1994)
a stat = static conditions (water unchanged for duration of test)
Sex differences were observed in the acute toxicity for bobwhite
quail (Colinus virginianus) of difenacoum. LD50 values for male
and female birds were 140 mg/kg body weight and 56 mg/kg body weight,
respectively (Ross et al., 1980c).
Birds seem to be relatively tolerant to diphacinone, the LD50
for mallard duck being 3158 mg/kg (Kosmin & Barlow, 1976).
The acute toxicity of brodifacoum for various species of birds is
presented in Table 9.
Table 9. The acute toxicity of brodifacoum to birds (Godfrey, 1985)
Species Acute oral LD50
(mg/kg)
Black-backed gull (Larus dominicans) < 0.75
Black-billed gull (L. bulleri) < 5.0
Canada goose (Branta canadensis canadensis) < 0.75
Pukeko (Porphyris porphyris melanotus) 0.95
Mallard duck (Anas platyrhynchus) 4.6
California quail (Lophortyx californica) 3.3
Blackbird (Turdus merula) > 3
House sparrow (Passer domesticus) > 6
Hedge sparrow (Prunella modularis occidentalis) > 3
Wax-eye (Zosterops lateralis) > 6
Harrier hawk (Circus approximans) 10.0
Ring-necked pheasant (Phasianus colchicus) 10.0
Paradise duck (Tadorna variegata) > 20
9.1.3.2 Primary toxicity
The subacute (5-day) dietary toxicity (LC50) of difenacoum for
the bobwhite quail was found to be between 0.25 and 7.00 mg/kg diet
(Ross et al., 1980a). The 5-day LC50 of difenacoum for the mallard
duck was calculated to be 18.9 mg/kg diet (Ross et al., 1980b).
Similarly to the acute toxicity studies, diphacinone was found to
be less toxic in 8-day dietary studies. The LC50 for the bobwhite
quail was 4485 mg/kg diet and for the mallard duck was > 10 000 mg/kg
diet (Kosmin & Barlow, 1976).
White Leghorn hens (4 per group) were fed warfarin,
coumatetralyl, bromadiolone, difenacoum or brodifacoum in baits as a
choice to non-poisonous chicken food for 15 days. No symptoms
appeared at a total warfarin intake of up to 171 mg/kg body weight.
Coumatetralyl and brodifacoum killed all hens after intakes of
79-137 mg/kg body weight of active ingredient and 7.1-15 mg/kg body
weight of active ingredient, respectively. Both bromadiolone and
difenacoum killed two of the hens, at 5.9 and 15.9 mg/kg body weight
of active ingredient for bromadiolone, and at 18.9 and 26.3 mg/kg
body weight active ingredient for difenacoum within 10-15 days (Lund,
1981).
Christopher et al. (1984) dosed male hybrid leghorn chickens with
anticoagulant rodenticides. No signs of poisoning were observed in
chickens fed 183.7 mg warfarin/kg (mean a.i. ingested) for 3 days. No
mortality occurred after the ingestion of a total dose of 36.9 mg
bromadiolone/kg over a 3-day period. Birds fed a diet containing
brodifacoum for 3 days ingested a mean of 28.9 mg/kg (active
ingredient). One bird died on day 4 while the other five birds
exposed had died by day 16.
Two-day no-choice tests in which chukar partridges ( Alectoris
graeca cypriotes Hartert) were fed 0.005% bromadiolone or difenacoum
resulted in no mortality. Ten-day no-choice feeding tests resulted in
6 out of 12 and 8 out of 12 dead partridges for bromadiolone and
difenacoum, respectively. The highest quantity of bait consumed by an
individual bird without any lethal effects was 411 g of bait with
bromadiolone (34.8 mg a.i./kg body weight) and 326.2 g of bait with
difenacoum (29.1 mg a.i./kg body weight) (Krambias & Hoppe, 1987).
9.1.3.3 Secondary toxicity
Barn owls (Tyto alba) were fed rats poisoned with diphacinone,
chlorophacinone, coumafuryl, difenacoum, bromadiolone or brodifacoum.
Five out of six owls died of haemorrhaging after feeding on rats
killed with brodifacoum after 8 to 11 days. Sublethal haemorrhaging,
but no mortality, occurred in owls fed rats killed with difenacoum.
One owl died following 10 days of treatment with bromadiolone-poisoned
rats, while five showed no symptoms. No abnormalities were observed
in two owls fed rats killed with diphacinone, coumafuryl or
chlorophacinone. Owls that died behaved normally until 24 h or less
before death, when they became lethargic and stopped eating
(Mendenhall & Pank, 1980).
Mice (weighing 35 g) were fed for 1 day (no choice) on a mixture
containing either 0.005% difenacoum or 0.002% brodifacoum. The mean
mass of residue on the day of death in a whole mouse was estimated to
be 10.17 µg for difenacoum and 15.36 µg for brodifacoum. Difenacoum-
and brodifacoumpoisoned mice were fed to captive barn owls for
successive periods of 1, 3 and 6 days. All of the owls fed on
difenacoum-poisoned mice survived the treatments and none showed
external bleeding. In contrast, four of the six owls fed
brodifacoum-poisoned mice died within 6-17 days after a 1-day dose.
The estimated lethal dose of brodifacoum was 0.15-0.18 mg/kg. After
death these owls had 0.63-1.25 mg brodifacoum/kg in their livers
(Newton et al., 1990).
Newton et al. (1994) fed barn owls flocoumafen-dosed mice for
consecutive periods of 1, 3 and 6 days. Four of the birds survived;
the fifth bird died from haemorrhaging 5 days after the final dose.
During the feeding trial birds received cumulative flocoumafen doses
ranging from 0.78 to 1.25 mg/kg.
Gray et al. (1992) investigated the toxicity of brodifacoum,
difenacoum and flocoumafen for barn owls fed poisoned mice. For each
rodenticide, the owls survived a cumulative dose of at least 1.9 mg/kg
owl weight over 15 days of treatment. All owls with cumulative doses
in excess of 1.9 mg/kg body weight showed multiple treatment-related
effects. The three rodenticides had approximately the same order of
magnitude of toxicity to barn owls. Gray et al. (1994) developed a
non-invasive method for monitoring the exposure of these birds to
second-generation rodenticides by measuring compounds in regurgitated
owl feed.
Radvanyi et al. (1988) fed American kestrels (Falco sparverius)
on meadow voles that had been maintained on 2% chlorophacinone. Voles
consumed approximately 53 mg of 2% chlorophacinone (1.14 mg a.i.)
before dying within 6 days. No kestrels fed poisoned mice, for up to
21 consecutive days, died. Haematomas were observed on the pectoral
muscles, lungs, liver and heart of exposed birds.
In a study by Townsend et al. (1981), captive-bred tawny owls
(Strix aluco) were given warfarin-treated mice for 3 months. No
behavioural changes were observed. Prothrombin levels decreased to
less than 10-12% of normal but returned to normal after 9 days.
Townsend et al. (1984) found that daily warfarin intakes
approaching 0.3 mg/kg body weight or 30 µg of warfarin/day can cause
the death of list weasels (Mustela nivalis) by secondary poisoning.
Lethal exposure occurred if mice were contaminated with 1.0-1.5 mg
warfarin/kg, an amount reached following exposure to 0.005 or 0.02%
sodium warfarin for about 3 days.
9.2 Field observations
9.2.1 Primary poisonings
There are few data available on the non-target hazard of
anticoagulant rodenticides in field conditions.
Greig-Smith et al. (1988) reported incidents in England and Wales
with barn owls and foxes. There were three incidents in which barn
owls were found to have residues of the anticoagulant rodenticide
brodifacoum in their livers. In two birds, residues were thought to
represent lethal poisoning (0.61 and 0.29 mg/kg) but in the third the
level was only 0.099 mg/kg. There were six separate incidents in
which dead foxes were found to contain residues of brodifacoum,
bromadiolone, coumatetralyl, difenacoum or warfarin. Residue levels
showed a wide range from 0.009 to 0.46 mg/kg tissue. Three of the
foxes contained more than one rodenticide, one animal revealing
residues of difenacoum, bromadiolone and warfarin.
Poisonings of pheasants and partridges by chlorophacinone used
against Microtus arvalis have been reported (Giban, 1974).
Reece et al. (1985) reported that, a few days after bromadiolone
was placed out for control of rodents, three pea fowl
(Pavo cristatus), a little raven (Corvus mellori) and an eastern
swamp hen (Porphyrio porphyrio melanotus) died. Necropsy showed
that the birds had been in good condition. One of the pea fowl and
the swamp hen had massive intra-abdominal haemorrhage without evidence
of external trauma. All had severe congestion of the liver and lungs.
The birds had negligible gut contents, so analysis for bromadiolone
could not be performed.
9.2.2 Secondary poisonings
Difenacoum and brodifacoum were detected in 15 of a total of 145
dead barn owls in Britain, and on postmortem the cause of death for
one owl was diagnosed as rodenticide poisoning (Newton et al., 1990,
see section 5.1).
A study of 35 active nests and the radio-telemetry of 34 barn
owls in an area where brodifacoum baits (0.005%) were used in and
around farm buildings in New Jersey, USA, showed no adverse effects of
the rodenticide on the owls studied. In this study, rats and house
mice were the target organisms and barn owls were found not to feed on
these animals to any extent. Chicks were fledged from at least eight
nests where poisoned rodents had been available during part of the
nesting and feeding period, but no rodenticide-induced mortality was
observed. Traces of brodifacoum (less than 0.05 mg/kg) were found in
a barn owl that had been accidentally electrocuted (Hegdal &
Blaskiewicz, 1984).
Merson & Byers (1984) monitored eastern screech owls (Otus asio)
using radio-transmitters following the use of 0.005% brodifacoum for
rodent control in a commercial orchard. Brodifacoum was detected in
screech owl pellets (see section 5.1). There were no owl deaths which
could be attributed to brodifacoum poisoning. Two owls were analysed
at the end of the study; one showed signs of haemorrhage and
contained 0.21 mg brodifacoum/kg and the other owl contained no
brodifacoum. In a larger study, Hegdal & Colvin (1988) monitored 38
eastern screech owls and several other species of owl following
brodifacoum application in an orchard. Minimum mortality was 58%
among screech owls for which more than 20% of the home range was
treated, as compared with 17% among those for which less than 10% of
the home range was treated. Secondary brodifacoum poisoning was the
most probable cause of death in six screech owls. Six radio-equipped
owls were collected 1-2 months post-treatment, and four contained
detectable concentrations of brodifacoum.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Anticoagulants, both hydroxycoumarins and indandiones, known also
as vitamin K antagonists, are widely used in urban rodent control and
against rodent pests in agriculture. They act by inhibiting the
vitamin K1 epoxide cycle, thereby depleting the active form of
vitamin K1 necessary for producing blood-clotting factors.
Since anticoagulant rodenticides are used in general as
low-concentration bait formulations and have low volatility, increased
levels in the air are unlikely. Being only slightly soluble in water
their use could not be a major source of water contamination.
Anticoagulant rodenticides are not intended for direct application to
growing crops. Hence no residues in plant foodstuffs are expected.
The controlled medicinal use of warfarin exposes more people to
higher concentrations over a longer period than would be expected to
occur as a result of accidental human exposure due to its use as a
rodenticide.
Occupational exposure may occur during manufacture, formulation
and bait application, but figures indicating the levels of exposure
are not available.
Anticoagulant rodenticides are readily absorbed through the
gastrointestinal tract, skin and respiratory system. The major route
of elimination in various species after oral administration is through
the faeces. The urine is a very minor route of elimination. The liver
is the major organ for accumulation and storage of anticoagulants.
The metabolism pattern of warfarin and indandiones mainly
involves hydroxylation. The second-generation hydroxycoumarins are
found principally as unchanged parent compounds. Their elimination
from the liver is slow with a biphasic rate, where a rapid initial
phase is followed by a prolonged second phase.
Most of the anticoagulants have high acute toxicity by oral,
percutaneous and inhalation routes of exposure. The second-generation
anticoagulants are more toxic than the first-generation one in the
sense that a single feeding may be lethal. Signs of poisoning in all
species, including humans, are associated with increased bleeding
tendency.
Many poisoning incidents (both intentional and unintentional)
have been reported. A few cases of intoxication from occupational
exposure to anticoagulants have also been observed.
The level of prothrombin concentration is a satisfactory guide to
the severity of acute intoxication and the effectiveness and duration
of the therapy. The specific antidote is vitamin K1. The high
retention of the second-generation anticoagulants in the liver means
that any treatment of intoxication should be prolonged to ensure that
anticoagulant activity does not recur.
The addition of bittering agents to anticoagulant rodenticides is
aimed at discouraging human consumption.
Warfarin has been found to be teratogenic in both rats and
humans. Second-generation anticoagulant rodenticides do not
demonstrate teratogenicity in laboratory animals. Developmental
effects in humans are observed when warfarin has been taken as a
therapeutic agent during pregnancy. No cases of embryopathy from
anticoagulants in their use as rodenticides have been reported.
Second-generation anticoagulants are not intended to be used as
therapeutic agents, and the risks associated with, for example,
warfarin will not apply.
There is no evidence to suggest that any anticoagulant
rodenticides are mutagenic, but there are insufficient data available
on some compounds to demonstrate an absence of mutagenicity.
There is experimental evidence that coumarin anticoagulants not
only inhibit the vitamin K cycle in the liver, but also in other
tissues such as bone. In humans it has been demonstrated that even
low levels of vitamin K deficiency form a risk factor for developing
osteoporosis (Hart et al., 1985; Szulc et al., 1993). Therefore, it
appears that long-term exposure to low levels of anticoagulant may
have an adverse effect on bone metabolism.
10.2 Evaluation of effects on the environment
Unlike conventional crop protection products, which must be
applied over relatively large crop areas, anticoagulant rodenticides
are applied to discrete sites in the form of low concentration baits.
Most of the anticoagulants are stable under normal conditions.
They are slightly soluble in water, and as bait formulations their use
is unlikely to be a source of water contamination. They appear to
bind rapidly in the soil, with very slow desorption and no leaching.
In general, anticoagulants are highly toxic to aquatic organisms when
tested as technical material.
Non-target organisms are potentially at risk in two ways: from
direct consumption of baits (primary hazard) and through eating
poisoned rodents (secondary hazard).
Bird species vary in their susceptibility to anticoagulant
rodenticides. Small pellets and whole grain baits are highly
attractive to birds. Wax block formulation appear to decrease the
attractiveness to the birds and so reduce the possibility of poisoning
incidents. It is difficult to assess the risks to birds due to direct
consumption of baits because most of the published studies are
toxicity trials in laboratory conditions.
The main cause for poisoning of domestic animals is through
direct consumption of anticoagulant baits.
Some of the anticoagulants show a similar range of acute toxicity
for non-target non-rodent mammals as for target rodents. The primary
hazard is usually expressed by the amount of finished bait which must
be consumed to approach the lethal dose. The bait concentration of
second-generation rodenticides is usually around 0.005%, whereas the
concentration of the first-generation rodenticides in the bait may be
5 to 10 times higher. Thus, to reach the toxic or lethal dose,
non-target animals must consume comparatively large amounts of bait
with low concentrations of active ingredient.
Secondary poisoning through the consumption of rats and mice
killed with anticoagulants may occur in dogs and cats in urban
situations but is more likely in farm situations.
Some secondary toxicity laboratory studies with wildlife have
shown that captive predators could be intoxicated by no-choice feeding
of anticoagulant-poisoned or dosed prey. The significance of these
results in terms of hazard under field conditions is difficult to
assess because the predators would not be expected to eat only
poisoned animals. However, where they occur, predators may take
poisoned, but not dead, small mammals preferentially. In areas close
to baiting, poisoned rodents may represent a high proportion of the
diet for individual birds. However, only a few individuals will be
affected except in situations of very widespread and constant use of
baits. Therefore, some kills of owls will be expected but no severe
population effects. This agrees with observed field effects with small
numbers of poisoned owls.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
a) Exposure of the general population to anticoagulant rodenticides
via food and drinking-water is unlikely and does not constitute a
significant health hazard.
b) Poisoning incidents may occur in cases of massive intentional or
unintentional ingestion or prolonged skin contact during
manufacture and formulation.
c) Anticoagulant rodenticides are relatively persistent in the
environment, but their specific use as low-concentration bait
formulations limits the potential for environmental
contamination.
d) Direct and secondary poisoning of birds, domestic and farm
animals, and wildlife may occur.
e) The major difference between the first- and second-generation
anticoagulant rodenticides is that the latter have longer body
retention, resulting in an increased tendency for bleeding over a
longer period of time.
f) The mode of action of anticoagulant rodenticides is known and an
effective antidote is available, i.e. vitamin K1.
g) There is no available evidence that this class of compound is
mutagenic or carcinogenic.
h) Only warfarin has been shown to possess some teratogenic
potential in both rats and humans.
11.2 Recommendations for protection of human health and the
environment
a) Exposed workers should receive appropriate biomonitoring and
health evaluation.
b) The inclusion of a bittering agent in formulations at appropriate
concentrations may reduce accidental ingestion.
c) For preventing primary poisoning, baits less attractive to birds
and domestic animals, as well as pulsating baiting, should be
used.
d) The location of bait placement should be carefully selected.
e) Killed rodents should be burned or buried to reduce the risk of
secondary poisoning of predators.
f) Training in the safe handling of rodenticides is essential.
12. FURTHER RESEARCH
a) Studies of exposed humans are required, particularly with regard
to possible teratogenic and embryotoxic effects.
b) More information is needed in order to evaluate the risks of
occupational exposure to anticoagulant rodenticides.
c) Methods more sensitive than prothrombin time determination need
to be developed for routinely assessing the absorption and
effects of anticoagulants.
d) More data are required to assess secondary effects on non-target
organism populations.
e) The extent to which second-generation anticoagulant rodenticides
are transferred across the placenta should be evaluated.
f) More information should be collected concerning the tissue
distribution of anticoagulant rodenticides after their ingestion
and their effects on physiological processes other than blood
coagulation, notably calcium and bone metabolism. This is
particularly necessary for long-term, low-level exposure to these
compounds.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by Hazard
(WHO, 1994), anticoagulant rodenticides were classified, according to
their acute oral LD50, as follows:
Class Ia (Extremely hazardous) Oral LD50 (mg/kg)
Brodifacoum 0.3
Bromadiolone 1.12
Chlorophacinone 3.1
Difenacoum 1.8
Difethialone 0.56
Diphacinone 2.3
Flocoumafen 0.25
Class Ib (Highly hazardous)
Coumachlor 33
Coumatetralyl 16
Warfarin 10
Class II (Moderately hazardous)
Pindone 50
A Poison Information Monograph for brodifacoum has been issued
(IPCS, 1992), and one on warfarin is in preparation.
REFERENCES
Allen JA, Proudlock RJ, & McCaffrey KJ (1986) Genotoxicity studies
with WL 108366 (rodenticide): in vivo chromosome studies with rat
bone marrow cells. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL 85/8610).
Alstad AD, Casper HH, & Johnson LJ (1985) Vitamin K treatment of sweet
clover poisoning in calves. J Am Med Assoc, 187: 729-731.
Anderson DR, Brill-Edwards P, & Walker I (1992) Warfarin-induced skin
necrosis in 2 patients with protein S deficiency: Successful
reinstatement of warfarin therapy. Haemostasis, 22: 124-128.
AMA (1980) AMA drug evaluations, 4th ed. Chicago, Illinois, American
Medical Association.
Anonymous (1965) Technical report on chlorophacinone. Lyon, France,
Lipha S.A.
Anonymous (1976) 'Ratak' containing difenacoum: A new rodenticide.
Haslemere, Surrey, Imperial Chemical Industries Ltd, Plant Protection
Division, 18 pp.
Anonymous (1985) Brodifacoum: safety in use. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division, 8 pp
(ICI Agrochemical Information Bulletin).
Anonymous (1988) The treatment of anticoagulant rodenticide poisoning.
Imperial Chemical Industries Ltd, Lipha S.A., Shell International
Chemical Company Ltd, and Sorex Ltd.
Antov G, Kasakova B, & Ajanova A (1985) Effect of warfarin on
experimentally induced sclerotic processes. In: Pathological models in
toxicological studies. Bucharest, Romania, Ministry of Chemical
Industry, Industrial Head-Office for Medicinal Drugs and Cosmetics,
pp 153-157.
Ashton AD, Jackson WB, & Peters H (1987) Comparative evaluation of
LD50 values for various anticoagulant rodenticides. In: Richards CGL
& Ku TY ed. Control of mammal pests. London, New York, Philadelphia,
Taylor & Francis, pp 187-197.
Avent ML, Canady BR, & Sawyer WT (1992) Warfarin-induced intramural
hematoma of the small intestine. Clin Pharm, 11: 632-635.
Bachmann KA & Sullivan TJ (1983) Dispositional and pharmacodynamic
characteristics of brodifacoum in warfarin-sensitive rats.
Pharmacology, 27: 281-288.
Barlow AM, Gay AL, & Park BK (1982) Difenacoum (Neosorexa) poisoning.
Br Med J, 285: 541.
Blair D (1984) Toxicology of a candidate rodenticide: The acute 4-hour
inhalation toxicity of manufacturing master mix (bait concentrate)
containing 0.5% m/m WL 108366. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 71 pp (Report No. SBGR.84.151).
Boermans HJ, Johnstone J, Black WD, & Murphy M (1991) Clinical signs,
laboratory changes and toxicokinetics of brodifacoum in horses. Can J
Vet Res, 55: 21-27.
Braithwaite GB (1982) Vitamin K and brodifacoum. J Am Vet Med Assoc,
181(6): 531, 534.
Bratt H (1987) Difenacoum: Elimination from tissues of rats following
administration of a single oral dose. Macclesfield, Surrey, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report
No. CTL/P/1592).
Braselton WE Jr, Neiger RD, & Poppenga RH (1992) Confirmation of
indandione rodenticide toxicoses by mass spectrometry/mass
spectrometry. J Vet Diagn Invest, 4, 441-446.
Brooks LW & Blais FX (1991) Coumarin-induced skin necrosis. J Am
Osteopath Assoc, 91: 601-605.
Brooks TM, Clare MG, & Wiggins DE (1984) Genotoxicity studies with
WL 108366 (candidate rodenticide). Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre, 64 pp (Report No. SBGR.84.160).
Brorson T, Björklund I, Svenstam G, & Lantz R (1994) Comparison of two
strategies for assessing ecotoxicological aspects of complex
wastewater from a chemical-pharmaceutical plant. Environ Toxicol Chem,
14(4): 543-552.
Buckle AP (1994) Rodent control methods in rodent pests and their
control. Wallingford, Oxon, Commonwealth Agriculture Bureau (CAB)
International.
Buitenhuis HC, Soute BAM, & Vermeer C (1990) Comparison of the
vitamins K1, K2 and K3 as cofactors for the hepatic vitamin
K-dependent carboxylase. Biochim Biophys Acta, 1034: 170-175.
Bull JO (1976) Laboratory and field investigations with difenacoum, a
promising new rodenticide. In: Proceedings of the 77th Vertebrate Pest
Conference, Monterey, California, March 1976. Davis, California,
University of California, pp 72-84.
Bullard RW, Thompson RD, & Holguin G (1976) Diphenadione residues in
tissues of cattle. J Agric Food Chem, 24(2): 261-263.
Burucoa Ch, Mura P, Robert R, Boinot C, Bouquet S, & Piriou A (1989)
Chlorophacinone intoxication: A biological and toxicological study.
Clin Toxicol, 27: 79-89.
Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, & Ind PW (1992)
Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol,
11: 553-554.
Callander RD (1984) Product registration. Brodifacoum: An evaluation
in the salmonella mutagenicity assay. Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V. (ICI CTL Report No. CTL/P/949).
Carson M & Reid M (1976) Warfarin and fetal abnormality. Lancet,
1: 1127.
Chalermchaikit T, Felice LJ, & Murphy MJ (1993) Simultaneous
determination of eight anticoagulant rodenticides in blood serum and
liver. J Anal Toxicol, 17: 56-61.
Chesterman H, Burford P, Harling RJ, & Heywood R (1984) WL 108366
rodenticide: Acute oral toxicity in Beagle dogs. Huntingdon, United
Kingdom, Huntingdon Research Centre (HRC Report No. SLL/72/84757).
Chong LL, Chan WK, & Ho CH (1986) A case of 'superwarfarin' poisoning.
Scand J Haematol, 36: 314-315.
Chow EY, Haley LP, Vickars LM, & Murphy MJ (1992) A case of
bromadiolone (superwarfarin) ingestion. Can Med Assoc J, 147: 60-62.
Christopher MJ, Balasubramanyam M, & Purushotham KR (1984) Toxicity of
three anticoagulant rodenticides to male hybrid Leghorns. Z Angew
Zool, 71(3): 275-281.
Cifone MA (1993) Mutagenicity test on bromadiolone technical in the
CHO/HGPRT forward mutation assay (HWA Study No. 15310-0-435). Vienna,
Virginia, Hazleton Washington Inc., 48 pp.
Clare MG & Wiggins DE (1986) In vitro mutagenicity studies with
WL 108366 (rodenticide) using cultured Chinese hamster V 79 cells.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research
Centre, 33 pp (Report No. SBGR.86.014).
Coveney PC & Forbes S (1987) Degradation of WL 108366 (Storm) in rat
baits, carcasses and faeces in field situations. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.87.171).
Cross M & Clay P (1984) Product registration. Brodifacoum: Assessment
of mutagenic potential using L5178Y mouse lymphoma cells. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/975).
Dodds WJ & Frantz SC (1984) Dog and cat poisonings. Pest Control
Technol, 12(3): 14.
Donovan JW, Ballard JO, & Murphy MJ (1990) Brodifacoum therapy with
activated charcoal: Effect on elimination kinetics. Vet Hum Toxicol,
32: 350.
Dreyfus M, Dubouch P, Pierson K, Neveu Y, Martin E, & Tchernia G
(1983) Warfarin-contaminated talc. Lancet, 1: 1110.
Dubock AC (1986) The evaluation of potential effects on non-target
vertebrate populations as a result of pesticide use. In: Proceedings
of the Second Symposium on Recent Advances in Rodent Control, Kuwait,
1986, pp 257-269.
Dusein P, Manigand G, & Taillandier J (1984) Hypoprothrombinémie
sévère et prolongée après intoxication par chlorphacinone. Presse Méd,
13(30): 1845.
DuVall MD, Murphy MJ, Ray AC, & Reagor JC (1989) Case studies on
second-generation anticoagulant rodenticide toxicities in non-target
species. J Vet Diagn Invest, 1: 66-68.
Eby CS (1993) Warfarin-induced skin necrosis. Hematol/Oncol Clin North
Am, 7: 1291-1300.
Elias DJ & Johns BE (1981) Response of rats to chronic ingestion of
diphacinone. Bull Environ Contam Toxicol, 27: 559-567.
Ellenhorn MJ & Barceloux DG (1988) Anticoagulant poisoning. In:
Medical toxicology, diagnosis and treatment of human poisoning.
Amsterdam, New York, Elsevier Science Publishing Company Inc.,
pp 211-221.
Erichsen C, Sudenaa K, Sreide JA, Andersen E, & Tysvoer A (1993)
Spontaneous liver hematomas induced by anti-coagulation therapy.
Hepato-Gastroenterology, 40: 402-406.
Exner DV, Brien WF, & Murphy MJ (1992) Superwarfarin ingestion. Can
Med Assoc J, 146(1): 34-35.
FAO (1979) Rodenticides: Analyses specifications, formulations. Rome,
Food and Agriculture Organization of the United Nations, pp 1-79 (FAO
Plant Production and Protection Paper 16).
Feinsod FM, Allam I, Ragai A, & Saah AJ (1986) Rodenticide-induced
signs simulating Rift Valley fever in sheep and goats in Egypt. Vet
Rec, 118: 270-272.
Felice LJ & Murphy MH (1989) The determination of the anticoagulant
rodenticide brodifacoum in blood serum by liquid chromatography with
fluorescence detection. J Anal Toxicol, 13: 229-231.
Felice LJ, Chalermchaikit T, & Murphy MJ (1991) Multicomponent
determination of 4-hydroxycoumarin anticoagulant rodenticides in blood
serum by liquid chromatography with fluorescence detection. J Anal
Toxicol, 15: 126-129.
Fiore CE, Tamburino C, Foti R, & Grimaldi D (1990) Reduced bone
mineral content in patients taking an oral anticoagulant. South Med J,
83: 538-542.
Forsey JD (1983a) The acute skin irritation of novel anticoagulants in
male rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 3 pp.
Forsey JD (1983b) Acute eye irritation of novel anticoagulants in male
rabbits. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 4 pp.
Forsey JD (1985) An evaluation of the long term sub-acute oral
toxicity of WL 108366 in Wistar rats. Widnes, United Kingdom, Sorex
Ltd, Agricultural Research Department, 10 pp.
Fristedt B & Sterner N (1965) Warfarin intoxication from percutaneous
absorption. Arch Environ Health, 11: 205-208.
Furie B & Furie BC (1990) Molecular basis of vitamin K-dependent
gamma-carboxylation. Blood, 75: 1753-1762.
Giban J (1974) Use of chlorophacinone in the struggle against the
common vole (Microtus arvalis Pallas) and against the musk rat
(Ondatra zibethicus). In: Proceedings of the 6th Vertebrate Pest
Conference, Sacramento, California. Davis, California, University of
California, pp 263-271.
Gijsbers BLMG, van Haarlem LJM, Soute BAM, Ebberink RHM, & Vermeer C
(1990) Characterization of a gla-containing protein from calcified
human atherosclerotic plaques. Arteriosclerosis, 10: 991-995.
Godfrey MER (1984) The toxicology of brodifacoum to target and
non-target species in New Zealand. Proceedings of a Conference on the
Organization and Practice of Vertebrate Pest Control, Elvetham Hall,
Hampshire, England, 30 August-3 September 1982. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division,
pp 631-637.
Godfrey MER (1985) Non-target and secondary poisoning hazards of
"second generation" anticoagulants. Acta Zool Fennica, 173: 209-212.
Godfrey MER, Laas FJ, & Rammell CG (1985) Acute toxicity of
brodifacoum to sheep. N Z J Exp Agric, 13: 23-25.
Gorenzel WP, Marsh RE, & Salmon TR (1982) Single-feeding
anticoagulants. Pest Control, 50(2): 32.
Grand M (1976) Données expérimentales sur un nouveau raticide
anticoagulant: le bromadiolone. Phytiatrie-Phytopharmacie, 25: 69-88.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1992) Toxicity of
second generation rodenticides to barn owls. In: Proceedings of the
Brighton Crop Protection Conference - Pests and Diseases, 1992,
vol 2, pp 781-786.
Gray A, Eadsforth CV, Dutton AJ, & Vaughan JA (1994) Non-invasive
method for monitoring the exposure of barn owls to second-generation
rodenticides. Pestic Sci, 41: 339-343.
Greeff MC, Mashile O, & MacDougall LG (1987) "Superwarfarin"
(bromadiolone) poisoning in two children resulting in prolonged
anticoagulation. Lancet, 1: 1269.
Greig-Smith PW, Fletcher MR, Hunter K, Quick MP, Ruthven AD, & Shaw IC
(1988) Pesticide poisoning of animals 1988: Investigations of
suspected incidents in Great Britain. A Report of the Environmental
Panel of the Advisory Committee on Pesticides.
Hadler MR (1974) Sub-acute five day oral toxicity of WBA 8119 to male
rats. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research.
Hadler MR (1975a) Acute oral toxicity of WBA 8119 to male rabbit.
Appendix 5. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural
Research, 3 pp.
Hadler MR (1975b) Acute oral toxicity of WBA 8119 to female Guinea
pig. Appendix 6. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research, 4 pp.
Hadler MR (1975c) Acute eye irritation of WBA 8119 to the rabbit.
Appendix 31. Widnes, Cheshire, Ward Blenkinsop & Co. Ltd,
Agricultural Research.
Hadler MR (1976) WBA 8119-12 week feeding study in rats. Widnes,
Cheshire, Ward Blenkinsop & Co. Ltd, Agricultural Research, 7 pp
(Laboratory report).
Hagan EC & Radomski JL (1953) The toxicity of 3-(acetonylbenzyl)-
4-hydroxycoumarin (warfarin) to laboratory animals. J Am Pharm Assoc,
42(6): 379-382.
Hall JG (1976) Warfarin and fetal abnormality. Lancet, 1: 1127.
Hall BE & Priestley I (1992) Brodifacoum: Metabolism in soil under
aerobic conditions (IRI Project No. 381441). Haslemere, Surrey, Zeneca
Agrochemicals, 63 pp (Report No. 8795).
Hall JG, Pauli RM, & Wilson KM (1980) Maternal and fetal sequelae of
anticoagulants during pregnancy. Am J Med, 68: 122-140.
Hall BE, Jackson R, & Priestley I (1992) Difenacoum: Photolysis in
buffered aqueous solutions (IRI Project No. 381614). Haslemere,
Surrey, Zeneca Agrochemicals, 123 pp (Report No. 8704).
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN,
Dodds RA, Bitensky L, & Chayen J (1985) Electrochemical detection of
depressed circulating levels of vitamin K1 in osteoporosis. J Clin
Endocrinol Metab, 60(6): 1268-1269.
Hegdal PL & Blaskiewicz RW (1984) Evaluation of the potential hazard
to barn owls of Talon (brodifacoum bait) used to control rats and
house mice. Environ Toxicol Chem, 3: 167-179.
Hegdal PL & Colvin BA (1988) Potential hazard to eastern screech-owls
and other raptors of brodifacoum bait used for vole control in
orchards. Environ Toxicol Chem, 7: 245-260.
Hill RW, Maddock BG, Hart B, & Bowles PF (1976) Determination of the
acute toxicity of PP 581 to Rainbow trout (Salmo gairdneri). Brixham,
Devon, Imperial Chemical Industries Ltd, Brixham Laboratory (Report
No. BL/B/1758).
Hodge MCE, Banham PB, Richards D, Weight TM, & Wilson J (1980a)
Product registration. Brodifacoum: Teratogenicity study in the rat.
Geneva, Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.
(ICI CTL Report No. CTL/P/437).
Hodge MCE, Banham PB, Richards D, & Weight TM (1980b) Product
registration. Brodifacoum: Teratogenicity study in the rabbit. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V. (ICI CTL
Report No. CTL/P/459).
Hodge MCE, Banham PB, Richards D & Weight TM (1980c) Product
registration. Brodifacoum: Teratogenicity study in the rabbit
(Appendices A-E). Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V. (ICI CTL Report No. CTL/P/459 S).
Hoffman RS, Smilkstein MJ, & Goldfrank LR (1988) Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose. Clin Toxicol, 26(3/4): 233-248.
Hollinger BR & Pastoor TP (1993) Case management and plasma half-life
in a case of brodifacoum poisoning. Arch Intern Med, 153: 1925-1928.
Hoogenboom JJL (1994) Anticoagulant poisoning cases 1977-1994.
Wellington, New Zealand Central Animal Health Laboratory.
Hoogenboom JJL & Rammell CG (1983) Improved HPLC method for
determining brodifacoum in animal tissues. J Environ Contam Toxicol,
31: 239-243.
Huckle KR & Warburton PA (1986a) Elimination, metabolism and
disposition of 14C-WL 108366 in the Fischer 344 rat following
repeated oral administration. Sittingbourne, Kent, Shell Research
Ltd, Sittingbourne Research Centre (Report No. SBGR.86.084).
Huckle KR & Warburton PA (1986b) Percutaneous absorption metabolism
and elimination of WL 108366 in the rat. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.218).
Huckle KR & Warburton PA (1986c) WL 108366: Absorption, metabolism and
disposition in Japanese quail (Coturnix coturnix Japonica) following a
single dose by intraperitoneal or oral administration. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.192).
Huckle KR, Hutson DH, & Warburton PA (1988) Elimination and
accumulation of the rodenticide flocoumafen in rats following repeated
oral administration. Xenobiotica, 18(12): 1465-1479.
Huckle KR, Hutson DH, Logan CJ, Morrison BJ, & Warburton PA (1989) The
fate of the rodenticide flocoumafen in the rat: Retention and
elimination of a single oral dose. Pestic Sci, 25: 297-312.
Huebner CF & Link KP (1941) Studies on the hemorrhagic sweet clover
disease. VI. The synthesis of the delta-diketone derived from the
hemorrhagic agent through alkaline degradation. J Biol Chem,
138: 529-534.
Hunter K (1983) Determination of coumarin anticoagulant rodenticide
residues in animal tissues by high-performance liquid chromatography:
Fluorescence detection using post-column techniques. J Chromatogr,
270: 267-276.
Hunter K (1985) High-performance liquid chromatographic strategies for
the determination and confirmation of anticoagulant rodenticides in
animal tissues. J Chromatogr, 321: 255-272.
Ikeda M, Conney AH, & Burns JJ (1968a) Stimulatory effect of
phenobarbital and insecticides on warfarin metabolism in the rat.
J Pharmacol Exp Ther, 162: 338-343.
Ikeda M, Ullrich V, & Staudinger H (1968b) Metabolism in vitro of
warfarin by enzymic and nonenzymic systems. Biochem Pharmacol,
17: 1663-1669.
IPCS (1992) IPCS/INTOX project. Poison Information Monograph:
Brodifacoum. Geneva, World Health Organization, 16 pp
(IPCS/INTOX/PIM.077).
Jackson R & Hall BE (1992) Aged soil leaching of [14C]-brodifacoum
(IRI Project No. 381986). Haslemere, Surrey, Zeneca Agrochemicals,
72 pp (Report No. 8879).
Jackson R, Priestley I, & Hall BE (1991) The determination of the
hydrolytic stability of [14C]-brodifacoum (IRI Project No. 381420).
Haslemere, Surrey, Zeneca Agrochemicals. 77 pp (Report No. 8330).
Jones EC, Growe GH, & Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. J Am Med Assoc, 252(21): 3005-3007.
Katona B & Wason S (1989) Superwarfarin poisoning. J Emerg Med,
7: 627-631.
Kaukeinen DE & Buckle AP (1992) Evaluations of aversive agents to
increase the selectivity of rodenticides, with emphasis on denatonium
benzoate (Bitrex(R)) bittering agent. In: Borrecco JE & Marsh RE ed.
Proceedings of the 15th Vertebrate Pest Conference. Davis, California,
University of California, pp 192-198.
Kelly MJ, Chambers J, & MacNicoll AD (1993) Simple and rapid method
for the determination of the diastereomers of difenacoum in blood and
liver using high-performance liquid chromatography with fluorescence
detection. J Chromatogr, 620: 105-112.
Kennelly JC (1990) Difenacoum: Assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 42 pp (Report No. CTL/P/3017.
Knapen MHJ, Jie K-SG, Hamulyák K, & Vermeer C (1993) Vitamin K-induced
changes in markers for osteoblast activity and urinary calcium loss.
Calcif Tissue Int, 53: 81-85.
Kosmin M & Barlow JN (1976) Rodent control using a novel formulation
of diphacinone, Ramik. Presented at the First Afro-Asian Vertebrate
Pest Conference, Cairo. Rosemont, Illinois, Velsicol Chemical
Corporation, 7 pp.
Krambias A & Hoppe AH (1987) The response of captive partridges to
dosing with anticoagulant rodenticides. In: Richards CGL & Ku TY ed.
Control of mammal pests. London, New York, Philadelphia, Taylor &
Francis, pp 181-186.
Kruse JA & Carlson RW (1992) Fatal rodenticide poisoning with
brodifacoum. Ann Emerg Med, 21: 331-336.
Laas FJ, Forss DA, & Goodfrey MER (1985) Retention of brodifacoum in
sheep tissues and excretion in faeces. N Z J Agric Res, 28: 357-359.
Lange PF & Terveer J (1954) Warfarin poisoning. US Armed Forces Med J,
5: 872-877.
Lawlor TE (1992) Mutagenicity test on bromadiolone in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test)
(HWA Study No. 15310-0-401). Vienna, Virginia, Hazleton Washington
Inc., 34 pp.
Lechevin JC & Grand M (1987) LM 2219 (proposed common name:
difethialone). Summary of the file submitted in the request for
approval (struggle against commensal rodents). Lyon, France, Lipha
S.A., Research and Development Centre, 15 pp (Report No. L-JCL/SP).
Leck JB & Park BK (1981) A comparative study of the effects of
warfarin and brodifacoum on the relationship between vitamin K1
metabolism and clotting factor activity in warfarin-susceptible and
warfarin-resistant rats. Biochem Pharmacol, 30: 123-128.
Lewis CJ (1992a) (14-C)-Difenacoum: A study of the degradation in two
soils. Haslemere, Surrey, Zeneca Agrochemicals (Report No. 6927).
Lewis CJ (1992b) (14C)-Difenacoum: Aged soil leaching. Haslemere,
Surrey, Zeneca Agrochemicals.
Lewis CJ (1992c) Difenacoum: Hydrolysis study. Haslemere, Surrey,
Zeneca Agrochemicals (Report No. 7031).
Lipton RA & Klass EM (1984) Human ingestion of a 'superwafarin'
rodenticide resulting in a prolonged anticoagulant effect. J Am Med
Assoc, 252: 3004-3005.
Litovitz TL, Clark LR, & Soloway RA (1994) 1993 Annual report of the
American Association of Poison Control Centers, Toxic Exposure
Surveillance System. Am J Emerg Med, 12: 546-584.
Locht H & Lindström FD (1993) Severe skin necrosis following warfarin
therapy in a patient with protein C deficiency. J Intern Med,
233: 287-289.
Lund M (1981) Hens, eggs and anticoagulants. Int Pest Control,
5: 126-128.
Lund M (1982) Annual report 1981. Lyngby, Danish Pest Infestation
Laboratory, pp 58-75.
Lund M (1984) Resistance to the second generation anticoagulant
rodenticides. In: Clark DO ed. Proceedings of the 11th Vertebrate Pest
Conference. Davis, California, University of California, pp 89-94.
Mackintosh CG, Laas FJ, Godfrey MER, & Turner K (1988) Vitamin K1
treatment of brodifacoum poisoning in dogs. In: Crabb AC & Marsh RE
ed. Proceedings of the 13th Vertebrate Pest Conference. Davis,
California, University of California, pp 86-90.
McSporran KD & Phillips CA (1983) Brodifacoum poisoning in a dog.
N Z Vet J, 31: 185-186.
Manfioletti G, Brancolini C, Avanzi G, & Schneider C (1993) The
protein encoded by a growth arrest-specific gene (gas6) is a new
member of the vitamin K-dependent proteins related to protein S, a
negative coregulator in the blood coagulation cascade. Mol Cell Biol,
13(5): 4976-4985.
Marsh RE (1985a) Are anticoagulant rodenticides a problem for
household pets? Pest Control, 53(8): 20-22.
Marsh RE (1985b) Anticoagulant rodenticides provide pet problems.
Part II. Pest Control, 53(9): 25-28.
Martin-Bouyer G, Linh PD, Tuan LC, Barin C, Khahn MB, Hoa DQ, Tourneau
J, & Guerbois H (1983) Epidemic of haemorrhagic disease in Vietnamese
infants caused by warfarin-contaminated talc. Lancet, 1: 230-232.
Mellano D (1984a) Product registration. In vitro study of chromosome
aberration induced by the article brodifacoum in cultured human
lymphocytes. Geneva, Switzerland, Zeneca Agrochemicals, Stauffer
Chemical B.V., 17 pp (Report No. CTL/C/1258).
Mellano D (1984b) Product registration. Study of the capacity of test
article brodifacoum to induce unscheduled DNA synthesis in cultured
Hela cells (autoradiographic method). Geneva, Switzerland, Zeneca
Agrochemicals, Stauffer Chemical B.V., 14 pp (Report No. CTL/C/1257).
Mendenhall VM & Pank LF (1980) Secondary poisoning of owls by
anticoagulant rodenticides. Wildl Soc Bull, 8(4): 311-315.
Merson MH & Byers RE (1984) Residues of the rodenticide brodifacoum in
voles and raptors after orchard treatment. J Wildl Manage,
48: 212-216.
Meyer AL & Wiggins DE (1986) Genotoxicity studies with WL 108366
(rodenticide): In vitro cell transformation studies. Sittingbourne,
Kent, Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.289).
Mirkova E & Antov G (1983) Experimental evaluation of the risk of
prenatal pathology under the effect of warfarin coumarine rodenticide.
Hig Zdrav, 25(6): 476-482.
Misenheimer TM, Lund ML, Baker EM, & Suttie JW (1994) Biochemical
basis of warfarin and bromadiolone resistance in the house mouse,
Mus musculus domesticus. Biochem Pharmacol, 47: 673-678.
Monnot G, Fave A, Illat T, & Briet Ph (1981) Phase 3 reformat of
MRID 0007-9375: Teratology study in the rat with LM 637 bromadiolone.
L'Arborasle, France, Institut Français de Recherches et Essais
Biologiques, 124 pp (Report No. MRID 0007-9375).
Morin M-F (1988) Study of the impact on the natural environment of
bromadiolone, an anticoagulant rodenticide: Evolution in aqueous
medium and bioaccumulation by terrestrial and aquatic organisms.
University of Poitiers (Thesis) ((Order No. 176).
Mosterd JJ & Thijssen HHW (1991) The long-term effects of the
rodenticide, brodifacoum, on blood coagulation and vitamin K
metabolism in rats. Br J Pharmacol, 104: 531-534.
Mount ME & Feldman BF (1983) Mechanism of diphacinone rodenticide
toxicosis in the dog and its therapeutic implications. Am J Vet Res,
44(11): 2009-2017.
Mount ME & Kass PH (1989) Diagnostic importance of vitamin K1 and
its epoxide measured in serum of dogs exposed to an anticoagulant
rodenticide. Am J Vet Res, 50: 1704-1709.
Mount ME, Feldman BF, & Buffington T (1982) Vitamin K and its
therapeutic importance. J Am Vet Med Assoc, 180(11): 1354-1356.
Murdoch DA (1983) Prolonged anticoagulation in chlorphacinone
poisoning. Lancet, 1: 355-356.
Murli H (1993) Mutagenicity test on bromadiolone, technical in an
in vivo mouse micronucleus assay (HWA Study No. 15310-0-455).
Washington, DC, Hazleton Laboratories.
Murphy MA & Nye DH (1991) Thoracic intramedullary haematoma as a
complication of warfarin: Case report and literature review. Aust N Z
J Surg, 61: 789-792.
Murphy MJ, Ray AC, & Bailey EM (1989) A high performance liquid
chromatography method for the detection of Brodifacoum in serum. Vet
Hum Toxicol, 31(3): 228-231.
Nehéz M, Kékes-Szabo A, Mazzag E, Selypes A, & Jarmay K (1985)
Histologic and cytogenetic effects of redentin on the spermiogenesis
and bone marrow cells of the mouse, an in vivo experiment. Regul
Toxicol Pharmacol, 5: 204-206.
Nelsestuen GL, Zytkovicz TH, & Howard JB (1974) The mode of action of
vitamin K: Identification of gamma-carboxyglutamic acid as a component
of prothrombin. J Biol Chem, 249: 6347-6350.
Newby S & White BG (1978) Brodifacoum: Adsorption and desorption in
soils measured under laboratory conditions. Haslemere, Surrey,
Imperial Chemical Industries Ltd, Plant Protection Division (Report
No TMJ 1764B).
Newton I, Wyllie I, & Freestone P (1990) Rodenticides in British barn
owls. Environ Pollut, 68: 101-117.
Newton I, Wyllie I, Gray A, Eadsforth CV (1994) The toxicity of the
rodenticide flocoumafen to barn owls and its elimination via pellets.
Pestic Sci, 41: 187-193.
Nighoghossian N, Ruel JH, French P, Froment JC, & Trouillas P (1990)
Hématome sous-dural cervico-dorsal par intoxication aux raticides
coumariniques. Rev Neurol (Paris), 146(3): 221-223.
Nilsson IM (1957) Recurrent hypoprothrombinaemia due to poisoning with
dicumarol-containing rat-killer. Acta Haematol, 17: 176-182.
O'Bryan SM & Constable DJC (1991) Quantification of brodifacoum in
plasma and liver tissue by HPLC. J Anal Toxicol, 15: 144-147.
O'Reilly RA, Aggeler PM, & Leong LS (1963) Studies on the coumarin
anticoagulant drugs: The pharmacodynamics of warfarin in man. J Clin
Invest, 4: 1542-1551.
O'Reilly RA, Aggeler PM, Haag MS, Leong LS, & Kropatkin ML (1964)
Hereditary transmission of exceptional resistance to coumarin
anticoagulant drugs. N Engl J Med, 271: 809-815.
Overman RS, Stahmann MA, Huebner CF, Sullivan WR, Spero L, Doherty DG,
Ikawa M, Graf L, Roseman S, & Link KP (1944) Studies on the
hemorrhagic sweet clover disease. XIII. Anticoagulant activity and
structure in the 4-hydroxycoumarin group. J Biol Chem, 153: 5-24.
Owren PA (1959) Thrombotest: A new method for controlling
anticoagulant therapy. Lancet, 2: 754-758.
Park BK & Leck JB (1982) A comparison of vitamin K antagonism by
warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol,
31(22): 3635-3639.
Park BK, Choonara IA, Haynes BP, Breckenridge AM, Malia RG, & Preston
FE (1986) Abnormal vitamin K metabolism in the presence of normal
clotting factor activity in factory workers exposed to
4-hydroxycoumarins. Br J Clin Pharmacol, 21: 289-294.
Parkinson GR (1975) WBA 8119: Acute oral toxicity. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 6 pp (Report No. CTL/P/216 (revised)).
Parkinson GR (1976) PP581: Acute oral toxicity to sheep. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, Central Toxicology
Laboratory, 3 pp (Report No. CTL/P/259 (revised)).
Parkinson GR (1979) PP 581: Acute oral toxicity and skin
sensitization. Macclesfield, Cheshire, Imperial Chemical Industries
Ltd, Central Toxicology Laboratory, 3 pp (Report No. CTL/P/260
(revised)).
Parmar G, Bratt H, Moore R, & Batten PL (1987) Evidence for common
binding site in vivo for the retention of anticoagulants in rat
liver. Hum Toxicol, 6: 431-432.
Pastoureau P, Vergnaud P, Meunier PJ, & Delmas PD (1993) Ostopenia and
bone-remodeling abnormalities in warfarin-treated lambs. J Bone Miner
Res, 8: 1417-1426.
Pauli RM & Hall JG (1979) Warfarin embryopathy. Lancet, 2: 144.
Pearson N (1984) WL 108366 manufacturing master mix: Acute toxicity to
Salmo gairdneri and Daphnia magna. Sittingbourne, Shell Research Ltd
(Report No. SBGR.84.274).
Pearson N & Wallace BG (1984) Technical WL 108366: Acute toxicity to
Salmo gairdneri, Cyprinus carpio, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Kent, Shell Research Ltd, Sittingbourne
Research Centre (Report No. SBGR.84.211).
Pelfrène AF (1991) Synthetic organic rodenticides. In: Hayes WJ Jr &
Laws ER Jr ed. Handbook of pesticide toxicology. New York, Academic
Press, vol 3, pp 1271-1316.
Persson H (1994) [Annual report 1993.] Stockholm, Swedish Poison
Information Centre, 24 pp (in Swedish).
Pribilla O (1966) Murder caused by warfarin. Arch Toxicol,
21: 235-249.
Price JB (1985a) Toxicology of rodenticides: The acute oral toxicity
of WL 108366 in rabbits and hamsters. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.85.189).
Price JB (1985b) Toxicology of rodenticides: The percutaneous toxicity
of WL 108366 corn oil manufacturing master mix. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.84.227).
Price JB (1985c) Toxicology of rodenticides - WL 108366: A five day
range finding feeding study in rats. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.84.279).
Price JB (1985d) WL 108366: A 28-day feeding study in rats.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.84.235).
Price JB (1986) Toxicology of rodenticides ("Storm"): The skin
sensitizing potential of WL 108366. Sittingbourne, Kent, Shell
Research Ltd, Sittingbourne Research Centre (Report No. SBGR.86.091).
Price PA, Williamson MK, Haba T, Dell RB, & Jee WS (1982) Excessive
mineralization with growth plate closure in rats on chronic warfarin
treatment. Proc Natl Acad Sci (USA), 79: 7734-7738.
Quick AJ (1935) The prothrombin in haemophilia and in obstructive
jaundice. J Biol Chem, 109: 73.
Radvanyi A, Weaver P, Massari C, Bid D, & Broughton E (1988) Effects
of chlorophacinone on captive kestrels. Bull Environ Contam Toxicol,
41: 441-448.
Rammell CG, Hoogenboom JJL, & Cotter M (1984) Brodifacoum residues in
target and non-target animals following rabbit poisoning trials. N Z J
Exp Agric, 12: 107-111.
Rauch AE, Weininger R, Pasquale D, Burkart PT, Dunn HG, Weissman C, &
Rydzak E (1994) Superwarfarin poisoning: A significant public health
problem. J Community Health, 19: 55-65.
Ray AC, Murphy MJ, DuVall MD, & Reagor JC (1989) Determination of
brodifacoum and bromadiolone residues in rodent and canine liver. Am J
Vet Res, 50(4): 546-550.
Redfern R, Gill JE, & Hadler MR (1976) Laboratory evaluation of WBA
8119 as a rodenticide for use against warfarin resistant and
non-resistant rats and mice. J Hyg Camb, 77: 419-426.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 16 November: 525-527.
Resch H, Pietschmann P, Krexner E, & Willvonseder R (1991) Decreased
peripheral bone mineral content in patients under anticoagulant
therapy with phenprocoumon. Eur Heart J, 12: 439-441.
Roberts NL, Cameron DM, & Street AE (1985a) WL 108366 - Acute oral
toxicity to pigs. Huntingdon, United Kingdom, Huntingdon Research
Centre (HRC Report No. SLL/76/851108).
Roberts NL, Fairley C, & Baldwin MK (1985b) The acute oral toxicity
(LD50) of WL 108366 to the Mallard duck. Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Report No. SLL 67/BT/84925).
Roberts NL, Fairley C, & Baldwin MK (1985c) The acute oral toxicity of
WL 108366 to the Mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Centre (HRC Report No. SLL/73/BT/8572).
Robinson DS & MacDonald MG (1966) The effect of phenobarbital
administration on the control of coagulation achieved during warfarin
therapy in man. J Pharmacol Exp Ther, 153: 250-253.
Ross DB, Roberts NL, & Cameron DM (1978) The acute oral toxicity
(LD50) of PP581 to the Mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No ICI 122WL/78507).
Ross DB, Roberts NL, & Phillips C (1979) The subacute toxicity of
difenacoum (Ratak) to pigs. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No.
ICI 262/79697).
Ross DB, Roberts NL, & Fairley C (1980a) The subacute dietary toxicity
(LC50) of difenacoum to Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 305/791197).
Ross DB, Roberts NL, & Fairley C (1980b) The subacute dietary toxicity
(LC50) of difenacoum to the mallard duck. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 304/791198).
Ross DB, Roberts NL & Fairley C (1980c) The acute oral toxicity
(LD50) of difenacoum to the Bobwhite quail. Macclesfield, Cheshire,
Imperial Chemical Industries Ltd, Central Toxicology Laboratory
(Report No. ICI 309/WL/8076).
Ross GS, Zacharski LR, Robert D, & Rabin DL (1992) An acquired
hemorrhagic disorder from long-acting rodenticide ingestion. Arch
Intern Med, 152: 410-412.
Schardein JL (1985) Anticoagulants. In: Chemically induced birth
defects. New York, Basel, Marcel Dekker Inc., pp 89-100.
Schulman A, Lusk R, Lippincott CL, & Ettinger SJ (1986)
Diphacinone-induced coagulopathy in the dog. J Am Vet Med Assoc,
188(4): 403-405.
Selypes A, Kékes-Szabo A, & Nehéz M (1984) Study of the mutagenic
effect of redentin on various species of animals. Acta Vet Hung,
32(3-4): 213-217.
Sharples R (1983a) The acute oral toxicity of a series of novel
anticoagulants in Wistar rats and C57BL/10 mice. Widnes, United
Kingdom, Sorex Ltd, Agricultural Research Department, 7 pp.
Sharples R (1983b) The acute oral toxicity of WL 108366 in
Dunkin-Hartley guinea pigs. Widnes, United Kingdom, Sorex Ltd,
Agricultural Research Department, 3 pp.
Sharples R (1984) The acute oral toxicity of WL 108366 in C57BL/10
mice. Widnes, United Kingdom, Sorex Ltd, Agricultural Research
Department, 6 pp.
Sheen SR, Spiller HA, & Grossman D (1994) Symptomatic brodifacoum
ingestion requiring high-dose phytonadione therapy. Vet Hum Toxicol,
36(3): 216-217.
Sheldon T, Richardson CR, & Shaw J (1984) Product registration. An
evaluation of brodifacoum in the mouse micronucleus test. Geneva,
Switzerland, Zeneca Agrochemicals, Stauffer Chemical B.V.,
19 pp (Report No. CTL/P/1006).
Smith MF & Cameron MD (1979) Warfarin as teratogen. Lancet, 1: 727.
Smolinske SC, Scherger DL, Kearns PS, Wruk KM, Kulig KW, & Rumack BH
(1989) Superwarfarin poisoning in children: A prospective study.
Pediatrics, 84: 490-494.
Spare WC (1982) Aqueous photodegradation of 14C-bromadiolone.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Spare WC (1992) Hydrolysis of bromadiolone. Beltsville, Maryland,
Biospherics Inc. (Unpublished report submitted to WHO by Lipha S.A.).
Spare WC & Olson SB (1980) 14C-Bromadiolone soil leaching.
Beltsville, Maryland, Biospherics Inc. (Unpublished report submitted
to WHO by Lipha S.A.).
Sridhara S & Krishnamurthy TR (1992) Potentiation of anticoagulant
toxicity to Rattus rattus by two non-steroid anti-inflammatory drugs.
In: Borrecco JE & Marsh RE ed. Proceedings of the 15th Vertebrate Pest
Conference. Davis, California, University of California, pp 212-217.
Stenflo J, Fernlund P, Egan W, & Roepstorff P (1974) Vitamin K
dependent modifications of glutamic acid residues in prothrombin. Proc
Natl Acad Sci (USA), 71(7): 2730-2733.
Stowe CM, Metz AL, Arendt TD, & Schulman J (1983) Apparent brodifacoum
poisoning in a dog. J Am Vet Med Assoc, 182(8): 817-818.
Sullivan MP, Dean BS, & Krenzelok EO (1989) Long-acting anticoagulant
rodenticides: An evaluation of 88 cases. Vet Hum Toxicol, 31: 361.
Szulc P, Chapuy M-C, Meunier PJ, & Delmas PD (1993) Serum
undercarboxylated osteocalcin is a marker of the risk of hip fracture
in elderly women. J Clin Invest, 91: 1769-1774.
Thomson WT (1988) Agricultural chemicals. Book III - Miscellaneous
chemicals: Fumigants, growth regulators, repellents, and rodenticides
(1988/89 Revision). Fresno, California, Thomson Publications.
Tomlin C ed. (1994) The pesticide manual incorporating the
agrochemical handbook, 10th ed. Farnham, Surrey, The British Crop
Protection Council.
Townsend MG, Fletcher MR, Odam EM, & Stanley PI (1981) An assessment
of the secondary poisoning hazard of warfarin to tawny owls. J Wildl
Manage, 45(1): 242-248.
Townsend MG, Bunyan PJ, Odam EM, Stanley PI, & Wardall HP (1984)
Assessment of secondary poisoning hazard of warfarin to least weasels.
J Wildl Manage, 48(2): 628-632.
Travis SF, Warfield W, Greenbaum BH, Mobkisher M, & Siegel JE (1993)
Spontaneous hemorrhage associated with accidental brodifacoum
poisoning in a child. J Pediatr, 122: 982-984.
Tuinman CP & Van Sittert NJ (1985) Biomedical monitoring of personnel
in Sorex Ltd (Widnes, UK) involved in a formulation run with the
rodenticide WL 108366. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report No. HSE 85.006).
Ullrich V & Staundinger H (1968) Metabolism in vitro of warfarin by
enzymic and nonenzymic systems. Biochem Pharmacol, 17: 1663-1669.
Ungvary G (1994) Anticoagulant poisoning incidents in Hungary.
Budapest, National Institute of Occupational Health (Unpublished
report).
Van Haarlem LJM, Knapen MHJ, Hamulyák K, & Vermeer C (1988)
Circulating osteocalcin during oral anticoagulant therapy. Thromb
Haemost, 60: 79-82.
Veenstra GE, Owen DE, & Huckle KR (1991) Metabolic and toxicological
studies on the anticoagulant rodenticide, flocoumafen. Arch Toxicol,
14(Suppl): 160-165.
Vermeer C (1990) gamma-Carboxyglutamate-containing proteins and the
vitamin K-dependent carboxylase. Biochem J, 266: 625-636.
Vermeer C & Soute BAM (1992) Comparison of rodenticide anticoagulant
action in vitro and in vivo. Sittingbourne, Kent, Shell Research
Ltd (Report No. HSE/50).
Virat M (1981) LM-637-Bromadiolone: Teratology study in the rabbit by
oral route. L'Arborasle, France, Institut Français de Recherches et
Essais Biologiques (Report No. 106206).
Vogel JJ, de Moerloose Ph, Bouvier CA, Gaspoz J, & Riant P (1988)
Anticoagulation prolongée lors d'une intoxication à la chlorphacinone.
Schweiz Med Wochenschr, 118: 1915-1917.
Völkl S & Galicia H (1992) 14C-Bromadiolone: Degradation and
metabolism in soils incubated under aerobic conditions. Itingen,
Switzerland, Umweltchemie AG (Unpublished report submitted to WHO by
Lipha S.A., Lyon).
Wallace S, Paull P, Worsnop C, & Mashford ML (1990) Covert self
poisoning with brodifacoum, a "superwarfarin". Aust N Z J Med,
20: 713-715.
Warburton PA & Huckle KR (1986) WL 108366 - Fate of a single oral dose
of 14C WL 108366 in rats. Part III: Metabolism. Sittingbourne, Kent,
Shell Research Ltd, Sittingbourne Research Centre (Report
No. SBGR.85.294).
Warburton PA & Hutson DH (1985) WL 108366 - Fate of a single oral dose
of 14C-WL 108366 in rats. Part I: Elimination and retention of
radioactivity and effect of WL 108366 on prothrombin time.
Sittingbourne, Kent, Shell Research Ltd, Sittingbourne Research Centre
(Report No. SBGR.85.053).
Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, Murphy
MJ, & Furie B (1990) Surreptitious ingestion of a long-acting vitamin
K antagonist/rodenticide, brodifacoum: Clinical and metabolic studies
of three cases. Blood, 76(12): 2555-2559.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, 64 pp (WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II). Clin Chem,
33(11): 2074-2078.
Winn MJ, Clegg JAD, & Park BK (1987) An investigation of sex-linked
differences to the toxic and to the pharmacological action of
difenacoum: Studies in mice and rats. J Pharm Pharmacol, 39: 219-222.
Wiswesser WJ (1976) Pesticide index, 5th ed. College Park, Maryland,
Entomological Society of America.
Woody BJ, Murphy MJ, Ray AC, & Green RA (1992) Coagulopathic effects
and therapy of brodifacoum toxicosis in dogs. J Vet Intern Med,
6: 23-28.
Yu CC, Atallah YH, & Whitacre DM (1982) Metabolism and disposition of
diphacinone in rats and mice. Drug Metab Dispos, 10: 645-648.
RESUME
1. Généralités
Les anticoagulants qui sont décrits dans la présente monographie
sont ceux que l'on utilise principalement en agriculture et pour la
destruction des rongeurs en milieu urbain. La warfarine, premier
rodenticide anticoagulant à connaître une large utilisation, était à
l'origine un médicament efficace pour le traitement des
thromboembolies chez l'homme.
Selon leur structure chimique, les rodenticides anticoagulants
peuvent se répartir en deux catégories, les hydroxycoumarines et les
indanediones, mais leur mode d'action est analogue.
2. Propriétés et méthodes d'analyse
Les rodenticides anticoagulants se présentent sous la forme de
solides cristallins ou de poudres et sont légèrement solubles dans
l'eau. La plupart d'entre eux sont stables dans les conditions
normales de conservation.
La plupart des méthodes de dosage des rodenticides anticoagulants
reposent sur la chromatographie en phase liquide à haute performance.
3. Sources d'exposition humaine et environnementale
Les hydroxycoumarines de la première génération ont commencé à
être utilisées comme rodenticides vers la fin des années 1940. Par la
suite, l'apparition d'une résistance à la warfarine et aux autres
anticoagulants de la première génération a conduit à mettre au point
des produits de deuxième génération, plus actifs. La concentration du
principe actif dans les appâts varie selon l'efficacité du
rodenticide.
4. Distribution, concentration et exposition dans l'environnement
Les rodenticides anticoagulants sont principalement utilisés sous
la forme d'appâts. Comme ils sont peu volatils, leur concentration
dans l'air est négligeable. De même n'étant que légèrement solubles
dans l'eau, il est peu probable que leur utilisation conduise à la
contamination des eaux.
Etant donné que les rodenticides anticoagulants ne sont pas
destinés à être appliqués directement sur les cultures, il n'y a pas
lieu de s'attendre à en trouver sous forme de résidus dans les
aliments d'origine végétale.
L'exposition aux rodenticides des vertébrés non visés peut se
produire directement par la consommation d'appâts empoisonnés et
indirectement par celle de rongeurs contaminés. Les petits granulés
et les appâts constitués de grains entiers attirent fortement les
oiseaux.
La warfarine est utilisé dans le traitement des thromboembolies.
Il existe une possibilité d'exposition professionnelle aux
rodenticides anticoagulants lors de leur fabrication ou formulation ou
encore lors de la pose d'appâts empoisonnés, mais on ne dispose pas de
données sur l'ampleur de cette exposition.
5. Mode d'action et métabolisme
Les rodenticides anticoagulants sont des antagonistes de la
vitamine K. Leur action est principalement localisée dans le foie où
plusieurs précurseurs de l'hémostase subissent un processus
post-traductionnel dépendant de la vitamine K avant d'être convertis
en zymogènes procoagulants. L'action proprement dite consiste dans
l'inhibition de la K1-époxyde-réductase.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives et pourraient l'être également à travers
la peau et dans les vois respiratoires. Après administration par voie
orale, c'est principalement dans les matières fécales que ces composés
sont éliminés chez diverses espèces.
Il est possible que la décomposition métabolique de la warfarine
et des indanediones chez le rat s'effectue principalement par
l'intermédiaire d'une hydroxylation. En revanche, les anti-coagulants
de la deuxième génération s'éliminent essentiellement tels quels. Le
faible taux d'excrétion urinaire empêche d'isoler les métabolites de
l'urine.
Le foie est le principal organe où s'accumulent les rodenticides
anticoagulants. Cette accumulation a lieu également dans les
graisses.
6. Effets sur les mammifères et les systèmes d'épreuve in vitro
Chez le rat et la souris, les signes d'intoxication consistent en
une tendance accrue au saignement.
La DL50 est très variable, et c'est par la voie orale que les
composés sont les plus toxiques. La toxicité est également élevée par
la voie percutanée et par la voie respiratoire.
Certains anticoagulants présentent une toxicité aiguë du même
ordre pour les mammifères non visés que pour les rongeurs à détruire,
toutefois leur spectre toxique peut varier d'une espèce à l'autre.
Après administration répétée par voie orale à des rats, les
principaux effets observés sont ceux qui résultent de l'activité
anticoagulante.
On ne possède que peu de données sur les expositions répétées aux
anticoagulants chez les espèces n'appartenant pas à l'ordre des
rongeurs.
Une étude relative aux effets de la warfarine sur le rat a fait
ressortir une action sur le développement de ces animaux. En dehors
de cela, rien n'indique que les anticoagulants aient une action
tératogène sur les animaux d'expérience.
Rien n'indique non plus que les rodenticides anticoagulants
soient mutagènes, toutefois les données relatives aux différents
composés sont insuffisantes pour qu'on puisse en conclure à l'absence
de mutagénicité. La souche, le sexe et le régime alimentaire sont des
facteurs importants qui influent sur la toxicité des anticoagulants
chez les rongeurs. On a fait état d'épisodes d'intoxication chez des
animaux domestiques qui avaient consommé des appâts additionnés
d'anticoagulants. Lorsqu'il y a issue fatale ou du moins un tableau
clinique grave, c'est généralement qu'il y a eu consommation
d'anticoagulants de la deuxième génération. La principale différence
entre la warfarine et les autres anticoagulants (qu'il s'agisse des
indanediones ou des hydroxycoumarines de la deuxième génération),
c'est que ceux-ci persistent plus longtemps dans l'organisme et ont
par conséquent un effet plus durable que la warfarine. Dans ces
conditions, devant une intoxication, il faut poursuivre plus longtemps
l'administration de l'antidote, c'est-à-dire de la vitamine K1.
7. Effets sur l'homme
De nombreuses intoxications (qu'elles soient intentionnelles on
non) ont été signalées. Il y a eu également quelques cas
d'intoxication imputables à une exposition professionnelle. En cas
d'intoxication aiguë par des rodenticides anticoagulants, les
symptômes vont de l'accroissement de la tendance au saignement en cas
d'intoxication minime ou modérée, à une hémorragie massive dans les
cas plus graves. Les signes cliniques se manifestent un à plusieurs
jours après l'absorption.
Chez l'homme, on attribue certaines malformations congénitales à
des traitements par la warfarine pendant la période de grossesse.
Aucune malformation de ce type n'a été observée par suite de
l'utilisation d'anticoagulants comme rodenticides.
La concentration de la prothrombine plasmatique donne une
indication de la gravité de l'intoxication. Elle est plus sensible
que des épreuves globales telles que le temps de Quick. En cas
d'exposition professionnelle répétée, le dosage direct des traces de
carboxyprothrombine circulante ou du 2,3-époxyde de la vitamine K peut
permettre un bilan plus sensible.
Le traitement d'une intoxication par des anticoagulants doit être
adapté à la gravité de l'intoxication. Il est basé sur l'action
pharmacologique spécifique de la vitamine K1 que l'on administre par
voie parentérale avec, dans les cas graves, administration simultanée
de constituants sanguins. La mesure du temps de Quick permet
d'apprécier l'efficacité du traitement et de déterminer sa durée.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On peut répartir en deux catégories les effets possibles de
rodenticides anticoagulants sur les organismes non visés: les effets
directs (par consommation d'appâts) et les effets indirects (par
consommation de rongeurs empoisonnés).
Sous forme de produit technique, les anticoagulants sont
extrêmement toxiques pour les poissons. Incorporés à des appâts, il
est peu probable qu'ils présentent un danger, du fait de leur faible
solubilité dans l'eau. C'est pourquoi, à moins d'une erreur de
manipulation, ils ne devraient pas parvenir jusqu'aux poissons.
Les différentes espèces d'oiseaux sont d'une sensibilité variable
aux rodenticides anticoagulants. Il est difficile d'apprécier les
risques que représente, pour les oiseaux, la consommation directe
d'appâts car la plupart des travaux publiés sont des études
toxicologiques effectuées au laboratoire. Le caractère attractif des
appâts constitués de grains entiers pour les petits oiseaux en accroît
le danger dans la nature.
Des études de toxicité indirecte effectuées en laboratoire sur
des animaux sauvages ont montré que des prédateurs captifs pouvaient
s'intoxiquer si on leur donnait de la nourriture empoisonnée sans
autre choix ou des proies empoisonnées. On a également constaté la
mort de prédateurs dans le milieu naturel.
9. Evaluation et conclusion
Les rodenticides anticoagulants bloquent le mécanisme normal de
l'hémostase, d'où une tendance accrue au saignement qui peut déboucher
sur une forte hémorragie.
Il est peu probable que la population générale puisse être
exposée involontairement à des rodenticides anticoagulants.
Il peut y avoir une exposition non négligeable par suite de
contacts lors de l'activité professionnelle. Ces contacts peuvent se
produire au cours des opérations de production et de formulation aussi
bien que lors de la préparation et de la pose des appâts.
Les rodenticides anticoagulants sont facilement absorbés au
niveau des voies digestives ainsi qu'à travers la peau et les voies
respiratoires. C'est principalement dans le foie qu'ils sont retenus
et s'accumulent. La concentration de prothrombine plasmatique est un
bon indicateur de la gravité d'une intoxication aiguë et permet
d'avoir une idée de l'efficacité et de la durée nécessaire du
traitement.
L'antidote spécifique est la vitamine K1.
La principale différence entre les rodenticides anticoagulants de
première et de deuxième génération tient au fait que ces derniers
séjournent plus longtemps dans l'organisme et ont donc tendance à
prolonger l'effet hémorragique.
La plupart des anticoagulants sont stables dans les conditions
normales d'utilisation. Comme ils sont peu solubles dans l'eau et que
les appâts n'en contiennent qu'une faible quantité, il est peu
probable qu'ils puissent contaminer les étendues d'eau. Par ailleurs,
ils se fixent rapidement aux particules du sol, ne s'en désorbent que
très lentement et ne sont pas lessivés.
Aucun organisme non visé ne court de risque d'intoxication
directe par consommation d'appâts, ni de risque d'intoxication
indirecte par consommation de rongeurs contaminés.
RESUMEN
1. Generalidades
Los anticoagulantes descritos en esta monografía son los
utilizados principalmente en agricultura y en la lucha contra los
roedores urbanos. La warfarina, el primer rodenticida anticoagulante
de uso generalizado, se introdujo como agente eficaz para el
tratamiento de la tromboembolia en el ser humano.
De acuerdo con su estructura química, los rodenticidas
anticoagulantes pueden agruparse en dos categorías, las
hidroxicumarinas y las indandonas, aunque su mecanismo de acción es
similar.
2. Propiedades y métodos analíticos
Los rodenticidas anticoagulantes se presentan en forma cristalina
sólida o en polvo, y son ligeramente solubles en agua. La mayoría de
ellos son estables en condiciones de almacenamiento normales.
La mayoría de los procedimientos para la determinación de los
rodenticidas anticoagulantes se basan en cromatografía líquida de alta
resolución.
3. Fuentes de exposición humana y ambiental
Las hidroxicumarinas de primera generación se introdujeron como
rodenticidas a finales de los años cuarenta. La aparición de
resistencia a la warfarina y a otros anticoagulantes de primera
generación dio lugar a la elaboración de anticoagulantes más potentes,
de segunda generación. Las concentraciones de componentes activos en
los cebos varían en función de la eficacia de los rodenticidas.
4. Distribución, niveles y exposición ambientales
Los rodenticidas anticoagulantes se utilizan principalmente como
formulaciones para cebo. Dada su baja volatilidad, las
concentraciones en el aire son insignificantes. Como son muy poco
solubles en agua, es improbable que su uso sea fuente de contaminación
del agua.
Como los rodenticidas anticoagulantes no están pensados para su
aplicación directa a cosechas en pie, no son de prever residuos en
alimentos vegetales.
Los vertebrados no destinatarios están expuestos a los
rodenticidas principalmente por medio del consumo del cebo y de forma
secundaria por el consumo de roedores envenenados. Los cebos en
bolitas y de grano entero son muy atractivos para las aves.
La warfarina se utiliza como agente terapéutico para la
tromboembolia.
Hay un potencial de exposición ocupacional a los rodenticidas
anticoagulantes durante la fabricación, formulación y aplicación del
cebo, pero no se dispone de datos sobre los niveles de exposición.
5. Modo de acción y metabolismo
Los rodenticidas anticoagulantes son antagonistas de la vitamina
K. Su lugar principal de acción es el hígado, donde varios de los
precursores de la coagulación de la sangre sufren un procesamiento
post-traslación dependiente de la vitamina K antes de convertirse en
los zimógenos procoagulantes respectivos. Parece que el mecanismo de
acción es la inhibición de la reductasa epoxídica K1.
Los rodenticidas anticoagulantes se absorben fácilmente por el
tracto intestinal, y también pueden absorberse por la piel y el
sistema respiratorio. Tras la administración oral, la principal vía
de eliminación en diversas especies son las heces.
La degradación metabólica de la warfarina y las indandonas en
ratas es principalmente la hidroxilación. Sin embargo, los
anticoagulantes de segunda generación se eliminan principalmente como
compuestos inalterados. El bajo nivel de excreción urinaria impide
aislar los metabolitos a partir de la orina.
El hígado es el órgano principal para la acumulación y
almacenamiento de anticoagulantes rodenticidas. La acumulación
también tiene lugar en la grasa.
6. Efectos en los mamíferos y en los sistemas de prueba in vitro
Los signos de envenenamiento en ratas y ratones son los asociados
a una mayor tendencia a la hemorragia.
Hay una gran variación en la DL50 de los rodenticidas
anticoagulantes, siendo máxima la toxicidad por vía oral. También es
alta la toxicidad cutánea y por inhalación.
Los márgenes de toxicidad aguda de algunos anticoagulantes son
similares en el caso de los mamíferos no destinatarios y de los
roedores de destino, pero los espectros de toxicidad para los
anticoagulantes pueden variar entre las especies.
Tras una administración oral repetida en ratas, los principales
efectos observados son los asociados a la acción anticoagulante.
Se dispone de pocos datos sobre la exposición repetida de
especies distintas de los roedores.
Un estudio sobre la warfarina en ratas ha indicado efectos sobre
el desarrollo. Por lo demás, no hay pruebas convincentes de que los
anticoagulantes sean teratogénicos en animales de experimentación.
No hay prueba que sugiera que los rodenticidas anticoagulantes
son mutagénicos, pero se dispone de datos insuficientes sobre los
compuestos individuales para demostrar la inexistencia de
mutagenicidad. La sobrecarga, el sexo y la alimentación son
importantes factores modificadores de la toxicidad de los
anticoagulantes en los roedores.
Se han dado casos de envenenamiento de animales domésticos tras
la ingestión de cebos anticoagulantes. Las muertes y los síndromes
clínicos graves se deben por lo general a anticoagulantes de segunda
generación. La diferencia principal entre la warfarina y los demás
anticoagulantes (tanto las indandonas como las hidroxicumarinas de
segunda generación) es que éstos tienen mayor tiempo de retención en
el organismo y por consiguiente un efecto más prolongado que la
warfarina. Por ello, en los casos de envenenamiento, el tratamiento
antídoto con vitamina K1 debe proseguir durante un periodo más
largo.
7. Efectos en el ser humano
Se han notificado muchos casos de envenenamiento (tanto
intencionados como no intencionados). También se han producido unos
pocos casos de intoxicación por exposición ocupacional a los
anticoagulantes. Los síntomas de intoxicación aguda por rodenticidas
anticoagulantes van desde una mayor tendencia a la hemorragia en el
envenenamiento leve o moderado a una hemorragia masiva en casos más
graves. Los signos de envenenamiento aparecen con un retraso de uno a
varios días después de la absorción.
La warfarina va asociada en el ser humano a la producción de
malformaciones del desarrollo cuando se toma como agente terapéutico
durante el embarazo. No se han notificado casos de defectos de
desarrollo tras el uso de anticoagulantes como rodenticidas.
La concentración de protrombina plasmática orienta sobre la
gravedad de la intoxicación. Es una indicación más sensible que
pruebas generales como el tiempo de protrombina. En la exposición
ocupacional repetida, la medición directa de cantidades ínfimas de
descarboxiprotrombina en circulación o de 2,3-epóxido de vitamina K en
circulación pueden constituir una evaluación más sensible.
El tratamiento del envenenamiento con anticoagulantes se gradúa
de acuerdo con la gravedad de la intoxicación. El tratamiento
farmacológico específico consiste en la administración parenteral de
vitamina K1 con administración simultánea, en los casos graves, de
componentes sanguíneos. La medición del tiempo de protrombina ayuda a
determinar la eficacia y la duración de tratamiento necesaria.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
Los posibles efectos de los rodenticidas en organismos no
destinatarios pueden dividirse en dos categorías: primarios
(envenenamiento directo por consumo de cebo) y secundario (por consumo
de roedores envenenados).
En la forma del producto técnico, los anticoagulantes son muy
tóxicos para los peces. Como formulaciones para cebo es improbable
que planteen riesgos en razón de su baja solubilidad en agua. Por
esta razón, a menos que se utilicen de forma indebida, no están al
alcance de los peces.
La susceptibilidad de las aves a los rodenticidas anticoagulantes
es variable. Es difícil evaluar los riesgos para las aves que entraña
el consumo directo porque la mayoría de los estudios publicados
consisten en ensayos de toxicidad en condiciones de laboratorio. El
atractivo del cebo de grano integral para las aves pequeñas aumenta el
riesgo en las condiciones de campo.
Los estudios en laboratorio de la toxicidad secundaria con la
fauna silvestre han mostrado que los predadores cautivos pueden
intoxicarse mediante alimentación sin otra elección con presas que se
han envenenado con anticoagulante o a las que se ha administrado este
producto. Se tiene noticias de algunas muertes de predadores en su
medio natural.
9. Evaluación y conclusión
Los rodenticidas anticoagulantes perturban los mecanismos
normales de coagulación de la sangre, determinando una mayor tendencia
a la hemorragia y, por último, una hemorragia abundante.
La exposición no intencionada de la población general a los
rodenticidas anticoagulantes es improbable.
El contacto ocupacional es una fuente potencial de exposición
significativa. Puede tener lugar durante la elaboración y
formulación, así como durante la preparación y aplicación del cebo.
Los compuestos de rodenticida anticoagulante se absorben
fácilmente por el tracto intestinal, y por la piel y el sistema
respiratorio. El hígado es el órgano principal de acumulación y
almacenamiento. La concentración de protrombina plasmática es una
buena orientación de la gravedad de la intoxicación aguda y de la
eficacia y la duración necesaria de la terapia.
El antídoto específico es la vitamina K1.
La principal diferencia entre los rodenticidas anticoagulantes de
la primera generación y los de la segunda es que éstos últimos tienen
una mayor retención en el organismo y por ello suelen dar lugar a un
periodo de hemorragia más prolongado.
La mayoría de los anticoagulantes son estables en condiciones de
uso normal. Su baja solubilidad en agua y baja concentración en los
cebos hace improbable que sean una fuente de contaminación del agua.
Parece que se asocian rápidamente a las partículas del suelo, con
desorción muy lenta y nula propiedad de lixiviación.
Los organismos no destinatarios pueden correr al riesgo de
consumir directamente cebos (riesgo primario) y de ingerir roedores
envenenados (riesgo secundario).
