
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 171
DIESEL FUEL AND EXHAUST EMISSIONS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 171
DIESEL FUEL AND EXHAUST EMISSIONS
First draft prepared by the staff members of the Fraunhofer Institute
of Toxicology and Aerosol Research, Germany, under the coordination of
Dr. G. Rosner
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework if the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Diesel Fuel and exhaust emission
(Environmental health criteria ; 171)
1.Air pollutants, Enviromental 2.Air pollution 3.Fueloils
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157171 3 (NLM Classification: WA 754)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
PREAMBLE
ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND EXHAUST EMISSIONS
WHO DRAFTING GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL
AND EXHAUST EMISSIONS
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND
EXHAUST EMISSIONS
PART A: DIESEL FUEL
A1. SUMMARY
A1.1 Identity, physical and chemical properties, and
analytical methods
A1.2 Sources of human and environmental exposure
A1.3 Environmental transport, distribution, and
transformation
A1.4 Environmental levels and human exposure
A1.5 Effects on laboratory mammals and in vitro test
systems
A1.6 Effects on humans
A1.7 Effects on other organisms in the laboratory and the
field
A1.8 Evaluation of human health risks
A1.9 Evaluation of effects on the environment
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
A2.1.1 Fuel components
A2.1.1.1 Alkanes
A2.1.1.2 Alkenes
A2.1.1.3 Aromatic compounds
A2.1.1.4 Sulfur
A2.1.2 Fuel additives
A2.1.2.1 Cetane number improvers
A2.1.2.2 Smoke suppressors
A2.1.2.3 Flow improvers
A2.1.2.4 Cloud-point depressors
A2.1.2.5 Wax anti-settling additives
A2.1.2.6 Other additives
A2.1.3 Quality aspects of diesel fuels
A2.1.3.1 Ignition performance and cetane
number
A2.1.3.2 Density
A2.1.3.3 Sulfur content
A2.1.3.4 Viscosity
A2.1.3.5 Cold-flow properties
A2.2 Physical and chemical properties
A2.3 Analytical methods
A2.4 Conversion factors
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
A3.2 Anthropogenic sources
A3.2.1 Production and use
A3.2.1.1 Production process
A3.2.1.2 Use
A3.2.1.3 Production and consumption
levels
A3.2.2 Emissions during production and use
A3.2.2.1 Air
A3.2.2.2 Water
A3.2.2.3 Soil
A3.2.3 Accidental releases to the environment
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A4.1 Transport and distribution between media
A4.1.1 Evaporation from and dissolution in the
aqueous phase
A4.1.2 Transport in and adsorption onto soil and
sediment
A4.1.2.1 Soil
A4.1.2.2 Sediment
A4.2 Transformation and removal
A4.2.1 Photooxidation
A4.2.2 Biodegradation
A4.2.2.1 Microbial degradation
A4.2.2.2 Phytoplankton and marine algae
A4.2.2.3 Invertebrates and vertebrates
A4.2.3 Bioaccumulation
A4.2.4 Tainting
A4.2.5 Entry into the food chain
A4.3 Ultimate fate after use
A4.3.1 Use in motor vehicles
A4.3.2 Spills
A4.3.3 Disposal
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
A5.2 Exposure of the general population
A5.3 Occupational exposure during manufacture,
formulation, or use
A6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A7.1 Single exposure
A7.2 Short-term exposure
A7.2.1 Subacute exposure
A7.2.1.1 Dermal exposure
A7.2.1.2 Inhalation
A7.2.2 Subchronic exposure
A7.2.2.1 Dermal exposure
A7.2.2.2 Inhalation
A7.3 Long-term exposure
A7.3.1 Dermal exposure
A7.3.2 Inhalation
A7.4 Dermal and ocular irritation; dermal sensitization
A7.4.1 Dermal irritation
A7.4.2 Ocular irritation
A7.4.3 Sensitization
A7.5 Reproductive toxicity, embryotoxicity, and
teratogenicity
A7.6 Mutagenicity and related end-points
A7.6.1 In vitro
A7.6.2 In vivo
A7.7 Carcinogenicity
A7.7.1 Dermal exposure
A7.7.2 Inhalation
A8. EFFECTS ON HUMANS
A8.1 Exposure of the general population
A8.2 Occupational exposure
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
A9.1 Laboratory experiments
A9.1.1 Microorganisms
A9.1.1.1 Water
A9.1.1.2 Soil
A9.1.2 Aquatic organisms
A9.1.2.1 Plants (phytoplankton)
A9.1.2.2 Invertebrates
A9.1.3 Terrestrial organisms
A9.1.3.1 Plants
A9.1.3.2 Invertebrates
A9.1.3.3 Vertebrates
A9.2 Field observations
A9.2.1 Microorganisms.
A9.2.1.1 Water
A9.2.1.2 Soil
A9.2.2 Aquatic organism
A9.2.3 Terrestrial organisms
A9.2.3.1 Plants
A9.2.3.2 Invertebrates
A9.2.3.3 Vertebrates
A10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
A10.1 Evaluation of human health risks
A10.1.1 Exposure of the general population
A10.1.2 Occupational exposure
A10.1.3 Non-neoplastic effects
A10.1.4 Neoplastic effects
A10.2 Evaluation of effects on the environment
A11. RECOMMENDATIONS
A11.1 Recommendations for the protection of human health
A11.2 Recommendation for the protection of the environment
A11.3 Recommendations for further research
A12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
PART B: DIESEL EXHAUST EMISSIONS
B1. SUMMARY
B1.1 Identity, physical and chemical properties, and
analytical methods
B1.2 Sources of human and environmental exposure
B1.3 Environmental transport, distribution, and
transformation
B1.4 Environmental levels and human exposure
B1.5 Kinetics and metabolism in laboratory animals and
humans
B1.5.1 Deposition
B1.5.2 Retention and clearance of particles
B1.5.3 Retention and clearance of polycyclic
aromatic hydrocarbons adsorbed onto diesel
soot
B1.5.4 Metabolism
B1.6 Effects on laboratory mammals and in vitro test
systems
B1.7 Effects on humans
B1.8 Effects on other organisms in the laboratory and the
field
B1.9 Evaluation of human health risks
B1.9.1 Non-neoplastic effects
B1.9.2 Neoplastic effects
B1.10 Evaluation of effects on the environment
B2. IDENTITY AND ANALYTICAL METHODS
B2.1 Identity
B2.1.1 Chemical composition of diesel exhaust
gases
B2.1.2 Type and composition of emitted particulate
matter
B2.2 Analytical methods
B2.2.1 Sampling
B2.2.1.1 Sampling from undiluted exhaust
gas (raw gas sampling)
B2.2.1.2 Sampling from diluted exhaust
(dilution tube sampling)
B2.2.2 Extraction from particles
B2.2.3 Clean-up and fractionation
B2.2.4 Chemical analysis
B2.3 Conversion factors
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Anthropogenic sources
B3.1.1 Diesel exhaust emissions
B3.1.1.1 Emission of chemical
constituents with the gaseous
portion of diesel exhaust
B3.1.1.2 Emission of particulate matter
and adsorbed components in
diesel exhaust gases
B3.1.2 Parameters that influence diesel exhaust
emissions
B3.1.2.1 Engine conditions
B3.1.2.2 Fuel specification
B3.1.2.3 Malfunction
B3.1.3 Total emissions by diesel engines
B3.1.4 Control of emissions
B3.1.4.1 Particle traps
B3.1.4.2 Catalytic converters
B3.2 Regulatory approaches
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
B4.1 Transport and distribution between media
B4.2 Transformation and removal
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
B5.1 Exposure of the general population
B5.2 Occupational exposure
B5.2.1 Truck drivers and mechanics
B5.2.2 Bus garage and other bus workers
B5.2.3 Fork-lift truck operators
B5.2.4 Railroad workers
B5.2.5 Mine workers
B5.2.6 Fire fighters
B5.3 Biomonitoring
B5.3.1 Urinary mutagenicity
B5.3.2 Other analyses
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
B6.1 Deposition
B6.2 Retention and clearance of particles
B6.3 Retention and clearance of polycyclic aromatic
hydrocarbons adsorbed onto diesel soot
B6.4 Metabolism
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
B7.1 Single exposure
B7.2 Short-term exposure
B7.3 Long-term exposure and studies of carcinogenicity
B7.3.1 Non-neoplastic effects
B7.3.2 Carcinogenicity
B7.3.2.1 Inhalation
B7.3.2.2 Other routes of exposure
B7.4 Dermal and ocular irritation; dermal sensitization
B7.5 Reproductive toxicity, embryotoxicity, and
teratogenicity
B7.5.1 Reproductive toxicity
B7.5.2 Embryotoxicity
B7.5.3 Teratogenicity
B7.6 Mutagenicity and related end-points
B7.6.1 In vitro
B7.6.2 In vivo
B7.6.3 DNA adduct formation
B7.7 Special studies
B7.7.1 Immunotoxicity
B7.7.2 Behavioural effects
B7.8 Factors that modify toxicity; toxicity of
metabolites
B7.9 Mechanisms of toxicity; mode of action
B7.9.1 Carcinogenic effects
B7.9.1.1 DNA-reactive mechanisms
B7.9.1.2 Cytotoxicity with regenerative
cell proliferation
B7.9.1.3 Effects of particles
B7.9.1.4 Effects of polycyclic aromatic
hydrocarbons
B7.9.2 Noncarcinogenic effects
B8. EFFECTS ON HUMANS
B8.1 General population
B8.1.1 Acute exposure: olfactory, nasal, and
ocular irritation
B8.1.2 Air pollution
B8.2 Occupational exposure
B8.2.1 Effects on the respiratory system
B8.2.1.1 Symptoms
B8.2.1.2 Acute changes in pulmonary
function
B8.2.1.3 Pulmonary effects
B8.2.2 Epidemiological studies (noncarcinogenic
effects)
B8.2.2.1 Effects on the respiratory
system
B8.2.2.2 Effects on the circulatory
system
B8.2.3 Epidemiological studies (carcinogenic
effects)
B8.2.3.1 Lung cancer
B8.2.3.2 Urinary bladder cancer
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
B10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
B10.1 Exposure of the general population
B10.2 Occupational exposure
B10.3 Non-neoplastic effects
B10.3.1 Hazard identification
B10.3.1.1 Humans
B10.3.1.1 Experimental animals
B10.3.2 Dose-response assessment
B10.3.2.1 Epidemiological studies
B10.3.2.2 Studies in experimental animals
B10.3.3 Exposure assessment
B10.3.4 Risk characterization
B10.3.4.1 Humans
B10.3.4.2 Experimental animals
B10.4 Neoplastic effects
B10.4.1 Hazard identification
B10.4.1.1 Lung cancer: occupational
exposure
B10.4.1.2 Urinary bladder cancer:
occupational exposure
B10.4.2 Dose-response assessment
B10.4.2.1 Lung cancer
B10.4.2.2 Urinary bladder cancer
B10.4.3 Exposure assessment
B10.4.4 Risk characterization
B10.4.4.1 Human lung cancer
B10.4.4.2 Human urinary bladder cancer
B10.4.4.3 Risk characterization based on
studies in experimental animals
Appendix B10.1 Construction of a biologically
based (alternative) model
Appendix B10.2 E-M algorithm
Appendix B10.3 A tumour growth model
B11. RECOMMENDATIONS
B11.1 Recommendations for the protection of human health
B11.2 Recommendation for the protection of the environment
B11.3 Recommendations for further research
B12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUMÉ
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the Criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 979 9111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
The WHO Environmental Health Criteria Programme was initiated in
1973, with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976; numerous assessments of chemicals and
of physical effects have since been produced. Many EHC monographs have
been devoted to toxicological methods, e.g. for genetic, neurotoxic,
teratogenic, and nephrotoxic effects. Other publications have been
concerned with e.g. epidemiological guidelines, evaluation of
short-term tests for carcinogens, biomarkers, and effects on the
elderly.
Since the time of its inauguration, the EHC Programme has widened
its scope, and the importance of environmental effects has been
increasingly emphasized in the total evaluation of chemicals, in
addition to their health effects.
The original impetus for the Programme came from resolutions of
the World Health Assembly and the recommendations of the 1972 United
Nations Conference on the Human Environment. Subsequently, the work
became an integral part of the International Programme on Chemical
Safety (IPCS), a cooperative programme of UNEP, ILO, and WHO. In this
manner, with the strong support of the new partners, the importance of
occupational health and environmental effects was fully recognized.
The EHC monographs have become widely established, used, and
recognized throughout the world.
The recommendations of the 1992 United Nations Conference on
Environment and Development and the subsequent establishment of the
Intergovernmental Forum on Chemical Safety, with priorities for action
in the six programme areas of Chapter 19, Agenda 21, lend further
weight to the need for EHC assessments of the risks of chemicals.
Scope
The Criteria monographs are intended to provide critical reviews
of the effect on human health and the environment of chemicals,
combinations of chemicals, and physical and biological agents. They
include reviews of studies that are of direct relevance for the
evaluation and do not describe every study that has been carried out.
Data obtained worldwide are used, and results are quoted from original
studies, not from abstracts or reviews. Both published and unpublished
reports are considered, and the authors are responsible for assessing
all of the articles cited; however, preference is always given to
published data, and unpublished data are used only when relevant
published data are absent or when the unpublished data are pivotal to
the risk assessment. A detailed policy statement is available that
describes the procedures used for citing unpublished proprietary data,
so that this information can be used in the evaluation without
compromising its confidential nature (WHO, 1990).
In the evaluation of human health risks, sound data on humans,
whenever available, are preferred to data on experimental animals.
Studies of animals and in vitro systems provide support and are used
mainly to supply evidence missing from human studies. It is mandatory
that research on human subjects be conducted in full accord with
ethical principles, including the provisions of the Helsinki
Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not in any sense recommendations for regulation or
setting standards. The latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary: a review of the salient facts and the risk evaluation of
the chemical
* Identity: physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution, and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in-vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and the field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g. the
International Agency for Research on Cancer, the Joint FAO/WHO
Expert Committee on Food Additives, and the Joint FAO/WHO Meeting
on Pesticide Residues
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of chemicals that are of
priority for subsequent evaluation. Such meetings have been held in
Ispra, Italy (1980); Oxford, United Kingdom (1984); Berlin, Germany
(1987); and North Carolina, United States of America (1995). The
selection of chemicals is based on the following criteria: the
existence of scientific evidence that the substance presents a hazard
to human health and/or the environment; the existence of evidence that
the possible use, persistence, accumulation, or degradation of the
substance involves significant human or environmental exposure; the
existence of evidence that the populations at risk (both human and
other species) and the risks for the environment are of a significant
size and nature; there is international concern, i.e. the substance is
of major interest to several countries; adequate data are available on
the hazards.
If it is proposed to write an EHC monograph on a chemical that is
not on the list of priorities, the IPCS Secretariat first consults
with the cooperating organizations and the participating institutions.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the following flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for the layout and language. The first draft, prepared by
consultants or, more usually, staff at an IPCS participating
institution is based initially on data provided from the International
Register of Potentially Toxic Chemicals and reference data bases such
as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the first draft acceptable,
it is distributed in its unedited form to over 150 EHC contact points
throughout the world for comment on its completeness and accuracy and,
where necessary, to provide additional material. The contact points,
usually designated by governments, may be participating institutions,
IPCS focal points, or individual scientists known for their particular
expertise. Generally, about four months are allowed before the
comments are considered by the RO and author(s). A second draft
incorporating the comments received and approved by the Director,
IPCS, is then distributed to Task Group members, who carry out a peer
review at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the risks to health and
the environment from exposure to the chemical. A summary and
recommendations for further research and improved safety are also
drawn up. The composition of the Task Group is dictated by the range
of expertise required for the subject of the meeting and by the need
for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations, so that
representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide valuable contributions to the process, they can speak only
at the invitation of the Chairperson. Observers do not participate in
the final evaluation of the chemical, which is the sole responsibility
of the Task Group members. The Task Group may meet in camera when it
considers that to be appropriate.
All individuals who participate in the preparation of an EHC
monograph as authors, consultants, or advisers must, in addition to
serving in their personal capacity as scientists, inform the RO if at
any time a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a statement to that
effect. This procedure ensures the transparency and probity of the
process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it is edited for language, the references are checked, and
camera-ready copy is prepared. After approval by the Director, IPCS,
the monograph is submitted to the WHO Office of Publications for
printing. At this time, a copy of the final draft is also sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern about
health or environmental effects of the agent because of greater
exposure; an appreciable time has elapsed since the last evaluation.
 All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting exhaust emissions of the documents. A comprehensive file of
all comments received on drafts of each EHC monograph is maintained
and is available on request. The chairpersons of task groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO DRAFTING GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL
AND EXHAUST EMISSIONS
WHO, Geneva, 6-9 December 1993
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United State
Dr R.P. Bos, University of Nijmegen, Nijmegen, Netherlands
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom (Joint Rapporteur)
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr I. Farkas, National Institute of Hygiene, Budapest, Hungary
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr P. Gustavsson, North Western Health Board, Stockholm, Sweden
Dr D. Guth, United States Environmental Protection Agency, Research
Triangle Park, NC, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr W. Pepelko, United States Environmental Protection Agency,
Washington DC, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Vice-Chairman)
Dr G. Rosner, Hazardous Substances Documentation Group, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr J. Roycroft, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, United States
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States (Chairman)
Dr B.H. Thomas, Environmental Health Directorate, Ottawa, Canada
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr. R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr P. Boffetta, International Agency for Research on Cancer, Lyon,
France (6-7 December 1993)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Representatives/Observers
CONCAWE
Dr R.H. McKee, Exxon Biomedical Sciences, East Millstone, NJ,
United States
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT
Dr P.T.C. Harrison, MRC Institute of Environment & Health,
Leicester, United Kingdom
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND
EXHAUST EMISSIONS
Fraunhofer Institute of Toxicology & Aerosol Research, Hanover
27 June-1 July 1994
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United States
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Dr Jun Kagawa, Tokyo Women's Medical College, Tokyo, Japan
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
Dr M. Roller, Medizinishes Institut für Umwelthyglene an der
Universität Düsseldorf, Düsseldorf, Germany Dr G. Rosner, Hazardous
Substances Documentation Group, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr H. Moller, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr M. Younes, European Centre for Environment and Health, Bilthoven,
Netherlands
Representatives/Observers
Dr Lutz Von Meyerinck, BP Oil Europe, Brussels, Belgium
GERMAN AUTOMOBILE ASSOCIATION
Dr N. Pelz, Mercedes-Benz AG, Stuttgart, Germany
ILSI
Dr I.T. Salmeen, Chemistry Department, Ford Motor Company,
Dearborn, MI, United States
IUTOX
Dr P. Montuschi, Department of Pharmacology, School of Medicine,
Catholic University of the Sacred Heart, Rome, Italy
ENVIRONMENTAL HEALTH CRIETERIA FOR DIESEL FUEL AND EXHAUST EMISSIONS
A WHO Task Group on Environmental Health Criteria for Diesel Fuel
and Exhaust Emissions met at the Fraunhofer Institute of Toxicology
and Aerosol Research, Hanover, Germany from 27 June to 1 July 1994.
Dr G. Rosner, Fraunhofer Institute, welcomed the participants on
behalf of the Institute and its Director, Professor U. Mohr, and
Dr E.M. Smith, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP, ILO, and WHO). The Task
Group reviewed and revised the draft and evaluated the risks for human
health and the environment from exposure to diesel fuel and exhaust
emissions.
The first draft of the monograph was prepared at the Fraunhofer
Institute. After international circulation for comment, this draft was
extensively revised by a Working/Drafting Group, convened at WHO,
Geneva, from 6 to 9 December 1993, and a second draft was prepared for
further international circulation for comment. The membership of the
drafting group is shown previously. A final draft, incorporating
comments received from the IPCS contact points for Environmental
Health Criteria monographs and new material, was completed at the
Fraunhofer Institute under the coordination of Dr G. Rosner, with
important contributions to the text from the following Institute staff
members:
Dr B. Bellman
Dr A. Boehnke
Dr O. Creutzenberg
Dr J. Kielhorn
Dr E.M. Smith of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs E. Heseltine, Lajarthe,
France, for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
PART A DIESEL FUEL
A1. SUMMARY
A1.1 Identity, physical and chemical properties, and analytical
methods
Diesel fuel is a complex mixture of normal, branched, and cyclic
alkanes (60 to > 90% by volume; hydrocarbon chain length, usually
between C9 and C30); aromatic compounds, especially alkylbenzenes
(5-40% by volume); and small amounts of alkenes (0-10% by volume)
obtained from the middle-distillate, gas-oil fraction during petroleum
separation. Benzene, toluene, ethylbenzene, and xylenes and polycyclic
aromatic hydrocarbons (PAHs), especially naphthalene and its
methyl-substituted derivatives, may be present at levels of parts per
million in diesel fuel. The sulfur content of diesel fuels depends on
the source of crude oil and the refinery process. It is regulated by
law in a number of countries and is usually between 0.05 and 0.5
weight percent. Additives are used to influence the flow, storage, and
combustion of diesel fuel, to differentiate products, and to meet
trademark specifications. At room temperature, diesel fuels are
generally moderately volatile, slightly viscous, flammable, brown
liquids with a kerosene-like odour. The boiling ranges are usually
between 140 and 385°C (> 588°C for marine diesel fuel); at 20°C, the
density is 0.87-1.0 g/cm3 and the water solubility is
0.2-5 mg/litre. The quality and composition of diesel fuel influence
the emissions of pollutants from diesel engines considerably.
Important variables are ignition behaviour (expressed in terms of
cetane number), density, viscosity, and sulfur content. The
specifications of commercial diesel fuel differ considerably in
different countries.
Heating fuels and some kerosene jet fuels produced during the
refining process may have a composition similar to that of diesel
fuel, although with different additives. Biological data on these
mixtures have therefore also been taken into account in the
assessments of toxicity and ecotoxicity.
Owing to the complexity of the diesel fuel mixture, there is no
specific analytical method, and the analytical techniques used in most
environmental assessments are suitable only for measuring the total
petroleum hydrocarbon mixture. The methods consist of preliminary
solvent extraction, a clean-up procedure to remove naturally occurring
hydrocarbons, and subsequent detection by gravimetry, infrared
spectroscopy or gas chromatography. Neither the gravimetric nor the
infrared technique provides useful qualitative or quantitative
information on contaminants and can thus be used only for screening.
Gas chromatography combined with detection techniques such as flame
ionization and mass spectrometry is the standard procedure for
analysing environmental samples. Many other methods are available for
the analysis of individual hydrocarbons in diesel fuels.
A1.2 Sources of human and environmental exposure
Diesel fuels are produced by refining crude oils. In order to
meet technical specifications for performance, diesel fuels are
generally blended; further formulation with additives improves their
properties for specific uses. Diesel fuels are widely used as
transportation fuels. The more volatile fuels, with low viscosity, are
required for high-speed engines and the heavier grades for railroad
and ship diesel engines. Much heavy-duty road transport is powered by
diesel engines. Passenger cars powered by diesel engines are becoming
increasingly more common in Europe and Japan (10-25%), whereas in
North America the percentage of diesel-fuelled passenger cars is about
1-2%, with a slightly decreasing tendency. Diesel fuel is used in
stationary engines and in boilers, e.g. reciprocating engines, gas
turbines, pipeline pumps, gas compressors, steam processing units in
electric power plants, burner installations, and industrial space and
water heating facilities.
Over the last five years, the worldwide demand for diesel fuels
has increased steadily. In 1985, the following amounts of diesel fuel
were consumed: about 170 000 kt per year in North America; about
160 000 kt per year, including gas oils, in the European Union; and
about 46 000 kt per year in Australia, Japan, and New Zealand,
equivalent to a total of 1062 kt per day. In 1990, world demand was
reported to be about 1110 kt per day.
No information is available on emissions during the production of
diesel fuels; however, this source would seem to be of minor
importance, because the refining process is carried out in closed
systems. Emissions may occur principally during storage and
transportation. Diesel fuels are released as a result of spills and at
filling stations during the refuelling of vehicles. The atmosphere and
the hydrosphere are the most heavily affected environmental
compartments. Soil contamination with diesel fuels may occur during
accidents and is also a problem in railroad yards. The numerous
techniques for cleaning soils contaminated with diesel fuel include
excavation, biological methods, and containment.
A1.3 Environmental transport, distribution, and transformation
Very few data are avilable on the environmental fate of diesel
fuels, but the mechanisms of their distribution and transformation are
considered to be comparable to those of heating fuels, such as No. 2
fuel oil, which have been well studied. Spills of diesel fuel on water
spread almost immediately to form a 'slick'. The polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, and the volatile components evaporate from the surface;
microbial degradation also begins. Chemical and biological weathering
alter the composition of the spill. These processes are dependent on
temperature; spills that occur in Arctic conditions are more
persistent than those that occur in temperate climates. In marine
environments, most of the low-relative-molecular-mass aromatic species
are dissolved into the water phase, but the primary branched alkanes,
cycloalkanes, and remaining aromatic compounds may remain in sediments
for more than a year.
Although no information is available on the photooxidation of
diesel fuels in water and air, evaporated oil components are degraded
photochemically. No. 2 fuel oil has been shown to be photooxidized
rapidly in water under environmental conditions.
The individual constituents of diesel fuel are inherently
biodegradable, to varying degrees and at different rates. The
n-alkane, n-alkylaromatic, and simple aromatic molecules in the C10-C22
range are the most readily degradable. Smaller molecules are generally
rapidly metabolized. Long-chain n-alkanes are more slowly degraded,
owing to their hydrophobicity and because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to biological breakdown, and PAHs are resistant. The overall
rates of degradation of hydrocarbons are limited by temperature, water
content, oxygen, pH, inorganic nutrients, and microbial metabolic
versatility.
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this actually occurs in
nature is poorly understood. Unlike microorganisms that use petroleum
carbons as a carbon source, animals generally oxidize and conjugate
products, rendering end-products that are more soluble and therefore
easier to excrete. All animal species tested can take up petroleum
hydrocarbons. PAHs, crude oil, and refined petroleum products are
known to induce cytochrome P450 enzymes and to increase the levels of
hydrocarbon metabolism in numerous marine and freshwater fish species.
Few data are available on the bioaccumulation of diesel fuel in
the laboratory, but there is plentiful evidence from studies of spills
and laboratory studies on other oils, particularly No. 2 fuel oil,
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient for diesel fuel is 3.3-7.06,
which suggests high potential bioaccumulation; however, many of the
lower-relative-molecular-mass compounds are readily metabolized, and
the actual bioaccumulation of higher-relative-molecular-mass compounds
is limited by their low water solubility and large molecular size.
Thus, actual bioaccumulation may be low.
Fish have been tainted by diesel fuel after spills. No data are
available on the biomagnification of diesel fuel.
No experimental data are available on the movement of diesel fuel
through the soil, although a direct correlation between the movement
and kinematic viscosity has been proposed. The movement of kerosene
through soil depends on the moisture content and nature of the soil.
A1.4 Environmental levels and human exposure
As diesel fuels are complex mixtures, the environmental levels
have not been measured. The individual constituents of diesel fuels
can be detected in almost all compartments of the environment,
although their source cannot be verified. The general population may
be exposed to diesel fuel at filling stations and as a result of
spills.
Occupational exposure to diesel fuel occurs in a large number of
activities. Because of their low volatility, diesel fuels should
generate only low concentrations of vapours at normal temperatures,
but high operating temperatures can result in significant
concentrations.
A1.5 Effects on laboratory mammals and in-vitro test systems
The acute toxicity of diesel fuels is low after oral or dermal
exposure or after inhalation. The oral LD50 value was > 5000 mg/kg
body weight in all species tested (mouse, rabbit, rat, guinea-pig).
Dermal application resulted in an LD50 value of > 5000 mg/kg body
weight in mice and rabbits, although values of > 2000 mg/kg body
weight were reported for some kerosenes and middle distillates, with
different protocols and lower limit doses. The LC0 value in rats
exposed by inhalation was about 5 mg/litre, except for one
straight-run middle distillate for which a value of 1.8 mg/litre was
seen.
In rabbits treated dermally with up to 8000 µl/kg body weight per
day and mice with up to 40 000 mg/kg body weight per day, acanthosis
and hyperkeratosis due to severe irritation were seen. Rabbits were
more sensitive than mice. Inhalation of diesel fuel was neuro-
depressive in mice at concentrations up to 0.2 mg/litre but not
in rats exposed to up to 6 mg/litre. Body and liver weights were
reduced in rats.
Mice, rats, and dogs did not show significant cumulative toxicity
after inhalation of up to 1.5 mg/litre subchronically. The specific
nephropathy syndrome seen in male rats is linked to an inherent
accumulation of hyalin droplets in the renal tubules.
The only effects of long-term exposures were ulceration after
dermal application to mice (250 or 500 mg/kg body weight per day) and
significant alterations in organ weight after inhalation of 1 or
5 mg/litre by rats. In both studies the mean body weights were
reduced.
Various types of diesel fuels were slightly to severely
irritating to the skin of rabbits. Diesel fuels do not irritate the
eye, but some kerosenes have been reported to have a slight irritating
effect. Diesel fuels do not cause skin sensitization.
Diesel and jet fuels (kerosene) were neither embryotoxic nor
teratogenic in two studies in rats exposed by inhalation to 100 or
400 ppm and in one study in which rats were given up to 2000 mg/kg
body weight per day by gavage. In the last study, reduced fetal weight
was observed.
Tests in Salmonella typhimurium did not provide clear evidence
of mutagenicity. Some positive findings in S. typhimurium and in
mouse lymphoma cells were considered to be equivocal owing to the
inconsistency of the results. Tests for genotoxicity in mice in vivo
(induction of micronuclei or chromosomal aberrations) also gave
equivocal or negative responses.
Diesel fuels induced a low level of dermal carcinogenicity. In
the present state of research, it cannot be concluded whether the
carcinogenic potency of diesel fuels is mediated by a genotoxic
mechanism or by chronic dermal damage.
A1.6 Effects on humans
Non-occupational exposure to diesel fuel can occur during manual
filling of fuel tanks. The primary source of dermal exposure is
accidental spills, which result in immediate high levels of exposure
but are of short duration.
After accidental dermal contact, anuria, renal failure, gastro-
intestinal symptoms, and cutaneous hyperkeratosis have been reported.
Toxic lung disease has been observed after accidental ingestion of
diesel fuel and subsequent aspiration. Persistent productive cough has
been reported after inhalation. In a case-control study of men exposed
to diesel fuel, an increased risk for cancer of the lung other than
adenocarcinoma was found; a positive association was also seen with
prostatic cancer, although a higher risk was noted for the group with
'nonsubstantial' exposure than for that with 'substantial' exposure.
In a cross-sectional study of factory workers exposed to kerosene jet
fuels, dizziness, headache, nausea, palpitation, pressure in the
chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of
vapour from the fuel in the breathing zone was estimated to be
128-423 mg/m3.
A1.7 Effects on other organisms in the laboratory and the field
Diesel fuel is more toxic than crude oil to aquatic organisms and
plants. The ecotoxicity of diesel fuel is generally attributed to
soluble aromatic compounds, but insoluble aliphatic hydrocarbons may
also be implicated. Of the aromatic compounds, monoaromatics are the
least toxic, their acute toxicity increasing with molecular mass up to
the four- to five-ring compounds, although these are poorly soluble in
seawater. In some animals, e.g. fish and birds, physical coating of
the body surface by the fuel can produce toxicity and mortality.
Laboratory experiments have been carried out on diesel fuel,
water-soluble fractions, oil-water dispersions, and microencapsulated
oil. Diesel fuel did not significantly reduce the growth in culture of
the green alga Euglena gracilis, whereas a low concentration (0.1%)
almost completely inhibited the growth of Scenedesmus quadricauda.
Light diesel fuel (0.05%) stimulated the growth, photosynthesis, and
chlorophyll asynthesis of Chlorella salina but slightly inhibited
respiration; at higher concentrations, the growth rate and
photosynthesis were greatly reduced. Long-term exposure inhibited the
growth of the benthic algae Ascophyllum nodosum and Laminaria
digitata. In blue-green algae, photosynthesis was reduced by the
aromatic and asphaltic fractions but not by the aliphatic fraction.
Diesel fuel was acutely toxic to Daphnia spp., chironomid
larvae, and the mollusc Viviparus bengalensis (Gastropoda). A
concentration of 0.1 ml/litre caused the death of tidepool copepods,
Tigriopus californicus, within five days.
Mytilus edulismussels accumulate diesel fuel, have markedly
reduced feeding and growth rates, and show reproductive toxicity after
chronic exposure to diesel fuel. The EC50 for spawning in mussels
exposed for 30 days was about 800 µg/litre. The LC50 of micro-
encapsulated diesel oil after exposure of maturing mussels for
30 days was about 5000 µg/litre. Diesel oil was more toxic to larvae
than to juveniles: 10 µg/litre had adverse effects on the growth of
larvae.
Freshwater crabs (Barytelphusa cunicularis) exposed to sublethal
concentrations of diesel fuel for up to 96 h generally reduced their
oxygen consumption, particularly at lower exposures up to 8 h. With
longer exposures, the oxygen consumption was equal to or higher than
that of the controls.
In 96-h tests of acute toxicity in juvenile salmonids under
static conditions, diesel fuel was more toxic to pink salmon,
Onchorhychus gorbuscha (LC50: 32-123 mg/litre), than to cohosalmon,
O. kisutch (LC50: 2186-3017 mg/litre), or rainbow trout,
O. mykiss (LC50: 3333-33 216 mg/litre), irrespective of water type.
The threshold for detection of behavioural responses of cod
( Gadus morhua L.) exposed to diesel fuel in seawater was within
100-400 ng/litre. The Antarctic fish Pagothenia borchgrevinki
withstood an undiluted water-soluble fraction of diesel fuel oil for
up to 72 h but showed signs of stress.
Birds are affected externally and internally by oil
contamination. Diesel fuel destroys the waterproof nature of the
birds' plumage and is ingested during preening. Diesel and fuel oil
fed by gavage at 2 ml/kg body weight to ducks caused lipid pneumonia,
extreme inflammation of the lungs, fatty infiltration of the liver,
and hepatic degeneration after 24 h. Administration of diesel or fuel
oil at 1 ml/kg caused severe irritation of the digestive tract and
toxic nephrosis. Higher doses resulted in adrenal enlargement (mainly
due to hyperplasia of cortical tissue), depression of plasma
cholinesterase levels, ataxia, and tremors. Doses up to 20 ml/kg body
weight were not fatal to healthy birds, but the LD50 for diesel and
fuel oil administered to birds under stress was 3-4 ml/kg body weight.
After spills of diesel fuel, zooplankton appear to be highly
vulnerable to dispersed and dissolved petroleum constituents but less
so to floating oils. Aquatic organisms may be affected in a number of
ways, including direct mortality (fish eggs, copepods, and mixed
plankton), external contamination by oil (chorions of fish eggs and
cuticles and feeding appendages of crustaceans), tissue contamination
by aromatic constituents, abnormal development of fish embryos, and
altered metabolic rates.
A1.8 Evaluation of human health risks
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, during the handling of such fuels, and during use of kerosene
for domestic cooking or heating. Workers can be exposed to diesel fuel
and other middle distillates while handling and discharging the fuel
at terminals, storage tanks, and filling stations; during the
manufacture, repair, maintenance, and testing of diesel engines and
other equipment; during use of diesel fuel as a cleaning agent or
solvent; and in handling and routine sampling of diesel fuel in the
laboratory. Owing to the low volatility of diesel fuel, only low
concentrations of vapour are likely to occur at room temperature,
although in confined spaces at high temperatures significant levels
may be found.
Exposure to vapour is minimal during the normal handling of
diesel fuel. The most likely effect on human health is dermatitis
after skin contact. Diesel fuel is a skin irritant but does not appear
to irritate the eye. Acute toxic effects on the kidney can occur after
dermal exposure, but the effects of long-term dermal absorption of low
concentrations are unknown.
Diesel fuels are toxic when ingested, sometimes resulting in
regurgitation and aspiration, which can cause chemical pneumonia; the
same is true for any hydrocarbon in a particular range of viscosity.
In rodents exposed by inhalation to diesel fuel at concentrations
up to 0.2 mg/litre, a neurodepressive effect was seen in mice but not
in rats at the higher concentrations. Subchronic exposure by
inhalation to various distillate fuels induced specific
alpha2-microglobulin nephropathy in male rats; this effect is
considered irrelevant for humans.
Diesel fuels were neither embryotoxic nor teratogenic in animals
exposed orally or by inhalation.
There is no clear evidence of mutagenic activity in bacteria, and
the results of other tests for genotoxicity in vitro and in vivo were
equivocal.
A case-control study of workers exposed to diesel fuel suggested
an increased risk for cancer of the lung other than adenocarcinoma and
for prostatic cancer. In neither case was there an exposure-response
relationship. In view of the small number of studies available, the
small number of cases, and the correspondingly wide confidence
intervals, no conclusion can be drawn about the carcinogenicity to
humans of diesel fuel.
In mice, dermally administered diesel fuels had weak carcinogenic
potential. In view of the absence of clear genotoxicity, cancer could
be induced by nongenotoxic mechanisms, e.g. by chronic dermal
irritation characterized by repeated cycles of skin lesions, causing
epidermal hyperplasia.
A1.9 Evaluation of effects on the environment
The environment can be polluted by accidental release of diesel
fuel on a large scale, such as during tanker disasters and pipeline
leaks, or on a smaller scale from contamination of soil around
factories or garages. In water, diesel fuel spreads almost
immediately, polar and low-relative-molecular-mass components dissolve
and leach out, volatile components evaporate from the water surface,
and microbial degradation begins. The extent to which 'weathering'
takes place depends on the temperature and on climatic conditions. The
chemical composition of spills changes with time: after spillage on
water, some fractions evaporate, and the evaporated diesel components
are degraded photochemically; in sediment, diesel fuel appears
generally to be delivered to bottom sediments by settling particles;
in soil, the components of diesel fuel migrate at different rates,
depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates of biodegradation depend heavily on
physical and climatic conditions and on microbial composition.
Aquatic organisms, in particular molluscs, bioaccumulate
hydro-carbons to varying extents, but the hydrocarbons are depurated
on transfer to clean water. Diesel fuel may be bioaccumulated; no data
are available about biomagnification.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota. Recolonization
may occur after about one year, depending on the animal or plant
species and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can be affected by
external oil contamination and tissue accumulation: abnormal
development and altered metabolic rates are signs of the resulting
stress.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Diesel fuels are a gas-oil fraction occurring during petroleum
separation and commonly known as middle distillates (International
Agency for Research on Cancer, 1989a). Gas oils are generally blended
materials formulated to meet technical specifications (CONCAWE, in
press). Commercial diesel fuels contain aliphatic, olefinic,
cycloparaffinic, and aromatic hydrocarbons (see section A2.1.1), and
additives (see section A2.1.2) to improve their fuelling properties
(Sandmeyer, 1981).
Four qualities of diesel fuel are available commercially: diesel
fuel (general), diesel fuel No. 1, diesel fuel No. 2, and diesel fuel
No. 4 (see Table 1). The composition of diesel fuels is comparable to
that of heating oils (e.g. No. 1 and No. 2 fuel oils), except for the
additives (International Agency for Research on Cancer, 1989a; Agency
for Toxic Substances and Disease Registry, 1995).
Diesel fuel No. 2 is used mainly as automobile fuel and
corresponds to diesel fuel (general). In Europe (countries of the
European Union (EU) and European Free Trade Area (EFTA)), the
specifications for diesel fuel for transportation purposes are given
in European standard EN 590 (European Committee for Standardization,
1993), which also provides for changes in the specifications to meet
the requirements of different climatic conditions. In Sweden, two
further qualities of on-road diesel fuel are available: environmental
class 1 and class 2 (city diesel), with sulfur contents of 0.05% and
0.001%, respectively (Standardization Board in Sweden, 1991). The
sulfur content of city diesel corresponds to that of kerosene. Diesel
fuel for ship engines is covered by the International Standards
Organization (ISO) standard 8217 (International Standards
Organization, 1987). In the United States of America, three grades of
diesel fuel are available: diesel fuel No. 1 (relatively high
volatility) for road vehicle engines subject to frequent speed and
load changes; diesel fuel No. 2 (lower volatility) for industrial or
heavy-duty, high-load engines running at uniform speed; and diesel
fuel No. 4 (viscous) for low- and medium-speed engines such as those
used in ships (American Society for Testing and Materials, 1988,
1992).
Several types of kerosene, also derived from the gas-oil fraction
of petroleum separation, are used as aviation turbine fuels (JP fuels,
jet fuels). Their hydrocarbon composition is comparable to that of
diesel fuels.
A2.1.1 Fuel components
A2.1.1.1 Alkanes
Normal, branched, and cyclic alkanes (paraffins) are the most
abundant components (about 65-85%) of diesel fuels. Pristane
(2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-
tetramethyl-hexadecane) are of particular interest environmentally, as
the ratios of pristane to heptadecane and of phytane to octadecane
make it possible to identify the source of a fuel spill; furthermore,
as these ratios increase during biological degradation, they can be
used to estimate the age of an environmental contamination and the
degree of elimination. Cycloalkanes and bicycloalkanes constitute a
significant portion of the mixture, but individual compounds are
present only at low levels and are difficult to analyse. Alkyl
derivatives of cyclopentane, cyclohexane, and cycloheptane are common
components (Block et al., 1991; Table 2).
A2.1.1.2 Alkenes
Alkenes are not common components of crude oil but may be
present in diesel fuel if converted products are added after cracking.
These alkenes have predominantly branched and cyclic structures (Block
et al., 1991). The total alkene content of diesel fuels is up to 10%
(CONCAWE, 1985) (see also Table 2).
A2.1.1.3 Aromatic compounds
Aromatic compounds constitute 5-30% of automotive diesel fuel,
5-40% of marine diesel fuel (CONCAWE, 1985), and 10-30% of diesel
fuel No. 2 (Block et al., 1991). Table 2 shows the specifications of
commercial diesel fuels in this regard.
Table 1. Synonyms and trade names of commercial diesel fuels
Name CAS CAS Registry Range of Synonyms
name number carbon
numbers
Diesel fuel Diesel oil 68334-30-5 C9-C20a Auto diesel, automotive
(general) C10-C28b diesel oil, DERV, diesel,
diesel fuel oil, diesel
oil, gas oil
Diesel fuel Not assigned C9-C16c Diesel fuel oil No. 1,
No. 1 (essentially C4-C16 (for diesel oil No. 1, No. 1
equivalent to wide-cut dieseld
kerosene, aviation)c
8008-20-6)
Diesel fuel No. 2 68476-34-6 Diesel fuel, diesel fuel
No. 2 diesel (applicable for oil No. 2, diesel oil
fuel specific No. 2, No. 2 diesele
viscosity
limits)
Diesel fuel 68476-31-3 C10-C30f Marine diesel fuel,
No. 4 distillate marine diesel
fuel
Adapted from International Agency for Research on Cancer (1989a) and supplemented
a From CONCAWE (in press)
b From CONCAWE (1985); automotive gas oil (automotive diesel fuel, DERV)
c From CONCAWE (1985, 1995)
d In Europe, fuels similar to US diesel No. 1 are commonly referred to as
'kerosene' or 'Arctic diesel'
e Term uncommon in Europe. In the United Kingdom, distillate fuels are frequently
categorized as Class A1 (road diesel) and A2 (off-highway diesel)
f From CONCAWE (1985); distillate marine diesel
Only trace quantities of toxicologically relevant benzene,
toluene, ethyl benzene, and xylene compounds (for physicochemical
properties, see Table 6) are present in diesel fuel No. 2, but
significant levels are found in diesel fuel No. 1 (Arctic diesel),
which has lower flash-point specifications (Block et al., 1991); the
International Agency for Research on Cancer (1989a) cites a
flash-point of 0.25-0.5%. The concentration of benzene in kerosenes is
< 0.01% by volume; wide-cut aviation kerosenes may have higher
levels, but they are usually < 1% by volume (IPCS, 1986; CONCAWE,
1995; IPCS, 1993).
Table 2. Hydrocarbon specifications of some commercial diesel fuel oils
Specification Diesel Keroseneb Distillate Diesel
fuela marine fueld
dieselc
Paraffins/naphthenes 65-95e 78-96 60-90
(volume %) (wide-cut
aviation, < 1)
n-Hexane (volume %) < 0.01
(wide-cut
aviation, < 1)
Saturates (volume %) 59.4-76.6
Olefins (volume %) 0-10 0-5 0-10 0.0-1.0
Aromatics (volume %) 5-30 4-25 5-40 23.4-39.6
(wide-cut
aviation,
6-25)
Aromaticity (weight %) 11-15
See also Table 1
a From CONCAWE (1985, in press); fuel oil similar to diesel fuel (general)
b From CONCAWE (1985, 1995); fuel oil similar to diesel fuel No. 1
c From CONCAWE (1985, in press); fuel oil similar to diesel fuel No. 4
d From German Scientific Association for Petroleum, Natural Gas, and Coal (1991);
three samples of diesel fuel (general)
e Depending on origin of crude oil
Alkyl benzenes (particularly C3 and C4) are common components of
diesel fuel. Polycyclic aromatic hydrocarbons (PAHs), e.g.
naphthalene, phenanthrene, acenaphthene, acenaphthylene, fluorene,
fluoranthene, and pyrene, are also present, as are alkyl- and
cycloalkyl-substituted homologues of these substances, the predominant
ones being naphthalene and its methyl-substituted derivatives (see
Table 6 for physicochemical properties) (Block et al., 1991).
As some PAHs and the benzene, toluene, ethyl benzene, and xylene
components have been shown to be toxic and ecotoxic, these classes of
compounds are usually included in analytical procedures for
environmental contamination by diesel fuel. The PAH content of diesel
fuels varies widely, the highest levels being found in low-quality
fuel blended for large users (frequently railroad companies). The
mid-range aromatic (and PAH) content of diesel blends is limited by
the cetane number specification (Block et al., 1991) (see section
A2.1.3).
The concentrations of total PAHs in diesel fuel are < 5% by
volume, although some marine diesel fuels may contain > 10% by volume
(CONCAWE, 1985; International Agency for Research on Cancer, 1989a).
In straight-run gas-oil components, which are the major blending
material of diesel fuel (see section A3.2.1.1), three-ring PAHs
predominate; use of heavier atmospheric vacuum or cracker gas oils in
a diesel-fuel mixture leads to an increasing content of four- to
six-ring PAHs (CONCAWE, in press).
The concentrations of these constituents in a commercial diesel
fuel (unspecified) and, for comparison, in No. 2 fuel oil are shown in
Table 3. It should be noted that the PAH content of No. 2 fuel oil is
not limited by cetane number specification, so that it may include a
larger proportion of these hydrocarbons (Block et al., 1991). Table 3
also gives the composition of the water-soluble fractions of diesel
and No. 2 fuel oils and indicates how the solubility of a compound
affects the composition of the fraction and of the whole oil.
A2.1.1.4 Sulfur
The sulfur content of middle distillates depends on the source of
crude oil (Booth & Reglitzky, 1991; CONCAWE, 1995). The sulfur content
of most diesel fuels is 0.1-0.5% by weight; it is higher than that of
gasoline, which is about 0.02% by weight (Scheepers & Bos, 1992a).
Only diesel fuel No. 4 (distillate marine diesel) has a sulfur level
> 1% by weight (CONCAWE, 1985, in press). ISO standard 8217
(International Standards Organization, 1987) specifies a sulfur
content of marine diesel fuel of 1.0-2.0% by weight.
The sulfur content of some diesel fuels ranges from 0.01 to > 3%
by weight. In Europe (countries of EU and EFTA), the sulfur content is
restricted to a maximum of 0.2-0.3% by weight, and, as of 1996, it
will be further reduced to 0.05% by weight (European Commission,
1993). In the United States in 1988, the maximal sulfur content
permitted was 0.5% by weight for diesel fuels No. 1 and 2 and 2.0% by
weight for diesel fuel No. 4 (American Society for Testing and
Table 3. Concentrations of toxicologically relevant aromatic hydrocarbons in diesel fuel and No. 2 fuel oil and in 10% water-soluble
fractions prepared from them
Compound Diesel fuela Diesel fuelb No. 2 fuelc No. 2 fueld Water-soluble fractions (µg/litre)
(% by wt) (% by wt) (% by wt) (% by wt)
No. 2 Diesel
fueld fuela
Benzene 0.1 > 0.02 0.006-0.008 NR 550 344
Toluene 0.7 0.25-0.5 0.01-0.08 NR 1040 777
Alkylbenzenese 970
Ethylbenzene 0.2 0.25-0.5 0.01-0.08 NR 139
Xylene 0.5 0.25-0.5 0.01-0.08 NR 875
Polycyclic aromatic hydrocarbons
Naphthalene 0.4 0.273 0.4 840 6.6
1-Methylnaphthalene NR NR 0.82 340 66.2
2-Methylnaphthalene NR 0.67 1.89 480 108
Dimethylnaphalenese NR NR 3.11 240 NR
Trimethylnaphthalenese NR NR 1.84 30 NR
Fluorenese NR NR 0.36 20 NR
Phenanthrenese NR 0.15 0.53 20 NR
NR, not reported
a From Dunlap & Beckmann (1988); the analytical method is not described in detail; the concentration of benzene seems to be
unusually high.
b From International Agency for Research on Cancer (1989a); according to CONCAWE (1985), some marine diesel fuels may contain
more than 10% polycyclic aromatic hydrocarbons.
c From Stone (1991); summary of several reports
d From Anderson et al. (1974); US National Research Council (1985)
e Total of several isomers
Materials, 1988); in October 1993, the maximum was reduced to 0.05% by
weight (US Environmental Protection Agency, 1992a; American Society
for Testing and Materials, 1992). The permitted sulfur level in Brazil
is > 3% by weight (A. Sivak, personal communication, 1993). In Japan,
the sulfur content of diesel fuels was reduced to 0.2% by weight in
1994, and a further reduction, to 0.05% by weight, is under discussion
(CONCAWE, 1990a). Blended marine diesel fuel may also contain up to
about 15% residual components, i.e. material with an initial
boiling-point above about 350°C (CONCAWE, in press).
A2.1.2 Fuel additives
Only agents that are added to fuels at a concentration < 1% are
described as 'additives'. A more appropriate term for substances
present at higher concentrations is 'fuel components'. Fuels are
treated with additives for a number of reasons (see Table 4 and
below); they also differentiate products and determine the trademark
quality of commercial fuels (Fabri et al., 1990).
A2.1.2.1 Cetane number improvers
Cetane number improvers upgrade the ignition characteristics of a
base fuel more economically than refinery processes (Fabri et al.,
1990) (see section A2.1.3). Primary alkyl nitrates (e.g. isooctyl
nitrate) are often used to improve cetane number. Polyethyleneglycol
dinitrates, although effective at much lower concentrations, have a
number of disadvantages, including their price and the fact that they
may not improve the performance of fuel in low-compression engines
(Russell, 1989).
A2.1.2.2 Smoke suppressors
Organometallic compounds containing barium, calcium, manganese,
or iron have been used to reduce diesel smoke. With barium-based
products, 85-95% of the metal is emitted as particulates in the
exhaust (Russell, 1989); however, barium and calcium compounds are no
longer used.
A2.1.2.3 Flow improvers
Cold-flow improvers increase the fluidity of the fuel by
modifying the growth of wax crystals formed by higher homologues of
paraffins at low temperatures. The wax content of diesel fuels is
influenced by the origin of the crude oil, the distillation range of
the fuel, and the source of blend components (Coley, 1989).
Table 4. Diesel fuel additives
Additive Material Concentration Effect
(ppm)
Ignition improvers, Organic nitratesa Enhancement of self-ignition qualitiesa
cetane enhancersa
Smoke suppressors, Organic compounds of Ca, Ba, Reduction of soot; increase in metal
combustion enhancersa or (sometimes)Mga sulfate emissionsa
Detergentsa Amines, imidazolines, Prevention and removal of coke deposits
succinimides, etc.b on fuel injector tips, etc.a
Flow improvers Olefin-ester copolymers 50-500 Interaction with wax crystals and
modification of their growth; prevention
of formation of agglomerates
Cloud-point depressors Olefin-ester copolymers About 1000 Depression of cloud-point; prevention of
formation of agglomerates
Wax anti-settlers Modified ethylene-vinyl 100-500c Reduction of crystal size and rate of
acetate copolymersc settling; prevention of formation of agglomerates
Anti-static agents Not reported Not reported Reduction of building up of charges of
static electricity
Anti-corrosion chemicals Alkenyl succinic acids and 5-50 Prevention of corrosion or rusting of
esters, dimer acids, amine storage tanks, pipelines, and metal fuel
salts system components
Table 4 (contd)
Additive Material Concentration Effect
(ppm)
Antioxidants Hindered phenols or amines 25-200 Prevention of aging processes
Anti-foam agents Silicones Up to 20 Reduction of foaming tendency; reduction
of risk of ground pollution from oil spills
Dehazers Quaternary amine salts 5-50 Reduction of formation of hazes;
acceleration of haze clearance
Biocides Imines, amines, About 200 Prevention of growth of bacteria and
imidazolines, etc. fungi in storage tank bottoms
Lubricants Surface-active agents such 50-500 Compensation of lower viscosity of fuels
as polyfunctional acids and in low-temperature regions
derivatives
Odour maskers Natural, identical substances, 10-100 Reduction or elimination of smell
such as vanillin and terpenesc
From Coley (1989), except as noted
a From Organisation for Economic Co-operation and Development (1993)
b From Russell (1989)
c From Fabri et al. (1990)
A2.1.2.4 Cloud-point depressors
Diesel fuels must be easily filterable, as wax crystals formed at
low temperatures can clog fuel filters. Cloud-point depressors are
therefore added (Fabri et al., 1990) which consist of substances with
lower cloud-points, e.g. kerosene. Addition of 10% kerosene lowers the
cloud-point of diesel fuel by about 2°C. Olefin-ester copolymers
depress the cloud-point by 3-4°C but are not currently in commercial
use (Coley, 1989).
A2.1.2.5 Wax anti-settling additives
These additives inhibit the tendency of wax to settle by reducing
the crystal size and slowing the settling rate. A five-fold reduction
in wax crystal size slows the settling rate by one-twenty-fifth. With
increasing temperature, small dispersed crystals redissolve more
readily than settled wax (Coley, 1989).
A2.1.2.6 Other additives
Detergents, including amines, amides, imidazolines, and
succinates, are used to reduce injector nozzle fouling. Detergents
such as polyalkenyl succinimides also improve fuel stability,
resistance to corrosion, and combustion efficiency (Russell, 1989).
Antistatic agents lower the risk of building up a charge of static
electricity during pumping at high rates at bulk terminals or in
large-capacity truck fuel tanks.
Other additives used are anti-oxidants (phenols, amines),
anti-corrosion chemicals (alkenyl succinic acids, esters, dimer acids,
amine salts), anti-foam agents (silicones), dehazers (anti-emulsion
agents) (quaternary ammonium salts), biocides, lubricants for cold
regions (surface-active polyfunctional acid derivatives), and odour
maskers (vanillin, terpenes) (Coley, 1989; Fabri et al., 1990).
A2.1.3 Quality of diesel fuels
A2.1.3.1 Ignition performance and cetane number
The cetane number determines the ignition performance of
transport fuels relative to a scale on which methyl naphthalene
corresponds to a combustion rate of 0 and cetane to one of 100
(Scheepers & Bos, 1992a). The cetane number is calculated by comparing
the ignition quality of a fuel with that of two reference fuel blends
of known cetane numbers under standard operating conditions (American
Society for Testing and Materials method D 613 CFR). A high cetane
number improves cold starting and engine durability and reduces noise,
fuel consumption, smoke emissions during warm-up, and exhaust
emissions (Russell, 1989). In Europe (countries of the EU and EFTA),
the minimal cetane number must be in the range 45-49, depending on the
climatic conditions (European Committee for Standardization, 1993);
cetane numbers are usually 49-53 (CONCAWE, 1987). In the United
States, the cetane number must be at least 30 for diesel fuel No. 4
and 40 for diesel fuels No. 1 and 2 (American Society for Testing and
Materials, 1988, 1992).
A2.1.3.2 Density
The density of diesel fuel influences engine performance: higher
density leads to enrichment of the fuel:air mixture, which results in
greater engine power output. Enrichment may, however, increase the
particulate content of exhaust gas emissions (Fabri et al., 1990) (see
section B3.1.2.2). A density range is specified in fuel standards in
some countries (American Society for Testing and Materials, 1992;
European Committee for Standardization, 1993).
A2.1.3.3 Sulfur content
Gas and particle emissions in diesel engine exhaust are
influenced by the sulfur content of the fuel; there is a direct
relationship between particle production and sulfur content (Hare,
1986) (see section B3.1.2.2).
A2.1.3.4 Viscosity
Too low a viscosity can lead to wear in the injection pump; too
high a viscosity impairs fuel injection and mixture formation (Fabri
et al., 1990). In Europe (countries of the EU and EFTA), the viscosity
of commercial diesel fuels at a temperature of 40°C must be
1.5-4.5 mm2/s (European Committee for Standardization, 1993). In the
United States, the permitted ranges of viscosity at 40°C are
1.3-2.4 mm2/s for diesel fuel No. 1, 1.9-4.1 mm2/s for diesel fuel
No. 2, and 5.5-45.0 mm2/s for diesel fuel No. 4 (American Society
for Testing and Materials, 1988, 1992).
A2.1.3.5 Cold-flow properties
Cloud-point and cold filter plugging point characterize the
behaviour of diesel fuels at low temperatures (Fabri et al., 1990).
These points are lowered by the addition of cloud-point depressors and
by special blending techniques, e.g. increasing the kerosene content
of the fuel (see section A2.1.2).
Changes in diesel fuel quality have been assessed. Wade & Jones
(1984) reported a deterioration in fuel quality with decreasing cetane
number and found that a 90% increase in boiling-point led to greater
emissions of particulates, nitrogen oxides and PAHs. A decline in
diesel fuel quality on the European market was predicted, as the
rising demand would lead to greater use of fuels from catalytic or
thermal cracking processes (CONCAWE, 1987) (see section A3.2.1.1). A
study by the Organisation for Economic Co-operation and Development
(1993) indicated an improvement in the quality of diesel fuel in the
United States due to a decline in aromaticity.
A2.2 Physical and chemical properties
Diesel fuel is a brown, slightly viscous, flammable liquid at
room temperature (Sandmeyer, 1981). It generally has a kerosene-like
odour (Agency for Toxic Substances and Disease Registry, 1995). Its
physical and chemical properties are listed in Table 5.
The water solubility of diesel fuels varies. The aqueous
solubility of crude and fuel oils in the environment is clearly
dependent on the salinity of the water and the age of the oil slick
(see section A4.1.1) and is of the same order of magnitude as the
solubility of fuel oils: at room temperature, 0.37-0.53 mg/litre in
sea water (Boehm & Quinn, 1974) and 0.7-11 mg/litre in tap water
(Lysyj & Russell, 1974). The solubility of a fuel slick decreases with
its age as the concentration of long-chain hydrocarbons increases; the
solubility of fresh crude oil is 29.3-32.3 mg/litre, whereas that of
weathered crude oil is 0.06-23.2 mg/litre at 25°C (Mackay & Shiu,
1976).
The physicochemical properties of the toxicologically relevant
benzene, toluene, ethyl benzene, xylene and PAH components are given
in Table 6.
A2.3 Analytical methods
Exhaustive identification and quantification of the individual
constituents of commercial diesel fuel (see section A2.1) is almost
impossible owing to their number and complexity. In most environmental
assessments, therefore, the mixture is analysed as total petroleum
hydrocarbon (Block et al., 1991). All such methods involve preliminary
solvent extraction of the matrix, with e.g. trichlorotrifluoroethane.
This step may also extract naturally occurring hydrocarbons, which
interfere with the analysis; however, some of these compounds are
polar and can be removed on silica gel. Three analytical methods are
available:
Table 5. Physicochemical properties of diesel fuels
Property Diesel fuel Diesel fuel Diesel fuel Diesel fuel
(general) No. 1 No. 2 No. 4
Melting-point (°C) - 34a 18a - 29-9a
Boiling range (°C) 160-190b 145-300 (wide-cut 282-338a 170-420d
143-384e aviation, 45-280)c 101- > 588a
193-293a
Flash-point (°C) > 56b > 21 - < 55 52 (closed cup)a > 56d
58-66e (wide-cut aviation, > 54 (closed cup)a
(Pensky-Martens) < 21)c
38 (closed cup)a
Autoignition temperature (°C) 177-329a 254-285a 263a
Density (g/cm3) 0.81-0.90 (15°C)b 0.805 (wide-cut 0.87-0.95 0.87-0.92 (15°C)d
0.82-0.84 (15°C)e aviation, 0.75-0.801) (20°C) 0.81-0.94 (15°C)
(15°C)c 0.81-0.94 1 (20°C)
(15°C)
Kinematic viscosity (mm2/s) 2-7.4 (40°C)b 1.5-2.5 (wide-cut 2-7.4 (40°C)d
2.20-3.25 (40°C)e aviation, about 1.1
(20°C)c
Vapour pressure (kPa) About 40 (40°C)b About 10 (wide-cut 2.83-35.2 2.83-35.2 (21°C)a
0.04f aviation, 140-210) (21°C)a
(Reid, 37.8°C)c
2.83-35.2 (21°C)a
Table 5 (contd)
Prpoerty Diesel fuel Diesel fuel Diesel fuel Diesel fuel
(general) No. 1 No. 2 No. 4
Water solubility (mg/litre) 1f About 5 (20°C)a About 5 (20°C)a About 5 (20°C)a
0.2f
Henry's law constant 4.3 × 103f 6.03-7.5 × 105a 6.03-7.5 × 105a 6.03-7.5 × 105a
(Pa × m3/mol) (20°C)
n-Octanol-water partition 3.3-7.06a 3.3-7.06a 3.3-7.06a
coefficient (log Kow)
Soil sorption coefficient 3.04f 3.0-6.7a 3.0-6.7a 3.0-6.7a
(log Koc)
Diffusion coefficient in air 4.63 × 10-2f
(cm2/s)
Odour threshold (ppm) 0.7a 0.5g
a From Agency for Toxic Substances and Disease Registry (1995)
b From CONCAWE (in press); automotive gas oil
c From CONCAWE (1985, 1995); kerosenes
d From CONCAWE (in press); distillate marine diesel
e From German Scientific Association for Petroleum, Natural Gas, and Coal (1991); average of three samples
f From Custance et al. (1993); water solubility measured, other data from literature; no further details
g From Fraser Williams (Scientific Systems) Ltd (1985)
-- Gravimetric detection: determination of the weight of residue
remaining after solvent evaporation. Although this method is
useful for measuring gross contamination, it cannot be used in
trace analysis.
-- Infrared detection: the absorbance of petroleum hydrocarbons is
detected in a solvent matrix at the maximal value, near
2930 cm-1. This carbon-hydrogen bond stretching absorbance is
directly related to the hydrocarbon concentration in the extract.
The results are dependent on the hydrocarbon standard used for
quantification.
-- Gas chromatographic detection: capillary gas chromatography
combined with flame ionization or mass spectrometric detection.
The gravimetric and infrared techniques are fast, relatively
simple and widely accepted by regulatory authorities; however, they do
not provide sufficient qualitative and quantitative information on
composition. Gas chromatography is therefore the standard procedure
used to identify and quantify fuel constituents in environmental
samples. Different distribution and degradation processes in
environmental compartments (see section A4) mean that the composition
of petroleum hydrocarbons in air, water, soil, and biota may differ
considerably from that of the original fuel. Newton et al. (1991)
developed an analytical method for identifying and quantifying traces
of diesel contamination in tinned fish products on the basis of the
n-alkane pattern.
Because of the suspected toxicity of aromatic components in
diesel fuel, a detailed analysis is often necessary. Two groups of
compounds are detected:
-- volatile aromatic compounds: Analysis of these minor components
of diesel fuel requires special enrichment techniques, such as
purge-trap gas chromatography, and methods of detection including
flame ionization and mass spectrometry.
-- PAHs: Gas chromatography with flame ionization or mass
spectrometric detection, or liquid chromatography, is used after
solvent extraction. As PAHs are poorly resolved from the diesel
fuel matrix, gas chromatography-mass spectrometry is necessary in
most cases to quantify the components. The target analytes of
commonly used analytical methods do not, however, include C3
and C4 alkyl-substituted benzenes or most alkyl-substituted
PAHs in diesel fuels.
Table 6. Physicochemical properties of aromatic components of diesel fuel
Aromatic compound Water solubility Henry's law constant Diffusion coefficient (cm2/s) Octanol-water partition
(mg/litre) (Pa × m3/mol) In air In water coefficient (log Pow)
Benzene 1791 550 0.087 9.8 × 10-6 1.99
Toluene 534.8 602 0.083 8.6 × 10-6 2.52
Ethylbenzene 161.0 855 0.076 7.8 × 10-6 2.94
Xylene 156.0 778 0.076 1 × 10-5 2.94
Polycyclic aromatic 0.067 0.007 0.067 2.12 × 10-6 5.30
hydrocarbons
Adapted from Custance et al. (1993)
A2.4 Conversion factors
As diesel fuel vapour is a complex mixture of gases, it is
impossible to give a conversion factor for converting parts per
million in the gaseous phase to SI units.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
Diesel fuels are derived from crude oil, which can be considered
a 'natural product'. Nevertheless, human and environmental exposure
results almost exclusively from anthropogenic activities.
A3.2 Anthropogenic sources
A3.2.1 Production and use
A3.2.1.1 Production process
Diesel fuel is produced during the refining of crude oils but is
generally blended to meet the specifications for technical
performance. The blending components may be produced by atmospheric
distillation of crude oil (straight-run atmospheric gas oil), vacuum
distillation of atmospheric residue (vacuum gas oil), thermal cracking
(thermally cracked gas oil), or catalytic cracking processes (e.g.
light catalytically cracked gas oil; cycle oil). The main components
are straight-run gas oils: 80% of European automotive diesel fuel is
made up of these components (Booth & Reglitzky, 1991). Secondary
processing of heavier fractions is increasingly necessary in order to
meet product demand (CONCAWE, in press).
Diesel fuel No. 1 is manufactured by a straight-run distillate
process (Agency for Toxic Substances and Disease Registry, 1995);
diesel fuel No. 2 is generally made by mixing straight-run and
catalytically cracked distillates; and diesel fuel No. 4 is produced
by adding blending stocks to distillation residues in order to meet
viscosity specifications (International Agency for Research on Cancer,
1989a). Further variations are introduced by formulation with
additives to improve fuel properties (see section A2.1.2).
A3.2.1.2 Use
Diesel fuel is widely used as a transport fuel for light- and
heavy-duty vehicles; the Organisation for Economic Co-operation and
Development (1993) classifies vehicles weighing < 3.5 t as light-duty
and those weighing > 3.5 or, occasionally, > 5 t as heavy-duty.
Diesel fuel No. 1 is suitable for engines that undergo frequent
changes in speed and load (Agency for Toxic Substances and Disease
Registry, 1995). Heavier grades (diesel fuels No. 2 and 4) are used
for trucks, railroad and marine diesel engines, and stationary engines
in continuous high-load service (Sandmeyer, 1981; International Agency
for Research on Cancer, 1989a). Diesel fuels are also used in
stationary gas turbines, e.g. to generate electric power during
peak-load periods. Residual fuel oils, such as diesel fuel No. 4, are
used to generate steam in electric power plants (International Agency
for Research on Cancer, 1989a), in commercial and industrial burner
installations without preheating facilities, in plants and factories
for space and water heating, for pipeline pumping, and in gas
compression; they are also sprayed on unmade roads to compact the
surface and are used in the manufacture of asphalt cement (Agency for
Toxic Substances and Disease Registry, 1995).
A3.2.1.3 Production and consumption levels
The demand for diesel fuel has increased worldwide over time. In
1990, world demand was about 1100 kt/day (Booth & Reglitzky, 1991).
The production and consumption of diesel fuel in different regions
over time are shown in Table 7.
Diesel-fuelled passenger cars are relatively common in western
Europe. The percentages of passenger cars with diesel engines in
various European countries over time are shown in Table 8. In many
European countries, taxi-cabs are equipped almost exclusively with
diesel engines. In 1985, diesel-fuelled heavy-duty trucks comprised
about 86% of the fleet in Norway, 89% in Denmark, 95% in Sweden, and
100% in the former Federal Republic of Germany. In some countries of
Europe, diesel fuel consumption has increased steadily over the last
decade, and consumption for heavy-duty vehicles is predicted to more
than double between 1980 and 2005 (Organisation for Economic
Co-operation and Development, 1993). In western Germany, diesel fuel
consumption was almost 60% higher in 1990 (about 5100 kt) than in 1984
(about 3200 kt) (Federal Ministry for Transport, 1992).
Diesel-fuelled passenger cars are less common in North America.
In the United States in 1986, about 1.6% of passenger cars were
diesel-fuelled, and the tendency was predicted to decrease slightly
(US Department of Energy, 1988). In contrast, 82% of heavy-duty trucks
were diesel-fuelled in 1985 (Organisation for Economic Co-operation
and Development, 1993), and 59300 kt of diesel fuel were consumed by
highway traffic in 1986, representing 14.8% of all the highway fuel
used and about one-half of United States diesel fuel consumption.
On-road use of diesel fuel was predicted to increase to 15.5% of all
highway fuel (66600 kt) in 1995. A difference in fuel use patterns is
seen between light- and heavy-duty vehicles. In 1986, about 4000 kt
were used by passenger cars and light trucks, with an estimate of
about 2300 kt for 1995, whereas in 1986 about 55300 kt were used by
heavy-duty trucks, with an estimate of 64300 kt for 1995 (US
Department of Energy, 1988). In Canada, only about 1% of passenger
cars are equipped with diesel engines. In 1992, 14126 kt of diesel
fuel were produced; 13508 kt were sold for domestic purposes (heating)
and about 10849 kt for on-road use (Thomas, personal communication,
1994).
The percentage of diesel-fuelled vehicles is increasing in Japan
(Table 9).
Table 7. Production and use of diesel fuel (including gas oils in
Europe) in different regions, and development over time
Region Production/ 1975 (kt) 1980 (kt) 1985 (kt)
Use
United States Production 134 967 138 323 135 181
Use 133 300 136 161 130 297
Canada Production 19 187
Use 19 130
OECD (all 24 Production 370 240 408 848 372 728
countries) Use 380 181 403 642 386 081
OECD (Europe) Production 173 474
Use 190 960
European Union Production 152 091
Use 163 132
Australia, Japan, Production 44 886
New Zealand Use 45 694
Adapted from International Agency for Research on Cancer (1989a);
OECD, Organisation for Economic Co-operation and Development
A3.2.2 Emissions during production and use
A3.2.2.1 Air
No data are available on emissions during the production and use
of diesel fuel. Release to the atmosphere during production in the
refining process is unlikely, as closed systems are used, but
volatilization may occur during storage and transport. Diesel fuel may
be released into the atmosphere as a result of spills during storage
and transport and at filling stations during bulk storage and vehicle
tank filling. The low-relative-molecular-mass constituents
(short-chain alkanes, benzene, toluene, ethyl benzene, and xylene
compounds) are the most likely to evaporate under environmental
conditions.
Table 8. Percentage of diesel passenger cars in western Europe, and
development over time
Country Diesel passenger cars (%)
1984 1988 1991 1992a
France 15.8 21.1 25.5 38
Germany (without former 16.9 15.1 15.3 14.9
German Democratic Republic) (1993)b
Germany (without former About 8 13.6 13
German Democratic Republic)c
Italy 18.7 11.9 7.4 NR
Spain (without station wagons) 15.3 10.5 14.6 NR
United Kingdom 1.1 4.5 11.3 12.5
United Kingdomd NR 5 9 19 (1993)
Adapted from American Automobile Manufacturers Association (1993),
unless otherwise stated; NR, not reported
a From Menne et al. (1994)
b With the former German Democratic Republic
c From German Institute for Scientific Research (1993)
d From Quality of Urban Air Review Group (1993)
Table 9. Percentage of diesel-fuelled vehicles in Japan and
development over time
Year Passenger cars (%) Trucks (%) Buses (%)
1984 3.8 23.7 49.5
1988 5.8 28.5 63.2
1991 8.3 40.7 81.7
Adapted from American Automobile Manufacturers Association (1993)
A3.2.2.2 Water
No data are available on effluents and emissions during diesel
fuel production. Diesel fuel oils may be released to surface waters as
a result of leakage from storage tanks or tankers (see section
A3.2.3). In the United States, the total volumes of spills of diesel
fuel oils in 1991 were (Agency for Toxic Substances and Disease
Registry, 1993): diesel fuel No. 1, 10.6 t (20 notations); diesel fuel
No. 2, 8.9 t (28 notations); and diesel fuel No. 4, 39.3 t (35
notations).
Groundwater can be contaminated with diesel fuel constituents by
leakage from underground bulk storage tanks, but quantitative data are
lacking. In the Province of New Brunswick, Canada (Thomas, personal
communication, 1993), of a total of 671 gasoline, diesel, and fuel oil
tanks examined in 1987, 11% leaked diesel or fuel oil; in 1989, 6% of
1085 tanks had leaks; and in 1991, 13% of 539 tanks leaked fuel. No
data were available about the amounts leaked, and diesel contamination
was determined on the basis of a cluster of 'middle-range peaks'
detected by gas chromatography.
On the basis of chronic petroleum inputs to sediments of
Narragansett Bay and Rhode Island Sound, United States, Van Vleet &
Quinn (1978) estimated that about 200 kt of petroleum hydrocarbons may
be released to surface waters worldwide from municipal treatment
plants. The precise source of the inputs cannot be verified in most
cases, but they are due, for example, to accidental discharge to sewer
systems, disposal of used crankcase and lubricating oils, oil washed
from roads, and atmospheric deposition of hydrocarbons.
No data were available from other countries.
A3.2.2.3 Soil
Diesel fuels may be released to soil as a result of accidental
spills (see section A3.2.3) and leakage from storage tanks or
pipelines. Diesel-contaminated soil is a major problem in railroad
yards, where diesel fuel is used in locomotives and as a solvent to
clean moving metal parts (the remaining paraffins give a waxy
anti-corrosive coat). Spillage during refuelling, engine maintenance,
and steam cleaning, leakage from fuel storage tanks, and absorption of
diesel fuel on sand used for traction are other possible sources of
soil contamination (Dineen, 1991); however, no quantitative data are
available.
A3.2.3 Accidental releases to the environment
Data on the accidental release of diesel fuels are summarized in
Table 10. The effects on the environment depend on both the amount and
environmental conditions.
Table 10. Diesel fuel spills and their effects on the environment (see also section A9.2)
Year Place Amount (t); type Effects Reference
of diesel fuela
Aquatic environment
1972 North of Puget Sound, > 600; diesel fuel Substantial mortality of some intertidal taxa; Woodin et al. (1972)
Washington State, USA No. 2 larval recruitment within six months
1973 East Lamma Channel 2000-3000; Almost total kill of meiofauna within four days; Wormald (1976);
near Hong Kong diesel fuel No. 4 heavy mortality among bivalves and molluscs, Stirling (1977)
Nerita albicilla and Monodonta labio, and in
marine fish farms; almost no effect on
Clypeomorus humilis or Planaxis sulcatus
1978 Svea, Mijenfjord, Norway 111 Most fuel trapped in ice; transport out of fjord Carstens &
during break-up; heavy mortality among shoreline Sendstad (1979)
invertebrates in some regions of fjord
1983 Yaquina Bay, Oregon, About 240 (with Decline in population of Rhepoxynius abronius Kemp et al. (1986)
USA Bunker C oil) by 75%; influence of spill not clear
1984 Queen Charlotte 130 (plus 30 t Low fuel levels in water during flood tide, high McLaren (1985)
Islands, Canada gasoline) levels during ebb as diesel was retained in
sediment; high mortality among barnacles
Table 10 (contd)
Year Place Amount (t); type Effects Reference
of diesel fuela
1987 Macquarie Island, 230; diesel fuel High mortality among one species of holothuroid, Pople et al. (1990)
Australia No. 4 one of isopod, one of limpet, and one of chiton;
decreased populations of crab, two species of
starfish, and gastropod; small number of dead
algae; slow recovery of organisms
1989 Arthur Harbor, 510; diesel fuel High mortality among limpets; partial recovery one Kennicutt et al.
Antarctica No. 1 year after spill; small effect on macroalgae (1991a,b,1992a,b)
Terrestrial environment
1983 San Bernadino County, 3 Hydrocarbon contamination up to 1500 mg/kg; Frankenberger et al.
CA, USA plume migrating towards surface water; in-situ (1989)
bioremediation (see also section A3.2.4)
NR Califonia, USA 193; diesel fuel Contamination at 6-34 m below surface; Peters et al. (1992)
No. 2 hydrocarbon levels at 18.3, about 1500 mg/kg;
remediation by flooding with surfactants
(see also section A3.2.4)
aWhen available
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few data are available on the environmental fate of diesel
fuels, but there are adequate data on the environmental behaviour of
individual hydrocarbon components. The transport, distribution, and
transformation of crude oils and some fuel oils (e.g. heating oils
No. 1 and 2) have been studied, and the mechanisms of distribution and
transformation are considered to be comparable to those of diesel
fuel.
The calculated half-lives for the toxicologically relevant PAH
components are listed in Table 11.
A4.1 Transport and distribution between media
Diesel fuels are released into the environment mainly during
storage, transport, and use (see section A3.2.3). The hydrosphere and
geosphere are the affected compartments.
A4.1.1 Evaporation from and dissolution in the aqueous phase
Oils and fuels spilled on water spread out almost immediately;
polar and low-relative-molecular-mass components dissolve and leach
out of the slick; and volatile components evaporate from the water
surface. With the start of microbial degradation the pattern of
substances changes, as was observed, for example, after a major oil
spill off the French coast (Atlas et al., 1981). The sum of these
processes is known as chemical and biological 'weathering'. At the
same time, the oil is emulsified to oil-in-water and water-in-oil
('mousse') emulsions (Atlas & Bartha, 1973). In laboratory experiments
with several diesel fuels, however, stable mousses were not formed at
any temperature (US National Research Council, 1985). Experiments
with South Louisiana crude oil showed that the evaporation rate was
more than twice the dissolution rate (Harrison et al., 1975). The loss
by evaporation of oil spread on water, which in oil spills amounts to
about 25% of the total released mass, is generally dependent on the
following factors (US National Research Council, 1985):
-- the exposed area, which increases with time as the film spreads;
-- the oil-phase vapour pressures, which decrease with time as the
low-relative-molecular-mass components evaporate. Components
with vapour pressures higher than that of n-octane evaporate
rapidly from the surface of spilled fuel oil, while those with
vapour pressures lower than that of n-octadecane persist and
form a viscous residue that retards the volatilization of other
constituents (Regnier & Scott, 1975);
-- the oil-air mass transfer coefficient, which depends on wind
speed and hydrocarbon diffusivity (for the diffusion coefficient
in air, see section A2.2, Table 5);
-- diffusion barriers, such as emulsions, or formation of a 'skin'
on the surface. Many surface waters have a thin layer of organic
material of natural origin on the surface, and these films may
reduce spread and volatilization, altering the persistence and
concentration of the oil in water (Edgerton et al., 1987).
Table 11. Calculated half-lives of polycyclic aromatic hydrocarbon
components in environmental compartments
Component Air Water Soil Sediment
Naphthalene 1 day 1 week 2 months 8 months
Acenaphthalene 2 days 3 weeks 8 months 2 years
Fluorene 2 days 3 weeks 8 months 2 years
Phenanthrene 2 days 3 weeks 8 months 2 years
Anthracene 2 days 3 weeks 8 months 2 years
Pyrene 1 week 2 months 2 years 6 years
Fluoranthene 1 week 2 months 2 years 6 years
Chrysene 1 week 2 months 2 years 6 years
Benz[a]anthracene 1 week 2 months 2 years 6 years
Benzo[a]fluoranthene 1 week 2 months 2 years 6 years
Benzo[a]pyrene 1 week 2 months 2 years 6 years
Perylene 1 week 2 months 2 years 6 years
Dibenz[a,h]anthracene 1 week 2 months 2 years 6 years
Adapted from Mackay et al. (1992)
On the basis of the constant of Henry's law (see section A2.2,
Table 5) and the classification of Thomas (1990), diesel fuels can be
considered to volatilize significantly from the aqueous phase. The
constant may change during volatilization, as the composition of the
mixture changes with time. Lockhart et al. (1987) observed a clear
decrease in the hydrocarbon composition of the 50% water-soluble
fraction of diesel fuel in open-aerated test cylinders. Within 48 h,
the concentration of hydrocarbons with a very low boiling-point
(< 115°C) decreased from 0.73 to 0.04 mg/litre and that of
hydrocarbons with a low boiling-point (115-270°C) from 3.2 to
0.17 mg/litre. Under sealed or open non-aerated conditions, there were
only minor changes. Regnier & Scott (1975) showed that n-decane
volatilizes from diesel fuel with a half-life of 1.65 h at 30°C,
whereas the half-life was 9.67 h at 5°C in a darkened chamber with a
constant wind speed of 21 km/h. Edgerton et al. (1987) reported that
diesel fuel spilled on ice had lost only 2-4% of its lightest fraction
after 10 days.
Dissolution of spilled oils and fuels in water is less important
from the viewpoint of mass loss than the ecotoxic effects of the
mixture. Lysyj & Russell (1974) conducted long-term experiments (42
days) on the dissolution of different fuel oils and gasoline in water.
After a period of stable concentration, during which physical
dissolution was probably the main mechanism for oil-to-water transfer,
the dissolution of organic compounds accelerated, probably because of
chemical modification by oxidation or microbial action of the
water-insoluble petroleum fraction.
Under equilibrium mass-transfer conditions, the levels of PAHs
(naphthalene and its methyl derivatives, acenaphthene, fluorene,
phenanthrene, anthracene, and fluoranthene) in the water phase in
contact with commercial automobile diesel fuel were about four to six
orders of magnitude lower than those in the diesel fuel itself, i.e.
in the low microgram per litre range. The concentrations of these
constituents were likely to be smaller under non-equilibrium
conditions but might be higher in the presence of surfactants,
emulsifiers, or co-solvents (Lee et al., 1992). When No. 2 fuel oil
and kerosene were placed in contact with drinking-water for 17 h,
although each fuel contained about 50% by weight of aliphatic
hydrocarbons, the water-soluble fractions contained primarily aromatic
compounds: total, > 93% by weight; proportion of benzene and
substituted derivatives, 19.4% by weight in No. 2 fuel oil and 53.2%
by weight in kerosene (Coleman et al., 1984). The chemical composition
of the water-soluble fractions of No. 2 fuel oil is compared with that
of the whole oil in Table 3.
During chemical weathering, other changes in composition (e.g.
autoxidation of n-alkanes) may occur that are not necessarily
associated with a net mass loss (Davis & Gibbs, 1975).
A4.1.2 Transport in and adsorption onto soil and sediment
A4.1.2.1 Soil
The movement of diesel fuel through soil is directly correlated
with its kinematic viscosity (see section A2.2, Table 5). As the
viscosity is about one-half that of water and about one-fourth to
one-fifth that of gasoline, the rate of percolation of water into soil
is about twice and that of gasoline about four to five times that of
diesel fuel (Stone, 1991). The retardation coefficient (Rd) is an
indicator of the migration of substances into groundwater. In the
aqueous phase, the maximal rate of movement of dissolved compounds is
that of groundwater, which is represented by an Rd of 1. Stone (1991)
calculated the Rd from the adsorption isotherms of individual diesel
constituents, and derived the following classification:
-- low mobility (Rd > 100): fluorene, phenanthrene, pyrene,
benzanthracene, benzo[ a]pyrene, fluoranthene;
-- medium mobility (100 > Rd > 10): naphthalene, dimethylbenzenes,
ethylbenzene, toluene;
-- high mobility (Rd < 10): benzene, quinoline, cresols, phenol.
In effect, soil contaminated with diesel fuel acts like a
chromatographic column, separating individual constituents by their
adsorption. Experimental data on the adsorption of diesel fuels on
soils and their transport are not available; however, the movement of
a synthetic kerosene through a clay-sand soil was dependent on the
moisture content. With increasing moisture content, the penetration
rate of nonvolatile components through the soil increased and the
adsorption of more volatile components decreased. In contrast, upward
mobility of the individual constituents (both volatile and
nonvolatile) of the fuel decreased with increasing moisture content.
In fully water-saturated soil, upward transport of kerosene was
completely inhibited (Acher et al., 1989). Desorption of a simulated
kerosene applied to three types of soil with the same moisture content
but different contents of organic matter was found to be complete
after 30 days under environmental conditions. The slowest desorption
was from the soil with the highest level of organic matter (Yaron et
al., 1989). Transport of kerosene through three types of sand was
influenced mainly by volatilization of C9-C13 hydrocarbon
constituents; the increase in the viscosity of the kerosene residue
was followed by a decrease in the infiltration rate (Galin et al.,
1990).
A4.1.2.2 Sediment
In marine environments, oils and fuels are generally delivered to
bottom sediments on settling particles. In areas with low
concentrations of suspended material (i.e. terrigenous minerals,
plankton, detrital particles, resuspended bottom sediments), the rates
of hydrocarbon sedimentation would be low (US National Research
Council, 1985).
No experimental data are available on diesel fuels in sediments.
In laboratory experiments with fuel oil, binding of the hydrocarbon
components to clay minerals of marine sediments was shown to be due to
physical adsorption of the van der Waals type. The compounds are
associated firmly because, once they are incorporated into sediments,
desorption to the overlying water is slow. Saturated hydrocarbon
constituents of fuel oils were transported slowly to the sediment
(Meyers & Quinn, 1973). In studies of a laboratory marine ecosystem to
which No. 2 fuel oil was added for 24 weeks, the concentrations in the
sediment remained low until 135 days after the start of the
experiment. Then, a steep increase was seen, leading to a level of 9%
by weight of the total fuel added (i.e. 12% by weight of the total
saturated hydrocarbons). The highest concentrations of saturated
hydrocarbons were found in the surface flocculent layer; 2-3 cm below
the sediment surface, the levels were below the detection limit. The
smaller particles (< 45 µm) of water-suspended material seemed to
adsorb about twice as much fuel oil as larger particles (> 45 µm)
(Wade & Quinn, 1980). In a comparable study in a model marine
ecosystem with No. 2 fuel oil, the particulate matter contained 40-50%
of the total amount of aliphatic hydrocarbons added and only 3-21% of
the aromatic fraction, indicating that most of the aromatic
hydrocarbons were dissolved in the water phase (see section A4.1.1)
(Gearing et al., 1980). Although biodegradation may remove many of the
soluble aromatic hydrocarbons (see section A4.2.2), 10-20% by weight
of the fuel oil, consisting primarily of branched alkanes,
cycloalkanes, and aromatics, may remain in the upper 2 cm of sediment
for over a year (Gearing et al., 1980; Oviatt et al., 1982).
A4.2 Transformation and removal
A4.2.1 Photooxidation
No data were available on the photooxidation of diesel fuel in
air or water. In the atmosphere, all evaporated oil components are
degraded photochemically by hydroxy radicals and other species within
hours or days to carbon monoxide, carbon dioxide, and oxygenated
compounds (US National Research Council, 1985; IPCS, 1986, 1993).
Under laboratory conditions, No. 2 fuel oil was rapidly photo-oxidized
in water. Two photooxidant pools were identified: hydrogen peroxide
and a mixture of indane and tetralin hydrogen peroxides, the first
being the faster acting (Herbes & Whitley, 1983). Peroxides are formed
by two mechanisms: the first is the reaction of free radicals with
triplet oxygen to produce peroxy radicals and hydroperoxides, which
continue the chain reaction; the second is addition of excited singlet
oxygen to reactive acceptors such as olefins. The photodegradation
products of fuel oils are phenols, naphthols, and carboxylic acids
(Larson et al., 1977, 1979).
A4.2.2 Biodegradation
A4.2.2.1 Microbial degradation
The individual constituents of spilled oil are inherently
degradable to varying degrees and at different rates. The n-alkane,
n-alkylaromatic, and simple aromatic molecules in the C10-C22
and the most readily degradable. Smaller molecules are generally range
are the least toxic rapidly metabolized, although they tend to be more
toxic. Long-chain n-alkanes are more slowly degraded because of
their hydrophobicity and also because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to attack. PAHs are the compounds most recalcitrant to
microbial degradation (Morgan & Watkinson, 1988).
A study of the breakdown of heating oils and diesel fuels in a
sub-soil demonstrated breakdown rates of 0.014-0.022 g/kg per day,
which were slower than the rates of topsoil degradation (Flowers et
al., 1984). The mineralization rates for heavy oils, sludges, and
crude oils were 0.02-0.6 gram of hydrocarbon per kilogram of soil per
day. Under Arctic conditions, the rates were considerably slower.
The relative contribution of bacteria and fungi to hydrocarbon
degradation is unclear. At acid pH, fungi are clearly more important,
but closer to neutrality hydrocarbon contamination tends to enhance
bacterial growth.
The overall rates of hydrocarbon degradation are limited by:
-- temperature (optimal temperature for biodegradation, 30-40°C);
-- water content (in soils, water contents of 50-80% capacity are
optimal for microbial activity);
-- oxygen (the metabolism of both aliphatic and aromatic
hydro-carbons normally requires oxygen);
-- pH (hydrocarbons are mineralized most rapidly at 6.5-8.0);
-- inorganic nutrients (as petroleum contaminants are extremely
deficient in nitrogen and phosphorus, a large quantity of carbon
sources tends to result in rapid depletion of the available pools
of major nutrients, i.e. enhancement of degradation in fertilized
soils);
-- microbial metabolic versatility (topsoils generally contain many
hydrogen-degrading bacteria, typically 105 - 106 per gram, but the
content of subsoils is unclear) (Morgan & Watkinson, 1988).
A remedial treatment consisting of liming, fertilization, and
tilling was evaluated on a laboratory scale for its effectiveness in
cleaning up a sand, a loam, and a clay-loam soil contaminated at
50-135 mg/g of soil by diesel and other oils. The disappearance of
hydrocarbons was maximal at 27°C (Song et al., 1990).
In sandy soils, 50-85% of the hydrocarbons were degraded within a
few weeks, depending on the experimental conditions. Degradation
efficiency increased with additional soil inoculation (Grundmann &
Rehm, 1991).
Frehland et al. (1992) examined the substrate specificity and
growth conditions of nine bacterial strains on samples of
diesel-contaminated soil under different conditions. The
biodegradative properties of Pseudomonas spp. were superior (59, 60,
and 80% in three samples) than those of Micrococcus, Bacillus,
Acinetobacter, and other species tested.
A4.2.2.2 Phytoplankton and marine algae
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this occurs in nature
is poorly understood. The ability to metabolize naphthalene is
widespread (US National Research Council, 1985).
A4.2.2.3 Invertebrates and vertebrates
Unlike microorganisms, which use petroleum as a source of carbon,
animals generally oxidize and conjugate products to render them more
soluble and therefore easier to excrete. All animal groups tested can
take up petroleum hydrocarbons, either through the body surface,
across respiratory surfaces, or via the gut, the route varying with
the organism and its feeding habits. In copepods (Calanus
helgolandicus), dietary uptake was more important than that from
water (Corner et al., 1976), but aromatic hydrocarbons accumulated by
the polychaete Neanthes arenaceodentata were derived from water and
not from sediment (Rossi, 1977). Hydrocarbons are stored in lipid-rich
tissues. The principal route of metabolism of hydrocarbons by
metazoans is conversion of lipophilic compounds by cytochrome
P450-dependent monooxygenases or mixed-function oxidases to
derivatives that are more water-soluble. Mixed-function oxygenase
activity has been found in all fish species studied. Lee (1981)
reported 18 marine invertebrate species belonging to four phyla
(Annelida, Arthropoda, Echinodermata, and Mollusca) that are known to
contain mixed-function oxidase activity in the hepatopancreas,
digestive gland, and other tissues. Sea birds and some other waterfowl
also have cytochrome P450 systems, but few estimates have been made of
their capacity for hydrocarbon metabolism. Some crude oils, PAHs, and
refined petroleum products are known to induce cytochrome P450 enzymes
and to increase the levels of hydrocarbon metabolism in marine and
freshwater fish, including embryonic and larval forms and polychaetes
(US National Research Council, 1985). Induction is usually described
in terms of the rate of metabolism of benzo[a]pyrene in vitro.
A4.2.3 Bioaccumulation
Few data are available on the bioaccumulation of diesel fuel
itself, although there is adequate evidence from studies on other oils
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient (log Pow) for diesel fuel is
3.3-7.1 (see section A2.2, Table 5). Although a higher bioaccumulation
potential might be expected from these log Pow values, the measured
bioconcentration factors are lower than predicted because many
compounds of low relative molecular mass, such as substituted
benzenes, are readily metabolized, and the actual bioaccumulation of
compounds of higher relative molecular mass is limited by their low
water solubility and large molecular size.
In a continuous-flow system, diesel fuel was taken up by
Mytilus edulis mussels exposed for 41 days to a concentration of
200-400 µg/litre. The hydrocarbon levels in tissue were about 1000
times higher after exposure than before. Depuration was rapid during
the first 15-20 days and then decreased rapidly to a minimum (Fossato
& Canzonier, 1976).
The accumulation of No. 2 fuel oil has been studied in a number
of organisms (Lee, 1977). When No. 2 fuel oil (38% aromatics) was
added to a commercial shrimp pond, the concentration of naphthalenes
in shrimps, clams, and oysters exceeded the level in ambient water
after 38 days. Depuration in clean water was complete after 10 days
for shrimps and after 47-96 days for oysters (Cox et al., 1975). In a
flow-through system to study accumulation of a No. 2 fuel oil-water
dispersion, aromatic petroleum hydrocarbons were accumulated at a rate
of 89.6 mg/kg tissue by Rangia cuneata (clams) and 311.7 mg/kg by
Crassostrea virginica (oysters) during 8 h of exposure. The
bioaccumulation factor for methyl naphthalenes in clams was 2.3-26.7
and increased with increasing alkylation of the naphthalene ring.
Depuration in clean water was complete after 28 days (Neff et al.,
1976a). The fuel components of a small spill of No. 2 fuel oil had the
following biological half-lives in mussels (Mytilus edulis): aliphatic
hydrocarbons (C16 and C23), 0.2 and 0.8 days; alkyl naphthalenes
(C2 and C3), 0.9 and 1.5 days; phenanthrene, 2.1 days (Farrington
et al., 1982). Lobsters exposed to a simulated small spill of No. 2.
fuel oil bioconcentrated PAH constituents in the hepato-pancreas and
muscle within three to four days after exposure. The levels remained
elevated for up to 10-11 days, and depuration was complete after 20-21
days.
The polychaetous annelid Neanthes arenaceodentata rapidly
accumulated 14C-naphthalene (to a biomagnification of 40 times) from
solution during exposure for 24 h and slowly released the accumulated
hydrocarbons within 300 h after return to clean sea water (Rossi,
1977).
Oral administration of South Louisiana crude oil to mallard ducks
(Anas platyrhynchus) at a dose of 5 ml daily led to detectable
aromatic petroleum hydrocarbon levels in each of the tissues examined:
liver, breast muscle, heart muscle, skin, brain, uropygial gland, and
blood. The skin and the underlying adipose tissue had accumulated far
more aromatic hydrocarbons than other tissues. The individual
components did not accumulate in the same relative concentrations as
in the crude oil, suggesting different uptake and/or metabolism; two-
and three-membered, condensed ring aromatic compounds were accumulated
to a greater extent than alkylbenzenes (Lawler et al., 1978).
A4.2.4 Tainting
Tainting (unpleasant off-flavours) of various aquatic organisms
by petroleum hydrocarbons has been reported, and diesel fuel has been
suspected of tainting fish in freshwater rivers. Mackie et al. (1972)
reported tainted rainbow trout up to at least nine days after a spill
of diesel fuel into water (Six Mile Water, Ireland). Ackman & Noble
(1973) described tainting of whitefish in the Athabaska River, Canada,
which was attributed to diesel fuel from a railroad yard.
A4.2.5 Entry into the food chain
No data are available on the effect of diesel fuel on the food
chain. The effects of petroleum compounds on the marine food web have
been studied, e.g. on zooplankton Calanus helgolandicus (Corner et
al., 1976), on phytoplankton (Mahoney & Haskin, 1980), and on
freshwater crayfish, one of the primary foods of waterfowl (Tarshis,
1981). In a study in situ on the distribution, biotransformation, and
flux of PAHs in the aquatic food chain (seston --> mussel [ Mytilus
edulis] --> eider duck [ Somateria molissima]), the PAH composition
changed significantly (Broman et al., 1990). The theoretical
inhalation of atmospheric PAHs was insignificant in comparison with
exposure from food. PAHs were biotransformed relatively rapidly. Their
concentrations did not increase with increasing trophic level.
A4.3 Ultimate fate after use
A4.3.1 Use in motor vehicles
Diesel fuel is combusted in motor vehicles and stationary engines
(see section A3.2.1.2). The resulting exhaust is discussed in Part B.
A4.3.2 Spills
The fate of diesel fuel after accidental spills is eventual
degradation by abiotic and biotic processes (see section A3.2.3)
A4.3.3 Disposal
In general, incineration is recommended for fuel oils (Fraser
Williams (Scientific Systems) Ltd, 1985). This procedure is also
useful for disposing of fuel production wastes.
Because diesel fuel is released during use, mainly accidentally
into water or onto soil (see sections A3.2.2.2, A3.2.2.3, and A3.2.3),
the affected compartments may have to be remediated. In general, oil
spills on beaches and into surface waters can be treated by burning
off; absorption onto straw, polyurethane foam, activated carbon, or
peat; or addition of sinking agents, gelling agents, or dispersants.
Mechanical systems can also be used (Fraser Williams (Scientific
Systems) Ltd, 1985). Various operations for treating soil affected by
diesel fuel have been reviewed (Dineen, 1991) and are as follows:
-- Disposal: excavation of contaminated soil and disposal at a
regulated landfill; the treatment used most commonly during the
last decade; advantageous for small volumes.
-- Biodegradation: The natural process of soil microorganisms,
which is stopped by a spill as a result of depletion of oxygen or
nutrients (mostly nitrogen and phosphorus), is restored by
providing sufficient amounts of the depleted substances. The
process can be carried out in situ (Frankenberger et al., 1989)
(see section A3.2.3, Table 10) or after excavation.
Biodegradation is the most widely applicable alternative to
landfill disposal, although its duration is uncertain and may be
relatively long and a large space is required (see sections
A9.1.1.2 and A9.1.3.1).
-- Thermal treatment: incineration of contaminated soil e.g. in
cement kilns or in specially designed thermal units, where the
soil is baked to remove the fuel, which is subsequently oxidized
(thermal desorption). The thermal desorption process is also
feasible in situ. In cement kilns, the desorbed diesel fuel is
used for heating the kiln, and the soil is incorporated into the
cement mixture.
-- Road base: The contaminated soil is spread on roads under
construction and is compacted and oiled before paving. If the
affected soil has a high proportion of expandable clays, it may
not be suitable as a road base.
-- Asphalt batching: The contaminated soil replaces part of the
sand and gravel component of asphalt; this is possible only if
the content of expandable clays is not too high.
-- Chemical oxidation: A strong oxidant, usually hydrogen
peroxide, in combination with a silica polymer stabilizer to
control the reaction is used to oxidize diesel hydrocarbons to
carbon dioxide and water. The reaction is highly exothermic, and
uncontrolled volatilization and leaching of some constituents may
occur.
-- Fixation: addition of a strong base to convert diesel
hydrocarbons to organic acids and subsequent treatment with a
dissolved silica polymer to effectively encapsulate the acids in
amorphous silica; immobilizes diesel fuel in situ and prevents
migration into surface or groundwater. The long-term stability of
the immobilized chemicals is, however, unknown.
-- Soil washing: Diesel fuel is washed out of soil by a
biodegradable, non-toxic surfactant (Peters et al., 1992). The
operation is feasible in situ and after excavation and is
particularly effective on materials with a low surface:volume
ratio (e.g. gravels and coarse sands). The major disadvantage is
the need to collect and treat or dispose of the
surfactant-diesel mixture after washing.
-- Containment: construction of a low-permeability surface
structure ('cap') that prevents infiltration of rain, snow, and
other moisture that could lead to leaching of diesel constituents
into groundwater. Long-term monitoring of the area of the spill
is necessary.
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
As diesel fuels are complex mixtures of a great variety of
hydrocarbons (see section A2.1), general 'environmental levels' cannot
be described. The individual constituents of diesel fuels should be
detectable in almost all compartments of the environment, but the
sources cannot be verified.
A5.2 Exposure of the general population
Nonoccupational exposure to diesel fuel occurs when fuel tanks
are filled manually. The primary source of dermal exposure is
accidental spills, although these exposures to high levels are of
short duration. Drinking-water can be contaminated by components of
petroleum products, including diesel fuel, after leakage into
groundwater from underground storage tanks. Specific data are not
available on the contribution of diesel fuel components to groundwater
contamination. Owing to the low volatility of diesel fuels at normal
temperatures, there is little exposure to vapours (International
Agency for Research on Cancer, 1989a).
A5.3 Occupational exposure during manufacture, formulation, or use
Workers are exposed to diesel fuel in a number of occupations,
including manual handling and discharge of fuels; loading and
unloading of diesel fuel; retailing at filling stations; manufacture,
repair, maintenance, and testing of diesel engines and other
equipment; use of diesel fuel as a cleaning agent or solvent; and
laboratory handling and routine sampling of diesel fuel (CONCAWE,
1985).
At normal temperatures, low concentrations of vapours are likely
to be generated from diesel fuels, owing to their low volatility. Only
in confined spaces or at high operating temperatures do significant
levels of vapour occur. Hydrogen sulfide gas may evolve from residual
fuel oils (CONCAWE, 1985).
A6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No quantitative data are available; however, the systemic and
local effects observed after exposure to diesel fuels by various
routes indicate that they are absorbed dermally, orally, and by
inhalation.
A7. EFFECTS ON LABORATORY MAMMALS AND IN-VITRO TEST SYSTEMS
A7.1 Single exposure
Diesel fuels have a low order of acute toxicity after
administration orally, dermally, or by inhalation. Acute LD50 Table
12. In rats and mice, the values after oral or dermal exposure were
values are summarized in generally > 5000 mg/kg body weight, and the
values in rabbits after dermal exposure were > 2000 mg/kg body
weight. Since the physicochemical properties of kerosenes are similar
to those of diesel fuels (see also section A2.1), the LD50 values
for various types of kerosene are included.
Beck et al. (1984) reported an oral LD50 value of 7500 mg/kg
body weight (9 ml/kg body weight) for a market-place sample of diesel
fuel in male and female rats. Gross necropsy of animals that died
during the test showed haemorrhagic gastroenteritis, gastrointestinal
tympani, and pneumonia with abscess formation. No deaths were seen
among rabbits treated dermally with 5000 mg/kg body weight under an
occlusive covering for 24 h.
After oral administration of 12 000 mg/kg body weight kerosene to
rats, no change was seen in heart weight or in the relative weights of
lung, spleen, and kidney. There were no histopathological changes
(Muralidhara et al., 1982). Exposure of rats for 8 h by inhalation to
about 0.1 mg/litre deodorized kerosene did not induce adverse effects.
Four male cats that inhaled 6.4 mg/litre for 6 h showed no toxic
effects on the central nervous system or body weight during the
exposure or during a subsequent 14-day observation period (Carpenter
et al., 1976).
Male Sprague-Dawley rats given JP-5 jet fuel at a dose of
18 912 mg/kg body weight (24 ml/kg body weight) orally had
vacuolization of periportal hepatocytes and serum enzyme alterations,
indicating hepatic damage, within two days (Parker et al., 1981). All
male and female B6C3F1 mice treated dermally with 5000-40000 mg/kg
body weight marine diesel fuel survived a 14-day observation period
(US National Toxicology Program, 1986).
Table 12. Acute toxicity (LD50 and LC50 values) of diesel and other fuels
Fuel Species Route LD50 or Dose Reference
LC50
Diesel fuels
Diesel fuel (market-place sample) Rat Oral LD50 7500 mg/kg bw Beck et al. (1984)
Diesel fuel (market-place sample) Rabbit Dermal LD50 > 5000 mg/kg bw Beck et al. (1984)
Home heating oil No. 2a Rat Oral LD50 14 500-21 100 µg/kg bw American Petroleum
Institute (1980a,b,c)
Home heating oil No. 2a Rabbit Dermal LD50 > 5000 µg/kg bw American Petroleum
Institute (1980a,b,c)
Marine diesel fuel, JP-5, JP-8 Mouse Oral LD50 > 16 000 mg/kg bw Schultz et al. (1981)
shale, or petroleum oila
Marine diesel fuel, shale, or Mouse Dermal LD50 > 16 000 mg/kg bw Schultz et al. (1981)
petroleum oila
Other fuels
Straight-run middle distillateb Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1985a)
Hydrodesulfurized middle distillateb Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1982a,b)
Straight-run middle distillateb Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985a)
Hydrodesulfurized middle distillateb Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1982a,b)
Straight-run middle distillateb Rat Inhalation LC50 1.8 mg/litre American Petroleum
Institute (1987a)
Table 12 (contd)
Fuel Species Route LD50 or Dose Reference
LC50
Hydrodesulfurized middle distillateb Rat Inhalation LC50 4.6 mg/litre American Petroleum
Institute (1983a)
Light catalytically cracked distillatec Rat Oral LD50 4660 mg/kg bw (m) American Petroleum
3200 mg/kg bw (f) Institute (1985b)
Light catalytically cracked distillatec Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985b)
Light catalytically cracked distillatec Rat Inhalation LC50 4.7-5.4 mg/litre American Petroleum
Institute (1986a,b)
Jet fuel Aa Rat Oral LD50 > 25 000 µl/kg bw American Petroleum
Institute (1980d)
JP-5 shale oild Mouse Dermal LD50 8000 mg/kg bw Schultz et al. (1981)
JP-5 petroleum oild Mouse Dermal LD50 11 200 mg/kg bw Schultz et al. (1981)
JP-8 shale oila Mouse Dermal LD50 6400 mg/kg bw Schultz et al. (1981)
JP-8 petroleum oila Mouse Dermal LD50 8000 mg/kg bw Schultz et al. (1981)
Jet fuel Aa Rabbit Dermal LD50 > 5000 µl/kg bw American Petroleum
Institute (1980d)
Kerosenea Rat Oral LD50 50 000 mg/kg bw Parker et al. (1981)
Kerosenea Guinea-pig Oral LD50 16 320 mg/kg bw Deichmann et al. (1944)
Kerosenea Rabbit Oral LD50 22 720 mg/kg bw Deichmann et al. (1944)
Straight-run kerosenea Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1985c)
Hydrodesulfurized kerosenea Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1982b)
Table 12 (contd)
Fuel Species Route LD50 or Dose Reference
LC50
Straight-run kerosenea Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985c)
Hydrodesulfurized kerosenea Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1982c)
Petroleum-derived hydrocarbona Rat Inhalation LC50 > 5.3 mg/litre American Petroleum
Institute (1987b)
Petroleum-derived hydrocarbona Rat Inhalation LC50 > 5.2 mg/litre American Petroleum
Institute (1983c)
Deodorized kerosenee Rat Inhalation LC50 > 0.1 mg/litre Carpenter et al. (1976)
(saturation)
a Hydrocarbon fraction similar to that of diesel fuel (see section A2.1)
b Hydrocarbon fractions from which also diesel fuels are gained during petroleum separation (see section A3.2.1.1)
c Blending component of diesel fuel
d Narrow boiling range
e Used as a solvent, not a fuel; very low aromaticity
Exposure of Sprague-Dawley rats to an aerosol of diesel fuel at a
concentration × time product of 8 mg.h/litre caused less than 1%
mortality and was estimated to represent the maximal tolerated
exposure. On this basis, 12 mg.h/litre was chosen as the highest
concentration in a study of repeated exposure (see section A7.2).
After exposure to 0.5 mg/litre for 2 h, a transient depression of the
startle reflex was observed. Further findings were a dose-related
decrease in respiratory frequency and influx of polymorphonuclear
cells into the lungs for several days. In a 6-h inhalation test, the
no-observed-adverse-effect level (NOAEL) was 2.7 mg/litre (Dalbey et
al., 1982, 1987).
A7.2 Short-term exposure
A7.2.1 Subacute exposure
A7.2.1.1 Dermal exposure
Dermal exposure to 0.1 ml/kg body weight per day kerosene for one
week was not lethal to male mice. Significant decreases in the
relative weights of thymus, spleen, adrenals, and abdominal lymph
nodes were observed (Upreti et al., 1989).
Eight rabbits (weighing 2.5-3.5 kg) were exposed dermally
(occlusive covering, 24 h/day, five days per week, two weeks) to 4 or
8 ml/kg body weight per day of a market-place sample of diesel fuel.
None of the animals given the low dose died, although there was a
weight loss of 0.3 kg, while five animals given the high dose died
with a weight loss of 0.5 kg; the control group had a weight gain of
0.2 kg. Severe chronic dermal irritation and anorexia were seen, which
were followed by cachexia and death (Beck et al., 1984).
Three doses between 1 and 10 ml/kg body weight per day of 10, 30,
or 50% fuel oil No. 2 were applied to the skin of New Zealand white
rabbits. After a 12-day treatment including a two-day rest, acute
dermal corrosion was seen in all animals treated with the 10% oil and
in animals given 10 ml/kg body weight per day of the 30 or 50% oil.
There was histopathological evidence of dermal toxicity in all treated
groups (American Petroleum Institute, 1980a,b,c).
Application of 8000 µl/kg body weight per day of jet fuel A to
four male and four female New Zealand white rabbits for 12 days,
including a two-day rest, induced acute dermal corrosion.
Histopathological evidence of dermal and hepatic toxicity and
hyperplastic changes in the urinary bladder transitional epithelium
were seen (American Petroleum Institute, 1980d).
Groups of five male and five female New Zealand white rabbits
were treated with 200, 1000, or 2000 mg/kg body weight per day of a
straight-run distillate, a light catalytically cracked distillate, or
two hydrodesulfurized distillates, three times per week for four
weeks. Moderate to severe irritation was seen, the degree of skin
irritation being directly related to the number of doses applied.
Microscopic examination of the skin at the application sites revealed
the presence of acanthosis and hyperkeratosis (American Petroleum
Institute, 1983a, 1984, 1985a,b).
In a study of marine diesel fuel containing 12.7% paraffins,
43.7% naphthalenes, and 43.6% aromatic compounds, groups of male and
female B6C3F1 mice received dermal applications (with an occlusive
covering) of 2000, 8000, 20000, or 40000 mg/kg body weight per day for
14 consecutive days. None of the animals at 20000 or 40000 mg/kg body
weight per day survived. Skin lesions were observed in all treated
groups, which consisted of moderate acanthosis, parakeratosis, and
hyperkeratosis. These findings were accompanied by a mixed cellular
inflammatory infiltrate in the upper dermis. The NOAEL was 8000 mg/kg
body weight per day. In the same study, JP-5 navy fuel containing
52.8% cycloparaffins, 30.8% paraffins, 15.9% aromatics, and 0.5%
olefins (see also Table 2) was applied at doses of 5000-40000 mg/kg
body weight per day for 14 days. All animals at 40000 mg/kg body
weight per day and females at 30000 mg/kg body weight per day group
died before the end of the study. Body weight loss was observed at
doses > 10 000 mg/kg body weight per day. The dermal findings were
similar to those in the study of marine diesel fuel. The NOAEL was
5000 mg/kg body weight per day (US National Toxicology Program, 1986).
A7.2.1.2 Inhalation
Rats (strain, age, and sex not specified) were exposed for
6 h/day to a droplet aerosol of deodorized kerosene at a mean
concentration of 7.7 mg/litre for four days. After a day with no
treatment, the skin of the extremities was dry and flakes had formed.
The body weight remained unchanged (Carpenter et al., 1976).
CD-1 mice were exposed for 8 h/day for five days to uncombusted
diesel vapour at concentrations of 0.065, 0.135, or 0.204 mg/litre.
The dose-response for neurotoxicity (measured by square-box activity)
was 50% of the control level at the high dose, 100% at the medium
dose, and 150% at the low dose. A drastic deterioration of motor
coordination was seen at the high dose in the rotarod test. In the hot
plate test, increased sensitivity followed by tolerance was seen at
the high dose, a slight increase at the medium dose, and a substantial
increase at the low dose; normalization occurred in all groups within
24 h. No effects were seen in the inclined plane test or the corneal
reflex test. Vasodilatation, poor grooming, and tremors were
described. In general, the most obvious effects were on the nervous
system, diesel fuel acting as a neurodepressant (Kainz & White, 1982,
1984).
Dalbey et al. (1982, 1987) investigated the toxic effects of
diesel fuel aerosols to assess the health risks of their military use
as smoke screens. Groups of male and female Sprague-Dawley rats were
exposed to concentrations of 1.3-6 mg/litre for 2 or 6 h, one or three
times per week, for a total of nine exposures; 12 mg.h/litre was
chosen as the highest concentration-time product that caused 6.25%
mortality. A significant depression of body weight and a slower growth
rate were seen initially, and liver weights were significantly
decreased. No neurotoxicity was seen. Histologically, there was focal
accumulation of free pulmonary cells and increased numbers of
leukocytes in bronchial lavage fluid. In pulmonary function tests, a
non-dose-dependent change in lung volume was seen, involving a
decrease in total and vital capacity and increases in functional
residual capacity and residual volume. No other organs were affected.
Groups of 20 male and 20 female Sprague-Dawley rats exposed by
inhalation to 0.025 mg/litre of one of three hydrodesulfurized middle
distillates for 6 h/day on five days per week for four weeks showed no
treatment-related changes. Mild subacute inflammatory changes were
observed in the nasal respiratory mucosa of the animals in one group
that had been exposed to distillate API 81-09 (American Petroleum
Institute, 1986c).
A7.2.2 Subchronic exposure
A7.2.2.1 Dermal exposure
Groups of 10 male and 10 female B6C3F1 mice received dermal
applications under an occlusive covering of 250 or 4000 mg/kg body
weight per day marine diesel fuel or 500-8000 mg/kg body weight per
day JP-5 navy fuel for five days per week for 13 weeks. The marine
diesel fuel caused no treatment-related deaths. Males had reduced body
weight gain; mild chronic active dermatitis at the site of application
was observed at the highest dose. At 1000 mg/kg body weight per day,
all animals survived and had weight gains similar to those of the
controls. Exposure to JP-5 navy fuel killed five males at the
high-dose group and caused dermatosis, splenic extramedullary
haematopoiesis, and liver karyomegaly in all groups (US National
Toxicology Program, 1986).
A7.2.2.2 Inhalation
The results of studies of subchronic inhalation of various
distillate fuels are summarized in Table 13.
Table 13. Studies of subchronic exposure to distillate fuels by inhalation
Fuel Species Exposure (high dose) Results Reference
Diesel fuels
Diesel fuel Rat 4 h/d, 2 d/week, 13 weeks + Reversible loss of body weight; Lock et al. (1984)
8 weeks' recovery (1.5 mg/litre) no other apparent effect
Marine diesel fuela Mouse, rat 90 d, continuous (0.05 and Nephrotoxic effects specific to Bruner (1984)
0.3 mg/litre) male rats
Other fuels
JP-8 jet fuelb Mouse, rat 24 h/d, 7 d/week,90 d + 24 Nephrotoxic effects specific to Mattie et al. (1991)
months' recovery (1 mg/litre) male rats
JP-5 jet fuelb Rat, dog 24 h/d, 7 d/week,90 d Nephrotoxic effects specific to Gaworski et al. (1984)
(0.75 mg/litre) male rats
JP-4 jet fuelb Mouse, rat 6 h/d, 5 d/week,12 months Nephrotoxic effects specific to Bruner et al. (1993)
(5 mg/litre) male rats; increased incidence
of hepatocellular adenomas in
female mice
Deodorized Rat, dog 6 h/d, 5 d/week,13 weeks No apparent effects Carpenter et al. (1976)
kerosenec (0.1 mg/litre)
a Hydrocarbon fraction similar to that of diesel fuel
b Narrow boiling range
c Not a fuel; very low aromaticity
Groups of 25 male rats and four male dogs were exposed to
deodorized kerosene at concentrations up to 0.1 mg/litre,
approximately the saturation limit at 25°C, for 6 h/day on five days
per week for about 13 weeks. Although dose-related changes were
observed in survival, body weight gain, organ weights, and
haematological and clinical chemical parameters, none of the findings
was statistically significantly different from those in controls. No
pathological change was seen in any of the organs examined (Carpenter
et al., 1976).
Groups of 12 male and 12 female Sprague-Dawley rats were exposed
in whole-body chambers for 4 h/day on two days per week for 13 weeks
to 0.25, 0.75, or 1.5 mg/litre aerosolized diesel fuel, followed by an
eight-week recovery period. There were no deaths. All treated animals
lost weight, indicating slight toxicity, during the first four weeks
of exposure, but the effect was reversed during the recovery period in
most cases. A slight depression of the startle reflex was seen at the
start of the study, and the number of macrophages in bronchial lavage
fluid was slightly elevated in all treated animals immediately after
cessation of exposure. Further tests of pulmonary function, gas
exchange, and dynamic lung function did not reveal any significant,
dose-related change. Overall, no significant cumulative toxicity was
seen (Lock et al., 1984).
A specific effect of diesel fuel in male rats, the hyaline
droplet nephropathy syndrome, was seen in several studies. Gaworski et
al. (1984) studied the effects of a 90-day exposure to 0.15 or
0.75 mg/litre JP-5 jet fuel derived from either petroleum or shale oil
for 24 h/day, in groups of three male and three female beagle dogs, 75
male and 75 female Fischer 344 rats, and 111-150 female C57Bl/6 mice.
The toxicity of the two types of fuel was not significantly different.
The only substantial effect at both concentrations was a renal tubular
lesion in male rats, probably caused by hyaline droplet accumulation
and unique to male rats. Other signs of toxicity were mild liver-cell
changes and mild nasal inflammation in rats. Cowan & Jenkins (1980)
studied petroleum and shale-oil marine diesel fuel using an identical
study design. Reduced body weight gain and hyaline droplet nephropathy
were reported in male rats. In another study, groups of 95 male and 75
female Fischer 344 rats and 100 male and 100 female C57Bl/6 mice were
exposed for 24 h/day on seven days per week to JP-8 jet fuel at
concentrations up to 1 mg/litre for 90 days, and then held for 20-21
months in order to assess the long-term consequences of exposure. The
main finding was accumulation of hyaline droplets in the kidneys of
male rats (Mattie et al., 1991). In a 90-day study, groups of 75 male
and 75 female Fischer 344 rats were exposed continuously to 0.05 or
0.3 mg/litre of marine diesel fuel. The main finding was again hyaline
droplet nephropathy in males at both levels (Bruner, 1984). In a study
of the carcinogenicity of JP-4 jet fuel vapour, hyaline droplet
nephropathy was observed in groups of 100 male Fischer 344 rats
exposed for 6 h/day, five days per week for 12 months to
concentrations up to 5 mg/litre (Bruner et al., 1993) (see also
section A7.7).
The hyaline droplet nephropathy syndrome is not considered to be
relevant for humans. It is linked to an inherent peculiarity of renal
protein. The histopathological sequence is: accumulation in the renal
proximal tubules of an excessive amount of hyaline droplets containing
alpha2-microglobulin, a special protein of male rats which is
synthesized in the liver; cytotoxicity and single-cell necrosis of the
tubular epithelium; sustained regenerative tubule-cell proliferation;
development of intraluminar granular casts from sloughed cell debris;
tubular dilatation and papillary mineralization; foci of tubule
hyperplasia; and renal tubule tumours (US Environmental Protection
Agency, 1991). Mechanistically, a globulin-substance complex that is
resistant to hydrolytic degradation is responsible for the protein
overload in kidneys.
A7.3 Long-term exposure
A7.3.1 Dermal exposure
Groups of 50 male and 50 female B6C3F1 mice received dermal
administrations of 250 or 500 mg/kg body weight per day marine diesel
fuel or JP-5 navy fuel on the clipped dorsal interscapular region on
five days per week for 103 weeks. (The doses were derived from studies
reported in section A7.2.) The high dose of marine diesel fuel caused
a dose- and time-dependent reduction in mean body weight in animals of
each sex, by up to 23% after week 40 in males and up to 20% after week
72 in females. Survival of treated females was decreased, and exposure
to the high dose was stopped at week 84 owing to severe ulceration.
Application of 500 mg/kg body weight per day JP-5 navy fuel resulted
in a time-dependent reduction in mean body weight in animals of each
sex: by up to 22% after week 76 in males and up to 25% after week 60
in females. Treatment of females with the high dose had to be
terminated at week 90. Overall, JP-5 navy fuel caused a lesser chronic
inflammatory response than marine diesel fuel (US National Toxicology
Program, 1986).
A7.3.2 Inhalation
Groups of 100 male and 100 female Fischer 344 rats and C57Bl/6
mice were exposed to JP-4 jet fuel vapour for 6 h/day on five days per
week for 12 months at concentrations of 1 or 5 mg/litre (see section
A7.7). The sex-specific alpha2-microglobulin nephropathy syndrome was
observed in 21% of male controls and in 20% of males at the low dose,
and 77% at the high dose (see also section A7.2). No significant,
dose-related alteration was seen in clinical findings, mortality,
haematological parameters, or clinical chemistry. All treated male
rats had a significant reduction in mean body weight. At the high
dose, male rats had significantly increased relative liver and kidney
weights and females had a significant decrease in relative spleen
weight. These alterations were not significant in comparison with
absolute organ weights (Bruner et al., 1993).
A7.4 Dermal and ocular irritation; dermal sensitization
A7.4.1 Dermal irritation
The results of studies of dermal irritation (Table 14) must be
interpreted critically, as different protocols were used, e.g. in
Europe and the United States. The main difference is in exposure time:
24 h in the American protocols and 4 h in those of the European Union
and the Organisation for Economic Co-operation and Development, which
may pose a problem when only mild irritation is observed, leading to a
classification of 'non-irritant' according to protocols of the latter
organizations.
A market-place sample of diesel fuel, tested at a dose of 0.5 ml
for 24 h in rabbits, was assessed as 'extremely irritating',
corresponding to a Draize score of 6.81 (Beck et al., 1984).
Topical applications three times a week to mice of various types
of kerosene and gas oil with boiling ranges similar to that of diesel
fuel produced degenerative changes in the skin surface, including
necrosis and hyperplasia (CONCAWE, 1991).
Four types of American military fuels were investigated:
petroleum JP-5 fuel, shale JP-5 fuel, petroleum marine diesel fuel,
and shale marine diesel fuel. None was active in the Draize test, in
which six female New Zealand white rabbits received 0.5 ml of
undiluted fuel on intact and abraded skin under an occlusive covering
for 24 h (Cowan & Jenkins, 1980). Applications to rabbit skin of
0.5 ml of various shale oil- and petroleum-derived fuels under
occlusive dressings for 4 h were not irritating (Schultz et al.,
1981).
Various modifications of fuel oil No. 2 (10, 30, and 50%) were
found to be moderately irritating to rabbit skin (American Petroleum
Institute, 1980a,b,c), and two hydrodesulfurized middle distillates
resulted in primary irritation indices of 4.3 and 5.9 (American
Petroleum Institute, 1982a,b). A straight-run middle distillate had a
moderately irritating effect, with an irritation index of 3.2
(American Petroleum Institute, 1985d). Jet fuel A was classified as
mildly irritating, with an irritation score of 1.96 (American
Petroleum Institute, 1980d). A hydrosulfurized kerosene was moderately
irritating, with an index of 4 (American Petroleum Institute, 1982c).
A light catalytically cracked distillate (American Petroleum
Institute, 1985d) and a straight-run kerosene (American Petroleum
Institute, 1985e) had severely irritating effects, with irritation
indexes of 5.5 and 5.6.
Table 14. Studies of dermal irritation in rabbits exposed for 24 h to various fuels
Fuel Classification of Reference
irritationa
Diesel fuel Extremely irritating Beck et al.
(1984)
Marine diesel fuel or marine Not irritating Cowan & Jenkins
shale fuel (1980)
Hydrodesulfurized middle Moderately irritating American Petroleum
distillate Institute (1982a,b)
Straight-run middle distillate Moderately irritating American Petroleum
Institute (1985d)
Light catalytically cracked Severely irritating American Petroleum
distillate Institute (1985d)
Heating oil No. 2 Moderately irritating American Petroleum
Institute (1980a,b,c)
Jet fuel A Mildly irritating American Petroleum
Institute (1980d)
Various kerosenes Slightly to severely CONCAWE (1995)
irritating
Shale oil- and Not irritating Schultz et al. (1981)
petroleum-derived fuels
Hydrodesulfurized kerosene Moderately irritating American Petroleum
Institute (1982c)
Straight-run kerosene Severely irritating American Petroleum
Institute (1985e)
a Note differences in protocols between regulations of the United States and
the Organisation for Economic Co-operation and Development (see section A7.4.1)
A7.4.2 Ocular irritation
All of the available reports indicate no or, at the most, a
slight irritating effect of diesel fuels and kerosenes on the rabbit
eye.
Four types of American military fuels (petroleum JP-5 fuel, shale
JP-5 fuel, petroleum marine diesel fuel, and shale marine diesel fuel)
gave generally negative responses in the Draize test, in which nine
female New Zealand white rabbits were given 0.1 ml of undiluted
substance (Cowan & Jenkins, 1980). A market-place sample of diesel
fuel (0.1 ml, undiluted) was also not irritating in the Draize test
(Beck et al., 1984). None of several kerosenes tested was more than
slightly irritating to the eye (CONCAWE, 1995), and none of the shale
oil- and petroleum-derived fuels tested caused visible irritation in
the rabbit eye (0.1 ml, undiluted) (Schultz et al., 1981).
Three modifications of fuel oil No. 2 (10, 30, and 50%) were
classified as mildly irritating in rabbits (American Petroleum
Institute, 1980a,b,c). Two hydrodesulfurized middle distillates did
not cause primary irritation in the Draize test (American Petroleum
Institute, 1982a,b); a straight-run middle distillate (American
Petroleum Institute, 1985d) and a straight-run kerosene also gave
negative results (American Petroleum Institute, 1985e). Jet fuel A was
classified as mildly irritating, with an average score of 2.67 after
one day and 0 after seven days (American Petroleum Institute, 1980d).
A hydrodesulfurized kerosene was not irritating (American Petroleum
Institute, 1982c).
A7.4.3 Sensitization
Three modifications of fuel oil No. 2 (10, 30, and 50%) were were
classified as non-sensitizing for the skin of guinea-pigs (American
Petroleum Institute, 1980a,b,c). A straight-run middle distillate
(American Petroleum Institute, 1985d), a straight-run kerosene
(American Petroleum Institute, 1985e), jet fuel A (American Petroleum
Institute, 1980d), a hydrodesulfurized middle distillate (American
Petroleum Institute, 1984), and a light catalytically cracked
distillate (American Petroleum Institute, 1985c) were also not
sensitizing.
Four types of fuels used in the United States Army, petroleum
JP-5 fuel, shale JP-5c fuel, petroleum marine diesel fuel, and shale
marine diesel fuel, were not sensitizing in a modified Landsteiner
test in groups of 24 guinea-pigs (Cowan & Jenkins, 1980). A
straight-run kerosene, hydrosulfurized kerosene, jet fuel A (CONCAWE,
1995), and shale oil- and petroleum-derived jet and diesel fuels
(Schultz et al., 1981) were also not sensitizing.
A7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Groups of 20 pregnant CRL:COBS rats were exposed by inhalation on
days 6-15 of gestation to 100 or 400 ppm of diesel fuel for 6 h/day
(American Petroleum Institute, 1979). A significant reduction in
maternal food consumption was seen at the high concentration, and
maternal body weight gain and fetal weights were reduced, although not
statistically significantly. Overall, no significant, dose-related
effects were observed in dams, and the compound had no teratogenic or
embryotoxic effect and no effect on fetal growth or development.
No signs of developmental or maternal toxicity were seen when
groups of 20 pregnant rats were exposed to jet fuel A or fuel oil for
6 h/day on days 6-15 of gestation (Beliles & Mecler, 1982).
Sprague-Dawley rats were fed 500, 1000, 1500, or 2000 mg/kg body
weight per day of JP-8 aviation fuel by gavage on days 6-15 of
gestation, and fetuses were examined on day 20. Significantly reduced
body weights were seen in dams at the two higher doses and in fetuses
at 1000-2000 mg/kg body weight per day, but the number of
malformations was not altered. The investigators concluded that JP-8
aviation fuel is a developmental toxin at doses > 1000 mg/kg body
weight per day but is not teratogenic (Cooper & Mattie, 1993).
A7.6 Mutagenicity and related end-points
A7.6.1 In vitro
Various samples of diesel and related fuels have been tested for
mutagenicity in Salmonella typhimurium (Henderson et al., 1981;
Conaway et al., 1984; Vandermeulen et al., 1985; US National
Toxicology Program, 1986; McKee et al., 1994). Negative results were
obtained in direct, unmodified Ames' assays (Conaway et al., 1984;
Vandermeulen et al., 1985; US National Toxicology Program, 1986). In
one study, refined shale oil, jet fuel, and shale oil- and
petroleum-derived marine diesel fuel were not mutagenic (Schultz et
al., 1981). Negative results were also found in studies of aliphatic
and aromatic fractions of diesel fuel in S. typhimurium TA98, TA100,
and TA1535 in the presence of an exogenous metabolic system (Henderson
et al., 1981).
The number of revertants of TA98 increased to 2.3-2.5 times the
background levels in a suspension assay (Conaway et al., 1984), but
increases were found at a limited number of doses. In a modified
Salmonella assay with an extraction step to concentrate the
mutagenic aromatic compounds, addition of an exogenous metabolic
system from Aroclor-induced hamster liver, and use of TA98 as the most
responsive strain, increased numbers of revertants were seen (McKee et
al., 1994); but the number never exceeded twice the background
response, and the results were considered to be equivocal.
A dimethyl sulfoxide extract of an intermediate, catalytically
cracked distillate (CAS No. 644741-60-2) was mutagenic in
S. typhimurium TA98 with an increased concentration of exogenous
metabolic system to optimize detection. Of two dimethyl sulfoxide
extracts of light hydrocracked distillate (CAS-No. 64741-77-7) tested
in the same system, one induced a weak, non-dose-dependent response,
while the other induced a clear dose-dependent positive response
(Blackburn et al., 1984). Under the same assay conditions, five
samples of cracked middle distillates also gave positive results
(German Scientific Association for Petroleum, Natural Gas, and Coal,
1991). These results in a series of assays in Salmonella with a number
of variations provide no clear evidence of mutagenic activity. In the
unicellular alga Chlamydomonas reinhardtii (streptomycin resistant)
tested in a natural aquatic environment, water-soluble fractions of
two crude and two refined oils did not induce forward mutations
(Vandermeulen & Lee, 1986). Neither aliphatic nor aromatic dimethyl
sulfoxide fractions of diesel fuel No. 7911 induced mutation in
S. typhimurium. After reaction with nitrogen dioxide, which may
activate the mutagenicity of diesel exhaust, it was mutagenic in
strain TA100, the aromatic fraction being 40-fold more active than the
aliphatic fraction (Henderson et al., 1981).
A market-place sample of diesel fuel did not induce mutation in
L5178Y tk+/- mouse lymphoma cells with or without metabolic
activation (Conaway et al., 1984). A hydrodesulfurized middle
distillate (CAS 64742-80-9) was active in the presence of metabolic
activation, but the concentrations were cytotoxic (American Petroleum
Institute, 1987c).
A7.6.2 In vivo
Undiluted diesel fuel No. 2 increased the frequency of
chromosomal aberrations in the bone marrow of Sprague-Dawley rats
given single or repeated (five days; 125, 417, or 1250 mg/kg body
weight) oral doses. Diesel fuel given intraperitoneally for one or
five days at 0.6, 2, or 6ml/kg body weight significantly increased the
number of aberrant cells at the highest dose (Conaway et al., 1984).
In order to assess these findings further, McKee et al. (1994)
evaluated micronucleus induction by diesel fuel No. 2 and related
materials in male and female CD-1 mice treated by gavage at up to
5 g/kg body weight. No effect was seen.
No effect was seen on the frequency of dominant lethal mutations
in male CD-1 mice exposed to 100 or 400 ppm of either diesel fuel or
jet fuel A for 6 h/day, five days per week for eight weeks (American
Petroleum Institute, 1980e, 1981).
A7.7 Carcinogenicity
A7.7.1 Dermal exposure
Diesel fuel has been shown to have at least weak carcinogenic
potency after dermal administration in a number of studies. As some of
the test samples contained low concentrations of carcinogenic PAHs, it
has been suggested (Biles et al., 1988) that the tumours induced may
have been a consequence of the dermal damage produced by repeated
treatment with these materials. The available studies are summarized
in Table 15.
Doses of 25 mg per animal of petroleum and shale fuels were
applied to groups of 25 male and 25 female C3H/HeN mice three times a
week, for 62 weeks for jet fuel A with subsequent observation up to
105 weeks, and for 104 weeks for JP-4 fuel. Controls received mineral
oil. The shale and petroleum diesel fuels were characterized for
boiling range (jet A, 171-271°C; JP-4, 60-249°C) and sulfur content
(jet A: petroleum, 3510 ppm; shale, 89 ppm; JP-4: petroleum, 1830 ppm;
shale, 199 ppm). After 60 weeks, increased incidences of squamous-cell
carcinomas and fibrosarcomas were reported (jet A: petroleum, 26%;
shale, 28%; JP-4: petroleum, 26%; shale, 50%; controls, 0-2%), but no
statistical analysis was reported (Clark et al., 1988).
Groups of 50 male and 50 female B6C3F1 mice were given 250 or
500 mg/kg body weight per day marine diesel fuel dermally, on five
days per week for 103 or 84 weeks (see section A7.3.). A control group
received the vehicle, acetone, only. The survival rates were 26/50
males and 29/50 females at the high dose at 84 weeks, 20/49 males and
12/50 females at the low dose at 104 weeks, and 30/50 male and 40/50
female controls. Males at the high dose had a significantly increased
number of hepatocellular adenomas or carcinomas (29%; control, 18%).
The total incidences of squamous-cell papillomas and carcinomas at the
application site and the adjacent inguinal site were: control, 1/50;
low dose, 2/49; high dose, 3/50 in males; and control, 0/50; low dose,
1/45; and high dose, 2/48 in females. No information was available on
historical frequencies in acetone-treated mice; the incidence of
papillomas and carcinomas in 3500 observations in untreated mice was
0.3-0.4%. In a parallel study with JP-5 navy fuel, 1/50 papillomas
were seen at the low dose and 4/49 carcinomas at the high dose in
males, and 0/49 carcinomas were seen at the low dose and 1/47 at the
high dose in females. The investigators concluded that there was
equivocal evidence for the carcinogenicity of marine diesel fuel and
no evidence for the carcinogenicity of JP-5 navy fuel (US National
Toxicology Program, 1986).
Two middle-distillate fractions of each of two crude oils
(boiling range, 170-370°C) were painted at a dose of 50 mg onto the
skin of male C3H mice twice a week for 18 months. The yield of benign
and malignant tumours (details not specified) was 2-19 % (Lewis,
1983).
Table 15. Studies of chronic dermal exposure of mice (usually 50 mice/group) to distillate fuels
Fuel Exposure Results Reference
Marine diesel fuel 0, 250, and 500 mg/kg bw, 1/50, 2/49, and 3/50 (m) and 0/50, 1/45, and 2/48 (f) US National Toxicology
5 d/week, 103 weeks with squamous-cell papillomas and carcinomas after Program (1986)
104 weeks
Straight-run gas oil 25 mg/animal, twice/week, Occasional ulcerations, scarring, epidermal dysplasia; American Petroleum
(laboratory sample)a up to 2 years after 2 years, 17/50 with tumours; controls, 0% Institute (1985c)
Straight-run middle 50 µl/animal, twice/week, Mild to moderate irritation (mild desquamation, American Petroleum
distillatea up to 2 years occasional scabbing); after 2 years, 10/50 with Institute (1989a)
squamous-cell carcinomas or fibrosarcomas;
controls, 0%
Straight-run 50 µl/animal, twice/week, 29/50 with malignant and 1/50 with benign tumours; American Petroleum
keroseneb > 2 years latency, 76 weeks; controls, 0% Institute (1989a)
Two cracked 50 µl/animal, twice/week, Mild to moderate irritation; after 24 months, 39/50 American Petroleum
distillatesc up to 2 years and 26/50 with tumours Institute (1989a)
Intermediate 50 mg/animal, twice/week, 42/43 with tumours; mean latency, 17 weeks; Blackburn et al. (1984)
catalytically cracked 80 weeks controls, 0/50
distillatec
Two hydrodesulfurized 50 µl/animal, twice/week, Acanthosis, fibrosis, hyperkeratosis, crusting; at end American Petroleum
middle distillatesa lifetime of study, 24/50 and 27/50 with tumours (no controls Institute (1989b)
reported)
Table 15 (contd)
Fuel Exposure Results Reference
Hydrodesulfurized 50 µl/animal, twice/week, 26 malignant and 1 benign tumours; latency, 77 American Petroleum
keroseneb > 2 years weeks (no controls reported) Institute (1989b)
Jet A and JP-4 fuels 25 mg/animal, three After 60 weeks, increased skin tumour rate; jet A, Clark et al. (1988)
from shale or times/ week, 62 weeks 26% petroleum, 28% shale; JP-4, 26% petroleum,
petroleum oilsb + observation (jet A) 50% shale
or 104 weeks (JP-4)
Two middle-distillate 50 mg/animal, twice/week, 2-19% tumour rate (no further information) Lewis (1983)
fractions 18 months
No. 2 fuel oilb 12, 25, or 50 µl/animal, Overall tumour rate, 10%; control, 0% Witschi et al. (1987)
three times/week, up to
100 weeks
Two heating oilsa 25 µl/animal, three 7/50 and 9/50 with tumours; control, 0/50 Biles et al. (1988)
times/week, lifetime
a Hydrocarbon fractions similar to that of diesel fuel (see section A2.1)
b Hydrocarbon fractions from which also diesel fuels are gained during petroleum separation (see section A3.2.1.1)
c Blending component of diesel fuel
Application of American Petroleum Institute No. 2 fuel oil
dermally to C3H mice at 12, 25, or 50 µl per animal, three times
weekly for up to 100 weeks induced an overall tumour incidence (type
not specified) of 10% (15/150 in the three treatment groups; controls,
0/100) (Witschi et al., 1987).
Ten samples of middle-distillate fuels were applied dermally to
C3H/HeJ mice at a dose of 25 µl, three times a week for life. A virgin
heating oil blending base (142-307°C) and a commercial No. 2 heating
oil (156-352°C) had aromatic contents closest to that of diesel fuel.
The blending base induced five skin carcinomas and two papillomas in
50 animals, and the commercial heating oil produced eight skin
carcinomas and one papilloma in 50 animals. There were no tumours in
the respective control groups (Biles et al., 1988).
A7.7.2 Inhalation
Fischer 344 rats and C57Bl/6 mice were exposed for 6 h/day on
five days per week for 12 months to 1 or 5 mg/litre of fuel vapours
(see section A7.3), followed by a 12-month recovery period. No
significant pulmonary neoplastic changes were seen. Female mice at the
high dose had a slightly but significantly increased incidence of
benign hepatocellular adenomas (10%; controls, 2%); however, the trend
was reversed in male mice. No conclusions about carcinogenic potency
were drawn from this study (Bruner et al., 1993).
A8. EFFECTS ON HUMANS
A8.1 Exposure of the general population
Like similar petroleum distillates, such as kerosene, diesel fuel
can cause dermal irritation and defatting after dermal exposure
(Sandmeyer, 1981). Considerable exposure and subsequent absorption may
also result in acute renal failure secondary to acute renal tubular
necrosis. A 28-year-old man developed progressive oliguria one day
after washing his hair with diesel fuel and had acute tubular necrosis
on renal biopsy (Barrientos et al., 1977). A 47-year-old man developed
acute tubular necrosis after cleaning his arms and hands with diesel
oil for several weeks (Crisp et al., 1979). Both recovered renal
function.
Accidental aspiration of diesel fuel resulted in immediate
coughing, followed by dyspnoea, cyanosis, and loss of consciousness
(Perez Rodriguez et al., 1976). X-Ray examination revealed diffuse
alveolar infiltrates. Mildly elevated levels of alkaline phosphatase,
serum glutamic-oxaloacetic transaminase, and serum glutamic-pyruvic
transaminase were also seen. After 37 days, residual infiltrates
remained, but the patient had improved. In another case, abdominal
pain and vomiting followed ingestion of a reported 1.5 litres of
diesel fuel. Pulmonary infiltrates were noted, presumably from
subsequent aspiration of diesel fuel (Boudet et al., 1983).
A8.2 Occupational exposure
The odour threshold values for diesel fuels No. 1 and 4 are
reported to be 0.7 and 0.5 ppm, respectively (see section A2.2, Table
5). Six volunteers exposed to 140 mg/m3 of deodorized kerosene for
15 min did not experience throat irritation. Olfactory fatigue was
induced in three subjects, and one reported taste sensation. The
investigators suggested that this concentration was the sensory
threshold for kerosene and estimated the odour threshold at
0.6 mg/m3 (Carpenter et al., 1976).
Acne and folliculitis developed on the arms and thighs of a man
who had worked in various automobile workshops for 15 years. He had
handled diesel oil with his bare hands and then wiped them on his
trousers (Das & Misra, 1988). Hyperkeratosis was noted in 320 drivers
working in Russian oil fields with regular dermal contact with diesel
fuel (Gusein-Zade, 1974). A 33-year-old man was exposed to diesel fuel
vapour (concentration not reported) for 10 days while driving a truck
with a fuel injector leak. He developed abdominal cramps, nausea,
vomiting, and then acute renal failure with anaemia and
thrombocytopenia (Reidenberg et al., 1964). Two aviators who were
exposed to JP-5 fuel vapour for 1 h while flying a small airplane
experienced eye irritation, difficulties in coordination and
concentration, and fatigue (Porter, 1990).
Siemiatycki et al. (1987) conducted a case-control study in
Montreal, Canada, and found an increased risk for squamous-cell
carcinoma of the lung in men exposed to diesel fuel. When all types of
lung cancer except adenocarcinoma were combined, an odds ratio
(adjusted for smoking) of 1.6 (90% confidence interval (CI), 1.0-2.4;
n = 39) was found for 'any' exposure. In four subcategories, the odds
ratios were 1.9 (short-low), 2.0 (short-high), 1.1 (long-low), and 2.0
(long-high). The possible effects of exposure to combustion products
were not taken into consideration. An association with prostatic
cancer was also observed, although there was no dose-response
relationship, with odds ratios of 2.3 (CI, 1.3-4.0; n = 17) for the
subgroup with nonsubstantial exposure and 1.4 (0.7-2.9; n = 8) in
the subgroup with substantial exposure.
In another case-control study, no association was found between
the occurrence of renal-cell cancer and occupational exposure to fuel
oils, including diesel fuel (levels and duration not quantified)
(Partanen et al., 1991).
In a cross-sectional epidemiological study, the effects of
long-term exposure of factory workers to jet fuels (not specified) was
investigated. Dizziness, headache, nausea, palpitation, pressure in
the chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of jet
fuel vapour in the breathing zone was estimated to be 128-423 mg/m3
(Knave et al., 1978).
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Comparison and evaluation of studies of the ecotoxicological
effects of diesel fuel are complicated by a number of factors:
(1) There are comparatively few ecotoxicological studies on
diesel fuel, and most work in the 1970s was concentrated on the
effects of No.2 fuel oil, which has a similar but not identical
chemical composition to diesel fuel No. 2 (see section A2). The
composition of marine diesel fuel differs greatly from both (see
Table 1). As described in the same section, diesel fuel itself has
various specifications in different countries; in particular, the
composition of non-hydrocarbon compounds from e.g. catalytic processes
or additives may vary widely. There may also be differences between
batches of the same oil (Hedtke & Puglisi, 1982) (see Table 20). The
amount of the chemical actually in solution is also pertinent to
ecotoxicological studies. Table 3 gives the concentration ranges of
toxicologically relevant constituents of diesel and No. 2 fuel oil as
a whole and as 10% water-soluble fractions.
(2) The chemical composition of fuel oils and, as a consequence,
their toxicity change with time, as oil can be modified by
biodegradation, photooxidation, and volatilization (see section A4).
Their toxicity also depends on whether they are mixed with water (sea
water or freshwater), sediment, or soil, the temperature (tropical or
Arctic), and physical conditions (e.g. storm conditions with continual
mixing and breaking up of oil). Oil modified by these processes is
usually less toxic than fresh oil (Baker, 1970; Morrow, 1973).
(3) A variety of methods have been used in ecotoxicological
studies on fuel oils. There is an obvious difference between
conditions in the laboratory and the field. Most of the field studies
that have been reported are related to fuel spills. In laboratory
studies, either water-soluble fractions (see below) or oil-water
dispersions have been used, and the methods of preparing them vary
among laboratories. Oil films and, more recently, microencapsulated
diesel oil have also been tested. In none of the laboratory studies
reported were test media prepared with 'water accommodated fractions',
as described and recommended by CONCAWE (1993) in laboratory protocols
for petroleum products. Thus, the amount of product that must be added
to a given volume of medium to produce the effects reported cannot be
derived from the results of these studies, making comparison of data
on ecotoxicity from different studies practically impossible.
Hedtke & Puglisi (1982) tested the lethality of No. 2 fuel oil in
two species of freshwater fish and two species of amphibians and found
variable LC50 values (see Table 20). Oil-water dispersions were very
toxic to fish. It is thought that these tiny oil droplets make contact
with the gills and directly damage the respiratory epithelium
(Engelhardt et al., 1981; Poirier et al., 1986). With floating oils,
the relationship between toxicity and oil-water contact is a function
of the equilibrium time for the soluble components of the oil and
water, death occurring more rapidly when the oil is added 96 h before
the organisms are exposed. Flow-through tests were more sensitive than
static tests, as, in the latter, the chemical nature and toxicity of
the oil change with time (Hedtke & Puglisi, 1982). Lockhart et al.
(1987) showed that the results of static tests in fish varied
considerably with aeration of test containers. Although flow-through
techniques give a better estimate of real exposure conditions, many of
the results reported in the literature are of static tests.
(4) The susceptibility of organisms to fuel oils and their
components depends not only on the species and strain but also on the
biological stage and the time of development within stages (Kühnhold,
1977; Winters et al., 1977). As the effects of No. 2 fuel oil and
diesel oil have been tested in a wide variety of organisms and life
stages under various conditions, direct comparison is difficult.
In spite of these difficulties, it can be concluded that diesel
oil is generally more toxic than crude oil, as seen, for example, in
barnacle nauplii (Winters et al., 1977), Daphnia (Cladocera),
chironomid larvae, and the mollusc Viviparus bengalensis (Gastropoda)
(Das & Konar, 1988). Crude oils themselves differ in toxicity,
depending on their source (Anderson, 1977a; Winters et al., 1977), as
do their corresponding products.
Rice et al. (1977a) concluded that the toxicity of fuel oil is
due to the chemical toxicity of soluble aromatic compounds rather than
to the physical toxicity of dispersed oil droplets. Physical coating
by oil of the gills of fish and the feathers of birds is also a cause
of toxicity. Monoaromatic compounds seem to be the least toxic, their
acute toxicity increasing with molecular size up to the four- to
five-ring compounds (e.g. chrysene and benzo[ a]pyrene) (Neff et al.,
1976b; Rice et al., 1977a), but they are not very soluble in sea water
(see Table 6). The toxicity of one-, two-, and three-ring compounds
(benzenes, naphthalenes, and phenanthrenes, respectively), seemed to
increase with increasing degrees of alkylation of the aromatic
nucleus. Neff et al. (1976b) reported 96-h LC50 values of 0.3-0.6 ppm
for 1-methylphenanthrene, fluoranthrene, and phenanthrene, indicating
the high toxicity of these polynuclear aromatic compounds for the
polychaete, Neanthes arenaceodentata. Of the pure compounds tested
by Winters et al. (1977), the most toxic to barnacle nauplii were
indan, naphthalene, xylene, and substituted benzenes and naphthalenes.
In four freshwater species, the water-soluble fraction was again
associated with the substituted benzenes, and naphthalenes were found
to be associated with the observed toxicity (Lockhart et al., 1987).
The concentrations of naphthalenes, methylnaphthalenes, and
dimethylnaphthalenes are particularly high in fuel oils (see Table 3),
and these compounds have been used as analytical markers in several
studies.
Experiments involving microencapsulated oil fractions have shown
that insoluble fractions also inhibit growth, e.g. of juvenile mussels
(Stromgren & Nielsen, 1991). Anderson (1979) indicated that the larval
stage might be the most sensitive to No. 2 fuel oil. Eggs of fish and
invertebrates were often tolerant, and adults were sometimes more
sensitive than juveniles.
Extensive studies have been carried out on lethal toxicity to
marine organisms under the same conditions. The 96-h LC50 values for
a wide range of invertebrates in static exposure conditions
(temperature, 18-22°C) were 1-20 ppm for crude oil in water and
0.4-6 ppm for No. 2 fuel oil (Anderson et al., 1974; Neff et al.,
1976b; Rossi et al., 1976). At 4-10°C, the 96-h LC50 values appeared
to be lower (Rice et al., 1976, 1977a). Table 16 gives the results of
lethality studies reported from several laboratories.
Table 16. Lethality (median lethal concentrations) to marine
zooplankton of physically dispersed and water-soluble
fractions of No. 2 fuel oil
Zooplankton Oil-water dispersions Water-soluble
(mg/litre) fractions (mg/litre)
Ctenophora 0.59 (1 day)
Mollusca
Clams
Embryos 0.43 (2 days)
Larvae 1.3 (2 days)
Larvae 0.53 (10 days)
Pteropods < 0.2 (2 days)
Crustacea
Barnacles 2.6 (1-h LC50)
Copepods 1.0 (3 days)
Amphipods 0.3 (2 days) 2.5 (3 days)
Decapods
Shrimp post-larvae 1.7; 9.4
Lobsters 1.2-6.6
Teleosts 1.5
Adapted from US National Research Council (1985). Values are for total
measured (extractable) hydrocarbons, calculated from initial
concentrations measured by spectroscopy and chromatography
No. 2 fuel oil (mostly the water-soluble fraction) has been found
to have sublethal effects in all phyla examined (Anderson, 1977a,b,
1979; US National Research Council, 1985). With increasing
concentrations, alterations were seen in the respiratory rate of the
fish Cyprinodon variegatus (Anderson et al., 1974) and Oncorhynchusm
gorbuscha (pink salmon) (Rice et al., 1977b), the crustaceans
Mysidopsis almyra and Penaeus aztecus, and the glass shrimp
Palaemoetes pugio (Anderson, 1979); and changes were found in the
pulse rate in embryonic estuarine fish, C. variegatus and Fundulus
heteroclitus (Anderson, 1977a) These effects have been used as a
measure of stress caused by exposure. Dysregulation of the ability to
regulate internal concentrations of solutes (osmotic and ionic
regulation) was demonstrated in the brown shrimp Penaeus aztecus
(Anderson et al., 1974; Cox, 1974; Anderson, 1979). The behaviour of
F. similus exposed to a 100% water-soluble fraction of No. 2 fuel
oil was correlated with the content of naphthalene in four organs
(Dixit & Anderson, 1977). Sublethal studies on No. 2. fuel oil showed
reduced growth rates, survival, and reproduction in a range of
organisms (Anderson, 1979; Table 17), including the annelid Neanthes
arenaceodentata (Rossi & Anderson, 1978) and the estuarine fish
F. grandis (Ernst et al., 1977).
A9.1 Laboratory experiments
Diesel fuel is a complex mixture of substances with a range of
solubilities in water (see section A2.2). In testing for aquatic
toxicity, it is important that the constituents in the medium be
characterized (Bennett et al., 1990), but these are seldom reported.
The results described in this section refer to diesel fuel, unless
otherwise stated.
As the solubility of diesel fuel in sea and freshwater is low,
investigations of the effects of diesel fuel on organisms in the
laboratory often involve preparation of a water-soluble fraction or an
oil-water dispersion. Water-soluble fractions can be prepared by
stirring one part of diesel fuel into nine parts of (sea) water for
20 h (Anderson et al., 1974) or one part of diesel fuel into eight
parts of sea water for 24 h (Pulich et al., 1974), and then extracting
the water phase. The resulting water phases contain 6-20 µg/ml of
total hydrocarbons; the 100% water-soluble fraction of No. 2 fuel oil
contains about 7 µg/ml of total hydrocarbons. Results are expressed as
the concentration (percent dilution of the water-soluble fraction)
and/or as the measured hydrocarbon concentration that causes the
specified level of effect. The results cannot, however, be correlated
with the original amount of fuel oil that was added to produce the
water-soluble fraction or oil-water dispersion (CONCAWE, 1993).
Table 17. Effects of No. 2 fuel oil on the growth and reproduction of marine animals
Species Exposure Concentration (ppm) Growth or reproduction Reference
(days)
TH TN TA
Fish
Cyprinodon variegatus 7 2.0 0.6 1.7 0% of eggs hatched Anderson et al. (1976)
Decapods
Rithropanopeus harrasii 27 1.0 0.3 0.9 Reduced survival and Neff et al. (1976b)
extended development to
megalopa
Palaemonetes pugio 12 0.9 0.3 0.8 Reduced growth rate of Tatem (1977)
larvae
3 1.4 0.6 Reduced viability of eggs
from exposed gravid females
Polychaetes
Neanthes arenaceodentata 22 1.0 0.3 0.9 Reduced growth of larvae Rossi & Anderson (1976)
28 0.3 0.1 Reduced growth of Anderson (1977a)
juveniles by 30%
Ctenodrilus serratus 28 2.2 0.5 1.4 Reduced survival and Carr & Reish (1977)
reproduction
Ophryotrocha sp. 28 1.3 0.3 0.9 Reduced survival and Carr & Reish (1977)
reproduction
From Anderson (1977b)
TH, total hydrocarbons; TN, total naphthalenes; TA, total aromatic compounds
Oil-water dispersions are produced by shaking a measured quantity
of oil in water for 5 min (Anderson et al., 1974) or 30 min (Pulich et
al., 1974). In some cases, the oil-water dispersion is maintained by
continuous mixing, but more usually it is allowed to settle, and the
aquatic organisms are exposed to the material in the water and to a
surface film. The effects seen in such tests can be due to both
physical action and toxicity, especially in small invertebrates such
as Daphnia.
Insoluble fractions of crude oil and other hydrocarbon
formulations strongly inhibited growth when they were
microencapsulated and ingested by juvenile mussels (Stromgren et al.,
1986; Stromgren & Reiersen, 1988) and larvae (Stromgren & Nielsen,
1991). In microcapsules with a thin acacia-gelatine structure, the
total oil is dispersed into particles of 1-10 µm, which are suitable
for ingestion by Mytilus larvae. The water-soluble components leak
through the wall, providing a low concentration, and are ingested by
the molluscs.
None of the studies involved use of the 'water accommodated
fractions' recommended by CONCAWE (1993), which allows the results to
be expressed as 'loading rate', defined as the amount of product that
must be equilibrated with the aqueous test medium in order to produce
a specified level of effect.
A9.1.1 Microorganisms
A9.1.1.1 Water
Marcus & Scott (1989) tested the effects of 0, 10, 20, and 40%
water-soluble fractions of a shale-diesel fuel mixture and a
petroleum-diesel fuel mixture on the growth of faecal coliform
bacteria. The 20 and 40% fractions of the petroleum-diesel fuel
mixture resulted in significantly lower bacterial densities than the
shale-diesel fuel mixture. The latter appeared to biostimulate or
mediate against toxicity, suggesting a difference in the chemical
characteristics of the two fuels.
The effects of diesel and furnace oils, petrol, kerosene, and
Assam crude oil and their paraffinic, aromatic, and asphaltic
fractions (higher relative molecular mass, poorly defined) on the
photosynthesis and respiration of blue-green algae (Cyanobacteria), in
particular Anabaena doliolum were investigated by Singh & Gaur (1990).
The chemical composition of the diesel and furnace oils was almost
identical, with 37 and 36% paraffins, 51 and 54% aromatic compounds,
and 6 and 0.6% asphaltic compounds, respectively. The aromatic
fractions of these oils were the most toxic, followed by the asphaltic
fractions; photosynthesis was reduced to 40% by the aromatic fractions
and to 50% by the asphaltic fractions of both oils at a concentration
of 5.0 mg/litre. The effects were dependent on concentration up to
10 mg/litre. Photosynthesis was stimulated 160% by 5 mg/litre of the
paraffinic fractions of crude oil , but only 100% by those of diesel
oil and 110% by those of furnace oil. In general, crude oil inhibited
photosynthesis and respiration by A. doliolum least and diesel and
furnace oils the most.
A9.1.1.2 Soil
In outdoor lysimeters, the toxicity of spills of 2.3 ml/cm2
diesel and heating fuels was investigated in soil microbes, using
Microtox(R) measurements (Wang & Bartha, 1990). (For parallel assays
of seed germination and plant growth, see section A9.1.3.1.) At time
0, both fuels were moderately toxic (EC50 = 80-90 mg of contaminated
soil); this was followed by a period of increased toxicity, which
started to decrease after 6-12 weeks. The values in soils treated by
biodegradation returned to background within 20 weeks, although diesel
fuel had significant residual toxicity in untreated soil. Seed
germination and plant growth were inhibited in a similar manner, and
diesel oil was more toxic and persistent than heating oil (section
A9.1.3.1). Biodegradation treatment, in which the conditions for
hydrocarbon degradation by microbes were optimized by mixing,
aeration, pH control, and addition of mineral nutrients (fertilizers),
strongly decreased fuel persistence and toxicity.
A9.1.2 Aquatic organisms
A9.1.2.1 Plants (phytoplankton)
Experiments in both the laboratory and the field have shown that
hydrocarbons can inhibit algal growth, although enhancement is
occasionally noted at lower concentrations of oil (US National
Research Council, 1985).
After 12 days of exposure to 10% diesel oil, the growth of cells
of Euglena gracilis was not significantly reduced, whereas a
concentration of 0.1% almost completely inhibited the growth of
Scenedesmus quadricauda. Cells of S. quadricauda grown in culture
media containing diesel fuel (concentration not given) became
chlorotic, suggesting damage to the photosynthesizing system
(Dennington et al., 1975).
A concentration of 0.05% light diesel fuel was found to stimulate
growth rate, growth yield, photosynthesis, and chlorophyll a
synthesis, while at the same time slightly inhibiting the respiration
of Chlorella salina CU-1 (Chan & Chiu, 1985). At higher
concentrations (0.5 or 5%), the growth rate, growth yield, and
photosynthesis were greatly reduced (to 50, 50, and 62% and 43, 32,
and 41% of the control levels, respectively), but the effect on algal
respiration was less severe (81% with 0.5% diesel and 86% with 5%).
Diesel fuel containing dispersants caused greater inhibition than did
the compounds separately.
The lengthwise growth of the benthic algae Ascophyllum nodosum
and Laminaria digitata and other rocky-shore communities was
measured while they were kept in 50-m3 concrete basins and exposed
continuously to diesel fuel for two years (Bokn, 1987). An average
hydrocarbon concentration of 130 µg/litre continuously inhibited
growth in both species, while a concentration of 30 µg/litre caused
periodic inhibition. Under oil-free conditions during the subsequent
season, the plants recovered completely.
Boiler fuel was more toxic than diesel fuel to the kelp
Macrocystis; both fuels reduced photosynthesis (Baker, 1970).
A9.1.2.2 Invertebrates
(a) Several species
The acute toxicity of diesel fuel to Daphnia (Cladocera),
chironomid (insect) larvae, and the mollusc Viviparus bengalensis
(Gastropoda) was tested after the organisms were acclimatized to
laboratory conditions for four, two, and four days, respectively. The
bioassays were conducted at 28 ± 2°C with unchlorinated borehole water
(pH 7.0 ± 0.2; oxygen, 8 mg/litre; free carbon dioxide, 1.2 mg/litre;
total alkalinity as calcium carbonate, 240 mg/litre; hardness,
260 mg/litre). The results are shown in Table 18. Plankton and insect
larvae exhibited erratic, uncoordinated movements before becoming
lethargic. In the mollusc, active avoidance and heavy secretion of
mucus were observed. The concentrations at which these behavioural
changes occurred were not specified (Das & Konar, 1988).
Fourteen species of five phyla (Echinodermeta, Mollusca,
Annelida, Arthropoda, and Urochordata) were exposed to 0.5% No. 2
diesel fuel in sea water. Only the larvae of the echinoderm
Crossaster, which were also the largest, survived up to eight days;
all of the other larvae died 3-72 h after being placed in the
fuel-water mixture (Chia, 1973).
The periwinkles Pachymelania aurita (Muller) and Tympanotonus
fuscatus (Linne) were exposed to refined diesel fuel films for 24
and 48h at concentrations of 2.5, 5.0, 7.0, or 10.0% and to emulsions
for 24 h at concentrations of 0.5, 1.5, 2.5, or 3.5%; they were then
shaken for 2 min with brackish water. After exposure to the emulsion,
the periwinkles were washed and reintroduced into oil-free, brackish
water for 24 h and their activity recorded. Both surface films and
emulsions were harmful to both gastropods, the emulsions being the
more toxic. T. fuscatus was less susceptible than P. aurita,
possibly because the former can retract further into its shell. The
mean percentage survival in the presence of oil film (7.5% diesel
fuel) was 100% at 24 h and 80% at 48 h for T. fuscatus and 80% at 24 h
and 50% at 48 h for P. aurita. The mean percentage survival in the
presence of 3.5% diesel fuel-water emulsion was 90% for T. fuscatus
and 80% for P. aurita (Dambo, 1993).
Table 18. Acute toxicity of diesel fuel to Daphnia (Cladocera),
chironomid larvae (insects), and the mollusc Viviparus
bengalensis (Gastropoda)
Species LC5 (mg/litre) LC50 (mg/litre) LC95 (mg/litre)
Daphnia magna 1.5 (0.8-2.5) 20 (19.2-21.0) 38.5 (37.5-39.5)
Chironomid 5.0 346.0 (238-455) 865.0 (750-1018)
larvae
Viviparus 2.0 254.0 (185-320) 637.0 (575-700)
bengalensis
From Das & Konar (1988)
(b) Molluscs
The feeding rate and growth of mussels ( Mytilus edilus L.) was
markedly reduced when they were exposed to 30 or 130 µg/litre diesel
fuel for eight months (Widdows et al., 1985). Recovery of
physiological responses (clearance rate, respiration, food absorption
efficiency, and ammonia excretion) after transfer to unpolluted water
was concomitant with the depuration of hydrocarbons from the tissues.
Mussels exposed to either dose recovered completely within about 55
days.
The effects on the nutrient storage system and reproductive
cell systems of the mussel M. edilus of exposure to a diesel
hydrocarbon-sea water emulsion and subsequent depuration were studied
in order to assess the capacity of these systems to recover after
discontinuation of exposure to the oil. After 144 days of exposure to
hydrocarbon concentrations of 27 and 128 units, the volume of storage
cell types was significantly reduced and there was a reduction in the
volume of ripe gametes. After 53 days of depuration, the volume of the
storage cells and of developing gametes increased. Exposure to either
concentration also increased the volume of atretic (degenerating)
gametes, but depuration allowed a return to normal (Lowe & Pipe,
1986). The authors suggested that exposure to the hydrocarbons reduced
the storage reserves and increased gamete atresia and resorption, so
that the storage pool was partially replenished, enabling the organism
to tolerate better the hydrocarbon insult.
The sensitivity of M. edulis to pollution with diesel fuel
depends on the salinity of the water (Tedengren & Kautsky, 1987). Low
salinity and diesel fuel acted synergistically to a greater degree in
Baltic than in North Sea mussels.
Adults and larvae of M. edulis were exposed to microencapsulated
diesel fuel at concentrations of 200, 600, 1000, 1300, or
5000 µg/litre and the effects on mortality, larval growth, and
spawning frequency were recorded (Stromgren & Nielsen, 1991). The EC50
for spawning in mussels exposed for 30 days was about 800 µg/litre,
and the LC50 (30days) for maturing mussels was about 5000 µg/litre.
The longitudinal growth of larvae was significantly reduced at
10 µg/litre; the EC50 for growth (10 days) corresponded to about
25-30 µg/litre (Stromgren & Nielsen, 1991), which is lower than the
EC50 of about 1000 µg/litre for growth of juvenile mussels reported
by Stromgren & Reiersen (1988).
Diesel fuel is therefore more toxic to mussel larvae than to
juveniles.
The EC50 values (10 days) for growth of larvae of the Quahog
clam, Mercenaria spp., exposed to water-soluble fractions of various
crude and refined fuels were 220-4200 µg/litre, indicating that the
insoluble fractions of hydrocarbons, ingested as microcapsules, are
far more toxic than the water-soluble fractions alone (Byrne & Calder,
1977). Stromgren et al. (1986) drew the same conclusion from their
results for the growth of juvenile mussels. Larval mortality during
exposure to diesel fuel increased steeply up to 50 µg/litre, at which
dose only 20-30% survived 10 days of exposure. At 500 µg/litre, there
was 100% mortality. Byrne & Calder (1977) reported LC50 values (10
days) of 50-2100 µg/litre for larvae of Mercenaria spp.
(c) Crustaceans
Freshwater crabs (Barytelphusa cunicularis) were exposed to
sublethal concentrations of diesel fuel (4.5, 3.7, 3.2, and 2.6 ppm)
for 24, 48, 72, and 96 h, respectively (Sarojini et al., 1989). The
oxygen consumption of the crabs was measured as an index of stress
caused by the fuel and compared with that of normal controls. The
crabs responded in general by lowering their oxygen consumption up to
8 h (down to 50% at 4 h), particularly at the lower doses; with longer
exposures, the oxygen consumption was higher than (approx. 120% at
24 h) or equal to (at 96 h) that of the controls.
Gammarus spp. (Crustacea, Amphipoda) were obtained from marine
and brackish water in the North and Baltic Seas and tested in two
laboratories for their sensitivity to diesel-fuel pollution (Tedengren
et al., 1988). G. oceanicus was obtained from the Fucus belt at a
depth of 1-3 m and G. duebeni from rock pools of various salinities.
The respiration, excretion, and atomic O:N and O:P ratios of the two
species were compared after a 6-h exposure to 10 mg/litre diesel fuel
emulsified in a syringe and injected into experimental aquaria; the
resulting hydrocarbons were not measured. Exposure to diesel fuel
generally resulted in decreased oxygen consumption and a rise in
nutrient excretion, leading to lowered O:N and O:P ratios.
G. oceanicus was the most sensitive, and the response was aggravated
by simultaneous lowering or raising of the salinity. No signs of
impaired swimming or other physical or mechanical effects were
detected in either species. The investigators concluded that
G. duebenihas a greater tolerance to pollutants and changes in
salinity, probably because it has broad physiological niches and has
evolved in and become adapted to more variable environments.
In a comparison of the effects of an oil-water dispersion of
diesel fuel, kerosene, gasoline, and benzene on the tidepool copepod
Tigriopus californicus, diesel fuel was the most detrimental: a
concentration of 0.10 ml/litre sea water caused 100% mortality within
five days (Barnett & Kontogiannis, 1975).
(d) Fish
Freshwater
Table 19 shows the results of a series of 96-h static tests for
acute toxicity with diesel fuel in freshwater on several species of
juvenile salmonids, Oncorhynchus kisutch (coho), O. gorbuscha
(pink), and O. mykiss (rainbow trout), exposed at 14°C in 16 h of
light and 8 h of darkness (Wan et al., 1990). The average loading
density was 250 mg/litre (range, 100-420 mg/litre) of Canadian diesel
fuel with a pour-point of -17.8°C. Three types of water were used for
dilution: soft acid city tapwater, hard alkaline lakewater, and
intermediate reconstituted deionized city tapwater. The LC50 values
varied between the species from 32 to 33216 mg/litre. Diesel fuel was
more toxic to pink salmon than to coho salmon or rainbow trout,
irrespective of the water type.
The LC values for diesel fuel in golden orfe (Leuciscus idus
melanotus) in a static test were: LC0 = 40-120 mg/litre; LC50 =
120-160 mg/litre; LC100 = 160-205 mg/litre (Juhnke & Lüdemann,
1978).
Various freshwater fish (Fundulus diaphanus, Roccus saxatilis,
Lepomis gibbosus, Roccus americanus, Anguilla rostrata, and Cyrinus
carpio) were exposed to No. 2 or No. 4 fuel oil (both dispersed) in
a static test at 19°C, pH 7.1, and 60 mg/litre hardness (Rehwoldt et
al., 1974). The 96-h LC50 values for No. 2 fuel oil ranged from
22.2 mg/litre for striped bass (Roccus saxatilis) to 49.1 mg/litre
for carp (Cyrinus carpio); those for No.4 fuel oil ranged from
21 mg/litre for banded killifish (Fundulus diaphanus) to 48.1 mg/litre
for carp. These concentrations represent total oil added and not the
oil dissolved in the water column.
Table 19. Acute toxicity of diesel fuel to juvenile Pacific salmonids in different
types of freshwater
Water type Salmonid LC50 (mg/litre
24-h 48-h 72-h 96-h
Soft Coho 28 972 28 972 12 345 10 299
Pink 1 972 276 84 74
Rainbow > 32 000 5 525 4 600 3 017
Intermediate Coho > 55 560 > 55 560 33 216 33 216
(reconstituted) Pink 1 829 376 133 123
Rainbow 168 363 168 363 28 787 2 186
Hard (lake) Coho > 26 743 26 743 4 845 3 333
Pink 1 404 302 48 32
Rainbow > 23 108 23 108 5 102 2 447
From Wan et al. (1990). Coho, Oncorhynchus kisutch; pink, O. gorbuscha; rainbow
trout, O. mykiss
The LC50 values of water-soluble fractions and emulsions and
the floating layer of No. 2 fuel oil for four freshwater species under
static and flow-through conditions are shown in Table 20 (Hedtke &
Puglisi, 1982).
Marine
In an investigation of the threshold doses at which cod (Gadus
morhua L.) detected samples of Ekofisk diesel fuel, sea water was
allowed to gravitate through the diesel fuel. The total content of
dissolved materials, estimated by gas chromatography, were 15%
benzenes, 2% toluenes, 6% xylenes, 15% naphthalenes, 3% phenols, 42%
n-alkanes (C10-C25), and 17% unidentified compounds. There were
no visible surface films or microdroplets in the samples, although the
authors suspected the presence of undissolved microdroplets. Behaviour
was observed with a sea-water olfactometer, and reaction patterns
after injection of samples (snapping, increased activity, coughing,
darting, backing) were noted. The most frequent reaction was snapping
of jaws followed by a short period of activity. None of the doses
produced alarm reactions. The mean activity scores increased with
increasing doses of oil compounds. In a set of four experiments, the
threshold for detection of diesel oil compounds was 100-400 ng/litre,
showing that cod detect fuel compounds at doses lower than those
hitherto observed (Hellstrom & Doving, 1983).
The effect of acute exposure to the water-soluble fraction of
Arctic diesel fuel (1:9 with sea water) on survival and metabolic rate
was studied in an Antarctic fish (Pagothenia borchgrevinki). The
fish proved to be extremely tolerant and withstood an undiluted
water-soluble fraction for at least 72 h. None died as a direct
consequence of contact with the water-soluble fraction, but they
showed signs of stress. In unpolluted water, little spontaneous
swimming activity was seen, but on transfer into water containing
diesel fuel they became agitated and increased their swimming activity
for a short time. Their ventilation rates increased but later
decreased; the depth of each ventilation increased considerably.
Coughing became apparent shortly after transfer and persisted for up
to 72 h. The haematocrit increased and remained elevated throughout
the experiment. Oxygen consumption was increased to about twice that
of the controls (Davison et al., 1992).
In further studies on P. borchgrevinki (Davison et al., 1993),
a water-soluble fraction was prepared (1:2 with sea water) and then
diluted to 33%; the fish were kept for seven days. On initial
placement, behaviour similar to that with the higher concentration in
the previous experiment was noted. After seven days, the only
noticeable differences between exposed and control fish of similar
weight and length were large amounts of mucus streaming from opercula,
increased coughing, and slightly deeper ventilation. Plasma chloride
and osmolarity were similar in the two groups. Haematocrit and
haemoglobin concentration were significantly higher in the treated
fish; spleen weights and oxygen consumption were similar. This
Antarctic fish can thus survive prolonged periods of exposure to low
levels of water-soluble hydrocarbons.
Table 20. 96-h LC50 values for No. 2 fuel oil on freshwater organisms under various conditions
Species Life stage Test conditions Effect Concentration
(µl/litre water)
Jordanella floridae (flagfish) Adult Water-soluble fraction of 10% No mortality No dilution
oil-water mixture; static
Emulsion; flow-through LC50 60.5
Pimephales promelas (fathead minnow) Adult Emulsion; flow-through LC50 38.6
Water-soluble fraction of 10% No mortality No dilution
oil-water mixture; static
Floating layer; static LC50 > 160 000
48 300 (2nd
sample)
Rana sylvatica (frog) Larvae Water-soluble fraction of LC50 413 000
10% oil-water mixture; static
Emulsion; static LC50 26.4
Emulsion; flow-through LC50 4.9
Floating layer; static LC50 < 5000
Ambystoma maculatum (salamander) Larvae Emulsion; flow-through LC50 86.4
From Hedtke & Puglisi (1982)
(e) Amphibians
Larvae of the frog Rana sylvatica were tested in 96-h static and
flow-through tests with No. 2 fuel oil as a water-soluble fraction, an
emulsion, or a floating layer (Hedtke & Puglisi, 1982) (Table 20).
Flow-through exposure to the emulsion was the most toxic (LC50 =
4.9 µl/litre), and static exposure to the water-soluble fraction was
the least toxic (LC50 = 413 000 µl/litre). The LC50 of No. 2 fuel
oil emulsion in larvae of the salamander Ambystoma maculatum in a
flow-through test was 86.4 µl/litre).
A9.1.3 Terrestrial organisms
A9.1.3.1 Plants
Seed germination and growth of soya beans and ryegrass were
inhibited by a diesel fuel spill of 2.3 ml/m2 (Wang & Bartha, 1990;
see sections A3.2.4 and A9.1.1.2). Four weeks after the spill,
biodegradation had at least partially restored the ability of the soil
to support seed germination and plant growth. Morphological effects,
such as reverse geotropism, were noted on some partially emerged
seedlings. In untreated diesel-contaminated soil, there was no
evidence of seed germination or plant growth. Parallel experiments
showed that heating oil was less toxic.
A9.1.3.2 Invertebrates
No data were available.
A9.1.3.3 Vertebrates
Birds are affected externally and internally by oil
contamination. The fuel rapidly destroys the waterproofing of the
birds' plumage, and as the birds attempt to preen the contaminant off
their feathers they ingest fuel. When No. 1 fuel oil was administered
by gavage at doses of 1-4 ml/kg body weight to ducks, a dose of
2 ml/kg caused lipid pneumonia, extreme inflammation of the lungs, and
fatty infiltration and degeneration of the liver after 24 h (Croxall,
1977). Administration of 1 ml of diesel or No. 1 fuel oil/kg produced
severe irritation of the digestive tract (enteritis), with diarrhoea
and bile pigments in faeces. Signs of toxic nephrosis were also noted.
Adrenal enlargement (mainly hyperplasia of cortical tissue),
depression of plasma cholinesterase levels, and incoordination,
ataxia, and tremors were reported at higher doses. The treatment was
not fatal to healthy birds, even at doses up to 20 ml/kg body weight;
however, when No. 1 fuel and diesel fuel were administered to birds
under stress, the LD50 was 3-4 ml/kg body weight (Hartung & Hunt,
1966).
A9.2 Field observations
The following information is derived mostly from observations
made after diesel spills (see Table 10).
A9.2.1 Microorganisms
A9.2.1.1 Water
No data were available.
A9.2.1.2 Soil
Six weeks after the ship Bahia Paraiso spilled Arctic diesel
fuel into the Antarctic Sea, the impact of the spill on microbial
ecology was investigated (Karl, 1992). The acute effects on the
metabolic activities of sedimentary microorganisms appeared to be
negligible, even in saturated sea water.
A9.2.2 Aquatic organisms
In field studies conducted after fuel spills to investigate the
effects on populations and ecosystems, zooplankton appeared to be
highly vulnerable to dispersed and dissolved petroleum constituents
but less so to floating oils. Field observations on oil spills in
general showed that individual organisms were affected in many ways,
including direct mortality (fish eggs, copepods, mixed plankton),
external contamination by fuel (chorion of fish eggs, cuticles, and
feeding appendages of crustacea), tissue contamination by aromatic
constituents, abnormal development of fish embryos, and altered
metabolic rates (US National Research Council, 1985). A spill of
No. 2 diesel fuel was extremely toxic: faunal repopulation of the
affected areas did not occur during the subsequent eight months
(Blumer et al., 1970). A spill of 750000 litres of No. 2 diesel fuel
caused substantial mortality in some taxa of the intertidal
population, whereas others showed little or no effect. There was
substantial recovery within six months, and there was no observable
mortality among the subtidal fauna or flora (Woodin et al., 1972).
After a spill of marine diesel fuel (2000-3000 t) off Hong Kong, much
of the oil was carried to land by onshore winds. Wormald (1976)
described the effects of the oil spill on intertidal meiofauna. Within
four days of the spill, there was almost total mortality: nematodes
and harpacticoid copepods were observed in an advanced state of decay,
and other meiofauna were not detected. The aromatic fractions probably
evaporated within four to six weeks but persisted longer in deeper
sediment. Harpacticoids and nematodes did not recolonize the beach
until nearly eight months after the spill. The density of meiofauna at
all stations observed reached a maximum 10 months after the spill and
subsequently showed large fluctuations, which were probably the result
of ecological successions during recovery of the beach fauna. One year
later, an increase in the number of macrofauna, especially polychaete
worms, was noted, which may have been responsible for the decrease in
meiofaunal populations. High rainfall 11 months after the spill may
have brought the soluble aromatic compounds nearer to the surface and
decreased the harpacticoids and nematode populations. After 15 months,
the meiofauna were again well established. Recovery at high shore
stations began only when the oil content of the sediment had decreased
by at least 50%, primarily through physical dispersion. Recolonization
of the lower shore had occurred earlier. Stirling (1977) studied the
effects of this spill on rocky shore fauna (see also Table 10),
concentrating on large, common species of gastropods (Monodonta labio,
Thais clavigera, Nerita albicilla, N. polita, Lunella coronata,
Clypeomorus humilis, and Planaxis sulcatus), bivalve molluscs (Donax
semignosus, Atactodea striata, Tapes philippinarum, and Circe
stutzeri), and crustaceans (shore and hermit crabs). Acute mortality
of gastropods was greatest at moderately contaminated sites where
oil-dispersing chemicals had been applied to floating oil slicks.
Long-term disturbances were most significant at the heavily polluted
sites where dispersants had not been used. Recovery of animals taken
from the oiled beaches was studied in clean salt water in the
laboratory. Bivalve molluscs and the gastropods Monodonta labioand
Thais clavigera were the most sensitive and Clypeomorus humilisand
Planaxis sulcatus the least sensitive. Field observations of acute
mortality were consistent with this order of sensitivity. Population
studies showed the greatest reductions in Monodonta labio and Nerita
albicilla, which were eliminated for at least 13 months from the
site of greatest oil pollution.
The release of 600 000 litres of Arctic diesel fuel from the
Bahia Paraiso in 1989 coated intertidal macroalgae, limpets, and
birds as well as sediments and shores (Kennicutt et al., 1991b).
Macroalgae (Leptosomia simplex and Monostroma spp.) were only
moderately affected. Intertidal limpet (Nacella concinna) populations
were reduced by 50% within the first few weeks after the spill
(Kennicutt et al., 1990), and a year later had still not fully
recovered (Kennicutt & Sweet, 1992). Bottom-feeding organisms, such as
clams (Laternula elliptica) and fish (Harpagifer anarcticus and
Notothenia coriiceps neglecta) were found to have PAHs in their gut
contents and muscle tissues (Kennicutt et al., 1991b). After the
spill, all of the chicks of the local population of South Polar skuas
(Catharacta maccormicki) died, although few of the adults or the
chicks died from direct contact with oil (Eppley, 1992). Within less
than two years, and at many locations within a few weeks, the PAH
content of sediment and intertidal organisms returned to background
levels (Kennicutt & Sweet, 1992), owing partly to the high volatility
of the spilled fluid and the high energy environment. Sampling of
intertidal limpets showed that the tissue contaminants were primarily
naphthalenes, but fluorenes and phenanthrenes were often the most
abundant aromatic contaminants in tissues with higher concentrations
of PAHs (Kennicutt & Sweet, 1992; Kennicutt et al., 1992b) After a
spill of 8000 litres of diesel fuel into an unspoiled freshwater
stream in California, United States, most animals were affected within
one to four days. Thousands of aquatic insects perished; some
crayfish, aquatic leeches, and freshwater planarians were killed; and
over 2500 fish, tadpoles (but no frogs), snakes, a turtle, and common
merganser (Mergus merganser) were found dead (Bury, 1972).
A9.2.3 Terrestrial organisms
A9.2.3.1 Plants
Diesel fuel was applied at 10 litres/m2 to the soil of five
common plant communities in north-east Greenland, comprising wet
marsh, grassland, and three types of dwarf-shrub heath. The plant
species were observed for up to three years after the application.
Within a few days, most species had lost chlorophyll or had abscissing
leaves. Species with xeromorphic leaf characteristics reacted more
slowly than species with orthophyllous leaves. During three successive
growing seasons, shrubs and forbs showed no signs of recovery.
Graminoids showed slight resistance and recovered in all mesic and wet
plots but were killed in dry plots. Three species of sedge (Carex
bigelowii, C. saxatilis, and C. stans) and mosses recovered to some
extent in all plots. Recovery of mosses was excellent in the wet
plots, good in the mesic plots, and poor in the dry plots. In general,
the phytotoxic effects of diesel fuel were much more pronounced than
those of crude oil in similar plots (Holt, 1987).
A9.2.3.2 Invertebrates
No data were available.
A9.2.3.3 Vertebrates
No data were available.
A10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
A10.1 Evaluation of human health risks
Diesel fuel is produced commercially in various qualities with
regard to volatility, aromaticity, cetane number, and sulfur content.
The composition of diesel fuel, which influences the type and amount
of compounds emitted in the exhaust, has not changed greatly during
the last few decades, although the cetane number has been slightly
increased, resulting in better ignition, and the sulfur content, which
influences the release of particulate matter, has been reduced in some
countries. Owing to the lack of data on diesel fuel, some data on
heating oils and kerosenes (jet fuels) with compositions similar to
that of diesel fuel are included in the evaluations of toxicological
and environmental effects.
A10.1.1 Exposure of the general population
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, when handling such fuels, or when using kerosene for domestic
cooking or heating. No data on exposure were available.
A10.1.2 Occupational exposure
Workers can be exposed to diesel fuel and other middle
distillates during manual handling and discharge of the fuel, i.e.
during retailing at filling stations; manufacture, repair,
maintenance, and testing of diesel engines and other equipment; in
jobs where diesel fuel is used as a cleaning agent or solvent; and in
the handling and routine sampling of diesel fuel in the laboratory. At
room temperature, very low concentrations of vapours are likely to be
generated from diesel fuel because of its low volatility; significant
levels of vapour are likely to occur only in confined spaces and at
high temperatures.
A10.1.3 Non-neoplastic effects
Diesel fuels are toxic when ingested, usually accidentally.
Ingestion may result in regurgitation and aspiration, which can cause
chemically induced pneumonia. The latter effect is, however, not
specific for diesel fuel and can occur with all hydrocarbons of a
particular viscosity range.
Exposure to vapours is minimal during normal handling of diesel
fuel. The most likely effect on human health is dermatitis as a result
of skin contact. Dermal absorption takes place and can result in acute
toxic effects on the kidney. The health effects of long-term
absorption of low levels are unknown, but the available data on acute
human toxicity indicate that practices such as washing hands in diesel
fuel should be avoided. Although groundwater contamination and entry
into drinking-water are potential sources of adverse health effects,
such contamination would be noticeable and affect palatability so that
inadvertent ingestion of contaminated drinking-water is unlikely.
In experimental animals, diesel fuel has little acute toxicity after
exposure orally, dermally, or by inhalation. The oral LD50 values in
rats were > 5000 mg/kg body weight and those in mice, rabbits, and
guinea-pigs even higher. After short-term dermal exposure of mice for
14 consecutive days, the NOAEL for two middle distillates (marine
diesel and JP-5 navy fuel) was 5000-8000 mg/kg body weight per day.
Inhalation of up to 0.2 mg/litre had a neurodepressive effect in mice
but not in rats. Subchronic exposure to various distillate fuels
induced mainly alpha2-microglobulin nephropathy in male rats, which is
not considered relevant for humans.
Female mice that received dermal applications of 250 or 500 mg/kg
body weight per day of marine diesel fuel or JP-5 navy fuel on five
days per week for 103 weeks showed decreased survival due to severe
ulceration. Rats and mice exposed by inhalation to 1 or 5 mg/litre had
significant alterations in organ weight.
Diesel fuel irritates the skin but not the eye.
Exposure to diesel or jet fuels orally or by inhalation was
neither embryotoxic nor teratogenic in rats; although doses toxic to
the dams reduced fetal weight, no effects on viability were seen.
No clear evidence of mutagenic activity was seen in a series of tests
in Salmonella typhimurium. A positive response achieved only under
special conditions appeared to be equivocal. Other tests for
genotoxicity in vitro or in vivo did not show clearly positive
responses.
Owing to lack of data, a quantitative risk assessment is not
possible.
A10.1.4 Neoplastic effects
A case-control study of cancers at several sites in relation to
exposure to diesel fuel suggested an increased risk for lung cancer
other than adenocarcinoma and for prostatic cancer. In neither case
was there an exposure-response relationship. As few studies were
available, the number of cases was small, and the confidence intervals
were correspondingly wide, no conclusion can be drawn at present about
the carcinogenicity to humans of diesel fuel.
Twelve studies of dermal carcinogenicity in animals demonstrated
that diesel fuels have weak carcinogenic potency in mice. As no clear
genotoxicity is seen, cancer may be induced by nongenotoxic
mechanisms, such as chronic dermal irritation characterized by
repeated cycles of skin lesions and epidermal hyperplasia.
A10.2 Evaluation of effects on the environment
Although there are numerous data on the environmental behaviour
of the individual components of diesel fuel, few data are available
on diesel fuel as a whole. The environment can be polluted by
accidental release of diesel fuel on a large scale, such as during
tanker disasters and pipeline leaks, or on a smaller scale, from
contamination of soil around factories and garages.
In water, diesel fuel spreads out almost immediately, polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, volatile components evaporate from the water surface, and
microbial degradation begins. The extent to which such 'weathering'
takes place depends on the temperature and climatic conditions, and
the chemical composition of spills changes with time. After spillage
on water, some fractions evaporate, and the evaporated diesel
components are degraded photochemically. In sediment, diesel fuel is
generally delivered to bottom sediments by settling particles. In
soil, the various components of diesel fuel migrate at different
rates, depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates depend greatly on the physical and
climatic conditions and microbial composition. PAHs are the most
recalcitrant molecules.
Aquatic organisms, in particular molluscs, bioaccumulate
hydrocarbons to various extents, but the hydrocarbons are depurated on
transfer to clean water. Bioaccumulation of diesel fuel may occur, but
there are few data to indicate biomagnification.
Although there have been a number of spills of diesel fuel, few
reports are available on their environmental effects. Generally,
diesel fuel is more toxic than crude oil to aquatic and plant species.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota; recolonization
occurs after about one year, depending on the animal and plant species
involved and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can still be
affected by external contamination and tissue accumulation; abnormal
development and altered metabolic rates are signs of such stress.
There are few data on the effects of diesel fuel on aquatic and
terrestrial organisms in experimental situations. In studies of
water-soluble fractions and emulsions of diesel fuel, the actual
chemical composition has rarely been analysed, although this is
crucial to an understanding of the toxicity of diesel fuel. No data
are available about flow-through conditions or mesocosms, which better
reflect environmental conditions
A11. RECOMMENDATIONS
A11.1 Recommendations for the protection of human health
Exposure to diesel fuel may cause irritation and dermatitis;
therefore, skin contact should be avoided, and diesel fuel should not
be used for cleaning purposes.
A11.2 Recommendation for the protection of the environment
As for all petroleum products, accidental releases to the
environment should be avoided and should be cleaned up as soon as
possible if they occur.
A11.3 Recommendations for further research
A basic data set on the aquatic toxicity of diesel fuels,
including complete analysis of test substances, should be generated.
The fate and environmental impact of diesel fuel spills and leakages
should be investigated in laboratory and controlled field studies.
The exposure of humans to diesel fuel in various situations should be
quantified.
The mechanism of the carcinogenic action of diesel fuel on the
skin of experimental animals should be clarified.
A12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks of diesel fuels for human beings were
evaluated by a working group convened by the International Agency for
Research on Cancer in 1988 (International Agency for Research on
Cancer, 1989a). It concluded that there was inadequate evidence for
the carcinogenicity of diesel fuels in humans, and there was limited
evidence for the carcinogenicity of marine diesel fuel in experimental
animals.
PART B
B1. SUMMARY
B1.1 Identity, physical and chemical properties, and analytical
methods
Diesel engine exhaust emissions contain hundreds of chemical
compounds, which are emitted partly in the gaseous phase and partly in
the particulate phase of the exhaust. The major gaseous products of
combustion are carbon dioxide, oxygen, nitrogen, and water vapour;
carbon monoxide, sulfur dioxide, nitrogen oxides, and hydrocarbons and
their derivatives are also present. Benzene and toluene are present in
the lower weight percent range in the gaseous part of the hydrocarbon
fraction. Other gaseous exhaust components are low-relative-molecular-
mass polycyclic aromatic hydrocarbons (PAHs).
A main characteristic of diesel exhaust is the release of
particles at a rate about 20 times greater than that from
gasoline-fuelled vehicles. The particles are composed of elemental
carbon, organic compounds adsorbed from fuel and lubricating oil,
sulfates from fuel sulfur, and traces of metallic components. Most of
the total particulate matter appears to occur in the submicrometre
range, between 0.02 and 0.5 µm. Agglomeration may occur during aging,
up to a maximal diameter of 30 µm. The emitted particles have a large
surface area. Organic compounds generally contribute 10-30% of the
total particulate matter, but poorly designed and maintained engines
may result in as much as 90%. Higher-relative-molecular-mass,
oxygenated and nitro-PAHs occur at concentrations of parts per million
in this fraction.
Specific transient or steady-state driving cycles are used in
measuring vehicle emissions. The exhaust can be sampled from undiluted
or diluted exhaust gas. It is difficult to obtain artefact-free
samples, as exhaust constituents can undergo chemical reactions,
adsorption and desorption processes, and condensation and diffusion.
The toxicologically relevant PAHs in diesel particulate matter are
usually determined by Soxhlet extraction, clean-up, and fractionation,
with subsequent analysis by high-performance liquid chromatography or
gas chromatography coupled with mass spectrometry.
B1.2 Sources of human and environmental exposure
Diesel engine exhausts are emitted mainly from motor vehicles;
other sources are stationary, railway locomotive, and ship diesel
engines. The emissions from diesel motor vehicles have been well
described, but the individual results are often not comparable owing
to differences in parameters such as driving cycle, engine type, and
fuel composition. The individual components are emitted in the
following quantities: carbon dioxide, about 1 kg/km; carbon monoxide,
nitrogen oxides, total gaseous hydrocarbons, and particulate matter,
0.1-20 g/km; and aliphatic compounds, alcohols, aldehydes, light
aromatics, and PAHs, micrograms per kilometer. The emissions of carbon
monoxide, nitrogen oxides, total gaseous hydrocarbons, and particulate
matter are regulated by law in a number of countries.
In principle, there is no difference between the quality and
quantity of exhaust emissions from light- and heavy-duty engines,
although heavy-duty vehicles release larger relative amounts of
particulate matter. Exhaust emissions depend on driving cycle
(transient or steady state), engine conditions (injection and
aspiration techniques, maintenance, total mileage), and fuel
composition (sulfur content, aromaticity, volatility); adjustment of
the engine plays a major role.
The release of particulate matter increases with decreasing
air:fuel ratio, increasing load, and increasing temperature. More
particulate is released from older, intensively used engines than from
new, low-mileage engines, probably because of a greater consumption of
lubricating oil. The emission of particles from light-duty diesel
vehicles is also correlated with the sulfur content of the fuel, as
the formation of metal sulfates increases the particle mass; particle
emissions from heavy-duty vehicles have not yet been established.
Increasing fuel aromaticity also increases particle emissions.
PAHs and oxygenated PAHs from diesel and spark-ignition engines
are qualitatively similar. Oxygenated and nitrated PAHs are emitted in
the low microgram per kilometer range, but the actual concentrations
of these compounds are uncertain, as decomposition and formation can
occur during sampling. PAH emissions increase with increasing load and
temperature and with the age of the engine, probably owing to
increased consumption of lubricating oil. PAH emissions also depend on
the injection technique of the engine: they increase with increasing
air:fuel ratio in engines with direct injection, whereas they decrease
in engines with indirect injection. The aromaticity and volatility of
the fuel are directly correlated with the emission of PAHs.
Malfunction of engine devices, especially the fuel injection system,
increases the emission of the main exhaust components. There are few
data on the contribution of diesel motor emissions to the total
man-made release of combustion products.
Diesel exhaust emissions can be reduced by improving engine
design and by use of particle traps (trap oxidizers) and catalytic
converters. While particle traps remove both the soot and soluble
organic compounds adsorbed onto the particles, catalytic converters
reduce the levels mainly of carbon monoxide and gaseous hydrocarbons.
In practice, it is dificult to regenerate particulate traps. Catalytic
converters require fuels with a low sulfur content, as sulfur poisons
the active centres of the catalyst.
B1.3 Environmental transport, distribution, and transformation
The compartment first affected by diesel exhaust emissions is the
atmosphere. The hydrosphere and geosphere are contaminated indirectly
by dry and wet deposition. The environmental fate of the individual
constituents of diesel exhaust is generally well known: Particles
behave like (non-reacting) gas molecules with regard to their
mechanical transport in the atmosphere; they may be transported over
long distances and even penetrate the stratosphere. The overall
removal rate of diesel particles is estimated to be low, resulting in
an atmospheric lifetime of several days. During aging, particles may
coagulate, with higher fall-out rates, thus reducing the total
airborne level. The elemental carbon of diesel particulates may act as
a catalyst in the formation of sulfuric acid by oxidation of sulfuric
dioxide. The organic components adsorbed on elemental carbon may
undergo a number of physical and chemical reactions with other
atmospheric compounds and during exposure to sunlight.
B1.4 Environmental levels and human exposure
As diesel exhaust is a complex mixture of a great variety of
compounds, general 'environmental levels' cannot be given. The
individual components of diesel exhaust should be detectable in all
compartments of the environment, although their source cannot usually
be verified. The environmental levels of most of the individual
constituents are known.
The general population is most likely to be exposed to diesel
exhaust in busy streets or parking areas, particularly underground.
The identification of sources is difficult, and the contribution of
diesel exhaust to total pollution by traffic combustion products is
generally calculated on the basis of emission factors and the
percentage of diesel-fuelled vehicles. The levels of exposure of the
general population and workers to diesel particulate matter are
toxicologically relevant. The daily average ambient concentrations of
diesel particulates near roads are 8-42 µg/m3. The estimated annual
average concentrations were 5-10 µg/m3 in urban areas and
< 1.5 µg/m3 in rural areas in Germany and 1-2 µg/m3 in urban
areas and 0.6-1 µg/m3 in rural areas in the United States of America.
The concentrations are directly correlated with traffic density and
decrease with increasing distance from roads. Identification of
sources may also be difficult in work-places, especially in mines,
where the total dust burden is high. Carbon core analysis has been
used to determine specific exposure to diesel exhaust in the
work-place, and the levels of particulate matter to which workers are
exposed have been reported to be 0.04-0.134 mg/m3 for truck drivers
and 0.004-0.192 mg/m3 for railroad workers. Total respirable
particles and total suspended particles have also been used as
measures of occupational exposure.
B1.5 Kinetics and metabolism in laboratory animals and humans
B1.5.1 Deposition
Diesel exhaust particles, with a mass median aerodynamic diameter
of about 0.2 µm, undergo some filtration in the nose, and the
deposition efficiency in the lung is only slightly greater than the
minimal value found for particles with a mass median aerodynamic
diameter of about 0.5 µm. Thus, 10-15% of inhaled diesel soot
particles are deposited in the alveolar region of the lung of rats and
guinea-pigs; in humans, about 10% is deposited in the alveolar region.
B1.5.2 Retention and clearance of particles
Mucociliary clearance of particles from ciliated airways is
almost complete within 24 h. The long-term clearance of diesel
particles from the alveolar region was measured in several studies in
rats exposed by inhalation to labelled diesel or test particles.
Half-times of 60-100 days were reported for controls with low lung
burdens (< 1 mg/lung), whereas the half-times increased to 100-600
days in rats with lung burdens increasing from 1 to 60 mg/lung. In
several studies, effects were seen to be caused by particle overload,
which has been described in a variety of species and with a number of
particulate materials. The phenomenon is generally observed when the
deposition rate of particles of low solubility and low acute toxicity
exceeds their clearance rate for a considerable time. The half-time of
unimpaired alveolar clearance in humans is several hundred days, which
is longer than that in rats.
A dosimetric lung model was developed on the basis of data on the
deposition and retention of diesel particles in rats after long-term
inhalation and of data on particle deposition and retention in humans.
The model can be used to predict retention of diesel particles and
adsorbed organic material in the lungs of people of different ages. No
data are available on changes in the retention of individual compounds
after prolonged exposure to diesel exhaust.
B1.5.2 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
PAHs in diesel soot adhere strongly to the surface of particles.
About 50% of the PAHs adsorbed onto diesel particles is cleared from
the lung within one day, but the retention half-times for the
remaining PAHs were 18-36 days. Studies with 3 H-benzo[ a]pyrene and
14C-nitropyrene show that when PAHs are associated with particulate
matter, their clearance from the lungs is significantly delayed in
comparison with the clearance of inhaled PAHs not associated with
particulate matter.
B1.5.4 Metabolism
Benzo[ a]pyrene coated on diesel exhaust particles was metabolized
by oxidation to benzo[ a]pyrene phenols, diols, and quinones in the
lung and in cell cultures of pulmonary macrophages. Nitropyrene
adsorbed to diesel particles was metabolized to acetylaminopyrene-
phenol after inhalation. More DNA adducts were found in the lungs and
type II cells of rats exposed to diesel exhaust than in controls. The
oxidative metabolism of some organic compounds to epoxides may be
responsible for the formation of these adducts, but adducts were found
only after exposure to particles. Certain organic substances in diesel
exhaust have been shown to be responsible for the formation of DNA
adducts; however, the carbonaceous core itself (without extractable
organic material) can also induce adducts, by chronic damage of
epithelial cells.
B1.6 Effects on laboratory mammals and in vitro test systems
The few data available suggest that diesel exhaust has little
acute toxicity. Mice treated intratracheally with diesel exhaust
particles died due to lung oedema; the LD50 was about 20 mg/kg body
weight. Methanol-extracted diesel exhaust particles were not lethal a
concentrations up to about 33 mg/kg body weight. In hamsters, the LD50
after intraperitoneal administration was 1280 mg/kg body weight. No
data are available for exposure by inhalation.
Exposure of rats, guinea-pigs, and cats to diesel exhaust with a
particle content of 6 mg/m3 for about four weeks altered lung
function, including a 35% increase in pulmonary flow resistance in
guinea-pigs and a 10% decrease in vital capacity (expiratory flow) in
cats. Histopathologically, focal thickening of alveolar walls, a
significantly increased type-II cell labelling index, and
accumulations of particleladen macrophages were found. The
accumulations were located near the terminal bronchioles and became
larger, due to macrophage attachment (sequestration), during a
subsequent recovery period.
Mice, rats, hamsters, cats, and monkeys did not have drastic
decreases in body weight or reduced survival times after long-term
inhalation of up to 4 mg/m3 . Dose-related toxic effects seen in all
species after long-term inhalation of diesel exhaust were: increases
up to 400% in lung weight; pulmonary inflammation measurable by
biochemical (cytoplasmic marker enzymes, collagen) and cytological
(increase in polymorphonuclear neutrophils) parameters; impairment of
lung mechanics; increasing numbers of particle-laden macrophages with
focal accumulations (sequestration) under overload conditions; and
subsequent proliferative alterations of epithelial cells and onset of
fibrosis.
Limited information on reproductive and developmental toxicity
suggests that inhalation of diesel exhaust is not critical. In most
experiments, no effects were seen in mice, rats, hamsters, rabbits, or
monkeys; however, after intraperitoneal injection, sperm abnormalities
were seen in mice given diesel exhaust particles and embryotoxicity
occurred in hamsters given diesel exhaust extract.
Most tests for genotoxicity in vitro have been performed with
diesel exhaust extracts rather than with total exhaust; positive
responses were found in the absence of metabolic activation, i.e. the
genotoxic effects appeared to be PAH-independent. About one-half of
investigations in vivo gave negative responses; the only positive
responses were sister chromatid exchange induction with total diesel
exhaust and with organic extracts and micronucleus induction with
organic extracts.
Immunotoxic effects were generally not seen after inhalation of
diesel exhaust; however, an enhanced anti-ovalbumin immunoglobulin E
antibody titre was seen in one study and an increased susceptibility
to infections in mice in two experiments. Studies in rats suggested
that inhalation of diesel exhaust affects behavioural and
neurophysiological status.
In studies of the carcinogenicity of diesel exhaust administered
by inhalation to rats, the gaseous phase (without the particulate
fraction) was not carcinogenic. In all valid studies in rats, diesel
exhaust was found to be carcinogenic at particle concentrations of >
2 mg/m3, corresponding to an equivalent continuous exposure of about
1mg/m3. No effect was seen in hamsters or mice. In studies by
intratracheal instillation, both diesel exhaust particles and carbon
black induced tumours, and the surface area of the carbonaceous
particles appeared to be correlated with the tumorigenic potency.
Long-term inhalation of carbon black virtually devoid of PAHs at
similar concentrations also resulted in lung tumours in rats.
It is not clear whether the carcinogenicity of diesel exhaust
involves DNA-reactive or non-DNA-reactive mechanisms (or a
combination). Various models have been used to elucidate the
carcinogenicity of diesel exhaust.
B1.7 Effects on humans
Diesel exhaust contributes to air pollution in general. Although
the role of diesel particles cannot be singled out in acute or chronic
studies, they may be partly responsible for the range of health
effects found to be associated with air pollution.
Typical diesel exhaust has a characteristic odour, which some
people find offensive, particularly at high concentrations. The
symptoms seen after acute and chronic exposure to diesel exhaust have
been described in studies and anecdotal reports of occupationally
exposed subjects. Acute exposure to diesel exhaust has been associated
with irritation of the ocular and nasal mucous membranes, and an
increased frequency of respiratory symptoms has been observed in
occupational cohorts; however, the contribution of diesel particulates
is not known. No consistent short-term effect on pulmonary functions
has been found, but asthma attacks have been reported.
In a controlled study in which eight healthy non-smoking
volunteers were exposed to diluted diesel exhaust in a chamber for
60 min, the phagocytosis rate of alveolar macrophages in broncho-
alveolar lavage fluid was reduced.
Some cross-sectional and longitudinal studies on workers with
long-term occupational exposure to diesel exhaust show decrements in
lung function and an increased prevalence of respiratory symptoms, but
these studies are limited by short exposure. Cohort studies in which
deaths from cardiovascular and/or cerebrovascular disease due to
diesel emissions were investigated did not show a significant excess.
The relationships between cancers of the lung and of the urinary
bladder and occupational exposure to diesel exhaust have been
evaluated in a number of epidemiological studies. Only those studies
that were considered relevant for evaluating the carcinogenic effects
of diesel exhaust are included in this monograph. The most relevant
studies with regard to lung cancer are those of railroad workers, bus
garage workers, and stevedores, who have definite exposure to diesel
exhaust. The four most informative studies all reported an increased
risk for lung cancer, with relative risks ranging from 1.4 for
railroad workers and 1.3-2.4 for bus garage workers (depending on
exposure category), to a three- to sixfold increase in risk for
stevedores (depending on the exposure assessment used, but with wide
confidence intervals). Adjustment for smoking was possible in one
case-control study of railroad workers and in the study of stevedores,
and in both cases, the effect of diesel exposure was not materially
influenced. In the three studies in which smoking could not be
adjusted for, the analysis was based on comparisons of subgroups of
the cohorts, so that confounding by tobacco smoking was less likely
than when external comparison groups were used.
Several case-control studies have been conducted to examine the
relationship between urinary bladder cancer and presumed exposure to
diesel exhaust. An increased risk was found, especially for truck
drivers; however, all of these studies are limited by poor
characterization of exposure. Hence, a causative association between
exposure to diesel exhaust and an increased risk for urinary bladder
cancer cannot be established.
B1.8 Effects on other organisms in the laboratory and the field
The effect of diesel emissions as such have been addressed in
only one study, of green algae.
B1.9 Evaluation of human health risks
The risk assessment paradigm of the United States National
Academy of Sciences (US National Research Council, 1983) was used to
assess the risks for both cancer and non-cancer end-points. The four
steps in this process comprise: (1) hazard identification, (2)
dose-response assessment, (3) exposure assessment, and (4) risk
characterization.
Epidemiological studies of long duration with well-defined
exposure and follow-up (> 20 years) are considered to be the most
informative. Four studies of lung cancer in occupationally exposed
individuals met these criteria. The relative risks for lung cancer
associated with exposure to diesel exhaust were generally low and were
susceptible to chance, to the effects of unmeasured confounding
factours, and to the difficulty in accurately adjusting for known
confounding factors. Studies with less precise definitions of exposure
support the conclusions of these studies. Overall, it is considered
that diesel exhaust is probably carcinogenic to humans; however, no
quantitative data are available for estimating human risk.
B1.9.1 Non-neoplastic effects
Two general approaches were used for risk characterization: a
no-observed-adverse effect level (NOAEL) divided by an uncertainty
factor; and a benchmark concentration. In both approaches, a
sophisticated dosimetric model was used which decreases any
uncertainty in interspecies extrapolation of dose.
The no-effect level of diesel exhaust particles in humans was
calculated to be 0.139 mg/m3. The guidance value for the general
population calculated from the dosimetric model was 5.6 µg/m3, and
that calculated without the model was 2.3 µg/m3.
The benchmark concentration approach takes into account the
entire exposure-response relationship rather than relying on a single
data point from studies by inhalation, as in the NOAEL approach. Three
sensitive end-points were identified: chronic alveolar inflammation,
impaired lung clearance, and hyperplastic lung lesions. The benchmark
concentrations, calculated from the same dosimetric model used in the
NOAEL approach, were 0.9-2 µg/m3for inflammation, 1.2-3 µg/m3 for
impaired lung clearance, and 6.3-14 µg/m3 for hyperplastic lesions.
B1.9.2 Neoplastic effects
A linearized multistage model was used to estimate unit risks due
to exposure to diesel exhaust. Because the results of the available
epidemiological studies were considered inadequate for a quantitative
estimate of unit risk, data from several studies of long-term
inhalation in rats were used in which carcinogenesis occurred at
concentrations >2 mg/m3. A unit risk of 3.4 × 10-5 µg/m3 (geometric
mean of four risk estimates) diesel exhaust particles was calculated.
An alternative biologically based model yielded a similar unit risk,
under the assumption that diesel particles affect cell initiation
and/or proliferation at low concentrations.
B1.10 Evaluation of effects on the environment
Insufficient information was available to evaluate the specific
effects of diesel exhaust emissions. The effects of combusted diesel
fuel should be similar to those of other fossil fuels and are related
to the consumption of diesel fuel.
B2. IDENTITY AND ANALYTICAL METHODS
B2.1 Identity
Diesel engine emissions contain hundreds of chemical compounds,
which are emitted partly in the gaseous phase and partly in the
particulate phase of the exhaust. These substances form particles or
contribute to the gaseous phase of the emissions, depending on vapour
pressure, temperature, and the concentration of individual species.
The amount and composition of diesel exhaust are related to engine
conditions and fuel specifications (see sections B3.1.2.1 and
B3.1.2.2). A total of 445 compounds has been identified or tentatively
identified as constituents of diesel exhaust emissions (Westerholm,
1987).
Diesel exhaust has an offensive odour (odour threshold, 320 ppm).
Compounds with a relative molecular mass > 80 mainly determine the
odour (Scheepers & Bos, 1992b). Partridge et al. (1987) concluded that
benzaldehyde and a methylbenzaldehyde isomer are the major substances
responsible; unburnt aromatic hydrocarbons may also contribute.
Aldehyde levels and odour emissions are directly correlated with the
total hydrocarbon content of the exhaust; decreased hydrocarbon levels
lead to diesel exhaust with less odour (Organisation for Economic
Co-operation and Development, 1993).
B2.1.1 Chemical composition of diesel exhaust gases
The main gaseous products of diesel exhaust are carbon dioxide,
oxygen, nitrogen, and water vapour; carbon monoxide, sulfur dioxide,
nitrogen oxides, and hydrocarbons and their derivatives are also
present. A representative composition of diesel exhaust gas
(light-duty engine) is shown in Table 21.
The levels of carbon monoxide, nitrogen oxides, total
hydrocarbons, and particulates emitted by diesel engines are regulated
by law in a number of countries (see section B3.2). The composition of
diesel exhaust gases is similar to that of gasoline engine gases, but
because of the relatively higher fuel:air ratio, carbon monoxide and
hydrocarbons occur in lower concentrations in diesel exhaust, and the
emission of nitrogen oxides, particulate matter, and sulfur compounds
is higher (the latter being due mainly to the higher sulfur content of
diesel fuels). The relative concentrations in the aldehyde fraction
are as follows: about 45% by weight formaldehyde, 17% by weight
acetaldehyde, 14% by weight acetone and acrolein, < 10% by weight
crotonaldehyde, propionaldehyde, isobutyraldehyde, benzaldehyde, and
hexanaldehyde; methylethylketone is also present (Volkswagen AG,
1989).
Table 21. Composition of light-duty diesel engine exhaust
Component Concentration
(% by weight)
Carbon dioxide 7.1
Water vapour 2.6
Oxygen 15.0
Nitrogen 75.2
Carbon monoxide 0.03
Hydrocarbons 0.0007
Nitrogen oxides 0.03
Hydrogen 0.002
Sulfur dioxide 0.01
Sulfates 0.00016
Aldehydes 0.0014
Ammonia 0.00005
Particulates 0.006
Adapted from Volkswagen AG (1989)
Diesel exhaust contains toxicologically relevant compounds such
as benzene, toluene, ethylbenzene, xylene, and PAHs. In the
hydrocarbon fraction, benzene and toluene are present in relative
concentrations of 11 and 4% by weight, respectively (Volkswagen AG,
1989).
Possible sources of PAHs in diesel exhaust are unburnt PAHs from
the fuel, pyrosynthesis of PAHs during combustion, crankcase oils, and
engine and/or exhaust system deposits (Scheepers & Bos, 1992a). These
constituents occur partly in the gaseous fraction of total
hydrocarbons (benzene, toluene, ethylbenzene, and xylene components,
PAHs of lower relative molecular mass) and partly in the particulate
fraction due to adsorption (PAHs of higher relative molecular mass).
The partitioning of constituents between the particulate and gas
phases has been measured by several investigators (Hampton et al.,
1983; Schuetzle, 1983; Schuetzle & Frazier, 1986). Most PAHs with five
or more rings and aliphatic hydrocarbons with more than 18 carbon
atoms are expected to be primarily adsorbed onto particles (Schuetzle,
1983). There is a direct relationship between adsorption behaviour and
vapour pressure (Hampton et al., 1983); compounds with vapour
pressures < 10-5 mbar (7.5 × 10-6 mm Hg; 2.09 kPa) at room
temperature occur predominantly in the particulate phase. An empirical
relationship between boiling-point and particle- to gas-phase
partition coefficients has been derived for PAHs and aliphatic
hydrocarbons in order to estimate gas-phase emission rates (Schuetzle
& Frazier, 1986). According to this equation, the concentration of
e.g. anthracene in the gaseous phase is about 20 times higher than
that in the particle phase, whereas the levels of e.g. pyrene and
fluoranthene are nearly equal in the two phases. Oxygenated PAHs are
found in diesel engine exhaust at concentrations as high (Volkswagen
AG, 1989) or twice as high (Jensen & Hites, 1983) as those of their
parent PAHs, perhaps because these engines have a larger air:fuel
ratio than gasoline motors (Scheepers & Bos, 1992a). 9-Fluorenone and
9,10-anthracenequinone are especially abundant in diesel exhaust
(Schuetzle & Frazier, 1986). In tests of diesel passenger cars by
Volkswagen AG (1989), phenalene-1-one, naphthalene dicarboxylic acid
anhydride, cyclopenta[ def]phenanthrene-4-one, monoaldehydes of
phenanthrene and anthracene, mono- and diketones of benzanthracenes
and benzo[cd]pyrene, and benzofluorenones were also found (for
artefact formation during sampling, see section B2.2.1; for emission
factors, see section B3.1.1.1).
Nitrogen-substituted PAHs are formed during combustion as a
result of either nitration of PAHs by nitric acid or addition of
nitrogen monoxide or dioxide to PAH free radicals generated during
combustion (Schuetzle, 1983). In the exhaust of a four-cylinder diesel
engine, nitrofluorene, dinitrofluorenone, nitroanthracene,
nitrofluoranthene, mono- and dinitropyrenes, and nitrobenzo[ a]pyrene
were detected at concentrations about one order of magnitude lower
than those of oxygenated PAHs (Volkswagen AG, 1989).
A number of investigators have found nitrated PAHs in extracts of
diesel engine exhaust particles (Handa et al., 1983; Hartung et al.
1984). Many three-, four-, and five-ring structures are involved
(Schuetzle et al., 1982). The concentration of 1-nitropyrene, one of
the prevalent species, has been reported to be 15-25 mg/kg particulate
matter, whereas benzo[ a]pyrene has been found at concentrations up
to 50 mg/kg. The nitrated PAHs are important in determining the health
effects of exposure to diesel exhaust, since they are effective
mutagens in microbial and human cell systems (Pederson & Siak, 1981;
Pitts et al., 1982; Patton et al., 1986). Some nitrated PAHs are
carcinogenic to animals (Ohgaki et al., 1985; Imaida et al., 1991).
Combustion products originating from fuel and lubricating oil
additives or from corrosion may also occur. For example, nitrate-based
cetane improvers (see also section A2.1.2) are assumed to form nitro-
PAHs (Organisation for Economic Co-operation and Development, 1993).
B2.1.2 Type and composition of emitted particulate matter
Diesel exhaust is characterized by a higher content (10-20-fold)
of particulate matter than that of gasoline-fuelled passenger cars
(Egebäck & Bertilsson, 1983; Volkswagen AG, 1989; Williams et al.,
1989; CONCAWE, 1990b; Hammerle et al., 1994a). How soot particles are
formed is still under discussion, but the precursor is probably
acetylene generated during combustion. As a result of the numerous
cracking, dehydration, and polymerization processes, quite large
amounts of carbon, hydrogen, hydroxyl, and oxygen radicals are
available in the combustion chamber, which can produce cyclic and
polycyclic aromatic hydrocarbons from acetylene by polymerization and
ring closure. With increasing polymerization, the carbon content of
the macromolecule increases, finally generating graphite-like soot
particles (Klingenberg et al., 1991).
Investigations of two four-cylinder engines with direct and
indirect injection (see also section B3.1.2.1) showed that the emitted
particles range in shape and size from spherical particles of
< 0.01 µm to cluster and chain agglomerates with maximal dimensions
of 30 µm (Dolan et al., 1980; Amann & Siegla, 1982; Klingenberg et
al., 1991). The formation of agglomerates (coagulation, 'aging') is
dependent not only on engine and exhaust conditions but also on
environmental factors, such as photochemical processes and humidity.
Between 50 and 80% of the total particulate emission occurs in the
range of 0.02-0.5 µm (Israel et al., 1982). According to Amann &
Siegla (1982), the emitted particles have a concentric, lamellar
structure arranged around the centre, similar to the structure of
carbon black.
Emitted soot particles are composed of elemental carbon, adsorbed
organic compounds (soluble organic fraction) from fuel and lubricating
oil, traces of metallic compounds (iron, calcium, and zinc at < 0.6%
by weight; Volkswagen AG, 1989), and sulfates. The organic components
of emitted particles are distributed over the surface. Numerous data
on the relative proportions of organic compounds are available
(Horvath et al., 1987; Williams et al., 1987; Volkswagen AG, 1989;
Williams et al., 1989; CONCAWE, 1992). Depending on the engine
conditions and testing cycle, the organic compounds comprise 10-90% of
the total particulate mass. The ranges of various constituents of
particulate matter in nine light-duty and four heavy-duty vehicles
(see also section B3.1.2.1) are shown in Table 22. The driving cycles
used were combined European Union tests (ECE-15 and 'high speed'
extra-urban driving cycle [EUDC]) for the light-duty vehicles and
modified ECE-R49 tests for the heavy-duty engines (see also section
B2.2.1); four fuels with different sulfur contents and an aromatic
content of about 25% by volume were used (CONCAWE, 1992). (For a
description of the influence of fuel composition on exhaust emissions,
see section B3.1.2.2.)
This study showed considerable variation, and no obvious
influence of fuel composition or engine conditions was discernible,
although a difference was seen between fuel and lubricating oil
hydrocarbons derived from light- and heavy-duty vehicles,
respectively. This is probably due to the different driving cycles:
transient in the case of light vehicles, steady state in the case of
heavy-duty engines. In the individual data, adjustment of the engine
appeared to play a major role. In the same study, PAHs were determined
in the soluble organic fraction of the exhausts of three light-duty
vehicles representing different engine conditions and of the four
heavy-duty engines. The data are shown in Table 23. The constituents
of the particulate matter of the exhaust are clearly dependent on the
driving cycle used. Under steady-state conditions (heavy-duty
engines), the PAH content is about one order of magnitude lower than
that under transient conditions (light-duty vehicles). In addition,
transient conditions lead to considerable variations in levels. In the
study of Volkswagen AG (1989) on a four-cylinder diesel engine in the
US-72-test, a hot start was used, and higher levels of fluoranthene,
pyrene, benzo[ ghi]fluoranthene and benzo[ c]phenanthrene were
found, perhaps due to either engine condition or driving cycle.
According to Volkswagen AG (1989), some of the PAHs, and especially
pyrene, benz[ a]anthracene and benzo[ a]pyrene, seemed to decompose
during sampling (see section B2.2.1).
Table 22. Particulate matter content of exhausts from light- and heavy-duty vehicles
Constitutent Particulate matter (% by weight) Reference
Light-duty vehiclesa Heavy-duty vehiclesa
Carbon 32.6-85.1 39.7-81.7 CONCAWE (1992)
82-88 (passenger 68-89 Williams et al.
cars) (1989)b
76-83 (light vans)
60-75 Hammerle et al.
(1994a)c
Fuel-derived 1.1-55.0 9.8-32.5 CONCAWE (1992)
hydrocarbons 6.0-18 Hammerle et al.
(1994a)c
Lubricating 4.2-54.4 4.0-25.9 CONCAWE (1992)
oil-derived 5.7-15 Hammerle et al.
hydrocarbons (1994a)c
Soluble organic 10.2-64.7 14.0-58.4 CONCAWE (1992)
fraction 14-26 (passenger 22-90 Williams et al.
cars) (1989)b
36-48 (light vans)
Sulfate (excluding 0.6-12.2 1.4-7.5 CONCAWE (1992)
bound water) 3.3 (passenger 0.3-6.9 Williams et al.
cars) (1989)b
2.5 (light vans) 1.5-2.0 Hammerle et al.
(1994a)c
a According to the Organisation for Economic Co-operation and Development (1993),
light-duty vehicles are generally classified as vehicles with a weight < 3.5 t,
occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b Measurements for four passenger cars and 15 light vans in different Australian
driving cycles corresponding roughly to US-FTP-72 and -75 tests for heavy-duty
vehicles, and 10 trucks and two buses in one Australian urban traffic driving cycle
c Five passenger cars and light vans in European Motor Vehicle Emissions group cycle
and US-FTP-75 test
Table 23. Polycyclic aromatic hydrocarbon content of the soluble organic fraction of
particulate matter in diesel exhaust
Constituent Soluble organic fraction Referenceb
(mg/kg particulate matter
Light-duty Heavy-duty
vehiclesa vehicles
Fluoranthene 8-238 4-52 CONCAWE (1992)
553-560 Volkswagen AG (1989)
Pyrene 11-287 7-90 CONCAWE (1992)
579-668 Volkswagen AG (1989)
Benzo[ghi]fluoranthene 36-61 3-6 CONCAWE (1992)
+ benzo[c]phenanthrene 163-179 Volkswagen AG (1989)
Benz[a]anthracene 9-285 1-16 CONCAWE (1992)
61-82 Volkswagen AG (1989)
Triphenylene and 14-138 4-13 CONCAWE (1992)
chrysene 117-138 Volkswagen AG (1989)
Benzo[b,j]fluoranthene 4-87 1-10 CONCAWE (1992)
180-193c Volkswagen AG (1989)
Benzo[a]fluoranthen 6-69 CONCAWE (1992)
Benzo[e]pyrene 8-40 1-2 CONCAWE (1992)
85-90 Volkswagen AG (1989)
Benzo[a]pyrene 2-48 1-3 CONCAWE (1992)
38-54 Volkswagen AG (1989)
Perylene 5-32 2 CONCAWE (1992)
4-8 Volkswagen AG (1989)
Dibenz[a,j]anthracene 10-78 CONCAWE (1992)
Indeno[123-cd]pyrene 6-54 CONCAWE (1992)
55-73 Volkswagen AG (1989)
Dinbenz[a,h]- or 3-40 CONCAWE (1992)
-[a,c]anthracene
Benzo[ghi]perylene 25-65 1-5 CONCAWE (1992)
75-82 Volkswagen AG (1989)
Anthracene 3-7 CONCAWE (1992)
Dibenz[a,i]pyrene 8-31 CONCAWE (1992)
Coronene and 9-52 CONCAWE (1992)
dibenzo[a,e]pyrene 33-48d Volkswagen AG (1989)
a According to the Organisation for Economic Co-operation and Development (1993),
light-duty vehicles are generally classified as vehicles with a weight < 3.5 t,
occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b From Volkswagen AG (1989): US-72 tests with one four-cylinder engine
c Including benzo[k]fluoranthene
d Excluding dibenz[a,e]pyrene
B2.2 Analytical methods
B2.2.1 Sampling
Driving cycles are specified to simulate on-road conditions for
measuring vehicle emissions (Grimmer et al., 1988). The test
procedures can be divided in two basic types: steady-state and
transient cycle tests. In the European Union and Japan, mainly
steady-state tests are used, in which emissions from engines are
measured at specified combinations of speed and load. The most widely
used official tests for light- and heavy-duty vehicles are as follows:
Light-duty vehicles:
* US FTP-75: transient cycle; cold start; driving distance, 17.8
km; 10-min break after 28.7 min (United States) (Volkswagen AG,
1989)
* US SET: transient cycle; 'sulfate emission test'; hot start;
simulating rush-hour freeway driving; driving distance, 21.7 km
(United States) (Volkswagen AG, 1989)
* ECE 15: transient cycle; cold start; simulating city-centre
driving; driving distance, 4.052 km (European Union) (CONCAWE,
1990a)
* US HDC: steady-state cycle; 'highway driving cycle'; hot start;
driving distance, 16.5 km; maximal speed, 96.4 km/h (United
States) (Volkswagen AG, 1989)
* EUDC: steady-state cycle; 'extra-urban driving cycle'; hot start
(after ECE 15); driving distance, 6.755 km; maximal speed,
120km/h (European Union) (CONCAWE, 1990a)
Heavy-duty vehicles:
* HD-FTP: transient cycle; 'heavy-duty federal test procedure';
simulating metropolitan driving (United States) (Organisation for
Economic Co-operation and Development, 1993);
* ECE 13: steady-state cycle at 13 different modes; identical to
ECE R49 (European Union) (Organisation for Economic Co-operation
and Development, 1993). Various bus cycles are also used to test
heavy-duty vehicles that are characterized by frequent
stop-and-go driving (Westerholm & Egebäck, 1991; Prakash et al.,
1992).
It is difficult to avoid artefacts in sampling. Exhaust gas
constituents can undergo chemical reactions, adsorption and desorption
processes, and condensation or diffusion. For example, oxygenated and
nitrated PAHs are chemically unstable, and artefacts may form on the
sampling device, especially if large sampling volumes are needed (Lee
& Schuetzle, 1983). Both decreases and increases in the concentrations
of PAHs and oxy-PAHs were observed during sampling in a study by
Volkswagen AG (1989); in particular, degradation of pyrene and
nitropyrene and formation of naphthalene dicarboxylic acid anhydride
on the filter occurred. PAHs can be converted into nitroarenes (Pitts
et al., 1978; Lee et al., 1980; Gibson et al., 1981; Lee & Schuetzle,
1983; Schuetzle & Perez, 1983; Gaddo et al., 1984; Levsen et al.,
1988).
The sampling techniques used for the collection of the gaseous
and particulate phases of diesel exhaust comprise dilution tube
sampling and raw gas sampling.
B2.2.1.1 Sampling from undiluted exhaust gas (raw gas sampling)
The device for sampling motor vehicle exhaust (total flow method;
Kraft & Lies, 1981; Grimmer et al., 1973) consists of a glass cooler,
a condensate separator, and a special micron filter of
paraffin-impregnated glass-fibre material providing separation of
> 99.9% of particles of 0.3-0.5 µm. The cooler is fed with cold tap
water, and the filter is placed on top of the cooler to remove any
uncondensed liquid or solid exhaust gas constituents that may pass
through the cooler coil. The particulate filter retains undissolved
particles of the exhaust gas, while the condensate consists of the
dissolved components (Volkswagen AG, 1989).
The US Environmental Protection Agency (Lies et al., 1986)
defines the 'particulate phase' as all materials (with the exception
of condensed water) which, at a temperature of < 51.7°C, are retained
on a special filter after dilution with air. Large volumes are
collected with this type of sampling, making analysis of trace
components difficult. The sample volumes are reduced by diverting the
exhaust emission flow and sampling only a part. It is difficult to
ensure a representative partial flow, as the amount of exhaust gas
produced varies considerably over time (Volkswagen AG, 1989).
B2.2.1.2 Sampling from diluted exhaust (dilution tube sampling)
The exhaust is mixed with ambient air in a dilution tunnel in a
constant volume sampler method (Behn et al., 1985). The total flow of
air and exhaust is kept constant with a suitable fan in a constant
volume sampler unit. This sampling technique more realistically
reflects the immediate dilution of exhaust leaving the tailpipe of
vehicles on the road. The concentrations of exhaust components are,
however, reduced in accordance with the degree of dilution (magnitude,
1:10), and components that are present only at low concentrations
cannot be determined.
There are various techniques for sampling gaseous constituents.
Substances present at sufficiently high concentrations are commonly
sampled in a plastic bag; adsorption onto solid materials, consisting
of extremely porous, tiny plastic pellets (e.g. Tenax; diameter,
0.1-0.2 mm; specific surface area, 20-50 m2/g), or absorption in
liquids are also used. Particulate constituents are sampled by
filtration (Lee & Schuetzle, 1983; Volkswagen AG, 1989).
B2.2.2 Extraction from particles
PAHs adsorbed onto diesel exhaust particles are of toxicological
relevance, and there are numerous methods for extracting these
constituents. The most frequently used method is Soxhlet extraction,
which permits extraction of 99% of the soluble organic fraction within
8 h; an additional 16 h are necessary to extract the remaining 1%.
Extraction is frequently carried out by sequentially changing the
solvents, from polar to nonpolar, e.g. toluene, toluene or n-propanol,
dichloromethane, and acetonitrile. PAHs may be further extracted by
refluxing the exhaust particulates in solvent for 1 h. Sublimation and
extraction by ultrasonic agitation are also used (Lee & Schuetzle,
1983; Levsen, 1988), and supercritical fluids such as carbon dioxide
are effective (Hawthorne & Miller, 1986). The extraction efficiency of
various solvents is still under discussion (Köhler & Eichhoff, 1967;
Swarin & Williams, 1980; Schuetzle & Perez, 1981; Grimmer et al.,
1982; Lee & Schuetzle, 1983; Schuetzle et al., 1985; Levsen, 1988).
Aromatic solvents and their combinations seem to be best suited for
extracting PAHs from diesel particulates (Levsen, 1988).
B2.2.3 Clean-up and fractionation
Complex fractionation schemes for separating the exhaust extract
into aliphatic compounds, aromatic compounds, and moderately and
highly polar fractions after the removal of acidic and basic fractions
have been described by Petersen & Chuang (1982) and Levsen (1992).
During fractionation, further artefacts may be produced by the
components of a complex sampling mixture like diesel exhaust,
especially with acidic and basic extraction and aggressive column
separation procedures.
B2.2.4 Chemical analysis
Chemical analysis of separated, cleaned compounds of diesel
exhaust involves numerous techniques, including thin-layer
chromatography, gas chromatography, gas chromatography with mass
spectrometric detection, and high-performance liquid chromatography
(US National Institute for Occupational Safety and Health, 1991).
Jinno et al. (1986) determined PAHs by supercritical fluid
chromatography with a photodiode array detector.
The detection methods vary widely and include ultra-violet,
visible, and fluorescence spectrophotometry, mass spectrometry, and
more or less specific devices, such as flame ionization, nitrogen
flame ionization, and sulfur-specific and electron-capture detectors
(US National Institute for Occupational Safety and Health, 1991). A
thermal optical method for determining elemental carbon is currently
being used as a surrogate for measurement of worker exposure in almost
all internal investigations at the US National Institute for
Occupational Safety and Health (Niemeier, personal communication,
1994).
Analytical methods are available for determining the organic
constituents of diesel particulate matter, with special emphasis on
the determination of PAHs and their nitrated and oxygenated
derivatives (Levsen, 1988).
B2.3 Conversion factors
Because diesel exhaust emissions are complex mixtures of
different gases and particles, it is impossible to give a conversion
factor for converting parts per million to SI units.
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Anthropogenic sources
Diesel exhaust emissions are exclusively man-made and are
distributed primarily in the atmosphere. The hydrosphere and geosphere
may also be affected owing to dry and wet deposition of the exhaust
constituents; however, determination of the source of deposited
compounds is difficult. No data are available on the individual
components of diesel exhaust and their deposition.
B3.1.1 Diesel exhaust emissions
Diesel fuels are widely used as transport fuels, and exhaust
emissions are released mainly by road traffic. Although many
investigations of these emissions have been reported, the individual
results are frequently not comparable because the experimental
parameters, e.g. driving cycles, are different, or different
dimensions were chosen. For example, the results of tests on emissions
from light-duty vehicles refer mainly to kilometres driven, whereas
those for heavy-duty vehicles are related to the engine power in
kilowatt-hours. Furthermore, the statistical power of studies of
emission factors is often limited by the small number of vehicles or
engines examined. Some investigators change more than one parameter
(e.g. sulfur content or aromaticity of the fuel) during their studies,
so that the specific cause of changes in exhaust composition is
difficult to assess. Adjustment of individual engine and fuel
parameters plays a major role in determining the quality and quantity
of exhaust emissions (see sections B3.1.2.1 and B3.1.2.2).
Further sources of diesel exhaust emissions include off-road and
stationary engines (reciprocating or gas turbine), electricity
generating plants, pipeline pumping, gas compression, commercial and
industrial furnace installations, and space and water heaters in
plants and factories (see section A3.2.1.2). Diesel exhaust is also
emitted from marine and inland ships' engines; residual fuels and
other heavy grades (for example Bunker C) are used in ocean shipping
(von Meyerinck, personal communication, 1994).
B3.1.1.1 Emission of chemical constituents with the gaseous portion
of diesel exhaust
The exhaust emissions from diesel-fuelled vehicles are generally
given as milligrams of component emitted per kilometre of driving
distance (Egebäck & Bertilsson, 1983; Westerholm et al., 1986;
Westerholm, 1987; Volkswagen AG, 1989; CONCAWE, 1990b; Westerholm &
Egebäck, 1991; Prakash et al., 1992; Scheepers & Bos, 1992a). Data for
verifying tests are issued by national regulatory bodies, e.g. the
German Federal Office for Motor Vehicles (1993, 1994). Depending on
the testing cycle, diesel engine, and fuel (see sections B3.1.1.1 and
B3.1.1.2), the data show considerable differences even though the
values are of the same order of magnitude. Emission factors used in
different regions to estimate the effects of regulated chemical
constituents (carbon monoxide, nitrogen oxides, and hydrocarbons) in
air are shown in Table 24.
Like all hydrocarbon fuel-burning engines, diesel engines release
significant amounts of carbon dioxide. Westerholm & Egebäck (1991)
measured emission factors of about 1 kg/km from a bus and a truck in
the US transient cycle and the bus cycle. In measurements of emissions
from six buses in four driving cycles (US transient cycle and three
bus cycles), Prakash et al. (1992) found emission factors up to about
4 kg/km, especially in the bus cycles. For five European diesel
passenger cars and one light van, Hammerle et al. (1994a) determined
emission factors of 162-252 g/km carbon dioxide in the European Motor
Vehicle Emissions cycle and the US FTP-75 test. Diesel engines are the
most efficient of all common types of combustion engines, with peak
thermal efficiencies typically > 40%, owing to the relatively large
air:fuel ratio during combustion (see section B3.1.2.1) (Organisation
for Economic Co-operation and Development, 1993).
Table 24. Emission factors for diesel vehicles in 1985 used as standards for estimating total impact
Region Passenger cars Commercial vehicles
g/km g/kg Average < 5 t > 5 t
g/km g/kg g/km g/kg g/km g/kg
Carbon monoxide
Denmark 1.0 2.0
Germanya 1.5 20 16
Netherlands 2.7 35 3.9 14 2.0 20 4.6 14
European Union 19 19 16
Austria 22.8 22.8 20
Sweden 10.5
Switzerland 4.0
United Statesb 3.1-6.2
Nitrogen oxides
Germanya 1.0 14 58
Netherlands 0.8 10 18.3 66 0.6 6 22.8 69
European Union 14 18 62
Sweden 39
Switzerland 14.0
United Statesb 3.1
Hydrocarbons
Germanya 0.5 7 12
Netherlands 0.6 10 4.6 17 0.9 9 6.3 19
Table 24 (contd)
Region Passenger cars Commercial vehicles
g/km g/kg Average < 5 t > 5 t
g/km g/kg g/km g/kg g/km g/kg
Hydrocarbons (contd)
European Union 10 13 9
Sweden 3.9
Switzerland 3.0
United Statesb 1.2
Adapted from Organisation for Economic Co-operation and Development (1993)
a Excluding former German Democratic Republic
b 1975-83, trucks tested in the US transient cycle
Cyanides, ammonia, and sulfur dioxide are detectable in the
gaseous portion of diesel exhaust. The average emission factors given
by Volkswagen AG (1989) for seven passenger diesel cars with four- and
five-cylinder engines in three driving cycles (US 75 test with cold
start, US sulfate emission test, US highway driving test) were
0.6 mg/km cyanides, 1.1 mg/km ammonia, and 233.0 mg/km sulfur dioxide.
The emission of individual organic components and classes of
compounds, summarized as total hydrocarbons, are shown in Table 25,
which provides an overview of the quantities of individual substances
emitted in diesel exhausts. Heavy-duty vehicles release more
low-boiling hydrocarbons than passenger cars; however, the difference
could be due to the driving cycle used, which involves frequent
changes of speed and load (see section B2.2.1). The emissions of
adsorbed organic components, such as PAHs (see section B3.1.1.2), are
correlated with the release of particulate matter.
B3.1.1.2 Emission of particulate matter and adsorbed components in
diesel exhaust gases
The emission of total particulate matter from diesel exhaust is
shown in Table 26. Analysis of the particulate matter released from
heavy-duty vehicles gave the following emission factors (Westerholm &
Egebäck, 1991): 170-370 mg/km carbon, 8-71 mg/km soluble organic
compounds derived from fuel, 56-167 mg/km soluble organic compounds
derived from lubricating oil, 1.4-37 mg/km soluble sulfates, and
0.6-3.7 mg/km nitrate. Comparable values were not available for
light-duty vehicles.
As PAHs and oxygenated PAHs from diesel and spark-ignition
engines are qualitatively similar, their differentiation in the
environment is difficult (Behymer & Hites, 1984); however,
benzo[ b]naphtho-[2,1-d]thiophene is specific for diesel exhaust and
can therefore be used as a marker (Grimmer et al., 1979). Emissions of
PAH components are shown in Table 27.
In experiments with one bus and one truck, Westerholm & Egebäck
(1991) detected 30-220 µg/km of particulate-associated PAHs. The
compounds measured included dibenzothiophene and its 4- and 3-methyl
derivatives, 2-methylfluorene, phenanthrene and its methylated
derivatives, anthracene and its 2-methyl derivative, fluoranthene,
pyrene and its 1- and 2-methyl derivatives, retene, benzo[ a]fluorene,
benzo[ ghi]fluoranthene, cyclopenta[ cd]pyrene, benzo[ a]anthracene,
chrysene or triphenylene, benzo[ b,k]fluoranthene, benzo[ e]pyrene,
benzo[ a]pyrene, perylene, indeno[1,2,3-cd]fluoranthene,
indeno[1,2,3-cd]pyrene, picene, benzo[ ghi]perylene, and coronene.
The emission factors for 1-nitropyrene were 0.13-7.3 µg/km.
Table 25. Emission factors for individual organic constituents and
classes of compounds in the gaseous portion of diesel
exhaust
Class of compound Emission factor (mg/km)
or constituent
Light-duty vehiclesa Heavy-duty vehiclesa
Straight-chain aliphatic hydrocarbons
Sum of C1-C4 5.3-6.6 (C1 + C2)b
< 1 - < 143c
Sum of > C4 < 1-5c
Olefins
Ethylene 25.6-90.7d 32-135e
13.5-16.9b 19.0-82.9f
< 1-31c
Propylene 7.3-22e
8.1-44.0f
1,3-Butadiene 0.2g
Acetylene 5.7-7.4b 27.9-106.9 (+ ethane)f
Alcohols Methanol 1.6d
Ethanol < 1.2-22.2d
Aldehydes
Formaldehyde 8.1b 60-255e
7.9-20.7d 140-150h
4.2-9.1g 19.0-207.4 f*
Acetaldehyde 3.1b 18-137e
< 22-36d 40-50h
2.0-4.4g
Crotonaldehyde 1.2b
Acrolein < 1-1.8d 8.5-36e
4h
Benzaldehyde 0.6b 10-14h
Light aromatic compounds
Benzene 2.6-4.3b 4.5-13.1e
ND-6c 6.9-30.5f
9.5-18.1d
0.15-3.47g
Table 25. (cont'd)
Class of compound Emission factor (mg/km)
or constituent
Light-duty vehiclesa Heavy-duty vehiclesa
Toluene 1.1-1.9b 2-13.7e
ND-2c 7.4-32.5f
< 1.0d
Ethylbenzene NDc
Xylenes NDc 7.8-41.7f
Phenols 0.7-1.5b
ND, not determined
a According to the Organisation for Economic Co-operation and
Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty
vehicles are those weighing > 5 t.
b From Volkswagen AG (1989): various numbers of diesel passenger cars
in three driving cycles (US-75 test with cold start, US sulfate
emission test, US highway driving test); determination of aldehydes
only in the US-75 test
c From Scheepers & Bos (1992a): two diesel passenger cars (< 50 000 and
> 100 000 km on odometer) in ECE test, 15 min at 90 km/h and 15 min
at 120 km/h
d From Egebäck & Bertilsson (1983): two passenger cars in US-78 test
e From Westerholm & Egebäck (1991): a bus and a truck in bus cycle with
different diesel fuels
f From Prakash et al. (1992): one bus in four driving cycles (US
transient cycle, three bus cycles) with the exception (*) of eight
buses in the same driving cycles
g From Hammerle et al. (1994a): five passenger cars and one light van
in European Motor Vehicle Emissions Group cycle
h From Westerholm et al. (1986): one heavy-duty vehicle on a chassis
dynamometer with two diesel fuels
i Mainly phenols, cresols and xylenols
Table 26. Emission factors for total particulate matter from diesel exhaust
Measurement conditions Emission factor (mg/km) Reference
Light-duty vehiclesa
Seven passenger cars in three driving cycles 166.4-238.7 Volkswagen AG (1989)
(US-FTP-75 test with cold start; US sulfate emission
test; US highway driving test)
Four passenger cars, 15 light vans in three 180-250 (passenger cars) Williams et al. (1989)
Australian driving cycles (corresponding roughly to 360-440 (light vans)
US-72 and US-75 tests)
Seven passenger cars, two light vans in combined 70-365 (passenger cars) CONCAWE (1990b)
ECE 15/EUDC test with four diesel fuels 233-609 (light vans)
Various Germanyb, 300 Organisation for Economic
Netherlands, 600 Co-operation and Development
Sweden, 400 (1993)
61 passenger cars and 30 light vans without catalyst 39-188 (mean, 104) Federal Office for Motor Vehicles
(optimally tuned) incombined ECE 15/EUDC test (1993)
73 passenger cars and 45 light vans with catalyst 48-127 (mean, 90) Federal Office for Motor Vehicles
(optimally tuned) in combined ECE 15/EUDC test (1993)
Table 26 (contd)
Measurement conditions Emission factor (mg/km) Reference
44 passenger cars and 8 light vans without catalyst 52-117 (mean, 95) Federal Office for Motor Vehicles
(optimally tuned) inUS FTP-75 test (1993)
118 passenger cars and 34 light vans with catalyst 50-118 (mean, 71) Federal Office for Motor Vehicles
(optimally tuned) inUS FTP-75 test (1993)
Five passenger cars and 1 light van in European Motor 52-117 Hammerle et al. (1994a)
Vehicle Emissions Group cycle and US FTP-75 test
Heavy-duty vehiclesa
One vehicle on chassis dynamometer (bus cycle); 680-1000 Westerholm et al. (1986)
two diesel fuels with different contents of sulfur and
aromatic compounds
10 trucks, 2 buses in one Australian driving cycle 800-7150 Williams et al. (1989)
simulating urban traffic
One bus, one truck in two driving cycles (bus cycle; 230-600 Westerholm & Egebäck (1991)
US transient cycle) with two diesel fuels
106 trucks and buses in ECE-R49 driving cycle 0.056-0.606 g/kWh Federal Office for Motor Vehicles
(1994)
Table 26 (contd)
Measurement conditions Emission factor (mg/km) Reference
Various Netherlands, 800 (< 5 t) Organisation for Economic
3300 (> 5 t) Co-operation and Development
Sweden, 400 (< 5 t) (1993)
1700 (> 5 t)
13 trucks and buses (three with particulate trap, 584-804 (without trap) Lowenthal et al. (1994)
10 without) in bus cycle with two diesel fuels 67-206 (with trap)
a According to the Organisation for Economic Co-operation and Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b Without the former German Democratic Republic
Table 27. Emission factors for polycyclic aromatic hydrocarbons in
diesel exhaust
Constituent Emission factor (µg/km)
Light-duty vehiclesa Heavy-duty vehiclesa
Anthracene 17-63b 0.9-3.2c; 9-26d;
3-14d+; 1.6e
Phenanthrene 140f 4.2-25c
295-524b 163-308d; 79-186d+;
12.2e
Fluoranthene 172f 10-39c
58-200g 27-34d; 14-17d+
60.4-79.7g 13.0e
Pyrene 186 15-102c
< 0.9-22 28-31d; 9-21d+
66.6-87.1 22.6e
Chrysene 40 9.5-26c
14-67 8-32d; 4-12d+
10.7-22.3 Not measurede
Benzo[b,k]fluoranthene 40 1.6-8.4c
2.6-47 9-12d,h; 2-8d,h+
10.3-15.2 5.6e
Benzo[ghi]fluoranthene 40f 1.4-13c; 6.9e
Cyclopenta[cd]pyrene 2f 2.5-11c
0.81ag* 1.4e
Benzo[e]pyrene 3-38b 1.6-23c
13.8-16.9 5-9d; 1-7d+; 2.6e
Benzo[a]pyrene 10 0.8-12c
< 1-19 2-3d; 0.4-2d+
4.0-5.3 1.3e
Benzo[a]anthracene 25 3.0-12c
8-43 5-12d; 1-4d+
2.7-3.9 3.6e
Indeno[123-cd]pyrene 15f 0.7-15c
3.5-4.4g
Benzo[ghi]perylene 20 0.6-30c
< 1-18 2-4d; 0.5-1d+
8.7-11.7 Not detected
(detection limit
not given)e
Table 27 (contd)
Constituent Emission factor (µg/km)
Light-duty vehiclesa Heavy-duty vehiclesa
Perylene < 1-2b Not measuredc
0.5-0.6g 1.0e
Coronene 10f < 0.1-12c
5.43g* Not detected
(detection limit
not given)e
a According to the Organisation for Economic Co-operation and
Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty
vehicles are those weighing > 5 t.
b From Scheepers & Bos (1992a): two diesel passenger cars (< 50 000
and > 100 000 km on odometer) in ECE test
c From Westerholm et al. (1986): one heavy-duty vehicle on a chassis
dynamometer (bus cycle)
d From Lowenthal et al. (1994): 13 trucks and buses (three with
particulate trap [d+], 10 without) in bus cycle
e From Rogge et al. (1993): two heavy-duty vehicles in special
driving cycle
f From Egebäck & Bertilsson (1983): average of two passenger cars in
US-78 test
g From Volkswagen AG (1989): seven passenger cars in three driving
cycles (US-75 test with cold start, US sulfate emission test, US
highway driving test) with the exception (*) of one four-cylinder
engine, US-72 test with hot start
h Isomers not specified
The emission of oxygenated PAHs by two diesel passenger cars (one
four-cylinder and one five-cylinder engine, US 72 test with hot start)
was 4-40 µg/km. In a comparable test (one four-cylinder engine, same
driving cycle), the emission of nitro-PAHs was 0.1-3.5 µg/km. As a
result of possible artefact formation during sampling (see section
B2.2.1), these measurements are uncertain (Volkswagen AG, 1989). It is
suspected that nitrate-based cetane improvers (see section A2.1.2)
could increase nitro-PAH emissions in exhaust (Organisation for
Economic Co-operation and Development, 1993).
There are no significant differences in the emissions of adsorbed
single substances between light- and heavy-duty vehicles, although
heavy-duty vehicles release larger relative amounts of particulate
matter (Salmeen et al., 1985).
The chlorine content of diesel fuel is typically < 1 ppm
(Oehme et al., 1991). Thus, no dioxins were found in the exhaust of
heavy vehicles, at a detection limit of 100 pg/litre of fuel (Marklund
et al., 1990). The dioxin emissions from one light-duty (indirect
injection) and one heavy-duty (direct injection) diesel engine under
different load conditions on a chassis dynamometer were 0.009-0.141 ng
of toxicity equivalents per litre of fuel consumed. The highest value
was reported in emissions from the light-duty engine at full load.
Overall, the emissions were of the same order of magnitude as the
dioxin emissions from a petrol-fuelled engine with a catalytic
converter (Schwind et al., 1991).
B3.1.2 Parameters that influence diesel exhaust emissions
B3.1.2.1 Engine conditions
In general, diesel engines are operated with excess air; the
air:fuel ratio is about 1.5, and at lower air:fuel ratios, much more
smoke is emitted (Organisation for Economic Co-operation and
Development, 1993). Wide local variations in the equivalence ratio and
the temperature of the mixture are caused by the heterogeneous nature
of the combustion process. To avoid over-rich mixtures and to improve
the air:fuel mixing processes, wall wetting and delay of fuel
injection are used (CONCAWE, 1986). In naturally aspirated engines,
the amount of air in the cylinder is independent of the power output.
For these engines, the maximal power output is limited by the amount
of fuel that can be injected without smoke formation. In turbocharged
engines, however, increasing the amount of fuel injected increases the
energy in the exhaust gas, and the turbocharger pumps more air into
the combustion chamber. For this reason, the power output of
turbocharged engines is not, in general, limited by smoke. Low
air:fuel ratios can occur only during transient acceleration. Most
turbocharged engines are further equipped with a cooling system
(intercooler) in which compressed air reduces adverse thermal effects.
In both types of engines, fuel is injected either directly into the
combustion chamber or indirectly into a separate pre-chamber where it
is mixed and partly burnt before reaching the main combustion chamber
(Organisation for Economic Co-operation and Development, 1993).
Scheepers & Bos (1992a) reported that most heavy-duty vehicles have
direct injection motors, and indirect injection is used for light-duty
vehicles.
The most important influences of engine conditions on diesel
exhaust emissions are summarized in Table 28. Contrasting results are
sometimes obtained with regard to the effect of engine conditions on
diesel exhaust emissions. Adjustment of the engine plays a major role
(CONCAWE, 1990b), which was slightly less in the US FTP-75 test cycle
than in the European cycle.
Table 28. Influence of engine conditions on diesel exhaust emissions
Condition Change Influence on exhaust emissions Reference
Air:fuel ratio Increase Increased emissions of hydrocarbons and particulate soluble Organisation for Economic
fraction; decreased particulate and nitrogen oxide emissions Co-operation and
from turbocharged and intercooled engines Development (1993)
Increased PAH emissions, decreased particulate emissions from Barbella et al. (1988)
direct injection engines; decreased PAH and particulate emissions
from indirect injection engines
Increased nitrogen oxide emissions, decreased particulate Iida et al. (1986)
emissions from direct injection engines
Injection timing Advance Increased particulate emissions from indirect injection engines Du et al. (1984); Williams et
al. (1989)
Decreased particulate and hydrocarbon emissions; increased Organisation for Economic
nitrogen oxide emissions Co-operation and Development (1993)
No influence on concentrations of PAHs and oxy-PAHs adsorbed Jensen & Hites (1983)
on particulate matter
Delay Decreased nitrogen oxide emissions, increased volumetric fuel Organisation for Economic
consumption Co-operation and Development (1993)
Table 28. Influence of engine conditions on diesel exhaust emissions
Condition Change Influence on exhaust emissions Reference
Injection timing Delay Decreased particulate emissions from indirect injection engines; Williams et al. (1989)
(contd) increased particulate emissions from direct injection engines
Small effect on PAH emissions from indirect injection engines Schuetzle & Frazier (1986)
Small effect on particulate emissions from direct injection engines Campbell et al. (1981a)
No influence on concentrations of PAHs and oxy-PAHs adsorbed Jensen & Hites (1983)
on particulate matter
Load and Increase Increased particulate and PAH emissions at high loads; decreased Pipho et al. (1986)
temperature particulate emissions at very high load from indirect injection
engines
Decreased concentrations of PAHs and oxy-PAHs adsorbed on Jensen & Hites (1983)
particulate matter
Decrease Decreased particulate and nitrogen oxide emissions; extremely Organisation for Economic
cold charge air may increase hydrocarbon emissions Co-operation and
Development (1993)
Lowest PAH emissions at medium load; enrichment of three- Pipho et al. (1986)
and four-ring PAHs during idling (indirect injection engines)
PAH, polycyclic aromatic hydrocarbons
(a) Engine aging
Increased emissions of hydrocarbons, particulates, and PAHs have
been reported from older, more intensively used light- and heavy-duty
vehicle engines in comparison with newer ones (Stenberg et al., 1983;
Braddock & Perry, 1986; Scheepers & Bos, 1992a). Stenberg et al.
(1983) proposed that the combustion of more lubricating oil in older
vehicles contributes to the enhanced emissions. Prakash et al. (1992)
measured the emission characteristics of several buses and reported
that a properly maintained engine would not emit significantly more
hydrocarbons and nitrogen oxides during its lifetime, whereas carbon
monoxide and particulate emissions could increase with age. These
findings are similar to those of Volkswagen AG (1989) on the influence
of distance travelled on exhaust emissions from one diesel passenger
car over a total distance of about 30 000 km in three driving cycles
(US FTP test, US sulfate emission test, US highway driving test). The
average deterioration factors, relative to the emissions at about
2500 km, were carbon monoxide, 0.97; nitrogen oxides, 0.96;
hydrocarbons, 0.91; particulates, 1.07; fluoranthene, 0.96; pyrene,
1.06; chrysene, 0.62; benzo[ b,k]fluoranthene, 1.11; benzo[ e]pyrene,
1.51; benzo[ a]pyrene, 0.68; perylene, 0.96; indeno[1,2,3-cd]pyrene,
2.91; and benzo[ ghi]perylene, 3.43.
(b) Lubricating oil
As a result of emission control techniques such as turbocharging,
intercooling, and injection timing, the relative importance of
emissions caused by lubricating oils has increased (Organisation for
Economic Co-operation and Development, 1993). Lubricating oils have
been hypothesized to be a sink for incomplete combustion products such
as PAHs and their derivatives, which are scrubbed from the exhaust
gas. When this 'enriched' lubricating oil leaks into the combustion
chamber, the constituents may be emitted in the exhaust (Scheepers &
Bos, 1992a; see section B2.1.1). McKee & Plutnick (1989) found,
however, that carcinogenic PAHs accumulate in gasoline- but not in
diesel-fuelled engine oils.
Oil consumption can be reduced by improving engine manufacturing
specifications and engine seals (Organisation for Economic
Co-operation and Development, 1993). The particle-bound hydrocarbons
derived from lubricating oil contribute up to about 50% by weight in
light-duty vehicles and to a maximum of about 26% by weight of the
total emitted particulate matter (see section B2.1.2, Table 22).
Experiments with 13C radiolabelled lubricating oil in three
light-duty vehicles showed that addition of up to 3% lubricating oil
increases the emitted particulate matter by about 50%, and the
particulates consist mainly of the soluble organic fraction adsorbed
onto the particles (Williams et al., 1989).
B3.1.2.2 Fuel specification
Fuel parameters influence the particulate emissions of diesel
engines but seem to be less important than other parameters like
engine adjustment, load, and maintenance (Weidmann et al., 1988).
The actual trend in legislation of the quality of diesel fuel, i.e. to
a lower sulfur and aromatic content, will improve emissions,
especially of particles. In addition, the more stringent emission
standards in many countries will decrease diesel-related emissions.
(a) Sulfur content
There have been numerous investigations on the influence of
sulfur content in diesel fuel on the release of particulate matter
(CONCAWE, 1990b; Van Beckhoven, 1991; Westerholm & Egebäck, 1991;
Scheepers & Bos, 1992a). The sulfur content is presumed to be directly
correlated with the release of particulate matter. About 3-5% of the
fuel sulfur in light-duty vehicles and 2-3% of that in heavy-duty
vehicles reacts with metal sulfates which are adsorbed onto the
particulate matter, increasing its mass and its hygroscopic
properties, as metal sulfates tend to absorb significant amounts of
water from the atmosphere (Organisation for Economic Co-operation and
Development, 1993).
A reduced sulfur content leads to decreased levels of
particulates in the exhaust of light-duty vehicles. For example,
investigations on seven passenger cars and two light vans in a
combined ECE 15/EUDC testing cycle with four diesel fuels of different
sulfur contents (0.055, 0.12, 0.22, and 0.31% by weight) showed a
statistically significant reduction in the emission of particulates up
to a maximum of 7.4%. The investigators noted variations in
particulate levels between the different vehicles, however, that were
about two orders of magnitude higher than could be attributed directly
to the influence of the sulfur content (CONCAWE, 1990b).
Reduction of the emission rates of heavy-duty vehicles by
lowering the fuel sulfur content is still under discussion. In a study
of four heavy-duty engines in the ECE R49-13 mode driving cycle with
the same four fuels as described above, no statistically significant
reduction in emitted particulate matte was seen (CONCAWE, 1990b). In
experiments with one heavy-duty vehicle on a chassis dynamometer bus
cycle with two different fuels, however, a considerable reduction in
the release of particles was found (Westerholm et al., 1986). This
result was confirmed by Westerholm & Egebäck (1991) who investigated
one bus and one truck in three driving cycles (ECE 13 test, bus cycle,
US transient test) with eight diesel fuels of different sulfur and
aromatic contents. Comparable results were found by Gairing et al.
(1994) in tests with one heavy-duty engine (direct injection) in ECE
R49 with 12 different fuels. Adjustment of the engines again appeared
to be a decisive factor.
(b) Aromaticity (density)
Aromaticity can be directly associated with density, as a higher
density is frequently achieved by a larger content of aromatic
compounds. Obviously, increasing the aromaticity leads to increased
emissions of particulate matter, although volumetric fuel consumption
is decreased (CONCAWE, 1986). This finding was confirmed by Hare
(1986) for light- and heavy-duty vehicles on the basis of data from
the automobile industry. The presumed increase in the release of
particulates is 0.5-1.9% for a 1% by volume change in aromatic
content. In a study of four light-duty vehicles in three Australian
driving cycles (resembling roughly the US 72 and 75 tests), Williams
et al. (1989) found reductions in particulate emissions of 22-27% at a
10% difference in fuel aromaticity. Unfortunately, the two fuels also
differed in sulfur content and volatility. Van Beckhoven (1991)
achieved comparable results with passenger cars with indirect
injection but found no significant influence for heavy-duty direct
injection engines. Experiments by Westerholm & Egebäck (1991) on one
bus and one truck showed a reduction in the emission of particulates
when fuel with low levels of sulfur and aromatics was used. The
increased aromatic content is probably also the source of higher
levels of PAHs and nitro-PAHs in the exhaust (Schuetzle & Frazier,
1986; Westerholm, 1987; Westerholm & Egebäck, 1991; Organisation for
Economic Co-operation and Development, 1993).
(c) Volatility
The volatility of diesel fuels is described by the boiling range,
and especially by the 10% boiling-point (low boiling end fraction) and
the 90% boiling-point (high boiling end fraction) of the mixture. The
influence of the boiling range, particularly the 90% boiling-point, on
the release of particulate matter is still under discussion. While
some investigators (Hare, 1986; Williams et al., 1989) assume that
emissions of particulate matter increase with an increased 90%
boiling-point, others have not been able to confirm this effect,
suggesting that other parameters, such as sulfur content and
aromaticity, are responsible for the increase (Westerholm & Egebäck,
1991; Organisation for Economic Co-operation and Development, 1993).
(d) Cetane number
An increase in cetane number lowers the emission of diesel
particulate matter. A slight decrease in cetane number, combined with
a small increase in the density of diesel fuels (see section A2.1.3),
was predicted in 1987 to result in an increase in emissions from 3 to
12% for carbon monoxide, and to maxima of 2% for nitrogen oxides, 7%
for hydrocarbons, and 6% for particles (CONCAWE, 1987).
B3.1.2.3 Malfunction
Malfunctions or defective components of the engine or exhaust gas
system may result in considerably higher emissions than those from a
properly maintained engine. Up to twofold increases in emissions of
carbon monoxide, hydrocarbons, and particulate matter have been
measured even in the absence of a significant effect on power or fuel
consumption (Ullman et al., 1984). Malfunction of the fuel injection
system may contributed markedly to higher emissions (Organisation for
Economic Co-operation and Development, 1993). Volkswagen AG (1989)
tested a diesel passenger car with defective injection nozzles in
three driving cycles (US FTP test, US sulfate emission test, US
highway driving cycle) and found statistically significant increases
in the emissions of carbon monoxide (up to about 25%), hydrocarbons
(up to about 45%), and particulate matter (up to about 15%), whereas
the release of nitrogen oxides was almost unaffected. The amounts of
benzene and PAHs emitted were slightly increased. Comparable results
were obtained by Williams et al. (1989) for the release of particles
by several light-duty vehicles equipped with defective injectors.
Malfunction of US medium- to heavy-duty vehicle diesel engines
increased the amount of nitrogen oxide emitted to about 4%, that of
hydrocarbons to about 75%, and that of particulates to about 140%
(Organisation for Economic Co-operation and Development, 1993).
B3.1.3 Total emissions by diesel engines
Few data are available on the emissions of different constituents
of diesel exhaust into the atmosphere and their contribution to the
total release by traffic and industry. The contribution of heavy-duty
vehicles seems to be much greater than that of passenger cars, despite
lower percent kilometres. For example, in westen Germany, only 8% of
the total annual urban mileage was attributable to heavy-duty vehicles
(Fromme, 1990). In contrast, in 1987, power plants contributed 15.3%
(85 000 t), industry 63.5% (360 000 t), domestic and minor consumers
8.1% (44 000 t), and total traffic 13% (72 000 t) to the particles
released (Metz, 1989). The contribution of diesel particulate matter
to the total particle burden of the atmosphere is highly dependent on
local conditions (Table 29).
Diesel vehicles, particularly in the light-duty category,
represent a smaller proportion of motor traffic in the United States
than in most European countries. Measurements from two heavy-duty
vehicles in 1982 indicated that light- and heavy-duty diesel vehicles
contributed about 6% to the total fine aerosol, organic carbon
emissions in the Los Angeles basin, about 1200 kg/day being emitted by
heavy-duty vehicles and 600-870 kg/day by light-duty vehicles
(Hildemann et al., 1991; Rogge et al., 1993).
B3.1.4 Control of emissions
The automotive industry has made efforts to reduce diesel exhaust
emissions (Obländer, 1991; Fortnagel, 1992; Hammerle et al., 1994b),
and technology is advancing.
B3.1.4.1 Particle traps
Particle traps are placed in the exhaust system to collect
particulate matter and to clean the filter by burning the trapped
particles (trap oxidizers). Most traps are based on cellular ceramic
monolith material or ceramic silica fibre coils. Many regeneration
techniques have been developed for trap oxidizers (Organisation for
Economic Co-operation and Development, 1993), including thermal
regeneration, which is the burning of collected particulate matter,
and catalytic regeneration, in which fuel catalysts, e.g. iron and
copper, containing organic compounds form finely dispersed metal
particles during combustion to reduce the burning temperature of the
diesel particles so that there is more efficient regeneration
(Volkswagen AG, 1989; Hammerle et al., 1994b). Traps remove the
soluble organic fraction adsorbed onto particles and the dry
carbonaceous soot, while catalysts can remove substances only from the
soluble organic fraction. In catalytic regeneration, over 95% of the
metal used as fuel catalyst is retained in the trap (Hammerle et al.,
1994b).
Studies of the emissions of one light-duty diesel passenger car
by Volkswagen AG (1989) showed that the release of particulates was
reduced by a thermal trap by at least 80% during the collection phase
and by about 60% during the regeneration phase; the reduction was 80%
for the whole cycle. Sulfate emissions decreased to about 50%;
however, emission of carbon monoxide increased by about 10%, probably
due to additional burning and/or incomplete oxidation of particulate
matter on the trap. Comparable experiments with a catalytically
regenerated trap, with addition of a manganese compound, also showed a
trap efficiency of about 80% for particulate matter. Hydrocarbon and
carbon monoxide emissions were slightly increased, probably due to
incomplete oxidation on the trap. Sulfate emissions were reduced to
less than half, emissions of PAHs were reduced to about 25%, and those
of phenol and aldehydes to about 25-33%. About 10% of the manganese
catalyst was detected in the exhaust.
Measurements with a heavy-duty vehicle on a chassis dynamometer
to test two particle traps showed decreases of 79 and 86% in the
release of particles, depending on the trap and fuel characteristics.
The emissions of hydrocarbon, carbon monoxide, aldehydes, and PAHs
were slightly reduced (Westerholm et al., 1986).
Table 29. Diesel exhaust emissions and their contribution to total anthropogenic emissions
Region Year Annual emissions (kt/year) Reference
NOx CO HC PM SO2
Light-duty vehicles
Germanya 1986 52 (3.3)b 76 (1.2)b 23 (2.2)b 15 (26.3)b NR Fromme (1990)
1987 NR NR NR 12 (2.2)c NR Metz (1989)
United Kingdom 1991 (0.7)c (0.4)c (1.1)c (2)c,d (0.2)c Quality of Urban Air Review
Group (1993)
Light- and heavy-duty vehicles
Europe NR NR NR NR NR (1.6)c OECD (1993)
United States 1984 NR NR NR NR 620 (3)c OECD (1993)
1986 NR NR NR 54.8 (20)b NR Carey (1987)
(4)c
Germanya 1989 640 240 150 55 NR Federal Environment
Agency(1991)
Heavy-duty vehicles
OECD (Europe) 1980 1548 (13)c NR 393 (4)c NR 361 (2)c OECD (1993)
Germanya 1986 502 (32.2)b 130 (2.1)b 104 (10.0)b 37 (64.7)b 35 (2)c OECD (1993); Fromme (1990)
(17)c (2)c (4)c (7)c
1987 NR NR NR 32.5 (5.9)c NR Metz (1989)
Table 29 (cont'd).
Region Year Annual emissions (kt/year) Reference
NOx CO HC PM SO2
Heavy-duty vehicles (contd)
Germany 1988 614 351 1.31 NR 38 Federal Environment Agency
(1992)
United Kingdom 1991 (19.9)c (1.9)c (5.4)c (14)c,d (1.0)c Quality of Urban Air
Review Group (1993)
Canadae 1985 265 104 37 20 21 Environment Canada
(1990)
NOx, nitrogen oxides; CO, carbon monoxide; HC, hydrocarbons; PM, particulate matter; SO2, sulfur dioxide; NR, not reported; OECD,
Organsiation for Economic Co-operation and Development
a Excluding the former German Democratic Republic
b Percentage of total traffic emissions
c Percentage of total man-made emissions
d PM10
e Sum of light- and heavy-duty trucks
The effect of a ceramic wall-flow monolith particulate trap was
studied on the emissions of a 1979 heavy-duty diesel engine with
direct injection in two steady-state modes. Reductions of 70% (50% at
full load) and 86% (75% at full load) in particle release were found.
There was also a decrease in the soluble organic fraction, whereas the
emitted portion of volatile organic carbons was only slightly reduced.
The trap had almost no effect on the release of nitrogen oxides (Dorie
et al., 1987).
The measurements of Lowenthal et al. (1994) on 13 trucks and
buses (three with particulate traps, 10 without) in a bus cycle showed
a similar reduction in the release of particles (Table 26). There was
a significant decrease in the emission of particle-bound PAHs.
B3.1.4.2 Catalytic converters
Diesel catalytic converters reduce gaseous and particle-bound
hydrocarbon constituents of the exhaust, e.g. aldehydes, PAHs, nitro-
PAHs, and carbon monoxide. Regeneration systems are not needed, as
particulate matter is not collected by common flow-through catalytic
converters. The efficiency in controlling particulates is, however,
much lower than that of traps. Disadvantages of catalytic converters
are further conversion of nitrogen monoxide to the more toxic nitrogen
dioxide and of sulfur dioxide to particulate sulfates (Organisation
for Economic Co-operation and Development, 1993). Catalysts that
selectively oxidize organic compounds and oxidize almost no sulfur
dioxide are currently in use. Removal of sulfur from diesel fuels
would allow the use of more active catalysts; use of catalysts in
diesel vehicles run on sulfur-containing fuel is limited because the
strong adsorbing tendency of sulfur partly inactivates the catalyst
(Hammerle et al., 1994b; see section A2.1.1).
B3.2 Regulatory approaches
The levels of carbon monoxide, nitrogen oxides, total
hydrocarbons, and particulates emitted by diesel engines are regulated
by law in a number of countries (Table 30). Most other industrialized
countries have adapted United States or European regulations (for
details, see CONCAWE, 1990a and Mercedes-Benz AG, 1994a).
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few data are available on the fate of diesel exhaust in the
atmosphere, which is the first environmental compartment affected. In
general, the effects on the overall environment of diesel fuel
combustion are typical of those of fossil fuel burning. The
contribution of diesel fuel emissions is proportional to diesel fuel
consumption both locally and globally.
Table 30. Limit values for components of diesel exhaust
Region Carbon Nitrogen Hydrocarbons Particulates Comments
monoxide oxides
Light-duty vehicles (g/km)
Austria 2.1 0.62 0.25 0.124 < 3.5 t; since 1991; from 1995,
adoption of European Union
standards planned
Canada 2.1 0.62 0.25 0.12 Since 1987
European Union 2.72 0.97 (with 0.14 Since 1992
hydrocarbons)
1.0 0.7 0.08 From 1996
Finland Since 1993
Japan 2.1 0.7 0.62 None Since 1986
2.1 0.5 0.4 0.2 Since 1994
Sweden, Norway 2.1 0.62 (city) 0.25 0.124 < 3.5 t; from motor year 1992
0.76 (highway)
Switzerland 2.1 0.62 (city) 0.25 0.124 < 3.5 t; since 1988; from 1995,
0.76 (highway) adoption of European Union
standards planned
USA (California) 2.1-5.2 0.2-0.6 0.2-0.3 (except 0.05 (up to Depending on mileage
methane) 31 000 km)
US Environmental 2.1-2.6 0.6-0.8 0.2 0.05-0.12 Depending on mileage
Protection Agency
Table 30 (contd)
Region Carbon Nitrogen Hydrocarbons Particulates Comments
monoxide oxides
Heavy-duty vehicles (g/kWh)
Austria 4.9 9.0 1.23 0.4
Canada 15.5 5.0 1.3 0.25 g/bhp-h
15.5 5.0 1.3 0.1 g/bhp-h; from 1995-97
European Union 4.5 8.0 1.1 0.36 Since 1992
4.0 7.0 1.1 0.15 From 1995-96
Japan 7.4 5.0 2.9 0.7 Indirect injection engines
7.4 6.0 2.9 0.7 Direct injection engines
Sweden 4.9 9.0 1.23 0.4
USA 15.5 5.0 1.3 0.07 g/bhp-h; bus
15.5 4.0 1.3 0.1 g/bhp-h; truck
15.5 5.0 1.3 0.05 g/bhp-h; bus; from 1998
15.5 4.0 1.3 0.1 g/bhp-h; truck; from 1998
Adapted from Mercedes-Benz AG (1994b); reference year, 1994, unless otherwise stated; bhp-h, brake horsepower-hour
Thus, the major components of diesel exhaust contribute to acid
deposition (nitrogen and sulfur oxides), tropospheric ozone formation
(carbon monoxide, nitrogen oxides, and hydrocarbons), and global
warming (carbon dioxide and particulates; carbon monoxide, nitrogen
oxides and hydrocarbons by ozone formation).
The environmental fate of released particulate matter and of
organic compounds, particularly the PAHs, is important environmentally.
The mechanisms of the distribution and transformation of individual
hydrocarbons in diesel exhaust have been well described (IPCS, 1986,
1993).
B4.1 Transport and distribution between media
The major fraction (50-80%) of the particulate emissions of
diesel engines is in the submicron size, ranging from 0.02 to 0.5 µm
(see section B2.1.2). Once particles have been emitted, their
mechanical transport in the atmosphere is like that of gas molecules
(nonreactive). Together with carbon particles from other combustion
processes, they may be transported over long distances and even
penetrate the stratosphere (Muhlbaier Dasch & Cadle, 1989).
Only one study was available on the agglomeration behaviour of
the particles in the atmosphere. Albrechcinski et al. (1985) measured
the aging behaviour of particulate matter from a 1978 diesel passenger
car in a 600-m3 model chamber with a nominal exhaust gas dilution of
150:1 and found peak mean aerosol diameters, based on volume size
distributions, of 0.1-0.18 µm for 'fresh' aerosol. The concentrations
of total particulate decreased during aging of the aerosol due to
coagulation, fall-out, and perhaps losses to the walls of the chamber.
The average decrease was 20% after 8 h and 60% after 24 h. Excess
nitrogen dioxide seemed to inhibit particle growth. Particles with an
average diameter of 1-10 µm constituted approximately 10% of the fresh
aerosol, but this percentage decreased during aging as a result of
apparently rapid fall-out of larger particles. It is not clear whether
these results are also representative of the particle emission pattern
of modern diesel passenger cars.
Model experiments of the gas-phase chemistry of nitrogen oxides,
total hydrocarbons, and sulfur dioxide from diesel exhaust were
performed by varying parameters such as irradiation, nitrogen dioxide
concentration, and ozone concentration (Albrechcinski et al., 1985).
Irradiation alone had only a minimal effect, but excess nitrogen
dioxide led to higher rates of decay of nitrogen monoxide in the dark
than under irradiation, probably because of photodissociation of
nitrogen dioxide to nitrogen monoxide. As nitrogen monoxide is rapidly
converted to nitrogen dioxide in the presence of excess ozone, the
nitrogen oxide concentration decreased as the ozone concentration
reached a maximum, presumably partly because of the formation of
nitric acid which was deposited onto the chamber walls. Since reactive
organic compounds were not added, the authors considered that the
experiments were not representative of urban atmospheres.
The hydrosphere and geosphere may be affected indirectly by
diesel exhaust emissions after dry or wet deposition of particulate
matter or individual constituents (see section B4.2). In experiments
with dysprosium-traced diesel fuel, Horvath et al. (1988) (see section
B5.1) found 0.012-0.07 g diesel particulate matter per gram of road
dust.
B4.2 Transformation and removal
Atmospheric removal of airborne carbon particles consists mainly
of dry deposition and scavenging by precipitation (wet deposition).
The rate of wet removal is directly correlated to the ratio of organic
to elemental carbon and is low for small ratios (Muhlbaier Dasch &
Cadle, 1989). As the overall removal rate of diesel particulates is
estimated to be low, the atmospheric life-time is several days
(Jaenicke, 1986).
The elemental carbon of diesel particulates may act as a catalyst
in the formation of sulfuric acid by oxidation of sulfur dioxide. The
oxidation reaction is assumed to occur on the surface of solid soot
particles or in liquid drops containing soot particles (Novakov, 1984;
Mészáros & Mészáros, 1989). Furthermore, diesel particles themselves
contain sulfate, adsorbed in the form of metal sulfates, at a
concentration of about 1-12% by weight, depending on the sulfur
content of the fuel (see section B2.1.2), which may be washed off if
the particles come into contact with precipitation. The organic
compounds adsorbed on the emitted particulate matter may undergo a
number of physical and chemical reactions (volatilization of
low-relative-molecular-mass compounds, formation of secondary
aerosols, oxidation, nitration) with other atmospheric compounds or
during exposure to sunlight (Pitts et al., 1985). The estimated
half-lives for unbound PAHs in the atmosphere range from one day
(naphthalene) to one week (e.g. pyrene, chrysene, benzofluoranthenes,
benzo[a]pyrene) (Mackay et al., 1992; see Table 11); however, PAHs
adsorbed onto particles may be more stable to nitrogen dioxide and
ozone than airborne PAHs (Gibson et al., 1981).
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Because diesel exhaust is a complex mixture of a great variety of
compounds (see also section B2.1), general 'environmental levels'
cannot be described. The individual constituents of diesel exhaust
should be detectable in all compartments of the environment. Since
diesel exhaust is only one of numerous other sources, the levels of
individual compounds cannot be correlated directly to diesel exhaust.
Particulate matter appears to be the most relevant fraction of
diesel exhaust for the general population and workers exposed to
diesel exhaust emissions from a toxicological point of view. The
concentration of particles is about 10- to 20-fold higher in diesel
exhaust than in the exhaust from gasoline-fuelled vehicles, and the
particle diameters are in the respirable range.
B5.1 Exposure of the general population
The general population is exposed to high levels of diesel
exhaust in certain microenvironments, such as busy streets and parking
areas. Exposure to the main gaseous components of diesel exhaust --
carbon dioxide, carbon monoxide, nitrogen oxides, and total
hydrocarbons -- can be estimated from the emission factors given in
section B3, the kilometrage of diesel vehicles in relation to total
traffic, and atmospheric dispersion models (Cuddihy et al., 1984;
Volkswagen AG, 1989). In view of the fact that measured emission
factors vary widely (see section B3.1.1) and are mainly dependent on
adjustment of individual engines, the validity of these estimations is
limited.
On a global basis, there are wide variations in the contribution
of diesel emissions to total airborne particulate matter, which
depends on the following factors: percentage of diesel vehicles in the
total volume of traffic; type, age, and maintenance of individual
engines (see section B3.1.2.1); fuel quality (e.g. sulfur content and
aromaticity; see section B3.1.2.2); and emission control techniques.
Particle concentrations in environmental air samples are usually given
as total particle concentrations, with no differentiation by source.
The contribution of diesel particulate matter is then estimated on the
basis of the percentage of diesel vehicle emissions in the total
emissions from traffic or total man-made emissions. Emissions from
running stationary diesel engines have up to now not been taken into
account. Data on emissions from light- and heavy-duty vehicles in some
industrialized countries are shown in Table 30 (section B3.1.3). The
proportion of the concentrations to which people are exposed that is
attributable to diesel vehicles varies widely, depending on traffic
flow and composition in the vicinity and the magnitude of background
pollution from other sources.
In a study in the United Kingdom (Waller et al., 1965),
measurements were made at a sampling site at the centre of a busy
street canyon, where traffic flows were typically 1200 gasoline and
600 diesel vehicles per hour, in order to assess the maximal
concentrations of traffic pollutants in urban areas. The contributions
of airborne particulate matter, expressed as seasonal means for
daytime working hours, were 200-421 µg/m3, after allowance for the
urban background measured at nearby control sites. The additional
particulate matter was attributed primarily to the diesel vehicles,
while the corresponding contribution to carbon monoxide, averaging
15 ppm (17 mg/m3), was associated mainly with the gasoline vehicles.
In France, Joumard & Perrin (1988) measured atmospheric
particulate matter (diameter, 0.01-25 µm) in a 30-40-m wide street
open to pedestrians, with two central lanes reserved for use by bus
services involving weekday traffic of more than 1000 vehicles per day
in each direction. The measurement point was in the immediate vicinity
of two bus stops, 3 m from the edge of the pavement and at a height of
2.5 m. The particle concentrations, determined by measuring ß
radiation, were 34 µg/m3 during the period 23.00-05.00 h and
67 µg/m3 during 07.00-19.30 h.
Airborne particle concentrations were calculated for an
eight-lane American freeway and for four- and six-lane European
autoroutes. The one-dimensional Gaussian model used neglects chemical
reactions during transport and possible deposition on the ground and
assumes turbulent mixing in the air. The calculated maximal
concentrations were 7-13 µg/m3 close to the road, and these
decreased with distance from the measuring point. About 100 m away,
the estimated particle concentration at a height of 1.5 m was three to
four times lower than that next to the motorway (Volkswagen AG, 1989).
Particle emissions, especially from diesel vehicles, were
estimated roughly for various locations by the lead surrogate method,
in which historical ambient lead concentrations in urban areas are
used as indices of mobile source pollutant levels. Particle emission
factors for light- and heavy-duty vehicles and the distribution
characteristics of lead and diesel particulate are also taken into
consideration (Rykowski & Brochu, 1986). The results are shown in
Table 31.
Concentrations of particles from diesel engines in the
environment were determined in Vienna, Austria, at several measuring
points during a four-week period. As there was only one distributor of
diesel fuel for the whole network of service stations in Vienna, the
rare earth element dysprosium was added as an indicator to the fuel at
this central point, which was distributed to diesel vehicles at
service stations and then emitted with the exhaust. Dust samples
collected from the air were subjected to neutron activation analysis
and the level of dysprosium was determined. The proportion of
dysprosium in diesel soot was calculated on the basis of emission
factors for diesel engines in the literature and from laboratory
experiments, assuming that the marker is totally transferred from fuel
to exhaust during combustion. At seven of the 11 measuring points for
different traffic densities, the concentrations were almost linearly
correlated to traffic density. Ambient particulate levels increased by
5.5 µg/m3 per 500 diesel vehicles per hour, from 10.3 in a residential
area to 23.6 µg/m3 near an urban freeway; the mean value was
14.4 µg/m3. The diesel particulate concentration in the centre of
Vienna was calculated to be 11 µg/m3 by correlating the measured
values and traffic density. At four measuring stations, significant
deviations from linearity were found which were due to local
conditions. The concentration of the marker was below the detection
limit before the testing period and one year later (Horvath et al.,
1988). A deficiency of the study is that the dysprosium concentrations
of diesel fuel before the addition were not taken into account, so
that the emission factors used may not reflect the true situation
(Holländer, personal communication, 1994). Nevertheless, the measured
ambient particle concentrations fit well with estimated data for
diesel exhaust emissions by other investigators.
Table 31. Estimated particle concentrations emitted from diesel
engine exhaust
Location and year Concentration
(µg/m3)
United Statesa, cities with > 1 million 1.3-3
residents (1984)
United Statesa, cities with 100 000-250 000 residents 0.7-1.7
Naples, Italy, urban area (1982) 7.2-35.1
Birmingham, United Kingdom, urban area (1982) 3.2-20.3
Birmingham, United Kingdom, university area (1982) 0.8-5.5
Stockholm, Sweden, inner city (1982) 2.5-16.4
From Rykowski & Brochu (1986)
a According to Cuddihy et al. (1984), the proportion of
diesel-fuelled light-duty vehicles in the United States was
about 2%.
In a programme to measure airborne diesel soot levels in
Düsseldorf, Germany, the total carbon content of fine dust (particle
diameter, < 7µm) at a busy crossing was 8-42 µm3 as a daily
average, 18 µg/m3 as an annual average, 21 µg/m3 on workdays, and
14 µg/m3 on weekends. The carbon content after solvent extraction
(elemental carbon) was 6-32 µg/m3 as a daily average, 16 µg/m3 on
workdays, and 10 µg/m3 on weekends. In a residential area in
Duisburg, Germany, the concentration of elemental carbon was 3 µg/m3.
The method of determination was as follows: a defined volume of air
was sucked through a fibreglass filter, and the respirable particle
fraction was separated. This fine dust was further separated into an
incinerable portion (according to German standard method TRGS 900),
total carbon, and the carbon content after liquid extraction
(elemental carbon). Total and elemental carbon were determined by
coulometry. By correlation with diurnal changes in carbon and nitrogen
monoxide concentrations, the main particle source was shown to be
automobile traffic at the sampling location. Correlation of a traffic
census with working day and weekend particle levels showed that the
airborne particulate matter was due primarily to diesel vehicles
(Elbers & Muratyan (1991). Further measurements at the same place in
1991 and 1992 confirmed the results (Elbers & Richter, 1994).
Validation of the analytical method gave detection limits of 0.39 for
elemental carbon (relative standard deviation, 14% or 4.4 µg/m3) and
0.65 µg/m3 for total carbon (relative standard deviation, 13% or
7.8 µg/m3).
The weekly average ambient concentrations of diesel particulate
in Germany were estimated to be 5-10 µg/m3 in urban areas, 15-25 µg/m3
in urban areas close to busy streets, and < 1.5 µg/m3 in rural
areas; however, the method used for the estimations was not reported
(State Committee for Immission Protection, 1992).
Estimates of the US Environmental Protection Agency for 1986 gave
annual mean ambient concentrations of diesel particle in the United
States of 2.6 µg/m3 in urban areas and 2.4 µg/m3 in rural areas. A
prediction for 1995 gave values of 1.5 µg/m3 for urban and 1.2 µg/m3
for rural areas (Mauderly, 1992). Higher concentrations were estimated
by the Motor Vehicles Manufacturing Association (Sienicki & Mago,
1992), which predicted a mean value of 2.7 µg/m3 for the city of Los
Angeles in 1995. Lower levels were estimated in a study by the US
Environmental Protection Agency (1993) in which national average
emission factors for diesel vehicles for 1988 were multiplied by urban
and rural mileage. For 1990 and 1995, the annual exposure levels were
estimated to be 2.0 and 1.2 µg/m3 in urban air and 1.1 and 0.6 µg/m3
in rural air, respectively. These estimates were confirmed by ambient
monitoring. Using data from the early 1980s, when the proportion of
diesel vehicles, the number of kilometres travelled, and emission
standards differed from present characteristics, McClellan (1986)
derived ambient diesel particulate concentrations of 10-30 µg/m3 on
American urban freeways and in urban street canyons.
B5.2 Occupational exposure
The levels of particulate matter to which workers are exposed
when their predominant exposure to exhaust is that from diesel engines
are described in this section. It is difficult to determine the
contribution of diesel exhaust to respirable particles, as they
include not only those from diesel engines but also others generated
in the worker's environment, such as sand, dirt, coal dust, and
cigarette smoke particles. Thus, the levels of total and respirable
particulates reported in jobs with potential exposure to diesel
exhaust may be significant overestimates of the actual exposure. Only
two occupational groups have been studied in this regard: railroad
workers (Woskie et al., 1988a,b) and mechanics and drivers in the
trucking industry (Zaebst et al., 1991). The specific exposures of
these groups to diesel particles range from 0.026 to 0.192 mg/m3. In
the railroad workers, cigarette smoke was found to account for a
substantial proportion of total respirable particulate matter, and the
geometric mean exposure to respirable particulates of workers not
exposed to diesel exhaust was reduced by over 80% when the
contribution of cigarette particulate was considered (Woskie et al.,
1988a).
The principal marker chosen to measure exposure of workers in the
trucking industry (including road drivers, local drivers, stevedores,
and mechanics) to diesel exhaust was elemental carbon, which is
derived from the core of the diesel exhaust particle and represents
about 20% of respirable particulate; cigarette smoke does not
contribute appreciable amounts of elemental carbon. Mechanics and
stevedores had the highest exposure to diesel exhaust, and truck
drivers had lower levels (Zaebst et al., 1991). The degree to which a
marker of diesel exhaust exposure actually represents true exposure
depends on the nature of all sources of respirable particles in the
work-place. Occupational exposure to particulates is summarized in
Table 32.
Table 32. Reported occupational exposure to particulates among
workers exposed to diesel exhaust
Occupational group Particles (mg/m3)
Total Respirable Diesel
Railroad workers 0.01-1.99 0.06-0.41 0.051-0.192a
Mine workers 0-23.0 0-16.1
Truck drivers 0.1-1.0 0.13-1.2 0.025b
Bus garage and bus 0.15-1.2 0.01-0.61
workers
Stevedores 0.157b
Truck mechanics 0.133b
a Estimated using nicotine to account for exposure to cigarette smoke
b Based on carbon core analysis, with an estimated 20% of respirable
particulate due to carbon (Steenland et al., 1992)
Because 1-nitropyrene is associated with the particle fraction of
diesel exhaust, this compound was used as an indicator of the
particulate fraction of diesel exhaust derived from various sources
(e.g. ships, railroad engines, heavy-duty road vehicles, fork-lift
trucks) (Scheepers et al., 1994a,b). The 1-nitropyrene content of
fresh and aged diesel exhaust was measured in small air samples
extracted with acetone by sonication and detected by gas
chromatography-mass spectrometry. The following concentrations of
respirable diesel exhaust were determined: 0.044-0.33 mg/m3 in
various work-places with fork-lift trucks, 0.10-0.37 mg/m3 in
railroad engine repair shops, 0.080mg/m3 in a river vessel, and 0.67
or 3.33 mg/m3 in the breathing zone of railroad workers.
B5.2.1 Truck drivers and mechanics
In a study of 15 truck drivers in Geneva, Switzerland, exposure
to particulate matter was measured during one working day with a
direct-reading instrument and by sampling beside the drivers in the
truck cabin (Guillemin et al., 1992). Local drivers had greater
exposure to particulate matter (0.3 mg/m3) than long-distance
drivers (0.1 mg/m3) and spent more time in polluted areas, such as
streets with heavy traffic and construction sites. Smoking did not
influence the exposure of professional truck drivers to particulate
matter, probably because the ventilation rate of the truck cabins was
relatively high, even on cold days.
Industrial hygiene surveys were conducted during warm and cold
weather at eight terminals and truck repair shops to determine
exposure to submicrometre particles of elemental carbon among American
workers in one of four jobs -- road drivers, local drivers, stevedores,
and mechanics -- all of whom were exposed mainly to diesel aerosol
(Zaebst et al., 1991). The overall arithmetic mean exposure was
5.1 µg/m3 for 72 long-distance road drivers, 5.4 µg/m3 for 66
local drivers, 26.6 µg/m3 for 80 mechanics, and 31.3 µg/m3 for 54
stevedores exposed to diesel fork-lift trucks. The background
concentrations in the same cities were 3.4 µg/m3 on 21 major
highways and 1.4 µg/m3 in 23 residential areas. The exposures of the
truck drivers were significantly higher than the background
concentrations in residential areas but could not be distinguished
from the background highway concentrations.
Area air sampling in an American truck repair shop in 1987 gave
the following results, depending on the sampling location: 0.225 and
0.32 µg/m3 submicrometre particles, 0.26 and 0.51 µg/m3 respirable
particles, and 0.63 and 0.66 µg/m3 total particulate matter. The
values for elemental carbon were 86-118 µg/m3 submicrometre
particles and 159-214 µg/m3 total carbon. In personal samples from
four mechanics, 79-193 µg/m3 submicrometre elemental carbon were
found (US National Institute for Occupational Safety and Health,
1990a).
The concentrations of particles collected on fibreglass filters
at 25 work-places in Germany where mainly fork-lift trucks and trucks
were used were 0.02-0.8 mg/m3 in area samples and 0.13-1.2 mg/m3
in personal samples (Lehmann et al., 1990). Area measurements in two
German truck repair garages (five samples from each) showed
concentrations of incinerable fine dust (according to German standard
method TRGS 900) of 0.11-0.40 mg/m3 (Blome et al., 1990).
Personal samples from truck drivers who loaded diesel-engined
trucks onto car ferries in Sweden showed particle concentrations of
0.1-1.0 mg/m3 (Ulfvarson et al., 1987). The drivers may also have
been exposed to diesel exhaust from the ferries.
B5.2.2 Bus garage and other bus workers
In area samples taken in an American bus garage in 1989, the
concentrations of particles were 0-0.1 mg/m3 submicrometre
particles, 0-0.3 mg/m3 respirable particles, 0-0.22 mg/m3 total
particulate matter, and 0.014-0.326 mg/m3 elemental carbon (US
National Institute for Occupational Safety and Health, 1990b).
The concentrations of smoke were measured in two diesel bus
garages in London, United Kingdom, from high-volume samplers for
successive periods through the day and night, which were linked to bus
movements and the exposure of the maintenance staff. The contribution
from the buses was assessed by subtracting the background values at
control sites outside the garages. In the most polluted garage,
0.3-1.2mg/m3 of smoke were measured during working periods (Waller
et al., 1985).
In Sweden, a particle concentration of 0.46 mg/m3 was found
during personal sampling in periods of high activity (arrival and
departure of buses) (Ulfvarson et al., 1987).
Area air sampling of the concentrations of incinerable fine dust
(according to German standard method TRGS 900) in two bus depots (six
samples in each) and one bus garage (three samples) in Germany showed
concentrations of 0.22-0.37 mg/m3 (Blome et al., 1990).
The respirable dust levels in bus garages in Italy and the United
States ranged from <0.01 to 0.73 mg/m3 (Gamble et al., 1987a). The
total dust levels in bus garages in Denver, Colorado, United States,
were 0.01-0.81 mg/m3 (Apol, 1983; Pryor, 1983).
B5.2.3 Fork-lift truck operators
Area samples taken in one American trucking terminal where
diesel-powered fork-lift trucks were used in 1987 contained
48-62 µg/m3 submicrometre particles, 34-57 µg/m3 respirable
particles, and 62-97 µg/m3 total particles (US National Institute
for Occupational Safety and Health, 1989a). The concentrations of
elemental carbon in area samples from another American trucking
terminal in 1988 were 40-44 µg/m3, and those in personal samples
from fork-lift operators were 33-77 µg/m3 (US National Institute for
Occupational Safety and Health, 1989b). Area samples taken in an
American freight dock contained concentrations of <12 and 294 µg/m3
respirable dust. The levels were significantly higher in areas where
unfiltered rather than filtered diesel motors were used (US National
Institute for Occupational Safety and Health, 1990c).
A mean concentration of 31.3 µg/m3 of submicrometre elemental
carbon was found in personal samples from stevedores operating
diesel-powered fork-lift trucks. About 10 times less was found for
operators of gasoline- or propane-powered trucks (Zaebst et al.,
1991).
The concentrations of particles in the breathing zone during use
of diesel-powered fork-lift trucks for loading, unloading, and
warehousing operations in American ammunition storage magazines were
< 0.01 to1.3 mg/m3 (Ungers, 1984) and 0.5-5.0 mg/m3 (Ungers, 1985).
B5.2.4 Railroad workers
The mean levels of total particulate matter were 0.38 mg/m3
(range, 0.1-0.8) in Finnish locomotive cabs and 1.99 mg/m3 (range,
0.07-8.7) in roundhouses (Heino et al., 1978). In three German engine
sheds, the ambient concentrations of incinerable fine dust were
0.1-0.41mg/m3 (Blome et al., 1990).
A summary of American literature showed time-weighted average
levels of 0.05-0.07 mg/m3 (8-h) in tunnels, 0.16 mg/m3 (7.5-h)
during freighting, 0.01 mg/m3 (5-h) in switchyards, and 0.27 mg/m3
(8-h) in cabooses (Hobbs et al., 1977). The arithmetic mean
concentrations of total respirable particles in personal samples from
shop workers, engineers, brakemen, conductors, and farmers in the
American railroad industry (after correction for the estimated
contribution of cigarette smoke particulates) were 51-192 µg/m3
(Woskie et al., 1988a).
B5.2.5 Mine workers
Diesel vehicles and machines have been used in nearly all
American mines since the 1960s (Wichmann & Brüske-Hohlfeld, 1991). The
mean concentration of diesel particulate matter in personal and area
samples in mines where diesel equipment was used was 4.6 mg/m3
(range, 0.2-14) (Holland, 1978). Total respirable dust levels of
0.6-1.7 mg/m3 were found by personal sampling (Wheeler et al.,
1981); average concentrations of 0.9-2.7 mg/m3 were found in
full-shift personal samples, and the range for full-shift area samples
was 0-16.1 mg/m3 (Reger et al., 1982). The 8-h time-weighted average
level of respirable particulate matter in personal samples taken in an
American molybdenum mine was 0.2-1.9 mg/m3 (Cornwell, 1982). Total
respirable dust concentrations of 1.1-4.4 mg/m3 were reported in an
American gold mine (Daniel, 1984). The US Mine Safety and Health
Administration reported that the concentrations of diesel particulate
matter in American mines other than coal mines were 0.3-1.5 mg/m3
(Mauderly, 1992).
Data from a salt mine in Canada showed a diesel particulate level
of about 0.5 mg/m3 (Johnson & Carlson, 1986).
In area samples from different work-places in eight German coal
mines, the levels of airborne diesel particulate matter (incinerable
fine dust) were 0.19-0.70 mg/m3 (Blome et al., 1990). In German
potash-salt mines, depending on the district, 0.2-0.4 mg/m3 of
diesel particles were measured (Bauer et al., 1990).
B5.2.6 Fire fighters
The average concentration of total particulate matter in personal
samples from fire-fighters in Boston, New York City, and Los Angeles,
United States, in 1985 was between < 0.1 and 0.48 mg/m3. On a typical
day in Boston or New York City, exposure to diesel exhaust particles
was estimated to be about 0.225 mg/m3 after correction for the
contributions of background and smoking. Simulation of 'worst-case'
exposures in Los Angeles fire stations gave a maximal concentration of
0.748 mg/m3 total particles (Froines et al., 1987).
B5.3 Biomonitoring
B5.3.1 Urinary mutagenicity
The occurrence of mutagens, measured by the Ames test, in the
urine and faeces of eight car mechanics occupationally exposed to
diesel exhaust was compared with that of a reference group of nine
office workers. The samples were collected over 48 h; information was
also gathered on dietary intake, smoking habits, and medication. No
enhancement of the incidence or degree of faecal or urinary
mutagenicity was seen (Willems et al., 1989).
Post-shift urinary mutagenicity was measured by the Ames test in
306 samples from 87 railroad workers with a range of exposures to
diesel exhaust (Schenker et al., 1992). Exposure was measured
throughout the work shift by constant-flow personal sampling pumps. In
separate analyses of smokers and nonsmokers, no independent
association was seen with exposure to diesel exhaust. The workers with
the highest median exposure to respirable particles attributable to
diesel exhaust (113 µg/m3) were railroad engineering shop workers.
In studies of the effect of diesel exhaust on stevedores on
'roll-on-roll-off' ships (Ulfvarson et al., 1987), no difference in
mutagenicity to Salmonella typhimurium TA98 or Escherichia coli WP
2 uvr A was seen in urine collected during periods of exposure and
of no exposure. Similarly, no increase in urinary mutagenicity was
found in samples from six volunteers before and after exposure to
diesel exhaust collected from an experimental automobile run for 3.7 h
at 60 km/h and 2580 rpm.
B5.3.2 Other analyses
Samples of total suspended particulate matter were collected in a
repair shop for train engines, and the diesel exhaust concentrations
were determined using 1-nitropyrene as a biomarker (see section B5.2).
Urinary metabolites and the corresponding nitro-substituted
derivatives were detected by immunoassay in the urine of three
diesel-engine mechanics. They had a significantly increased excretion
of metabolites in comparison with two office clerks (Scheepers et al.,
1994b).
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
B6.1 Deposition
The mass median aerodynamic diameter of diesel exhaust particles
in long-term studies in animals exposed by inhalation was 0.17-0.25 µm
(Lewis et al., 1986; Lee et al., 1987; Wolff et al., 1987;
Creutzenberg et al., 1990). The deposition efficiency of particles of
this size range is lower than that of smaller particles, for which the
deposition by diffusion increases with decreasing diameter. The
deposition efficiency of particles with a diameter > 0.5 µm increases
with the aerodynamic diameter, owing to sedimentation and impaction.
The nose has hardly any filter effect for diesel particles because the
fraction of particles < 0.4 µm deposited in the nose is very low
(Heyder et al., 1986).
After short-term inhalation by rats of 0.29-µm fused
aluminosilicate particles, 8% was deposited in the bronchi and 13% in
the lungs (Raabe et al., 1988). A total of 10-20% diesel exhaust
particles was deposited in rats and guinea-pigs (Chan et al., 1981;
Lee et al., 1983; Dutcher et al., 1984). In humans, the alveolar
deposition efficiency of 0.2-µm particles was 10-20%, and no bronchial
deposition was detected (Heyder et al., 1986).
These experimental results agree well with those of a predictive
model for deposition of inhaled diesel exhaust particles in laboratory
animals and humans (Yu & Xu, 1987). In humans, the deposition
efficiency was about 7% in the alveolar region and 4% in the
tracheobronchial region. The model can also predict the fractions that
will be deposited in different airway generations; maximal deposition
occurs in the alveolar region (twentieth generation) of adults and of
children over two years of age.
The length and concentration of exposure had no significant
effect on deposition in rats exposed to 0, 0.4, 3.5, or 7.1 mg/m3
diesel exhaust particles for 6, 12, 18, or 24 months, when deposition
of 0.1-µm gallium oxide particles was used as the indicator (Wolff et
al., 1987).
B6.2 Retention and clearance of particles
The kinetics of inhaled substances are conveniently described by
their pulmonary clearance and retention. These terms are often used
interchangeably, but it should be kept in mind that the fraction
cleared from the lung plus the fraction retained in the lung account
for the total deposited amount (retention = deposition - clearance).
Thus, in the following paragraphs, the term 'clearance' refers to a
clearance rate ( r, per unit of time) and the term 'retention' to a
biological half-time ( t ´), provided there is a constant rate of
exponential clearance (ln2/ t ´).
Clearance of diesel particles from the alveolar region is
important in the long-term effects of particles on cells, as it is
more than two orders of magnitude slower than mucociliary clearance,
measured in several studies in rats administered labelled diesel or
surrogate particles by inhalation. The pulmonary retention half-times
in studies in which the lung burden of diesel soot was also reported
are summarized in Table 33. The equivalent continuous exposure
concentration is calculated by multiplying the actual concentration by
the number of hours of weekly exposure divided by 168 h. This figure
allows a comparison of exposure levels in studies with different
exposure protocols. The assumption is that Habers' law ( c × t =
constant) is applicable. This is probably valid until a certain burden
of particulate is reached in the lung; however, when that level is
exceeded (particle overload), the calculation is increasingly invalid.
The retention half-time in these studies was 60-100 days in
control rats with low lung burdens and up to 600 days in animals with
high lung burdens. In a double logarithmic plot of retention
half-times and lung burden, a linear relationship can be seen for lung
burdens of diesel particles above 1mg per lung (Figure 1).
The gaseous phase of diesel exhaust appears to have no effect on
alveolar clearance in rats or hamsters (Heinrich et al., 1986a).
The retardation of lung clearance is not specific to diesel
exhaust but is known as 'lung overloading by particles'. This effect
is characterized by (i) an overwhelming of normal alveolar
macrophage-mediated clearance processes under certain conditions of
exposure, (ii) resulting in lung burdens greater than predicted from
deposition kinetics at low concentrations, (iii) with associated
pathophysiological changes including altered macrophage function,
inflammation, and pulmonary fibrosis, and (iv) an uncertain
association with an increased incidence of lung tumours in rats
(McClellan, 1990).
The effect of overloading lung clearance mechanisms was
investigated in NMRI and C57Bl mice, Wistar and Fischer 344 rats, and
Syrian golden hamsters exposed to particulates, including test toner
(polymer pigmented with carbon black), polyvinyl chloride, carbon
black, diesel exhaust, and two crystalline forms of titanium dioxide
(anatase and rutile), at concentrations of 0.8-64 mg/m3 for up to
two years. In rats, lung overloading generally occurred after exposure
to 0.5-1.5 mg per lung or about 1 mg of material per gram of lung
tissue. A two- to 10-fold decrease in the clearance rate was seen
after heavy particulate loading in all of these rodent species (Muhle
et al., 1990).
In comparisons of the effects of particles of different
densities, the retained volume of particles appears to be a more
useful parameter than the retained mass, as alveolar macrophages have
an upper volumetric uptake limit. The particulate overload effect
appears to be initiated in rats when the average particle volume per
alveolar macrophage exceeds about 60m3, and alveolar
macrophage-mediated particle clearance virtually ceases when the
phagacytosed particulate volume exceeds an average of 600m3 (Morrow,
1988).
At a lung burden above threshold, signs of lung overloading in
Fischer 344 rats persisted for 15 months after cessation of exposure
(Bellmann et al., 1992). Retardation of alveolar clearance was also
observed in hamsters and mice at a lung burden above about 1 mg per
lung. For mice, no data were available with regard to lung burdens
below 1 mg per lung, but retardation of alveolar clearance is probably
already present at lower lung burdens. Lung overloading is thus seen
in a variety of species with various materials. It is generally
observed after the threshold lung burden of particles of low
solubility and low acute toxicity is exceeded for a considerable time
(Muhle et al., 1990). The effects of overloading should be considered
in extrapolating the health effects seen at high concentrations in
experiental animals to the low concentrations occurring in the
environment or the work-place.
The alveolar clearance rates of highly insoluble particles differ
among species, with retention half-times ranging from 50 to 100 days
in rodents to several hundred days in dogs and man (Figure 2). In
human lung, the retention half-time of insoluble, nontoxic particles
may be >500 days (Bailey et al., 1985a,b).
Table 33. Pulmonary retention of insoluble particles in rats after exposure to diesel exhaust, and lung burden of diesel soot
Strain and sex No./group Duration of Exposure Pulmonary retention Reference
exposure
(h/week)
Concentration (mg/m3) Duration Lung burden Material Half-time
(weeks) (mg/lung) studied (days)
Actual Equivalent
continuous
Fischer 344, 4 140 0 0 0 0 14C-Diesel 77 Chan et al.
male 0.25 0.21 7 0.2 exhaust 90 (1984)
0.25 0.21 16 0.5 92
6.00 5.00 1 0.7 166
6.00 5.00 9 6.5 562
6.00 5.00 16 11.8 (> 1000)
Wistar, female 5-7 90 0 0 13 0 59Fe2O3 61 Creutzenberg
0.84 0.45 0.63 (0.3 µm) 94 et al. (1990);
2.5 1.34 2.5 119 Heinrich et
6.98 3.74 8.2 330 al. (1995)
0 0 52 0 72
0.84 0.45 2.8 121
2.5 1.34 10.7 254
6.98 3.74 35.7 541
0 0 78 0 96
0.84 0.45 5.5a 221
2.5 1.34 12.2 272
6.98 3.74 58.8 687
Table 33 (contd)
Strain and sex No./group Duration of Exposure Pulmonary retention Reference
exposure
(h/week)
Concentration (mg/m3) Duration Lung burden Material Half-time
(weeks) (mg/lung) studied (days)
Actual Equivalent
continuous
Fischer 344, 8 35 0.15 0.03 18 0.035 Diesel 87 Griffis et al.
male and 0.94 0.20 0.22 exhaust 99 (1983)
female 4.10 0.85 1.89 165
Fischer 344, 4 140 6.00 5.00 1 1.7 14C-Diesel 61 Lee et al.
male 6.00 5.00 3 4.4 exhaust 124 (1987)
6.00 5.00 6 10.4 192
Fischer 344, 15-16 (lung 35 0 0 104 0 134Cs-Fused 79 Wolff et al.
male and burden) 0.35 0.07 0.6 aluminosilicate 81 (1987)
female 10 3.50 0.73 11.5 particles 264
(retention) 7.00 1.46 20.5 (2 µm) 240
a Measured; value derived by interpolation from data at 52 and 104 weeks
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting exhaust emissions of the documents. A comprehensive file of
all comments received on drafts of each EHC monograph is maintained
and is available on request. The chairpersons of task groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO DRAFTING GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL
AND EXHAUST EMISSIONS
WHO, Geneva, 6-9 December 1993
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United State
Dr R.P. Bos, University of Nijmegen, Nijmegen, Netherlands
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom (Joint Rapporteur)
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr I. Farkas, National Institute of Hygiene, Budapest, Hungary
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr P. Gustavsson, North Western Health Board, Stockholm, Sweden
Dr D. Guth, United States Environmental Protection Agency, Research
Triangle Park, NC, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr W. Pepelko, United States Environmental Protection Agency,
Washington DC, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Vice-Chairman)
Dr G. Rosner, Hazardous Substances Documentation Group, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr J. Roycroft, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, United States
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States (Chairman)
Dr B.H. Thomas, Environmental Health Directorate, Ottawa, Canada
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr. R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr P. Boffetta, International Agency for Research on Cancer, Lyon,
France (6-7 December 1993)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Representatives/Observers
CONCAWE
Dr R.H. McKee, Exxon Biomedical Sciences, East Millstone, NJ,
United States
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT
Dr P.T.C. Harrison, MRC Institute of Environment & Health,
Leicester, United Kingdom
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND
EXHAUST EMISSIONS
Fraunhofer Institute of Toxicology & Aerosol Research, Hanover
27 June-1 July 1994
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United States
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Dr Jun Kagawa, Tokyo Women's Medical College, Tokyo, Japan
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
Dr M. Roller, Medizinishes Institut für Umwelthyglene an der
Universität Düsseldorf, Düsseldorf, Germany Dr G. Rosner, Hazardous
Substances Documentation Group, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr H. Moller, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr M. Younes, European Centre for Environment and Health, Bilthoven,
Netherlands
Representatives/Observers
Dr Lutz Von Meyerinck, BP Oil Europe, Brussels, Belgium
GERMAN AUTOMOBILE ASSOCIATION
Dr N. Pelz, Mercedes-Benz AG, Stuttgart, Germany
ILSI
Dr I.T. Salmeen, Chemistry Department, Ford Motor Company,
Dearborn, MI, United States
IUTOX
Dr P. Montuschi, Department of Pharmacology, School of Medicine,
Catholic University of the Sacred Heart, Rome, Italy
ENVIRONMENTAL HEALTH CRIETERIA FOR DIESEL FUEL AND EXHAUST EMISSIONS
A WHO Task Group on Environmental Health Criteria for Diesel Fuel
and Exhaust Emissions met at the Fraunhofer Institute of Toxicology
and Aerosol Research, Hanover, Germany from 27 June to 1 July 1994.
Dr G. Rosner, Fraunhofer Institute, welcomed the participants on
behalf of the Institute and its Director, Professor U. Mohr, and
Dr E.M. Smith, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP, ILO, and WHO). The Task
Group reviewed and revised the draft and evaluated the risks for human
health and the environment from exposure to diesel fuel and exhaust
emissions.
The first draft of the monograph was prepared at the Fraunhofer
Institute. After international circulation for comment, this draft was
extensively revised by a Working/Drafting Group, convened at WHO,
Geneva, from 6 to 9 December 1993, and a second draft was prepared for
further international circulation for comment. The membership of the
drafting group is shown previously. A final draft, incorporating
comments received from the IPCS contact points for Environmental
Health Criteria monographs and new material, was completed at the
Fraunhofer Institute under the coordination of Dr G. Rosner, with
important contributions to the text from the following Institute staff
members:
Dr B. Bellman
Dr A. Boehnke
Dr O. Creutzenberg
Dr J. Kielhorn
Dr E.M. Smith of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs E. Heseltine, Lajarthe,
France, for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
PART A DIESEL FUEL
A1. SUMMARY
A1.1 Identity, physical and chemical properties, and analytical
methods
Diesel fuel is a complex mixture of normal, branched, and cyclic
alkanes (60 to > 90% by volume; hydrocarbon chain length, usually
between C9 and C30); aromatic compounds, especially alkylbenzenes
(5-40% by volume); and small amounts of alkenes (0-10% by volume)
obtained from the middle-distillate, gas-oil fraction during petroleum
separation. Benzene, toluene, ethylbenzene, and xylenes and polycyclic
aromatic hydrocarbons (PAHs), especially naphthalene and its
methyl-substituted derivatives, may be present at levels of parts per
million in diesel fuel. The sulfur content of diesel fuels depends on
the source of crude oil and the refinery process. It is regulated by
law in a number of countries and is usually between 0.05 and 0.5
weight percent. Additives are used to influence the flow, storage, and
combustion of diesel fuel, to differentiate products, and to meet
trademark specifications. At room temperature, diesel fuels are
generally moderately volatile, slightly viscous, flammable, brown
liquids with a kerosene-like odour. The boiling ranges are usually
between 140 and 385°C (> 588°C for marine diesel fuel); at 20°C, the
density is 0.87-1.0 g/cm3 and the water solubility is
0.2-5 mg/litre. The quality and composition of diesel fuel influence
the emissions of pollutants from diesel engines considerably.
Important variables are ignition behaviour (expressed in terms of
cetane number), density, viscosity, and sulfur content. The
specifications of commercial diesel fuel differ considerably in
different countries.
Heating fuels and some kerosene jet fuels produced during the
refining process may have a composition similar to that of diesel
fuel, although with different additives. Biological data on these
mixtures have therefore also been taken into account in the
assessments of toxicity and ecotoxicity.
Owing to the complexity of the diesel fuel mixture, there is no
specific analytical method, and the analytical techniques used in most
environmental assessments are suitable only for measuring the total
petroleum hydrocarbon mixture. The methods consist of preliminary
solvent extraction, a clean-up procedure to remove naturally occurring
hydrocarbons, and subsequent detection by gravimetry, infrared
spectroscopy or gas chromatography. Neither the gravimetric nor the
infrared technique provides useful qualitative or quantitative
information on contaminants and can thus be used only for screening.
Gas chromatography combined with detection techniques such as flame
ionization and mass spectrometry is the standard procedure for
analysing environmental samples. Many other methods are available for
the analysis of individual hydrocarbons in diesel fuels.
A1.2 Sources of human and environmental exposure
Diesel fuels are produced by refining crude oils. In order to
meet technical specifications for performance, diesel fuels are
generally blended; further formulation with additives improves their
properties for specific uses. Diesel fuels are widely used as
transportation fuels. The more volatile fuels, with low viscosity, are
required for high-speed engines and the heavier grades for railroad
and ship diesel engines. Much heavy-duty road transport is powered by
diesel engines. Passenger cars powered by diesel engines are becoming
increasingly more common in Europe and Japan (10-25%), whereas in
North America the percentage of diesel-fuelled passenger cars is about
1-2%, with a slightly decreasing tendency. Diesel fuel is used in
stationary engines and in boilers, e.g. reciprocating engines, gas
turbines, pipeline pumps, gas compressors, steam processing units in
electric power plants, burner installations, and industrial space and
water heating facilities.
Over the last five years, the worldwide demand for diesel fuels
has increased steadily. In 1985, the following amounts of diesel fuel
were consumed: about 170 000 kt per year in North America; about
160 000 kt per year, including gas oils, in the European Union; and
about 46 000 kt per year in Australia, Japan, and New Zealand,
equivalent to a total of 1062 kt per day. In 1990, world demand was
reported to be about 1110 kt per day.
No information is available on emissions during the production of
diesel fuels; however, this source would seem to be of minor
importance, because the refining process is carried out in closed
systems. Emissions may occur principally during storage and
transportation. Diesel fuels are released as a result of spills and at
filling stations during the refuelling of vehicles. The atmosphere and
the hydrosphere are the most heavily affected environmental
compartments. Soil contamination with diesel fuels may occur during
accidents and is also a problem in railroad yards. The numerous
techniques for cleaning soils contaminated with diesel fuel include
excavation, biological methods, and containment.
A1.3 Environmental transport, distribution, and transformation
Very few data are avilable on the environmental fate of diesel
fuels, but the mechanisms of their distribution and transformation are
considered to be comparable to those of heating fuels, such as No. 2
fuel oil, which have been well studied. Spills of diesel fuel on water
spread almost immediately to form a 'slick'. The polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, and the volatile components evaporate from the surface;
microbial degradation also begins. Chemical and biological weathering
alter the composition of the spill. These processes are dependent on
temperature; spills that occur in Arctic conditions are more
persistent than those that occur in temperate climates. In marine
environments, most of the low-relative-molecular-mass aromatic species
are dissolved into the water phase, but the primary branched alkanes,
cycloalkanes, and remaining aromatic compounds may remain in sediments
for more than a year.
Although no information is available on the photooxidation of
diesel fuels in water and air, evaporated oil components are degraded
photochemically. No. 2 fuel oil has been shown to be photooxidized
rapidly in water under environmental conditions.
The individual constituents of diesel fuel are inherently
biodegradable, to varying degrees and at different rates. The
n-alkane, n-alkylaromatic, and simple aromatic molecules in the C10-C22
range are the most readily degradable. Smaller molecules are generally
rapidly metabolized. Long-chain n-alkanes are more slowly degraded,
owing to their hydrophobicity and because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to biological breakdown, and PAHs are resistant. The overall
rates of degradation of hydrocarbons are limited by temperature, water
content, oxygen, pH, inorganic nutrients, and microbial metabolic
versatility.
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this actually occurs in
nature is poorly understood. Unlike microorganisms that use petroleum
carbons as a carbon source, animals generally oxidize and conjugate
products, rendering end-products that are more soluble and therefore
easier to excrete. All animal species tested can take up petroleum
hydrocarbons. PAHs, crude oil, and refined petroleum products are
known to induce cytochrome P450 enzymes and to increase the levels of
hydrocarbon metabolism in numerous marine and freshwater fish species.
Few data are available on the bioaccumulation of diesel fuel in
the laboratory, but there is plentiful evidence from studies of spills
and laboratory studies on other oils, particularly No. 2 fuel oil,
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient for diesel fuel is 3.3-7.06,
which suggests high potential bioaccumulation; however, many of the
lower-relative-molecular-mass compounds are readily metabolized, and
the actual bioaccumulation of higher-relative-molecular-mass compounds
is limited by their low water solubility and large molecular size.
Thus, actual bioaccumulation may be low.
Fish have been tainted by diesel fuel after spills. No data are
available on the biomagnification of diesel fuel.
No experimental data are available on the movement of diesel fuel
through the soil, although a direct correlation between the movement
and kinematic viscosity has been proposed. The movement of kerosene
through soil depends on the moisture content and nature of the soil.
A1.4 Environmental levels and human exposure
As diesel fuels are complex mixtures, the environmental levels
have not been measured. The individual constituents of diesel fuels
can be detected in almost all compartments of the environment,
although their source cannot be verified. The general population may
be exposed to diesel fuel at filling stations and as a result of
spills.
Occupational exposure to diesel fuel occurs in a large number of
activities. Because of their low volatility, diesel fuels should
generate only low concentrations of vapours at normal temperatures,
but high operating temperatures can result in significant
concentrations.
A1.5 Effects on laboratory mammals and in-vitro test systems
The acute toxicity of diesel fuels is low after oral or dermal
exposure or after inhalation. The oral LD50 value was > 5000 mg/kg
body weight in all species tested (mouse, rabbit, rat, guinea-pig).
Dermal application resulted in an LD50 value of > 5000 mg/kg body
weight in mice and rabbits, although values of > 2000 mg/kg body
weight were reported for some kerosenes and middle distillates, with
different protocols and lower limit doses. The LC0 value in rats
exposed by inhalation was about 5 mg/litre, except for one
straight-run middle distillate for which a value of 1.8 mg/litre was
seen.
In rabbits treated dermally with up to 8000 µl/kg body weight per
day and mice with up to 40 000 mg/kg body weight per day, acanthosis
and hyperkeratosis due to severe irritation were seen. Rabbits were
more sensitive than mice. Inhalation of diesel fuel was neuro-
depressive in mice at concentrations up to 0.2 mg/litre but not
in rats exposed to up to 6 mg/litre. Body and liver weights were
reduced in rats.
Mice, rats, and dogs did not show significant cumulative toxicity
after inhalation of up to 1.5 mg/litre subchronically. The specific
nephropathy syndrome seen in male rats is linked to an inherent
accumulation of hyalin droplets in the renal tubules.
The only effects of long-term exposures were ulceration after
dermal application to mice (250 or 500 mg/kg body weight per day) and
significant alterations in organ weight after inhalation of 1 or
5 mg/litre by rats. In both studies the mean body weights were
reduced.
Various types of diesel fuels were slightly to severely
irritating to the skin of rabbits. Diesel fuels do not irritate the
eye, but some kerosenes have been reported to have a slight irritating
effect. Diesel fuels do not cause skin sensitization.
Diesel and jet fuels (kerosene) were neither embryotoxic nor
teratogenic in two studies in rats exposed by inhalation to 100 or
400 ppm and in one study in which rats were given up to 2000 mg/kg
body weight per day by gavage. In the last study, reduced fetal weight
was observed.
Tests in Salmonella typhimurium did not provide clear evidence
of mutagenicity. Some positive findings in S. typhimurium and in
mouse lymphoma cells were considered to be equivocal owing to the
inconsistency of the results. Tests for genotoxicity in mice in vivo
(induction of micronuclei or chromosomal aberrations) also gave
equivocal or negative responses.
Diesel fuels induced a low level of dermal carcinogenicity. In
the present state of research, it cannot be concluded whether the
carcinogenic potency of diesel fuels is mediated by a genotoxic
mechanism or by chronic dermal damage.
A1.6 Effects on humans
Non-occupational exposure to diesel fuel can occur during manual
filling of fuel tanks. The primary source of dermal exposure is
accidental spills, which result in immediate high levels of exposure
but are of short duration.
After accidental dermal contact, anuria, renal failure, gastro-
intestinal symptoms, and cutaneous hyperkeratosis have been reported.
Toxic lung disease has been observed after accidental ingestion of
diesel fuel and subsequent aspiration. Persistent productive cough has
been reported after inhalation. In a case-control study of men exposed
to diesel fuel, an increased risk for cancer of the lung other than
adenocarcinoma was found; a positive association was also seen with
prostatic cancer, although a higher risk was noted for the group with
'nonsubstantial' exposure than for that with 'substantial' exposure.
In a cross-sectional study of factory workers exposed to kerosene jet
fuels, dizziness, headache, nausea, palpitation, pressure in the
chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of
vapour from the fuel in the breathing zone was estimated to be
128-423 mg/m3.
A1.7 Effects on other organisms in the laboratory and the field
Diesel fuel is more toxic than crude oil to aquatic organisms and
plants. The ecotoxicity of diesel fuel is generally attributed to
soluble aromatic compounds, but insoluble aliphatic hydrocarbons may
also be implicated. Of the aromatic compounds, monoaromatics are the
least toxic, their acute toxicity increasing with molecular mass up to
the four- to five-ring compounds, although these are poorly soluble in
seawater. In some animals, e.g. fish and birds, physical coating of
the body surface by the fuel can produce toxicity and mortality.
Laboratory experiments have been carried out on diesel fuel,
water-soluble fractions, oil-water dispersions, and microencapsulated
oil. Diesel fuel did not significantly reduce the growth in culture of
the green alga Euglena gracilis, whereas a low concentration (0.1%)
almost completely inhibited the growth of Scenedesmus quadricauda.
Light diesel fuel (0.05%) stimulated the growth, photosynthesis, and
chlorophyll asynthesis of Chlorella salina but slightly inhibited
respiration; at higher concentrations, the growth rate and
photosynthesis were greatly reduced. Long-term exposure inhibited the
growth of the benthic algae Ascophyllum nodosum and Laminaria
digitata. In blue-green algae, photosynthesis was reduced by the
aromatic and asphaltic fractions but not by the aliphatic fraction.
Diesel fuel was acutely toxic to Daphnia spp., chironomid
larvae, and the mollusc Viviparus bengalensis (Gastropoda). A
concentration of 0.1 ml/litre caused the death of tidepool copepods,
Tigriopus californicus, within five days.
Mytilus edulismussels accumulate diesel fuel, have markedly
reduced feeding and growth rates, and show reproductive toxicity after
chronic exposure to diesel fuel. The EC50 for spawning in mussels
exposed for 30 days was about 800 µg/litre. The LC50 of micro-
encapsulated diesel oil after exposure of maturing mussels for
30 days was about 5000 µg/litre. Diesel oil was more toxic to larvae
than to juveniles: 10 µg/litre had adverse effects on the growth of
larvae.
Freshwater crabs (Barytelphusa cunicularis) exposed to sublethal
concentrations of diesel fuel for up to 96 h generally reduced their
oxygen consumption, particularly at lower exposures up to 8 h. With
longer exposures, the oxygen consumption was equal to or higher than
that of the controls.
In 96-h tests of acute toxicity in juvenile salmonids under
static conditions, diesel fuel was more toxic to pink salmon,
Onchorhychus gorbuscha (LC50: 32-123 mg/litre), than to cohosalmon,
O. kisutch (LC50: 2186-3017 mg/litre), or rainbow trout,
O. mykiss (LC50: 3333-33 216 mg/litre), irrespective of water type.
The threshold for detection of behavioural responses of cod
( Gadus morhua L.) exposed to diesel fuel in seawater was within
100-400 ng/litre. The Antarctic fish Pagothenia borchgrevinki
withstood an undiluted water-soluble fraction of diesel fuel oil for
up to 72 h but showed signs of stress.
Birds are affected externally and internally by oil
contamination. Diesel fuel destroys the waterproof nature of the
birds' plumage and is ingested during preening. Diesel and fuel oil
fed by gavage at 2 ml/kg body weight to ducks caused lipid pneumonia,
extreme inflammation of the lungs, fatty infiltration of the liver,
and hepatic degeneration after 24 h. Administration of diesel or fuel
oil at 1 ml/kg caused severe irritation of the digestive tract and
toxic nephrosis. Higher doses resulted in adrenal enlargement (mainly
due to hyperplasia of cortical tissue), depression of plasma
cholinesterase levels, ataxia, and tremors. Doses up to 20 ml/kg body
weight were not fatal to healthy birds, but the LD50 for diesel and
fuel oil administered to birds under stress was 3-4 ml/kg body weight.
After spills of diesel fuel, zooplankton appear to be highly
vulnerable to dispersed and dissolved petroleum constituents but less
so to floating oils. Aquatic organisms may be affected in a number of
ways, including direct mortality (fish eggs, copepods, and mixed
plankton), external contamination by oil (chorions of fish eggs and
cuticles and feeding appendages of crustaceans), tissue contamination
by aromatic constituents, abnormal development of fish embryos, and
altered metabolic rates.
A1.8 Evaluation of human health risks
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, during the handling of such fuels, and during use of kerosene
for domestic cooking or heating. Workers can be exposed to diesel fuel
and other middle distillates while handling and discharging the fuel
at terminals, storage tanks, and filling stations; during the
manufacture, repair, maintenance, and testing of diesel engines and
other equipment; during use of diesel fuel as a cleaning agent or
solvent; and in handling and routine sampling of diesel fuel in the
laboratory. Owing to the low volatility of diesel fuel, only low
concentrations of vapour are likely to occur at room temperature,
although in confined spaces at high temperatures significant levels
may be found.
Exposure to vapour is minimal during the normal handling of
diesel fuel. The most likely effect on human health is dermatitis
after skin contact. Diesel fuel is a skin irritant but does not appear
to irritate the eye. Acute toxic effects on the kidney can occur after
dermal exposure, but the effects of long-term dermal absorption of low
concentrations are unknown.
Diesel fuels are toxic when ingested, sometimes resulting in
regurgitation and aspiration, which can cause chemical pneumonia; the
same is true for any hydrocarbon in a particular range of viscosity.
In rodents exposed by inhalation to diesel fuel at concentrations
up to 0.2 mg/litre, a neurodepressive effect was seen in mice but not
in rats at the higher concentrations. Subchronic exposure by
inhalation to various distillate fuels induced specific
alpha2-microglobulin nephropathy in male rats; this effect is
considered irrelevant for humans.
Diesel fuels were neither embryotoxic nor teratogenic in animals
exposed orally or by inhalation.
There is no clear evidence of mutagenic activity in bacteria, and
the results of other tests for genotoxicity in vitro and in vivo were
equivocal.
A case-control study of workers exposed to diesel fuel suggested
an increased risk for cancer of the lung other than adenocarcinoma and
for prostatic cancer. In neither case was there an exposure-response
relationship. In view of the small number of studies available, the
small number of cases, and the correspondingly wide confidence
intervals, no conclusion can be drawn about the carcinogenicity to
humans of diesel fuel.
In mice, dermally administered diesel fuels had weak carcinogenic
potential. In view of the absence of clear genotoxicity, cancer could
be induced by nongenotoxic mechanisms, e.g. by chronic dermal
irritation characterized by repeated cycles of skin lesions, causing
epidermal hyperplasia.
A1.9 Evaluation of effects on the environment
The environment can be polluted by accidental release of diesel
fuel on a large scale, such as during tanker disasters and pipeline
leaks, or on a smaller scale from contamination of soil around
factories or garages. In water, diesel fuel spreads almost
immediately, polar and low-relative-molecular-mass components dissolve
and leach out, volatile components evaporate from the water surface,
and microbial degradation begins. The extent to which 'weathering'
takes place depends on the temperature and on climatic conditions. The
chemical composition of spills changes with time: after spillage on
water, some fractions evaporate, and the evaporated diesel components
are degraded photochemically; in sediment, diesel fuel appears
generally to be delivered to bottom sediments by settling particles;
in soil, the components of diesel fuel migrate at different rates,
depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates of biodegradation depend heavily on
physical and climatic conditions and on microbial composition.
Aquatic organisms, in particular molluscs, bioaccumulate
hydro-carbons to varying extents, but the hydrocarbons are depurated
on transfer to clean water. Diesel fuel may be bioaccumulated; no data
are available about biomagnification.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota. Recolonization
may occur after about one year, depending on the animal or plant
species and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can be affected by
external oil contamination and tissue accumulation: abnormal
development and altered metabolic rates are signs of the resulting
stress.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Diesel fuels are a gas-oil fraction occurring during petroleum
separation and commonly known as middle distillates (International
Agency for Research on Cancer, 1989a). Gas oils are generally blended
materials formulated to meet technical specifications (CONCAWE, in
press). Commercial diesel fuels contain aliphatic, olefinic,
cycloparaffinic, and aromatic hydrocarbons (see section A2.1.1), and
additives (see section A2.1.2) to improve their fuelling properties
(Sandmeyer, 1981).
Four qualities of diesel fuel are available commercially: diesel
fuel (general), diesel fuel No. 1, diesel fuel No. 2, and diesel fuel
No. 4 (see Table 1). The composition of diesel fuels is comparable to
that of heating oils (e.g. No. 1 and No. 2 fuel oils), except for the
additives (International Agency for Research on Cancer, 1989a; Agency
for Toxic Substances and Disease Registry, 1995).
Diesel fuel No. 2 is used mainly as automobile fuel and
corresponds to diesel fuel (general). In Europe (countries of the
European Union (EU) and European Free Trade Area (EFTA)), the
specifications for diesel fuel for transportation purposes are given
in European standard EN 590 (European Committee for Standardization,
1993), which also provides for changes in the specifications to meet
the requirements of different climatic conditions. In Sweden, two
further qualities of on-road diesel fuel are available: environmental
class 1 and class 2 (city diesel), with sulfur contents of 0.05% and
0.001%, respectively (Standardization Board in Sweden, 1991). The
sulfur content of city diesel corresponds to that of kerosene. Diesel
fuel for ship engines is covered by the International Standards
Organization (ISO) standard 8217 (International Standards
Organization, 1987). In the United States of America, three grades of
diesel fuel are available: diesel fuel No. 1 (relatively high
volatility) for road vehicle engines subject to frequent speed and
load changes; diesel fuel No. 2 (lower volatility) for industrial or
heavy-duty, high-load engines running at uniform speed; and diesel
fuel No. 4 (viscous) for low- and medium-speed engines such as those
used in ships (American Society for Testing and Materials, 1988,
1992).
Several types of kerosene, also derived from the gas-oil fraction
of petroleum separation, are used as aviation turbine fuels (JP fuels,
jet fuels). Their hydrocarbon composition is comparable to that of
diesel fuels.
A2.1.1 Fuel components
A2.1.1.1 Alkanes
Normal, branched, and cyclic alkanes (paraffins) are the most
abundant components (about 65-85%) of diesel fuels. Pristane
(2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-
tetramethyl-hexadecane) are of particular interest environmentally, as
the ratios of pristane to heptadecane and of phytane to octadecane
make it possible to identify the source of a fuel spill; furthermore,
as these ratios increase during biological degradation, they can be
used to estimate the age of an environmental contamination and the
degree of elimination. Cycloalkanes and bicycloalkanes constitute a
significant portion of the mixture, but individual compounds are
present only at low levels and are difficult to analyse. Alkyl
derivatives of cyclopentane, cyclohexane, and cycloheptane are common
components (Block et al., 1991; Table 2).
A2.1.1.2 Alkenes
Alkenes are not common components of crude oil but may be
present in diesel fuel if converted products are added after cracking.
These alkenes have predominantly branched and cyclic structures (Block
et al., 1991). The total alkene content of diesel fuels is up to 10%
(CONCAWE, 1985) (see also Table 2).
A2.1.1.3 Aromatic compounds
Aromatic compounds constitute 5-30% of automotive diesel fuel,
5-40% of marine diesel fuel (CONCAWE, 1985), and 10-30% of diesel
fuel No. 2 (Block et al., 1991). Table 2 shows the specifications of
commercial diesel fuels in this regard.
Table 1. Synonyms and trade names of commercial diesel fuels
Name CAS CAS Registry Range of Synonyms
name number carbon
numbers
Diesel fuel Diesel oil 68334-30-5 C9-C20a Auto diesel, automotive
(general) C10-C28b diesel oil, DERV, diesel,
diesel fuel oil, diesel
oil, gas oil
Diesel fuel Not assigned C9-C16c Diesel fuel oil No. 1,
No. 1 (essentially C4-C16 (for diesel oil No. 1, No. 1
equivalent to wide-cut dieseld
kerosene, aviation)c
8008-20-6)
Diesel fuel No. 2 68476-34-6 Diesel fuel, diesel fuel
No. 2 diesel (applicable for oil No. 2, diesel oil
fuel specific No. 2, No. 2 diesele
viscosity
limits)
Diesel fuel 68476-31-3 C10-C30f Marine diesel fuel,
No. 4 distillate marine diesel
fuel
Adapted from International Agency for Research on Cancer (1989a) and supplemented
a From CONCAWE (in press)
b From CONCAWE (1985); automotive gas oil (automotive diesel fuel, DERV)
c From CONCAWE (1985, 1995)
d In Europe, fuels similar to US diesel No. 1 are commonly referred to as
'kerosene' or 'Arctic diesel'
e Term uncommon in Europe. In the United Kingdom, distillate fuels are frequently
categorized as Class A1 (road diesel) and A2 (off-highway diesel)
f From CONCAWE (1985); distillate marine diesel
Only trace quantities of toxicologically relevant benzene,
toluene, ethyl benzene, and xylene compounds (for physicochemical
properties, see Table 6) are present in diesel fuel No. 2, but
significant levels are found in diesel fuel No. 1 (Arctic diesel),
which has lower flash-point specifications (Block et al., 1991); the
International Agency for Research on Cancer (1989a) cites a
flash-point of 0.25-0.5%. The concentration of benzene in kerosenes is
< 0.01% by volume; wide-cut aviation kerosenes may have higher
levels, but they are usually < 1% by volume (IPCS, 1986; CONCAWE,
1995; IPCS, 1993).
Table 2. Hydrocarbon specifications of some commercial diesel fuel oils
Specification Diesel Keroseneb Distillate Diesel
fuela marine fueld
dieselc
Paraffins/naphthenes 65-95e 78-96 60-90
(volume %) (wide-cut
aviation, < 1)
n-Hexane (volume %) < 0.01
(wide-cut
aviation, < 1)
Saturates (volume %) 59.4-76.6
Olefins (volume %) 0-10 0-5 0-10 0.0-1.0
Aromatics (volume %) 5-30 4-25 5-40 23.4-39.6
(wide-cut
aviation,
6-25)
Aromaticity (weight %) 11-15
See also Table 1
a From CONCAWE (1985, in press); fuel oil similar to diesel fuel (general)
b From CONCAWE (1985, 1995); fuel oil similar to diesel fuel No. 1
c From CONCAWE (1985, in press); fuel oil similar to diesel fuel No. 4
d From German Scientific Association for Petroleum, Natural Gas, and Coal (1991);
three samples of diesel fuel (general)
e Depending on origin of crude oil
Alkyl benzenes (particularly C3 and C4) are common components of
diesel fuel. Polycyclic aromatic hydrocarbons (PAHs), e.g.
naphthalene, phenanthrene, acenaphthene, acenaphthylene, fluorene,
fluoranthene, and pyrene, are also present, as are alkyl- and
cycloalkyl-substituted homologues of these substances, the predominant
ones being naphthalene and its methyl-substituted derivatives (see
Table 6 for physicochemical properties) (Block et al., 1991).
As some PAHs and the benzene, toluene, ethyl benzene, and xylene
components have been shown to be toxic and ecotoxic, these classes of
compounds are usually included in analytical procedures for
environmental contamination by diesel fuel. The PAH content of diesel
fuels varies widely, the highest levels being found in low-quality
fuel blended for large users (frequently railroad companies). The
mid-range aromatic (and PAH) content of diesel blends is limited by
the cetane number specification (Block et al., 1991) (see section
A2.1.3).
The concentrations of total PAHs in diesel fuel are < 5% by
volume, although some marine diesel fuels may contain > 10% by volume
(CONCAWE, 1985; International Agency for Research on Cancer, 1989a).
In straight-run gas-oil components, which are the major blending
material of diesel fuel (see section A3.2.1.1), three-ring PAHs
predominate; use of heavier atmospheric vacuum or cracker gas oils in
a diesel-fuel mixture leads to an increasing content of four- to
six-ring PAHs (CONCAWE, in press).
The concentrations of these constituents in a commercial diesel
fuel (unspecified) and, for comparison, in No. 2 fuel oil are shown in
Table 3. It should be noted that the PAH content of No. 2 fuel oil is
not limited by cetane number specification, so that it may include a
larger proportion of these hydrocarbons (Block et al., 1991). Table 3
also gives the composition of the water-soluble fractions of diesel
and No. 2 fuel oils and indicates how the solubility of a compound
affects the composition of the fraction and of the whole oil.
A2.1.1.4 Sulfur
The sulfur content of middle distillates depends on the source of
crude oil (Booth & Reglitzky, 1991; CONCAWE, 1995). The sulfur content
of most diesel fuels is 0.1-0.5% by weight; it is higher than that of
gasoline, which is about 0.02% by weight (Scheepers & Bos, 1992a).
Only diesel fuel No. 4 (distillate marine diesel) has a sulfur level
> 1% by weight (CONCAWE, 1985, in press). ISO standard 8217
(International Standards Organization, 1987) specifies a sulfur
content of marine diesel fuel of 1.0-2.0% by weight.
The sulfur content of some diesel fuels ranges from 0.01 to > 3%
by weight. In Europe (countries of EU and EFTA), the sulfur content is
restricted to a maximum of 0.2-0.3% by weight, and, as of 1996, it
will be further reduced to 0.05% by weight (European Commission,
1993). In the United States in 1988, the maximal sulfur content
permitted was 0.5% by weight for diesel fuels No. 1 and 2 and 2.0% by
weight for diesel fuel No. 4 (American Society for Testing and
Table 3. Concentrations of toxicologically relevant aromatic hydrocarbons in diesel fuel and No. 2 fuel oil and in 10% water-soluble
fractions prepared from them
Compound Diesel fuela Diesel fuelb No. 2 fuelc No. 2 fueld Water-soluble fractions (µg/litre)
(% by wt) (% by wt) (% by wt) (% by wt)
No. 2 Diesel
fueld fuela
Benzene 0.1 > 0.02 0.006-0.008 NR 550 344
Toluene 0.7 0.25-0.5 0.01-0.08 NR 1040 777
Alkylbenzenese 970
Ethylbenzene 0.2 0.25-0.5 0.01-0.08 NR 139
Xylene 0.5 0.25-0.5 0.01-0.08 NR 875
Polycyclic aromatic hydrocarbons
Naphthalene 0.4 0.273 0.4 840 6.6
1-Methylnaphthalene NR NR 0.82 340 66.2
2-Methylnaphthalene NR 0.67 1.89 480 108
Dimethylnaphalenese NR NR 3.11 240 NR
Trimethylnaphthalenese NR NR 1.84 30 NR
Fluorenese NR NR 0.36 20 NR
Phenanthrenese NR 0.15 0.53 20 NR
NR, not reported
a From Dunlap & Beckmann (1988); the analytical method is not described in detail; the concentration of benzene seems to be
unusually high.
b From International Agency for Research on Cancer (1989a); according to CONCAWE (1985), some marine diesel fuels may contain
more than 10% polycyclic aromatic hydrocarbons.
c From Stone (1991); summary of several reports
d From Anderson et al. (1974); US National Research Council (1985)
e Total of several isomers
Materials, 1988); in October 1993, the maximum was reduced to 0.05% by
weight (US Environmental Protection Agency, 1992a; American Society
for Testing and Materials, 1992). The permitted sulfur level in Brazil
is > 3% by weight (A. Sivak, personal communication, 1993). In Japan,
the sulfur content of diesel fuels was reduced to 0.2% by weight in
1994, and a further reduction, to 0.05% by weight, is under discussion
(CONCAWE, 1990a). Blended marine diesel fuel may also contain up to
about 15% residual components, i.e. material with an initial
boiling-point above about 350°C (CONCAWE, in press).
A2.1.2 Fuel additives
Only agents that are added to fuels at a concentration < 1% are
described as 'additives'. A more appropriate term for substances
present at higher concentrations is 'fuel components'. Fuels are
treated with additives for a number of reasons (see Table 4 and
below); they also differentiate products and determine the trademark
quality of commercial fuels (Fabri et al., 1990).
A2.1.2.1 Cetane number improvers
Cetane number improvers upgrade the ignition characteristics of a
base fuel more economically than refinery processes (Fabri et al.,
1990) (see section A2.1.3). Primary alkyl nitrates (e.g. isooctyl
nitrate) are often used to improve cetane number. Polyethyleneglycol
dinitrates, although effective at much lower concentrations, have a
number of disadvantages, including their price and the fact that they
may not improve the performance of fuel in low-compression engines
(Russell, 1989).
A2.1.2.2 Smoke suppressors
Organometallic compounds containing barium, calcium, manganese,
or iron have been used to reduce diesel smoke. With barium-based
products, 85-95% of the metal is emitted as particulates in the
exhaust (Russell, 1989); however, barium and calcium compounds are no
longer used.
A2.1.2.3 Flow improvers
Cold-flow improvers increase the fluidity of the fuel by
modifying the growth of wax crystals formed by higher homologues of
paraffins at low temperatures. The wax content of diesel fuels is
influenced by the origin of the crude oil, the distillation range of
the fuel, and the source of blend components (Coley, 1989).
Table 4. Diesel fuel additives
Additive Material Concentration Effect
(ppm)
Ignition improvers, Organic nitratesa Enhancement of self-ignition qualitiesa
cetane enhancersa
Smoke suppressors, Organic compounds of Ca, Ba, Reduction of soot; increase in metal
combustion enhancersa or (sometimes)Mga sulfate emissionsa
Detergentsa Amines, imidazolines, Prevention and removal of coke deposits
succinimides, etc.b on fuel injector tips, etc.a
Flow improvers Olefin-ester copolymers 50-500 Interaction with wax crystals and
modification of their growth; prevention
of formation of agglomerates
Cloud-point depressors Olefin-ester copolymers About 1000 Depression of cloud-point; prevention of
formation of agglomerates
Wax anti-settlers Modified ethylene-vinyl 100-500c Reduction of crystal size and rate of
acetate copolymersc settling; prevention of formation of agglomerates
Anti-static agents Not reported Not reported Reduction of building up of charges of
static electricity
Anti-corrosion chemicals Alkenyl succinic acids and 5-50 Prevention of corrosion or rusting of
esters, dimer acids, amine storage tanks, pipelines, and metal fuel
salts system components
Table 4 (contd)
Additive Material Concentration Effect
(ppm)
Antioxidants Hindered phenols or amines 25-200 Prevention of aging processes
Anti-foam agents Silicones Up to 20 Reduction of foaming tendency; reduction
of risk of ground pollution from oil spills
Dehazers Quaternary amine salts 5-50 Reduction of formation of hazes;
acceleration of haze clearance
Biocides Imines, amines, About 200 Prevention of growth of bacteria and
imidazolines, etc. fungi in storage tank bottoms
Lubricants Surface-active agents such 50-500 Compensation of lower viscosity of fuels
as polyfunctional acids and in low-temperature regions
derivatives
Odour maskers Natural, identical substances, 10-100 Reduction or elimination of smell
such as vanillin and terpenesc
From Coley (1989), except as noted
a From Organisation for Economic Co-operation and Development (1993)
b From Russell (1989)
c From Fabri et al. (1990)
A2.1.2.4 Cloud-point depressors
Diesel fuels must be easily filterable, as wax crystals formed at
low temperatures can clog fuel filters. Cloud-point depressors are
therefore added (Fabri et al., 1990) which consist of substances with
lower cloud-points, e.g. kerosene. Addition of 10% kerosene lowers the
cloud-point of diesel fuel by about 2°C. Olefin-ester copolymers
depress the cloud-point by 3-4°C but are not currently in commercial
use (Coley, 1989).
A2.1.2.5 Wax anti-settling additives
These additives inhibit the tendency of wax to settle by reducing
the crystal size and slowing the settling rate. A five-fold reduction
in wax crystal size slows the settling rate by one-twenty-fifth. With
increasing temperature, small dispersed crystals redissolve more
readily than settled wax (Coley, 1989).
A2.1.2.6 Other additives
Detergents, including amines, amides, imidazolines, and
succinates, are used to reduce injector nozzle fouling. Detergents
such as polyalkenyl succinimides also improve fuel stability,
resistance to corrosion, and combustion efficiency (Russell, 1989).
Antistatic agents lower the risk of building up a charge of static
electricity during pumping at high rates at bulk terminals or in
large-capacity truck fuel tanks.
Other additives used are anti-oxidants (phenols, amines),
anti-corrosion chemicals (alkenyl succinic acids, esters, dimer acids,
amine salts), anti-foam agents (silicones), dehazers (anti-emulsion
agents) (quaternary ammonium salts), biocides, lubricants for cold
regions (surface-active polyfunctional acid derivatives), and odour
maskers (vanillin, terpenes) (Coley, 1989; Fabri et al., 1990).
A2.1.3 Quality of diesel fuels
A2.1.3.1 Ignition performance and cetane number
The cetane number determines the ignition performance of
transport fuels relative to a scale on which methyl naphthalene
corresponds to a combustion rate of 0 and cetane to one of 100
(Scheepers & Bos, 1992a). The cetane number is calculated by comparing
the ignition quality of a fuel with that of two reference fuel blends
of known cetane numbers under standard operating conditions (American
Society for Testing and Materials method D 613 CFR). A high cetane
number improves cold starting and engine durability and reduces noise,
fuel consumption, smoke emissions during warm-up, and exhaust
emissions (Russell, 1989). In Europe (countries of the EU and EFTA),
the minimal cetane number must be in the range 45-49, depending on the
climatic conditions (European Committee for Standardization, 1993);
cetane numbers are usually 49-53 (CONCAWE, 1987). In the United
States, the cetane number must be at least 30 for diesel fuel No. 4
and 40 for diesel fuels No. 1 and 2 (American Society for Testing and
Materials, 1988, 1992).
A2.1.3.2 Density
The density of diesel fuel influences engine performance: higher
density leads to enrichment of the fuel:air mixture, which results in
greater engine power output. Enrichment may, however, increase the
particulate content of exhaust gas emissions (Fabri et al., 1990) (see
section B3.1.2.2). A density range is specified in fuel standards in
some countries (American Society for Testing and Materials, 1992;
European Committee for Standardization, 1993).
A2.1.3.3 Sulfur content
Gas and particle emissions in diesel engine exhaust are
influenced by the sulfur content of the fuel; there is a direct
relationship between particle production and sulfur content (Hare,
1986) (see section B3.1.2.2).
A2.1.3.4 Viscosity
Too low a viscosity can lead to wear in the injection pump; too
high a viscosity impairs fuel injection and mixture formation (Fabri
et al., 1990). In Europe (countries of the EU and EFTA), the viscosity
of commercial diesel fuels at a temperature of 40°C must be
1.5-4.5 mm2/s (European Committee for Standardization, 1993). In the
United States, the permitted ranges of viscosity at 40°C are
1.3-2.4 mm2/s for diesel fuel No. 1, 1.9-4.1 mm2/s for diesel fuel
No. 2, and 5.5-45.0 mm2/s for diesel fuel No. 4 (American Society
for Testing and Materials, 1988, 1992).
A2.1.3.5 Cold-flow properties
Cloud-point and cold filter plugging point characterize the
behaviour of diesel fuels at low temperatures (Fabri et al., 1990).
These points are lowered by the addition of cloud-point depressors and
by special blending techniques, e.g. increasing the kerosene content
of the fuel (see section A2.1.2).
Changes in diesel fuel quality have been assessed. Wade & Jones
(1984) reported a deterioration in fuel quality with decreasing cetane
number and found that a 90% increase in boiling-point led to greater
emissions of particulates, nitrogen oxides and PAHs. A decline in
diesel fuel quality on the European market was predicted, as the
rising demand would lead to greater use of fuels from catalytic or
thermal cracking processes (CONCAWE, 1987) (see section A3.2.1.1). A
study by the Organisation for Economic Co-operation and Development
(1993) indicated an improvement in the quality of diesel fuel in the
United States due to a decline in aromaticity.
A2.2 Physical and chemical properties
Diesel fuel is a brown, slightly viscous, flammable liquid at
room temperature (Sandmeyer, 1981). It generally has a kerosene-like
odour (Agency for Toxic Substances and Disease Registry, 1995). Its
physical and chemical properties are listed in Table 5.
The water solubility of diesel fuels varies. The aqueous
solubility of crude and fuel oils in the environment is clearly
dependent on the salinity of the water and the age of the oil slick
(see section A4.1.1) and is of the same order of magnitude as the
solubility of fuel oils: at room temperature, 0.37-0.53 mg/litre in
sea water (Boehm & Quinn, 1974) and 0.7-11 mg/litre in tap water
(Lysyj & Russell, 1974). The solubility of a fuel slick decreases with
its age as the concentration of long-chain hydrocarbons increases; the
solubility of fresh crude oil is 29.3-32.3 mg/litre, whereas that of
weathered crude oil is 0.06-23.2 mg/litre at 25°C (Mackay & Shiu,
1976).
The physicochemical properties of the toxicologically relevant
benzene, toluene, ethyl benzene, xylene and PAH components are given
in Table 6.
A2.3 Analytical methods
Exhaustive identification and quantification of the individual
constituents of commercial diesel fuel (see section A2.1) is almost
impossible owing to their number and complexity. In most environmental
assessments, therefore, the mixture is analysed as total petroleum
hydrocarbon (Block et al., 1991). All such methods involve preliminary
solvent extraction of the matrix, with e.g. trichlorotrifluoroethane.
This step may also extract naturally occurring hydrocarbons, which
interfere with the analysis; however, some of these compounds are
polar and can be removed on silica gel. Three analytical methods are
available:
Table 5. Physicochemical properties of diesel fuels
Property Diesel fuel Diesel fuel Diesel fuel Diesel fuel
(general) No. 1 No. 2 No. 4
Melting-point (°C) - 34a 18a - 29-9a
Boiling range (°C) 160-190b 145-300 (wide-cut 282-338a 170-420d
143-384e aviation, 45-280)c 101- > 588a
193-293a
Flash-point (°C) > 56b > 21 - < 55 52 (closed cup)a > 56d
58-66e (wide-cut aviation, > 54 (closed cup)a
(Pensky-Martens) < 21)c
38 (closed cup)a
Autoignition temperature (°C) 177-329a 254-285a 263a
Density (g/cm3) 0.81-0.90 (15°C)b 0.805 (wide-cut 0.87-0.95 0.87-0.92 (15°C)d
0.82-0.84 (15°C)e aviation, 0.75-0.801) (20°C) 0.81-0.94 (15°C)
(15°C)c 0.81-0.94 1 (20°C)
(15°C)
Kinematic viscosity (mm2/s) 2-7.4 (40°C)b 1.5-2.5 (wide-cut 2-7.4 (40°C)d
2.20-3.25 (40°C)e aviation, about 1.1
(20°C)c
Vapour pressure (kPa) About 40 (40°C)b About 10 (wide-cut 2.83-35.2 2.83-35.2 (21°C)a
0.04f aviation, 140-210) (21°C)a
(Reid, 37.8°C)c
2.83-35.2 (21°C)a
Table 5 (contd)
Prpoerty Diesel fuel Diesel fuel Diesel fuel Diesel fuel
(general) No. 1 No. 2 No. 4
Water solubility (mg/litre) 1f About 5 (20°C)a About 5 (20°C)a About 5 (20°C)a
0.2f
Henry's law constant 4.3 × 103f 6.03-7.5 × 105a 6.03-7.5 × 105a 6.03-7.5 × 105a
(Pa × m3/mol) (20°C)
n-Octanol-water partition 3.3-7.06a 3.3-7.06a 3.3-7.06a
coefficient (log Kow)
Soil sorption coefficient 3.04f 3.0-6.7a 3.0-6.7a 3.0-6.7a
(log Koc)
Diffusion coefficient in air 4.63 × 10-2f
(cm2/s)
Odour threshold (ppm) 0.7a 0.5g
a From Agency for Toxic Substances and Disease Registry (1995)
b From CONCAWE (in press); automotive gas oil
c From CONCAWE (1985, 1995); kerosenes
d From CONCAWE (in press); distillate marine diesel
e From German Scientific Association for Petroleum, Natural Gas, and Coal (1991); average of three samples
f From Custance et al. (1993); water solubility measured, other data from literature; no further details
g From Fraser Williams (Scientific Systems) Ltd (1985)
-- Gravimetric detection: determination of the weight of residue
remaining after solvent evaporation. Although this method is
useful for measuring gross contamination, it cannot be used in
trace analysis.
-- Infrared detection: the absorbance of petroleum hydrocarbons is
detected in a solvent matrix at the maximal value, near
2930 cm-1. This carbon-hydrogen bond stretching absorbance is
directly related to the hydrocarbon concentration in the extract.
The results are dependent on the hydrocarbon standard used for
quantification.
-- Gas chromatographic detection: capillary gas chromatography
combined with flame ionization or mass spectrometric detection.
The gravimetric and infrared techniques are fast, relatively
simple and widely accepted by regulatory authorities; however, they do
not provide sufficient qualitative and quantitative information on
composition. Gas chromatography is therefore the standard procedure
used to identify and quantify fuel constituents in environmental
samples. Different distribution and degradation processes in
environmental compartments (see section A4) mean that the composition
of petroleum hydrocarbons in air, water, soil, and biota may differ
considerably from that of the original fuel. Newton et al. (1991)
developed an analytical method for identifying and quantifying traces
of diesel contamination in tinned fish products on the basis of the
n-alkane pattern.
Because of the suspected toxicity of aromatic components in
diesel fuel, a detailed analysis is often necessary. Two groups of
compounds are detected:
-- volatile aromatic compounds: Analysis of these minor components
of diesel fuel requires special enrichment techniques, such as
purge-trap gas chromatography, and methods of detection including
flame ionization and mass spectrometry.
-- PAHs: Gas chromatography with flame ionization or mass
spectrometric detection, or liquid chromatography, is used after
solvent extraction. As PAHs are poorly resolved from the diesel
fuel matrix, gas chromatography-mass spectrometry is necessary in
most cases to quantify the components. The target analytes of
commonly used analytical methods do not, however, include C3
and C4 alkyl-substituted benzenes or most alkyl-substituted
PAHs in diesel fuels.
Table 6. Physicochemical properties of aromatic components of diesel fuel
Aromatic compound Water solubility Henry's law constant Diffusion coefficient (cm2/s) Octanol-water partition
(mg/litre) (Pa × m3/mol) In air In water coefficient (log Pow)
Benzene 1791 550 0.087 9.8 × 10-6 1.99
Toluene 534.8 602 0.083 8.6 × 10-6 2.52
Ethylbenzene 161.0 855 0.076 7.8 × 10-6 2.94
Xylene 156.0 778 0.076 1 × 10-5 2.94
Polycyclic aromatic 0.067 0.007 0.067 2.12 × 10-6 5.30
hydrocarbons
Adapted from Custance et al. (1993)
A2.4 Conversion factors
As diesel fuel vapour is a complex mixture of gases, it is
impossible to give a conversion factor for converting parts per
million in the gaseous phase to SI units.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
Diesel fuels are derived from crude oil, which can be considered
a 'natural product'. Nevertheless, human and environmental exposure
results almost exclusively from anthropogenic activities.
A3.2 Anthropogenic sources
A3.2.1 Production and use
A3.2.1.1 Production process
Diesel fuel is produced during the refining of crude oils but is
generally blended to meet the specifications for technical
performance. The blending components may be produced by atmospheric
distillation of crude oil (straight-run atmospheric gas oil), vacuum
distillation of atmospheric residue (vacuum gas oil), thermal cracking
(thermally cracked gas oil), or catalytic cracking processes (e.g.
light catalytically cracked gas oil; cycle oil). The main components
are straight-run gas oils: 80% of European automotive diesel fuel is
made up of these components (Booth & Reglitzky, 1991). Secondary
processing of heavier fractions is increasingly necessary in order to
meet product demand (CONCAWE, in press).
Diesel fuel No. 1 is manufactured by a straight-run distillate
process (Agency for Toxic Substances and Disease Registry, 1995);
diesel fuel No. 2 is generally made by mixing straight-run and
catalytically cracked distillates; and diesel fuel No. 4 is produced
by adding blending stocks to distillation residues in order to meet
viscosity specifications (International Agency for Research on Cancer,
1989a). Further variations are introduced by formulation with
additives to improve fuel properties (see section A2.1.2).
A3.2.1.2 Use
Diesel fuel is widely used as a transport fuel for light- and
heavy-duty vehicles; the Organisation for Economic Co-operation and
Development (1993) classifies vehicles weighing < 3.5 t as light-duty
and those weighing > 3.5 or, occasionally, > 5 t as heavy-duty.
Diesel fuel No. 1 is suitable for engines that undergo frequent
changes in speed and load (Agency for Toxic Substances and Disease
Registry, 1995). Heavier grades (diesel fuels No. 2 and 4) are used
for trucks, railroad and marine diesel engines, and stationary engines
in continuous high-load service (Sandmeyer, 1981; International Agency
for Research on Cancer, 1989a). Diesel fuels are also used in
stationary gas turbines, e.g. to generate electric power during
peak-load periods. Residual fuel oils, such as diesel fuel No. 4, are
used to generate steam in electric power plants (International Agency
for Research on Cancer, 1989a), in commercial and industrial burner
installations without preheating facilities, in plants and factories
for space and water heating, for pipeline pumping, and in gas
compression; they are also sprayed on unmade roads to compact the
surface and are used in the manufacture of asphalt cement (Agency for
Toxic Substances and Disease Registry, 1995).
A3.2.1.3 Production and consumption levels
The demand for diesel fuel has increased worldwide over time. In
1990, world demand was about 1100 kt/day (Booth & Reglitzky, 1991).
The production and consumption of diesel fuel in different regions
over time are shown in Table 7.
Diesel-fuelled passenger cars are relatively common in western
Europe. The percentages of passenger cars with diesel engines in
various European countries over time are shown in Table 8. In many
European countries, taxi-cabs are equipped almost exclusively with
diesel engines. In 1985, diesel-fuelled heavy-duty trucks comprised
about 86% of the fleet in Norway, 89% in Denmark, 95% in Sweden, and
100% in the former Federal Republic of Germany. In some countries of
Europe, diesel fuel consumption has increased steadily over the last
decade, and consumption for heavy-duty vehicles is predicted to more
than double between 1980 and 2005 (Organisation for Economic
Co-operation and Development, 1993). In western Germany, diesel fuel
consumption was almost 60% higher in 1990 (about 5100 kt) than in 1984
(about 3200 kt) (Federal Ministry for Transport, 1992).
Diesel-fuelled passenger cars are less common in North America.
In the United States in 1986, about 1.6% of passenger cars were
diesel-fuelled, and the tendency was predicted to decrease slightly
(US Department of Energy, 1988). In contrast, 82% of heavy-duty trucks
were diesel-fuelled in 1985 (Organisation for Economic Co-operation
and Development, 1993), and 59300 kt of diesel fuel were consumed by
highway traffic in 1986, representing 14.8% of all the highway fuel
used and about one-half of United States diesel fuel consumption.
On-road use of diesel fuel was predicted to increase to 15.5% of all
highway fuel (66600 kt) in 1995. A difference in fuel use patterns is
seen between light- and heavy-duty vehicles. In 1986, about 4000 kt
were used by passenger cars and light trucks, with an estimate of
about 2300 kt for 1995, whereas in 1986 about 55300 kt were used by
heavy-duty trucks, with an estimate of 64300 kt for 1995 (US
Department of Energy, 1988). In Canada, only about 1% of passenger
cars are equipped with diesel engines. In 1992, 14126 kt of diesel
fuel were produced; 13508 kt were sold for domestic purposes (heating)
and about 10849 kt for on-road use (Thomas, personal communication,
1994).
The percentage of diesel-fuelled vehicles is increasing in Japan
(Table 9).
Table 7. Production and use of diesel fuel (including gas oils in
Europe) in different regions, and development over time
Region Production/ 1975 (kt) 1980 (kt) 1985 (kt)
Use
United States Production 134 967 138 323 135 181
Use 133 300 136 161 130 297
Canada Production 19 187
Use 19 130
OECD (all 24 Production 370 240 408 848 372 728
countries) Use 380 181 403 642 386 081
OECD (Europe) Production 173 474
Use 190 960
European Union Production 152 091
Use 163 132
Australia, Japan, Production 44 886
New Zealand Use 45 694
Adapted from International Agency for Research on Cancer (1989a);
OECD, Organisation for Economic Co-operation and Development
A3.2.2 Emissions during production and use
A3.2.2.1 Air
No data are available on emissions during the production and use
of diesel fuel. Release to the atmosphere during production in the
refining process is unlikely, as closed systems are used, but
volatilization may occur during storage and transport. Diesel fuel may
be released into the atmosphere as a result of spills during storage
and transport and at filling stations during bulk storage and vehicle
tank filling. The low-relative-molecular-mass constituents
(short-chain alkanes, benzene, toluene, ethyl benzene, and xylene
compounds) are the most likely to evaporate under environmental
conditions.
Table 8. Percentage of diesel passenger cars in western Europe, and
development over time
Country Diesel passenger cars (%)
1984 1988 1991 1992a
France 15.8 21.1 25.5 38
Germany (without former 16.9 15.1 15.3 14.9
German Democratic Republic) (1993)b
Germany (without former About 8 13.6 13
German Democratic Republic)c
Italy 18.7 11.9 7.4 NR
Spain (without station wagons) 15.3 10.5 14.6 NR
United Kingdom 1.1 4.5 11.3 12.5
United Kingdomd NR 5 9 19 (1993)
Adapted from American Automobile Manufacturers Association (1993),
unless otherwise stated; NR, not reported
a From Menne et al. (1994)
b With the former German Democratic Republic
c From German Institute for Scientific Research (1993)
d From Quality of Urban Air Review Group (1993)
Table 9. Percentage of diesel-fuelled vehicles in Japan and
development over time
Year Passenger cars (%) Trucks (%) Buses (%)
1984 3.8 23.7 49.5
1988 5.8 28.5 63.2
1991 8.3 40.7 81.7
Adapted from American Automobile Manufacturers Association (1993)
A3.2.2.2 Water
No data are available on effluents and emissions during diesel
fuel production. Diesel fuel oils may be released to surface waters as
a result of leakage from storage tanks or tankers (see section
A3.2.3). In the United States, the total volumes of spills of diesel
fuel oils in 1991 were (Agency for Toxic Substances and Disease
Registry, 1993): diesel fuel No. 1, 10.6 t (20 notations); diesel fuel
No. 2, 8.9 t (28 notations); and diesel fuel No. 4, 39.3 t (35
notations).
Groundwater can be contaminated with diesel fuel constituents by
leakage from underground bulk storage tanks, but quantitative data are
lacking. In the Province of New Brunswick, Canada (Thomas, personal
communication, 1993), of a total of 671 gasoline, diesel, and fuel oil
tanks examined in 1987, 11% leaked diesel or fuel oil; in 1989, 6% of
1085 tanks had leaks; and in 1991, 13% of 539 tanks leaked fuel. No
data were available about the amounts leaked, and diesel contamination
was determined on the basis of a cluster of 'middle-range peaks'
detected by gas chromatography.
On the basis of chronic petroleum inputs to sediments of
Narragansett Bay and Rhode Island Sound, United States, Van Vleet &
Quinn (1978) estimated that about 200 kt of petroleum hydrocarbons may
be released to surface waters worldwide from municipal treatment
plants. The precise source of the inputs cannot be verified in most
cases, but they are due, for example, to accidental discharge to sewer
systems, disposal of used crankcase and lubricating oils, oil washed
from roads, and atmospheric deposition of hydrocarbons.
No data were available from other countries.
A3.2.2.3 Soil
Diesel fuels may be released to soil as a result of accidental
spills (see section A3.2.3) and leakage from storage tanks or
pipelines. Diesel-contaminated soil is a major problem in railroad
yards, where diesel fuel is used in locomotives and as a solvent to
clean moving metal parts (the remaining paraffins give a waxy
anti-corrosive coat). Spillage during refuelling, engine maintenance,
and steam cleaning, leakage from fuel storage tanks, and absorption of
diesel fuel on sand used for traction are other possible sources of
soil contamination (Dineen, 1991); however, no quantitative data are
available.
A3.2.3 Accidental releases to the environment
Data on the accidental release of diesel fuels are summarized in
Table 10. The effects on the environment depend on both the amount and
environmental conditions.
Table 10. Diesel fuel spills and their effects on the environment (see also section A9.2)
Year Place Amount (t); type Effects Reference
of diesel fuela
Aquatic environment
1972 North of Puget Sound, > 600; diesel fuel Substantial mortality of some intertidal taxa; Woodin et al. (1972)
Washington State, USA No. 2 larval recruitment within six months
1973 East Lamma Channel 2000-3000; Almost total kill of meiofauna within four days; Wormald (1976);
near Hong Kong diesel fuel No. 4 heavy mortality among bivalves and molluscs, Stirling (1977)
Nerita albicilla and Monodonta labio, and in
marine fish farms; almost no effect on
Clypeomorus humilis or Planaxis sulcatus
1978 Svea, Mijenfjord, Norway 111 Most fuel trapped in ice; transport out of fjord Carstens &
during break-up; heavy mortality among shoreline Sendstad (1979)
invertebrates in some regions of fjord
1983 Yaquina Bay, Oregon, About 240 (with Decline in population of Rhepoxynius abronius Kemp et al. (1986)
USA Bunker C oil) by 75%; influence of spill not clear
1984 Queen Charlotte 130 (plus 30 t Low fuel levels in water during flood tide, high McLaren (1985)
Islands, Canada gasoline) levels during ebb as diesel was retained in
sediment; high mortality among barnacles
Table 10 (contd)
Year Place Amount (t); type Effects Reference
of diesel fuela
1987 Macquarie Island, 230; diesel fuel High mortality among one species of holothuroid, Pople et al. (1990)
Australia No. 4 one of isopod, one of limpet, and one of chiton;
decreased populations of crab, two species of
starfish, and gastropod; small number of dead
algae; slow recovery of organisms
1989 Arthur Harbor, 510; diesel fuel High mortality among limpets; partial recovery one Kennicutt et al.
Antarctica No. 1 year after spill; small effect on macroalgae (1991a,b,1992a,b)
Terrestrial environment
1983 San Bernadino County, 3 Hydrocarbon contamination up to 1500 mg/kg; Frankenberger et al.
CA, USA plume migrating towards surface water; in-situ (1989)
bioremediation (see also section A3.2.4)
NR Califonia, USA 193; diesel fuel Contamination at 6-34 m below surface; Peters et al. (1992)
No. 2 hydrocarbon levels at 18.3, about 1500 mg/kg;
remediation by flooding with surfactants
(see also section A3.2.4)
aWhen available
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few data are available on the environmental fate of diesel
fuels, but there are adequate data on the environmental behaviour of
individual hydrocarbon components. The transport, distribution, and
transformation of crude oils and some fuel oils (e.g. heating oils
No. 1 and 2) have been studied, and the mechanisms of distribution and
transformation are considered to be comparable to those of diesel
fuel.
The calculated half-lives for the toxicologically relevant PAH
components are listed in Table 11.
A4.1 Transport and distribution between media
Diesel fuels are released into the environment mainly during
storage, transport, and use (see section A3.2.3). The hydrosphere and
geosphere are the affected compartments.
A4.1.1 Evaporation from and dissolution in the aqueous phase
Oils and fuels spilled on water spread out almost immediately;
polar and low-relative-molecular-mass components dissolve and leach
out of the slick; and volatile components evaporate from the water
surface. With the start of microbial degradation the pattern of
substances changes, as was observed, for example, after a major oil
spill off the French coast (Atlas et al., 1981). The sum of these
processes is known as chemical and biological 'weathering'. At the
same time, the oil is emulsified to oil-in-water and water-in-oil
('mousse') emulsions (Atlas & Bartha, 1973). In laboratory experiments
with several diesel fuels, however, stable mousses were not formed at
any temperature (US National Research Council, 1985). Experiments
with South Louisiana crude oil showed that the evaporation rate was
more than twice the dissolution rate (Harrison et al., 1975). The loss
by evaporation of oil spread on water, which in oil spills amounts to
about 25% of the total released mass, is generally dependent on the
following factors (US National Research Council, 1985):
-- the exposed area, which increases with time as the film spreads;
-- the oil-phase vapour pressures, which decrease with time as the
low-relative-molecular-mass components evaporate. Components
with vapour pressures higher than that of n-octane evaporate
rapidly from the surface of spilled fuel oil, while those with
vapour pressures lower than that of n-octadecane persist and
form a viscous residue that retards the volatilization of other
constituents (Regnier & Scott, 1975);
-- the oil-air mass transfer coefficient, which depends on wind
speed and hydrocarbon diffusivity (for the diffusion coefficient
in air, see section A2.2, Table 5);
-- diffusion barriers, such as emulsions, or formation of a 'skin'
on the surface. Many surface waters have a thin layer of organic
material of natural origin on the surface, and these films may
reduce spread and volatilization, altering the persistence and
concentration of the oil in water (Edgerton et al., 1987).
Table 11. Calculated half-lives of polycyclic aromatic hydrocarbon
components in environmental compartments
Component Air Water Soil Sediment
Naphthalene 1 day 1 week 2 months 8 months
Acenaphthalene 2 days 3 weeks 8 months 2 years
Fluorene 2 days 3 weeks 8 months 2 years
Phenanthrene 2 days 3 weeks 8 months 2 years
Anthracene 2 days 3 weeks 8 months 2 years
Pyrene 1 week 2 months 2 years 6 years
Fluoranthene 1 week 2 months 2 years 6 years
Chrysene 1 week 2 months 2 years 6 years
Benz[a]anthracene 1 week 2 months 2 years 6 years
Benzo[a]fluoranthene 1 week 2 months 2 years 6 years
Benzo[a]pyrene 1 week 2 months 2 years 6 years
Perylene 1 week 2 months 2 years 6 years
Dibenz[a,h]anthracene 1 week 2 months 2 years 6 years
Adapted from Mackay et al. (1992)
On the basis of the constant of Henry's law (see section A2.2,
Table 5) and the classification of Thomas (1990), diesel fuels can be
considered to volatilize significantly from the aqueous phase. The
constant may change during volatilization, as the composition of the
mixture changes with time. Lockhart et al. (1987) observed a clear
decrease in the hydrocarbon composition of the 50% water-soluble
fraction of diesel fuel in open-aerated test cylinders. Within 48 h,
the concentration of hydrocarbons with a very low boiling-point
(< 115°C) decreased from 0.73 to 0.04 mg/litre and that of
hydrocarbons with a low boiling-point (115-270°C) from 3.2 to
0.17 mg/litre. Under sealed or open non-aerated conditions, there were
only minor changes. Regnier & Scott (1975) showed that n-decane
volatilizes from diesel fuel with a half-life of 1.65 h at 30°C,
whereas the half-life was 9.67 h at 5°C in a darkened chamber with a
constant wind speed of 21 km/h. Edgerton et al. (1987) reported that
diesel fuel spilled on ice had lost only 2-4% of its lightest fraction
after 10 days.
Dissolution of spilled oils and fuels in water is less important
from the viewpoint of mass loss than the ecotoxic effects of the
mixture. Lysyj & Russell (1974) conducted long-term experiments (42
days) on the dissolution of different fuel oils and gasoline in water.
After a period of stable concentration, during which physical
dissolution was probably the main mechanism for oil-to-water transfer,
the dissolution of organic compounds accelerated, probably because of
chemical modification by oxidation or microbial action of the
water-insoluble petroleum fraction.
Under equilibrium mass-transfer conditions, the levels of PAHs
(naphthalene and its methyl derivatives, acenaphthene, fluorene,
phenanthrene, anthracene, and fluoranthene) in the water phase in
contact with commercial automobile diesel fuel were about four to six
orders of magnitude lower than those in the diesel fuel itself, i.e.
in the low microgram per litre range. The concentrations of these
constituents were likely to be smaller under non-equilibrium
conditions but might be higher in the presence of surfactants,
emulsifiers, or co-solvents (Lee et al., 1992). When No. 2 fuel oil
and kerosene were placed in contact with drinking-water for 17 h,
although each fuel contained about 50% by weight of aliphatic
hydrocarbons, the water-soluble fractions contained primarily aromatic
compounds: total, > 93% by weight; proportion of benzene and
substituted derivatives, 19.4% by weight in No. 2 fuel oil and 53.2%
by weight in kerosene (Coleman et al., 1984). The chemical composition
of the water-soluble fractions of No. 2 fuel oil is compared with that
of the whole oil in Table 3.
During chemical weathering, other changes in composition (e.g.
autoxidation of n-alkanes) may occur that are not necessarily
associated with a net mass loss (Davis & Gibbs, 1975).
A4.1.2 Transport in and adsorption onto soil and sediment
A4.1.2.1 Soil
The movement of diesel fuel through soil is directly correlated
with its kinematic viscosity (see section A2.2, Table 5). As the
viscosity is about one-half that of water and about one-fourth to
one-fifth that of gasoline, the rate of percolation of water into soil
is about twice and that of gasoline about four to five times that of
diesel fuel (Stone, 1991). The retardation coefficient (Rd) is an
indicator of the migration of substances into groundwater. In the
aqueous phase, the maximal rate of movement of dissolved compounds is
that of groundwater, which is represented by an Rd of 1. Stone (1991)
calculated the Rd from the adsorption isotherms of individual diesel
constituents, and derived the following classification:
-- low mobility (Rd > 100): fluorene, phenanthrene, pyrene,
benzanthracene, benzo[ a]pyrene, fluoranthene;
-- medium mobility (100 > Rd > 10): naphthalene, dimethylbenzenes,
ethylbenzene, toluene;
-- high mobility (Rd < 10): benzene, quinoline, cresols, phenol.
In effect, soil contaminated with diesel fuel acts like a
chromatographic column, separating individual constituents by their
adsorption. Experimental data on the adsorption of diesel fuels on
soils and their transport are not available; however, the movement of
a synthetic kerosene through a clay-sand soil was dependent on the
moisture content. With increasing moisture content, the penetration
rate of nonvolatile components through the soil increased and the
adsorption of more volatile components decreased. In contrast, upward
mobility of the individual constituents (both volatile and
nonvolatile) of the fuel decreased with increasing moisture content.
In fully water-saturated soil, upward transport of kerosene was
completely inhibited (Acher et al., 1989). Desorption of a simulated
kerosene applied to three types of soil with the same moisture content
but different contents of organic matter was found to be complete
after 30 days under environmental conditions. The slowest desorption
was from the soil with the highest level of organic matter (Yaron et
al., 1989). Transport of kerosene through three types of sand was
influenced mainly by volatilization of C9-C13 hydrocarbon
constituents; the increase in the viscosity of the kerosene residue
was followed by a decrease in the infiltration rate (Galin et al.,
1990).
A4.1.2.2 Sediment
In marine environments, oils and fuels are generally delivered to
bottom sediments on settling particles. In areas with low
concentrations of suspended material (i.e. terrigenous minerals,
plankton, detrital particles, resuspended bottom sediments), the rates
of hydrocarbon sedimentation would be low (US National Research
Council, 1985).
No experimental data are available on diesel fuels in sediments.
In laboratory experiments with fuel oil, binding of the hydrocarbon
components to clay minerals of marine sediments was shown to be due to
physical adsorption of the van der Waals type. The compounds are
associated firmly because, once they are incorporated into sediments,
desorption to the overlying water is slow. Saturated hydrocarbon
constituents of fuel oils were transported slowly to the sediment
(Meyers & Quinn, 1973). In studies of a laboratory marine ecosystem to
which No. 2 fuel oil was added for 24 weeks, the concentrations in the
sediment remained low until 135 days after the start of the
experiment. Then, a steep increase was seen, leading to a level of 9%
by weight of the total fuel added (i.e. 12% by weight of the total
saturated hydrocarbons). The highest concentrations of saturated
hydrocarbons were found in the surface flocculent layer; 2-3 cm below
the sediment surface, the levels were below the detection limit. The
smaller particles (< 45 µm) of water-suspended material seemed to
adsorb about twice as much fuel oil as larger particles (> 45 µm)
(Wade & Quinn, 1980). In a comparable study in a model marine
ecosystem with No. 2 fuel oil, the particulate matter contained 40-50%
of the total amount of aliphatic hydrocarbons added and only 3-21% of
the aromatic fraction, indicating that most of the aromatic
hydrocarbons were dissolved in the water phase (see section A4.1.1)
(Gearing et al., 1980). Although biodegradation may remove many of the
soluble aromatic hydrocarbons (see section A4.2.2), 10-20% by weight
of the fuel oil, consisting primarily of branched alkanes,
cycloalkanes, and aromatics, may remain in the upper 2 cm of sediment
for over a year (Gearing et al., 1980; Oviatt et al., 1982).
A4.2 Transformation and removal
A4.2.1 Photooxidation
No data were available on the photooxidation of diesel fuel in
air or water. In the atmosphere, all evaporated oil components are
degraded photochemically by hydroxy radicals and other species within
hours or days to carbon monoxide, carbon dioxide, and oxygenated
compounds (US National Research Council, 1985; IPCS, 1986, 1993).
Under laboratory conditions, No. 2 fuel oil was rapidly photo-oxidized
in water. Two photooxidant pools were identified: hydrogen peroxide
and a mixture of indane and tetralin hydrogen peroxides, the first
being the faster acting (Herbes & Whitley, 1983). Peroxides are formed
by two mechanisms: the first is the reaction of free radicals with
triplet oxygen to produce peroxy radicals and hydroperoxides, which
continue the chain reaction; the second is addition of excited singlet
oxygen to reactive acceptors such as olefins. The photodegradation
products of fuel oils are phenols, naphthols, and carboxylic acids
(Larson et al., 1977, 1979).
A4.2.2 Biodegradation
A4.2.2.1 Microbial degradation
The individual constituents of spilled oil are inherently
degradable to varying degrees and at different rates. The n-alkane,
n-alkylaromatic, and simple aromatic molecules in the C10-C22
and the most readily degradable. Smaller molecules are generally range
are the least toxic rapidly metabolized, although they tend to be more
toxic. Long-chain n-alkanes are more slowly degraded because of
their hydrophobicity and also because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to attack. PAHs are the compounds most recalcitrant to
microbial degradation (Morgan & Watkinson, 1988).
A study of the breakdown of heating oils and diesel fuels in a
sub-soil demonstrated breakdown rates of 0.014-0.022 g/kg per day,
which were slower than the rates of topsoil degradation (Flowers et
al., 1984). The mineralization rates for heavy oils, sludges, and
crude oils were 0.02-0.6 gram of hydrocarbon per kilogram of soil per
day. Under Arctic conditions, the rates were considerably slower.
The relative contribution of bacteria and fungi to hydrocarbon
degradation is unclear. At acid pH, fungi are clearly more important,
but closer to neutrality hydrocarbon contamination tends to enhance
bacterial growth.
The overall rates of hydrocarbon degradation are limited by:
-- temperature (optimal temperature for biodegradation, 30-40°C);
-- water content (in soils, water contents of 50-80% capacity are
optimal for microbial activity);
-- oxygen (the metabolism of both aliphatic and aromatic
hydro-carbons normally requires oxygen);
-- pH (hydrocarbons are mineralized most rapidly at 6.5-8.0);
-- inorganic nutrients (as petroleum contaminants are extremely
deficient in nitrogen and phosphorus, a large quantity of carbon
sources tends to result in rapid depletion of the available pools
of major nutrients, i.e. enhancement of degradation in fertilized
soils);
-- microbial metabolic versatility (topsoils generally contain many
hydrogen-degrading bacteria, typically 105 - 106 per gram, but the
content of subsoils is unclear) (Morgan & Watkinson, 1988).
A remedial treatment consisting of liming, fertilization, and
tilling was evaluated on a laboratory scale for its effectiveness in
cleaning up a sand, a loam, and a clay-loam soil contaminated at
50-135 mg/g of soil by diesel and other oils. The disappearance of
hydrocarbons was maximal at 27°C (Song et al., 1990).
In sandy soils, 50-85% of the hydrocarbons were degraded within a
few weeks, depending on the experimental conditions. Degradation
efficiency increased with additional soil inoculation (Grundmann &
Rehm, 1991).
Frehland et al. (1992) examined the substrate specificity and
growth conditions of nine bacterial strains on samples of
diesel-contaminated soil under different conditions. The
biodegradative properties of Pseudomonas spp. were superior (59, 60,
and 80% in three samples) than those of Micrococcus, Bacillus,
Acinetobacter, and other species tested.
A4.2.2.2 Phytoplankton and marine algae
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this occurs in nature
is poorly understood. The ability to metabolize naphthalene is
widespread (US National Research Council, 1985).
A4.2.2.3 Invertebrates and vertebrates
Unlike microorganisms, which use petroleum as a source of carbon,
animals generally oxidize and conjugate products to render them more
soluble and therefore easier to excrete. All animal groups tested can
take up petroleum hydrocarbons, either through the body surface,
across respiratory surfaces, or via the gut, the route varying with
the organism and its feeding habits. In copepods (Calanus
helgolandicus), dietary uptake was more important than that from
water (Corner et al., 1976), but aromatic hydrocarbons accumulated by
the polychaete Neanthes arenaceodentata were derived from water and
not from sediment (Rossi, 1977). Hydrocarbons are stored in lipid-rich
tissues. The principal route of metabolism of hydrocarbons by
metazoans is conversion of lipophilic compounds by cytochrome
P450-dependent monooxygenases or mixed-function oxidases to
derivatives that are more water-soluble. Mixed-function oxygenase
activity has been found in all fish species studied. Lee (1981)
reported 18 marine invertebrate species belonging to four phyla
(Annelida, Arthropoda, Echinodermata, and Mollusca) that are known to
contain mixed-function oxidase activity in the hepatopancreas,
digestive gland, and other tissues. Sea birds and some other waterfowl
also have cytochrome P450 systems, but few estimates have been made of
their capacity for hydrocarbon metabolism. Some crude oils, PAHs, and
refined petroleum products are known to induce cytochrome P450 enzymes
and to increase the levels of hydrocarbon metabolism in marine and
freshwater fish, including embryonic and larval forms and polychaetes
(US National Research Council, 1985). Induction is usually described
in terms of the rate of metabolism of benzo[a]pyrene in vitro.
A4.2.3 Bioaccumulation
Few data are available on the bioaccumulation of diesel fuel
itself, although there is adequate evidence from studies on other oils
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient (log Pow) for diesel fuel is
3.3-7.1 (see section A2.2, Table 5). Although a higher bioaccumulation
potential might be expected from these log Pow values, the measured
bioconcentration factors are lower than predicted because many
compounds of low relative molecular mass, such as substituted
benzenes, are readily metabolized, and the actual bioaccumulation of
compounds of higher relative molecular mass is limited by their low
water solubility and large molecular size.
In a continuous-flow system, diesel fuel was taken up by
Mytilus edulis mussels exposed for 41 days to a concentration of
200-400 µg/litre. The hydrocarbon levels in tissue were about 1000
times higher after exposure than before. Depuration was rapid during
the first 15-20 days and then decreased rapidly to a minimum (Fossato
& Canzonier, 1976).
The accumulation of No. 2 fuel oil has been studied in a number
of organisms (Lee, 1977). When No. 2 fuel oil (38% aromatics) was
added to a commercial shrimp pond, the concentration of naphthalenes
in shrimps, clams, and oysters exceeded the level in ambient water
after 38 days. Depuration in clean water was complete after 10 days
for shrimps and after 47-96 days for oysters (Cox et al., 1975). In a
flow-through system to study accumulation of a No. 2 fuel oil-water
dispersion, aromatic petroleum hydrocarbons were accumulated at a rate
of 89.6 mg/kg tissue by Rangia cuneata (clams) and 311.7 mg/kg by
Crassostrea virginica (oysters) during 8 h of exposure. The
bioaccumulation factor for methyl naphthalenes in clams was 2.3-26.7
and increased with increasing alkylation of the naphthalene ring.
Depuration in clean water was complete after 28 days (Neff et al.,
1976a). The fuel components of a small spill of No. 2 fuel oil had the
following biological half-lives in mussels (Mytilus edulis): aliphatic
hydrocarbons (C16 and C23), 0.2 and 0.8 days; alkyl naphthalenes
(C2 and C3), 0.9 and 1.5 days; phenanthrene, 2.1 days (Farrington
et al., 1982). Lobsters exposed to a simulated small spill of No. 2.
fuel oil bioconcentrated PAH constituents in the hepato-pancreas and
muscle within three to four days after exposure. The levels remained
elevated for up to 10-11 days, and depuration was complete after 20-21
days.
The polychaetous annelid Neanthes arenaceodentata rapidly
accumulated 14C-naphthalene (to a biomagnification of 40 times) from
solution during exposure for 24 h and slowly released the accumulated
hydrocarbons within 300 h after return to clean sea water (Rossi,
1977).
Oral administration of South Louisiana crude oil to mallard ducks
(Anas platyrhynchus) at a dose of 5 ml daily led to detectable
aromatic petroleum hydrocarbon levels in each of the tissues examined:
liver, breast muscle, heart muscle, skin, brain, uropygial gland, and
blood. The skin and the underlying adipose tissue had accumulated far
more aromatic hydrocarbons than other tissues. The individual
components did not accumulate in the same relative concentrations as
in the crude oil, suggesting different uptake and/or metabolism; two-
and three-membered, condensed ring aromatic compounds were accumulated
to a greater extent than alkylbenzenes (Lawler et al., 1978).
A4.2.4 Tainting
Tainting (unpleasant off-flavours) of various aquatic organisms
by petroleum hydrocarbons has been reported, and diesel fuel has been
suspected of tainting fish in freshwater rivers. Mackie et al. (1972)
reported tainted rainbow trout up to at least nine days after a spill
of diesel fuel into water (Six Mile Water, Ireland). Ackman & Noble
(1973) described tainting of whitefish in the Athabaska River, Canada,
which was attributed to diesel fuel from a railroad yard.
A4.2.5 Entry into the food chain
No data are available on the effect of diesel fuel on the food
chain. The effects of petroleum compounds on the marine food web have
been studied, e.g. on zooplankton Calanus helgolandicus (Corner et
al., 1976), on phytoplankton (Mahoney & Haskin, 1980), and on
freshwater crayfish, one of the primary foods of waterfowl (Tarshis,
1981). In a study in situ on the distribution, biotransformation, and
flux of PAHs in the aquatic food chain (seston --> mussel [ Mytilus
edulis] --> eider duck [ Somateria molissima]), the PAH composition
changed significantly (Broman et al., 1990). The theoretical
inhalation of atmospheric PAHs was insignificant in comparison with
exposure from food. PAHs were biotransformed relatively rapidly. Their
concentrations did not increase with increasing trophic level.
A4.3 Ultimate fate after use
A4.3.1 Use in motor vehicles
Diesel fuel is combusted in motor vehicles and stationary engines
(see section A3.2.1.2). The resulting exhaust is discussed in Part B.
A4.3.2 Spills
The fate of diesel fuel after accidental spills is eventual
degradation by abiotic and biotic processes (see section A3.2.3)
A4.3.3 Disposal
In general, incineration is recommended for fuel oils (Fraser
Williams (Scientific Systems) Ltd, 1985). This procedure is also
useful for disposing of fuel production wastes.
Because diesel fuel is released during use, mainly accidentally
into water or onto soil (see sections A3.2.2.2, A3.2.2.3, and A3.2.3),
the affected compartments may have to be remediated. In general, oil
spills on beaches and into surface waters can be treated by burning
off; absorption onto straw, polyurethane foam, activated carbon, or
peat; or addition of sinking agents, gelling agents, or dispersants.
Mechanical systems can also be used (Fraser Williams (Scientific
Systems) Ltd, 1985). Various operations for treating soil affected by
diesel fuel have been reviewed (Dineen, 1991) and are as follows:
-- Disposal: excavation of contaminated soil and disposal at a
regulated landfill; the treatment used most commonly during the
last decade; advantageous for small volumes.
-- Biodegradation: The natural process of soil microorganisms,
which is stopped by a spill as a result of depletion of oxygen or
nutrients (mostly nitrogen and phosphorus), is restored by
providing sufficient amounts of the depleted substances. The
process can be carried out in situ (Frankenberger et al., 1989)
(see section A3.2.3, Table 10) or after excavation.
Biodegradation is the most widely applicable alternative to
landfill disposal, although its duration is uncertain and may be
relatively long and a large space is required (see sections
A9.1.1.2 and A9.1.3.1).
-- Thermal treatment: incineration of contaminated soil e.g. in
cement kilns or in specially designed thermal units, where the
soil is baked to remove the fuel, which is subsequently oxidized
(thermal desorption). The thermal desorption process is also
feasible in situ. In cement kilns, the desorbed diesel fuel is
used for heating the kiln, and the soil is incorporated into the
cement mixture.
-- Road base: The contaminated soil is spread on roads under
construction and is compacted and oiled before paving. If the
affected soil has a high proportion of expandable clays, it may
not be suitable as a road base.
-- Asphalt batching: The contaminated soil replaces part of the
sand and gravel component of asphalt; this is possible only if
the content of expandable clays is not too high.
-- Chemical oxidation: A strong oxidant, usually hydrogen
peroxide, in combination with a silica polymer stabilizer to
control the reaction is used to oxidize diesel hydrocarbons to
carbon dioxide and water. The reaction is highly exothermic, and
uncontrolled volatilization and leaching of some constituents may
occur.
-- Fixation: addition of a strong base to convert diesel
hydrocarbons to organic acids and subsequent treatment with a
dissolved silica polymer to effectively encapsulate the acids in
amorphous silica; immobilizes diesel fuel in situ and prevents
migration into surface or groundwater. The long-term stability of
the immobilized chemicals is, however, unknown.
-- Soil washing: Diesel fuel is washed out of soil by a
biodegradable, non-toxic surfactant (Peters et al., 1992). The
operation is feasible in situ and after excavation and is
particularly effective on materials with a low surface:volume
ratio (e.g. gravels and coarse sands). The major disadvantage is
the need to collect and treat or dispose of the
surfactant-diesel mixture after washing.
-- Containment: construction of a low-permeability surface
structure ('cap') that prevents infiltration of rain, snow, and
other moisture that could lead to leaching of diesel constituents
into groundwater. Long-term monitoring of the area of the spill
is necessary.
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
As diesel fuels are complex mixtures of a great variety of
hydrocarbons (see section A2.1), general 'environmental levels' cannot
be described. The individual constituents of diesel fuels should be
detectable in almost all compartments of the environment, but the
sources cannot be verified.
A5.2 Exposure of the general population
Nonoccupational exposure to diesel fuel occurs when fuel tanks
are filled manually. The primary source of dermal exposure is
accidental spills, although these exposures to high levels are of
short duration. Drinking-water can be contaminated by components of
petroleum products, including diesel fuel, after leakage into
groundwater from underground storage tanks. Specific data are not
available on the contribution of diesel fuel components to groundwater
contamination. Owing to the low volatility of diesel fuels at normal
temperatures, there is little exposure to vapours (International
Agency for Research on Cancer, 1989a).
A5.3 Occupational exposure during manufacture, formulation, or use
Workers are exposed to diesel fuel in a number of occupations,
including manual handling and discharge of fuels; loading and
unloading of diesel fuel; retailing at filling stations; manufacture,
repair, maintenance, and testing of diesel engines and other
equipment; use of diesel fuel as a cleaning agent or solvent; and
laboratory handling and routine sampling of diesel fuel (CONCAWE,
1985).
At normal temperatures, low concentrations of vapours are likely
to be generated from diesel fuels, owing to their low volatility. Only
in confined spaces or at high operating temperatures do significant
levels of vapour occur. Hydrogen sulfide gas may evolve from residual
fuel oils (CONCAWE, 1985).
A6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No quantitative data are available; however, the systemic and
local effects observed after exposure to diesel fuels by various
routes indicate that they are absorbed dermally, orally, and by
inhalation.
A7. EFFECTS ON LABORATORY MAMMALS AND IN-VITRO TEST SYSTEMS
A7.1 Single exposure
Diesel fuels have a low order of acute toxicity after
administration orally, dermally, or by inhalation. Acute LD50 Table
12. In rats and mice, the values after oral or dermal exposure were
values are summarized in generally > 5000 mg/kg body weight, and the
values in rabbits after dermal exposure were > 2000 mg/kg body
weight. Since the physicochemical properties of kerosenes are similar
to those of diesel fuels (see also section A2.1), the LD50 values
for various types of kerosene are included.
Beck et al. (1984) reported an oral LD50 value of 7500 mg/kg
body weight (9 ml/kg body weight) for a market-place sample of diesel
fuel in male and female rats. Gross necropsy of animals that died
during the test showed haemorrhagic gastroenteritis, gastrointestinal
tympani, and pneumonia with abscess formation. No deaths were seen
among rabbits treated dermally with 5000 mg/kg body weight under an
occlusive covering for 24 h.
After oral administration of 12 000 mg/kg body weight kerosene to
rats, no change was seen in heart weight or in the relative weights of
lung, spleen, and kidney. There were no histopathological changes
(Muralidhara et al., 1982). Exposure of rats for 8 h by inhalation to
about 0.1 mg/litre deodorized kerosene did not induce adverse effects.
Four male cats that inhaled 6.4 mg/litre for 6 h showed no toxic
effects on the central nervous system or body weight during the
exposure or during a subsequent 14-day observation period (Carpenter
et al., 1976).
Male Sprague-Dawley rats given JP-5 jet fuel at a dose of
18 912 mg/kg body weight (24 ml/kg body weight) orally had
vacuolization of periportal hepatocytes and serum enzyme alterations,
indicating hepatic damage, within two days (Parker et al., 1981). All
male and female B6C3F1 mice treated dermally with 5000-40000 mg/kg
body weight marine diesel fuel survived a 14-day observation period
(US National Toxicology Program, 1986).
Table 12. Acute toxicity (LD50 and LC50 values) of diesel and other fuels
Fuel Species Route LD50 or Dose Reference
LC50
Diesel fuels
Diesel fuel (market-place sample) Rat Oral LD50 7500 mg/kg bw Beck et al. (1984)
Diesel fuel (market-place sample) Rabbit Dermal LD50 > 5000 mg/kg bw Beck et al. (1984)
Home heating oil No. 2a Rat Oral LD50 14 500-21 100 µg/kg bw American Petroleum
Institute (1980a,b,c)
Home heating oil No. 2a Rabbit Dermal LD50 > 5000 µg/kg bw American Petroleum
Institute (1980a,b,c)
Marine diesel fuel, JP-5, JP-8 Mouse Oral LD50 > 16 000 mg/kg bw Schultz et al. (1981)
shale, or petroleum oila
Marine diesel fuel, shale, or Mouse Dermal LD50 > 16 000 mg/kg bw Schultz et al. (1981)
petroleum oila
Other fuels
Straight-run middle distillateb Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1985a)
Hydrodesulfurized middle distillateb Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1982a,b)
Straight-run middle distillateb Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985a)
Hydrodesulfurized middle distillateb Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1982a,b)
Straight-run middle distillateb Rat Inhalation LC50 1.8 mg/litre American Petroleum
Institute (1987a)
Table 12 (contd)
Fuel Species Route LD50 or Dose Reference
LC50
Hydrodesulfurized middle distillateb Rat Inhalation LC50 4.6 mg/litre American Petroleum
Institute (1983a)
Light catalytically cracked distillatec Rat Oral LD50 4660 mg/kg bw (m) American Petroleum
3200 mg/kg bw (f) Institute (1985b)
Light catalytically cracked distillatec Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985b)
Light catalytically cracked distillatec Rat Inhalation LC50 4.7-5.4 mg/litre American Petroleum
Institute (1986a,b)
Jet fuel Aa Rat Oral LD50 > 25 000 µl/kg bw American Petroleum
Institute (1980d)
JP-5 shale oild Mouse Dermal LD50 8000 mg/kg bw Schultz et al. (1981)
JP-5 petroleum oild Mouse Dermal LD50 11 200 mg/kg bw Schultz et al. (1981)
JP-8 shale oila Mouse Dermal LD50 6400 mg/kg bw Schultz et al. (1981)
JP-8 petroleum oila Mouse Dermal LD50 8000 mg/kg bw Schultz et al. (1981)
Jet fuel Aa Rabbit Dermal LD50 > 5000 µl/kg bw American Petroleum
Institute (1980d)
Kerosenea Rat Oral LD50 50 000 mg/kg bw Parker et al. (1981)
Kerosenea Guinea-pig Oral LD50 16 320 mg/kg bw Deichmann et al. (1944)
Kerosenea Rabbit Oral LD50 22 720 mg/kg bw Deichmann et al. (1944)
Straight-run kerosenea Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1985c)
Hydrodesulfurized kerosenea Rat Oral LD50 > 5000 mg/kg bw American Petroleum
Institute (1982b)
Table 12 (contd)
Fuel Species Route LD50 or Dose Reference
LC50
Straight-run kerosenea Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1985c)
Hydrodesulfurized kerosenea Rabbit Dermal LD50 > 2000 mg/kg bw American Petroleum
Institute (1982c)
Petroleum-derived hydrocarbona Rat Inhalation LC50 > 5.3 mg/litre American Petroleum
Institute (1987b)
Petroleum-derived hydrocarbona Rat Inhalation LC50 > 5.2 mg/litre American Petroleum
Institute (1983c)
Deodorized kerosenee Rat Inhalation LC50 > 0.1 mg/litre Carpenter et al. (1976)
(saturation)
a Hydrocarbon fraction similar to that of diesel fuel (see section A2.1)
b Hydrocarbon fractions from which also diesel fuels are gained during petroleum separation (see section A3.2.1.1)
c Blending component of diesel fuel
d Narrow boiling range
e Used as a solvent, not a fuel; very low aromaticity
Exposure of Sprague-Dawley rats to an aerosol of diesel fuel at a
concentration × time product of 8 mg.h/litre caused less than 1%
mortality and was estimated to represent the maximal tolerated
exposure. On this basis, 12 mg.h/litre was chosen as the highest
concentration in a study of repeated exposure (see section A7.2).
After exposure to 0.5 mg/litre for 2 h, a transient depression of the
startle reflex was observed. Further findings were a dose-related
decrease in respiratory frequency and influx of polymorphonuclear
cells into the lungs for several days. In a 6-h inhalation test, the
no-observed-adverse-effect level (NOAEL) was 2.7 mg/litre (Dalbey et
al., 1982, 1987).
A7.2 Short-term exposure
A7.2.1 Subacute exposure
A7.2.1.1 Dermal exposure
Dermal exposure to 0.1 ml/kg body weight per day kerosene for one
week was not lethal to male mice. Significant decreases in the
relative weights of thymus, spleen, adrenals, and abdominal lymph
nodes were observed (Upreti et al., 1989).
Eight rabbits (weighing 2.5-3.5 kg) were exposed dermally
(occlusive covering, 24 h/day, five days per week, two weeks) to 4 or
8 ml/kg body weight per day of a market-place sample of diesel fuel.
None of the animals given the low dose died, although there was a
weight loss of 0.3 kg, while five animals given the high dose died
with a weight loss of 0.5 kg; the control group had a weight gain of
0.2 kg. Severe chronic dermal irritation and anorexia were seen, which
were followed by cachexia and death (Beck et al., 1984).
Three doses between 1 and 10 ml/kg body weight per day of 10, 30,
or 50% fuel oil No. 2 were applied to the skin of New Zealand white
rabbits. After a 12-day treatment including a two-day rest, acute
dermal corrosion was seen in all animals treated with the 10% oil and
in animals given 10 ml/kg body weight per day of the 30 or 50% oil.
There was histopathological evidence of dermal toxicity in all treated
groups (American Petroleum Institute, 1980a,b,c).
Application of 8000 µl/kg body weight per day of jet fuel A to
four male and four female New Zealand white rabbits for 12 days,
including a two-day rest, induced acute dermal corrosion.
Histopathological evidence of dermal and hepatic toxicity and
hyperplastic changes in the urinary bladder transitional epithelium
were seen (American Petroleum Institute, 1980d).
Groups of five male and five female New Zealand white rabbits
were treated with 200, 1000, or 2000 mg/kg body weight per day of a
straight-run distillate, a light catalytically cracked distillate, or
two hydrodesulfurized distillates, three times per week for four
weeks. Moderate to severe irritation was seen, the degree of skin
irritation being directly related to the number of doses applied.
Microscopic examination of the skin at the application sites revealed
the presence of acanthosis and hyperkeratosis (American Petroleum
Institute, 1983a, 1984, 1985a,b).
In a study of marine diesel fuel containing 12.7% paraffins,
43.7% naphthalenes, and 43.6% aromatic compounds, groups of male and
female B6C3F1 mice received dermal applications (with an occlusive
covering) of 2000, 8000, 20000, or 40000 mg/kg body weight per day for
14 consecutive days. None of the animals at 20000 or 40000 mg/kg body
weight per day survived. Skin lesions were observed in all treated
groups, which consisted of moderate acanthosis, parakeratosis, and
hyperkeratosis. These findings were accompanied by a mixed cellular
inflammatory infiltrate in the upper dermis. The NOAEL was 8000 mg/kg
body weight per day. In the same study, JP-5 navy fuel containing
52.8% cycloparaffins, 30.8% paraffins, 15.9% aromatics, and 0.5%
olefins (see also Table 2) was applied at doses of 5000-40000 mg/kg
body weight per day for 14 days. All animals at 40000 mg/kg body
weight per day and females at 30000 mg/kg body weight per day group
died before the end of the study. Body weight loss was observed at
doses > 10 000 mg/kg body weight per day. The dermal findings were
similar to those in the study of marine diesel fuel. The NOAEL was
5000 mg/kg body weight per day (US National Toxicology Program, 1986).
A7.2.1.2 Inhalation
Rats (strain, age, and sex not specified) were exposed for
6 h/day to a droplet aerosol of deodorized kerosene at a mean
concentration of 7.7 mg/litre for four days. After a day with no
treatment, the skin of the extremities was dry and flakes had formed.
The body weight remained unchanged (Carpenter et al., 1976).
CD-1 mice were exposed for 8 h/day for five days to uncombusted
diesel vapour at concentrations of 0.065, 0.135, or 0.204 mg/litre.
The dose-response for neurotoxicity (measured by square-box activity)
was 50% of the control level at the high dose, 100% at the medium
dose, and 150% at the low dose. A drastic deterioration of motor
coordination was seen at the high dose in the rotarod test. In the hot
plate test, increased sensitivity followed by tolerance was seen at
the high dose, a slight increase at the medium dose, and a substantial
increase at the low dose; normalization occurred in all groups within
24 h. No effects were seen in the inclined plane test or the corneal
reflex test. Vasodilatation, poor grooming, and tremors were
described. In general, the most obvious effects were on the nervous
system, diesel fuel acting as a neurodepressant (Kainz & White, 1982,
1984).
Dalbey et al. (1982, 1987) investigated the toxic effects of
diesel fuel aerosols to assess the health risks of their military use
as smoke screens. Groups of male and female Sprague-Dawley rats were
exposed to concentrations of 1.3-6 mg/litre for 2 or 6 h, one or three
times per week, for a total of nine exposures; 12 mg.h/litre was
chosen as the highest concentration-time product that caused 6.25%
mortality. A significant depression of body weight and a slower growth
rate were seen initially, and liver weights were significantly
decreased. No neurotoxicity was seen. Histologically, there was focal
accumulation of free pulmonary cells and increased numbers of
leukocytes in bronchial lavage fluid. In pulmonary function tests, a
non-dose-dependent change in lung volume was seen, involving a
decrease in total and vital capacity and increases in functional
residual capacity and residual volume. No other organs were affected.
Groups of 20 male and 20 female Sprague-Dawley rats exposed by
inhalation to 0.025 mg/litre of one of three hydrodesulfurized middle
distillates for 6 h/day on five days per week for four weeks showed no
treatment-related changes. Mild subacute inflammatory changes were
observed in the nasal respiratory mucosa of the animals in one group
that had been exposed to distillate API 81-09 (American Petroleum
Institute, 1986c).
A7.2.2 Subchronic exposure
A7.2.2.1 Dermal exposure
Groups of 10 male and 10 female B6C3F1 mice received dermal
applications under an occlusive covering of 250 or 4000 mg/kg body
weight per day marine diesel fuel or 500-8000 mg/kg body weight per
day JP-5 navy fuel for five days per week for 13 weeks. The marine
diesel fuel caused no treatment-related deaths. Males had reduced body
weight gain; mild chronic active dermatitis at the site of application
was observed at the highest dose. At 1000 mg/kg body weight per day,
all animals survived and had weight gains similar to those of the
controls. Exposure to JP-5 navy fuel killed five males at the
high-dose group and caused dermatosis, splenic extramedullary
haematopoiesis, and liver karyomegaly in all groups (US National
Toxicology Program, 1986).
A7.2.2.2 Inhalation
The results of studies of subchronic inhalation of various
distillate fuels are summarized in Table 13.
Table 13. Studies of subchronic exposure to distillate fuels by inhalation
Fuel Species Exposure (high dose) Results Reference
Diesel fuels
Diesel fuel Rat 4 h/d, 2 d/week, 13 weeks + Reversible loss of body weight; Lock et al. (1984)
8 weeks' recovery (1.5 mg/litre) no other apparent effect
Marine diesel fuela Mouse, rat 90 d, continuous (0.05 and Nephrotoxic effects specific to Bruner (1984)
0.3 mg/litre) male rats
Other fuels
JP-8 jet fuelb Mouse, rat 24 h/d, 7 d/week,90 d + 24 Nephrotoxic effects specific to Mattie et al. (1991)
months' recovery (1 mg/litre) male rats
JP-5 jet fuelb Rat, dog 24 h/d, 7 d/week,90 d Nephrotoxic effects specific to Gaworski et al. (1984)
(0.75 mg/litre) male rats
JP-4 jet fuelb Mouse, rat 6 h/d, 5 d/week,12 months Nephrotoxic effects specific to Bruner et al. (1993)
(5 mg/litre) male rats; increased incidence
of hepatocellular adenomas in
female mice
Deodorized Rat, dog 6 h/d, 5 d/week,13 weeks No apparent effects Carpenter et al. (1976)
kerosenec (0.1 mg/litre)
a Hydrocarbon fraction similar to that of diesel fuel
b Narrow boiling range
c Not a fuel; very low aromaticity
Groups of 25 male rats and four male dogs were exposed to
deodorized kerosene at concentrations up to 0.1 mg/litre,
approximately the saturation limit at 25°C, for 6 h/day on five days
per week for about 13 weeks. Although dose-related changes were
observed in survival, body weight gain, organ weights, and
haematological and clinical chemical parameters, none of the findings
was statistically significantly different from those in controls. No
pathological change was seen in any of the organs examined (Carpenter
et al., 1976).
Groups of 12 male and 12 female Sprague-Dawley rats were exposed
in whole-body chambers for 4 h/day on two days per week for 13 weeks
to 0.25, 0.75, or 1.5 mg/litre aerosolized diesel fuel, followed by an
eight-week recovery period. There were no deaths. All treated animals
lost weight, indicating slight toxicity, during the first four weeks
of exposure, but the effect was reversed during the recovery period in
most cases. A slight depression of the startle reflex was seen at the
start of the study, and the number of macrophages in bronchial lavage
fluid was slightly elevated in all treated animals immediately after
cessation of exposure. Further tests of pulmonary function, gas
exchange, and dynamic lung function did not reveal any significant,
dose-related change. Overall, no significant cumulative toxicity was
seen (Lock et al., 1984).
A specific effect of diesel fuel in male rats, the hyaline
droplet nephropathy syndrome, was seen in several studies. Gaworski et
al. (1984) studied the effects of a 90-day exposure to 0.15 or
0.75 mg/litre JP-5 jet fuel derived from either petroleum or shale oil
for 24 h/day, in groups of three male and three female beagle dogs, 75
male and 75 female Fischer 344 rats, and 111-150 female C57Bl/6 mice.
The toxicity of the two types of fuel was not significantly different.
The only substantial effect at both concentrations was a renal tubular
lesion in male rats, probably caused by hyaline droplet accumulation
and unique to male rats. Other signs of toxicity were mild liver-cell
changes and mild nasal inflammation in rats. Cowan & Jenkins (1980)
studied petroleum and shale-oil marine diesel fuel using an identical
study design. Reduced body weight gain and hyaline droplet nephropathy
were reported in male rats. In another study, groups of 95 male and 75
female Fischer 344 rats and 100 male and 100 female C57Bl/6 mice were
exposed for 24 h/day on seven days per week to JP-8 jet fuel at
concentrations up to 1 mg/litre for 90 days, and then held for 20-21
months in order to assess the long-term consequences of exposure. The
main finding was accumulation of hyaline droplets in the kidneys of
male rats (Mattie et al., 1991). In a 90-day study, groups of 75 male
and 75 female Fischer 344 rats were exposed continuously to 0.05 or
0.3 mg/litre of marine diesel fuel. The main finding was again hyaline
droplet nephropathy in males at both levels (Bruner, 1984). In a study
of the carcinogenicity of JP-4 jet fuel vapour, hyaline droplet
nephropathy was observed in groups of 100 male Fischer 344 rats
exposed for 6 h/day, five days per week for 12 months to
concentrations up to 5 mg/litre (Bruner et al., 1993) (see also
section A7.7).
The hyaline droplet nephropathy syndrome is not considered to be
relevant for humans. It is linked to an inherent peculiarity of renal
protein. The histopathological sequence is: accumulation in the renal
proximal tubules of an excessive amount of hyaline droplets containing
alpha2-microglobulin, a special protein of male rats which is
synthesized in the liver; cytotoxicity and single-cell necrosis of the
tubular epithelium; sustained regenerative tubule-cell proliferation;
development of intraluminar granular casts from sloughed cell debris;
tubular dilatation and papillary mineralization; foci of tubule
hyperplasia; and renal tubule tumours (US Environmental Protection
Agency, 1991). Mechanistically, a globulin-substance complex that is
resistant to hydrolytic degradation is responsible for the protein
overload in kidneys.
A7.3 Long-term exposure
A7.3.1 Dermal exposure
Groups of 50 male and 50 female B6C3F1 mice received dermal
administrations of 250 or 500 mg/kg body weight per day marine diesel
fuel or JP-5 navy fuel on the clipped dorsal interscapular region on
five days per week for 103 weeks. (The doses were derived from studies
reported in section A7.2.) The high dose of marine diesel fuel caused
a dose- and time-dependent reduction in mean body weight in animals of
each sex, by up to 23% after week 40 in males and up to 20% after week
72 in females. Survival of treated females was decreased, and exposure
to the high dose was stopped at week 84 owing to severe ulceration.
Application of 500 mg/kg body weight per day JP-5 navy fuel resulted
in a time-dependent reduction in mean body weight in animals of each
sex: by up to 22% after week 76 in males and up to 25% after week 60
in females. Treatment of females with the high dose had to be
terminated at week 90. Overall, JP-5 navy fuel caused a lesser chronic
inflammatory response than marine diesel fuel (US National Toxicology
Program, 1986).
A7.3.2 Inhalation
Groups of 100 male and 100 female Fischer 344 rats and C57Bl/6
mice were exposed to JP-4 jet fuel vapour for 6 h/day on five days per
week for 12 months at concentrations of 1 or 5 mg/litre (see section
A7.7). The sex-specific alpha2-microglobulin nephropathy syndrome was
observed in 21% of male controls and in 20% of males at the low dose,
and 77% at the high dose (see also section A7.2). No significant,
dose-related alteration was seen in clinical findings, mortality,
haematological parameters, or clinical chemistry. All treated male
rats had a significant reduction in mean body weight. At the high
dose, male rats had significantly increased relative liver and kidney
weights and females had a significant decrease in relative spleen
weight. These alterations were not significant in comparison with
absolute organ weights (Bruner et al., 1993).
A7.4 Dermal and ocular irritation; dermal sensitization
A7.4.1 Dermal irritation
The results of studies of dermal irritation (Table 14) must be
interpreted critically, as different protocols were used, e.g. in
Europe and the United States. The main difference is in exposure time:
24 h in the American protocols and 4 h in those of the European Union
and the Organisation for Economic Co-operation and Development, which
may pose a problem when only mild irritation is observed, leading to a
classification of 'non-irritant' according to protocols of the latter
organizations.
A market-place sample of diesel fuel, tested at a dose of 0.5 ml
for 24 h in rabbits, was assessed as 'extremely irritating',
corresponding to a Draize score of 6.81 (Beck et al., 1984).
Topical applications three times a week to mice of various types
of kerosene and gas oil with boiling ranges similar to that of diesel
fuel produced degenerative changes in the skin surface, including
necrosis and hyperplasia (CONCAWE, 1991).
Four types of American military fuels were investigated:
petroleum JP-5 fuel, shale JP-5 fuel, petroleum marine diesel fuel,
and shale marine diesel fuel. None was active in the Draize test, in
which six female New Zealand white rabbits received 0.5 ml of
undiluted fuel on intact and abraded skin under an occlusive covering
for 24 h (Cowan & Jenkins, 1980). Applications to rabbit skin of
0.5 ml of various shale oil- and petroleum-derived fuels under
occlusive dressings for 4 h were not irritating (Schultz et al.,
1981).
Various modifications of fuel oil No. 2 (10, 30, and 50%) were
found to be moderately irritating to rabbit skin (American Petroleum
Institute, 1980a,b,c), and two hydrodesulfurized middle distillates
resulted in primary irritation indices of 4.3 and 5.9 (American
Petroleum Institute, 1982a,b). A straight-run middle distillate had a
moderately irritating effect, with an irritation index of 3.2
(American Petroleum Institute, 1985d). Jet fuel A was classified as
mildly irritating, with an irritation score of 1.96 (American
Petroleum Institute, 1980d). A hydrosulfurized kerosene was moderately
irritating, with an index of 4 (American Petroleum Institute, 1982c).
A light catalytically cracked distillate (American Petroleum
Institute, 1985d) and a straight-run kerosene (American Petroleum
Institute, 1985e) had severely irritating effects, with irritation
indexes of 5.5 and 5.6.
Table 14. Studies of dermal irritation in rabbits exposed for 24 h to various fuels
Fuel Classification of Reference
irritationa
Diesel fuel Extremely irritating Beck et al.
(1984)
Marine diesel fuel or marine Not irritating Cowan & Jenkins
shale fuel (1980)
Hydrodesulfurized middle Moderately irritating American Petroleum
distillate Institute (1982a,b)
Straight-run middle distillate Moderately irritating American Petroleum
Institute (1985d)
Light catalytically cracked Severely irritating American Petroleum
distillate Institute (1985d)
Heating oil No. 2 Moderately irritating American Petroleum
Institute (1980a,b,c)
Jet fuel A Mildly irritating American Petroleum
Institute (1980d)
Various kerosenes Slightly to severely CONCAWE (1995)
irritating
Shale oil- and Not irritating Schultz et al. (1981)
petroleum-derived fuels
Hydrodesulfurized kerosene Moderately irritating American Petroleum
Institute (1982c)
Straight-run kerosene Severely irritating American Petroleum
Institute (1985e)
a Note differences in protocols between regulations of the United States and
the Organisation for Economic Co-operation and Development (see section A7.4.1)
A7.4.2 Ocular irritation
All of the available reports indicate no or, at the most, a
slight irritating effect of diesel fuels and kerosenes on the rabbit
eye.
Four types of American military fuels (petroleum JP-5 fuel, shale
JP-5 fuel, petroleum marine diesel fuel, and shale marine diesel fuel)
gave generally negative responses in the Draize test, in which nine
female New Zealand white rabbits were given 0.1 ml of undiluted
substance (Cowan & Jenkins, 1980). A market-place sample of diesel
fuel (0.1 ml, undiluted) was also not irritating in the Draize test
(Beck et al., 1984). None of several kerosenes tested was more than
slightly irritating to the eye (CONCAWE, 1995), and none of the shale
oil- and petroleum-derived fuels tested caused visible irritation in
the rabbit eye (0.1 ml, undiluted) (Schultz et al., 1981).
Three modifications of fuel oil No. 2 (10, 30, and 50%) were
classified as mildly irritating in rabbits (American Petroleum
Institute, 1980a,b,c). Two hydrodesulfurized middle distillates did
not cause primary irritation in the Draize test (American Petroleum
Institute, 1982a,b); a straight-run middle distillate (American
Petroleum Institute, 1985d) and a straight-run kerosene also gave
negative results (American Petroleum Institute, 1985e). Jet fuel A was
classified as mildly irritating, with an average score of 2.67 after
one day and 0 after seven days (American Petroleum Institute, 1980d).
A hydrodesulfurized kerosene was not irritating (American Petroleum
Institute, 1982c).
A7.4.3 Sensitization
Three modifications of fuel oil No. 2 (10, 30, and 50%) were were
classified as non-sensitizing for the skin of guinea-pigs (American
Petroleum Institute, 1980a,b,c). A straight-run middle distillate
(American Petroleum Institute, 1985d), a straight-run kerosene
(American Petroleum Institute, 1985e), jet fuel A (American Petroleum
Institute, 1980d), a hydrodesulfurized middle distillate (American
Petroleum Institute, 1984), and a light catalytically cracked
distillate (American Petroleum Institute, 1985c) were also not
sensitizing.
Four types of fuels used in the United States Army, petroleum
JP-5 fuel, shale JP-5c fuel, petroleum marine diesel fuel, and shale
marine diesel fuel, were not sensitizing in a modified Landsteiner
test in groups of 24 guinea-pigs (Cowan & Jenkins, 1980). A
straight-run kerosene, hydrosulfurized kerosene, jet fuel A (CONCAWE,
1995), and shale oil- and petroleum-derived jet and diesel fuels
(Schultz et al., 1981) were also not sensitizing.
A7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Groups of 20 pregnant CRL:COBS rats were exposed by inhalation on
days 6-15 of gestation to 100 or 400 ppm of diesel fuel for 6 h/day
(American Petroleum Institute, 1979). A significant reduction in
maternal food consumption was seen at the high concentration, and
maternal body weight gain and fetal weights were reduced, although not
statistically significantly. Overall, no significant, dose-related
effects were observed in dams, and the compound had no teratogenic or
embryotoxic effect and no effect on fetal growth or development.
No signs of developmental or maternal toxicity were seen when
groups of 20 pregnant rats were exposed to jet fuel A or fuel oil for
6 h/day on days 6-15 of gestation (Beliles & Mecler, 1982).
Sprague-Dawley rats were fed 500, 1000, 1500, or 2000 mg/kg body
weight per day of JP-8 aviation fuel by gavage on days 6-15 of
gestation, and fetuses were examined on day 20. Significantly reduced
body weights were seen in dams at the two higher doses and in fetuses
at 1000-2000 mg/kg body weight per day, but the number of
malformations was not altered. The investigators concluded that JP-8
aviation fuel is a developmental toxin at doses > 1000 mg/kg body
weight per day but is not teratogenic (Cooper & Mattie, 1993).
A7.6 Mutagenicity and related end-points
A7.6.1 In vitro
Various samples of diesel and related fuels have been tested for
mutagenicity in Salmonella typhimurium (Henderson et al., 1981;
Conaway et al., 1984; Vandermeulen et al., 1985; US National
Toxicology Program, 1986; McKee et al., 1994). Negative results were
obtained in direct, unmodified Ames' assays (Conaway et al., 1984;
Vandermeulen et al., 1985; US National Toxicology Program, 1986). In
one study, refined shale oil, jet fuel, and shale oil- and
petroleum-derived marine diesel fuel were not mutagenic (Schultz et
al., 1981). Negative results were also found in studies of aliphatic
and aromatic fractions of diesel fuel in S. typhimurium TA98, TA100,
and TA1535 in the presence of an exogenous metabolic system (Henderson
et al., 1981).
The number of revertants of TA98 increased to 2.3-2.5 times the
background levels in a suspension assay (Conaway et al., 1984), but
increases were found at a limited number of doses. In a modified
Salmonella assay with an extraction step to concentrate the
mutagenic aromatic compounds, addition of an exogenous metabolic
system from Aroclor-induced hamster liver, and use of TA98 as the most
responsive strain, increased numbers of revertants were seen (McKee et
al., 1994); but the number never exceeded twice the background
response, and the results were considered to be equivocal.
A dimethyl sulfoxide extract of an intermediate, catalytically
cracked distillate (CAS No. 644741-60-2) was mutagenic in
S. typhimurium TA98 with an increased concentration of exogenous
metabolic system to optimize detection. Of two dimethyl sulfoxide
extracts of light hydrocracked distillate (CAS-No. 64741-77-7) tested
in the same system, one induced a weak, non-dose-dependent response,
while the other induced a clear dose-dependent positive response
(Blackburn et al., 1984). Under the same assay conditions, five
samples of cracked middle distillates also gave positive results
(German Scientific Association for Petroleum, Natural Gas, and Coal,
1991). These results in a series of assays in Salmonella with a number
of variations provide no clear evidence of mutagenic activity. In the
unicellular alga Chlamydomonas reinhardtii (streptomycin resistant)
tested in a natural aquatic environment, water-soluble fractions of
two crude and two refined oils did not induce forward mutations
(Vandermeulen & Lee, 1986). Neither aliphatic nor aromatic dimethyl
sulfoxide fractions of diesel fuel No. 7911 induced mutation in
S. typhimurium. After reaction with nitrogen dioxide, which may
activate the mutagenicity of diesel exhaust, it was mutagenic in
strain TA100, the aromatic fraction being 40-fold more active than the
aliphatic fraction (Henderson et al., 1981).
A market-place sample of diesel fuel did not induce mutation in
L5178Y tk+/- mouse lymphoma cells with or without metabolic
activation (Conaway et al., 1984). A hydrodesulfurized middle
distillate (CAS 64742-80-9) was active in the presence of metabolic
activation, but the concentrations were cytotoxic (American Petroleum
Institute, 1987c).
A7.6.2 In vivo
Undiluted diesel fuel No. 2 increased the frequency of
chromosomal aberrations in the bone marrow of Sprague-Dawley rats
given single or repeated (five days; 125, 417, or 1250 mg/kg body
weight) oral doses. Diesel fuel given intraperitoneally for one or
five days at 0.6, 2, or 6ml/kg body weight significantly increased the
number of aberrant cells at the highest dose (Conaway et al., 1984).
In order to assess these findings further, McKee et al. (1994)
evaluated micronucleus induction by diesel fuel No. 2 and related
materials in male and female CD-1 mice treated by gavage at up to
5 g/kg body weight. No effect was seen.
No effect was seen on the frequency of dominant lethal mutations
in male CD-1 mice exposed to 100 or 400 ppm of either diesel fuel or
jet fuel A for 6 h/day, five days per week for eight weeks (American
Petroleum Institute, 1980e, 1981).
A7.7 Carcinogenicity
A7.7.1 Dermal exposure
Diesel fuel has been shown to have at least weak carcinogenic
potency after dermal administration in a number of studies. As some of
the test samples contained low concentrations of carcinogenic PAHs, it
has been suggested (Biles et al., 1988) that the tumours induced may
have been a consequence of the dermal damage produced by repeated
treatment with these materials. The available studies are summarized
in Table 15.
Doses of 25 mg per animal of petroleum and shale fuels were
applied to groups of 25 male and 25 female C3H/HeN mice three times a
week, for 62 weeks for jet fuel A with subsequent observation up to
105 weeks, and for 104 weeks for JP-4 fuel. Controls received mineral
oil. The shale and petroleum diesel fuels were characterized for
boiling range (jet A, 171-271°C; JP-4, 60-249°C) and sulfur content
(jet A: petroleum, 3510 ppm; shale, 89 ppm; JP-4: petroleum, 1830 ppm;
shale, 199 ppm). After 60 weeks, increased incidences of squamous-cell
carcinomas and fibrosarcomas were reported (jet A: petroleum, 26%;
shale, 28%; JP-4: petroleum, 26%; shale, 50%; controls, 0-2%), but no
statistical analysis was reported (Clark et al., 1988).
Groups of 50 male and 50 female B6C3F1 mice were given 250 or
500 mg/kg body weight per day marine diesel fuel dermally, on five
days per week for 103 or 84 weeks (see section A7.3.). A control group
received the vehicle, acetone, only. The survival rates were 26/50
males and 29/50 females at the high dose at 84 weeks, 20/49 males and
12/50 females at the low dose at 104 weeks, and 30/50 male and 40/50
female controls. Males at the high dose had a significantly increased
number of hepatocellular adenomas or carcinomas (29%; control, 18%).
The total incidences of squamous-cell papillomas and carcinomas at the
application site and the adjacent inguinal site were: control, 1/50;
low dose, 2/49; high dose, 3/50 in males; and control, 0/50; low dose,
1/45; and high dose, 2/48 in females. No information was available on
historical frequencies in acetone-treated mice; the incidence of
papillomas and carcinomas in 3500 observations in untreated mice was
0.3-0.4%. In a parallel study with JP-5 navy fuel, 1/50 papillomas
were seen at the low dose and 4/49 carcinomas at the high dose in
males, and 0/49 carcinomas were seen at the low dose and 1/47 at the
high dose in females. The investigators concluded that there was
equivocal evidence for the carcinogenicity of marine diesel fuel and
no evidence for the carcinogenicity of JP-5 navy fuel (US National
Toxicology Program, 1986).
Two middle-distillate fractions of each of two crude oils
(boiling range, 170-370°C) were painted at a dose of 50 mg onto the
skin of male C3H mice twice a week for 18 months. The yield of benign
and malignant tumours (details not specified) was 2-19 % (Lewis,
1983).
Table 15. Studies of chronic dermal exposure of mice (usually 50 mice/group) to distillate fuels
Fuel Exposure Results Reference
Marine diesel fuel 0, 250, and 500 mg/kg bw, 1/50, 2/49, and 3/50 (m) and 0/50, 1/45, and 2/48 (f) US National Toxicology
5 d/week, 103 weeks with squamous-cell papillomas and carcinomas after Program (1986)
104 weeks
Straight-run gas oil 25 mg/animal, twice/week, Occasional ulcerations, scarring, epidermal dysplasia; American Petroleum
(laboratory sample)a up to 2 years after 2 years, 17/50 with tumours; controls, 0% Institute (1985c)
Straight-run middle 50 µl/animal, twice/week, Mild to moderate irritation (mild desquamation, American Petroleum
distillatea up to 2 years occasional scabbing); after 2 years, 10/50 with Institute (1989a)
squamous-cell carcinomas or fibrosarcomas;
controls, 0%
Straight-run 50 µl/animal, twice/week, 29/50 with malignant and 1/50 with benign tumours; American Petroleum
keroseneb > 2 years latency, 76 weeks; controls, 0% Institute (1989a)
Two cracked 50 µl/animal, twice/week, Mild to moderate irritation; after 24 months, 39/50 American Petroleum
distillatesc up to 2 years and 26/50 with tumours Institute (1989a)
Intermediate 50 mg/animal, twice/week, 42/43 with tumours; mean latency, 17 weeks; Blackburn et al. (1984)
catalytically cracked 80 weeks controls, 0/50
distillatec
Two hydrodesulfurized 50 µl/animal, twice/week, Acanthosis, fibrosis, hyperkeratosis, crusting; at end American Petroleum
middle distillatesa lifetime of study, 24/50 and 27/50 with tumours (no controls Institute (1989b)
reported)
Table 15 (contd)
Fuel Exposure Results Reference
Hydrodesulfurized 50 µl/animal, twice/week, 26 malignant and 1 benign tumours; latency, 77 American Petroleum
keroseneb > 2 years weeks (no controls reported) Institute (1989b)
Jet A and JP-4 fuels 25 mg/animal, three After 60 weeks, increased skin tumour rate; jet A, Clark et al. (1988)
from shale or times/ week, 62 weeks 26% petroleum, 28% shale; JP-4, 26% petroleum,
petroleum oilsb + observation (jet A) 50% shale
or 104 weeks (JP-4)
Two middle-distillate 50 mg/animal, twice/week, 2-19% tumour rate (no further information) Lewis (1983)
fractions 18 months
No. 2 fuel oilb 12, 25, or 50 µl/animal, Overall tumour rate, 10%; control, 0% Witschi et al. (1987)
three times/week, up to
100 weeks
Two heating oilsa 25 µl/animal, three 7/50 and 9/50 with tumours; control, 0/50 Biles et al. (1988)
times/week, lifetime
a Hydrocarbon fractions similar to that of diesel fuel (see section A2.1)
b Hydrocarbon fractions from which also diesel fuels are gained during petroleum separation (see section A3.2.1.1)
c Blending component of diesel fuel
Application of American Petroleum Institute No. 2 fuel oil
dermally to C3H mice at 12, 25, or 50 µl per animal, three times
weekly for up to 100 weeks induced an overall tumour incidence (type
not specified) of 10% (15/150 in the three treatment groups; controls,
0/100) (Witschi et al., 1987).
Ten samples of middle-distillate fuels were applied dermally to
C3H/HeJ mice at a dose of 25 µl, three times a week for life. A virgin
heating oil blending base (142-307°C) and a commercial No. 2 heating
oil (156-352°C) had aromatic contents closest to that of diesel fuel.
The blending base induced five skin carcinomas and two papillomas in
50 animals, and the commercial heating oil produced eight skin
carcinomas and one papilloma in 50 animals. There were no tumours in
the respective control groups (Biles et al., 1988).
A7.7.2 Inhalation
Fischer 344 rats and C57Bl/6 mice were exposed for 6 h/day on
five days per week for 12 months to 1 or 5 mg/litre of fuel vapours
(see section A7.3), followed by a 12-month recovery period. No
significant pulmonary neoplastic changes were seen. Female mice at the
high dose had a slightly but significantly increased incidence of
benign hepatocellular adenomas (10%; controls, 2%); however, the trend
was reversed in male mice. No conclusions about carcinogenic potency
were drawn from this study (Bruner et al., 1993).
A8. EFFECTS ON HUMANS
A8.1 Exposure of the general population
Like similar petroleum distillates, such as kerosene, diesel fuel
can cause dermal irritation and defatting after dermal exposure
(Sandmeyer, 1981). Considerable exposure and subsequent absorption may
also result in acute renal failure secondary to acute renal tubular
necrosis. A 28-year-old man developed progressive oliguria one day
after washing his hair with diesel fuel and had acute tubular necrosis
on renal biopsy (Barrientos et al., 1977). A 47-year-old man developed
acute tubular necrosis after cleaning his arms and hands with diesel
oil for several weeks (Crisp et al., 1979). Both recovered renal
function.
Accidental aspiration of diesel fuel resulted in immediate
coughing, followed by dyspnoea, cyanosis, and loss of consciousness
(Perez Rodriguez et al., 1976). X-Ray examination revealed diffuse
alveolar infiltrates. Mildly elevated levels of alkaline phosphatase,
serum glutamic-oxaloacetic transaminase, and serum glutamic-pyruvic
transaminase were also seen. After 37 days, residual infiltrates
remained, but the patient had improved. In another case, abdominal
pain and vomiting followed ingestion of a reported 1.5 litres of
diesel fuel. Pulmonary infiltrates were noted, presumably from
subsequent aspiration of diesel fuel (Boudet et al., 1983).
A8.2 Occupational exposure
The odour threshold values for diesel fuels No. 1 and 4 are
reported to be 0.7 and 0.5 ppm, respectively (see section A2.2, Table
5). Six volunteers exposed to 140 mg/m3 of deodorized kerosene for
15 min did not experience throat irritation. Olfactory fatigue was
induced in three subjects, and one reported taste sensation. The
investigators suggested that this concentration was the sensory
threshold for kerosene and estimated the odour threshold at
0.6 mg/m3 (Carpenter et al., 1976).
Acne and folliculitis developed on the arms and thighs of a man
who had worked in various automobile workshops for 15 years. He had
handled diesel oil with his bare hands and then wiped them on his
trousers (Das & Misra, 1988). Hyperkeratosis was noted in 320 drivers
working in Russian oil fields with regular dermal contact with diesel
fuel (Gusein-Zade, 1974). A 33-year-old man was exposed to diesel fuel
vapour (concentration not reported) for 10 days while driving a truck
with a fuel injector leak. He developed abdominal cramps, nausea,
vomiting, and then acute renal failure with anaemia and
thrombocytopenia (Reidenberg et al., 1964). Two aviators who were
exposed to JP-5 fuel vapour for 1 h while flying a small airplane
experienced eye irritation, difficulties in coordination and
concentration, and fatigue (Porter, 1990).
Siemiatycki et al. (1987) conducted a case-control study in
Montreal, Canada, and found an increased risk for squamous-cell
carcinoma of the lung in men exposed to diesel fuel. When all types of
lung cancer except adenocarcinoma were combined, an odds ratio
(adjusted for smoking) of 1.6 (90% confidence interval (CI), 1.0-2.4;
n = 39) was found for 'any' exposure. In four subcategories, the odds
ratios were 1.9 (short-low), 2.0 (short-high), 1.1 (long-low), and 2.0
(long-high). The possible effects of exposure to combustion products
were not taken into consideration. An association with prostatic
cancer was also observed, although there was no dose-response
relationship, with odds ratios of 2.3 (CI, 1.3-4.0; n = 17) for the
subgroup with nonsubstantial exposure and 1.4 (0.7-2.9; n = 8) in
the subgroup with substantial exposure.
In another case-control study, no association was found between
the occurrence of renal-cell cancer and occupational exposure to fuel
oils, including diesel fuel (levels and duration not quantified)
(Partanen et al., 1991).
In a cross-sectional epidemiological study, the effects of
long-term exposure of factory workers to jet fuels (not specified) was
investigated. Dizziness, headache, nausea, palpitation, pressure in
the chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of jet
fuel vapour in the breathing zone was estimated to be 128-423 mg/m3
(Knave et al., 1978).
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Comparison and evaluation of studies of the ecotoxicological
effects of diesel fuel are complicated by a number of factors:
(1) There are comparatively few ecotoxicological studies on
diesel fuel, and most work in the 1970s was concentrated on the
effects of No.2 fuel oil, which has a similar but not identical
chemical composition to diesel fuel No. 2 (see section A2). The
composition of marine diesel fuel differs greatly from both (see
Table 1). As described in the same section, diesel fuel itself has
various specifications in different countries; in particular, the
composition of non-hydrocarbon compounds from e.g. catalytic processes
or additives may vary widely. There may also be differences between
batches of the same oil (Hedtke & Puglisi, 1982) (see Table 20). The
amount of the chemical actually in solution is also pertinent to
ecotoxicological studies. Table 3 gives the concentration ranges of
toxicologically relevant constituents of diesel and No. 2 fuel oil as
a whole and as 10% water-soluble fractions.
(2) The chemical composition of fuel oils and, as a consequence,
their toxicity change with time, as oil can be modified by
biodegradation, photooxidation, and volatilization (see section A4).
Their toxicity also depends on whether they are mixed with water (sea
water or freshwater), sediment, or soil, the temperature (tropical or
Arctic), and physical conditions (e.g. storm conditions with continual
mixing and breaking up of oil). Oil modified by these processes is
usually less toxic than fresh oil (Baker, 1970; Morrow, 1973).
(3) A variety of methods have been used in ecotoxicological
studies on fuel oils. There is an obvious difference between
conditions in the laboratory and the field. Most of the field studies
that have been reported are related to fuel spills. In laboratory
studies, either water-soluble fractions (see below) or oil-water
dispersions have been used, and the methods of preparing them vary
among laboratories. Oil films and, more recently, microencapsulated
diesel oil have also been tested. In none of the laboratory studies
reported were test media prepared with 'water accommodated fractions',
as described and recommended by CONCAWE (1993) in laboratory protocols
for petroleum products. Thus, the amount of product that must be added
to a given volume of medium to produce the effects reported cannot be
derived from the results of these studies, making comparison of data
on ecotoxicity from different studies practically impossible.
Hedtke & Puglisi (1982) tested the lethality of No. 2 fuel oil in
two species of freshwater fish and two species of amphibians and found
variable LC50 values (see Table 20). Oil-water dispersions were very
toxic to fish. It is thought that these tiny oil droplets make contact
with the gills and directly damage the respiratory epithelium
(Engelhardt et al., 1981; Poirier et al., 1986). With floating oils,
the relationship between toxicity and oil-water contact is a function
of the equilibrium time for the soluble components of the oil and
water, death occurring more rapidly when the oil is added 96 h before
the organisms are exposed. Flow-through tests were more sensitive than
static tests, as, in the latter, the chemical nature and toxicity of
the oil change with time (Hedtke & Puglisi, 1982). Lockhart et al.
(1987) showed that the results of static tests in fish varied
considerably with aeration of test containers. Although flow-through
techniques give a better estimate of real exposure conditions, many of
the results reported in the literature are of static tests.
(4) The susceptibility of organisms to fuel oils and their
components depends not only on the species and strain but also on the
biological stage and the time of development within stages (Kühnhold,
1977; Winters et al., 1977). As the effects of No. 2 fuel oil and
diesel oil have been tested in a wide variety of organisms and life
stages under various conditions, direct comparison is difficult.
In spite of these difficulties, it can be concluded that diesel
oil is generally more toxic than crude oil, as seen, for example, in
barnacle nauplii (Winters et al., 1977), Daphnia (Cladocera),
chironomid larvae, and the mollusc Viviparus bengalensis (Gastropoda)
(Das & Konar, 1988). Crude oils themselves differ in toxicity,
depending on their source (Anderson, 1977a; Winters et al., 1977), as
do their corresponding products.
Rice et al. (1977a) concluded that the toxicity of fuel oil is
due to the chemical toxicity of soluble aromatic compounds rather than
to the physical toxicity of dispersed oil droplets. Physical coating
by oil of the gills of fish and the feathers of birds is also a cause
of toxicity. Monoaromatic compounds seem to be the least toxic, their
acute toxicity increasing with molecular size up to the four- to
five-ring compounds (e.g. chrysene and benzo[ a]pyrene) (Neff et al.,
1976b; Rice et al., 1977a), but they are not very soluble in sea water
(see Table 6). The toxicity of one-, two-, and three-ring compounds
(benzenes, naphthalenes, and phenanthrenes, respectively), seemed to
increase with increasing degrees of alkylation of the aromatic
nucleus. Neff et al. (1976b) reported 96-h LC50 values of 0.3-0.6 ppm
for 1-methylphenanthrene, fluoranthrene, and phenanthrene, indicating
the high toxicity of these polynuclear aromatic compounds for the
polychaete, Neanthes arenaceodentata. Of the pure compounds tested
by Winters et al. (1977), the most toxic to barnacle nauplii were
indan, naphthalene, xylene, and substituted benzenes and naphthalenes.
In four freshwater species, the water-soluble fraction was again
associated with the substituted benzenes, and naphthalenes were found
to be associated with the observed toxicity (Lockhart et al., 1987).
The concentrations of naphthalenes, methylnaphthalenes, and
dimethylnaphthalenes are particularly high in fuel oils (see Table 3),
and these compounds have been used as analytical markers in several
studies.
Experiments involving microencapsulated oil fractions have shown
that insoluble fractions also inhibit growth, e.g. of juvenile mussels
(Stromgren & Nielsen, 1991). Anderson (1979) indicated that the larval
stage might be the most sensitive to No. 2 fuel oil. Eggs of fish and
invertebrates were often tolerant, and adults were sometimes more
sensitive than juveniles.
Extensive studies have been carried out on lethal toxicity to
marine organisms under the same conditions. The 96-h LC50 values for
a wide range of invertebrates in static exposure conditions
(temperature, 18-22°C) were 1-20 ppm for crude oil in water and
0.4-6 ppm for No. 2 fuel oil (Anderson et al., 1974; Neff et al.,
1976b; Rossi et al., 1976). At 4-10°C, the 96-h LC50 values appeared
to be lower (Rice et al., 1976, 1977a). Table 16 gives the results of
lethality studies reported from several laboratories.
Table 16. Lethality (median lethal concentrations) to marine
zooplankton of physically dispersed and water-soluble
fractions of No. 2 fuel oil
Zooplankton Oil-water dispersions Water-soluble
(mg/litre) fractions (mg/litre)
Ctenophora 0.59 (1 day)
Mollusca
Clams
Embryos 0.43 (2 days)
Larvae 1.3 (2 days)
Larvae 0.53 (10 days)
Pteropods < 0.2 (2 days)
Crustacea
Barnacles 2.6 (1-h LC50)
Copepods 1.0 (3 days)
Amphipods 0.3 (2 days) 2.5 (3 days)
Decapods
Shrimp post-larvae 1.7; 9.4
Lobsters 1.2-6.6
Teleosts 1.5
Adapted from US National Research Council (1985). Values are for total
measured (extractable) hydrocarbons, calculated from initial
concentrations measured by spectroscopy and chromatography
No. 2 fuel oil (mostly the water-soluble fraction) has been found
to have sublethal effects in all phyla examined (Anderson, 1977a,b,
1979; US National Research Council, 1985). With increasing
concentrations, alterations were seen in the respiratory rate of the
fish Cyprinodon variegatus (Anderson et al., 1974) and Oncorhynchusm
gorbuscha (pink salmon) (Rice et al., 1977b), the crustaceans
Mysidopsis almyra and Penaeus aztecus, and the glass shrimp
Palaemoetes pugio (Anderson, 1979); and changes were found in the
pulse rate in embryonic estuarine fish, C. variegatus and Fundulus
heteroclitus (Anderson, 1977a) These effects have been used as a
measure of stress caused by exposure. Dysregulation of the ability to
regulate internal concentrations of solutes (osmotic and ionic
regulation) was demonstrated in the brown shrimp Penaeus aztecus
(Anderson et al., 1974; Cox, 1974; Anderson, 1979). The behaviour of
F. similus exposed to a 100% water-soluble fraction of No. 2 fuel
oil was correlated with the content of naphthalene in four organs
(Dixit & Anderson, 1977). Sublethal studies on No. 2. fuel oil showed
reduced growth rates, survival, and reproduction in a range of
organisms (Anderson, 1979; Table 17), including the annelid Neanthes
arenaceodentata (Rossi & Anderson, 1978) and the estuarine fish
F. grandis (Ernst et al., 1977).
A9.1 Laboratory experiments
Diesel fuel is a complex mixture of substances with a range of
solubilities in water (see section A2.2). In testing for aquatic
toxicity, it is important that the constituents in the medium be
characterized (Bennett et al., 1990), but these are seldom reported.
The results described in this section refer to diesel fuel, unless
otherwise stated.
As the solubility of diesel fuel in sea and freshwater is low,
investigations of the effects of diesel fuel on organisms in the
laboratory often involve preparation of a water-soluble fraction or an
oil-water dispersion. Water-soluble fractions can be prepared by
stirring one part of diesel fuel into nine parts of (sea) water for
20 h (Anderson et al., 1974) or one part of diesel fuel into eight
parts of sea water for 24 h (Pulich et al., 1974), and then extracting
the water phase. The resulting water phases contain 6-20 µg/ml of
total hydrocarbons; the 100% water-soluble fraction of No. 2 fuel oil
contains about 7 µg/ml of total hydrocarbons. Results are expressed as
the concentration (percent dilution of the water-soluble fraction)
and/or as the measured hydrocarbon concentration that causes the
specified level of effect. The results cannot, however, be correlated
with the original amount of fuel oil that was added to produce the
water-soluble fraction or oil-water dispersion (CONCAWE, 1993).
Table 17. Effects of No. 2 fuel oil on the growth and reproduction of marine animals
Species Exposure Concentration (ppm) Growth or reproduction Reference
(days)
TH TN TA
Fish
Cyprinodon variegatus 7 2.0 0.6 1.7 0% of eggs hatched Anderson et al. (1976)
Decapods
Rithropanopeus harrasii 27 1.0 0.3 0.9 Reduced survival and Neff et al. (1976b)
extended development to
megalopa
Palaemonetes pugio 12 0.9 0.3 0.8 Reduced growth rate of Tatem (1977)
larvae
3 1.4 0.6 Reduced viability of eggs
from exposed gravid females
Polychaetes
Neanthes arenaceodentata 22 1.0 0.3 0.9 Reduced growth of larvae Rossi & Anderson (1976)
28 0.3 0.1 Reduced growth of Anderson (1977a)
juveniles by 30%
Ctenodrilus serratus 28 2.2 0.5 1.4 Reduced survival and Carr & Reish (1977)
reproduction
Ophryotrocha sp. 28 1.3 0.3 0.9 Reduced survival and Carr & Reish (1977)
reproduction
From Anderson (1977b)
TH, total hydrocarbons; TN, total naphthalenes; TA, total aromatic compounds
Oil-water dispersions are produced by shaking a measured quantity
of oil in water for 5 min (Anderson et al., 1974) or 30 min (Pulich et
al., 1974). In some cases, the oil-water dispersion is maintained by
continuous mixing, but more usually it is allowed to settle, and the
aquatic organisms are exposed to the material in the water and to a
surface film. The effects seen in such tests can be due to both
physical action and toxicity, especially in small invertebrates such
as Daphnia.
Insoluble fractions of crude oil and other hydrocarbon
formulations strongly inhibited growth when they were
microencapsulated and ingested by juvenile mussels (Stromgren et al.,
1986; Stromgren & Reiersen, 1988) and larvae (Stromgren & Nielsen,
1991). In microcapsules with a thin acacia-gelatine structure, the
total oil is dispersed into particles of 1-10 µm, which are suitable
for ingestion by Mytilus larvae. The water-soluble components leak
through the wall, providing a low concentration, and are ingested by
the molluscs.
None of the studies involved use of the 'water accommodated
fractions' recommended by CONCAWE (1993), which allows the results to
be expressed as 'loading rate', defined as the amount of product that
must be equilibrated with the aqueous test medium in order to produce
a specified level of effect.
A9.1.1 Microorganisms
A9.1.1.1 Water
Marcus & Scott (1989) tested the effects of 0, 10, 20, and 40%
water-soluble fractions of a shale-diesel fuel mixture and a
petroleum-diesel fuel mixture on the growth of faecal coliform
bacteria. The 20 and 40% fractions of the petroleum-diesel fuel
mixture resulted in significantly lower bacterial densities than the
shale-diesel fuel mixture. The latter appeared to biostimulate or
mediate against toxicity, suggesting a difference in the chemical
characteristics of the two fuels.
The effects of diesel and furnace oils, petrol, kerosene, and
Assam crude oil and their paraffinic, aromatic, and asphaltic
fractions (higher relative molecular mass, poorly defined) on the
photosynthesis and respiration of blue-green algae (Cyanobacteria), in
particular Anabaena doliolum were investigated by Singh & Gaur (1990).
The chemical composition of the diesel and furnace oils was almost
identical, with 37 and 36% paraffins, 51 and 54% aromatic compounds,
and 6 and 0.6% asphaltic compounds, respectively. The aromatic
fractions of these oils were the most toxic, followed by the asphaltic
fractions; photosynthesis was reduced to 40% by the aromatic fractions
and to 50% by the asphaltic fractions of both oils at a concentration
of 5.0 mg/litre. The effects were dependent on concentration up to
10 mg/litre. Photosynthesis was stimulated 160% by 5 mg/litre of the
paraffinic fractions of crude oil , but only 100% by those of diesel
oil and 110% by those of furnace oil. In general, crude oil inhibited
photosynthesis and respiration by A. doliolum least and diesel and
furnace oils the most.
A9.1.1.2 Soil
In outdoor lysimeters, the toxicity of spills of 2.3 ml/cm2
diesel and heating fuels was investigated in soil microbes, using
Microtox(R) measurements (Wang & Bartha, 1990). (For parallel assays
of seed germination and plant growth, see section A9.1.3.1.) At time
0, both fuels were moderately toxic (EC50 = 80-90 mg of contaminated
soil); this was followed by a period of increased toxicity, which
started to decrease after 6-12 weeks. The values in soils treated by
biodegradation returned to background within 20 weeks, although diesel
fuel had significant residual toxicity in untreated soil. Seed
germination and plant growth were inhibited in a similar manner, and
diesel oil was more toxic and persistent than heating oil (section
A9.1.3.1). Biodegradation treatment, in which the conditions for
hydrocarbon degradation by microbes were optimized by mixing,
aeration, pH control, and addition of mineral nutrients (fertilizers),
strongly decreased fuel persistence and toxicity.
A9.1.2 Aquatic organisms
A9.1.2.1 Plants (phytoplankton)
Experiments in both the laboratory and the field have shown that
hydrocarbons can inhibit algal growth, although enhancement is
occasionally noted at lower concentrations of oil (US National
Research Council, 1985).
After 12 days of exposure to 10% diesel oil, the growth of cells
of Euglena gracilis was not significantly reduced, whereas a
concentration of 0.1% almost completely inhibited the growth of
Scenedesmus quadricauda. Cells of S. quadricauda grown in culture
media containing diesel fuel (concentration not given) became
chlorotic, suggesting damage to the photosynthesizing system
(Dennington et al., 1975).
A concentration of 0.05% light diesel fuel was found to stimulate
growth rate, growth yield, photosynthesis, and chlorophyll a
synthesis, while at the same time slightly inhibiting the respiration
of Chlorella salina CU-1 (Chan & Chiu, 1985). At higher
concentrations (0.5 or 5%), the growth rate, growth yield, and
photosynthesis were greatly reduced (to 50, 50, and 62% and 43, 32,
and 41% of the control levels, respectively), but the effect on algal
respiration was less severe (81% with 0.5% diesel and 86% with 5%).
Diesel fuel containing dispersants caused greater inhibition than did
the compounds separately.
The lengthwise growth of the benthic algae Ascophyllum nodosum
and Laminaria digitata and other rocky-shore communities was
measured while they were kept in 50-m3 concrete basins and exposed
continuously to diesel fuel for two years (Bokn, 1987). An average
hydrocarbon concentration of 130 µg/litre continuously inhibited
growth in both species, while a concentration of 30 µg/litre caused
periodic inhibition. Under oil-free conditions during the subsequent
season, the plants recovered completely.
Boiler fuel was more toxic than diesel fuel to the kelp
Macrocystis; both fuels reduced photosynthesis (Baker, 1970).
A9.1.2.2 Invertebrates
(a) Several species
The acute toxicity of diesel fuel to Daphnia (Cladocera),
chironomid (insect) larvae, and the mollusc Viviparus bengalensis
(Gastropoda) was tested after the organisms were acclimatized to
laboratory conditions for four, two, and four days, respectively. The
bioassays were conducted at 28 ± 2°C with unchlorinated borehole water
(pH 7.0 ± 0.2; oxygen, 8 mg/litre; free carbon dioxide, 1.2 mg/litre;
total alkalinity as calcium carbonate, 240 mg/litre; hardness,
260 mg/litre). The results are shown in Table 18. Plankton and insect
larvae exhibited erratic, uncoordinated movements before becoming
lethargic. In the mollusc, active avoidance and heavy secretion of
mucus were observed. The concentrations at which these behavioural
changes occurred were not specified (Das & Konar, 1988).
Fourteen species of five phyla (Echinodermeta, Mollusca,
Annelida, Arthropoda, and Urochordata) were exposed to 0.5% No. 2
diesel fuel in sea water. Only the larvae of the echinoderm
Crossaster, which were also the largest, survived up to eight days;
all of the other larvae died 3-72 h after being placed in the
fuel-water mixture (Chia, 1973).
The periwinkles Pachymelania aurita (Muller) and Tympanotonus
fuscatus (Linne) were exposed to refined diesel fuel films for 24
and 48h at concentrations of 2.5, 5.0, 7.0, or 10.0% and to emulsions
for 24 h at concentrations of 0.5, 1.5, 2.5, or 3.5%; they were then
shaken for 2 min with brackish water. After exposure to the emulsion,
the periwinkles were washed and reintroduced into oil-free, brackish
water for 24 h and their activity recorded. Both surface films and
emulsions were harmful to both gastropods, the emulsions being the
more toxic. T. fuscatus was less susceptible than P. aurita,
possibly because the former can retract further into its shell. The
mean percentage survival in the presence of oil film (7.5% diesel
fuel) was 100% at 24 h and 80% at 48 h for T. fuscatus and 80% at 24 h
and 50% at 48 h for P. aurita. The mean percentage survival in the
presence of 3.5% diesel fuel-water emulsion was 90% for T. fuscatus
and 80% for P. aurita (Dambo, 1993).
Table 18. Acute toxicity of diesel fuel to Daphnia (Cladocera),
chironomid larvae (insects), and the mollusc Viviparus
bengalensis (Gastropoda)
Species LC5 (mg/litre) LC50 (mg/litre) LC95 (mg/litre)
Daphnia magna 1.5 (0.8-2.5) 20 (19.2-21.0) 38.5 (37.5-39.5)
Chironomid 5.0 346.0 (238-455) 865.0 (750-1018)
larvae
Viviparus 2.0 254.0 (185-320) 637.0 (575-700)
bengalensis
From Das & Konar (1988)
(b) Molluscs
The feeding rate and growth of mussels ( Mytilus edilus L.) was
markedly reduced when they were exposed to 30 or 130 µg/litre diesel
fuel for eight months (Widdows et al., 1985). Recovery of
physiological responses (clearance rate, respiration, food absorption
efficiency, and ammonia excretion) after transfer to unpolluted water
was concomitant with the depuration of hydrocarbons from the tissues.
Mussels exposed to either dose recovered completely within about 55
days.
The effects on the nutrient storage system and reproductive
cell systems of the mussel M. edilus of exposure to a diesel
hydrocarbon-sea water emulsion and subsequent depuration were studied
in order to assess the capacity of these systems to recover after
discontinuation of exposure to the oil. After 144 days of exposure to
hydrocarbon concentrations of 27 and 128 units, the volume of storage
cell types was significantly reduced and there was a reduction in the
volume of ripe gametes. After 53 days of depuration, the volume of the
storage cells and of developing gametes increased. Exposure to either
concentration also increased the volume of atretic (degenerating)
gametes, but depuration allowed a return to normal (Lowe & Pipe,
1986). The authors suggested that exposure to the hydrocarbons reduced
the storage reserves and increased gamete atresia and resorption, so
that the storage pool was partially replenished, enabling the organism
to tolerate better the hydrocarbon insult.
The sensitivity of M. edulis to pollution with diesel fuel
depends on the salinity of the water (Tedengren & Kautsky, 1987). Low
salinity and diesel fuel acted synergistically to a greater degree in
Baltic than in North Sea mussels.
Adults and larvae of M. edulis were exposed to microencapsulated
diesel fuel at concentrations of 200, 600, 1000, 1300, or
5000 µg/litre and the effects on mortality, larval growth, and
spawning frequency were recorded (Stromgren & Nielsen, 1991). The EC50
for spawning in mussels exposed for 30 days was about 800 µg/litre,
and the LC50 (30days) for maturing mussels was about 5000 µg/litre.
The longitudinal growth of larvae was significantly reduced at
10 µg/litre; the EC50 for growth (10 days) corresponded to about
25-30 µg/litre (Stromgren & Nielsen, 1991), which is lower than the
EC50 of about 1000 µg/litre for growth of juvenile mussels reported
by Stromgren & Reiersen (1988).
Diesel fuel is therefore more toxic to mussel larvae than to
juveniles.
The EC50 values (10 days) for growth of larvae of the Quahog
clam, Mercenaria spp., exposed to water-soluble fractions of various
crude and refined fuels were 220-4200 µg/litre, indicating that the
insoluble fractions of hydrocarbons, ingested as microcapsules, are
far more toxic than the water-soluble fractions alone (Byrne & Calder,
1977). Stromgren et al. (1986) drew the same conclusion from their
results for the growth of juvenile mussels. Larval mortality during
exposure to diesel fuel increased steeply up to 50 µg/litre, at which
dose only 20-30% survived 10 days of exposure. At 500 µg/litre, there
was 100% mortality. Byrne & Calder (1977) reported LC50 values (10
days) of 50-2100 µg/litre for larvae of Mercenaria spp.
(c) Crustaceans
Freshwater crabs (Barytelphusa cunicularis) were exposed to
sublethal concentrations of diesel fuel (4.5, 3.7, 3.2, and 2.6 ppm)
for 24, 48, 72, and 96 h, respectively (Sarojini et al., 1989). The
oxygen consumption of the crabs was measured as an index of stress
caused by the fuel and compared with that of normal controls. The
crabs responded in general by lowering their oxygen consumption up to
8 h (down to 50% at 4 h), particularly at the lower doses; with longer
exposures, the oxygen consumption was higher than (approx. 120% at
24 h) or equal to (at 96 h) that of the controls.
Gammarus spp. (Crustacea, Amphipoda) were obtained from marine
and brackish water in the North and Baltic Seas and tested in two
laboratories for their sensitivity to diesel-fuel pollution (Tedengren
et al., 1988). G. oceanicus was obtained from the Fucus belt at a
depth of 1-3 m and G. duebeni from rock pools of various salinities.
The respiration, excretion, and atomic O:N and O:P ratios of the two
species were compared after a 6-h exposure to 10 mg/litre diesel fuel
emulsified in a syringe and injected into experimental aquaria; the
resulting hydrocarbons were not measured. Exposure to diesel fuel
generally resulted in decreased oxygen consumption and a rise in
nutrient excretion, leading to lowered O:N and O:P ratios.
G. oceanicus was the most sensitive, and the response was aggravated
by simultaneous lowering or raising of the salinity. No signs of
impaired swimming or other physical or mechanical effects were
detected in either species. The investigators concluded that
G. duebenihas a greater tolerance to pollutants and changes in
salinity, probably because it has broad physiological niches and has
evolved in and become adapted to more variable environments.
In a comparison of the effects of an oil-water dispersion of
diesel fuel, kerosene, gasoline, and benzene on the tidepool copepod
Tigriopus californicus, diesel fuel was the most detrimental: a
concentration of 0.10 ml/litre sea water caused 100% mortality within
five days (Barnett & Kontogiannis, 1975).
(d) Fish
Freshwater
Table 19 shows the results of a series of 96-h static tests for
acute toxicity with diesel fuel in freshwater on several species of
juvenile salmonids, Oncorhynchus kisutch (coho), O. gorbuscha
(pink), and O. mykiss (rainbow trout), exposed at 14°C in 16 h of
light and 8 h of darkness (Wan et al., 1990). The average loading
density was 250 mg/litre (range, 100-420 mg/litre) of Canadian diesel
fuel with a pour-point of -17.8°C. Three types of water were used for
dilution: soft acid city tapwater, hard alkaline lakewater, and
intermediate reconstituted deionized city tapwater. The LC50 values
varied between the species from 32 to 33216 mg/litre. Diesel fuel was
more toxic to pink salmon than to coho salmon or rainbow trout,
irrespective of the water type.
The LC values for diesel fuel in golden orfe (Leuciscus idus
melanotus) in a static test were: LC0 = 40-120 mg/litre; LC50 =
120-160 mg/litre; LC100 = 160-205 mg/litre (Juhnke & Lüdemann,
1978).
Various freshwater fish (Fundulus diaphanus, Roccus saxatilis,
Lepomis gibbosus, Roccus americanus, Anguilla rostrata, and Cyrinus
carpio) were exposed to No. 2 or No. 4 fuel oil (both dispersed) in
a static test at 19°C, pH 7.1, and 60 mg/litre hardness (Rehwoldt et
al., 1974). The 96-h LC50 values for No. 2 fuel oil ranged from
22.2 mg/litre for striped bass (Roccus saxatilis) to 49.1 mg/litre
for carp (Cyrinus carpio); those for No.4 fuel oil ranged from
21 mg/litre for banded killifish (Fundulus diaphanus) to 48.1 mg/litre
for carp. These concentrations represent total oil added and not the
oil dissolved in the water column.
Table 19. Acute toxicity of diesel fuel to juvenile Pacific salmonids in different
types of freshwater
Water type Salmonid LC50 (mg/litre
24-h 48-h 72-h 96-h
Soft Coho 28 972 28 972 12 345 10 299
Pink 1 972 276 84 74
Rainbow > 32 000 5 525 4 600 3 017
Intermediate Coho > 55 560 > 55 560 33 216 33 216
(reconstituted) Pink 1 829 376 133 123
Rainbow 168 363 168 363 28 787 2 186
Hard (lake) Coho > 26 743 26 743 4 845 3 333
Pink 1 404 302 48 32
Rainbow > 23 108 23 108 5 102 2 447
From Wan et al. (1990). Coho, Oncorhynchus kisutch; pink, O. gorbuscha; rainbow
trout, O. mykiss
The LC50 values of water-soluble fractions and emulsions and
the floating layer of No. 2 fuel oil for four freshwater species under
static and flow-through conditions are shown in Table 20 (Hedtke &
Puglisi, 1982).
Marine
In an investigation of the threshold doses at which cod (Gadus
morhua L.) detected samples of Ekofisk diesel fuel, sea water was
allowed to gravitate through the diesel fuel. The total content of
dissolved materials, estimated by gas chromatography, were 15%
benzenes, 2% toluenes, 6% xylenes, 15% naphthalenes, 3% phenols, 42%
n-alkanes (C10-C25), and 17% unidentified compounds. There were
no visible surface films or microdroplets in the samples, although the
authors suspected the presence of undissolved microdroplets. Behaviour
was observed with a sea-water olfactometer, and reaction patterns
after injection of samples (snapping, increased activity, coughing,
darting, backing) were noted. The most frequent reaction was snapping
of jaws followed by a short period of activity. None of the doses
produced alarm reactions. The mean activity scores increased with
increasing doses of oil compounds. In a set of four experiments, the
threshold for detection of diesel oil compounds was 100-400 ng/litre,
showing that cod detect fuel compounds at doses lower than those
hitherto observed (Hellstrom & Doving, 1983).
The effect of acute exposure to the water-soluble fraction of
Arctic diesel fuel (1:9 with sea water) on survival and metabolic rate
was studied in an Antarctic fish (Pagothenia borchgrevinki). The
fish proved to be extremely tolerant and withstood an undiluted
water-soluble fraction for at least 72 h. None died as a direct
consequence of contact with the water-soluble fraction, but they
showed signs of stress. In unpolluted water, little spontaneous
swimming activity was seen, but on transfer into water containing
diesel fuel they became agitated and increased their swimming activity
for a short time. Their ventilation rates increased but later
decreased; the depth of each ventilation increased considerably.
Coughing became apparent shortly after transfer and persisted for up
to 72 h. The haematocrit increased and remained elevated throughout
the experiment. Oxygen consumption was increased to about twice that
of the controls (Davison et al., 1992).
In further studies on P. borchgrevinki (Davison et al., 1993),
a water-soluble fraction was prepared (1:2 with sea water) and then
diluted to 33%; the fish were kept for seven days. On initial
placement, behaviour similar to that with the higher concentration in
the previous experiment was noted. After seven days, the only
noticeable differences between exposed and control fish of similar
weight and length were large amounts of mucus streaming from opercula,
increased coughing, and slightly deeper ventilation. Plasma chloride
and osmolarity were similar in the two groups. Haematocrit and
haemoglobin concentration were significantly higher in the treated
fish; spleen weights and oxygen consumption were similar. This
Antarctic fish can thus survive prolonged periods of exposure to low
levels of water-soluble hydrocarbons.
Table 20. 96-h LC50 values for No. 2 fuel oil on freshwater organisms under various conditions
Species Life stage Test conditions Effect Concentration
(µl/litre water)
Jordanella floridae (flagfish) Adult Water-soluble fraction of 10% No mortality No dilution
oil-water mixture; static
Emulsion; flow-through LC50 60.5
Pimephales promelas (fathead minnow) Adult Emulsion; flow-through LC50 38.6
Water-soluble fraction of 10% No mortality No dilution
oil-water mixture; static
Floating layer; static LC50 > 160 000
48 300 (2nd
sample)
Rana sylvatica (frog) Larvae Water-soluble fraction of LC50 413 000
10% oil-water mixture; static
Emulsion; static LC50 26.4
Emulsion; flow-through LC50 4.9
Floating layer; static LC50 < 5000
Ambystoma maculatum (salamander) Larvae Emulsion; flow-through LC50 86.4
From Hedtke & Puglisi (1982)
(e) Amphibians
Larvae of the frog Rana sylvatica were tested in 96-h static and
flow-through tests with No. 2 fuel oil as a water-soluble fraction, an
emulsion, or a floating layer (Hedtke & Puglisi, 1982) (Table 20).
Flow-through exposure to the emulsion was the most toxic (LC50 =
4.9 µl/litre), and static exposure to the water-soluble fraction was
the least toxic (LC50 = 413 000 µl/litre). The LC50 of No. 2 fuel
oil emulsion in larvae of the salamander Ambystoma maculatum in a
flow-through test was 86.4 µl/litre).
A9.1.3 Terrestrial organisms
A9.1.3.1 Plants
Seed germination and growth of soya beans and ryegrass were
inhibited by a diesel fuel spill of 2.3 ml/m2 (Wang & Bartha, 1990;
see sections A3.2.4 and A9.1.1.2). Four weeks after the spill,
biodegradation had at least partially restored the ability of the soil
to support seed germination and plant growth. Morphological effects,
such as reverse geotropism, were noted on some partially emerged
seedlings. In untreated diesel-contaminated soil, there was no
evidence of seed germination or plant growth. Parallel experiments
showed that heating oil was less toxic.
A9.1.3.2 Invertebrates
No data were available.
A9.1.3.3 Vertebrates
Birds are affected externally and internally by oil
contamination. The fuel rapidly destroys the waterproofing of the
birds' plumage, and as the birds attempt to preen the contaminant off
their feathers they ingest fuel. When No. 1 fuel oil was administered
by gavage at doses of 1-4 ml/kg body weight to ducks, a dose of
2 ml/kg caused lipid pneumonia, extreme inflammation of the lungs, and
fatty infiltration and degeneration of the liver after 24 h (Croxall,
1977). Administration of 1 ml of diesel or No. 1 fuel oil/kg produced
severe irritation of the digestive tract (enteritis), with diarrhoea
and bile pigments in faeces. Signs of toxic nephrosis were also noted.
Adrenal enlargement (mainly hyperplasia of cortical tissue),
depression of plasma cholinesterase levels, and incoordination,
ataxia, and tremors were reported at higher doses. The treatment was
not fatal to healthy birds, even at doses up to 20 ml/kg body weight;
however, when No. 1 fuel and diesel fuel were administered to birds
under stress, the LD50 was 3-4 ml/kg body weight (Hartung & Hunt,
1966).
A9.2 Field observations
The following information is derived mostly from observations
made after diesel spills (see Table 10).
A9.2.1 Microorganisms
A9.2.1.1 Water
No data were available.
A9.2.1.2 Soil
Six weeks after the ship Bahia Paraiso spilled Arctic diesel
fuel into the Antarctic Sea, the impact of the spill on microbial
ecology was investigated (Karl, 1992). The acute effects on the
metabolic activities of sedimentary microorganisms appeared to be
negligible, even in saturated sea water.
A9.2.2 Aquatic organisms
In field studies conducted after fuel spills to investigate the
effects on populations and ecosystems, zooplankton appeared to be
highly vulnerable to dispersed and dissolved petroleum constituents
but less so to floating oils. Field observations on oil spills in
general showed that individual organisms were affected in many ways,
including direct mortality (fish eggs, copepods, mixed plankton),
external contamination by fuel (chorion of fish eggs, cuticles, and
feeding appendages of crustacea), tissue contamination by aromatic
constituents, abnormal development of fish embryos, and altered
metabolic rates (US National Research Council, 1985). A spill of
No. 2 diesel fuel was extremely toxic: faunal repopulation of the
affected areas did not occur during the subsequent eight months
(Blumer et al., 1970). A spill of 750000 litres of No. 2 diesel fuel
caused substantial mortality in some taxa of the intertidal
population, whereas others showed little or no effect. There was
substantial recovery within six months, and there was no observable
mortality among the subtidal fauna or flora (Woodin et al., 1972).
After a spill of marine diesel fuel (2000-3000 t) off Hong Kong, much
of the oil was carried to land by onshore winds. Wormald (1976)
described the effects of the oil spill on intertidal meiofauna. Within
four days of the spill, there was almost total mortality: nematodes
and harpacticoid copepods were observed in an advanced state of decay,
and other meiofauna were not detected. The aromatic fractions probably
evaporated within four to six weeks but persisted longer in deeper
sediment. Harpacticoids and nematodes did not recolonize the beach
until nearly eight months after the spill. The density of meiofauna at
all stations observed reached a maximum 10 months after the spill and
subsequently showed large fluctuations, which were probably the result
of ecological successions during recovery of the beach fauna. One year
later, an increase in the number of macrofauna, especially polychaete
worms, was noted, which may have been responsible for the decrease in
meiofaunal populations. High rainfall 11 months after the spill may
have brought the soluble aromatic compounds nearer to the surface and
decreased the harpacticoids and nematode populations. After 15 months,
the meiofauna were again well established. Recovery at high shore
stations began only when the oil content of the sediment had decreased
by at least 50%, primarily through physical dispersion. Recolonization
of the lower shore had occurred earlier. Stirling (1977) studied the
effects of this spill on rocky shore fauna (see also Table 10),
concentrating on large, common species of gastropods (Monodonta labio,
Thais clavigera, Nerita albicilla, N. polita, Lunella coronata,
Clypeomorus humilis, and Planaxis sulcatus), bivalve molluscs (Donax
semignosus, Atactodea striata, Tapes philippinarum, and Circe
stutzeri), and crustaceans (shore and hermit crabs). Acute mortality
of gastropods was greatest at moderately contaminated sites where
oil-dispersing chemicals had been applied to floating oil slicks.
Long-term disturbances were most significant at the heavily polluted
sites where dispersants had not been used. Recovery of animals taken
from the oiled beaches was studied in clean salt water in the
laboratory. Bivalve molluscs and the gastropods Monodonta labioand
Thais clavigera were the most sensitive and Clypeomorus humilisand
Planaxis sulcatus the least sensitive. Field observations of acute
mortality were consistent with this order of sensitivity. Population
studies showed the greatest reductions in Monodonta labio and Nerita
albicilla, which were eliminated for at least 13 months from the
site of greatest oil pollution.
The release of 600 000 litres of Arctic diesel fuel from the
Bahia Paraiso in 1989 coated intertidal macroalgae, limpets, and
birds as well as sediments and shores (Kennicutt et al., 1991b).
Macroalgae (Leptosomia simplex and Monostroma spp.) were only
moderately affected. Intertidal limpet (Nacella concinna) populations
were reduced by 50% within the first few weeks after the spill
(Kennicutt et al., 1990), and a year later had still not fully
recovered (Kennicutt & Sweet, 1992). Bottom-feeding organisms, such as
clams (Laternula elliptica) and fish (Harpagifer anarcticus and
Notothenia coriiceps neglecta) were found to have PAHs in their gut
contents and muscle tissues (Kennicutt et al., 1991b). After the
spill, all of the chicks of the local population of South Polar skuas
(Catharacta maccormicki) died, although few of the adults or the
chicks died from direct contact with oil (Eppley, 1992). Within less
than two years, and at many locations within a few weeks, the PAH
content of sediment and intertidal organisms returned to background
levels (Kennicutt & Sweet, 1992), owing partly to the high volatility
of the spilled fluid and the high energy environment. Sampling of
intertidal limpets showed that the tissue contaminants were primarily
naphthalenes, but fluorenes and phenanthrenes were often the most
abundant aromatic contaminants in tissues with higher concentrations
of PAHs (Kennicutt & Sweet, 1992; Kennicutt et al., 1992b) After a
spill of 8000 litres of diesel fuel into an unspoiled freshwater
stream in California, United States, most animals were affected within
one to four days. Thousands of aquatic insects perished; some
crayfish, aquatic leeches, and freshwater planarians were killed; and
over 2500 fish, tadpoles (but no frogs), snakes, a turtle, and common
merganser (Mergus merganser) were found dead (Bury, 1972).
A9.2.3 Terrestrial organisms
A9.2.3.1 Plants
Diesel fuel was applied at 10 litres/m2 to the soil of five
common plant communities in north-east Greenland, comprising wet
marsh, grassland, and three types of dwarf-shrub heath. The plant
species were observed for up to three years after the application.
Within a few days, most species had lost chlorophyll or had abscissing
leaves. Species with xeromorphic leaf characteristics reacted more
slowly than species with orthophyllous leaves. During three successive
growing seasons, shrubs and forbs showed no signs of recovery.
Graminoids showed slight resistance and recovered in all mesic and wet
plots but were killed in dry plots. Three species of sedge (Carex
bigelowii, C. saxatilis, and C. stans) and mosses recovered to some
extent in all plots. Recovery of mosses was excellent in the wet
plots, good in the mesic plots, and poor in the dry plots. In general,
the phytotoxic effects of diesel fuel were much more pronounced than
those of crude oil in similar plots (Holt, 1987).
A9.2.3.2 Invertebrates
No data were available.
A9.2.3.3 Vertebrates
No data were available.
A10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
A10.1 Evaluation of human health risks
Diesel fuel is produced commercially in various qualities with
regard to volatility, aromaticity, cetane number, and sulfur content.
The composition of diesel fuel, which influences the type and amount
of compounds emitted in the exhaust, has not changed greatly during
the last few decades, although the cetane number has been slightly
increased, resulting in better ignition, and the sulfur content, which
influences the release of particulate matter, has been reduced in some
countries. Owing to the lack of data on diesel fuel, some data on
heating oils and kerosenes (jet fuels) with compositions similar to
that of diesel fuel are included in the evaluations of toxicological
and environmental effects.
A10.1.1 Exposure of the general population
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, when handling such fuels, or when using kerosene for domestic
cooking or heating. No data on exposure were available.
A10.1.2 Occupational exposure
Workers can be exposed to diesel fuel and other middle
distillates during manual handling and discharge of the fuel, i.e.
during retailing at filling stations; manufacture, repair,
maintenance, and testing of diesel engines and other equipment; in
jobs where diesel fuel is used as a cleaning agent or solvent; and in
the handling and routine sampling of diesel fuel in the laboratory. At
room temperature, very low concentrations of vapours are likely to be
generated from diesel fuel because of its low volatility; significant
levels of vapour are likely to occur only in confined spaces and at
high temperatures.
A10.1.3 Non-neoplastic effects
Diesel fuels are toxic when ingested, usually accidentally.
Ingestion may result in regurgitation and aspiration, which can cause
chemically induced pneumonia. The latter effect is, however, not
specific for diesel fuel and can occur with all hydrocarbons of a
particular viscosity range.
Exposure to vapours is minimal during normal handling of diesel
fuel. The most likely effect on human health is dermatitis as a result
of skin contact. Dermal absorption takes place and can result in acute
toxic effects on the kidney. The health effects of long-term
absorption of low levels are unknown, but the available data on acute
human toxicity indicate that practices such as washing hands in diesel
fuel should be avoided. Although groundwater contamination and entry
into drinking-water are potential sources of adverse health effects,
such contamination would be noticeable and affect palatability so that
inadvertent ingestion of contaminated drinking-water is unlikely.
In experimental animals, diesel fuel has little acute toxicity after
exposure orally, dermally, or by inhalation. The oral LD50 values in
rats were > 5000 mg/kg body weight and those in mice, rabbits, and
guinea-pigs even higher. After short-term dermal exposure of mice for
14 consecutive days, the NOAEL for two middle distillates (marine
diesel and JP-5 navy fuel) was 5000-8000 mg/kg body weight per day.
Inhalation of up to 0.2 mg/litre had a neurodepressive effect in mice
but not in rats. Subchronic exposure to various distillate fuels
induced mainly alpha2-microglobulin nephropathy in male rats, which is
not considered relevant for humans.
Female mice that received dermal applications of 250 or 500 mg/kg
body weight per day of marine diesel fuel or JP-5 navy fuel on five
days per week for 103 weeks showed decreased survival due to severe
ulceration. Rats and mice exposed by inhalation to 1 or 5 mg/litre had
significant alterations in organ weight.
Diesel fuel irritates the skin but not the eye.
Exposure to diesel or jet fuels orally or by inhalation was
neither embryotoxic nor teratogenic in rats; although doses toxic to
the dams reduced fetal weight, no effects on viability were seen.
No clear evidence of mutagenic activity was seen in a series of tests
in Salmonella typhimurium. A positive response achieved only under
special conditions appeared to be equivocal. Other tests for
genotoxicity in vitro or in vivo did not show clearly positive
responses.
Owing to lack of data, a quantitative risk assessment is not
possible.
A10.1.4 Neoplastic effects
A case-control study of cancers at several sites in relation to
exposure to diesel fuel suggested an increased risk for lung cancer
other than adenocarcinoma and for prostatic cancer. In neither case
was there an exposure-response relationship. As few studies were
available, the number of cases was small, and the confidence intervals
were correspondingly wide, no conclusion can be drawn at present about
the carcinogenicity to humans of diesel fuel.
Twelve studies of dermal carcinogenicity in animals demonstrated
that diesel fuels have weak carcinogenic potency in mice. As no clear
genotoxicity is seen, cancer may be induced by nongenotoxic
mechanisms, such as chronic dermal irritation characterized by
repeated cycles of skin lesions and epidermal hyperplasia.
A10.2 Evaluation of effects on the environment
Although there are numerous data on the environmental behaviour
of the individual components of diesel fuel, few data are available
on diesel fuel as a whole. The environment can be polluted by
accidental release of diesel fuel on a large scale, such as during
tanker disasters and pipeline leaks, or on a smaller scale, from
contamination of soil around factories and garages.
In water, diesel fuel spreads out almost immediately, polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, volatile components evaporate from the water surface, and
microbial degradation begins. The extent to which such 'weathering'
takes place depends on the temperature and climatic conditions, and
the chemical composition of spills changes with time. After spillage
on water, some fractions evaporate, and the evaporated diesel
components are degraded photochemically. In sediment, diesel fuel is
generally delivered to bottom sediments by settling particles. In
soil, the various components of diesel fuel migrate at different
rates, depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates depend greatly on the physical and
climatic conditions and microbial composition. PAHs are the most
recalcitrant molecules.
Aquatic organisms, in particular molluscs, bioaccumulate
hydrocarbons to various extents, but the hydrocarbons are depurated on
transfer to clean water. Bioaccumulation of diesel fuel may occur, but
there are few data to indicate biomagnification.
Although there have been a number of spills of diesel fuel, few
reports are available on their environmental effects. Generally,
diesel fuel is more toxic than crude oil to aquatic and plant species.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota; recolonization
occurs after about one year, depending on the animal and plant species
involved and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can still be
affected by external contamination and tissue accumulation; abnormal
development and altered metabolic rates are signs of such stress.
There are few data on the effects of diesel fuel on aquatic and
terrestrial organisms in experimental situations. In studies of
water-soluble fractions and emulsions of diesel fuel, the actual
chemical composition has rarely been analysed, although this is
crucial to an understanding of the toxicity of diesel fuel. No data
are available about flow-through conditions or mesocosms, which better
reflect environmental conditions
A11. RECOMMENDATIONS
A11.1 Recommendations for the protection of human health
Exposure to diesel fuel may cause irritation and dermatitis;
therefore, skin contact should be avoided, and diesel fuel should not
be used for cleaning purposes.
A11.2 Recommendation for the protection of the environment
As for all petroleum products, accidental releases to the
environment should be avoided and should be cleaned up as soon as
possible if they occur.
A11.3 Recommendations for further research
A basic data set on the aquatic toxicity of diesel fuels,
including complete analysis of test substances, should be generated.
The fate and environmental impact of diesel fuel spills and leakages
should be investigated in laboratory and controlled field studies.
The exposure of humans to diesel fuel in various situations should be
quantified.
The mechanism of the carcinogenic action of diesel fuel on the
skin of experimental animals should be clarified.
A12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks of diesel fuels for human beings were
evaluated by a working group convened by the International Agency for
Research on Cancer in 1988 (International Agency for Research on
Cancer, 1989a). It concluded that there was inadequate evidence for
the carcinogenicity of diesel fuels in humans, and there was limited
evidence for the carcinogenicity of marine diesel fuel in experimental
animals.
PART B
B1. SUMMARY
B1.1 Identity, physical and chemical properties, and analytical
methods
Diesel engine exhaust emissions contain hundreds of chemical
compounds, which are emitted partly in the gaseous phase and partly in
the particulate phase of the exhaust. The major gaseous products of
combustion are carbon dioxide, oxygen, nitrogen, and water vapour;
carbon monoxide, sulfur dioxide, nitrogen oxides, and hydrocarbons and
their derivatives are also present. Benzene and toluene are present in
the lower weight percent range in the gaseous part of the hydrocarbon
fraction. Other gaseous exhaust components are low-relative-molecular-
mass polycyclic aromatic hydrocarbons (PAHs).
A main characteristic of diesel exhaust is the release of
particles at a rate about 20 times greater than that from
gasoline-fuelled vehicles. The particles are composed of elemental
carbon, organic compounds adsorbed from fuel and lubricating oil,
sulfates from fuel sulfur, and traces of metallic components. Most of
the total particulate matter appears to occur in the submicrometre
range, between 0.02 and 0.5 µm. Agglomeration may occur during aging,
up to a maximal diameter of 30 µm. The emitted particles have a large
surface area. Organic compounds generally contribute 10-30% of the
total particulate matter, but poorly designed and maintained engines
may result in as much as 90%. Higher-relative-molecular-mass,
oxygenated and nitro-PAHs occur at concentrations of parts per million
in this fraction.
Specific transient or steady-state driving cycles are used in
measuring vehicle emissions. The exhaust can be sampled from undiluted
or diluted exhaust gas. It is difficult to obtain artefact-free
samples, as exhaust constituents can undergo chemical reactions,
adsorption and desorption processes, and condensation and diffusion.
The toxicologically relevant PAHs in diesel particulate matter are
usually determined by Soxhlet extraction, clean-up, and fractionation,
with subsequent analysis by high-performance liquid chromatography or
gas chromatography coupled with mass spectrometry.
B1.2 Sources of human and environmental exposure
Diesel engine exhausts are emitted mainly from motor vehicles;
other sources are stationary, railway locomotive, and ship diesel
engines. The emissions from diesel motor vehicles have been well
described, but the individual results are often not comparable owing
to differences in parameters such as driving cycle, engine type, and
fuel composition. The individual components are emitted in the
following quantities: carbon dioxide, about 1 kg/km; carbon monoxide,
nitrogen oxides, total gaseous hydrocarbons, and particulate matter,
0.1-20 g/km; and aliphatic compounds, alcohols, aldehydes, light
aromatics, and PAHs, micrograms per kilometer. The emissions of carbon
monoxide, nitrogen oxides, total gaseous hydrocarbons, and particulate
matter are regulated by law in a number of countries.
In principle, there is no difference between the quality and
quantity of exhaust emissions from light- and heavy-duty engines,
although heavy-duty vehicles release larger relative amounts of
particulate matter. Exhaust emissions depend on driving cycle
(transient or steady state), engine conditions (injection and
aspiration techniques, maintenance, total mileage), and fuel
composition (sulfur content, aromaticity, volatility); adjustment of
the engine plays a major role.
The release of particulate matter increases with decreasing
air:fuel ratio, increasing load, and increasing temperature. More
particulate is released from older, intensively used engines than from
new, low-mileage engines, probably because of a greater consumption of
lubricating oil. The emission of particles from light-duty diesel
vehicles is also correlated with the sulfur content of the fuel, as
the formation of metal sulfates increases the particle mass; particle
emissions from heavy-duty vehicles have not yet been established.
Increasing fuel aromaticity also increases particle emissions.
PAHs and oxygenated PAHs from diesel and spark-ignition engines
are qualitatively similar. Oxygenated and nitrated PAHs are emitted in
the low microgram per kilometer range, but the actual concentrations
of these compounds are uncertain, as decomposition and formation can
occur during sampling. PAH emissions increase with increasing load and
temperature and with the age of the engine, probably owing to
increased consumption of lubricating oil. PAH emissions also depend on
the injection technique of the engine: they increase with increasing
air:fuel ratio in engines with direct injection, whereas they decrease
in engines with indirect injection. The aromaticity and volatility of
the fuel are directly correlated with the emission of PAHs.
Malfunction of engine devices, especially the fuel injection system,
increases the emission of the main exhaust components. There are few
data on the contribution of diesel motor emissions to the total
man-made release of combustion products.
Diesel exhaust emissions can be reduced by improving engine
design and by use of particle traps (trap oxidizers) and catalytic
converters. While particle traps remove both the soot and soluble
organic compounds adsorbed onto the particles, catalytic converters
reduce the levels mainly of carbon monoxide and gaseous hydrocarbons.
In practice, it is dificult to regenerate particulate traps. Catalytic
converters require fuels with a low sulfur content, as sulfur poisons
the active centres of the catalyst.
B1.3 Environmental transport, distribution, and transformation
The compartment first affected by diesel exhaust emissions is the
atmosphere. The hydrosphere and geosphere are contaminated indirectly
by dry and wet deposition. The environmental fate of the individual
constituents of diesel exhaust is generally well known: Particles
behave like (non-reacting) gas molecules with regard to their
mechanical transport in the atmosphere; they may be transported over
long distances and even penetrate the stratosphere. The overall
removal rate of diesel particles is estimated to be low, resulting in
an atmospheric lifetime of several days. During aging, particles may
coagulate, with higher fall-out rates, thus reducing the total
airborne level. The elemental carbon of diesel particulates may act as
a catalyst in the formation of sulfuric acid by oxidation of sulfuric
dioxide. The organic components adsorbed on elemental carbon may
undergo a number of physical and chemical reactions with other
atmospheric compounds and during exposure to sunlight.
B1.4 Environmental levels and human exposure
As diesel exhaust is a complex mixture of a great variety of
compounds, general 'environmental levels' cannot be given. The
individual components of diesel exhaust should be detectable in all
compartments of the environment, although their source cannot usually
be verified. The environmental levels of most of the individual
constituents are known.
The general population is most likely to be exposed to diesel
exhaust in busy streets or parking areas, particularly underground.
The identification of sources is difficult, and the contribution of
diesel exhaust to total pollution by traffic combustion products is
generally calculated on the basis of emission factors and the
percentage of diesel-fuelled vehicles. The levels of exposure of the
general population and workers to diesel particulate matter are
toxicologically relevant. The daily average ambient concentrations of
diesel particulates near roads are 8-42 µg/m3. The estimated annual
average concentrations were 5-10 µg/m3 in urban areas and
< 1.5 µg/m3 in rural areas in Germany and 1-2 µg/m3 in urban
areas and 0.6-1 µg/m3 in rural areas in the United States of America.
The concentrations are directly correlated with traffic density and
decrease with increasing distance from roads. Identification of
sources may also be difficult in work-places, especially in mines,
where the total dust burden is high. Carbon core analysis has been
used to determine specific exposure to diesel exhaust in the
work-place, and the levels of particulate matter to which workers are
exposed have been reported to be 0.04-0.134 mg/m3 for truck drivers
and 0.004-0.192 mg/m3 for railroad workers. Total respirable
particles and total suspended particles have also been used as
measures of occupational exposure.
B1.5 Kinetics and metabolism in laboratory animals and humans
B1.5.1 Deposition
Diesel exhaust particles, with a mass median aerodynamic diameter
of about 0.2 µm, undergo some filtration in the nose, and the
deposition efficiency in the lung is only slightly greater than the
minimal value found for particles with a mass median aerodynamic
diameter of about 0.5 µm. Thus, 10-15% of inhaled diesel soot
particles are deposited in the alveolar region of the lung of rats and
guinea-pigs; in humans, about 10% is deposited in the alveolar region.
B1.5.2 Retention and clearance of particles
Mucociliary clearance of particles from ciliated airways is
almost complete within 24 h. The long-term clearance of diesel
particles from the alveolar region was measured in several studies in
rats exposed by inhalation to labelled diesel or test particles.
Half-times of 60-100 days were reported for controls with low lung
burdens (< 1 mg/lung), whereas the half-times increased to 100-600
days in rats with lung burdens increasing from 1 to 60 mg/lung. In
several studies, effects were seen to be caused by particle overload,
which has been described in a variety of species and with a number of
particulate materials. The phenomenon is generally observed when the
deposition rate of particles of low solubility and low acute toxicity
exceeds their clearance rate for a considerable time. The half-time of
unimpaired alveolar clearance in humans is several hundred days, which
is longer than that in rats.
A dosimetric lung model was developed on the basis of data on the
deposition and retention of diesel particles in rats after long-term
inhalation and of data on particle deposition and retention in humans.
The model can be used to predict retention of diesel particles and
adsorbed organic material in the lungs of people of different ages. No
data are available on changes in the retention of individual compounds
after prolonged exposure to diesel exhaust.
B1.5.2 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
PAHs in diesel soot adhere strongly to the surface of particles.
About 50% of the PAHs adsorbed onto diesel particles is cleared from
the lung within one day, but the retention half-times for the
remaining PAHs were 18-36 days. Studies with 3 H-benzo[ a]pyrene and
14C-nitropyrene show that when PAHs are associated with particulate
matter, their clearance from the lungs is significantly delayed in
comparison with the clearance of inhaled PAHs not associated with
particulate matter.
B1.5.4 Metabolism
Benzo[ a]pyrene coated on diesel exhaust particles was metabolized
by oxidation to benzo[ a]pyrene phenols, diols, and quinones in the
lung and in cell cultures of pulmonary macrophages. Nitropyrene
adsorbed to diesel particles was metabolized to acetylaminopyrene-
phenol after inhalation. More DNA adducts were found in the lungs and
type II cells of rats exposed to diesel exhaust than in controls. The
oxidative metabolism of some organic compounds to epoxides may be
responsible for the formation of these adducts, but adducts were found
only after exposure to particles. Certain organic substances in diesel
exhaust have been shown to be responsible for the formation of DNA
adducts; however, the carbonaceous core itself (without extractable
organic material) can also induce adducts, by chronic damage of
epithelial cells.
B1.6 Effects on laboratory mammals and in vitro test systems
The few data available suggest that diesel exhaust has little
acute toxicity. Mice treated intratracheally with diesel exhaust
particles died due to lung oedema; the LD50 was about 20 mg/kg body
weight. Methanol-extracted diesel exhaust particles were not lethal a
concentrations up to about 33 mg/kg body weight. In hamsters, the LD50
after intraperitoneal administration was 1280 mg/kg body weight. No
data are available for exposure by inhalation.
Exposure of rats, guinea-pigs, and cats to diesel exhaust with a
particle content of 6 mg/m3 for about four weeks altered lung
function, including a 35% increase in pulmonary flow resistance in
guinea-pigs and a 10% decrease in vital capacity (expiratory flow) in
cats. Histopathologically, focal thickening of alveolar walls, a
significantly increased type-II cell labelling index, and
accumulations of particleladen macrophages were found. The
accumulations were located near the terminal bronchioles and became
larger, due to macrophage attachment (sequestration), during a
subsequent recovery period.
Mice, rats, hamsters, cats, and monkeys did not have drastic
decreases in body weight or reduced survival times after long-term
inhalation of up to 4 mg/m3 . Dose-related toxic effects seen in all
species after long-term inhalation of diesel exhaust were: increases
up to 400% in lung weight; pulmonary inflammation measurable by
biochemical (cytoplasmic marker enzymes, collagen) and cytological
(increase in polymorphonuclear neutrophils) parameters; impairment of
lung mechanics; increasing numbers of particle-laden macrophages with
focal accumulations (sequestration) under overload conditions; and
subsequent proliferative alterations of epithelial cells and onset of
fibrosis.
Limited information on reproductive and developmental toxicity
suggests that inhalation of diesel exhaust is not critical. In most
experiments, no effects were seen in mice, rats, hamsters, rabbits, or
monkeys; however, after intraperitoneal injection, sperm abnormalities
were seen in mice given diesel exhaust particles and embryotoxicity
occurred in hamsters given diesel exhaust extract.
Most tests for genotoxicity in vitro have been performed with
diesel exhaust extracts rather than with total exhaust; positive
responses were found in the absence of metabolic activation, i.e. the
genotoxic effects appeared to be PAH-independent. About one-half of
investigations in vivo gave negative responses; the only positive
responses were sister chromatid exchange induction with total diesel
exhaust and with organic extracts and micronucleus induction with
organic extracts.
Immunotoxic effects were generally not seen after inhalation of
diesel exhaust; however, an enhanced anti-ovalbumin immunoglobulin E
antibody titre was seen in one study and an increased susceptibility
to infections in mice in two experiments. Studies in rats suggested
that inhalation of diesel exhaust affects behavioural and
neurophysiological status.
In studies of the carcinogenicity of diesel exhaust administered
by inhalation to rats, the gaseous phase (without the particulate
fraction) was not carcinogenic. In all valid studies in rats, diesel
exhaust was found to be carcinogenic at particle concentrations of >
2 mg/m3, corresponding to an equivalent continuous exposure of about
1mg/m3. No effect was seen in hamsters or mice. In studies by
intratracheal instillation, both diesel exhaust particles and carbon
black induced tumours, and the surface area of the carbonaceous
particles appeared to be correlated with the tumorigenic potency.
Long-term inhalation of carbon black virtually devoid of PAHs at
similar concentrations also resulted in lung tumours in rats.
It is not clear whether the carcinogenicity of diesel exhaust
involves DNA-reactive or non-DNA-reactive mechanisms (or a
combination). Various models have been used to elucidate the
carcinogenicity of diesel exhaust.
B1.7 Effects on humans
Diesel exhaust contributes to air pollution in general. Although
the role of diesel particles cannot be singled out in acute or chronic
studies, they may be partly responsible for the range of health
effects found to be associated with air pollution.
Typical diesel exhaust has a characteristic odour, which some
people find offensive, particularly at high concentrations. The
symptoms seen after acute and chronic exposure to diesel exhaust have
been described in studies and anecdotal reports of occupationally
exposed subjects. Acute exposure to diesel exhaust has been associated
with irritation of the ocular and nasal mucous membranes, and an
increased frequency of respiratory symptoms has been observed in
occupational cohorts; however, the contribution of diesel particulates
is not known. No consistent short-term effect on pulmonary functions
has been found, but asthma attacks have been reported.
In a controlled study in which eight healthy non-smoking
volunteers were exposed to diluted diesel exhaust in a chamber for
60 min, the phagocytosis rate of alveolar macrophages in broncho-
alveolar lavage fluid was reduced.
Some cross-sectional and longitudinal studies on workers with
long-term occupational exposure to diesel exhaust show decrements in
lung function and an increased prevalence of respiratory symptoms, but
these studies are limited by short exposure. Cohort studies in which
deaths from cardiovascular and/or cerebrovascular disease due to
diesel emissions were investigated did not show a significant excess.
The relationships between cancers of the lung and of the urinary
bladder and occupational exposure to diesel exhaust have been
evaluated in a number of epidemiological studies. Only those studies
that were considered relevant for evaluating the carcinogenic effects
of diesel exhaust are included in this monograph. The most relevant
studies with regard to lung cancer are those of railroad workers, bus
garage workers, and stevedores, who have definite exposure to diesel
exhaust. The four most informative studies all reported an increased
risk for lung cancer, with relative risks ranging from 1.4 for
railroad workers and 1.3-2.4 for bus garage workers (depending on
exposure category), to a three- to sixfold increase in risk for
stevedores (depending on the exposure assessment used, but with wide
confidence intervals). Adjustment for smoking was possible in one
case-control study of railroad workers and in the study of stevedores,
and in both cases, the effect of diesel exposure was not materially
influenced. In the three studies in which smoking could not be
adjusted for, the analysis was based on comparisons of subgroups of
the cohorts, so that confounding by tobacco smoking was less likely
than when external comparison groups were used.
Several case-control studies have been conducted to examine the
relationship between urinary bladder cancer and presumed exposure to
diesel exhaust. An increased risk was found, especially for truck
drivers; however, all of these studies are limited by poor
characterization of exposure. Hence, a causative association between
exposure to diesel exhaust and an increased risk for urinary bladder
cancer cannot be established.
B1.8 Effects on other organisms in the laboratory and the field
The effect of diesel emissions as such have been addressed in
only one study, of green algae.
B1.9 Evaluation of human health risks
The risk assessment paradigm of the United States National
Academy of Sciences (US National Research Council, 1983) was used to
assess the risks for both cancer and non-cancer end-points. The four
steps in this process comprise: (1) hazard identification, (2)
dose-response assessment, (3) exposure assessment, and (4) risk
characterization.
Epidemiological studies of long duration with well-defined
exposure and follow-up (> 20 years) are considered to be the most
informative. Four studies of lung cancer in occupationally exposed
individuals met these criteria. The relative risks for lung cancer
associated with exposure to diesel exhaust were generally low and were
susceptible to chance, to the effects of unmeasured confounding
factours, and to the difficulty in accurately adjusting for known
confounding factors. Studies with less precise definitions of exposure
support the conclusions of these studies. Overall, it is considered
that diesel exhaust is probably carcinogenic to humans; however, no
quantitative data are available for estimating human risk.
B1.9.1 Non-neoplastic effects
Two general approaches were used for risk characterization: a
no-observed-adverse effect level (NOAEL) divided by an uncertainty
factor; and a benchmark concentration. In both approaches, a
sophisticated dosimetric model was used which decreases any
uncertainty in interspecies extrapolation of dose.
The no-effect level of diesel exhaust particles in humans was
calculated to be 0.139 mg/m3. The guidance value for the general
population calculated from the dosimetric model was 5.6 µg/m3, and
that calculated without the model was 2.3 µg/m3.
The benchmark concentration approach takes into account the
entire exposure-response relationship rather than relying on a single
data point from studies by inhalation, as in the NOAEL approach. Three
sensitive end-points were identified: chronic alveolar inflammation,
impaired lung clearance, and hyperplastic lung lesions. The benchmark
concentrations, calculated from the same dosimetric model used in the
NOAEL approach, were 0.9-2 µg/m3for inflammation, 1.2-3 µg/m3 for
impaired lung clearance, and 6.3-14 µg/m3 for hyperplastic lesions.
B1.9.2 Neoplastic effects
A linearized multistage model was used to estimate unit risks due
to exposure to diesel exhaust. Because the results of the available
epidemiological studies were considered inadequate for a quantitative
estimate of unit risk, data from several studies of long-term
inhalation in rats were used in which carcinogenesis occurred at
concentrations >2 mg/m3. A unit risk of 3.4 × 10-5 µg/m3 (geometric
mean of four risk estimates) diesel exhaust particles was calculated.
An alternative biologically based model yielded a similar unit risk,
under the assumption that diesel particles affect cell initiation
and/or proliferation at low concentrations.
B1.10 Evaluation of effects on the environment
Insufficient information was available to evaluate the specific
effects of diesel exhaust emissions. The effects of combusted diesel
fuel should be similar to those of other fossil fuels and are related
to the consumption of diesel fuel.
B2. IDENTITY AND ANALYTICAL METHODS
B2.1 Identity
Diesel engine emissions contain hundreds of chemical compounds,
which are emitted partly in the gaseous phase and partly in the
particulate phase of the exhaust. These substances form particles or
contribute to the gaseous phase of the emissions, depending on vapour
pressure, temperature, and the concentration of individual species.
The amount and composition of diesel exhaust are related to engine
conditions and fuel specifications (see sections B3.1.2.1 and
B3.1.2.2). A total of 445 compounds has been identified or tentatively
identified as constituents of diesel exhaust emissions (Westerholm,
1987).
Diesel exhaust has an offensive odour (odour threshold, 320 ppm).
Compounds with a relative molecular mass > 80 mainly determine the
odour (Scheepers & Bos, 1992b). Partridge et al. (1987) concluded that
benzaldehyde and a methylbenzaldehyde isomer are the major substances
responsible; unburnt aromatic hydrocarbons may also contribute.
Aldehyde levels and odour emissions are directly correlated with the
total hydrocarbon content of the exhaust; decreased hydrocarbon levels
lead to diesel exhaust with less odour (Organisation for Economic
Co-operation and Development, 1993).
B2.1.1 Chemical composition of diesel exhaust gases
The main gaseous products of diesel exhaust are carbon dioxide,
oxygen, nitrogen, and water vapour; carbon monoxide, sulfur dioxide,
nitrogen oxides, and hydrocarbons and their derivatives are also
present. A representative composition of diesel exhaust gas
(light-duty engine) is shown in Table 21.
The levels of carbon monoxide, nitrogen oxides, total
hydrocarbons, and particulates emitted by diesel engines are regulated
by law in a number of countries (see section B3.2). The composition of
diesel exhaust gases is similar to that of gasoline engine gases, but
because of the relatively higher fuel:air ratio, carbon monoxide and
hydrocarbons occur in lower concentrations in diesel exhaust, and the
emission of nitrogen oxides, particulate matter, and sulfur compounds
is higher (the latter being due mainly to the higher sulfur content of
diesel fuels). The relative concentrations in the aldehyde fraction
are as follows: about 45% by weight formaldehyde, 17% by weight
acetaldehyde, 14% by weight acetone and acrolein, < 10% by weight
crotonaldehyde, propionaldehyde, isobutyraldehyde, benzaldehyde, and
hexanaldehyde; methylethylketone is also present (Volkswagen AG,
1989).
Table 21. Composition of light-duty diesel engine exhaust
Component Concentration
(% by weight)
Carbon dioxide 7.1
Water vapour 2.6
Oxygen 15.0
Nitrogen 75.2
Carbon monoxide 0.03
Hydrocarbons 0.0007
Nitrogen oxides 0.03
Hydrogen 0.002
Sulfur dioxide 0.01
Sulfates 0.00016
Aldehydes 0.0014
Ammonia 0.00005
Particulates 0.006
Adapted from Volkswagen AG (1989)
Diesel exhaust contains toxicologically relevant compounds such
as benzene, toluene, ethylbenzene, xylene, and PAHs. In the
hydrocarbon fraction, benzene and toluene are present in relative
concentrations of 11 and 4% by weight, respectively (Volkswagen AG,
1989).
Possible sources of PAHs in diesel exhaust are unburnt PAHs from
the fuel, pyrosynthesis of PAHs during combustion, crankcase oils, and
engine and/or exhaust system deposits (Scheepers & Bos, 1992a). These
constituents occur partly in the gaseous fraction of total
hydrocarbons (benzene, toluene, ethylbenzene, and xylene components,
PAHs of lower relative molecular mass) and partly in the particulate
fraction due to adsorption (PAHs of higher relative molecular mass).
The partitioning of constituents between the particulate and gas
phases has been measured by several investigators (Hampton et al.,
1983; Schuetzle, 1983; Schuetzle & Frazier, 1986). Most PAHs with five
or more rings and aliphatic hydrocarbons with more than 18 carbon
atoms are expected to be primarily adsorbed onto particles (Schuetzle,
1983). There is a direct relationship between adsorption behaviour and
vapour pressure (Hampton et al., 1983); compounds with vapour
pressures < 10-5 mbar (7.5 × 10-6 mm Hg; 2.09 kPa) at room
temperature occur predominantly in the particulate phase. An empirical
relationship between boiling-point and particle- to gas-phase
partition coefficients has been derived for PAHs and aliphatic
hydrocarbons in order to estimate gas-phase emission rates (Schuetzle
& Frazier, 1986). According to this equation, the concentration of
e.g. anthracene in the gaseous phase is about 20 times higher than
that in the particle phase, whereas the levels of e.g. pyrene and
fluoranthene are nearly equal in the two phases. Oxygenated PAHs are
found in diesel engine exhaust at concentrations as high (Volkswagen
AG, 1989) or twice as high (Jensen & Hites, 1983) as those of their
parent PAHs, perhaps because these engines have a larger air:fuel
ratio than gasoline motors (Scheepers & Bos, 1992a). 9-Fluorenone and
9,10-anthracenequinone are especially abundant in diesel exhaust
(Schuetzle & Frazier, 1986). In tests of diesel passenger cars by
Volkswagen AG (1989), phenalene-1-one, naphthalene dicarboxylic acid
anhydride, cyclopenta[ def]phenanthrene-4-one, monoaldehydes of
phenanthrene and anthracene, mono- and diketones of benzanthracenes
and benzo[cd]pyrene, and benzofluorenones were also found (for
artefact formation during sampling, see section B2.2.1; for emission
factors, see section B3.1.1.1).
Nitrogen-substituted PAHs are formed during combustion as a
result of either nitration of PAHs by nitric acid or addition of
nitrogen monoxide or dioxide to PAH free radicals generated during
combustion (Schuetzle, 1983). In the exhaust of a four-cylinder diesel
engine, nitrofluorene, dinitrofluorenone, nitroanthracene,
nitrofluoranthene, mono- and dinitropyrenes, and nitrobenzo[ a]pyrene
were detected at concentrations about one order of magnitude lower
than those of oxygenated PAHs (Volkswagen AG, 1989).
A number of investigators have found nitrated PAHs in extracts of
diesel engine exhaust particles (Handa et al., 1983; Hartung et al.
1984). Many three-, four-, and five-ring structures are involved
(Schuetzle et al., 1982). The concentration of 1-nitropyrene, one of
the prevalent species, has been reported to be 15-25 mg/kg particulate
matter, whereas benzo[ a]pyrene has been found at concentrations up
to 50 mg/kg. The nitrated PAHs are important in determining the health
effects of exposure to diesel exhaust, since they are effective
mutagens in microbial and human cell systems (Pederson & Siak, 1981;
Pitts et al., 1982; Patton et al., 1986). Some nitrated PAHs are
carcinogenic to animals (Ohgaki et al., 1985; Imaida et al., 1991).
Combustion products originating from fuel and lubricating oil
additives or from corrosion may also occur. For example, nitrate-based
cetane improvers (see also section A2.1.2) are assumed to form nitro-
PAHs (Organisation for Economic Co-operation and Development, 1993).
B2.1.2 Type and composition of emitted particulate matter
Diesel exhaust is characterized by a higher content (10-20-fold)
of particulate matter than that of gasoline-fuelled passenger cars
(Egebäck & Bertilsson, 1983; Volkswagen AG, 1989; Williams et al.,
1989; CONCAWE, 1990b; Hammerle et al., 1994a). How soot particles are
formed is still under discussion, but the precursor is probably
acetylene generated during combustion. As a result of the numerous
cracking, dehydration, and polymerization processes, quite large
amounts of carbon, hydrogen, hydroxyl, and oxygen radicals are
available in the combustion chamber, which can produce cyclic and
polycyclic aromatic hydrocarbons from acetylene by polymerization and
ring closure. With increasing polymerization, the carbon content of
the macromolecule increases, finally generating graphite-like soot
particles (Klingenberg et al., 1991).
Investigations of two four-cylinder engines with direct and
indirect injection (see also section B3.1.2.1) showed that the emitted
particles range in shape and size from spherical particles of
< 0.01 µm to cluster and chain agglomerates with maximal dimensions
of 30 µm (Dolan et al., 1980; Amann & Siegla, 1982; Klingenberg et
al., 1991). The formation of agglomerates (coagulation, 'aging') is
dependent not only on engine and exhaust conditions but also on
environmental factors, such as photochemical processes and humidity.
Between 50 and 80% of the total particulate emission occurs in the
range of 0.02-0.5 µm (Israel et al., 1982). According to Amann &
Siegla (1982), the emitted particles have a concentric, lamellar
structure arranged around the centre, similar to the structure of
carbon black.
Emitted soot particles are composed of elemental carbon, adsorbed
organic compounds (soluble organic fraction) from fuel and lubricating
oil, traces of metallic compounds (iron, calcium, and zinc at < 0.6%
by weight; Volkswagen AG, 1989), and sulfates. The organic components
of emitted particles are distributed over the surface. Numerous data
on the relative proportions of organic compounds are available
(Horvath et al., 1987; Williams et al., 1987; Volkswagen AG, 1989;
Williams et al., 1989; CONCAWE, 1992). Depending on the engine
conditions and testing cycle, the organic compounds comprise 10-90% of
the total particulate mass. The ranges of various constituents of
particulate matter in nine light-duty and four heavy-duty vehicles
(see also section B3.1.2.1) are shown in Table 22. The driving cycles
used were combined European Union tests (ECE-15 and 'high speed'
extra-urban driving cycle [EUDC]) for the light-duty vehicles and
modified ECE-R49 tests for the heavy-duty engines (see also section
B2.2.1); four fuels with different sulfur contents and an aromatic
content of about 25% by volume were used (CONCAWE, 1992). (For a
description of the influence of fuel composition on exhaust emissions,
see section B3.1.2.2.)
This study showed considerable variation, and no obvious
influence of fuel composition or engine conditions was discernible,
although a difference was seen between fuel and lubricating oil
hydrocarbons derived from light- and heavy-duty vehicles,
respectively. This is probably due to the different driving cycles:
transient in the case of light vehicles, steady state in the case of
heavy-duty engines. In the individual data, adjustment of the engine
appeared to play a major role. In the same study, PAHs were determined
in the soluble organic fraction of the exhausts of three light-duty
vehicles representing different engine conditions and of the four
heavy-duty engines. The data are shown in Table 23. The constituents
of the particulate matter of the exhaust are clearly dependent on the
driving cycle used. Under steady-state conditions (heavy-duty
engines), the PAH content is about one order of magnitude lower than
that under transient conditions (light-duty vehicles). In addition,
transient conditions lead to considerable variations in levels. In the
study of Volkswagen AG (1989) on a four-cylinder diesel engine in the
US-72-test, a hot start was used, and higher levels of fluoranthene,
pyrene, benzo[ ghi]fluoranthene and benzo[ c]phenanthrene were
found, perhaps due to either engine condition or driving cycle.
According to Volkswagen AG (1989), some of the PAHs, and especially
pyrene, benz[ a]anthracene and benzo[ a]pyrene, seemed to decompose
during sampling (see section B2.2.1).
Table 22. Particulate matter content of exhausts from light- and heavy-duty vehicles
Constitutent Particulate matter (% by weight) Reference
Light-duty vehiclesa Heavy-duty vehiclesa
Carbon 32.6-85.1 39.7-81.7 CONCAWE (1992)
82-88 (passenger 68-89 Williams et al.
cars) (1989)b
76-83 (light vans)
60-75 Hammerle et al.
(1994a)c
Fuel-derived 1.1-55.0 9.8-32.5 CONCAWE (1992)
hydrocarbons 6.0-18 Hammerle et al.
(1994a)c
Lubricating 4.2-54.4 4.0-25.9 CONCAWE (1992)
oil-derived 5.7-15 Hammerle et al.
hydrocarbons (1994a)c
Soluble organic 10.2-64.7 14.0-58.4 CONCAWE (1992)
fraction 14-26 (passenger 22-90 Williams et al.
cars) (1989)b
36-48 (light vans)
Sulfate (excluding 0.6-12.2 1.4-7.5 CONCAWE (1992)
bound water) 3.3 (passenger 0.3-6.9 Williams et al.
cars) (1989)b
2.5 (light vans) 1.5-2.0 Hammerle et al.
(1994a)c
a According to the Organisation for Economic Co-operation and Development (1993),
light-duty vehicles are generally classified as vehicles with a weight < 3.5 t,
occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b Measurements for four passenger cars and 15 light vans in different Australian
driving cycles corresponding roughly to US-FTP-72 and -75 tests for heavy-duty
vehicles, and 10 trucks and two buses in one Australian urban traffic driving cycle
c Five passenger cars and light vans in European Motor Vehicle Emissions group cycle
and US-FTP-75 test
Table 23. Polycyclic aromatic hydrocarbon content of the soluble organic fraction of
particulate matter in diesel exhaust
Constituent Soluble organic fraction Referenceb
(mg/kg particulate matter
Light-duty Heavy-duty
vehiclesa vehicles
Fluoranthene 8-238 4-52 CONCAWE (1992)
553-560 Volkswagen AG (1989)
Pyrene 11-287 7-90 CONCAWE (1992)
579-668 Volkswagen AG (1989)
Benzo[ghi]fluoranthene 36-61 3-6 CONCAWE (1992)
+ benzo[c]phenanthrene 163-179 Volkswagen AG (1989)
Benz[a]anthracene 9-285 1-16 CONCAWE (1992)
61-82 Volkswagen AG (1989)
Triphenylene and 14-138 4-13 CONCAWE (1992)
chrysene 117-138 Volkswagen AG (1989)
Benzo[b,j]fluoranthene 4-87 1-10 CONCAWE (1992)
180-193c Volkswagen AG (1989)
Benzo[a]fluoranthen 6-69 CONCAWE (1992)
Benzo[e]pyrene 8-40 1-2 CONCAWE (1992)
85-90 Volkswagen AG (1989)
Benzo[a]pyrene 2-48 1-3 CONCAWE (1992)
38-54 Volkswagen AG (1989)
Perylene 5-32 2 CONCAWE (1992)
4-8 Volkswagen AG (1989)
Dibenz[a,j]anthracene 10-78 CONCAWE (1992)
Indeno[123-cd]pyrene 6-54 CONCAWE (1992)
55-73 Volkswagen AG (1989)
Dinbenz[a,h]- or 3-40 CONCAWE (1992)
-[a,c]anthracene
Benzo[ghi]perylene 25-65 1-5 CONCAWE (1992)
75-82 Volkswagen AG (1989)
Anthracene 3-7 CONCAWE (1992)
Dibenz[a,i]pyrene 8-31 CONCAWE (1992)
Coronene and 9-52 CONCAWE (1992)
dibenzo[a,e]pyrene 33-48d Volkswagen AG (1989)
a According to the Organisation for Economic Co-operation and Development (1993),
light-duty vehicles are generally classified as vehicles with a weight < 3.5 t,
occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b From Volkswagen AG (1989): US-72 tests with one four-cylinder engine
c Including benzo[k]fluoranthene
d Excluding dibenz[a,e]pyrene
B2.2 Analytical methods
B2.2.1 Sampling
Driving cycles are specified to simulate on-road conditions for
measuring vehicle emissions (Grimmer et al., 1988). The test
procedures can be divided in two basic types: steady-state and
transient cycle tests. In the European Union and Japan, mainly
steady-state tests are used, in which emissions from engines are
measured at specified combinations of speed and load. The most widely
used official tests for light- and heavy-duty vehicles are as follows:
Light-duty vehicles:
* US FTP-75: transient cycle; cold start; driving distance, 17.8
km; 10-min break after 28.7 min (United States) (Volkswagen AG,
1989)
* US SET: transient cycle; 'sulfate emission test'; hot start;
simulating rush-hour freeway driving; driving distance, 21.7 km
(United States) (Volkswagen AG, 1989)
* ECE 15: transient cycle; cold start; simulating city-centre
driving; driving distance, 4.052 km (European Union) (CONCAWE,
1990a)
* US HDC: steady-state cycle; 'highway driving cycle'; hot start;
driving distance, 16.5 km; maximal speed, 96.4 km/h (United
States) (Volkswagen AG, 1989)
* EUDC: steady-state cycle; 'extra-urban driving cycle'; hot start
(after ECE 15); driving distance, 6.755 km; maximal speed,
120km/h (European Union) (CONCAWE, 1990a)
Heavy-duty vehicles:
* HD-FTP: transient cycle; 'heavy-duty federal test procedure';
simulating metropolitan driving (United States) (Organisation for
Economic Co-operation and Development, 1993);
* ECE 13: steady-state cycle at 13 different modes; identical to
ECE R49 (European Union) (Organisation for Economic Co-operation
and Development, 1993). Various bus cycles are also used to test
heavy-duty vehicles that are characterized by frequent
stop-and-go driving (Westerholm & Egebäck, 1991; Prakash et al.,
1992).
It is difficult to avoid artefacts in sampling. Exhaust gas
constituents can undergo chemical reactions, adsorption and desorption
processes, and condensation or diffusion. For example, oxygenated and
nitrated PAHs are chemically unstable, and artefacts may form on the
sampling device, especially if large sampling volumes are needed (Lee
& Schuetzle, 1983). Both decreases and increases in the concentrations
of PAHs and oxy-PAHs were observed during sampling in a study by
Volkswagen AG (1989); in particular, degradation of pyrene and
nitropyrene and formation of naphthalene dicarboxylic acid anhydride
on the filter occurred. PAHs can be converted into nitroarenes (Pitts
et al., 1978; Lee et al., 1980; Gibson et al., 1981; Lee & Schuetzle,
1983; Schuetzle & Perez, 1983; Gaddo et al., 1984; Levsen et al.,
1988).
The sampling techniques used for the collection of the gaseous
and particulate phases of diesel exhaust comprise dilution tube
sampling and raw gas sampling.
B2.2.1.1 Sampling from undiluted exhaust gas (raw gas sampling)
The device for sampling motor vehicle exhaust (total flow method;
Kraft & Lies, 1981; Grimmer et al., 1973) consists of a glass cooler,
a condensate separator, and a special micron filter of
paraffin-impregnated glass-fibre material providing separation of
> 99.9% of particles of 0.3-0.5 µm. The cooler is fed with cold tap
water, and the filter is placed on top of the cooler to remove any
uncondensed liquid or solid exhaust gas constituents that may pass
through the cooler coil. The particulate filter retains undissolved
particles of the exhaust gas, while the condensate consists of the
dissolved components (Volkswagen AG, 1989).
The US Environmental Protection Agency (Lies et al., 1986)
defines the 'particulate phase' as all materials (with the exception
of condensed water) which, at a temperature of < 51.7°C, are retained
on a special filter after dilution with air. Large volumes are
collected with this type of sampling, making analysis of trace
components difficult. The sample volumes are reduced by diverting the
exhaust emission flow and sampling only a part. It is difficult to
ensure a representative partial flow, as the amount of exhaust gas
produced varies considerably over time (Volkswagen AG, 1989).
B2.2.1.2 Sampling from diluted exhaust (dilution tube sampling)
The exhaust is mixed with ambient air in a dilution tunnel in a
constant volume sampler method (Behn et al., 1985). The total flow of
air and exhaust is kept constant with a suitable fan in a constant
volume sampler unit. This sampling technique more realistically
reflects the immediate dilution of exhaust leaving the tailpipe of
vehicles on the road. The concentrations of exhaust components are,
however, reduced in accordance with the degree of dilution (magnitude,
1:10), and components that are present only at low concentrations
cannot be determined.
There are various techniques for sampling gaseous constituents.
Substances present at sufficiently high concentrations are commonly
sampled in a plastic bag; adsorption onto solid materials, consisting
of extremely porous, tiny plastic pellets (e.g. Tenax; diameter,
0.1-0.2 mm; specific surface area, 20-50 m2/g), or absorption in
liquids are also used. Particulate constituents are sampled by
filtration (Lee & Schuetzle, 1983; Volkswagen AG, 1989).
B2.2.2 Extraction from particles
PAHs adsorbed onto diesel exhaust particles are of toxicological
relevance, and there are numerous methods for extracting these
constituents. The most frequently used method is Soxhlet extraction,
which permits extraction of 99% of the soluble organic fraction within
8 h; an additional 16 h are necessary to extract the remaining 1%.
Extraction is frequently carried out by sequentially changing the
solvents, from polar to nonpolar, e.g. toluene, toluene or n-propanol,
dichloromethane, and acetonitrile. PAHs may be further extracted by
refluxing the exhaust particulates in solvent for 1 h. Sublimation and
extraction by ultrasonic agitation are also used (Lee & Schuetzle,
1983; Levsen, 1988), and supercritical fluids such as carbon dioxide
are effective (Hawthorne & Miller, 1986). The extraction efficiency of
various solvents is still under discussion (Köhler & Eichhoff, 1967;
Swarin & Williams, 1980; Schuetzle & Perez, 1981; Grimmer et al.,
1982; Lee & Schuetzle, 1983; Schuetzle et al., 1985; Levsen, 1988).
Aromatic solvents and their combinations seem to be best suited for
extracting PAHs from diesel particulates (Levsen, 1988).
B2.2.3 Clean-up and fractionation
Complex fractionation schemes for separating the exhaust extract
into aliphatic compounds, aromatic compounds, and moderately and
highly polar fractions after the removal of acidic and basic fractions
have been described by Petersen & Chuang (1982) and Levsen (1992).
During fractionation, further artefacts may be produced by the
components of a complex sampling mixture like diesel exhaust,
especially with acidic and basic extraction and aggressive column
separation procedures.
B2.2.4 Chemical analysis
Chemical analysis of separated, cleaned compounds of diesel
exhaust involves numerous techniques, including thin-layer
chromatography, gas chromatography, gas chromatography with mass
spectrometric detection, and high-performance liquid chromatography
(US National Institute for Occupational Safety and Health, 1991).
Jinno et al. (1986) determined PAHs by supercritical fluid
chromatography with a photodiode array detector.
The detection methods vary widely and include ultra-violet,
visible, and fluorescence spectrophotometry, mass spectrometry, and
more or less specific devices, such as flame ionization, nitrogen
flame ionization, and sulfur-specific and electron-capture detectors
(US National Institute for Occupational Safety and Health, 1991). A
thermal optical method for determining elemental carbon is currently
being used as a surrogate for measurement of worker exposure in almost
all internal investigations at the US National Institute for
Occupational Safety and Health (Niemeier, personal communication,
1994).
Analytical methods are available for determining the organic
constituents of diesel particulate matter, with special emphasis on
the determination of PAHs and their nitrated and oxygenated
derivatives (Levsen, 1988).
B2.3 Conversion factors
Because diesel exhaust emissions are complex mixtures of
different gases and particles, it is impossible to give a conversion
factor for converting parts per million to SI units.
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Anthropogenic sources
Diesel exhaust emissions are exclusively man-made and are
distributed primarily in the atmosphere. The hydrosphere and geosphere
may also be affected owing to dry and wet deposition of the exhaust
constituents; however, determination of the source of deposited
compounds is difficult. No data are available on the individual
components of diesel exhaust and their deposition.
B3.1.1 Diesel exhaust emissions
Diesel fuels are widely used as transport fuels, and exhaust
emissions are released mainly by road traffic. Although many
investigations of these emissions have been reported, the individual
results are frequently not comparable because the experimental
parameters, e.g. driving cycles, are different, or different
dimensions were chosen. For example, the results of tests on emissions
from light-duty vehicles refer mainly to kilometres driven, whereas
those for heavy-duty vehicles are related to the engine power in
kilowatt-hours. Furthermore, the statistical power of studies of
emission factors is often limited by the small number of vehicles or
engines examined. Some investigators change more than one parameter
(e.g. sulfur content or aromaticity of the fuel) during their studies,
so that the specific cause of changes in exhaust composition is
difficult to assess. Adjustment of individual engine and fuel
parameters plays a major role in determining the quality and quantity
of exhaust emissions (see sections B3.1.2.1 and B3.1.2.2).
Further sources of diesel exhaust emissions include off-road and
stationary engines (reciprocating or gas turbine), electricity
generating plants, pipeline pumping, gas compression, commercial and
industrial furnace installations, and space and water heaters in
plants and factories (see section A3.2.1.2). Diesel exhaust is also
emitted from marine and inland ships' engines; residual fuels and
other heavy grades (for example Bunker C) are used in ocean shipping
(von Meyerinck, personal communication, 1994).
B3.1.1.1 Emission of chemical constituents with the gaseous portion
of diesel exhaust
The exhaust emissions from diesel-fuelled vehicles are generally
given as milligrams of component emitted per kilometre of driving
distance (Egebäck & Bertilsson, 1983; Westerholm et al., 1986;
Westerholm, 1987; Volkswagen AG, 1989; CONCAWE, 1990b; Westerholm &
Egebäck, 1991; Prakash et al., 1992; Scheepers & Bos, 1992a). Data for
verifying tests are issued by national regulatory bodies, e.g. the
German Federal Office for Motor Vehicles (1993, 1994). Depending on
the testing cycle, diesel engine, and fuel (see sections B3.1.1.1 and
B3.1.1.2), the data show considerable differences even though the
values are of the same order of magnitude. Emission factors used in
different regions to estimate the effects of regulated chemical
constituents (carbon monoxide, nitrogen oxides, and hydrocarbons) in
air are shown in Table 24.
Like all hydrocarbon fuel-burning engines, diesel engines release
significant amounts of carbon dioxide. Westerholm & Egebäck (1991)
measured emission factors of about 1 kg/km from a bus and a truck in
the US transient cycle and the bus cycle. In measurements of emissions
from six buses in four driving cycles (US transient cycle and three
bus cycles), Prakash et al. (1992) found emission factors up to about
4 kg/km, especially in the bus cycles. For five European diesel
passenger cars and one light van, Hammerle et al. (1994a) determined
emission factors of 162-252 g/km carbon dioxide in the European Motor
Vehicle Emissions cycle and the US FTP-75 test. Diesel engines are the
most efficient of all common types of combustion engines, with peak
thermal efficiencies typically > 40%, owing to the relatively large
air:fuel ratio during combustion (see section B3.1.2.1) (Organisation
for Economic Co-operation and Development, 1993).
Table 24. Emission factors for diesel vehicles in 1985 used as standards for estimating total impact
Region Passenger cars Commercial vehicles
g/km g/kg Average < 5 t > 5 t
g/km g/kg g/km g/kg g/km g/kg
Carbon monoxide
Denmark 1.0 2.0
Germanya 1.5 20 16
Netherlands 2.7 35 3.9 14 2.0 20 4.6 14
European Union 19 19 16
Austria 22.8 22.8 20
Sweden 10.5
Switzerland 4.0
United Statesb 3.1-6.2
Nitrogen oxides
Germanya 1.0 14 58
Netherlands 0.8 10 18.3 66 0.6 6 22.8 69
European Union 14 18 62
Sweden 39
Switzerland 14.0
United Statesb 3.1
Hydrocarbons
Germanya 0.5 7 12
Netherlands 0.6 10 4.6 17 0.9 9 6.3 19
Table 24 (contd)
Region Passenger cars Commercial vehicles
g/km g/kg Average < 5 t > 5 t
g/km g/kg g/km g/kg g/km g/kg
Hydrocarbons (contd)
European Union 10 13 9
Sweden 3.9
Switzerland 3.0
United Statesb 1.2
Adapted from Organisation for Economic Co-operation and Development (1993)
a Excluding former German Democratic Republic
b 1975-83, trucks tested in the US transient cycle
Cyanides, ammonia, and sulfur dioxide are detectable in the
gaseous portion of diesel exhaust. The average emission factors given
by Volkswagen AG (1989) for seven passenger diesel cars with four- and
five-cylinder engines in three driving cycles (US 75 test with cold
start, US sulfate emission test, US highway driving test) were
0.6 mg/km cyanides, 1.1 mg/km ammonia, and 233.0 mg/km sulfur dioxide.
The emission of individual organic components and classes of
compounds, summarized as total hydrocarbons, are shown in Table 25,
which provides an overview of the quantities of individual substances
emitted in diesel exhausts. Heavy-duty vehicles release more
low-boiling hydrocarbons than passenger cars; however, the difference
could be due to the driving cycle used, which involves frequent
changes of speed and load (see section B2.2.1). The emissions of
adsorbed organic components, such as PAHs (see section B3.1.1.2), are
correlated with the release of particulate matter.
B3.1.1.2 Emission of particulate matter and adsorbed components in
diesel exhaust gases
The emission of total particulate matter from diesel exhaust is
shown in Table 26. Analysis of the particulate matter released from
heavy-duty vehicles gave the following emission factors (Westerholm &
Egebäck, 1991): 170-370 mg/km carbon, 8-71 mg/km soluble organic
compounds derived from fuel, 56-167 mg/km soluble organic compounds
derived from lubricating oil, 1.4-37 mg/km soluble sulfates, and
0.6-3.7 mg/km nitrate. Comparable values were not available for
light-duty vehicles.
As PAHs and oxygenated PAHs from diesel and spark-ignition
engines are qualitatively similar, their differentiation in the
environment is difficult (Behymer & Hites, 1984); however,
benzo[ b]naphtho-[2,1-d]thiophene is specific for diesel exhaust and
can therefore be used as a marker (Grimmer et al., 1979). Emissions of
PAH components are shown in Table 27.
In experiments with one bus and one truck, Westerholm & Egebäck
(1991) detected 30-220 µg/km of particulate-associated PAHs. The
compounds measured included dibenzothiophene and its 4- and 3-methyl
derivatives, 2-methylfluorene, phenanthrene and its methylated
derivatives, anthracene and its 2-methyl derivative, fluoranthene,
pyrene and its 1- and 2-methyl derivatives, retene, benzo[ a]fluorene,
benzo[ ghi]fluoranthene, cyclopenta[ cd]pyrene, benzo[ a]anthracene,
chrysene or triphenylene, benzo[ b,k]fluoranthene, benzo[ e]pyrene,
benzo[ a]pyrene, perylene, indeno[1,2,3-cd]fluoranthene,
indeno[1,2,3-cd]pyrene, picene, benzo[ ghi]perylene, and coronene.
The emission factors for 1-nitropyrene were 0.13-7.3 µg/km.
Table 25. Emission factors for individual organic constituents and
classes of compounds in the gaseous portion of diesel
exhaust
Class of compound Emission factor (mg/km)
or constituent
Light-duty vehiclesa Heavy-duty vehiclesa
Straight-chain aliphatic hydrocarbons
Sum of C1-C4 5.3-6.6 (C1 + C2)b
< 1 - < 143c
Sum of > C4 < 1-5c
Olefins
Ethylene 25.6-90.7d 32-135e
13.5-16.9b 19.0-82.9f
< 1-31c
Propylene 7.3-22e
8.1-44.0f
1,3-Butadiene 0.2g
Acetylene 5.7-7.4b 27.9-106.9 (+ ethane)f
Alcohols Methanol 1.6d
Ethanol < 1.2-22.2d
Aldehydes
Formaldehyde 8.1b 60-255e
7.9-20.7d 140-150h
4.2-9.1g 19.0-207.4 f*
Acetaldehyde 3.1b 18-137e
< 22-36d 40-50h
2.0-4.4g
Crotonaldehyde 1.2b
Acrolein < 1-1.8d 8.5-36e
4h
Benzaldehyde 0.6b 10-14h
Light aromatic compounds
Benzene 2.6-4.3b 4.5-13.1e
ND-6c 6.9-30.5f
9.5-18.1d
0.15-3.47g
Table 25. (cont'd)
Class of compound Emission factor (mg/km)
or constituent
Light-duty vehiclesa Heavy-duty vehiclesa
Toluene 1.1-1.9b 2-13.7e
ND-2c 7.4-32.5f
< 1.0d
Ethylbenzene NDc
Xylenes NDc 7.8-41.7f
Phenols 0.7-1.5b
ND, not determined
a According to the Organisation for Economic Co-operation and
Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty
vehicles are those weighing > 5 t.
b From Volkswagen AG (1989): various numbers of diesel passenger cars
in three driving cycles (US-75 test with cold start, US sulfate
emission test, US highway driving test); determination of aldehydes
only in the US-75 test
c From Scheepers & Bos (1992a): two diesel passenger cars (< 50 000 and
> 100 000 km on odometer) in ECE test, 15 min at 90 km/h and 15 min
at 120 km/h
d From Egebäck & Bertilsson (1983): two passenger cars in US-78 test
e From Westerholm & Egebäck (1991): a bus and a truck in bus cycle with
different diesel fuels
f From Prakash et al. (1992): one bus in four driving cycles (US
transient cycle, three bus cycles) with the exception (*) of eight
buses in the same driving cycles
g From Hammerle et al. (1994a): five passenger cars and one light van
in European Motor Vehicle Emissions Group cycle
h From Westerholm et al. (1986): one heavy-duty vehicle on a chassis
dynamometer with two diesel fuels
i Mainly phenols, cresols and xylenols
Table 26. Emission factors for total particulate matter from diesel exhaust
Measurement conditions Emission factor (mg/km) Reference
Light-duty vehiclesa
Seven passenger cars in three driving cycles 166.4-238.7 Volkswagen AG (1989)
(US-FTP-75 test with cold start; US sulfate emission
test; US highway driving test)
Four passenger cars, 15 light vans in three 180-250 (passenger cars) Williams et al. (1989)
Australian driving cycles (corresponding roughly to 360-440 (light vans)
US-72 and US-75 tests)
Seven passenger cars, two light vans in combined 70-365 (passenger cars) CONCAWE (1990b)
ECE 15/EUDC test with four diesel fuels 233-609 (light vans)
Various Germanyb, 300 Organisation for Economic
Netherlands, 600 Co-operation and Development
Sweden, 400 (1993)
61 passenger cars and 30 light vans without catalyst 39-188 (mean, 104) Federal Office for Motor Vehicles
(optimally tuned) incombined ECE 15/EUDC test (1993)
73 passenger cars and 45 light vans with catalyst 48-127 (mean, 90) Federal Office for Motor Vehicles
(optimally tuned) in combined ECE 15/EUDC test (1993)
Table 26 (contd)
Measurement conditions Emission factor (mg/km) Reference
44 passenger cars and 8 light vans without catalyst 52-117 (mean, 95) Federal Office for Motor Vehicles
(optimally tuned) inUS FTP-75 test (1993)
118 passenger cars and 34 light vans with catalyst 50-118 (mean, 71) Federal Office for Motor Vehicles
(optimally tuned) inUS FTP-75 test (1993)
Five passenger cars and 1 light van in European Motor 52-117 Hammerle et al. (1994a)
Vehicle Emissions Group cycle and US FTP-75 test
Heavy-duty vehiclesa
One vehicle on chassis dynamometer (bus cycle); 680-1000 Westerholm et al. (1986)
two diesel fuels with different contents of sulfur and
aromatic compounds
10 trucks, 2 buses in one Australian driving cycle 800-7150 Williams et al. (1989)
simulating urban traffic
One bus, one truck in two driving cycles (bus cycle; 230-600 Westerholm & Egebäck (1991)
US transient cycle) with two diesel fuels
106 trucks and buses in ECE-R49 driving cycle 0.056-0.606 g/kWh Federal Office for Motor Vehicles
(1994)
Table 26 (contd)
Measurement conditions Emission factor (mg/km) Reference
Various Netherlands, 800 (< 5 t) Organisation for Economic
3300 (> 5 t) Co-operation and Development
Sweden, 400 (< 5 t) (1993)
1700 (> 5 t)
13 trucks and buses (three with particulate trap, 584-804 (without trap) Lowenthal et al. (1994)
10 without) in bus cycle with two diesel fuels 67-206 (with trap)
a According to the Organisation for Economic Co-operation and Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty vehicles are those weighing > 5 t.
b Without the former German Democratic Republic
Table 27. Emission factors for polycyclic aromatic hydrocarbons in
diesel exhaust
Constituent Emission factor (µg/km)
Light-duty vehiclesa Heavy-duty vehiclesa
Anthracene 17-63b 0.9-3.2c; 9-26d;
3-14d+; 1.6e
Phenanthrene 140f 4.2-25c
295-524b 163-308d; 79-186d+;
12.2e
Fluoranthene 172f 10-39c
58-200g 27-34d; 14-17d+
60.4-79.7g 13.0e
Pyrene 186 15-102c
< 0.9-22 28-31d; 9-21d+
66.6-87.1 22.6e
Chrysene 40 9.5-26c
14-67 8-32d; 4-12d+
10.7-22.3 Not measurede
Benzo[b,k]fluoranthene 40 1.6-8.4c
2.6-47 9-12d,h; 2-8d,h+
10.3-15.2 5.6e
Benzo[ghi]fluoranthene 40f 1.4-13c; 6.9e
Cyclopenta[cd]pyrene 2f 2.5-11c
0.81ag* 1.4e
Benzo[e]pyrene 3-38b 1.6-23c
13.8-16.9 5-9d; 1-7d+; 2.6e
Benzo[a]pyrene 10 0.8-12c
< 1-19 2-3d; 0.4-2d+
4.0-5.3 1.3e
Benzo[a]anthracene 25 3.0-12c
8-43 5-12d; 1-4d+
2.7-3.9 3.6e
Indeno[123-cd]pyrene 15f 0.7-15c
3.5-4.4g
Benzo[ghi]perylene 20 0.6-30c
< 1-18 2-4d; 0.5-1d+
8.7-11.7 Not detected
(detection limit
not given)e
Table 27 (contd)
Constituent Emission factor (µg/km)
Light-duty vehiclesa Heavy-duty vehiclesa
Perylene < 1-2b Not measuredc
0.5-0.6g 1.0e
Coronene 10f < 0.1-12c
5.43g* Not detected
(detection limit
not given)e
a According to the Organisation for Economic Co-operation and
Development (1993), light-duty vehicles are generally classified as
vehicles with a weight < 3.5 t, occasionally < 5 t; heavy-duty
vehicles are those weighing > 5 t.
b From Scheepers & Bos (1992a): two diesel passenger cars (< 50 000
and > 100 000 km on odometer) in ECE test
c From Westerholm et al. (1986): one heavy-duty vehicle on a chassis
dynamometer (bus cycle)
d From Lowenthal et al. (1994): 13 trucks and buses (three with
particulate trap [d+], 10 without) in bus cycle
e From Rogge et al. (1993): two heavy-duty vehicles in special
driving cycle
f From Egebäck & Bertilsson (1983): average of two passenger cars in
US-78 test
g From Volkswagen AG (1989): seven passenger cars in three driving
cycles (US-75 test with cold start, US sulfate emission test, US
highway driving test) with the exception (*) of one four-cylinder
engine, US-72 test with hot start
h Isomers not specified
The emission of oxygenated PAHs by two diesel passenger cars (one
four-cylinder and one five-cylinder engine, US 72 test with hot start)
was 4-40 µg/km. In a comparable test (one four-cylinder engine, same
driving cycle), the emission of nitro-PAHs was 0.1-3.5 µg/km. As a
result of possible artefact formation during sampling (see section
B2.2.1), these measurements are uncertain (Volkswagen AG, 1989). It is
suspected that nitrate-based cetane improvers (see section A2.1.2)
could increase nitro-PAH emissions in exhaust (Organisation for
Economic Co-operation and Development, 1993).
There are no significant differences in the emissions of adsorbed
single substances between light- and heavy-duty vehicles, although
heavy-duty vehicles release larger relative amounts of particulate
matter (Salmeen et al., 1985).
The chlorine content of diesel fuel is typically < 1 ppm
(Oehme et al., 1991). Thus, no dioxins were found in the exhaust of
heavy vehicles, at a detection limit of 100 pg/litre of fuel (Marklund
et al., 1990). The dioxin emissions from one light-duty (indirect
injection) and one heavy-duty (direct injection) diesel engine under
different load conditions on a chassis dynamometer were 0.009-0.141 ng
of toxicity equivalents per litre of fuel consumed. The highest value
was reported in emissions from the light-duty engine at full load.
Overall, the emissions were of the same order of magnitude as the
dioxin emissions from a petrol-fuelled engine with a catalytic
converter (Schwind et al., 1991).
B3.1.2 Parameters that influence diesel exhaust emissions
B3.1.2.1 Engine conditions
In general, diesel engines are operated with excess air; the
air:fuel ratio is about 1.5, and at lower air:fuel ratios, much more
smoke is emitted (Organisation for Economic Co-operation and
Development, 1993). Wide local variations in the equivalence ratio and
the temperature of the mixture are caused by the heterogeneous nature
of the combustion process. To avoid over-rich mixtures and to improve
the air:fuel mixing processes, wall wetting and delay of fuel
injection are used (CONCAWE, 1986). In naturally aspirated engines,
the amount of air in the cylinder is independent of the power output.
For these engines, the maximal power output is limited by the amount
of fuel that can be injected without smoke formation. In turbocharged
engines, however, increasing the amount of fuel injected increases the
energy in the exhaust gas, and the turbocharger pumps more air into
the combustion chamber. For this reason, the power output of
turbocharged engines is not, in general, limited by smoke. Low
air:fuel ratios can occur only during transient acceleration. Most
turbocharged engines are further equipped with a cooling system
(intercooler) in which compressed air reduces adverse thermal effects.
In both types of engines, fuel is injected either directly into the
combustion chamber or indirectly into a separate pre-chamber where it
is mixed and partly burnt before reaching the main combustion chamber
(Organisation for Economic Co-operation and Development, 1993).
Scheepers & Bos (1992a) reported that most heavy-duty vehicles have
direct injection motors, and indirect injection is used for light-duty
vehicles.
The most important influences of engine conditions on diesel
exhaust emissions are summarized in Table 28. Contrasting results are
sometimes obtained with regard to the effect of engine conditions on
diesel exhaust emissions. Adjustment of the engine plays a major role
(CONCAWE, 1990b), which was slightly less in the US FTP-75 test cycle
than in the European cycle.
Table 28. Influence of engine conditions on diesel exhaust emissions
Condition Change Influence on exhaust emissions Reference
Air:fuel ratio Increase Increased emissions of hydrocarbons and particulate soluble Organisation for Economic
fraction; decreased particulate and nitrogen oxide emissions Co-operation and
from turbocharged and intercooled engines Development (1993)
Increased PAH emissions, decreased particulate emissions from Barbella et al. (1988)
direct injection engines; decreased PAH and particulate emissions
from indirect injection engines
Increased nitrogen oxide emissions, decreased particulate Iida et al. (1986)
emissions from direct injection engines
Injection timing Advance Increased particulate emissions from indirect injection engines Du et al. (1984); Williams et
al. (1989)
Decreased particulate and hydrocarbon emissions; increased Organisation for Economic
nitrogen oxide emissions Co-operation and Development (1993)
No influence on concentrations of PAHs and oxy-PAHs adsorbed Jensen & Hites (1983)
on particulate matter
Delay Decreased nitrogen oxide emissions, increased volumetric fuel Organisation for Economic
consumption Co-operation and Development (1993)
Table 28. Influence of engine conditions on diesel exhaust emissions
Condition Change Influence on exhaust emissions Reference
Injection timing Delay Decreased particulate emissions from indirect injection engines; Williams et al. (1989)
(contd) increased particulate emissions from direct injection engines
Small effect on PAH emissions from indirect injection engines Schuetzle & Frazier (1986)
Small effect on particulate emissions from direct injection engines Campbell et al. (1981a)
No influence on concentrations of PAHs and oxy-PAHs adsorbed Jensen & Hites (1983)
on particulate matter
Load and Increase Increased particulate and PAH emissions at high loads; decreased Pipho et al. (1986)
temperature particulate emissions at very high load from indirect injection
engines
Decreased concentrations of PAHs and oxy-PAHs adsorbed on Jensen & Hites (1983)
particulate matter
Decrease Decreased particulate and nitrogen oxide emissions; extremely Organisation for Economic
cold charge air may increase hydrocarbon emissions Co-operation and
Development (1993)
Lowest PAH emissions at medium load; enrichment of three- Pipho et al. (1986)
and four-ring PAHs during idling (indirect injection engines)
PAH, polycyclic aromatic hydrocarbons
(a) Engine aging
Increased emissions of hydrocarbons, particulates, and PAHs have
been reported from older, more intensively used light- and heavy-duty
vehicle engines in comparison with newer ones (Stenberg et al., 1983;
Braddock & Perry, 1986; Scheepers & Bos, 1992a). Stenberg et al.
(1983) proposed that the combustion of more lubricating oil in older
vehicles contributes to the enhanced emissions. Prakash et al. (1992)
measured the emission characteristics of several buses and reported
that a properly maintained engine would not emit significantly more
hydrocarbons and nitrogen oxides during its lifetime, whereas carbon
monoxide and particulate emissions could increase with age. These
findings are similar to those of Volkswagen AG (1989) on the influence
of distance travelled on exhaust emissions from one diesel passenger
car over a total distance of about 30 000 km in three driving cycles
(US FTP test, US sulfate emission test, US highway driving test). The
average deterioration factors, relative to the emissions at about
2500 km, were carbon monoxide, 0.97; nitrogen oxides, 0.96;
hydrocarbons, 0.91; particulates, 1.07; fluoranthene, 0.96; pyrene,
1.06; chrysene, 0.62; benzo[ b,k]fluoranthene, 1.11; benzo[ e]pyrene,
1.51; benzo[ a]pyrene, 0.68; perylene, 0.96; indeno[1,2,3-cd]pyrene,
2.91; and benzo[ ghi]perylene, 3.43.
(b) Lubricating oil
As a result of emission control techniques such as turbocharging,
intercooling, and injection timing, the relative importance of
emissions caused by lubricating oils has increased (Organisation for
Economic Co-operation and Development, 1993). Lubricating oils have
been hypothesized to be a sink for incomplete combustion products such
as PAHs and their derivatives, which are scrubbed from the exhaust
gas. When this 'enriched' lubricating oil leaks into the combustion
chamber, the constituents may be emitted in the exhaust (Scheepers &
Bos, 1992a; see section B2.1.1). McKee & Plutnick (1989) found,
however, that carcinogenic PAHs accumulate in gasoline- but not in
diesel-fuelled engine oils.
Oil consumption can be reduced by improving engine manufacturing
specifications and engine seals (Organisation for Economic
Co-operation and Development, 1993). The particle-bound hydrocarbons
derived from lubricating oil contribute up to about 50% by weight in
light-duty vehicles and to a maximum of about 26% by weight of the
total emitted particulate matter (see section B2.1.2, Table 22).
Experiments with 13C radiolabelled lubricating oil in three
light-duty vehicles showed that addition of up to 3% lubricating oil
increases the emitted particulate matter by about 50%, and the
particulates consist mainly of the soluble organic fraction adsorbed
onto the particles (Williams et al., 1989).
B3.1.2.2 Fuel specification
Fuel parameters influence the particulate emissions of diesel
engines but seem to be less important than other parameters like
engine adjustment, load, and maintenance (Weidmann et al., 1988).
The actual trend in legislation of the quality of diesel fuel, i.e. to
a lower sulfur and aromatic content, will improve emissions,
especially of particles. In addition, the more stringent emission
standards in many countries will decrease diesel-related emissions.
(a) Sulfur content
There have been numerous investigations on the influence of
sulfur content in diesel fuel on the release of particulate matter
(CONCAWE, 1990b; Van Beckhoven, 1991; Westerholm & Egebäck, 1991;
Scheepers & Bos, 1992a). The sulfur content is presumed to be directly
correlated with the release of particulate matter. About 3-5% of the
fuel sulfur in light-duty vehicles and 2-3% of that in heavy-duty
vehicles reacts with metal sulfates which are adsorbed onto the
particulate matter, increasing its mass and its hygroscopic
properties, as metal sulfates tend to absorb significant amounts of
water from the atmosphere (Organisation for Economic Co-operation and
Development, 1993).
A reduced sulfur content leads to decreased levels of
particulates in the exhaust of light-duty vehicles. For example,
investigations on seven passenger cars and two light vans in a
combined ECE 15/EUDC testing cycle with four diesel fuels of different
sulfur contents (0.055, 0.12, 0.22, and 0.31% by weight) showed a
statistically significant reduction in the emission of particulates up
to a maximum of 7.4%. The investigators noted variations in
particulate levels between the different vehicles, however, that were
about two orders of magnitude higher than could be attributed directly
to the influence of the sulfur content (CONCAWE, 1990b).
Reduction of the emission rates of heavy-duty vehicles by
lowering the fuel sulfur content is still under discussion. In a study
of four heavy-duty engines in the ECE R49-13 mode driving cycle with
the same four fuels as described above, no statistically significant
reduction in emitted particulate matte was seen (CONCAWE, 1990b). In
experiments with one heavy-duty vehicle on a chassis dynamometer bus
cycle with two different fuels, however, a considerable reduction in
the release of particles was found (Westerholm et al., 1986). This
result was confirmed by Westerholm & Egebäck (1991) who investigated
one bus and one truck in three driving cycles (ECE 13 test, bus cycle,
US transient test) with eight diesel fuels of different sulfur and
aromatic contents. Comparable results were found by Gairing et al.
(1994) in tests with one heavy-duty engine (direct injection) in ECE
R49 with 12 different fuels. Adjustment of the engines again appeared
to be a decisive factor.
(b) Aromaticity (density)
Aromaticity can be directly associated with density, as a higher
density is frequently achieved by a larger content of aromatic
compounds. Obviously, increasing the aromaticity leads to increased
emissions of particulate matter, although volumetric fuel consumption
is decreased (CONCAWE, 1986). This finding was confirmed by Hare
(1986) for light- and heavy-duty vehicles on the basis of data from
the automobile industry. The presumed increase in the release of
particulates is 0.5-1.9% for a 1% by volume change in aromatic
content. In a study of four light-duty vehicles in three Australian
driving cycles (resembling roughly the US 72 and 75 tests), Williams
et al. (1989) found reductions in particulate emissions of 22-27% at a
10% difference in fuel aromaticity. Unfortunately, the two fuels also
differed in sulfur content and volatility. Van Beckhoven (1991)
achieved comparable results with passenger cars with indirect
injection but found no significant influence for heavy-duty direct
injection engines. Experiments by Westerholm & Egebäck (1991) on one
bus and one truck showed a reduction in the emission of particulates
when fuel with low levels of sulfur and aromatics was used. The
increased aromatic content is probably also the source of higher
levels of PAHs and nitro-PAHs in the exhaust (Schuetzle & Frazier,
1986; Westerholm, 1987; Westerholm & Egebäck, 1991; Organisation for
Economic Co-operation and Development, 1993).
(c) Volatility
The volatility of diesel fuels is described by the boiling range,
and especially by the 10% boiling-point (low boiling end fraction) and
the 90% boiling-point (high boiling end fraction) of the mixture. The
influence of the boiling range, particularly the 90% boiling-point, on
the release of particulate matter is still under discussion. While
some investigators (Hare, 1986; Williams et al., 1989) assume that
emissions of particulate matter increase with an increased 90%
boiling-point, others have not been able to confirm this effect,
suggesting that other parameters, such as sulfur content and
aromaticity, are responsible for the increase (Westerholm & Egebäck,
1991; Organisation for Economic Co-operation and Development, 1993).
(d) Cetane number
An increase in cetane number lowers the emission of diesel
particulate matter. A slight decrease in cetane number, combined with
a small increase in the density of diesel fuels (see section A2.1.3),
was predicted in 1987 to result in an increase in emissions from 3 to
12% for carbon monoxide, and to maxima of 2% for nitrogen oxides, 7%
for hydrocarbons, and 6% for particles (CONCAWE, 1987).
B3.1.2.3 Malfunction
Malfunctions or defective components of the engine or exhaust gas
system may result in considerably higher emissions than those from a
properly maintained engine. Up to twofold increases in emissions of
carbon monoxide, hydrocarbons, and particulate matter have been
measured even in the absence of a significant effect on power or fuel
consumption (Ullman et al., 1984). Malfunction of the fuel injection
system may contributed markedly to higher emissions (Organisation for
Economic Co-operation and Development, 1993). Volkswagen AG (1989)
tested a diesel passenger car with defective injection nozzles in
three driving cycles (US FTP test, US sulfate emission test, US
highway driving cycle) and found statistically significant increases
in the emissions of carbon monoxide (up to about 25%), hydrocarbons
(up to about 45%), and particulate matter (up to about 15%), whereas
the release of nitrogen oxides was almost unaffected. The amounts of
benzene and PAHs emitted were slightly increased. Comparable results
were obtained by Williams et al. (1989) for the release of particles
by several light-duty vehicles equipped with defective injectors.
Malfunction of US medium- to heavy-duty vehicle diesel engines
increased the amount of nitrogen oxide emitted to about 4%, that of
hydrocarbons to about 75%, and that of particulates to about 140%
(Organisation for Economic Co-operation and Development, 1993).
B3.1.3 Total emissions by diesel engines
Few data are available on the emissions of different constituents
of diesel exhaust into the atmosphere and their contribution to the
total release by traffic and industry. The contribution of heavy-duty
vehicles seems to be much greater than that of passenger cars, despite
lower percent kilometres. For example, in westen Germany, only 8% of
the total annual urban mileage was attributable to heavy-duty vehicles
(Fromme, 1990). In contrast, in 1987, power plants contributed 15.3%
(85 000 t), industry 63.5% (360 000 t), domestic and minor consumers
8.1% (44 000 t), and total traffic 13% (72 000 t) to the particles
released (Metz, 1989). The contribution of diesel particulate matter
to the total particle burden of the atmosphere is highly dependent on
local conditions (Table 29).
Diesel vehicles, particularly in the light-duty category,
represent a smaller proportion of motor traffic in the United States
than in most European countries. Measurements from two heavy-duty
vehicles in 1982 indicated that light- and heavy-duty diesel vehicles
contributed about 6% to the total fine aerosol, organic carbon
emissions in the Los Angeles basin, about 1200 kg/day being emitted by
heavy-duty vehicles and 600-870 kg/day by light-duty vehicles
(Hildemann et al., 1991; Rogge et al., 1993).
B3.1.4 Control of emissions
The automotive industry has made efforts to reduce diesel exhaust
emissions (Obländer, 1991; Fortnagel, 1992; Hammerle et al., 1994b),
and technology is advancing.
B3.1.4.1 Particle traps
Particle traps are placed in the exhaust system to collect
particulate matter and to clean the filter by burning the trapped
particles (trap oxidizers). Most traps are based on cellular ceramic
monolith material or ceramic silica fibre coils. Many regeneration
techniques have been developed for trap oxidizers (Organisation for
Economic Co-operation and Development, 1993), including thermal
regeneration, which is the burning of collected particulate matter,
and catalytic regeneration, in which fuel catalysts, e.g. iron and
copper, containing organic compounds form finely dispersed metal
particles during combustion to reduce the burning temperature of the
diesel particles so that there is more efficient regeneration
(Volkswagen AG, 1989; Hammerle et al., 1994b). Traps remove the
soluble organic fraction adsorbed onto particles and the dry
carbonaceous soot, while catalysts can remove substances only from the
soluble organic fraction. In catalytic regeneration, over 95% of the
metal used as fuel catalyst is retained in the trap (Hammerle et al.,
1994b).
Studies of the emissions of one light-duty diesel passenger car
by Volkswagen AG (1989) showed that the release of particulates was
reduced by a thermal trap by at least 80% during the collection phase
and by about 60% during the regeneration phase; the reduction was 80%
for the whole cycle. Sulfate emissions decreased to about 50%;
however, emission of carbon monoxide increased by about 10%, probably
due to additional burning and/or incomplete oxidation of particulate
matter on the trap. Comparable experiments with a catalytically
regenerated trap, with addition of a manganese compound, also showed a
trap efficiency of about 80% for particulate matter. Hydrocarbon and
carbon monoxide emissions were slightly increased, probably due to
incomplete oxidation on the trap. Sulfate emissions were reduced to
less than half, emissions of PAHs were reduced to about 25%, and those
of phenol and aldehydes to about 25-33%. About 10% of the manganese
catalyst was detected in the exhaust.
Measurements with a heavy-duty vehicle on a chassis dynamometer
to test two particle traps showed decreases of 79 and 86% in the
release of particles, depending on the trap and fuel characteristics.
The emissions of hydrocarbon, carbon monoxide, aldehydes, and PAHs
were slightly reduced (Westerholm et al., 1986).
Table 29. Diesel exhaust emissions and their contribution to total anthropogenic emissions
Region Year Annual emissions (kt/year) Reference
NOx CO HC PM SO2
Light-duty vehicles
Germanya 1986 52 (3.3)b 76 (1.2)b 23 (2.2)b 15 (26.3)b NR Fromme (1990)
1987 NR NR NR 12 (2.2)c NR Metz (1989)
United Kingdom 1991 (0.7)c (0.4)c (1.1)c (2)c,d (0.2)c Quality of Urban Air Review
Group (1993)
Light- and heavy-duty vehicles
Europe NR NR NR NR NR (1.6)c OECD (1993)
United States 1984 NR NR NR NR 620 (3)c OECD (1993)
1986 NR NR NR 54.8 (20)b NR Carey (1987)
(4)c
Germanya 1989 640 240 150 55 NR Federal Environment
Agency(1991)
Heavy-duty vehicles
OECD (Europe) 1980 1548 (13)c NR 393 (4)c NR 361 (2)c OECD (1993)
Germanya 1986 502 (32.2)b 130 (2.1)b 104 (10.0)b 37 (64.7)b 35 (2)c OECD (1993); Fromme (1990)
(17)c (2)c (4)c (7)c
1987 NR NR NR 32.5 (5.9)c NR Metz (1989)
Table 29 (cont'd).
Region Year Annual emissions (kt/year) Reference
NOx CO HC PM SO2
Heavy-duty vehicles (contd)
Germany 1988 614 351 1.31 NR 38 Federal Environment Agency
(1992)
United Kingdom 1991 (19.9)c (1.9)c (5.4)c (14)c,d (1.0)c Quality of Urban Air
Review Group (1993)
Canadae 1985 265 104 37 20 21 Environment Canada
(1990)
NOx, nitrogen oxides; CO, carbon monoxide; HC, hydrocarbons; PM, particulate matter; SO2, sulfur dioxide; NR, not reported; OECD,
Organsiation for Economic Co-operation and Development
a Excluding the former German Democratic Republic
b Percentage of total traffic emissions
c Percentage of total man-made emissions
d PM10
e Sum of light- and heavy-duty trucks
The effect of a ceramic wall-flow monolith particulate trap was
studied on the emissions of a 1979 heavy-duty diesel engine with
direct injection in two steady-state modes. Reductions of 70% (50% at
full load) and 86% (75% at full load) in particle release were found.
There was also a decrease in the soluble organic fraction, whereas the
emitted portion of volatile organic carbons was only slightly reduced.
The trap had almost no effect on the release of nitrogen oxides (Dorie
et al., 1987).
The measurements of Lowenthal et al. (1994) on 13 trucks and
buses (three with particulate traps, 10 without) in a bus cycle showed
a similar reduction in the release of particles (Table 26). There was
a significant decrease in the emission of particle-bound PAHs.
B3.1.4.2 Catalytic converters
Diesel catalytic converters reduce gaseous and particle-bound
hydrocarbon constituents of the exhaust, e.g. aldehydes, PAHs, nitro-
PAHs, and carbon monoxide. Regeneration systems are not needed, as
particulate matter is not collected by common flow-through catalytic
converters. The efficiency in controlling particulates is, however,
much lower than that of traps. Disadvantages of catalytic converters
are further conversion of nitrogen monoxide to the more toxic nitrogen
dioxide and of sulfur dioxide to particulate sulfates (Organisation
for Economic Co-operation and Development, 1993). Catalysts that
selectively oxidize organic compounds and oxidize almost no sulfur
dioxide are currently in use. Removal of sulfur from diesel fuels
would allow the use of more active catalysts; use of catalysts in
diesel vehicles run on sulfur-containing fuel is limited because the
strong adsorbing tendency of sulfur partly inactivates the catalyst
(Hammerle et al., 1994b; see section A2.1.1).
B3.2 Regulatory approaches
The levels of carbon monoxide, nitrogen oxides, total
hydrocarbons, and particulates emitted by diesel engines are regulated
by law in a number of countries (Table 30). Most other industrialized
countries have adapted United States or European regulations (for
details, see CONCAWE, 1990a and Mercedes-Benz AG, 1994a).
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few data are available on the fate of diesel exhaust in the
atmosphere, which is the first environmental compartment affected. In
general, the effects on the overall environment of diesel fuel
combustion are typical of those of fossil fuel burning. The
contribution of diesel fuel emissions is proportional to diesel fuel
consumption both locally and globally.
Table 30. Limit values for components of diesel exhaust
Region Carbon Nitrogen Hydrocarbons Particulates Comments
monoxide oxides
Light-duty vehicles (g/km)
Austria 2.1 0.62 0.25 0.124 < 3.5 t; since 1991; from 1995,
adoption of European Union
standards planned
Canada 2.1 0.62 0.25 0.12 Since 1987
European Union 2.72 0.97 (with 0.14 Since 1992
hydrocarbons)
1.0 0.7 0.08 From 1996
Finland Since 1993
Japan 2.1 0.7 0.62 None Since 1986
2.1 0.5 0.4 0.2 Since 1994
Sweden, Norway 2.1 0.62 (city) 0.25 0.124 < 3.5 t; from motor year 1992
0.76 (highway)
Switzerland 2.1 0.62 (city) 0.25 0.124 < 3.5 t; since 1988; from 1995,
0.76 (highway) adoption of European Union
standards planned
USA (California) 2.1-5.2 0.2-0.6 0.2-0.3 (except 0.05 (up to Depending on mileage
methane) 31 000 km)
US Environmental 2.1-2.6 0.6-0.8 0.2 0.05-0.12 Depending on mileage
Protection Agency
Table 30 (contd)
Region Carbon Nitrogen Hydrocarbons Particulates Comments
monoxide oxides
Heavy-duty vehicles (g/kWh)
Austria 4.9 9.0 1.23 0.4
Canada 15.5 5.0 1.3 0.25 g/bhp-h
15.5 5.0 1.3 0.1 g/bhp-h; from 1995-97
European Union 4.5 8.0 1.1 0.36 Since 1992
4.0 7.0 1.1 0.15 From 1995-96
Japan 7.4 5.0 2.9 0.7 Indirect injection engines
7.4 6.0 2.9 0.7 Direct injection engines
Sweden 4.9 9.0 1.23 0.4
USA 15.5 5.0 1.3 0.07 g/bhp-h; bus
15.5 4.0 1.3 0.1 g/bhp-h; truck
15.5 5.0 1.3 0.05 g/bhp-h; bus; from 1998
15.5 4.0 1.3 0.1 g/bhp-h; truck; from 1998
Adapted from Mercedes-Benz AG (1994b); reference year, 1994, unless otherwise stated; bhp-h, brake horsepower-hour
Thus, the major components of diesel exhaust contribute to acid
deposition (nitrogen and sulfur oxides), tropospheric ozone formation
(carbon monoxide, nitrogen oxides, and hydrocarbons), and global
warming (carbon dioxide and particulates; carbon monoxide, nitrogen
oxides and hydrocarbons by ozone formation).
The environmental fate of released particulate matter and of
organic compounds, particularly the PAHs, is important environmentally.
The mechanisms of the distribution and transformation of individual
hydrocarbons in diesel exhaust have been well described (IPCS, 1986,
1993).
B4.1 Transport and distribution between media
The major fraction (50-80%) of the particulate emissions of
diesel engines is in the submicron size, ranging from 0.02 to 0.5 µm
(see section B2.1.2). Once particles have been emitted, their
mechanical transport in the atmosphere is like that of gas molecules
(nonreactive). Together with carbon particles from other combustion
processes, they may be transported over long distances and even
penetrate the stratosphere (Muhlbaier Dasch & Cadle, 1989).
Only one study was available on the agglomeration behaviour of
the particles in the atmosphere. Albrechcinski et al. (1985) measured
the aging behaviour of particulate matter from a 1978 diesel passenger
car in a 600-m3 model chamber with a nominal exhaust gas dilution of
150:1 and found peak mean aerosol diameters, based on volume size
distributions, of 0.1-0.18 µm for 'fresh' aerosol. The concentrations
of total particulate decreased during aging of the aerosol due to
coagulation, fall-out, and perhaps losses to the walls of the chamber.
The average decrease was 20% after 8 h and 60% after 24 h. Excess
nitrogen dioxide seemed to inhibit particle growth. Particles with an
average diameter of 1-10 µm constituted approximately 10% of the fresh
aerosol, but this percentage decreased during aging as a result of
apparently rapid fall-out of larger particles. It is not clear whether
these results are also representative of the particle emission pattern
of modern diesel passenger cars.
Model experiments of the gas-phase chemistry of nitrogen oxides,
total hydrocarbons, and sulfur dioxide from diesel exhaust were
performed by varying parameters such as irradiation, nitrogen dioxide
concentration, and ozone concentration (Albrechcinski et al., 1985).
Irradiation alone had only a minimal effect, but excess nitrogen
dioxide led to higher rates of decay of nitrogen monoxide in the dark
than under irradiation, probably because of photodissociation of
nitrogen dioxide to nitrogen monoxide. As nitrogen monoxide is rapidly
converted to nitrogen dioxide in the presence of excess ozone, the
nitrogen oxide concentration decreased as the ozone concentration
reached a maximum, presumably partly because of the formation of
nitric acid which was deposited onto the chamber walls. Since reactive
organic compounds were not added, the authors considered that the
experiments were not representative of urban atmospheres.
The hydrosphere and geosphere may be affected indirectly by
diesel exhaust emissions after dry or wet deposition of particulate
matter or individual constituents (see section B4.2). In experiments
with dysprosium-traced diesel fuel, Horvath et al. (1988) (see section
B5.1) found 0.012-0.07 g diesel particulate matter per gram of road
dust.
B4.2 Transformation and removal
Atmospheric removal of airborne carbon particles consists mainly
of dry deposition and scavenging by precipitation (wet deposition).
The rate of wet removal is directly correlated to the ratio of organic
to elemental carbon and is low for small ratios (Muhlbaier Dasch &
Cadle, 1989). As the overall removal rate of diesel particulates is
estimated to be low, the atmospheric life-time is several days
(Jaenicke, 1986).
The elemental carbon of diesel particulates may act as a catalyst
in the formation of sulfuric acid by oxidation of sulfur dioxide. The
oxidation reaction is assumed to occur on the surface of solid soot
particles or in liquid drops containing soot particles (Novakov, 1984;
Mészáros & Mészáros, 1989). Furthermore, diesel particles themselves
contain sulfate, adsorbed in the form of metal sulfates, at a
concentration of about 1-12% by weight, depending on the sulfur
content of the fuel (see section B2.1.2), which may be washed off if
the particles come into contact with precipitation. The organic
compounds adsorbed on the emitted particulate matter may undergo a
number of physical and chemical reactions (volatilization of
low-relative-molecular-mass compounds, formation of secondary
aerosols, oxidation, nitration) with other atmospheric compounds or
during exposure to sunlight (Pitts et al., 1985). The estimated
half-lives for unbound PAHs in the atmosphere range from one day
(naphthalene) to one week (e.g. pyrene, chrysene, benzofluoranthenes,
benzo[a]pyrene) (Mackay et al., 1992; see Table 11); however, PAHs
adsorbed onto particles may be more stable to nitrogen dioxide and
ozone than airborne PAHs (Gibson et al., 1981).
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Because diesel exhaust is a complex mixture of a great variety of
compounds (see also section B2.1), general 'environmental levels'
cannot be described. The individual constituents of diesel exhaust
should be detectable in all compartments of the environment. Since
diesel exhaust is only one of numerous other sources, the levels of
individual compounds cannot be correlated directly to diesel exhaust.
Particulate matter appears to be the most relevant fraction of
diesel exhaust for the general population and workers exposed to
diesel exhaust emissions from a toxicological point of view. The
concentration of particles is about 10- to 20-fold higher in diesel
exhaust than in the exhaust from gasoline-fuelled vehicles, and the
particle diameters are in the respirable range.
B5.1 Exposure of the general population
The general population is exposed to high levels of diesel
exhaust in certain microenvironments, such as busy streets and parking
areas. Exposure to the main gaseous components of diesel exhaust --
carbon dioxide, carbon monoxide, nitrogen oxides, and total
hydrocarbons -- can be estimated from the emission factors given in
section B3, the kilometrage of diesel vehicles in relation to total
traffic, and atmospheric dispersion models (Cuddihy et al., 1984;
Volkswagen AG, 1989). In view of the fact that measured emission
factors vary widely (see section B3.1.1) and are mainly dependent on
adjustment of individual engines, the validity of these estimations is
limited.
On a global basis, there are wide variations in the contribution
of diesel emissions to total airborne particulate matter, which
depends on the following factors: percentage of diesel vehicles in the
total volume of traffic; type, age, and maintenance of individual
engines (see section B3.1.2.1); fuel quality (e.g. sulfur content and
aromaticity; see section B3.1.2.2); and emission control techniques.
Particle concentrations in environmental air samples are usually given
as total particle concentrations, with no differentiation by source.
The contribution of diesel particulate matter is then estimated on the
basis of the percentage of diesel vehicle emissions in the total
emissions from traffic or total man-made emissions. Emissions from
running stationary diesel engines have up to now not been taken into
account. Data on emissions from light- and heavy-duty vehicles in some
industrialized countries are shown in Table 30 (section B3.1.3). The
proportion of the concentrations to which people are exposed that is
attributable to diesel vehicles varies widely, depending on traffic
flow and composition in the vicinity and the magnitude of background
pollution from other sources.
In a study in the United Kingdom (Waller et al., 1965),
measurements were made at a sampling site at the centre of a busy
street canyon, where traffic flows were typically 1200 gasoline and
600 diesel vehicles per hour, in order to assess the maximal
concentrations of traffic pollutants in urban areas. The contributions
of airborne particulate matter, expressed as seasonal means for
daytime working hours, were 200-421 µg/m3, after allowance for the
urban background measured at nearby control sites. The additional
particulate matter was attributed primarily to the diesel vehicles,
while the corresponding contribution to carbon monoxide, averaging
15 ppm (17 mg/m3), was associated mainly with the gasoline vehicles.
In France, Joumard & Perrin (1988) measured atmospheric
particulate matter (diameter, 0.01-25 µm) in a 30-40-m wide street
open to pedestrians, with two central lanes reserved for use by bus
services involving weekday traffic of more than 1000 vehicles per day
in each direction. The measurement point was in the immediate vicinity
of two bus stops, 3 m from the edge of the pavement and at a height of
2.5 m. The particle concentrations, determined by measuring ß
radiation, were 34 µg/m3 during the period 23.00-05.00 h and
67 µg/m3 during 07.00-19.30 h.
Airborne particle concentrations were calculated for an
eight-lane American freeway and for four- and six-lane European
autoroutes. The one-dimensional Gaussian model used neglects chemical
reactions during transport and possible deposition on the ground and
assumes turbulent mixing in the air. The calculated maximal
concentrations were 7-13 µg/m3 close to the road, and these
decreased with distance from the measuring point. About 100 m away,
the estimated particle concentration at a height of 1.5 m was three to
four times lower than that next to the motorway (Volkswagen AG, 1989).
Particle emissions, especially from diesel vehicles, were
estimated roughly for various locations by the lead surrogate method,
in which historical ambient lead concentrations in urban areas are
used as indices of mobile source pollutant levels. Particle emission
factors for light- and heavy-duty vehicles and the distribution
characteristics of lead and diesel particulate are also taken into
consideration (Rykowski & Brochu, 1986). The results are shown in
Table 31.
Concentrations of particles from diesel engines in the
environment were determined in Vienna, Austria, at several measuring
points during a four-week period. As there was only one distributor of
diesel fuel for the whole network of service stations in Vienna, the
rare earth element dysprosium was added as an indicator to the fuel at
this central point, which was distributed to diesel vehicles at
service stations and then emitted with the exhaust. Dust samples
collected from the air were subjected to neutron activation analysis
and the level of dysprosium was determined. The proportion of
dysprosium in diesel soot was calculated on the basis of emission
factors for diesel engines in the literature and from laboratory
experiments, assuming that the marker is totally transferred from fuel
to exhaust during combustion. At seven of the 11 measuring points for
different traffic densities, the concentrations were almost linearly
correlated to traffic density. Ambient particulate levels increased by
5.5 µg/m3 per 500 diesel vehicles per hour, from 10.3 in a residential
area to 23.6 µg/m3 near an urban freeway; the mean value was
14.4 µg/m3. The diesel particulate concentration in the centre of
Vienna was calculated to be 11 µg/m3 by correlating the measured
values and traffic density. At four measuring stations, significant
deviations from linearity were found which were due to local
conditions. The concentration of the marker was below the detection
limit before the testing period and one year later (Horvath et al.,
1988). A deficiency of the study is that the dysprosium concentrations
of diesel fuel before the addition were not taken into account, so
that the emission factors used may not reflect the true situation
(Holländer, personal communication, 1994). Nevertheless, the measured
ambient particle concentrations fit well with estimated data for
diesel exhaust emissions by other investigators.
Table 31. Estimated particle concentrations emitted from diesel
engine exhaust
Location and year Concentration
(µg/m3)
United Statesa, cities with > 1 million 1.3-3
residents (1984)
United Statesa, cities with 100 000-250 000 residents 0.7-1.7
Naples, Italy, urban area (1982) 7.2-35.1
Birmingham, United Kingdom, urban area (1982) 3.2-20.3
Birmingham, United Kingdom, university area (1982) 0.8-5.5
Stockholm, Sweden, inner city (1982) 2.5-16.4
From Rykowski & Brochu (1986)
a According to Cuddihy et al. (1984), the proportion of
diesel-fuelled light-duty vehicles in the United States was
about 2%.
In a programme to measure airborne diesel soot levels in
Düsseldorf, Germany, the total carbon content of fine dust (particle
diameter, < 7µm) at a busy crossing was 8-42 µm3 as a daily
average, 18 µg/m3 as an annual average, 21 µg/m3 on workdays, and
14 µg/m3 on weekends. The carbon content after solvent extraction
(elemental carbon) was 6-32 µg/m3 as a daily average, 16 µg/m3 on
workdays, and 10 µg/m3 on weekends. In a residential area in
Duisburg, Germany, the concentration of elemental carbon was 3 µg/m3.
The method of determination was as follows: a defined volume of air
was sucked through a fibreglass filter, and the respirable particle
fraction was separated. This fine dust was further separated into an
incinerable portion (according to German standard method TRGS 900),
total carbon, and the carbon content after liquid extraction
(elemental carbon). Total and elemental carbon were determined by
coulometry. By correlation with diurnal changes in carbon and nitrogen
monoxide concentrations, the main particle source was shown to be
automobile traffic at the sampling location. Correlation of a traffic
census with working day and weekend particle levels showed that the
airborne particulate matter was due primarily to diesel vehicles
(Elbers & Muratyan (1991). Further measurements at the same place in
1991 and 1992 confirmed the results (Elbers & Richter, 1994).
Validation of the analytical method gave detection limits of 0.39 for
elemental carbon (relative standard deviation, 14% or 4.4 µg/m3) and
0.65 µg/m3 for total carbon (relative standard deviation, 13% or
7.8 µg/m3).
The weekly average ambient concentrations of diesel particulate
in Germany were estimated to be 5-10 µg/m3 in urban areas, 15-25 µg/m3
in urban areas close to busy streets, and < 1.5 µg/m3 in rural
areas; however, the method used for the estimations was not reported
(State Committee for Immission Protection, 1992).
Estimates of the US Environmental Protection Agency for 1986 gave
annual mean ambient concentrations of diesel particle in the United
States of 2.6 µg/m3 in urban areas and 2.4 µg/m3 in rural areas. A
prediction for 1995 gave values of 1.5 µg/m3 for urban and 1.2 µg/m3
for rural areas (Mauderly, 1992). Higher concentrations were estimated
by the Motor Vehicles Manufacturing Association (Sienicki & Mago,
1992), which predicted a mean value of 2.7 µg/m3 for the city of Los
Angeles in 1995. Lower levels were estimated in a study by the US
Environmental Protection Agency (1993) in which national average
emission factors for diesel vehicles for 1988 were multiplied by urban
and rural mileage. For 1990 and 1995, the annual exposure levels were
estimated to be 2.0 and 1.2 µg/m3 in urban air and 1.1 and 0.6 µg/m3
in rural air, respectively. These estimates were confirmed by ambient
monitoring. Using data from the early 1980s, when the proportion of
diesel vehicles, the number of kilometres travelled, and emission
standards differed from present characteristics, McClellan (1986)
derived ambient diesel particulate concentrations of 10-30 µg/m3 on
American urban freeways and in urban street canyons.
B5.2 Occupational exposure
The levels of particulate matter to which workers are exposed
when their predominant exposure to exhaust is that from diesel engines
are described in this section. It is difficult to determine the
contribution of diesel exhaust to respirable particles, as they
include not only those from diesel engines but also others generated
in the worker's environment, such as sand, dirt, coal dust, and
cigarette smoke particles. Thus, the levels of total and respirable
particulates reported in jobs with potential exposure to diesel
exhaust may be significant overestimates of the actual exposure. Only
two occupational groups have been studied in this regard: railroad
workers (Woskie et al., 1988a,b) and mechanics and drivers in the
trucking industry (Zaebst et al., 1991). The specific exposures of
these groups to diesel particles range from 0.026 to 0.192 mg/m3. In
the railroad workers, cigarette smoke was found to account for a
substantial proportion of total respirable particulate matter, and the
geometric mean exposure to respirable particulates of workers not
exposed to diesel exhaust was reduced by over 80% when the
contribution of cigarette particulate was considered (Woskie et al.,
1988a).
The principal marker chosen to measure exposure of workers in the
trucking industry (including road drivers, local drivers, stevedores,
and mechanics) to diesel exhaust was elemental carbon, which is
derived from the core of the diesel exhaust particle and represents
about 20% of respirable particulate; cigarette smoke does not
contribute appreciable amounts of elemental carbon. Mechanics and
stevedores had the highest exposure to diesel exhaust, and truck
drivers had lower levels (Zaebst et al., 1991). The degree to which a
marker of diesel exhaust exposure actually represents true exposure
depends on the nature of all sources of respirable particles in the
work-place. Occupational exposure to particulates is summarized in
Table 32.
Table 32. Reported occupational exposure to particulates among
workers exposed to diesel exhaust
Occupational group Particles (mg/m3)
Total Respirable Diesel
Railroad workers 0.01-1.99 0.06-0.41 0.051-0.192a
Mine workers 0-23.0 0-16.1
Truck drivers 0.1-1.0 0.13-1.2 0.025b
Bus garage and bus 0.15-1.2 0.01-0.61
workers
Stevedores 0.157b
Truck mechanics 0.133b
a Estimated using nicotine to account for exposure to cigarette smoke
b Based on carbon core analysis, with an estimated 20% of respirable
particulate due to carbon (Steenland et al., 1992)
Because 1-nitropyrene is associated with the particle fraction of
diesel exhaust, this compound was used as an indicator of the
particulate fraction of diesel exhaust derived from various sources
(e.g. ships, railroad engines, heavy-duty road vehicles, fork-lift
trucks) (Scheepers et al., 1994a,b). The 1-nitropyrene content of
fresh and aged diesel exhaust was measured in small air samples
extracted with acetone by sonication and detected by gas
chromatography-mass spectrometry. The following concentrations of
respirable diesel exhaust were determined: 0.044-0.33 mg/m3 in
various work-places with fork-lift trucks, 0.10-0.37 mg/m3 in
railroad engine repair shops, 0.080mg/m3 in a river vessel, and 0.67
or 3.33 mg/m3 in the breathing zone of railroad workers.
B5.2.1 Truck drivers and mechanics
In a study of 15 truck drivers in Geneva, Switzerland, exposure
to particulate matter was measured during one working day with a
direct-reading instrument and by sampling beside the drivers in the
truck cabin (Guillemin et al., 1992). Local drivers had greater
exposure to particulate matter (0.3 mg/m3) than long-distance
drivers (0.1 mg/m3) and spent more time in polluted areas, such as
streets with heavy traffic and construction sites. Smoking did not
influence the exposure of professional truck drivers to particulate
matter, probably because the ventilation rate of the truck cabins was
relatively high, even on cold days.
Industrial hygiene surveys were conducted during warm and cold
weather at eight terminals and truck repair shops to determine
exposure to submicrometre particles of elemental carbon among American
workers in one of four jobs -- road drivers, local drivers, stevedores,
and mechanics -- all of whom were exposed mainly to diesel aerosol
(Zaebst et al., 1991). The overall arithmetic mean exposure was
5.1 µg/m3 for 72 long-distance road drivers, 5.4 µg/m3 for 66
local drivers, 26.6 µg/m3 for 80 mechanics, and 31.3 µg/m3 for 54
stevedores exposed to diesel fork-lift trucks. The background
concentrations in the same cities were 3.4 µg/m3 on 21 major
highways and 1.4 µg/m3 in 23 residential areas. The exposures of the
truck drivers were significantly higher than the background
concentrations in residential areas but could not be distinguished
from the background highway concentrations.
Area air sampling in an American truck repair shop in 1987 gave
the following results, depending on the sampling location: 0.225 and
0.32 µg/m3 submicrometre particles, 0.26 and 0.51 µg/m3 respirable
particles, and 0.63 and 0.66 µg/m3 total particulate matter. The
values for elemental carbon were 86-118 µg/m3 submicrometre
particles and 159-214 µg/m3 total carbon. In personal samples from
four mechanics, 79-193 µg/m3 submicrometre elemental carbon were
found (US National Institute for Occupational Safety and Health,
1990a).
The concentrations of particles collected on fibreglass filters
at 25 work-places in Germany where mainly fork-lift trucks and trucks
were used were 0.02-0.8 mg/m3 in area samples and 0.13-1.2 mg/m3
in personal samples (Lehmann et al., 1990). Area measurements in two
German truck repair garages (five samples from each) showed
concentrations of incinerable fine dust (according to German standard
method TRGS 900) of 0.11-0.40 mg/m3 (Blome et al., 1990).
Personal samples from truck drivers who loaded diesel-engined
trucks onto car ferries in Sweden showed particle concentrations of
0.1-1.0 mg/m3 (Ulfvarson et al., 1987). The drivers may also have
been exposed to diesel exhaust from the ferries.
B5.2.2 Bus garage and other bus workers
In area samples taken in an American bus garage in 1989, the
concentrations of particles were 0-0.1 mg/m3 submicrometre
particles, 0-0.3 mg/m3 respirable particles, 0-0.22 mg/m3 total
particulate matter, and 0.014-0.326 mg/m3 elemental carbon (US
National Institute for Occupational Safety and Health, 1990b).
The concentrations of smoke were measured in two diesel bus
garages in London, United Kingdom, from high-volume samplers for
successive periods through the day and night, which were linked to bus
movements and the exposure of the maintenance staff. The contribution
from the buses was assessed by subtracting the background values at
control sites outside the garages. In the most polluted garage,
0.3-1.2mg/m3 of smoke were measured during working periods (Waller
et al., 1985).
In Sweden, a particle concentration of 0.46 mg/m3 was found
during personal sampling in periods of high activity (arrival and
departure of buses) (Ulfvarson et al., 1987).
Area air sampling of the concentrations of incinerable fine dust
(according to German standard method TRGS 900) in two bus depots (six
samples in each) and one bus garage (three samples) in Germany showed
concentrations of 0.22-0.37 mg/m3 (Blome et al., 1990).
The respirable dust levels in bus garages in Italy and the United
States ranged from <0.01 to 0.73 mg/m3 (Gamble et al., 1987a). The
total dust levels in bus garages in Denver, Colorado, United States,
were 0.01-0.81 mg/m3 (Apol, 1983; Pryor, 1983).
B5.2.3 Fork-lift truck operators
Area samples taken in one American trucking terminal where
diesel-powered fork-lift trucks were used in 1987 contained
48-62 µg/m3 submicrometre particles, 34-57 µg/m3 respirable
particles, and 62-97 µg/m3 total particles (US National Institute
for Occupational Safety and Health, 1989a). The concentrations of
elemental carbon in area samples from another American trucking
terminal in 1988 were 40-44 µg/m3, and those in personal samples
from fork-lift operators were 33-77 µg/m3 (US National Institute for
Occupational Safety and Health, 1989b). Area samples taken in an
American freight dock contained concentrations of <12 and 294 µg/m3
respirable dust. The levels were significantly higher in areas where
unfiltered rather than filtered diesel motors were used (US National
Institute for Occupational Safety and Health, 1990c).
A mean concentration of 31.3 µg/m3 of submicrometre elemental
carbon was found in personal samples from stevedores operating
diesel-powered fork-lift trucks. About 10 times less was found for
operators of gasoline- or propane-powered trucks (Zaebst et al.,
1991).
The concentrations of particles in the breathing zone during use
of diesel-powered fork-lift trucks for loading, unloading, and
warehousing operations in American ammunition storage magazines were
< 0.01 to1.3 mg/m3 (Ungers, 1984) and 0.5-5.0 mg/m3 (Ungers, 1985).
B5.2.4 Railroad workers
The mean levels of total particulate matter were 0.38 mg/m3
(range, 0.1-0.8) in Finnish locomotive cabs and 1.99 mg/m3 (range,
0.07-8.7) in roundhouses (Heino et al., 1978). In three German engine
sheds, the ambient concentrations of incinerable fine dust were
0.1-0.41mg/m3 (Blome et al., 1990).
A summary of American literature showed time-weighted average
levels of 0.05-0.07 mg/m3 (8-h) in tunnels, 0.16 mg/m3 (7.5-h)
during freighting, 0.01 mg/m3 (5-h) in switchyards, and 0.27 mg/m3
(8-h) in cabooses (Hobbs et al., 1977). The arithmetic mean
concentrations of total respirable particles in personal samples from
shop workers, engineers, brakemen, conductors, and farmers in the
American railroad industry (after correction for the estimated
contribution of cigarette smoke particulates) were 51-192 µg/m3
(Woskie et al., 1988a).
B5.2.5 Mine workers
Diesel vehicles and machines have been used in nearly all
American mines since the 1960s (Wichmann & Brüske-Hohlfeld, 1991). The
mean concentration of diesel particulate matter in personal and area
samples in mines where diesel equipment was used was 4.6 mg/m3
(range, 0.2-14) (Holland, 1978). Total respirable dust levels of
0.6-1.7 mg/m3 were found by personal sampling (Wheeler et al.,
1981); average concentrations of 0.9-2.7 mg/m3 were found in
full-shift personal samples, and the range for full-shift area samples
was 0-16.1 mg/m3 (Reger et al., 1982). The 8-h time-weighted average
level of respirable particulate matter in personal samples taken in an
American molybdenum mine was 0.2-1.9 mg/m3 (Cornwell, 1982). Total
respirable dust concentrations of 1.1-4.4 mg/m3 were reported in an
American gold mine (Daniel, 1984). The US Mine Safety and Health
Administration reported that the concentrations of diesel particulate
matter in American mines other than coal mines were 0.3-1.5 mg/m3
(Mauderly, 1992).
Data from a salt mine in Canada showed a diesel particulate level
of about 0.5 mg/m3 (Johnson & Carlson, 1986).
In area samples from different work-places in eight German coal
mines, the levels of airborne diesel particulate matter (incinerable
fine dust) were 0.19-0.70 mg/m3 (Blome et al., 1990). In German
potash-salt mines, depending on the district, 0.2-0.4 mg/m3 of
diesel particles were measured (Bauer et al., 1990).
B5.2.6 Fire fighters
The average concentration of total particulate matter in personal
samples from fire-fighters in Boston, New York City, and Los Angeles,
United States, in 1985 was between < 0.1 and 0.48 mg/m3. On a typical
day in Boston or New York City, exposure to diesel exhaust particles
was estimated to be about 0.225 mg/m3 after correction for the
contributions of background and smoking. Simulation of 'worst-case'
exposures in Los Angeles fire stations gave a maximal concentration of
0.748 mg/m3 total particles (Froines et al., 1987).
B5.3 Biomonitoring
B5.3.1 Urinary mutagenicity
The occurrence of mutagens, measured by the Ames test, in the
urine and faeces of eight car mechanics occupationally exposed to
diesel exhaust was compared with that of a reference group of nine
office workers. The samples were collected over 48 h; information was
also gathered on dietary intake, smoking habits, and medication. No
enhancement of the incidence or degree of faecal or urinary
mutagenicity was seen (Willems et al., 1989).
Post-shift urinary mutagenicity was measured by the Ames test in
306 samples from 87 railroad workers with a range of exposures to
diesel exhaust (Schenker et al., 1992). Exposure was measured
throughout the work shift by constant-flow personal sampling pumps. In
separate analyses of smokers and nonsmokers, no independent
association was seen with exposure to diesel exhaust. The workers with
the highest median exposure to respirable particles attributable to
diesel exhaust (113 µg/m3) were railroad engineering shop workers.
In studies of the effect of diesel exhaust on stevedores on
'roll-on-roll-off' ships (Ulfvarson et al., 1987), no difference in
mutagenicity to Salmonella typhimurium TA98 or Escherichia coli WP
2 uvr A was seen in urine collected during periods of exposure and
of no exposure. Similarly, no increase in urinary mutagenicity was
found in samples from six volunteers before and after exposure to
diesel exhaust collected from an experimental automobile run for 3.7 h
at 60 km/h and 2580 rpm.
B5.3.2 Other analyses
Samples of total suspended particulate matter were collected in a
repair shop for train engines, and the diesel exhaust concentrations
were determined using 1-nitropyrene as a biomarker (see section B5.2).
Urinary metabolites and the corresponding nitro-substituted
derivatives were detected by immunoassay in the urine of three
diesel-engine mechanics. They had a significantly increased excretion
of metabolites in comparison with two office clerks (Scheepers et al.,
1994b).
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
B6.1 Deposition
The mass median aerodynamic diameter of diesel exhaust particles
in long-term studies in animals exposed by inhalation was 0.17-0.25 µm
(Lewis et al., 1986; Lee et al., 1987; Wolff et al., 1987;
Creutzenberg et al., 1990). The deposition efficiency of particles of
this size range is lower than that of smaller particles, for which the
deposition by diffusion increases with decreasing diameter. The
deposition efficiency of particles with a diameter > 0.5 µm increases
with the aerodynamic diameter, owing to sedimentation and impaction.
The nose has hardly any filter effect for diesel particles because the
fraction of particles < 0.4 µm deposited in the nose is very low
(Heyder et al., 1986).
After short-term inhalation by rats of 0.29-µm fused
aluminosilicate particles, 8% was deposited in the bronchi and 13% in
the lungs (Raabe et al., 1988). A total of 10-20% diesel exhaust
particles was deposited in rats and guinea-pigs (Chan et al., 1981;
Lee et al., 1983; Dutcher et al., 1984). In humans, the alveolar
deposition efficiency of 0.2-µm particles was 10-20%, and no bronchial
deposition was detected (Heyder et al., 1986).
These experimental results agree well with those of a predictive
model for deposition of inhaled diesel exhaust particles in laboratory
animals and humans (Yu & Xu, 1987). In humans, the deposition
efficiency was about 7% in the alveolar region and 4% in the
tracheobronchial region. The model can also predict the fractions that
will be deposited in different airway generations; maximal deposition
occurs in the alveolar region (twentieth generation) of adults and of
children over two years of age.
The length and concentration of exposure had no significant
effect on deposition in rats exposed to 0, 0.4, 3.5, or 7.1 mg/m3
diesel exhaust particles for 6, 12, 18, or 24 months, when deposition
of 0.1-µm gallium oxide particles was used as the indicator (Wolff et
al., 1987).
B6.2 Retention and clearance of particles
The kinetics of inhaled substances are conveniently described by
their pulmonary clearance and retention. These terms are often used
interchangeably, but it should be kept in mind that the fraction
cleared from the lung plus the fraction retained in the lung account
for the total deposited amount (retention = deposition - clearance).
Thus, in the following paragraphs, the term 'clearance' refers to a
clearance rate ( r, per unit of time) and the term 'retention' to a
biological half-time ( t ´), provided there is a constant rate of
exponential clearance (ln2/ t ´).
Clearance of diesel particles from the alveolar region is
important in the long-term effects of particles on cells, as it is
more than two orders of magnitude slower than mucociliary clearance,
measured in several studies in rats administered labelled diesel or
surrogate particles by inhalation. The pulmonary retention half-times
in studies in which the lung burden of diesel soot was also reported
are summarized in Table 33. The equivalent continuous exposure
concentration is calculated by multiplying the actual concentration by
the number of hours of weekly exposure divided by 168 h. This figure
allows a comparison of exposure levels in studies with different
exposure protocols. The assumption is that Habers' law ( c × t =
constant) is applicable. This is probably valid until a certain burden
of particulate is reached in the lung; however, when that level is
exceeded (particle overload), the calculation is increasingly invalid.
The retention half-time in these studies was 60-100 days in
control rats with low lung burdens and up to 600 days in animals with
high lung burdens. In a double logarithmic plot of retention
half-times and lung burden, a linear relationship can be seen for lung
burdens of diesel particles above 1mg per lung (Figure 1).
The gaseous phase of diesel exhaust appears to have no effect on
alveolar clearance in rats or hamsters (Heinrich et al., 1986a).
The retardation of lung clearance is not specific to diesel
exhaust but is known as 'lung overloading by particles'. This effect
is characterized by (i) an overwhelming of normal alveolar
macrophage-mediated clearance processes under certain conditions of
exposure, (ii) resulting in lung burdens greater than predicted from
deposition kinetics at low concentrations, (iii) with associated
pathophysiological changes including altered macrophage function,
inflammation, and pulmonary fibrosis, and (iv) an uncertain
association with an increased incidence of lung tumours in rats
(McClellan, 1990).
The effect of overloading lung clearance mechanisms was
investigated in NMRI and C57Bl mice, Wistar and Fischer 344 rats, and
Syrian golden hamsters exposed to particulates, including test toner
(polymer pigmented with carbon black), polyvinyl chloride, carbon
black, diesel exhaust, and two crystalline forms of titanium dioxide
(anatase and rutile), at concentrations of 0.8-64 mg/m3 for up to
two years. In rats, lung overloading generally occurred after exposure
to 0.5-1.5 mg per lung or about 1 mg of material per gram of lung
tissue. A two- to 10-fold decrease in the clearance rate was seen
after heavy particulate loading in all of these rodent species (Muhle
et al., 1990).
In comparisons of the effects of particles of different
densities, the retained volume of particles appears to be a more
useful parameter than the retained mass, as alveolar macrophages have
an upper volumetric uptake limit. The particulate overload effect
appears to be initiated in rats when the average particle volume per
alveolar macrophage exceeds about 60m3, and alveolar
macrophage-mediated particle clearance virtually ceases when the
phagacytosed particulate volume exceeds an average of 600m3 (Morrow,
1988).
At a lung burden above threshold, signs of lung overloading in
Fischer 344 rats persisted for 15 months after cessation of exposure
(Bellmann et al., 1992). Retardation of alveolar clearance was also
observed in hamsters and mice at a lung burden above about 1 mg per
lung. For mice, no data were available with regard to lung burdens
below 1 mg per lung, but retardation of alveolar clearance is probably
already present at lower lung burdens. Lung overloading is thus seen
in a variety of species with various materials. It is generally
observed after the threshold lung burden of particles of low
solubility and low acute toxicity is exceeded for a considerable time
(Muhle et al., 1990). The effects of overloading should be considered
in extrapolating the health effects seen at high concentrations in
experiental animals to the low concentrations occurring in the
environment or the work-place.
The alveolar clearance rates of highly insoluble particles differ
among species, with retention half-times ranging from 50 to 100 days
in rodents to several hundred days in dogs and man (Figure 2). In
human lung, the retention half-time of insoluble, nontoxic particles
may be >500 days (Bailey et al., 1985a,b).
Table 33. Pulmonary retention of insoluble particles in rats after exposure to diesel exhaust, and lung burden of diesel soot
Strain and sex No./group Duration of Exposure Pulmonary retention Reference
exposure
(h/week)
Concentration (mg/m3) Duration Lung burden Material Half-time
(weeks) (mg/lung) studied (days)
Actual Equivalent
continuous
Fischer 344, 4 140 0 0 0 0 14C-Diesel 77 Chan et al.
male 0.25 0.21 7 0.2 exhaust 90 (1984)
0.25 0.21 16 0.5 92
6.00 5.00 1 0.7 166
6.00 5.00 9 6.5 562
6.00 5.00 16 11.8 (> 1000)
Wistar, female 5-7 90 0 0 13 0 59Fe2O3 61 Creutzenberg
0.84 0.45 0.63 (0.3 µm) 94 et al. (1990);
2.5 1.34 2.5 119 Heinrich et
6.98 3.74 8.2 330 al. (1995)
0 0 52 0 72
0.84 0.45 2.8 121
2.5 1.34 10.7 254
6.98 3.74 35.7 541
0 0 78 0 96
0.84 0.45 5.5a 221
2.5 1.34 12.2 272
6.98 3.74 58.8 687
Table 33 (contd)
Strain and sex No./group Duration of Exposure Pulmonary retention Reference
exposure
(h/week)
Concentration (mg/m3) Duration Lung burden Material Half-time
(weeks) (mg/lung) studied (days)
Actual Equivalent
continuous
Fischer 344, 8 35 0.15 0.03 18 0.035 Diesel 87 Griffis et al.
male and 0.94 0.20 0.22 exhaust 99 (1983)
female 4.10 0.85 1.89 165
Fischer 344, 4 140 6.00 5.00 1 1.7 14C-Diesel 61 Lee et al.
male 6.00 5.00 3 4.4 exhaust 124 (1987)
6.00 5.00 6 10.4 192
Fischer 344, 15-16 (lung 35 0 0 104 0 134Cs-Fused 79 Wolff et al.
male and burden) 0.35 0.07 0.6 aluminosilicate 81 (1987)
female 10 3.50 0.73 11.5 particles 264
(retention) 7.00 1.46 20.5 (2 µm) 240
a Measured; value derived by interpolation from data at 52 and 104 weeks
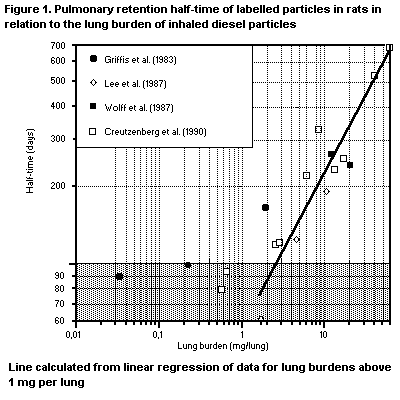
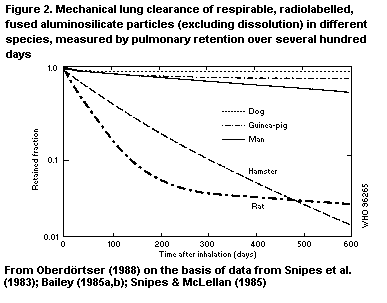 Retention of diesel particles in the lungs and lymph nodes has
been reported in Fischer 344 and Wistar rats after long-term
inhalation (Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly
et al., 1987; Strom et al., 1988; Creutzenberg et al., 1990; Strom et
al., 1990). Some results with regard to lung burden are included in
Table 33. Because clearance of lung burdens above about 1 mg per lung
was impaired after long-term, continuous inhalation of a concentration
equivalent to about 0.5mg/m3, no steady-state lung burden was found
at serial sacrifice at up to two years. About 20% of the lung burden
of diesel particles was found in lung-associated lymph nodes at that
time.
Mathematical models have been developed to calculate the
retention of diesel particles in rats. Yu & Yoon (1990) and Yu et al.
(1991) developed a dosimetry model to predict the retention of diesel
particles and associated organic compounds in rats and humans of
different ages. The model indicates that lung clearance would not be
impaired in humans exposed to a concentration < 0.05 mg/m3 for
24 h/day. The highest lung burden per unit of lung weight was
calculated for five-year-old children. This model was used to assess
the risk of inhaling diesel particles (Pepelko & Chen, 1993).
B6.3 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
The clearance of particles from ciliated airways by the
mucociliary escalator after bronchial deposition is virtually complete
within 24 h. For soluble organic material like PAHs on the surface of
carbon particles, diffusion-limited clearance through the airway
epithelium to capillary blood was found to be slower in the bronchial
region than in the alveoli (Gerde et al., 1991a, 1993a,b).
PAHs in diesel soot adhere strongly to the surface of the
particles. Several studies have shown that the association of PAHs
with particles significantly slows the clearance of the PAHs from
lungs in comparison with the clearance of inhaled PAHs unassociated
with particulate matter.
After inhalation by Fischer 344 rats of 3H-benzo[ a]pyrene
adsorbed onto diesel soot (0.1% by mass) for 30 min, biphasic lung
clearance was noted. In a first, fast phase, about 50% of the inhaled
compound was cleared with a half-time of about 1 h, predominantly by
mucociliary clearance. At the end of exposure, about 15% of the 3H
label was found in blood, liver, and kidney, indicating rapid, direct
absorption of this fraction of 3H-benzo[ a]pyrene and its metabolites
into blood. The remaining 50% was retained in the lung, with a
half-time of 18 days; this is one-third of the retention half-time of
diesel particles. Thus, removal of benzo[ a]pyrene and its metabolites
from the particle carrier may be the rate-limiting step of this
clearance process (Sun et al., 1984). In contrast, more than 95% of
pure 3H-benzo[a]pyrene was cleared from the lung within 12 h after
inhalation (Sun et al., 1982).
These results were confirmed and extended to another PAH,
1-nitropyrene (Bond et al., 1986a,b), when the clearance of
14C-labelled compound adsorbed to diesel soot was determined by the
same method in Fischer 344 rats. After exposure to 490 ng/litre of
pure labelled compound, about 90% was cleared in the first, fast
clearance phase, with a retention half-time of 1 h; after exposure to
650 ng/litre of 14C-1-nitropyrene adsorbed onto diesel particles,
45% of the labelled compound was cleared from the lungs in the first
phase, with a half-time of about 2 h. The remaining 55% was retained
with a half-time of 36 days, which was twice the corresponding value
for 3H-benzo[ a]pyrene in the study of Sun et al. (1984).
On the basis of their data on the clearance of 3H-benzo[ a]pyrene
and 14C-1-nitropyrene, Bond et al. (1986b) predicted equilibrium
lung burdens in humans after long-term inhalation. Assuming inhalation
of 3.5 g/m3 of particles containing 0.1% benzo[ a]pyrene and
1-nitropyrene over an 8-h working day, they predicted equilibria of
160 ng benzo[ a]pyrene and 31 ng 1-nitropyrene per lung. No data are
available on changes in the retention of individual compounds after
prolonged exposure to diesel exhaust.
The clearance of a very low concentration (2.6 g/g particles) of
3H-benzo[ a]pyrene adsorbed onto diesel particles was measured in
Sprague-Dawley rats after intratracheal instillation (Bevan & Ruggio,
1991). Only 25% was cleared in the early, fast phase; a long-term
retention half-time of eight days was calculated for the remainder
during a three-day follow-up, which was similar to the clearance in
that period in the study of Sun et al. (1984).
B6.4 Metabolism
In the studies of Sun et al. (1984) and Bond et al. (1986a,b),
the levels of 3H-benzo[ a]pyrene and 14C-1-nitropyrene and their
metabolites in extracts of lung homogenates after inhalation of diesel
exhaust particles were measured by high-performance liquid
chromatography. Sun et al. detected oxidized metabolites 30 min after
inhalation: 35% of the 3H-benzo[ a]pyrene had been metabolized in
equal amounts to phenols (3-hydroxy- and 9-hydroxybenzo[ a]pyrene)
and quinones (1,6 and 3,6 isomers). Twenty days later, only 13%
phenols and 5% quinones were detected. In the study of Bond et al.
(1986b), about 30% of the 3H-1-nitro-pyrene had been metabolized to
acetylaminopyrenephenol (6 and 8 isomers) and about 40% remained
unmetabolized 1 h after inhalation. Oxidative metabolism of
14C-benzo[ a]pyrene, in solution or adsorbed onto diesel particles
at a concentration of 500 pmol/106 cells, was also detected in cell
cultures of pulmonary macrophages obtained from beagle dogs. The
quantity of metabolites increased with incubation time up to 45 and
125 pmol/106 cells in extracts and in the media, respectively, after
48 h of incubation. After incubation for 6 h, the major metabolites
found in extracts of cells were benzo[ a]pyrene-7,8-diol (0.5pmol/106
cells) and benzo[a]pyrene-4,5-diol (0.2 pmol/106 cells); those in
extracts of culture medium were benzo[ a]pyrene-7,8-diol (1.7 pmol/106
cells) and benzo[ a]pyrene-9,10-diol (1.4 pmol/106 cells), in
comparison with a total concentration of metabolites of 2.5 pmol/106
cells in the extracts and 6 pmol/10 6 cells in the medium. Minor
metabolites were benzo[ a]pyrene phenols (3-hydroxy- and
9-hydroxy-benzo[ a]pyrene) and benzo[ a]pyrene quinones (6,12, 1,5,
and 3,5 isomers). The quantities of metabolite were similar when
macrophages were incubated with benzo[ a]pyrene in solution or
adsorbed onto diesel particles (Bond et al., 1984).
Using the 32P-postlabelling technique, Wong et al. (1986) found
increased DNA adduct formation in the lungs of Fischer 344 rats after
exposure for 31 months to diesel exhaust containing 7.1 mg/m3
particles. After exposure of Fischer 344 rats and Syrian golden
hamsters to dilutions of diesel engine exhaust for six months to two
years, the level of haemoglobin adducts (2-hydroxyethylvaline and
2-hydroxypropylvaline), corresponding to the metabolic conversion of
5-10% of inhaled ethylene and propylene to its oxides, increased
dose-dependently (Törnqvist et al., 1988). An adduct of
benzo[ a]pyrenediol epoxide with deoxyguanosine was identified after
inhalation of benzo[a]pyrene adsorbed onto carbon black (20 mg/g)
(Wolff et al., 1989), demonstrating the production of epoxides of PAHs
by oxidative metabolism.
Methods for extracting diesel soot before testing for
genotoxicity in vitro have been discussed. As dichloromethane and
dimethyl sulfoxide do not correspond to leaching conditions in the
lung, it can be assumed that the bioavailability of genotoxic
materials is lower in lungs than in vitro. An investigation of
dipalmitoyl phosphatidylcholine, the primary component of
physiological surfactant, demonstrated that the genotoxicity
associated with diesel particles inhaled into the lung can be
activated by the solubilization and dispersion properties of pulmonary
surfactants. This finding was confirmed in mammalian cells for sister
chromatid induction (Keane et al., 1991) and for chromosomal
aberrations and micronucleus formation (Gu et al., 1991, 1992).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
B7.1 Single exposure
Data on the acute toxicity of diesel exhaust are very limited;
the two available studies suggest that diesel exhaust particles and
their extracts have little acute toxicity.
Male ICR mice were administered diesel exhaust particles by
intratracheal instillation, and mortality due to lung oedema was
assessed for up to 24 h. At a dose of 0.9 mg per animal, all animals
died within 24 h. The LD50 value was 0.6 mg per animal, equivalent
to about 20 mg/kg body weight. Diesel exhaust particles washed three
times with methanol did not cause death at a dose of 1 mg per mouse,
suggesting that the primary source of toxicity is the extractable
organic compounds rather than the particles themselves. These
compounds may produce superoxide radicals that damage endothelial
cells (Sagai et al., 1993).
The LD50 value for diesel exhaust extract in adult Syrian
golden hamsters after intraperitoneal injection was 1280 mg/kg body
weight (Pereira et al., 1982).
B7.2 Short-term exposure
Tracer-monitored DNA synthesis, indicating a proliferative
response, was observed in whole lung tissue of groups of 3-10 Fischer
344 rats exposed to diesel exhaust by inhalation at 6 mg/m3 for
20h/day on seven days per week for periods of 1-14 days. The response
was maximal after two days of exposure, showing a fourfold increase.
The type II cell labelling index was significantly higher (fourfold)
than that in controls after two and three days of exposure to diesel
exhaust. Both values returned to control levels after a one-week
recovery period. Experiments involving incorporation of 14C-palmitic
acid revealed transient changes in lipid metabolism after one day of
exposure, with a threefold increase in the amount of phosphatidyl-
choline in lung tissue (Wright, 1986).
Exposure of various species by inhalation to 6 mg/m3 resulted
in slight alterations in lung function and histopathological changes.
Groups of 10 male Chinese hamsters were exposed for 8 h/day on
seven days per week for six months to particle concentrations of
either 6 or 12 mg/m3 diesel exhaust.
Measurements of pulmonary function indicated dose-related
increases in lung weight and dose-related decreases in vital and
diffusion capacity. Pathological evaluation revealed oedema,
thickening of the alveolar lining, a possible increase in collagen,
and the presence of numerous particle-laden macrophages, which almost
filled the alveolar spaces (Vinegar et al., 1981).
Groups of 41-51 male and female Hartley guinea-pigs were exposed
for 20 h/day on seven days per week to exhaust from a light-duty
diesel engine diluted to attain a concentration of 6 mg/m3
particulate matter. Separate groups were exposed to raw exhaust and
exhaust that had been irradiated with ultra-violet light for an
average of about 1 h. Pulmonary function was measured, and
electrocardiography was conducted on animals exposed for four weeks;
those examined for histological changes were exposed for eight weeks.
The only changes noted after four weeks were a 35% increase in
pulmonary flow resistance and a small, but significant decrease in
heart rate in animals exposed to irradiated exhaust. No changes in the
electrocardiogram were noted. The pathological changes were limited to
hyperplasia of alveolar lining cells and slight focal thickening of
the interstitium in occasional alveoli with accumulation of
particle-laden macrophages. Growth rates were unaffected (Wiester et
al., 1980).
As part of the same study, cats (Pepelko et al., 1981) and male
rats (15 per group) (Pepelko, 1982) were exposed for four weeks to
unirradiated exhaust under identical conditions. The pulmonary
function of rats was unchanged; the only changes were significant
increases in vital and total lung capacity, but these differences were
quite small and of questionable significance. The only functional
change noted in the cats was a decrease in maximal expiratory flow
rate at 10% of vital capacity. The pathological changes were again
limited to focal thickening of the alveolar walls near accumulations
of particle-laden macrophages. In general, the functional and
pathological changes were limited under these experimental conditions.
Kaplan et al. (1982) exposed male Fischer 344 rats (168 per
group), A/J mice (672 per group), and male Syrian golden hamsters (236
per group) to 1.5 mg/m3 of diesel exhaust particles for 20 h/day on
seven days per week for three months or to clean air. Animals were
killed at the end of exposure and after an additional six-month
recovery period. Mortality was not increased during the observation
period, and body weight was not significantly altered in any species.
At the end of exposure, the relative weight of the lung was
significantly increased in rats. In mice, the pulmonary adenoma
response was increased but not significantly. In all three species,
grey to black discolouration of the lungs and lung-associated lymph
nodes were observed. Microscopically, most of the particulate was
found in macrophages, but some was free. After six months' exposure to
clean air, the lungs of all species had considerably less pigment
accumulated in foci.
Sixteen male Fischer 344 rats were exposed to 6 mg/m3 diesel
exhaust for periods ranging from 6 h to 63 days, with subsequent
histological examination of the lungs. The numbers of diesel
particulate-containing alveolar macrophages, type II alveolar cells,
and inflammatory cells were found to be increased over those in eight
controls. After a nine-week exposure, accumulation of macrophages was
observed near the terminal bronchioles (White & Garg, 1981). In rats
that inhaled 6 mg/m3 diesel exhaust for two weeks and were either
killed immediately or allowed to recover for six weeks before
sacrifice, there was no statistical difference in the number of
macrophage aggregates (240 000 and 220 000 per lung); however, the
aggregates in the recovery group had significantly higher average
diameter and volume, demonstrating macrophage attachment after the end
of exposure (Garg, 1983).
The observations of Wiester et al. (1980) and Garg (1983)
demonstrate the importance of alveolar macrophages in the clearance of
particles of low solubility. These are engulfed by alveolar
macrophages and eliminated via the mucociliary escalator and
gastrointestinal tract, or, in the case of lung particle overloading,
increasing fractions are sequestered in focal areas of the lung tissue
and transferred to the lymph nodes (Muhle et al., 1990).
B7.3 Long-term exposure and studies of carcinogenicity
B7.3.1 Non-neoplastic effects
No obvious signs of toxicity were observed in groups of 183 male
and 183 female Fischer 344 rats exposed by inhalation to diesel
exhaust at 0.35, 3.5, or 7.1 mg/m3 for 7 h/day on five days per week
for up to 30 months (Mauderly et al., 1987). The body weights did not
differ significantly from those of controls, and the exposures did not
affect the life span of animals of either sex. Focal fibrotic and
proliferative lung disease was observed in parallel with progressive
accumulation of diesel soot in the lung. Henderson et al. (1988)
reported the results of biochemical and cytological analyses of lung
lavage fluid and lung tissue from rats and mice exposed in the same
study, including determinations of lactic dehydrogenase, glutathione
reductase, ß-glucuronidase, glutathione, and hydroxyproline, and the
numbers of macrophages and neutrophils. No changes were seen in rats
or mice exposed to 0.35 mg/m3 , but at higher concentrations dose-
and duration-dependent increases in biochemical parameters and cell
numbers in lavage fluid were observed, indicating a chronic
inflammatory response. Lung weights were also increased in male and
female mice exposed to the two higher levels for any length of time,
in rats exposed to 7.0 mg/m3 for 12 months or longer, in female rats
exposed to 3.5 mg/m3 for 18 months, and in males exposed to
3.5 mg/m3 for 24 months. Tissue enzyme levels, measured in lung
lavage fluid as an indicator of damage, were consistently raised in
rats and mice at the two higher exposure levels and in lung tissue of
rats and mice at various times (most pronounced at 7 mg/m3). These
changes probably indicate an increase in lung tissue due to increased
inflammatory cells and epithelial cell proliferation.
In the same study, Mauderly et al. (1988) described focal
proliferative, fibrotic, and emphysematous changes in the lungs of
animals at the higher doses. Measurements in vivo revealed a
reduction in lung volume and compliance.
In a follow-up study, Mauderly et al. (1990) exposed male Fischer
344 rats that were either normal or had pre-existing pulmonary
emphysema (elastase-treated) to diesel soot at 3.5 mg/m3 for 7 h/day
on five days per week for 24 months, to determine whether
emphysematous rats were more susceptible to diesel exhaust. No
increase in susceptibility was seen. The emphysematous lungs had a
lower accumulation of particles than the normal lungs, which prevented
an overproportional response of the compromised animals.
Male and female Fischer 344 rats exposed to diesel exhaust
particles at 0.7, 2.2, or 6.6 mg/m3 for 16 h/day on five days per
week for two years or to filtered diesel exhaust showed an
exposure-related reduction in the rate of weight gain throughout the
study at the highest dose and an increase in absolute lung weight at
the two higher doses (Brightwell et al., 1986, 1989).
Groups of 72 male and 72 female Fischer 344 rats exposed to
diesel exhaust particles at 2 mg/m3 for 7 h/day on five days per
week for 24 months showed alveolar type II cell hyperplasia,
inhibition of long-term clearance of particles and pulmonary
lipidosis. No immunological abnormalities, no induction of pulmonary
or hepatic xenobiotic metabolizing enzymes, and no effects on
mortality, body weight gain, organ:body weight ratios or clinical
chemical parameters were seen (Lewis et al., 1986, 1989). In the same
study, a dose-related depression in phagocytic activity was seen in
alveolar macrophages (Castranova et al., 1985), and a trend to
increased pulmonary arterial wall thickness was observed in comparison
with controls (Vallyathan et al., 1986).
In groups of 15 male monkeys, exposure to diesel exhaust (as
described above) resulted in mild obstructive airway disease, but
there was no evidence of restrictive lung disease. The effects were
observed throughout the exposure period, after 6, 12, 18, and 24
months (Lewis et al., 1989).
Reduced body weight, significantly increased lung:body weight
ratios, and type II cell proliferation were seen in female Fischer 344
rats exposed to whole-fraction diesel exhaust at 5 mg/m3 for 8 h/day
on seven days per week for 24 months (Iwai et al., 1986).
Groups of 96 male and 96 female Syrian golden hamsters, female
NMRI mice, and female Wistar rats were exposed to diesel engine
emissions at 4.4 mg/m3 for 19 h/day on five days per week for
120-140 weeks (Heinrich et al., 1986a; Stöber, 1986). The body weights
of mice were significantly reduced, by about 12%. Lung weights were
increased after two years of exposure, by two- to threefold in rats
and mice and by 50-70% in hamsters. Different levels of histological
response were seen in the three species. Hamsters had thickened
alveolar septa and bronchioalveolar hyperplasia. Of the mice, 64% had
bronchioalveolar hyperplasia (controls, 5%), 71% had multifocal
alveolar lipoproteinosis, and 43% had multifocal interstitial fibrosis
(controls, 4%). The nose, larynx, and trachea were not affected.
Treated rats showed severe inflammatory changes in the lungs,
thickened septa, foci of macrophages, and hyperplastic and metaplastic
lesions, but no significant changes in respiratory rate, minute
volume, compliance, or resistance were seen in 14 exposed rats.
Significantly increased airway resistance and a significant decrease
in dynamic lung compliance were observed in rats after two years of
exposure. The hamsters had a significant increase in airway resistance
and a nonsignificant reduction in lung compliance at termination of a
one-year exposure; these changes persisted during the second year of
exposure. In the same experiments, filtered diesel exhaust did not
have the same effects as total exhaust in any of the three species
(Heinrich et al., 1986a).
Groups of 27 female Fischer 344 rats and about 50 male and 50
female C57Bl/6N and ICR mice were exposed to particle concentrations
of 2-4 mg/m3 for 4 h/day on four days per week for up to 24 months.
After 12 months, the incidence of alveolar hyperplasia was increased
in all animals. Exposed rats had more severe inflammation than
controls, with inflammatory exudate into the nose, and bronchitis and
pneumonia, but this effect was ascribed to the housing, which was not
specific pathogen-free (Takemoto et al., 1986).
Groups of nine male Hartley guinea-pigs were exposed at
concentrations of 0.25, 0.75, or 1.5 mg/m3 for 20 h/day on 5.5 days
per week for up to two years. An analysis of the alveolar capillary
membrane by quantitative morphometry showed that it was thickened as a
result of an increase in the absolute tissue volume of interstitium
and type II cells. Exposure to 0.75 mg/m3 for six months caused
fibrosis in regions of macrophage clusters and focal type II cell
proliferation, as observed under the light microscope. No appreciable
change in morphometric parameters was seen after exposure to the
lowest dose, but the higher doses increased the thickness of alveolar
septa and the numbers of various types of alveolar cells (Barnhart et
al., 1981, 1982).
As part of this investigation, Fischer 344 rats were exposed by
inhalation to 0.25 or 1.5 mg/m3 diesel exhaust particles, and
various biochemical and cytological studies were conducted up to 36
weeks (Misiorowski et al., 1980). At the high dose, normalized lung
weight, the rate of collagen synthesis, and the cellular and lipid
content of lung tissue were significantly increased after six months.
It was concluded that the reactivity of fibrogenic cells had been
enhanced. In a long-term study of respiratory end-points in male
guinea-pigs, including light and electron microscopy, lavage cytology,
and lung tissue biochemistry, the lowest-observable-adverse-effect
level (LOAEL) was 0.796 and the no-observable-adverse-effect level
(NOAEL) was 0.258 mg/m3 (US Environmental Protection Agency, 1992b).
Groups of 120 male Fischer 344 rats, 450 male A/J mice, and 120
male Syrian hamsters were exposed to 0.25, 0.75, or 1.5 mg/m3 diesel
exhaust particles for 20 h/day on seven days per week for 15 months.
Mortality was not increased in any species. A dose-related deposition
of pigment was observed histopathologically in alveolar macrophages
and regional lymph nodes. Accumulation of alveolar macrophages was
associated with an increase in connective tissue in alveolar walls,
with mild fibrosis and proliferation of type II pneumocytes; these
effects were termed pneumoconiosis. No treatment-related responses
were found in organs other than the respiratory tract. The LOAEL was
0.735 and the NOAEL, 0.242 mg/m3 (Kaplan et al., 1983).
Groups of 120 male and 95 female Fischer 344/Jcl rats were
exposed to light-duty diesel engine exhaust particulates at 0, 0.1,
0.4, 1.1, or 2.3 mg/m3 or heavy-duty diesel engine exhaust
particulates at 0, 0.5, 1.0, 1.8, or 3.7 mg/m3 for 16 h/day on six
days per week for up to 30 months (Ishinishi et al., 1986, 1988;
Suzuki et al., 1990). The body weights of females exposed to the
highest dose of heavy-duty engine exhaust were decreased throughout
the study by 15-20% in comparison with controls. No changes were
observed histopathologically in the lungs at any concentration.
Accumulation of particle-laden macrophages was observed at levels
> 0.4 mg/m3. In areas of macrophage accumulation, bronchiolar
epithelium replaced alveolar epithelium in the ducts. Proliferation of
bronchiolar epithelium and type II cells and oedematous thickening and
fibrosis of the alveolar septum were observed, which developed into
small fibrotic lesions classified as hyperplastic. The incidences of
hyperplastic lesions were 4, 4, 6, 12, and 87 per 124 rats exposed to
light-duty engine exhaust and 1, 3, 7, 14, and 25 per 124 rats in
those exposed to heavy-duty engine exhaust. The LOAELs were
1.2 mg/m3 for light-duty and 1.0 mg/m3 for heavy-duty exhaust, and
the NOAELs were 0.4 and 0.5 mg/m3, respectively (US Environmental
Protection Agency, 1992b).
In the same experimental series, Kato et al. (1992) studied
non-neoplastic lesions in the respiratory organs of the rats every six
months. The marked changes consisted of intake of diesel particles by
type I epithelium, hypertrophy, and glandular proliferation of type II
epithelium, with many extended microvilli and increased numbers and
size of lamellar inclusion bodies, focal increase of collagen fibres
in the interstitium, and infiltration of particle-laden macrophages,
neutrophils, mast cells, and plasma cells into the interstitium of
alveolar septa. The most marked changes in the airway were focal
shortening of cilia and protrusions of nonciliated cells. These
morphological changes appeared in all groups exposed to particle
concentrations > 1 mg/m3 after six months of exposure and were more
marked with increasing particle concentration and with time.
Exposure of Syrian golden hamsters to 4 or 11 mg/m3 diesel
exhaust for 7 h/day on five days per week for five months resulted in
enlargement of liver sinusoids and loss of cristae in mitochondria
(Meiss et al., 1981).
The induction of pulmonary adenomas and carcinomas in rats after
long-term inhalation of carbon black is proposed to be due partly to
inflammatory cells and their mediators (Driscoll et al., 1995;
Oberdörster et al., 1995; Driscoll et al., in press). After exposure
of rats to carbon black at 1, 7, or 50 mg/m3 for 6 h/day on five
days per week for 13 weeks, 353, 1923, and 7112 µg of carbon,
respectively, were deposited per lung. After exposure to air or to
1 mg/m3 of carbon black, no macrophage inflammatory protein-2
(MIP-2) mRNA expression and no increase in inflammatory cells was
observed; with 7 or 50mg/m3, both MIP-2 mRNA and the number of
neutrophils in bronchoalveolar lavage fluid were increased, and the
hprt frequencies were 3.2 and 4.2 times greater than in controls.
Groups of 25 cats were exposed to either clean air or diesel
exhaust for 8 h/day on seven days per week for two years at a
concentration of 6 mg/m3 through week 62 and to 12 mg/m3 for weeks
62-124. Some of the cats were then held in clean air for an additional
six months. No response was seen after 61 weeks, but after 123 weeks
signs of restrictive lung disease were observed. Total lung capacity,
forced vital capacity, functional residual capacity, peak expiratory
flow rates, and diffusion capacity were significantly decreased
(Moorman et al., 1985). Morphological evaluation revealed
exposure-related lesions in the centriacinar region of the lungs,
bronchiolar epithelial metaplasia associated with the presence of
ciliated and basal cells, and alveolar macrophages containing diesel
particle-like inclusions. After six months' recovery in clean air, the
metaplasia had disappeared but the fibrosis had progressed (Hyde et
al., 1985). In exposed cats, the epithelium of the terminal and
respiratory bronchioles consisted of ciliated, basal, and Clara cells,
whereas controls had only Clara cells (Plopper et al., 1983). These
observations indicate that the foci of the lesions were the
epithelium, the interstitial compartments, and the centriacinar region
of the lung. The metaplastic changes suggest that cancer may be
induced by continuous exposure, while the other major effect is
pulmonary fibrosis resulting in restrictive disease which is not
ameliorated and in fact progresses after removal from exposure.
Progression of effects is probably due to continued lung overloading
with particulate matter, despite cessation of exposure.
The dose-dependent effects on lung morphology, histopathology,
biochemistry, and cytology after long-term exposure to diesel exhaust
described above are reflected in the following findings seen in rats
at a lung burden of about 0.5 mg/lung (Henderson et al., 1988; Muhle
et al., 1990):
-- The wet and dry weights of lungs increase by up to fourfold in
comparison with those of concurrent controls. This is due to
severe functional overloading in the lung, resulting in enhanced
leukocyte influx and tissue proliferation.
-- Pulmonary inflammation is manifested by cytological (increased
numbers of polymorphonuclear neutrophils) and biochemical (lactic
dehydrogenase, collagen in lung lavage fluid) parameters.
-- The retention of material is characterized by an increasing
number of particle-laden macrophages and by black discolouration
of lungs due to sequestration of accumulated particles.
Additionally, increased transfer of material to the
lung-associated lymph nodes is observed.
-- Later findings during inhalation are proliferative alterations of
the epithelial cells and onset of fibrosis.
B7.3.2 Carcinogenicity
B7.3.2.1 Inhalation
Rats were exposed in several studies to diesel emissions
containing > 2 mg/m3 of particles for more than two years,
resulting in the induction of pulmonary tumours (Table 34). Many
organic and inorganic gaseous components were also present in the test
atmospheres, and the relatively high concentration of some pulmonary
irritants like nitrogen dioxide, aldehydes, and sulfur dioxide may
have contributed to the inflammatory response and to epithelial cell
damage and subsequent cell proliferation. Exposure to gaseous PAHs,
like naphthalene which is toxic to Clara cells, may influence the
metabolic state of pulmonary cells. It is not clear however, whether
the combined exposure to particles and gases in these experiments
favoured tumour induction. It should be noted that Brightwell et al.
(1986), Heinrich et al. (1986a), Iwai et al. (1986), Ishinishi et al.
(1986), and Takaki et al. (1989) have reported that the gaseous phase,
i.e. the proportion of exhaust without particles, does not induce
pulmonary tumours.
Table 34. Carcinogenicity of diesel engine exhaust in rats
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 40 m 8.3 1.5 6 h/d, 5 d/week, up NR 1 17c NR Karagianes
40 m Clean air to 20 months 0 et al.
(20 months) (1981)
Fischer 72 f 6.6 3.1 16 h/d, 5 d/week, 24 NR 39 54 38.5 < 0.1 Brightwell
344 71 m months (30 months) 16 23 et al.
72 f 2.2 1.1 11 15 9.7 < 0.1 (1986, 1989)
72 m 3 4
71 f 0.7 0.4 0 0.7
72 m 1 1
Filtered exhaust 0
0
142 f Clean air 1 1 1.4
140 m 3 2
Wistar 95 f 4.4 2.5 19 h/d, 5 d/week, 32 NR 17 18 < 0.1 Heinrich et
92 f Filtered exhaust months (up to death) 0 al. (1986a)
96 f Clean air 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer
344/Jcl
Light-duty 60 f 2 1.1 16 h/d, 6 d/week, 30 NR 1 2 2.4 NR Ishinishi
engine 64 m months (30 months) 2 3 et al.
59 f 1 0.6 2 3 4.1 (1986,1988;
64 m 3 5 Takaki et al.
61 f 0.4 0.2 0 0.8 (1989)
64 m 1 2
59 f 0.1 0.06 2 3 1.6
64 m 0
59 f Clean air 2 3 3.3
64 m 2 3
Heavy-duty 60 f 4 2.3 3 5 6.5 1.8
engine 64 m 5 8
59 f 2 1.1 1 2 3.3
64 m 3 5
61 f 1 0.6 0 0
64 m 0
59 f 0.4 0.2 1 2 0.8
64 m 0
59 f Clean air 1 2 0.8
64 m 0
16 m Filtered exhaust 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer 19 f 4.9 1.6 8 h/d, 7 d/week, 24 NR 8 42 < 1d Iwai et al.
344 16 f Filtered exhaust months (24 or 30 0 (1986)
22 f Clean air months) 1 5
Fischer 15 f 2.0-4.0 0.2-0.4 4 h/d, 4 d/week, 24 NR 0 NR Takemoto
344 15 f Clean air months (24 months) 0 et al.
(1986)
Fischer 69 f 7 1.5 7 h/d, 5 d/week, 30 NR 13 19 16.1 < 0.1 (f) Mauderly et
344/Crl 74 m months (up to death) 10 14 1.7 (m) al. (1986)
68 f 3.5 0.7 2 3 4.6
63 m 4 6
68 f 0.35 0.07 0 0.7
70 m 1 1
68 f Clean air 0 1.4
73 m 2 3
Fischer 227 f+m 7 1.5 7 h/d, 5 d/week, 30 20.8 12.8 < 0.1 Mauderly et
344/N Crl 221 f+m 3.5 0.7 months (up to death) 11.5 3.6 4.8 al. (1987)
223 f+m 0.35 0.07 0.6 1.3
230 f+m Clean air (24 months) 0.9
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 100 f 7.0 3.8 18 h/d, 5 d/week, 24 63.9 22 22.0 < 0.1 Heinrich et
200 f 2.5 1.3 months (30 months) 23.7 11 5.5 < 1 al. (1992,
198 f 0.8 0.5 6.3 0 1995);
217 f Clean air (24 months) NR 1 0.5 Heinrich
(1994)
Fischer 212 f+m 6.3 3.0 16 h/d, 5 d/week, 24 85.4 38 17.9 17.9 NR Nikula et al.
344/N 210 f+m 2.4 1.1 months (24 months) 40.7 13 6.2 6.2 (1994)
214 f+m Clean air (23 months) 3 1.4 1.4
Fischer 49 f 4.7 1.3 15 h/d, 3 d/week, 24, NR 6 12.2 NR Kawabata
344/N 42 f 4.7 1.3 12, or 6 months 8 19.0 et al.
45 f 4.7 1.3 1 2.2 (1993)
48 f Clean air 5 10.4
Fischer 183 f+m 2.0 0.4 7 h/d, 5 d/week, 24 NR 8 4.4 4.4 NR Lewis et al.
344/N 180 f+m Clean air months 6 3.3 3.3 (1989)
From Pott & Heinrich (1987) and supplemented; NR, not reported
a Mass median aerodynamic diameter, 0.17-0.25 µm; 'Continuous', equivalent continuous value
b Unilateral Fisher's exact test
c Only six animals investigated
d With Yates' correction
No difference in lung tumour incidence was seen in groups of male
and female rats exposed for 7 h/day on five days per week for 24
months either to diesel exhaust particulates at 2 mg/m3 or to
filtered air (Lewis et al., 1989).
Exposure of six male Wistar rats to diesel exhaust soot at
8.3 mg/m3 and coal dust for up to 20 months gave inconclusive
results (Karagianes et al., 1981). The study had little statistical
power owing to the small group size, and the insufficient duration of
exposure may have prevented detection of tumours, although one mammary
fibroadenoma and one lung bronchiolar adenoma were detected. One
subcutal fibrosarcoma and one renal lymphoma were seen in controls.
Eight bronchioalveolar adenomas and nine squamous-cell tumours
were seen in 95 rats (18%) that had inhaled 4.4 mg/m3 diesel exhaust
for 19 h/day on five days per week for 32 months. No tumours were
observed in controls (Heinrich et al., 1986a). The dose-dependent
carcinogenic potency of diesel exhaust was confirmed in a study in
which rats were exposed to diesel exhaust at concentrations of 0.8,
2.5, or 7.0 mg/m3 for 18 h/day on five days per week for 24 months
(Heinrich et al., 1992; Heinrich, 1994; Heinrich et al., 1995).
In male and female rats exposed to diesel exhaust at 6.6 mg/m3,
a high incidence of lung tumours was observed in animals dying or
sacrificed after 24 months (24/25 females, 12/27 males). The incidence
of lung tumours (Table 34) was derived by pooling pulmonary adenomas;
squamous-cell carcinomas; adenocarcinomas; mixed ademomas,
adenocarcinomas, and squamous-cell carcinomas; and mesotheliomas.
Non-neoplastic pulmonary lesions were not described (Brightwell et
al., 1986, 1989).
Groups of 24 female Fischer 344 rats were exposed to clean air,
to whole-fraction (4.9 mg/m3 particulates) fuel, or to filtered
diesel exhaust for 8 h/day on seven days per week for 24 months. After
six months of exposure, the rats exposed to whole exhaust had lower
body weights than those in the other two groups; after 18 months, both
exposed groups showed body weight reduction. The relative lung weight
was increased after 12 months' exposure to whole exhaust, and
epithelial proliferation and significantly elevated numbers of lung
tumours were seen in animals exposed to the whole fraction after six
months. Increased incidences of leukaemia and mammary gland tumours
were also reported in animals exposed to whole or filtered exhaust;
however, because of the small numbers of animals, the significance of
the results cannot be determined (Iwai et al., 1986).
A total of 140 female and 140 male Fischer 344/N rats (in groups
of three males and three females, five males and five females, or
eight males and eight females, depending on the end-point) were
exposed for 16 h/day on five days per week for up to 24 months,
beginning at eight weeks of age, to particles of diesel exhaust or
carbon black at 2.5 or 6.5 mg/m3 of air, or to clean air (Mauderly
et al., 1994). Rats were killed after 3, 6, 12, 18, or 23 months of
exposure for histopathological assessment and measurement of lung and
lung-associated lymph node burdens of particles, lung weight,
bronchoalveolar lavage indicators of inflammation, DNA adducts in
whole lung and alveolar type II cells, and chromosomal anomalies in
circulating lymphocytes. Diesel exhaust and carbon black had nearly
identical effects, with a similar relation to exposure. There was a
dose-related slowing of particle clearance after three months, with
progressive accumulation of particles in lungs and lymph nodes.
Inflammation, epithelial proliferation, and fibrosis were progressive
and related to dose. Diesel exhaust slightly increased the number of
lung DNA adducts, and the numbers of DNA adducts in type II cells were
increased by both diesel exhaust and carbon black. No exposure-related
chromosomal damage was found in circulating lymphocytes. The incidence
of primary lung neoplasms was increased significantly and was related
to the doses of both diesel exhaust and carbon black. The types of
neoplasms were identical and included benign adenomas, malignant
adenocarcinomas, squamous-cell carcinomas, and adenosquamous
carcinomas; squamous cysts were also seen. The neoplasms had similar
growth characteristics when transplanted; 50-67% of the transplanted
squamous-cell carcinomas and 25-40% of the transplanted
adenocarcinomas grew after injection into athymic mice (Table 35),
whereas the squamous cysts did not.
Fischer 344/Jcl rats were exposed to a range of concentrations of
exhaust emission particulates from light-duty (up to 2 mg/m3) or
heavy-duty diesel engines (up to 4 mg/m3) for 16 h/day on six days
per week for up to 30 months. After 18 months, marked hyperplasia of
type II cells was noted in the groups at higher exposures. After 30
months, treatment-related pulmonary hyperplastic lesions were
detected, and a dose-response relationship was seen for tumours in the
groups exposed to heavy-duty but not light-duty diesel exhaust. The
lung tumours were diagnosed as adenocarcinomas, squamous-cell
carcinomas, or adenosquamous carcinomas. A significant correlation was
observed between the incidence of anthracosis and areas with
hyperplastic lesions; furthermore, anthracosis and hyperplastic
lesions did not appear in lungs exposed to particle-free exhaust. The
authors concluded that the occurrence of hyperplastic lesions was
dependent on the presence of carbon particles (Ishinishi et al., 1986,
1988; Takaki et al., 1989).
Table 35. Growth in athymic mice of lung tumours from rats exposed to
diesel exhaust and carbon black
Type of tumour Carbon black Diesel exhaust
Number Growing Number Growing
implanted No. % implanted No. %
Adenocarcinom 8 2 25 10 4 40
Squamous-cell 2 1 50 3 2 67
carcinoma
Squamous cyst 19 0 0 7 0 0
From Mauderly et al. (1994)
In a large-scale study of unfiltered diesel exhaust, 1097 Fischer
344 rats were exposed for up to 30 months to 0.35, 3.5, or 7.0 mg/m3
of diesel soot. A group of 365 rats exposed to clean air served as
controls. Exposure to exhaust did not cause overt signs of toxicity,
and no significant, exposure-related alterations in body weight were
observed. Rats were killed at six-month intervals, examined for their
lung burdens of diesel soot, and subjected to complete necropsy.
Diesel soot accumulated at all doses but minimally in the low-dose
group. An increased incidence of tumours was observed in the groups at
the medium and high doses; most (81%) of the tumours were observed
after two years of exposure. The inhaled soot concentration and the
lung burden of diesel soot were highly significantly related to tumour
incidence ( P < 0.001) (Mauderly et al., 1987). In a follow-up study
with longer daily exposure (16 h/day), the dose-dependency of the
tumour incidence was reproduced (Nikula et al., 1994). In this study,
the carcinogenicity of diesel exhaust soot (8% extractable organic
material; 2.5 mg/m3) was compared with that of carbon black (0.12%
extractable organic material; 6.5 mg/m3 ) in 1152 male and female
Fischer 344 rats exposed for 16 h/day on five days per week for up to
24 months and examined histopathologically. The lung burden of
particles was greater after exposure to diesel exhaust than to carbon
black, but the neoplastic response in the lung was similar and usually
occurred late in the study. When the lung burden of particles was used
as the dose parameter, the carcinogenicity of carbon black was
greater than that of diesel exhaust soot.
Groups of 50 four-week-old female Fischer 344 rats were exposed
to diesel soot at a mean concentration of 4.73 mg/m3 for 15 h/day
three times per week for 6, 12, or 24 months, and animals that died
after 18-30 months were evaluated for tumours. After 30 months, all
animals, including controls, were sacrificed and examined for lung
tumours. The incidences of lung tumours were 10.4% in controls and
2.2% after six months' exposure to diesel exhaust, 19.0% after 12
months, and 12.2% after 24 months. No statistical analysis was
presented, but it seems unlikely that there was a significant effect
(Kawabata et al., 1993).
Concurrent administration of known carcinogens with diesel
exhaust has also been studied. Exposure of rats to diesel exhaust
given an intraperitoneal injection of N-nitrosodiisopropylamine or
N-nitrosodiethylamine did not influence the rates of tumours induced
by the nitrosamines (Takemoto et al., 1986). The effects of treatment
of hamsters with N-nitrosodiethylamine or benzo[ a]pyrene were not
influenced by subsequent administration of diesel exhaust, whereas
pretreatment of mice with benzo[ a]pyrene or dibenzanthracene followed
by diesel exhaust gave inconsistent results. Exposure to diesel
exhaust did not affect the total tumour rates in rats pretreated with
6.25 or 12.5 g/kg body weight of N-nitrosodipentylamine; however,
the incidence of squamous-cell tumours was increased in both
pretreated groups (Heinrich et al., 1986a).
Hamsters were exposed for two years to unfiltered diesel exhaust
at 0, 0.7, 2.2, or 6.6 mg/m3 or to filtered diesel exhaust, and
groups of control and treated hamsters were administered
N-nitrosodiethylamine subcutaneously before the beginning of
exposure. The rate of pulmonary tumours was not increased by diesel
exhaust, with or without pretreatment with the nitrosamine. Several
hamsters that developed 'wet tail' infection after six months on test,
resulting in significant mortality (46%), were treated with
oxytetracycline, chloramphenicol, and dimetridazole (Brightwell et
al., 1986, 1989).
Overall, the only clear effect in these studies of
co-carcinogenicity in rats was an increase in the percentage of
malignant tumours in animals treated with diesel exhaust plus
carcinogens in comparison with the carcinogen alone (Heinrich et al.,
1986a).
The results of studies in other species (mice, hamsters, and
monkeys) treated by inhalation are given in Table 36. Heinrich et al.
(1982) found significant increases in lung tumour incidence in mice,
but this result could not be repeated in later studies (Heinrich et
al., 1986a, 1992, 1995). Small but statistically significant increases
in lung tumour incidence were reported in ICR and C57Bl mice (Takemoto
et al., 1986) and in female Sencar mice (Pepelko & Peirano, 1983);
however, because only small increases in non-malignant tumours were
seen only in females in the study of Pepelko & Peirano (1983), the
results must be considered to be weakly positive or equivocal.
Hamsters showed no response to diesel exhaust.
Table 36. Long-term studies of diesel exhaust by inhalation in species other than rats
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI mouse 2 × 96 f (a) Exhaust 4 19 h/d, 5 d/week, Lung tumours (adenomas and Heinrich et al.
(b) Filtered 27-28 months adenocarcinomas) (a) 24/76 (32%), (1986a)
(c) Clean air including 13 (17%) adenocarcinomas;
(b) 29/93 (31%), including 18 (19%)
adenocarcinomas; (c) 11/84 (13%),
including 2 (2.4%) adenocarcinomas
Incidence in historical controls,
< 32%
ICR and 315 ICR, Exhaust or 2.0-4.0 4 h/d, 4 d/week, Lung tumours (adenomas and Takemoto et al.
C57Bl/6N 297 C57Bl/6N clean air 13-28 months adenocarcinomas). ICR: 14/56; (1986)
mice, newborn (sex 7/60 in controls; C57Bl/6N:
unspecified) 17/150; 1/51 in controls
Sencar mouse 2 × 130 f (a) Exhaust 12 8 h/d, 7 d/week, Adenomas and carcinomas Pepelko &
2 × 130 m (b) Clean air to 15 months of (a) m: 5.9%; f: 16.3% Peirano (1983)
age (from (b) m: 3.8%; f: 7.2%
conception to
sacrifice)
NMRI mouse 2 × 80 f (a) Exhaust 7.5 18 h/d, 5 d/week, Lung tumours Heinrich et al.
(b) Clean air 13.5 months, 9.5 (a) 32.1% (1992, 1995)
months' recovery (b) 30%
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI and 3 × 120 f (a) Exhaust 4.5 18 h/d, 5 d/week; Lung tumours Heinrich et al.
C57Bl/6N mice 3 × 120 f (b) Filtered NMRI, 23 months; NMRI: (a) 23.3% (1992, 1995)
(c) Clean air C57Bl, 24 months (b) 46.7%
+ 6 months' (c) 30%
recovery C57Bl: (a) 8.5%
(b) 3.6%
(c) 5.1%
Syrian golden 3 × 48 f (a) Exhaust 3.9 ± 0.5 7-8 h/d, 5 d/week, No effect on survival, no lung Heinrich et al.
hamster (b) Filtered up to 30 months tumours (1982)
(c) Clean air
Syrian golden 3 × 48 f (a) Exhaust 4 19 h/d, 5 d/week, Normal life span, no lung tumours Heinrich et al.
hamster 3 × 48 m (b) Filtered 27-28 months (1986a)
(c) Clean air
Syrian golden 52 f + 52 m (a) Exhaust 0.7, 2.2, 16 h/d, 5 d/week, No significant increase in Brightwell
hamster 104 f + 104 m (b) Filtered 6.6 24 months frequency of tumours in et al.
(controls) (c) Clean air respiratory tract (1986, 1989)
Cat 2 × 25 m (a) Exhaust 6 (1st 8 h/d, 7 d/week, Lung function: decrease in closing Pepelko &
yr), 12 24 months volume after 1 year; reduction Peraino (1983)
(2nd yr) in inspiratory, vital, and total
(b) Clean air lung capacity after 2 years;
diagnosis: pulmonary fibrosis of
the interstitial or
intra-alveolar type
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
Cynomolgus 4 × 15 m (a) Coal dust 2.0 7 h/d, 5 d/week, No difference in tumour incidence Lewis et al.
monkey (b) Exhaust 2.0 24 months among groups (1986)
(c) Coal dust 1.0
+ exhaust 1.0
(d) Clean air
It is clear that repeated inhalation of diesel exhaust at
concentrations of more than about 2 mg/m3 (actual value),
corresponding to a calculated equivalent continuous concentration of
about 1 mg/m3, increases the incidence of pulmonary tumours. These
tumours occur late in life after exposures of 24 months.
B7.3.2.2 Other routes of exposure
Studies have also been conducted by intratracheal instillation in
rats and hamsters and by painting on mouse skin (Table 37). While the
former are especially useful for hazard identification and for
evaluating specific mechanistic aspects, they are less useful for the
purposes of risk characterization and no attempt was made to identify
critical studies. The results of the studies by intratracheal
instillation, however, confirm that both diesel exhaust particles and
carbon black induce lung tumours and demonstrate that the specific
surface area of carbonaceous particles is correlated with the
tumorigenic potency. Both of these findings support the suggestion
that a nonspecific particle effect is of crucial importance for the
induction of lung tumours by diesel exhaust. The dermal experiments,
conducted by Nesnow et al. (1982a,b, 1983), have been used to estimate
risk quantitatively, by the comparative potency method.
B7.4 Dermal and ocular irritation; dermal sensitization
No data were available.
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
B7.5.1 Reproductive toxicity
In a two-generation study of reproduction, 100 male and 100
female CD-1 mice were exposed by inhalation to exhaust from a
light-duty diesel engine for 8 h/day on seven days per week, at a
concentration of 12 mg/m3. Most treatment-related effects were
minimal. Overall fertility and survival rates were not significantly
altered (Pepelko & Peirano, 1983).
(C57Bl/6 × C3H)F mice (number not given) showed sperm
abnormalities (reduced sperm count and weight of testis;
teratospermia) after daily intraperitoneal injections of 50, 100, or
200 mg/kg body weight of diesel exhaust particles for five days. The
highest dose caused an eightfold increase in abnormalities over that
in controls and a significant decrease in sperm number (Quinto & De
Marinis, 1984).
Table 37. Carcinogenicity of diesel exhaust after exposure other than by inhalation
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Fischer 344 (a) 31 f Intratracheal (a) Activated carbon 1/animal 10/week, Survival rate, 71-83% Kawabata
rat (b) 59 f instillation (b) Diesel particles 1/animal 30 months' (lowest ingroup b). Malignant et al.
(c) 53 f (c) None observation lung tumours: (a) 7/23; (1986)
(d) 27 f (d) Vehicle (b) 20/42; (c) 0/44; (d) 1/23;
P < 0.01. Benign and
malignant tumours combined:
(a) 11/23; (b) 31/42
Osborne- Groups Lung Organic material from Observation (a) 1 bronchioalveolar adenoma; Grimmer
Mendel rat of 35 f implant diesel exhaust: until (b) 5 squamous-cell carcinomas; et al.
(a) Hydrophilic fraction 6.7 spontaneous (c) 1 bronchioalveolar adenoma; (1987)
(b) Hydrophobic fraction 20.0 death (d) 6 carcinomas;
(c) Nonaromatic compounds 19.2 (e) No tumours
+ PAHs (2 or (f) 1 carcinoma
3 rings) (g) 7 carcinomas, 1 adenoma
(d) PAHs ( > 4 rings) 0.2 (h) No tumours
(e) Polar PAHs 0.3 (i) 1 adenoma
(f) Nitro-PAHs 0.2
(g) Reconstituted
hydrophobic fraction 19.9
(h) None
(i) Vehicle
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Wistar rat (a) 40 f Intratracheal (a) Diesel soot (34 m2/g) (a) 3 × 15 Primary lung tumours (%) Pott &
(b) 58 f instillation (b) Diesel soot (70 m2/g) (b) 3 × 10 (a) 65 Roller
(c) 38 f (c) Diesel soot (70 m2/g) (c) 3 × 20 (b) 60 (1994);
(d) 37 f (d) Carbon black (270 (d) 3 × 15 (c) 66 Pott et
m2/g) (d) 65 al.(1994)
(e) 37 f (e) Activated charcoal (e) 3 × 10
(f) 39 f (860 m2/g) 3 × 20 (e) 27
(g) 40 f (f) NaCl solution (f) 3 × 10 (f) 0
(g) NaCl solution (g) 0.4 × 20 until (g) 0
ml spontaneous
death or 131
weeks
Wistar rat Groups Intratracheal (a) Diesel soot (native) (a) 1 × 15 Primary lung tumours (%): Heinrich
of 48 f instillation (b) Diesel soot (toluene (b) 2 × 15 (a) 17 (1994)
extract; 130 m2/g) 1 × 15
(c) Carbon black (c) 1 × 15 (b) 23
(toluene extract; (d) 1 × 15 4
270 m2/g; primary (e) Vehicle control (c) 21
particle, 15 nm)
(d) Carbon black (toluene (d) 8
extract; 270 m2/g; (e) 0
primary particle,
15 nm)
(e) NaCl solution
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Syrian Groups Intratracheal (a) Diesel particles 1.25, 1/week, 15 1 lung adenoma at high dose Shefner
golden of 50 m; instillation (b) Diesel particles + 2.5, in groups (a) and (c) after 61 et al.
hamster various same amounts of weeks weeks; no lung tumours in (1982
controls ferric oxide controls
(c) Diesel particle
extract
+ ferric oxide
Syrian (a) 3 × Intratracheal (a) Exhaust extract 0.1, 0.5, 1/week, 15 Survival rates: (a) 95, 92, Kunitake
golden 62 m instillation (b) Vehicle 1 weeks 71%; (b) 98%. No difference in et al.
hamster (b) 59 m (c) 0.5 mg tumour incidence between (a) (1986)
(c) 62 m benzo[a]pyrene and (b); 88% respiratory
tumours in positive controls
C57Bl 12 m, Dermal Acetone extract of 0.5 ml 3 times/week 16 mice dead by 10 weeks, 33 Kotin
mouse 40 f, particles for life or skin tumours by 13 months, 2 et al.
69 22-23 months skin papillomas, no tumours in (1955)
controls controls
A mouse 50 m, Dermal Acetone extract of 0.5 ml 3 times/week Males: 8 skin tumours by 16
25 f, particles for life or 1 papilloma, 3 squamous-cell
34 22-23 months carcinomas. Females: 20 skin
controls tumours by 13 months,17 tumours
at 13-17 months, no tumours in
controls
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Sencar Groups Dermal Dichloromethane 0.1, 0.5, 1/week, 50-52 Skin carcinomas: Nesnow
mouse of 40 m extracts of particles 1.0, 2.0, weeks Engine A: 3% (m), 5% (f) at et al.
+ 40 f from engines (A, B, E) or 4.0 4 mg Engine B: 3% (m) at 0.5 mg (1982a,b,
Benzo[a]pyrene 12.6- Engine E: 3% (f) at 0.1 mg 1983)
202 µg Positive control: 10-90%
C57Bl/6N Groups Subcutaneous Particles in olive oil 10, 25, 1/week, 5 First tumours palpated in: Kunitake
mouse of injection containing 5% dimethyl 50, 100, weeks; 18 week 47 at 25 mg/kg bw et al.
15-30 f sulfoxide 200, or months' week 30 at 50 mg/kg bw (1986)
500 mg/kg observvation week 27 at 100 mg/kg bw
bw week 39 at 200 or 500 mg/kg bw
Malignant fibrous histiocytomas
in 5/22 mice at 500 mg/kg bw;
0/38 in controls
PAH, polycyclic aromatic hydrocarbon; TPA, 12-O-tetradecanoylphorbol 13-acetate
Groups of 15 male cynomolgus monkeys were exposed by inhalation
to 2 mg/m3 diesel particulates for 7 h/day on five days per week for
two years. Sperm motility and velocity were similar to those of
controls (Lewis et al., 1989).
In a test for dominant lethal mutation, male Fischer 344 rats
inhaled 2 mg/m3 diesel exhaust for 7 h/day on five days per week for
six months and were subsequently mated with untreated females. Live
and dead implants and preimplantation losses were analysed on days
19-20 of gestation: no significant effects were observed (Lewis et
al., 1989). In a similar test, 100 male and 54 female T stock mice
were exposed to 6 mg/m3 diesel exhaust for 8 h/day on seven days per
week for seven weeks. There were no significant dominant lethal
effects in males or females. A reduced number of corpora lutea
(reproductive function) was the only significant result (Pepelko &
Peirano, 1983).
B7.5.2 Embryotoxicity
No increase in the frequency of sister chromatid exchange was
seen in the livers of fetuses of Syrian golden hamsters that inhaled
diesel exhaust particles at 12 mg/m3 for 8 h/day on days 5-13 of
gestation. An intraperitoneal injection of 300 mg/kg body weight of
diesel particulates on day 12 of gestation also had no significant
effect on this end-point; however, intraperitoneal injection of 23%
particulate mass extracted with methylene chloride on day 12 resulted
in a dose-dependent increase in sister chromatid exchange frequency in
fetal liver on day 13, and a doubling was seen at 320 mg/kg body
weight. It was concluded that chemicals must be eluted from diesel
particles in order for the genotoxic material to cross the placenta
(Pereira et al., 1982).
B7.5.3 Teratogenicity
Sprague-Dawley rats and New Zealand white rabbits were exposed by
inhalation to exhaust from a light-duty diesel engine, at a
particulate matter concentration of 6 mg/m3, for 8 h/day on seven
days per week.
Twenty rats were exposed on days 5-16 of gestation and 20 rabbits
on days 6-18. The numbers of viable fetuses per litter, dead fetuses
per litter, resorptions per litter, implantation sites per litter,
corpora lutea per litter, and average fetal weight did not differ
significantly from those of controls (Pepelko & Peirano, 1983).
Reproductive and developmental toxicity are considered unlikely
to be critical end-points for diesel exhaust.
B7.6 Mutagenicity and related end-points
B7.6.1 In vitro
The genetic effects of particles and particle extracts have been
described (Lewtas & Williams, 1986; Morimoto et al., 1986; Henschler,
1987). Diesel exhaust extracts rather than particles were used in most
of these studies. Point mutations were observed in vitro in bacteria
and mammalian cells. Extracts caused chromosomal aberrations, DNA
damage, sister chromatid exchange, and cell transformation (Table 38).
The mutagenic potency of diesel engine exhaust depended in several
studies on the characteristics of the diesel fuel and the type, age,
and operating conditions of the engine (Henschler, 1987). As a rule,
organic extracts of particles were mutagenic in the absence of an
exogenous metabolic activating system (S9) (Ball et al., 1990);
addition of S9 decreased (Lewtas, 1983) or suppressed (Morimoto et
al., 1986) the mutagenic activity, perhaps by increasing protein
binding of mutagenic material or by metabolic detoxification of
directly acting mutagens, such as the nitroarenes, in the Ames test.
It can therefore be concluded that PAHs and thioarenes, which must be
metabolically activated, do not account for the mutagenic potency of
diesel particles. Salmeen et al. (1985) and Beland et al. (1985)
showed that nitroarenes are the main genotoxic agents in Salmonella
typhimurium T98 in the absence of S9. A number of other
investigators have reported the presence of nitrated PAHs in extracts
of diesel engine exhaust particles (Handa et al., 1983), including
many three-, four-, and five-ring structures (Schuetzle et al., 1982).
The concentration of 1-nitropyrene, one of the prevalent species, has
been reported to be 15-25 mg/kg particulate matter, whereas
benzo[ a]pyrene has been found at concentrations up to 50 mg/kg. The
nitrated PAHs are important in the health effects of diesel exhaust
since they are effective mutagens in microbial and human cell systems
(Patton et al., 1986). Some nitrated PAHs are also carcinogenic in
animals (Imaida et al., 1991). Ball & Young (1992) found that strain
T102, which does not respond to the mutagenic action of nitroarenes,
responds to a class of oxidizing compounds that can interact with DNA;
these compounds are not nitroarenes or typical PAHs.
The effects of diesel soot particles with and without adsorbed
organic substances, diesel soot extract without particles, an isolated
fraction of PAHs, and titanium dioxide and carbon black (Printex 90)
as reference particles were investigated in vitro on hamster lung
epithelial cells (Mohr & Riebe-Imre, 1992). The substances were added
to the cells at concentrations of 100-300 mg/litre. Diesel soot
extract, but not the PAH moiety isolated from the extract, stimulated
mixed-function oxygenases. The diesel soot extract was more cytotoxic
than the PAH fraction for the epithelium of the hamster respiratory
tract, but both mixtures induced the development of micronuclei. Under
the study conditions, only the PAH fraction led to transformation of
cells. The respiratory epithelium of hamsters was more sensitive than
human lung epithelial cells investigated in parallel, perhaps because
hamster cells are generally more easily transformed than human cells.
Diesel soot particles, titanium dioxide, and Printex 90 had hardly any
cytotoxic effect but triggered transformation. The authors concluded
that a combination of effects of particles and PAHs are responsible
for the degeneration of cells.
Schiffmann & Henschler (1992) studied the effects of diesel soot
extract and isolated fractions of PAHs, oxy-PAHs, and nitro-PAHs on
hamster embryo fibroblasts in vitro. The end-points investigated
were induction of micronuclei, unscheduled DNA synthesis, and cell
transformation. Diesel soot extract induced micronuclei at
concentrations of 80-400 mg/litre, whereas the fractions of various
PAHs were strongly cytotoxic. Diesel soot extract and the PAH fraction
transformed the fibroblasts; the nitro- and oxy-PAH fractions had a
weaker transforming effect, but their cytotoxic potency was clearly
stronger. Kinetochore analysis showed a high percentage (37%) of
chromosomes in micronuclei. No unscheduled DNA synthesis was induced.
Micronuclei were also induced in human embryonic lung fibroblasts
treated with diesel soot extract under comparable experimental
conditions.
B7.6.2 In vivo
The genotoxicity of total diesel exhaust, diesel particles, and
diesel soot extracts in vivo has been investigated in somatic cells
(Table 38). All of the substances induced genotoxic responses. Total
exhaust induced only sister chromatid exchange in Syrian golden
hamsters. The frequency of micronuclei was not increased significantly
in mice, even at high doses (up to 640 mg/kg body weight). Organic
extracts had clear genotoxic effects in various assays (Henschler,
1987).
No mutagenic activity was found in urine taken from rats exposed
to diesel exhaust emission by inhalation at 2 mg/m3 for 7 h/day on
five days per week for up to two years. A slight but nonsignificant
increase in micronuclei was observed in the bone marrow of mice that
had been exposed for six months; no increase in micronuclei was
detected in rats over a 24-month period (Ong et al., 1985).
In studies of heritable mutations in T stock mice, males were
examined for point mutations, induction of dominant lethal mutations,
translocations, and spermatogonial survival; females were examined for
oocyte death and dominant lethal mutations. No significant effect was
seen on germ cells. A reduced number of corpora lutea (reproductive
function) was the only significant result (Pepelko & Peirano, 1983).
B7.6.3 DNA adduct formation
Extensive research has been done to determine whether the DNA
adducts induced in lungs by diesel exhaust are related to later
tumorigenesis.
In rats that inhaled benzo[ a]pyrene absorbed to carbon black
(20 mg/g), an adduct of the diol epoxide with deoxyguanosine was
identified (Wolff et al., 1989), demonstrating that epoxides of PAHs
are produced by oxidative metabolism. Haemoglobin and albumin adducts
were also investigated.
Methods have been developed to detect DNA adducts of
1-nitropyrene or 1,6-dinitropyrene, which are markers of diesel engine
exhaust. The adducts are analysed in rat tissue or peripheral blood
lymphocytes by the 32P-postlabelling method and may be useful for
the dosimetry of 1-nitropyrene or diesel exhaust particulates in
occupational settings (El-Bayoumy et al., 1994). Studies on adduct
formation in lung DNA induced by inhaled diesel exhaust have been
conducted by Wong et al. (1986), Wolff et al. (1990), Bond et al.
(1988, 1989, 1990a,b), and Gallagher et al. (1993, 1994), all
involving the 32P-postlabelling technique. In general, these studies
suggest that DNA adducts are good measures of the 'effective dose' of
carcinogenic compounds.
Wong et al. (1986) found increased DNA adduct formation in the
lungs of Fischer 344 rats exposed to diesel exhaust particles at
7.1 mg/m3 for 31 months. After Fischer 344 rats and Syrian golden
hamsters were exposed to dilutions of diesel engine exhaust for six
months to two years, the level of haemoglobin adducts (2-hydroxy-
ethylvaline and 2-hydroxypropylvaline) increased dose-dependently,
corresponding to metabolic conversion of 5-10% of inhaled ethylene and
propylene to their oxides (Törnqvist et al., 1988).
Bond et al. (1988) designed experiments to determine the location
of DNA adducts in the respiratory tract of rats exposed to exhaust.
Fischer 344 rats were exposed for 12 weeks to diesel exhaust soot at
10 mg/m3; they were then sacrificed, and various regions of the
respiratory tract were removed and analysed for DNA adducts. Adducts
were found only in peripheral lung tissue and nasal tissue; the total
levels were highest in the peripheral tissue (about 18 adducts per 109
bases). Thus, the levels of total DNA adducts and exhaust-induced
adducts are highest in the region of the rat respiratory tract where
tumours are formed after exposure to a carcinogenic concentration of
diesel exhaust (Mauderly et al., 1987).
Bond et al. (1990c) exposed groups of Fischer 344 rats to soot at
0.35, 3.5, 7.0, or 10 mg/m3 for 12 weeks and found that the levels
of DNA adducts were similar, at about 14 adducts per 109 bases. This
level is nearly twice as high as that found in sham-exposed rats.
Thus, DNA adduct formation in lungs is independent of the
concentration of exhaust at the levels tested. One explanation for
these results (Bond et al., 1990a) is that the lung enzymes
responsible for the metabolism of soot-associated chemicals to
metabolites that bind to DNA were saturated at the concentrations
tested. These data also suggest that other factors are important in
the carcinogenicity of diesel exhaust, since the number of adducts was
elevated at an exposure level (0.35 mg/m3) that did not increase
lung tumour incidence. The induction of DNA adducts at low
concentrations is likely to result in tumour initiation by the organic
compounds present; at higher concentrations, the particles will induce
cell death, and subsequent proliferation may well act as a promotional
event. The combination may then result in detectable increases in
tumour incidence.
Bond et al. (1990a) also investigated the time course for DNA
adduct formation and persistence. Fischer 344 rats were exposed to
soot at 7 mg/m3 for up to 12 weeks and were sacrificed at 2, 4, 8,
12, 14, and 16 weeks after the start of exposure. DNA adducts
accumulated slowly in the lung, and the number was highest at the end
of exposure, representing about 160% of the level seen in controls
exposed to air only. The levels declined rapidly after termination of
exposure and were not significantly different from those of controls
four weeks later. Thus, steady-state levels of DNA adducts would be
reached during long-term exposure to diesel exhaust.
Bond et al. (1989, 1990c) and Wolff et al. (1990) investigated
whether exposure to carbon particles without associated mutagenic
organic chemicals also increases DNA adduct levels. Rats were exposed
either to diesel exhaust or carbon black at 0, 3.5, or 10 mg/m3 for
12 weeks. Solvent-extractable organic compounds made up about 30% of
the diesel soot but only about 0.04% of the carbon black particles.
Exposure to the highest levels of both carbon black and diesel exhaust
increased the DNA adduct levels in lungs, although the levels in rats
exposed to diesel exhaust were about 30% higher than those in rats
exposed to carbon black. Heavy exposure to carbon particles can
therefore increase DNA adduct formation, although the concentrations
are lower than those of exhaust-related adducts.
Bond et al. (1990b) investigated whether DNA adducts could be
formed in specific cells of the rat lung, i.e. alveolar type II cells.
After exposure of rats to 6.2 mg/m3 diesel exhaust or carbon black
particles for 12 weeks (16 h/day on five days per week), type II cells
were isolated and their DNA analysed for adducts. Both diesel exhaust
and carbon black resulted in about a fourfold increase in total DNA
adducts in type II cells. DNA adduct levels in peripheral lung tissue
were increased by 60-80% in male and female Fischer 344 rats and
female cynomolgus monkeys but not in female B6C3F1 mice or female
Syrian hamsters after inhalation of diesel soot at 8.1 mg/m3 for 12
weeks (Bond et al., 1989, 1990c).
Gallagher et al. (1993, 1994) exposed female Wistar rats to
diesel soot at 7.5 mg/m3 and to carbon black at 11.3 mg/m3 for 24
months and measured DNA adducts in lungs. The mean adduct levels were
similar in the two groups but were not significantly greater than
those in controls. Time-course studies indicated that the levels in
the lungs of rats exposed to diesel exhaust were lower at 24 months
than after two or six months of exposure, presumably as a result of
dilution due to increased cell proliferation. The level of a single
DNA adduct, thought to be derived from a nitro-PAH in diesel exhaust
and not observed in rats exposed to carbon black or titanium oxide,
was elevated over that in controls after two, six, and 24 months. The
DNA adduct levels in control rats increased over the duration of the
24-month study as an effect of age.
Gallagher et al. (1993) compared DNA adduct formation after
exposure to diesel emissions in vitro and in vivo. After exposure
of human lymphocytes to diesel extract, five major DNA adducts were
detected, one of which was characterized as an adduct with
benzo[a]pyrene. One was also detectable in reaction products of calf
thymus DNA and diesel particle extract in vitro and in skin and lung
DNA from mice treated dermally with 50 mg diesel extract in vivo. The
differences in DNA patterns in vitro and in vivo (skin and lung)
may reflect differences in metabolic pathways.
Taken together, the studies of DNA adducts suggest that some
organic chemicals in diesel exhaust can form DNA adducts in lung
tissue and may play a role in the carcinogenic effects. As pointed out
by Bond et al. (1989), however, DNA adducts alone cannot explain the
carcinogenicity of diesel exhaust, and other factors, such as chronic
inflammation and cell proliferation, are also important.
B7.7 Special studies
B7.7.1 Immunotoxicity
During the clearance process, one possible pathway is
translocation of diesel particulates into the lymphatic channels.
After male guinea-pigs were exposed to diesel exhaust at a particle
concentration of 1.5mg/m3 for four or eight weeks, the B- and T-cell
counts in lymph nodes were not altered, and there were no significant
changes in blood or spleen (Dziedzic, 1981).
Fischer 344 rats were exposed to 2 mg/m3 diesel exhaust for
7h/day on five days per week for 12 or 24 months and the immunological
function of splenic B and T cells was measured by enumerating
antibody-producing cells in the spleen or monitoring the proliferative
response. No changes were observed (Mentnech et al., 1984).
Table 38. Genotoxicity of diesel exhaust, particles, and extracts
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity
Bacteria
S. typhimurium Point mutation (his) + + Huisingh et al. (1978)
S. typhimurium Point mutation (his) + + Loprieno et al. (1980)
S. typhimurium Point mutation (his) + Clark & Vigil (1980)
S. typhimurium Point mutation (his) + + Li & Royer (1982)
S. typhimurium Point mutation (his) + Salmeen et al. (1984)
S. typhimurium Point mutation (his) + Ong et al. (1985)
S. typhimurium Point mutation (his) + Bechtold et al. (1986)
S. typhimurium Point mutation (his; + Whong et al (1986)
SOS umu test)
S. typhimurium Point mutation (his) + Wallace et al. (1987)
S. typhimurium Point mutation (his) +a Wallace et al. (1990)
S. typhimurium Point mutation (his) + Lewis et al. (1989)
S. typhimurium Point mutation (his) + Rasmussen (1990)
S. typhimurium Point mutation (his) + + Keane et al. (1991)
S. typhimurium, E. coli Point mutation (trp) + Crebelli et al. (1991)
E. coli Point mutation (trp) + Lewtas (1983)
Mammalian cells
L5178Y mouse Point mutation (tk) + Lewtas (1983)
lymphoma cells
L5178Y mouse Point mutation (tk) + Mitchell et al. (1981)
lymphoma cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity (contd)
Chinese hamster Point mutation (hprt) + Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Casto et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt, + Morimoto et al. (1986)
lung V79 cells ATPase)
Human xeroderma Point mutation (hprt) + + McCormick et al. (1980)
pigmentosum fibroblasts
Human lymphoblast Point mutation (tk) + Barfknecht et al. (1981)
TK6 cells
Balb/c3T3 mouse Point mutation (ATPase) + (+) Curren et al. (1981)
fibroblasts
DNA damage
Syrian hamster DNA chain breaks - Casto et al. (1981)
embryo cells (alkaline elution)
Human xeroderma DNA damage + + McCormick et al. (1980)
pigmentosum fibroblasts
Rat primary Unscheduled DNA + Lewtas (1983)
hepatocytes synthesis
Chinese hamster lung Unscheduled DNA +a + Gu et al. (1994)
V79 cells synthesis
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Chromosomal effects
Chinese hamster Sister chromatid exchange + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations +b Hasegawa et al. (1988)
lung V79 cells -c
Chinese hamster Sister chromatid exchange + Hasegawa et al. (1988)
lung V79 cells
Chinese hamster Sister chromatid exchange + + Keane et al. (1991)
lung V79 cells
Human lymphocytes Chromosomal aberrations + Lewtas (1983)
Chinese hamster Sister chromatid exchange + Morimoto et al. (1986)
lung V79 cells
Human lymphocytes Sister chromatid exchange (+) Tucker et al. (1986)
Human lymhoblastoid Sister chromatid exchange + Morimoto et al. (1986)
cells
Hamster embryo Micronuclei + Schiffmann & Henschler
fibroblasts (1992)
Chinese hamster lung Micronuclei +a + Gu et al. (1992)
V79 and ovary cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Cell transformation
Balb/c3T3 mouse fibroblasts Cell transformation (+) Curren et al. (1981)
Balb/c3T3 mouse fibroblasts Cell transformation +b Hasegawa et al. (1988)
(+)c
Hamster lung epithelial cells Cell transformation + + Mohr & Riebre-Imre (1992)
Hamster embryo fibroblasts Cell transformation + Schiffmann & Henschler (1992)
In vivoa
Mouse Micronuclei, bone marrow - - + Pereira (1982)
Mouse Micronuclei, bone marrow - Lewis et al. (1989)
Mouse Micronuclei, bone marrow - Morimoto et al. (1986)
Mouse Micronuclei, bone marrow - - - Pepelko & Peraino (1983)
Mouse Micronuclei, bone marrow (+) Ong et al. (1985)
Rat Micronuclei, bone marrow - Ishinishi et al. (1988)
Rat Micronuclei, bone marrow - Ong et al. (1985)
Chinese hamster Micronuclei, bone marrow (+) - - Pepelko & Peraino (1983)
Mouse Sister chromatid exchange, - + + Pereira (1982)
bone marrow
Rat Sister chromatid exchange, - Ishinishi et al. (1988)
bone marrow
Rat Sister chromatid exchange, - Morimoto et al. (1986)
bone marrow
Rat Sister chromatid exchange, - Ong et al. (1985)
peripheral blood leukocytes
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Rat Sister chromatid exchange, - Lewis et al. (1989)
lymphocytes
Syrian golden Sister chromatid exchange, + Pereira (1982)
hamster lung cells (inhalation)
Syrian golden Sister chromatid exchange, + Guerrero et al. (1981)
hamster lung cells (instillation)
Syrian golden Sister cromatid exchange, + + Pereira (1982)
hamster lung cells (instillation)
Syrian golden Sister chromatid exchange + Morimoto et al. (1986)
hamster liver cells (transplacental)
Syrian golden hprt mutation + Morimoto et al. (1986)
hamster (transplacental)
Mouse Mutation, S. typhimurium - Morimoto et al. (1986)
(host-mediated assay)
Mouse Heritable effects on germ - Pepelko & Peirano (1983)
cells (various assays; m/f)
Drosophila Heritable effects (sex-linked Schuler & Niemeier (1981)
melanogaster recessive lethal mutation) -
his, histidine independence; trp, tryptophane independence; hprt, hypoxanthine-guanine-phosphoryltransferase (8-aza- or
6-thioguanine resistance); tk, thymidine kinase (bromodeoxyuridine or trifluorothymidine resistance); ATPase, Na+/K+-ATPase
(ouabain resistance); +, positive; (+), weakly positive
a Dispersed in artificial surfactant
b Light-duty engine
c Heavy-duty engine
d Modified from Henschler (1987) and supplemented
Fischer 344 rats and CD-1 mice were exposed to diesel exhaust
particles at 0.35, 3.5, or 7 mg/m3 for 6, 12, 18, or 24 months to
investigate whether the accumulation of diesel particulates in lymph
nodes (indicated by black discolouration) influences the subsequent
response to immunization by sheep red blood cells, evaluated by the
presence of immunoglobulin (Ig) M antibody-forming cells. In rats, the
total number of lymphoid and antibody-forming cells was significantly
increased at the medium and high aerosol concentrations. In mice, the
number of lymphoid cells was increased only at the high concentration.
The authors concluded that diesel exhaust particles have only a
minimal effect on the immune and antigen filtration functions in
lung-associated lymph nodes because the relative numbers of
antibody-forming cells and specific IgM, IgG, and IgA antibodies in
rat sera were not significantly changed (Bice et al., 1985).
Five intranasal inoculations of various doses of a suspension of
diesel engine exhaust particles in ovalbumin were administered to BDF1
mice at intervals of three weeks. Ovalbumin IgE antibody titres,
assayed by passive cutaneous anaphylaxis, were enhanced by doses as
low as 1 µg (Takafuji et al., 1987, 1989). Similarly, primary IgE
responses were increased after intraperitoneal administration to mice
of ovalbumin or cedar pollen allergen mixed with diesel exhaust
particulates (Muranaka et al., 1986).
Female CR/CD-1 mice were exposed by inhalation to 6-7 mg/m3
diesel exhaust for 2-6 h (acute), 8 h/day for 2-16 days (subacute),
or 8h/day for about 300 days (chronic). Immediately after the end
of exposure, animals were exposed to the infectious aerosols
S. typhimurium, Streptococcus pyogenes, or A/PR8-34 influenza virus.
Increased susceptibility to post-infection mortality was seen with the
bacterial but not the viral pathogens. Nitrogen dioxide and acrolein
vapour had specific effects (Campbell et al., 1981b).
CD-1 mice were exposed to 2 mg/m3 diesel exhaust for one to six
months. After three months of exposure and subsequent infection with
influenza virus, an enhanced severity of response was seen, involving
significant lung consolidation and focal macular collections of
particle-laden macrophages (Hahon et al., 1985).
B7.7.2 Behavioural effects
Sprague-Dawley rats were exposed for 8 h/day on seven days per
week for 16 weeks to diesel exhaust diluted to a particulate matter
concentration of 6 mg/m3. Spontaneous locomotor activity, measured
weekly on Wahman LC-34 running wheels, was significantly decreased
during 8, 9, 11, and 12 weeks of exposure. In rats exposed for
20 h/day on seven days per week for six weeks, forced activity on a
motorized treadmill was measured during the last week. Exhaustion
occurred in less than half the time that it occurred in control
animals. Groups of 10 rats were exposed to diesel exhaust at 6 mg/m3
for 20 h/day on days 1-7 post partum and were then held in clean air
until 15 months of age. In bar pressing acquisition training carried
out at five-day intervals for the next 42 days, the rate of learning
was much slower than in control rats. The difference was highly
statistically significant (Pepelko & Peirano, 1983).
As these three studies were conducted at a high concentration,
6 mg/m3, the practical consequences of the effects observed are not
known. The results, while limited in scope, indicate, however, that
behavioural and neurophysiological effects may be important toxic
end-points which should be investigated further.
B7.8 Factors that modify toxicity; toxicity of metabolites
Little information is available, but chemicals and other factors
may modify the toxicity of diesel emissions. For instance,
chlorophyllin, a derivative of the green pigment chlorophyll, has been
reported to inhibit or reduce the mutagenic activity of diesel
particulate extracts in the Ames test (Ong et al., 1986).
B7.9 Mechanisms of toxicity; mode of action
It is not clear whether DNA-reactive or non-DNA-reactive
mechanisms, or a combination of the two, are responsible for the
carcinogenic action of inhaled diesel exhaust in laboratory animals.
The results of several studies indicated that many PAHs (e.g.
benzo[ a]pyrene) cause a carcinogenic response in rodents, but it is
not known whether the same PAHs when associated with diesel exhaust
also induce a carcinogenic response. One hypothesis for the mechanism
of the tumorigenic response is that organic chemicals (e.g. PAHs,
nitro-PAHs) desorb from soot particles, are metabolized to reactive
metabolites, and interact with lung DNA to initiate carcinogenesis.
This would be a predominantly DNA-reactive mechanism. The observation
that organic chemicals associated with diesel soot can be metabolized
by lung cells to metabolites that can form DNA adducts after long-term
exposure to diesel exhaust supports this hypothesis. Studies with
carbon black essentially devoid of PAHs but which also form DNA
adducts do not, however, corroborate the PAH-DNA-reactive concept.
Another hypothesis is that the lung tumours that arise in rats
exposed to high levels of diesel exhaust are due to overloading of the
normal lung particle clearance mechanisms, accumulation of soot
particles, and cell damage followed by regenerative cell
proliferation. Enhanced cell proliferation may increase the mutation
frequency of key target genes. In this hypothesis, genotoxic chemicals
may not be causative factors in tumorigenicity. This view is supported
by the observations that lung cancer can be induced in rodents by
inhalation of highly insoluble particles of low toxicity that are
virtually devoid of organic chemicals (e.g. talc, carbon black, coal
dust, titanium dioxide), although high concentrations of these
particles (overload) are typically necessary.
A third hypothesis, which draws upon the above two mechanisms, is
that carcinogenesis is initiated by exposure to organic compounds
associated with diesel soot and promoted by the chronic inflammation,
cytotoxicity, and cell proliferation arising from the high
concentrations of particles deposited and retained in the lungs.
Again, however, the tumorigenic response observed with particles alone
(carbon black, titanium dioxide) does not support this hypothesis.
A key consideration is the relative contribution of different
mechanisms at different levels of exposure. As discussed later in this
section, high concentrations of particles, including diesel exhaust,
leading to high lung burdens, compromise normal clearance mechanisms.
Therefore, certain mechanisms may be invoked at high lung burdens that
may not occur at lower lung burdens. Genotoxic aromatic compounds may
take on increasing importance at lower concentrations.
Figure 3 is a diagram outlining the possible mechanism of action
of diesel exhaust, including the effects of overload conditions.
B7.9.1 Carcinogenic effects
B7.9.1.1 DNA-reactive mechanisms
Diesel exhaust contains hundreds of chemicals, including PAHs,
nitro-PAHs, alkyl-PAHs, oxy-PAHs, oxy-nitro-PAHs, and aldehydes
(Scheepers & Bos, 1992b), many of which are known mutagens and
carcinogens in laboratory animals. For example, inhaled
benzo[ a]pyrene (in tar and pitch) is carcinogenic in rat lung
(Heinrich et al., 1994). Nitro-PAHs are potent mutagens and, in some
instances, carcinogenic. In pharmacokinetic studies cited in this
monograph, clearance of PAHs and nitro-PAHs associated with the diesel
particulate fraction is retarded or delayed. Other studies have shown
clearly that the organic compounds associated with diesel soot are
bioavailable to lung cells. Mechanisms for the delayed clearance of
the PAHs have been discussed (Bond et al., 1986b; Gerde et al.,
1991b). PAHs require metabolic activation to metabolites (e.g.
epoxides) that can potentially bind to DNA. In the case of the PAHs
associated with diesel soot, it is likely that alveolar lung cells
(e.g. type II cells) are responsible for their metabolic activation,
so that they can bind to lung cell DNA (Bond et al., 1983).
Additional evidence for a DNA-reactive mechanism in the
carcinogenicity of diesel exhaust is that exposure of laboratory
animals, including rats and monkeys, to diesel exhaust results in the
formation of DNA adducts in lung cells (see section B7.6). DNA adducts
and subsequent DNA replication can result in mutations that play a key
Retention of diesel particles in the lungs and lymph nodes has
been reported in Fischer 344 and Wistar rats after long-term
inhalation (Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly
et al., 1987; Strom et al., 1988; Creutzenberg et al., 1990; Strom et
al., 1990). Some results with regard to lung burden are included in
Table 33. Because clearance of lung burdens above about 1 mg per lung
was impaired after long-term, continuous inhalation of a concentration
equivalent to about 0.5mg/m3, no steady-state lung burden was found
at serial sacrifice at up to two years. About 20% of the lung burden
of diesel particles was found in lung-associated lymph nodes at that
time.
Mathematical models have been developed to calculate the
retention of diesel particles in rats. Yu & Yoon (1990) and Yu et al.
(1991) developed a dosimetry model to predict the retention of diesel
particles and associated organic compounds in rats and humans of
different ages. The model indicates that lung clearance would not be
impaired in humans exposed to a concentration < 0.05 mg/m3 for
24 h/day. The highest lung burden per unit of lung weight was
calculated for five-year-old children. This model was used to assess
the risk of inhaling diesel particles (Pepelko & Chen, 1993).
B6.3 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
The clearance of particles from ciliated airways by the
mucociliary escalator after bronchial deposition is virtually complete
within 24 h. For soluble organic material like PAHs on the surface of
carbon particles, diffusion-limited clearance through the airway
epithelium to capillary blood was found to be slower in the bronchial
region than in the alveoli (Gerde et al., 1991a, 1993a,b).
PAHs in diesel soot adhere strongly to the surface of the
particles. Several studies have shown that the association of PAHs
with particles significantly slows the clearance of the PAHs from
lungs in comparison with the clearance of inhaled PAHs unassociated
with particulate matter.
After inhalation by Fischer 344 rats of 3H-benzo[ a]pyrene
adsorbed onto diesel soot (0.1% by mass) for 30 min, biphasic lung
clearance was noted. In a first, fast phase, about 50% of the inhaled
compound was cleared with a half-time of about 1 h, predominantly by
mucociliary clearance. At the end of exposure, about 15% of the 3H
label was found in blood, liver, and kidney, indicating rapid, direct
absorption of this fraction of 3H-benzo[ a]pyrene and its metabolites
into blood. The remaining 50% was retained in the lung, with a
half-time of 18 days; this is one-third of the retention half-time of
diesel particles. Thus, removal of benzo[ a]pyrene and its metabolites
from the particle carrier may be the rate-limiting step of this
clearance process (Sun et al., 1984). In contrast, more than 95% of
pure 3H-benzo[a]pyrene was cleared from the lung within 12 h after
inhalation (Sun et al., 1982).
These results were confirmed and extended to another PAH,
1-nitropyrene (Bond et al., 1986a,b), when the clearance of
14C-labelled compound adsorbed to diesel soot was determined by the
same method in Fischer 344 rats. After exposure to 490 ng/litre of
pure labelled compound, about 90% was cleared in the first, fast
clearance phase, with a retention half-time of 1 h; after exposure to
650 ng/litre of 14C-1-nitropyrene adsorbed onto diesel particles,
45% of the labelled compound was cleared from the lungs in the first
phase, with a half-time of about 2 h. The remaining 55% was retained
with a half-time of 36 days, which was twice the corresponding value
for 3H-benzo[ a]pyrene in the study of Sun et al. (1984).
On the basis of their data on the clearance of 3H-benzo[ a]pyrene
and 14C-1-nitropyrene, Bond et al. (1986b) predicted equilibrium
lung burdens in humans after long-term inhalation. Assuming inhalation
of 3.5 g/m3 of particles containing 0.1% benzo[ a]pyrene and
1-nitropyrene over an 8-h working day, they predicted equilibria of
160 ng benzo[ a]pyrene and 31 ng 1-nitropyrene per lung. No data are
available on changes in the retention of individual compounds after
prolonged exposure to diesel exhaust.
The clearance of a very low concentration (2.6 g/g particles) of
3H-benzo[ a]pyrene adsorbed onto diesel particles was measured in
Sprague-Dawley rats after intratracheal instillation (Bevan & Ruggio,
1991). Only 25% was cleared in the early, fast phase; a long-term
retention half-time of eight days was calculated for the remainder
during a three-day follow-up, which was similar to the clearance in
that period in the study of Sun et al. (1984).
B6.4 Metabolism
In the studies of Sun et al. (1984) and Bond et al. (1986a,b),
the levels of 3H-benzo[ a]pyrene and 14C-1-nitropyrene and their
metabolites in extracts of lung homogenates after inhalation of diesel
exhaust particles were measured by high-performance liquid
chromatography. Sun et al. detected oxidized metabolites 30 min after
inhalation: 35% of the 3H-benzo[ a]pyrene had been metabolized in
equal amounts to phenols (3-hydroxy- and 9-hydroxybenzo[ a]pyrene)
and quinones (1,6 and 3,6 isomers). Twenty days later, only 13%
phenols and 5% quinones were detected. In the study of Bond et al.
(1986b), about 30% of the 3H-1-nitro-pyrene had been metabolized to
acetylaminopyrenephenol (6 and 8 isomers) and about 40% remained
unmetabolized 1 h after inhalation. Oxidative metabolism of
14C-benzo[ a]pyrene, in solution or adsorbed onto diesel particles
at a concentration of 500 pmol/106 cells, was also detected in cell
cultures of pulmonary macrophages obtained from beagle dogs. The
quantity of metabolites increased with incubation time up to 45 and
125 pmol/106 cells in extracts and in the media, respectively, after
48 h of incubation. After incubation for 6 h, the major metabolites
found in extracts of cells were benzo[ a]pyrene-7,8-diol (0.5pmol/106
cells) and benzo[a]pyrene-4,5-diol (0.2 pmol/106 cells); those in
extracts of culture medium were benzo[ a]pyrene-7,8-diol (1.7 pmol/106
cells) and benzo[ a]pyrene-9,10-diol (1.4 pmol/106 cells), in
comparison with a total concentration of metabolites of 2.5 pmol/106
cells in the extracts and 6 pmol/10 6 cells in the medium. Minor
metabolites were benzo[ a]pyrene phenols (3-hydroxy- and
9-hydroxy-benzo[ a]pyrene) and benzo[ a]pyrene quinones (6,12, 1,5,
and 3,5 isomers). The quantities of metabolite were similar when
macrophages were incubated with benzo[ a]pyrene in solution or
adsorbed onto diesel particles (Bond et al., 1984).
Using the 32P-postlabelling technique, Wong et al. (1986) found
increased DNA adduct formation in the lungs of Fischer 344 rats after
exposure for 31 months to diesel exhaust containing 7.1 mg/m3
particles. After exposure of Fischer 344 rats and Syrian golden
hamsters to dilutions of diesel engine exhaust for six months to two
years, the level of haemoglobin adducts (2-hydroxyethylvaline and
2-hydroxypropylvaline), corresponding to the metabolic conversion of
5-10% of inhaled ethylene and propylene to its oxides, increased
dose-dependently (Törnqvist et al., 1988). An adduct of
benzo[ a]pyrenediol epoxide with deoxyguanosine was identified after
inhalation of benzo[a]pyrene adsorbed onto carbon black (20 mg/g)
(Wolff et al., 1989), demonstrating the production of epoxides of PAHs
by oxidative metabolism.
Methods for extracting diesel soot before testing for
genotoxicity in vitro have been discussed. As dichloromethane and
dimethyl sulfoxide do not correspond to leaching conditions in the
lung, it can be assumed that the bioavailability of genotoxic
materials is lower in lungs than in vitro. An investigation of
dipalmitoyl phosphatidylcholine, the primary component of
physiological surfactant, demonstrated that the genotoxicity
associated with diesel particles inhaled into the lung can be
activated by the solubilization and dispersion properties of pulmonary
surfactants. This finding was confirmed in mammalian cells for sister
chromatid induction (Keane et al., 1991) and for chromosomal
aberrations and micronucleus formation (Gu et al., 1991, 1992).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
B7.1 Single exposure
Data on the acute toxicity of diesel exhaust are very limited;
the two available studies suggest that diesel exhaust particles and
their extracts have little acute toxicity.
Male ICR mice were administered diesel exhaust particles by
intratracheal instillation, and mortality due to lung oedema was
assessed for up to 24 h. At a dose of 0.9 mg per animal, all animals
died within 24 h. The LD50 value was 0.6 mg per animal, equivalent
to about 20 mg/kg body weight. Diesel exhaust particles washed three
times with methanol did not cause death at a dose of 1 mg per mouse,
suggesting that the primary source of toxicity is the extractable
organic compounds rather than the particles themselves. These
compounds may produce superoxide radicals that damage endothelial
cells (Sagai et al., 1993).
The LD50 value for diesel exhaust extract in adult Syrian
golden hamsters after intraperitoneal injection was 1280 mg/kg body
weight (Pereira et al., 1982).
B7.2 Short-term exposure
Tracer-monitored DNA synthesis, indicating a proliferative
response, was observed in whole lung tissue of groups of 3-10 Fischer
344 rats exposed to diesel exhaust by inhalation at 6 mg/m3 for
20h/day on seven days per week for periods of 1-14 days. The response
was maximal after two days of exposure, showing a fourfold increase.
The type II cell labelling index was significantly higher (fourfold)
than that in controls after two and three days of exposure to diesel
exhaust. Both values returned to control levels after a one-week
recovery period. Experiments involving incorporation of 14C-palmitic
acid revealed transient changes in lipid metabolism after one day of
exposure, with a threefold increase in the amount of phosphatidyl-
choline in lung tissue (Wright, 1986).
Exposure of various species by inhalation to 6 mg/m3 resulted
in slight alterations in lung function and histopathological changes.
Groups of 10 male Chinese hamsters were exposed for 8 h/day on
seven days per week for six months to particle concentrations of
either 6 or 12 mg/m3 diesel exhaust.
Measurements of pulmonary function indicated dose-related
increases in lung weight and dose-related decreases in vital and
diffusion capacity. Pathological evaluation revealed oedema,
thickening of the alveolar lining, a possible increase in collagen,
and the presence of numerous particle-laden macrophages, which almost
filled the alveolar spaces (Vinegar et al., 1981).
Groups of 41-51 male and female Hartley guinea-pigs were exposed
for 20 h/day on seven days per week to exhaust from a light-duty
diesel engine diluted to attain a concentration of 6 mg/m3
particulate matter. Separate groups were exposed to raw exhaust and
exhaust that had been irradiated with ultra-violet light for an
average of about 1 h. Pulmonary function was measured, and
electrocardiography was conducted on animals exposed for four weeks;
those examined for histological changes were exposed for eight weeks.
The only changes noted after four weeks were a 35% increase in
pulmonary flow resistance and a small, but significant decrease in
heart rate in animals exposed to irradiated exhaust. No changes in the
electrocardiogram were noted. The pathological changes were limited to
hyperplasia of alveolar lining cells and slight focal thickening of
the interstitium in occasional alveoli with accumulation of
particle-laden macrophages. Growth rates were unaffected (Wiester et
al., 1980).
As part of the same study, cats (Pepelko et al., 1981) and male
rats (15 per group) (Pepelko, 1982) were exposed for four weeks to
unirradiated exhaust under identical conditions. The pulmonary
function of rats was unchanged; the only changes were significant
increases in vital and total lung capacity, but these differences were
quite small and of questionable significance. The only functional
change noted in the cats was a decrease in maximal expiratory flow
rate at 10% of vital capacity. The pathological changes were again
limited to focal thickening of the alveolar walls near accumulations
of particle-laden macrophages. In general, the functional and
pathological changes were limited under these experimental conditions.
Kaplan et al. (1982) exposed male Fischer 344 rats (168 per
group), A/J mice (672 per group), and male Syrian golden hamsters (236
per group) to 1.5 mg/m3 of diesel exhaust particles for 20 h/day on
seven days per week for three months or to clean air. Animals were
killed at the end of exposure and after an additional six-month
recovery period. Mortality was not increased during the observation
period, and body weight was not significantly altered in any species.
At the end of exposure, the relative weight of the lung was
significantly increased in rats. In mice, the pulmonary adenoma
response was increased but not significantly. In all three species,
grey to black discolouration of the lungs and lung-associated lymph
nodes were observed. Microscopically, most of the particulate was
found in macrophages, but some was free. After six months' exposure to
clean air, the lungs of all species had considerably less pigment
accumulated in foci.
Sixteen male Fischer 344 rats were exposed to 6 mg/m3 diesel
exhaust for periods ranging from 6 h to 63 days, with subsequent
histological examination of the lungs. The numbers of diesel
particulate-containing alveolar macrophages, type II alveolar cells,
and inflammatory cells were found to be increased over those in eight
controls. After a nine-week exposure, accumulation of macrophages was
observed near the terminal bronchioles (White & Garg, 1981). In rats
that inhaled 6 mg/m3 diesel exhaust for two weeks and were either
killed immediately or allowed to recover for six weeks before
sacrifice, there was no statistical difference in the number of
macrophage aggregates (240 000 and 220 000 per lung); however, the
aggregates in the recovery group had significantly higher average
diameter and volume, demonstrating macrophage attachment after the end
of exposure (Garg, 1983).
The observations of Wiester et al. (1980) and Garg (1983)
demonstrate the importance of alveolar macrophages in the clearance of
particles of low solubility. These are engulfed by alveolar
macrophages and eliminated via the mucociliary escalator and
gastrointestinal tract, or, in the case of lung particle overloading,
increasing fractions are sequestered in focal areas of the lung tissue
and transferred to the lymph nodes (Muhle et al., 1990).
B7.3 Long-term exposure and studies of carcinogenicity
B7.3.1 Non-neoplastic effects
No obvious signs of toxicity were observed in groups of 183 male
and 183 female Fischer 344 rats exposed by inhalation to diesel
exhaust at 0.35, 3.5, or 7.1 mg/m3 for 7 h/day on five days per week
for up to 30 months (Mauderly et al., 1987). The body weights did not
differ significantly from those of controls, and the exposures did not
affect the life span of animals of either sex. Focal fibrotic and
proliferative lung disease was observed in parallel with progressive
accumulation of diesel soot in the lung. Henderson et al. (1988)
reported the results of biochemical and cytological analyses of lung
lavage fluid and lung tissue from rats and mice exposed in the same
study, including determinations of lactic dehydrogenase, glutathione
reductase, ß-glucuronidase, glutathione, and hydroxyproline, and the
numbers of macrophages and neutrophils. No changes were seen in rats
or mice exposed to 0.35 mg/m3 , but at higher concentrations dose-
and duration-dependent increases in biochemical parameters and cell
numbers in lavage fluid were observed, indicating a chronic
inflammatory response. Lung weights were also increased in male and
female mice exposed to the two higher levels for any length of time,
in rats exposed to 7.0 mg/m3 for 12 months or longer, in female rats
exposed to 3.5 mg/m3 for 18 months, and in males exposed to
3.5 mg/m3 for 24 months. Tissue enzyme levels, measured in lung
lavage fluid as an indicator of damage, were consistently raised in
rats and mice at the two higher exposure levels and in lung tissue of
rats and mice at various times (most pronounced at 7 mg/m3). These
changes probably indicate an increase in lung tissue due to increased
inflammatory cells and epithelial cell proliferation.
In the same study, Mauderly et al. (1988) described focal
proliferative, fibrotic, and emphysematous changes in the lungs of
animals at the higher doses. Measurements in vivo revealed a
reduction in lung volume and compliance.
In a follow-up study, Mauderly et al. (1990) exposed male Fischer
344 rats that were either normal or had pre-existing pulmonary
emphysema (elastase-treated) to diesel soot at 3.5 mg/m3 for 7 h/day
on five days per week for 24 months, to determine whether
emphysematous rats were more susceptible to diesel exhaust. No
increase in susceptibility was seen. The emphysematous lungs had a
lower accumulation of particles than the normal lungs, which prevented
an overproportional response of the compromised animals.
Male and female Fischer 344 rats exposed to diesel exhaust
particles at 0.7, 2.2, or 6.6 mg/m3 for 16 h/day on five days per
week for two years or to filtered diesel exhaust showed an
exposure-related reduction in the rate of weight gain throughout the
study at the highest dose and an increase in absolute lung weight at
the two higher doses (Brightwell et al., 1986, 1989).
Groups of 72 male and 72 female Fischer 344 rats exposed to
diesel exhaust particles at 2 mg/m3 for 7 h/day on five days per
week for 24 months showed alveolar type II cell hyperplasia,
inhibition of long-term clearance of particles and pulmonary
lipidosis. No immunological abnormalities, no induction of pulmonary
or hepatic xenobiotic metabolizing enzymes, and no effects on
mortality, body weight gain, organ:body weight ratios or clinical
chemical parameters were seen (Lewis et al., 1986, 1989). In the same
study, a dose-related depression in phagocytic activity was seen in
alveolar macrophages (Castranova et al., 1985), and a trend to
increased pulmonary arterial wall thickness was observed in comparison
with controls (Vallyathan et al., 1986).
In groups of 15 male monkeys, exposure to diesel exhaust (as
described above) resulted in mild obstructive airway disease, but
there was no evidence of restrictive lung disease. The effects were
observed throughout the exposure period, after 6, 12, 18, and 24
months (Lewis et al., 1989).
Reduced body weight, significantly increased lung:body weight
ratios, and type II cell proliferation were seen in female Fischer 344
rats exposed to whole-fraction diesel exhaust at 5 mg/m3 for 8 h/day
on seven days per week for 24 months (Iwai et al., 1986).
Groups of 96 male and 96 female Syrian golden hamsters, female
NMRI mice, and female Wistar rats were exposed to diesel engine
emissions at 4.4 mg/m3 for 19 h/day on five days per week for
120-140 weeks (Heinrich et al., 1986a; Stöber, 1986). The body weights
of mice were significantly reduced, by about 12%. Lung weights were
increased after two years of exposure, by two- to threefold in rats
and mice and by 50-70% in hamsters. Different levels of histological
response were seen in the three species. Hamsters had thickened
alveolar septa and bronchioalveolar hyperplasia. Of the mice, 64% had
bronchioalveolar hyperplasia (controls, 5%), 71% had multifocal
alveolar lipoproteinosis, and 43% had multifocal interstitial fibrosis
(controls, 4%). The nose, larynx, and trachea were not affected.
Treated rats showed severe inflammatory changes in the lungs,
thickened septa, foci of macrophages, and hyperplastic and metaplastic
lesions, but no significant changes in respiratory rate, minute
volume, compliance, or resistance were seen in 14 exposed rats.
Significantly increased airway resistance and a significant decrease
in dynamic lung compliance were observed in rats after two years of
exposure. The hamsters had a significant increase in airway resistance
and a nonsignificant reduction in lung compliance at termination of a
one-year exposure; these changes persisted during the second year of
exposure. In the same experiments, filtered diesel exhaust did not
have the same effects as total exhaust in any of the three species
(Heinrich et al., 1986a).
Groups of 27 female Fischer 344 rats and about 50 male and 50
female C57Bl/6N and ICR mice were exposed to particle concentrations
of 2-4 mg/m3 for 4 h/day on four days per week for up to 24 months.
After 12 months, the incidence of alveolar hyperplasia was increased
in all animals. Exposed rats had more severe inflammation than
controls, with inflammatory exudate into the nose, and bronchitis and
pneumonia, but this effect was ascribed to the housing, which was not
specific pathogen-free (Takemoto et al., 1986).
Groups of nine male Hartley guinea-pigs were exposed at
concentrations of 0.25, 0.75, or 1.5 mg/m3 for 20 h/day on 5.5 days
per week for up to two years. An analysis of the alveolar capillary
membrane by quantitative morphometry showed that it was thickened as a
result of an increase in the absolute tissue volume of interstitium
and type II cells. Exposure to 0.75 mg/m3 for six months caused
fibrosis in regions of macrophage clusters and focal type II cell
proliferation, as observed under the light microscope. No appreciable
change in morphometric parameters was seen after exposure to the
lowest dose, but the higher doses increased the thickness of alveolar
septa and the numbers of various types of alveolar cells (Barnhart et
al., 1981, 1982).
As part of this investigation, Fischer 344 rats were exposed by
inhalation to 0.25 or 1.5 mg/m3 diesel exhaust particles, and
various biochemical and cytological studies were conducted up to 36
weeks (Misiorowski et al., 1980). At the high dose, normalized lung
weight, the rate of collagen synthesis, and the cellular and lipid
content of lung tissue were significantly increased after six months.
It was concluded that the reactivity of fibrogenic cells had been
enhanced. In a long-term study of respiratory end-points in male
guinea-pigs, including light and electron microscopy, lavage cytology,
and lung tissue biochemistry, the lowest-observable-adverse-effect
level (LOAEL) was 0.796 and the no-observable-adverse-effect level
(NOAEL) was 0.258 mg/m3 (US Environmental Protection Agency, 1992b).
Groups of 120 male Fischer 344 rats, 450 male A/J mice, and 120
male Syrian hamsters were exposed to 0.25, 0.75, or 1.5 mg/m3 diesel
exhaust particles for 20 h/day on seven days per week for 15 months.
Mortality was not increased in any species. A dose-related deposition
of pigment was observed histopathologically in alveolar macrophages
and regional lymph nodes. Accumulation of alveolar macrophages was
associated with an increase in connective tissue in alveolar walls,
with mild fibrosis and proliferation of type II pneumocytes; these
effects were termed pneumoconiosis. No treatment-related responses
were found in organs other than the respiratory tract. The LOAEL was
0.735 and the NOAEL, 0.242 mg/m3 (Kaplan et al., 1983).
Groups of 120 male and 95 female Fischer 344/Jcl rats were
exposed to light-duty diesel engine exhaust particulates at 0, 0.1,
0.4, 1.1, or 2.3 mg/m3 or heavy-duty diesel engine exhaust
particulates at 0, 0.5, 1.0, 1.8, or 3.7 mg/m3 for 16 h/day on six
days per week for up to 30 months (Ishinishi et al., 1986, 1988;
Suzuki et al., 1990). The body weights of females exposed to the
highest dose of heavy-duty engine exhaust were decreased throughout
the study by 15-20% in comparison with controls. No changes were
observed histopathologically in the lungs at any concentration.
Accumulation of particle-laden macrophages was observed at levels
> 0.4 mg/m3. In areas of macrophage accumulation, bronchiolar
epithelium replaced alveolar epithelium in the ducts. Proliferation of
bronchiolar epithelium and type II cells and oedematous thickening and
fibrosis of the alveolar septum were observed, which developed into
small fibrotic lesions classified as hyperplastic. The incidences of
hyperplastic lesions were 4, 4, 6, 12, and 87 per 124 rats exposed to
light-duty engine exhaust and 1, 3, 7, 14, and 25 per 124 rats in
those exposed to heavy-duty engine exhaust. The LOAELs were
1.2 mg/m3 for light-duty and 1.0 mg/m3 for heavy-duty exhaust, and
the NOAELs were 0.4 and 0.5 mg/m3, respectively (US Environmental
Protection Agency, 1992b).
In the same experimental series, Kato et al. (1992) studied
non-neoplastic lesions in the respiratory organs of the rats every six
months. The marked changes consisted of intake of diesel particles by
type I epithelium, hypertrophy, and glandular proliferation of type II
epithelium, with many extended microvilli and increased numbers and
size of lamellar inclusion bodies, focal increase of collagen fibres
in the interstitium, and infiltration of particle-laden macrophages,
neutrophils, mast cells, and plasma cells into the interstitium of
alveolar septa. The most marked changes in the airway were focal
shortening of cilia and protrusions of nonciliated cells. These
morphological changes appeared in all groups exposed to particle
concentrations > 1 mg/m3 after six months of exposure and were more
marked with increasing particle concentration and with time.
Exposure of Syrian golden hamsters to 4 or 11 mg/m3 diesel
exhaust for 7 h/day on five days per week for five months resulted in
enlargement of liver sinusoids and loss of cristae in mitochondria
(Meiss et al., 1981).
The induction of pulmonary adenomas and carcinomas in rats after
long-term inhalation of carbon black is proposed to be due partly to
inflammatory cells and their mediators (Driscoll et al., 1995;
Oberdörster et al., 1995; Driscoll et al., in press). After exposure
of rats to carbon black at 1, 7, or 50 mg/m3 for 6 h/day on five
days per week for 13 weeks, 353, 1923, and 7112 µg of carbon,
respectively, were deposited per lung. After exposure to air or to
1 mg/m3 of carbon black, no macrophage inflammatory protein-2
(MIP-2) mRNA expression and no increase in inflammatory cells was
observed; with 7 or 50mg/m3, both MIP-2 mRNA and the number of
neutrophils in bronchoalveolar lavage fluid were increased, and the
hprt frequencies were 3.2 and 4.2 times greater than in controls.
Groups of 25 cats were exposed to either clean air or diesel
exhaust for 8 h/day on seven days per week for two years at a
concentration of 6 mg/m3 through week 62 and to 12 mg/m3 for weeks
62-124. Some of the cats were then held in clean air for an additional
six months. No response was seen after 61 weeks, but after 123 weeks
signs of restrictive lung disease were observed. Total lung capacity,
forced vital capacity, functional residual capacity, peak expiratory
flow rates, and diffusion capacity were significantly decreased
(Moorman et al., 1985). Morphological evaluation revealed
exposure-related lesions in the centriacinar region of the lungs,
bronchiolar epithelial metaplasia associated with the presence of
ciliated and basal cells, and alveolar macrophages containing diesel
particle-like inclusions. After six months' recovery in clean air, the
metaplasia had disappeared but the fibrosis had progressed (Hyde et
al., 1985). In exposed cats, the epithelium of the terminal and
respiratory bronchioles consisted of ciliated, basal, and Clara cells,
whereas controls had only Clara cells (Plopper et al., 1983). These
observations indicate that the foci of the lesions were the
epithelium, the interstitial compartments, and the centriacinar region
of the lung. The metaplastic changes suggest that cancer may be
induced by continuous exposure, while the other major effect is
pulmonary fibrosis resulting in restrictive disease which is not
ameliorated and in fact progresses after removal from exposure.
Progression of effects is probably due to continued lung overloading
with particulate matter, despite cessation of exposure.
The dose-dependent effects on lung morphology, histopathology,
biochemistry, and cytology after long-term exposure to diesel exhaust
described above are reflected in the following findings seen in rats
at a lung burden of about 0.5 mg/lung (Henderson et al., 1988; Muhle
et al., 1990):
-- The wet and dry weights of lungs increase by up to fourfold in
comparison with those of concurrent controls. This is due to
severe functional overloading in the lung, resulting in enhanced
leukocyte influx and tissue proliferation.
-- Pulmonary inflammation is manifested by cytological (increased
numbers of polymorphonuclear neutrophils) and biochemical (lactic
dehydrogenase, collagen in lung lavage fluid) parameters.
-- The retention of material is characterized by an increasing
number of particle-laden macrophages and by black discolouration
of lungs due to sequestration of accumulated particles.
Additionally, increased transfer of material to the
lung-associated lymph nodes is observed.
-- Later findings during inhalation are proliferative alterations of
the epithelial cells and onset of fibrosis.
B7.3.2 Carcinogenicity
B7.3.2.1 Inhalation
Rats were exposed in several studies to diesel emissions
containing > 2 mg/m3 of particles for more than two years,
resulting in the induction of pulmonary tumours (Table 34). Many
organic and inorganic gaseous components were also present in the test
atmospheres, and the relatively high concentration of some pulmonary
irritants like nitrogen dioxide, aldehydes, and sulfur dioxide may
have contributed to the inflammatory response and to epithelial cell
damage and subsequent cell proliferation. Exposure to gaseous PAHs,
like naphthalene which is toxic to Clara cells, may influence the
metabolic state of pulmonary cells. It is not clear however, whether
the combined exposure to particles and gases in these experiments
favoured tumour induction. It should be noted that Brightwell et al.
(1986), Heinrich et al. (1986a), Iwai et al. (1986), Ishinishi et al.
(1986), and Takaki et al. (1989) have reported that the gaseous phase,
i.e. the proportion of exhaust without particles, does not induce
pulmonary tumours.
Table 34. Carcinogenicity of diesel engine exhaust in rats
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 40 m 8.3 1.5 6 h/d, 5 d/week, up NR 1 17c NR Karagianes
40 m Clean air to 20 months 0 et al.
(20 months) (1981)
Fischer 72 f 6.6 3.1 16 h/d, 5 d/week, 24 NR 39 54 38.5 < 0.1 Brightwell
344 71 m months (30 months) 16 23 et al.
72 f 2.2 1.1 11 15 9.7 < 0.1 (1986, 1989)
72 m 3 4
71 f 0.7 0.4 0 0.7
72 m 1 1
Filtered exhaust 0
0
142 f Clean air 1 1 1.4
140 m 3 2
Wistar 95 f 4.4 2.5 19 h/d, 5 d/week, 32 NR 17 18 < 0.1 Heinrich et
92 f Filtered exhaust months (up to death) 0 al. (1986a)
96 f Clean air 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer
344/Jcl
Light-duty 60 f 2 1.1 16 h/d, 6 d/week, 30 NR 1 2 2.4 NR Ishinishi
engine 64 m months (30 months) 2 3 et al.
59 f 1 0.6 2 3 4.1 (1986,1988;
64 m 3 5 Takaki et al.
61 f 0.4 0.2 0 0.8 (1989)
64 m 1 2
59 f 0.1 0.06 2 3 1.6
64 m 0
59 f Clean air 2 3 3.3
64 m 2 3
Heavy-duty 60 f 4 2.3 3 5 6.5 1.8
engine 64 m 5 8
59 f 2 1.1 1 2 3.3
64 m 3 5
61 f 1 0.6 0 0
64 m 0
59 f 0.4 0.2 1 2 0.8
64 m 0
59 f Clean air 1 2 0.8
64 m 0
16 m Filtered exhaust 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer 19 f 4.9 1.6 8 h/d, 7 d/week, 24 NR 8 42 < 1d Iwai et al.
344 16 f Filtered exhaust months (24 or 30 0 (1986)
22 f Clean air months) 1 5
Fischer 15 f 2.0-4.0 0.2-0.4 4 h/d, 4 d/week, 24 NR 0 NR Takemoto
344 15 f Clean air months (24 months) 0 et al.
(1986)
Fischer 69 f 7 1.5 7 h/d, 5 d/week, 30 NR 13 19 16.1 < 0.1 (f) Mauderly et
344/Crl 74 m months (up to death) 10 14 1.7 (m) al. (1986)
68 f 3.5 0.7 2 3 4.6
63 m 4 6
68 f 0.35 0.07 0 0.7
70 m 1 1
68 f Clean air 0 1.4
73 m 2 3
Fischer 227 f+m 7 1.5 7 h/d, 5 d/week, 30 20.8 12.8 < 0.1 Mauderly et
344/N Crl 221 f+m 3.5 0.7 months (up to death) 11.5 3.6 4.8 al. (1987)
223 f+m 0.35 0.07 0.6 1.3
230 f+m Clean air (24 months) 0.9
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 100 f 7.0 3.8 18 h/d, 5 d/week, 24 63.9 22 22.0 < 0.1 Heinrich et
200 f 2.5 1.3 months (30 months) 23.7 11 5.5 < 1 al. (1992,
198 f 0.8 0.5 6.3 0 1995);
217 f Clean air (24 months) NR 1 0.5 Heinrich
(1994)
Fischer 212 f+m 6.3 3.0 16 h/d, 5 d/week, 24 85.4 38 17.9 17.9 NR Nikula et al.
344/N 210 f+m 2.4 1.1 months (24 months) 40.7 13 6.2 6.2 (1994)
214 f+m Clean air (23 months) 3 1.4 1.4
Fischer 49 f 4.7 1.3 15 h/d, 3 d/week, 24, NR 6 12.2 NR Kawabata
344/N 42 f 4.7 1.3 12, or 6 months 8 19.0 et al.
45 f 4.7 1.3 1 2.2 (1993)
48 f Clean air 5 10.4
Fischer 183 f+m 2.0 0.4 7 h/d, 5 d/week, 24 NR 8 4.4 4.4 NR Lewis et al.
344/N 180 f+m Clean air months 6 3.3 3.3 (1989)
From Pott & Heinrich (1987) and supplemented; NR, not reported
a Mass median aerodynamic diameter, 0.17-0.25 µm; 'Continuous', equivalent continuous value
b Unilateral Fisher's exact test
c Only six animals investigated
d With Yates' correction
No difference in lung tumour incidence was seen in groups of male
and female rats exposed for 7 h/day on five days per week for 24
months either to diesel exhaust particulates at 2 mg/m3 or to
filtered air (Lewis et al., 1989).
Exposure of six male Wistar rats to diesel exhaust soot at
8.3 mg/m3 and coal dust for up to 20 months gave inconclusive
results (Karagianes et al., 1981). The study had little statistical
power owing to the small group size, and the insufficient duration of
exposure may have prevented detection of tumours, although one mammary
fibroadenoma and one lung bronchiolar adenoma were detected. One
subcutal fibrosarcoma and one renal lymphoma were seen in controls.
Eight bronchioalveolar adenomas and nine squamous-cell tumours
were seen in 95 rats (18%) that had inhaled 4.4 mg/m3 diesel exhaust
for 19 h/day on five days per week for 32 months. No tumours were
observed in controls (Heinrich et al., 1986a). The dose-dependent
carcinogenic potency of diesel exhaust was confirmed in a study in
which rats were exposed to diesel exhaust at concentrations of 0.8,
2.5, or 7.0 mg/m3 for 18 h/day on five days per week for 24 months
(Heinrich et al., 1992; Heinrich, 1994; Heinrich et al., 1995).
In male and female rats exposed to diesel exhaust at 6.6 mg/m3,
a high incidence of lung tumours was observed in animals dying or
sacrificed after 24 months (24/25 females, 12/27 males). The incidence
of lung tumours (Table 34) was derived by pooling pulmonary adenomas;
squamous-cell carcinomas; adenocarcinomas; mixed ademomas,
adenocarcinomas, and squamous-cell carcinomas; and mesotheliomas.
Non-neoplastic pulmonary lesions were not described (Brightwell et
al., 1986, 1989).
Groups of 24 female Fischer 344 rats were exposed to clean air,
to whole-fraction (4.9 mg/m3 particulates) fuel, or to filtered
diesel exhaust for 8 h/day on seven days per week for 24 months. After
six months of exposure, the rats exposed to whole exhaust had lower
body weights than those in the other two groups; after 18 months, both
exposed groups showed body weight reduction. The relative lung weight
was increased after 12 months' exposure to whole exhaust, and
epithelial proliferation and significantly elevated numbers of lung
tumours were seen in animals exposed to the whole fraction after six
months. Increased incidences of leukaemia and mammary gland tumours
were also reported in animals exposed to whole or filtered exhaust;
however, because of the small numbers of animals, the significance of
the results cannot be determined (Iwai et al., 1986).
A total of 140 female and 140 male Fischer 344/N rats (in groups
of three males and three females, five males and five females, or
eight males and eight females, depending on the end-point) were
exposed for 16 h/day on five days per week for up to 24 months,
beginning at eight weeks of age, to particles of diesel exhaust or
carbon black at 2.5 or 6.5 mg/m3 of air, or to clean air (Mauderly
et al., 1994). Rats were killed after 3, 6, 12, 18, or 23 months of
exposure for histopathological assessment and measurement of lung and
lung-associated lymph node burdens of particles, lung weight,
bronchoalveolar lavage indicators of inflammation, DNA adducts in
whole lung and alveolar type II cells, and chromosomal anomalies in
circulating lymphocytes. Diesel exhaust and carbon black had nearly
identical effects, with a similar relation to exposure. There was a
dose-related slowing of particle clearance after three months, with
progressive accumulation of particles in lungs and lymph nodes.
Inflammation, epithelial proliferation, and fibrosis were progressive
and related to dose. Diesel exhaust slightly increased the number of
lung DNA adducts, and the numbers of DNA adducts in type II cells were
increased by both diesel exhaust and carbon black. No exposure-related
chromosomal damage was found in circulating lymphocytes. The incidence
of primary lung neoplasms was increased significantly and was related
to the doses of both diesel exhaust and carbon black. The types of
neoplasms were identical and included benign adenomas, malignant
adenocarcinomas, squamous-cell carcinomas, and adenosquamous
carcinomas; squamous cysts were also seen. The neoplasms had similar
growth characteristics when transplanted; 50-67% of the transplanted
squamous-cell carcinomas and 25-40% of the transplanted
adenocarcinomas grew after injection into athymic mice (Table 35),
whereas the squamous cysts did not.
Fischer 344/Jcl rats were exposed to a range of concentrations of
exhaust emission particulates from light-duty (up to 2 mg/m3) or
heavy-duty diesel engines (up to 4 mg/m3) for 16 h/day on six days
per week for up to 30 months. After 18 months, marked hyperplasia of
type II cells was noted in the groups at higher exposures. After 30
months, treatment-related pulmonary hyperplastic lesions were
detected, and a dose-response relationship was seen for tumours in the
groups exposed to heavy-duty but not light-duty diesel exhaust. The
lung tumours were diagnosed as adenocarcinomas, squamous-cell
carcinomas, or adenosquamous carcinomas. A significant correlation was
observed between the incidence of anthracosis and areas with
hyperplastic lesions; furthermore, anthracosis and hyperplastic
lesions did not appear in lungs exposed to particle-free exhaust. The
authors concluded that the occurrence of hyperplastic lesions was
dependent on the presence of carbon particles (Ishinishi et al., 1986,
1988; Takaki et al., 1989).
Table 35. Growth in athymic mice of lung tumours from rats exposed to
diesel exhaust and carbon black
Type of tumour Carbon black Diesel exhaust
Number Growing Number Growing
implanted No. % implanted No. %
Adenocarcinom 8 2 25 10 4 40
Squamous-cell 2 1 50 3 2 67
carcinoma
Squamous cyst 19 0 0 7 0 0
From Mauderly et al. (1994)
In a large-scale study of unfiltered diesel exhaust, 1097 Fischer
344 rats were exposed for up to 30 months to 0.35, 3.5, or 7.0 mg/m3
of diesel soot. A group of 365 rats exposed to clean air served as
controls. Exposure to exhaust did not cause overt signs of toxicity,
and no significant, exposure-related alterations in body weight were
observed. Rats were killed at six-month intervals, examined for their
lung burdens of diesel soot, and subjected to complete necropsy.
Diesel soot accumulated at all doses but minimally in the low-dose
group. An increased incidence of tumours was observed in the groups at
the medium and high doses; most (81%) of the tumours were observed
after two years of exposure. The inhaled soot concentration and the
lung burden of diesel soot were highly significantly related to tumour
incidence ( P < 0.001) (Mauderly et al., 1987). In a follow-up study
with longer daily exposure (16 h/day), the dose-dependency of the
tumour incidence was reproduced (Nikula et al., 1994). In this study,
the carcinogenicity of diesel exhaust soot (8% extractable organic
material; 2.5 mg/m3) was compared with that of carbon black (0.12%
extractable organic material; 6.5 mg/m3 ) in 1152 male and female
Fischer 344 rats exposed for 16 h/day on five days per week for up to
24 months and examined histopathologically. The lung burden of
particles was greater after exposure to diesel exhaust than to carbon
black, but the neoplastic response in the lung was similar and usually
occurred late in the study. When the lung burden of particles was used
as the dose parameter, the carcinogenicity of carbon black was
greater than that of diesel exhaust soot.
Groups of 50 four-week-old female Fischer 344 rats were exposed
to diesel soot at a mean concentration of 4.73 mg/m3 for 15 h/day
three times per week for 6, 12, or 24 months, and animals that died
after 18-30 months were evaluated for tumours. After 30 months, all
animals, including controls, were sacrificed and examined for lung
tumours. The incidences of lung tumours were 10.4% in controls and
2.2% after six months' exposure to diesel exhaust, 19.0% after 12
months, and 12.2% after 24 months. No statistical analysis was
presented, but it seems unlikely that there was a significant effect
(Kawabata et al., 1993).
Concurrent administration of known carcinogens with diesel
exhaust has also been studied. Exposure of rats to diesel exhaust
given an intraperitoneal injection of N-nitrosodiisopropylamine or
N-nitrosodiethylamine did not influence the rates of tumours induced
by the nitrosamines (Takemoto et al., 1986). The effects of treatment
of hamsters with N-nitrosodiethylamine or benzo[ a]pyrene were not
influenced by subsequent administration of diesel exhaust, whereas
pretreatment of mice with benzo[ a]pyrene or dibenzanthracene followed
by diesel exhaust gave inconsistent results. Exposure to diesel
exhaust did not affect the total tumour rates in rats pretreated with
6.25 or 12.5 g/kg body weight of N-nitrosodipentylamine; however,
the incidence of squamous-cell tumours was increased in both
pretreated groups (Heinrich et al., 1986a).
Hamsters were exposed for two years to unfiltered diesel exhaust
at 0, 0.7, 2.2, or 6.6 mg/m3 or to filtered diesel exhaust, and
groups of control and treated hamsters were administered
N-nitrosodiethylamine subcutaneously before the beginning of
exposure. The rate of pulmonary tumours was not increased by diesel
exhaust, with or without pretreatment with the nitrosamine. Several
hamsters that developed 'wet tail' infection after six months on test,
resulting in significant mortality (46%), were treated with
oxytetracycline, chloramphenicol, and dimetridazole (Brightwell et
al., 1986, 1989).
Overall, the only clear effect in these studies of
co-carcinogenicity in rats was an increase in the percentage of
malignant tumours in animals treated with diesel exhaust plus
carcinogens in comparison with the carcinogen alone (Heinrich et al.,
1986a).
The results of studies in other species (mice, hamsters, and
monkeys) treated by inhalation are given in Table 36. Heinrich et al.
(1982) found significant increases in lung tumour incidence in mice,
but this result could not be repeated in later studies (Heinrich et
al., 1986a, 1992, 1995). Small but statistically significant increases
in lung tumour incidence were reported in ICR and C57Bl mice (Takemoto
et al., 1986) and in female Sencar mice (Pepelko & Peirano, 1983);
however, because only small increases in non-malignant tumours were
seen only in females in the study of Pepelko & Peirano (1983), the
results must be considered to be weakly positive or equivocal.
Hamsters showed no response to diesel exhaust.
Table 36. Long-term studies of diesel exhaust by inhalation in species other than rats
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI mouse 2 × 96 f (a) Exhaust 4 19 h/d, 5 d/week, Lung tumours (adenomas and Heinrich et al.
(b) Filtered 27-28 months adenocarcinomas) (a) 24/76 (32%), (1986a)
(c) Clean air including 13 (17%) adenocarcinomas;
(b) 29/93 (31%), including 18 (19%)
adenocarcinomas; (c) 11/84 (13%),
including 2 (2.4%) adenocarcinomas
Incidence in historical controls,
< 32%
ICR and 315 ICR, Exhaust or 2.0-4.0 4 h/d, 4 d/week, Lung tumours (adenomas and Takemoto et al.
C57Bl/6N 297 C57Bl/6N clean air 13-28 months adenocarcinomas). ICR: 14/56; (1986)
mice, newborn (sex 7/60 in controls; C57Bl/6N:
unspecified) 17/150; 1/51 in controls
Sencar mouse 2 × 130 f (a) Exhaust 12 8 h/d, 7 d/week, Adenomas and carcinomas Pepelko &
2 × 130 m (b) Clean air to 15 months of (a) m: 5.9%; f: 16.3% Peirano (1983)
age (from (b) m: 3.8%; f: 7.2%
conception to
sacrifice)
NMRI mouse 2 × 80 f (a) Exhaust 7.5 18 h/d, 5 d/week, Lung tumours Heinrich et al.
(b) Clean air 13.5 months, 9.5 (a) 32.1% (1992, 1995)
months' recovery (b) 30%
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI and 3 × 120 f (a) Exhaust 4.5 18 h/d, 5 d/week; Lung tumours Heinrich et al.
C57Bl/6N mice 3 × 120 f (b) Filtered NMRI, 23 months; NMRI: (a) 23.3% (1992, 1995)
(c) Clean air C57Bl, 24 months (b) 46.7%
+ 6 months' (c) 30%
recovery C57Bl: (a) 8.5%
(b) 3.6%
(c) 5.1%
Syrian golden 3 × 48 f (a) Exhaust 3.9 ± 0.5 7-8 h/d, 5 d/week, No effect on survival, no lung Heinrich et al.
hamster (b) Filtered up to 30 months tumours (1982)
(c) Clean air
Syrian golden 3 × 48 f (a) Exhaust 4 19 h/d, 5 d/week, Normal life span, no lung tumours Heinrich et al.
hamster 3 × 48 m (b) Filtered 27-28 months (1986a)
(c) Clean air
Syrian golden 52 f + 52 m (a) Exhaust 0.7, 2.2, 16 h/d, 5 d/week, No significant increase in Brightwell
hamster 104 f + 104 m (b) Filtered 6.6 24 months frequency of tumours in et al.
(controls) (c) Clean air respiratory tract (1986, 1989)
Cat 2 × 25 m (a) Exhaust 6 (1st 8 h/d, 7 d/week, Lung function: decrease in closing Pepelko &
yr), 12 24 months volume after 1 year; reduction Peraino (1983)
(2nd yr) in inspiratory, vital, and total
(b) Clean air lung capacity after 2 years;
diagnosis: pulmonary fibrosis of
the interstitial or
intra-alveolar type
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
Cynomolgus 4 × 15 m (a) Coal dust 2.0 7 h/d, 5 d/week, No difference in tumour incidence Lewis et al.
monkey (b) Exhaust 2.0 24 months among groups (1986)
(c) Coal dust 1.0
+ exhaust 1.0
(d) Clean air
It is clear that repeated inhalation of diesel exhaust at
concentrations of more than about 2 mg/m3 (actual value),
corresponding to a calculated equivalent continuous concentration of
about 1 mg/m3, increases the incidence of pulmonary tumours. These
tumours occur late in life after exposures of 24 months.
B7.3.2.2 Other routes of exposure
Studies have also been conducted by intratracheal instillation in
rats and hamsters and by painting on mouse skin (Table 37). While the
former are especially useful for hazard identification and for
evaluating specific mechanistic aspects, they are less useful for the
purposes of risk characterization and no attempt was made to identify
critical studies. The results of the studies by intratracheal
instillation, however, confirm that both diesel exhaust particles and
carbon black induce lung tumours and demonstrate that the specific
surface area of carbonaceous particles is correlated with the
tumorigenic potency. Both of these findings support the suggestion
that a nonspecific particle effect is of crucial importance for the
induction of lung tumours by diesel exhaust. The dermal experiments,
conducted by Nesnow et al. (1982a,b, 1983), have been used to estimate
risk quantitatively, by the comparative potency method.
B7.4 Dermal and ocular irritation; dermal sensitization
No data were available.
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
B7.5.1 Reproductive toxicity
In a two-generation study of reproduction, 100 male and 100
female CD-1 mice were exposed by inhalation to exhaust from a
light-duty diesel engine for 8 h/day on seven days per week, at a
concentration of 12 mg/m3. Most treatment-related effects were
minimal. Overall fertility and survival rates were not significantly
altered (Pepelko & Peirano, 1983).
(C57Bl/6 × C3H)F mice (number not given) showed sperm
abnormalities (reduced sperm count and weight of testis;
teratospermia) after daily intraperitoneal injections of 50, 100, or
200 mg/kg body weight of diesel exhaust particles for five days. The
highest dose caused an eightfold increase in abnormalities over that
in controls and a significant decrease in sperm number (Quinto & De
Marinis, 1984).
Table 37. Carcinogenicity of diesel exhaust after exposure other than by inhalation
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Fischer 344 (a) 31 f Intratracheal (a) Activated carbon 1/animal 10/week, Survival rate, 71-83% Kawabata
rat (b) 59 f instillation (b) Diesel particles 1/animal 30 months' (lowest ingroup b). Malignant et al.
(c) 53 f (c) None observation lung tumours: (a) 7/23; (1986)
(d) 27 f (d) Vehicle (b) 20/42; (c) 0/44; (d) 1/23;
P < 0.01. Benign and
malignant tumours combined:
(a) 11/23; (b) 31/42
Osborne- Groups Lung Organic material from Observation (a) 1 bronchioalveolar adenoma; Grimmer
Mendel rat of 35 f implant diesel exhaust: until (b) 5 squamous-cell carcinomas; et al.
(a) Hydrophilic fraction 6.7 spontaneous (c) 1 bronchioalveolar adenoma; (1987)
(b) Hydrophobic fraction 20.0 death (d) 6 carcinomas;
(c) Nonaromatic compounds 19.2 (e) No tumours
+ PAHs (2 or (f) 1 carcinoma
3 rings) (g) 7 carcinomas, 1 adenoma
(d) PAHs ( > 4 rings) 0.2 (h) No tumours
(e) Polar PAHs 0.3 (i) 1 adenoma
(f) Nitro-PAHs 0.2
(g) Reconstituted
hydrophobic fraction 19.9
(h) None
(i) Vehicle
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Wistar rat (a) 40 f Intratracheal (a) Diesel soot (34 m2/g) (a) 3 × 15 Primary lung tumours (%) Pott &
(b) 58 f instillation (b) Diesel soot (70 m2/g) (b) 3 × 10 (a) 65 Roller
(c) 38 f (c) Diesel soot (70 m2/g) (c) 3 × 20 (b) 60 (1994);
(d) 37 f (d) Carbon black (270 (d) 3 × 15 (c) 66 Pott et
m2/g) (d) 65 al.(1994)
(e) 37 f (e) Activated charcoal (e) 3 × 10
(f) 39 f (860 m2/g) 3 × 20 (e) 27
(g) 40 f (f) NaCl solution (f) 3 × 10 (f) 0
(g) NaCl solution (g) 0.4 × 20 until (g) 0
ml spontaneous
death or 131
weeks
Wistar rat Groups Intratracheal (a) Diesel soot (native) (a) 1 × 15 Primary lung tumours (%): Heinrich
of 48 f instillation (b) Diesel soot (toluene (b) 2 × 15 (a) 17 (1994)
extract; 130 m2/g) 1 × 15
(c) Carbon black (c) 1 × 15 (b) 23
(toluene extract; (d) 1 × 15 4
270 m2/g; primary (e) Vehicle control (c) 21
particle, 15 nm)
(d) Carbon black (toluene (d) 8
extract; 270 m2/g; (e) 0
primary particle,
15 nm)
(e) NaCl solution
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Syrian Groups Intratracheal (a) Diesel particles 1.25, 1/week, 15 1 lung adenoma at high dose Shefner
golden of 50 m; instillation (b) Diesel particles + 2.5, in groups (a) and (c) after 61 et al.
hamster various same amounts of weeks weeks; no lung tumours in (1982
controls ferric oxide controls
(c) Diesel particle
extract
+ ferric oxide
Syrian (a) 3 × Intratracheal (a) Exhaust extract 0.1, 0.5, 1/week, 15 Survival rates: (a) 95, 92, Kunitake
golden 62 m instillation (b) Vehicle 1 weeks 71%; (b) 98%. No difference in et al.
hamster (b) 59 m (c) 0.5 mg tumour incidence between (a) (1986)
(c) 62 m benzo[a]pyrene and (b); 88% respiratory
tumours in positive controls
C57Bl 12 m, Dermal Acetone extract of 0.5 ml 3 times/week 16 mice dead by 10 weeks, 33 Kotin
mouse 40 f, particles for life or skin tumours by 13 months, 2 et al.
69 22-23 months skin papillomas, no tumours in (1955)
controls controls
A mouse 50 m, Dermal Acetone extract of 0.5 ml 3 times/week Males: 8 skin tumours by 16
25 f, particles for life or 1 papilloma, 3 squamous-cell
34 22-23 months carcinomas. Females: 20 skin
controls tumours by 13 months,17 tumours
at 13-17 months, no tumours in
controls
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Sencar Groups Dermal Dichloromethane 0.1, 0.5, 1/week, 50-52 Skin carcinomas: Nesnow
mouse of 40 m extracts of particles 1.0, 2.0, weeks Engine A: 3% (m), 5% (f) at et al.
+ 40 f from engines (A, B, E) or 4.0 4 mg Engine B: 3% (m) at 0.5 mg (1982a,b,
Benzo[a]pyrene 12.6- Engine E: 3% (f) at 0.1 mg 1983)
202 µg Positive control: 10-90%
C57Bl/6N Groups Subcutaneous Particles in olive oil 10, 25, 1/week, 5 First tumours palpated in: Kunitake
mouse of injection containing 5% dimethyl 50, 100, weeks; 18 week 47 at 25 mg/kg bw et al.
15-30 f sulfoxide 200, or months' week 30 at 50 mg/kg bw (1986)
500 mg/kg observvation week 27 at 100 mg/kg bw
bw week 39 at 200 or 500 mg/kg bw
Malignant fibrous histiocytomas
in 5/22 mice at 500 mg/kg bw;
0/38 in controls
PAH, polycyclic aromatic hydrocarbon; TPA, 12-O-tetradecanoylphorbol 13-acetate
Groups of 15 male cynomolgus monkeys were exposed by inhalation
to 2 mg/m3 diesel particulates for 7 h/day on five days per week for
two years. Sperm motility and velocity were similar to those of
controls (Lewis et al., 1989).
In a test for dominant lethal mutation, male Fischer 344 rats
inhaled 2 mg/m3 diesel exhaust for 7 h/day on five days per week for
six months and were subsequently mated with untreated females. Live
and dead implants and preimplantation losses were analysed on days
19-20 of gestation: no significant effects were observed (Lewis et
al., 1989). In a similar test, 100 male and 54 female T stock mice
were exposed to 6 mg/m3 diesel exhaust for 8 h/day on seven days per
week for seven weeks. There were no significant dominant lethal
effects in males or females. A reduced number of corpora lutea
(reproductive function) was the only significant result (Pepelko &
Peirano, 1983).
B7.5.2 Embryotoxicity
No increase in the frequency of sister chromatid exchange was
seen in the livers of fetuses of Syrian golden hamsters that inhaled
diesel exhaust particles at 12 mg/m3 for 8 h/day on days 5-13 of
gestation. An intraperitoneal injection of 300 mg/kg body weight of
diesel particulates on day 12 of gestation also had no significant
effect on this end-point; however, intraperitoneal injection of 23%
particulate mass extracted with methylene chloride on day 12 resulted
in a dose-dependent increase in sister chromatid exchange frequency in
fetal liver on day 13, and a doubling was seen at 320 mg/kg body
weight. It was concluded that chemicals must be eluted from diesel
particles in order for the genotoxic material to cross the placenta
(Pereira et al., 1982).
B7.5.3 Teratogenicity
Sprague-Dawley rats and New Zealand white rabbits were exposed by
inhalation to exhaust from a light-duty diesel engine, at a
particulate matter concentration of 6 mg/m3, for 8 h/day on seven
days per week.
Twenty rats were exposed on days 5-16 of gestation and 20 rabbits
on days 6-18. The numbers of viable fetuses per litter, dead fetuses
per litter, resorptions per litter, implantation sites per litter,
corpora lutea per litter, and average fetal weight did not differ
significantly from those of controls (Pepelko & Peirano, 1983).
Reproductive and developmental toxicity are considered unlikely
to be critical end-points for diesel exhaust.
B7.6 Mutagenicity and related end-points
B7.6.1 In vitro
The genetic effects of particles and particle extracts have been
described (Lewtas & Williams, 1986; Morimoto et al., 1986; Henschler,
1987). Diesel exhaust extracts rather than particles were used in most
of these studies. Point mutations were observed in vitro in bacteria
and mammalian cells. Extracts caused chromosomal aberrations, DNA
damage, sister chromatid exchange, and cell transformation (Table 38).
The mutagenic potency of diesel engine exhaust depended in several
studies on the characteristics of the diesel fuel and the type, age,
and operating conditions of the engine (Henschler, 1987). As a rule,
organic extracts of particles were mutagenic in the absence of an
exogenous metabolic activating system (S9) (Ball et al., 1990);
addition of S9 decreased (Lewtas, 1983) or suppressed (Morimoto et
al., 1986) the mutagenic activity, perhaps by increasing protein
binding of mutagenic material or by metabolic detoxification of
directly acting mutagens, such as the nitroarenes, in the Ames test.
It can therefore be concluded that PAHs and thioarenes, which must be
metabolically activated, do not account for the mutagenic potency of
diesel particles. Salmeen et al. (1985) and Beland et al. (1985)
showed that nitroarenes are the main genotoxic agents in Salmonella
typhimurium T98 in the absence of S9. A number of other
investigators have reported the presence of nitrated PAHs in extracts
of diesel engine exhaust particles (Handa et al., 1983), including
many three-, four-, and five-ring structures (Schuetzle et al., 1982).
The concentration of 1-nitropyrene, one of the prevalent species, has
been reported to be 15-25 mg/kg particulate matter, whereas
benzo[ a]pyrene has been found at concentrations up to 50 mg/kg. The
nitrated PAHs are important in the health effects of diesel exhaust
since they are effective mutagens in microbial and human cell systems
(Patton et al., 1986). Some nitrated PAHs are also carcinogenic in
animals (Imaida et al., 1991). Ball & Young (1992) found that strain
T102, which does not respond to the mutagenic action of nitroarenes,
responds to a class of oxidizing compounds that can interact with DNA;
these compounds are not nitroarenes or typical PAHs.
The effects of diesel soot particles with and without adsorbed
organic substances, diesel soot extract without particles, an isolated
fraction of PAHs, and titanium dioxide and carbon black (Printex 90)
as reference particles were investigated in vitro on hamster lung
epithelial cells (Mohr & Riebe-Imre, 1992). The substances were added
to the cells at concentrations of 100-300 mg/litre. Diesel soot
extract, but not the PAH moiety isolated from the extract, stimulated
mixed-function oxygenases. The diesel soot extract was more cytotoxic
than the PAH fraction for the epithelium of the hamster respiratory
tract, but both mixtures induced the development of micronuclei. Under
the study conditions, only the PAH fraction led to transformation of
cells. The respiratory epithelium of hamsters was more sensitive than
human lung epithelial cells investigated in parallel, perhaps because
hamster cells are generally more easily transformed than human cells.
Diesel soot particles, titanium dioxide, and Printex 90 had hardly any
cytotoxic effect but triggered transformation. The authors concluded
that a combination of effects of particles and PAHs are responsible
for the degeneration of cells.
Schiffmann & Henschler (1992) studied the effects of diesel soot
extract and isolated fractions of PAHs, oxy-PAHs, and nitro-PAHs on
hamster embryo fibroblasts in vitro. The end-points investigated
were induction of micronuclei, unscheduled DNA synthesis, and cell
transformation. Diesel soot extract induced micronuclei at
concentrations of 80-400 mg/litre, whereas the fractions of various
PAHs were strongly cytotoxic. Diesel soot extract and the PAH fraction
transformed the fibroblasts; the nitro- and oxy-PAH fractions had a
weaker transforming effect, but their cytotoxic potency was clearly
stronger. Kinetochore analysis showed a high percentage (37%) of
chromosomes in micronuclei. No unscheduled DNA synthesis was induced.
Micronuclei were also induced in human embryonic lung fibroblasts
treated with diesel soot extract under comparable experimental
conditions.
B7.6.2 In vivo
The genotoxicity of total diesel exhaust, diesel particles, and
diesel soot extracts in vivo has been investigated in somatic cells
(Table 38). All of the substances induced genotoxic responses. Total
exhaust induced only sister chromatid exchange in Syrian golden
hamsters. The frequency of micronuclei was not increased significantly
in mice, even at high doses (up to 640 mg/kg body weight). Organic
extracts had clear genotoxic effects in various assays (Henschler,
1987).
No mutagenic activity was found in urine taken from rats exposed
to diesel exhaust emission by inhalation at 2 mg/m3 for 7 h/day on
five days per week for up to two years. A slight but nonsignificant
increase in micronuclei was observed in the bone marrow of mice that
had been exposed for six months; no increase in micronuclei was
detected in rats over a 24-month period (Ong et al., 1985).
In studies of heritable mutations in T stock mice, males were
examined for point mutations, induction of dominant lethal mutations,
translocations, and spermatogonial survival; females were examined for
oocyte death and dominant lethal mutations. No significant effect was
seen on germ cells. A reduced number of corpora lutea (reproductive
function) was the only significant result (Pepelko & Peirano, 1983).
B7.6.3 DNA adduct formation
Extensive research has been done to determine whether the DNA
adducts induced in lungs by diesel exhaust are related to later
tumorigenesis.
In rats that inhaled benzo[ a]pyrene absorbed to carbon black
(20 mg/g), an adduct of the diol epoxide with deoxyguanosine was
identified (Wolff et al., 1989), demonstrating that epoxides of PAHs
are produced by oxidative metabolism. Haemoglobin and albumin adducts
were also investigated.
Methods have been developed to detect DNA adducts of
1-nitropyrene or 1,6-dinitropyrene, which are markers of diesel engine
exhaust. The adducts are analysed in rat tissue or peripheral blood
lymphocytes by the 32P-postlabelling method and may be useful for
the dosimetry of 1-nitropyrene or diesel exhaust particulates in
occupational settings (El-Bayoumy et al., 1994). Studies on adduct
formation in lung DNA induced by inhaled diesel exhaust have been
conducted by Wong et al. (1986), Wolff et al. (1990), Bond et al.
(1988, 1989, 1990a,b), and Gallagher et al. (1993, 1994), all
involving the 32P-postlabelling technique. In general, these studies
suggest that DNA adducts are good measures of the 'effective dose' of
carcinogenic compounds.
Wong et al. (1986) found increased DNA adduct formation in the
lungs of Fischer 344 rats exposed to diesel exhaust particles at
7.1 mg/m3 for 31 months. After Fischer 344 rats and Syrian golden
hamsters were exposed to dilutions of diesel engine exhaust for six
months to two years, the level of haemoglobin adducts (2-hydroxy-
ethylvaline and 2-hydroxypropylvaline) increased dose-dependently,
corresponding to metabolic conversion of 5-10% of inhaled ethylene and
propylene to their oxides (Törnqvist et al., 1988).
Bond et al. (1988) designed experiments to determine the location
of DNA adducts in the respiratory tract of rats exposed to exhaust.
Fischer 344 rats were exposed for 12 weeks to diesel exhaust soot at
10 mg/m3; they were then sacrificed, and various regions of the
respiratory tract were removed and analysed for DNA adducts. Adducts
were found only in peripheral lung tissue and nasal tissue; the total
levels were highest in the peripheral tissue (about 18 adducts per 109
bases). Thus, the levels of total DNA adducts and exhaust-induced
adducts are highest in the region of the rat respiratory tract where
tumours are formed after exposure to a carcinogenic concentration of
diesel exhaust (Mauderly et al., 1987).
Bond et al. (1990c) exposed groups of Fischer 344 rats to soot at
0.35, 3.5, 7.0, or 10 mg/m3 for 12 weeks and found that the levels
of DNA adducts were similar, at about 14 adducts per 109 bases. This
level is nearly twice as high as that found in sham-exposed rats.
Thus, DNA adduct formation in lungs is independent of the
concentration of exhaust at the levels tested. One explanation for
these results (Bond et al., 1990a) is that the lung enzymes
responsible for the metabolism of soot-associated chemicals to
metabolites that bind to DNA were saturated at the concentrations
tested. These data also suggest that other factors are important in
the carcinogenicity of diesel exhaust, since the number of adducts was
elevated at an exposure level (0.35 mg/m3) that did not increase
lung tumour incidence. The induction of DNA adducts at low
concentrations is likely to result in tumour initiation by the organic
compounds present; at higher concentrations, the particles will induce
cell death, and subsequent proliferation may well act as a promotional
event. The combination may then result in detectable increases in
tumour incidence.
Bond et al. (1990a) also investigated the time course for DNA
adduct formation and persistence. Fischer 344 rats were exposed to
soot at 7 mg/m3 for up to 12 weeks and were sacrificed at 2, 4, 8,
12, 14, and 16 weeks after the start of exposure. DNA adducts
accumulated slowly in the lung, and the number was highest at the end
of exposure, representing about 160% of the level seen in controls
exposed to air only. The levels declined rapidly after termination of
exposure and were not significantly different from those of controls
four weeks later. Thus, steady-state levels of DNA adducts would be
reached during long-term exposure to diesel exhaust.
Bond et al. (1989, 1990c) and Wolff et al. (1990) investigated
whether exposure to carbon particles without associated mutagenic
organic chemicals also increases DNA adduct levels. Rats were exposed
either to diesel exhaust or carbon black at 0, 3.5, or 10 mg/m3 for
12 weeks. Solvent-extractable organic compounds made up about 30% of
the diesel soot but only about 0.04% of the carbon black particles.
Exposure to the highest levels of both carbon black and diesel exhaust
increased the DNA adduct levels in lungs, although the levels in rats
exposed to diesel exhaust were about 30% higher than those in rats
exposed to carbon black. Heavy exposure to carbon particles can
therefore increase DNA adduct formation, although the concentrations
are lower than those of exhaust-related adducts.
Bond et al. (1990b) investigated whether DNA adducts could be
formed in specific cells of the rat lung, i.e. alveolar type II cells.
After exposure of rats to 6.2 mg/m3 diesel exhaust or carbon black
particles for 12 weeks (16 h/day on five days per week), type II cells
were isolated and their DNA analysed for adducts. Both diesel exhaust
and carbon black resulted in about a fourfold increase in total DNA
adducts in type II cells. DNA adduct levels in peripheral lung tissue
were increased by 60-80% in male and female Fischer 344 rats and
female cynomolgus monkeys but not in female B6C3F1 mice or female
Syrian hamsters after inhalation of diesel soot at 8.1 mg/m3 for 12
weeks (Bond et al., 1989, 1990c).
Gallagher et al. (1993, 1994) exposed female Wistar rats to
diesel soot at 7.5 mg/m3 and to carbon black at 11.3 mg/m3 for 24
months and measured DNA adducts in lungs. The mean adduct levels were
similar in the two groups but were not significantly greater than
those in controls. Time-course studies indicated that the levels in
the lungs of rats exposed to diesel exhaust were lower at 24 months
than after two or six months of exposure, presumably as a result of
dilution due to increased cell proliferation. The level of a single
DNA adduct, thought to be derived from a nitro-PAH in diesel exhaust
and not observed in rats exposed to carbon black or titanium oxide,
was elevated over that in controls after two, six, and 24 months. The
DNA adduct levels in control rats increased over the duration of the
24-month study as an effect of age.
Gallagher et al. (1993) compared DNA adduct formation after
exposure to diesel emissions in vitro and in vivo. After exposure
of human lymphocytes to diesel extract, five major DNA adducts were
detected, one of which was characterized as an adduct with
benzo[a]pyrene. One was also detectable in reaction products of calf
thymus DNA and diesel particle extract in vitro and in skin and lung
DNA from mice treated dermally with 50 mg diesel extract in vivo. The
differences in DNA patterns in vitro and in vivo (skin and lung)
may reflect differences in metabolic pathways.
Taken together, the studies of DNA adducts suggest that some
organic chemicals in diesel exhaust can form DNA adducts in lung
tissue and may play a role in the carcinogenic effects. As pointed out
by Bond et al. (1989), however, DNA adducts alone cannot explain the
carcinogenicity of diesel exhaust, and other factors, such as chronic
inflammation and cell proliferation, are also important.
B7.7 Special studies
B7.7.1 Immunotoxicity
During the clearance process, one possible pathway is
translocation of diesel particulates into the lymphatic channels.
After male guinea-pigs were exposed to diesel exhaust at a particle
concentration of 1.5mg/m3 for four or eight weeks, the B- and T-cell
counts in lymph nodes were not altered, and there were no significant
changes in blood or spleen (Dziedzic, 1981).
Fischer 344 rats were exposed to 2 mg/m3 diesel exhaust for
7h/day on five days per week for 12 or 24 months and the immunological
function of splenic B and T cells was measured by enumerating
antibody-producing cells in the spleen or monitoring the proliferative
response. No changes were observed (Mentnech et al., 1984).
Table 38. Genotoxicity of diesel exhaust, particles, and extracts
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity
Bacteria
S. typhimurium Point mutation (his) + + Huisingh et al. (1978)
S. typhimurium Point mutation (his) + + Loprieno et al. (1980)
S. typhimurium Point mutation (his) + Clark & Vigil (1980)
S. typhimurium Point mutation (his) + + Li & Royer (1982)
S. typhimurium Point mutation (his) + Salmeen et al. (1984)
S. typhimurium Point mutation (his) + Ong et al. (1985)
S. typhimurium Point mutation (his) + Bechtold et al. (1986)
S. typhimurium Point mutation (his; + Whong et al (1986)
SOS umu test)
S. typhimurium Point mutation (his) + Wallace et al. (1987)
S. typhimurium Point mutation (his) +a Wallace et al. (1990)
S. typhimurium Point mutation (his) + Lewis et al. (1989)
S. typhimurium Point mutation (his) + Rasmussen (1990)
S. typhimurium Point mutation (his) + + Keane et al. (1991)
S. typhimurium, E. coli Point mutation (trp) + Crebelli et al. (1991)
E. coli Point mutation (trp) + Lewtas (1983)
Mammalian cells
L5178Y mouse Point mutation (tk) + Lewtas (1983)
lymphoma cells
L5178Y mouse Point mutation (tk) + Mitchell et al. (1981)
lymphoma cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity (contd)
Chinese hamster Point mutation (hprt) + Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Casto et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt, + Morimoto et al. (1986)
lung V79 cells ATPase)
Human xeroderma Point mutation (hprt) + + McCormick et al. (1980)
pigmentosum fibroblasts
Human lymphoblast Point mutation (tk) + Barfknecht et al. (1981)
TK6 cells
Balb/c3T3 mouse Point mutation (ATPase) + (+) Curren et al. (1981)
fibroblasts
DNA damage
Syrian hamster DNA chain breaks - Casto et al. (1981)
embryo cells (alkaline elution)
Human xeroderma DNA damage + + McCormick et al. (1980)
pigmentosum fibroblasts
Rat primary Unscheduled DNA + Lewtas (1983)
hepatocytes synthesis
Chinese hamster lung Unscheduled DNA +a + Gu et al. (1994)
V79 cells synthesis
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Chromosomal effects
Chinese hamster Sister chromatid exchange + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations +b Hasegawa et al. (1988)
lung V79 cells -c
Chinese hamster Sister chromatid exchange + Hasegawa et al. (1988)
lung V79 cells
Chinese hamster Sister chromatid exchange + + Keane et al. (1991)
lung V79 cells
Human lymphocytes Chromosomal aberrations + Lewtas (1983)
Chinese hamster Sister chromatid exchange + Morimoto et al. (1986)
lung V79 cells
Human lymphocytes Sister chromatid exchange (+) Tucker et al. (1986)
Human lymhoblastoid Sister chromatid exchange + Morimoto et al. (1986)
cells
Hamster embryo Micronuclei + Schiffmann & Henschler
fibroblasts (1992)
Chinese hamster lung Micronuclei +a + Gu et al. (1992)
V79 and ovary cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Cell transformation
Balb/c3T3 mouse fibroblasts Cell transformation (+) Curren et al. (1981)
Balb/c3T3 mouse fibroblasts Cell transformation +b Hasegawa et al. (1988)
(+)c
Hamster lung epithelial cells Cell transformation + + Mohr & Riebre-Imre (1992)
Hamster embryo fibroblasts Cell transformation + Schiffmann & Henschler (1992)
In vivoa
Mouse Micronuclei, bone marrow - - + Pereira (1982)
Mouse Micronuclei, bone marrow - Lewis et al. (1989)
Mouse Micronuclei, bone marrow - Morimoto et al. (1986)
Mouse Micronuclei, bone marrow - - - Pepelko & Peraino (1983)
Mouse Micronuclei, bone marrow (+) Ong et al. (1985)
Rat Micronuclei, bone marrow - Ishinishi et al. (1988)
Rat Micronuclei, bone marrow - Ong et al. (1985)
Chinese hamster Micronuclei, bone marrow (+) - - Pepelko & Peraino (1983)
Mouse Sister chromatid exchange, - + + Pereira (1982)
bone marrow
Rat Sister chromatid exchange, - Ishinishi et al. (1988)
bone marrow
Rat Sister chromatid exchange, - Morimoto et al. (1986)
bone marrow
Rat Sister chromatid exchange, - Ong et al. (1985)
peripheral blood leukocytes
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Rat Sister chromatid exchange, - Lewis et al. (1989)
lymphocytes
Syrian golden Sister chromatid exchange, + Pereira (1982)
hamster lung cells (inhalation)
Syrian golden Sister chromatid exchange, + Guerrero et al. (1981)
hamster lung cells (instillation)
Syrian golden Sister cromatid exchange, + + Pereira (1982)
hamster lung cells (instillation)
Syrian golden Sister chromatid exchange + Morimoto et al. (1986)
hamster liver cells (transplacental)
Syrian golden hprt mutation + Morimoto et al. (1986)
hamster (transplacental)
Mouse Mutation, S. typhimurium - Morimoto et al. (1986)
(host-mediated assay)
Mouse Heritable effects on germ - Pepelko & Peirano (1983)
cells (various assays; m/f)
Drosophila Heritable effects (sex-linked Schuler & Niemeier (1981)
melanogaster recessive lethal mutation) -
his, histidine independence; trp, tryptophane independence; hprt, hypoxanthine-guanine-phosphoryltransferase (8-aza- or
6-thioguanine resistance); tk, thymidine kinase (bromodeoxyuridine or trifluorothymidine resistance); ATPase, Na+/K+-ATPase
(ouabain resistance); +, positive; (+), weakly positive
a Dispersed in artificial surfactant
b Light-duty engine
c Heavy-duty engine
d Modified from Henschler (1987) and supplemented
Fischer 344 rats and CD-1 mice were exposed to diesel exhaust
particles at 0.35, 3.5, or 7 mg/m3 for 6, 12, 18, or 24 months to
investigate whether the accumulation of diesel particulates in lymph
nodes (indicated by black discolouration) influences the subsequent
response to immunization by sheep red blood cells, evaluated by the
presence of immunoglobulin (Ig) M antibody-forming cells. In rats, the
total number of lymphoid and antibody-forming cells was significantly
increased at the medium and high aerosol concentrations. In mice, the
number of lymphoid cells was increased only at the high concentration.
The authors concluded that diesel exhaust particles have only a
minimal effect on the immune and antigen filtration functions in
lung-associated lymph nodes because the relative numbers of
antibody-forming cells and specific IgM, IgG, and IgA antibodies in
rat sera were not significantly changed (Bice et al., 1985).
Five intranasal inoculations of various doses of a suspension of
diesel engine exhaust particles in ovalbumin were administered to BDF1
mice at intervals of three weeks. Ovalbumin IgE antibody titres,
assayed by passive cutaneous anaphylaxis, were enhanced by doses as
low as 1 µg (Takafuji et al., 1987, 1989). Similarly, primary IgE
responses were increased after intraperitoneal administration to mice
of ovalbumin or cedar pollen allergen mixed with diesel exhaust
particulates (Muranaka et al., 1986).
Female CR/CD-1 mice were exposed by inhalation to 6-7 mg/m3
diesel exhaust for 2-6 h (acute), 8 h/day for 2-16 days (subacute),
or 8h/day for about 300 days (chronic). Immediately after the end
of exposure, animals were exposed to the infectious aerosols
S. typhimurium, Streptococcus pyogenes, or A/PR8-34 influenza virus.
Increased susceptibility to post-infection mortality was seen with the
bacterial but not the viral pathogens. Nitrogen dioxide and acrolein
vapour had specific effects (Campbell et al., 1981b).
CD-1 mice were exposed to 2 mg/m3 diesel exhaust for one to six
months. After three months of exposure and subsequent infection with
influenza virus, an enhanced severity of response was seen, involving
significant lung consolidation and focal macular collections of
particle-laden macrophages (Hahon et al., 1985).
B7.7.2 Behavioural effects
Sprague-Dawley rats were exposed for 8 h/day on seven days per
week for 16 weeks to diesel exhaust diluted to a particulate matter
concentration of 6 mg/m3. Spontaneous locomotor activity, measured
weekly on Wahman LC-34 running wheels, was significantly decreased
during 8, 9, 11, and 12 weeks of exposure. In rats exposed for
20 h/day on seven days per week for six weeks, forced activity on a
motorized treadmill was measured during the last week. Exhaustion
occurred in less than half the time that it occurred in control
animals. Groups of 10 rats were exposed to diesel exhaust at 6 mg/m3
for 20 h/day on days 1-7 post partum and were then held in clean air
until 15 months of age. In bar pressing acquisition training carried
out at five-day intervals for the next 42 days, the rate of learning
was much slower than in control rats. The difference was highly
statistically significant (Pepelko & Peirano, 1983).
As these three studies were conducted at a high concentration,
6 mg/m3, the practical consequences of the effects observed are not
known. The results, while limited in scope, indicate, however, that
behavioural and neurophysiological effects may be important toxic
end-points which should be investigated further.
B7.8 Factors that modify toxicity; toxicity of metabolites
Little information is available, but chemicals and other factors
may modify the toxicity of diesel emissions. For instance,
chlorophyllin, a derivative of the green pigment chlorophyll, has been
reported to inhibit or reduce the mutagenic activity of diesel
particulate extracts in the Ames test (Ong et al., 1986).
B7.9 Mechanisms of toxicity; mode of action
It is not clear whether DNA-reactive or non-DNA-reactive
mechanisms, or a combination of the two, are responsible for the
carcinogenic action of inhaled diesel exhaust in laboratory animals.
The results of several studies indicated that many PAHs (e.g.
benzo[ a]pyrene) cause a carcinogenic response in rodents, but it is
not known whether the same PAHs when associated with diesel exhaust
also induce a carcinogenic response. One hypothesis for the mechanism
of the tumorigenic response is that organic chemicals (e.g. PAHs,
nitro-PAHs) desorb from soot particles, are metabolized to reactive
metabolites, and interact with lung DNA to initiate carcinogenesis.
This would be a predominantly DNA-reactive mechanism. The observation
that organic chemicals associated with diesel soot can be metabolized
by lung cells to metabolites that can form DNA adducts after long-term
exposure to diesel exhaust supports this hypothesis. Studies with
carbon black essentially devoid of PAHs but which also form DNA
adducts do not, however, corroborate the PAH-DNA-reactive concept.
Another hypothesis is that the lung tumours that arise in rats
exposed to high levels of diesel exhaust are due to overloading of the
normal lung particle clearance mechanisms, accumulation of soot
particles, and cell damage followed by regenerative cell
proliferation. Enhanced cell proliferation may increase the mutation
frequency of key target genes. In this hypothesis, genotoxic chemicals
may not be causative factors in tumorigenicity. This view is supported
by the observations that lung cancer can be induced in rodents by
inhalation of highly insoluble particles of low toxicity that are
virtually devoid of organic chemicals (e.g. talc, carbon black, coal
dust, titanium dioxide), although high concentrations of these
particles (overload) are typically necessary.
A third hypothesis, which draws upon the above two mechanisms, is
that carcinogenesis is initiated by exposure to organic compounds
associated with diesel soot and promoted by the chronic inflammation,
cytotoxicity, and cell proliferation arising from the high
concentrations of particles deposited and retained in the lungs.
Again, however, the tumorigenic response observed with particles alone
(carbon black, titanium dioxide) does not support this hypothesis.
A key consideration is the relative contribution of different
mechanisms at different levels of exposure. As discussed later in this
section, high concentrations of particles, including diesel exhaust,
leading to high lung burdens, compromise normal clearance mechanisms.
Therefore, certain mechanisms may be invoked at high lung burdens that
may not occur at lower lung burdens. Genotoxic aromatic compounds may
take on increasing importance at lower concentrations.
Figure 3 is a diagram outlining the possible mechanism of action
of diesel exhaust, including the effects of overload conditions.
B7.9.1 Carcinogenic effects
B7.9.1.1 DNA-reactive mechanisms
Diesel exhaust contains hundreds of chemicals, including PAHs,
nitro-PAHs, alkyl-PAHs, oxy-PAHs, oxy-nitro-PAHs, and aldehydes
(Scheepers & Bos, 1992b), many of which are known mutagens and
carcinogens in laboratory animals. For example, inhaled
benzo[ a]pyrene (in tar and pitch) is carcinogenic in rat lung
(Heinrich et al., 1994). Nitro-PAHs are potent mutagens and, in some
instances, carcinogenic. In pharmacokinetic studies cited in this
monograph, clearance of PAHs and nitro-PAHs associated with the diesel
particulate fraction is retarded or delayed. Other studies have shown
clearly that the organic compounds associated with diesel soot are
bioavailable to lung cells. Mechanisms for the delayed clearance of
the PAHs have been discussed (Bond et al., 1986b; Gerde et al.,
1991b). PAHs require metabolic activation to metabolites (e.g.
epoxides) that can potentially bind to DNA. In the case of the PAHs
associated with diesel soot, it is likely that alveolar lung cells
(e.g. type II cells) are responsible for their metabolic activation,
so that they can bind to lung cell DNA (Bond et al., 1983).
Additional evidence for a DNA-reactive mechanism in the
carcinogenicity of diesel exhaust is that exposure of laboratory
animals, including rats and monkeys, to diesel exhaust results in the
formation of DNA adducts in lung cells (see section B7.6). DNA adducts
and subsequent DNA replication can result in mutations that play a key
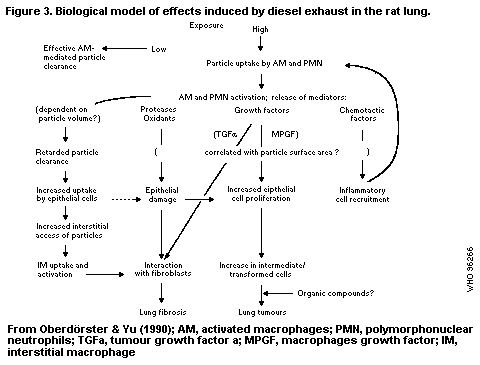 role in initiating the carcinogenic response. These mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes. There may be some as yet unidentified chemicals in diesel
exhaust with high mutagenic potential; furthermore, chemical
interactions (additive or inhibitory) may occur among the components
of diesel exhaust (Ball & Young, 1992).
B7.9.1.2 Cytotoxicity with regenerative cell proliferation
Another mechanism by which diesel exhaust may induce
carcinogenesis involves cell killing, or cytotoxicity, by the
particles or the reactive gases in exhaust, followed by regenerative
proliferation of lung cells. Enhanced cell replication may result in
an increased frequency of spontaneous mutations. Mutations could also
arise from PAH-DNA adducts or from oxidative DNA damage caused by the
reactive oxygen and nitrogen species in diesel soot, resulting in the
inflammatory response. Driscoll et al. (1994) showed that the hprt
gene mutation frequency in rat lung epithelial cells was significantly
increased when the cells were incubated in vitro with inflammatory
cells obtained by bronchoalveolar lavage from rats exposed to quartz
particles. This appears to be an important mechanism in lung tumour
induction by particles that elicit a chronic inflammatory response in
the lung. Sagai et al. (1993) showed that diesel exhaust particles
could produce superoxide anions and hydroxyl radicals in the absence
of biological activation (detection by cytochrome c and electron spin
resonance). Reactive oxygen species may induce specific DNA adducts,
such as 8-hydroxydeoxyguanosine. As mentioned above, mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes.
Several data sets cited in this monograph support this
hypothesis. For example, carbon black particles, which are virtually
devoid of PAHs but are morphologically similar to diesel soot
particles, induce lung cancer in rats. High particle burdens in rodent
lungs result in an inflammatory response (Henderson et al., 1988).
Release of cytokines and reactive oxygen and nitrogen species during
the inflammatory response to particles may result in cell death. Some
evidence exists (Wright, 1986) that diesel exhaust enhances lung cell
replication.
B7.9.1.3 Effects of particles
The importance of pulmonary particle burden on lung tumour
induction has been demonstrated clearly in long-term studies by
inhalation in rats. As reviewed above, rats have similarly increased
lung tumour incidences when exposed to diesel exhaust or carbon black
particles by inhalation for 24 months. Similarly, carbon black
particles practically devoid of PAHs induce pulmonary tumours after
intratracheal instillation. The very large surface area of carbon
black and of diesel exhaust particles after desorption of adsorbed
organic compounds in vivo may be involved mechanistically in a
tumorigenic effect. Heinrich (1994) showed that the tumour response to
different types of carbon black particles instilled intratracheally
correlated well with their respective surface areas. Pott (1991) and
Pott et al. (1993) suggested that particles with a large surface area
have greater effects in humans than in rats because insoluble
particles with no specific toxicity remain significantly longer in the
terminal airways of humans than of rats (retention half-time, about 70
days in rats and about 500 days in humans).
The correlation between particle surface area and lung tumour
incidence was examined (Oberdörster & Yu, 1990) by evaluating
published studies of inhalation of diesel and other particles. Tumour
induction in rats was best correlated with the surface area of the
particles retained in the lung rather than with the particle mass,
particle volume, or number of particles, regardless of the PAH
content. It was suggested that particle surface area and surface
properties play a decisive role ('critical surface area') and that
absorbed PAHs are not responsible for the tumour response in rats
exposed to diesel exhaust. In the human situation, however, it could
not be excluded that organic compounds and gas-phase components are
also involved, since the human particulate lung burden is much lower
than those achieved in rats after long-term inhalation.
Inhalation of diesel engine exhaust can result not only in
pulmonary tumours but also in inflammation and fibrosis and in a delay
in alveolar (not bronchial) pulmonary clearance. The mechanism by
which tumours develop due to particle overload and its associated
pathological and anatomical changes may be restricted to rats and may
not occur under environmental conditions in humans, since the lung
burdens of humans do not reach the levels that induce lung tumours in
rats. This is of importance for quantitative risk assessment. Only
occupational exposure to diesel exhaust may result in lung burdens
near or at overload conditions, particularly if the lung is already
compromised by exposure to other dusts. Bohning et al. (1982) reported
retarded particle clearance in smokers; in these people, additional
exposure to diesel exhaust may induce overload and associated toxic
effects. It is not known, however, whether the mechanisms of
particle-induced lung tumours are the same in rats and humans, and
this information is necessary for extrapolating data from rats to
humans.
Not only diesel soot (0.8, 2.5, or 7.0 mg/m3) but also carbon
black nearly completely devoid of organic compounds (Printex 90,
7.5-12 mg/m3; particle size, 10 nm) and ultrafine titanium dioxide
particles (7.5-15 mg/m3; particle size, 20 nm) caused lung tumours
in female Wistar rats exposed by inhalation for 18 h/day on five days
per week for 24 months. The tumour rate increased with increasing
particle concentrations, independently of the type of particles
inhaled. No lung tumours were observed in the rats exposed to the
lowest concentration of diesel particles. The authors concluded that
the carcinogenic component of diesel exhaust is in the inner part of
the diesel soot particle, the carbon core, and is not the relatively
small amount of carcinogenic PAHs (3.9 g benzo[ a]pyrene per gram of
diesel soot) (Heinrich et al., 1992; Heinrich, 1994; Heinrich et al.,
1995). These results were confirmed in another two-year study, in
which male and female rats were exposed by inhalation to various
concentrations of carbon black and diesel exhaust. Lung tumours were
observed with both particle types (Nikula et al., 1994).
Diesel soot does not appear to have a specific carcinogenic
effect in rats; rather, there is a nonspecific effect of particles.
B7.9.1.4 Effects of polycyclic aromatic hydrocarbons
Exposure of rats by inhalation to 2.6 mg/m3 of an aerosol of
tar-pitch condensate with no carbon core but containing 50 µg/m3
benzo[ a]pyrene and other PAHs for 10 months caused lung tumours at a
rate of 39%. The same amount of tar-pitch vapour condensed onto the
surface of carbon black particles at 2 and 6 mg/m3 resulted in
tumour rates that were roughly two times higher (89 and 72%). Since
exposure to 6 mg/m3 carbon black almost devoid of extractable
organic material caused a lung tumour rate of 18%, the tumour rate of
72% seen after combined exposure to tar-pitch vapour and carbon black
particles indicates a syncarcinogenic effect of PAHs and carbon black.
A possible mechanism is an effect of deposition of PAHs (Heinrich et
al., 1994). As the level of benzo[ a]pyrene in the coal-tar pitch was
about three orders of magnitude greater than those in diesel soot,
PAHs may play a negligible role in the tumorigenicity of diesel soot
in rats. The PAH profile in diesel soot is, however, quite different
from that in coal-tar pitch, as diesel soot contains highly mutagenic,
carcinogenic nitro-PAHs and other poorly characterized mutagens that
are not present in coal-tar pitch or on some of the carbon black
particles used in experimental studies (Heinrich et al., 1994; Nikula
et al., 1994).
In a study of various extracts of diesel exhaust particles,
30-40% of the total mutagenicity could be attributed to a group of six
nitroarenes (Salmeen et al., 1984).
A diesel exhaust particle extract was separated into a water- and
a lipid-soluble fraction, and the latter was further separated into a
PAH-free, a PAH-containing, and a polar fraction by column
chromatography. These fractions were then tested in Osborne-Mendel
rats by pulmonary implantation at doses corresponding to the
composition of the original diesel exhaust. The water-soluble fraction
did not induce tumours; the incidences induced by the lipid-soluble
fractions were 0% with the PAH-free fraction, 25% with the
PAH-containing fraction, and 0% with the polar fraction. The
PAH-containing fraction, comprising only 1% by weight of the total
extract, was shown to be responsible for the carcinogenic activity
(Grimmer et al., 1991).
Various dichloromethane extracts, each representing a complex
mixture, were obtained from particulate emissions of four
diesel-fuelled and one gasoline-fuelled automobiles (combustion), a
coke oven battery (pyrolysis), and a roofing tar pot (evaporation),
and their tumorigenic potency was compared in Sencar mice. It was
concluded that the benzo[ a]pyrene content alone could not explain
the tumorigenic activity of the mixtures (Nesnow et al., 1982a, 1983).
The lung tumour rates in rats exposed to atmospheres containing
PAHs depend not only on the PAH concentrations of the exhaust gas but
also on parameters such as the composition of the carrier particle
(mass ratio of carbon core to adsorbed layer of organics), the
dissolution rate of particle-attached organic compounds, the retention
half-time, and the cytotoxic effect of the carrier particle in the
lung (Heinrich et al., 1991).
Extracted diesel soot given intratracheally to rats induced lung
tumours, but native, unchanged diesel soot resulted in higher tumour
rates than extracted soot. Carbon black also caused tumours after
intratracheal administration, and the rate increased with decreasing
size of the primary particles (Heinrich, 1994).
Carbon black particles almost completely devoid of organic
compounds (< 0.046% extractable organic compounds and 0.6 ng/g
benzo[ a]pyrene) caused tumours in the lungs of 17% of rats after
exposure to a concentration of 6 mg/m3 for 18 h/day on five days per
week for 10 months. No lung tumours occurred in controls exposed to
clean air. Thus, the particle effect may be responsible for induction
of lung tumours in rats exposed to diesel engine exhaust, and the
effect may be related to the surface area of the carbon particle. A
PAH depot effect could lead, however, to retarded dissolution of PAHs
from the carrier, resulting in very efficient use of the extremely
small amount of carcinogenic PAHs retained in the lung after exposure
to diluted diesel engine exhaust, which contained benzo[ a]pyrene at
about 10 ng/m3 (Heinrich et al., 1991). Since diesel particles
induce DNA adducts at lower concentrations than carbon black (Bond et
al., 1989, 1990c; Wolff et al., 1990), PAHs may have tumour initiating
properties that are too weak to be detected at low doses; at higher
doses, promotional effects of the particles, perhaps with additional
initiation, may synergize to produce detectable carcinogenic effects.
B7.9.2 Non-carcinogenic effects
Diesel exhaust contains various respiratory irritants in the gas
phase and in particulate matter. Both can induce inflammatory
responses in the airways and alveolar regions of the lung. Airway
inflammation involves damage to epithelial cells, including lipid
peroxidation of cell membranes by oxidizing gaseous pollutants such as
nitrogen dioxide. Indirect effects of particles, resulting from
phagocytosis, can include the formation and release of various
mediators, including oxidants, such as superoxide anions and hydroxyl
radicals, and cytokines. These mediators may play a role in focal loss
or shortening of cilia, type II cell hypertrophy, and hyperplasia. The
latter changes can lead to the hyperplastic lesions seen in animals
exposed to diesel exahaust (Ishinishi et al., 1986, 1988; Suzuki et
al., 1990; Kato et al., 1992). Phagocytosis and subsequent clearance
of particles by alveolar macrophages can be compromised by high
particle burdens (Wolff et al., 1989; Creutzenberg et al., 1990),
which may also increase the access of particles to the interstitium,
leading to focal fibrosis (Henderson et al., 1988).
Inflammation, altered lung clearance, and hyperplastic lesions
can be considered early markers of exposure to diesel exhaust and are
the basis of the non-neoplastic effects used to determine both the
NOAEL and the 'benchmark concentration' described in section B10.1.3.
B8. EFFECTS ON HUMANS
B8.1 General population
B8.1.1 Acute exposure: olfactory, nasal, and ocular irritation
Acute exposure to diesel exhaust has been associated with
irritation of the eyes and nose. Signs and symptoms reported after
acute exposure to diesel exhaust are described in section B8.2.1.
The exhaust from e.g. poorly maintained engines or engines under
load may be visible as a black, smoky cloud. WHO (1987) considered
that sensory effects were parameters that could be used in setting
occupational exposure limits. The characteristic odour of diesel
exhaust provides a warning of its presence. At higher concentrations
and under certain operating conditions, the odour can be offensive.
The odour-causing agents are not known; however, compounds with a
relative molecular mass > 80 are probably those mainly responsible,
and aliphatic olefins (C1-C6, including acetylene), hydrocarbons
with more than six carbons, and aliphatic aldehydes are probably not
involved. Substances with low odour thresholds, such as nitrogen
dioxide (0.3 ppm) and acrolein (0.5 ppm), appear to contribute to the
odour of diesel exhaust to minor extents (5.3 and 0.25%, respectively)
(Oelert & Florian, 1972).
The ability to detect the odour and the irritating effects of
diesel exhaust vary. For example, when six subjects smelled diesel
exhaust diluted with air, the dilution factors needed to achieve the
odour detection threshold varied from 140 to 475. The concentrations
of different constituents of the exhaust at the odour threshold also
varied: formaldehyde, 0.012-0.088 ppm; acrolein, 0.011-0.046 ppm; and
nitrogen dioxide, 0.11-2.28 ppm (Linnell & Scott, 1962). When six
subjects were exposed to three concentrations of diesel exhaust for
10 min, the mean concentrations at the odour threshold were 1.3-4.2 ppm
nitrogen dioxide, 0.2-1 ppm sulfur dioxide, < 0.1 ppm formaldehyde,
and < 0.05 ppm acrolein. Three of the subjects exposed to the highest
level discontinued exposure before 10 min, while none at the lowest
level discontinued exposure although some experienced eye irritation
(Battigelli, 1965).
The health effects of inorganic gases present in diesel exhaust
are not specific and are therefore not discussed in detail; however,
it must be noted that these gases contribute to the environmental
burden.
B8.1.2 Air pollution
The concentrations of some air pollutants, such as particulates
and sulfur dioxide from the burning of coal for domestic heating and
industrial purposes, are in many parts of the world much lower than
they were several decades ago, and the associated adverse health
effects have diminished accordingly. A number of epidemiological
studies have demonstrated, however, that the relatively low remaining
levels of air pollutants are associated with a range of health
indices, including day-to-day changes in mortality (Schwartz, 1993),
visits to hospital emergency departments (Schwartz et al., 1993), and
changes in measures of lung function (Pope & Dockery, 1992). In most
studies, the associations have been strongest with the fine
particulate component of pollution, some of which may be derived from
diesel exhausts.
Vehicle traffic, both gasoline and diesel, also contributes to
the nitrogen dioxide content of urban air, and there is evidence,
primarily from experimental studies (Bauer et al., 1986; Koenig et
al., 1988; Grant et al., 1993) that it can induce short-term
decrements in lung function in asthmatic and non-asthmatic patients.
Nitrogen dioxide and volatile hydrocarbons are involved in the
formation of ozone and associated photochemical pollutants.
Associations have also been demonstrated between long-term
exposure to urban air pollutants and death rates from certain chronic
conditions. Confounding factors often make interpretation difficult,
but in an investigation conducted in the United States (Dockery et
al., 1993), air pollution attributable to fine particles was
positively associated with lung cancer and cardiopulmonary disease,
after adjustment for smoking and other relevant risk factors, but not
with other causes considered collectively.
In neither short- nor long-term studies can the possible role of
diesel particulates be specified, but diesel emissions contribute to
urban particulates, especially in Europe and developing countries. The
acidic and sulfate components of particulates, which are derived
primarily from stationary fuel-burning sources, appeared to be
implicated in a number of studies.
B8.2 Occupational exposure
B8.2.1 Effects on the respiratory system
B8.2.1.1 Symptoms
Most of the information about symptoms after acute exposure to
diesel exhaust comes from anecdotal reports of occupationally exposed
individuals. It was noted in a bus garage in London, United Kingdom,
that the buses produce a 'lachrymatory mist' when they were started up
from cold (Commins et al., 1956). In a review of 13 cases of acute
overexposure to diesel exhaust in five underground coal mines in Utah
and Colorado, United States, between 1974 and 1985, interviews in 1986
with the miners revealed that 12 had experienced symptoms of mucous
membrane irritation, headache, and light-headedness; eight had
reported nausea, and four reported a sensation of unreality and
'heartburn' (Kahn et al., 1988).
In a study designed to investigate the acute effects of diesel
exhaust on respiratory symptoms, a questionnaire was administered to
232 male workers in four diesel bus garages, and personal samples of
nitrogen dioxide and respirable particulate were obtained. After
adjustment for age and smoking (current, ex-, never), workers with the
highest exposure to respirable particulate (0.31 mg/m3) reported
significantly more cough, itchy or burning eyes, headache, difficult
or laboured breathing, a feeling of chest constriction, and wheeze
than workers with lower exposures. Those exposed to nitrogen dioxide
at >0.4 mg/m3 (> 0.3 ppm) also more frequently reported itchy or
burning eyes, difficult or laboured breathing, a feeling of chest
constriction, and wheezing. In comparison with a group of lead acid
battery workers, the reporting of the following symptoms as
'sometimes' or 'often' was significantly more prevalent among the
workers in the diesel bus garage: eyes itch, burn, or water (49.5
versus 23.5%), headache (24.2 versus 12.1%), difficult or laboured
breathing (13.5 versus 3.6%), nausea (13.5 versus 4.5%), and wheeze
(13.7 versus 4.5%) (Gamble et al., 1987a).
In comparison with 11 office workers, 17 ferry stevedores had a
greater prevalance of wheezing (24 versus 9%), chest tightness (29
versus 9%), nasal complaints (47 versus 0%), chest pain (24 versus
0%), and eye irritation (59 versus 27%); however, more of the
stevedores smoked. The concentrations of nitrogen dioxide (0.3 mg/m3)
and sulfur dioxide (0.7mg/m3) were both < 0.25 ppm; respirable
particulate was not measured (Purdham et al., 1987). In a study in an
iron ore mine where diesel engines were used underground, more workers
who were smokers reported pressure over the chest or difficulty in
getting air than the nonsmokers. No such difference was observed among
smoking and nonsmoking surface workers. The underground workers who
were smokers also had more episodes of a productive cough lasting
three weeks during several winters (Jorgensen & Svensson, 1970).
B8.2.1.2 Acute changes in pulmonary function
The pulmonary function of 60 coal miners who worked in mines
equipped with diesel engines was compared with that of 90 coal miners
not exposed to diesel exhaust. There were similar proportions of
current smokers (45% in the exposed group and 43% in the unexposed
group). Measurements made with personal dust samplers and passive
nitrogen dioxide dosimeters showed average concentrations of
0.04 mg/m3 (0.2 ppm) nitrogen dioxide, 0.4mg/m3 (0.3 ppm)
formaldehyde, and 2.0 mg/m3 respirable dust in the exposed group;
the unexposed group was exposed to 1.4 mg/m3 respirable dust. Forced
vital capacity (FVC) and forced expiratory volume in 1 sec (FEV) were
reduced during the work shift for both the diesel-exposed and
unexposed groups. The reduction was slightly but not significantly
greater in smokers than in ex- and nonsmokers. An additional analysis
with adjustment for age, exposure to respirable dust, and years of
underground mining still revealed no difference in shift-related
pulmonary function between miners exposed and unexposed to diesel
exhaust (Ames et al., 1982).
No significant shift-related change in FVC or FEV was seen among
workers on ferries transporting diesel trucks and other vehicles in
comparison with controls (Purdham et al., 1987), and no significant
change in spirometry measured over a shift was seen in 232 workers in
four diesel bus garages (Gamble et al., 1987a). Ulfvarson et al.
(1987), however, found significant decrements in FVC and FEV1 across
a shift in 23 ferry workers who had had no exposure to diesel for 10
days, with no difference between smokers and nonsmokers. The
concentration range of total particles was 0.13-1 mg/m3, that of
formaldehyde 0.04-0.6mg/m3 (< 0.03-0.5 ppm), and that of nitrogen
dioxide 0.12-4.6mg/m3 (0.06-2.3 ppm). In a repetition of the study,
a significant shift-related decrement was noted in FVC but not in
FEV1. In a later study in which filters were put on the diesel
trucks, a shift-related decrement in FVC was seen, but no subsequent
shift-related changes in FEV were noted, with or without filters
(Ulfvarson & Alexandersson, 19901).
Three male railroad workers developed asthma after heavy exposure
to locomotive emissions (Wade & Newman, 1993). The workers, none of
whom were current smokers, had no previous history of asthma or of any
respiratory disease, except one who had seasonal rhinitis. They were
riding immediately behind the lead engines of the trains, where diesel
exhaust was blown almost continually into the cab. In two, the acute
onset of asthma occurred within the first hours of exposure.
B8.2.1.3 Pulmonary effects
In a study to investigate the effects of diesel exhaust on the
cells found in bronchoalveolar lavage (BAL) fluid (Rudell et al.,
1990), eight healthy, nonsmoking volunteers were exposed to diesel
exhaust (for 60 min according to Rudell et al., 1989) at least three
weeks after an initial BAL. The median steady-state concentrations
measured in the exhaust were 4.6mg/m3 (3.7 ppm) nitric oxide,
3.1 mg/m3 (1.6 ppm) nitrogen dioxide, 22.5mg/m3 (27 ppm) carbon
monoxide, 0.5 mg/m3 formaldehyde, and 4.3 × 105 particles/cm3;
according to Rudell et al. (1989), this particle concentration
corresponds to a mass concentration of approximately 100 µg/m3. BAL
was performed again 18 h after exposure. A significant reduction
(P < 0.02) in the total number of mast cells was observed; the number
of neutrophils was slightly but significantly (P < 0.05) increased in
comparison with the values before exposure. The ratio of
T-helper:suppressor cytotoxic cells was elevated ( P < 0.02), and the
rate of phagocytosis of opsonized yeast cells by alveolar macrophages
in vitro was reduced (P < 0.02). The number of lymphocytes remained
unchanged.
B8.2.2 Epidemiological studies (noncarcinogenic effects)
Some but not all of the results from cross-sectional and
longitudinal studies on workers with occupational exposure to low
levels of diesel engine exhaust show decrements in lung function and
an increased prevalence of respiratory symptoms.
B8.2.2.1 Effects on the respiratory system
A group of 550 underground workers and 273 surface workers in six
coal mines where diesel-powered equipment was used were matched for
smoking status, age, height, and years of underground mining to
workers at other underground coal mines where diesel units were not
used. The workers at the mines with diesel engines reported
significantly more persistent cough (23.6%) than controls (16.5%) and
more frequently had exacerbations of cough and phlegm (21.7 and 16.2%,
respectively). Pulmonary function (FVC and FEV ) was decreased in both
surface and underground workers in mines where diesel engines were
used in comparison with surface and underground miners in other mines.
There was no consistent relationship between years of underground
mining and respiratory symptoms and pulmonary function; however, the
mean time spent in underground mining was only 4.7 years. Full-shift
area samples showed concentrations of respirable dust ranging from 0.4
to 16.1 mg/m3 for various jobs; the mean values from personal
samples were 0.93-2.73 mg/m3, and the nitrogen dioxide levels in
personal samples were 0.16-0.26 mg/m3 (0.13-0.22 ppm) (Reger et al.,
1982).
Pulmonary function and respiratory symptoms were studied in 1976
in 630 miners in six potash mines where diesel-powered vehicles had
been introduced between 1950 and 1964. There was no consistent
relationship with exposure to diesel exhaust, considered in several
ways, including years of exposure, cumulative exposure to dust,
nitrogen dioxide concentration, and prevalence of diesel use in the
mine. The mean length of exposure to diesel engines was, however,
relatively short: 5-14 years across mines. In addition, cumulative
exposure was measured only to total dust rather than respirable dust.
The mean values for total dust in the mines ranged from 9 to
23 mg/m3 (personal sampling); the ratio of total dust to respirable
dust ranged from 2 to 11 (area sampling), and the nitrogen dioxide
concentration was 0.2-6.3mg/m3 (0.1-3.3 ppm) (personal sampling)
(Attfield et al., 1982).
A total of 259 miners in five salt mines where diesel equipment
was used in some of the mines were studied with regard to the number
of years worked underground and cumulative exposure to respirable
particulate and nitrogen dioxide. These parameters were associated
with phlegm after adjustment for age and smoking but not cough. The
average exposure to respirable particulate in the five mines was
0.2-0.7 mg/m3, and the average exposure to nitrogen dioxide was
0.6-4.8mg/m3 (0.3-2.5 ppm). Pulmonary function (FEV 1, FVC, peak
flow) was not related to the three parameters but was slightly reduced
in comparison with that of other blue-collar workers. In another
analysis in the same population, phlegm but not pulmonary function was
associated with years of work underground in mines where diesel
engines were used, after adjustment for age and smoking. There was a
nonsignificant trend for an association between cough and shortness of
breath with years of exposure (Gamble & Jones, 1983).
Longitudinal changes in pulmonary function and chronic
respiratory symptoms were studied between 1977 and 1982 in 280 miners
exposed to diesel exhaust and 838 miners not exposed to diesel exhaust
in American underground coal mines. The mean number of years of
underground work ranged from 6.6 to 17.4 years across mines. Area
samples taken in 1977 revealed 'low' levels of dust and diesel exhaust
constituents, which were reported not to exceed 25% of current
standards, but the actual values were not given. The exposed workers
had smaller average decrements in FVC and FEV1 than workers not
exposed to diesel exhaust after adjustment for age, smoking, and years
of underground work. Additional analysis revealed no relationship with
cumulative years of underground work, but the level of pulmonary
function was not considered as a predictor of longitudinal change. The
exposed miners also had a lower five-year incidence of cough, phlegm,
and breathlessness than the miners not exposed to exhaust (Ames et
al., 1984).
Pulmonary function and respiratory symptoms were studied in 283
male diesel bus workers in four garages in two cities. The number of
years worked was a significant predictor of lower FVC and FEV1
adjustment for age, height, race, and smoking status. In comparison
after with another blue-collar population, the bus garage workers had
a higher prevalence of cough and phlegm after adjustment for age and
smoking. The prevalence of these symptoms was not related to the
number of years worked (Gamble et al., 1987b).
B8.2.2.2 Effects on the circulatory system
No consistent excess of deaths due to cardiovascular disease has
been identified in cohort studies of workers with potential exposure
to diesel emissions. Motor vehicle examiners (Stern et al., 1981), who
are probably exposed to exhaust from a variety of vehicles, had a
slight, nonsignificant increase in deaths due to cardiovascular
disease (standardized mortality ratio [SMR] = 105). No difference in
mortality was seen among men working in potash mines with
diesel-powered equipment in comparison with men in potash mines with
no diesel engines (Waxweiler et al., 1973). In a pilot study of 129
men employed in a bus company in 1951-59 for whom mortality was
ascertained until 1978 (Edling & Axelson, 1984), a fourfold increase
in cardiovascular deaths was seen in garage workers. The increase was
calculated on the basis of at least 15 years since first exposure and
10 or more years of exposure, but was based on only four deaths. This
finding was not confirmed in a follow-up of 694 men through 1983
(Edling et al., 1987). In a study of 8490 garage maintenance workers
with at least one continuous year of service between 1967 and 1975, a
deficit of deaths due to cardiovascular disease was seen (Rushton et
al., 1983).
B8.2.3 Epidemiological studies (carcinogenic effects)
The relationship between cancers of the lung and bladder and
occupational exposure to diesel exhaust has been evaluated in a number
of epidemiological studies. The occupations examined included diesel
truck drivers, bus garage workers, railroad workers, heavy equipment
operators, and stevedores. The risk for lung cancer in relation to
exposure to diesel exhaust, either self-reported or assumed from
occupational categories, has also been evaluated in case-control
studies. Only those studies that were considered relevant for an
evaluation of the carcinogenic effects of diesel exposure are included
in this assessment.
B8.2.3.1 Lung cancer
The epidemiological studies are summarized in Tables 39-41. Table
39 is a summary of nine case-control and cohort studies of workers
exposed to diesel exhaust, Table 40 summarizes nine population- and
hospital-based studies, and Table 41 presents two studies based on
information on exposure derived from registries (surveillance
studies).
Until recently, the epidemiological study of lung cancer and
exposure to diesel exhaust was limited by failure to consider the
latency and duration of exposure necessary for the development of lung
cancer, both generally considered to be about 20 years (Schenker &
Speizer, 1979). Studies of exposed workers have also been limited by a
lack of worker- and industry-specific data on exposure. Such data were
available only for the American railroad and trucking industries. For
other occupational groups, such as bus garage workers and stevedores,
indices of exposure were derived from vehicle use, fuel consumption,
or years of exposure, or simply job title unsubstantiated by exposure
assessment. In studies based in hospitals, the general population, or
registries, information on usual work in a job with exposure to
vehicle exhaust was derived from self- or surrogate reporting;
self-reporting of exposure to diesel fumes has also been used. Studies
with short durations of exposure and follow-up are not useful for
determining whether diesel exhaust is a human carcinogen. Studies in
which there is an imprecise definition of exposure to diesel exhaust
(such as self-reported job title) do not allow detection of an effect,
since the extent of exposure is unknown. In studies in which an
elevated risk for lung cancer is noted, such imprecision can result in
wide confidence intervals.
The major potential confounding factor in occupational studies of
lung cancer is tobacco smoking. Such a factor must be related not only
to the outcome but also to the exposure. In order to assess whether
tobacco use is a true confounder in a study of occupation, the smoking
rates among individuals exposed and unexposed to diesel exhaust must
be known; however, owing to the retrospective nature of many
occupational studies, smoking histories are often not available. It is
unlikely that the smoking habits of workers exposed and unexposed to
diesel exhaust within the same occupational cohort are differentially
related to the exposure, but when the effect of exposure is small, in
the order of a relative risk of 1.5 (such has been found for diesel
exhaust and lung cancer in most studies), differences in smoking that
are unaccounted for could reduce the relative risk attributable to
exposure to diesel exhaust. Interpretation of the results of studies
lacking specific information about smoking is more uncertain when
small relative risks are seen. Despite imprecise exposure histories,
one strength of hospital- and registry-based studies of lung cancer is
that smoking histories are often available.
(a) Occupationally based cohort and case-control studies
Three studies have been conducted of railroad workers exposed to
diesel exhaust. The transition from steam- to diesel-powered
locomotives occurred in the United States during the 1950s. By 1959,
95% of the locomotives in service were diesel powered. In a
retrospective cohort study of American railroad workers, the mortality
of 55 407 white male workers aged 40-64 in 1959 who had worked on the
railroads for 10-20 years was ascertained through 1980, providing a
22-year period of follow-up (Garshick et al., 1988). A yearly
three-digit job code up to the time of death or retirement was
available from the Railroad Retirement Board. The workers had held
jobs in 39 selected categories, and an industrial hygiene survey was
conducted to categorize the job codes into those associated with
regular exposure to diesel exhaust (engineers, conductors, firemen,
brakemen, and diesel locomotive repair shop workers) and those not
exposed to diesel exhaust (clerks and signal maintainers). The
concentration of respirable particulate, adjusted for cigarette smoke,
was used as an indicator of exposure to diesel exhaust (Woskie et al.,
1988a,b). A potentially confounding exposure in the railroad industry
is asbestos, which was used to insulate the steam locomotives run
previously. Thus, workers in steam locomotive repair shops would have
been exposed to asbestos. Workers who had the longest potential
duration of exposure, i.e. those aged 40-44 in jobs with exposure to
diesel exhaust in 1959, had a relative risk of dying of lung cancer of
1.45 (95% confidence interval [CI] = 1.11-1.89). Workers aged 45-49 in
1959, who would have had slightly less exposure, had a relative risk
of 1.33 (95% CI = 1.03-1.73). Exclusion of the workers with potential
past exposure to asbestos did not appreciably change these relative
risks.
The US Environmental Protection Agency has supported an effort to
obtain more detailed information on exposure in this study in order to
estimate unit risk (Pepelko & Chen, 1993). Adequate exposure-response
curves could not be drawn for years of exposure or for measurements of
current exposure. Possible reasons include an imprecise assessment of
past exposure, since only current measurements of exposure were
available; changes in exposure over time, since improved ventilation
and new diesel locomotives have been introduced, resulting in reduced
exposure; and the lack of exposure histories for the workers who would
have been exposed to diesel exhaust before 1959. In addition, the
industrial hygiene survey was performed in four smaller railroads,
where exposure may not reflect that throughout the industry; the
presence of non-diesel particulate in the respirable samples collected
could have led to exposure misclassification. Nevertheless, the
industrial hygiene survey validated the classification of jobs into
those associated and not associated with exposure.
In a case-control study of American railroad workers by the same
investigators (Garshick et al., 1987), deaths occurring between 1
March 1981 and 28 February 1982 were recorded, and 1256 from lung
cancer were matched by age to deaths from causes other than cancer or
accidents. Smoking histories were obtained from next-of-kin. After
adjustment for smoking and past exposure to asbestos, workers aged 64
at the time of death and with 20 years of work in a job with exposure
to diesel exhaust had an odds ratio of 1.41 (95% CI = 1.06-1.88).
A cohort study addressed mortality between 1965 and 1977 among
all 43 826 male railroad workers who had retired from the Canadian
National Railway Company before 1965 but who were still alive and
those who retired between 1965 and 1977. Occupation at the time of
retirement was used to classify workers as unexposed, possibly
exposed, or probably exposed to diesel fume. The relative risk of
dying of lung cancer was 1.20 for workers with possible exposure
(P = 0.013) and 1.35 for those probably exposed (P < 0.001). Similar
relative risks were obtained after exclusion of workers involved in
locomotive repair, who were most likely to have had past exposure to
asbestos. These results are consistent with those of the studies of
American railroad workers, although the degree to which the workers
were actually exposed to diesel fumes is not known. Only retired
workers were studied, excluding younger, active workers who would have
had the most exposure. No information was given about the duration of
exposure of the retired workers to diesel exhaust since many would
have worked primarily before the transition to diesel locomotives
(Howe et al., 1983).
Table 39. Occupation-based case-control and cohort studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
43 826 male pensioners of the Exposure classified by RR = 1.20 (P = 0.013) Incomplete exposure Howe et al.
Canadian National Railway experts on occupation at and 1.35 (P = 0.001) for assessment owing to (1983)
time of retirement: possible and probable lack of lifetime occupational
unexposed, possibly exposure, respectively. history. Difficult to
exposed, probably Significant trend in RR separate combustion products
exposed with increasing likelihood from diesel exhaust,
of exposure since the cohort would
have worked in both the
steam and diesel eras;
many would have had
relatively short exposure
to diesel. No data on
smoking; method of
categorizing exposure
not validated or described
in detail
Incident deaths between Personal exposure Adjusted for smoking and Possible misclassification Garshick et al.
1 March 1981 and 28 assessed by industrial to asbestos, OR = 1.41 of exposure to diesel (1987)
February 1982 in US railroad hygiene sampling in 39 (1.06-1.88) (< 64 years of exhaust since job title might
workers. 1256 cases of job categories; yearly age) for 20 years of work not reflect exposure in each
lung cancer and 2385 matched job title used to in a diesel-exposed job. case; however, this should
controls (up to two each, on dichotomize exposure For > 20 years of work in a reduce the OR.
age and date of death). Cases into exposed and diesel-exposed job relative
and controls had worked for unexposed between 1959 to 0-4 years, OR = 1.64
the railroad for > 10 years. and retirement (1.18-2.29)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
55 407 white male railroad Industrial hygiene data RR = 1.45 (1.11-1.89) in No data on smoking Garshick et al.
workers, aged 40-64 and used to categorize jobs workers aged 40-44 in (1988)
with 10-20 years of work in as exposed or unexposed 1959; RR = 1.33 (1.03-
1959. Mortality due to lung to diesel exhaust; jobs 1.73) in workers aged 45-
cancer (1694 deaths) well categorized for 49. Unexplained by exposure
determined retrospectively most of the cohort to asbestos. Workers with
through 1980 highest potential
cumulative exposure to
diesel exhaust had highest
risk for cancer
About 20 000 male London Five job categories used SMRs for all five job Duration of work not Waller (1981)
Transport workers aged 45-64. to define exposure, categories < 100 for lung considered; impossible to
667 cases of lung cancer validated by cancer (healthy worker follow individuals after
ascertained among active environmental effect), but highest SMR in retirement; no adjustment for
workers > 25 years (1950-74) concentrations of highest exposure group, not smoking
benzo[a]pyrene and significantly greater than
particles measured least exposed (underground
in 1957 and 1979 train crew)
8490 male London Transport 100 job titles grouped SMR = 101 overall, but job No validation of exposure Rushton et al.
maintenance workers (cohort into 20 broad categories category 'general hand' by air sampling; short (1983)
mortality study). Mortality of showed increased SMR for (average, 6 years) follow-up;
workers employed for > 1 year lung cancer with no no adjustment for smoking
between 1 January 1967 apparent link to diesel
and 12 March 1975 exhaust
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
695 workers in bus garages Intensity of exposure to SMR = 122 (71-196); for No adjustment for smoking Gustavsson et
who had worked for > 6 months exhaust and asbestos increasing exposure index, al. (1990)
in 1945-70. 20 deaths from according to industrial RR = 1.34 (1.09-1.64), 1.81
lung cancer identified (nested hygiene estimate; (1.20-2.71), 2.43
case-control study) duration of exposure (1.32-4.47)
based on company records
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases among non-smokers, Walrath et al.
veterans alive in 1954 and driver) among people who had but SMR calculated from (1985)
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates
given
994 male lung cancer deaths Longest job held: diesel OR = 1.55 (0.97-2.47) for Extent of actual exposure Steenland et
in 1982-83 and 1085 controls truck driver, gasoline long-haul drivers with > 18 to diesel exhaust unknown al. (1990, 1992)
(excluding lung cancer, truck driver, both years of employment; OR =
bladder cancer, accidents). types, truck mechanic, 1.89 (1.04-3.42) for diesel
Cases and controls from stevedore. Exposure truck drivers with > 35
Teamsters Union who had filed validated by industrial years of employment
claims (requires 20-year hygiene survey (adjusted for smoking)
tenure)
Retrospective cohort study of Cohort assumed to be Lung cancer: SMR = 168 No adjustment for smoking; Gustafsson et
stevedores in Sweden first exposed on basis of job (136-207) for incidence; exposure assumptions not al. (1986)
employed before 1974 for a duties, but no formal SMR = 132 (105-166) for fully justified; no
continuous period of 6 months. assessment made mortality calculations done to explore
6071 workers employed in 1961 employment time as a surrogate
with mortality determined for exposure
through 1980. 89 cases of
lung cancer noted in Swedish
Cancer Registry (71 deaths)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
Stevedores in Swedish Estimated from use of Adjusted for smoking Crude adjustment for Emmelin et al.
population defined by fuel in each port and (yes/no) depending on smoking; no industrial (1993)
Gustafsson et al. but limited years of work since use index of exposure used; hygiene data to validate
to workers in ports for which of diesel equipment exposure-response estimates of exposure to
data data on diesel fuel relationship noted, diesel exhaust
consumption were available. relative odds for medium
Case-control study with 50 exposure ranging from 1.5
cases of lung cancer detected to 2.7 and that for high
in 1960-82 matched to 154 exposure ranging from 2.9
controls on port and date of to 6.8. Lower limit of 90%
birth confidence interval < 1,
except for one
high-exposure category
RR, relative risk; OR, crude odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless
otherwise stated
Three studies reported findings among bus garage workers. Waller
(1981) studied lung cancer occurrence among London Transport staff on
the basis of job category. Diesel buses were introduced in the 1930s,
gradually replacing gasoline-powered vehicles and, in the 1950s,
electric trolley-buses. Mortality from lung cancer was ascertained for
active workers aged 45-64 between 1950 and 1974. A lower risk was seen
for people in all job categories in comparison with the general
population of the London area, indicating a 'healthy worker effect'.
The highest SMR was seen for workers who had the most exposure to
diesel exhaust (bus garage engineers), although the SMR was not
significantly different in workers with no exposure. Although this
study showed no relationship between job category and mortality from
lung cancer, the study had significant limitations. No retired workers
were followed up, so that workers who would have had significant
exposure were excluded. Since the results were not presented on the
basis of years of work (years of exposure to diesel fumes), workers
with little exposure were grouped with those with long exposure,
making it more difficult to detect an effect of diesel exhaust.
Rushton et al. (1983) ascertained the mortality of 8490 London
Transport bus garage workers who had been employed for at least one
year between 1967 and 1975. One hundred job titles were grouped into
20 broad categories. The overall SMR for lung cancer was not elevated,
and there was no relationship with any job with exposure to diesel
exhaust; however, the SMRs were not presented on the basis of years of
work in a job with exposure, and the duration of follow-up was short,
with a mean of 5.9 years.
Using a nested case-control design, Gustavsson et al. (1990)
studied a cohort of 695 men who had worked in five bus garages in
Stockholm, Sweden, in 1945-70. Diesel-powered buses were first
introduced in Stockholm in the 1930s, and after 1945 all of the
internal combustion engines in buses were diesel-powered. No
measurements of exposure were available, so exposure to diesel exhaust
was estimated by industrial hygienists on the basis of garage
ventilation, work practices, and the number of buses in the garages
and was graded on a five-point scale, each increase corresponding to a
50% increase in intensity. An index of cumulative exposure to diesel
exhaust was calculated for each worker by multiplying the exposure
level for each work period by the duration in years. Past exposure to
asbestos was estimated on the basis of historical measurements of
asbestos fibres obtained during brake-repair operations. Twenty cases
of lung cancer were identified and matched by age to six controls. The
relative risk for lung cancer increased with increasing index of
exposure to diesel exhaust: That at the lowest exposure level was 1.34
(95% CI = 1.09-1.74), that at an intermediate level was 1.81
(95% CI = 1.20-2.71), and that at the highest level was 2.43 (95%
CI = 1.32-4.47). No such increase was observed with a similar index of
exposure to asbestos. No information was available on smoking.
Diesel-powered trucks were introduced in the United States in the
1950s and 1960s. Trucking companies had completed the transition to
diesel trucks by 1960, while independent drivers and non-trucking
companies would have completed the transition later. Steenland et al.
(1990, 1992) studied 994 deaths from lung cancer occurring in 1982 and
1983 among male members of the Teamsters Union who had filed claims
for pension benefits (requiring at least 20 years of membership). Work
histories and smoking histories were obtained from next-of-kin; job
categories were also available from the Union records. The odds ratio
for lung cancer among long-haul drivers, who drove mostly diesel
trucks, with 18 or more years of employment after 1959 was 1.55 (95%
CI = 0.97-2.47) after adjustment for age, smoking, and exposure to
asbestos. Teamsters with 35 years or more of employment whose main job
was a diesel truck driver had a relative risk for lung cancer of 1.89
(95% CI = 1.04-3.42). For drivers of gasoline trucks, the relative
risk was only 1.34 and did not achieve conventional levels of
significance (95% CI = 0.81-2.22).
In an industrial hygiene survey (Zaebst et al., 1991; Steenland
et al., 1992) that accompanied this study, measurements of elemental
carbon were used to estimate exposure to diesel exhaust. The truck
drivers appeared to have received much of their current exposure to
diesel exhaust from background levels on the road rather than directly
from their engines. No historical measurements of exposure were
available.
Gustafsson et al. (1986) studied the mortality of 6071 stevedores
in Sweden who had been employed for at least six months between 1961
and 1974 through 1980. The stevedores had been exposed to both
gasoline and diesel exhausts; diesel-powered trucks were first used in
Swedish ports in the late 1950s, with a rapid increase in use in the
1960s. The SMR for lung cancer was 132 (95% CI = 105-166). The
year-specific lung cancer rates in the stevedores between 1961 and
1980 were greater than those in the Swedish male population.
In order to refine the assessment of exposure among the
stevedores and to adjust for smoking, a case-control study was
conducted in this cohort (Emmelin et al., 1993). Cases and controls
were selected from among male stevedores who had been employed for at
least six months in 1950-74; the cases were those occurring in
1960-82. On the basis of 50 cases and 154 controls, with adjustment
for smoking (yes/no), the odds ratio for lung cancer was seen to
increase with increases in three indices of exposure: years since
diesel equipment was used in the port, estimates of cumulative fuel
consumption, and years that fuel use was above a minimal level in the
port. The increases were consistent with an exposure-response
relationship. Only one of the point estimates of the odds ratios for
lung cancer reached statistical significance.
Table 40. Population- and hospital-based studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
7518 (3539 men, 3979 Occupation determined OR = 1.52 for truck drivers Exposure estimate based on Williams
women) incident invasive at interview (P > 0.05) self-reporting; not validated et al.
cancers from the Third 47% non-response; controls (1977)
National Cancer Survey. Lung consisted of other cancers,
cancer cases: 432 in men, probably diluting risk
128 in women. Combined other estimate; few cause-specific
cancer sites used as cancers and individual
controls occupations
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases in nonsmokers, Walrath
veterans alive in 1954 and driver) among people who had but SMR calculated using et al.
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates (1985)
589 cases of lung cancer Job title from next-of-kin Adjustment for smoking; No validation of exposure Damber &
reported to the Swedish for occupations held for (0.6-2.2) for professional groupings, so actual exposure Larsson
Cancer registry 1972-77 with > 1 year drivers (> 20 years of to diesel exhaust uncertain (1987)
death before 1979; 562 employment) with dead
matched dead controls (sex, controls; OR = 1.1 (0.6-2.2)
age, year of death, with living controls
municipality) drawn from
National Registry of Causes
of Death; matched living
controls (sex, year of birth,
municipality) drawn from
National Population Registry
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Montreal, Canada, Interview with subject; Adjustment for smoking; Job title used to indicate Siemiatycki
hospital-based cases and job title translated into OR = 1.2 for squamous-cell exposure to diesel exhaust; et al.
controls; 857 cases of lung likely exposure to lung carcinoma (90% CI, not validated; exposure (1988)
cancer and 1523 controls (other combustion products by 1.0-1.5) for any exposure ill-defined
cancers than lung) industrial hygienist but not elevated for other
cell types. No relationship
with years of exposure or
intensity
461 981 male volunteers Self-reported occupation Adjustment for smoking; Exposure information Boffetta
enrolled in the American and exposure to diesel > 16 years' exposure to based on self-reporting; et al.
Cancer Society prospective exhaust diesel exhaust, OR = 1.21 not validated (1988)
mortality study in 1982; aged (0.94-1.56); for 1-15 years,
40-79 at enrolment; 2-year OR = 1.05 (0.80-1.39).
follow-up; 378 622 men with Lung cancer mortality also
known exposure to diesel elevated in miners and heavy
exhaust equipment operators
1260 cases of lung cancer Job title obtained from For motor vehicle drivers Crude classification of Benhamou
and 2084 controls matched occupational history (adjusted for smoking), exposure et al.
for age, sex, hospital obtained at interview; OR = 1.42 (1.07-1.89); for (1988)
admission in France in 1976-80 exposure based on any all transport equipment
work in a job operators, OR = 1.35
(1.05-1.75). Increase in risk
with duration of employment
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Pooled data from three Job title from interviews Smoking-adjusted OR = No validation of exposure Hayes
case-control studies with with subjects or 1.5 (1.1-1.9) for truck classification; unclear if et al.
2291 male lung cancer cases next-of-kin; years of work drivers with > 10 years of most exposure was to (1989)
and 2570 controls. Analysis in motor exhaust-related work and job history obtained diesel exhaust or if job
limited to 1444 cases and occupation calculated by direct interview. For all titles selected as 'exposed'
1893 controls who provided motor exhaust-related were actually exposed to
information at interview occupations and > 10 years diesel exhaust
of work, OR = 1.5 (1.2-1.9)
Hospital-based study of 2584 Occupational titles and Smoking-adjusted OR for Exposure histories not Boffetta
cases in 18 hospitals in six self-reporting of exposure occupations with probable validated et al.
US cities; 5099 controls exposure to diesel exhaust (1990)
= 0.95 (0.78-1.16). For
self-reported exposure,
OR = 1.21 (0.78-2.02)
Detroit, USA, area; 3792 Interview to obtain full For drivers of heavy trucks Exposure histories not Swanson
cases and 1966 controls occupational history; with > 20 years of work, validated et al.
identified through cancer analysis by job title OR = 2.5 (1.4-4.4), with (1993)
surveillance system adjustment for smoking;
exposure-response
relationship with years of
work. For drivers of light
trucks with > 20 years of
work, OR = 2.1 (0.9-4.6)
OR, odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless otherwise stated
(b) Population- and hospital-based studies
In a study of 107 563 American veterans who were alive in 1954
and were followed to the end of 1969, the SMR among smokers who
reported their occupation as truck or tractor driver was 153, based on
23 cases. Most of these individuals would have had little exposure to
diesel exhaust, however, since diesel trucks were introduced in the
United States only in the 1950s and 1960s (Walrath et al., 1985).
In the Third National Cancer Survey in the United States,
self-reporting of work as a truck driver and adjustment for smoking
resulted in an odds ratio of 1.52, which was not statistically
significant (Williams et al., 1977). In a study of 589 cases of lung
cancer reported to the Swedish Cancer Registry between 1972 and 1977
among people who had died before 1979, the odds ratio for > 20 years
of work as a professional driver was 1.2 (95% CI = 0.6-2.2) after
adjustment for smoking (Damber & Larsson, 1987). In a hospital-based
study of 857 cases of lung cancer and controls, occupational histories
obtained by interview were interpreted by an industrial hygienist.
After adjustment for smoking, the odds ratio for squamous-cell
carcinoma of the lung was 1.2 (90% CI = 1.0-1.5) in association with
any exposure to diesel exhaust (Siemiatycki et al., 1988). The
mortality of 378 622 men between 1982 and 1984 was analysed in the
American Cancer Society prospective study of cancer on the basis of
self-reporting of exposure to diesel exhaust. After adjustment for
age, smoking, and other occupational exposures, including asbestos,
coal-tar and pitch, and gasoline exhaust, the relative risk for lung
cancer for men with 16 years of exposure was 1.21 (95% CI =
0.94-1.56). For truck drivers who reported exposure to diesel exhaust,
the relative risk was 1.22 (95% CI = 0.77-1.95) (Boffetta et al.,
1988).
In a study in France, 1260 male cases of lung cancer and 2084
controls matched on age and hospital were collected between 1976 and
1980; job titles and smoking histories were obtained by interview, and
odds ratios were calculated on the basis of any work in a particular
occupation. After adjustment for smoking, the odds ratios were 1.35
(95% CI = 1.05-1.75) for transport equipment operators and 1.42 (95%
CI = 1.07-1.89) for motor vehicle drivers. There was no increase in
risk with increasing years of work in a job, but details of the
analysis were not presented (Benhamou et al., 1988).
The data from three case-control studies carried out by the
American National Cancer Institute between 1976 and 1983 were pooled
in order to study lung cancer in people with occupations associated
with motor vehicles. The analysis was limited to 1444 male patients
with lung cancer and 1893 controls who provided information on smoking
and occupation at an interview. The odds ratio for lung cancer for 10
or more years of employment in any occupation related to exhaust from
either diesel or non-diesel engines was 1.5 (95% CI = 1.2-1.9), after
adjustment for age and smoking. In truck drivers, the odds ratio was
1.5 (95% CI = 1.1-1.9) (Hayes et al., 1989).
In a case-control study of patients with lung cancer in 18
hospitals in six American cities, 2584 cases seen between 1969 and the
late 1980s were matched to at least one control on the basis of age,
sex, hospital, and year of interview. A smoking history and usual
occupation were obtained by interview. After 1985, reports of
self-reported exposure to diesel exhaust were obtained for 477 lung
cancer patients and 946 controls. Exposure was graded as 'probable' if
the individual had usually worked on railroads (the specific job was
not used) or in a variety of jobs related to motor vehicles. For these
individuals, the odds ratio for lung cancer was 1.31 (95% CI =
1.09-1.57); for those reporting usual occupation as a truck driver,
the odds ratio was 1.31 (95% CI = 1.03-1.67). After adjustment for
cigarette use, age, race, and date of interview, however, the odds
ratios for lung cancer were reduced to 0.95 (95% CI = 0.78-1.16) for
individuals with probable exposure and 0.88 (95% CI = 0.67-1.15) for
truck drivers. For self-reported exposure to diesel exhaust, the odds
ratio was 1.21, after adjustment for smoking, age, and other potential
confounding variables including exposure to asbestos, education, and
race, but did not achieve statistical significance (95% CI =
0.73-2.02) (Boffetta et al., 1990).
In a study of 3792 cases of lung cancer in men in Detroit, United
States, newly diagnosed in 1984-87, an occupational history and
information on smoking were collected by interview with the subject or
a surrogate. Controls were men with colon or rectal cancer. After
adjustment for smoking, the odds ratio for lung cancer was 2.5 (95% CI
= 1.4-4.4) among white men who had driven heavy trucks for 20 years
and 2.1 (95% CI = 0.9-4.6) for drivers of light trucks for 20 years.
There was a significant increase in the odds ratio for lung cancer
with increasing years of work for drivers of both types of truck
(Swanson et al., 1993).
(c) Registry-based, surveillance studies
Table 41 summarizes two studies in which information on exposure
was based on occupational titles obtained from censuses. No
information was available on smoking or on length of employment. Both
studies reported an elevated risk for lung cancer. Ahlberg et al.
(1981) found a relative risk of 1.33 (95% CI = 1.13-1.56) for
professional drivers, and Hansen (1993) found an SMR for all
respiratory cancer in truck drivers of 160 (95% CI = 128-198).
Table 41. Surveillance studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
Swedish census-based cancer Listing of occupation in RR = 1.33 (1.13-1.56) No validation of exposure; Ahlberg et al.
incidence registry; 154 cases census no adjustment for smoking (1981)
among professional drivers
Retrospective cohort study of Job title in census 84 cancers in truck drivers; No adjustment for smoking; Hansen (1993)
14 225 truck drivers in 1970; SMR =160 (128-198); information on specific
mortality determined until expected number based exposure or length of
1980. Referent group of persons on rates in referent employment
with no exposure to combustion population
products on basis of census
job title
RR, relative risk; SMR, standardized mortality ratio; in parentheses, 95% confidence interval
Table 42. Case-control studies of urinary bladder cancer in individuals with possible exposure to diesel exhaust
Population Exposure assessment Results Reference
Incident cases in 480 men Any exposure to diesel and Based on 15 cases and controls with discordant Howe et al.
and 152 women in three traffic fumes obtained at exposure histories, OR for exposure to diesel and (1980)
Canadian provinces, April interview traffic fumes = 2.8 (0.8-11.8); not adjusted for
1974-June 1976; each case smoking
matched by age and sex to
a neighbourhood control
Incident cases in 303 white Occupation and industry For any employment in trucking, RR = 2.2 (1.1-4.4) Silverman et al.
men in metropolitan Detroit, obtained at interview based on 28 cases and 13 controls. For any work in (1983)
USA, December 1977-November railroad and railway express services, RR = 1.9
1978; 2986 controls (0.9-3.8) based on 22 cases and 12 controls, not
selected by random-digit adjusted for smoking. For any employment as a truck
dialling but of same age driver (42 cases and 18 controls), adjusted for
distribution as cases smoking, RR = 2.1 (1.4-4.4). For > 10 years as truck
driver, smoking-adjusted RR = 5.5 (1.8-17.3), and for
truck drivers who ever drove vehicles with diesel
engines, smoking-adjusted RR = 11.9 (2.3-61.1).
Elevated risk for truck drivers with employment after
rather than before 1950, when diesel truck use became
more prevalent
Table 42 (contd)
Population Exposure assessment Results Reference
White residents of New Occupation and exposure For any employment as a truck driver (35 cases, 53 Hoar & Hoover
Hampshire and Vermont, to diesel fuel or engines controls), OR = 1.5 (0.9-2.6), not adjusted for smoking. (1985)
USA, who died of bladder by next-of-kin Those employed 1930-49 had the highest OR (2.6;
cancer 1975-79 (on death 1.3-5.1) after adjustment for coffee drinking and
certificate) matched to one smoking. For 26 cases and 39 controls who reported
control with cause of death exposure to diesel fuel or engines, OR = 1.5 (0.8-2.8),
other than suicide, matched not adjusted for smoking. Significant trend for
on state, sex, race, and age, increased relative odds up to 39 years of exposure
and a second control with to diesel exhaust
same criteria but also matched
on county. Information obtained
for 325 cases and 673 controls
Cases in 512 men in Turin, Occupation obtained at In truck drivers, based on 16 cases and 16 controls, Vineis &
Italy, matched by age to 596 interview OR = 1.2 (0.6-2.5), not adjusted for smoking Magnani (1985)
hospital controls without cancer
Cases in 194 men in 18 hospitals Usual occupation obtained; Only 16 cases in occupations with potential exposure Wynder et al.
in six US cities, January likelihood of exposure to to diesel exhaust. ORs all < 1 for warehousemen, bus (1985)
1981-May 1983. Controls diesel exhaust determined and truck drivers, and heavy equipment operators. In
were persons hospitalized at on basis of estimates of railroad workers, based on two cases and one control,
the same time as the case but percentage of workers with OR = 2. For any exposure, with adjustment for smoking,
for diseases not related to potential exposure in that OR = 0.87 (0.47-1.58). For high exposure, OR = 1.68
cigarette smoking. Each case occupation: high exposure, (0.49-5.73), not adjusted for smoking
matched to three controls > 20% of workers; moderate
(total, 582) by age, race, year exposure, 10-19% of workers
of interview, and hospital of
admission
Table 42 (contd)
Population Exposure assessment Results Reference
1909 new cases in white men Occupation obtained at After adjustment for smoking, RR for men usually Silverman
from 10 metropolitan areas in interview working as a truck driver = 1.5 (1.1-2.0), based on 99 et al. (1986)
the USA, 1977-78. Up to two cases and 123 controls. For those ever employed as
controls (total, 3569) selected truck driver, increased risk with increasing years of
by random-digit dialling and employment; however, truck drivers first employed
matched for age and < 40 years since diagnosis had no increased risk. OR
geographic area with case for bus driver as usual occupation = 1.5 (0.6-3.9),
based on 9 cases and 13 controls, adjusted for smoking
Cases in 99 men in La Plata, Occupational history Based on 20 cases ever employed as a truck or railway Iscovich et al.
Argentina, matched on age to obtained at interview driver, RR = 4.31 (P < 0.05); reduced whenr (1987)
99 hospital controls without adjusted for smoking, but no details provided
cancer and 99 neighbourhood
controls
Cases in 371 men and women Occupational history After adjustment for age and sex, RR = 1.55 (1.06-2.28) Jensen et al.
in the Danish Cancer Registry. at interview any employment as a land transport worker; based (1987)
Controls (771) selected randomly on 51 cases and 73 controls. After adjustment for
from general population smoking, age, and sex, years of work as a land transport
worker, and years of work as a bus, taxi, or truck
driver significantly associated with increased risk
Deaths in 731 men in Ohio, Yearly listing of Work as a truck driver > 20 years, OR = 12 (P < 0.01), Steenland
USA, 1960-82, identified on occupation obtained from six cases and one control; for > 20 years as a railroad et al. (1987)
death certificates and city commercial directories worker, OR = 2.21 (P < 0.01), based on 22 cases, not
matched to six controls without adjusted for smoking
tumours of the urinary tract or
pneumonia on age, year of
death, and race
Table 42 (contd)
Population Exposure assessment Results Reference
Cases in 826 men and women Occupational history and For any exposure of men to exhausts, OR adjusted for Risch et al.
diagnosed 1979-82 in exposure to 'exhausts' cigarette smoking = 1.16 (0.91-1.48). For men ever (1988)
Edmonton, Calgary, Toronto, obtained at interview exposed 8-28 years before diagnosis, OR = 1.21
and Kingston, Canada, (0.93-1.58). For men in jobs with any contact with
matched by age, sex, and diesel or traffic fumes 8-28 years before diagnosis,
area of residence to 792 adjusted for smoking, OR = 1.69 (1.24-2.31);
randomly selected population significant trend with increasing years of exposure
controls
Cases in 486 men and women Interview with subject; After adjustment for smoking, OR = 1.0 (90% CI, Siemiatycki
in Montreal, Canada, hospitals job title translated into 0.8-1.2) for any exposure to diesel exhaust, based et al. (1988)
and 2196 controls with likely exposure to on 82 cases with exposure
diseases other than lung combustion products by
or kidney cancer industrial hygienists
Cases in 136 men in 18 Usual occupation and For any exposure, OR = 1.24 (0.77-2.00), based on Iyer et al.
hospitals in six US cities. self-reporting of exposure 41 cases, with adjustment for smoking (1990)
Two controls without to diesel exhaust obtained
tobacco-related diseases matched at interview; grouped into
to each case on age (< 2 years), probable and possible
race, hospital, and year of exposure
interview
Table 42 (contd)
Population Exposure assessment Results Reference
256 cases and 287 controls in Job titles for all jobs held For any exposure to diesel exhaust, RR = 1.7 Steineck et al.
population-based study of all obtained by postal (0.9-3.3), based on 25 cases and 19 controls, adjusted (1990)
urothelial cancer in Stockholm, questionnaire; industrial for year of birth and smoking. For subjects considered
Sweden, 1985-87, among hygeienists reviewed titles to have moderate or high exposure, RR = 1.1 (0.3-4.3),
men born 1911-45 and categorized subjects as but based on only four cases and five controls
exposed to various
substances
Cases in 658 men in seven Interview with subject; job After adjustment for smoking, OR for work in road Cordier et al.
French hospitals, 1984-87, title and duties translated transport = 1.02 (0.62-1.69), based on 36 cases and (1993)
matched for age, race, and into likely exposure to 35 controls. For stevedores, OR = 1.31 (0.87-1.98);
place of residence to 658 diesel exhaust by industrial for material-handling equipment operator, OR = 7.67
controls selected randomly hygienists (0.96-61.4); and for transport equipment operators,
among patients admitted OR = 0.88 (0.62-1.26). For diesel fumes, OR = 0.99
for diagnoses other than (0.32-3.03), based on seven cases and two controls
cancer
Cases in 153 men in one Occupational history For any work as a road transport worker, Notani et al.
hospital in Bombay, India, obtained at interview smoking-adjusted OR = 1.36 (0.5-3.7), based on eight (1993)
1986-90, matched by age to cases and nine controls
212 controls with oral or
pharyngeal cancer or benign
oral disease
OR, odds ratio; RR, relative risk; in parentheses, 95% confidence interval (CI), unless otherwise stated
B8.2.3.2 Urinary bladder cancer
Fifteen case-control studies of urinary bladder cancer in
relation to presumed exposure to diesel exhaust are summarized in
Table 42. The limitation of all of these studies is that exposure to
diesel exhaust was not clearly characterized. Howe et al. (1980) used
any self-reported exposure to fumes that included diesel fumes, while
Hoar & Hoover (1985) used reports by next-of-kin of work with any
diesel fuel or engines or work as a truck driver. Risch et al. (1988)
obtained a history of exposure to exhaust by interview. Wynder et al.
(1985) estimated the likelihood of exposure to diesel exhaust during a
worker's lifetime on the basis of usual occupation, obtained at
interview, and attempted to divide exposure into high and moderate. In
a later study, self-reported exposure to diesel exhaust was recorded
(Iyer et al., 1990). Silverman et al. (1983), Vineis & Magnani (1985),
Silverman et al. (1986), Jensen et al. (1987), Iscovich et al. (1987),
and Notani et al. (1993) based their analyses of the risk for bladder
cancer on employment in an occupational category with a high
likelihood of exposure to diesel exhaust, such as truck driver,
railroad worker, transport worker, bus driver, and other related
occupations. In the studies of Siemiatycki et al. (1988) and Steineck
et al. (1990), occupational histories were reviewed by industrial
hygienists to link occupational titles to possible exposure. Cordier
et al. (1993) also used expert review of occupational histories to
estimate exposure to diesel exhaust on the basis of job title, but
additionally examined specific job titles. Furthermore, exposure was
usually assumed to have occurred if the person had ever been employed
in an occupation or had ever been exposed to diesel exhaust or other
fumes. Silverman et al. (1983, 1986) and Jensen et al. (1987) used
self-reported years of work as a truck driver. Self-reporting of
exposure poses a potential problem of differential recall among cases
and controls, as cases may recall exposure to diesel fumes or work in
a diesel-exposed job more readily than controls. Steenland et al.
(1987) used city directories listing occupation to obtain independent
occupational histories.
As the available occupational histories were crude, it was also
difficult to assess latency or duration of exposure. Silverman et al.
(1983) found that the relative risk (adjusted for smoking) was 5.5
(95% CI = 1.8-17.3) for truck drivers with 10 or more years of work
experience and 11.9 (95% CI = 2.3-61.1) for those with a history of
driving vehicles with diesel engines. Truck drivers employed after
1950, when diesel truck use became prevalent, had the highest risk. In
a later, larger study (Silverman et al., 1986), men who usually worked
as a truck driver or deliveryman had a relative risk of 1.5 (95% CI =
1.1-2.0), adjusted for smoking. The risk increased with increasing
years of work; however, truck drivers who had started work fewer than
40 years before diagnosis, who would have driven primarily diesel
engines, did not have an increased risk for bladder cancer. Hoar &
Hoover (1985) also noted increased relative odds with increasing years
of employment in jobs with reported exposure to diesel exhaust.
An additional limitation of the 15 case-control studies is that
results indicating an effect of presumed exposure to diesel exhaust or
a specific occupation are based on relatively few cases and controls,
even when conventional levels of statistical significance were
achieved. The study of Silverman et al. (1986), in which exposure was
defined as working as a truck driver or deliveryman, had the largest
number of exposed individuals (99 cases and 123 controls). Four
additional cohort studies not listed in the table (Howe et al., 1983;
Rushton et al., 1983; Schenker et al., 1984; Boffetta et al., 1988),
which were designed mainly to examine lung cancer and exposure to
diesel exhaust, also had too few cases of bladder cancer on which to
base meaningful conclusions. One cohort study of Danish bus drivers
(Netterstrom, 1988) showed an elevated SMR of 153, which was of
borderline statistical significance (95% CI = 91-217), but this result
was based on 13 cases.
Misclassification of exposure to diesel exhaust would hide any
increase in risk for bladder cancer, since many unexposed individuals
would be included with those classified as exposed. Diesel exhaust is
responsible for most of the respirable particles in mixed vehicle
exhausts, and exposure to respirable particles with adsorbed PAHs is
presumed to result ultimately in an increased risk for bladder cancer
due to metabolic transformation and urinary excretion of carcinogens.
The risk for bladder cancer is also increased in cigarette smokers and
in workers exposed to dyes containing aromatic amines (Ruder et al.,
1990). Coffee has been suggested to be a risk factor for bladder
cancer but has not been clearly implicated (Howe et al., 1980). The
classification of exposure to diesel exhaust is sufficiently crude in
the papers summarized in Table 42, however, that even when smoking was
considered, the effect of other, unmeasured confounding exposures
cannot be excluded. The actual contribution of diesel exhaust to an
increased risk of bladder cancer is uncertain, even when an effect of
presumed exposure was noted (Silverman et al., 1983; Hoar & Hoover,
1985; Silverman et al., 1986; Iscovich et al., 1987; Steenland et al.,
1987). Although the literature suggests that truck drivers have an
increased risk for bladder cancer, the specific exposure responsible
has not been determined.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Virtually no data are available relating specifically to the
effects of diesel fuel exhaust emissions.
The growth and photosynthesis of liquid cultures of the green
alga Chlamydomonas reinhardtii were examined after incubation with
iso-octane extracts of diesel particulate exhaust containing 51
compounds (mainly polynuclear aromatic ketones and pure PAHs)
identified by gas chromatographic-mass spectrometric analysis. At
concentrations up to 0.125% by volume, dose-dependent growth
retardation of the algae was observed. Higher concentrations (no
details given) caused death. The algae adapted to sublethal
concentrations of the extract over a period of several days;
thereafter, toxic extracts at concentrations up to 2.5% by volume
affected neither growth nor photosynthesis. It was noted that the
amounts of certain components decreased during incubation, suggesting
uptake into the cells (Liebe & Fock, 1992).
B10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Hundreds of chemical compounds are released during the combustion
of diesel fuel. The characteristics and amount of exhaust depend on
the type of diesel engine, its operating conditions and adjustment,
and the composition of the fuel. The following evaluation focuses
mainly on the risks to human health associated with exposure to diesel
particulate matter, since the large amount of soot particles emitted
is the main characteristic of diesel exhaust.
In general, the risk assessment paradigm proposed by the National
Academy of Sciences of the United States (US National Reserach
Council, 1983) will be followed. The four steps in this process are:
hazard identification, dose-response assessment, exposure assessment,
and risk characterization. The outcome of these individual steps is
critical for taking risk management decisions, including setting
exposure standards that have consequences for public health, and for
social, economic, and political issues.
The epidemiological studies in humans and the studies in
experimental animals considered useful for human health risk
assessment are discussed in this section. Both carcinogenic and other
effects are considered, but a threshold is assumed to exist only for
non-cancer effects. An IPCS method for deriving health-based guidance
exposure limits provides further details (IPCS, 1993).
B10.1 Exposure of the general population
The exposure of the general population to diesel exhaust varies
with proximity to diesel-powered vehicles. For example, higher levels
of diesel exhaust constituents are present in busy streets and parking
areas than in rural areas. The concentrations of the main gaseous
components resulting from diesel exhaust can be estimated from
specific emission factors, the contribution of diesel vehicles to
total traffic, and atmospheric dispersion models. As assumptions are
necessary to attribute the emissions of the gaseous components to
diesel sources, the validity of such estimates is considered to be
limited.
On a global basis, the contribution of diesel exhaust to the
total emissions of particulate matter varies widely, depending on the
percentage of diesel vehicles in the total volume of traffic,
maintenance of individual engines, fuel quality, and emission control
techniques. Such factors also lead to wide local variations in the
concentrations to which humans are exposed.
Measurements and calculations of ambient concentrations of diesel
particulates near roads provide a daily average range of 8-42 µg/m3.
The estimated annual average concentrations in Germany are 5-10 µg/m3
in urban areas and 1.5 µg/m3 in rural areas, and those in the United
States are 1-2 µg/m3 in urban areas and 0.6-1 µg/m3 in rural
areas. As the proportion of diesel vehicles is smaller in the United
States than in Europe, the concentrations in 1990, estimated from
national averages, were 2.0 µg/m3 in an urban area and 1.1 µg/m3
in a rural area, with predictions of 1.2 g/m3 for urban areas and
0.6 µg/m3 for rural areas in 1995.
Diesel particulates form part of the fine particle range
(< 2.5 µm diameter). The average concentration of this fraction of
suspended particulate matter as a whole, measured in six cities in the
United States during 1979-85, was 11-30 µg/m3 or, in terms of
inhalable particles (<10-15 µm diameter; equivalent to particulate
matter with a diameter < 10 µm [PM10]), 18-46 µg/m3 (Dockery et
al., 1993). In these locations, diesel particulates probably
represented less than 10% of the suspended particulates measured as
PM10; considerably higher proportions were found in London and other
large cities in the United Kingdom on the basis of data on emissions
(Quality of Urban Air Review Group, 1993), but few data are available
that are related directly to ambient concentrations of the diesel
component. The one experimental study that has been reported (Horvath
et al., 1985), carried out in Vienna, Austria, using chemically
labelled fuel, yielded a value for the background concentration of
diesel particulates within the city of 11 µg/m3.
B10.2 Occupational exposure
Occupational exposure to airborne diesel particulate matter has
been well determined for only two occupational groups, railroad
workers (Howe et al., 1983; Garshick et al., 1987, 1988) and workers
in the trucking industry (stevedores, local and road truck drivers,
and mechanics) (Emmelin et al., 1993). The levels of exposure during
work shifts are about 51-192 µg/m3 for railroad workers exposed to
diesel locomotive exhaust, roughly 25 µg/m3 for truck drivers, and
156 µg/m3 for stevedores (assuming that elemental carbon represented
20% of respirable particulate). These levels would be expected to vary
with the amount of ventilation available. Exposure to diesel exhaust
can also vary over a worker's lifetime, even within the same job. In
the railroad industry, exposure to diesel exhaust was greater in the
years after the introduction of diesel equipment. Reports of working
conditions in the 1950s and 1960s in diesel repair shops cited 'smoky'
atmospheres that were probably related to use of diesel equipment and
poor ventilation (Woskie et al., 1988b). No direct information is
available that allows reconstruction of accurate levels of past
exposure.
Reports of concentrations of diesel exhaust in other industries
are not accurate because of the presence of other dusts, although
attempts have been made to measure the contribution of diesel exhaust
(Daniel, 1984; Lehmann et al., 1990). Even for American railroad
workers (Woskie et al., 1988a), the contribution of other dusts could
not be determined, although cigarette smoke particulate was taken into
consideration. Consequently, the concentrations of diesel exhaust
reported in some jobs on railroads where other dusts (such as sand)
were present might have been lower.
B10.3 Non-neoplastic effects
B10.3.1 Hazard identification
B10.3.1.1 Humans
Diesel exhaust contains gases that irritate the nose, throat, and
eyes. It also contains particles which, together with the gases, can
cause airway irritation. Diesel exhaust may be recognized by its
characteristic odour. After acute exposure, it may induce mucous
membrane irritation, headache, and light-headedness. When volunteers
were exposed to diesel exhaust with an estimated particle
concentration of 100 µg/m3 for 1 h, several indicators of an
inflammatory response were observed in bronchioalveolar lavage fluid
(see section B8.2.1). In a well-conducted study on diesel-bus garage
workers, those with the highest exposures to respirable particulate
(> 0.31 mg/m3) reported significantly more cough, itchy or burning
eyes, headache, and wheeze, after adjustment for age and smoking, than
people with lower exposure. The contribution of diesel exhaust to the
respirable particulate is not known. Exposure to nitrogen dioxide at
> 0.3 ppm also resulted in more eye irritation and wheeze. Studies of
exposure indicate, however, variability in the ability of individuals
to detect the odour and the irritating effects of diesel exhaust.
Several cases of persistent asthma and asthma attacks have been
reported after acute exposure.
No consistent change in pulmonary function has been reported over
a work shift, although in one study of ferry workers, a decline in FVC
and FEV1 was noted. Specific measurements of diesel exhaust are
lacking in these studies, although total dust and respirable dust were
sometimes measured. Cases of persistent asthma have been reported in
railroad workers acutely exposed to apparently high levels of whole
exhaust, but the mechanism is unknown.
Studies of the possible chronic effects of exposure on
respiratory parameters are limited to occupational cohorts who are
still at work and thus have relatively short durations of exposure.
Such cohorts are likely to be healthier than older cohorts, which
include retirees who had longer exposure. Although excess respiratory
symptoms and reduced pulmonary function have been reported in some
studies, it is not clear whether these are long-term effects of
exposure.
B10.3.1.2 Experimental animals
Long-term studies in laboratory animals are described in section
B7.3. Many end-points were measured in the respiratory system of
several species of rodent after two or more years of exposure and at
several intermediate times. Adverse effects were seen after a
sufficient particle load had accumulated in the lung; and the
appearance of the effects is determined by both the concentration and
the duration of exposure. The results of these studies illustrate the
response of the respiratory tract in terms of biochemistry,
histopathology, cytology, pulmonary function, inflammation, and
clearance of particles. Early events in the pathogenesis of this
response include phagocytosis of inhaled particles. As the lung burden
increases, particle-filled macrophages are observed in the alveoli. At
lung burdens associated with particle overload, migration of these
macrophages out of the lung appears to be inhibited, and an early
event in the development of lung damage is the accumulation and
aggregation of clusters of particle-filled macrophages. Degeneration
of macrophages is observed, and the numbers of macrophages and
neutrophils in the alveolar and interstitial spaces increase.
Damage to the alveolar epithelium in areas surrounding these
macrophage clusters includes cell damage and proliferation.
Biochemical changes are seen in lavage fluid and lung tissue. The
alterations to particle-filled macrophages result in decreased
function and impaired particle clearance, the latter leading to a more
rapid increase in the lung burden of particles. Late effects include
inflammatory and proliferative responses, leading to fibrotic
responses. The sequence of these events has not been distinguished
clearly in experimental work; they are likely to be interrelated and
to occur, to some extent, concurrently.
The possible mechanism of action of diesel exhaust (discussed in
section B7.9) includes a role of the gas phase, as opposed to the
particle phase, in causing lung damage. The non-carcinogenic toxicity
of diesel exhaust is considered to be due to the particle content,
since no effects are seen in rodents exposed to diesel exhaust that
has been filtered to remove particulate matter. Another issue in the
dosimetry of diesel exhaust is the role of the insoluble carbon core
in relation to that of the adsorbed organic material. As discussed in
section B7.9, studies that show similarities in non-carcinogenic
responses to exposure to diesel exhaust and to carbon particles
without an organic component indicate a role of long-term retention of
the insoluble carbon core.
B10.3.2 Dose-response assessment
B10.3.2.1 Epidemiological studies
Variability in the reporting of odour and irritation indicates
that a subsegment of the population is sensitive to diesel exhaust;
however, the proportion of the population with this characteristic
cannot be estimated. Chronic effects have not been seen in
occupationally exposed people, but excess respiratory symptoms have
been reported after acute exposure. It is not possible to determine
the dose of diesel exhaust (on the basis of exposure to gas or
particles) that produces these symptoms.
B10.3.2.2 Studies in experimental animals
In order to select the pivotal study for establishing the NOAEL
or uncertainty factor, the most sensitive, relevant studies must be
identified. All of the long-term studies focus on effects on the
respiratory tract, which are clearly those most relevant. Studies of
relatively low concentrations are listed in Table 43, which gives the
target and actual exposure concentrations, the equivalent continuous
exposure of the animal, the equivalent continuous human exposure, and
the effect level. The equivalent continuous exposure of animals is
calculated by multiplying the actual exposure concentration by the
(number of hours of daily exposure/24) and by the (number of days per
week of exposure/7). This value is necessary for comparing exposure
concentrations among studies with different protocols, i.e. different
numbers of hours of exposure per day and/or days per week.
The equivalent continuous human exposure is calculated from the
particle deposition-clearance model of Yu & Yoon (1990), discussed in
section B6.2. This model is based on studies of rats since only those
studies provide data on the exposure-response relationship for
inhibition of particle clearance.
The model of Yu & Yoon (1990) is a sophisticated approach to
defining the relationship between the inhaled concentration and the
dose in lung tissue. It combines detailed models of rat and human lung
structure, aerodynamic models of the air flow in the airways, particle
deposition dynamics, and information on clearance of particles from
rat and human lung. The model is specific to diesel particles because
the characteristics of the particles used in the model are derived
from studies of diesel particles and because the information on lung
clearance is based on studies of diesel exhaust in rats. The model has
been adapted for diesel exhaust particles by allowing evaluation of
the insoluble carbon core and of the tightly and loosely bound organic
compounds separately. Values for deposition and clearance are combined
with input from experimental studies to calculate the burden of
particles in the lungs of the animals at the end of the study. The
continuous concentration that would result in the same lung burden in
humans is then calculated, as the mass of particles per unit of
alveolar surface area or unit of lung weight. This lung burden is
assumed to result in a similar effect in humans and in rats. With
these considerations, the equivalent human concentrations are
predicted on the basis of the assumption that the retained particle
mass per unit alveolar surface area is the appropriate dose measure
for extrapolation between species. The effect level used in the risk
assessment is based on an evaluation of the adversity of the effect.
For the purposes of risk characterization, the NOAEL or, if that
is not available, the LOAEL for the critical effect is related to
exposure. The critical effect is the first adverse effect that appears
when the critical concentration is reached at the target site. A
decision about whether an effect is critical is a matter of expert
judgement. An adverse effect is defined as a change in morphology,
physiology, growth, development, or the life span of an organism which
results in impairment of functional capacity or of capacity to
compensate for additional stress or an increase in susceptibility to
the harmful effects of other environmental influences (International
Union for Pure and Applied Chemistry, 1993).
The NOAEL is defined as the greatest concentration or amount of a
substance, found by experiment or observation, that causes no
alteration in morphology, functional capacity, growth, development, or
the life span of target organisms that are distinguishable from those
observed in normal (control) organisms of the same species and strain
under the same defined conditions of exposure (International Union for
Pure and Applied Chemistry, 1993).
The LOAEL is defined as the lowest concentration or amount of a
substance, found by experiment or observation, that causes an adverse
alteration in morphology, functional capacity, growth, development, or
the life span of a target organism distinguishable from those of
normal (control) organisms of the same species and strain under
defined conditions of exposure (International Union for Pure and
Applied Chemistry, 1993).
Table 43. Exposure of experimental animals, equivalent continuous human exposure, and effect levels of long-term inhalation in studies
of rats described in section B7.3
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
7 h/d, HP, LL, 0.35 0.353 0.0735 0.042 N Mauderly
5 d/week, LTB, TC, 3.5 3.47 0.723 0.360 A et al. (1987)
30 months LB 7 7.08 1.47 0.582 A
Light-duty, HP 0.1 0.11 0.063 0.038 N Ishinishi
16 h/d, 0.4 0.41 0.23 0.139 N et al. (1986,
6 d/week, 1 1.18c 0.67 0.359 A 1988)
30 months 2 2.32 1.3 0.571 A
Heavy-duty, HP 0.4 0.46 0.26 0.155 N Ishinishi
16 h/d, 1 0.96 0.55 0.303 A et al. (1986,
6 d/week, 2 1.84 1.05 0.493 A 1988)
30 months 4 3.72 2.13 0.911 A
110 h/week, HP 0.25 0.258 0.17 NA N Barnhart
6 months 0.75 0.796 0.52 NA A et al. (1981)
1.5 1.53 1.0 NA A
7 h/d, HP, SB 2 1.95 0.57 0.336 A Lewis et al.
5 d/week, (1989)
24 months
Table 43 (contd)
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
19 h/d, HP, LL, 4 4.24 2.40 1.02 A Heinrich et
5 d/week, TC, LB al. (1986a)
32 months
18 h/d, HP, LL, 0.8 0.84 0.45 0.23 A Heinrich et
5 d/week, TC, LB 2.5 2.50 1.34 0.59 A al. (1995)
30 months 7.5 6.98 3.74 1.56 A
NA, not available (guinea-pigs were used)
a HP, histopathological examination; LL, lung lavage fluid; LTB, lung tissue biochemistry; TC, tracer clearance; SB, serum biochemistry;
LB, lung burden
b N, no effect; A, adverse effect
c According to Suzuki et al. (1990), 1.08 mg/m3
The selected NOAEL is divided by uncertainty factors to account
for data gaps. For these data, uncertainty exists in two areas:
extrapolation from animals to humans and accounting for sensitive
subpopulations. The factor for accounting for sensitive subpopulations
is a default value of 10, as it is considered that there are no data
suggesting a different factor. An additional default value of 10 is
usually applied for interspecies extrapolation, which is nominally
considered to consist of 100.4 (2.5) for toxicodynamics and 100.6
(4.0) for toxicokinetics (IPCS, 1994).
The LOAELs and NOAELs are selected on the basis of the following
reasoning. The LOAEL is the lowest level at which adverse effects are
seen in studies of adequate quality. The NOAEL, the highest level at
which no effect is seen in the available studies, must be lower than
the selected LOAEL. The LOAELs and NOAELs are identified by a
comparison with the equivalent continuous human exposure level. The
LOAELs in these studies are strikingly similar, with values of 0.360,
0.359, 0.303, 0.336, and 0.23 mg/m3 in studies from the Inhalation
Toxicology Research Institute (United States; Mauderly et al., 1987),
the Health Effects Research Program on light- and heavy-duty diesel
engine exhausts (Japan; Ishinishi et al., 1986, 1988), the National
Institute for Occupational Safety and Health (United States; Lewis et
al., 1989), and the Fraunhofer Institute for Toxicology and Aerosol
Research (Germany; Heinrich et al., 1986a, 1995), respectively (see
Table 43). The consistency of these levels of effect, in view of the
diversity of end-points represented, gives a high level of confidence
in the end-points.
The study at the Inhalation Toxicology Research Institute was
selected as the most representative because it covers the greatest
variety of end-points, including histopathology, bronchiolar lavage
biochemistry and cytology, lung tissue biochemistry, and particle
clearance; LOAELs were observed for all of these end-points. The
studies of the Health Effects Research Program showed a similar LOAEL
but only for histological changes in the lung. The value for
equivalent continuous human exposure is not available from the study
carried out by the General Motors Corporation (Barnhart et al., 1981)
because the main results were for guinea-pigs; although rats were
included in the study, detailed results were not presented. The
appropriate NOAEL in these studies is not selected on the basis of the
LOAEL. As close dosing intervals were used in the studies of of the
Health Effects Research Program, the NOAEL was selected on the basis
of studies of light-duty diesel engines, as 0.139 mg/m3.
B10.3.3 Exposure assessment
Exposure to diesel exhaust is discussed in sections B10.1 and
B10.2.
B10.3.4 Risk characterization
B10.3.4.1 Humans
A quantitative assessment of the risk for humans of
non-carcinogenic effects of exposure to diesel exhaust cannot be made
on the basis of studies in humans. A substantial volume of literature
shows an association between acute exposure to fine particulates and
morbidity and mortality in the general population. It is somewhat
uncertain whether there is a direct causal link and, if so, whether it
is related to a specific component of the suspended particulates.
Attention has been focused on fractions of suspended particulate
matter, currently measured as PM10 (diameter < 10 µm) or PM2.5
(diameter < 2.5 µm), comprised mainly of diesel particulates in the
submicrometre range, which represent an increasing proportion in some
countries. There is also evidence that exposure to particulates is
involved in respiratory symptoms and in mortality from certain chronic
respiratory conditions.
B10.3.4.2 Experimental animals
Three approaches were used in assessing the risk for
non-carcinogenic effects of exposure to diesel exhaust, on the basis of
exposure (dose)-response assessment in experimental studies (see
section B10.3.2.2), and for deriving guidance values for exposure.
Approaches 1 and 2 are based on the NOAEL from studies in rats. The
difference between the two is that approach 1 involves a dosimetric
extrapolation model (Yu & Yoon, 1990) for converting the actual
concentration to which animals were exposed to an equivalent
continuous human exposure, thereby reducing the uncertainty in
interspecies extrapolation.
In approach 2, no dosimetric conversion is performed but the
usual uncertainty factors for interspecies and intraspecies
extrapolation are applied. In contrast to these NOAEL-defined
approaches, approach 3 is based on the principle of the 'benchmark
dose', in which a concentration of exposure to diesel exhaust is
derived from dose-response relationships observed in rats.
The model of Yu & Yoon (1990) takes into consideration the
specific characteristics of particle deposition and retention in rat
and human lung. Application of the model to studies of inhalation in
experimental animals is shown schematically in Figure 4. The deposited
and retained doses in the lung are calculated from the concentrations
in rats and expressed per unit lung weight or per unit alveolar
surface area. Under the assumption that the retained dose per lung
will lead to the same effect in rodent and human lung, a human
equivalent concentration can be calculated (as shown in Table 43), to
obtain an equivalent continuous human exposure. The equivalent
continuous exposure of animals shown in Table 43 was derived either by
calculating the continuous exposure at which the lung burden attained
in a study by inhalation (if measured) would have been reached, or by
calculating the lung burden in rats at the end of exposure on the
basis of the actual exposure parameters (also shown in Table 43) and
then calculating as above. The correlation between continuous exposure
in animals and humans in the model of Yu & Yoon (1990), assuming that
overload induces prolonged lung clearance, is shown in Figure 5. Use
of this approach, rather than simple adjustment of discontinuous to
continuous exposure by the c x t constant used in other assessments,
should reduce the uncertainty associated with the transformation.
Approach 1. The NOAEL of 0.41 mg/m3 from the study in rats
exposed by inhalation to light-duty engine exhaust (Ishinishi et al.,
1986, 1988; Table 43) is converted to an equivalent continuous
exposure of 0.23 mg/m3 in rats and then to an equivalent continuous
exposure of 0.139 mg/m3 , assumed to be the NOAEL in humans.
Application of a sophisticated dosimetric model decreases the
uncertainty in interspecies extrapolation from 10 to 100.4 (IPCS,
1994). Application of the usual uncertainty factor of 10 for
intraspecies differences results in a total uncertainty factor of
10 × 100.4 = 25.
The guidance value derived from this approach is
0.139 mg/m3 = 5.6 µg/m3.
---------------
25
No additional correction factor is required, resulting in a guidance
value (for the general population) of 5.6 µg/m3.
The inherent uncertainties in the dosimetric model are due to the
assumptions that must be made. A principal assumption is necessary to
estimate inhibition of clearance in humans, since data on this aspect
do not exist. It is assumed that clearance in humans is inhibited at
the same lung burden (mass per alveolar surface area) as in rats. The
other principal assumption is that the correct dose measure for lung
damage is mass of particle core per alveolar surface area. Since the
damage is localized to specific areas, another measure may be more
appropriate.
role in initiating the carcinogenic response. These mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes. There may be some as yet unidentified chemicals in diesel
exhaust with high mutagenic potential; furthermore, chemical
interactions (additive or inhibitory) may occur among the components
of diesel exhaust (Ball & Young, 1992).
B7.9.1.2 Cytotoxicity with regenerative cell proliferation
Another mechanism by which diesel exhaust may induce
carcinogenesis involves cell killing, or cytotoxicity, by the
particles or the reactive gases in exhaust, followed by regenerative
proliferation of lung cells. Enhanced cell replication may result in
an increased frequency of spontaneous mutations. Mutations could also
arise from PAH-DNA adducts or from oxidative DNA damage caused by the
reactive oxygen and nitrogen species in diesel soot, resulting in the
inflammatory response. Driscoll et al. (1994) showed that the hprt
gene mutation frequency in rat lung epithelial cells was significantly
increased when the cells were incubated in vitro with inflammatory
cells obtained by bronchoalveolar lavage from rats exposed to quartz
particles. This appears to be an important mechanism in lung tumour
induction by particles that elicit a chronic inflammatory response in
the lung. Sagai et al. (1993) showed that diesel exhaust particles
could produce superoxide anions and hydroxyl radicals in the absence
of biological activation (detection by cytochrome c and electron spin
resonance). Reactive oxygen species may induce specific DNA adducts,
such as 8-hydroxydeoxyguanosine. As mentioned above, mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes.
Several data sets cited in this monograph support this
hypothesis. For example, carbon black particles, which are virtually
devoid of PAHs but are morphologically similar to diesel soot
particles, induce lung cancer in rats. High particle burdens in rodent
lungs result in an inflammatory response (Henderson et al., 1988).
Release of cytokines and reactive oxygen and nitrogen species during
the inflammatory response to particles may result in cell death. Some
evidence exists (Wright, 1986) that diesel exhaust enhances lung cell
replication.
B7.9.1.3 Effects of particles
The importance of pulmonary particle burden on lung tumour
induction has been demonstrated clearly in long-term studies by
inhalation in rats. As reviewed above, rats have similarly increased
lung tumour incidences when exposed to diesel exhaust or carbon black
particles by inhalation for 24 months. Similarly, carbon black
particles practically devoid of PAHs induce pulmonary tumours after
intratracheal instillation. The very large surface area of carbon
black and of diesel exhaust particles after desorption of adsorbed
organic compounds in vivo may be involved mechanistically in a
tumorigenic effect. Heinrich (1994) showed that the tumour response to
different types of carbon black particles instilled intratracheally
correlated well with their respective surface areas. Pott (1991) and
Pott et al. (1993) suggested that particles with a large surface area
have greater effects in humans than in rats because insoluble
particles with no specific toxicity remain significantly longer in the
terminal airways of humans than of rats (retention half-time, about 70
days in rats and about 500 days in humans).
The correlation between particle surface area and lung tumour
incidence was examined (Oberdörster & Yu, 1990) by evaluating
published studies of inhalation of diesel and other particles. Tumour
induction in rats was best correlated with the surface area of the
particles retained in the lung rather than with the particle mass,
particle volume, or number of particles, regardless of the PAH
content. It was suggested that particle surface area and surface
properties play a decisive role ('critical surface area') and that
absorbed PAHs are not responsible for the tumour response in rats
exposed to diesel exhaust. In the human situation, however, it could
not be excluded that organic compounds and gas-phase components are
also involved, since the human particulate lung burden is much lower
than those achieved in rats after long-term inhalation.
Inhalation of diesel engine exhaust can result not only in
pulmonary tumours but also in inflammation and fibrosis and in a delay
in alveolar (not bronchial) pulmonary clearance. The mechanism by
which tumours develop due to particle overload and its associated
pathological and anatomical changes may be restricted to rats and may
not occur under environmental conditions in humans, since the lung
burdens of humans do not reach the levels that induce lung tumours in
rats. This is of importance for quantitative risk assessment. Only
occupational exposure to diesel exhaust may result in lung burdens
near or at overload conditions, particularly if the lung is already
compromised by exposure to other dusts. Bohning et al. (1982) reported
retarded particle clearance in smokers; in these people, additional
exposure to diesel exhaust may induce overload and associated toxic
effects. It is not known, however, whether the mechanisms of
particle-induced lung tumours are the same in rats and humans, and
this information is necessary for extrapolating data from rats to
humans.
Not only diesel soot (0.8, 2.5, or 7.0 mg/m3) but also carbon
black nearly completely devoid of organic compounds (Printex 90,
7.5-12 mg/m3; particle size, 10 nm) and ultrafine titanium dioxide
particles (7.5-15 mg/m3; particle size, 20 nm) caused lung tumours
in female Wistar rats exposed by inhalation for 18 h/day on five days
per week for 24 months. The tumour rate increased with increasing
particle concentrations, independently of the type of particles
inhaled. No lung tumours were observed in the rats exposed to the
lowest concentration of diesel particles. The authors concluded that
the carcinogenic component of diesel exhaust is in the inner part of
the diesel soot particle, the carbon core, and is not the relatively
small amount of carcinogenic PAHs (3.9 g benzo[ a]pyrene per gram of
diesel soot) (Heinrich et al., 1992; Heinrich, 1994; Heinrich et al.,
1995). These results were confirmed in another two-year study, in
which male and female rats were exposed by inhalation to various
concentrations of carbon black and diesel exhaust. Lung tumours were
observed with both particle types (Nikula et al., 1994).
Diesel soot does not appear to have a specific carcinogenic
effect in rats; rather, there is a nonspecific effect of particles.
B7.9.1.4 Effects of polycyclic aromatic hydrocarbons
Exposure of rats by inhalation to 2.6 mg/m3 of an aerosol of
tar-pitch condensate with no carbon core but containing 50 µg/m3
benzo[ a]pyrene and other PAHs for 10 months caused lung tumours at a
rate of 39%. The same amount of tar-pitch vapour condensed onto the
surface of carbon black particles at 2 and 6 mg/m3 resulted in
tumour rates that were roughly two times higher (89 and 72%). Since
exposure to 6 mg/m3 carbon black almost devoid of extractable
organic material caused a lung tumour rate of 18%, the tumour rate of
72% seen after combined exposure to tar-pitch vapour and carbon black
particles indicates a syncarcinogenic effect of PAHs and carbon black.
A possible mechanism is an effect of deposition of PAHs (Heinrich et
al., 1994). As the level of benzo[ a]pyrene in the coal-tar pitch was
about three orders of magnitude greater than those in diesel soot,
PAHs may play a negligible role in the tumorigenicity of diesel soot
in rats. The PAH profile in diesel soot is, however, quite different
from that in coal-tar pitch, as diesel soot contains highly mutagenic,
carcinogenic nitro-PAHs and other poorly characterized mutagens that
are not present in coal-tar pitch or on some of the carbon black
particles used in experimental studies (Heinrich et al., 1994; Nikula
et al., 1994).
In a study of various extracts of diesel exhaust particles,
30-40% of the total mutagenicity could be attributed to a group of six
nitroarenes (Salmeen et al., 1984).
A diesel exhaust particle extract was separated into a water- and
a lipid-soluble fraction, and the latter was further separated into a
PAH-free, a PAH-containing, and a polar fraction by column
chromatography. These fractions were then tested in Osborne-Mendel
rats by pulmonary implantation at doses corresponding to the
composition of the original diesel exhaust. The water-soluble fraction
did not induce tumours; the incidences induced by the lipid-soluble
fractions were 0% with the PAH-free fraction, 25% with the
PAH-containing fraction, and 0% with the polar fraction. The
PAH-containing fraction, comprising only 1% by weight of the total
extract, was shown to be responsible for the carcinogenic activity
(Grimmer et al., 1991).
Various dichloromethane extracts, each representing a complex
mixture, were obtained from particulate emissions of four
diesel-fuelled and one gasoline-fuelled automobiles (combustion), a
coke oven battery (pyrolysis), and a roofing tar pot (evaporation),
and their tumorigenic potency was compared in Sencar mice. It was
concluded that the benzo[ a]pyrene content alone could not explain
the tumorigenic activity of the mixtures (Nesnow et al., 1982a, 1983).
The lung tumour rates in rats exposed to atmospheres containing
PAHs depend not only on the PAH concentrations of the exhaust gas but
also on parameters such as the composition of the carrier particle
(mass ratio of carbon core to adsorbed layer of organics), the
dissolution rate of particle-attached organic compounds, the retention
half-time, and the cytotoxic effect of the carrier particle in the
lung (Heinrich et al., 1991).
Extracted diesel soot given intratracheally to rats induced lung
tumours, but native, unchanged diesel soot resulted in higher tumour
rates than extracted soot. Carbon black also caused tumours after
intratracheal administration, and the rate increased with decreasing
size of the primary particles (Heinrich, 1994).
Carbon black particles almost completely devoid of organic
compounds (< 0.046% extractable organic compounds and 0.6 ng/g
benzo[ a]pyrene) caused tumours in the lungs of 17% of rats after
exposure to a concentration of 6 mg/m3 for 18 h/day on five days per
week for 10 months. No lung tumours occurred in controls exposed to
clean air. Thus, the particle effect may be responsible for induction
of lung tumours in rats exposed to diesel engine exhaust, and the
effect may be related to the surface area of the carbon particle. A
PAH depot effect could lead, however, to retarded dissolution of PAHs
from the carrier, resulting in very efficient use of the extremely
small amount of carcinogenic PAHs retained in the lung after exposure
to diluted diesel engine exhaust, which contained benzo[ a]pyrene at
about 10 ng/m3 (Heinrich et al., 1991). Since diesel particles
induce DNA adducts at lower concentrations than carbon black (Bond et
al., 1989, 1990c; Wolff et al., 1990), PAHs may have tumour initiating
properties that are too weak to be detected at low doses; at higher
doses, promotional effects of the particles, perhaps with additional
initiation, may synergize to produce detectable carcinogenic effects.
B7.9.2 Non-carcinogenic effects
Diesel exhaust contains various respiratory irritants in the gas
phase and in particulate matter. Both can induce inflammatory
responses in the airways and alveolar regions of the lung. Airway
inflammation involves damage to epithelial cells, including lipid
peroxidation of cell membranes by oxidizing gaseous pollutants such as
nitrogen dioxide. Indirect effects of particles, resulting from
phagocytosis, can include the formation and release of various
mediators, including oxidants, such as superoxide anions and hydroxyl
radicals, and cytokines. These mediators may play a role in focal loss
or shortening of cilia, type II cell hypertrophy, and hyperplasia. The
latter changes can lead to the hyperplastic lesions seen in animals
exposed to diesel exahaust (Ishinishi et al., 1986, 1988; Suzuki et
al., 1990; Kato et al., 1992). Phagocytosis and subsequent clearance
of particles by alveolar macrophages can be compromised by high
particle burdens (Wolff et al., 1989; Creutzenberg et al., 1990),
which may also increase the access of particles to the interstitium,
leading to focal fibrosis (Henderson et al., 1988).
Inflammation, altered lung clearance, and hyperplastic lesions
can be considered early markers of exposure to diesel exhaust and are
the basis of the non-neoplastic effects used to determine both the
NOAEL and the 'benchmark concentration' described in section B10.1.3.
B8. EFFECTS ON HUMANS
B8.1 General population
B8.1.1 Acute exposure: olfactory, nasal, and ocular irritation
Acute exposure to diesel exhaust has been associated with
irritation of the eyes and nose. Signs and symptoms reported after
acute exposure to diesel exhaust are described in section B8.2.1.
The exhaust from e.g. poorly maintained engines or engines under
load may be visible as a black, smoky cloud. WHO (1987) considered
that sensory effects were parameters that could be used in setting
occupational exposure limits. The characteristic odour of diesel
exhaust provides a warning of its presence. At higher concentrations
and under certain operating conditions, the odour can be offensive.
The odour-causing agents are not known; however, compounds with a
relative molecular mass > 80 are probably those mainly responsible,
and aliphatic olefins (C1-C6, including acetylene), hydrocarbons
with more than six carbons, and aliphatic aldehydes are probably not
involved. Substances with low odour thresholds, such as nitrogen
dioxide (0.3 ppm) and acrolein (0.5 ppm), appear to contribute to the
odour of diesel exhaust to minor extents (5.3 and 0.25%, respectively)
(Oelert & Florian, 1972).
The ability to detect the odour and the irritating effects of
diesel exhaust vary. For example, when six subjects smelled diesel
exhaust diluted with air, the dilution factors needed to achieve the
odour detection threshold varied from 140 to 475. The concentrations
of different constituents of the exhaust at the odour threshold also
varied: formaldehyde, 0.012-0.088 ppm; acrolein, 0.011-0.046 ppm; and
nitrogen dioxide, 0.11-2.28 ppm (Linnell & Scott, 1962). When six
subjects were exposed to three concentrations of diesel exhaust for
10 min, the mean concentrations at the odour threshold were 1.3-4.2 ppm
nitrogen dioxide, 0.2-1 ppm sulfur dioxide, < 0.1 ppm formaldehyde,
and < 0.05 ppm acrolein. Three of the subjects exposed to the highest
level discontinued exposure before 10 min, while none at the lowest
level discontinued exposure although some experienced eye irritation
(Battigelli, 1965).
The health effects of inorganic gases present in diesel exhaust
are not specific and are therefore not discussed in detail; however,
it must be noted that these gases contribute to the environmental
burden.
B8.1.2 Air pollution
The concentrations of some air pollutants, such as particulates
and sulfur dioxide from the burning of coal for domestic heating and
industrial purposes, are in many parts of the world much lower than
they were several decades ago, and the associated adverse health
effects have diminished accordingly. A number of epidemiological
studies have demonstrated, however, that the relatively low remaining
levels of air pollutants are associated with a range of health
indices, including day-to-day changes in mortality (Schwartz, 1993),
visits to hospital emergency departments (Schwartz et al., 1993), and
changes in measures of lung function (Pope & Dockery, 1992). In most
studies, the associations have been strongest with the fine
particulate component of pollution, some of which may be derived from
diesel exhausts.
Vehicle traffic, both gasoline and diesel, also contributes to
the nitrogen dioxide content of urban air, and there is evidence,
primarily from experimental studies (Bauer et al., 1986; Koenig et
al., 1988; Grant et al., 1993) that it can induce short-term
decrements in lung function in asthmatic and non-asthmatic patients.
Nitrogen dioxide and volatile hydrocarbons are involved in the
formation of ozone and associated photochemical pollutants.
Associations have also been demonstrated between long-term
exposure to urban air pollutants and death rates from certain chronic
conditions. Confounding factors often make interpretation difficult,
but in an investigation conducted in the United States (Dockery et
al., 1993), air pollution attributable to fine particles was
positively associated with lung cancer and cardiopulmonary disease,
after adjustment for smoking and other relevant risk factors, but not
with other causes considered collectively.
In neither short- nor long-term studies can the possible role of
diesel particulates be specified, but diesel emissions contribute to
urban particulates, especially in Europe and developing countries. The
acidic and sulfate components of particulates, which are derived
primarily from stationary fuel-burning sources, appeared to be
implicated in a number of studies.
B8.2 Occupational exposure
B8.2.1 Effects on the respiratory system
B8.2.1.1 Symptoms
Most of the information about symptoms after acute exposure to
diesel exhaust comes from anecdotal reports of occupationally exposed
individuals. It was noted in a bus garage in London, United Kingdom,
that the buses produce a 'lachrymatory mist' when they were started up
from cold (Commins et al., 1956). In a review of 13 cases of acute
overexposure to diesel exhaust in five underground coal mines in Utah
and Colorado, United States, between 1974 and 1985, interviews in 1986
with the miners revealed that 12 had experienced symptoms of mucous
membrane irritation, headache, and light-headedness; eight had
reported nausea, and four reported a sensation of unreality and
'heartburn' (Kahn et al., 1988).
In a study designed to investigate the acute effects of diesel
exhaust on respiratory symptoms, a questionnaire was administered to
232 male workers in four diesel bus garages, and personal samples of
nitrogen dioxide and respirable particulate were obtained. After
adjustment for age and smoking (current, ex-, never), workers with the
highest exposure to respirable particulate (0.31 mg/m3) reported
significantly more cough, itchy or burning eyes, headache, difficult
or laboured breathing, a feeling of chest constriction, and wheeze
than workers with lower exposures. Those exposed to nitrogen dioxide
at >0.4 mg/m3 (> 0.3 ppm) also more frequently reported itchy or
burning eyes, difficult or laboured breathing, a feeling of chest
constriction, and wheezing. In comparison with a group of lead acid
battery workers, the reporting of the following symptoms as
'sometimes' or 'often' was significantly more prevalent among the
workers in the diesel bus garage: eyes itch, burn, or water (49.5
versus 23.5%), headache (24.2 versus 12.1%), difficult or laboured
breathing (13.5 versus 3.6%), nausea (13.5 versus 4.5%), and wheeze
(13.7 versus 4.5%) (Gamble et al., 1987a).
In comparison with 11 office workers, 17 ferry stevedores had a
greater prevalance of wheezing (24 versus 9%), chest tightness (29
versus 9%), nasal complaints (47 versus 0%), chest pain (24 versus
0%), and eye irritation (59 versus 27%); however, more of the
stevedores smoked. The concentrations of nitrogen dioxide (0.3 mg/m3)
and sulfur dioxide (0.7mg/m3) were both < 0.25 ppm; respirable
particulate was not measured (Purdham et al., 1987). In a study in an
iron ore mine where diesel engines were used underground, more workers
who were smokers reported pressure over the chest or difficulty in
getting air than the nonsmokers. No such difference was observed among
smoking and nonsmoking surface workers. The underground workers who
were smokers also had more episodes of a productive cough lasting
three weeks during several winters (Jorgensen & Svensson, 1970).
B8.2.1.2 Acute changes in pulmonary function
The pulmonary function of 60 coal miners who worked in mines
equipped with diesel engines was compared with that of 90 coal miners
not exposed to diesel exhaust. There were similar proportions of
current smokers (45% in the exposed group and 43% in the unexposed
group). Measurements made with personal dust samplers and passive
nitrogen dioxide dosimeters showed average concentrations of
0.04 mg/m3 (0.2 ppm) nitrogen dioxide, 0.4mg/m3 (0.3 ppm)
formaldehyde, and 2.0 mg/m3 respirable dust in the exposed group;
the unexposed group was exposed to 1.4 mg/m3 respirable dust. Forced
vital capacity (FVC) and forced expiratory volume in 1 sec (FEV) were
reduced during the work shift for both the diesel-exposed and
unexposed groups. The reduction was slightly but not significantly
greater in smokers than in ex- and nonsmokers. An additional analysis
with adjustment for age, exposure to respirable dust, and years of
underground mining still revealed no difference in shift-related
pulmonary function between miners exposed and unexposed to diesel
exhaust (Ames et al., 1982).
No significant shift-related change in FVC or FEV was seen among
workers on ferries transporting diesel trucks and other vehicles in
comparison with controls (Purdham et al., 1987), and no significant
change in spirometry measured over a shift was seen in 232 workers in
four diesel bus garages (Gamble et al., 1987a). Ulfvarson et al.
(1987), however, found significant decrements in FVC and FEV1 across
a shift in 23 ferry workers who had had no exposure to diesel for 10
days, with no difference between smokers and nonsmokers. The
concentration range of total particles was 0.13-1 mg/m3, that of
formaldehyde 0.04-0.6mg/m3 (< 0.03-0.5 ppm), and that of nitrogen
dioxide 0.12-4.6mg/m3 (0.06-2.3 ppm). In a repetition of the study,
a significant shift-related decrement was noted in FVC but not in
FEV1. In a later study in which filters were put on the diesel
trucks, a shift-related decrement in FVC was seen, but no subsequent
shift-related changes in FEV were noted, with or without filters
(Ulfvarson & Alexandersson, 19901).
Three male railroad workers developed asthma after heavy exposure
to locomotive emissions (Wade & Newman, 1993). The workers, none of
whom were current smokers, had no previous history of asthma or of any
respiratory disease, except one who had seasonal rhinitis. They were
riding immediately behind the lead engines of the trains, where diesel
exhaust was blown almost continually into the cab. In two, the acute
onset of asthma occurred within the first hours of exposure.
B8.2.1.3 Pulmonary effects
In a study to investigate the effects of diesel exhaust on the
cells found in bronchoalveolar lavage (BAL) fluid (Rudell et al.,
1990), eight healthy, nonsmoking volunteers were exposed to diesel
exhaust (for 60 min according to Rudell et al., 1989) at least three
weeks after an initial BAL. The median steady-state concentrations
measured in the exhaust were 4.6mg/m3 (3.7 ppm) nitric oxide,
3.1 mg/m3 (1.6 ppm) nitrogen dioxide, 22.5mg/m3 (27 ppm) carbon
monoxide, 0.5 mg/m3 formaldehyde, and 4.3 × 105 particles/cm3;
according to Rudell et al. (1989), this particle concentration
corresponds to a mass concentration of approximately 100 µg/m3. BAL
was performed again 18 h after exposure. A significant reduction
(P < 0.02) in the total number of mast cells was observed; the number
of neutrophils was slightly but significantly (P < 0.05) increased in
comparison with the values before exposure. The ratio of
T-helper:suppressor cytotoxic cells was elevated ( P < 0.02), and the
rate of phagocytosis of opsonized yeast cells by alveolar macrophages
in vitro was reduced (P < 0.02). The number of lymphocytes remained
unchanged.
B8.2.2 Epidemiological studies (noncarcinogenic effects)
Some but not all of the results from cross-sectional and
longitudinal studies on workers with occupational exposure to low
levels of diesel engine exhaust show decrements in lung function and
an increased prevalence of respiratory symptoms.
B8.2.2.1 Effects on the respiratory system
A group of 550 underground workers and 273 surface workers in six
coal mines where diesel-powered equipment was used were matched for
smoking status, age, height, and years of underground mining to
workers at other underground coal mines where diesel units were not
used. The workers at the mines with diesel engines reported
significantly more persistent cough (23.6%) than controls (16.5%) and
more frequently had exacerbations of cough and phlegm (21.7 and 16.2%,
respectively). Pulmonary function (FVC and FEV ) was decreased in both
surface and underground workers in mines where diesel engines were
used in comparison with surface and underground miners in other mines.
There was no consistent relationship between years of underground
mining and respiratory symptoms and pulmonary function; however, the
mean time spent in underground mining was only 4.7 years. Full-shift
area samples showed concentrations of respirable dust ranging from 0.4
to 16.1 mg/m3 for various jobs; the mean values from personal
samples were 0.93-2.73 mg/m3, and the nitrogen dioxide levels in
personal samples were 0.16-0.26 mg/m3 (0.13-0.22 ppm) (Reger et al.,
1982).
Pulmonary function and respiratory symptoms were studied in 1976
in 630 miners in six potash mines where diesel-powered vehicles had
been introduced between 1950 and 1964. There was no consistent
relationship with exposure to diesel exhaust, considered in several
ways, including years of exposure, cumulative exposure to dust,
nitrogen dioxide concentration, and prevalence of diesel use in the
mine. The mean length of exposure to diesel engines was, however,
relatively short: 5-14 years across mines. In addition, cumulative
exposure was measured only to total dust rather than respirable dust.
The mean values for total dust in the mines ranged from 9 to
23 mg/m3 (personal sampling); the ratio of total dust to respirable
dust ranged from 2 to 11 (area sampling), and the nitrogen dioxide
concentration was 0.2-6.3mg/m3 (0.1-3.3 ppm) (personal sampling)
(Attfield et al., 1982).
A total of 259 miners in five salt mines where diesel equipment
was used in some of the mines were studied with regard to the number
of years worked underground and cumulative exposure to respirable
particulate and nitrogen dioxide. These parameters were associated
with phlegm after adjustment for age and smoking but not cough. The
average exposure to respirable particulate in the five mines was
0.2-0.7 mg/m3, and the average exposure to nitrogen dioxide was
0.6-4.8mg/m3 (0.3-2.5 ppm). Pulmonary function (FEV 1, FVC, peak
flow) was not related to the three parameters but was slightly reduced
in comparison with that of other blue-collar workers. In another
analysis in the same population, phlegm but not pulmonary function was
associated with years of work underground in mines where diesel
engines were used, after adjustment for age and smoking. There was a
nonsignificant trend for an association between cough and shortness of
breath with years of exposure (Gamble & Jones, 1983).
Longitudinal changes in pulmonary function and chronic
respiratory symptoms were studied between 1977 and 1982 in 280 miners
exposed to diesel exhaust and 838 miners not exposed to diesel exhaust
in American underground coal mines. The mean number of years of
underground work ranged from 6.6 to 17.4 years across mines. Area
samples taken in 1977 revealed 'low' levels of dust and diesel exhaust
constituents, which were reported not to exceed 25% of current
standards, but the actual values were not given. The exposed workers
had smaller average decrements in FVC and FEV1 than workers not
exposed to diesel exhaust after adjustment for age, smoking, and years
of underground work. Additional analysis revealed no relationship with
cumulative years of underground work, but the level of pulmonary
function was not considered as a predictor of longitudinal change. The
exposed miners also had a lower five-year incidence of cough, phlegm,
and breathlessness than the miners not exposed to exhaust (Ames et
al., 1984).
Pulmonary function and respiratory symptoms were studied in 283
male diesel bus workers in four garages in two cities. The number of
years worked was a significant predictor of lower FVC and FEV1
adjustment for age, height, race, and smoking status. In comparison
after with another blue-collar population, the bus garage workers had
a higher prevalence of cough and phlegm after adjustment for age and
smoking. The prevalence of these symptoms was not related to the
number of years worked (Gamble et al., 1987b).
B8.2.2.2 Effects on the circulatory system
No consistent excess of deaths due to cardiovascular disease has
been identified in cohort studies of workers with potential exposure
to diesel emissions. Motor vehicle examiners (Stern et al., 1981), who
are probably exposed to exhaust from a variety of vehicles, had a
slight, nonsignificant increase in deaths due to cardiovascular
disease (standardized mortality ratio [SMR] = 105). No difference in
mortality was seen among men working in potash mines with
diesel-powered equipment in comparison with men in potash mines with
no diesel engines (Waxweiler et al., 1973). In a pilot study of 129
men employed in a bus company in 1951-59 for whom mortality was
ascertained until 1978 (Edling & Axelson, 1984), a fourfold increase
in cardiovascular deaths was seen in garage workers. The increase was
calculated on the basis of at least 15 years since first exposure and
10 or more years of exposure, but was based on only four deaths. This
finding was not confirmed in a follow-up of 694 men through 1983
(Edling et al., 1987). In a study of 8490 garage maintenance workers
with at least one continuous year of service between 1967 and 1975, a
deficit of deaths due to cardiovascular disease was seen (Rushton et
al., 1983).
B8.2.3 Epidemiological studies (carcinogenic effects)
The relationship between cancers of the lung and bladder and
occupational exposure to diesel exhaust has been evaluated in a number
of epidemiological studies. The occupations examined included diesel
truck drivers, bus garage workers, railroad workers, heavy equipment
operators, and stevedores. The risk for lung cancer in relation to
exposure to diesel exhaust, either self-reported or assumed from
occupational categories, has also been evaluated in case-control
studies. Only those studies that were considered relevant for an
evaluation of the carcinogenic effects of diesel exposure are included
in this assessment.
B8.2.3.1 Lung cancer
The epidemiological studies are summarized in Tables 39-41. Table
39 is a summary of nine case-control and cohort studies of workers
exposed to diesel exhaust, Table 40 summarizes nine population- and
hospital-based studies, and Table 41 presents two studies based on
information on exposure derived from registries (surveillance
studies).
Until recently, the epidemiological study of lung cancer and
exposure to diesel exhaust was limited by failure to consider the
latency and duration of exposure necessary for the development of lung
cancer, both generally considered to be about 20 years (Schenker &
Speizer, 1979). Studies of exposed workers have also been limited by a
lack of worker- and industry-specific data on exposure. Such data were
available only for the American railroad and trucking industries. For
other occupational groups, such as bus garage workers and stevedores,
indices of exposure were derived from vehicle use, fuel consumption,
or years of exposure, or simply job title unsubstantiated by exposure
assessment. In studies based in hospitals, the general population, or
registries, information on usual work in a job with exposure to
vehicle exhaust was derived from self- or surrogate reporting;
self-reporting of exposure to diesel fumes has also been used. Studies
with short durations of exposure and follow-up are not useful for
determining whether diesel exhaust is a human carcinogen. Studies in
which there is an imprecise definition of exposure to diesel exhaust
(such as self-reported job title) do not allow detection of an effect,
since the extent of exposure is unknown. In studies in which an
elevated risk for lung cancer is noted, such imprecision can result in
wide confidence intervals.
The major potential confounding factor in occupational studies of
lung cancer is tobacco smoking. Such a factor must be related not only
to the outcome but also to the exposure. In order to assess whether
tobacco use is a true confounder in a study of occupation, the smoking
rates among individuals exposed and unexposed to diesel exhaust must
be known; however, owing to the retrospective nature of many
occupational studies, smoking histories are often not available. It is
unlikely that the smoking habits of workers exposed and unexposed to
diesel exhaust within the same occupational cohort are differentially
related to the exposure, but when the effect of exposure is small, in
the order of a relative risk of 1.5 (such has been found for diesel
exhaust and lung cancer in most studies), differences in smoking that
are unaccounted for could reduce the relative risk attributable to
exposure to diesel exhaust. Interpretation of the results of studies
lacking specific information about smoking is more uncertain when
small relative risks are seen. Despite imprecise exposure histories,
one strength of hospital- and registry-based studies of lung cancer is
that smoking histories are often available.
(a) Occupationally based cohort and case-control studies
Three studies have been conducted of railroad workers exposed to
diesel exhaust. The transition from steam- to diesel-powered
locomotives occurred in the United States during the 1950s. By 1959,
95% of the locomotives in service were diesel powered. In a
retrospective cohort study of American railroad workers, the mortality
of 55 407 white male workers aged 40-64 in 1959 who had worked on the
railroads for 10-20 years was ascertained through 1980, providing a
22-year period of follow-up (Garshick et al., 1988). A yearly
three-digit job code up to the time of death or retirement was
available from the Railroad Retirement Board. The workers had held
jobs in 39 selected categories, and an industrial hygiene survey was
conducted to categorize the job codes into those associated with
regular exposure to diesel exhaust (engineers, conductors, firemen,
brakemen, and diesel locomotive repair shop workers) and those not
exposed to diesel exhaust (clerks and signal maintainers). The
concentration of respirable particulate, adjusted for cigarette smoke,
was used as an indicator of exposure to diesel exhaust (Woskie et al.,
1988a,b). A potentially confounding exposure in the railroad industry
is asbestos, which was used to insulate the steam locomotives run
previously. Thus, workers in steam locomotive repair shops would have
been exposed to asbestos. Workers who had the longest potential
duration of exposure, i.e. those aged 40-44 in jobs with exposure to
diesel exhaust in 1959, had a relative risk of dying of lung cancer of
1.45 (95% confidence interval [CI] = 1.11-1.89). Workers aged 45-49 in
1959, who would have had slightly less exposure, had a relative risk
of 1.33 (95% CI = 1.03-1.73). Exclusion of the workers with potential
past exposure to asbestos did not appreciably change these relative
risks.
The US Environmental Protection Agency has supported an effort to
obtain more detailed information on exposure in this study in order to
estimate unit risk (Pepelko & Chen, 1993). Adequate exposure-response
curves could not be drawn for years of exposure or for measurements of
current exposure. Possible reasons include an imprecise assessment of
past exposure, since only current measurements of exposure were
available; changes in exposure over time, since improved ventilation
and new diesel locomotives have been introduced, resulting in reduced
exposure; and the lack of exposure histories for the workers who would
have been exposed to diesel exhaust before 1959. In addition, the
industrial hygiene survey was performed in four smaller railroads,
where exposure may not reflect that throughout the industry; the
presence of non-diesel particulate in the respirable samples collected
could have led to exposure misclassification. Nevertheless, the
industrial hygiene survey validated the classification of jobs into
those associated and not associated with exposure.
In a case-control study of American railroad workers by the same
investigators (Garshick et al., 1987), deaths occurring between 1
March 1981 and 28 February 1982 were recorded, and 1256 from lung
cancer were matched by age to deaths from causes other than cancer or
accidents. Smoking histories were obtained from next-of-kin. After
adjustment for smoking and past exposure to asbestos, workers aged 64
at the time of death and with 20 years of work in a job with exposure
to diesel exhaust had an odds ratio of 1.41 (95% CI = 1.06-1.88).
A cohort study addressed mortality between 1965 and 1977 among
all 43 826 male railroad workers who had retired from the Canadian
National Railway Company before 1965 but who were still alive and
those who retired between 1965 and 1977. Occupation at the time of
retirement was used to classify workers as unexposed, possibly
exposed, or probably exposed to diesel fume. The relative risk of
dying of lung cancer was 1.20 for workers with possible exposure
(P = 0.013) and 1.35 for those probably exposed (P < 0.001). Similar
relative risks were obtained after exclusion of workers involved in
locomotive repair, who were most likely to have had past exposure to
asbestos. These results are consistent with those of the studies of
American railroad workers, although the degree to which the workers
were actually exposed to diesel fumes is not known. Only retired
workers were studied, excluding younger, active workers who would have
had the most exposure. No information was given about the duration of
exposure of the retired workers to diesel exhaust since many would
have worked primarily before the transition to diesel locomotives
(Howe et al., 1983).
Table 39. Occupation-based case-control and cohort studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
43 826 male pensioners of the Exposure classified by RR = 1.20 (P = 0.013) Incomplete exposure Howe et al.
Canadian National Railway experts on occupation at and 1.35 (P = 0.001) for assessment owing to (1983)
time of retirement: possible and probable lack of lifetime occupational
unexposed, possibly exposure, respectively. history. Difficult to
exposed, probably Significant trend in RR separate combustion products
exposed with increasing likelihood from diesel exhaust,
of exposure since the cohort would
have worked in both the
steam and diesel eras;
many would have had
relatively short exposure
to diesel. No data on
smoking; method of
categorizing exposure
not validated or described
in detail
Incident deaths between Personal exposure Adjusted for smoking and Possible misclassification Garshick et al.
1 March 1981 and 28 assessed by industrial to asbestos, OR = 1.41 of exposure to diesel (1987)
February 1982 in US railroad hygiene sampling in 39 (1.06-1.88) (< 64 years of exhaust since job title might
workers. 1256 cases of job categories; yearly age) for 20 years of work not reflect exposure in each
lung cancer and 2385 matched job title used to in a diesel-exposed job. case; however, this should
controls (up to two each, on dichotomize exposure For > 20 years of work in a reduce the OR.
age and date of death). Cases into exposed and diesel-exposed job relative
and controls had worked for unexposed between 1959 to 0-4 years, OR = 1.64
the railroad for > 10 years. and retirement (1.18-2.29)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
55 407 white male railroad Industrial hygiene data RR = 1.45 (1.11-1.89) in No data on smoking Garshick et al.
workers, aged 40-64 and used to categorize jobs workers aged 40-44 in (1988)
with 10-20 years of work in as exposed or unexposed 1959; RR = 1.33 (1.03-
1959. Mortality due to lung to diesel exhaust; jobs 1.73) in workers aged 45-
cancer (1694 deaths) well categorized for 49. Unexplained by exposure
determined retrospectively most of the cohort to asbestos. Workers with
through 1980 highest potential
cumulative exposure to
diesel exhaust had highest
risk for cancer
About 20 000 male London Five job categories used SMRs for all five job Duration of work not Waller (1981)
Transport workers aged 45-64. to define exposure, categories < 100 for lung considered; impossible to
667 cases of lung cancer validated by cancer (healthy worker follow individuals after
ascertained among active environmental effect), but highest SMR in retirement; no adjustment for
workers > 25 years (1950-74) concentrations of highest exposure group, not smoking
benzo[a]pyrene and significantly greater than
particles measured least exposed (underground
in 1957 and 1979 train crew)
8490 male London Transport 100 job titles grouped SMR = 101 overall, but job No validation of exposure Rushton et al.
maintenance workers (cohort into 20 broad categories category 'general hand' by air sampling; short (1983)
mortality study). Mortality of showed increased SMR for (average, 6 years) follow-up;
workers employed for > 1 year lung cancer with no no adjustment for smoking
between 1 January 1967 apparent link to diesel
and 12 March 1975 exhaust
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
695 workers in bus garages Intensity of exposure to SMR = 122 (71-196); for No adjustment for smoking Gustavsson et
who had worked for > 6 months exhaust and asbestos increasing exposure index, al. (1990)
in 1945-70. 20 deaths from according to industrial RR = 1.34 (1.09-1.64), 1.81
lung cancer identified (nested hygiene estimate; (1.20-2.71), 2.43
case-control study) duration of exposure (1.32-4.47)
based on company records
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases among non-smokers, Walrath et al.
veterans alive in 1954 and driver) among people who had but SMR calculated from (1985)
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates
given
994 male lung cancer deaths Longest job held: diesel OR = 1.55 (0.97-2.47) for Extent of actual exposure Steenland et
in 1982-83 and 1085 controls truck driver, gasoline long-haul drivers with > 18 to diesel exhaust unknown al. (1990, 1992)
(excluding lung cancer, truck driver, both years of employment; OR =
bladder cancer, accidents). types, truck mechanic, 1.89 (1.04-3.42) for diesel
Cases and controls from stevedore. Exposure truck drivers with > 35
Teamsters Union who had filed validated by industrial years of employment
claims (requires 20-year hygiene survey (adjusted for smoking)
tenure)
Retrospective cohort study of Cohort assumed to be Lung cancer: SMR = 168 No adjustment for smoking; Gustafsson et
stevedores in Sweden first exposed on basis of job (136-207) for incidence; exposure assumptions not al. (1986)
employed before 1974 for a duties, but no formal SMR = 132 (105-166) for fully justified; no
continuous period of 6 months. assessment made mortality calculations done to explore
6071 workers employed in 1961 employment time as a surrogate
with mortality determined for exposure
through 1980. 89 cases of
lung cancer noted in Swedish
Cancer Registry (71 deaths)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
Stevedores in Swedish Estimated from use of Adjusted for smoking Crude adjustment for Emmelin et al.
population defined by fuel in each port and (yes/no) depending on smoking; no industrial (1993)
Gustafsson et al. but limited years of work since use index of exposure used; hygiene data to validate
to workers in ports for which of diesel equipment exposure-response estimates of exposure to
data data on diesel fuel relationship noted, diesel exhaust
consumption were available. relative odds for medium
Case-control study with 50 exposure ranging from 1.5
cases of lung cancer detected to 2.7 and that for high
in 1960-82 matched to 154 exposure ranging from 2.9
controls on port and date of to 6.8. Lower limit of 90%
birth confidence interval < 1,
except for one
high-exposure category
RR, relative risk; OR, crude odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless
otherwise stated
Three studies reported findings among bus garage workers. Waller
(1981) studied lung cancer occurrence among London Transport staff on
the basis of job category. Diesel buses were introduced in the 1930s,
gradually replacing gasoline-powered vehicles and, in the 1950s,
electric trolley-buses. Mortality from lung cancer was ascertained for
active workers aged 45-64 between 1950 and 1974. A lower risk was seen
for people in all job categories in comparison with the general
population of the London area, indicating a 'healthy worker effect'.
The highest SMR was seen for workers who had the most exposure to
diesel exhaust (bus garage engineers), although the SMR was not
significantly different in workers with no exposure. Although this
study showed no relationship between job category and mortality from
lung cancer, the study had significant limitations. No retired workers
were followed up, so that workers who would have had significant
exposure were excluded. Since the results were not presented on the
basis of years of work (years of exposure to diesel fumes), workers
with little exposure were grouped with those with long exposure,
making it more difficult to detect an effect of diesel exhaust.
Rushton et al. (1983) ascertained the mortality of 8490 London
Transport bus garage workers who had been employed for at least one
year between 1967 and 1975. One hundred job titles were grouped into
20 broad categories. The overall SMR for lung cancer was not elevated,
and there was no relationship with any job with exposure to diesel
exhaust; however, the SMRs were not presented on the basis of years of
work in a job with exposure, and the duration of follow-up was short,
with a mean of 5.9 years.
Using a nested case-control design, Gustavsson et al. (1990)
studied a cohort of 695 men who had worked in five bus garages in
Stockholm, Sweden, in 1945-70. Diesel-powered buses were first
introduced in Stockholm in the 1930s, and after 1945 all of the
internal combustion engines in buses were diesel-powered. No
measurements of exposure were available, so exposure to diesel exhaust
was estimated by industrial hygienists on the basis of garage
ventilation, work practices, and the number of buses in the garages
and was graded on a five-point scale, each increase corresponding to a
50% increase in intensity. An index of cumulative exposure to diesel
exhaust was calculated for each worker by multiplying the exposure
level for each work period by the duration in years. Past exposure to
asbestos was estimated on the basis of historical measurements of
asbestos fibres obtained during brake-repair operations. Twenty cases
of lung cancer were identified and matched by age to six controls. The
relative risk for lung cancer increased with increasing index of
exposure to diesel exhaust: That at the lowest exposure level was 1.34
(95% CI = 1.09-1.74), that at an intermediate level was 1.81
(95% CI = 1.20-2.71), and that at the highest level was 2.43 (95%
CI = 1.32-4.47). No such increase was observed with a similar index of
exposure to asbestos. No information was available on smoking.
Diesel-powered trucks were introduced in the United States in the
1950s and 1960s. Trucking companies had completed the transition to
diesel trucks by 1960, while independent drivers and non-trucking
companies would have completed the transition later. Steenland et al.
(1990, 1992) studied 994 deaths from lung cancer occurring in 1982 and
1983 among male members of the Teamsters Union who had filed claims
for pension benefits (requiring at least 20 years of membership). Work
histories and smoking histories were obtained from next-of-kin; job
categories were also available from the Union records. The odds ratio
for lung cancer among long-haul drivers, who drove mostly diesel
trucks, with 18 or more years of employment after 1959 was 1.55 (95%
CI = 0.97-2.47) after adjustment for age, smoking, and exposure to
asbestos. Teamsters with 35 years or more of employment whose main job
was a diesel truck driver had a relative risk for lung cancer of 1.89
(95% CI = 1.04-3.42). For drivers of gasoline trucks, the relative
risk was only 1.34 and did not achieve conventional levels of
significance (95% CI = 0.81-2.22).
In an industrial hygiene survey (Zaebst et al., 1991; Steenland
et al., 1992) that accompanied this study, measurements of elemental
carbon were used to estimate exposure to diesel exhaust. The truck
drivers appeared to have received much of their current exposure to
diesel exhaust from background levels on the road rather than directly
from their engines. No historical measurements of exposure were
available.
Gustafsson et al. (1986) studied the mortality of 6071 stevedores
in Sweden who had been employed for at least six months between 1961
and 1974 through 1980. The stevedores had been exposed to both
gasoline and diesel exhausts; diesel-powered trucks were first used in
Swedish ports in the late 1950s, with a rapid increase in use in the
1960s. The SMR for lung cancer was 132 (95% CI = 105-166). The
year-specific lung cancer rates in the stevedores between 1961 and
1980 were greater than those in the Swedish male population.
In order to refine the assessment of exposure among the
stevedores and to adjust for smoking, a case-control study was
conducted in this cohort (Emmelin et al., 1993). Cases and controls
were selected from among male stevedores who had been employed for at
least six months in 1950-74; the cases were those occurring in
1960-82. On the basis of 50 cases and 154 controls, with adjustment
for smoking (yes/no), the odds ratio for lung cancer was seen to
increase with increases in three indices of exposure: years since
diesel equipment was used in the port, estimates of cumulative fuel
consumption, and years that fuel use was above a minimal level in the
port. The increases were consistent with an exposure-response
relationship. Only one of the point estimates of the odds ratios for
lung cancer reached statistical significance.
Table 40. Population- and hospital-based studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
7518 (3539 men, 3979 Occupation determined OR = 1.52 for truck drivers Exposure estimate based on Williams
women) incident invasive at interview (P > 0.05) self-reporting; not validated et al.
cancers from the Third 47% non-response; controls (1977)
National Cancer Survey. Lung consisted of other cancers,
cancer cases: 432 in men, probably diluting risk
128 in women. Combined other estimate; few cause-specific
cancer sites used as cancers and individual
controls occupations
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases in nonsmokers, Walrath
veterans alive in 1954 and driver) among people who had but SMR calculated using et al.
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates (1985)
589 cases of lung cancer Job title from next-of-kin Adjustment for smoking; No validation of exposure Damber &
reported to the Swedish for occupations held for (0.6-2.2) for professional groupings, so actual exposure Larsson
Cancer registry 1972-77 with > 1 year drivers (> 20 years of to diesel exhaust uncertain (1987)
death before 1979; 562 employment) with dead
matched dead controls (sex, controls; OR = 1.1 (0.6-2.2)
age, year of death, with living controls
municipality) drawn from
National Registry of Causes
of Death; matched living
controls (sex, year of birth,
municipality) drawn from
National Population Registry
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Montreal, Canada, Interview with subject; Adjustment for smoking; Job title used to indicate Siemiatycki
hospital-based cases and job title translated into OR = 1.2 for squamous-cell exposure to diesel exhaust; et al.
controls; 857 cases of lung likely exposure to lung carcinoma (90% CI, not validated; exposure (1988)
cancer and 1523 controls (other combustion products by 1.0-1.5) for any exposure ill-defined
cancers than lung) industrial hygienist but not elevated for other
cell types. No relationship
with years of exposure or
intensity
461 981 male volunteers Self-reported occupation Adjustment for smoking; Exposure information Boffetta
enrolled in the American and exposure to diesel > 16 years' exposure to based on self-reporting; et al.
Cancer Society prospective exhaust diesel exhaust, OR = 1.21 not validated (1988)
mortality study in 1982; aged (0.94-1.56); for 1-15 years,
40-79 at enrolment; 2-year OR = 1.05 (0.80-1.39).
follow-up; 378 622 men with Lung cancer mortality also
known exposure to diesel elevated in miners and heavy
exhaust equipment operators
1260 cases of lung cancer Job title obtained from For motor vehicle drivers Crude classification of Benhamou
and 2084 controls matched occupational history (adjusted for smoking), exposure et al.
for age, sex, hospital obtained at interview; OR = 1.42 (1.07-1.89); for (1988)
admission in France in 1976-80 exposure based on any all transport equipment
work in a job operators, OR = 1.35
(1.05-1.75). Increase in risk
with duration of employment
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Pooled data from three Job title from interviews Smoking-adjusted OR = No validation of exposure Hayes
case-control studies with with subjects or 1.5 (1.1-1.9) for truck classification; unclear if et al.
2291 male lung cancer cases next-of-kin; years of work drivers with > 10 years of most exposure was to (1989)
and 2570 controls. Analysis in motor exhaust-related work and job history obtained diesel exhaust or if job
limited to 1444 cases and occupation calculated by direct interview. For all titles selected as 'exposed'
1893 controls who provided motor exhaust-related were actually exposed to
information at interview occupations and > 10 years diesel exhaust
of work, OR = 1.5 (1.2-1.9)
Hospital-based study of 2584 Occupational titles and Smoking-adjusted OR for Exposure histories not Boffetta
cases in 18 hospitals in six self-reporting of exposure occupations with probable validated et al.
US cities; 5099 controls exposure to diesel exhaust (1990)
= 0.95 (0.78-1.16). For
self-reported exposure,
OR = 1.21 (0.78-2.02)
Detroit, USA, area; 3792 Interview to obtain full For drivers of heavy trucks Exposure histories not Swanson
cases and 1966 controls occupational history; with > 20 years of work, validated et al.
identified through cancer analysis by job title OR = 2.5 (1.4-4.4), with (1993)
surveillance system adjustment for smoking;
exposure-response
relationship with years of
work. For drivers of light
trucks with > 20 years of
work, OR = 2.1 (0.9-4.6)
OR, odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless otherwise stated
(b) Population- and hospital-based studies
In a study of 107 563 American veterans who were alive in 1954
and were followed to the end of 1969, the SMR among smokers who
reported their occupation as truck or tractor driver was 153, based on
23 cases. Most of these individuals would have had little exposure to
diesel exhaust, however, since diesel trucks were introduced in the
United States only in the 1950s and 1960s (Walrath et al., 1985).
In the Third National Cancer Survey in the United States,
self-reporting of work as a truck driver and adjustment for smoking
resulted in an odds ratio of 1.52, which was not statistically
significant (Williams et al., 1977). In a study of 589 cases of lung
cancer reported to the Swedish Cancer Registry between 1972 and 1977
among people who had died before 1979, the odds ratio for > 20 years
of work as a professional driver was 1.2 (95% CI = 0.6-2.2) after
adjustment for smoking (Damber & Larsson, 1987). In a hospital-based
study of 857 cases of lung cancer and controls, occupational histories
obtained by interview were interpreted by an industrial hygienist.
After adjustment for smoking, the odds ratio for squamous-cell
carcinoma of the lung was 1.2 (90% CI = 1.0-1.5) in association with
any exposure to diesel exhaust (Siemiatycki et al., 1988). The
mortality of 378 622 men between 1982 and 1984 was analysed in the
American Cancer Society prospective study of cancer on the basis of
self-reporting of exposure to diesel exhaust. After adjustment for
age, smoking, and other occupational exposures, including asbestos,
coal-tar and pitch, and gasoline exhaust, the relative risk for lung
cancer for men with 16 years of exposure was 1.21 (95% CI =
0.94-1.56). For truck drivers who reported exposure to diesel exhaust,
the relative risk was 1.22 (95% CI = 0.77-1.95) (Boffetta et al.,
1988).
In a study in France, 1260 male cases of lung cancer and 2084
controls matched on age and hospital were collected between 1976 and
1980; job titles and smoking histories were obtained by interview, and
odds ratios were calculated on the basis of any work in a particular
occupation. After adjustment for smoking, the odds ratios were 1.35
(95% CI = 1.05-1.75) for transport equipment operators and 1.42 (95%
CI = 1.07-1.89) for motor vehicle drivers. There was no increase in
risk with increasing years of work in a job, but details of the
analysis were not presented (Benhamou et al., 1988).
The data from three case-control studies carried out by the
American National Cancer Institute between 1976 and 1983 were pooled
in order to study lung cancer in people with occupations associated
with motor vehicles. The analysis was limited to 1444 male patients
with lung cancer and 1893 controls who provided information on smoking
and occupation at an interview. The odds ratio for lung cancer for 10
or more years of employment in any occupation related to exhaust from
either diesel or non-diesel engines was 1.5 (95% CI = 1.2-1.9), after
adjustment for age and smoking. In truck drivers, the odds ratio was
1.5 (95% CI = 1.1-1.9) (Hayes et al., 1989).
In a case-control study of patients with lung cancer in 18
hospitals in six American cities, 2584 cases seen between 1969 and the
late 1980s were matched to at least one control on the basis of age,
sex, hospital, and year of interview. A smoking history and usual
occupation were obtained by interview. After 1985, reports of
self-reported exposure to diesel exhaust were obtained for 477 lung
cancer patients and 946 controls. Exposure was graded as 'probable' if
the individual had usually worked on railroads (the specific job was
not used) or in a variety of jobs related to motor vehicles. For these
individuals, the odds ratio for lung cancer was 1.31 (95% CI =
1.09-1.57); for those reporting usual occupation as a truck driver,
the odds ratio was 1.31 (95% CI = 1.03-1.67). After adjustment for
cigarette use, age, race, and date of interview, however, the odds
ratios for lung cancer were reduced to 0.95 (95% CI = 0.78-1.16) for
individuals with probable exposure and 0.88 (95% CI = 0.67-1.15) for
truck drivers. For self-reported exposure to diesel exhaust, the odds
ratio was 1.21, after adjustment for smoking, age, and other potential
confounding variables including exposure to asbestos, education, and
race, but did not achieve statistical significance (95% CI =
0.73-2.02) (Boffetta et al., 1990).
In a study of 3792 cases of lung cancer in men in Detroit, United
States, newly diagnosed in 1984-87, an occupational history and
information on smoking were collected by interview with the subject or
a surrogate. Controls were men with colon or rectal cancer. After
adjustment for smoking, the odds ratio for lung cancer was 2.5 (95% CI
= 1.4-4.4) among white men who had driven heavy trucks for 20 years
and 2.1 (95% CI = 0.9-4.6) for drivers of light trucks for 20 years.
There was a significant increase in the odds ratio for lung cancer
with increasing years of work for drivers of both types of truck
(Swanson et al., 1993).
(c) Registry-based, surveillance studies
Table 41 summarizes two studies in which information on exposure
was based on occupational titles obtained from censuses. No
information was available on smoking or on length of employment. Both
studies reported an elevated risk for lung cancer. Ahlberg et al.
(1981) found a relative risk of 1.33 (95% CI = 1.13-1.56) for
professional drivers, and Hansen (1993) found an SMR for all
respiratory cancer in truck drivers of 160 (95% CI = 128-198).
Table 41. Surveillance studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
Swedish census-based cancer Listing of occupation in RR = 1.33 (1.13-1.56) No validation of exposure; Ahlberg et al.
incidence registry; 154 cases census no adjustment for smoking (1981)
among professional drivers
Retrospective cohort study of Job title in census 84 cancers in truck drivers; No adjustment for smoking; Hansen (1993)
14 225 truck drivers in 1970; SMR =160 (128-198); information on specific
mortality determined until expected number based exposure or length of
1980. Referent group of persons on rates in referent employment
with no exposure to combustion population
products on basis of census
job title
RR, relative risk; SMR, standardized mortality ratio; in parentheses, 95% confidence interval
Table 42. Case-control studies of urinary bladder cancer in individuals with possible exposure to diesel exhaust
Population Exposure assessment Results Reference
Incident cases in 480 men Any exposure to diesel and Based on 15 cases and controls with discordant Howe et al.
and 152 women in three traffic fumes obtained at exposure histories, OR for exposure to diesel and (1980)
Canadian provinces, April interview traffic fumes = 2.8 (0.8-11.8); not adjusted for
1974-June 1976; each case smoking
matched by age and sex to
a neighbourhood control
Incident cases in 303 white Occupation and industry For any employment in trucking, RR = 2.2 (1.1-4.4) Silverman et al.
men in metropolitan Detroit, obtained at interview based on 28 cases and 13 controls. For any work in (1983)
USA, December 1977-November railroad and railway express services, RR = 1.9
1978; 2986 controls (0.9-3.8) based on 22 cases and 12 controls, not
selected by random-digit adjusted for smoking. For any employment as a truck
dialling but of same age driver (42 cases and 18 controls), adjusted for
distribution as cases smoking, RR = 2.1 (1.4-4.4). For > 10 years as truck
driver, smoking-adjusted RR = 5.5 (1.8-17.3), and for
truck drivers who ever drove vehicles with diesel
engines, smoking-adjusted RR = 11.9 (2.3-61.1).
Elevated risk for truck drivers with employment after
rather than before 1950, when diesel truck use became
more prevalent
Table 42 (contd)
Population Exposure assessment Results Reference
White residents of New Occupation and exposure For any employment as a truck driver (35 cases, 53 Hoar & Hoover
Hampshire and Vermont, to diesel fuel or engines controls), OR = 1.5 (0.9-2.6), not adjusted for smoking. (1985)
USA, who died of bladder by next-of-kin Those employed 1930-49 had the highest OR (2.6;
cancer 1975-79 (on death 1.3-5.1) after adjustment for coffee drinking and
certificate) matched to one smoking. For 26 cases and 39 controls who reported
control with cause of death exposure to diesel fuel or engines, OR = 1.5 (0.8-2.8),
other than suicide, matched not adjusted for smoking. Significant trend for
on state, sex, race, and age, increased relative odds up to 39 years of exposure
and a second control with to diesel exhaust
same criteria but also matched
on county. Information obtained
for 325 cases and 673 controls
Cases in 512 men in Turin, Occupation obtained at In truck drivers, based on 16 cases and 16 controls, Vineis &
Italy, matched by age to 596 interview OR = 1.2 (0.6-2.5), not adjusted for smoking Magnani (1985)
hospital controls without cancer
Cases in 194 men in 18 hospitals Usual occupation obtained; Only 16 cases in occupations with potential exposure Wynder et al.
in six US cities, January likelihood of exposure to to diesel exhaust. ORs all < 1 for warehousemen, bus (1985)
1981-May 1983. Controls diesel exhaust determined and truck drivers, and heavy equipment operators. In
were persons hospitalized at on basis of estimates of railroad workers, based on two cases and one control,
the same time as the case but percentage of workers with OR = 2. For any exposure, with adjustment for smoking,
for diseases not related to potential exposure in that OR = 0.87 (0.47-1.58). For high exposure, OR = 1.68
cigarette smoking. Each case occupation: high exposure, (0.49-5.73), not adjusted for smoking
matched to three controls > 20% of workers; moderate
(total, 582) by age, race, year exposure, 10-19% of workers
of interview, and hospital of
admission
Table 42 (contd)
Population Exposure assessment Results Reference
1909 new cases in white men Occupation obtained at After adjustment for smoking, RR for men usually Silverman
from 10 metropolitan areas in interview working as a truck driver = 1.5 (1.1-2.0), based on 99 et al. (1986)
the USA, 1977-78. Up to two cases and 123 controls. For those ever employed as
controls (total, 3569) selected truck driver, increased risk with increasing years of
by random-digit dialling and employment; however, truck drivers first employed
matched for age and < 40 years since diagnosis had no increased risk. OR
geographic area with case for bus driver as usual occupation = 1.5 (0.6-3.9),
based on 9 cases and 13 controls, adjusted for smoking
Cases in 99 men in La Plata, Occupational history Based on 20 cases ever employed as a truck or railway Iscovich et al.
Argentina, matched on age to obtained at interview driver, RR = 4.31 (P < 0.05); reduced whenr (1987)
99 hospital controls without adjusted for smoking, but no details provided
cancer and 99 neighbourhood
controls
Cases in 371 men and women Occupational history After adjustment for age and sex, RR = 1.55 (1.06-2.28) Jensen et al.
in the Danish Cancer Registry. at interview any employment as a land transport worker; based (1987)
Controls (771) selected randomly on 51 cases and 73 controls. After adjustment for
from general population smoking, age, and sex, years of work as a land transport
worker, and years of work as a bus, taxi, or truck
driver significantly associated with increased risk
Deaths in 731 men in Ohio, Yearly listing of Work as a truck driver > 20 years, OR = 12 (P < 0.01), Steenland
USA, 1960-82, identified on occupation obtained from six cases and one control; for > 20 years as a railroad et al. (1987)
death certificates and city commercial directories worker, OR = 2.21 (P < 0.01), based on 22 cases, not
matched to six controls without adjusted for smoking
tumours of the urinary tract or
pneumonia on age, year of
death, and race
Table 42 (contd)
Population Exposure assessment Results Reference
Cases in 826 men and women Occupational history and For any exposure of men to exhausts, OR adjusted for Risch et al.
diagnosed 1979-82 in exposure to 'exhausts' cigarette smoking = 1.16 (0.91-1.48). For men ever (1988)
Edmonton, Calgary, Toronto, obtained at interview exposed 8-28 years before diagnosis, OR = 1.21
and Kingston, Canada, (0.93-1.58). For men in jobs with any contact with
matched by age, sex, and diesel or traffic fumes 8-28 years before diagnosis,
area of residence to 792 adjusted for smoking, OR = 1.69 (1.24-2.31);
randomly selected population significant trend with increasing years of exposure
controls
Cases in 486 men and women Interview with subject; After adjustment for smoking, OR = 1.0 (90% CI, Siemiatycki
in Montreal, Canada, hospitals job title translated into 0.8-1.2) for any exposure to diesel exhaust, based et al. (1988)
and 2196 controls with likely exposure to on 82 cases with exposure
diseases other than lung combustion products by
or kidney cancer industrial hygienists
Cases in 136 men in 18 Usual occupation and For any exposure, OR = 1.24 (0.77-2.00), based on Iyer et al.
hospitals in six US cities. self-reporting of exposure 41 cases, with adjustment for smoking (1990)
Two controls without to diesel exhaust obtained
tobacco-related diseases matched at interview; grouped into
to each case on age (< 2 years), probable and possible
race, hospital, and year of exposure
interview
Table 42 (contd)
Population Exposure assessment Results Reference
256 cases and 287 controls in Job titles for all jobs held For any exposure to diesel exhaust, RR = 1.7 Steineck et al.
population-based study of all obtained by postal (0.9-3.3), based on 25 cases and 19 controls, adjusted (1990)
urothelial cancer in Stockholm, questionnaire; industrial for year of birth and smoking. For subjects considered
Sweden, 1985-87, among hygeienists reviewed titles to have moderate or high exposure, RR = 1.1 (0.3-4.3),
men born 1911-45 and categorized subjects as but based on only four cases and five controls
exposed to various
substances
Cases in 658 men in seven Interview with subject; job After adjustment for smoking, OR for work in road Cordier et al.
French hospitals, 1984-87, title and duties translated transport = 1.02 (0.62-1.69), based on 36 cases and (1993)
matched for age, race, and into likely exposure to 35 controls. For stevedores, OR = 1.31 (0.87-1.98);
place of residence to 658 diesel exhaust by industrial for material-handling equipment operator, OR = 7.67
controls selected randomly hygienists (0.96-61.4); and for transport equipment operators,
among patients admitted OR = 0.88 (0.62-1.26). For diesel fumes, OR = 0.99
for diagnoses other than (0.32-3.03), based on seven cases and two controls
cancer
Cases in 153 men in one Occupational history For any work as a road transport worker, Notani et al.
hospital in Bombay, India, obtained at interview smoking-adjusted OR = 1.36 (0.5-3.7), based on eight (1993)
1986-90, matched by age to cases and nine controls
212 controls with oral or
pharyngeal cancer or benign
oral disease
OR, odds ratio; RR, relative risk; in parentheses, 95% confidence interval (CI), unless otherwise stated
B8.2.3.2 Urinary bladder cancer
Fifteen case-control studies of urinary bladder cancer in
relation to presumed exposure to diesel exhaust are summarized in
Table 42. The limitation of all of these studies is that exposure to
diesel exhaust was not clearly characterized. Howe et al. (1980) used
any self-reported exposure to fumes that included diesel fumes, while
Hoar & Hoover (1985) used reports by next-of-kin of work with any
diesel fuel or engines or work as a truck driver. Risch et al. (1988)
obtained a history of exposure to exhaust by interview. Wynder et al.
(1985) estimated the likelihood of exposure to diesel exhaust during a
worker's lifetime on the basis of usual occupation, obtained at
interview, and attempted to divide exposure into high and moderate. In
a later study, self-reported exposure to diesel exhaust was recorded
(Iyer et al., 1990). Silverman et al. (1983), Vineis & Magnani (1985),
Silverman et al. (1986), Jensen et al. (1987), Iscovich et al. (1987),
and Notani et al. (1993) based their analyses of the risk for bladder
cancer on employment in an occupational category with a high
likelihood of exposure to diesel exhaust, such as truck driver,
railroad worker, transport worker, bus driver, and other related
occupations. In the studies of Siemiatycki et al. (1988) and Steineck
et al. (1990), occupational histories were reviewed by industrial
hygienists to link occupational titles to possible exposure. Cordier
et al. (1993) also used expert review of occupational histories to
estimate exposure to diesel exhaust on the basis of job title, but
additionally examined specific job titles. Furthermore, exposure was
usually assumed to have occurred if the person had ever been employed
in an occupation or had ever been exposed to diesel exhaust or other
fumes. Silverman et al. (1983, 1986) and Jensen et al. (1987) used
self-reported years of work as a truck driver. Self-reporting of
exposure poses a potential problem of differential recall among cases
and controls, as cases may recall exposure to diesel fumes or work in
a diesel-exposed job more readily than controls. Steenland et al.
(1987) used city directories listing occupation to obtain independent
occupational histories.
As the available occupational histories were crude, it was also
difficult to assess latency or duration of exposure. Silverman et al.
(1983) found that the relative risk (adjusted for smoking) was 5.5
(95% CI = 1.8-17.3) for truck drivers with 10 or more years of work
experience and 11.9 (95% CI = 2.3-61.1) for those with a history of
driving vehicles with diesel engines. Truck drivers employed after
1950, when diesel truck use became prevalent, had the highest risk. In
a later, larger study (Silverman et al., 1986), men who usually worked
as a truck driver or deliveryman had a relative risk of 1.5 (95% CI =
1.1-2.0), adjusted for smoking. The risk increased with increasing
years of work; however, truck drivers who had started work fewer than
40 years before diagnosis, who would have driven primarily diesel
engines, did not have an increased risk for bladder cancer. Hoar &
Hoover (1985) also noted increased relative odds with increasing years
of employment in jobs with reported exposure to diesel exhaust.
An additional limitation of the 15 case-control studies is that
results indicating an effect of presumed exposure to diesel exhaust or
a specific occupation are based on relatively few cases and controls,
even when conventional levels of statistical significance were
achieved. The study of Silverman et al. (1986), in which exposure was
defined as working as a truck driver or deliveryman, had the largest
number of exposed individuals (99 cases and 123 controls). Four
additional cohort studies not listed in the table (Howe et al., 1983;
Rushton et al., 1983; Schenker et al., 1984; Boffetta et al., 1988),
which were designed mainly to examine lung cancer and exposure to
diesel exhaust, also had too few cases of bladder cancer on which to
base meaningful conclusions. One cohort study of Danish bus drivers
(Netterstrom, 1988) showed an elevated SMR of 153, which was of
borderline statistical significance (95% CI = 91-217), but this result
was based on 13 cases.
Misclassification of exposure to diesel exhaust would hide any
increase in risk for bladder cancer, since many unexposed individuals
would be included with those classified as exposed. Diesel exhaust is
responsible for most of the respirable particles in mixed vehicle
exhausts, and exposure to respirable particles with adsorbed PAHs is
presumed to result ultimately in an increased risk for bladder cancer
due to metabolic transformation and urinary excretion of carcinogens.
The risk for bladder cancer is also increased in cigarette smokers and
in workers exposed to dyes containing aromatic amines (Ruder et al.,
1990). Coffee has been suggested to be a risk factor for bladder
cancer but has not been clearly implicated (Howe et al., 1980). The
classification of exposure to diesel exhaust is sufficiently crude in
the papers summarized in Table 42, however, that even when smoking was
considered, the effect of other, unmeasured confounding exposures
cannot be excluded. The actual contribution of diesel exhaust to an
increased risk of bladder cancer is uncertain, even when an effect of
presumed exposure was noted (Silverman et al., 1983; Hoar & Hoover,
1985; Silverman et al., 1986; Iscovich et al., 1987; Steenland et al.,
1987). Although the literature suggests that truck drivers have an
increased risk for bladder cancer, the specific exposure responsible
has not been determined.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Virtually no data are available relating specifically to the
effects of diesel fuel exhaust emissions.
The growth and photosynthesis of liquid cultures of the green
alga Chlamydomonas reinhardtii were examined after incubation with
iso-octane extracts of diesel particulate exhaust containing 51
compounds (mainly polynuclear aromatic ketones and pure PAHs)
identified by gas chromatographic-mass spectrometric analysis. At
concentrations up to 0.125% by volume, dose-dependent growth
retardation of the algae was observed. Higher concentrations (no
details given) caused death. The algae adapted to sublethal
concentrations of the extract over a period of several days;
thereafter, toxic extracts at concentrations up to 2.5% by volume
affected neither growth nor photosynthesis. It was noted that the
amounts of certain components decreased during incubation, suggesting
uptake into the cells (Liebe & Fock, 1992).
B10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Hundreds of chemical compounds are released during the combustion
of diesel fuel. The characteristics and amount of exhaust depend on
the type of diesel engine, its operating conditions and adjustment,
and the composition of the fuel. The following evaluation focuses
mainly on the risks to human health associated with exposure to diesel
particulate matter, since the large amount of soot particles emitted
is the main characteristic of diesel exhaust.
In general, the risk assessment paradigm proposed by the National
Academy of Sciences of the United States (US National Reserach
Council, 1983) will be followed. The four steps in this process are:
hazard identification, dose-response assessment, exposure assessment,
and risk characterization. The outcome of these individual steps is
critical for taking risk management decisions, including setting
exposure standards that have consequences for public health, and for
social, economic, and political issues.
The epidemiological studies in humans and the studies in
experimental animals considered useful for human health risk
assessment are discussed in this section. Both carcinogenic and other
effects are considered, but a threshold is assumed to exist only for
non-cancer effects. An IPCS method for deriving health-based guidance
exposure limits provides further details (IPCS, 1993).
B10.1 Exposure of the general population
The exposure of the general population to diesel exhaust varies
with proximity to diesel-powered vehicles. For example, higher levels
of diesel exhaust constituents are present in busy streets and parking
areas than in rural areas. The concentrations of the main gaseous
components resulting from diesel exhaust can be estimated from
specific emission factors, the contribution of diesel vehicles to
total traffic, and atmospheric dispersion models. As assumptions are
necessary to attribute the emissions of the gaseous components to
diesel sources, the validity of such estimates is considered to be
limited.
On a global basis, the contribution of diesel exhaust to the
total emissions of particulate matter varies widely, depending on the
percentage of diesel vehicles in the total volume of traffic,
maintenance of individual engines, fuel quality, and emission control
techniques. Such factors also lead to wide local variations in the
concentrations to which humans are exposed.
Measurements and calculations of ambient concentrations of diesel
particulates near roads provide a daily average range of 8-42 µg/m3.
The estimated annual average concentrations in Germany are 5-10 µg/m3
in urban areas and 1.5 µg/m3 in rural areas, and those in the United
States are 1-2 µg/m3 in urban areas and 0.6-1 µg/m3 in rural
areas. As the proportion of diesel vehicles is smaller in the United
States than in Europe, the concentrations in 1990, estimated from
national averages, were 2.0 µg/m3 in an urban area and 1.1 µg/m3
in a rural area, with predictions of 1.2 g/m3 for urban areas and
0.6 µg/m3 for rural areas in 1995.
Diesel particulates form part of the fine particle range
(< 2.5 µm diameter). The average concentration of this fraction of
suspended particulate matter as a whole, measured in six cities in the
United States during 1979-85, was 11-30 µg/m3 or, in terms of
inhalable particles (<10-15 µm diameter; equivalent to particulate
matter with a diameter < 10 µm [PM10]), 18-46 µg/m3 (Dockery et
al., 1993). In these locations, diesel particulates probably
represented less than 10% of the suspended particulates measured as
PM10; considerably higher proportions were found in London and other
large cities in the United Kingdom on the basis of data on emissions
(Quality of Urban Air Review Group, 1993), but few data are available
that are related directly to ambient concentrations of the diesel
component. The one experimental study that has been reported (Horvath
et al., 1985), carried out in Vienna, Austria, using chemically
labelled fuel, yielded a value for the background concentration of
diesel particulates within the city of 11 µg/m3.
B10.2 Occupational exposure
Occupational exposure to airborne diesel particulate matter has
been well determined for only two occupational groups, railroad
workers (Howe et al., 1983; Garshick et al., 1987, 1988) and workers
in the trucking industry (stevedores, local and road truck drivers,
and mechanics) (Emmelin et al., 1993). The levels of exposure during
work shifts are about 51-192 µg/m3 for railroad workers exposed to
diesel locomotive exhaust, roughly 25 µg/m3 for truck drivers, and
156 µg/m3 for stevedores (assuming that elemental carbon represented
20% of respirable particulate). These levels would be expected to vary
with the amount of ventilation available. Exposure to diesel exhaust
can also vary over a worker's lifetime, even within the same job. In
the railroad industry, exposure to diesel exhaust was greater in the
years after the introduction of diesel equipment. Reports of working
conditions in the 1950s and 1960s in diesel repair shops cited 'smoky'
atmospheres that were probably related to use of diesel equipment and
poor ventilation (Woskie et al., 1988b). No direct information is
available that allows reconstruction of accurate levels of past
exposure.
Reports of concentrations of diesel exhaust in other industries
are not accurate because of the presence of other dusts, although
attempts have been made to measure the contribution of diesel exhaust
(Daniel, 1984; Lehmann et al., 1990). Even for American railroad
workers (Woskie et al., 1988a), the contribution of other dusts could
not be determined, although cigarette smoke particulate was taken into
consideration. Consequently, the concentrations of diesel exhaust
reported in some jobs on railroads where other dusts (such as sand)
were present might have been lower.
B10.3 Non-neoplastic effects
B10.3.1 Hazard identification
B10.3.1.1 Humans
Diesel exhaust contains gases that irritate the nose, throat, and
eyes. It also contains particles which, together with the gases, can
cause airway irritation. Diesel exhaust may be recognized by its
characteristic odour. After acute exposure, it may induce mucous
membrane irritation, headache, and light-headedness. When volunteers
were exposed to diesel exhaust with an estimated particle
concentration of 100 µg/m3 for 1 h, several indicators of an
inflammatory response were observed in bronchioalveolar lavage fluid
(see section B8.2.1). In a well-conducted study on diesel-bus garage
workers, those with the highest exposures to respirable particulate
(> 0.31 mg/m3) reported significantly more cough, itchy or burning
eyes, headache, and wheeze, after adjustment for age and smoking, than
people with lower exposure. The contribution of diesel exhaust to the
respirable particulate is not known. Exposure to nitrogen dioxide at
> 0.3 ppm also resulted in more eye irritation and wheeze. Studies of
exposure indicate, however, variability in the ability of individuals
to detect the odour and the irritating effects of diesel exhaust.
Several cases of persistent asthma and asthma attacks have been
reported after acute exposure.
No consistent change in pulmonary function has been reported over
a work shift, although in one study of ferry workers, a decline in FVC
and FEV1 was noted. Specific measurements of diesel exhaust are
lacking in these studies, although total dust and respirable dust were
sometimes measured. Cases of persistent asthma have been reported in
railroad workers acutely exposed to apparently high levels of whole
exhaust, but the mechanism is unknown.
Studies of the possible chronic effects of exposure on
respiratory parameters are limited to occupational cohorts who are
still at work and thus have relatively short durations of exposure.
Such cohorts are likely to be healthier than older cohorts, which
include retirees who had longer exposure. Although excess respiratory
symptoms and reduced pulmonary function have been reported in some
studies, it is not clear whether these are long-term effects of
exposure.
B10.3.1.2 Experimental animals
Long-term studies in laboratory animals are described in section
B7.3. Many end-points were measured in the respiratory system of
several species of rodent after two or more years of exposure and at
several intermediate times. Adverse effects were seen after a
sufficient particle load had accumulated in the lung; and the
appearance of the effects is determined by both the concentration and
the duration of exposure. The results of these studies illustrate the
response of the respiratory tract in terms of biochemistry,
histopathology, cytology, pulmonary function, inflammation, and
clearance of particles. Early events in the pathogenesis of this
response include phagocytosis of inhaled particles. As the lung burden
increases, particle-filled macrophages are observed in the alveoli. At
lung burdens associated with particle overload, migration of these
macrophages out of the lung appears to be inhibited, and an early
event in the development of lung damage is the accumulation and
aggregation of clusters of particle-filled macrophages. Degeneration
of macrophages is observed, and the numbers of macrophages and
neutrophils in the alveolar and interstitial spaces increase.
Damage to the alveolar epithelium in areas surrounding these
macrophage clusters includes cell damage and proliferation.
Biochemical changes are seen in lavage fluid and lung tissue. The
alterations to particle-filled macrophages result in decreased
function and impaired particle clearance, the latter leading to a more
rapid increase in the lung burden of particles. Late effects include
inflammatory and proliferative responses, leading to fibrotic
responses. The sequence of these events has not been distinguished
clearly in experimental work; they are likely to be interrelated and
to occur, to some extent, concurrently.
The possible mechanism of action of diesel exhaust (discussed in
section B7.9) includes a role of the gas phase, as opposed to the
particle phase, in causing lung damage. The non-carcinogenic toxicity
of diesel exhaust is considered to be due to the particle content,
since no effects are seen in rodents exposed to diesel exhaust that
has been filtered to remove particulate matter. Another issue in the
dosimetry of diesel exhaust is the role of the insoluble carbon core
in relation to that of the adsorbed organic material. As discussed in
section B7.9, studies that show similarities in non-carcinogenic
responses to exposure to diesel exhaust and to carbon particles
without an organic component indicate a role of long-term retention of
the insoluble carbon core.
B10.3.2 Dose-response assessment
B10.3.2.1 Epidemiological studies
Variability in the reporting of odour and irritation indicates
that a subsegment of the population is sensitive to diesel exhaust;
however, the proportion of the population with this characteristic
cannot be estimated. Chronic effects have not been seen in
occupationally exposed people, but excess respiratory symptoms have
been reported after acute exposure. It is not possible to determine
the dose of diesel exhaust (on the basis of exposure to gas or
particles) that produces these symptoms.
B10.3.2.2 Studies in experimental animals
In order to select the pivotal study for establishing the NOAEL
or uncertainty factor, the most sensitive, relevant studies must be
identified. All of the long-term studies focus on effects on the
respiratory tract, which are clearly those most relevant. Studies of
relatively low concentrations are listed in Table 43, which gives the
target and actual exposure concentrations, the equivalent continuous
exposure of the animal, the equivalent continuous human exposure, and
the effect level. The equivalent continuous exposure of animals is
calculated by multiplying the actual exposure concentration by the
(number of hours of daily exposure/24) and by the (number of days per
week of exposure/7). This value is necessary for comparing exposure
concentrations among studies with different protocols, i.e. different
numbers of hours of exposure per day and/or days per week.
The equivalent continuous human exposure is calculated from the
particle deposition-clearance model of Yu & Yoon (1990), discussed in
section B6.2. This model is based on studies of rats since only those
studies provide data on the exposure-response relationship for
inhibition of particle clearance.
The model of Yu & Yoon (1990) is a sophisticated approach to
defining the relationship between the inhaled concentration and the
dose in lung tissue. It combines detailed models of rat and human lung
structure, aerodynamic models of the air flow in the airways, particle
deposition dynamics, and information on clearance of particles from
rat and human lung. The model is specific to diesel particles because
the characteristics of the particles used in the model are derived
from studies of diesel particles and because the information on lung
clearance is based on studies of diesel exhaust in rats. The model has
been adapted for diesel exhaust particles by allowing evaluation of
the insoluble carbon core and of the tightly and loosely bound organic
compounds separately. Values for deposition and clearance are combined
with input from experimental studies to calculate the burden of
particles in the lungs of the animals at the end of the study. The
continuous concentration that would result in the same lung burden in
humans is then calculated, as the mass of particles per unit of
alveolar surface area or unit of lung weight. This lung burden is
assumed to result in a similar effect in humans and in rats. With
these considerations, the equivalent human concentrations are
predicted on the basis of the assumption that the retained particle
mass per unit alveolar surface area is the appropriate dose measure
for extrapolation between species. The effect level used in the risk
assessment is based on an evaluation of the adversity of the effect.
For the purposes of risk characterization, the NOAEL or, if that
is not available, the LOAEL for the critical effect is related to
exposure. The critical effect is the first adverse effect that appears
when the critical concentration is reached at the target site. A
decision about whether an effect is critical is a matter of expert
judgement. An adverse effect is defined as a change in morphology,
physiology, growth, development, or the life span of an organism which
results in impairment of functional capacity or of capacity to
compensate for additional stress or an increase in susceptibility to
the harmful effects of other environmental influences (International
Union for Pure and Applied Chemistry, 1993).
The NOAEL is defined as the greatest concentration or amount of a
substance, found by experiment or observation, that causes no
alteration in morphology, functional capacity, growth, development, or
the life span of target organisms that are distinguishable from those
observed in normal (control) organisms of the same species and strain
under the same defined conditions of exposure (International Union for
Pure and Applied Chemistry, 1993).
The LOAEL is defined as the lowest concentration or amount of a
substance, found by experiment or observation, that causes an adverse
alteration in morphology, functional capacity, growth, development, or
the life span of a target organism distinguishable from those of
normal (control) organisms of the same species and strain under
defined conditions of exposure (International Union for Pure and
Applied Chemistry, 1993).
Table 43. Exposure of experimental animals, equivalent continuous human exposure, and effect levels of long-term inhalation in studies
of rats described in section B7.3
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
7 h/d, HP, LL, 0.35 0.353 0.0735 0.042 N Mauderly
5 d/week, LTB, TC, 3.5 3.47 0.723 0.360 A et al. (1987)
30 months LB 7 7.08 1.47 0.582 A
Light-duty, HP 0.1 0.11 0.063 0.038 N Ishinishi
16 h/d, 0.4 0.41 0.23 0.139 N et al. (1986,
6 d/week, 1 1.18c 0.67 0.359 A 1988)
30 months 2 2.32 1.3 0.571 A
Heavy-duty, HP 0.4 0.46 0.26 0.155 N Ishinishi
16 h/d, 1 0.96 0.55 0.303 A et al. (1986,
6 d/week, 2 1.84 1.05 0.493 A 1988)
30 months 4 3.72 2.13 0.911 A
110 h/week, HP 0.25 0.258 0.17 NA N Barnhart
6 months 0.75 0.796 0.52 NA A et al. (1981)
1.5 1.53 1.0 NA A
7 h/d, HP, SB 2 1.95 0.57 0.336 A Lewis et al.
5 d/week, (1989)
24 months
Table 43 (contd)
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
19 h/d, HP, LL, 4 4.24 2.40 1.02 A Heinrich et
5 d/week, TC, LB al. (1986a)
32 months
18 h/d, HP, LL, 0.8 0.84 0.45 0.23 A Heinrich et
5 d/week, TC, LB 2.5 2.50 1.34 0.59 A al. (1995)
30 months 7.5 6.98 3.74 1.56 A
NA, not available (guinea-pigs were used)
a HP, histopathological examination; LL, lung lavage fluid; LTB, lung tissue biochemistry; TC, tracer clearance; SB, serum biochemistry;
LB, lung burden
b N, no effect; A, adverse effect
c According to Suzuki et al. (1990), 1.08 mg/m3
The selected NOAEL is divided by uncertainty factors to account
for data gaps. For these data, uncertainty exists in two areas:
extrapolation from animals to humans and accounting for sensitive
subpopulations. The factor for accounting for sensitive subpopulations
is a default value of 10, as it is considered that there are no data
suggesting a different factor. An additional default value of 10 is
usually applied for interspecies extrapolation, which is nominally
considered to consist of 100.4 (2.5) for toxicodynamics and 100.6
(4.0) for toxicokinetics (IPCS, 1994).
The LOAELs and NOAELs are selected on the basis of the following
reasoning. The LOAEL is the lowest level at which adverse effects are
seen in studies of adequate quality. The NOAEL, the highest level at
which no effect is seen in the available studies, must be lower than
the selected LOAEL. The LOAELs and NOAELs are identified by a
comparison with the equivalent continuous human exposure level. The
LOAELs in these studies are strikingly similar, with values of 0.360,
0.359, 0.303, 0.336, and 0.23 mg/m3 in studies from the Inhalation
Toxicology Research Institute (United States; Mauderly et al., 1987),
the Health Effects Research Program on light- and heavy-duty diesel
engine exhausts (Japan; Ishinishi et al., 1986, 1988), the National
Institute for Occupational Safety and Health (United States; Lewis et
al., 1989), and the Fraunhofer Institute for Toxicology and Aerosol
Research (Germany; Heinrich et al., 1986a, 1995), respectively (see
Table 43). The consistency of these levels of effect, in view of the
diversity of end-points represented, gives a high level of confidence
in the end-points.
The study at the Inhalation Toxicology Research Institute was
selected as the most representative because it covers the greatest
variety of end-points, including histopathology, bronchiolar lavage
biochemistry and cytology, lung tissue biochemistry, and particle
clearance; LOAELs were observed for all of these end-points. The
studies of the Health Effects Research Program showed a similar LOAEL
but only for histological changes in the lung. The value for
equivalent continuous human exposure is not available from the study
carried out by the General Motors Corporation (Barnhart et al., 1981)
because the main results were for guinea-pigs; although rats were
included in the study, detailed results were not presented. The
appropriate NOAEL in these studies is not selected on the basis of the
LOAEL. As close dosing intervals were used in the studies of of the
Health Effects Research Program, the NOAEL was selected on the basis
of studies of light-duty diesel engines, as 0.139 mg/m3.
B10.3.3 Exposure assessment
Exposure to diesel exhaust is discussed in sections B10.1 and
B10.2.
B10.3.4 Risk characterization
B10.3.4.1 Humans
A quantitative assessment of the risk for humans of
non-carcinogenic effects of exposure to diesel exhaust cannot be made
on the basis of studies in humans. A substantial volume of literature
shows an association between acute exposure to fine particulates and
morbidity and mortality in the general population. It is somewhat
uncertain whether there is a direct causal link and, if so, whether it
is related to a specific component of the suspended particulates.
Attention has been focused on fractions of suspended particulate
matter, currently measured as PM10 (diameter < 10 µm) or PM2.5
(diameter < 2.5 µm), comprised mainly of diesel particulates in the
submicrometre range, which represent an increasing proportion in some
countries. There is also evidence that exposure to particulates is
involved in respiratory symptoms and in mortality from certain chronic
respiratory conditions.
B10.3.4.2 Experimental animals
Three approaches were used in assessing the risk for
non-carcinogenic effects of exposure to diesel exhaust, on the basis of
exposure (dose)-response assessment in experimental studies (see
section B10.3.2.2), and for deriving guidance values for exposure.
Approaches 1 and 2 are based on the NOAEL from studies in rats. The
difference between the two is that approach 1 involves a dosimetric
extrapolation model (Yu & Yoon, 1990) for converting the actual
concentration to which animals were exposed to an equivalent
continuous human exposure, thereby reducing the uncertainty in
interspecies extrapolation.
In approach 2, no dosimetric conversion is performed but the
usual uncertainty factors for interspecies and intraspecies
extrapolation are applied. In contrast to these NOAEL-defined
approaches, approach 3 is based on the principle of the 'benchmark
dose', in which a concentration of exposure to diesel exhaust is
derived from dose-response relationships observed in rats.
The model of Yu & Yoon (1990) takes into consideration the
specific characteristics of particle deposition and retention in rat
and human lung. Application of the model to studies of inhalation in
experimental animals is shown schematically in Figure 4. The deposited
and retained doses in the lung are calculated from the concentrations
in rats and expressed per unit lung weight or per unit alveolar
surface area. Under the assumption that the retained dose per lung
will lead to the same effect in rodent and human lung, a human
equivalent concentration can be calculated (as shown in Table 43), to
obtain an equivalent continuous human exposure. The equivalent
continuous exposure of animals shown in Table 43 was derived either by
calculating the continuous exposure at which the lung burden attained
in a study by inhalation (if measured) would have been reached, or by
calculating the lung burden in rats at the end of exposure on the
basis of the actual exposure parameters (also shown in Table 43) and
then calculating as above. The correlation between continuous exposure
in animals and humans in the model of Yu & Yoon (1990), assuming that
overload induces prolonged lung clearance, is shown in Figure 5. Use
of this approach, rather than simple adjustment of discontinuous to
continuous exposure by the c x t constant used in other assessments,
should reduce the uncertainty associated with the transformation.
Approach 1. The NOAEL of 0.41 mg/m3 from the study in rats
exposed by inhalation to light-duty engine exhaust (Ishinishi et al.,
1986, 1988; Table 43) is converted to an equivalent continuous
exposure of 0.23 mg/m3 in rats and then to an equivalent continuous
exposure of 0.139 mg/m3 , assumed to be the NOAEL in humans.
Application of a sophisticated dosimetric model decreases the
uncertainty in interspecies extrapolation from 10 to 100.4 (IPCS,
1994). Application of the usual uncertainty factor of 10 for
intraspecies differences results in a total uncertainty factor of
10 × 100.4 = 25.
The guidance value derived from this approach is
0.139 mg/m3 = 5.6 µg/m3.
---------------
25
No additional correction factor is required, resulting in a guidance
value (for the general population) of 5.6 µg/m3.
The inherent uncertainties in the dosimetric model are due to the
assumptions that must be made. A principal assumption is necessary to
estimate inhibition of clearance in humans, since data on this aspect
do not exist. It is assumed that clearance in humans is inhibited at
the same lung burden (mass per alveolar surface area) as in rats. The
other principal assumption is that the correct dose measure for lung
damage is mass of particle core per alveolar surface area. Since the
damage is localized to specific areas, another measure may be more
appropriate.
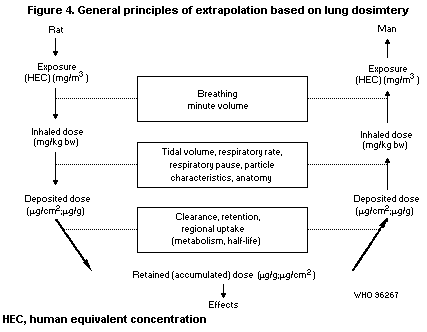 Approach 2. In this approach, the equivalent continuous animal
exposure based on the NOAEL of 0.41 in rats (light-duty diesel
exhaust; Ishinishi et al., 1986, 1988; Table 47) is used to derive the
guideline value. Because the dosimetric model is not used, the default
value of 10 is applied for interspecies uncertainty and an uncertainty
factor of 10 is added for intraspecies differences. The guidance value
obtained with this approach is
0.23 mg/m3
------------- = 2.3 µg/m3
100
Approach 3. An alternative to using the NOAEL is to derive a
'benchmark dose', as described by Crump (1984). In studies by
inhalation, the concentration rather than the dose is considered to be
more precise, and the 'benchmark concentration' is used. Like the
benchmark dose, this term covers the entire exposure-response
relationship in a given study rather than relying on only one data
point representing the NOAEL or LOAEL. This approach reduces the
uncertainty inherent in defining an NOAEL or LOAEL by considering the
upper 95% confidence interval of the full exposure-response curve from
a study in experimental animals, in which the lower 95% confidence
limit of a concentration corresponds to a 1, 5, or 10% increase in
response, defined as the percentage of animals responding in a
specific group. This exposure concentration is then the benchmark
concentration of the study. It is converted to the human benchmark
concentration on the basis of differences in pulmonary dosimetry
between the two species, which additionally reduces the use of
uncertainty factors. Because the full range of experimental results
from a specific study is used, the benchmark concentration approach
reduces statistical uncertainty.
In principle, this approach involves three steps. The first is
selection of the appropriate experimental study and end-point for
establishing an exposure-response curve. The second is calculation of
the benchmark concentration for the animals from a mathematical
description of the exposure-response curve and determination of the
95% confidence interval. Thirdly, the human benchmark concentration is
calculated; for exposure by inhalation, the exposure of the animals is
extrapolated dosimetrically to the human situation, with application
of uncertainty factors the size of which is determined as discussed
above. These factors generally consist of one for interspecies
extrapolation and one for sensitive subpopulations. The first two
steps of the benchmark concentration approach are shown schematically
in Figure 6.
Approach 2. In this approach, the equivalent continuous animal
exposure based on the NOAEL of 0.41 in rats (light-duty diesel
exhaust; Ishinishi et al., 1986, 1988; Table 47) is used to derive the
guideline value. Because the dosimetric model is not used, the default
value of 10 is applied for interspecies uncertainty and an uncertainty
factor of 10 is added for intraspecies differences. The guidance value
obtained with this approach is
0.23 mg/m3
------------- = 2.3 µg/m3
100
Approach 3. An alternative to using the NOAEL is to derive a
'benchmark dose', as described by Crump (1984). In studies by
inhalation, the concentration rather than the dose is considered to be
more precise, and the 'benchmark concentration' is used. Like the
benchmark dose, this term covers the entire exposure-response
relationship in a given study rather than relying on only one data
point representing the NOAEL or LOAEL. This approach reduces the
uncertainty inherent in defining an NOAEL or LOAEL by considering the
upper 95% confidence interval of the full exposure-response curve from
a study in experimental animals, in which the lower 95% confidence
limit of a concentration corresponds to a 1, 5, or 10% increase in
response, defined as the percentage of animals responding in a
specific group. This exposure concentration is then the benchmark
concentration of the study. It is converted to the human benchmark
concentration on the basis of differences in pulmonary dosimetry
between the two species, which additionally reduces the use of
uncertainty factors. Because the full range of experimental results
from a specific study is used, the benchmark concentration approach
reduces statistical uncertainty.
In principle, this approach involves three steps. The first is
selection of the appropriate experimental study and end-point for
establishing an exposure-response curve. The second is calculation of
the benchmark concentration for the animals from a mathematical
description of the exposure-response curve and determination of the
95% confidence interval. Thirdly, the human benchmark concentration is
calculated; for exposure by inhalation, the exposure of the animals is
extrapolated dosimetrically to the human situation, with application
of uncertainty factors the size of which is determined as discussed
above. These factors generally consist of one for interspecies
extrapolation and one for sensitive subpopulations. The first two
steps of the benchmark concentration approach are shown schematically
in Figure 6.
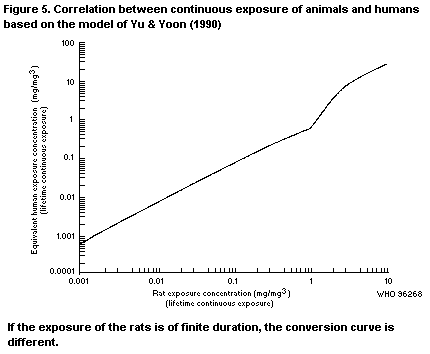
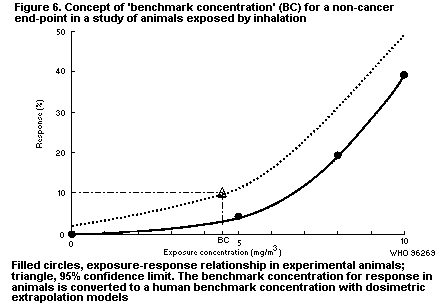 Two sets of data were used to derive the benchmark concentration
for diesel exhaust. The first was that of Ishinishi et al. (1988) from
a study in which rats were exposed to exhaust from a light-duty diesel
engine for two years at four concentrations. Hyperplastic lesions,
shown histopathologically to be a sensitive indicator of lung damage,
were used to establish a well-described exposure-response
relationship. The second data set was that of Creutzenberg et al.
(1990) for female Wistar rats exposed for 96 h per week for a total of
78 weeks to three concentrations of diesel exhaust. The most sensitive
end-points for exposure-related non-neoplastic changes were lung
clearance of particles and the occurrence of polymorphonuclear
neutrophils (PMN) in lung lavage fluid as indicators of inflammation.
Since these measurements are laborious, however, they were performed
only in a subset of the exposed animals, so that only six to eight
rats were studied per group.
The responses in the study of Ishinishi et al. (1988) were
expressed in terms of individual animals affected per total number of
exposed animals in each group, whereas the data of Creutzenberg et al.
(1990) were reported as group mean values plus or minus the standard
deviation, which is less useful for the benchmark concentration
approach. Individual responses in the latter study were, however,
provided by Bellmann (personal communication), so that the data from
this study could also be expressed in terms of percentage of animals
with impaired lung clearance. Impairment of lung function was
considered to be significant when the calculated pulmonary retention
half-time of the administered particles was at least 3.5 times greater
than their normal average half-time in the lungs of control animals.
Evaluation of the end-point chronic inflammation (PMN in lung
lavage fluid) proved to be more difficult, since all of the rats at
the lowest exposure level (0.8 mg/m3) in the study of Creutzenberg
et al. (1990) had increased PMN levels (100% response). Lung lavage
was performed at 22 and 24 months of exposure, and since the responses
at these two times were similar, the data were combined to increase
the numbers of animals per group to 11-14. The individual responses
(percentage of PMN among the total number of cells in the lavage
fluid) were used to derive the curve and the 95% confidence interval.
An excess of PMN of up to 3% of the total number of cells over the
background level was used to define the benchmark concentration. The
highest value observed in the control group was 2.75% PMN after
22 months of exposure.
The three data sets and the results of the probability function,
using a Weibull model to calculate the benchmark concentration, are
given in Tables 44, 45, and 46 and are illustrated in Figures 7 and 8.
Table 44. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Ishinishi et al.
(1988); end-point: hyperplastic lesions of the lung
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 4/125 4.6 3.2
0.063 4/125 4.6 3.25
0.23 6/125 4.7 4.8
0.67 12/123 12.1 9.76
1.3 87/124 87.0 70.2
Probability function [p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 *
Conc)K]
A0 = 3.7196 × 10-2
A1 = 3.740
K = 4.3504
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.634
0.05 0.511
0.01 0.313
The respective benchmark concentrations from the data sets for rats
are 0.634, 0.119, and 0.090 mg/m3, corresponding to a 10% response
(the lower 95% confidence limit on the exposure concentration for
hyperplastic lung injury and impaired lung clearance) or a 3% excess
of PMN in lavage fluid (the lower 95% confidence limit on the exposure
concentration for chronic alveolar inflammation).
The resulting human benchmark concentrations for the three
end-points were calculated from the model of Yu & Yoon (1990),
applying uncertainty factors of 10 to account for sensitive
subpopulations (human intraspecies differences) and 100.4 (2.5) for
potentially different toxicodynamics (see above), to be: 14 µg/m3
for hyperplastic lung lesions, 3 µg/m3 for impaired lung clearance,
and 2 µg/m3 for chronic alveolar inflammation. Although the
mathematical description of the exposure-response relationships in
rats is very good for all three sets of data, they should be viewed as
hypothetical since they are based on dosimetric conversions from rats
to humans and on the application of uncertainty factors.
Table 45. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Creutzenberg
et al. (1990); end-point: impaired lung clearance
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 0/6 0 0
0.45 2/8 1.7 25
1.34 3/6 3.0 50
3.74 5/6 5.2 83.3
Probability function
[p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 * Conc - D0)A2]
A0 = 0.0
A1 = 0.522
A2 = 1.00
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.119
0.05 0.058
0.01 0.011
The non-cancer guidance values and the benchmark concentrations
derived from these approaches are summarized in Table 47.
More sensitive end-points with respect to the adverse effects of
diesel exhaust on the lower respiratory tract are impaired clearance
in the deep lung and chronic alveolar inflammation, rather than
hyperplastic lung lesions. Chronic alveolar inflammation was also a
significant finding in the long-term study of rats exposed to diesel
exhaust by Henderson et al. (1988), who found a significantly
increased percentage of PMN in lavage fluid from the group with the
lowest exposure (0.35 mg/m3) after two years. For all three types of
end-points, however, there may be a threshold below which no change is
to be expected. The threshold concept is not included in the
mathematical model, and it would be difficult to do so at this point
in the absence of specific experimental data and data on variation
between human subjects.
These estimates are for the effects of long-term exposures, and
the concentrations are expressed as annual means over a lifetime. They
could apply to long-term effects on health, such as the association
between mortality from certain chronic conditions and exposure to
particulates in air pollution seen in a study in six cities in the
United States (Dockery et al., 1993). Their relevance to human
experience must also be considered in the context of particulate air
pollutants in general, however, and they have no direct bearing on the
acute effects of exposure to particulates, for which guidance values
are required in terms of 24-h mean concentrations.
B10.4 Neoplastic effects
B10.4.1 Hazard identification
B10.4.1.1 Lung cancer: occupational exposure
In the 1970s and 1980s, the recognition that diesel exhaust
contains respirable particles and that known carcinogenic substances
are adsorbed on the surface of these particles led to the hypothesis
that inhalation of diesel exhaust could result in lung cancer in
humans. As lung cancer develops slowly, over many years, studies of
individuals with long, well-defined exposure and follow-up (> 20
years) were considered to be the most informative. Four studies of
occupationally exposed individuals meet these criteria (Garshick et
al., 1987, 1988; Gustavsson et al., 1990; Emmelin et al., 1993; see
Table 43).
Table 46. Benchmark concentration for rats after long-term exposure to
diesel exhaust by inhalation in study by Creutzenberg et al.
(1990); end-point: chronic alveolar inflammation
Concentration (mg/m3) PMN in lung lavage fluid (%) No. rats
(equivalent continuous responding
exposure of rats Actual/observed Predicted per no.
exposed
0 0.7 0 0/11
0.45 11.1 11.7 13/13
1.34 29.9 27.6 14/14
3.74 50.8 51.9 14/14
Dose-response function: F(dose) = Q(0) × (Dose - (Dose - D0)Q(2)
Dose = log(1 + d) (d = rat exposure concentration)
Q(0) = 0.6.53
Q(1) = 77.010
Q(2) = 1.056
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
excess polymorphonuclear neutrophil (PMN) response (% of total lavaged
cells):
PMN in excess Rat BC
of control (%) (mg/m3)
3 0.090
2 0.059
1 0.029
Transition from steam to diesel locomotives occurred in the
American railroad industry during the 1950s. In a case-control study
of American railroad workers (Garshick et al., 1987), exposure was
estimated on the basis of yearly job (exposed or unexposed to diesel
exhaust) from 1959 to death or retirement; deaths were identified for
12 months in 1981-82. In an additional study of these workers
(Garshick et al., 1988), a retrospective cohort was defined on the
basis of work in a job with exposure to diesel exhaust in 1959, and
Two sets of data were used to derive the benchmark concentration
for diesel exhaust. The first was that of Ishinishi et al. (1988) from
a study in which rats were exposed to exhaust from a light-duty diesel
engine for two years at four concentrations. Hyperplastic lesions,
shown histopathologically to be a sensitive indicator of lung damage,
were used to establish a well-described exposure-response
relationship. The second data set was that of Creutzenberg et al.
(1990) for female Wistar rats exposed for 96 h per week for a total of
78 weeks to three concentrations of diesel exhaust. The most sensitive
end-points for exposure-related non-neoplastic changes were lung
clearance of particles and the occurrence of polymorphonuclear
neutrophils (PMN) in lung lavage fluid as indicators of inflammation.
Since these measurements are laborious, however, they were performed
only in a subset of the exposed animals, so that only six to eight
rats were studied per group.
The responses in the study of Ishinishi et al. (1988) were
expressed in terms of individual animals affected per total number of
exposed animals in each group, whereas the data of Creutzenberg et al.
(1990) were reported as group mean values plus or minus the standard
deviation, which is less useful for the benchmark concentration
approach. Individual responses in the latter study were, however,
provided by Bellmann (personal communication), so that the data from
this study could also be expressed in terms of percentage of animals
with impaired lung clearance. Impairment of lung function was
considered to be significant when the calculated pulmonary retention
half-time of the administered particles was at least 3.5 times greater
than their normal average half-time in the lungs of control animals.
Evaluation of the end-point chronic inflammation (PMN in lung
lavage fluid) proved to be more difficult, since all of the rats at
the lowest exposure level (0.8 mg/m3) in the study of Creutzenberg
et al. (1990) had increased PMN levels (100% response). Lung lavage
was performed at 22 and 24 months of exposure, and since the responses
at these two times were similar, the data were combined to increase
the numbers of animals per group to 11-14. The individual responses
(percentage of PMN among the total number of cells in the lavage
fluid) were used to derive the curve and the 95% confidence interval.
An excess of PMN of up to 3% of the total number of cells over the
background level was used to define the benchmark concentration. The
highest value observed in the control group was 2.75% PMN after
22 months of exposure.
The three data sets and the results of the probability function,
using a Weibull model to calculate the benchmark concentration, are
given in Tables 44, 45, and 46 and are illustrated in Figures 7 and 8.
Table 44. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Ishinishi et al.
(1988); end-point: hyperplastic lesions of the lung
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 4/125 4.6 3.2
0.063 4/125 4.6 3.25
0.23 6/125 4.7 4.8
0.67 12/123 12.1 9.76
1.3 87/124 87.0 70.2
Probability function [p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 *
Conc)K]
A0 = 3.7196 × 10-2
A1 = 3.740
K = 4.3504
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.634
0.05 0.511
0.01 0.313
The respective benchmark concentrations from the data sets for rats
are 0.634, 0.119, and 0.090 mg/m3, corresponding to a 10% response
(the lower 95% confidence limit on the exposure concentration for
hyperplastic lung injury and impaired lung clearance) or a 3% excess
of PMN in lavage fluid (the lower 95% confidence limit on the exposure
concentration for chronic alveolar inflammation).
The resulting human benchmark concentrations for the three
end-points were calculated from the model of Yu & Yoon (1990),
applying uncertainty factors of 10 to account for sensitive
subpopulations (human intraspecies differences) and 100.4 (2.5) for
potentially different toxicodynamics (see above), to be: 14 µg/m3
for hyperplastic lung lesions, 3 µg/m3 for impaired lung clearance,
and 2 µg/m3 for chronic alveolar inflammation. Although the
mathematical description of the exposure-response relationships in
rats is very good for all three sets of data, they should be viewed as
hypothetical since they are based on dosimetric conversions from rats
to humans and on the application of uncertainty factors.
Table 45. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Creutzenberg
et al. (1990); end-point: impaired lung clearance
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 0/6 0 0
0.45 2/8 1.7 25
1.34 3/6 3.0 50
3.74 5/6 5.2 83.3
Probability function
[p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 * Conc - D0)A2]
A0 = 0.0
A1 = 0.522
A2 = 1.00
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.119
0.05 0.058
0.01 0.011
The non-cancer guidance values and the benchmark concentrations
derived from these approaches are summarized in Table 47.
More sensitive end-points with respect to the adverse effects of
diesel exhaust on the lower respiratory tract are impaired clearance
in the deep lung and chronic alveolar inflammation, rather than
hyperplastic lung lesions. Chronic alveolar inflammation was also a
significant finding in the long-term study of rats exposed to diesel
exhaust by Henderson et al. (1988), who found a significantly
increased percentage of PMN in lavage fluid from the group with the
lowest exposure (0.35 mg/m3) after two years. For all three types of
end-points, however, there may be a threshold below which no change is
to be expected. The threshold concept is not included in the
mathematical model, and it would be difficult to do so at this point
in the absence of specific experimental data and data on variation
between human subjects.
These estimates are for the effects of long-term exposures, and
the concentrations are expressed as annual means over a lifetime. They
could apply to long-term effects on health, such as the association
between mortality from certain chronic conditions and exposure to
particulates in air pollution seen in a study in six cities in the
United States (Dockery et al., 1993). Their relevance to human
experience must also be considered in the context of particulate air
pollutants in general, however, and they have no direct bearing on the
acute effects of exposure to particulates, for which guidance values
are required in terms of 24-h mean concentrations.
B10.4 Neoplastic effects
B10.4.1 Hazard identification
B10.4.1.1 Lung cancer: occupational exposure
In the 1970s and 1980s, the recognition that diesel exhaust
contains respirable particles and that known carcinogenic substances
are adsorbed on the surface of these particles led to the hypothesis
that inhalation of diesel exhaust could result in lung cancer in
humans. As lung cancer develops slowly, over many years, studies of
individuals with long, well-defined exposure and follow-up (> 20
years) were considered to be the most informative. Four studies of
occupationally exposed individuals meet these criteria (Garshick et
al., 1987, 1988; Gustavsson et al., 1990; Emmelin et al., 1993; see
Table 43).
Table 46. Benchmark concentration for rats after long-term exposure to
diesel exhaust by inhalation in study by Creutzenberg et al.
(1990); end-point: chronic alveolar inflammation
Concentration (mg/m3) PMN in lung lavage fluid (%) No. rats
(equivalent continuous responding
exposure of rats Actual/observed Predicted per no.
exposed
0 0.7 0 0/11
0.45 11.1 11.7 13/13
1.34 29.9 27.6 14/14
3.74 50.8 51.9 14/14
Dose-response function: F(dose) = Q(0) × (Dose - (Dose - D0)Q(2)
Dose = log(1 + d) (d = rat exposure concentration)
Q(0) = 0.6.53
Q(1) = 77.010
Q(2) = 1.056
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
excess polymorphonuclear neutrophil (PMN) response (% of total lavaged
cells):
PMN in excess Rat BC
of control (%) (mg/m3)
3 0.090
2 0.059
1 0.029
Transition from steam to diesel locomotives occurred in the
American railroad industry during the 1950s. In a case-control study
of American railroad workers (Garshick et al., 1987), exposure was
estimated on the basis of yearly job (exposed or unexposed to diesel
exhaust) from 1959 to death or retirement; deaths were identified for
12 months in 1981-82. In an additional study of these workers
(Garshick et al., 1988), a retrospective cohort was defined on the
basis of work in a job with exposure to diesel exhaust in 1959, and
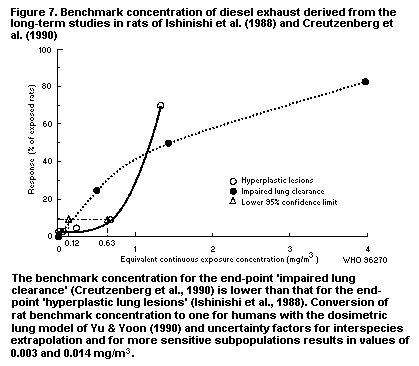
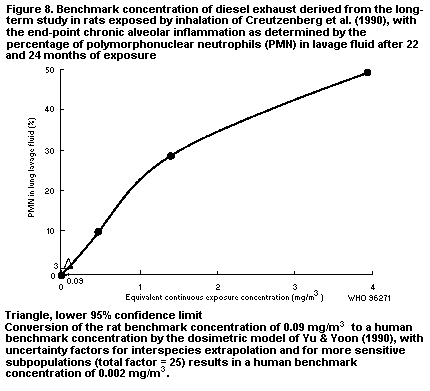 deaths were recorded for 1959-80. Although the previous exposure
levels of the railroad workers were not available, the classification
of workers into groups on the basis of exposure was validated by an
industrial hygienist and a review of work practices. Air sampling was
conducted to establish current exposure levels in various jobs
(Hammond et al., 1988; Woskie et al., 1988a,b).
Gustavsson et al. (1990) studied lung cancer among bus garage
workers in Stockholm, Sweden, where all buses with internal combustion
engines have been diesel-fuelled since 1945. A cohort was established
of people who had worked in bus garages for at least six months
between 1945 and 1970, and mortality was determined for 1952-86. A
nested case-control study was performed within this cohort. Although
actual exposure was not measured, relative exposure to diesel exhaust
was graded on the basis of a scale established by an industrial
hygienist in a review of work practices, bus engine operation, and
shop ventilation.
Table 47. Summary of non-cancer guidance values and benchmark
concentrations
Approach Guidance value
or benchmark
concentration
(µg/m3)
NOAEL with dosimetric conversion from rats to humans 5.6a
NOAEL without dosimetric conversion from rats to 2.3
humans
Benchmark concentration with dosimetric conversion
from rats to humans
Chronic alveolar inflammation 2a
Impaired lung clearance 3a
Hyperplastic lesions 14a
Benchmark concentration without dosimetric
conversion from rats to humans
Chronic alveolar inflammation 0.9
Impaired lung clearance 1.2
Hyperplastic lesions 6.3
NOAEL, no-observed-adverse-effect level
Normalized for lung surface area in rats and humans; after
normalization for lung weight, the benchmark concentration increases
by a factor of 4.
Emmelin et al. (1993) studied Swedish stevedores in 15 ports
where diesel equipment had been introduced between 1957 and 1963; they
also performed a nested case-control study on cases of lung cancer
identified between 1960 and 1982. Exposure to diesel exhaust was
estimated on the basis of three indices derived from estimated diesel
fuel consumption and years of work after the introduction of diesel
equipment in each port.
All four studies showed an increased risk for lung cancer with
exposure to diesel exhaust. The relative risks reported ranged from
about 1.4 for railroad workers with the longest duration of exposure
in both studies of such populations to 1.3-2.4 for bus garage workers,
depending on the exposure category. The stevedores also had an
increased relative odds ratio for lung cancer with increasing exposure
with respect to all three indices of exposure examined, with an
increase of three- to sixfold in the highest exposure categories and
1.5-2.7-fold in the lowest exposure category. The point estimates
were, however, imprecise and had wide confidence intervals: for all
but one exposure category, the lower limit of the 90% confidence
intervals presented by the authors included 1.0.
The effects of cigarette smoking could be adjusted for in the
case-control studies of railroad workers (Garshick et al., 1987) and
stevedores (Emmelin et al., 1993). The smoking histories of the
railroad workers were obtained from next-of-kin. Various regression
models (including both dose and duration of smoking) were examined in
detail in order to adjust adequately for the effects of smoking. No
matter how it was accounted for, the risk for lung cancer based on
work in a job with exposure to diesel exhaust was similar to the
unadjusted odds ratio. The study of stevedores (Emmelin et al., 1993)
was smaller than that of railroad workers, and in order to reduce the
number of strata for analysis workers were stratified only as smoker
or nonsmoker. In the two studies in which information on smoking was
not available (Garshick et al., 1988; Gustavsson et al., 1990), the
analysis was based on a comparison with workers in the same
occupational cohort, making confounding by smoking less likely.
The results of most of the other studies summarized in Tables
39-41 support those of the four studies discussed above. The relative
risks were generally in the range 1.2-1.9 but did not always achieve
statistical significance. Furthermore, exposure to diesel exhaust was
less precisely defined in these studies; it was usually based on
self-reporting, the report of a surrogate, or a census report of
occupation (Table 41). Self-reporting or the report of a surrogate
would reflect actual exposure to diesel exhaust or work in a job in
which exposure was likely; however, defining exposure in this less
precise fashion make it harder to detect an effect of the exposure and
leads to lower relative risks and wider confidence intervals.
In three other studies, job titles obtained from an employer were
used to define exposure. In the study of Howe et al. (1983), the job
held at the time of retirement from the railroads was used to
categorize exposure, and mortality was assessed for 1965-77.
Significantly elevated risks for lung cancer were noted among workers
who had probably been exposed to diesel exhaust (relative risk = 1.35)
and among those who had possibly been exposed (1.20); however, since
only retired workers were studied, actual exposure to diesel exhaust
would have been short for many workers. Rushton et al. (1983) and
Waller (1981) used job titles within the London Transport organization
to indicate potential exposure to diesel exhaust. Neither study showed
an elevated risk for lung cancer, but the study of Rushton and
coworkers was characterized by a short duration of exposure and
follow-up, whereas in the study of Waller, deaths occurring after
employment were not included.
The relative risks for lung cancer as a result of exposure to
diesel exhaust are generally low, and risks of this magnitude are more
susceptible to chance and to the effects of unmeasured confounding
factors and imprecision in adjusting for known confounding factors. As
discussed above, the elevated risk for lung cancer observed in the
four most informative studies is unlikely to be due to confounding by
cigarette smoking and is probably due to exposure to diesel exhaust.
Other studies, although limited primarily by the exposure
ascertainment, support this assessment.
B10.4.1.2 Urinary bladder cancer: occupational exposure
The hypothesis that exposure to diesel exhaust results in cancer
of the urinary bladder is based on the fact that individuals who smoke
cigarettes, a source of PAHs (as is diesel particulate), excrete
mutagenic substances in their urine, and cigarette smoking is
associated with an increased risk for bladder cancer. In a recent
study of railroad workers with current exposure to diesel exhaust
(median concentration of respirable particulates over a work shift,
54-113 µg/m3; adjusted for smoking), mutagenicity in urine at the
end of a shift was not associated with exposure (Schenker et al.,
1992).
The evidence that bladder cancer is associated with exposure to
diesel exhaust is based on an association between work in industries
or occupations with potential exposure to diesel exhaust. The relative
risks reported (some of which are adjusted for smoking) are generally
in the range 1.2-2 (Hoar & Hoover, 1985; Silverman et al., 1986),
although higher risks have been reported. Exposure was assessed mainly
by self-reporting and reporting of occupation at interview. The main
occupation in which there was considered to be potential exposure to
diesel exhaust was truck driving. Measurements of exposure of truck
drivers by Zaebst et al. (1991) indicate that current exposure is
likely to be low, but there is no knowledge about past exposure
levels.
Thus, although work in occupations related to motor vehicles has
been associated with bladder cancer, the extent of exposure of these
workers to diesel exhaust is unknown.
B10.4.2 Dose-response assessment
B10.4.2.1 Lung cancer
Job category and work in an occupation with exposure to diesel
exhaust have been used as indicators of exposure. When years of
exposure and derived indices of exposure have been used, an
exposure-response relationship has generally been found. Measurements
of total and respirable dust in exposed workers do not reflect actual
exposure to diesel exhaust particles, however, since there are other
sources of dust in an occupational environment. The exposure of only
two occupational groups, American railroad workers (Woskie et al.,
1988b) and truck drivers (Zaebst et al., 1991), to diesel exhaust
particles has been quantified (see section B5.2). Current exposure was
measured for both these groups, but past exposure levels could not be
determined (Woskie et al., 1988b), so that studies of workers do not
permit determination of a dose-response relationship for inhaled
diesel particles.
B10.4.2.2 Urinary bladder cancer
Years of work in an occupation related to motor vehicles (mainly
truck driving) was examined in some, but not all, studies, but no
measurements were available to estimate exposure to diesel exhaust in
these settings.
B10.4.3 Exposure assessment
The ranges of exposures observed for workers are presented in
sections B5.2 and B10.2.
B10.4.4 Risk characterization
B10.4.4.1 Human lung cancer
Thus, historical measurements of exposure to diesel exhaust are
unreliable and exist only for current workers in two industries. A
quantitative risk assessment cannot be conducted on the basis of
epidemiological data in which job title was used as a surrogate of
exposure. Attempts to obtain estimates of risk (presented in section
B10.4.1) in the retrospective cohort study of American railroad
workers (Garshick et al., 1988) were not successful (see below for
discussion). Consequently, there are no human data suitable for
estimating unit risk.
B10.4.4.2 Human urinary bladder cancer
The risk for urinary bladder cancer cannot be assessed from the
available epidemiological data.
B10.4.4.3 Risk characterization based on studies in experimental
animals
(a) Introduction
Most human tumours arise in the bronchial region, and although
the frequency of adenocarcinomas has increased in recent years
(Martini, 1993), squamous bronchogenic carcinomas still represent the
majority (about 60%) of lung tumours in humans (Auerbach & Garfinkel,
1991; Devesa et al., 1991; Campobosso et al., 1993). The tumours
observed in rats are located almost exclusively in the bronchoalveolar
regions. This difference raises the question of whether the rat is the
appropriate species for extrapolating to humans. Although it may be
the wrong model, however, studies of hamsters and monkeys give
negative results, so the rat is the most appropriate model from a
conservative viewpoint and is therefore used for quantitative risk
extrapolation.
The objective is to assess quantitatively the potential risk for
lung cancer in humans posed by exposure to diesel exhaust emissions in
the ambient air. Ideally, human risk due to exposure to an
environmental pollutant should be predicted on the basis of human
experience. Although several epidemiological studies are available on
bus and railroad workers, these results alone are not adequate to
assess the potential risk of cancer in humans because of the lack of
reliable information on the conditions of exposure of these workers.
The challenge is to assess the risk of exposure to diesel exhaust on
the basis of all of the available information, for both experimental
animals and humans. In contrast to the sparse human data, there is a
rich pool of information on diesel-induced lung tumours in two strains
of rat. One approach to integrating this information is to make a
quantitative risk assessment on the basis of information from
bioassays and on the relevant biological mechanism and then to
evaluate these animal-based results against available human
experience. This approach was followed in this monograph.
Although, the available experimental data on the possible impact
of extractable organic matter and PAHs on the lung tumour response
associated with exposure to diesel soot are not strong (Heinrich et
al., 1991; Heinrich, 1994; Heinrich et al., 1995), a mathematical
approach to assessing the risk of exposure to diesel exhaust should
take into account the effects of both particles (carbon core) and
extractable organic matter, because: (i) organic compounds include a
variety of PAHs and nitroaromatic compounds, many of which are known
to be carcinogenic; (ii) the results of recent studies on inert
particles and carbon black in rats (Mauderly et al., 1991; Heinrich,
1994; Heinrich et al., 1994) strongly support the hypothesis that the
carbon cores of diesel particles are the component primarily
responsible for the induction of lung cancer; (iii) PAHs alone are
unlikely to be responsible for the tumour response; (iv) the
disproportionately high tumour incidence in animals with heavy
exposure coincides with a disproportionate increase in the cumulative
lung burden of diesel particles. Although a qualitative description of
the biological mechanism of diesel exhaust-induced tumours is
plausible, however, the lack of quantitative information on the
dynamics of tumour initiation, promotion, and progression vitiates the
construction of a biologically based dose-response model.
The crudest dose-response model is obtained by fitting observed
dose-response data to a mathematical function that is a monotonic,
non-decreasing function of exposure, using the inhaled concentration
and observed tumour incidence, without considering information on
pharmacokinetics and the mechanism of carcinogenesis. This approach
may result in uncertain estimates of risk at low doses, because, while
the model may adequately fit the dose-response data for high doses at
which a tumour response is observed, it may greatly underestimate or
overestimate the risk at low doses.
Most previous risk estimates of diesel exhaust-induced cancer
risk were developed before the results of many of the bioassays became
available (Pepelko & Chen, 1993). Nevertheless, most of the estimates
are similar (within an order of magnitude), except for those
calculated on the basis of human data, which are higher than those
calculated from other databases. Because of great uncertainties
associated with the available human data, it is considered more
appropriate to calculate risk on the basis of animal data and then use
human data to evaluate their validity.
The approach adopted in this assessment is the linearized
multistage model (Anderson et al., 1983), in which burden per lung
surface area (milligrams per square centimetre) is used as an
equivalent dose in animals and humans. The use of lung (alveolar)
surface area is justified by the fact that the lung tumours observed
in rats are derived exclusively from epithelial cells in the alveolar
region of the lung. Normalization on the basis of lung weight can also
be done by using a factor of 4, for increasing the dose or decreasing
the risk, for the difference in the ratio of rat:human lung surface
area and lung weight. The risk prediction calculated by this approach
is presumably conservative (i.e. overpredicts the risk) because the
dose-response function is assumed to be non-threshold and linear at
low doses.
An alternative model that incorporates a biological mechanism is
constructed using statistically estimated parameters. The model
assumes that carcinogenic agents present in the organic fraction act
directly on the target cells, primarily via initiation. It is further
assumed that the majority of the particles are ingested by
macrophages. Particle-laden macrophages are then induced to secrete a
variety of mediators (e.g. reactive oxygen species and cytokines),
which diffuse to the target cells and induce initiation,
proliferation, and conversion of initiated cells to malignant cells.
This alternative model can be used only as a tool to evaluate the
impact of various biological assumptions and not for risk prediction,
because the parameters of the model are not measured in the
laboratory.
(b) Approaches to quantification of human risk from exposure to
diesel exhaust
Several issues must be addressed in estimating the risk of
exposure to diesel exhaust, including the critical target site, the
fraction of exhaust responsible for tumour induction, the availability
of dosimetric methods for accurately extrapolating dose from that for
experimental animals chronically exposed to high concentrations of
exhaust to that of humans exposed at ambient concentrations, and the
most suitable low-dose extrapolation model.
The critical target organ was considered to be the lung. Although
Iwai et al. (1986) reported induction of both malignant lymphomas and
lung tumours in rats exposed to diesel exhaust, the lung was the only
target site in other experimental studies of this species. Although
potentially carcinogenic agents present in diesel exhaust may be
absorbed from the lungs, enter the bloodstream, and be transported
systemically, no data are available to evaluate this possibility.
Organic compounds adsorbed on particles may also reach systemic
targets via the gastrointestinal tract, as particles deposited in the
conducting airways are cleared rapidly to the oropharynx and
swallowed. A considerable volume of particles is also likely to be
ingested as a result of grooming when the whole animal is exposed
(Wolff et. al., 1982); however, because the half-times for the elution
of organic compounds from particles are considerably longer than their
passage through the gastrointestinal tract, the fraction absorbed is
expected to be small. In any case, there is little evidence for any
systemic effects of diesel exhaust.
The site of action in the lungs is assumed to be the epithelial
lining of the alveoli and small airways. According to Mauderly et al.
(1987), inflammation and tumours appear to arise from this tissue.
Although interstitial events (e.g. fibrosis) have been suggested to be
associated with induction of lung tumours by particles (Kuschner,
1968), no data are available to support this view with respect to
diesel exhaust.
Accurate extrapolation of dose from studies of animals exposed to
high concentrations of exhaust to humans exposed to ambient
concentrations requires a variety of adjustments for species
differences in deposition efficiency and respiratory ventilation
rates. As the normal retention half-times in the alveolar region are
several times longer in humans than rats (Chan et al., 1981; Bohning
et al., 1982), the lung burden of humans may be underestimated if this
difference is not taken into account. The high exposure concentrations
used in some experimental studies, however, result in greatly slowed
or even completely inhibited clearance (Griffis et al., 1983). In
order to extrapolate dose accurately from experimental studies to
humans, the detailed dosimetric model developed by Yu et al. (1991)
was used, which accounts for species differences in respiratory
ventilation rates, deposition efficiency, normal particle clearance
rates, transport of particles to lung-associated lymph nodes, and lung
surface area. It also accounts for inhibition of particle clearance
due to lung overload. In this model, dose is estimated in terms of
particle concentration per unit of lung surface area.
Two approaches were used to derive unit risk estimates: the
linearized, multistage, low-dose extrapolation model and a model based
on the biological mechanism discussed above. In the linearized
multistage model, the lung burden of carbon core per lung surface area
is used as the dosimetric parameter. A particle-based assessment was
considered to be reasonable for two principal reasons: (i) exposure to
the vapour phase alone did not result in detectable tumour induction
in rats (Brightwell et al., 1986; Iwai et al., 1986; Heinrich et al.,
1986a); and (ii) exposure to carbon black, which is similar in
composition to the carbon core of whole diesel exhaust but contains
only negligible amounts of organic compounds, was about as effective
in inducing lung cancer as whole diesel exhaust (Mauderly et al.,
1991; Nikula et al., 1994; Heinrich et al., 1995). Although use of the
carbon core as a dosimetric parameter implies that it is primarily the
insoluble carbon core of diesel particles that is responsible for the
carcinogenic effects of diesel exhaust, it can also be viewed simply
as a marker of exposure to diesel exhaust, with no biological
implication. Similarly, organic compounds could be used as markers of
exposure for the dose-response calculation. When this is done, the
resulting unit risk estimates (not presented here) are very similar
(within 25%) to those calculated with the carbon core as the surrogate
of exposure.
The second approach is based on the assumption that, even though
the concentration of carcinogenic compounds on diesel particles is
low, they nevertheless can act in concert with the particles to induce
carcinogenesis. An alternative low-dose extrapolation model was
therefore developed that allows for the possibility that various PAHs
and nitroaromatic compounds may induce organ-specific adducts that
contribute to cell initiation. This model is described in Appendix A1.
Both approaches incorporate the detailed dosimetric model of Yu
et al. (1991) to estimate dose at the airway and alveolar surfaces.
Risk is based on the assumption that an equivalent concentration
(dose) per unit of alveolar surface area results in equivalent risks
in humans and rats.
(c) Evidence to support a carcinogenic effect of the carbon core
The study of Heinrich et al. (1994) on carbon black and coal-tar
pitch provides information useful for evaluating the assumptions used
to construct the model that reflects current thinking about the
mechanism of action of diesel exhaust in inducing lung tumours. One
relevant question about diesel exhaust is whether lung tumours are
induced by the carbon core alone or in combination with organic
compounds.
In this study, female Wistar rats were exposed to carbon black or
tar pitch, or both for 10 months (43 weeks) or 20 months (86 weeks)
and followed until the end of the study, which lasted for
two-and-a-half years. No lung tumours were observed in the control
group. For each exposed group, the probability of dying from lung
cancer was calculated by a Kaplan-Meier survival analysis. Since these
data are used solely for interpretation and not for risk prediction,
it is statistically more appropriate to use only data on mortality,
excluding those tumours found at terminal sacrifice. Figure 9 shows
the probability of dying from lung cancer for the rats that were
exposed either to 6 mg/m3 of carbon black, or to coal-tar pitch
containing 50 µg/m3 of benzo[a]pyrene, or to a combination of the
two for 10 months, and then followed until the end of the study. In
Figure 10, each plot represents the time at which a tumour occurred.
To provide a better visual presentation, the probabilities at these
points are connected by straight lines. (Note: It is statistically
more appropriate to present these probabilities by a step function
rather than by interpolation when the Kaplan-Meier procedure is used.)
Two conclusions can be drawn from Figure 10: (1) Tumours appeared
much earlier in animals exposed to coal-tar pitch alone or in
combination with carbon black than in animals exposed to carbon black
alone. For instance, the first tumour occurred 665 days after the
start of exposure to carbon black, 406 days after exposure to coal-tar
pitch, and only 310 days after exposure to the combination. (2) Carbon
black and coal-tar pitch together caused a higher rate of mortality
from tumours than the sum of mortality caused by carbon black and
coal-tar pitch separately. The probability of dying from a lung tumour
before terminal sacrifice at 912 days was 0.28 for carbon black, 0.52
for coal-tar pitch, and 0.99 for the combination. These two
observations suggest that carbon black and coal-tar pitch act on
different stages of tumorigenesis. They are consistent with the
hypothesis that carbon black can increase the proliferation rates of
normal and cells at different stages of differentiation initiated
spontaneously or by coal-tar pitch.
When the rate of mortality from tumours among rats exposed to
diesel exhaust is compared with that of groups exposed to carbon
black, coal-tar pitch, or the combination (Figure 9), the rate among
the animals exposed to diesel exhaust is comaprable to that of rats
exposd to carbon black for 10 to 20 months. This implies that carbon
black alone can induce a tumour response similar to that induced by
diesel exhaust. Figure 9 also shows that doubling the duration of
exposure to carbon black from 10 to 20 months does not increase the
probability of dying from a lung tumour before terminal sacrifice;
however, doubling the duration of exposure to coal-tar pitch
significantly increases the probability of dying from a lung tumour.
Figure 11 shows that the shapes of the curves for age-dependent
mortality from cancer for animals exposed to diesel exhaust and
coal-tar pitch for 20 months are similar. It should be kept in mind
that the PAH content of the atmosphere containing both carbon black
and coal-tar pitch was about 1000 times higher than that of diesel
engine exhaust. These observations may have profound implications for
elucidating the mechanisms of induction of lung tumours by diesel
exhaust, but more refined analyses are necessary before any conjecture
can be made.
(d) Data available for calculating risk
Seven bioassays have shown lung tumour responses in rats
(Brightwell et al., 1986; Heinrich et al., 1986b; Ishinishi et al.,
1986; Iwai et al., 1986; Mauderly et al., 1987; Nikula et al., 1994;
Heinrich et al., 1995). Four studies were chosen for use in
calculating unit risk (Table 48) because they involved multiple
exposure groups. Data on time to event (death with or without a
tumour) are available in the studies of both Mauderly et al. (1987)
and Heinrich et al. (1995) and were used in all of the risk
calculations.
(e) Calculation of unit risks
The unit risk from exposure to an air pollutant is defined as the
95% upper bound of the increased lifetime cancer risk for an
individual continuously exposed to a concentration of 1 µg/m3 in
ambient air. Unit risk is a convenient tool for calculating lifetime
risk due to exposure to a pollutant when low-dose linearity is
assumed. Under this assumption, the risk due to exposure to d µg/m3
of pollutant can be calculated from u × d if the pollutant has a
unit risk of u/µg per m3. This calculation is valid, however, only
when the level of risk is low. The results of unit risk calculations
are summarized in Table 49.
deaths were recorded for 1959-80. Although the previous exposure
levels of the railroad workers were not available, the classification
of workers into groups on the basis of exposure was validated by an
industrial hygienist and a review of work practices. Air sampling was
conducted to establish current exposure levels in various jobs
(Hammond et al., 1988; Woskie et al., 1988a,b).
Gustavsson et al. (1990) studied lung cancer among bus garage
workers in Stockholm, Sweden, where all buses with internal combustion
engines have been diesel-fuelled since 1945. A cohort was established
of people who had worked in bus garages for at least six months
between 1945 and 1970, and mortality was determined for 1952-86. A
nested case-control study was performed within this cohort. Although
actual exposure was not measured, relative exposure to diesel exhaust
was graded on the basis of a scale established by an industrial
hygienist in a review of work practices, bus engine operation, and
shop ventilation.
Table 47. Summary of non-cancer guidance values and benchmark
concentrations
Approach Guidance value
or benchmark
concentration
(µg/m3)
NOAEL with dosimetric conversion from rats to humans 5.6a
NOAEL without dosimetric conversion from rats to 2.3
humans
Benchmark concentration with dosimetric conversion
from rats to humans
Chronic alveolar inflammation 2a
Impaired lung clearance 3a
Hyperplastic lesions 14a
Benchmark concentration without dosimetric
conversion from rats to humans
Chronic alveolar inflammation 0.9
Impaired lung clearance 1.2
Hyperplastic lesions 6.3
NOAEL, no-observed-adverse-effect level
Normalized for lung surface area in rats and humans; after
normalization for lung weight, the benchmark concentration increases
by a factor of 4.
Emmelin et al. (1993) studied Swedish stevedores in 15 ports
where diesel equipment had been introduced between 1957 and 1963; they
also performed a nested case-control study on cases of lung cancer
identified between 1960 and 1982. Exposure to diesel exhaust was
estimated on the basis of three indices derived from estimated diesel
fuel consumption and years of work after the introduction of diesel
equipment in each port.
All four studies showed an increased risk for lung cancer with
exposure to diesel exhaust. The relative risks reported ranged from
about 1.4 for railroad workers with the longest duration of exposure
in both studies of such populations to 1.3-2.4 for bus garage workers,
depending on the exposure category. The stevedores also had an
increased relative odds ratio for lung cancer with increasing exposure
with respect to all three indices of exposure examined, with an
increase of three- to sixfold in the highest exposure categories and
1.5-2.7-fold in the lowest exposure category. The point estimates
were, however, imprecise and had wide confidence intervals: for all
but one exposure category, the lower limit of the 90% confidence
intervals presented by the authors included 1.0.
The effects of cigarette smoking could be adjusted for in the
case-control studies of railroad workers (Garshick et al., 1987) and
stevedores (Emmelin et al., 1993). The smoking histories of the
railroad workers were obtained from next-of-kin. Various regression
models (including both dose and duration of smoking) were examined in
detail in order to adjust adequately for the effects of smoking. No
matter how it was accounted for, the risk for lung cancer based on
work in a job with exposure to diesel exhaust was similar to the
unadjusted odds ratio. The study of stevedores (Emmelin et al., 1993)
was smaller than that of railroad workers, and in order to reduce the
number of strata for analysis workers were stratified only as smoker
or nonsmoker. In the two studies in which information on smoking was
not available (Garshick et al., 1988; Gustavsson et al., 1990), the
analysis was based on a comparison with workers in the same
occupational cohort, making confounding by smoking less likely.
The results of most of the other studies summarized in Tables
39-41 support those of the four studies discussed above. The relative
risks were generally in the range 1.2-1.9 but did not always achieve
statistical significance. Furthermore, exposure to diesel exhaust was
less precisely defined in these studies; it was usually based on
self-reporting, the report of a surrogate, or a census report of
occupation (Table 41). Self-reporting or the report of a surrogate
would reflect actual exposure to diesel exhaust or work in a job in
which exposure was likely; however, defining exposure in this less
precise fashion make it harder to detect an effect of the exposure and
leads to lower relative risks and wider confidence intervals.
In three other studies, job titles obtained from an employer were
used to define exposure. In the study of Howe et al. (1983), the job
held at the time of retirement from the railroads was used to
categorize exposure, and mortality was assessed for 1965-77.
Significantly elevated risks for lung cancer were noted among workers
who had probably been exposed to diesel exhaust (relative risk = 1.35)
and among those who had possibly been exposed (1.20); however, since
only retired workers were studied, actual exposure to diesel exhaust
would have been short for many workers. Rushton et al. (1983) and
Waller (1981) used job titles within the London Transport organization
to indicate potential exposure to diesel exhaust. Neither study showed
an elevated risk for lung cancer, but the study of Rushton and
coworkers was characterized by a short duration of exposure and
follow-up, whereas in the study of Waller, deaths occurring after
employment were not included.
The relative risks for lung cancer as a result of exposure to
diesel exhaust are generally low, and risks of this magnitude are more
susceptible to chance and to the effects of unmeasured confounding
factors and imprecision in adjusting for known confounding factors. As
discussed above, the elevated risk for lung cancer observed in the
four most informative studies is unlikely to be due to confounding by
cigarette smoking and is probably due to exposure to diesel exhaust.
Other studies, although limited primarily by the exposure
ascertainment, support this assessment.
B10.4.1.2 Urinary bladder cancer: occupational exposure
The hypothesis that exposure to diesel exhaust results in cancer
of the urinary bladder is based on the fact that individuals who smoke
cigarettes, a source of PAHs (as is diesel particulate), excrete
mutagenic substances in their urine, and cigarette smoking is
associated with an increased risk for bladder cancer. In a recent
study of railroad workers with current exposure to diesel exhaust
(median concentration of respirable particulates over a work shift,
54-113 µg/m3; adjusted for smoking), mutagenicity in urine at the
end of a shift was not associated with exposure (Schenker et al.,
1992).
The evidence that bladder cancer is associated with exposure to
diesel exhaust is based on an association between work in industries
or occupations with potential exposure to diesel exhaust. The relative
risks reported (some of which are adjusted for smoking) are generally
in the range 1.2-2 (Hoar & Hoover, 1985; Silverman et al., 1986),
although higher risks have been reported. Exposure was assessed mainly
by self-reporting and reporting of occupation at interview. The main
occupation in which there was considered to be potential exposure to
diesel exhaust was truck driving. Measurements of exposure of truck
drivers by Zaebst et al. (1991) indicate that current exposure is
likely to be low, but there is no knowledge about past exposure
levels.
Thus, although work in occupations related to motor vehicles has
been associated with bladder cancer, the extent of exposure of these
workers to diesel exhaust is unknown.
B10.4.2 Dose-response assessment
B10.4.2.1 Lung cancer
Job category and work in an occupation with exposure to diesel
exhaust have been used as indicators of exposure. When years of
exposure and derived indices of exposure have been used, an
exposure-response relationship has generally been found. Measurements
of total and respirable dust in exposed workers do not reflect actual
exposure to diesel exhaust particles, however, since there are other
sources of dust in an occupational environment. The exposure of only
two occupational groups, American railroad workers (Woskie et al.,
1988b) and truck drivers (Zaebst et al., 1991), to diesel exhaust
particles has been quantified (see section B5.2). Current exposure was
measured for both these groups, but past exposure levels could not be
determined (Woskie et al., 1988b), so that studies of workers do not
permit determination of a dose-response relationship for inhaled
diesel particles.
B10.4.2.2 Urinary bladder cancer
Years of work in an occupation related to motor vehicles (mainly
truck driving) was examined in some, but not all, studies, but no
measurements were available to estimate exposure to diesel exhaust in
these settings.
B10.4.3 Exposure assessment
The ranges of exposures observed for workers are presented in
sections B5.2 and B10.2.
B10.4.4 Risk characterization
B10.4.4.1 Human lung cancer
Thus, historical measurements of exposure to diesel exhaust are
unreliable and exist only for current workers in two industries. A
quantitative risk assessment cannot be conducted on the basis of
epidemiological data in which job title was used as a surrogate of
exposure. Attempts to obtain estimates of risk (presented in section
B10.4.1) in the retrospective cohort study of American railroad
workers (Garshick et al., 1988) were not successful (see below for
discussion). Consequently, there are no human data suitable for
estimating unit risk.
B10.4.4.2 Human urinary bladder cancer
The risk for urinary bladder cancer cannot be assessed from the
available epidemiological data.
B10.4.4.3 Risk characterization based on studies in experimental
animals
(a) Introduction
Most human tumours arise in the bronchial region, and although
the frequency of adenocarcinomas has increased in recent years
(Martini, 1993), squamous bronchogenic carcinomas still represent the
majority (about 60%) of lung tumours in humans (Auerbach & Garfinkel,
1991; Devesa et al., 1991; Campobosso et al., 1993). The tumours
observed in rats are located almost exclusively in the bronchoalveolar
regions. This difference raises the question of whether the rat is the
appropriate species for extrapolating to humans. Although it may be
the wrong model, however, studies of hamsters and monkeys give
negative results, so the rat is the most appropriate model from a
conservative viewpoint and is therefore used for quantitative risk
extrapolation.
The objective is to assess quantitatively the potential risk for
lung cancer in humans posed by exposure to diesel exhaust emissions in
the ambient air. Ideally, human risk due to exposure to an
environmental pollutant should be predicted on the basis of human
experience. Although several epidemiological studies are available on
bus and railroad workers, these results alone are not adequate to
assess the potential risk of cancer in humans because of the lack of
reliable information on the conditions of exposure of these workers.
The challenge is to assess the risk of exposure to diesel exhaust on
the basis of all of the available information, for both experimental
animals and humans. In contrast to the sparse human data, there is a
rich pool of information on diesel-induced lung tumours in two strains
of rat. One approach to integrating this information is to make a
quantitative risk assessment on the basis of information from
bioassays and on the relevant biological mechanism and then to
evaluate these animal-based results against available human
experience. This approach was followed in this monograph.
Although, the available experimental data on the possible impact
of extractable organic matter and PAHs on the lung tumour response
associated with exposure to diesel soot are not strong (Heinrich et
al., 1991; Heinrich, 1994; Heinrich et al., 1995), a mathematical
approach to assessing the risk of exposure to diesel exhaust should
take into account the effects of both particles (carbon core) and
extractable organic matter, because: (i) organic compounds include a
variety of PAHs and nitroaromatic compounds, many of which are known
to be carcinogenic; (ii) the results of recent studies on inert
particles and carbon black in rats (Mauderly et al., 1991; Heinrich,
1994; Heinrich et al., 1994) strongly support the hypothesis that the
carbon cores of diesel particles are the component primarily
responsible for the induction of lung cancer; (iii) PAHs alone are
unlikely to be responsible for the tumour response; (iv) the
disproportionately high tumour incidence in animals with heavy
exposure coincides with a disproportionate increase in the cumulative
lung burden of diesel particles. Although a qualitative description of
the biological mechanism of diesel exhaust-induced tumours is
plausible, however, the lack of quantitative information on the
dynamics of tumour initiation, promotion, and progression vitiates the
construction of a biologically based dose-response model.
The crudest dose-response model is obtained by fitting observed
dose-response data to a mathematical function that is a monotonic,
non-decreasing function of exposure, using the inhaled concentration
and observed tumour incidence, without considering information on
pharmacokinetics and the mechanism of carcinogenesis. This approach
may result in uncertain estimates of risk at low doses, because, while
the model may adequately fit the dose-response data for high doses at
which a tumour response is observed, it may greatly underestimate or
overestimate the risk at low doses.
Most previous risk estimates of diesel exhaust-induced cancer
risk were developed before the results of many of the bioassays became
available (Pepelko & Chen, 1993). Nevertheless, most of the estimates
are similar (within an order of magnitude), except for those
calculated on the basis of human data, which are higher than those
calculated from other databases. Because of great uncertainties
associated with the available human data, it is considered more
appropriate to calculate risk on the basis of animal data and then use
human data to evaluate their validity.
The approach adopted in this assessment is the linearized
multistage model (Anderson et al., 1983), in which burden per lung
surface area (milligrams per square centimetre) is used as an
equivalent dose in animals and humans. The use of lung (alveolar)
surface area is justified by the fact that the lung tumours observed
in rats are derived exclusively from epithelial cells in the alveolar
region of the lung. Normalization on the basis of lung weight can also
be done by using a factor of 4, for increasing the dose or decreasing
the risk, for the difference in the ratio of rat:human lung surface
area and lung weight. The risk prediction calculated by this approach
is presumably conservative (i.e. overpredicts the risk) because the
dose-response function is assumed to be non-threshold and linear at
low doses.
An alternative model that incorporates a biological mechanism is
constructed using statistically estimated parameters. The model
assumes that carcinogenic agents present in the organic fraction act
directly on the target cells, primarily via initiation. It is further
assumed that the majority of the particles are ingested by
macrophages. Particle-laden macrophages are then induced to secrete a
variety of mediators (e.g. reactive oxygen species and cytokines),
which diffuse to the target cells and induce initiation,
proliferation, and conversion of initiated cells to malignant cells.
This alternative model can be used only as a tool to evaluate the
impact of various biological assumptions and not for risk prediction,
because the parameters of the model are not measured in the
laboratory.
(b) Approaches to quantification of human risk from exposure to
diesel exhaust
Several issues must be addressed in estimating the risk of
exposure to diesel exhaust, including the critical target site, the
fraction of exhaust responsible for tumour induction, the availability
of dosimetric methods for accurately extrapolating dose from that for
experimental animals chronically exposed to high concentrations of
exhaust to that of humans exposed at ambient concentrations, and the
most suitable low-dose extrapolation model.
The critical target organ was considered to be the lung. Although
Iwai et al. (1986) reported induction of both malignant lymphomas and
lung tumours in rats exposed to diesel exhaust, the lung was the only
target site in other experimental studies of this species. Although
potentially carcinogenic agents present in diesel exhaust may be
absorbed from the lungs, enter the bloodstream, and be transported
systemically, no data are available to evaluate this possibility.
Organic compounds adsorbed on particles may also reach systemic
targets via the gastrointestinal tract, as particles deposited in the
conducting airways are cleared rapidly to the oropharynx and
swallowed. A considerable volume of particles is also likely to be
ingested as a result of grooming when the whole animal is exposed
(Wolff et. al., 1982); however, because the half-times for the elution
of organic compounds from particles are considerably longer than their
passage through the gastrointestinal tract, the fraction absorbed is
expected to be small. In any case, there is little evidence for any
systemic effects of diesel exhaust.
The site of action in the lungs is assumed to be the epithelial
lining of the alveoli and small airways. According to Mauderly et al.
(1987), inflammation and tumours appear to arise from this tissue.
Although interstitial events (e.g. fibrosis) have been suggested to be
associated with induction of lung tumours by particles (Kuschner,
1968), no data are available to support this view with respect to
diesel exhaust.
Accurate extrapolation of dose from studies of animals exposed to
high concentrations of exhaust to humans exposed to ambient
concentrations requires a variety of adjustments for species
differences in deposition efficiency and respiratory ventilation
rates. As the normal retention half-times in the alveolar region are
several times longer in humans than rats (Chan et al., 1981; Bohning
et al., 1982), the lung burden of humans may be underestimated if this
difference is not taken into account. The high exposure concentrations
used in some experimental studies, however, result in greatly slowed
or even completely inhibited clearance (Griffis et al., 1983). In
order to extrapolate dose accurately from experimental studies to
humans, the detailed dosimetric model developed by Yu et al. (1991)
was used, which accounts for species differences in respiratory
ventilation rates, deposition efficiency, normal particle clearance
rates, transport of particles to lung-associated lymph nodes, and lung
surface area. It also accounts for inhibition of particle clearance
due to lung overload. In this model, dose is estimated in terms of
particle concentration per unit of lung surface area.
Two approaches were used to derive unit risk estimates: the
linearized, multistage, low-dose extrapolation model and a model based
on the biological mechanism discussed above. In the linearized
multistage model, the lung burden of carbon core per lung surface area
is used as the dosimetric parameter. A particle-based assessment was
considered to be reasonable for two principal reasons: (i) exposure to
the vapour phase alone did not result in detectable tumour induction
in rats (Brightwell et al., 1986; Iwai et al., 1986; Heinrich et al.,
1986a); and (ii) exposure to carbon black, which is similar in
composition to the carbon core of whole diesel exhaust but contains
only negligible amounts of organic compounds, was about as effective
in inducing lung cancer as whole diesel exhaust (Mauderly et al.,
1991; Nikula et al., 1994; Heinrich et al., 1995). Although use of the
carbon core as a dosimetric parameter implies that it is primarily the
insoluble carbon core of diesel particles that is responsible for the
carcinogenic effects of diesel exhaust, it can also be viewed simply
as a marker of exposure to diesel exhaust, with no biological
implication. Similarly, organic compounds could be used as markers of
exposure for the dose-response calculation. When this is done, the
resulting unit risk estimates (not presented here) are very similar
(within 25%) to those calculated with the carbon core as the surrogate
of exposure.
The second approach is based on the assumption that, even though
the concentration of carcinogenic compounds on diesel particles is
low, they nevertheless can act in concert with the particles to induce
carcinogenesis. An alternative low-dose extrapolation model was
therefore developed that allows for the possibility that various PAHs
and nitroaromatic compounds may induce organ-specific adducts that
contribute to cell initiation. This model is described in Appendix A1.
Both approaches incorporate the detailed dosimetric model of Yu
et al. (1991) to estimate dose at the airway and alveolar surfaces.
Risk is based on the assumption that an equivalent concentration
(dose) per unit of alveolar surface area results in equivalent risks
in humans and rats.
(c) Evidence to support a carcinogenic effect of the carbon core
The study of Heinrich et al. (1994) on carbon black and coal-tar
pitch provides information useful for evaluating the assumptions used
to construct the model that reflects current thinking about the
mechanism of action of diesel exhaust in inducing lung tumours. One
relevant question about diesel exhaust is whether lung tumours are
induced by the carbon core alone or in combination with organic
compounds.
In this study, female Wistar rats were exposed to carbon black or
tar pitch, or both for 10 months (43 weeks) or 20 months (86 weeks)
and followed until the end of the study, which lasted for
two-and-a-half years. No lung tumours were observed in the control
group. For each exposed group, the probability of dying from lung
cancer was calculated by a Kaplan-Meier survival analysis. Since these
data are used solely for interpretation and not for risk prediction,
it is statistically more appropriate to use only data on mortality,
excluding those tumours found at terminal sacrifice. Figure 9 shows
the probability of dying from lung cancer for the rats that were
exposed either to 6 mg/m3 of carbon black, or to coal-tar pitch
containing 50 µg/m3 of benzo[a]pyrene, or to a combination of the
two for 10 months, and then followed until the end of the study. In
Figure 10, each plot represents the time at which a tumour occurred.
To provide a better visual presentation, the probabilities at these
points are connected by straight lines. (Note: It is statistically
more appropriate to present these probabilities by a step function
rather than by interpolation when the Kaplan-Meier procedure is used.)
Two conclusions can be drawn from Figure 10: (1) Tumours appeared
much earlier in animals exposed to coal-tar pitch alone or in
combination with carbon black than in animals exposed to carbon black
alone. For instance, the first tumour occurred 665 days after the
start of exposure to carbon black, 406 days after exposure to coal-tar
pitch, and only 310 days after exposure to the combination. (2) Carbon
black and coal-tar pitch together caused a higher rate of mortality
from tumours than the sum of mortality caused by carbon black and
coal-tar pitch separately. The probability of dying from a lung tumour
before terminal sacrifice at 912 days was 0.28 for carbon black, 0.52
for coal-tar pitch, and 0.99 for the combination. These two
observations suggest that carbon black and coal-tar pitch act on
different stages of tumorigenesis. They are consistent with the
hypothesis that carbon black can increase the proliferation rates of
normal and cells at different stages of differentiation initiated
spontaneously or by coal-tar pitch.
When the rate of mortality from tumours among rats exposed to
diesel exhaust is compared with that of groups exposed to carbon
black, coal-tar pitch, or the combination (Figure 9), the rate among
the animals exposed to diesel exhaust is comaprable to that of rats
exposd to carbon black for 10 to 20 months. This implies that carbon
black alone can induce a tumour response similar to that induced by
diesel exhaust. Figure 9 also shows that doubling the duration of
exposure to carbon black from 10 to 20 months does not increase the
probability of dying from a lung tumour before terminal sacrifice;
however, doubling the duration of exposure to coal-tar pitch
significantly increases the probability of dying from a lung tumour.
Figure 11 shows that the shapes of the curves for age-dependent
mortality from cancer for animals exposed to diesel exhaust and
coal-tar pitch for 20 months are similar. It should be kept in mind
that the PAH content of the atmosphere containing both carbon black
and coal-tar pitch was about 1000 times higher than that of diesel
engine exhaust. These observations may have profound implications for
elucidating the mechanisms of induction of lung tumours by diesel
exhaust, but more refined analyses are necessary before any conjecture
can be made.
(d) Data available for calculating risk
Seven bioassays have shown lung tumour responses in rats
(Brightwell et al., 1986; Heinrich et al., 1986b; Ishinishi et al.,
1986; Iwai et al., 1986; Mauderly et al., 1987; Nikula et al., 1994;
Heinrich et al., 1995). Four studies were chosen for use in
calculating unit risk (Table 48) because they involved multiple
exposure groups. Data on time to event (death with or without a
tumour) are available in the studies of both Mauderly et al. (1987)
and Heinrich et al. (1995) and were used in all of the risk
calculations.
(e) Calculation of unit risks
The unit risk from exposure to an air pollutant is defined as the
95% upper bound of the increased lifetime cancer risk for an
individual continuously exposed to a concentration of 1 µg/m3 in
ambient air. Unit risk is a convenient tool for calculating lifetime
risk due to exposure to a pollutant when low-dose linearity is
assumed. Under this assumption, the risk due to exposure to d µg/m3
of pollutant can be calculated from u × d if the pollutant has a
unit risk of u/µg per m3. This calculation is valid, however, only
when the level of risk is low. The results of unit risk calculations
are summarized in Table 49.
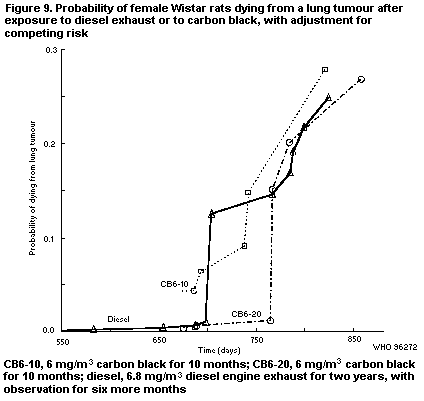
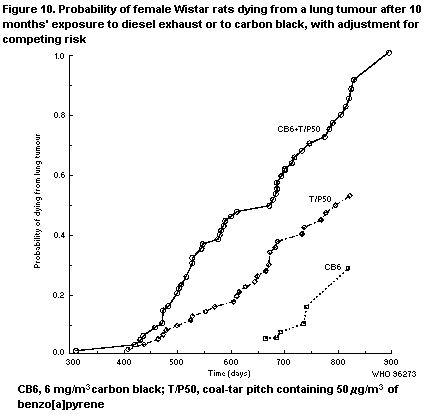
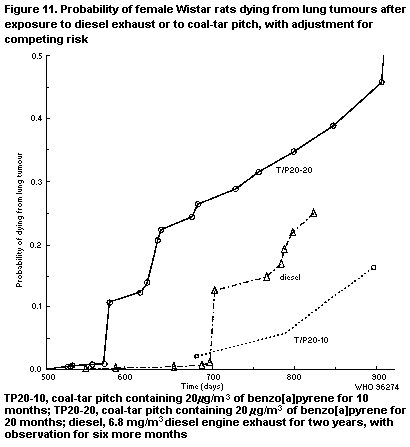 Table 48. Incidence of lung tumours in rats exposed to diesel engine exhaust
Strain Exposure Dose metric Lung tumour Reference
(sex) incidence
Schedule Concentration Weekly exposure Lung particle burden
(mg/m3) (mg/m3 × h) (mg/cm2 lung surface)a
Fischer 7 h/d, 0 0 0 2/230 Mauderly et al.
344 5 d/week 0.35 12 6.4 × 10-5 3/223 (1987)
(f+m) 3.50 122 2.8 × 10-3 8/222
7.08 248 6.0 × 10-3 29/227
Wistar 18 h/d, 0 0 0 1/217 Heinrich et al.
(f) 5 d/week, 0.84 76 5.8 × 10-4 0/198 (1995)
2 years, 2.50 225 5.2 × 10-3 11/200
< 6 months 6.98 628 1.5 × 10-2 22/100
follow-up
Fischer 16 h/d, 0 0 0 1/123 Ishinishi et al.
344 6 d/weekb 0.46 44 2.5 × 10-4 1/123 (1986)
(f+m) 0.96 92 2.0 × 10-3 0/125
1.84 177 4.2 × 10-3 4/123
3.72 357 8.8 × 10-3 8/124
Fischer 16 h/d, 0 0 0 4/250 Brightwell et al.
344 5 d/week 0.7 56 3.5 × 10-4 1/112 (1986)
(f+m) 2.2 176 4.2 × 10-3 14/112
6.6 528 1.3 × 10-2 55/111
a Calculated from the model of Yu et al. (1991)
b Data on heavy-duty diesel engine exhaust used because those for light-duty engines showed no statistically significant difference
Particle-based model: The linearized multistage model, which is
used as a conservative approach to estimating risk, has the
mathematical form P = 1 exp(-Z), where Z is either q0 + q1 × d + ...
+ qm × dm of a polynomial concentration d, or ( Q0 + Q1 × d + ...
+ Qm × dm) × tk, a polynomial of concentration d multiplied by a time
factor, tk, when data on time to event are used. In this case, the
lifetime risk is calculated from actuarial life tables using the
survival probability of control Fischer 344 rats within the National
Toxicology Program, provided by Portier et al. (1986). The range of
extrapolation is about three orders of magnitude in the present
assessment. Denote P0 the lifetime cancer risk at concentration 0.
Because the extra risk (P-P0)/(1-P0) is dominated by the linear term
q1 × d at low concentrations, the 95% upper bound of q1 is used
to represent unit risk.
In extrapolating risk from animals to humans, a dose metric that
induces the same tumour incidence rate in animals and humans must be
assumed (i.e. dose equivalence). To calculate equivalent doses, a
mathematical model is used to adjust for the dosimetric parameters
that determine the lung burden of particulate matter in rats and
humans and to correct to dose per unit lung surface area. This dose is
considered to be equally potent in inducing lung tumours in animals
and in humans. The model accounts for differences between animals and
humans in regional deposition efficiency, particle clearance rates at
low doses and at doses that result in impaired clearance, and lung
surface area. In these calculations, the mass fraction of organic
compounds adsorbed on particles is assumed to be 20%; one-half of the
mass is assumed to be composed of slowly eluted organic compounds
(half-life, 30 days) and the other half of rapidly eluted organic
compounds (half-life, 1.3 h). The remainder is considered to be
inorganic carbon. At higher concentrations, particle clearance slows
and may even stop, and the lung particle burdens increase continually
during exposure. The organic constituents, however, are eluted from
the particles fairly quickly and reach a steady state even during
continued exposure to high concentrations. The lung burdens of organic
compounds are therefore less affected by inhibition of clearance by
overloading.
In the linearized multistage model, determination of the dose of
the carbon core is problematic because the lung burdens after low and
high exposures differ drastically over time. This difference suggests
that use of a lung burden at a fixed time (e.g. one year after the
start of exposure) to represent dose may not be appropriate. In this
assessment, the average lung burden is used as the dose at the target
organ. The average lung burden is calculated by dividing the area
under the curve of lung burden over time by the corresponding period
of the experiment for which the curve was calculated. Figure 12 shows
a comparison of the lung burden predicted from the model and that
observed in the laboratory (Muhle et al., 1994) at specific times. It
suggests that the model used to calculate lung burden is adequate.
Table 49. Estimates of unit risk per microgram of particles per
cubic metre of diesel exhaust
Upper 95% confidence limit of cancer Study
risk due to exposure to 1 µg/m3 of
diesel particulate matter
3.4 × 10-5 Mauderly et al. (1987)
1.6 × 10-5a Ishinishi et al. (1988)
7.1 × 10-5 Brightwell et al. (1986)
3.4 × 10-5 Heinrich et al. (1995)
3.4 × 10-5 Geometric mean of
four studies
If milligrams per lung weight are used instead of milligrams per lung
surface as the equivalent dose, the risk estimates are reduced by a
factor of 4.
a Heavy-duty diesel engine
Biologically based model: A second approach to estimating risk
was used because it was considered more desirable to base risk on a
biologically based dose-response model. Although the data are at
present insufficient to replace the linearized multistage model, which
is considered more conservative, the implications of hypothetical
mechanisms of cancer induction by diesel particles can nevertheless be
investigated. The biological issues considered include the effects on
the carcinogenic process of particle-adsorbed organic compounds and of
a variety of mediators secreted by particle-laden macrophages. A
stochastic model was developed, which:
-- accounts for the possible effects of both the carbon particles
and their associated organic compounds:
-- allows evaluation of the contribution to tumour induction of
both carbon particles and organic compounds;
-- allows for changing parameters with increasing lung burden;
and
-- assumes that cell proliferation and tumour induction are
stochastic. (For instance, it is not appropriate to assume that
all cells divide at the same age.)
Unlike the linearized multistage model, this approach does not require
a constant dose metric but allows for varying lung burden over time.
Table 48. Incidence of lung tumours in rats exposed to diesel engine exhaust
Strain Exposure Dose metric Lung tumour Reference
(sex) incidence
Schedule Concentration Weekly exposure Lung particle burden
(mg/m3) (mg/m3 × h) (mg/cm2 lung surface)a
Fischer 7 h/d, 0 0 0 2/230 Mauderly et al.
344 5 d/week 0.35 12 6.4 × 10-5 3/223 (1987)
(f+m) 3.50 122 2.8 × 10-3 8/222
7.08 248 6.0 × 10-3 29/227
Wistar 18 h/d, 0 0 0 1/217 Heinrich et al.
(f) 5 d/week, 0.84 76 5.8 × 10-4 0/198 (1995)
2 years, 2.50 225 5.2 × 10-3 11/200
< 6 months 6.98 628 1.5 × 10-2 22/100
follow-up
Fischer 16 h/d, 0 0 0 1/123 Ishinishi et al.
344 6 d/weekb 0.46 44 2.5 × 10-4 1/123 (1986)
(f+m) 0.96 92 2.0 × 10-3 0/125
1.84 177 4.2 × 10-3 4/123
3.72 357 8.8 × 10-3 8/124
Fischer 16 h/d, 0 0 0 4/250 Brightwell et al.
344 5 d/week 0.7 56 3.5 × 10-4 1/112 (1986)
(f+m) 2.2 176 4.2 × 10-3 14/112
6.6 528 1.3 × 10-2 55/111
a Calculated from the model of Yu et al. (1991)
b Data on heavy-duty diesel engine exhaust used because those for light-duty engines showed no statistically significant difference
Particle-based model: The linearized multistage model, which is
used as a conservative approach to estimating risk, has the
mathematical form P = 1 exp(-Z), where Z is either q0 + q1 × d + ...
+ qm × dm of a polynomial concentration d, or ( Q0 + Q1 × d + ...
+ Qm × dm) × tk, a polynomial of concentration d multiplied by a time
factor, tk, when data on time to event are used. In this case, the
lifetime risk is calculated from actuarial life tables using the
survival probability of control Fischer 344 rats within the National
Toxicology Program, provided by Portier et al. (1986). The range of
extrapolation is about three orders of magnitude in the present
assessment. Denote P0 the lifetime cancer risk at concentration 0.
Because the extra risk (P-P0)/(1-P0) is dominated by the linear term
q1 × d at low concentrations, the 95% upper bound of q1 is used
to represent unit risk.
In extrapolating risk from animals to humans, a dose metric that
induces the same tumour incidence rate in animals and humans must be
assumed (i.e. dose equivalence). To calculate equivalent doses, a
mathematical model is used to adjust for the dosimetric parameters
that determine the lung burden of particulate matter in rats and
humans and to correct to dose per unit lung surface area. This dose is
considered to be equally potent in inducing lung tumours in animals
and in humans. The model accounts for differences between animals and
humans in regional deposition efficiency, particle clearance rates at
low doses and at doses that result in impaired clearance, and lung
surface area. In these calculations, the mass fraction of organic
compounds adsorbed on particles is assumed to be 20%; one-half of the
mass is assumed to be composed of slowly eluted organic compounds
(half-life, 30 days) and the other half of rapidly eluted organic
compounds (half-life, 1.3 h). The remainder is considered to be
inorganic carbon. At higher concentrations, particle clearance slows
and may even stop, and the lung particle burdens increase continually
during exposure. The organic constituents, however, are eluted from
the particles fairly quickly and reach a steady state even during
continued exposure to high concentrations. The lung burdens of organic
compounds are therefore less affected by inhibition of clearance by
overloading.
In the linearized multistage model, determination of the dose of
the carbon core is problematic because the lung burdens after low and
high exposures differ drastically over time. This difference suggests
that use of a lung burden at a fixed time (e.g. one year after the
start of exposure) to represent dose may not be appropriate. In this
assessment, the average lung burden is used as the dose at the target
organ. The average lung burden is calculated by dividing the area
under the curve of lung burden over time by the corresponding period
of the experiment for which the curve was calculated. Figure 12 shows
a comparison of the lung burden predicted from the model and that
observed in the laboratory (Muhle et al., 1994) at specific times. It
suggests that the model used to calculate lung burden is adequate.
Table 49. Estimates of unit risk per microgram of particles per
cubic metre of diesel exhaust
Upper 95% confidence limit of cancer Study
risk due to exposure to 1 µg/m3 of
diesel particulate matter
3.4 × 10-5 Mauderly et al. (1987)
1.6 × 10-5a Ishinishi et al. (1988)
7.1 × 10-5 Brightwell et al. (1986)
3.4 × 10-5 Heinrich et al. (1995)
3.4 × 10-5 Geometric mean of
four studies
If milligrams per lung weight are used instead of milligrams per lung
surface as the equivalent dose, the risk estimates are reduced by a
factor of 4.
a Heavy-duty diesel engine
Biologically based model: A second approach to estimating risk
was used because it was considered more desirable to base risk on a
biologically based dose-response model. Although the data are at
present insufficient to replace the linearized multistage model, which
is considered more conservative, the implications of hypothetical
mechanisms of cancer induction by diesel particles can nevertheless be
investigated. The biological issues considered include the effects on
the carcinogenic process of particle-adsorbed organic compounds and of
a variety of mediators secreted by particle-laden macrophages. A
stochastic model was developed, which:
-- accounts for the possible effects of both the carbon particles
and their associated organic compounds:
-- allows evaluation of the contribution to tumour induction of
both carbon particles and organic compounds;
-- allows for changing parameters with increasing lung burden;
and
-- assumes that cell proliferation and tumour induction are
stochastic. (For instance, it is not appropriate to assume that
all cells divide at the same age.)
Unlike the linearized multistage model, this approach does not require
a constant dose metric but allows for varying lung burden over time.
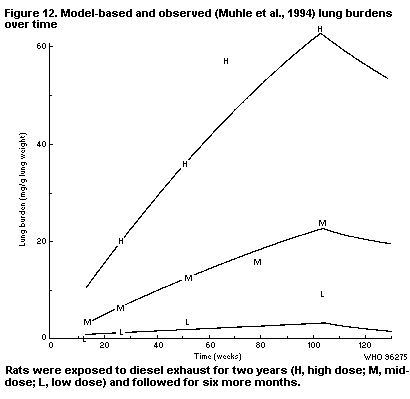 The model, which is described in Appendix B10.3, allows for
initiation by both the carbon and the organic fraction and for the
proliferative effects of the carbon fraction. Although these
mechanisms remain to be proven, it is assumed that carcinogenic agents
present in the organic fraction act directly on the target cells,
primarily by initiation. It is further assumed that most of the
particles are ingested by macrophages. Particle-laden macrophages are
then induced to secrete a variety of mediators (e.g. reactive oxygen
species and cytokines), which diffuse to the target cells, inducing
initiation, proliferation, and conversion of initiated cells to
malignant cells.
The results reported by Mauderly et al. (1987) for tumour
induction were used to estimate the model parameters. These are based
on the development of malignant tumours rather than all tumours as in
the first method. This was necessary in order to ensure that the data
used to estimate the parameters represented the same biological
mechanism. Lung burdens were calculated from the same dosimetry model
used in the linearized multistage model.
(f) Results of unit risk calculations
Particle-based model: Unit risks were derived from the linearized
multistage approach for the tumour incidences seen in four bioassays
(Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly et al.,
1987; Heinrich et al., 1995) and the corresponding equivalent doses
(Table 48). The resulting unit risk estimates, listed in Table 49,
range from 1.6 to 7.1 × 10-5/µg particles per m3 with a geometric
mean of 3.4 × 10-5/µg per m3.
In these calculations, the relationship between air concentration
(micrograms per cubic metre) and lung burden (milligrams) in humans is
used to determine the lung burden resulting from lifetime exposure to
1 g/m3 of diesel exhaust particulate matter. The particle burden in
terms of mass per unit lung surface area is then multiplied by the
slope derived from the bioassay data. For instance, when the data of
Mauderly et al. (1987) are used, the slope of the curve for
carcinogenicity in rats (i.e. the upper 95% confidence limit of the
linear coefficient in the multistage model), expressed in terms of
equivalent dose (micrograms of carbon particulate matter per square
centimetre) is 1.7 × 10-2/µg per cm2. According to the dosimetry
model, an air concentration of 1 µg/m3 of particulate matter
corresponds to a mass of 1230 µg of carbon particles per human lung.
Because the lung epithelial surface area, including the alveolar
region and conducting airways, is assumed to be 627 000 cm2, a unit
risk of 3.4 × 10-5/µg per m3 is derived from 1.7 × 10-2/µg per cm2
× 1230 µg/627 000 cm2.
Biologically based model: The unit risk estimated by the
alternative model, based on the data on malignant tumours from the
study of Mauderly et al. (1987), is equal to 1.65 × 10-5/µg particles
per m3. This is lower than the estimate of 3.4 × 10-5/µg per m3
derived from the same study with the linearized multistage approach.
Application of the linearized multistage model only to malignant
tumours seen in that study, rather than all lung tumours, resulted in
a unit risk estimate of 1.74 × 10-5/µg particles per m3 (Table 50).
Thus, the unit risk estimates obtained by the two approaches are
virtually identical. The estimated risks may differ somewhat with
increasing doses, because the slopes are not identical at all exposure
levels. It should be noted, however, that the unit risk predicted by
the alternative model is derived under the assumption that particles
continue to exert an effect on cell initiation or proliferation (or
both) at low doses. There is considerable uncertainty about the
effects of particles at low doses. It has been claimed that particles
do not induce initiation or proliferation at low doses (Vostal, 1986).
At present, the evidence is inadequate to support or refute this
claim. Moreover, even if macrophage overload is required, because of
uneven distribution of particles, some macrophages may become
overloaded even at low concentrations. Because of this uncertainty,
the biologically based model, like the linearized multistage model,
does not depart from linearity at low doses; however, if initiation
and proliferation do not occur at low doses, the risk may be much
smaller.
In an estimate of unit risk for rats, based on the results of
seven studies by inhalation and using linear interpolation and the
linearized multistage model, the risks were 7 × 10-5/µg of diesel
particles per m3 and 10 × 10-5/µg carbon core particles per m3
(Csicsaky et al., 1993; Pott et al., 1993; Roller & Pott, 1994).
(g) Results and implications of the biologically based model
On the assumption that particles continue to affect cell
initiation or proliferation at low doses, the risks calculated with
this model are comparable to those obtained with the linearized
multistage model. Excess risks due to various exposures are shown in
Tables 50 and 51. Those in Table 50 are the risks predicted for humans
exposed continuously (24 h/day) from the two models: it is interesting
to note that the results are similar. Table 51 shows the excess risks
due to exposure to 2.6 µg/m3 of diesel particulate emission for
16 h/day on seven days per week and to 15 µg/m3 for 8 h/day on five
days per week. The first concentration was reported by the US
Environmental Protection Agency Office of Mobile Sources to be the
annual mean exposure of the American population to diesel particulate
matter in 1986; the second concentration was reported to be that to
which workers are exposed on urban freeways (Carey, 1987). The
retention half-time for insoluble particles after exposure to
Table 50. Comparison of excess risk for humans due to continuous exposure to
various concentrations of diesel exhaust emissions using two
different models
Exposure concentration Biologically based (alternative) Linearized (µg/m3)
multistage
Maximum likelihood Upper 95% model
estimate bound estimate
0.01 7.68 × 10-8 1.35 × 10-7 1.71 × 10-7
0.1 8.12 × 10-7 1.71 × 10-6 1.72 × 10-7
1.0 (unit risk) 8.16 × 10-6 1.65 × 10-5 1.74 × 10-5
100 5.58 × 10-4 9.63 × 10-4 1.74 × 10-4
1000 2.60 × 10-2 4.22 × 10-2 3.33 × 10-2
a Slope - 9.04 per mg/cm2 of lung surface, using carbon core as dosimetric.
Only malignant tumours are used in the calculations.
2.6 µg/m3 is shown to increase from 296 days for members of the
general population with normal respiratory function to 519 days for
those with a smoking history of 20 pack-years (Bohning et al., 1982).
This information was used to reduce the alveolar clearance rate for
the dosimetric calculations to that used for other risk calculations.
Interactive effects of smoking and diesel exhaust are not considered
in the risk calculations owing to lack of data. The studies of carbon
black and coal-tar pitch (Heinrich, 1994; Heinrich et al., 1994)
indicate that smokers have a higher risk for lung cancer than
nonsmokers when they are exposed to diesel exhaust.
The excess lifetime risks shown in Tables 50 and 51 are
standardized by the actuarial life-table approach, using the survival
probability of control animals in the US National Toxicology Program
provided by Portier et al. (1986). This approach gives a weighted
average of the probability of cancer occurrence over an entire
lifetime, weighted by survival probability.
Table 51. Excess lifetime risk for humans due to exposure to diesel
exhaust emissions under various exposure scenarios
Exposure pattern Biologically based model Linearized
multistage
Maximum Upper 95% modela
likelihood bound
estimate estimate
General population (normal 1.41 × 10-5 2.44 × 10-5
respiratory function;
nonsmoker): 2.6 µg/m3,
16 h/day, seven days
per week
General population 2.32 × 10-5 3.61 × 10-5 5.38 × 10-5
(20-pack-year smoker)b:
2.6 µg/m3, 16 h/day,
seven days per week
Occupationally exposed: 3.12 × 10-5 5.17 × 10-5 6.18 × 10-5
15 µg/m3 8 h/day, five
days per week
a Calculated using carbon core as dosimetric; only malignant
tumours are used.
b In this calculation, smoking affects only lung clearance rate;
biological interaction between smoking and exposure to diesel
exhaust is not considered. The retention half-times for
insoluble particles increased from 296 days for persons with
normal respiratory function to 519 days for 20-pack-year smokers
(Bohning et al., 1982).
Some implications of the alternative model are:
(1) At a low exposure concentration, the decrease in diesel-induced
initiation results in a greater reduction of risk; that is, the
number of initiated cells (cancer risk) is reduced more
efficiently with a low than with a high exposure. If only organic
compounds induce initiation when the concentration is low, they
play a more important role than particles in inducing tumours at
low concentrations, whereas the roles are reversed when the
concentration is high.
(2) Although cells initiated by diesel exhaust play an important role
in cancer induction, either organic compounds or the carbon core
alone could induce initiation by increasing their respective
proportions. Thus, although initiated cells are important for
tumour induction, they may be induced by any agent that initiates
tumours (e.g. smoking).
(3) A small change in the rate of proliferation induced by diesel
exhaust could disproportionately change cancer risk. As this
parameter is assumed to be a function of the dose of carbon core,
lung overloading has a significant effect on cancer incidence. In
the absence of better information, it is assumed in this
assessment that the carbon core continues to have a proliferating
effect at low doses.
These observations suggest that, although the effect of particle
overload on cell proliferation is important, initiation by the carbon
core or organic compounds or both is also essential. Although this
conclusion is only tentative, because the model parameters are
estimated statistically on the basis of bioassays conducted at high
concentrations, it does suggest the importance of studying the role of
the carbon core and organic compounds in initiation and promotion at
low and high exposure concentrations. Does the relative initiation
potential of organic compounds and the carbon core differ with
concentration? These observations also suggest that a subcohort of
workers who were smokers and were exposed to high concentrations of
diesel exhaust for a long time would have a greater risk of dying from
lung cancer.
(h) Comparison of risk estimates derived from experimental studies
and human experience
The bioassay-based risk estimates, which range from 1.6 × 10-5 to
7.1 × 10-5, with a geometric mean of 3.4 x 10-5, can be compared with
human experience on the basis of three data sets: those of an
epidemiological study conducted on London Transport employees (Waller,
1981) and a subsequent analysis (Harris, 1983) and those on American
railroad workers (Garshick et al., 1987, 1988). Although these data
cannot be used to calculate unit risk, mainly because of a lack of
reliable information on exposure, they can be used to evaluate the
validity of unit risk estimates based on the results of experimental
studies.
Attempts have already been made to estimate the potential cancer
risk due to exposure to diesel exhaust on the basis of epidemiological
data. From the results of the study of London Transport workers,
Harris (1983) estimated that the increase in the relative risk for
lung cancer associated with exposure to 1 µg/m3-year of diesel exhaust
was 1.2 × 10-4, with an upper bound of the 95% confidence limit of
4.8 × 10-4. (Generally, when data from an epidemiological study with
negative results are used to estimate cancer risk, the upper bound is
used to calculate unit risk.) The resulting unit risk estimate is 2 ×
10-3, which is about 60 times higher than the mean unit risk estimate
derived from bioassays, 3.4 × 10-5, and about 30 times higher than the
upper end of the range of unit risk estimates from bioassays, 7.1 ×
10-5. Therefore, the risk estimate based on bioassays is not
inconsistent with that for humans, since 2 × 10-3 is the estimated
upper bound in an epidemiological study with negative results.
McClellan et al. (1989) reported risk estimates based on the
study of Garshick et al. (1987), in which lung cancers in railroad
workers were evaluated. Assuming exposure to concentrations of 500 and
125 µg/m3, the upper bounds of the 95% confidence interval for
lifetime cancer risk were estimated to be 6 × 10-4 and 2 × 10-3,
respectively. The lower of the two unit risk estimates is only about
one order of magnitude higher than the risk estimates based on
bioassays.
An epidemiological study potentially more suitable for
quantitative risk assessment was reported by Garshick et al. (1988),
which was based on a large number of subjects; a small but significant
increase in the rate of mortality from lung cancer was seen in some
subcohorts. The US Environmental Protection Agency has supported an
effort to derive a unit risk estimate from this study, and data on
exposure were estimated by Woskie et al. (1988a,b) and Hammond et al.
(1988). These data were analysed in a variety of ways, using relative
risk and absolute risk dose-response models and by classifying
individuals into various exposure categories, including job, duration
of employment, age, and exposure markers. Even though at least 50
analyses were carried out, an adequate dose-response relationship
could not be obtained; these data were therefore not used to estimate
unit risk.
The lack of a dose-response relationship is not totally
unexpected, given the low increase in mortality rate in the study of
Garshick et al. (1988) and the uncertain estimates of exposure. The
data on exposure were derived from air samples collected during a
limited period (1981-83) on four small railroads operating in a
limited geographical area (northern United States), in which
concentrations of respirable particulate matter were measured rather
than diesel exhaust per se; the measured concentrations were then
adjusted to derive markers of exposure. The data were used to estimate
exposure to diesel exhaust of railroad workers throughout the United
States 30 or more years earlier. Diesel equipment and working
conditions have, however, changed since the 1940s when diesel engines
first began to be used in large numbers. Woskie et al. (1988b)
summarized anecdotal reports of smoky working conditions in diesel
repair shops during the 1950s and 1960s and reported that the limited
data available on levels of nitrogen oxide during those periods
indicated high levels of diesel exhaust. By the time samples were
collected, however, the smoky conditions would have been largely
mitigated by improved ventilation and the advent of less smoky diesel
engines. The study of Garshick et al. (1988) gives the relative risks
for dying from lung cancer in exposed as opposed to unexposed railroad
workers classified into five subcohorts by age in 1959. The risks
ranged from 0.96 (95% CI, 0.74-1.33) to 1.45 (1.11-1.89). The highest
relative risk, 1.45, which was observed in workers who were 40-45
years old in 1959, was used here to evaluate the validity of unit risk
based on biossays results. Assuming that this subcohort of workers was
exposed to diesel exhaust for 8 h/day on five days per week from age
35 to age 65, the background mortality rate from lung cancer in this
subcohort would be 0.038 (0.63 × 0.06), as the unexposed workers in
the same age group had a relative risk of 0.63 and the corresponding
lifetime mortality rate from lung cancer in the general American white
male population is about 0.06. If the lung cancer risk due to
1 µg/m3 is assumed to be 3.4 × 10-5, the concentration of diesel
exhaust in the working environment was at least 400 µg/m3, calculated
as follows: The risk due to 1 µg/cm2 of particles is 0.017 (which
results in a unit risk of 3.4 × 10-5). For a lower bound of the
relative risk of 1.1, a lung burden, d, that satisfies the
relationship 0.1 × 0.038 = 0.017 d would be needed; that is, d =
0.22 µg/cm2, which is equivalent to an air concentration of
0.4 mg/m3 by the dosimetry model of Yu et al. (1991). If the highest
unit risk estimate derived from Brightwell et al. (1986), 7.1 ×
10-5, is used, the required minimal air concentration would be about
0.2 mg/m3. These calculations imply that a diesel exhaust
concentration of at least 0.2 mg/m3 was necessary to observe a
statistically significant increase in the mortality rate from lung
cancer in this study. This concentration appears to be reasonable in
the light of the working conditions described by Woskie et al.
(1988a).
Although the risk estimates derived from bioassays are lower than
those derived from human data and may possibly over-predict risk,
since the non-linearity of the human response is not considered in
this model, they are not inconsistent, for the following reasons:
(1) The risk estimates based on epidemiological studies are not
derived from all of the available data but on only a subset with
the highest response. The estimates are therefore higher than
those that would be derived from the whole data set.
(2) When a single data point (i.e. an overall relative risk and an
averaged exposure concentration) is used in the calculations, the
resulting slope for potency will be overestimated if the
dose-response relationship is not linear over all exposure
concentrations. Assume, for example, that the response follows
the simple multistage model P(d) = 1 - exp[-( q0 + qd + q2 d2)].
The relative risk, R, at concentration d is R(d) = P(d)/P0.
Using this mathematical expression, it is easy to demonstrate
that the slope factor calculated from [ P(d) - 1] P0/ d is
greater at high doses (including the averaged concentration used
in the risk calculation) than at low doses where the
dose-response function is dominated by q1. Epidemiologists
have long had a similar (but not identical) concern about the use
of averaged data, since ecological associations are not
necessarily consistent with those measured at the individual
level (see Cohen, 1994; Greenland & Robins, 1994; Piantadosi,
1994).
(3) Some occupational groups were exposed to considerably higher
concentrations of diesel exhaust in the past than presently. For
example, the average particle concentration in a Finnish
roundhouse was reported to be 2 mg/m3 (Heino et al., 1978). The
lung burdens would thus be greater than those predicted on the
basis of current exposures, and the unit risk estimates would be
higher
(i) Summary and conclusions
A dosimetric model that accounts for differences between
experimental animals and humans in lung deposition efficiency, lung
particle clearance rates, lung surface area, ventilation, and the
rates of elution of organic chemicals from the particle surface was
used to calculate equivalent human doses as particle concentration per
unit lung surface area. After dosimetric adjustment, four risk
estimates were derived by a linearized multistage model from three
bioassays with Fischer 344 rats and one with female Wistar rats, which
ranged from 1.6 to 7.1 × 10-5/µg particles per m3, with a geometric
mean of 3.4 × 10-5. This quantitative assessment of the carcinogenic
risk due to exposure to diesel engine emissions is reasonable,
because:
-- The estimates are based on several well-designed, well-executed,
long-term bioassays.
-- Epidemiological studies indicate that humans are susceptible to
the induction of lung cancer after inhalation of diesel exhaust.
-- Dosimetry modelling, especially to account for inhibition of
particle clearance at high doses, has allowed accurate
extrapolation of doses from animals to humans.
-- The doses are based on actual concentrations of particulate
matter per unit of lung surface area.
-- Use of an alternative model that attempts to account for the
possible biological effects of the organic and carbon core
fractions did not appreciably change the unit risk estimate.
-- The risk estimates based on the results of bioassyas are not
inconsistent with the available human experience.
Nevertheless, a number of uncertainties remain, the most
significant of which are:
-- In any interspecies extrapolation there may be an inherent
difference in sensitivity to the agent being assessed.
-- The assumption of equivalent sensitivity across species is based
on concentration per unit of lung surface area, and use of other
assumptions of dose equivalence may lead to different estimates
of risk; however, the estimates should not vary by more than one
order of magnitude.
-- Although linearized low-dose extrapolation methods are used, it
is still uncertain whether inflammatory cells secrete mediators
that induce cancer in lung epithelial cells when the particle
burden is smaller than that necessary to inhibit clearance. Even
if macrophages are activated at low particle burdens, it is
uncertain whether the responses of epithelial cells are linear at
very low concentrations; however, non-linear carcinogenic
responses have been observed in bioassays.
-- Although the unit risk estimate is corroborated in the
alternative model, uncertainty about the response at low doses
remains because the estimates in the alternative model are based
on the assumption that particles continue to induce cell
initiation and/or proliferation at low doses. The actual risk
would be much lower if this assumption does not hold.
The risk at low doses derived from the linearized multistage
model may thus be overestimated if particles no longer induce cell
initiation and/or proliferation. The model was selected for
calculating risk because a conservative model was needed in order to
ensure protection of public health and because adequate data were not
available to use fully the alternative model constructed for this
assessment. Thus, the risk derived from the bioassays should be viewed
as hypothetical.
These unit risk estimates should not be used to evaluate the
carcinogenic risk of other types of particulate matter present in
ambient air, which may have different solubilities, surface areas, and
free radical contents, which factors greatly affect carcinogenic
potency.
It could be argued that since the types and location of tumours
seen in rats after exposure to particles are different from those
found in humans, the experimental data are not relevant for humans.
The tumours diagnosed in rats after long-term exposure to carbon black
particles and diesel exhaust include benign adenomas and malignant
adenocarcinomas, squamous-cell carcinomas, adenosquamous carcinomas,
and squamous cysts (Mauderly et al., 1994; see section B7.3.2). After
injection into athymic mice, cells from 50-67% of squamous-cell
carcinomas and 25-40% of adenocarcinomas were found to grow (Table
35), whereas those from squamous cysts did not (Mauderly et al.,
1994).
Squamous cysts have been defined by other authors as benign
cystic keratinizing squamous-cell tumours (Kittel et al., 1993;
Dungworth et al., 1994; Heinrich et al., 1995) and found not to grow
after transplantation (Heinrich et al., 1995). As pointed out by Mohr
(1992), there is, however, controversy about the correct terminology
of this type of lesion. In 1995, an international group of
pathologists re-evaluated lung tissue sections from rats exposed to
particles by inhalation and identified four distinct lesions:
squamous-cell metaplasia, pulmonary keratinizing cyst, cystic
keratinizing epithelioma (considered to be benign), and squamous-cell
carcinoma (G. Oberdörster, personal communication).
Regardless of the correct classification of lesions, the
important fact is that malignant tumours are induced in rats after
long-term inhalation of deisel exhaust and carbon black. The tumour
response of human beings may not, however, be the same, either
qualitatively or quantitatively. In fact, the response of rats to
long-term exposure to high concentrations of particles differs from
that of other species, including mice and hamsters. In the absence of
sufficient information about specific mechanisms unique to rats,
however, there is no justification for excluding data on this species
from extrapolations to the human situation (US Environmental
Protection Agency, 1986).
With respect to the mechanisms of induction of lung tumours by
particles in rats, it has been shown that particles devoid of
polyaromatic compounds can increase mutation rates in pulmonary
epithelial cells, which is an important step in tumour development via
cell transformation. In a recent study in which rats were exposed to
carbon black for 13 weeks, a significant influx of inflammatory cells
was seen at concentrations of 7 and 50 mg/m3 but not at 1 mg/m3
(Oberdörster et al., 1995), and a significant, three- to fourfold
increase in the frequency of hprt mutations was seen in alveolar
epithelial cells (Driscoll et al., 1995 and in press; see section
B7.6). Thus, burdens of particles of low toxicity per se, similar to
those reached in long-term studies in rats, can be mutagenic, possibly
through the involvement of DNA damaging oxidants derived from
inflammatory cells.
Appendix B10.1 Construction of a biologically based (alternative)
model
1. Preliminary considerations
In order to evaluate the effects of various biological
assumptions on the assessments of the risk of exposure to diesel
exhaust, a mathematical dose-response model must be constructed that
takes into account the proposed biological mechanisms. As a
significant issue in assessing the risk of diesel exhaust is the
effect of lung overloading on tumour induction, the model should have
the following properties:
-- It should depend on dose metrics for both organic compounds and
the carbon core, and it should account for the contributions of
each to tumour induction and formation both separately and
jointly.
-- It should allow for changes in the model parameters with time due
to increasing lung burden during exposure.
-- It should view cell proliferation and tumour induction and
formation stochastically: it is not realistic to assume
deterministic clonal growth. For instance, it should not be
assumed that all cells divide at the same age.
It is therefore assumed that a normal cell can be initiated by
both organic compounds and the carbon core. The initiation rate is
denoted by µ1, which is a function of the background rate and that
induced by diesel exhaust (as specified below). Because an initiated
cell eventually either dies or enters the cell cycle (including cells
in quiescence, G0), it is reasonable to assume that the lifetime of
an initiated cell follows a certain probability distribution. In this
model, a cell in G0 phase is equivalent to one with a certain
probability of a very long lifetime (i.e. in the right-hand tail of
the distribution of the cell's lifetime). At the end of its lifetime,
it either dies (death) with probability b, divides into two daughter
cells (birth) with probability a, or divides into one initiated cell
and one malignant cell (second transition) with probability µ2;
alpha + ß + µ2 = 1. Instead of assuming that a single malignant cell
is equivalent to a tumour, as in the model proposed by Moolgavkar &
Venzon (1979) and Moolgavkar & Knudson (1981), it is assumed here that
a malignant cell has a certain probability of becoming a tumour; this
probability is assumed to be dose-dependent, thus allowing for an
evaluation of the effect of dose on tumour progression. It should be
noted that the proposed model does not exclude the possibility that
there may be more than one step in the 'initiation' of a normal cell.
The rate of initiation used in the model should be viewed as a net
rate that represents several genetic alterations and repairs.
2. Mathematical model and relationship of parameters to lung burden
A dose-response function P(t:d,D) is constructed for the
probability of cancer by time (age) t, which depends on both organic
compounds, d, and particles (carbon core), D, and incorporates the
biologically based concept discussed above. Because the model
parameters that are not observed directly in the laboratory can be
estimated statistically only from the results of bioassays at high
concentrations, the model should not be considered a real model of
diesel-induced carcinogenesis; uncertainty about extrapolation to low
doses remains.
A model with the features presented above was originally proposed
by Chen & Farland (1991) and was extended into one with time-variable
parameters by Tan & Chen (1992). This model was used as the basis for
constructing a biologically based dose-response model. A brief
mathematical description is presented in Appendix B10.3.
The data on time to event from Mauderly et al. (1987) were used
to estimate model parameters. These data are useful because they
contain information on natural mortality and serial sacrifice of
animals with and without (malignant) tumours, which is valuable for
estimating tumour latency. In order to use this information to
calculate maximum likelihood estimates of parameters, an 'E-M'
algorithm was derived. In the E-M algorithm, each iteration involves
an 'expectation' step (E) and a 'maximization' step (M) (see Appendix
B10.2).
(a) Model parameters and notations
The following parameters are incorporated into the dose-response
model, which includes the rate of initiation (µ1), the rate of
proliferation (gammaalpha), the rate of conversion (gammaµ2), and
the probability of progression ( q). The rate of death of the
initiated cells is implicitly defined by gamma(1-µ2-alpha). The
parameters are all dose dependent.
D: dose of carbon core in milligrams per square centimetre of
lung epithelial surface; varies over time;
d: dose of organic compounds in milligrams per square centimetre
of lung epithelial surface;
µ1: dose-related initiation rate (per cell per day); depends on
µ0 (background rate), d, and D by µ1 = µ0 (1 + ad + bD), where
a and b are parameters to be estimated statistically;
µ2: probability that a malignant cell will be produced by the end
of the lifetime of an initiated cell;
alpha: probability that an initiated cell will divide into two
daughter cells by the end of its lifetime;
q: probability that a single malignant cell will develop into a
malignant tumour;
gamma: 1/gamma is the mean lifetime of an initiated cell in days; the
lifetime ends when the cell goes into mitosis or dies. If it
is assumed that the probability that a cell will go into
mitosis is about the same as the probability that it will die,
the mean cell lifetime can be conveniently interpreted as time
to mitosis (i.e. cell turnover time); thus, a shorter cell
lifetime implies more frequent cell division. Time to mitosis
is a random variable, not a fixed constant as in the
assumption of the model of Greenfield et al. (1984), which was
used by Cohen & Ellwein (1988) to analyse the results of
bioassays to dectect urinary bladder cancer.
N: number of (normal) target cells.
(b) Practical considerations
The E-M algorithm developed in Appendix B10.2 is an elegant
procedure that can be used by statistical theory alone to test
hypotheses about whether a particular parameter is influenced by
organic compounds and the carbon core, individually or together. For
instance, it could be postulated that the parameter the reciprocal of
which represents mean cell lifetime is given by gamma( d,Di) = gamma0 +
gamma11 d + gamma12 Di, and then proceed to test the null hypothesis
that gamma11 = 0, i.e. no effect of organic compounds on the cell
lifetime. This temptation must, however, be resisted, because too many
parameters would have to be be estimated. Therefore, the biologically
plausible assumption that parameters q and gamma depend only on the
lung burden of the carbon core, D,
is used.
The study of Mauderly et al. (1987) lasted about 940 days. In
order to construct a dose-response model including time-dependent lung
burden, the time interval (0-940) is divided into five sub-intervals,
each spanning six months, except for the last, which spans 730-940
days. The deposition-retention model developed by Yu et al. (1991),
corresponding to a concentration of diesel exhaust emissions in
ambient air of milligrams per cubic metre, is used to calculate
( d,Di), where i = 1, 2, ..., 5; the dose of organic compounds,
d, does not change with time because it reaches a steady state soon
after exposure begins; and Di is the lung burden of carbon core
during the ith sub-interval.
The assumptions for the dose-parameters relationship are:
-- The rate of initiation associated with a lung burden { d,Di, i
= 1, 2, ..., 5} is given by µi( d,Di) = µ0(1 + a * d + b * Di) for
i = 1, 2, ..., 5. This is the only parameter that is assumed to
depend on both d and D.
-- The probability of tumour formation from a malignant cell is
assumed to be dependent on lung burden, D, from q( Di) = q0
q0 Di, i = 1, 2, ..., 5. In order to simplify calculation, the
possibility that q0 is also dependent on organic compounds, d, is
not considered.
-- The cell lifetime, gamma, is assumed to be related nonlinearly to
lung burden, D, from gamma( Di) = gamma0 + gamma1 Log(1 + Di),
i = 1, 2, ..., 5.
In order to reduce the number of parameters that are to be
estimated from the data of Mauderly et al. (1987), some of the
background parameters for the dose-response model (µ0, q0, and
gamma0) are estimated from the historical control rates for Fischer
344 rats in the US National Toxicology Program (reconstructed from
Portier et al., 1986). The dose-related parameters are then estimated
with the E-M algorithm, which is described in Appendix B10.2. The
parameters estimated for the model resulting from the tumour response
data of Mauderly et al. (1987) and the corresponding dosimetric
parameters (Table 52) are given in Table 53.
Table 52. Dosimetric parameters (milligrams per square centimetre of lung surface) used
in modelling
Exposure d D1 D2 D3 D4 D5
concn
(mg/m3)
0.35 2.5 × 10-6 6.2 × 10-5 8.8 × 10-5 9.0 × 10-5 9.0 × 10-5 9.0 × 10-5
3.50 3.6 × 10-5 7.5 × 10-4 2.4 × 10-3 3.9 × 10-3 5.3 × 10-3 6.3 × 10-3
7.08 7.3 × 10-5 2.0 × 10-3 5.5 × 10-3 8.6 × 10-3 1.1 × 10-2 1.4 × 10-2
d, organic compounds; Di, i = 1, 2, ..., 5, are average lung burdens of carbon core over
five time intervals. Values calculated from the retention model of Yu et al. (1991)
Table 53. Maximum likelihood estimates for model parameters
Parameter Estimate
µ 1.033 × 10-7
a 1.103 × 104
b 3.214 × 102
µ2 7.907 × 10-7
q0 1.035 × 10-1
q1 5.332 × 10-2
gamma0 1.662 × 10-2
gamma1 2.647 × 10-2
alpha 5.443 × 10-1
N (given) 8.80 × 107
For definitions of parameters, see text. Background parameters µ0, q0,
and gamma0 are estimated separately from historical control data from
the US National Toxicology Program. The number of target cells, N, is
assumed to be 10 times the number of type II cells in mice, which is
given by Kauffman (1974). It is not essential that N be given
accurately because Nµ0 appears as a single term in the model; the
estimated µ0 will compensate for the underestimation or overestimation
of N.
Appendix B10.2 E-M algorithm
The E-M algorithm, derived below, was used to calculate maximum
likelihood estimates of the parameters for the alternative model. The
data used were taken from Mauderly et al. (1987) and include the time
when an animal died, naturally or at sacrifice, with or without
(malignant) tumours. The theory of the algorithm is given by Dempster
et al. (1977).
Assume that the distinct times at which animals died are t1
< t2 < ...< tm. The observations can be classified as follows:
a1x (i): observed number of natural deaths without tumour at time
ti in treatment group x (The four groups are x =
1, 2, 3, 4.)
a2x (i): observed number of natural deaths with tumour at time
ti in treatment group x
b1x (i): series sacrificed at time ti without tumour in
treatment group x
b2x (i): series sacrificed at time ti with tumours in
treatment group x.
Let Td represent the time an animal died and T the time a
tumour developed.
alphax (i) = Pr Td = ti | Td > ti, T > ti,, x} (conditional probability
of death without tumour)
ßx( i | u) = Pr Td = ti | Td > ti, T Epsolin ( tu-1, tu], x} (related
to deaths with tumours)
Define
The model, which is described in Appendix B10.3, allows for
initiation by both the carbon and the organic fraction and for the
proliferative effects of the carbon fraction. Although these
mechanisms remain to be proven, it is assumed that carcinogenic agents
present in the organic fraction act directly on the target cells,
primarily by initiation. It is further assumed that most of the
particles are ingested by macrophages. Particle-laden macrophages are
then induced to secrete a variety of mediators (e.g. reactive oxygen
species and cytokines), which diffuse to the target cells, inducing
initiation, proliferation, and conversion of initiated cells to
malignant cells.
The results reported by Mauderly et al. (1987) for tumour
induction were used to estimate the model parameters. These are based
on the development of malignant tumours rather than all tumours as in
the first method. This was necessary in order to ensure that the data
used to estimate the parameters represented the same biological
mechanism. Lung burdens were calculated from the same dosimetry model
used in the linearized multistage model.
(f) Results of unit risk calculations
Particle-based model: Unit risks were derived from the linearized
multistage approach for the tumour incidences seen in four bioassays
(Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly et al.,
1987; Heinrich et al., 1995) and the corresponding equivalent doses
(Table 48). The resulting unit risk estimates, listed in Table 49,
range from 1.6 to 7.1 × 10-5/µg particles per m3 with a geometric
mean of 3.4 × 10-5/µg per m3.
In these calculations, the relationship between air concentration
(micrograms per cubic metre) and lung burden (milligrams) in humans is
used to determine the lung burden resulting from lifetime exposure to
1 g/m3 of diesel exhaust particulate matter. The particle burden in
terms of mass per unit lung surface area is then multiplied by the
slope derived from the bioassay data. For instance, when the data of
Mauderly et al. (1987) are used, the slope of the curve for
carcinogenicity in rats (i.e. the upper 95% confidence limit of the
linear coefficient in the multistage model), expressed in terms of
equivalent dose (micrograms of carbon particulate matter per square
centimetre) is 1.7 × 10-2/µg per cm2. According to the dosimetry
model, an air concentration of 1 µg/m3 of particulate matter
corresponds to a mass of 1230 µg of carbon particles per human lung.
Because the lung epithelial surface area, including the alveolar
region and conducting airways, is assumed to be 627 000 cm2, a unit
risk of 3.4 × 10-5/µg per m3 is derived from 1.7 × 10-2/µg per cm2
× 1230 µg/627 000 cm2.
Biologically based model: The unit risk estimated by the
alternative model, based on the data on malignant tumours from the
study of Mauderly et al. (1987), is equal to 1.65 × 10-5/µg particles
per m3. This is lower than the estimate of 3.4 × 10-5/µg per m3
derived from the same study with the linearized multistage approach.
Application of the linearized multistage model only to malignant
tumours seen in that study, rather than all lung tumours, resulted in
a unit risk estimate of 1.74 × 10-5/µg particles per m3 (Table 50).
Thus, the unit risk estimates obtained by the two approaches are
virtually identical. The estimated risks may differ somewhat with
increasing doses, because the slopes are not identical at all exposure
levels. It should be noted, however, that the unit risk predicted by
the alternative model is derived under the assumption that particles
continue to exert an effect on cell initiation or proliferation (or
both) at low doses. There is considerable uncertainty about the
effects of particles at low doses. It has been claimed that particles
do not induce initiation or proliferation at low doses (Vostal, 1986).
At present, the evidence is inadequate to support or refute this
claim. Moreover, even if macrophage overload is required, because of
uneven distribution of particles, some macrophages may become
overloaded even at low concentrations. Because of this uncertainty,
the biologically based model, like the linearized multistage model,
does not depart from linearity at low doses; however, if initiation
and proliferation do not occur at low doses, the risk may be much
smaller.
In an estimate of unit risk for rats, based on the results of
seven studies by inhalation and using linear interpolation and the
linearized multistage model, the risks were 7 × 10-5/µg of diesel
particles per m3 and 10 × 10-5/µg carbon core particles per m3
(Csicsaky et al., 1993; Pott et al., 1993; Roller & Pott, 1994).
(g) Results and implications of the biologically based model
On the assumption that particles continue to affect cell
initiation or proliferation at low doses, the risks calculated with
this model are comparable to those obtained with the linearized
multistage model. Excess risks due to various exposures are shown in
Tables 50 and 51. Those in Table 50 are the risks predicted for humans
exposed continuously (24 h/day) from the two models: it is interesting
to note that the results are similar. Table 51 shows the excess risks
due to exposure to 2.6 µg/m3 of diesel particulate emission for
16 h/day on seven days per week and to 15 µg/m3 for 8 h/day on five
days per week. The first concentration was reported by the US
Environmental Protection Agency Office of Mobile Sources to be the
annual mean exposure of the American population to diesel particulate
matter in 1986; the second concentration was reported to be that to
which workers are exposed on urban freeways (Carey, 1987). The
retention half-time for insoluble particles after exposure to
Table 50. Comparison of excess risk for humans due to continuous exposure to
various concentrations of diesel exhaust emissions using two
different models
Exposure concentration Biologically based (alternative) Linearized (µg/m3)
multistage
Maximum likelihood Upper 95% model
estimate bound estimate
0.01 7.68 × 10-8 1.35 × 10-7 1.71 × 10-7
0.1 8.12 × 10-7 1.71 × 10-6 1.72 × 10-7
1.0 (unit risk) 8.16 × 10-6 1.65 × 10-5 1.74 × 10-5
100 5.58 × 10-4 9.63 × 10-4 1.74 × 10-4
1000 2.60 × 10-2 4.22 × 10-2 3.33 × 10-2
a Slope - 9.04 per mg/cm2 of lung surface, using carbon core as dosimetric.
Only malignant tumours are used in the calculations.
2.6 µg/m3 is shown to increase from 296 days for members of the
general population with normal respiratory function to 519 days for
those with a smoking history of 20 pack-years (Bohning et al., 1982).
This information was used to reduce the alveolar clearance rate for
the dosimetric calculations to that used for other risk calculations.
Interactive effects of smoking and diesel exhaust are not considered
in the risk calculations owing to lack of data. The studies of carbon
black and coal-tar pitch (Heinrich, 1994; Heinrich et al., 1994)
indicate that smokers have a higher risk for lung cancer than
nonsmokers when they are exposed to diesel exhaust.
The excess lifetime risks shown in Tables 50 and 51 are
standardized by the actuarial life-table approach, using the survival
probability of control animals in the US National Toxicology Program
provided by Portier et al. (1986). This approach gives a weighted
average of the probability of cancer occurrence over an entire
lifetime, weighted by survival probability.
Table 51. Excess lifetime risk for humans due to exposure to diesel
exhaust emissions under various exposure scenarios
Exposure pattern Biologically based model Linearized
multistage
Maximum Upper 95% modela
likelihood bound
estimate estimate
General population (normal 1.41 × 10-5 2.44 × 10-5
respiratory function;
nonsmoker): 2.6 µg/m3,
16 h/day, seven days
per week
General population 2.32 × 10-5 3.61 × 10-5 5.38 × 10-5
(20-pack-year smoker)b:
2.6 µg/m3, 16 h/day,
seven days per week
Occupationally exposed: 3.12 × 10-5 5.17 × 10-5 6.18 × 10-5
15 µg/m3 8 h/day, five
days per week
a Calculated using carbon core as dosimetric; only malignant
tumours are used.
b In this calculation, smoking affects only lung clearance rate;
biological interaction between smoking and exposure to diesel
exhaust is not considered. The retention half-times for
insoluble particles increased from 296 days for persons with
normal respiratory function to 519 days for 20-pack-year smokers
(Bohning et al., 1982).
Some implications of the alternative model are:
(1) At a low exposure concentration, the decrease in diesel-induced
initiation results in a greater reduction of risk; that is, the
number of initiated cells (cancer risk) is reduced more
efficiently with a low than with a high exposure. If only organic
compounds induce initiation when the concentration is low, they
play a more important role than particles in inducing tumours at
low concentrations, whereas the roles are reversed when the
concentration is high.
(2) Although cells initiated by diesel exhaust play an important role
in cancer induction, either organic compounds or the carbon core
alone could induce initiation by increasing their respective
proportions. Thus, although initiated cells are important for
tumour induction, they may be induced by any agent that initiates
tumours (e.g. smoking).
(3) A small change in the rate of proliferation induced by diesel
exhaust could disproportionately change cancer risk. As this
parameter is assumed to be a function of the dose of carbon core,
lung overloading has a significant effect on cancer incidence. In
the absence of better information, it is assumed in this
assessment that the carbon core continues to have a proliferating
effect at low doses.
These observations suggest that, although the effect of particle
overload on cell proliferation is important, initiation by the carbon
core or organic compounds or both is also essential. Although this
conclusion is only tentative, because the model parameters are
estimated statistically on the basis of bioassays conducted at high
concentrations, it does suggest the importance of studying the role of
the carbon core and organic compounds in initiation and promotion at
low and high exposure concentrations. Does the relative initiation
potential of organic compounds and the carbon core differ with
concentration? These observations also suggest that a subcohort of
workers who were smokers and were exposed to high concentrations of
diesel exhaust for a long time would have a greater risk of dying from
lung cancer.
(h) Comparison of risk estimates derived from experimental studies
and human experience
The bioassay-based risk estimates, which range from 1.6 × 10-5 to
7.1 × 10-5, with a geometric mean of 3.4 x 10-5, can be compared with
human experience on the basis of three data sets: those of an
epidemiological study conducted on London Transport employees (Waller,
1981) and a subsequent analysis (Harris, 1983) and those on American
railroad workers (Garshick et al., 1987, 1988). Although these data
cannot be used to calculate unit risk, mainly because of a lack of
reliable information on exposure, they can be used to evaluate the
validity of unit risk estimates based on the results of experimental
studies.
Attempts have already been made to estimate the potential cancer
risk due to exposure to diesel exhaust on the basis of epidemiological
data. From the results of the study of London Transport workers,
Harris (1983) estimated that the increase in the relative risk for
lung cancer associated with exposure to 1 µg/m3-year of diesel exhaust
was 1.2 × 10-4, with an upper bound of the 95% confidence limit of
4.8 × 10-4. (Generally, when data from an epidemiological study with
negative results are used to estimate cancer risk, the upper bound is
used to calculate unit risk.) The resulting unit risk estimate is 2 ×
10-3, which is about 60 times higher than the mean unit risk estimate
derived from bioassays, 3.4 × 10-5, and about 30 times higher than the
upper end of the range of unit risk estimates from bioassays, 7.1 ×
10-5. Therefore, the risk estimate based on bioassays is not
inconsistent with that for humans, since 2 × 10-3 is the estimated
upper bound in an epidemiological study with negative results.
McClellan et al. (1989) reported risk estimates based on the
study of Garshick et al. (1987), in which lung cancers in railroad
workers were evaluated. Assuming exposure to concentrations of 500 and
125 µg/m3, the upper bounds of the 95% confidence interval for
lifetime cancer risk were estimated to be 6 × 10-4 and 2 × 10-3,
respectively. The lower of the two unit risk estimates is only about
one order of magnitude higher than the risk estimates based on
bioassays.
An epidemiological study potentially more suitable for
quantitative risk assessment was reported by Garshick et al. (1988),
which was based on a large number of subjects; a small but significant
increase in the rate of mortality from lung cancer was seen in some
subcohorts. The US Environmental Protection Agency has supported an
effort to derive a unit risk estimate from this study, and data on
exposure were estimated by Woskie et al. (1988a,b) and Hammond et al.
(1988). These data were analysed in a variety of ways, using relative
risk and absolute risk dose-response models and by classifying
individuals into various exposure categories, including job, duration
of employment, age, and exposure markers. Even though at least 50
analyses were carried out, an adequate dose-response relationship
could not be obtained; these data were therefore not used to estimate
unit risk.
The lack of a dose-response relationship is not totally
unexpected, given the low increase in mortality rate in the study of
Garshick et al. (1988) and the uncertain estimates of exposure. The
data on exposure were derived from air samples collected during a
limited period (1981-83) on four small railroads operating in a
limited geographical area (northern United States), in which
concentrations of respirable particulate matter were measured rather
than diesel exhaust per se; the measured concentrations were then
adjusted to derive markers of exposure. The data were used to estimate
exposure to diesel exhaust of railroad workers throughout the United
States 30 or more years earlier. Diesel equipment and working
conditions have, however, changed since the 1940s when diesel engines
first began to be used in large numbers. Woskie et al. (1988b)
summarized anecdotal reports of smoky working conditions in diesel
repair shops during the 1950s and 1960s and reported that the limited
data available on levels of nitrogen oxide during those periods
indicated high levels of diesel exhaust. By the time samples were
collected, however, the smoky conditions would have been largely
mitigated by improved ventilation and the advent of less smoky diesel
engines. The study of Garshick et al. (1988) gives the relative risks
for dying from lung cancer in exposed as opposed to unexposed railroad
workers classified into five subcohorts by age in 1959. The risks
ranged from 0.96 (95% CI, 0.74-1.33) to 1.45 (1.11-1.89). The highest
relative risk, 1.45, which was observed in workers who were 40-45
years old in 1959, was used here to evaluate the validity of unit risk
based on biossays results. Assuming that this subcohort of workers was
exposed to diesel exhaust for 8 h/day on five days per week from age
35 to age 65, the background mortality rate from lung cancer in this
subcohort would be 0.038 (0.63 × 0.06), as the unexposed workers in
the same age group had a relative risk of 0.63 and the corresponding
lifetime mortality rate from lung cancer in the general American white
male population is about 0.06. If the lung cancer risk due to
1 µg/m3 is assumed to be 3.4 × 10-5, the concentration of diesel
exhaust in the working environment was at least 400 µg/m3, calculated
as follows: The risk due to 1 µg/cm2 of particles is 0.017 (which
results in a unit risk of 3.4 × 10-5). For a lower bound of the
relative risk of 1.1, a lung burden, d, that satisfies the
relationship 0.1 × 0.038 = 0.017 d would be needed; that is, d =
0.22 µg/cm2, which is equivalent to an air concentration of
0.4 mg/m3 by the dosimetry model of Yu et al. (1991). If the highest
unit risk estimate derived from Brightwell et al. (1986), 7.1 ×
10-5, is used, the required minimal air concentration would be about
0.2 mg/m3. These calculations imply that a diesel exhaust
concentration of at least 0.2 mg/m3 was necessary to observe a
statistically significant increase in the mortality rate from lung
cancer in this study. This concentration appears to be reasonable in
the light of the working conditions described by Woskie et al.
(1988a).
Although the risk estimates derived from bioassays are lower than
those derived from human data and may possibly over-predict risk,
since the non-linearity of the human response is not considered in
this model, they are not inconsistent, for the following reasons:
(1) The risk estimates based on epidemiological studies are not
derived from all of the available data but on only a subset with
the highest response. The estimates are therefore higher than
those that would be derived from the whole data set.
(2) When a single data point (i.e. an overall relative risk and an
averaged exposure concentration) is used in the calculations, the
resulting slope for potency will be overestimated if the
dose-response relationship is not linear over all exposure
concentrations. Assume, for example, that the response follows
the simple multistage model P(d) = 1 - exp[-( q0 + qd + q2 d2)].
The relative risk, R, at concentration d is R(d) = P(d)/P0.
Using this mathematical expression, it is easy to demonstrate
that the slope factor calculated from [ P(d) - 1] P0/ d is
greater at high doses (including the averaged concentration used
in the risk calculation) than at low doses where the
dose-response function is dominated by q1. Epidemiologists
have long had a similar (but not identical) concern about the use
of averaged data, since ecological associations are not
necessarily consistent with those measured at the individual
level (see Cohen, 1994; Greenland & Robins, 1994; Piantadosi,
1994).
(3) Some occupational groups were exposed to considerably higher
concentrations of diesel exhaust in the past than presently. For
example, the average particle concentration in a Finnish
roundhouse was reported to be 2 mg/m3 (Heino et al., 1978). The
lung burdens would thus be greater than those predicted on the
basis of current exposures, and the unit risk estimates would be
higher
(i) Summary and conclusions
A dosimetric model that accounts for differences between
experimental animals and humans in lung deposition efficiency, lung
particle clearance rates, lung surface area, ventilation, and the
rates of elution of organic chemicals from the particle surface was
used to calculate equivalent human doses as particle concentration per
unit lung surface area. After dosimetric adjustment, four risk
estimates were derived by a linearized multistage model from three
bioassays with Fischer 344 rats and one with female Wistar rats, which
ranged from 1.6 to 7.1 × 10-5/µg particles per m3, with a geometric
mean of 3.4 × 10-5. This quantitative assessment of the carcinogenic
risk due to exposure to diesel engine emissions is reasonable,
because:
-- The estimates are based on several well-designed, well-executed,
long-term bioassays.
-- Epidemiological studies indicate that humans are susceptible to
the induction of lung cancer after inhalation of diesel exhaust.
-- Dosimetry modelling, especially to account for inhibition of
particle clearance at high doses, has allowed accurate
extrapolation of doses from animals to humans.
-- The doses are based on actual concentrations of particulate
matter per unit of lung surface area.
-- Use of an alternative model that attempts to account for the
possible biological effects of the organic and carbon core
fractions did not appreciably change the unit risk estimate.
-- The risk estimates based on the results of bioassyas are not
inconsistent with the available human experience.
Nevertheless, a number of uncertainties remain, the most
significant of which are:
-- In any interspecies extrapolation there may be an inherent
difference in sensitivity to the agent being assessed.
-- The assumption of equivalent sensitivity across species is based
on concentration per unit of lung surface area, and use of other
assumptions of dose equivalence may lead to different estimates
of risk; however, the estimates should not vary by more than one
order of magnitude.
-- Although linearized low-dose extrapolation methods are used, it
is still uncertain whether inflammatory cells secrete mediators
that induce cancer in lung epithelial cells when the particle
burden is smaller than that necessary to inhibit clearance. Even
if macrophages are activated at low particle burdens, it is
uncertain whether the responses of epithelial cells are linear at
very low concentrations; however, non-linear carcinogenic
responses have been observed in bioassays.
-- Although the unit risk estimate is corroborated in the
alternative model, uncertainty about the response at low doses
remains because the estimates in the alternative model are based
on the assumption that particles continue to induce cell
initiation and/or proliferation at low doses. The actual risk
would be much lower if this assumption does not hold.
The risk at low doses derived from the linearized multistage
model may thus be overestimated if particles no longer induce cell
initiation and/or proliferation. The model was selected for
calculating risk because a conservative model was needed in order to
ensure protection of public health and because adequate data were not
available to use fully the alternative model constructed for this
assessment. Thus, the risk derived from the bioassays should be viewed
as hypothetical.
These unit risk estimates should not be used to evaluate the
carcinogenic risk of other types of particulate matter present in
ambient air, which may have different solubilities, surface areas, and
free radical contents, which factors greatly affect carcinogenic
potency.
It could be argued that since the types and location of tumours
seen in rats after exposure to particles are different from those
found in humans, the experimental data are not relevant for humans.
The tumours diagnosed in rats after long-term exposure to carbon black
particles and diesel exhaust include benign adenomas and malignant
adenocarcinomas, squamous-cell carcinomas, adenosquamous carcinomas,
and squamous cysts (Mauderly et al., 1994; see section B7.3.2). After
injection into athymic mice, cells from 50-67% of squamous-cell
carcinomas and 25-40% of adenocarcinomas were found to grow (Table
35), whereas those from squamous cysts did not (Mauderly et al.,
1994).
Squamous cysts have been defined by other authors as benign
cystic keratinizing squamous-cell tumours (Kittel et al., 1993;
Dungworth et al., 1994; Heinrich et al., 1995) and found not to grow
after transplantation (Heinrich et al., 1995). As pointed out by Mohr
(1992), there is, however, controversy about the correct terminology
of this type of lesion. In 1995, an international group of
pathologists re-evaluated lung tissue sections from rats exposed to
particles by inhalation and identified four distinct lesions:
squamous-cell metaplasia, pulmonary keratinizing cyst, cystic
keratinizing epithelioma (considered to be benign), and squamous-cell
carcinoma (G. Oberdörster, personal communication).
Regardless of the correct classification of lesions, the
important fact is that malignant tumours are induced in rats after
long-term inhalation of deisel exhaust and carbon black. The tumour
response of human beings may not, however, be the same, either
qualitatively or quantitatively. In fact, the response of rats to
long-term exposure to high concentrations of particles differs from
that of other species, including mice and hamsters. In the absence of
sufficient information about specific mechanisms unique to rats,
however, there is no justification for excluding data on this species
from extrapolations to the human situation (US Environmental
Protection Agency, 1986).
With respect to the mechanisms of induction of lung tumours by
particles in rats, it has been shown that particles devoid of
polyaromatic compounds can increase mutation rates in pulmonary
epithelial cells, which is an important step in tumour development via
cell transformation. In a recent study in which rats were exposed to
carbon black for 13 weeks, a significant influx of inflammatory cells
was seen at concentrations of 7 and 50 mg/m3 but not at 1 mg/m3
(Oberdörster et al., 1995), and a significant, three- to fourfold
increase in the frequency of hprt mutations was seen in alveolar
epithelial cells (Driscoll et al., 1995 and in press; see section
B7.6). Thus, burdens of particles of low toxicity per se, similar to
those reached in long-term studies in rats, can be mutagenic, possibly
through the involvement of DNA damaging oxidants derived from
inflammatory cells.
Appendix B10.1 Construction of a biologically based (alternative)
model
1. Preliminary considerations
In order to evaluate the effects of various biological
assumptions on the assessments of the risk of exposure to diesel
exhaust, a mathematical dose-response model must be constructed that
takes into account the proposed biological mechanisms. As a
significant issue in assessing the risk of diesel exhaust is the
effect of lung overloading on tumour induction, the model should have
the following properties:
-- It should depend on dose metrics for both organic compounds and
the carbon core, and it should account for the contributions of
each to tumour induction and formation both separately and
jointly.
-- It should allow for changes in the model parameters with time due
to increasing lung burden during exposure.
-- It should view cell proliferation and tumour induction and
formation stochastically: it is not realistic to assume
deterministic clonal growth. For instance, it should not be
assumed that all cells divide at the same age.
It is therefore assumed that a normal cell can be initiated by
both organic compounds and the carbon core. The initiation rate is
denoted by µ1, which is a function of the background rate and that
induced by diesel exhaust (as specified below). Because an initiated
cell eventually either dies or enters the cell cycle (including cells
in quiescence, G0), it is reasonable to assume that the lifetime of
an initiated cell follows a certain probability distribution. In this
model, a cell in G0 phase is equivalent to one with a certain
probability of a very long lifetime (i.e. in the right-hand tail of
the distribution of the cell's lifetime). At the end of its lifetime,
it either dies (death) with probability b, divides into two daughter
cells (birth) with probability a, or divides into one initiated cell
and one malignant cell (second transition) with probability µ2;
alpha + ß + µ2 = 1. Instead of assuming that a single malignant cell
is equivalent to a tumour, as in the model proposed by Moolgavkar &
Venzon (1979) and Moolgavkar & Knudson (1981), it is assumed here that
a malignant cell has a certain probability of becoming a tumour; this
probability is assumed to be dose-dependent, thus allowing for an
evaluation of the effect of dose on tumour progression. It should be
noted that the proposed model does not exclude the possibility that
there may be more than one step in the 'initiation' of a normal cell.
The rate of initiation used in the model should be viewed as a net
rate that represents several genetic alterations and repairs.
2. Mathematical model and relationship of parameters to lung burden
A dose-response function P(t:d,D) is constructed for the
probability of cancer by time (age) t, which depends on both organic
compounds, d, and particles (carbon core), D, and incorporates the
biologically based concept discussed above. Because the model
parameters that are not observed directly in the laboratory can be
estimated statistically only from the results of bioassays at high
concentrations, the model should not be considered a real model of
diesel-induced carcinogenesis; uncertainty about extrapolation to low
doses remains.
A model with the features presented above was originally proposed
by Chen & Farland (1991) and was extended into one with time-variable
parameters by Tan & Chen (1992). This model was used as the basis for
constructing a biologically based dose-response model. A brief
mathematical description is presented in Appendix B10.3.
The data on time to event from Mauderly et al. (1987) were used
to estimate model parameters. These data are useful because they
contain information on natural mortality and serial sacrifice of
animals with and without (malignant) tumours, which is valuable for
estimating tumour latency. In order to use this information to
calculate maximum likelihood estimates of parameters, an 'E-M'
algorithm was derived. In the E-M algorithm, each iteration involves
an 'expectation' step (E) and a 'maximization' step (M) (see Appendix
B10.2).
(a) Model parameters and notations
The following parameters are incorporated into the dose-response
model, which includes the rate of initiation (µ1), the rate of
proliferation (gammaalpha), the rate of conversion (gammaµ2), and
the probability of progression ( q). The rate of death of the
initiated cells is implicitly defined by gamma(1-µ2-alpha). The
parameters are all dose dependent.
D: dose of carbon core in milligrams per square centimetre of
lung epithelial surface; varies over time;
d: dose of organic compounds in milligrams per square centimetre
of lung epithelial surface;
µ1: dose-related initiation rate (per cell per day); depends on
µ0 (background rate), d, and D by µ1 = µ0 (1 + ad + bD), where
a and b are parameters to be estimated statistically;
µ2: probability that a malignant cell will be produced by the end
of the lifetime of an initiated cell;
alpha: probability that an initiated cell will divide into two
daughter cells by the end of its lifetime;
q: probability that a single malignant cell will develop into a
malignant tumour;
gamma: 1/gamma is the mean lifetime of an initiated cell in days; the
lifetime ends when the cell goes into mitosis or dies. If it
is assumed that the probability that a cell will go into
mitosis is about the same as the probability that it will die,
the mean cell lifetime can be conveniently interpreted as time
to mitosis (i.e. cell turnover time); thus, a shorter cell
lifetime implies more frequent cell division. Time to mitosis
is a random variable, not a fixed constant as in the
assumption of the model of Greenfield et al. (1984), which was
used by Cohen & Ellwein (1988) to analyse the results of
bioassays to dectect urinary bladder cancer.
N: number of (normal) target cells.
(b) Practical considerations
The E-M algorithm developed in Appendix B10.2 is an elegant
procedure that can be used by statistical theory alone to test
hypotheses about whether a particular parameter is influenced by
organic compounds and the carbon core, individually or together. For
instance, it could be postulated that the parameter the reciprocal of
which represents mean cell lifetime is given by gamma( d,Di) = gamma0 +
gamma11 d + gamma12 Di, and then proceed to test the null hypothesis
that gamma11 = 0, i.e. no effect of organic compounds on the cell
lifetime. This temptation must, however, be resisted, because too many
parameters would have to be be estimated. Therefore, the biologically
plausible assumption that parameters q and gamma depend only on the
lung burden of the carbon core, D,
is used.
The study of Mauderly et al. (1987) lasted about 940 days. In
order to construct a dose-response model including time-dependent lung
burden, the time interval (0-940) is divided into five sub-intervals,
each spanning six months, except for the last, which spans 730-940
days. The deposition-retention model developed by Yu et al. (1991),
corresponding to a concentration of diesel exhaust emissions in
ambient air of milligrams per cubic metre, is used to calculate
( d,Di), where i = 1, 2, ..., 5; the dose of organic compounds,
d, does not change with time because it reaches a steady state soon
after exposure begins; and Di is the lung burden of carbon core
during the ith sub-interval.
The assumptions for the dose-parameters relationship are:
-- The rate of initiation associated with a lung burden { d,Di, i
= 1, 2, ..., 5} is given by µi( d,Di) = µ0(1 + a * d + b * Di) for
i = 1, 2, ..., 5. This is the only parameter that is assumed to
depend on both d and D.
-- The probability of tumour formation from a malignant cell is
assumed to be dependent on lung burden, D, from q( Di) = q0
q0 Di, i = 1, 2, ..., 5. In order to simplify calculation, the
possibility that q0 is also dependent on organic compounds, d, is
not considered.
-- The cell lifetime, gamma, is assumed to be related nonlinearly to
lung burden, D, from gamma( Di) = gamma0 + gamma1 Log(1 + Di),
i = 1, 2, ..., 5.
In order to reduce the number of parameters that are to be
estimated from the data of Mauderly et al. (1987), some of the
background parameters for the dose-response model (µ0, q0, and
gamma0) are estimated from the historical control rates for Fischer
344 rats in the US National Toxicology Program (reconstructed from
Portier et al., 1986). The dose-related parameters are then estimated
with the E-M algorithm, which is described in Appendix B10.2. The
parameters estimated for the model resulting from the tumour response
data of Mauderly et al. (1987) and the corresponding dosimetric
parameters (Table 52) are given in Table 53.
Table 52. Dosimetric parameters (milligrams per square centimetre of lung surface) used
in modelling
Exposure d D1 D2 D3 D4 D5
concn
(mg/m3)
0.35 2.5 × 10-6 6.2 × 10-5 8.8 × 10-5 9.0 × 10-5 9.0 × 10-5 9.0 × 10-5
3.50 3.6 × 10-5 7.5 × 10-4 2.4 × 10-3 3.9 × 10-3 5.3 × 10-3 6.3 × 10-3
7.08 7.3 × 10-5 2.0 × 10-3 5.5 × 10-3 8.6 × 10-3 1.1 × 10-2 1.4 × 10-2
d, organic compounds; Di, i = 1, 2, ..., 5, are average lung burdens of carbon core over
five time intervals. Values calculated from the retention model of Yu et al. (1991)
Table 53. Maximum likelihood estimates for model parameters
Parameter Estimate
µ 1.033 × 10-7
a 1.103 × 104
b 3.214 × 102
µ2 7.907 × 10-7
q0 1.035 × 10-1
q1 5.332 × 10-2
gamma0 1.662 × 10-2
gamma1 2.647 × 10-2
alpha 5.443 × 10-1
N (given) 8.80 × 107
For definitions of parameters, see text. Background parameters µ0, q0,
and gamma0 are estimated separately from historical control data from
the US National Toxicology Program. The number of target cells, N, is
assumed to be 10 times the number of type II cells in mice, which is
given by Kauffman (1974). It is not essential that N be given
accurately because Nµ0 appears as a single term in the model; the
estimated µ0 will compensate for the underestimation or overestimation
of N.
Appendix B10.2 E-M algorithm
The E-M algorithm, derived below, was used to calculate maximum
likelihood estimates of the parameters for the alternative model. The
data used were taken from Mauderly et al. (1987) and include the time
when an animal died, naturally or at sacrifice, with or without
(malignant) tumours. The theory of the algorithm is given by Dempster
et al. (1977).
Assume that the distinct times at which animals died are t1
< t2 < ...< tm. The observations can be classified as follows:
a1x (i): observed number of natural deaths without tumour at time
ti in treatment group x (The four groups are x =
1, 2, 3, 4.)
a2x (i): observed number of natural deaths with tumour at time
ti in treatment group x
b1x (i): series sacrificed at time ti without tumour in
treatment group x
b2x (i): series sacrificed at time ti with tumours in
treatment group x.
Let Td represent the time an animal died and T the time a
tumour developed.
alphax (i) = Pr Td = ti | Td > ti, T > ti,, x} (conditional probability
of death without tumour)
ßx( i | u) = Pr Td = ti | Td > ti, T Epsolin ( tu-1, tu], x} (related
to deaths with tumours)
Define
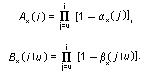 and
Sx (t) = Pr T > t | x} = exp[- t0integralt h(x)dx].
The function Sx (t) is the probability of being tumour-free by
time t.
The exact forms of the hazard function, h(x), and Sx (t) are
given in Appendix B10.3.
Let
a2x( i | u) = number of natural deaths at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i,
b2x( i | u) = number of animals sacrificed at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i.
Then
and
Sx (t) = Pr T > t | x} = exp[- t0integralt h(x)dx].
The function Sx (t) is the probability of being tumour-free by
time t.
The exact forms of the hazard function, h(x), and Sx (t) are
given in Appendix B10.3.
Let
a2x( i | u) = number of natural deaths at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i,
b2x( i | u) = number of animals sacrificed at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i.
Then
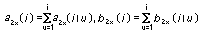 Let
Let
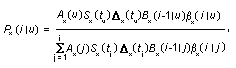 and
and
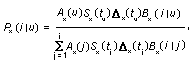 where
where
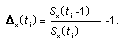 Given a2x( x), { a2x( i | u), u = 1, ..., i} is an ( i-1)-dimension
multinomial with parameter { a2x( i), Px( i | u), u = 1, ..., i}.
Thus,
E[ a2x( i | u) | a2x( i)] = a2x( i) Px( i | u).
Similarly, { b2x( i | u), u = 1, ..., i}, is an ( i - 1)-dimension
multinomial with parameters { b2x( i), Qx( i | u), u = 1, ..., i}, and
E[ b2x( i | u) | b2x( i)] = b2x( i) Qx( i | u).
It can be shown that the likelihood function is proportional to
Given a2x( x), { a2x( i | u), u = 1, ..., i} is an ( i-1)-dimension
multinomial with parameter { a2x( i), Px( i | u), u = 1, ..., i}.
Thus,
E[ a2x( i | u) | a2x( i)] = a2x( i) Px( i | u).
Similarly, { b2x( i | u), u = 1, ..., i}, is an ( i - 1)-dimension
multinomial with parameters { b2x( i), Qx( i | u), u = 1, ..., i}, and
E[ b2x( i | u) | b2x( i)] = b2x( i) Qx( i | u).
It can be shown that the likelihood function is proportional to
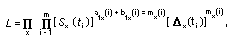 where
where
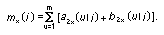 Let
Let
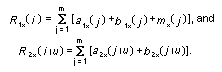 Let
Let
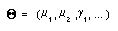 be a vector of parameters in function S;
alphax = [alphax(1), alphax(2), ..., alphax( m)], and
ßx( u) = [ßx(1 | u), ßx(2 | u, ..., ßx( m | u)]
be vectors of parameters related to conditional probabilities of death
with and without tumours. These parameters and those in thetax were
estimated by the E-M algorithm described below.
The M step:
Given initial values a2x( i | u) and b2x( i | u), estimate
be a vector of parameters in function S;
alphax = [alphax(1), alphax(2), ..., alphax( m)], and
ßx( u) = [ßx(1 | u), ßx(2 | u, ..., ßx( m | u)]
be vectors of parameters related to conditional probabilities of death
with and without tumours. These parameters and those in thetax were
estimated by the E-M algorithm described below.
The M step:
Given initial values a2x( i | u) and b2x( i | u), estimate
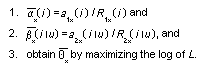 The E step:
Given the estimated values of alphax( i), ßx( i), and thetax from the M
step, compute Px( i | u) and Qx( i | u) and obtain estimates of
a2x( i | u) and b2x( i | u) by
The E step:
Given the estimated values of alphax( i), ßx( i), and thetax from the M
step, compute Px( i | u) and Qx( i | u) and obtain estimates of
a2x( i | u) and b2x( i | u) by
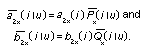 With the estimated values of a2x( i | u) and b2x( i | u) available
from the E step, go back to the M step and repeat the process until
the estimates are stable.
Appendix B10.3 A tumour growth model
A tumour growth model with piece-wise constant parameters taken
from Tan & Chen (1992) is an extension of a stochastic model developed
by Chen & Farland (1991). The biological justification of this model
is similar to that of the two-stage model proposed by Greenfield et
al. (1984), which was used by Cohen & Ellwein (1988) to analyse
urinary bladder tumour occurrence. The two models differ, however,
with respect to their mathematical formulations; the one adopted in
this report is a stochastic model, whereas the other is a
deterministic model and does not allow for estimation of parameters
because it does not have complete mathematical expression.
Although its most general form is not used here because of lack
of data, the stochastic model of Chen & Farland (1991) has two
desirable features: (i) it allows for any cell growth distribution
(e.g. Gompertz), rather than only exponential distribution as in other
models; and (ii) it incorporates the birth and death of tumour cells,
rather than assuming that a tumour is born once a single tumour cell
occurs, as did Moolgavkar & Venzon (1979) and Moolgavkar & Knudson
(1981). Therefore, if information on cell lifetime distribution and
the progression of tumour development is available, a reasonably
realistic model can be constructed.
For completeness, a brief description of the model is presented
here. The following notations are required:
N(t): number of normal (target) cells at time t,
µ1: rate of initiation, and
contour the probability density function for the lifetime of an
integral (t): an initiated cell.
At the end of its lifetime, an initiated cell either divides
(mitosis) or dies (programmed or nonprogrammed death). If it enters
mitosis, it either divides either into two initiated cells with
probability a or into one initiated cell and one malignant cell with
probability µ2. At the end of a cell's lifetime, the probability of
it dying is ß = 1 - alpha - µ2. A similar set-up (to allow for any
cell lifetime distribution) can be made for a malignant cell; however,
we confined ourselves to a simpler version, assuming that the lifetime
of a malignant cell follows an exponential distribution. Thus, we
assume that a malignant cell follows a simple birth-death process; it
can either divide into two malignant cells at a rate alpham or die
at a rate ßm.
When the parameters are constant over time (age), the hazard
function is given by
h(t) = µ1µ2 0integralt N(s)m( t - s) ds
where
With the estimated values of a2x( i | u) and b2x( i | u) available
from the E step, go back to the M step and repeat the process until
the estimates are stable.
Appendix B10.3 A tumour growth model
A tumour growth model with piece-wise constant parameters taken
from Tan & Chen (1992) is an extension of a stochastic model developed
by Chen & Farland (1991). The biological justification of this model
is similar to that of the two-stage model proposed by Greenfield et
al. (1984), which was used by Cohen & Ellwein (1988) to analyse
urinary bladder tumour occurrence. The two models differ, however,
with respect to their mathematical formulations; the one adopted in
this report is a stochastic model, whereas the other is a
deterministic model and does not allow for estimation of parameters
because it does not have complete mathematical expression.
Although its most general form is not used here because of lack
of data, the stochastic model of Chen & Farland (1991) has two
desirable features: (i) it allows for any cell growth distribution
(e.g. Gompertz), rather than only exponential distribution as in other
models; and (ii) it incorporates the birth and death of tumour cells,
rather than assuming that a tumour is born once a single tumour cell
occurs, as did Moolgavkar & Venzon (1979) and Moolgavkar & Knudson
(1981). Therefore, if information on cell lifetime distribution and
the progression of tumour development is available, a reasonably
realistic model can be constructed.
For completeness, a brief description of the model is presented
here. The following notations are required:
N(t): number of normal (target) cells at time t,
µ1: rate of initiation, and
contour the probability density function for the lifetime of an
integral (t): an initiated cell.
At the end of its lifetime, an initiated cell either divides
(mitosis) or dies (programmed or nonprogrammed death). If it enters
mitosis, it either divides either into two initiated cells with
probability a or into one initiated cell and one malignant cell with
probability µ2. At the end of a cell's lifetime, the probability of
it dying is ß = 1 - alpha - µ2. A similar set-up (to allow for any
cell lifetime distribution) can be made for a malignant cell; however,
we confined ourselves to a simpler version, assuming that the lifetime
of a malignant cell follows an exponential distribution. Thus, we
assume that a malignant cell follows a simple birth-death process; it
can either divide into two malignant cells at a rate alpham or die
at a rate ßm.
When the parameters are constant over time (age), the hazard
function is given by
h(t) = µ1µ2 0integralt N(s)m( t - s) ds
where
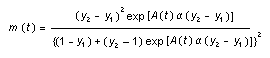 where y1 < y2 are two real roots of alpha y2 - (alpha + ß + µ2 q) y +
ß = 0; alpha + ß + µ2
= 1; q = 1 - ßm/alpham; A(t) = / 0integralt a(x)d x, where a(t) =
contour integral( t)/[1 - F(t)] is the hazard function of the cell
lifetime and F(t) is the cumulative function of contour integral (t).
Two cases of special interest are a(t) = gamma when an exponential
distribution is assumed and a(t) = exp(-gamma t), when the Gompertz
distribution is assumed.
When an exponential distribution (i.e. a(t) = gamma or A(t) =
gamma t) and q = 1 are assumed, the model is equivalent to the
model of Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981). A
special case that may be more appropriate than the exponential
distribution is that when the Gompertz distribution is assumed (i.e.
A(t) = [1 - exp(-gamma t)]/gamma).
For the model with time-dependent parameters, assume that the
study begins at time t0. Divide the time scale ( t0, t] into
k sub-intervals Lj = ( tj-1, tj], j = 1, 2, ... k-1 and Lk = ( tk-1, tk]
where tk = t. (Note that these sub-intervals may not be the same as
those defined by deaths or sacrifice previously.) The parameters that
vary over sub-intervals ( ti-1, ti], i = 1, 2, ..., k are µ1j, alphaj,
ßj, µ2j, N, and those parameters related to contour integral( t). The
hazard functionis given by
where y1 < y2 are two real roots of alpha y2 - (alpha + ß + µ2 q) y +
ß = 0; alpha + ß + µ2
= 1; q = 1 - ßm/alpham; A(t) = / 0integralt a(x)d x, where a(t) =
contour integral( t)/[1 - F(t)] is the hazard function of the cell
lifetime and F(t) is the cumulative function of contour integral (t).
Two cases of special interest are a(t) = gamma when an exponential
distribution is assumed and a(t) = exp(-gamma t), when the Gompertz
distribution is assumed.
When an exponential distribution (i.e. a(t) = gamma or A(t) =
gamma t) and q = 1 are assumed, the model is equivalent to the
model of Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981). A
special case that may be more appropriate than the exponential
distribution is that when the Gompertz distribution is assumed (i.e.
A(t) = [1 - exp(-gamma t)]/gamma).
For the model with time-dependent parameters, assume that the
study begins at time t0. Divide the time scale ( t0, t] into
k sub-intervals Lj = ( tj-1, tj], j = 1, 2, ... k-1 and Lk = ( tk-1, tk]
where tk = t. (Note that these sub-intervals may not be the same as
those defined by deaths or sacrifice previously.) The parameters that
vary over sub-intervals ( ti-1, ti], i = 1, 2, ..., k are µ1j, alphaj,
ßj, µ2j, N, and those parameters related to contour integral( t). The
hazard functionis given by
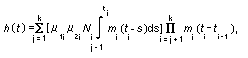 where
where
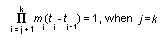 and
and
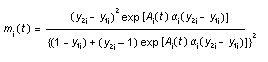 where y1j < y2j are two real roots of a alpha y2 - (alphaj + ßj +
µ2j qj) y + ßj = 0; alphaj + ßj + µ2j = 1; qj = 1 - ßmj/alphamj, j = 1,
2, ..., k.
When an exponential distribution (i.e. Aj( t) = gammaj( t) and
qj = 1) is assumed, the model is equivalent to the model of
Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981), with
piece-wise constant parameters. A special case that may be more
appropriate than the exponential distribution is that when the
Gompertz distribution is assumed (i.e. Aj( t) = {1 - exp[-gammaj t)]}
/gammaj).
In the alternative model for exposure to diesel exhaust, in which
the total time is divided into five (i.e. k = 5) sub-intervals, Tan
& Chen (1992) showed that, under the assumption of exponential cell
lifetime distribution, the tumour-free distribution function, Sx( t),
can be written:
where y1j < y2j are two real roots of a alpha y2 - (alphaj + ßj +
µ2j qj) y + ßj = 0; alphaj + ßj + µ2j = 1; qj = 1 - ßmj/alphamj, j = 1,
2, ..., k.
When an exponential distribution (i.e. Aj( t) = gammaj( t) and
qj = 1) is assumed, the model is equivalent to the model of
Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981), with
piece-wise constant parameters. A special case that may be more
appropriate than the exponential distribution is that when the
Gompertz distribution is assumed (i.e. Aj( t) = {1 - exp[-gammaj t)]}
/gammaj).
In the alternative model for exposure to diesel exhaust, in which
the total time is divided into five (i.e. k = 5) sub-intervals, Tan
& Chen (1992) showed that, under the assumption of exponential cell
lifetime distribution, the tumour-free distribution function, Sx( t),
can be written:
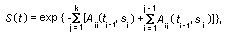 where sj = tj if j < k and sj = t if j = k, and
where sj = tj if j < k and sj = t if j = k, and
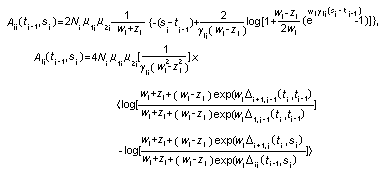 where,
WI = [alpha + ß + µ2 q)2 - 4alphaß]´
ZI = alpha - ß - µ2 q, and
DeltaI( s,t) = gammai( t - s) if both s and t are in the same closed
sub-interval [ ti-1, ti] and
where,
WI = [alpha + ß + µ2 q)2 - 4alphaß]´
ZI = alpha - ß - µ2 q, and
DeltaI( s,t) = gammai( t - s) if both s and t are in the same closed
sub-interval [ ti-1, ti] and
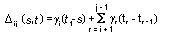 if sepsilon Li, tepsilon Lj with tj < tj.
B11. RECOMMENDATIONS
B11.1 Recommendations for the protection of human health
Diesel exhaust contributes to the total effect of combustion
products on the environment. The data reviewed in this monograph
support the conclusion that inhalation of diesel exhaust is of concern
with respect to both neoplastic and non-neoplastic diseases. The
particulate phase appears to have the greatest effect on health, and
both the particle core and the associated organic materials have
biological activity, although the gas-phase components cannot be
disregarded. The following actions are recommended for the protection
of human health.
-- Diesel exhaust emissions should be controlled as part of the
overall control of atmospheric pollution, particularly in urban
environments.
-- Emissions should be controlled strictly by regulatory inspections
and prompt remedial action.
-- Urgent efforts should be made to reduce emissions, specifically
of particulates, by changing exhaust train techniques, engine
design, and fuel composition.
-- In the occupational environment, good work practices should be
encouraged, and adequate ventilation must be provided to prevent
excessive exposure.
B11.2 Recommendations for the protection of the environment
Too few data are available on which to base specific suggestions
with regard to diesel exhaust, except as part of the general control
of emissions.
B11.3 Recommendations for further research
The following recommendations are intended to help reduce the
uncertainty associated with assessing the risks of exposure to diesel
exhaust for human health.
-- Research is required on methods for determining concentrations of
diesel particulates in the presence of fine particles from other
sources, in order to improve assessments of the exposures of
occupational groups and the general population indoors and
outdoors. The quality and quantity of emissions from engines that
are not properly maintained or tuned should be investigated as
part of these studies.
-- The effects of diesel exhaust emissions on lung clearance should
be studied in animals and humans at concentrations including
those likely to be encountered by humans.
-- The mechanisms involved in the etiology of tumours induced in
rats exposed to particulate must be investigated by all available
techniques.
-- The relative roles of the gas and particulate phases of diesel
exhaust in causing adverse effects after short- and long-term
exposure should be investigated.
-- Epidemiological investigations into the effects of diesel exhaust
should be continued in order to assess issues of dose and latency
in human populations with respect to lung cancer; and longer-term
studies should be conducted in populations exposed to diesel
exhaust with respect to noncarcinogenic effects.
-- Further steps should be undertaken to improve the combustion and
exhaust emission characteristics of diesel engines.
B12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks for human beings were evaluated by a
working group convened by the International Agency for Research on
Cancer in 1988 (International Agency for Research on Cancer, 1989b).
The conclusions were:
'There is sufficient evidence for the carcinogenicity in
experimental animals of whole diesel engine exhaust.
'There is inadequate evidence for the carcinogenicity in
experimental animals of gas-phase diesel engine exhaust (with
particles removed).
'There is sufficient evidence for the carcinogenicity in
experimental animals of extracts of diesel engine exhaust particles.
'There is limited evidence for the carcinogenicity in humans of
diesel engine exhaust.
'There is limited evidencefor the carcinogenicity in humans of
engine exhausts (unspecified as from diesel or gasoline engines).
'Overall evaluation
'Diesel engine exhaust is probably carcinogenic to humans (Group2A).'
REFERENCES
Acher AJ, Boderie P, & Yaron B (1989) Soil pollution by petroleum
products: I. Multiphase migration of kerosene components in soil
columns. J Contam Hydrol, 4: 333-345.
Ackman RG & Noble D (1973) Steam distillation: A simple technique for
recovery of petroleum hydrocarbons from tainted fish. J Fish Resour
Board Can, 30: 711-714.
Agency for Toxic Substances and Disease Registry (1995) Toxicological
profile for fuel oils. Atlanta, GA, 204 pp.
Ahlberg J, Ahlbom A, Lipping H, Norell S, & Österblom L (1981) [Cancer
among professional drivers.] Laekartidn, 78: 1545-1546 (in Danish).
Albrechcinski TM, Michalovic JG, Wattle BJ, & Wilkinson EP (1985) A
laboratory investigation of the fate of diesel emissions in the
atmosphere. Task 2 (Final Report No. CRC-APRAC-CAPA-13-76-05;
PB 86-215837). Buffalo, NY, Calspan Corp., 163 pp.
Amann CA & Siegla DC (1982) Diesel particulates -- What they are and
why. Aerosol Sci Technol, 1: 73-101.
American Automobile Manufacturers Association (1993) World motor
vehicle data. Detroit, pp 9, 50, 51, 335.
American Petroleum Institute (1979) Teratology study in rats, diesel
fuel (Publication No. 27-32174). Washington DC, 23 pp.
American Petroleum Institute (1980a) Acute toxicity tests:
API 78-3, No. 2 home heating oil (10% cat.) (Publication
No. 27-32773). Washington DC, 52 pp.
American Petroleum Institute (1980b) Acute toxicity tests:
API 78-2, No. 2 home heating oil (30% cat.) (Publication
No. 27-32771). Washington DC, 48 pp.
American Petroleum Institute (1980c) Acute toxicity tests:
API 78-4, No. 2 home heating oil (50% cat.) (Publication
No. 27-32068). Washington DC, 52 pp.
American Petroleum Institute (1980d) Acute toxicity tests. API jet
fuel A (Publication no. 27-32815). Washington DC, 48 pp.
American Petroleum Institute (1980e) Mutagenicity evaluation of jet
fuel A in the mouse dominant lethal assay. Final report (Publication
No. 28-31345). Washington DC, 36 pp.
American Petroleum Institute (1981) Mutagenicity evaluation of diesel
fuel in the mouse dominant lethal assay, final report (Publication
No. 33-30493). Washington DC, 9 pp.
American Petroleum Institute (1982a) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32347). Washington DC, 24 pp.
American Petroleum Institute (1982b) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-10 (Publication
No. 30-32348). Washington DC, 26 pp.
American Petroleum Institute (1982c) Acute toxicity studies
hydrodesulfurized kerosine. Sample 81-07 (Publication No. 30-31986).
Washington DC, 112 pp.
American Petroleum Institute (1983a) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32856). Washington DC, 47 pp.
American Petroleum Institute (1983b) 28-Day dermal toxicity study in
the rabbit: Hydrodesulfurized middle distillate, API sample 81-10
(Publication No. 30-32298). Washington DC, 13 pp.
American Petroleum Institute (1983c) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats (Publication
no. 30-32855). Washington DC, 32 pp.
American Petroleum Institute (1984) Dermal sensitization study in
guinea pig, closed patch technique: Hydrodesulfurized middle
distillate, sample 81-10 (Publication No. 31-31414). Washington DC,
12 pp.
American Petroleum Institute (1985a) 28-Day dermal toxicity study in
the rabbit: API 83-08, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 32-32753). Washington DC, 27 pp.
American Petroleum Institute (1985b) 28-Day dermal toxicity study in
the rabbit: API 83-11, straight run middle distillate (CAS 64741-59-9)
(Publication No. 32-32747). Washington DC, 29 pp.
American Petroleum Institute (1985c) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs: API 83-07, light
catalytically cracked distillate (CAS 64741-59-9) (Publication
No. 33-30162). Washington DC, 41 pp.
American Petroleum Institute (1985d) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs, API 83-11, straight run
middle distillate (CAS 64741-44-2) (Publication No. 32-32857).
Washington DC, 34 pp.
American Petroleum Institute (1985e) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs. API 83-09, straight run
kerosine (CAS No. 8008-20-6) (Publication No. 32-32858). Washington
DC, 39 pp.
American Petroleum Institute (1985f) The evaluation of the
carcinogenicity of certain petroleum fractions (Publication
No. 32-30964). Washington DC, 148 pp.
American Petroleum Institute (1986a) Acute inhalation toxicity
evaluation in rats: API 83-07, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 33-30549). Washington DC, 15 pp.
American Petroleum Institute (1986b) Acute inhalation toxicity
evaluation in rats: API 83-08, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 33-30444). Washington DC, 16 pp.
American Petroleum Institute (1986c) Four week subchronic inhalation
toxicity study in rats -- final report: API 81-07, hydrodesulfurized
kerosene (petroleum) (CAS 64742-81-0); API 81-09, hydrodesulfurized
middle distillate (petroleum) (CAS 64742-80-9); API 81-10,
hydrodesulfurized middle distillate (petroleum) (CAS 64742-80-9)
(Publication No. 33-32724). Washington DC, 31 pp.
American Petroleum Institute (1987a) Acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats: API 83-11,
straight run middle distillate (CAS 64741-59-9) (Publication
No. 34-30635). Washington DC, 53 pp.
American Petroleum Institute (1987b) Acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats -- API 83-09.
Final report (Publication No. 34-30634). Washington DC, 10 pp.
American Petroleum Institute (1987c) Mutagenicity of API 81-10,
hydrode-sulfurized middle distillate (CAS 64742-80-9) (coded as
API 86-10) in a mouse lymphoma mutation assay. Final report
(Publication No. 34-32643). Washington DC, 21 pp.
American Petroleum Institute (1989a) Twenty-four month dermal
carcinogenesis/chronic toxicity screening bioassay of refinery streams
in C3H/ Hej mice. API 190r. Final report: lifetime carcinogenicity
evaluation (Publication No. 36-33220). Washington DC, 26 pp.
American Petroleum Institute (1989b) Lifetime dermal
carcinogenesis/chronic toxicity screening bioassay of refinery streams
in C3H/HE mice. API 135r. Final report: lifetime carcinogenicity
evaluation (Publication No. 36-31364). Washington DC, 41 pp.
American Society for Testing and Materials (1988) ASTM Designation:
D 975-81. Standard specification for diesel fuel oils. Philadelphia,
Committee on Standards, pp 351-361.
American Society for Testing and Materials (1992) ASTM Designation:
D 975-92a. Standard specification for diesel fuel oils. Philadelphia,
Committee on Standards, pp 310-311.
Ames RG, Attfield MD, Hankinson JL, Hearl FJ, & Reger RB (1982) Acute
respiratory effects of exposure to diesel emissions in coal miners.
Am Rev Respir Dis, 125: 39-42.
Ames RG, Hall DS, & Reger RB (1984) Chronic respiratory effects of
exposure to diesel emissions in coal mines. Arch Environ Health,
39: 389-394.
Anderson JW (1977a) Effects of petroleum hydrocarbons on the growth of
marine organisms. In: McIntyre AD & Whittle KJ, eds, Petroleum
hydrocarbons in the marine environment (Rapports et proces-verbaux des
réunions, Vol. 171). Copenhagen, International Council for the
Exploration of the Sea, pp 157-165.
Anderson JW (1977b) Responses to sublethal levels of petroleum
hydrocarbons: Are they sensitive indicators and do they correlate with
tissue contamination? In: Wolfe DA, ed., Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, Pergamon
Press, pp 95-114.
Anderson JW (1979) An assessment of knowledge concerning the fate and
effects of petroleum hydrocarbons in the marine environment. In:
Vernberg WB, Calabrese A, Thurberg FP, & Vernberg FJ, eds, Marine
pollution: Functional responses. New York, Academic Press, pp 3-21.
Anderson JW, Neff JM, Cox BA, Tatem HE, & Hightower GM (1974)
Characteristics of dispersions and water-soluble extracts of crude and
refined oils and their toxicity to estuarine crustaceans and fish. Mar
Biol, 27: 75-88.
Anderson JW, Dixit DB, Ward GS, & Foster RS (1976) Effects of
petroleum hydrocarbons on the rate of heart beat and hatching success
of estuarine fish embryos. In: Vernberg FJ & Vernberg WB, eds,
Pollution and physiology of marine organisms, Vol. 2. New York,
Academic Press, pp 241-258.
Anderson E, Albert R, Anderson L, Bayliss D, Bayard S, Chen C, Chu M,
Gibb H, Haberman B, Hiremath C, McGaughy R, Singh D, & Thorslund T
(1983) Quantitative approaches in use to assess cancer risk.
Risk Anal, 3: 277-295.
Apol A (1983) Health hazard evaluation. Regional transportation
district; Denver (Report No. HETA 82-137-1264; PB84-209386).
Cincinnati, OH, National Institute for Occupational Safety and Health,
pp 1-23.
Atlas RM & Bartha R (1973) Fate and effects of polluting petroleum in
the marine environment. Res Rev, 49: 49-84.
Atlas RM, Boehm PD, & Calder JA (1981) Chemical and biological
weathering of oil, from the Amoco Cadiz oil spillage, within the
littoral zone. Estuarine Coastal Mar Sci, 12: 589-608.
Attfield MD, Trabant GD, & Wheeler RW (1982) Exposure to diesel fumes
and dust at six potash mines. Ann Occup Hyg, 26: 817-831.
Auerbach O & Garfinkel L (1991) The changing pattern of lung
carcinoma. Cancer, 68: 1973-1977.
Bailey MR, Fry FA, & James AC (1985a) Long-term retention of particles
in the human respiratory tract. J Aerosol Sci, 16: 295-305.
Bailey MR, Hodgson A, & Smith H (1985b) Respiratory tract retention of
relatively insoluble particles in rodents. J Aerosol Sci, 16: 279-293.
Baker JM (1970) The effect of fuel oils on plants. Environ Pollut,
1: 27-44.
Ball JC & Young WC (1992) Evidence for a new class of mutagens in
diesel particulate extracts. Environ Sci Technol, 26: 2181-2186.
Ball JC, Greene B, Young WC, Richert JFO, & Salmeen IT (1990)
S9-Activated Ames assays of diesel-particle extracts. Detecting
indirect-acting mutagens in samples that are direct-acting. Environ
Sci Technol, 24: 890-894.
Barbella R, Bertoli C, Ciajolo A, & D'Anna A (1988) Soot and unburnt
liquid hydrocarbon emissions from diesel engines. Combust Sci Technol,
59: 183-198.
Barfknecht TR, Andon BM, Thilly WG, & Hites RA (1981) Soot and
mutation in bacteria and human cells. In: Cooke M & Dennis AJ, eds,
Chemical analysis and biological fate: Polynuclear aromatic
hydrocarbons. Columbus, OH, Battelle Press, pp 231-242.
Barnett CJ & Kontogiannis JE (1975) The effect of crude oil fractions
on the survival of a tidepool copepod, Tigriopus californicus.
Environ Pollut, 8: 45-54.
Barnhart MI, Chen S-T, Salley SO, & Puro H (1981) Ultrastructure and
morphometry of the alveolar lung of guinea pigs chronically exposed to
diesel engine exhaust: six months' experience. J Appl Toxicol,
1: 88-103.
Barnhart MI, Salley SO, Chen S-T, & Puro H (1982) Morphometric
ultrastructural analysis of alveolar lungs of guinea pigs chronically
exposed by inhalation to diesel exhaust (DE). In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. New York,
Elsevier, pp 183-200.
Barrientos A, Ortuno MT, Morales JM, Martinez Tello F, & Rodicio JL
(1977) Acute renal failure after use of diesel fuel as shampoo. Arch
Int Med, 137: 1217.
Battigelli MC (1965) Effects of diesel exhaust. Arch Environ Health,
10: 165-167.
Bauer MA, Utell MJ, Morrow PE, Speers DM, & Gibb FR (1986) Inhalation
of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm
in asthmatics. Am Rev Respir Dis, 134: 1203-1208.
Bauer HD, Dahmann D, Kollmeier FW, & Lindecke B (1990) [Diesel engine
emissions in potash-salt mines.] Kompass, 100: 516-524 (in German).
Bechtold WE, Henderson TR, & Brooks AL (1986) Isolation,
identification and bacterial mutagenicity of 2-nitro-9-fluorenone from
diesel-exhaust particle extracts. Mutat Res, 173: 105-109.
Beck LS, Hepler DI, & Hansen KL (1984) The acute toxicology of
selected petroleum hydrocarbons. In: MacFarland HN, Holdsworth CE,
MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the symposium:
The toxicology of petroleum hydrocarbons, May 1984. Washington DC,
American Petroleum Institute, pp 1-12.
Behn U, Kaschani DT, Lützke K Rentel S & Burk HD (1985) ['PAH-sample
system' for industrial plants. Trial and first results.] Erdöl Kohle
Erdgas Petrochem, 38: 131 (in German).
Behymer T & Hites R (1984) Similarity of some organic compounds in
spark-ignition and diesel engine particulate extracts. Environ Sci
Technol, 18: 203-206
Beland FA, Heflich RH, Howard PC, & Fu PP (1985) The in vitrometabolic
activation of nitro polycyclic aromatic hydrocarbons. In: Harvey RG,
ed., Polycyclic hydrocarbons and carcinogenesis. Washington DC,
American Chemical Society, pp 371-396.
Beliles RP & Mecler FJ (1982) Inhalation teratology of jet fuel A,
fuel oil and petroleum naphtha in rats. In: MacFarland HN, Holdsworth
CE, MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the
symposium: The toxicology of petroleum hydrocarbons, May 1982.
Washington DC, American Petroleum Institute, pp 234-238.
Bellmann B, Muhle H, Creutzenberg O, & Mermelstein R (1992)
Irreversible pulmonary changes induced in rat lung by dust overload.
Environ Health Perspectives, 97: 189-191.
Benhamou S, Benhamou E, & Flamant R (1988) Occupational risk factors
of lung cancer in a French case-control study. Br J Ind Med,
45: 231-233.
Bennett D, Girling AE, & Bounds A (1990) Ecotoxicology of oil
products: Preparation and characterisation of aqueous test media.
Chemosphere, 21: 659-669.
Bevan DR & Ruggio DM (1991) Bioavailability in vivo of benzo[ a]pyrene
adsorbed to diesel particulate. Toxicol Ind Health, 7: 125-139.
Bice DE, Mauderly JL, Jones RK, & McClellan RO (1985) Effects of
inhaled diesel exhaust on immune responses after lung immunization.
Fundam Appl Toxicol, 5: 1075-1086.
Biles R, McKee RH, Lewis SC, Scala RA, & DePass LR (1988) Dermal
carcinogenic activity of petroleum-derived middle distillate fuels.
Toxicology, 53: 301-314.
Blackburn GR, Deitch RA, Schreiner CA, Mehlman MA, & Mackerer CR
(1984) Estimation of the dermal carcinogenic activity of petroleum
fractions using a modified Ames assay. Cell Biol Toxicol, 1: 67-80.
Block RN, Allworth N, & Bishop M (1991) Assessment of diesel
contamination in soil. In: Calabrese EJ & Kostecki PT, eds,
Hydrocarbon contaminated soils, Vol. I. Remediation techniques,
environmental fate, risk assessment, analytical methodologies,
regulatory considerations. Chelsea, MI, Lewis Publishers, pp 135-148.
Blome H, Heidermanns G, & Timmer J (1990) [Assessment of workplace
atmospheres in case of diesel motor vehicles.] Staub Reinhalt Luft,
50: 93-97 (in German).
Blumer M, Souza G, & Sass J (1970) Hydrocarbon pollution of edible
shellfish by an oil spill. Mar Biol, 5: 195-202.
Boehm PD & Quinn JG (1974) The solubility behavior of No. 2 fuel oil
in sea water. Mar Pollut Bull, 5: 101-105.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Boffetta P, Harris RE, & Wynder EL (1990) Case-control study on
occupational exposure to diesel exhaust and lung cancer risk. Am J Ind
Med, 17: 577-591.
Bohning D, Atkins H, & Cohn S (1982) Long-term particle clearance in
man: Normal and impaired. Ann Occup Hyg, 26: 259-271.
Bokn T (1987) Effects of diesel oil and subsequent recovery of
commercial benthic algae. Hydrobiology, 151-152: 277-284.
Bond JA, Mitchell CE, & Li AP (1983) Metabolism and macromolecular
covalent binding of benzo[ a]pyrene in cultured Fischer-344 rat lung
type II epithelial cells. Biochem Pharmacol, 32: 3771-3776.
Bond JA, Butler MM, Medinsky MA, Muggenburg BA, & McClellan RO (1984)
Dog pulmonary macrophage metabolism of free and particle-associated
[14C]benzo[a]pyrene. J Toxicol Environ Health, 14: 181-189.
Bond JA, Sun JD, Medinsky MA, Jones RK, & Yeh HC (1986a) Deposition,
metabolism, and excretion of 1-[14C]nitropyrene and 1-[14C]nitropyrene
coated on diesel exhaust particles as influenced by exposure
concentration. Toxicol Appl Pharmacol, 85: 102-117.
Bond JA, Sun JD, Mitchell CE, Dutcher JS, Wolff RK, & McClellan RO
(1986b) Biological fate of inhaled organic compounds associated with
particulate matter. In: Lee SD, Schneider D, Grant JD, & Verkerk PJ,
eds, Aerosols. Chelsea, MI, Lewis Publishers, pp 579-592.
Bond JA, Wolff RK, Harkema JR, Mauderly JL, Henderson RF, Griffith WC,
& McClellan RO (1988) Distribution of DNA adducts in the respiratory
tract of rats exposed to diesel exhaust. Toxicol Appl Pharmacol,
96: 336-346.
Bond JA, Harkema JR, Henderson RF, Mauderly JL, McClellan RO, & Wolff
RK (1989) Molecular dosimetry of inhaled diesel exhaust. In: Mohr U,
Bates DV, Dungworth DL, Lee PN, McLellan RO, & Roa FJC, eds,
Assessment of inhalation hazards: Integration and extrapolation using
diverse data. Berlin, Springer, pp 315-324.
Bond JA, Johnson NF, Snipes MB, & Mauderly JL (1990a) DNA adduct
formation in rat alveolar type II cells: Cells potentially at risk for
inhaled diesel exhaust. Environ Mol Mutag, 16: 64-69.
Bond JA, Harkema JR, Henderson RF, Mauderly JL, McClellan RO, & Wolff
RK (1990b) The role of DNA adducts in diesel exhaust-induced pulmonary
carcinogenesis. Prog Clin Biol Res, 340C: 259-269.
Bond JA, Mauderly JL, & Wolff RK (1990c) Concentration- and
time-dependent formation of DNA adducts in lungs of rats exposed to
diesel exhaust. Toxicology, 60: 127-135.
Booth M & Reglitzky AA (1991) Diesel fuel quality in an
environmentally conscious world. In: The diesel engine -- energy stake
and environmental constraints. Cologne, TÜV Rheinland-OPET,
pp 289-299.
Boudet F, Fabre M, Boe M, Delon M, Ruiz J, & Lareng L (1983) Toxic
pneumopathy after voluntary ingestion of one-and-a-half litres of
diesel fuel. Toxicol Eur Res, 5: 247-249.
Braddock JN & Perry NK Jr (1986) Gaseous and particulate emissions
from gasoline- and diesel-powered heavy duty trucks (Paper
No. 860617). In: International Congress and Exposition, Detroit, MI,
24-28 February 1986. Warrendale, PA, Society of Automotive Engineers,
39 pp.
Brightwell J, Fouillet X, Cassano-Zoppi A-L, Gatz R, & Duchosal F
(1986) Neoplastic and functional changes in rodents after chronic
inhalation of engine exhaust emissions. In: Ishinishi N, Koizumi A,
McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects of
diesel engine exhaust. Amsterdam, Elsevier, pp 471-485.
Brightwell J, Fouillet X, Cassano-Zoppi A-L, Bernstein D, Crawley F,
Duchosal F, Gatz R, Perczel S, & Pfeifer, H (1989) Tumours of the
respiratory tract in rats and hamsters following chronic inhalation of
engine exhaust emissions. J Appl Toxicol, 9: 23-31.
Broman D, Näf C, Lundbergh I, & Zebühr Y (1990) An in situ study on
the distribution, biotransformation and flux of polycyclic aromatic
hydrocarbons (PAHs) in an aquatic food chain (seston Mytilus edulis L.
Somateria mollissima L.) from the Baltic: An ecotoxicological
perspective. Environ Toxicol Chem, 9: 429-442.
Bruner RH (1984) Pathologic findings in laboratory animals exposed to
hydrocarbon fuels of military interest. In: Mehlman MA, Hemstreet GP,
Thorpe JT, & Weaver NK, eds, Renal effects of petroleum hydrocarbons
(Advances in modern environmental toxicology, Vol. 7). Princeton, NJ,
Princeton Scientific Publishers, pp 133-140.
Bruner RH, Kinkead ER, O'Neill TP, Flemming CD, Mattie DR, Russell CA,
& Wall HG (1993) The toxicologic and oncogenic potential of JP-4 jet
fuel vapors in rats and mice: 12-month intermittent inhalation
exposures. Fundam Appl Toxicol, 20: 97-110.
Bury RB (1972) The effects of diesel fuel on a stream fauna. Calif
Fish Game, 58: 291-295.
Byrne CJ & Calder JA (1977) Effect of the water-soluble fractions of
crude, refined and waste oils on the embryonic and larval stages of
the Quahog clam Mercenaria sp. Mar Biol, 40: 225-231.
Campbell J, Scholl J, Hibbler F, Bagley S, Leddy D, Abata D, & Johnson
J (1981a) The effect of fuel injection and timing on the physical,
chemical, and biological character of particulate emissions from a
direct injection diesel. In: Diesel combustion and emissions,
Part III, SP-495. International Off-Highway Meeting and Exposition,
Milwaukee, Wisconsin, 14-17 September 1981. Warrendale, PA, Society of
Automotive Engineers, pp 71-108.
Campbell KI, George EI, & Washington IS Jr (1981b) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ Int, 5: 377-382.
Campobosso O, Andrion A, Ribotta M & Ronco G (1993) The value of the
1981 WHO histological classification in inter-observer reproducibility
and changing pattern of lung cancer. Int J Cancer, 53:205-208
Carey PM (1987) Air toxics emissions from motor vehicles (Technical
report) (EPA-AA-TSS-PA-86-5). Ann Arbor, MI, US Environmental
Protection Agency, 129 pp.
Carpenter CP, Geary DL Jr, Myers RC, Nachreiner DJ, Sullivan LJ, &
King JM (1976) Petroleum hydrocarbon toxicity studies. XI. Animal and
human response to vapors of deodorized kerosene. Toxicol Appl
Pharmacol, 36: 443-456.
Carr RS & Reish DJ (1977) The effect of petroleum hydrocarbons on the
survival and life history of polychaetous annelids. In: Wolfe D, ed.,
Fate and effects of petroleum hydrocarbons in marine ecosystems and
organisms. Oxford, Pergamon Press, pp 168-173.
Carstens T & Sendstad E (1979) Oil spill on the shore of an
ice-covered fjord in Spitzbergen. In: Proceedings of the 79th
international conference on port and ocean engineering under Arctic
conditions, Trondheim, 13-18 August. Trondheim, Norwegian Institute of
Technology, pp 1227-1242.
Casto BC, Hatch GG, Huang SL, Lewtas J, Nesnow S, & Waters MD (1981)
Mutagenic and carcinogenic potency of extracts of diesel and related
environmental emissions: In vitro mutagenesis and oncogenic
transformation. Environ Int, 5: 403-409.
Castranova V, Bowman L, Reasor MJ, Lewis T, Tucker J, & Miles PR
(1985) The response of rat alveolar macrophages to chronic inhalation
of coal dust and/or diesel exhaust. Environ Res, 36: 405-419.
Chan K-Y & Chiu SY (1985) The effects of diesel oil and oil
dispersants on growth, photosynthesis, and respiration of Chlorella
salina. Arch Environ Contam Toxicol, 14: 325-331.
Chan TL, Lee PS, & Hering WE (1981) Deposition and clearance of
inhaled diesel exhaust particles in the respiratory tract of Fischer
rats. J Appl Toxicol, 1: 77-82.
Chan TL, Lee PS, & Hering WE (1984) Pulmonary retention of inhaled
diesel particles after prolonged exposures to diesel exhaust. Fundam
Appl Toxicol, 4: 624-631.
Chen B & Farland W (1991) Incorporating cell proliferation in
quantitative cancer risk assessment: Approach, issues, and
uncertainties. In: Butterworth B, Slaga T, Farland W, & McClain M,
eds, Chemically induced cell proliferation: Implications for risk
assessment. New York, Wiley-Liss, pp 481-499.
Chescheir GM, Garrett NE, Shelburne JD, Huisingh JL, & Waters MD
(1981) Mutagenic effects of environmental particulates in the
CHO\HGPRT system In: Waters MD, Sandhu SS, Huisingh JL, Claxton L, &
Nesnow S, eds, Short-term bioassays in the analysis of complex
environmental mixtures II, Vol. 2. New York, Plenum Press, pp 337-350.
Chia F-S (1973) Killing of marine larvae by diesel oil. Mar Pollut
Bull, 4: 29-30.
Clark CR & Vigil CL (1980) Influence of rat lung and liver homogenates
on the mutagenicity of diesel exhaust particulate extracts. Toxicol
Appl Pharmacol, 56: 110-115.
Clark CR, Walter MK, Ferguson PW, & Katchen, M (1988) Comparative
dermal carcinogenesis of shale and petroleum-derived distillates.
Toxicol Ind Health, 4: 11-22.
Cohen BL (1994) Invited commentary: In defense of ecologic studies for
testing a linear no threshold theory. Am J Epidemiol, 139: 765-768.
Cohen S & Ellwein L (1988) Cell growth dynamics in long-term bladder
carcinogenesis. Toxicol Lett, 43: 151-173.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
The identification and measurement of components in gasoline,
kerosene, and No. 2 fuel oil that partition into the aqueous phase
after mixing. Arch Environ Contam Toxicol, 13: 171-178.
Coley TR (1989) Diesel fuel additives influencing flow and storage
properties. In: Owen K, ed., Gasoline and diesel fuel additives. New
York, John Wiley & Sons, pp 105-132.
Commins BT, Waller RE, & Lawther PJ (1956) Smoke in a London diesel
bus garage. An interim report. Br Med J, ii: 753-754.
Conaway CC, Schreiner CA, & Cragg ST (1984) Mutagenicity evaluation of
petroleum hydrocarbons. In: MacFarland HN, Holdsworth CE, MacGregor
JA, Call RW, & Lane ML, eds, Applied toxicology of petroleum
hydrocarbons (Advances in modern environmental toxicology, Vol 6).
Princeton, NJ, Princeton Scientific Publishers, pp 89-107.
CONCAWE (1985) Health aspects of petroleum fuels. Potential hazards
and precautions for individual classes of fuels (Report No 85/51). The
Hague, 54 pp.
CONCAWE (1986) The relationship between automotive diesel fuel
characteristics and engine performance (Report No. 86/65). The Hague,
30 pp.
CONCAWE (1987) Diesel fuel quality and its relationship with emissions
from diesel engines (Report No. 10/87). The Hague, 21 pp.
CONCAWE (1990a) Motor vehicle emission regulations and fuel
specifications -- 1990 update (Report No. 2/90). Brussels, Health
Management Group, 92 pp.
CONCAWE (1990b) The sulphur content of diesel fuel and its
relationship with particulate emissions from diesel engines (Report
No. 90/54). Brussels, Health Management Group, 42 pp.
CONCAWE (1991) Middle distillates -- A review of the results of a
CONCAWE programme of short-term biological studies (Report No. 91/51).
Brussels, Health Management Group, pp 1-13.
CONCAWE (1992) The chemical composition of diesel particulate
emissions (Report No. 92/51). Brussels, Health Management Group,
24 pp.
CONCAWE (1993) Ecotoxicological testing of petroleum products: test
methodology (Report No. 92/56). Brussels, Petroleum Products Ecology
Group, 18 pp.
CONCAWE (1995) Kerosines/jet fuels (Product No. 94/106). Brussels,
Petroleum Products and Health Managements Groups, 13 pp.
CONCAWE (in press) Gas oils (diesel fuels/heating oils) Brussels,
Petroleum Products and Health Management Groups, 45 pp.
Cooper JR & Mattie DR (1993) Abstracts of the 32nd Annual Meeting of
the Society of Toxicology. Toxicologist, 13: 78.
Cordier S, Clavel J, Limaset JC, Boccon-Gibod L, Le Moual N, Mandereau
L, & Hermon D (1993) Occupational risks of bladder cancer in France: A
multicentre case-control study. Int J Epidemiol, 22: 403-411.
Corner EDS, Harris RP, Kilvington CC, & O'Hara SCM (1976) Petroleum
compounds in the marine food web: Short-term experiments on the fate
of naphthalene in Calanus. J Mar Biol, 56: 121-133.
Cornwell RJ (1982) Health hazard evaluation determination report.
Climax Molybdenum Company, Climax, CO (PB84-14850 1; Report
No MHETA-81-108-9004). Morgantown, WV, National Institute for
Occupational Safety and Health, 23 pp.
Cowan MJ & Jenkins, LJ (1980) Navy toxicity study of shale and
petroleum JP-5 aviation fuel and diesel fuel marine. In: Griest WH,
Guerin MR, & Coffin DL, eds, Health effects investigation of oil shale
development. Ann Arbor, MI, Ann Arbor Science Publishers, pp 129-139.
Cox BA (1974) Responses of the marine crustaceans Mysidopsis almyra
Bowman, Penaeus aztecus Ives, and Penaeus setiferus (L.) to
petroleum hydrocarbons. Diss Abstr Int, B35: 3739.
Cox BA, Anderson JW, & Parker JC (1975) An experimental oil spill: The
distribution of aromatic hydrocarbons in the water, sediment, and
animal tissues within a shrimp pond. In: Proceedings of the Conference
on Prevention and Control of Oil Pollution, 25-27 March 1975, San
Francisco, California. Washington DC, American Petroleum Institute,
pp 607-612.
Crebelli R, Fuselli S, Conti G, Conti L, & Carere A (1991)
Mutagenicity spectra in bacterial strains of airborne and engine
exhaust particulate extracts. Mutat Res, 261: 237-248.
Creutzenberg O, Bellmann B, Heinrich U, Fuhst R, Koch W, & Muhle H
(1990) Clearance and retention of inhaled diesel exhaust particles,
carbon black, and titanium dioxide in rats at lung overload
conditions. J Aerosol Sci, 21: 455-458.
Crisp AJ, Bhalla AK, & Hoffbrand BI (1979) Acute tubular necrosis
after exposure to diesel oil. Br Med J, ii: 177.
Croxall JP (1977) The effects of oil on seabirds. In: McIntyre AD &
Whittle KJ, eds, Petroleum hydrocarbons in the marine environment
(Rapports et proces-verbaux des réunions, Vol. 171). Copenhagen,
International Council for Exploration of the Sea, pp 191-195.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Csicsaky M, Roller M, & Pott F (1993) [Quantitative risk assessment of
selected carcinogenic substances in the work area (Special publication
No. S/31)]. Dortmund, Federal Institute for Labour Protection,
pp 18-29 (in German).
Cuddihy RG, Griffith WC, & McClellan RO (1984) Health risks from
light-duty diesel vehicles. Low risk of lung cancer from exposure to
diesel exhaust is projected from laboratory and epidemiologic data.
Environ Sci Technol, 18: 14A-21A.
Curren RD, Kouri RE, Kim CM, & Schechtman LM (1981) Mutagenic and
carcinogenic potency of extracts from diesel related environmental
emissions: Simultaneous morphological transformation and mutagenesis
in BALB/c3T3 cells. Environ Int, 5: 411-415.
Custance SR, Sullivan MJ, McCaw PA, & Kopf AC (1993) Environmental
fate of the chemical mixtures: Crude oil, JP-5, mineral spirits, and
diesel fuel. In: Kostecki PT, & Calabrese EJ, eds, Hydrocarbon
contaminated soils and groundwater, Vol. 3. Boca Raton, FL, Lewis
Publishers, pp 205-212.
Dalbey W, Lock S, Garfinkel S, Jenkins R, Holmberg R, & Guerin M
(1982) Inhalation exposures of rats to aerosolized diesel fuel. In:
MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Kane ML, eds,
Proceedings of the symposium on toxicology of petroleum hydrocarbons.
Washington DC, American Petroleum Institute, pp 13-25.
Dalbey W, Henry M, Holmberg R, Moneyhun J, Schmoyer R, & Lock S (1987)
Role of exposure parameters in toxicity of aerosolized diesel fuel in
the rat. J Appl Toxicol, 7: 265-275.
Damber LA & Larsson LG (1987) Occupation and male lung cancer: A
case-control study in northern Sweden. Br J Ind Med, 44: 446-453.
Dambo WB (1993) Tolerance of the periwinkles Pachymelania aurita
(Muller) and Tympanotonus fuscatus (Linne) to refined oils. Environ
Pollut, 79: 293-296.
Daniel JH Jr (1984) Diesels in underground mining: A review and an
evaluation of an air quality monitoring methodology (Document
No. Rl-8884; US NTIS PB84-214444). Washington DC, US Department of the
Interior, Bureau of Mines.
Das PKMK & Konar SK (1988) Acute toxicity of petroleum products, crude
oil and oil refinery effluent on plankton, benthic invertebrates and
fish. Environ Ecol, 6: 885-891.
Das M & Misra MP (1988) Acne and folliculitis due to diesel oil.
Contact Derm, 18: 120-121.
Davis SJ & Gibbs CF (1975) The effect of weathering on a crude oil
residue exposed at sea. Water Res, 9: 275-285.
Davison W, Franklin CE, McKenzie JC, & Dougan MCR (1992) The effects
of acute exposure to the water soluble fraction of diesel fuel oil on
survival and metabolic rate of an Antarctic fish ( Pagothenia
borchgrevinki). Comp Biochem Physiol C, 102: 185-188.
Davison W, Franklin CE, McKenzie JC, & Carey PW (1993) The effects of
chronic exposure to the water soluble fraction of fuel oil on an
Antarctic fish Pagothenia borchgrevinki. Comp Biochem Physiol,
C104: 67-70.
Deichmann WB, Kitzmiller KV, Witherup S, & Johansmann R (1944)
Kerosene intoxication. Ann Intern Med, 21: 803-823.
Dempster A, Laird N, & Rubin D (1977) Maximum likelihood from
incomplete data via the EM algorithm. R Stat Soc, B39: 1-38.
Dennington VN, George JJ, & Wyborn CHE (1975) The effects of oils on
growth of freshwater phytoplankton. Environ Pollut, 8: 233-237.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Cancer Epidemiol Biomarkers Prev,
1: 29-34
Dineen D (1991) Remediation options for diesel-contaminated soil. In:
Calabrese EJ & Kostecki PT, eds, Hydrocarbon contaminated soils,
Vol. 1. Remediation techniques, environmental fate, risk assessment,
analytical methodologies, regulatory considerations. Chelsea, MI,
Lewis Publishers Inc., pp 181-191.
Dixit D & Anderson JW (1977) Distribution of naphthalenes within
exposed Fundulus similus and correlations with stress behavior. In:
Proceedings of 1977 oil spill conference, New Orleans, March 1977.
Washington DC, American Petroleum Institute.
Dockery DW, Arden Pope C III, Xu X, Spengler JD, Ware JH, Fay ME,
Ferris BG Jr, & Speizer FE (1993) An association between air pollution
and mortality in six US cities. New Engl J Med, 329: 1753-1759.
Dolan DF, Kittelson DB, & Pui, DYH (1980) Diesel exhaust particle size
distribution measurement techniques (Paper No. 800187). Warrendale,
PA, Society of Automotive Engineers, pp 149-164.
Dorie LD, Bagley ST, Leddy DG, & Johnson JH (1987) Characterization of
mutagenic subfractions of diesel exhaust modified by ceramic
particulate traps. Environ Sci Technol, 21: 757-765.
Driscoll KE, Carter JM, Howard BW, & Hassenbein DG (1994) Mutagenesis
in rat lung epithelial cells after in vivo silica exposure or
ex vivo exposure to inflammatory cells. Am J Resp Crit Care Med,
149: A553.
Driscoll KE, Carter JM, Oberdörster G, Howard BW, & Hassenbein DG
(1995) Inflammation, cytokine expression and mutagenesis in rat lung
after carbon black inhalation. Toxicologist, 15: 46.
Driscoll KE, Carter JM, Howard BW, Hassenbein D, Pepelko W, Baggs RB,
& Oberdörster G (in press) Pulmonary inflammatory, chemokine and
mutagenic responses in rats after subchronic inhalation of carbon
black. Toxicol Appl Pharmacol.
Du C-J, Kittelson DB, & Zweidinger RB (1984) Measurements of
polycyclic aromatic compounds in the cylinder of an operating diesel
engine (SAE Technical Paper No. 840364). In: International congress
and exposition, Detroit, Michigan, 27 February-2 March 1984.
Warrendale, PA, Society of Automotive Engineers, Engineering Resource
for Advancing Mobility, 12 pp.
Dungworth DL, Mohr U, Heinrich U, Ernst H, & Kittel B (1994)
Pathological effects of inhaled particles in rat lungs: Associations
between inflammatory and neoplastic processes. In: Mohr U, Dungworth
DL, Mauderly JL, & Oberdörster, eds, Toxic and carcinogenic effects of
solid particles in the respiratory tract. Washington DC, International
Life Sciences Institute Press, pp 75-98.
Dunlap LE & Beckmann DD (1988) Soluble hydrocarbons analysis from
kerosene/diesel type hydrocarbons. In: Proceedings of the conference
on petroleum hydrocarbons and organic chemicals in ground water:
Prevention, detection and restoration, 9-11 November 1988, The Westin
Galleria, Houston, Texas. Dublin, OH, National Water Well Association,
pp 37-45
Dutcher JS, Sun JD, Lopez JA, Wolf I, Wolff RK, & McClellan RO (1984)
Generation and characterization of radiolabeled diesel exhaust. Am Ind
Hyg Assoc J, 45: 491-498.
Dziedzic D (1981) Differential counts of B and T lymphocytes in the
lymph nodes, circulating blood and spleen after inhalation of high
concentrations of diesel exhaust. J Appl Toxicol, 1: 111-115.
Edgerton SA, Coutant RW, & Henley MV (1987) Hydrocarbon fuel spill
dispersion on water: a literature review. Chemosphere, 16: 1475-1487.
Edling C & Axelson O (1984) Risk factors of coronary heart disease
among personnel in a bus company. Int Arch Occup Environ Health,
54: 181-183.
Edling C, Anjou C-G, Axelson O, & Kling H (1987) Mortality among
personnel exposed to diesel exhaust. Int Arch Occup Environ Health,
59: 559-565.
Egebäck KE & Bertilsson BM (1983) Chemical and biological
characterization of exhaust emissions from vehicles fueled with
gasoline, alcohol, LPG and diesel (Report No. SNV PM 1635). Solna,
National Swedish Environment Protection Board, 188 pp.
El-Bayoumy K, Johnson BE, Roy AK, Upadhyaya P, & Partian SJ (1994)
Biomonitoring of nitropolynuclear aromatic hydrocarbons via protein
and DNA adducts (Research report No. 64). Cambridge, MA, Health
Effects Institute, pp 1-39.
Elbers G & Muratyan S (1991) [Problems of traffic-related outdoor air
measurement of particles (diesel soot) (VDI Report No. 888)]. In:
[Carcinogenic substances in the environment: Origin, measurement,
risk, minimization.
Meeting of the Commission for Clean Air of the Society of German
Engineers (VDI) and the German Standards Institute (DIN), Mannheim,
23-25 April]. Düsseldorf, Society of German Engineers Press,
pp 143-170 (in German).
Elbers G & Richter J (1994) [Measurement of traffic-induced soot
immissions.] Staub Reinhalt Luft, 54: 19-24 (in German).
Emmelin A, Nyström L, & Wall S (1993) Diesel exhaust exposure and
smoking: A case-referent study of lung cancer among Swedish dock
workers. Epidemiology, 4: 237-244.
Engelhardt FR, Wong MP, & Duey ME (1981) Hydromineral balance and gill
morphology in rainbow trout Salmo gairdneri, acclimated to fresh and
sea water, as affected by petroleum exposure. Aquat Toxicol,
1: 175-186.
Environment Canada (1990) Canadian emissions inventory of common air
contaminants -- 1985 (Environmental Protection Series No. EPS/5/AP/3),
Ottawa.
Environment Canada (1992) Summary of Canadian new vehicle emissions
standards, June 1992. Unpublished results.
Eppley ZA (1992) Assessing indirect effects of oil in the presence of
natural variation: The problem of reproductive failure in South Polar
skuas during the Bahia Paraiso oil spill. Mar Pollut Bull,
25: 307-312.
Ernst VV, Neff JM, & Anderson JW (1977) The effects of the
water-soluble fractions of No. 2 fuel oil on the early development of
the estuarine fish, Fundulus grandis Baird and Girard. Environ
Pollut, 14: 25-35.
European Commission (1993) Guideline 93/12/EEC of the Council from 23
March 1993 on sulfur content of certain liquid fuels (EEC Directive,
L74/81), Luxembourg, 3 pp.
European Committee for Standardization (1993) [Automotive fuels,
diesel. Requirements and methods of test. European Standard EN 590.]
Brussels, 5 pp (in German).
Fabri J, Dabelstein W, & Reglitzky A (1990) Motor fuels. In: Elvers B,
Hawkins S, & Schulz G, eds, Ullmann's encyclopedia of industrial
chemistry, 5th Ed, Vol A16. Weinheim, VCH, pp 719-753.
Farrington JW, Davis AC, Frew NM, & Rabin KS (1982) No. 2 fuel oil
compounds in Mytilus edulis. Retention and release after an oil
spill. Mar Biol, 66: 15-26.
Federal Environment Agency (1991) [Air and noise levels caused by
traffic -- emissions, immissions and effects (Report No. 40/91)].
Berlin, 194 pp (in German).
Federal Environment Agency (1992) [Transport in Germany-Energy
consumption and emissions of air pollutants due to transport in German
Democratic Republic, Berlin (East) and the Federal Republic of Germany
in 1988 and in Germany in 2005 (UBA-FB 50473/85; Report No. 5/92)].
Berlin (in German).
Federal Ministry for Transport (1992) [Traffic in statistical
numbers.] Berlin, German Institute for Economic Research,
pp 132, 133, 280, 281 (in German).
Federal Office for Motor Vehicles (1993) [Emission and noise values of
passenger cars, light vans and station wagons with EEC licence], 3rd
Ed. Flensburg, 260 pp (in German).
Federal Office for Motor Vehicles (1994) [Emission values of vehicles
with EEC licence], 4th Ed. Flensburg, 192 pp (in German).
Flowers TH, Pulford ID, & Duncan HJ (1984) Studies on the breakdown of
oil in soil. Environ Pollut Ser, B8: 71.
Fortnagel M (1992) [Possibilities of the reduction of harmful
substances of diesel engines from the view of the automotive
industry.] In: [Effects of diesel engine exhausts on health]. Munich,
Society for Research on Radiation and the Environment, Research Centre
for Environment and Health, pp 53-56 (in German).
Fossato VU & Canzonier WJ (1976) Hydrocarbon uptake and loss by the
mussel Mytilus edulis. Mar Biol, 36: 243-250.
Frankenberger WT Jr, Emerson KD, & Turner DW (1989) In situ
bioremediation of an underground diesel fuel spill: A case history.
Environ Manage, 13: 325-332.
Fraser Williams (Scientific Systems) Ltd (1985) Oil and hazardous
materials: technical assistance data system (OHM/TADS;
Version 5.00/9.5-December, 1985) Poynton, Cheshire.
Frehland E, Weiss A, & Meduna V (1992) [Microbial communities in
diesel contaminated soil]. In: Behrens D & Wiesner J, eds, [Microbial
cleaning of soil].
Frankfurt/Main, German Society for Chemical Apparatus, Chemical
Technology and Biotechnology, pp 347-349.
Froines JR, Hinds WC, Duffy RM, Lafuente EJ, & Liu WCV (1987) Exposure
of firefighters to diesel emissions in fire stations. Am Ind Hyg
Assoc J, 48: 202-207.
Fromme H (1990) [The importance of diesel engine exhaust gases for
ecological health protection and hygienic environmental protection.]
Öff Gesundheitswesen, 52: 625-629 (in German).
Gaddo P, Settis M, & Giacomelli L (1984) Artifact formation during
diesel particulate collection. Orbassano, Fiat Engineering, Ecological
Service, pp 437-449.
Gairing M, Naber D, Schäfer A, Lange W, Reglitzky A & Le'Jeune A
(1994) [The influence of fuel property on the exhaust emissions of
modern diesel motors from Mercedes-Benz]. Motortech Z, 55: 3-11
(in German, with English abstract)
Galin T, McDowell C, & Yaron B. (1990) The effect of volatilization on
the mass flow of a non-aqueous pollutant liquid mixture in an inert
porous medium: Experiments with kerosene. J. Soil Sci, 41: 631-641.
Gallagher J, George M, Kohan M, Thompson C, Shank T, & Lewtas J (1993)
Detection and comparison of DNA adducts after in vitro and in vivo
diesel emission exposures. Environ Health Perspectives, 99: 225-228.
Gallagher J, Heinrich U, George M, Hendee L, Philips DH, & Lewtas J
(1994) Formation of DNA adducts in rat lung following chronic
inhalation of diesel emissions, carbon black and titanium dioxide
particles. Carcinogenesis, 15: 1291-1299.
Gamble JF & Jones WG (1983) Respiratory effects of diesel exhaust in
salt miners. Am Rev Respir Dis, 128: 389-394.
Gamble J, Jones W, & Minshall S (1987a) Epidemiological-environmental
study of diesel bus garage workers: Acute effects of NO2 and
respirable particulate on the respiratory system. Environ Res,
42: 201-214.
Gamble J, Jones W, & Minshall S (1987b) Epidemiological-environmental
study of diesel bus garage workers: Chronic effects of diesel exhaust
on the respiratory system. Environ Res, 44: 6-17.
Garg BD (1983) Histologic quantitation of macrophage aggregates in the
lungs of diesel exhaust exposed rats. Acta Stereol, 2
(Suppl. 1): 235-238.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Gaworski CL, MacEwen JD, Vernot EH, Bruner RH, & Cowan MJ Jr (1984)
Comparison of the subchronic inhalation toxicity of petroleum and oil
shale JP-5 jet fuels. In: MacFarland HN, Holdsworth CE, MacGregor JA,
Call RW, Kane ML, eds, Applied toxicology of petroleum hydrocarbons
(Advances in modern environmental toxicology, Vol. 6). Princeton, NJ,
Princeton Scientific Publishers, pp 33-47.
Gearing PJ, Gearing JN, Pruell RJ, Wade TL, & Quinn JG (1980)
Partitioning of No. 2 fuel oil in controlled estuarine ecosystems.
Sediments and suspended particulate matter. Environ Sci Technol,
14: 1129-1136.
Gerde P, Medinsky A, & Bond JA (1991a) The retention of polycyclic
aromatic hydrocarbons in the bronchial airways and in the alveolar
region. A theoretical comparison. Toxicol Appl Pharmacol,
107: 239-252.
Gerde P, Medinsky MA, & Bond JA (1991b) Contemporary issues in
toxicology. Particle-associated polycyclic aromatic hydrocarbons. A
reappraisal of their possible role in pulmonary carcinogenesis.
Toxicol Appl Pharmacol, 108: 1-13.
Gerde P, Muggenburg BA, Sabourin PJ, Harkema JR, Hotchkiss JA, Hoover
MD, & Henderson RF (1993a) Disposition of polycyclic aromatic
hydrocarbons in the respiratory tract of the beagle dog. II. The
conducting airways. Toxicol Appl Pharmacol, 121: 319-327.
Gerde P, Muggenburg BA, & Henderson RF (1993b) Disposition of
polycyclic aromatic hydrocarbons in the respiratory tract of the
beagle dog. III. Mechanisms of the dosimetry. Toxicol Appl Pharmacol,
121: 328-334.
German Scientific Association for Petroleum, Natural Gas, and Coal
(1991) [Middle distillates. Analytical investigations and mutagenicity
studies. Research report No. 412-1.] Hamburg, 65 pp (in German).
German Institute for Scientific Research (1993) [Transport in
figures]. Berlin, p 133
Gibson TL, Ricci AI, & Williams RL (1981) Measurement of polynuclear
aromatic hydrocarbons, their derivatives, and their reactivity in
diesel automobile exhaust. In: Cooke M & Dennis AJ, eds, Polynuclear
aromatic hydrocarbons, fifth international symposium on chemical
analysis and biological fate. Columbus, OH, Battelle Press,
pp 707-717.
Grant LD, Raub JA, Graham JA, Kotchmar DJ & Tilton BE (1993) Health
effects of motor vehicle-related criteria air pollutants. In:
Proceedings of the IPCS/Australia international workshop on human
health and environmental effects of motor vehicle fuels and their
exhaust emissions, Sydney, 6-10 April 1992. Canberra, Commonwealth
Department of Health, Housing, Local Government and Community
Services, pp 101-173.
Greenfield R, Ellwein L & Cohen S (1984) A general probabilistic model
of carcinogenic analysis: Experimental bladder cancer. Carcinogenesis,
5: 437-445.
Greenland S & Robins J (1994) Invited commentary: Ecologic
studies -- biases, misconceptions, and counterexamples. Am J Epidemiol,
139: 747-760.
Griffis LC, Wolff RK, Henderson RF, Griffith WC, Mokler BV, &
McClellan RO (1983) Clearance of diesel soot particles from rat lung
after a subchronic diesel exhaust exposure. Fundam Appl Toxicol,
3: 99-103.
Grimmer G, Hildebrandt A, & Böhnke H (1973) Investigations on the
carcinogenic burden by air pollution in man. II. Sampling and
analytics of polycyclic aromatic hydrocarbons in automobile exhaust
gas. 1. Optimization of the collecting arrangement. Zbl Bakt Hyg I Abt
Orig B, 158: 22-34.
Grimmer G, Naujack K-W, & Schneider D (1979) Changes in PAH-profiles
in different areas of a city during the year. In: Björseth A & Dennis
A, eds, Polynuclear aromatic hydrocarbons: Chemistry and biological
effects. Fourth international symposium on polynuclear aromatic
hydrocarbons. Columbus, OH, Battelle Press, pp 107-125.
Grimmer G, Naujack K-W, & Schneider D (1982) Profile analysis of
polycyclic aromatic hydrocarbons by glass capillary gas chromatography
in atmospheric suspended particulate matter in the nanogram range
collecting 10 m3 of air. Fresenius Z Anal Chem, 311: 475-484.
Grimmer G, Brune H, Deutsch-Wenzel R, Dettbarn G, Jacob J, Naujack
K-W, Mohr U, & Ernst H (1987) Contribution of polycyclic aromatic
hydrocarbons and nitro-derivatives to the carcinogenic impact of
diesel engine exhaust condensate evaluated by implantation into the
lungs of rats. Cancer Lett, 37: 173-180.
Grimmer G, Jacob J, Dettbarn G, & Naujack K-W (1988) Effect of the
pH-value of diesel exhaust on the amount of filter-collected
nitro-PAH. In: Cooke M & Dennis AJ, eds, Polynuclear aromatic
hydrocarbons. Tenth international symposium: A decade of progress.
Columbus, OH, Battelle Press, pp 341-351.
Grimmer G, Brune H, Dettborn G, Jacob J, Misfeld J, Mohr U, Naujack
K-W, Timm J, & Wenzel-Hartung R (1991) Relevance of polycyclic
aromatic hydrocarbons as environmental carcinogens. Fresenius J Anal
Chem, 339: 792- 795.
Grundmann R & Rehm HJ (1991) Biodegradation of diesel-fuel. Use of
free and immobilized mixed cultures in soils. Erdöl Kohle Erdgas
Petrochem 44: 149-150.
Gu Z-W, Zhong B-Z, Whong W-Z, Wallace WE, & Ong T (1991) Mutagenicity
and clastogenicity studies of diesel emission particles in cultured
mammalian cells. Environ Mol Mutag, 17 (Suppl. 19): 28.
Gu Z-W, Zhong B-Z, Nath B, Whong W-Z, Wallace WE, & Ong T-M (1992)
Micronucleus induction and phagocytosis in mammalian cells treated
with diesel emission particles. Mutat Res, 279: 55-60.
Gu Z-W, Zhong B-Z, Keane MJ, Whong W-Z, Wallace WE, & Ong T (1994)
Induction of unscheduled DNA synthesis in V79 cells by diesel emission
particles dispersed in simulated pulmonary surfactant. Ann Occup Hyg,
38 (Suppl 1): 345-349.
Guerrero RR, Rounds DE, & Orthoefer J (1981) Genotoxicity of Syrian
hamster lung cells treated in vivo with diesel exhaust particulates.
Environ Int, 5: 445-454.
Guillemin MP, Herrera H, Huynh CK, Droz P-O, & Vu Duc T (1992)
Occupational exposure of truck drivers to dust and polynuclear
aromatic hydrocarbons: A pilot study in Geneva, Switzerland. Int Arch
Occup Environ Health, 63: 439-447.
Gusein-Zade KM (1974) [The results of dermatological examinations of
engine drivers working in oil fields of Apsheron.] Vestn Dermatol
Venereol, 10: 47-49 (in Russian).
Gustafsson L, Wall S, Larsson L-G, & Skog B (1986) Mortality and
cancer incidence among Swedish dock workers -- A retrospective cohort
study. Scand J Work Environ Health, 12: 22-26.
Gustavsson P Plato N Lidström E-B, & Hogstedt C (1990) Lung cancer and
exposure to diesel exhaust among bus garage workers. Scand J Work
Environ Health, 16: 348-354.
Hahon N, Booth JA, Green F, & Lewis TR (1985) Influenza virus
infection in mice after exposure to coal dust and diesel engine
emissions. Environ Res, 37: 44-60.
Hammerle RH, Ketcher DA, Horrocks RW, Lepperhoff G, Hüthwohl G, &
Lüers B (1994a) Emissions from current diesel vehicles (SAE Technical
Paper Series No. 942943). Warrendale, PA, Society of Automotive
Engineers.
Hammerle R, Schuetzle D, & Adams W (1994b) A perspective on the
potential development of 'environmentally acceptable' light-duty
diesel vehicles. Environ Health Perspectives, 101: 1-20.
Hammond S, Smith T, Woskie S, Leaderer B, & Bettinger N (1988) Markers
of exposure to diesel exhaust and cigarette smoke in railroad workers.
Am Ind Hyg Assoc J, 49: 516-522.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Handa T, Yamanuchi T, Ohnishi M, Hisamatsu Y, & Ishii T (1983)
Detection and average content levels of carcinogenic and mutagenic
compounds from the particulates on diesel and gasoline engine
mufflers. Environ Int, 9: 335-341.
Hansen ES (1993) A follow-up study on the mortality of truck drivers.
Am J Ind Med, 23: 811-821.
Hare CT (1986) The effects of diesel fuel properties on particulate
emissions. In: Lee SD, Schneider T, Grant LD, & Verkerk PJ, eds,
Aerosols: Research, risk assessment and control strategies. Chelsea,
MI, Lewis Publishers, pp 501-514.
Harris J (1983) Diesel emissions and lung cancer. Risk Anal,
3: 83-100.
Harrison W, Winnik MA, Kwong PTY, & MacKay D (1975) Crude oil spills.
Disappearence of aromatic and aliphatic components from small
sea-surface slicks. Environ Sci Technol, 9: 231-234.
Hartung R & Hunt GS (1966) Toxicity of some oils to waterfowl. J Wildl
Manage, 30: 564-570.
Hartung A, Kraft J, Schulze J, Kiess H, & Lies K-H (1984)
Identification of nitrated polycyclic aromatic hydrocarbons in diesel
particulate extracts and their potential formation as artifacts during
particulate collection. Chromatographia, 19: 269-273.
Hasegawa MM, Nishi Y, Tsuda H, Inui N, & Morimoto K (1988) Effects of
diesel exhaust particles on chromosome aberration, sister chromatid
exchange and morphological transformation in cultured mammalian cells.
Cancer Lett, 42: 61-66.
Hawthorne SB & Miller DJ (1986) Extraction and recovery of organic
pollutants from environmental solids and Tenax-GC using supercritical
CO2. J Chromatogr Sci, 24: 258-264.
Hayes RB, Thomas T, Silverman DT, Vineis P, Blot WJ, Mason TJ, Pickle
LW, Correa P, Fontham ETH, & Schoenberg JB (1989) Lung cancer in motor
exhaust-related occupations. Am J Ind Med, 16: 685-695.
Hedtke SF & Puglisi FA (1982) Short-term toxicity of five oils to four
freshwater species. Arch Environ Contam Toxicol, 11: 425-430.
Heino M, Ketola R, Mäkelä P, Mäkinen R, Niemelä R, Starck J, &
Partanen T (1978) Work conditions and health of locomotive engineers.
1. Noise, vibration, thermal climate, diesel exhaust constituents,
ergonomics. Scand J Work Environ Health, 4: 3-14.
Heinrich U (1994) Carcinogenic effects of solid particles. In: Mohr U,
Dungworth DL, Mauderly JL, & Oberdörster G, eds, Toxic and
carcinogenic effects of solid particles in the respiratory tract.
Washington DC, International Life Science Institute Press, pp 57-73.
Heinrich U, Peters L, Funcke W, Pott F, Mohr U, & Stöber W (1982)
Investigation of toxic and carcinogenic effects of diesel exhaust in
long-term inhalation exposure of rodents. In: Lewtas S, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 225-242.
Heinrich U, Muhle H, Takenaka S, Ernst H, Fuhst R, Mohr U, Pott F, &
Stöber W (1986a) Chronic effects on the respiratory tract of hamsters,
mice and rats after long-term inhalation of high concentrations of
filtered and unfiltered diesel engine emissions. J Appl Toxicol,
6: 383-395.
Heinrich U Pott F & Rittinghausen S (1986b) Comparison of chronic
inhalation effects in rodents after long-term exposure to either coal
oven flue gas mixed with pyrolized pitch or diesel engine exhaust. In:
Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic
and mutagenic effects of diesel engine exhaust. Amsterdam, Elsevier,
pp 441-457.
Heinrich U, Pott F, & Roller M (1991) [Polycyclic aromatic
hydrocarbons -- Animal results and epidemiological findings relevant to
risk assessment]. In: [Carcinogenic substances in the environment:
Origin, measurement, risk, minimization. Meeting of the Commission for
Clean Air of the Society of German Engineers (VDI) and the German
Standards Institute (DIN), Mannheim, 23-25 April]. Düsseldorf, Society
of German Engineers Press, pp 71-92 (in German).
Heinrich U, Fuhst R, & Mohr U (1992) [Experimental inhalation studies
with laboratory animals on the question of the tumour-inducing effect
of diesel engine exhausts and two test dusts.] In: Effects of diesel
engine exhausts on health. Munich, GSF Research Centre for Environment
and Health Inc., pp 21-30 (in German).
Heinrich U, Peters L, Creutzenberg O, Dasenbrock C, & Hoymann HG
(1994) Inhalation exposure of rats to tar/pitch condensation aerosol
or carbon black alone or in combination with irritant gases. In: Mohr
U, Dungworth DL, Mauderly JL, & Oberdörster G, eds, Toxic and
carcinogenic effects of solid particles in the respiratory tract.
Washington DC, International Life Sciences Institute Press,
pp 433-441.
Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch
W, & Levsen K (1995) Chronic inhalation exposure of Wistar rats, and
two different strains of mice to diesel engine exhaust, carbon black
and titanium dioxide. Inhal Toxicol, 7: 533-556.
Hellstrom T & Doving KB (1983) Perception of diesel oil by cod
( Gadus morhua L.). Aquat Toxicol, 4: 303-315.
Henderson TR, Li AP, Royer RE, & Clark CR (1981) Increased
cytotoxicity and mutagenicity of diesel fuel after reaction with
NO2. Environ Mutag, 3: 211-220.
Henderson RF, Pickrell JA, Jones RK, Sun JD, Benson JM, Mauderly JL, &
McClellan RO (1988) Response of rodents to inhaled diluted diesel
exhaust: Biochemical and cytological changes in bronchoalveolar lavage
fluid and in lung tissue. Fundam Appl Toxicol, 11: 546-567.
Henschler D ed. (1987) [Diesel engine emissions]. In: [Maximal working
place concentration. Chemical compounds in the work area hazardous to
health]. Weinheim, VCH, 25 pp (in German).
Herbes SE & Whitley TA (1983) Characterization and toxicity of
water-soluble photooxidants produced during irradiation of coal
liquids by sunlight. Environ Pollut, 6: 221-240.
Heyder J, Gebhart J, Rudolf G, Schiller CF, & Stahlhofen W (1986)
Deposition of particles in the human respiratory tract in the size
range 0.005-15 µm. J Aerosol Sci, 17: 811-825.
Hildemann LM, Markowski GR, & Cass GR (1991) Chemical composition of
emissions from urban sources of fine organic aerosol. Environ Sci
Technol, 25: 744-759.
Hoar SK & Hoover R (1985) Truck driving and bladder cancer mortality
in rural New England. J Natl Cancer Inst, 74: 771-774.
Hobbs JR, Walter RA, Hard T, & Devoe D (1977) Train generated air
contaminants in the train crew's working environment (FRA/ORD-77/08:
US NTIS PB 265-355). Springfield, VA, National Technical Information
Service, pp 1-46.
Holland WD (1978) Determination of breathing zone concentrations of
contaminants from emissions from diesel powered vehicles in
underground mines (BuMines OFR 24-80; US NTIS PB80-150766).
Springfield, VA, National Technical Information Service, pp 1-127.
Holt S (1987) The effects of crude and diesel oil spills on plant
communities at Mesters Vig, northeast Greenland. Arct Alp Res,
19: 490-497. Horvath et al. (1985)
Horvath H, Kreiner I, & Norek C (1987) The role of the diesel aerosol
in the air pollution of Vienna. J Aerosol Sci, 18: 817-819.
Horvath H, Kreiner I, Norek C, Preining O, & Georgi B (1988) Diesel
emissions in Vienna. Atmos Environ, 22: 1255-1269.
Howe GR, Burch JD, Miller AB, Cook GM, Estève J, Morrison B, Gordon P,
Chambers LW, Fodor G, & Winsor GM (1980) Tobacco use, occupation,
coffee, various nutrients, and bladder cancer. J Natl Cancer Inst,
64: 701-713.
Howe GR, Fraser D, Lindsay J, Presnal B, & Zhang Yu S (1983) Cancer
mortality (1965-77) in relation to diesel fume and coal exposure in a
cohort of retired railway workers. J Natl Canc Inst, 6: 1015-1019.
Huisingh J, Bradow R, Jungers R, Claxton L, Zweidinger R, Tejada S,
Bumgarner J, Duffield F, Waters MD, Simmon VF, Hare C, Rodriguez C, &
Snow L (1978) Application of bioassay to the characterization of
diesel particle emissions. In: Waters MD, Nesnow S, Huisingh JL,
Sandhu SS, & Claxton L, eds, Application of short-term bioassays in
the fractionation and analysis of complex environmental mixtures. New
York, Plenum Press, pp 383-418.
Hyde DM, Plopper CG, Weir AJ, Murnane RD, Warren DL, Last JA, &
Pepelko WE (1985) Peribronchiolar fibrosis in lungs of cats
chronically exposed to diesel exhaust. Lab Invest, 52: 195-206.
Iida N, Suzuki Y, Sato GT, & Sawada T (1986) Effects of intake oxygen
concentration on the characteristics of particulate emissions from a
DI diesel engine (Paper No. 861233). Warrendale, PA, Society of
Automotive Engineers.
Imaida K, Hirose M, Tay L, Lee M-S, Wang Y, & King CM (1991)
Comparative carcinogenicities of 1-, 2-, and 4-nitropyrene and
structurally related compounds in the female CD rat. Cancer Res,
51: 2902-2907.
International Agency for Research on Cancer (1989a) Diesel fuels. In:
IARC monographs on the evaluation of the carcinogenic risk of
chemicals to humans, Vol. 45, Occupational exposures in petroleum
refining; crude oil and major petroleum fuels. Lyon, pp 219-237.
International Agency for Research on Cancer (1989b) Diesel and
gasoline engine exhausts. In: IARC monographs on the evaluation of the
carcinogenic risk of chemicals to humans, Vol. 46, Diesel and gasoline
engine exhaust and some nitroarenes. Lyon, pp 41-185.
International Standards Organization (1987) Petroleum products -- Fuels
(class F) -- Specifications of marine fuels (No. ISO 8217-1987/E).
Geneva, 7 pp.
International Union for Pure and Applied Chemistry (1993) Glossary for
chemists of terms used in toxicology (IUPAC Recommendations 1993).
Pure Appl Chem, 65: 2003-2122.
IPCS (1986) Environmental health criteria, 52: Toluene. Geneva, WHO.
IPCS (1993) Environmental health criteria, 150: Benzene. Geneva, WHO,
156 pp.
IPCS (1994) Environmental health criteria, 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO, 73 pp.
Iscovich J, Castelletto R, Estève J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Ishinishi N, Kuwabara N, Nagase S, Suzuki T, Ishiwata S, & Kohno T
(1986) Long-term inhalation studies on effects of exhaust from heavy
and light duty diesel engines on F344 rats. In: Ishinishi N, Koizumi
A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects
of diesel engine exhaust. Amsterdam, Elsevier, pp 329-348.
Ishinishi N, Kuwabara N, Takaki Y, Nagase S, Suzuki T, Nakajima T,
Maejima K, Kato A, & Nakamura M (1988) Long-term inhalation
experiments on diesel exhaust. In: Diesel exhaust and health risk.
Results of the HERP studies. Tsukuba, Health Effects Research
Programme, pp 11-84.
Israel GW, Zierock KH, & Mollenhauer K (1982) [Distribution and
chemical composition of particle emissions from different diesel
motors] (VDI Report No. 429). Düsseldorf, Association of German
Engineers Press, pp 279-286 (in German).
Iwai K, Udagawa T, Yamagishi M, & Yamada H (1986) Long-term inhalation
studies of diesel exhaust on F344 SFP rats. Incidence of lung cancer
and lymphoma. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 349-360.
Iyer V, Harris RE, & Wynder EL (1990) Diesel exhaust exposure and
bladder cancer risk. Eur J Epidemiol, 6: 49-54.
Jaenicke R (1986) Physical characterization of aerosols. In: Lee SD,
Schneider T, Grant LD, & Verkerk PJ, eds, Aerosols: Research, risk
assessment and control strategies. Chelsea, MI, Lewis Publishers,
pp 97-106.
Jensen TE & Hites RA (1983) Aromatic diesel emissions as a function of
engine conditions. Anal Chem, 55: 594-599.
Jensen OM, Wahrendorf J, Knudsen JB, & Sorensen BL (1987) The
Copenhagen case-referent study on bladder cancer. Risks among drivers,
painters and certain other occupations. Scand J Work Environ Health,
13: 129-134.
Jinno K, Hoshino T, Hondo T, Saito M, & Senda M (1986) Identification
of polycyclic aromatic hydrocarbons in extracts of diesel particulate
matter by supercritical fluid chromatography coupled with an
ultraviolet multichannel detector. Anal Chem, 58: 2696-2699.
Johnson JH & Carlson DH (1986) The application of advanced measurement
and control technology to diesel-powered vehicles in an underground
salt mine. Montreal, Canadian Institute of Mining and Metallurgy,
pp 206-237.
Jorgensen H & Svensson Å (1970) Studies on pulmonary function and
respiratory tract symptoms of workers in an iron ore mine where diesel
trucks are used underground. J Occup Med, 12: 348-354.
Joumard R & Perrin M-L (1988) Measurement of particle and gaseous
pollution of the atmosphere due to buses. Sci Total Environ,
76: 55-62.
Juhnke I & Lüdemann D (1978) [Results of a study of 200 chemical
compounds for acute fish toxicity with the golden orfe test.] Z Wasser
Abwasser Forsch, 11: 161-164 (in German).
Kahn G, Orris P, & Weeks J (1988) Acute over exposure to diesel
exhaust: Report of 13 cases. Am J Ind Med, 13: 405-406.
Kainz RJ & White LE (1982) Consequences associated with the inhalation
of uncombusted diesel vapor. In: MacFarland HN, Holdsworth CE,
MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the symposium:
The toxicology of petroleum hydrocarbons, May 1982. Washington DC,
American Petroleum Institute, pp 320-327.
Kainz RJ & White LE (1984) Depressant effects associated with the
inhalation of uncombusted diesel vapor. In: MacFarland HN, Holdsworth
CE, MacGregor JA, Call RW, & Kane ML, eds, Applied toxicology of
petroleum hydrocarbons (Advances in modern environmental toxicology,
Vol. 6). Princeton, NJ, Princeton Scientific Publishers, pp 233-243.
Kaplan HL, MacKenzie WF, Springer KJ, Schreck RM, & Vostal JJ (1982) A
subchronic study of the effects of exposure of three species of
rodents to diesel exhaust. In: Lewtas J, ed., Toxicological effects of
emissions from diesel engines. Amsterdam, Elsevier, pp 161-182.
Kaplan HL, Springer KJ, & MacKenzie WF (1983) Studies of potential
health effects of long-term exposure to diesel exhaust emissions.
Final report (SwRI Project No. 01-0750-103; SFRE Project No. 1239).
San Antonio, TX, Southwest Research Institute, 59 pp.
Karagianes MT, Palmer RF, & Busch RH (1981) Effects of inhaled diesel
emissions and coal dust in rats. Am Ind Hyg Assoc J, 42: 382-391.
Karl DM (1992) The grounding of the Bahia Paraiso: Microbial ecology
of the 1989 Antarctic oil spill. Microbiol Ecol, 24: 77-89.
Kato A, Kyono H, & Kuwabara N (1992) [Electron-microscopic
observations on rat lungs after long term inhalation of diesel
emissions -- Non-neoplastic lesions]. Jpn J Chest Dis, 30: 238-247
(in Japanese, with English abstract).
Kauffman S (1974) Kinetics of alveolar epithelial hyperplasia in lungs
of mice exposed to urethane. I. Quantitative analysis of cell
populations. Lab Invest, 30: 170-175.
Kawabata Y, Iwai K, Udagawa T, Tukagoshi K, & Higuchi K (1986) Effects
of diesel soot on unscheduled DNA synthesis of tracheal epithelium and
lung tumor formation. In: Ishinishi N, Koizumi A, McClellan RO, &
Stöber W, eds, Carcinogenic and mutagenic effects of diesel engine
exhaust. Amsterdam, Elsevier, pp 213-222.
Kawabata Y, Udagawa T, Higuchi K, Yamada H, & Iwai K (1993) One year
exposure to diesel engine exhaust causes lung tumors. In: Mohr U,
Dungworth DL, Mauderly JL & Oberdörster G, eds, Toxic and carcinogenic
effects of solid articles in the respiratory tract. Washington DC,
International Life Sciences Institute Press, pp 429-431.
Keane MJ, Xing S-G, Harrison JC, Ong T, & Wallace WE (1991)
Genotoxicity of diesel-exhaust particles dispersed in simulated
pulmonary surfactant. Mutat Res, 260: 233-238.
Kemp PF, Swartz RC, & Lamberson JO (1986) Response of the
phoxocephalid amphipod, Rhepoxynius abronius, to a small oil spill
in Yaquina Bay, Oregon. Estuaries, 9: 340-347.
Kennicutt MC II, (1990) Oil spillage in Antarctica: Initial report of
the National Science Foundation-sponsored quick response team on the
grounding of the Bahia Paraiso. Environ Sci Technol, 24: 620-624.
Kennicutt MC II & Sweet ST (1992) Hydrocarbon contamination on the
Antarctic peninsula: III. The Bahia Paraiso -- Two years after the
spill. Mar Pollut Bull, 25: 303-306.
Kennicutt MC II, Sweet ST, Fraser WR, Stockton WL, & Culver M (1991a)
Grounding of the Bahia Paraiso at Arthur Harbor, Antarctica. 1.
Distribution and fate of oil spill related hydrocarbons. Environ Sci
Technol, 25: 509-518.
Kennicutt MC II, Sweet ST, Fraser WR, Culver M, & Stockton WL (1991b)
The fate of diesel fuel spilled by the Bahia Paraiso in Arthur
Harbour, Antarctica. In: Proceedings 1991 international oil spill
conference. (Prevention, behavior, control, cleanup), 4-7 March 1991,
San Diego, California. Washington DC, American Petroleum Institute,
pp 493-500.
Kennicutt MC II, McDonald TJ, Denoux GJ, & McDonald SJ (1992a)
Hydrocarbon contamination on the Antarctic peninsula. I. Arthur
Harbor -- Subtidal sediments. Mar Pollut Bull, 24: 499-506.
Kennicutt MC II, McDonald TJ, Denoux GJ, & McDonald SJ (1992b)
Hydrocarbon contamination on the Antarctic peninsula. II. Arthur
Harbor -- Inter- and subtidal limpets (Nacella concinna). Mar Pollut
Bull, 24: 506-511.
Kittel B, Ernst H, Dungworth DL, Rittinghausen S, Nolte T, Kamino K,
Stuart B, Lake SG, Cardesa A, Moraweitz G, & Mohr U (1993)
Morphological comparison between benign keratinizing cystic squamous
cell tumours of the lung and squamous lesions of the skin in rats.
Exp Toxicol Pathol, 45: 257-267
Klingenberg H, Schürmann D, & Lies KH (1991) [Diesel motor
exhaust -- Formation and determination]. In: [Carcinogenic substances in
the environment: Origin, measurement, risk, minimization. Meeting of
the Commission for Clean Air of the Society of German Engineers (VDI)
and the German Standards Institute (DIN), Mannheim, 23-25 April].
Düsseldorf, Society of German Engineers Press, pp 119-131 (in German)
Knave B, Olson BA, Elofson S, Gamberale F, Isaksson A, Mindus P,
Persson HE, Struwe G, Wennberg A, & Westerholm P (1978) Long term
exposure to jet fuel. II. A cross-sectional epidemiologic
investigation on occupationally exposed industrial workers with
special reference to the nervous system. Scand J Work Environ Health,
4: 19-45.
Koenig JQ, Covert DS, Marshall SSG, Van Belle G, & Pierson WE (1988)
The pulmonary effects of ozone and nitrogen dioxide alone and combined
in healthy and asthmatic adolescent subjects. Toxicol Ind Health,
4: 521-532
Köhler M & Eichhoff HJ (1967) [Quick method for determination of
aromatic hydrocarbons in air dust.] Z Anal Chem, 232: 401-409
(in German).
Kotin P, Falk HL, & Thomas M (1955) Aromatic hydrocarbons. III.
Presence in the particulate phase of diesel-engine exhausts and the
carcinogenicity of exhaust extracts. Arch Ind Health, 11: 113-120.
Kraft J & Lies K-H (1981) Polycyclic aromatic hydrocarbons in the
exhaust of gasoline and diesel vehicles (Technical paper No. 810082).
Warrendale, PA, Society of Automotive Engineers, pp 1-11.
Kühnhold WW (1977) The effect of mineral oils on the development of
eggs and larvae of marine species. A review and comparison of
experimental data in regard to possible damage at sea. In: McIntyre AD
& Whittle KJ, eds, Petroleum hydrocarbons in the marine environment
(Rapports et proces-verbaux des réunions, Vol. 171). Copenhagen,
International Council for Exploration of the Sea, pp 175-183.
Kunitake E, Shimamura K, Katayama H, Takemoto K, Yamamoto A, Hisanaga
A, Ohyama S, & Ishinishi N (1986) Studies concerning carcinogenesis of
diesel particulate extracts following intratracheal instillation,
subcutaneous injection, or skin application. In: Ishinishi N, Koizumi
A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects
of diesel engine exhaust. Amsterdam, Elsevier, pp 235-252.
Kuschner M (1968) The causes of cancer. Am Rev Respir Dis,
98: 573-590.
Larson RA, Hunt LL, & Blankenship DW (1977) Formation of toxic
products from a No. 2 fuel oil by photooxidation. Environ Sci Technol,
11: 492-496.
Larson RA, Bott TL, Hunt LL, & Rogenmuser K (1979) Photooxidation
products of a fuel oil and their antimicrobial activity. Environ Sci
Technol, 13: 965-969.
Lawler GC, Loong W-A, & Laseter JL (1978) Accumulation of aromatic
hydrocarbons in tissues of petroleum-exposed mallard ducks
(Anas platyrhynchos). Environ Sci Technol, 12: 51-54.
Lee RF (1977) Accumulation and turnover of petroleum in marine
organisms. In: Wolfe DA, ed., Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, Pergamon
Press, pp 60-70.
Lee RF (1981) Mixed function oxygenases (MFO) in marine invertebrates.
Mar Biol Lett, 2: 87-105.
Lee FS-C & Schuetzle D (1983) Sampling, extraction and analysis of
polycyclic aromatic hydrocarbons from internal combustion engines. In:
Björseth A, ed., Handbook of polycyclic aromatic hydrocarbons. New
York, Marcel Dekker, pp 27-94.
Lee FS-C, Pierson WR, & Ezike J (1980) The problem of PAH degradation
during filter collection of airborne particulates -- An evaluation of
several commonly used filter media. In: Björseth A & Dennis AJ, eds,
Polynuclear aromatic hydrocarbons, 4th International Symposium:
Chemistry and biological effects. Dearborn, MI, Engineering and
Research Staff Research, Ford Motor Co., pp 543-563.
Lee PS, Chan TL, & Hering WE (1983) Long-term clearance of inhaled
diesel exhaust particles in rodents. J Toxicol Environ Health,
12: 801-813.
Lee PS, Gorski RA, Hering WE, & Chan TL (1987) Lung clearance of
inhaled particles after exposure to carbon black generated from a
resuspension system. Environ Res, 43: 364-373.
Lee LS, Hagwall M, Delfino JJ, & Rao PSC (1992) Partitioning of
polycyclic aromatic hycrocarbons from diesel fuel into water. Environ
Sci Technol, 26: 2104-2110.
Lehmann E, Rentel K-H, Allescher W, & Hohmann R (1990) [Measurement of
diesel exhaust at the workplace.] Zentralbl Arbeitsmed Arbeitsschutz
Prophyl Ergon, 40: 2-11 (in German).
Levsen K (1988) The analysis of diesel particulate. Fresenius Z Anal
Chem, 331: 467-478.
Levsen K (1992) [Production and analysis of total diesel exhaust for
efficiency research.] In: [Effects of diesel engine exhausts on
health]. Munich, GSF Research Centre for Environment and Health Inc.,
pp 16-20 (in German).
Levsen K, Puttins U, Schilhabel J, & Priess B (1988) Artefacts during
the sampling of nitro PAHs of diesel exhaust. Fresenius Z Anal Chem,
330: 527-528.
Lewis SC (1983) Crude petroleum and selected fractions. Prog Exp Tumor
Res, 26: 68-84.
Lewis TR, Green FHY, Moorman WJ, Burg JAR, & Lynch DW (1986) A chronic
inhalation toxicity study of diesel engine emissions and coal dust,
alone and combined. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber
W, eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 361-380.
Lewis TR, Green FHY, Moorman WJ, Burg JR, & Lynch DW (1989) A chronic
inhalation toxicity study of diesel engine emissions and coal dust,
alone and combined. J Am Coll Toxicol, 8: 345-375.
Lewtas J (1983) Evaluation of the mutagenicity and carcinogenicity of
motor vehicle emissions in short-term bioassays. Environ Health
Perspectives, 47: 141- 152.
Lewtas J & Williams K (1986) A retrospective view of the value of
short-term genetic bioassays in predicting the chronic effects of
diesel soot. In: Ishinishi N, Koizumi A, McClellan R, & Stöber W, eds,
Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 119-140.
Li AP & Royer RE (1982) Diesel-exhaust-particle extract enhancement of
chemical-induced mutagenesis in cultured Chinese hamster ovary cells:
Possible interaction of diesel exhaust with environmental carcinogens.
Mutat Res, 103: 349-355.
Liebe B & Fock HP (1992) Growth and adaptation of the green algae
Chlamydomonas reinhardtii on diesel exhaust particle extracts. J Gen
Microbiol, 138: 973-978.
Lies K-H, Hartung, A Postulka, A, Gring H, & Schulze J (1986)
Composition of diesel exhaust with particular reference to particle
bound organics including formation of artifacts. In: Ishinishi N,
Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic
effects of diesel engine exhaust. Amsterdam, Elsevier, pp 65-82.
Linnell RH & Scott WE (1962) Diesel exhaust composition and odor
studies. J Am Poll Control Assoc, 12: 510-515.
Lock S, Dalbey W, Schmoyer R, & Griesemer R (1984) Inhalation
toxicology of diesel fuel obscurant aerosol in Sprague-Dawley rats:
Final report, phase 3, subchronic exposures (TN AD-A150 100). Oak
Ridge, TN, Oak Ridge National Laboratory.
Lockhart WL, Danell RW, & Murray DAJ (1987) Acute toxicity bioassays
with petroleum products: Influence of exposure conditions. In:
Vandermeulen JH & Hrudey SE, eds, Oil in freshwater: Chemistry,
biology, countermeasure technology. New York, Pergamon, pp 335-409.
Loprieno N, DeLorenzo F, Cornetti GM, & Biaggini G (1980) Mutagenicity
studies on diesel particles and particulate extracts. Health effects
of diesel engine emissions (EPA NO 600/9-80-057a). In: Proceedings of
an international symposium, Vol 1. Washington DC, US Environmental
Protection Agency, pp 276-308.
Lowe DM & Pipe RK (1986) Hydrocarbon exposure in mussels: A
quantitative study of the responses in the reproductive and nutrient
storage cell systems. Aquat Toxicol, 8: 265-272.
Lowenthal DH, Zielinska B, Chow JC, Watson JG, Gautan M, Ferguson DH,
Neuroth GR, & Stevens KD (1994) Characterization of heavy-duty diesel
vehicle emissions. Atmos Environ, 28: 731-743.
Lysyj I & Russell EC (1974) Dissolution of petroleum-derived products
in water. Water Res, 8: 863-868.
Mackay D & Shiu WY (1976) Aqueous solubilities of weathered northern
crude oils. Bull Environ Contam Toxicol, 15: 101-109.
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. 2. Polynuclear aromatic hydrocarbons, polychlorinated
dioxins and dibenzofurans. Ann Arbor, MI, Lewis Publishers Inc.,
p 253.
Mackie PR, McGill AS, & Hardy R (1972) Diesel oil contamination of
brown trout ( Salmo trutta L.). Environ Pollut, 3: 9-16.
Mahoney BM & Haskin HH (1980) The effects of petroleum hydrocarbons on
the growth of phytoplankton recognised as food forms for the eastern
oyster, Crassostrea virginica Gmelin. Environ Pollut, A22: 123-132.
Marcus JM & Scott GI (1989) Effects of two diesel fuel mixtures on
fecal coliform bacteria densities. Bull Environ Contam Toxicol,
42: 395-401.
Marklund S, Andersson R, Tysklind M, Rappe C, Egebäck K-E, Björkman E,
& Grigoriadis V (1990) Emissions of PCDDs and PCDFs in gasoline and
diesel fueled cars. Chemosphere, 20: 553-561.
Martini N (1993) Operable lung cancers. CA Cancer J Clin, 43: 201-204.
Mattie DR, Alden CL, Newell TK, Gaworski CL, & Flemming CD (1991) A
90-day continuous vapor inhalation toxicity study of JP-8 jet fuel
followed by 20 or 21 months of recovery in Fischer 344 rats and
C57BL/6 mice. Toxicol Pathol, 19: 77-87
Mauderly JL (1992) Diesel exhaust. In: Lippmann M, ed., Environmental
toxicants. Human exposures and their health effects. New York, Van
Nostrand Reinhold, pp 119-162.
Mauderly JL, Jones RK, McClellan RO, Henderson RF, & Griffith WC
(1986) Carcinogenicity of diesel exhaust inhaled chronically by rats.
In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds,
Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 397-409.
Mauderly JL, Jones RK, Griffith WC, Henderson RF, & McClellan RO
(1987) Diesel exhaust is a pulmonary carcinogen in rats exposed
chronically by inhalation. Fundam Appl Toxicol, 9: 208-221.
Mauderly JL, Gillett NA, Henderson RF, Jones RK, & McClellan RO (1988)
Relationships of lung structural and functional changes to
accumulation of diesel exhaust particles. Ann Occup Hyg, 32: 659-669.
Mauderly JL, Bice DE, Cheng YS, Gillett NA, Griffith WC, Henderson RF,
Pickrell JA, & Wolff RK (1990) Influence of preexisting pulmonary
emphysema on susceptibility of rats to inhaled diesel exhaust. Am Rev
Respir Dis, 141: 1333-1341.
Mauderly JL, Snipes MB, Barr EB, Bechtold WE, Bellinsky SA, Henderson
RF, Mitchell CE, Nikula KJ, & Thomassen DG (1991) Influence of
particle-associated organic compounds on carcinogenicity of diesel
exhaust. In: Eighth Health Effects Institute Annual Conference,
21-24 April 1991, Colorado Springs, CO. Cambridge, MA, Health Effects
Institute, 13 pp.
Mauderly JL, Snipes MB, Barr EB, Belinsky SA, Bond JA, Brooks AL,
Change IY, Cheng YS, Gillett NA, Griffith WC, Henderson RF, Mitchell
CE, Nikula KJ, & Thomassen DG (1994) Pulmonary toxicity of inhaled
diesel exhaust and carbon black in chronically exposed rats. (HEI
Research Report No. 68). Cambridge, MA, Health Effects Institute,
97 pp.
McClellan RO (1986) Health effects of diesel exhaust: A case study in
risk assessment. Am Ind Hyg Assoc J, 47: 1-13.
McClellan RO (1990) Particle overload in the lung: Approaches to
improving our knowledge. J Aerosol Med, 3: 197-207.
McClellan R, Cuddihy R, Griffith W, & Mauderly J (1989) Integrating
diverse data sets to assess the risks of airborne pollutants. In: Mohr
U, ed., Assessment of inhalation hazards. New York, Springer, pp 3-22.
McCormick JJ, Zator RM, DaGue BB, & Maher VM (1980) Studies on the
effects of diesel particulate on normal and xeroderma pigmentosum
cells (EPA-NO 600/9-80-057a). In: Pepelko WE, Danner RM, & Clark NA,
eds, Health effects of diesel engine emissions. Cincinnati, OH, US
Environmental Protection Agency, pp 413-415.
McKee RH & Plutnick RT (1989) Carcinogenic potential of gasoline and
diesel engine oils. Fundam Appl Toxicol, 13: 545-553.
McKee RH, Amoruso MA, & Freeman JJ (1994) Evaluation of the genetic
toxicity of middle distillate fuels. Environ Mol Mutag, 23: 234-238.
McLaren P (1985) Behaviour of diesel fuel on a high energy beach. Mar
Pollut Bull, 16: 191-196.
Meiss R, Robenek H, Schubert M, Themann H, & Heinrich U (1981)
Ultrastructural alterations in the livers of golden hamsters following
experimental chronic inhalation of diluted diesel exhaust emission.
Int Arch Occup Environ Health, 48: 147-157.
Menne RJ, Rechs M, Horrocks R & Harbolla B (1994) [Current and future
trends of passenger car diesel engines]. Motortech Z, 55: 323
(in German, with English abstract).
Mentnech MS, Lewis DM, Olenchock SA, Mull JC, & Koller WA (1984)
Effects of coal dust and diesel exhaust on immune competence in rats.
J Toxicol Environ Health, 13: 31-41.
Mercedes-Benz AG (1994a) [Exhaust emissions: Threshold limits,
regulations and measurement of exhaust emissions; calculation of the
fuel use from the exhaust test; passenger cars (May 1994)
Mercedes-Benz]. Stuttgart, 19 pp (in German).
Mercedes-Benz AG (1994b) Limit values for diesel exhaust components.
Data sheet. Stuttgart, 7 pp.
Mészáros A & Mészáros E (1989) Sulfate formation on elemental carbon
particulate. J Aerosol Sci Technol, 10: 337-342.
Metz N (1989) Diesel particulate emission in Germany (West). Exp
Pathol, 37: 15-22.
Meyers PA & Quinn JG (1973) Association of hydrocarbons and mineral
particles in saline solution. Nature, 244: 23-24.
Misiorowski RL, Strom KA, Vostal JJ, & Chvapil M (1980) Lung
biochemistry of rats chronically exposed to diesel particulates. In:
Pepelko WE, Danner RM, & Clarke NA, eds, Health effects of diesel
engine emissions, Vol 1 (EPA-600/9-80-057a). Cincinnatti, OH, US
Environmental Protection Agency, pp 465-480.
Mitchell AD, Evans EL, Jotz MM, Riccio ES, Mortelmans KE, & Simmon VF
(1981) Mutagenic and carcinogenic potency of extracts of diesel and
related environmental emissions: in vitro mutagenesis and DNA
damage. Environ Int, 5: 393-401.
Mohr, U, ed. (1992) International classification of rodent tumours.
Part 1. The rat (IARC Scientific Publications No. 122). Lyon,
International Agency for Research on Cancer.
Mohr U & Riebe-Imre M (1992) [Tests on the in vitro transformation
of epithelial cells of the respiratory tract by automotive exhaust
gases]. In: [Effects of diesel engine exhausts on health.] Munich, GSF
Research Centre for Environment and Health Inc., pp 31-38 (in German).
Moolgavkar S & Knudson A (1981) Mutation and cancer: A model for human
carcinogenesis. J Natl Cancer Inst, 66: 1037-1052.
Moolgavkar S & Venzon D (1979) Two-event models for carcinogenesis:
Incidence curves for childhood and adult tumors. Math Biosci,
47: 55-77.
Moorman WJ, Clark JC, Pepelko WE, & Mattox J (1985) Pulmonary function
responses in cats following long-term exposure to diesel exhaust.
J Appl Toxicol, 5: 301-305.
Morgan P & Watkinson RJ (1988) Hydrocarbon degradation in soils and
methods for soil biotreatment. Crit Rev Biotechnol, 8: 305-333.
Morimoto K, Kitamura M, Kondo H, & Koizumi A (1986) Genotoxicity of
diesel exhaust emissions in a battery of in-vitro short-term and
in-vivo bioassays. In: Ishinishi N, Koizumi A, McClellan RO, &
Stöber W, eds, Carcinogenic and mutagenic effects of diesel engine
exhaust. Amsterdam, Elsevier, pp 85-101.
Morrow JE (1973) Oil-induced mortalities in juvenile coho and sock-eye
salmon. J Mar Res, 31: 135-141.
Morrow PE (1988) Possible mechanisms to explain dust overloading of
the lungs. Fundam Appl Toxicol, 10: 369-384.
Muhlbaier Dasch J & Cadle SH (1989) Atmospheric carbon particle in the
Detroit urban area: Wintertime sources and sinks. Aerosol Sci Technol,
10: 236-248.
Muhle H, Creutzenberg O, Bellmann B, Heinrich U, & Mermelstein R
(1990) Dust overloading of lungs: Investigations of various materials,
species differences, and irreversibility of effects. J Aerosol Med,
3: 111-128.
Muhle H, Bellmann B, & Creutzenberg O (1994) Toxicokinetics of solid
particles in chronic rat studies using diesel soot, carbon black,
toner, titanium dioxide, and quartz. In: Mohr U, Dungworth DL,
Mauderly JL, & Oberdörster G, eds, Toxic and carcinogenic effects of
solid particles in the respiratory tract. Washington DC, International
Life Sciences Institute Press, pp 29-41.
Muralidhara KMK, Krishnakumari HP, Ramesh HP, & Majumder SK (1982)
Toxicity of some petroleum fractions used in pesticidal emulsions to
albino rats. J Food Sci Technol, 19: 260-262.
Muranaka M, Suzuki S, Koizumi K, Takafuji S, Miyamoto T, Ikemori R, &
Tokiwa H (1986) Adjuvant activity of diesel-exhaust particulates for
the production of IgE antibody in mice. J Allergy Immunol,
77: 616-623.
Neff JM, Cox BA, Dixit D, & Anderson JW (1976a) Accumulation and
release of petroleum-derived aromatic hydrocarbons by four species of
marine animals. Mar Biol, 38: 279-289.
Neff JM, Anderson JW, Cox BA, Laughlin RB Jr, Rossi SS, & Tatem HE
(1976b) Effects of petroleum on survival, respiration and growth of
marine animals. In: Sources, effects and sinks of hydrocarbons in the
aquatic environment. Proceedings of the symposium, American
University, Washington DC, 9-11 August 1976. Washington DC, American
Institute of Biological Sciences, pp 516-539.
Nesnow S, Triplett LL, & Slaga TJ (1982a) Comparative tumor-initiating
activity of complex mixtures from environmental particulate emissions
on SENCAR mouse skin. J Natl Cancer Inst, 68: 829-834.
Nesnow S, Evans C, Stead A, Creason J, Slaga TJ, & Triplett LL (1982b)
Skin carcinogenesis studies of emission extracts. In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 295-320.
Nesnow S, Triplett LL, & Slaga TJ (1983) Mouse skin tumor
initiation-promotion and complete carcinogenesis bioassays: Mechanisms
and biological activities of emission samples. Environ Health
Perspectives, 47: 255-268.
Netterstrom B (1988) Cancer incidence among urban bus drivers in
Denmark. Int Arch Occup Environ Health, 61: 217-221.
Newton JM, Rothman BS, & Walker FA (1991) Separation and determination
of diesel contaminants in various fish products by capillary gas
chromatography. J Assoc Off Anal Chem, 74: 986-990.
Nikula KJ Snipes MB Barr EB Griffith WC Henderson RF & Mauderly JL
(1994) Influence of particle-associated organic compounds on the
carcinogenicity of diesel exhaust. In: Mohr U, Dungworth DL, Mauderly
JL, & Oberdörster G, eds, Toxic and carcinogenic effects of solid
particles in the respiratory tract. Washington DC, International Life
Science Institute Press, pp 565-568.
Notani PN, Shah P, Jayant K, & Balakrishnan V (1993) Occupation and
cancers of the lung and bladder: A case-control study in Bombay.
Int J Epidemiol, 22: 185-191.
Novakov T (1984) The role of soot and primary oxidants in atmospheric
chemistry. Sci Total Environ, 36: 1-10.
Oberdörster G (1988) Lung clearence of inhaled insoluble and soluble
particles. J Aerosol Med, 1: 289-330.
Oberdörster G & Yu CP (1990) The carcinogenic potential of inhaled
diesel exhaust: A particle effect? J Aerosol Sci, 21: 397-401.
Oberdörster G, Baggs R, Gelein R, Corson N, Mercer P, Nguyen K, &
Pepelko W (1995) Pulmonary effects of inhaled carbon black in rats.
Toxicologist, 15: 46.
Obländer K (1991) [Possibility of pollutant risk reduction in diesel
engines.] In: [Carcinogenic substances in the environment: Origin,
measurement, risk, minimization. Meeting of the Commission for Clean
Air of the Society of German Engineers (VDI) and the German Standards
Institute (DIN), Mannheim, 23-25 April]. Düsseldorf, Society of German
Engineers Press, pp 245-253 (in German).
Oehme M, Larssen S, & Brevik EM (1991) Emisson factors of PCDD and
PCDF for road vehicles obtained by tunnel experiment. Chemosphere,
23: 1699-1708.
Oelert HH & Florian T (1972) [Registration and assessment of the odour
intensity of diesel exhaust.] Staub Reinhalt Luft, 32: 400-407
(in German).
Ohgaki H, Hasegawa H, Kata T, Negishi C, Sato S, & Sugimura T (1985)
Absence of carcinogenicity of 1-nitropyrene, correction of previous
results, and new demostration of carcinogenicity of 1,6-dinitropyrene
in rats. Cancer Lett, 25: 239-245.
Ong T, Whong W-Z, Xu J, Burchell B, Green FHY & Lewis T (1985)
Genotoxicity studies of rodents exposed to coal dust and diesel
emission particulates. Environ Res, 37: 399-409.
Ong T, Whong W-Z, Steward J & Brockman HE (1986) Chlorophyllin: A
potent antimutagen against environmental and dietary complex mixtures.
Mutat Res, 173: 111-115.
Organisation for Economic Co-operation and Development (1993) Control
of emissions from heavy-duty vehicles (OECD Environment Monographs
No. 55). Paris, 121 pp.
Oviatt C, Frithsen J, Gearing J, & Gearing P (1982) Low chronic
additions of No. 2 fuel oil: Chemical behavior, biological impact and
recovery in a simulated estuarine environment. Mar Ecol Prog Ser,
9: 121-136.
Parker GA, Bogo V, & Young RW (1981) Acute toxicity of conventional
versus shale-derived JP-5 jet fuel: Light microscopic, hematologic,
and serum chemistry studies. Toxicol Appl Pharmacol, 57: 302-317
Partanen T, Heikkilä P, Hernberg S, Kauppinen T, Moneta G, & Ojajärvi
A (1991) Renal cell cancer and occupational exposure to chemical
agents. Scand J Work Environ Health, 17: 231-239.
Partridge PA, Shala FJ, Cernansky NP, & Suffet IH (1987)
Characterization and analysis of diesel exhaust odor. Environ Sci
Technol, 21: 403-408.
Patton JD, Maher VM, & McCormick JJ (1986) Cytostatic and mutagenic
effects of 1-nitropyrene and 1-nitrosopyrene in diploid human
fibroblasts. Carcinogenesis, 7: 89-93.
Pederson TJ & Siak JS (1981) The role of nitroaromatic compounds in
the direct-acting mutagenicity of diesel particle extracts. J Appl
Toxicol, 1: 54-60.
Pepelko WE (1982) Effects of 28 days exposure to diesel engine
emissions in rats. Environ Res, 27: 16-23.
Pepelko WE & Chen C (1993) Quantitative assessment of cancer risk from
exposure to diesel engine emissions. Regul Toxicol Pharmacol,
17: 52-65.
Pepelko WE & Peirano WB (1983) Health effects of exposure to diesel
engine emissions. A summary of animal studies conducted by the US
Environmental Protection Agency's Health Effects Research Laboratories
at Cincinnati, Ohio. J Am Coll Toxicol, 2: 253-306.
Pepelko WE, Mattox J, Moorman WJ, & Clark JC (1981) Pulmonary function
evaluation of cats after one year of exposure to diesel exhaust.
Environ Int, 5: 373-376.
Pereira MA (1982) Genotoxicity of diesel exhaust emissions in
laboratory animals. In: Lewtas J, ed., Toxicological effects of
emissions from diesel engines. Amsterdam, Elsevier, pp 265-276.
Pereira MA, McMillan L, Kaur P, Gulati DK, & Sabharwal PS (1982)
Effect of diesel exhaust emissions, particulates, and extract on
sister chromatid exchange in transplacentally exposed fetal hamster
liver. Environ Mutag, 4: 215-220.
Perez Rodriguez E, Latour Perez J, Aller Alvarez JL, Alix J, & Alix Y
(1976) [Diesel fuel pneumonia. Presentation of a case.] Rev Clin Esp,
143: 397-400 (in Spanish).
Peters RW, Montemagno CD, & Shem L (1992) Surfactant screening of
diesel-contaminated soil. Hazard Waste Mater, 9: 113-136.
Petersen BA & Chuang CC (1982) Methodology of fractionation and
partition of diesel exhaust particulate samples. In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 51-67.
Piantadosi S (1994) Invited commentary: Ecologic biases. Am J
Epidemiol, 139: 761-764.
Pipho MJ, Ambs JL, & Kittelson DB (1986) In-cylinder measurements of
particulate formation in an indirect injection diesel engine (SAE
Technical Paper No. 860024). Warrendale, PA, Society of Automotive
Engineers, 9 pp.
Pitts JN Jr, Van Cauwenberghe KA, Grosjean D, Schmid JP, Fitz DR,
Belser WL Jr, Knudson GB, & Hynds PM (1978) Atmospheric reactions of
polycyclic aromatic hydrocarbons: Facile formation of mutagenic nitro
derivatives. Science, 202: 515-519.
Pitts JN Jr, Lokensgard DM, Harger W, Fisher TS, Mejia V, Schuler JJ,
Scorziell GM, & Katzenstein YA (1982) Mutagens in diesel exhaust
particulate. Identification and direct activities of
6-nitrobenzo( a)pyrene, 9-nitroanthracene, 1-nitropyrene and
5 H-phenanthrene(4,5- bcd)pyran-5-one. Mutat Res, 103: 241-249.
Pitts JN Jr, Sweetman JA, Zielinska B, Winer AM, & Atkinson R (1985)
Determination of 2-nitrofluoranthene and 2-nitropyrene in ambient
particulate organic matter: Evidence for atmospheric reactions. Atmos
Environ, 19: 1601-1608.
Plopper CG, Hyde DM, & Weir AJ (1983) Centriacinar alterations in
lungs of cats chronically exposed to diesel exhaust. Lab Invest,
49: 391-399.
Poirier A, Baudin Laurencin F, Bodennec G, & Quentel C (1986)
[Experimental poisoning of the rainbow trout, Salmo gairdneri
Richardson, by diesel engine fuel: Haematological changes, histology].
Aquaculture, 55: 115-137 (in French).
Pope CA III & Dockery DW (1992) Acute health effects of PM10 pollution
on symptomatic and asymptomatic children. Am Rev Respir Dis,
145: 1123-1128.
Pople A, Simpson RD, & Cairns SC (1990) An incident of southern ocean
oil pollution: Effects of a spillage of diesel fuel on the rocky shore
of Macquarie Island (sub-Antarctic). Aust J Mar Freshwater Res,
41: 603-620.
Porter HO (1990) Aviators intoxicated by inhalation of JP-5 fuel
vapors. Aviat Space Environ Med, 61: 654-656.
Portier C, Hedges J, & Hoel D (1986) Age-specific models of mortality
and tumor onset for historical control animals in the National
Toxicology Program's carcinogenicity experiments. Cancer Res,
46: 4372-4378.
Pott F (1991) [Diesel engine exhaust -- results of laboratory animal
experiments relative to risk estimation]. In: [Carcinogenic substances
in the environment: Origin, measurement, risk, minimization. Meeting
of the Commission for Clean Air of the Society of German Engineers
(VDI) and the German Standards Institute (DIN), Mannheim, 23-25
April]. Düsseldorf, Society of German Engineers Press, pp 211-244
(in German).
Pott F & Heinrich U (1987) [Diesel engine exhaust and lung cancer.
Data from animal experiments and their evaluation with respect to the
hazard for humans]. Düsseldorf, Association for the promotion of
Environmental Health and Silicosis Research, Vol. 19, pp 130-167
(in German).
Pott F & Roller M (1994) Relevance of non-physiologic exposure routes
for carcinogenicity studies of solid particles. In: Mohr U, Dungworth
DL, Mauderly JL, & Oberdörster G, eds, Toxic and carcinogenic effects
of solid particles in the respiratory tract. Washington DC,
International Life Sciences Institute Press, pp 109-125.
Pott F, Heinrich U & Roller M (1993) [Diesel engine emissions.] In:
Wichmann HE, Schlipköter HW, & Fülgraff G, eds, [Handbook of
environmental medicine]. Landsberg, Lech, Ecomed mbH, 77 pp
(in German).
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann PG,
Roller M, Rippe RM, & Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Ann Occup Hyg,
38 (Suppl 1): 357-363.
Prakash CB, Rideout GB, & Kirshenblatt M (1992) Exhaust emissions from
diesel powered urban transit buses (Paper No. IU-17A). In: Proceedings
of the 9th World Clean Air Congress and Exhibition, Montreal, 30
August-4 September 1992, Ottawa, Environment Canada, Vol. 2, 22 pp.
Pryor P (1983) Health Hazard Evaluation. Trailways Bus System, Denver,
CO (PB85-102952, Report No HETA 81-416-1334). Cincinnati, OH, National
Institute for Occupational Safety and Health, 11 pp.
Pulich WM Jr, Winters K, & Van Baalen C (1974) The effects of a No. 2
fuel oil and two crude oils on the growth and photosynthesis of
microalgae. Mar Biol, 28: 87-94.
Purdham JI, Holness DL, & Pilger CW (1987) Environmental and medical
assessment of stevedores employed in ferry operations. Appl Ind Hyg,
2: 133- 139.
Quality of Urban Air Review Group (1993) Diesel vehicle emissions and
urban air quality. Birmingham, 88 pp.
Quinto I & De Marinis E (1984) Sperm abnormalities in mice exposed to
diesel particulate. Mutat Res, 130: 242.
Raabe OG, Al-Bayati MA, Teague SV, & Rasolt A (1988) Regional
deposition of inhaled monodisperse coarse and fine aerosol particles
in small laboratory animals. Ann Occup Hyg, 32 (Suppl 1): 53-63.
Rasmussen RE (1990) Effect of fuel properties on mutagenic activity in
extracts of heavy-duty diesel exhaust particulate. J Air Waste Manage
Assoc, 40: 1391-1396.
Reger R, Hancock J, Hankinson J, Hearl F, & Merchant J (1982) Coal
miners exposed to diesel exhaust emissions. Ann Occup Hyg,
26: 799-815.
Regnier ZR & Scott BF (1975) Evaporation rates of oil components.
Environ Sci Technol, 9: 469-472.
Reidenberg MM, Powers DV, Sevy RW, & Bello CT (1964) Acute renal
failure due to nephrotoxins. Am J Med Sci, 247: 25-29.
Rice SD, Short JW, & Karinen JF (1976) Toxicity of Cook Inlet crude
oil and No. 2 fuel oil to several Alaskan marine fishes and
invertebrates. In: Sources, effects and sinks of hydrocarbons in the
aquatic environment. Washington DC, American Institute of Biological
Sciences, pp 395-406.
Rice SD, Short JW, & Karinen JF (1977a) Comparative oil toxicity and
comparative animal sensitivity. In: Wolfe DA, Anderson JW, Button DK,
Malins, DC, Roubal T, & Varanasi U, eds, Fate and effects of petroleum
hydrocarbons in marine organisms and ecosystems. Oxford, Pergamon
Press, pp 78-94.
Rice SD, Thomas RE, & Short JW (1977b) Effect of petroleum
hydrocarbons on breathing and coughing rates and hydrocarbons
uptake-depuration in pink salmon fry. In: Verberg JF, Calabrese A,
Thurberg FP, & Vernberg WB, eds, Physiological responses of marine
biota to pollutants. New York, Academic Press, pp 259-277.
Risch HA, Burch JD, Miller AB, Hill GB, Steele R, & Howe GR (1988)
Occupational factors and the incidence of cancer of the bladder in
Canada. Br J Ind Med, 45: 361-367.
Rogge WF, Hildemann LM, Mazurek MA, Cass GR & Simonelt BRT (1993)
Sources of fine organic aerosol 2. Noncatalyst and catalyst-equipped
automobiles and heavy-duty diesel trucks. Environ Sci Technol,
27: 636-651.
Roller M & Pott F (1994) Use of experimental data for risk assessment
of fibrous dusts and other aerosols. Inf Biom Epidemiol Med Biol,
25: 301-311.
Rossi SS (1977) Bioavailability of petroleum hydrocarbons from water,
sediments and detritus to the marine annelid Neanthes arenaceodentata.
In: Proceedings, 1977 Oil Spill Conference. Washington DC, American
Petroleum Institute, pp 621-625.
Rossi SS & Anderson JW (1976) Toxicity of water-soluble fractions of
No. 2 fuel oil and South Louisiana crude oil to selected stages in the
life history of the polychaete, Neanthes arenaceodentata. Bull Environ
Contam Toxicol, 16: 18-24.
Rossi SS & Anderson JW (1978) Effects of No. 2 fuel oil
water-soluble-fractions on growth and reproduction in Neanthes
arenaceodentata (Polychaeta: Annelida). Water Air Soil Pollut,
9: 155-170.
Rossi SS, Anderson JW, & Ward GS (1976) Toxicity of water-soluble
fractions of four test oils for the polychaetous annelids Neanthes
arenaceodentata and Capitella capitata. Environ Pollut, 10: 9-18.
Rudell B, Marqvardsen MD, Lindström O, & Kolmodin-Hedman B (1989) A
method for controlled exposure to diesel exhaust from a continuously
idling diesel engine. J Aerosol Sci, 20: 1281-1284.
Rudell B, Sandström T, Stjernberg N, & Kolmodin-Hedman B (1990)
Controlled diesel exhaust exposure in an exposure chamber: Pulmonary
effects investigated with bronchoalveolar lavage. J Aerosol Sci,
21: 411-414.
Ruder AM, Fine LJ, & Sundin DS (1990) National estimates of
occupational exposure to animal bladder-tumorigens. J Occup Med,
32: 797-805.
Rushton L, Alderson MR, & Nagarajah CR (1983) Epidemiological survey
of maintenance workers in London transport executive bus garages and
Chiswick Works. Br J Ind Med, 40: 340-345.
Russell TJ (1989) Additives influencing diesel fuel combustion. In:
Owen K, ed., Gasoline and diesel fuel additives (Critical reports on
applied chemistry, Vol 25). Chichester, John Wiley & Sons, pp 65-104.
Rykowski RA & Brochu AJ (1986) Diesel particulate emissions: The
United States experience, with extrapolations to Europe and Japan. In:
Lee SD, Schneider I, Grant LD, & Verkerk PJ, eds, Aerosols. Chelsea,
MI, Lewis Publishers Inc., pp443-457.
Sagai M, Saito H, Ichinose T, Kodama M, & Mori Y (1993) Biological
effects of diesel exhaust particles. I. In vitro production of
superoxide and in vivo toxicity in mouse. Free Radicals Biol Med,
14: 37-47.
Salmeen IT, Pero AM, Zator R, Schuetzle D, & Riley TL (1984) Ames
assay chromatograms and the identification of mutagens in diesel
particle extracts. Environ Sci Technol, 18: 375-382.
Salmeen IT, Gorse Jr RA, & Pierson WR (1985) Ames assay chromatograms
of extracts of diesel exhaust particles from heavy-duty trucks on the
road and from passenger cars on a dynamometer. Environ Sci Technol,
19: 270-273.
Sandmeyer EE (1981) Aliphatic hydrocarbons. In: Clayton GD & Clayton
FE, eds, Patty's industrial hygiene and toxicology, Vol. 2B,
Toxicology. New York, John Wiley & Sons, pp 3175-3431.
Sarojini R, Khan AK, & Nagabhushanam R (1989) Effect of petroleum
hydrocarbons (petrol and diesel) on the physiology of the crab,
Barytelphusa cunicularis I -- oxygen consumption. J Environ Biol,
10: 363-365.
Scheepers PTJ & Bos RP (1992a) Combustion of diesel fuel from a
toxicological perspective. I. Origin of incomplete combustion
products. Int Arch Occup Environ Health, 64: 149-161.
Scheepers PTJ & Bos RP (1992b) Combustion of diesel fuel from a
toxicological perspective. II. Toxicity. Int Arch Occup Environ
Health, 64: 163-177.
Scheepers PTJ, Velders DD, Martens MHJ, Noordhoek J, & Bos RP (1994a)
Gas chromatographic-mass spectrometric determination of nitro
polycyclic aromatic hydrocarbons in airborne particulate matter from
workplace atmospheres contaminated with diesel exhaust. J Chromatogr,
677: 107-121.
Scheepers PTJ, Thuis HJTM, Martens MHJ, & Bos RP (1994b) Assessment of
occupational exposure to diesel exhaust. The use of an immunoassay for
the determination of urinary metabolites of nitroarenes and polycyclic
aromatic hydrocarbons. Toxicol Lett, 72: 191-198.
Schenker MB & Speizer FE (1979) A retrospective cohort study of diesel
exhaust exposure in railroad workers: Study design and methodologic
issues. In: Pepelko WE, Danner RB, & Clark NA, eds, Health effects of
diesel engine emissions (EPA-600/9-80-057b). Cincinnati, OH,
Environmental Protection Agency.
Schenker MB, Smith T, Muñoz A, Woskie S, & Speizer FE (1984) Diesel
exposure and mortality among railway workers: Results of a pilot
study. Br J Ind Med, 41: 320-327.
Schenker MB, Kado NY, Hammond SK, Samuels SJ, Woskie SR, & Smith TJ
(1992) Urinary mutagenic activity in workers exposed to diesel
exhaust. Environ Res, 57: 133-148.
Schiffmann D & Henschler D (1992) [Studies of diesel engine exhaust
fractions on the genotoxic and cell-transforming properties with the
model of Syrian hamster embryo fibroblasts and with lung cells]. In:
[Effects of diesel engine exhausts on health]. Munich, GSF Research
Centre for Environment and Health Inc. pp 39-42 (in German).
Schuetzle D (1983) Sampling of vehicle emissions for chemical analysis
and biological testing. Environ Health Perspectives, 47: 65-80.
Schuetzle D & Frazier JA (1986) Factors influencing the emission of
vapor and particulate phase components from diesel engines. In:
Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic
and mutagenic effects of diesel engine exhaust. Amsterdam, Elsevier,
pp 41-63.
Schuetzle D & Perez JM (1981) A CRC cooperative comparison of
extraction and HPLC techniques for diesel particulate emissions (Paper
81-564). In: Proceedings of the 74th Annual Meeting of the Air
Pollution Control Association, 16-21 June 1981, Philadelphia.
Philadelphia, PA, Air Pollution Control Association, pp 1-32.
Schuetzle D & Perez JM (1983) Factors influencing the emissions of
nitrated-polynuclear aromatic hydrocarbons (nitro-PAH) from diesel
engines. J Air Pollut Control Assoc, 33: 751-755.
Schuetzle D, Riley TL, Prater TJ, Harvey TM, & Hunt DF (1982) Analysis
of nitrated polycyclic aromatic hydrocarbons in diesel particulates.
Anal Chem, 54: 265-271.
Schuetzle D, Jensen TE, & Ball JC (1985) Polar polynuclear aromatic
hydrocarbon derivatives in extracts of particulates: Biological
characterization and techniques for chemical analysis. Environ Int,
11: 169-181.
Schuler RL & Niemeier RW (1981) A study of diesel emissions on
Drosophila. Environ Int, 5: 431-434.
Schultz T, Witschi H, Smith L, Haschek W, Holland J, Epler J, Fry R,
Rao T, Larimer F, & Dumont J (1981) Health effects research in oil
shale development (ORNL/TM-8034). Oak Ridge, TN, Oak Ridge National
Laboratories, 56 pp.
Schwartz J (1993) Air pollution and daily mortality in Birmingham,
Alabama. Am J Epidemiol, 136: 1136-1147.
Schwartz J, Slater D, Larson TV, Pierson WE, & Koenig JO (1993)
Particulate air pollution and hospital emergency room visits for
asthma in Seattle. Am Rev Respir Dis, 147: 826-831.
Schwind KH, Thoma H, Hutzinger O, Dawidowsky N, Weberuss, Hagenmaier
H, Bühler U, Greiner R, Essers U, & Bessey E (1991) [Emission of
halogenated dibenzodioxins (PXDD) and dibenzofurans (PXDF) from
internal combustion engines]. Z Umweltchem Ökotox 3: 291-298
(in German).
Shefner AM, Collins BR, Dooley L, Fiks A, Graf JL, & Preache MM (1982)
Respiratory carcinogenicity of diesel fuel emissions. Interim results.
In: Lewtas J, ed., Toxicological effects of emissions from diesel
engines. Amsterdam, Elsevier, pp 329-350.
Siemiatycki J, Dewar R, Nadon L, Gérin M, Richardson L, & Wacholder S
(1987) Associations between several sites of cancer and twelve
petroleum-derived liquids: Results from a case-referent study in
Montreal. Scand J Work Environ Health, 13: 493-504.
Siemiatycki J, Gérin M, Stewart P, Nadon L, Dewar R, & Richardson L
(1988) Associations between several sites of cancer and ten types of
exhaust and combustion products. Scand J Environ Health, 14: 79-90.
Sienicki EJ & Mago RS (1992) Re-evaluation of diesel engine
particulate emission inventories. In: Toxic air pollutants from mobile
sources. Pittsburgh, PA, Air and Waste Management Association,
pp 151-164.
Silverman DT, Hoover RN, Albert S, & Graff KM (1983) Occupation and
cancer of the lower urinary tract in Detroit. J Natl Cancer Inst,
70: 237-245.
Silverman DT, Hoover RN, Mason TJ, & Swanson GM (1986) Motor
exhaust-related occupations and bladder cancer. Cancer Res,
46: 2113-2116.
Singh AK, & Gaur JP (1990) Effects of petroleum oils and their
paraffinic, asphaltic, and aromatic fractions on photosynthesis and
respiration of microalgae. Ecotoxicol Environ Saf, 19: 8-16.
Snipes MB & McClellan RO (1985) Retention of 14C-labeled
aluminosilicate particles inhaled by dogs and guinea pigs -- simulation
model projections for humans. In: Inhalation Toxicology Research
Institute Annual report 1984-1985, Albuquerque, NM, pp 96-99.
Snipes MB, Boecker BB, & McClellan RO (1983) Retention of monodisperse
or polydisperse aluminosilicate particle inhaled by dogs, rats, and
mice. Toxicol Appl Pharmacol, 69: 345-362.
Song H-G, Wang X, & Bartha R (1990) Bioremediation potential of
terrestrial fuel spills. Appl Environ Microbiol, 56: 652-656.
Standardization Board in Sweden (1991) Swedish Standard SS 15 54 35:
Diesel fuel oil for high-speed diesel engines. Stockholm.
State Committee for Immission Protection (1992) [Diesel motor
emissions (DME)]. In: [Cancer risk through polluted air. Development
of 'Evaluation standards for cancerogenic air pollution' commissioned
by the Conference for Ministers of the Environment], Düsseldorf,
pp 89-93 (in German).
Steenland K, Burnett C, & Osorio AM (1987) A case-control study of
bladder cancer using city directories as a source of occupational
data. Am J Epidemiol, 126: 247-257.
Steenland NK, Silverman DT, & Hornung RW (1990) Case-control study of
lung cancer and truck driving in the Teamsters Union. Am J Public
Health, 80: 670- 674.
Steenland K, Silverman D, & Zaebst D (1992) Exposure to diesel exhaust
in the trucking industry and possible relationships with lung cancer.
Am J Ind Med, 21: 887-890.
Steineck G, Plato N, Gerhardsson M, Norell SE, & Hogstedt C (1990)
Increased risk of urothelial cancer in Stockholm during 1985-87 after
exposure to benzene and exhausts. Int J Cancer, 45: 1012-1017.
Stenberg U, Alsberg T, & Westerholm W (1983) Emission of carcinogenic
components with automobile exhausts. Environ Health Perspectives,
47: 53-56.
Stern FB, Curtis RA, & Lemen RA (1981) Exposure of motor vehicle
examiners to carbon monoxide: A historical prospective mortality
study. Arch Environ Health, 36: 59-66.
Stirling HP (1977) Effects of a spill of marine diesel oil on the
rocky shore fauna of Lamma Island, Hong Kong. Environ Pollut,
12: 93-117.
Stöber W (1986) Experimental induction of tumors in hamsters, mice and
rats after long-term inhalation of filtered and unfiltered diesel
engine exhaust. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 421-439.
Stone WA (1991) Assessing health risks associated with diesel
contaminated soils and groundwater. In: Calabrese EJ & Kostecki PT,
eds, Hydrocarbon contaminated soils, Vol. 1, Remediation techniques,
environmental fate, risk assessment, analytical methodologies,
regulatory considerations. Chelsea, MI, Lewis Publishers, pp 167-179.
Strom KA, Chan TL, & Johnson JT (1988) Pulmonary retention of inhaled
submicron particles in rats: Diesel exhaust exposures and lung
retention model. Ann Occup Hyg, 32: 645-657.
Strom KA, Garg BD, Johnson JT, D'Arcy JB, & Smiler KL (1990) Inhaled
particle retention in rats receiving low exposures of diesel exhaust.
J Toxicol Environ Health, 29: 377-398.
Stromgren T & Nielsen MV (1991) Spawning frequency, growth and
mortality of Mytilus edulis larvae, exposed to copper and diesel
oil. Aquat Toxicol, 21: 171-180.
Stromgren T & Reiersen LO. (1988) A new method for testing toxicity of
drilling fluid; effect on growth of mussels. Oil Chem Pollut,
4: 127-138.
Stromgren T, Nielsen MV, & Ueland K (1986) Short-term effect of
microencapsulated hydrocarbons on shell growth of Mytilus edilus.
Mar Biol, 91: 33-39.
Sun JD, Wolff RD, & Kanapilly GM (1982) Deposition, retention, and
biological fate of inhaled benzo[ a]pyrene adsorbed onto ultrafine
particles and as a pure aerosol. Toxicol Appl Pharmacol,
65: 231-244.
Sun JD, Wolff RK, Kanapilly GM, & McClellan RO (1984) Lung retention
and metabolic fate of inhaled benzo( a)pyrene associated with diesel
exhaust particles. Toxicol Appl Pharmacol, 73: 48-59.
Suzuki T, Nakajima T, Maejima K, Kato A, Takaki Y, Kuwabara N,
Hisanaga A, & Ishinishi N (1990) [Long-term inhalation experiments on
the health effects of diesel exhaust]. Taiki Osen Gatukai-shi,
25: 192-205 (in Japanese, with English summary).
Swanson GM, Lin C-S, & Burns PB (1993) Diversity in the association
between occupation and lung cancer among black and white men. Cancer
Epidemiol Biomarkers Prev, 2: 313-320.
Swarin SJ & Williams RL (1980) Liquid chromatographic determination of
benzo[ a]pyrene in diesel exhaust particulate: Verification of the
collection and analytical methods. In: Björseth A & Dennis AJ, eds,
Polynuclear aromatic hydrocarbons, 4th International Symposium:
Chemistry and biological effects. Columbus, OH, Battelle Press,
pp 771-806.
Takafuji S, Suzuki S, Koizumi K, Tadokoro K, Miyamoto T, Ikemori R, &
Muranaka M (1987) Diesel-exhaust particulates inoculated by the
intranasal route have an adjuvant activity for IgE production in mice.
J Allergy Clin Immunol, 79: 639-645.
Takafuji S, Suzuki S, Muranaka M, & Miyamoto T (1989) Influence of
environmental factors on IgE production. In: Mast cells and the
allergic response (Ciba Foundation Symposium 147). Chichester, John
Wiley & Sons, pp 188-204.
Takaki Y, Kitamura S, Kuwabara N, & Fukuda Y (1989) Long-term
inhalation studies of exhaust from the diesel engine in F-344 rats:
The quantitative relationship between pulmonary hyperplasia and
anthracosis. Exp Pathol, 37: 56-61.
Takemoto K, Yoshimura H, & Katayama H (1986) Effects of chronic
inhalation exposure to diesel exhaust on the development of lung
tumors in di-isopropanol-nitrosamine-treated F344 rats and newborn
C57BL and ICR mice. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber
W, eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 311-327.
Tan W & Chen C. (1992) A nonhomogeneous stochastic model of
carcinogenesis and its applications. In: Arino O, Axelrod D, & Kimmel
M, eds, Mathematical population dynamics 2. Winnipeg, Manitoba, Wuerz
Publishing Co., pp 49-67.
Tarshis IB (1981) Uptake and depuration of petroleum hydrocarbons by
crayfish. Arch Environ Contam Toxicol, 10: 79-86.
Tatem HE (1977) Accumulation of naphthalenes by grass shrimp: Effects
on respiration, hatching, and larval growth. In: Wolfe DA, Anderson
JW, Button DK, Malins DC, Roubel T, & Varanasi U, eds, Fate and effect
of petroleum hydrocarbons in marine ecosystems and organisms. Oxford,
Pergamon Press, pp 201-209.
Tedengren M & Kautsky N (1987) Comparative stress response to diesel
oil and salinity changes of the blue mussel, Mytilus edilus, from
the Baltic and North seas. Ophelia, 28: 1-9.
Tedengren M, Arnér M, & Kautsky N (1988) Ecophysiology and stress
response of marine and brackish water Gammarus species (Crustacea
amphipoda) to changes in salinity and exposure to cadmium and
diesel-oil. Mar Ecol Prog Ser, 47: 107-116.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF, &
Rosenblatt DH, eds, Handbook of chemical property estimation methods.
Environmental behaviour of organic compounds. New York, McGraw-Hill
Book Co., pp 15:1-15:34.
Törnqvist M, Kautiainen A, Gatz RN, & Ehrenberg L (1988) Hemoglobin
adducts in animals exposed to gasoline and diesel exhausts. I.
Alkenes. J Appl Toxicol, 8: 159-170.
Tucker JD, Xu J, Stewart J, Baciu PC, & Ong T (1986) Detection of
sister chromatid exchanges induced by volatile genotoxicants. Teratog
Carcinog Mutag, 6: 15-21.
Ulfvarson U & Alexandersson R (1990) Reduction in adverse effect on
pulmonary function after exposure to filtered diesel exhaust. Am J Ind
Med, 17: 341-347.
Ulfvarson U, Alexandersson R, Aringer L, Svensson E, Hedenstierna G,
Hogstedt C, Holmberg B, Rosén G, & Sorsa M (1987) Effects of exposure
to vehicle exhaust on health. Scand J Work Environ Health,
13: 505-512.
Ullman TL, Hare CT, & Baines TM (1984) Influence of maladjustment on
emissions from two heavy-duty diesel bus engines (SAE Paper
No. 840416). Warrendale, PA, Society of Automotive Engineers, 11 pp.
Ungers LJ (1984) Measurement of exhaust emissions from diesel-powered
forklifts during operations in ammunition storage magazines (phase I)
(Report No. AD-A141 792). Cincinnati, OH, PEDCO Environmental Inc.,
57 pp.
Ungers LJ (1985) Measurement of exhaust emissions from diesel-powered
forklifts during operations in ammunition storage magazines (phase
II). Final report for the period July 23, 1984 to March 15, 1985
(Report No. AD-A153-092). Cincinnati, OH, PEI Associates, 47 pp.
Upreti RK, Das M, & Shanker R (1989) Dermal exposure to kerosene. Vet
Hum Toxicol, 31: 16-20.
US Department of Energy (1988) The motor fuel consumption model
fourteenth periodical report (DOE/OR/21400 -- H12, DE 89-011358).
Arlingston, VA, Energy and Environmental Analysis Inc., 16 pp.
US Environmental Protection Agency (1986) The risk assessment
guidelines of 1986 (EPA/600/8-87/045). Washington DC, Office of Health
and Environmental Assessment.
US Environmental Protection Agency (1991) Alpha 2µ-globulin:
Association with chemically induced renal toxicity and neoplasia in
the male rat (Risk Assessment Forum Series) (EPA/625/3-91/019F).
Washington DC, 118 pp.
US Environmental Protection Agency (1992a) Integrated risk information
system (IRIS). Reference concentration (RfC) file for inhalation
exposure for diesel engine emissions. Online (Verification date
6/25/92). Cincinnati, OH, Office of Health and Environmental
Assessment, 25 pp.
US Environmental Protection Agency (1992b) Regulation of fuels and
fuel additives: Standards for highway diesel fuel quality-sulfur
content. Final rule. Fed Reg, 57: 19535-19537.
US Environmental Protection Agency (1993) Motor vehicle-related air
toxics study (EPA 420-R-93-005). Ann Arbor, MI, Office of Mobile
Sources, pp 4.1-4.17, 9.1-9.62.
US National Institute for Occupational Safety and Health (1989a)
Health Hazard Evaluation Report. Consolidated Freightways Pocono
Summit, Pennsylvania (HETA 87-232-1948). Cincinnatti, OH, US
Department of Health and Human Services, 20 pp.
US National Institute for Occupational Safety and Health (1989b)
Health Hazard Evaluation Report. Consolidated Freightways Peru,
Illinois (HETA 88-077-1969). Cincinnatti, OH, US Department of Health
and Human Services, 31 pp.
US National Institute for Occupational Safety and Health (1990a) Final
report of the NIOSH health hazard evaluation (HHE) conducted at
Benedict Enterprises Dayton, Ohio (October 2 and November 5, 1987)
(HETA 87-237). Cincinnatti, OH, US Department of Health and Human
Services, 33 pp.
US National Institute for Occupational Safety and Health (1990b) Final
report of the NIOSH health hazard evaluation (HHE) conducted at
Madison Metro's Transit System's Maintenance and Administration
Facility (January 10-13, 1989) (HETA 88-218). Cincinnatti, OH, US
Department of Health and Human Services, 38 pp.
US National Institute for Occupational Safety and Health (1990c)
Health Hazard Evaluation Report. Yellow Freight System, Inc.,
Columbus, Ohio, Maybrook, New York (HETA 90-088-2110). Cincinnatti,
OH, US Department of Health and Human Services, 127 pp.
US National Institute for Occupational Safety and Health (1991)
Research plan. A sampling and analytical method for airborne
diesel-exhaust particels. Cincinnatti, OH, Centers for Disease
Control, National Institute for Occupational and Health, pp 1-34.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Committee on the Institutional Means
for Assessment of Risks to Public Health. Washington DC, National
Academy Press, pp 1-50.
US National Research Council (1985) Oil in the sea. Inputs, fates, and
effects. Washington DC, National Academy Press, 587 pp.
US National Toxicology Program (1986) Toxicology and carcinogenesis
studies of marine diesel fuel and JP-5 navy fuel (CAS-No 8008-20-6) in
B6C3F1 mice (dermal studies) (Technical report series No. 310).
Research Triangle Park, NC, 206 pp.
Vallyathan V, Virmani R, Rochlani S, Green FHY, & Lewis T (1986)
Effect of diesel emissions and coal dust inhalation on heart and
pulmonary arteries of rats. J Toxicol Environ Health, 19: 33-41.
Van Beckhoven LC (1991) Effects of fuel properties on diesel engine
emissions -- A review of information available to EEC-MVEG group (SAE
Technical Paper No. 910608). Warrendale, PA, Society of Automotive
Engineers, pp 237-249.
Vandermeulen JH & Lee RW (1986) Lack of mutagenic activity of crude
and refined oils in the unicellular alga Chlamydomonas reinhardtii.
Bull Environ Contam Toxicol, 36: 250-253.
Vandermeulen JH, Foda A, & Stuttard C (1985) Toxicity vs mutagenicity
of some crude oils, distillates and their water soluble fractions.
Water Res, 19: 1283-1289.
Van Vleet ES & Quinn JG (1978) Contribution of chronic petroleum
inputs to Narragansett Bay and Rhode Island Sound sediments. J Fish
Resour Board Can, 35: 536-543.
Vinegar A, Carson A, & Pepelko WE (1981) Pulmonary function changes in
Chinese hamster exposed 6 months to diesel exhaust. Environ Int,
5: 369-371.
Vineis P & Magnani C (1985) Occupation and bladder cancer in males: A
case-control study. Int J Cancer, 35: 599-606.
Volkswagen AG (1989) Unregulated motor vehicle exhaust gas components.
Wolfsburg, Research and Development, pp 1-128.
Vostal JJ (1986) Factors limiting the evidence for chemical
carcinogenicity of diesel emissions in long-term inhalation
experiments. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp. 381-396.
Wade WR & Jones CM (1984) Current and future light duty diesel engines
and their fuels (Paper No. 840105). In: International congress and
exposition, Detroit, Michigan, 27 February-2 March 1984. Warrendale,
PA, Society of Automotive Engineers.
Wade JF III & Newman LS (1993) Diesel asthma. Reactive airways disease
following overexposure to locomotive exhaust. J Occup Med,
35: 149-154.
Wade TL & Quinn JG (1980) Incorporation, distribution, and fate of
saturated petroleum hydrocarbons in sediments from a controlled marine
ecosystem. Mar Environ Res, 3: 15-34.
Wallace WE, Keane MJ, Hill CA, Xu J, & Ong T (1987) Mutagenicity of
diesel exhaust particles and oil shale particles dispersed in lecithin
surfactant. J Toxicol Environ Health, 21: 163-171.
Wallace WE, Keane MJ, Xing S, & Ong T (1990) Mutagenicity of diesel
exhaust soot dispersed in phospholipid surfactants. In: Seemayer NH &
Hadnagy W, eds, Environmental hygiene II. Berlin, Springer, pp 7-10.
Waller RE (1981) Trends in lung cancer in London in relation to
exposure to diesel fumes. Environ Int, 5: 479-483
Waller RE, Commins BT, & Lawther PJ (1965) Air pollution in a city
street. Br J Ind Med, 22: 128-138.
Waller RE, Hampton L, & Lawther PJ (1985) A further study of air
pollution in diesel bus garages. Br J Ind Med, 42: 824-830.
Walrath J, Rogot E, Murray J, & Blair A (1985) Mortality patterns
among US veterans by occupation and smoking status, Vol. 1 (NIH
Publication No. 85-2756). Washington DC, US Government Printing
Office, p 206.
Wan MT, Watts RG, & Moul DJ (1990) Acute toxicity to juvenile Pacific
salmonids and rainbow trout of butoxyethyl esters of 2,4-D, 2,4-DP and
their formulated product: Weedone CB and its carrier. Bull Environ
Contam Toxicol, 45: 604-611.
Wang X & Bartha R (1990) Effects of bioremediation on residues,
activity and toxicity in soil contaminated by fuel spills. Soil Biol
Biochem, 22: 501-505.
Waxweiler RJ, Wagoner JK, & Archer VE (1973) Mortality of potash
workers. J Occup Med, 15: 486-489.
Weidmann K, Menrad H, Reders K, & Hutcheson RC (1988) Diesel fuel
quality effects on exhaust emissions (SAE Technical Paper No. 881649).
Warrendale, PA, Society of Automotive Engineers, 11 pp.
Westerholm R (1987) Inorganic and organic compounds in emissions from
diesel powered vehicles. A literature survey (Report No; 3389).
Stockholm, National Swedish Environmental Protection Board.
Westerholm R & Egebäck KE (1991) Impact of fuels on diesel exhaust
emissions. A chemical and biological characterization (Report
No. 3968). Solna, National Swedish Environmental Protection Agency,
61 pp.
Westerholm R, Alsberg T, Strandell M, Frommelin A, Grigoriadis V,
Hantzaridou A, Maitra G, Winquist L, Rannug U, Egebäck KE, &
Bertilsson (1986) Chemical analysis and biological testing of
emissions from a heavy duty diesel truck with and without two
different particulate traps (SAE Technical Paper No. 860014).
Warrendale, PA, Society of Automotive Engineers, pp 73-83.
Wheeler RW, Hearl FJ, & McCawley M (1981) An industrial hygiene
characterization of exposure to diesel emissions in an underground
coal mine. Environ Int, 5: 485-488.
White HJ & Garg BD (1981) Early pulmonary response of the rat lung to
inhalation of high concentration of diesel particles. J Appl Toxicol,
1: 104-110.
WHO (1987) Air quality guidelines for Europe (WHO Regional
Publications, European Series No. 23). Copenhagen, Regional Office for
Europe.
WHO (1990) Revised guidelines for the preparation of Environmental
Health Criteria monographs (PCS/90.69). Geneva.
Whong W-Z, Wen Y-F, Stewart J, & Ong T-M (1986) Validation of the
SOS/umu test with mutagenic complex mixtures. Mutat Res, 175: 139-144.
Wichmann HE & Brüske-Hohlfeld I (1991) [Epidemiological findings on
the cancer risk due to diesel engine exhausts]. In: [Carcinogenic
substances in the environment: origin, measurement, risk,
minimization. Meeting of the Commission for Clean Air of the Society
of German Engineers (VDI) and the German Standards Institute (DIN),
Mannheim, 23-25 April]. Düsseldorf, Society of German Engineers Press,
pp 171-209 (in German).
Widdows J, Donkin P, & Evans SV (1985) Recovery of Mytilus edulis L.
from chronic oil exposure. Mar Environ Res, 17: 250-253.
Wiester MJ, Iltis R, & Moore W (1980) Altered function and histology
in guinea pigs after inhalation of diesel exhaust. Environ Res,
22: 285-297.
Willems MI, de Raat WK, Wesstra JA, Bakker GL, Dubois G, & van Dokkum
W (1989) Urinary and faecal mutagenicity in car mechanics exposed to
diesel exhaust and in unexposed office workers. Mutat Res,
222: 375-391.
Williams RR Stegens NL & Goldsmith JR (1977) Associations of cancer
site and type with occupation and industry from the Third National
Cancer Survey Interview. J Natl Cancer Inst, 59: 1147-1185.
Williams PT, Andrews GE, & Bartle KD (1987) The role of lubricating
oil in diesel particulate and particulate PAH emissions (SAE Technical
Paper No. 872084). Warrendale, PA, Society of Automotive Engineers,
pp 1-9.
Williams DJ, Milne JW, Quigley SM, Roberts DB, & Kimberlee MC (1989)
Particulate emissions from 'in-use' motor vehicles. II. Diesel
vehicles. Atmos Environ, 23: 2647-2661.
Winters K, Van Baalen C, & Nicol JAC (1977) Water soluble extractives
from petroleum oils: Chemical characterization and effects on
microalgae and marine animals. In: McIntyre AD & Whittle KJ, eds,
Petroleum hydrocarbons in the marine environment (Rapports et
procés-verbaux des réunions, Vol. 171). Copenhagen, International
Council for Exporation of the Sea, pp 166-174.
Witschi HP, Smith LH, Frome EL, Pequet-Goad ME, Griest WH, Ho CH, &
Guerin MR (1987) Skin tumorigenic potential of crude and refined coal
liquids and analogous petroleum products. Fundam Appl Toxicol,
9: 297-303.
Wolff RK, Griffis LC, Hobbs CH, & McClellan RO (1982) Deposition and
retention of 0.1 µm 67Ga2 O3 aggregate aerosols in rats
following whole body exposures. Fundam Appl Toxicol, 2: 195-200.
Wolff RK, Henderson RF, Snipes MB, Griffith WC, Mauderly JL, Cuddihy
RG, & McClellan RO (1987) Alterations in particle accumulation and
clearance in lungs of rats chronically exposed to diesel exhaust.
Fundam Appl Toxicol, 9: 154-166.
Wolff RK, Bond JA, Sun JD, Henderson RF, Harkema JR, Griffith WC,
Mauderly JL, & McClellan RO (1989) Effects of adsorption of
benzo[ a]pyrene onto carbon black particles on levels of DNA adducts
in lungs of rats exposed by inhalation. Toxicol Appl Pharmacol,
97: 289-299.
Wolff RK, Bond JA, Henderson RF, Harkema JR, & Mauderly JL (1990)
Pulmonary inflammation and DNA adducts in rats inhaling diesel exhaust
or carbon black. Inhal Toxicol, 2: 241-254.
Wong O, Mitchell LE, Wolff RK, Mauderly JL, & Jeffrey AM (1986)
Identification of DNA damage as a result of exposure of rats to diesel
engine exhaust. Carcinogenesis, 7: 1595-1597.
Woodin SA, Nyblade CF, & Chia F-S (1972) Effect of diesel oil spill on
invertebrates. Mar Pollut Bull, 3: 139-143.
Wormald AP (1976) Effects of a spill of marine diesel oil on the
meiofauna of a sandy beach at Picnic Bay, Hong Kong. Environ Pollut,
11: 117-130.
Woskie SR, Smith TJ, Hammond SK, Schenker MB, Garshick E, & Speizer FE
(1988a) Estimation of the diesel exhaust exposures of railroad
workers: I. Current exposures. Am J Ind Med, 13: 381-394.
Woskie SR, Smith TJ, Hammond SK, Schenker MB, Garshick E, & Speizer FE
(1988b) Estimation of the diesel exhaust exposures of railroad
workers: II. National and historical exposures. Am J Ind Med,
13: 395-404.
Wright ES (1986) Effects of short-term exposure to diesel exhaust on
lung cell proliferation and phospholipid metabolism. Exp Lung Res,
10: 39-55.
Wynder EL, Dieck GS, Hall NEL, & Lahti H (1985) A case-control study
of diesel exhaust exposure and bladder cancer. Environ Res,
37: 475-489.
Yaron B, Sutherland P, Galin T, & Acher AJ (1989) Soil pollution by
petroleum products: II. Adsorption-desorption of 'kerosene' vapors on
soils. J Contam Hydrol, 4: 347-358.
Yu CP & Xu GB (1987) Predictive models for depositon of inhaled diesel
exhaust particles in humans and laboratory species (Report No. 10).
Cambridge, MA, Health Effects Institute, 27 pp.
Yu CP & Yoon KJ (1990) Retention modeling of diesel exhaust particles
in rats and humans (Report No. 40). Cambridge, MA, Health Effects
Institute, 33 pp.
Yu CP, Yoon KJ, & Chen YK (1991) Retention modeling of diesel exhaust
particles in rats and humans. J Aerosol Med, 4: 79-115.
Zaebst DD, Clapp DE, Blade LM, Marlow DA, Steenland K, Hornung RW,
Scheutzle D, & Butler J (1991) Quantitative determination of trucking
industry workers' exposures to diesel exhaust particles. Am Ind Hyg
Assoc J, 52: 529-541.
PARTIE A COMBUSTIBLE DIESEL
A1. RESUME
A1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le combustible diesel est un mélange complexe d'alcanes normaux
ramifiés ou cycliques (de 60 à plus de 90% de volume; longueur de la
chaîne hydrocarbonée, généralement comprise entre C9 et C30), de
composés aromatiques, surtout des alkyl benzènes, (5 à 40% en volume)
et de petites quantités d'alcènes (0 à 10% en volume) obtenu lors de
la distillation fractionnée du pétrole, à partir du distillat moyen
qui correspond à la fraction gazole. Le combustible diesel peut
contenir, à des concentrations de quelques parties par million, du
benzène, du toluène, de l'éthylbenzène, du xylène et des hydrocarbures
aromatiques polycycliques (HAP), en particulier du naphtalène et
certains de ses dérivés méthylés. La teneur en soufre du combustible
diesel dépend de l'origine du pétrole et du procédé de raffinage
utilisé. Dans un certain nombre de pays, elle est soumise à
réglementation et se situe en général entre 0,05 et 0,5% en poids. On
utilise un certain nombre d'additifs pour modifier la viscosité, la
conservation et la combustion, pour différencier les différents
produits et également pour satisfaire aux spécifications commerciales.
A la température ambiante, le combustible diesel est en général
modérément volatile, légèrement visqueux, inflammable et se présente
sous la forme d'un liquide brun à odeur de kérosène. L'intervalle
d'ébullition est habituellement de 140°C à 385°C (plus de 588°C pour
le carburant destiné aux moteurs marins); à 20°C, la densité est de
0,87-1,0 g/cm3 et la solubilité dans l'eau de 0,2-5 mg/litre. La
qualité et la composition du combustible diesel influent
considérablement sur l'émission de polluants par les moteurs diesel.
Les conditions d'allumage (exprimées au moyen de l'indice de cétane),
la densité, la viscosité et la teneur en soufre sont des variables
importantes. Les spécifications du combustible diesel commercial
varient fortement d'un pays à l'autre.
Les différents types de mazout destinés au chauffage ou de
kérosène pour avions à réaction produits lors du raffinage du pétrole
peuvent avoir une composition analogue à celle du combustible diesel,
mais avec des additifs différents. Les données biologiques relatives à
ces mélanges ont donc été prises en considération lors de l'évaluation
toxicologique et écotoxicologique.
En raison de la complexité du mélange, il n'existe pas de méthode
d'analyse spécifique pour le combustible diesel et les techniques
utilisées pour la plupart des études d'impact dur l'environnement ne
se prêtent qu'au dosage des hydrocarbures totaux. Elles consistent
tout d'abord à effectuer une extraction par solvant, puis à éliminer
les hydrocarbures d'origine naturelle et enfin à procéder au dosage
par gravimétrie, spectrophotométrie infrarouge ou chromatographie en
phase gazeuse. Ni la méthode gravimétrique, ni la spectrophotométrie
infrarouge ne donnent de renseignements qualitatifs ou quantitatifs
utiles et ne peuvent donc être utilisées que pour un premier tri. La
chromatographie en phase gazeuse couplée à des techniques de détection
telles que l'ionisation de flamme ou la spectrométrie de masse, est la
méthode classique d'analyse des échantillons prélevés dans
l'environnement. Il existe de nombreuses autres méthodes pour la
recherche et le dosage des divers hydrocarbures présents dans le
combustible diesel.
A1.2 Sources d'exposition humaine et environnementale
Le combustible diesel est produit par raffinage du pétrole brut.
Afin de satisfaire aux spécifications techniques relatives au
rendement des moteurs, ces combustibles sont généralement mélangés;
l'adjonction ultérieure d'additifs en améliore également l'adaptation
à diverses utilisations particulières. Ces combustibles sont largement
utilisés dans les transports. Les plus volatils d'entre eux, qui
présentent une faible viscosité, sont destinés aux moteurs tournant à
haut régime, les combustibles plus lourds étant réservés aux
transports ferroviaires et maritimes. Une grande partie des véhicules
lourds de transport routier sont mus par des moteurs diesel. Les cars
à moteur diesel destinés au transport des voyageurs se répandent de
plus en plus en Europe et au Japon (10 à 25%) alors qu'en Amérique du
Nord, la proportion de cars assurant les transports en commun est
d'environ 1 à 2%, et a même une légère tendance à se tasser. On
utilise également le combustible diesel pour alimenter les chaudières
et les moteurs fixes, qu'il s'agisse de moteurs à pistons, de turbines
à gaz, de pompes d'oléoducs, de compresseurs, de générateurs de vapeur
pour centrales thermiques, de brûleurs, et d'installations de
chauffage industriel par convection ou circulation d'eau.
La demande mondiale de combustible diesel a régulièrement
progressé au cours des 5 dernières années. En 1985, les chiffres de
consommation étaient les suivants: environ 170 000 kilotonnes par an
en Amérique du Nord, environ 160 000 kilotonnes par an, gazole y
compris, dans l'Union européenne, environ 46 000 kilotonnes par an en
Australie, au Japon et en Nouvelle-Zélande, soit l'équivalent de
1062 kilotonnes par jour. En 1990, la demande mondiale a été estimée à
environ 1110 kilotonnes par jour.
On ne dispose d'aucun renseignement sur les émissions qui se
produisent lors de la production des combustibles diesel; toutefois,
il semble que cette source soit d'importance secondaire, étant donné
que le raffinage s'effectue en vase clos. Si des émissions doivent se
produire, c'est surtout au cours du stockage et du transport. Ces
combustibles peuvent également être répandus dans l'environnement par
suite de déversements accidentels ou dans des stations service lorsque
l'on fait de plein des véhicules. L'atmosphère et l'hydrosphère sont
les compartiments du milieu les plus fortement affectés par ces
décharges accidentelles. Il peut y avoir contamination du sol par du
combustible diesel à la faveur d'accidents et ce type de contamination
pose également un problème dans les gares de triage.
Parmi les nombreuses techniques utilisables pour nettoyer les
sols contaminés par du combustible diesel, on peut citer l'excavation,
les méthodes biologiques et le confinement.
A1.3 Transport, distribution et transformation dans
l'environnement
On ne dispose que très rares données sur la destinée du
combustible diesel dans l'environnement mais on suppose que son mode
de distribution et de transformation est comparable à celui des huiles
lourdes destinées au chauffage comme le No 2 qui a été bien étudié. En
cas de déversement dans l'eau, il se forme presque immédiatement une
nappe de mazout. Les constituants polaires et ceux dont la masse
moléculaire est relativement faible se dissolvent et s'éliminent de la
nappe par lessivage; les constituants volatils s'évaporent en surface
et il y a un début de dégradation microbienne. Par attaque chimique et
biologique, la composition de la nappe se modifie. Ces processus
dépendent de la température; les déversements qui se produisent en
milieu arctique conduisent à des nappes plus durables que sous les
climats tempérés. En milieu marin, la plupart des composés aromatiques
de masse moléculaire relative faible se dissolvent dans la phase
aqueuse, mais les alcanes normaux, les alcanes ramifiés, les
cycloalcanes et les composés aromatiques restantes peuvent demeurer
dans les sédiments pendant plus d'un an.
Bien qu'on ne dispose d'aucune donnée sur la photo-oxydation du
combustible diesel dans l'air et l'eau, on sait que les composants qui
s'évaporent subissent une décomposition photochimique. Ainsi, on a
montré que l'huile lourde No 2 subissait une photo-oxydation rapide en
milieu aqueux dans les conditions naturelles.
Les différents constituants du combustible diesel sont
intrinsèquement biodégradables, mais à des degrés et à des
vitesses variables. Les alcanes normaux ainsi que les dérivés
n-alkylaromatiques et les molécules aromatiques simples en
C10-C22, sont les plus facilement dégradables. Les petites
molécules se métabolisent en général rapidement. Les n-alcanes à
longue chaîne sont plus lentement dégradés en raison de leur
hydrophobicité et du fait qu'ils sont visqueux ou solides à la
température ambiante. Les alcanes ramifiés et les cycloalcanes sont
relativement résistants à la décomposition biologique et les
hydrocarbures aromatiques polycycliques, franchement résistants. La
vitesse globale de dégradation des hydrocarbures est limitée par la
température, la teneur en eau et en oxygène, le pH, la présence de
nutriments inorganiques et la versatilité métabolique microbienne.
Les algues unicellulaires peuvent fixer et métaboliser les
hydrocarbures aliphatiques et aromatiques mais on connaît mal
l'ampleur de ce phénomène. Contrairement aux microorganismes qui
utilisent les hydrocarbures du pétrole comme source de carbone, le
métabolisme animal a généralement tendance à oxyder et à conjuguer les
produits pour les transformer en substances plus solubles et donc plus
faciles à excréter. Toutes les espèces animales étudiées sont capables
de fixer des hydrocarbures du pétrole. On sait que les hydrocarbures
aromatiques polycycliques, le pétrole brut et les produits pétroliers
raffinés induisent les enzymes du cytochrome P450 et chez de
nombreuses espèces de poissons de mer et d'eau douce, on constate
qu'il y a accroissement du métabolisme des hydrocarbures.
On n'a que peu de données sur la bioaccumulation du combustible
diesel dans les conditions du laboratoire mais il est largement
prouvé, par l'étude des nappes de mazout et d'autres études de
laboratoire consacrées à ce genre de produits, en particulier le No 2,
que les organismes aquatiques concentrent les hydrocarbures. Le
coefficient de partage du combustible diesel entre le n-octanol et
l'eau est égal à 3,3-7,06, ce qui incite à penser que son potentiel de
bioaccumulation est élevé; quoi qu'il en soit, de nombreux composés de
faible masse moléculaire relative sont facilement métabolisés et la
bioaccumulation effective des produits de masse moléculaire relative
plus élevée est limitée par leur faible solubilité dans l'eau et les
dimensions importantes de leur molécule. Il en résulte donc que la
bioaccumulation peut en réalité être faible.
On a constaté qu'après des déversements de combustible diesel, le
poisson pouvait devenir inconsommable. On ne dispose d'aucune donnée
sur la bioamplification du combustible diesel.
On ne possède non plus aucune donnée expérimentale sur le
déplacement du combustible diesel à travers le sol, encore qu'il ait
été avancé qu'il existait une corrélation directe entre ce déplacement
et la viscosité cinématique du produit. Le déplacement du kérosène
dans un sol dépend de la teneur en eau et de la nature de ce sol.
A1.4 Concentrations dans l'environnement et exposition humaine
Les divers types de combustible diesel étant constitués de
mélanges complexes, on n'en n'a pas mesuré la concentration dans
l'environnement. On peut mettre en évidence la présence de leurs
divers constituants dans presque tous les compartiments du milieu,
même s'il n'est pas possible d'en vérifier l'origine. Lorsqu'il y a
exposition de la population générale, elle se produit dans les
stations service ou par suite de déversements.
Il peut y avoir exposition professionnelle au combustible diesel
à la faveur d'un grand nombre d'activités. Du fait de leur faible
volatilité, ces combustibles ne devraient produire qu'une vapeur assez
ténue à la température normale mais si l'on opère à température
élevée, la concentration peut augmenter sensiblement.
A1.5 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Les combustibles diesel présentent une faible toxicité aiguë
après administration par voie orale, percutanée ou respiratoire. Chez
toutes les espèce étudiées (souris, lapin, rat, cobaye) on a obtenu
pour la DL50 par voie orale une valeur > 5000 mg/kg de poids
corporel. En application cutanée, on obtient une valeur de la DL50
également > 5000 mg/kg de poids corporel chez la souris et le lapin,
mais des valeurs > 2000 mg/kg de poids corporel ont été relevées pour
certains types de kérosène et de distillats moyens, selon le protocole
d'application et la limite inférieure de la dose. Chez des rats
exposés par la voie respiratoire, on a obtenu une valeur de la CL50
d'environ 5 mg/litre, sauf dans le cas d'un distillat moyen de
première distillation pour lequel on a obtenu une valeur de
1,8 mg/litre.
Chez des lapins badigeonnés avec du combustible diesel à des
doses allant jusqu'à 8000 µl/kg de poids corporel et par jour et chez
des souris traitées dans les mêmes conditions avec des doses
quotidiennes atteignant 40 000 mg/kg de poids corporel, on a observé
une acanthose et une hyperkératose dues à une grave irritation. Les
lapins se sont révélés plus sensibles que les souris. Chez la souris,
l'inhalation de combustible diesel a provoqué des effets
neurodépresseurs à des concentrations allant jusqu'à 0,2 mg/litre; en
revanche ces effets n'ont pas été observés chez des rats exposés à des
doses allant jusqu'à 6 mg/litre. Chez les rats, on a observé une
réduction du poids corporel et du poids du foie.
Après inhalation de doses de combustible diesel allant jusqu'à
1,5 mg/litre dans des conditions de subchronicité, des souris, des
rats et des chiens n'ont pas présenté de signes sensibles de toxicité
cumulative. Le syndrome néphropathique spécifique observé chez les
rats mâles est lié à l'accumulation intrinsèque d'inclusions hyalines
dans les tubules rénaux.
Les seuls effets de l'exposition à long terme ont été des
ulcérations après application cutanée de combustible diesel à des
souris (doses quotidiennes: 250 ou 500 mg/kg de poids corporel) et une
modification importante du poids des organes après inhalation par des
rats de ce même produit à la dose de 1 ou 5 mg/litre. Dans les deux
études, on a constaté une réduction du poids corporel moyen.
Les divers types de combustible diesel se révèlent légèrement à
fortement irritants pour la peau du lapin. Ils ne produisent pas
d'irritation oculaire mais dans le cas d'un certain nombre de
kérosènes, on a fait état d'un léger effet irritant. Il n'y a pas de
sensibilisation cutanée.
Le combustible diesel et les carburéacteurs (kérosène) ne se sont
révélés ni embryotoxiques, ni tératogènes lors de deux études
effectuées sur des rats à qui l'on avait fait inhaler ces produits aux
doses de 100 ou 400 ppm ainsi que dans une autre étude au cours de
laquelle des rats avaient reçu par gavage des doses quotidiennes de
ces produits allant jusqu'à 2000 mg/kg de poids corporel. Dans la
dernière étude, on a observé une réduction du poids des foetus.
Les épreuves effectuées sur Salmonella typhimurium n'ont pas
permis de prouver de manière nette l'existence d'une activité
mutagène. Des résultats positifs ont bien été observés chez
S. typhimurium ainsi que sur des cellules lymphomateuses de souris
mais leur caractère contradictoire les a fait considérer comme
équivoques. Les tests de génotoxicité effectués sur des souris
in vivo (induction de la formation de micronoyaux ou d'aberrations
chromosomiques) ont également donné des résultats équivoques ou
négatifs.
Les combustibles diesel présentent un faible pouvoir cancérogène
au niveau cutané. Dans l'état actuel de la recherche, on ne peut
déterminer si l'activité cancérogène de ces produits est due à leur
génotoxicité ou aux lésions chroniques qu'ils induisent dans le derme.
A1.6 Effets sur l'homme
Il peut y avoir exposition non professionnelle aux combustibles
diesel lors du remplissage manuel de citernes. C'est principalement
lors de déversements accidentels que la peau peut se trouver fortement
exposée à ces produits, encore que pour une brève durée.
A la suite de contacts directs avec le peau on a observé de
l'anurie, une insuffisance rénale, des symptômes gastro-intestinaux
ainsi qu'une hyperkératose cutanée. Des pneumopathies d'aspiration
d'origine toxique ont été observées par suite de l'ingestion
accidentelle de combustible diesel. Après inhalation, on peut observer
une toux grasse persistante. Lors d'une étude cas-témoins portant sur
des hommes exposés à du combustible diesel, on a constaté qu'ils
courraient un risque accru de cancers du poumon, autres que les
adénocarcinomes; on a également observé une association positive avec
le cancer de la prostate, encore que le risque ait été plus important
dans le groupe soumis à une exposition 'non substantielle' que dans
celui qui était soumis à une exposition 'substantielle'.
Lors d'une étude transversale portant sur des ouvriers d'une
usine exposés à des carburéacteurs, on a constaté qu'ils étaient plus
fréquemment sujets à des étourdissements, des maux de tête, des
nausées, des palpitations, une sensation d'oppression thoracique et
une irritation oculaire que les témoins non exposés. La concentration
moyenne pondérée par rapport au temps de la vapeur dégagée par le
combustible au niveau de la zone de respiration avait été estimée à
128-423 mg/m3.
A1.7 Effets sur les autres êtres vivants au laboratoire et dans
leur milieu naturel
Le combustible diesel est plus toxique que le pétrole brut pour
les animaux et les plantes aquatiques. L'écotoxicité du combustible
diesel est généralement attribuée à la présence de composés
aromatiques solubles, mais les hydrocarbures aliphatiques insolubles
peuvent également jouer un rôle. Parmi les composés aromatiques, les
dérivés monocycliques sont les moins toxiques, leur toxicité aiguë
augmentant avec la masse moléculaire jusqu'aux composés tétra- ou
pentacycliques, encore que ces derniers soient peu solubles dans l'eau
de mer. Certains animaux, comme les poissons et les oiseaux, peuvent
avoir le corps enduit de produit, avec des effets toxiques parfois
mortels.
On a étudié en laboratoire le combustible diesel et notamment ses
fractions solubles dans l'eau, les dispersions huile-eau et le pétrole
microencapsulé. Il apparaît que le combustible diesel ne réduit pas
sensiblement la croissance des cultures d'algues vertes du genre
Euglena gracilis, mais à faible concentration (0,1%), il inhibe
presque complètement la croissance de Scenedesmus quadricauda. Le
diesel léger (0,05%) stimule la croissance, la photosynthèse et la
synthèse de la chlorophylle a chez Chlorella salina, mais il en
inhibe légèrement la respiration. A concentration plus élevée, le taux
de croissance et la photosynthèse sont fortement réduits. Une
exposition de longue durée inhibe la croissance des algues benthiques
comme Ascophyllum nodosum et Laminaria digitata. Chez les algues
bleues, la photosynthèse est réduite par les fractions aromatiques et
asphaltiques, mais pas par la fraction aliphatique.
Le combustible diesel est fortement toxique pour les daphnies,
pour les larves de chironomidés et pour le mollusque Viviparus
bengalensis (un gastéropode). A la concentration de 0,1 ml/litre, il
a provoqué la mort de copépodes du genre Tigriopus californicus,
en l'espace de cinq jours.
Les moules du genre Mytilus edulis, accumulent le combustible
diesel, avec pour conséquence une réduction marquée de leur rythme
d'alimentation et de croissance; leur reproduction souffre également
d'une exposition de longue durée à ce produit. La CE50 relative au
frai des moules exposées pendant 30 jours à du combustible diesel, a
été trouvée égale à 800 µg/litre. La CL50 de gazole microencapsulé a
été égale à 5000 µg/litre pour des moules en maturation exposées
pendant 30 jours. Le gazole s'est révélé plus toxique pour les larves
que pour les jeunes moules: il avait un effet nocif sur la croissance
des larves à la dose de 10 µg/litre.
Des crabes d'eau douce (Barytelphusa cunicularis) exposés à des
concentrations sublétales de combustible diesel pendant des durées
allant jusqu'à 96 heures, ont généralement réduit leur consommation
d'oxygène, en particulier aux concentrations les plus faibles et pour
des durées d'exposition allant jusqu'à 8 heures. Lorsque l'exposition
se prolongeait, la consommation d'oxygène était égale ou supérieure à
celle des animaux témoins.
Lors d'épreuves de 96 heures visant à évaluer la toxicité aiguë
du produit sur des alevins de salmonidés dans des conditions
statiques, on a constaté que le combustible diesel était plus toxique
pour Onchorhychus gorbuscha (CL50: 32-123 mg/litre), que pour
O. kisutch (CL50: 2186-3017mg/litre) ou pour la truite arc-en-ciel
O. mykiss (CL50: 3333-33 216 mg/litre) quel que soit le type
d'eau.
Le seuil de détection des réactions comportementales de la morue
( Gadus marhua L.), exposée à du combustible diesel dans de l'eau de
mer, a été trouvé égal à 100-400 ng/litre. Un poisson de
l'antarctique, Pagothenia borchgrevinki, a résisté pendant des
durées allant jusqu'à 72 heures à la fraction hydrosoluble non diluée
du combustible diesel, tout en présentant cependant des signes de
stress.
Dans le cas des oiseaux, il peut y avoir contamination externe ou
interne par les produits pétroliers. Le combustible diesel supprime
l'hydrophobicité du plumage et peut être ingéré lorsque l'oiseau lisse
ses plumes. Du combustible diesel et du mazout administrés par gavage
à des canards à la dose de 2 ml/kg de poids corporel, a provoqué au
bout de 24 heures une stéatose, une inflammation extrême des poumons,
une infiltration graisseuse du foie et une dégénérescence hépatique.
L'administration de combustible diesel ou de mazout à la dose de
1 ml/kg a également entraîné une grave irritation des voies digestives
et une néphrose toxique. A doses plus élevées, on a observé une
hypertrophie des surrénales (due principalement à une hyperplasie du
tissu cortical), une chute du taux de la cholinestérase plasmatique,
de l'ataxie et des tremblements. Jusqu'à 20 ml/kg de poids corporel,
la contamination n'a pas été mortelle pour les oiseaux en bonne santé
mais la DL50 s'abaissait à 3-4 ml/kg de poids corporel lorsque le
combustible diesel ou le mazout était administré à des oiseaux
stressés.
En cas de déversement de combustible diesel, le zooplancton se
révèle extrêmement vulnérable aux constituants dispersés ou dissous du
pétrole, mais moins aux nappes d'huile flottante. Pour les organismes
aquatiques, la nocivité de ces produits peut se manifester de diverses
manières: mortalité directe (oeufs de poisson, copépodes et plancton),
contamination externe (chorions des oeufs de poisson ou cuticules et
appendices buccaux des crustacés), contamination tissulaire par des
constituants aromatiques, développement anormal des embryons de
poisson et perturbation du métabolisme.
A1.8 Evaluation des risques pour la santé humaine
Il peut y avoir exposition de la population générale au
combustible diesel et à d'autres distillats moyens sur les lieux et
dans les circonstances suivants: dans les stations service, lors de
déversements accidentels, lors de la manipulation de ces combustibles
et lors de l'utilisation de pétrole lampant pour la cuisine ou le
chauffage. Il peut y avoir exposition des travailleurs à ces produits
lors de la manipulation et du transvasement du combustible dans les
terminaux, les citernes et les stations services; lors de la
fabrication, de la réparation, de l'entretien et de l'essai des
moteurs diesel et autres matériels; lors de l'utilisation de
combustible diesel pour le nettoyage ou comme solvant ou encore lors
de la manipulation des prélèvements de routine au laboratoire. En
raison de sa faible volatilité, le produit ne devrait pas émettre de
vapeurs très denses à la température ambiante, encore que dans un
espace confiné et à température élevée, la vapeur puisse être plus
concentrée.
Lors de la manipulation normale du combustible diesel,
l'exposition aux vapeurs est minime. L'effet le plus probable sur la
santé humaine consiste en une dermatite de contact. Le combustible
diesel est irritant pour la peau mais il ne semble pas irriter la
muqueuse oculaire. Des effets toxiques aigus au niveau rénal peuvent
s'observer après exposition cutanée, mais on ignore quels peuvent être
les effets à long terme d'une absorption percutanée de faibles
concentrations.
L'ingestion de combustible diesel peut avoir des effets toxiques
qui se traduisent quelquefois par une régurgitation et une aspiration
pouvant provoquer une pneumonie chimique, comme dans le cas de tout
hydrocarbure entre certaines limites de viscosité.
Chez des rongeurs exposés par la voie respiratoire à du
combustible diesel à des concentrations allant jusqu'à 0,2 mg/litre,
on a observé un effet neurodépresseur dans le cas de souris, mais pas
chez des rats, mêmes aux concentrations les plus élevées. Chez des
rats mâles, une exposition subchronique par la voie respiratoire à
divers distillats a produit une néphropathie spécifique à
alpha2-microglobulines; cette observation n'est pas considérée comme
transposable à l'homme.
Les combustibles diesel ne se sont révélés ni embryotoxiques ni
tératogènes chez les animaux exposés par la voie orale ou par la voie
respiratoire.
Rien n'indique de façon nette qu'il existe une activité mutagène
chez les bactéries, mais les résultats d'autres tests de génotoxicité
in vitro et in vivo sont plutôt équivoques.
Un étude cas-témoins portant sur des ouvriers exposés à du
combustible diesel incitent à penser qu'il existe un risque accru de
cancers du poumon n'appartenant pas au type épithélium glandulaire
ainsi que de ce cancer de la prostate. Il n'a pas été possible
d'établir de relation dose-réponse pour l'un ou l'autre de ces cas. En
raison du petit nombre d'études disponibles, du petit nombre de cas et
par voie de conséquence, de l'étendue de l'intervalle de confiance, il
n'est pas possible de tirer la moindre conclusion de ces données au
sujet de la cancérogénicité du produit pour l'homme. Chez la souris,
du combustible diesel administré par voie intradermique a présenté une
faible activité cancérogène. En raison de l'absence d'une activité
génotoxique avérée, il est possible d'invoquer un mécanisme non
génotoxique pour ces cancers; il pourrait s'agir par exemple d'une
irritation dermique chronique caractérisée par la répétition de
lésions cutanées entraînant une hyperplasie de l'épiderme.
A1.9 Evaluation des effets sur l'environnement
Il peut y avoir pollution de l'environnement par libération
accidentelle à grande échelle de combustible diesel, comme cela peut
se produire lors catastrophes affectant des réservoirs ou encore en
cas de fuites d'oléoduc ou, à plus petite échelle, lorsqu'il y a
contamination du sol aux alentours d'une usine ou d'un garage. Dans
l'eau, le combustible diesel s'étale presque immédiatement, les
composants polaires de masse moléculaire relative peu élevée se
dissolvent et disparaissent pas lessivage, les constituants volatils
s'évaporent et la décomposition microbienne s'amorece. L'élimination
des différents constituants dépend de la température et des conditions
climatiques. La composition chimique de la nappe varie dans le temps:
une fois répandues dans l'eau, certaines fractions s'évaporent et
subissent une décomposition photochimique; les fractions lourdes,
transportées par les particules qui se déposent, parviennent jusqu'aux
sédiments du fond; dans le sol les constituants du combustible diesel
migrent à des vitesses variables, en fonction de la nature de ce sol.
Les divers constituants du combustible diesel sont
intrinsèquement biodégradables mais leur vitesse de biodécomposition
dépend largement des conditions physiques et climatiques et de la
composition microbiologique du milieu.
Des organismes aquatiques et en particulier les mollusques,
accumulent les hydrocarbures à des degrés divers mais ceux-ci peuvent
être éliminés par passage dans l'eau claire. Le combustible diesel
peut subir une bioaccumulation; en revanche on ne dispose d'aucune
donnée sur une bioamplification éventuelle.
Tout déversement de combustible diesel a un effet nocif immédiat
sur l'environnement, qui se traduit par une mortalité notable pour la
faune et la flore. Il peut y avoir recolonisation au bout d'une année,
selon l'espèce animale ou végétale en cause et selon la composition
chimique et physique des résidus de produits pétroliers.
Les organismes aquatiques qui survivent aux déversements de
combustible diesel peuvent cependant subir une contamination externe
par ces produits et en accumuler dans leurs tissus: le stress qui en
résulte se traduit par un développement anormal et une modification du
métabolisme
PARTIE B GAZ D'ECHAPPEMENT DES MOTEURS DIESEL
B1. RESUME
B1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Les gaz d'échappement des moteurs diesel contiennent des
centaines de composés chimiques qui sont émis en partie dans la phase
gazeuse proprement dite et en partie dans la phase particulaire. Les
principaux produits gazeux de la combustion sont le dioxyde de
carbone, l'oxygène, l'azote et la vapeur d'eau: on trouve également du
monoxyde de carbone dixoyde de soufre, des oxydes d'azote et des
hydrocarbures et leurs dérivés. La phase gazeuse de la fraction
hydrocarbonée contient également un faible pourcentage en poids de
benzène et de toluène. Les autres constituants des gaz d'échappement
sont des hydrocarbures aromatiques polycycliques (HAP) de faible masse
moléculaire relative.
L'échappement des moteurs diesel est principalement caractérisé
par l'émission de particules dans une proportion environ 20 fois
supérieure à celle des moteurs à essence. Ces particules sont
composées de carbone élémentaire, de dérivés organiques adsorbés
provenant du combustible et de l'huile lubrifiante, de sulfates formés
à partir du soufre contenu dans le combustible et de dérivés
métalliques à l'état de traces. La granulométrie globale de ces
particules les situe en dessous du micron, entre 0,02 et 0,5 µm. Le
vieillissement peut conduire à une agglomération qui les amène à un
diamètre maximal de 30 µm. Les particules émises ont une aire
superficielle importante. Les composés organiques constituent
généralement 10 à 30% des particules totales mais avec des moteurs mal
conçus et mal entretenus cette proportion peut atteindre 90%. Dans
cette fraction on trouve également des hydrocarbures aromatiques
polycycliques de masse moléculaire élevée, entre autres, sous forme
oxygénée et nitrée, à des concentrations de l'ordre de plusieurs
parties par million.
Pour mesurer les émissions des véhicules on travaille en régime
transitoire ou stationnaire. L'échantillonnage peut se faire à partir
de gaz d'échappement dilués ou non dilués. Il est difficile d'obtenir
des échantillons débarrassés de tout artéfact car les constituants
peuvent subir des réactions chimiques, être adsorbés ou désorbés, ou
encore soumis à une condensation ou à une diffusion. Les hydrocarbures
aromatiques polycycliques d'importance toxicologique qui sont présents
dans les particules émises par les moteurs diesel sont généralement
dosées selon la procédure suivante: extraction au Soxhlet,
purification et fractionnement puis analyse finale par chromatographie
liquide à haute performance ou chromatographie en phase gazeuse
couplée à la spectométrie de masse.
B1.2 Sources d'exposition humaine et environnementale
Les gaz d'échappement des moteurs diesel proviennent
essentiellement des véhicules à moteur; parmi les autres source on
peut citer les installations fixes, le matériel de traction
ferroviaire et les machines de navires. Les émissions de ces véhicules
à moteurs diesel ont été bien décrites mais les résultats obtenus pour
chaque catégorie particulière ne sont souvent pas comparables en
raison de différences concernant certains paramètres comme le cycle de
fonctionnement, le type de moteur et la composition du combustible.
Les divers constituants sont émis dans les proportions suivantes:
dioxyde de carbone, environ 1 kg/km; monoxyde de carbone, oxydes
d'azote, hydrocarbures gazeux totaux et particules 0,1-20 g/km;
dérivés aliphatiques, alcools, aldédhydes, hydrocarbures aromatiques
légers et hydrocarbures aromatiques polycycliques, quelques
microgrammes/km. Dans un certain nombre de pays, il y a réglementation
des émissions de monoxyde de carbone, d'oxyde d'azote, d'hydrocarbures
gazeux totaux et de particules.
En principe, il n'y a aucune différence entre les moteurs de
faible ou de forte puissance pour ce qui est de la nature et de la
quantité des émissions, encore que les véhicules lourds aient tendance
à dégager une quantité relativement plus importante de particules. Les
émissions dépendent du cycle de fonctionnement du moteur (régime
transitoire ou régime stationnaire), du type et de l'état du moteur
(injection ou aspiration, entretien, kilométrage total) et de la
composition du combustible (teneur en soufre, teneur en composés
aromatiques, volatilité); le réglage du moteur jour un rôle très
important.
Le dégagement de particules augmente en raison inverse du rapport
air/combustible, mais en raison directe de la charge et de la
température. Les moteurs vieillissants, soumis à une utilisation
intense, libèrent plus de particules que les moteurs récents, qui ont
peu de kilométrage, probablement du fait de la plus grande
consommation d'huile lubrifiante. Dans le cas des véhicules légers à
moteur diesel, il y a également une corrélation entre l'émission de
particules et la teneur en soufre du combustible, car la formation de
sulfates métalliques accroît la masse des particules. Dans le cas des
véhicules lourds, ce type de corrélation n'a pas encore été établi.
Entre outre, plus la teneur du combustible en dérivés aromatiques est
élevée, plus le moteur dégage de particules.
Les hydrocarbures aromatiques polycycliques, oxygénés ou non,
émis par les moteurs diesel et les moteurs à allumage commandé, sont
de nature similaire. Des hydrocarbures aromatiques polycycliques
oxygénés ou nitrés sont émis à raison de quelques microgrammes/km,
mais la concentration effective de ces composés n'est pas connue avec
certitude car il peut y avoir décomposition ou formation lors de
l'échantillonnage. Les émissions de HAP augmentent avec la charge et
la température ainsi qu'avec l'âge du moteur, probablement du fait
d'une consommation plus importante d'huile lubrifiante. Les émissions
d'HAP dépendent également de la technique d'injection utilisée dans le
moteur: ces émissions sont proportionnelles au rapport air/combustible
dans les moteurs à injection directe mais la situation est inversée
dans le cas des moteurs à injection indirecte. La teneur en dérivés
aromatiques et la volatilité du combustible sont directement liées aux
émissions de HAP. En cas de mauvais fonctionnement de certains
éléments du moteur, en particulier des injecteurs, il y a augmentation
de l'émission des principaux constituants des gaz d'échappement. On ne
possède guère de données sur la contribution des moteurs diesel aux
émissions totales de produits de combustion d'origine artificielle.
Les émissions des moteurs diesel peuvent être réduites moyennant
une meilleure conception du moteur, la pose de pièges à particules
(pièges oxydants) et de convertisseurs catalytiques. Alors que les
pièges à particules éliminent la suie et les composés organiques
solubles adsorbés sur les particules, les convertisseurs catalytiques
réduisent principalement la teneur en oxyde de carbone et
hydrocarbures gazeux. En pratique, il est difficile de régénérer les
pièges à particules. Les convertisseurs catalytiques nécessitent
l'utilisation de combustibles à faible teneur en soufre car le soufre
empoisonne les centres actifs du catalyseur.
B1.3 Transport, distribution et transformation dans l'environnement
C'est principalement l'atmosphère qui est affectée par les
émissions de moteurs diesel. L'hydrosphère et la géosphère peuvent
être contaminées indirectement par des dépôts secs ou humides. La
destinée environnementale des divers constituants des gaz
d'échappement des moteurs diesel est en général bien connue: les
particules se comportent comme les molécules de gaz non réactives pour
ce qui est de leur transport mécanique dans l'atmosphère; elles
peuvent être transportées sur de longues distances et même pénétrer
dans la stratosphère. On pense que leur vitesse d'élimination est
faible, ce qui fait qu'elles séjournent plusieurs jours dans
l'atmosphère. Avec le temps, ces particules peuvent s'agglomérer, et
leur vitesse de chute augmentant, la quantité restante aéroportée
diminue. Le carbone élémentaire présent dans des particules émises par
les moteurs diesel peut catalyser la formation d'acide sulfurique par
oxydation du dioxyde de soufre. Les constituants organiques adsorbés à
la surface des particules de carbone élémentaire peuvent subir un
certain nombre de transformations et de rections chimiques avec
d'autres composés atmosphériques ou par exposition à la lumière
solaire.
B1.4 Concentrations dans l'environnement et exposition humaine
Etant donné que les gaz d'échappement des moteurs diesel sont des
mélanges complexes contenant des composés très divers, on ne peut pas
définir de 'concentration dans l'environnement'. On devrait pouvoir
mettre en évidence la présence de ces divers constituants dans tous
les compartiments de l'environnement, encore qu'il ne soit pas
généralement possible d'en déterminer l'origine. On connaît la
concentration dans l'environnement de la plupart de ces constituants.
C'est le plus probablement dans les rues très passantes ou dans
les parkings, en particulier souterrains, que la population générale a
le plus de chances d'être exposée aux émissions des moteurs diesel. Il
est difficile d'identifier les sources de pollution et on calcule
généralement la contribution des gaz d'échappement des moteurs diesel
à la pollution totale due à la circulation des véhicules à moteur, sur
la base des facteurs d'émission et du pourcentage de véhicules à
moteur diesel dans le parc automobile général. Le niveau d'exposition
de la population générale et des travailleurs aux particules émises
par les moteurs diesel est toxicologiquement significatif.
Les concentrations quotidiennes moyennes ambiantes de particules
à proximité des voies de communication sont de 8-42 µg/m3. On a
calculé que les concentrations annuelles moyennes de particules
étaient de 5 à 10 µg/m3 dans les zones urbaines et < 1,5 µg/m3
dans les zones rurales d'Allemagne et de 1 à 2 µg/m3 dans les zones
urbaines et 0,6-1 µg/m3 dans les zones rurales des Etats-Unis
d'Amérique. Ces concentrations sont directement corrélées à la densité
de la circulation automobile et diminuent à mesure qu'on s'éloigne des
routes.
Il peut être également difficile de déterminer l'origine de la
pollution sur les lieux de travail, en particulier dans les mines où
la quantité totale de poussière est élevée. Le dosage du carbone
particulaire a permis de déterminer l'exposition spécifique aux
émissions de moteurs diesel sur les lieux de travail et il en ressort
que les travailleurs seraient exposés à des concentrations de
particules de l'ordre de 0,04 à 0,134 mg/m3 pour les camionneurs et
de 0,004 à 0,192 mg/m3 pour les cheminots. On a également eu recours
au dosage des particules respirables totales et des particules totales
en suspension pour évaluer l'exposition professionnelle.
B1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
B1.5.1 Dépôt
Les particules émises par les moteurs diesel, dont le diamètre
aérodynamique médian massique est égal à environ 0,2 µm, sont quelque
peu filtrés par le nez et leur aptitude au dépôt dans les poumons
n'est que légèrement supérieure à la valeur minimale trouvée pour les
particules de diamètre aérodynamique médian massique égal à environ
0,5 µm. Autrement dit, lorsque les particules de suie émises par les
moteurs diesel sont inhalées, 10 à 15% d'entre elles se déposent au
niveau des alvéoles chez le rat et le cobaye, le dépôt au niveau des
alvéoles étant d'environ 10% chez l'homme.
B1.5.2 Rétention et élimination des particules
L'élimination des particules par l'ascenseur muco-ciliaire est
pratiquement complète au bout de 24 heures. L'élimination à long terme
des particules déposées dans la zone alvéolaire a fait l'objet de
plusieurs études sur des rats qui avaient été exposés par la voie
respiratoire à deux types de particules: les particules émises par un
moteur diesel et les particules servant de référence. Dans le cas des
témoins chez lesquels la charge pulmonaire était faible (< 1 mg par
poumon), on a obtenu des temps de demi-élimination de l'ordre de 60 à
100 jours, alors que chez les rats présentant une charge pulmonaire
allant de 1 à 60 mg/poumon, le temps de demi-élimination était de
l'ordre de 100 à 600 jours. Dans plusieurs études, on a constaté que
les effets observés étaient dus à une surcharge en particules,
laquelle a été décrite chez diverses espèces et pour un certain nombre
de matériaux particulaires. Ce phénomène s'observe généralement
lorsque la vitesse de dépôt des particules de faible solubilité et de
faible toxicité aiguë reste supérieure à leur vitesse d'élimination
pendant une très longue durée. Chez l'homme, le temps de
demi-élimination alvéolaire normal est de plusieurs centaines de
jours, c'est-à-dire plus long que chez le rat.
On a mis au point un modèle pulmonaire dosimétrique sur la base
des données de dépôt et de rétention des particules diesel chez le rat
après inhalation de longue durée et des mêmes données chez l'homme. Ce
modèle peut être utilisé pour prévoir la rétention des particules
diesel et des composés organiques adsorbés dans les poumons de
personnes d'âges divers. On ne dispose d'aucune donnée sur la manière
dont la rétention de différents composés évolue après une exposition
prolongée aux gaz d'échappement de moteurs diesel.
B1.5.3 Rétention et élimination des hydrocarbures aromatiques
polycycliques adsorbés sur la suie de moteurs diesel
Les hydrocarbures aromatiques polycycliques présents dans la suie
émise par les moteurs diesel adhèrent fortement à la surface des
particules. Environ 50% des HAP adsorbés sur les particules sont
éliminés par les poumons dans l'espace d'une journée, mais le temps de
demi-rétention de la fraction restante s'est révélé égal à 18-36
jours. Des études portant sur du 3H-benzo[ a]pyrène et du
14C-nitropyrène ont montré que lorsqu'ils sont associés à des
particules, les HAP sont sensiblement plus longs à être éliminés des
poumons que leurs homologues libres.
B1.5.4 Métabolisme
Déposé sur des particules émises par un moteur diesel, du
benzo[ a]pyrène a subi une métabolisation oxydative au niveau du
poumon et dans des cultures cellulaires de macrophages pulmonaires
aboutissant à la formation de phénols, de diols et de quinones
substituant le noyau benzo[ a]pyrène. Du nitropyrène adsorbé sur des
particules diesel a été métabolisé en acétylaminopyrène-phénol après
inhalation. Chez des rats exposés à des émissions de moteur diesel on
a constaté, dans les poumons et les cellules de type II, un
accroissement des adduits de l'ADN par rapport aux témoins. Le
métabolisme oxydatif de certains composés organiques qui conduit à des
époxydes pourrait être responsable de la formation des adduits, mais
il est vrai qu'on ne les trouve qu'après exposition à des particules.
Certains dérivés organiques présents dans les émissions de moteur
diesel se sont révélés capables de former des adduits avec l'ADN;
toutefois le noyau carboné lui-même (sans composés organiques
extractibles) peut également conduire à la formation d'adduits, en
provoquant des lésions chroniques au niveau des cellules épithéliales.
B1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Les quelques données disponibles incitent à penser que les
émissions de moteur diesel ne présentent qu'une faible toxicité aiguë.
Des souris auxquelles on avait administré par voie intratrachéale, des
particules émises par un moteur diesel, sont mortes des suites d'une
oedème du poumon. La DL50 était égale à environ 20 mg/kg de poids
corporel. Des particules du même type, extraites au méthanol, n'ont
entraîné aucune mortalité jusqu'à une concentration d'environ 33 mg/kg
de poids corporel. Chez le hamster, on a obtenu une DL50 de
1280 mg/kg de poids corporel après administration par voie
intrapéritonéale. On ne dispose d'aucune donnée concernant
l'exposition par la voie respiratoire.
Après exposition de rats, de cobayes et de chats pendant environ
quatre semaines à des émissions de moteur diesel ayant une teneur en
particules de 6 mg/m3, on a observé une altération de la fonction
pulmonaire, et notamment une augmentation de 35% de la résistance
ventilatoire chez les cobayes et une diminution de 10% de la capacité
vitale chez les chats. Du point de vue histopathologique, on a observé
un épaississement focal des parois alvéolaires, un accroissement
significatif de l'indice de marquage des cellules de type II et des
accumulations de macrophages chargés de particules. Ces accumulations
se situaient au niveau des bronchioles terminales et elles ont
augmenté de volume lors de la période de récupération postérieure à
l'expérience en raison de la fixation des macrophages (séquestration).
Après avoir inhalé pendant de longues périodes des émissions de
moteur diesel à des concentrations atteignant 4 mg/m3, des rats, des
souris, des hamsters, des chats et des singes n'ont pas présenté de
chute spectaculaire de leur poids corporel ni de réduction de leur
longévité. Les effets toxiques liés à la dose qui ont été observés
chez toutes les espèces après une longue période d'inhalation étaient
les suivants: augmentation du poids des poumons pouvant aller jusqu'à
400%; inflammation pulmonaire objectivée par des paramètres
biochimiques (marqueurs enzymatiques cytoplasmiques, collagène) et
cytologiques (augmentation des neutrophiles polynucléaires);
perturbation de la mécanique pulmonaire; accroissement du nombre de
macrophages chargés de particules avec accumulations focales
(séquestration) dans des conditions de surcharge; enfin, altérations
prolifératives des cellules épithéliales et début de fibrose.
D'après les données limitées dont on dispose sur la toxicité des
émissions de moteurs diesel pour la fonction de reproduction et le
développement, on peut penser qu'il n'y a pas d'effets toxiques
déterminants. Dans la plupart des expériences, on n'a pas constaté
d'effets chez des souris, des rats, des hamsters, des lapins ou des
singes; toutefois, après injection par voie intrapéritonéale, on a
observé chez des souris qui avaient reçu de particules émises pas des
moteurs diesel, des anomalies touchant les spermatozoïdes et constaté
une certaine embryotoxicité chez des hamsters auxquels on avait
administré des extraits d'émissions de ces mêmes moteurs.
La plupart des épreuves de génotoxicité in vitro ont été
réalisées avec des extraits d'émissions de moteurs diesel plutôt
qu'avec les émissions totales elles-mêmes et l'on a obtenu des
réactions positives en l'absence d'activation métabolique. Autrement
dit, il semble que ces effets génotoxiques soient indépendants de la
présence d'HAP. Environ 50% des études in vivo ont donné des
résultats négatifs; les seuls résultats positifs obtenus consistaient
en échanges de chromatides soeurs dans le cas des émissions totales et
des extraits organiques, et dans la présence de micronoyaux dans le
cas des extraits organiques.
On n'a généralement pas observé d'effets immunotoxiques après
inhalation d'émissions de moteurs diesel; toutefois on a observé dans
une étude une augmentation du titre des immunoglobulines
anti-ovalbumine et deux autres expériences ont mis en évidence un
accroissement de la sensibilité aux infections chez des souris.
Des études effectuées sur des rats incitent à penser que
l'inhalation de gaz d'échappement de moteurs diesel affecte l'état
comportemental et neurophysiologique.
Lors d'études sur la cancérogénicité des émissions de moteur
diesel, au cours desquelles des rats étaient exposés à ces émissions
par la voie respiratoire, on a constaté que la phase gazeuse
(c'est-à-dire dépourvue de particules) n'était pas cancérogène. Toutes
les études sur des rats qui ont été validées ont montré que les
émissions de moteurs diesel avaient un effet cancérogène lorsque la
concentration en particules était supérieure à 2 mg/m3, soit
l'équivalent d'une exposition continue à une concentration d'environ
1 mg/m3. Aucun effet n'a été observé en revanche chez les hamsters
et les souris. Lors d'études au cours desquelles on a pratiqué une
instillation intratrachéale, on a constaté que les particules
présentes dans les émissions de moteurs diesel et le noir de fumée
produisaient des tumeurs; en outre on a observé l'existence d'une
corrélation entre l'aire superficielle des particules carbonées et
leur activité tumorigène.
L'inhalation, pendant une longue période, de noir de fumée
pratiquement dépourvu d'hydrocarbures aromatiques polycycliques à ces
concentrations, a également provoqué des tumeurs pulmonaires chez le
rat.
On ne sait pas avec certitude si la cancérogénicité des émissions
de moteurs diesel est due à un mécanisme faisant intervenir ou non des
réactions au niveau de l'ADN (ou à un mécanisme mixte). Différents
modèles ont été proposés pour élucider la cancérogénicité des
émissions de moteurs diesel.
B1.7 Effets sur l'homme
Les émissions de moteurs diesel contribuent à la pollution
globale de l'air. Les études toxicologiques, qu'elles cherchent à
mettre en évidence des effets aigus ou des effets chroniques, ne
permettent pas d'assigner un rôle particulier à ces particules mais il
apparaît qu'elles pourraient être en partie responsables d'un certain
nombre d'effets attribués à la pollution de l'air.
Les émissions de moteur diesel ont des caractéristiques qu'un
certain nombre de personnes trouvent agressives, en particulier à
fortes concentrations. Les symptômes observés après exposition de
brève ou de longue durée à ce type d'émissions ont été décrits dans un
certain nombre d'études et de rapports concernant des personnes
exposées de par leur profession. Les effets aigus consistent en
irritation des muqueuses oculaires et nasales et l'on a observé dans
les cohortes professionnelles une augmentation de la fréquence des
symptômes respiratoires; toutefois on ignore qu'elle est la
contribution exacte des particules émises par les moteurs diesel à ce
type de symptômes. Aucun effet à court terme n'a été régulièrement
observé au niveau de fonction pulmonaire, mais on a signalé des crises
d'asthme.
Lors d'une étude contrôlée au cours de laquelle huit volontaires
non fumeurs, en bonne santé, ont été exposés à des émissions diluées
de moteurs diesel, pendant 60 minutes dans une chambre fermée, on a
constaté une réduction du taux de phagocytose chez des macrophages
alvéolaires recueillis par lavage broncho-alvéolaire.
Un certain nombre d'études transversales et longitudinales
portant sur des ouvriers longtemps exposés de part leur profession à
des émissions de moteur diesel, ont révélé une altération de la
fonction pulmonaire et une augmentation de la prévalence des symptômes
respiratoires, toutefois la brièveté des épisodes d'exposition limite
la portée de ces études. Aucune surmortalité n'a été constatée lors
d'études de cohorte au cours desquelles on s'est efforcé d'étudier des
décès par maladie cardio-vasculaire ou accident vasculaire cérébral,
imputables à l'exposition des émissions de moteurs diesel.
Lors d'un certain nombre d'études épidémiologiques on a tenté
d'établir des relations entre certains cancers du poumon et de la
vessie et une exposition professionnelle à des émissions de moteurs
diesel. Seules les études jugées utiles pour l'évaluation des effets
cancérogènes de ces émissions ont été prises en considération dans la
présente monographie. Celles qui sont les plus intéressantes pour ce
qui est du cancer du poumon, concernent des cheminots, des personnes
travaillant dans des garages de cars et des débardeurs qui constituent
des cohortes dont les membres sont effectivement exposés aux émissions
de moteur diesel. Les quatre études les plus informatives font toutes
état d'un risque accru de cancer du poumon, avec un risque relatif
allant de 1,4 pour les cheminots, et de 1,3-2,4 pour les ouvriers des
garages (selon le type d'exposition), jusqu'à un risque trois à six
fois plus élevé pour les débardeurs (en fonction du mode d'évaluation
de l'exposition, mais avec un large intervalle de confiance). Il a été
possible de tenir compte du tabagisme dans une étude cas-témoins sur
des cheminots et dans une étude sur des débardeurs. Dans les deux cas,
cette correction pour tenir compte du tabagisme n'a eu aucune
influence sur l'effet de l'exposition aux émissions diesel. Dans les
trois études pour lesquelles on n'a pas pu tenir compte du tabagisme,
l'analyse était basée sur des comparaisons entre les différents
sous-groupes de ces cohortes, de sorte que l'effet de confusion créé
par le tabagisme avait moins de chance d'être gênant qu'en cas de
comparaison avec des groupes extérieurs.
Plusieurs études cas-témoins ont été menées afin d'examiner la
relation pouvant exister entre les cancers de la vessie et une
exposition supposée à des émissions de moteurs diesel. On a constaté
un accroissement du risque, en particulier pour le chauffeurs de
camion; toutefois toutes ces études souffrent d'une caractérisation
insuffisante de l'exposition. De la sorte, il n'est pas possible
d'affirmer qu'une exposition aux émissions de moteurs diesel entraîne
un risque accru de cancer vésical.
B1.8 Effets sur les autres êtres vivants au laboratoire et dans
leur milieu naturel
Les effets des émissions de moteur diesel n'ont été abordés que
dans une seule étude, portant sur des algues vertes.
B1.9 Evaluation des risques pour la santé humaine
L'évaluation des risques de cancer et de maladies non malignes a
été menée conformément au modèle d'évaluation des risques établi par
l'Académie nationale des sciences des Etats-Unis (National Research
Council, 1983). Ce modèle d'évaluation comporte quatre étapes: (1)
identification du danger; (2) évaluation de la relation dose-réponse;
(3) évaluation de l'exposition et (4) caractérisation du risque.
On estime que les travaux les plus intéressants sont les études
épidémiologiques de longue durée dans lesquelles l'exposition est bien
définie et le suivi supérieur à 20 ans. Quatre études portant sur le
cancer du poumon chez des personnes professionnellement exposées
satisfont à ces critères. Il apparait que le risque relatif de cancer
du poumon lié à une exposition à des émissions de moteur diesel est
généralement faible et qu'il est influencé par les circonstances, les
effets des facteurs de confusion non évalués et la difficulté de tenir
suffisamment compte des facteurs de confusion reconnus. D'autres
études, où l'exposition est définie avec une moindre précision,
corroborent les conclusions des études précédentes. Globalement, on
estime que les émissions de moteurs diesel sont probablement
cancérogènes pour l'homme; toutefois on ne dispose d'aucune donnée
quantitative permettant d'évaluer le risque.
B.1.9.1 Effets non néoplasiques
Pour caractériser le risque, on a procédé de deux manières:
premièrement une concentration sans effets nocifs observables que l'on
divise par un coefficient d'incertitude; deuxièmement, une
concentration de référence. Dans les deux cas, on a utilisé un modèle
dosimétrique élaboré qui permet de réduire l'imprécision due à
l'extrapolation interspécifique des doses.
La dose de particules sans effet pour l'homme a été estimée à
0,139 mg/m3. La valeur guide pour la population générale calculée
d'après le modèle dosimétrique a été établie à 5,6 µg/m3, la valeur
calculée sans recours au modèle étant de 2,3 µg/m3.
La méthode basée sur l'utilisation d'une concentration de
référence prend en compte l'ensemble de la relation exposition-réponse
de préférence aux données ponctuelles fournies par les études
d'inhalation, comme c'est le cas dans la méthode de la dose sans
effets observables. On a identifié trois paramètres biologiques
sensibles: l'inflammation alvéolaire chronique, la diminution
d'efficacité de l'ascenseur mucociliaire et les lésions pulmonaires
hyperplasiques. Les concentrations de référence calculées à partir du
même modèle dosimétrique que dans le cas de la méthode de la dose sans
effets observables, étaient de 0,9 à 2 µg/m3 pour l'inflammation, de
1,2 à 3 2 µg/m3 pour la dégradation de l'ascenseur muco-ciliaire et
de 6,3 à 14,2 µg/m3 pour les lésions hyperplasiques.
B1.9.2 Effets néoplasiques
Pour évaluer le risque d'une exposition aux émissions de moteur
diesel, on a eu recours à un modèle linéarisé en plusieurs étapes.
Comme les résultats des études épidémiologiques avaient été jugés
insuffisants pour une évaluation quantitative du risque unitaire, on a
eu recours à des données provenant de plusieurs études d'inhalation à
long terme chez des rats, études qui montraient l'existence d'une
cancérogénèse à partir de 2 mg/m3.
On a ainsi établi par le calcul que le risque unitaire était
de 3,4 × 10-5 µg/m3 (moyenne géométrique de quatre estimations).
Un autre modèle de type biologique a fourni une valeur similaire pour
le risque unitaire, en prenant pour hypothèse que les particules
émises par les moteurs diesel agissent à faibles concentrations sur la
prolifération cellulaire ou l'initiation du processus de
transformation.
B1.10 Evaluation des effets sur l'environnement
On ne dispose pas d'informations suffisantes pour évaluer les
effets spécifiques des émissions de moteurs diesel. La combustion du
carburant diesel devrait avoir des effets similaires à celle des
autres combustibles fossiles, effets qui sont liés à la consommation
de ce type de combustible.
PARTE A DIESEL
A1. RESUMEN
A1.1 Identidad, propiedades físicas y químicas y métodos
analíticos
El diesel es una mezcla compleja de alcanos normales, ramificados
y cíclicos (60%-> 90% en volumen; hidrocarburos de longitud de cadena
comprendida entre C9 y C30); compuestos aromáticos, especialmente
alquilbencenos (5%-40% en volumen), y pequeñas cantidades de alquenos
(0%-10% en volumen) obtenidos a partir de la fracción de gasóleo del
destilado medio durante la separación del petróleo. El diesel puede
contener benceno, tolueno, etilbenceno, y xilenos e hidrocarburos
aromáticos policíclicos (HAP), sobre todo naftaleno y derivados del
mismo con metilos sustituyentes, a niveles de partes por millón. El
contenido de azufre de los combustibles diesel depende del origen del
crudo y del proceso de refino. En un cierto número de países está
reglamentado por ley, y suele estar comprendido entre 0,05% y 0,5% en
peso. Se utilizan aditivos para influir en el flujo, el almacenamiento
y la combustión del diesel, para diferenciar productos y para
satisfacer las especificaciones comerciales. A temperatura ambiente,
los combustibles diesel suelen ser líquidos de color marrón
moderadamente volátiles, ligeramente viscosos, inflamables y de olor
parecido al del queroseno. Los márgenes de ebullición están
comprendidos por lo general entre 140°C y 385°C (> 588°C en el caso
del diesel marino); a 20°C la densidad es de 0,87-1,0 g/cm3, y la
hidrosolubilidad de 0,2-5 mg/litro. La calidad y composición del
diesel influye considerablemente en los contaminantes emitidos por los
motores diesel. Otras variables importantes son el proceso de
encendido (expresado en términos del índice de cetano), la densidad,
la viscosidad y el contenido de azufre. Las especificaciones del
diesel comercial varían considerablemente de un país a otro.
Los combustibles de calefacción y algunos combustibles de
aviación a base de queroseno producidos durante el proceso de refino
tienen a veces una composición parecida a la del diesel, aunque con
distintos aditivos. Por ello, a la hora de evaluar la toxicidad y la
ecotoxicidad se han tenido también en cuenta los datos biológicos
sobre estas mezclas.
Debido a la complejidad de las mezclas diesel, no existe un
método analítico específico para ellas, y las técnicas analíticas
empleadas en la mayoría de las evaluaciones ambientales sólo permiten
determinar la mezcla total de hidrocarburos de petróleo. Los métodos
consisten en una extracción previa con solventes, un proceso de
limpieza para eliminar los hidrocarburos naturales, y la posterior
detección mediante gravimetría, espectroscopía infrarroja o
cromatografía de gases.
Ni la gravimetría ni la técnica infrarroja proporcionan
información cualitativa o cuantitativa de utilidad sobre los
contaminantes, por lo que sólo pueden usarse con fines de cribado.
El método utilizado habitualmente para analizar muestras
ambientales es la cromatografía de gases unida a técnicas de detección
tales como la ionización por conductor y la espectrometría de masas.
Existen otros muchos métodos para analizar hidrocarburos particulares
en los combustibles diesel.
A1.2 Fuentes de exposición humana y ambiental
Los combustibles diesel se producen por refinación del petróleo bruto.
Para satisfacer las especificaciones técnicas en cuanto al
rendimiento, normalmente se combinan varios componentes para
obtenerlos; la ulterior inclusión de aditivos mejora sus propiedades
para usos específicos. Los combustibles diesel son muy utilizados con
fines de transporte. En los motores de alta velocidad se deben emplear
los más volátiles y de menor viscosidad, y los de cadena más larga se
reservan para los motores diesel de ferrocarriles y barcos. Una gran
parte del transporte pesado por carretera está propulsado por motores
diesel.
Los automóviles para pasajeros propulsados por motores diesel son
cada vez más frecuentes en Europa y el Japón (10%-25%), mientras que
en América del Norte el porcentaje de ese tipo de automóviles es de
aproximadamente 1%-2%, con una ligera tendencia a la baja. El diesel
es utilizado en motores fijos y en calderas, como por ejemplo motores
de pistones, turbinas de gas, bombas de oleoductos, compresores de
gas, unidades de procesamiento de vapor de centrales de energía
eléctrica, quemadores e instalaciones industriales de calefacción de
espacios y de agua.
En los últimos cinco años la demanda mundial de combustibles
diesel ha aumentado constantemente. En 1985 se consumieron las
siguientes cantidades: unas 170 000 kilotoneladas al año en América
del Norte; unas 160 000 kilotoneladas al año, gasóleos incluidos, en
la Unión Europea; y aproximadamente 46 000 kilotoneladas anuales en
Australia, el Japón y Nueva Zelandia, lo que equivale a un total de
1062 kilotoneladas diarias. En 1990 se notificó que la demanda mundial
ascendía aproximadamente a 1110 kilotoneladas al día.
No se dispone de información sobre las emisiones a que da lugar
la producción de combustibles diesel; no obstante, al parecer esta
fuente tiene una importancia secundaria, dado que el proceso de
refinación se lleva a cabo en sistemas cerrados. Las emisiones se
producen sobre todo durante el almacenamiento y el transporte. Se
libera diesel como resultado de derrames y durante el reabastecimiento
de los vehículos en las gasolineras.
La atmósfera y la hidrosfera son los compartimentos ambientales
más gravemente afectados. En algunos accidentes se produce
contaminación del suelo por combustibles diesel, problema que también
afecta a las playas ferroviarias. Entre las numerosas técnicas
empleadas para limpiar los suelos contaminados por diesel, cabe citar
la excavación, los métodos biológicos y el confinamiento.
A1.3 Transporte, distribución y transformación en el medio
ambiente
Apenas hay datos sobre el destino ambiental de los combustibles
diesel, pero se considera que los mecanismos de su distribución y
transformación son comparables a los de combustibles de calefacción
que han sido bien estudiados, como por ejemplo el fuel-oil No 2. Los
derrames de diesel en agua se extienden casi de inmediato para formar
una 'capa aceitosa'. Los componentes polares y de bajo peso molecular
relativo se disuelven y se separan por lixiviación de dicha capa,
mientras que los componentes volátiles se evaporan a partir de la
superficie; además da comienzo la degradación microbiana. La
degradación química y biológica altera la composición del derrame.
Estos procesos dependen de la temperatura; así, los derrames que se
producen en el Artico duran más que los que se producen en zonas de
clima templado. En entornos marinos, la mayoría de los agentes
aromáticos de bajo peso molecular relativo se disuelven en la fase
acuosa, pero los alcanos ramificados, los cicloalcanos y los restantes
compuestos aromáticos primarios pueden persistir en los sedimentos
durante más de un año.
No se dispone de información sobre la fotooxidación de los
combustibles diesel en el agua y el aire, pero se sabe que los
componentes del petróleo evaporados se degradan fotoquímicamente. Se
ha demostrado que el fuel-oil No 2 se fotooxida rápidamente en el agua
en condiciones ambientales.
Los distintos componentes del diesel son de por sí
biodegradables, en diversa medida y a distinta velocidad. Los
n-alcano, los compuestos n-alquilaromáticos y las moléculas
aromáticas simples comprendidas en el margen de C10 a C22 son los
que más fácilmente se degradan. Las moléculas más pequeñas son
metabolizadas por lo general rápidamente. Los n-alcanos de cadena
larga se degradan más lentamente, debido a su hidrofobicidad y a que
son viscosos o sólidos a temperatura ambiente. Los alcanos ramificados
y los cicloalcanos son relativamente resistentes a la degradación
biológica, y los HAP son resistentes. La velocidad global de
degradación de los hidrocarburos depende de la temperatura, el
contenido de agua, el oxígeno, el pH, los nutrientes inorgánicos y la
variabilidad metabólica microbiana.
Las algas unicelulares pueden incorporar y metabolizar
hidrocarburos tanto alifáticos como aromáticos, pero no se sabe con
seguridad en qué medida ocurre realmente tal cosa en la naturaleza. A
diferencia de los microorganismos que utilizan carbonos de petróleo
como fuente de carbono, los animales generalmente oxidan y conjugan
los productos, generando productos finales que son más solubles y por
consiguiente más fáciles de excretar. Todas las especies animales
analizadas pueden incorporar hidrocarburos de petróleo. Se sabe que
los HAP, el petróleo bruto y los productos refinados derivados del
petróleo son inductores de las enzimas del citocromo P450 y aumentan
el metabolismo de los hidrocarburos en numerosas especies de peces
marinos y de agua dulce.
Hay pocos datos disponibles sobre la bioacumulación de diesel en
condiciones de laboratorio, pero diversos estudios sobre derrames y
estudios de laboratorio sobre otros tipos de petróleo, en particular
el fuel-oil No 2, han aportado pruebas abundantes de que los
organismos acuáticos bioconcentran los hidrocarburos. El coeficiente
de reparto n-octanol-agua para el diesel es de 3,3-7,06, valores que
indican una alta bioacumulación potencial; no obstante, muchos de los
compuestos de bajo peso molecular relativo son metabolizados
fácilmente, y la bioacumulación real de los compuestos de mayor peso
molecular relativo se ve limitada por su escasa hidrosolubilidad y su
gran tamaño molecular. Así pues, la bioacumulación real posiblemente
es baja.
Se han descubierto peces envenenados por diesel después de
derrames, pero no se dispone de datos sobre la biomagnificación del
combustible. No hay datos experimentales sobre el movimiento del
diesel a través del suelo, aunque se ha señalado que existe quizá una
correlación directa entre el movimiento y la viscosidad cinemática. El
movimiento del queroseno a través del suelo depende del contenido de
humedad y de las características del suelo.
A1.4 Niveles ambientales y exposición humana
Debido a su carácter de mezclas complejas, no se han determinado
los niveles ambientales de los combustibles diesel. Se pueden detectar
componentes individuales del diesel en casi todos los compartimentos
del medio ambiente, pero no es posible averiguar su origen. La
población general puede verse expuesta al diesel en las gasolineras y
de resultas de los derrames.
Hay muchas actividades que acarrean exposición ocupacional al
diesel. Dada su escasa volatilidad, en principio estos combustibles
sólo suelen generar bajas concentraciones de vapores a temperaturas
normales, pero a temperaturas altas las concentraciones pueden llegar
a ser importantes.
A1.5 Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
La toxicidad aguda de los combustibles diesel es baja en caso de
exposición oral o cutánea o de inhalación. El valor de la DL50 oral
fue superior a 5000 mg/kg de peso corporal en todas las especies
analizadas (ratón, conejo, rata, cobayo). La DL50 del producto
aplicado a nivel cutáneo fue superior a 5000 mg/kg de peso corporal en
el ratón y el conejo, si bien se notificaron valores de más de
2000 mg/k de peso corporal para algunos querosenos y destilados
medios, con distintos protocolos y dosis límite menores. La CL50
observada en ratas expuestas por inhalación fue de aproximadamente
5 mg/litro, con excepción de un destilado medio directo para el que se
halló un valor de 1,8 mg/litro.
En conejos y en ratones tratados cutáneamente con cantidades
diarias de hasta 8000 µl/kg de peso corporal y hasta 40 000 mg/kg de
peso corporal respectivamente, se observó la aparición de acantosis e
hiperqueratosis como consecuencia de una irritación grave. Los conejos
eran más sensibles que los ratones. La inhalación de diesel tuvo
efectos neurodepresivos en ratones a concentraciones de hasta
0,2 mg/litro, pero no así en ratas expuestas a concentraciones de
hasta 6 mg/litro. En ratas se observó una disminución del peso
corporal y del peso del hígado.
No se observaron signos importantes de toxicidad acumulativa en
ratones, ratas y perros que habían inhalado hasta 1,5 mg/litro de
forma subcrónica. El síndrome de nefropatía específico observado en
ratas macho se asocia a una acumulación inherente de gotas hialinas en
los túbulos renales.
Los únicos efectos de las exposiciones a largo plazo fueron la
ulceración por aplicación cutánea en ratones (250 ó 500 mg/kg de peso
corporal al día) y alteraciones importantes del peso de los órganos
tras la inhalación de 1 ó 5 mg/litro en ratas. En los dos estudios el
peso corporal medio disminuyó.
Diversos tipos de diesel se revelaron entre leves y gravemente
irritantes para la piel del conejo. Los combustibles diesel no son
irritantes para el ojo, si bien se ha observado que algunos querosenos
tienen un ligero efecto de esa naturaleza. Los combustibles diesel no
causan sensibilización cutánea.
El diesel y los combustibles de avión (queroseno) no tuvieron
efectos embriotóxicos ni teratogénicos en dos estudios realizados en
ratas expuestas por inhalación a concentraciones de 100 ó 400 ppm y en
un estudio en que las ratas recibieron hasta 2000 mg/kg de peso
corporal diarios mediante sonda. En este último estudio se observó una
disminución del peso fetal.
Las pruebas realizadas con Salmonella typhimurium no aportaron
indicios claros de mutagenicidad. Algunos datos positivos obtenidos
con S. typhimurium y en células de linfoma de ratón se consideraron
equívocos dada la incoherencia de los resultados. Las pruebas de
genotoxicidad realizadas con ratones in vivo (inducción de
micronúcleos o aberraciones cromosómicas) también dieron resultados
equívocos o negativos.
Se ha observado la inducción de un bajo nivel de carcinogenicidad
cutánea por combustibles diesel. En el estado actual de las
investigaciones, es imposible discernir si la actividad carcinogénica
del diesel se debe a un mecanismo genotóxico o a la aparición de
lesiones cutáneas crónicas.
A1.6 Efectos en el ser humano
Una posible fuente de exposición no ocupacional al combustible
diesel es el llenado manual de depósitos de combustible. La fuente
principal de exposición cutánea son los derrames accidentales, que dan
lugar de inmediato a niveles altos de exposición, si bien de corta
duración.
Como efectos del contacto cutáneo accidental se han notificado
anuria, insuficiencia renal, síntomas gastrointestinales e
hiperqueratosis cutánea. Se ha observado un cuadro pulmonar tóxico
tras la ingestión accidental de diesel y su sucesiva aspiración. Se ha
notificado el desarrollo de tos productiva persistente tras la
inhalación del producto.
En un estudio de casos y testigos realizado en hombres expuestos
al diesel, se observó un aumento del riesgo de cáncer de pulmón
distinto del adenocarcinoma; se detectó asimismo una correlación
positiva con el cáncer de próstata, si bien se halló un riesgo mayor
para el grupo sometido a una exposición 'no considerable' que para el
sometido a una exposición 'considerable'.
En un estudio transversal realizado entre obreros expuestos a
combustibles de avión a base de queroseno se observó que los
trabajadores presentaban mareos, cefaleas, náuseas, palpitaciones,
presión torácica e irritación ocular con mayor frecuencia que los
testigos no expuestos. Se calculó que la concentración promedio
ponderada por el tiempo del vapor desprendido por el combustible en la
zona en que se respiraba era de 128-423 mg/m3
A1.7 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El diesel es más tóxico que el petróleo bruto para los organismos
y las plantas acuáticas. La ecotoxicidad del diesel se atribuye por lo
general a compuestos aromáticos solubles, pero es posible que también
intervengan hidrocarburos alifáticos insolubles. Entre los compuestos
aromáticos, los monoaromáticos son los menos tóxicos, y su toxicidad
aguda aumenta con el peso molecular hasta los compuestos de cuatro a
cinco anillos, si bien estos últimos son escasamente solubles en agua
de mar. En algunos animales, como por ejemplo peces y aves, el
recubrimiento físico de la superficie corporal por el combustible
puede ser causa de toxicidad y mortalidad.
Se han llevado a cabo experimentos de laboratorio con combustible
diesel, fracciones hidrosolubles, dispersiones de petróleo-agua y
petróleo microencapsulado. El diesel no redujo significativamente el
crecimiento en cultivo del alga verde Euglena gracilis, mientras que
una concentración baja (0,1%) inhibió casi por completo el crecimiento
de Scenedesmus quadricauda. La presencia de diesel a baja
concentración (0,05%) estimuló el crecimiento, la fotosíntesis y la
síntesis de clorofila a de Chlorella salina, pero inhibió
ligeramente su respiración; a concentraciones superiores el ritmo de
crecimiento y la fotosíntesis se vieron considerablemente reducidos.
La exposición prolongada inhibió el crecimiento de las algas
bentónicas Ascophyllum nodosum y Laminaria digitata. En algas
verdeazuladas, la fotosíntesis se vio reducida por las fracciones
aromática y asfáltica, pero no por la fracción alifática.
El diesel tuvo efectos tóxicos agudos en Daphnia, en larvas de
quironómidos, y en el molusco Viviparus bengalensis (gasterópodos).
Una concentración de 0,1 ml/litro causó la muerte de copépodos de
charca Tigriopus californicus en el término de cinco días.
Los mejillones Mytilus edulis acumulan diesel, presentan
índices de alimentación y crecimiento considerablemente reducidos y
acusan efectos de toxicidad reproductiva tras la exposición crónica a
dicho combustible. La CE50 para el desove en mejillones expuestos
durante 30 días fue de aproximadamente 800 µg/litro. La CL50 de
gasóleo microencapsulado en mejillones en fase de maduración expuestos
durante 30 días al producto fue de unos 5000 µg/litro. El gasóleo fue
más tóxico para las larvas que para los ejemplares jóvenes:
10 µg/litro tenían efectos adversos para el crecimiento de las larvas.
Cangrejos de agua dulce (Barytelphusa cunicularis) expuestos a
concentraciones subletales de diesel por espacio de hasta 96 horas
redujeron por lo general su consumo de oxígeno, sobre todo a
exposiciones bajas de hasta ocho horas. A exposiciones más largas el
consumo de oxígeno fue similar o superior al de los controles.
En las pruebas de toxicidad aguda de 96 horas llevadas a cabo en
salmónidos jóvenes en condiciones estáticas, el diesel tuvo efectos
más tóxicos para el salmón rosado Onchorhychus gorbuscha
(CL50: 32-123mg/litro) que para el salmón plateado O. kisutch
(CL50: 2186-3017 mg/litro) o para la trucha arco iris O. mykiss
(CL50: 3333-33216 mg/litro), con independencia del tipo de agua.
El umbral de detección de efectos comportamentales en bacalaos
( Gadus morhua L.) expuestos a diesel en agua de mar fue de
100-400 ng/litro. El pez antártico Pagothenia borchgrevinki soportó
una fracción hidrosoluble no diluida de combustible diesel durante
72 horas, si bien mostró signos de estrés.
Las aves se ven afectadas externa e internamente por los
petróleos contaminantes. El diesel destruye la impermeabilidad del
plumaje de las aves y éstas lo ingieren cuando se limpian las plumas.
El diesel y el fuel-oil administrados mediante sonda a razón de
2 ml/kg de peso corporal a patos causó neumonía lipídica, inflamación
extrema de los pulmones, infiltración grasa del hígado y degeneración
hepática al cabo de 24 horas. La administración de diesel o fuel-oil a
dosis de 1 ml/kg causó irritación grave del tracto digestivo y
nefrosis tóxica. Dosis mayores causaron aumento de las glándulas
suprarrenales (debido sobre todo a una hiperplasia del tejido
cortical), disminución de los niveles plasmáticos de colinesterasa,
ataxia y temblores. La administración de estos productos a dosis de
hasta 20 ml/kg de peso corporal no tuvieron efectos mortales en
pájaros sanos, pero la DL50 del diesel y el fuel-oil administrados a
aves estresadas fue de 3-4 ml/kg de peso corporal.
Tras los derrames de diesel, el zooplancton parece altamente
vulnerable a los componentes dispersos y disueltos del petróleo, pero
no tanto al petróleo flotante. Los organismos acuáticos pueden verse
afectados de varias formas: mortalidad directa (huevos de pescados,
copépodos y plancton mixto), contaminación externa por petróleo
(corion de huevos de pescados y cutículas y apéndices de alimentación
de los crustáceos), contaminación tisular por componentes aromáticos,
desarrollo anormal de embriones de peces, y alteración de las tasas
metabólicas.
A1.8 Evaluación de los riesgos para la salud humana
La población general puede verse expuesta al diesel y a otros
destilados medios en las gasolineras de resultas de derrames
accidentales, durante la manipulación de dichos combustibles y con
motivo del uso de queroseno en la cocina o la calefacción domésticas.
Los trabajadores pueden verse expuestos al diesel o a otros destilados
medios al manipular y descargar el combustible en las terminales, los
tanques de almacenamiento y las gasolineras; durante la fabricación,
la reparación, el mantenimiento y el ensayo de motores y demás equipo
a base de diesel; durante la utilización del diesel como agente
limpiador o disolvente; y durante el manejo y el muestreo sistemáticos
de combustible diesel en el laboratorio. Debido a su baja volatilidad
a temperatura ambiente sólo suelen producirse concentraciones bajas de
vapor, si bien en espacios cerrados y a altas temperaturas se pueden
alcanzar niveles importantes.
La exposición al vapor desprendido es mínima durante la
manipulación habitual del diesel. El efecto más probable en la salud
humana es la dermatitis resultante del contacto cutáneo. El diesel es
irritante para la piel, pero al parecer no para el ojo. La exposición
cutánea puede dar lugar a efectos tóxicos agudos a nivel renal, pero
se desconoce qué efectos tiene la absorción cutánea prolongada de
bajas concentraciones.
La ingestión de combustibles diesel tiene efectos tóxicos, y en
ocasiones se produce regurgitación y aspiración, posible causa de
neumonía química, al igual que puede ocurrir con cualquier
hidrocarburo que tenga un determinado grado de viscosidad.
En roedores, la exposición por inhalación a concentraciones de
diesel de hasta 0,2 mg/litro tuvo efectos neurodepresivos en ratones,
pero no en cambio en ratas a las concentraciones más altas. La
exposición subcrónica por inhalación a diversos destilados
combustibles indujo una nefropatía específica para la
alpha2-microglobulina en ratas macho; se consideró que este efecto era
irrelevante para el hombre.
No se observaron efectos embriotóxicos ni teratogénicos en
animales expuestos oralmente o por inhalación a combustibles diesel.
No hay indicios claros de una actividad mutagénica en bacterias,
y los resultados de otras pruebas de genotoxicidad
in vitro e in vivo fueron ambiguos.
Los resultados de un estudio de casos y testigos realizado entre
trabajadores expuestos a diesel llevaron a pensar en un aumento del
riesgo de cáncer de pulmón distinto del adenocarcinoma y de cáncer de
próstata. En ninguno de los casos se observó una relación exposición-
respuesta. En vista de los pocos estudios disponibles, del reducido
número de casos y de la consiguiente amplitud de los intervalos de
confianza, no es posible extraer conclusión alguna acerca de la
carcinogenicidad del diesel para el hombre.
En ratones, la administración cutánea de combustibles diesel fue
débilmente carcinógena. Dado que no existe una genotoxicidad clara, es
posible que la inducción de cáncer se deba a mecanismos no
genotóxicos, por ejemplo a una irritación cutánea crónica
caracterizada por ciclos repetidos de lesiones cutáneas, con la
consiguiente hiperplasia epidérmica.
A1.9 Evaluación de los efectos en el medio ambiente
El medio puede contaminarse de resultas de la liberación
accidental de diesel en gran escala, como la asociada a desastres en
depósitos o escapes en oleoductos, o a menor escala como resultado de
la contaminación del suelo de las inmediaciones de fábricas o garajes.
En el agua, el diesel se propaga de forma casi inmediata, los
componentes polares y de bajo peso molecular relativo se disuelven y
se separan por lixiviación, los componentes volátiles se evaporan a
partir de la superficie del agua, y da comienzo la degradación
microbiana. La intensidad de la degradación depende de la temperatura
y de las condiciones climáticas. La composición química de los
derrames cambia con el tiempo: después de un vertido al agua, algunas
fracciones se evaporan, y los componentes de diesel evaporados se
degradan fotoquímicamente; en el sedimento, parece ser que el diesel
es transportado en general a los sedimentos del fondo por las
partículas que se depositan; en el suelo, los componentes del diesel
migran a distintas velocidades, en función del tipo de suelo.
Los componentes individuales son de por sí biodegradables, pero
la velocidad de biodegradación depende estrechamente de las
condiciones físicas y climáticas y de la composición de la población
microbiana.
Los organismos acuáticos, en particular los moluscos, bioacumulan
hidrocarburos en diversa medida, pero se produce una depuración de los
hidrocarburos al transferirlos a agua limpia. Puede darse una
bioacumulación de diesel; ahora bien, no hay datos sobre su
biomagnificación.
Los derrames de diesel tienen efectos nocivos inmediatos en el
medio, donde causan una considerable mortalidad de la biota. Puede
producirse una recolonización al cabo de aproximadamente un año, en
función de la especie de animal o planta y del contenido químico y
físico de los restos del derrame. Los organismos acuáticos que
sobreviven a los derrames de diesel pueden verse afectados por la
contaminación externa y por la acumulación del producto en los
tejidos: un desarrollo anormal y la presencia de tasas metabólicas
alteradas son indicios del estrés resultante.
PARTE B EMISIONES DE GASES DE ESCAPE DE MOTORES DIESEL
B1. RESUMEN
B1.1 Identidad, propiedades físicas y químicas y métodos
analíticos
Las emisiones de gases de escape de los motores diesel contienen
cientos de compuestos químicos, que son emitidos en parte en la fase
gaseosa y en parte en la fase de materia particulada de los gases de
escape. Los principales productos gaseosos de la combustión son los
siguientes: dióxido de carbono, oxígeno, nitrógeno y vapor de agua;
hay también monóxido de carbono, dióxido de azufre, óxidos de
nitrógeno, e hidrocarburos y sus derivados. Se detectan benceno y
tolueno en el margen porcentual correspondiente a los compuestos de
menor peso en la parte gaseosa de la fracción de hidrocarburos. Otros
componentes de los gases de escape son los hidrocarburos aromáticos
policíclicos (HAP) de bajo peso molecular relativo.
Una importante característica de las emisiones de estos gases de
escape es que la liberación de partículas es 20 veces más intensa que
en los vehículos propulsados por gasolina. Las partículas están
compuestas de carbono elemental, compuestos orgánicos adsorbidos del
combustible y el aceite lubricante, sulfatos derivados del azufre del
combustible, y cantidades mínimas de componentes metálicos. La mayor
parte de la materia particulada total tiene al parecer dimensiones del
orden de la submicra: entre 0,02 y 0,5 µm. Con el paso del tiempo
puede producirse aglomeración, hasta un diámetro máximo de 30 µm. Las
partículas emitidas presentan una gran superficie. Los compuestos
orgánicos representan por lo general un 10%-30% de la materia
particulada total, pero los motores mal diseñados o mantenidos pueden
generar hasta un 90%. En esta fracción se encuentran también, a
concentraciones de partes por millón, HAP, HAP oxigenados y nitro-HAP
de alto peso molecular relativo.
Para medir las emisiones de los vehículos se utilizan ciclos de
pruebas transitorios y estables específicos. Pueden tomarse muestras
de gases de escape diluidos o no diluidos. Es difícil conseguir
muestras sin artefactos, ya que los componentes de los gases de escape
pueden sufrir reacciones químicas, procesos de adsorción y desorción,
y condensación y difusión. Los HAP de mayor interés toxicológico de la
materia particulada del diesel se determinan por lo general mediante
la extracción de Soxhlet, limpieza y fraccionamiento, seguidos de
análisis por cromatografía líquida de alta resolución o cromatografía
de gases unida a espectrometría de masas.
B1.2 Fuentes de exposición humana y ambiental
La principal fuente de gases de escape de motores diesel son los
vehículos de motor; otras fuentes son los motores diesel fijos, de
locomotoras de ferrocarril y de barco. Las emisiones de los vehículos
de motor diesel han sido bien caracterizadas, pero a menudo los
resultados de los estudios no se prestan a comparación, debido a
diferencias que afectan a parámetros tales como los ciclos de pruebas,
el tipo de motor y la composición del combustible. Las cantidades
emitidas de los diversos componentes son las siguientes: dióxido de
carbono, aproximadamente 1 kg/km; monóxido de carbono, óxidos de
nitrógeno, hidrocarburos gaseosos totales y materia particulada,
0,1-20 g/km; y compuestos alifáticos, alcoholes, aldehídos, compuestos
aromáticos ligeros y HAP, microgramos por kilómetro. Las emisiones de
monóxido de carbono, óxidos de nitrógeno, hidrocarburos gaseosos
totales y materia particulada están reglamentadas por ley en varios
países.
En lo que respecta a la calidad y cantidad de los gases de
escape, no suele haber diferencias entre los motores de baja y de gran
potencia, si bien los vehículos pesados liberan cantidades
relativamente mayores de materia particulada. La naturaleza de los
gases de escape depende del ciclo de pruebas (transitorio o estable),
de las características del motor (técnicas de inyección y de
aspiración, mantenimiento, kilometraje), y de la composición del
combustible (contenido de azufre, aromaticidad, volatilidad); el
ajuste del motor es muy importante en este sentido.
La liberación de materia particulada aumenta con la disminución
de la proporción aire/combustible, el aumento de la carga y el aumento
de la temperatura. Emiten más partículas los motores viejos y muy
usados que los nuevos y de bajo kilometraje, probablemente debido a un
mayor consumo de aceites lubricantes. La emisión de partículas por los
vehículos diesel de poca potencia también está correlacionada con el
contenido de azufre del combustible, ya que la formación de sulfatos
de metal se traduce en un aumento de la masa de partículas; no se ha
determinado la emisión de partículas por parte de vehículos de gran
potencia. El aumento de la aromaticidad del combustible también
conlleva una mayor emisión de partículas.
Los HAP y los HAP oxigenados emitidos por los motores diesel y de
encendido por chispa son parecidos cualitativamente. Los HAP
oxigenados y nitrados se emiten a niveles del orden de pocos
microgramos por kilómetro, pero no se sabe con seguridad qué
concentración real alcanzan estos compuestos, ya que pueden
descomponerse y formarse durante el muestreo. Las emisiones de HAP
aumentan con la carga y con la temperatura, así como con a antigüedad
del motor, a causa probablemente de un mayor consumo de aceites
lubricantes. La emisión de HAP depende también de la técnica de
inyección del motor: las emisiones de HAP aumentan al mismo tiempo que
la proporción aire/combustible en los motores de inyección directa,
mientras que disminuyen en los motores de inyección indirecta. La
aromaticidad y la volatilidad del combustible guardan relación directa
con la emisión de HAP. El mal funcionamiento de los dispositivos del
motor, sobre todo del sistema de inyección de combustible, aumenta la
emisión de los principales componentes de escape. Se dispone de pocos
datos sobre la contribución de las emisiones de los motores diesel a
la liberación total de productos de combustión por el hombre.
Es posible reducir las emisiones de gases de escape producidos
por el diesel mejorando el diseño de los motores, los captores de
partículas (oxidantes de captores) y los catalizadores. Mientras que
los captores de partículas eliminan tanto el hollín como los
compuestos orgánicos solubles adsorbidos en las partículas, los
catalizadores reducen sobre todo los niveles de monóxido de carbono y
de hidrocarburos gaseosos. En la práctica es difícil regenerar los
captores de partículas. Los catalizadores exigen combustibles de bajo
contenido de azufre, ya que este elemento daña los centros activos del
catalizador.
B1.3 Transporte, distribución y transformación en el medio
ambiente
El compartimento que antes se ve afectado por las emisiones de
gases de escape de los motores diesel es la atmósfera. La hidrosfera y
la geosfera se ven contaminadas indirectamente por depósitos secos y
húmedos. El destino ambiental de los distintos componentes de estos
gases de escape es en general bien conocido: las partículas se
comportan como moléculas de gas (no reactivas) por lo que se refiere a
su transporte mecánico en la atmósfera; pueden ser transportadas a
gran distancia y penetrar incluso en la estratosfera.
Se estima que el índice global de eliminación de las partículas
diesel es bajo, lo que se traduce en una permanencia de varios días en
la atmósfera. Con el tiempo se puede producir una coagulación de las
partículas, con lo que aumenta la precipitación de las mismas y
disminuye su concentración total en el aire. El carbono elemental de
las partículas diesel puede actuar como catalizador de la formación de
ácido sulfúrico por oxidación del dióxido de azufre. Los componentes
orgánicos adsorbidos en el carbono elemental pueden experimentar
varias reacciones físicas y químicas con otros compuestos atmosféricos
y durante la exposición a la luz solar.
B1.4 Niveles ambientales y exposición humana
Dado que los gases de escape diesel son una mezcla compleja de
gran diversidad de compuestos, por lo general no es posible precisar
los 'niveles ambientales'. Los distintos componentes de los gases de
escape diesel se pueden detectar en principio en todos los
compartimentos del medio ambiente, aunque por lo general no es posible
averiguar su origen. Se conocen los niveles ambientales de la mayoría
de los distintos componentes.
La exposición de la población general a los gases de escape de
motores diesel se produce especialmente en las calles transitadas y en
los aparcamientos, sobre todo los subterráneos. La identificación de
las fuentes resulta difícil, y la contribución de esos gases a la
contaminación total por productos de combustión del tráfico se calcula
en general a partir de los coeficientes de emisión y del porcentaje de
vehículos propulsados por diesel. Los niveles de exposición de la
población general y de los trabajadores a la materia particulada de
los motores diesel tienen importancia toxicológica. Las
concentraciones ambientales diarias de partículas diesel cerca de las
carreteras oscilan como promedio entre 8 y 42 µg/m3. Las
concentraciones promedio anuales estimadas fueron de 5-10 µg/m3 en
zonas urbanas y < 1,5 µg/m3 en zonas rurales de Alemania, y de
1-2 µg/m3 en zonas urbanas y 0,6-1 µg/m3 en zonas rurales de los
Estados Unidos de América. Las concentraciones están directamente
relacionadas con la densidad del tráfico y son tanto menores cuanto
más lejos están las carreteras.
La identificación de las fuentes también puede revestir
dificultad en los lugares de trabajo, sobre todo en las minas, donde
la carga total de polvo es elevada. Se han hecho análisis de muestras
de carbono para determinar la exposición específica a gases de escape
de motores diesel en el lugar de trabajo y, según se ha notificado,
los niveles de materia particulada a que están expuestos los
trabajadores son del orden de 0,04-0,134 mg/m3 para los conductores
de camiones y de 0,004-0,192mg/m3 para los ferroviarios. Como
medidas de la exposición ocupacional se han utilizado también las
partículas respirables totales y las partículas suspendidas totales.
B1.5 Cinética y metabolismo en animales de laboratorio y en el
hombre
B1.5.1 Depósito
Las partículas de los gases de escape de motores diesel, con un
diámetro aerodinámico mediano de aproximadamente 0,2 µm, son filtradas
en cierta medida por la nariz, y la eficiencia de depósito en el
pulmón es sólo ligeramente superior al valor mínimo hallado para las
partículas que tienen un diámetro aerodinámico mediano de
aproximadamente 0,5 µm. Así, un 10%-15% de las partículas de hollín
diesel inhaladas se depositan en la región alveolar de los pulmones de
la rata y el cobayo; en el hombre se deposita en la región alveolar un
10%.
B1.5.2 Retención y eliminación de las partículas
La eliminación mucociliar de partículas en las vías respiratorias
ciliadas es casi completa en un plazo de 24 horas. La eliminación a
largo plazo de las partículas diesel en la región alveolar se ha
determinado mediante varios estudios realizados en ratas expuestas por
inhalación a partículas diesel o control marcadas. Se notificaron
periodos de semieliminación de 60-100 días en testigos con cargas
pulmonares bajas (< 1 mg/pulmón), mientras que los periodos de
semieliminación aumentaron a 100-600 días en ratas con cargas
pulmonares crecientes de 1 a 60 mg/pulmón. En varios estudios se halló
que los efectos se debían a una sobrecarga de partículas, fenómeno
descrito en diversas especies y con varios tipos de materia
particulada. Este hecho se observa por lo general cuando la velocidad
de depósito de las partículas de baja solubilidad y baja toxicidad
aguda supera su velocidad de eliminación durante un tiempo
considerable. El periodo de semieliminación alveolar libre en el
hombre es de varios cientos de días, mayor que en la rata.
Se desarrolló un modelo pulmonar dosimétrico a partir de datos
relativos al depósito y retención de las partículas diesel en ratas
sometidas a inhalación prolongada, así como de datos referentes al
depósito y retención de partículas en el hombre. Este modelo puede
utilizarse para predecir la retención de partículas diesel y de
material orgánico adsorbido en los pulmones de personas de diferentes
edades. No se dispone de datos sobre la variación de la retención de
distintos componentes tras la exposición prolongada a gases de escape
de motores diesel.
B1.5.3 Retención y eliminación de hidrocarburos aromáticos
policíclicos adsorbidos en hollín de diesel
Los HAP del hollín de diesel se adhieren fuertemente a la
superficie de las partículas. Aproximadamente un 50% de los HAP
adsorbidos en las partículas diesel desaparece de los pulmones en el
plazo de un día, pero los periodos de semieliminación de los restantes
HAP fueron del orden de 18-36 días. Estudios realizados con
3H-benzo[ a]pireno y 14C-nitropireno han demostrado que, cuando
los HAP están asociados a materia particulada, su eliminación de los
pulmones se retrasa significativamente en comparación con los HAP
inhalados no asociados a materia particulada.
B1.5.4 Metabolismo
Se ha observado que el benzo[ a]pireno adsorbido en partículas
de gases de escape de motores diesel se metaboliza por oxidación en
fenoles, dioles y quinonas de benzo[ a]pireno en el pulmón y en
cultivos celulares de macrófagos pulmonares.
El nitropireno adsorbido en partículas diesel se metabolizó en
acetilaminopirenofenol después de su inhalación. Se han hallado más
aductos de ADN en los pulmones y las células de tipo II de ratas
expuestas a gases de escape de motores diesel que en las testigo. El
metabolismo oxidativo de algunos compuestos orgánicos en epóxidos
podría ser la causa de la formación de esos aductos, pero únicamente
se hallaron aductos tras la exposición a partículas. Se ha demostrado
que algunas sustancias orgánicas de los gases de escape de motores
diesel dan lugar a la formación de aductos de ADN; sin embargo, el
núcleo carbonáceo por sí mismo (sin material orgánico extraíble)
también puede inducir aductos, por lesión crónica de las células
epiteliales.
B1.6 Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
Los escasos datos disponibles parecen indicar que la toxicidad
aguda de los gases de escape de motores diesel es escasa. Ratones
tratados intratraquealmente con partículas de tales gases murieron de
edema pulmonar; la DL50 fue de aproximadamente 20 mg/kg de peso
corporal. Partículas extraídas con metanol no tuvieron efectos letales
a concentraciones de hasta aproximadamente 33 mg/kg de peso corporal.
En hámsters, la DL50tras administración intraperitoneal fue de
1280 mg/kg de peso corporal. No hay datos respecto a la exposición por
inhalación.
La exposición de ratas, cobayos y gatos a gases de escape de
motores diesel con un contenido de partículas de 6 mg/m3 durante
aproximadamente cuatro semanas alteró la función pulmonar, causando en
particular un aumento del 35% de la resistencia al flujo pulmonar en
los cobayos y una disminución del 10% de la capacidad vital (flujo
espiratorio) en los gatos. El análisis histopatológico reveló un
espesamiento focal de las paredes alveolares, un aumento significativo
del índice de marcaje de las células de tipo II, y acumulaciones de
macrófagos cargados de partículas. Estas acumulaciones se hallaban
cerca de los bronquiolos terminales, y aumentaron de tamaño, debido a
la adhesión (secuestro) de macrófagos, durante el periodo ulterior de
recuperación.
No se observaron disminuciones importantes del peso corporal ni
acortamiento alguno del tiempo de supervivencia en ratones, ratas,
hámsters, gatos y monos después de un periodo prolongado de inhalación
de concentraciones de hasta 4 mg/m3. En todas las especies sometidas
a inhalación prolongada de gases de escape de motores diesel se
observaron los siguientes efectos tóxicos relacionados con la dosis:
aumentos de hasta un 400% del peso pulmonar; inflamación pulmonar
cuantificable mediante parámetros bioquímicos (enzimas citoplasmáticas
marcadoras, colágeno) y citológicos (aumento de los neutrófilos
polimorfonucleares); trastornos de la mecánica pulmonar; aumento del
número de macrófagos cargados de partículas con acumulaciones focales
(secuestro) en condiciones de sobrecarga; y ulteriores cambios
proliferativos de las células epiteliales, con inicio de fibrosis.
La limitada información disponible sobre la toxicidad para la
reproducción y el desarrollo lleva a pensar que la inhalación de gases
de escape de motores diesel no reviste gran importancia. En la mayoría
de los experimentos no se observaron efectos en ratones, ratas,
hámsters, conejos o monos; no obstante, tras inyección intraperitoneal
se observaron anomalías en el semen de ratones sometidos a partículas
de esos gases de escape, y embriotoxicidad en hámsters sometidos a
extractos de tales gases.
La mayoría de las pruebas de genotoxicidad in vitro se han
llevado a cabo con extractos de gases de escape de motores diesel en
lugar de con la mezcla total de tales gases; se detectaron respuestas
positivas en ausencia de activación metabólica, es decir: los efectos
genotóxicos eran al parecer independientes de los HAP. Aproximadamente
la mitad de las investigaciones in vivo arrojaron resultados
negativos; los únicos resultados positivos fueron la inducción del
intercambio de cromátides hermanas en respuesta a la mezcla total de
gases de escape y a extractos orgánicos, y la inducción de
micronúcleos en respuesta a extractos orgánicos.
Por lo general no se observaron efectos inmunotóxicos después de
la inhalación de gases de escape de motores diesel; sin embargo, en un
estudio se halló un mayor título de anticuerpos IgE antiovoalbúmina, y
en dos experimentos, una mayor susceptibilidad a las infecciones en
ratones. Algunos estudios realizados en la rata parecen indicar que la
inhalación de estos gases de escape tiene efectos sobre el
comportamiento y el estado neurofisiológico.
En estudios de determinación de la carcinogenicidad de los gases
de escape de motores diesel administrados por inhalación a ratas se
observó que la fase gaseosa (sin la fracción particulada) no era
carcinogénica. En todos los estudios válidos realizados en la rata se
halló que los gases eran carcinogénicos a concentraciones de
partículas superiores a 2 mg/m3, lo que equivale a una exposición
continua de aproximadamente 1 mg/m3. No se observaron efectos ni en
el hámster ni en el ratón. En estudios realizados mediante instilación
intratraqueal, tanto las partículas de los gases de escape como el
negro de humo indujeron la aparición de tumores, y se observó una
aparente correlación entre la superficie de las partículas carbonosas
y la actividad tumorigénica. La inhalación prolongada de negro de humo
prácticamente desprovisto de HAP a concentraciones similares también
provocó la aparición de tumores pulmonares en ratas.
No está claro si en los mecanismos de carcinogenicidad de los
gases de escape de motores diesel intervienen reacciones del ADN o de
otro tipo (o quizá ambas). Se han utilizado diversos modelos para
dilucidar la carcinogenicidad de esos gases.
B1.7 Efectos en el ser humano
Los gases de escape de los motores diesel contribuyen a la
contaminación atmosférica en general. Aunque en los estudios agudos o
crónicos no es posible diferenciar el papel de las partículas diesel,
éstas podrían ser parcialmente responsables de los diversos efectos
sobre la salud asociados a la contaminación atmosférica.
Los gases de escape habituales de los motores diesel tienen un
olor peculiar, que algunas personas consideran desagradable, sobre
todo a altas concentraciones. Los síntomas que siguen a la exposición
aguda y crónica a esos gases han sido descritos en estudios y en
referencias anecdóticas de personas expuestas en el lugar de trabajo.
Se ha observado la aparición de irritación de las membranas mucosas
oculares y nasales en respuesta a la exposición aguda a tales gases,
así como un aumento de la frecuencia de síntomas respiratorios en
cohortes ocupacionales; no obstante, se ignora la contribución de las
partículas diesel. No se han detectado efectos sistemáticos a corto
plazo en la función pulmonar, pero se han notificado ataques de asma.
En un estudio controlado en el que se expuso a ocho voluntarios
sanos no fumadores en una cámara durante 60 minutos a gases de escape
de motores diesel diluidos, se detectó una disminución de la
fagocitosis en los macrófagos alveolares del líquido de lavado
broncoalveolar.
En algunos estudios transversales y longitudinales realizados
entre trabajadores expuestos de forma prolongada en su entorno laboral
a esos gases se han hallado decrementos de la función pulmonar y una
mayor frecuencia de síntomas respiratorios, pero estos estudios
presentan la limitación de haberse realizado con una exposición breve.
No se observaron aumentos importantes en estudios de cohortes
realizados para investigar las defunciones por trastornos
cardiovasculares y/o cerebrovasculares debidos a las emisiones diesel.
En varios estudios epidemiológicos se ha evaluado la asociación
entre cánceres de pulmón y de la vejiga urinaria y la exposición
ocupacional a gases de escape de motores diesel. En esta monografía se
incluyen sólo los estudios considerados pertinentes para evaluar los
efectos carcinogénicos de esos gases. Los estudios más importantes por
lo que se refiere al cáncer de pulmón son los realizados en
ferroviarios, trabajadores de garajes de autobuses y estibadores, esto
es, cohortes de individuos cuya actividad laboral no deja lugar a
dudas sobre la exposición a gases de escape diesel. La totalidad de
los cuatro estudios más informativos pusieron de manifiesto un mayor
riesgo de cáncer de pulmón, con riesgos relativos que van desde 1,4
para los ferroviarios, pasando por 1,3-2,4 para los trabajadores de
garajes de autobuses (según el grado de exposición), hasta 3-6 para
los estibadores (según el método empleado para evaluar la exposición,
pero con amplios intervalos de confianza). En un estudio de casos y
testigos realizados entre ferroviarios y en el estudio de los
estibadores fue posible introducir ajustes en función del consumo de
tabaco. En los dos casos, el efecto de la exposición al diesel no se
vio materialmente alterado por el ajuste realizado en función del
consumo de tabaco. En los tres estudios en que no pudo hacerse ese
ajuste, el análisis se basó en comparaciones de subgrupos de las
cohortes, de manera que la probabilidad de influencia del tabaco como
factor de confusión fue menor que al utilizar grupos de comparación
externos.
Se han llevado a cabo varios estudios de casos y testigos para
examinar la relación entre el cáncer de vejiga urinaria y la presunta
exposición a gases de escape de motores diesel. Se detectó un aumento
del riesgo, sobre todo para los conductores de camión; no obstante,
todos estos estudios adolecían de una deficiente caracterización de la
exposición. Por ello, no es posible establecer una asociación causal
entre la exposición a los gases de escape diesel y un aumento del
riesgo de cáncer de vejiga urinaria
B1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El único estudio sobre los efectos de las emisiones de motores
diesel propiamente dichas es un trabajo realizado con algas verdes.
B1.9 Evaluación de los riesgos para la salud humana
Se utilizó el paradigma de evaluación de riesgos de la Academia
Nacional de Ciencias de los Estados Unidos (National Research Council,
1983) para evaluar los riesgos asociados a variables de valoración
relacionadas y no relacionadas con el cáncer. Este proceso consta de
los cuatro pasos siguientes: 1) identificación del riesgo, 2)
evaluación de la relación dosis-respuesta, 3) evaluación de la
exposición, y 4) caracterización del riesgo.
Se considera que los estudios más informativos son los
epidemiológicos de larga duración realizados con una exposición bien
definida y con seguimiento ulterior (> 20 años). Satisfacían esos
criterios cuatro estudios sobre el cáncer de pulmón en personas
expuestas en su entorno de trabajo. Los riesgos relativos de cáncer de
pulmón asociado a la exposición a gases de escape de motores diesel
fueron por lo general bajos y podían haberse debido al azar, a los
efectos de factores de confusión no determinados y a la dificultad
para corregir con precisión la influencia de los factores de confusión
conocidos. Las conclusiones de estos estudios están avaladas por los
resultados de otros estudios menos precisos en cuanto a la definición
de la exposición. Globalmente se considera que los gases de escape de
los motores diesel son probablemente carcinogénicos para el hombre;
sin embargo, no se dispone de datos cuantitativos que permitan estimar
el riesgo para el ser humano.
B1.9.1 Efectos no neoplásicos
Para caracterizar los riesgos se utilizaron dos métodos
generales: el nivel sin efectos adversos observados (NOAEL) dividido
por un factor de incertidumbre y una concentración de referencia. En
los dos casos se empleó un sofisticado modelo dosimétrico para reducir
la incertidumbre asociada a la extrapolación interespecies de las
dosis.
Se calculó que el nivel sin efecto de las partículas de los gases
de escape de los motores diesel en el hombre era de 0,139 mg/m3. El
valor orientativo para la población general calculado a partir del
modelo dosimétrico fue de 5,6 µg/m3, y el calculado sin el modelo,
de 2,3 µg/m3.
El método de la concentración de referencia tiene en cuenta la
totalidad de la relación exposición-respuesta en lugar de puntos
únicos obtenidos en estudios de inhalación, como ocurre con el método
del NOAEL. Se identificaron tres variables de evaluación sensibles:
inflamación alveolar crónica, eliminación pulmonar alterada, y
lesiones pulmonares hiperplásicas. Las concentraciones de referencia,
calculadas mediante el mismo modelo dosimétrico utilizado para el
método del NOAEL, fueron de 0,9-2 µg/m3 para la inflamación,
1,2-3 µg/m3 para la disminución de la eliminación pulmonar, y
6,3-14 µg/m3 para las lesiones hiperplásicas.
B1.9.2 Efectos neoplásicos
Se utilizó un modelo polietápico linearizado para hacer
estimaciones del riesgo unitario asociado a la exposición a gases de
escape de motores diesel. Se consideró que los resultados de los
estudios epidemiológicos disponibles eran insuficientes para hacer una
estimación cuantitativa del riesgo unitario, por lo que se decidió
utilizar datos de varios estudios de inhalación prolongada en los que
se había detectado carcinogénesis a concentraciones superiores a
2 mg/m3. Se calculó un riesgo unitario de 3,4 × 10-5 µg/m3
(media geométrica de cuatro estimaciones del riesgo) para las
partículas de los gases de escape. Usando otro modelo biológico se
obtuvo un riesgo unitario parecido, en el supuesto de que las
partículas diesel influyen en la iniciación y/o proliferación celular
a bajas concentraciones.
B1.10 Evaluación de los efectos en el medio ambiente
No se disponía de suficiente información para evaluar los efectos
específicos de las emisiones de gases de escape de motores diesel. Los
efectos del combustible diesel quemado deben de ser parecidos a los de
otros combustibles fósiles y están relacionados con el consumo de
diesel.
if sepsilon Li, tepsilon Lj with tj < tj.
B11. RECOMMENDATIONS
B11.1 Recommendations for the protection of human health
Diesel exhaust contributes to the total effect of combustion
products on the environment. The data reviewed in this monograph
support the conclusion that inhalation of diesel exhaust is of concern
with respect to both neoplastic and non-neoplastic diseases. The
particulate phase appears to have the greatest effect on health, and
both the particle core and the associated organic materials have
biological activity, although the gas-phase components cannot be
disregarded. The following actions are recommended for the protection
of human health.
-- Diesel exhaust emissions should be controlled as part of the
overall control of atmospheric pollution, particularly in urban
environments.
-- Emissions should be controlled strictly by regulatory inspections
and prompt remedial action.
-- Urgent efforts should be made to reduce emissions, specifically
of particulates, by changing exhaust train techniques, engine
design, and fuel composition.
-- In the occupational environment, good work practices should be
encouraged, and adequate ventilation must be provided to prevent
excessive exposure.
B11.2 Recommendations for the protection of the environment
Too few data are available on which to base specific suggestions
with regard to diesel exhaust, except as part of the general control
of emissions.
B11.3 Recommendations for further research
The following recommendations are intended to help reduce the
uncertainty associated with assessing the risks of exposure to diesel
exhaust for human health.
-- Research is required on methods for determining concentrations of
diesel particulates in the presence of fine particles from other
sources, in order to improve assessments of the exposures of
occupational groups and the general population indoors and
outdoors. The quality and quantity of emissions from engines that
are not properly maintained or tuned should be investigated as
part of these studies.
-- The effects of diesel exhaust emissions on lung clearance should
be studied in animals and humans at concentrations including
those likely to be encountered by humans.
-- The mechanisms involved in the etiology of tumours induced in
rats exposed to particulate must be investigated by all available
techniques.
-- The relative roles of the gas and particulate phases of diesel
exhaust in causing adverse effects after short- and long-term
exposure should be investigated.
-- Epidemiological investigations into the effects of diesel exhaust
should be continued in order to assess issues of dose and latency
in human populations with respect to lung cancer; and longer-term
studies should be conducted in populations exposed to diesel
exhaust with respect to noncarcinogenic effects.
-- Further steps should be undertaken to improve the combustion and
exhaust emission characteristics of diesel engines.
B12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks for human beings were evaluated by a
working group convened by the International Agency for Research on
Cancer in 1988 (International Agency for Research on Cancer, 1989b).
The conclusions were:
'There is sufficient evidence for the carcinogenicity in
experimental animals of whole diesel engine exhaust.
'There is inadequate evidence for the carcinogenicity in
experimental animals of gas-phase diesel engine exhaust (with
particles removed).
'There is sufficient evidence for the carcinogenicity in
experimental animals of extracts of diesel engine exhaust particles.
'There is limited evidence for the carcinogenicity in humans of
diesel engine exhaust.
'There is limited evidencefor the carcinogenicity in humans of
engine exhausts (unspecified as from diesel or gasoline engines).
'Overall evaluation
'Diesel engine exhaust is probably carcinogenic to humans (Group2A).'
REFERENCES
Acher AJ, Boderie P, & Yaron B (1989) Soil pollution by petroleum
products: I. Multiphase migration of kerosene components in soil
columns. J Contam Hydrol, 4: 333-345.
Ackman RG & Noble D (1973) Steam distillation: A simple technique for
recovery of petroleum hydrocarbons from tainted fish. J Fish Resour
Board Can, 30: 711-714.
Agency for Toxic Substances and Disease Registry (1995) Toxicological
profile for fuel oils. Atlanta, GA, 204 pp.
Ahlberg J, Ahlbom A, Lipping H, Norell S, & Österblom L (1981) [Cancer
among professional drivers.] Laekartidn, 78: 1545-1546 (in Danish).
Albrechcinski TM, Michalovic JG, Wattle BJ, & Wilkinson EP (1985) A
laboratory investigation of the fate of diesel emissions in the
atmosphere. Task 2 (Final Report No. CRC-APRAC-CAPA-13-76-05;
PB 86-215837). Buffalo, NY, Calspan Corp., 163 pp.
Amann CA & Siegla DC (1982) Diesel particulates -- What they are and
why. Aerosol Sci Technol, 1: 73-101.
American Automobile Manufacturers Association (1993) World motor
vehicle data. Detroit, pp 9, 50, 51, 335.
American Petroleum Institute (1979) Teratology study in rats, diesel
fuel (Publication No. 27-32174). Washington DC, 23 pp.
American Petroleum Institute (1980a) Acute toxicity tests:
API 78-3, No. 2 home heating oil (10% cat.) (Publication
No. 27-32773). Washington DC, 52 pp.
American Petroleum Institute (1980b) Acute toxicity tests:
API 78-2, No. 2 home heating oil (30% cat.) (Publication
No. 27-32771). Washington DC, 48 pp.
American Petroleum Institute (1980c) Acute toxicity tests:
API 78-4, No. 2 home heating oil (50% cat.) (Publication
No. 27-32068). Washington DC, 52 pp.
American Petroleum Institute (1980d) Acute toxicity tests. API jet
fuel A (Publication no. 27-32815). Washington DC, 48 pp.
American Petroleum Institute (1980e) Mutagenicity evaluation of jet
fuel A in the mouse dominant lethal assay. Final report (Publication
No. 28-31345). Washington DC, 36 pp.
American Petroleum Institute (1981) Mutagenicity evaluation of diesel
fuel in the mouse dominant lethal assay, final report (Publication
No. 33-30493). Washington DC, 9 pp.
American Petroleum Institute (1982a) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32347). Washington DC, 24 pp.
American Petroleum Institute (1982b) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-10 (Publication
No. 30-32348). Washington DC, 26 pp.
American Petroleum Institute (1982c) Acute toxicity studies
hydrodesulfurized kerosine. Sample 81-07 (Publication No. 30-31986).
Washington DC, 112 pp.
American Petroleum Institute (1983a) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32856). Washington DC, 47 pp.
American Petroleum Institute (1983b) 28-Day dermal toxicity study in
the rabbit: Hydrodesulfurized middle distillate, API sample 81-10
(Publication No. 30-32298). Washington DC, 13 pp.
American Petroleum Institute (1983c) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats (Publication
no. 30-32855). Washington DC, 32 pp.
American Petroleum Institute (1984) Dermal sensitization study in
guinea pig, closed patch technique: Hydrodesulfurized middle
distillate, sample 81-10 (Publication No. 31-31414). Washington DC,
12 pp.
American Petroleum Institute (1985a) 28-Day dermal toxicity study in
the rabbit: API 83-08, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 32-32753). Washington DC, 27 pp.
American Petroleum Institute (1985b) 28-Day dermal toxicity study in
the rabbit: API 83-11, straight run middle distillate (CAS 64741-59-9)
(Publication No. 32-32747). Washington DC, 29 pp.
American Petroleum Institute (1985c) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs: API 83-07, light
catalytically cracked distillate (CAS 64741-59-9) (Publication
No. 33-30162). Washington DC, 41 pp.
American Petroleum Institute (1985d) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs, API 83-11, straight run
middle distillate (CAS 64741-44-2) (Publication No. 32-32857).
Washington DC, 34 pp.
American Petroleum Institute (1985e) Acute oral toxicity study in
rats, acute dermal toxicity study in rabbits, primary dermal
irritation study in rabbits, primary eye irritation study in rabbits,
dermal sensitization study in guinea pigs. API 83-09, straight run
kerosine (CAS No. 8008-20-6) (Publication No. 32-32858). Washington
DC, 39 pp.
American Petroleum Institute (1985f) The evaluation of the
carcinogenicity of certain petroleum fractions (Publication
No. 32-30964). Washington DC, 148 pp.
American Petroleum Institute (1986a) Acute inhalation toxicity
evaluation in rats: API 83-07, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 33-30549). Washington DC, 15 pp.
American Petroleum Institute (1986b) Acute inhalation toxicity
evaluation in rats: API 83-08, light catalytically cracked distillate
(CAS 64741-59-9) (Publication No. 33-30444). Washington DC, 16 pp.
American Petroleum Institute (1986c) Four week subchronic inhalation
toxicity study in rats -- final report: API 81-07, hydrodesulfurized
kerosene (petroleum) (CAS 64742-81-0); API 81-09, hydrodesulfurized
middle distillate (petroleum) (CAS 64742-80-9); API 81-10,
hydrodesulfurized middle distillate (petroleum) (CAS 64742-80-9)
(Publication No. 33-32724). Washington DC, 31 pp.
American Petroleum Institute (1987a) Acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats: API 83-11,
straight run middle distillate (CAS 64741-59-9) (Publication
No. 34-30635). Washington DC, 53 pp.
American Petroleum Institute (1987b) Acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats -- API 83-09.
Final report (Publication No. 34-30634). Washington DC, 10 pp.
American Petroleum Institute (1987c) Mutagenicity of API 81-10,
hydrode-sulfurized middle distillate (CAS 64742-80-9) (coded as
API 86-10) in a mouse lymphoma mutation assay. Final report
(Publication No. 34-32643). Washington DC, 21 pp.
American Petroleum Institute (1989a) Twenty-four month dermal
carcinogenesis/chronic toxicity screening bioassay of refinery streams
in C3H/ Hej mice. API 190r. Final report: lifetime carcinogenicity
evaluation (Publication No. 36-33220). Washington DC, 26 pp.
American Petroleum Institute (1989b) Lifetime dermal
carcinogenesis/chronic toxicity screening bioassay of refinery streams
in C3H/HE mice. API 135r. Final report: lifetime carcinogenicity
evaluation (Publication No. 36-31364). Washington DC, 41 pp.
American Society for Testing and Materials (1988) ASTM Designation:
D 975-81. Standard specification for diesel fuel oils. Philadelphia,
Committee on Standards, pp 351-361.
American Society for Testing and Materials (1992) ASTM Designation:
D 975-92a. Standard specification for diesel fuel oils. Philadelphia,
Committee on Standards, pp 310-311.
Ames RG, Attfield MD, Hankinson JL, Hearl FJ, & Reger RB (1982) Acute
respiratory effects of exposure to diesel emissions in coal miners.
Am Rev Respir Dis, 125: 39-42.
Ames RG, Hall DS, & Reger RB (1984) Chronic respiratory effects of
exposure to diesel emissions in coal mines. Arch Environ Health,
39: 389-394.
Anderson JW (1977a) Effects of petroleum hydrocarbons on the growth of
marine organisms. In: McIntyre AD & Whittle KJ, eds, Petroleum
hydrocarbons in the marine environment (Rapports et proces-verbaux des
réunions, Vol. 171). Copenhagen, International Council for the
Exploration of the Sea, pp 157-165.
Anderson JW (1977b) Responses to sublethal levels of petroleum
hydrocarbons: Are they sensitive indicators and do they correlate with
tissue contamination? In: Wolfe DA, ed., Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, Pergamon
Press, pp 95-114.
Anderson JW (1979) An assessment of knowledge concerning the fate and
effects of petroleum hydrocarbons in the marine environment. In:
Vernberg WB, Calabrese A, Thurberg FP, & Vernberg FJ, eds, Marine
pollution: Functional responses. New York, Academic Press, pp 3-21.
Anderson JW, Neff JM, Cox BA, Tatem HE, & Hightower GM (1974)
Characteristics of dispersions and water-soluble extracts of crude and
refined oils and their toxicity to estuarine crustaceans and fish. Mar
Biol, 27: 75-88.
Anderson JW, Dixit DB, Ward GS, & Foster RS (1976) Effects of
petroleum hydrocarbons on the rate of heart beat and hatching success
of estuarine fish embryos. In: Vernberg FJ & Vernberg WB, eds,
Pollution and physiology of marine organisms, Vol. 2. New York,
Academic Press, pp 241-258.
Anderson E, Albert R, Anderson L, Bayliss D, Bayard S, Chen C, Chu M,
Gibb H, Haberman B, Hiremath C, McGaughy R, Singh D, & Thorslund T
(1983) Quantitative approaches in use to assess cancer risk.
Risk Anal, 3: 277-295.
Apol A (1983) Health hazard evaluation. Regional transportation
district; Denver (Report No. HETA 82-137-1264; PB84-209386).
Cincinnati, OH, National Institute for Occupational Safety and Health,
pp 1-23.
Atlas RM & Bartha R (1973) Fate and effects of polluting petroleum in
the marine environment. Res Rev, 49: 49-84.
Atlas RM, Boehm PD, & Calder JA (1981) Chemical and biological
weathering of oil, from the Amoco Cadiz oil spillage, within the
littoral zone. Estuarine Coastal Mar Sci, 12: 589-608.
Attfield MD, Trabant GD, & Wheeler RW (1982) Exposure to diesel fumes
and dust at six potash mines. Ann Occup Hyg, 26: 817-831.
Auerbach O & Garfinkel L (1991) The changing pattern of lung
carcinoma. Cancer, 68: 1973-1977.
Bailey MR, Fry FA, & James AC (1985a) Long-term retention of particles
in the human respiratory tract. J Aerosol Sci, 16: 295-305.
Bailey MR, Hodgson A, & Smith H (1985b) Respiratory tract retention of
relatively insoluble particles in rodents. J Aerosol Sci, 16: 279-293.
Baker JM (1970) The effect of fuel oils on plants. Environ Pollut,
1: 27-44.
Ball JC & Young WC (1992) Evidence for a new class of mutagens in
diesel particulate extracts. Environ Sci Technol, 26: 2181-2186.
Ball JC, Greene B, Young WC, Richert JFO, & Salmeen IT (1990)
S9-Activated Ames assays of diesel-particle extracts. Detecting
indirect-acting mutagens in samples that are direct-acting. Environ
Sci Technol, 24: 890-894.
Barbella R, Bertoli C, Ciajolo A, & D'Anna A (1988) Soot and unburnt
liquid hydrocarbon emissions from diesel engines. Combust Sci Technol,
59: 183-198.
Barfknecht TR, Andon BM, Thilly WG, & Hites RA (1981) Soot and
mutation in bacteria and human cells. In: Cooke M & Dennis AJ, eds,
Chemical analysis and biological fate: Polynuclear aromatic
hydrocarbons. Columbus, OH, Battelle Press, pp 231-242.
Barnett CJ & Kontogiannis JE (1975) The effect of crude oil fractions
on the survival of a tidepool copepod, Tigriopus californicus.
Environ Pollut, 8: 45-54.
Barnhart MI, Chen S-T, Salley SO, & Puro H (1981) Ultrastructure and
morphometry of the alveolar lung of guinea pigs chronically exposed to
diesel engine exhaust: six months' experience. J Appl Toxicol,
1: 88-103.
Barnhart MI, Salley SO, Chen S-T, & Puro H (1982) Morphometric
ultrastructural analysis of alveolar lungs of guinea pigs chronically
exposed by inhalation to diesel exhaust (DE). In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. New York,
Elsevier, pp 183-200.
Barrientos A, Ortuno MT, Morales JM, Martinez Tello F, & Rodicio JL
(1977) Acute renal failure after use of diesel fuel as shampoo. Arch
Int Med, 137: 1217.
Battigelli MC (1965) Effects of diesel exhaust. Arch Environ Health,
10: 165-167.
Bauer MA, Utell MJ, Morrow PE, Speers DM, & Gibb FR (1986) Inhalation
of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm
in asthmatics. Am Rev Respir Dis, 134: 1203-1208.
Bauer HD, Dahmann D, Kollmeier FW, & Lindecke B (1990) [Diesel engine
emissions in potash-salt mines.] Kompass, 100: 516-524 (in German).
Bechtold WE, Henderson TR, & Brooks AL (1986) Isolation,
identification and bacterial mutagenicity of 2-nitro-9-fluorenone from
diesel-exhaust particle extracts. Mutat Res, 173: 105-109.
Beck LS, Hepler DI, & Hansen KL (1984) The acute toxicology of
selected petroleum hydrocarbons. In: MacFarland HN, Holdsworth CE,
MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the symposium:
The toxicology of petroleum hydrocarbons, May 1984. Washington DC,
American Petroleum Institute, pp 1-12.
Behn U, Kaschani DT, Lützke K Rentel S & Burk HD (1985) ['PAH-sample
system' for industrial plants. Trial and first results.] Erdöl Kohle
Erdgas Petrochem, 38: 131 (in German).
Behymer T & Hites R (1984) Similarity of some organic compounds in
spark-ignition and diesel engine particulate extracts. Environ Sci
Technol, 18: 203-206
Beland FA, Heflich RH, Howard PC, & Fu PP (1985) The in vitrometabolic
activation of nitro polycyclic aromatic hydrocarbons. In: Harvey RG,
ed., Polycyclic hydrocarbons and carcinogenesis. Washington DC,
American Chemical Society, pp 371-396.
Beliles RP & Mecler FJ (1982) Inhalation teratology of jet fuel A,
fuel oil and petroleum naphtha in rats. In: MacFarland HN, Holdsworth
CE, MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the
symposium: The toxicology of petroleum hydrocarbons, May 1982.
Washington DC, American Petroleum Institute, pp 234-238.
Bellmann B, Muhle H, Creutzenberg O, & Mermelstein R (1992)
Irreversible pulmonary changes induced in rat lung by dust overload.
Environ Health Perspectives, 97: 189-191.
Benhamou S, Benhamou E, & Flamant R (1988) Occupational risk factors
of lung cancer in a French case-control study. Br J Ind Med,
45: 231-233.
Bennett D, Girling AE, & Bounds A (1990) Ecotoxicology of oil
products: Preparation and characterisation of aqueous test media.
Chemosphere, 21: 659-669.
Bevan DR & Ruggio DM (1991) Bioavailability in vivo of benzo[ a]pyrene
adsorbed to diesel particulate. Toxicol Ind Health, 7: 125-139.
Bice DE, Mauderly JL, Jones RK, & McClellan RO (1985) Effects of
inhaled diesel exhaust on immune responses after lung immunization.
Fundam Appl Toxicol, 5: 1075-1086.
Biles R, McKee RH, Lewis SC, Scala RA, & DePass LR (1988) Dermal
carcinogenic activity of petroleum-derived middle distillate fuels.
Toxicology, 53: 301-314.
Blackburn GR, Deitch RA, Schreiner CA, Mehlman MA, & Mackerer CR
(1984) Estimation of the dermal carcinogenic activity of petroleum
fractions using a modified Ames assay. Cell Biol Toxicol, 1: 67-80.
Block RN, Allworth N, & Bishop M (1991) Assessment of diesel
contamination in soil. In: Calabrese EJ & Kostecki PT, eds,
Hydrocarbon contaminated soils, Vol. I. Remediation techniques,
environmental fate, risk assessment, analytical methodologies,
regulatory considerations. Chelsea, MI, Lewis Publishers, pp 135-148.
Blome H, Heidermanns G, & Timmer J (1990) [Assessment of workplace
atmospheres in case of diesel motor vehicles.] Staub Reinhalt Luft,
50: 93-97 (in German).
Blumer M, Souza G, & Sass J (1970) Hydrocarbon pollution of edible
shellfish by an oil spill. Mar Biol, 5: 195-202.
Boehm PD & Quinn JG (1974) The solubility behavior of No. 2 fuel oil
in sea water. Mar Pollut Bull, 5: 101-105.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Boffetta P, Harris RE, & Wynder EL (1990) Case-control study on
occupational exposure to diesel exhaust and lung cancer risk. Am J Ind
Med, 17: 577-591.
Bohning D, Atkins H, & Cohn S (1982) Long-term particle clearance in
man: Normal and impaired. Ann Occup Hyg, 26: 259-271.
Bokn T (1987) Effects of diesel oil and subsequent recovery of
commercial benthic algae. Hydrobiology, 151-152: 277-284.
Bond JA, Mitchell CE, & Li AP (1983) Metabolism and macromolecular
covalent binding of benzo[ a]pyrene in cultured Fischer-344 rat lung
type II epithelial cells. Biochem Pharmacol, 32: 3771-3776.
Bond JA, Butler MM, Medinsky MA, Muggenburg BA, & McClellan RO (1984)
Dog pulmonary macrophage metabolism of free and particle-associated
[14C]benzo[a]pyrene. J Toxicol Environ Health, 14: 181-189.
Bond JA, Sun JD, Medinsky MA, Jones RK, & Yeh HC (1986a) Deposition,
metabolism, and excretion of 1-[14C]nitropyrene and 1-[14C]nitropyrene
coated on diesel exhaust particles as influenced by exposure
concentration. Toxicol Appl Pharmacol, 85: 102-117.
Bond JA, Sun JD, Mitchell CE, Dutcher JS, Wolff RK, & McClellan RO
(1986b) Biological fate of inhaled organic compounds associated with
particulate matter. In: Lee SD, Schneider D, Grant JD, & Verkerk PJ,
eds, Aerosols. Chelsea, MI, Lewis Publishers, pp 579-592.
Bond JA, Wolff RK, Harkema JR, Mauderly JL, Henderson RF, Griffith WC,
& McClellan RO (1988) Distribution of DNA adducts in the respiratory
tract of rats exposed to diesel exhaust. Toxicol Appl Pharmacol,
96: 336-346.
Bond JA, Harkema JR, Henderson RF, Mauderly JL, McClellan RO, & Wolff
RK (1989) Molecular dosimetry of inhaled diesel exhaust. In: Mohr U,
Bates DV, Dungworth DL, Lee PN, McLellan RO, & Roa FJC, eds,
Assessment of inhalation hazards: Integration and extrapolation using
diverse data. Berlin, Springer, pp 315-324.
Bond JA, Johnson NF, Snipes MB, & Mauderly JL (1990a) DNA adduct
formation in rat alveolar type II cells: Cells potentially at risk for
inhaled diesel exhaust. Environ Mol Mutag, 16: 64-69.
Bond JA, Harkema JR, Henderson RF, Mauderly JL, McClellan RO, & Wolff
RK (1990b) The role of DNA adducts in diesel exhaust-induced pulmonary
carcinogenesis. Prog Clin Biol Res, 340C: 259-269.
Bond JA, Mauderly JL, & Wolff RK (1990c) Concentration- and
time-dependent formation of DNA adducts in lungs of rats exposed to
diesel exhaust. Toxicology, 60: 127-135.
Booth M & Reglitzky AA (1991) Diesel fuel quality in an
environmentally conscious world. In: The diesel engine -- energy stake
and environmental constraints. Cologne, TÜV Rheinland-OPET,
pp 289-299.
Boudet F, Fabre M, Boe M, Delon M, Ruiz J, & Lareng L (1983) Toxic
pneumopathy after voluntary ingestion of one-and-a-half litres of
diesel fuel. Toxicol Eur Res, 5: 247-249.
Braddock JN & Perry NK Jr (1986) Gaseous and particulate emissions
from gasoline- and diesel-powered heavy duty trucks (Paper
No. 860617). In: International Congress and Exposition, Detroit, MI,
24-28 February 1986. Warrendale, PA, Society of Automotive Engineers,
39 pp.
Brightwell J, Fouillet X, Cassano-Zoppi A-L, Gatz R, & Duchosal F
(1986) Neoplastic and functional changes in rodents after chronic
inhalation of engine exhaust emissions. In: Ishinishi N, Koizumi A,
McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects of
diesel engine exhaust. Amsterdam, Elsevier, pp 471-485.
Brightwell J, Fouillet X, Cassano-Zoppi A-L, Bernstein D, Crawley F,
Duchosal F, Gatz R, Perczel S, & Pfeifer, H (1989) Tumours of the
respiratory tract in rats and hamsters following chronic inhalation of
engine exhaust emissions. J Appl Toxicol, 9: 23-31.
Broman D, Näf C, Lundbergh I, & Zebühr Y (1990) An in situ study on
the distribution, biotransformation and flux of polycyclic aromatic
hydrocarbons (PAHs) in an aquatic food chain (seston Mytilus edulis L.
Somateria mollissima L.) from the Baltic: An ecotoxicological
perspective. Environ Toxicol Chem, 9: 429-442.
Bruner RH (1984) Pathologic findings in laboratory animals exposed to
hydrocarbon fuels of military interest. In: Mehlman MA, Hemstreet GP,
Thorpe JT, & Weaver NK, eds, Renal effects of petroleum hydrocarbons
(Advances in modern environmental toxicology, Vol. 7). Princeton, NJ,
Princeton Scientific Publishers, pp 133-140.
Bruner RH, Kinkead ER, O'Neill TP, Flemming CD, Mattie DR, Russell CA,
& Wall HG (1993) The toxicologic and oncogenic potential of JP-4 jet
fuel vapors in rats and mice: 12-month intermittent inhalation
exposures. Fundam Appl Toxicol, 20: 97-110.
Bury RB (1972) The effects of diesel fuel on a stream fauna. Calif
Fish Game, 58: 291-295.
Byrne CJ & Calder JA (1977) Effect of the water-soluble fractions of
crude, refined and waste oils on the embryonic and larval stages of
the Quahog clam Mercenaria sp. Mar Biol, 40: 225-231.
Campbell J, Scholl J, Hibbler F, Bagley S, Leddy D, Abata D, & Johnson
J (1981a) The effect of fuel injection and timing on the physical,
chemical, and biological character of particulate emissions from a
direct injection diesel. In: Diesel combustion and emissions,
Part III, SP-495. International Off-Highway Meeting and Exposition,
Milwaukee, Wisconsin, 14-17 September 1981. Warrendale, PA, Society of
Automotive Engineers, pp 71-108.
Campbell KI, George EI, & Washington IS Jr (1981b) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ Int, 5: 377-382.
Campobosso O, Andrion A, Ribotta M & Ronco G (1993) The value of the
1981 WHO histological classification in inter-observer reproducibility
and changing pattern of lung cancer. Int J Cancer, 53:205-208
Carey PM (1987) Air toxics emissions from motor vehicles (Technical
report) (EPA-AA-TSS-PA-86-5). Ann Arbor, MI, US Environmental
Protection Agency, 129 pp.
Carpenter CP, Geary DL Jr, Myers RC, Nachreiner DJ, Sullivan LJ, &
King JM (1976) Petroleum hydrocarbon toxicity studies. XI. Animal and
human response to vapors of deodorized kerosene. Toxicol Appl
Pharmacol, 36: 443-456.
Carr RS & Reish DJ (1977) The effect of petroleum hydrocarbons on the
survival and life history of polychaetous annelids. In: Wolfe D, ed.,
Fate and effects of petroleum hydrocarbons in marine ecosystems and
organisms. Oxford, Pergamon Press, pp 168-173.
Carstens T & Sendstad E (1979) Oil spill on the shore of an
ice-covered fjord in Spitzbergen. In: Proceedings of the 79th
international conference on port and ocean engineering under Arctic
conditions, Trondheim, 13-18 August. Trondheim, Norwegian Institute of
Technology, pp 1227-1242.
Casto BC, Hatch GG, Huang SL, Lewtas J, Nesnow S, & Waters MD (1981)
Mutagenic and carcinogenic potency of extracts of diesel and related
environmental emissions: In vitro mutagenesis and oncogenic
transformation. Environ Int, 5: 403-409.
Castranova V, Bowman L, Reasor MJ, Lewis T, Tucker J, & Miles PR
(1985) The response of rat alveolar macrophages to chronic inhalation
of coal dust and/or diesel exhaust. Environ Res, 36: 405-419.
Chan K-Y & Chiu SY (1985) The effects of diesel oil and oil
dispersants on growth, photosynthesis, and respiration of Chlorella
salina. Arch Environ Contam Toxicol, 14: 325-331.
Chan TL, Lee PS, & Hering WE (1981) Deposition and clearance of
inhaled diesel exhaust particles in the respiratory tract of Fischer
rats. J Appl Toxicol, 1: 77-82.
Chan TL, Lee PS, & Hering WE (1984) Pulmonary retention of inhaled
diesel particles after prolonged exposures to diesel exhaust. Fundam
Appl Toxicol, 4: 624-631.
Chen B & Farland W (1991) Incorporating cell proliferation in
quantitative cancer risk assessment: Approach, issues, and
uncertainties. In: Butterworth B, Slaga T, Farland W, & McClain M,
eds, Chemically induced cell proliferation: Implications for risk
assessment. New York, Wiley-Liss, pp 481-499.
Chescheir GM, Garrett NE, Shelburne JD, Huisingh JL, & Waters MD
(1981) Mutagenic effects of environmental particulates in the
CHO\HGPRT system In: Waters MD, Sandhu SS, Huisingh JL, Claxton L, &
Nesnow S, eds, Short-term bioassays in the analysis of complex
environmental mixtures II, Vol. 2. New York, Plenum Press, pp 337-350.
Chia F-S (1973) Killing of marine larvae by diesel oil. Mar Pollut
Bull, 4: 29-30.
Clark CR & Vigil CL (1980) Influence of rat lung and liver homogenates
on the mutagenicity of diesel exhaust particulate extracts. Toxicol
Appl Pharmacol, 56: 110-115.
Clark CR, Walter MK, Ferguson PW, & Katchen, M (1988) Comparative
dermal carcinogenesis of shale and petroleum-derived distillates.
Toxicol Ind Health, 4: 11-22.
Cohen BL (1994) Invited commentary: In defense of ecologic studies for
testing a linear no threshold theory. Am J Epidemiol, 139: 765-768.
Cohen S & Ellwein L (1988) Cell growth dynamics in long-term bladder
carcinogenesis. Toxicol Lett, 43: 151-173.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
The identification and measurement of components in gasoline,
kerosene, and No. 2 fuel oil that partition into the aqueous phase
after mixing. Arch Environ Contam Toxicol, 13: 171-178.
Coley TR (1989) Diesel fuel additives influencing flow and storage
properties. In: Owen K, ed., Gasoline and diesel fuel additives. New
York, John Wiley & Sons, pp 105-132.
Commins BT, Waller RE, & Lawther PJ (1956) Smoke in a London diesel
bus garage. An interim report. Br Med J, ii: 753-754.
Conaway CC, Schreiner CA, & Cragg ST (1984) Mutagenicity evaluation of
petroleum hydrocarbons. In: MacFarland HN, Holdsworth CE, MacGregor
JA, Call RW, & Lane ML, eds, Applied toxicology of petroleum
hydrocarbons (Advances in modern environmental toxicology, Vol 6).
Princeton, NJ, Princeton Scientific Publishers, pp 89-107.
CONCAWE (1985) Health aspects of petroleum fuels. Potential hazards
and precautions for individual classes of fuels (Report No 85/51). The
Hague, 54 pp.
CONCAWE (1986) The relationship between automotive diesel fuel
characteristics and engine performance (Report No. 86/65). The Hague,
30 pp.
CONCAWE (1987) Diesel fuel quality and its relationship with emissions
from diesel engines (Report No. 10/87). The Hague, 21 pp.
CONCAWE (1990a) Motor vehicle emission regulations and fuel
specifications -- 1990 update (Report No. 2/90). Brussels, Health
Management Group, 92 pp.
CONCAWE (1990b) The sulphur content of diesel fuel and its
relationship with particulate emissions from diesel engines (Report
No. 90/54). Brussels, Health Management Group, 42 pp.
CONCAWE (1991) Middle distillates -- A review of the results of a
CONCAWE programme of short-term biological studies (Report No. 91/51).
Brussels, Health Management Group, pp 1-13.
CONCAWE (1992) The chemical composition of diesel particulate
emissions (Report No. 92/51). Brussels, Health Management Group,
24 pp.
CONCAWE (1993) Ecotoxicological testing of petroleum products: test
methodology (Report No. 92/56). Brussels, Petroleum Products Ecology
Group, 18 pp.
CONCAWE (1995) Kerosines/jet fuels (Product No. 94/106). Brussels,
Petroleum Products and Health Managements Groups, 13 pp.
CONCAWE (in press) Gas oils (diesel fuels/heating oils) Brussels,
Petroleum Products and Health Management Groups, 45 pp.
Cooper JR & Mattie DR (1993) Abstracts of the 32nd Annual Meeting of
the Society of Toxicology. Toxicologist, 13: 78.
Cordier S, Clavel J, Limaset JC, Boccon-Gibod L, Le Moual N, Mandereau
L, & Hermon D (1993) Occupational risks of bladder cancer in France: A
multicentre case-control study. Int J Epidemiol, 22: 403-411.
Corner EDS, Harris RP, Kilvington CC, & O'Hara SCM (1976) Petroleum
compounds in the marine food web: Short-term experiments on the fate
of naphthalene in Calanus. J Mar Biol, 56: 121-133.
Cornwell RJ (1982) Health hazard evaluation determination report.
Climax Molybdenum Company, Climax, CO (PB84-14850 1; Report
No MHETA-81-108-9004). Morgantown, WV, National Institute for
Occupational Safety and Health, 23 pp.
Cowan MJ & Jenkins, LJ (1980) Navy toxicity study of shale and
petroleum JP-5 aviation fuel and diesel fuel marine. In: Griest WH,
Guerin MR, & Coffin DL, eds, Health effects investigation of oil shale
development. Ann Arbor, MI, Ann Arbor Science Publishers, pp 129-139.
Cox BA (1974) Responses of the marine crustaceans Mysidopsis almyra
Bowman, Penaeus aztecus Ives, and Penaeus setiferus (L.) to
petroleum hydrocarbons. Diss Abstr Int, B35: 3739.
Cox BA, Anderson JW, & Parker JC (1975) An experimental oil spill: The
distribution of aromatic hydrocarbons in the water, sediment, and
animal tissues within a shrimp pond. In: Proceedings of the Conference
on Prevention and Control of Oil Pollution, 25-27 March 1975, San
Francisco, California. Washington DC, American Petroleum Institute,
pp 607-612.
Crebelli R, Fuselli S, Conti G, Conti L, & Carere A (1991)
Mutagenicity spectra in bacterial strains of airborne and engine
exhaust particulate extracts. Mutat Res, 261: 237-248.
Creutzenberg O, Bellmann B, Heinrich U, Fuhst R, Koch W, & Muhle H
(1990) Clearance and retention of inhaled diesel exhaust particles,
carbon black, and titanium dioxide in rats at lung overload
conditions. J Aerosol Sci, 21: 455-458.
Crisp AJ, Bhalla AK, & Hoffbrand BI (1979) Acute tubular necrosis
after exposure to diesel oil. Br Med J, ii: 177.
Croxall JP (1977) The effects of oil on seabirds. In: McIntyre AD &
Whittle KJ, eds, Petroleum hydrocarbons in the marine environment
(Rapports et proces-verbaux des réunions, Vol. 171). Copenhagen,
International Council for Exploration of the Sea, pp 191-195.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Csicsaky M, Roller M, & Pott F (1993) [Quantitative risk assessment of
selected carcinogenic substances in the work area (Special publication
No. S/31)]. Dortmund, Federal Institute for Labour Protection,
pp 18-29 (in German).
Cuddihy RG, Griffith WC, & McClellan RO (1984) Health risks from
light-duty diesel vehicles. Low risk of lung cancer from exposure to
diesel exhaust is projected from laboratory and epidemiologic data.
Environ Sci Technol, 18: 14A-21A.
Curren RD, Kouri RE, Kim CM, & Schechtman LM (1981) Mutagenic and
carcinogenic potency of extracts from diesel related environmental
emissions: Simultaneous morphological transformation and mutagenesis
in BALB/c3T3 cells. Environ Int, 5: 411-415.
Custance SR, Sullivan MJ, McCaw PA, & Kopf AC (1993) Environmental
fate of the chemical mixtures: Crude oil, JP-5, mineral spirits, and
diesel fuel. In: Kostecki PT, & Calabrese EJ, eds, Hydrocarbon
contaminated soils and groundwater, Vol. 3. Boca Raton, FL, Lewis
Publishers, pp 205-212.
Dalbey W, Lock S, Garfinkel S, Jenkins R, Holmberg R, & Guerin M
(1982) Inhalation exposures of rats to aerosolized diesel fuel. In:
MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Kane ML, eds,
Proceedings of the symposium on toxicology of petroleum hydrocarbons.
Washington DC, American Petroleum Institute, pp 13-25.
Dalbey W, Henry M, Holmberg R, Moneyhun J, Schmoyer R, & Lock S (1987)
Role of exposure parameters in toxicity of aerosolized diesel fuel in
the rat. J Appl Toxicol, 7: 265-275.
Damber LA & Larsson LG (1987) Occupation and male lung cancer: A
case-control study in northern Sweden. Br J Ind Med, 44: 446-453.
Dambo WB (1993) Tolerance of the periwinkles Pachymelania aurita
(Muller) and Tympanotonus fuscatus (Linne) to refined oils. Environ
Pollut, 79: 293-296.
Daniel JH Jr (1984) Diesels in underground mining: A review and an
evaluation of an air quality monitoring methodology (Document
No. Rl-8884; US NTIS PB84-214444). Washington DC, US Department of the
Interior, Bureau of Mines.
Das PKMK & Konar SK (1988) Acute toxicity of petroleum products, crude
oil and oil refinery effluent on plankton, benthic invertebrates and
fish. Environ Ecol, 6: 885-891.
Das M & Misra MP (1988) Acne and folliculitis due to diesel oil.
Contact Derm, 18: 120-121.
Davis SJ & Gibbs CF (1975) The effect of weathering on a crude oil
residue exposed at sea. Water Res, 9: 275-285.
Davison W, Franklin CE, McKenzie JC, & Dougan MCR (1992) The effects
of acute exposure to the water soluble fraction of diesel fuel oil on
survival and metabolic rate of an Antarctic fish ( Pagothenia
borchgrevinki). Comp Biochem Physiol C, 102: 185-188.
Davison W, Franklin CE, McKenzie JC, & Carey PW (1993) The effects of
chronic exposure to the water soluble fraction of fuel oil on an
Antarctic fish Pagothenia borchgrevinki. Comp Biochem Physiol,
C104: 67-70.
Deichmann WB, Kitzmiller KV, Witherup S, & Johansmann R (1944)
Kerosene intoxication. Ann Intern Med, 21: 803-823.
Dempster A, Laird N, & Rubin D (1977) Maximum likelihood from
incomplete data via the EM algorithm. R Stat Soc, B39: 1-38.
Dennington VN, George JJ, & Wyborn CHE (1975) The effects of oils on
growth of freshwater phytoplankton. Environ Pollut, 8: 233-237.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Cancer Epidemiol Biomarkers Prev,
1: 29-34
Dineen D (1991) Remediation options for diesel-contaminated soil. In:
Calabrese EJ & Kostecki PT, eds, Hydrocarbon contaminated soils,
Vol. 1. Remediation techniques, environmental fate, risk assessment,
analytical methodologies, regulatory considerations. Chelsea, MI,
Lewis Publishers Inc., pp 181-191.
Dixit D & Anderson JW (1977) Distribution of naphthalenes within
exposed Fundulus similus and correlations with stress behavior. In:
Proceedings of 1977 oil spill conference, New Orleans, March 1977.
Washington DC, American Petroleum Institute.
Dockery DW, Arden Pope C III, Xu X, Spengler JD, Ware JH, Fay ME,
Ferris BG Jr, & Speizer FE (1993) An association between air pollution
and mortality in six US cities. New Engl J Med, 329: 1753-1759.
Dolan DF, Kittelson DB, & Pui, DYH (1980) Diesel exhaust particle size
distribution measurement techniques (Paper No. 800187). Warrendale,
PA, Society of Automotive Engineers, pp 149-164.
Dorie LD, Bagley ST, Leddy DG, & Johnson JH (1987) Characterization of
mutagenic subfractions of diesel exhaust modified by ceramic
particulate traps. Environ Sci Technol, 21: 757-765.
Driscoll KE, Carter JM, Howard BW, & Hassenbein DG (1994) Mutagenesis
in rat lung epithelial cells after in vivo silica exposure or
ex vivo exposure to inflammatory cells. Am J Resp Crit Care Med,
149: A553.
Driscoll KE, Carter JM, Oberdörster G, Howard BW, & Hassenbein DG
(1995) Inflammation, cytokine expression and mutagenesis in rat lung
after carbon black inhalation. Toxicologist, 15: 46.
Driscoll KE, Carter JM, Howard BW, Hassenbein D, Pepelko W, Baggs RB,
& Oberdörster G (in press) Pulmonary inflammatory, chemokine and
mutagenic responses in rats after subchronic inhalation of carbon
black. Toxicol Appl Pharmacol.
Du C-J, Kittelson DB, & Zweidinger RB (1984) Measurements of
polycyclic aromatic compounds in the cylinder of an operating diesel
engine (SAE Technical Paper No. 840364). In: International congress
and exposition, Detroit, Michigan, 27 February-2 March 1984.
Warrendale, PA, Society of Automotive Engineers, Engineering Resource
for Advancing Mobility, 12 pp.
Dungworth DL, Mohr U, Heinrich U, Ernst H, & Kittel B (1994)
Pathological effects of inhaled particles in rat lungs: Associations
between inflammatory and neoplastic processes. In: Mohr U, Dungworth
DL, Mauderly JL, & Oberdörster, eds, Toxic and carcinogenic effects of
solid particles in the respiratory tract. Washington DC, International
Life Sciences Institute Press, pp 75-98.
Dunlap LE & Beckmann DD (1988) Soluble hydrocarbons analysis from
kerosene/diesel type hydrocarbons. In: Proceedings of the conference
on petroleum hydrocarbons and organic chemicals in ground water:
Prevention, detection and restoration, 9-11 November 1988, The Westin
Galleria, Houston, Texas. Dublin, OH, National Water Well Association,
pp 37-45
Dutcher JS, Sun JD, Lopez JA, Wolf I, Wolff RK, & McClellan RO (1984)
Generation and characterization of radiolabeled diesel exhaust. Am Ind
Hyg Assoc J, 45: 491-498.
Dziedzic D (1981) Differential counts of B and T lymphocytes in the
lymph nodes, circulating blood and spleen after inhalation of high
concentrations of diesel exhaust. J Appl Toxicol, 1: 111-115.
Edgerton SA, Coutant RW, & Henley MV (1987) Hydrocarbon fuel spill
dispersion on water: a literature review. Chemosphere, 16: 1475-1487.
Edling C & Axelson O (1984) Risk factors of coronary heart disease
among personnel in a bus company. Int Arch Occup Environ Health,
54: 181-183.
Edling C, Anjou C-G, Axelson O, & Kling H (1987) Mortality among
personnel exposed to diesel exhaust. Int Arch Occup Environ Health,
59: 559-565.
Egebäck KE & Bertilsson BM (1983) Chemical and biological
characterization of exhaust emissions from vehicles fueled with
gasoline, alcohol, LPG and diesel (Report No. SNV PM 1635). Solna,
National Swedish Environment Protection Board, 188 pp.
El-Bayoumy K, Johnson BE, Roy AK, Upadhyaya P, & Partian SJ (1994)
Biomonitoring of nitropolynuclear aromatic hydrocarbons via protein
and DNA adducts (Research report No. 64). Cambridge, MA, Health
Effects Institute, pp 1-39.
Elbers G & Muratyan S (1991) [Problems of traffic-related outdoor air
measurement of particles (diesel soot) (VDI Report No. 888)]. In:
[Carcinogenic substances in the environment: Origin, measurement,
risk, minimization.
Meeting of the Commission for Clean Air of the Society of German
Engineers (VDI) and the German Standards Institute (DIN), Mannheim,
23-25 April]. Düsseldorf, Society of German Engineers Press,
pp 143-170 (in German).
Elbers G & Richter J (1994) [Measurement of traffic-induced soot
immissions.] Staub Reinhalt Luft, 54: 19-24 (in German).
Emmelin A, Nyström L, & Wall S (1993) Diesel exhaust exposure and
smoking: A case-referent study of lung cancer among Swedish dock
workers. Epidemiology, 4: 237-244.
Engelhardt FR, Wong MP, & Duey ME (1981) Hydromineral balance and gill
morphology in rainbow trout Salmo gairdneri, acclimated to fresh and
sea water, as affected by petroleum exposure. Aquat Toxicol,
1: 175-186.
Environment Canada (1990) Canadian emissions inventory of common air
contaminants -- 1985 (Environmental Protection Series No. EPS/5/AP/3),
Ottawa.
Environment Canada (1992) Summary of Canadian new vehicle emissions
standards, June 1992. Unpublished results.
Eppley ZA (1992) Assessing indirect effects of oil in the presence of
natural variation: The problem of reproductive failure in South Polar
skuas during the Bahia Paraiso oil spill. Mar Pollut Bull,
25: 307-312.
Ernst VV, Neff JM, & Anderson JW (1977) The effects of the
water-soluble fractions of No. 2 fuel oil on the early development of
the estuarine fish, Fundulus grandis Baird and Girard. Environ
Pollut, 14: 25-35.
European Commission (1993) Guideline 93/12/EEC of the Council from 23
March 1993 on sulfur content of certain liquid fuels (EEC Directive,
L74/81), Luxembourg, 3 pp.
European Committee for Standardization (1993) [Automotive fuels,
diesel. Requirements and methods of test. European Standard EN 590.]
Brussels, 5 pp (in German).
Fabri J, Dabelstein W, & Reglitzky A (1990) Motor fuels. In: Elvers B,
Hawkins S, & Schulz G, eds, Ullmann's encyclopedia of industrial
chemistry, 5th Ed, Vol A16. Weinheim, VCH, pp 719-753.
Farrington JW, Davis AC, Frew NM, & Rabin KS (1982) No. 2 fuel oil
compounds in Mytilus edulis. Retention and release after an oil
spill. Mar Biol, 66: 15-26.
Federal Environment Agency (1991) [Air and noise levels caused by
traffic -- emissions, immissions and effects (Report No. 40/91)].
Berlin, 194 pp (in German).
Federal Environment Agency (1992) [Transport in Germany-Energy
consumption and emissions of air pollutants due to transport in German
Democratic Republic, Berlin (East) and the Federal Republic of Germany
in 1988 and in Germany in 2005 (UBA-FB 50473/85; Report No. 5/92)].
Berlin (in German).
Federal Ministry for Transport (1992) [Traffic in statistical
numbers.] Berlin, German Institute for Economic Research,
pp 132, 133, 280, 281 (in German).
Federal Office for Motor Vehicles (1993) [Emission and noise values of
passenger cars, light vans and station wagons with EEC licence], 3rd
Ed. Flensburg, 260 pp (in German).
Federal Office for Motor Vehicles (1994) [Emission values of vehicles
with EEC licence], 4th Ed. Flensburg, 192 pp (in German).
Flowers TH, Pulford ID, & Duncan HJ (1984) Studies on the breakdown of
oil in soil. Environ Pollut Ser, B8: 71.
Fortnagel M (1992) [Possibilities of the reduction of harmful
substances of diesel engines from the view of the automotive
industry.] In: [Effects of diesel engine exhausts on health]. Munich,
Society for Research on Radiation and the Environment, Research Centre
for Environment and Health, pp 53-56 (in German).
Fossato VU & Canzonier WJ (1976) Hydrocarbon uptake and loss by the
mussel Mytilus edulis. Mar Biol, 36: 243-250.
Frankenberger WT Jr, Emerson KD, & Turner DW (1989) In situ
bioremediation of an underground diesel fuel spill: A case history.
Environ Manage, 13: 325-332.
Fraser Williams (Scientific Systems) Ltd (1985) Oil and hazardous
materials: technical assistance data system (OHM/TADS;
Version 5.00/9.5-December, 1985) Poynton, Cheshire.
Frehland E, Weiss A, & Meduna V (1992) [Microbial communities in
diesel contaminated soil]. In: Behrens D & Wiesner J, eds, [Microbial
cleaning of soil].
Frankfurt/Main, German Society for Chemical Apparatus, Chemical
Technology and Biotechnology, pp 347-349.
Froines JR, Hinds WC, Duffy RM, Lafuente EJ, & Liu WCV (1987) Exposure
of firefighters to diesel emissions in fire stations. Am Ind Hyg
Assoc J, 48: 202-207.
Fromme H (1990) [The importance of diesel engine exhaust gases for
ecological health protection and hygienic environmental protection.]
Öff Gesundheitswesen, 52: 625-629 (in German).
Gaddo P, Settis M, & Giacomelli L (1984) Artifact formation during
diesel particulate collection. Orbassano, Fiat Engineering, Ecological
Service, pp 437-449.
Gairing M, Naber D, Schäfer A, Lange W, Reglitzky A & Le'Jeune A
(1994) [The influence of fuel property on the exhaust emissions of
modern diesel motors from Mercedes-Benz]. Motortech Z, 55: 3-11
(in German, with English abstract)
Galin T, McDowell C, & Yaron B. (1990) The effect of volatilization on
the mass flow of a non-aqueous pollutant liquid mixture in an inert
porous medium: Experiments with kerosene. J. Soil Sci, 41: 631-641.
Gallagher J, George M, Kohan M, Thompson C, Shank T, & Lewtas J (1993)
Detection and comparison of DNA adducts after in vitro and in vivo
diesel emission exposures. Environ Health Perspectives, 99: 225-228.
Gallagher J, Heinrich U, George M, Hendee L, Philips DH, & Lewtas J
(1994) Formation of DNA adducts in rat lung following chronic
inhalation of diesel emissions, carbon black and titanium dioxide
particles. Carcinogenesis, 15: 1291-1299.
Gamble JF & Jones WG (1983) Respiratory effects of diesel exhaust in
salt miners. Am Rev Respir Dis, 128: 389-394.
Gamble J, Jones W, & Minshall S (1987a) Epidemiological-environmental
study of diesel bus garage workers: Acute effects of NO2 and
respirable particulate on the respiratory system. Environ Res,
42: 201-214.
Gamble J, Jones W, & Minshall S (1987b) Epidemiological-environmental
study of diesel bus garage workers: Chronic effects of diesel exhaust
on the respiratory system. Environ Res, 44: 6-17.
Garg BD (1983) Histologic quantitation of macrophage aggregates in the
lungs of diesel exhaust exposed rats. Acta Stereol, 2
(Suppl. 1): 235-238.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Gaworski CL, MacEwen JD, Vernot EH, Bruner RH, & Cowan MJ Jr (1984)
Comparison of the subchronic inhalation toxicity of petroleum and oil
shale JP-5 jet fuels. In: MacFarland HN, Holdsworth CE, MacGregor JA,
Call RW, Kane ML, eds, Applied toxicology of petroleum hydrocarbons
(Advances in modern environmental toxicology, Vol. 6). Princeton, NJ,
Princeton Scientific Publishers, pp 33-47.
Gearing PJ, Gearing JN, Pruell RJ, Wade TL, & Quinn JG (1980)
Partitioning of No. 2 fuel oil in controlled estuarine ecosystems.
Sediments and suspended particulate matter. Environ Sci Technol,
14: 1129-1136.
Gerde P, Medinsky A, & Bond JA (1991a) The retention of polycyclic
aromatic hydrocarbons in the bronchial airways and in the alveolar
region. A theoretical comparison. Toxicol Appl Pharmacol,
107: 239-252.
Gerde P, Medinsky MA, & Bond JA (1991b) Contemporary issues in
toxicology. Particle-associated polycyclic aromatic hydrocarbons. A
reappraisal of their possible role in pulmonary carcinogenesis.
Toxicol Appl Pharmacol, 108: 1-13.
Gerde P, Muggenburg BA, Sabourin PJ, Harkema JR, Hotchkiss JA, Hoover
MD, & Henderson RF (1993a) Disposition of polycyclic aromatic
hydrocarbons in the respiratory tract of the beagle dog. II. The
conducting airways. Toxicol Appl Pharmacol, 121: 319-327.
Gerde P, Muggenburg BA, & Henderson RF (1993b) Disposition of
polycyclic aromatic hydrocarbons in the respiratory tract of the
beagle dog. III. Mechanisms of the dosimetry. Toxicol Appl Pharmacol,
121: 328-334.
German Scientific Association for Petroleum, Natural Gas, and Coal
(1991) [Middle distillates. Analytical investigations and mutagenicity
studies. Research report No. 412-1.] Hamburg, 65 pp (in German).
German Institute for Scientific Research (1993) [Transport in
figures]. Berlin, p 133
Gibson TL, Ricci AI, & Williams RL (1981) Measurement of polynuclear
aromatic hydrocarbons, their derivatives, and their reactivity in
diesel automobile exhaust. In: Cooke M & Dennis AJ, eds, Polynuclear
aromatic hydrocarbons, fifth international symposium on chemical
analysis and biological fate. Columbus, OH, Battelle Press,
pp 707-717.
Grant LD, Raub JA, Graham JA, Kotchmar DJ & Tilton BE (1993) Health
effects of motor vehicle-related criteria air pollutants. In:
Proceedings of the IPCS/Australia international workshop on human
health and environmental effects of motor vehicle fuels and their
exhaust emissions, Sydney, 6-10 April 1992. Canberra, Commonwealth
Department of Health, Housing, Local Government and Community
Services, pp 101-173.
Greenfield R, Ellwein L & Cohen S (1984) A general probabilistic model
of carcinogenic analysis: Experimental bladder cancer. Carcinogenesis,
5: 437-445.
Greenland S & Robins J (1994) Invited commentary: Ecologic
studies -- biases, misconceptions, and counterexamples. Am J Epidemiol,
139: 747-760.
Griffis LC, Wolff RK, Henderson RF, Griffith WC, Mokler BV, &
McClellan RO (1983) Clearance of diesel soot particles from rat lung
after a subchronic diesel exhaust exposure. Fundam Appl Toxicol,
3: 99-103.
Grimmer G, Hildebrandt A, & Böhnke H (1973) Investigations on the
carcinogenic burden by air pollution in man. II. Sampling and
analytics of polycyclic aromatic hydrocarbons in automobile exhaust
gas. 1. Optimization of the collecting arrangement. Zbl Bakt Hyg I Abt
Orig B, 158: 22-34.
Grimmer G, Naujack K-W, & Schneider D (1979) Changes in PAH-profiles
in different areas of a city during the year. In: Björseth A & Dennis
A, eds, Polynuclear aromatic hydrocarbons: Chemistry and biological
effects. Fourth international symposium on polynuclear aromatic
hydrocarbons. Columbus, OH, Battelle Press, pp 107-125.
Grimmer G, Naujack K-W, & Schneider D (1982) Profile analysis of
polycyclic aromatic hydrocarbons by glass capillary gas chromatography
in atmospheric suspended particulate matter in the nanogram range
collecting 10 m3 of air. Fresenius Z Anal Chem, 311: 475-484.
Grimmer G, Brune H, Deutsch-Wenzel R, Dettbarn G, Jacob J, Naujack
K-W, Mohr U, & Ernst H (1987) Contribution of polycyclic aromatic
hydrocarbons and nitro-derivatives to the carcinogenic impact of
diesel engine exhaust condensate evaluated by implantation into the
lungs of rats. Cancer Lett, 37: 173-180.
Grimmer G, Jacob J, Dettbarn G, & Naujack K-W (1988) Effect of the
pH-value of diesel exhaust on the amount of filter-collected
nitro-PAH. In: Cooke M & Dennis AJ, eds, Polynuclear aromatic
hydrocarbons. Tenth international symposium: A decade of progress.
Columbus, OH, Battelle Press, pp 341-351.
Grimmer G, Brune H, Dettborn G, Jacob J, Misfeld J, Mohr U, Naujack
K-W, Timm J, & Wenzel-Hartung R (1991) Relevance of polycyclic
aromatic hydrocarbons as environmental carcinogens. Fresenius J Anal
Chem, 339: 792- 795.
Grundmann R & Rehm HJ (1991) Biodegradation of diesel-fuel. Use of
free and immobilized mixed cultures in soils. Erdöl Kohle Erdgas
Petrochem 44: 149-150.
Gu Z-W, Zhong B-Z, Whong W-Z, Wallace WE, & Ong T (1991) Mutagenicity
and clastogenicity studies of diesel emission particles in cultured
mammalian cells. Environ Mol Mutag, 17 (Suppl. 19): 28.
Gu Z-W, Zhong B-Z, Nath B, Whong W-Z, Wallace WE, & Ong T-M (1992)
Micronucleus induction and phagocytosis in mammalian cells treated
with diesel emission particles. Mutat Res, 279: 55-60.
Gu Z-W, Zhong B-Z, Keane MJ, Whong W-Z, Wallace WE, & Ong T (1994)
Induction of unscheduled DNA synthesis in V79 cells by diesel emission
particles dispersed in simulated pulmonary surfactant. Ann Occup Hyg,
38 (Suppl 1): 345-349.
Guerrero RR, Rounds DE, & Orthoefer J (1981) Genotoxicity of Syrian
hamster lung cells treated in vivo with diesel exhaust particulates.
Environ Int, 5: 445-454.
Guillemin MP, Herrera H, Huynh CK, Droz P-O, & Vu Duc T (1992)
Occupational exposure of truck drivers to dust and polynuclear
aromatic hydrocarbons: A pilot study in Geneva, Switzerland. Int Arch
Occup Environ Health, 63: 439-447.
Gusein-Zade KM (1974) [The results of dermatological examinations of
engine drivers working in oil fields of Apsheron.] Vestn Dermatol
Venereol, 10: 47-49 (in Russian).
Gustafsson L, Wall S, Larsson L-G, & Skog B (1986) Mortality and
cancer incidence among Swedish dock workers -- A retrospective cohort
study. Scand J Work Environ Health, 12: 22-26.
Gustavsson P Plato N Lidström E-B, & Hogstedt C (1990) Lung cancer and
exposure to diesel exhaust among bus garage workers. Scand J Work
Environ Health, 16: 348-354.
Hahon N, Booth JA, Green F, & Lewis TR (1985) Influenza virus
infection in mice after exposure to coal dust and diesel engine
emissions. Environ Res, 37: 44-60.
Hammerle RH, Ketcher DA, Horrocks RW, Lepperhoff G, Hüthwohl G, &
Lüers B (1994a) Emissions from current diesel vehicles (SAE Technical
Paper Series No. 942943). Warrendale, PA, Society of Automotive
Engineers.
Hammerle R, Schuetzle D, & Adams W (1994b) A perspective on the
potential development of 'environmentally acceptable' light-duty
diesel vehicles. Environ Health Perspectives, 101: 1-20.
Hammond S, Smith T, Woskie S, Leaderer B, & Bettinger N (1988) Markers
of exposure to diesel exhaust and cigarette smoke in railroad workers.
Am Ind Hyg Assoc J, 49: 516-522.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Handa T, Yamanuchi T, Ohnishi M, Hisamatsu Y, & Ishii T (1983)
Detection and average content levels of carcinogenic and mutagenic
compounds from the particulates on diesel and gasoline engine
mufflers. Environ Int, 9: 335-341.
Hansen ES (1993) A follow-up study on the mortality of truck drivers.
Am J Ind Med, 23: 811-821.
Hare CT (1986) The effects of diesel fuel properties on particulate
emissions. In: Lee SD, Schneider T, Grant LD, & Verkerk PJ, eds,
Aerosols: Research, risk assessment and control strategies. Chelsea,
MI, Lewis Publishers, pp 501-514.
Harris J (1983) Diesel emissions and lung cancer. Risk Anal,
3: 83-100.
Harrison W, Winnik MA, Kwong PTY, & MacKay D (1975) Crude oil spills.
Disappearence of aromatic and aliphatic components from small
sea-surface slicks. Environ Sci Technol, 9: 231-234.
Hartung R & Hunt GS (1966) Toxicity of some oils to waterfowl. J Wildl
Manage, 30: 564-570.
Hartung A, Kraft J, Schulze J, Kiess H, & Lies K-H (1984)
Identification of nitrated polycyclic aromatic hydrocarbons in diesel
particulate extracts and their potential formation as artifacts during
particulate collection. Chromatographia, 19: 269-273.
Hasegawa MM, Nishi Y, Tsuda H, Inui N, & Morimoto K (1988) Effects of
diesel exhaust particles on chromosome aberration, sister chromatid
exchange and morphological transformation in cultured mammalian cells.
Cancer Lett, 42: 61-66.
Hawthorne SB & Miller DJ (1986) Extraction and recovery of organic
pollutants from environmental solids and Tenax-GC using supercritical
CO2. J Chromatogr Sci, 24: 258-264.
Hayes RB, Thomas T, Silverman DT, Vineis P, Blot WJ, Mason TJ, Pickle
LW, Correa P, Fontham ETH, & Schoenberg JB (1989) Lung cancer in motor
exhaust-related occupations. Am J Ind Med, 16: 685-695.
Hedtke SF & Puglisi FA (1982) Short-term toxicity of five oils to four
freshwater species. Arch Environ Contam Toxicol, 11: 425-430.
Heino M, Ketola R, Mäkelä P, Mäkinen R, Niemelä R, Starck J, &
Partanen T (1978) Work conditions and health of locomotive engineers.
1. Noise, vibration, thermal climate, diesel exhaust constituents,
ergonomics. Scand J Work Environ Health, 4: 3-14.
Heinrich U (1994) Carcinogenic effects of solid particles. In: Mohr U,
Dungworth DL, Mauderly JL, & Oberdörster G, eds, Toxic and
carcinogenic effects of solid particles in the respiratory tract.
Washington DC, International Life Science Institute Press, pp 57-73.
Heinrich U, Peters L, Funcke W, Pott F, Mohr U, & Stöber W (1982)
Investigation of toxic and carcinogenic effects of diesel exhaust in
long-term inhalation exposure of rodents. In: Lewtas S, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 225-242.
Heinrich U, Muhle H, Takenaka S, Ernst H, Fuhst R, Mohr U, Pott F, &
Stöber W (1986a) Chronic effects on the respiratory tract of hamsters,
mice and rats after long-term inhalation of high concentrations of
filtered and unfiltered diesel engine emissions. J Appl Toxicol,
6: 383-395.
Heinrich U Pott F & Rittinghausen S (1986b) Comparison of chronic
inhalation effects in rodents after long-term exposure to either coal
oven flue gas mixed with pyrolized pitch or diesel engine exhaust. In:
Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic
and mutagenic effects of diesel engine exhaust. Amsterdam, Elsevier,
pp 441-457.
Heinrich U, Pott F, & Roller M (1991) [Polycyclic aromatic
hydrocarbons -- Animal results and epidemiological findings relevant to
risk assessment]. In: [Carcinogenic substances in the environment:
Origin, measurement, risk, minimization. Meeting of the Commission for
Clean Air of the Society of German Engineers (VDI) and the German
Standards Institute (DIN), Mannheim, 23-25 April]. Düsseldorf, Society
of German Engineers Press, pp 71-92 (in German).
Heinrich U, Fuhst R, & Mohr U (1992) [Experimental inhalation studies
with laboratory animals on the question of the tumour-inducing effect
of diesel engine exhausts and two test dusts.] In: Effects of diesel
engine exhausts on health. Munich, GSF Research Centre for Environment
and Health Inc., pp 21-30 (in German).
Heinrich U, Peters L, Creutzenberg O, Dasenbrock C, & Hoymann HG
(1994) Inhalation exposure of rats to tar/pitch condensation aerosol
or carbon black alone or in combination with irritant gases. In: Mohr
U, Dungworth DL, Mauderly JL, & Oberdörster G, eds, Toxic and
carcinogenic effects of solid particles in the respiratory tract.
Washington DC, International Life Sciences Institute Press,
pp 433-441.
Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch
W, & Levsen K (1995) Chronic inhalation exposure of Wistar rats, and
two different strains of mice to diesel engine exhaust, carbon black
and titanium dioxide. Inhal Toxicol, 7: 533-556.
Hellstrom T & Doving KB (1983) Perception of diesel oil by cod
( Gadus morhua L.). Aquat Toxicol, 4: 303-315.
Henderson TR, Li AP, Royer RE, & Clark CR (1981) Increased
cytotoxicity and mutagenicity of diesel fuel after reaction with
NO2. Environ Mutag, 3: 211-220.
Henderson RF, Pickrell JA, Jones RK, Sun JD, Benson JM, Mauderly JL, &
McClellan RO (1988) Response of rodents to inhaled diluted diesel
exhaust: Biochemical and cytological changes in bronchoalveolar lavage
fluid and in lung tissue. Fundam Appl Toxicol, 11: 546-567.
Henschler D ed. (1987) [Diesel engine emissions]. In: [Maximal working
place concentration. Chemical compounds in the work area hazardous to
health]. Weinheim, VCH, 25 pp (in German).
Herbes SE & Whitley TA (1983) Characterization and toxicity of
water-soluble photooxidants produced during irradiation of coal
liquids by sunlight. Environ Pollut, 6: 221-240.
Heyder J, Gebhart J, Rudolf G, Schiller CF, & Stahlhofen W (1986)
Deposition of particles in the human respiratory tract in the size
range 0.005-15 µm. J Aerosol Sci, 17: 811-825.
Hildemann LM, Markowski GR, & Cass GR (1991) Chemical composition of
emissions from urban sources of fine organic aerosol. Environ Sci
Technol, 25: 744-759.
Hoar SK & Hoover R (1985) Truck driving and bladder cancer mortality
in rural New England. J Natl Cancer Inst, 74: 771-774.
Hobbs JR, Walter RA, Hard T, & Devoe D (1977) Train generated air
contaminants in the train crew's working environment (FRA/ORD-77/08:
US NTIS PB 265-355). Springfield, VA, National Technical Information
Service, pp 1-46.
Holland WD (1978) Determination of breathing zone concentrations of
contaminants from emissions from diesel powered vehicles in
underground mines (BuMines OFR 24-80; US NTIS PB80-150766).
Springfield, VA, National Technical Information Service, pp 1-127.
Holt S (1987) The effects of crude and diesel oil spills on plant
communities at Mesters Vig, northeast Greenland. Arct Alp Res,
19: 490-497. Horvath et al. (1985)
Horvath H, Kreiner I, & Norek C (1987) The role of the diesel aerosol
in the air pollution of Vienna. J Aerosol Sci, 18: 817-819.
Horvath H, Kreiner I, Norek C, Preining O, & Georgi B (1988) Diesel
emissions in Vienna. Atmos Environ, 22: 1255-1269.
Howe GR, Burch JD, Miller AB, Cook GM, Estève J, Morrison B, Gordon P,
Chambers LW, Fodor G, & Winsor GM (1980) Tobacco use, occupation,
coffee, various nutrients, and bladder cancer. J Natl Cancer Inst,
64: 701-713.
Howe GR, Fraser D, Lindsay J, Presnal B, & Zhang Yu S (1983) Cancer
mortality (1965-77) in relation to diesel fume and coal exposure in a
cohort of retired railway workers. J Natl Canc Inst, 6: 1015-1019.
Huisingh J, Bradow R, Jungers R, Claxton L, Zweidinger R, Tejada S,
Bumgarner J, Duffield F, Waters MD, Simmon VF, Hare C, Rodriguez C, &
Snow L (1978) Application of bioassay to the characterization of
diesel particle emissions. In: Waters MD, Nesnow S, Huisingh JL,
Sandhu SS, & Claxton L, eds, Application of short-term bioassays in
the fractionation and analysis of complex environmental mixtures. New
York, Plenum Press, pp 383-418.
Hyde DM, Plopper CG, Weir AJ, Murnane RD, Warren DL, Last JA, &
Pepelko WE (1985) Peribronchiolar fibrosis in lungs of cats
chronically exposed to diesel exhaust. Lab Invest, 52: 195-206.
Iida N, Suzuki Y, Sato GT, & Sawada T (1986) Effects of intake oxygen
concentration on the characteristics of particulate emissions from a
DI diesel engine (Paper No. 861233). Warrendale, PA, Society of
Automotive Engineers.
Imaida K, Hirose M, Tay L, Lee M-S, Wang Y, & King CM (1991)
Comparative carcinogenicities of 1-, 2-, and 4-nitropyrene and
structurally related compounds in the female CD rat. Cancer Res,
51: 2902-2907.
International Agency for Research on Cancer (1989a) Diesel fuels. In:
IARC monographs on the evaluation of the carcinogenic risk of
chemicals to humans, Vol. 45, Occupational exposures in petroleum
refining; crude oil and major petroleum fuels. Lyon, pp 219-237.
International Agency for Research on Cancer (1989b) Diesel and
gasoline engine exhausts. In: IARC monographs on the evaluation of the
carcinogenic risk of chemicals to humans, Vol. 46, Diesel and gasoline
engine exhaust and some nitroarenes. Lyon, pp 41-185.
International Standards Organization (1987) Petroleum products -- Fuels
(class F) -- Specifications of marine fuels (No. ISO 8217-1987/E).
Geneva, 7 pp.
International Union for Pure and Applied Chemistry (1993) Glossary for
chemists of terms used in toxicology (IUPAC Recommendations 1993).
Pure Appl Chem, 65: 2003-2122.
IPCS (1986) Environmental health criteria, 52: Toluene. Geneva, WHO.
IPCS (1993) Environmental health criteria, 150: Benzene. Geneva, WHO,
156 pp.
IPCS (1994) Environmental health criteria, 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, WHO, 73 pp.
Iscovich J, Castelletto R, Estève J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Ishinishi N, Kuwabara N, Nagase S, Suzuki T, Ishiwata S, & Kohno T
(1986) Long-term inhalation studies on effects of exhaust from heavy
and light duty diesel engines on F344 rats. In: Ishinishi N, Koizumi
A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects
of diesel engine exhaust. Amsterdam, Elsevier, pp 329-348.
Ishinishi N, Kuwabara N, Takaki Y, Nagase S, Suzuki T, Nakajima T,
Maejima K, Kato A, & Nakamura M (1988) Long-term inhalation
experiments on diesel exhaust. In: Diesel exhaust and health risk.
Results of the HERP studies. Tsukuba, Health Effects Research
Programme, pp 11-84.
Israel GW, Zierock KH, & Mollenhauer K (1982) [Distribution and
chemical composition of particle emissions from different diesel
motors] (VDI Report No. 429). Düsseldorf, Association of German
Engineers Press, pp 279-286 (in German).
Iwai K, Udagawa T, Yamagishi M, & Yamada H (1986) Long-term inhalation
studies of diesel exhaust on F344 SFP rats. Incidence of lung cancer
and lymphoma. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 349-360.
Iyer V, Harris RE, & Wynder EL (1990) Diesel exhaust exposure and
bladder cancer risk. Eur J Epidemiol, 6: 49-54.
Jaenicke R (1986) Physical characterization of aerosols. In: Lee SD,
Schneider T, Grant LD, & Verkerk PJ, eds, Aerosols: Research, risk
assessment and control strategies. Chelsea, MI, Lewis Publishers,
pp 97-106.
Jensen TE & Hites RA (1983) Aromatic diesel emissions as a function of
engine conditions. Anal Chem, 55: 594-599.
Jensen OM, Wahrendorf J, Knudsen JB, & Sorensen BL (1987) The
Copenhagen case-referent study on bladder cancer. Risks among drivers,
painters and certain other occupations. Scand J Work Environ Health,
13: 129-134.
Jinno K, Hoshino T, Hondo T, Saito M, & Senda M (1986) Identification
of polycyclic aromatic hydrocarbons in extracts of diesel particulate
matter by supercritical fluid chromatography coupled with an
ultraviolet multichannel detector. Anal Chem, 58: 2696-2699.
Johnson JH & Carlson DH (1986) The application of advanced measurement
and control technology to diesel-powered vehicles in an underground
salt mine. Montreal, Canadian Institute of Mining and Metallurgy,
pp 206-237.
Jorgensen H & Svensson Å (1970) Studies on pulmonary function and
respiratory tract symptoms of workers in an iron ore mine where diesel
trucks are used underground. J Occup Med, 12: 348-354.
Joumard R & Perrin M-L (1988) Measurement of particle and gaseous
pollution of the atmosphere due to buses. Sci Total Environ,
76: 55-62.
Juhnke I & Lüdemann D (1978) [Results of a study of 200 chemical
compounds for acute fish toxicity with the golden orfe test.] Z Wasser
Abwasser Forsch, 11: 161-164 (in German).
Kahn G, Orris P, & Weeks J (1988) Acute over exposure to diesel
exhaust: Report of 13 cases. Am J Ind Med, 13: 405-406.
Kainz RJ & White LE (1982) Consequences associated with the inhalation
of uncombusted diesel vapor. In: MacFarland HN, Holdsworth CE,
MacGregor JA, Call RW, & Kane ML, eds, Proceedings of the symposium:
The toxicology of petroleum hydrocarbons, May 1982. Washington DC,
American Petroleum Institute, pp 320-327.
Kainz RJ & White LE (1984) Depressant effects associated with the
inhalation of uncombusted diesel vapor. In: MacFarland HN, Holdsworth
CE, MacGregor JA, Call RW, & Kane ML, eds, Applied toxicology of
petroleum hydrocarbons (Advances in modern environmental toxicology,
Vol. 6). Princeton, NJ, Princeton Scientific Publishers, pp 233-243.
Kaplan HL, MacKenzie WF, Springer KJ, Schreck RM, & Vostal JJ (1982) A
subchronic study of the effects of exposure of three species of
rodents to diesel exhaust. In: Lewtas J, ed., Toxicological effects of
emissions from diesel engines. Amsterdam, Elsevier, pp 161-182.
Kaplan HL, Springer KJ, & MacKenzie WF (1983) Studies of potential
health effects of long-term exposure to diesel exhaust emissions.
Final report (SwRI Project No. 01-0750-103; SFRE Project No. 1239).
San Antonio, TX, Southwest Research Institute, 59 pp.
Karagianes MT, Palmer RF, & Busch RH (1981) Effects of inhaled diesel
emissions and coal dust in rats. Am Ind Hyg Assoc J, 42: 382-391.
Karl DM (1992) The grounding of the Bahia Paraiso: Microbial ecology
of the 1989 Antarctic oil spill. Microbiol Ecol, 24: 77-89.
Kato A, Kyono H, & Kuwabara N (1992) [Electron-microscopic
observations on rat lungs after long term inhalation of diesel
emissions -- Non-neoplastic lesions]. Jpn J Chest Dis, 30: 238-247
(in Japanese, with English abstract).
Kauffman S (1974) Kinetics of alveolar epithelial hyperplasia in lungs
of mice exposed to urethane. I. Quantitative analysis of cell
populations. Lab Invest, 30: 170-175.
Kawabata Y, Iwai K, Udagawa T, Tukagoshi K, & Higuchi K (1986) Effects
of diesel soot on unscheduled DNA synthesis of tracheal epithelium and
lung tumor formation. In: Ishinishi N, Koizumi A, McClellan RO, &
Stöber W, eds, Carcinogenic and mutagenic effects of diesel engine
exhaust. Amsterdam, Elsevier, pp 213-222.
Kawabata Y, Udagawa T, Higuchi K, Yamada H, & Iwai K (1993) One year
exposure to diesel engine exhaust causes lung tumors. In: Mohr U,
Dungworth DL, Mauderly JL & Oberdörster G, eds, Toxic and carcinogenic
effects of solid articles in the respiratory tract. Washington DC,
International Life Sciences Institute Press, pp 429-431.
Keane MJ, Xing S-G, Harrison JC, Ong T, & Wallace WE (1991)
Genotoxicity of diesel-exhaust particles dispersed in simulated
pulmonary surfactant. Mutat Res, 260: 233-238.
Kemp PF, Swartz RC, & Lamberson JO (1986) Response of the
phoxocephalid amphipod, Rhepoxynius abronius, to a small oil spill
in Yaquina Bay, Oregon. Estuaries, 9: 340-347.
Kennicutt MC II, (1990) Oil spillage in Antarctica: Initial report of
the National Science Foundation-sponsored quick response team on the
grounding of the Bahia Paraiso. Environ Sci Technol, 24: 620-624.
Kennicutt MC II & Sweet ST (1992) Hydrocarbon contamination on the
Antarctic peninsula: III. The Bahia Paraiso -- Two years after the
spill. Mar Pollut Bull, 25: 303-306.
Kennicutt MC II, Sweet ST, Fraser WR, Stockton WL, & Culver M (1991a)
Grounding of the Bahia Paraiso at Arthur Harbor, Antarctica. 1.
Distribution and fate of oil spill related hydrocarbons. Environ Sci
Technol, 25: 509-518.
Kennicutt MC II, Sweet ST, Fraser WR, Culver M, & Stockton WL (1991b)
The fate of diesel fuel spilled by the Bahia Paraiso in Arthur
Harbour, Antarctica. In: Proceedings 1991 international oil spill
conference. (Prevention, behavior, control, cleanup), 4-7 March 1991,
San Diego, California. Washington DC, American Petroleum Institute,
pp 493-500.
Kennicutt MC II, McDonald TJ, Denoux GJ, & McDonald SJ (1992a)
Hydrocarbon contamination on the Antarctic peninsula. I. Arthur
Harbor -- Subtidal sediments. Mar Pollut Bull, 24: 499-506.
Kennicutt MC II, McDonald TJ, Denoux GJ, & McDonald SJ (1992b)
Hydrocarbon contamination on the Antarctic peninsula. II. Arthur
Harbor -- Inter- and subtidal limpets (Nacella concinna). Mar Pollut
Bull, 24: 506-511.
Kittel B, Ernst H, Dungworth DL, Rittinghausen S, Nolte T, Kamino K,
Stuart B, Lake SG, Cardesa A, Moraweitz G, & Mohr U (1993)
Morphological comparison between benign keratinizing cystic squamous
cell tumours of the lung and squamous lesions of the skin in rats.
Exp Toxicol Pathol, 45: 257-267
Klingenberg H, Schürmann D, & Lies KH (1991) [Diesel motor
exhaust -- Formation and determination]. In: [Carcinogenic substances in
the environment: Origin, measurement, risk, minimization. Meeting of
the Commission for Clean Air of the Society of German Engineers (VDI)
and the German Standards Institute (DIN), Mannheim, 23-25 April].
Düsseldorf, Society of German Engineers Press, pp 119-131 (in German)
Knave B, Olson BA, Elofson S, Gamberale F, Isaksson A, Mindus P,
Persson HE, Struwe G, Wennberg A, & Westerholm P (1978) Long term
exposure to jet fuel. II. A cross-sectional epidemiologic
investigation on occupationally exposed industrial workers with
special reference to the nervous system. Scand J Work Environ Health,
4: 19-45.
Koenig JQ, Covert DS, Marshall SSG, Van Belle G, & Pierson WE (1988)
The pulmonary effects of ozone and nitrogen dioxide alone and combined
in healthy and asthmatic adolescent subjects. Toxicol Ind Health,
4: 521-532
Köhler M & Eichhoff HJ (1967) [Quick method for determination of
aromatic hydrocarbons in air dust.] Z Anal Chem, 232: 401-409
(in German).
Kotin P, Falk HL, & Thomas M (1955) Aromatic hydrocarbons. III.
Presence in the particulate phase of diesel-engine exhausts and the
carcinogenicity of exhaust extracts. Arch Ind Health, 11: 113-120.
Kraft J & Lies K-H (1981) Polycyclic aromatic hydrocarbons in the
exhaust of gasoline and diesel vehicles (Technical paper No. 810082).
Warrendale, PA, Society of Automotive Engineers, pp 1-11.
Kühnhold WW (1977) The effect of mineral oils on the development of
eggs and larvae of marine species. A review and comparison of
experimental data in regard to possible damage at sea. In: McIntyre AD
& Whittle KJ, eds, Petroleum hydrocarbons in the marine environment
(Rapports et proces-verbaux des réunions, Vol. 171). Copenhagen,
International Council for Exploration of the Sea, pp 175-183.
Kunitake E, Shimamura K, Katayama H, Takemoto K, Yamamoto A, Hisanaga
A, Ohyama S, & Ishinishi N (1986) Studies concerning carcinogenesis of
diesel particulate extracts following intratracheal instillation,
subcutaneous injection, or skin application. In: Ishinishi N, Koizumi
A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic effects
of diesel engine exhaust. Amsterdam, Elsevier, pp 235-252.
Kuschner M (1968) The causes of cancer. Am Rev Respir Dis,
98: 573-590.
Larson RA, Hunt LL, & Blankenship DW (1977) Formation of toxic
products from a No. 2 fuel oil by photooxidation. Environ Sci Technol,
11: 492-496.
Larson RA, Bott TL, Hunt LL, & Rogenmuser K (1979) Photooxidation
products of a fuel oil and their antimicrobial activity. Environ Sci
Technol, 13: 965-969.
Lawler GC, Loong W-A, & Laseter JL (1978) Accumulation of aromatic
hydrocarbons in tissues of petroleum-exposed mallard ducks
(Anas platyrhynchos). Environ Sci Technol, 12: 51-54.
Lee RF (1977) Accumulation and turnover of petroleum in marine
organisms. In: Wolfe DA, ed., Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, Pergamon
Press, pp 60-70.
Lee RF (1981) Mixed function oxygenases (MFO) in marine invertebrates.
Mar Biol Lett, 2: 87-105.
Lee FS-C & Schuetzle D (1983) Sampling, extraction and analysis of
polycyclic aromatic hydrocarbons from internal combustion engines. In:
Björseth A, ed., Handbook of polycyclic aromatic hydrocarbons. New
York, Marcel Dekker, pp 27-94.
Lee FS-C, Pierson WR, & Ezike J (1980) The problem of PAH degradation
during filter collection of airborne particulates -- An evaluation of
several commonly used filter media. In: Björseth A & Dennis AJ, eds,
Polynuclear aromatic hydrocarbons, 4th International Symposium:
Chemistry and biological effects. Dearborn, MI, Engineering and
Research Staff Research, Ford Motor Co., pp 543-563.
Lee PS, Chan TL, & Hering WE (1983) Long-term clearance of inhaled
diesel exhaust particles in rodents. J Toxicol Environ Health,
12: 801-813.
Lee PS, Gorski RA, Hering WE, & Chan TL (1987) Lung clearance of
inhaled particles after exposure to carbon black generated from a
resuspension system. Environ Res, 43: 364-373.
Lee LS, Hagwall M, Delfino JJ, & Rao PSC (1992) Partitioning of
polycyclic aromatic hycrocarbons from diesel fuel into water. Environ
Sci Technol, 26: 2104-2110.
Lehmann E, Rentel K-H, Allescher W, & Hohmann R (1990) [Measurement of
diesel exhaust at the workplace.] Zentralbl Arbeitsmed Arbeitsschutz
Prophyl Ergon, 40: 2-11 (in German).
Levsen K (1988) The analysis of diesel particulate. Fresenius Z Anal
Chem, 331: 467-478.
Levsen K (1992) [Production and analysis of total diesel exhaust for
efficiency research.] In: [Effects of diesel engine exhausts on
health]. Munich, GSF Research Centre for Environment and Health Inc.,
pp 16-20 (in German).
Levsen K, Puttins U, Schilhabel J, & Priess B (1988) Artefacts during
the sampling of nitro PAHs of diesel exhaust. Fresenius Z Anal Chem,
330: 527-528.
Lewis SC (1983) Crude petroleum and selected fractions. Prog Exp Tumor
Res, 26: 68-84.
Lewis TR, Green FHY, Moorman WJ, Burg JAR, & Lynch DW (1986) A chronic
inhalation toxicity study of diesel engine emissions and coal dust,
alone and combined. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber
W, eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 361-380.
Lewis TR, Green FHY, Moorman WJ, Burg JR, & Lynch DW (1989) A chronic
inhalation toxicity study of diesel engine emissions and coal dust,
alone and combined. J Am Coll Toxicol, 8: 345-375.
Lewtas J (1983) Evaluation of the mutagenicity and carcinogenicity of
motor vehicle emissions in short-term bioassays. Environ Health
Perspectives, 47: 141- 152.
Lewtas J & Williams K (1986) A retrospective view of the value of
short-term genetic bioassays in predicting the chronic effects of
diesel soot. In: Ishinishi N, Koizumi A, McClellan R, & Stöber W, eds,
Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 119-140.
Li AP & Royer RE (1982) Diesel-exhaust-particle extract enhancement of
chemical-induced mutagenesis in cultured Chinese hamster ovary cells:
Possible interaction of diesel exhaust with environmental carcinogens.
Mutat Res, 103: 349-355.
Liebe B & Fock HP (1992) Growth and adaptation of the green algae
Chlamydomonas reinhardtii on diesel exhaust particle extracts. J Gen
Microbiol, 138: 973-978.
Lies K-H, Hartung, A Postulka, A, Gring H, & Schulze J (1986)
Composition of diesel exhaust with particular reference to particle
bound organics including formation of artifacts. In: Ishinishi N,
Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic and mutagenic
effects of diesel engine exhaust. Amsterdam, Elsevier, pp 65-82.
Linnell RH & Scott WE (1962) Diesel exhaust composition and odor
studies. J Am Poll Control Assoc, 12: 510-515.
Lock S, Dalbey W, Schmoyer R, & Griesemer R (1984) Inhalation
toxicology of diesel fuel obscurant aerosol in Sprague-Dawley rats:
Final report, phase 3, subchronic exposures (TN AD-A150 100). Oak
Ridge, TN, Oak Ridge National Laboratory.
Lockhart WL, Danell RW, & Murray DAJ (1987) Acute toxicity bioassays
with petroleum products: Influence of exposure conditions. In:
Vandermeulen JH & Hrudey SE, eds, Oil in freshwater: Chemistry,
biology, countermeasure technology. New York, Pergamon, pp 335-409.
Loprieno N, DeLorenzo F, Cornetti GM, & Biaggini G (1980) Mutagenicity
studies on diesel particles and particulate extracts. Health effects
of diesel engine emissions (EPA NO 600/9-80-057a). In: Proceedings of
an international symposium, Vol 1. Washington DC, US Environmental
Protection Agency, pp 276-308.
Lowe DM & Pipe RK (1986) Hydrocarbon exposure in mussels: A
quantitative study of the responses in the reproductive and nutrient
storage cell systems. Aquat Toxicol, 8: 265-272.
Lowenthal DH, Zielinska B, Chow JC, Watson JG, Gautan M, Ferguson DH,
Neuroth GR, & Stevens KD (1994) Characterization of heavy-duty diesel
vehicle emissions. Atmos Environ, 28: 731-743.
Lysyj I & Russell EC (1974) Dissolution of petroleum-derived products
in water. Water Res, 8: 863-868.
Mackay D & Shiu WY (1976) Aqueous solubilities of weathered northern
crude oils. Bull Environ Contam Toxicol, 15: 101-109.
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. 2. Polynuclear aromatic hydrocarbons, polychlorinated
dioxins and dibenzofurans. Ann Arbor, MI, Lewis Publishers Inc.,
p 253.
Mackie PR, McGill AS, & Hardy R (1972) Diesel oil contamination of
brown trout ( Salmo trutta L.). Environ Pollut, 3: 9-16.
Mahoney BM & Haskin HH (1980) The effects of petroleum hydrocarbons on
the growth of phytoplankton recognised as food forms for the eastern
oyster, Crassostrea virginica Gmelin. Environ Pollut, A22: 123-132.
Marcus JM & Scott GI (1989) Effects of two diesel fuel mixtures on
fecal coliform bacteria densities. Bull Environ Contam Toxicol,
42: 395-401.
Marklund S, Andersson R, Tysklind M, Rappe C, Egebäck K-E, Björkman E,
& Grigoriadis V (1990) Emissions of PCDDs and PCDFs in gasoline and
diesel fueled cars. Chemosphere, 20: 553-561.
Martini N (1993) Operable lung cancers. CA Cancer J Clin, 43: 201-204.
Mattie DR, Alden CL, Newell TK, Gaworski CL, & Flemming CD (1991) A
90-day continuous vapor inhalation toxicity study of JP-8 jet fuel
followed by 20 or 21 months of recovery in Fischer 344 rats and
C57BL/6 mice. Toxicol Pathol, 19: 77-87
Mauderly JL (1992) Diesel exhaust. In: Lippmann M, ed., Environmental
toxicants. Human exposures and their health effects. New York, Van
Nostrand Reinhold, pp 119-162.
Mauderly JL, Jones RK, McClellan RO, Henderson RF, & Griffith WC
(1986) Carcinogenicity of diesel exhaust inhaled chronically by rats.
In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds,
Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 397-409.
Mauderly JL, Jones RK, Griffith WC, Henderson RF, & McClellan RO
(1987) Diesel exhaust is a pulmonary carcinogen in rats exposed
chronically by inhalation. Fundam Appl Toxicol, 9: 208-221.
Mauderly JL, Gillett NA, Henderson RF, Jones RK, & McClellan RO (1988)
Relationships of lung structural and functional changes to
accumulation of diesel exhaust particles. Ann Occup Hyg, 32: 659-669.
Mauderly JL, Bice DE, Cheng YS, Gillett NA, Griffith WC, Henderson RF,
Pickrell JA, & Wolff RK (1990) Influence of preexisting pulmonary
emphysema on susceptibility of rats to inhaled diesel exhaust. Am Rev
Respir Dis, 141: 1333-1341.
Mauderly JL, Snipes MB, Barr EB, Bechtold WE, Bellinsky SA, Henderson
RF, Mitchell CE, Nikula KJ, & Thomassen DG (1991) Influence of
particle-associated organic compounds on carcinogenicity of diesel
exhaust. In: Eighth Health Effects Institute Annual Conference,
21-24 April 1991, Colorado Springs, CO. Cambridge, MA, Health Effects
Institute, 13 pp.
Mauderly JL, Snipes MB, Barr EB, Belinsky SA, Bond JA, Brooks AL,
Change IY, Cheng YS, Gillett NA, Griffith WC, Henderson RF, Mitchell
CE, Nikula KJ, & Thomassen DG (1994) Pulmonary toxicity of inhaled
diesel exhaust and carbon black in chronically exposed rats. (HEI
Research Report No. 68). Cambridge, MA, Health Effects Institute,
97 pp.
McClellan RO (1986) Health effects of diesel exhaust: A case study in
risk assessment. Am Ind Hyg Assoc J, 47: 1-13.
McClellan RO (1990) Particle overload in the lung: Approaches to
improving our knowledge. J Aerosol Med, 3: 197-207.
McClellan R, Cuddihy R, Griffith W, & Mauderly J (1989) Integrating
diverse data sets to assess the risks of airborne pollutants. In: Mohr
U, ed., Assessment of inhalation hazards. New York, Springer, pp 3-22.
McCormick JJ, Zator RM, DaGue BB, & Maher VM (1980) Studies on the
effects of diesel particulate on normal and xeroderma pigmentosum
cells (EPA-NO 600/9-80-057a). In: Pepelko WE, Danner RM, & Clark NA,
eds, Health effects of diesel engine emissions. Cincinnati, OH, US
Environmental Protection Agency, pp 413-415.
McKee RH & Plutnick RT (1989) Carcinogenic potential of gasoline and
diesel engine oils. Fundam Appl Toxicol, 13: 545-553.
McKee RH, Amoruso MA, & Freeman JJ (1994) Evaluation of the genetic
toxicity of middle distillate fuels. Environ Mol Mutag, 23: 234-238.
McLaren P (1985) Behaviour of diesel fuel on a high energy beach. Mar
Pollut Bull, 16: 191-196.
Meiss R, Robenek H, Schubert M, Themann H, & Heinrich U (1981)
Ultrastructural alterations in the livers of golden hamsters following
experimental chronic inhalation of diluted diesel exhaust emission.
Int Arch Occup Environ Health, 48: 147-157.
Menne RJ, Rechs M, Horrocks R & Harbolla B (1994) [Current and future
trends of passenger car diesel engines]. Motortech Z, 55: 323
(in German, with English abstract).
Mentnech MS, Lewis DM, Olenchock SA, Mull JC, & Koller WA (1984)
Effects of coal dust and diesel exhaust on immune competence in rats.
J Toxicol Environ Health, 13: 31-41.
Mercedes-Benz AG (1994a) [Exhaust emissions: Threshold limits,
regulations and measurement of exhaust emissions; calculation of the
fuel use from the exhaust test; passenger cars (May 1994)
Mercedes-Benz]. Stuttgart, 19 pp (in German).
Mercedes-Benz AG (1994b) Limit values for diesel exhaust components.
Data sheet. Stuttgart, 7 pp.
Mészáros A & Mészáros E (1989) Sulfate formation on elemental carbon
particulate. J Aerosol Sci Technol, 10: 337-342.
Metz N (1989) Diesel particulate emission in Germany (West). Exp
Pathol, 37: 15-22.
Meyers PA & Quinn JG (1973) Association of hydrocarbons and mineral
particles in saline solution. Nature, 244: 23-24.
Misiorowski RL, Strom KA, Vostal JJ, & Chvapil M (1980) Lung
biochemistry of rats chronically exposed to diesel particulates. In:
Pepelko WE, Danner RM, & Clarke NA, eds, Health effects of diesel
engine emissions, Vol 1 (EPA-600/9-80-057a). Cincinnatti, OH, US
Environmental Protection Agency, pp 465-480.
Mitchell AD, Evans EL, Jotz MM, Riccio ES, Mortelmans KE, & Simmon VF
(1981) Mutagenic and carcinogenic potency of extracts of diesel and
related environmental emissions: in vitro mutagenesis and DNA
damage. Environ Int, 5: 393-401.
Mohr, U, ed. (1992) International classification of rodent tumours.
Part 1. The rat (IARC Scientific Publications No. 122). Lyon,
International Agency for Research on Cancer.
Mohr U & Riebe-Imre M (1992) [Tests on the in vitro transformation
of epithelial cells of the respiratory tract by automotive exhaust
gases]. In: [Effects of diesel engine exhausts on health.] Munich, GSF
Research Centre for Environment and Health Inc., pp 31-38 (in German).
Moolgavkar S & Knudson A (1981) Mutation and cancer: A model for human
carcinogenesis. J Natl Cancer Inst, 66: 1037-1052.
Moolgavkar S & Venzon D (1979) Two-event models for carcinogenesis:
Incidence curves for childhood and adult tumors. Math Biosci,
47: 55-77.
Moorman WJ, Clark JC, Pepelko WE, & Mattox J (1985) Pulmonary function
responses in cats following long-term exposure to diesel exhaust.
J Appl Toxicol, 5: 301-305.
Morgan P & Watkinson RJ (1988) Hydrocarbon degradation in soils and
methods for soil biotreatment. Crit Rev Biotechnol, 8: 305-333.
Morimoto K, Kitamura M, Kondo H, & Koizumi A (1986) Genotoxicity of
diesel exhaust emissions in a battery of in-vitro short-term and
in-vivo bioassays. In: Ishinishi N, Koizumi A, McClellan RO, &
Stöber W, eds, Carcinogenic and mutagenic effects of diesel engine
exhaust. Amsterdam, Elsevier, pp 85-101.
Morrow JE (1973) Oil-induced mortalities in juvenile coho and sock-eye
salmon. J Mar Res, 31: 135-141.
Morrow PE (1988) Possible mechanisms to explain dust overloading of
the lungs. Fundam Appl Toxicol, 10: 369-384.
Muhlbaier Dasch J & Cadle SH (1989) Atmospheric carbon particle in the
Detroit urban area: Wintertime sources and sinks. Aerosol Sci Technol,
10: 236-248.
Muhle H, Creutzenberg O, Bellmann B, Heinrich U, & Mermelstein R
(1990) Dust overloading of lungs: Investigations of various materials,
species differences, and irreversibility of effects. J Aerosol Med,
3: 111-128.
Muhle H, Bellmann B, & Creutzenberg O (1994) Toxicokinetics of solid
particles in chronic rat studies using diesel soot, carbon black,
toner, titanium dioxide, and quartz. In: Mohr U, Dungworth DL,
Mauderly JL, & Oberdörster G, eds, Toxic and carcinogenic effects of
solid particles in the respiratory tract. Washington DC, International
Life Sciences Institute Press, pp 29-41.
Muralidhara KMK, Krishnakumari HP, Ramesh HP, & Majumder SK (1982)
Toxicity of some petroleum fractions used in pesticidal emulsions to
albino rats. J Food Sci Technol, 19: 260-262.
Muranaka M, Suzuki S, Koizumi K, Takafuji S, Miyamoto T, Ikemori R, &
Tokiwa H (1986) Adjuvant activity of diesel-exhaust particulates for
the production of IgE antibody in mice. J Allergy Immunol,
77: 616-623.
Neff JM, Cox BA, Dixit D, & Anderson JW (1976a) Accumulation and
release of petroleum-derived aromatic hydrocarbons by four species of
marine animals. Mar Biol, 38: 279-289.
Neff JM, Anderson JW, Cox BA, Laughlin RB Jr, Rossi SS, & Tatem HE
(1976b) Effects of petroleum on survival, respiration and growth of
marine animals. In: Sources, effects and sinks of hydrocarbons in the
aquatic environment. Proceedings of the symposium, American
University, Washington DC, 9-11 August 1976. Washington DC, American
Institute of Biological Sciences, pp 516-539.
Nesnow S, Triplett LL, & Slaga TJ (1982a) Comparative tumor-initiating
activity of complex mixtures from environmental particulate emissions
on SENCAR mouse skin. J Natl Cancer Inst, 68: 829-834.
Nesnow S, Evans C, Stead A, Creason J, Slaga TJ, & Triplett LL (1982b)
Skin carcinogenesis studies of emission extracts. In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 295-320.
Nesnow S, Triplett LL, & Slaga TJ (1983) Mouse skin tumor
initiation-promotion and complete carcinogenesis bioassays: Mechanisms
and biological activities of emission samples. Environ Health
Perspectives, 47: 255-268.
Netterstrom B (1988) Cancer incidence among urban bus drivers in
Denmark. Int Arch Occup Environ Health, 61: 217-221.
Newton JM, Rothman BS, & Walker FA (1991) Separation and determination
of diesel contaminants in various fish products by capillary gas
chromatography. J Assoc Off Anal Chem, 74: 986-990.
Nikula KJ Snipes MB Barr EB Griffith WC Henderson RF & Mauderly JL
(1994) Influence of particle-associated organic compounds on the
carcinogenicity of diesel exhaust. In: Mohr U, Dungworth DL, Mauderly
JL, & Oberdörster G, eds, Toxic and carcinogenic effects of solid
particles in the respiratory tract. Washington DC, International Life
Science Institute Press, pp 565-568.
Notani PN, Shah P, Jayant K, & Balakrishnan V (1993) Occupation and
cancers of the lung and bladder: A case-control study in Bombay.
Int J Epidemiol, 22: 185-191.
Novakov T (1984) The role of soot and primary oxidants in atmospheric
chemistry. Sci Total Environ, 36: 1-10.
Oberdörster G (1988) Lung clearence of inhaled insoluble and soluble
particles. J Aerosol Med, 1: 289-330.
Oberdörster G & Yu CP (1990) The carcinogenic potential of inhaled
diesel exhaust: A particle effect? J Aerosol Sci, 21: 397-401.
Oberdörster G, Baggs R, Gelein R, Corson N, Mercer P, Nguyen K, &
Pepelko W (1995) Pulmonary effects of inhaled carbon black in rats.
Toxicologist, 15: 46.
Obländer K (1991) [Possibility of pollutant risk reduction in diesel
engines.] In: [Carcinogenic substances in the environment: Origin,
measurement, risk, minimization. Meeting of the Commission for Clean
Air of the Society of German Engineers (VDI) and the German Standards
Institute (DIN), Mannheim, 23-25 April]. Düsseldorf, Society of German
Engineers Press, pp 245-253 (in German).
Oehme M, Larssen S, & Brevik EM (1991) Emisson factors of PCDD and
PCDF for road vehicles obtained by tunnel experiment. Chemosphere,
23: 1699-1708.
Oelert HH & Florian T (1972) [Registration and assessment of the odour
intensity of diesel exhaust.] Staub Reinhalt Luft, 32: 400-407
(in German).
Ohgaki H, Hasegawa H, Kata T, Negishi C, Sato S, & Sugimura T (1985)
Absence of carcinogenicity of 1-nitropyrene, correction of previous
results, and new demostration of carcinogenicity of 1,6-dinitropyrene
in rats. Cancer Lett, 25: 239-245.
Ong T, Whong W-Z, Xu J, Burchell B, Green FHY & Lewis T (1985)
Genotoxicity studies of rodents exposed to coal dust and diesel
emission particulates. Environ Res, 37: 399-409.
Ong T, Whong W-Z, Steward J & Brockman HE (1986) Chlorophyllin: A
potent antimutagen against environmental and dietary complex mixtures.
Mutat Res, 173: 111-115.
Organisation for Economic Co-operation and Development (1993) Control
of emissions from heavy-duty vehicles (OECD Environment Monographs
No. 55). Paris, 121 pp.
Oviatt C, Frithsen J, Gearing J, & Gearing P (1982) Low chronic
additions of No. 2 fuel oil: Chemical behavior, biological impact and
recovery in a simulated estuarine environment. Mar Ecol Prog Ser,
9: 121-136.
Parker GA, Bogo V, & Young RW (1981) Acute toxicity of conventional
versus shale-derived JP-5 jet fuel: Light microscopic, hematologic,
and serum chemistry studies. Toxicol Appl Pharmacol, 57: 302-317
Partanen T, Heikkilä P, Hernberg S, Kauppinen T, Moneta G, & Ojajärvi
A (1991) Renal cell cancer and occupational exposure to chemical
agents. Scand J Work Environ Health, 17: 231-239.
Partridge PA, Shala FJ, Cernansky NP, & Suffet IH (1987)
Characterization and analysis of diesel exhaust odor. Environ Sci
Technol, 21: 403-408.
Patton JD, Maher VM, & McCormick JJ (1986) Cytostatic and mutagenic
effects of 1-nitropyrene and 1-nitrosopyrene in diploid human
fibroblasts. Carcinogenesis, 7: 89-93.
Pederson TJ & Siak JS (1981) The role of nitroaromatic compounds in
the direct-acting mutagenicity of diesel particle extracts. J Appl
Toxicol, 1: 54-60.
Pepelko WE (1982) Effects of 28 days exposure to diesel engine
emissions in rats. Environ Res, 27: 16-23.
Pepelko WE & Chen C (1993) Quantitative assessment of cancer risk from
exposure to diesel engine emissions. Regul Toxicol Pharmacol,
17: 52-65.
Pepelko WE & Peirano WB (1983) Health effects of exposure to diesel
engine emissions. A summary of animal studies conducted by the US
Environmental Protection Agency's Health Effects Research Laboratories
at Cincinnati, Ohio. J Am Coll Toxicol, 2: 253-306.
Pepelko WE, Mattox J, Moorman WJ, & Clark JC (1981) Pulmonary function
evaluation of cats after one year of exposure to diesel exhaust.
Environ Int, 5: 373-376.
Pereira MA (1982) Genotoxicity of diesel exhaust emissions in
laboratory animals. In: Lewtas J, ed., Toxicological effects of
emissions from diesel engines. Amsterdam, Elsevier, pp 265-276.
Pereira MA, McMillan L, Kaur P, Gulati DK, & Sabharwal PS (1982)
Effect of diesel exhaust emissions, particulates, and extract on
sister chromatid exchange in transplacentally exposed fetal hamster
liver. Environ Mutag, 4: 215-220.
Perez Rodriguez E, Latour Perez J, Aller Alvarez JL, Alix J, & Alix Y
(1976) [Diesel fuel pneumonia. Presentation of a case.] Rev Clin Esp,
143: 397-400 (in Spanish).
Peters RW, Montemagno CD, & Shem L (1992) Surfactant screening of
diesel-contaminated soil. Hazard Waste Mater, 9: 113-136.
Petersen BA & Chuang CC (1982) Methodology of fractionation and
partition of diesel exhaust particulate samples. In: Lewtas J, ed.,
Toxicological effects of emissions from diesel engines. Amsterdam,
Elsevier, pp 51-67.
Piantadosi S (1994) Invited commentary: Ecologic biases. Am J
Epidemiol, 139: 761-764.
Pipho MJ, Ambs JL, & Kittelson DB (1986) In-cylinder measurements of
particulate formation in an indirect injection diesel engine (SAE
Technical Paper No. 860024). Warrendale, PA, Society of Automotive
Engineers, 9 pp.
Pitts JN Jr, Van Cauwenberghe KA, Grosjean D, Schmid JP, Fitz DR,
Belser WL Jr, Knudson GB, & Hynds PM (1978) Atmospheric reactions of
polycyclic aromatic hydrocarbons: Facile formation of mutagenic nitro
derivatives. Science, 202: 515-519.
Pitts JN Jr, Lokensgard DM, Harger W, Fisher TS, Mejia V, Schuler JJ,
Scorziell GM, & Katzenstein YA (1982) Mutagens in diesel exhaust
particulate. Identification and direct activities of
6-nitrobenzo( a)pyrene, 9-nitroanthracene, 1-nitropyrene and
5 H-phenanthrene(4,5- bcd)pyran-5-one. Mutat Res, 103: 241-249.
Pitts JN Jr, Sweetman JA, Zielinska B, Winer AM, & Atkinson R (1985)
Determination of 2-nitrofluoranthene and 2-nitropyrene in ambient
particulate organic matter: Evidence for atmospheric reactions. Atmos
Environ, 19: 1601-1608.
Plopper CG, Hyde DM, & Weir AJ (1983) Centriacinar alterations in
lungs of cats chronically exposed to diesel exhaust. Lab Invest,
49: 391-399.
Poirier A, Baudin Laurencin F, Bodennec G, & Quentel C (1986)
[Experimental poisoning of the rainbow trout, Salmo gairdneri
Richardson, by diesel engine fuel: Haematological changes, histology].
Aquaculture, 55: 115-137 (in French).
Pope CA III & Dockery DW (1992) Acute health effects of PM10 pollution
on symptomatic and asymptomatic children. Am Rev Respir Dis,
145: 1123-1128.
Pople A, Simpson RD, & Cairns SC (1990) An incident of southern ocean
oil pollution: Effects of a spillage of diesel fuel on the rocky shore
of Macquarie Island (sub-Antarctic). Aust J Mar Freshwater Res,
41: 603-620.
Porter HO (1990) Aviators intoxicated by inhalation of JP-5 fuel
vapors. Aviat Space Environ Med, 61: 654-656.
Portier C, Hedges J, & Hoel D (1986) Age-specific models of mortality
and tumor onset for historical control animals in the National
Toxicology Program's carcinogenicity experiments. Cancer Res,
46: 4372-4378.
Pott F (1991) [Diesel engine exhaust -- results of laboratory animal
experiments relative to risk estimation]. In: [Carcinogenic substances
in the environment: Origin, measurement, risk, minimization. Meeting
of the Commission for Clean Air of the Society of German Engineers
(VDI) and the German Standards Institute (DIN), Mannheim, 23-25
April]. Düsseldorf, Society of German Engineers Press, pp 211-244
(in German).
Pott F & Heinrich U (1987) [Diesel engine exhaust and lung cancer.
Data from animal experiments and their evaluation with respect to the
hazard for humans]. Düsseldorf, Association for the promotion of
Environmental Health and Silicosis Research, Vol. 19, pp 130-167
(in German).
Pott F & Roller M (1994) Relevance of non-physiologic exposure routes
for carcinogenicity studies of solid particles. In: Mohr U, Dungworth
DL, Mauderly JL, & Oberdörster G, eds, Toxic and carcinogenic effects
of solid particles in the respiratory tract. Washington DC,
International Life Sciences Institute Press, pp 109-125.
Pott F, Heinrich U & Roller M (1993) [Diesel engine emissions.] In:
Wichmann HE, Schlipköter HW, & Fülgraff G, eds, [Handbook of
environmental medicine]. Landsberg, Lech, Ecomed mbH, 77 pp
(in German).
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann PG,
Roller M, Rippe RM, & Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Ann Occup Hyg,
38 (Suppl 1): 357-363.
Prakash CB, Rideout GB, & Kirshenblatt M (1992) Exhaust emissions from
diesel powered urban transit buses (Paper No. IU-17A). In: Proceedings
of the 9th World Clean Air Congress and Exhibition, Montreal, 30
August-4 September 1992, Ottawa, Environment Canada, Vol. 2, 22 pp.
Pryor P (1983) Health Hazard Evaluation. Trailways Bus System, Denver,
CO (PB85-102952, Report No HETA 81-416-1334). Cincinnati, OH, National
Institute for Occupational Safety and Health, 11 pp.
Pulich WM Jr, Winters K, & Van Baalen C (1974) The effects of a No. 2
fuel oil and two crude oils on the growth and photosynthesis of
microalgae. Mar Biol, 28: 87-94.
Purdham JI, Holness DL, & Pilger CW (1987) Environmental and medical
assessment of stevedores employed in ferry operations. Appl Ind Hyg,
2: 133- 139.
Quality of Urban Air Review Group (1993) Diesel vehicle emissions and
urban air quality. Birmingham, 88 pp.
Quinto I & De Marinis E (1984) Sperm abnormalities in mice exposed to
diesel particulate. Mutat Res, 130: 242.
Raabe OG, Al-Bayati MA, Teague SV, & Rasolt A (1988) Regional
deposition of inhaled monodisperse coarse and fine aerosol particles
in small laboratory animals. Ann Occup Hyg, 32 (Suppl 1): 53-63.
Rasmussen RE (1990) Effect of fuel properties on mutagenic activity in
extracts of heavy-duty diesel exhaust particulate. J Air Waste Manage
Assoc, 40: 1391-1396.
Reger R, Hancock J, Hankinson J, Hearl F, & Merchant J (1982) Coal
miners exposed to diesel exhaust emissions. Ann Occup Hyg,
26: 799-815.
Regnier ZR & Scott BF (1975) Evaporation rates of oil components.
Environ Sci Technol, 9: 469-472.
Reidenberg MM, Powers DV, Sevy RW, & Bello CT (1964) Acute renal
failure due to nephrotoxins. Am J Med Sci, 247: 25-29.
Rice SD, Short JW, & Karinen JF (1976) Toxicity of Cook Inlet crude
oil and No. 2 fuel oil to several Alaskan marine fishes and
invertebrates. In: Sources, effects and sinks of hydrocarbons in the
aquatic environment. Washington DC, American Institute of Biological
Sciences, pp 395-406.
Rice SD, Short JW, & Karinen JF (1977a) Comparative oil toxicity and
comparative animal sensitivity. In: Wolfe DA, Anderson JW, Button DK,
Malins, DC, Roubal T, & Varanasi U, eds, Fate and effects of petroleum
hydrocarbons in marine organisms and ecosystems. Oxford, Pergamon
Press, pp 78-94.
Rice SD, Thomas RE, & Short JW (1977b) Effect of petroleum
hydrocarbons on breathing and coughing rates and hydrocarbons
uptake-depuration in pink salmon fry. In: Verberg JF, Calabrese A,
Thurberg FP, & Vernberg WB, eds, Physiological responses of marine
biota to pollutants. New York, Academic Press, pp 259-277.
Risch HA, Burch JD, Miller AB, Hill GB, Steele R, & Howe GR (1988)
Occupational factors and the incidence of cancer of the bladder in
Canada. Br J Ind Med, 45: 361-367.
Rogge WF, Hildemann LM, Mazurek MA, Cass GR & Simonelt BRT (1993)
Sources of fine organic aerosol 2. Noncatalyst and catalyst-equipped
automobiles and heavy-duty diesel trucks. Environ Sci Technol,
27: 636-651.
Roller M & Pott F (1994) Use of experimental data for risk assessment
of fibrous dusts and other aerosols. Inf Biom Epidemiol Med Biol,
25: 301-311.
Rossi SS (1977) Bioavailability of petroleum hydrocarbons from water,
sediments and detritus to the marine annelid Neanthes arenaceodentata.
In: Proceedings, 1977 Oil Spill Conference. Washington DC, American
Petroleum Institute, pp 621-625.
Rossi SS & Anderson JW (1976) Toxicity of water-soluble fractions of
No. 2 fuel oil and South Louisiana crude oil to selected stages in the
life history of the polychaete, Neanthes arenaceodentata. Bull Environ
Contam Toxicol, 16: 18-24.
Rossi SS & Anderson JW (1978) Effects of No. 2 fuel oil
water-soluble-fractions on growth and reproduction in Neanthes
arenaceodentata (Polychaeta: Annelida). Water Air Soil Pollut,
9: 155-170.
Rossi SS, Anderson JW, & Ward GS (1976) Toxicity of water-soluble
fractions of four test oils for the polychaetous annelids Neanthes
arenaceodentata and Capitella capitata. Environ Pollut, 10: 9-18.
Rudell B, Marqvardsen MD, Lindström O, & Kolmodin-Hedman B (1989) A
method for controlled exposure to diesel exhaust from a continuously
idling diesel engine. J Aerosol Sci, 20: 1281-1284.
Rudell B, Sandström T, Stjernberg N, & Kolmodin-Hedman B (1990)
Controlled diesel exhaust exposure in an exposure chamber: Pulmonary
effects investigated with bronchoalveolar lavage. J Aerosol Sci,
21: 411-414.
Ruder AM, Fine LJ, & Sundin DS (1990) National estimates of
occupational exposure to animal bladder-tumorigens. J Occup Med,
32: 797-805.
Rushton L, Alderson MR, & Nagarajah CR (1983) Epidemiological survey
of maintenance workers in London transport executive bus garages and
Chiswick Works. Br J Ind Med, 40: 340-345.
Russell TJ (1989) Additives influencing diesel fuel combustion. In:
Owen K, ed., Gasoline and diesel fuel additives (Critical reports on
applied chemistry, Vol 25). Chichester, John Wiley & Sons, pp 65-104.
Rykowski RA & Brochu AJ (1986) Diesel particulate emissions: The
United States experience, with extrapolations to Europe and Japan. In:
Lee SD, Schneider I, Grant LD, & Verkerk PJ, eds, Aerosols. Chelsea,
MI, Lewis Publishers Inc., pp443-457.
Sagai M, Saito H, Ichinose T, Kodama M, & Mori Y (1993) Biological
effects of diesel exhaust particles. I. In vitro production of
superoxide and in vivo toxicity in mouse. Free Radicals Biol Med,
14: 37-47.
Salmeen IT, Pero AM, Zator R, Schuetzle D, & Riley TL (1984) Ames
assay chromatograms and the identification of mutagens in diesel
particle extracts. Environ Sci Technol, 18: 375-382.
Salmeen IT, Gorse Jr RA, & Pierson WR (1985) Ames assay chromatograms
of extracts of diesel exhaust particles from heavy-duty trucks on the
road and from passenger cars on a dynamometer. Environ Sci Technol,
19: 270-273.
Sandmeyer EE (1981) Aliphatic hydrocarbons. In: Clayton GD & Clayton
FE, eds, Patty's industrial hygiene and toxicology, Vol. 2B,
Toxicology. New York, John Wiley & Sons, pp 3175-3431.
Sarojini R, Khan AK, & Nagabhushanam R (1989) Effect of petroleum
hydrocarbons (petrol and diesel) on the physiology of the crab,
Barytelphusa cunicularis I -- oxygen consumption. J Environ Biol,
10: 363-365.
Scheepers PTJ & Bos RP (1992a) Combustion of diesel fuel from a
toxicological perspective. I. Origin of incomplete combustion
products. Int Arch Occup Environ Health, 64: 149-161.
Scheepers PTJ & Bos RP (1992b) Combustion of diesel fuel from a
toxicological perspective. II. Toxicity. Int Arch Occup Environ
Health, 64: 163-177.
Scheepers PTJ, Velders DD, Martens MHJ, Noordhoek J, & Bos RP (1994a)
Gas chromatographic-mass spectrometric determination of nitro
polycyclic aromatic hydrocarbons in airborne particulate matter from
workplace atmospheres contaminated with diesel exhaust. J Chromatogr,
677: 107-121.
Scheepers PTJ, Thuis HJTM, Martens MHJ, & Bos RP (1994b) Assessment of
occupational exposure to diesel exhaust. The use of an immunoassay for
the determination of urinary metabolites of nitroarenes and polycyclic
aromatic hydrocarbons. Toxicol Lett, 72: 191-198.
Schenker MB & Speizer FE (1979) A retrospective cohort study of diesel
exhaust exposure in railroad workers: Study design and methodologic
issues. In: Pepelko WE, Danner RB, & Clark NA, eds, Health effects of
diesel engine emissions (EPA-600/9-80-057b). Cincinnati, OH,
Environmental Protection Agency.
Schenker MB, Smith T, Muñoz A, Woskie S, & Speizer FE (1984) Diesel
exposure and mortality among railway workers: Results of a pilot
study. Br J Ind Med, 41: 320-327.
Schenker MB, Kado NY, Hammond SK, Samuels SJ, Woskie SR, & Smith TJ
(1992) Urinary mutagenic activity in workers exposed to diesel
exhaust. Environ Res, 57: 133-148.
Schiffmann D & Henschler D (1992) [Studies of diesel engine exhaust
fractions on the genotoxic and cell-transforming properties with the
model of Syrian hamster embryo fibroblasts and with lung cells]. In:
[Effects of diesel engine exhausts on health]. Munich, GSF Research
Centre for Environment and Health Inc. pp 39-42 (in German).
Schuetzle D (1983) Sampling of vehicle emissions for chemical analysis
and biological testing. Environ Health Perspectives, 47: 65-80.
Schuetzle D & Frazier JA (1986) Factors influencing the emission of
vapor and particulate phase components from diesel engines. In:
Ishinishi N, Koizumi A, McClellan RO, & Stöber W, eds, Carcinogenic
and mutagenic effects of diesel engine exhaust. Amsterdam, Elsevier,
pp 41-63.
Schuetzle D & Perez JM (1981) A CRC cooperative comparison of
extraction and HPLC techniques for diesel particulate emissions (Paper
81-564). In: Proceedings of the 74th Annual Meeting of the Air
Pollution Control Association, 16-21 June 1981, Philadelphia.
Philadelphia, PA, Air Pollution Control Association, pp 1-32.
Schuetzle D & Perez JM (1983) Factors influencing the emissions of
nitrated-polynuclear aromatic hydrocarbons (nitro-PAH) from diesel
engines. J Air Pollut Control Assoc, 33: 751-755.
Schuetzle D, Riley TL, Prater TJ, Harvey TM, & Hunt DF (1982) Analysis
of nitrated polycyclic aromatic hydrocarbons in diesel particulates.
Anal Chem, 54: 265-271.
Schuetzle D, Jensen TE, & Ball JC (1985) Polar polynuclear aromatic
hydrocarbon derivatives in extracts of particulates: Biological
characterization and techniques for chemical analysis. Environ Int,
11: 169-181.
Schuler RL & Niemeier RW (1981) A study of diesel emissions on
Drosophila. Environ Int, 5: 431-434.
Schultz T, Witschi H, Smith L, Haschek W, Holland J, Epler J, Fry R,
Rao T, Larimer F, & Dumont J (1981) Health effects research in oil
shale development (ORNL/TM-8034). Oak Ridge, TN, Oak Ridge National
Laboratories, 56 pp.
Schwartz J (1993) Air pollution and daily mortality in Birmingham,
Alabama. Am J Epidemiol, 136: 1136-1147.
Schwartz J, Slater D, Larson TV, Pierson WE, & Koenig JO (1993)
Particulate air pollution and hospital emergency room visits for
asthma in Seattle. Am Rev Respir Dis, 147: 826-831.
Schwind KH, Thoma H, Hutzinger O, Dawidowsky N, Weberuss, Hagenmaier
H, Bühler U, Greiner R, Essers U, & Bessey E (1991) [Emission of
halogenated dibenzodioxins (PXDD) and dibenzofurans (PXDF) from
internal combustion engines]. Z Umweltchem Ökotox 3: 291-298
(in German).
Shefner AM, Collins BR, Dooley L, Fiks A, Graf JL, & Preache MM (1982)
Respiratory carcinogenicity of diesel fuel emissions. Interim results.
In: Lewtas J, ed., Toxicological effects of emissions from diesel
engines. Amsterdam, Elsevier, pp 329-350.
Siemiatycki J, Dewar R, Nadon L, Gérin M, Richardson L, & Wacholder S
(1987) Associations between several sites of cancer and twelve
petroleum-derived liquids: Results from a case-referent study in
Montreal. Scand J Work Environ Health, 13: 493-504.
Siemiatycki J, Gérin M, Stewart P, Nadon L, Dewar R, & Richardson L
(1988) Associations between several sites of cancer and ten types of
exhaust and combustion products. Scand J Environ Health, 14: 79-90.
Sienicki EJ & Mago RS (1992) Re-evaluation of diesel engine
particulate emission inventories. In: Toxic air pollutants from mobile
sources. Pittsburgh, PA, Air and Waste Management Association,
pp 151-164.
Silverman DT, Hoover RN, Albert S, & Graff KM (1983) Occupation and
cancer of the lower urinary tract in Detroit. J Natl Cancer Inst,
70: 237-245.
Silverman DT, Hoover RN, Mason TJ, & Swanson GM (1986) Motor
exhaust-related occupations and bladder cancer. Cancer Res,
46: 2113-2116.
Singh AK, & Gaur JP (1990) Effects of petroleum oils and their
paraffinic, asphaltic, and aromatic fractions on photosynthesis and
respiration of microalgae. Ecotoxicol Environ Saf, 19: 8-16.
Snipes MB & McClellan RO (1985) Retention of 14C-labeled
aluminosilicate particles inhaled by dogs and guinea pigs -- simulation
model projections for humans. In: Inhalation Toxicology Research
Institute Annual report 1984-1985, Albuquerque, NM, pp 96-99.
Snipes MB, Boecker BB, & McClellan RO (1983) Retention of monodisperse
or polydisperse aluminosilicate particle inhaled by dogs, rats, and
mice. Toxicol Appl Pharmacol, 69: 345-362.
Song H-G, Wang X, & Bartha R (1990) Bioremediation potential of
terrestrial fuel spills. Appl Environ Microbiol, 56: 652-656.
Standardization Board in Sweden (1991) Swedish Standard SS 15 54 35:
Diesel fuel oil for high-speed diesel engines. Stockholm.
State Committee for Immission Protection (1992) [Diesel motor
emissions (DME)]. In: [Cancer risk through polluted air. Development
of 'Evaluation standards for cancerogenic air pollution' commissioned
by the Conference for Ministers of the Environment], Düsseldorf,
pp 89-93 (in German).
Steenland K, Burnett C, & Osorio AM (1987) A case-control study of
bladder cancer using city directories as a source of occupational
data. Am J Epidemiol, 126: 247-257.
Steenland NK, Silverman DT, & Hornung RW (1990) Case-control study of
lung cancer and truck driving in the Teamsters Union. Am J Public
Health, 80: 670- 674.
Steenland K, Silverman D, & Zaebst D (1992) Exposure to diesel exhaust
in the trucking industry and possible relationships with lung cancer.
Am J Ind Med, 21: 887-890.
Steineck G, Plato N, Gerhardsson M, Norell SE, & Hogstedt C (1990)
Increased risk of urothelial cancer in Stockholm during 1985-87 after
exposure to benzene and exhausts. Int J Cancer, 45: 1012-1017.
Stenberg U, Alsberg T, & Westerholm W (1983) Emission of carcinogenic
components with automobile exhausts. Environ Health Perspectives,
47: 53-56.
Stern FB, Curtis RA, & Lemen RA (1981) Exposure of motor vehicle
examiners to carbon monoxide: A historical prospective mortality
study. Arch Environ Health, 36: 59-66.
Stirling HP (1977) Effects of a spill of marine diesel oil on the
rocky shore fauna of Lamma Island, Hong Kong. Environ Pollut,
12: 93-117.
Stöber W (1986) Experimental induction of tumors in hamsters, mice and
rats after long-term inhalation of filtered and unfiltered diesel
engine exhaust. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 421-439.
Stone WA (1991) Assessing health risks associated with diesel
contaminated soils and groundwater. In: Calabrese EJ & Kostecki PT,
eds, Hydrocarbon contaminated soils, Vol. 1, Remediation techniques,
environmental fate, risk assessment, analytical methodologies,
regulatory considerations. Chelsea, MI, Lewis Publishers, pp 167-179.
Strom KA, Chan TL, & Johnson JT (1988) Pulmonary retention of inhaled
submicron particles in rats: Diesel exhaust exposures and lung
retention model. Ann Occup Hyg, 32: 645-657.
Strom KA, Garg BD, Johnson JT, D'Arcy JB, & Smiler KL (1990) Inhaled
particle retention in rats receiving low exposures of diesel exhaust.
J Toxicol Environ Health, 29: 377-398.
Stromgren T & Nielsen MV (1991) Spawning frequency, growth and
mortality of Mytilus edulis larvae, exposed to copper and diesel
oil. Aquat Toxicol, 21: 171-180.
Stromgren T & Reiersen LO. (1988) A new method for testing toxicity of
drilling fluid; effect on growth of mussels. Oil Chem Pollut,
4: 127-138.
Stromgren T, Nielsen MV, & Ueland K (1986) Short-term effect of
microencapsulated hydrocarbons on shell growth of Mytilus edilus.
Mar Biol, 91: 33-39.
Sun JD, Wolff RD, & Kanapilly GM (1982) Deposition, retention, and
biological fate of inhaled benzo[ a]pyrene adsorbed onto ultrafine
particles and as a pure aerosol. Toxicol Appl Pharmacol,
65: 231-244.
Sun JD, Wolff RK, Kanapilly GM, & McClellan RO (1984) Lung retention
and metabolic fate of inhaled benzo( a)pyrene associated with diesel
exhaust particles. Toxicol Appl Pharmacol, 73: 48-59.
Suzuki T, Nakajima T, Maejima K, Kato A, Takaki Y, Kuwabara N,
Hisanaga A, & Ishinishi N (1990) [Long-term inhalation experiments on
the health effects of diesel exhaust]. Taiki Osen Gatukai-shi,
25: 192-205 (in Japanese, with English summary).
Swanson GM, Lin C-S, & Burns PB (1993) Diversity in the association
between occupation and lung cancer among black and white men. Cancer
Epidemiol Biomarkers Prev, 2: 313-320.
Swarin SJ & Williams RL (1980) Liquid chromatographic determination of
benzo[ a]pyrene in diesel exhaust particulate: Verification of the
collection and analytical methods. In: Björseth A & Dennis AJ, eds,
Polynuclear aromatic hydrocarbons, 4th International Symposium:
Chemistry and biological effects. Columbus, OH, Battelle Press,
pp 771-806.
Takafuji S, Suzuki S, Koizumi K, Tadokoro K, Miyamoto T, Ikemori R, &
Muranaka M (1987) Diesel-exhaust particulates inoculated by the
intranasal route have an adjuvant activity for IgE production in mice.
J Allergy Clin Immunol, 79: 639-645.
Takafuji S, Suzuki S, Muranaka M, & Miyamoto T (1989) Influence of
environmental factors on IgE production. In: Mast cells and the
allergic response (Ciba Foundation Symposium 147). Chichester, John
Wiley & Sons, pp 188-204.
Takaki Y, Kitamura S, Kuwabara N, & Fukuda Y (1989) Long-term
inhalation studies of exhaust from the diesel engine in F-344 rats:
The quantitative relationship between pulmonary hyperplasia and
anthracosis. Exp Pathol, 37: 56-61.
Takemoto K, Yoshimura H, & Katayama H (1986) Effects of chronic
inhalation exposure to diesel exhaust on the development of lung
tumors in di-isopropanol-nitrosamine-treated F344 rats and newborn
C57BL and ICR mice. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber
W, eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp 311-327.
Tan W & Chen C. (1992) A nonhomogeneous stochastic model of
carcinogenesis and its applications. In: Arino O, Axelrod D, & Kimmel
M, eds, Mathematical population dynamics 2. Winnipeg, Manitoba, Wuerz
Publishing Co., pp 49-67.
Tarshis IB (1981) Uptake and depuration of petroleum hydrocarbons by
crayfish. Arch Environ Contam Toxicol, 10: 79-86.
Tatem HE (1977) Accumulation of naphthalenes by grass shrimp: Effects
on respiration, hatching, and larval growth. In: Wolfe DA, Anderson
JW, Button DK, Malins DC, Roubel T, & Varanasi U, eds, Fate and effect
of petroleum hydrocarbons in marine ecosystems and organisms. Oxford,
Pergamon Press, pp 201-209.
Tedengren M & Kautsky N (1987) Comparative stress response to diesel
oil and salinity changes of the blue mussel, Mytilus edilus, from
the Baltic and North seas. Ophelia, 28: 1-9.
Tedengren M, Arnér M, & Kautsky N (1988) Ecophysiology and stress
response of marine and brackish water Gammarus species (Crustacea
amphipoda) to changes in salinity and exposure to cadmium and
diesel-oil. Mar Ecol Prog Ser, 47: 107-116.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF, &
Rosenblatt DH, eds, Handbook of chemical property estimation methods.
Environmental behaviour of organic compounds. New York, McGraw-Hill
Book Co., pp 15:1-15:34.
Törnqvist M, Kautiainen A, Gatz RN, & Ehrenberg L (1988) Hemoglobin
adducts in animals exposed to gasoline and diesel exhausts. I.
Alkenes. J Appl Toxicol, 8: 159-170.
Tucker JD, Xu J, Stewart J, Baciu PC, & Ong T (1986) Detection of
sister chromatid exchanges induced by volatile genotoxicants. Teratog
Carcinog Mutag, 6: 15-21.
Ulfvarson U & Alexandersson R (1990) Reduction in adverse effect on
pulmonary function after exposure to filtered diesel exhaust. Am J Ind
Med, 17: 341-347.
Ulfvarson U, Alexandersson R, Aringer L, Svensson E, Hedenstierna G,
Hogstedt C, Holmberg B, Rosén G, & Sorsa M (1987) Effects of exposure
to vehicle exhaust on health. Scand J Work Environ Health,
13: 505-512.
Ullman TL, Hare CT, & Baines TM (1984) Influence of maladjustment on
emissions from two heavy-duty diesel bus engines (SAE Paper
No. 840416). Warrendale, PA, Society of Automotive Engineers, 11 pp.
Ungers LJ (1984) Measurement of exhaust emissions from diesel-powered
forklifts during operations in ammunition storage magazines (phase I)
(Report No. AD-A141 792). Cincinnati, OH, PEDCO Environmental Inc.,
57 pp.
Ungers LJ (1985) Measurement of exhaust emissions from diesel-powered
forklifts during operations in ammunition storage magazines (phase
II). Final report for the period July 23, 1984 to March 15, 1985
(Report No. AD-A153-092). Cincinnati, OH, PEI Associates, 47 pp.
Upreti RK, Das M, & Shanker R (1989) Dermal exposure to kerosene. Vet
Hum Toxicol, 31: 16-20.
US Department of Energy (1988) The motor fuel consumption model
fourteenth periodical report (DOE/OR/21400 -- H12, DE 89-011358).
Arlingston, VA, Energy and Environmental Analysis Inc., 16 pp.
US Environmental Protection Agency (1986) The risk assessment
guidelines of 1986 (EPA/600/8-87/045). Washington DC, Office of Health
and Environmental Assessment.
US Environmental Protection Agency (1991) Alpha 2µ-globulin:
Association with chemically induced renal toxicity and neoplasia in
the male rat (Risk Assessment Forum Series) (EPA/625/3-91/019F).
Washington DC, 118 pp.
US Environmental Protection Agency (1992a) Integrated risk information
system (IRIS). Reference concentration (RfC) file for inhalation
exposure for diesel engine emissions. Online (Verification date
6/25/92). Cincinnati, OH, Office of Health and Environmental
Assessment, 25 pp.
US Environmental Protection Agency (1992b) Regulation of fuels and
fuel additives: Standards for highway diesel fuel quality-sulfur
content. Final rule. Fed Reg, 57: 19535-19537.
US Environmental Protection Agency (1993) Motor vehicle-related air
toxics study (EPA 420-R-93-005). Ann Arbor, MI, Office of Mobile
Sources, pp 4.1-4.17, 9.1-9.62.
US National Institute for Occupational Safety and Health (1989a)
Health Hazard Evaluation Report. Consolidated Freightways Pocono
Summit, Pennsylvania (HETA 87-232-1948). Cincinnatti, OH, US
Department of Health and Human Services, 20 pp.
US National Institute for Occupational Safety and Health (1989b)
Health Hazard Evaluation Report. Consolidated Freightways Peru,
Illinois (HETA 88-077-1969). Cincinnatti, OH, US Department of Health
and Human Services, 31 pp.
US National Institute for Occupational Safety and Health (1990a) Final
report of the NIOSH health hazard evaluation (HHE) conducted at
Benedict Enterprises Dayton, Ohio (October 2 and November 5, 1987)
(HETA 87-237). Cincinnatti, OH, US Department of Health and Human
Services, 33 pp.
US National Institute for Occupational Safety and Health (1990b) Final
report of the NIOSH health hazard evaluation (HHE) conducted at
Madison Metro's Transit System's Maintenance and Administration
Facility (January 10-13, 1989) (HETA 88-218). Cincinnatti, OH, US
Department of Health and Human Services, 38 pp.
US National Institute for Occupational Safety and Health (1990c)
Health Hazard Evaluation Report. Yellow Freight System, Inc.,
Columbus, Ohio, Maybrook, New York (HETA 90-088-2110). Cincinnatti,
OH, US Department of Health and Human Services, 127 pp.
US National Institute for Occupational Safety and Health (1991)
Research plan. A sampling and analytical method for airborne
diesel-exhaust particels. Cincinnatti, OH, Centers for Disease
Control, National Institute for Occupational and Health, pp 1-34.
US National Research Council (1983) Risk assessment in the Federal
Government: Managing the process. Committee on the Institutional Means
for Assessment of Risks to Public Health. Washington DC, National
Academy Press, pp 1-50.
US National Research Council (1985) Oil in the sea. Inputs, fates, and
effects. Washington DC, National Academy Press, 587 pp.
US National Toxicology Program (1986) Toxicology and carcinogenesis
studies of marine diesel fuel and JP-5 navy fuel (CAS-No 8008-20-6) in
B6C3F1 mice (dermal studies) (Technical report series No. 310).
Research Triangle Park, NC, 206 pp.
Vallyathan V, Virmani R, Rochlani S, Green FHY, & Lewis T (1986)
Effect of diesel emissions and coal dust inhalation on heart and
pulmonary arteries of rats. J Toxicol Environ Health, 19: 33-41.
Van Beckhoven LC (1991) Effects of fuel properties on diesel engine
emissions -- A review of information available to EEC-MVEG group (SAE
Technical Paper No. 910608). Warrendale, PA, Society of Automotive
Engineers, pp 237-249.
Vandermeulen JH & Lee RW (1986) Lack of mutagenic activity of crude
and refined oils in the unicellular alga Chlamydomonas reinhardtii.
Bull Environ Contam Toxicol, 36: 250-253.
Vandermeulen JH, Foda A, & Stuttard C (1985) Toxicity vs mutagenicity
of some crude oils, distillates and their water soluble fractions.
Water Res, 19: 1283-1289.
Van Vleet ES & Quinn JG (1978) Contribution of chronic petroleum
inputs to Narragansett Bay and Rhode Island Sound sediments. J Fish
Resour Board Can, 35: 536-543.
Vinegar A, Carson A, & Pepelko WE (1981) Pulmonary function changes in
Chinese hamster exposed 6 months to diesel exhaust. Environ Int,
5: 369-371.
Vineis P & Magnani C (1985) Occupation and bladder cancer in males: A
case-control study. Int J Cancer, 35: 599-606.
Volkswagen AG (1989) Unregulated motor vehicle exhaust gas components.
Wolfsburg, Research and Development, pp 1-128.
Vostal JJ (1986) Factors limiting the evidence for chemical
carcinogenicity of diesel emissions in long-term inhalation
experiments. In: Ishinishi N, Koizumi A, McClellan RO, & Stöber W,
eds, Carcinogenic and mutagenic effects of diesel engine exhaust.
Amsterdam, Elsevier, pp. 381-396.
Wade WR & Jones CM (1984) Current and future light duty diesel engines
and their fuels (Paper No. 840105). In: International congress and
exposition, Detroit, Michigan, 27 February-2 March 1984. Warrendale,
PA, Society of Automotive Engineers.
Wade JF III & Newman LS (1993) Diesel asthma. Reactive airways disease
following overexposure to locomotive exhaust. J Occup Med,
35: 149-154.
Wade TL & Quinn JG (1980) Incorporation, distribution, and fate of
saturated petroleum hydrocarbons in sediments from a controlled marine
ecosystem. Mar Environ Res, 3: 15-34.
Wallace WE, Keane MJ, Hill CA, Xu J, & Ong T (1987) Mutagenicity of
diesel exhaust particles and oil shale particles dispersed in lecithin
surfactant. J Toxicol Environ Health, 21: 163-171.
Wallace WE, Keane MJ, Xing S, & Ong T (1990) Mutagenicity of diesel
exhaust soot dispersed in phospholipid surfactants. In: Seemayer NH &
Hadnagy W, eds, Environmental hygiene II. Berlin, Springer, pp 7-10.
Waller RE (1981) Trends in lung cancer in London in relation to
exposure to diesel fumes. Environ Int, 5: 479-483
Waller RE, Commins BT, & Lawther PJ (1965) Air pollution in a city
street. Br J Ind Med, 22: 128-138.
Waller RE, Hampton L, & Lawther PJ (1985) A further study of air
pollution in diesel bus garages. Br J Ind Med, 42: 824-830.
Walrath J, Rogot E, Murray J, & Blair A (1985) Mortality patterns
among US veterans by occupation and smoking status, Vol. 1 (NIH
Publication No. 85-2756). Washington DC, US Government Printing
Office, p 206.
Wan MT, Watts RG, & Moul DJ (1990) Acute toxicity to juvenile Pacific
salmonids and rainbow trout of butoxyethyl esters of 2,4-D, 2,4-DP and
their formulated product: Weedone CB and its carrier. Bull Environ
Contam Toxicol, 45: 604-611.
Wang X & Bartha R (1990) Effects of bioremediation on residues,
activity and toxicity in soil contaminated by fuel spills. Soil Biol
Biochem, 22: 501-505.
Waxweiler RJ, Wagoner JK, & Archer VE (1973) Mortality of potash
workers. J Occup Med, 15: 486-489.
Weidmann K, Menrad H, Reders K, & Hutcheson RC (1988) Diesel fuel
quality effects on exhaust emissions (SAE Technical Paper No. 881649).
Warrendale, PA, Society of Automotive Engineers, 11 pp.
Westerholm R (1987) Inorganic and organic compounds in emissions from
diesel powered vehicles. A literature survey (Report No; 3389).
Stockholm, National Swedish Environmental Protection Board.
Westerholm R & Egebäck KE (1991) Impact of fuels on diesel exhaust
emissions. A chemical and biological characterization (Report
No. 3968). Solna, National Swedish Environmental Protection Agency,
61 pp.
Westerholm R, Alsberg T, Strandell M, Frommelin A, Grigoriadis V,
Hantzaridou A, Maitra G, Winquist L, Rannug U, Egebäck KE, &
Bertilsson (1986) Chemical analysis and biological testing of
emissions from a heavy duty diesel truck with and without two
different particulate traps (SAE Technical Paper No. 860014).
Warrendale, PA, Society of Automotive Engineers, pp 73-83.
Wheeler RW, Hearl FJ, & McCawley M (1981) An industrial hygiene
characterization of exposure to diesel emissions in an underground
coal mine. Environ Int, 5: 485-488.
White HJ & Garg BD (1981) Early pulmonary response of the rat lung to
inhalation of high concentration of diesel particles. J Appl Toxicol,
1: 104-110.
WHO (1987) Air quality guidelines for Europe (WHO Regional
Publications, European Series No. 23). Copenhagen, Regional Office for
Europe.
WHO (1990) Revised guidelines for the preparation of Environmental
Health Criteria monographs (PCS/90.69). Geneva.
Whong W-Z, Wen Y-F, Stewart J, & Ong T-M (1986) Validation of the
SOS/umu test with mutagenic complex mixtures. Mutat Res, 175: 139-144.
Wichmann HE & Brüske-Hohlfeld I (1991) [Epidemiological findings on
the cancer risk due to diesel engine exhausts]. In: [Carcinogenic
substances in the environment: origin, measurement, risk,
minimization. Meeting of the Commission for Clean Air of the Society
of German Engineers (VDI) and the German Standards Institute (DIN),
Mannheim, 23-25 April]. Düsseldorf, Society of German Engineers Press,
pp 171-209 (in German).
Widdows J, Donkin P, & Evans SV (1985) Recovery of Mytilus edulis L.
from chronic oil exposure. Mar Environ Res, 17: 250-253.
Wiester MJ, Iltis R, & Moore W (1980) Altered function and histology
in guinea pigs after inhalation of diesel exhaust. Environ Res,
22: 285-297.
Willems MI, de Raat WK, Wesstra JA, Bakker GL, Dubois G, & van Dokkum
W (1989) Urinary and faecal mutagenicity in car mechanics exposed to
diesel exhaust and in unexposed office workers. Mutat Res,
222: 375-391.
Williams RR Stegens NL & Goldsmith JR (1977) Associations of cancer
site and type with occupation and industry from the Third National
Cancer Survey Interview. J Natl Cancer Inst, 59: 1147-1185.
Williams PT, Andrews GE, & Bartle KD (1987) The role of lubricating
oil in diesel particulate and particulate PAH emissions (SAE Technical
Paper No. 872084). Warrendale, PA, Society of Automotive Engineers,
pp 1-9.
Williams DJ, Milne JW, Quigley SM, Roberts DB, & Kimberlee MC (1989)
Particulate emissions from 'in-use' motor vehicles. II. Diesel
vehicles. Atmos Environ, 23: 2647-2661.
Winters K, Van Baalen C, & Nicol JAC (1977) Water soluble extractives
from petroleum oils: Chemical characterization and effects on
microalgae and marine animals. In: McIntyre AD & Whittle KJ, eds,
Petroleum hydrocarbons in the marine environment (Rapports et
procés-verbaux des réunions, Vol. 171). Copenhagen, International
Council for Exporation of the Sea, pp 166-174.
Witschi HP, Smith LH, Frome EL, Pequet-Goad ME, Griest WH, Ho CH, &
Guerin MR (1987) Skin tumorigenic potential of crude and refined coal
liquids and analogous petroleum products. Fundam Appl Toxicol,
9: 297-303.
Wolff RK, Griffis LC, Hobbs CH, & McClellan RO (1982) Deposition and
retention of 0.1 µm 67Ga2 O3 aggregate aerosols in rats
following whole body exposures. Fundam Appl Toxicol, 2: 195-200.
Wolff RK, Henderson RF, Snipes MB, Griffith WC, Mauderly JL, Cuddihy
RG, & McClellan RO (1987) Alterations in particle accumulation and
clearance in lungs of rats chronically exposed to diesel exhaust.
Fundam Appl Toxicol, 9: 154-166.
Wolff RK, Bond JA, Sun JD, Henderson RF, Harkema JR, Griffith WC,
Mauderly JL, & McClellan RO (1989) Effects of adsorption of
benzo[ a]pyrene onto carbon black particles on levels of DNA adducts
in lungs of rats exposed by inhalation. Toxicol Appl Pharmacol,
97: 289-299.
Wolff RK, Bond JA, Henderson RF, Harkema JR, & Mauderly JL (1990)
Pulmonary inflammation and DNA adducts in rats inhaling diesel exhaust
or carbon black. Inhal Toxicol, 2: 241-254.
Wong O, Mitchell LE, Wolff RK, Mauderly JL, & Jeffrey AM (1986)
Identification of DNA damage as a result of exposure of rats to diesel
engine exhaust. Carcinogenesis, 7: 1595-1597.
Woodin SA, Nyblade CF, & Chia F-S (1972) Effect of diesel oil spill on
invertebrates. Mar Pollut Bull, 3: 139-143.
Wormald AP (1976) Effects of a spill of marine diesel oil on the
meiofauna of a sandy beach at Picnic Bay, Hong Kong. Environ Pollut,
11: 117-130.
Woskie SR, Smith TJ, Hammond SK, Schenker MB, Garshick E, & Speizer FE
(1988a) Estimation of the diesel exhaust exposures of railroad
workers: I. Current exposures. Am J Ind Med, 13: 381-394.
Woskie SR, Smith TJ, Hammond SK, Schenker MB, Garshick E, & Speizer FE
(1988b) Estimation of the diesel exhaust exposures of railroad
workers: II. National and historical exposures. Am J Ind Med,
13: 395-404.
Wright ES (1986) Effects of short-term exposure to diesel exhaust on
lung cell proliferation and phospholipid metabolism. Exp Lung Res,
10: 39-55.
Wynder EL, Dieck GS, Hall NEL, & Lahti H (1985) A case-control study
of diesel exhaust exposure and bladder cancer. Environ Res,
37: 475-489.
Yaron B, Sutherland P, Galin T, & Acher AJ (1989) Soil pollution by
petroleum products: II. Adsorption-desorption of 'kerosene' vapors on
soils. J Contam Hydrol, 4: 347-358.
Yu CP & Xu GB (1987) Predictive models for depositon of inhaled diesel
exhaust particles in humans and laboratory species (Report No. 10).
Cambridge, MA, Health Effects Institute, 27 pp.
Yu CP & Yoon KJ (1990) Retention modeling of diesel exhaust particles
in rats and humans (Report No. 40). Cambridge, MA, Health Effects
Institute, 33 pp.
Yu CP, Yoon KJ, & Chen YK (1991) Retention modeling of diesel exhaust
particles in rats and humans. J Aerosol Med, 4: 79-115.
Zaebst DD, Clapp DE, Blade LM, Marlow DA, Steenland K, Hornung RW,
Scheutzle D, & Butler J (1991) Quantitative determination of trucking
industry workers' exposures to diesel exhaust particles. Am Ind Hyg
Assoc J, 52: 529-541.
PARTIE A COMBUSTIBLE DIESEL
A1. RESUME
A1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le combustible diesel est un mélange complexe d'alcanes normaux
ramifiés ou cycliques (de 60 à plus de 90% de volume; longueur de la
chaîne hydrocarbonée, généralement comprise entre C9 et C30), de
composés aromatiques, surtout des alkyl benzènes, (5 à 40% en volume)
et de petites quantités d'alcènes (0 à 10% en volume) obtenu lors de
la distillation fractionnée du pétrole, à partir du distillat moyen
qui correspond à la fraction gazole. Le combustible diesel peut
contenir, à des concentrations de quelques parties par million, du
benzène, du toluène, de l'éthylbenzène, du xylène et des hydrocarbures
aromatiques polycycliques (HAP), en particulier du naphtalène et
certains de ses dérivés méthylés. La teneur en soufre du combustible
diesel dépend de l'origine du pétrole et du procédé de raffinage
utilisé. Dans un certain nombre de pays, elle est soumise à
réglementation et se situe en général entre 0,05 et 0,5% en poids. On
utilise un certain nombre d'additifs pour modifier la viscosité, la
conservation et la combustion, pour différencier les différents
produits et également pour satisfaire aux spécifications commerciales.
A la température ambiante, le combustible diesel est en général
modérément volatile, légèrement visqueux, inflammable et se présente
sous la forme d'un liquide brun à odeur de kérosène. L'intervalle
d'ébullition est habituellement de 140°C à 385°C (plus de 588°C pour
le carburant destiné aux moteurs marins); à 20°C, la densité est de
0,87-1,0 g/cm3 et la solubilité dans l'eau de 0,2-5 mg/litre. La
qualité et la composition du combustible diesel influent
considérablement sur l'émission de polluants par les moteurs diesel.
Les conditions d'allumage (exprimées au moyen de l'indice de cétane),
la densité, la viscosité et la teneur en soufre sont des variables
importantes. Les spécifications du combustible diesel commercial
varient fortement d'un pays à l'autre.
Les différents types de mazout destinés au chauffage ou de
kérosène pour avions à réaction produits lors du raffinage du pétrole
peuvent avoir une composition analogue à celle du combustible diesel,
mais avec des additifs différents. Les données biologiques relatives à
ces mélanges ont donc été prises en considération lors de l'évaluation
toxicologique et écotoxicologique.
En raison de la complexité du mélange, il n'existe pas de méthode
d'analyse spécifique pour le combustible diesel et les techniques
utilisées pour la plupart des études d'impact dur l'environnement ne
se prêtent qu'au dosage des hydrocarbures totaux. Elles consistent
tout d'abord à effectuer une extraction par solvant, puis à éliminer
les hydrocarbures d'origine naturelle et enfin à procéder au dosage
par gravimétrie, spectrophotométrie infrarouge ou chromatographie en
phase gazeuse. Ni la méthode gravimétrique, ni la spectrophotométrie
infrarouge ne donnent de renseignements qualitatifs ou quantitatifs
utiles et ne peuvent donc être utilisées que pour un premier tri. La
chromatographie en phase gazeuse couplée à des techniques de détection
telles que l'ionisation de flamme ou la spectrométrie de masse, est la
méthode classique d'analyse des échantillons prélevés dans
l'environnement. Il existe de nombreuses autres méthodes pour la
recherche et le dosage des divers hydrocarbures présents dans le
combustible diesel.
A1.2 Sources d'exposition humaine et environnementale
Le combustible diesel est produit par raffinage du pétrole brut.
Afin de satisfaire aux spécifications techniques relatives au
rendement des moteurs, ces combustibles sont généralement mélangés;
l'adjonction ultérieure d'additifs en améliore également l'adaptation
à diverses utilisations particulières. Ces combustibles sont largement
utilisés dans les transports. Les plus volatils d'entre eux, qui
présentent une faible viscosité, sont destinés aux moteurs tournant à
haut régime, les combustibles plus lourds étant réservés aux
transports ferroviaires et maritimes. Une grande partie des véhicules
lourds de transport routier sont mus par des moteurs diesel. Les cars
à moteur diesel destinés au transport des voyageurs se répandent de
plus en plus en Europe et au Japon (10 à 25%) alors qu'en Amérique du
Nord, la proportion de cars assurant les transports en commun est
d'environ 1 à 2%, et a même une légère tendance à se tasser. On
utilise également le combustible diesel pour alimenter les chaudières
et les moteurs fixes, qu'il s'agisse de moteurs à pistons, de turbines
à gaz, de pompes d'oléoducs, de compresseurs, de générateurs de vapeur
pour centrales thermiques, de brûleurs, et d'installations de
chauffage industriel par convection ou circulation d'eau.
La demande mondiale de combustible diesel a régulièrement
progressé au cours des 5 dernières années. En 1985, les chiffres de
consommation étaient les suivants: environ 170 000 kilotonnes par an
en Amérique du Nord, environ 160 000 kilotonnes par an, gazole y
compris, dans l'Union européenne, environ 46 000 kilotonnes par an en
Australie, au Japon et en Nouvelle-Zélande, soit l'équivalent de
1062 kilotonnes par jour. En 1990, la demande mondiale a été estimée à
environ 1110 kilotonnes par jour.
On ne dispose d'aucun renseignement sur les émissions qui se
produisent lors de la production des combustibles diesel; toutefois,
il semble que cette source soit d'importance secondaire, étant donné
que le raffinage s'effectue en vase clos. Si des émissions doivent se
produire, c'est surtout au cours du stockage et du transport. Ces
combustibles peuvent également être répandus dans l'environnement par
suite de déversements accidentels ou dans des stations service lorsque
l'on fait de plein des véhicules. L'atmosphère et l'hydrosphère sont
les compartiments du milieu les plus fortement affectés par ces
décharges accidentelles. Il peut y avoir contamination du sol par du
combustible diesel à la faveur d'accidents et ce type de contamination
pose également un problème dans les gares de triage.
Parmi les nombreuses techniques utilisables pour nettoyer les
sols contaminés par du combustible diesel, on peut citer l'excavation,
les méthodes biologiques et le confinement.
A1.3 Transport, distribution et transformation dans
l'environnement
On ne dispose que très rares données sur la destinée du
combustible diesel dans l'environnement mais on suppose que son mode
de distribution et de transformation est comparable à celui des huiles
lourdes destinées au chauffage comme le No 2 qui a été bien étudié. En
cas de déversement dans l'eau, il se forme presque immédiatement une
nappe de mazout. Les constituants polaires et ceux dont la masse
moléculaire est relativement faible se dissolvent et s'éliminent de la
nappe par lessivage; les constituants volatils s'évaporent en surface
et il y a un début de dégradation microbienne. Par attaque chimique et
biologique, la composition de la nappe se modifie. Ces processus
dépendent de la température; les déversements qui se produisent en
milieu arctique conduisent à des nappes plus durables que sous les
climats tempérés. En milieu marin, la plupart des composés aromatiques
de masse moléculaire relative faible se dissolvent dans la phase
aqueuse, mais les alcanes normaux, les alcanes ramifiés, les
cycloalcanes et les composés aromatiques restantes peuvent demeurer
dans les sédiments pendant plus d'un an.
Bien qu'on ne dispose d'aucune donnée sur la photo-oxydation du
combustible diesel dans l'air et l'eau, on sait que les composants qui
s'évaporent subissent une décomposition photochimique. Ainsi, on a
montré que l'huile lourde No 2 subissait une photo-oxydation rapide en
milieu aqueux dans les conditions naturelles.
Les différents constituants du combustible diesel sont
intrinsèquement biodégradables, mais à des degrés et à des
vitesses variables. Les alcanes normaux ainsi que les dérivés
n-alkylaromatiques et les molécules aromatiques simples en
C10-C22, sont les plus facilement dégradables. Les petites
molécules se métabolisent en général rapidement. Les n-alcanes à
longue chaîne sont plus lentement dégradés en raison de leur
hydrophobicité et du fait qu'ils sont visqueux ou solides à la
température ambiante. Les alcanes ramifiés et les cycloalcanes sont
relativement résistants à la décomposition biologique et les
hydrocarbures aromatiques polycycliques, franchement résistants. La
vitesse globale de dégradation des hydrocarbures est limitée par la
température, la teneur en eau et en oxygène, le pH, la présence de
nutriments inorganiques et la versatilité métabolique microbienne.
Les algues unicellulaires peuvent fixer et métaboliser les
hydrocarbures aliphatiques et aromatiques mais on connaît mal
l'ampleur de ce phénomène. Contrairement aux microorganismes qui
utilisent les hydrocarbures du pétrole comme source de carbone, le
métabolisme animal a généralement tendance à oxyder et à conjuguer les
produits pour les transformer en substances plus solubles et donc plus
faciles à excréter. Toutes les espèces animales étudiées sont capables
de fixer des hydrocarbures du pétrole. On sait que les hydrocarbures
aromatiques polycycliques, le pétrole brut et les produits pétroliers
raffinés induisent les enzymes du cytochrome P450 et chez de
nombreuses espèces de poissons de mer et d'eau douce, on constate
qu'il y a accroissement du métabolisme des hydrocarbures.
On n'a que peu de données sur la bioaccumulation du combustible
diesel dans les conditions du laboratoire mais il est largement
prouvé, par l'étude des nappes de mazout et d'autres études de
laboratoire consacrées à ce genre de produits, en particulier le No 2,
que les organismes aquatiques concentrent les hydrocarbures. Le
coefficient de partage du combustible diesel entre le n-octanol et
l'eau est égal à 3,3-7,06, ce qui incite à penser que son potentiel de
bioaccumulation est élevé; quoi qu'il en soit, de nombreux composés de
faible masse moléculaire relative sont facilement métabolisés et la
bioaccumulation effective des produits de masse moléculaire relative
plus élevée est limitée par leur faible solubilité dans l'eau et les
dimensions importantes de leur molécule. Il en résulte donc que la
bioaccumulation peut en réalité être faible.
On a constaté qu'après des déversements de combustible diesel, le
poisson pouvait devenir inconsommable. On ne dispose d'aucune donnée
sur la bioamplification du combustible diesel.
On ne possède non plus aucune donnée expérimentale sur le
déplacement du combustible diesel à travers le sol, encore qu'il ait
été avancé qu'il existait une corrélation directe entre ce déplacement
et la viscosité cinématique du produit. Le déplacement du kérosène
dans un sol dépend de la teneur en eau et de la nature de ce sol.
A1.4 Concentrations dans l'environnement et exposition humaine
Les divers types de combustible diesel étant constitués de
mélanges complexes, on n'en n'a pas mesuré la concentration dans
l'environnement. On peut mettre en évidence la présence de leurs
divers constituants dans presque tous les compartiments du milieu,
même s'il n'est pas possible d'en vérifier l'origine. Lorsqu'il y a
exposition de la population générale, elle se produit dans les
stations service ou par suite de déversements.
Il peut y avoir exposition professionnelle au combustible diesel
à la faveur d'un grand nombre d'activités. Du fait de leur faible
volatilité, ces combustibles ne devraient produire qu'une vapeur assez
ténue à la température normale mais si l'on opère à température
élevée, la concentration peut augmenter sensiblement.
A1.5 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Les combustibles diesel présentent une faible toxicité aiguë
après administration par voie orale, percutanée ou respiratoire. Chez
toutes les espèce étudiées (souris, lapin, rat, cobaye) on a obtenu
pour la DL50 par voie orale une valeur > 5000 mg/kg de poids
corporel. En application cutanée, on obtient une valeur de la DL50
également > 5000 mg/kg de poids corporel chez la souris et le lapin,
mais des valeurs > 2000 mg/kg de poids corporel ont été relevées pour
certains types de kérosène et de distillats moyens, selon le protocole
d'application et la limite inférieure de la dose. Chez des rats
exposés par la voie respiratoire, on a obtenu une valeur de la CL50
d'environ 5 mg/litre, sauf dans le cas d'un distillat moyen de
première distillation pour lequel on a obtenu une valeur de
1,8 mg/litre.
Chez des lapins badigeonnés avec du combustible diesel à des
doses allant jusqu'à 8000 µl/kg de poids corporel et par jour et chez
des souris traitées dans les mêmes conditions avec des doses
quotidiennes atteignant 40 000 mg/kg de poids corporel, on a observé
une acanthose et une hyperkératose dues à une grave irritation. Les
lapins se sont révélés plus sensibles que les souris. Chez la souris,
l'inhalation de combustible diesel a provoqué des effets
neurodépresseurs à des concentrations allant jusqu'à 0,2 mg/litre; en
revanche ces effets n'ont pas été observés chez des rats exposés à des
doses allant jusqu'à 6 mg/litre. Chez les rats, on a observé une
réduction du poids corporel et du poids du foie.
Après inhalation de doses de combustible diesel allant jusqu'à
1,5 mg/litre dans des conditions de subchronicité, des souris, des
rats et des chiens n'ont pas présenté de signes sensibles de toxicité
cumulative. Le syndrome néphropathique spécifique observé chez les
rats mâles est lié à l'accumulation intrinsèque d'inclusions hyalines
dans les tubules rénaux.
Les seuls effets de l'exposition à long terme ont été des
ulcérations après application cutanée de combustible diesel à des
souris (doses quotidiennes: 250 ou 500 mg/kg de poids corporel) et une
modification importante du poids des organes après inhalation par des
rats de ce même produit à la dose de 1 ou 5 mg/litre. Dans les deux
études, on a constaté une réduction du poids corporel moyen.
Les divers types de combustible diesel se révèlent légèrement à
fortement irritants pour la peau du lapin. Ils ne produisent pas
d'irritation oculaire mais dans le cas d'un certain nombre de
kérosènes, on a fait état d'un léger effet irritant. Il n'y a pas de
sensibilisation cutanée.
Le combustible diesel et les carburéacteurs (kérosène) ne se sont
révélés ni embryotoxiques, ni tératogènes lors de deux études
effectuées sur des rats à qui l'on avait fait inhaler ces produits aux
doses de 100 ou 400 ppm ainsi que dans une autre étude au cours de
laquelle des rats avaient reçu par gavage des doses quotidiennes de
ces produits allant jusqu'à 2000 mg/kg de poids corporel. Dans la
dernière étude, on a observé une réduction du poids des foetus.
Les épreuves effectuées sur Salmonella typhimurium n'ont pas
permis de prouver de manière nette l'existence d'une activité
mutagène. Des résultats positifs ont bien été observés chez
S. typhimurium ainsi que sur des cellules lymphomateuses de souris
mais leur caractère contradictoire les a fait considérer comme
équivoques. Les tests de génotoxicité effectués sur des souris
in vivo (induction de la formation de micronoyaux ou d'aberrations
chromosomiques) ont également donné des résultats équivoques ou
négatifs.
Les combustibles diesel présentent un faible pouvoir cancérogène
au niveau cutané. Dans l'état actuel de la recherche, on ne peut
déterminer si l'activité cancérogène de ces produits est due à leur
génotoxicité ou aux lésions chroniques qu'ils induisent dans le derme.
A1.6 Effets sur l'homme
Il peut y avoir exposition non professionnelle aux combustibles
diesel lors du remplissage manuel de citernes. C'est principalement
lors de déversements accidentels que la peau peut se trouver fortement
exposée à ces produits, encore que pour une brève durée.
A la suite de contacts directs avec le peau on a observé de
l'anurie, une insuffisance rénale, des symptômes gastro-intestinaux
ainsi qu'une hyperkératose cutanée. Des pneumopathies d'aspiration
d'origine toxique ont été observées par suite de l'ingestion
accidentelle de combustible diesel. Après inhalation, on peut observer
une toux grasse persistante. Lors d'une étude cas-témoins portant sur
des hommes exposés à du combustible diesel, on a constaté qu'ils
courraient un risque accru de cancers du poumon, autres que les
adénocarcinomes; on a également observé une association positive avec
le cancer de la prostate, encore que le risque ait été plus important
dans le groupe soumis à une exposition 'non substantielle' que dans
celui qui était soumis à une exposition 'substantielle'.
Lors d'une étude transversale portant sur des ouvriers d'une
usine exposés à des carburéacteurs, on a constaté qu'ils étaient plus
fréquemment sujets à des étourdissements, des maux de tête, des
nausées, des palpitations, une sensation d'oppression thoracique et
une irritation oculaire que les témoins non exposés. La concentration
moyenne pondérée par rapport au temps de la vapeur dégagée par le
combustible au niveau de la zone de respiration avait été estimée à
128-423 mg/m3.
A1.7 Effets sur les autres êtres vivants au laboratoire et dans
leur milieu naturel
Le combustible diesel est plus toxique que le pétrole brut pour
les animaux et les plantes aquatiques. L'écotoxicité du combustible
diesel est généralement attribuée à la présence de composés
aromatiques solubles, mais les hydrocarbures aliphatiques insolubles
peuvent également jouer un rôle. Parmi les composés aromatiques, les
dérivés monocycliques sont les moins toxiques, leur toxicité aiguë
augmentant avec la masse moléculaire jusqu'aux composés tétra- ou
pentacycliques, encore que ces derniers soient peu solubles dans l'eau
de mer. Certains animaux, comme les poissons et les oiseaux, peuvent
avoir le corps enduit de produit, avec des effets toxiques parfois
mortels.
On a étudié en laboratoire le combustible diesel et notamment ses
fractions solubles dans l'eau, les dispersions huile-eau et le pétrole
microencapsulé. Il apparaît que le combustible diesel ne réduit pas
sensiblement la croissance des cultures d'algues vertes du genre
Euglena gracilis, mais à faible concentration (0,1%), il inhibe
presque complètement la croissance de Scenedesmus quadricauda. Le
diesel léger (0,05%) stimule la croissance, la photosynthèse et la
synthèse de la chlorophylle a chez Chlorella salina, mais il en
inhibe légèrement la respiration. A concentration plus élevée, le taux
de croissance et la photosynthèse sont fortement réduits. Une
exposition de longue durée inhibe la croissance des algues benthiques
comme Ascophyllum nodosum et Laminaria digitata. Chez les algues
bleues, la photosynthèse est réduite par les fractions aromatiques et
asphaltiques, mais pas par la fraction aliphatique.
Le combustible diesel est fortement toxique pour les daphnies,
pour les larves de chironomidés et pour le mollusque Viviparus
bengalensis (un gastéropode). A la concentration de 0,1 ml/litre, il
a provoqué la mort de copépodes du genre Tigriopus californicus,
en l'espace de cinq jours.
Les moules du genre Mytilus edulis, accumulent le combustible
diesel, avec pour conséquence une réduction marquée de leur rythme
d'alimentation et de croissance; leur reproduction souffre également
d'une exposition de longue durée à ce produit. La CE50 relative au
frai des moules exposées pendant 30 jours à du combustible diesel, a
été trouvée égale à 800 µg/litre. La CL50 de gazole microencapsulé a
été égale à 5000 µg/litre pour des moules en maturation exposées
pendant 30 jours. Le gazole s'est révélé plus toxique pour les larves
que pour les jeunes moules: il avait un effet nocif sur la croissance
des larves à la dose de 10 µg/litre.
Des crabes d'eau douce (Barytelphusa cunicularis) exposés à des
concentrations sublétales de combustible diesel pendant des durées
allant jusqu'à 96 heures, ont généralement réduit leur consommation
d'oxygène, en particulier aux concentrations les plus faibles et pour
des durées d'exposition allant jusqu'à 8 heures. Lorsque l'exposition
se prolongeait, la consommation d'oxygène était égale ou supérieure à
celle des animaux témoins.
Lors d'épreuves de 96 heures visant à évaluer la toxicité aiguë
du produit sur des alevins de salmonidés dans des conditions
statiques, on a constaté que le combustible diesel était plus toxique
pour Onchorhychus gorbuscha (CL50: 32-123 mg/litre), que pour
O. kisutch (CL50: 2186-3017mg/litre) ou pour la truite arc-en-ciel
O. mykiss (CL50: 3333-33 216 mg/litre) quel que soit le type
d'eau.
Le seuil de détection des réactions comportementales de la morue
( Gadus marhua L.), exposée à du combustible diesel dans de l'eau de
mer, a été trouvé égal à 100-400 ng/litre. Un poisson de
l'antarctique, Pagothenia borchgrevinki, a résisté pendant des
durées allant jusqu'à 72 heures à la fraction hydrosoluble non diluée
du combustible diesel, tout en présentant cependant des signes de
stress.
Dans le cas des oiseaux, il peut y avoir contamination externe ou
interne par les produits pétroliers. Le combustible diesel supprime
l'hydrophobicité du plumage et peut être ingéré lorsque l'oiseau lisse
ses plumes. Du combustible diesel et du mazout administrés par gavage
à des canards à la dose de 2 ml/kg de poids corporel, a provoqué au
bout de 24 heures une stéatose, une inflammation extrême des poumons,
une infiltration graisseuse du foie et une dégénérescence hépatique.
L'administration de combustible diesel ou de mazout à la dose de
1 ml/kg a également entraîné une grave irritation des voies digestives
et une néphrose toxique. A doses plus élevées, on a observé une
hypertrophie des surrénales (due principalement à une hyperplasie du
tissu cortical), une chute du taux de la cholinestérase plasmatique,
de l'ataxie et des tremblements. Jusqu'à 20 ml/kg de poids corporel,
la contamination n'a pas été mortelle pour les oiseaux en bonne santé
mais la DL50 s'abaissait à 3-4 ml/kg de poids corporel lorsque le
combustible diesel ou le mazout était administré à des oiseaux
stressés.
En cas de déversement de combustible diesel, le zooplancton se
révèle extrêmement vulnérable aux constituants dispersés ou dissous du
pétrole, mais moins aux nappes d'huile flottante. Pour les organismes
aquatiques, la nocivité de ces produits peut se manifester de diverses
manières: mortalité directe (oeufs de poisson, copépodes et plancton),
contamination externe (chorions des oeufs de poisson ou cuticules et
appendices buccaux des crustacés), contamination tissulaire par des
constituants aromatiques, développement anormal des embryons de
poisson et perturbation du métabolisme.
A1.8 Evaluation des risques pour la santé humaine
Il peut y avoir exposition de la population générale au
combustible diesel et à d'autres distillats moyens sur les lieux et
dans les circonstances suivants: dans les stations service, lors de
déversements accidentels, lors de la manipulation de ces combustibles
et lors de l'utilisation de pétrole lampant pour la cuisine ou le
chauffage. Il peut y avoir exposition des travailleurs à ces produits
lors de la manipulation et du transvasement du combustible dans les
terminaux, les citernes et les stations services; lors de la
fabrication, de la réparation, de l'entretien et de l'essai des
moteurs diesel et autres matériels; lors de l'utilisation de
combustible diesel pour le nettoyage ou comme solvant ou encore lors
de la manipulation des prélèvements de routine au laboratoire. En
raison de sa faible volatilité, le produit ne devrait pas émettre de
vapeurs très denses à la température ambiante, encore que dans un
espace confiné et à température élevée, la vapeur puisse être plus
concentrée.
Lors de la manipulation normale du combustible diesel,
l'exposition aux vapeurs est minime. L'effet le plus probable sur la
santé humaine consiste en une dermatite de contact. Le combustible
diesel est irritant pour la peau mais il ne semble pas irriter la
muqueuse oculaire. Des effets toxiques aigus au niveau rénal peuvent
s'observer après exposition cutanée, mais on ignore quels peuvent être
les effets à long terme d'une absorption percutanée de faibles
concentrations.
L'ingestion de combustible diesel peut avoir des effets toxiques
qui se traduisent quelquefois par une régurgitation et une aspiration
pouvant provoquer une pneumonie chimique, comme dans le cas de tout
hydrocarbure entre certaines limites de viscosité.
Chez des rongeurs exposés par la voie respiratoire à du
combustible diesel à des concentrations allant jusqu'à 0,2 mg/litre,
on a observé un effet neurodépresseur dans le cas de souris, mais pas
chez des rats, mêmes aux concentrations les plus élevées. Chez des
rats mâles, une exposition subchronique par la voie respiratoire à
divers distillats a produit une néphropathie spécifique à
alpha2-microglobulines; cette observation n'est pas considérée comme
transposable à l'homme.
Les combustibles diesel ne se sont révélés ni embryotoxiques ni
tératogènes chez les animaux exposés par la voie orale ou par la voie
respiratoire.
Rien n'indique de façon nette qu'il existe une activité mutagène
chez les bactéries, mais les résultats d'autres tests de génotoxicité
in vitro et in vivo sont plutôt équivoques.
Un étude cas-témoins portant sur des ouvriers exposés à du
combustible diesel incitent à penser qu'il existe un risque accru de
cancers du poumon n'appartenant pas au type épithélium glandulaire
ainsi que de ce cancer de la prostate. Il n'a pas été possible
d'établir de relation dose-réponse pour l'un ou l'autre de ces cas. En
raison du petit nombre d'études disponibles, du petit nombre de cas et
par voie de conséquence, de l'étendue de l'intervalle de confiance, il
n'est pas possible de tirer la moindre conclusion de ces données au
sujet de la cancérogénicité du produit pour l'homme. Chez la souris,
du combustible diesel administré par voie intradermique a présenté une
faible activité cancérogène. En raison de l'absence d'une activité
génotoxique avérée, il est possible d'invoquer un mécanisme non
génotoxique pour ces cancers; il pourrait s'agir par exemple d'une
irritation dermique chronique caractérisée par la répétition de
lésions cutanées entraînant une hyperplasie de l'épiderme.
A1.9 Evaluation des effets sur l'environnement
Il peut y avoir pollution de l'environnement par libération
accidentelle à grande échelle de combustible diesel, comme cela peut
se produire lors catastrophes affectant des réservoirs ou encore en
cas de fuites d'oléoduc ou, à plus petite échelle, lorsqu'il y a
contamination du sol aux alentours d'une usine ou d'un garage. Dans
l'eau, le combustible diesel s'étale presque immédiatement, les
composants polaires de masse moléculaire relative peu élevée se
dissolvent et disparaissent pas lessivage, les constituants volatils
s'évaporent et la décomposition microbienne s'amorece. L'élimination
des différents constituants dépend de la température et des conditions
climatiques. La composition chimique de la nappe varie dans le temps:
une fois répandues dans l'eau, certaines fractions s'évaporent et
subissent une décomposition photochimique; les fractions lourdes,
transportées par les particules qui se déposent, parviennent jusqu'aux
sédiments du fond; dans le sol les constituants du combustible diesel
migrent à des vitesses variables, en fonction de la nature de ce sol.
Les divers constituants du combustible diesel sont
intrinsèquement biodégradables mais leur vitesse de biodécomposition
dépend largement des conditions physiques et climatiques et de la
composition microbiologique du milieu.
Des organismes aquatiques et en particulier les mollusques,
accumulent les hydrocarbures à des degrés divers mais ceux-ci peuvent
être éliminés par passage dans l'eau claire. Le combustible diesel
peut subir une bioaccumulation; en revanche on ne dispose d'aucune
donnée sur une bioamplification éventuelle.
Tout déversement de combustible diesel a un effet nocif immédiat
sur l'environnement, qui se traduit par une mortalité notable pour la
faune et la flore. Il peut y avoir recolonisation au bout d'une année,
selon l'espèce animale ou végétale en cause et selon la composition
chimique et physique des résidus de produits pétroliers.
Les organismes aquatiques qui survivent aux déversements de
combustible diesel peuvent cependant subir une contamination externe
par ces produits et en accumuler dans leurs tissus: le stress qui en
résulte se traduit par un développement anormal et une modification du
métabolisme
PARTIE B GAZ D'ECHAPPEMENT DES MOTEURS DIESEL
B1. RESUME
B1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Les gaz d'échappement des moteurs diesel contiennent des
centaines de composés chimiques qui sont émis en partie dans la phase
gazeuse proprement dite et en partie dans la phase particulaire. Les
principaux produits gazeux de la combustion sont le dioxyde de
carbone, l'oxygène, l'azote et la vapeur d'eau: on trouve également du
monoxyde de carbone dixoyde de soufre, des oxydes d'azote et des
hydrocarbures et leurs dérivés. La phase gazeuse de la fraction
hydrocarbonée contient également un faible pourcentage en poids de
benzène et de toluène. Les autres constituants des gaz d'échappement
sont des hydrocarbures aromatiques polycycliques (HAP) de faible masse
moléculaire relative.
L'échappement des moteurs diesel est principalement caractérisé
par l'émission de particules dans une proportion environ 20 fois
supérieure à celle des moteurs à essence. Ces particules sont
composées de carbone élémentaire, de dérivés organiques adsorbés
provenant du combustible et de l'huile lubrifiante, de sulfates formés
à partir du soufre contenu dans le combustible et de dérivés
métalliques à l'état de traces. La granulométrie globale de ces
particules les situe en dessous du micron, entre 0,02 et 0,5 µm. Le
vieillissement peut conduire à une agglomération qui les amène à un
diamètre maximal de 30 µm. Les particules émises ont une aire
superficielle importante. Les composés organiques constituent
généralement 10 à 30% des particules totales mais avec des moteurs mal
conçus et mal entretenus cette proportion peut atteindre 90%. Dans
cette fraction on trouve également des hydrocarbures aromatiques
polycycliques de masse moléculaire élevée, entre autres, sous forme
oxygénée et nitrée, à des concentrations de l'ordre de plusieurs
parties par million.
Pour mesurer les émissions des véhicules on travaille en régime
transitoire ou stationnaire. L'échantillonnage peut se faire à partir
de gaz d'échappement dilués ou non dilués. Il est difficile d'obtenir
des échantillons débarrassés de tout artéfact car les constituants
peuvent subir des réactions chimiques, être adsorbés ou désorbés, ou
encore soumis à une condensation ou à une diffusion. Les hydrocarbures
aromatiques polycycliques d'importance toxicologique qui sont présents
dans les particules émises par les moteurs diesel sont généralement
dosées selon la procédure suivante: extraction au Soxhlet,
purification et fractionnement puis analyse finale par chromatographie
liquide à haute performance ou chromatographie en phase gazeuse
couplée à la spectométrie de masse.
B1.2 Sources d'exposition humaine et environnementale
Les gaz d'échappement des moteurs diesel proviennent
essentiellement des véhicules à moteur; parmi les autres source on
peut citer les installations fixes, le matériel de traction
ferroviaire et les machines de navires. Les émissions de ces véhicules
à moteurs diesel ont été bien décrites mais les résultats obtenus pour
chaque catégorie particulière ne sont souvent pas comparables en
raison de différences concernant certains paramètres comme le cycle de
fonctionnement, le type de moteur et la composition du combustible.
Les divers constituants sont émis dans les proportions suivantes:
dioxyde de carbone, environ 1 kg/km; monoxyde de carbone, oxydes
d'azote, hydrocarbures gazeux totaux et particules 0,1-20 g/km;
dérivés aliphatiques, alcools, aldédhydes, hydrocarbures aromatiques
légers et hydrocarbures aromatiques polycycliques, quelques
microgrammes/km. Dans un certain nombre de pays, il y a réglementation
des émissions de monoxyde de carbone, d'oxyde d'azote, d'hydrocarbures
gazeux totaux et de particules.
En principe, il n'y a aucune différence entre les moteurs de
faible ou de forte puissance pour ce qui est de la nature et de la
quantité des émissions, encore que les véhicules lourds aient tendance
à dégager une quantité relativement plus importante de particules. Les
émissions dépendent du cycle de fonctionnement du moteur (régime
transitoire ou régime stationnaire), du type et de l'état du moteur
(injection ou aspiration, entretien, kilométrage total) et de la
composition du combustible (teneur en soufre, teneur en composés
aromatiques, volatilité); le réglage du moteur jour un rôle très
important.
Le dégagement de particules augmente en raison inverse du rapport
air/combustible, mais en raison directe de la charge et de la
température. Les moteurs vieillissants, soumis à une utilisation
intense, libèrent plus de particules que les moteurs récents, qui ont
peu de kilométrage, probablement du fait de la plus grande
consommation d'huile lubrifiante. Dans le cas des véhicules légers à
moteur diesel, il y a également une corrélation entre l'émission de
particules et la teneur en soufre du combustible, car la formation de
sulfates métalliques accroît la masse des particules. Dans le cas des
véhicules lourds, ce type de corrélation n'a pas encore été établi.
Entre outre, plus la teneur du combustible en dérivés aromatiques est
élevée, plus le moteur dégage de particules.
Les hydrocarbures aromatiques polycycliques, oxygénés ou non,
émis par les moteurs diesel et les moteurs à allumage commandé, sont
de nature similaire. Des hydrocarbures aromatiques polycycliques
oxygénés ou nitrés sont émis à raison de quelques microgrammes/km,
mais la concentration effective de ces composés n'est pas connue avec
certitude car il peut y avoir décomposition ou formation lors de
l'échantillonnage. Les émissions de HAP augmentent avec la charge et
la température ainsi qu'avec l'âge du moteur, probablement du fait
d'une consommation plus importante d'huile lubrifiante. Les émissions
d'HAP dépendent également de la technique d'injection utilisée dans le
moteur: ces émissions sont proportionnelles au rapport air/combustible
dans les moteurs à injection directe mais la situation est inversée
dans le cas des moteurs à injection indirecte. La teneur en dérivés
aromatiques et la volatilité du combustible sont directement liées aux
émissions de HAP. En cas de mauvais fonctionnement de certains
éléments du moteur, en particulier des injecteurs, il y a augmentation
de l'émission des principaux constituants des gaz d'échappement. On ne
possède guère de données sur la contribution des moteurs diesel aux
émissions totales de produits de combustion d'origine artificielle.
Les émissions des moteurs diesel peuvent être réduites moyennant
une meilleure conception du moteur, la pose de pièges à particules
(pièges oxydants) et de convertisseurs catalytiques. Alors que les
pièges à particules éliminent la suie et les composés organiques
solubles adsorbés sur les particules, les convertisseurs catalytiques
réduisent principalement la teneur en oxyde de carbone et
hydrocarbures gazeux. En pratique, il est difficile de régénérer les
pièges à particules. Les convertisseurs catalytiques nécessitent
l'utilisation de combustibles à faible teneur en soufre car le soufre
empoisonne les centres actifs du catalyseur.
B1.3 Transport, distribution et transformation dans l'environnement
C'est principalement l'atmosphère qui est affectée par les
émissions de moteurs diesel. L'hydrosphère et la géosphère peuvent
être contaminées indirectement par des dépôts secs ou humides. La
destinée environnementale des divers constituants des gaz
d'échappement des moteurs diesel est en général bien connue: les
particules se comportent comme les molécules de gaz non réactives pour
ce qui est de leur transport mécanique dans l'atmosphère; elles
peuvent être transportées sur de longues distances et même pénétrer
dans la stratosphère. On pense que leur vitesse d'élimination est
faible, ce qui fait qu'elles séjournent plusieurs jours dans
l'atmosphère. Avec le temps, ces particules peuvent s'agglomérer, et
leur vitesse de chute augmentant, la quantité restante aéroportée
diminue. Le carbone élémentaire présent dans des particules émises par
les moteurs diesel peut catalyser la formation d'acide sulfurique par
oxydation du dioxyde de soufre. Les constituants organiques adsorbés à
la surface des particules de carbone élémentaire peuvent subir un
certain nombre de transformations et de rections chimiques avec
d'autres composés atmosphériques ou par exposition à la lumière
solaire.
B1.4 Concentrations dans l'environnement et exposition humaine
Etant donné que les gaz d'échappement des moteurs diesel sont des
mélanges complexes contenant des composés très divers, on ne peut pas
définir de 'concentration dans l'environnement'. On devrait pouvoir
mettre en évidence la présence de ces divers constituants dans tous
les compartiments de l'environnement, encore qu'il ne soit pas
généralement possible d'en déterminer l'origine. On connaît la
concentration dans l'environnement de la plupart de ces constituants.
C'est le plus probablement dans les rues très passantes ou dans
les parkings, en particulier souterrains, que la population générale a
le plus de chances d'être exposée aux émissions des moteurs diesel. Il
est difficile d'identifier les sources de pollution et on calcule
généralement la contribution des gaz d'échappement des moteurs diesel
à la pollution totale due à la circulation des véhicules à moteur, sur
la base des facteurs d'émission et du pourcentage de véhicules à
moteur diesel dans le parc automobile général. Le niveau d'exposition
de la population générale et des travailleurs aux particules émises
par les moteurs diesel est toxicologiquement significatif.
Les concentrations quotidiennes moyennes ambiantes de particules
à proximité des voies de communication sont de 8-42 µg/m3. On a
calculé que les concentrations annuelles moyennes de particules
étaient de 5 à 10 µg/m3 dans les zones urbaines et < 1,5 µg/m3
dans les zones rurales d'Allemagne et de 1 à 2 µg/m3 dans les zones
urbaines et 0,6-1 µg/m3 dans les zones rurales des Etats-Unis
d'Amérique. Ces concentrations sont directement corrélées à la densité
de la circulation automobile et diminuent à mesure qu'on s'éloigne des
routes.
Il peut être également difficile de déterminer l'origine de la
pollution sur les lieux de travail, en particulier dans les mines où
la quantité totale de poussière est élevée. Le dosage du carbone
particulaire a permis de déterminer l'exposition spécifique aux
émissions de moteurs diesel sur les lieux de travail et il en ressort
que les travailleurs seraient exposés à des concentrations de
particules de l'ordre de 0,04 à 0,134 mg/m3 pour les camionneurs et
de 0,004 à 0,192 mg/m3 pour les cheminots. On a également eu recours
au dosage des particules respirables totales et des particules totales
en suspension pour évaluer l'exposition professionnelle.
B1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
B1.5.1 Dépôt
Les particules émises par les moteurs diesel, dont le diamètre
aérodynamique médian massique est égal à environ 0,2 µm, sont quelque
peu filtrés par le nez et leur aptitude au dépôt dans les poumons
n'est que légèrement supérieure à la valeur minimale trouvée pour les
particules de diamètre aérodynamique médian massique égal à environ
0,5 µm. Autrement dit, lorsque les particules de suie émises par les
moteurs diesel sont inhalées, 10 à 15% d'entre elles se déposent au
niveau des alvéoles chez le rat et le cobaye, le dépôt au niveau des
alvéoles étant d'environ 10% chez l'homme.
B1.5.2 Rétention et élimination des particules
L'élimination des particules par l'ascenseur muco-ciliaire est
pratiquement complète au bout de 24 heures. L'élimination à long terme
des particules déposées dans la zone alvéolaire a fait l'objet de
plusieurs études sur des rats qui avaient été exposés par la voie
respiratoire à deux types de particules: les particules émises par un
moteur diesel et les particules servant de référence. Dans le cas des
témoins chez lesquels la charge pulmonaire était faible (< 1 mg par
poumon), on a obtenu des temps de demi-élimination de l'ordre de 60 à
100 jours, alors que chez les rats présentant une charge pulmonaire
allant de 1 à 60 mg/poumon, le temps de demi-élimination était de
l'ordre de 100 à 600 jours. Dans plusieurs études, on a constaté que
les effets observés étaient dus à une surcharge en particules,
laquelle a été décrite chez diverses espèces et pour un certain nombre
de matériaux particulaires. Ce phénomène s'observe généralement
lorsque la vitesse de dépôt des particules de faible solubilité et de
faible toxicité aiguë reste supérieure à leur vitesse d'élimination
pendant une très longue durée. Chez l'homme, le temps de
demi-élimination alvéolaire normal est de plusieurs centaines de
jours, c'est-à-dire plus long que chez le rat.
On a mis au point un modèle pulmonaire dosimétrique sur la base
des données de dépôt et de rétention des particules diesel chez le rat
après inhalation de longue durée et des mêmes données chez l'homme. Ce
modèle peut être utilisé pour prévoir la rétention des particules
diesel et des composés organiques adsorbés dans les poumons de
personnes d'âges divers. On ne dispose d'aucune donnée sur la manière
dont la rétention de différents composés évolue après une exposition
prolongée aux gaz d'échappement de moteurs diesel.
B1.5.3 Rétention et élimination des hydrocarbures aromatiques
polycycliques adsorbés sur la suie de moteurs diesel
Les hydrocarbures aromatiques polycycliques présents dans la suie
émise par les moteurs diesel adhèrent fortement à la surface des
particules. Environ 50% des HAP adsorbés sur les particules sont
éliminés par les poumons dans l'espace d'une journée, mais le temps de
demi-rétention de la fraction restante s'est révélé égal à 18-36
jours. Des études portant sur du 3H-benzo[ a]pyrène et du
14C-nitropyrène ont montré que lorsqu'ils sont associés à des
particules, les HAP sont sensiblement plus longs à être éliminés des
poumons que leurs homologues libres.
B1.5.4 Métabolisme
Déposé sur des particules émises par un moteur diesel, du
benzo[ a]pyrène a subi une métabolisation oxydative au niveau du
poumon et dans des cultures cellulaires de macrophages pulmonaires
aboutissant à la formation de phénols, de diols et de quinones
substituant le noyau benzo[ a]pyrène. Du nitropyrène adsorbé sur des
particules diesel a été métabolisé en acétylaminopyrène-phénol après
inhalation. Chez des rats exposés à des émissions de moteur diesel on
a constaté, dans les poumons et les cellules de type II, un
accroissement des adduits de l'ADN par rapport aux témoins. Le
métabolisme oxydatif de certains composés organiques qui conduit à des
époxydes pourrait être responsable de la formation des adduits, mais
il est vrai qu'on ne les trouve qu'après exposition à des particules.
Certains dérivés organiques présents dans les émissions de moteur
diesel se sont révélés capables de former des adduits avec l'ADN;
toutefois le noyau carboné lui-même (sans composés organiques
extractibles) peut également conduire à la formation d'adduits, en
provoquant des lésions chroniques au niveau des cellules épithéliales.
B1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Les quelques données disponibles incitent à penser que les
émissions de moteur diesel ne présentent qu'une faible toxicité aiguë.
Des souris auxquelles on avait administré par voie intratrachéale, des
particules émises par un moteur diesel, sont mortes des suites d'une
oedème du poumon. La DL50 était égale à environ 20 mg/kg de poids
corporel. Des particules du même type, extraites au méthanol, n'ont
entraîné aucune mortalité jusqu'à une concentration d'environ 33 mg/kg
de poids corporel. Chez le hamster, on a obtenu une DL50 de
1280 mg/kg de poids corporel après administration par voie
intrapéritonéale. On ne dispose d'aucune donnée concernant
l'exposition par la voie respiratoire.
Après exposition de rats, de cobayes et de chats pendant environ
quatre semaines à des émissions de moteur diesel ayant une teneur en
particules de 6 mg/m3, on a observé une altération de la fonction
pulmonaire, et notamment une augmentation de 35% de la résistance
ventilatoire chez les cobayes et une diminution de 10% de la capacité
vitale chez les chats. Du point de vue histopathologique, on a observé
un épaississement focal des parois alvéolaires, un accroissement
significatif de l'indice de marquage des cellules de type II et des
accumulations de macrophages chargés de particules. Ces accumulations
se situaient au niveau des bronchioles terminales et elles ont
augmenté de volume lors de la période de récupération postérieure à
l'expérience en raison de la fixation des macrophages (séquestration).
Après avoir inhalé pendant de longues périodes des émissions de
moteur diesel à des concentrations atteignant 4 mg/m3, des rats, des
souris, des hamsters, des chats et des singes n'ont pas présenté de
chute spectaculaire de leur poids corporel ni de réduction de leur
longévité. Les effets toxiques liés à la dose qui ont été observés
chez toutes les espèces après une longue période d'inhalation étaient
les suivants: augmentation du poids des poumons pouvant aller jusqu'à
400%; inflammation pulmonaire objectivée par des paramètres
biochimiques (marqueurs enzymatiques cytoplasmiques, collagène) et
cytologiques (augmentation des neutrophiles polynucléaires);
perturbation de la mécanique pulmonaire; accroissement du nombre de
macrophages chargés de particules avec accumulations focales
(séquestration) dans des conditions de surcharge; enfin, altérations
prolifératives des cellules épithéliales et début de fibrose.
D'après les données limitées dont on dispose sur la toxicité des
émissions de moteurs diesel pour la fonction de reproduction et le
développement, on peut penser qu'il n'y a pas d'effets toxiques
déterminants. Dans la plupart des expériences, on n'a pas constaté
d'effets chez des souris, des rats, des hamsters, des lapins ou des
singes; toutefois, après injection par voie intrapéritonéale, on a
observé chez des souris qui avaient reçu de particules émises pas des
moteurs diesel, des anomalies touchant les spermatozoïdes et constaté
une certaine embryotoxicité chez des hamsters auxquels on avait
administré des extraits d'émissions de ces mêmes moteurs.
La plupart des épreuves de génotoxicité in vitro ont été
réalisées avec des extraits d'émissions de moteurs diesel plutôt
qu'avec les émissions totales elles-mêmes et l'on a obtenu des
réactions positives en l'absence d'activation métabolique. Autrement
dit, il semble que ces effets génotoxiques soient indépendants de la
présence d'HAP. Environ 50% des études in vivo ont donné des
résultats négatifs; les seuls résultats positifs obtenus consistaient
en échanges de chromatides soeurs dans le cas des émissions totales et
des extraits organiques, et dans la présence de micronoyaux dans le
cas des extraits organiques.
On n'a généralement pas observé d'effets immunotoxiques après
inhalation d'émissions de moteurs diesel; toutefois on a observé dans
une étude une augmentation du titre des immunoglobulines
anti-ovalbumine et deux autres expériences ont mis en évidence un
accroissement de la sensibilité aux infections chez des souris.
Des études effectuées sur des rats incitent à penser que
l'inhalation de gaz d'échappement de moteurs diesel affecte l'état
comportemental et neurophysiologique.
Lors d'études sur la cancérogénicité des émissions de moteur
diesel, au cours desquelles des rats étaient exposés à ces émissions
par la voie respiratoire, on a constaté que la phase gazeuse
(c'est-à-dire dépourvue de particules) n'était pas cancérogène. Toutes
les études sur des rats qui ont été validées ont montré que les
émissions de moteurs diesel avaient un effet cancérogène lorsque la
concentration en particules était supérieure à 2 mg/m3, soit
l'équivalent d'une exposition continue à une concentration d'environ
1 mg/m3. Aucun effet n'a été observé en revanche chez les hamsters
et les souris. Lors d'études au cours desquelles on a pratiqué une
instillation intratrachéale, on a constaté que les particules
présentes dans les émissions de moteurs diesel et le noir de fumée
produisaient des tumeurs; en outre on a observé l'existence d'une
corrélation entre l'aire superficielle des particules carbonées et
leur activité tumorigène.
L'inhalation, pendant une longue période, de noir de fumée
pratiquement dépourvu d'hydrocarbures aromatiques polycycliques à ces
concentrations, a également provoqué des tumeurs pulmonaires chez le
rat.
On ne sait pas avec certitude si la cancérogénicité des émissions
de moteurs diesel est due à un mécanisme faisant intervenir ou non des
réactions au niveau de l'ADN (ou à un mécanisme mixte). Différents
modèles ont été proposés pour élucider la cancérogénicité des
émissions de moteurs diesel.
B1.7 Effets sur l'homme
Les émissions de moteurs diesel contribuent à la pollution
globale de l'air. Les études toxicologiques, qu'elles cherchent à
mettre en évidence des effets aigus ou des effets chroniques, ne
permettent pas d'assigner un rôle particulier à ces particules mais il
apparaît qu'elles pourraient être en partie responsables d'un certain
nombre d'effets attribués à la pollution de l'air.
Les émissions de moteur diesel ont des caractéristiques qu'un
certain nombre de personnes trouvent agressives, en particulier à
fortes concentrations. Les symptômes observés après exposition de
brève ou de longue durée à ce type d'émissions ont été décrits dans un
certain nombre d'études et de rapports concernant des personnes
exposées de par leur profession. Les effets aigus consistent en
irritation des muqueuses oculaires et nasales et l'on a observé dans
les cohortes professionnelles une augmentation de la fréquence des
symptômes respiratoires; toutefois on ignore qu'elle est la
contribution exacte des particules émises par les moteurs diesel à ce
type de symptômes. Aucun effet à court terme n'a été régulièrement
observé au niveau de fonction pulmonaire, mais on a signalé des crises
d'asthme.
Lors d'une étude contrôlée au cours de laquelle huit volontaires
non fumeurs, en bonne santé, ont été exposés à des émissions diluées
de moteurs diesel, pendant 60 minutes dans une chambre fermée, on a
constaté une réduction du taux de phagocytose chez des macrophages
alvéolaires recueillis par lavage broncho-alvéolaire.
Un certain nombre d'études transversales et longitudinales
portant sur des ouvriers longtemps exposés de part leur profession à
des émissions de moteur diesel, ont révélé une altération de la
fonction pulmonaire et une augmentation de la prévalence des symptômes
respiratoires, toutefois la brièveté des épisodes d'exposition limite
la portée de ces études. Aucune surmortalité n'a été constatée lors
d'études de cohorte au cours desquelles on s'est efforcé d'étudier des
décès par maladie cardio-vasculaire ou accident vasculaire cérébral,
imputables à l'exposition des émissions de moteurs diesel.
Lors d'un certain nombre d'études épidémiologiques on a tenté
d'établir des relations entre certains cancers du poumon et de la
vessie et une exposition professionnelle à des émissions de moteurs
diesel. Seules les études jugées utiles pour l'évaluation des effets
cancérogènes de ces émissions ont été prises en considération dans la
présente monographie. Celles qui sont les plus intéressantes pour ce
qui est du cancer du poumon, concernent des cheminots, des personnes
travaillant dans des garages de cars et des débardeurs qui constituent
des cohortes dont les membres sont effectivement exposés aux émissions
de moteur diesel. Les quatre études les plus informatives font toutes
état d'un risque accru de cancer du poumon, avec un risque relatif
allant de 1,4 pour les cheminots, et de 1,3-2,4 pour les ouvriers des
garages (selon le type d'exposition), jusqu'à un risque trois à six
fois plus élevé pour les débardeurs (en fonction du mode d'évaluation
de l'exposition, mais avec un large intervalle de confiance). Il a été
possible de tenir compte du tabagisme dans une étude cas-témoins sur
des cheminots et dans une étude sur des débardeurs. Dans les deux cas,
cette correction pour tenir compte du tabagisme n'a eu aucune
influence sur l'effet de l'exposition aux émissions diesel. Dans les
trois études pour lesquelles on n'a pas pu tenir compte du tabagisme,
l'analyse était basée sur des comparaisons entre les différents
sous-groupes de ces cohortes, de sorte que l'effet de confusion créé
par le tabagisme avait moins de chance d'être gênant qu'en cas de
comparaison avec des groupes extérieurs.
Plusieurs études cas-témoins ont été menées afin d'examiner la
relation pouvant exister entre les cancers de la vessie et une
exposition supposée à des émissions de moteurs diesel. On a constaté
un accroissement du risque, en particulier pour le chauffeurs de
camion; toutefois toutes ces études souffrent d'une caractérisation
insuffisante de l'exposition. De la sorte, il n'est pas possible
d'affirmer qu'une exposition aux émissions de moteurs diesel entraîne
un risque accru de cancer vésical.
B1.8 Effets sur les autres êtres vivants au laboratoire et dans
leur milieu naturel
Les effets des émissions de moteur diesel n'ont été abordés que
dans une seule étude, portant sur des algues vertes.
B1.9 Evaluation des risques pour la santé humaine
L'évaluation des risques de cancer et de maladies non malignes a
été menée conformément au modèle d'évaluation des risques établi par
l'Académie nationale des sciences des Etats-Unis (National Research
Council, 1983). Ce modèle d'évaluation comporte quatre étapes: (1)
identification du danger; (2) évaluation de la relation dose-réponse;
(3) évaluation de l'exposition et (4) caractérisation du risque.
On estime que les travaux les plus intéressants sont les études
épidémiologiques de longue durée dans lesquelles l'exposition est bien
définie et le suivi supérieur à 20 ans. Quatre études portant sur le
cancer du poumon chez des personnes professionnellement exposées
satisfont à ces critères. Il apparait que le risque relatif de cancer
du poumon lié à une exposition à des émissions de moteur diesel est
généralement faible et qu'il est influencé par les circonstances, les
effets des facteurs de confusion non évalués et la difficulté de tenir
suffisamment compte des facteurs de confusion reconnus. D'autres
études, où l'exposition est définie avec une moindre précision,
corroborent les conclusions des études précédentes. Globalement, on
estime que les émissions de moteurs diesel sont probablement
cancérogènes pour l'homme; toutefois on ne dispose d'aucune donnée
quantitative permettant d'évaluer le risque.
B.1.9.1 Effets non néoplasiques
Pour caractériser le risque, on a procédé de deux manières:
premièrement une concentration sans effets nocifs observables que l'on
divise par un coefficient d'incertitude; deuxièmement, une
concentration de référence. Dans les deux cas, on a utilisé un modèle
dosimétrique élaboré qui permet de réduire l'imprécision due à
l'extrapolation interspécifique des doses.
La dose de particules sans effet pour l'homme a été estimée à
0,139 mg/m3. La valeur guide pour la population générale calculée
d'après le modèle dosimétrique a été établie à 5,6 µg/m3, la valeur
calculée sans recours au modèle étant de 2,3 µg/m3.
La méthode basée sur l'utilisation d'une concentration de
référence prend en compte l'ensemble de la relation exposition-réponse
de préférence aux données ponctuelles fournies par les études
d'inhalation, comme c'est le cas dans la méthode de la dose sans
effets observables. On a identifié trois paramètres biologiques
sensibles: l'inflammation alvéolaire chronique, la diminution
d'efficacité de l'ascenseur mucociliaire et les lésions pulmonaires
hyperplasiques. Les concentrations de référence calculées à partir du
même modèle dosimétrique que dans le cas de la méthode de la dose sans
effets observables, étaient de 0,9 à 2 µg/m3 pour l'inflammation, de
1,2 à 3 2 µg/m3 pour la dégradation de l'ascenseur muco-ciliaire et
de 6,3 à 14,2 µg/m3 pour les lésions hyperplasiques.
B1.9.2 Effets néoplasiques
Pour évaluer le risque d'une exposition aux émissions de moteur
diesel, on a eu recours à un modèle linéarisé en plusieurs étapes.
Comme les résultats des études épidémiologiques avaient été jugés
insuffisants pour une évaluation quantitative du risque unitaire, on a
eu recours à des données provenant de plusieurs études d'inhalation à
long terme chez des rats, études qui montraient l'existence d'une
cancérogénèse à partir de 2 mg/m3.
On a ainsi établi par le calcul que le risque unitaire était
de 3,4 × 10-5 µg/m3 (moyenne géométrique de quatre estimations).
Un autre modèle de type biologique a fourni une valeur similaire pour
le risque unitaire, en prenant pour hypothèse que les particules
émises par les moteurs diesel agissent à faibles concentrations sur la
prolifération cellulaire ou l'initiation du processus de
transformation.
B1.10 Evaluation des effets sur l'environnement
On ne dispose pas d'informations suffisantes pour évaluer les
effets spécifiques des émissions de moteurs diesel. La combustion du
carburant diesel devrait avoir des effets similaires à celle des
autres combustibles fossiles, effets qui sont liés à la consommation
de ce type de combustible.
PARTE A DIESEL
A1. RESUMEN
A1.1 Identidad, propiedades físicas y químicas y métodos
analíticos
El diesel es una mezcla compleja de alcanos normales, ramificados
y cíclicos (60%-> 90% en volumen; hidrocarburos de longitud de cadena
comprendida entre C9 y C30); compuestos aromáticos, especialmente
alquilbencenos (5%-40% en volumen), y pequeñas cantidades de alquenos
(0%-10% en volumen) obtenidos a partir de la fracción de gasóleo del
destilado medio durante la separación del petróleo. El diesel puede
contener benceno, tolueno, etilbenceno, y xilenos e hidrocarburos
aromáticos policíclicos (HAP), sobre todo naftaleno y derivados del
mismo con metilos sustituyentes, a niveles de partes por millón. El
contenido de azufre de los combustibles diesel depende del origen del
crudo y del proceso de refino. En un cierto número de países está
reglamentado por ley, y suele estar comprendido entre 0,05% y 0,5% en
peso. Se utilizan aditivos para influir en el flujo, el almacenamiento
y la combustión del diesel, para diferenciar productos y para
satisfacer las especificaciones comerciales. A temperatura ambiente,
los combustibles diesel suelen ser líquidos de color marrón
moderadamente volátiles, ligeramente viscosos, inflamables y de olor
parecido al del queroseno. Los márgenes de ebullición están
comprendidos por lo general entre 140°C y 385°C (> 588°C en el caso
del diesel marino); a 20°C la densidad es de 0,87-1,0 g/cm3, y la
hidrosolubilidad de 0,2-5 mg/litro. La calidad y composición del
diesel influye considerablemente en los contaminantes emitidos por los
motores diesel. Otras variables importantes son el proceso de
encendido (expresado en términos del índice de cetano), la densidad,
la viscosidad y el contenido de azufre. Las especificaciones del
diesel comercial varían considerablemente de un país a otro.
Los combustibles de calefacción y algunos combustibles de
aviación a base de queroseno producidos durante el proceso de refino
tienen a veces una composición parecida a la del diesel, aunque con
distintos aditivos. Por ello, a la hora de evaluar la toxicidad y la
ecotoxicidad se han tenido también en cuenta los datos biológicos
sobre estas mezclas.
Debido a la complejidad de las mezclas diesel, no existe un
método analítico específico para ellas, y las técnicas analíticas
empleadas en la mayoría de las evaluaciones ambientales sólo permiten
determinar la mezcla total de hidrocarburos de petróleo. Los métodos
consisten en una extracción previa con solventes, un proceso de
limpieza para eliminar los hidrocarburos naturales, y la posterior
detección mediante gravimetría, espectroscopía infrarroja o
cromatografía de gases.
Ni la gravimetría ni la técnica infrarroja proporcionan
información cualitativa o cuantitativa de utilidad sobre los
contaminantes, por lo que sólo pueden usarse con fines de cribado.
El método utilizado habitualmente para analizar muestras
ambientales es la cromatografía de gases unida a técnicas de detección
tales como la ionización por conductor y la espectrometría de masas.
Existen otros muchos métodos para analizar hidrocarburos particulares
en los combustibles diesel.
A1.2 Fuentes de exposición humana y ambiental
Los combustibles diesel se producen por refinación del petróleo bruto.
Para satisfacer las especificaciones técnicas en cuanto al
rendimiento, normalmente se combinan varios componentes para
obtenerlos; la ulterior inclusión de aditivos mejora sus propiedades
para usos específicos. Los combustibles diesel son muy utilizados con
fines de transporte. En los motores de alta velocidad se deben emplear
los más volátiles y de menor viscosidad, y los de cadena más larga se
reservan para los motores diesel de ferrocarriles y barcos. Una gran
parte del transporte pesado por carretera está propulsado por motores
diesel.
Los automóviles para pasajeros propulsados por motores diesel son
cada vez más frecuentes en Europa y el Japón (10%-25%), mientras que
en América del Norte el porcentaje de ese tipo de automóviles es de
aproximadamente 1%-2%, con una ligera tendencia a la baja. El diesel
es utilizado en motores fijos y en calderas, como por ejemplo motores
de pistones, turbinas de gas, bombas de oleoductos, compresores de
gas, unidades de procesamiento de vapor de centrales de energía
eléctrica, quemadores e instalaciones industriales de calefacción de
espacios y de agua.
En los últimos cinco años la demanda mundial de combustibles
diesel ha aumentado constantemente. En 1985 se consumieron las
siguientes cantidades: unas 170 000 kilotoneladas al año en América
del Norte; unas 160 000 kilotoneladas al año, gasóleos incluidos, en
la Unión Europea; y aproximadamente 46 000 kilotoneladas anuales en
Australia, el Japón y Nueva Zelandia, lo que equivale a un total de
1062 kilotoneladas diarias. En 1990 se notificó que la demanda mundial
ascendía aproximadamente a 1110 kilotoneladas al día.
No se dispone de información sobre las emisiones a que da lugar
la producción de combustibles diesel; no obstante, al parecer esta
fuente tiene una importancia secundaria, dado que el proceso de
refinación se lleva a cabo en sistemas cerrados. Las emisiones se
producen sobre todo durante el almacenamiento y el transporte. Se
libera diesel como resultado de derrames y durante el reabastecimiento
de los vehículos en las gasolineras.
La atmósfera y la hidrosfera son los compartimentos ambientales
más gravemente afectados. En algunos accidentes se produce
contaminación del suelo por combustibles diesel, problema que también
afecta a las playas ferroviarias. Entre las numerosas técnicas
empleadas para limpiar los suelos contaminados por diesel, cabe citar
la excavación, los métodos biológicos y el confinamiento.
A1.3 Transporte, distribución y transformación en el medio
ambiente
Apenas hay datos sobre el destino ambiental de los combustibles
diesel, pero se considera que los mecanismos de su distribución y
transformación son comparables a los de combustibles de calefacción
que han sido bien estudiados, como por ejemplo el fuel-oil No 2. Los
derrames de diesel en agua se extienden casi de inmediato para formar
una 'capa aceitosa'. Los componentes polares y de bajo peso molecular
relativo se disuelven y se separan por lixiviación de dicha capa,
mientras que los componentes volátiles se evaporan a partir de la
superficie; además da comienzo la degradación microbiana. La
degradación química y biológica altera la composición del derrame.
Estos procesos dependen de la temperatura; así, los derrames que se
producen en el Artico duran más que los que se producen en zonas de
clima templado. En entornos marinos, la mayoría de los agentes
aromáticos de bajo peso molecular relativo se disuelven en la fase
acuosa, pero los alcanos ramificados, los cicloalcanos y los restantes
compuestos aromáticos primarios pueden persistir en los sedimentos
durante más de un año.
No se dispone de información sobre la fotooxidación de los
combustibles diesel en el agua y el aire, pero se sabe que los
componentes del petróleo evaporados se degradan fotoquímicamente. Se
ha demostrado que el fuel-oil No 2 se fotooxida rápidamente en el agua
en condiciones ambientales.
Los distintos componentes del diesel son de por sí
biodegradables, en diversa medida y a distinta velocidad. Los
n-alcano, los compuestos n-alquilaromáticos y las moléculas
aromáticas simples comprendidas en el margen de C10 a C22 son los
que más fácilmente se degradan. Las moléculas más pequeñas son
metabolizadas por lo general rápidamente. Los n-alcanos de cadena
larga se degradan más lentamente, debido a su hidrofobicidad y a que
son viscosos o sólidos a temperatura ambiente. Los alcanos ramificados
y los cicloalcanos son relativamente resistentes a la degradación
biológica, y los HAP son resistentes. La velocidad global de
degradación de los hidrocarburos depende de la temperatura, el
contenido de agua, el oxígeno, el pH, los nutrientes inorgánicos y la
variabilidad metabólica microbiana.
Las algas unicelulares pueden incorporar y metabolizar
hidrocarburos tanto alifáticos como aromáticos, pero no se sabe con
seguridad en qué medida ocurre realmente tal cosa en la naturaleza. A
diferencia de los microorganismos que utilizan carbonos de petróleo
como fuente de carbono, los animales generalmente oxidan y conjugan
los productos, generando productos finales que son más solubles y por
consiguiente más fáciles de excretar. Todas las especies animales
analizadas pueden incorporar hidrocarburos de petróleo. Se sabe que
los HAP, el petróleo bruto y los productos refinados derivados del
petróleo son inductores de las enzimas del citocromo P450 y aumentan
el metabolismo de los hidrocarburos en numerosas especies de peces
marinos y de agua dulce.
Hay pocos datos disponibles sobre la bioacumulación de diesel en
condiciones de laboratorio, pero diversos estudios sobre derrames y
estudios de laboratorio sobre otros tipos de petróleo, en particular
el fuel-oil No 2, han aportado pruebas abundantes de que los
organismos acuáticos bioconcentran los hidrocarburos. El coeficiente
de reparto n-octanol-agua para el diesel es de 3,3-7,06, valores que
indican una alta bioacumulación potencial; no obstante, muchos de los
compuestos de bajo peso molecular relativo son metabolizados
fácilmente, y la bioacumulación real de los compuestos de mayor peso
molecular relativo se ve limitada por su escasa hidrosolubilidad y su
gran tamaño molecular. Así pues, la bioacumulación real posiblemente
es baja.
Se han descubierto peces envenenados por diesel después de
derrames, pero no se dispone de datos sobre la biomagnificación del
combustible. No hay datos experimentales sobre el movimiento del
diesel a través del suelo, aunque se ha señalado que existe quizá una
correlación directa entre el movimiento y la viscosidad cinemática. El
movimiento del queroseno a través del suelo depende del contenido de
humedad y de las características del suelo.
A1.4 Niveles ambientales y exposición humana
Debido a su carácter de mezclas complejas, no se han determinado
los niveles ambientales de los combustibles diesel. Se pueden detectar
componentes individuales del diesel en casi todos los compartimentos
del medio ambiente, pero no es posible averiguar su origen. La
población general puede verse expuesta al diesel en las gasolineras y
de resultas de los derrames.
Hay muchas actividades que acarrean exposición ocupacional al
diesel. Dada su escasa volatilidad, en principio estos combustibles
sólo suelen generar bajas concentraciones de vapores a temperaturas
normales, pero a temperaturas altas las concentraciones pueden llegar
a ser importantes.
A1.5 Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
La toxicidad aguda de los combustibles diesel es baja en caso de
exposición oral o cutánea o de inhalación. El valor de la DL50 oral
fue superior a 5000 mg/kg de peso corporal en todas las especies
analizadas (ratón, conejo, rata, cobayo). La DL50 del producto
aplicado a nivel cutáneo fue superior a 5000 mg/kg de peso corporal en
el ratón y el conejo, si bien se notificaron valores de más de
2000 mg/k de peso corporal para algunos querosenos y destilados
medios, con distintos protocolos y dosis límite menores. La CL50
observada en ratas expuestas por inhalación fue de aproximadamente
5 mg/litro, con excepción de un destilado medio directo para el que se
halló un valor de 1,8 mg/litro.
En conejos y en ratones tratados cutáneamente con cantidades
diarias de hasta 8000 µl/kg de peso corporal y hasta 40 000 mg/kg de
peso corporal respectivamente, se observó la aparición de acantosis e
hiperqueratosis como consecuencia de una irritación grave. Los conejos
eran más sensibles que los ratones. La inhalación de diesel tuvo
efectos neurodepresivos en ratones a concentraciones de hasta
0,2 mg/litro, pero no así en ratas expuestas a concentraciones de
hasta 6 mg/litro. En ratas se observó una disminución del peso
corporal y del peso del hígado.
No se observaron signos importantes de toxicidad acumulativa en
ratones, ratas y perros que habían inhalado hasta 1,5 mg/litro de
forma subcrónica. El síndrome de nefropatía específico observado en
ratas macho se asocia a una acumulación inherente de gotas hialinas en
los túbulos renales.
Los únicos efectos de las exposiciones a largo plazo fueron la
ulceración por aplicación cutánea en ratones (250 ó 500 mg/kg de peso
corporal al día) y alteraciones importantes del peso de los órganos
tras la inhalación de 1 ó 5 mg/litro en ratas. En los dos estudios el
peso corporal medio disminuyó.
Diversos tipos de diesel se revelaron entre leves y gravemente
irritantes para la piel del conejo. Los combustibles diesel no son
irritantes para el ojo, si bien se ha observado que algunos querosenos
tienen un ligero efecto de esa naturaleza. Los combustibles diesel no
causan sensibilización cutánea.
El diesel y los combustibles de avión (queroseno) no tuvieron
efectos embriotóxicos ni teratogénicos en dos estudios realizados en
ratas expuestas por inhalación a concentraciones de 100 ó 400 ppm y en
un estudio en que las ratas recibieron hasta 2000 mg/kg de peso
corporal diarios mediante sonda. En este último estudio se observó una
disminución del peso fetal.
Las pruebas realizadas con Salmonella typhimurium no aportaron
indicios claros de mutagenicidad. Algunos datos positivos obtenidos
con S. typhimurium y en células de linfoma de ratón se consideraron
equívocos dada la incoherencia de los resultados. Las pruebas de
genotoxicidad realizadas con ratones in vivo (inducción de
micronúcleos o aberraciones cromosómicas) también dieron resultados
equívocos o negativos.
Se ha observado la inducción de un bajo nivel de carcinogenicidad
cutánea por combustibles diesel. En el estado actual de las
investigaciones, es imposible discernir si la actividad carcinogénica
del diesel se debe a un mecanismo genotóxico o a la aparición de
lesiones cutáneas crónicas.
A1.6 Efectos en el ser humano
Una posible fuente de exposición no ocupacional al combustible
diesel es el llenado manual de depósitos de combustible. La fuente
principal de exposición cutánea son los derrames accidentales, que dan
lugar de inmediato a niveles altos de exposición, si bien de corta
duración.
Como efectos del contacto cutáneo accidental se han notificado
anuria, insuficiencia renal, síntomas gastrointestinales e
hiperqueratosis cutánea. Se ha observado un cuadro pulmonar tóxico
tras la ingestión accidental de diesel y su sucesiva aspiración. Se ha
notificado el desarrollo de tos productiva persistente tras la
inhalación del producto.
En un estudio de casos y testigos realizado en hombres expuestos
al diesel, se observó un aumento del riesgo de cáncer de pulmón
distinto del adenocarcinoma; se detectó asimismo una correlación
positiva con el cáncer de próstata, si bien se halló un riesgo mayor
para el grupo sometido a una exposición 'no considerable' que para el
sometido a una exposición 'considerable'.
En un estudio transversal realizado entre obreros expuestos a
combustibles de avión a base de queroseno se observó que los
trabajadores presentaban mareos, cefaleas, náuseas, palpitaciones,
presión torácica e irritación ocular con mayor frecuencia que los
testigos no expuestos. Se calculó que la concentración promedio
ponderada por el tiempo del vapor desprendido por el combustible en la
zona en que se respiraba era de 128-423 mg/m3
A1.7 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El diesel es más tóxico que el petróleo bruto para los organismos
y las plantas acuáticas. La ecotoxicidad del diesel se atribuye por lo
general a compuestos aromáticos solubles, pero es posible que también
intervengan hidrocarburos alifáticos insolubles. Entre los compuestos
aromáticos, los monoaromáticos son los menos tóxicos, y su toxicidad
aguda aumenta con el peso molecular hasta los compuestos de cuatro a
cinco anillos, si bien estos últimos son escasamente solubles en agua
de mar. En algunos animales, como por ejemplo peces y aves, el
recubrimiento físico de la superficie corporal por el combustible
puede ser causa de toxicidad y mortalidad.
Se han llevado a cabo experimentos de laboratorio con combustible
diesel, fracciones hidrosolubles, dispersiones de petróleo-agua y
petróleo microencapsulado. El diesel no redujo significativamente el
crecimiento en cultivo del alga verde Euglena gracilis, mientras que
una concentración baja (0,1%) inhibió casi por completo el crecimiento
de Scenedesmus quadricauda. La presencia de diesel a baja
concentración (0,05%) estimuló el crecimiento, la fotosíntesis y la
síntesis de clorofila a de Chlorella salina, pero inhibió
ligeramente su respiración; a concentraciones superiores el ritmo de
crecimiento y la fotosíntesis se vieron considerablemente reducidos.
La exposición prolongada inhibió el crecimiento de las algas
bentónicas Ascophyllum nodosum y Laminaria digitata. En algas
verdeazuladas, la fotosíntesis se vio reducida por las fracciones
aromática y asfáltica, pero no por la fracción alifática.
El diesel tuvo efectos tóxicos agudos en Daphnia, en larvas de
quironómidos, y en el molusco Viviparus bengalensis (gasterópodos).
Una concentración de 0,1 ml/litro causó la muerte de copépodos de
charca Tigriopus californicus en el término de cinco días.
Los mejillones Mytilus edulis acumulan diesel, presentan
índices de alimentación y crecimiento considerablemente reducidos y
acusan efectos de toxicidad reproductiva tras la exposición crónica a
dicho combustible. La CE50 para el desove en mejillones expuestos
durante 30 días fue de aproximadamente 800 µg/litro. La CL50 de
gasóleo microencapsulado en mejillones en fase de maduración expuestos
durante 30 días al producto fue de unos 5000 µg/litro. El gasóleo fue
más tóxico para las larvas que para los ejemplares jóvenes:
10 µg/litro tenían efectos adversos para el crecimiento de las larvas.
Cangrejos de agua dulce (Barytelphusa cunicularis) expuestos a
concentraciones subletales de diesel por espacio de hasta 96 horas
redujeron por lo general su consumo de oxígeno, sobre todo a
exposiciones bajas de hasta ocho horas. A exposiciones más largas el
consumo de oxígeno fue similar o superior al de los controles.
En las pruebas de toxicidad aguda de 96 horas llevadas a cabo en
salmónidos jóvenes en condiciones estáticas, el diesel tuvo efectos
más tóxicos para el salmón rosado Onchorhychus gorbuscha
(CL50: 32-123mg/litro) que para el salmón plateado O. kisutch
(CL50: 2186-3017 mg/litro) o para la trucha arco iris O. mykiss
(CL50: 3333-33216 mg/litro), con independencia del tipo de agua.
El umbral de detección de efectos comportamentales en bacalaos
( Gadus morhua L.) expuestos a diesel en agua de mar fue de
100-400 ng/litro. El pez antártico Pagothenia borchgrevinki soportó
una fracción hidrosoluble no diluida de combustible diesel durante
72 horas, si bien mostró signos de estrés.
Las aves se ven afectadas externa e internamente por los
petróleos contaminantes. El diesel destruye la impermeabilidad del
plumaje de las aves y éstas lo ingieren cuando se limpian las plumas.
El diesel y el fuel-oil administrados mediante sonda a razón de
2 ml/kg de peso corporal a patos causó neumonía lipídica, inflamación
extrema de los pulmones, infiltración grasa del hígado y degeneración
hepática al cabo de 24 horas. La administración de diesel o fuel-oil a
dosis de 1 ml/kg causó irritación grave del tracto digestivo y
nefrosis tóxica. Dosis mayores causaron aumento de las glándulas
suprarrenales (debido sobre todo a una hiperplasia del tejido
cortical), disminución de los niveles plasmáticos de colinesterasa,
ataxia y temblores. La administración de estos productos a dosis de
hasta 20 ml/kg de peso corporal no tuvieron efectos mortales en
pájaros sanos, pero la DL50 del diesel y el fuel-oil administrados a
aves estresadas fue de 3-4 ml/kg de peso corporal.
Tras los derrames de diesel, el zooplancton parece altamente
vulnerable a los componentes dispersos y disueltos del petróleo, pero
no tanto al petróleo flotante. Los organismos acuáticos pueden verse
afectados de varias formas: mortalidad directa (huevos de pescados,
copépodos y plancton mixto), contaminación externa por petróleo
(corion de huevos de pescados y cutículas y apéndices de alimentación
de los crustáceos), contaminación tisular por componentes aromáticos,
desarrollo anormal de embriones de peces, y alteración de las tasas
metabólicas.
A1.8 Evaluación de los riesgos para la salud humana
La población general puede verse expuesta al diesel y a otros
destilados medios en las gasolineras de resultas de derrames
accidentales, durante la manipulación de dichos combustibles y con
motivo del uso de queroseno en la cocina o la calefacción domésticas.
Los trabajadores pueden verse expuestos al diesel o a otros destilados
medios al manipular y descargar el combustible en las terminales, los
tanques de almacenamiento y las gasolineras; durante la fabricación,
la reparación, el mantenimiento y el ensayo de motores y demás equipo
a base de diesel; durante la utilización del diesel como agente
limpiador o disolvente; y durante el manejo y el muestreo sistemáticos
de combustible diesel en el laboratorio. Debido a su baja volatilidad
a temperatura ambiente sólo suelen producirse concentraciones bajas de
vapor, si bien en espacios cerrados y a altas temperaturas se pueden
alcanzar niveles importantes.
La exposición al vapor desprendido es mínima durante la
manipulación habitual del diesel. El efecto más probable en la salud
humana es la dermatitis resultante del contacto cutáneo. El diesel es
irritante para la piel, pero al parecer no para el ojo. La exposición
cutánea puede dar lugar a efectos tóxicos agudos a nivel renal, pero
se desconoce qué efectos tiene la absorción cutánea prolongada de
bajas concentraciones.
La ingestión de combustibles diesel tiene efectos tóxicos, y en
ocasiones se produce regurgitación y aspiración, posible causa de
neumonía química, al igual que puede ocurrir con cualquier
hidrocarburo que tenga un determinado grado de viscosidad.
En roedores, la exposición por inhalación a concentraciones de
diesel de hasta 0,2 mg/litro tuvo efectos neurodepresivos en ratones,
pero no en cambio en ratas a las concentraciones más altas. La
exposición subcrónica por inhalación a diversos destilados
combustibles indujo una nefropatía específica para la
alpha2-microglobulina en ratas macho; se consideró que este efecto era
irrelevante para el hombre.
No se observaron efectos embriotóxicos ni teratogénicos en
animales expuestos oralmente o por inhalación a combustibles diesel.
No hay indicios claros de una actividad mutagénica en bacterias,
y los resultados de otras pruebas de genotoxicidad
in vitro e in vivo fueron ambiguos.
Los resultados de un estudio de casos y testigos realizado entre
trabajadores expuestos a diesel llevaron a pensar en un aumento del
riesgo de cáncer de pulmón distinto del adenocarcinoma y de cáncer de
próstata. En ninguno de los casos se observó una relación exposición-
respuesta. En vista de los pocos estudios disponibles, del reducido
número de casos y de la consiguiente amplitud de los intervalos de
confianza, no es posible extraer conclusión alguna acerca de la
carcinogenicidad del diesel para el hombre.
En ratones, la administración cutánea de combustibles diesel fue
débilmente carcinógena. Dado que no existe una genotoxicidad clara, es
posible que la inducción de cáncer se deba a mecanismos no
genotóxicos, por ejemplo a una irritación cutánea crónica
caracterizada por ciclos repetidos de lesiones cutáneas, con la
consiguiente hiperplasia epidérmica.
A1.9 Evaluación de los efectos en el medio ambiente
El medio puede contaminarse de resultas de la liberación
accidental de diesel en gran escala, como la asociada a desastres en
depósitos o escapes en oleoductos, o a menor escala como resultado de
la contaminación del suelo de las inmediaciones de fábricas o garajes.
En el agua, el diesel se propaga de forma casi inmediata, los
componentes polares y de bajo peso molecular relativo se disuelven y
se separan por lixiviación, los componentes volátiles se evaporan a
partir de la superficie del agua, y da comienzo la degradación
microbiana. La intensidad de la degradación depende de la temperatura
y de las condiciones climáticas. La composición química de los
derrames cambia con el tiempo: después de un vertido al agua, algunas
fracciones se evaporan, y los componentes de diesel evaporados se
degradan fotoquímicamente; en el sedimento, parece ser que el diesel
es transportado en general a los sedimentos del fondo por las
partículas que se depositan; en el suelo, los componentes del diesel
migran a distintas velocidades, en función del tipo de suelo.
Los componentes individuales son de por sí biodegradables, pero
la velocidad de biodegradación depende estrechamente de las
condiciones físicas y climáticas y de la composición de la población
microbiana.
Los organismos acuáticos, en particular los moluscos, bioacumulan
hidrocarburos en diversa medida, pero se produce una depuración de los
hidrocarburos al transferirlos a agua limpia. Puede darse una
bioacumulación de diesel; ahora bien, no hay datos sobre su
biomagnificación.
Los derrames de diesel tienen efectos nocivos inmediatos en el
medio, donde causan una considerable mortalidad de la biota. Puede
producirse una recolonización al cabo de aproximadamente un año, en
función de la especie de animal o planta y del contenido químico y
físico de los restos del derrame. Los organismos acuáticos que
sobreviven a los derrames de diesel pueden verse afectados por la
contaminación externa y por la acumulación del producto en los
tejidos: un desarrollo anormal y la presencia de tasas metabólicas
alteradas son indicios del estrés resultante.
PARTE B EMISIONES DE GASES DE ESCAPE DE MOTORES DIESEL
B1. RESUMEN
B1.1 Identidad, propiedades físicas y químicas y métodos
analíticos
Las emisiones de gases de escape de los motores diesel contienen
cientos de compuestos químicos, que son emitidos en parte en la fase
gaseosa y en parte en la fase de materia particulada de los gases de
escape. Los principales productos gaseosos de la combustión son los
siguientes: dióxido de carbono, oxígeno, nitrógeno y vapor de agua;
hay también monóxido de carbono, dióxido de azufre, óxidos de
nitrógeno, e hidrocarburos y sus derivados. Se detectan benceno y
tolueno en el margen porcentual correspondiente a los compuestos de
menor peso en la parte gaseosa de la fracción de hidrocarburos. Otros
componentes de los gases de escape son los hidrocarburos aromáticos
policíclicos (HAP) de bajo peso molecular relativo.
Una importante característica de las emisiones de estos gases de
escape es que la liberación de partículas es 20 veces más intensa que
en los vehículos propulsados por gasolina. Las partículas están
compuestas de carbono elemental, compuestos orgánicos adsorbidos del
combustible y el aceite lubricante, sulfatos derivados del azufre del
combustible, y cantidades mínimas de componentes metálicos. La mayor
parte de la materia particulada total tiene al parecer dimensiones del
orden de la submicra: entre 0,02 y 0,5 µm. Con el paso del tiempo
puede producirse aglomeración, hasta un diámetro máximo de 30 µm. Las
partículas emitidas presentan una gran superficie. Los compuestos
orgánicos representan por lo general un 10%-30% de la materia
particulada total, pero los motores mal diseñados o mantenidos pueden
generar hasta un 90%. En esta fracción se encuentran también, a
concentraciones de partes por millón, HAP, HAP oxigenados y nitro-HAP
de alto peso molecular relativo.
Para medir las emisiones de los vehículos se utilizan ciclos de
pruebas transitorios y estables específicos. Pueden tomarse muestras
de gases de escape diluidos o no diluidos. Es difícil conseguir
muestras sin artefactos, ya que los componentes de los gases de escape
pueden sufrir reacciones químicas, procesos de adsorción y desorción,
y condensación y difusión. Los HAP de mayor interés toxicológico de la
materia particulada del diesel se determinan por lo general mediante
la extracción de Soxhlet, limpieza y fraccionamiento, seguidos de
análisis por cromatografía líquida de alta resolución o cromatografía
de gases unida a espectrometría de masas.
B1.2 Fuentes de exposición humana y ambiental
La principal fuente de gases de escape de motores diesel son los
vehículos de motor; otras fuentes son los motores diesel fijos, de
locomotoras de ferrocarril y de barco. Las emisiones de los vehículos
de motor diesel han sido bien caracterizadas, pero a menudo los
resultados de los estudios no se prestan a comparación, debido a
diferencias que afectan a parámetros tales como los ciclos de pruebas,
el tipo de motor y la composición del combustible. Las cantidades
emitidas de los diversos componentes son las siguientes: dióxido de
carbono, aproximadamente 1 kg/km; monóxido de carbono, óxidos de
nitrógeno, hidrocarburos gaseosos totales y materia particulada,
0,1-20 g/km; y compuestos alifáticos, alcoholes, aldehídos, compuestos
aromáticos ligeros y HAP, microgramos por kilómetro. Las emisiones de
monóxido de carbono, óxidos de nitrógeno, hidrocarburos gaseosos
totales y materia particulada están reglamentadas por ley en varios
países.
En lo que respecta a la calidad y cantidad de los gases de
escape, no suele haber diferencias entre los motores de baja y de gran
potencia, si bien los vehículos pesados liberan cantidades
relativamente mayores de materia particulada. La naturaleza de los
gases de escape depende del ciclo de pruebas (transitorio o estable),
de las características del motor (técnicas de inyección y de
aspiración, mantenimiento, kilometraje), y de la composición del
combustible (contenido de azufre, aromaticidad, volatilidad); el
ajuste del motor es muy importante en este sentido.
La liberación de materia particulada aumenta con la disminución
de la proporción aire/combustible, el aumento de la carga y el aumento
de la temperatura. Emiten más partículas los motores viejos y muy
usados que los nuevos y de bajo kilometraje, probablemente debido a un
mayor consumo de aceites lubricantes. La emisión de partículas por los
vehículos diesel de poca potencia también está correlacionada con el
contenido de azufre del combustible, ya que la formación de sulfatos
de metal se traduce en un aumento de la masa de partículas; no se ha
determinado la emisión de partículas por parte de vehículos de gran
potencia. El aumento de la aromaticidad del combustible también
conlleva una mayor emisión de partículas.
Los HAP y los HAP oxigenados emitidos por los motores diesel y de
encendido por chispa son parecidos cualitativamente. Los HAP
oxigenados y nitrados se emiten a niveles del orden de pocos
microgramos por kilómetro, pero no se sabe con seguridad qué
concentración real alcanzan estos compuestos, ya que pueden
descomponerse y formarse durante el muestreo. Las emisiones de HAP
aumentan con la carga y con la temperatura, así como con a antigüedad
del motor, a causa probablemente de un mayor consumo de aceites
lubricantes. La emisión de HAP depende también de la técnica de
inyección del motor: las emisiones de HAP aumentan al mismo tiempo que
la proporción aire/combustible en los motores de inyección directa,
mientras que disminuyen en los motores de inyección indirecta. La
aromaticidad y la volatilidad del combustible guardan relación directa
con la emisión de HAP. El mal funcionamiento de los dispositivos del
motor, sobre todo del sistema de inyección de combustible, aumenta la
emisión de los principales componentes de escape. Se dispone de pocos
datos sobre la contribución de las emisiones de los motores diesel a
la liberación total de productos de combustión por el hombre.
Es posible reducir las emisiones de gases de escape producidos
por el diesel mejorando el diseño de los motores, los captores de
partículas (oxidantes de captores) y los catalizadores. Mientras que
los captores de partículas eliminan tanto el hollín como los
compuestos orgánicos solubles adsorbidos en las partículas, los
catalizadores reducen sobre todo los niveles de monóxido de carbono y
de hidrocarburos gaseosos. En la práctica es difícil regenerar los
captores de partículas. Los catalizadores exigen combustibles de bajo
contenido de azufre, ya que este elemento daña los centros activos del
catalizador.
B1.3 Transporte, distribución y transformación en el medio
ambiente
El compartimento que antes se ve afectado por las emisiones de
gases de escape de los motores diesel es la atmósfera. La hidrosfera y
la geosfera se ven contaminadas indirectamente por depósitos secos y
húmedos. El destino ambiental de los distintos componentes de estos
gases de escape es en general bien conocido: las partículas se
comportan como moléculas de gas (no reactivas) por lo que se refiere a
su transporte mecánico en la atmósfera; pueden ser transportadas a
gran distancia y penetrar incluso en la estratosfera.
Se estima que el índice global de eliminación de las partículas
diesel es bajo, lo que se traduce en una permanencia de varios días en
la atmósfera. Con el tiempo se puede producir una coagulación de las
partículas, con lo que aumenta la precipitación de las mismas y
disminuye su concentración total en el aire. El carbono elemental de
las partículas diesel puede actuar como catalizador de la formación de
ácido sulfúrico por oxidación del dióxido de azufre. Los componentes
orgánicos adsorbidos en el carbono elemental pueden experimentar
varias reacciones físicas y químicas con otros compuestos atmosféricos
y durante la exposición a la luz solar.
B1.4 Niveles ambientales y exposición humana
Dado que los gases de escape diesel son una mezcla compleja de
gran diversidad de compuestos, por lo general no es posible precisar
los 'niveles ambientales'. Los distintos componentes de los gases de
escape diesel se pueden detectar en principio en todos los
compartimentos del medio ambiente, aunque por lo general no es posible
averiguar su origen. Se conocen los niveles ambientales de la mayoría
de los distintos componentes.
La exposición de la población general a los gases de escape de
motores diesel se produce especialmente en las calles transitadas y en
los aparcamientos, sobre todo los subterráneos. La identificación de
las fuentes resulta difícil, y la contribución de esos gases a la
contaminación total por productos de combustión del tráfico se calcula
en general a partir de los coeficientes de emisión y del porcentaje de
vehículos propulsados por diesel. Los niveles de exposición de la
población general y de los trabajadores a la materia particulada de
los motores diesel tienen importancia toxicológica. Las
concentraciones ambientales diarias de partículas diesel cerca de las
carreteras oscilan como promedio entre 8 y 42 µg/m3. Las
concentraciones promedio anuales estimadas fueron de 5-10 µg/m3 en
zonas urbanas y < 1,5 µg/m3 en zonas rurales de Alemania, y de
1-2 µg/m3 en zonas urbanas y 0,6-1 µg/m3 en zonas rurales de los
Estados Unidos de América. Las concentraciones están directamente
relacionadas con la densidad del tráfico y son tanto menores cuanto
más lejos están las carreteras.
La identificación de las fuentes también puede revestir
dificultad en los lugares de trabajo, sobre todo en las minas, donde
la carga total de polvo es elevada. Se han hecho análisis de muestras
de carbono para determinar la exposición específica a gases de escape
de motores diesel en el lugar de trabajo y, según se ha notificado,
los niveles de materia particulada a que están expuestos los
trabajadores son del orden de 0,04-0,134 mg/m3 para los conductores
de camiones y de 0,004-0,192mg/m3 para los ferroviarios. Como
medidas de la exposición ocupacional se han utilizado también las
partículas respirables totales y las partículas suspendidas totales.
B1.5 Cinética y metabolismo en animales de laboratorio y en el
hombre
B1.5.1 Depósito
Las partículas de los gases de escape de motores diesel, con un
diámetro aerodinámico mediano de aproximadamente 0,2 µm, son filtradas
en cierta medida por la nariz, y la eficiencia de depósito en el
pulmón es sólo ligeramente superior al valor mínimo hallado para las
partículas que tienen un diámetro aerodinámico mediano de
aproximadamente 0,5 µm. Así, un 10%-15% de las partículas de hollín
diesel inhaladas se depositan en la región alveolar de los pulmones de
la rata y el cobayo; en el hombre se deposita en la región alveolar un
10%.
B1.5.2 Retención y eliminación de las partículas
La eliminación mucociliar de partículas en las vías respiratorias
ciliadas es casi completa en un plazo de 24 horas. La eliminación a
largo plazo de las partículas diesel en la región alveolar se ha
determinado mediante varios estudios realizados en ratas expuestas por
inhalación a partículas diesel o control marcadas. Se notificaron
periodos de semieliminación de 60-100 días en testigos con cargas
pulmonares bajas (< 1 mg/pulmón), mientras que los periodos de
semieliminación aumentaron a 100-600 días en ratas con cargas
pulmonares crecientes de 1 a 60 mg/pulmón. En varios estudios se halló
que los efectos se debían a una sobrecarga de partículas, fenómeno
descrito en diversas especies y con varios tipos de materia
particulada. Este hecho se observa por lo general cuando la velocidad
de depósito de las partículas de baja solubilidad y baja toxicidad
aguda supera su velocidad de eliminación durante un tiempo
considerable. El periodo de semieliminación alveolar libre en el
hombre es de varios cientos de días, mayor que en la rata.
Se desarrolló un modelo pulmonar dosimétrico a partir de datos
relativos al depósito y retención de las partículas diesel en ratas
sometidas a inhalación prolongada, así como de datos referentes al
depósito y retención de partículas en el hombre. Este modelo puede
utilizarse para predecir la retención de partículas diesel y de
material orgánico adsorbido en los pulmones de personas de diferentes
edades. No se dispone de datos sobre la variación de la retención de
distintos componentes tras la exposición prolongada a gases de escape
de motores diesel.
B1.5.3 Retención y eliminación de hidrocarburos aromáticos
policíclicos adsorbidos en hollín de diesel
Los HAP del hollín de diesel se adhieren fuertemente a la
superficie de las partículas. Aproximadamente un 50% de los HAP
adsorbidos en las partículas diesel desaparece de los pulmones en el
plazo de un día, pero los periodos de semieliminación de los restantes
HAP fueron del orden de 18-36 días. Estudios realizados con
3H-benzo[ a]pireno y 14C-nitropireno han demostrado que, cuando
los HAP están asociados a materia particulada, su eliminación de los
pulmones se retrasa significativamente en comparación con los HAP
inhalados no asociados a materia particulada.
B1.5.4 Metabolismo
Se ha observado que el benzo[ a]pireno adsorbido en partículas
de gases de escape de motores diesel se metaboliza por oxidación en
fenoles, dioles y quinonas de benzo[ a]pireno en el pulmón y en
cultivos celulares de macrófagos pulmonares.
El nitropireno adsorbido en partículas diesel se metabolizó en
acetilaminopirenofenol después de su inhalación. Se han hallado más
aductos de ADN en los pulmones y las células de tipo II de ratas
expuestas a gases de escape de motores diesel que en las testigo. El
metabolismo oxidativo de algunos compuestos orgánicos en epóxidos
podría ser la causa de la formación de esos aductos, pero únicamente
se hallaron aductos tras la exposición a partículas. Se ha demostrado
que algunas sustancias orgánicas de los gases de escape de motores
diesel dan lugar a la formación de aductos de ADN; sin embargo, el
núcleo carbonáceo por sí mismo (sin material orgánico extraíble)
también puede inducir aductos, por lesión crónica de las células
epiteliales.
B1.6 Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
Los escasos datos disponibles parecen indicar que la toxicidad
aguda de los gases de escape de motores diesel es escasa. Ratones
tratados intratraquealmente con partículas de tales gases murieron de
edema pulmonar; la DL50 fue de aproximadamente 20 mg/kg de peso
corporal. Partículas extraídas con metanol no tuvieron efectos letales
a concentraciones de hasta aproximadamente 33 mg/kg de peso corporal.
En hámsters, la DL50tras administración intraperitoneal fue de
1280 mg/kg de peso corporal. No hay datos respecto a la exposición por
inhalación.
La exposición de ratas, cobayos y gatos a gases de escape de
motores diesel con un contenido de partículas de 6 mg/m3 durante
aproximadamente cuatro semanas alteró la función pulmonar, causando en
particular un aumento del 35% de la resistencia al flujo pulmonar en
los cobayos y una disminución del 10% de la capacidad vital (flujo
espiratorio) en los gatos. El análisis histopatológico reveló un
espesamiento focal de las paredes alveolares, un aumento significativo
del índice de marcaje de las células de tipo II, y acumulaciones de
macrófagos cargados de partículas. Estas acumulaciones se hallaban
cerca de los bronquiolos terminales, y aumentaron de tamaño, debido a
la adhesión (secuestro) de macrófagos, durante el periodo ulterior de
recuperación.
No se observaron disminuciones importantes del peso corporal ni
acortamiento alguno del tiempo de supervivencia en ratones, ratas,
hámsters, gatos y monos después de un periodo prolongado de inhalación
de concentraciones de hasta 4 mg/m3. En todas las especies sometidas
a inhalación prolongada de gases de escape de motores diesel se
observaron los siguientes efectos tóxicos relacionados con la dosis:
aumentos de hasta un 400% del peso pulmonar; inflamación pulmonar
cuantificable mediante parámetros bioquímicos (enzimas citoplasmáticas
marcadoras, colágeno) y citológicos (aumento de los neutrófilos
polimorfonucleares); trastornos de la mecánica pulmonar; aumento del
número de macrófagos cargados de partículas con acumulaciones focales
(secuestro) en condiciones de sobrecarga; y ulteriores cambios
proliferativos de las células epiteliales, con inicio de fibrosis.
La limitada información disponible sobre la toxicidad para la
reproducción y el desarrollo lleva a pensar que la inhalación de gases
de escape de motores diesel no reviste gran importancia. En la mayoría
de los experimentos no se observaron efectos en ratones, ratas,
hámsters, conejos o monos; no obstante, tras inyección intraperitoneal
se observaron anomalías en el semen de ratones sometidos a partículas
de esos gases de escape, y embriotoxicidad en hámsters sometidos a
extractos de tales gases.
La mayoría de las pruebas de genotoxicidad in vitro se han
llevado a cabo con extractos de gases de escape de motores diesel en
lugar de con la mezcla total de tales gases; se detectaron respuestas
positivas en ausencia de activación metabólica, es decir: los efectos
genotóxicos eran al parecer independientes de los HAP. Aproximadamente
la mitad de las investigaciones in vivo arrojaron resultados
negativos; los únicos resultados positivos fueron la inducción del
intercambio de cromátides hermanas en respuesta a la mezcla total de
gases de escape y a extractos orgánicos, y la inducción de
micronúcleos en respuesta a extractos orgánicos.
Por lo general no se observaron efectos inmunotóxicos después de
la inhalación de gases de escape de motores diesel; sin embargo, en un
estudio se halló un mayor título de anticuerpos IgE antiovoalbúmina, y
en dos experimentos, una mayor susceptibilidad a las infecciones en
ratones. Algunos estudios realizados en la rata parecen indicar que la
inhalación de estos gases de escape tiene efectos sobre el
comportamiento y el estado neurofisiológico.
En estudios de determinación de la carcinogenicidad de los gases
de escape de motores diesel administrados por inhalación a ratas se
observó que la fase gaseosa (sin la fracción particulada) no era
carcinogénica. En todos los estudios válidos realizados en la rata se
halló que los gases eran carcinogénicos a concentraciones de
partículas superiores a 2 mg/m3, lo que equivale a una exposición
continua de aproximadamente 1 mg/m3. No se observaron efectos ni en
el hámster ni en el ratón. En estudios realizados mediante instilación
intratraqueal, tanto las partículas de los gases de escape como el
negro de humo indujeron la aparición de tumores, y se observó una
aparente correlación entre la superficie de las partículas carbonosas
y la actividad tumorigénica. La inhalación prolongada de negro de humo
prácticamente desprovisto de HAP a concentraciones similares también
provocó la aparición de tumores pulmonares en ratas.
No está claro si en los mecanismos de carcinogenicidad de los
gases de escape de motores diesel intervienen reacciones del ADN o de
otro tipo (o quizá ambas). Se han utilizado diversos modelos para
dilucidar la carcinogenicidad de esos gases.
B1.7 Efectos en el ser humano
Los gases de escape de los motores diesel contribuyen a la
contaminación atmosférica en general. Aunque en los estudios agudos o
crónicos no es posible diferenciar el papel de las partículas diesel,
éstas podrían ser parcialmente responsables de los diversos efectos
sobre la salud asociados a la contaminación atmosférica.
Los gases de escape habituales de los motores diesel tienen un
olor peculiar, que algunas personas consideran desagradable, sobre
todo a altas concentraciones. Los síntomas que siguen a la exposición
aguda y crónica a esos gases han sido descritos en estudios y en
referencias anecdóticas de personas expuestas en el lugar de trabajo.
Se ha observado la aparición de irritación de las membranas mucosas
oculares y nasales en respuesta a la exposición aguda a tales gases,
así como un aumento de la frecuencia de síntomas respiratorios en
cohortes ocupacionales; no obstante, se ignora la contribución de las
partículas diesel. No se han detectado efectos sistemáticos a corto
plazo en la función pulmonar, pero se han notificado ataques de asma.
En un estudio controlado en el que se expuso a ocho voluntarios
sanos no fumadores en una cámara durante 60 minutos a gases de escape
de motores diesel diluidos, se detectó una disminución de la
fagocitosis en los macrófagos alveolares del líquido de lavado
broncoalveolar.
En algunos estudios transversales y longitudinales realizados
entre trabajadores expuestos de forma prolongada en su entorno laboral
a esos gases se han hallado decrementos de la función pulmonar y una
mayor frecuencia de síntomas respiratorios, pero estos estudios
presentan la limitación de haberse realizado con una exposición breve.
No se observaron aumentos importantes en estudios de cohortes
realizados para investigar las defunciones por trastornos
cardiovasculares y/o cerebrovasculares debidos a las emisiones diesel.
En varios estudios epidemiológicos se ha evaluado la asociación
entre cánceres de pulmón y de la vejiga urinaria y la exposición
ocupacional a gases de escape de motores diesel. En esta monografía se
incluyen sólo los estudios considerados pertinentes para evaluar los
efectos carcinogénicos de esos gases. Los estudios más importantes por
lo que se refiere al cáncer de pulmón son los realizados en
ferroviarios, trabajadores de garajes de autobuses y estibadores, esto
es, cohortes de individuos cuya actividad laboral no deja lugar a
dudas sobre la exposición a gases de escape diesel. La totalidad de
los cuatro estudios más informativos pusieron de manifiesto un mayor
riesgo de cáncer de pulmón, con riesgos relativos que van desde 1,4
para los ferroviarios, pasando por 1,3-2,4 para los trabajadores de
garajes de autobuses (según el grado de exposición), hasta 3-6 para
los estibadores (según el método empleado para evaluar la exposición,
pero con amplios intervalos de confianza). En un estudio de casos y
testigos realizados entre ferroviarios y en el estudio de los
estibadores fue posible introducir ajustes en función del consumo de
tabaco. En los dos casos, el efecto de la exposición al diesel no se
vio materialmente alterado por el ajuste realizado en función del
consumo de tabaco. En los tres estudios en que no pudo hacerse ese
ajuste, el análisis se basó en comparaciones de subgrupos de las
cohortes, de manera que la probabilidad de influencia del tabaco como
factor de confusión fue menor que al utilizar grupos de comparación
externos.
Se han llevado a cabo varios estudios de casos y testigos para
examinar la relación entre el cáncer de vejiga urinaria y la presunta
exposición a gases de escape de motores diesel. Se detectó un aumento
del riesgo, sobre todo para los conductores de camión; no obstante,
todos estos estudios adolecían de una deficiente caracterización de la
exposición. Por ello, no es posible establecer una asociación causal
entre la exposición a los gases de escape diesel y un aumento del
riesgo de cáncer de vejiga urinaria
B1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El único estudio sobre los efectos de las emisiones de motores
diesel propiamente dichas es un trabajo realizado con algas verdes.
B1.9 Evaluación de los riesgos para la salud humana
Se utilizó el paradigma de evaluación de riesgos de la Academia
Nacional de Ciencias de los Estados Unidos (National Research Council,
1983) para evaluar los riesgos asociados a variables de valoración
relacionadas y no relacionadas con el cáncer. Este proceso consta de
los cuatro pasos siguientes: 1) identificación del riesgo, 2)
evaluación de la relación dosis-respuesta, 3) evaluación de la
exposición, y 4) caracterización del riesgo.
Se considera que los estudios más informativos son los
epidemiológicos de larga duración realizados con una exposición bien
definida y con seguimiento ulterior (> 20 años). Satisfacían esos
criterios cuatro estudios sobre el cáncer de pulmón en personas
expuestas en su entorno de trabajo. Los riesgos relativos de cáncer de
pulmón asociado a la exposición a gases de escape de motores diesel
fueron por lo general bajos y podían haberse debido al azar, a los
efectos de factores de confusión no determinados y a la dificultad
para corregir con precisión la influencia de los factores de confusión
conocidos. Las conclusiones de estos estudios están avaladas por los
resultados de otros estudios menos precisos en cuanto a la definición
de la exposición. Globalmente se considera que los gases de escape de
los motores diesel son probablemente carcinogénicos para el hombre;
sin embargo, no se dispone de datos cuantitativos que permitan estimar
el riesgo para el ser humano.
B1.9.1 Efectos no neoplásicos
Para caracterizar los riesgos se utilizaron dos métodos
generales: el nivel sin efectos adversos observados (NOAEL) dividido
por un factor de incertidumbre y una concentración de referencia. En
los dos casos se empleó un sofisticado modelo dosimétrico para reducir
la incertidumbre asociada a la extrapolación interespecies de las
dosis.
Se calculó que el nivel sin efecto de las partículas de los gases
de escape de los motores diesel en el hombre era de 0,139 mg/m3. El
valor orientativo para la población general calculado a partir del
modelo dosimétrico fue de 5,6 µg/m3, y el calculado sin el modelo,
de 2,3 µg/m3.
El método de la concentración de referencia tiene en cuenta la
totalidad de la relación exposición-respuesta en lugar de puntos
únicos obtenidos en estudios de inhalación, como ocurre con el método
del NOAEL. Se identificaron tres variables de evaluación sensibles:
inflamación alveolar crónica, eliminación pulmonar alterada, y
lesiones pulmonares hiperplásicas. Las concentraciones de referencia,
calculadas mediante el mismo modelo dosimétrico utilizado para el
método del NOAEL, fueron de 0,9-2 µg/m3 para la inflamación,
1,2-3 µg/m3 para la disminución de la eliminación pulmonar, y
6,3-14 µg/m3 para las lesiones hiperplásicas.
B1.9.2 Efectos neoplásicos
Se utilizó un modelo polietápico linearizado para hacer
estimaciones del riesgo unitario asociado a la exposición a gases de
escape de motores diesel. Se consideró que los resultados de los
estudios epidemiológicos disponibles eran insuficientes para hacer una
estimación cuantitativa del riesgo unitario, por lo que se decidió
utilizar datos de varios estudios de inhalación prolongada en los que
se había detectado carcinogénesis a concentraciones superiores a
2 mg/m3. Se calculó un riesgo unitario de 3,4 × 10-5 µg/m3
(media geométrica de cuatro estimaciones del riesgo) para las
partículas de los gases de escape. Usando otro modelo biológico se
obtuvo un riesgo unitario parecido, en el supuesto de que las
partículas diesel influyen en la iniciación y/o proliferación celular
a bajas concentraciones.
B1.10 Evaluación de los efectos en el medio ambiente
No se disponía de suficiente información para evaluar los efectos
específicos de las emisiones de gases de escape de motores diesel. Los
efectos del combustible diesel quemado deben de ser parecidos a los de
otros combustibles fósiles y están relacionados con el consumo de
diesel.
See Also:
Toxicological Abbreviations
 All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting exhaust emissions of the documents. A comprehensive file of
all comments received on drafts of each EHC monograph is maintained
and is available on request. The chairpersons of task groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO DRAFTING GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL
AND EXHAUST EMISSIONS
WHO, Geneva, 6-9 December 1993
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United State
Dr R.P. Bos, University of Nijmegen, Nijmegen, Netherlands
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom (Joint Rapporteur)
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr I. Farkas, National Institute of Hygiene, Budapest, Hungary
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr P. Gustavsson, North Western Health Board, Stockholm, Sweden
Dr D. Guth, United States Environmental Protection Agency, Research
Triangle Park, NC, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr W. Pepelko, United States Environmental Protection Agency,
Washington DC, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Vice-Chairman)
Dr G. Rosner, Hazardous Substances Documentation Group, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr J. Roycroft, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, United States
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States (Chairman)
Dr B.H. Thomas, Environmental Health Directorate, Ottawa, Canada
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr. R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr P. Boffetta, International Agency for Research on Cancer, Lyon,
France (6-7 December 1993)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Representatives/Observers
CONCAWE
Dr R.H. McKee, Exxon Biomedical Sciences, East Millstone, NJ,
United States
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT
Dr P.T.C. Harrison, MRC Institute of Environment & Health,
Leicester, United Kingdom
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND
EXHAUST EMISSIONS
Fraunhofer Institute of Toxicology & Aerosol Research, Hanover
27 June-1 July 1994
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United States
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Dr Jun Kagawa, Tokyo Women's Medical College, Tokyo, Japan
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
Dr M. Roller, Medizinishes Institut für Umwelthyglene an der
Universität Düsseldorf, Düsseldorf, Germany Dr G. Rosner, Hazardous
Substances Documentation Group, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr H. Moller, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr M. Younes, European Centre for Environment and Health, Bilthoven,
Netherlands
Representatives/Observers
Dr Lutz Von Meyerinck, BP Oil Europe, Brussels, Belgium
GERMAN AUTOMOBILE ASSOCIATION
Dr N. Pelz, Mercedes-Benz AG, Stuttgart, Germany
ILSI
Dr I.T. Salmeen, Chemistry Department, Ford Motor Company,
Dearborn, MI, United States
IUTOX
Dr P. Montuschi, Department of Pharmacology, School of Medicine,
Catholic University of the Sacred Heart, Rome, Italy
ENVIRONMENTAL HEALTH CRIETERIA FOR DIESEL FUEL AND EXHAUST EMISSIONS
A WHO Task Group on Environmental Health Criteria for Diesel Fuel
and Exhaust Emissions met at the Fraunhofer Institute of Toxicology
and Aerosol Research, Hanover, Germany from 27 June to 1 July 1994.
Dr G. Rosner, Fraunhofer Institute, welcomed the participants on
behalf of the Institute and its Director, Professor U. Mohr, and
Dr E.M. Smith, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP, ILO, and WHO). The Task
Group reviewed and revised the draft and evaluated the risks for human
health and the environment from exposure to diesel fuel and exhaust
emissions.
The first draft of the monograph was prepared at the Fraunhofer
Institute. After international circulation for comment, this draft was
extensively revised by a Working/Drafting Group, convened at WHO,
Geneva, from 6 to 9 December 1993, and a second draft was prepared for
further international circulation for comment. The membership of the
drafting group is shown previously. A final draft, incorporating
comments received from the IPCS contact points for Environmental
Health Criteria monographs and new material, was completed at the
Fraunhofer Institute under the coordination of Dr G. Rosner, with
important contributions to the text from the following Institute staff
members:
Dr B. Bellman
Dr A. Boehnke
Dr O. Creutzenberg
Dr J. Kielhorn
Dr E.M. Smith of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs E. Heseltine, Lajarthe,
France, for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
PART A DIESEL FUEL
A1. SUMMARY
A1.1 Identity, physical and chemical properties, and analytical
methods
Diesel fuel is a complex mixture of normal, branched, and cyclic
alkanes (60 to > 90% by volume; hydrocarbon chain length, usually
between C9 and C30); aromatic compounds, especially alkylbenzenes
(5-40% by volume); and small amounts of alkenes (0-10% by volume)
obtained from the middle-distillate, gas-oil fraction during petroleum
separation. Benzene, toluene, ethylbenzene, and xylenes and polycyclic
aromatic hydrocarbons (PAHs), especially naphthalene and its
methyl-substituted derivatives, may be present at levels of parts per
million in diesel fuel. The sulfur content of diesel fuels depends on
the source of crude oil and the refinery process. It is regulated by
law in a number of countries and is usually between 0.05 and 0.5
weight percent. Additives are used to influence the flow, storage, and
combustion of diesel fuel, to differentiate products, and to meet
trademark specifications. At room temperature, diesel fuels are
generally moderately volatile, slightly viscous, flammable, brown
liquids with a kerosene-like odour. The boiling ranges are usually
between 140 and 385°C (> 588°C for marine diesel fuel); at 20°C, the
density is 0.87-1.0 g/cm3 and the water solubility is
0.2-5 mg/litre. The quality and composition of diesel fuel influence
the emissions of pollutants from diesel engines considerably.
Important variables are ignition behaviour (expressed in terms of
cetane number), density, viscosity, and sulfur content. The
specifications of commercial diesel fuel differ considerably in
different countries.
Heating fuels and some kerosene jet fuels produced during the
refining process may have a composition similar to that of diesel
fuel, although with different additives. Biological data on these
mixtures have therefore also been taken into account in the
assessments of toxicity and ecotoxicity.
Owing to the complexity of the diesel fuel mixture, there is no
specific analytical method, and the analytical techniques used in most
environmental assessments are suitable only for measuring the total
petroleum hydrocarbon mixture. The methods consist of preliminary
solvent extraction, a clean-up procedure to remove naturally occurring
hydrocarbons, and subsequent detection by gravimetry, infrared
spectroscopy or gas chromatography. Neither the gravimetric nor the
infrared technique provides useful qualitative or quantitative
information on contaminants and can thus be used only for screening.
Gas chromatography combined with detection techniques such as flame
ionization and mass spectrometry is the standard procedure for
analysing environmental samples. Many other methods are available for
the analysis of individual hydrocarbons in diesel fuels.
A1.2 Sources of human and environmental exposure
Diesel fuels are produced by refining crude oils. In order to
meet technical specifications for performance, diesel fuels are
generally blended; further formulation with additives improves their
properties for specific uses. Diesel fuels are widely used as
transportation fuels. The more volatile fuels, with low viscosity, are
required for high-speed engines and the heavier grades for railroad
and ship diesel engines. Much heavy-duty road transport is powered by
diesel engines. Passenger cars powered by diesel engines are becoming
increasingly more common in Europe and Japan (10-25%), whereas in
North America the percentage of diesel-fuelled passenger cars is about
1-2%, with a slightly decreasing tendency. Diesel fuel is used in
stationary engines and in boilers, e.g. reciprocating engines, gas
turbines, pipeline pumps, gas compressors, steam processing units in
electric power plants, burner installations, and industrial space and
water heating facilities.
Over the last five years, the worldwide demand for diesel fuels
has increased steadily. In 1985, the following amounts of diesel fuel
were consumed: about 170 000 kt per year in North America; about
160 000 kt per year, including gas oils, in the European Union; and
about 46 000 kt per year in Australia, Japan, and New Zealand,
equivalent to a total of 1062 kt per day. In 1990, world demand was
reported to be about 1110 kt per day.
No information is available on emissions during the production of
diesel fuels; however, this source would seem to be of minor
importance, because the refining process is carried out in closed
systems. Emissions may occur principally during storage and
transportation. Diesel fuels are released as a result of spills and at
filling stations during the refuelling of vehicles. The atmosphere and
the hydrosphere are the most heavily affected environmental
compartments. Soil contamination with diesel fuels may occur during
accidents and is also a problem in railroad yards. The numerous
techniques for cleaning soils contaminated with diesel fuel include
excavation, biological methods, and containment.
A1.3 Environmental transport, distribution, and transformation
Very few data are avilable on the environmental fate of diesel
fuels, but the mechanisms of their distribution and transformation are
considered to be comparable to those of heating fuels, such as No. 2
fuel oil, which have been well studied. Spills of diesel fuel on water
spread almost immediately to form a 'slick'. The polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, and the volatile components evaporate from the surface;
microbial degradation also begins. Chemical and biological weathering
alter the composition of the spill. These processes are dependent on
temperature; spills that occur in Arctic conditions are more
persistent than those that occur in temperate climates. In marine
environments, most of the low-relative-molecular-mass aromatic species
are dissolved into the water phase, but the primary branched alkanes,
cycloalkanes, and remaining aromatic compounds may remain in sediments
for more than a year.
Although no information is available on the photooxidation of
diesel fuels in water and air, evaporated oil components are degraded
photochemically. No. 2 fuel oil has been shown to be photooxidized
rapidly in water under environmental conditions.
The individual constituents of diesel fuel are inherently
biodegradable, to varying degrees and at different rates. The
n-alkane, n-alkylaromatic, and simple aromatic molecules in the C10-C22
range are the most readily degradable. Smaller molecules are generally
rapidly metabolized. Long-chain n-alkanes are more slowly degraded,
owing to their hydrophobicity and because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to biological breakdown, and PAHs are resistant. The overall
rates of degradation of hydrocarbons are limited by temperature, water
content, oxygen, pH, inorganic nutrients, and microbial metabolic
versatility.
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this actually occurs in
nature is poorly understood. Unlike microorganisms that use petroleum
carbons as a carbon source, animals generally oxidize and conjugate
products, rendering end-products that are more soluble and therefore
easier to excrete. All animal species tested can take up petroleum
hydrocarbons. PAHs, crude oil, and refined petroleum products are
known to induce cytochrome P450 enzymes and to increase the levels of
hydrocarbon metabolism in numerous marine and freshwater fish species.
Few data are available on the bioaccumulation of diesel fuel in
the laboratory, but there is plentiful evidence from studies of spills
and laboratory studies on other oils, particularly No. 2 fuel oil,
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient for diesel fuel is 3.3-7.06,
which suggests high potential bioaccumulation; however, many of the
lower-relative-molecular-mass compounds are readily metabolized, and
the actual bioaccumulation of higher-relative-molecular-mass compounds
is limited by their low water solubility and large molecular size.
Thus, actual bioaccumulation may be low.
Fish have been tainted by diesel fuel after spills. No data are
available on the biomagnification of diesel fuel.
No experimental data are available on the movement of diesel fuel
through the soil, although a direct correlation between the movement
and kinematic viscosity has been proposed. The movement of kerosene
through soil depends on the moisture content and nature of the soil.
A1.4 Environmental levels and human exposure
As diesel fuels are complex mixtures, the environmental levels
have not been measured. The individual constituents of diesel fuels
can be detected in almost all compartments of the environment,
although their source cannot be verified. The general population may
be exposed to diesel fuel at filling stations and as a result of
spills.
Occupational exposure to diesel fuel occurs in a large number of
activities. Because of their low volatility, diesel fuels should
generate only low concentrations of vapours at normal temperatures,
but high operating temperatures can result in significant
concentrations.
A1.5 Effects on laboratory mammals and in-vitro test systems
The acute toxicity of diesel fuels is low after oral or dermal
exposure or after inhalation. The oral LD50 value was > 5000 mg/kg
body weight in all species tested (mouse, rabbit, rat, guinea-pig).
Dermal application resulted in an LD50 value of > 5000 mg/kg body
weight in mice and rabbits, although values of > 2000 mg/kg body
weight were reported for some kerosenes and middle distillates, with
different protocols and lower limit doses. The LC0 value in rats
exposed by inhalation was about 5 mg/litre, except for one
straight-run middle distillate for which a value of 1.8 mg/litre was
seen.
In rabbits treated dermally with up to 8000 µl/kg body weight per
day and mice with up to 40 000 mg/kg body weight per day, acanthosis
and hyperkeratosis due to severe irritation were seen. Rabbits were
more sensitive than mice. Inhalation of diesel fuel was neuro-
depressive in mice at concentrations up to 0.2 mg/litre but not
in rats exposed to up to 6 mg/litre. Body and liver weights were
reduced in rats.
Mice, rats, and dogs did not show significant cumulative toxicity
after inhalation of up to 1.5 mg/litre subchronically. The specific
nephropathy syndrome seen in male rats is linked to an inherent
accumulation of hyalin droplets in the renal tubules.
The only effects of long-term exposures were ulceration after
dermal application to mice (250 or 500 mg/kg body weight per day) and
significant alterations in organ weight after inhalation of 1 or
5 mg/litre by rats. In both studies the mean body weights were
reduced.
Various types of diesel fuels were slightly to severely
irritating to the skin of rabbits. Diesel fuels do not irritate the
eye, but some kerosenes have been reported to have a slight irritating
effect. Diesel fuels do not cause skin sensitization.
Diesel and jet fuels (kerosene) were neither embryotoxic nor
teratogenic in two studies in rats exposed by inhalation to 100 or
400 ppm and in one study in which rats were given up to 2000 mg/kg
body weight per day by gavage. In the last study, reduced fetal weight
was observed.
Tests in Salmonella typhimurium did not provide clear evidence
of mutagenicity. Some positive findings in S. typhimurium and in
mouse lymphoma cells were considered to be equivocal owing to the
inconsistency of the results. Tests for genotoxicity in mice in vivo
(induction of micronuclei or chromosomal aberrations) also gave
equivocal or negative responses.
Diesel fuels induced a low level of dermal carcinogenicity. In
the present state of research, it cannot be concluded whether the
carcinogenic potency of diesel fuels is mediated by a genotoxic
mechanism or by chronic dermal damage.
A1.6 Effects on humans
Non-occupational exposure to diesel fuel can occur during manual
filling of fuel tanks. The primary source of dermal exposure is
accidental spills, which result in immediate high levels of exposure
but are of short duration.
After accidental dermal contact, anuria, renal failure, gastro-
intestinal symptoms, and cutaneous hyperkeratosis have been reported.
Toxic lung disease has been observed after accidental ingestion of
diesel fuel and subsequent aspiration. Persistent productive cough has
been reported after inhalation. In a case-control study of men exposed
to diesel fuel, an increased risk for cancer of the lung other than
adenocarcinoma was found; a positive association was also seen with
prostatic cancer, although a higher risk was noted for the group with
'nonsubstantial' exposure than for that with 'substantial' exposure.
In a cross-sectional study of factory workers exposed to kerosene jet
fuels, dizziness, headache, nausea, palpitation, pressure in the
chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of
vapour from the fuel in the breathing zone was estimated to be
128-423 mg/m3.
A1.7 Effects on other organisms in the laboratory and the field
Diesel fuel is more toxic than crude oil to aquatic organisms and
plants. The ecotoxicity of diesel fuel is generally attributed to
soluble aromatic compounds, but insoluble aliphatic hydrocarbons may
also be implicated. Of the aromatic compounds, monoaromatics are the
least toxic, their acute toxicity increasing with molecular mass up to
the four- to five-ring compounds, although these are poorly soluble in
seawater. In some animals, e.g. fish and birds, physical coating of
the body surface by the fuel can produce toxicity and mortality.
Laboratory experiments have been carried out on diesel fuel,
water-soluble fractions, oil-water dispersions, and microencapsulated
oil. Diesel fuel did not significantly reduce the growth in culture of
the green alga Euglena gracilis, whereas a low concentration (0.1%)
almost completely inhibited the growth of Scenedesmus quadricauda.
Light diesel fuel (0.05%) stimulated the growth, photosynthesis, and
chlorophyll asynthesis of Chlorella salina but slightly inhibited
respiration; at higher concentrations, the growth rate and
photosynthesis were greatly reduced. Long-term exposure inhibited the
growth of the benthic algae Ascophyllum nodosum and Laminaria
digitata. In blue-green algae, photosynthesis was reduced by the
aromatic and asphaltic fractions but not by the aliphatic fraction.
Diesel fuel was acutely toxic to Daphnia spp., chironomid
larvae, and the mollusc Viviparus bengalensis (Gastropoda). A
concentration of 0.1 ml/litre caused the death of tidepool copepods,
Tigriopus californicus, within five days.
Mytilus edulismussels accumulate diesel fuel, have markedly
reduced feeding and growth rates, and show reproductive toxicity after
chronic exposure to diesel fuel. The EC50 for spawning in mussels
exposed for 30 days was about 800 µg/litre. The LC50 of micro-
encapsulated diesel oil after exposure of maturing mussels for
30 days was about 5000 µg/litre. Diesel oil was more toxic to larvae
than to juveniles: 10 µg/litre had adverse effects on the growth of
larvae.
Freshwater crabs (Barytelphusa cunicularis) exposed to sublethal
concentrations of diesel fuel for up to 96 h generally reduced their
oxygen consumption, particularly at lower exposures up to 8 h. With
longer exposures, the oxygen consumption was equal to or higher than
that of the controls.
In 96-h tests of acute toxicity in juvenile salmonids under
static conditions, diesel fuel was more toxic to pink salmon,
Onchorhychus gorbuscha (LC50: 32-123 mg/litre), than to cohosalmon,
O. kisutch (LC50: 2186-3017 mg/litre), or rainbow trout,
O. mykiss (LC50: 3333-33 216 mg/litre), irrespective of water type.
The threshold for detection of behavioural responses of cod
( Gadus morhua L.) exposed to diesel fuel in seawater was within
100-400 ng/litre. The Antarctic fish Pagothenia borchgrevinki
withstood an undiluted water-soluble fraction of diesel fuel oil for
up to 72 h but showed signs of stress.
Birds are affected externally and internally by oil
contamination. Diesel fuel destroys the waterproof nature of the
birds' plumage and is ingested during preening. Diesel and fuel oil
fed by gavage at 2 ml/kg body weight to ducks caused lipid pneumonia,
extreme inflammation of the lungs, fatty infiltration of the liver,
and hepatic degeneration after 24 h. Administration of diesel or fuel
oil at 1 ml/kg caused severe irritation of the digestive tract and
toxic nephrosis. Higher doses resulted in adrenal enlargement (mainly
due to hyperplasia of cortical tissue), depression of plasma
cholinesterase levels, ataxia, and tremors. Doses up to 20 ml/kg body
weight were not fatal to healthy birds, but the LD50 for diesel and
fuel oil administered to birds under stress was 3-4 ml/kg body weight.
After spills of diesel fuel, zooplankton appear to be highly
vulnerable to dispersed and dissolved petroleum constituents but less
so to floating oils. Aquatic organisms may be affected in a number of
ways, including direct mortality (fish eggs, copepods, and mixed
plankton), external contamination by oil (chorions of fish eggs and
cuticles and feeding appendages of crustaceans), tissue contamination
by aromatic constituents, abnormal development of fish embryos, and
altered metabolic rates.
A1.8 Evaluation of human health risks
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, during the handling of such fuels, and during use of kerosene
for domestic cooking or heating. Workers can be exposed to diesel fuel
and other middle distillates while handling and discharging the fuel
at terminals, storage tanks, and filling stations; during the
manufacture, repair, maintenance, and testing of diesel engines and
other equipment; during use of diesel fuel as a cleaning agent or
solvent; and in handling and routine sampling of diesel fuel in the
laboratory. Owing to the low volatility of diesel fuel, only low
concentrations of vapour are likely to occur at room temperature,
although in confined spaces at high temperatures significant levels
may be found.
Exposure to vapour is minimal during the normal handling of
diesel fuel. The most likely effect on human health is dermatitis
after skin contact. Diesel fuel is a skin irritant but does not appear
to irritate the eye. Acute toxic effects on the kidney can occur after
dermal exposure, but the effects of long-term dermal absorption of low
concentrations are unknown.
Diesel fuels are toxic when ingested, sometimes resulting in
regurgitation and aspiration, which can cause chemical pneumonia; the
same is true for any hydrocarbon in a particular range of viscosity.
In rodents exposed by inhalation to diesel fuel at concentrations
up to 0.2 mg/litre, a neurodepressive effect was seen in mice but not
in rats at the higher concentrations. Subchronic exposure by
inhalation to various distillate fuels induced specific
alpha2-microglobulin nephropathy in male rats; this effect is
considered irrelevant for humans.
Diesel fuels were neither embryotoxic nor teratogenic in animals
exposed orally or by inhalation.
There is no clear evidence of mutagenic activity in bacteria, and
the results of other tests for genotoxicity in vitro and in vivo were
equivocal.
A case-control study of workers exposed to diesel fuel suggested
an increased risk for cancer of the lung other than adenocarcinoma and
for prostatic cancer. In neither case was there an exposure-response
relationship. In view of the small number of studies available, the
small number of cases, and the correspondingly wide confidence
intervals, no conclusion can be drawn about the carcinogenicity to
humans of diesel fuel.
In mice, dermally administered diesel fuels had weak carcinogenic
potential. In view of the absence of clear genotoxicity, cancer could
be induced by nongenotoxic mechanisms, e.g. by chronic dermal
irritation characterized by repeated cycles of skin lesions, causing
epidermal hyperplasia.
A1.9 Evaluation of effects on the environment
The environment can be polluted by accidental release of diesel
fuel on a large scale, such as during tanker disasters and pipeline
leaks, or on a smaller scale from contamination of soil around
factories or garages. In water, diesel fuel spreads almost
immediately, polar and low-relative-molecular-mass components dissolve
and leach out, volatile components evaporate from the water surface,
and microbial degradation begins. The extent to which 'weathering'
takes place depends on the temperature and on climatic conditions. The
chemical composition of spills changes with time: after spillage on
water, some fractions evaporate, and the evaporated diesel components
are degraded photochemically; in sediment, diesel fuel appears
generally to be delivered to bottom sediments by settling particles;
in soil, the components of diesel fuel migrate at different rates,
depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates of biodegradation depend heavily on
physical and climatic conditions and on microbial composition.
Aquatic organisms, in particular molluscs, bioaccumulate
hydro-carbons to varying extents, but the hydrocarbons are depurated
on transfer to clean water. Diesel fuel may be bioaccumulated; no data
are available about biomagnification.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota. Recolonization
may occur after about one year, depending on the animal or plant
species and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can be affected by
external oil contamination and tissue accumulation: abnormal
development and altered metabolic rates are signs of the resulting
stress.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Diesel fuels are a gas-oil fraction occurring during petroleum
separation and commonly known as middle distillates (International
Agency for Research on Cancer, 1989a). Gas oils are generally blended
materials formulated to meet technical specifications (CONCAWE, in
press). Commercial diesel fuels contain aliphatic, olefinic,
cycloparaffinic, and aromatic hydrocarbons (see section A2.1.1), and
additives (see section A2.1.2) to improve their fuelling properties
(Sandmeyer, 1981).
Four qualities of diesel fuel are available commercially: diesel
fuel (general), diesel fuel No. 1, diesel fuel No. 2, and diesel fuel
No. 4 (see Table 1). The composition of diesel fuels is comparable to
that of heating oils (e.g. No. 1 and No. 2 fuel oils), except for the
additives (International Agency for Research on Cancer, 1989a; Agency
for Toxic Substances and Disease Registry, 1995).
Diesel fuel No. 2 is used mainly as automobile fuel and
corresponds to diesel fuel (general). In Europe (countries of the
European Union (EU) and European Free Trade Area (EFTA)), the
specifications for diesel fuel for transportation purposes are given
in European standard EN 590 (European Committee for Standardization,
1993), which also provides for changes in the specifications to meet
the requirements of different climatic conditions. In Sweden, two
further qualities of on-road diesel fuel are available: environmental
class 1 and class 2 (city diesel), with sulfur contents of 0.05% and
0.001%, respectively (Standardization Board in Sweden, 1991). The
sulfur content of city diesel corresponds to that of kerosene. Diesel
fuel for ship engines is covered by the International Standards
Organization (ISO) standard 8217 (International Standards
Organization, 1987). In the United States of America, three grades of
diesel fuel are available: diesel fuel No. 1 (relatively high
volatility) for road vehicle engines subject to frequent speed and
load changes; diesel fuel No. 2 (lower volatility) for industrial or
heavy-duty, high-load engines running at uniform speed; and diesel
fuel No. 4 (viscous) for low- and medium-speed engines such as those
used in ships (American Society for Testing and Materials, 1988,
1992).
Several types of kerosene, also derived from the gas-oil fraction
of petroleum separation, are used as aviation turbine fuels (JP fuels,
jet fuels). Their hydrocarbon composition is comparable to that of
diesel fuels.
A2.1.1 Fuel components
A2.1.1.1 Alkanes
Normal, branched, and cyclic alkanes (paraffins) are the most
abundant components (about 65-85%) of diesel fuels. Pristane
(2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-
tetramethyl-hexadecane) are of particular interest environmentally, as
the ratios of pristane to heptadecane and of phytane to octadecane
make it possible to identify the source of a fuel spill; furthermore,
as these ratios increase during biological degradation, they can be
used to estimate the age of an environmental contamination and the
degree of elimination. Cycloalkanes and bicycloalkanes constitute a
significant portion of the mixture, but individual compounds are
present only at low levels and are difficult to analyse. Alkyl
derivatives of cyclopentane, cyclohexane, and cycloheptane are common
components (Block et al., 1991; Table 2).
A2.1.1.2 Alkenes
Alkenes are not common components of crude oil but may be
present in diesel fuel if converted products are added after cracking.
These alkenes have predominantly branched and cyclic structures (Block
et al., 1991). The total alkene content of diesel fuels is up to 10%
(CONCAWE, 1985) (see also Table 2).
A2.1.1.3 Aromatic compounds
Aromatic compounds constitute 5-30% of automotive diesel fuel,
5-40% of marine diesel fuel (CONCAWE, 1985), and 10-30% of diesel
fuel No. 2 (Block et al., 1991). Table 2 shows the specifications of
commercial diesel fuels in this regard.
Table 1. Synonyms and trade names of commercial diesel fuels
Name CAS CAS Registry Range of Synonyms
name number carbon
numbers
Diesel fuel Diesel oil
All participating institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting exhaust emissions of the documents. A comprehensive file of
all comments received on drafts of each EHC monograph is maintained
and is available on request. The chairpersons of task groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO DRAFTING GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL
AND EXHAUST EMISSIONS
WHO, Geneva, 6-9 December 1993
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United State
Dr R.P. Bos, University of Nijmegen, Nijmegen, Netherlands
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom (Joint Rapporteur)
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr I. Farkas, National Institute of Hygiene, Budapest, Hungary
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr P. Gustavsson, North Western Health Board, Stockholm, Sweden
Dr D. Guth, United States Environmental Protection Agency, Research
Triangle Park, NC, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr W. Pepelko, United States Environmental Protection Agency,
Washington DC, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
(Vice-Chairman)
Dr G. Rosner, Hazardous Substances Documentation Group, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr J. Roycroft, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, United States
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States (Chairman)
Dr B.H. Thomas, Environmental Health Directorate, Ottawa, Canada
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr. R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr P. Boffetta, International Agency for Research on Cancer, Lyon,
France (6-7 December 1993)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Representatives/Observers
CONCAWE
Dr R.H. McKee, Exxon Biomedical Sciences, East Millstone, NJ,
United States
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT
Dr P.T.C. Harrison, MRC Institute of Environment & Health,
Leicester, United Kingdom
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIESEL FUEL AND
EXHAUST EMISSIONS
Fraunhofer Institute of Toxicology & Aerosol Research, Hanover
27 June-1 July 1994
Members
Dr J.A. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, NC, United States
Dr R. Brown, Medical Research Council Toxicology Unit, University of
Leicester, Leicester, United Kingdom
Dr Chao Chen, Human Health Assessment Group, United States
Environmental Protection Agency, Washington DC, United States
Dr E. Garshick, Pulmonary Section, Brockton/West Roxbury VA Medical
Center, West Roxbury, MA, United States
Dr U. Heinrich, Department of Experimental Hygiene, Fraunhofer
Institute of Toxicology and Aerosol Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Office, Bundesgesundheitsamt, Berlin,
Germany
Dr Jun Kagawa, Tokyo Women's Medical College, Tokyo, Japan
Professor G. Oberdörster, Department of Environmental Medicine,
University of Rochester Medical Center, Rochester, NY, United States
Dr P.J.A. Rombout, Laboratory of Toxicology, National Institute of
Public Health and Environmental Protection, Bilthoven, Netherlands
Dr M. Roller, Medizinishes Institut für Umwelthyglene an der
Universität Düsseldorf, Düsseldorf, Germany Dr G. Rosner, Hazardous
Substances Documentation Group, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Dr A. Sivak, Environmental Health Sciences, Saint Augustine, FL,
United States
Dr L. Turrio, Istituto Superiore di Sanita, Laboratorio Tossicologia
Comparata e Ecotossicologia, Rome, Italy
Mr R. Waller, Department of Health, London, United Kingdom
Secretariat
Dr H. Moller, International Agency for Research on Cancer, Lyon,
France
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr M. Younes, European Centre for Environment and Health, Bilthoven,
Netherlands
Representatives/Observers
Dr Lutz Von Meyerinck, BP Oil Europe, Brussels, Belgium
GERMAN AUTOMOBILE ASSOCIATION
Dr N. Pelz, Mercedes-Benz AG, Stuttgart, Germany
ILSI
Dr I.T. Salmeen, Chemistry Department, Ford Motor Company,
Dearborn, MI, United States
IUTOX
Dr P. Montuschi, Department of Pharmacology, School of Medicine,
Catholic University of the Sacred Heart, Rome, Italy
ENVIRONMENTAL HEALTH CRIETERIA FOR DIESEL FUEL AND EXHAUST EMISSIONS
A WHO Task Group on Environmental Health Criteria for Diesel Fuel
and Exhaust Emissions met at the Fraunhofer Institute of Toxicology
and Aerosol Research, Hanover, Germany from 27 June to 1 July 1994.
Dr G. Rosner, Fraunhofer Institute, welcomed the participants on
behalf of the Institute and its Director, Professor U. Mohr, and
Dr E.M. Smith, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS, and on behalf of the heads of the
three IPCS cooperating organizations (UNEP, ILO, and WHO). The Task
Group reviewed and revised the draft and evaluated the risks for human
health and the environment from exposure to diesel fuel and exhaust
emissions.
The first draft of the monograph was prepared at the Fraunhofer
Institute. After international circulation for comment, this draft was
extensively revised by a Working/Drafting Group, convened at WHO,
Geneva, from 6 to 9 December 1993, and a second draft was prepared for
further international circulation for comment. The membership of the
drafting group is shown previously. A final draft, incorporating
comments received from the IPCS contact points for Environmental
Health Criteria monographs and new material, was completed at the
Fraunhofer Institute under the coordination of Dr G. Rosner, with
important contributions to the text from the following Institute staff
members:
Dr B. Bellman
Dr A. Boehnke
Dr O. Creutzenberg
Dr J. Kielhorn
Dr E.M. Smith of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs E. Heseltine, Lajarthe,
France, for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
PART A DIESEL FUEL
A1. SUMMARY
A1.1 Identity, physical and chemical properties, and analytical
methods
Diesel fuel is a complex mixture of normal, branched, and cyclic
alkanes (60 to > 90% by volume; hydrocarbon chain length, usually
between C9 and C30); aromatic compounds, especially alkylbenzenes
(5-40% by volume); and small amounts of alkenes (0-10% by volume)
obtained from the middle-distillate, gas-oil fraction during petroleum
separation. Benzene, toluene, ethylbenzene, and xylenes and polycyclic
aromatic hydrocarbons (PAHs), especially naphthalene and its
methyl-substituted derivatives, may be present at levels of parts per
million in diesel fuel. The sulfur content of diesel fuels depends on
the source of crude oil and the refinery process. It is regulated by
law in a number of countries and is usually between 0.05 and 0.5
weight percent. Additives are used to influence the flow, storage, and
combustion of diesel fuel, to differentiate products, and to meet
trademark specifications. At room temperature, diesel fuels are
generally moderately volatile, slightly viscous, flammable, brown
liquids with a kerosene-like odour. The boiling ranges are usually
between 140 and 385°C (> 588°C for marine diesel fuel); at 20°C, the
density is 0.87-1.0 g/cm3 and the water solubility is
0.2-5 mg/litre. The quality and composition of diesel fuel influence
the emissions of pollutants from diesel engines considerably.
Important variables are ignition behaviour (expressed in terms of
cetane number), density, viscosity, and sulfur content. The
specifications of commercial diesel fuel differ considerably in
different countries.
Heating fuels and some kerosene jet fuels produced during the
refining process may have a composition similar to that of diesel
fuel, although with different additives. Biological data on these
mixtures have therefore also been taken into account in the
assessments of toxicity and ecotoxicity.
Owing to the complexity of the diesel fuel mixture, there is no
specific analytical method, and the analytical techniques used in most
environmental assessments are suitable only for measuring the total
petroleum hydrocarbon mixture. The methods consist of preliminary
solvent extraction, a clean-up procedure to remove naturally occurring
hydrocarbons, and subsequent detection by gravimetry, infrared
spectroscopy or gas chromatography. Neither the gravimetric nor the
infrared technique provides useful qualitative or quantitative
information on contaminants and can thus be used only for screening.
Gas chromatography combined with detection techniques such as flame
ionization and mass spectrometry is the standard procedure for
analysing environmental samples. Many other methods are available for
the analysis of individual hydrocarbons in diesel fuels.
A1.2 Sources of human and environmental exposure
Diesel fuels are produced by refining crude oils. In order to
meet technical specifications for performance, diesel fuels are
generally blended; further formulation with additives improves their
properties for specific uses. Diesel fuels are widely used as
transportation fuels. The more volatile fuels, with low viscosity, are
required for high-speed engines and the heavier grades for railroad
and ship diesel engines. Much heavy-duty road transport is powered by
diesel engines. Passenger cars powered by diesel engines are becoming
increasingly more common in Europe and Japan (10-25%), whereas in
North America the percentage of diesel-fuelled passenger cars is about
1-2%, with a slightly decreasing tendency. Diesel fuel is used in
stationary engines and in boilers, e.g. reciprocating engines, gas
turbines, pipeline pumps, gas compressors, steam processing units in
electric power plants, burner installations, and industrial space and
water heating facilities.
Over the last five years, the worldwide demand for diesel fuels
has increased steadily. In 1985, the following amounts of diesel fuel
were consumed: about 170 000 kt per year in North America; about
160 000 kt per year, including gas oils, in the European Union; and
about 46 000 kt per year in Australia, Japan, and New Zealand,
equivalent to a total of 1062 kt per day. In 1990, world demand was
reported to be about 1110 kt per day.
No information is available on emissions during the production of
diesel fuels; however, this source would seem to be of minor
importance, because the refining process is carried out in closed
systems. Emissions may occur principally during storage and
transportation. Diesel fuels are released as a result of spills and at
filling stations during the refuelling of vehicles. The atmosphere and
the hydrosphere are the most heavily affected environmental
compartments. Soil contamination with diesel fuels may occur during
accidents and is also a problem in railroad yards. The numerous
techniques for cleaning soils contaminated with diesel fuel include
excavation, biological methods, and containment.
A1.3 Environmental transport, distribution, and transformation
Very few data are avilable on the environmental fate of diesel
fuels, but the mechanisms of their distribution and transformation are
considered to be comparable to those of heating fuels, such as No. 2
fuel oil, which have been well studied. Spills of diesel fuel on water
spread almost immediately to form a 'slick'. The polar and
low-relative-molecular-mass components dissolve and leach out of the
slick, and the volatile components evaporate from the surface;
microbial degradation also begins. Chemical and biological weathering
alter the composition of the spill. These processes are dependent on
temperature; spills that occur in Arctic conditions are more
persistent than those that occur in temperate climates. In marine
environments, most of the low-relative-molecular-mass aromatic species
are dissolved into the water phase, but the primary branched alkanes,
cycloalkanes, and remaining aromatic compounds may remain in sediments
for more than a year.
Although no information is available on the photooxidation of
diesel fuels in water and air, evaporated oil components are degraded
photochemically. No. 2 fuel oil has been shown to be photooxidized
rapidly in water under environmental conditions.
The individual constituents of diesel fuel are inherently
biodegradable, to varying degrees and at different rates. The
n-alkane, n-alkylaromatic, and simple aromatic molecules in the C10-C22
range are the most readily degradable. Smaller molecules are generally
rapidly metabolized. Long-chain n-alkanes are more slowly degraded,
owing to their hydrophobicity and because they are viscous or solid at
ambient temperatures. Branched alkanes and cycloalkanes are relatively
resistant to biological breakdown, and PAHs are resistant. The overall
rates of degradation of hydrocarbons are limited by temperature, water
content, oxygen, pH, inorganic nutrients, and microbial metabolic
versatility.
Unicellular algae can take up and metabolize both aliphatic and
aromatic hydrocarbons, but the extent to which this actually occurs in
nature is poorly understood. Unlike microorganisms that use petroleum
carbons as a carbon source, animals generally oxidize and conjugate
products, rendering end-products that are more soluble and therefore
easier to excrete. All animal species tested can take up petroleum
hydrocarbons. PAHs, crude oil, and refined petroleum products are
known to induce cytochrome P450 enzymes and to increase the levels of
hydrocarbon metabolism in numerous marine and freshwater fish species.
Few data are available on the bioaccumulation of diesel fuel in
the laboratory, but there is plentiful evidence from studies of spills
and laboratory studies on other oils, particularly No. 2 fuel oil,
that aquatic organisms bioconcentrate hydrocarbons. The
n-octanol-water partition coefficient for diesel fuel is 3.3-7.06,
which suggests high potential bioaccumulation; however, many of the
lower-relative-molecular-mass compounds are readily metabolized, and
the actual bioaccumulation of higher-relative-molecular-mass compounds
is limited by their low water solubility and large molecular size.
Thus, actual bioaccumulation may be low.
Fish have been tainted by diesel fuel after spills. No data are
available on the biomagnification of diesel fuel.
No experimental data are available on the movement of diesel fuel
through the soil, although a direct correlation between the movement
and kinematic viscosity has been proposed. The movement of kerosene
through soil depends on the moisture content and nature of the soil.
A1.4 Environmental levels and human exposure
As diesel fuels are complex mixtures, the environmental levels
have not been measured. The individual constituents of diesel fuels
can be detected in almost all compartments of the environment,
although their source cannot be verified. The general population may
be exposed to diesel fuel at filling stations and as a result of
spills.
Occupational exposure to diesel fuel occurs in a large number of
activities. Because of their low volatility, diesel fuels should
generate only low concentrations of vapours at normal temperatures,
but high operating temperatures can result in significant
concentrations.
A1.5 Effects on laboratory mammals and in-vitro test systems
The acute toxicity of diesel fuels is low after oral or dermal
exposure or after inhalation. The oral LD50 value was > 5000 mg/kg
body weight in all species tested (mouse, rabbit, rat, guinea-pig).
Dermal application resulted in an LD50 value of > 5000 mg/kg body
weight in mice and rabbits, although values of > 2000 mg/kg body
weight were reported for some kerosenes and middle distillates, with
different protocols and lower limit doses. The LC0 value in rats
exposed by inhalation was about 5 mg/litre, except for one
straight-run middle distillate for which a value of 1.8 mg/litre was
seen.
In rabbits treated dermally with up to 8000 µl/kg body weight per
day and mice with up to 40 000 mg/kg body weight per day, acanthosis
and hyperkeratosis due to severe irritation were seen. Rabbits were
more sensitive than mice. Inhalation of diesel fuel was neuro-
depressive in mice at concentrations up to 0.2 mg/litre but not
in rats exposed to up to 6 mg/litre. Body and liver weights were
reduced in rats.
Mice, rats, and dogs did not show significant cumulative toxicity
after inhalation of up to 1.5 mg/litre subchronically. The specific
nephropathy syndrome seen in male rats is linked to an inherent
accumulation of hyalin droplets in the renal tubules.
The only effects of long-term exposures were ulceration after
dermal application to mice (250 or 500 mg/kg body weight per day) and
significant alterations in organ weight after inhalation of 1 or
5 mg/litre by rats. In both studies the mean body weights were
reduced.
Various types of diesel fuels were slightly to severely
irritating to the skin of rabbits. Diesel fuels do not irritate the
eye, but some kerosenes have been reported to have a slight irritating
effect. Diesel fuels do not cause skin sensitization.
Diesel and jet fuels (kerosene) were neither embryotoxic nor
teratogenic in two studies in rats exposed by inhalation to 100 or
400 ppm and in one study in which rats were given up to 2000 mg/kg
body weight per day by gavage. In the last study, reduced fetal weight
was observed.
Tests in Salmonella typhimurium did not provide clear evidence
of mutagenicity. Some positive findings in S. typhimurium and in
mouse lymphoma cells were considered to be equivocal owing to the
inconsistency of the results. Tests for genotoxicity in mice in vivo
(induction of micronuclei or chromosomal aberrations) also gave
equivocal or negative responses.
Diesel fuels induced a low level of dermal carcinogenicity. In
the present state of research, it cannot be concluded whether the
carcinogenic potency of diesel fuels is mediated by a genotoxic
mechanism or by chronic dermal damage.
A1.6 Effects on humans
Non-occupational exposure to diesel fuel can occur during manual
filling of fuel tanks. The primary source of dermal exposure is
accidental spills, which result in immediate high levels of exposure
but are of short duration.
After accidental dermal contact, anuria, renal failure, gastro-
intestinal symptoms, and cutaneous hyperkeratosis have been reported.
Toxic lung disease has been observed after accidental ingestion of
diesel fuel and subsequent aspiration. Persistent productive cough has
been reported after inhalation. In a case-control study of men exposed
to diesel fuel, an increased risk for cancer of the lung other than
adenocarcinoma was found; a positive association was also seen with
prostatic cancer, although a higher risk was noted for the group with
'nonsubstantial' exposure than for that with 'substantial' exposure.
In a cross-sectional study of factory workers exposed to kerosene jet
fuels, dizziness, headache, nausea, palpitation, pressure in the
chest, and eye irritation were found to be more prevalent than in
unexposed controls. The time-weighted average concentration of
vapour from the fuel in the breathing zone was estimated to be
128-423 mg/m3.
A1.7 Effects on other organisms in the laboratory and the field
Diesel fuel is more toxic than crude oil to aquatic organisms and
plants. The ecotoxicity of diesel fuel is generally attributed to
soluble aromatic compounds, but insoluble aliphatic hydrocarbons may
also be implicated. Of the aromatic compounds, monoaromatics are the
least toxic, their acute toxicity increasing with molecular mass up to
the four- to five-ring compounds, although these are poorly soluble in
seawater. In some animals, e.g. fish and birds, physical coating of
the body surface by the fuel can produce toxicity and mortality.
Laboratory experiments have been carried out on diesel fuel,
water-soluble fractions, oil-water dispersions, and microencapsulated
oil. Diesel fuel did not significantly reduce the growth in culture of
the green alga Euglena gracilis, whereas a low concentration (0.1%)
almost completely inhibited the growth of Scenedesmus quadricauda.
Light diesel fuel (0.05%) stimulated the growth, photosynthesis, and
chlorophyll asynthesis of Chlorella salina but slightly inhibited
respiration; at higher concentrations, the growth rate and
photosynthesis were greatly reduced. Long-term exposure inhibited the
growth of the benthic algae Ascophyllum nodosum and Laminaria
digitata. In blue-green algae, photosynthesis was reduced by the
aromatic and asphaltic fractions but not by the aliphatic fraction.
Diesel fuel was acutely toxic to Daphnia spp., chironomid
larvae, and the mollusc Viviparus bengalensis (Gastropoda). A
concentration of 0.1 ml/litre caused the death of tidepool copepods,
Tigriopus californicus, within five days.
Mytilus edulismussels accumulate diesel fuel, have markedly
reduced feeding and growth rates, and show reproductive toxicity after
chronic exposure to diesel fuel. The EC50 for spawning in mussels
exposed for 30 days was about 800 µg/litre. The LC50 of micro-
encapsulated diesel oil after exposure of maturing mussels for
30 days was about 5000 µg/litre. Diesel oil was more toxic to larvae
than to juveniles: 10 µg/litre had adverse effects on the growth of
larvae.
Freshwater crabs (Barytelphusa cunicularis) exposed to sublethal
concentrations of diesel fuel for up to 96 h generally reduced their
oxygen consumption, particularly at lower exposures up to 8 h. With
longer exposures, the oxygen consumption was equal to or higher than
that of the controls.
In 96-h tests of acute toxicity in juvenile salmonids under
static conditions, diesel fuel was more toxic to pink salmon,
Onchorhychus gorbuscha (LC50: 32-123 mg/litre), than to cohosalmon,
O. kisutch (LC50: 2186-3017 mg/litre), or rainbow trout,
O. mykiss (LC50: 3333-33 216 mg/litre), irrespective of water type.
The threshold for detection of behavioural responses of cod
( Gadus morhua L.) exposed to diesel fuel in seawater was within
100-400 ng/litre. The Antarctic fish Pagothenia borchgrevinki
withstood an undiluted water-soluble fraction of diesel fuel oil for
up to 72 h but showed signs of stress.
Birds are affected externally and internally by oil
contamination. Diesel fuel destroys the waterproof nature of the
birds' plumage and is ingested during preening. Diesel and fuel oil
fed by gavage at 2 ml/kg body weight to ducks caused lipid pneumonia,
extreme inflammation of the lungs, fatty infiltration of the liver,
and hepatic degeneration after 24 h. Administration of diesel or fuel
oil at 1 ml/kg caused severe irritation of the digestive tract and
toxic nephrosis. Higher doses resulted in adrenal enlargement (mainly
due to hyperplasia of cortical tissue), depression of plasma
cholinesterase levels, ataxia, and tremors. Doses up to 20 ml/kg body
weight were not fatal to healthy birds, but the LD50 for diesel and
fuel oil administered to birds under stress was 3-4 ml/kg body weight.
After spills of diesel fuel, zooplankton appear to be highly
vulnerable to dispersed and dissolved petroleum constituents but less
so to floating oils. Aquatic organisms may be affected in a number of
ways, including direct mortality (fish eggs, copepods, and mixed
plankton), external contamination by oil (chorions of fish eggs and
cuticles and feeding appendages of crustaceans), tissue contamination
by aromatic constituents, abnormal development of fish embryos, and
altered metabolic rates.
A1.8 Evaluation of human health risks
The general population can be exposed to diesel fuel and other
middle distillates at filling stations, as a result of accidental
spills, during the handling of such fuels, and during use of kerosene
for domestic cooking or heating. Workers can be exposed to diesel fuel
and other middle distillates while handling and discharging the fuel
at terminals, storage tanks, and filling stations; during the
manufacture, repair, maintenance, and testing of diesel engines and
other equipment; during use of diesel fuel as a cleaning agent or
solvent; and in handling and routine sampling of diesel fuel in the
laboratory. Owing to the low volatility of diesel fuel, only low
concentrations of vapour are likely to occur at room temperature,
although in confined spaces at high temperatures significant levels
may be found.
Exposure to vapour is minimal during the normal handling of
diesel fuel. The most likely effect on human health is dermatitis
after skin contact. Diesel fuel is a skin irritant but does not appear
to irritate the eye. Acute toxic effects on the kidney can occur after
dermal exposure, but the effects of long-term dermal absorption of low
concentrations are unknown.
Diesel fuels are toxic when ingested, sometimes resulting in
regurgitation and aspiration, which can cause chemical pneumonia; the
same is true for any hydrocarbon in a particular range of viscosity.
In rodents exposed by inhalation to diesel fuel at concentrations
up to 0.2 mg/litre, a neurodepressive effect was seen in mice but not
in rats at the higher concentrations. Subchronic exposure by
inhalation to various distillate fuels induced specific
alpha2-microglobulin nephropathy in male rats; this effect is
considered irrelevant for humans.
Diesel fuels were neither embryotoxic nor teratogenic in animals
exposed orally or by inhalation.
There is no clear evidence of mutagenic activity in bacteria, and
the results of other tests for genotoxicity in vitro and in vivo were
equivocal.
A case-control study of workers exposed to diesel fuel suggested
an increased risk for cancer of the lung other than adenocarcinoma and
for prostatic cancer. In neither case was there an exposure-response
relationship. In view of the small number of studies available, the
small number of cases, and the correspondingly wide confidence
intervals, no conclusion can be drawn about the carcinogenicity to
humans of diesel fuel.
In mice, dermally administered diesel fuels had weak carcinogenic
potential. In view of the absence of clear genotoxicity, cancer could
be induced by nongenotoxic mechanisms, e.g. by chronic dermal
irritation characterized by repeated cycles of skin lesions, causing
epidermal hyperplasia.
A1.9 Evaluation of effects on the environment
The environment can be polluted by accidental release of diesel
fuel on a large scale, such as during tanker disasters and pipeline
leaks, or on a smaller scale from contamination of soil around
factories or garages. In water, diesel fuel spreads almost
immediately, polar and low-relative-molecular-mass components dissolve
and leach out, volatile components evaporate from the water surface,
and microbial degradation begins. The extent to which 'weathering'
takes place depends on the temperature and on climatic conditions. The
chemical composition of spills changes with time: after spillage on
water, some fractions evaporate, and the evaporated diesel components
are degraded photochemically; in sediment, diesel fuel appears
generally to be delivered to bottom sediments by settling particles;
in soil, the components of diesel fuel migrate at different rates,
depending on the soil type.
The individual constituents of diesel fuel are inherently
biodegradable, but the rates of biodegradation depend heavily on
physical and climatic conditions and on microbial composition.
Aquatic organisms, in particular molluscs, bioaccumulate
hydro-carbons to varying extents, but the hydrocarbons are depurated
on transfer to clean water. Diesel fuel may be bioaccumulated; no data
are available about biomagnification.
Spills of diesel fuel have an immediate detrimental effect on the
environment, causing substantial mortality of biota. Recolonization
may occur after about one year, depending on the animal or plant
species and the chemical and physical content of the spill residues.
Aquatic organisms that survive diesel fuel spills can be affected by
external oil contamination and tissue accumulation: abnormal
development and altered metabolic rates are signs of the resulting
stress.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Diesel fuels are a gas-oil fraction occurring during petroleum
separation and commonly known as middle distillates (International
Agency for Research on Cancer, 1989a). Gas oils are generally blended
materials formulated to meet technical specifications (CONCAWE, in
press). Commercial diesel fuels contain aliphatic, olefinic,
cycloparaffinic, and aromatic hydrocarbons (see section A2.1.1), and
additives (see section A2.1.2) to improve their fuelling properties
(Sandmeyer, 1981).
Four qualities of diesel fuel are available commercially: diesel
fuel (general), diesel fuel No. 1, diesel fuel No. 2, and diesel fuel
No. 4 (see Table 1). The composition of diesel fuels is comparable to
that of heating oils (e.g. No. 1 and No. 2 fuel oils), except for the
additives (International Agency for Research on Cancer, 1989a; Agency
for Toxic Substances and Disease Registry, 1995).
Diesel fuel No. 2 is used mainly as automobile fuel and
corresponds to diesel fuel (general). In Europe (countries of the
European Union (EU) and European Free Trade Area (EFTA)), the
specifications for diesel fuel for transportation purposes are given
in European standard EN 590 (European Committee for Standardization,
1993), which also provides for changes in the specifications to meet
the requirements of different climatic conditions. In Sweden, two
further qualities of on-road diesel fuel are available: environmental
class 1 and class 2 (city diesel), with sulfur contents of 0.05% and
0.001%, respectively (Standardization Board in Sweden, 1991). The
sulfur content of city diesel corresponds to that of kerosene. Diesel
fuel for ship engines is covered by the International Standards
Organization (ISO) standard 8217 (International Standards
Organization, 1987). In the United States of America, three grades of
diesel fuel are available: diesel fuel No. 1 (relatively high
volatility) for road vehicle engines subject to frequent speed and
load changes; diesel fuel No. 2 (lower volatility) for industrial or
heavy-duty, high-load engines running at uniform speed; and diesel
fuel No. 4 (viscous) for low- and medium-speed engines such as those
used in ships (American Society for Testing and Materials, 1988,
1992).
Several types of kerosene, also derived from the gas-oil fraction
of petroleum separation, are used as aviation turbine fuels (JP fuels,
jet fuels). Their hydrocarbon composition is comparable to that of
diesel fuels.
A2.1.1 Fuel components
A2.1.1.1 Alkanes
Normal, branched, and cyclic alkanes (paraffins) are the most
abundant components (about 65-85%) of diesel fuels. Pristane
(2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-
tetramethyl-hexadecane) are of particular interest environmentally, as
the ratios of pristane to heptadecane and of phytane to octadecane
make it possible to identify the source of a fuel spill; furthermore,
as these ratios increase during biological degradation, they can be
used to estimate the age of an environmental contamination and the
degree of elimination. Cycloalkanes and bicycloalkanes constitute a
significant portion of the mixture, but individual compounds are
present only at low levels and are difficult to analyse. Alkyl
derivatives of cyclopentane, cyclohexane, and cycloheptane are common
components (Block et al., 1991; Table 2).
A2.1.1.2 Alkenes
Alkenes are not common components of crude oil but may be
present in diesel fuel if converted products are added after cracking.
These alkenes have predominantly branched and cyclic structures (Block
et al., 1991). The total alkene content of diesel fuels is up to 10%
(CONCAWE, 1985) (see also Table 2).
A2.1.1.3 Aromatic compounds
Aromatic compounds constitute 5-30% of automotive diesel fuel,
5-40% of marine diesel fuel (CONCAWE, 1985), and 10-30% of diesel
fuel No. 2 (Block et al., 1991). Table 2 shows the specifications of
commercial diesel fuels in this regard.
Table 1. Synonyms and trade names of commercial diesel fuels
Name CAS CAS Registry Range of Synonyms
name number carbon
numbers
Diesel fuel Diesel oil 
 Retention of diesel particles in the lungs and lymph nodes has
been reported in Fischer 344 and Wistar rats after long-term
inhalation (Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly
et al., 1987; Strom et al., 1988; Creutzenberg et al., 1990; Strom et
al., 1990). Some results with regard to lung burden are included in
Table 33. Because clearance of lung burdens above about 1 mg per lung
was impaired after long-term, continuous inhalation of a concentration
equivalent to about 0.5mg/m3, no steady-state lung burden was found
at serial sacrifice at up to two years. About 20% of the lung burden
of diesel particles was found in lung-associated lymph nodes at that
time.
Mathematical models have been developed to calculate the
retention of diesel particles in rats. Yu & Yoon (1990) and Yu et al.
(1991) developed a dosimetry model to predict the retention of diesel
particles and associated organic compounds in rats and humans of
different ages. The model indicates that lung clearance would not be
impaired in humans exposed to a concentration < 0.05 mg/m3 for
24 h/day. The highest lung burden per unit of lung weight was
calculated for five-year-old children. This model was used to assess
the risk of inhaling diesel particles (Pepelko & Chen, 1993).
B6.3 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
The clearance of particles from ciliated airways by the
mucociliary escalator after bronchial deposition is virtually complete
within 24 h. For soluble organic material like PAHs on the surface of
carbon particles, diffusion-limited clearance through the airway
epithelium to capillary blood was found to be slower in the bronchial
region than in the alveoli (Gerde et al., 1991a, 1993a,b).
PAHs in diesel soot adhere strongly to the surface of the
particles. Several studies have shown that the association of PAHs
with particles significantly slows the clearance of the PAHs from
lungs in comparison with the clearance of inhaled PAHs unassociated
with particulate matter.
After inhalation by Fischer 344 rats of 3H-benzo[ a]pyrene
adsorbed onto diesel soot (0.1% by mass) for 30 min, biphasic lung
clearance was noted. In a first, fast phase, about 50% of the inhaled
compound was cleared with a half-time of about 1 h, predominantly by
mucociliary clearance. At the end of exposure, about 15% of the 3H
label was found in blood, liver, and kidney, indicating rapid, direct
absorption of this fraction of 3H-benzo[ a]pyrene and its metabolites
into blood. The remaining 50% was retained in the lung, with a
half-time of 18 days; this is one-third of the retention half-time of
diesel particles. Thus, removal of benzo[ a]pyrene and its metabolites
from the particle carrier may be the rate-limiting step of this
clearance process (Sun et al., 1984). In contrast, more than 95% of
pure 3H-benzo[a]pyrene was cleared from the lung within 12 h after
inhalation (Sun et al., 1982).
These results were confirmed and extended to another PAH,
1-nitropyrene (Bond et al., 1986a,b), when the clearance of
14C-labelled compound adsorbed to diesel soot was determined by the
same method in Fischer 344 rats. After exposure to 490 ng/litre of
pure labelled compound, about 90% was cleared in the first, fast
clearance phase, with a retention half-time of 1 h; after exposure to
650 ng/litre of 14C-1-nitropyrene adsorbed onto diesel particles,
45% of the labelled compound was cleared from the lungs in the first
phase, with a half-time of about 2 h. The remaining 55% was retained
with a half-time of 36 days, which was twice the corresponding value
for 3H-benzo[ a]pyrene in the study of Sun et al. (1984).
On the basis of their data on the clearance of 3H-benzo[ a]pyrene
and 14C-1-nitropyrene, Bond et al. (1986b) predicted equilibrium
lung burdens in humans after long-term inhalation. Assuming inhalation
of 3.5 g/m3 of particles containing 0.1% benzo[ a]pyrene and
1-nitropyrene over an 8-h working day, they predicted equilibria of
160 ng benzo[ a]pyrene and 31 ng 1-nitropyrene per lung. No data are
available on changes in the retention of individual compounds after
prolonged exposure to diesel exhaust.
The clearance of a very low concentration (2.6 g/g particles) of
3H-benzo[ a]pyrene adsorbed onto diesel particles was measured in
Sprague-Dawley rats after intratracheal instillation (Bevan & Ruggio,
1991). Only 25% was cleared in the early, fast phase; a long-term
retention half-time of eight days was calculated for the remainder
during a three-day follow-up, which was similar to the clearance in
that period in the study of Sun et al. (1984).
B6.4 Metabolism
In the studies of Sun et al. (1984) and Bond et al. (1986a,b),
the levels of 3H-benzo[ a]pyrene and 14C-1-nitropyrene and their
metabolites in extracts of lung homogenates after inhalation of diesel
exhaust particles were measured by high-performance liquid
chromatography. Sun et al. detected oxidized metabolites 30 min after
inhalation: 35% of the 3H-benzo[ a]pyrene had been metabolized in
equal amounts to phenols (3-hydroxy- and 9-hydroxybenzo[ a]pyrene)
and quinones (1,6 and 3,6 isomers). Twenty days later, only 13%
phenols and 5% quinones were detected. In the study of Bond et al.
(1986b), about 30% of the 3H-1-nitro-pyrene had been metabolized to
acetylaminopyrenephenol (6 and 8 isomers) and about 40% remained
unmetabolized 1 h after inhalation. Oxidative metabolism of
14C-benzo[ a]pyrene, in solution or adsorbed onto diesel particles
at a concentration of 500 pmol/106 cells, was also detected in cell
cultures of pulmonary macrophages obtained from beagle dogs. The
quantity of metabolites increased with incubation time up to 45 and
125 pmol/106 cells in extracts and in the media, respectively, after
48 h of incubation. After incubation for 6 h, the major metabolites
found in extracts of cells were benzo[ a]pyrene-7,8-diol (0.5pmol/106
cells) and benzo[a]pyrene-4,5-diol (0.2 pmol/106 cells); those in
extracts of culture medium were benzo[ a]pyrene-7,8-diol (1.7 pmol/106
cells) and benzo[ a]pyrene-9,10-diol (1.4 pmol/106 cells), in
comparison with a total concentration of metabolites of 2.5 pmol/106
cells in the extracts and 6 pmol/10 6 cells in the medium. Minor
metabolites were benzo[ a]pyrene phenols (3-hydroxy- and
9-hydroxy-benzo[ a]pyrene) and benzo[ a]pyrene quinones (6,12, 1,5,
and 3,5 isomers). The quantities of metabolite were similar when
macrophages were incubated with benzo[ a]pyrene in solution or
adsorbed onto diesel particles (Bond et al., 1984).
Using the 32P-postlabelling technique, Wong et al. (1986) found
increased DNA adduct formation in the lungs of Fischer 344 rats after
exposure for 31 months to diesel exhaust containing 7.1 mg/m3
particles. After exposure of Fischer 344 rats and Syrian golden
hamsters to dilutions of diesel engine exhaust for six months to two
years, the level of haemoglobin adducts (2-hydroxyethylvaline and
2-hydroxypropylvaline), corresponding to the metabolic conversion of
5-10% of inhaled ethylene and propylene to its oxides, increased
dose-dependently (Törnqvist et al., 1988). An adduct of
benzo[ a]pyrenediol epoxide with deoxyguanosine was identified after
inhalation of benzo[a]pyrene adsorbed onto carbon black (20 mg/g)
(Wolff et al., 1989), demonstrating the production of epoxides of PAHs
by oxidative metabolism.
Methods for extracting diesel soot before testing for
genotoxicity in vitro have been discussed. As dichloromethane and
dimethyl sulfoxide do not correspond to leaching conditions in the
lung, it can be assumed that the bioavailability of genotoxic
materials is lower in lungs than in vitro. An investigation of
dipalmitoyl phosphatidylcholine, the primary component of
physiological surfactant, demonstrated that the genotoxicity
associated with diesel particles inhaled into the lung can be
activated by the solubilization and dispersion properties of pulmonary
surfactants. This finding was confirmed in mammalian cells for sister
chromatid induction (Keane et al., 1991) and for chromosomal
aberrations and micronucleus formation (Gu et al., 1991, 1992).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
B7.1 Single exposure
Data on the acute toxicity of diesel exhaust are very limited;
the two available studies suggest that diesel exhaust particles and
their extracts have little acute toxicity.
Male ICR mice were administered diesel exhaust particles by
intratracheal instillation, and mortality due to lung oedema was
assessed for up to 24 h. At a dose of 0.9 mg per animal, all animals
died within 24 h. The LD50 value was 0.6 mg per animal, equivalent
to about 20 mg/kg body weight. Diesel exhaust particles washed three
times with methanol did not cause death at a dose of 1 mg per mouse,
suggesting that the primary source of toxicity is the extractable
organic compounds rather than the particles themselves. These
compounds may produce superoxide radicals that damage endothelial
cells (Sagai et al., 1993).
The LD50 value for diesel exhaust extract in adult Syrian
golden hamsters after intraperitoneal injection was 1280 mg/kg body
weight (Pereira et al., 1982).
B7.2 Short-term exposure
Tracer-monitored DNA synthesis, indicating a proliferative
response, was observed in whole lung tissue of groups of 3-10 Fischer
344 rats exposed to diesel exhaust by inhalation at 6 mg/m3 for
20h/day on seven days per week for periods of 1-14 days. The response
was maximal after two days of exposure, showing a fourfold increase.
The type II cell labelling index was significantly higher (fourfold)
than that in controls after two and three days of exposure to diesel
exhaust. Both values returned to control levels after a one-week
recovery period. Experiments involving incorporation of 14C-palmitic
acid revealed transient changes in lipid metabolism after one day of
exposure, with a threefold increase in the amount of phosphatidyl-
choline in lung tissue (Wright, 1986).
Exposure of various species by inhalation to 6 mg/m3 resulted
in slight alterations in lung function and histopathological changes.
Groups of 10 male Chinese hamsters were exposed for 8 h/day on
seven days per week for six months to particle concentrations of
either 6 or 12 mg/m3 diesel exhaust.
Measurements of pulmonary function indicated dose-related
increases in lung weight and dose-related decreases in vital and
diffusion capacity. Pathological evaluation revealed oedema,
thickening of the alveolar lining, a possible increase in collagen,
and the presence of numerous particle-laden macrophages, which almost
filled the alveolar spaces (Vinegar et al., 1981).
Groups of 41-51 male and female Hartley guinea-pigs were exposed
for 20 h/day on seven days per week to exhaust from a light-duty
diesel engine diluted to attain a concentration of 6 mg/m3
particulate matter. Separate groups were exposed to raw exhaust and
exhaust that had been irradiated with ultra-violet light for an
average of about 1 h. Pulmonary function was measured, and
electrocardiography was conducted on animals exposed for four weeks;
those examined for histological changes were exposed for eight weeks.
The only changes noted after four weeks were a 35% increase in
pulmonary flow resistance and a small, but significant decrease in
heart rate in animals exposed to irradiated exhaust. No changes in the
electrocardiogram were noted. The pathological changes were limited to
hyperplasia of alveolar lining cells and slight focal thickening of
the interstitium in occasional alveoli with accumulation of
particle-laden macrophages. Growth rates were unaffected (Wiester et
al., 1980).
As part of the same study, cats (Pepelko et al., 1981) and male
rats (15 per group) (Pepelko, 1982) were exposed for four weeks to
unirradiated exhaust under identical conditions. The pulmonary
function of rats was unchanged; the only changes were significant
increases in vital and total lung capacity, but these differences were
quite small and of questionable significance. The only functional
change noted in the cats was a decrease in maximal expiratory flow
rate at 10% of vital capacity. The pathological changes were again
limited to focal thickening of the alveolar walls near accumulations
of particle-laden macrophages. In general, the functional and
pathological changes were limited under these experimental conditions.
Kaplan et al. (1982) exposed male Fischer 344 rats (168 per
group), A/J mice (672 per group), and male Syrian golden hamsters (236
per group) to 1.5 mg/m3 of diesel exhaust particles for 20 h/day on
seven days per week for three months or to clean air. Animals were
killed at the end of exposure and after an additional six-month
recovery period. Mortality was not increased during the observation
period, and body weight was not significantly altered in any species.
At the end of exposure, the relative weight of the lung was
significantly increased in rats. In mice, the pulmonary adenoma
response was increased but not significantly. In all three species,
grey to black discolouration of the lungs and lung-associated lymph
nodes were observed. Microscopically, most of the particulate was
found in macrophages, but some was free. After six months' exposure to
clean air, the lungs of all species had considerably less pigment
accumulated in foci.
Sixteen male Fischer 344 rats were exposed to 6 mg/m3 diesel
exhaust for periods ranging from 6 h to 63 days, with subsequent
histological examination of the lungs. The numbers of diesel
particulate-containing alveolar macrophages, type II alveolar cells,
and inflammatory cells were found to be increased over those in eight
controls. After a nine-week exposure, accumulation of macrophages was
observed near the terminal bronchioles (White & Garg, 1981). In rats
that inhaled 6 mg/m3 diesel exhaust for two weeks and were either
killed immediately or allowed to recover for six weeks before
sacrifice, there was no statistical difference in the number of
macrophage aggregates (240 000 and 220 000 per lung); however, the
aggregates in the recovery group had significantly higher average
diameter and volume, demonstrating macrophage attachment after the end
of exposure (Garg, 1983).
The observations of Wiester et al. (1980) and Garg (1983)
demonstrate the importance of alveolar macrophages in the clearance of
particles of low solubility. These are engulfed by alveolar
macrophages and eliminated via the mucociliary escalator and
gastrointestinal tract, or, in the case of lung particle overloading,
increasing fractions are sequestered in focal areas of the lung tissue
and transferred to the lymph nodes (Muhle et al., 1990).
B7.3 Long-term exposure and studies of carcinogenicity
B7.3.1 Non-neoplastic effects
No obvious signs of toxicity were observed in groups of 183 male
and 183 female Fischer 344 rats exposed by inhalation to diesel
exhaust at 0.35, 3.5, or 7.1 mg/m3 for 7 h/day on five days per week
for up to 30 months (Mauderly et al., 1987). The body weights did not
differ significantly from those of controls, and the exposures did not
affect the life span of animals of either sex. Focal fibrotic and
proliferative lung disease was observed in parallel with progressive
accumulation of diesel soot in the lung. Henderson et al. (1988)
reported the results of biochemical and cytological analyses of lung
lavage fluid and lung tissue from rats and mice exposed in the same
study, including determinations of lactic dehydrogenase, glutathione
reductase, ß-glucuronidase, glutathione, and hydroxyproline, and the
numbers of macrophages and neutrophils. No changes were seen in rats
or mice exposed to 0.35 mg/m3 , but at higher concentrations dose-
and duration-dependent increases in biochemical parameters and cell
numbers in lavage fluid were observed, indicating a chronic
inflammatory response. Lung weights were also increased in male and
female mice exposed to the two higher levels for any length of time,
in rats exposed to 7.0 mg/m3 for 12 months or longer, in female rats
exposed to 3.5 mg/m3 for 18 months, and in males exposed to
3.5 mg/m3 for 24 months. Tissue enzyme levels, measured in lung
lavage fluid as an indicator of damage, were consistently raised in
rats and mice at the two higher exposure levels and in lung tissue of
rats and mice at various times (most pronounced at 7 mg/m3). These
changes probably indicate an increase in lung tissue due to increased
inflammatory cells and epithelial cell proliferation.
In the same study, Mauderly et al. (1988) described focal
proliferative, fibrotic, and emphysematous changes in the lungs of
animals at the higher doses. Measurements in vivo revealed a
reduction in lung volume and compliance.
In a follow-up study, Mauderly et al. (1990) exposed male Fischer
344 rats that were either normal or had pre-existing pulmonary
emphysema (elastase-treated) to diesel soot at 3.5 mg/m3 for 7 h/day
on five days per week for 24 months, to determine whether
emphysematous rats were more susceptible to diesel exhaust. No
increase in susceptibility was seen. The emphysematous lungs had a
lower accumulation of particles than the normal lungs, which prevented
an overproportional response of the compromised animals.
Male and female Fischer 344 rats exposed to diesel exhaust
particles at 0.7, 2.2, or 6.6 mg/m3 for 16 h/day on five days per
week for two years or to filtered diesel exhaust showed an
exposure-related reduction in the rate of weight gain throughout the
study at the highest dose and an increase in absolute lung weight at
the two higher doses (Brightwell et al., 1986, 1989).
Groups of 72 male and 72 female Fischer 344 rats exposed to
diesel exhaust particles at 2 mg/m3 for 7 h/day on five days per
week for 24 months showed alveolar type II cell hyperplasia,
inhibition of long-term clearance of particles and pulmonary
lipidosis. No immunological abnormalities, no induction of pulmonary
or hepatic xenobiotic metabolizing enzymes, and no effects on
mortality, body weight gain, organ:body weight ratios or clinical
chemical parameters were seen (Lewis et al., 1986, 1989). In the same
study, a dose-related depression in phagocytic activity was seen in
alveolar macrophages (Castranova et al., 1985), and a trend to
increased pulmonary arterial wall thickness was observed in comparison
with controls (Vallyathan et al., 1986).
In groups of 15 male monkeys, exposure to diesel exhaust (as
described above) resulted in mild obstructive airway disease, but
there was no evidence of restrictive lung disease. The effects were
observed throughout the exposure period, after 6, 12, 18, and 24
months (Lewis et al., 1989).
Reduced body weight, significantly increased lung:body weight
ratios, and type II cell proliferation were seen in female Fischer 344
rats exposed to whole-fraction diesel exhaust at 5 mg/m3 for 8 h/day
on seven days per week for 24 months (Iwai et al., 1986).
Groups of 96 male and 96 female Syrian golden hamsters, female
NMRI mice, and female Wistar rats were exposed to diesel engine
emissions at 4.4 mg/m3 for 19 h/day on five days per week for
120-140 weeks (Heinrich et al., 1986a; Stöber, 1986). The body weights
of mice were significantly reduced, by about 12%. Lung weights were
increased after two years of exposure, by two- to threefold in rats
and mice and by 50-70% in hamsters. Different levels of histological
response were seen in the three species. Hamsters had thickened
alveolar septa and bronchioalveolar hyperplasia. Of the mice, 64% had
bronchioalveolar hyperplasia (controls, 5%), 71% had multifocal
alveolar lipoproteinosis, and 43% had multifocal interstitial fibrosis
(controls, 4%). The nose, larynx, and trachea were not affected.
Treated rats showed severe inflammatory changes in the lungs,
thickened septa, foci of macrophages, and hyperplastic and metaplastic
lesions, but no significant changes in respiratory rate, minute
volume, compliance, or resistance were seen in 14 exposed rats.
Significantly increased airway resistance and a significant decrease
in dynamic lung compliance were observed in rats after two years of
exposure. The hamsters had a significant increase in airway resistance
and a nonsignificant reduction in lung compliance at termination of a
one-year exposure; these changes persisted during the second year of
exposure. In the same experiments, filtered diesel exhaust did not
have the same effects as total exhaust in any of the three species
(Heinrich et al., 1986a).
Groups of 27 female Fischer 344 rats and about 50 male and 50
female C57Bl/6N and ICR mice were exposed to particle concentrations
of 2-4 mg/m3 for 4 h/day on four days per week for up to 24 months.
After 12 months, the incidence of alveolar hyperplasia was increased
in all animals. Exposed rats had more severe inflammation than
controls, with inflammatory exudate into the nose, and bronchitis and
pneumonia, but this effect was ascribed to the housing, which was not
specific pathogen-free (Takemoto et al., 1986).
Groups of nine male Hartley guinea-pigs were exposed at
concentrations of 0.25, 0.75, or 1.5 mg/m3 for 20 h/day on 5.5 days
per week for up to two years. An analysis of the alveolar capillary
membrane by quantitative morphometry showed that it was thickened as a
result of an increase in the absolute tissue volume of interstitium
and type II cells. Exposure to 0.75 mg/m3 for six months caused
fibrosis in regions of macrophage clusters and focal type II cell
proliferation, as observed under the light microscope. No appreciable
change in morphometric parameters was seen after exposure to the
lowest dose, but the higher doses increased the thickness of alveolar
septa and the numbers of various types of alveolar cells (Barnhart et
al., 1981, 1982).
As part of this investigation, Fischer 344 rats were exposed by
inhalation to 0.25 or 1.5 mg/m3 diesel exhaust particles, and
various biochemical and cytological studies were conducted up to 36
weeks (Misiorowski et al., 1980). At the high dose, normalized lung
weight, the rate of collagen synthesis, and the cellular and lipid
content of lung tissue were significantly increased after six months.
It was concluded that the reactivity of fibrogenic cells had been
enhanced. In a long-term study of respiratory end-points in male
guinea-pigs, including light and electron microscopy, lavage cytology,
and lung tissue biochemistry, the lowest-observable-adverse-effect
level (LOAEL) was 0.796 and the no-observable-adverse-effect level
(NOAEL) was 0.258 mg/m3 (US Environmental Protection Agency, 1992b).
Groups of 120 male Fischer 344 rats, 450 male A/J mice, and 120
male Syrian hamsters were exposed to 0.25, 0.75, or 1.5 mg/m3 diesel
exhaust particles for 20 h/day on seven days per week for 15 months.
Mortality was not increased in any species. A dose-related deposition
of pigment was observed histopathologically in alveolar macrophages
and regional lymph nodes. Accumulation of alveolar macrophages was
associated with an increase in connective tissue in alveolar walls,
with mild fibrosis and proliferation of type II pneumocytes; these
effects were termed pneumoconiosis. No treatment-related responses
were found in organs other than the respiratory tract. The LOAEL was
0.735 and the NOAEL, 0.242 mg/m3 (Kaplan et al., 1983).
Groups of 120 male and 95 female Fischer 344/Jcl rats were
exposed to light-duty diesel engine exhaust particulates at 0, 0.1,
0.4, 1.1, or 2.3 mg/m3 or heavy-duty diesel engine exhaust
particulates at 0, 0.5, 1.0, 1.8, or 3.7 mg/m3 for 16 h/day on six
days per week for up to 30 months (Ishinishi et al., 1986, 1988;
Suzuki et al., 1990). The body weights of females exposed to the
highest dose of heavy-duty engine exhaust were decreased throughout
the study by 15-20% in comparison with controls. No changes were
observed histopathologically in the lungs at any concentration.
Accumulation of particle-laden macrophages was observed at levels
> 0.4 mg/m3. In areas of macrophage accumulation, bronchiolar
epithelium replaced alveolar epithelium in the ducts. Proliferation of
bronchiolar epithelium and type II cells and oedematous thickening and
fibrosis of the alveolar septum were observed, which developed into
small fibrotic lesions classified as hyperplastic. The incidences of
hyperplastic lesions were 4, 4, 6, 12, and 87 per 124 rats exposed to
light-duty engine exhaust and 1, 3, 7, 14, and 25 per 124 rats in
those exposed to heavy-duty engine exhaust. The LOAELs were
1.2 mg/m3 for light-duty and 1.0 mg/m3 for heavy-duty exhaust, and
the NOAELs were 0.4 and 0.5 mg/m3, respectively (US Environmental
Protection Agency, 1992b).
In the same experimental series, Kato et al. (1992) studied
non-neoplastic lesions in the respiratory organs of the rats every six
months. The marked changes consisted of intake of diesel particles by
type I epithelium, hypertrophy, and glandular proliferation of type II
epithelium, with many extended microvilli and increased numbers and
size of lamellar inclusion bodies, focal increase of collagen fibres
in the interstitium, and infiltration of particle-laden macrophages,
neutrophils, mast cells, and plasma cells into the interstitium of
alveolar septa. The most marked changes in the airway were focal
shortening of cilia and protrusions of nonciliated cells. These
morphological changes appeared in all groups exposed to particle
concentrations > 1 mg/m3 after six months of exposure and were more
marked with increasing particle concentration and with time.
Exposure of Syrian golden hamsters to 4 or 11 mg/m3 diesel
exhaust for 7 h/day on five days per week for five months resulted in
enlargement of liver sinusoids and loss of cristae in mitochondria
(Meiss et al., 1981).
The induction of pulmonary adenomas and carcinomas in rats after
long-term inhalation of carbon black is proposed to be due partly to
inflammatory cells and their mediators (Driscoll et al., 1995;
Oberdörster et al., 1995; Driscoll et al., in press). After exposure
of rats to carbon black at 1, 7, or 50 mg/m3 for 6 h/day on five
days per week for 13 weeks, 353, 1923, and 7112 µg of carbon,
respectively, were deposited per lung. After exposure to air or to
1 mg/m3 of carbon black, no macrophage inflammatory protein-2
(MIP-2) mRNA expression and no increase in inflammatory cells was
observed; with 7 or 50mg/m3, both MIP-2 mRNA and the number of
neutrophils in bronchoalveolar lavage fluid were increased, and the
hprt frequencies were 3.2 and 4.2 times greater than in controls.
Groups of 25 cats were exposed to either clean air or diesel
exhaust for 8 h/day on seven days per week for two years at a
concentration of 6 mg/m3 through week 62 and to 12 mg/m3 for weeks
62-124. Some of the cats were then held in clean air for an additional
six months. No response was seen after 61 weeks, but after 123 weeks
signs of restrictive lung disease were observed. Total lung capacity,
forced vital capacity, functional residual capacity, peak expiratory
flow rates, and diffusion capacity were significantly decreased
(Moorman et al., 1985). Morphological evaluation revealed
exposure-related lesions in the centriacinar region of the lungs,
bronchiolar epithelial metaplasia associated with the presence of
ciliated and basal cells, and alveolar macrophages containing diesel
particle-like inclusions. After six months' recovery in clean air, the
metaplasia had disappeared but the fibrosis had progressed (Hyde et
al., 1985). In exposed cats, the epithelium of the terminal and
respiratory bronchioles consisted of ciliated, basal, and Clara cells,
whereas controls had only Clara cells (Plopper et al., 1983). These
observations indicate that the foci of the lesions were the
epithelium, the interstitial compartments, and the centriacinar region
of the lung. The metaplastic changes suggest that cancer may be
induced by continuous exposure, while the other major effect is
pulmonary fibrosis resulting in restrictive disease which is not
ameliorated and in fact progresses after removal from exposure.
Progression of effects is probably due to continued lung overloading
with particulate matter, despite cessation of exposure.
The dose-dependent effects on lung morphology, histopathology,
biochemistry, and cytology after long-term exposure to diesel exhaust
described above are reflected in the following findings seen in rats
at a lung burden of about 0.5 mg/lung (Henderson et al., 1988; Muhle
et al., 1990):
-- The wet and dry weights of lungs increase by up to fourfold in
comparison with those of concurrent controls. This is due to
severe functional overloading in the lung, resulting in enhanced
leukocyte influx and tissue proliferation.
-- Pulmonary inflammation is manifested by cytological (increased
numbers of polymorphonuclear neutrophils) and biochemical (lactic
dehydrogenase, collagen in lung lavage fluid) parameters.
-- The retention of material is characterized by an increasing
number of particle-laden macrophages and by black discolouration
of lungs due to sequestration of accumulated particles.
Additionally, increased transfer of material to the
lung-associated lymph nodes is observed.
-- Later findings during inhalation are proliferative alterations of
the epithelial cells and onset of fibrosis.
B7.3.2 Carcinogenicity
B7.3.2.1 Inhalation
Rats were exposed in several studies to diesel emissions
containing > 2 mg/m3 of particles for more than two years,
resulting in the induction of pulmonary tumours (Table 34). Many
organic and inorganic gaseous components were also present in the test
atmospheres, and the relatively high concentration of some pulmonary
irritants like nitrogen dioxide, aldehydes, and sulfur dioxide may
have contributed to the inflammatory response and to epithelial cell
damage and subsequent cell proliferation. Exposure to gaseous PAHs,
like naphthalene which is toxic to Clara cells, may influence the
metabolic state of pulmonary cells. It is not clear however, whether
the combined exposure to particles and gases in these experiments
favoured tumour induction. It should be noted that Brightwell et al.
(1986), Heinrich et al. (1986a), Iwai et al. (1986), Ishinishi et al.
(1986), and Takaki et al. (1989) have reported that the gaseous phase,
i.e. the proportion of exhaust without particles, does not induce
pulmonary tumours.
Table 34. Carcinogenicity of diesel engine exhaust in rats
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 40 m 8.3 1.5 6 h/d, 5 d/week, up NR 1 17c NR Karagianes
40 m Clean air to 20 months 0 et al.
(20 months) (1981)
Fischer 72 f 6.6 3.1 16 h/d, 5 d/week, 24 NR 39 54 38.5 < 0.1 Brightwell
344 71 m months (30 months) 16 23 et al.
72 f 2.2 1.1 11 15 9.7 < 0.1 (1986, 1989)
72 m 3 4
71 f 0.7 0.4 0 0.7
72 m 1 1
Filtered exhaust 0
0
142 f Clean air 1 1 1.4
140 m 3 2
Wistar 95 f 4.4 2.5 19 h/d, 5 d/week, 32 NR 17 18 < 0.1 Heinrich et
92 f Filtered exhaust months (up to death) 0 al. (1986a)
96 f Clean air 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer
344/Jcl
Light-duty 60 f 2 1.1 16 h/d, 6 d/week, 30 NR 1 2 2.4 NR Ishinishi
engine 64 m months (30 months) 2 3 et al.
59 f 1 0.6 2 3 4.1 (1986,1988;
64 m 3 5 Takaki et al.
61 f 0.4 0.2 0 0.8 (1989)
64 m 1 2
59 f 0.1 0.06 2 3 1.6
64 m 0
59 f Clean air 2 3 3.3
64 m 2 3
Heavy-duty 60 f 4 2.3 3 5 6.5 1.8
engine 64 m 5 8
59 f 2 1.1 1 2 3.3
64 m 3 5
61 f 1 0.6 0 0
64 m 0
59 f 0.4 0.2 1 2 0.8
64 m 0
59 f Clean air 1 2 0.8
64 m 0
16 m Filtered exhaust 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer 19 f 4.9 1.6 8 h/d, 7 d/week, 24 NR 8 42 < 1d Iwai et al.
344 16 f Filtered exhaust months (24 or 30 0 (1986)
22 f Clean air months) 1 5
Fischer 15 f 2.0-4.0 0.2-0.4 4 h/d, 4 d/week, 24 NR 0 NR Takemoto
344 15 f Clean air months (24 months) 0 et al.
(1986)
Fischer 69 f 7 1.5 7 h/d, 5 d/week, 30 NR 13 19 16.1 < 0.1 (f) Mauderly et
344/Crl 74 m months (up to death) 10 14 1.7 (m) al. (1986)
68 f 3.5 0.7 2 3 4.6
63 m 4 6
68 f 0.35 0.07 0 0.7
70 m 1 1
68 f Clean air 0 1.4
73 m 2 3
Fischer 227 f+m 7 1.5 7 h/d, 5 d/week, 30 20.8 12.8 < 0.1 Mauderly et
344/N Crl 221 f+m 3.5 0.7 months (up to death) 11.5 3.6 4.8 al. (1987)
223 f+m 0.35 0.07 0.6 1.3
230 f+m Clean air (24 months) 0.9
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 100 f 7.0 3.8 18 h/d, 5 d/week, 24 63.9 22 22.0 < 0.1 Heinrich et
200 f 2.5 1.3 months (30 months) 23.7 11 5.5 < 1 al. (1992,
198 f 0.8 0.5 6.3 0 1995);
217 f Clean air (24 months) NR 1 0.5 Heinrich
(1994)
Fischer 212 f+m 6.3 3.0 16 h/d, 5 d/week, 24 85.4 38 17.9 17.9 NR Nikula et al.
344/N 210 f+m 2.4 1.1 months (24 months) 40.7 13 6.2 6.2 (1994)
214 f+m Clean air (23 months) 3 1.4 1.4
Fischer 49 f 4.7 1.3 15 h/d, 3 d/week, 24, NR 6 12.2 NR Kawabata
344/N 42 f 4.7 1.3 12, or 6 months 8 19.0 et al.
45 f 4.7 1.3 1 2.2 (1993)
48 f Clean air 5 10.4
Fischer 183 f+m 2.0 0.4 7 h/d, 5 d/week, 24 NR 8 4.4 4.4 NR Lewis et al.
344/N 180 f+m Clean air months 6 3.3 3.3 (1989)
From Pott & Heinrich (1987) and supplemented; NR, not reported
a Mass median aerodynamic diameter, 0.17-0.25 µm; 'Continuous', equivalent continuous value
b Unilateral Fisher's exact test
c Only six animals investigated
d With Yates' correction
No difference in lung tumour incidence was seen in groups of male
and female rats exposed for 7 h/day on five days per week for 24
months either to diesel exhaust particulates at 2 mg/m3 or to
filtered air (Lewis et al., 1989).
Exposure of six male Wistar rats to diesel exhaust soot at
8.3 mg/m3 and coal dust for up to 20 months gave inconclusive
results (Karagianes et al., 1981). The study had little statistical
power owing to the small group size, and the insufficient duration of
exposure may have prevented detection of tumours, although one mammary
fibroadenoma and one lung bronchiolar adenoma were detected. One
subcutal fibrosarcoma and one renal lymphoma were seen in controls.
Eight bronchioalveolar adenomas and nine squamous-cell tumours
were seen in 95 rats (18%) that had inhaled 4.4 mg/m3 diesel exhaust
for 19 h/day on five days per week for 32 months. No tumours were
observed in controls (Heinrich et al., 1986a). The dose-dependent
carcinogenic potency of diesel exhaust was confirmed in a study in
which rats were exposed to diesel exhaust at concentrations of 0.8,
2.5, or 7.0 mg/m3 for 18 h/day on five days per week for 24 months
(Heinrich et al., 1992; Heinrich, 1994; Heinrich et al., 1995).
In male and female rats exposed to diesel exhaust at 6.6 mg/m3,
a high incidence of lung tumours was observed in animals dying or
sacrificed after 24 months (24/25 females, 12/27 males). The incidence
of lung tumours (Table 34) was derived by pooling pulmonary adenomas;
squamous-cell carcinomas; adenocarcinomas; mixed ademomas,
adenocarcinomas, and squamous-cell carcinomas; and mesotheliomas.
Non-neoplastic pulmonary lesions were not described (Brightwell et
al., 1986, 1989).
Groups of 24 female Fischer 344 rats were exposed to clean air,
to whole-fraction (4.9 mg/m3 particulates) fuel, or to filtered
diesel exhaust for 8 h/day on seven days per week for 24 months. After
six months of exposure, the rats exposed to whole exhaust had lower
body weights than those in the other two groups; after 18 months, both
exposed groups showed body weight reduction. The relative lung weight
was increased after 12 months' exposure to whole exhaust, and
epithelial proliferation and significantly elevated numbers of lung
tumours were seen in animals exposed to the whole fraction after six
months. Increased incidences of leukaemia and mammary gland tumours
were also reported in animals exposed to whole or filtered exhaust;
however, because of the small numbers of animals, the significance of
the results cannot be determined (Iwai et al., 1986).
A total of 140 female and 140 male Fischer 344/N rats (in groups
of three males and three females, five males and five females, or
eight males and eight females, depending on the end-point) were
exposed for 16 h/day on five days per week for up to 24 months,
beginning at eight weeks of age, to particles of diesel exhaust or
carbon black at 2.5 or 6.5 mg/m3 of air, or to clean air (Mauderly
et al., 1994). Rats were killed after 3, 6, 12, 18, or 23 months of
exposure for histopathological assessment and measurement of lung and
lung-associated lymph node burdens of particles, lung weight,
bronchoalveolar lavage indicators of inflammation, DNA adducts in
whole lung and alveolar type II cells, and chromosomal anomalies in
circulating lymphocytes. Diesel exhaust and carbon black had nearly
identical effects, with a similar relation to exposure. There was a
dose-related slowing of particle clearance after three months, with
progressive accumulation of particles in lungs and lymph nodes.
Inflammation, epithelial proliferation, and fibrosis were progressive
and related to dose. Diesel exhaust slightly increased the number of
lung DNA adducts, and the numbers of DNA adducts in type II cells were
increased by both diesel exhaust and carbon black. No exposure-related
chromosomal damage was found in circulating lymphocytes. The incidence
of primary lung neoplasms was increased significantly and was related
to the doses of both diesel exhaust and carbon black. The types of
neoplasms were identical and included benign adenomas, malignant
adenocarcinomas, squamous-cell carcinomas, and adenosquamous
carcinomas; squamous cysts were also seen. The neoplasms had similar
growth characteristics when transplanted; 50-67% of the transplanted
squamous-cell carcinomas and 25-40% of the transplanted
adenocarcinomas grew after injection into athymic mice (Table 35),
whereas the squamous cysts did not.
Fischer 344/Jcl rats were exposed to a range of concentrations of
exhaust emission particulates from light-duty (up to 2 mg/m3) or
heavy-duty diesel engines (up to 4 mg/m3) for 16 h/day on six days
per week for up to 30 months. After 18 months, marked hyperplasia of
type II cells was noted in the groups at higher exposures. After 30
months, treatment-related pulmonary hyperplastic lesions were
detected, and a dose-response relationship was seen for tumours in the
groups exposed to heavy-duty but not light-duty diesel exhaust. The
lung tumours were diagnosed as adenocarcinomas, squamous-cell
carcinomas, or adenosquamous carcinomas. A significant correlation was
observed between the incidence of anthracosis and areas with
hyperplastic lesions; furthermore, anthracosis and hyperplastic
lesions did not appear in lungs exposed to particle-free exhaust. The
authors concluded that the occurrence of hyperplastic lesions was
dependent on the presence of carbon particles (Ishinishi et al., 1986,
1988; Takaki et al., 1989).
Table 35. Growth in athymic mice of lung tumours from rats exposed to
diesel exhaust and carbon black
Type of tumour Carbon black Diesel exhaust
Number Growing Number Growing
implanted No. % implanted No. %
Adenocarcinom 8 2 25 10 4 40
Squamous-cell 2 1 50 3 2 67
carcinoma
Squamous cyst 19 0 0 7 0 0
From Mauderly et al. (1994)
In a large-scale study of unfiltered diesel exhaust, 1097 Fischer
344 rats were exposed for up to 30 months to 0.35, 3.5, or 7.0 mg/m3
of diesel soot. A group of 365 rats exposed to clean air served as
controls. Exposure to exhaust did not cause overt signs of toxicity,
and no significant, exposure-related alterations in body weight were
observed. Rats were killed at six-month intervals, examined for their
lung burdens of diesel soot, and subjected to complete necropsy.
Diesel soot accumulated at all doses but minimally in the low-dose
group. An increased incidence of tumours was observed in the groups at
the medium and high doses; most (81%) of the tumours were observed
after two years of exposure. The inhaled soot concentration and the
lung burden of diesel soot were highly significantly related to tumour
incidence ( P < 0.001) (Mauderly et al., 1987). In a follow-up study
with longer daily exposure (16 h/day), the dose-dependency of the
tumour incidence was reproduced (Nikula et al., 1994). In this study,
the carcinogenicity of diesel exhaust soot (8% extractable organic
material; 2.5 mg/m3) was compared with that of carbon black (0.12%
extractable organic material; 6.5 mg/m3 ) in 1152 male and female
Fischer 344 rats exposed for 16 h/day on five days per week for up to
24 months and examined histopathologically. The lung burden of
particles was greater after exposure to diesel exhaust than to carbon
black, but the neoplastic response in the lung was similar and usually
occurred late in the study. When the lung burden of particles was used
as the dose parameter, the carcinogenicity of carbon black was
greater than that of diesel exhaust soot.
Groups of 50 four-week-old female Fischer 344 rats were exposed
to diesel soot at a mean concentration of 4.73 mg/m3 for 15 h/day
three times per week for 6, 12, or 24 months, and animals that died
after 18-30 months were evaluated for tumours. After 30 months, all
animals, including controls, were sacrificed and examined for lung
tumours. The incidences of lung tumours were 10.4% in controls and
2.2% after six months' exposure to diesel exhaust, 19.0% after 12
months, and 12.2% after 24 months. No statistical analysis was
presented, but it seems unlikely that there was a significant effect
(Kawabata et al., 1993).
Concurrent administration of known carcinogens with diesel
exhaust has also been studied. Exposure of rats to diesel exhaust
given an intraperitoneal injection of N-nitrosodiisopropylamine or
N-nitrosodiethylamine did not influence the rates of tumours induced
by the nitrosamines (Takemoto et al., 1986). The effects of treatment
of hamsters with N-nitrosodiethylamine or benzo[ a]pyrene were not
influenced by subsequent administration of diesel exhaust, whereas
pretreatment of mice with benzo[ a]pyrene or dibenzanthracene followed
by diesel exhaust gave inconsistent results. Exposure to diesel
exhaust did not affect the total tumour rates in rats pretreated with
6.25 or 12.5 g/kg body weight of N-nitrosodipentylamine; however,
the incidence of squamous-cell tumours was increased in both
pretreated groups (Heinrich et al., 1986a).
Hamsters were exposed for two years to unfiltered diesel exhaust
at 0, 0.7, 2.2, or 6.6 mg/m3 or to filtered diesel exhaust, and
groups of control and treated hamsters were administered
N-nitrosodiethylamine subcutaneously before the beginning of
exposure. The rate of pulmonary tumours was not increased by diesel
exhaust, with or without pretreatment with the nitrosamine. Several
hamsters that developed 'wet tail' infection after six months on test,
resulting in significant mortality (46%), were treated with
oxytetracycline, chloramphenicol, and dimetridazole (Brightwell et
al., 1986, 1989).
Overall, the only clear effect in these studies of
co-carcinogenicity in rats was an increase in the percentage of
malignant tumours in animals treated with diesel exhaust plus
carcinogens in comparison with the carcinogen alone (Heinrich et al.,
1986a).
The results of studies in other species (mice, hamsters, and
monkeys) treated by inhalation are given in Table 36. Heinrich et al.
(1982) found significant increases in lung tumour incidence in mice,
but this result could not be repeated in later studies (Heinrich et
al., 1986a, 1992, 1995). Small but statistically significant increases
in lung tumour incidence were reported in ICR and C57Bl mice (Takemoto
et al., 1986) and in female Sencar mice (Pepelko & Peirano, 1983);
however, because only small increases in non-malignant tumours were
seen only in females in the study of Pepelko & Peirano (1983), the
results must be considered to be weakly positive or equivocal.
Hamsters showed no response to diesel exhaust.
Table 36. Long-term studies of diesel exhaust by inhalation in species other than rats
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI mouse 2 × 96 f (a) Exhaust 4 19 h/d, 5 d/week, Lung tumours (adenomas and Heinrich et al.
(b) Filtered 27-28 months adenocarcinomas) (a) 24/76 (32%), (1986a)
(c) Clean air including 13 (17%) adenocarcinomas;
(b) 29/93 (31%), including 18 (19%)
adenocarcinomas; (c) 11/84 (13%),
including 2 (2.4%) adenocarcinomas
Incidence in historical controls,
< 32%
ICR and 315 ICR, Exhaust or 2.0-4.0 4 h/d, 4 d/week, Lung tumours (adenomas and Takemoto et al.
C57Bl/6N 297 C57Bl/6N clean air 13-28 months adenocarcinomas). ICR: 14/56; (1986)
mice, newborn (sex 7/60 in controls; C57Bl/6N:
unspecified) 17/150; 1/51 in controls
Sencar mouse 2 × 130 f (a) Exhaust 12 8 h/d, 7 d/week, Adenomas and carcinomas Pepelko &
2 × 130 m (b) Clean air to 15 months of (a) m: 5.9%; f: 16.3% Peirano (1983)
age (from (b) m: 3.8%; f: 7.2%
conception to
sacrifice)
NMRI mouse 2 × 80 f (a) Exhaust 7.5 18 h/d, 5 d/week, Lung tumours Heinrich et al.
(b) Clean air 13.5 months, 9.5 (a) 32.1% (1992, 1995)
months' recovery (b) 30%
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI and 3 × 120 f (a) Exhaust 4.5 18 h/d, 5 d/week; Lung tumours Heinrich et al.
C57Bl/6N mice 3 × 120 f (b) Filtered NMRI, 23 months; NMRI: (a) 23.3% (1992, 1995)
(c) Clean air C57Bl, 24 months (b) 46.7%
+ 6 months' (c) 30%
recovery C57Bl: (a) 8.5%
(b) 3.6%
(c) 5.1%
Syrian golden 3 × 48 f (a) Exhaust 3.9 ± 0.5 7-8 h/d, 5 d/week, No effect on survival, no lung Heinrich et al.
hamster (b) Filtered up to 30 months tumours (1982)
(c) Clean air
Syrian golden 3 × 48 f (a) Exhaust 4 19 h/d, 5 d/week, Normal life span, no lung tumours Heinrich et al.
hamster 3 × 48 m (b) Filtered 27-28 months (1986a)
(c) Clean air
Syrian golden 52 f + 52 m (a) Exhaust 0.7, 2.2, 16 h/d, 5 d/week, No significant increase in Brightwell
hamster 104 f + 104 m (b) Filtered 6.6 24 months frequency of tumours in et al.
(controls) (c) Clean air respiratory tract (1986, 1989)
Cat 2 × 25 m (a) Exhaust 6 (1st 8 h/d, 7 d/week, Lung function: decrease in closing Pepelko &
yr), 12 24 months volume after 1 year; reduction Peraino (1983)
(2nd yr) in inspiratory, vital, and total
(b) Clean air lung capacity after 2 years;
diagnosis: pulmonary fibrosis of
the interstitial or
intra-alveolar type
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
Cynomolgus 4 × 15 m (a) Coal dust 2.0 7 h/d, 5 d/week, No difference in tumour incidence Lewis et al.
monkey (b) Exhaust 2.0 24 months among groups (1986)
(c) Coal dust 1.0
+ exhaust 1.0
(d) Clean air
It is clear that repeated inhalation of diesel exhaust at
concentrations of more than about 2 mg/m3 (actual value),
corresponding to a calculated equivalent continuous concentration of
about 1 mg/m3, increases the incidence of pulmonary tumours. These
tumours occur late in life after exposures of 24 months.
B7.3.2.2 Other routes of exposure
Studies have also been conducted by intratracheal instillation in
rats and hamsters and by painting on mouse skin (Table 37). While the
former are especially useful for hazard identification and for
evaluating specific mechanistic aspects, they are less useful for the
purposes of risk characterization and no attempt was made to identify
critical studies. The results of the studies by intratracheal
instillation, however, confirm that both diesel exhaust particles and
carbon black induce lung tumours and demonstrate that the specific
surface area of carbonaceous particles is correlated with the
tumorigenic potency. Both of these findings support the suggestion
that a nonspecific particle effect is of crucial importance for the
induction of lung tumours by diesel exhaust. The dermal experiments,
conducted by Nesnow et al. (1982a,b, 1983), have been used to estimate
risk quantitatively, by the comparative potency method.
B7.4 Dermal and ocular irritation; dermal sensitization
No data were available.
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
B7.5.1 Reproductive toxicity
In a two-generation study of reproduction, 100 male and 100
female CD-1 mice were exposed by inhalation to exhaust from a
light-duty diesel engine for 8 h/day on seven days per week, at a
concentration of 12 mg/m3. Most treatment-related effects were
minimal. Overall fertility and survival rates were not significantly
altered (Pepelko & Peirano, 1983).
(C57Bl/6 × C3H)F mice (number not given) showed sperm
abnormalities (reduced sperm count and weight of testis;
teratospermia) after daily intraperitoneal injections of 50, 100, or
200 mg/kg body weight of diesel exhaust particles for five days. The
highest dose caused an eightfold increase in abnormalities over that
in controls and a significant decrease in sperm number (Quinto & De
Marinis, 1984).
Table 37. Carcinogenicity of diesel exhaust after exposure other than by inhalation
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Fischer 344 (a) 31 f Intratracheal (a) Activated carbon 1/animal 10/week, Survival rate, 71-83% Kawabata
rat (b) 59 f instillation (b) Diesel particles 1/animal 30 months' (lowest ingroup b). Malignant et al.
(c) 53 f (c) None observation lung tumours: (a) 7/23; (1986)
(d) 27 f (d) Vehicle (b) 20/42; (c) 0/44; (d) 1/23;
P < 0.01. Benign and
malignant tumours combined:
(a) 11/23; (b) 31/42
Osborne- Groups Lung Organic material from Observation (a) 1 bronchioalveolar adenoma; Grimmer
Mendel rat of 35 f implant diesel exhaust: until (b) 5 squamous-cell carcinomas; et al.
(a) Hydrophilic fraction 6.7 spontaneous (c) 1 bronchioalveolar adenoma; (1987)
(b) Hydrophobic fraction 20.0 death (d) 6 carcinomas;
(c) Nonaromatic compounds 19.2 (e) No tumours
+ PAHs (2 or (f) 1 carcinoma
3 rings) (g) 7 carcinomas, 1 adenoma
(d) PAHs ( > 4 rings) 0.2 (h) No tumours
(e) Polar PAHs 0.3 (i) 1 adenoma
(f) Nitro-PAHs 0.2
(g) Reconstituted
hydrophobic fraction 19.9
(h) None
(i) Vehicle
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Wistar rat (a) 40 f Intratracheal (a) Diesel soot (34 m2/g) (a) 3 × 15 Primary lung tumours (%) Pott &
(b) 58 f instillation (b) Diesel soot (70 m2/g) (b) 3 × 10 (a) 65 Roller
(c) 38 f (c) Diesel soot (70 m2/g) (c) 3 × 20 (b) 60 (1994);
(d) 37 f (d) Carbon black (270 (d) 3 × 15 (c) 66 Pott et
m2/g) (d) 65 al.(1994)
(e) 37 f (e) Activated charcoal (e) 3 × 10
(f) 39 f (860 m2/g) 3 × 20 (e) 27
(g) 40 f (f) NaCl solution (f) 3 × 10 (f) 0
(g) NaCl solution (g) 0.4 × 20 until (g) 0
ml spontaneous
death or 131
weeks
Wistar rat Groups Intratracheal (a) Diesel soot (native) (a) 1 × 15 Primary lung tumours (%): Heinrich
of 48 f instillation (b) Diesel soot (toluene (b) 2 × 15 (a) 17 (1994)
extract; 130 m2/g) 1 × 15
(c) Carbon black (c) 1 × 15 (b) 23
(toluene extract; (d) 1 × 15 4
270 m2/g; primary (e) Vehicle control (c) 21
particle, 15 nm)
(d) Carbon black (toluene (d) 8
extract; 270 m2/g; (e) 0
primary particle,
15 nm)
(e) NaCl solution
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Syrian Groups Intratracheal (a) Diesel particles 1.25, 1/week, 15 1 lung adenoma at high dose Shefner
golden of 50 m; instillation (b) Diesel particles + 2.5, in groups (a) and (c) after 61 et al.
hamster various same amounts of weeks weeks; no lung tumours in (1982
controls ferric oxide controls
(c) Diesel particle
extract
+ ferric oxide
Syrian (a) 3 × Intratracheal (a) Exhaust extract 0.1, 0.5, 1/week, 15 Survival rates: (a) 95, 92, Kunitake
golden 62 m instillation (b) Vehicle 1 weeks 71%; (b) 98%. No difference in et al.
hamster (b) 59 m (c) 0.5 mg tumour incidence between (a) (1986)
(c) 62 m benzo[a]pyrene and (b); 88% respiratory
tumours in positive controls
C57Bl 12 m, Dermal Acetone extract of 0.5 ml 3 times/week 16 mice dead by 10 weeks, 33 Kotin
mouse 40 f, particles for life or skin tumours by 13 months, 2 et al.
69 22-23 months skin papillomas, no tumours in (1955)
controls controls
A mouse 50 m, Dermal Acetone extract of 0.5 ml 3 times/week Males: 8 skin tumours by 16
25 f, particles for life or 1 papilloma, 3 squamous-cell
34 22-23 months carcinomas. Females: 20 skin
controls tumours by 13 months,17 tumours
at 13-17 months, no tumours in
controls
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Sencar Groups Dermal Dichloromethane 0.1, 0.5, 1/week, 50-52 Skin carcinomas: Nesnow
mouse of 40 m extracts of particles 1.0, 2.0, weeks Engine A: 3% (m), 5% (f) at et al.
+ 40 f from engines (A, B, E) or 4.0 4 mg Engine B: 3% (m) at 0.5 mg (1982a,b,
Benzo[a]pyrene 12.6- Engine E: 3% (f) at 0.1 mg 1983)
202 µg Positive control: 10-90%
C57Bl/6N Groups Subcutaneous Particles in olive oil 10, 25, 1/week, 5 First tumours palpated in: Kunitake
mouse of injection containing 5% dimethyl 50, 100, weeks; 18 week 47 at 25 mg/kg bw et al.
15-30 f sulfoxide 200, or months' week 30 at 50 mg/kg bw (1986)
500 mg/kg observvation week 27 at 100 mg/kg bw
bw week 39 at 200 or 500 mg/kg bw
Malignant fibrous histiocytomas
in 5/22 mice at 500 mg/kg bw;
0/38 in controls
PAH, polycyclic aromatic hydrocarbon; TPA, 12-O-tetradecanoylphorbol 13-acetate
Groups of 15 male cynomolgus monkeys were exposed by inhalation
to 2 mg/m3 diesel particulates for 7 h/day on five days per week for
two years. Sperm motility and velocity were similar to those of
controls (Lewis et al., 1989).
In a test for dominant lethal mutation, male Fischer 344 rats
inhaled 2 mg/m3 diesel exhaust for 7 h/day on five days per week for
six months and were subsequently mated with untreated females. Live
and dead implants and preimplantation losses were analysed on days
19-20 of gestation: no significant effects were observed (Lewis et
al., 1989). In a similar test, 100 male and 54 female T stock mice
were exposed to 6 mg/m3 diesel exhaust for 8 h/day on seven days per
week for seven weeks. There were no significant dominant lethal
effects in males or females. A reduced number of corpora lutea
(reproductive function) was the only significant result (Pepelko &
Peirano, 1983).
B7.5.2 Embryotoxicity
No increase in the frequency of sister chromatid exchange was
seen in the livers of fetuses of Syrian golden hamsters that inhaled
diesel exhaust particles at 12 mg/m3 for 8 h/day on days 5-13 of
gestation. An intraperitoneal injection of 300 mg/kg body weight of
diesel particulates on day 12 of gestation also had no significant
effect on this end-point; however, intraperitoneal injection of 23%
particulate mass extracted with methylene chloride on day 12 resulted
in a dose-dependent increase in sister chromatid exchange frequency in
fetal liver on day 13, and a doubling was seen at 320 mg/kg body
weight. It was concluded that chemicals must be eluted from diesel
particles in order for the genotoxic material to cross the placenta
(Pereira et al., 1982).
B7.5.3 Teratogenicity
Sprague-Dawley rats and New Zealand white rabbits were exposed by
inhalation to exhaust from a light-duty diesel engine, at a
particulate matter concentration of 6 mg/m3, for 8 h/day on seven
days per week.
Twenty rats were exposed on days 5-16 of gestation and 20 rabbits
on days 6-18. The numbers of viable fetuses per litter, dead fetuses
per litter, resorptions per litter, implantation sites per litter,
corpora lutea per litter, and average fetal weight did not differ
significantly from those of controls (Pepelko & Peirano, 1983).
Reproductive and developmental toxicity are considered unlikely
to be critical end-points for diesel exhaust.
B7.6 Mutagenicity and related end-points
B7.6.1 In vitro
The genetic effects of particles and particle extracts have been
described (Lewtas & Williams, 1986; Morimoto et al., 1986; Henschler,
1987). Diesel exhaust extracts rather than particles were used in most
of these studies. Point mutations were observed in vitro in bacteria
and mammalian cells. Extracts caused chromosomal aberrations, DNA
damage, sister chromatid exchange, and cell transformation (Table 38).
The mutagenic potency of diesel engine exhaust depended in several
studies on the characteristics of the diesel fuel and the type, age,
and operating conditions of the engine (Henschler, 1987). As a rule,
organic extracts of particles were mutagenic in the absence of an
exogenous metabolic activating system (S9) (Ball et al., 1990);
addition of S9 decreased (Lewtas, 1983) or suppressed (Morimoto et
al., 1986) the mutagenic activity, perhaps by increasing protein
binding of mutagenic material or by metabolic detoxification of
directly acting mutagens, such as the nitroarenes, in the Ames test.
It can therefore be concluded that PAHs and thioarenes, which must be
metabolically activated, do not account for the mutagenic potency of
diesel particles. Salmeen et al. (1985) and Beland et al. (1985)
showed that nitroarenes are the main genotoxic agents in Salmonella
typhimurium T98 in the absence of S9. A number of other
investigators have reported the presence of nitrated PAHs in extracts
of diesel engine exhaust particles (Handa et al., 1983), including
many three-, four-, and five-ring structures (Schuetzle et al., 1982).
The concentration of 1-nitropyrene, one of the prevalent species, has
been reported to be 15-25 mg/kg particulate matter, whereas
benzo[ a]pyrene has been found at concentrations up to 50 mg/kg. The
nitrated PAHs are important in the health effects of diesel exhaust
since they are effective mutagens in microbial and human cell systems
(Patton et al., 1986). Some nitrated PAHs are also carcinogenic in
animals (Imaida et al., 1991). Ball & Young (1992) found that strain
T102, which does not respond to the mutagenic action of nitroarenes,
responds to a class of oxidizing compounds that can interact with DNA;
these compounds are not nitroarenes or typical PAHs.
The effects of diesel soot particles with and without adsorbed
organic substances, diesel soot extract without particles, an isolated
fraction of PAHs, and titanium dioxide and carbon black (Printex 90)
as reference particles were investigated in vitro on hamster lung
epithelial cells (Mohr & Riebe-Imre, 1992). The substances were added
to the cells at concentrations of 100-300 mg/litre. Diesel soot
extract, but not the PAH moiety isolated from the extract, stimulated
mixed-function oxygenases. The diesel soot extract was more cytotoxic
than the PAH fraction for the epithelium of the hamster respiratory
tract, but both mixtures induced the development of micronuclei. Under
the study conditions, only the PAH fraction led to transformation of
cells. The respiratory epithelium of hamsters was more sensitive than
human lung epithelial cells investigated in parallel, perhaps because
hamster cells are generally more easily transformed than human cells.
Diesel soot particles, titanium dioxide, and Printex 90 had hardly any
cytotoxic effect but triggered transformation. The authors concluded
that a combination of effects of particles and PAHs are responsible
for the degeneration of cells.
Schiffmann & Henschler (1992) studied the effects of diesel soot
extract and isolated fractions of PAHs, oxy-PAHs, and nitro-PAHs on
hamster embryo fibroblasts in vitro. The end-points investigated
were induction of micronuclei, unscheduled DNA synthesis, and cell
transformation. Diesel soot extract induced micronuclei at
concentrations of 80-400 mg/litre, whereas the fractions of various
PAHs were strongly cytotoxic. Diesel soot extract and the PAH fraction
transformed the fibroblasts; the nitro- and oxy-PAH fractions had a
weaker transforming effect, but their cytotoxic potency was clearly
stronger. Kinetochore analysis showed a high percentage (37%) of
chromosomes in micronuclei. No unscheduled DNA synthesis was induced.
Micronuclei were also induced in human embryonic lung fibroblasts
treated with diesel soot extract under comparable experimental
conditions.
B7.6.2 In vivo
The genotoxicity of total diesel exhaust, diesel particles, and
diesel soot extracts in vivo has been investigated in somatic cells
(Table 38). All of the substances induced genotoxic responses. Total
exhaust induced only sister chromatid exchange in Syrian golden
hamsters. The frequency of micronuclei was not increased significantly
in mice, even at high doses (up to 640 mg/kg body weight). Organic
extracts had clear genotoxic effects in various assays (Henschler,
1987).
No mutagenic activity was found in urine taken from rats exposed
to diesel exhaust emission by inhalation at 2 mg/m3 for 7 h/day on
five days per week for up to two years. A slight but nonsignificant
increase in micronuclei was observed in the bone marrow of mice that
had been exposed for six months; no increase in micronuclei was
detected in rats over a 24-month period (Ong et al., 1985).
In studies of heritable mutations in T stock mice, males were
examined for point mutations, induction of dominant lethal mutations,
translocations, and spermatogonial survival; females were examined for
oocyte death and dominant lethal mutations. No significant effect was
seen on germ cells. A reduced number of corpora lutea (reproductive
function) was the only significant result (Pepelko & Peirano, 1983).
B7.6.3 DNA adduct formation
Extensive research has been done to determine whether the DNA
adducts induced in lungs by diesel exhaust are related to later
tumorigenesis.
In rats that inhaled benzo[ a]pyrene absorbed to carbon black
(20 mg/g), an adduct of the diol epoxide with deoxyguanosine was
identified (Wolff et al., 1989), demonstrating that epoxides of PAHs
are produced by oxidative metabolism. Haemoglobin and albumin adducts
were also investigated.
Methods have been developed to detect DNA adducts of
1-nitropyrene or 1,6-dinitropyrene, which are markers of diesel engine
exhaust. The adducts are analysed in rat tissue or peripheral blood
lymphocytes by the 32P-postlabelling method and may be useful for
the dosimetry of 1-nitropyrene or diesel exhaust particulates in
occupational settings (El-Bayoumy et al., 1994). Studies on adduct
formation in lung DNA induced by inhaled diesel exhaust have been
conducted by Wong et al. (1986), Wolff et al. (1990), Bond et al.
(1988, 1989, 1990a,b), and Gallagher et al. (1993, 1994), all
involving the 32P-postlabelling technique. In general, these studies
suggest that DNA adducts are good measures of the 'effective dose' of
carcinogenic compounds.
Wong et al. (1986) found increased DNA adduct formation in the
lungs of Fischer 344 rats exposed to diesel exhaust particles at
7.1 mg/m3 for 31 months. After Fischer 344 rats and Syrian golden
hamsters were exposed to dilutions of diesel engine exhaust for six
months to two years, the level of haemoglobin adducts (2-hydroxy-
ethylvaline and 2-hydroxypropylvaline) increased dose-dependently,
corresponding to metabolic conversion of 5-10% of inhaled ethylene and
propylene to their oxides (Törnqvist et al., 1988).
Bond et al. (1988) designed experiments to determine the location
of DNA adducts in the respiratory tract of rats exposed to exhaust.
Fischer 344 rats were exposed for 12 weeks to diesel exhaust soot at
10 mg/m3; they were then sacrificed, and various regions of the
respiratory tract were removed and analysed for DNA adducts. Adducts
were found only in peripheral lung tissue and nasal tissue; the total
levels were highest in the peripheral tissue (about 18 adducts per 109
bases). Thus, the levels of total DNA adducts and exhaust-induced
adducts are highest in the region of the rat respiratory tract where
tumours are formed after exposure to a carcinogenic concentration of
diesel exhaust (Mauderly et al., 1987).
Bond et al. (1990c) exposed groups of Fischer 344 rats to soot at
0.35, 3.5, 7.0, or 10 mg/m3 for 12 weeks and found that the levels
of DNA adducts were similar, at about 14 adducts per 109 bases. This
level is nearly twice as high as that found in sham-exposed rats.
Thus, DNA adduct formation in lungs is independent of the
concentration of exhaust at the levels tested. One explanation for
these results (Bond et al., 1990a) is that the lung enzymes
responsible for the metabolism of soot-associated chemicals to
metabolites that bind to DNA were saturated at the concentrations
tested. These data also suggest that other factors are important in
the carcinogenicity of diesel exhaust, since the number of adducts was
elevated at an exposure level (0.35 mg/m3) that did not increase
lung tumour incidence. The induction of DNA adducts at low
concentrations is likely to result in tumour initiation by the organic
compounds present; at higher concentrations, the particles will induce
cell death, and subsequent proliferation may well act as a promotional
event. The combination may then result in detectable increases in
tumour incidence.
Bond et al. (1990a) also investigated the time course for DNA
adduct formation and persistence. Fischer 344 rats were exposed to
soot at 7 mg/m3 for up to 12 weeks and were sacrificed at 2, 4, 8,
12, 14, and 16 weeks after the start of exposure. DNA adducts
accumulated slowly in the lung, and the number was highest at the end
of exposure, representing about 160% of the level seen in controls
exposed to air only. The levels declined rapidly after termination of
exposure and were not significantly different from those of controls
four weeks later. Thus, steady-state levels of DNA adducts would be
reached during long-term exposure to diesel exhaust.
Bond et al. (1989, 1990c) and Wolff et al. (1990) investigated
whether exposure to carbon particles without associated mutagenic
organic chemicals also increases DNA adduct levels. Rats were exposed
either to diesel exhaust or carbon black at 0, 3.5, or 10 mg/m3 for
12 weeks. Solvent-extractable organic compounds made up about 30% of
the diesel soot but only about 0.04% of the carbon black particles.
Exposure to the highest levels of both carbon black and diesel exhaust
increased the DNA adduct levels in lungs, although the levels in rats
exposed to diesel exhaust were about 30% higher than those in rats
exposed to carbon black. Heavy exposure to carbon particles can
therefore increase DNA adduct formation, although the concentrations
are lower than those of exhaust-related adducts.
Bond et al. (1990b) investigated whether DNA adducts could be
formed in specific cells of the rat lung, i.e. alveolar type II cells.
After exposure of rats to 6.2 mg/m3 diesel exhaust or carbon black
particles for 12 weeks (16 h/day on five days per week), type II cells
were isolated and their DNA analysed for adducts. Both diesel exhaust
and carbon black resulted in about a fourfold increase in total DNA
adducts in type II cells. DNA adduct levels in peripheral lung tissue
were increased by 60-80% in male and female Fischer 344 rats and
female cynomolgus monkeys but not in female B6C3F1 mice or female
Syrian hamsters after inhalation of diesel soot at 8.1 mg/m3 for 12
weeks (Bond et al., 1989, 1990c).
Gallagher et al. (1993, 1994) exposed female Wistar rats to
diesel soot at 7.5 mg/m3 and to carbon black at 11.3 mg/m3 for 24
months and measured DNA adducts in lungs. The mean adduct levels were
similar in the two groups but were not significantly greater than
those in controls. Time-course studies indicated that the levels in
the lungs of rats exposed to diesel exhaust were lower at 24 months
than after two or six months of exposure, presumably as a result of
dilution due to increased cell proliferation. The level of a single
DNA adduct, thought to be derived from a nitro-PAH in diesel exhaust
and not observed in rats exposed to carbon black or titanium oxide,
was elevated over that in controls after two, six, and 24 months. The
DNA adduct levels in control rats increased over the duration of the
24-month study as an effect of age.
Gallagher et al. (1993) compared DNA adduct formation after
exposure to diesel emissions in vitro and in vivo. After exposure
of human lymphocytes to diesel extract, five major DNA adducts were
detected, one of which was characterized as an adduct with
benzo[a]pyrene. One was also detectable in reaction products of calf
thymus DNA and diesel particle extract in vitro and in skin and lung
DNA from mice treated dermally with 50 mg diesel extract in vivo. The
differences in DNA patterns in vitro and in vivo (skin and lung)
may reflect differences in metabolic pathways.
Taken together, the studies of DNA adducts suggest that some
organic chemicals in diesel exhaust can form DNA adducts in lung
tissue and may play a role in the carcinogenic effects. As pointed out
by Bond et al. (1989), however, DNA adducts alone cannot explain the
carcinogenicity of diesel exhaust, and other factors, such as chronic
inflammation and cell proliferation, are also important.
B7.7 Special studies
B7.7.1 Immunotoxicity
During the clearance process, one possible pathway is
translocation of diesel particulates into the lymphatic channels.
After male guinea-pigs were exposed to diesel exhaust at a particle
concentration of 1.5mg/m3 for four or eight weeks, the B- and T-cell
counts in lymph nodes were not altered, and there were no significant
changes in blood or spleen (Dziedzic, 1981).
Fischer 344 rats were exposed to 2 mg/m3 diesel exhaust for
7h/day on five days per week for 12 or 24 months and the immunological
function of splenic B and T cells was measured by enumerating
antibody-producing cells in the spleen or monitoring the proliferative
response. No changes were observed (Mentnech et al., 1984).
Table 38. Genotoxicity of diesel exhaust, particles, and extracts
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity
Bacteria
S. typhimurium Point mutation (his) + + Huisingh et al. (1978)
S. typhimurium Point mutation (his) + + Loprieno et al. (1980)
S. typhimurium Point mutation (his) + Clark & Vigil (1980)
S. typhimurium Point mutation (his) + + Li & Royer (1982)
S. typhimurium Point mutation (his) + Salmeen et al. (1984)
S. typhimurium Point mutation (his) + Ong et al. (1985)
S. typhimurium Point mutation (his) + Bechtold et al. (1986)
S. typhimurium Point mutation (his; + Whong et al (1986)
SOS umu test)
S. typhimurium Point mutation (his) + Wallace et al. (1987)
S. typhimurium Point mutation (his) +a Wallace et al. (1990)
S. typhimurium Point mutation (his) + Lewis et al. (1989)
S. typhimurium Point mutation (his) + Rasmussen (1990)
S. typhimurium Point mutation (his) + + Keane et al. (1991)
S. typhimurium, E. coli Point mutation (trp) + Crebelli et al. (1991)
E. coli Point mutation (trp) + Lewtas (1983)
Mammalian cells
L5178Y mouse Point mutation (tk) + Lewtas (1983)
lymphoma cells
L5178Y mouse Point mutation (tk) + Mitchell et al. (1981)
lymphoma cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity (contd)
Chinese hamster Point mutation (hprt) + Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Casto et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt, + Morimoto et al. (1986)
lung V79 cells ATPase)
Human xeroderma Point mutation (hprt) + + McCormick et al. (1980)
pigmentosum fibroblasts
Human lymphoblast Point mutation (tk) + Barfknecht et al. (1981)
TK6 cells
Balb/c3T3 mouse Point mutation (ATPase) + (+) Curren et al. (1981)
fibroblasts
DNA damage
Syrian hamster DNA chain breaks - Casto et al. (1981)
embryo cells (alkaline elution)
Human xeroderma DNA damage + + McCormick et al. (1980)
pigmentosum fibroblasts
Rat primary Unscheduled DNA + Lewtas (1983)
hepatocytes synthesis
Chinese hamster lung Unscheduled DNA +a + Gu et al. (1994)
V79 cells synthesis
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Chromosomal effects
Chinese hamster Sister chromatid exchange + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations +b Hasegawa et al. (1988)
lung V79 cells -c
Chinese hamster Sister chromatid exchange + Hasegawa et al. (1988)
lung V79 cells
Chinese hamster Sister chromatid exchange + + Keane et al. (1991)
lung V79 cells
Human lymphocytes Chromosomal aberrations + Lewtas (1983)
Chinese hamster Sister chromatid exchange + Morimoto et al. (1986)
lung V79 cells
Human lymphocytes Sister chromatid exchange (+) Tucker et al. (1986)
Human lymhoblastoid Sister chromatid exchange + Morimoto et al. (1986)
cells
Hamster embryo Micronuclei + Schiffmann & Henschler
fibroblasts (1992)
Chinese hamster lung Micronuclei +a + Gu et al. (1992)
V79 and ovary cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Cell transformation
Balb/c3T3 mouse fibroblasts Cell transformation (+) Curren et al. (1981)
Balb/c3T3 mouse fibroblasts Cell transformation +b Hasegawa et al. (1988)
(+)c
Hamster lung epithelial cells Cell transformation + + Mohr & Riebre-Imre (1992)
Hamster embryo fibroblasts Cell transformation + Schiffmann & Henschler (1992)
In vivoa
Mouse Micronuclei, bone marrow - - + Pereira (1982)
Mouse Micronuclei, bone marrow - Lewis et al. (1989)
Mouse Micronuclei, bone marrow - Morimoto et al. (1986)
Mouse Micronuclei, bone marrow - - - Pepelko & Peraino (1983)
Mouse Micronuclei, bone marrow (+) Ong et al. (1985)
Rat Micronuclei, bone marrow - Ishinishi et al. (1988)
Rat Micronuclei, bone marrow - Ong et al. (1985)
Chinese hamster Micronuclei, bone marrow (+) - - Pepelko & Peraino (1983)
Mouse Sister chromatid exchange, - + + Pereira (1982)
bone marrow
Rat Sister chromatid exchange, - Ishinishi et al. (1988)
bone marrow
Rat Sister chromatid exchange, - Morimoto et al. (1986)
bone marrow
Rat Sister chromatid exchange, - Ong et al. (1985)
peripheral blood leukocytes
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Rat Sister chromatid exchange, - Lewis et al. (1989)
lymphocytes
Syrian golden Sister chromatid exchange, + Pereira (1982)
hamster lung cells (inhalation)
Syrian golden Sister chromatid exchange, + Guerrero et al. (1981)
hamster lung cells (instillation)
Syrian golden Sister cromatid exchange, + + Pereira (1982)
hamster lung cells (instillation)
Syrian golden Sister chromatid exchange + Morimoto et al. (1986)
hamster liver cells (transplacental)
Syrian golden hprt mutation + Morimoto et al. (1986)
hamster (transplacental)
Mouse Mutation, S. typhimurium - Morimoto et al. (1986)
(host-mediated assay)
Mouse Heritable effects on germ - Pepelko & Peirano (1983)
cells (various assays; m/f)
Drosophila Heritable effects (sex-linked Schuler & Niemeier (1981)
melanogaster recessive lethal mutation) -
his, histidine independence; trp, tryptophane independence; hprt, hypoxanthine-guanine-phosphoryltransferase (8-aza- or
6-thioguanine resistance); tk, thymidine kinase (bromodeoxyuridine or trifluorothymidine resistance); ATPase, Na+/K+-ATPase
(ouabain resistance); +, positive; (+), weakly positive
a Dispersed in artificial surfactant
b Light-duty engine
c Heavy-duty engine
d Modified from Henschler (1987) and supplemented
Fischer 344 rats and CD-1 mice were exposed to diesel exhaust
particles at 0.35, 3.5, or 7 mg/m3 for 6, 12, 18, or 24 months to
investigate whether the accumulation of diesel particulates in lymph
nodes (indicated by black discolouration) influences the subsequent
response to immunization by sheep red blood cells, evaluated by the
presence of immunoglobulin (Ig) M antibody-forming cells. In rats, the
total number of lymphoid and antibody-forming cells was significantly
increased at the medium and high aerosol concentrations. In mice, the
number of lymphoid cells was increased only at the high concentration.
The authors concluded that diesel exhaust particles have only a
minimal effect on the immune and antigen filtration functions in
lung-associated lymph nodes because the relative numbers of
antibody-forming cells and specific IgM, IgG, and IgA antibodies in
rat sera were not significantly changed (Bice et al., 1985).
Five intranasal inoculations of various doses of a suspension of
diesel engine exhaust particles in ovalbumin were administered to BDF1
mice at intervals of three weeks. Ovalbumin IgE antibody titres,
assayed by passive cutaneous anaphylaxis, were enhanced by doses as
low as 1 µg (Takafuji et al., 1987, 1989). Similarly, primary IgE
responses were increased after intraperitoneal administration to mice
of ovalbumin or cedar pollen allergen mixed with diesel exhaust
particulates (Muranaka et al., 1986).
Female CR/CD-1 mice were exposed by inhalation to 6-7 mg/m3
diesel exhaust for 2-6 h (acute), 8 h/day for 2-16 days (subacute),
or 8h/day for about 300 days (chronic). Immediately after the end
of exposure, animals were exposed to the infectious aerosols
S. typhimurium, Streptococcus pyogenes, or A/PR8-34 influenza virus.
Increased susceptibility to post-infection mortality was seen with the
bacterial but not the viral pathogens. Nitrogen dioxide and acrolein
vapour had specific effects (Campbell et al., 1981b).
CD-1 mice were exposed to 2 mg/m3 diesel exhaust for one to six
months. After three months of exposure and subsequent infection with
influenza virus, an enhanced severity of response was seen, involving
significant lung consolidation and focal macular collections of
particle-laden macrophages (Hahon et al., 1985).
B7.7.2 Behavioural effects
Sprague-Dawley rats were exposed for 8 h/day on seven days per
week for 16 weeks to diesel exhaust diluted to a particulate matter
concentration of 6 mg/m3. Spontaneous locomotor activity, measured
weekly on Wahman LC-34 running wheels, was significantly decreased
during 8, 9, 11, and 12 weeks of exposure. In rats exposed for
20 h/day on seven days per week for six weeks, forced activity on a
motorized treadmill was measured during the last week. Exhaustion
occurred in less than half the time that it occurred in control
animals. Groups of 10 rats were exposed to diesel exhaust at 6 mg/m3
for 20 h/day on days 1-7 post partum and were then held in clean air
until 15 months of age. In bar pressing acquisition training carried
out at five-day intervals for the next 42 days, the rate of learning
was much slower than in control rats. The difference was highly
statistically significant (Pepelko & Peirano, 1983).
As these three studies were conducted at a high concentration,
6 mg/m3, the practical consequences of the effects observed are not
known. The results, while limited in scope, indicate, however, that
behavioural and neurophysiological effects may be important toxic
end-points which should be investigated further.
B7.8 Factors that modify toxicity; toxicity of metabolites
Little information is available, but chemicals and other factors
may modify the toxicity of diesel emissions. For instance,
chlorophyllin, a derivative of the green pigment chlorophyll, has been
reported to inhibit or reduce the mutagenic activity of diesel
particulate extracts in the Ames test (Ong et al., 1986).
B7.9 Mechanisms of toxicity; mode of action
It is not clear whether DNA-reactive or non-DNA-reactive
mechanisms, or a combination of the two, are responsible for the
carcinogenic action of inhaled diesel exhaust in laboratory animals.
The results of several studies indicated that many PAHs (e.g.
benzo[ a]pyrene) cause a carcinogenic response in rodents, but it is
not known whether the same PAHs when associated with diesel exhaust
also induce a carcinogenic response. One hypothesis for the mechanism
of the tumorigenic response is that organic chemicals (e.g. PAHs,
nitro-PAHs) desorb from soot particles, are metabolized to reactive
metabolites, and interact with lung DNA to initiate carcinogenesis.
This would be a predominantly DNA-reactive mechanism. The observation
that organic chemicals associated with diesel soot can be metabolized
by lung cells to metabolites that can form DNA adducts after long-term
exposure to diesel exhaust supports this hypothesis. Studies with
carbon black essentially devoid of PAHs but which also form DNA
adducts do not, however, corroborate the PAH-DNA-reactive concept.
Another hypothesis is that the lung tumours that arise in rats
exposed to high levels of diesel exhaust are due to overloading of the
normal lung particle clearance mechanisms, accumulation of soot
particles, and cell damage followed by regenerative cell
proliferation. Enhanced cell proliferation may increase the mutation
frequency of key target genes. In this hypothesis, genotoxic chemicals
may not be causative factors in tumorigenicity. This view is supported
by the observations that lung cancer can be induced in rodents by
inhalation of highly insoluble particles of low toxicity that are
virtually devoid of organic chemicals (e.g. talc, carbon black, coal
dust, titanium dioxide), although high concentrations of these
particles (overload) are typically necessary.
A third hypothesis, which draws upon the above two mechanisms, is
that carcinogenesis is initiated by exposure to organic compounds
associated with diesel soot and promoted by the chronic inflammation,
cytotoxicity, and cell proliferation arising from the high
concentrations of particles deposited and retained in the lungs.
Again, however, the tumorigenic response observed with particles alone
(carbon black, titanium dioxide) does not support this hypothesis.
A key consideration is the relative contribution of different
mechanisms at different levels of exposure. As discussed later in this
section, high concentrations of particles, including diesel exhaust,
leading to high lung burdens, compromise normal clearance mechanisms.
Therefore, certain mechanisms may be invoked at high lung burdens that
may not occur at lower lung burdens. Genotoxic aromatic compounds may
take on increasing importance at lower concentrations.
Figure 3 is a diagram outlining the possible mechanism of action
of diesel exhaust, including the effects of overload conditions.
B7.9.1 Carcinogenic effects
B7.9.1.1 DNA-reactive mechanisms
Diesel exhaust contains hundreds of chemicals, including PAHs,
nitro-PAHs, alkyl-PAHs, oxy-PAHs, oxy-nitro-PAHs, and aldehydes
(Scheepers & Bos, 1992b), many of which are known mutagens and
carcinogens in laboratory animals. For example, inhaled
benzo[ a]pyrene (in tar and pitch) is carcinogenic in rat lung
(Heinrich et al., 1994). Nitro-PAHs are potent mutagens and, in some
instances, carcinogenic. In pharmacokinetic studies cited in this
monograph, clearance of PAHs and nitro-PAHs associated with the diesel
particulate fraction is retarded or delayed. Other studies have shown
clearly that the organic compounds associated with diesel soot are
bioavailable to lung cells. Mechanisms for the delayed clearance of
the PAHs have been discussed (Bond et al., 1986b; Gerde et al.,
1991b). PAHs require metabolic activation to metabolites (e.g.
epoxides) that can potentially bind to DNA. In the case of the PAHs
associated with diesel soot, it is likely that alveolar lung cells
(e.g. type II cells) are responsible for their metabolic activation,
so that they can bind to lung cell DNA (Bond et al., 1983).
Additional evidence for a DNA-reactive mechanism in the
carcinogenicity of diesel exhaust is that exposure of laboratory
animals, including rats and monkeys, to diesel exhaust results in the
formation of DNA adducts in lung cells (see section B7.6). DNA adducts
and subsequent DNA replication can result in mutations that play a key
Retention of diesel particles in the lungs and lymph nodes has
been reported in Fischer 344 and Wistar rats after long-term
inhalation (Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly
et al., 1987; Strom et al., 1988; Creutzenberg et al., 1990; Strom et
al., 1990). Some results with regard to lung burden are included in
Table 33. Because clearance of lung burdens above about 1 mg per lung
was impaired after long-term, continuous inhalation of a concentration
equivalent to about 0.5mg/m3, no steady-state lung burden was found
at serial sacrifice at up to two years. About 20% of the lung burden
of diesel particles was found in lung-associated lymph nodes at that
time.
Mathematical models have been developed to calculate the
retention of diesel particles in rats. Yu & Yoon (1990) and Yu et al.
(1991) developed a dosimetry model to predict the retention of diesel
particles and associated organic compounds in rats and humans of
different ages. The model indicates that lung clearance would not be
impaired in humans exposed to a concentration < 0.05 mg/m3 for
24 h/day. The highest lung burden per unit of lung weight was
calculated for five-year-old children. This model was used to assess
the risk of inhaling diesel particles (Pepelko & Chen, 1993).
B6.3 Retention and clearance of polycyclic aromatic hydrocarbons
adsorbed onto diesel soot
The clearance of particles from ciliated airways by the
mucociliary escalator after bronchial deposition is virtually complete
within 24 h. For soluble organic material like PAHs on the surface of
carbon particles, diffusion-limited clearance through the airway
epithelium to capillary blood was found to be slower in the bronchial
region than in the alveoli (Gerde et al., 1991a, 1993a,b).
PAHs in diesel soot adhere strongly to the surface of the
particles. Several studies have shown that the association of PAHs
with particles significantly slows the clearance of the PAHs from
lungs in comparison with the clearance of inhaled PAHs unassociated
with particulate matter.
After inhalation by Fischer 344 rats of 3H-benzo[ a]pyrene
adsorbed onto diesel soot (0.1% by mass) for 30 min, biphasic lung
clearance was noted. In a first, fast phase, about 50% of the inhaled
compound was cleared with a half-time of about 1 h, predominantly by
mucociliary clearance. At the end of exposure, about 15% of the 3H
label was found in blood, liver, and kidney, indicating rapid, direct
absorption of this fraction of 3H-benzo[ a]pyrene and its metabolites
into blood. The remaining 50% was retained in the lung, with a
half-time of 18 days; this is one-third of the retention half-time of
diesel particles. Thus, removal of benzo[ a]pyrene and its metabolites
from the particle carrier may be the rate-limiting step of this
clearance process (Sun et al., 1984). In contrast, more than 95% of
pure 3H-benzo[a]pyrene was cleared from the lung within 12 h after
inhalation (Sun et al., 1982).
These results were confirmed and extended to another PAH,
1-nitropyrene (Bond et al., 1986a,b), when the clearance of
14C-labelled compound adsorbed to diesel soot was determined by the
same method in Fischer 344 rats. After exposure to 490 ng/litre of
pure labelled compound, about 90% was cleared in the first, fast
clearance phase, with a retention half-time of 1 h; after exposure to
650 ng/litre of 14C-1-nitropyrene adsorbed onto diesel particles,
45% of the labelled compound was cleared from the lungs in the first
phase, with a half-time of about 2 h. The remaining 55% was retained
with a half-time of 36 days, which was twice the corresponding value
for 3H-benzo[ a]pyrene in the study of Sun et al. (1984).
On the basis of their data on the clearance of 3H-benzo[ a]pyrene
and 14C-1-nitropyrene, Bond et al. (1986b) predicted equilibrium
lung burdens in humans after long-term inhalation. Assuming inhalation
of 3.5 g/m3 of particles containing 0.1% benzo[ a]pyrene and
1-nitropyrene over an 8-h working day, they predicted equilibria of
160 ng benzo[ a]pyrene and 31 ng 1-nitropyrene per lung. No data are
available on changes in the retention of individual compounds after
prolonged exposure to diesel exhaust.
The clearance of a very low concentration (2.6 g/g particles) of
3H-benzo[ a]pyrene adsorbed onto diesel particles was measured in
Sprague-Dawley rats after intratracheal instillation (Bevan & Ruggio,
1991). Only 25% was cleared in the early, fast phase; a long-term
retention half-time of eight days was calculated for the remainder
during a three-day follow-up, which was similar to the clearance in
that period in the study of Sun et al. (1984).
B6.4 Metabolism
In the studies of Sun et al. (1984) and Bond et al. (1986a,b),
the levels of 3H-benzo[ a]pyrene and 14C-1-nitropyrene and their
metabolites in extracts of lung homogenates after inhalation of diesel
exhaust particles were measured by high-performance liquid
chromatography. Sun et al. detected oxidized metabolites 30 min after
inhalation: 35% of the 3H-benzo[ a]pyrene had been metabolized in
equal amounts to phenols (3-hydroxy- and 9-hydroxybenzo[ a]pyrene)
and quinones (1,6 and 3,6 isomers). Twenty days later, only 13%
phenols and 5% quinones were detected. In the study of Bond et al.
(1986b), about 30% of the 3H-1-nitro-pyrene had been metabolized to
acetylaminopyrenephenol (6 and 8 isomers) and about 40% remained
unmetabolized 1 h after inhalation. Oxidative metabolism of
14C-benzo[ a]pyrene, in solution or adsorbed onto diesel particles
at a concentration of 500 pmol/106 cells, was also detected in cell
cultures of pulmonary macrophages obtained from beagle dogs. The
quantity of metabolites increased with incubation time up to 45 and
125 pmol/106 cells in extracts and in the media, respectively, after
48 h of incubation. After incubation for 6 h, the major metabolites
found in extracts of cells were benzo[ a]pyrene-7,8-diol (0.5pmol/106
cells) and benzo[a]pyrene-4,5-diol (0.2 pmol/106 cells); those in
extracts of culture medium were benzo[ a]pyrene-7,8-diol (1.7 pmol/106
cells) and benzo[ a]pyrene-9,10-diol (1.4 pmol/106 cells), in
comparison with a total concentration of metabolites of 2.5 pmol/106
cells in the extracts and 6 pmol/10 6 cells in the medium. Minor
metabolites were benzo[ a]pyrene phenols (3-hydroxy- and
9-hydroxy-benzo[ a]pyrene) and benzo[ a]pyrene quinones (6,12, 1,5,
and 3,5 isomers). The quantities of metabolite were similar when
macrophages were incubated with benzo[ a]pyrene in solution or
adsorbed onto diesel particles (Bond et al., 1984).
Using the 32P-postlabelling technique, Wong et al. (1986) found
increased DNA adduct formation in the lungs of Fischer 344 rats after
exposure for 31 months to diesel exhaust containing 7.1 mg/m3
particles. After exposure of Fischer 344 rats and Syrian golden
hamsters to dilutions of diesel engine exhaust for six months to two
years, the level of haemoglobin adducts (2-hydroxyethylvaline and
2-hydroxypropylvaline), corresponding to the metabolic conversion of
5-10% of inhaled ethylene and propylene to its oxides, increased
dose-dependently (Törnqvist et al., 1988). An adduct of
benzo[ a]pyrenediol epoxide with deoxyguanosine was identified after
inhalation of benzo[a]pyrene adsorbed onto carbon black (20 mg/g)
(Wolff et al., 1989), demonstrating the production of epoxides of PAHs
by oxidative metabolism.
Methods for extracting diesel soot before testing for
genotoxicity in vitro have been discussed. As dichloromethane and
dimethyl sulfoxide do not correspond to leaching conditions in the
lung, it can be assumed that the bioavailability of genotoxic
materials is lower in lungs than in vitro. An investigation of
dipalmitoyl phosphatidylcholine, the primary component of
physiological surfactant, demonstrated that the genotoxicity
associated with diesel particles inhaled into the lung can be
activated by the solubilization and dispersion properties of pulmonary
surfactants. This finding was confirmed in mammalian cells for sister
chromatid induction (Keane et al., 1991) and for chromosomal
aberrations and micronucleus formation (Gu et al., 1991, 1992).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
B7.1 Single exposure
Data on the acute toxicity of diesel exhaust are very limited;
the two available studies suggest that diesel exhaust particles and
their extracts have little acute toxicity.
Male ICR mice were administered diesel exhaust particles by
intratracheal instillation, and mortality due to lung oedema was
assessed for up to 24 h. At a dose of 0.9 mg per animal, all animals
died within 24 h. The LD50 value was 0.6 mg per animal, equivalent
to about 20 mg/kg body weight. Diesel exhaust particles washed three
times with methanol did not cause death at a dose of 1 mg per mouse,
suggesting that the primary source of toxicity is the extractable
organic compounds rather than the particles themselves. These
compounds may produce superoxide radicals that damage endothelial
cells (Sagai et al., 1993).
The LD50 value for diesel exhaust extract in adult Syrian
golden hamsters after intraperitoneal injection was 1280 mg/kg body
weight (Pereira et al., 1982).
B7.2 Short-term exposure
Tracer-monitored DNA synthesis, indicating a proliferative
response, was observed in whole lung tissue of groups of 3-10 Fischer
344 rats exposed to diesel exhaust by inhalation at 6 mg/m3 for
20h/day on seven days per week for periods of 1-14 days. The response
was maximal after two days of exposure, showing a fourfold increase.
The type II cell labelling index was significantly higher (fourfold)
than that in controls after two and three days of exposure to diesel
exhaust. Both values returned to control levels after a one-week
recovery period. Experiments involving incorporation of 14C-palmitic
acid revealed transient changes in lipid metabolism after one day of
exposure, with a threefold increase in the amount of phosphatidyl-
choline in lung tissue (Wright, 1986).
Exposure of various species by inhalation to 6 mg/m3 resulted
in slight alterations in lung function and histopathological changes.
Groups of 10 male Chinese hamsters were exposed for 8 h/day on
seven days per week for six months to particle concentrations of
either 6 or 12 mg/m3 diesel exhaust.
Measurements of pulmonary function indicated dose-related
increases in lung weight and dose-related decreases in vital and
diffusion capacity. Pathological evaluation revealed oedema,
thickening of the alveolar lining, a possible increase in collagen,
and the presence of numerous particle-laden macrophages, which almost
filled the alveolar spaces (Vinegar et al., 1981).
Groups of 41-51 male and female Hartley guinea-pigs were exposed
for 20 h/day on seven days per week to exhaust from a light-duty
diesel engine diluted to attain a concentration of 6 mg/m3
particulate matter. Separate groups were exposed to raw exhaust and
exhaust that had been irradiated with ultra-violet light for an
average of about 1 h. Pulmonary function was measured, and
electrocardiography was conducted on animals exposed for four weeks;
those examined for histological changes were exposed for eight weeks.
The only changes noted after four weeks were a 35% increase in
pulmonary flow resistance and a small, but significant decrease in
heart rate in animals exposed to irradiated exhaust. No changes in the
electrocardiogram were noted. The pathological changes were limited to
hyperplasia of alveolar lining cells and slight focal thickening of
the interstitium in occasional alveoli with accumulation of
particle-laden macrophages. Growth rates were unaffected (Wiester et
al., 1980).
As part of the same study, cats (Pepelko et al., 1981) and male
rats (15 per group) (Pepelko, 1982) were exposed for four weeks to
unirradiated exhaust under identical conditions. The pulmonary
function of rats was unchanged; the only changes were significant
increases in vital and total lung capacity, but these differences were
quite small and of questionable significance. The only functional
change noted in the cats was a decrease in maximal expiratory flow
rate at 10% of vital capacity. The pathological changes were again
limited to focal thickening of the alveolar walls near accumulations
of particle-laden macrophages. In general, the functional and
pathological changes were limited under these experimental conditions.
Kaplan et al. (1982) exposed male Fischer 344 rats (168 per
group), A/J mice (672 per group), and male Syrian golden hamsters (236
per group) to 1.5 mg/m3 of diesel exhaust particles for 20 h/day on
seven days per week for three months or to clean air. Animals were
killed at the end of exposure and after an additional six-month
recovery period. Mortality was not increased during the observation
period, and body weight was not significantly altered in any species.
At the end of exposure, the relative weight of the lung was
significantly increased in rats. In mice, the pulmonary adenoma
response was increased but not significantly. In all three species,
grey to black discolouration of the lungs and lung-associated lymph
nodes were observed. Microscopically, most of the particulate was
found in macrophages, but some was free. After six months' exposure to
clean air, the lungs of all species had considerably less pigment
accumulated in foci.
Sixteen male Fischer 344 rats were exposed to 6 mg/m3 diesel
exhaust for periods ranging from 6 h to 63 days, with subsequent
histological examination of the lungs. The numbers of diesel
particulate-containing alveolar macrophages, type II alveolar cells,
and inflammatory cells were found to be increased over those in eight
controls. After a nine-week exposure, accumulation of macrophages was
observed near the terminal bronchioles (White & Garg, 1981). In rats
that inhaled 6 mg/m3 diesel exhaust for two weeks and were either
killed immediately or allowed to recover for six weeks before
sacrifice, there was no statistical difference in the number of
macrophage aggregates (240 000 and 220 000 per lung); however, the
aggregates in the recovery group had significantly higher average
diameter and volume, demonstrating macrophage attachment after the end
of exposure (Garg, 1983).
The observations of Wiester et al. (1980) and Garg (1983)
demonstrate the importance of alveolar macrophages in the clearance of
particles of low solubility. These are engulfed by alveolar
macrophages and eliminated via the mucociliary escalator and
gastrointestinal tract, or, in the case of lung particle overloading,
increasing fractions are sequestered in focal areas of the lung tissue
and transferred to the lymph nodes (Muhle et al., 1990).
B7.3 Long-term exposure and studies of carcinogenicity
B7.3.1 Non-neoplastic effects
No obvious signs of toxicity were observed in groups of 183 male
and 183 female Fischer 344 rats exposed by inhalation to diesel
exhaust at 0.35, 3.5, or 7.1 mg/m3 for 7 h/day on five days per week
for up to 30 months (Mauderly et al., 1987). The body weights did not
differ significantly from those of controls, and the exposures did not
affect the life span of animals of either sex. Focal fibrotic and
proliferative lung disease was observed in parallel with progressive
accumulation of diesel soot in the lung. Henderson et al. (1988)
reported the results of biochemical and cytological analyses of lung
lavage fluid and lung tissue from rats and mice exposed in the same
study, including determinations of lactic dehydrogenase, glutathione
reductase, ß-glucuronidase, glutathione, and hydroxyproline, and the
numbers of macrophages and neutrophils. No changes were seen in rats
or mice exposed to 0.35 mg/m3 , but at higher concentrations dose-
and duration-dependent increases in biochemical parameters and cell
numbers in lavage fluid were observed, indicating a chronic
inflammatory response. Lung weights were also increased in male and
female mice exposed to the two higher levels for any length of time,
in rats exposed to 7.0 mg/m3 for 12 months or longer, in female rats
exposed to 3.5 mg/m3 for 18 months, and in males exposed to
3.5 mg/m3 for 24 months. Tissue enzyme levels, measured in lung
lavage fluid as an indicator of damage, were consistently raised in
rats and mice at the two higher exposure levels and in lung tissue of
rats and mice at various times (most pronounced at 7 mg/m3). These
changes probably indicate an increase in lung tissue due to increased
inflammatory cells and epithelial cell proliferation.
In the same study, Mauderly et al. (1988) described focal
proliferative, fibrotic, and emphysematous changes in the lungs of
animals at the higher doses. Measurements in vivo revealed a
reduction in lung volume and compliance.
In a follow-up study, Mauderly et al. (1990) exposed male Fischer
344 rats that were either normal or had pre-existing pulmonary
emphysema (elastase-treated) to diesel soot at 3.5 mg/m3 for 7 h/day
on five days per week for 24 months, to determine whether
emphysematous rats were more susceptible to diesel exhaust. No
increase in susceptibility was seen. The emphysematous lungs had a
lower accumulation of particles than the normal lungs, which prevented
an overproportional response of the compromised animals.
Male and female Fischer 344 rats exposed to diesel exhaust
particles at 0.7, 2.2, or 6.6 mg/m3 for 16 h/day on five days per
week for two years or to filtered diesel exhaust showed an
exposure-related reduction in the rate of weight gain throughout the
study at the highest dose and an increase in absolute lung weight at
the two higher doses (Brightwell et al., 1986, 1989).
Groups of 72 male and 72 female Fischer 344 rats exposed to
diesel exhaust particles at 2 mg/m3 for 7 h/day on five days per
week for 24 months showed alveolar type II cell hyperplasia,
inhibition of long-term clearance of particles and pulmonary
lipidosis. No immunological abnormalities, no induction of pulmonary
or hepatic xenobiotic metabolizing enzymes, and no effects on
mortality, body weight gain, organ:body weight ratios or clinical
chemical parameters were seen (Lewis et al., 1986, 1989). In the same
study, a dose-related depression in phagocytic activity was seen in
alveolar macrophages (Castranova et al., 1985), and a trend to
increased pulmonary arterial wall thickness was observed in comparison
with controls (Vallyathan et al., 1986).
In groups of 15 male monkeys, exposure to diesel exhaust (as
described above) resulted in mild obstructive airway disease, but
there was no evidence of restrictive lung disease. The effects were
observed throughout the exposure period, after 6, 12, 18, and 24
months (Lewis et al., 1989).
Reduced body weight, significantly increased lung:body weight
ratios, and type II cell proliferation were seen in female Fischer 344
rats exposed to whole-fraction diesel exhaust at 5 mg/m3 for 8 h/day
on seven days per week for 24 months (Iwai et al., 1986).
Groups of 96 male and 96 female Syrian golden hamsters, female
NMRI mice, and female Wistar rats were exposed to diesel engine
emissions at 4.4 mg/m3 for 19 h/day on five days per week for
120-140 weeks (Heinrich et al., 1986a; Stöber, 1986). The body weights
of mice were significantly reduced, by about 12%. Lung weights were
increased after two years of exposure, by two- to threefold in rats
and mice and by 50-70% in hamsters. Different levels of histological
response were seen in the three species. Hamsters had thickened
alveolar septa and bronchioalveolar hyperplasia. Of the mice, 64% had
bronchioalveolar hyperplasia (controls, 5%), 71% had multifocal
alveolar lipoproteinosis, and 43% had multifocal interstitial fibrosis
(controls, 4%). The nose, larynx, and trachea were not affected.
Treated rats showed severe inflammatory changes in the lungs,
thickened septa, foci of macrophages, and hyperplastic and metaplastic
lesions, but no significant changes in respiratory rate, minute
volume, compliance, or resistance were seen in 14 exposed rats.
Significantly increased airway resistance and a significant decrease
in dynamic lung compliance were observed in rats after two years of
exposure. The hamsters had a significant increase in airway resistance
and a nonsignificant reduction in lung compliance at termination of a
one-year exposure; these changes persisted during the second year of
exposure. In the same experiments, filtered diesel exhaust did not
have the same effects as total exhaust in any of the three species
(Heinrich et al., 1986a).
Groups of 27 female Fischer 344 rats and about 50 male and 50
female C57Bl/6N and ICR mice were exposed to particle concentrations
of 2-4 mg/m3 for 4 h/day on four days per week for up to 24 months.
After 12 months, the incidence of alveolar hyperplasia was increased
in all animals. Exposed rats had more severe inflammation than
controls, with inflammatory exudate into the nose, and bronchitis and
pneumonia, but this effect was ascribed to the housing, which was not
specific pathogen-free (Takemoto et al., 1986).
Groups of nine male Hartley guinea-pigs were exposed at
concentrations of 0.25, 0.75, or 1.5 mg/m3 for 20 h/day on 5.5 days
per week for up to two years. An analysis of the alveolar capillary
membrane by quantitative morphometry showed that it was thickened as a
result of an increase in the absolute tissue volume of interstitium
and type II cells. Exposure to 0.75 mg/m3 for six months caused
fibrosis in regions of macrophage clusters and focal type II cell
proliferation, as observed under the light microscope. No appreciable
change in morphometric parameters was seen after exposure to the
lowest dose, but the higher doses increased the thickness of alveolar
septa and the numbers of various types of alveolar cells (Barnhart et
al., 1981, 1982).
As part of this investigation, Fischer 344 rats were exposed by
inhalation to 0.25 or 1.5 mg/m3 diesel exhaust particles, and
various biochemical and cytological studies were conducted up to 36
weeks (Misiorowski et al., 1980). At the high dose, normalized lung
weight, the rate of collagen synthesis, and the cellular and lipid
content of lung tissue were significantly increased after six months.
It was concluded that the reactivity of fibrogenic cells had been
enhanced. In a long-term study of respiratory end-points in male
guinea-pigs, including light and electron microscopy, lavage cytology,
and lung tissue biochemistry, the lowest-observable-adverse-effect
level (LOAEL) was 0.796 and the no-observable-adverse-effect level
(NOAEL) was 0.258 mg/m3 (US Environmental Protection Agency, 1992b).
Groups of 120 male Fischer 344 rats, 450 male A/J mice, and 120
male Syrian hamsters were exposed to 0.25, 0.75, or 1.5 mg/m3 diesel
exhaust particles for 20 h/day on seven days per week for 15 months.
Mortality was not increased in any species. A dose-related deposition
of pigment was observed histopathologically in alveolar macrophages
and regional lymph nodes. Accumulation of alveolar macrophages was
associated with an increase in connective tissue in alveolar walls,
with mild fibrosis and proliferation of type II pneumocytes; these
effects were termed pneumoconiosis. No treatment-related responses
were found in organs other than the respiratory tract. The LOAEL was
0.735 and the NOAEL, 0.242 mg/m3 (Kaplan et al., 1983).
Groups of 120 male and 95 female Fischer 344/Jcl rats were
exposed to light-duty diesel engine exhaust particulates at 0, 0.1,
0.4, 1.1, or 2.3 mg/m3 or heavy-duty diesel engine exhaust
particulates at 0, 0.5, 1.0, 1.8, or 3.7 mg/m3 for 16 h/day on six
days per week for up to 30 months (Ishinishi et al., 1986, 1988;
Suzuki et al., 1990). The body weights of females exposed to the
highest dose of heavy-duty engine exhaust were decreased throughout
the study by 15-20% in comparison with controls. No changes were
observed histopathologically in the lungs at any concentration.
Accumulation of particle-laden macrophages was observed at levels
> 0.4 mg/m3. In areas of macrophage accumulation, bronchiolar
epithelium replaced alveolar epithelium in the ducts. Proliferation of
bronchiolar epithelium and type II cells and oedematous thickening and
fibrosis of the alveolar septum were observed, which developed into
small fibrotic lesions classified as hyperplastic. The incidences of
hyperplastic lesions were 4, 4, 6, 12, and 87 per 124 rats exposed to
light-duty engine exhaust and 1, 3, 7, 14, and 25 per 124 rats in
those exposed to heavy-duty engine exhaust. The LOAELs were
1.2 mg/m3 for light-duty and 1.0 mg/m3 for heavy-duty exhaust, and
the NOAELs were 0.4 and 0.5 mg/m3, respectively (US Environmental
Protection Agency, 1992b).
In the same experimental series, Kato et al. (1992) studied
non-neoplastic lesions in the respiratory organs of the rats every six
months. The marked changes consisted of intake of diesel particles by
type I epithelium, hypertrophy, and glandular proliferation of type II
epithelium, with many extended microvilli and increased numbers and
size of lamellar inclusion bodies, focal increase of collagen fibres
in the interstitium, and infiltration of particle-laden macrophages,
neutrophils, mast cells, and plasma cells into the interstitium of
alveolar septa. The most marked changes in the airway were focal
shortening of cilia and protrusions of nonciliated cells. These
morphological changes appeared in all groups exposed to particle
concentrations > 1 mg/m3 after six months of exposure and were more
marked with increasing particle concentration and with time.
Exposure of Syrian golden hamsters to 4 or 11 mg/m3 diesel
exhaust for 7 h/day on five days per week for five months resulted in
enlargement of liver sinusoids and loss of cristae in mitochondria
(Meiss et al., 1981).
The induction of pulmonary adenomas and carcinomas in rats after
long-term inhalation of carbon black is proposed to be due partly to
inflammatory cells and their mediators (Driscoll et al., 1995;
Oberdörster et al., 1995; Driscoll et al., in press). After exposure
of rats to carbon black at 1, 7, or 50 mg/m3 for 6 h/day on five
days per week for 13 weeks, 353, 1923, and 7112 µg of carbon,
respectively, were deposited per lung. After exposure to air or to
1 mg/m3 of carbon black, no macrophage inflammatory protein-2
(MIP-2) mRNA expression and no increase in inflammatory cells was
observed; with 7 or 50mg/m3, both MIP-2 mRNA and the number of
neutrophils in bronchoalveolar lavage fluid were increased, and the
hprt frequencies were 3.2 and 4.2 times greater than in controls.
Groups of 25 cats were exposed to either clean air or diesel
exhaust for 8 h/day on seven days per week for two years at a
concentration of 6 mg/m3 through week 62 and to 12 mg/m3 for weeks
62-124. Some of the cats were then held in clean air for an additional
six months. No response was seen after 61 weeks, but after 123 weeks
signs of restrictive lung disease were observed. Total lung capacity,
forced vital capacity, functional residual capacity, peak expiratory
flow rates, and diffusion capacity were significantly decreased
(Moorman et al., 1985). Morphological evaluation revealed
exposure-related lesions in the centriacinar region of the lungs,
bronchiolar epithelial metaplasia associated with the presence of
ciliated and basal cells, and alveolar macrophages containing diesel
particle-like inclusions. After six months' recovery in clean air, the
metaplasia had disappeared but the fibrosis had progressed (Hyde et
al., 1985). In exposed cats, the epithelium of the terminal and
respiratory bronchioles consisted of ciliated, basal, and Clara cells,
whereas controls had only Clara cells (Plopper et al., 1983). These
observations indicate that the foci of the lesions were the
epithelium, the interstitial compartments, and the centriacinar region
of the lung. The metaplastic changes suggest that cancer may be
induced by continuous exposure, while the other major effect is
pulmonary fibrosis resulting in restrictive disease which is not
ameliorated and in fact progresses after removal from exposure.
Progression of effects is probably due to continued lung overloading
with particulate matter, despite cessation of exposure.
The dose-dependent effects on lung morphology, histopathology,
biochemistry, and cytology after long-term exposure to diesel exhaust
described above are reflected in the following findings seen in rats
at a lung burden of about 0.5 mg/lung (Henderson et al., 1988; Muhle
et al., 1990):
-- The wet and dry weights of lungs increase by up to fourfold in
comparison with those of concurrent controls. This is due to
severe functional overloading in the lung, resulting in enhanced
leukocyte influx and tissue proliferation.
-- Pulmonary inflammation is manifested by cytological (increased
numbers of polymorphonuclear neutrophils) and biochemical (lactic
dehydrogenase, collagen in lung lavage fluid) parameters.
-- The retention of material is characterized by an increasing
number of particle-laden macrophages and by black discolouration
of lungs due to sequestration of accumulated particles.
Additionally, increased transfer of material to the
lung-associated lymph nodes is observed.
-- Later findings during inhalation are proliferative alterations of
the epithelial cells and onset of fibrosis.
B7.3.2 Carcinogenicity
B7.3.2.1 Inhalation
Rats were exposed in several studies to diesel emissions
containing > 2 mg/m3 of particles for more than two years,
resulting in the induction of pulmonary tumours (Table 34). Many
organic and inorganic gaseous components were also present in the test
atmospheres, and the relatively high concentration of some pulmonary
irritants like nitrogen dioxide, aldehydes, and sulfur dioxide may
have contributed to the inflammatory response and to epithelial cell
damage and subsequent cell proliferation. Exposure to gaseous PAHs,
like naphthalene which is toxic to Clara cells, may influence the
metabolic state of pulmonary cells. It is not clear however, whether
the combined exposure to particles and gases in these experiments
favoured tumour induction. It should be noted that Brightwell et al.
(1986), Heinrich et al. (1986a), Iwai et al. (1986), Ishinishi et al.
(1986), and Takaki et al. (1989) have reported that the gaseous phase,
i.e. the proportion of exhaust without particles, does not induce
pulmonary tumours.
Table 34. Carcinogenicity of diesel engine exhaust in rats
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 40 m 8.3 1.5 6 h/d, 5 d/week, up NR 1 17c NR Karagianes
40 m Clean air to 20 months 0 et al.
(20 months) (1981)
Fischer 72 f 6.6 3.1 16 h/d, 5 d/week, 24 NR 39 54 38.5 < 0.1 Brightwell
344 71 m months (30 months) 16 23 et al.
72 f 2.2 1.1 11 15 9.7 < 0.1 (1986, 1989)
72 m 3 4
71 f 0.7 0.4 0 0.7
72 m 1 1
Filtered exhaust 0
0
142 f Clean air 1 1 1.4
140 m 3 2
Wistar 95 f 4.4 2.5 19 h/d, 5 d/week, 32 NR 17 18 < 0.1 Heinrich et
92 f Filtered exhaust months (up to death) 0 al. (1986a)
96 f Clean air 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer
344/Jcl
Light-duty 60 f 2 1.1 16 h/d, 6 d/week, 30 NR 1 2 2.4 NR Ishinishi
engine 64 m months (30 months) 2 3 et al.
59 f 1 0.6 2 3 4.1 (1986,1988;
64 m 3 5 Takaki et al.
61 f 0.4 0.2 0 0.8 (1989)
64 m 1 2
59 f 0.1 0.06 2 3 1.6
64 m 0
59 f Clean air 2 3 3.3
64 m 2 3
Heavy-duty 60 f 4 2.3 3 5 6.5 1.8
engine 64 m 5 8
59 f 2 1.1 1 2 3.3
64 m 3 5
61 f 1 0.6 0 0
64 m 0
59 f 0.4 0.2 1 2 0.8
64 m 0
59 f Clean air 1 2 0.8
64 m 0
16 m Filtered exhaust 0
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Fischer 19 f 4.9 1.6 8 h/d, 7 d/week, 24 NR 8 42 < 1d Iwai et al.
344 16 f Filtered exhaust months (24 or 30 0 (1986)
22 f Clean air months) 1 5
Fischer 15 f 2.0-4.0 0.2-0.4 4 h/d, 4 d/week, 24 NR 0 NR Takemoto
344 15 f Clean air months (24 months) 0 et al.
(1986)
Fischer 69 f 7 1.5 7 h/d, 5 d/week, 30 NR 13 19 16.1 < 0.1 (f) Mauderly et
344/Crl 74 m months (up to death) 10 14 1.7 (m) al. (1986)
68 f 3.5 0.7 2 3 4.6
63 m 4 6
68 f 0.35 0.07 0 0.7
70 m 1 1
68 f Clean air 0 1.4
73 m 2 3
Fischer 227 f+m 7 1.5 7 h/d, 5 d/week, 30 20.8 12.8 < 0.1 Mauderly et
344/N Crl 221 f+m 3.5 0.7 months (up to death) 11.5 3.6 4.8 al. (1987)
223 f+m 0.35 0.07 0.6 1.3
230 f+m Clean air (24 months) 0.9
Table 34 (contd)
Strain No. and Exposure concentration Duration Lung Pulmonary tumours Probability Reference
sex (mg/m3)a (observation burden of error (%)b
period) (mg/lung) No. % Mean
Actual Continuous (f+m) (%)
Wistar 100 f 7.0 3.8 18 h/d, 5 d/week, 24 63.9 22 22.0 < 0.1 Heinrich et
200 f 2.5 1.3 months (30 months) 23.7 11 5.5 < 1 al. (1992,
198 f 0.8 0.5 6.3 0 1995);
217 f Clean air (24 months) NR 1 0.5 Heinrich
(1994)
Fischer 212 f+m 6.3 3.0 16 h/d, 5 d/week, 24 85.4 38 17.9 17.9 NR Nikula et al.
344/N 210 f+m 2.4 1.1 months (24 months) 40.7 13 6.2 6.2 (1994)
214 f+m Clean air (23 months) 3 1.4 1.4
Fischer 49 f 4.7 1.3 15 h/d, 3 d/week, 24, NR 6 12.2 NR Kawabata
344/N 42 f 4.7 1.3 12, or 6 months 8 19.0 et al.
45 f 4.7 1.3 1 2.2 (1993)
48 f Clean air 5 10.4
Fischer 183 f+m 2.0 0.4 7 h/d, 5 d/week, 24 NR 8 4.4 4.4 NR Lewis et al.
344/N 180 f+m Clean air months 6 3.3 3.3 (1989)
From Pott & Heinrich (1987) and supplemented; NR, not reported
a Mass median aerodynamic diameter, 0.17-0.25 µm; 'Continuous', equivalent continuous value
b Unilateral Fisher's exact test
c Only six animals investigated
d With Yates' correction
No difference in lung tumour incidence was seen in groups of male
and female rats exposed for 7 h/day on five days per week for 24
months either to diesel exhaust particulates at 2 mg/m3 or to
filtered air (Lewis et al., 1989).
Exposure of six male Wistar rats to diesel exhaust soot at
8.3 mg/m3 and coal dust for up to 20 months gave inconclusive
results (Karagianes et al., 1981). The study had little statistical
power owing to the small group size, and the insufficient duration of
exposure may have prevented detection of tumours, although one mammary
fibroadenoma and one lung bronchiolar adenoma were detected. One
subcutal fibrosarcoma and one renal lymphoma were seen in controls.
Eight bronchioalveolar adenomas and nine squamous-cell tumours
were seen in 95 rats (18%) that had inhaled 4.4 mg/m3 diesel exhaust
for 19 h/day on five days per week for 32 months. No tumours were
observed in controls (Heinrich et al., 1986a). The dose-dependent
carcinogenic potency of diesel exhaust was confirmed in a study in
which rats were exposed to diesel exhaust at concentrations of 0.8,
2.5, or 7.0 mg/m3 for 18 h/day on five days per week for 24 months
(Heinrich et al., 1992; Heinrich, 1994; Heinrich et al., 1995).
In male and female rats exposed to diesel exhaust at 6.6 mg/m3,
a high incidence of lung tumours was observed in animals dying or
sacrificed after 24 months (24/25 females, 12/27 males). The incidence
of lung tumours (Table 34) was derived by pooling pulmonary adenomas;
squamous-cell carcinomas; adenocarcinomas; mixed ademomas,
adenocarcinomas, and squamous-cell carcinomas; and mesotheliomas.
Non-neoplastic pulmonary lesions were not described (Brightwell et
al., 1986, 1989).
Groups of 24 female Fischer 344 rats were exposed to clean air,
to whole-fraction (4.9 mg/m3 particulates) fuel, or to filtered
diesel exhaust for 8 h/day on seven days per week for 24 months. After
six months of exposure, the rats exposed to whole exhaust had lower
body weights than those in the other two groups; after 18 months, both
exposed groups showed body weight reduction. The relative lung weight
was increased after 12 months' exposure to whole exhaust, and
epithelial proliferation and significantly elevated numbers of lung
tumours were seen in animals exposed to the whole fraction after six
months. Increased incidences of leukaemia and mammary gland tumours
were also reported in animals exposed to whole or filtered exhaust;
however, because of the small numbers of animals, the significance of
the results cannot be determined (Iwai et al., 1986).
A total of 140 female and 140 male Fischer 344/N rats (in groups
of three males and three females, five males and five females, or
eight males and eight females, depending on the end-point) were
exposed for 16 h/day on five days per week for up to 24 months,
beginning at eight weeks of age, to particles of diesel exhaust or
carbon black at 2.5 or 6.5 mg/m3 of air, or to clean air (Mauderly
et al., 1994). Rats were killed after 3, 6, 12, 18, or 23 months of
exposure for histopathological assessment and measurement of lung and
lung-associated lymph node burdens of particles, lung weight,
bronchoalveolar lavage indicators of inflammation, DNA adducts in
whole lung and alveolar type II cells, and chromosomal anomalies in
circulating lymphocytes. Diesel exhaust and carbon black had nearly
identical effects, with a similar relation to exposure. There was a
dose-related slowing of particle clearance after three months, with
progressive accumulation of particles in lungs and lymph nodes.
Inflammation, epithelial proliferation, and fibrosis were progressive
and related to dose. Diesel exhaust slightly increased the number of
lung DNA adducts, and the numbers of DNA adducts in type II cells were
increased by both diesel exhaust and carbon black. No exposure-related
chromosomal damage was found in circulating lymphocytes. The incidence
of primary lung neoplasms was increased significantly and was related
to the doses of both diesel exhaust and carbon black. The types of
neoplasms were identical and included benign adenomas, malignant
adenocarcinomas, squamous-cell carcinomas, and adenosquamous
carcinomas; squamous cysts were also seen. The neoplasms had similar
growth characteristics when transplanted; 50-67% of the transplanted
squamous-cell carcinomas and 25-40% of the transplanted
adenocarcinomas grew after injection into athymic mice (Table 35),
whereas the squamous cysts did not.
Fischer 344/Jcl rats were exposed to a range of concentrations of
exhaust emission particulates from light-duty (up to 2 mg/m3) or
heavy-duty diesel engines (up to 4 mg/m3) for 16 h/day on six days
per week for up to 30 months. After 18 months, marked hyperplasia of
type II cells was noted in the groups at higher exposures. After 30
months, treatment-related pulmonary hyperplastic lesions were
detected, and a dose-response relationship was seen for tumours in the
groups exposed to heavy-duty but not light-duty diesel exhaust. The
lung tumours were diagnosed as adenocarcinomas, squamous-cell
carcinomas, or adenosquamous carcinomas. A significant correlation was
observed between the incidence of anthracosis and areas with
hyperplastic lesions; furthermore, anthracosis and hyperplastic
lesions did not appear in lungs exposed to particle-free exhaust. The
authors concluded that the occurrence of hyperplastic lesions was
dependent on the presence of carbon particles (Ishinishi et al., 1986,
1988; Takaki et al., 1989).
Table 35. Growth in athymic mice of lung tumours from rats exposed to
diesel exhaust and carbon black
Type of tumour Carbon black Diesel exhaust
Number Growing Number Growing
implanted No. % implanted No. %
Adenocarcinom 8 2 25 10 4 40
Squamous-cell 2 1 50 3 2 67
carcinoma
Squamous cyst 19 0 0 7 0 0
From Mauderly et al. (1994)
In a large-scale study of unfiltered diesel exhaust, 1097 Fischer
344 rats were exposed for up to 30 months to 0.35, 3.5, or 7.0 mg/m3
of diesel soot. A group of 365 rats exposed to clean air served as
controls. Exposure to exhaust did not cause overt signs of toxicity,
and no significant, exposure-related alterations in body weight were
observed. Rats were killed at six-month intervals, examined for their
lung burdens of diesel soot, and subjected to complete necropsy.
Diesel soot accumulated at all doses but minimally in the low-dose
group. An increased incidence of tumours was observed in the groups at
the medium and high doses; most (81%) of the tumours were observed
after two years of exposure. The inhaled soot concentration and the
lung burden of diesel soot were highly significantly related to tumour
incidence ( P < 0.001) (Mauderly et al., 1987). In a follow-up study
with longer daily exposure (16 h/day), the dose-dependency of the
tumour incidence was reproduced (Nikula et al., 1994). In this study,
the carcinogenicity of diesel exhaust soot (8% extractable organic
material; 2.5 mg/m3) was compared with that of carbon black (0.12%
extractable organic material; 6.5 mg/m3 ) in 1152 male and female
Fischer 344 rats exposed for 16 h/day on five days per week for up to
24 months and examined histopathologically. The lung burden of
particles was greater after exposure to diesel exhaust than to carbon
black, but the neoplastic response in the lung was similar and usually
occurred late in the study. When the lung burden of particles was used
as the dose parameter, the carcinogenicity of carbon black was
greater than that of diesel exhaust soot.
Groups of 50 four-week-old female Fischer 344 rats were exposed
to diesel soot at a mean concentration of 4.73 mg/m3 for 15 h/day
three times per week for 6, 12, or 24 months, and animals that died
after 18-30 months were evaluated for tumours. After 30 months, all
animals, including controls, were sacrificed and examined for lung
tumours. The incidences of lung tumours were 10.4% in controls and
2.2% after six months' exposure to diesel exhaust, 19.0% after 12
months, and 12.2% after 24 months. No statistical analysis was
presented, but it seems unlikely that there was a significant effect
(Kawabata et al., 1993).
Concurrent administration of known carcinogens with diesel
exhaust has also been studied. Exposure of rats to diesel exhaust
given an intraperitoneal injection of N-nitrosodiisopropylamine or
N-nitrosodiethylamine did not influence the rates of tumours induced
by the nitrosamines (Takemoto et al., 1986). The effects of treatment
of hamsters with N-nitrosodiethylamine or benzo[ a]pyrene were not
influenced by subsequent administration of diesel exhaust, whereas
pretreatment of mice with benzo[ a]pyrene or dibenzanthracene followed
by diesel exhaust gave inconsistent results. Exposure to diesel
exhaust did not affect the total tumour rates in rats pretreated with
6.25 or 12.5 g/kg body weight of N-nitrosodipentylamine; however,
the incidence of squamous-cell tumours was increased in both
pretreated groups (Heinrich et al., 1986a).
Hamsters were exposed for two years to unfiltered diesel exhaust
at 0, 0.7, 2.2, or 6.6 mg/m3 or to filtered diesel exhaust, and
groups of control and treated hamsters were administered
N-nitrosodiethylamine subcutaneously before the beginning of
exposure. The rate of pulmonary tumours was not increased by diesel
exhaust, with or without pretreatment with the nitrosamine. Several
hamsters that developed 'wet tail' infection after six months on test,
resulting in significant mortality (46%), were treated with
oxytetracycline, chloramphenicol, and dimetridazole (Brightwell et
al., 1986, 1989).
Overall, the only clear effect in these studies of
co-carcinogenicity in rats was an increase in the percentage of
malignant tumours in animals treated with diesel exhaust plus
carcinogens in comparison with the carcinogen alone (Heinrich et al.,
1986a).
The results of studies in other species (mice, hamsters, and
monkeys) treated by inhalation are given in Table 36. Heinrich et al.
(1982) found significant increases in lung tumour incidence in mice,
but this result could not be repeated in later studies (Heinrich et
al., 1986a, 1992, 1995). Small but statistically significant increases
in lung tumour incidence were reported in ICR and C57Bl mice (Takemoto
et al., 1986) and in female Sencar mice (Pepelko & Peirano, 1983);
however, because only small increases in non-malignant tumours were
seen only in females in the study of Pepelko & Peirano (1983), the
results must be considered to be weakly positive or equivocal.
Hamsters showed no response to diesel exhaust.
Table 36. Long-term studies of diesel exhaust by inhalation in species other than rats
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI mouse 2 × 96 f (a) Exhaust 4 19 h/d, 5 d/week, Lung tumours (adenomas and Heinrich et al.
(b) Filtered 27-28 months adenocarcinomas) (a) 24/76 (32%), (1986a)
(c) Clean air including 13 (17%) adenocarcinomas;
(b) 29/93 (31%), including 18 (19%)
adenocarcinomas; (c) 11/84 (13%),
including 2 (2.4%) adenocarcinomas
Incidence in historical controls,
< 32%
ICR and 315 ICR, Exhaust or 2.0-4.0 4 h/d, 4 d/week, Lung tumours (adenomas and Takemoto et al.
C57Bl/6N 297 C57Bl/6N clean air 13-28 months adenocarcinomas). ICR: 14/56; (1986)
mice, newborn (sex 7/60 in controls; C57Bl/6N:
unspecified) 17/150; 1/51 in controls
Sencar mouse 2 × 130 f (a) Exhaust 12 8 h/d, 7 d/week, Adenomas and carcinomas Pepelko &
2 × 130 m (b) Clean air to 15 months of (a) m: 5.9%; f: 16.3% Peirano (1983)
age (from (b) m: 3.8%; f: 7.2%
conception to
sacrifice)
NMRI mouse 2 × 80 f (a) Exhaust 7.5 18 h/d, 5 d/week, Lung tumours Heinrich et al.
(b) Clean air 13.5 months, 9.5 (a) 32.1% (1992, 1995)
months' recovery (b) 30%
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
NMRI and 3 × 120 f (a) Exhaust 4.5 18 h/d, 5 d/week; Lung tumours Heinrich et al.
C57Bl/6N mice 3 × 120 f (b) Filtered NMRI, 23 months; NMRI: (a) 23.3% (1992, 1995)
(c) Clean air C57Bl, 24 months (b) 46.7%
+ 6 months' (c) 30%
recovery C57Bl: (a) 8.5%
(b) 3.6%
(c) 5.1%
Syrian golden 3 × 48 f (a) Exhaust 3.9 ± 0.5 7-8 h/d, 5 d/week, No effect on survival, no lung Heinrich et al.
hamster (b) Filtered up to 30 months tumours (1982)
(c) Clean air
Syrian golden 3 × 48 f (a) Exhaust 4 19 h/d, 5 d/week, Normal life span, no lung tumours Heinrich et al.
hamster 3 × 48 m (b) Filtered 27-28 months (1986a)
(c) Clean air
Syrian golden 52 f + 52 m (a) Exhaust 0.7, 2.2, 16 h/d, 5 d/week, No significant increase in Brightwell
hamster 104 f + 104 m (b) Filtered 6.6 24 months frequency of tumours in et al.
(controls) (c) Clean air respiratory tract (1986, 1989)
Cat 2 × 25 m (a) Exhaust 6 (1st 8 h/d, 7 d/week, Lung function: decrease in closing Pepelko &
yr), 12 24 months volume after 1 year; reduction Peraino (1983)
(2nd yr) in inspiratory, vital, and total
(b) Clean air lung capacity after 2 years;
diagnosis: pulmonary fibrosis of
the interstitial or
intra-alveolar type
Table 36 (contd)
Strain, species No. and sex Exposure Results Reference
Material Particles Duration
(mg/m3)
Cynomolgus 4 × 15 m (a) Coal dust 2.0 7 h/d, 5 d/week, No difference in tumour incidence Lewis et al.
monkey (b) Exhaust 2.0 24 months among groups (1986)
(c) Coal dust 1.0
+ exhaust 1.0
(d) Clean air
It is clear that repeated inhalation of diesel exhaust at
concentrations of more than about 2 mg/m3 (actual value),
corresponding to a calculated equivalent continuous concentration of
about 1 mg/m3, increases the incidence of pulmonary tumours. These
tumours occur late in life after exposures of 24 months.
B7.3.2.2 Other routes of exposure
Studies have also been conducted by intratracheal instillation in
rats and hamsters and by painting on mouse skin (Table 37). While the
former are especially useful for hazard identification and for
evaluating specific mechanistic aspects, they are less useful for the
purposes of risk characterization and no attempt was made to identify
critical studies. The results of the studies by intratracheal
instillation, however, confirm that both diesel exhaust particles and
carbon black induce lung tumours and demonstrate that the specific
surface area of carbonaceous particles is correlated with the
tumorigenic potency. Both of these findings support the suggestion
that a nonspecific particle effect is of crucial importance for the
induction of lung tumours by diesel exhaust. The dermal experiments,
conducted by Nesnow et al. (1982a,b, 1983), have been used to estimate
risk quantitatively, by the comparative potency method.
B7.4 Dermal and ocular irritation; dermal sensitization
No data were available.
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
B7.5.1 Reproductive toxicity
In a two-generation study of reproduction, 100 male and 100
female CD-1 mice were exposed by inhalation to exhaust from a
light-duty diesel engine for 8 h/day on seven days per week, at a
concentration of 12 mg/m3. Most treatment-related effects were
minimal. Overall fertility and survival rates were not significantly
altered (Pepelko & Peirano, 1983).
(C57Bl/6 × C3H)F mice (number not given) showed sperm
abnormalities (reduced sperm count and weight of testis;
teratospermia) after daily intraperitoneal injections of 50, 100, or
200 mg/kg body weight of diesel exhaust particles for five days. The
highest dose caused an eightfold increase in abnormalities over that
in controls and a significant decrease in sperm number (Quinto & De
Marinis, 1984).
Table 37. Carcinogenicity of diesel exhaust after exposure other than by inhalation
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Fischer 344 (a) 31 f Intratracheal (a) Activated carbon 1/animal 10/week, Survival rate, 71-83% Kawabata
rat (b) 59 f instillation (b) Diesel particles 1/animal 30 months' (lowest ingroup b). Malignant et al.
(c) 53 f (c) None observation lung tumours: (a) 7/23; (1986)
(d) 27 f (d) Vehicle (b) 20/42; (c) 0/44; (d) 1/23;
P < 0.01. Benign and
malignant tumours combined:
(a) 11/23; (b) 31/42
Osborne- Groups Lung Organic material from Observation (a) 1 bronchioalveolar adenoma; Grimmer
Mendel rat of 35 f implant diesel exhaust: until (b) 5 squamous-cell carcinomas; et al.
(a) Hydrophilic fraction 6.7 spontaneous (c) 1 bronchioalveolar adenoma; (1987)
(b) Hydrophobic fraction 20.0 death (d) 6 carcinomas;
(c) Nonaromatic compounds 19.2 (e) No tumours
+ PAHs (2 or (f) 1 carcinoma
3 rings) (g) 7 carcinomas, 1 adenoma
(d) PAHs ( > 4 rings) 0.2 (h) No tumours
(e) Polar PAHs 0.3 (i) 1 adenoma
(f) Nitro-PAHs 0.2
(g) Reconstituted
hydrophobic fraction 19.9
(h) None
(i) Vehicle
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Wistar rat (a) 40 f Intratracheal (a) Diesel soot (34 m2/g) (a) 3 × 15 Primary lung tumours (%) Pott &
(b) 58 f instillation (b) Diesel soot (70 m2/g) (b) 3 × 10 (a) 65 Roller
(c) 38 f (c) Diesel soot (70 m2/g) (c) 3 × 20 (b) 60 (1994);
(d) 37 f (d) Carbon black (270 (d) 3 × 15 (c) 66 Pott et
m2/g) (d) 65 al.(1994)
(e) 37 f (e) Activated charcoal (e) 3 × 10
(f) 39 f (860 m2/g) 3 × 20 (e) 27
(g) 40 f (f) NaCl solution (f) 3 × 10 (f) 0
(g) NaCl solution (g) 0.4 × 20 until (g) 0
ml spontaneous
death or 131
weeks
Wistar rat Groups Intratracheal (a) Diesel soot (native) (a) 1 × 15 Primary lung tumours (%): Heinrich
of 48 f instillation (b) Diesel soot (toluene (b) 2 × 15 (a) 17 (1994)
extract; 130 m2/g) 1 × 15
(c) Carbon black (c) 1 × 15 (b) 23
(toluene extract; (d) 1 × 15 4
270 m2/g; primary (e) Vehicle control (c) 21
particle, 15 nm)
(d) Carbon black (toluene (d) 8
extract; 270 m2/g; (e) 0
primary particle,
15 nm)
(e) NaCl solution
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Syrian Groups Intratracheal (a) Diesel particles 1.25, 1/week, 15 1 lung adenoma at high dose Shefner
golden of 50 m; instillation (b) Diesel particles + 2.5, in groups (a) and (c) after 61 et al.
hamster various same amounts of weeks weeks; no lung tumours in (1982
controls ferric oxide controls
(c) Diesel particle
extract
+ ferric oxide
Syrian (a) 3 × Intratracheal (a) Exhaust extract 0.1, 0.5, 1/week, 15 Survival rates: (a) 95, 92, Kunitake
golden 62 m instillation (b) Vehicle 1 weeks 71%; (b) 98%. No difference in et al.
hamster (b) 59 m (c) 0.5 mg tumour incidence between (a) (1986)
(c) 62 m benzo[a]pyrene and (b); 88% respiratory
tumours in positive controls
C57Bl 12 m, Dermal Acetone extract of 0.5 ml 3 times/week 16 mice dead by 10 weeks, 33 Kotin
mouse 40 f, particles for life or skin tumours by 13 months, 2 et al.
69 22-23 months skin papillomas, no tumours in (1955)
controls controls
A mouse 50 m, Dermal Acetone extract of 0.5 ml 3 times/week Males: 8 skin tumours by 16
25 f, particles for life or 1 papilloma, 3 squamous-cell
34 22-23 months carcinomas. Females: 20 skin
controls tumours by 13 months,17 tumours
at 13-17 months, no tumours in
controls
Table 37 (contd)
Strain, No. and Exposure Schedule, Results Reference
species sex duration
Route Material Dose
(mg)
Sencar Groups Dermal Dichloromethane 0.1, 0.5, 1/week, 50-52 Skin carcinomas: Nesnow
mouse of 40 m extracts of particles 1.0, 2.0, weeks Engine A: 3% (m), 5% (f) at et al.
+ 40 f from engines (A, B, E) or 4.0 4 mg Engine B: 3% (m) at 0.5 mg (1982a,b,
Benzo[a]pyrene 12.6- Engine E: 3% (f) at 0.1 mg 1983)
202 µg Positive control: 10-90%
C57Bl/6N Groups Subcutaneous Particles in olive oil 10, 25, 1/week, 5 First tumours palpated in: Kunitake
mouse of injection containing 5% dimethyl 50, 100, weeks; 18 week 47 at 25 mg/kg bw et al.
15-30 f sulfoxide 200, or months' week 30 at 50 mg/kg bw (1986)
500 mg/kg observvation week 27 at 100 mg/kg bw
bw week 39 at 200 or 500 mg/kg bw
Malignant fibrous histiocytomas
in 5/22 mice at 500 mg/kg bw;
0/38 in controls
PAH, polycyclic aromatic hydrocarbon; TPA, 12-O-tetradecanoylphorbol 13-acetate
Groups of 15 male cynomolgus monkeys were exposed by inhalation
to 2 mg/m3 diesel particulates for 7 h/day on five days per week for
two years. Sperm motility and velocity were similar to those of
controls (Lewis et al., 1989).
In a test for dominant lethal mutation, male Fischer 344 rats
inhaled 2 mg/m3 diesel exhaust for 7 h/day on five days per week for
six months and were subsequently mated with untreated females. Live
and dead implants and preimplantation losses were analysed on days
19-20 of gestation: no significant effects were observed (Lewis et
al., 1989). In a similar test, 100 male and 54 female T stock mice
were exposed to 6 mg/m3 diesel exhaust for 8 h/day on seven days per
week for seven weeks. There were no significant dominant lethal
effects in males or females. A reduced number of corpora lutea
(reproductive function) was the only significant result (Pepelko &
Peirano, 1983).
B7.5.2 Embryotoxicity
No increase in the frequency of sister chromatid exchange was
seen in the livers of fetuses of Syrian golden hamsters that inhaled
diesel exhaust particles at 12 mg/m3 for 8 h/day on days 5-13 of
gestation. An intraperitoneal injection of 300 mg/kg body weight of
diesel particulates on day 12 of gestation also had no significant
effect on this end-point; however, intraperitoneal injection of 23%
particulate mass extracted with methylene chloride on day 12 resulted
in a dose-dependent increase in sister chromatid exchange frequency in
fetal liver on day 13, and a doubling was seen at 320 mg/kg body
weight. It was concluded that chemicals must be eluted from diesel
particles in order for the genotoxic material to cross the placenta
(Pereira et al., 1982).
B7.5.3 Teratogenicity
Sprague-Dawley rats and New Zealand white rabbits were exposed by
inhalation to exhaust from a light-duty diesel engine, at a
particulate matter concentration of 6 mg/m3, for 8 h/day on seven
days per week.
Twenty rats were exposed on days 5-16 of gestation and 20 rabbits
on days 6-18. The numbers of viable fetuses per litter, dead fetuses
per litter, resorptions per litter, implantation sites per litter,
corpora lutea per litter, and average fetal weight did not differ
significantly from those of controls (Pepelko & Peirano, 1983).
Reproductive and developmental toxicity are considered unlikely
to be critical end-points for diesel exhaust.
B7.6 Mutagenicity and related end-points
B7.6.1 In vitro
The genetic effects of particles and particle extracts have been
described (Lewtas & Williams, 1986; Morimoto et al., 1986; Henschler,
1987). Diesel exhaust extracts rather than particles were used in most
of these studies. Point mutations were observed in vitro in bacteria
and mammalian cells. Extracts caused chromosomal aberrations, DNA
damage, sister chromatid exchange, and cell transformation (Table 38).
The mutagenic potency of diesel engine exhaust depended in several
studies on the characteristics of the diesel fuel and the type, age,
and operating conditions of the engine (Henschler, 1987). As a rule,
organic extracts of particles were mutagenic in the absence of an
exogenous metabolic activating system (S9) (Ball et al., 1990);
addition of S9 decreased (Lewtas, 1983) or suppressed (Morimoto et
al., 1986) the mutagenic activity, perhaps by increasing protein
binding of mutagenic material or by metabolic detoxification of
directly acting mutagens, such as the nitroarenes, in the Ames test.
It can therefore be concluded that PAHs and thioarenes, which must be
metabolically activated, do not account for the mutagenic potency of
diesel particles. Salmeen et al. (1985) and Beland et al. (1985)
showed that nitroarenes are the main genotoxic agents in Salmonella
typhimurium T98 in the absence of S9. A number of other
investigators have reported the presence of nitrated PAHs in extracts
of diesel engine exhaust particles (Handa et al., 1983), including
many three-, four-, and five-ring structures (Schuetzle et al., 1982).
The concentration of 1-nitropyrene, one of the prevalent species, has
been reported to be 15-25 mg/kg particulate matter, whereas
benzo[ a]pyrene has been found at concentrations up to 50 mg/kg. The
nitrated PAHs are important in the health effects of diesel exhaust
since they are effective mutagens in microbial and human cell systems
(Patton et al., 1986). Some nitrated PAHs are also carcinogenic in
animals (Imaida et al., 1991). Ball & Young (1992) found that strain
T102, which does not respond to the mutagenic action of nitroarenes,
responds to a class of oxidizing compounds that can interact with DNA;
these compounds are not nitroarenes or typical PAHs.
The effects of diesel soot particles with and without adsorbed
organic substances, diesel soot extract without particles, an isolated
fraction of PAHs, and titanium dioxide and carbon black (Printex 90)
as reference particles were investigated in vitro on hamster lung
epithelial cells (Mohr & Riebe-Imre, 1992). The substances were added
to the cells at concentrations of 100-300 mg/litre. Diesel soot
extract, but not the PAH moiety isolated from the extract, stimulated
mixed-function oxygenases. The diesel soot extract was more cytotoxic
than the PAH fraction for the epithelium of the hamster respiratory
tract, but both mixtures induced the development of micronuclei. Under
the study conditions, only the PAH fraction led to transformation of
cells. The respiratory epithelium of hamsters was more sensitive than
human lung epithelial cells investigated in parallel, perhaps because
hamster cells are generally more easily transformed than human cells.
Diesel soot particles, titanium dioxide, and Printex 90 had hardly any
cytotoxic effect but triggered transformation. The authors concluded
that a combination of effects of particles and PAHs are responsible
for the degeneration of cells.
Schiffmann & Henschler (1992) studied the effects of diesel soot
extract and isolated fractions of PAHs, oxy-PAHs, and nitro-PAHs on
hamster embryo fibroblasts in vitro. The end-points investigated
were induction of micronuclei, unscheduled DNA synthesis, and cell
transformation. Diesel soot extract induced micronuclei at
concentrations of 80-400 mg/litre, whereas the fractions of various
PAHs were strongly cytotoxic. Diesel soot extract and the PAH fraction
transformed the fibroblasts; the nitro- and oxy-PAH fractions had a
weaker transforming effect, but their cytotoxic potency was clearly
stronger. Kinetochore analysis showed a high percentage (37%) of
chromosomes in micronuclei. No unscheduled DNA synthesis was induced.
Micronuclei were also induced in human embryonic lung fibroblasts
treated with diesel soot extract under comparable experimental
conditions.
B7.6.2 In vivo
The genotoxicity of total diesel exhaust, diesel particles, and
diesel soot extracts in vivo has been investigated in somatic cells
(Table 38). All of the substances induced genotoxic responses. Total
exhaust induced only sister chromatid exchange in Syrian golden
hamsters. The frequency of micronuclei was not increased significantly
in mice, even at high doses (up to 640 mg/kg body weight). Organic
extracts had clear genotoxic effects in various assays (Henschler,
1987).
No mutagenic activity was found in urine taken from rats exposed
to diesel exhaust emission by inhalation at 2 mg/m3 for 7 h/day on
five days per week for up to two years. A slight but nonsignificant
increase in micronuclei was observed in the bone marrow of mice that
had been exposed for six months; no increase in micronuclei was
detected in rats over a 24-month period (Ong et al., 1985).
In studies of heritable mutations in T stock mice, males were
examined for point mutations, induction of dominant lethal mutations,
translocations, and spermatogonial survival; females were examined for
oocyte death and dominant lethal mutations. No significant effect was
seen on germ cells. A reduced number of corpora lutea (reproductive
function) was the only significant result (Pepelko & Peirano, 1983).
B7.6.3 DNA adduct formation
Extensive research has been done to determine whether the DNA
adducts induced in lungs by diesel exhaust are related to later
tumorigenesis.
In rats that inhaled benzo[ a]pyrene absorbed to carbon black
(20 mg/g), an adduct of the diol epoxide with deoxyguanosine was
identified (Wolff et al., 1989), demonstrating that epoxides of PAHs
are produced by oxidative metabolism. Haemoglobin and albumin adducts
were also investigated.
Methods have been developed to detect DNA adducts of
1-nitropyrene or 1,6-dinitropyrene, which are markers of diesel engine
exhaust. The adducts are analysed in rat tissue or peripheral blood
lymphocytes by the 32P-postlabelling method and may be useful for
the dosimetry of 1-nitropyrene or diesel exhaust particulates in
occupational settings (El-Bayoumy et al., 1994). Studies on adduct
formation in lung DNA induced by inhaled diesel exhaust have been
conducted by Wong et al. (1986), Wolff et al. (1990), Bond et al.
(1988, 1989, 1990a,b), and Gallagher et al. (1993, 1994), all
involving the 32P-postlabelling technique. In general, these studies
suggest that DNA adducts are good measures of the 'effective dose' of
carcinogenic compounds.
Wong et al. (1986) found increased DNA adduct formation in the
lungs of Fischer 344 rats exposed to diesel exhaust particles at
7.1 mg/m3 for 31 months. After Fischer 344 rats and Syrian golden
hamsters were exposed to dilutions of diesel engine exhaust for six
months to two years, the level of haemoglobin adducts (2-hydroxy-
ethylvaline and 2-hydroxypropylvaline) increased dose-dependently,
corresponding to metabolic conversion of 5-10% of inhaled ethylene and
propylene to their oxides (Törnqvist et al., 1988).
Bond et al. (1988) designed experiments to determine the location
of DNA adducts in the respiratory tract of rats exposed to exhaust.
Fischer 344 rats were exposed for 12 weeks to diesel exhaust soot at
10 mg/m3; they were then sacrificed, and various regions of the
respiratory tract were removed and analysed for DNA adducts. Adducts
were found only in peripheral lung tissue and nasal tissue; the total
levels were highest in the peripheral tissue (about 18 adducts per 109
bases). Thus, the levels of total DNA adducts and exhaust-induced
adducts are highest in the region of the rat respiratory tract where
tumours are formed after exposure to a carcinogenic concentration of
diesel exhaust (Mauderly et al., 1987).
Bond et al. (1990c) exposed groups of Fischer 344 rats to soot at
0.35, 3.5, 7.0, or 10 mg/m3 for 12 weeks and found that the levels
of DNA adducts were similar, at about 14 adducts per 109 bases. This
level is nearly twice as high as that found in sham-exposed rats.
Thus, DNA adduct formation in lungs is independent of the
concentration of exhaust at the levels tested. One explanation for
these results (Bond et al., 1990a) is that the lung enzymes
responsible for the metabolism of soot-associated chemicals to
metabolites that bind to DNA were saturated at the concentrations
tested. These data also suggest that other factors are important in
the carcinogenicity of diesel exhaust, since the number of adducts was
elevated at an exposure level (0.35 mg/m3) that did not increase
lung tumour incidence. The induction of DNA adducts at low
concentrations is likely to result in tumour initiation by the organic
compounds present; at higher concentrations, the particles will induce
cell death, and subsequent proliferation may well act as a promotional
event. The combination may then result in detectable increases in
tumour incidence.
Bond et al. (1990a) also investigated the time course for DNA
adduct formation and persistence. Fischer 344 rats were exposed to
soot at 7 mg/m3 for up to 12 weeks and were sacrificed at 2, 4, 8,
12, 14, and 16 weeks after the start of exposure. DNA adducts
accumulated slowly in the lung, and the number was highest at the end
of exposure, representing about 160% of the level seen in controls
exposed to air only. The levels declined rapidly after termination of
exposure and were not significantly different from those of controls
four weeks later. Thus, steady-state levels of DNA adducts would be
reached during long-term exposure to diesel exhaust.
Bond et al. (1989, 1990c) and Wolff et al. (1990) investigated
whether exposure to carbon particles without associated mutagenic
organic chemicals also increases DNA adduct levels. Rats were exposed
either to diesel exhaust or carbon black at 0, 3.5, or 10 mg/m3 for
12 weeks. Solvent-extractable organic compounds made up about 30% of
the diesel soot but only about 0.04% of the carbon black particles.
Exposure to the highest levels of both carbon black and diesel exhaust
increased the DNA adduct levels in lungs, although the levels in rats
exposed to diesel exhaust were about 30% higher than those in rats
exposed to carbon black. Heavy exposure to carbon particles can
therefore increase DNA adduct formation, although the concentrations
are lower than those of exhaust-related adducts.
Bond et al. (1990b) investigated whether DNA adducts could be
formed in specific cells of the rat lung, i.e. alveolar type II cells.
After exposure of rats to 6.2 mg/m3 diesel exhaust or carbon black
particles for 12 weeks (16 h/day on five days per week), type II cells
were isolated and their DNA analysed for adducts. Both diesel exhaust
and carbon black resulted in about a fourfold increase in total DNA
adducts in type II cells. DNA adduct levels in peripheral lung tissue
were increased by 60-80% in male and female Fischer 344 rats and
female cynomolgus monkeys but not in female B6C3F1 mice or female
Syrian hamsters after inhalation of diesel soot at 8.1 mg/m3 for 12
weeks (Bond et al., 1989, 1990c).
Gallagher et al. (1993, 1994) exposed female Wistar rats to
diesel soot at 7.5 mg/m3 and to carbon black at 11.3 mg/m3 for 24
months and measured DNA adducts in lungs. The mean adduct levels were
similar in the two groups but were not significantly greater than
those in controls. Time-course studies indicated that the levels in
the lungs of rats exposed to diesel exhaust were lower at 24 months
than after two or six months of exposure, presumably as a result of
dilution due to increased cell proliferation. The level of a single
DNA adduct, thought to be derived from a nitro-PAH in diesel exhaust
and not observed in rats exposed to carbon black or titanium oxide,
was elevated over that in controls after two, six, and 24 months. The
DNA adduct levels in control rats increased over the duration of the
24-month study as an effect of age.
Gallagher et al. (1993) compared DNA adduct formation after
exposure to diesel emissions in vitro and in vivo. After exposure
of human lymphocytes to diesel extract, five major DNA adducts were
detected, one of which was characterized as an adduct with
benzo[a]pyrene. One was also detectable in reaction products of calf
thymus DNA and diesel particle extract in vitro and in skin and lung
DNA from mice treated dermally with 50 mg diesel extract in vivo. The
differences in DNA patterns in vitro and in vivo (skin and lung)
may reflect differences in metabolic pathways.
Taken together, the studies of DNA adducts suggest that some
organic chemicals in diesel exhaust can form DNA adducts in lung
tissue and may play a role in the carcinogenic effects. As pointed out
by Bond et al. (1989), however, DNA adducts alone cannot explain the
carcinogenicity of diesel exhaust, and other factors, such as chronic
inflammation and cell proliferation, are also important.
B7.7 Special studies
B7.7.1 Immunotoxicity
During the clearance process, one possible pathway is
translocation of diesel particulates into the lymphatic channels.
After male guinea-pigs were exposed to diesel exhaust at a particle
concentration of 1.5mg/m3 for four or eight weeks, the B- and T-cell
counts in lymph nodes were not altered, and there were no significant
changes in blood or spleen (Dziedzic, 1981).
Fischer 344 rats were exposed to 2 mg/m3 diesel exhaust for
7h/day on five days per week for 12 or 24 months and the immunological
function of splenic B and T cells was measured by enumerating
antibody-producing cells in the spleen or monitoring the proliferative
response. No changes were observed (Mentnech et al., 1984).
Table 38. Genotoxicity of diesel exhaust, particles, and extracts
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity
Bacteria
S. typhimurium Point mutation (his) + + Huisingh et al. (1978)
S. typhimurium Point mutation (his) + + Loprieno et al. (1980)
S. typhimurium Point mutation (his) + Clark & Vigil (1980)
S. typhimurium Point mutation (his) + + Li & Royer (1982)
S. typhimurium Point mutation (his) + Salmeen et al. (1984)
S. typhimurium Point mutation (his) + Ong et al. (1985)
S. typhimurium Point mutation (his) + Bechtold et al. (1986)
S. typhimurium Point mutation (his; + Whong et al (1986)
SOS umu test)
S. typhimurium Point mutation (his) + Wallace et al. (1987)
S. typhimurium Point mutation (his) +a Wallace et al. (1990)
S. typhimurium Point mutation (his) + Lewis et al. (1989)
S. typhimurium Point mutation (his) + Rasmussen (1990)
S. typhimurium Point mutation (his) + + Keane et al. (1991)
S. typhimurium, E. coli Point mutation (trp) + Crebelli et al. (1991)
E. coli Point mutation (trp) + Lewtas (1983)
Mammalian cells
L5178Y mouse Point mutation (tk) + Lewtas (1983)
lymphoma cells
L5178Y mouse Point mutation (tk) + Mitchell et al. (1981)
lymphoma cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Mutagenicity (contd)
Chinese hamster Point mutation (hprt) + Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Chescheir et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt) (+) Casto et al. (1981)
ovary cells
Chinese hamster Point mutation (hprt, + Morimoto et al. (1986)
lung V79 cells ATPase)
Human xeroderma Point mutation (hprt) + + McCormick et al. (1980)
pigmentosum fibroblasts
Human lymphoblast Point mutation (tk) + Barfknecht et al. (1981)
TK6 cells
Balb/c3T3 mouse Point mutation (ATPase) + (+) Curren et al. (1981)
fibroblasts
DNA damage
Syrian hamster DNA chain breaks - Casto et al. (1981)
embryo cells (alkaline elution)
Human xeroderma DNA damage + + McCormick et al. (1980)
pigmentosum fibroblasts
Rat primary Unscheduled DNA + Lewtas (1983)
hepatocytes synthesis
Chinese hamster lung Unscheduled DNA +a + Gu et al. (1994)
V79 cells synthesis
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Chromosomal effects
Chinese hamster Sister chromatid exchange + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations + Lewtas (1983)
ovary cells
Chinese hamster Chromosomal aberrations +b Hasegawa et al. (1988)
lung V79 cells -c
Chinese hamster Sister chromatid exchange + Hasegawa et al. (1988)
lung V79 cells
Chinese hamster Sister chromatid exchange + + Keane et al. (1991)
lung V79 cells
Human lymphocytes Chromosomal aberrations + Lewtas (1983)
Chinese hamster Sister chromatid exchange + Morimoto et al. (1986)
lung V79 cells
Human lymphocytes Sister chromatid exchange (+) Tucker et al. (1986)
Human lymhoblastoid Sister chromatid exchange + Morimoto et al. (1986)
cells
Hamster embryo Micronuclei + Schiffmann & Henschler
fibroblasts (1992)
Chinese hamster lung Micronuclei +a + Gu et al. (1992)
V79 and ovary cells
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Cell transformation
Balb/c3T3 mouse fibroblasts Cell transformation (+) Curren et al. (1981)
Balb/c3T3 mouse fibroblasts Cell transformation +b Hasegawa et al. (1988)
(+)c
Hamster lung epithelial cells Cell transformation + + Mohr & Riebre-Imre (1992)
Hamster embryo fibroblasts Cell transformation + Schiffmann & Henschler (1992)
In vivoa
Mouse Micronuclei, bone marrow - - + Pereira (1982)
Mouse Micronuclei, bone marrow - Lewis et al. (1989)
Mouse Micronuclei, bone marrow - Morimoto et al. (1986)
Mouse Micronuclei, bone marrow - - - Pepelko & Peraino (1983)
Mouse Micronuclei, bone marrow (+) Ong et al. (1985)
Rat Micronuclei, bone marrow - Ishinishi et al. (1988)
Rat Micronuclei, bone marrow - Ong et al. (1985)
Chinese hamster Micronuclei, bone marrow (+) - - Pepelko & Peraino (1983)
Mouse Sister chromatid exchange, - + + Pereira (1982)
bone marrow
Rat Sister chromatid exchange, - Ishinishi et al. (1988)
bone marrow
Rat Sister chromatid exchange, - Morimoto et al. (1986)
bone marrow
Rat Sister chromatid exchange, - Ong et al. (1985)
peripheral blood leukocytes
Table 38 (contd)
Test organism End-point Exhaust Particles Extract Reference
Rat Sister chromatid exchange, - Lewis et al. (1989)
lymphocytes
Syrian golden Sister chromatid exchange, + Pereira (1982)
hamster lung cells (inhalation)
Syrian golden Sister chromatid exchange, + Guerrero et al. (1981)
hamster lung cells (instillation)
Syrian golden Sister cromatid exchange, + + Pereira (1982)
hamster lung cells (instillation)
Syrian golden Sister chromatid exchange + Morimoto et al. (1986)
hamster liver cells (transplacental)
Syrian golden hprt mutation + Morimoto et al. (1986)
hamster (transplacental)
Mouse Mutation, S. typhimurium - Morimoto et al. (1986)
(host-mediated assay)
Mouse Heritable effects on germ - Pepelko & Peirano (1983)
cells (various assays; m/f)
Drosophila Heritable effects (sex-linked Schuler & Niemeier (1981)
melanogaster recessive lethal mutation) -
his, histidine independence; trp, tryptophane independence; hprt, hypoxanthine-guanine-phosphoryltransferase (8-aza- or
6-thioguanine resistance); tk, thymidine kinase (bromodeoxyuridine or trifluorothymidine resistance); ATPase, Na+/K+-ATPase
(ouabain resistance); +, positive; (+), weakly positive
a Dispersed in artificial surfactant
b Light-duty engine
c Heavy-duty engine
d Modified from Henschler (1987) and supplemented
Fischer 344 rats and CD-1 mice were exposed to diesel exhaust
particles at 0.35, 3.5, or 7 mg/m3 for 6, 12, 18, or 24 months to
investigate whether the accumulation of diesel particulates in lymph
nodes (indicated by black discolouration) influences the subsequent
response to immunization by sheep red blood cells, evaluated by the
presence of immunoglobulin (Ig) M antibody-forming cells. In rats, the
total number of lymphoid and antibody-forming cells was significantly
increased at the medium and high aerosol concentrations. In mice, the
number of lymphoid cells was increased only at the high concentration.
The authors concluded that diesel exhaust particles have only a
minimal effect on the immune and antigen filtration functions in
lung-associated lymph nodes because the relative numbers of
antibody-forming cells and specific IgM, IgG, and IgA antibodies in
rat sera were not significantly changed (Bice et al., 1985).
Five intranasal inoculations of various doses of a suspension of
diesel engine exhaust particles in ovalbumin were administered to BDF1
mice at intervals of three weeks. Ovalbumin IgE antibody titres,
assayed by passive cutaneous anaphylaxis, were enhanced by doses as
low as 1 µg (Takafuji et al., 1987, 1989). Similarly, primary IgE
responses were increased after intraperitoneal administration to mice
of ovalbumin or cedar pollen allergen mixed with diesel exhaust
particulates (Muranaka et al., 1986).
Female CR/CD-1 mice were exposed by inhalation to 6-7 mg/m3
diesel exhaust for 2-6 h (acute), 8 h/day for 2-16 days (subacute),
or 8h/day for about 300 days (chronic). Immediately after the end
of exposure, animals were exposed to the infectious aerosols
S. typhimurium, Streptococcus pyogenes, or A/PR8-34 influenza virus.
Increased susceptibility to post-infection mortality was seen with the
bacterial but not the viral pathogens. Nitrogen dioxide and acrolein
vapour had specific effects (Campbell et al., 1981b).
CD-1 mice were exposed to 2 mg/m3 diesel exhaust for one to six
months. After three months of exposure and subsequent infection with
influenza virus, an enhanced severity of response was seen, involving
significant lung consolidation and focal macular collections of
particle-laden macrophages (Hahon et al., 1985).
B7.7.2 Behavioural effects
Sprague-Dawley rats were exposed for 8 h/day on seven days per
week for 16 weeks to diesel exhaust diluted to a particulate matter
concentration of 6 mg/m3. Spontaneous locomotor activity, measured
weekly on Wahman LC-34 running wheels, was significantly decreased
during 8, 9, 11, and 12 weeks of exposure. In rats exposed for
20 h/day on seven days per week for six weeks, forced activity on a
motorized treadmill was measured during the last week. Exhaustion
occurred in less than half the time that it occurred in control
animals. Groups of 10 rats were exposed to diesel exhaust at 6 mg/m3
for 20 h/day on days 1-7 post partum and were then held in clean air
until 15 months of age. In bar pressing acquisition training carried
out at five-day intervals for the next 42 days, the rate of learning
was much slower than in control rats. The difference was highly
statistically significant (Pepelko & Peirano, 1983).
As these three studies were conducted at a high concentration,
6 mg/m3, the practical consequences of the effects observed are not
known. The results, while limited in scope, indicate, however, that
behavioural and neurophysiological effects may be important toxic
end-points which should be investigated further.
B7.8 Factors that modify toxicity; toxicity of metabolites
Little information is available, but chemicals and other factors
may modify the toxicity of diesel emissions. For instance,
chlorophyllin, a derivative of the green pigment chlorophyll, has been
reported to inhibit or reduce the mutagenic activity of diesel
particulate extracts in the Ames test (Ong et al., 1986).
B7.9 Mechanisms of toxicity; mode of action
It is not clear whether DNA-reactive or non-DNA-reactive
mechanisms, or a combination of the two, are responsible for the
carcinogenic action of inhaled diesel exhaust in laboratory animals.
The results of several studies indicated that many PAHs (e.g.
benzo[ a]pyrene) cause a carcinogenic response in rodents, but it is
not known whether the same PAHs when associated with diesel exhaust
also induce a carcinogenic response. One hypothesis for the mechanism
of the tumorigenic response is that organic chemicals (e.g. PAHs,
nitro-PAHs) desorb from soot particles, are metabolized to reactive
metabolites, and interact with lung DNA to initiate carcinogenesis.
This would be a predominantly DNA-reactive mechanism. The observation
that organic chemicals associated with diesel soot can be metabolized
by lung cells to metabolites that can form DNA adducts after long-term
exposure to diesel exhaust supports this hypothesis. Studies with
carbon black essentially devoid of PAHs but which also form DNA
adducts do not, however, corroborate the PAH-DNA-reactive concept.
Another hypothesis is that the lung tumours that arise in rats
exposed to high levels of diesel exhaust are due to overloading of the
normal lung particle clearance mechanisms, accumulation of soot
particles, and cell damage followed by regenerative cell
proliferation. Enhanced cell proliferation may increase the mutation
frequency of key target genes. In this hypothesis, genotoxic chemicals
may not be causative factors in tumorigenicity. This view is supported
by the observations that lung cancer can be induced in rodents by
inhalation of highly insoluble particles of low toxicity that are
virtually devoid of organic chemicals (e.g. talc, carbon black, coal
dust, titanium dioxide), although high concentrations of these
particles (overload) are typically necessary.
A third hypothesis, which draws upon the above two mechanisms, is
that carcinogenesis is initiated by exposure to organic compounds
associated with diesel soot and promoted by the chronic inflammation,
cytotoxicity, and cell proliferation arising from the high
concentrations of particles deposited and retained in the lungs.
Again, however, the tumorigenic response observed with particles alone
(carbon black, titanium dioxide) does not support this hypothesis.
A key consideration is the relative contribution of different
mechanisms at different levels of exposure. As discussed later in this
section, high concentrations of particles, including diesel exhaust,
leading to high lung burdens, compromise normal clearance mechanisms.
Therefore, certain mechanisms may be invoked at high lung burdens that
may not occur at lower lung burdens. Genotoxic aromatic compounds may
take on increasing importance at lower concentrations.
Figure 3 is a diagram outlining the possible mechanism of action
of diesel exhaust, including the effects of overload conditions.
B7.9.1 Carcinogenic effects
B7.9.1.1 DNA-reactive mechanisms
Diesel exhaust contains hundreds of chemicals, including PAHs,
nitro-PAHs, alkyl-PAHs, oxy-PAHs, oxy-nitro-PAHs, and aldehydes
(Scheepers & Bos, 1992b), many of which are known mutagens and
carcinogens in laboratory animals. For example, inhaled
benzo[ a]pyrene (in tar and pitch) is carcinogenic in rat lung
(Heinrich et al., 1994). Nitro-PAHs are potent mutagens and, in some
instances, carcinogenic. In pharmacokinetic studies cited in this
monograph, clearance of PAHs and nitro-PAHs associated with the diesel
particulate fraction is retarded or delayed. Other studies have shown
clearly that the organic compounds associated with diesel soot are
bioavailable to lung cells. Mechanisms for the delayed clearance of
the PAHs have been discussed (Bond et al., 1986b; Gerde et al.,
1991b). PAHs require metabolic activation to metabolites (e.g.
epoxides) that can potentially bind to DNA. In the case of the PAHs
associated with diesel soot, it is likely that alveolar lung cells
(e.g. type II cells) are responsible for their metabolic activation,
so that they can bind to lung cell DNA (Bond et al., 1983).
Additional evidence for a DNA-reactive mechanism in the
carcinogenicity of diesel exhaust is that exposure of laboratory
animals, including rats and monkeys, to diesel exhaust results in the
formation of DNA adducts in lung cells (see section B7.6). DNA adducts
and subsequent DNA replication can result in mutations that play a key
 role in initiating the carcinogenic response. These mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes. There may be some as yet unidentified chemicals in diesel
exhaust with high mutagenic potential; furthermore, chemical
interactions (additive or inhibitory) may occur among the components
of diesel exhaust (Ball & Young, 1992).
B7.9.1.2 Cytotoxicity with regenerative cell proliferation
Another mechanism by which diesel exhaust may induce
carcinogenesis involves cell killing, or cytotoxicity, by the
particles or the reactive gases in exhaust, followed by regenerative
proliferation of lung cells. Enhanced cell replication may result in
an increased frequency of spontaneous mutations. Mutations could also
arise from PAH-DNA adducts or from oxidative DNA damage caused by the
reactive oxygen and nitrogen species in diesel soot, resulting in the
inflammatory response. Driscoll et al. (1994) showed that the hprt
gene mutation frequency in rat lung epithelial cells was significantly
increased when the cells were incubated in vitro with inflammatory
cells obtained by bronchoalveolar lavage from rats exposed to quartz
particles. This appears to be an important mechanism in lung tumour
induction by particles that elicit a chronic inflammatory response in
the lung. Sagai et al. (1993) showed that diesel exhaust particles
could produce superoxide anions and hydroxyl radicals in the absence
of biological activation (detection by cytochrome c and electron spin
resonance). Reactive oxygen species may induce specific DNA adducts,
such as 8-hydroxydeoxyguanosine. As mentioned above, mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes.
Several data sets cited in this monograph support this
hypothesis. For example, carbon black particles, which are virtually
devoid of PAHs but are morphologically similar to diesel soot
particles, induce lung cancer in rats. High particle burdens in rodent
lungs result in an inflammatory response (Henderson et al., 1988).
Release of cytokines and reactive oxygen and nitrogen species during
the inflammatory response to particles may result in cell death. Some
evidence exists (Wright, 1986) that diesel exhaust enhances lung cell
replication.
B7.9.1.3 Effects of particles
The importance of pulmonary particle burden on lung tumour
induction has been demonstrated clearly in long-term studies by
inhalation in rats. As reviewed above, rats have similarly increased
lung tumour incidences when exposed to diesel exhaust or carbon black
particles by inhalation for 24 months. Similarly, carbon black
particles practically devoid of PAHs induce pulmonary tumours after
intratracheal instillation. The very large surface area of carbon
black and of diesel exhaust particles after desorption of adsorbed
organic compounds in vivo may be involved mechanistically in a
tumorigenic effect. Heinrich (1994) showed that the tumour response to
different types of carbon black particles instilled intratracheally
correlated well with their respective surface areas. Pott (1991) and
Pott et al. (1993) suggested that particles with a large surface area
have greater effects in humans than in rats because insoluble
particles with no specific toxicity remain significantly longer in the
terminal airways of humans than of rats (retention half-time, about 70
days in rats and about 500 days in humans).
The correlation between particle surface area and lung tumour
incidence was examined (Oberdörster & Yu, 1990) by evaluating
published studies of inhalation of diesel and other particles. Tumour
induction in rats was best correlated with the surface area of the
particles retained in the lung rather than with the particle mass,
particle volume, or number of particles, regardless of the PAH
content. It was suggested that particle surface area and surface
properties play a decisive role ('critical surface area') and that
absorbed PAHs are not responsible for the tumour response in rats
exposed to diesel exhaust. In the human situation, however, it could
not be excluded that organic compounds and gas-phase components are
also involved, since the human particulate lung burden is much lower
than those achieved in rats after long-term inhalation.
Inhalation of diesel engine exhaust can result not only in
pulmonary tumours but also in inflammation and fibrosis and in a delay
in alveolar (not bronchial) pulmonary clearance. The mechanism by
which tumours develop due to particle overload and its associated
pathological and anatomical changes may be restricted to rats and may
not occur under environmental conditions in humans, since the lung
burdens of humans do not reach the levels that induce lung tumours in
rats. This is of importance for quantitative risk assessment. Only
occupational exposure to diesel exhaust may result in lung burdens
near or at overload conditions, particularly if the lung is already
compromised by exposure to other dusts. Bohning et al. (1982) reported
retarded particle clearance in smokers; in these people, additional
exposure to diesel exhaust may induce overload and associated toxic
effects. It is not known, however, whether the mechanisms of
particle-induced lung tumours are the same in rats and humans, and
this information is necessary for extrapolating data from rats to
humans.
Not only diesel soot (0.8, 2.5, or 7.0 mg/m3) but also carbon
black nearly completely devoid of organic compounds (Printex 90,
7.5-12 mg/m3; particle size, 10 nm) and ultrafine titanium dioxide
particles (7.5-15 mg/m3; particle size, 20 nm) caused lung tumours
in female Wistar rats exposed by inhalation for 18 h/day on five days
per week for 24 months. The tumour rate increased with increasing
particle concentrations, independently of the type of particles
inhaled. No lung tumours were observed in the rats exposed to the
lowest concentration of diesel particles. The authors concluded that
the carcinogenic component of diesel exhaust is in the inner part of
the diesel soot particle, the carbon core, and is not the relatively
small amount of carcinogenic PAHs (3.9 g benzo[ a]pyrene per gram of
diesel soot) (Heinrich et al., 1992; Heinrich, 1994; Heinrich et al.,
1995). These results were confirmed in another two-year study, in
which male and female rats were exposed by inhalation to various
concentrations of carbon black and diesel exhaust. Lung tumours were
observed with both particle types (Nikula et al., 1994).
Diesel soot does not appear to have a specific carcinogenic
effect in rats; rather, there is a nonspecific effect of particles.
B7.9.1.4 Effects of polycyclic aromatic hydrocarbons
Exposure of rats by inhalation to 2.6 mg/m3 of an aerosol of
tar-pitch condensate with no carbon core but containing 50 µg/m3
benzo[ a]pyrene and other PAHs for 10 months caused lung tumours at a
rate of 39%. The same amount of tar-pitch vapour condensed onto the
surface of carbon black particles at 2 and 6 mg/m3 resulted in
tumour rates that were roughly two times higher (89 and 72%). Since
exposure to 6 mg/m3 carbon black almost devoid of extractable
organic material caused a lung tumour rate of 18%, the tumour rate of
72% seen after combined exposure to tar-pitch vapour and carbon black
particles indicates a syncarcinogenic effect of PAHs and carbon black.
A possible mechanism is an effect of deposition of PAHs (Heinrich et
al., 1994). As the level of benzo[ a]pyrene in the coal-tar pitch was
about three orders of magnitude greater than those in diesel soot,
PAHs may play a negligible role in the tumorigenicity of diesel soot
in rats. The PAH profile in diesel soot is, however, quite different
from that in coal-tar pitch, as diesel soot contains highly mutagenic,
carcinogenic nitro-PAHs and other poorly characterized mutagens that
are not present in coal-tar pitch or on some of the carbon black
particles used in experimental studies (Heinrich et al., 1994; Nikula
et al., 1994).
In a study of various extracts of diesel exhaust particles,
30-40% of the total mutagenicity could be attributed to a group of six
nitroarenes (Salmeen et al., 1984).
A diesel exhaust particle extract was separated into a water- and
a lipid-soluble fraction, and the latter was further separated into a
PAH-free, a PAH-containing, and a polar fraction by column
chromatography. These fractions were then tested in Osborne-Mendel
rats by pulmonary implantation at doses corresponding to the
composition of the original diesel exhaust. The water-soluble fraction
did not induce tumours; the incidences induced by the lipid-soluble
fractions were 0% with the PAH-free fraction, 25% with the
PAH-containing fraction, and 0% with the polar fraction. The
PAH-containing fraction, comprising only 1% by weight of the total
extract, was shown to be responsible for the carcinogenic activity
(Grimmer et al., 1991).
Various dichloromethane extracts, each representing a complex
mixture, were obtained from particulate emissions of four
diesel-fuelled and one gasoline-fuelled automobiles (combustion), a
coke oven battery (pyrolysis), and a roofing tar pot (evaporation),
and their tumorigenic potency was compared in Sencar mice. It was
concluded that the benzo[ a]pyrene content alone could not explain
the tumorigenic activity of the mixtures (Nesnow et al., 1982a, 1983).
The lung tumour rates in rats exposed to atmospheres containing
PAHs depend not only on the PAH concentrations of the exhaust gas but
also on parameters such as the composition of the carrier particle
(mass ratio of carbon core to adsorbed layer of organics), the
dissolution rate of particle-attached organic compounds, the retention
half-time, and the cytotoxic effect of the carrier particle in the
lung (Heinrich et al., 1991).
Extracted diesel soot given intratracheally to rats induced lung
tumours, but native, unchanged diesel soot resulted in higher tumour
rates than extracted soot. Carbon black also caused tumours after
intratracheal administration, and the rate increased with decreasing
size of the primary particles (Heinrich, 1994).
Carbon black particles almost completely devoid of organic
compounds (< 0.046% extractable organic compounds and 0.6 ng/g
benzo[ a]pyrene) caused tumours in the lungs of 17% of rats after
exposure to a concentration of 6 mg/m3 for 18 h/day on five days per
week for 10 months. No lung tumours occurred in controls exposed to
clean air. Thus, the particle effect may be responsible for induction
of lung tumours in rats exposed to diesel engine exhaust, and the
effect may be related to the surface area of the carbon particle. A
PAH depot effect could lead, however, to retarded dissolution of PAHs
from the carrier, resulting in very efficient use of the extremely
small amount of carcinogenic PAHs retained in the lung after exposure
to diluted diesel engine exhaust, which contained benzo[ a]pyrene at
about 10 ng/m3 (Heinrich et al., 1991). Since diesel particles
induce DNA adducts at lower concentrations than carbon black (Bond et
al., 1989, 1990c; Wolff et al., 1990), PAHs may have tumour initiating
properties that are too weak to be detected at low doses; at higher
doses, promotional effects of the particles, perhaps with additional
initiation, may synergize to produce detectable carcinogenic effects.
B7.9.2 Non-carcinogenic effects
Diesel exhaust contains various respiratory irritants in the gas
phase and in particulate matter. Both can induce inflammatory
responses in the airways and alveolar regions of the lung. Airway
inflammation involves damage to epithelial cells, including lipid
peroxidation of cell membranes by oxidizing gaseous pollutants such as
nitrogen dioxide. Indirect effects of particles, resulting from
phagocytosis, can include the formation and release of various
mediators, including oxidants, such as superoxide anions and hydroxyl
radicals, and cytokines. These mediators may play a role in focal loss
or shortening of cilia, type II cell hypertrophy, and hyperplasia. The
latter changes can lead to the hyperplastic lesions seen in animals
exposed to diesel exahaust (Ishinishi et al., 1986, 1988; Suzuki et
al., 1990; Kato et al., 1992). Phagocytosis and subsequent clearance
of particles by alveolar macrophages can be compromised by high
particle burdens (Wolff et al., 1989; Creutzenberg et al., 1990),
which may also increase the access of particles to the interstitium,
leading to focal fibrosis (Henderson et al., 1988).
Inflammation, altered lung clearance, and hyperplastic lesions
can be considered early markers of exposure to diesel exhaust and are
the basis of the non-neoplastic effects used to determine both the
NOAEL and the 'benchmark concentration' described in section B10.1.3.
B8. EFFECTS ON HUMANS
B8.1 General population
B8.1.1 Acute exposure: olfactory, nasal, and ocular irritation
Acute exposure to diesel exhaust has been associated with
irritation of the eyes and nose. Signs and symptoms reported after
acute exposure to diesel exhaust are described in section B8.2.1.
The exhaust from e.g. poorly maintained engines or engines under
load may be visible as a black, smoky cloud. WHO (1987) considered
that sensory effects were parameters that could be used in setting
occupational exposure limits. The characteristic odour of diesel
exhaust provides a warning of its presence. At higher concentrations
and under certain operating conditions, the odour can be offensive.
The odour-causing agents are not known; however, compounds with a
relative molecular mass > 80 are probably those mainly responsible,
and aliphatic olefins (C1-C6, including acetylene), hydrocarbons
with more than six carbons, and aliphatic aldehydes are probably not
involved. Substances with low odour thresholds, such as nitrogen
dioxide (0.3 ppm) and acrolein (0.5 ppm), appear to contribute to the
odour of diesel exhaust to minor extents (5.3 and 0.25%, respectively)
(Oelert & Florian, 1972).
The ability to detect the odour and the irritating effects of
diesel exhaust vary. For example, when six subjects smelled diesel
exhaust diluted with air, the dilution factors needed to achieve the
odour detection threshold varied from 140 to 475. The concentrations
of different constituents of the exhaust at the odour threshold also
varied: formaldehyde, 0.012-0.088 ppm; acrolein, 0.011-0.046 ppm; and
nitrogen dioxide, 0.11-2.28 ppm (Linnell & Scott, 1962). When six
subjects were exposed to three concentrations of diesel exhaust for
10 min, the mean concentrations at the odour threshold were 1.3-4.2 ppm
nitrogen dioxide, 0.2-1 ppm sulfur dioxide, < 0.1 ppm formaldehyde,
and < 0.05 ppm acrolein. Three of the subjects exposed to the highest
level discontinued exposure before 10 min, while none at the lowest
level discontinued exposure although some experienced eye irritation
(Battigelli, 1965).
The health effects of inorganic gases present in diesel exhaust
are not specific and are therefore not discussed in detail; however,
it must be noted that these gases contribute to the environmental
burden.
B8.1.2 Air pollution
The concentrations of some air pollutants, such as particulates
and sulfur dioxide from the burning of coal for domestic heating and
industrial purposes, are in many parts of the world much lower than
they were several decades ago, and the associated adverse health
effects have diminished accordingly. A number of epidemiological
studies have demonstrated, however, that the relatively low remaining
levels of air pollutants are associated with a range of health
indices, including day-to-day changes in mortality (Schwartz, 1993),
visits to hospital emergency departments (Schwartz et al., 1993), and
changes in measures of lung function (Pope & Dockery, 1992). In most
studies, the associations have been strongest with the fine
particulate component of pollution, some of which may be derived from
diesel exhausts.
Vehicle traffic, both gasoline and diesel, also contributes to
the nitrogen dioxide content of urban air, and there is evidence,
primarily from experimental studies (Bauer et al., 1986; Koenig et
al., 1988; Grant et al., 1993) that it can induce short-term
decrements in lung function in asthmatic and non-asthmatic patients.
Nitrogen dioxide and volatile hydrocarbons are involved in the
formation of ozone and associated photochemical pollutants.
Associations have also been demonstrated between long-term
exposure to urban air pollutants and death rates from certain chronic
conditions. Confounding factors often make interpretation difficult,
but in an investigation conducted in the United States (Dockery et
al., 1993), air pollution attributable to fine particles was
positively associated with lung cancer and cardiopulmonary disease,
after adjustment for smoking and other relevant risk factors, but not
with other causes considered collectively.
In neither short- nor long-term studies can the possible role of
diesel particulates be specified, but diesel emissions contribute to
urban particulates, especially in Europe and developing countries. The
acidic and sulfate components of particulates, which are derived
primarily from stationary fuel-burning sources, appeared to be
implicated in a number of studies.
B8.2 Occupational exposure
B8.2.1 Effects on the respiratory system
B8.2.1.1 Symptoms
Most of the information about symptoms after acute exposure to
diesel exhaust comes from anecdotal reports of occupationally exposed
individuals. It was noted in a bus garage in London, United Kingdom,
that the buses produce a 'lachrymatory mist' when they were started up
from cold (Commins et al., 1956). In a review of 13 cases of acute
overexposure to diesel exhaust in five underground coal mines in Utah
and Colorado, United States, between 1974 and 1985, interviews in 1986
with the miners revealed that 12 had experienced symptoms of mucous
membrane irritation, headache, and light-headedness; eight had
reported nausea, and four reported a sensation of unreality and
'heartburn' (Kahn et al., 1988).
In a study designed to investigate the acute effects of diesel
exhaust on respiratory symptoms, a questionnaire was administered to
232 male workers in four diesel bus garages, and personal samples of
nitrogen dioxide and respirable particulate were obtained. After
adjustment for age and smoking (current, ex-, never), workers with the
highest exposure to respirable particulate (0.31 mg/m3) reported
significantly more cough, itchy or burning eyes, headache, difficult
or laboured breathing, a feeling of chest constriction, and wheeze
than workers with lower exposures. Those exposed to nitrogen dioxide
at >0.4 mg/m3 (> 0.3 ppm) also more frequently reported itchy or
burning eyes, difficult or laboured breathing, a feeling of chest
constriction, and wheezing. In comparison with a group of lead acid
battery workers, the reporting of the following symptoms as
'sometimes' or 'often' was significantly more prevalent among the
workers in the diesel bus garage: eyes itch, burn, or water (49.5
versus 23.5%), headache (24.2 versus 12.1%), difficult or laboured
breathing (13.5 versus 3.6%), nausea (13.5 versus 4.5%), and wheeze
(13.7 versus 4.5%) (Gamble et al., 1987a).
In comparison with 11 office workers, 17 ferry stevedores had a
greater prevalance of wheezing (24 versus 9%), chest tightness (29
versus 9%), nasal complaints (47 versus 0%), chest pain (24 versus
0%), and eye irritation (59 versus 27%); however, more of the
stevedores smoked. The concentrations of nitrogen dioxide (0.3 mg/m3)
and sulfur dioxide (0.7mg/m3) were both < 0.25 ppm; respirable
particulate was not measured (Purdham et al., 1987). In a study in an
iron ore mine where diesel engines were used underground, more workers
who were smokers reported pressure over the chest or difficulty in
getting air than the nonsmokers. No such difference was observed among
smoking and nonsmoking surface workers. The underground workers who
were smokers also had more episodes of a productive cough lasting
three weeks during several winters (Jorgensen & Svensson, 1970).
B8.2.1.2 Acute changes in pulmonary function
The pulmonary function of 60 coal miners who worked in mines
equipped with diesel engines was compared with that of 90 coal miners
not exposed to diesel exhaust. There were similar proportions of
current smokers (45% in the exposed group and 43% in the unexposed
group). Measurements made with personal dust samplers and passive
nitrogen dioxide dosimeters showed average concentrations of
0.04 mg/m3 (0.2 ppm) nitrogen dioxide, 0.4mg/m3 (0.3 ppm)
formaldehyde, and 2.0 mg/m3 respirable dust in the exposed group;
the unexposed group was exposed to 1.4 mg/m3 respirable dust. Forced
vital capacity (FVC) and forced expiratory volume in 1 sec (FEV) were
reduced during the work shift for both the diesel-exposed and
unexposed groups. The reduction was slightly but not significantly
greater in smokers than in ex- and nonsmokers. An additional analysis
with adjustment for age, exposure to respirable dust, and years of
underground mining still revealed no difference in shift-related
pulmonary function between miners exposed and unexposed to diesel
exhaust (Ames et al., 1982).
No significant shift-related change in FVC or FEV was seen among
workers on ferries transporting diesel trucks and other vehicles in
comparison with controls (Purdham et al., 1987), and no significant
change in spirometry measured over a shift was seen in 232 workers in
four diesel bus garages (Gamble et al., 1987a). Ulfvarson et al.
(1987), however, found significant decrements in FVC and FEV1 across
a shift in 23 ferry workers who had had no exposure to diesel for 10
days, with no difference between smokers and nonsmokers. The
concentration range of total particles was 0.13-1 mg/m3, that of
formaldehyde 0.04-0.6mg/m3 (< 0.03-0.5 ppm), and that of nitrogen
dioxide 0.12-4.6mg/m3 (0.06-2.3 ppm). In a repetition of the study,
a significant shift-related decrement was noted in FVC but not in
FEV1. In a later study in which filters were put on the diesel
trucks, a shift-related decrement in FVC was seen, but no subsequent
shift-related changes in FEV were noted, with or without filters
(Ulfvarson & Alexandersson, 19901).
Three male railroad workers developed asthma after heavy exposure
to locomotive emissions (Wade & Newman, 1993). The workers, none of
whom were current smokers, had no previous history of asthma or of any
respiratory disease, except one who had seasonal rhinitis. They were
riding immediately behind the lead engines of the trains, where diesel
exhaust was blown almost continually into the cab. In two, the acute
onset of asthma occurred within the first hours of exposure.
B8.2.1.3 Pulmonary effects
In a study to investigate the effects of diesel exhaust on the
cells found in bronchoalveolar lavage (BAL) fluid (Rudell et al.,
1990), eight healthy, nonsmoking volunteers were exposed to diesel
exhaust (for 60 min according to Rudell et al., 1989) at least three
weeks after an initial BAL. The median steady-state concentrations
measured in the exhaust were 4.6mg/m3 (3.7 ppm) nitric oxide,
3.1 mg/m3 (1.6 ppm) nitrogen dioxide, 22.5mg/m3 (27 ppm) carbon
monoxide, 0.5 mg/m3 formaldehyde, and 4.3 × 105 particles/cm3;
according to Rudell et al. (1989), this particle concentration
corresponds to a mass concentration of approximately 100 µg/m3. BAL
was performed again 18 h after exposure. A significant reduction
(P < 0.02) in the total number of mast cells was observed; the number
of neutrophils was slightly but significantly (P < 0.05) increased in
comparison with the values before exposure. The ratio of
T-helper:suppressor cytotoxic cells was elevated ( P < 0.02), and the
rate of phagocytosis of opsonized yeast cells by alveolar macrophages
in vitro was reduced (P < 0.02). The number of lymphocytes remained
unchanged.
B8.2.2 Epidemiological studies (noncarcinogenic effects)
Some but not all of the results from cross-sectional and
longitudinal studies on workers with occupational exposure to low
levels of diesel engine exhaust show decrements in lung function and
an increased prevalence of respiratory symptoms.
B8.2.2.1 Effects on the respiratory system
A group of 550 underground workers and 273 surface workers in six
coal mines where diesel-powered equipment was used were matched for
smoking status, age, height, and years of underground mining to
workers at other underground coal mines where diesel units were not
used. The workers at the mines with diesel engines reported
significantly more persistent cough (23.6%) than controls (16.5%) and
more frequently had exacerbations of cough and phlegm (21.7 and 16.2%,
respectively). Pulmonary function (FVC and FEV ) was decreased in both
surface and underground workers in mines where diesel engines were
used in comparison with surface and underground miners in other mines.
There was no consistent relationship between years of underground
mining and respiratory symptoms and pulmonary function; however, the
mean time spent in underground mining was only 4.7 years. Full-shift
area samples showed concentrations of respirable dust ranging from 0.4
to 16.1 mg/m3 for various jobs; the mean values from personal
samples were 0.93-2.73 mg/m3, and the nitrogen dioxide levels in
personal samples were 0.16-0.26 mg/m3 (0.13-0.22 ppm) (Reger et al.,
1982).
Pulmonary function and respiratory symptoms were studied in 1976
in 630 miners in six potash mines where diesel-powered vehicles had
been introduced between 1950 and 1964. There was no consistent
relationship with exposure to diesel exhaust, considered in several
ways, including years of exposure, cumulative exposure to dust,
nitrogen dioxide concentration, and prevalence of diesel use in the
mine. The mean length of exposure to diesel engines was, however,
relatively short: 5-14 years across mines. In addition, cumulative
exposure was measured only to total dust rather than respirable dust.
The mean values for total dust in the mines ranged from 9 to
23 mg/m3 (personal sampling); the ratio of total dust to respirable
dust ranged from 2 to 11 (area sampling), and the nitrogen dioxide
concentration was 0.2-6.3mg/m3 (0.1-3.3 ppm) (personal sampling)
(Attfield et al., 1982).
A total of 259 miners in five salt mines where diesel equipment
was used in some of the mines were studied with regard to the number
of years worked underground and cumulative exposure to respirable
particulate and nitrogen dioxide. These parameters were associated
with phlegm after adjustment for age and smoking but not cough. The
average exposure to respirable particulate in the five mines was
0.2-0.7 mg/m3, and the average exposure to nitrogen dioxide was
0.6-4.8mg/m3 (0.3-2.5 ppm). Pulmonary function (FEV 1, FVC, peak
flow) was not related to the three parameters but was slightly reduced
in comparison with that of other blue-collar workers. In another
analysis in the same population, phlegm but not pulmonary function was
associated with years of work underground in mines where diesel
engines were used, after adjustment for age and smoking. There was a
nonsignificant trend for an association between cough and shortness of
breath with years of exposure (Gamble & Jones, 1983).
Longitudinal changes in pulmonary function and chronic
respiratory symptoms were studied between 1977 and 1982 in 280 miners
exposed to diesel exhaust and 838 miners not exposed to diesel exhaust
in American underground coal mines. The mean number of years of
underground work ranged from 6.6 to 17.4 years across mines. Area
samples taken in 1977 revealed 'low' levels of dust and diesel exhaust
constituents, which were reported not to exceed 25% of current
standards, but the actual values were not given. The exposed workers
had smaller average decrements in FVC and FEV1 than workers not
exposed to diesel exhaust after adjustment for age, smoking, and years
of underground work. Additional analysis revealed no relationship with
cumulative years of underground work, but the level of pulmonary
function was not considered as a predictor of longitudinal change. The
exposed miners also had a lower five-year incidence of cough, phlegm,
and breathlessness than the miners not exposed to exhaust (Ames et
al., 1984).
Pulmonary function and respiratory symptoms were studied in 283
male diesel bus workers in four garages in two cities. The number of
years worked was a significant predictor of lower FVC and FEV1
adjustment for age, height, race, and smoking status. In comparison
after with another blue-collar population, the bus garage workers had
a higher prevalence of cough and phlegm after adjustment for age and
smoking. The prevalence of these symptoms was not related to the
number of years worked (Gamble et al., 1987b).
B8.2.2.2 Effects on the circulatory system
No consistent excess of deaths due to cardiovascular disease has
been identified in cohort studies of workers with potential exposure
to diesel emissions. Motor vehicle examiners (Stern et al., 1981), who
are probably exposed to exhaust from a variety of vehicles, had a
slight, nonsignificant increase in deaths due to cardiovascular
disease (standardized mortality ratio [SMR] = 105). No difference in
mortality was seen among men working in potash mines with
diesel-powered equipment in comparison with men in potash mines with
no diesel engines (Waxweiler et al., 1973). In a pilot study of 129
men employed in a bus company in 1951-59 for whom mortality was
ascertained until 1978 (Edling & Axelson, 1984), a fourfold increase
in cardiovascular deaths was seen in garage workers. The increase was
calculated on the basis of at least 15 years since first exposure and
10 or more years of exposure, but was based on only four deaths. This
finding was not confirmed in a follow-up of 694 men through 1983
(Edling et al., 1987). In a study of 8490 garage maintenance workers
with at least one continuous year of service between 1967 and 1975, a
deficit of deaths due to cardiovascular disease was seen (Rushton et
al., 1983).
B8.2.3 Epidemiological studies (carcinogenic effects)
The relationship between cancers of the lung and bladder and
occupational exposure to diesel exhaust has been evaluated in a number
of epidemiological studies. The occupations examined included diesel
truck drivers, bus garage workers, railroad workers, heavy equipment
operators, and stevedores. The risk for lung cancer in relation to
exposure to diesel exhaust, either self-reported or assumed from
occupational categories, has also been evaluated in case-control
studies. Only those studies that were considered relevant for an
evaluation of the carcinogenic effects of diesel exposure are included
in this assessment.
B8.2.3.1 Lung cancer
The epidemiological studies are summarized in Tables 39-41. Table
39 is a summary of nine case-control and cohort studies of workers
exposed to diesel exhaust, Table 40 summarizes nine population- and
hospital-based studies, and Table 41 presents two studies based on
information on exposure derived from registries (surveillance
studies).
Until recently, the epidemiological study of lung cancer and
exposure to diesel exhaust was limited by failure to consider the
latency and duration of exposure necessary for the development of lung
cancer, both generally considered to be about 20 years (Schenker &
Speizer, 1979). Studies of exposed workers have also been limited by a
lack of worker- and industry-specific data on exposure. Such data were
available only for the American railroad and trucking industries. For
other occupational groups, such as bus garage workers and stevedores,
indices of exposure were derived from vehicle use, fuel consumption,
or years of exposure, or simply job title unsubstantiated by exposure
assessment. In studies based in hospitals, the general population, or
registries, information on usual work in a job with exposure to
vehicle exhaust was derived from self- or surrogate reporting;
self-reporting of exposure to diesel fumes has also been used. Studies
with short durations of exposure and follow-up are not useful for
determining whether diesel exhaust is a human carcinogen. Studies in
which there is an imprecise definition of exposure to diesel exhaust
(such as self-reported job title) do not allow detection of an effect,
since the extent of exposure is unknown. In studies in which an
elevated risk for lung cancer is noted, such imprecision can result in
wide confidence intervals.
The major potential confounding factor in occupational studies of
lung cancer is tobacco smoking. Such a factor must be related not only
to the outcome but also to the exposure. In order to assess whether
tobacco use is a true confounder in a study of occupation, the smoking
rates among individuals exposed and unexposed to diesel exhaust must
be known; however, owing to the retrospective nature of many
occupational studies, smoking histories are often not available. It is
unlikely that the smoking habits of workers exposed and unexposed to
diesel exhaust within the same occupational cohort are differentially
related to the exposure, but when the effect of exposure is small, in
the order of a relative risk of 1.5 (such has been found for diesel
exhaust and lung cancer in most studies), differences in smoking that
are unaccounted for could reduce the relative risk attributable to
exposure to diesel exhaust. Interpretation of the results of studies
lacking specific information about smoking is more uncertain when
small relative risks are seen. Despite imprecise exposure histories,
one strength of hospital- and registry-based studies of lung cancer is
that smoking histories are often available.
(a) Occupationally based cohort and case-control studies
Three studies have been conducted of railroad workers exposed to
diesel exhaust. The transition from steam- to diesel-powered
locomotives occurred in the United States during the 1950s. By 1959,
95% of the locomotives in service were diesel powered. In a
retrospective cohort study of American railroad workers, the mortality
of 55 407 white male workers aged 40-64 in 1959 who had worked on the
railroads for 10-20 years was ascertained through 1980, providing a
22-year period of follow-up (Garshick et al., 1988). A yearly
three-digit job code up to the time of death or retirement was
available from the Railroad Retirement Board. The workers had held
jobs in 39 selected categories, and an industrial hygiene survey was
conducted to categorize the job codes into those associated with
regular exposure to diesel exhaust (engineers, conductors, firemen,
brakemen, and diesel locomotive repair shop workers) and those not
exposed to diesel exhaust (clerks and signal maintainers). The
concentration of respirable particulate, adjusted for cigarette smoke,
was used as an indicator of exposure to diesel exhaust (Woskie et al.,
1988a,b). A potentially confounding exposure in the railroad industry
is asbestos, which was used to insulate the steam locomotives run
previously. Thus, workers in steam locomotive repair shops would have
been exposed to asbestos. Workers who had the longest potential
duration of exposure, i.e. those aged 40-44 in jobs with exposure to
diesel exhaust in 1959, had a relative risk of dying of lung cancer of
1.45 (95% confidence interval [CI] = 1.11-1.89). Workers aged 45-49 in
1959, who would have had slightly less exposure, had a relative risk
of 1.33 (95% CI = 1.03-1.73). Exclusion of the workers with potential
past exposure to asbestos did not appreciably change these relative
risks.
The US Environmental Protection Agency has supported an effort to
obtain more detailed information on exposure in this study in order to
estimate unit risk (Pepelko & Chen, 1993). Adequate exposure-response
curves could not be drawn for years of exposure or for measurements of
current exposure. Possible reasons include an imprecise assessment of
past exposure, since only current measurements of exposure were
available; changes in exposure over time, since improved ventilation
and new diesel locomotives have been introduced, resulting in reduced
exposure; and the lack of exposure histories for the workers who would
have been exposed to diesel exhaust before 1959. In addition, the
industrial hygiene survey was performed in four smaller railroads,
where exposure may not reflect that throughout the industry; the
presence of non-diesel particulate in the respirable samples collected
could have led to exposure misclassification. Nevertheless, the
industrial hygiene survey validated the classification of jobs into
those associated and not associated with exposure.
In a case-control study of American railroad workers by the same
investigators (Garshick et al., 1987), deaths occurring between 1
March 1981 and 28 February 1982 were recorded, and 1256 from lung
cancer were matched by age to deaths from causes other than cancer or
accidents. Smoking histories were obtained from next-of-kin. After
adjustment for smoking and past exposure to asbestos, workers aged 64
at the time of death and with 20 years of work in a job with exposure
to diesel exhaust had an odds ratio of 1.41 (95% CI = 1.06-1.88).
A cohort study addressed mortality between 1965 and 1977 among
all 43 826 male railroad workers who had retired from the Canadian
National Railway Company before 1965 but who were still alive and
those who retired between 1965 and 1977. Occupation at the time of
retirement was used to classify workers as unexposed, possibly
exposed, or probably exposed to diesel fume. The relative risk of
dying of lung cancer was 1.20 for workers with possible exposure
(P = 0.013) and 1.35 for those probably exposed (P < 0.001). Similar
relative risks were obtained after exclusion of workers involved in
locomotive repair, who were most likely to have had past exposure to
asbestos. These results are consistent with those of the studies of
American railroad workers, although the degree to which the workers
were actually exposed to diesel fumes is not known. Only retired
workers were studied, excluding younger, active workers who would have
had the most exposure. No information was given about the duration of
exposure of the retired workers to diesel exhaust since many would
have worked primarily before the transition to diesel locomotives
(Howe et al., 1983).
Table 39. Occupation-based case-control and cohort studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
43 826 male pensioners of the Exposure classified by RR = 1.20 (P = 0.013) Incomplete exposure Howe et al.
Canadian National Railway experts on occupation at and 1.35 (P = 0.001) for assessment owing to (1983)
time of retirement: possible and probable lack of lifetime occupational
unexposed, possibly exposure, respectively. history. Difficult to
exposed, probably Significant trend in RR separate combustion products
exposed with increasing likelihood from diesel exhaust,
of exposure since the cohort would
have worked in both the
steam and diesel eras;
many would have had
relatively short exposure
to diesel. No data on
smoking; method of
categorizing exposure
not validated or described
in detail
Incident deaths between Personal exposure Adjusted for smoking and Possible misclassification Garshick et al.
1 March 1981 and 28 assessed by industrial to asbestos, OR = 1.41 of exposure to diesel (1987)
February 1982 in US railroad hygiene sampling in 39 (1.06-1.88) (< 64 years of exhaust since job title might
workers. 1256 cases of job categories; yearly age) for 20 years of work not reflect exposure in each
lung cancer and 2385 matched job title used to in a diesel-exposed job. case; however, this should
controls (up to two each, on dichotomize exposure For > 20 years of work in a reduce the OR.
age and date of death). Cases into exposed and diesel-exposed job relative
and controls had worked for unexposed between 1959 to 0-4 years, OR = 1.64
the railroad for > 10 years. and retirement (1.18-2.29)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
55 407 white male railroad Industrial hygiene data RR = 1.45 (1.11-1.89) in No data on smoking Garshick et al.
workers, aged 40-64 and used to categorize jobs workers aged 40-44 in (1988)
with 10-20 years of work in as exposed or unexposed 1959; RR = 1.33 (1.03-
1959. Mortality due to lung to diesel exhaust; jobs 1.73) in workers aged 45-
cancer (1694 deaths) well categorized for 49. Unexplained by exposure
determined retrospectively most of the cohort to asbestos. Workers with
through 1980 highest potential
cumulative exposure to
diesel exhaust had highest
risk for cancer
About 20 000 male London Five job categories used SMRs for all five job Duration of work not Waller (1981)
Transport workers aged 45-64. to define exposure, categories < 100 for lung considered; impossible to
667 cases of lung cancer validated by cancer (healthy worker follow individuals after
ascertained among active environmental effect), but highest SMR in retirement; no adjustment for
workers > 25 years (1950-74) concentrations of highest exposure group, not smoking
benzo[a]pyrene and significantly greater than
particles measured least exposed (underground
in 1957 and 1979 train crew)
8490 male London Transport 100 job titles grouped SMR = 101 overall, but job No validation of exposure Rushton et al.
maintenance workers (cohort into 20 broad categories category 'general hand' by air sampling; short (1983)
mortality study). Mortality of showed increased SMR for (average, 6 years) follow-up;
workers employed for > 1 year lung cancer with no no adjustment for smoking
between 1 January 1967 apparent link to diesel
and 12 March 1975 exhaust
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
695 workers in bus garages Intensity of exposure to SMR = 122 (71-196); for No adjustment for smoking Gustavsson et
who had worked for > 6 months exhaust and asbestos increasing exposure index, al. (1990)
in 1945-70. 20 deaths from according to industrial RR = 1.34 (1.09-1.64), 1.81
lung cancer identified (nested hygiene estimate; (1.20-2.71), 2.43
case-control study) duration of exposure (1.32-4.47)
based on company records
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases among non-smokers, Walrath et al.
veterans alive in 1954 and driver) among people who had but SMR calculated from (1985)
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates
given
994 male lung cancer deaths Longest job held: diesel OR = 1.55 (0.97-2.47) for Extent of actual exposure Steenland et
in 1982-83 and 1085 controls truck driver, gasoline long-haul drivers with > 18 to diesel exhaust unknown al. (1990, 1992)
(excluding lung cancer, truck driver, both years of employment; OR =
bladder cancer, accidents). types, truck mechanic, 1.89 (1.04-3.42) for diesel
Cases and controls from stevedore. Exposure truck drivers with > 35
Teamsters Union who had filed validated by industrial years of employment
claims (requires 20-year hygiene survey (adjusted for smoking)
tenure)
Retrospective cohort study of Cohort assumed to be Lung cancer: SMR = 168 No adjustment for smoking; Gustafsson et
stevedores in Sweden first exposed on basis of job (136-207) for incidence; exposure assumptions not al. (1986)
employed before 1974 for a duties, but no formal SMR = 132 (105-166) for fully justified; no
continuous period of 6 months. assessment made mortality calculations done to explore
6071 workers employed in 1961 employment time as a surrogate
with mortality determined for exposure
through 1980. 89 cases of
lung cancer noted in Swedish
Cancer Registry (71 deaths)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
Stevedores in Swedish Estimated from use of Adjusted for smoking Crude adjustment for Emmelin et al.
population defined by fuel in each port and (yes/no) depending on smoking; no industrial (1993)
Gustafsson et al. but limited years of work since use index of exposure used; hygiene data to validate
to workers in ports for which of diesel equipment exposure-response estimates of exposure to
data data on diesel fuel relationship noted, diesel exhaust
consumption were available. relative odds for medium
Case-control study with 50 exposure ranging from 1.5
cases of lung cancer detected to 2.7 and that for high
in 1960-82 matched to 154 exposure ranging from 2.9
controls on port and date of to 6.8. Lower limit of 90%
birth confidence interval < 1,
except for one
high-exposure category
RR, relative risk; OR, crude odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless
otherwise stated
Three studies reported findings among bus garage workers. Waller
(1981) studied lung cancer occurrence among London Transport staff on
the basis of job category. Diesel buses were introduced in the 1930s,
gradually replacing gasoline-powered vehicles and, in the 1950s,
electric trolley-buses. Mortality from lung cancer was ascertained for
active workers aged 45-64 between 1950 and 1974. A lower risk was seen
for people in all job categories in comparison with the general
population of the London area, indicating a 'healthy worker effect'.
The highest SMR was seen for workers who had the most exposure to
diesel exhaust (bus garage engineers), although the SMR was not
significantly different in workers with no exposure. Although this
study showed no relationship between job category and mortality from
lung cancer, the study had significant limitations. No retired workers
were followed up, so that workers who would have had significant
exposure were excluded. Since the results were not presented on the
basis of years of work (years of exposure to diesel fumes), workers
with little exposure were grouped with those with long exposure,
making it more difficult to detect an effect of diesel exhaust.
Rushton et al. (1983) ascertained the mortality of 8490 London
Transport bus garage workers who had been employed for at least one
year between 1967 and 1975. One hundred job titles were grouped into
20 broad categories. The overall SMR for lung cancer was not elevated,
and there was no relationship with any job with exposure to diesel
exhaust; however, the SMRs were not presented on the basis of years of
work in a job with exposure, and the duration of follow-up was short,
with a mean of 5.9 years.
Using a nested case-control design, Gustavsson et al. (1990)
studied a cohort of 695 men who had worked in five bus garages in
Stockholm, Sweden, in 1945-70. Diesel-powered buses were first
introduced in Stockholm in the 1930s, and after 1945 all of the
internal combustion engines in buses were diesel-powered. No
measurements of exposure were available, so exposure to diesel exhaust
was estimated by industrial hygienists on the basis of garage
ventilation, work practices, and the number of buses in the garages
and was graded on a five-point scale, each increase corresponding to a
50% increase in intensity. An index of cumulative exposure to diesel
exhaust was calculated for each worker by multiplying the exposure
level for each work period by the duration in years. Past exposure to
asbestos was estimated on the basis of historical measurements of
asbestos fibres obtained during brake-repair operations. Twenty cases
of lung cancer were identified and matched by age to six controls. The
relative risk for lung cancer increased with increasing index of
exposure to diesel exhaust: That at the lowest exposure level was 1.34
(95% CI = 1.09-1.74), that at an intermediate level was 1.81
(95% CI = 1.20-2.71), and that at the highest level was 2.43 (95%
CI = 1.32-4.47). No such increase was observed with a similar index of
exposure to asbestos. No information was available on smoking.
Diesel-powered trucks were introduced in the United States in the
1950s and 1960s. Trucking companies had completed the transition to
diesel trucks by 1960, while independent drivers and non-trucking
companies would have completed the transition later. Steenland et al.
(1990, 1992) studied 994 deaths from lung cancer occurring in 1982 and
1983 among male members of the Teamsters Union who had filed claims
for pension benefits (requiring at least 20 years of membership). Work
histories and smoking histories were obtained from next-of-kin; job
categories were also available from the Union records. The odds ratio
for lung cancer among long-haul drivers, who drove mostly diesel
trucks, with 18 or more years of employment after 1959 was 1.55 (95%
CI = 0.97-2.47) after adjustment for age, smoking, and exposure to
asbestos. Teamsters with 35 years or more of employment whose main job
was a diesel truck driver had a relative risk for lung cancer of 1.89
(95% CI = 1.04-3.42). For drivers of gasoline trucks, the relative
risk was only 1.34 and did not achieve conventional levels of
significance (95% CI = 0.81-2.22).
In an industrial hygiene survey (Zaebst et al., 1991; Steenland
et al., 1992) that accompanied this study, measurements of elemental
carbon were used to estimate exposure to diesel exhaust. The truck
drivers appeared to have received much of their current exposure to
diesel exhaust from background levels on the road rather than directly
from their engines. No historical measurements of exposure were
available.
Gustafsson et al. (1986) studied the mortality of 6071 stevedores
in Sweden who had been employed for at least six months between 1961
and 1974 through 1980. The stevedores had been exposed to both
gasoline and diesel exhausts; diesel-powered trucks were first used in
Swedish ports in the late 1950s, with a rapid increase in use in the
1960s. The SMR for lung cancer was 132 (95% CI = 105-166). The
year-specific lung cancer rates in the stevedores between 1961 and
1980 were greater than those in the Swedish male population.
In order to refine the assessment of exposure among the
stevedores and to adjust for smoking, a case-control study was
conducted in this cohort (Emmelin et al., 1993). Cases and controls
were selected from among male stevedores who had been employed for at
least six months in 1950-74; the cases were those occurring in
1960-82. On the basis of 50 cases and 154 controls, with adjustment
for smoking (yes/no), the odds ratio for lung cancer was seen to
increase with increases in three indices of exposure: years since
diesel equipment was used in the port, estimates of cumulative fuel
consumption, and years that fuel use was above a minimal level in the
port. The increases were consistent with an exposure-response
relationship. Only one of the point estimates of the odds ratios for
lung cancer reached statistical significance.
Table 40. Population- and hospital-based studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
7518 (3539 men, 3979 Occupation determined OR = 1.52 for truck drivers Exposure estimate based on Williams
women) incident invasive at interview (P > 0.05) self-reporting; not validated et al.
cancers from the Third 47% non-response; controls (1977)
National Cancer Survey. Lung consisted of other cancers,
cancer cases: 432 in men, probably diluting risk
128 in women. Combined other estimate; few cause-specific
cancer sites used as cancers and individual
controls occupations
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases in nonsmokers, Walrath
veterans alive in 1954 and driver) among people who had but SMR calculated using et al.
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates (1985)
589 cases of lung cancer Job title from next-of-kin Adjustment for smoking; No validation of exposure Damber &
reported to the Swedish for occupations held for (0.6-2.2) for professional groupings, so actual exposure Larsson
Cancer registry 1972-77 with > 1 year drivers (> 20 years of to diesel exhaust uncertain (1987)
death before 1979; 562 employment) with dead
matched dead controls (sex, controls; OR = 1.1 (0.6-2.2)
age, year of death, with living controls
municipality) drawn from
National Registry of Causes
of Death; matched living
controls (sex, year of birth,
municipality) drawn from
National Population Registry
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Montreal, Canada, Interview with subject; Adjustment for smoking; Job title used to indicate Siemiatycki
hospital-based cases and job title translated into OR = 1.2 for squamous-cell exposure to diesel exhaust; et al.
controls; 857 cases of lung likely exposure to lung carcinoma (90% CI, not validated; exposure (1988)
cancer and 1523 controls (other combustion products by 1.0-1.5) for any exposure ill-defined
cancers than lung) industrial hygienist but not elevated for other
cell types. No relationship
with years of exposure or
intensity
461 981 male volunteers Self-reported occupation Adjustment for smoking; Exposure information Boffetta
enrolled in the American and exposure to diesel > 16 years' exposure to based on self-reporting; et al.
Cancer Society prospective exhaust diesel exhaust, OR = 1.21 not validated (1988)
mortality study in 1982; aged (0.94-1.56); for 1-15 years,
40-79 at enrolment; 2-year OR = 1.05 (0.80-1.39).
follow-up; 378 622 men with Lung cancer mortality also
known exposure to diesel elevated in miners and heavy
exhaust equipment operators
1260 cases of lung cancer Job title obtained from For motor vehicle drivers Crude classification of Benhamou
and 2084 controls matched occupational history (adjusted for smoking), exposure et al.
for age, sex, hospital obtained at interview; OR = 1.42 (1.07-1.89); for (1988)
admission in France in 1976-80 exposure based on any all transport equipment
work in a job operators, OR = 1.35
(1.05-1.75). Increase in risk
with duration of employment
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Pooled data from three Job title from interviews Smoking-adjusted OR = No validation of exposure Hayes
case-control studies with with subjects or 1.5 (1.1-1.9) for truck classification; unclear if et al.
2291 male lung cancer cases next-of-kin; years of work drivers with > 10 years of most exposure was to (1989)
and 2570 controls. Analysis in motor exhaust-related work and job history obtained diesel exhaust or if job
limited to 1444 cases and occupation calculated by direct interview. For all titles selected as 'exposed'
1893 controls who provided motor exhaust-related were actually exposed to
information at interview occupations and > 10 years diesel exhaust
of work, OR = 1.5 (1.2-1.9)
Hospital-based study of 2584 Occupational titles and Smoking-adjusted OR for Exposure histories not Boffetta
cases in 18 hospitals in six self-reporting of exposure occupations with probable validated et al.
US cities; 5099 controls exposure to diesel exhaust (1990)
= 0.95 (0.78-1.16). For
self-reported exposure,
OR = 1.21 (0.78-2.02)
Detroit, USA, area; 3792 Interview to obtain full For drivers of heavy trucks Exposure histories not Swanson
cases and 1966 controls occupational history; with > 20 years of work, validated et al.
identified through cancer analysis by job title OR = 2.5 (1.4-4.4), with (1993)
surveillance system adjustment for smoking;
exposure-response
relationship with years of
work. For drivers of light
trucks with > 20 years of
work, OR = 2.1 (0.9-4.6)
OR, odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless otherwise stated
(b) Population- and hospital-based studies
In a study of 107 563 American veterans who were alive in 1954
and were followed to the end of 1969, the SMR among smokers who
reported their occupation as truck or tractor driver was 153, based on
23 cases. Most of these individuals would have had little exposure to
diesel exhaust, however, since diesel trucks were introduced in the
United States only in the 1950s and 1960s (Walrath et al., 1985).
In the Third National Cancer Survey in the United States,
self-reporting of work as a truck driver and adjustment for smoking
resulted in an odds ratio of 1.52, which was not statistically
significant (Williams et al., 1977). In a study of 589 cases of lung
cancer reported to the Swedish Cancer Registry between 1972 and 1977
among people who had died before 1979, the odds ratio for > 20 years
of work as a professional driver was 1.2 (95% CI = 0.6-2.2) after
adjustment for smoking (Damber & Larsson, 1987). In a hospital-based
study of 857 cases of lung cancer and controls, occupational histories
obtained by interview were interpreted by an industrial hygienist.
After adjustment for smoking, the odds ratio for squamous-cell
carcinoma of the lung was 1.2 (90% CI = 1.0-1.5) in association with
any exposure to diesel exhaust (Siemiatycki et al., 1988). The
mortality of 378 622 men between 1982 and 1984 was analysed in the
American Cancer Society prospective study of cancer on the basis of
self-reporting of exposure to diesel exhaust. After adjustment for
age, smoking, and other occupational exposures, including asbestos,
coal-tar and pitch, and gasoline exhaust, the relative risk for lung
cancer for men with 16 years of exposure was 1.21 (95% CI =
0.94-1.56). For truck drivers who reported exposure to diesel exhaust,
the relative risk was 1.22 (95% CI = 0.77-1.95) (Boffetta et al.,
1988).
In a study in France, 1260 male cases of lung cancer and 2084
controls matched on age and hospital were collected between 1976 and
1980; job titles and smoking histories were obtained by interview, and
odds ratios were calculated on the basis of any work in a particular
occupation. After adjustment for smoking, the odds ratios were 1.35
(95% CI = 1.05-1.75) for transport equipment operators and 1.42 (95%
CI = 1.07-1.89) for motor vehicle drivers. There was no increase in
risk with increasing years of work in a job, but details of the
analysis were not presented (Benhamou et al., 1988).
The data from three case-control studies carried out by the
American National Cancer Institute between 1976 and 1983 were pooled
in order to study lung cancer in people with occupations associated
with motor vehicles. The analysis was limited to 1444 male patients
with lung cancer and 1893 controls who provided information on smoking
and occupation at an interview. The odds ratio for lung cancer for 10
or more years of employment in any occupation related to exhaust from
either diesel or non-diesel engines was 1.5 (95% CI = 1.2-1.9), after
adjustment for age and smoking. In truck drivers, the odds ratio was
1.5 (95% CI = 1.1-1.9) (Hayes et al., 1989).
In a case-control study of patients with lung cancer in 18
hospitals in six American cities, 2584 cases seen between 1969 and the
late 1980s were matched to at least one control on the basis of age,
sex, hospital, and year of interview. A smoking history and usual
occupation were obtained by interview. After 1985, reports of
self-reported exposure to diesel exhaust were obtained for 477 lung
cancer patients and 946 controls. Exposure was graded as 'probable' if
the individual had usually worked on railroads (the specific job was
not used) or in a variety of jobs related to motor vehicles. For these
individuals, the odds ratio for lung cancer was 1.31 (95% CI =
1.09-1.57); for those reporting usual occupation as a truck driver,
the odds ratio was 1.31 (95% CI = 1.03-1.67). After adjustment for
cigarette use, age, race, and date of interview, however, the odds
ratios for lung cancer were reduced to 0.95 (95% CI = 0.78-1.16) for
individuals with probable exposure and 0.88 (95% CI = 0.67-1.15) for
truck drivers. For self-reported exposure to diesel exhaust, the odds
ratio was 1.21, after adjustment for smoking, age, and other potential
confounding variables including exposure to asbestos, education, and
race, but did not achieve statistical significance (95% CI =
0.73-2.02) (Boffetta et al., 1990).
In a study of 3792 cases of lung cancer in men in Detroit, United
States, newly diagnosed in 1984-87, an occupational history and
information on smoking were collected by interview with the subject or
a surrogate. Controls were men with colon or rectal cancer. After
adjustment for smoking, the odds ratio for lung cancer was 2.5 (95% CI
= 1.4-4.4) among white men who had driven heavy trucks for 20 years
and 2.1 (95% CI = 0.9-4.6) for drivers of light trucks for 20 years.
There was a significant increase in the odds ratio for lung cancer
with increasing years of work for drivers of both types of truck
(Swanson et al., 1993).
(c) Registry-based, surveillance studies
Table 41 summarizes two studies in which information on exposure
was based on occupational titles obtained from censuses. No
information was available on smoking or on length of employment. Both
studies reported an elevated risk for lung cancer. Ahlberg et al.
(1981) found a relative risk of 1.33 (95% CI = 1.13-1.56) for
professional drivers, and Hansen (1993) found an SMR for all
respiratory cancer in truck drivers of 160 (95% CI = 128-198).
Table 41. Surveillance studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
Swedish census-based cancer Listing of occupation in RR = 1.33 (1.13-1.56) No validation of exposure; Ahlberg et al.
incidence registry; 154 cases census no adjustment for smoking (1981)
among professional drivers
Retrospective cohort study of Job title in census 84 cancers in truck drivers; No adjustment for smoking; Hansen (1993)
14 225 truck drivers in 1970; SMR =160 (128-198); information on specific
mortality determined until expected number based exposure or length of
1980. Referent group of persons on rates in referent employment
with no exposure to combustion population
products on basis of census
job title
RR, relative risk; SMR, standardized mortality ratio; in parentheses, 95% confidence interval
Table 42. Case-control studies of urinary bladder cancer in individuals with possible exposure to diesel exhaust
Population Exposure assessment Results Reference
Incident cases in 480 men Any exposure to diesel and Based on 15 cases and controls with discordant Howe et al.
and 152 women in three traffic fumes obtained at exposure histories, OR for exposure to diesel and (1980)
Canadian provinces, April interview traffic fumes = 2.8 (0.8-11.8); not adjusted for
1974-June 1976; each case smoking
matched by age and sex to
a neighbourhood control
Incident cases in 303 white Occupation and industry For any employment in trucking, RR = 2.2 (1.1-4.4) Silverman et al.
men in metropolitan Detroit, obtained at interview based on 28 cases and 13 controls. For any work in (1983)
USA, December 1977-November railroad and railway express services, RR = 1.9
1978; 2986 controls (0.9-3.8) based on 22 cases and 12 controls, not
selected by random-digit adjusted for smoking. For any employment as a truck
dialling but of same age driver (42 cases and 18 controls), adjusted for
distribution as cases smoking, RR = 2.1 (1.4-4.4). For > 10 years as truck
driver, smoking-adjusted RR = 5.5 (1.8-17.3), and for
truck drivers who ever drove vehicles with diesel
engines, smoking-adjusted RR = 11.9 (2.3-61.1).
Elevated risk for truck drivers with employment after
rather than before 1950, when diesel truck use became
more prevalent
Table 42 (contd)
Population Exposure assessment Results Reference
White residents of New Occupation and exposure For any employment as a truck driver (35 cases, 53 Hoar & Hoover
Hampshire and Vermont, to diesel fuel or engines controls), OR = 1.5 (0.9-2.6), not adjusted for smoking. (1985)
USA, who died of bladder by next-of-kin Those employed 1930-49 had the highest OR (2.6;
cancer 1975-79 (on death 1.3-5.1) after adjustment for coffee drinking and
certificate) matched to one smoking. For 26 cases and 39 controls who reported
control with cause of death exposure to diesel fuel or engines, OR = 1.5 (0.8-2.8),
other than suicide, matched not adjusted for smoking. Significant trend for
on state, sex, race, and age, increased relative odds up to 39 years of exposure
and a second control with to diesel exhaust
same criteria but also matched
on county. Information obtained
for 325 cases and 673 controls
Cases in 512 men in Turin, Occupation obtained at In truck drivers, based on 16 cases and 16 controls, Vineis &
Italy, matched by age to 596 interview OR = 1.2 (0.6-2.5), not adjusted for smoking Magnani (1985)
hospital controls without cancer
Cases in 194 men in 18 hospitals Usual occupation obtained; Only 16 cases in occupations with potential exposure Wynder et al.
in six US cities, January likelihood of exposure to to diesel exhaust. ORs all < 1 for warehousemen, bus (1985)
1981-May 1983. Controls diesel exhaust determined and truck drivers, and heavy equipment operators. In
were persons hospitalized at on basis of estimates of railroad workers, based on two cases and one control,
the same time as the case but percentage of workers with OR = 2. For any exposure, with adjustment for smoking,
for diseases not related to potential exposure in that OR = 0.87 (0.47-1.58). For high exposure, OR = 1.68
cigarette smoking. Each case occupation: high exposure, (0.49-5.73), not adjusted for smoking
matched to three controls > 20% of workers; moderate
(total, 582) by age, race, year exposure, 10-19% of workers
of interview, and hospital of
admission
Table 42 (contd)
Population Exposure assessment Results Reference
1909 new cases in white men Occupation obtained at After adjustment for smoking, RR for men usually Silverman
from 10 metropolitan areas in interview working as a truck driver = 1.5 (1.1-2.0), based on 99 et al. (1986)
the USA, 1977-78. Up to two cases and 123 controls. For those ever employed as
controls (total, 3569) selected truck driver, increased risk with increasing years of
by random-digit dialling and employment; however, truck drivers first employed
matched for age and < 40 years since diagnosis had no increased risk. OR
geographic area with case for bus driver as usual occupation = 1.5 (0.6-3.9),
based on 9 cases and 13 controls, adjusted for smoking
Cases in 99 men in La Plata, Occupational history Based on 20 cases ever employed as a truck or railway Iscovich et al.
Argentina, matched on age to obtained at interview driver, RR = 4.31 (P < 0.05); reduced whenr (1987)
99 hospital controls without adjusted for smoking, but no details provided
cancer and 99 neighbourhood
controls
Cases in 371 men and women Occupational history After adjustment for age and sex, RR = 1.55 (1.06-2.28) Jensen et al.
in the Danish Cancer Registry. at interview any employment as a land transport worker; based (1987)
Controls (771) selected randomly on 51 cases and 73 controls. After adjustment for
from general population smoking, age, and sex, years of work as a land transport
worker, and years of work as a bus, taxi, or truck
driver significantly associated with increased risk
Deaths in 731 men in Ohio, Yearly listing of Work as a truck driver > 20 years, OR = 12 (P < 0.01), Steenland
USA, 1960-82, identified on occupation obtained from six cases and one control; for > 20 years as a railroad et al. (1987)
death certificates and city commercial directories worker, OR = 2.21 (P < 0.01), based on 22 cases, not
matched to six controls without adjusted for smoking
tumours of the urinary tract or
pneumonia on age, year of
death, and race
Table 42 (contd)
Population Exposure assessment Results Reference
Cases in 826 men and women Occupational history and For any exposure of men to exhausts, OR adjusted for Risch et al.
diagnosed 1979-82 in exposure to 'exhausts' cigarette smoking = 1.16 (0.91-1.48). For men ever (1988)
Edmonton, Calgary, Toronto, obtained at interview exposed 8-28 years before diagnosis, OR = 1.21
and Kingston, Canada, (0.93-1.58). For men in jobs with any contact with
matched by age, sex, and diesel or traffic fumes 8-28 years before diagnosis,
area of residence to 792 adjusted for smoking, OR = 1.69 (1.24-2.31);
randomly selected population significant trend with increasing years of exposure
controls
Cases in 486 men and women Interview with subject; After adjustment for smoking, OR = 1.0 (90% CI, Siemiatycki
in Montreal, Canada, hospitals job title translated into 0.8-1.2) for any exposure to diesel exhaust, based et al. (1988)
and 2196 controls with likely exposure to on 82 cases with exposure
diseases other than lung combustion products by
or kidney cancer industrial hygienists
Cases in 136 men in 18 Usual occupation and For any exposure, OR = 1.24 (0.77-2.00), based on Iyer et al.
hospitals in six US cities. self-reporting of exposure 41 cases, with adjustment for smoking (1990)
Two controls without to diesel exhaust obtained
tobacco-related diseases matched at interview; grouped into
to each case on age (< 2 years), probable and possible
race, hospital, and year of exposure
interview
Table 42 (contd)
Population Exposure assessment Results Reference
256 cases and 287 controls in Job titles for all jobs held For any exposure to diesel exhaust, RR = 1.7 Steineck et al.
population-based study of all obtained by postal (0.9-3.3), based on 25 cases and 19 controls, adjusted (1990)
urothelial cancer in Stockholm, questionnaire; industrial for year of birth and smoking. For subjects considered
Sweden, 1985-87, among hygeienists reviewed titles to have moderate or high exposure, RR = 1.1 (0.3-4.3),
men born 1911-45 and categorized subjects as but based on only four cases and five controls
exposed to various
substances
Cases in 658 men in seven Interview with subject; job After adjustment for smoking, OR for work in road Cordier et al.
French hospitals, 1984-87, title and duties translated transport = 1.02 (0.62-1.69), based on 36 cases and (1993)
matched for age, race, and into likely exposure to 35 controls. For stevedores, OR = 1.31 (0.87-1.98);
place of residence to 658 diesel exhaust by industrial for material-handling equipment operator, OR = 7.67
controls selected randomly hygienists (0.96-61.4); and for transport equipment operators,
among patients admitted OR = 0.88 (0.62-1.26). For diesel fumes, OR = 0.99
for diagnoses other than (0.32-3.03), based on seven cases and two controls
cancer
Cases in 153 men in one Occupational history For any work as a road transport worker, Notani et al.
hospital in Bombay, India, obtained at interview smoking-adjusted OR = 1.36 (0.5-3.7), based on eight (1993)
1986-90, matched by age to cases and nine controls
212 controls with oral or
pharyngeal cancer or benign
oral disease
OR, odds ratio; RR, relative risk; in parentheses, 95% confidence interval (CI), unless otherwise stated
B8.2.3.2 Urinary bladder cancer
Fifteen case-control studies of urinary bladder cancer in
relation to presumed exposure to diesel exhaust are summarized in
Table 42. The limitation of all of these studies is that exposure to
diesel exhaust was not clearly characterized. Howe et al. (1980) used
any self-reported exposure to fumes that included diesel fumes, while
Hoar & Hoover (1985) used reports by next-of-kin of work with any
diesel fuel or engines or work as a truck driver. Risch et al. (1988)
obtained a history of exposure to exhaust by interview. Wynder et al.
(1985) estimated the likelihood of exposure to diesel exhaust during a
worker's lifetime on the basis of usual occupation, obtained at
interview, and attempted to divide exposure into high and moderate. In
a later study, self-reported exposure to diesel exhaust was recorded
(Iyer et al., 1990). Silverman et al. (1983), Vineis & Magnani (1985),
Silverman et al. (1986), Jensen et al. (1987), Iscovich et al. (1987),
and Notani et al. (1993) based their analyses of the risk for bladder
cancer on employment in an occupational category with a high
likelihood of exposure to diesel exhaust, such as truck driver,
railroad worker, transport worker, bus driver, and other related
occupations. In the studies of Siemiatycki et al. (1988) and Steineck
et al. (1990), occupational histories were reviewed by industrial
hygienists to link occupational titles to possible exposure. Cordier
et al. (1993) also used expert review of occupational histories to
estimate exposure to diesel exhaust on the basis of job title, but
additionally examined specific job titles. Furthermore, exposure was
usually assumed to have occurred if the person had ever been employed
in an occupation or had ever been exposed to diesel exhaust or other
fumes. Silverman et al. (1983, 1986) and Jensen et al. (1987) used
self-reported years of work as a truck driver. Self-reporting of
exposure poses a potential problem of differential recall among cases
and controls, as cases may recall exposure to diesel fumes or work in
a diesel-exposed job more readily than controls. Steenland et al.
(1987) used city directories listing occupation to obtain independent
occupational histories.
As the available occupational histories were crude, it was also
difficult to assess latency or duration of exposure. Silverman et al.
(1983) found that the relative risk (adjusted for smoking) was 5.5
(95% CI = 1.8-17.3) for truck drivers with 10 or more years of work
experience and 11.9 (95% CI = 2.3-61.1) for those with a history of
driving vehicles with diesel engines. Truck drivers employed after
1950, when diesel truck use became prevalent, had the highest risk. In
a later, larger study (Silverman et al., 1986), men who usually worked
as a truck driver or deliveryman had a relative risk of 1.5 (95% CI =
1.1-2.0), adjusted for smoking. The risk increased with increasing
years of work; however, truck drivers who had started work fewer than
40 years before diagnosis, who would have driven primarily diesel
engines, did not have an increased risk for bladder cancer. Hoar &
Hoover (1985) also noted increased relative odds with increasing years
of employment in jobs with reported exposure to diesel exhaust.
An additional limitation of the 15 case-control studies is that
results indicating an effect of presumed exposure to diesel exhaust or
a specific occupation are based on relatively few cases and controls,
even when conventional levels of statistical significance were
achieved. The study of Silverman et al. (1986), in which exposure was
defined as working as a truck driver or deliveryman, had the largest
number of exposed individuals (99 cases and 123 controls). Four
additional cohort studies not listed in the table (Howe et al., 1983;
Rushton et al., 1983; Schenker et al., 1984; Boffetta et al., 1988),
which were designed mainly to examine lung cancer and exposure to
diesel exhaust, also had too few cases of bladder cancer on which to
base meaningful conclusions. One cohort study of Danish bus drivers
(Netterstrom, 1988) showed an elevated SMR of 153, which was of
borderline statistical significance (95% CI = 91-217), but this result
was based on 13 cases.
Misclassification of exposure to diesel exhaust would hide any
increase in risk for bladder cancer, since many unexposed individuals
would be included with those classified as exposed. Diesel exhaust is
responsible for most of the respirable particles in mixed vehicle
exhausts, and exposure to respirable particles with adsorbed PAHs is
presumed to result ultimately in an increased risk for bladder cancer
due to metabolic transformation and urinary excretion of carcinogens.
The risk for bladder cancer is also increased in cigarette smokers and
in workers exposed to dyes containing aromatic amines (Ruder et al.,
1990). Coffee has been suggested to be a risk factor for bladder
cancer but has not been clearly implicated (Howe et al., 1980). The
classification of exposure to diesel exhaust is sufficiently crude in
the papers summarized in Table 42, however, that even when smoking was
considered, the effect of other, unmeasured confounding exposures
cannot be excluded. The actual contribution of diesel exhaust to an
increased risk of bladder cancer is uncertain, even when an effect of
presumed exposure was noted (Silverman et al., 1983; Hoar & Hoover,
1985; Silverman et al., 1986; Iscovich et al., 1987; Steenland et al.,
1987). Although the literature suggests that truck drivers have an
increased risk for bladder cancer, the specific exposure responsible
has not been determined.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Virtually no data are available relating specifically to the
effects of diesel fuel exhaust emissions.
The growth and photosynthesis of liquid cultures of the green
alga Chlamydomonas reinhardtii were examined after incubation with
iso-octane extracts of diesel particulate exhaust containing 51
compounds (mainly polynuclear aromatic ketones and pure PAHs)
identified by gas chromatographic-mass spectrometric analysis. At
concentrations up to 0.125% by volume, dose-dependent growth
retardation of the algae was observed. Higher concentrations (no
details given) caused death. The algae adapted to sublethal
concentrations of the extract over a period of several days;
thereafter, toxic extracts at concentrations up to 2.5% by volume
affected neither growth nor photosynthesis. It was noted that the
amounts of certain components decreased during incubation, suggesting
uptake into the cells (Liebe & Fock, 1992).
B10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Hundreds of chemical compounds are released during the combustion
of diesel fuel. The characteristics and amount of exhaust depend on
the type of diesel engine, its operating conditions and adjustment,
and the composition of the fuel. The following evaluation focuses
mainly on the risks to human health associated with exposure to diesel
particulate matter, since the large amount of soot particles emitted
is the main characteristic of diesel exhaust.
In general, the risk assessment paradigm proposed by the National
Academy of Sciences of the United States (US National Reserach
Council, 1983) will be followed. The four steps in this process are:
hazard identification, dose-response assessment, exposure assessment,
and risk characterization. The outcome of these individual steps is
critical for taking risk management decisions, including setting
exposure standards that have consequences for public health, and for
social, economic, and political issues.
The epidemiological studies in humans and the studies in
experimental animals considered useful for human health risk
assessment are discussed in this section. Both carcinogenic and other
effects are considered, but a threshold is assumed to exist only for
non-cancer effects. An IPCS method for deriving health-based guidance
exposure limits provides further details (IPCS, 1993).
B10.1 Exposure of the general population
The exposure of the general population to diesel exhaust varies
with proximity to diesel-powered vehicles. For example, higher levels
of diesel exhaust constituents are present in busy streets and parking
areas than in rural areas. The concentrations of the main gaseous
components resulting from diesel exhaust can be estimated from
specific emission factors, the contribution of diesel vehicles to
total traffic, and atmospheric dispersion models. As assumptions are
necessary to attribute the emissions of the gaseous components to
diesel sources, the validity of such estimates is considered to be
limited.
On a global basis, the contribution of diesel exhaust to the
total emissions of particulate matter varies widely, depending on the
percentage of diesel vehicles in the total volume of traffic,
maintenance of individual engines, fuel quality, and emission control
techniques. Such factors also lead to wide local variations in the
concentrations to which humans are exposed.
Measurements and calculations of ambient concentrations of diesel
particulates near roads provide a daily average range of 8-42 µg/m3.
The estimated annual average concentrations in Germany are 5-10 µg/m3
in urban areas and 1.5 µg/m3 in rural areas, and those in the United
States are 1-2 µg/m3 in urban areas and 0.6-1 µg/m3 in rural
areas. As the proportion of diesel vehicles is smaller in the United
States than in Europe, the concentrations in 1990, estimated from
national averages, were 2.0 µg/m3 in an urban area and 1.1 µg/m3
in a rural area, with predictions of 1.2 g/m3 for urban areas and
0.6 µg/m3 for rural areas in 1995.
Diesel particulates form part of the fine particle range
(< 2.5 µm diameter). The average concentration of this fraction of
suspended particulate matter as a whole, measured in six cities in the
United States during 1979-85, was 11-30 µg/m3 or, in terms of
inhalable particles (<10-15 µm diameter; equivalent to particulate
matter with a diameter < 10 µm [PM10]), 18-46 µg/m3 (Dockery et
al., 1993). In these locations, diesel particulates probably
represented less than 10% of the suspended particulates measured as
PM10; considerably higher proportions were found in London and other
large cities in the United Kingdom on the basis of data on emissions
(Quality of Urban Air Review Group, 1993), but few data are available
that are related directly to ambient concentrations of the diesel
component. The one experimental study that has been reported (Horvath
et al., 1985), carried out in Vienna, Austria, using chemically
labelled fuel, yielded a value for the background concentration of
diesel particulates within the city of 11 µg/m3.
B10.2 Occupational exposure
Occupational exposure to airborne diesel particulate matter has
been well determined for only two occupational groups, railroad
workers (Howe et al., 1983; Garshick et al., 1987, 1988) and workers
in the trucking industry (stevedores, local and road truck drivers,
and mechanics) (Emmelin et al., 1993). The levels of exposure during
work shifts are about 51-192 µg/m3 for railroad workers exposed to
diesel locomotive exhaust, roughly 25 µg/m3 for truck drivers, and
156 µg/m3 for stevedores (assuming that elemental carbon represented
20% of respirable particulate). These levels would be expected to vary
with the amount of ventilation available. Exposure to diesel exhaust
can also vary over a worker's lifetime, even within the same job. In
the railroad industry, exposure to diesel exhaust was greater in the
years after the introduction of diesel equipment. Reports of working
conditions in the 1950s and 1960s in diesel repair shops cited 'smoky'
atmospheres that were probably related to use of diesel equipment and
poor ventilation (Woskie et al., 1988b). No direct information is
available that allows reconstruction of accurate levels of past
exposure.
Reports of concentrations of diesel exhaust in other industries
are not accurate because of the presence of other dusts, although
attempts have been made to measure the contribution of diesel exhaust
(Daniel, 1984; Lehmann et al., 1990). Even for American railroad
workers (Woskie et al., 1988a), the contribution of other dusts could
not be determined, although cigarette smoke particulate was taken into
consideration. Consequently, the concentrations of diesel exhaust
reported in some jobs on railroads where other dusts (such as sand)
were present might have been lower.
B10.3 Non-neoplastic effects
B10.3.1 Hazard identification
B10.3.1.1 Humans
Diesel exhaust contains gases that irritate the nose, throat, and
eyes. It also contains particles which, together with the gases, can
cause airway irritation. Diesel exhaust may be recognized by its
characteristic odour. After acute exposure, it may induce mucous
membrane irritation, headache, and light-headedness. When volunteers
were exposed to diesel exhaust with an estimated particle
concentration of 100 µg/m3 for 1 h, several indicators of an
inflammatory response were observed in bronchioalveolar lavage fluid
(see section B8.2.1). In a well-conducted study on diesel-bus garage
workers, those with the highest exposures to respirable particulate
(> 0.31 mg/m3) reported significantly more cough, itchy or burning
eyes, headache, and wheeze, after adjustment for age and smoking, than
people with lower exposure. The contribution of diesel exhaust to the
respirable particulate is not known. Exposure to nitrogen dioxide at
> 0.3 ppm also resulted in more eye irritation and wheeze. Studies of
exposure indicate, however, variability in the ability of individuals
to detect the odour and the irritating effects of diesel exhaust.
Several cases of persistent asthma and asthma attacks have been
reported after acute exposure.
No consistent change in pulmonary function has been reported over
a work shift, although in one study of ferry workers, a decline in FVC
and FEV1 was noted. Specific measurements of diesel exhaust are
lacking in these studies, although total dust and respirable dust were
sometimes measured. Cases of persistent asthma have been reported in
railroad workers acutely exposed to apparently high levels of whole
exhaust, but the mechanism is unknown.
Studies of the possible chronic effects of exposure on
respiratory parameters are limited to occupational cohorts who are
still at work and thus have relatively short durations of exposure.
Such cohorts are likely to be healthier than older cohorts, which
include retirees who had longer exposure. Although excess respiratory
symptoms and reduced pulmonary function have been reported in some
studies, it is not clear whether these are long-term effects of
exposure.
B10.3.1.2 Experimental animals
Long-term studies in laboratory animals are described in section
B7.3. Many end-points were measured in the respiratory system of
several species of rodent after two or more years of exposure and at
several intermediate times. Adverse effects were seen after a
sufficient particle load had accumulated in the lung; and the
appearance of the effects is determined by both the concentration and
the duration of exposure. The results of these studies illustrate the
response of the respiratory tract in terms of biochemistry,
histopathology, cytology, pulmonary function, inflammation, and
clearance of particles. Early events in the pathogenesis of this
response include phagocytosis of inhaled particles. As the lung burden
increases, particle-filled macrophages are observed in the alveoli. At
lung burdens associated with particle overload, migration of these
macrophages out of the lung appears to be inhibited, and an early
event in the development of lung damage is the accumulation and
aggregation of clusters of particle-filled macrophages. Degeneration
of macrophages is observed, and the numbers of macrophages and
neutrophils in the alveolar and interstitial spaces increase.
Damage to the alveolar epithelium in areas surrounding these
macrophage clusters includes cell damage and proliferation.
Biochemical changes are seen in lavage fluid and lung tissue. The
alterations to particle-filled macrophages result in decreased
function and impaired particle clearance, the latter leading to a more
rapid increase in the lung burden of particles. Late effects include
inflammatory and proliferative responses, leading to fibrotic
responses. The sequence of these events has not been distinguished
clearly in experimental work; they are likely to be interrelated and
to occur, to some extent, concurrently.
The possible mechanism of action of diesel exhaust (discussed in
section B7.9) includes a role of the gas phase, as opposed to the
particle phase, in causing lung damage. The non-carcinogenic toxicity
of diesel exhaust is considered to be due to the particle content,
since no effects are seen in rodents exposed to diesel exhaust that
has been filtered to remove particulate matter. Another issue in the
dosimetry of diesel exhaust is the role of the insoluble carbon core
in relation to that of the adsorbed organic material. As discussed in
section B7.9, studies that show similarities in non-carcinogenic
responses to exposure to diesel exhaust and to carbon particles
without an organic component indicate a role of long-term retention of
the insoluble carbon core.
B10.3.2 Dose-response assessment
B10.3.2.1 Epidemiological studies
Variability in the reporting of odour and irritation indicates
that a subsegment of the population is sensitive to diesel exhaust;
however, the proportion of the population with this characteristic
cannot be estimated. Chronic effects have not been seen in
occupationally exposed people, but excess respiratory symptoms have
been reported after acute exposure. It is not possible to determine
the dose of diesel exhaust (on the basis of exposure to gas or
particles) that produces these symptoms.
B10.3.2.2 Studies in experimental animals
In order to select the pivotal study for establishing the NOAEL
or uncertainty factor, the most sensitive, relevant studies must be
identified. All of the long-term studies focus on effects on the
respiratory tract, which are clearly those most relevant. Studies of
relatively low concentrations are listed in Table 43, which gives the
target and actual exposure concentrations, the equivalent continuous
exposure of the animal, the equivalent continuous human exposure, and
the effect level. The equivalent continuous exposure of animals is
calculated by multiplying the actual exposure concentration by the
(number of hours of daily exposure/24) and by the (number of days per
week of exposure/7). This value is necessary for comparing exposure
concentrations among studies with different protocols, i.e. different
numbers of hours of exposure per day and/or days per week.
The equivalent continuous human exposure is calculated from the
particle deposition-clearance model of Yu & Yoon (1990), discussed in
section B6.2. This model is based on studies of rats since only those
studies provide data on the exposure-response relationship for
inhibition of particle clearance.
The model of Yu & Yoon (1990) is a sophisticated approach to
defining the relationship between the inhaled concentration and the
dose in lung tissue. It combines detailed models of rat and human lung
structure, aerodynamic models of the air flow in the airways, particle
deposition dynamics, and information on clearance of particles from
rat and human lung. The model is specific to diesel particles because
the characteristics of the particles used in the model are derived
from studies of diesel particles and because the information on lung
clearance is based on studies of diesel exhaust in rats. The model has
been adapted for diesel exhaust particles by allowing evaluation of
the insoluble carbon core and of the tightly and loosely bound organic
compounds separately. Values for deposition and clearance are combined
with input from experimental studies to calculate the burden of
particles in the lungs of the animals at the end of the study. The
continuous concentration that would result in the same lung burden in
humans is then calculated, as the mass of particles per unit of
alveolar surface area or unit of lung weight. This lung burden is
assumed to result in a similar effect in humans and in rats. With
these considerations, the equivalent human concentrations are
predicted on the basis of the assumption that the retained particle
mass per unit alveolar surface area is the appropriate dose measure
for extrapolation between species. The effect level used in the risk
assessment is based on an evaluation of the adversity of the effect.
For the purposes of risk characterization, the NOAEL or, if that
is not available, the LOAEL for the critical effect is related to
exposure. The critical effect is the first adverse effect that appears
when the critical concentration is reached at the target site. A
decision about whether an effect is critical is a matter of expert
judgement. An adverse effect is defined as a change in morphology,
physiology, growth, development, or the life span of an organism which
results in impairment of functional capacity or of capacity to
compensate for additional stress or an increase in susceptibility to
the harmful effects of other environmental influences (International
Union for Pure and Applied Chemistry, 1993).
The NOAEL is defined as the greatest concentration or amount of a
substance, found by experiment or observation, that causes no
alteration in morphology, functional capacity, growth, development, or
the life span of target organisms that are distinguishable from those
observed in normal (control) organisms of the same species and strain
under the same defined conditions of exposure (International Union for
Pure and Applied Chemistry, 1993).
The LOAEL is defined as the lowest concentration or amount of a
substance, found by experiment or observation, that causes an adverse
alteration in morphology, functional capacity, growth, development, or
the life span of a target organism distinguishable from those of
normal (control) organisms of the same species and strain under
defined conditions of exposure (International Union for Pure and
Applied Chemistry, 1993).
Table 43. Exposure of experimental animals, equivalent continuous human exposure, and effect levels of long-term inhalation in studies
of rats described in section B7.3
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
7 h/d, HP, LL, 0.35 0.353 0.0735 0.042 N Mauderly
5 d/week, LTB, TC, 3.5 3.47 0.723 0.360 A et al. (1987)
30 months LB 7 7.08 1.47 0.582 A
Light-duty, HP 0.1 0.11 0.063 0.038 N Ishinishi
16 h/d, 0.4 0.41 0.23 0.139 N et al. (1986,
6 d/week, 1 1.18c 0.67 0.359 A 1988)
30 months 2 2.32 1.3 0.571 A
Heavy-duty, HP 0.4 0.46 0.26 0.155 N Ishinishi
16 h/d, 1 0.96 0.55 0.303 A et al. (1986,
6 d/week, 2 1.84 1.05 0.493 A 1988)
30 months 4 3.72 2.13 0.911 A
110 h/week, HP 0.25 0.258 0.17 NA N Barnhart
6 months 0.75 0.796 0.52 NA A et al. (1981)
1.5 1.53 1.0 NA A
7 h/d, HP, SB 2 1.95 0.57 0.336 A Lewis et al.
5 d/week, (1989)
24 months
Table 43 (contd)
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
19 h/d, HP, LL, 4 4.24 2.40 1.02 A Heinrich et
5 d/week, TC, LB al. (1986a)
32 months
18 h/d, HP, LL, 0.8 0.84 0.45 0.23 A Heinrich et
5 d/week, TC, LB 2.5 2.50 1.34 0.59 A al. (1995)
30 months 7.5 6.98 3.74 1.56 A
NA, not available (guinea-pigs were used)
a HP, histopathological examination; LL, lung lavage fluid; LTB, lung tissue biochemistry; TC, tracer clearance; SB, serum biochemistry;
LB, lung burden
b N, no effect; A, adverse effect
c According to Suzuki et al. (1990), 1.08 mg/m3
The selected NOAEL is divided by uncertainty factors to account
for data gaps. For these data, uncertainty exists in two areas:
extrapolation from animals to humans and accounting for sensitive
subpopulations. The factor for accounting for sensitive subpopulations
is a default value of 10, as it is considered that there are no data
suggesting a different factor. An additional default value of 10 is
usually applied for interspecies extrapolation, which is nominally
considered to consist of 100.4 (2.5) for toxicodynamics and 100.6
(4.0) for toxicokinetics (IPCS, 1994).
The LOAELs and NOAELs are selected on the basis of the following
reasoning. The LOAEL is the lowest level at which adverse effects are
seen in studies of adequate quality. The NOAEL, the highest level at
which no effect is seen in the available studies, must be lower than
the selected LOAEL. The LOAELs and NOAELs are identified by a
comparison with the equivalent continuous human exposure level. The
LOAELs in these studies are strikingly similar, with values of 0.360,
0.359, 0.303, 0.336, and 0.23 mg/m3 in studies from the Inhalation
Toxicology Research Institute (United States; Mauderly et al., 1987),
the Health Effects Research Program on light- and heavy-duty diesel
engine exhausts (Japan; Ishinishi et al., 1986, 1988), the National
Institute for Occupational Safety and Health (United States; Lewis et
al., 1989), and the Fraunhofer Institute for Toxicology and Aerosol
Research (Germany; Heinrich et al., 1986a, 1995), respectively (see
Table 43). The consistency of these levels of effect, in view of the
diversity of end-points represented, gives a high level of confidence
in the end-points.
The study at the Inhalation Toxicology Research Institute was
selected as the most representative because it covers the greatest
variety of end-points, including histopathology, bronchiolar lavage
biochemistry and cytology, lung tissue biochemistry, and particle
clearance; LOAELs were observed for all of these end-points. The
studies of the Health Effects Research Program showed a similar LOAEL
but only for histological changes in the lung. The value for
equivalent continuous human exposure is not available from the study
carried out by the General Motors Corporation (Barnhart et al., 1981)
because the main results were for guinea-pigs; although rats were
included in the study, detailed results were not presented. The
appropriate NOAEL in these studies is not selected on the basis of the
LOAEL. As close dosing intervals were used in the studies of of the
Health Effects Research Program, the NOAEL was selected on the basis
of studies of light-duty diesel engines, as 0.139 mg/m3.
B10.3.3 Exposure assessment
Exposure to diesel exhaust is discussed in sections B10.1 and
B10.2.
B10.3.4 Risk characterization
B10.3.4.1 Humans
A quantitative assessment of the risk for humans of
non-carcinogenic effects of exposure to diesel exhaust cannot be made
on the basis of studies in humans. A substantial volume of literature
shows an association between acute exposure to fine particulates and
morbidity and mortality in the general population. It is somewhat
uncertain whether there is a direct causal link and, if so, whether it
is related to a specific component of the suspended particulates.
Attention has been focused on fractions of suspended particulate
matter, currently measured as PM10 (diameter < 10 µm) or PM2.5
(diameter < 2.5 µm), comprised mainly of diesel particulates in the
submicrometre range, which represent an increasing proportion in some
countries. There is also evidence that exposure to particulates is
involved in respiratory symptoms and in mortality from certain chronic
respiratory conditions.
B10.3.4.2 Experimental animals
Three approaches were used in assessing the risk for
non-carcinogenic effects of exposure to diesel exhaust, on the basis of
exposure (dose)-response assessment in experimental studies (see
section B10.3.2.2), and for deriving guidance values for exposure.
Approaches 1 and 2 are based on the NOAEL from studies in rats. The
difference between the two is that approach 1 involves a dosimetric
extrapolation model (Yu & Yoon, 1990) for converting the actual
concentration to which animals were exposed to an equivalent
continuous human exposure, thereby reducing the uncertainty in
interspecies extrapolation.
In approach 2, no dosimetric conversion is performed but the
usual uncertainty factors for interspecies and intraspecies
extrapolation are applied. In contrast to these NOAEL-defined
approaches, approach 3 is based on the principle of the 'benchmark
dose', in which a concentration of exposure to diesel exhaust is
derived from dose-response relationships observed in rats.
The model of Yu & Yoon (1990) takes into consideration the
specific characteristics of particle deposition and retention in rat
and human lung. Application of the model to studies of inhalation in
experimental animals is shown schematically in Figure 4. The deposited
and retained doses in the lung are calculated from the concentrations
in rats and expressed per unit lung weight or per unit alveolar
surface area. Under the assumption that the retained dose per lung
will lead to the same effect in rodent and human lung, a human
equivalent concentration can be calculated (as shown in Table 43), to
obtain an equivalent continuous human exposure. The equivalent
continuous exposure of animals shown in Table 43 was derived either by
calculating the continuous exposure at which the lung burden attained
in a study by inhalation (if measured) would have been reached, or by
calculating the lung burden in rats at the end of exposure on the
basis of the actual exposure parameters (also shown in Table 43) and
then calculating as above. The correlation between continuous exposure
in animals and humans in the model of Yu & Yoon (1990), assuming that
overload induces prolonged lung clearance, is shown in Figure 5. Use
of this approach, rather than simple adjustment of discontinuous to
continuous exposure by the c x t constant used in other assessments,
should reduce the uncertainty associated with the transformation.
Approach 1. The NOAEL of 0.41 mg/m3 from the study in rats
exposed by inhalation to light-duty engine exhaust (Ishinishi et al.,
1986, 1988; Table 43) is converted to an equivalent continuous
exposure of 0.23 mg/m3 in rats and then to an equivalent continuous
exposure of 0.139 mg/m3 , assumed to be the NOAEL in humans.
Application of a sophisticated dosimetric model decreases the
uncertainty in interspecies extrapolation from 10 to 100.4 (IPCS,
1994). Application of the usual uncertainty factor of 10 for
intraspecies differences results in a total uncertainty factor of
10 × 100.4 = 25.
The guidance value derived from this approach is
0.139 mg/m3 = 5.6 µg/m3.
---------------
25
No additional correction factor is required, resulting in a guidance
value (for the general population) of 5.6 µg/m3.
The inherent uncertainties in the dosimetric model are due to the
assumptions that must be made. A principal assumption is necessary to
estimate inhibition of clearance in humans, since data on this aspect
do not exist. It is assumed that clearance in humans is inhibited at
the same lung burden (mass per alveolar surface area) as in rats. The
other principal assumption is that the correct dose measure for lung
damage is mass of particle core per alveolar surface area. Since the
damage is localized to specific areas, another measure may be more
appropriate.
role in initiating the carcinogenic response. These mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes. There may be some as yet unidentified chemicals in diesel
exhaust with high mutagenic potential; furthermore, chemical
interactions (additive or inhibitory) may occur among the components
of diesel exhaust (Ball & Young, 1992).
B7.9.1.2 Cytotoxicity with regenerative cell proliferation
Another mechanism by which diesel exhaust may induce
carcinogenesis involves cell killing, or cytotoxicity, by the
particles or the reactive gases in exhaust, followed by regenerative
proliferation of lung cells. Enhanced cell replication may result in
an increased frequency of spontaneous mutations. Mutations could also
arise from PAH-DNA adducts or from oxidative DNA damage caused by the
reactive oxygen and nitrogen species in diesel soot, resulting in the
inflammatory response. Driscoll et al. (1994) showed that the hprt
gene mutation frequency in rat lung epithelial cells was significantly
increased when the cells were incubated in vitro with inflammatory
cells obtained by bronchoalveolar lavage from rats exposed to quartz
particles. This appears to be an important mechanism in lung tumour
induction by particles that elicit a chronic inflammatory response in
the lung. Sagai et al. (1993) showed that diesel exhaust particles
could produce superoxide anions and hydroxyl radicals in the absence
of biological activation (detection by cytochrome c and electron spin
resonance). Reactive oxygen species may induce specific DNA adducts,
such as 8-hydroxydeoxyguanosine. As mentioned above, mutations could
involve activation of oncogenes or inactivation of tumour suppressor
genes.
Several data sets cited in this monograph support this
hypothesis. For example, carbon black particles, which are virtually
devoid of PAHs but are morphologically similar to diesel soot
particles, induce lung cancer in rats. High particle burdens in rodent
lungs result in an inflammatory response (Henderson et al., 1988).
Release of cytokines and reactive oxygen and nitrogen species during
the inflammatory response to particles may result in cell death. Some
evidence exists (Wright, 1986) that diesel exhaust enhances lung cell
replication.
B7.9.1.3 Effects of particles
The importance of pulmonary particle burden on lung tumour
induction has been demonstrated clearly in long-term studies by
inhalation in rats. As reviewed above, rats have similarly increased
lung tumour incidences when exposed to diesel exhaust or carbon black
particles by inhalation for 24 months. Similarly, carbon black
particles practically devoid of PAHs induce pulmonary tumours after
intratracheal instillation. The very large surface area of carbon
black and of diesel exhaust particles after desorption of adsorbed
organic compounds in vivo may be involved mechanistically in a
tumorigenic effect. Heinrich (1994) showed that the tumour response to
different types of carbon black particles instilled intratracheally
correlated well with their respective surface areas. Pott (1991) and
Pott et al. (1993) suggested that particles with a large surface area
have greater effects in humans than in rats because insoluble
particles with no specific toxicity remain significantly longer in the
terminal airways of humans than of rats (retention half-time, about 70
days in rats and about 500 days in humans).
The correlation between particle surface area and lung tumour
incidence was examined (Oberdörster & Yu, 1990) by evaluating
published studies of inhalation of diesel and other particles. Tumour
induction in rats was best correlated with the surface area of the
particles retained in the lung rather than with the particle mass,
particle volume, or number of particles, regardless of the PAH
content. It was suggested that particle surface area and surface
properties play a decisive role ('critical surface area') and that
absorbed PAHs are not responsible for the tumour response in rats
exposed to diesel exhaust. In the human situation, however, it could
not be excluded that organic compounds and gas-phase components are
also involved, since the human particulate lung burden is much lower
than those achieved in rats after long-term inhalation.
Inhalation of diesel engine exhaust can result not only in
pulmonary tumours but also in inflammation and fibrosis and in a delay
in alveolar (not bronchial) pulmonary clearance. The mechanism by
which tumours develop due to particle overload and its associated
pathological and anatomical changes may be restricted to rats and may
not occur under environmental conditions in humans, since the lung
burdens of humans do not reach the levels that induce lung tumours in
rats. This is of importance for quantitative risk assessment. Only
occupational exposure to diesel exhaust may result in lung burdens
near or at overload conditions, particularly if the lung is already
compromised by exposure to other dusts. Bohning et al. (1982) reported
retarded particle clearance in smokers; in these people, additional
exposure to diesel exhaust may induce overload and associated toxic
effects. It is not known, however, whether the mechanisms of
particle-induced lung tumours are the same in rats and humans, and
this information is necessary for extrapolating data from rats to
humans.
Not only diesel soot (0.8, 2.5, or 7.0 mg/m3) but also carbon
black nearly completely devoid of organic compounds (Printex 90,
7.5-12 mg/m3; particle size, 10 nm) and ultrafine titanium dioxide
particles (7.5-15 mg/m3; particle size, 20 nm) caused lung tumours
in female Wistar rats exposed by inhalation for 18 h/day on five days
per week for 24 months. The tumour rate increased with increasing
particle concentrations, independently of the type of particles
inhaled. No lung tumours were observed in the rats exposed to the
lowest concentration of diesel particles. The authors concluded that
the carcinogenic component of diesel exhaust is in the inner part of
the diesel soot particle, the carbon core, and is not the relatively
small amount of carcinogenic PAHs (3.9 g benzo[ a]pyrene per gram of
diesel soot) (Heinrich et al., 1992; Heinrich, 1994; Heinrich et al.,
1995). These results were confirmed in another two-year study, in
which male and female rats were exposed by inhalation to various
concentrations of carbon black and diesel exhaust. Lung tumours were
observed with both particle types (Nikula et al., 1994).
Diesel soot does not appear to have a specific carcinogenic
effect in rats; rather, there is a nonspecific effect of particles.
B7.9.1.4 Effects of polycyclic aromatic hydrocarbons
Exposure of rats by inhalation to 2.6 mg/m3 of an aerosol of
tar-pitch condensate with no carbon core but containing 50 µg/m3
benzo[ a]pyrene and other PAHs for 10 months caused lung tumours at a
rate of 39%. The same amount of tar-pitch vapour condensed onto the
surface of carbon black particles at 2 and 6 mg/m3 resulted in
tumour rates that were roughly two times higher (89 and 72%). Since
exposure to 6 mg/m3 carbon black almost devoid of extractable
organic material caused a lung tumour rate of 18%, the tumour rate of
72% seen after combined exposure to tar-pitch vapour and carbon black
particles indicates a syncarcinogenic effect of PAHs and carbon black.
A possible mechanism is an effect of deposition of PAHs (Heinrich et
al., 1994). As the level of benzo[ a]pyrene in the coal-tar pitch was
about three orders of magnitude greater than those in diesel soot,
PAHs may play a negligible role in the tumorigenicity of diesel soot
in rats. The PAH profile in diesel soot is, however, quite different
from that in coal-tar pitch, as diesel soot contains highly mutagenic,
carcinogenic nitro-PAHs and other poorly characterized mutagens that
are not present in coal-tar pitch or on some of the carbon black
particles used in experimental studies (Heinrich et al., 1994; Nikula
et al., 1994).
In a study of various extracts of diesel exhaust particles,
30-40% of the total mutagenicity could be attributed to a group of six
nitroarenes (Salmeen et al., 1984).
A diesel exhaust particle extract was separated into a water- and
a lipid-soluble fraction, and the latter was further separated into a
PAH-free, a PAH-containing, and a polar fraction by column
chromatography. These fractions were then tested in Osborne-Mendel
rats by pulmonary implantation at doses corresponding to the
composition of the original diesel exhaust. The water-soluble fraction
did not induce tumours; the incidences induced by the lipid-soluble
fractions were 0% with the PAH-free fraction, 25% with the
PAH-containing fraction, and 0% with the polar fraction. The
PAH-containing fraction, comprising only 1% by weight of the total
extract, was shown to be responsible for the carcinogenic activity
(Grimmer et al., 1991).
Various dichloromethane extracts, each representing a complex
mixture, were obtained from particulate emissions of four
diesel-fuelled and one gasoline-fuelled automobiles (combustion), a
coke oven battery (pyrolysis), and a roofing tar pot (evaporation),
and their tumorigenic potency was compared in Sencar mice. It was
concluded that the benzo[ a]pyrene content alone could not explain
the tumorigenic activity of the mixtures (Nesnow et al., 1982a, 1983).
The lung tumour rates in rats exposed to atmospheres containing
PAHs depend not only on the PAH concentrations of the exhaust gas but
also on parameters such as the composition of the carrier particle
(mass ratio of carbon core to adsorbed layer of organics), the
dissolution rate of particle-attached organic compounds, the retention
half-time, and the cytotoxic effect of the carrier particle in the
lung (Heinrich et al., 1991).
Extracted diesel soot given intratracheally to rats induced lung
tumours, but native, unchanged diesel soot resulted in higher tumour
rates than extracted soot. Carbon black also caused tumours after
intratracheal administration, and the rate increased with decreasing
size of the primary particles (Heinrich, 1994).
Carbon black particles almost completely devoid of organic
compounds (< 0.046% extractable organic compounds and 0.6 ng/g
benzo[ a]pyrene) caused tumours in the lungs of 17% of rats after
exposure to a concentration of 6 mg/m3 for 18 h/day on five days per
week for 10 months. No lung tumours occurred in controls exposed to
clean air. Thus, the particle effect may be responsible for induction
of lung tumours in rats exposed to diesel engine exhaust, and the
effect may be related to the surface area of the carbon particle. A
PAH depot effect could lead, however, to retarded dissolution of PAHs
from the carrier, resulting in very efficient use of the extremely
small amount of carcinogenic PAHs retained in the lung after exposure
to diluted diesel engine exhaust, which contained benzo[ a]pyrene at
about 10 ng/m3 (Heinrich et al., 1991). Since diesel particles
induce DNA adducts at lower concentrations than carbon black (Bond et
al., 1989, 1990c; Wolff et al., 1990), PAHs may have tumour initiating
properties that are too weak to be detected at low doses; at higher
doses, promotional effects of the particles, perhaps with additional
initiation, may synergize to produce detectable carcinogenic effects.
B7.9.2 Non-carcinogenic effects
Diesel exhaust contains various respiratory irritants in the gas
phase and in particulate matter. Both can induce inflammatory
responses in the airways and alveolar regions of the lung. Airway
inflammation involves damage to epithelial cells, including lipid
peroxidation of cell membranes by oxidizing gaseous pollutants such as
nitrogen dioxide. Indirect effects of particles, resulting from
phagocytosis, can include the formation and release of various
mediators, including oxidants, such as superoxide anions and hydroxyl
radicals, and cytokines. These mediators may play a role in focal loss
or shortening of cilia, type II cell hypertrophy, and hyperplasia. The
latter changes can lead to the hyperplastic lesions seen in animals
exposed to diesel exahaust (Ishinishi et al., 1986, 1988; Suzuki et
al., 1990; Kato et al., 1992). Phagocytosis and subsequent clearance
of particles by alveolar macrophages can be compromised by high
particle burdens (Wolff et al., 1989; Creutzenberg et al., 1990),
which may also increase the access of particles to the interstitium,
leading to focal fibrosis (Henderson et al., 1988).
Inflammation, altered lung clearance, and hyperplastic lesions
can be considered early markers of exposure to diesel exhaust and are
the basis of the non-neoplastic effects used to determine both the
NOAEL and the 'benchmark concentration' described in section B10.1.3.
B8. EFFECTS ON HUMANS
B8.1 General population
B8.1.1 Acute exposure: olfactory, nasal, and ocular irritation
Acute exposure to diesel exhaust has been associated with
irritation of the eyes and nose. Signs and symptoms reported after
acute exposure to diesel exhaust are described in section B8.2.1.
The exhaust from e.g. poorly maintained engines or engines under
load may be visible as a black, smoky cloud. WHO (1987) considered
that sensory effects were parameters that could be used in setting
occupational exposure limits. The characteristic odour of diesel
exhaust provides a warning of its presence. At higher concentrations
and under certain operating conditions, the odour can be offensive.
The odour-causing agents are not known; however, compounds with a
relative molecular mass > 80 are probably those mainly responsible,
and aliphatic olefins (C1-C6, including acetylene), hydrocarbons
with more than six carbons, and aliphatic aldehydes are probably not
involved. Substances with low odour thresholds, such as nitrogen
dioxide (0.3 ppm) and acrolein (0.5 ppm), appear to contribute to the
odour of diesel exhaust to minor extents (5.3 and 0.25%, respectively)
(Oelert & Florian, 1972).
The ability to detect the odour and the irritating effects of
diesel exhaust vary. For example, when six subjects smelled diesel
exhaust diluted with air, the dilution factors needed to achieve the
odour detection threshold varied from 140 to 475. The concentrations
of different constituents of the exhaust at the odour threshold also
varied: formaldehyde, 0.012-0.088 ppm; acrolein, 0.011-0.046 ppm; and
nitrogen dioxide, 0.11-2.28 ppm (Linnell & Scott, 1962). When six
subjects were exposed to three concentrations of diesel exhaust for
10 min, the mean concentrations at the odour threshold were 1.3-4.2 ppm
nitrogen dioxide, 0.2-1 ppm sulfur dioxide, < 0.1 ppm formaldehyde,
and < 0.05 ppm acrolein. Three of the subjects exposed to the highest
level discontinued exposure before 10 min, while none at the lowest
level discontinued exposure although some experienced eye irritation
(Battigelli, 1965).
The health effects of inorganic gases present in diesel exhaust
are not specific and are therefore not discussed in detail; however,
it must be noted that these gases contribute to the environmental
burden.
B8.1.2 Air pollution
The concentrations of some air pollutants, such as particulates
and sulfur dioxide from the burning of coal for domestic heating and
industrial purposes, are in many parts of the world much lower than
they were several decades ago, and the associated adverse health
effects have diminished accordingly. A number of epidemiological
studies have demonstrated, however, that the relatively low remaining
levels of air pollutants are associated with a range of health
indices, including day-to-day changes in mortality (Schwartz, 1993),
visits to hospital emergency departments (Schwartz et al., 1993), and
changes in measures of lung function (Pope & Dockery, 1992). In most
studies, the associations have been strongest with the fine
particulate component of pollution, some of which may be derived from
diesel exhausts.
Vehicle traffic, both gasoline and diesel, also contributes to
the nitrogen dioxide content of urban air, and there is evidence,
primarily from experimental studies (Bauer et al., 1986; Koenig et
al., 1988; Grant et al., 1993) that it can induce short-term
decrements in lung function in asthmatic and non-asthmatic patients.
Nitrogen dioxide and volatile hydrocarbons are involved in the
formation of ozone and associated photochemical pollutants.
Associations have also been demonstrated between long-term
exposure to urban air pollutants and death rates from certain chronic
conditions. Confounding factors often make interpretation difficult,
but in an investigation conducted in the United States (Dockery et
al., 1993), air pollution attributable to fine particles was
positively associated with lung cancer and cardiopulmonary disease,
after adjustment for smoking and other relevant risk factors, but not
with other causes considered collectively.
In neither short- nor long-term studies can the possible role of
diesel particulates be specified, but diesel emissions contribute to
urban particulates, especially in Europe and developing countries. The
acidic and sulfate components of particulates, which are derived
primarily from stationary fuel-burning sources, appeared to be
implicated in a number of studies.
B8.2 Occupational exposure
B8.2.1 Effects on the respiratory system
B8.2.1.1 Symptoms
Most of the information about symptoms after acute exposure to
diesel exhaust comes from anecdotal reports of occupationally exposed
individuals. It was noted in a bus garage in London, United Kingdom,
that the buses produce a 'lachrymatory mist' when they were started up
from cold (Commins et al., 1956). In a review of 13 cases of acute
overexposure to diesel exhaust in five underground coal mines in Utah
and Colorado, United States, between 1974 and 1985, interviews in 1986
with the miners revealed that 12 had experienced symptoms of mucous
membrane irritation, headache, and light-headedness; eight had
reported nausea, and four reported a sensation of unreality and
'heartburn' (Kahn et al., 1988).
In a study designed to investigate the acute effects of diesel
exhaust on respiratory symptoms, a questionnaire was administered to
232 male workers in four diesel bus garages, and personal samples of
nitrogen dioxide and respirable particulate were obtained. After
adjustment for age and smoking (current, ex-, never), workers with the
highest exposure to respirable particulate (0.31 mg/m3) reported
significantly more cough, itchy or burning eyes, headache, difficult
or laboured breathing, a feeling of chest constriction, and wheeze
than workers with lower exposures. Those exposed to nitrogen dioxide
at >0.4 mg/m3 (> 0.3 ppm) also more frequently reported itchy or
burning eyes, difficult or laboured breathing, a feeling of chest
constriction, and wheezing. In comparison with a group of lead acid
battery workers, the reporting of the following symptoms as
'sometimes' or 'often' was significantly more prevalent among the
workers in the diesel bus garage: eyes itch, burn, or water (49.5
versus 23.5%), headache (24.2 versus 12.1%), difficult or laboured
breathing (13.5 versus 3.6%), nausea (13.5 versus 4.5%), and wheeze
(13.7 versus 4.5%) (Gamble et al., 1987a).
In comparison with 11 office workers, 17 ferry stevedores had a
greater prevalance of wheezing (24 versus 9%), chest tightness (29
versus 9%), nasal complaints (47 versus 0%), chest pain (24 versus
0%), and eye irritation (59 versus 27%); however, more of the
stevedores smoked. The concentrations of nitrogen dioxide (0.3 mg/m3)
and sulfur dioxide (0.7mg/m3) were both < 0.25 ppm; respirable
particulate was not measured (Purdham et al., 1987). In a study in an
iron ore mine where diesel engines were used underground, more workers
who were smokers reported pressure over the chest or difficulty in
getting air than the nonsmokers. No such difference was observed among
smoking and nonsmoking surface workers. The underground workers who
were smokers also had more episodes of a productive cough lasting
three weeks during several winters (Jorgensen & Svensson, 1970).
B8.2.1.2 Acute changes in pulmonary function
The pulmonary function of 60 coal miners who worked in mines
equipped with diesel engines was compared with that of 90 coal miners
not exposed to diesel exhaust. There were similar proportions of
current smokers (45% in the exposed group and 43% in the unexposed
group). Measurements made with personal dust samplers and passive
nitrogen dioxide dosimeters showed average concentrations of
0.04 mg/m3 (0.2 ppm) nitrogen dioxide, 0.4mg/m3 (0.3 ppm)
formaldehyde, and 2.0 mg/m3 respirable dust in the exposed group;
the unexposed group was exposed to 1.4 mg/m3 respirable dust. Forced
vital capacity (FVC) and forced expiratory volume in 1 sec (FEV) were
reduced during the work shift for both the diesel-exposed and
unexposed groups. The reduction was slightly but not significantly
greater in smokers than in ex- and nonsmokers. An additional analysis
with adjustment for age, exposure to respirable dust, and years of
underground mining still revealed no difference in shift-related
pulmonary function between miners exposed and unexposed to diesel
exhaust (Ames et al., 1982).
No significant shift-related change in FVC or FEV was seen among
workers on ferries transporting diesel trucks and other vehicles in
comparison with controls (Purdham et al., 1987), and no significant
change in spirometry measured over a shift was seen in 232 workers in
four diesel bus garages (Gamble et al., 1987a). Ulfvarson et al.
(1987), however, found significant decrements in FVC and FEV1 across
a shift in 23 ferry workers who had had no exposure to diesel for 10
days, with no difference between smokers and nonsmokers. The
concentration range of total particles was 0.13-1 mg/m3, that of
formaldehyde 0.04-0.6mg/m3 (< 0.03-0.5 ppm), and that of nitrogen
dioxide 0.12-4.6mg/m3 (0.06-2.3 ppm). In a repetition of the study,
a significant shift-related decrement was noted in FVC but not in
FEV1. In a later study in which filters were put on the diesel
trucks, a shift-related decrement in FVC was seen, but no subsequent
shift-related changes in FEV were noted, with or without filters
(Ulfvarson & Alexandersson, 19901).
Three male railroad workers developed asthma after heavy exposure
to locomotive emissions (Wade & Newman, 1993). The workers, none of
whom were current smokers, had no previous history of asthma or of any
respiratory disease, except one who had seasonal rhinitis. They were
riding immediately behind the lead engines of the trains, where diesel
exhaust was blown almost continually into the cab. In two, the acute
onset of asthma occurred within the first hours of exposure.
B8.2.1.3 Pulmonary effects
In a study to investigate the effects of diesel exhaust on the
cells found in bronchoalveolar lavage (BAL) fluid (Rudell et al.,
1990), eight healthy, nonsmoking volunteers were exposed to diesel
exhaust (for 60 min according to Rudell et al., 1989) at least three
weeks after an initial BAL. The median steady-state concentrations
measured in the exhaust were 4.6mg/m3 (3.7 ppm) nitric oxide,
3.1 mg/m3 (1.6 ppm) nitrogen dioxide, 22.5mg/m3 (27 ppm) carbon
monoxide, 0.5 mg/m3 formaldehyde, and 4.3 × 105 particles/cm3;
according to Rudell et al. (1989), this particle concentration
corresponds to a mass concentration of approximately 100 µg/m3. BAL
was performed again 18 h after exposure. A significant reduction
(P < 0.02) in the total number of mast cells was observed; the number
of neutrophils was slightly but significantly (P < 0.05) increased in
comparison with the values before exposure. The ratio of
T-helper:suppressor cytotoxic cells was elevated ( P < 0.02), and the
rate of phagocytosis of opsonized yeast cells by alveolar macrophages
in vitro was reduced (P < 0.02). The number of lymphocytes remained
unchanged.
B8.2.2 Epidemiological studies (noncarcinogenic effects)
Some but not all of the results from cross-sectional and
longitudinal studies on workers with occupational exposure to low
levels of diesel engine exhaust show decrements in lung function and
an increased prevalence of respiratory symptoms.
B8.2.2.1 Effects on the respiratory system
A group of 550 underground workers and 273 surface workers in six
coal mines where diesel-powered equipment was used were matched for
smoking status, age, height, and years of underground mining to
workers at other underground coal mines where diesel units were not
used. The workers at the mines with diesel engines reported
significantly more persistent cough (23.6%) than controls (16.5%) and
more frequently had exacerbations of cough and phlegm (21.7 and 16.2%,
respectively). Pulmonary function (FVC and FEV ) was decreased in both
surface and underground workers in mines where diesel engines were
used in comparison with surface and underground miners in other mines.
There was no consistent relationship between years of underground
mining and respiratory symptoms and pulmonary function; however, the
mean time spent in underground mining was only 4.7 years. Full-shift
area samples showed concentrations of respirable dust ranging from 0.4
to 16.1 mg/m3 for various jobs; the mean values from personal
samples were 0.93-2.73 mg/m3, and the nitrogen dioxide levels in
personal samples were 0.16-0.26 mg/m3 (0.13-0.22 ppm) (Reger et al.,
1982).
Pulmonary function and respiratory symptoms were studied in 1976
in 630 miners in six potash mines where diesel-powered vehicles had
been introduced between 1950 and 1964. There was no consistent
relationship with exposure to diesel exhaust, considered in several
ways, including years of exposure, cumulative exposure to dust,
nitrogen dioxide concentration, and prevalence of diesel use in the
mine. The mean length of exposure to diesel engines was, however,
relatively short: 5-14 years across mines. In addition, cumulative
exposure was measured only to total dust rather than respirable dust.
The mean values for total dust in the mines ranged from 9 to
23 mg/m3 (personal sampling); the ratio of total dust to respirable
dust ranged from 2 to 11 (area sampling), and the nitrogen dioxide
concentration was 0.2-6.3mg/m3 (0.1-3.3 ppm) (personal sampling)
(Attfield et al., 1982).
A total of 259 miners in five salt mines where diesel equipment
was used in some of the mines were studied with regard to the number
of years worked underground and cumulative exposure to respirable
particulate and nitrogen dioxide. These parameters were associated
with phlegm after adjustment for age and smoking but not cough. The
average exposure to respirable particulate in the five mines was
0.2-0.7 mg/m3, and the average exposure to nitrogen dioxide was
0.6-4.8mg/m3 (0.3-2.5 ppm). Pulmonary function (FEV 1, FVC, peak
flow) was not related to the three parameters but was slightly reduced
in comparison with that of other blue-collar workers. In another
analysis in the same population, phlegm but not pulmonary function was
associated with years of work underground in mines where diesel
engines were used, after adjustment for age and smoking. There was a
nonsignificant trend for an association between cough and shortness of
breath with years of exposure (Gamble & Jones, 1983).
Longitudinal changes in pulmonary function and chronic
respiratory symptoms were studied between 1977 and 1982 in 280 miners
exposed to diesel exhaust and 838 miners not exposed to diesel exhaust
in American underground coal mines. The mean number of years of
underground work ranged from 6.6 to 17.4 years across mines. Area
samples taken in 1977 revealed 'low' levels of dust and diesel exhaust
constituents, which were reported not to exceed 25% of current
standards, but the actual values were not given. The exposed workers
had smaller average decrements in FVC and FEV1 than workers not
exposed to diesel exhaust after adjustment for age, smoking, and years
of underground work. Additional analysis revealed no relationship with
cumulative years of underground work, but the level of pulmonary
function was not considered as a predictor of longitudinal change. The
exposed miners also had a lower five-year incidence of cough, phlegm,
and breathlessness than the miners not exposed to exhaust (Ames et
al., 1984).
Pulmonary function and respiratory symptoms were studied in 283
male diesel bus workers in four garages in two cities. The number of
years worked was a significant predictor of lower FVC and FEV1
adjustment for age, height, race, and smoking status. In comparison
after with another blue-collar population, the bus garage workers had
a higher prevalence of cough and phlegm after adjustment for age and
smoking. The prevalence of these symptoms was not related to the
number of years worked (Gamble et al., 1987b).
B8.2.2.2 Effects on the circulatory system
No consistent excess of deaths due to cardiovascular disease has
been identified in cohort studies of workers with potential exposure
to diesel emissions. Motor vehicle examiners (Stern et al., 1981), who
are probably exposed to exhaust from a variety of vehicles, had a
slight, nonsignificant increase in deaths due to cardiovascular
disease (standardized mortality ratio [SMR] = 105). No difference in
mortality was seen among men working in potash mines with
diesel-powered equipment in comparison with men in potash mines with
no diesel engines (Waxweiler et al., 1973). In a pilot study of 129
men employed in a bus company in 1951-59 for whom mortality was
ascertained until 1978 (Edling & Axelson, 1984), a fourfold increase
in cardiovascular deaths was seen in garage workers. The increase was
calculated on the basis of at least 15 years since first exposure and
10 or more years of exposure, but was based on only four deaths. This
finding was not confirmed in a follow-up of 694 men through 1983
(Edling et al., 1987). In a study of 8490 garage maintenance workers
with at least one continuous year of service between 1967 and 1975, a
deficit of deaths due to cardiovascular disease was seen (Rushton et
al., 1983).
B8.2.3 Epidemiological studies (carcinogenic effects)
The relationship between cancers of the lung and bladder and
occupational exposure to diesel exhaust has been evaluated in a number
of epidemiological studies. The occupations examined included diesel
truck drivers, bus garage workers, railroad workers, heavy equipment
operators, and stevedores. The risk for lung cancer in relation to
exposure to diesel exhaust, either self-reported or assumed from
occupational categories, has also been evaluated in case-control
studies. Only those studies that were considered relevant for an
evaluation of the carcinogenic effects of diesel exposure are included
in this assessment.
B8.2.3.1 Lung cancer
The epidemiological studies are summarized in Tables 39-41. Table
39 is a summary of nine case-control and cohort studies of workers
exposed to diesel exhaust, Table 40 summarizes nine population- and
hospital-based studies, and Table 41 presents two studies based on
information on exposure derived from registries (surveillance
studies).
Until recently, the epidemiological study of lung cancer and
exposure to diesel exhaust was limited by failure to consider the
latency and duration of exposure necessary for the development of lung
cancer, both generally considered to be about 20 years (Schenker &
Speizer, 1979). Studies of exposed workers have also been limited by a
lack of worker- and industry-specific data on exposure. Such data were
available only for the American railroad and trucking industries. For
other occupational groups, such as bus garage workers and stevedores,
indices of exposure were derived from vehicle use, fuel consumption,
or years of exposure, or simply job title unsubstantiated by exposure
assessment. In studies based in hospitals, the general population, or
registries, information on usual work in a job with exposure to
vehicle exhaust was derived from self- or surrogate reporting;
self-reporting of exposure to diesel fumes has also been used. Studies
with short durations of exposure and follow-up are not useful for
determining whether diesel exhaust is a human carcinogen. Studies in
which there is an imprecise definition of exposure to diesel exhaust
(such as self-reported job title) do not allow detection of an effect,
since the extent of exposure is unknown. In studies in which an
elevated risk for lung cancer is noted, such imprecision can result in
wide confidence intervals.
The major potential confounding factor in occupational studies of
lung cancer is tobacco smoking. Such a factor must be related not only
to the outcome but also to the exposure. In order to assess whether
tobacco use is a true confounder in a study of occupation, the smoking
rates among individuals exposed and unexposed to diesel exhaust must
be known; however, owing to the retrospective nature of many
occupational studies, smoking histories are often not available. It is
unlikely that the smoking habits of workers exposed and unexposed to
diesel exhaust within the same occupational cohort are differentially
related to the exposure, but when the effect of exposure is small, in
the order of a relative risk of 1.5 (such has been found for diesel
exhaust and lung cancer in most studies), differences in smoking that
are unaccounted for could reduce the relative risk attributable to
exposure to diesel exhaust. Interpretation of the results of studies
lacking specific information about smoking is more uncertain when
small relative risks are seen. Despite imprecise exposure histories,
one strength of hospital- and registry-based studies of lung cancer is
that smoking histories are often available.
(a) Occupationally based cohort and case-control studies
Three studies have been conducted of railroad workers exposed to
diesel exhaust. The transition from steam- to diesel-powered
locomotives occurred in the United States during the 1950s. By 1959,
95% of the locomotives in service were diesel powered. In a
retrospective cohort study of American railroad workers, the mortality
of 55 407 white male workers aged 40-64 in 1959 who had worked on the
railroads for 10-20 years was ascertained through 1980, providing a
22-year period of follow-up (Garshick et al., 1988). A yearly
three-digit job code up to the time of death or retirement was
available from the Railroad Retirement Board. The workers had held
jobs in 39 selected categories, and an industrial hygiene survey was
conducted to categorize the job codes into those associated with
regular exposure to diesel exhaust (engineers, conductors, firemen,
brakemen, and diesel locomotive repair shop workers) and those not
exposed to diesel exhaust (clerks and signal maintainers). The
concentration of respirable particulate, adjusted for cigarette smoke,
was used as an indicator of exposure to diesel exhaust (Woskie et al.,
1988a,b). A potentially confounding exposure in the railroad industry
is asbestos, which was used to insulate the steam locomotives run
previously. Thus, workers in steam locomotive repair shops would have
been exposed to asbestos. Workers who had the longest potential
duration of exposure, i.e. those aged 40-44 in jobs with exposure to
diesel exhaust in 1959, had a relative risk of dying of lung cancer of
1.45 (95% confidence interval [CI] = 1.11-1.89). Workers aged 45-49 in
1959, who would have had slightly less exposure, had a relative risk
of 1.33 (95% CI = 1.03-1.73). Exclusion of the workers with potential
past exposure to asbestos did not appreciably change these relative
risks.
The US Environmental Protection Agency has supported an effort to
obtain more detailed information on exposure in this study in order to
estimate unit risk (Pepelko & Chen, 1993). Adequate exposure-response
curves could not be drawn for years of exposure or for measurements of
current exposure. Possible reasons include an imprecise assessment of
past exposure, since only current measurements of exposure were
available; changes in exposure over time, since improved ventilation
and new diesel locomotives have been introduced, resulting in reduced
exposure; and the lack of exposure histories for the workers who would
have been exposed to diesel exhaust before 1959. In addition, the
industrial hygiene survey was performed in four smaller railroads,
where exposure may not reflect that throughout the industry; the
presence of non-diesel particulate in the respirable samples collected
could have led to exposure misclassification. Nevertheless, the
industrial hygiene survey validated the classification of jobs into
those associated and not associated with exposure.
In a case-control study of American railroad workers by the same
investigators (Garshick et al., 1987), deaths occurring between 1
March 1981 and 28 February 1982 were recorded, and 1256 from lung
cancer were matched by age to deaths from causes other than cancer or
accidents. Smoking histories were obtained from next-of-kin. After
adjustment for smoking and past exposure to asbestos, workers aged 64
at the time of death and with 20 years of work in a job with exposure
to diesel exhaust had an odds ratio of 1.41 (95% CI = 1.06-1.88).
A cohort study addressed mortality between 1965 and 1977 among
all 43 826 male railroad workers who had retired from the Canadian
National Railway Company before 1965 but who were still alive and
those who retired between 1965 and 1977. Occupation at the time of
retirement was used to classify workers as unexposed, possibly
exposed, or probably exposed to diesel fume. The relative risk of
dying of lung cancer was 1.20 for workers with possible exposure
(P = 0.013) and 1.35 for those probably exposed (P < 0.001). Similar
relative risks were obtained after exclusion of workers involved in
locomotive repair, who were most likely to have had past exposure to
asbestos. These results are consistent with those of the studies of
American railroad workers, although the degree to which the workers
were actually exposed to diesel fumes is not known. Only retired
workers were studied, excluding younger, active workers who would have
had the most exposure. No information was given about the duration of
exposure of the retired workers to diesel exhaust since many would
have worked primarily before the transition to diesel locomotives
(Howe et al., 1983).
Table 39. Occupation-based case-control and cohort studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
43 826 male pensioners of the Exposure classified by RR = 1.20 (P = 0.013) Incomplete exposure Howe et al.
Canadian National Railway experts on occupation at and 1.35 (P = 0.001) for assessment owing to (1983)
time of retirement: possible and probable lack of lifetime occupational
unexposed, possibly exposure, respectively. history. Difficult to
exposed, probably Significant trend in RR separate combustion products
exposed with increasing likelihood from diesel exhaust,
of exposure since the cohort would
have worked in both the
steam and diesel eras;
many would have had
relatively short exposure
to diesel. No data on
smoking; method of
categorizing exposure
not validated or described
in detail
Incident deaths between Personal exposure Adjusted for smoking and Possible misclassification Garshick et al.
1 March 1981 and 28 assessed by industrial to asbestos, OR = 1.41 of exposure to diesel (1987)
February 1982 in US railroad hygiene sampling in 39 (1.06-1.88) (< 64 years of exhaust since job title might
workers. 1256 cases of job categories; yearly age) for 20 years of work not reflect exposure in each
lung cancer and 2385 matched job title used to in a diesel-exposed job. case; however, this should
controls (up to two each, on dichotomize exposure For > 20 years of work in a reduce the OR.
age and date of death). Cases into exposed and diesel-exposed job relative
and controls had worked for unexposed between 1959 to 0-4 years, OR = 1.64
the railroad for > 10 years. and retirement (1.18-2.29)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
55 407 white male railroad Industrial hygiene data RR = 1.45 (1.11-1.89) in No data on smoking Garshick et al.
workers, aged 40-64 and used to categorize jobs workers aged 40-44 in (1988)
with 10-20 years of work in as exposed or unexposed 1959; RR = 1.33 (1.03-
1959. Mortality due to lung to diesel exhaust; jobs 1.73) in workers aged 45-
cancer (1694 deaths) well categorized for 49. Unexplained by exposure
determined retrospectively most of the cohort to asbestos. Workers with
through 1980 highest potential
cumulative exposure to
diesel exhaust had highest
risk for cancer
About 20 000 male London Five job categories used SMRs for all five job Duration of work not Waller (1981)
Transport workers aged 45-64. to define exposure, categories < 100 for lung considered; impossible to
667 cases of lung cancer validated by cancer (healthy worker follow individuals after
ascertained among active environmental effect), but highest SMR in retirement; no adjustment for
workers > 25 years (1950-74) concentrations of highest exposure group, not smoking
benzo[a]pyrene and significantly greater than
particles measured least exposed (underground
in 1957 and 1979 train crew)
8490 male London Transport 100 job titles grouped SMR = 101 overall, but job No validation of exposure Rushton et al.
maintenance workers (cohort into 20 broad categories category 'general hand' by air sampling; short (1983)
mortality study). Mortality of showed increased SMR for (average, 6 years) follow-up;
workers employed for > 1 year lung cancer with no no adjustment for smoking
between 1 January 1967 apparent link to diesel
and 12 March 1975 exhaust
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
695 workers in bus garages Intensity of exposure to SMR = 122 (71-196); for No adjustment for smoking Gustavsson et
who had worked for > 6 months exhaust and asbestos increasing exposure index, al. (1990)
in 1945-70. 20 deaths from according to industrial RR = 1.34 (1.09-1.64), 1.81
lung cancer identified (nested hygiene estimate; (1.20-2.71), 2.43
case-control study) duration of exposure (1.32-4.47)
based on company records
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases among non-smokers, Walrath et al.
veterans alive in 1954 and driver) among people who had but SMR calculated from (1985)
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates
given
994 male lung cancer deaths Longest job held: diesel OR = 1.55 (0.97-2.47) for Extent of actual exposure Steenland et
in 1982-83 and 1085 controls truck driver, gasoline long-haul drivers with > 18 to diesel exhaust unknown al. (1990, 1992)
(excluding lung cancer, truck driver, both years of employment; OR =
bladder cancer, accidents). types, truck mechanic, 1.89 (1.04-3.42) for diesel
Cases and controls from stevedore. Exposure truck drivers with > 35
Teamsters Union who had filed validated by industrial years of employment
claims (requires 20-year hygiene survey (adjusted for smoking)
tenure)
Retrospective cohort study of Cohort assumed to be Lung cancer: SMR = 168 No adjustment for smoking; Gustafsson et
stevedores in Sweden first exposed on basis of job (136-207) for incidence; exposure assumptions not al. (1986)
employed before 1974 for a duties, but no formal SMR = 132 (105-166) for fully justified; no
continuous period of 6 months. assessment made mortality calculations done to explore
6071 workers employed in 1961 employment time as a surrogate
with mortality determined for exposure
through 1980. 89 cases of
lung cancer noted in Swedish
Cancer Registry (71 deaths)
Table 39 (contd)
Population Exposure assessment Results Limitations Reference
Stevedores in Swedish Estimated from use of Adjusted for smoking Crude adjustment for Emmelin et al.
population defined by fuel in each port and (yes/no) depending on smoking; no industrial (1993)
Gustafsson et al. but limited years of work since use index of exposure used; hygiene data to validate
to workers in ports for which of diesel equipment exposure-response estimates of exposure to
data data on diesel fuel relationship noted, diesel exhaust
consumption were available. relative odds for medium
Case-control study with 50 exposure ranging from 1.5
cases of lung cancer detected to 2.7 and that for high
in 1960-82 matched to 154 exposure ranging from 2.9
controls on port and date of to 6.8. Lower limit of 90%
birth confidence interval < 1,
except for one
high-exposure category
RR, relative risk; OR, crude odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless
otherwise stated
Three studies reported findings among bus garage workers. Waller
(1981) studied lung cancer occurrence among London Transport staff on
the basis of job category. Diesel buses were introduced in the 1930s,
gradually replacing gasoline-powered vehicles and, in the 1950s,
electric trolley-buses. Mortality from lung cancer was ascertained for
active workers aged 45-64 between 1950 and 1974. A lower risk was seen
for people in all job categories in comparison with the general
population of the London area, indicating a 'healthy worker effect'.
The highest SMR was seen for workers who had the most exposure to
diesel exhaust (bus garage engineers), although the SMR was not
significantly different in workers with no exposure. Although this
study showed no relationship between job category and mortality from
lung cancer, the study had significant limitations. No retired workers
were followed up, so that workers who would have had significant
exposure were excluded. Since the results were not presented on the
basis of years of work (years of exposure to diesel fumes), workers
with little exposure were grouped with those with long exposure,
making it more difficult to detect an effect of diesel exhaust.
Rushton et al. (1983) ascertained the mortality of 8490 London
Transport bus garage workers who had been employed for at least one
year between 1967 and 1975. One hundred job titles were grouped into
20 broad categories. The overall SMR for lung cancer was not elevated,
and there was no relationship with any job with exposure to diesel
exhaust; however, the SMRs were not presented on the basis of years of
work in a job with exposure, and the duration of follow-up was short,
with a mean of 5.9 years.
Using a nested case-control design, Gustavsson et al. (1990)
studied a cohort of 695 men who had worked in five bus garages in
Stockholm, Sweden, in 1945-70. Diesel-powered buses were first
introduced in Stockholm in the 1930s, and after 1945 all of the
internal combustion engines in buses were diesel-powered. No
measurements of exposure were available, so exposure to diesel exhaust
was estimated by industrial hygienists on the basis of garage
ventilation, work practices, and the number of buses in the garages
and was graded on a five-point scale, each increase corresponding to a
50% increase in intensity. An index of cumulative exposure to diesel
exhaust was calculated for each worker by multiplying the exposure
level for each work period by the duration in years. Past exposure to
asbestos was estimated on the basis of historical measurements of
asbestos fibres obtained during brake-repair operations. Twenty cases
of lung cancer were identified and matched by age to six controls. The
relative risk for lung cancer increased with increasing index of
exposure to diesel exhaust: That at the lowest exposure level was 1.34
(95% CI = 1.09-1.74), that at an intermediate level was 1.81
(95% CI = 1.20-2.71), and that at the highest level was 2.43 (95%
CI = 1.32-4.47). No such increase was observed with a similar index of
exposure to asbestos. No information was available on smoking.
Diesel-powered trucks were introduced in the United States in the
1950s and 1960s. Trucking companies had completed the transition to
diesel trucks by 1960, while independent drivers and non-trucking
companies would have completed the transition later. Steenland et al.
(1990, 1992) studied 994 deaths from lung cancer occurring in 1982 and
1983 among male members of the Teamsters Union who had filed claims
for pension benefits (requiring at least 20 years of membership). Work
histories and smoking histories were obtained from next-of-kin; job
categories were also available from the Union records. The odds ratio
for lung cancer among long-haul drivers, who drove mostly diesel
trucks, with 18 or more years of employment after 1959 was 1.55 (95%
CI = 0.97-2.47) after adjustment for age, smoking, and exposure to
asbestos. Teamsters with 35 years or more of employment whose main job
was a diesel truck driver had a relative risk for lung cancer of 1.89
(95% CI = 1.04-3.42). For drivers of gasoline trucks, the relative
risk was only 1.34 and did not achieve conventional levels of
significance (95% CI = 0.81-2.22).
In an industrial hygiene survey (Zaebst et al., 1991; Steenland
et al., 1992) that accompanied this study, measurements of elemental
carbon were used to estimate exposure to diesel exhaust. The truck
drivers appeared to have received much of their current exposure to
diesel exhaust from background levels on the road rather than directly
from their engines. No historical measurements of exposure were
available.
Gustafsson et al. (1986) studied the mortality of 6071 stevedores
in Sweden who had been employed for at least six months between 1961
and 1974 through 1980. The stevedores had been exposed to both
gasoline and diesel exhausts; diesel-powered trucks were first used in
Swedish ports in the late 1950s, with a rapid increase in use in the
1960s. The SMR for lung cancer was 132 (95% CI = 105-166). The
year-specific lung cancer rates in the stevedores between 1961 and
1980 were greater than those in the Swedish male population.
In order to refine the assessment of exposure among the
stevedores and to adjust for smoking, a case-control study was
conducted in this cohort (Emmelin et al., 1993). Cases and controls
were selected from among male stevedores who had been employed for at
least six months in 1950-74; the cases were those occurring in
1960-82. On the basis of 50 cases and 154 controls, with adjustment
for smoking (yes/no), the odds ratio for lung cancer was seen to
increase with increases in three indices of exposure: years since
diesel equipment was used in the port, estimates of cumulative fuel
consumption, and years that fuel use was above a minimal level in the
port. The increases were consistent with an exposure-response
relationship. Only one of the point estimates of the odds ratios for
lung cancer reached statistical significance.
Table 40. Population- and hospital-based studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
7518 (3539 men, 3979 Occupation determined OR = 1.52 for truck drivers Exposure estimate based on Williams
women) incident invasive at interview (P > 0.05) self-reporting; not validated et al.
cancers from the Third 47% non-response; controls (1977)
National Cancer Survey. Lung consisted of other cancers,
cancer cases: 432 in men, probably diluting risk
128 in women. Combined other estimate; few cause-specific
cancer sites used as cancers and individual
controls occupations
Cohort study of 107 563 US Usual occupation (truck SMR = 153 (23 cases) No cases in nonsmokers, Walrath
veterans alive in 1954 and driver) among people who had but SMR calculated using et al.
followed-up to 1 January 1970 ever smoked; no statistics smoking-specific rates (1985)
589 cases of lung cancer Job title from next-of-kin Adjustment for smoking; No validation of exposure Damber &
reported to the Swedish for occupations held for (0.6-2.2) for professional groupings, so actual exposure Larsson
Cancer registry 1972-77 with > 1 year drivers (> 20 years of to diesel exhaust uncertain (1987)
death before 1979; 562 employment) with dead
matched dead controls (sex, controls; OR = 1.1 (0.6-2.2)
age, year of death, with living controls
municipality) drawn from
National Registry of Causes
of Death; matched living
controls (sex, year of birth,
municipality) drawn from
National Population Registry
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Montreal, Canada, Interview with subject; Adjustment for smoking; Job title used to indicate Siemiatycki
hospital-based cases and job title translated into OR = 1.2 for squamous-cell exposure to diesel exhaust; et al.
controls; 857 cases of lung likely exposure to lung carcinoma (90% CI, not validated; exposure (1988)
cancer and 1523 controls (other combustion products by 1.0-1.5) for any exposure ill-defined
cancers than lung) industrial hygienist but not elevated for other
cell types. No relationship
with years of exposure or
intensity
461 981 male volunteers Self-reported occupation Adjustment for smoking; Exposure information Boffetta
enrolled in the American and exposure to diesel > 16 years' exposure to based on self-reporting; et al.
Cancer Society prospective exhaust diesel exhaust, OR = 1.21 not validated (1988)
mortality study in 1982; aged (0.94-1.56); for 1-15 years,
40-79 at enrolment; 2-year OR = 1.05 (0.80-1.39).
follow-up; 378 622 men with Lung cancer mortality also
known exposure to diesel elevated in miners and heavy
exhaust equipment operators
1260 cases of lung cancer Job title obtained from For motor vehicle drivers Crude classification of Benhamou
and 2084 controls matched occupational history (adjusted for smoking), exposure et al.
for age, sex, hospital obtained at interview; OR = 1.42 (1.07-1.89); for (1988)
admission in France in 1976-80 exposure based on any all transport equipment
work in a job operators, OR = 1.35
(1.05-1.75). Increase in risk
with duration of employment
Table 40 (contd)
Population Exposure assessment Results Limitations Reference
Pooled data from three Job title from interviews Smoking-adjusted OR = No validation of exposure Hayes
case-control studies with with subjects or 1.5 (1.1-1.9) for truck classification; unclear if et al.
2291 male lung cancer cases next-of-kin; years of work drivers with > 10 years of most exposure was to (1989)
and 2570 controls. Analysis in motor exhaust-related work and job history obtained diesel exhaust or if job
limited to 1444 cases and occupation calculated by direct interview. For all titles selected as 'exposed'
1893 controls who provided motor exhaust-related were actually exposed to
information at interview occupations and > 10 years diesel exhaust
of work, OR = 1.5 (1.2-1.9)
Hospital-based study of 2584 Occupational titles and Smoking-adjusted OR for Exposure histories not Boffetta
cases in 18 hospitals in six self-reporting of exposure occupations with probable validated et al.
US cities; 5099 controls exposure to diesel exhaust (1990)
= 0.95 (0.78-1.16). For
self-reported exposure,
OR = 1.21 (0.78-2.02)
Detroit, USA, area; 3792 Interview to obtain full For drivers of heavy trucks Exposure histories not Swanson
cases and 1966 controls occupational history; with > 20 years of work, validated et al.
identified through cancer analysis by job title OR = 2.5 (1.4-4.4), with (1993)
surveillance system adjustment for smoking;
exposure-response
relationship with years of
work. For drivers of light
trucks with > 20 years of
work, OR = 2.1 (0.9-4.6)
OR, odds ratio; SMR, standardized mortality ratio; in parentheses, 95% confidence interval (CI), unless otherwise stated
(b) Population- and hospital-based studies
In a study of 107 563 American veterans who were alive in 1954
and were followed to the end of 1969, the SMR among smokers who
reported their occupation as truck or tractor driver was 153, based on
23 cases. Most of these individuals would have had little exposure to
diesel exhaust, however, since diesel trucks were introduced in the
United States only in the 1950s and 1960s (Walrath et al., 1985).
In the Third National Cancer Survey in the United States,
self-reporting of work as a truck driver and adjustment for smoking
resulted in an odds ratio of 1.52, which was not statistically
significant (Williams et al., 1977). In a study of 589 cases of lung
cancer reported to the Swedish Cancer Registry between 1972 and 1977
among people who had died before 1979, the odds ratio for > 20 years
of work as a professional driver was 1.2 (95% CI = 0.6-2.2) after
adjustment for smoking (Damber & Larsson, 1987). In a hospital-based
study of 857 cases of lung cancer and controls, occupational histories
obtained by interview were interpreted by an industrial hygienist.
After adjustment for smoking, the odds ratio for squamous-cell
carcinoma of the lung was 1.2 (90% CI = 1.0-1.5) in association with
any exposure to diesel exhaust (Siemiatycki et al., 1988). The
mortality of 378 622 men between 1982 and 1984 was analysed in the
American Cancer Society prospective study of cancer on the basis of
self-reporting of exposure to diesel exhaust. After adjustment for
age, smoking, and other occupational exposures, including asbestos,
coal-tar and pitch, and gasoline exhaust, the relative risk for lung
cancer for men with 16 years of exposure was 1.21 (95% CI =
0.94-1.56). For truck drivers who reported exposure to diesel exhaust,
the relative risk was 1.22 (95% CI = 0.77-1.95) (Boffetta et al.,
1988).
In a study in France, 1260 male cases of lung cancer and 2084
controls matched on age and hospital were collected between 1976 and
1980; job titles and smoking histories were obtained by interview, and
odds ratios were calculated on the basis of any work in a particular
occupation. After adjustment for smoking, the odds ratios were 1.35
(95% CI = 1.05-1.75) for transport equipment operators and 1.42 (95%
CI = 1.07-1.89) for motor vehicle drivers. There was no increase in
risk with increasing years of work in a job, but details of the
analysis were not presented (Benhamou et al., 1988).
The data from three case-control studies carried out by the
American National Cancer Institute between 1976 and 1983 were pooled
in order to study lung cancer in people with occupations associated
with motor vehicles. The analysis was limited to 1444 male patients
with lung cancer and 1893 controls who provided information on smoking
and occupation at an interview. The odds ratio for lung cancer for 10
or more years of employment in any occupation related to exhaust from
either diesel or non-diesel engines was 1.5 (95% CI = 1.2-1.9), after
adjustment for age and smoking. In truck drivers, the odds ratio was
1.5 (95% CI = 1.1-1.9) (Hayes et al., 1989).
In a case-control study of patients with lung cancer in 18
hospitals in six American cities, 2584 cases seen between 1969 and the
late 1980s were matched to at least one control on the basis of age,
sex, hospital, and year of interview. A smoking history and usual
occupation were obtained by interview. After 1985, reports of
self-reported exposure to diesel exhaust were obtained for 477 lung
cancer patients and 946 controls. Exposure was graded as 'probable' if
the individual had usually worked on railroads (the specific job was
not used) or in a variety of jobs related to motor vehicles. For these
individuals, the odds ratio for lung cancer was 1.31 (95% CI =
1.09-1.57); for those reporting usual occupation as a truck driver,
the odds ratio was 1.31 (95% CI = 1.03-1.67). After adjustment for
cigarette use, age, race, and date of interview, however, the odds
ratios for lung cancer were reduced to 0.95 (95% CI = 0.78-1.16) for
individuals with probable exposure and 0.88 (95% CI = 0.67-1.15) for
truck drivers. For self-reported exposure to diesel exhaust, the odds
ratio was 1.21, after adjustment for smoking, age, and other potential
confounding variables including exposure to asbestos, education, and
race, but did not achieve statistical significance (95% CI =
0.73-2.02) (Boffetta et al., 1990).
In a study of 3792 cases of lung cancer in men in Detroit, United
States, newly diagnosed in 1984-87, an occupational history and
information on smoking were collected by interview with the subject or
a surrogate. Controls were men with colon or rectal cancer. After
adjustment for smoking, the odds ratio for lung cancer was 2.5 (95% CI
= 1.4-4.4) among white men who had driven heavy trucks for 20 years
and 2.1 (95% CI = 0.9-4.6) for drivers of light trucks for 20 years.
There was a significant increase in the odds ratio for lung cancer
with increasing years of work for drivers of both types of truck
(Swanson et al., 1993).
(c) Registry-based, surveillance studies
Table 41 summarizes two studies in which information on exposure
was based on occupational titles obtained from censuses. No
information was available on smoking or on length of employment. Both
studies reported an elevated risk for lung cancer. Ahlberg et al.
(1981) found a relative risk of 1.33 (95% CI = 1.13-1.56) for
professional drivers, and Hansen (1993) found an SMR for all
respiratory cancer in truck drivers of 160 (95% CI = 128-198).
Table 41. Surveillance studies of lung cancer in groups exposed to diesel engine exhaust
Population Exposure assessment Results Limitations Reference
Swedish census-based cancer Listing of occupation in RR = 1.33 (1.13-1.56) No validation of exposure; Ahlberg et al.
incidence registry; 154 cases census no adjustment for smoking (1981)
among professional drivers
Retrospective cohort study of Job title in census 84 cancers in truck drivers; No adjustment for smoking; Hansen (1993)
14 225 truck drivers in 1970; SMR =160 (128-198); information on specific
mortality determined until expected number based exposure or length of
1980. Referent group of persons on rates in referent employment
with no exposure to combustion population
products on basis of census
job title
RR, relative risk; SMR, standardized mortality ratio; in parentheses, 95% confidence interval
Table 42. Case-control studies of urinary bladder cancer in individuals with possible exposure to diesel exhaust
Population Exposure assessment Results Reference
Incident cases in 480 men Any exposure to diesel and Based on 15 cases and controls with discordant Howe et al.
and 152 women in three traffic fumes obtained at exposure histories, OR for exposure to diesel and (1980)
Canadian provinces, April interview traffic fumes = 2.8 (0.8-11.8); not adjusted for
1974-June 1976; each case smoking
matched by age and sex to
a neighbourhood control
Incident cases in 303 white Occupation and industry For any employment in trucking, RR = 2.2 (1.1-4.4) Silverman et al.
men in metropolitan Detroit, obtained at interview based on 28 cases and 13 controls. For any work in (1983)
USA, December 1977-November railroad and railway express services, RR = 1.9
1978; 2986 controls (0.9-3.8) based on 22 cases and 12 controls, not
selected by random-digit adjusted for smoking. For any employment as a truck
dialling but of same age driver (42 cases and 18 controls), adjusted for
distribution as cases smoking, RR = 2.1 (1.4-4.4). For > 10 years as truck
driver, smoking-adjusted RR = 5.5 (1.8-17.3), and for
truck drivers who ever drove vehicles with diesel
engines, smoking-adjusted RR = 11.9 (2.3-61.1).
Elevated risk for truck drivers with employment after
rather than before 1950, when diesel truck use became
more prevalent
Table 42 (contd)
Population Exposure assessment Results Reference
White residents of New Occupation and exposure For any employment as a truck driver (35 cases, 53 Hoar & Hoover
Hampshire and Vermont, to diesel fuel or engines controls), OR = 1.5 (0.9-2.6), not adjusted for smoking. (1985)
USA, who died of bladder by next-of-kin Those employed 1930-49 had the highest OR (2.6;
cancer 1975-79 (on death 1.3-5.1) after adjustment for coffee drinking and
certificate) matched to one smoking. For 26 cases and 39 controls who reported
control with cause of death exposure to diesel fuel or engines, OR = 1.5 (0.8-2.8),
other than suicide, matched not adjusted for smoking. Significant trend for
on state, sex, race, and age, increased relative odds up to 39 years of exposure
and a second control with to diesel exhaust
same criteria but also matched
on county. Information obtained
for 325 cases and 673 controls
Cases in 512 men in Turin, Occupation obtained at In truck drivers, based on 16 cases and 16 controls, Vineis &
Italy, matched by age to 596 interview OR = 1.2 (0.6-2.5), not adjusted for smoking Magnani (1985)
hospital controls without cancer
Cases in 194 men in 18 hospitals Usual occupation obtained; Only 16 cases in occupations with potential exposure Wynder et al.
in six US cities, January likelihood of exposure to to diesel exhaust. ORs all < 1 for warehousemen, bus (1985)
1981-May 1983. Controls diesel exhaust determined and truck drivers, and heavy equipment operators. In
were persons hospitalized at on basis of estimates of railroad workers, based on two cases and one control,
the same time as the case but percentage of workers with OR = 2. For any exposure, with adjustment for smoking,
for diseases not related to potential exposure in that OR = 0.87 (0.47-1.58). For high exposure, OR = 1.68
cigarette smoking. Each case occupation: high exposure, (0.49-5.73), not adjusted for smoking
matched to three controls > 20% of workers; moderate
(total, 582) by age, race, year exposure, 10-19% of workers
of interview, and hospital of
admission
Table 42 (contd)
Population Exposure assessment Results Reference
1909 new cases in white men Occupation obtained at After adjustment for smoking, RR for men usually Silverman
from 10 metropolitan areas in interview working as a truck driver = 1.5 (1.1-2.0), based on 99 et al. (1986)
the USA, 1977-78. Up to two cases and 123 controls. For those ever employed as
controls (total, 3569) selected truck driver, increased risk with increasing years of
by random-digit dialling and employment; however, truck drivers first employed
matched for age and < 40 years since diagnosis had no increased risk. OR
geographic area with case for bus driver as usual occupation = 1.5 (0.6-3.9),
based on 9 cases and 13 controls, adjusted for smoking
Cases in 99 men in La Plata, Occupational history Based on 20 cases ever employed as a truck or railway Iscovich et al.
Argentina, matched on age to obtained at interview driver, RR = 4.31 (P < 0.05); reduced whenr (1987)
99 hospital controls without adjusted for smoking, but no details provided
cancer and 99 neighbourhood
controls
Cases in 371 men and women Occupational history After adjustment for age and sex, RR = 1.55 (1.06-2.28) Jensen et al.
in the Danish Cancer Registry. at interview any employment as a land transport worker; based (1987)
Controls (771) selected randomly on 51 cases and 73 controls. After adjustment for
from general population smoking, age, and sex, years of work as a land transport
worker, and years of work as a bus, taxi, or truck
driver significantly associated with increased risk
Deaths in 731 men in Ohio, Yearly listing of Work as a truck driver > 20 years, OR = 12 (P < 0.01), Steenland
USA, 1960-82, identified on occupation obtained from six cases and one control; for > 20 years as a railroad et al. (1987)
death certificates and city commercial directories worker, OR = 2.21 (P < 0.01), based on 22 cases, not
matched to six controls without adjusted for smoking
tumours of the urinary tract or
pneumonia on age, year of
death, and race
Table 42 (contd)
Population Exposure assessment Results Reference
Cases in 826 men and women Occupational history and For any exposure of men to exhausts, OR adjusted for Risch et al.
diagnosed 1979-82 in exposure to 'exhausts' cigarette smoking = 1.16 (0.91-1.48). For men ever (1988)
Edmonton, Calgary, Toronto, obtained at interview exposed 8-28 years before diagnosis, OR = 1.21
and Kingston, Canada, (0.93-1.58). For men in jobs with any contact with
matched by age, sex, and diesel or traffic fumes 8-28 years before diagnosis,
area of residence to 792 adjusted for smoking, OR = 1.69 (1.24-2.31);
randomly selected population significant trend with increasing years of exposure
controls
Cases in 486 men and women Interview with subject; After adjustment for smoking, OR = 1.0 (90% CI, Siemiatycki
in Montreal, Canada, hospitals job title translated into 0.8-1.2) for any exposure to diesel exhaust, based et al. (1988)
and 2196 controls with likely exposure to on 82 cases with exposure
diseases other than lung combustion products by
or kidney cancer industrial hygienists
Cases in 136 men in 18 Usual occupation and For any exposure, OR = 1.24 (0.77-2.00), based on Iyer et al.
hospitals in six US cities. self-reporting of exposure 41 cases, with adjustment for smoking (1990)
Two controls without to diesel exhaust obtained
tobacco-related diseases matched at interview; grouped into
to each case on age (< 2 years), probable and possible
race, hospital, and year of exposure
interview
Table 42 (contd)
Population Exposure assessment Results Reference
256 cases and 287 controls in Job titles for all jobs held For any exposure to diesel exhaust, RR = 1.7 Steineck et al.
population-based study of all obtained by postal (0.9-3.3), based on 25 cases and 19 controls, adjusted (1990)
urothelial cancer in Stockholm, questionnaire; industrial for year of birth and smoking. For subjects considered
Sweden, 1985-87, among hygeienists reviewed titles to have moderate or high exposure, RR = 1.1 (0.3-4.3),
men born 1911-45 and categorized subjects as but based on only four cases and five controls
exposed to various
substances
Cases in 658 men in seven Interview with subject; job After adjustment for smoking, OR for work in road Cordier et al.
French hospitals, 1984-87, title and duties translated transport = 1.02 (0.62-1.69), based on 36 cases and (1993)
matched for age, race, and into likely exposure to 35 controls. For stevedores, OR = 1.31 (0.87-1.98);
place of residence to 658 diesel exhaust by industrial for material-handling equipment operator, OR = 7.67
controls selected randomly hygienists (0.96-61.4); and for transport equipment operators,
among patients admitted OR = 0.88 (0.62-1.26). For diesel fumes, OR = 0.99
for diagnoses other than (0.32-3.03), based on seven cases and two controls
cancer
Cases in 153 men in one Occupational history For any work as a road transport worker, Notani et al.
hospital in Bombay, India, obtained at interview smoking-adjusted OR = 1.36 (0.5-3.7), based on eight (1993)
1986-90, matched by age to cases and nine controls
212 controls with oral or
pharyngeal cancer or benign
oral disease
OR, odds ratio; RR, relative risk; in parentheses, 95% confidence interval (CI), unless otherwise stated
B8.2.3.2 Urinary bladder cancer
Fifteen case-control studies of urinary bladder cancer in
relation to presumed exposure to diesel exhaust are summarized in
Table 42. The limitation of all of these studies is that exposure to
diesel exhaust was not clearly characterized. Howe et al. (1980) used
any self-reported exposure to fumes that included diesel fumes, while
Hoar & Hoover (1985) used reports by next-of-kin of work with any
diesel fuel or engines or work as a truck driver. Risch et al. (1988)
obtained a history of exposure to exhaust by interview. Wynder et al.
(1985) estimated the likelihood of exposure to diesel exhaust during a
worker's lifetime on the basis of usual occupation, obtained at
interview, and attempted to divide exposure into high and moderate. In
a later study, self-reported exposure to diesel exhaust was recorded
(Iyer et al., 1990). Silverman et al. (1983), Vineis & Magnani (1985),
Silverman et al. (1986), Jensen et al. (1987), Iscovich et al. (1987),
and Notani et al. (1993) based their analyses of the risk for bladder
cancer on employment in an occupational category with a high
likelihood of exposure to diesel exhaust, such as truck driver,
railroad worker, transport worker, bus driver, and other related
occupations. In the studies of Siemiatycki et al. (1988) and Steineck
et al. (1990), occupational histories were reviewed by industrial
hygienists to link occupational titles to possible exposure. Cordier
et al. (1993) also used expert review of occupational histories to
estimate exposure to diesel exhaust on the basis of job title, but
additionally examined specific job titles. Furthermore, exposure was
usually assumed to have occurred if the person had ever been employed
in an occupation or had ever been exposed to diesel exhaust or other
fumes. Silverman et al. (1983, 1986) and Jensen et al. (1987) used
self-reported years of work as a truck driver. Self-reporting of
exposure poses a potential problem of differential recall among cases
and controls, as cases may recall exposure to diesel fumes or work in
a diesel-exposed job more readily than controls. Steenland et al.
(1987) used city directories listing occupation to obtain independent
occupational histories.
As the available occupational histories were crude, it was also
difficult to assess latency or duration of exposure. Silverman et al.
(1983) found that the relative risk (adjusted for smoking) was 5.5
(95% CI = 1.8-17.3) for truck drivers with 10 or more years of work
experience and 11.9 (95% CI = 2.3-61.1) for those with a history of
driving vehicles with diesel engines. Truck drivers employed after
1950, when diesel truck use became prevalent, had the highest risk. In
a later, larger study (Silverman et al., 1986), men who usually worked
as a truck driver or deliveryman had a relative risk of 1.5 (95% CI =
1.1-2.0), adjusted for smoking. The risk increased with increasing
years of work; however, truck drivers who had started work fewer than
40 years before diagnosis, who would have driven primarily diesel
engines, did not have an increased risk for bladder cancer. Hoar &
Hoover (1985) also noted increased relative odds with increasing years
of employment in jobs with reported exposure to diesel exhaust.
An additional limitation of the 15 case-control studies is that
results indicating an effect of presumed exposure to diesel exhaust or
a specific occupation are based on relatively few cases and controls,
even when conventional levels of statistical significance were
achieved. The study of Silverman et al. (1986), in which exposure was
defined as working as a truck driver or deliveryman, had the largest
number of exposed individuals (99 cases and 123 controls). Four
additional cohort studies not listed in the table (Howe et al., 1983;
Rushton et al., 1983; Schenker et al., 1984; Boffetta et al., 1988),
which were designed mainly to examine lung cancer and exposure to
diesel exhaust, also had too few cases of bladder cancer on which to
base meaningful conclusions. One cohort study of Danish bus drivers
(Netterstrom, 1988) showed an elevated SMR of 153, which was of
borderline statistical significance (95% CI = 91-217), but this result
was based on 13 cases.
Misclassification of exposure to diesel exhaust would hide any
increase in risk for bladder cancer, since many unexposed individuals
would be included with those classified as exposed. Diesel exhaust is
responsible for most of the respirable particles in mixed vehicle
exhausts, and exposure to respirable particles with adsorbed PAHs is
presumed to result ultimately in an increased risk for bladder cancer
due to metabolic transformation and urinary excretion of carcinogens.
The risk for bladder cancer is also increased in cigarette smokers and
in workers exposed to dyes containing aromatic amines (Ruder et al.,
1990). Coffee has been suggested to be a risk factor for bladder
cancer but has not been clearly implicated (Howe et al., 1980). The
classification of exposure to diesel exhaust is sufficiently crude in
the papers summarized in Table 42, however, that even when smoking was
considered, the effect of other, unmeasured confounding exposures
cannot be excluded. The actual contribution of diesel exhaust to an
increased risk of bladder cancer is uncertain, even when an effect of
presumed exposure was noted (Silverman et al., 1983; Hoar & Hoover,
1985; Silverman et al., 1986; Iscovich et al., 1987; Steenland et al.,
1987). Although the literature suggests that truck drivers have an
increased risk for bladder cancer, the specific exposure responsible
has not been determined.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Virtually no data are available relating specifically to the
effects of diesel fuel exhaust emissions.
The growth and photosynthesis of liquid cultures of the green
alga Chlamydomonas reinhardtii were examined after incubation with
iso-octane extracts of diesel particulate exhaust containing 51
compounds (mainly polynuclear aromatic ketones and pure PAHs)
identified by gas chromatographic-mass spectrometric analysis. At
concentrations up to 0.125% by volume, dose-dependent growth
retardation of the algae was observed. Higher concentrations (no
details given) caused death. The algae adapted to sublethal
concentrations of the extract over a period of several days;
thereafter, toxic extracts at concentrations up to 2.5% by volume
affected neither growth nor photosynthesis. It was noted that the
amounts of certain components decreased during incubation, suggesting
uptake into the cells (Liebe & Fock, 1992).
B10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Hundreds of chemical compounds are released during the combustion
of diesel fuel. The characteristics and amount of exhaust depend on
the type of diesel engine, its operating conditions and adjustment,
and the composition of the fuel. The following evaluation focuses
mainly on the risks to human health associated with exposure to diesel
particulate matter, since the large amount of soot particles emitted
is the main characteristic of diesel exhaust.
In general, the risk assessment paradigm proposed by the National
Academy of Sciences of the United States (US National Reserach
Council, 1983) will be followed. The four steps in this process are:
hazard identification, dose-response assessment, exposure assessment,
and risk characterization. The outcome of these individual steps is
critical for taking risk management decisions, including setting
exposure standards that have consequences for public health, and for
social, economic, and political issues.
The epidemiological studies in humans and the studies in
experimental animals considered useful for human health risk
assessment are discussed in this section. Both carcinogenic and other
effects are considered, but a threshold is assumed to exist only for
non-cancer effects. An IPCS method for deriving health-based guidance
exposure limits provides further details (IPCS, 1993).
B10.1 Exposure of the general population
The exposure of the general population to diesel exhaust varies
with proximity to diesel-powered vehicles. For example, higher levels
of diesel exhaust constituents are present in busy streets and parking
areas than in rural areas. The concentrations of the main gaseous
components resulting from diesel exhaust can be estimated from
specific emission factors, the contribution of diesel vehicles to
total traffic, and atmospheric dispersion models. As assumptions are
necessary to attribute the emissions of the gaseous components to
diesel sources, the validity of such estimates is considered to be
limited.
On a global basis, the contribution of diesel exhaust to the
total emissions of particulate matter varies widely, depending on the
percentage of diesel vehicles in the total volume of traffic,
maintenance of individual engines, fuel quality, and emission control
techniques. Such factors also lead to wide local variations in the
concentrations to which humans are exposed.
Measurements and calculations of ambient concentrations of diesel
particulates near roads provide a daily average range of 8-42 µg/m3.
The estimated annual average concentrations in Germany are 5-10 µg/m3
in urban areas and 1.5 µg/m3 in rural areas, and those in the United
States are 1-2 µg/m3 in urban areas and 0.6-1 µg/m3 in rural
areas. As the proportion of diesel vehicles is smaller in the United
States than in Europe, the concentrations in 1990, estimated from
national averages, were 2.0 µg/m3 in an urban area and 1.1 µg/m3
in a rural area, with predictions of 1.2 g/m3 for urban areas and
0.6 µg/m3 for rural areas in 1995.
Diesel particulates form part of the fine particle range
(< 2.5 µm diameter). The average concentration of this fraction of
suspended particulate matter as a whole, measured in six cities in the
United States during 1979-85, was 11-30 µg/m3 or, in terms of
inhalable particles (<10-15 µm diameter; equivalent to particulate
matter with a diameter < 10 µm [PM10]), 18-46 µg/m3 (Dockery et
al., 1993). In these locations, diesel particulates probably
represented less than 10% of the suspended particulates measured as
PM10; considerably higher proportions were found in London and other
large cities in the United Kingdom on the basis of data on emissions
(Quality of Urban Air Review Group, 1993), but few data are available
that are related directly to ambient concentrations of the diesel
component. The one experimental study that has been reported (Horvath
et al., 1985), carried out in Vienna, Austria, using chemically
labelled fuel, yielded a value for the background concentration of
diesel particulates within the city of 11 µg/m3.
B10.2 Occupational exposure
Occupational exposure to airborne diesel particulate matter has
been well determined for only two occupational groups, railroad
workers (Howe et al., 1983; Garshick et al., 1987, 1988) and workers
in the trucking industry (stevedores, local and road truck drivers,
and mechanics) (Emmelin et al., 1993). The levels of exposure during
work shifts are about 51-192 µg/m3 for railroad workers exposed to
diesel locomotive exhaust, roughly 25 µg/m3 for truck drivers, and
156 µg/m3 for stevedores (assuming that elemental carbon represented
20% of respirable particulate). These levels would be expected to vary
with the amount of ventilation available. Exposure to diesel exhaust
can also vary over a worker's lifetime, even within the same job. In
the railroad industry, exposure to diesel exhaust was greater in the
years after the introduction of diesel equipment. Reports of working
conditions in the 1950s and 1960s in diesel repair shops cited 'smoky'
atmospheres that were probably related to use of diesel equipment and
poor ventilation (Woskie et al., 1988b). No direct information is
available that allows reconstruction of accurate levels of past
exposure.
Reports of concentrations of diesel exhaust in other industries
are not accurate because of the presence of other dusts, although
attempts have been made to measure the contribution of diesel exhaust
(Daniel, 1984; Lehmann et al., 1990). Even for American railroad
workers (Woskie et al., 1988a), the contribution of other dusts could
not be determined, although cigarette smoke particulate was taken into
consideration. Consequently, the concentrations of diesel exhaust
reported in some jobs on railroads where other dusts (such as sand)
were present might have been lower.
B10.3 Non-neoplastic effects
B10.3.1 Hazard identification
B10.3.1.1 Humans
Diesel exhaust contains gases that irritate the nose, throat, and
eyes. It also contains particles which, together with the gases, can
cause airway irritation. Diesel exhaust may be recognized by its
characteristic odour. After acute exposure, it may induce mucous
membrane irritation, headache, and light-headedness. When volunteers
were exposed to diesel exhaust with an estimated particle
concentration of 100 µg/m3 for 1 h, several indicators of an
inflammatory response were observed in bronchioalveolar lavage fluid
(see section B8.2.1). In a well-conducted study on diesel-bus garage
workers, those with the highest exposures to respirable particulate
(> 0.31 mg/m3) reported significantly more cough, itchy or burning
eyes, headache, and wheeze, after adjustment for age and smoking, than
people with lower exposure. The contribution of diesel exhaust to the
respirable particulate is not known. Exposure to nitrogen dioxide at
> 0.3 ppm also resulted in more eye irritation and wheeze. Studies of
exposure indicate, however, variability in the ability of individuals
to detect the odour and the irritating effects of diesel exhaust.
Several cases of persistent asthma and asthma attacks have been
reported after acute exposure.
No consistent change in pulmonary function has been reported over
a work shift, although in one study of ferry workers, a decline in FVC
and FEV1 was noted. Specific measurements of diesel exhaust are
lacking in these studies, although total dust and respirable dust were
sometimes measured. Cases of persistent asthma have been reported in
railroad workers acutely exposed to apparently high levels of whole
exhaust, but the mechanism is unknown.
Studies of the possible chronic effects of exposure on
respiratory parameters are limited to occupational cohorts who are
still at work and thus have relatively short durations of exposure.
Such cohorts are likely to be healthier than older cohorts, which
include retirees who had longer exposure. Although excess respiratory
symptoms and reduced pulmonary function have been reported in some
studies, it is not clear whether these are long-term effects of
exposure.
B10.3.1.2 Experimental animals
Long-term studies in laboratory animals are described in section
B7.3. Many end-points were measured in the respiratory system of
several species of rodent after two or more years of exposure and at
several intermediate times. Adverse effects were seen after a
sufficient particle load had accumulated in the lung; and the
appearance of the effects is determined by both the concentration and
the duration of exposure. The results of these studies illustrate the
response of the respiratory tract in terms of biochemistry,
histopathology, cytology, pulmonary function, inflammation, and
clearance of particles. Early events in the pathogenesis of this
response include phagocytosis of inhaled particles. As the lung burden
increases, particle-filled macrophages are observed in the alveoli. At
lung burdens associated with particle overload, migration of these
macrophages out of the lung appears to be inhibited, and an early
event in the development of lung damage is the accumulation and
aggregation of clusters of particle-filled macrophages. Degeneration
of macrophages is observed, and the numbers of macrophages and
neutrophils in the alveolar and interstitial spaces increase.
Damage to the alveolar epithelium in areas surrounding these
macrophage clusters includes cell damage and proliferation.
Biochemical changes are seen in lavage fluid and lung tissue. The
alterations to particle-filled macrophages result in decreased
function and impaired particle clearance, the latter leading to a more
rapid increase in the lung burden of particles. Late effects include
inflammatory and proliferative responses, leading to fibrotic
responses. The sequence of these events has not been distinguished
clearly in experimental work; they are likely to be interrelated and
to occur, to some extent, concurrently.
The possible mechanism of action of diesel exhaust (discussed in
section B7.9) includes a role of the gas phase, as opposed to the
particle phase, in causing lung damage. The non-carcinogenic toxicity
of diesel exhaust is considered to be due to the particle content,
since no effects are seen in rodents exposed to diesel exhaust that
has been filtered to remove particulate matter. Another issue in the
dosimetry of diesel exhaust is the role of the insoluble carbon core
in relation to that of the adsorbed organic material. As discussed in
section B7.9, studies that show similarities in non-carcinogenic
responses to exposure to diesel exhaust and to carbon particles
without an organic component indicate a role of long-term retention of
the insoluble carbon core.
B10.3.2 Dose-response assessment
B10.3.2.1 Epidemiological studies
Variability in the reporting of odour and irritation indicates
that a subsegment of the population is sensitive to diesel exhaust;
however, the proportion of the population with this characteristic
cannot be estimated. Chronic effects have not been seen in
occupationally exposed people, but excess respiratory symptoms have
been reported after acute exposure. It is not possible to determine
the dose of diesel exhaust (on the basis of exposure to gas or
particles) that produces these symptoms.
B10.3.2.2 Studies in experimental animals
In order to select the pivotal study for establishing the NOAEL
or uncertainty factor, the most sensitive, relevant studies must be
identified. All of the long-term studies focus on effects on the
respiratory tract, which are clearly those most relevant. Studies of
relatively low concentrations are listed in Table 43, which gives the
target and actual exposure concentrations, the equivalent continuous
exposure of the animal, the equivalent continuous human exposure, and
the effect level. The equivalent continuous exposure of animals is
calculated by multiplying the actual exposure concentration by the
(number of hours of daily exposure/24) and by the (number of days per
week of exposure/7). This value is necessary for comparing exposure
concentrations among studies with different protocols, i.e. different
numbers of hours of exposure per day and/or days per week.
The equivalent continuous human exposure is calculated from the
particle deposition-clearance model of Yu & Yoon (1990), discussed in
section B6.2. This model is based on studies of rats since only those
studies provide data on the exposure-response relationship for
inhibition of particle clearance.
The model of Yu & Yoon (1990) is a sophisticated approach to
defining the relationship between the inhaled concentration and the
dose in lung tissue. It combines detailed models of rat and human lung
structure, aerodynamic models of the air flow in the airways, particle
deposition dynamics, and information on clearance of particles from
rat and human lung. The model is specific to diesel particles because
the characteristics of the particles used in the model are derived
from studies of diesel particles and because the information on lung
clearance is based on studies of diesel exhaust in rats. The model has
been adapted for diesel exhaust particles by allowing evaluation of
the insoluble carbon core and of the tightly and loosely bound organic
compounds separately. Values for deposition and clearance are combined
with input from experimental studies to calculate the burden of
particles in the lungs of the animals at the end of the study. The
continuous concentration that would result in the same lung burden in
humans is then calculated, as the mass of particles per unit of
alveolar surface area or unit of lung weight. This lung burden is
assumed to result in a similar effect in humans and in rats. With
these considerations, the equivalent human concentrations are
predicted on the basis of the assumption that the retained particle
mass per unit alveolar surface area is the appropriate dose measure
for extrapolation between species. The effect level used in the risk
assessment is based on an evaluation of the adversity of the effect.
For the purposes of risk characterization, the NOAEL or, if that
is not available, the LOAEL for the critical effect is related to
exposure. The critical effect is the first adverse effect that appears
when the critical concentration is reached at the target site. A
decision about whether an effect is critical is a matter of expert
judgement. An adverse effect is defined as a change in morphology,
physiology, growth, development, or the life span of an organism which
results in impairment of functional capacity or of capacity to
compensate for additional stress or an increase in susceptibility to
the harmful effects of other environmental influences (International
Union for Pure and Applied Chemistry, 1993).
The NOAEL is defined as the greatest concentration or amount of a
substance, found by experiment or observation, that causes no
alteration in morphology, functional capacity, growth, development, or
the life span of target organisms that are distinguishable from those
observed in normal (control) organisms of the same species and strain
under the same defined conditions of exposure (International Union for
Pure and Applied Chemistry, 1993).
The LOAEL is defined as the lowest concentration or amount of a
substance, found by experiment or observation, that causes an adverse
alteration in morphology, functional capacity, growth, development, or
the life span of a target organism distinguishable from those of
normal (control) organisms of the same species and strain under
defined conditions of exposure (International Union for Pure and
Applied Chemistry, 1993).
Table 43. Exposure of experimental animals, equivalent continuous human exposure, and effect levels of long-term inhalation in studies
of rats described in section B7.3
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
7 h/d, HP, LL, 0.35 0.353 0.0735 0.042 N Mauderly
5 d/week, LTB, TC, 3.5 3.47 0.723 0.360 A et al. (1987)
30 months LB 7 7.08 1.47 0.582 A
Light-duty, HP 0.1 0.11 0.063 0.038 N Ishinishi
16 h/d, 0.4 0.41 0.23 0.139 N et al. (1986,
6 d/week, 1 1.18c 0.67 0.359 A 1988)
30 months 2 2.32 1.3 0.571 A
Heavy-duty, HP 0.4 0.46 0.26 0.155 N Ishinishi
16 h/d, 1 0.96 0.55 0.303 A et al. (1986,
6 d/week, 2 1.84 1.05 0.493 A 1988)
30 months 4 3.72 2.13 0.911 A
110 h/week, HP 0.25 0.258 0.17 NA N Barnhart
6 months 0.75 0.796 0.52 NA A et al. (1981)
1.5 1.53 1.0 NA A
7 h/d, HP, SB 2 1.95 0.57 0.336 A Lewis et al.
5 d/week, (1989)
24 months
Table 43 (contd)
Length of End-points Target Actual Equivalent Equivalent Effect Study
exposure evaluateda exposure exposure continuous continuous levelb
(mg/m3) (mg/m3) animal exposure human exposure
(mg/m3) (mg/m3)
19 h/d, HP, LL, 4 4.24 2.40 1.02 A Heinrich et
5 d/week, TC, LB al. (1986a)
32 months
18 h/d, HP, LL, 0.8 0.84 0.45 0.23 A Heinrich et
5 d/week, TC, LB 2.5 2.50 1.34 0.59 A al. (1995)
30 months 7.5 6.98 3.74 1.56 A
NA, not available (guinea-pigs were used)
a HP, histopathological examination; LL, lung lavage fluid; LTB, lung tissue biochemistry; TC, tracer clearance; SB, serum biochemistry;
LB, lung burden
b N, no effect; A, adverse effect
c According to Suzuki et al. (1990), 1.08 mg/m3
The selected NOAEL is divided by uncertainty factors to account
for data gaps. For these data, uncertainty exists in two areas:
extrapolation from animals to humans and accounting for sensitive
subpopulations. The factor for accounting for sensitive subpopulations
is a default value of 10, as it is considered that there are no data
suggesting a different factor. An additional default value of 10 is
usually applied for interspecies extrapolation, which is nominally
considered to consist of 100.4 (2.5) for toxicodynamics and 100.6
(4.0) for toxicokinetics (IPCS, 1994).
The LOAELs and NOAELs are selected on the basis of the following
reasoning. The LOAEL is the lowest level at which adverse effects are
seen in studies of adequate quality. The NOAEL, the highest level at
which no effect is seen in the available studies, must be lower than
the selected LOAEL. The LOAELs and NOAELs are identified by a
comparison with the equivalent continuous human exposure level. The
LOAELs in these studies are strikingly similar, with values of 0.360,
0.359, 0.303, 0.336, and 0.23 mg/m3 in studies from the Inhalation
Toxicology Research Institute (United States; Mauderly et al., 1987),
the Health Effects Research Program on light- and heavy-duty diesel
engine exhausts (Japan; Ishinishi et al., 1986, 1988), the National
Institute for Occupational Safety and Health (United States; Lewis et
al., 1989), and the Fraunhofer Institute for Toxicology and Aerosol
Research (Germany; Heinrich et al., 1986a, 1995), respectively (see
Table 43). The consistency of these levels of effect, in view of the
diversity of end-points represented, gives a high level of confidence
in the end-points.
The study at the Inhalation Toxicology Research Institute was
selected as the most representative because it covers the greatest
variety of end-points, including histopathology, bronchiolar lavage
biochemistry and cytology, lung tissue biochemistry, and particle
clearance; LOAELs were observed for all of these end-points. The
studies of the Health Effects Research Program showed a similar LOAEL
but only for histological changes in the lung. The value for
equivalent continuous human exposure is not available from the study
carried out by the General Motors Corporation (Barnhart et al., 1981)
because the main results were for guinea-pigs; although rats were
included in the study, detailed results were not presented. The
appropriate NOAEL in these studies is not selected on the basis of the
LOAEL. As close dosing intervals were used in the studies of of the
Health Effects Research Program, the NOAEL was selected on the basis
of studies of light-duty diesel engines, as 0.139 mg/m3.
B10.3.3 Exposure assessment
Exposure to diesel exhaust is discussed in sections B10.1 and
B10.2.
B10.3.4 Risk characterization
B10.3.4.1 Humans
A quantitative assessment of the risk for humans of
non-carcinogenic effects of exposure to diesel exhaust cannot be made
on the basis of studies in humans. A substantial volume of literature
shows an association between acute exposure to fine particulates and
morbidity and mortality in the general population. It is somewhat
uncertain whether there is a direct causal link and, if so, whether it
is related to a specific component of the suspended particulates.
Attention has been focused on fractions of suspended particulate
matter, currently measured as PM10 (diameter < 10 µm) or PM2.5
(diameter < 2.5 µm), comprised mainly of diesel particulates in the
submicrometre range, which represent an increasing proportion in some
countries. There is also evidence that exposure to particulates is
involved in respiratory symptoms and in mortality from certain chronic
respiratory conditions.
B10.3.4.2 Experimental animals
Three approaches were used in assessing the risk for
non-carcinogenic effects of exposure to diesel exhaust, on the basis of
exposure (dose)-response assessment in experimental studies (see
section B10.3.2.2), and for deriving guidance values for exposure.
Approaches 1 and 2 are based on the NOAEL from studies in rats. The
difference between the two is that approach 1 involves a dosimetric
extrapolation model (Yu & Yoon, 1990) for converting the actual
concentration to which animals were exposed to an equivalent
continuous human exposure, thereby reducing the uncertainty in
interspecies extrapolation.
In approach 2, no dosimetric conversion is performed but the
usual uncertainty factors for interspecies and intraspecies
extrapolation are applied. In contrast to these NOAEL-defined
approaches, approach 3 is based on the principle of the 'benchmark
dose', in which a concentration of exposure to diesel exhaust is
derived from dose-response relationships observed in rats.
The model of Yu & Yoon (1990) takes into consideration the
specific characteristics of particle deposition and retention in rat
and human lung. Application of the model to studies of inhalation in
experimental animals is shown schematically in Figure 4. The deposited
and retained doses in the lung are calculated from the concentrations
in rats and expressed per unit lung weight or per unit alveolar
surface area. Under the assumption that the retained dose per lung
will lead to the same effect in rodent and human lung, a human
equivalent concentration can be calculated (as shown in Table 43), to
obtain an equivalent continuous human exposure. The equivalent
continuous exposure of animals shown in Table 43 was derived either by
calculating the continuous exposure at which the lung burden attained
in a study by inhalation (if measured) would have been reached, or by
calculating the lung burden in rats at the end of exposure on the
basis of the actual exposure parameters (also shown in Table 43) and
then calculating as above. The correlation between continuous exposure
in animals and humans in the model of Yu & Yoon (1990), assuming that
overload induces prolonged lung clearance, is shown in Figure 5. Use
of this approach, rather than simple adjustment of discontinuous to
continuous exposure by the c x t constant used in other assessments,
should reduce the uncertainty associated with the transformation.
Approach 1. The NOAEL of 0.41 mg/m3 from the study in rats
exposed by inhalation to light-duty engine exhaust (Ishinishi et al.,
1986, 1988; Table 43) is converted to an equivalent continuous
exposure of 0.23 mg/m3 in rats and then to an equivalent continuous
exposure of 0.139 mg/m3 , assumed to be the NOAEL in humans.
Application of a sophisticated dosimetric model decreases the
uncertainty in interspecies extrapolation from 10 to 100.4 (IPCS,
1994). Application of the usual uncertainty factor of 10 for
intraspecies differences results in a total uncertainty factor of
10 × 100.4 = 25.
The guidance value derived from this approach is
0.139 mg/m3 = 5.6 µg/m3.
---------------
25
No additional correction factor is required, resulting in a guidance
value (for the general population) of 5.6 µg/m3.
The inherent uncertainties in the dosimetric model are due to the
assumptions that must be made. A principal assumption is necessary to
estimate inhibition of clearance in humans, since data on this aspect
do not exist. It is assumed that clearance in humans is inhibited at
the same lung burden (mass per alveolar surface area) as in rats. The
other principal assumption is that the correct dose measure for lung
damage is mass of particle core per alveolar surface area. Since the
damage is localized to specific areas, another measure may be more
appropriate.
 Approach 2. In this approach, the equivalent continuous animal
exposure based on the NOAEL of 0.41 in rats (light-duty diesel
exhaust; Ishinishi et al., 1986, 1988; Table 47) is used to derive the
guideline value. Because the dosimetric model is not used, the default
value of 10 is applied for interspecies uncertainty and an uncertainty
factor of 10 is added for intraspecies differences. The guidance value
obtained with this approach is
0.23 mg/m3
------------- = 2.3 µg/m3
100
Approach 3. An alternative to using the NOAEL is to derive a
'benchmark dose', as described by Crump (1984). In studies by
inhalation, the concentration rather than the dose is considered to be
more precise, and the 'benchmark concentration' is used. Like the
benchmark dose, this term covers the entire exposure-response
relationship in a given study rather than relying on only one data
point representing the NOAEL or LOAEL. This approach reduces the
uncertainty inherent in defining an NOAEL or LOAEL by considering the
upper 95% confidence interval of the full exposure-response curve from
a study in experimental animals, in which the lower 95% confidence
limit of a concentration corresponds to a 1, 5, or 10% increase in
response, defined as the percentage of animals responding in a
specific group. This exposure concentration is then the benchmark
concentration of the study. It is converted to the human benchmark
concentration on the basis of differences in pulmonary dosimetry
between the two species, which additionally reduces the use of
uncertainty factors. Because the full range of experimental results
from a specific study is used, the benchmark concentration approach
reduces statistical uncertainty.
In principle, this approach involves three steps. The first is
selection of the appropriate experimental study and end-point for
establishing an exposure-response curve. The second is calculation of
the benchmark concentration for the animals from a mathematical
description of the exposure-response curve and determination of the
95% confidence interval. Thirdly, the human benchmark concentration is
calculated; for exposure by inhalation, the exposure of the animals is
extrapolated dosimetrically to the human situation, with application
of uncertainty factors the size of which is determined as discussed
above. These factors generally consist of one for interspecies
extrapolation and one for sensitive subpopulations. The first two
steps of the benchmark concentration approach are shown schematically
in Figure 6.
Approach 2. In this approach, the equivalent continuous animal
exposure based on the NOAEL of 0.41 in rats (light-duty diesel
exhaust; Ishinishi et al., 1986, 1988; Table 47) is used to derive the
guideline value. Because the dosimetric model is not used, the default
value of 10 is applied for interspecies uncertainty and an uncertainty
factor of 10 is added for intraspecies differences. The guidance value
obtained with this approach is
0.23 mg/m3
------------- = 2.3 µg/m3
100
Approach 3. An alternative to using the NOAEL is to derive a
'benchmark dose', as described by Crump (1984). In studies by
inhalation, the concentration rather than the dose is considered to be
more precise, and the 'benchmark concentration' is used. Like the
benchmark dose, this term covers the entire exposure-response
relationship in a given study rather than relying on only one data
point representing the NOAEL or LOAEL. This approach reduces the
uncertainty inherent in defining an NOAEL or LOAEL by considering the
upper 95% confidence interval of the full exposure-response curve from
a study in experimental animals, in which the lower 95% confidence
limit of a concentration corresponds to a 1, 5, or 10% increase in
response, defined as the percentage of animals responding in a
specific group. This exposure concentration is then the benchmark
concentration of the study. It is converted to the human benchmark
concentration on the basis of differences in pulmonary dosimetry
between the two species, which additionally reduces the use of
uncertainty factors. Because the full range of experimental results
from a specific study is used, the benchmark concentration approach
reduces statistical uncertainty.
In principle, this approach involves three steps. The first is
selection of the appropriate experimental study and end-point for
establishing an exposure-response curve. The second is calculation of
the benchmark concentration for the animals from a mathematical
description of the exposure-response curve and determination of the
95% confidence interval. Thirdly, the human benchmark concentration is
calculated; for exposure by inhalation, the exposure of the animals is
extrapolated dosimetrically to the human situation, with application
of uncertainty factors the size of which is determined as discussed
above. These factors generally consist of one for interspecies
extrapolation and one for sensitive subpopulations. The first two
steps of the benchmark concentration approach are shown schematically
in Figure 6.

 Two sets of data were used to derive the benchmark concentration
for diesel exhaust. The first was that of Ishinishi et al. (1988) from
a study in which rats were exposed to exhaust from a light-duty diesel
engine for two years at four concentrations. Hyperplastic lesions,
shown histopathologically to be a sensitive indicator of lung damage,
were used to establish a well-described exposure-response
relationship. The second data set was that of Creutzenberg et al.
(1990) for female Wistar rats exposed for 96 h per week for a total of
78 weeks to three concentrations of diesel exhaust. The most sensitive
end-points for exposure-related non-neoplastic changes were lung
clearance of particles and the occurrence of polymorphonuclear
neutrophils (PMN) in lung lavage fluid as indicators of inflammation.
Since these measurements are laborious, however, they were performed
only in a subset of the exposed animals, so that only six to eight
rats were studied per group.
The responses in the study of Ishinishi et al. (1988) were
expressed in terms of individual animals affected per total number of
exposed animals in each group, whereas the data of Creutzenberg et al.
(1990) were reported as group mean values plus or minus the standard
deviation, which is less useful for the benchmark concentration
approach. Individual responses in the latter study were, however,
provided by Bellmann (personal communication), so that the data from
this study could also be expressed in terms of percentage of animals
with impaired lung clearance. Impairment of lung function was
considered to be significant when the calculated pulmonary retention
half-time of the administered particles was at least 3.5 times greater
than their normal average half-time in the lungs of control animals.
Evaluation of the end-point chronic inflammation (PMN in lung
lavage fluid) proved to be more difficult, since all of the rats at
the lowest exposure level (0.8 mg/m3) in the study of Creutzenberg
et al. (1990) had increased PMN levels (100% response). Lung lavage
was performed at 22 and 24 months of exposure, and since the responses
at these two times were similar, the data were combined to increase
the numbers of animals per group to 11-14. The individual responses
(percentage of PMN among the total number of cells in the lavage
fluid) were used to derive the curve and the 95% confidence interval.
An excess of PMN of up to 3% of the total number of cells over the
background level was used to define the benchmark concentration. The
highest value observed in the control group was 2.75% PMN after
22 months of exposure.
The three data sets and the results of the probability function,
using a Weibull model to calculate the benchmark concentration, are
given in Tables 44, 45, and 46 and are illustrated in Figures 7 and 8.
Table 44. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Ishinishi et al.
(1988); end-point: hyperplastic lesions of the lung
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 4/125 4.6 3.2
0.063 4/125 4.6 3.25
0.23 6/125 4.7 4.8
0.67 12/123 12.1 9.76
1.3 87/124 87.0 70.2
Probability function [p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 *
Conc)K]
A0 = 3.7196 × 10-2
A1 = 3.740
K = 4.3504
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.634
0.05 0.511
0.01 0.313
The respective benchmark concentrations from the data sets for rats
are 0.634, 0.119, and 0.090 mg/m3, corresponding to a 10% response
(the lower 95% confidence limit on the exposure concentration for
hyperplastic lung injury and impaired lung clearance) or a 3% excess
of PMN in lavage fluid (the lower 95% confidence limit on the exposure
concentration for chronic alveolar inflammation).
The resulting human benchmark concentrations for the three
end-points were calculated from the model of Yu & Yoon (1990),
applying uncertainty factors of 10 to account for sensitive
subpopulations (human intraspecies differences) and 100.4 (2.5) for
potentially different toxicodynamics (see above), to be: 14 µg/m3
for hyperplastic lung lesions, 3 µg/m3 for impaired lung clearance,
and 2 µg/m3 for chronic alveolar inflammation. Although the
mathematical description of the exposure-response relationships in
rats is very good for all three sets of data, they should be viewed as
hypothetical since they are based on dosimetric conversions from rats
to humans and on the application of uncertainty factors.
Table 45. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Creutzenberg
et al. (1990); end-point: impaired lung clearance
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 0/6 0 0
0.45 2/8 1.7 25
1.34 3/6 3.0 50
3.74 5/6 5.2 83.3
Probability function
[p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 * Conc - D0)A2]
A0 = 0.0
A1 = 0.522
A2 = 1.00
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.119
0.05 0.058
0.01 0.011
The non-cancer guidance values and the benchmark concentrations
derived from these approaches are summarized in Table 47.
More sensitive end-points with respect to the adverse effects of
diesel exhaust on the lower respiratory tract are impaired clearance
in the deep lung and chronic alveolar inflammation, rather than
hyperplastic lung lesions. Chronic alveolar inflammation was also a
significant finding in the long-term study of rats exposed to diesel
exhaust by Henderson et al. (1988), who found a significantly
increased percentage of PMN in lavage fluid from the group with the
lowest exposure (0.35 mg/m3) after two years. For all three types of
end-points, however, there may be a threshold below which no change is
to be expected. The threshold concept is not included in the
mathematical model, and it would be difficult to do so at this point
in the absence of specific experimental data and data on variation
between human subjects.
These estimates are for the effects of long-term exposures, and
the concentrations are expressed as annual means over a lifetime. They
could apply to long-term effects on health, such as the association
between mortality from certain chronic conditions and exposure to
particulates in air pollution seen in a study in six cities in the
United States (Dockery et al., 1993). Their relevance to human
experience must also be considered in the context of particulate air
pollutants in general, however, and they have no direct bearing on the
acute effects of exposure to particulates, for which guidance values
are required in terms of 24-h mean concentrations.
B10.4 Neoplastic effects
B10.4.1 Hazard identification
B10.4.1.1 Lung cancer: occupational exposure
In the 1970s and 1980s, the recognition that diesel exhaust
contains respirable particles and that known carcinogenic substances
are adsorbed on the surface of these particles led to the hypothesis
that inhalation of diesel exhaust could result in lung cancer in
humans. As lung cancer develops slowly, over many years, studies of
individuals with long, well-defined exposure and follow-up (> 20
years) were considered to be the most informative. Four studies of
occupationally exposed individuals meet these criteria (Garshick et
al., 1987, 1988; Gustavsson et al., 1990; Emmelin et al., 1993; see
Table 43).
Table 46. Benchmark concentration for rats after long-term exposure to
diesel exhaust by inhalation in study by Creutzenberg et al.
(1990); end-point: chronic alveolar inflammation
Concentration (mg/m3) PMN in lung lavage fluid (%) No. rats
(equivalent continuous responding
exposure of rats Actual/observed Predicted per no.
exposed
0 0.7 0 0/11
0.45 11.1 11.7 13/13
1.34 29.9 27.6 14/14
3.74 50.8 51.9 14/14
Dose-response function: F(dose) = Q(0) × (Dose - (Dose - D0)Q(2)
Dose = log(1 + d) (d = rat exposure concentration)
Q(0) = 0.6.53
Q(1) = 77.010
Q(2) = 1.056
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
excess polymorphonuclear neutrophil (PMN) response (% of total lavaged
cells):
PMN in excess Rat BC
of control (%) (mg/m3)
3 0.090
2 0.059
1 0.029
Transition from steam to diesel locomotives occurred in the
American railroad industry during the 1950s. In a case-control study
of American railroad workers (Garshick et al., 1987), exposure was
estimated on the basis of yearly job (exposed or unexposed to diesel
exhaust) from 1959 to death or retirement; deaths were identified for
12 months in 1981-82. In an additional study of these workers
(Garshick et al., 1988), a retrospective cohort was defined on the
basis of work in a job with exposure to diesel exhaust in 1959, and
Two sets of data were used to derive the benchmark concentration
for diesel exhaust. The first was that of Ishinishi et al. (1988) from
a study in which rats were exposed to exhaust from a light-duty diesel
engine for two years at four concentrations. Hyperplastic lesions,
shown histopathologically to be a sensitive indicator of lung damage,
were used to establish a well-described exposure-response
relationship. The second data set was that of Creutzenberg et al.
(1990) for female Wistar rats exposed for 96 h per week for a total of
78 weeks to three concentrations of diesel exhaust. The most sensitive
end-points for exposure-related non-neoplastic changes were lung
clearance of particles and the occurrence of polymorphonuclear
neutrophils (PMN) in lung lavage fluid as indicators of inflammation.
Since these measurements are laborious, however, they were performed
only in a subset of the exposed animals, so that only six to eight
rats were studied per group.
The responses in the study of Ishinishi et al. (1988) were
expressed in terms of individual animals affected per total number of
exposed animals in each group, whereas the data of Creutzenberg et al.
(1990) were reported as group mean values plus or minus the standard
deviation, which is less useful for the benchmark concentration
approach. Individual responses in the latter study were, however,
provided by Bellmann (personal communication), so that the data from
this study could also be expressed in terms of percentage of animals
with impaired lung clearance. Impairment of lung function was
considered to be significant when the calculated pulmonary retention
half-time of the administered particles was at least 3.5 times greater
than their normal average half-time in the lungs of control animals.
Evaluation of the end-point chronic inflammation (PMN in lung
lavage fluid) proved to be more difficult, since all of the rats at
the lowest exposure level (0.8 mg/m3) in the study of Creutzenberg
et al. (1990) had increased PMN levels (100% response). Lung lavage
was performed at 22 and 24 months of exposure, and since the responses
at these two times were similar, the data were combined to increase
the numbers of animals per group to 11-14. The individual responses
(percentage of PMN among the total number of cells in the lavage
fluid) were used to derive the curve and the 95% confidence interval.
An excess of PMN of up to 3% of the total number of cells over the
background level was used to define the benchmark concentration. The
highest value observed in the control group was 2.75% PMN after
22 months of exposure.
The three data sets and the results of the probability function,
using a Weibull model to calculate the benchmark concentration, are
given in Tables 44, 45, and 46 and are illustrated in Figures 7 and 8.
Table 44. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Ishinishi et al.
(1988); end-point: hyperplastic lesions of the lung
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 4/125 4.6 3.2
0.063 4/125 4.6 3.25
0.23 6/125 4.7 4.8
0.67 12/123 12.1 9.76
1.3 87/124 87.0 70.2
Probability function [p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 *
Conc)K]
A0 = 3.7196 × 10-2
A1 = 3.740
K = 4.3504
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.634
0.05 0.511
0.01 0.313
The respective benchmark concentrations from the data sets for rats
are 0.634, 0.119, and 0.090 mg/m3, corresponding to a 10% response
(the lower 95% confidence limit on the exposure concentration for
hyperplastic lung injury and impaired lung clearance) or a 3% excess
of PMN in lavage fluid (the lower 95% confidence limit on the exposure
concentration for chronic alveolar inflammation).
The resulting human benchmark concentrations for the three
end-points were calculated from the model of Yu & Yoon (1990),
applying uncertainty factors of 10 to account for sensitive
subpopulations (human intraspecies differences) and 100.4 (2.5) for
potentially different toxicodynamics (see above), to be: 14 µg/m3
for hyperplastic lung lesions, 3 µg/m3 for impaired lung clearance,
and 2 µg/m3 for chronic alveolar inflammation. Although the
mathematical description of the exposure-response relationships in
rats is very good for all three sets of data, they should be viewed as
hypothetical since they are based on dosimetric conversions from rats
to humans and on the application of uncertainty factors.
Table 45. Benchmark concentration for rats after long-term exposure
to diesel exhaust by inhalation in study by Creutzenberg
et al. (1990); end-point: impaired lung clearance
Concentration (mg/m3) Rats that responded
(equivalent continuous
exposure of rats) Actal/observed Predicted Percent
numbers number
0 0/6 0 0
0.45 2/8 1.7 25
1.34 3/6 3.0 50
3.74 5/6 5.2 83.3
Probability function
[p(conc)] - A0]/(1 - A0) = [1 - exp(-A1 * Conc - D0)A2]
A0 = 0.0
A1 = 0.522
A2 = 1.00
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
risk:
Risk Rat BC
(mg/m3)
0.1 0.119
0.05 0.058
0.01 0.011
The non-cancer guidance values and the benchmark concentrations
derived from these approaches are summarized in Table 47.
More sensitive end-points with respect to the adverse effects of
diesel exhaust on the lower respiratory tract are impaired clearance
in the deep lung and chronic alveolar inflammation, rather than
hyperplastic lung lesions. Chronic alveolar inflammation was also a
significant finding in the long-term study of rats exposed to diesel
exhaust by Henderson et al. (1988), who found a significantly
increased percentage of PMN in lavage fluid from the group with the
lowest exposure (0.35 mg/m3) after two years. For all three types of
end-points, however, there may be a threshold below which no change is
to be expected. The threshold concept is not included in the
mathematical model, and it would be difficult to do so at this point
in the absence of specific experimental data and data on variation
between human subjects.
These estimates are for the effects of long-term exposures, and
the concentrations are expressed as annual means over a lifetime. They
could apply to long-term effects on health, such as the association
between mortality from certain chronic conditions and exposure to
particulates in air pollution seen in a study in six cities in the
United States (Dockery et al., 1993). Their relevance to human
experience must also be considered in the context of particulate air
pollutants in general, however, and they have no direct bearing on the
acute effects of exposure to particulates, for which guidance values
are required in terms of 24-h mean concentrations.
B10.4 Neoplastic effects
B10.4.1 Hazard identification
B10.4.1.1 Lung cancer: occupational exposure
In the 1970s and 1980s, the recognition that diesel exhaust
contains respirable particles and that known carcinogenic substances
are adsorbed on the surface of these particles led to the hypothesis
that inhalation of diesel exhaust could result in lung cancer in
humans. As lung cancer develops slowly, over many years, studies of
individuals with long, well-defined exposure and follow-up (> 20
years) were considered to be the most informative. Four studies of
occupationally exposed individuals meet these criteria (Garshick et
al., 1987, 1988; Gustavsson et al., 1990; Emmelin et al., 1993; see
Table 43).
Table 46. Benchmark concentration for rats after long-term exposure to
diesel exhaust by inhalation in study by Creutzenberg et al.
(1990); end-point: chronic alveolar inflammation
Concentration (mg/m3) PMN in lung lavage fluid (%) No. rats
(equivalent continuous responding
exposure of rats Actual/observed Predicted per no.
exposed
0 0.7 0 0/11
0.45 11.1 11.7 13/13
1.34 29.9 27.6 14/14
3.74 50.8 51.9 14/14
Dose-response function: F(dose) = Q(0) × (Dose - (Dose - D0)Q(2)
Dose = log(1 + d) (d = rat exposure concentration)
Q(0) = 0.6.53
Q(1) = 77.010
Q(2) = 1.056
D0 = 0.0 (threshold)
Lower confidence limit of the benchmark concentration (BC) for a given
excess polymorphonuclear neutrophil (PMN) response (% of total lavaged
cells):
PMN in excess Rat BC
of control (%) (mg/m3)
3 0.090
2 0.059
1 0.029
Transition from steam to diesel locomotives occurred in the
American railroad industry during the 1950s. In a case-control study
of American railroad workers (Garshick et al., 1987), exposure was
estimated on the basis of yearly job (exposed or unexposed to diesel
exhaust) from 1959 to death or retirement; deaths were identified for
12 months in 1981-82. In an additional study of these workers
(Garshick et al., 1988), a retrospective cohort was defined on the
basis of work in a job with exposure to diesel exhaust in 1959, and

 deaths were recorded for 1959-80. Although the previous exposure
levels of the railroad workers were not available, the classification
of workers into groups on the basis of exposure was validated by an
industrial hygienist and a review of work practices. Air sampling was
conducted to establish current exposure levels in various jobs
(Hammond et al., 1988; Woskie et al., 1988a,b).
Gustavsson et al. (1990) studied lung cancer among bus garage
workers in Stockholm, Sweden, where all buses with internal combustion
engines have been diesel-fuelled since 1945. A cohort was established
of people who had worked in bus garages for at least six months
between 1945 and 1970, and mortality was determined for 1952-86. A
nested case-control study was performed within this cohort. Although
actual exposure was not measured, relative exposure to diesel exhaust
was graded on the basis of a scale established by an industrial
hygienist in a review of work practices, bus engine operation, and
shop ventilation.
Table 47. Summary of non-cancer guidance values and benchmark
concentrations
Approach Guidance value
or benchmark
concentration
(µg/m3)
NOAEL with dosimetric conversion from rats to humans 5.6a
NOAEL without dosimetric conversion from rats to 2.3
humans
Benchmark concentration with dosimetric conversion
from rats to humans
Chronic alveolar inflammation 2a
Impaired lung clearance 3a
Hyperplastic lesions 14a
Benchmark concentration without dosimetric
conversion from rats to humans
Chronic alveolar inflammation 0.9
Impaired lung clearance 1.2
Hyperplastic lesions 6.3
NOAEL, no-observed-adverse-effect level
Normalized for lung surface area in rats and humans; after
normalization for lung weight, the benchmark concentration increases
by a factor of 4.
Emmelin et al. (1993) studied Swedish stevedores in 15 ports
where diesel equipment had been introduced between 1957 and 1963; they
also performed a nested case-control study on cases of lung cancer
identified between 1960 and 1982. Exposure to diesel exhaust was
estimated on the basis of three indices derived from estimated diesel
fuel consumption and years of work after the introduction of diesel
equipment in each port.
All four studies showed an increased risk for lung cancer with
exposure to diesel exhaust. The relative risks reported ranged from
about 1.4 for railroad workers with the longest duration of exposure
in both studies of such populations to 1.3-2.4 for bus garage workers,
depending on the exposure category. The stevedores also had an
increased relative odds ratio for lung cancer with increasing exposure
with respect to all three indices of exposure examined, with an
increase of three- to sixfold in the highest exposure categories and
1.5-2.7-fold in the lowest exposure category. The point estimates
were, however, imprecise and had wide confidence intervals: for all
but one exposure category, the lower limit of the 90% confidence
intervals presented by the authors included 1.0.
The effects of cigarette smoking could be adjusted for in the
case-control studies of railroad workers (Garshick et al., 1987) and
stevedores (Emmelin et al., 1993). The smoking histories of the
railroad workers were obtained from next-of-kin. Various regression
models (including both dose and duration of smoking) were examined in
detail in order to adjust adequately for the effects of smoking. No
matter how it was accounted for, the risk for lung cancer based on
work in a job with exposure to diesel exhaust was similar to the
unadjusted odds ratio. The study of stevedores (Emmelin et al., 1993)
was smaller than that of railroad workers, and in order to reduce the
number of strata for analysis workers were stratified only as smoker
or nonsmoker. In the two studies in which information on smoking was
not available (Garshick et al., 1988; Gustavsson et al., 1990), the
analysis was based on a comparison with workers in the same
occupational cohort, making confounding by smoking less likely.
The results of most of the other studies summarized in Tables
39-41 support those of the four studies discussed above. The relative
risks were generally in the range 1.2-1.9 but did not always achieve
statistical significance. Furthermore, exposure to diesel exhaust was
less precisely defined in these studies; it was usually based on
self-reporting, the report of a surrogate, or a census report of
occupation (Table 41). Self-reporting or the report of a surrogate
would reflect actual exposure to diesel exhaust or work in a job in
which exposure was likely; however, defining exposure in this less
precise fashion make it harder to detect an effect of the exposure and
leads to lower relative risks and wider confidence intervals.
In three other studies, job titles obtained from an employer were
used to define exposure. In the study of Howe et al. (1983), the job
held at the time of retirement from the railroads was used to
categorize exposure, and mortality was assessed for 1965-77.
Significantly elevated risks for lung cancer were noted among workers
who had probably been exposed to diesel exhaust (relative risk = 1.35)
and among those who had possibly been exposed (1.20); however, since
only retired workers were studied, actual exposure to diesel exhaust
would have been short for many workers. Rushton et al. (1983) and
Waller (1981) used job titles within the London Transport organization
to indicate potential exposure to diesel exhaust. Neither study showed
an elevated risk for lung cancer, but the study of Rushton and
coworkers was characterized by a short duration of exposure and
follow-up, whereas in the study of Waller, deaths occurring after
employment were not included.
The relative risks for lung cancer as a result of exposure to
diesel exhaust are generally low, and risks of this magnitude are more
susceptible to chance and to the effects of unmeasured confounding
factors and imprecision in adjusting for known confounding factors. As
discussed above, the elevated risk for lung cancer observed in the
four most informative studies is unlikely to be due to confounding by
cigarette smoking and is probably due to exposure to diesel exhaust.
Other studies, although limited primarily by the exposure
ascertainment, support this assessment.
B10.4.1.2 Urinary bladder cancer: occupational exposure
The hypothesis that exposure to diesel exhaust results in cancer
of the urinary bladder is based on the fact that individuals who smoke
cigarettes, a source of PAHs (as is diesel particulate), excrete
mutagenic substances in their urine, and cigarette smoking is
associated with an increased risk for bladder cancer. In a recent
study of railroad workers with current exposure to diesel exhaust
(median concentration of respirable particulates over a work shift,
54-113 µg/m3; adjusted for smoking), mutagenicity in urine at the
end of a shift was not associated with exposure (Schenker et al.,
1992).
The evidence that bladder cancer is associated with exposure to
diesel exhaust is based on an association between work in industries
or occupations with potential exposure to diesel exhaust. The relative
risks reported (some of which are adjusted for smoking) are generally
in the range 1.2-2 (Hoar & Hoover, 1985; Silverman et al., 1986),
although higher risks have been reported. Exposure was assessed mainly
by self-reporting and reporting of occupation at interview. The main
occupation in which there was considered to be potential exposure to
diesel exhaust was truck driving. Measurements of exposure of truck
drivers by Zaebst et al. (1991) indicate that current exposure is
likely to be low, but there is no knowledge about past exposure
levels.
Thus, although work in occupations related to motor vehicles has
been associated with bladder cancer, the extent of exposure of these
workers to diesel exhaust is unknown.
B10.4.2 Dose-response assessment
B10.4.2.1 Lung cancer
Job category and work in an occupation with exposure to diesel
exhaust have been used as indicators of exposure. When years of
exposure and derived indices of exposure have been used, an
exposure-response relationship has generally been found. Measurements
of total and respirable dust in exposed workers do not reflect actual
exposure to diesel exhaust particles, however, since there are other
sources of dust in an occupational environment. The exposure of only
two occupational groups, American railroad workers (Woskie et al.,
1988b) and truck drivers (Zaebst et al., 1991), to diesel exhaust
particles has been quantified (see section B5.2). Current exposure was
measured for both these groups, but past exposure levels could not be
determined (Woskie et al., 1988b), so that studies of workers do not
permit determination of a dose-response relationship for inhaled
diesel particles.
B10.4.2.2 Urinary bladder cancer
Years of work in an occupation related to motor vehicles (mainly
truck driving) was examined in some, but not all, studies, but no
measurements were available to estimate exposure to diesel exhaust in
these settings.
B10.4.3 Exposure assessment
The ranges of exposures observed for workers are presented in
sections B5.2 and B10.2.
B10.4.4 Risk characterization
B10.4.4.1 Human lung cancer
Thus, historical measurements of exposure to diesel exhaust are
unreliable and exist only for current workers in two industries. A
quantitative risk assessment cannot be conducted on the basis of
epidemiological data in which job title was used as a surrogate of
exposure. Attempts to obtain estimates of risk (presented in section
B10.4.1) in the retrospective cohort study of American railroad
workers (Garshick et al., 1988) were not successful (see below for
discussion). Consequently, there are no human data suitable for
estimating unit risk.
B10.4.4.2 Human urinary bladder cancer
The risk for urinary bladder cancer cannot be assessed from the
available epidemiological data.
B10.4.4.3 Risk characterization based on studies in experimental
animals
(a) Introduction
Most human tumours arise in the bronchial region, and although
the frequency of adenocarcinomas has increased in recent years
(Martini, 1993), squamous bronchogenic carcinomas still represent the
majority (about 60%) of lung tumours in humans (Auerbach & Garfinkel,
1991; Devesa et al., 1991; Campobosso et al., 1993). The tumours
observed in rats are located almost exclusively in the bronchoalveolar
regions. This difference raises the question of whether the rat is the
appropriate species for extrapolating to humans. Although it may be
the wrong model, however, studies of hamsters and monkeys give
negative results, so the rat is the most appropriate model from a
conservative viewpoint and is therefore used for quantitative risk
extrapolation.
The objective is to assess quantitatively the potential risk for
lung cancer in humans posed by exposure to diesel exhaust emissions in
the ambient air. Ideally, human risk due to exposure to an
environmental pollutant should be predicted on the basis of human
experience. Although several epidemiological studies are available on
bus and railroad workers, these results alone are not adequate to
assess the potential risk of cancer in humans because of the lack of
reliable information on the conditions of exposure of these workers.
The challenge is to assess the risk of exposure to diesel exhaust on
the basis of all of the available information, for both experimental
animals and humans. In contrast to the sparse human data, there is a
rich pool of information on diesel-induced lung tumours in two strains
of rat. One approach to integrating this information is to make a
quantitative risk assessment on the basis of information from
bioassays and on the relevant biological mechanism and then to
evaluate these animal-based results against available human
experience. This approach was followed in this monograph.
Although, the available experimental data on the possible impact
of extractable organic matter and PAHs on the lung tumour response
associated with exposure to diesel soot are not strong (Heinrich et
al., 1991; Heinrich, 1994; Heinrich et al., 1995), a mathematical
approach to assessing the risk of exposure to diesel exhaust should
take into account the effects of both particles (carbon core) and
extractable organic matter, because: (i) organic compounds include a
variety of PAHs and nitroaromatic compounds, many of which are known
to be carcinogenic; (ii) the results of recent studies on inert
particles and carbon black in rats (Mauderly et al., 1991; Heinrich,
1994; Heinrich et al., 1994) strongly support the hypothesis that the
carbon cores of diesel particles are the component primarily
responsible for the induction of lung cancer; (iii) PAHs alone are
unlikely to be responsible for the tumour response; (iv) the
disproportionately high tumour incidence in animals with heavy
exposure coincides with a disproportionate increase in the cumulative
lung burden of diesel particles. Although a qualitative description of
the biological mechanism of diesel exhaust-induced tumours is
plausible, however, the lack of quantitative information on the
dynamics of tumour initiation, promotion, and progression vitiates the
construction of a biologically based dose-response model.
The crudest dose-response model is obtained by fitting observed
dose-response data to a mathematical function that is a monotonic,
non-decreasing function of exposure, using the inhaled concentration
and observed tumour incidence, without considering information on
pharmacokinetics and the mechanism of carcinogenesis. This approach
may result in uncertain estimates of risk at low doses, because, while
the model may adequately fit the dose-response data for high doses at
which a tumour response is observed, it may greatly underestimate or
overestimate the risk at low doses.
Most previous risk estimates of diesel exhaust-induced cancer
risk were developed before the results of many of the bioassays became
available (Pepelko & Chen, 1993). Nevertheless, most of the estimates
are similar (within an order of magnitude), except for those
calculated on the basis of human data, which are higher than those
calculated from other databases. Because of great uncertainties
associated with the available human data, it is considered more
appropriate to calculate risk on the basis of animal data and then use
human data to evaluate their validity.
The approach adopted in this assessment is the linearized
multistage model (Anderson et al., 1983), in which burden per lung
surface area (milligrams per square centimetre) is used as an
equivalent dose in animals and humans. The use of lung (alveolar)
surface area is justified by the fact that the lung tumours observed
in rats are derived exclusively from epithelial cells in the alveolar
region of the lung. Normalization on the basis of lung weight can also
be done by using a factor of 4, for increasing the dose or decreasing
the risk, for the difference in the ratio of rat:human lung surface
area and lung weight. The risk prediction calculated by this approach
is presumably conservative (i.e. overpredicts the risk) because the
dose-response function is assumed to be non-threshold and linear at
low doses.
An alternative model that incorporates a biological mechanism is
constructed using statistically estimated parameters. The model
assumes that carcinogenic agents present in the organic fraction act
directly on the target cells, primarily via initiation. It is further
assumed that the majority of the particles are ingested by
macrophages. Particle-laden macrophages are then induced to secrete a
variety of mediators (e.g. reactive oxygen species and cytokines),
which diffuse to the target cells and induce initiation,
proliferation, and conversion of initiated cells to malignant cells.
This alternative model can be used only as a tool to evaluate the
impact of various biological assumptions and not for risk prediction,
because the parameters of the model are not measured in the
laboratory.
(b) Approaches to quantification of human risk from exposure to
diesel exhaust
Several issues must be addressed in estimating the risk of
exposure to diesel exhaust, including the critical target site, the
fraction of exhaust responsible for tumour induction, the availability
of dosimetric methods for accurately extrapolating dose from that for
experimental animals chronically exposed to high concentrations of
exhaust to that of humans exposed at ambient concentrations, and the
most suitable low-dose extrapolation model.
The critical target organ was considered to be the lung. Although
Iwai et al. (1986) reported induction of both malignant lymphomas and
lung tumours in rats exposed to diesel exhaust, the lung was the only
target site in other experimental studies of this species. Although
potentially carcinogenic agents present in diesel exhaust may be
absorbed from the lungs, enter the bloodstream, and be transported
systemically, no data are available to evaluate this possibility.
Organic compounds adsorbed on particles may also reach systemic
targets via the gastrointestinal tract, as particles deposited in the
conducting airways are cleared rapidly to the oropharynx and
swallowed. A considerable volume of particles is also likely to be
ingested as a result of grooming when the whole animal is exposed
(Wolff et. al., 1982); however, because the half-times for the elution
of organic compounds from particles are considerably longer than their
passage through the gastrointestinal tract, the fraction absorbed is
expected to be small. In any case, there is little evidence for any
systemic effects of diesel exhaust.
The site of action in the lungs is assumed to be the epithelial
lining of the alveoli and small airways. According to Mauderly et al.
(1987), inflammation and tumours appear to arise from this tissue.
Although interstitial events (e.g. fibrosis) have been suggested to be
associated with induction of lung tumours by particles (Kuschner,
1968), no data are available to support this view with respect to
diesel exhaust.
Accurate extrapolation of dose from studies of animals exposed to
high concentrations of exhaust to humans exposed to ambient
concentrations requires a variety of adjustments for species
differences in deposition efficiency and respiratory ventilation
rates. As the normal retention half-times in the alveolar region are
several times longer in humans than rats (Chan et al., 1981; Bohning
et al., 1982), the lung burden of humans may be underestimated if this
difference is not taken into account. The high exposure concentrations
used in some experimental studies, however, result in greatly slowed
or even completely inhibited clearance (Griffis et al., 1983). In
order to extrapolate dose accurately from experimental studies to
humans, the detailed dosimetric model developed by Yu et al. (1991)
was used, which accounts for species differences in respiratory
ventilation rates, deposition efficiency, normal particle clearance
rates, transport of particles to lung-associated lymph nodes, and lung
surface area. It also accounts for inhibition of particle clearance
due to lung overload. In this model, dose is estimated in terms of
particle concentration per unit of lung surface area.
Two approaches were used to derive unit risk estimates: the
linearized, multistage, low-dose extrapolation model and a model based
on the biological mechanism discussed above. In the linearized
multistage model, the lung burden of carbon core per lung surface area
is used as the dosimetric parameter. A particle-based assessment was
considered to be reasonable for two principal reasons: (i) exposure to
the vapour phase alone did not result in detectable tumour induction
in rats (Brightwell et al., 1986; Iwai et al., 1986; Heinrich et al.,
1986a); and (ii) exposure to carbon black, which is similar in
composition to the carbon core of whole diesel exhaust but contains
only negligible amounts of organic compounds, was about as effective
in inducing lung cancer as whole diesel exhaust (Mauderly et al.,
1991; Nikula et al., 1994; Heinrich et al., 1995). Although use of the
carbon core as a dosimetric parameter implies that it is primarily the
insoluble carbon core of diesel particles that is responsible for the
carcinogenic effects of diesel exhaust, it can also be viewed simply
as a marker of exposure to diesel exhaust, with no biological
implication. Similarly, organic compounds could be used as markers of
exposure for the dose-response calculation. When this is done, the
resulting unit risk estimates (not presented here) are very similar
(within 25%) to those calculated with the carbon core as the surrogate
of exposure.
The second approach is based on the assumption that, even though
the concentration of carcinogenic compounds on diesel particles is
low, they nevertheless can act in concert with the particles to induce
carcinogenesis. An alternative low-dose extrapolation model was
therefore developed that allows for the possibility that various PAHs
and nitroaromatic compounds may induce organ-specific adducts that
contribute to cell initiation. This model is described in Appendix A1.
Both approaches incorporate the detailed dosimetric model of Yu
et al. (1991) to estimate dose at the airway and alveolar surfaces.
Risk is based on the assumption that an equivalent concentration
(dose) per unit of alveolar surface area results in equivalent risks
in humans and rats.
(c) Evidence to support a carcinogenic effect of the carbon core
The study of Heinrich et al. (1994) on carbon black and coal-tar
pitch provides information useful for evaluating the assumptions used
to construct the model that reflects current thinking about the
mechanism of action of diesel exhaust in inducing lung tumours. One
relevant question about diesel exhaust is whether lung tumours are
induced by the carbon core alone or in combination with organic
compounds.
In this study, female Wistar rats were exposed to carbon black or
tar pitch, or both for 10 months (43 weeks) or 20 months (86 weeks)
and followed until the end of the study, which lasted for
two-and-a-half years. No lung tumours were observed in the control
group. For each exposed group, the probability of dying from lung
cancer was calculated by a Kaplan-Meier survival analysis. Since these
data are used solely for interpretation and not for risk prediction,
it is statistically more appropriate to use only data on mortality,
excluding those tumours found at terminal sacrifice. Figure 9 shows
the probability of dying from lung cancer for the rats that were
exposed either to 6 mg/m3 of carbon black, or to coal-tar pitch
containing 50 µg/m3 of benzo[a]pyrene, or to a combination of the
two for 10 months, and then followed until the end of the study. In
Figure 10, each plot represents the time at which a tumour occurred.
To provide a better visual presentation, the probabilities at these
points are connected by straight lines. (Note: It is statistically
more appropriate to present these probabilities by a step function
rather than by interpolation when the Kaplan-Meier procedure is used.)
Two conclusions can be drawn from Figure 10: (1) Tumours appeared
much earlier in animals exposed to coal-tar pitch alone or in
combination with carbon black than in animals exposed to carbon black
alone. For instance, the first tumour occurred 665 days after the
start of exposure to carbon black, 406 days after exposure to coal-tar
pitch, and only 310 days after exposure to the combination. (2) Carbon
black and coal-tar pitch together caused a higher rate of mortality
from tumours than the sum of mortality caused by carbon black and
coal-tar pitch separately. The probability of dying from a lung tumour
before terminal sacrifice at 912 days was 0.28 for carbon black, 0.52
for coal-tar pitch, and 0.99 for the combination. These two
observations suggest that carbon black and coal-tar pitch act on
different stages of tumorigenesis. They are consistent with the
hypothesis that carbon black can increase the proliferation rates of
normal and cells at different stages of differentiation initiated
spontaneously or by coal-tar pitch.
When the rate of mortality from tumours among rats exposed to
diesel exhaust is compared with that of groups exposed to carbon
black, coal-tar pitch, or the combination (Figure 9), the rate among
the animals exposed to diesel exhaust is comaprable to that of rats
exposd to carbon black for 10 to 20 months. This implies that carbon
black alone can induce a tumour response similar to that induced by
diesel exhaust. Figure 9 also shows that doubling the duration of
exposure to carbon black from 10 to 20 months does not increase the
probability of dying from a lung tumour before terminal sacrifice;
however, doubling the duration of exposure to coal-tar pitch
significantly increases the probability of dying from a lung tumour.
Figure 11 shows that the shapes of the curves for age-dependent
mortality from cancer for animals exposed to diesel exhaust and
coal-tar pitch for 20 months are similar. It should be kept in mind
that the PAH content of the atmosphere containing both carbon black
and coal-tar pitch was about 1000 times higher than that of diesel
engine exhaust. These observations may have profound implications for
elucidating the mechanisms of induction of lung tumours by diesel
exhaust, but more refined analyses are necessary before any conjecture
can be made.
(d) Data available for calculating risk
Seven bioassays have shown lung tumour responses in rats
(Brightwell et al., 1986; Heinrich et al., 1986b; Ishinishi et al.,
1986; Iwai et al., 1986; Mauderly et al., 1987; Nikula et al., 1994;
Heinrich et al., 1995). Four studies were chosen for use in
calculating unit risk (Table 48) because they involved multiple
exposure groups. Data on time to event (death with or without a
tumour) are available in the studies of both Mauderly et al. (1987)
and Heinrich et al. (1995) and were used in all of the risk
calculations.
(e) Calculation of unit risks
The unit risk from exposure to an air pollutant is defined as the
95% upper bound of the increased lifetime cancer risk for an
individual continuously exposed to a concentration of 1 µg/m3 in
ambient air. Unit risk is a convenient tool for calculating lifetime
risk due to exposure to a pollutant when low-dose linearity is
assumed. Under this assumption, the risk due to exposure to d µg/m3
of pollutant can be calculated from u × d if the pollutant has a
unit risk of u/µg per m3. This calculation is valid, however, only
when the level of risk is low. The results of unit risk calculations
are summarized in Table 49.
deaths were recorded for 1959-80. Although the previous exposure
levels of the railroad workers were not available, the classification
of workers into groups on the basis of exposure was validated by an
industrial hygienist and a review of work practices. Air sampling was
conducted to establish current exposure levels in various jobs
(Hammond et al., 1988; Woskie et al., 1988a,b).
Gustavsson et al. (1990) studied lung cancer among bus garage
workers in Stockholm, Sweden, where all buses with internal combustion
engines have been diesel-fuelled since 1945. A cohort was established
of people who had worked in bus garages for at least six months
between 1945 and 1970, and mortality was determined for 1952-86. A
nested case-control study was performed within this cohort. Although
actual exposure was not measured, relative exposure to diesel exhaust
was graded on the basis of a scale established by an industrial
hygienist in a review of work practices, bus engine operation, and
shop ventilation.
Table 47. Summary of non-cancer guidance values and benchmark
concentrations
Approach Guidance value
or benchmark
concentration
(µg/m3)
NOAEL with dosimetric conversion from rats to humans 5.6a
NOAEL without dosimetric conversion from rats to 2.3
humans
Benchmark concentration with dosimetric conversion
from rats to humans
Chronic alveolar inflammation 2a
Impaired lung clearance 3a
Hyperplastic lesions 14a
Benchmark concentration without dosimetric
conversion from rats to humans
Chronic alveolar inflammation 0.9
Impaired lung clearance 1.2
Hyperplastic lesions 6.3
NOAEL, no-observed-adverse-effect level
Normalized for lung surface area in rats and humans; after
normalization for lung weight, the benchmark concentration increases
by a factor of 4.
Emmelin et al. (1993) studied Swedish stevedores in 15 ports
where diesel equipment had been introduced between 1957 and 1963; they
also performed a nested case-control study on cases of lung cancer
identified between 1960 and 1982. Exposure to diesel exhaust was
estimated on the basis of three indices derived from estimated diesel
fuel consumption and years of work after the introduction of diesel
equipment in each port.
All four studies showed an increased risk for lung cancer with
exposure to diesel exhaust. The relative risks reported ranged from
about 1.4 for railroad workers with the longest duration of exposure
in both studies of such populations to 1.3-2.4 for bus garage workers,
depending on the exposure category. The stevedores also had an
increased relative odds ratio for lung cancer with increasing exposure
with respect to all three indices of exposure examined, with an
increase of three- to sixfold in the highest exposure categories and
1.5-2.7-fold in the lowest exposure category. The point estimates
were, however, imprecise and had wide confidence intervals: for all
but one exposure category, the lower limit of the 90% confidence
intervals presented by the authors included 1.0.
The effects of cigarette smoking could be adjusted for in the
case-control studies of railroad workers (Garshick et al., 1987) and
stevedores (Emmelin et al., 1993). The smoking histories of the
railroad workers were obtained from next-of-kin. Various regression
models (including both dose and duration of smoking) were examined in
detail in order to adjust adequately for the effects of smoking. No
matter how it was accounted for, the risk for lung cancer based on
work in a job with exposure to diesel exhaust was similar to the
unadjusted odds ratio. The study of stevedores (Emmelin et al., 1993)
was smaller than that of railroad workers, and in order to reduce the
number of strata for analysis workers were stratified only as smoker
or nonsmoker. In the two studies in which information on smoking was
not available (Garshick et al., 1988; Gustavsson et al., 1990), the
analysis was based on a comparison with workers in the same
occupational cohort, making confounding by smoking less likely.
The results of most of the other studies summarized in Tables
39-41 support those of the four studies discussed above. The relative
risks were generally in the range 1.2-1.9 but did not always achieve
statistical significance. Furthermore, exposure to diesel exhaust was
less precisely defined in these studies; it was usually based on
self-reporting, the report of a surrogate, or a census report of
occupation (Table 41). Self-reporting or the report of a surrogate
would reflect actual exposure to diesel exhaust or work in a job in
which exposure was likely; however, defining exposure in this less
precise fashion make it harder to detect an effect of the exposure and
leads to lower relative risks and wider confidence intervals.
In three other studies, job titles obtained from an employer were
used to define exposure. In the study of Howe et al. (1983), the job
held at the time of retirement from the railroads was used to
categorize exposure, and mortality was assessed for 1965-77.
Significantly elevated risks for lung cancer were noted among workers
who had probably been exposed to diesel exhaust (relative risk = 1.35)
and among those who had possibly been exposed (1.20); however, since
only retired workers were studied, actual exposure to diesel exhaust
would have been short for many workers. Rushton et al. (1983) and
Waller (1981) used job titles within the London Transport organization
to indicate potential exposure to diesel exhaust. Neither study showed
an elevated risk for lung cancer, but the study of Rushton and
coworkers was characterized by a short duration of exposure and
follow-up, whereas in the study of Waller, deaths occurring after
employment were not included.
The relative risks for lung cancer as a result of exposure to
diesel exhaust are generally low, and risks of this magnitude are more
susceptible to chance and to the effects of unmeasured confounding
factors and imprecision in adjusting for known confounding factors. As
discussed above, the elevated risk for lung cancer observed in the
four most informative studies is unlikely to be due to confounding by
cigarette smoking and is probably due to exposure to diesel exhaust.
Other studies, although limited primarily by the exposure
ascertainment, support this assessment.
B10.4.1.2 Urinary bladder cancer: occupational exposure
The hypothesis that exposure to diesel exhaust results in cancer
of the urinary bladder is based on the fact that individuals who smoke
cigarettes, a source of PAHs (as is diesel particulate), excrete
mutagenic substances in their urine, and cigarette smoking is
associated with an increased risk for bladder cancer. In a recent
study of railroad workers with current exposure to diesel exhaust
(median concentration of respirable particulates over a work shift,
54-113 µg/m3; adjusted for smoking), mutagenicity in urine at the
end of a shift was not associated with exposure (Schenker et al.,
1992).
The evidence that bladder cancer is associated with exposure to
diesel exhaust is based on an association between work in industries
or occupations with potential exposure to diesel exhaust. The relative
risks reported (some of which are adjusted for smoking) are generally
in the range 1.2-2 (Hoar & Hoover, 1985; Silverman et al., 1986),
although higher risks have been reported. Exposure was assessed mainly
by self-reporting and reporting of occupation at interview. The main
occupation in which there was considered to be potential exposure to
diesel exhaust was truck driving. Measurements of exposure of truck
drivers by Zaebst et al. (1991) indicate that current exposure is
likely to be low, but there is no knowledge about past exposure
levels.
Thus, although work in occupations related to motor vehicles has
been associated with bladder cancer, the extent of exposure of these
workers to diesel exhaust is unknown.
B10.4.2 Dose-response assessment
B10.4.2.1 Lung cancer
Job category and work in an occupation with exposure to diesel
exhaust have been used as indicators of exposure. When years of
exposure and derived indices of exposure have been used, an
exposure-response relationship has generally been found. Measurements
of total and respirable dust in exposed workers do not reflect actual
exposure to diesel exhaust particles, however, since there are other
sources of dust in an occupational environment. The exposure of only
two occupational groups, American railroad workers (Woskie et al.,
1988b) and truck drivers (Zaebst et al., 1991), to diesel exhaust
particles has been quantified (see section B5.2). Current exposure was
measured for both these groups, but past exposure levels could not be
determined (Woskie et al., 1988b), so that studies of workers do not
permit determination of a dose-response relationship for inhaled
diesel particles.
B10.4.2.2 Urinary bladder cancer
Years of work in an occupation related to motor vehicles (mainly
truck driving) was examined in some, but not all, studies, but no
measurements were available to estimate exposure to diesel exhaust in
these settings.
B10.4.3 Exposure assessment
The ranges of exposures observed for workers are presented in
sections B5.2 and B10.2.
B10.4.4 Risk characterization
B10.4.4.1 Human lung cancer
Thus, historical measurements of exposure to diesel exhaust are
unreliable and exist only for current workers in two industries. A
quantitative risk assessment cannot be conducted on the basis of
epidemiological data in which job title was used as a surrogate of
exposure. Attempts to obtain estimates of risk (presented in section
B10.4.1) in the retrospective cohort study of American railroad
workers (Garshick et al., 1988) were not successful (see below for
discussion). Consequently, there are no human data suitable for
estimating unit risk.
B10.4.4.2 Human urinary bladder cancer
The risk for urinary bladder cancer cannot be assessed from the
available epidemiological data.
B10.4.4.3 Risk characterization based on studies in experimental
animals
(a) Introduction
Most human tumours arise in the bronchial region, and although
the frequency of adenocarcinomas has increased in recent years
(Martini, 1993), squamous bronchogenic carcinomas still represent the
majority (about 60%) of lung tumours in humans (Auerbach & Garfinkel,
1991; Devesa et al., 1991; Campobosso et al., 1993). The tumours
observed in rats are located almost exclusively in the bronchoalveolar
regions. This difference raises the question of whether the rat is the
appropriate species for extrapolating to humans. Although it may be
the wrong model, however, studies of hamsters and monkeys give
negative results, so the rat is the most appropriate model from a
conservative viewpoint and is therefore used for quantitative risk
extrapolation.
The objective is to assess quantitatively the potential risk for
lung cancer in humans posed by exposure to diesel exhaust emissions in
the ambient air. Ideally, human risk due to exposure to an
environmental pollutant should be predicted on the basis of human
experience. Although several epidemiological studies are available on
bus and railroad workers, these results alone are not adequate to
assess the potential risk of cancer in humans because of the lack of
reliable information on the conditions of exposure of these workers.
The challenge is to assess the risk of exposure to diesel exhaust on
the basis of all of the available information, for both experimental
animals and humans. In contrast to the sparse human data, there is a
rich pool of information on diesel-induced lung tumours in two strains
of rat. One approach to integrating this information is to make a
quantitative risk assessment on the basis of information from
bioassays and on the relevant biological mechanism and then to
evaluate these animal-based results against available human
experience. This approach was followed in this monograph.
Although, the available experimental data on the possible impact
of extractable organic matter and PAHs on the lung tumour response
associated with exposure to diesel soot are not strong (Heinrich et
al., 1991; Heinrich, 1994; Heinrich et al., 1995), a mathematical
approach to assessing the risk of exposure to diesel exhaust should
take into account the effects of both particles (carbon core) and
extractable organic matter, because: (i) organic compounds include a
variety of PAHs and nitroaromatic compounds, many of which are known
to be carcinogenic; (ii) the results of recent studies on inert
particles and carbon black in rats (Mauderly et al., 1991; Heinrich,
1994; Heinrich et al., 1994) strongly support the hypothesis that the
carbon cores of diesel particles are the component primarily
responsible for the induction of lung cancer; (iii) PAHs alone are
unlikely to be responsible for the tumour response; (iv) the
disproportionately high tumour incidence in animals with heavy
exposure coincides with a disproportionate increase in the cumulative
lung burden of diesel particles. Although a qualitative description of
the biological mechanism of diesel exhaust-induced tumours is
plausible, however, the lack of quantitative information on the
dynamics of tumour initiation, promotion, and progression vitiates the
construction of a biologically based dose-response model.
The crudest dose-response model is obtained by fitting observed
dose-response data to a mathematical function that is a monotonic,
non-decreasing function of exposure, using the inhaled concentration
and observed tumour incidence, without considering information on
pharmacokinetics and the mechanism of carcinogenesis. This approach
may result in uncertain estimates of risk at low doses, because, while
the model may adequately fit the dose-response data for high doses at
which a tumour response is observed, it may greatly underestimate or
overestimate the risk at low doses.
Most previous risk estimates of diesel exhaust-induced cancer
risk were developed before the results of many of the bioassays became
available (Pepelko & Chen, 1993). Nevertheless, most of the estimates
are similar (within an order of magnitude), except for those
calculated on the basis of human data, which are higher than those
calculated from other databases. Because of great uncertainties
associated with the available human data, it is considered more
appropriate to calculate risk on the basis of animal data and then use
human data to evaluate their validity.
The approach adopted in this assessment is the linearized
multistage model (Anderson et al., 1983), in which burden per lung
surface area (milligrams per square centimetre) is used as an
equivalent dose in animals and humans. The use of lung (alveolar)
surface area is justified by the fact that the lung tumours observed
in rats are derived exclusively from epithelial cells in the alveolar
region of the lung. Normalization on the basis of lung weight can also
be done by using a factor of 4, for increasing the dose or decreasing
the risk, for the difference in the ratio of rat:human lung surface
area and lung weight. The risk prediction calculated by this approach
is presumably conservative (i.e. overpredicts the risk) because the
dose-response function is assumed to be non-threshold and linear at
low doses.
An alternative model that incorporates a biological mechanism is
constructed using statistically estimated parameters. The model
assumes that carcinogenic agents present in the organic fraction act
directly on the target cells, primarily via initiation. It is further
assumed that the majority of the particles are ingested by
macrophages. Particle-laden macrophages are then induced to secrete a
variety of mediators (e.g. reactive oxygen species and cytokines),
which diffuse to the target cells and induce initiation,
proliferation, and conversion of initiated cells to malignant cells.
This alternative model can be used only as a tool to evaluate the
impact of various biological assumptions and not for risk prediction,
because the parameters of the model are not measured in the
laboratory.
(b) Approaches to quantification of human risk from exposure to
diesel exhaust
Several issues must be addressed in estimating the risk of
exposure to diesel exhaust, including the critical target site, the
fraction of exhaust responsible for tumour induction, the availability
of dosimetric methods for accurately extrapolating dose from that for
experimental animals chronically exposed to high concentrations of
exhaust to that of humans exposed at ambient concentrations, and the
most suitable low-dose extrapolation model.
The critical target organ was considered to be the lung. Although
Iwai et al. (1986) reported induction of both malignant lymphomas and
lung tumours in rats exposed to diesel exhaust, the lung was the only
target site in other experimental studies of this species. Although
potentially carcinogenic agents present in diesel exhaust may be
absorbed from the lungs, enter the bloodstream, and be transported
systemically, no data are available to evaluate this possibility.
Organic compounds adsorbed on particles may also reach systemic
targets via the gastrointestinal tract, as particles deposited in the
conducting airways are cleared rapidly to the oropharynx and
swallowed. A considerable volume of particles is also likely to be
ingested as a result of grooming when the whole animal is exposed
(Wolff et. al., 1982); however, because the half-times for the elution
of organic compounds from particles are considerably longer than their
passage through the gastrointestinal tract, the fraction absorbed is
expected to be small. In any case, there is little evidence for any
systemic effects of diesel exhaust.
The site of action in the lungs is assumed to be the epithelial
lining of the alveoli and small airways. According to Mauderly et al.
(1987), inflammation and tumours appear to arise from this tissue.
Although interstitial events (e.g. fibrosis) have been suggested to be
associated with induction of lung tumours by particles (Kuschner,
1968), no data are available to support this view with respect to
diesel exhaust.
Accurate extrapolation of dose from studies of animals exposed to
high concentrations of exhaust to humans exposed to ambient
concentrations requires a variety of adjustments for species
differences in deposition efficiency and respiratory ventilation
rates. As the normal retention half-times in the alveolar region are
several times longer in humans than rats (Chan et al., 1981; Bohning
et al., 1982), the lung burden of humans may be underestimated if this
difference is not taken into account. The high exposure concentrations
used in some experimental studies, however, result in greatly slowed
or even completely inhibited clearance (Griffis et al., 1983). In
order to extrapolate dose accurately from experimental studies to
humans, the detailed dosimetric model developed by Yu et al. (1991)
was used, which accounts for species differences in respiratory
ventilation rates, deposition efficiency, normal particle clearance
rates, transport of particles to lung-associated lymph nodes, and lung
surface area. It also accounts for inhibition of particle clearance
due to lung overload. In this model, dose is estimated in terms of
particle concentration per unit of lung surface area.
Two approaches were used to derive unit risk estimates: the
linearized, multistage, low-dose extrapolation model and a model based
on the biological mechanism discussed above. In the linearized
multistage model, the lung burden of carbon core per lung surface area
is used as the dosimetric parameter. A particle-based assessment was
considered to be reasonable for two principal reasons: (i) exposure to
the vapour phase alone did not result in detectable tumour induction
in rats (Brightwell et al., 1986; Iwai et al., 1986; Heinrich et al.,
1986a); and (ii) exposure to carbon black, which is similar in
composition to the carbon core of whole diesel exhaust but contains
only negligible amounts of organic compounds, was about as effective
in inducing lung cancer as whole diesel exhaust (Mauderly et al.,
1991; Nikula et al., 1994; Heinrich et al., 1995). Although use of the
carbon core as a dosimetric parameter implies that it is primarily the
insoluble carbon core of diesel particles that is responsible for the
carcinogenic effects of diesel exhaust, it can also be viewed simply
as a marker of exposure to diesel exhaust, with no biological
implication. Similarly, organic compounds could be used as markers of
exposure for the dose-response calculation. When this is done, the
resulting unit risk estimates (not presented here) are very similar
(within 25%) to those calculated with the carbon core as the surrogate
of exposure.
The second approach is based on the assumption that, even though
the concentration of carcinogenic compounds on diesel particles is
low, they nevertheless can act in concert with the particles to induce
carcinogenesis. An alternative low-dose extrapolation model was
therefore developed that allows for the possibility that various PAHs
and nitroaromatic compounds may induce organ-specific adducts that
contribute to cell initiation. This model is described in Appendix A1.
Both approaches incorporate the detailed dosimetric model of Yu
et al. (1991) to estimate dose at the airway and alveolar surfaces.
Risk is based on the assumption that an equivalent concentration
(dose) per unit of alveolar surface area results in equivalent risks
in humans and rats.
(c) Evidence to support a carcinogenic effect of the carbon core
The study of Heinrich et al. (1994) on carbon black and coal-tar
pitch provides information useful for evaluating the assumptions used
to construct the model that reflects current thinking about the
mechanism of action of diesel exhaust in inducing lung tumours. One
relevant question about diesel exhaust is whether lung tumours are
induced by the carbon core alone or in combination with organic
compounds.
In this study, female Wistar rats were exposed to carbon black or
tar pitch, or both for 10 months (43 weeks) or 20 months (86 weeks)
and followed until the end of the study, which lasted for
two-and-a-half years. No lung tumours were observed in the control
group. For each exposed group, the probability of dying from lung
cancer was calculated by a Kaplan-Meier survival analysis. Since these
data are used solely for interpretation and not for risk prediction,
it is statistically more appropriate to use only data on mortality,
excluding those tumours found at terminal sacrifice. Figure 9 shows
the probability of dying from lung cancer for the rats that were
exposed either to 6 mg/m3 of carbon black, or to coal-tar pitch
containing 50 µg/m3 of benzo[a]pyrene, or to a combination of the
two for 10 months, and then followed until the end of the study. In
Figure 10, each plot represents the time at which a tumour occurred.
To provide a better visual presentation, the probabilities at these
points are connected by straight lines. (Note: It is statistically
more appropriate to present these probabilities by a step function
rather than by interpolation when the Kaplan-Meier procedure is used.)
Two conclusions can be drawn from Figure 10: (1) Tumours appeared
much earlier in animals exposed to coal-tar pitch alone or in
combination with carbon black than in animals exposed to carbon black
alone. For instance, the first tumour occurred 665 days after the
start of exposure to carbon black, 406 days after exposure to coal-tar
pitch, and only 310 days after exposure to the combination. (2) Carbon
black and coal-tar pitch together caused a higher rate of mortality
from tumours than the sum of mortality caused by carbon black and
coal-tar pitch separately. The probability of dying from a lung tumour
before terminal sacrifice at 912 days was 0.28 for carbon black, 0.52
for coal-tar pitch, and 0.99 for the combination. These two
observations suggest that carbon black and coal-tar pitch act on
different stages of tumorigenesis. They are consistent with the
hypothesis that carbon black can increase the proliferation rates of
normal and cells at different stages of differentiation initiated
spontaneously or by coal-tar pitch.
When the rate of mortality from tumours among rats exposed to
diesel exhaust is compared with that of groups exposed to carbon
black, coal-tar pitch, or the combination (Figure 9), the rate among
the animals exposed to diesel exhaust is comaprable to that of rats
exposd to carbon black for 10 to 20 months. This implies that carbon
black alone can induce a tumour response similar to that induced by
diesel exhaust. Figure 9 also shows that doubling the duration of
exposure to carbon black from 10 to 20 months does not increase the
probability of dying from a lung tumour before terminal sacrifice;
however, doubling the duration of exposure to coal-tar pitch
significantly increases the probability of dying from a lung tumour.
Figure 11 shows that the shapes of the curves for age-dependent
mortality from cancer for animals exposed to diesel exhaust and
coal-tar pitch for 20 months are similar. It should be kept in mind
that the PAH content of the atmosphere containing both carbon black
and coal-tar pitch was about 1000 times higher than that of diesel
engine exhaust. These observations may have profound implications for
elucidating the mechanisms of induction of lung tumours by diesel
exhaust, but more refined analyses are necessary before any conjecture
can be made.
(d) Data available for calculating risk
Seven bioassays have shown lung tumour responses in rats
(Brightwell et al., 1986; Heinrich et al., 1986b; Ishinishi et al.,
1986; Iwai et al., 1986; Mauderly et al., 1987; Nikula et al., 1994;
Heinrich et al., 1995). Four studies were chosen for use in
calculating unit risk (Table 48) because they involved multiple
exposure groups. Data on time to event (death with or without a
tumour) are available in the studies of both Mauderly et al. (1987)
and Heinrich et al. (1995) and were used in all of the risk
calculations.
(e) Calculation of unit risks
The unit risk from exposure to an air pollutant is defined as the
95% upper bound of the increased lifetime cancer risk for an
individual continuously exposed to a concentration of 1 µg/m3 in
ambient air. Unit risk is a convenient tool for calculating lifetime
risk due to exposure to a pollutant when low-dose linearity is
assumed. Under this assumption, the risk due to exposure to d µg/m3
of pollutant can be calculated from u × d if the pollutant has a
unit risk of u/µg per m3. This calculation is valid, however, only
when the level of risk is low. The results of unit risk calculations
are summarized in Table 49.


 Table 48. Incidence of lung tumours in rats exposed to diesel engine exhaust
Strain Exposure Dose metric Lung tumour Reference
(sex) incidence
Schedule Concentration Weekly exposure Lung particle burden
(mg/m3) (mg/m3 × h) (mg/cm2 lung surface)a
Fischer 7 h/d, 0 0 0 2/230 Mauderly et al.
344 5 d/week 0.35 12 6.4 × 10-5 3/223 (1987)
(f+m) 3.50 122 2.8 × 10-3 8/222
7.08 248 6.0 × 10-3 29/227
Wistar 18 h/d, 0 0 0 1/217 Heinrich et al.
(f) 5 d/week, 0.84 76 5.8 × 10-4 0/198 (1995)
2 years, 2.50 225 5.2 × 10-3 11/200
< 6 months 6.98 628 1.5 × 10-2 22/100
follow-up
Fischer 16 h/d, 0 0 0 1/123 Ishinishi et al.
344 6 d/weekb 0.46 44 2.5 × 10-4 1/123 (1986)
(f+m) 0.96 92 2.0 × 10-3 0/125
1.84 177 4.2 × 10-3 4/123
3.72 357 8.8 × 10-3 8/124
Fischer 16 h/d, 0 0 0 4/250 Brightwell et al.
344 5 d/week 0.7 56 3.5 × 10-4 1/112 (1986)
(f+m) 2.2 176 4.2 × 10-3 14/112
6.6 528 1.3 × 10-2 55/111
a Calculated from the model of Yu et al. (1991)
b Data on heavy-duty diesel engine exhaust used because those for light-duty engines showed no statistically significant difference
Particle-based model: The linearized multistage model, which is
used as a conservative approach to estimating risk, has the
mathematical form P = 1 exp(-Z), where Z is either q0 + q1 × d + ...
+ qm × dm of a polynomial concentration d, or ( Q0 + Q1 × d + ...
+ Qm × dm) × tk, a polynomial of concentration d multiplied by a time
factor, tk, when data on time to event are used. In this case, the
lifetime risk is calculated from actuarial life tables using the
survival probability of control Fischer 344 rats within the National
Toxicology Program, provided by Portier et al. (1986). The range of
extrapolation is about three orders of magnitude in the present
assessment. Denote P0 the lifetime cancer risk at concentration 0.
Because the extra risk (P-P0)/(1-P0) is dominated by the linear term
q1 × d at low concentrations, the 95% upper bound of q1 is used
to represent unit risk.
In extrapolating risk from animals to humans, a dose metric that
induces the same tumour incidence rate in animals and humans must be
assumed (i.e. dose equivalence). To calculate equivalent doses, a
mathematical model is used to adjust for the dosimetric parameters
that determine the lung burden of particulate matter in rats and
humans and to correct to dose per unit lung surface area. This dose is
considered to be equally potent in inducing lung tumours in animals
and in humans. The model accounts for differences between animals and
humans in regional deposition efficiency, particle clearance rates at
low doses and at doses that result in impaired clearance, and lung
surface area. In these calculations, the mass fraction of organic
compounds adsorbed on particles is assumed to be 20%; one-half of the
mass is assumed to be composed of slowly eluted organic compounds
(half-life, 30 days) and the other half of rapidly eluted organic
compounds (half-life, 1.3 h). The remainder is considered to be
inorganic carbon. At higher concentrations, particle clearance slows
and may even stop, and the lung particle burdens increase continually
during exposure. The organic constituents, however, are eluted from
the particles fairly quickly and reach a steady state even during
continued exposure to high concentrations. The lung burdens of organic
compounds are therefore less affected by inhibition of clearance by
overloading.
In the linearized multistage model, determination of the dose of
the carbon core is problematic because the lung burdens after low and
high exposures differ drastically over time. This difference suggests
that use of a lung burden at a fixed time (e.g. one year after the
start of exposure) to represent dose may not be appropriate. In this
assessment, the average lung burden is used as the dose at the target
organ. The average lung burden is calculated by dividing the area
under the curve of lung burden over time by the corresponding period
of the experiment for which the curve was calculated. Figure 12 shows
a comparison of the lung burden predicted from the model and that
observed in the laboratory (Muhle et al., 1994) at specific times. It
suggests that the model used to calculate lung burden is adequate.
Table 49. Estimates of unit risk per microgram of particles per
cubic metre of diesel exhaust
Upper 95% confidence limit of cancer Study
risk due to exposure to 1 µg/m3 of
diesel particulate matter
3.4 × 10-5 Mauderly et al. (1987)
1.6 × 10-5a Ishinishi et al. (1988)
7.1 × 10-5 Brightwell et al. (1986)
3.4 × 10-5 Heinrich et al. (1995)
3.4 × 10-5 Geometric mean of
four studies
If milligrams per lung weight are used instead of milligrams per lung
surface as the equivalent dose, the risk estimates are reduced by a
factor of 4.
a Heavy-duty diesel engine
Biologically based model: A second approach to estimating risk
was used because it was considered more desirable to base risk on a
biologically based dose-response model. Although the data are at
present insufficient to replace the linearized multistage model, which
is considered more conservative, the implications of hypothetical
mechanisms of cancer induction by diesel particles can nevertheless be
investigated. The biological issues considered include the effects on
the carcinogenic process of particle-adsorbed organic compounds and of
a variety of mediators secreted by particle-laden macrophages. A
stochastic model was developed, which:
-- accounts for the possible effects of both the carbon particles
and their associated organic compounds:
-- allows evaluation of the contribution to tumour induction of
both carbon particles and organic compounds;
-- allows for changing parameters with increasing lung burden;
and
-- assumes that cell proliferation and tumour induction are
stochastic. (For instance, it is not appropriate to assume that
all cells divide at the same age.)
Unlike the linearized multistage model, this approach does not require
a constant dose metric but allows for varying lung burden over time.
Table 48. Incidence of lung tumours in rats exposed to diesel engine exhaust
Strain Exposure Dose metric Lung tumour Reference
(sex) incidence
Schedule Concentration Weekly exposure Lung particle burden
(mg/m3) (mg/m3 × h) (mg/cm2 lung surface)a
Fischer 7 h/d, 0 0 0 2/230 Mauderly et al.
344 5 d/week 0.35 12 6.4 × 10-5 3/223 (1987)
(f+m) 3.50 122 2.8 × 10-3 8/222
7.08 248 6.0 × 10-3 29/227
Wistar 18 h/d, 0 0 0 1/217 Heinrich et al.
(f) 5 d/week, 0.84 76 5.8 × 10-4 0/198 (1995)
2 years, 2.50 225 5.2 × 10-3 11/200
< 6 months 6.98 628 1.5 × 10-2 22/100
follow-up
Fischer 16 h/d, 0 0 0 1/123 Ishinishi et al.
344 6 d/weekb 0.46 44 2.5 × 10-4 1/123 (1986)
(f+m) 0.96 92 2.0 × 10-3 0/125
1.84 177 4.2 × 10-3 4/123
3.72 357 8.8 × 10-3 8/124
Fischer 16 h/d, 0 0 0 4/250 Brightwell et al.
344 5 d/week 0.7 56 3.5 × 10-4 1/112 (1986)
(f+m) 2.2 176 4.2 × 10-3 14/112
6.6 528 1.3 × 10-2 55/111
a Calculated from the model of Yu et al. (1991)
b Data on heavy-duty diesel engine exhaust used because those for light-duty engines showed no statistically significant difference
Particle-based model: The linearized multistage model, which is
used as a conservative approach to estimating risk, has the
mathematical form P = 1 exp(-Z), where Z is either q0 + q1 × d + ...
+ qm × dm of a polynomial concentration d, or ( Q0 + Q1 × d + ...
+ Qm × dm) × tk, a polynomial of concentration d multiplied by a time
factor, tk, when data on time to event are used. In this case, the
lifetime risk is calculated from actuarial life tables using the
survival probability of control Fischer 344 rats within the National
Toxicology Program, provided by Portier et al. (1986). The range of
extrapolation is about three orders of magnitude in the present
assessment. Denote P0 the lifetime cancer risk at concentration 0.
Because the extra risk (P-P0)/(1-P0) is dominated by the linear term
q1 × d at low concentrations, the 95% upper bound of q1 is used
to represent unit risk.
In extrapolating risk from animals to humans, a dose metric that
induces the same tumour incidence rate in animals and humans must be
assumed (i.e. dose equivalence). To calculate equivalent doses, a
mathematical model is used to adjust for the dosimetric parameters
that determine the lung burden of particulate matter in rats and
humans and to correct to dose per unit lung surface area. This dose is
considered to be equally potent in inducing lung tumours in animals
and in humans. The model accounts for differences between animals and
humans in regional deposition efficiency, particle clearance rates at
low doses and at doses that result in impaired clearance, and lung
surface area. In these calculations, the mass fraction of organic
compounds adsorbed on particles is assumed to be 20%; one-half of the
mass is assumed to be composed of slowly eluted organic compounds
(half-life, 30 days) and the other half of rapidly eluted organic
compounds (half-life, 1.3 h). The remainder is considered to be
inorganic carbon. At higher concentrations, particle clearance slows
and may even stop, and the lung particle burdens increase continually
during exposure. The organic constituents, however, are eluted from
the particles fairly quickly and reach a steady state even during
continued exposure to high concentrations. The lung burdens of organic
compounds are therefore less affected by inhibition of clearance by
overloading.
In the linearized multistage model, determination of the dose of
the carbon core is problematic because the lung burdens after low and
high exposures differ drastically over time. This difference suggests
that use of a lung burden at a fixed time (e.g. one year after the
start of exposure) to represent dose may not be appropriate. In this
assessment, the average lung burden is used as the dose at the target
organ. The average lung burden is calculated by dividing the area
under the curve of lung burden over time by the corresponding period
of the experiment for which the curve was calculated. Figure 12 shows
a comparison of the lung burden predicted from the model and that
observed in the laboratory (Muhle et al., 1994) at specific times. It
suggests that the model used to calculate lung burden is adequate.
Table 49. Estimates of unit risk per microgram of particles per
cubic metre of diesel exhaust
Upper 95% confidence limit of cancer Study
risk due to exposure to 1 µg/m3 of
diesel particulate matter
3.4 × 10-5 Mauderly et al. (1987)
1.6 × 10-5a Ishinishi et al. (1988)
7.1 × 10-5 Brightwell et al. (1986)
3.4 × 10-5 Heinrich et al. (1995)
3.4 × 10-5 Geometric mean of
four studies
If milligrams per lung weight are used instead of milligrams per lung
surface as the equivalent dose, the risk estimates are reduced by a
factor of 4.
a Heavy-duty diesel engine
Biologically based model: A second approach to estimating risk
was used because it was considered more desirable to base risk on a
biologically based dose-response model. Although the data are at
present insufficient to replace the linearized multistage model, which
is considered more conservative, the implications of hypothetical
mechanisms of cancer induction by diesel particles can nevertheless be
investigated. The biological issues considered include the effects on
the carcinogenic process of particle-adsorbed organic compounds and of
a variety of mediators secreted by particle-laden macrophages. A
stochastic model was developed, which:
-- accounts for the possible effects of both the carbon particles
and their associated organic compounds:
-- allows evaluation of the contribution to tumour induction of
both carbon particles and organic compounds;
-- allows for changing parameters with increasing lung burden;
and
-- assumes that cell proliferation and tumour induction are
stochastic. (For instance, it is not appropriate to assume that
all cells divide at the same age.)
Unlike the linearized multistage model, this approach does not require
a constant dose metric but allows for varying lung burden over time.
 The model, which is described in Appendix B10.3, allows for
initiation by both the carbon and the organic fraction and for the
proliferative effects of the carbon fraction. Although these
mechanisms remain to be proven, it is assumed that carcinogenic agents
present in the organic fraction act directly on the target cells,
primarily by initiation. It is further assumed that most of the
particles are ingested by macrophages. Particle-laden macrophages are
then induced to secrete a variety of mediators (e.g. reactive oxygen
species and cytokines), which diffuse to the target cells, inducing
initiation, proliferation, and conversion of initiated cells to
malignant cells.
The results reported by Mauderly et al. (1987) for tumour
induction were used to estimate the model parameters. These are based
on the development of malignant tumours rather than all tumours as in
the first method. This was necessary in order to ensure that the data
used to estimate the parameters represented the same biological
mechanism. Lung burdens were calculated from the same dosimetry model
used in the linearized multistage model.
(f) Results of unit risk calculations
Particle-based model: Unit risks were derived from the linearized
multistage approach for the tumour incidences seen in four bioassays
(Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly et al.,
1987; Heinrich et al., 1995) and the corresponding equivalent doses
(Table 48). The resulting unit risk estimates, listed in Table 49,
range from 1.6 to 7.1 × 10-5/µg particles per m3 with a geometric
mean of 3.4 × 10-5/µg per m3.
In these calculations, the relationship between air concentration
(micrograms per cubic metre) and lung burden (milligrams) in humans is
used to determine the lung burden resulting from lifetime exposure to
1 g/m3 of diesel exhaust particulate matter. The particle burden in
terms of mass per unit lung surface area is then multiplied by the
slope derived from the bioassay data. For instance, when the data of
Mauderly et al. (1987) are used, the slope of the curve for
carcinogenicity in rats (i.e. the upper 95% confidence limit of the
linear coefficient in the multistage model), expressed in terms of
equivalent dose (micrograms of carbon particulate matter per square
centimetre) is 1.7 × 10-2/µg per cm2. According to the dosimetry
model, an air concentration of 1 µg/m3 of particulate matter
corresponds to a mass of 1230 µg of carbon particles per human lung.
Because the lung epithelial surface area, including the alveolar
region and conducting airways, is assumed to be 627 000 cm2, a unit
risk of 3.4 × 10-5/µg per m3 is derived from 1.7 × 10-2/µg per cm2
× 1230 µg/627 000 cm2.
Biologically based model: The unit risk estimated by the
alternative model, based on the data on malignant tumours from the
study of Mauderly et al. (1987), is equal to 1.65 × 10-5/µg particles
per m3. This is lower than the estimate of 3.4 × 10-5/µg per m3
derived from the same study with the linearized multistage approach.
Application of the linearized multistage model only to malignant
tumours seen in that study, rather than all lung tumours, resulted in
a unit risk estimate of 1.74 × 10-5/µg particles per m3 (Table 50).
Thus, the unit risk estimates obtained by the two approaches are
virtually identical. The estimated risks may differ somewhat with
increasing doses, because the slopes are not identical at all exposure
levels. It should be noted, however, that the unit risk predicted by
the alternative model is derived under the assumption that particles
continue to exert an effect on cell initiation or proliferation (or
both) at low doses. There is considerable uncertainty about the
effects of particles at low doses. It has been claimed that particles
do not induce initiation or proliferation at low doses (Vostal, 1986).
At present, the evidence is inadequate to support or refute this
claim. Moreover, even if macrophage overload is required, because of
uneven distribution of particles, some macrophages may become
overloaded even at low concentrations. Because of this uncertainty,
the biologically based model, like the linearized multistage model,
does not depart from linearity at low doses; however, if initiation
and proliferation do not occur at low doses, the risk may be much
smaller.
In an estimate of unit risk for rats, based on the results of
seven studies by inhalation and using linear interpolation and the
linearized multistage model, the risks were 7 × 10-5/µg of diesel
particles per m3 and 10 × 10-5/µg carbon core particles per m3
(Csicsaky et al., 1993; Pott et al., 1993; Roller & Pott, 1994).
(g) Results and implications of the biologically based model
On the assumption that particles continue to affect cell
initiation or proliferation at low doses, the risks calculated with
this model are comparable to those obtained with the linearized
multistage model. Excess risks due to various exposures are shown in
Tables 50 and 51. Those in Table 50 are the risks predicted for humans
exposed continuously (24 h/day) from the two models: it is interesting
to note that the results are similar. Table 51 shows the excess risks
due to exposure to 2.6 µg/m3 of diesel particulate emission for
16 h/day on seven days per week and to 15 µg/m3 for 8 h/day on five
days per week. The first concentration was reported by the US
Environmental Protection Agency Office of Mobile Sources to be the
annual mean exposure of the American population to diesel particulate
matter in 1986; the second concentration was reported to be that to
which workers are exposed on urban freeways (Carey, 1987). The
retention half-time for insoluble particles after exposure to
Table 50. Comparison of excess risk for humans due to continuous exposure to
various concentrations of diesel exhaust emissions using two
different models
Exposure concentration Biologically based (alternative) Linearized (µg/m3)
multistage
Maximum likelihood Upper 95% model
estimate bound estimate
0.01 7.68 × 10-8 1.35 × 10-7 1.71 × 10-7
0.1 8.12 × 10-7 1.71 × 10-6 1.72 × 10-7
1.0 (unit risk) 8.16 × 10-6 1.65 × 10-5 1.74 × 10-5
100 5.58 × 10-4 9.63 × 10-4 1.74 × 10-4
1000 2.60 × 10-2 4.22 × 10-2 3.33 × 10-2
a Slope - 9.04 per mg/cm2 of lung surface, using carbon core as dosimetric.
Only malignant tumours are used in the calculations.
2.6 µg/m3 is shown to increase from 296 days for members of the
general population with normal respiratory function to 519 days for
those with a smoking history of 20 pack-years (Bohning et al., 1982).
This information was used to reduce the alveolar clearance rate for
the dosimetric calculations to that used for other risk calculations.
Interactive effects of smoking and diesel exhaust are not considered
in the risk calculations owing to lack of data. The studies of carbon
black and coal-tar pitch (Heinrich, 1994; Heinrich et al., 1994)
indicate that smokers have a higher risk for lung cancer than
nonsmokers when they are exposed to diesel exhaust.
The excess lifetime risks shown in Tables 50 and 51 are
standardized by the actuarial life-table approach, using the survival
probability of control animals in the US National Toxicology Program
provided by Portier et al. (1986). This approach gives a weighted
average of the probability of cancer occurrence over an entire
lifetime, weighted by survival probability.
Table 51. Excess lifetime risk for humans due to exposure to diesel
exhaust emissions under various exposure scenarios
Exposure pattern Biologically based model Linearized
multistage
Maximum Upper 95% modela
likelihood bound
estimate estimate
General population (normal 1.41 × 10-5 2.44 × 10-5
respiratory function;
nonsmoker): 2.6 µg/m3,
16 h/day, seven days
per week
General population 2.32 × 10-5 3.61 × 10-5 5.38 × 10-5
(20-pack-year smoker)b:
2.6 µg/m3, 16 h/day,
seven days per week
Occupationally exposed: 3.12 × 10-5 5.17 × 10-5 6.18 × 10-5
15 µg/m3 8 h/day, five
days per week
a Calculated using carbon core as dosimetric; only malignant
tumours are used.
b In this calculation, smoking affects only lung clearance rate;
biological interaction between smoking and exposure to diesel
exhaust is not considered. The retention half-times for
insoluble particles increased from 296 days for persons with
normal respiratory function to 519 days for 20-pack-year smokers
(Bohning et al., 1982).
Some implications of the alternative model are:
(1) At a low exposure concentration, the decrease in diesel-induced
initiation results in a greater reduction of risk; that is, the
number of initiated cells (cancer risk) is reduced more
efficiently with a low than with a high exposure. If only organic
compounds induce initiation when the concentration is low, they
play a more important role than particles in inducing tumours at
low concentrations, whereas the roles are reversed when the
concentration is high.
(2) Although cells initiated by diesel exhaust play an important role
in cancer induction, either organic compounds or the carbon core
alone could induce initiation by increasing their respective
proportions. Thus, although initiated cells are important for
tumour induction, they may be induced by any agent that initiates
tumours (e.g. smoking).
(3) A small change in the rate of proliferation induced by diesel
exhaust could disproportionately change cancer risk. As this
parameter is assumed to be a function of the dose of carbon core,
lung overloading has a significant effect on cancer incidence. In
the absence of better information, it is assumed in this
assessment that the carbon core continues to have a proliferating
effect at low doses.
These observations suggest that, although the effect of particle
overload on cell proliferation is important, initiation by the carbon
core or organic compounds or both is also essential. Although this
conclusion is only tentative, because the model parameters are
estimated statistically on the basis of bioassays conducted at high
concentrations, it does suggest the importance of studying the role of
the carbon core and organic compounds in initiation and promotion at
low and high exposure concentrations. Does the relative initiation
potential of organic compounds and the carbon core differ with
concentration? These observations also suggest that a subcohort of
workers who were smokers and were exposed to high concentrations of
diesel exhaust for a long time would have a greater risk of dying from
lung cancer.
(h) Comparison of risk estimates derived from experimental studies
and human experience
The bioassay-based risk estimates, which range from 1.6 × 10-5 to
7.1 × 10-5, with a geometric mean of 3.4 x 10-5, can be compared with
human experience on the basis of three data sets: those of an
epidemiological study conducted on London Transport employees (Waller,
1981) and a subsequent analysis (Harris, 1983) and those on American
railroad workers (Garshick et al., 1987, 1988). Although these data
cannot be used to calculate unit risk, mainly because of a lack of
reliable information on exposure, they can be used to evaluate the
validity of unit risk estimates based on the results of experimental
studies.
Attempts have already been made to estimate the potential cancer
risk due to exposure to diesel exhaust on the basis of epidemiological
data. From the results of the study of London Transport workers,
Harris (1983) estimated that the increase in the relative risk for
lung cancer associated with exposure to 1 µg/m3-year of diesel exhaust
was 1.2 × 10-4, with an upper bound of the 95% confidence limit of
4.8 × 10-4. (Generally, when data from an epidemiological study with
negative results are used to estimate cancer risk, the upper bound is
used to calculate unit risk.) The resulting unit risk estimate is 2 ×
10-3, which is about 60 times higher than the mean unit risk estimate
derived from bioassays, 3.4 × 10-5, and about 30 times higher than the
upper end of the range of unit risk estimates from bioassays, 7.1 ×
10-5. Therefore, the risk estimate based on bioassays is not
inconsistent with that for humans, since 2 × 10-3 is the estimated
upper bound in an epidemiological study with negative results.
McClellan et al. (1989) reported risk estimates based on the
study of Garshick et al. (1987), in which lung cancers in railroad
workers were evaluated. Assuming exposure to concentrations of 500 and
125 µg/m3, the upper bounds of the 95% confidence interval for
lifetime cancer risk were estimated to be 6 × 10-4 and 2 × 10-3,
respectively. The lower of the two unit risk estimates is only about
one order of magnitude higher than the risk estimates based on
bioassays.
An epidemiological study potentially more suitable for
quantitative risk assessment was reported by Garshick et al. (1988),
which was based on a large number of subjects; a small but significant
increase in the rate of mortality from lung cancer was seen in some
subcohorts. The US Environmental Protection Agency has supported an
effort to derive a unit risk estimate from this study, and data on
exposure were estimated by Woskie et al. (1988a,b) and Hammond et al.
(1988). These data were analysed in a variety of ways, using relative
risk and absolute risk dose-response models and by classifying
individuals into various exposure categories, including job, duration
of employment, age, and exposure markers. Even though at least 50
analyses were carried out, an adequate dose-response relationship
could not be obtained; these data were therefore not used to estimate
unit risk.
The lack of a dose-response relationship is not totally
unexpected, given the low increase in mortality rate in the study of
Garshick et al. (1988) and the uncertain estimates of exposure. The
data on exposure were derived from air samples collected during a
limited period (1981-83) on four small railroads operating in a
limited geographical area (northern United States), in which
concentrations of respirable particulate matter were measured rather
than diesel exhaust per se; the measured concentrations were then
adjusted to derive markers of exposure. The data were used to estimate
exposure to diesel exhaust of railroad workers throughout the United
States 30 or more years earlier. Diesel equipment and working
conditions have, however, changed since the 1940s when diesel engines
first began to be used in large numbers. Woskie et al. (1988b)
summarized anecdotal reports of smoky working conditions in diesel
repair shops during the 1950s and 1960s and reported that the limited
data available on levels of nitrogen oxide during those periods
indicated high levels of diesel exhaust. By the time samples were
collected, however, the smoky conditions would have been largely
mitigated by improved ventilation and the advent of less smoky diesel
engines. The study of Garshick et al. (1988) gives the relative risks
for dying from lung cancer in exposed as opposed to unexposed railroad
workers classified into five subcohorts by age in 1959. The risks
ranged from 0.96 (95% CI, 0.74-1.33) to 1.45 (1.11-1.89). The highest
relative risk, 1.45, which was observed in workers who were 40-45
years old in 1959, was used here to evaluate the validity of unit risk
based on biossays results. Assuming that this subcohort of workers was
exposed to diesel exhaust for 8 h/day on five days per week from age
35 to age 65, the background mortality rate from lung cancer in this
subcohort would be 0.038 (0.63 × 0.06), as the unexposed workers in
the same age group had a relative risk of 0.63 and the corresponding
lifetime mortality rate from lung cancer in the general American white
male population is about 0.06. If the lung cancer risk due to
1 µg/m3 is assumed to be 3.4 × 10-5, the concentration of diesel
exhaust in the working environment was at least 400 µg/m3, calculated
as follows: The risk due to 1 µg/cm2 of particles is 0.017 (which
results in a unit risk of 3.4 × 10-5). For a lower bound of the
relative risk of 1.1, a lung burden, d, that satisfies the
relationship 0.1 × 0.038 = 0.017 d would be needed; that is, d =
0.22 µg/cm2, which is equivalent to an air concentration of
0.4 mg/m3 by the dosimetry model of Yu et al. (1991). If the highest
unit risk estimate derived from Brightwell et al. (1986), 7.1 ×
10-5, is used, the required minimal air concentration would be about
0.2 mg/m3. These calculations imply that a diesel exhaust
concentration of at least 0.2 mg/m3 was necessary to observe a
statistically significant increase in the mortality rate from lung
cancer in this study. This concentration appears to be reasonable in
the light of the working conditions described by Woskie et al.
(1988a).
Although the risk estimates derived from bioassays are lower than
those derived from human data and may possibly over-predict risk,
since the non-linearity of the human response is not considered in
this model, they are not inconsistent, for the following reasons:
(1) The risk estimates based on epidemiological studies are not
derived from all of the available data but on only a subset with
the highest response. The estimates are therefore higher than
those that would be derived from the whole data set.
(2) When a single data point (i.e. an overall relative risk and an
averaged exposure concentration) is used in the calculations, the
resulting slope for potency will be overestimated if the
dose-response relationship is not linear over all exposure
concentrations. Assume, for example, that the response follows
the simple multistage model P(d) = 1 - exp[-( q0 + qd + q2 d2)].
The relative risk, R, at concentration d is R(d) = P(d)/P0.
Using this mathematical expression, it is easy to demonstrate
that the slope factor calculated from [ P(d) - 1] P0/ d is
greater at high doses (including the averaged concentration used
in the risk calculation) than at low doses where the
dose-response function is dominated by q1. Epidemiologists
have long had a similar (but not identical) concern about the use
of averaged data, since ecological associations are not
necessarily consistent with those measured at the individual
level (see Cohen, 1994; Greenland & Robins, 1994; Piantadosi,
1994).
(3) Some occupational groups were exposed to considerably higher
concentrations of diesel exhaust in the past than presently. For
example, the average particle concentration in a Finnish
roundhouse was reported to be 2 mg/m3 (Heino et al., 1978). The
lung burdens would thus be greater than those predicted on the
basis of current exposures, and the unit risk estimates would be
higher
(i) Summary and conclusions
A dosimetric model that accounts for differences between
experimental animals and humans in lung deposition efficiency, lung
particle clearance rates, lung surface area, ventilation, and the
rates of elution of organic chemicals from the particle surface was
used to calculate equivalent human doses as particle concentration per
unit lung surface area. After dosimetric adjustment, four risk
estimates were derived by a linearized multistage model from three
bioassays with Fischer 344 rats and one with female Wistar rats, which
ranged from 1.6 to 7.1 × 10-5/µg particles per m3, with a geometric
mean of 3.4 × 10-5. This quantitative assessment of the carcinogenic
risk due to exposure to diesel engine emissions is reasonable,
because:
-- The estimates are based on several well-designed, well-executed,
long-term bioassays.
-- Epidemiological studies indicate that humans are susceptible to
the induction of lung cancer after inhalation of diesel exhaust.
-- Dosimetry modelling, especially to account for inhibition of
particle clearance at high doses, has allowed accurate
extrapolation of doses from animals to humans.
-- The doses are based on actual concentrations of particulate
matter per unit of lung surface area.
-- Use of an alternative model that attempts to account for the
possible biological effects of the organic and carbon core
fractions did not appreciably change the unit risk estimate.
-- The risk estimates based on the results of bioassyas are not
inconsistent with the available human experience.
Nevertheless, a number of uncertainties remain, the most
significant of which are:
-- In any interspecies extrapolation there may be an inherent
difference in sensitivity to the agent being assessed.
-- The assumption of equivalent sensitivity across species is based
on concentration per unit of lung surface area, and use of other
assumptions of dose equivalence may lead to different estimates
of risk; however, the estimates should not vary by more than one
order of magnitude.
-- Although linearized low-dose extrapolation methods are used, it
is still uncertain whether inflammatory cells secrete mediators
that induce cancer in lung epithelial cells when the particle
burden is smaller than that necessary to inhibit clearance. Even
if macrophages are activated at low particle burdens, it is
uncertain whether the responses of epithelial cells are linear at
very low concentrations; however, non-linear carcinogenic
responses have been observed in bioassays.
-- Although the unit risk estimate is corroborated in the
alternative model, uncertainty about the response at low doses
remains because the estimates in the alternative model are based
on the assumption that particles continue to induce cell
initiation and/or proliferation at low doses. The actual risk
would be much lower if this assumption does not hold.
The risk at low doses derived from the linearized multistage
model may thus be overestimated if particles no longer induce cell
initiation and/or proliferation. The model was selected for
calculating risk because a conservative model was needed in order to
ensure protection of public health and because adequate data were not
available to use fully the alternative model constructed for this
assessment. Thus, the risk derived from the bioassays should be viewed
as hypothetical.
These unit risk estimates should not be used to evaluate the
carcinogenic risk of other types of particulate matter present in
ambient air, which may have different solubilities, surface areas, and
free radical contents, which factors greatly affect carcinogenic
potency.
It could be argued that since the types and location of tumours
seen in rats after exposure to particles are different from those
found in humans, the experimental data are not relevant for humans.
The tumours diagnosed in rats after long-term exposure to carbon black
particles and diesel exhaust include benign adenomas and malignant
adenocarcinomas, squamous-cell carcinomas, adenosquamous carcinomas,
and squamous cysts (Mauderly et al., 1994; see section B7.3.2). After
injection into athymic mice, cells from 50-67% of squamous-cell
carcinomas and 25-40% of adenocarcinomas were found to grow (Table
35), whereas those from squamous cysts did not (Mauderly et al.,
1994).
Squamous cysts have been defined by other authors as benign
cystic keratinizing squamous-cell tumours (Kittel et al., 1993;
Dungworth et al., 1994; Heinrich et al., 1995) and found not to grow
after transplantation (Heinrich et al., 1995). As pointed out by Mohr
(1992), there is, however, controversy about the correct terminology
of this type of lesion. In 1995, an international group of
pathologists re-evaluated lung tissue sections from rats exposed to
particles by inhalation and identified four distinct lesions:
squamous-cell metaplasia, pulmonary keratinizing cyst, cystic
keratinizing epithelioma (considered to be benign), and squamous-cell
carcinoma (G. Oberdörster, personal communication).
Regardless of the correct classification of lesions, the
important fact is that malignant tumours are induced in rats after
long-term inhalation of deisel exhaust and carbon black. The tumour
response of human beings may not, however, be the same, either
qualitatively or quantitatively. In fact, the response of rats to
long-term exposure to high concentrations of particles differs from
that of other species, including mice and hamsters. In the absence of
sufficient information about specific mechanisms unique to rats,
however, there is no justification for excluding data on this species
from extrapolations to the human situation (US Environmental
Protection Agency, 1986).
With respect to the mechanisms of induction of lung tumours by
particles in rats, it has been shown that particles devoid of
polyaromatic compounds can increase mutation rates in pulmonary
epithelial cells, which is an important step in tumour development via
cell transformation. In a recent study in which rats were exposed to
carbon black for 13 weeks, a significant influx of inflammatory cells
was seen at concentrations of 7 and 50 mg/m3 but not at 1 mg/m3
(Oberdörster et al., 1995), and a significant, three- to fourfold
increase in the frequency of hprt mutations was seen in alveolar
epithelial cells (Driscoll et al., 1995 and in press; see section
B7.6). Thus, burdens of particles of low toxicity per se, similar to
those reached in long-term studies in rats, can be mutagenic, possibly
through the involvement of DNA damaging oxidants derived from
inflammatory cells.
Appendix B10.1 Construction of a biologically based (alternative)
model
1. Preliminary considerations
In order to evaluate the effects of various biological
assumptions on the assessments of the risk of exposure to diesel
exhaust, a mathematical dose-response model must be constructed that
takes into account the proposed biological mechanisms. As a
significant issue in assessing the risk of diesel exhaust is the
effect of lung overloading on tumour induction, the model should have
the following properties:
-- It should depend on dose metrics for both organic compounds and
the carbon core, and it should account for the contributions of
each to tumour induction and formation both separately and
jointly.
-- It should allow for changes in the model parameters with time due
to increasing lung burden during exposure.
-- It should view cell proliferation and tumour induction and
formation stochastically: it is not realistic to assume
deterministic clonal growth. For instance, it should not be
assumed that all cells divide at the same age.
It is therefore assumed that a normal cell can be initiated by
both organic compounds and the carbon core. The initiation rate is
denoted by µ1, which is a function of the background rate and that
induced by diesel exhaust (as specified below). Because an initiated
cell eventually either dies or enters the cell cycle (including cells
in quiescence, G0), it is reasonable to assume that the lifetime of
an initiated cell follows a certain probability distribution. In this
model, a cell in G0 phase is equivalent to one with a certain
probability of a very long lifetime (i.e. in the right-hand tail of
the distribution of the cell's lifetime). At the end of its lifetime,
it either dies (death) with probability b, divides into two daughter
cells (birth) with probability a, or divides into one initiated cell
and one malignant cell (second transition) with probability µ2;
alpha + ß + µ2 = 1. Instead of assuming that a single malignant cell
is equivalent to a tumour, as in the model proposed by Moolgavkar &
Venzon (1979) and Moolgavkar & Knudson (1981), it is assumed here that
a malignant cell has a certain probability of becoming a tumour; this
probability is assumed to be dose-dependent, thus allowing for an
evaluation of the effect of dose on tumour progression. It should be
noted that the proposed model does not exclude the possibility that
there may be more than one step in the 'initiation' of a normal cell.
The rate of initiation used in the model should be viewed as a net
rate that represents several genetic alterations and repairs.
2. Mathematical model and relationship of parameters to lung burden
A dose-response function P(t:d,D) is constructed for the
probability of cancer by time (age) t, which depends on both organic
compounds, d, and particles (carbon core), D, and incorporates the
biologically based concept discussed above. Because the model
parameters that are not observed directly in the laboratory can be
estimated statistically only from the results of bioassays at high
concentrations, the model should not be considered a real model of
diesel-induced carcinogenesis; uncertainty about extrapolation to low
doses remains.
A model with the features presented above was originally proposed
by Chen & Farland (1991) and was extended into one with time-variable
parameters by Tan & Chen (1992). This model was used as the basis for
constructing a biologically based dose-response model. A brief
mathematical description is presented in Appendix B10.3.
The data on time to event from Mauderly et al. (1987) were used
to estimate model parameters. These data are useful because they
contain information on natural mortality and serial sacrifice of
animals with and without (malignant) tumours, which is valuable for
estimating tumour latency. In order to use this information to
calculate maximum likelihood estimates of parameters, an 'E-M'
algorithm was derived. In the E-M algorithm, each iteration involves
an 'expectation' step (E) and a 'maximization' step (M) (see Appendix
B10.2).
(a) Model parameters and notations
The following parameters are incorporated into the dose-response
model, which includes the rate of initiation (µ1), the rate of
proliferation (gammaalpha), the rate of conversion (gammaµ2), and
the probability of progression ( q). The rate of death of the
initiated cells is implicitly defined by gamma(1-µ2-alpha). The
parameters are all dose dependent.
D: dose of carbon core in milligrams per square centimetre of
lung epithelial surface; varies over time;
d: dose of organic compounds in milligrams per square centimetre
of lung epithelial surface;
µ1: dose-related initiation rate (per cell per day); depends on
µ0 (background rate), d, and D by µ1 = µ0 (1 + ad + bD), where
a and b are parameters to be estimated statistically;
µ2: probability that a malignant cell will be produced by the end
of the lifetime of an initiated cell;
alpha: probability that an initiated cell will divide into two
daughter cells by the end of its lifetime;
q: probability that a single malignant cell will develop into a
malignant tumour;
gamma: 1/gamma is the mean lifetime of an initiated cell in days; the
lifetime ends when the cell goes into mitosis or dies. If it
is assumed that the probability that a cell will go into
mitosis is about the same as the probability that it will die,
the mean cell lifetime can be conveniently interpreted as time
to mitosis (i.e. cell turnover time); thus, a shorter cell
lifetime implies more frequent cell division. Time to mitosis
is a random variable, not a fixed constant as in the
assumption of the model of Greenfield et al. (1984), which was
used by Cohen & Ellwein (1988) to analyse the results of
bioassays to dectect urinary bladder cancer.
N: number of (normal) target cells.
(b) Practical considerations
The E-M algorithm developed in Appendix B10.2 is an elegant
procedure that can be used by statistical theory alone to test
hypotheses about whether a particular parameter is influenced by
organic compounds and the carbon core, individually or together. For
instance, it could be postulated that the parameter the reciprocal of
which represents mean cell lifetime is given by gamma( d,Di) = gamma0 +
gamma11 d + gamma12 Di, and then proceed to test the null hypothesis
that gamma11 = 0, i.e. no effect of organic compounds on the cell
lifetime. This temptation must, however, be resisted, because too many
parameters would have to be be estimated. Therefore, the biologically
plausible assumption that parameters q and gamma depend only on the
lung burden of the carbon core, D,
is used.
The study of Mauderly et al. (1987) lasted about 940 days. In
order to construct a dose-response model including time-dependent lung
burden, the time interval (0-940) is divided into five sub-intervals,
each spanning six months, except for the last, which spans 730-940
days. The deposition-retention model developed by Yu et al. (1991),
corresponding to a concentration of diesel exhaust emissions in
ambient air of milligrams per cubic metre, is used to calculate
( d,Di), where i = 1, 2, ..., 5; the dose of organic compounds,
d, does not change with time because it reaches a steady state soon
after exposure begins; and Di is the lung burden of carbon core
during the ith sub-interval.
The assumptions for the dose-parameters relationship are:
-- The rate of initiation associated with a lung burden { d,Di, i
= 1, 2, ..., 5} is given by µi( d,Di) = µ0(1 + a * d + b * Di) for
i = 1, 2, ..., 5. This is the only parameter that is assumed to
depend on both d and D.
-- The probability of tumour formation from a malignant cell is
assumed to be dependent on lung burden, D, from q( Di) = q0
q0 Di, i = 1, 2, ..., 5. In order to simplify calculation, the
possibility that q0 is also dependent on organic compounds, d, is
not considered.
-- The cell lifetime, gamma, is assumed to be related nonlinearly to
lung burden, D, from gamma( Di) = gamma0 + gamma1 Log(1 + Di),
i = 1, 2, ..., 5.
In order to reduce the number of parameters that are to be
estimated from the data of Mauderly et al. (1987), some of the
background parameters for the dose-response model (µ0, q0, and
gamma0) are estimated from the historical control rates for Fischer
344 rats in the US National Toxicology Program (reconstructed from
Portier et al., 1986). The dose-related parameters are then estimated
with the E-M algorithm, which is described in Appendix B10.2. The
parameters estimated for the model resulting from the tumour response
data of Mauderly et al. (1987) and the corresponding dosimetric
parameters (Table 52) are given in Table 53.
Table 52. Dosimetric parameters (milligrams per square centimetre of lung surface) used
in modelling
Exposure d D1 D2 D3 D4 D5
concn
(mg/m3)
0.35 2.5 × 10-6 6.2 × 10-5 8.8 × 10-5 9.0 × 10-5 9.0 × 10-5 9.0 × 10-5
3.50 3.6 × 10-5 7.5 × 10-4 2.4 × 10-3 3.9 × 10-3 5.3 × 10-3 6.3 × 10-3
7.08 7.3 × 10-5 2.0 × 10-3 5.5 × 10-3 8.6 × 10-3 1.1 × 10-2 1.4 × 10-2
d, organic compounds; Di, i = 1, 2, ..., 5, are average lung burdens of carbon core over
five time intervals. Values calculated from the retention model of Yu et al. (1991)
Table 53. Maximum likelihood estimates for model parameters
Parameter Estimate
µ 1.033 × 10-7
a 1.103 × 104
b 3.214 × 102
µ2 7.907 × 10-7
q0 1.035 × 10-1
q1 5.332 × 10-2
gamma0 1.662 × 10-2
gamma1 2.647 × 10-2
alpha 5.443 × 10-1
N (given) 8.80 × 107
For definitions of parameters, see text. Background parameters µ0, q0,
and gamma0 are estimated separately from historical control data from
the US National Toxicology Program. The number of target cells, N, is
assumed to be 10 times the number of type II cells in mice, which is
given by Kauffman (1974). It is not essential that N be given
accurately because Nµ0 appears as a single term in the model; the
estimated µ0 will compensate for the underestimation or overestimation
of N.
Appendix B10.2 E-M algorithm
The E-M algorithm, derived below, was used to calculate maximum
likelihood estimates of the parameters for the alternative model. The
data used were taken from Mauderly et al. (1987) and include the time
when an animal died, naturally or at sacrifice, with or without
(malignant) tumours. The theory of the algorithm is given by Dempster
et al. (1977).
Assume that the distinct times at which animals died are t1
< t2 < ...< tm. The observations can be classified as follows:
a1x (i): observed number of natural deaths without tumour at time
ti in treatment group x (The four groups are x =
1, 2, 3, 4.)
a2x (i): observed number of natural deaths with tumour at time
ti in treatment group x
b1x (i): series sacrificed at time ti without tumour in
treatment group x
b2x (i): series sacrificed at time ti with tumours in
treatment group x.
Let Td represent the time an animal died and T the time a
tumour developed.
alphax (i) = Pr Td = ti | Td > ti, T > ti,, x} (conditional probability
of death without tumour)
ßx( i | u) = Pr Td = ti | Td > ti, T Epsolin ( tu-1, tu], x} (related
to deaths with tumours)
Define
The model, which is described in Appendix B10.3, allows for
initiation by both the carbon and the organic fraction and for the
proliferative effects of the carbon fraction. Although these
mechanisms remain to be proven, it is assumed that carcinogenic agents
present in the organic fraction act directly on the target cells,
primarily by initiation. It is further assumed that most of the
particles are ingested by macrophages. Particle-laden macrophages are
then induced to secrete a variety of mediators (e.g. reactive oxygen
species and cytokines), which diffuse to the target cells, inducing
initiation, proliferation, and conversion of initiated cells to
malignant cells.
The results reported by Mauderly et al. (1987) for tumour
induction were used to estimate the model parameters. These are based
on the development of malignant tumours rather than all tumours as in
the first method. This was necessary in order to ensure that the data
used to estimate the parameters represented the same biological
mechanism. Lung burdens were calculated from the same dosimetry model
used in the linearized multistage model.
(f) Results of unit risk calculations
Particle-based model: Unit risks were derived from the linearized
multistage approach for the tumour incidences seen in four bioassays
(Brightwell et al., 1986; Ishinishi et al., 1986; Mauderly et al.,
1987; Heinrich et al., 1995) and the corresponding equivalent doses
(Table 48). The resulting unit risk estimates, listed in Table 49,
range from 1.6 to 7.1 × 10-5/µg particles per m3 with a geometric
mean of 3.4 × 10-5/µg per m3.
In these calculations, the relationship between air concentration
(micrograms per cubic metre) and lung burden (milligrams) in humans is
used to determine the lung burden resulting from lifetime exposure to
1 g/m3 of diesel exhaust particulate matter. The particle burden in
terms of mass per unit lung surface area is then multiplied by the
slope derived from the bioassay data. For instance, when the data of
Mauderly et al. (1987) are used, the slope of the curve for
carcinogenicity in rats (i.e. the upper 95% confidence limit of the
linear coefficient in the multistage model), expressed in terms of
equivalent dose (micrograms of carbon particulate matter per square
centimetre) is 1.7 × 10-2/µg per cm2. According to the dosimetry
model, an air concentration of 1 µg/m3 of particulate matter
corresponds to a mass of 1230 µg of carbon particles per human lung.
Because the lung epithelial surface area, including the alveolar
region and conducting airways, is assumed to be 627 000 cm2, a unit
risk of 3.4 × 10-5/µg per m3 is derived from 1.7 × 10-2/µg per cm2
× 1230 µg/627 000 cm2.
Biologically based model: The unit risk estimated by the
alternative model, based on the data on malignant tumours from the
study of Mauderly et al. (1987), is equal to 1.65 × 10-5/µg particles
per m3. This is lower than the estimate of 3.4 × 10-5/µg per m3
derived from the same study with the linearized multistage approach.
Application of the linearized multistage model only to malignant
tumours seen in that study, rather than all lung tumours, resulted in
a unit risk estimate of 1.74 × 10-5/µg particles per m3 (Table 50).
Thus, the unit risk estimates obtained by the two approaches are
virtually identical. The estimated risks may differ somewhat with
increasing doses, because the slopes are not identical at all exposure
levels. It should be noted, however, that the unit risk predicted by
the alternative model is derived under the assumption that particles
continue to exert an effect on cell initiation or proliferation (or
both) at low doses. There is considerable uncertainty about the
effects of particles at low doses. It has been claimed that particles
do not induce initiation or proliferation at low doses (Vostal, 1986).
At present, the evidence is inadequate to support or refute this
claim. Moreover, even if macrophage overload is required, because of
uneven distribution of particles, some macrophages may become
overloaded even at low concentrations. Because of this uncertainty,
the biologically based model, like the linearized multistage model,
does not depart from linearity at low doses; however, if initiation
and proliferation do not occur at low doses, the risk may be much
smaller.
In an estimate of unit risk for rats, based on the results of
seven studies by inhalation and using linear interpolation and the
linearized multistage model, the risks were 7 × 10-5/µg of diesel
particles per m3 and 10 × 10-5/µg carbon core particles per m3
(Csicsaky et al., 1993; Pott et al., 1993; Roller & Pott, 1994).
(g) Results and implications of the biologically based model
On the assumption that particles continue to affect cell
initiation or proliferation at low doses, the risks calculated with
this model are comparable to those obtained with the linearized
multistage model. Excess risks due to various exposures are shown in
Tables 50 and 51. Those in Table 50 are the risks predicted for humans
exposed continuously (24 h/day) from the two models: it is interesting
to note that the results are similar. Table 51 shows the excess risks
due to exposure to 2.6 µg/m3 of diesel particulate emission for
16 h/day on seven days per week and to 15 µg/m3 for 8 h/day on five
days per week. The first concentration was reported by the US
Environmental Protection Agency Office of Mobile Sources to be the
annual mean exposure of the American population to diesel particulate
matter in 1986; the second concentration was reported to be that to
which workers are exposed on urban freeways (Carey, 1987). The
retention half-time for insoluble particles after exposure to
Table 50. Comparison of excess risk for humans due to continuous exposure to
various concentrations of diesel exhaust emissions using two
different models
Exposure concentration Biologically based (alternative) Linearized (µg/m3)
multistage
Maximum likelihood Upper 95% model
estimate bound estimate
0.01 7.68 × 10-8 1.35 × 10-7 1.71 × 10-7
0.1 8.12 × 10-7 1.71 × 10-6 1.72 × 10-7
1.0 (unit risk) 8.16 × 10-6 1.65 × 10-5 1.74 × 10-5
100 5.58 × 10-4 9.63 × 10-4 1.74 × 10-4
1000 2.60 × 10-2 4.22 × 10-2 3.33 × 10-2
a Slope - 9.04 per mg/cm2 of lung surface, using carbon core as dosimetric.
Only malignant tumours are used in the calculations.
2.6 µg/m3 is shown to increase from 296 days for members of the
general population with normal respiratory function to 519 days for
those with a smoking history of 20 pack-years (Bohning et al., 1982).
This information was used to reduce the alveolar clearance rate for
the dosimetric calculations to that used for other risk calculations.
Interactive effects of smoking and diesel exhaust are not considered
in the risk calculations owing to lack of data. The studies of carbon
black and coal-tar pitch (Heinrich, 1994; Heinrich et al., 1994)
indicate that smokers have a higher risk for lung cancer than
nonsmokers when they are exposed to diesel exhaust.
The excess lifetime risks shown in Tables 50 and 51 are
standardized by the actuarial life-table approach, using the survival
probability of control animals in the US National Toxicology Program
provided by Portier et al. (1986). This approach gives a weighted
average of the probability of cancer occurrence over an entire
lifetime, weighted by survival probability.
Table 51. Excess lifetime risk for humans due to exposure to diesel
exhaust emissions under various exposure scenarios
Exposure pattern Biologically based model Linearized
multistage
Maximum Upper 95% modela
likelihood bound
estimate estimate
General population (normal 1.41 × 10-5 2.44 × 10-5
respiratory function;
nonsmoker): 2.6 µg/m3,
16 h/day, seven days
per week
General population 2.32 × 10-5 3.61 × 10-5 5.38 × 10-5
(20-pack-year smoker)b:
2.6 µg/m3, 16 h/day,
seven days per week
Occupationally exposed: 3.12 × 10-5 5.17 × 10-5 6.18 × 10-5
15 µg/m3 8 h/day, five
days per week
a Calculated using carbon core as dosimetric; only malignant
tumours are used.
b In this calculation, smoking affects only lung clearance rate;
biological interaction between smoking and exposure to diesel
exhaust is not considered. The retention half-times for
insoluble particles increased from 296 days for persons with
normal respiratory function to 519 days for 20-pack-year smokers
(Bohning et al., 1982).
Some implications of the alternative model are:
(1) At a low exposure concentration, the decrease in diesel-induced
initiation results in a greater reduction of risk; that is, the
number of initiated cells (cancer risk) is reduced more
efficiently with a low than with a high exposure. If only organic
compounds induce initiation when the concentration is low, they
play a more important role than particles in inducing tumours at
low concentrations, whereas the roles are reversed when the
concentration is high.
(2) Although cells initiated by diesel exhaust play an important role
in cancer induction, either organic compounds or the carbon core
alone could induce initiation by increasing their respective
proportions. Thus, although initiated cells are important for
tumour induction, they may be induced by any agent that initiates
tumours (e.g. smoking).
(3) A small change in the rate of proliferation induced by diesel
exhaust could disproportionately change cancer risk. As this
parameter is assumed to be a function of the dose of carbon core,
lung overloading has a significant effect on cancer incidence. In
the absence of better information, it is assumed in this
assessment that the carbon core continues to have a proliferating
effect at low doses.
These observations suggest that, although the effect of particle
overload on cell proliferation is important, initiation by the carbon
core or organic compounds or both is also essential. Although this
conclusion is only tentative, because the model parameters are
estimated statistically on the basis of bioassays conducted at high
concentrations, it does suggest the importance of studying the role of
the carbon core and organic compounds in initiation and promotion at
low and high exposure concentrations. Does the relative initiation
potential of organic compounds and the carbon core differ with
concentration? These observations also suggest that a subcohort of
workers who were smokers and were exposed to high concentrations of
diesel exhaust for a long time would have a greater risk of dying from
lung cancer.
(h) Comparison of risk estimates derived from experimental studies
and human experience
The bioassay-based risk estimates, which range from 1.6 × 10-5 to
7.1 × 10-5, with a geometric mean of 3.4 x 10-5, can be compared with
human experience on the basis of three data sets: those of an
epidemiological study conducted on London Transport employees (Waller,
1981) and a subsequent analysis (Harris, 1983) and those on American
railroad workers (Garshick et al., 1987, 1988). Although these data
cannot be used to calculate unit risk, mainly because of a lack of
reliable information on exposure, they can be used to evaluate the
validity of unit risk estimates based on the results of experimental
studies.
Attempts have already been made to estimate the potential cancer
risk due to exposure to diesel exhaust on the basis of epidemiological
data. From the results of the study of London Transport workers,
Harris (1983) estimated that the increase in the relative risk for
lung cancer associated with exposure to 1 µg/m3-year of diesel exhaust
was 1.2 × 10-4, with an upper bound of the 95% confidence limit of
4.8 × 10-4. (Generally, when data from an epidemiological study with
negative results are used to estimate cancer risk, the upper bound is
used to calculate unit risk.) The resulting unit risk estimate is 2 ×
10-3, which is about 60 times higher than the mean unit risk estimate
derived from bioassays, 3.4 × 10-5, and about 30 times higher than the
upper end of the range of unit risk estimates from bioassays, 7.1 ×
10-5. Therefore, the risk estimate based on bioassays is not
inconsistent with that for humans, since 2 × 10-3 is the estimated
upper bound in an epidemiological study with negative results.
McClellan et al. (1989) reported risk estimates based on the
study of Garshick et al. (1987), in which lung cancers in railroad
workers were evaluated. Assuming exposure to concentrations of 500 and
125 µg/m3, the upper bounds of the 95% confidence interval for
lifetime cancer risk were estimated to be 6 × 10-4 and 2 × 10-3,
respectively. The lower of the two unit risk estimates is only about
one order of magnitude higher than the risk estimates based on
bioassays.
An epidemiological study potentially more suitable for
quantitative risk assessment was reported by Garshick et al. (1988),
which was based on a large number of subjects; a small but significant
increase in the rate of mortality from lung cancer was seen in some
subcohorts. The US Environmental Protection Agency has supported an
effort to derive a unit risk estimate from this study, and data on
exposure were estimated by Woskie et al. (1988a,b) and Hammond et al.
(1988). These data were analysed in a variety of ways, using relative
risk and absolute risk dose-response models and by classifying
individuals into various exposure categories, including job, duration
of employment, age, and exposure markers. Even though at least 50
analyses were carried out, an adequate dose-response relationship
could not be obtained; these data were therefore not used to estimate
unit risk.
The lack of a dose-response relationship is not totally
unexpected, given the low increase in mortality rate in the study of
Garshick et al. (1988) and the uncertain estimates of exposure. The
data on exposure were derived from air samples collected during a
limited period (1981-83) on four small railroads operating in a
limited geographical area (northern United States), in which
concentrations of respirable particulate matter were measured rather
than diesel exhaust per se; the measured concentrations were then
adjusted to derive markers of exposure. The data were used to estimate
exposure to diesel exhaust of railroad workers throughout the United
States 30 or more years earlier. Diesel equipment and working
conditions have, however, changed since the 1940s when diesel engines
first began to be used in large numbers. Woskie et al. (1988b)
summarized anecdotal reports of smoky working conditions in diesel
repair shops during the 1950s and 1960s and reported that the limited
data available on levels of nitrogen oxide during those periods
indicated high levels of diesel exhaust. By the time samples were
collected, however, the smoky conditions would have been largely
mitigated by improved ventilation and the advent of less smoky diesel
engines. The study of Garshick et al. (1988) gives the relative risks
for dying from lung cancer in exposed as opposed to unexposed railroad
workers classified into five subcohorts by age in 1959. The risks
ranged from 0.96 (95% CI, 0.74-1.33) to 1.45 (1.11-1.89). The highest
relative risk, 1.45, which was observed in workers who were 40-45
years old in 1959, was used here to evaluate the validity of unit risk
based on biossays results. Assuming that this subcohort of workers was
exposed to diesel exhaust for 8 h/day on five days per week from age
35 to age 65, the background mortality rate from lung cancer in this
subcohort would be 0.038 (0.63 × 0.06), as the unexposed workers in
the same age group had a relative risk of 0.63 and the corresponding
lifetime mortality rate from lung cancer in the general American white
male population is about 0.06. If the lung cancer risk due to
1 µg/m3 is assumed to be 3.4 × 10-5, the concentration of diesel
exhaust in the working environment was at least 400 µg/m3, calculated
as follows: The risk due to 1 µg/cm2 of particles is 0.017 (which
results in a unit risk of 3.4 × 10-5). For a lower bound of the
relative risk of 1.1, a lung burden, d, that satisfies the
relationship 0.1 × 0.038 = 0.017 d would be needed; that is, d =
0.22 µg/cm2, which is equivalent to an air concentration of
0.4 mg/m3 by the dosimetry model of Yu et al. (1991). If the highest
unit risk estimate derived from Brightwell et al. (1986), 7.1 ×
10-5, is used, the required minimal air concentration would be about
0.2 mg/m3. These calculations imply that a diesel exhaust
concentration of at least 0.2 mg/m3 was necessary to observe a
statistically significant increase in the mortality rate from lung
cancer in this study. This concentration appears to be reasonable in
the light of the working conditions described by Woskie et al.
(1988a).
Although the risk estimates derived from bioassays are lower than
those derived from human data and may possibly over-predict risk,
since the non-linearity of the human response is not considered in
this model, they are not inconsistent, for the following reasons:
(1) The risk estimates based on epidemiological studies are not
derived from all of the available data but on only a subset with
the highest response. The estimates are therefore higher than
those that would be derived from the whole data set.
(2) When a single data point (i.e. an overall relative risk and an
averaged exposure concentration) is used in the calculations, the
resulting slope for potency will be overestimated if the
dose-response relationship is not linear over all exposure
concentrations. Assume, for example, that the response follows
the simple multistage model P(d) = 1 - exp[-( q0 + qd + q2 d2)].
The relative risk, R, at concentration d is R(d) = P(d)/P0.
Using this mathematical expression, it is easy to demonstrate
that the slope factor calculated from [ P(d) - 1] P0/ d is
greater at high doses (including the averaged concentration used
in the risk calculation) than at low doses where the
dose-response function is dominated by q1. Epidemiologists
have long had a similar (but not identical) concern about the use
of averaged data, since ecological associations are not
necessarily consistent with those measured at the individual
level (see Cohen, 1994; Greenland & Robins, 1994; Piantadosi,
1994).
(3) Some occupational groups were exposed to considerably higher
concentrations of diesel exhaust in the past than presently. For
example, the average particle concentration in a Finnish
roundhouse was reported to be 2 mg/m3 (Heino et al., 1978). The
lung burdens would thus be greater than those predicted on the
basis of current exposures, and the unit risk estimates would be
higher
(i) Summary and conclusions
A dosimetric model that accounts for differences between
experimental animals and humans in lung deposition efficiency, lung
particle clearance rates, lung surface area, ventilation, and the
rates of elution of organic chemicals from the particle surface was
used to calculate equivalent human doses as particle concentration per
unit lung surface area. After dosimetric adjustment, four risk
estimates were derived by a linearized multistage model from three
bioassays with Fischer 344 rats and one with female Wistar rats, which
ranged from 1.6 to 7.1 × 10-5/µg particles per m3, with a geometric
mean of 3.4 × 10-5. This quantitative assessment of the carcinogenic
risk due to exposure to diesel engine emissions is reasonable,
because:
-- The estimates are based on several well-designed, well-executed,
long-term bioassays.
-- Epidemiological studies indicate that humans are susceptible to
the induction of lung cancer after inhalation of diesel exhaust.
-- Dosimetry modelling, especially to account for inhibition of
particle clearance at high doses, has allowed accurate
extrapolation of doses from animals to humans.
-- The doses are based on actual concentrations of particulate
matter per unit of lung surface area.
-- Use of an alternative model that attempts to account for the
possible biological effects of the organic and carbon core
fractions did not appreciably change the unit risk estimate.
-- The risk estimates based on the results of bioassyas are not
inconsistent with the available human experience.
Nevertheless, a number of uncertainties remain, the most
significant of which are:
-- In any interspecies extrapolation there may be an inherent
difference in sensitivity to the agent being assessed.
-- The assumption of equivalent sensitivity across species is based
on concentration per unit of lung surface area, and use of other
assumptions of dose equivalence may lead to different estimates
of risk; however, the estimates should not vary by more than one
order of magnitude.
-- Although linearized low-dose extrapolation methods are used, it
is still uncertain whether inflammatory cells secrete mediators
that induce cancer in lung epithelial cells when the particle
burden is smaller than that necessary to inhibit clearance. Even
if macrophages are activated at low particle burdens, it is
uncertain whether the responses of epithelial cells are linear at
very low concentrations; however, non-linear carcinogenic
responses have been observed in bioassays.
-- Although the unit risk estimate is corroborated in the
alternative model, uncertainty about the response at low doses
remains because the estimates in the alternative model are based
on the assumption that particles continue to induce cell
initiation and/or proliferation at low doses. The actual risk
would be much lower if this assumption does not hold.
The risk at low doses derived from the linearized multistage
model may thus be overestimated if particles no longer induce cell
initiation and/or proliferation. The model was selected for
calculating risk because a conservative model was needed in order to
ensure protection of public health and because adequate data were not
available to use fully the alternative model constructed for this
assessment. Thus, the risk derived from the bioassays should be viewed
as hypothetical.
These unit risk estimates should not be used to evaluate the
carcinogenic risk of other types of particulate matter present in
ambient air, which may have different solubilities, surface areas, and
free radical contents, which factors greatly affect carcinogenic
potency.
It could be argued that since the types and location of tumours
seen in rats after exposure to particles are different from those
found in humans, the experimental data are not relevant for humans.
The tumours diagnosed in rats after long-term exposure to carbon black
particles and diesel exhaust include benign adenomas and malignant
adenocarcinomas, squamous-cell carcinomas, adenosquamous carcinomas,
and squamous cysts (Mauderly et al., 1994; see section B7.3.2). After
injection into athymic mice, cells from 50-67% of squamous-cell
carcinomas and 25-40% of adenocarcinomas were found to grow (Table
35), whereas those from squamous cysts did not (Mauderly et al.,
1994).
Squamous cysts have been defined by other authors as benign
cystic keratinizing squamous-cell tumours (Kittel et al., 1993;
Dungworth et al., 1994; Heinrich et al., 1995) and found not to grow
after transplantation (Heinrich et al., 1995). As pointed out by Mohr
(1992), there is, however, controversy about the correct terminology
of this type of lesion. In 1995, an international group of
pathologists re-evaluated lung tissue sections from rats exposed to
particles by inhalation and identified four distinct lesions:
squamous-cell metaplasia, pulmonary keratinizing cyst, cystic
keratinizing epithelioma (considered to be benign), and squamous-cell
carcinoma (G. Oberdörster, personal communication).
Regardless of the correct classification of lesions, the
important fact is that malignant tumours are induced in rats after
long-term inhalation of deisel exhaust and carbon black. The tumour
response of human beings may not, however, be the same, either
qualitatively or quantitatively. In fact, the response of rats to
long-term exposure to high concentrations of particles differs from
that of other species, including mice and hamsters. In the absence of
sufficient information about specific mechanisms unique to rats,
however, there is no justification for excluding data on this species
from extrapolations to the human situation (US Environmental
Protection Agency, 1986).
With respect to the mechanisms of induction of lung tumours by
particles in rats, it has been shown that particles devoid of
polyaromatic compounds can increase mutation rates in pulmonary
epithelial cells, which is an important step in tumour development via
cell transformation. In a recent study in which rats were exposed to
carbon black for 13 weeks, a significant influx of inflammatory cells
was seen at concentrations of 7 and 50 mg/m3 but not at 1 mg/m3
(Oberdörster et al., 1995), and a significant, three- to fourfold
increase in the frequency of hprt mutations was seen in alveolar
epithelial cells (Driscoll et al., 1995 and in press; see section
B7.6). Thus, burdens of particles of low toxicity per se, similar to
those reached in long-term studies in rats, can be mutagenic, possibly
through the involvement of DNA damaging oxidants derived from
inflammatory cells.
Appendix B10.1 Construction of a biologically based (alternative)
model
1. Preliminary considerations
In order to evaluate the effects of various biological
assumptions on the assessments of the risk of exposure to diesel
exhaust, a mathematical dose-response model must be constructed that
takes into account the proposed biological mechanisms. As a
significant issue in assessing the risk of diesel exhaust is the
effect of lung overloading on tumour induction, the model should have
the following properties:
-- It should depend on dose metrics for both organic compounds and
the carbon core, and it should account for the contributions of
each to tumour induction and formation both separately and
jointly.
-- It should allow for changes in the model parameters with time due
to increasing lung burden during exposure.
-- It should view cell proliferation and tumour induction and
formation stochastically: it is not realistic to assume
deterministic clonal growth. For instance, it should not be
assumed that all cells divide at the same age.
It is therefore assumed that a normal cell can be initiated by
both organic compounds and the carbon core. The initiation rate is
denoted by µ1, which is a function of the background rate and that
induced by diesel exhaust (as specified below). Because an initiated
cell eventually either dies or enters the cell cycle (including cells
in quiescence, G0), it is reasonable to assume that the lifetime of
an initiated cell follows a certain probability distribution. In this
model, a cell in G0 phase is equivalent to one with a certain
probability of a very long lifetime (i.e. in the right-hand tail of
the distribution of the cell's lifetime). At the end of its lifetime,
it either dies (death) with probability b, divides into two daughter
cells (birth) with probability a, or divides into one initiated cell
and one malignant cell (second transition) with probability µ2;
alpha + ß + µ2 = 1. Instead of assuming that a single malignant cell
is equivalent to a tumour, as in the model proposed by Moolgavkar &
Venzon (1979) and Moolgavkar & Knudson (1981), it is assumed here that
a malignant cell has a certain probability of becoming a tumour; this
probability is assumed to be dose-dependent, thus allowing for an
evaluation of the effect of dose on tumour progression. It should be
noted that the proposed model does not exclude the possibility that
there may be more than one step in the 'initiation' of a normal cell.
The rate of initiation used in the model should be viewed as a net
rate that represents several genetic alterations and repairs.
2. Mathematical model and relationship of parameters to lung burden
A dose-response function P(t:d,D) is constructed for the
probability of cancer by time (age) t, which depends on both organic
compounds, d, and particles (carbon core), D, and incorporates the
biologically based concept discussed above. Because the model
parameters that are not observed directly in the laboratory can be
estimated statistically only from the results of bioassays at high
concentrations, the model should not be considered a real model of
diesel-induced carcinogenesis; uncertainty about extrapolation to low
doses remains.
A model with the features presented above was originally proposed
by Chen & Farland (1991) and was extended into one with time-variable
parameters by Tan & Chen (1992). This model was used as the basis for
constructing a biologically based dose-response model. A brief
mathematical description is presented in Appendix B10.3.
The data on time to event from Mauderly et al. (1987) were used
to estimate model parameters. These data are useful because they
contain information on natural mortality and serial sacrifice of
animals with and without (malignant) tumours, which is valuable for
estimating tumour latency. In order to use this information to
calculate maximum likelihood estimates of parameters, an 'E-M'
algorithm was derived. In the E-M algorithm, each iteration involves
an 'expectation' step (E) and a 'maximization' step (M) (see Appendix
B10.2).
(a) Model parameters and notations
The following parameters are incorporated into the dose-response
model, which includes the rate of initiation (µ1), the rate of
proliferation (gammaalpha), the rate of conversion (gammaµ2), and
the probability of progression ( q). The rate of death of the
initiated cells is implicitly defined by gamma(1-µ2-alpha). The
parameters are all dose dependent.
D: dose of carbon core in milligrams per square centimetre of
lung epithelial surface; varies over time;
d: dose of organic compounds in milligrams per square centimetre
of lung epithelial surface;
µ1: dose-related initiation rate (per cell per day); depends on
µ0 (background rate), d, and D by µ1 = µ0 (1 + ad + bD), where
a and b are parameters to be estimated statistically;
µ2: probability that a malignant cell will be produced by the end
of the lifetime of an initiated cell;
alpha: probability that an initiated cell will divide into two
daughter cells by the end of its lifetime;
q: probability that a single malignant cell will develop into a
malignant tumour;
gamma: 1/gamma is the mean lifetime of an initiated cell in days; the
lifetime ends when the cell goes into mitosis or dies. If it
is assumed that the probability that a cell will go into
mitosis is about the same as the probability that it will die,
the mean cell lifetime can be conveniently interpreted as time
to mitosis (i.e. cell turnover time); thus, a shorter cell
lifetime implies more frequent cell division. Time to mitosis
is a random variable, not a fixed constant as in the
assumption of the model of Greenfield et al. (1984), which was
used by Cohen & Ellwein (1988) to analyse the results of
bioassays to dectect urinary bladder cancer.
N: number of (normal) target cells.
(b) Practical considerations
The E-M algorithm developed in Appendix B10.2 is an elegant
procedure that can be used by statistical theory alone to test
hypotheses about whether a particular parameter is influenced by
organic compounds and the carbon core, individually or together. For
instance, it could be postulated that the parameter the reciprocal of
which represents mean cell lifetime is given by gamma( d,Di) = gamma0 +
gamma11 d + gamma12 Di, and then proceed to test the null hypothesis
that gamma11 = 0, i.e. no effect of organic compounds on the cell
lifetime. This temptation must, however, be resisted, because too many
parameters would have to be be estimated. Therefore, the biologically
plausible assumption that parameters q and gamma depend only on the
lung burden of the carbon core, D,
is used.
The study of Mauderly et al. (1987) lasted about 940 days. In
order to construct a dose-response model including time-dependent lung
burden, the time interval (0-940) is divided into five sub-intervals,
each spanning six months, except for the last, which spans 730-940
days. The deposition-retention model developed by Yu et al. (1991),
corresponding to a concentration of diesel exhaust emissions in
ambient air of milligrams per cubic metre, is used to calculate
( d,Di), where i = 1, 2, ..., 5; the dose of organic compounds,
d, does not change with time because it reaches a steady state soon
after exposure begins; and Di is the lung burden of carbon core
during the ith sub-interval.
The assumptions for the dose-parameters relationship are:
-- The rate of initiation associated with a lung burden { d,Di, i
= 1, 2, ..., 5} is given by µi( d,Di) = µ0(1 + a * d + b * Di) for
i = 1, 2, ..., 5. This is the only parameter that is assumed to
depend on both d and D.
-- The probability of tumour formation from a malignant cell is
assumed to be dependent on lung burden, D, from q( Di) = q0
q0 Di, i = 1, 2, ..., 5. In order to simplify calculation, the
possibility that q0 is also dependent on organic compounds, d, is
not considered.
-- The cell lifetime, gamma, is assumed to be related nonlinearly to
lung burden, D, from gamma( Di) = gamma0 + gamma1 Log(1 + Di),
i = 1, 2, ..., 5.
In order to reduce the number of parameters that are to be
estimated from the data of Mauderly et al. (1987), some of the
background parameters for the dose-response model (µ0, q0, and
gamma0) are estimated from the historical control rates for Fischer
344 rats in the US National Toxicology Program (reconstructed from
Portier et al., 1986). The dose-related parameters are then estimated
with the E-M algorithm, which is described in Appendix B10.2. The
parameters estimated for the model resulting from the tumour response
data of Mauderly et al. (1987) and the corresponding dosimetric
parameters (Table 52) are given in Table 53.
Table 52. Dosimetric parameters (milligrams per square centimetre of lung surface) used
in modelling
Exposure d D1 D2 D3 D4 D5
concn
(mg/m3)
0.35 2.5 × 10-6 6.2 × 10-5 8.8 × 10-5 9.0 × 10-5 9.0 × 10-5 9.0 × 10-5
3.50 3.6 × 10-5 7.5 × 10-4 2.4 × 10-3 3.9 × 10-3 5.3 × 10-3 6.3 × 10-3
7.08 7.3 × 10-5 2.0 × 10-3 5.5 × 10-3 8.6 × 10-3 1.1 × 10-2 1.4 × 10-2
d, organic compounds; Di, i = 1, 2, ..., 5, are average lung burdens of carbon core over
five time intervals. Values calculated from the retention model of Yu et al. (1991)
Table 53. Maximum likelihood estimates for model parameters
Parameter Estimate
µ 1.033 × 10-7
a 1.103 × 104
b 3.214 × 102
µ2 7.907 × 10-7
q0 1.035 × 10-1
q1 5.332 × 10-2
gamma0 1.662 × 10-2
gamma1 2.647 × 10-2
alpha 5.443 × 10-1
N (given) 8.80 × 107
For definitions of parameters, see text. Background parameters µ0, q0,
and gamma0 are estimated separately from historical control data from
the US National Toxicology Program. The number of target cells, N, is
assumed to be 10 times the number of type II cells in mice, which is
given by Kauffman (1974). It is not essential that N be given
accurately because Nµ0 appears as a single term in the model; the
estimated µ0 will compensate for the underestimation or overestimation
of N.
Appendix B10.2 E-M algorithm
The E-M algorithm, derived below, was used to calculate maximum
likelihood estimates of the parameters for the alternative model. The
data used were taken from Mauderly et al. (1987) and include the time
when an animal died, naturally or at sacrifice, with or without
(malignant) tumours. The theory of the algorithm is given by Dempster
et al. (1977).
Assume that the distinct times at which animals died are t1
< t2 < ...< tm. The observations can be classified as follows:
a1x (i): observed number of natural deaths without tumour at time
ti in treatment group x (The four groups are x =
1, 2, 3, 4.)
a2x (i): observed number of natural deaths with tumour at time
ti in treatment group x
b1x (i): series sacrificed at time ti without tumour in
treatment group x
b2x (i): series sacrificed at time ti with tumours in
treatment group x.
Let Td represent the time an animal died and T the time a
tumour developed.
alphax (i) = Pr Td = ti | Td > ti, T > ti,, x} (conditional probability
of death without tumour)
ßx( i | u) = Pr Td = ti | Td > ti, T Epsolin ( tu-1, tu], x} (related
to deaths with tumours)
Define
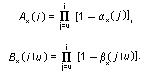 and
Sx (t) = Pr T > t | x} = exp[- t0integralt h(x)dx].
The function Sx (t) is the probability of being tumour-free by
time t.
The exact forms of the hazard function, h(x), and Sx (t) are
given in Appendix B10.3.
Let
a2x( i | u) = number of natural deaths at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i,
b2x( i | u) = number of animals sacrificed at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i.
Then
and
Sx (t) = Pr T > t | x} = exp[- t0integralt h(x)dx].
The function Sx (t) is the probability of being tumour-free by
time t.
The exact forms of the hazard function, h(x), and Sx (t) are
given in Appendix B10.3.
Let
a2x( i | u) = number of natural deaths at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i,
b2x( i | u) = number of animals sacrificed at ti, with a tumour
developing during ( tu-1, tu], in treatment group
x, u < i.
Then
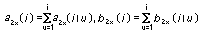 Let
Let
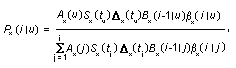 and
and
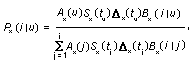 where
where
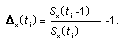 Given a2x( x), { a2x( i | u), u = 1, ..., i} is an ( i-1)-dimension
multinomial with parameter { a2x( i), Px( i | u), u = 1, ..., i}.
Thus,
E[ a2x( i | u) | a2x( i)] = a2x( i) Px( i | u).
Similarly, { b2x( i | u), u = 1, ..., i}, is an ( i - 1)-dimension
multinomial with parameters { b2x( i), Qx( i | u), u = 1, ..., i}, and
E[ b2x( i | u) | b2x( i)] = b2x( i) Qx( i | u).
It can be shown that the likelihood function is proportional to
Given a2x( x), { a2x( i | u), u = 1, ..., i} is an ( i-1)-dimension
multinomial with parameter { a2x( i), Px( i | u), u = 1, ..., i}.
Thus,
E[ a2x( i | u) | a2x( i)] = a2x( i) Px( i | u).
Similarly, { b2x( i | u), u = 1, ..., i}, is an ( i - 1)-dimension
multinomial with parameters { b2x( i), Qx( i | u), u = 1, ..., i}, and
E[ b2x( i | u) | b2x( i)] = b2x( i) Qx( i | u).
It can be shown that the likelihood function is proportional to
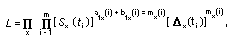 where
where
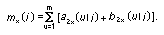 Let
Let
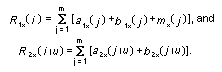 Let
Let
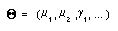 be a vector of parameters in function S;
alphax = [alphax(1), alphax(2), ..., alphax( m)], and
ßx( u) = [ßx(1 | u), ßx(2 | u, ..., ßx( m | u)]
be vectors of parameters related to conditional probabilities of death
with and without tumours. These parameters and those in thetax were
estimated by the E-M algorithm described below.
The M step:
Given initial values a2x( i | u) and b2x( i | u), estimate
be a vector of parameters in function S;
alphax = [alphax(1), alphax(2), ..., alphax( m)], and
ßx( u) = [ßx(1 | u), ßx(2 | u, ..., ßx( m | u)]
be vectors of parameters related to conditional probabilities of death
with and without tumours. These parameters and those in thetax were
estimated by the E-M algorithm described below.
The M step:
Given initial values a2x( i | u) and b2x( i | u), estimate
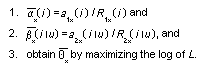 The E step:
Given the estimated values of alphax( i), ßx( i), and thetax from the M
step, compute Px( i | u) and Qx( i | u) and obtain estimates of
a2x( i | u) and b2x( i | u) by
The E step:
Given the estimated values of alphax( i), ßx( i), and thetax from the M
step, compute Px( i | u) and Qx( i | u) and obtain estimates of
a2x( i | u) and b2x( i | u) by
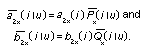 With the estimated values of a2x( i | u) and b2x( i | u) available
from the E step, go back to the M step and repeat the process until
the estimates are stable.
Appendix B10.3 A tumour growth model
A tumour growth model with piece-wise constant parameters taken
from Tan & Chen (1992) is an extension of a stochastic model developed
by Chen & Farland (1991). The biological justification of this model
is similar to that of the two-stage model proposed by Greenfield et
al. (1984), which was used by Cohen & Ellwein (1988) to analyse
urinary bladder tumour occurrence. The two models differ, however,
with respect to their mathematical formulations; the one adopted in
this report is a stochastic model, whereas the other is a
deterministic model and does not allow for estimation of parameters
because it does not have complete mathematical expression.
Although its most general form is not used here because of lack
of data, the stochastic model of Chen & Farland (1991) has two
desirable features: (i) it allows for any cell growth distribution
(e.g. Gompertz), rather than only exponential distribution as in other
models; and (ii) it incorporates the birth and death of tumour cells,
rather than assuming that a tumour is born once a single tumour cell
occurs, as did Moolgavkar & Venzon (1979) and Moolgavkar & Knudson
(1981). Therefore, if information on cell lifetime distribution and
the progression of tumour development is available, a reasonably
realistic model can be constructed.
For completeness, a brief description of the model is presented
here. The following notations are required:
N(t): number of normal (target) cells at time t,
µ1: rate of initiation, and
contour the probability density function for the lifetime of an
integral (t): an initiated cell.
At the end of its lifetime, an initiated cell either divides
(mitosis) or dies (programmed or nonprogrammed death). If it enters
mitosis, it either divides either into two initiated cells with
probability a or into one initiated cell and one malignant cell with
probability µ2. At the end of a cell's lifetime, the probability of
it dying is ß = 1 - alpha - µ2. A similar set-up (to allow for any
cell lifetime distribution) can be made for a malignant cell; however,
we confined ourselves to a simpler version, assuming that the lifetime
of a malignant cell follows an exponential distribution. Thus, we
assume that a malignant cell follows a simple birth-death process; it
can either divide into two malignant cells at a rate alpham or die
at a rate ßm.
When the parameters are constant over time (age), the hazard
function is given by
h(t) = µ1µ2 0integralt N(s)m( t - s) ds
where
With the estimated values of a2x( i | u) and b2x( i | u) available
from the E step, go back to the M step and repeat the process until
the estimates are stable.
Appendix B10.3 A tumour growth model
A tumour growth model with piece-wise constant parameters taken
from Tan & Chen (1992) is an extension of a stochastic model developed
by Chen & Farland (1991). The biological justification of this model
is similar to that of the two-stage model proposed by Greenfield et
al. (1984), which was used by Cohen & Ellwein (1988) to analyse
urinary bladder tumour occurrence. The two models differ, however,
with respect to their mathematical formulations; the one adopted in
this report is a stochastic model, whereas the other is a
deterministic model and does not allow for estimation of parameters
because it does not have complete mathematical expression.
Although its most general form is not used here because of lack
of data, the stochastic model of Chen & Farland (1991) has two
desirable features: (i) it allows for any cell growth distribution
(e.g. Gompertz), rather than only exponential distribution as in other
models; and (ii) it incorporates the birth and death of tumour cells,
rather than assuming that a tumour is born once a single tumour cell
occurs, as did Moolgavkar & Venzon (1979) and Moolgavkar & Knudson
(1981). Therefore, if information on cell lifetime distribution and
the progression of tumour development is available, a reasonably
realistic model can be constructed.
For completeness, a brief description of the model is presented
here. The following notations are required:
N(t): number of normal (target) cells at time t,
µ1: rate of initiation, and
contour the probability density function for the lifetime of an
integral (t): an initiated cell.
At the end of its lifetime, an initiated cell either divides
(mitosis) or dies (programmed or nonprogrammed death). If it enters
mitosis, it either divides either into two initiated cells with
probability a or into one initiated cell and one malignant cell with
probability µ2. At the end of a cell's lifetime, the probability of
it dying is ß = 1 - alpha - µ2. A similar set-up (to allow for any
cell lifetime distribution) can be made for a malignant cell; however,
we confined ourselves to a simpler version, assuming that the lifetime
of a malignant cell follows an exponential distribution. Thus, we
assume that a malignant cell follows a simple birth-death process; it
can either divide into two malignant cells at a rate alpham or die
at a rate ßm.
When the parameters are constant over time (age), the hazard
function is given by
h(t) = µ1µ2 0integralt N(s)m( t - s) ds
where
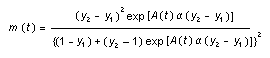 where y1 < y2 are two real roots of alpha y2 - (alpha + ß + µ2 q) y +
ß = 0; alpha + ß + µ2
= 1; q = 1 - ßm/alpham; A(t) = / 0integralt a(x)d x, where a(t) =
contour integral( t)/[1 - F(t)] is the hazard function of the cell
lifetime and F(t) is the cumulative function of contour integral (t).
Two cases of special interest are a(t) = gamma when an exponential
distribution is assumed and a(t) = exp(-gamma t), when the Gompertz
distribution is assumed.
When an exponential distribution (i.e. a(t) = gamma or A(t) =
gamma t) and q = 1 are assumed, the model is equivalent to the
model of Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981). A
special case that may be more appropriate than the exponential
distribution is that when the Gompertz distribution is assumed (i.e.
A(t) = [1 - exp(-gamma t)]/gamma).
For the model with time-dependent parameters, assume that the
study begins at time t0. Divide the time scale ( t0, t] into
k sub-intervals Lj = ( tj-1, tj], j = 1, 2, ... k-1 and Lk = ( tk-1, tk]
where tk = t. (Note that these sub-intervals may not be the same as
those defined by deaths or sacrifice previously.) The parameters that
vary over sub-intervals ( ti-1, ti], i = 1, 2, ..., k are µ1j, alphaj,
ßj, µ2j, N, and those parameters related to contour integral( t). The
hazard functionis given by
where y1 < y2 are two real roots of alpha y2 - (alpha + ß + µ2 q) y +
ß = 0; alpha + ß + µ2
= 1; q = 1 - ßm/alpham; A(t) = / 0integralt a(x)d x, where a(t) =
contour integral( t)/[1 - F(t)] is the hazard function of the cell
lifetime and F(t) is the cumulative function of contour integral (t).
Two cases of special interest are a(t) = gamma when an exponential
distribution is assumed and a(t) = exp(-gamma t), when the Gompertz
distribution is assumed.
When an exponential distribution (i.e. a(t) = gamma or A(t) =
gamma t) and q = 1 are assumed, the model is equivalent to the
model of Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981). A
special case that may be more appropriate than the exponential
distribution is that when the Gompertz distribution is assumed (i.e.
A(t) = [1 - exp(-gamma t)]/gamma).
For the model with time-dependent parameters, assume that the
study begins at time t0. Divide the time scale ( t0, t] into
k sub-intervals Lj = ( tj-1, tj], j = 1, 2, ... k-1 and Lk = ( tk-1, tk]
where tk = t. (Note that these sub-intervals may not be the same as
those defined by deaths or sacrifice previously.) The parameters that
vary over sub-intervals ( ti-1, ti], i = 1, 2, ..., k are µ1j, alphaj,
ßj, µ2j, N, and those parameters related to contour integral( t). The
hazard functionis given by
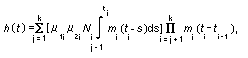 where
where
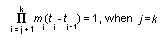 and
and
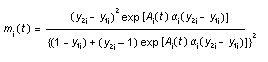 where y1j < y2j are two real roots of a alpha y2 - (alphaj + ßj +
µ2j qj) y + ßj = 0; alphaj + ßj + µ2j = 1; qj = 1 - ßmj/alphamj, j = 1,
2, ..., k.
When an exponential distribution (i.e. Aj( t) = gammaj( t) and
qj = 1) is assumed, the model is equivalent to the model of
Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981), with
piece-wise constant parameters. A special case that may be more
appropriate than the exponential distribution is that when the
Gompertz distribution is assumed (i.e. Aj( t) = {1 - exp[-gammaj t)]}
/gammaj).
In the alternative model for exposure to diesel exhaust, in which
the total time is divided into five (i.e. k = 5) sub-intervals, Tan
& Chen (1992) showed that, under the assumption of exponential cell
lifetime distribution, the tumour-free distribution function, Sx( t),
can be written:
where y1j < y2j are two real roots of a alpha y2 - (alphaj + ßj +
µ2j qj) y + ßj = 0; alphaj + ßj + µ2j = 1; qj = 1 - ßmj/alphamj, j = 1,
2, ..., k.
When an exponential distribution (i.e. Aj( t) = gammaj( t) and
qj = 1) is assumed, the model is equivalent to the model of
Moolgavkar & Venzon (1979) and Moolgavkar & Knudson (1981), with
piece-wise constant parameters. A special case that may be more
appropriate than the exponential distribution is that when the
Gompertz distribution is assumed (i.e. Aj( t) = {1 - exp[-gammaj t)]}
/gammaj).
In the alternative model for exposure to diesel exhaust, in which
the total time is divided into five (i.e. k = 5) sub-intervals, Tan
& Chen (1992) showed that, under the assumption of exponential cell
lifetime distribution, the tumour-free distribution function, Sx( t),
can be written:
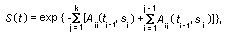 where sj = tj if j < k and sj = t if j = k, and
where sj = tj if j < k and sj = t if j = k, and
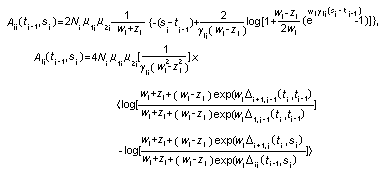 where,
WI = [alpha + ß + µ2 q)2 - 4alphaß]´
ZI = alpha - ß - µ2 q, and
DeltaI( s,t) = gammai( t - s) if both s and t are in the same closed
sub-interval [ ti-1, ti] and
where,
WI = [alpha + ß + µ2 q)2 - 4alphaß]´
ZI = alpha - ß - µ2 q, and
DeltaI( s,t) = gammai( t - s) if both s and t are in the same closed
sub-interval [ ti-1, ti] and
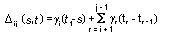 if sepsilon Li, tepsilon Lj with tj < tj.
B11. RECOMMENDATIONS
B11.1 Recommendations for the protection of human health
Diesel exhaust contributes to the total effect of combustion
products on the environment. The data reviewed in this monograph
support the conclusion that inhalation of diesel exhaust is of concern
with respect to both neoplastic and non-neoplastic diseases. The
particulate phase appears to have the greatest effect on health, and
both the particle core and the associated organic materials have
biological activity, although the gas-phase components cannot be
disregarded. The following actions are recommended for the protection
of human health.
-- Diesel exhaust emissions should be controlled as part of the
overall control of atmospheric pollution, particularly in urban
environments.
-- Emissions should be controlled strictly by regulatory inspections
and prompt remedial action.
-- Urgent efforts should be made to reduce emissions, specifically
of particulates, by changing exhaust train techniques, engine
design, and fuel composition.
-- In the occupational environment, good work practices should be
encouraged, and adequate ventilation must be provided to prevent
excessive exposure.
B11.2 Recommendations for the protection of the environment
Too few data are available on which to base specific suggestions
with regard to diesel exhaust, except as part of the general control
of emissions.
B11.3 Recommendations for further research
The following recommendations are intended to help reduce the
uncertainty associated with assessing the risks of exposure to diesel
exhaust for human health.
-- Research is required on methods for determining concentrations of
diesel particulates in the presence of fine particles from other
sources, in order to improve assessments of the exposures of
occupational groups and the general population indoors and
outdoors. The quality and quantity of emissions from engines that
are not properly maintained or tuned should be investigated as
part of these studies.
-- The effects of diesel exhaust emissions on lung clearance should
be studied in animals and humans at concentrations including
those likely to be encountered by humans.
-- The mechanisms involved in the etiology of tumours induced in
rats exposed to particulate must be investigated by all available
techniques.
-- The relative roles of the gas and particulate phases of diesel
exhaust in causing adverse effects after short- and long-term
exposure should be investigated.
-- Epidemiological investigations into the effects of diesel exhaust
should be continued in order to assess issues of dose and latency
in human populations with respect to lung cancer; and longer-term
studies should be conducted in populations exposed to diesel
exhaust with respect to noncarcinogenic effects.
-- Further steps should be undertaken to improve the combustion and
exhaust emission characteristics of diesel engines.
B12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks for human beings were evaluated by a
working group convened by the International Agency for Research on
Cancer in 1988 (International Agency for Research on Cancer, 1989b).
The conclusions were:
'There is sufficient evidence for the carcinogenicity in
experimental animals of whole diesel engine exhaust.
'There is inadequate evidence for the carcinogenicity in
experimental animals of gas-phase diesel engine exhaust (with
particles removed).
'There is sufficient evidence for the carcinogenicity in
experimental animals of extracts of diesel engine exhaust particles.
'There is limited evidence for the carcinogenicity in humans of
diesel engine exhaust.
'There is limited evidencefor the carcinogenicity in humans of
engine exhausts (unspecified as from diesel or gasoline engines).
'Overall evaluation
'Diesel engine exhaust is probably carcinogenic to humans (Group2A).'
REFERENCES
Acher AJ, Boderie P, & Yaron B (1989) Soil pollution by petroleum
products: I. Multiphase migration of kerosene components in soil
columns. J Contam Hydrol, 4: 333-345.
Ackman RG & Noble D (1973) Steam distillation: A simple technique for
recovery of petroleum hydrocarbons from tainted fish. J Fish Resour
Board Can, 30: 711-714.
Agency for Toxic Substances and Disease Registry (1995) Toxicological
profile for fuel oils. Atlanta, GA, 204 pp.
Ahlberg J, Ahlbom A, Lipping H, Norell S, & Österblom L (1981) [Cancer
among professional drivers.] Laekartidn, 78: 1545-1546 (in Danish).
Albrechcinski TM, Michalovic JG, Wattle BJ, & Wilkinson EP (1985) A
laboratory investigation of the fate of diesel emissions in the
atmosphere. Task 2 (Final Report No. CRC-APRAC-CAPA-13-76-05;
PB 86-215837). Buffalo, NY, Calspan Corp., 163 pp.
Amann CA & Siegla DC (1982) Diesel particulates -- What they are and
why. Aerosol Sci Technol, 1: 73-101.
American Automobile Manufacturers Association (1993) World motor
vehicle data. Detroit, pp 9, 50, 51, 335.
American Petroleum Institute (1979) Teratology study in rats, diesel
fuel (Publication No. 27-32174). Washington DC, 23 pp.
American Petroleum Institute (1980a) Acute toxicity tests:
API 78-3, No. 2 home heating oil (10% cat.) (Publication
No. 27-32773). Washington DC, 52 pp.
American Petroleum Institute (1980b) Acute toxicity tests:
API 78-2, No. 2 home heating oil (30% cat.) (Publication
No. 27-32771). Washington DC, 48 pp.
American Petroleum Institute (1980c) Acute toxicity tests:
API 78-4, No. 2 home heating oil (50% cat.) (Publication
No. 27-32068). Washington DC, 52 pp.
American Petroleum Institute (1980d) Acute toxicity tests. API jet
fuel A (Publication no. 27-32815). Washington DC, 48 pp.
American Petroleum Institute (1980e) Mutagenicity evaluation of jet
fuel A in the mouse dominant lethal assay. Final report (Publication
No. 28-31345). Washington DC, 36 pp.
American Petroleum Institute (1981) Mutagenicity evaluation of diesel
fuel in the mouse dominant lethal assay, final report (Publication
No. 33-30493). Washington DC, 9 pp.
American Petroleum Institute (1982a) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32347). Washington DC, 24 pp.
American Petroleum Institute (1982b) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-10 (Publication
No. 30-32348). Washington DC, 26 pp.
American Petroleum Institute (1982c) Acute toxicity studies
hydrodesulfurized kerosine. Sample 81-07 (Publication No. 30-31986).
Washington DC, 112 pp.
American Petroleum Institute (1983a) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32856). Washington DC, 47 pp.
American Petroleum Institute (1983b) 28-Day dermal toxicity study in
the rabbit: Hydrodesulfurized middle distillate, API sample 81-10
(Publication No. 30-32298). Washington DC, 13 pp.
American Petroleum Institute (1983c) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats (Publication
no. 30-32855). Washington DC, 32 pp.
American Petroleum Institute (1984) Dermal sensitization study in
guinea pig, closed patch technique: Hydrodesulfurized middle
distillate, sample 81-10 (Publication No. 31-31414). Washington DC,
12 pp.
American Petroleum Institute (1985a) 28-Day dermal toxicity study in
the rabbit: API 83-08, light catalytically cracked distillate
(CAS
if sepsilon Li, tepsilon Lj with tj < tj.
B11. RECOMMENDATIONS
B11.1 Recommendations for the protection of human health
Diesel exhaust contributes to the total effect of combustion
products on the environment. The data reviewed in this monograph
support the conclusion that inhalation of diesel exhaust is of concern
with respect to both neoplastic and non-neoplastic diseases. The
particulate phase appears to have the greatest effect on health, and
both the particle core and the associated organic materials have
biological activity, although the gas-phase components cannot be
disregarded. The following actions are recommended for the protection
of human health.
-- Diesel exhaust emissions should be controlled as part of the
overall control of atmospheric pollution, particularly in urban
environments.
-- Emissions should be controlled strictly by regulatory inspections
and prompt remedial action.
-- Urgent efforts should be made to reduce emissions, specifically
of particulates, by changing exhaust train techniques, engine
design, and fuel composition.
-- In the occupational environment, good work practices should be
encouraged, and adequate ventilation must be provided to prevent
excessive exposure.
B11.2 Recommendations for the protection of the environment
Too few data are available on which to base specific suggestions
with regard to diesel exhaust, except as part of the general control
of emissions.
B11.3 Recommendations for further research
The following recommendations are intended to help reduce the
uncertainty associated with assessing the risks of exposure to diesel
exhaust for human health.
-- Research is required on methods for determining concentrations of
diesel particulates in the presence of fine particles from other
sources, in order to improve assessments of the exposures of
occupational groups and the general population indoors and
outdoors. The quality and quantity of emissions from engines that
are not properly maintained or tuned should be investigated as
part of these studies.
-- The effects of diesel exhaust emissions on lung clearance should
be studied in animals and humans at concentrations including
those likely to be encountered by humans.
-- The mechanisms involved in the etiology of tumours induced in
rats exposed to particulate must be investigated by all available
techniques.
-- The relative roles of the gas and particulate phases of diesel
exhaust in causing adverse effects after short- and long-term
exposure should be investigated.
-- Epidemiological investigations into the effects of diesel exhaust
should be continued in order to assess issues of dose and latency
in human populations with respect to lung cancer; and longer-term
studies should be conducted in populations exposed to diesel
exhaust with respect to noncarcinogenic effects.
-- Further steps should be undertaken to improve the combustion and
exhaust emission characteristics of diesel engines.
B12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risks for human beings were evaluated by a
working group convened by the International Agency for Research on
Cancer in 1988 (International Agency for Research on Cancer, 1989b).
The conclusions were:
'There is sufficient evidence for the carcinogenicity in
experimental animals of whole diesel engine exhaust.
'There is inadequate evidence for the carcinogenicity in
experimental animals of gas-phase diesel engine exhaust (with
particles removed).
'There is sufficient evidence for the carcinogenicity in
experimental animals of extracts of diesel engine exhaust particles.
'There is limited evidence for the carcinogenicity in humans of
diesel engine exhaust.
'There is limited evidencefor the carcinogenicity in humans of
engine exhausts (unspecified as from diesel or gasoline engines).
'Overall evaluation
'Diesel engine exhaust is probably carcinogenic to humans (Group2A).'
REFERENCES
Acher AJ, Boderie P, & Yaron B (1989) Soil pollution by petroleum
products: I. Multiphase migration of kerosene components in soil
columns. J Contam Hydrol, 4: 333-345.
Ackman RG & Noble D (1973) Steam distillation: A simple technique for
recovery of petroleum hydrocarbons from tainted fish. J Fish Resour
Board Can, 30: 711-714.
Agency for Toxic Substances and Disease Registry (1995) Toxicological
profile for fuel oils. Atlanta, GA, 204 pp.
Ahlberg J, Ahlbom A, Lipping H, Norell S, & Österblom L (1981) [Cancer
among professional drivers.] Laekartidn, 78: 1545-1546 (in Danish).
Albrechcinski TM, Michalovic JG, Wattle BJ, & Wilkinson EP (1985) A
laboratory investigation of the fate of diesel emissions in the
atmosphere. Task 2 (Final Report No. CRC-APRAC-CAPA-13-76-05;
PB 86-215837). Buffalo, NY, Calspan Corp., 163 pp.
Amann CA & Siegla DC (1982) Diesel particulates -- What they are and
why. Aerosol Sci Technol, 1: 73-101.
American Automobile Manufacturers Association (1993) World motor
vehicle data. Detroit, pp 9, 50, 51, 335.
American Petroleum Institute (1979) Teratology study in rats, diesel
fuel (Publication No. 27-32174). Washington DC, 23 pp.
American Petroleum Institute (1980a) Acute toxicity tests:
API 78-3, No. 2 home heating oil (10% cat.) (Publication
No. 27-32773). Washington DC, 52 pp.
American Petroleum Institute (1980b) Acute toxicity tests:
API 78-2, No. 2 home heating oil (30% cat.) (Publication
No. 27-32771). Washington DC, 48 pp.
American Petroleum Institute (1980c) Acute toxicity tests:
API 78-4, No. 2 home heating oil (50% cat.) (Publication
No. 27-32068). Washington DC, 52 pp.
American Petroleum Institute (1980d) Acute toxicity tests. API jet
fuel A (Publication no. 27-32815). Washington DC, 48 pp.
American Petroleum Institute (1980e) Mutagenicity evaluation of jet
fuel A in the mouse dominant lethal assay. Final report (Publication
No. 28-31345). Washington DC, 36 pp.
American Petroleum Institute (1981) Mutagenicity evaluation of diesel
fuel in the mouse dominant lethal assay, final report (Publication
No. 33-30493). Washington DC, 9 pp.
American Petroleum Institute (1982a) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32347). Washington DC, 24 pp.
American Petroleum Institute (1982b) Acute toxicity studies:
Hydrodesulfurized middle distillate, API sample 81-10 (Publication
No. 30-32348). Washington DC, 26 pp.
American Petroleum Institute (1982c) Acute toxicity studies
hydrodesulfurized kerosine. Sample 81-07 (Publication No. 30-31986).
Washington DC, 112 pp.
American Petroleum Institute (1983a) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats:
Hydrodesulfurized middle distillate, API sample 81-09 (Publication
No. 30-32856). Washington DC, 47 pp.
American Petroleum Institute (1983b) 28-Day dermal toxicity study in
the rabbit: Hydrodesulfurized middle distillate, API sample 81-10
(Publication No. 30-32298). Washington DC, 13 pp.
American Petroleum Institute (1983c) LC50 acute inhalation toxicity
evaluation of a petroleum derived hydrocarbon in rats (Publication
no. 30-32855). Washington DC, 32 pp.
American Petroleum Institute (1984) Dermal sensitization study in
guinea pig, closed patch technique: Hydrodesulfurized middle
distillate, sample 81-10 (Publication No. 31-31414). Washington DC,
12 pp.
American Petroleum Institute (1985a) 28-Day dermal toxicity study in
the rabbit: API 83-08, light catalytically cracked distillate
(CAS 