
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 174
Isophorone
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared at the National Institute of Health Sciences,
Tokyo, Japan, and the Institute of Terrestrial Ecology, Monk's Wood,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Linear Alkylbenzene Sulfonates and Related Compounds.
(Environmental health criteria ; 174)
1.Cyclohexanones 2.Environmental exposure
3.Occupational exposure 4.Solvents I.Series
ISBN 92 4 157174 8 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
Preamble
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Physical and chemical properties
1.1.2. Production and use
1.1.3. Environmental transport, distribution
and transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals
and humans
1.1.6. Effects on laboratory mammals and in vitro
test systems
1.1.7. Effect on humans
1.1.8. Effects on other organisms in the laboratory
and field
1.2. Conclusions
1.2.1. General population
1.2.2. Occupational exposure
1.2.3. The environment
1.3. Recommendations
1.3.1. Protection of human health and the environment
1.3.2. Further research
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Environmental distribution
4.2. Biotransformation and environmental fate
4.2.1. Atmospheric fate
4.2.2. Aquatic fate
4.2.3. Terrestrial fate
4.2.4. Biodegradation
4.2.5. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil and sediment
5.1.4. Terrestrial organisms
5.1.4.1 Plants
5.1.4.2 Animals
5.1.5. Aquatic organisms
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Human
6.2. Laboratory mammals
7. EFFECTS ON LABORATORY MAMMALS, AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity
7.1.1. Oral
7.1.1.1 ß-Isophorone
7.1.2. Dermal
7.1.3. Inhalation
7.2. Skin, eye and respiratory irritation, sensitization
7.2.1. Skin irritation
7.2.1.1 ß-Isophorone
7.2.2. Eye irritation
7.2.2.1 ß-Isophorone
7.2.3. Respiratory irritation
7.2.4. Sensitization
7.3. Subchronic toxicity
7.3.1. Inhalation
7.3.2. Oral
7.3.3. Dermal
7.4. Mutagenicity
7.4.1. Gene mutation in bacteria (Ames tests)
7.4.1.1 Dihydroisophorone
7.4.2. Gene mutation in mammalian cells
7.4.3. Chromosome aberrations and sister chromatid
exchange
7.4.3.1 Chromosome aberrations
7.4.3.2 Sister chromatid exchange
7.4.4. Micronucleus test
7.4.5. Primary DNA damage
7.4.5.1 Bacterial tests
7.4.5.2 Unscheduled DNA synthesis
7.4.5.3 DNA binding
7.4.6. Morphological transformation
7.5. Chronic toxicity and carcinogenicity
7.6. Mechanisms of toxicity
7.7. Appraisal for mutagenicity/carcinogenicity
7.8. Reproduction, embryotoxicity, teratogenicity
7.9. Neurotoxicity
7.10. Other special studies
8. EFFECTS ON HUMANS
8.1. Acute
8.2. Sub-chronic
8.3. Irritation and sensitization
8.3.1. Eye and respiratory irritation
8.4. Chronic toxicity and carcinogenicity
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and epidemio-
logical methods in order to have internationally comparable
results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
ofthe chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
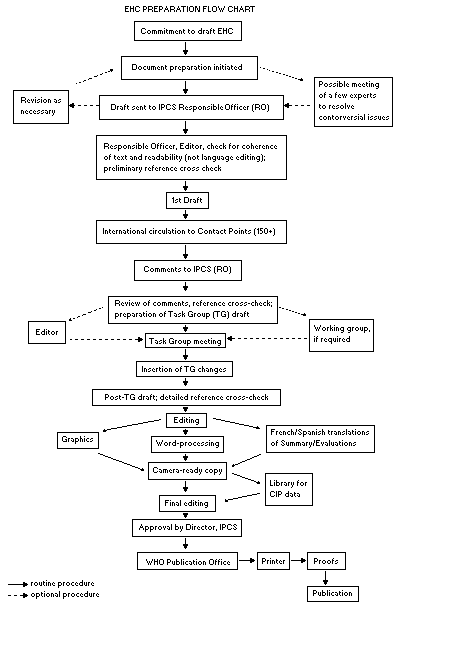
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
 All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico (Vice-Chairman)
Dr G.J. van Esch, Bilthoven, Netherlands
Dr S.K. Kashyap, National Institute of Occupational Health Ahmedabad,
India
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood
Experimental Station Huntingdon, United Kingdom (part-time)
Dr K. Peltonen, Institute of Occupational Health, Helsinki, Finland
Professor Wai-On Phoon, Worksafe Australia, and Department of
Occupational Health, University of Sydney, Sydney, Australia
(Chairman)
Mr D.J. Reisman, US Environmental Protection Agency, Cincinnati, USA
Dr E. Soderlund, National Institute of Public Health, Oslo, Norway
(Rapporteur)
Observer
Dr H. Certa, Hüls AG, Marl, Germany
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer (IARC),
Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
A WHO Task Group on Environmental Health Criteria for Isophorone
met at the World Health Organization, Geneva, from 12 to 16 December
1994. Dr K.W. Jager of the IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to isophorone.
The first draft of the monograph was prepared by Dr H.J. Wiegand
(Hüls), Dr J.F. Regnier (Atochem) and Dr P.L. Mason (British
Petroleum), and appeared as ECETOC-JACC Report No. 10. The second
draft, incorporating comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria, was prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The fact that industry made available to the IPCS and the Task
Group their proprietary toxicological information on isophorone is
gratefully acknowledged. This allowed the Task Group to make its
evaluation on a more complete data base.
The effort of all who helped in the preparation and the
finalization of the document is gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluatione
1.1.1 Physical and chemical properties
Isophorone is a colourless liquid with a peppermint-like odour.
It is soluble in water (12 g/litre) and miscible with most organic
solvents. Its freezing point is -8.1°C and its boiling point 215°C.
Its vapour pressure at 20°C is in the order of 40 Pa, and its vapour
density (air = 1) is 4.7. It is a stable substance.
Commercial samples of technical grade isophorone contain 1-3% of
the isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one); the sum
of alpha and isomers exceeds 99%.
1.1.2 Production and use
Isophorone is widely used as a solvent for a number of synthetic
resins and polymers, as well as in special application paints and
printing inks. It is also a chemical intermediate and a solvent in
certain pesticide formulations.
Its worldwide production was estimated to be in the order of
92 000 tonnes per year in 1988.
1.1.3 Environmental transport, distribution and transformation
Isophorone may enter the environment from numerous industries,
waste and wastewater disposal and its use as a solvent and a pesticide
carrier. Following release to water or soil, environmental
concentrations will decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded to the extent of approximately 70% within 14 days and 95%
within 28 days. The results of biodegradation studies are variable
and limited. Water solubility, soil adsorption coefficients and
polarity indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and
the physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
1.1.4 Environmental levels and human exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg has been reported.
Isophorone has been identified in surface waters (0.6 to 3 µg/litre),
groundwater (10 µg/litre), urban run-off (10 µg/litre) and landfill
leachate (29 µg/litre).
Isophorone has been found in industrial wastewater at a
concentration of 100 µg/litre. After classical secondary treatment,
the concentration of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6 to 12 µg/kg
dry weight) and in the tissues of several species of fish at
concentrations up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice or sugar beet following application as a pesticide carrier.
1.1.5 Kinetics and metabolism in laboratory animals and humans
Distribution studies in rats using 14C-isophorone showed that
93% of orally administered radioactivity appeared mainly in urine and
expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney and preputial
glands.
The metabolites from oral administration of isophorone identified
in rabbits' urine resulted from oxidation of the 3-methyl group,
reduction of the keto group and hydrogenation of the double bond of
the cyclohexene ring, and were eliminated as such or as glucuronide
derivatives in the case of the alcohols.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, with oral LD50 values
being > 1500 mg/kg in the rat, > 2200 mg/kg in the mouse and
> 2000 mg/kg in the rabbit. Dermal LD50 values were 1700 mg/kg in
the rat and > 1200 mg/kg in the rabbit. Acute effects from dermal
exposure in rats and rabbits ranged from mild erythema to scabs.
Conjunctivitis and corneal damage have been reported following direct
application to the eye or exposure to high concentrations of
isophorone. No skin sensitization was reported in guinea-pigs using
the Magnusson-Kligman test.
In acute and short-term oral studies on rodents at high doses
(> 1000 mg/kg), degenerative effects were seen in the liver as well
as CNS depression and some deaths. In 90-day studies, a NOEL in rats
and mice of 500 mg/kg body weight per day was determined. In a 90-day
oral study in beagle dogs (with limited numbers), no effects were seen
at doses of up to 150 mg/kg body weight per day.
In the acute and short-term inhalation experiments which were
reviewed, eye and respiratory irritation, haematological effects and
decreased body weights were noted. Since the study designs were
inadequate, no NOEL could be determined and no inference regarding
human health can be made.
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, DNA repair in primary rat
hepatocytes, or bone-marrow micronuclei in mice. Positive effects were
observed only in the absence of an exogenous metabolic system in
L5178Y TK +/- mouse lymphoma mutagenesis assays as well as in a sister
chromatid exchange assay. Isophorone induced morphological
transformation in vitro in the absence of an exogenous metabolism
system. It did not induce sex-linked recessive lethal mutations in
Drosophila. The weight of evidence of all mutagenicity data
supports the contention that isophorone is not a potent DNA-reactive
compound. In an in vivo assay, no DNA binding was observed in the
liver and kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies in mice and rats, male rats
showed several lesions of the kidney, including nephropathy, tubular
cell hyperplasia and low incidence of tubular cell adenomas and
adenocarcinomas. The role of alpha2u-globulin accumulation in the
etiology of these lesions has been recognized. Since significant
amounts of alpha2u-globulin have not been detected in humans this
mechanism of carcinogenesis appears not to be relevant to humans.
Preputial gland carcinomas were observed in five high-dose male rats,
and two clitoral gland adenomas were seen in low-dose female rats
following exposure to isophorone. These tumours may also be related
to alpha2u-globulin accumulation. Isophorone exposure was
associated with some neoplastic lesions of the liver, and the
integumentary and lymphoreticular systems of male mice, as well as
non-neoplastic liver and adrenal cortex lesions, but this was not
observed in dosed female mice.
In the only available long-term inhalation study in rats and
rabbits, irritation to eye and nasal mucosa, and lung and liver
changes, were observed at approx. 1427 mg/m3 (approx. 250 ppm).
However, it may have been due to limitations in the study.
Very limited studies in rats and mice indicate that isophorone
does not affect fertility nor does it cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural despair
swimming test.
1.1.7 Effect on humans
The odour of isophorone can be detected at a concentration as low
as 1.14 mg/m3 (0.2 ppm). Eye, nose and throat irritation has been
reported at concentrations below 28.55 mg/m3 (5 ppm); above
1142 mg/m3 (200 ppm) nausea, headache, dizziness, faintness and
inebriation have been reported.
1.1.8 Effects on other organisms in the laboratory and field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and
marine species. The 96-h EC50 values (based on cell count and
chlorophyll) range from 105 to 126 mg/litre. 48-h LC50 values for
Daphnia magna range from 117 to 120 mg/litre, and 96-h LC50 values
for freshwater fish range from 145 to 255 mg/litre.
The 96-h LC50 values for marine invertebrates range from 12.9
to 430 mg/litre, while the 96-h LC50 for a single marine fish
species was between 170 and 300 mg/litre. Data from studies with
measured exposure concentrations did not differ from studies with
nominal concentrations. NOEL values for Pimephales promelas tested
in different laboratories ranged from 14 to 45.4 mg/litre.
The available data suggest that isophorone has a low toxicity to
aquatic organisms.
1.2 Conclusions
1.2.1 General population
Isophorone is used as a solvent for resins, polymers and
pesticides formulations. Dermal and inhalation exposure may occur,
but will most likely be minimal. Data show that isophorone can occur
in µg/litre (kg) concentrations in drinking-water and fish. In view
of low toxicity in experimental studies and low levels of exposure
from environmental sources, the risk to the general population appears
to be minimal.
1.2.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin and respiratory irritation. At
higher concentrations other health effects may occur. No studies on
long-term health effects in workers were available for review by the
Task Group.
1.2.3 The environment
Isophorone may be released into the environment following its use
as a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, although it has a low environmental persistence due to
biodegradation, volatilization and photochemical oxidation processes.
The available data suggest that isophorone has low toxicity to aquatic
organisms.
1.3 Recommendations
1.3.1 Protection of human health and the environment
Care should be taken to prevent contamination of groundwater and
air.
Workers manufacturing or using isophorone should be protected
from exposure by means of adequate engineering controls and
appropriate industrial hygiene measures. Their occupational exposure
should be kept within acceptable levels and monitored regularly.
1.3.2 Further research
a) Health surveillance of exposed workers should be conducted.
b) Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
c) Adequate short-term/long-term inhalation studies in experimental
animals should be conducted in order to determine safe levels of
occupational exposure.
d) Information on anaerobic biodegradation of isophorone is needed,
especially as it has been identified in landfill leachate.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Common name: Isophorone
Synonyms: 2-cyclohexen-1-one, 3,5,5,-trimethyl;
3,5,5-trimethyl-2-cyclohexene-1-one;
1,1,3-trimethyl-3-cyclohexene-5-one;
alpha-isophorone; isoacetophorone; isoforone;
izoforon; 1,5,5-trimethyl-3-oxo-cyclohexene
Empirical formula: C9H14O
Chemical structure:
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico (Vice-Chairman)
Dr G.J. van Esch, Bilthoven, Netherlands
Dr S.K. Kashyap, National Institute of Occupational Health Ahmedabad,
India
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood
Experimental Station Huntingdon, United Kingdom (part-time)
Dr K. Peltonen, Institute of Occupational Health, Helsinki, Finland
Professor Wai-On Phoon, Worksafe Australia, and Department of
Occupational Health, University of Sydney, Sydney, Australia
(Chairman)
Mr D.J. Reisman, US Environmental Protection Agency, Cincinnati, USA
Dr E. Soderlund, National Institute of Public Health, Oslo, Norway
(Rapporteur)
Observer
Dr H. Certa, Hüls AG, Marl, Germany
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer (IARC),
Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
A WHO Task Group on Environmental Health Criteria for Isophorone
met at the World Health Organization, Geneva, from 12 to 16 December
1994. Dr K.W. Jager of the IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to isophorone.
The first draft of the monograph was prepared by Dr H.J. Wiegand
(Hüls), Dr J.F. Regnier (Atochem) and Dr P.L. Mason (British
Petroleum), and appeared as ECETOC-JACC Report No. 10. The second
draft, incorporating comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria, was prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The fact that industry made available to the IPCS and the Task
Group their proprietary toxicological information on isophorone is
gratefully acknowledged. This allowed the Task Group to make its
evaluation on a more complete data base.
The effort of all who helped in the preparation and the
finalization of the document is gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluatione
1.1.1 Physical and chemical properties
Isophorone is a colourless liquid with a peppermint-like odour.
It is soluble in water (12 g/litre) and miscible with most organic
solvents. Its freezing point is -8.1°C and its boiling point 215°C.
Its vapour pressure at 20°C is in the order of 40 Pa, and its vapour
density (air = 1) is 4.7. It is a stable substance.
Commercial samples of technical grade isophorone contain 1-3% of
the isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one); the sum
of alpha and isomers exceeds 99%.
1.1.2 Production and use
Isophorone is widely used as a solvent for a number of synthetic
resins and polymers, as well as in special application paints and
printing inks. It is also a chemical intermediate and a solvent in
certain pesticide formulations.
Its worldwide production was estimated to be in the order of
92 000 tonnes per year in 1988.
1.1.3 Environmental transport, distribution and transformation
Isophorone may enter the environment from numerous industries,
waste and wastewater disposal and its use as a solvent and a pesticide
carrier. Following release to water or soil, environmental
concentrations will decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded to the extent of approximately 70% within 14 days and 95%
within 28 days. The results of biodegradation studies are variable
and limited. Water solubility, soil adsorption coefficients and
polarity indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and
the physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
1.1.4 Environmental levels and human exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg has been reported.
Isophorone has been identified in surface waters (0.6 to 3 µg/litre),
groundwater (10 µg/litre), urban run-off (10 µg/litre) and landfill
leachate (29 µg/litre).
Isophorone has been found in industrial wastewater at a
concentration of 100 µg/litre. After classical secondary treatment,
the concentration of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6 to 12 µg/kg
dry weight) and in the tissues of several species of fish at
concentrations up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice or sugar beet following application as a pesticide carrier.
1.1.5 Kinetics and metabolism in laboratory animals and humans
Distribution studies in rats using 14C-isophorone showed that
93% of orally administered radioactivity appeared mainly in urine and
expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney and preputial
glands.
The metabolites from oral administration of isophorone identified
in rabbits' urine resulted from oxidation of the 3-methyl group,
reduction of the keto group and hydrogenation of the double bond of
the cyclohexene ring, and were eliminated as such or as glucuronide
derivatives in the case of the alcohols.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, with oral LD50 values
being > 1500 mg/kg in the rat, > 2200 mg/kg in the mouse and
> 2000 mg/kg in the rabbit. Dermal LD50 values were 1700 mg/kg in
the rat and > 1200 mg/kg in the rabbit. Acute effects from dermal
exposure in rats and rabbits ranged from mild erythema to scabs.
Conjunctivitis and corneal damage have been reported following direct
application to the eye or exposure to high concentrations of
isophorone. No skin sensitization was reported in guinea-pigs using
the Magnusson-Kligman test.
In acute and short-term oral studies on rodents at high doses
(> 1000 mg/kg), degenerative effects were seen in the liver as well
as CNS depression and some deaths. In 90-day studies, a NOEL in rats
and mice of 500 mg/kg body weight per day was determined. In a 90-day
oral study in beagle dogs (with limited numbers), no effects were seen
at doses of up to 150 mg/kg body weight per day.
In the acute and short-term inhalation experiments which were
reviewed, eye and respiratory irritation, haematological effects and
decreased body weights were noted. Since the study designs were
inadequate, no NOEL could be determined and no inference regarding
human health can be made.
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, DNA repair in primary rat
hepatocytes, or bone-marrow micronuclei in mice. Positive effects were
observed only in the absence of an exogenous metabolic system in
L5178Y TK +/- mouse lymphoma mutagenesis assays as well as in a sister
chromatid exchange assay. Isophorone induced morphological
transformation in vitro in the absence of an exogenous metabolism
system. It did not induce sex-linked recessive lethal mutations in
Drosophila. The weight of evidence of all mutagenicity data
supports the contention that isophorone is not a potent DNA-reactive
compound. In an in vivo assay, no DNA binding was observed in the
liver and kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies in mice and rats, male rats
showed several lesions of the kidney, including nephropathy, tubular
cell hyperplasia and low incidence of tubular cell adenomas and
adenocarcinomas. The role of alpha2u-globulin accumulation in the
etiology of these lesions has been recognized. Since significant
amounts of alpha2u-globulin have not been detected in humans this
mechanism of carcinogenesis appears not to be relevant to humans.
Preputial gland carcinomas were observed in five high-dose male rats,
and two clitoral gland adenomas were seen in low-dose female rats
following exposure to isophorone. These tumours may also be related
to alpha2u-globulin accumulation. Isophorone exposure was
associated with some neoplastic lesions of the liver, and the
integumentary and lymphoreticular systems of male mice, as well as
non-neoplastic liver and adrenal cortex lesions, but this was not
observed in dosed female mice.
In the only available long-term inhalation study in rats and
rabbits, irritation to eye and nasal mucosa, and lung and liver
changes, were observed at approx. 1427 mg/m3 (approx. 250 ppm).
However, it may have been due to limitations in the study.
Very limited studies in rats and mice indicate that isophorone
does not affect fertility nor does it cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural despair
swimming test.
1.1.7 Effect on humans
The odour of isophorone can be detected at a concentration as low
as 1.14 mg/m3 (0.2 ppm). Eye, nose and throat irritation has been
reported at concentrations below 28.55 mg/m3 (5 ppm); above
1142 mg/m3 (200 ppm) nausea, headache, dizziness, faintness and
inebriation have been reported.
1.1.8 Effects on other organisms in the laboratory and field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and
marine species. The 96-h EC50 values (based on cell count and
chlorophyll) range from 105 to 126 mg/litre. 48-h LC50 values for
Daphnia magna range from 117 to 120 mg/litre, and 96-h LC50 values
for freshwater fish range from 145 to 255 mg/litre.
The 96-h LC50 values for marine invertebrates range from 12.9
to 430 mg/litre, while the 96-h LC50 for a single marine fish
species was between 170 and 300 mg/litre. Data from studies with
measured exposure concentrations did not differ from studies with
nominal concentrations. NOEL values for Pimephales promelas tested
in different laboratories ranged from 14 to 45.4 mg/litre.
The available data suggest that isophorone has a low toxicity to
aquatic organisms.
1.2 Conclusions
1.2.1 General population
Isophorone is used as a solvent for resins, polymers and
pesticides formulations. Dermal and inhalation exposure may occur,
but will most likely be minimal. Data show that isophorone can occur
in µg/litre (kg) concentrations in drinking-water and fish. In view
of low toxicity in experimental studies and low levels of exposure
from environmental sources, the risk to the general population appears
to be minimal.
1.2.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin and respiratory irritation. At
higher concentrations other health effects may occur. No studies on
long-term health effects in workers were available for review by the
Task Group.
1.2.3 The environment
Isophorone may be released into the environment following its use
as a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, although it has a low environmental persistence due to
biodegradation, volatilization and photochemical oxidation processes.
The available data suggest that isophorone has low toxicity to aquatic
organisms.
1.3 Recommendations
1.3.1 Protection of human health and the environment
Care should be taken to prevent contamination of groundwater and
air.
Workers manufacturing or using isophorone should be protected
from exposure by means of adequate engineering controls and
appropriate industrial hygiene measures. Their occupational exposure
should be kept within acceptable levels and monitored regularly.
1.3.2 Further research
a) Health surveillance of exposed workers should be conducted.
b) Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
c) Adequate short-term/long-term inhalation studies in experimental
animals should be conducted in order to determine safe levels of
occupational exposure.
d) Information on anaerobic biodegradation of isophorone is needed,
especially as it has been identified in landfill leachate.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Common name: Isophorone
Synonyms: 2-cyclohexen-1-one, 3,5,5,-trimethyl;
3,5,5-trimethyl-2-cyclohexene-1-one;
1,1,3-trimethyl-3-cyclohexene-5-one;
alpha-isophorone; isoacetophorone; isoforone;
izoforon; 1,5,5-trimethyl-3-oxo-cyclohexene
Empirical formula: C9H14O
Chemical structure: 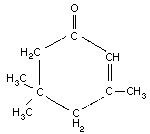 Relative molecular
mass: 138.2
CAS registry
number: 78-59-1
RTECs registry
number: GW7700000
EEC No: 606-012-00-8
EINECS No: 1011260
2.2 Physical and chemical properties
Isophorone is a colourless liquid. Its odour has been described
as being similar to peppermint and camphor. It is soluble in water
and is miscible in all proportions with aliphatic and aromatic
hydrocarbons, alcohols, ethers, esters, ketones and chlorinated
hydrocarbons. Its physical and chemical data are summarized in Table
1.
A typical commercial sample of isophorone may contain 1-3% of the
isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) with the sum
of alpha- and ß-isomers exceeding 99% (Hüls, 1981; Atochem, 1986).
Isophorone is stable and may be stored in steel or aluminium
containers. Prolonged periods of storage may lead to slight
yellowing.
2.3 Conversion factors
The following conversion factors have been calculated for 22°C
and 1013 hPa:
1 ppm = 5.71 mg/m3
1 mg/m3 = 0.175 ppm
2.4 Analytical methods
The purity of technical isophorone may be determined by capillary
gas chromatography (GC) with a flame ionization detector (FID).
Recommended conditions are shown in Table 2.
Earlier methods for determining isophorone in air were based on
adsorption on charcoal (White et al., 1970; US NIOSH, 1977). However,
it has been found that isophorone adsorbed on charcoal decomposes
during storage. More recent methods involve adsorption on polymers
such as XAD resins (Levin & Carleborg, 1987) or Tenax-GC (Brown &
Purnell, 1979), followed by desorption and analysis by capillary GC
with FID.
The analysis of isophorone present in wastewater samples and fish
tissues may be achieved by solvent extraction, clean up by gel
permeation chromatography and analysis by GC/MS, in both the electron
impact and chemical ionization modes (Jungclaus et al., 1976; US EPA,
1979; Sheldon & Hites, 1979).
Table 1. Physical and chemical data of isophorone
Value Reference
Specific gravity (20°C/4°C) 0.922 Bartholomé et al. (1977)
Boiling point at 1013 hPa 215°C Bartholomé et al. (1977)
Freezing point -8.1°C Cheminfo (1988)
Refractive index (n20D) 1.4775 Bartholomé et al. (1977)
Viscosity at 20°C 2.6 mPa Hüls (1981)
Coefficient of cubical expansion 0.00085°C-1 BP (1988b)
at 20°C 0.00078°C-1 Atochem (1986)
Surface tension at 20°C 30 mN/m BP (1988b)
Vapour pressure 40 Pa (20°C) Bartholomé et al. (1977)
34.7 Pa (25°C) BIBRA (1991)
Vapour density (air = 1) 4.7 Cheminfo (1988)
Concentration in saturated air
at 20°C and 1013 hPa 1941 mg/m3 Cheminfo (1988)
Solubility at 20°C
- Isophorone in water 12.0 g/litre Lyman et al. (1982)
17.5 g/litrea
- Water in isophorone 53 g/litrea
Log Kow (20°C) 1.67 (measured) Veith et al. (1978)
1.7 (estimated) Callahan et al. (1979)
Table 1 (cont'd)
Value Reference
Solubility parameters (Hansen)
delta 19.2 (J/cm3)1/2 Hüls (1981)
deltaD 16.6 (J/cm3)1/2 Hüls (1981)
deltaP 8.2 (J/cm3)1/2 Hüls (1981)
deltaH 7.4 (J/cm3)1/2 Hüls (1981)
Hydrogen bonding parameter,
gamma 14.9 Hüls (1981)
Flash point, closed cup 85°C Cheminfo (1988)
Explosion limits in air 0.8-3.8 vol-% Bartholomé et al. (1977)
Ignition temperature 470°C Bartholomé et al. (1977)
455°C BIBRA (1991)
Heat of evaporation at 215°C 349.2 kJ/kg Bartholomé et al. (1977)
Heat of combustion
at 20°C 38 100 kJ/kg Bartholomé et al. (1977)
Relative permittivity at 20°C 19.9 Hüls (1981)
Specific resistivity 1 × 107 ohm × cm Atochem (1986)
a Communication from Hüls AG, Marl, Germany, 1989.
Table 2. Gas chromatographic conditions for the analysis of technical isophorone
Column Fused silica capillarya Macroboreb
Coating OV-1701 CP Wax 52CB
Dimensions 60 m/0.25 mm 25 m/0.53 mm
Injector temperature 240°C 250°C
Temperature-programme 6 min 70°C to 10 min 105°C to 120°C
220°C at 4°/min at 6°/min
2 min 120°C to 150°C
at 10 °/min
From: a Hüls (1988c); b Atochem (1988)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Isophorone has tentatively been identified as a component of the
essential oil of Thymus cariensis (Baser et al., 1992).
Isophorone is produced commercially by catalytic condensation of
acetone at elevated temperature and pressure and is purified by
distillation. Worldwide annual production capacity was estimated to be
92 000 tonnes in 1988 (Personal communication from Hüls AG, Marl,
Germany, 1989, to the IPCS).
Isophorone is a solvent for a number of natural and synthetic
resins and polymers such as polyvinyl chlorides and acetates,
cellulose derivatives, epoxy and alkyd resins and polyacrylates. It
is therefore used as a high boiling solvent in industrial air drying
and stoving paints, nitro emulsion leather finishes and the
manufacture of vinyl resin based printing inks for plastic surfaces.
Isophorone is also used as a solvent for some pesticide formulations,
especially for emulsifiable concentrates of anilides and carbamates.
Isophorone is used as a chemical intermediate for the synthesis
of a variety of organic chemicals (Hüls, 1981; Thier & Xu, 1990).
Coal-burning power plants may be a source of atmospheric
isophorone as it was detected in the fly ash from an electrostatic
precipitator (Harrison et al., 1985). It has also been found in the
breathing zones and area samples of work sites (US NIOSH, 1980, 1984)
and in wastewater from industrial processes (Jungclaus et al., 1976).
Thus, some manufacturing processes may be an environmental source of
isophorone.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental distribution
In view of its widespread use as a solvent for polymers, resins,
waxes, oils and pesticides, there is a possibility of a wide
distribution into the environment. Isophorone has been detected in
river, surface, and groundwaters and in finished drinking-water (Thier
& Xu, 1990) (see section 5.1.2.), in effluents from latex and chemical
plants (Shackelford & Keith, 1976), in wastewater from a tyre
manufacturing plant (Jungclaus et al., 1976), and in air during
manufacturing operations (US NIOSH, 1980, 1984). The detection of
isophorone in coal fly ash suggests that it may also be found in
ambient air.
4.2 Biotransformation and environmental fate
4.2.1 Atmospheric fate
By virtue of its vapour pressure of 40 Pa at 20°C, atmospheric
isophorone will exist mainly in the vapour state (ECETOC, 1988). The
Graphical Exposure Modelling System (GEMS) predicts that the half-life
for reaction with both ambient ozone and photo-chemically generated
hydroxyl radicals will be approximately 30 min. This estimate assumes
a concentration of 8 × 105 molecules per cm3 and a reaction rate
constant of 8.14 × 10-11 cm3 molecule-1 sec-1 at 25°C for
hydroxyl radicals and 1.0 × 1012 molecules per cm3 with a reaction
rate constant of 5 × 10-16 cm3 molecule-1 sec-1 at 25°C for
ozone (US EPA, 1986).
4.2.2 Aquatic fate
From an estimated Henry's Law constant of 5.8 × 10-6 atm m3
mole-1, based upon a water solubility of 12 g/litre at 20°C and a
vapour pressure of 40 Pa at 20°C, the volatilization half-life in a
model river flowing at 1 m/sec was calculated to be 7.5 days (Lyman et
al., 1982). Based on a water solubility of 17.5 g/litre the
volatilization half-life would be 11 days (Personal communication from
Hüls AG, Marl, Germany, 1989, to the IPCS).
The oxidation of isophorone by alkylperoxy radicals or singlet
oxygen in water is unlikely to be significant in the environment
(Mabey, 1981). Although dimerization has been reported in water
irradiated at wave-lengths > 200 nm and in organic solvents at
> 300 nm, such products are also considered unlikely at the levels
existing in the environment (Callahan et al., 1979).
Evidence that isophorone is photo-oxidized is provided by Borup &
Middlebrooks (1986). Treatment with hydrogen peroxide (250 mg/litre)
followed by UV radiation reduced an isophorone concentration of
62 mg/litre to < 0.05 mg/litre in 60 min.
Isophorone has been shown to be converted to a compound(s)
mutagenic to Salmonella typhimurium TA100 by aqueous chlorination
under conditions of pH and reactant concentrations that may be
relevant to wastewater and drinking-water chlorination (Cheh, 1986).
4.2.3 Terrestrial fate
Loss from soil, as in the case of surface water, will be by
volatilization and biodegradation. In view of the vapour pressure and
Henry's Law constant, volatilization from both wet and dry soil
surfaces would be slow.
Based on a log Kow of 2.22 and assuming a water solubility of
12 g/litre at 20°C, a soil adsorption coefficient (Koc) of 25 has
been estimated (Lyman et al., 1982). These values suggest that
isophorone would be mobile in soil and that adsorption on suspended
solids and sediment in water would be insignificant (Swann et al.,
1983).
4.2.4 Biodegradation
Tabak et al. (1981a,b) reported that isophorone (concentrations
of 5 and 10 mg/litre) was rapidly degraded over 7 days by adapted
microorganisms based on an aerobic-static culture procedure
incorporating settled domestic wastewater as the microbial inoculum.
Aerobic incubation of 100 mg/litre with activated sludge
(30 mg/litre) for 2 weeks resulted in < 30% degradation (Kawasaki,
1980; Sasaki, 1980). Price et al. (1974) reported the removal (using
a BOD procedure) of 9 and 42% isophorone from salt and fresh water,
respectively, following incubation for 20 days with a settled non-
adapted domestic wastewater inoculum.
The losses of isophorone from wastewater treated using a
trickling filter, activated sludge, aerated lagoon and facultative
lagoon were 19, 98, 24 and 30%, respectively (Hannah et al., 1986).
Intermediate degradation products of isophorone identified by Mikami
et al. (1981) after incubation with Aspergillus niger were 3,5,5-
trimethyl-2-cyclohexene-1,4-dione; 3,5,5-trimethylcyclo-hexane-1,4-
dione; (S)-4-hydroxy-3,5,5-trimethyl-2-cyclohexene-1-one; and
3-hydroxymethyl-5,5-dimethyl-2-cyclohexene-1-one.
In a Die-away test, the breakdown of isophorone after 14 days was
approximately 70%, whereas 95% was broken down within 28 days
(Schöberl, 1992).
4.2.5 Bioaccumulation
Barrows et al. (1980) reported a measured bioconcentration factor
of 7 for the bluegill sunfish (Lepomis macrochirus) exposed for 14
days to a mean concentration of 92.4 (± 10.5) µg/litre. The half-life
of isophorone in the tissues of this species was 1 day. The
bioconcentration factor was expressed as the quotient of the mean
measured residues of the compound in fish tissues (whole body) during
the equilibrium period divided by the mean measured concentration of
the compound in water. The half-life of the compound in tissues was
the time in days required for mean measured residue concentration in
tissues to be reduced to half that which was measured during the
equilibrium period in the uptake phase (Barrows et al., 1980). Thus,
it is assumed that isophorone will not bioconcentrate in aquatic
organisms.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Harrison et al. (1985) identified isophorone at a level of
490 µg/kg by GC/MS in electrostatically precipitated coal fly ash,
suggesting that coal-fired power stations may be a source of emission
to the atmosphere.
5.1.2 Water
The available data on isophorone concentrations in water are
presented in Table 3.
Isophorone was detected in 1% of 795 surface water samples
(Hauser & Bromberg, 1982). Concentrations of up to 3 µg/litre were
reported in the Delaware river (Sheldon & Hites, 1979) and a
concentration of 10 µg/litre was measured in urban run-off in
Washington, DC, USA (Cole et al., 1984).
The US Environmental Protection Agency has identified isophorone
in finished drinking-water at concentrations ranging from 0.02 to
9.5 µg/litre (US EPA, 1980). A maximum concentration of 10 µg/litre
has been reported in groundwater in the Netherlands (Zoeteman et al.,
1981); the specific sources of this contamination were not identified.
Isophorone was not detected in 36 water samples during the 1981
Environmental Survey of Chemicals in Japan (Japan Environment Agency,
1983).
5.1.3 Soil and sediment
Isophorone was identified in sediment and soil taken from Love
Canal, New York, USA (Hauser & Bromberg, 1982) and in sediment taken
from Lake Pontchartrain, Louisiana, USA (McFall et al., 1985). The
concentrations in the latter were 0.9-12 µg/kg dry weight.
Isophorone was detected in 18 out of 36 bottom sediments, at
concentrations ranging from 0.6 to 6.6 µg/kg dry weight, in the 1981
Environmental Survey of Chemicals in Japan (Japan Environment Agency,
1983).
5.1.4 Terrestrial organisms
5.1.4.1 Plants
To estimate the decline of isophorone concentration in plants
treated with pesticides containing isophorone as a carrier,
14C-isophorone was sprayed on bean plants and rice at a rate
Table 3. Isophorone concentrations in water
Media Location Number Analytical Range Samples with Reference
of samples method (µg/litre) detectable
residues (%)
Surface water Delaware River, USA GC-MS 0.6-3 Hites (1979)
Delaware River, USA GC-MS 3 Sheldon & Hites (1979)
Drinking-water USA 0.02-9.5 US EPA (1980)
Philadelphia, USA 12 GC-MS 17 Suffet et al. (1980)
Ground water Netherlands 10 Zoeteman et al. (1981)
Industrial effluent manufacturing effluent GC-MS 40 Jungclaus et al. (1976)
industrial effluent GC-MS 100 Hites (1979)
treated effluent GC-MS 10 Hites (1979)
Urban run-off Washington, DC, USA 86 10 4 Cole et al. (1984)
Leachate hazardous waste landfill 8 GC-MS 29 12.5 Ghassemi et al. (1984)
equivalent to 7.5 kg/ha. Samples of the plants were taken
periodically and assayed for total radioactivity. No attempt was made
to characterize metabolites or degradation products. In bean plants
total residues declined rapidly from 16 mg/kg one hour after
application to below 0.1 mg/kg on day 42. Beans harvested on day 56
did not contain detectable radioactivity. Residues in rice plants
declined from 7.3 mg/kg one hour after application to 3.1 mg/kg on day
35 and 0.12 mg/kg on day 128. Immature rice heads did not contain
radioactivity on days 110 and 128. The relatively slow decay in rice
plants was considered by the authors to be due to unfavourable growing
conditions in this particular study (Rohm & Haas, 1972).
In a similar study, sugar beet was sprayed at the 2-leaf stage
with a herbicide containing 14C-isophorone. Total radioactivity
found on day 30 was reported to be 10% of the initial value. On day
90, residues in the plants were below 0.01 mg/kg except in dry leaves
where 0.07 mg/kg were found. The results suggested some uptake of
radiolabel from the soil from day 60 onwards; this was considered
likely to be due to the uptake of small carbon fragments or 14CO2
resulting from degradation of isophorone in the soil (Schering, 1974).
5.1.4.2 Animals
No data are available.
5.1.5 Aquatic organisms
Isophorone was found in three samples of commercial mussels
(Mytilus edulis) collected near Holbaek, Denmark, but no
concentrations were reported (Rasmussen et al., 1993).
Isophorone was detected in fish sampled from several tributary
rivers of Lake Michigan, Canada. Composite samples of several fish
carcasses were analysed using GC-MS, and the following concentration
ranges (mg/kg wet weight) were reported: common carp, < 0.02-3.13;
smallmouth bass, 0.74-3.61; largemouth bass, 0.72; small bass,
0.74-3.61; pumpkinseed, 0.4; bowfin, < 0.02-0.76; northern pike,
< 0.02-0.48; rock bass, < 0.02-1.44; and lake trout, 2.38.
Isophorone was not detected in the channel catfish sample (Camanzo et
al., 1987).
5.2 General population exposure
No data were available.
5.3 Occupational exposure
In the USA, the ACGIH (1986) have adopted a short-term (15 min)
ceiling value of 28 mg/m3 (5 ppm) and the NIOSH recommended a 10-h
TWA exposure limit of 23 mg/m3 (4 ppm) based primarily on
unpublished reports, supplied to the TLV committee, of fatigue and
malaise in workers exposed to concentrations of 28-46 mg/m3
(5-8 ppm) for 1 month. On lowering the concentration to between 5.7
and 22.8 mg/m3 (1 and 4 ppm) no further complaints were received
(ACGIH, 1986). Exposure to isophorone has been determined in a screen
printing plant, where several environmental conditions favoured
evaporation of this solvent and where working conditions increased the
risk of employee exposure. The highest exposures in screen printers
were reported to be 131 ± 31 mg/m3 (23 ± 5.4 ppm) (50-90 min TWA) in
the breathing zone of printing press workers. Other workers also
received significant exposures: paint mixers, 102 ± 31 mg/m3
(17.8 ± 5.5 ppm); manual drying, 86 ± 23 mg/m3 (15 ± 4.1 ppm);
automatic drying, 54 ± 19 mg/m3 (9.5 ± 3.3 ppm); and screen washer,
47 ± 32 mg/m3 (8.3 ± 5.6 ppm) (Samimi, 1982).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found workshift (6´ h) average
exposures of printers to isophorone of 4 and 80 mg/m3 (0.7 and
14 ppm). Symptoms of respiratory tract and eye irritation reported by
workers were attributed to the antistatic agent containing principally
isophorone.
A NIOSH evaluation (US NIOSH, 1984) conducted at another screen
printing operation in 1984 found no detectable (< 2.8 mg/m3,
0.5 ppm) exposure to isophorone.
Isophorone has been found in 6 out of 29 samples of printer's
inks from different European manufacturers. The method of analysis
was headspace gas chromatography and mass spectrometry. All of the
samples in the present study were for serigraphy on electric and
electronic articles (Rastogi, 1991).
6. KINETICS AND METABOLISM
6.1 Human
No data are available.
6.2 Laboratory mammals
Isophorone is absorbed by oral, dermal and inhalation routes.
Following a single oral administration of isophorone to rats
(4 g/kg) and rabbits (1 g/kg), the substance was distributed rapidly
in the body and detected in the stomach, pancreas, adrenals, spleen
and liver. Following inhalation (2284 mg/m3, 400 ppm for 4 h)
isophorone was detected in the kidney, adrenals, liver, pancreas and
brain of rats (Dutertre-Catella, 1976).
Strasser et al. (1988) studied the distribution of isophorone in
male rats following administration by gavage of a single dose
(3.6 mmol/kg) of 14C-isophorone (in corn oil) containing 177 µCi/kg.
Determination of radiolabel distribution of 14C-isophorone 24 h
after dosing showed that 93% of the label had been excreted in the
urine, faeces and expired air (approximate ratio 1200:1:67). The
remainder of the radiolabel was concentrated in the liver, kidney and
preputial glands, which contained 3.7, 1.1 and 0.7%, respectively, of
the original dose. The high concentration of radiolabel in the
preputial gland may have been due to the high concentrations of
alpha2u-globulin to which it could bind (see also section 7.6).
Following oral administration of isophorone to rats and rabbits,
the substance was partly eliminated unchanged in expired air and
urine; the remainder was metabolized (see Fig. 1) to:
a) 5,5-dimethyl-cyclohex-1-en-3-one-1-carboxylic acid (i), derived
from isophorone by methyl-oxidation;
b) isophorol (3,5,5-trimethyl-cyclohex-2-en-1-ol) (ii), formed by
the reduction of the ketonic group to a secondary alcohol and
eliminated as a glucuronide; and
c) dihydroisophorone (3,5,5-trimethyl-cyclohexanone) (iii),
proceeding from the hydrogenation of the cyclohexene ring, and
small quantities of cis- and trans-3,5,5-trimethyl-
cyclohexanol-1 (iv), likely to have been formed from
dihydroisophorone.
The amounts of the identified metabolites as a proportion of the
administered dose were not reported (Truhaut et al., 1970; Dutertre-
Catella et al., 1978).
A single oral isophorone dose of 500 mg/kg to male SD rats has
been reported to cause significant depletion of hepatic, testicular
and epididymal glutathione. Evidence was subsequently presented for
enhanced ethyl methane sulfonate (EMS)-induced alkylation of DNA taken
from epididymal spermatozoa (Gandy et al., 1990). Although individual
compounds were not identified, the data suggest glutathione may play a
significant role in the elimination of isophorone and its metabolites.
Quantitative data regarding the excretion of isophorone are not
available. In the study of Dutertre-Catella et al. (1978), unchanged
isophorone, isophorol (ii), dihydroisophorone (iii), 3-carboxy-5,5-
dimethyl-2-cyclohexene-1-one (i), and cis- and trans-3,5,5-
trimethyl-cyclohexanols (iv) were detected in the urine of rats and
rabbits 24 h after an oral dose of isophorone. The expired air
contained unchanged isophorone 6 h after dosing.
Relative molecular
mass: 138.2
CAS registry
number: 78-59-1
RTECs registry
number: GW7700000
EEC No: 606-012-00-8
EINECS No: 1011260
2.2 Physical and chemical properties
Isophorone is a colourless liquid. Its odour has been described
as being similar to peppermint and camphor. It is soluble in water
and is miscible in all proportions with aliphatic and aromatic
hydrocarbons, alcohols, ethers, esters, ketones and chlorinated
hydrocarbons. Its physical and chemical data are summarized in Table
1.
A typical commercial sample of isophorone may contain 1-3% of the
isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) with the sum
of alpha- and ß-isomers exceeding 99% (Hüls, 1981; Atochem, 1986).
Isophorone is stable and may be stored in steel or aluminium
containers. Prolonged periods of storage may lead to slight
yellowing.
2.3 Conversion factors
The following conversion factors have been calculated for 22°C
and 1013 hPa:
1 ppm = 5.71 mg/m3
1 mg/m3 = 0.175 ppm
2.4 Analytical methods
The purity of technical isophorone may be determined by capillary
gas chromatography (GC) with a flame ionization detector (FID).
Recommended conditions are shown in Table 2.
Earlier methods for determining isophorone in air were based on
adsorption on charcoal (White et al., 1970; US NIOSH, 1977). However,
it has been found that isophorone adsorbed on charcoal decomposes
during storage. More recent methods involve adsorption on polymers
such as XAD resins (Levin & Carleborg, 1987) or Tenax-GC (Brown &
Purnell, 1979), followed by desorption and analysis by capillary GC
with FID.
The analysis of isophorone present in wastewater samples and fish
tissues may be achieved by solvent extraction, clean up by gel
permeation chromatography and analysis by GC/MS, in both the electron
impact and chemical ionization modes (Jungclaus et al., 1976; US EPA,
1979; Sheldon & Hites, 1979).
Table 1. Physical and chemical data of isophorone
Value Reference
Specific gravity (20°C/4°C) 0.922 Bartholomé et al. (1977)
Boiling point at 1013 hPa 215°C Bartholomé et al. (1977)
Freezing point -8.1°C Cheminfo (1988)
Refractive index (n20D) 1.4775 Bartholomé et al. (1977)
Viscosity at 20°C 2.6 mPa Hüls (1981)
Coefficient of cubical expansion 0.00085°C-1 BP (1988b)
at 20°C 0.00078°C-1 Atochem (1986)
Surface tension at 20°C 30 mN/m BP (1988b)
Vapour pressure 40 Pa (20°C) Bartholomé et al. (1977)
34.7 Pa (25°C) BIBRA (1991)
Vapour density (air = 1) 4.7 Cheminfo (1988)
Concentration in saturated air
at 20°C and 1013 hPa 1941 mg/m3 Cheminfo (1988)
Solubility at 20°C
- Isophorone in water 12.0 g/litre Lyman et al. (1982)
17.5 g/litrea
- Water in isophorone 53 g/litrea
Log Kow (20°C) 1.67 (measured) Veith et al. (1978)
1.7 (estimated) Callahan et al. (1979)
Table 1 (cont'd)
Value Reference
Solubility parameters (Hansen)
delta 19.2 (J/cm3)1/2 Hüls (1981)
deltaD 16.6 (J/cm3)1/2 Hüls (1981)
deltaP 8.2 (J/cm3)1/2 Hüls (1981)
deltaH 7.4 (J/cm3)1/2 Hüls (1981)
Hydrogen bonding parameter,
gamma 14.9 Hüls (1981)
Flash point, closed cup 85°C Cheminfo (1988)
Explosion limits in air 0.8-3.8 vol-% Bartholomé et al. (1977)
Ignition temperature 470°C Bartholomé et al. (1977)
455°C BIBRA (1991)
Heat of evaporation at 215°C 349.2 kJ/kg Bartholomé et al. (1977)
Heat of combustion
at 20°C 38 100 kJ/kg Bartholomé et al. (1977)
Relative permittivity at 20°C 19.9 Hüls (1981)
Specific resistivity 1 × 107 ohm × cm Atochem (1986)
a Communication from Hüls AG, Marl, Germany, 1989.
Table 2. Gas chromatographic conditions for the analysis of technical isophorone
Column Fused silica capillarya Macroboreb
Coating OV-1701 CP Wax 52CB
Dimensions 60 m/0.25 mm 25 m/0.53 mm
Injector temperature 240°C 250°C
Temperature-programme 6 min 70°C to 10 min 105°C to 120°C
220°C at 4°/min at 6°/min
2 min 120°C to 150°C
at 10 °/min
From: a Hüls (1988c); b Atochem (1988)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Isophorone has tentatively been identified as a component of the
essential oil of Thymus cariensis (Baser et al., 1992).
Isophorone is produced commercially by catalytic condensation of
acetone at elevated temperature and pressure and is purified by
distillation. Worldwide annual production capacity was estimated to be
92 000 tonnes in 1988 (Personal communication from Hüls AG, Marl,
Germany, 1989, to the IPCS).
Isophorone is a solvent for a number of natural and synthetic
resins and polymers such as polyvinyl chlorides and acetates,
cellulose derivatives, epoxy and alkyd resins and polyacrylates. It
is therefore used as a high boiling solvent in industrial air drying
and stoving paints, nitro emulsion leather finishes and the
manufacture of vinyl resin based printing inks for plastic surfaces.
Isophorone is also used as a solvent for some pesticide formulations,
especially for emulsifiable concentrates of anilides and carbamates.
Isophorone is used as a chemical intermediate for the synthesis
of a variety of organic chemicals (Hüls, 1981; Thier & Xu, 1990).
Coal-burning power plants may be a source of atmospheric
isophorone as it was detected in the fly ash from an electrostatic
precipitator (Harrison et al., 1985). It has also been found in the
breathing zones and area samples of work sites (US NIOSH, 1980, 1984)
and in wastewater from industrial processes (Jungclaus et al., 1976).
Thus, some manufacturing processes may be an environmental source of
isophorone.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental distribution
In view of its widespread use as a solvent for polymers, resins,
waxes, oils and pesticides, there is a possibility of a wide
distribution into the environment. Isophorone has been detected in
river, surface, and groundwaters and in finished drinking-water (Thier
& Xu, 1990) (see section 5.1.2.), in effluents from latex and chemical
plants (Shackelford & Keith, 1976), in wastewater from a tyre
manufacturing plant (Jungclaus et al., 1976), and in air during
manufacturing operations (US NIOSH, 1980, 1984). The detection of
isophorone in coal fly ash suggests that it may also be found in
ambient air.
4.2 Biotransformation and environmental fate
4.2.1 Atmospheric fate
By virtue of its vapour pressure of 40 Pa at 20°C, atmospheric
isophorone will exist mainly in the vapour state (ECETOC, 1988). The
Graphical Exposure Modelling System (GEMS) predicts that the half-life
for reaction with both ambient ozone and photo-chemically generated
hydroxyl radicals will be approximately 30 min. This estimate assumes
a concentration of 8 × 105 molecules per cm3 and a reaction rate
constant of 8.14 × 10-11 cm3 molecule-1 sec-1 at 25°C for
hydroxyl radicals and 1.0 × 1012 molecules per cm3 with a reaction
rate constant of 5 × 10-16 cm3 molecule-1 sec-1 at 25°C for
ozone (US EPA, 1986).
4.2.2 Aquatic fate
From an estimated Henry's Law constant of 5.8 × 10-6 atm m3
mole-1, based upon a water solubility of 12 g/litre at 20°C and a
vapour pressure of 40 Pa at 20°C, the volatilization half-life in a
model river flowing at 1 m/sec was calculated to be 7.5 days (Lyman et
al., 1982). Based on a water solubility of 17.5 g/litre the
volatilization half-life would be 11 days (Personal communication from
Hüls AG, Marl, Germany, 1989, to the IPCS).
The oxidation of isophorone by alkylperoxy radicals or singlet
oxygen in water is unlikely to be significant in the environment
(Mabey, 1981). Although dimerization has been reported in water
irradiated at wave-lengths > 200 nm and in organic solvents at
> 300 nm, such products are also considered unlikely at the levels
existing in the environment (Callahan et al., 1979).
Evidence that isophorone is photo-oxidized is provided by Borup &
Middlebrooks (1986). Treatment with hydrogen peroxide (250 mg/litre)
followed by UV radiation reduced an isophorone concentration of
62 mg/litre to < 0.05 mg/litre in 60 min.
Isophorone has been shown to be converted to a compound(s)
mutagenic to Salmonella typhimurium TA100 by aqueous chlorination
under conditions of pH and reactant concentrations that may be
relevant to wastewater and drinking-water chlorination (Cheh, 1986).
4.2.3 Terrestrial fate
Loss from soil, as in the case of surface water, will be by
volatilization and biodegradation. In view of the vapour pressure and
Henry's Law constant, volatilization from both wet and dry soil
surfaces would be slow.
Based on a log Kow of 2.22 and assuming a water solubility of
12 g/litre at 20°C, a soil adsorption coefficient (Koc) of 25 has
been estimated (Lyman et al., 1982). These values suggest that
isophorone would be mobile in soil and that adsorption on suspended
solids and sediment in water would be insignificant (Swann et al.,
1983).
4.2.4 Biodegradation
Tabak et al. (1981a,b) reported that isophorone (concentrations
of 5 and 10 mg/litre) was rapidly degraded over 7 days by adapted
microorganisms based on an aerobic-static culture procedure
incorporating settled domestic wastewater as the microbial inoculum.
Aerobic incubation of 100 mg/litre with activated sludge
(30 mg/litre) for 2 weeks resulted in < 30% degradation (Kawasaki,
1980; Sasaki, 1980). Price et al. (1974) reported the removal (using
a BOD procedure) of 9 and 42% isophorone from salt and fresh water,
respectively, following incubation for 20 days with a settled non-
adapted domestic wastewater inoculum.
The losses of isophorone from wastewater treated using a
trickling filter, activated sludge, aerated lagoon and facultative
lagoon were 19, 98, 24 and 30%, respectively (Hannah et al., 1986).
Intermediate degradation products of isophorone identified by Mikami
et al. (1981) after incubation with Aspergillus niger were 3,5,5-
trimethyl-2-cyclohexene-1,4-dione; 3,5,5-trimethylcyclo-hexane-1,4-
dione; (S)-4-hydroxy-3,5,5-trimethyl-2-cyclohexene-1-one; and
3-hydroxymethyl-5,5-dimethyl-2-cyclohexene-1-one.
In a Die-away test, the breakdown of isophorone after 14 days was
approximately 70%, whereas 95% was broken down within 28 days
(Schöberl, 1992).
4.2.5 Bioaccumulation
Barrows et al. (1980) reported a measured bioconcentration factor
of 7 for the bluegill sunfish (Lepomis macrochirus) exposed for 14
days to a mean concentration of 92.4 (± 10.5) µg/litre. The half-life
of isophorone in the tissues of this species was 1 day. The
bioconcentration factor was expressed as the quotient of the mean
measured residues of the compound in fish tissues (whole body) during
the equilibrium period divided by the mean measured concentration of
the compound in water. The half-life of the compound in tissues was
the time in days required for mean measured residue concentration in
tissues to be reduced to half that which was measured during the
equilibrium period in the uptake phase (Barrows et al., 1980). Thus,
it is assumed that isophorone will not bioconcentrate in aquatic
organisms.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Harrison et al. (1985) identified isophorone at a level of
490 µg/kg by GC/MS in electrostatically precipitated coal fly ash,
suggesting that coal-fired power stations may be a source of emission
to the atmosphere.
5.1.2 Water
The available data on isophorone concentrations in water are
presented in Table 3.
Isophorone was detected in 1% of 795 surface water samples
(Hauser & Bromberg, 1982). Concentrations of up to 3 µg/litre were
reported in the Delaware river (Sheldon & Hites, 1979) and a
concentration of 10 µg/litre was measured in urban run-off in
Washington, DC, USA (Cole et al., 1984).
The US Environmental Protection Agency has identified isophorone
in finished drinking-water at concentrations ranging from 0.02 to
9.5 µg/litre (US EPA, 1980). A maximum concentration of 10 µg/litre
has been reported in groundwater in the Netherlands (Zoeteman et al.,
1981); the specific sources of this contamination were not identified.
Isophorone was not detected in 36 water samples during the 1981
Environmental Survey of Chemicals in Japan (Japan Environment Agency,
1983).
5.1.3 Soil and sediment
Isophorone was identified in sediment and soil taken from Love
Canal, New York, USA (Hauser & Bromberg, 1982) and in sediment taken
from Lake Pontchartrain, Louisiana, USA (McFall et al., 1985). The
concentrations in the latter were 0.9-12 µg/kg dry weight.
Isophorone was detected in 18 out of 36 bottom sediments, at
concentrations ranging from 0.6 to 6.6 µg/kg dry weight, in the 1981
Environmental Survey of Chemicals in Japan (Japan Environment Agency,
1983).
5.1.4 Terrestrial organisms
5.1.4.1 Plants
To estimate the decline of isophorone concentration in plants
treated with pesticides containing isophorone as a carrier,
14C-isophorone was sprayed on bean plants and rice at a rate
Table 3. Isophorone concentrations in water
Media Location Number Analytical Range Samples with Reference
of samples method (µg/litre) detectable
residues (%)
Surface water Delaware River, USA GC-MS 0.6-3 Hites (1979)
Delaware River, USA GC-MS 3 Sheldon & Hites (1979)
Drinking-water USA 0.02-9.5 US EPA (1980)
Philadelphia, USA 12 GC-MS 17 Suffet et al. (1980)
Ground water Netherlands 10 Zoeteman et al. (1981)
Industrial effluent manufacturing effluent GC-MS 40 Jungclaus et al. (1976)
industrial effluent GC-MS 100 Hites (1979)
treated effluent GC-MS 10 Hites (1979)
Urban run-off Washington, DC, USA 86 10 4 Cole et al. (1984)
Leachate hazardous waste landfill 8 GC-MS 29 12.5 Ghassemi et al. (1984)
equivalent to 7.5 kg/ha. Samples of the plants were taken
periodically and assayed for total radioactivity. No attempt was made
to characterize metabolites or degradation products. In bean plants
total residues declined rapidly from 16 mg/kg one hour after
application to below 0.1 mg/kg on day 42. Beans harvested on day 56
did not contain detectable radioactivity. Residues in rice plants
declined from 7.3 mg/kg one hour after application to 3.1 mg/kg on day
35 and 0.12 mg/kg on day 128. Immature rice heads did not contain
radioactivity on days 110 and 128. The relatively slow decay in rice
plants was considered by the authors to be due to unfavourable growing
conditions in this particular study (Rohm & Haas, 1972).
In a similar study, sugar beet was sprayed at the 2-leaf stage
with a herbicide containing 14C-isophorone. Total radioactivity
found on day 30 was reported to be 10% of the initial value. On day
90, residues in the plants were below 0.01 mg/kg except in dry leaves
where 0.07 mg/kg were found. The results suggested some uptake of
radiolabel from the soil from day 60 onwards; this was considered
likely to be due to the uptake of small carbon fragments or 14CO2
resulting from degradation of isophorone in the soil (Schering, 1974).
5.1.4.2 Animals
No data are available.
5.1.5 Aquatic organisms
Isophorone was found in three samples of commercial mussels
(Mytilus edulis) collected near Holbaek, Denmark, but no
concentrations were reported (Rasmussen et al., 1993).
Isophorone was detected in fish sampled from several tributary
rivers of Lake Michigan, Canada. Composite samples of several fish
carcasses were analysed using GC-MS, and the following concentration
ranges (mg/kg wet weight) were reported: common carp, < 0.02-3.13;
smallmouth bass, 0.74-3.61; largemouth bass, 0.72; small bass,
0.74-3.61; pumpkinseed, 0.4; bowfin, < 0.02-0.76; northern pike,
< 0.02-0.48; rock bass, < 0.02-1.44; and lake trout, 2.38.
Isophorone was not detected in the channel catfish sample (Camanzo et
al., 1987).
5.2 General population exposure
No data were available.
5.3 Occupational exposure
In the USA, the ACGIH (1986) have adopted a short-term (15 min)
ceiling value of 28 mg/m3 (5 ppm) and the NIOSH recommended a 10-h
TWA exposure limit of 23 mg/m3 (4 ppm) based primarily on
unpublished reports, supplied to the TLV committee, of fatigue and
malaise in workers exposed to concentrations of 28-46 mg/m3
(5-8 ppm) for 1 month. On lowering the concentration to between 5.7
and 22.8 mg/m3 (1 and 4 ppm) no further complaints were received
(ACGIH, 1986). Exposure to isophorone has been determined in a screen
printing plant, where several environmental conditions favoured
evaporation of this solvent and where working conditions increased the
risk of employee exposure. The highest exposures in screen printers
were reported to be 131 ± 31 mg/m3 (23 ± 5.4 ppm) (50-90 min TWA) in
the breathing zone of printing press workers. Other workers also
received significant exposures: paint mixers, 102 ± 31 mg/m3
(17.8 ± 5.5 ppm); manual drying, 86 ± 23 mg/m3 (15 ± 4.1 ppm);
automatic drying, 54 ± 19 mg/m3 (9.5 ± 3.3 ppm); and screen washer,
47 ± 32 mg/m3 (8.3 ± 5.6 ppm) (Samimi, 1982).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found workshift (6´ h) average
exposures of printers to isophorone of 4 and 80 mg/m3 (0.7 and
14 ppm). Symptoms of respiratory tract and eye irritation reported by
workers were attributed to the antistatic agent containing principally
isophorone.
A NIOSH evaluation (US NIOSH, 1984) conducted at another screen
printing operation in 1984 found no detectable (< 2.8 mg/m3,
0.5 ppm) exposure to isophorone.
Isophorone has been found in 6 out of 29 samples of printer's
inks from different European manufacturers. The method of analysis
was headspace gas chromatography and mass spectrometry. All of the
samples in the present study were for serigraphy on electric and
electronic articles (Rastogi, 1991).
6. KINETICS AND METABOLISM
6.1 Human
No data are available.
6.2 Laboratory mammals
Isophorone is absorbed by oral, dermal and inhalation routes.
Following a single oral administration of isophorone to rats
(4 g/kg) and rabbits (1 g/kg), the substance was distributed rapidly
in the body and detected in the stomach, pancreas, adrenals, spleen
and liver. Following inhalation (2284 mg/m3, 400 ppm for 4 h)
isophorone was detected in the kidney, adrenals, liver, pancreas and
brain of rats (Dutertre-Catella, 1976).
Strasser et al. (1988) studied the distribution of isophorone in
male rats following administration by gavage of a single dose
(3.6 mmol/kg) of 14C-isophorone (in corn oil) containing 177 µCi/kg.
Determination of radiolabel distribution of 14C-isophorone 24 h
after dosing showed that 93% of the label had been excreted in the
urine, faeces and expired air (approximate ratio 1200:1:67). The
remainder of the radiolabel was concentrated in the liver, kidney and
preputial glands, which contained 3.7, 1.1 and 0.7%, respectively, of
the original dose. The high concentration of radiolabel in the
preputial gland may have been due to the high concentrations of
alpha2u-globulin to which it could bind (see also section 7.6).
Following oral administration of isophorone to rats and rabbits,
the substance was partly eliminated unchanged in expired air and
urine; the remainder was metabolized (see Fig. 1) to:
a) 5,5-dimethyl-cyclohex-1-en-3-one-1-carboxylic acid (i), derived
from isophorone by methyl-oxidation;
b) isophorol (3,5,5-trimethyl-cyclohex-2-en-1-ol) (ii), formed by
the reduction of the ketonic group to a secondary alcohol and
eliminated as a glucuronide; and
c) dihydroisophorone (3,5,5-trimethyl-cyclohexanone) (iii),
proceeding from the hydrogenation of the cyclohexene ring, and
small quantities of cis- and trans-3,5,5-trimethyl-
cyclohexanol-1 (iv), likely to have been formed from
dihydroisophorone.
The amounts of the identified metabolites as a proportion of the
administered dose were not reported (Truhaut et al., 1970; Dutertre-
Catella et al., 1978).
A single oral isophorone dose of 500 mg/kg to male SD rats has
been reported to cause significant depletion of hepatic, testicular
and epididymal glutathione. Evidence was subsequently presented for
enhanced ethyl methane sulfonate (EMS)-induced alkylation of DNA taken
from epididymal spermatozoa (Gandy et al., 1990). Although individual
compounds were not identified, the data suggest glutathione may play a
significant role in the elimination of isophorone and its metabolites.
Quantitative data regarding the excretion of isophorone are not
available. In the study of Dutertre-Catella et al. (1978), unchanged
isophorone, isophorol (ii), dihydroisophorone (iii), 3-carboxy-5,5-
dimethyl-2-cyclohexene-1-one (i), and cis- and trans-3,5,5-
trimethyl-cyclohexanols (iv) were detected in the urine of rats and
rabbits 24 h after an oral dose of isophorone. The expired air
contained unchanged isophorone 6 h after dosing.
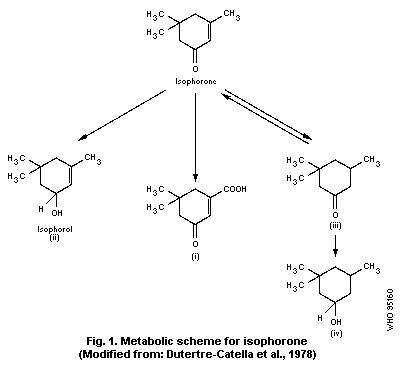 7. EFFECTS ON LABORATORY MAMMALS, AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity
The acute LD50 values for various routes of exposure are
presented in Table 4.
Table 4. Acute LD50 values for technical (commercial) grade isophorone
Route Species Sex LD50 Reference
(mg/kg)
Oral rat male and female 1500 Schering (1968)
female 2104 Smyth et al. (1969)
male 2700 Dutertre-Catella (1976)
female 2100 Dutertre-Catella (1976)
mouse male and female 2200 Dutertre-Catella (1976)
rabbit male and female 2000 Dutertre-Catella (1976)
female 2000 Smyth et al. (1969)
Dermal rat male and female 1700 Schering (1968)
rabbit male and female 1200 Dutertre-Catella (1976)
7.1.1 Oral
Median lethal doses for isophorone in laboratory mammals ranging
from 1500 to 2700 mg/kg body weight have been reported. The signs of
toxicity were similar to those of solvents and narcotics, prostration
being followed rapidly by coma. Deaths occurred within 24 h,
otherwise recovery was complete. Degenerative changes in the liver
were reported in animals that died (Dutertre-Catella, 1976).
7.1.1.1 ß-Isophorone
An oral LD50 of 2950 mg/kg in rats has been reported for
ß-isophorone (purity 97.5%, containing 2.5% alpha-isophorone). The
predominant systemic effect was non-specific CNS depression shortly
after dosing. Cirrhosis-like changes on the surface of the liver and
severe irritation of the stomach were observed in the animals that
died following dosing (Hüls, 1988d).
7.1.2 Dermal
Dermal LD50 values indicate that isophorone is rapidly absorbed
through the skin under occlusion. During the first 6 h of occluded
application an increase in respiratory rate, followed by prostration
and narcosis, was reported in rabbits exposed to 500-1000 mg/kg.
Occluded skin contact for 24 h resulted in erythema, followed after
several days by scarring. Skin damage was still evident after 14
days. In this study, 10-25 ml isophorone was applied to skin as
compared to 0.5 ml in the skin irritation test (see section 7.2.1)
(Dutertre-Catella, 1976).
7.1.3 Inhalation
Rats and guinea-pigs were exposed to atmospheres containing
isophorone concentrations of 1713 or 4282 mg/m3 (300 or 750 ppm) for
24 h, of 5025 mg/m3 (880 ppm) for 12 h, or of 7823 to 26 266 mg/m3
(1370 to 4600 ppm) for 8 h. In both animal species, in the order of
development, eye and respiratory irritation, lachrymation, ataxia,
dyspnoea, diarrhoea, light narcosis and death were observed.
Postmortem examination of rats dying after exposure to isophorone
showed haemorrhage in the lungs, congestion of the stomach and liver,
peritoneal effusion and discoloration of the kidney and spleen (Smyth
& Seaton, 1940). However, it would appear that some of the
atmospheric concentrations could not have been achieved with pure
isophorone, and therefore the results reported in the study are of
little relevance (Rowe & Wolf, 1963).
Groups of six rabbits and rats were exposed to isophorone for
5 h. The observation period was 2 weeks. At a concentration of
39.9 g/m3 (7000 ppm), 10% of the rats and 30% of the rabbits died.
No LC50 value could be established from this study (Dutertre-
Catella, 1976).
7.2 Skin, eye and respiratory irritation, sensitization
7.2.1 Skin irritation
The skin irritation of isophorone was studied by Truhaut et al.
(1972). A single application of 0.5 ml isophorone under an occlusive
patch for a period of 24 h on the shaved or scarified skin of six
rabbits produced a light erythema which disappeared rapidly after
exposure. Microscopical examination did not show any
histopathological changes.
In the rabbit, occlusive and semi-occlusive contact with 0.5 ml
neat isophorone for 1 or 4 h was non-irritating (Potokar et al.,
1985).
7.2.1.1 ß-Isophorone
A single application of 0.5 ml ß-isophorone (containing 2.5%
alpha-isophorone) under a semi-occlusive patch over a period of 4 h on
the shaved skin of three rabbits produced moderate erythema and
swelling (Hüls, 1988e).
7.2.2 Eye irritation
A single instillation of 0.1 ml isophorone in the eyes of six
rabbits caused opacity in four animals, which in some instances
covered the entire area of the cornea, inflammation of the conjunctiva
and purulent discharge. In a supplementary experiment in which the
rabbits' eyes were washed with 20 ml of warm water 2-4 seconds after
the introduction of 0.1 ml isophorone, considerable recovery from
these effects occurred over 7 days (Dutertre-Catella, 1976).
Grant (1974) reported that application of one drop of isophorone
to rabbit cornea caused mild transient injury, graded 4 on a scale of
1 to 10 after 24 h. Pronounced irritation of eyes and nose occurred
in rats and guinea-pigs exposed to atmospheres containing isophorone
(see section 7.1.3) (Smyth & Seaton, 1940; Smyth et al., 1942).
7.2.2.1 ß-Isophorone
A single instillation of 0.1 ml in the eyes of three rabbits
produced moderate conjunctival and corneal opacities. Iridial changes
were also reported in two animals. Although the corneal and iridial
changes had resolved by 7 days, minor conjunctival irritation was
still in evidence (Hüls, 1988f).
7.2.3 Respiratory irritation
In a study of sensory irritation, De Ceaurriz et al. (1981)
estimated the concentration of various chemicals causing a 50%
decrease in respiratory rate (measured using individual
plethysmographs) in mice (RD50). The RD50 for isophorone was
158.7 mg/m3 (27.8 ppm) for a 5-min exposure. For comparison, the
RD50 values for toluene diisocyanate and acetone were 0.24 and
23 480 ppm, respectively.
Exposure of rats to airborne concentrations of 383-514 mg/m3
(67 or 90 ppm) for a single 4-h period produced statistically
significant reductions in circulating leukocytes. This leukopenia was
considered to be due to the stress-induced release of cortico-steroids
resulting from sensory irritation (Brondeau et al., 1990).
7.2.4 Sensitization
Isophorone, administered at a concentration of 10% intradermally
(in maize germ oil) and 100% topically to female guinea-pigs in the
Magnusson-Kligman test, showed no sensitizing potential (Hüls, 1988a).
7.3 Subchronic toxicity
7.3.1 Inhalation
The effects of repeated whole body exposure to isophorone vapour
were reported by Smyth et al. (1942). Groups of male Wistar rats and
guinea-pigs of both sexes, 16-20 animals per group, were exposed to
isophorone concentrations up to approximately 2855 mg/m3 (500 ppm)
8 h/day, 5 days/week, for 6 weeks. As in the Smyth & Seaton (1940)
study (section 7.1.3), the air concentrations of isophorone could not
have been attained under conditions employed by Smyth et al. (1942).
In the presentation of these results no distinction was made between
the two species and no control data were presented. Growth
retardation was noted in all animals exposed to higher concentrations.
Postmortem examination of animals dying after exposure revealed
severely injured kidneys and lungs. The kidneys of surviving animals
were congested with dilation of Bowman's capsules and cloudy swelling
of tubular cells. The lungs and liver were also reported to be
congested with desquamation of the bronchial epithelium in the lungs
and cloudy swelling in the liver cells.
Dutertre-Catella (1976) exposed groups of 10 male and 10 female
rats to atmospheres reported to contain 2855 mg/m3 8 h/day, 5
days/week for 6 and 4 months, respectively. Two males and one female
exposed to isophorone died; there were no deaths in the control
animals. The only reported effects were irritation of the eyes and
nose.
Groups of 10 male and 10 female young adult Charles River CD rats
were exposed to isophorone at air concentrations of 0 or 250 mg/m3,
6 h/day, 5 days/week, for 4 weeks. Results of daily spectroscopic
determinations indicated that the average daily exposure was
208 mg/m3. Body weight measurements and haematological studies were
made before exposure and after 4 weeks. The rats were killed and
gross necropsy was performed. Organ weights were determined for
lungs, liver, kidneys, adrenals and spleen. Histological examination
of those tissues were performed in three males and three females per
group. The following effects were observed: transient nasal bleeding,
increased percentages of lymphocytes, decreased percentages of
neutrophils and increased haemoglobin concentration in males and
females and significantly lower terminal body weights and
significantly decreased absolute and relative liver weights of exposed
males, compared with controls (Littlefield, 1968).
7.3.2 Oral
Four groups of 20 male and 20 female albino rats were fed diets
containing 0, 750, 1500 or 3000 mg isophorone/kg (0, 37.5, 75 or
150 mg/kg body weight) for 90 days. Isophorone in corn oil (ratio
1:2) was blended with the diet; fresh diets were prepared each week.
Haematology, serum chemistry and urine analyses were carried out on
five animals of each sex from each group at week 4 and at termination.
Comprehensive histopathological examination was confined to five
animals of each sex from the control and high dose groups. The liver
and kidney from five animals of the intermediate dose levels were also
examined histopathologically. Under the conditions of this study, no
effects on the general appearance of the test animals, on their
behaviour, on body weight gain or on food consumption were observed at
a dietary level of 1500 mg/kg or less. Isophorone did not alter the
composition of the formed elements of the blood, nor did it interfere
with the general metabolism or with liver and kidney function. No
detectable gross or microscopic pathological changes were noted in any
of the animals examined after 28 or 90 days of feeding. Organ/body
weight ratios for vital organs were not changed. The only adverse
finding reported was a reduction in body weight gain in the male rats
receiving the highest dose (Affiliated Medical Enterprises, 1972a).
As part of a preliminary investigation prior to an NTP
carcinogenicity study, five rats (F-344) and five mice (B6C3F1) of
each sex were given by gavage 12 oral doses of up to 2000 mg
isophorone/kg body weight administered in corn oil over a 16-day
period (US NTP, 1986). Lethargy was reported in all rats following
dosing while in mice uncertain locomotion was reported among animals
receiving 1000 mg/kg. All mice and 50% of the rats receiving
2000 mg/kg died. The weight gain of animals surviving 2000 mg/kg and
all animals receiving 1000 mg/kg was reduced. As part of the same
programme, 10 rats and mice of each sex were given by gavage daily
doses of 0, 62.5, 125, 250, 500 or 1000 mg isophorone/kg body weight
for 90 days (US NTP, 1986). The rats were drowsy and lethargic
following administration. At the highest dose level, one female rat
and three female mice died. No macroscopic or microscopic changes
were observed in the organs examined from either study. A subsequent
histopathological review of the kidney, which included re-sectioning
and additional staining, also failed to reveal any treatment-related
effects. The no-observed-effect level in this study was considered to
be 500 mg/kg body weight per day.
Groups of four male and four female beagle dogs were given 90
daily oral doses of isophorone in gelatin capsules at dosage rates of
35, 75 or 150 mg/kg per day. Comprehensive haematology, clinical
chemistry and urine analyses were carried out initially and at 1, 2
and 3 months. Apart from a mild intermittent incidence of soft stools
in animals of the high-dose group, there was no observable effect as
demonstrated by the data on general appearance and conditions as well
as those from haematological or biochemical investigations. At
autopsy, no changes in the organ/body weight ratios and no
histopathological changes were observed. No toxic effect was found at
any of the dose levels used. It was concluded that the no-observed-
effect level for isophorone in the dog was 150 mg/kg per day
(Affiliated Medical Enterprises, 1972b).
7.3.3 Dermal
The daily occluded application on shaved and abraded skin of 0.1
or 0.2 ml of isophorone to rats for 8 weeks produced erythema and
scabs at the site of application (Dutertre-Catella, 1976). The only
apparent systemic effect reported was an 8% reduction in mean weight
gain in the females compared with controls; the dose levels at which
this occurred were not given.
7.4 Mutagenicity
The results of mutagenicity tests are summarized in Table 5.
7.4.1 Gene mutation in bacteria (Ames tests)
The mutagenic potential of isophorone was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100, following a preincubation protocol. The test substance
concentrations used were between 10 and 5000 µg per plate. There was
no increase in the number of revertants at any of the concentrations
tested with and without rat liver S9 fraction (Hüls, 1988b).
Two further studies were conducted on Salmonella typhimurium
TA1535, TA1537 and TA1538 strains (1 to 1000 µg/plate) (Atochem,
1978a) and TA98, TA100, TA1535 and TA1537 strains (100 to
10 000 µg/plate) (US NTP, 1986). No evidence of mutagenic potential
was provided in the presence or absence of rat or hamster liver S9
fractions.
Salmonella strains TA1535, TA1537, TA97, TA98 and TA100 were
used to test isophorone (up to 10 000 µg/plate) with and without
Aroclor 1254-induced rat and hamster metabolic activation systems
(male Sprague-Dawley rats and male Syrian hamsters). When negative
results were obtained in the initial assay, the chemicals were
retested in all strains with and without activation. Isophorone
showed no mutagenic activity (Mortelmans et al., 1986).
7.4.1.1 Dihydroisophorone
The mutagenic potential of dihydroisophorone (iii in Fig. 1, see
section 6.2), a metabolite of isophorone, was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100 at concentrations of 25 to 2500 µg per plate. There was no
increase in the number of revertants at the concentrations tested,
either with or without rat liver S9 fraction (BP, 1988a).
Table 5. Isophorone genotoxicity tests
Test system Results Reference
Gene Ames test (with and negative Atochem (1978a); US NTP
mutation without S9) (1986); Mortelmans et
al. (1986); Hüls (1988b)
mouse lymphoma assay positive US NTP (1986)
(without S9)
(with and without S9) negative Microbiological Associates
(1984a)
(with and without S9) positive McGregor et al. (1988)
(with and without S9) negative O'Donoghue et al. (1988)
positive Mitchell (1993)
sex-linked recessive negative Foureman et al. (1994)
lethal in Drosophila
melanogaster
Chromosome CHO cells (in vitro) negative US NTP (1986)
aberrations (with and without S9) negative Gulati et al. (1989)
Micronucleus mouse (in vivo) negative Atochem (1978b); Microbio-
test logical Associates (1984b);
O'Donoghue et al. (1988)
Sister CHO cells (in vitro)
chromatid (without S9) positive US NTP (1986)
exchanges (with S9) negative US NTP (1986)
(without S9) positive Gulati et al. (1989)
Direct DNA unscheduled DNA negative Microbiological Associates
damage synthesis (primary rat (1984c); O'Donoghue et al.
hepatocyte culture) (1988)
DNA binding liver and kidney DNA negative Thier et al. (1990);
in vivo (mouse and rat) Thier & Xu (1990)
Morphological BALB/c-3T3 cells positive Matthews et al. (1993)
transformation (without S9)
7.4.2 Gene mutation in mammalian cells
Isophorone was tested in the L5178Y TK +/- mouse lymphoma
mutagenesis assay (MLA) in the presence and absence of rat liver S9
fraction. The experiment was performed only once and all doses were
tested in duplicate. In the absence of S9, isophorone concentrations
of 0.13 to 1.3 ml/litre produced total growth of 111% to 12% compared
to the control. In the presence of S9, isophorone concentrations of
0.067 to 0.89 ml/litre produced total growth of 86 to 9% compared to
the control. None of these cultures exhibited mutation frequencies
which were significantly greater than the mean mutation frequency of
the solvent control (Microbiological Associates, 1984a; O'Donoghue et
al., 1988). The authors pointed out that while the results of the MLA
without metabolic activation were negative in the present study, these
assays were positive in the NTP study and thus were not reproducible
between laboratories. In contrast to O'Donoghue et al. (1988),
McGregor et al. (1988) reported isophorone to be positive in the MLA
both with and without rat liver S9 mix (from male Fischer-344 rats).
Isophorone was toxic to the cultures only at doses of 600 ml/litre or
more. The authors viewed the experiment with reservations since the
cloning efficiency was low.
Another MLA study employing concentrations of 400 to
1200 mg/litre was carried out in the absence of rat liver S9 fraction.
Duplicate experiments produced gradations of total growth relative to
control values of 112 or 118% for the low concentrations to 7 or 14%
for the high concentrations. Dose-related increases in mutation
frequencies compared with control were observed; at the highest dose
level the increase was 4-fold (US NTP, 1986).
Mitchell (1993) compared the induction of mutation frequencies by
the in situ variant (ISV) approach with the standard MLA.
Isophorone was one of the compounds tested, and showed a higher
induced mutation frequency with the ISV approach than in the standard
MLA.
Isophorone was tested for its ability to induce sex-linked
recessive lethal mutations in post-meiotic and meiotic germ cells of
male Drosophila melanogaster. No induction was observed at 2000 ppm
by feeding or at 15 000 ppm by injection (Foureman et al., 1994).
7.4.3 Chromosome aberrations and sister chromatid exchange
7.4.3.1 Chromosome aberrations
Isophorone was tested in an in vitro cytogenetic assay using
Chinese hamster ovary (CHO) cell cultures. Treatments were performed
both in the absence and presence of rat liver S9 fraction. Under the
conditions of this test, isophorone did not induce chromosomal
aberrations (ABS) at concentrations up to 1600 or 1500 mg/litre,
respectively (US NTP, 1986).
Twenty-seven chemicals (including isophorone), previously tested
in rodent carcinogenicity assays were tested for induction of ABS in
CHO cells as part of a more extensive analysis of the correlation
between results of in vitro genetic toxicity assays and
carcinogenicity bioassays. Chemicals were tested up to toxic doses
both with and without exogenous metabolic activation. A liver
fraction (S9) prepared from Aroclor 1254-induced male Sprague-Dawley
rats was used to provide exogenous metabolic activation. No ABS were
observed in CHO cells following incubation with isophorone in either
the presence or the absence of S9 (Gulati et al., 1989).
7.4.3.2 Sister chromatid exchange
The ability of isophorone to induce sister chromatid exchange
(SCE) was studied in CHO cells, in the presence and absence of rat
liver S9 fraction at concentrations up to 1000 mg isophorone/litre.
Without S9, isophorone induced a small but dose-related increase in
the frequencies of SCE. No effect was observed in the presence of S9
(US NTP, 1986).
A significant increase in SCE frequency with isophorone was
observed only in the absence of S9, at doses of 500-1000 mg/litre
(Gulati et al., 1989 - see also section 7.4.3.1). At these high dose
levels, isophorone is extremely cytostatic; therefore, the increase in
SCE frequency was observed only after delayed harvest (6-13 h
additional culture time).
7.4.4 Micronucleus test
Isophorone doses of 450, 900 and 1800 mg/kg body weight were
administered by gavage to CFLP mice of both sexes in two equal doses
separated by an interval of 24 h. Six hours after the last treatment
the mean micronucleated cell counts and the bone marrow cytotoxicity
were similar in all test groups and controls (Atochem, 1978b).
Male and female CD-1 mice were treated by intraperitoneal
injection of isophorone (0.54 ml/kg body weight). There was no
evidence of micronuclei or cytotoxicity in bone marrow samples
collected 12, 24 and 48 h after administration (Microbiological
Associates, 1984b; O'Donoghue et al., 1988).
7.4.5 Primary DNA damage
7.4.5.1 Bacterial tests
In the Bacillus subtilis (strain H17) rec-assay, isophorone
showed DNA-damaging potential without metabolic activation and a
reverse effect with activation (Matsui et al., 1989).
Isophorone did not induce the SOS function responses in the umu
test using Salmonella typhimurium strain TA1535/pSK1002 (Ono et al.,
1991).
7.4.5.2 Unscheduled DNA synthesis
Isophorone was tested at dose levels ranging from 0.005 to
0.40 ml/litre using primary rat cultures of hepatocytes. There was no
increase in the mean nuclear grain count compared to the controls or
in the incidence of cells undergoing repair at any dose level
(Microbiological Associates, 1984c; O'Donoghue et al., 1988).
7.4.5.3 DNA binding
A DNA-binding study was performed with radiolabelled 1,3,5-
14C-isophorone (Thier et al., 1990; Thier & Xu, 1990). Male and
female F-344 rats and male and female B6C3F1 mice received doses
(500 mg/kg body weight) of unlabelled isophorone containing 0.4 mCi
(per rat) and 0.8 mCi (per mouse) labelled isophorone in neutral oil
by gavage. No binding of the radioactivity to liver or kidney DNA was
observed in either species.
7.4.6 Morphological transformation
Isophorone was positive in a standard transformation assay using
BALB/c-3T3 cells without exogenous activation at concentrations of
1.07 g/litre (7.76 mmol/litre) (Matthews et al., 1993).
7.5 Chronic toxicity and carcinogenicity
In 18-month inhalation studies, groups of 10 rats and 2 rabbits
of each sex were exposed to atmospheres containing 1413 mg
isophorone/m3 (250 ppm) for 6 h/day, 5 days/week. Slight
conjunctivitis and irritation of the nasal mucosa with a bloody
discharge were observed. In the lungs of the animals, frequent
haemorrhages were found with oedema in the alveoli. Vacuolization was
found in the liver of the treated animals (Dutertre-Catella, 1976).
Toxicological and carcinogenesis studies of isophorone (more than
94% pure) were conducted by administering 0, 250 or 500 mg
isophorone/kg body weight per day by gavage in corn oil to groups of
50 F-344/N rats and 50 B6C3F1 mice of each sex, 5 days per week for
103 weeks. Throughout the 2-year study, the mean body weights of the
high-dose male rats were on average 5% less than those of the vehicle
controls. During the second year, the mean body weights of the female
high-dose rats were on average 8% less than those of the vehicle
controls, and the high-dose female mice were 5% lower. The survival
of high-dose male rats was significantly lower than that of the
vehicle controls after week 96 (final survival: vehicle control,
33/50; low dose, 33/50; high dose, 14/50). The survival of dosed
female rats was poor (30/50; 23/50; 20/50), due in part to 20 gavage-
related accidental deaths of dosed animals. The survival of male mice
was also low (16/50; 16/50; 19/50), but there was a significant trend
toward increased survival of dosed female mice relative to that of the
vehicle controls (26/50; 35/50; 34/50). Dosed male rats showed a
variety of proliferative lesions of the kidney (tubular cell
hyperplasia: 0/50; 1/50; 4/50; tubular cell adenoma: 0/50; 0/50; 2/50;
tubular cell adenocarcinoma: 0/50; 3/50; 1/50; epithelial hyperplasia
of the renal pelvis: 0/50; 5/50; 5/50). Dosed male rats also
exhibited increased mineralization of the medullary collecting ducts
(1/50; 31/50; 20/50), and low-dose male rats showed a more severe
nephropathy than is commonly seen in ageing F-344/N rats. Carcinomas
of the preputial gland were increased in high-dose male rats (0/50;
0/50; 5/50). With the exception of a moderate increase in nephropathy
(21/50; 39/50; 32/50), female rats did not show chemically related
increased incidences of neoplastic or non-neoplastic lesions. In
high-dose male mice, isophorone exposure was associated with increased
incidences of hepatocellular adenomas and carcinomas (18/48; 13/50;
29/50) and of mesenchymal tumours of the integumentary system
(fibroma, fibrosarcoma, neurofibrosarcoma or sarcoma: 6/48; 8/50;
14/50). An increased incidence of lymphomas or leukaemias was noted
in low-dose male mice (8/48; 18/50; 5/50). Coagulative necrosis
(3/48; 10/50; 11/50) and hepatocytomegaly (23/48; 39/50; 37/50) were
observed more frequently in the livers of dosed male mice than in
vehicle controls. No compound-related neoplastic or non-neoplastic
lesions associated with isophorone exposure were seen in female mice.
Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenicity of isophorone in male F-344/N rats as
shown by the occurrence of renal tubular cell adenomas and
adenocarcinomas in animals given 250 or 500 mg/kg per day; carcinomas
of the preputial gland were also observed at increased incidence in
male rats given 500 mg/kg. There was no evidence of carcinogenicity
in female F-344/N rats given 250 or 500 mg/kg per day. For male
B6C3F1 mice, there was equivocal evidence of carcinogenicity of
isophorone as shown by an increased incidence of hepatocellular
adenomas or carcinomas (combined) and of mesenchymal tumours in the
integumentary system in animals given 500 mg/kg per day and by an
increase in malignant lymphomas in animals given 250 mg/kg per day.
There was no evidence of carcinogenicity of isophorone in female
B6C3F1 mice given 250 or 500 mg/kg per day (US NTP, 1986).
In the above NTP study, preputial gland carcinomas were observed
in five high-dose male rats. The apparent absence of this tumour in
vehicle controls or in the low-dose group, and the very low incidence
(12/1094, i.e. 1%) in corn oil vehicle controls in previous 2-year
studies, suggest that this may be a chemical-related effect. No
preputial gland tumours were observed in male mice, but two
histogenetically related clitoral gland adenomas were seen in low-dose
female rats, providing some support for an association of isophorone
exposure with this tumour type (Bucher et al., 1986).
7.6 Mechanisms of toxicity
Swenberg et al. (1992) and US EPA (1991a,b) reviewed the
mechanisms involved in alpha2u-globulin nephropathy and renal
carcinogenesis of several chemicals in various animal, biochemical and
molecular modelling systems. All of the data are consistent with the
hypothesis that reversible binding of chemicals or their metabolites
to this abundant protein is causally related to the induction of
disease. Alpha2u-globulin, found mainly in male rats, is
synthesized by the liver and subsequently transported to the kidney.
It is normally present in the cytoplasm of the proximal convoluted
tubules of untreated animals in the form of hyaline droplets, visible
by light microscopy. Xenobiotics, or their metabolites, are bound to
the alpha2u-globulin in the liver of animals; this conjugate is even
more difficult to hydrolyse than the alpha2u-globulin itself and
induces the formation of the hyaline droplets which accumulate in the
tubules (Swenberg et al., 1989). Accumulation of the chemical/
alpha2u-globulin complex causes lysomal protein overload and
necrosis of the cells with subsequent cellular regeneration. Thus
cellular proliferation may contribute to the development of renal
tumours.
In order to determine whether isophorone and other compounds that
cause alpha2u-globulin to accumulate have the same binding
characteristics, binding studies were conducted with kidney cytosol
preparations from male Fischer-344 rats. The inhibition constant
value (Ki) for isophorone was in the range of 10-6 to
10-7 mol/litre, while those for d-limonene, 1,4-dichlorobenzene
and 2,5-dichlorophenol were higher (around 10-4 mol/litre). This
suggests that other factors besides binding are involved in the
accumulation of alpha2u-globulin. Results so far indicate that
binding is dependent on both hydrophobic interactions and hydrogen
bonding (Borghoff et al., 1991).
Investigations (Charbonneau et al., 1988; Strasser et al., 1988)
have shown that isophorone, isophorol and dihydroisophorone bind to
alpha2u-globulin, resulting in an increased accumulation of hyaline
droplets in renal tubular cells. These findings suggest the same
sequence of events may be responsible for the small increase in the
incidence of renal tubular neoplasias seen in male rats.
alpha2u-Globulin was not detected at significant levels in plasma
and urine of female rats or either sex of mice and humans (Swenberg et
al., 1989; Olson et al., 1990). In addition, other laboratory
rodents, dogs and primates do not develop hydrocarbon-related
nephropathy (Swenberg et al., 1989). For these reasons, it was
concluded that the low increase in the incidence of tubular adenomas
and adenocarcinomas observed in male rats following isophorone
administration was attributable to the accumulation of
alpha2u-globulin and was sex- and species-specific (Charbonneau &
Swenberg, 1988; Strasser et al., 1988; Swenberg et al., 1989).
Further evidence to support this view was gained by a study of
Dietrich & Swenberg (1991) using male animals of the NCI Black Reiter
(NBR) strain, which does not synthesize alpha2u-globulin. It was
shown that an oral dose (gavage) of 1000 mg isophorone/kg on four
consecutive days failed to produce the early features of the
alpha2u-globulin-related nephropathy syndrome (i.e. hyaline droplets
or alpha2u-globulin) in this strain.
In terms of human risk assessment, renal tubule tumours produced
in male rats in association with chemicals inducing alpha2u-globulin
accumulation should be distinguished from renal tubule tumours of
other origin (Borghoff et al., 1990; Swenberg, 1991; US EPA, 1991a,b).
It has been recognized that the induction of such tumours does not
necessarily indicate a potential carcinogenic hazard to humans. The
significance of this information with respect to the etiology of
pathological changes is uncertain at present.
Studies have shown that high levels of alpha2u-globulin and its
messenger RNA (mRNA) are present in the preputial gland of both male
and female rats. The preputial gland in both sexes contained about 3
times more alpha2u-globulin mRNA and about 300 times more
alpha2u-globulin than the male rat liver (Murty et al., 1987). It
was claimed that this high content of alpha2u-globulin was primarily
due to the cellular and ductal accumulation of the protein in the
preputial gland but did not reflect a difference in the rate of
transcription of the alpha2u-globulin gene. The high amount of
radiolabel derived from 14C-isophorone found in the preputial gland
of the male rats following single gavage dosing with 5 ml/kg (Strasser
et al., 1988) may therefore be related to its high content of
alpha2u-globulin.
7.7 Appraisal for mutagenicity/carcinogenicity
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, bone-marrow micronuclei in mice or
DNA repair in primary cultures of rat hepatocytes. No DNA binding in
vivo was observed in a DNA binding study using 1,3,5-
14C-isophorone. In one study in the absence and in another study in
the absence and presence of metabolic activation, weak mutagenic
responses were obtained in three out of five L5178Y TK +/- mouse
lymphoma mutagenesis assays and a small increase of sister chromatid
exchange (SCE) was found only without metabolic activation in CHO
cells. Another study with SCE showed no increases in chromosomal
aberrations (for details see section 7.4). Isophorone was negative in
a test for sex-linked recessive lethal mutations in Drosophila. It
induced morphological transformation in mouse cells without
activation.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound.
There was no DNA binding in the liver and kidneys (sites affected in
the carcinogenicity bioassays).
The available data on in vitro mutagenicity and related end-
points do not suggest that isophorone is genotoxic.
When tested in a two-year carcinogenicity bioassay, isophorone
produced a variety of lesions of the kidneys, such as severe
nephropathy and tubular cell hyperplasia. A low but statistically
significant increase in the frequency of renal tubular cell adenomas
and adenocarcinomas was found in male F-344 rats dosed at levels of
250 and 500 mg/kg per day (see section 7.5).
An increase in the number of carcinomas of the preputial gland
was found only in male rats given 500 mg/kg per day. In high-dose
male B6C3F1 mice hepatocellular adenomas and carcinomas were
observed. No compound related neoplastic lesions were seen in female
rats (for details, see section 7.5).
The induction of these two types of tumours in male rats may be
associated with nephropathy, epithelial hyperplasia and
alpha2u-globulin formation and accumulation of the isophorone/
alpha2u-globulin complex, causing lysomal protein overload and
necrosis of the kidney cells with subsequent cellular regeneration
(for details see section 7.6).
7.8 Reproduction, embryotoxicity, teratogenicity
The teratogenicity of isophorone to rats and mice was studied by
Traul et al. (1984). Groups of 22 confirmed mated females of each
species were exposed 6 h/day on days 6-15 of gestation to atmospheres
containing 0, 143, 285 or 656 mg/m3 (0, 25, 50 or 115 ppm)
isophorone. At the highest atmospheric concentration there was
evidence of maternal toxicity which showed as reduced food
consumption, alopecia and cervical or anogenital staining in the rats
and reduced body weights in the mice. Comprehensive uterine and fetal
examinations did not show any significant differences between animals
exposed to isophorone and their respective controls. From the results
of this study, it can be concluded that no teratogenic or fetotoxic
effect was observed with 656 mg/m3 in F-344 rats and CD-1 mice.
In a study by Dutertre-Catella (1976), groups of 10 rats of each
sex, exposed 6 h/day, 5 days/week to 2855 mg/m3 (500 ppm) isophorone
for 3 months, were mated to exposed animals or to controls. All
females were reported to have delivered 7-10 pups. Anatomo-
pathological examination did not show any abnormalities. However,
only one isophorone concentration was used, the group size was small,
and no information was provided on reproductive success and maternal
survival.
7.9 Neurotoxicity
Central nervous system depression is a characteristic feature of
isophorone intoxication in experimental animals (Smyth & Seaton, 1940;
Dutertre-Catella, 1976; De Ceaurriz et al., 1981).
Isophorone and a number of aliphatic ketones have been studied
using the mouse behavioural despair swimming test (De Ceaurriz et al.,
1984). This test, which was developed for screening antidepressant
drugs, is based on the duration of the periods of immobility exhibited
by mice when placed in water. Mice were exposed to isophorone at
atmospheric concentrations of 508-782 mg/m3 (89-137 ppm) for 4 h.
The ID50 (an estimated concentration producing a 50% reduction in
the immobility time) for animals thus exposed was 628 mg/m3
(110 ppm).
7.10 Other special studies
One of the current research approaches for assessing cytotoxicity
is to monitor the respiratory activity of the mitochondrion, a
sensitive, nonspecific subcellular target site. Detected changes in
mitochondrial function after the addition of a test chemical could be
correlated to toxic effects. In this case, rat (male, Charles River)
liver mitochondria were used, and isophorone was shown to induce no
observable effects on rat liver mitochondria respiratory activity over
the tested concentration range of 0.273-113.8 mg/litre
(1.98-825 µmol/litre) (Haubenstricker et al., 1990a).
Isophorone was tested for its ability to perturb glutathione
(GSH) levels in the testes and epididymides, as well as liver,
following single acute dosages to rats. Groups of four mature male
Sprague-Dawley rats were administered isophorone intraperitoneally
(2 ml/kg). Concurrent control groups were maintained for all time
points examined to account for possible circadian fluctuation of GSH
throughout the day. Animals were sacrificed at 1, 2, 4, 8 or 16 h
after administration of the test compound. Liver, testes and
epididymides were excised for determination of total GSH content by
spectrophotometry. Isophorone (500 mg/kg) significantly reduced GSH
in the liver and in both reproductive organs examined. The ability of
isophorone to enhance the covalent binding of tritiated ethyl
methanesulfonate (3H-EMS) to spermatocytes was assessed.
Perturbation of reproductive tract GSH by isophorone treatment
significantly enhanced the extent of 3H-EMS-induced binding to sperm
heads. The authors postulate that chemical-induced lowering of GSH in
the male reproductive tract may be a mechanism for potentiation of
chemical-induced germ-cell mutations (Gandy et al., 1990).
8. EFFECTS ON HUMANS
8.1 Acute
Groups of 11 or 12 subjects exposed for a few minutes to
atmospheric isophorone concentrations of 228 mg/m3 (40 ppm) or more
experienced irritation of the eyes, nose and throat. A few complaints
of nausea, headache, dizziness, faintness, inebriation and a feeling
of suffocation occurred at concentrations 1142 mg/m3 (> 200 ppm).
The symptoms of irritation and narcosis were said to be less intensive
following exposure to concentrations below 1142 mg/m3 (Smyth &
Seaton 1940). However, this study has been criticized because of
uncertain actual concentrations and the use of impure substances (Rowe
& Wolf, 1963).
8.2 Sub-chronic
Complaints of fatigue and malaise were reported among workers
exposed for 1 month to atmospheres containing 28.5 to 48 mg
isophorone/m3 (5 to 8 ppm) (Communication from Ware, GD, to
Chairman, TLV Committee, 1973). No further complaints were received
following a reduction of the concentration to between 5.7 and
22.8 mg/m3 (1 and 4 ppm). On the basis of these data, the American
Conference of Governmental Industrial Hygienists (ACGIH, 1986)
recommended a ceiling limit (15 min) of 29 mg/m3 (5 ppm) for
isophorone.
8.3 Irritation and sensitization
No report on skin irritation or skin sensitization has been
published.
8.3.1 Eye and respiratory irritation
Smyth & Seaton (1940) reported eye, nose and throat irritation
following exposure to isophorone levels of 228 mg/m3 (40 ppm) or
more.
Silverman et al. (1946) estimated the sensory threshold of a
number of ketones including isophorone. An average of 12 subjects of
both sexes were used for each solvent exposure. The time of exposure
was 15 min. Irritation of the eyes, nose and throat was experienced
at 142.7 mg/m3 (25 ppm) isophorone; the highest concentration
considered acceptable by the majority of subjects for an 8-h exposure
was 57.1 mg/m3 (10 ppm).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found that the workshift (6´ h)
average exposures of printers to isophorone were 4 and 80 mg/m3
(0.7 and 14 ppm). Symptoms of respiratory tract and eye irritation
reported by workers were attributed to the antistatic agent containing
principally isophorone.
Amoore & Hautala (1983) reported an air odour threshold for
isophorone of 1.14 mg/m3 (0.2 ppm). The "odour safety factor",
which was defined as the threshold limit value 28.5 mg/m3 (5 ppm)
divided by the odour threshold, was 25. From the magnitude of this
value the authors predicted that 50% of exposed persons would perceive
sensory warning of the TLV (28.5 mg/m3, 5 ppm).
8.4 Chronic toxicity and carcinogenicity
No long-term surveys have been carried out on occupationally
exposed workers or other potentially exposed populations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Yoshioka et al. (1985) studied the acute toxicity of isophorone
in Tetrahymena pyriformis. The EC50 after a 24-h exposure was
420 mg/litre. Yoshioka et al. (1986) found that the EC50 in the
Activated Sludge Respiration Inhibition Test was 100 mg/litre.
In a 2-day laboratory test with the marine red alga Champia
parvula, 38.3 mg isophorone/litre caused 50% reduction in cystocarp
formation. Above 138.5 mg/litre no cystocarps were formed, indicating
absence of sexual reproduction. In a 2-week laboratory test,
107.3 mg/litre completely inhibited cystocarp formation (Thursby &
Steele, 1986).
Isophorone was tested in air-saturated media with Saccharomyces
cerevisiae at concentrations of 0.272 to 113.7 mg/litre
(1.97-824 µmol/litre). These produced no detectable effect on the
rate of yeast respiration as measured with a dissolved oxygen
electrode (Haubenstricker et al., 1990b).
9.2 Aquatic organisms
The acute toxicity of isophorone to fish, crustaceans and algae
is summarized in Table 6. With the exception of Mysidopsis bahia
(US EPA, 1978) all acute LC50 values were above 100 mg/litre,
indicating a relatively low aquatic toxicity. This was consistent
with the results of a subacute (14 day) study with the marine red alga
Champia parvula. The toxicity of isophorone was determined by means
of various biological end-points, namely vegetative growth, formation
of tetrasporangia (asexual reproduction) and production of cystocarps
(sexual reproduction). Depending on the toxicological end-point, the
lowest concentration resulting in a significant difference from
controls ranged between 50 and 138 mg/litre (Thursby et al., 1985).
In addition to the results of acute toxicity tests shown in Table
6, an early life stage toxicity test was conducted with the freshwater
fathead minnow Pimephales promelas (Cairns & Nebeker, 1982) using
concentrations of 11, 19, 30, 56 and 112 mg isophorone/litre.
Survival was affected at a concentration of 112 mg/litre, but not at
56 mg/litre or less; fork length was affected at 30 mg/litre but not
at 19 mg/litre or less; body weight gain was decreased at 19 mg/litre
or more but not at 11 mg/litre. The authors calculated a no-observed-
effect concentration of 14 mg/litre. Using the same test with the
same species, Lemke et al. (1983) found a no-observed-effect level of
19.5 mg/litre, and in an interlaboratory (6 laboratories) comparison
no-observed-effect levels of up to 45.4 mg/litre were found (Lemke,
1983).
9.3 Terrestrial organisms
No data are available concerning the effects of isophorone on
terrestrial organisms.
Table 6. Acute toxicity of technical isophorone to aquatic species
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Fish
Bluegill sunfish LC50 96 static 220 (n) US EPA (1978)
(Lepomis macrochirus) LC50 24 static 240 (n) Buccafusco et al. (1981)
Fathead minnow LC50 96 flow 145-255 (m) Cairns & Nebeker (1982)
(Pimephales promelas) LC50 96 228 Geiger et al. (1990)
Golden orfe LC50 72 204 Scheubel & Scholz (1989)
(Leuciscus idus melanotus)
Sheepshead minnow LC50 96 140 Ward et al. (1981)
(Cyprinodon variegatus) LC50 96 static > 170, < 300 (n) Heitmuller et al. (1981)
Invertebrates
Brine shrimp LC50 24 430 Price et al. (1974)
(Artemia salina)
Mysid shrimp LC50 96 static 12.9 US EPA (1978)
(Mysidopsis bahia)
Water flea immobilization: EC50 24 static 430 (n) LeBlanc (1980)
(Daphnia magna) Immobilization: EC50 24 static 254-277 Scholz (1988a)
immobilization: EC50 48 static 120 (n) LeBlanc (1980)
immobilization: EC50 48 static 117 US EPA (1978)
Algae
Selenastrum capricornutum cell count: EC50 96 static 122 US EPA (1978)
chlorophyll: EC50 96 static 126 US EPA (1978)
Table 6 (cont'd)
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Skeletonema costatum cell count: EC50 96 static 105 US EPA (1978)
chlorophyll: EC50 96 static 110 US EPA (1978)
Scenedesmus subspicatus cell count: EC50 72 475-476 Scholz (1988b)
chlorophyll: EC50 72 524-527 Scholz (1988c)
Red alga reproduction EC50 24 38.3 Thursby & Steele (1986)
(Champia parvula)
a static = static exposure; flow = flow-through exposure
b n = nominal exposure concentration; m = measured exposure concentration
REFERENCES
ACGIH (1986) Documentation on the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
Affiliated Medical Enterprises Inc. (1972a) 90-day sub-chronic
toxicity of isophorone in the rat. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Affiliated Medical Enterprises Inc. (1972b) 90-day sub-chronic
toxicity of isophorone in the dog. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Amoore JE & Hautala E (1983) Odor as an aid for chemical safety: Odor
threshold limit values and volatilities for 214 industrial chemicals
in air and water dilution. J Appl Toxicol, 3: 272-290.
Atochem (1978a) Ames metabolic activation test to assess the potential
mutagenic effect of isophorone. Paris, Atochem (Report No. UKM
52/78204, prepared by Huntingdon Research Centre).
Atochem (1978b) Micronucleus test on isophorone. Paris, Atochem
(Report No. UKM 52/78457, prepared by Huntingdon Research Centre).
Atochem (1986) Data sheet on isophorone. Paris, Atochem.
Atochem (1988) Analytical method No. LCH 200 131/GC. Paris, Atochem.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Baser KHC, Özek T, & Tümen G (1992) Essential oils of Thymus
cariensis and Thymus haussknechtii, two endemic species in Turkey.
J Essent Oil Res, 4: 659-661.
Bartholomé E, Biekert E, Hellmann H, Ley H, Weigert M, & Weise E ed.
(1977) [Ullmann's encyclopaedia of technical chemistry], 4th ed.
Weinheim, New York, Verlag Chemie, vol 14, pp 210-211 (in German).
BIBRA (1991) Toxicity profile: Isophorone. Carshalton, United Kingdom,
The British Industrial Biological Research Association.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha-2 µ-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 349-367.
Borghoff SJ, Miller AB, Bowen JP, & Swenberg JA (1991) Characteristics
of chemical binding to alpha-2 µ-globulin in vitro - evaluating
structure-activity relationships. Toxicol Appl Pharmacol,
107: 228-238.
Borup MB & Middlebrooks EJ (1986) Photocatalyzed oxidation of organic
wastestreams. In: Toxic and hazardous wastes. Proceedings of the 18th
Mid-Atlantic Industrial Waste Conference, 29 June-1 July 1986.
Lancaster, Pennsylvania, Technimic Publishers Co., Inc., pp 554-568.
BP (1988a) Dihydroisophorone: Assessment of mutagenic potential in
histidine auxotrophs of Salmonella typhimurium (the Ames test).
Guildford, United Kingdom, BP International Ltd (LSR Report 88/0501).
BP (1988b) Material safety data sheet: Isophorone. Guildford, United
Kingdom, BP International Ltd.
Brondeau MT, Bonnet P, Guenier JP, Simon P, & De Ceaurriz J (1990)
Adrenal-dependent leucopenia after short-term exposure to various
airborne irritants in rats. J Appl Toxicol, 10(2): 83-86.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Bucher JR, Huff J, & Kluwe WM (1986) Toxicology and carcinogenesis
studies of isophorone in F344 rats and B6C3F1 mice. Toxicology,
39: 207-219.
Cairns MA & Nebeker AV (1982) Toxicity of acenaphthene and isophorone
to early life stages of fathead minnows. Arch Environ Contam Toxicol,
11: 703-707.
Callahan MA, Slimak MW, & Gabel NW (1979) Water-related environmental
fate of 129 priority pollutants. Washington, DC, US Environmental
Protection Agency (EPA 440/4-79-029B).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments. J Great Lakes Res, 13: 296-309.
Charbonneau M & Swenberg JA (1988) Studies on the biochemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT Act,
8(6): 1-5.
Charbonneau M, Strasser J, Lock EA, Turner MR, & Swenberg JA (1988)
1,4-Dichlorobenzene (1,4-DCB)-induced nephrotoxicity: Similarity with
unleaded gasoline (UG)-induced renal effects. In: Bach PH & Lock EH
ed. Nephrotoxicity: Extrapolation from in vitro to in vivo and
from animal to man. New York, London, Plenum Press.
Cheh AM (1986) Mutagen production by chlorination of methylated alpha,
ß-unsaturated ketones. Mutat Res, 169: 1-9.
Cheminfo (1988) Database of the Canadian Centre for Occupational
Health and Safety: Record No. 22E: Isophorone. Ottawa, Canada,
Canadian Centre for Occupational Health and Safety.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff programme. J Water Pollut Control Fed,
56: 898-908.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz JC, Micillino JC, Marignac B, Bonnet P, Muller J, &
Guenier JP (1984) Quantitative evaluation of sensory-irritating and
neurobehavioural properties of aliphatic ketones in mice. Food Chem
Toxicol, 22: 545-549.
Dietrich DR & Swenberg JA (1991) NCI-Black-Reiter (NBR) male rats fail
to develop renal disease following exposure to agents that induce
alpha-2 µ-globulin nephropathy. Fundam Appl Toxicol, 16: 749-762.
Dutertre-Catella H (1976) Contribution à l'étude analytique
toxicologique et biochimique de l'isophorone. Paris, Université René
Descartes (Thesis for doctorate in pharmacology).
Dutertre-Catella H, Nguyen PL, Dang Quoc Q, & Truhaut R (1978)
Transformations métaboliques de la triméthyl-3,5,5-cyclohexène-2,
one-1 (isophorone). Toxicol Eur Res, 1: 209-216.
ECETOC (1988) Concentrations of industrial organic chemicals measured
in the environment: The influence of physico-chemical properties,
tonnage and use pattern. Brussels, European Chemical Industry Ecology
and Toxicology Centre (Technical Report No. 29).
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: X. Results of 70 coded chemicals
tested for the National Toxicology Program. Environ Mol Mutagen,
23(3): 208-227.
Gandy J, Millner GC, Bates HK, Casciano DA, & Harbison RD (1990)
Effects of selected chemicals on the glutathione status in the male
reproductive system of rats. J Toxicol Environ Health, 29: 45-57.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows. Superior, Wisconsin, Center for Lake
Superior Environmental Studies, University of Wisconsin, 332 pp.
Ghassemi M, Quinlivan S, & Bachmaier J (1984) Characteristics of
leachates from hazardous waste landfills. J Environ Sci Health,
A19(5): 579-620.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Illinois,
Charles C. Thomas, pp 608-609.
Gulati DK, Witt K, Anderson B, Zeiger E, & Shelby MD (1989) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. III. Results with 27 chemicals. Environ Mol
Mutagen, 13: 133-193.
Hannah SA, Austern BM, Eralp AE, & Wise RH (1986) Comparative removal
of toxic pollutants by six wastewater treatment processes. J Water
Pollut Control Fed, 58: 27-34.
Harrison FL, Bishop DJ, & Mallon BJ (1985) Comparison of organic
combustion products in fly ash collected by a Venturi wet scrubber and
an electrostatic precipitator at a coal-fired power station. Environ
Sci Technol, 19: 186-193.
Haubenstricker ME, Holodnick SE, Mancy KH, & Brabec MJ (1990a) Rapid
toxicity testing based on mitochondrial respiratory activity. Bull
Environ Contam Toxicol, 44: 675-680.
Haubenstricker ME, Meier PG, Mancy KH, & Brabec MJ (1990b) Rapid
toxicity testing based on yeast respiratory activity. Bull Environ
Contam Toxicol, 44: 669-674.
Hauser TR & Bromberg SM (1982) EPA's monitoring program at Love Canal
1980. Environ Monit Assess, 2: 249-272.
Heitmuller PT, Hollister TA, & Parish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hites R (1979) Sources and fates of industrial organic chemicals; a
case study. Sludge Manage, 8: 107-119.
Hüls (1981) Data sheet No. 2257: Isophorone. Marl, Germany, Hüls AG.
Hüls (1988a) Test of the skin sensitizing effect of isophorone for
guinea-pig. Marl, Germany, Hüls AG (Internal report No. 1278).
Hüls (1988b) Mutagenicity of isophorone by means of the Ames
Salmonella typhimurium/microsomes mutagenicity test. Marl, Germany,
Hüls AG (Internal report No. 88/300).
Hüls (1988c) Analytical method for determining isophorone. Marl,
Germany, Hüls AG (Unpublished information).
Hüls (1988d) [Acute oral toxicity of beta-isophorone (3,5,5-
trimethyl-3-cyclohexene-1-one) for rats.] Marl, Germany, Hüls AG
(Report No. 1273) (in German).
Hüls (1988e) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the skin.] Marl,
Germany, Hüls AG (Report No. 1274) (in German).
Hüls (1988f) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the eyes and
mucosa.] Marl, Germany, Hüls AG (Report No. 1275) (in German).
Japan Environment Agency (1983) Environmental monitoring of chemicals:
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Department
of Environmental Health, Japan Environment Agency.
Jungclaus GA, Games LM, & Hites RA (1976) Identification of trace
organic compounds in tire manufacturing plant wastewaters. Anal Chem,
48: 1894-1896.
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemke AE (1983) Interlaboratory comparison of continuous flow, early
life stage testing with fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory, 26
pp (EPA-600/3-84-005; NTIS PB84-129493).
Lemke AE, Durham E, & Felhaber T (1983) Evaluation of a fathead minnow
embryo-larval test guideline using acenaphtene and isophorone. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory, 26 pp (EPA-600/3-83-062; NTIS PB83-243436).
Levin J-O & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Littlefield HA (1968) Assessment and comparison of subacute inhalation
toxicities of three ketones. Falls Church, Virginia (Unpublished
studies done for Esso Research and Engineering Company, Linden, New
Jersey).
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemistry:
Property estimation methods. New York, McGraw-Hill, pp 4-9.
Mabey WR (1981) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA-440/4-81-014).
McFall JA, Antoine SR, & DeLeon IR (1985) Base-neutral extractable
organic pollutants in biota and sediments from Lake Pontchartrain.
Chemosphere, 14(10): 1561-1569.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus subtilis/
microsome rec-assay for the detection of DNA damaging substances which
may occur in chlorinated and ozonated waters. Brighton. Water Sci
Technol, 21: 875-887.
Matthews EJ, Spalding JW, & Tennant RW (1993) Transformation of
BALB/C-3T3 cells: V. Transformation responses of 168 chemicals
compared with mutagenicity in Salmonella and carcinogenicity in
rodent bioassays. Environ Health Perspect, 101(Suppl 2): 347-482.
Microbiological Associates (1984a) L5178Y TK +/- mouse lymphoma
mutagenesis assay on isophorone. Bethesda, Maryland, Microbiological
Associates, Inc. (Report No. T2408.701005, prepared for the Chemical
Manufacturers' Association, Washington).
Microbiological Associates (1984b) Activity of isophorone in the
micronucleus cytogenetic assay in mice. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.121001, prepared
for the Chemical Manufacturers' Association, Washington).
Microbiological Associates (1984c) Unscheduled DNA synthesis in rat
primary hepatocytes with isophorone. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.380003, prepared
for the Chemical Manufacturers' Association, Washington).
Mikami Y, Fukunaga Y, Arita M, Obi Y, & Kisaki T (1981) Preparation of
aroma compounds by microbial transformation of isophorone with
Aspergillus niger. Agric Biol Chem, 45: 791-793.
Mitchell AD (1993) Does the in situ approach yield a more accurate
assessment of induced mutation frequencies than the standard L5178Y
tk+/- tk -/- mouse lymphoma assay? Environ Mol Mutagen, 21
(Suppl 22): 49.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Murty CVR, Sarkar FH, Mancini MA, & Roy AK (1987) Sex-independent
synthesis of alpha-2 µ-globulin and its messenger ribonucleic acid in
the rat preputial gland: Biochemical and immunocyto-chemical analyses.
Endocrinology, 121: 1000-1005.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putman DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Olson CT, Yu KO, & Serve MP (1986) Metabolism of cis- and trans-
decalin in F344 rats. J Toxicol Environ Health, 18: 285-292.
Olson MJ, Johnson JT, & Reidy CA (1990) A comparison of male rat and
human urinary proteins: Implications for human resistance to hyaline
droplet nephropathy. Toxicol Appl Pharmacol, 102: 524-536.
Ono Y, Somiya I, & Kawamura M (1991) The evaluation of genotoxicity
using DNA repairing test for chemicals produced in chlorination and
ozonation processes. Water Sci Technol, 23: 329-338.
Potokar M, Grundler OJ, Heusener A, Jung R, Mürmann P, Schöbel C,
Suberg H, & Zechel HJ (1985) Studies on the design of animal tests for
the corrosiveness of industrial chemicals. Food Chem Toxicol, 23:
615-617.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Rasmussen T, Anthoni U, Christophersen C, & Nielsen PH (1993) Volatile
compounds from the marine indicator organism Mytilus edulis.
Chemosphere, 27(11): 2123-2125.
Rastogi SC (1991) Levels of organic solvents in printer's inks. Arch
Environ Contam Toxicol, 20: 543-547.
Rohm & Haas (1972) A residue decline study (in bean and rice plants)
using 14C-isophorone. Philadelphia, Pennsylvania, Rohm & Haas
Company.
Rowe VK & Wolf MA (1963) Ketones. In: Patty FA ed. Industrial hygiene
and toxicology, 2nd ed. New York, Interscience, vol II, pp 1722-1724,
1763-1765.
Samimi B (1982) Exposure to isophorone and other organic solvents in a
screen printing plant. Am Ind Hyg Assoc J, 43: 43-48.
Sasaki S (1980) Transformation and biological effects. In: Aquatic
pollutants. Oxford, New York, Pergamon Press.
Schering (1968) [Isophorone - Systemic tolerability following single
administration (LD50, oral).] Berlin, Germany, Schering AG
(in German).
Schering (1974) Dissipation of isophorone in sugar beets. Berlin,
Germany, Schering AG.
Scheubel JB & Scholz N (1989) [Acute toxicity test with isophorone in
the Golden Orfe.] Marl, Germany, Hüls AG (Unpublished study No. F 120)
(in German).
Schöberl P (1992) [Determination of the biodegradability of isophorone
in the DOC-DIE AWAY test.] Marl, Germany, Hüls AG (Unpublished report)
(in German).
Scholz N (1988a) [Acute toxicity of isophorone to Daphnia magna
strauss]. Marl, Germany, Hüls AG (Unpublished study No. D 318)
(in German).
Scholz N (1988b) [Growth retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. AW 146) (in German).
Scholz N (1988c) [Assimilation retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. A 115) (in German).
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency (EPA 600/4-76-062).
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Smyth HF Jr & Seaton J (1940) Acute response of guinea-pigs and rats
to inhalation of the vapours of isophorone. J Ind Hyg Toxicol,
22: 477-483.
Smyth HF Jr, Seaton J, & Fischer L (1942) Response of guinea-pigs and
rats to repeated inhalation of vapours of mesityl oxide and
isophorone. J Ind Hyg Toxicol, 4: 46-50.
Smyth HF Jr, Weil CS, West JS, & Carpenter CP (1969) An exploration of
joint toxic action: Twenty-seven industrial chemicals intubated in
rats in all possible pairs. Toxicol Appl Pharmacol, 14: 340-347.
Strasser J Jr, Charbonneau M, Borghoff SJ, Turner MJ, & Swenberg JA
(1988) Renal protein droplet formation in male F344 rats after
isophorone (IPH) treatment. Toxicologist, 8: 136.
Suffet IH, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking water during a 2-year period. Water
Res, 14: 853-867.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residues Rev,
85: 17-28.
Swenberg JA (1991) Risk assessment of chemicals causing alpha-2
µ-globulin nephropathy. Regul Toxicol Pharmacol, 13: 1-2.
Swenberg JA, Short B, Borghoff S, Strasser J, & Charbonneau M (1989)
The comparative pathobiology of alpha-2 µ-globulin nephropathy.
Toxicol Appl Pharmacol, 97: 35-46.
Swenberg JA, Dietrich DR, McClain RM, & Cohen SM (1992) Species-
specific mechanisms of carcinogenesis. In: Vainio H, Magee PN,
McGregor DB, & McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 477-500.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981a) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981b) Biodegradability
studies for predicting the environmental fate of organic priority
pollutants. Washington, DC, Association of Official Analytical
Chemists.
Thier R, Peter HP, Wiegand HJ, & Bolt HM (1990) DNA binding study of
isophorone in rats and mice. Arch Toxicol, 64: 684-685.
Thier R & Xu DG (1990) Urinary excretion of isophorone metabolites by
male rats. Naunyn-Schmiedeberg's Arch Pharmacol, 341: R11 (Abstract
No. 41).
Thursby GB & Steele RL (1986) Comparison of short- and long-term
sexual reproduction with the marine red alga Champia parvula.
Environ Toxicol Chem, 5: 1013-1018.
Thursby GB, Steele RL, & Kane ME (1985) Effect of organic chemicals on
growth and reproduction in the marine red alga Champia parvula.
Environ Toxicol Chem, 4: 797-805.
Traul KA, Hinz JP, & Kapp RW (1984) Inhalation teratology study in
rats and mice - MRD-83-237 (isophorone) (Project No. 32377). East
Millstone, New Jersey, Bio/dynamics Inc., East Laboratory (Report
submitted to Chemical Manufacturers Association).
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1970) Premiers
résultats de l'étude du métabolisme chez le lapin d'un solvent
industriel : l'isophorone. C R Acad Sci (Paris), 271: 1333-1336.
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1972) Etude de la
toxicité d'un solvant industriel: l'isophorone - pouvoir irritant
vis-à-vis des téguments et des muqueuses. J Eur Toxicol, 5: 31-37.
US EPA (1978) Isophorone: Ambient water quality criteria. Washington,
DC, US Environmental Protection Agency (NTIS PB-296 798).
US EPA (1979) Standard operating procedure: Analysis of non volatile
extractable organic compounds. Sample extraction and screening by gas
chromatography. Method 625. Fed Reg, 44: 223.
US EPA (1980) Ambient water quality criteria for isophorone.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-056;
NTIS PB81-11767).
US EPA (1986) Graphical exposure modelling system (GEMS). Fate of
atmospheric pollutants. Washington, DC, US Environmental Protection
Agency.
US EPA (1991a) Alpha-2 µ-globulin: Association with chemically induced
renal toxicity and neoplasia in the male rat. Washington, DC, US
Environmental Protection Agency (Risk Assessment Forum).
US EPA (1991b) Report of the EPA peer review workshop on alpha-2
µ-globulin: Association with renal toxicity and neoplasia in the male
rat. Washington, DC, US Environmental Protection Agency
(EPA/625/3-91/021).
US NIOSH (1977) Manual of analytical methods: Method S367, 2nd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Cincinnati, vol 3).
US NIOSH (1980) Health hazard evaluation report HHE-80-103-827.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NIOSH (1984) Health hazard evaluation report HHE-84-299-1543.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NTP (1986) Toxicological and carcinogenesis studies of isophorone
(CAS No. 78-59-1) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, North Carolina, National Toxicology Program
(Technical Report Series No. 291).
Veith GD, Macek KJ, Betrocelli SR, & Carroll J (1978) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. Paper
presented at the 3rd Symposium on Aquatic Toxicology, New Orleans,
17-18 October 1978.
Ward GS, Parrish PR, & Rigby RA (1981) Early life stage toxicity tests
with a saltwater fish: effects of eight chemicals on survival, growth,
and development of sheepshead minnows (Cyprinodon variegatus).
J Toxicol Environ Health, 8: 225-240.
White LD, Taylor DG, Mauer PA, & Kuper RE (1970) A convenient
optimized method for the analysis of selected solvent vapours in the
industrial atmosphere. Am Ind Hyg Assoc J, 31: 225-232.
Yoshioka Y, Ose Y, & Sato T (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci Total Environ,
43: 149-157.
Yoshioka Y, Nagase H, Ose Y, & Sato T (1986) Evaluation of the test
method "activated sludge, respiration inhibition test" proposed by the
OECD. Ecotoxicol Environ Saf, 12(3): 206-212.
Zoeteman BCJ, De Greef E, & Brinkmann FJJ (1981) Persistency of
organic contaminants in groundwater; lessons from soil pollution
incidents in The Netherlands. Sci Total Environ, 21: 187-202.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés physiques et chimiques
L'isophorone est un liquide incolore d'odeur mentholée. Elle est
soluble dans l'eau (12 g/litre) et miscible à la plupart des solvants
organiques. Son point de congélation est de -8,1°C et son point
d'ébullition de 215°C. San tension de vapeur à 20°C est de l'ordre
40 Pa et sa densité de vapeur par rapport à l'air est de 4,7. C'est
une substance stable.
L'isophorone de qualité technique vendue dans le commerce
contient 1 à 3% d'isomère ß-(3,5,5-triméthyl-3-cyclohexène-1-one); la
somme des isomères alpha et ß-dépasse 99%.
1.2 Production et usage
L'isophorone est largement utilisée comme solvant pour un grand
nombre de résines et de polymères de synthèse ainsi que dans certaines
peintures spéciales et certaines encres d'imprimerie. Elle joue le
rôle de produit intermédiaire en synthèse et on l'utilise comme
solvant dans certaines formulations de pesticides.
On estime qu'en 1988, la production mondiale d'isophorone était
de l'ordre de 92 000 tonnes par an.
1.3 Transport, distribution et transformation dans l'environnement
L'isophorone peut pénétrer dans l'environnement du fait de son
utilisation par de nombreuses industries, lors du rejet de déchets ou
d'eaux usées et par suite de son utilisation comme solvant, notamment
dans les formulations de pesticides. Une fois libérée dans l'eau ou
le sol, sa volatilisation et sa biodégradation entraînent une baisse
de la concentration. L'isophorone présente dans l'atmosphère en est
éliminée par des processus photochimiques avec une demi-vie estimative
d'environ 30 minutes (selon un modèle mathématique). On a constaté
que la biodécomposition de l'isophorone atteignait environ 70% dans
les 14 jours et 95% dans les 28 jours. Les résultats des études de
biodécomposition sont variables et limités. D'après la solubilité
dans l'eau, les coefficients d'adsorption au sol et la polarité de ce
composé, il paraît improbable qu'il soit adsorbé en quantité notable
sur les matières solides en suspension et les sédiments.
Bien qu'on ait trouvé de l'isophorone dans des tissus pisciaires,
les données concernant ce composé et notamment ses propriétés
physiques et chimiques, incitent à penser qu'il a peu de chances de
subir une bioconcentration. On a mesuré une demi-vie d'un jour chez
une espèce de poisson.
1.4 Concentrations dans l'environnement et exposition humaine
On n'a pas procédé au dosage de l'isophorone dans l'air ambiant.
On a fait état d'une concentration d'isophorone dans des cendres
volantes de houille égale à 490 µg/kg. On a également mis en évidence
la présence d'isophorone dans des eaux de surface (0,6 à 3 µg/litre),
des eaux souterraines (10 µg/litre), des eaux de ruissellement
urbaines (10 µg/litre) et des eaux de lessivage de décharge
(29 µg/litre).
De l'isophorone a été trouvée à la concentration de 100 µg/litre
dans des eaux usées industrielles. Après un traitement secondaire
classique, la concentration d'isophorone dans l'effluent était tombée
à 10 µg/litre.
La présence d'isophorone a été observée dans des sédiments
lacustres (0,6 à 12 µg/kg de poids sec) ainsi que dans les tissus de
plusieurs espèces de poisson à des concentrations allant jusqu'à
3,61 µg/kg de poids frais.
On n'a pas constaté la présence d'isophorone dans les parties
comestibles de plants de haricots, de riz ou dans des betteraves à
sucre, après application sous la forme de véhicule pour pesticide.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Des études de distribution effectuées sur des rats au moyen de
14C-isophorone ont montré que 93% de la radioactivité administrée
par voie orale, réapparaissaient principalement dans les urines et
l'air expiré au bout de 24 heures. Les organes dans lesquels
subsistait au bout de cette période la plus forte concentration
d'isophorone étaient le foie, les reins et les glandes préputiales.
Après administration par voie orale d'isophorone à des lapins, on
a retrouvé dans l'urine de ces derniers des métabolites résultant
d'une oxydation du groupe méthyle en position 3, de la réduction du
groupe carbonyle et de l'hydrogénation de la double liaison du cycle
cyclohexénique; ces métabolites ont été éliminés tels quels ou sous
forme de glucuronides dans le cas des alcools.
Les valeurs de la DL50 percutanée indiquent que l'isophorone
passe rapidement à travers la peau.
1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
La toxicité aiguë de l'isophorone est faible, les valeurs de
DL50 par voie orale étant > 1500 mg/kg chez le rat, à > 2200 mg/kg
chez la souris et à > 2000 mg/kg chez le lapin. On a obtenu pour la
DL50 par voie percutanée des valeurs de 1700 mg/kg chez le rat et de
> 1200 mg/kg chez le lapin. Au niveau cutané les effets aigus d'une
exposition vont, chez le rat et le lapin, d'un léger érythème à la
formation de croûtes. On a signalé une conjonctivite et des lésions
cornéennes après instillation directe dans l'oeil ou exposition à de
fortes concentrations d'isophorone. Le test de Magnusson-Kligman
pratiqué sur des cobayes n'a pas permis de mettre en évidence un effet
de sensibilisation cutanée.
Les études de toxicité aiguë et celles au cours desquelles on a
administré de l'isophorone par voie orale pendant de brèves périodes à
des rongeurs ont montré qu'à fortes doses (> 1000 mg/kg), ce
composé provoquait des effets dégénératifs au niveau du foie ainsi
qu'une dépression du système nerveux central et une certaine
mortalité. Lors d'études de 90 jours, on a évalué à 500 mg/kg de
poids corporel, la dose quotidienne sans effets observables pour le
rat et la souris. Une autre étude de 90 jours, au cours de laquelle
on a administré de l'isophorone par voie orale à des chiens beagle (en
nombre limité), n'a révélé aucun effet à des doses quotidiennes allant
jusqu'à 150 mg/kg de poids corporel.
Lors d'études de toxicité aiguë au cours desquelles on a fait
inhaler pendant de brèves périodes de l'isophorone aux animaux de
laboratoire, on a observé une irritation oculaire et respiratoire, des
effets hématologiques et une diminution du poids corporel. Etant
donné que la conception de ces études laissait à désirer, il n'a pas
été possible de déterminer la dose sans effets observables et on ne
peut en tirer aucune conclusion en ce qui concerne la santé humaine.
L'isophorone ne provoque pas de mutations géniques chez les
bactéries ni d'aberrations chromosomiques in vitro; on n'observe pas
non plus de réparation de l'ADN dans des cultures primaires
d'hépatocytes de rat, ni la présence de micro-noyaux dans des cellules
de moelle osseuse de souris. Des effets positifs ont été observés,
lors d'essais de mutagénèse, sur des cellules de lymphomes murins
L5178Y TK +/-, mais uniquement en l'absence d'un système métabolique
exogène. Le même phénomène a été observé en ce qui concerne les
échanges de chromatides soeurs. L'isophorone a produit une
transformation morphologique in vitro, mais là encore, en l'absence
de système métabolique exogène. En revanche, elle n'a pas produit de
mutations létales récessives liées au sexe chez la drosophile. Les
données de mutagénicité ont un poids expérimental suffisant pour qu'on
puisse soutenir que l'isophorone n'est pas un composé qui réagit
énergiquement avec l'ADN. D'ailleurs, lors d'une épreuve in vivo,
on n'a pas observé de lésion de l'ADN dans des cellules hépatiques et
rénales (alors que ces organes sont ceux où l'on observe des lésions
dans les épreuves de cancérogénicité).
Les études toxicologiques au cours desquelles on a administré
pendant une longue période de l'isophorone par voie orale à des souris
et à des rats ont révélé la présence de plusieurs lésions rénales
prolifératives chez les rats males, comprenant une néphropathie, une
hyperplasie tubulaire et une faible incidence des adénomes affectant
les tubules et des adénocarcinomes. Il est admis que l'accumulation
d'alpha2u-globuline joue un rôle dans l'étiologie de ces lésions.
Etant donné que l'on n'a pas décelé chez l'homme la présence
d'alpha2u-globuline en quantités appréciables, il ne semble pas que
ce type de cancérogénèse soit à envisager dans l'espèce humaine. Chez
cinq rats males soumis à des fortes doses d'isophorone, on a observé
des carcinomes de la glande préputiale, et deux adénomes de la glande
clitoridienne ont été observés chez les rattes soumises à de faibles
doses d'isophorone. Là encore, il est possible que l'accumulation
d'alpha2u-globuline soit en cause. On a également attribué à
l'isophorone la présence d'un certain nombre de lésions néoplasiques
du foie, des téguments et du système lymphoréticulaire, observées chez
les rats males, de même d'ailleurs que des lésions bénignes du foie et
du cortex surrénalien, lésions qui n'ont cependant pas été observées
chez les souris femelles.
La seule étude d'inhalation à long terme dont on connaisse les
résultats chez le rat et le lapin, a permis d'observer une irritation
de la muqueuse oculaire et de la muqueuse nasale et des altérations au
niveau pulmonaire et hépatique, à une dose de approx.1427 mg/m3
(environ 250 ppm). Toutefois ces résultats peuvent s'expliquer par le
fait que les études en question n'offraient pas toutes les garanties
de rigueur.
Des études très limitées effectuées sur des rats et des souris
indiquent que l'isophorone n'affecte pas la fécondité ni le
développement chez les animaux de laboratoire.
Le fait qu'une dépression du système nerveux central se produise
chez les animaux de laboratoire par suite d'exposition à de
l'isophorone, pourrait être le signe d'un effet neurotoxique. Lors
d'une épreuve comportementale de nage désespérée, l'isophorone a
également produit un effet positif.
1.7 Effets sur l'homme
On peut déceler une odeur d'isophorone à une concentration ne
dépassant pas 1,14 mg/m3 (0,2 ppm). A des concentrations
inférieures à 28,55 mg/m3 (5 ppm), on a signalé une irritation des
yeux, du nez et de la gorge; au-delà de 1142 mg/m3 (200 ppm) on a
fait état de nausées, de maux de tête, d'étourdissements, de faiblesse
et de sensation ébrieuse.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
En ce qui concerne les effets aigus sur un certain nombre
d'espèces marines ou dulçaquicoles, on possède plusieurs valeurs de la
CL50. Les valeurs de CE50 à 96 heures (basées sur la numération
cellulaire et la chlorophylle) s'échelonnent de 105 à 126 mg/litre.
Pour Daphnia magna, les valeurs de la CL50 à 48 heures vont de 177
à 120 mg/litre et pour les poissons d'eau douce; celles de la CL50 à
96 heures s'étagent de 145 à 255 mg/litre.
Les valeurs de la CL50 à 96 heurs pour les invertébrés marins
vont de 12,9 à 430 mg/litre et pour une espèce de poisson de mer,
cette valeur se situe entre 170 et 300 mg/litre. Les données fournies
par les études au cours desquelles on a utilisé des concentrations
mesurées ne diffèrent pas de celles où ce sont les concentrations
nominales dont on s'est servi. Les épreuves effectuées par différents
laboratoire sur Pimephales promelas ont fait ressortir, pour la dose
sans effets nocifs observables, des valeurs allant de 14 à
45,4 mg/litre.
Les données disponibles incitent à penser que l'isophorone n'est
que faiblement toxique pour les organismes aquatiques.
2. Conclusions
2.1 Population générale
L'isophorone est utilisée comme solvant pour les résines, les
polymères et certaines formulations de pesticides. Il peut y avoir
exposition par voie cutanée ou respiratoire, mais il y a de grandes
chances pour qu'elle reste minime. Les données disponibles montrent
que l'isophorone peut être présente à des concentrations de l'ordre du
µg/litre (ou par kg) dans l'eau de boisson et dans le poisson. Les
études expérimentales ayant montré que ce composé était faiblement
toxique et du fait que l'exposition aux sources d'isophorone présentes
dans l'environnement est peu importante, on peut considérer que le
risque pour la population générale est minime.
2.2 Exposition professionnelle
Faute de contrôles techniques suffisants et de mesures d'hygiène
industrielle convenables, il est possible que l'exposition
professionnelle à l'isophorone dépasse les limites acceptables et
provoque une irritation oculaire, cutanée ou respiratoire. A plus
fortes concentrations, d'autres effets nocifs peuvent se produire. Le
groupe de travail ne disposait pas d'études sur les effets à long
terme de ce composé chez les ouvriers.
2.3 Environnement
Il est possible que l'isophorone soit libérée dans
l'environnement lorsqu'on l'utilise comme véhicule de pesticides et du
fait de son emploi généralisé comme solvant. On en a trouvé de
faibles concentrations dans plusieurs compartiments du milieu, mais sa
persistance est faible par suite des processus de biodécomposition,
volatilisation et oxydation photochimique qu'elle subit. D'après les
données disponibles, il semble que l'isophorone soit peu toxique pour
les organismes aquatiques.
3. Recommandations
3.1 Protection de la santé humaine et de l'environnement
Des précautions sont à prendre pour éviter la pollution des eaux
souterraines et de l'air.
Les travailleurs qui sont employés à la production d'isophorone
doivent se prémunir contre l'exposition à ce composé grâce à des
mesures de contrôle technique suffisantes et à des précautions
d'hygiène industrielle appropriées. L'exposition professionnelle doit
rester dans des limites acceptables et être régulièrement contrôlée.
3.2 Recherches futures
a) Surveillance médicale des travailleurs exposés.
b) Détermination de la concentration effective d'isophorone dans les
eaux à l'entour des zones industrielles.
c) Etudes d'inhalation satisfaisantes à court et à long terme sur
des animaux de laboratoire afin de déterminer les limites de
sécurité pour l'exposition professionnelle.
d) Nécessité d'obtenir des données sur la biodécomposition anaérobie
de l'isophorone, notamment du fait qu'on en a observé la présence
dans les lixiviats de décharges.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades físicas y químicas
La isoforona es un líquido incoloro con un olor parecido al de la
menta. Es soluble en agua (12 g/litro) y se mezcla con la mayoría de
disolventes orgánicos. Su punto de congelación es -8,1°C y su punto
de ebullición 215°C. Su presión en vapor a 20°C es del orden de
40 Pa, y su densidad en vapor (aire = 1) es 4,7. Es una sustancia
estable.
Las muestras comerciales de isoforona de calidad técnica
contienen 1-3% del isómero ß-isoforona (3,5,5-trimetil-3-ciclo-
hexeno-1-1); la suma de isómeros alpha y supera el 99%.
1.2 Producción y utilización
La isoforona se utiliza mucho como disolvente para cierto número
de resinas y polímeros sintéticos, así como en pinturas y tintas de
imprenta para aplicaciones especiales. Es también un producto químico
intermedio y un disolvente en determinadas formulaciones de
plaguicidas.
Se ha estimado que en 1988 su producción mundial fue del orden de
92 000 toneladas.
1.3 Transporte, distribución y transformación en el medio ambiente
La isoforona puede introducirse en el medio ambiente teniendo
como procedencia numerosas industrias, la evacuación de desechos y de
aguas residuales y a raíz de su utilización como disolvente y como
portador de plaguicida. Tras su descarga en el agua o el suelo, la
concentración ambiental disminuye a consecuencia de la volatilización
y la biodegradación. La isoforona de la atmósfera se elimina por
procesos fotoquímicos con una semivida estimada de unos 30 minutos
(sobre la base de un modelo matemático). En una prueba de estimación,
la isoforona se biodegradó hasta aproximadamente un 70% en 14 días y
un 95% en 28 días. Los resultados de los estudios de biodegradación
son variables y limitados. Los coeficientes de solubilidad en agua y
de adsorción en el suelo y la polaridad indican que es improbable que
tenga lugar una adsorción significativa por sólidos en suspensión y
sedimentos.
Aunque se ha hallado isoforona en tejidos de peces, los datos y
las propiedades fisicas y químicas parecen indicar que es improbable
una bioconcentración significativa. Se ha medido una semivida de un
día en una única especie de peces.
1.4 Niveles ambientales y exposición humana
No se ha medido isoforona en el aire ambiente. Se ha notificado
una concentración de isoforona en ceniza volátil de carbón de
490 µg/kg. Se ha identificado isoforona en aguas superficiales (0,6 a
3 µg/litro), en aguas subterráneas (10 µg/litro), en aguas de
escorrentía urbanas (10 µg/litro) y en lixiviado de terraplenados
(29 µg/litros).
Se ha hallado isoforona en aguas residuales industriales en una
concentración de 100 µg/litro. Tras un tratamiento secundario
clásico, la concentración de isoforona en el efluente fue de
10 µg/litro.
Se ha identificado isoforona en sedimentos lacustres (0,6 a
12 µg/kg de peso en seco) y en los tejidos de varias especies de peces
en concentraciones de hasta 3,61 mg/kg de peso en húmedo.
No se detectó isoforona en las partes comestibles de las plantas
del frijol, del arroz o de la remolacha azucarera tras la aplicación
de un portador de plaguicida.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
Los estudios de distribución en ratas utilizando 14C-isoforona
mostraron que el 93% de la radiactividad administrada por vía oral
aparecía principalmente en la orina y el aire espirado en 24 horas.
Los tejidos que retuvieron la mayor concentración tras ese periodo
fueron el hígado, los riñones y las glándulas prepuciales.
Los metabolitos tras administración oral de isoforona
identificados en la orina de conejos fueron resultado de la oxidación
del grupo 3-metilo, la reducción del grupo keto y la hidrogenación del
enlace doble del anillo de ciclohexeno, y se eliminaron como tales o
como derivados de glucuronida en el caso de los alcoholes.
Los valores de la DL50 percutáneas indican que la isoforona se
absorbe rápidamente a través de la piel.
1.6 Efectos en mamíferos de laboratorio y en sistemas de prueba in
vitro
La toxicidad aguda de la isoforona es baja, con valores de DL50
oral > 1500 mg/kg en la rata, > de 2200 mg/kg en el ratón y
> 2000 mg/kg en el conejo. Los valores de la DL50 cutáneas fueron
1700 mg/kg en la rata y > 1200 mg/kg en el conejo. Los efectos
agudos por exposición cutánea en ratas y conejos oscilaron entre
eritema leve y escaras. Se han notificado casos de conjuntivitis y
lesión corneana tras la aplicación directa al ojo o la exposición a
concentraciones elevadas de isoforona, pero ningún caso de
sensibilización de la piel en cobayos utilizando la prueba Magnusson-
Kligman.
En estudios de administración por vía oral sobre efectos agudos y
a corto plazo a roedores en dosis altas (> 1000 mg/kg) se
observaron efectos degenerativos en el hígado, así como depresión del
sistema nervioso central y algunas defunciones. En estudios de 90
días, se determinó un NOEL en ratas y ratones de 500 mg/kg de peso
corporal por día. En un estudio de administración por vía oral de 90
días a perros pachón (en número limitado) no se apreciaron efectos en
dosis de hasta 150 mg/kg de peso corporal por día.
En los experimentos examinados de inhalación aguda y a corto
plazo, se observaron irritación ocular y respiratoria, efectos
hematológicos y reducción del peso corporal. Como el diseño de los
estudios era inadecuado, no pudo determinarse NOEL y no puede hacerse
ninguna deducción con respecto a la salud humana.
La isoforona no induce mutaciones genéticas en bacterias,
aberraciones cromosómicas in vitro, reparación del ADN en los
hepatocitos primarios de la rata, ni micronúcleos de médula ósea en
los ratones. Se observaron efectos positivos únicamente en ausencia
de un sistema metabólico exógeno en valoraciones L5178Y TK+/- de los
mutagénesis de linfoma del ratón, así como en una valoración de
intercambio de cromátides hermanos. La isoforona indujo
transformación morfológica in vitro en ausencia de un sistema
metabólico exógeno. No indujo mutaciones recesivas letales ligadas al
sexo en Drosophila. El peso probatorio de conjunto de datos sobre
mutagenicidad avala la tesis de que la isoforona no es un potente
compuesto ADN-reactivo. En una valoración in vivo no se observó
enlace con el ADN en el hígado y los riñones (órganos afectados en las
biovaloraciones de carcinogenicidad).
En estudios de toxicidad por administración oral a largo plazo en
ratones y ratas, las ratas macho mostraron varias lesiones del riñón,
incluidas nefropatía, hiperplasia de las células tubulares y una baja
incidencia de adenomas y adenocarcinomas de ese tipo de células. Se
ha reconocido el papel que desempeña la acumulación de
alpha2u-globulina en la etiología de estas lesiones. Como no se han
detectado cantidades significativas de alpha2u-globulina en el
hombre, este mecanismo de carcinogénesis no parece ser importante en
la especie humana. Se observaron carcinomas de la glándula prepucial
en cinco ratas macho sometidas a dosis elevadas y dos adenomas de la
glándula clitorídea en ratas hembra expuestas a bajas dosis de
isoforona. Estos tumores pueden estar relacionados también con la
acumulación de alpha2u-globulina. La exposición a la isoforona se
asoció con algunas lesiones neoplásicas del hígado, y de los sistemas
integumentario y linforreticular de los ratones macho, así como con
lesiones no neoplásicas del hígado y de la corteza suprarrenal, pero
esto no se observó en los ratones hembra a los que se administraron
estas dosis.
En el único estudio a largo plazo disponible sobre inhalación en
ratas y conejos, se observaron irritación de los ojos y de la mucosa
nasal, así como cambios pulmonares y hepáticos, a dosis de
approx.1427 mg/m3 (approx. 250 ppm). Sin embargo, estos resultados
pueden haberse debido a las limitaciones del estudio.
Estudios muy limitados en ratas y ratones indican que la
isoforona no afecta a la fecundidad ni produce toxicidad para el
desarrollo en los animales de experimentación.
El hecho de que en los animales de experimentación se produzca
depresión del sistema nervioso central podría indicar un posible
efecto neurotóxico. La isoforona también produjo un efecto positivo
en la prueba de comportamiento de natación desesperada.
1.7 Efectos en el ser humano
El olor de la isoforona puede detectarse a concentraciones de
sólo 1,14 mg/m3 (0,2 ppm). Se ha observado irritación de los ojos,
la nariz y la garganta a concentraciones inferiores a 28,55 mg/m3
(5 ppm); y por encima de 1142 mg/m3 (200 ppm), náuseas, cefalea,
vértigo, desmayo y embriaguez.
1.8 Efectos en otros organismos en laboratorio y sobre el terreno
No se dispuso de datos sobre animales terrestres.
Se dispone de valores de LC50 agudos en varias especies de agua
dulce y marinas. Los valores EC50 en 96 horas (basados en recuento
celular y clorofila) oscilan entre 105 y 126 mg/litro. Los valores
LC50 en 48 horas para Daphnia magna oscilan entre 117 y
120 mg/litro, y los valores LC50 en 96 horas para peces de agua
dulce, entre 145 y 255 mg/litro.
Los valores de la LC50 en 96 horas para animales invertebrados
marinos oscilan entre 12,9 y 430 mg/litro, y el valor de la LC50 en
96 horas para una única especie de pez marino, entre 170 y
300 mg/litro. Los datos de estudios a concentraciones de exposición
medida no fueron diferentes de los obtenidos en estudios a
concentraciones nominales. Los valores NOEL para Pimephales promelas
sometidos a prueba en diferentes laboratorios oscilaron entre 14 y
45,4 mg/litro.
Los datos disponibles parecen indicar que la isoforona tiene una
baja toxicidad para los organismos acuáticos.
2. Conclusiones
2.1 Población general
La isoforona se utiliza como disolvente para formulaciones de
resinas, polímeros y plaguicidas. Puede producirse una exposición
cutánea y por inhalación, pero lo más probable es que sea
insignificante. Los datos muestran que la isoforona puede aparecer en
concentraciones de µg/litro (kg) en el agua de bebida y en los peces.
En vista de la baja toxicidad en los estudios experimentales y de los
bajos niveles de exposición a partir de fuentes ambientales, parece
ser mínimo el riesgo para la población general.
2.2 Exposición ocupacional
A falta de controles técnicos adecuados y de medidas de higiene
industrial, la exposición ocupacional a la isoforona puede superar los
niveles aceptables y producir irritación ocular, cutánea y
respiratoria. En concentraciones superiores pueden producirse otros
efectos sobre la salud. El Grupo Especial no dispuso de estudios
sobre los efectos a largo plazo en la salud de los trabajadores.
2.3 El medio ambiente
La isoforona puede pasar al medio ambiente tras su utilización
como portador de plaguicida y su uso generalizado como disolvente. Se
han identificado concentraciones bajas en varios compartimientos
ambientales, aunque tiene una baja persistencia ambiental a causa de
los procesos de biodegradación, volatilización y oxidación
fotoquímica. Los datos disponibles parecen indicar que la isoforona
tiene una baja toxicidad para los organismos acuáticos.
3. Recomendaciones
3.1 Protección de la salud humana y del medio ambiente
Hay que tener cuidado en prevenir la contaminación de las aguas
subterráneas y del aire.
Los trabajadores que fabrican o utilizan isoforona deben
protegerse de la exposición a ésta por medio de controles técnicos y
medidas de higiene industrial adecuados. Su exposición ocupacional
debe mantenerse dentro de niveles aceptables y vigilarse de forma
regular.
3.2 Investigaciones ulteriores
a) Debe procederse a la vigilancia sanitaria de los trabajadores
expuestos.
b) Deben determinarse los niveles efectivos de isoforona en las
aguas que rodean a las zonas industriales.
c) Deben realizarse estudios adecuados a corto y largo plazo sobre
inhalación en animales de experimentación para determinar los
niveles inocuos de exposición ocupacional.
d) Es necesaria información sobre la biodegradación anaerobia de la
isoforona, especialmente por haberse identificado en la
lixiviación de terraplenados.
7. EFFECTS ON LABORATORY MAMMALS, AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity
The acute LD50 values for various routes of exposure are
presented in Table 4.
Table 4. Acute LD50 values for technical (commercial) grade isophorone
Route Species Sex LD50 Reference
(mg/kg)
Oral rat male and female 1500 Schering (1968)
female 2104 Smyth et al. (1969)
male 2700 Dutertre-Catella (1976)
female 2100 Dutertre-Catella (1976)
mouse male and female 2200 Dutertre-Catella (1976)
rabbit male and female 2000 Dutertre-Catella (1976)
female 2000 Smyth et al. (1969)
Dermal rat male and female 1700 Schering (1968)
rabbit male and female 1200 Dutertre-Catella (1976)
7.1.1 Oral
Median lethal doses for isophorone in laboratory mammals ranging
from 1500 to 2700 mg/kg body weight have been reported. The signs of
toxicity were similar to those of solvents and narcotics, prostration
being followed rapidly by coma. Deaths occurred within 24 h,
otherwise recovery was complete. Degenerative changes in the liver
were reported in animals that died (Dutertre-Catella, 1976).
7.1.1.1 ß-Isophorone
An oral LD50 of 2950 mg/kg in rats has been reported for
ß-isophorone (purity 97.5%, containing 2.5% alpha-isophorone). The
predominant systemic effect was non-specific CNS depression shortly
after dosing. Cirrhosis-like changes on the surface of the liver and
severe irritation of the stomach were observed in the animals that
died following dosing (Hüls, 1988d).
7.1.2 Dermal
Dermal LD50 values indicate that isophorone is rapidly absorbed
through the skin under occlusion. During the first 6 h of occluded
application an increase in respiratory rate, followed by prostration
and narcosis, was reported in rabbits exposed to 500-1000 mg/kg.
Occluded skin contact for 24 h resulted in erythema, followed after
several days by scarring. Skin damage was still evident after 14
days. In this study, 10-25 ml isophorone was applied to skin as
compared to 0.5 ml in the skin irritation test (see section 7.2.1)
(Dutertre-Catella, 1976).
7.1.3 Inhalation
Rats and guinea-pigs were exposed to atmospheres containing
isophorone concentrations of 1713 or 4282 mg/m3 (300 or 750 ppm) for
24 h, of 5025 mg/m3 (880 ppm) for 12 h, or of 7823 to 26 266 mg/m3
(1370 to 4600 ppm) for 8 h. In both animal species, in the order of
development, eye and respiratory irritation, lachrymation, ataxia,
dyspnoea, diarrhoea, light narcosis and death were observed.
Postmortem examination of rats dying after exposure to isophorone
showed haemorrhage in the lungs, congestion of the stomach and liver,
peritoneal effusion and discoloration of the kidney and spleen (Smyth
& Seaton, 1940). However, it would appear that some of the
atmospheric concentrations could not have been achieved with pure
isophorone, and therefore the results reported in the study are of
little relevance (Rowe & Wolf, 1963).
Groups of six rabbits and rats were exposed to isophorone for
5 h. The observation period was 2 weeks. At a concentration of
39.9 g/m3 (7000 ppm), 10% of the rats and 30% of the rabbits died.
No LC50 value could be established from this study (Dutertre-
Catella, 1976).
7.2 Skin, eye and respiratory irritation, sensitization
7.2.1 Skin irritation
The skin irritation of isophorone was studied by Truhaut et al.
(1972). A single application of 0.5 ml isophorone under an occlusive
patch for a period of 24 h on the shaved or scarified skin of six
rabbits produced a light erythema which disappeared rapidly after
exposure. Microscopical examination did not show any
histopathological changes.
In the rabbit, occlusive and semi-occlusive contact with 0.5 ml
neat isophorone for 1 or 4 h was non-irritating (Potokar et al.,
1985).
7.2.1.1 ß-Isophorone
A single application of 0.5 ml ß-isophorone (containing 2.5%
alpha-isophorone) under a semi-occlusive patch over a period of 4 h on
the shaved skin of three rabbits produced moderate erythema and
swelling (Hüls, 1988e).
7.2.2 Eye irritation
A single instillation of 0.1 ml isophorone in the eyes of six
rabbits caused opacity in four animals, which in some instances
covered the entire area of the cornea, inflammation of the conjunctiva
and purulent discharge. In a supplementary experiment in which the
rabbits' eyes were washed with 20 ml of warm water 2-4 seconds after
the introduction of 0.1 ml isophorone, considerable recovery from
these effects occurred over 7 days (Dutertre-Catella, 1976).
Grant (1974) reported that application of one drop of isophorone
to rabbit cornea caused mild transient injury, graded 4 on a scale of
1 to 10 after 24 h. Pronounced irritation of eyes and nose occurred
in rats and guinea-pigs exposed to atmospheres containing isophorone
(see section 7.1.3) (Smyth & Seaton, 1940; Smyth et al., 1942).
7.2.2.1 ß-Isophorone
A single instillation of 0.1 ml in the eyes of three rabbits
produced moderate conjunctival and corneal opacities. Iridial changes
were also reported in two animals. Although the corneal and iridial
changes had resolved by 7 days, minor conjunctival irritation was
still in evidence (Hüls, 1988f).
7.2.3 Respiratory irritation
In a study of sensory irritation, De Ceaurriz et al. (1981)
estimated the concentration of various chemicals causing a 50%
decrease in respiratory rate (measured using individual
plethysmographs) in mice (RD50). The RD50 for isophorone was
158.7 mg/m3 (27.8 ppm) for a 5-min exposure. For comparison, the
RD50 values for toluene diisocyanate and acetone were 0.24 and
23 480 ppm, respectively.
Exposure of rats to airborne concentrations of 383-514 mg/m3
(67 or 90 ppm) for a single 4-h period produced statistically
significant reductions in circulating leukocytes. This leukopenia was
considered to be due to the stress-induced release of cortico-steroids
resulting from sensory irritation (Brondeau et al., 1990).
7.2.4 Sensitization
Isophorone, administered at a concentration of 10% intradermally
(in maize germ oil) and 100% topically to female guinea-pigs in the
Magnusson-Kligman test, showed no sensitizing potential (Hüls, 1988a).
7.3 Subchronic toxicity
7.3.1 Inhalation
The effects of repeated whole body exposure to isophorone vapour
were reported by Smyth et al. (1942). Groups of male Wistar rats and
guinea-pigs of both sexes, 16-20 animals per group, were exposed to
isophorone concentrations up to approximately 2855 mg/m3 (500 ppm)
8 h/day, 5 days/week, for 6 weeks. As in the Smyth & Seaton (1940)
study (section 7.1.3), the air concentrations of isophorone could not
have been attained under conditions employed by Smyth et al. (1942).
In the presentation of these results no distinction was made between
the two species and no control data were presented. Growth
retardation was noted in all animals exposed to higher concentrations.
Postmortem examination of animals dying after exposure revealed
severely injured kidneys and lungs. The kidneys of surviving animals
were congested with dilation of Bowman's capsules and cloudy swelling
of tubular cells. The lungs and liver were also reported to be
congested with desquamation of the bronchial epithelium in the lungs
and cloudy swelling in the liver cells.
Dutertre-Catella (1976) exposed groups of 10 male and 10 female
rats to atmospheres reported to contain 2855 mg/m3 8 h/day, 5
days/week for 6 and 4 months, respectively. Two males and one female
exposed to isophorone died; there were no deaths in the control
animals. The only reported effects were irritation of the eyes and
nose.
Groups of 10 male and 10 female young adult Charles River CD rats
were exposed to isophorone at air concentrations of 0 or 250 mg/m3,
6 h/day, 5 days/week, for 4 weeks. Results of daily spectroscopic
determinations indicated that the average daily exposure was
208 mg/m3. Body weight measurements and haematological studies were
made before exposure and after 4 weeks. The rats were killed and
gross necropsy was performed. Organ weights were determined for
lungs, liver, kidneys, adrenals and spleen. Histological examination
of those tissues were performed in three males and three females per
group. The following effects were observed: transient nasal bleeding,
increased percentages of lymphocytes, decreased percentages of
neutrophils and increased haemoglobin concentration in males and
females and significantly lower terminal body weights and
significantly decreased absolute and relative liver weights of exposed
males, compared with controls (Littlefield, 1968).
7.3.2 Oral
Four groups of 20 male and 20 female albino rats were fed diets
containing 0, 750, 1500 or 3000 mg isophorone/kg (0, 37.5, 75 or
150 mg/kg body weight) for 90 days. Isophorone in corn oil (ratio
1:2) was blended with the diet; fresh diets were prepared each week.
Haematology, serum chemistry and urine analyses were carried out on
five animals of each sex from each group at week 4 and at termination.
Comprehensive histopathological examination was confined to five
animals of each sex from the control and high dose groups. The liver
and kidney from five animals of the intermediate dose levels were also
examined histopathologically. Under the conditions of this study, no
effects on the general appearance of the test animals, on their
behaviour, on body weight gain or on food consumption were observed at
a dietary level of 1500 mg/kg or less. Isophorone did not alter the
composition of the formed elements of the blood, nor did it interfere
with the general metabolism or with liver and kidney function. No
detectable gross or microscopic pathological changes were noted in any
of the animals examined after 28 or 90 days of feeding. Organ/body
weight ratios for vital organs were not changed. The only adverse
finding reported was a reduction in body weight gain in the male rats
receiving the highest dose (Affiliated Medical Enterprises, 1972a).
As part of a preliminary investigation prior to an NTP
carcinogenicity study, five rats (F-344) and five mice (B6C3F1) of
each sex were given by gavage 12 oral doses of up to 2000 mg
isophorone/kg body weight administered in corn oil over a 16-day
period (US NTP, 1986). Lethargy was reported in all rats following
dosing while in mice uncertain locomotion was reported among animals
receiving 1000 mg/kg. All mice and 50% of the rats receiving
2000 mg/kg died. The weight gain of animals surviving 2000 mg/kg and
all animals receiving 1000 mg/kg was reduced. As part of the same
programme, 10 rats and mice of each sex were given by gavage daily
doses of 0, 62.5, 125, 250, 500 or 1000 mg isophorone/kg body weight
for 90 days (US NTP, 1986). The rats were drowsy and lethargic
following administration. At the highest dose level, one female rat
and three female mice died. No macroscopic or microscopic changes
were observed in the organs examined from either study. A subsequent
histopathological review of the kidney, which included re-sectioning
and additional staining, also failed to reveal any treatment-related
effects. The no-observed-effect level in this study was considered to
be 500 mg/kg body weight per day.
Groups of four male and four female beagle dogs were given 90
daily oral doses of isophorone in gelatin capsules at dosage rates of
35, 75 or 150 mg/kg per day. Comprehensive haematology, clinical
chemistry and urine analyses were carried out initially and at 1, 2
and 3 months. Apart from a mild intermittent incidence of soft stools
in animals of the high-dose group, there was no observable effect as
demonstrated by the data on general appearance and conditions as well
as those from haematological or biochemical investigations. At
autopsy, no changes in the organ/body weight ratios and no
histopathological changes were observed. No toxic effect was found at
any of the dose levels used. It was concluded that the no-observed-
effect level for isophorone in the dog was 150 mg/kg per day
(Affiliated Medical Enterprises, 1972b).
7.3.3 Dermal
The daily occluded application on shaved and abraded skin of 0.1
or 0.2 ml of isophorone to rats for 8 weeks produced erythema and
scabs at the site of application (Dutertre-Catella, 1976). The only
apparent systemic effect reported was an 8% reduction in mean weight
gain in the females compared with controls; the dose levels at which
this occurred were not given.
7.4 Mutagenicity
The results of mutagenicity tests are summarized in Table 5.
7.4.1 Gene mutation in bacteria (Ames tests)
The mutagenic potential of isophorone was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100, following a preincubation protocol. The test substance
concentrations used were between 10 and 5000 µg per plate. There was
no increase in the number of revertants at any of the concentrations
tested with and without rat liver S9 fraction (Hüls, 1988b).
Two further studies were conducted on Salmonella typhimurium
TA1535, TA1537 and TA1538 strains (1 to 1000 µg/plate) (Atochem,
1978a) and TA98, TA100, TA1535 and TA1537 strains (100 to
10 000 µg/plate) (US NTP, 1986). No evidence of mutagenic potential
was provided in the presence or absence of rat or hamster liver S9
fractions.
Salmonella strains TA1535, TA1537, TA97, TA98 and TA100 were
used to test isophorone (up to 10 000 µg/plate) with and without
Aroclor 1254-induced rat and hamster metabolic activation systems
(male Sprague-Dawley rats and male Syrian hamsters). When negative
results were obtained in the initial assay, the chemicals were
retested in all strains with and without activation. Isophorone
showed no mutagenic activity (Mortelmans et al., 1986).
7.4.1.1 Dihydroisophorone
The mutagenic potential of dihydroisophorone (iii in Fig. 1, see
section 6.2), a metabolite of isophorone, was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100 at concentrations of 25 to 2500 µg per plate. There was no
increase in the number of revertants at the concentrations tested,
either with or without rat liver S9 fraction (BP, 1988a).
Table 5. Isophorone genotoxicity tests
Test system Results Reference
Gene Ames test (with and negative Atochem (1978a); US NTP
mutation without S9) (1986); Mortelmans et
al. (1986); Hüls (1988b)
mouse lymphoma assay positive US NTP (1986)
(without S9)
(with and without S9) negative Microbiological Associates
(1984a)
(with and without S9) positive McGregor et al. (1988)
(with and without S9) negative O'Donoghue et al. (1988)
positive Mitchell (1993)
sex-linked recessive negative Foureman et al. (1994)
lethal in Drosophila
melanogaster
Chromosome CHO cells (in vitro) negative US NTP (1986)
aberrations (with and without S9) negative Gulati et al. (1989)
Micronucleus mouse (in vivo) negative Atochem (1978b); Microbio-
test logical Associates (1984b);
O'Donoghue et al. (1988)
Sister CHO cells (in vitro)
chromatid (without S9) positive US NTP (1986)
exchanges (with S9) negative US NTP (1986)
(without S9) positive Gulati et al. (1989)
Direct DNA unscheduled DNA negative Microbiological Associates
damage synthesis (primary rat (1984c); O'Donoghue et al.
hepatocyte culture) (1988)
DNA binding liver and kidney DNA negative Thier et al. (1990);
in vivo (mouse and rat) Thier & Xu (1990)
Morphological BALB/c-3T3 cells positive Matthews et al. (1993)
transformation (without S9)
7.4.2 Gene mutation in mammalian cells
Isophorone was tested in the L5178Y TK +/- mouse lymphoma
mutagenesis assay (MLA) in the presence and absence of rat liver S9
fraction. The experiment was performed only once and all doses were
tested in duplicate. In the absence of S9, isophorone concentrations
of 0.13 to 1.3 ml/litre produced total growth of 111% to 12% compared
to the control. In the presence of S9, isophorone concentrations of
0.067 to 0.89 ml/litre produced total growth of 86 to 9% compared to
the control. None of these cultures exhibited mutation frequencies
which were significantly greater than the mean mutation frequency of
the solvent control (Microbiological Associates, 1984a; O'Donoghue et
al., 1988). The authors pointed out that while the results of the MLA
without metabolic activation were negative in the present study, these
assays were positive in the NTP study and thus were not reproducible
between laboratories. In contrast to O'Donoghue et al. (1988),
McGregor et al. (1988) reported isophorone to be positive in the MLA
both with and without rat liver S9 mix (from male Fischer-344 rats).
Isophorone was toxic to the cultures only at doses of 600 ml/litre or
more. The authors viewed the experiment with reservations since the
cloning efficiency was low.
Another MLA study employing concentrations of 400 to
1200 mg/litre was carried out in the absence of rat liver S9 fraction.
Duplicate experiments produced gradations of total growth relative to
control values of 112 or 118% for the low concentrations to 7 or 14%
for the high concentrations. Dose-related increases in mutation
frequencies compared with control were observed; at the highest dose
level the increase was 4-fold (US NTP, 1986).
Mitchell (1993) compared the induction of mutation frequencies by
the in situ variant (ISV) approach with the standard MLA.
Isophorone was one of the compounds tested, and showed a higher
induced mutation frequency with the ISV approach than in the standard
MLA.
Isophorone was tested for its ability to induce sex-linked
recessive lethal mutations in post-meiotic and meiotic germ cells of
male Drosophila melanogaster. No induction was observed at 2000 ppm
by feeding or at 15 000 ppm by injection (Foureman et al., 1994).
7.4.3 Chromosome aberrations and sister chromatid exchange
7.4.3.1 Chromosome aberrations
Isophorone was tested in an in vitro cytogenetic assay using
Chinese hamster ovary (CHO) cell cultures. Treatments were performed
both in the absence and presence of rat liver S9 fraction. Under the
conditions of this test, isophorone did not induce chromosomal
aberrations (ABS) at concentrations up to 1600 or 1500 mg/litre,
respectively (US NTP, 1986).
Twenty-seven chemicals (including isophorone), previously tested
in rodent carcinogenicity assays were tested for induction of ABS in
CHO cells as part of a more extensive analysis of the correlation
between results of in vitro genetic toxicity assays and
carcinogenicity bioassays. Chemicals were tested up to toxic doses
both with and without exogenous metabolic activation. A liver
fraction (S9) prepared from Aroclor 1254-induced male Sprague-Dawley
rats was used to provide exogenous metabolic activation. No ABS were
observed in CHO cells following incubation with isophorone in either
the presence or the absence of S9 (Gulati et al., 1989).
7.4.3.2 Sister chromatid exchange
The ability of isophorone to induce sister chromatid exchange
(SCE) was studied in CHO cells, in the presence and absence of rat
liver S9 fraction at concentrations up to 1000 mg isophorone/litre.
Without S9, isophorone induced a small but dose-related increase in
the frequencies of SCE. No effect was observed in the presence of S9
(US NTP, 1986).
A significant increase in SCE frequency with isophorone was
observed only in the absence of S9, at doses of 500-1000 mg/litre
(Gulati et al., 1989 - see also section 7.4.3.1). At these high dose
levels, isophorone is extremely cytostatic; therefore, the increase in
SCE frequency was observed only after delayed harvest (6-13 h
additional culture time).
7.4.4 Micronucleus test
Isophorone doses of 450, 900 and 1800 mg/kg body weight were
administered by gavage to CFLP mice of both sexes in two equal doses
separated by an interval of 24 h. Six hours after the last treatment
the mean micronucleated cell counts and the bone marrow cytotoxicity
were similar in all test groups and controls (Atochem, 1978b).
Male and female CD-1 mice were treated by intraperitoneal
injection of isophorone (0.54 ml/kg body weight). There was no
evidence of micronuclei or cytotoxicity in bone marrow samples
collected 12, 24 and 48 h after administration (Microbiological
Associates, 1984b; O'Donoghue et al., 1988).
7.4.5 Primary DNA damage
7.4.5.1 Bacterial tests
In the Bacillus subtilis (strain H17) rec-assay, isophorone
showed DNA-damaging potential without metabolic activation and a
reverse effect with activation (Matsui et al., 1989).
Isophorone did not induce the SOS function responses in the umu
test using Salmonella typhimurium strain TA1535/pSK1002 (Ono et al.,
1991).
7.4.5.2 Unscheduled DNA synthesis
Isophorone was tested at dose levels ranging from 0.005 to
0.40 ml/litre using primary rat cultures of hepatocytes. There was no
increase in the mean nuclear grain count compared to the controls or
in the incidence of cells undergoing repair at any dose level
(Microbiological Associates, 1984c; O'Donoghue et al., 1988).
7.4.5.3 DNA binding
A DNA-binding study was performed with radiolabelled 1,3,5-
14C-isophorone (Thier et al., 1990; Thier & Xu, 1990). Male and
female F-344 rats and male and female B6C3F1 mice received doses
(500 mg/kg body weight) of unlabelled isophorone containing 0.4 mCi
(per rat) and 0.8 mCi (per mouse) labelled isophorone in neutral oil
by gavage. No binding of the radioactivity to liver or kidney DNA was
observed in either species.
7.4.6 Morphological transformation
Isophorone was positive in a standard transformation assay using
BALB/c-3T3 cells without exogenous activation at concentrations of
1.07 g/litre (7.76 mmol/litre) (Matthews et al., 1993).
7.5 Chronic toxicity and carcinogenicity
In 18-month inhalation studies, groups of 10 rats and 2 rabbits
of each sex were exposed to atmospheres containing 1413 mg
isophorone/m3 (250 ppm) for 6 h/day, 5 days/week. Slight
conjunctivitis and irritation of the nasal mucosa with a bloody
discharge were observed. In the lungs of the animals, frequent
haemorrhages were found with oedema in the alveoli. Vacuolization was
found in the liver of the treated animals (Dutertre-Catella, 1976).
Toxicological and carcinogenesis studies of isophorone (more than
94% pure) were conducted by administering 0, 250 or 500 mg
isophorone/kg body weight per day by gavage in corn oil to groups of
50 F-344/N rats and 50 B6C3F1 mice of each sex, 5 days per week for
103 weeks. Throughout the 2-year study, the mean body weights of the
high-dose male rats were on average 5% less than those of the vehicle
controls. During the second year, the mean body weights of the female
high-dose rats were on average 8% less than those of the vehicle
controls, and the high-dose female mice were 5% lower. The survival
of high-dose male rats was significantly lower than that of the
vehicle controls after week 96 (final survival: vehicle control,
33/50; low dose, 33/50; high dose, 14/50). The survival of dosed
female rats was poor (30/50; 23/50; 20/50), due in part to 20 gavage-
related accidental deaths of dosed animals. The survival of male mice
was also low (16/50; 16/50; 19/50), but there was a significant trend
toward increased survival of dosed female mice relative to that of the
vehicle controls (26/50; 35/50; 34/50). Dosed male rats showed a
variety of proliferative lesions of the kidney (tubular cell
hyperplasia: 0/50; 1/50; 4/50; tubular cell adenoma: 0/50; 0/50; 2/50;
tubular cell adenocarcinoma: 0/50; 3/50; 1/50; epithelial hyperplasia
of the renal pelvis: 0/50; 5/50; 5/50). Dosed male rats also
exhibited increased mineralization of the medullary collecting ducts
(1/50; 31/50; 20/50), and low-dose male rats showed a more severe
nephropathy than is commonly seen in ageing F-344/N rats. Carcinomas
of the preputial gland were increased in high-dose male rats (0/50;
0/50; 5/50). With the exception of a moderate increase in nephropathy
(21/50; 39/50; 32/50), female rats did not show chemically related
increased incidences of neoplastic or non-neoplastic lesions. In
high-dose male mice, isophorone exposure was associated with increased
incidences of hepatocellular adenomas and carcinomas (18/48; 13/50;
29/50) and of mesenchymal tumours of the integumentary system
(fibroma, fibrosarcoma, neurofibrosarcoma or sarcoma: 6/48; 8/50;
14/50). An increased incidence of lymphomas or leukaemias was noted
in low-dose male mice (8/48; 18/50; 5/50). Coagulative necrosis
(3/48; 10/50; 11/50) and hepatocytomegaly (23/48; 39/50; 37/50) were
observed more frequently in the livers of dosed male mice than in
vehicle controls. No compound-related neoplastic or non-neoplastic
lesions associated with isophorone exposure were seen in female mice.
Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenicity of isophorone in male F-344/N rats as
shown by the occurrence of renal tubular cell adenomas and
adenocarcinomas in animals given 250 or 500 mg/kg per day; carcinomas
of the preputial gland were also observed at increased incidence in
male rats given 500 mg/kg. There was no evidence of carcinogenicity
in female F-344/N rats given 250 or 500 mg/kg per day. For male
B6C3F1 mice, there was equivocal evidence of carcinogenicity of
isophorone as shown by an increased incidence of hepatocellular
adenomas or carcinomas (combined) and of mesenchymal tumours in the
integumentary system in animals given 500 mg/kg per day and by an
increase in malignant lymphomas in animals given 250 mg/kg per day.
There was no evidence of carcinogenicity of isophorone in female
B6C3F1 mice given 250 or 500 mg/kg per day (US NTP, 1986).
In the above NTP study, preputial gland carcinomas were observed
in five high-dose male rats. The apparent absence of this tumour in
vehicle controls or in the low-dose group, and the very low incidence
(12/1094, i.e. 1%) in corn oil vehicle controls in previous 2-year
studies, suggest that this may be a chemical-related effect. No
preputial gland tumours were observed in male mice, but two
histogenetically related clitoral gland adenomas were seen in low-dose
female rats, providing some support for an association of isophorone
exposure with this tumour type (Bucher et al., 1986).
7.6 Mechanisms of toxicity
Swenberg et al. (1992) and US EPA (1991a,b) reviewed the
mechanisms involved in alpha2u-globulin nephropathy and renal
carcinogenesis of several chemicals in various animal, biochemical and
molecular modelling systems. All of the data are consistent with the
hypothesis that reversible binding of chemicals or their metabolites
to this abundant protein is causally related to the induction of
disease. Alpha2u-globulin, found mainly in male rats, is
synthesized by the liver and subsequently transported to the kidney.
It is normally present in the cytoplasm of the proximal convoluted
tubules of untreated animals in the form of hyaline droplets, visible
by light microscopy. Xenobiotics, or their metabolites, are bound to
the alpha2u-globulin in the liver of animals; this conjugate is even
more difficult to hydrolyse than the alpha2u-globulin itself and
induces the formation of the hyaline droplets which accumulate in the
tubules (Swenberg et al., 1989). Accumulation of the chemical/
alpha2u-globulin complex causes lysomal protein overload and
necrosis of the cells with subsequent cellular regeneration. Thus
cellular proliferation may contribute to the development of renal
tumours.
In order to determine whether isophorone and other compounds that
cause alpha2u-globulin to accumulate have the same binding
characteristics, binding studies were conducted with kidney cytosol
preparations from male Fischer-344 rats. The inhibition constant
value (Ki) for isophorone was in the range of 10-6 to
10-7 mol/litre, while those for d-limonene, 1,4-dichlorobenzene
and 2,5-dichlorophenol were higher (around 10-4 mol/litre). This
suggests that other factors besides binding are involved in the
accumulation of alpha2u-globulin. Results so far indicate that
binding is dependent on both hydrophobic interactions and hydrogen
bonding (Borghoff et al., 1991).
Investigations (Charbonneau et al., 1988; Strasser et al., 1988)
have shown that isophorone, isophorol and dihydroisophorone bind to
alpha2u-globulin, resulting in an increased accumulation of hyaline
droplets in renal tubular cells. These findings suggest the same
sequence of events may be responsible for the small increase in the
incidence of renal tubular neoplasias seen in male rats.
alpha2u-Globulin was not detected at significant levels in plasma
and urine of female rats or either sex of mice and humans (Swenberg et
al., 1989; Olson et al., 1990). In addition, other laboratory
rodents, dogs and primates do not develop hydrocarbon-related
nephropathy (Swenberg et al., 1989). For these reasons, it was
concluded that the low increase in the incidence of tubular adenomas
and adenocarcinomas observed in male rats following isophorone
administration was attributable to the accumulation of
alpha2u-globulin and was sex- and species-specific (Charbonneau &
Swenberg, 1988; Strasser et al., 1988; Swenberg et al., 1989).
Further evidence to support this view was gained by a study of
Dietrich & Swenberg (1991) using male animals of the NCI Black Reiter
(NBR) strain, which does not synthesize alpha2u-globulin. It was
shown that an oral dose (gavage) of 1000 mg isophorone/kg on four
consecutive days failed to produce the early features of the
alpha2u-globulin-related nephropathy syndrome (i.e. hyaline droplets
or alpha2u-globulin) in this strain.
In terms of human risk assessment, renal tubule tumours produced
in male rats in association with chemicals inducing alpha2u-globulin
accumulation should be distinguished from renal tubule tumours of
other origin (Borghoff et al., 1990; Swenberg, 1991; US EPA, 1991a,b).
It has been recognized that the induction of such tumours does not
necessarily indicate a potential carcinogenic hazard to humans. The
significance of this information with respect to the etiology of
pathological changes is uncertain at present.
Studies have shown that high levels of alpha2u-globulin and its
messenger RNA (mRNA) are present in the preputial gland of both male
and female rats. The preputial gland in both sexes contained about 3
times more alpha2u-globulin mRNA and about 300 times more
alpha2u-globulin than the male rat liver (Murty et al., 1987). It
was claimed that this high content of alpha2u-globulin was primarily
due to the cellular and ductal accumulation of the protein in the
preputial gland but did not reflect a difference in the rate of
transcription of the alpha2u-globulin gene. The high amount of
radiolabel derived from 14C-isophorone found in the preputial gland
of the male rats following single gavage dosing with 5 ml/kg (Strasser
et al., 1988) may therefore be related to its high content of
alpha2u-globulin.
7.7 Appraisal for mutagenicity/carcinogenicity
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, bone-marrow micronuclei in mice or
DNA repair in primary cultures of rat hepatocytes. No DNA binding in
vivo was observed in a DNA binding study using 1,3,5-
14C-isophorone. In one study in the absence and in another study in
the absence and presence of metabolic activation, weak mutagenic
responses were obtained in three out of five L5178Y TK +/- mouse
lymphoma mutagenesis assays and a small increase of sister chromatid
exchange (SCE) was found only without metabolic activation in CHO
cells. Another study with SCE showed no increases in chromosomal
aberrations (for details see section 7.4). Isophorone was negative in
a test for sex-linked recessive lethal mutations in Drosophila. It
induced morphological transformation in mouse cells without
activation.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound.
There was no DNA binding in the liver and kidneys (sites affected in
the carcinogenicity bioassays).
The available data on in vitro mutagenicity and related end-
points do not suggest that isophorone is genotoxic.
When tested in a two-year carcinogenicity bioassay, isophorone
produced a variety of lesions of the kidneys, such as severe
nephropathy and tubular cell hyperplasia. A low but statistically
significant increase in the frequency of renal tubular cell adenomas
and adenocarcinomas was found in male F-344 rats dosed at levels of
250 and 500 mg/kg per day (see section 7.5).
An increase in the number of carcinomas of the preputial gland
was found only in male rats given 500 mg/kg per day. In high-dose
male B6C3F1 mice hepatocellular adenomas and carcinomas were
observed. No compound related neoplastic lesions were seen in female
rats (for details, see section 7.5).
The induction of these two types of tumours in male rats may be
associated with nephropathy, epithelial hyperplasia and
alpha2u-globulin formation and accumulation of the isophorone/
alpha2u-globulin complex, causing lysomal protein overload and
necrosis of the kidney cells with subsequent cellular regeneration
(for details see section 7.6).
7.8 Reproduction, embryotoxicity, teratogenicity
The teratogenicity of isophorone to rats and mice was studied by
Traul et al. (1984). Groups of 22 confirmed mated females of each
species were exposed 6 h/day on days 6-15 of gestation to atmospheres
containing 0, 143, 285 or 656 mg/m3 (0, 25, 50 or 115 ppm)
isophorone. At the highest atmospheric concentration there was
evidence of maternal toxicity which showed as reduced food
consumption, alopecia and cervical or anogenital staining in the rats
and reduced body weights in the mice. Comprehensive uterine and fetal
examinations did not show any significant differences between animals
exposed to isophorone and their respective controls. From the results
of this study, it can be concluded that no teratogenic or fetotoxic
effect was observed with 656 mg/m3 in F-344 rats and CD-1 mice.
In a study by Dutertre-Catella (1976), groups of 10 rats of each
sex, exposed 6 h/day, 5 days/week to 2855 mg/m3 (500 ppm) isophorone
for 3 months, were mated to exposed animals or to controls. All
females were reported to have delivered 7-10 pups. Anatomo-
pathological examination did not show any abnormalities. However,
only one isophorone concentration was used, the group size was small,
and no information was provided on reproductive success and maternal
survival.
7.9 Neurotoxicity
Central nervous system depression is a characteristic feature of
isophorone intoxication in experimental animals (Smyth & Seaton, 1940;
Dutertre-Catella, 1976; De Ceaurriz et al., 1981).
Isophorone and a number of aliphatic ketones have been studied
using the mouse behavioural despair swimming test (De Ceaurriz et al.,
1984). This test, which was developed for screening antidepressant
drugs, is based on the duration of the periods of immobility exhibited
by mice when placed in water. Mice were exposed to isophorone at
atmospheric concentrations of 508-782 mg/m3 (89-137 ppm) for 4 h.
The ID50 (an estimated concentration producing a 50% reduction in
the immobility time) for animals thus exposed was 628 mg/m3
(110 ppm).
7.10 Other special studies
One of the current research approaches for assessing cytotoxicity
is to monitor the respiratory activity of the mitochondrion, a
sensitive, nonspecific subcellular target site. Detected changes in
mitochondrial function after the addition of a test chemical could be
correlated to toxic effects. In this case, rat (male, Charles River)
liver mitochondria were used, and isophorone was shown to induce no
observable effects on rat liver mitochondria respiratory activity over
the tested concentration range of 0.273-113.8 mg/litre
(1.98-825 µmol/litre) (Haubenstricker et al., 1990a).
Isophorone was tested for its ability to perturb glutathione
(GSH) levels in the testes and epididymides, as well as liver,
following single acute dosages to rats. Groups of four mature male
Sprague-Dawley rats were administered isophorone intraperitoneally
(2 ml/kg). Concurrent control groups were maintained for all time
points examined to account for possible circadian fluctuation of GSH
throughout the day. Animals were sacrificed at 1, 2, 4, 8 or 16 h
after administration of the test compound. Liver, testes and
epididymides were excised for determination of total GSH content by
spectrophotometry. Isophorone (500 mg/kg) significantly reduced GSH
in the liver and in both reproductive organs examined. The ability of
isophorone to enhance the covalent binding of tritiated ethyl
methanesulfonate (3H-EMS) to spermatocytes was assessed.
Perturbation of reproductive tract GSH by isophorone treatment
significantly enhanced the extent of 3H-EMS-induced binding to sperm
heads. The authors postulate that chemical-induced lowering of GSH in
the male reproductive tract may be a mechanism for potentiation of
chemical-induced germ-cell mutations (Gandy et al., 1990).
8. EFFECTS ON HUMANS
8.1 Acute
Groups of 11 or 12 subjects exposed for a few minutes to
atmospheric isophorone concentrations of 228 mg/m3 (40 ppm) or more
experienced irritation of the eyes, nose and throat. A few complaints
of nausea, headache, dizziness, faintness, inebriation and a feeling
of suffocation occurred at concentrations 1142 mg/m3 (> 200 ppm).
The symptoms of irritation and narcosis were said to be less intensive
following exposure to concentrations below 1142 mg/m3 (Smyth &
Seaton 1940). However, this study has been criticized because of
uncertain actual concentrations and the use of impure substances (Rowe
& Wolf, 1963).
8.2 Sub-chronic
Complaints of fatigue and malaise were reported among workers
exposed for 1 month to atmospheres containing 28.5 to 48 mg
isophorone/m3 (5 to 8 ppm) (Communication from Ware, GD, to
Chairman, TLV Committee, 1973). No further complaints were received
following a reduction of the concentration to between 5.7 and
22.8 mg/m3 (1 and 4 ppm). On the basis of these data, the American
Conference of Governmental Industrial Hygienists (ACGIH, 1986)
recommended a ceiling limit (15 min) of 29 mg/m3 (5 ppm) for
isophorone.
8.3 Irritation and sensitization
No report on skin irritation or skin sensitization has been
published.
8.3.1 Eye and respiratory irritation
Smyth & Seaton (1940) reported eye, nose and throat irritation
following exposure to isophorone levels of 228 mg/m3 (40 ppm) or
more.
Silverman et al. (1946) estimated the sensory threshold of a
number of ketones including isophorone. An average of 12 subjects of
both sexes were used for each solvent exposure. The time of exposure
was 15 min. Irritation of the eyes, nose and throat was experienced
at 142.7 mg/m3 (25 ppm) isophorone; the highest concentration
considered acceptable by the majority of subjects for an 8-h exposure
was 57.1 mg/m3 (10 ppm).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found that the workshift (6´ h)
average exposures of printers to isophorone were 4 and 80 mg/m3
(0.7 and 14 ppm). Symptoms of respiratory tract and eye irritation
reported by workers were attributed to the antistatic agent containing
principally isophorone.
Amoore & Hautala (1983) reported an air odour threshold for
isophorone of 1.14 mg/m3 (0.2 ppm). The "odour safety factor",
which was defined as the threshold limit value 28.5 mg/m3 (5 ppm)
divided by the odour threshold, was 25. From the magnitude of this
value the authors predicted that 50% of exposed persons would perceive
sensory warning of the TLV (28.5 mg/m3, 5 ppm).
8.4 Chronic toxicity and carcinogenicity
No long-term surveys have been carried out on occupationally
exposed workers or other potentially exposed populations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Yoshioka et al. (1985) studied the acute toxicity of isophorone
in Tetrahymena pyriformis. The EC50 after a 24-h exposure was
420 mg/litre. Yoshioka et al. (1986) found that the EC50 in the
Activated Sludge Respiration Inhibition Test was 100 mg/litre.
In a 2-day laboratory test with the marine red alga Champia
parvula, 38.3 mg isophorone/litre caused 50% reduction in cystocarp
formation. Above 138.5 mg/litre no cystocarps were formed, indicating
absence of sexual reproduction. In a 2-week laboratory test,
107.3 mg/litre completely inhibited cystocarp formation (Thursby &
Steele, 1986).
Isophorone was tested in air-saturated media with Saccharomyces
cerevisiae at concentrations of 0.272 to 113.7 mg/litre
(1.97-824 µmol/litre). These produced no detectable effect on the
rate of yeast respiration as measured with a dissolved oxygen
electrode (Haubenstricker et al., 1990b).
9.2 Aquatic organisms
The acute toxicity of isophorone to fish, crustaceans and algae
is summarized in Table 6. With the exception of Mysidopsis bahia
(US EPA, 1978) all acute LC50 values were above 100 mg/litre,
indicating a relatively low aquatic toxicity. This was consistent
with the results of a subacute (14 day) study with the marine red alga
Champia parvula. The toxicity of isophorone was determined by means
of various biological end-points, namely vegetative growth, formation
of tetrasporangia (asexual reproduction) and production of cystocarps
(sexual reproduction). Depending on the toxicological end-point, the
lowest concentration resulting in a significant difference from
controls ranged between 50 and 138 mg/litre (Thursby et al., 1985).
In addition to the results of acute toxicity tests shown in Table
6, an early life stage toxicity test was conducted with the freshwater
fathead minnow Pimephales promelas (Cairns & Nebeker, 1982) using
concentrations of 11, 19, 30, 56 and 112 mg isophorone/litre.
Survival was affected at a concentration of 112 mg/litre, but not at
56 mg/litre or less; fork length was affected at 30 mg/litre but not
at 19 mg/litre or less; body weight gain was decreased at 19 mg/litre
or more but not at 11 mg/litre. The authors calculated a no-observed-
effect concentration of 14 mg/litre. Using the same test with the
same species, Lemke et al. (1983) found a no-observed-effect level of
19.5 mg/litre, and in an interlaboratory (6 laboratories) comparison
no-observed-effect levels of up to 45.4 mg/litre were found (Lemke,
1983).
9.3 Terrestrial organisms
No data are available concerning the effects of isophorone on
terrestrial organisms.
Table 6. Acute toxicity of technical isophorone to aquatic species
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Fish
Bluegill sunfish LC50 96 static 220 (n) US EPA (1978)
(Lepomis macrochirus) LC50 24 static 240 (n) Buccafusco et al. (1981)
Fathead minnow LC50 96 flow 145-255 (m) Cairns & Nebeker (1982)
(Pimephales promelas) LC50 96 228 Geiger et al. (1990)
Golden orfe LC50 72 204 Scheubel & Scholz (1989)
(Leuciscus idus melanotus)
Sheepshead minnow LC50 96 140 Ward et al. (1981)
(Cyprinodon variegatus) LC50 96 static > 170, < 300 (n) Heitmuller et al. (1981)
Invertebrates
Brine shrimp LC50 24 430 Price et al. (1974)
(Artemia salina)
Mysid shrimp LC50 96 static 12.9 US EPA (1978)
(Mysidopsis bahia)
Water flea immobilization: EC50 24 static 430 (n) LeBlanc (1980)
(Daphnia magna) Immobilization: EC50 24 static 254-277 Scholz (1988a)
immobilization: EC50 48 static 120 (n) LeBlanc (1980)
immobilization: EC50 48 static 117 US EPA (1978)
Algae
Selenastrum capricornutum cell count: EC50 96 static 122 US EPA (1978)
chlorophyll: EC50 96 static 126 US EPA (1978)
Table 6 (cont'd)
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Skeletonema costatum cell count: EC50 96 static 105 US EPA (1978)
chlorophyll: EC50 96 static 110 US EPA (1978)
Scenedesmus subspicatus cell count: EC50 72 475-476 Scholz (1988b)
chlorophyll: EC50 72 524-527 Scholz (1988c)
Red alga reproduction EC50 24 38.3 Thursby & Steele (1986)
(Champia parvula)
a static = static exposure; flow = flow-through exposure
b n = nominal exposure concentration; m = measured exposure concentration
REFERENCES
ACGIH (1986) Documentation on the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
Affiliated Medical Enterprises Inc. (1972a) 90-day sub-chronic
toxicity of isophorone in the rat. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Affiliated Medical Enterprises Inc. (1972b) 90-day sub-chronic
toxicity of isophorone in the dog. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Amoore JE & Hautala E (1983) Odor as an aid for chemical safety: Odor
threshold limit values and volatilities for 214 industrial chemicals
in air and water dilution. J Appl Toxicol, 3: 272-290.
Atochem (1978a) Ames metabolic activation test to assess the potential
mutagenic effect of isophorone. Paris, Atochem (Report No. UKM
52/78204, prepared by Huntingdon Research Centre).
Atochem (1978b) Micronucleus test on isophorone. Paris, Atochem
(Report No. UKM 52/78457, prepared by Huntingdon Research Centre).
Atochem (1986) Data sheet on isophorone. Paris, Atochem.
Atochem (1988) Analytical method No. LCH 200 131/GC. Paris, Atochem.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Baser KHC, Özek T, & Tümen G (1992) Essential oils of Thymus
cariensis and Thymus haussknechtii, two endemic species in Turkey.
J Essent Oil Res, 4: 659-661.
Bartholomé E, Biekert E, Hellmann H, Ley H, Weigert M, & Weise E ed.
(1977) [Ullmann's encyclopaedia of technical chemistry], 4th ed.
Weinheim, New York, Verlag Chemie, vol 14, pp 210-211 (in German).
BIBRA (1991) Toxicity profile: Isophorone. Carshalton, United Kingdom,
The British Industrial Biological Research Association.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha-2 µ-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 349-367.
Borghoff SJ, Miller AB, Bowen JP, & Swenberg JA (1991) Characteristics
of chemical binding to alpha-2 µ-globulin in vitro - evaluating
structure-activity relationships. Toxicol Appl Pharmacol,
107: 228-238.
Borup MB & Middlebrooks EJ (1986) Photocatalyzed oxidation of organic
wastestreams. In: Toxic and hazardous wastes. Proceedings of the 18th
Mid-Atlantic Industrial Waste Conference, 29 June-1 July 1986.
Lancaster, Pennsylvania, Technimic Publishers Co., Inc., pp 554-568.
BP (1988a) Dihydroisophorone: Assessment of mutagenic potential in
histidine auxotrophs of Salmonella typhimurium (the Ames test).
Guildford, United Kingdom, BP International Ltd (LSR Report 88/0501).
BP (1988b) Material safety data sheet: Isophorone. Guildford, United
Kingdom, BP International Ltd.
Brondeau MT, Bonnet P, Guenier JP, Simon P, & De Ceaurriz J (1990)
Adrenal-dependent leucopenia after short-term exposure to various
airborne irritants in rats. J Appl Toxicol, 10(2): 83-86.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Bucher JR, Huff J, & Kluwe WM (1986) Toxicology and carcinogenesis
studies of isophorone in F344 rats and B6C3F1 mice. Toxicology,
39: 207-219.
Cairns MA & Nebeker AV (1982) Toxicity of acenaphthene and isophorone
to early life stages of fathead minnows. Arch Environ Contam Toxicol,
11: 703-707.
Callahan MA, Slimak MW, & Gabel NW (1979) Water-related environmental
fate of 129 priority pollutants. Washington, DC, US Environmental
Protection Agency (EPA 440/4-79-029B).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments. J Great Lakes Res, 13: 296-309.
Charbonneau M & Swenberg JA (1988) Studies on the biochemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT Act,
8(6): 1-5.
Charbonneau M, Strasser J, Lock EA, Turner MR, & Swenberg JA (1988)
1,4-Dichlorobenzene (1,4-DCB)-induced nephrotoxicity: Similarity with
unleaded gasoline (UG)-induced renal effects. In: Bach PH & Lock EH
ed. Nephrotoxicity: Extrapolation from in vitro to in vivo and
from animal to man. New York, London, Plenum Press.
Cheh AM (1986) Mutagen production by chlorination of methylated alpha,
ß-unsaturated ketones. Mutat Res, 169: 1-9.
Cheminfo (1988) Database of the Canadian Centre for Occupational
Health and Safety: Record No. 22E: Isophorone. Ottawa, Canada,
Canadian Centre for Occupational Health and Safety.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff programme. J Water Pollut Control Fed,
56: 898-908.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz JC, Micillino JC, Marignac B, Bonnet P, Muller J, &
Guenier JP (1984) Quantitative evaluation of sensory-irritating and
neurobehavioural properties of aliphatic ketones in mice. Food Chem
Toxicol, 22: 545-549.
Dietrich DR & Swenberg JA (1991) NCI-Black-Reiter (NBR) male rats fail
to develop renal disease following exposure to agents that induce
alpha-2 µ-globulin nephropathy. Fundam Appl Toxicol, 16: 749-762.
Dutertre-Catella H (1976) Contribution à l'étude analytique
toxicologique et biochimique de l'isophorone. Paris, Université René
Descartes (Thesis for doctorate in pharmacology).
Dutertre-Catella H, Nguyen PL, Dang Quoc Q, & Truhaut R (1978)
Transformations métaboliques de la triméthyl-3,5,5-cyclohexène-2,
one-1 (isophorone). Toxicol Eur Res, 1: 209-216.
ECETOC (1988) Concentrations of industrial organic chemicals measured
in the environment: The influence of physico-chemical properties,
tonnage and use pattern. Brussels, European Chemical Industry Ecology
and Toxicology Centre (Technical Report No. 29).
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: X. Results of 70 coded chemicals
tested for the National Toxicology Program. Environ Mol Mutagen,
23(3): 208-227.
Gandy J, Millner GC, Bates HK, Casciano DA, & Harbison RD (1990)
Effects of selected chemicals on the glutathione status in the male
reproductive system of rats. J Toxicol Environ Health, 29: 45-57.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows. Superior, Wisconsin, Center for Lake
Superior Environmental Studies, University of Wisconsin, 332 pp.
Ghassemi M, Quinlivan S, & Bachmaier J (1984) Characteristics of
leachates from hazardous waste landfills. J Environ Sci Health,
A19(5): 579-620.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Illinois,
Charles C. Thomas, pp 608-609.
Gulati DK, Witt K, Anderson B, Zeiger E, & Shelby MD (1989) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. III. Results with 27 chemicals. Environ Mol
Mutagen, 13: 133-193.
Hannah SA, Austern BM, Eralp AE, & Wise RH (1986) Comparative removal
of toxic pollutants by six wastewater treatment processes. J Water
Pollut Control Fed, 58: 27-34.
Harrison FL, Bishop DJ, & Mallon BJ (1985) Comparison of organic
combustion products in fly ash collected by a Venturi wet scrubber and
an electrostatic precipitator at a coal-fired power station. Environ
Sci Technol, 19: 186-193.
Haubenstricker ME, Holodnick SE, Mancy KH, & Brabec MJ (1990a) Rapid
toxicity testing based on mitochondrial respiratory activity. Bull
Environ Contam Toxicol, 44: 675-680.
Haubenstricker ME, Meier PG, Mancy KH, & Brabec MJ (1990b) Rapid
toxicity testing based on yeast respiratory activity. Bull Environ
Contam Toxicol, 44: 669-674.
Hauser TR & Bromberg SM (1982) EPA's monitoring program at Love Canal
1980. Environ Monit Assess, 2: 249-272.
Heitmuller PT, Hollister TA, & Parish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hites R (1979) Sources and fates of industrial organic chemicals; a
case study. Sludge Manage, 8: 107-119.
Hüls (1981) Data sheet No. 2257: Isophorone. Marl, Germany, Hüls AG.
Hüls (1988a) Test of the skin sensitizing effect of isophorone for
guinea-pig. Marl, Germany, Hüls AG (Internal report No. 1278).
Hüls (1988b) Mutagenicity of isophorone by means of the Ames
Salmonella typhimurium/microsomes mutagenicity test. Marl, Germany,
Hüls AG (Internal report No. 88/300).
Hüls (1988c) Analytical method for determining isophorone. Marl,
Germany, Hüls AG (Unpublished information).
Hüls (1988d) [Acute oral toxicity of beta-isophorone (3,5,5-
trimethyl-3-cyclohexene-1-one) for rats.] Marl, Germany, Hüls AG
(Report No. 1273) (in German).
Hüls (1988e) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the skin.] Marl,
Germany, Hüls AG (Report No. 1274) (in German).
Hüls (1988f) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the eyes and
mucosa.] Marl, Germany, Hüls AG (Report No. 1275) (in German).
Japan Environment Agency (1983) Environmental monitoring of chemicals:
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Department
of Environmental Health, Japan Environment Agency.
Jungclaus GA, Games LM, & Hites RA (1976) Identification of trace
organic compounds in tire manufacturing plant wastewaters. Anal Chem,
48: 1894-1896.
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemke AE (1983) Interlaboratory comparison of continuous flow, early
life stage testing with fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory, 26
pp (EPA-600/3-84-005; NTIS PB84-129493).
Lemke AE, Durham E, & Felhaber T (1983) Evaluation of a fathead minnow
embryo-larval test guideline using acenaphtene and isophorone. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory, 26 pp (EPA-600/3-83-062; NTIS PB83-243436).
Levin J-O & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Littlefield HA (1968) Assessment and comparison of subacute inhalation
toxicities of three ketones. Falls Church, Virginia (Unpublished
studies done for Esso Research and Engineering Company, Linden, New
Jersey).
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemistry:
Property estimation methods. New York, McGraw-Hill, pp 4-9.
Mabey WR (1981) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA-440/4-81-014).
McFall JA, Antoine SR, & DeLeon IR (1985) Base-neutral extractable
organic pollutants in biota and sediments from Lake Pontchartrain.
Chemosphere, 14(10): 1561-1569.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus subtilis/
microsome rec-assay for the detection of DNA damaging substances which
may occur in chlorinated and ozonated waters. Brighton. Water Sci
Technol, 21: 875-887.
Matthews EJ, Spalding JW, & Tennant RW (1993) Transformation of
BALB/C-3T3 cells: V. Transformation responses of 168 chemicals
compared with mutagenicity in Salmonella and carcinogenicity in
rodent bioassays. Environ Health Perspect, 101(Suppl 2): 347-482.
Microbiological Associates (1984a) L5178Y TK +/- mouse lymphoma
mutagenesis assay on isophorone. Bethesda, Maryland, Microbiological
Associates, Inc. (Report No. T2408.701005, prepared for the Chemical
Manufacturers' Association, Washington).
Microbiological Associates (1984b) Activity of isophorone in the
micronucleus cytogenetic assay in mice. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.121001, prepared
for the Chemical Manufacturers' Association, Washington).
Microbiological Associates (1984c) Unscheduled DNA synthesis in rat
primary hepatocytes with isophorone. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.380003, prepared
for the Chemical Manufacturers' Association, Washington).
Mikami Y, Fukunaga Y, Arita M, Obi Y, & Kisaki T (1981) Preparation of
aroma compounds by microbial transformation of isophorone with
Aspergillus niger. Agric Biol Chem, 45: 791-793.
Mitchell AD (1993) Does the in situ approach yield a more accurate
assessment of induced mutation frequencies than the standard L5178Y
tk+/- tk -/- mouse lymphoma assay? Environ Mol Mutagen, 21
(Suppl 22): 49.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Murty CVR, Sarkar FH, Mancini MA, & Roy AK (1987) Sex-independent
synthesis of alpha-2 µ-globulin and its messenger ribonucleic acid in
the rat preputial gland: Biochemical and immunocyto-chemical analyses.
Endocrinology, 121: 1000-1005.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putman DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Olson CT, Yu KO, & Serve MP (1986) Metabolism of cis- and trans-
decalin in F344 rats. J Toxicol Environ Health, 18: 285-292.
Olson MJ, Johnson JT, & Reidy CA (1990) A comparison of male rat and
human urinary proteins: Implications for human resistance to hyaline
droplet nephropathy. Toxicol Appl Pharmacol, 102: 524-536.
Ono Y, Somiya I, & Kawamura M (1991) The evaluation of genotoxicity
using DNA repairing test for chemicals produced in chlorination and
ozonation processes. Water Sci Technol, 23: 329-338.
Potokar M, Grundler OJ, Heusener A, Jung R, Mürmann P, Schöbel C,
Suberg H, & Zechel HJ (1985) Studies on the design of animal tests for
the corrosiveness of industrial chemicals. Food Chem Toxicol, 23:
615-617.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Rasmussen T, Anthoni U, Christophersen C, & Nielsen PH (1993) Volatile
compounds from the marine indicator organism Mytilus edulis.
Chemosphere, 27(11): 2123-2125.
Rastogi SC (1991) Levels of organic solvents in printer's inks. Arch
Environ Contam Toxicol, 20: 543-547.
Rohm & Haas (1972) A residue decline study (in bean and rice plants)
using 14C-isophorone. Philadelphia, Pennsylvania, Rohm & Haas
Company.
Rowe VK & Wolf MA (1963) Ketones. In: Patty FA ed. Industrial hygiene
and toxicology, 2nd ed. New York, Interscience, vol II, pp 1722-1724,
1763-1765.
Samimi B (1982) Exposure to isophorone and other organic solvents in a
screen printing plant. Am Ind Hyg Assoc J, 43: 43-48.
Sasaki S (1980) Transformation and biological effects. In: Aquatic
pollutants. Oxford, New York, Pergamon Press.
Schering (1968) [Isophorone - Systemic tolerability following single
administration (LD50, oral).] Berlin, Germany, Schering AG
(in German).
Schering (1974) Dissipation of isophorone in sugar beets. Berlin,
Germany, Schering AG.
Scheubel JB & Scholz N (1989) [Acute toxicity test with isophorone in
the Golden Orfe.] Marl, Germany, Hüls AG (Unpublished study No. F 120)
(in German).
Schöberl P (1992) [Determination of the biodegradability of isophorone
in the DOC-DIE AWAY test.] Marl, Germany, Hüls AG (Unpublished report)
(in German).
Scholz N (1988a) [Acute toxicity of isophorone to Daphnia magna
strauss]. Marl, Germany, Hüls AG (Unpublished study No. D 318)
(in German).
Scholz N (1988b) [Growth retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. AW 146) (in German).
Scholz N (1988c) [Assimilation retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. A 115) (in German).
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency (EPA 600/4-76-062).
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Smyth HF Jr & Seaton J (1940) Acute response of guinea-pigs and rats
to inhalation of the vapours of isophorone. J Ind Hyg Toxicol,
22: 477-483.
Smyth HF Jr, Seaton J, & Fischer L (1942) Response of guinea-pigs and
rats to repeated inhalation of vapours of mesityl oxide and
isophorone. J Ind Hyg Toxicol, 4: 46-50.
Smyth HF Jr, Weil CS, West JS, & Carpenter CP (1969) An exploration of
joint toxic action: Twenty-seven industrial chemicals intubated in
rats in all possible pairs. Toxicol Appl Pharmacol, 14: 340-347.
Strasser J Jr, Charbonneau M, Borghoff SJ, Turner MJ, & Swenberg JA
(1988) Renal protein droplet formation in male F344 rats after
isophorone (IPH) treatment. Toxicologist, 8: 136.
Suffet IH, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking water during a 2-year period. Water
Res, 14: 853-867.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residues Rev,
85: 17-28.
Swenberg JA (1991) Risk assessment of chemicals causing alpha-2
µ-globulin nephropathy. Regul Toxicol Pharmacol, 13: 1-2.
Swenberg JA, Short B, Borghoff S, Strasser J, & Charbonneau M (1989)
The comparative pathobiology of alpha-2 µ-globulin nephropathy.
Toxicol Appl Pharmacol, 97: 35-46.
Swenberg JA, Dietrich DR, McClain RM, & Cohen SM (1992) Species-
specific mechanisms of carcinogenesis. In: Vainio H, Magee PN,
McGregor DB, & McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 477-500.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981a) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981b) Biodegradability
studies for predicting the environmental fate of organic priority
pollutants. Washington, DC, Association of Official Analytical
Chemists.
Thier R, Peter HP, Wiegand HJ, & Bolt HM (1990) DNA binding study of
isophorone in rats and mice. Arch Toxicol, 64: 684-685.
Thier R & Xu DG (1990) Urinary excretion of isophorone metabolites by
male rats. Naunyn-Schmiedeberg's Arch Pharmacol, 341: R11 (Abstract
No. 41).
Thursby GB & Steele RL (1986) Comparison of short- and long-term
sexual reproduction with the marine red alga Champia parvula.
Environ Toxicol Chem, 5: 1013-1018.
Thursby GB, Steele RL, & Kane ME (1985) Effect of organic chemicals on
growth and reproduction in the marine red alga Champia parvula.
Environ Toxicol Chem, 4: 797-805.
Traul KA, Hinz JP, & Kapp RW (1984) Inhalation teratology study in
rats and mice - MRD-83-237 (isophorone) (Project No. 32377). East
Millstone, New Jersey, Bio/dynamics Inc., East Laboratory (Report
submitted to Chemical Manufacturers Association).
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1970) Premiers
résultats de l'étude du métabolisme chez le lapin d'un solvent
industriel : l'isophorone. C R Acad Sci (Paris), 271: 1333-1336.
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1972) Etude de la
toxicité d'un solvant industriel: l'isophorone - pouvoir irritant
vis-à-vis des téguments et des muqueuses. J Eur Toxicol, 5: 31-37.
US EPA (1978) Isophorone: Ambient water quality criteria. Washington,
DC, US Environmental Protection Agency (NTIS PB-296 798).
US EPA (1979) Standard operating procedure: Analysis of non volatile
extractable organic compounds. Sample extraction and screening by gas
chromatography. Method 625. Fed Reg, 44: 223.
US EPA (1980) Ambient water quality criteria for isophorone.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-056;
NTIS PB81-11767).
US EPA (1986) Graphical exposure modelling system (GEMS). Fate of
atmospheric pollutants. Washington, DC, US Environmental Protection
Agency.
US EPA (1991a) Alpha-2 µ-globulin: Association with chemically induced
renal toxicity and neoplasia in the male rat. Washington, DC, US
Environmental Protection Agency (Risk Assessment Forum).
US EPA (1991b) Report of the EPA peer review workshop on alpha-2
µ-globulin: Association with renal toxicity and neoplasia in the male
rat. Washington, DC, US Environmental Protection Agency
(EPA/625/3-91/021).
US NIOSH (1977) Manual of analytical methods: Method S367, 2nd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Cincinnati, vol 3).
US NIOSH (1980) Health hazard evaluation report HHE-80-103-827.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NIOSH (1984) Health hazard evaluation report HHE-84-299-1543.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NTP (1986) Toxicological and carcinogenesis studies of isophorone
(CAS No. 78-59-1) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, North Carolina, National Toxicology Program
(Technical Report Series No. 291).
Veith GD, Macek KJ, Betrocelli SR, & Carroll J (1978) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. Paper
presented at the 3rd Symposium on Aquatic Toxicology, New Orleans,
17-18 October 1978.
Ward GS, Parrish PR, & Rigby RA (1981) Early life stage toxicity tests
with a saltwater fish: effects of eight chemicals on survival, growth,
and development of sheepshead minnows (Cyprinodon variegatus).
J Toxicol Environ Health, 8: 225-240.
White LD, Taylor DG, Mauer PA, & Kuper RE (1970) A convenient
optimized method for the analysis of selected solvent vapours in the
industrial atmosphere. Am Ind Hyg Assoc J, 31: 225-232.
Yoshioka Y, Ose Y, & Sato T (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci Total Environ,
43: 149-157.
Yoshioka Y, Nagase H, Ose Y, & Sato T (1986) Evaluation of the test
method "activated sludge, respiration inhibition test" proposed by the
OECD. Ecotoxicol Environ Saf, 12(3): 206-212.
Zoeteman BCJ, De Greef E, & Brinkmann FJJ (1981) Persistency of
organic contaminants in groundwater; lessons from soil pollution
incidents in The Netherlands. Sci Total Environ, 21: 187-202.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés physiques et chimiques
L'isophorone est un liquide incolore d'odeur mentholée. Elle est
soluble dans l'eau (12 g/litre) et miscible à la plupart des solvants
organiques. Son point de congélation est de -8,1°C et son point
d'ébullition de 215°C. San tension de vapeur à 20°C est de l'ordre
40 Pa et sa densité de vapeur par rapport à l'air est de 4,7. C'est
une substance stable.
L'isophorone de qualité technique vendue dans le commerce
contient 1 à 3% d'isomère ß-(3,5,5-triméthyl-3-cyclohexène-1-one); la
somme des isomères alpha et ß-dépasse 99%.
1.2 Production et usage
L'isophorone est largement utilisée comme solvant pour un grand
nombre de résines et de polymères de synthèse ainsi que dans certaines
peintures spéciales et certaines encres d'imprimerie. Elle joue le
rôle de produit intermédiaire en synthèse et on l'utilise comme
solvant dans certaines formulations de pesticides.
On estime qu'en 1988, la production mondiale d'isophorone était
de l'ordre de 92 000 tonnes par an.
1.3 Transport, distribution et transformation dans l'environnement
L'isophorone peut pénétrer dans l'environnement du fait de son
utilisation par de nombreuses industries, lors du rejet de déchets ou
d'eaux usées et par suite de son utilisation comme solvant, notamment
dans les formulations de pesticides. Une fois libérée dans l'eau ou
le sol, sa volatilisation et sa biodégradation entraînent une baisse
de la concentration. L'isophorone présente dans l'atmosphère en est
éliminée par des processus photochimiques avec une demi-vie estimative
d'environ 30 minutes (selon un modèle mathématique). On a constaté
que la biodécomposition de l'isophorone atteignait environ 70% dans
les 14 jours et 95% dans les 28 jours. Les résultats des études de
biodécomposition sont variables et limités. D'après la solubilité
dans l'eau, les coefficients d'adsorption au sol et la polarité de ce
composé, il paraît improbable qu'il soit adsorbé en quantité notable
sur les matières solides en suspension et les sédiments.
Bien qu'on ait trouvé de l'isophorone dans des tissus pisciaires,
les données concernant ce composé et notamment ses propriétés
physiques et chimiques, incitent à penser qu'il a peu de chances de
subir une bioconcentration. On a mesuré une demi-vie d'un jour chez
une espèce de poisson.
1.4 Concentrations dans l'environnement et exposition humaine
On n'a pas procédé au dosage de l'isophorone dans l'air ambiant.
On a fait état d'une concentration d'isophorone dans des cendres
volantes de houille égale à 490 µg/kg. On a également mis en évidence
la présence d'isophorone dans des eaux de surface (0,6 à 3 µg/litre),
des eaux souterraines (10 µg/litre), des eaux de ruissellement
urbaines (10 µg/litre) et des eaux de lessivage de décharge
(29 µg/litre).
De l'isophorone a été trouvée à la concentration de 100 µg/litre
dans des eaux usées industrielles. Après un traitement secondaire
classique, la concentration d'isophorone dans l'effluent était tombée
à 10 µg/litre.
La présence d'isophorone a été observée dans des sédiments
lacustres (0,6 à 12 µg/kg de poids sec) ainsi que dans les tissus de
plusieurs espèces de poisson à des concentrations allant jusqu'à
3,61 µg/kg de poids frais.
On n'a pas constaté la présence d'isophorone dans les parties
comestibles de plants de haricots, de riz ou dans des betteraves à
sucre, après application sous la forme de véhicule pour pesticide.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Des études de distribution effectuées sur des rats au moyen de
14C-isophorone ont montré que 93% de la radioactivité administrée
par voie orale, réapparaissaient principalement dans les urines et
l'air expiré au bout de 24 heures. Les organes dans lesquels
subsistait au bout de cette période la plus forte concentration
d'isophorone étaient le foie, les reins et les glandes préputiales.
Après administration par voie orale d'isophorone à des lapins, on
a retrouvé dans l'urine de ces derniers des métabolites résultant
d'une oxydation du groupe méthyle en position 3, de la réduction du
groupe carbonyle et de l'hydrogénation de la double liaison du cycle
cyclohexénique; ces métabolites ont été éliminés tels quels ou sous
forme de glucuronides dans le cas des alcools.
Les valeurs de la DL50 percutanée indiquent que l'isophorone
passe rapidement à travers la peau.
1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
La toxicité aiguë de l'isophorone est faible, les valeurs de
DL50 par voie orale étant > 1500 mg/kg chez le rat, à > 2200 mg/kg
chez la souris et à > 2000 mg/kg chez le lapin. On a obtenu pour la
DL50 par voie percutanée des valeurs de 1700 mg/kg chez le rat et de
> 1200 mg/kg chez le lapin. Au niveau cutané les effets aigus d'une
exposition vont, chez le rat et le lapin, d'un léger érythème à la
formation de croûtes. On a signalé une conjonctivite et des lésions
cornéennes après instillation directe dans l'oeil ou exposition à de
fortes concentrations d'isophorone. Le test de Magnusson-Kligman
pratiqué sur des cobayes n'a pas permis de mettre en évidence un effet
de sensibilisation cutanée.
Les études de toxicité aiguë et celles au cours desquelles on a
administré de l'isophorone par voie orale pendant de brèves périodes à
des rongeurs ont montré qu'à fortes doses (> 1000 mg/kg), ce
composé provoquait des effets dégénératifs au niveau du foie ainsi
qu'une dépression du système nerveux central et une certaine
mortalité. Lors d'études de 90 jours, on a évalué à 500 mg/kg de
poids corporel, la dose quotidienne sans effets observables pour le
rat et la souris. Une autre étude de 90 jours, au cours de laquelle
on a administré de l'isophorone par voie orale à des chiens beagle (en
nombre limité), n'a révélé aucun effet à des doses quotidiennes allant
jusqu'à 150 mg/kg de poids corporel.
Lors d'études de toxicité aiguë au cours desquelles on a fait
inhaler pendant de brèves périodes de l'isophorone aux animaux de
laboratoire, on a observé une irritation oculaire et respiratoire, des
effets hématologiques et une diminution du poids corporel. Etant
donné que la conception de ces études laissait à désirer, il n'a pas
été possible de déterminer la dose sans effets observables et on ne
peut en tirer aucune conclusion en ce qui concerne la santé humaine.
L'isophorone ne provoque pas de mutations géniques chez les
bactéries ni d'aberrations chromosomiques in vitro; on n'observe pas
non plus de réparation de l'ADN dans des cultures primaires
d'hépatocytes de rat, ni la présence de micro-noyaux dans des cellules
de moelle osseuse de souris. Des effets positifs ont été observés,
lors d'essais de mutagénèse, sur des cellules de lymphomes murins
L5178Y TK +/-, mais uniquement en l'absence d'un système métabolique
exogène. Le même phénomène a été observé en ce qui concerne les
échanges de chromatides soeurs. L'isophorone a produit une
transformation morphologique in vitro, mais là encore, en l'absence
de système métabolique exogène. En revanche, elle n'a pas produit de
mutations létales récessives liées au sexe chez la drosophile. Les
données de mutagénicité ont un poids expérimental suffisant pour qu'on
puisse soutenir que l'isophorone n'est pas un composé qui réagit
énergiquement avec l'ADN. D'ailleurs, lors d'une épreuve in vivo,
on n'a pas observé de lésion de l'ADN dans des cellules hépatiques et
rénales (alors que ces organes sont ceux où l'on observe des lésions
dans les épreuves de cancérogénicité).
Les études toxicologiques au cours desquelles on a administré
pendant une longue période de l'isophorone par voie orale à des souris
et à des rats ont révélé la présence de plusieurs lésions rénales
prolifératives chez les rats males, comprenant une néphropathie, une
hyperplasie tubulaire et une faible incidence des adénomes affectant
les tubules et des adénocarcinomes. Il est admis que l'accumulation
d'alpha2u-globuline joue un rôle dans l'étiologie de ces lésions.
Etant donné que l'on n'a pas décelé chez l'homme la présence
d'alpha2u-globuline en quantités appréciables, il ne semble pas que
ce type de cancérogénèse soit à envisager dans l'espèce humaine. Chez
cinq rats males soumis à des fortes doses d'isophorone, on a observé
des carcinomes de la glande préputiale, et deux adénomes de la glande
clitoridienne ont été observés chez les rattes soumises à de faibles
doses d'isophorone. Là encore, il est possible que l'accumulation
d'alpha2u-globuline soit en cause. On a également attribué à
l'isophorone la présence d'un certain nombre de lésions néoplasiques
du foie, des téguments et du système lymphoréticulaire, observées chez
les rats males, de même d'ailleurs que des lésions bénignes du foie et
du cortex surrénalien, lésions qui n'ont cependant pas été observées
chez les souris femelles.
La seule étude d'inhalation à long terme dont on connaisse les
résultats chez le rat et le lapin, a permis d'observer une irritation
de la muqueuse oculaire et de la muqueuse nasale et des altérations au
niveau pulmonaire et hépatique, à une dose de approx.1427 mg/m3
(environ 250 ppm). Toutefois ces résultats peuvent s'expliquer par le
fait que les études en question n'offraient pas toutes les garanties
de rigueur.
Des études très limitées effectuées sur des rats et des souris
indiquent que l'isophorone n'affecte pas la fécondité ni le
développement chez les animaux de laboratoire.
Le fait qu'une dépression du système nerveux central se produise
chez les animaux de laboratoire par suite d'exposition à de
l'isophorone, pourrait être le signe d'un effet neurotoxique. Lors
d'une épreuve comportementale de nage désespérée, l'isophorone a
également produit un effet positif.
1.7 Effets sur l'homme
On peut déceler une odeur d'isophorone à une concentration ne
dépassant pas 1,14 mg/m3 (0,2 ppm). A des concentrations
inférieures à 28,55 mg/m3 (5 ppm), on a signalé une irritation des
yeux, du nez et de la gorge; au-delà de 1142 mg/m3 (200 ppm) on a
fait état de nausées, de maux de tête, d'étourdissements, de faiblesse
et de sensation ébrieuse.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
En ce qui concerne les effets aigus sur un certain nombre
d'espèces marines ou dulçaquicoles, on possède plusieurs valeurs de la
CL50. Les valeurs de CE50 à 96 heures (basées sur la numération
cellulaire et la chlorophylle) s'échelonnent de 105 à 126 mg/litre.
Pour Daphnia magna, les valeurs de la CL50 à 48 heures vont de 177
à 120 mg/litre et pour les poissons d'eau douce; celles de la CL50 à
96 heures s'étagent de 145 à 255 mg/litre.
Les valeurs de la CL50 à 96 heurs pour les invertébrés marins
vont de 12,9 à 430 mg/litre et pour une espèce de poisson de mer,
cette valeur se situe entre 170 et 300 mg/litre. Les données fournies
par les études au cours desquelles on a utilisé des concentrations
mesurées ne diffèrent pas de celles où ce sont les concentrations
nominales dont on s'est servi. Les épreuves effectuées par différents
laboratoire sur Pimephales promelas ont fait ressortir, pour la dose
sans effets nocifs observables, des valeurs allant de 14 à
45,4 mg/litre.
Les données disponibles incitent à penser que l'isophorone n'est
que faiblement toxique pour les organismes aquatiques.
2. Conclusions
2.1 Population générale
L'isophorone est utilisée comme solvant pour les résines, les
polymères et certaines formulations de pesticides. Il peut y avoir
exposition par voie cutanée ou respiratoire, mais il y a de grandes
chances pour qu'elle reste minime. Les données disponibles montrent
que l'isophorone peut être présente à des concentrations de l'ordre du
µg/litre (ou par kg) dans l'eau de boisson et dans le poisson. Les
études expérimentales ayant montré que ce composé était faiblement
toxique et du fait que l'exposition aux sources d'isophorone présentes
dans l'environnement est peu importante, on peut considérer que le
risque pour la population générale est minime.
2.2 Exposition professionnelle
Faute de contrôles techniques suffisants et de mesures d'hygiène
industrielle convenables, il est possible que l'exposition
professionnelle à l'isophorone dépasse les limites acceptables et
provoque une irritation oculaire, cutanée ou respiratoire. A plus
fortes concentrations, d'autres effets nocifs peuvent se produire. Le
groupe de travail ne disposait pas d'études sur les effets à long
terme de ce composé chez les ouvriers.
2.3 Environnement
Il est possible que l'isophorone soit libérée dans
l'environnement lorsqu'on l'utilise comme véhicule de pesticides et du
fait de son emploi généralisé comme solvant. On en a trouvé de
faibles concentrations dans plusieurs compartiments du milieu, mais sa
persistance est faible par suite des processus de biodécomposition,
volatilisation et oxydation photochimique qu'elle subit. D'après les
données disponibles, il semble que l'isophorone soit peu toxique pour
les organismes aquatiques.
3. Recommandations
3.1 Protection de la santé humaine et de l'environnement
Des précautions sont à prendre pour éviter la pollution des eaux
souterraines et de l'air.
Les travailleurs qui sont employés à la production d'isophorone
doivent se prémunir contre l'exposition à ce composé grâce à des
mesures de contrôle technique suffisantes et à des précautions
d'hygiène industrielle appropriées. L'exposition professionnelle doit
rester dans des limites acceptables et être régulièrement contrôlée.
3.2 Recherches futures
a) Surveillance médicale des travailleurs exposés.
b) Détermination de la concentration effective d'isophorone dans les
eaux à l'entour des zones industrielles.
c) Etudes d'inhalation satisfaisantes à court et à long terme sur
des animaux de laboratoire afin de déterminer les limites de
sécurité pour l'exposition professionnelle.
d) Nécessité d'obtenir des données sur la biodécomposition anaérobie
de l'isophorone, notamment du fait qu'on en a observé la présence
dans les lixiviats de décharges.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades físicas y químicas
La isoforona es un líquido incoloro con un olor parecido al de la
menta. Es soluble en agua (12 g/litro) y se mezcla con la mayoría de
disolventes orgánicos. Su punto de congelación es -8,1°C y su punto
de ebullición 215°C. Su presión en vapor a 20°C es del orden de
40 Pa, y su densidad en vapor (aire = 1) es 4,7. Es una sustancia
estable.
Las muestras comerciales de isoforona de calidad técnica
contienen 1-3% del isómero ß-isoforona (3,5,5-trimetil-3-ciclo-
hexeno-1-1); la suma de isómeros alpha y supera el 99%.
1.2 Producción y utilización
La isoforona se utiliza mucho como disolvente para cierto número
de resinas y polímeros sintéticos, así como en pinturas y tintas de
imprenta para aplicaciones especiales. Es también un producto químico
intermedio y un disolvente en determinadas formulaciones de
plaguicidas.
Se ha estimado que en 1988 su producción mundial fue del orden de
92 000 toneladas.
1.3 Transporte, distribución y transformación en el medio ambiente
La isoforona puede introducirse en el medio ambiente teniendo
como procedencia numerosas industrias, la evacuación de desechos y de
aguas residuales y a raíz de su utilización como disolvente y como
portador de plaguicida. Tras su descarga en el agua o el suelo, la
concentración ambiental disminuye a consecuencia de la volatilización
y la biodegradación. La isoforona de la atmósfera se elimina por
procesos fotoquímicos con una semivida estimada de unos 30 minutos
(sobre la base de un modelo matemático). En una prueba de estimación,
la isoforona se biodegradó hasta aproximadamente un 70% en 14 días y
un 95% en 28 días. Los resultados de los estudios de biodegradación
son variables y limitados. Los coeficientes de solubilidad en agua y
de adsorción en el suelo y la polaridad indican que es improbable que
tenga lugar una adsorción significativa por sólidos en suspensión y
sedimentos.
Aunque se ha hallado isoforona en tejidos de peces, los datos y
las propiedades fisicas y químicas parecen indicar que es improbable
una bioconcentración significativa. Se ha medido una semivida de un
día en una única especie de peces.
1.4 Niveles ambientales y exposición humana
No se ha medido isoforona en el aire ambiente. Se ha notificado
una concentración de isoforona en ceniza volátil de carbón de
490 µg/kg. Se ha identificado isoforona en aguas superficiales (0,6 a
3 µg/litro), en aguas subterráneas (10 µg/litro), en aguas de
escorrentía urbanas (10 µg/litro) y en lixiviado de terraplenados
(29 µg/litros).
Se ha hallado isoforona en aguas residuales industriales en una
concentración de 100 µg/litro. Tras un tratamiento secundario
clásico, la concentración de isoforona en el efluente fue de
10 µg/litro.
Se ha identificado isoforona en sedimentos lacustres (0,6 a
12 µg/kg de peso en seco) y en los tejidos de varias especies de peces
en concentraciones de hasta 3,61 mg/kg de peso en húmedo.
No se detectó isoforona en las partes comestibles de las plantas
del frijol, del arroz o de la remolacha azucarera tras la aplicación
de un portador de plaguicida.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
Los estudios de distribución en ratas utilizando 14C-isoforona
mostraron que el 93% de la radiactividad administrada por vía oral
aparecía principalmente en la orina y el aire espirado en 24 horas.
Los tejidos que retuvieron la mayor concentración tras ese periodo
fueron el hígado, los riñones y las glándulas prepuciales.
Los metabolitos tras administración oral de isoforona
identificados en la orina de conejos fueron resultado de la oxidación
del grupo 3-metilo, la reducción del grupo keto y la hidrogenación del
enlace doble del anillo de ciclohexeno, y se eliminaron como tales o
como derivados de glucuronida en el caso de los alcoholes.
Los valores de la DL50 percutáneas indican que la isoforona se
absorbe rápidamente a través de la piel.
1.6 Efectos en mamíferos de laboratorio y en sistemas de prueba in
vitro
La toxicidad aguda de la isoforona es baja, con valores de DL50
oral > 1500 mg/kg en la rata, > de 2200 mg/kg en el ratón y
> 2000 mg/kg en el conejo. Los valores de la DL50 cutáneas fueron
1700 mg/kg en la rata y > 1200 mg/kg en el conejo. Los efectos
agudos por exposición cutánea en ratas y conejos oscilaron entre
eritema leve y escaras. Se han notificado casos de conjuntivitis y
lesión corneana tras la aplicación directa al ojo o la exposición a
concentraciones elevadas de isoforona, pero ningún caso de
sensibilización de la piel en cobayos utilizando la prueba Magnusson-
Kligman.
En estudios de administración por vía oral sobre efectos agudos y
a corto plazo a roedores en dosis altas (> 1000 mg/kg) se
observaron efectos degenerativos en el hígado, así como depresión del
sistema nervioso central y algunas defunciones. En estudios de 90
días, se determinó un NOEL en ratas y ratones de 500 mg/kg de peso
corporal por día. En un estudio de administración por vía oral de 90
días a perros pachón (en número limitado) no se apreciaron efectos en
dosis de hasta 150 mg/kg de peso corporal por día.
En los experimentos examinados de inhalación aguda y a corto
plazo, se observaron irritación ocular y respiratoria, efectos
hematológicos y reducción del peso corporal. Como el diseño de los
estudios era inadecuado, no pudo determinarse NOEL y no puede hacerse
ninguna deducción con respecto a la salud humana.
La isoforona no induce mutaciones genéticas en bacterias,
aberraciones cromosómicas in vitro, reparación del ADN en los
hepatocitos primarios de la rata, ni micronúcleos de médula ósea en
los ratones. Se observaron efectos positivos únicamente en ausencia
de un sistema metabólico exógeno en valoraciones L5178Y TK+/- de los
mutagénesis de linfoma del ratón, así como en una valoración de
intercambio de cromátides hermanos. La isoforona indujo
transformación morfológica in vitro en ausencia de un sistema
metabólico exógeno. No indujo mutaciones recesivas letales ligadas al
sexo en Drosophila. El peso probatorio de conjunto de datos sobre
mutagenicidad avala la tesis de que la isoforona no es un potente
compuesto ADN-reactivo. En una valoración in vivo no se observó
enlace con el ADN en el hígado y los riñones (órganos afectados en las
biovaloraciones de carcinogenicidad).
En estudios de toxicidad por administración oral a largo plazo en
ratones y ratas, las ratas macho mostraron varias lesiones del riñón,
incluidas nefropatía, hiperplasia de las células tubulares y una baja
incidencia de adenomas y adenocarcinomas de ese tipo de células. Se
ha reconocido el papel que desempeña la acumulación de
alpha2u-globulina en la etiología de estas lesiones. Como no se han
detectado cantidades significativas de alpha2u-globulina en el
hombre, este mecanismo de carcinogénesis no parece ser importante en
la especie humana. Se observaron carcinomas de la glándula prepucial
en cinco ratas macho sometidas a dosis elevadas y dos adenomas de la
glándula clitorídea en ratas hembra expuestas a bajas dosis de
isoforona. Estos tumores pueden estar relacionados también con la
acumulación de alpha2u-globulina. La exposición a la isoforona se
asoció con algunas lesiones neoplásicas del hígado, y de los sistemas
integumentario y linforreticular de los ratones macho, así como con
lesiones no neoplásicas del hígado y de la corteza suprarrenal, pero
esto no se observó en los ratones hembra a los que se administraron
estas dosis.
En el único estudio a largo plazo disponible sobre inhalación en
ratas y conejos, se observaron irritación de los ojos y de la mucosa
nasal, así como cambios pulmonares y hepáticos, a dosis de
approx.1427 mg/m3 (approx. 250 ppm). Sin embargo, estos resultados
pueden haberse debido a las limitaciones del estudio.
Estudios muy limitados en ratas y ratones indican que la
isoforona no afecta a la fecundidad ni produce toxicidad para el
desarrollo en los animales de experimentación.
El hecho de que en los animales de experimentación se produzca
depresión del sistema nervioso central podría indicar un posible
efecto neurotóxico. La isoforona también produjo un efecto positivo
en la prueba de comportamiento de natación desesperada.
1.7 Efectos en el ser humano
El olor de la isoforona puede detectarse a concentraciones de
sólo 1,14 mg/m3 (0,2 ppm). Se ha observado irritación de los ojos,
la nariz y la garganta a concentraciones inferiores a 28,55 mg/m3
(5 ppm); y por encima de 1142 mg/m3 (200 ppm), náuseas, cefalea,
vértigo, desmayo y embriaguez.
1.8 Efectos en otros organismos en laboratorio y sobre el terreno
No se dispuso de datos sobre animales terrestres.
Se dispone de valores de LC50 agudos en varias especies de agua
dulce y marinas. Los valores EC50 en 96 horas (basados en recuento
celular y clorofila) oscilan entre 105 y 126 mg/litro. Los valores
LC50 en 48 horas para Daphnia magna oscilan entre 117 y
120 mg/litro, y los valores LC50 en 96 horas para peces de agua
dulce, entre 145 y 255 mg/litro.
Los valores de la LC50 en 96 horas para animales invertebrados
marinos oscilan entre 12,9 y 430 mg/litro, y el valor de la LC50 en
96 horas para una única especie de pez marino, entre 170 y
300 mg/litro. Los datos de estudios a concentraciones de exposición
medida no fueron diferentes de los obtenidos en estudios a
concentraciones nominales. Los valores NOEL para Pimephales promelas
sometidos a prueba en diferentes laboratorios oscilaron entre 14 y
45,4 mg/litro.
Los datos disponibles parecen indicar que la isoforona tiene una
baja toxicidad para los organismos acuáticos.
2. Conclusiones
2.1 Población general
La isoforona se utiliza como disolvente para formulaciones de
resinas, polímeros y plaguicidas. Puede producirse una exposición
cutánea y por inhalación, pero lo más probable es que sea
insignificante. Los datos muestran que la isoforona puede aparecer en
concentraciones de µg/litro (kg) en el agua de bebida y en los peces.
En vista de la baja toxicidad en los estudios experimentales y de los
bajos niveles de exposición a partir de fuentes ambientales, parece
ser mínimo el riesgo para la población general.
2.2 Exposición ocupacional
A falta de controles técnicos adecuados y de medidas de higiene
industrial, la exposición ocupacional a la isoforona puede superar los
niveles aceptables y producir irritación ocular, cutánea y
respiratoria. En concentraciones superiores pueden producirse otros
efectos sobre la salud. El Grupo Especial no dispuso de estudios
sobre los efectos a largo plazo en la salud de los trabajadores.
2.3 El medio ambiente
La isoforona puede pasar al medio ambiente tras su utilización
como portador de plaguicida y su uso generalizado como disolvente. Se
han identificado concentraciones bajas en varios compartimientos
ambientales, aunque tiene una baja persistencia ambiental a causa de
los procesos de biodegradación, volatilización y oxidación
fotoquímica. Los datos disponibles parecen indicar que la isoforona
tiene una baja toxicidad para los organismos acuáticos.
3. Recomendaciones
3.1 Protección de la salud humana y del medio ambiente
Hay que tener cuidado en prevenir la contaminación de las aguas
subterráneas y del aire.
Los trabajadores que fabrican o utilizan isoforona deben
protegerse de la exposición a ésta por medio de controles técnicos y
medidas de higiene industrial adecuados. Su exposición ocupacional
debe mantenerse dentro de niveles aceptables y vigilarse de forma
regular.
3.2 Investigaciones ulteriores
a) Debe procederse a la vigilancia sanitaria de los trabajadores
expuestos.
b) Deben determinarse los niveles efectivos de isoforona en las
aguas que rodean a las zonas industriales.
c) Deben realizarse estudios adecuados a corto y largo plazo sobre
inhalación en animales de experimentación para determinar los
niveles inocuos de exposición ocupacional.
d) Es necesaria información sobre la biodegradación anaerobia de la
isoforona, especialmente por haberse identificado en la
lixiviación de terraplenados.
 All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico (Vice-Chairman)
Dr G.J. van Esch, Bilthoven, Netherlands
Dr S.K. Kashyap, National Institute of Occupational Health Ahmedabad,
India
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood
Experimental Station Huntingdon, United Kingdom (part-time)
Dr K. Peltonen, Institute of Occupational Health, Helsinki, Finland
Professor Wai-On Phoon, Worksafe Australia, and Department of
Occupational Health, University of Sydney, Sydney, Australia
(Chairman)
Mr D.J. Reisman, US Environmental Protection Agency, Cincinnati, USA
Dr E. Soderlund, National Institute of Public Health, Oslo, Norway
(Rapporteur)
Observer
Dr H. Certa, Hüls AG, Marl, Germany
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer (IARC),
Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
A WHO Task Group on Environmental Health Criteria for Isophorone
met at the World Health Organization, Geneva, from 12 to 16 December
1994. Dr K.W. Jager of the IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to isophorone.
The first draft of the monograph was prepared by Dr H.J. Wiegand
(Hüls), Dr J.F. Regnier (Atochem) and Dr P.L. Mason (British
Petroleum), and appeared as ECETOC-JACC Report No. 10. The second
draft, incorporating comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria, was prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The fact that industry made available to the IPCS and the Task
Group their proprietary toxicological information on isophorone is
gratefully acknowledged. This allowed the Task Group to make its
evaluation on a more complete data base.
The effort of all who helped in the preparation and the
finalization of the document is gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluatione
1.1.1 Physical and chemical properties
Isophorone is a colourless liquid with a peppermint-like odour.
It is soluble in water (12 g/litre) and miscible with most organic
solvents. Its freezing point is -8.1°C and its boiling point 215°C.
Its vapour pressure at 20°C is in the order of 40 Pa, and its vapour
density (air = 1) is 4.7. It is a stable substance.
Commercial samples of technical grade isophorone contain 1-3% of
the isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one); the sum
of alpha and isomers exceeds 99%.
1.1.2 Production and use
Isophorone is widely used as a solvent for a number of synthetic
resins and polymers, as well as in special application paints and
printing inks. It is also a chemical intermediate and a solvent in
certain pesticide formulations.
Its worldwide production was estimated to be in the order of
92 000 tonnes per year in 1988.
1.1.3 Environmental transport, distribution and transformation
Isophorone may enter the environment from numerous industries,
waste and wastewater disposal and its use as a solvent and a pesticide
carrier. Following release to water or soil, environmental
concentrations will decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded to the extent of approximately 70% within 14 days and 95%
within 28 days. The results of biodegradation studies are variable
and limited. Water solubility, soil adsorption coefficients and
polarity indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and
the physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
1.1.4 Environmental levels and human exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg has been reported.
Isophorone has been identified in surface waters (0.6 to 3 µg/litre),
groundwater (10 µg/litre), urban run-off (10 µg/litre) and landfill
leachate (29 µg/litre).
Isophorone has been found in industrial wastewater at a
concentration of 100 µg/litre. After classical secondary treatment,
the concentration of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6 to 12 µg/kg
dry weight) and in the tissues of several species of fish at
concentrations up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice or sugar beet following application as a pesticide carrier.
1.1.5 Kinetics and metabolism in laboratory animals and humans
Distribution studies in rats using 14C-isophorone showed that
93% of orally administered radioactivity appeared mainly in urine and
expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney and preputial
glands.
The metabolites from oral administration of isophorone identified
in rabbits' urine resulted from oxidation of the 3-methyl group,
reduction of the keto group and hydrogenation of the double bond of
the cyclohexene ring, and were eliminated as such or as glucuronide
derivatives in the case of the alcohols.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, with oral LD50 values
being > 1500 mg/kg in the rat, > 2200 mg/kg in the mouse and
> 2000 mg/kg in the rabbit. Dermal LD50 values were 1700 mg/kg in
the rat and > 1200 mg/kg in the rabbit. Acute effects from dermal
exposure in rats and rabbits ranged from mild erythema to scabs.
Conjunctivitis and corneal damage have been reported following direct
application to the eye or exposure to high concentrations of
isophorone. No skin sensitization was reported in guinea-pigs using
the Magnusson-Kligman test.
In acute and short-term oral studies on rodents at high doses
(> 1000 mg/kg), degenerative effects were seen in the liver as well
as CNS depression and some deaths. In 90-day studies, a NOEL in rats
and mice of 500 mg/kg body weight per day was determined. In a 90-day
oral study in beagle dogs (with limited numbers), no effects were seen
at doses of up to 150 mg/kg body weight per day.
In the acute and short-term inhalation experiments which were
reviewed, eye and respiratory irritation, haematological effects and
decreased body weights were noted. Since the study designs were
inadequate, no NOEL could be determined and no inference regarding
human health can be made.
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, DNA repair in primary rat
hepatocytes, or bone-marrow micronuclei in mice. Positive effects were
observed only in the absence of an exogenous metabolic system in
L5178Y TK +/- mouse lymphoma mutagenesis assays as well as in a sister
chromatid exchange assay. Isophorone induced morphological
transformation in vitro in the absence of an exogenous metabolism
system. It did not induce sex-linked recessive lethal mutations in
Drosophila. The weight of evidence of all mutagenicity data
supports the contention that isophorone is not a potent DNA-reactive
compound. In an in vivo assay, no DNA binding was observed in the
liver and kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies in mice and rats, male rats
showed several lesions of the kidney, including nephropathy, tubular
cell hyperplasia and low incidence of tubular cell adenomas and
adenocarcinomas. The role of alpha2u-globulin accumulation in the
etiology of these lesions has been recognized. Since significant
amounts of alpha2u-globulin have not been detected in humans this
mechanism of carcinogenesis appears not to be relevant to humans.
Preputial gland carcinomas were observed in five high-dose male rats,
and two clitoral gland adenomas were seen in low-dose female rats
following exposure to isophorone. These tumours may also be related
to alpha2u-globulin accumulation. Isophorone exposure was
associated with some neoplastic lesions of the liver, and the
integumentary and lymphoreticular systems of male mice, as well as
non-neoplastic liver and adrenal cortex lesions, but this was not
observed in dosed female mice.
In the only available long-term inhalation study in rats and
rabbits, irritation to eye and nasal mucosa, and lung and liver
changes, were observed at approx. 1427 mg/m3 (approx. 250 ppm).
However, it may have been due to limitations in the study.
Very limited studies in rats and mice indicate that isophorone
does not affect fertility nor does it cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural despair
swimming test.
1.1.7 Effect on humans
The odour of isophorone can be detected at a concentration as low
as 1.14 mg/m3 (0.2 ppm). Eye, nose and throat irritation has been
reported at concentrations below 28.55 mg/m3 (5 ppm); above
1142 mg/m3 (200 ppm) nausea, headache, dizziness, faintness and
inebriation have been reported.
1.1.8 Effects on other organisms in the laboratory and field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and
marine species. The 96-h EC50 values (based on cell count and
chlorophyll) range from 105 to 126 mg/litre. 48-h LC50 values for
Daphnia magna range from 117 to 120 mg/litre, and 96-h LC50 values
for freshwater fish range from 145 to 255 mg/litre.
The 96-h LC50 values for marine invertebrates range from 12.9
to 430 mg/litre, while the 96-h LC50 for a single marine fish
species was between 170 and 300 mg/litre. Data from studies with
measured exposure concentrations did not differ from studies with
nominal concentrations. NOEL values for Pimephales promelas tested
in different laboratories ranged from 14 to 45.4 mg/litre.
The available data suggest that isophorone has a low toxicity to
aquatic organisms.
1.2 Conclusions
1.2.1 General population
Isophorone is used as a solvent for resins, polymers and
pesticides formulations. Dermal and inhalation exposure may occur,
but will most likely be minimal. Data show that isophorone can occur
in µg/litre (kg) concentrations in drinking-water and fish. In view
of low toxicity in experimental studies and low levels of exposure
from environmental sources, the risk to the general population appears
to be minimal.
1.2.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin and respiratory irritation. At
higher concentrations other health effects may occur. No studies on
long-term health effects in workers were available for review by the
Task Group.
1.2.3 The environment
Isophorone may be released into the environment following its use
as a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, although it has a low environmental persistence due to
biodegradation, volatilization and photochemical oxidation processes.
The available data suggest that isophorone has low toxicity to aquatic
organisms.
1.3 Recommendations
1.3.1 Protection of human health and the environment
Care should be taken to prevent contamination of groundwater and
air.
Workers manufacturing or using isophorone should be protected
from exposure by means of adequate engineering controls and
appropriate industrial hygiene measures. Their occupational exposure
should be kept within acceptable levels and monitored regularly.
1.3.2 Further research
a) Health surveillance of exposed workers should be conducted.
b) Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
c) Adequate short-term/long-term inhalation studies in experimental
animals should be conducted in order to determine safe levels of
occupational exposure.
d) Information on anaerobic biodegradation of isophorone is needed,
especially as it has been identified in landfill leachate.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Common name: Isophorone
Synonyms: 2-cyclohexen-1-one, 3,5,5,-trimethyl;
3,5,5-trimethyl-2-cyclohexene-1-one;
1,1,3-trimethyl-3-cyclohexene-5-one;
alpha-isophorone; isoacetophorone; isoforone;
izoforon; 1,5,5-trimethyl-3-oxo-cyclohexene
Empirical formula: C9H14O
Chemical structure:
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico (Vice-Chairman)
Dr G.J. van Esch, Bilthoven, Netherlands
Dr S.K. Kashyap, National Institute of Occupational Health Ahmedabad,
India
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood
Experimental Station Huntingdon, United Kingdom (part-time)
Dr K. Peltonen, Institute of Occupational Health, Helsinki, Finland
Professor Wai-On Phoon, Worksafe Australia, and Department of
Occupational Health, University of Sydney, Sydney, Australia
(Chairman)
Mr D.J. Reisman, US Environmental Protection Agency, Cincinnati, USA
Dr E. Soderlund, National Institute of Public Health, Oslo, Norway
(Rapporteur)
Observer
Dr H. Certa, Hüls AG, Marl, Germany
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer (IARC),
Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR ISOPHORONE
A WHO Task Group on Environmental Health Criteria for Isophorone
met at the World Health Organization, Geneva, from 12 to 16 December
1994. Dr K.W. Jager of the IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to isophorone.
The first draft of the monograph was prepared by Dr H.J. Wiegand
(Hüls), Dr J.F. Regnier (Atochem) and Dr P.L. Mason (British
Petroleum), and appeared as ECETOC-JACC Report No. 10. The second
draft, incorporating comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria, was prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The fact that industry made available to the IPCS and the Task
Group their proprietary toxicological information on isophorone is
gratefully acknowledged. This allowed the Task Group to make its
evaluation on a more complete data base.
The effort of all who helped in the preparation and the
finalization of the document is gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluatione
1.1.1 Physical and chemical properties
Isophorone is a colourless liquid with a peppermint-like odour.
It is soluble in water (12 g/litre) and miscible with most organic
solvents. Its freezing point is -8.1°C and its boiling point 215°C.
Its vapour pressure at 20°C is in the order of 40 Pa, and its vapour
density (air = 1) is 4.7. It is a stable substance.
Commercial samples of technical grade isophorone contain 1-3% of
the isomer ß-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one); the sum
of alpha and isomers exceeds 99%.
1.1.2 Production and use
Isophorone is widely used as a solvent for a number of synthetic
resins and polymers, as well as in special application paints and
printing inks. It is also a chemical intermediate and a solvent in
certain pesticide formulations.
Its worldwide production was estimated to be in the order of
92 000 tonnes per year in 1988.
1.1.3 Environmental transport, distribution and transformation
Isophorone may enter the environment from numerous industries,
waste and wastewater disposal and its use as a solvent and a pesticide
carrier. Following release to water or soil, environmental
concentrations will decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded to the extent of approximately 70% within 14 days and 95%
within 28 days. The results of biodegradation studies are variable
and limited. Water solubility, soil adsorption coefficients and
polarity indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and
the physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
1.1.4 Environmental levels and human exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg has been reported.
Isophorone has been identified in surface waters (0.6 to 3 µg/litre),
groundwater (10 µg/litre), urban run-off (10 µg/litre) and landfill
leachate (29 µg/litre).
Isophorone has been found in industrial wastewater at a
concentration of 100 µg/litre. After classical secondary treatment,
the concentration of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6 to 12 µg/kg
dry weight) and in the tissues of several species of fish at
concentrations up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice or sugar beet following application as a pesticide carrier.
1.1.5 Kinetics and metabolism in laboratory animals and humans
Distribution studies in rats using 14C-isophorone showed that
93% of orally administered radioactivity appeared mainly in urine and
expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney and preputial
glands.
The metabolites from oral administration of isophorone identified
in rabbits' urine resulted from oxidation of the 3-methyl group,
reduction of the keto group and hydrogenation of the double bond of
the cyclohexene ring, and were eliminated as such or as glucuronide
derivatives in the case of the alcohols.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, with oral LD50 values
being > 1500 mg/kg in the rat, > 2200 mg/kg in the mouse and
> 2000 mg/kg in the rabbit. Dermal LD50 values were 1700 mg/kg in
the rat and > 1200 mg/kg in the rabbit. Acute effects from dermal
exposure in rats and rabbits ranged from mild erythema to scabs.
Conjunctivitis and corneal damage have been reported following direct
application to the eye or exposure to high concentrations of
isophorone. No skin sensitization was reported in guinea-pigs using
the Magnusson-Kligman test.
In acute and short-term oral studies on rodents at high doses
(> 1000 mg/kg), degenerative effects were seen in the liver as well
as CNS depression and some deaths. In 90-day studies, a NOEL in rats
and mice of 500 mg/kg body weight per day was determined. In a 90-day
oral study in beagle dogs (with limited numbers), no effects were seen
at doses of up to 150 mg/kg body weight per day.
In the acute and short-term inhalation experiments which were
reviewed, eye and respiratory irritation, haematological effects and
decreased body weights were noted. Since the study designs were
inadequate, no NOEL could be determined and no inference regarding
human health can be made.
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, DNA repair in primary rat
hepatocytes, or bone-marrow micronuclei in mice. Positive effects were
observed only in the absence of an exogenous metabolic system in
L5178Y TK +/- mouse lymphoma mutagenesis assays as well as in a sister
chromatid exchange assay. Isophorone induced morphological
transformation in vitro in the absence of an exogenous metabolism
system. It did not induce sex-linked recessive lethal mutations in
Drosophila. The weight of evidence of all mutagenicity data
supports the contention that isophorone is not a potent DNA-reactive
compound. In an in vivo assay, no DNA binding was observed in the
liver and kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies in mice and rats, male rats
showed several lesions of the kidney, including nephropathy, tubular
cell hyperplasia and low incidence of tubular cell adenomas and
adenocarcinomas. The role of alpha2u-globulin accumulation in the
etiology of these lesions has been recognized. Since significant
amounts of alpha2u-globulin have not been detected in humans this
mechanism of carcinogenesis appears not to be relevant to humans.
Preputial gland carcinomas were observed in five high-dose male rats,
and two clitoral gland adenomas were seen in low-dose female rats
following exposure to isophorone. These tumours may also be related
to alpha2u-globulin accumulation. Isophorone exposure was
associated with some neoplastic lesions of the liver, and the
integumentary and lymphoreticular systems of male mice, as well as
non-neoplastic liver and adrenal cortex lesions, but this was not
observed in dosed female mice.
In the only available long-term inhalation study in rats and
rabbits, irritation to eye and nasal mucosa, and lung and liver
changes, were observed at approx. 1427 mg/m3 (approx. 250 ppm).
However, it may have been due to limitations in the study.
Very limited studies in rats and mice indicate that isophorone
does not affect fertility nor does it cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural despair
swimming test.
1.1.7 Effect on humans
The odour of isophorone can be detected at a concentration as low
as 1.14 mg/m3 (0.2 ppm). Eye, nose and throat irritation has been
reported at concentrations below 28.55 mg/m3 (5 ppm); above
1142 mg/m3 (200 ppm) nausea, headache, dizziness, faintness and
inebriation have been reported.
1.1.8 Effects on other organisms in the laboratory and field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and
marine species. The 96-h EC50 values (based on cell count and
chlorophyll) range from 105 to 126 mg/litre. 48-h LC50 values for
Daphnia magna range from 117 to 120 mg/litre, and 96-h LC50 values
for freshwater fish range from 145 to 255 mg/litre.
The 96-h LC50 values for marine invertebrates range from 12.9
to 430 mg/litre, while the 96-h LC50 for a single marine fish
species was between 170 and 300 mg/litre. Data from studies with
measured exposure concentrations did not differ from studies with
nominal concentrations. NOEL values for Pimephales promelas tested
in different laboratories ranged from 14 to 45.4 mg/litre.
The available data suggest that isophorone has a low toxicity to
aquatic organisms.
1.2 Conclusions
1.2.1 General population
Isophorone is used as a solvent for resins, polymers and
pesticides formulations. Dermal and inhalation exposure may occur,
but will most likely be minimal. Data show that isophorone can occur
in µg/litre (kg) concentrations in drinking-water and fish. In view
of low toxicity in experimental studies and low levels of exposure
from environmental sources, the risk to the general population appears
to be minimal.
1.2.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin and respiratory irritation. At
higher concentrations other health effects may occur. No studies on
long-term health effects in workers were available for review by the
Task Group.
1.2.3 The environment
Isophorone may be released into the environment following its use
as a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, although it has a low environmental persistence due to
biodegradation, volatilization and photochemical oxidation processes.
The available data suggest that isophorone has low toxicity to aquatic
organisms.
1.3 Recommendations
1.3.1 Protection of human health and the environment
Care should be taken to prevent contamination of groundwater and
air.
Workers manufacturing or using isophorone should be protected
from exposure by means of adequate engineering controls and
appropriate industrial hygiene measures. Their occupational exposure
should be kept within acceptable levels and monitored regularly.
1.3.2 Further research
a) Health surveillance of exposed workers should be conducted.
b) Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
c) Adequate short-term/long-term inhalation studies in experimental
animals should be conducted in order to determine safe levels of
occupational exposure.
d) Information on anaerobic biodegradation of isophorone is needed,
especially as it has been identified in landfill leachate.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Common name: Isophorone
Synonyms: 2-cyclohexen-1-one, 3,5,5,-trimethyl;
3,5,5-trimethyl-2-cyclohexene-1-one;
1,1,3-trimethyl-3-cyclohexene-5-one;
alpha-isophorone; isoacetophorone; isoforone;
izoforon; 1,5,5-trimethyl-3-oxo-cyclohexene
Empirical formula: C9H14O
Chemical structure:  Relative molecular
mass: 138.2
CAS registry
number:
Relative molecular
mass: 138.2
CAS registry
number:  7. EFFECTS ON LABORATORY MAMMALS, AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity
The acute LD50 values for various routes of exposure are
presented in Table 4.
Table 4. Acute LD50 values for technical (commercial) grade isophorone
Route Species Sex LD50 Reference
(mg/kg)
Oral rat male and female 1500 Schering (1968)
female 2104 Smyth et al. (1969)
male 2700 Dutertre-Catella (1976)
female 2100 Dutertre-Catella (1976)
mouse male and female 2200 Dutertre-Catella (1976)
rabbit male and female 2000 Dutertre-Catella (1976)
female 2000 Smyth et al. (1969)
Dermal rat male and female 1700 Schering (1968)
rabbit male and female 1200 Dutertre-Catella (1976)
7.1.1 Oral
Median lethal doses for isophorone in laboratory mammals ranging
from 1500 to 2700 mg/kg body weight have been reported. The signs of
toxicity were similar to those of solvents and narcotics, prostration
being followed rapidly by coma. Deaths occurred within 24 h,
otherwise recovery was complete. Degenerative changes in the liver
were reported in animals that died (Dutertre-Catella, 1976).
7.1.1.1 ß-Isophorone
An oral LD50 of 2950 mg/kg in rats has been reported for
ß-isophorone (purity 97.5%, containing 2.5% alpha-isophorone). The
predominant systemic effect was non-specific CNS depression shortly
after dosing. Cirrhosis-like changes on the surface of the liver and
severe irritation of the stomach were observed in the animals that
died following dosing (Hüls, 1988d).
7.1.2 Dermal
Dermal LD50 values indicate that isophorone is rapidly absorbed
through the skin under occlusion. During the first 6 h of occluded
application an increase in respiratory rate, followed by prostration
and narcosis, was reported in rabbits exposed to 500-1000 mg/kg.
Occluded skin contact for 24 h resulted in erythema, followed after
several days by scarring. Skin damage was still evident after 14
days. In this study, 10-25 ml isophorone was applied to skin as
compared to 0.5 ml in the skin irritation test (see section 7.2.1)
(Dutertre-Catella, 1976).
7.1.3 Inhalation
Rats and guinea-pigs were exposed to atmospheres containing
isophorone concentrations of 1713 or 4282 mg/m3 (300 or 750 ppm) for
24 h, of 5025 mg/m3 (880 ppm) for 12 h, or of 7823 to 26 266 mg/m3
(1370 to 4600 ppm) for 8 h. In both animal species, in the order of
development, eye and respiratory irritation, lachrymation, ataxia,
dyspnoea, diarrhoea, light narcosis and death were observed.
Postmortem examination of rats dying after exposure to isophorone
showed haemorrhage in the lungs, congestion of the stomach and liver,
peritoneal effusion and discoloration of the kidney and spleen (Smyth
& Seaton, 1940). However, it would appear that some of the
atmospheric concentrations could not have been achieved with pure
isophorone, and therefore the results reported in the study are of
little relevance (Rowe & Wolf, 1963).
Groups of six rabbits and rats were exposed to isophorone for
5 h. The observation period was 2 weeks. At a concentration of
39.9 g/m3 (7000 ppm), 10% of the rats and 30% of the rabbits died.
No LC50 value could be established from this study (Dutertre-
Catella, 1976).
7.2 Skin, eye and respiratory irritation, sensitization
7.2.1 Skin irritation
The skin irritation of isophorone was studied by Truhaut et al.
(1972). A single application of 0.5 ml isophorone under an occlusive
patch for a period of 24 h on the shaved or scarified skin of six
rabbits produced a light erythema which disappeared rapidly after
exposure. Microscopical examination did not show any
histopathological changes.
In the rabbit, occlusive and semi-occlusive contact with 0.5 ml
neat isophorone for 1 or 4 h was non-irritating (Potokar et al.,
1985).
7.2.1.1 ß-Isophorone
A single application of 0.5 ml ß-isophorone (containing 2.5%
alpha-isophorone) under a semi-occlusive patch over a period of 4 h on
the shaved skin of three rabbits produced moderate erythema and
swelling (Hüls, 1988e).
7.2.2 Eye irritation
A single instillation of 0.1 ml isophorone in the eyes of six
rabbits caused opacity in four animals, which in some instances
covered the entire area of the cornea, inflammation of the conjunctiva
and purulent discharge. In a supplementary experiment in which the
rabbits' eyes were washed with 20 ml of warm water 2-4 seconds after
the introduction of 0.1 ml isophorone, considerable recovery from
these effects occurred over 7 days (Dutertre-Catella, 1976).
Grant (1974) reported that application of one drop of isophorone
to rabbit cornea caused mild transient injury, graded 4 on a scale of
1 to 10 after 24 h. Pronounced irritation of eyes and nose occurred
in rats and guinea-pigs exposed to atmospheres containing isophorone
(see section 7.1.3) (Smyth & Seaton, 1940; Smyth et al., 1942).
7.2.2.1 ß-Isophorone
A single instillation of 0.1 ml in the eyes of three rabbits
produced moderate conjunctival and corneal opacities. Iridial changes
were also reported in two animals. Although the corneal and iridial
changes had resolved by 7 days, minor conjunctival irritation was
still in evidence (Hüls, 1988f).
7.2.3 Respiratory irritation
In a study of sensory irritation, De Ceaurriz et al. (1981)
estimated the concentration of various chemicals causing a 50%
decrease in respiratory rate (measured using individual
plethysmographs) in mice (RD50). The RD50 for isophorone was
158.7 mg/m3 (27.8 ppm) for a 5-min exposure. For comparison, the
RD50 values for toluene diisocyanate and acetone were 0.24 and
23 480 ppm, respectively.
Exposure of rats to airborne concentrations of 383-514 mg/m3
(67 or 90 ppm) for a single 4-h period produced statistically
significant reductions in circulating leukocytes. This leukopenia was
considered to be due to the stress-induced release of cortico-steroids
resulting from sensory irritation (Brondeau et al., 1990).
7.2.4 Sensitization
Isophorone, administered at a concentration of 10% intradermally
(in maize germ oil) and 100% topically to female guinea-pigs in the
Magnusson-Kligman test, showed no sensitizing potential (Hüls, 1988a).
7.3 Subchronic toxicity
7.3.1 Inhalation
The effects of repeated whole body exposure to isophorone vapour
were reported by Smyth et al. (1942). Groups of male Wistar rats and
guinea-pigs of both sexes, 16-20 animals per group, were exposed to
isophorone concentrations up to approximately 2855 mg/m3 (500 ppm)
8 h/day, 5 days/week, for 6 weeks. As in the Smyth & Seaton (1940)
study (section 7.1.3), the air concentrations of isophorone could not
have been attained under conditions employed by Smyth et al. (1942).
In the presentation of these results no distinction was made between
the two species and no control data were presented. Growth
retardation was noted in all animals exposed to higher concentrations.
Postmortem examination of animals dying after exposure revealed
severely injured kidneys and lungs. The kidneys of surviving animals
were congested with dilation of Bowman's capsules and cloudy swelling
of tubular cells. The lungs and liver were also reported to be
congested with desquamation of the bronchial epithelium in the lungs
and cloudy swelling in the liver cells.
Dutertre-Catella (1976) exposed groups of 10 male and 10 female
rats to atmospheres reported to contain 2855 mg/m3 8 h/day, 5
days/week for 6 and 4 months, respectively. Two males and one female
exposed to isophorone died; there were no deaths in the control
animals. The only reported effects were irritation of the eyes and
nose.
Groups of 10 male and 10 female young adult Charles River CD rats
were exposed to isophorone at air concentrations of 0 or 250 mg/m3,
6 h/day, 5 days/week, for 4 weeks. Results of daily spectroscopic
determinations indicated that the average daily exposure was
208 mg/m3. Body weight measurements and haematological studies were
made before exposure and after 4 weeks. The rats were killed and
gross necropsy was performed. Organ weights were determined for
lungs, liver, kidneys, adrenals and spleen. Histological examination
of those tissues were performed in three males and three females per
group. The following effects were observed: transient nasal bleeding,
increased percentages of lymphocytes, decreased percentages of
neutrophils and increased haemoglobin concentration in males and
females and significantly lower terminal body weights and
significantly decreased absolute and relative liver weights of exposed
males, compared with controls (Littlefield, 1968).
7.3.2 Oral
Four groups of 20 male and 20 female albino rats were fed diets
containing 0, 750, 1500 or 3000 mg isophorone/kg (0, 37.5, 75 or
150 mg/kg body weight) for 90 days. Isophorone in corn oil (ratio
1:2) was blended with the diet; fresh diets were prepared each week.
Haematology, serum chemistry and urine analyses were carried out on
five animals of each sex from each group at week 4 and at termination.
Comprehensive histopathological examination was confined to five
animals of each sex from the control and high dose groups. The liver
and kidney from five animals of the intermediate dose levels were also
examined histopathologically. Under the conditions of this study, no
effects on the general appearance of the test animals, on their
behaviour, on body weight gain or on food consumption were observed at
a dietary level of 1500 mg/kg or less. Isophorone did not alter the
composition of the formed elements of the blood, nor did it interfere
with the general metabolism or with liver and kidney function. No
detectable gross or microscopic pathological changes were noted in any
of the animals examined after 28 or 90 days of feeding. Organ/body
weight ratios for vital organs were not changed. The only adverse
finding reported was a reduction in body weight gain in the male rats
receiving the highest dose (Affiliated Medical Enterprises, 1972a).
As part of a preliminary investigation prior to an NTP
carcinogenicity study, five rats (F-344) and five mice (B6C3F1) of
each sex were given by gavage 12 oral doses of up to 2000 mg
isophorone/kg body weight administered in corn oil over a 16-day
period (US NTP, 1986). Lethargy was reported in all rats following
dosing while in mice uncertain locomotion was reported among animals
receiving 1000 mg/kg. All mice and 50% of the rats receiving
2000 mg/kg died. The weight gain of animals surviving 2000 mg/kg and
all animals receiving 1000 mg/kg was reduced. As part of the same
programme, 10 rats and mice of each sex were given by gavage daily
doses of 0, 62.5, 125, 250, 500 or 1000 mg isophorone/kg body weight
for 90 days (US NTP, 1986). The rats were drowsy and lethargic
following administration. At the highest dose level, one female rat
and three female mice died. No macroscopic or microscopic changes
were observed in the organs examined from either study. A subsequent
histopathological review of the kidney, which included re-sectioning
and additional staining, also failed to reveal any treatment-related
effects. The no-observed-effect level in this study was considered to
be 500 mg/kg body weight per day.
Groups of four male and four female beagle dogs were given 90
daily oral doses of isophorone in gelatin capsules at dosage rates of
35, 75 or 150 mg/kg per day. Comprehensive haematology, clinical
chemistry and urine analyses were carried out initially and at 1, 2
and 3 months. Apart from a mild intermittent incidence of soft stools
in animals of the high-dose group, there was no observable effect as
demonstrated by the data on general appearance and conditions as well
as those from haematological or biochemical investigations. At
autopsy, no changes in the organ/body weight ratios and no
histopathological changes were observed. No toxic effect was found at
any of the dose levels used. It was concluded that the no-observed-
effect level for isophorone in the dog was 150 mg/kg per day
(Affiliated Medical Enterprises, 1972b).
7.3.3 Dermal
The daily occluded application on shaved and abraded skin of 0.1
or 0.2 ml of isophorone to rats for 8 weeks produced erythema and
scabs at the site of application (Dutertre-Catella, 1976). The only
apparent systemic effect reported was an 8% reduction in mean weight
gain in the females compared with controls; the dose levels at which
this occurred were not given.
7.4 Mutagenicity
The results of mutagenicity tests are summarized in Table 5.
7.4.1 Gene mutation in bacteria (Ames tests)
The mutagenic potential of isophorone was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100, following a preincubation protocol. The test substance
concentrations used were between 10 and 5000 µg per plate. There was
no increase in the number of revertants at any of the concentrations
tested with and without rat liver S9 fraction (Hüls, 1988b).
Two further studies were conducted on Salmonella typhimurium
TA1535, TA1537 and TA1538 strains (1 to 1000 µg/plate) (Atochem,
1978a) and TA98, TA100, TA1535 and TA1537 strains (100 to
10 000 µg/plate) (US NTP, 1986). No evidence of mutagenic potential
was provided in the presence or absence of rat or hamster liver S9
fractions.
Salmonella strains TA1535, TA1537, TA97, TA98 and TA100 were
used to test isophorone (up to 10 000 µg/plate) with and without
Aroclor 1254-induced rat and hamster metabolic activation systems
(male Sprague-Dawley rats and male Syrian hamsters). When negative
results were obtained in the initial assay, the chemicals were
retested in all strains with and without activation. Isophorone
showed no mutagenic activity (Mortelmans et al., 1986).
7.4.1.1 Dihydroisophorone
The mutagenic potential of dihydroisophorone (iii in Fig. 1, see
section 6.2), a metabolite of isophorone, was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100 at concentrations of 25 to 2500 µg per plate. There was no
increase in the number of revertants at the concentrations tested,
either with or without rat liver S9 fraction (BP, 1988a).
Table 5. Isophorone genotoxicity tests
Test system Results Reference
Gene Ames test (with and negative Atochem (1978a); US NTP
mutation without S9) (1986); Mortelmans et
al. (1986); Hüls (1988b)
mouse lymphoma assay positive US NTP (1986)
(without S9)
(with and without S9) negative Microbiological Associates
(1984a)
(with and without S9) positive McGregor et al. (1988)
(with and without S9) negative O'Donoghue et al. (1988)
positive Mitchell (1993)
sex-linked recessive negative Foureman et al. (1994)
lethal in Drosophila
melanogaster
Chromosome CHO cells (in vitro) negative US NTP (1986)
aberrations (with and without S9) negative Gulati et al. (1989)
Micronucleus mouse (in vivo) negative Atochem (1978b); Microbio-
test logical Associates (1984b);
O'Donoghue et al. (1988)
Sister CHO cells (in vitro)
chromatid (without S9) positive US NTP (1986)
exchanges (with S9) negative US NTP (1986)
(without S9) positive Gulati et al. (1989)
Direct DNA unscheduled DNA negative Microbiological Associates
damage synthesis (primary rat (1984c); O'Donoghue et al.
hepatocyte culture) (1988)
DNA binding liver and kidney DNA negative Thier et al. (1990);
in vivo (mouse and rat) Thier & Xu (1990)
Morphological BALB/c-3T3 cells positive Matthews et al. (1993)
transformation (without S9)
7.4.2 Gene mutation in mammalian cells
Isophorone was tested in the L5178Y TK +/- mouse lymphoma
mutagenesis assay (MLA) in the presence and absence of rat liver S9
fraction. The experiment was performed only once and all doses were
tested in duplicate. In the absence of S9, isophorone concentrations
of 0.13 to 1.3 ml/litre produced total growth of 111% to 12% compared
to the control. In the presence of S9, isophorone concentrations of
0.067 to 0.89 ml/litre produced total growth of 86 to 9% compared to
the control. None of these cultures exhibited mutation frequencies
which were significantly greater than the mean mutation frequency of
the solvent control (Microbiological Associates, 1984a; O'Donoghue et
al., 1988). The authors pointed out that while the results of the MLA
without metabolic activation were negative in the present study, these
assays were positive in the NTP study and thus were not reproducible
between laboratories. In contrast to O'Donoghue et al. (1988),
McGregor et al. (1988) reported isophorone to be positive in the MLA
both with and without rat liver S9 mix (from male Fischer-344 rats).
Isophorone was toxic to the cultures only at doses of 600 ml/litre or
more. The authors viewed the experiment with reservations since the
cloning efficiency was low.
Another MLA study employing concentrations of 400 to
1200 mg/litre was carried out in the absence of rat liver S9 fraction.
Duplicate experiments produced gradations of total growth relative to
control values of 112 or 118% for the low concentrations to 7 or 14%
for the high concentrations. Dose-related increases in mutation
frequencies compared with control were observed; at the highest dose
level the increase was 4-fold (US NTP, 1986).
Mitchell (1993) compared the induction of mutation frequencies by
the in situ variant (ISV) approach with the standard MLA.
Isophorone was one of the compounds tested, and showed a higher
induced mutation frequency with the ISV approach than in the standard
MLA.
Isophorone was tested for its ability to induce sex-linked
recessive lethal mutations in post-meiotic and meiotic germ cells of
male Drosophila melanogaster. No induction was observed at 2000 ppm
by feeding or at 15 000 ppm by injection (Foureman et al., 1994).
7.4.3 Chromosome aberrations and sister chromatid exchange
7.4.3.1 Chromosome aberrations
Isophorone was tested in an in vitro cytogenetic assay using
Chinese hamster ovary (CHO) cell cultures. Treatments were performed
both in the absence and presence of rat liver S9 fraction. Under the
conditions of this test, isophorone did not induce chromosomal
aberrations (ABS) at concentrations up to 1600 or 1500 mg/litre,
respectively (US NTP, 1986).
Twenty-seven chemicals (including isophorone), previously tested
in rodent carcinogenicity assays were tested for induction of ABS in
CHO cells as part of a more extensive analysis of the correlation
between results of in vitro genetic toxicity assays and
carcinogenicity bioassays. Chemicals were tested up to toxic doses
both with and without exogenous metabolic activation. A liver
fraction (S9) prepared from Aroclor 1254-induced male Sprague-Dawley
rats was used to provide exogenous metabolic activation. No ABS were
observed in CHO cells following incubation with isophorone in either
the presence or the absence of S9 (Gulati et al., 1989).
7.4.3.2 Sister chromatid exchange
The ability of isophorone to induce sister chromatid exchange
(SCE) was studied in CHO cells, in the presence and absence of rat
liver S9 fraction at concentrations up to 1000 mg isophorone/litre.
Without S9, isophorone induced a small but dose-related increase in
the frequencies of SCE. No effect was observed in the presence of S9
(US NTP, 1986).
A significant increase in SCE frequency with isophorone was
observed only in the absence of S9, at doses of 500-1000 mg/litre
(Gulati et al., 1989 - see also section 7.4.3.1). At these high dose
levels, isophorone is extremely cytostatic; therefore, the increase in
SCE frequency was observed only after delayed harvest (6-13 h
additional culture time).
7.4.4 Micronucleus test
Isophorone doses of 450, 900 and 1800 mg/kg body weight were
administered by gavage to CFLP mice of both sexes in two equal doses
separated by an interval of 24 h. Six hours after the last treatment
the mean micronucleated cell counts and the bone marrow cytotoxicity
were similar in all test groups and controls (Atochem, 1978b).
Male and female CD-1 mice were treated by intraperitoneal
injection of isophorone (0.54 ml/kg body weight). There was no
evidence of micronuclei or cytotoxicity in bone marrow samples
collected 12, 24 and 48 h after administration (Microbiological
Associates, 1984b; O'Donoghue et al., 1988).
7.4.5 Primary DNA damage
7.4.5.1 Bacterial tests
In the Bacillus subtilis (strain H17) rec-assay, isophorone
showed DNA-damaging potential without metabolic activation and a
reverse effect with activation (Matsui et al., 1989).
Isophorone did not induce the SOS function responses in the umu
test using Salmonella typhimurium strain TA1535/pSK1002 (Ono et al.,
1991).
7.4.5.2 Unscheduled DNA synthesis
Isophorone was tested at dose levels ranging from 0.005 to
0.40 ml/litre using primary rat cultures of hepatocytes. There was no
increase in the mean nuclear grain count compared to the controls or
in the incidence of cells undergoing repair at any dose level
(Microbiological Associates, 1984c; O'Donoghue et al., 1988).
7.4.5.3 DNA binding
A DNA-binding study was performed with radiolabelled 1,3,5-
14C-isophorone (Thier et al., 1990; Thier & Xu, 1990). Male and
female F-344 rats and male and female B6C3F1 mice received doses
(500 mg/kg body weight) of unlabelled isophorone containing 0.4 mCi
(per rat) and 0.8 mCi (per mouse) labelled isophorone in neutral oil
by gavage. No binding of the radioactivity to liver or kidney DNA was
observed in either species.
7.4.6 Morphological transformation
Isophorone was positive in a standard transformation assay using
BALB/c-3T3 cells without exogenous activation at concentrations of
1.07 g/litre (7.76 mmol/litre) (Matthews et al., 1993).
7.5 Chronic toxicity and carcinogenicity
In 18-month inhalation studies, groups of 10 rats and 2 rabbits
of each sex were exposed to atmospheres containing 1413 mg
isophorone/m3 (250 ppm) for 6 h/day, 5 days/week. Slight
conjunctivitis and irritation of the nasal mucosa with a bloody
discharge were observed. In the lungs of the animals, frequent
haemorrhages were found with oedema in the alveoli. Vacuolization was
found in the liver of the treated animals (Dutertre-Catella, 1976).
Toxicological and carcinogenesis studies of isophorone (more than
94% pure) were conducted by administering 0, 250 or 500 mg
isophorone/kg body weight per day by gavage in corn oil to groups of
50 F-344/N rats and 50 B6C3F1 mice of each sex, 5 days per week for
103 weeks. Throughout the 2-year study, the mean body weights of the
high-dose male rats were on average 5% less than those of the vehicle
controls. During the second year, the mean body weights of the female
high-dose rats were on average 8% less than those of the vehicle
controls, and the high-dose female mice were 5% lower. The survival
of high-dose male rats was significantly lower than that of the
vehicle controls after week 96 (final survival: vehicle control,
33/50; low dose, 33/50; high dose, 14/50). The survival of dosed
female rats was poor (30/50; 23/50; 20/50), due in part to 20 gavage-
related accidental deaths of dosed animals. The survival of male mice
was also low (16/50; 16/50; 19/50), but there was a significant trend
toward increased survival of dosed female mice relative to that of the
vehicle controls (26/50; 35/50; 34/50). Dosed male rats showed a
variety of proliferative lesions of the kidney (tubular cell
hyperplasia: 0/50; 1/50; 4/50; tubular cell adenoma: 0/50; 0/50; 2/50;
tubular cell adenocarcinoma: 0/50; 3/50; 1/50; epithelial hyperplasia
of the renal pelvis: 0/50; 5/50; 5/50). Dosed male rats also
exhibited increased mineralization of the medullary collecting ducts
(1/50; 31/50; 20/50), and low-dose male rats showed a more severe
nephropathy than is commonly seen in ageing F-344/N rats. Carcinomas
of the preputial gland were increased in high-dose male rats (0/50;
0/50; 5/50). With the exception of a moderate increase in nephropathy
(21/50; 39/50; 32/50), female rats did not show chemically related
increased incidences of neoplastic or non-neoplastic lesions. In
high-dose male mice, isophorone exposure was associated with increased
incidences of hepatocellular adenomas and carcinomas (18/48; 13/50;
29/50) and of mesenchymal tumours of the integumentary system
(fibroma, fibrosarcoma, neurofibrosarcoma or sarcoma: 6/48; 8/50;
14/50). An increased incidence of lymphomas or leukaemias was noted
in low-dose male mice (8/48; 18/50; 5/50). Coagulative necrosis
(3/48; 10/50; 11/50) and hepatocytomegaly (23/48; 39/50; 37/50) were
observed more frequently in the livers of dosed male mice than in
vehicle controls. No compound-related neoplastic or non-neoplastic
lesions associated with isophorone exposure were seen in female mice.
Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenicity of isophorone in male F-344/N rats as
shown by the occurrence of renal tubular cell adenomas and
adenocarcinomas in animals given 250 or 500 mg/kg per day; carcinomas
of the preputial gland were also observed at increased incidence in
male rats given 500 mg/kg. There was no evidence of carcinogenicity
in female F-344/N rats given 250 or 500 mg/kg per day. For male
B6C3F1 mice, there was equivocal evidence of carcinogenicity of
isophorone as shown by an increased incidence of hepatocellular
adenomas or carcinomas (combined) and of mesenchymal tumours in the
integumentary system in animals given 500 mg/kg per day and by an
increase in malignant lymphomas in animals given 250 mg/kg per day.
There was no evidence of carcinogenicity of isophorone in female
B6C3F1 mice given 250 or 500 mg/kg per day (US NTP, 1986).
In the above NTP study, preputial gland carcinomas were observed
in five high-dose male rats. The apparent absence of this tumour in
vehicle controls or in the low-dose group, and the very low incidence
(12/1094, i.e. 1%) in corn oil vehicle controls in previous 2-year
studies, suggest that this may be a chemical-related effect. No
preputial gland tumours were observed in male mice, but two
histogenetically related clitoral gland adenomas were seen in low-dose
female rats, providing some support for an association of isophorone
exposure with this tumour type (Bucher et al., 1986).
7.6 Mechanisms of toxicity
Swenberg et al. (1992) and US EPA (1991a,b) reviewed the
mechanisms involved in alpha2u-globulin nephropathy and renal
carcinogenesis of several chemicals in various animal, biochemical and
molecular modelling systems. All of the data are consistent with the
hypothesis that reversible binding of chemicals or their metabolites
to this abundant protein is causally related to the induction of
disease. Alpha2u-globulin, found mainly in male rats, is
synthesized by the liver and subsequently transported to the kidney.
It is normally present in the cytoplasm of the proximal convoluted
tubules of untreated animals in the form of hyaline droplets, visible
by light microscopy. Xenobiotics, or their metabolites, are bound to
the alpha2u-globulin in the liver of animals; this conjugate is even
more difficult to hydrolyse than the alpha2u-globulin itself and
induces the formation of the hyaline droplets which accumulate in the
tubules (Swenberg et al., 1989). Accumulation of the chemical/
alpha2u-globulin complex causes lysomal protein overload and
necrosis of the cells with subsequent cellular regeneration. Thus
cellular proliferation may contribute to the development of renal
tumours.
In order to determine whether isophorone and other compounds that
cause alpha2u-globulin to accumulate have the same binding
characteristics, binding studies were conducted with kidney cytosol
preparations from male Fischer-344 rats. The inhibition constant
value (Ki) for isophorone was in the range of 10-6 to
10-7 mol/litre, while those for d-limonene, 1,4-dichlorobenzene
and 2,5-dichlorophenol were higher (around 10-4 mol/litre). This
suggests that other factors besides binding are involved in the
accumulation of alpha2u-globulin. Results so far indicate that
binding is dependent on both hydrophobic interactions and hydrogen
bonding (Borghoff et al., 1991).
Investigations (Charbonneau et al., 1988; Strasser et al., 1988)
have shown that isophorone, isophorol and dihydroisophorone bind to
alpha2u-globulin, resulting in an increased accumulation of hyaline
droplets in renal tubular cells. These findings suggest the same
sequence of events may be responsible for the small increase in the
incidence of renal tubular neoplasias seen in male rats.
alpha2u-Globulin was not detected at significant levels in plasma
and urine of female rats or either sex of mice and humans (Swenberg et
al., 1989; Olson et al., 1990). In addition, other laboratory
rodents, dogs and primates do not develop hydrocarbon-related
nephropathy (Swenberg et al., 1989). For these reasons, it was
concluded that the low increase in the incidence of tubular adenomas
and adenocarcinomas observed in male rats following isophorone
administration was attributable to the accumulation of
alpha2u-globulin and was sex- and species-specific (Charbonneau &
Swenberg, 1988; Strasser et al., 1988; Swenberg et al., 1989).
Further evidence to support this view was gained by a study of
Dietrich & Swenberg (1991) using male animals of the NCI Black Reiter
(NBR) strain, which does not synthesize alpha2u-globulin. It was
shown that an oral dose (gavage) of 1000 mg isophorone/kg on four
consecutive days failed to produce the early features of the
alpha2u-globulin-related nephropathy syndrome (i.e. hyaline droplets
or alpha2u-globulin) in this strain.
In terms of human risk assessment, renal tubule tumours produced
in male rats in association with chemicals inducing alpha2u-globulin
accumulation should be distinguished from renal tubule tumours of
other origin (Borghoff et al., 1990; Swenberg, 1991; US EPA, 1991a,b).
It has been recognized that the induction of such tumours does not
necessarily indicate a potential carcinogenic hazard to humans. The
significance of this information with respect to the etiology of
pathological changes is uncertain at present.
Studies have shown that high levels of alpha2u-globulin and its
messenger RNA (mRNA) are present in the preputial gland of both male
and female rats. The preputial gland in both sexes contained about 3
times more alpha2u-globulin mRNA and about 300 times more
alpha2u-globulin than the male rat liver (Murty et al., 1987). It
was claimed that this high content of alpha2u-globulin was primarily
due to the cellular and ductal accumulation of the protein in the
preputial gland but did not reflect a difference in the rate of
transcription of the alpha2u-globulin gene. The high amount of
radiolabel derived from 14C-isophorone found in the preputial gland
of the male rats following single gavage dosing with 5 ml/kg (Strasser
et al., 1988) may therefore be related to its high content of
alpha2u-globulin.
7.7 Appraisal for mutagenicity/carcinogenicity
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, bone-marrow micronuclei in mice or
DNA repair in primary cultures of rat hepatocytes. No DNA binding in
vivo was observed in a DNA binding study using 1,3,5-
14C-isophorone. In one study in the absence and in another study in
the absence and presence of metabolic activation, weak mutagenic
responses were obtained in three out of five L5178Y TK +/- mouse
lymphoma mutagenesis assays and a small increase of sister chromatid
exchange (SCE) was found only without metabolic activation in CHO
cells. Another study with SCE showed no increases in chromosomal
aberrations (for details see section 7.4). Isophorone was negative in
a test for sex-linked recessive lethal mutations in Drosophila. It
induced morphological transformation in mouse cells without
activation.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound.
There was no DNA binding in the liver and kidneys (sites affected in
the carcinogenicity bioassays).
The available data on in vitro mutagenicity and related end-
points do not suggest that isophorone is genotoxic.
When tested in a two-year carcinogenicity bioassay, isophorone
produced a variety of lesions of the kidneys, such as severe
nephropathy and tubular cell hyperplasia. A low but statistically
significant increase in the frequency of renal tubular cell adenomas
and adenocarcinomas was found in male F-344 rats dosed at levels of
250 and 500 mg/kg per day (see section 7.5).
An increase in the number of carcinomas of the preputial gland
was found only in male rats given 500 mg/kg per day. In high-dose
male B6C3F1 mice hepatocellular adenomas and carcinomas were
observed. No compound related neoplastic lesions were seen in female
rats (for details, see section 7.5).
The induction of these two types of tumours in male rats may be
associated with nephropathy, epithelial hyperplasia and
alpha2u-globulin formation and accumulation of the isophorone/
alpha2u-globulin complex, causing lysomal protein overload and
necrosis of the kidney cells with subsequent cellular regeneration
(for details see section 7.6).
7.8 Reproduction, embryotoxicity, teratogenicity
The teratogenicity of isophorone to rats and mice was studied by
Traul et al. (1984). Groups of 22 confirmed mated females of each
species were exposed 6 h/day on days 6-15 of gestation to atmospheres
containing 0, 143, 285 or 656 mg/m3 (0, 25, 50 or 115 ppm)
isophorone. At the highest atmospheric concentration there was
evidence of maternal toxicity which showed as reduced food
consumption, alopecia and cervical or anogenital staining in the rats
and reduced body weights in the mice. Comprehensive uterine and fetal
examinations did not show any significant differences between animals
exposed to isophorone and their respective controls. From the results
of this study, it can be concluded that no teratogenic or fetotoxic
effect was observed with 656 mg/m3 in F-344 rats and CD-1 mice.
In a study by Dutertre-Catella (1976), groups of 10 rats of each
sex, exposed 6 h/day, 5 days/week to 2855 mg/m3 (500 ppm) isophorone
for 3 months, were mated to exposed animals or to controls. All
females were reported to have delivered 7-10 pups. Anatomo-
pathological examination did not show any abnormalities. However,
only one isophorone concentration was used, the group size was small,
and no information was provided on reproductive success and maternal
survival.
7.9 Neurotoxicity
Central nervous system depression is a characteristic feature of
isophorone intoxication in experimental animals (Smyth & Seaton, 1940;
Dutertre-Catella, 1976; De Ceaurriz et al., 1981).
Isophorone and a number of aliphatic ketones have been studied
using the mouse behavioural despair swimming test (De Ceaurriz et al.,
1984). This test, which was developed for screening antidepressant
drugs, is based on the duration of the periods of immobility exhibited
by mice when placed in water. Mice were exposed to isophorone at
atmospheric concentrations of 508-782 mg/m3 (89-137 ppm) for 4 h.
The ID50 (an estimated concentration producing a 50% reduction in
the immobility time) for animals thus exposed was 628 mg/m3
(110 ppm).
7.10 Other special studies
One of the current research approaches for assessing cytotoxicity
is to monitor the respiratory activity of the mitochondrion, a
sensitive, nonspecific subcellular target site. Detected changes in
mitochondrial function after the addition of a test chemical could be
correlated to toxic effects. In this case, rat (male, Charles River)
liver mitochondria were used, and isophorone was shown to induce no
observable effects on rat liver mitochondria respiratory activity over
the tested concentration range of 0.273-113.8 mg/litre
(1.98-825 µmol/litre) (Haubenstricker et al., 1990a).
Isophorone was tested for its ability to perturb glutathione
(GSH) levels in the testes and epididymides, as well as liver,
following single acute dosages to rats. Groups of four mature male
Sprague-Dawley rats were administered isophorone intraperitoneally
(2 ml/kg). Concurrent control groups were maintained for all time
points examined to account for possible circadian fluctuation of GSH
throughout the day. Animals were sacrificed at 1, 2, 4, 8 or 16 h
after administration of the test compound. Liver, testes and
epididymides were excised for determination of total GSH content by
spectrophotometry. Isophorone (500 mg/kg) significantly reduced GSH
in the liver and in both reproductive organs examined. The ability of
isophorone to enhance the covalent binding of tritiated ethyl
methanesulfonate (3H-EMS) to spermatocytes was assessed.
Perturbation of reproductive tract GSH by isophorone treatment
significantly enhanced the extent of 3H-EMS-induced binding to sperm
heads. The authors postulate that chemical-induced lowering of GSH in
the male reproductive tract may be a mechanism for potentiation of
chemical-induced germ-cell mutations (Gandy et al., 1990).
8. EFFECTS ON HUMANS
8.1 Acute
Groups of 11 or 12 subjects exposed for a few minutes to
atmospheric isophorone concentrations of 228 mg/m3 (40 ppm) or more
experienced irritation of the eyes, nose and throat. A few complaints
of nausea, headache, dizziness, faintness, inebriation and a feeling
of suffocation occurred at concentrations 1142 mg/m3 (> 200 ppm).
The symptoms of irritation and narcosis were said to be less intensive
following exposure to concentrations below 1142 mg/m3 (Smyth &
Seaton 1940). However, this study has been criticized because of
uncertain actual concentrations and the use of impure substances (Rowe
& Wolf, 1963).
8.2 Sub-chronic
Complaints of fatigue and malaise were reported among workers
exposed for 1 month to atmospheres containing 28.5 to 48 mg
isophorone/m3 (5 to 8 ppm) (Communication from Ware, GD, to
Chairman, TLV Committee, 1973). No further complaints were received
following a reduction of the concentration to between 5.7 and
22.8 mg/m3 (1 and 4 ppm). On the basis of these data, the American
Conference of Governmental Industrial Hygienists (ACGIH, 1986)
recommended a ceiling limit (15 min) of 29 mg/m3 (5 ppm) for
isophorone.
8.3 Irritation and sensitization
No report on skin irritation or skin sensitization has been
published.
8.3.1 Eye and respiratory irritation
Smyth & Seaton (1940) reported eye, nose and throat irritation
following exposure to isophorone levels of 228 mg/m3 (40 ppm) or
more.
Silverman et al. (1946) estimated the sensory threshold of a
number of ketones including isophorone. An average of 12 subjects of
both sexes were used for each solvent exposure. The time of exposure
was 15 min. Irritation of the eyes, nose and throat was experienced
at 142.7 mg/m3 (25 ppm) isophorone; the highest concentration
considered acceptable by the majority of subjects for an 8-h exposure
was 57.1 mg/m3 (10 ppm).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found that the workshift (6´ h)
average exposures of printers to isophorone were 4 and 80 mg/m3
(0.7 and 14 ppm). Symptoms of respiratory tract and eye irritation
reported by workers were attributed to the antistatic agent containing
principally isophorone.
Amoore & Hautala (1983) reported an air odour threshold for
isophorone of 1.14 mg/m3 (0.2 ppm). The "odour safety factor",
which was defined as the threshold limit value 28.5 mg/m3 (5 ppm)
divided by the odour threshold, was 25. From the magnitude of this
value the authors predicted that 50% of exposed persons would perceive
sensory warning of the TLV (28.5 mg/m3, 5 ppm).
8.4 Chronic toxicity and carcinogenicity
No long-term surveys have been carried out on occupationally
exposed workers or other potentially exposed populations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Yoshioka et al. (1985) studied the acute toxicity of isophorone
in Tetrahymena pyriformis. The EC50 after a 24-h exposure was
420 mg/litre. Yoshioka et al. (1986) found that the EC50 in the
Activated Sludge Respiration Inhibition Test was 100 mg/litre.
In a 2-day laboratory test with the marine red alga Champia
parvula, 38.3 mg isophorone/litre caused 50% reduction in cystocarp
formation. Above 138.5 mg/litre no cystocarps were formed, indicating
absence of sexual reproduction. In a 2-week laboratory test,
107.3 mg/litre completely inhibited cystocarp formation (Thursby &
Steele, 1986).
Isophorone was tested in air-saturated media with Saccharomyces
cerevisiae at concentrations of 0.272 to 113.7 mg/litre
(1.97-824 µmol/litre). These produced no detectable effect on the
rate of yeast respiration as measured with a dissolved oxygen
electrode (Haubenstricker et al., 1990b).
9.2 Aquatic organisms
The acute toxicity of isophorone to fish, crustaceans and algae
is summarized in Table 6. With the exception of Mysidopsis bahia
(US EPA, 1978) all acute LC50 values were above 100 mg/litre,
indicating a relatively low aquatic toxicity. This was consistent
with the results of a subacute (14 day) study with the marine red alga
Champia parvula. The toxicity of isophorone was determined by means
of various biological end-points, namely vegetative growth, formation
of tetrasporangia (asexual reproduction) and production of cystocarps
(sexual reproduction). Depending on the toxicological end-point, the
lowest concentration resulting in a significant difference from
controls ranged between 50 and 138 mg/litre (Thursby et al., 1985).
In addition to the results of acute toxicity tests shown in Table
6, an early life stage toxicity test was conducted with the freshwater
fathead minnow Pimephales promelas (Cairns & Nebeker, 1982) using
concentrations of 11, 19, 30, 56 and 112 mg isophorone/litre.
Survival was affected at a concentration of 112 mg/litre, but not at
56 mg/litre or less; fork length was affected at 30 mg/litre but not
at 19 mg/litre or less; body weight gain was decreased at 19 mg/litre
or more but not at 11 mg/litre. The authors calculated a no-observed-
effect concentration of 14 mg/litre. Using the same test with the
same species, Lemke et al. (1983) found a no-observed-effect level of
19.5 mg/litre, and in an interlaboratory (6 laboratories) comparison
no-observed-effect levels of up to 45.4 mg/litre were found (Lemke,
1983).
9.3 Terrestrial organisms
No data are available concerning the effects of isophorone on
terrestrial organisms.
Table 6. Acute toxicity of technical isophorone to aquatic species
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Fish
Bluegill sunfish LC50 96 static 220 (n) US EPA (1978)
(Lepomis macrochirus) LC50 24 static 240 (n) Buccafusco et al. (1981)
Fathead minnow LC50 96 flow 145-255 (m) Cairns & Nebeker (1982)
(Pimephales promelas) LC50 96 228 Geiger et al. (1990)
Golden orfe LC50 72 204 Scheubel & Scholz (1989)
(Leuciscus idus melanotus)
Sheepshead minnow LC50 96 140 Ward et al. (1981)
(Cyprinodon variegatus) LC50 96 static > 170, < 300 (n) Heitmuller et al. (1981)
Invertebrates
Brine shrimp LC50 24 430 Price et al. (1974)
(Artemia salina)
Mysid shrimp LC50 96 static 12.9 US EPA (1978)
(Mysidopsis bahia)
Water flea immobilization: EC50 24 static 430 (n) LeBlanc (1980)
(Daphnia magna) Immobilization: EC50 24 static 254-277 Scholz (1988a)
immobilization: EC50 48 static 120 (n) LeBlanc (1980)
immobilization: EC50 48 static 117 US EPA (1978)
Algae
Selenastrum capricornutum cell count: EC50 96 static 122 US EPA (1978)
chlorophyll: EC50 96 static 126 US EPA (1978)
Table 6 (cont'd)
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Skeletonema costatum cell count: EC50 96 static 105 US EPA (1978)
chlorophyll: EC50 96 static 110 US EPA (1978)
Scenedesmus subspicatus cell count: EC50 72 475-476 Scholz (1988b)
chlorophyll: EC50 72 524-527 Scholz (1988c)
Red alga reproduction EC50 24 38.3 Thursby & Steele (1986)
(Champia parvula)
a static = static exposure; flow = flow-through exposure
b n = nominal exposure concentration; m = measured exposure concentration
REFERENCES
ACGIH (1986) Documentation on the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
Affiliated Medical Enterprises Inc. (1972a) 90-day sub-chronic
toxicity of isophorone in the rat. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Affiliated Medical Enterprises Inc. (1972b) 90-day sub-chronic
toxicity of isophorone in the dog. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Amoore JE & Hautala E (1983) Odor as an aid for chemical safety: Odor
threshold limit values and volatilities for 214 industrial chemicals
in air and water dilution. J Appl Toxicol, 3: 272-290.
Atochem (1978a) Ames metabolic activation test to assess the potential
mutagenic effect of isophorone. Paris, Atochem (Report No. UKM
52/78204, prepared by Huntingdon Research Centre).
Atochem (1978b) Micronucleus test on isophorone. Paris, Atochem
(Report No. UKM 52/78457, prepared by Huntingdon Research Centre).
Atochem (1986) Data sheet on isophorone. Paris, Atochem.
Atochem (1988) Analytical method No. LCH 200 131/GC. Paris, Atochem.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Baser KHC, Özek T, & Tümen G (1992) Essential oils of Thymus
cariensis and Thymus haussknechtii, two endemic species in Turkey.
J Essent Oil Res, 4: 659-661.
Bartholomé E, Biekert E, Hellmann H, Ley H, Weigert M, & Weise E ed.
(1977) [Ullmann's encyclopaedia of technical chemistry], 4th ed.
Weinheim, New York, Verlag Chemie, vol 14, pp 210-211 (in German).
BIBRA (1991) Toxicity profile: Isophorone. Carshalton, United Kingdom,
The British Industrial Biological Research Association.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha-2 µ-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 349-367.
Borghoff SJ, Miller AB, Bowen JP, & Swenberg JA (1991) Characteristics
of chemical binding to alpha-2 µ-globulin in vitro - evaluating
structure-activity relationships. Toxicol Appl Pharmacol,
107: 228-238.
Borup MB & Middlebrooks EJ (1986) Photocatalyzed oxidation of organic
wastestreams. In: Toxic and hazardous wastes. Proceedings of the 18th
Mid-Atlantic Industrial Waste Conference, 29 June-1 July 1986.
Lancaster, Pennsylvania, Technimic Publishers Co., Inc., pp 554-568.
BP (1988a) Dihydroisophorone: Assessment of mutagenic potential in
histidine auxotrophs of Salmonella typhimurium (the Ames test).
Guildford, United Kingdom, BP International Ltd (LSR Report 88/0501).
BP (1988b) Material safety data sheet: Isophorone. Guildford, United
Kingdom, BP International Ltd.
Brondeau MT, Bonnet P, Guenier JP, Simon P, & De Ceaurriz J (1990)
Adrenal-dependent leucopenia after short-term exposure to various
airborne irritants in rats. J Appl Toxicol, 10(2): 83-86.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Bucher JR, Huff J, & Kluwe WM (1986) Toxicology and carcinogenesis
studies of isophorone in F344 rats and B6C3F1 mice. Toxicology,
39: 207-219.
Cairns MA & Nebeker AV (1982) Toxicity of acenaphthene and isophorone
to early life stages of fathead minnows. Arch Environ Contam Toxicol,
11: 703-707.
Callahan MA, Slimak MW, & Gabel NW (1979) Water-related environmental
fate of 129 priority pollutants. Washington, DC, US Environmental
Protection Agency (EPA 440/4-79-029B).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments. J Great Lakes Res, 13: 296-309.
Charbonneau M & Swenberg JA (1988) Studies on the biochemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT Act,
8(6): 1-5.
Charbonneau M, Strasser J, Lock EA, Turner MR, & Swenberg JA (1988)
1,4-Dichlorobenzene (1,4-DCB)-induced nephrotoxicity: Similarity with
unleaded gasoline (UG)-induced renal effects. In: Bach PH & Lock EH
ed. Nephrotoxicity: Extrapolation from in vitro to in vivo and
from animal to man. New York, London, Plenum Press.
Cheh AM (1986) Mutagen production by chlorination of methylated alpha,
ß-unsaturated ketones. Mutat Res, 169: 1-9.
Cheminfo (1988) Database of the Canadian Centre for Occupational
Health and Safety: Record No. 22E: Isophorone. Ottawa, Canada,
Canadian Centre for Occupational Health and Safety.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff programme. J Water Pollut Control Fed,
56: 898-908.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz JC, Micillino JC, Marignac B, Bonnet P, Muller J, &
Guenier JP (1984) Quantitative evaluation of sensory-irritating and
neurobehavioural properties of aliphatic ketones in mice. Food Chem
Toxicol, 22: 545-549.
Dietrich DR & Swenberg JA (1991) NCI-Black-Reiter (NBR) male rats fail
to develop renal disease following exposure to agents that induce
alpha-2 µ-globulin nephropathy. Fundam Appl Toxicol, 16: 749-762.
Dutertre-Catella H (1976) Contribution à l'étude analytique
toxicologique et biochimique de l'isophorone. Paris, Université René
Descartes (Thesis for doctorate in pharmacology).
Dutertre-Catella H, Nguyen PL, Dang Quoc Q, & Truhaut R (1978)
Transformations métaboliques de la triméthyl-3,5,5-cyclohexène-2,
one-1 (isophorone). Toxicol Eur Res, 1: 209-216.
ECETOC (1988) Concentrations of industrial organic chemicals measured
in the environment: The influence of physico-chemical properties,
tonnage and use pattern. Brussels, European Chemical Industry Ecology
and Toxicology Centre (Technical Report No. 29).
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: X. Results of 70 coded chemicals
tested for the National Toxicology Program. Environ Mol Mutagen,
23(3): 208-227.
Gandy J, Millner GC, Bates HK, Casciano DA, & Harbison RD (1990)
Effects of selected chemicals on the glutathione status in the male
reproductive system of rats. J Toxicol Environ Health, 29: 45-57.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows. Superior, Wisconsin, Center for Lake
Superior Environmental Studies, University of Wisconsin, 332 pp.
Ghassemi M, Quinlivan S, & Bachmaier J (1984) Characteristics of
leachates from hazardous waste landfills. J Environ Sci Health,
A19(5): 579-620.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Illinois,
Charles C. Thomas, pp 608-609.
Gulati DK, Witt K, Anderson B, Zeiger E, & Shelby MD (1989) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. III. Results with 27 chemicals. Environ Mol
Mutagen, 13: 133-193.
Hannah SA, Austern BM, Eralp AE, & Wise RH (1986) Comparative removal
of toxic pollutants by six wastewater treatment processes. J Water
Pollut Control Fed, 58: 27-34.
Harrison FL, Bishop DJ, & Mallon BJ (1985) Comparison of organic
combustion products in fly ash collected by a Venturi wet scrubber and
an electrostatic precipitator at a coal-fired power station. Environ
Sci Technol, 19: 186-193.
Haubenstricker ME, Holodnick SE, Mancy KH, & Brabec MJ (1990a) Rapid
toxicity testing based on mitochondrial respiratory activity. Bull
Environ Contam Toxicol, 44: 675-680.
Haubenstricker ME, Meier PG, Mancy KH, & Brabec MJ (1990b) Rapid
toxicity testing based on yeast respiratory activity. Bull Environ
Contam Toxicol, 44: 669-674.
Hauser TR & Bromberg SM (1982) EPA's monitoring program at Love Canal
1980. Environ Monit Assess, 2: 249-272.
Heitmuller PT, Hollister TA, & Parish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hites R (1979) Sources and fates of industrial organic chemicals; a
case study. Sludge Manage, 8: 107-119.
Hüls (1981) Data sheet No. 2257: Isophorone. Marl, Germany, Hüls AG.
Hüls (1988a) Test of the skin sensitizing effect of isophorone for
guinea-pig. Marl, Germany, Hüls AG (Internal report No. 1278).
Hüls (1988b) Mutagenicity of isophorone by means of the Ames
Salmonella typhimurium/microsomes mutagenicity test. Marl, Germany,
Hüls AG (Internal report No. 88/300).
Hüls (1988c) Analytical method for determining isophorone. Marl,
Germany, Hüls AG (Unpublished information).
Hüls (1988d) [Acute oral toxicity of beta-isophorone (3,5,5-
trimethyl-3-cyclohexene-1-one) for rats.] Marl, Germany, Hüls AG
(Report No. 1273) (in German).
Hüls (1988e) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the skin.] Marl,
Germany, Hüls AG (Report No. 1274) (in German).
Hüls (1988f) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the eyes and
mucosa.] Marl, Germany, Hüls AG (Report No. 1275) (in German).
Japan Environment Agency (1983) Environmental monitoring of chemicals:
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Department
of Environmental Health, Japan Environment Agency.
Jungclaus GA, Games LM, & Hites RA (1976) Identification of trace
organic compounds in tire manufacturing plant wastewaters. Anal Chem,
48: 1894-1896.
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemke AE (1983) Interlaboratory comparison of continuous flow, early
life stage testing with fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory, 26
pp (EPA-600/3-84-005; NTIS PB84-129493).
Lemke AE, Durham E, & Felhaber T (1983) Evaluation of a fathead minnow
embryo-larval test guideline using acenaphtene and isophorone. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory, 26 pp (EPA-600/3-83-062; NTIS PB83-243436).
Levin J-O & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Littlefield HA (1968) Assessment and comparison of subacute inhalation
toxicities of three ketones. Falls Church, Virginia (Unpublished
studies done for Esso Research and Engineering Company, Linden, New
Jersey).
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemistry:
Property estimation methods. New York, McGraw-Hill, pp 4-9.
Mabey WR (1981) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA-440/4-81-014).
McFall JA, Antoine SR, & DeLeon IR (1985) Base-neutral extractable
organic pollutants in biota and sediments from Lake Pontchartrain.
Chemosphere, 14(10): 1561-1569.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus subtilis/
microsome rec-assay for the detection of DNA damaging substances which
may occur in chlorinated and ozonated waters. Brighton. Water Sci
Technol, 21: 875-887.
Matthews EJ, Spalding JW, & Tennant RW (1993) Transformation of
BALB/C-3T3 cells: V. Transformation responses of 168 chemicals
compared with mutagenicity in Salmonella and carcinogenicity in
rodent bioassays. Environ Health Perspect, 101(Suppl 2): 347-482.
Microbiological Associates (1984a) L5178Y TK +/- mouse lymphoma
mutagenesis assay on isophorone. Bethesda, Maryland, Microbiological
Associates, Inc. (Report No. T2408.701005, prepared for the Chemical
Manufacturers' Association, Washington).
Microbiological Associates (1984b) Activity of isophorone in the
micronucleus cytogenetic assay in mice. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.121001, prepared
for the Chemical Manufacturers' Association, Washington).
Microbiological Associates (1984c) Unscheduled DNA synthesis in rat
primary hepatocytes with isophorone. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.380003, prepared
for the Chemical Manufacturers' Association, Washington).
Mikami Y, Fukunaga Y, Arita M, Obi Y, & Kisaki T (1981) Preparation of
aroma compounds by microbial transformation of isophorone with
Aspergillus niger. Agric Biol Chem, 45: 791-793.
Mitchell AD (1993) Does the in situ approach yield a more accurate
assessment of induced mutation frequencies than the standard L5178Y
tk+/- tk -/- mouse lymphoma assay? Environ Mol Mutagen, 21
(Suppl 22): 49.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Murty CVR, Sarkar FH, Mancini MA, & Roy AK (1987) Sex-independent
synthesis of alpha-2 µ-globulin and its messenger ribonucleic acid in
the rat preputial gland: Biochemical and immunocyto-chemical analyses.
Endocrinology, 121: 1000-1005.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putman DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Olson CT, Yu KO, & Serve MP (1986) Metabolism of cis- and trans-
decalin in F344 rats. J Toxicol Environ Health, 18: 285-292.
Olson MJ, Johnson JT, & Reidy CA (1990) A comparison of male rat and
human urinary proteins: Implications for human resistance to hyaline
droplet nephropathy. Toxicol Appl Pharmacol, 102: 524-536.
Ono Y, Somiya I, & Kawamura M (1991) The evaluation of genotoxicity
using DNA repairing test for chemicals produced in chlorination and
ozonation processes. Water Sci Technol, 23: 329-338.
Potokar M, Grundler OJ, Heusener A, Jung R, Mürmann P, Schöbel C,
Suberg H, & Zechel HJ (1985) Studies on the design of animal tests for
the corrosiveness of industrial chemicals. Food Chem Toxicol, 23:
615-617.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Rasmussen T, Anthoni U, Christophersen C, & Nielsen PH (1993) Volatile
compounds from the marine indicator organism Mytilus edulis.
Chemosphere, 27(11): 2123-2125.
Rastogi SC (1991) Levels of organic solvents in printer's inks. Arch
Environ Contam Toxicol, 20: 543-547.
Rohm & Haas (1972) A residue decline study (in bean and rice plants)
using 14C-isophorone. Philadelphia, Pennsylvania, Rohm & Haas
Company.
Rowe VK & Wolf MA (1963) Ketones. In: Patty FA ed. Industrial hygiene
and toxicology, 2nd ed. New York, Interscience, vol II, pp 1722-1724,
1763-1765.
Samimi B (1982) Exposure to isophorone and other organic solvents in a
screen printing plant. Am Ind Hyg Assoc J, 43: 43-48.
Sasaki S (1980) Transformation and biological effects. In: Aquatic
pollutants. Oxford, New York, Pergamon Press.
Schering (1968) [Isophorone - Systemic tolerability following single
administration (LD50, oral).] Berlin, Germany, Schering AG
(in German).
Schering (1974) Dissipation of isophorone in sugar beets. Berlin,
Germany, Schering AG.
Scheubel JB & Scholz N (1989) [Acute toxicity test with isophorone in
the Golden Orfe.] Marl, Germany, Hüls AG (Unpublished study No. F 120)
(in German).
Schöberl P (1992) [Determination of the biodegradability of isophorone
in the DOC-DIE AWAY test.] Marl, Germany, Hüls AG (Unpublished report)
(in German).
Scholz N (1988a) [Acute toxicity of isophorone to Daphnia magna
strauss]. Marl, Germany, Hüls AG (Unpublished study No. D 318)
(in German).
Scholz N (1988b) [Growth retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. AW 146) (in German).
Scholz N (1988c) [Assimilation retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. A 115) (in German).
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency (EPA 600/4-76-062).
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Smyth HF Jr & Seaton J (1940) Acute response of guinea-pigs and rats
to inhalation of the vapours of isophorone. J Ind Hyg Toxicol,
22: 477-483.
Smyth HF Jr, Seaton J, & Fischer L (1942) Response of guinea-pigs and
rats to repeated inhalation of vapours of mesityl oxide and
isophorone. J Ind Hyg Toxicol, 4: 46-50.
Smyth HF Jr, Weil CS, West JS, & Carpenter CP (1969) An exploration of
joint toxic action: Twenty-seven industrial chemicals intubated in
rats in all possible pairs. Toxicol Appl Pharmacol, 14: 340-347.
Strasser J Jr, Charbonneau M, Borghoff SJ, Turner MJ, & Swenberg JA
(1988) Renal protein droplet formation in male F344 rats after
isophorone (IPH) treatment. Toxicologist, 8: 136.
Suffet IH, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking water during a 2-year period. Water
Res, 14: 853-867.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residues Rev,
85: 17-28.
Swenberg JA (1991) Risk assessment of chemicals causing alpha-2
µ-globulin nephropathy. Regul Toxicol Pharmacol, 13: 1-2.
Swenberg JA, Short B, Borghoff S, Strasser J, & Charbonneau M (1989)
The comparative pathobiology of alpha-2 µ-globulin nephropathy.
Toxicol Appl Pharmacol, 97: 35-46.
Swenberg JA, Dietrich DR, McClain RM, & Cohen SM (1992) Species-
specific mechanisms of carcinogenesis. In: Vainio H, Magee PN,
McGregor DB, & McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 477-500.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981a) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981b) Biodegradability
studies for predicting the environmental fate of organic priority
pollutants. Washington, DC, Association of Official Analytical
Chemists.
Thier R, Peter HP, Wiegand HJ, & Bolt HM (1990) DNA binding study of
isophorone in rats and mice. Arch Toxicol, 64: 684-685.
Thier R & Xu DG (1990) Urinary excretion of isophorone metabolites by
male rats. Naunyn-Schmiedeberg's Arch Pharmacol, 341: R11 (Abstract
No. 41).
Thursby GB & Steele RL (1986) Comparison of short- and long-term
sexual reproduction with the marine red alga Champia parvula.
Environ Toxicol Chem, 5: 1013-1018.
Thursby GB, Steele RL, & Kane ME (1985) Effect of organic chemicals on
growth and reproduction in the marine red alga Champia parvula.
Environ Toxicol Chem, 4: 797-805.
Traul KA, Hinz JP, & Kapp RW (1984) Inhalation teratology study in
rats and mice - MRD-83-237 (isophorone) (Project No. 32377). East
Millstone, New Jersey, Bio/dynamics Inc., East Laboratory (Report
submitted to Chemical Manufacturers Association).
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1970) Premiers
résultats de l'étude du métabolisme chez le lapin d'un solvent
industriel : l'isophorone. C R Acad Sci (Paris), 271: 1333-1336.
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1972) Etude de la
toxicité d'un solvant industriel: l'isophorone - pouvoir irritant
vis-à-vis des téguments et des muqueuses. J Eur Toxicol, 5: 31-37.
US EPA (1978) Isophorone: Ambient water quality criteria. Washington,
DC, US Environmental Protection Agency (NTIS PB-296 798).
US EPA (1979) Standard operating procedure: Analysis of non volatile
extractable organic compounds. Sample extraction and screening by gas
chromatography. Method 625. Fed Reg, 44: 223.
US EPA (1980) Ambient water quality criteria for isophorone.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-056;
NTIS PB81-11767).
US EPA (1986) Graphical exposure modelling system (GEMS). Fate of
atmospheric pollutants. Washington, DC, US Environmental Protection
Agency.
US EPA (1991a) Alpha-2 µ-globulin: Association with chemically induced
renal toxicity and neoplasia in the male rat. Washington, DC, US
Environmental Protection Agency (Risk Assessment Forum).
US EPA (1991b) Report of the EPA peer review workshop on alpha-2
µ-globulin: Association with renal toxicity and neoplasia in the male
rat. Washington, DC, US Environmental Protection Agency
(EPA/625/3-91/021).
US NIOSH (1977) Manual of analytical methods: Method S367, 2nd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Cincinnati, vol 3).
US NIOSH (1980) Health hazard evaluation report HHE-80-103-827.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NIOSH (1984) Health hazard evaluation report HHE-84-299-1543.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NTP (1986) Toxicological and carcinogenesis studies of isophorone
(CAS No.
7. EFFECTS ON LABORATORY MAMMALS, AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity
The acute LD50 values for various routes of exposure are
presented in Table 4.
Table 4. Acute LD50 values for technical (commercial) grade isophorone
Route Species Sex LD50 Reference
(mg/kg)
Oral rat male and female 1500 Schering (1968)
female 2104 Smyth et al. (1969)
male 2700 Dutertre-Catella (1976)
female 2100 Dutertre-Catella (1976)
mouse male and female 2200 Dutertre-Catella (1976)
rabbit male and female 2000 Dutertre-Catella (1976)
female 2000 Smyth et al. (1969)
Dermal rat male and female 1700 Schering (1968)
rabbit male and female 1200 Dutertre-Catella (1976)
7.1.1 Oral
Median lethal doses for isophorone in laboratory mammals ranging
from 1500 to 2700 mg/kg body weight have been reported. The signs of
toxicity were similar to those of solvents and narcotics, prostration
being followed rapidly by coma. Deaths occurred within 24 h,
otherwise recovery was complete. Degenerative changes in the liver
were reported in animals that died (Dutertre-Catella, 1976).
7.1.1.1 ß-Isophorone
An oral LD50 of 2950 mg/kg in rats has been reported for
ß-isophorone (purity 97.5%, containing 2.5% alpha-isophorone). The
predominant systemic effect was non-specific CNS depression shortly
after dosing. Cirrhosis-like changes on the surface of the liver and
severe irritation of the stomach were observed in the animals that
died following dosing (Hüls, 1988d).
7.1.2 Dermal
Dermal LD50 values indicate that isophorone is rapidly absorbed
through the skin under occlusion. During the first 6 h of occluded
application an increase in respiratory rate, followed by prostration
and narcosis, was reported in rabbits exposed to 500-1000 mg/kg.
Occluded skin contact for 24 h resulted in erythema, followed after
several days by scarring. Skin damage was still evident after 14
days. In this study, 10-25 ml isophorone was applied to skin as
compared to 0.5 ml in the skin irritation test (see section 7.2.1)
(Dutertre-Catella, 1976).
7.1.3 Inhalation
Rats and guinea-pigs were exposed to atmospheres containing
isophorone concentrations of 1713 or 4282 mg/m3 (300 or 750 ppm) for
24 h, of 5025 mg/m3 (880 ppm) for 12 h, or of 7823 to 26 266 mg/m3
(1370 to 4600 ppm) for 8 h. In both animal species, in the order of
development, eye and respiratory irritation, lachrymation, ataxia,
dyspnoea, diarrhoea, light narcosis and death were observed.
Postmortem examination of rats dying after exposure to isophorone
showed haemorrhage in the lungs, congestion of the stomach and liver,
peritoneal effusion and discoloration of the kidney and spleen (Smyth
& Seaton, 1940). However, it would appear that some of the
atmospheric concentrations could not have been achieved with pure
isophorone, and therefore the results reported in the study are of
little relevance (Rowe & Wolf, 1963).
Groups of six rabbits and rats were exposed to isophorone for
5 h. The observation period was 2 weeks. At a concentration of
39.9 g/m3 (7000 ppm), 10% of the rats and 30% of the rabbits died.
No LC50 value could be established from this study (Dutertre-
Catella, 1976).
7.2 Skin, eye and respiratory irritation, sensitization
7.2.1 Skin irritation
The skin irritation of isophorone was studied by Truhaut et al.
(1972). A single application of 0.5 ml isophorone under an occlusive
patch for a period of 24 h on the shaved or scarified skin of six
rabbits produced a light erythema which disappeared rapidly after
exposure. Microscopical examination did not show any
histopathological changes.
In the rabbit, occlusive and semi-occlusive contact with 0.5 ml
neat isophorone for 1 or 4 h was non-irritating (Potokar et al.,
1985).
7.2.1.1 ß-Isophorone
A single application of 0.5 ml ß-isophorone (containing 2.5%
alpha-isophorone) under a semi-occlusive patch over a period of 4 h on
the shaved skin of three rabbits produced moderate erythema and
swelling (Hüls, 1988e).
7.2.2 Eye irritation
A single instillation of 0.1 ml isophorone in the eyes of six
rabbits caused opacity in four animals, which in some instances
covered the entire area of the cornea, inflammation of the conjunctiva
and purulent discharge. In a supplementary experiment in which the
rabbits' eyes were washed with 20 ml of warm water 2-4 seconds after
the introduction of 0.1 ml isophorone, considerable recovery from
these effects occurred over 7 days (Dutertre-Catella, 1976).
Grant (1974) reported that application of one drop of isophorone
to rabbit cornea caused mild transient injury, graded 4 on a scale of
1 to 10 after 24 h. Pronounced irritation of eyes and nose occurred
in rats and guinea-pigs exposed to atmospheres containing isophorone
(see section 7.1.3) (Smyth & Seaton, 1940; Smyth et al., 1942).
7.2.2.1 ß-Isophorone
A single instillation of 0.1 ml in the eyes of three rabbits
produced moderate conjunctival and corneal opacities. Iridial changes
were also reported in two animals. Although the corneal and iridial
changes had resolved by 7 days, minor conjunctival irritation was
still in evidence (Hüls, 1988f).
7.2.3 Respiratory irritation
In a study of sensory irritation, De Ceaurriz et al. (1981)
estimated the concentration of various chemicals causing a 50%
decrease in respiratory rate (measured using individual
plethysmographs) in mice (RD50). The RD50 for isophorone was
158.7 mg/m3 (27.8 ppm) for a 5-min exposure. For comparison, the
RD50 values for toluene diisocyanate and acetone were 0.24 and
23 480 ppm, respectively.
Exposure of rats to airborne concentrations of 383-514 mg/m3
(67 or 90 ppm) for a single 4-h period produced statistically
significant reductions in circulating leukocytes. This leukopenia was
considered to be due to the stress-induced release of cortico-steroids
resulting from sensory irritation (Brondeau et al., 1990).
7.2.4 Sensitization
Isophorone, administered at a concentration of 10% intradermally
(in maize germ oil) and 100% topically to female guinea-pigs in the
Magnusson-Kligman test, showed no sensitizing potential (Hüls, 1988a).
7.3 Subchronic toxicity
7.3.1 Inhalation
The effects of repeated whole body exposure to isophorone vapour
were reported by Smyth et al. (1942). Groups of male Wistar rats and
guinea-pigs of both sexes, 16-20 animals per group, were exposed to
isophorone concentrations up to approximately 2855 mg/m3 (500 ppm)
8 h/day, 5 days/week, for 6 weeks. As in the Smyth & Seaton (1940)
study (section 7.1.3), the air concentrations of isophorone could not
have been attained under conditions employed by Smyth et al. (1942).
In the presentation of these results no distinction was made between
the two species and no control data were presented. Growth
retardation was noted in all animals exposed to higher concentrations.
Postmortem examination of animals dying after exposure revealed
severely injured kidneys and lungs. The kidneys of surviving animals
were congested with dilation of Bowman's capsules and cloudy swelling
of tubular cells. The lungs and liver were also reported to be
congested with desquamation of the bronchial epithelium in the lungs
and cloudy swelling in the liver cells.
Dutertre-Catella (1976) exposed groups of 10 male and 10 female
rats to atmospheres reported to contain 2855 mg/m3 8 h/day, 5
days/week for 6 and 4 months, respectively. Two males and one female
exposed to isophorone died; there were no deaths in the control
animals. The only reported effects were irritation of the eyes and
nose.
Groups of 10 male and 10 female young adult Charles River CD rats
were exposed to isophorone at air concentrations of 0 or 250 mg/m3,
6 h/day, 5 days/week, for 4 weeks. Results of daily spectroscopic
determinations indicated that the average daily exposure was
208 mg/m3. Body weight measurements and haematological studies were
made before exposure and after 4 weeks. The rats were killed and
gross necropsy was performed. Organ weights were determined for
lungs, liver, kidneys, adrenals and spleen. Histological examination
of those tissues were performed in three males and three females per
group. The following effects were observed: transient nasal bleeding,
increased percentages of lymphocytes, decreased percentages of
neutrophils and increased haemoglobin concentration in males and
females and significantly lower terminal body weights and
significantly decreased absolute and relative liver weights of exposed
males, compared with controls (Littlefield, 1968).
7.3.2 Oral
Four groups of 20 male and 20 female albino rats were fed diets
containing 0, 750, 1500 or 3000 mg isophorone/kg (0, 37.5, 75 or
150 mg/kg body weight) for 90 days. Isophorone in corn oil (ratio
1:2) was blended with the diet; fresh diets were prepared each week.
Haematology, serum chemistry and urine analyses were carried out on
five animals of each sex from each group at week 4 and at termination.
Comprehensive histopathological examination was confined to five
animals of each sex from the control and high dose groups. The liver
and kidney from five animals of the intermediate dose levels were also
examined histopathologically. Under the conditions of this study, no
effects on the general appearance of the test animals, on their
behaviour, on body weight gain or on food consumption were observed at
a dietary level of 1500 mg/kg or less. Isophorone did not alter the
composition of the formed elements of the blood, nor did it interfere
with the general metabolism or with liver and kidney function. No
detectable gross or microscopic pathological changes were noted in any
of the animals examined after 28 or 90 days of feeding. Organ/body
weight ratios for vital organs were not changed. The only adverse
finding reported was a reduction in body weight gain in the male rats
receiving the highest dose (Affiliated Medical Enterprises, 1972a).
As part of a preliminary investigation prior to an NTP
carcinogenicity study, five rats (F-344) and five mice (B6C3F1) of
each sex were given by gavage 12 oral doses of up to 2000 mg
isophorone/kg body weight administered in corn oil over a 16-day
period (US NTP, 1986). Lethargy was reported in all rats following
dosing while in mice uncertain locomotion was reported among animals
receiving 1000 mg/kg. All mice and 50% of the rats receiving
2000 mg/kg died. The weight gain of animals surviving 2000 mg/kg and
all animals receiving 1000 mg/kg was reduced. As part of the same
programme, 10 rats and mice of each sex were given by gavage daily
doses of 0, 62.5, 125, 250, 500 or 1000 mg isophorone/kg body weight
for 90 days (US NTP, 1986). The rats were drowsy and lethargic
following administration. At the highest dose level, one female rat
and three female mice died. No macroscopic or microscopic changes
were observed in the organs examined from either study. A subsequent
histopathological review of the kidney, which included re-sectioning
and additional staining, also failed to reveal any treatment-related
effects. The no-observed-effect level in this study was considered to
be 500 mg/kg body weight per day.
Groups of four male and four female beagle dogs were given 90
daily oral doses of isophorone in gelatin capsules at dosage rates of
35, 75 or 150 mg/kg per day. Comprehensive haematology, clinical
chemistry and urine analyses were carried out initially and at 1, 2
and 3 months. Apart from a mild intermittent incidence of soft stools
in animals of the high-dose group, there was no observable effect as
demonstrated by the data on general appearance and conditions as well
as those from haematological or biochemical investigations. At
autopsy, no changes in the organ/body weight ratios and no
histopathological changes were observed. No toxic effect was found at
any of the dose levels used. It was concluded that the no-observed-
effect level for isophorone in the dog was 150 mg/kg per day
(Affiliated Medical Enterprises, 1972b).
7.3.3 Dermal
The daily occluded application on shaved and abraded skin of 0.1
or 0.2 ml of isophorone to rats for 8 weeks produced erythema and
scabs at the site of application (Dutertre-Catella, 1976). The only
apparent systemic effect reported was an 8% reduction in mean weight
gain in the females compared with controls; the dose levels at which
this occurred were not given.
7.4 Mutagenicity
The results of mutagenicity tests are summarized in Table 5.
7.4.1 Gene mutation in bacteria (Ames tests)
The mutagenic potential of isophorone was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100, following a preincubation protocol. The test substance
concentrations used were between 10 and 5000 µg per plate. There was
no increase in the number of revertants at any of the concentrations
tested with and without rat liver S9 fraction (Hüls, 1988b).
Two further studies were conducted on Salmonella typhimurium
TA1535, TA1537 and TA1538 strains (1 to 1000 µg/plate) (Atochem,
1978a) and TA98, TA100, TA1535 and TA1537 strains (100 to
10 000 µg/plate) (US NTP, 1986). No evidence of mutagenic potential
was provided in the presence or absence of rat or hamster liver S9
fractions.
Salmonella strains TA1535, TA1537, TA97, TA98 and TA100 were
used to test isophorone (up to 10 000 µg/plate) with and without
Aroclor 1254-induced rat and hamster metabolic activation systems
(male Sprague-Dawley rats and male Syrian hamsters). When negative
results were obtained in the initial assay, the chemicals were
retested in all strains with and without activation. Isophorone
showed no mutagenic activity (Mortelmans et al., 1986).
7.4.1.1 Dihydroisophorone
The mutagenic potential of dihydroisophorone (iii in Fig. 1, see
section 6.2), a metabolite of isophorone, was examined using
Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98 and
TA100 at concentrations of 25 to 2500 µg per plate. There was no
increase in the number of revertants at the concentrations tested,
either with or without rat liver S9 fraction (BP, 1988a).
Table 5. Isophorone genotoxicity tests
Test system Results Reference
Gene Ames test (with and negative Atochem (1978a); US NTP
mutation without S9) (1986); Mortelmans et
al. (1986); Hüls (1988b)
mouse lymphoma assay positive US NTP (1986)
(without S9)
(with and without S9) negative Microbiological Associates
(1984a)
(with and without S9) positive McGregor et al. (1988)
(with and without S9) negative O'Donoghue et al. (1988)
positive Mitchell (1993)
sex-linked recessive negative Foureman et al. (1994)
lethal in Drosophila
melanogaster
Chromosome CHO cells (in vitro) negative US NTP (1986)
aberrations (with and without S9) negative Gulati et al. (1989)
Micronucleus mouse (in vivo) negative Atochem (1978b); Microbio-
test logical Associates (1984b);
O'Donoghue et al. (1988)
Sister CHO cells (in vitro)
chromatid (without S9) positive US NTP (1986)
exchanges (with S9) negative US NTP (1986)
(without S9) positive Gulati et al. (1989)
Direct DNA unscheduled DNA negative Microbiological Associates
damage synthesis (primary rat (1984c); O'Donoghue et al.
hepatocyte culture) (1988)
DNA binding liver and kidney DNA negative Thier et al. (1990);
in vivo (mouse and rat) Thier & Xu (1990)
Morphological BALB/c-3T3 cells positive Matthews et al. (1993)
transformation (without S9)
7.4.2 Gene mutation in mammalian cells
Isophorone was tested in the L5178Y TK +/- mouse lymphoma
mutagenesis assay (MLA) in the presence and absence of rat liver S9
fraction. The experiment was performed only once and all doses were
tested in duplicate. In the absence of S9, isophorone concentrations
of 0.13 to 1.3 ml/litre produced total growth of 111% to 12% compared
to the control. In the presence of S9, isophorone concentrations of
0.067 to 0.89 ml/litre produced total growth of 86 to 9% compared to
the control. None of these cultures exhibited mutation frequencies
which were significantly greater than the mean mutation frequency of
the solvent control (Microbiological Associates, 1984a; O'Donoghue et
al., 1988). The authors pointed out that while the results of the MLA
without metabolic activation were negative in the present study, these
assays were positive in the NTP study and thus were not reproducible
between laboratories. In contrast to O'Donoghue et al. (1988),
McGregor et al. (1988) reported isophorone to be positive in the MLA
both with and without rat liver S9 mix (from male Fischer-344 rats).
Isophorone was toxic to the cultures only at doses of 600 ml/litre or
more. The authors viewed the experiment with reservations since the
cloning efficiency was low.
Another MLA study employing concentrations of 400 to
1200 mg/litre was carried out in the absence of rat liver S9 fraction.
Duplicate experiments produced gradations of total growth relative to
control values of 112 or 118% for the low concentrations to 7 or 14%
for the high concentrations. Dose-related increases in mutation
frequencies compared with control were observed; at the highest dose
level the increase was 4-fold (US NTP, 1986).
Mitchell (1993) compared the induction of mutation frequencies by
the in situ variant (ISV) approach with the standard MLA.
Isophorone was one of the compounds tested, and showed a higher
induced mutation frequency with the ISV approach than in the standard
MLA.
Isophorone was tested for its ability to induce sex-linked
recessive lethal mutations in post-meiotic and meiotic germ cells of
male Drosophila melanogaster. No induction was observed at 2000 ppm
by feeding or at 15 000 ppm by injection (Foureman et al., 1994).
7.4.3 Chromosome aberrations and sister chromatid exchange
7.4.3.1 Chromosome aberrations
Isophorone was tested in an in vitro cytogenetic assay using
Chinese hamster ovary (CHO) cell cultures. Treatments were performed
both in the absence and presence of rat liver S9 fraction. Under the
conditions of this test, isophorone did not induce chromosomal
aberrations (ABS) at concentrations up to 1600 or 1500 mg/litre,
respectively (US NTP, 1986).
Twenty-seven chemicals (including isophorone), previously tested
in rodent carcinogenicity assays were tested for induction of ABS in
CHO cells as part of a more extensive analysis of the correlation
between results of in vitro genetic toxicity assays and
carcinogenicity bioassays. Chemicals were tested up to toxic doses
both with and without exogenous metabolic activation. A liver
fraction (S9) prepared from Aroclor 1254-induced male Sprague-Dawley
rats was used to provide exogenous metabolic activation. No ABS were
observed in CHO cells following incubation with isophorone in either
the presence or the absence of S9 (Gulati et al., 1989).
7.4.3.2 Sister chromatid exchange
The ability of isophorone to induce sister chromatid exchange
(SCE) was studied in CHO cells, in the presence and absence of rat
liver S9 fraction at concentrations up to 1000 mg isophorone/litre.
Without S9, isophorone induced a small but dose-related increase in
the frequencies of SCE. No effect was observed in the presence of S9
(US NTP, 1986).
A significant increase in SCE frequency with isophorone was
observed only in the absence of S9, at doses of 500-1000 mg/litre
(Gulati et al., 1989 - see also section 7.4.3.1). At these high dose
levels, isophorone is extremely cytostatic; therefore, the increase in
SCE frequency was observed only after delayed harvest (6-13 h
additional culture time).
7.4.4 Micronucleus test
Isophorone doses of 450, 900 and 1800 mg/kg body weight were
administered by gavage to CFLP mice of both sexes in two equal doses
separated by an interval of 24 h. Six hours after the last treatment
the mean micronucleated cell counts and the bone marrow cytotoxicity
were similar in all test groups and controls (Atochem, 1978b).
Male and female CD-1 mice were treated by intraperitoneal
injection of isophorone (0.54 ml/kg body weight). There was no
evidence of micronuclei or cytotoxicity in bone marrow samples
collected 12, 24 and 48 h after administration (Microbiological
Associates, 1984b; O'Donoghue et al., 1988).
7.4.5 Primary DNA damage
7.4.5.1 Bacterial tests
In the Bacillus subtilis (strain H17) rec-assay, isophorone
showed DNA-damaging potential without metabolic activation and a
reverse effect with activation (Matsui et al., 1989).
Isophorone did not induce the SOS function responses in the umu
test using Salmonella typhimurium strain TA1535/pSK1002 (Ono et al.,
1991).
7.4.5.2 Unscheduled DNA synthesis
Isophorone was tested at dose levels ranging from 0.005 to
0.40 ml/litre using primary rat cultures of hepatocytes. There was no
increase in the mean nuclear grain count compared to the controls or
in the incidence of cells undergoing repair at any dose level
(Microbiological Associates, 1984c; O'Donoghue et al., 1988).
7.4.5.3 DNA binding
A DNA-binding study was performed with radiolabelled 1,3,5-
14C-isophorone (Thier et al., 1990; Thier & Xu, 1990). Male and
female F-344 rats and male and female B6C3F1 mice received doses
(500 mg/kg body weight) of unlabelled isophorone containing 0.4 mCi
(per rat) and 0.8 mCi (per mouse) labelled isophorone in neutral oil
by gavage. No binding of the radioactivity to liver or kidney DNA was
observed in either species.
7.4.6 Morphological transformation
Isophorone was positive in a standard transformation assay using
BALB/c-3T3 cells without exogenous activation at concentrations of
1.07 g/litre (7.76 mmol/litre) (Matthews et al., 1993).
7.5 Chronic toxicity and carcinogenicity
In 18-month inhalation studies, groups of 10 rats and 2 rabbits
of each sex were exposed to atmospheres containing 1413 mg
isophorone/m3 (250 ppm) for 6 h/day, 5 days/week. Slight
conjunctivitis and irritation of the nasal mucosa with a bloody
discharge were observed. In the lungs of the animals, frequent
haemorrhages were found with oedema in the alveoli. Vacuolization was
found in the liver of the treated animals (Dutertre-Catella, 1976).
Toxicological and carcinogenesis studies of isophorone (more than
94% pure) were conducted by administering 0, 250 or 500 mg
isophorone/kg body weight per day by gavage in corn oil to groups of
50 F-344/N rats and 50 B6C3F1 mice of each sex, 5 days per week for
103 weeks. Throughout the 2-year study, the mean body weights of the
high-dose male rats were on average 5% less than those of the vehicle
controls. During the second year, the mean body weights of the female
high-dose rats were on average 8% less than those of the vehicle
controls, and the high-dose female mice were 5% lower. The survival
of high-dose male rats was significantly lower than that of the
vehicle controls after week 96 (final survival: vehicle control,
33/50; low dose, 33/50; high dose, 14/50). The survival of dosed
female rats was poor (30/50; 23/50; 20/50), due in part to 20 gavage-
related accidental deaths of dosed animals. The survival of male mice
was also low (16/50; 16/50; 19/50), but there was a significant trend
toward increased survival of dosed female mice relative to that of the
vehicle controls (26/50; 35/50; 34/50). Dosed male rats showed a
variety of proliferative lesions of the kidney (tubular cell
hyperplasia: 0/50; 1/50; 4/50; tubular cell adenoma: 0/50; 0/50; 2/50;
tubular cell adenocarcinoma: 0/50; 3/50; 1/50; epithelial hyperplasia
of the renal pelvis: 0/50; 5/50; 5/50). Dosed male rats also
exhibited increased mineralization of the medullary collecting ducts
(1/50; 31/50; 20/50), and low-dose male rats showed a more severe
nephropathy than is commonly seen in ageing F-344/N rats. Carcinomas
of the preputial gland were increased in high-dose male rats (0/50;
0/50; 5/50). With the exception of a moderate increase in nephropathy
(21/50; 39/50; 32/50), female rats did not show chemically related
increased incidences of neoplastic or non-neoplastic lesions. In
high-dose male mice, isophorone exposure was associated with increased
incidences of hepatocellular adenomas and carcinomas (18/48; 13/50;
29/50) and of mesenchymal tumours of the integumentary system
(fibroma, fibrosarcoma, neurofibrosarcoma or sarcoma: 6/48; 8/50;
14/50). An increased incidence of lymphomas or leukaemias was noted
in low-dose male mice (8/48; 18/50; 5/50). Coagulative necrosis
(3/48; 10/50; 11/50) and hepatocytomegaly (23/48; 39/50; 37/50) were
observed more frequently in the livers of dosed male mice than in
vehicle controls. No compound-related neoplastic or non-neoplastic
lesions associated with isophorone exposure were seen in female mice.
Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenicity of isophorone in male F-344/N rats as
shown by the occurrence of renal tubular cell adenomas and
adenocarcinomas in animals given 250 or 500 mg/kg per day; carcinomas
of the preputial gland were also observed at increased incidence in
male rats given 500 mg/kg. There was no evidence of carcinogenicity
in female F-344/N rats given 250 or 500 mg/kg per day. For male
B6C3F1 mice, there was equivocal evidence of carcinogenicity of
isophorone as shown by an increased incidence of hepatocellular
adenomas or carcinomas (combined) and of mesenchymal tumours in the
integumentary system in animals given 500 mg/kg per day and by an
increase in malignant lymphomas in animals given 250 mg/kg per day.
There was no evidence of carcinogenicity of isophorone in female
B6C3F1 mice given 250 or 500 mg/kg per day (US NTP, 1986).
In the above NTP study, preputial gland carcinomas were observed
in five high-dose male rats. The apparent absence of this tumour in
vehicle controls or in the low-dose group, and the very low incidence
(12/1094, i.e. 1%) in corn oil vehicle controls in previous 2-year
studies, suggest that this may be a chemical-related effect. No
preputial gland tumours were observed in male mice, but two
histogenetically related clitoral gland adenomas were seen in low-dose
female rats, providing some support for an association of isophorone
exposure with this tumour type (Bucher et al., 1986).
7.6 Mechanisms of toxicity
Swenberg et al. (1992) and US EPA (1991a,b) reviewed the
mechanisms involved in alpha2u-globulin nephropathy and renal
carcinogenesis of several chemicals in various animal, biochemical and
molecular modelling systems. All of the data are consistent with the
hypothesis that reversible binding of chemicals or their metabolites
to this abundant protein is causally related to the induction of
disease. Alpha2u-globulin, found mainly in male rats, is
synthesized by the liver and subsequently transported to the kidney.
It is normally present in the cytoplasm of the proximal convoluted
tubules of untreated animals in the form of hyaline droplets, visible
by light microscopy. Xenobiotics, or their metabolites, are bound to
the alpha2u-globulin in the liver of animals; this conjugate is even
more difficult to hydrolyse than the alpha2u-globulin itself and
induces the formation of the hyaline droplets which accumulate in the
tubules (Swenberg et al., 1989). Accumulation of the chemical/
alpha2u-globulin complex causes lysomal protein overload and
necrosis of the cells with subsequent cellular regeneration. Thus
cellular proliferation may contribute to the development of renal
tumours.
In order to determine whether isophorone and other compounds that
cause alpha2u-globulin to accumulate have the same binding
characteristics, binding studies were conducted with kidney cytosol
preparations from male Fischer-344 rats. The inhibition constant
value (Ki) for isophorone was in the range of 10-6 to
10-7 mol/litre, while those for d-limonene, 1,4-dichlorobenzene
and 2,5-dichlorophenol were higher (around 10-4 mol/litre). This
suggests that other factors besides binding are involved in the
accumulation of alpha2u-globulin. Results so far indicate that
binding is dependent on both hydrophobic interactions and hydrogen
bonding (Borghoff et al., 1991).
Investigations (Charbonneau et al., 1988; Strasser et al., 1988)
have shown that isophorone, isophorol and dihydroisophorone bind to
alpha2u-globulin, resulting in an increased accumulation of hyaline
droplets in renal tubular cells. These findings suggest the same
sequence of events may be responsible for the small increase in the
incidence of renal tubular neoplasias seen in male rats.
alpha2u-Globulin was not detected at significant levels in plasma
and urine of female rats or either sex of mice and humans (Swenberg et
al., 1989; Olson et al., 1990). In addition, other laboratory
rodents, dogs and primates do not develop hydrocarbon-related
nephropathy (Swenberg et al., 1989). For these reasons, it was
concluded that the low increase in the incidence of tubular adenomas
and adenocarcinomas observed in male rats following isophorone
administration was attributable to the accumulation of
alpha2u-globulin and was sex- and species-specific (Charbonneau &
Swenberg, 1988; Strasser et al., 1988; Swenberg et al., 1989).
Further evidence to support this view was gained by a study of
Dietrich & Swenberg (1991) using male animals of the NCI Black Reiter
(NBR) strain, which does not synthesize alpha2u-globulin. It was
shown that an oral dose (gavage) of 1000 mg isophorone/kg on four
consecutive days failed to produce the early features of the
alpha2u-globulin-related nephropathy syndrome (i.e. hyaline droplets
or alpha2u-globulin) in this strain.
In terms of human risk assessment, renal tubule tumours produced
in male rats in association with chemicals inducing alpha2u-globulin
accumulation should be distinguished from renal tubule tumours of
other origin (Borghoff et al., 1990; Swenberg, 1991; US EPA, 1991a,b).
It has been recognized that the induction of such tumours does not
necessarily indicate a potential carcinogenic hazard to humans. The
significance of this information with respect to the etiology of
pathological changes is uncertain at present.
Studies have shown that high levels of alpha2u-globulin and its
messenger RNA (mRNA) are present in the preputial gland of both male
and female rats. The preputial gland in both sexes contained about 3
times more alpha2u-globulin mRNA and about 300 times more
alpha2u-globulin than the male rat liver (Murty et al., 1987). It
was claimed that this high content of alpha2u-globulin was primarily
due to the cellular and ductal accumulation of the protein in the
preputial gland but did not reflect a difference in the rate of
transcription of the alpha2u-globulin gene. The high amount of
radiolabel derived from 14C-isophorone found in the preputial gland
of the male rats following single gavage dosing with 5 ml/kg (Strasser
et al., 1988) may therefore be related to its high content of
alpha2u-globulin.
7.7 Appraisal for mutagenicity/carcinogenicity
Isophorone does not induce gene mutations in bacteria,
chromosomal aberrations in vitro, bone-marrow micronuclei in mice or
DNA repair in primary cultures of rat hepatocytes. No DNA binding in
vivo was observed in a DNA binding study using 1,3,5-
14C-isophorone. In one study in the absence and in another study in
the absence and presence of metabolic activation, weak mutagenic
responses were obtained in three out of five L5178Y TK +/- mouse
lymphoma mutagenesis assays and a small increase of sister chromatid
exchange (SCE) was found only without metabolic activation in CHO
cells. Another study with SCE showed no increases in chromosomal
aberrations (for details see section 7.4). Isophorone was negative in
a test for sex-linked recessive lethal mutations in Drosophila. It
induced morphological transformation in mouse cells without
activation.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound.
There was no DNA binding in the liver and kidneys (sites affected in
the carcinogenicity bioassays).
The available data on in vitro mutagenicity and related end-
points do not suggest that isophorone is genotoxic.
When tested in a two-year carcinogenicity bioassay, isophorone
produced a variety of lesions of the kidneys, such as severe
nephropathy and tubular cell hyperplasia. A low but statistically
significant increase in the frequency of renal tubular cell adenomas
and adenocarcinomas was found in male F-344 rats dosed at levels of
250 and 500 mg/kg per day (see section 7.5).
An increase in the number of carcinomas of the preputial gland
was found only in male rats given 500 mg/kg per day. In high-dose
male B6C3F1 mice hepatocellular adenomas and carcinomas were
observed. No compound related neoplastic lesions were seen in female
rats (for details, see section 7.5).
The induction of these two types of tumours in male rats may be
associated with nephropathy, epithelial hyperplasia and
alpha2u-globulin formation and accumulation of the isophorone/
alpha2u-globulin complex, causing lysomal protein overload and
necrosis of the kidney cells with subsequent cellular regeneration
(for details see section 7.6).
7.8 Reproduction, embryotoxicity, teratogenicity
The teratogenicity of isophorone to rats and mice was studied by
Traul et al. (1984). Groups of 22 confirmed mated females of each
species were exposed 6 h/day on days 6-15 of gestation to atmospheres
containing 0, 143, 285 or 656 mg/m3 (0, 25, 50 or 115 ppm)
isophorone. At the highest atmospheric concentration there was
evidence of maternal toxicity which showed as reduced food
consumption, alopecia and cervical or anogenital staining in the rats
and reduced body weights in the mice. Comprehensive uterine and fetal
examinations did not show any significant differences between animals
exposed to isophorone and their respective controls. From the results
of this study, it can be concluded that no teratogenic or fetotoxic
effect was observed with 656 mg/m3 in F-344 rats and CD-1 mice.
In a study by Dutertre-Catella (1976), groups of 10 rats of each
sex, exposed 6 h/day, 5 days/week to 2855 mg/m3 (500 ppm) isophorone
for 3 months, were mated to exposed animals or to controls. All
females were reported to have delivered 7-10 pups. Anatomo-
pathological examination did not show any abnormalities. However,
only one isophorone concentration was used, the group size was small,
and no information was provided on reproductive success and maternal
survival.
7.9 Neurotoxicity
Central nervous system depression is a characteristic feature of
isophorone intoxication in experimental animals (Smyth & Seaton, 1940;
Dutertre-Catella, 1976; De Ceaurriz et al., 1981).
Isophorone and a number of aliphatic ketones have been studied
using the mouse behavioural despair swimming test (De Ceaurriz et al.,
1984). This test, which was developed for screening antidepressant
drugs, is based on the duration of the periods of immobility exhibited
by mice when placed in water. Mice were exposed to isophorone at
atmospheric concentrations of 508-782 mg/m3 (89-137 ppm) for 4 h.
The ID50 (an estimated concentration producing a 50% reduction in
the immobility time) for animals thus exposed was 628 mg/m3
(110 ppm).
7.10 Other special studies
One of the current research approaches for assessing cytotoxicity
is to monitor the respiratory activity of the mitochondrion, a
sensitive, nonspecific subcellular target site. Detected changes in
mitochondrial function after the addition of a test chemical could be
correlated to toxic effects. In this case, rat (male, Charles River)
liver mitochondria were used, and isophorone was shown to induce no
observable effects on rat liver mitochondria respiratory activity over
the tested concentration range of 0.273-113.8 mg/litre
(1.98-825 µmol/litre) (Haubenstricker et al., 1990a).
Isophorone was tested for its ability to perturb glutathione
(GSH) levels in the testes and epididymides, as well as liver,
following single acute dosages to rats. Groups of four mature male
Sprague-Dawley rats were administered isophorone intraperitoneally
(2 ml/kg). Concurrent control groups were maintained for all time
points examined to account for possible circadian fluctuation of GSH
throughout the day. Animals were sacrificed at 1, 2, 4, 8 or 16 h
after administration of the test compound. Liver, testes and
epididymides were excised for determination of total GSH content by
spectrophotometry. Isophorone (500 mg/kg) significantly reduced GSH
in the liver and in both reproductive organs examined. The ability of
isophorone to enhance the covalent binding of tritiated ethyl
methanesulfonate (3H-EMS) to spermatocytes was assessed.
Perturbation of reproductive tract GSH by isophorone treatment
significantly enhanced the extent of 3H-EMS-induced binding to sperm
heads. The authors postulate that chemical-induced lowering of GSH in
the male reproductive tract may be a mechanism for potentiation of
chemical-induced germ-cell mutations (Gandy et al., 1990).
8. EFFECTS ON HUMANS
8.1 Acute
Groups of 11 or 12 subjects exposed for a few minutes to
atmospheric isophorone concentrations of 228 mg/m3 (40 ppm) or more
experienced irritation of the eyes, nose and throat. A few complaints
of nausea, headache, dizziness, faintness, inebriation and a feeling
of suffocation occurred at concentrations 1142 mg/m3 (> 200 ppm).
The symptoms of irritation and narcosis were said to be less intensive
following exposure to concentrations below 1142 mg/m3 (Smyth &
Seaton 1940). However, this study has been criticized because of
uncertain actual concentrations and the use of impure substances (Rowe
& Wolf, 1963).
8.2 Sub-chronic
Complaints of fatigue and malaise were reported among workers
exposed for 1 month to atmospheres containing 28.5 to 48 mg
isophorone/m3 (5 to 8 ppm) (Communication from Ware, GD, to
Chairman, TLV Committee, 1973). No further complaints were received
following a reduction of the concentration to between 5.7 and
22.8 mg/m3 (1 and 4 ppm). On the basis of these data, the American
Conference of Governmental Industrial Hygienists (ACGIH, 1986)
recommended a ceiling limit (15 min) of 29 mg/m3 (5 ppm) for
isophorone.
8.3 Irritation and sensitization
No report on skin irritation or skin sensitization has been
published.
8.3.1 Eye and respiratory irritation
Smyth & Seaton (1940) reported eye, nose and throat irritation
following exposure to isophorone levels of 228 mg/m3 (40 ppm) or
more.
Silverman et al. (1946) estimated the sensory threshold of a
number of ketones including isophorone. An average of 12 subjects of
both sexes were used for each solvent exposure. The time of exposure
was 15 min. Irritation of the eyes, nose and throat was experienced
at 142.7 mg/m3 (25 ppm) isophorone; the highest concentration
considered acceptable by the majority of subjects for an 8-h exposure
was 57.1 mg/m3 (10 ppm).
A NIOSH health hazard evaluation (US NIOSH, 1980) conducted at a
screen printing process in 1980 found that the workshift (6´ h)
average exposures of printers to isophorone were 4 and 80 mg/m3
(0.7 and 14 ppm). Symptoms of respiratory tract and eye irritation
reported by workers were attributed to the antistatic agent containing
principally isophorone.
Amoore & Hautala (1983) reported an air odour threshold for
isophorone of 1.14 mg/m3 (0.2 ppm). The "odour safety factor",
which was defined as the threshold limit value 28.5 mg/m3 (5 ppm)
divided by the odour threshold, was 25. From the magnitude of this
value the authors predicted that 50% of exposed persons would perceive
sensory warning of the TLV (28.5 mg/m3, 5 ppm).
8.4 Chronic toxicity and carcinogenicity
No long-term surveys have been carried out on occupationally
exposed workers or other potentially exposed populations.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Yoshioka et al. (1985) studied the acute toxicity of isophorone
in Tetrahymena pyriformis. The EC50 after a 24-h exposure was
420 mg/litre. Yoshioka et al. (1986) found that the EC50 in the
Activated Sludge Respiration Inhibition Test was 100 mg/litre.
In a 2-day laboratory test with the marine red alga Champia
parvula, 38.3 mg isophorone/litre caused 50% reduction in cystocarp
formation. Above 138.5 mg/litre no cystocarps were formed, indicating
absence of sexual reproduction. In a 2-week laboratory test,
107.3 mg/litre completely inhibited cystocarp formation (Thursby &
Steele, 1986).
Isophorone was tested in air-saturated media with Saccharomyces
cerevisiae at concentrations of 0.272 to 113.7 mg/litre
(1.97-824 µmol/litre). These produced no detectable effect on the
rate of yeast respiration as measured with a dissolved oxygen
electrode (Haubenstricker et al., 1990b).
9.2 Aquatic organisms
The acute toxicity of isophorone to fish, crustaceans and algae
is summarized in Table 6. With the exception of Mysidopsis bahia
(US EPA, 1978) all acute LC50 values were above 100 mg/litre,
indicating a relatively low aquatic toxicity. This was consistent
with the results of a subacute (14 day) study with the marine red alga
Champia parvula. The toxicity of isophorone was determined by means
of various biological end-points, namely vegetative growth, formation
of tetrasporangia (asexual reproduction) and production of cystocarps
(sexual reproduction). Depending on the toxicological end-point, the
lowest concentration resulting in a significant difference from
controls ranged between 50 and 138 mg/litre (Thursby et al., 1985).
In addition to the results of acute toxicity tests shown in Table
6, an early life stage toxicity test was conducted with the freshwater
fathead minnow Pimephales promelas (Cairns & Nebeker, 1982) using
concentrations of 11, 19, 30, 56 and 112 mg isophorone/litre.
Survival was affected at a concentration of 112 mg/litre, but not at
56 mg/litre or less; fork length was affected at 30 mg/litre but not
at 19 mg/litre or less; body weight gain was decreased at 19 mg/litre
or more but not at 11 mg/litre. The authors calculated a no-observed-
effect concentration of 14 mg/litre. Using the same test with the
same species, Lemke et al. (1983) found a no-observed-effect level of
19.5 mg/litre, and in an interlaboratory (6 laboratories) comparison
no-observed-effect levels of up to 45.4 mg/litre were found (Lemke,
1983).
9.3 Terrestrial organisms
No data are available concerning the effects of isophorone on
terrestrial organisms.
Table 6. Acute toxicity of technical isophorone to aquatic species
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Fish
Bluegill sunfish LC50 96 static 220 (n) US EPA (1978)
(Lepomis macrochirus) LC50 24 static 240 (n) Buccafusco et al. (1981)
Fathead minnow LC50 96 flow 145-255 (m) Cairns & Nebeker (1982)
(Pimephales promelas) LC50 96 228 Geiger et al. (1990)
Golden orfe LC50 72 204 Scheubel & Scholz (1989)
(Leuciscus idus melanotus)
Sheepshead minnow LC50 96 140 Ward et al. (1981)
(Cyprinodon variegatus) LC50 96 static > 170, < 300 (n) Heitmuller et al. (1981)
Invertebrates
Brine shrimp LC50 24 430 Price et al. (1974)
(Artemia salina)
Mysid shrimp LC50 96 static 12.9 US EPA (1978)
(Mysidopsis bahia)
Water flea immobilization: EC50 24 static 430 (n) LeBlanc (1980)
(Daphnia magna) Immobilization: EC50 24 static 254-277 Scholz (1988a)
immobilization: EC50 48 static 120 (n) LeBlanc (1980)
immobilization: EC50 48 static 117 US EPA (1978)
Algae
Selenastrum capricornutum cell count: EC50 96 static 122 US EPA (1978)
chlorophyll: EC50 96 static 126 US EPA (1978)
Table 6 (cont'd)
Test species Parameter Duration Static/flowa Resultsb Reference
(h) (mg/litre)
Skeletonema costatum cell count: EC50 96 static 105 US EPA (1978)
chlorophyll: EC50 96 static 110 US EPA (1978)
Scenedesmus subspicatus cell count: EC50 72 475-476 Scholz (1988b)
chlorophyll: EC50 72 524-527 Scholz (1988c)
Red alga reproduction EC50 24 38.3 Thursby & Steele (1986)
(Champia parvula)
a static = static exposure; flow = flow-through exposure
b n = nominal exposure concentration; m = measured exposure concentration
REFERENCES
ACGIH (1986) Documentation on the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
Affiliated Medical Enterprises Inc. (1972a) 90-day sub-chronic
toxicity of isophorone in the rat. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Affiliated Medical Enterprises Inc. (1972b) 90-day sub-chronic
toxicity of isophorone in the dog. Princeton, New Jersey, Affiliated
Medical Enterprises Inc. (Report prepared for Rohm & Haas Co.,
Philadelphia).
Amoore JE & Hautala E (1983) Odor as an aid for chemical safety: Odor
threshold limit values and volatilities for 214 industrial chemicals
in air and water dilution. J Appl Toxicol, 3: 272-290.
Atochem (1978a) Ames metabolic activation test to assess the potential
mutagenic effect of isophorone. Paris, Atochem (Report No. UKM
52/78204, prepared by Huntingdon Research Centre).
Atochem (1978b) Micronucleus test on isophorone. Paris, Atochem
(Report No. UKM 52/78457, prepared by Huntingdon Research Centre).
Atochem (1986) Data sheet on isophorone. Paris, Atochem.
Atochem (1988) Analytical method No. LCH 200 131/GC. Paris, Atochem.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Baser KHC, Özek T, & Tümen G (1992) Essential oils of Thymus
cariensis and Thymus haussknechtii, two endemic species in Turkey.
J Essent Oil Res, 4: 659-661.
Bartholomé E, Biekert E, Hellmann H, Ley H, Weigert M, & Weise E ed.
(1977) [Ullmann's encyclopaedia of technical chemistry], 4th ed.
Weinheim, New York, Verlag Chemie, vol 14, pp 210-211 (in German).
BIBRA (1991) Toxicity profile: Isophorone. Carshalton, United Kingdom,
The British Industrial Biological Research Association.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha-2 µ-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 349-367.
Borghoff SJ, Miller AB, Bowen JP, & Swenberg JA (1991) Characteristics
of chemical binding to alpha-2 µ-globulin in vitro - evaluating
structure-activity relationships. Toxicol Appl Pharmacol,
107: 228-238.
Borup MB & Middlebrooks EJ (1986) Photocatalyzed oxidation of organic
wastestreams. In: Toxic and hazardous wastes. Proceedings of the 18th
Mid-Atlantic Industrial Waste Conference, 29 June-1 July 1986.
Lancaster, Pennsylvania, Technimic Publishers Co., Inc., pp 554-568.
BP (1988a) Dihydroisophorone: Assessment of mutagenic potential in
histidine auxotrophs of Salmonella typhimurium (the Ames test).
Guildford, United Kingdom, BP International Ltd (LSR Report 88/0501).
BP (1988b) Material safety data sheet: Isophorone. Guildford, United
Kingdom, BP International Ltd.
Brondeau MT, Bonnet P, Guenier JP, Simon P, & De Ceaurriz J (1990)
Adrenal-dependent leucopenia after short-term exposure to various
airborne irritants in rats. J Appl Toxicol, 10(2): 83-86.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Bucher JR, Huff J, & Kluwe WM (1986) Toxicology and carcinogenesis
studies of isophorone in F344 rats and B6C3F1 mice. Toxicology,
39: 207-219.
Cairns MA & Nebeker AV (1982) Toxicity of acenaphthene and isophorone
to early life stages of fathead minnows. Arch Environ Contam Toxicol,
11: 703-707.
Callahan MA, Slimak MW, & Gabel NW (1979) Water-related environmental
fate of 129 priority pollutants. Washington, DC, US Environmental
Protection Agency (EPA 440/4-79-029B).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments. J Great Lakes Res, 13: 296-309.
Charbonneau M & Swenberg JA (1988) Studies on the biochemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT Act,
8(6): 1-5.
Charbonneau M, Strasser J, Lock EA, Turner MR, & Swenberg JA (1988)
1,4-Dichlorobenzene (1,4-DCB)-induced nephrotoxicity: Similarity with
unleaded gasoline (UG)-induced renal effects. In: Bach PH & Lock EH
ed. Nephrotoxicity: Extrapolation from in vitro to in vivo and
from animal to man. New York, London, Plenum Press.
Cheh AM (1986) Mutagen production by chlorination of methylated alpha,
ß-unsaturated ketones. Mutat Res, 169: 1-9.
Cheminfo (1988) Database of the Canadian Centre for Occupational
Health and Safety: Record No. 22E: Isophorone. Ottawa, Canada,
Canadian Centre for Occupational Health and Safety.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff programme. J Water Pollut Control Fed,
56: 898-908.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz JC, Micillino JC, Marignac B, Bonnet P, Muller J, &
Guenier JP (1984) Quantitative evaluation of sensory-irritating and
neurobehavioural properties of aliphatic ketones in mice. Food Chem
Toxicol, 22: 545-549.
Dietrich DR & Swenberg JA (1991) NCI-Black-Reiter (NBR) male rats fail
to develop renal disease following exposure to agents that induce
alpha-2 µ-globulin nephropathy. Fundam Appl Toxicol, 16: 749-762.
Dutertre-Catella H (1976) Contribution à l'étude analytique
toxicologique et biochimique de l'isophorone. Paris, Université René
Descartes (Thesis for doctorate in pharmacology).
Dutertre-Catella H, Nguyen PL, Dang Quoc Q, & Truhaut R (1978)
Transformations métaboliques de la triméthyl-3,5,5-cyclohexène-2,
one-1 (isophorone). Toxicol Eur Res, 1: 209-216.
ECETOC (1988) Concentrations of industrial organic chemicals measured
in the environment: The influence of physico-chemical properties,
tonnage and use pattern. Brussels, European Chemical Industry Ecology
and Toxicology Centre (Technical Report No. 29).
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: X. Results of 70 coded chemicals
tested for the National Toxicology Program. Environ Mol Mutagen,
23(3): 208-227.
Gandy J, Millner GC, Bates HK, Casciano DA, & Harbison RD (1990)
Effects of selected chemicals on the glutathione status in the male
reproductive system of rats. J Toxicol Environ Health, 29: 45-57.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows. Superior, Wisconsin, Center for Lake
Superior Environmental Studies, University of Wisconsin, 332 pp.
Ghassemi M, Quinlivan S, & Bachmaier J (1984) Characteristics of
leachates from hazardous waste landfills. J Environ Sci Health,
A19(5): 579-620.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Illinois,
Charles C. Thomas, pp 608-609.
Gulati DK, Witt K, Anderson B, Zeiger E, & Shelby MD (1989) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. III. Results with 27 chemicals. Environ Mol
Mutagen, 13: 133-193.
Hannah SA, Austern BM, Eralp AE, & Wise RH (1986) Comparative removal
of toxic pollutants by six wastewater treatment processes. J Water
Pollut Control Fed, 58: 27-34.
Harrison FL, Bishop DJ, & Mallon BJ (1985) Comparison of organic
combustion products in fly ash collected by a Venturi wet scrubber and
an electrostatic precipitator at a coal-fired power station. Environ
Sci Technol, 19: 186-193.
Haubenstricker ME, Holodnick SE, Mancy KH, & Brabec MJ (1990a) Rapid
toxicity testing based on mitochondrial respiratory activity. Bull
Environ Contam Toxicol, 44: 675-680.
Haubenstricker ME, Meier PG, Mancy KH, & Brabec MJ (1990b) Rapid
toxicity testing based on yeast respiratory activity. Bull Environ
Contam Toxicol, 44: 669-674.
Hauser TR & Bromberg SM (1982) EPA's monitoring program at Love Canal
1980. Environ Monit Assess, 2: 249-272.
Heitmuller PT, Hollister TA, & Parish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hites R (1979) Sources and fates of industrial organic chemicals; a
case study. Sludge Manage, 8: 107-119.
Hüls (1981) Data sheet No. 2257: Isophorone. Marl, Germany, Hüls AG.
Hüls (1988a) Test of the skin sensitizing effect of isophorone for
guinea-pig. Marl, Germany, Hüls AG (Internal report No. 1278).
Hüls (1988b) Mutagenicity of isophorone by means of the Ames
Salmonella typhimurium/microsomes mutagenicity test. Marl, Germany,
Hüls AG (Internal report No. 88/300).
Hüls (1988c) Analytical method for determining isophorone. Marl,
Germany, Hüls AG (Unpublished information).
Hüls (1988d) [Acute oral toxicity of beta-isophorone (3,5,5-
trimethyl-3-cyclohexene-1-one) for rats.] Marl, Germany, Hüls AG
(Report No. 1273) (in German).
Hüls (1988e) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the skin.] Marl,
Germany, Hüls AG (Report No. 1274) (in German).
Hüls (1988f) [Testing of the acute irritant effect of beta-
isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) on the eyes and
mucosa.] Marl, Germany, Hüls AG (Report No. 1275) (in German).
Japan Environment Agency (1983) Environmental monitoring of chemicals:
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Department
of Environmental Health, Japan Environment Agency.
Jungclaus GA, Games LM, & Hites RA (1976) Identification of trace
organic compounds in tire manufacturing plant wastewaters. Anal Chem,
48: 1894-1896.
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemke AE (1983) Interlaboratory comparison of continuous flow, early
life stage testing with fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory, 26
pp (EPA-600/3-84-005; NTIS PB84-129493).
Lemke AE, Durham E, & Felhaber T (1983) Evaluation of a fathead minnow
embryo-larval test guideline using acenaphtene and isophorone. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory, 26 pp (EPA-600/3-83-062; NTIS PB83-243436).
Levin J-O & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Littlefield HA (1968) Assessment and comparison of subacute inhalation
toxicities of three ketones. Falls Church, Virginia (Unpublished
studies done for Esso Research and Engineering Company, Linden, New
Jersey).
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemistry:
Property estimation methods. New York, McGraw-Hill, pp 4-9.
Mabey WR (1981) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA-440/4-81-014).
McFall JA, Antoine SR, & DeLeon IR (1985) Base-neutral extractable
organic pollutants in biota and sediments from Lake Pontchartrain.
Chemosphere, 14(10): 1561-1569.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus subtilis/
microsome rec-assay for the detection of DNA damaging substances which
may occur in chlorinated and ozonated waters. Brighton. Water Sci
Technol, 21: 875-887.
Matthews EJ, Spalding JW, & Tennant RW (1993) Transformation of
BALB/C-3T3 cells: V. Transformation responses of 168 chemicals
compared with mutagenicity in Salmonella and carcinogenicity in
rodent bioassays. Environ Health Perspect, 101(Suppl 2): 347-482.
Microbiological Associates (1984a) L5178Y TK +/- mouse lymphoma
mutagenesis assay on isophorone. Bethesda, Maryland, Microbiological
Associates, Inc. (Report No. T2408.701005, prepared for the Chemical
Manufacturers' Association, Washington).
Microbiological Associates (1984b) Activity of isophorone in the
micronucleus cytogenetic assay in mice. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.121001, prepared
for the Chemical Manufacturers' Association, Washington).
Microbiological Associates (1984c) Unscheduled DNA synthesis in rat
primary hepatocytes with isophorone. Bethesda, Maryland,
Microbiological Associates, Inc. (Report No. T2408.380003, prepared
for the Chemical Manufacturers' Association, Washington).
Mikami Y, Fukunaga Y, Arita M, Obi Y, & Kisaki T (1981) Preparation of
aroma compounds by microbial transformation of isophorone with
Aspergillus niger. Agric Biol Chem, 45: 791-793.
Mitchell AD (1993) Does the in situ approach yield a more accurate
assessment of induced mutation frequencies than the standard L5178Y
tk+/- tk -/- mouse lymphoma assay? Environ Mol Mutagen, 21
(Suppl 22): 49.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Murty CVR, Sarkar FH, Mancini MA, & Roy AK (1987) Sex-independent
synthesis of alpha-2 µ-globulin and its messenger ribonucleic acid in
the rat preputial gland: Biochemical and immunocyto-chemical analyses.
Endocrinology, 121: 1000-1005.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putman DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Olson CT, Yu KO, & Serve MP (1986) Metabolism of cis- and trans-
decalin in F344 rats. J Toxicol Environ Health, 18: 285-292.
Olson MJ, Johnson JT, & Reidy CA (1990) A comparison of male rat and
human urinary proteins: Implications for human resistance to hyaline
droplet nephropathy. Toxicol Appl Pharmacol, 102: 524-536.
Ono Y, Somiya I, & Kawamura M (1991) The evaluation of genotoxicity
using DNA repairing test for chemicals produced in chlorination and
ozonation processes. Water Sci Technol, 23: 329-338.
Potokar M, Grundler OJ, Heusener A, Jung R, Mürmann P, Schöbel C,
Suberg H, & Zechel HJ (1985) Studies on the design of animal tests for
the corrosiveness of industrial chemicals. Food Chem Toxicol, 23:
615-617.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Rasmussen T, Anthoni U, Christophersen C, & Nielsen PH (1993) Volatile
compounds from the marine indicator organism Mytilus edulis.
Chemosphere, 27(11): 2123-2125.
Rastogi SC (1991) Levels of organic solvents in printer's inks. Arch
Environ Contam Toxicol, 20: 543-547.
Rohm & Haas (1972) A residue decline study (in bean and rice plants)
using 14C-isophorone. Philadelphia, Pennsylvania, Rohm & Haas
Company.
Rowe VK & Wolf MA (1963) Ketones. In: Patty FA ed. Industrial hygiene
and toxicology, 2nd ed. New York, Interscience, vol II, pp 1722-1724,
1763-1765.
Samimi B (1982) Exposure to isophorone and other organic solvents in a
screen printing plant. Am Ind Hyg Assoc J, 43: 43-48.
Sasaki S (1980) Transformation and biological effects. In: Aquatic
pollutants. Oxford, New York, Pergamon Press.
Schering (1968) [Isophorone - Systemic tolerability following single
administration (LD50, oral).] Berlin, Germany, Schering AG
(in German).
Schering (1974) Dissipation of isophorone in sugar beets. Berlin,
Germany, Schering AG.
Scheubel JB & Scholz N (1989) [Acute toxicity test with isophorone in
the Golden Orfe.] Marl, Germany, Hüls AG (Unpublished study No. F 120)
(in German).
Schöberl P (1992) [Determination of the biodegradability of isophorone
in the DOC-DIE AWAY test.] Marl, Germany, Hüls AG (Unpublished report)
(in German).
Scholz N (1988a) [Acute toxicity of isophorone to Daphnia magna
strauss]. Marl, Germany, Hüls AG (Unpublished study No. D 318)
(in German).
Scholz N (1988b) [Growth retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. AW 146) (in German).
Scholz N (1988c) [Assimilation retardation test with isophorone in
Scenedesmus subspicatus.] Marl, Germany, Hüls AG (Unpublished study
No. A 115) (in German).
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency (EPA 600/4-76-062).
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Smyth HF Jr & Seaton J (1940) Acute response of guinea-pigs and rats
to inhalation of the vapours of isophorone. J Ind Hyg Toxicol,
22: 477-483.
Smyth HF Jr, Seaton J, & Fischer L (1942) Response of guinea-pigs and
rats to repeated inhalation of vapours of mesityl oxide and
isophorone. J Ind Hyg Toxicol, 4: 46-50.
Smyth HF Jr, Weil CS, West JS, & Carpenter CP (1969) An exploration of
joint toxic action: Twenty-seven industrial chemicals intubated in
rats in all possible pairs. Toxicol Appl Pharmacol, 14: 340-347.
Strasser J Jr, Charbonneau M, Borghoff SJ, Turner MJ, & Swenberg JA
(1988) Renal protein droplet formation in male F344 rats after
isophorone (IPH) treatment. Toxicologist, 8: 136.
Suffet IH, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking water during a 2-year period. Water
Res, 14: 853-867.
Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, & Dishburger HJ
(1983) A rapid method for the estimation of the environmental
parameters octanol/water partition coefficient, soil sorption
constant, water to air ratio, and water solubility. Residues Rev,
85: 17-28.
Swenberg JA (1991) Risk assessment of chemicals causing alpha-2
µ-globulin nephropathy. Regul Toxicol Pharmacol, 13: 1-2.
Swenberg JA, Short B, Borghoff S, Strasser J, & Charbonneau M (1989)
The comparative pathobiology of alpha-2 µ-globulin nephropathy.
Toxicol Appl Pharmacol, 97: 35-46.
Swenberg JA, Dietrich DR, McClain RM, & Cohen SM (1992) Species-
specific mechanisms of carcinogenesis. In: Vainio H, Magee PN,
McGregor DB, & McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 477-500.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981a) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981b) Biodegradability
studies for predicting the environmental fate of organic priority
pollutants. Washington, DC, Association of Official Analytical
Chemists.
Thier R, Peter HP, Wiegand HJ, & Bolt HM (1990) DNA binding study of
isophorone in rats and mice. Arch Toxicol, 64: 684-685.
Thier R & Xu DG (1990) Urinary excretion of isophorone metabolites by
male rats. Naunyn-Schmiedeberg's Arch Pharmacol, 341: R11 (Abstract
No. 41).
Thursby GB & Steele RL (1986) Comparison of short- and long-term
sexual reproduction with the marine red alga Champia parvula.
Environ Toxicol Chem, 5: 1013-1018.
Thursby GB, Steele RL, & Kane ME (1985) Effect of organic chemicals on
growth and reproduction in the marine red alga Champia parvula.
Environ Toxicol Chem, 4: 797-805.
Traul KA, Hinz JP, & Kapp RW (1984) Inhalation teratology study in
rats and mice - MRD-83-237 (isophorone) (Project No. 32377). East
Millstone, New Jersey, Bio/dynamics Inc., East Laboratory (Report
submitted to Chemical Manufacturers Association).
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1970) Premiers
résultats de l'étude du métabolisme chez le lapin d'un solvent
industriel : l'isophorone. C R Acad Sci (Paris), 271: 1333-1336.
Truhaut R, Dutertre-Catella H, & N'Guyen Phu-Lich (1972) Etude de la
toxicité d'un solvant industriel: l'isophorone - pouvoir irritant
vis-à-vis des téguments et des muqueuses. J Eur Toxicol, 5: 31-37.
US EPA (1978) Isophorone: Ambient water quality criteria. Washington,
DC, US Environmental Protection Agency (NTIS PB-296 798).
US EPA (1979) Standard operating procedure: Analysis of non volatile
extractable organic compounds. Sample extraction and screening by gas
chromatography. Method 625. Fed Reg, 44: 223.
US EPA (1980) Ambient water quality criteria for isophorone.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-056;
NTIS PB81-11767).
US EPA (1986) Graphical exposure modelling system (GEMS). Fate of
atmospheric pollutants. Washington, DC, US Environmental Protection
Agency.
US EPA (1991a) Alpha-2 µ-globulin: Association with chemically induced
renal toxicity and neoplasia in the male rat. Washington, DC, US
Environmental Protection Agency (Risk Assessment Forum).
US EPA (1991b) Report of the EPA peer review workshop on alpha-2
µ-globulin: Association with renal toxicity and neoplasia in the male
rat. Washington, DC, US Environmental Protection Agency
(EPA/625/3-91/021).
US NIOSH (1977) Manual of analytical methods: Method S367, 2nd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Cincinnati, vol 3).
US NIOSH (1980) Health hazard evaluation report HHE-80-103-827.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NIOSH (1984) Health hazard evaluation report HHE-84-299-1543.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
US NTP (1986) Toxicological and carcinogenesis studies of isophorone
(CAS No. 