
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 169
LINEAR ALKYLBENZENE SULFONATES
AND RELATED COMPOUNDS
This report contains the collective views of an international group
of experts and does not necessarily represent the decisions or the
stated policy of the United Nations Environment Programme, the
International Labour Organisation, or the World Health Organization.
First draft prepared at the National Institute of Health Sciences,
Tokyo, Japan, and the Institute of Terrestrial Ecology, Monk's Wood,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and
the World Health Organization
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Linear Alkylbenzene Sulfonates and Related Compounds.
(Environmental health criteria ; 169)
1.Alkane sulfonates - adverse effects 2.Environmental exposure
3.Guidelines I.Series
ISBN 92 4 157169 1 (NLM Classification: QU 98)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
cWorld Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The
designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area
or of its authorities, or concerning the delimitation of its
frontiers or boundaries. The mention of specific companies or of
certain manufacturers' products does not imply that they are
endorsed or recommended by the World Health Organization in
preference to others of a similar nature that are not mentioned.
Errors and omissions excepted, the names of proprietary products are
distinguished by initial capital letters.
CONTENTS
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR LINEAR
ALKYLBENZENE SULFONATES AND RELATED COMPOUNDS
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
ENVIRONMENTAL HEALTH CRITERIA FOR LINEAR ALKYLBENZENE SULFONATES AND
RELATED COMPOUNDS
1. OVERALL SUMMARY, EVALUATION, AND RECOMMENDATIONS
1.1. Identity and analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental concentrations
1.3.1. Linear alklylbenzene sulfonates
1.3.2. alpha-Olefin sulfonates and alkyl sulfates
1.4. Environmental transport, distribution, and transformation
1.4.1. Linear alklylbenzene sulfonates
1.4.2. alpha-Olefin sulfonates
1.4.3. Alkyl sulfates
1.5. Kinetics
1.6. Effects on experimental animals and in vitro
test systems
1.7. Effects on humans
1.8. Environmental effects
1.8.1. Linear alklylbenzene sulfonates
1.8.1.1 Aquatic environment
1.8.1.2 Terrestrial environment
1.8.1.3 Birds
1.8.2. alpha-Olefin sulfonates
1.8.2.1 Aquatic environment
1.8.2.2 Terrestrial environment
1.8.3. Alkyl sulfates
1.8.3.1 Aquatic environment
1.8.3.2 Terrestrial environment
1.9. Human health risk evaluation
1.10. Evaluation of effects on the environment
1.11. Recommendations for protection of human health
and the environment
1.12. Recommendations for further research
A. Linear alkylbenzene sulfonates and their salts.
A1. SUMMARY
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
A2.1 Identity (sodium salt)
A2.2 Physical and chemical properties
A2.3 Analysis
A2.3.1 Isolation
A2.3.2 Analytical methods
A2.3.2.1 Nonspecific methods
A2.3.2.2 Specific methods
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
A3.2.2 Uses
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
A4.1 Transport and distribution between media
A4.1.1 Wastewater treatment
A4.1.2 Surface waters, sediments, and soils
A4.1.3 Fate models
A4.2 Environmental transformation
A4.2.1 Biodegradation
A4.2.1.1 Aerobic degradation
A4.2.1.2 Anaerobic degradation
A4.2.2 Abiotic degradation
A4.2.2.1 Photodegradation
A4.2.2.2 Cobalt-60 irradiation
A4.2.3 Bioaccumulation and biomagnification
A4.2.3.1 Aquatic organisms
A4.2.3.2 Terrestrial plants
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
A5.1 Environmental levels
A5.1.1 Wastewater, sewage effluent, and sludge
A5.1.2 Sediment
A5.1.3 Surface water
A5.1.4 Soil and groundwater
A5.1.5 Drinking-water
A5.1.6 Biota
A5.2 Environmental processes that influence concentrations
of linear alkylbenzene sulfonates
A5.2.1 Changes in chain length distribution during
environmental removal of linear alkylbenzene
sulfonates
A5.2.2 Specification of linear alkylbenzene sulfonates
in surface waters
A5.3 Estimation of human intake
A6. KINETICS
Section summary
A6.1 Absorption, distribution, and excretion
A6.2 Biotransformation
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
A7.1 Single exposures
A7.2 Short-term exposure
A7.2.1 Mouse
A7.2.2 Rat
A7.2.2.1 Administration in the diet
A7.2.2.2 Administration by gavage
A7.2.2.3 Dermal application
A7.2.2.4 Subcutaneous injection
A7.2.3 Guinea-pig
A7.2.4 Monkey
A7.3 Long-term exposure; carcinogenicity
A7.3.1 Mouse
A7.3.1.1 Administration in the diet
A7.3.1.2 Administration in the drinking-water.
A7.3.2 Rat
A7.3.2.1 Administration in the diet
A7.3.2.2 Administration in the drinking-water.
A7.3.2.3 Administration by gavage
A7.3.2.4 Dermal application
A7.4 Skin and eye irritation; sensitization
A7.4.1 Studies of skin
A7.4.2 Studies of the eye
A7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
A7.6 Mutagenicity and related end-points
A7.6.1 Studies in vitro
A7.6.2 Studies in vivo
A7.7 Special studies
A7.7.1 Studies in vitro
A7.7.2 Biochemical effects
A8. EFFECTS ON HUMANS
Section summary
A8.1 Exposure of the general population
A8.2 Clinical studies
A8.2.1 Skin irritation and sensitization
A8.2.2 Effects on the epidermis
A8.2.3 Hand eczema
A8.2.4 Occupational exposure
A8.2.5 Accidental or suicidal ingestion
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
A9.1 Effect of chain length on the toxicity of linear
alkylbenzene sulfonates
A9.2 Microorganisms
A9.3 Aquatic organisms
A9.3.1 Aquatic plants
A9.3.1.1 Freshwater algae and cyanobacteria
A9.3.1.2 Marine algae
A9.3.1.3 Macrophytes
A9.3.2 Aquatic invertebrates
A9.3.2.1 Acute toxicity
A9.3.2.2 Short-term and long-term toxicity
A9.3.2.3 Biochemical and physiological effects
A9.3.3 Fish
A9.3.3.1 Acute toxicity
A9.3.3.2 Chronic toxicity
A9.3.3.3 Biochemical and physiological effects
A9.3.3.4 Behavioural effects
A9.3.3.5 Interactive effects with other
chemicals
A9.3.4 Amphibia
A9.3.5 Studies of the mesocosm and communities
A9.3.6 Field studies
A9.3.7 Toxicity of biodegradation intermediates and
impurities of linear alkylbenzene sulfonates
A9.3.7.1 Individual compounds
A9.3.7.2 Effluents
A9.4 Terrestrial organisms
A9.4.1 Terrestrial plants
A9.4.2 Terrestrial invertebrates
A9.4.3 Birds
B. alpha-Olefin sulfonates
B1. SUMMARY
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
B2.1 Identity
B2.2 Physical and chemical properties
B2.3 Analytical methods
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
B3.2.2 Uses
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
B4.1 Transport and distribution between media
B4.2 Biotransformation
B4.2.1 Biodegradation
B4.2.1.1 Aerobic biodegradation
B4.2.1.2 Anaerobic degradation
B4.2.2 Abiotic degradation
B4.2.3 Bioaccumulation and biomagnification
B4.3 Interaction with other physical, chemical, and
biological factors
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
B6. KINETICS
Section summary
B6.1 Absorption, distribution, and excretion
B6.2 Biotransformation
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
B7.1 Single exposures
B7.2 Short-term exposure
B7.3 Long-term exposure; carcinogenicity
B7.3.1 Mouse
B7.3.2 Rat
B7.4 Skin and eye irritation; sensitization
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
B7.6 Mutagenicity and related end-points
B7.7 Special studies
B8. EFFECTS ON HUMANS
Section summary
B8.1 Exposure of the general population
B8.2 Clinical studies
B8.2.1 Skin irritation and sensitization
B8.2.2 Effect on the epidermis
B8.2.3 Hand eczema
B8.2.4 Accidental or suicidal ingestion
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
B9.1 Microorganisms
B9.2 Aquatic organisms
B9.2.1 Aquatic plants
B9.2.2 Aquatic invertebrates
B9.2.3 Fish
B9.3 Terrestrial organisms
B9.3.1 Terrestrial plants
B9.3.2 Terrestrial invertebrates
B9.3.3 Birds
C. Alkyl sulfates
C1. SUMMARY
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
C2.1 Identity
C2.2 Physical and chemical properties
C2.3 Analysis
C2.3.1 Isolation
C2.3.2 Analytical methods
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Section summary
C3.1 Natural occurrence
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
C3.2.2 Uses
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
C4.1 Transport and distribution between media
C4.2 Biotransformation
C4.2.1 Biodegradation
C4.2.1.1 Biodegradation pathway; mechanism
C4.2.1.2 Biodegradation in the environment
C4.2.1.3 Anaerobic degradation
C4.2.2 Abiotic degradation
C4.2.3 Bioaccumulation and biomagnification
C4.3 Interaction with other physical, chemical,
and biological factors
C4.4 Ultimate fate following use
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
Environmental levels
C6. KINETICS
Section summary
C6.1 Absorption, distribution, and excretion
C6.2 Biotransformation
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
C7.1 Single exposures
C7.2 Short-term exposure
C7.2.1 Rat
C7.2.1.1 Administration in the diet
C7.2.1.2 Administration in the drinking-water
C7.2.1.3 Dermal application
C7.2.2 Rabbit
C7.3 Long-term exposure; carcinogenicity
C7.3.1 Mouse
C7.3.2 Rat
C7.3.2.1 Administration in the diet
C7.3.2.2 Administration in the drinking-water
C7.4 Skin and eye irritation; sensitization
C7.4.1 Local irritation
C7.4.1.1 Skin
C7.4.1.2 Eye
C7.4.2 Skin sensitization
C7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
C7.6 Mutagenicity and related end-points
C7.7 Special studies
C8. EFFECTS ON HUMANS
Section summary
C8.1 Exposure of the general population
C8.2 Clinical studies
C8.2.1 Skin irritation and sensitization
C8.2.2 Effects on the epidermis
C8.2.3 Hand eczema
C8.2.4 Accidental or suicidal ingestion
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
C9.1 Microorganisms
C9.2 Aquatic organisms
C9.2.1 Aquatic plants
C9.2.1.1 Freshwater algae
C9.2.1.2 Macrophytes
C9.2.2 Aquatic invertebrates
C9.2.3 Fish
C9.2.4 Tests in biocenoses
C9.3 Terrestrial organisms
APPENDIX I
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR LINEAR
ALKYLBENZENE SULFONATES AND RELATED COMPOUNDS
Members
Dr R.S. Chhabra, National Institutes of Health, Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr A. Granmo, University of Göteborg, Marine Research Station at
Kristineberg, Fiskebackskil, Sweden
Ms K. Hughes, Priority Substances Section, Health and Welfare
Canada, Ottawa, Ontario, Canada
Mr H. Malcolm, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr E. van der Plassche, Toxicology Advisory Centre, National
Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Dr J. Sekizawa, Division of Information on Chemical Safety, National
Institute of Hygienic Sciences, Tokyo, Japan
Ms R. Takei, Research Planning and Administration Department, Lion
Corporation, Tokyo, Japan
Dr D.G. Van Ormer, Health Effects Division, Office of Pesticides
Programs, Environmental Protection Agency, Washington DC, USA
Professor P.N. Viswanathan, Industrial Toxicology Research Centre,
Lucknow, India
Representatives/Observers
IUTOX
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy
CEFIC
Dr J.L. Berna, Petresa, Madrid, Spain (20-21 October)
Dr L. Cavalli, Enichem Augusta Industriale Srl, Milan, Italy
(18-19 October)
IASD
Dr G. Holland, UNILEVER Ltd, Environmental Safety Laboratory,
Sharnbrook, United Kingdom
Dr M. Stalmans, Procter & Gamble ETC, 100 Temselaan,
Strombeek-Bever, Belgium
Secretariat
Dr H.-J. Poremski, Umweltbundesamt, Berlin, Germany (21 October)
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr B. Wittann, Umweltbundesamt, Berlin, Germany (18-20 October)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria monographs, readers are requested to
communicate any errors that may have occurred to the Director of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
Postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone no.
979 9111).
* * *
This publication was made possible by financial support from the
US Environmental Protection Agency, USA, and from the European
Commission.
ENVIRONMENTAL HEALTH CRITERIA FOR LINEAR ALKYLBENZENE SULFONATES AND
RELATED COMPOUNDS
A WHO Task Group on Environmental Health Criteria for Linear
Alkylbenzene Sulfonates and Related Compounds met at the World
Health Organization, Geneva, on 18-22 October 1993. Dr E. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
of IPCS, and of the three IPCS cooperating organizations (UNEP, ILO,
and WHO). The Group reviewed and revised a draft document and
evaluated the risks for human health and the environment of exposure
to linear alkylbenzene sulfonates, a-olefin sulfonates, and alkyl
sulfonates.
The sections of the first draft on toxicology and human health
were prepared at the National Institute of Health Sciences (NIHS),
Tokyo, Japan, and the sections on the environment at the Institute
of Terrestrial Ecology (ITE), Monks Wood, United Kingdom.
Dr E. Smith of the IPCS Central Unit was responsible for
the scientific content of the monograph and Mrs E. Heseltine,
St Léon-sur-Vézère, France, for the editing.
The authors who contributed to the first draft were:
Dr S. Dobson, ITE, Monks Wood, United Kingdom
Dr R. Hasegawa, NIHS, Tokyo, Japan
Dr Y. Hayashi, NIHS, Tokyo, Japan
Dr K. Hiraga, Public Health Research Laboratory, Tokyo, Japan
Dr P. Howe, ITE, Monks Wood, United Kingdom
Dr Y. Ikeda, NIHS, Tokyo, Japan
Dr Y. Kurokawa, NIHS, Tokyo, Japan
Dr H. Malcolm, ITE, Monks Wood, United Kingdom
Dr A. Matsuoka, NIHS, Tokyo, Japan
Dr K. Morimoto, NIHS, Tokyo, Japan
Dr M. Nakadate, NIHS, Tokyo, Japan
Dr K. Oba, Lion Chemical Corporation, Tokyo, Japan
Dr J. Sekizawa, NIHS, Tokyo, Japan
Dr T. Sohuni, NIHS, Tokyo, Japan
Dr M. Takahashi, NIHS, Tokyo, Japan
Dr R. Takei, Lion Chemical Corporation, Tokyo, Japan
Dr S. Tanaka, NIHS, Tokyo, Japan
Dr S. Tomiyama, Lion Chemical Corporation, Tokyo, Japan
Dr T. Yamaha, NIHS, Tokyo, Japan
Dr S. Yoshikawa, Environmental Research Institute, Kawasaki, Japan
Dr M. Wakabayashi, Water Quality Management Centre, Tokyo, Japan
Dr Y. Watanabe, Central Railway Hospital, Tokyo, Japan
Dr P. Howe, Dr H. Malcolm, and Dr J Sekizawa also contributed to
the second draft.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
1. OVERALL SUMMARY, EVALUATION, AND RECOMMENDATIONS
1.1 Identity and analytical methods
Linear alkylbenzene sulfonates (LAS), alpha-olefin sulfonates
(AOS), and alkyl sulfates (AS) are anionic surfactants with
molecules characterized by a hydrophobic and a hydrophilic (polar)
group. Commercial mixtures consist of isomers and homologues of
related compounds, which differ in physicochemical properties,
resulting in formulations for various applications.
LAS, AOS, and AS can be analysed by nonspecific methods. The
assay usually used is one for substances that react with methylene
blue, which responds to any compound containing an anionic and
hydrophobic group. It thus suffers from analytical interference if
used for environmental samples; furthermore, the sensitivity of this
method is about 0.02 mg/litre. Although nonspecific alternatives to
this method have been developed, they are not commonly used.
Specific methods for environmental analysis are available only for
LAS and AS. An improved method based on methylene blue reactivity
and high-performance liquid chromatography (HPLC) is available for
analysis of AOS.
LAS are nonvolatile compounds produced by sulfonation of linear
alkylbenzene. Commercial products are always mixtures of homologues
of different alkyl chain lengths (C10-C13 or C14) and isomers
differing in the phenyl ring positions (2 to 5 phenyl). All of the
homologues and isomers of LAS can be determined in environmental
samples and other matrices by specific analytical methods such as
HPLC, gas chromatography, and gas chromatography-mass spectrometry.
AOS are nonvolatile compounds produced by sulfonation of
alpha-olefins. They are mixtures of two compounds, sodium alkene
sulfonate and hydroxyalkane sulfonate, with alkyl chain lengths of
C14-C18.
AS are nonvolatile compounds produced by sulfation of
oleochemical or petrochemical alcohols. They are mixtures of
homologues with alkyl chain lengths of C10-C18. Specific
analytical methods are being developed for environmental monitoring.
1.2 Sources of human and environmental exposure
LAS, AOS, and AS are used as active ingredients in household and
personal care products and in specialized applications. After use,
such detergent compounds are discharged into the environment in
wastewater.
There is occupational exposure to these compounds. The exposure
of the general human population and of environmental organisms
depends on the application of LAS, AOS, and AS (and other
surfactants), on local sewage treatment practices, and on the
characteristics of the receiving environment.
In 1990, worldwide consumption figures were about 2 million
tonnes of LAS, 86 000 tonnes of AOS, and 289 000 tonnes of AS.
1.3 Environmental concentrations
1.3.1 Linear alkylbenzene sulfonates
Concentrations of LAS have been quantified by means of a
specific, sensitive analytical method in almost every environmental
compartment in which they might be present. The concentrations
decrease progressively in the order wastewater > treated effluent
> surface waters > the sea.
In areas where LAS are the predominant surfactants used, the
concentrations are usually 1-10 mg/litre in wastewater,
0.05-0.1 mg/litre in effluents treated biologically,
0.05-0.6 mg/litre in effluents treated with a percolating filter,
0.005-0.05 mg/litre in surface waters below sewage outfalls (with
concentrations decreasing rapidly to 0.01 mg/litre downstream of the
outfall), < 1-10 mg/kg in river sediments (< 100 mg/kg in highly
polluted sediments near discharge zones), 1-10 g/kg in sewage
sludge, and < 1-5 mg/kg in sludge-amended soils (initially
5-10 mg/kg; - 50 mg/kg have been reported after atypically high
applications of sludge). The concentrations of LAS in estuarine
waters are 0.001-0.01 mg/litre, although higher levels occur where
wastewater is discharged directly. The concentrations in offshore
marine waters are < 0.001-0.002 mg/litre.
It should be noted that the environmental concentrations of LAS
vary widely. This variation is due to differences in analytical
methods, in the characteristics of sampling sites (ranging from
highly polluted areas with inadequate sewage treatment to areas
where sewage undergoes extensive treatment), in season (which can
account for a difference of twofold), and in consumption of LAS.
Environmental monitoring shows that there has been no
accumulation of LAS in environmental compartments over time. The
concentrations in soil do not increase with time but decrease owing
to mineralization. As LAS do not degrade under strictly anaerobic
conditions (to generate methane), it cannot be concluded that they
are mineralized in anaerobic sediments. With current use, the rate
of assimilation of LAS in all receiving environmental compartments
is equal to the rate of input, implying a steady state.
1.3.2 alpha-Olefin sulfonates and alkyl sulfates
Limited data are available on the concentrations of AOS in the
environment owing to the difficulty of analysing them in
environmental samples. Nonspecific colorimetric methods (such as
that based on methylene blue) allow detection of anionic surfactants
in general, but they suffer from analytical interferences and are
not suitable for determining specific concentrations of AOS. A
specific method is being developed for measuring AS in environmental
samples.
Studies conducted in the laboratory indicate that AOS and AS are
mineralized rapidly in all environmental compartments and are
virtually entirely removed from sewage during treatment. The
concentrations in surface water, sediments, soil, estuarine water,
and the marine environment are probably low. The levels of AOS in
river water have been found to be low.
1.4 Environmental transport, distribution, and transformation
At te mperatures below 5-10°C, the biodegradation kinetics of
LAS, AOS, and AS is reduced because of a reduction in microbial
activity.
1.4.1 Linear alkylbenzene sulfonates
The routes by which LAS enter the environment vary among
countries, but the main route is via discharge from sewage treatment
works. When wastewater treatment facilities are absent or
inadequate, sewage may be discharged directly into rivers, lakes,
and the sea. Another route of entry of LAS to the environment is by
the spreading of sewage sludge on agricultural land.
Throughout their passage into the environment, LAS are removed
by a combination of adsorption and primary and ultimate
bio-degradation. LAS are adsorbed onto colloidal surfaces and onto
suspended particles, with measured adsorption coefficients of
40-5200 litres/kg depending on the media and the structure of the
LAS. They biodegrade in surface water (half-life, 1-2 days), aerobic
sediments (1-3 days), and marine and estuarine systems (5-10 days).
During primary sewage treatment, about 25% of LAS (range,
10-40%) are adsorbed onto and removed with waste sludge. They are
not removed during anaerobic sludge digestion but are removed during
aerobic treatment of sludge, with a half-life of about 10 days.
After application of sludge to soil, 90% of LAS are generally
degraded within three months, with a half-life of 5-30 days.
The whole-body concentration factors for LAS range from 100 to
300, for the sum of 14C-LAS and 14C metabolites. Uptake by fish
occurs mainly through the gills, with subsequent distribution to the
liver and gall-bladder after biotransformation. LAS are excreted
rapidly, and there is therefore no evidence that they undergo
biomagnification.
1.4.2 alpha-Olefin sulfonates
Fewer data are available on the environmental transport,
distribution, and transformation of AOS than for LAS. It can be
inferred that AOS are transported into the environment in a manner
similar to that established for LAS, AS and other detergent
surfactants, and the environmental fate of AOS is similar to that of
LAS and AS. It is readily biodegraded under aerobic conditions, and
primary biodegradation is complete within 2-10 days, depending on
the temperature. Limited data are available on the bioaccumulation
of AOS; no bioaccumulation was observed in fish. There are no data
on abiotic degradation.
1.4.3 Alkyl sulfates
AS are transported into the environment by mechanisms similar to
those that operate for LAS and AOS. They are readily biodegradable
under aerobic and anaerobic conditions in the laboratory and under
environmental conditions; primary biodegradation is complete within
2-5 days. The whole-body bioconcentration factor ranges from 2 to 73
and varies with the chain length of alkyl sulfate homologues. AS are
taken up, distributed, biotransformed, and excreted by fish in the
same way as LAS and are not bioconcentrated in aquatic organisms.
1.5 Kinetics
LAS, AOS, and AS are readily absorbed by the gastrointestinal
tract, widely distributed throughout the body, and extensively
metabolized. LAS undergo omega- and ß-oxidation. The parent
compounds and metabolites are excreted mainly through the kidney,
although a proportion of an absorbed dose may be excreted as
metabolites in the faeces by biliary excretion. Only minimal amounts
of LAS, AOS, and AS appear to be absorbed through intact skin,
although prolonged contact may compromise the integrity of the
epidermal barrier, thereby permitting greater absorption; high
concentrations may reduce the time required for penetration.
1.6 Effects on experimental animals and in vitro test systems
The oral LD50 values for sodium salts of LAS were 404-1470
mg/kg body weight in rats and 1259-2300 mg/kg body weight in mice,
suggesting that rats are more sensitive than mice to the toxicity of
LAS. An oral LD50 of 3000 mg/kg body weight was measured for a
sodium salt of AOS in mice. The oral LD50 values of AS in rats
were 1000-4120 mg/kg body weight. LAS, AOS, and AS irritate the skin
and eye.
Minimal effects, including biochemical alterations and
histo-pathological changes in the liver, have been reported in
subchronic studies in which rats were administered LAS in the diet
or drinking-water at concentrations equivalent to doses greater than
120 mg/kg body weight per day. Although ultrastructural changes were
observed in liver cells at lower doses in one study, the changes
appeared to be reversible. Effects were not seen at similar doses in
other studies, but the organs may have been examined more closely in
the initial study. Reproductive effects, including decreased
pregnancy rate and litter loss, have been reported in animals
administered doses > 300 mg/kg per day. Histopathological and
biochemical changes were observed after long-term dermal application
to rats of solutions of > 5% LAS, and after 30 days' application to
the skin of guinea-pigs of 60 mg/kg body weight. Repeated dermal
application of > 0.3% solutions of LAS induced fetotoxic and
reproductive effects, but also induced maternal toxicity. Few data
are available from studies in experimental animals that allow
evaluation of the potential effects of AOS in humans. No effects
were observed in rats administered oral doses of 250 mg/kg body
weight per day chronically, but fetotoxicity was seen in rabbits
administered a maternally toxic dose of 300 mg/kg body weight per
day. Application of AOS to the skin and eyes of experimental animals
induced local effects.
Although the effects of short- and long-term exposure of animals
to AS have been investigated in several studies, most suffered from
inadequate histopathological examination or small group sizes;
furthermore, the highest doses used in the long-term studies did not
produce any toxic effects, so that an NOAEL could not be
established. Effects have, however, been reported consistently in
rats administered AS in the diet or drinking-water at concentrations
equivalent to 200 mg/kg body weight per day or more. Local effects
have been observed on the skin and eyes after topical application of
concentrations of about 0.5% AS or more. Maternally toxic and
fetotoxic effects have been observed at higher concentrations.
Most of the long-term studies are inadequate to evaluate the
carcinogenic potential of LAS, AOS, and AS in experimental animals,
owing to factors such as small numbers of animals, limited numbers
of doses, absence of a maximal tolerated dose, and limited
histo-pathological examination in the majority of studies. In those
studies in which the pathological findings were adequately reported,
maximal tolerated doses were not used, and the doses did not produce
toxic effects. Subject to these limitations, however, the studies in
which animals were administered LAS, AOS, or AS orally gave no
evidence of carcinogenicity; long-term studies in which AOS was
applied by skin painting studies also showed no effect.
On the basis of limited data, these compounds do not appear to
be genotoxic in vivo or in vitro.
1.7 Effects on humans
The results of patch tests show that human skin can tolerate
contact with solutions containing up to 1% LAS, AOS, or AS for 24 h
with only mild irritation reactions. These surfactants caused
delipidation of the skin surface, elution of natural moisturizing
factor, denaturation of the proteins of the outer epidermal layer,
and increased permeability and swelling of the outer layer. Neither
LAS, AOS, nor AS induced skin sensitization in volunteers, and there
is no conclusive evidence that they induce eczema. No serious
injuries or fatalities have been reported following accidental
ingestion of these surfactant by humans.
1.8 Environmental effects
1.8.1 Linear alkylbenzene sulfonates
1.8.1.1 Aquatic environment
LAS have been studied extensively both in the laboratory (short-
and long-term studies) and under more realistic conditions (micro-
and mesocosm and field studies). In general, a decrease in alkyl
chain length or greater internalization of the phenyl group is
accompanied by a decrease in toxicity. Observations in fish and
Daphnia indicate that a decrease in chain length of one unit (e.g.
C12 to C11) results in an approximately twofold decrease in
toxicity.
The results of laboratory tests are as follows:
-- Microorganisms: The results are highly variable owing to
the use of a variety of test systems (e.g. inhibition of activated
sludge; mixed cultures and individual species). The EC50 values
range from 0.5 mg/litre (single species) to > 1000 mg/litre. For
microorganisms, there is no linear relationship between chain length
and toxicity.
-- Aquatic plants: The results are highly species dependent.
For freshwater organisms, the EC50 values are 10-235 mg/litre
(C10-C14) in green algae, 5-56 mg/litre (C11.1-C13) in blue
algae, 1.4-50 mg/litre (C11.6-C13) in diatoms, and
2.7-4.9 mg/litre (C11.8) in macrophytes; marine algae appear to be
even more sensitive. In algae, there is probably no linear
relationship between chain length and toxicity.
-- Invertebrates: The acute L(E)C50 values for at least 22
freshwater species are 4.6-200 mg/litre (chain length not specified;
C13) for molluscs; 0.12-27 mg/litre (not specified; C11.2-C18)
for crustaceans; 1.7-16 mg/litre (not specified; C11.8) for worms,
and 1.4-270 mg/litre (C10-C15) for insects. The chronic L(E)C50
values are 2.2 mg/litre (C11.8) for insects and 1.1-2.3 mg/litre
(C11.8-C13) for crustaceans. The chronic no-observed-effect
concentration (NOEC; based on lethality or reproductive effects) is
0.2-10 mg/litre (not specified; C11.8) for crusta-ceans. Marine
invertebrates appear to be more sensitive, with LC50 values of 1
to >100 mg/litre (almost all C12) for 13 species, and NOECs of
0.025-0.4 mg/litre (not specified for all tests) for seven species
tested.
-- Fish: The acute LC50 values are 0.1-125 mg/litre
(C8-C15) for 21 freshwater species; the chronic L(E)C50 values
are 2.4 and 11 mg/litre (not specified; C11.7) for two species;
and the NOECs are 0.11-8.4 to 1.8 mg/litre (not specified;
C11.2-C13) for two species. Again, marine fish appear to be more
sensitive, with acute LC50 values of 0.05-7 mg/litre (not
specified; C11.7) for six species and chronic LC50 values of
0.01-1 mg/litre (not specified) for two species. In most of the
reports, the chain length was not reported. An NOEC of <
0.02 mg/litre (C12) was reported for marine species.
The average chain length of products commonly used commercially
is C12. Compounds of many different chain lengths have been tested
in Daphnia magna and fish, but the length tested in other
freshwater organisms has usually been C11.8. The typical acute
L(E)C50 values for C12 LAS are 3-6 mg/litre in Daphnia magna
and 2-15 mg/litre in freshwater fish, and the typical chronic NOECs
are 1.2-3.2 mg/litre for Daphnia and 0.48-0.9 mg/litre for
freshwater fish. The typical acute LC50 values for LAS of this
chain length in marine fish are < 1-6.7 mg/litre.
Saltwater organisms, especially invertebrates, appear to be more
sensitive to LAS than freshwater organisms. In invertebrates, the
sequestering action of LAS on calcium may affect the availability of
this ion for morphogenesis. LAS have a general effect on ion
transport. Biodegradation products and by-products of LAS are 10-100
times less toxic than the parent compounds.
The results obtained under more realistic conditions are as
follows: LAS have been tested in all freshwater tests at several
trophic levels, including enclosures in lakes (lower organisms),
model ecosystems (sediment and water systems), rivers below and
above the outfall of wastewater treatment plants, and in
experimental streams. C12 LAS were used in almost all cases. Algae
appear to be more sensitive in summer than in winter, as the 3-h
EC50 values were 0.2-8.1 mg/litre after photosynthesis, whereas in
model ecosystems no effects were seen on the relative abundance of
algal communities at 0.35 mg/litre. The no-effect levels in these
studies were 0.24-5 mg/litre, depending on the organism and
parameter tested. These results agree fairly well with those of
laboratory tests.
1.8.1.2 Terrestrial environment
Information is available for plants and earthworms. The
NOECs for seven plant species tested in nutrient solutions are
< 10-20 mg/litre; that for three species tested in soils, based
on growth, was 100 mg/kg (C10-C13). The 14-day LC50 for earthworms
was > 1000 mg/kg.
1.8.1.3 Birds
One study of chickens treated in the diet resulted in an NOEC
(based on egg quality) of > 200 mg/kg.
1.8.2 alpha-Olefin sulfonates
There are limited data on the effects of AOS on aquatic and
terrestrial organisms.
1.8.2.1 Aquatic environment
Only the results of laboratory tests are available:
-- Algae: EC50 values of > 20-65 mg/litre (C16-C18)
have been reported for green algae.
-- Invertebrates: LC50 values of 19 and 26 mg/litre (C16-C18)
have been reported for Daphnia.
-- Fish: The acute LC50 values are 0.3-6.8 mg/litre (C12-C18)
for nine species of fish. On the basis of short-term studies in
brown trout (Salmo trutta), golden orfe (Idus melanotus), and
harlequin fish (Rasbora heteromorpha), it can be concluded that
the toxicity of C14-C16 compounds is about five times lower than
that of C16-C18, with LC50 values (all measured concentrations)
of 0.5-3.1 (C16-C18) and 2.5-5.0 mg/litre (C14-C16). Two
long-term studies in rainbow trout showed that growth is the most
sensitive parameter, resulting in an EC50 of 0.35 mg/litre. In a
marine fish, the grey mullet (Mugal cephalus), the 96-h LC50 value
was 0.70 mg/litre.
1.8.2.2 Terrestrial environment
One study of plants in nutrient solutions showed NOECs of
32-56 mg/litre. In a study of chickens treated in the diet, an NOEC
(based on egg quality) of > 200 mg/kg was reported.
1.8.3 Alkyl sulfates
1.8.3.1 Aquatic environment
AS have been studied in short- and long-term studies and in one
study under more realistic conditions. Their toxicity is again
dependent on the alkyl chain length; no information was available on
any difference in toxicity between linear and branched AS.
The results of the laboratory tests are as follows:
-- Microorganisms: The EC50 values in a marine community
were 2.1-4.1 mg/litre (C12). The NOECs in Pseudomonas putida were
35-550 mg/litre (C16-C18).
-- Aquatic plants: The EC50 values were > 20-65 mg/litre
(C12-C13) in green algae and 18-43 mg/litre (C12) in
macrophytes. The NOECs were 14-26 mg/litre (C12-C16/C18) in
green algae.
-- Invertebrates: The LC50 and EC50 values were 4-140 mg/litre
(C12/C15-C16/C18) in freshwater species and 1.7-56 mg/litre
(all C12) in marine species. The chronic NOEC in Daphnia magna was
16.5 mg/litre (C16/C18) and those in marine species were
0.29-0.73 mg/litre (chain length not specified).
-- Fish: The LC50 values were 0.5-5.1 mg/litre (not
specified; C12-C16) in freshwater species and 6.4-16 mg/litre
(all C12) in marine species. No long-term studies were available.
It should be noted that many of these studies were carried out
under static conditions. As AS are readily biodegradable, their
toxicity may have been underestimated. In a 48-h study with Oryzias
latipes, the LC50 values were 46, 2.5 and 0.61 mg/litre
(measured concentrations) for C12, C14, and C16 compounds,
respectively. This and other studies indicate that toxicity differs
by a factor of five for two units of chain length. In a flow-through
biocenosis study with compounds of C16-C18, an NOEC of
0.55 mg/litre was observed.
1.8.3.2 Terrestrial environment
NOEC values of > 1000 mg/kg (C16-C18) were reported for
earthworms and turnips.
1.9 Human health risk evaluation
LAS are the most widely used surfactants in detergents and
cleaning products; AOS and AS are also used in detergents and
personal care products. The primary route of human exposure is,
therefore, through dermal contact. Minor amounts of LAS, AOS, and AS
may be ingested in drinking-water and as a result of residues on
utensils and food. Although limited information is available, the
daily intake of LAS via these media can be estimated to be about
5 mg/person. Occupational exposure to LAS, AOS, and AS may occur
during the formulation of various products, but no data are
available on the effects in humans of chronic exposure to these
compounds.
LAS, AOS, and AS can irritate the skin after repeated or
prolonged dermal contact with concentrations similar to those found
in undiluted products. In guinea-pigs, AOS can induce skin
sensitization when the level of gamma-unsaturated sultone exceeds
about 10 ppm.
The available long-term studies in experimental animals are
inadequate to evaluate the carcinogenic potential of LAS, AOS, and
AS, owing to factors such as study design, use of small numbers of
animals, testing of insufficient doses, and limited
histopathological examination. In the limited studies available in
which animals were administered LAS, AOS, or AS orally, there was no
evidence of carcinogenicity; the results of long-term studies in
which AOS were administered by skin painting were also negative.
These compounds do not appear to be genotoxic in vivo or
in vitro, although few studies have been reported.
Minimal effects, including biochemical alterations and
histopathological changes in the liver, have been reported in
subchronic studies of rats administered LAS in the diet or
drinking-water at concentrations equivalent to a dose of about
120 mg/kg body weight per day, although no effects were observed in
studies in which animals were exposed to higher doses for longer
periods. Dermal application of LAS caused both systemic toxicity and
local effects.
The average daily intake of LAS by the general population, on
the basis of limited estimates of exposure via drinking-water,
utensils, and food, is probably much lower (about three orders of
magnitude) than the levels shown to induce minor effects in
experimental animals.
The effects of AOS in humans observed in the few studies
available are similar to those reported in animals exposed to LAS.
As insufficient data are available to estimate the average daily
intake of AOS by the general population and on the levels that
induce effects in humans and animals, it is not possible to evaluate
with confidence whether exposure to AOS in the environment presents
a risk to human health. The levels of AOS in media to which humans
may be exposed are likely to be lower than those of LAS, however, as
AOS are used less.
Effects have been reported consistently in a few, limited
studies in rats administered AS in the diet or drinking-water at
concentrations equivalent to doses of 200 mg/kg body weight per day
or more. Local effects on the skin and eyes have been observed after
repeated or prolonged topical application. The available data are
insufficient to estimate the average daily intake of AS by the
general population. Since AS surfactants are not used as extensively
as those containing LAS, however, intake of AS is likely to be at
least three orders of magnitude lower than the doses shown to induce
effects in animals.
1.10 Evaluation of effects on the environment
LAS, AS, and AOS are used in large quantities and are released
into the environment via wastewater. Risk assessment requires
comparison of exposure concentrations with concentrations that cause
no adverse effects, and this can be done for several environmental
compartments. For anionic surfactants in general, the most important
compartments are sewage water treatment plants, surface waters,
sediment- and sludge-amended soils, and estuarine and marine
environments. Both biodegradation (primary and ultimate) and
adsorption occur, resulting in decreased environmental
concentrations and bioavailability. Reduction in chain length and
loss of the parent structure both result in compounds that are less
toxic than the parent compound. It is important that these
considerations be taken into account when the results of laboratory
tests are compared with potential effects on the environment.
Furthermore, in assessing the risk associated with environmental
exposure to these three anionic compounds, comparisons should be
made with the results of tests for toxicity of compounds of the same
chain length.
The effects of LAS on aquatic organisms have been tested
extensively. In laboratory tests in freshwater, fish appeared to be
the most sensitive species; the NOEC for fathead minnow was about
0.5 mg/litre (C12), and these results were confirmed in tests
under more realistic conditions. Differences have been observed
among phyto-plankton: in acute 3-h assays on phytoplankton, the
EC50 values were 0.2-0.1 mg/litre (C12-C13), whereas no
effects on relative abundance were found in other tests at
0.24 mg/litre (C11.8). Marine species appeared to be slightly more
sensitive than most other taxonomic groups.
A broad range of concentrations of all three anionic compounds
occurs in the environment, as shown by extensive measurements of
LAS. Owing to this broad range, no generally applicable
environmental risk assessment can be made for these compounds. A
risk assessment must involve appropriate understanding of the
exposure and effect concentrations in the ecosystem of interest.
Accurate data on exposure to AS and AOS are needed before an
environmental risk assessment can be made. Models are therefore
being used to assess exposure concentrations in the receiving
environmental compartments. Data on the toxicity of AS and AOS to
aquatic organisms, especially after chronic exposure to stable
concentrations, are relatively scarce. The available data show that
the toxicity of AOS and AS is similar to that of other anionic
surfactants.
Saltwater organisms appear to be more sensitive than freshwater
organisms to these compounds; however, their concentrations are
lower in seawater, except near wastewater outlets. The fate and
effects of these compounds in sewage in seawater have not been
investigated in detail.
For an evaluation of the environmental safety of surfactants
such as LAS, AOS, and AS, actual environmental concentrations must
be compared with no-effect concentrations. Research requirements are
determined not only by the intrinsic properties of a chemical but
also by its pattern or trend of consumption. As these can vary
considerably among geographic areas, assessment and evaluation must
be carried out regionally.
1.11 Recommendations for protection of human health and
the environment
1. As exposure to dusts may occur in the workplace (during
processing and formulation), standard occupational hygiene practices
should be used to ensure protection of workers' health.
2. The composition of formulations for consumer and industrial use
should be designed to avoid hazard, particularly for formulations
that are used for cleaning or laundering by hand.
3. Environmental exposure and effects should be appropriately
monitored to provide early indications of any overloading of
relevant environmental compartments.
1.12 Recommendations for further research
Human health
1. Since the skin is the primary route of human exposure to LAS,
AOS, and AS and since no adequate long-term studies of dermal
toxicity or carcinogenicity in experimental animals are available,
it is recommended that suitably designed long-term studies in which
these compounds are applied dermally be conducted.
2. In view of the lack of definitive data on the genotoxicity of
AOS and AS, additional studies should be performed in vivo and
in vitro.
3. In view of the inadequacies of the available studies on
reproductive and developmental toxicity, definitive studies should
be carried out in laboratory animals to obtain data on the effects
and on the effect and no-effect levels of LAS, AOS, and AS.
4. As exposure to LAS, AOS, and AS is not adequately defined, the
exposure of the general population should be monitored, particularly
when these surfactants are used for cleaning and laundering by hand.
5. Since LAS, AOS, and AS may enhance the transport of other
chemicals in environmental media and modulate their bioavailability
and toxicity in surface waters, river sediments, and soils to which
humans may be exposed, interactions with other environmental
chemicals and the consequences for humans should be investigated.
Environmental safety
6. Additional studies should be carried out on the mechanisms of
adsorption and desorption of AOS and AS. Studies should also be done
on the partitioning of LAS, AOS, and AS between dissolved and
suspended colloidal particles in water. Mathematical models of
sorption coefficients should be developed and validated on the basis
of physical-chemical parameters.
7. Studies of the biodegradation of AOS and AS in sludge-amended
soils and river sediments should be carried out when exposure
occurs. Studies in river sediments (aerobic and anaerobic zones)
should be performed downstream of treated and untreated wastewater
and sewage outfalls.
8. Environmental concentrations of LAS, AOS, and AS should be
monitored regionally and nationally in order to obtain information
on exposure. Analytical methods should be developed for detecting
low levels of AOS and AS in relevant environmental compartments.
9. National databases should be developed on the concentrations of
LAS, AOS, and AS in wastewater and rivers and on the types,
efficiency, and location of wastewater treatment plants, in order to
facilitate an assessment of the impact of discharges of these
surfactants to the environment.
10. Long-term studies of the toxicity of AOS and AS to fish
(freshwater and marine) and aquatic invertebrates should be
conducted in order to establish the relative sensitivity of these
species.
A. Linear alkylbenzene sulfonates and their salts
A1. SUMMARY
See Overall Summary, Evaluation, and Recommendations (pp. 7-21).
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
A2.1 Identity (sodium salt)
Chemical formula: CnH2n-1O3S Na ( n: 16-20) (for
current commercial products)
Chemical structure:
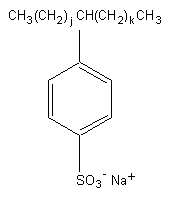 j,k: integers ( j + k = 7-11)
Common name: Sodium linear alkylbenzenesulfonate
Common synonyms: LAS, LAS sodium salt, linear
alkylbenzene-sulfonic acid sodium salt,
linear dodecyl-benzenesulfonic acid
sodium salt, sodium straight chain
alkylbenzenesulfonate
CAS Registry number: 68411-30-3 (LAS sodium salt, C10-13
alkyl)
Common trade names: Ablusol DBC, Agrilan WP, Alkasurf CA,
Arylan, Atlas G-3300B, Atlox, Biosoft,
Berol, Calsoft, Demelan CB-30, Elecut
S-507, Elfan, Emulphor ECB, Emulsogen
Brands, Gardilene, Hexaryl, Idet,
Kllen, Lutopon SN, Manro, Marlopon,
Marlon A, Nacconol 90 F, Nansa HS 80,
Nansa Lutersit, Neopelex, Sandozin AM,
Sipex, Sulfamin, Sulframin, Surfax 495,
Teepol, Tersapol, Tersaryl, Ufaryl DL
80P, Witconate (McCutcheon, 1993)
Abbreviations: LAS, LAS-Na
Specification: LAS are anionic surfactants which were
introduced in the 1960s as more
biodegradable replacements for highly
branched alkyl-benzene sulfonates. LAS
are produced by sulfonation of linear
alkylbenzene (LAB) with sulfur trioxide
(SO3), usually on a falling film
reactor or with oleum in batch
reactors. The corresponding sulfonic
acid is subsequently neutralized with
an alkali such as caustic soda. The
hydrocarbon intermediate, LAB, is
currently produced mainly by alkylation
of benzene with n-olefins or
n-chloroparaffins using hydrogen
fluoride (HF) or aluminium chloride
(AlCl3) as a catalyst, and the LAS
derivatives are thus generally referred
to in that context (Cavalli et al.,
1993a). Currently, 74% of world
production of LAB is via HF and 26% via
AlCl3 (Berna et al., 1993a).
LAS are a mixture of homologues and phenyl positional isomers,
each containing an aromatic ring sulfonated at the para position
and attached to a linear alkyl chain of C10-C14 (in Europe,
predominantly C10-C13) at any position except the terminal one.
The product is generally used in detergents in the form of the
sodium salt.
Some of the typical characteristics of LAS, including the
distribution of alkyl chain lengths and the positions of the phenyl
rings in the two types of LAS used in laundry detergents, are shown
in the box below. The United States Toxic Substances Control Act
inventory lists LAS homologues with chain lengths up to C18
(Tables 1 and 2), but these products are not currently used for
commercial purposes.
A2.2 Physical and chemical properties
The properties of LAS differ greatly depending on the alkyl
chain length. Table 3 shows the Krafft points (temperature at which
1 g of LAS dissolve in 100 ml of water) and the relative critical
micelle concentrations of the single homologues.
Typical characteristics of linear alkylbenzene sulfonates used in laundry
detergents:
Appearance (commercial product): White paste (containing water)
Average length of alkyl carbon chain: 11.8
Average relative molecular mass: 342
Unsulfonated matter: 1-2%
Alkyl chain distribution:
C10 10-15%
C11 25-35%
C12 25-35%
C13 15-30%
C14 0-5%
Phenyl ring position LAS (LAB-HFa) LAS (LAB-AlCl3b)
2-phenyl 18 28
3-phenyl 16 19
4-phenyl 17 17
5-phenyl 24 18
6-phenyl 25 18
From Cavalli et al. (1993a)
a Hydrofluoric acid-catalysed process
b Aluminium chloride-catalysed process
Table 1. Mixtures of linear alkylbenzene sulfonates and their salts found in the
United States Toxic Substances Control Act inventory
Generic benzene- CAS number
sulfonic acid groups
Acid Salts
(C10-13)Alkyl-a 68411-30-3 (sodium salt)
(C10-16)Alkyl- 68584-22-5 68584-23-6 (calcium salt)
68584-26-9 (magnesium salt)
68584-27-0 (potassium salt)
Mono (C6-12)alkyl- 68608-87-7 (sodium salt)
Mono(C7-17)alkyl- 68953-91-3 (calcium salt)
68953-94-6 (potassium salt)
Mono(C9-12)alkyl- 68953-95-7 (sodium salt)
Mono(C10-16)alkyl- 68910-31-6 (ammonium salt)
68081-81-2 (sodium salt)
Mono(C12-18)alkyl- 68648-97-5 (potassium salt)
a There may be more than one alkyl substituent per benzene ring (United
States Environmental Protection Agency, 1981).
Table 2. Individual linear alkylbenzene sulfonates (LAS) found in the United States Toxic Substances Control Act inventory
Parent sulfonic acid Empirical CAS Registry number
(abbreviation) formula
Acids Sodium salts Other salts
Dodecylbenzene C16H26O3S 1322-98-1 1322-98-1
(C10 LAS) (140-60-3)a (2627-06-7)a
Undecylbenzene C17H28O3S 50854-94-9 27636-75-5 NH4 salt, 61931-75-7
(C11 LAS)
Dodecylbenzene C18H30O3S 27176-87-0 25155-30-0 Al salt, 29756-98-7; NH4 salt, 1331-61-9;
(C12 LAS) (2211-98-5)a Ca salt, 26264-06-2; K salt, 27177-77-1;
(68628-60-4)b also numerous salts with alkyl amines
(18777-54-3)c
Tridecylbenzene C19H32O3S 25496-01-9 26248-24-8 Also salts with alkyl amines
(C13 LAS)
Tetradecylbenzene C20H34O3S 30776-59-1 28348-61-0
(C14 LAS) (47377-10-2)a (1797-33-7)a
Pentadecylbenzene C21H36O3S 61215-89-2 K salt, 64716-02-5
(C15 LAS)
Hexadecylbenzene C22H38O3S (16722-32-0)a K salt, 64716-00-3
(C16 LAS)
Heptadecylbenzene C23H40O3S 39735-13-2
(C17 LAS)
From United States Environmental Protection Agency (1981)
a Specifies para substitution
b Specifies para substitution at second position on alkyl chain
Table 3. Relationship between alkyl chain length, Krafft point,
and critical micelle concentration (CMC) of linear
alkylbenzene sulfonates
Alkyl chain length Krafft point (°C) CMC × 10-3 (25°C)
10 -1 5.8
12 3 1.1
14 8 0.24
15 - 0.11
16 13 -
From Ohki & Tokiwa (1970)
The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature. For
surfactants, a distinct, sharp bend (break point) is observed in the
solubility/temperature curve. The steep rise in solubility above the
sharp bend is caused by micelle formation. The point of intersection
of the solubility and critical micelle curves plotted as a function
of temperature is referred to as the Krafft point, which is a triple
point at which surfactant molecules coexist as monomers, micelles,
and hydrated solids. The temperature corresponding to the Krafft
point is called the Krafft temperature. Above the Krafft temperature
and critical micelle concentration, a micellar solution is formed
and higher than aqueous solubility may be obtained.
As commercial LAS are a mixture of homologues and phenyl-
positional isomers, their properties may differ. Even some products
with the same alkyl chain distribution (same average carbon number)
have different properties, depending on the 2-phenyl isomer content.
The solubility in water of commercial LAS used for detergents
(average alkyl carbon length, 11.8), for example, which is important
for liquid formulations, is typically about 25% at 25°C for LAS (LAB
via HF) and about 38% at 25°C for LAS (LAB via AlCl3) (Cavalli et
al., 1993a).
As LAS are anionic surfactants, they lower the surface tension
of water so that it can wet and penetrate fabrics more easily to
loosen and remove soils and stains. Micelles, which are formed at
low concentrations, solubilize oil and stains effectively (Ohki &
Tokiwa, 1969). Other important properties of LAS are detergency,
foaming, sensitivity to Ca and Mg ions, wetting, and surface
tension, which reach their optimal values generally when the alkyl
chain length is about C12 (Yamane et al., 1970).
A physico-chemical property often used in environmental
modelling is the octanol-water partition coefficient (Kow).
Although it is impossible to measure the Kow for surface-active
compounds like LAS, it can be calculated. Roberts (1989) modified
the fragment method of Leo & Hansch (1979) in order to take the
branching of position into account. He thus defined a function, log
( CP + 1), where CP is found by pairing off carbon atoms along
the two branches up to the terminus of the shorter branch. (In the
case of LAS, CP is the carbon number of the shorter of the
integers j and k noted in section 2.1.) This gave the formula:
log Kow = ALK-1.44 log ( CP + 1),
where ALK is log Kow calculated without a branch factor.
In order to calculate log Kow for multicomponent materials
like LAS, the calculated Kow for each component is multiplied by
the mole fraction of the corresponding component, the products are
summed, and the logarithm is calculated to give log WAK ( WA,
weighted average).
A2.3 Analysis
A2.3.1 Isolation
A number of analytical methods are available for the
determination of LAS in water, but the primary method is assay as
methylene blue-active substances (MBAS). The methylene blue reaction
responds to any compound containing an anionic centre and a
hydrophobic centre, because such compounds tend to form an
extractable ion pair when they combine with cationic dyes such as
methylene blue; as only the oxidized form is blue, many positive
interferences may occur. Negative interference in MBAS analysis is
seen in the presence of cationic substances such as proteins and
amines (Swisher, 1970, 1987). Therefore, isolation of LAS from a
sample is one of the most important aspects of their analysis. Most
analytical methods include appropriate procedures for isolation.
A2.3.2 Analytical methods
The analytical methods available for determining LAS in water
include nonspecific methods, involving colorimetric, fluorimetric,
and atomic adsorption techniques, and specific methods involving
techniques such as high-performance liquid chromatography (HPLC),
gas chromatography (GC) and GC-mass spectrometry (MS).
A2.3.2.1 Nonspecific methods
The simplest procedure for the determination of LAS in aqueous
solution is a two-phase titration method. LAS are titrated in a
mixed aqueous chloroform medium with a standard solution of a
cationic reagent, such as benzethonium chloride (Hyamine 1622), and
a small amount of indicator, such as a mixture of dimidium bromide
and acid blue. The end-point is determined by a change in the colour
of the organic solvent (ISO 2271, 1972).
The main nonspecific analytical method used is assay for MBAS,
described above. Colorimetric techniques are routinely used to
determine low concentrations of anionic surfactants, including LAS,
in aqueous samples and have been used extensively in testing and
environmental monitoring of these materials. The colorimetric
methods have the same common analytical basis, that is, formation of
solvent extractable compounds between the anionic surfactant and an
intensely coloured cationic species. The most commonly used cationic
reagent for this purpose is methylene blue (Swisher, 1970, 1987).
The same principle has been used as the basis of many other
procedures for the determination of anionic surfactants.
It has been shown or predicted that organic sulfates,
sulfonates, carboxylates, phenols, and even simple inorganic anions
such as cyanide, nitrate, thiocyanate, and sulfate can be methylene
blue-reactive (Swisher, 1970, 1987). The negative interferences that
can occur as a result of direct competition of other 'cationic'
materials are generally considered to be less important than
positive interferences, and the entities detected by the analysis
are correctly referred to as MBAS.
The procedure developed by Longwell & Maniece (1955) and the
improved version of Abbott (1962) are considered to be the best
methods for the determination of MBAS in aqueous samples. The
sensitivity of these procedures is such that levels of
0.01-0.02 mg/litre MBAS can be determined.
The MBAS response can be used as an acceptable overestimate of
the synthetic anionics present in domestic wastewaters, but these
materials may comprise only a small proportion of the total MBAS in
surface waters (Waters & Garrigan, 1983; Matthijs & De Henau, 1987).
Berna et al. (1991) found that LAS contributed 75% of the MBAS in
integrated sewage and 50% in treated water. Direct methylene blue
analysis of extracts derived from sludge, sediment, and soil
invariably leads to highly inflated estimates of LAS (Matthijs & De
Henau, 1987). Numerous attempts have been made to improve the
specificity of methylene blue analysis, by using a variety of
separation steps before the usual colorimetric estimation. Such
indirect procedures are usually lengthy, difficult, and still
susceptible to interference. A number of analytical methods for the
determination of LAS involving extraction and methylene blue are
summarized in Table 4.
Table 4. Analytical methods for anionic surfactants in environmental water using methylene
blue and extraction
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Absorption Extract LAS in water 50-300 Urea, Jones (1945)
photometry into chloroform as thiocyanate,
ion-pair with MB; measure chloride
absorption of chloroform
solution at 650 nm
Extract from alkaline 10-100 As above Longwell &
solution, wash with Maniece
cidic MB (1955)
Remove impurities 0.1-1 As above Abbot (1962)
from MBreagent by
chloroformextraction
Remove MBAS by TLC 0.1-1 Oba & Yoshida
(1965)
Remove MBAS on Takeshita &
polymer bead column Yoshida
(1975)
Remove MBAS on ion 0.02 Yasuda
exchange column (1980)
UV absorption Re-extract LAS into 1 Uchiyama
photometry water; measure UV (1977)
absorption at 222 nm
Table 4 (contd)
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Infra-red Use to reduce 1000 Ambe &
spectometry interference from MBAS Hanya
(1972)
Gas Convert into fluorine 0.02 Tsukioka &
chromatography derivative; measure Murakami
by ECD (1983)
HPLC Remove MB by cation 0.1 Hashimoto et
exchange, HPLC al. (1976)
Remove MB by anion 0.02 Saito et al.
exchange, HPLC (1982)
LAS, linear alkylbenzene sulfonates; MB, methylene blue; MBAS, methylene blue-active
substances; TLC, thin-layer chromatography; UV, ultraviolet radiation; ECD, electron
capture detection; HPLC, high-performance liquid chromatography
Many other cationic dyes and metal chelates have been used as
colorimetric (and fluorimetric) reagents for the determination of
anionic surfactants, including LAS. Use of the cationic metal
chelates has also led to the development of sensitive atomic
absorption methods for indirect determination of anionic surfactants
in fresh, estuarine, and marine waters. Although these alternative
systems may offer some advantages over the methylene blue cation
method, they cannot match the wide experience gained with methylene
blue analysis. Some examples of analytical methods based on the use
of alternative cationic reagents are shown in Table 5.
A2.3.2.2 Specific methods
Good progress has been made towards developing methods for the
specific determination of the many homologues and phenyl-positional
isomers of LAS in almost all laboratory and environmental matrices
(liquid and solid) at concentrations down to micrograms per litre.
High-resolution GC techniques have allowed determination of all the
major components of LAS (homologues and phenyl-positional isomers)
in environmental samples. Waters & Garrigan (1983) and Osburn (1986)
reported improved microdesulfonation-GC procedures for the
determination of LAS in both liquid and solid matrices.
Derivatization techniques offer an alternative approach to
desulfonation for increasing the volatility of LAS for GC (or GC-MS)
analysis (Hon-nami & Hanya, 1980a; McEvoy & Giger, 1986; Trehy et
al., 1990. The GC-MS technique was also applied, after ion-pair,
supercritical fluid extraction and derivatization, to five sewage
sludges, and the LAS were found to occur at 3.83-7.51 g/kg on a
daily basis (Field et al., 1992). These GC procedures, however,
involve extensive sample pre-treatment and depend on conversion of
the isolated LAS into a suitably volatile form for GC determination;
they are therefore time-consuming.
HPLC offers a more convenient means for determining homologues
of LAS in all types of environmental matrices routinely. Several
researchers have reported HPLC procedures for LAS which involve
trace enrichment of the surfactant as the first step (Kikuchi et
al., 1986; Matthijs & De Henau, 1987; Castles et al., 1989; Di
Corcia et al., 1991). Takita & Oba (1985) developed a modified
analytical method based on MBAS-HPLC measurement. Further HPLC
methods, some requiring no sample preparation, are listed in
Table 6.
Table 5. Analytical methods involving reagents other than methylene blue
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Absorption 1-Methyl-4-(4-diethyl- 0.04 Fe[III] Higuchi et al.
photometry aminophenylazo)pyridinium (1982)
iodide; measure
chloroform solution at
564 nm
Bis[2-(5-chloro-2- 0.06 Taguchi et al.
pyridylazo)-5-diethyl- (1981);
aminophenolato]Co Kobayashi
[III] chloride; measure et al. (1986)
benzene solution at
560 nm
Ethylviolet; measure 0.01 Motomizu et
benzene or toluene al. (1982);
solution at 540 nm Yamamoto &
Motomizu (1987)
Atomic Bis[2-(5-chloro-2-
absorption pyridylazo)-5- 1 × 10-3 Hydro- Adachi &
spectrometry diethylaminophenolato] chlorite Kobayashi
Co [III] chloride; measure ion (1982)
Co by atomic absorption
spectrometry
Potassium dibenzo- 0.05 Alkali, Nakamura et al.
18-crown-6; measure K alkaline (1983)
earth
metals
Table 5 (contd)
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Atomic Cu[II] ethylenediamine 0.03 × 10-3 Gagnon (1979);
absorption derivatives; measure Cu Sawada et al.
spectrometry (1983)
Absorption Bis(ethylenediamine)Cu; 5 × 10-3 Rama Bhat
photometry determine Cu after et al. (1980)
addition of 1-(2-
pyridylazo)-2-naphthol
at 560 nm
GC-MS Extract solid phase on 1 × 10-3 Trehy et al.
C8 column; derivatize (1990)
LAS with sulfonyl
chloride for GC-MS
LAS, linear alkylbenzene sulfonates; GC-MS, gas chromatography-mass spectrometry
Table 6. Analytical methods for linear alkylbenzene sulfonates (LAS) by specific analysees
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Recover LAS on column Column, silica gel, mobile phase 0.02-0.03 Takano et al. (1975)
chromatograph packed hexane:ethanol containing
with polymer beads sulfuric acid; UV at 225 nm
Extract LAS with methylisobutyl Column, ODS; mobile phase, 0.05 Matsueda et al.
ketone ethanol:water; UV at 225 nm (1982)
Recover LAS by ionexchange Column, cyanopropyl-modified silica; 0.04 Saito et al.
column mobile phase, ethanol:water; (1982)
chromatography UV at 225 nm
Direct analysis Column, ODS; mobile phase, methanol: 0.1 Nakae et al.
water with sodium perchlorate; (1980)
fluorescence detector capable of
determining alkyl homologue distribution
Extract LAS using Column, ODS; mobile phase, acetonitrile: 0.1 × 10-3 Kikuchi et al.
mini-column water with sodium perchlorate; (1986)
fluorescence detector
Concentrate LAS using Column, ODS; mobile phase, acetonitrile: 3 × 10-3 Takami et al.
mini-cartridge column water with sodium perchlorate; (1987)
connected in sequence with fluorescence detector
HPLC system
Table 6 (contd)
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Extract LAS with methylisobutyl Column, ODS; mobile phase, acetonitrile: NR Inaba & Amano
ketone water (gradient elution to sharpen peak) (1988)
with sodium perchlorate; UV at 222 nm
Extract from solids with Column, octyl-modified silica; 0.8 Marcomini &
methanol on Soxhlet mobile phase, 2-propanol:water: (injected Giger
acetonitrile (gradient elution) weight) (1987)
with sodium perchlorate; fluorescence
detector
Two-step solid phase Column, C1 Sphesorb; mobile phase, 7 × 10-3 Castles et al. (1989)
extraction with C2 and THF:water with sodium perchlorate;
SAX cartridges fluorescence detector
Extract LAS using Column, C8-DB (Supelco); mobile 0.8 × 10-3 Di Corcia et al.
Carbopack B (graphitized phase, methanol:water with sodium (1991)
carbon black) cartridge perchlorate; fluorescence detector
Concentrate LAS on Column, Wakosil 5C4; mobile phase, 10 × 10-3 Yokoyama & Sato
anion-exchange pre-column acetonitrile:water with sodium (1991)
connected to HPLC system perchlorate; UV at 220 nm
Table 6 (contd)
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Ion-pair extraction under Column, capillary gas chromatography, NR Field et al.
SFE conditions using 20 m; mass spectrometry with electron (1992)
tetralhyl-ammonium ion impact ionization operating in
pair reagents, coupled with selected ion mode
ion-pair derivatization
Solid-phase extraction for HPLC column, Bandapat C18 10 × 10-3 Matthijs & De Henau
purification and gradient elution water:acetonitrile (water phase) (1987)
concentration and 0.15 mol/litre NaClOn 0.1 (solid phase)
UV, ultraviolet spectrometry; ODS, octadecyl silica; HPLC, high-performance liquid chromatography; NR, not reported;
SFE, supercritical fluid extraction
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
LAS do not occur naturally.
A3.2 Anthropogenic sources
LAS are synthetic surfactants that were introduced as prime
components of almost all types of household surfactant products in
the early 1960s to replace alkylbenzene sulfonates (ABS), which were
then in widespread use. The change-over from ABS to LAS took place
gradually, starting in the United Kingdom (1960) and then spreading
to Germany (1961), the United States of America (1963), Japan (1965)
and to other European countries (Brenner, 1968; Husmann, 1968;
Waldmeyer, 1968; Tomiyama, 1972).
After use, LAS are discharged into wastewater. As the surfactant
components of the detergent products are soluble, they eventually
reach raw sewage at concentrations of 1-7 mg/litre (Rapaport et al.,
1987). Unlike ABS, which has a branched alkyl chain structure, LAS
with a linear, straight alkyl chain structure are readily
biodegradable. Their use has alleviated significant environmental
hazards such as foaming and residual surfactant in water.
A3.2.1 Production levels and processes
Annual world production of surfactants, excluding soap, in 1990
was estimated to be about 7 million tonnes (Colin A. Houston &
Associates, Inc., 1990; Richtler & Knaut, 1991). World consumption
of LAS in 1989 was about 2.43 million tonnes, 50% of which was used
in North America, western Europe, and Japan (Hewin International
Inc., 1992). Worldwide consumption of LAS in 1990 was about 2
million tonnes, with the following geographical distribution:
western Europe, 23%; North America, 19%, eastern Asia, 16%,
South-east Asia, 12%; eastern Europe, 11%; western Asia, 7%; South
America, 7%; and Africa, 5% (CEFIC, 1992). Berna et al. (1993a)
reported that, in 1990, 380 000 tonnes were used in western Europe,
180 000 tonnes in eastern Europe, 110 000 in Africa, 100 000
tonnes in western Asia, 305 000 in eastern Asia, 180 000 in
South-east Asia, 295 000 in North America, and 140 000 in Latin
America. An additional demand of 650 000 tonnes is expected by the
year 2000. The estimates for 1990 show an increase over 1987, when
LAS production in the United States, Japan, and western Europe was
about 1.4 million tonnes, on the basis of global demand for linear
alkylbenzene (Painter & Zabel, 1988), and consumption of LAS was
about 307 500 tonnes in the United States, 485 000 tonnes in western
Europe, and 145 000 tonnes in Japan (Richtler & Knaut, 1988).
LAS are complex mixtures of isomers and homologues in
proportions dictated by the starting materials and reaction
conditions. LAS are manufactured by reacting the parent
alkylbenzenes with sulfuric acid or sulfur trioxide to give the
corresponding sulfonic acid, which is then neutralized to the
desired salt. This is usually the sodium salt but ammonium, calcium,
potassium, and triethanolamine salts are also made. The reactions
are smooth and the yields nearly quantitative. Commercial LAS
contain linear alkyl chains 10-14 carbons in length, with phenyl
groups placed at various internal positions on the alkyl chain, with
the exception of 1-phenyl (Painter & Zabel, 1988).
LAS are manufactured in an enclosed process; under normal
conditions, therefore, exposure can occur only at the stage of
detergent formulation, by inhalation or dermally. Dermal exposure is
generally short and accidental, whereas exposure by inhalation can
occur continually.
The concentration of surfactants in water from washing machines
is 0.2-0.6%. LAS are estimated to represent 5-25% of the total
surfactant mixture.
In Germany in 1988, when annual consumption of LAS in the
western states was about 85 000 tonnes, daily consumption was 3.8 g
per inhabitant per day. As consumption of drinking-water was 190
litres per inhabitant per day, the average LAS concentration in
sewage was 20 mg/litre. Consumption of LAS per capita in other
countries is shown in Table 7 (Huber, 1989).
A3.2.2 Uses
LAS are the most widely used surfactants in detergent and
cleaning products, in both liquid and powder preparations and for
household and industrial use. The amount of LAS in a product depends
on several factors, including the type of application (washing-up
products, light- and heavy-duty powders and liquids) and the
formulation, but is usually 5-25%. Small amounts of LAS are used in
non-detergent applications, but these represent less than 5% of
total world consumption.
Table 7. Specific consumption of linear alkylbenzene sulfonates (LAS)
in various countries
Country Water usage LAS usage Reference
(litres per capita (g per capita
per day) per day)
Germany - 3.8 Huber (1989)
185 2.2 Wagner (1978)
United States 560 2.6a, 2.1b Rapaport et al.
(1987)
United Kingdom 208 3.5c, 2.7c Standing Technical
Committee on
Synthetic Detergents
(1978, 1989)
Spain - 5.6a, 2.6b Berna et al. (1989)
Japan 493 2.7 Ministry of Health and
Welfare (1992);
Hewin International
Inc. (1992)
a Calculated from sales
b Calculated by analysis
c Methylene blue-active substances
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
The way in which LAS enter the environment varies between
countries, but the major route is via discharge from sewage
treatment works. Direct discharge of sewage to rivers, lakes, and
the sea occurs when wastewater treatment facilities are absent or
inadequate. Another route of entry of LAS into the environment is
via disposal of sewage sludge on agricultural land.
Throughout their journey into the environment, LAS are removed
by a combination of adsorption and primary or ultimate
biodegradation. LAS adsorb onto colloidal surfaces and suspended
particles, with measured adsorption coefficients of 40-5200
litres/kg, depending on the medium and structure of the LAS. LAS
undergo primary biodegradation in all environmentally relevant
compartments, such as raw sewage, sewage treatment water, surface
waters, sediments, and soils. They are readily and ultimately
mineralized under aerobic conditions in the laboratory and the
field. They tend not to be biodegraded under methanogenic conditions
or if the initial LAS concentration is so high that microbial
degradation is inhibited (> 20-30 mg/litre). Typical half-lives for
aerobic biodegradation of LAS are 1-8 days in river water, 1-2 days
in sediments, and 5-10 days in marine systems. The rate of
biodegradation depends on temperature: biodegradation is rapid
between 10 and 25°C; at lower temperatures, biodegradation kinetics
are reduced, in close association with microbial activity. During
primary sewage treatment, LAS are partially adsorbed onto and
removed with waste sludge to an extent of about 25% (range, 10-40%).
LAS are not removed during anaerobic sludge digestion but are
removed during aerobic treatment with a half-life of about 10 days.
Application of the sludge to soil generally results in 90%
degradation within three months, with a half-life of 5-30 days.
LAS are not bioconcentrated or biomagnified in aquatic
organisms. They are readily absorbed through the gills and body
surface of fish and are distributed via the blood to the systemic
organs. Most LAS-related compounds (parent compound and metabolites)
have been detected in the gall-bladder and hepatopancreas of fish.
They are usually cleared rapidly, with a half-life of two to three
days.
A4.1 Transport and distribution between media
Detergent chemicals such as LAS are normally discharged after
use into sewers in communal wastewater. The proportion of wastewater
that is subjected to sewage treatment varies widely between
countries. In most advanced countries, 50 to > 90% may be treated,
whereas in less developed countries the proportion may be as little
as 5-30% (Eurostat, 1991). In countries where there is no or
inadequate sewage treatment, LAS are removed from the environment
via adsorption and mineralization in the receiving surface waters.
Anionic surfactants such as LAS can adsorb onto the solid
substrates associated with sewage, sludge, sediment, and soil; the
extent of adsorption is dependent on the composition and physical
nature of the solid matrix. Measured values of the adsorption
constant (Kd) for LAS on a range of solid substrates were compiled
by Painter & Zabel (1989), who reported Kd values of 590-1400
litres/kg for primary sludge, 660-5200 1itres/kg for activated
sludge, and 40-360 1itres/kg for river water sediment.
A4.1.1 Wastewater treatment
Under certain conditions, up to 50% of the LAS present can be
biodegraded in sewers before entering sewage treatment (Moreno et
al., 1990). In large-volume batch biodegradation tests with
acclimatized sludge, the MBAS levels decreased to 10% of the initial
concentration within 15 days. During biodegradation, the toxicity of
the test solution decreased in parallel with the reduction in MBAS.
A relative enrichment of the shorter chain homologues was observed
by GC analysis concurrently with the decrease in MBAS levels,
indicating preferential removal of the higher homologues (Dolan &
Hendricks, 1976).
The distribution and fate of LAS have been established in the
course of mass balance studies at sewage treatment plants in Spain
(Berna et al., 1989), Italy (Cavalli et al., 1991), Switzerland
(Giger et al., 1989), Germany (De Henau et al., 1989), and the
United States (Rapaport & Eckhoff, 1990; McAvoy et al., 1993).
Efficient, well-operated activated sludge plants generally remove
most of the LAS during aerobic treatment, and the overall removal of
LAS in primary settlement and secondary aerobic treatment stages can
be < 98% (Berna et al., 1991). Smaller amounts of LAS were
removed (77 ± 15%) in less efficient, trickling filter plants
(McAvoy et al., 1993).
The main mechanism for removal of LAS during sewage treatment is
biodegradation (Berna et al., 1991), but a significant fraction (on
average, 20-30%) of the LAS entering sewage treatment plants may be
removed on primary sewage solids and do not undergo aerobic sewage
treatment (Giger et al., 1989). Instead, the sludge is digested
under anaerobic conditions, and in some countries a high proportion
may then be applied raw or digested to agricultural land as a source
of plant nutrients (Berna et al., 1991). In Germany and the United
Kingdom, 40-45% of sewage sludge is disposed of in this way (Waters
et al., 1989). Since LAS do not undergo significant anaerobic
biodegradation under methanogenic conditions, concentrations of
3-12 g/kg can be found on dried solids in sludge (see Section 5,
Table 10). Any LAS in sludge applied to agricultural soil should
then be rapidly biodegraded, since the receiving soil environment is
aerobic. In Germany and the United Kingdom a typical application of
digested sludge was estimated to add LAS at a rate of 7-16 mg/kg
soil (Waters et al., 1989).
Adsorption can account for 15-40% of the removal of LAS from raw
sewage during the primary settlement stage of treatment (Berna et
al., 1989; Giger et al., 1989). Berna et al. (1989) reported that
precipitation and adsorption were particularly important in removing
LAS from wastewater containing high concentrations of calcium and
magnesium ions.
The percentage adsorption of C10, C11, C12, and C13 LAS
onto activated sludge, Amazon clay, and various bacteria and algae
was directly related to the chain length and phenyl position: longer
homologues and more terminal phenyl isomers were adsorbed much more
readily than other forms. Adsorption of LAS at a concentration of
23 mg/litre was found to be pH-dependent, with adsorption increasing
as the pH decreased from 7 to 3 (Yoshimura et al., 1984a).
A4.1.2 Surface waters, sediments, and soils
The half-life for the removal of LAS by combined sorption and
settling < 12 km below a sewage outfall in Rapid Creek, South
Dakota, United States, was 0.25 days. The biodegradation half-life
was 1.5 days (Rapaport & Eckhoff, 1990). The partition coefficient
of LAS between natural water and sediment was reported to increase
with increasing chain length and with the position of the phenyl
nearer to the end of the chain. Adsorption was increased when either
the concentration of suspended solids or fractional organic carbon
was increased (Amano & Fukushima, 1993).
Freshwater pearl oysters are cultivated in Lake Nishinoko,
Japan, which has an area of 2.8 km2, an average depth of 1.5 m,
and a residence period of 27 days. The water of the lake was found
to contain total concentrations of MBAS of 0.01-0.02 mg/litre and
LAS concentrations of 0.005-0.018 mg/litre. The partition
coefficients of LAS (Kd) were 70 litres/kg for bottom
sediment:water and 11 litres/kg for oyster:water (Sueishi et al.,
1988). The authors concluded that when a river flows into a
semi-enclosed lagoon, the fate of the surfactants is dominated by
mass transfer between media and transformation due to degradation
rather than spatial transportation.
LAS were present in Swiss soils that had been treated with
sludge for 10 years; however, the application rates were six times
higher than normal. The reported half-lives were 5-80 days. The
authors noted that it is not entirely correct to use half-life to
describe the loss of LAS from soils, because there is competition
between biodegradation and sorption on and into soil particulates,
and LAS may persist at very low 'threshold' levels. During the
330-day study, the levels of LAS decreased from 45 mg/kg dry soil to
a residual level of 5 mg/kg (Giger et al., 1989).
A comparison of the measured concentration of LAS with detailed
records on the amount of sludge applied on 51 fields in England
indicated that loss of LAS exceeded 98% in fields that had not
recently been sprayed with sludge; losses from fields that had been
sprayed recently were calculated to be 70-99% of the estimated
cumulative load. The calculated half-lives for removal of LAS from
soil sprayed with sludge were 7-22 days. Examination of the
distribution of homologues suggested that loss of LAS is the result
of biodegradation and not leaching (Holt et al., 1989). In a study
of the disappearance of LAS from sludge-amended soils at two
locations, the average half-lives were 26 days when sludge was
applied at a rate of 1.6 kg dry sludge per m2 (giving a
concentration of LAS of 16.4 mg/kg soil) and 33 days when sludge was
applied at 5 kg wet sludge per m2 (concentration, 52.5 mg/kg soil)
(Berna et al., 1989). In another study, the half-life for LAS in
soil was more than three months; there was no evidence that they
accumulate in soil over time (Rapaport & Eckhoff, 1990).
When C13 LAS were applied to various soil (surface) types at a
concentration of 0.05 mg/kg under laboratory test conditions, the
half-lives were 1-5 days, with an average of two days. There was no
significant variation with regard to soil type. In a second
experiment, the average half-life of C12 LAS applied to subsurface
soil was 20 days (Larson et al., 1989).
After grass, radishes, and garden beans were grown for 76 days
in soil treated with 14C-LAS at a rate of approximately
1.2 g/m2, 98% of radioactive residues were recovered, with 63.6%
released to the atmosphere, 26.8% found in the soil, 6.6% in plant
biomass, and 0.9% leached out in percolated water. When potatoes
were grown on the soil for 106 days, 97.9% of the radioactivity was
recovered, and 72.3% was released to the atmosphere, 18.3% to the
soil, 5.9% in plant biomass, and 1.4% leached into percolated water
(Figge & Schoberl, 1989).
LAS in a plume of contaminated groundwater on Cape Cod, United
States, were degraded rapidly and was found only within 0.6 km of
the sewage disposal bed (Thurman et al., 1986).
Effects on the biodegradation of LAS applied at 50 mg/litre of
aqueous dispersion were studied in three Japanese soil types
inoculated with sewage. The rate of sludge application used in this
study was not typical of that found in the environment. Primary
degradation, as measured by the presence of MBAS, reached 70% within
16 days. Addition of andosol (allophane) and weathered granite
(kaolin and illite) both reduced primary degradation, and 40-50% of
the LAS was still present after 30 days, indicating that the rate of
microbial degradation of LAS adsorbed onto soils containing large
amounts of allophane and/or sesquioxides was reduced. A
montmorillonite soil did not affect the rate of degradation (Inoue
et al., 1978).
The behaviour of C10-C13 LAS and C12 LAS at concentrations
of 50 and 100 mg/litre was studied by HPLC in perfusion tests on two
types of soil, a clay loam and a sandy loam. The sandy loam, with a
lower content of humus and clay, adsorbed less LAS with a longer
lag. During the first three days of perfusion, only adsorption
occurred, 50% being adsorbed; after nine days, decomposition was
observed and only 16.6% of the LAS remained; after 15 days, the LAS
had almost completely disappeared (Abe & Seno, 1987).
LAS were applied at a rate of 5 g/m2 to three soil types:
loamy orthic luvisol under agricultural land, sandy acidic dystric
combisol under a pine forest, and combisol irrigated with
wastewater. The half-life in loamy soil was five days; 80% was
degraded after 12 days, and none was detectable after 28 days. With
45 mm of precipitation, about 8% of the LAS percolated to a depth of
10-30 cm. The LAS moved significantly more slowly than radioactively
labelled water. LAS were less mobile in the sandy soil, with a
maximal percolation depth of 5 cm after two weeks, whereas water
percolated 15 cm. The half-life in the sandy soil was 10 days, with
80% degradation after 19 days and total degradation after 28 days.
The LAS were bound to organic material in the humic litter, which
probably slowed degradation and reduced mobility. In combisol
irrigated with wastewater, the LAS were bound mainly in the upper
5 cm, with some percolation to 10-30 cm after an application of
180 mm of wastewater. The half-life was 12 days, and 80% was
degraded within 21 days; however, there was no further degradation
after 28 days, and the remaining LAS were tightly bound. Increasing
the application rate to 50 g/m2 had no effect on percolation;
however, the half-life was doubled. Samples collected during the
winter showed much slower degradation, with half-lives of 68-117
days. Percolation was also much deeper; the authors suggest this was
due to a higher rate of precipitation and extensive evaporation
(Litz et al., 1987).
In a study of the adsorption of LAS in aqueous solution onto
clay grumusol and sandy regosol soils, a linear adsorption isotherm
was obtained. The release of the surfactant was proportional to the
initial adsorption and the soil type, suggesting ready desorption.
More LAS was adsorbed by the clay soil than by the sandy soil (Acher
& Yaron, 1977).
Hydroxy aluminium and iron adsorbed LAS more readily and with a
much larger capacity than other soil constituents, such as organic
matter, silica gel, layer silicates, and calcium carbonate (Volk &
Jackson, 1968).
In a study of the adsorption of LAS applied at a concentration
of 2 mg/litre to a variety of Wisconsin (United States) soils, a
highly significant correlation was found between adsorption and
organic matter content (including the iron and aluminium
components), phosphate fixing capacity, and aluminium content. The
removal of sesquioxides reduced the adsorption of LAS to zero;
however treatment of montmorillonitic soils with H2O2 and
Na2S2O4 increased adsorption by oxidizing and removing the
organic matter, indicating that montmorillonite can adsorb LAS.
Treatment of soils with H2O2 increased adsorption because iron
and aluminium were released from organic chelates (Krishna Murti et
al., 1966).
Adsorption of LAS to microorganisms was found on the basis of
calculated adsorption isotherms to be more important than adsorption
to humic substances (Urano et al., 1984; Urano & Saito, 1985).
A4.1.3 Fate models
One model of the fate of LAS predicted the sorption coefficient
to within one order of magnitude. The sorption distribution
coefficient was consistently underpredicted, so that when the
concentrations of LAS in interstitial and overlying water were
predicted from concentrations in sediment they were overestimated.
The model thus provided conservative estimates for assessing safety
in aquatic media (Hand et al., 1990).
The reported concentrations of LAS in Rapid Creek, South Dakota,
United States, were compared with expected concentrations generated
by the quantitative water-air-sediment interaction fugacity model,
which is based on physical, chemical, reactive, and transport
properties and emission rates into rivers. In general, close
agreement was reached: in both cases, LAS had a residence time of
about two days. The authors pointed out that the results might
differ if the model were applied in situations that differed
hydrodynamically (Holysh et al., 1986).
A model to predict surface water concentrations of LAS in German
and American rivers included the following parameters: river flow
and velocity, sewage treatment plant location and type, discharge
volume, and connected population. The values obtained were in
general agreement with those measured. The authors also investigated
a septic tank discharge at a Canadian site by applying a groundwater
model, which was based on hydrogeological, biodegradation, and
sorption data. The predicted and measured concentrations were in
good agreement (Hennes & Rapaport, 1989).
A mathematical model was derived to explain a downstream
decrease in the concentration of LAS in the Lake Teganuma estuary,
Japan. The model included the adsorption coefficient, the
biodegradation rate constant, and the rate of transport (diffusive
and settling) flux of LAS between water and sediment. The model
predictions and laboratory findings were used to confirm that
biodegradation is the predominant mechanism for removal of LAS from
the estuary (Amano et al., 1991).
A model based on data from the Lake Biwa basin was devised to
predict the fate of LAS in Japanese rivers, assuming that complete
mixing occurs in any given cross-section of a river. The parameters
included the cross-sectional mean concentration of LAS, time
elapsed, flow velocity, longitudinal dispersion coefficient, decay
due to biodegradation and sedimentation, water depth, and river
width (Sueishi et al., 1988).
The measured concentrations of LAS in United States river water
under critical flow conditions were mirrored by the predictions of a
simple dilution model, which predicts chemical concentrations below
the mixing zone of wastewater treatment plants. The model is based
on three large databases, which link river flow, treatment type and
wastewater discharge volume; the output of the model is a frequency
distribution of concentrations just below the mixing zone of
treatment plant outfalls. The model predicted that 95% of the river
waters below that point would have concentrations of LAS of <
0.35 mg/litre during critical low-flow periods. The sampling sites
selected for this study were reported to have a low dilution factor
for mixing effluent with surface water, however. The predictions
therefore represent a 'worst-case scenario', since the 95 percentile
value represents critical low-flow periods, in which the lowest ever
recorded flow is used for a consecutive period of seven days within
10 years (McAvoy et al., 1993).
A4.2 Environmental transformation
A4.2.1 Biodegradation
A4.2.1.1 Aerobic degradation
Studies on aerobic biodegradation of LAS can be divided into
those of primary degradation and those of ultimate degradation.
Primary degradation of LAS occurs during the initial reactions in
the metabolic pathway, and the products are often shorter-chain
homologues. The ultimate degradation of LAS is that of the entire
molecule to its biodegradation end-products, CO2, H2O, and
NH4. These products are used in cell synthesis or, in the case of
CO2, excreted. The ultimate degradation of LAS normally requires
the action of several species of bacteria.
The degradation pathway of LAS has been described (Huddleston &
Allred, 1963; Swisher, 1963). The steps, shown in Figure 1, are:
omega-oxidation of the end of the alkyl chain, rapid ß-oxidation of
the chain, and oxidation of the ring.
Figure 1. Postulated metabolic pathway of linear alkylbenzene sulfonates
omega-oxidation
CH3(CH2)nCH(C6H4SO3H)(CH2)mCH ------------------> COOH(CH2)nCH(C6H4SO3H)CH2)mCH3
(n>m) |
|
| ß-oxidation
|
v
ß-oxidation
COOHCH(C6H4SO3H)(CH2)mCH3 <------------------ COOH(CH2)n-2CH(C6H4SO3H)(CH2)mCH3
|
| Ring
| dihydroxylation
|
v
COOHCH(C6H2SO3H)CH2CH3 ------------------> ring fission at the 1-2 position
of the ring, then desulfonation to
aliphatic products and sulphate.
From Painter (1992)
Swisher (1981) pointed out that ultimate biodegradation (at
least 80%) is achievable under the correct conditions, which
include:
(i) the presence of mixed bacterial species,
(ii) free access to new bacteria during the test,
(iii) acclimatization,
(iv) enough growth factors and food, and
(v) limitation of the LAS concentration to that found in the
environment.
Biodegradation of LAS begins at the terminus of the alkyl chain
with an omega-oxidation and is followed by successive cleavage of
C2 fragments (ß-oxidation) (Huddleston & Allred, 1963; Swisher,
1963). The resulting sulfocarboxylic acids have a chain length of
four to five carbon atoms (Schöberl, 1989). These intermediates are
further biodegraded by oxidative scission of the aromatic ring and
cleavage of the sulfonate group (Setzkorn & Huddleston, 1965;
Swisher, 1967). Catabolites of further oxidation steps are fed into
the central metabolic pathways, i.e. the Krebs cycle and glyoxylate
cycle (Schöberl, 1989).
LAS degradation begins at the longest end of the linear alkyl
chain, with omega- and ß-oxidation, and proceeds up to the
sulfophenylmono-carboxylic acids (one to two CH2 groups) (Divo &
Cardini, 1980). Under mild conditions, as in river water,
intermediates such as sulfo-phenylcarboxylic acids are often not
degraded, as the greater distance between sulfophenyl groups and the
far end of the hydrophobic group increases the speed of primary
biodegradation (Swisher, 1976). Once the terminal methyl group has
been attacked, primary biodegradation is rapid (Swisher, 1970;
Gledhill, 1975). Short-chain sulfophenylmonocarboxylic acids were
not degraded by Pseudomonas but were degraded by mixed cultures of
microorganisms (Leidner et al., 1976). The initial attack that opens
the aromatic ring is the rate limiting step for ultimate
biodegradation: once the ring is opened, degradation is rapid.
Enzymological methods were used to show that the same sequence
of steps occurs when ring degradation proceeds via the catechol
derivative. A variety of microorganisms isolated from soil, sewage,
and river water showed at least five distinct metabolic routes for
the degradation of LAS: omega- and ß-oxidation of the side-chain;
oxidation and desulfonation followed by cleavage of the aromatic
nucleus; reductive desulfonation of the ring; and metabolic
alpha-oxidation of the side-chain, followed by ß-oxidation and
desulfonation. Metabolism of alkyl chains shorter than four carbons
was initiated through the aromatic nucleus by hydrolytic or
reductive desulfonation of the ring (Cain et al., 1971). LAS may
also be cleaved by biochemical mechanisms (Schöberl, 1989).
Primary degradation
(i) Low levels of biomass
Measurement of MBAS was compared with measurement of total
organic carbon for detecting biodegradation in shake cultures. With
the MBAS method, LAS had lost 98% of their activity within five
days, whereas 34% of the total carbon had disappeared by that time,
and 70% was lost by the end of the 31-day test (Sekiguchi et al.,
1975a).
In a modification of the screening test of the Organisation for
Economic Co-operation and Development (OECD), accepted by the
European Commission, the percentage of dissolved organic carbon was
found to have decreased by more than 80% within four weeks. The
authors cautioned that the decrease in LAS may not have been due
solely to biological degradation, since 40-50% of organic carbon was
also removed from abiotic controls, suggesting that adsorption may
account for part of the removal of LAS (Canton & Slooff, 1982). When
aerobic biodegradation of 10 mg/litre LAS was followed during a
10-day incubation period at 27°C, primary degradation, measured by
the MBAS method, was complete within 8-10 days, and the theoretical
CO2 production reached 20-25% within 10 days. At a concentration
of 20 mg/litre, no degradation was observed, but this elevated
concenration may have inhibited the microbial inoculum (Itoh et al.,
1979).
The rate and degree of biodegradation of LAS are dependent on
temperature. In an unacclimatized microbial population, no more than
25% biodegradation was achieved at 5°C during a 28-day test,whereas
at 15, 25, and 35°C about 90% degradation was achieved within 7-14
days. At 45°C, the microbial population degraded 75% of the LAS
within 14 days, but this rate of degradation was not maintained,
probably because of loss of the acclimatized seed due to the high
temperature. A clearer effect of temperature was observed when the
microorganisms were acclimatized to LAS before the test. Under these
conditions, the rate of biodegradation increased steadily with
increasing temperature from 15 to 35°C (Hollis et al., 1976).
(ii) Wastewater treatment
In the OECD screening test, there was 95% loss of LAS, measured
by the MBAS method, and similar losses were measured in OECD
confirmatory test No. 1 with 20 mg/litre LAS. In the closed-bottle
test with a concentration of LAS of 2 mg/litre, there was 90-95%
analytical loss (by the MBAS method) and 60-65% loss of biochemical
oxygen demand. Coupled-unit tests with 10 mg/litre LAS and a mean
hydraulic retention time of 6 h showed 94% removal of chemical
oxygen demand (values > 73% indicate benzene ring opening) (Fischer
& Gerike, 1975). In activated sludge, 80-90% of dissolved organic
carbon and benzene rings disappeared within 6 h (Swisher, 1972). A
bacterium similar to Klebsiella pneumoniae, isolated from sewage,
degraded 93% of a concentration of LAS reported as 1% (10 g/litre),
as measured by the MBAS method (Hong et al., 1984). A direct
correlation was found between the rate of primary degradation of
1.5 mg/litre C11.7 LAS and the initial bacterial population size
(Yediler et al., 1989).
The biodegradation of C9-C13 LAS at concentrations of 25,
50, and 65 mg/litre was monitored in activated sludge at
100 mg/litre over a period of 12 days. Four methods were used: MBAS,
chemical oxygen demand, dissolved organic carbon, and ultra-violet
spectrophotometry. The results obtained with the MBAS method showed
a percentage loss of 94-97% for the three concentrations of LAS,
whereas the other methods showed losses of approx. 50% at
25 mg/litre LAS and approx. 70% at 50 and 65 mg/litre. The specific
rate of biodegradation was calculated to be 3.6 mg/g per h, on the
basis of loss of chemical oxygen demand (Pitter & Fuka, 1979).
The degradation ratio (biochemical oxygen demand:total oxygen
demand) for LAS by a synthetic sewage solution after five days was
0.81 for a concentration of 3 mg/litre and 0.14 for 10 mg/litre.
Concentrations of 30 and 100 mg/litre LAS were not degraded during
the 14-day test. Even after acclimatization to a concentration of
5 mg/litre LAS for one month, the two higher concentrations were not
degraded, probably because these levels inhibited the microbial
inoculum (Urano & Saito, 1985).
The percentage removal of biochemical oxygen demand and of LAS
were found to be significantly correlated in activated sludge and in
a trickling filter system under laboratory and field conditions,
implying that a well-functioning sewage treatment plant effectively
removes LAS (Tang, 1974).
LAS at a concentration of 150 mg/litre were inoculated into
sewage water collected from French water treatment plants. In six
out of eight experiments, primary degradation was almost complete
(90%) within seven days, but in the other two experiments only
45-55% degradation was achieved. The authors concluded that rapid
biodegradation of LAS requires the presence of a community of
several bacterial species, including Flavobacterium, Pseudomonas,
and Acinetobacter (Gard-Terech & Palla, 1986).
In an extended aeration activated sludge plant, 95-99% of LAS
was removed. Degradation of LAS and reduction of biochemical oxygen
demand were strongly correlated, in a 1:1 ratio (Knopp et al.,
1965). In long-term laboratory tests, 95-97% of LAS was removed by
activated sludge (Janicke & Hilge, 1979).
In a wastewater treatment plant where the input water had an
MBAS concentration of 6.2-9.4 mg/litre, at least 99% of the LAS
present was removed during treatment, biodegradation accounting for
85%. The relative composition of long-chain (C12-C13) homologues
adsorbed on the suspended solids was increased in comparison with
the relative incidence of short-chain (C10-C11) homologues
detected in the aqueous phases. Sulfophenylcarboxylates were
identified as intermediates of the biodegradation of LAS but were
detected only in the aqueous and not in the adsorbed phases (Cavalli
et al., 1993b).
Biodegradation of LAS in field trials with trickling filter
sewage was 86-95%, and average biochemical oxygen demand removal was
93.8%. Thus, the LAS appeared to be removed almost as rapidly as the
naturally occurring organic material. The linear correlation between
degradation and temperature (7.5-17.5°C) was highly significant.
Further degradation (94-99%) took place after additional aeration
(Mann & Reid, 1971).
MBAS degradation did not correspond to biodegradation of LAS
(20-200 mg/litre) in laboratory sludge units, because of the
presence of intermediates not accounted for by analysis of MBAS
(Janicke, 1971).
(iii) Surface waters
Primary degradation, measured by HPLC, of 5 mg/litre C11 LAS
in a static lake microcosm was complete within 18 days. The
sulfo-phenylcarboxylic acid intermediates produced were completely
degraded within 22 days (Eggert et al., 1979).
Aerobic degradation of 5 mg/litre LAS in river water, measured
by MBAS levels, was 100% after seven days at 25°C. Under
microaerophilic conditions at 25 and 35°C), no degradation took
place within 10 days (Maurer et al., 1971; Cordon et al., 1972).
In die-away tests with water from various sites on the Tama
River, Japan, primary biodegradation (measured by the MBAS method)
was complete within 7-15 days, but total organic carbon was
completely removed within an incubation time of 45 days. In a study
of LAS in seawater collected from the mouth of the Tama River,
degradation was only 50% complete within 60 days, as measured by
total organic carbon (Sekiguchi et al., 1975b). In a study to
monitor detergent-degrading bacteria from the Han River, Republic of
Korea, the lowest density was found in January and the highest in
July; the dominant group throughout the year was Pseudomonas (Bae
et al., 1982). Mixed and pure isomers of LAS were metabolized
readily (97.5%) by bacteria collected during the summer from a
sewage lagoon, but bacteria collected from under the ice during the
winter were not able to metabolize LAS (Halvorson & Ishaque, 1969).
Primary biodegradation of C10-C13 LAS was dependent on
incubation temperature in die-away tests with water from the Tama
River, Japan: primary biodegradation was complete within two days at
27°C, within six days at 15°C, and within three days at 21°C; at a
water temperature of 10°C, however, only 20% of the LAS had been
degraded within the nine-day test (Kikuchi, 1985). The optimal
temperature for the biodegradation of LAS in a river water die-away
test was found to be 25°C (Yoshimura et al., 1984b).
Degradation of 10 mg/litre LAS in a simulated river model was
found to be almost complete within 20 days, on the basis of MBAS
levels in water and sludge; however, ultra-violet spectrophotometry
showed that 40% of the LAS remained in the water and 25% in the
sludge. LAS with an alkyl chain length of C10 were degraded more
slowly than those with a chain length of C14, and LAS compounds
with sulfylphenyl groups near the terminal part of the alkyl chains
were degraded more easily than those with such groups further from
the end (Fujiwara et al., 1975).
In a study of the biodegradation of LAS (10 mg/litre) and a 1:1
LAS:ABS (10 mg/litre) mixture in canal water with an unaerated or
aerated system, LAS were rapidly degraded in the unaerated system,
by 14.9% within two days and 40.7% within seven days. Biodegradation
was more rapid in the aerated tanks, with 40.4% degraded within two
days and 74.5% after seven days. Addition of sewage to the test
system further increased the rate of degradation in the aerated
system: addition of 0.5 ml/litre sewage resulted in degradation of
78.2% after two days and 89.4% after seven days, and addition of
1.0 ml/litre sewage resulted in degradation of 89.7% after two days
and 99.8% within three days. No results were reported for the
unaerated system. The LAS-ABS mixture was degraded more slowly than
pure LAS: after two days, 12.3% was degraded without aeration, 36.4%
with aeration, 60.1% with addition of 0.5 ml/litre sewage, and 78.3%
after addition of 1.0 ml/litre sewage. The corresponding
degradations calculated after seven days were 32.5, 66.0, 80.7, and
87.3%. The authors concluded that degradation of these detergents
was increased by aerating the tank and by increasing the number of
microflora by adding sewage (Abdel-Shafy et al., 1988).
In the Lake Teganuma estuary (Japan), an average of 66% of LAS
is removed, with seasonal variability, ranging from 28% in winter to
100% in summer. Laboratory studies (based on HPLC methods) of
estuarine water showed that LAS degraded with a half-life of eight
days at 5°C and 0.2 days at 25°C. Model calculations and field
monitoring showed that biodegradation is 10 times more important in
the removal of LAS from the estuary during summer than is the
settling of solids or adsorption to bottom sediments. At lower
temperatures, biodegradation and the other removal mechanisms are of
equal importance (Amano et al., 1991).
In well water, biodegradation of all LAS homologues
(C10-C13) and isomers (maximal concentration, 2.5 mg/litre)
after an acclimatization period of one day was reported to follow
zero-order kinetics (Yakabe et al., 1992).
In seawater, primary biodegradation of 20 mg/litre LAS was 70%
after 10 days; the half-life was six to nine days (Vives-Rego et
al., 1987).
(iv) Soil
In soil degradation tests, levels of 2.5 mg/kg MBAS were reached
within 15 days of the addition of 20 mg/kg LAS (Cordon et al.,
1972). The biodegradation of LAS in soil was studied by measuring
the amounts of ferroin reagent-active substance and total organic
carbon. At 50 mg/litre LAS, total organic carbon disappeared within
50 days, whereas total ferroin reagent-active substance was
completely lost after only 10 days. Both chemical and physical
properties of the soils affected the loss of LAS: more LAS was
adsorbed onto clay loam than sandy loam, and biodegradation occurred
more readily in the clay loam (Abe, 1984). In a further study
(initial concentration not given), loss of C10-C13 and C12
LAS was complete within 15 days when measured as ferroin
reagent-active substances; however, when measured as total organic
carbon, residues remained until day 50 in the clay loam and beyond
day 60 in the sandy loam (Abe & Seno, 1987).
Ultimate degradation
A number of studies have been conducted of the biodegradation of
phenyl-radiolabelled LAS, in which 14CO2 production was measured.
(i) Screening tests
In a simple shake-flask system with LAS, CO2 evolution reached
60% or more of the theoretical value (Gledhill, 1975).
Four gram-negative bacteria synergistically mineralized
10 mg/litre 14C-LAS. After 13 days of incubation, 29% of the
14C-LAS was mineralized to 14CO2. Pure cultures were unable to
mineralize the LAS, although three of them carried out primary
biodegradation, measured by the MBAS method (Jimenez et al., 1991).
Pseudomonas, Alcaligenes, Necromonas, and Moraxella spp. isolated
from activated sludge and river water degraded the alkyl chains of
C12 LAS, while a group of unidentified Gram-negative bacteria cleaved
the benzene ring. A mixture of the two groups degraded LAS completely
(Yoshimura et al., 1984b).
(ii) Wastewater treatment
Mixed cultures of microorganisms found under natural conditions
or in sewage treatment plants can readily degrade LAS, to 95% of
MBAS and > 80% of dissolved organic carbon (Schöberl, 1989).
During a 19-day OECD screening test for the biodegradation of
14C-LAS, there was a high degree of ring mineralization, as seen
by the evolution of 55% as 14CO2. In a continuous system, 80% of
the LAS was evolved as CO2, with a mean retention time of 3 h;
2-3% remained as unaltered surfactant and 15-25% as the
sulfophenylcarboxylic acid intermediates (Steber, 1979).
Loss of MBAS (primary biodegradation) and ring cleavage were
found to be nearly complete (> 98%) during simulated waste
treatment of 14C-LAS. During simulated secondary waste treatment,
62% of alkyl and ring carbon was converted to CO2, 28-30% was
assimilated into biomass, and 8-10% remained as soluble residue. In
die-away tests, 85-100% of the substrates of LAS were converted to
CO2 within 91 days (Nielsen et al., 1980; Nielsen & Huddleston,
1981).
Continuous-flow experiments were conducted with mixed bacterial
cultures isolated from a detergent plant wastewater containing five
species of Achromobacter and two species of Acinetobacter. All
were more efficient at primary degradation than ultimate degradation
of LAS at concentrations of 20 and 50 mg/litre. One species of each
genus could effect primary degradation even after isolation (Hrsak
et al., 1982).
In a semi-continuous activated sludge method, 95% of the phenyl
ring of radiolabelled LAS was cleaved, indicating near complete
biodegradation of the whole molecule. Complete primary degradation
(MBAS method) of C10, C12, and C14 LAS was followed by 99-100%
ultimate degradation (HPLC and ultra-violet fluorescence). In
die-away tests with 10 mg/litre of C10, C12, and C14 LAS,
primary degradation was rapid and complete; 100% of C12 LAS was
removed within four days. Almost complete ultimate degradation was
observed within the 80-day test, with 90% ring cleavage of C10 LAS
and C11 LAS within 10 days and 70% ring cleavage of C14 LAS
within 30 days; however, no HPLC analysis was carried out on C14
LAS after day 30 (Huddleston & Nielsen, 1979).
The biodegradation of LAS (C9-C14) by a mixed bacterial
culture was studied in river water, forest soil, and wastewater from
a detergent plant. The bacteria were acclimatized to 10 mg/litre
LAS. Under continuous-flow conditions, LAS at a concentration of
20.8 mg/litre were 96% degraded, and a concentration of 46 mg/litre
was 64% degraded. Only 8-10% of the breakdown products were
completely mineralized; however, under the flow-through conditions
of this test, water-soluble compounds were usually removed via the
aqueous effluent and were not present long enough to allow
mineralization. Acclimatization considerably increased the kinetics
of mineralization (Hrsak et al., 1981).
(iii) Surface water and sediment
Detritus is a significant site of surfactant removal, and LAS
were found to be the most sorptive of the surfactants tested. In
wastewater from a pond containing submerged oak leaves, degradation
followed first-order kinetics, with a half-life of 12.6 days. LAS
were mineralized more slowly by leaves from a control pond, and an
S-shaped pattern of degradation was seen (Federle & Ventullo, 1990).
In river water in which the biomass levels were 10-100 times
higher below than above a sewage outfall, primary degradation of
added C11.6 LAS (11 mg/litre) and background LAS (0.37 mg/litre)
was rapid in water taken from below the outfall, with a half-life of
0.23 days (based on measurements of MBAS). Mineralization of the
benzene ring was rapid in water from below the outfall containing
sediment (500 mg/litre), with a half-life of 0.7 days. Water taken
above the sewage outfall also underwent ring mineralization, but the
rate of degradation was about 25% of that seen for water from below
the outfall, with a half-life of 2.7 days. When samples were
incubated in the absence of sediment, ring degradation was much
slower, with half-lives of 1.4 days in water taken from below the
outfall and approx.14 days in water taken above it. In all cases,
degradation was immediate in water taken below the outfall, but
occurred after a three-day lag in water taken above (Larson & Payne,
1981).
Degradation of C10-C14 homologues of LAS at concentrations
of 10 or 100 µg/litre followed first-order kinetics in both river
water and river water plus sediment; the half-time for
mineralization of the benzene ring was 15-33 h. The length of the
alkyl chain and the phenyl position had no significant effect, and
there was no effect of suspended sediment or competing homologues
(Larson, 1990).
LAS were degraded in leaf litter, creek water, periphyton, and
sediment at temperatures as low as 4°C, with half-lives of 6-11
days. Temperature changes altered the dependence of the
biodegradation of LAS: the half-lives increased by less than a
factor of two over an 18°C temperature range. Under realistic
conditions, temperature had less effect than was predicted on the
basis of classical thermodynamic studies in the laboratory
(Palmisano et al., 1991). The dependence of the biodegradation of
LAS follows a classical Arrhenius relationship down to about 12°C,
with a tenfold increase in reaction kinetics for every 2°C drop in
temperature (Larson, 1990).
Mineralization of LAS in saturated subsurface sediment from a
wastewater pond and in a pristine pond was monitored by amending the
sediment with 14C-LAS and measuring the evolution of 14CO2.
Mineralization in both sediments exhibited first-order kinetics. LAS
were mineralized without a lag in wastewater sediment, with
half-lives of 3.2-16.5 days. In the control pond, LAS were
mineralized much more slowly, with half-lives of 5.2-1540 days, and
only after a lag of 2-40 days; the lag tended to increase with
increasing depth. These findings confirm the assumption that
acclimatization considerably increases the kinetics of LAS
mineralization (Federle & Pastwa, 1988).
A study was conducted of the biodegradation of LAS by
microorganisms associated with the roots of two aquatic plants,
duckweed (Lemna minor) and cattail (Typha latifola).
Microorganisms from the roots of cattail mineralized 14C-LAS
without a lag, attaining 17% evolution of 14CO2 within the
35-day experiment. Microbiota associated with duckweed roots did not
mineralize LAS. The fact that the plants came from a pristine pond
or from a wastewater pond had no effect on the ability of the
microorganisms to mineralize LAS (Federle & Schwab, 1989).
More than 70% of parent LAS (20 mg/litre) in natural seawater at
22°C was biodegraded within 10 days, with an estimated half-life of
6-9 days (Vives-Rego et al., 1987). In an investigation of the
primary biodegradation kinetics of LAS (10 mg/litre) in natural
seawater in the presence of sediments (250 g/litre), 60% remained
after 20 days at 15°C and almost 100% of LAS at 5°C; however, at 20
and 25°C, only a small percentage of the original concentration
remained (Sales et al., 1987). In another study in seawater, 97% of
parent LAS (10 mg/litre) was biodegraded within two weeks (von Bock
& Mann, 1971).
More than 85% of LAS (C11.8) in estuarine water underwent
primary biodegradation, measured as MBAS removal, after 11 days
(Arthur D. Little Inc., 1991). In water from Chesapeake Bay, United
States, 75% of MBAS were removed within three days (Cook & Goldman,
1974). In a study of effluent-exposed estuarine waters, with
phenyl-radiolabelled C13 LAS, production of 14CO2 represented
42% of the added label. Addition of sediment from the site
(1 g/litre) increased the 14CO2 yield to 60%. In both tests, the
half-life for mineralization of LAS was about seven days. Up to 54%
of a radiolabelled control chemical, glucose, was mineralized. Thus,
mineralization of LAS occurs rapidly in pre-exposed estuarine
systems, with half-lives shorter than the typical hydraulic
residence times of such estuaries (Shimp, 1989).
(iv) Soils and groundwater
A simple shake-flask system was used to determine CO2
evolution in a test to assess the ultimate biodegradability of LAS
by microorganisms in soil and sewage. At 30 mg/litre, high
relative-molecular-mass LAS were biodegraded more slowly than those
with a low relative molecular mass. Ultimate biodegradation could
not be assessed precisely within the 28-day test period, but CO2
removal was 37-77% and dissolved organic carbon removal was 59-84%.
Ultimate biodegradation of the entire molecule (total CO2)
occurred concomitantly with biodegradation of the benzene ring
(14CO2). Ring desulfonation, measured as 35S-LAS, was rapid
and occurred mainly after primary biodegradation (MBAS method)
(Gledhill, 1975).
The kinetics of the ultimate biodegradation of C10-C14 LAS
to CO2 was studied in a sludge-amended soil at 0.1-10 times
environmental concentrations. All four homologues underwent rapid
degradation, with half-lives for the breakdown of the benzene ring
of 18-26 days (Ward & Larson, 1989).
Microbial mineralization of 50 µg/kg 14C-LAS was examined in
soil types ranging from a loamy sand impacted with sewage effluent
to a highly organic alpine soil, by monitoring the evolution of
14CO2. LAS were mineralized without a lag in all soils;
mineralization exhibited first-order kinetics in nine of the 11 soil
types. Asymptotic yields of CO2 ranged from 16 to 70%; the
half-lives were 1.1-3.7 days. The degradation rates were not
correlated with microbial activity, pH, total organic content, or
previous exposure (Knaebel et al., 1990).
After 14C calcium and sodium salts of LAS were applied to two
silty loam soils, the distribution of 14C was similar. After 60
days, 31-47% of the applied 14C had evolved as 14CO2 and
31-40% was present as soil residue, possibly as a combination of
parent and metabolized surfactant (Kawashima & Takeno, 1982).
A4.2.1.2 Anaerobic degradation
Degradation of LAS (measured as MBAS) was much slower under
anaerobic conditions in activated sludge than under aerobic
conditions. No degradation had taken place after one day; up to 20%
had been degraded between days 3 and 21, and 36% after 28 days. When
soil and wastewater were used, only 20% of the MBAS had disappeared
within 28 days (Oba et al., 1967). No significant removal of LAS was
reported in an anaerobic sludge digester at a Swiss sewage treatment
plant (Giger at al., 1989).
In a review of the fate of LAS in anaerobic and aerobic sewage
treatment plants, it was concluded that drying anaerobic sludge on
open beds considerably reduces the LAS content. Anaerobic
degradation of LAS is, however, limited, as the addition of LAS at
15 g/kg raw sewage (about 15 g/litre raw sewage) may inhibit
anaerobic degradation. In the laboratory, digestion of LAS was
impaired at concentrations of > 15-20 g/kg, and a concentration of
20 g/kg seriously inhibited gas production, especially when other
potentially inhibitory compounds were present. The concentration of
LAS normally found in sewage (5-10 g/kg) is, however, unlikely to
inhibit anaerobic degradation (Painter & Zabel, 1989). About 15-35%
of LAS in raw sewage is physically removed in primary settlers in
sewage treatment plants, accounting for most of the LAS found in
anaerobic sludge. Precipitation of LAS is correlated with water
hardness, since the solubilities of the calcium and magnesium salts
of LAS are very low; the solubility products ranged from
2.2 × 10-10 for C10 LAS to 6.2 × 10-13 for C13 LAS (Berna et
al., 1989). The effect of water hardness was confirmed by mass
balance analysis of Na+, Ca2+, and Mg2+ (Berna et al., 1993b).
The content of total calcium and magnesium in anaerobically digested
sludge was 43 times higher than that in water. High contents of LAS
in the sludge (up to 30 g/kg) did not inhibit the anaerobic
digestion process (Painter & Mobey, 1992), probably because LAS were
present as calcium and magnesium salts and therefore had reduced
bioavailability.
LAS were not degraded in an anaerobic sediment from a pond
receiving wastewater from a laundromat. Despite an exposure period
of 25 years, no anaerobic degradation was reported (Federle &
Schwab, 1992).
Pre-aerobic treatment of LAS may cause changes in the molecule
that permit subsequent degradation under anaerobic conditions (Ward,
1986).
A4.2.2 Abiotic degradation
The mechanisms of abiotic degradation of LAS reported below are
not of environmental significance, since biodegradation and sorption
are rapid, effective removal mechanisms.
A4.2.2.1 Photodegradation
In a study of the kinetics of the photodecomposition of C12
LAS, using a continuous-flow reactor, the initial concentrations
were 60-182 mg/litre and the radiation wavelength was 200-450 nm.
Conversion of LAS to intermediate products occurred within 1 min,
yielding 7 mol CO2 per mol LAS, and was complete within 20 min.
The reaction rate was increased by two orders of magnitude by ferric
perchlorate (Matsuura & Smith, 1970).
Rapid photodegradation of LAS (50 mg/litre) occurred within
1-2 h in an aqueous, aerated titanium dioxide suspension without
noble metal catalysts. There was rapid decomposition of the aromatic
ring and slower oxidation of the aliphatic ring. Photodegradation
was dependent on the simultaneous presence of titanium dioxide,
oxygen, and light (Hidaka et al., 1985).
A4.2.2.2 Cobalt-60 irradiation
The decomposition of LAS was studied in distilled water
irradiated with cobalt-60 gamma rays, which react with water to
produce oxygen, peroxide, hydrogen peroxide, and other strong
oxidizing agents. A concentration of 10 mg/litre LAS was reduced to
7.8 mg/litre by absorption of 10 Gy and to 0.9 mg/litre by
absorption of 100 Gy. The rate of irradiation was found to be less
important than the total absorbed energy (Rohrer & Woodbridge,
1975).
A4.2.3 Bioaccumulation and biomagnification
Studies of the bioaccumulation potential of LAS have all been
carried out with LAS labelled with 14C or 35S. It should be
noted that as these techniques do not usually allow consideration of
metabolic transformation the actual bioaccumulation of the parent
compound may be overestimated. Toxic concentrations of the breakdown
products of LAS are discussed in section A9.3.7.
A4.2.3.1 Aquatic organisms
Bioaccumulation has been studied in daphnids and fish (Table 8).
LAS are readily absorbed through the gills and body surface of fish
and are subsequently distributed via the blood to the organs and
tissues; most LAS accumulate in the gall-bladder and hepatopancreas.
Clearance is usually rapid, with a half-life of two to three days.
Short-chain LAS are accumulated to a lesser degree than long-chain
LAS.
Only 1% of 0.5 mg/litre LAS added to water was retained in
Daphnia magna within three or four days after transfer to 'clean'
water. Almost all of the chemical was in the form of intact LAS. In
fathead minnows (Pimephales promelas), metabolic transformation
occurred. All tissues monitored showed some uptake, with
concentration factors ranging from 79-372 in muscle to 21 000-70 000
in gall-bladder. Within four days of transfer to 'clean' water, 85%
of the LAS had been lost, and almost 100% was lost within 10 days
(Comotto et al., 1979).
Table 8. Bioconcentration factors for linear alkylbenzene sulfonates in aquatic invertebrates and fish
Organism Static/flow Exposure Duration Chain Steady Bioconcentration Tissue Reference
concentration of test length state factor
(mg/litre) (days)
Daphnia magna Flow 0.07 3 C12 ? 490 Comotto et al.
(1979)
0.11 560
044 720
0.09 3 C13 Yes 1250
0.11 1050
0.41 1325
Cyprinus carpio Static 61.1 1 C12 Yes 4.1 Skin surface Kikuchi et al.
(1978)
1000 Gall-bladder
Flow 0.5 4 C12 Yes 20 Whole body Wakabayashi
30 Hepatopancreas et al. (1978)
9000 Gall-bladder
0.0091 5 C12 Yes 16 Whole body Wakabayashi
et al. (1981)
0.3 400
1.0 300
Pimephales Flow 0.1 11 C12 Yes 551 Whole body Comotto et al.
promelas C13 1223 (1979)
C12, C13 269
Table 8 (contd)
Organism Static/flow Exposure Duration Chain Steady Bioconcentration Tissue Reference
concentration of test length state factor
(mg/litre) (days)
Lepomis Flow 0.063 28 C12 Yes 260 Whole body Bishop &
macrochinus 0.064 120 Maki (1980)
Flow 0.5 35 C11.7 Yes 107 Whole body Kimerle et al.
5000 Gall-bladder (1981)
Static, water unchanged for the duration of the test; flow, concentration in water maintained continuously
In an experiment in which the aqueous concentrations of an
initial concentration of 1.1 mg/litre LAS decreased by 20% during
the test, the compounds were concentrated in the gills of carp
(Cyprinus carpio) within 2 h of exposure, with a concentration
factor of 40. Skin surface, muscle, brain, kidney, hepatopancreas,
and gall-bladder showed more gradual uptake of LAS over the 24 h of
exposure, with concentration factors ranging from 4.1 for skin
surface to 1000 for gall-bladder. Blood, gonads, and spleen also
took up LAS but were not monitored throughout the period of
exposure. LAS was lost rapidly from all tissues except the
gall-bladder during 48 h in 'clean' water (Kikuchi et al., 1978).
In the bluegill (Lepomis macrochirus), a steady state was
reached within 120-168 h. The bioconcentration factor was calculated
by a kinetic method to be 286 for a concentration of LAS of
0.8 mg/litre and 132 for 0.08 mg/litre. LAS were cleared rapidly
after the fish were transferred to 'clean' water, with 99%
eliminated within 336 h; the time for clearance was 29-30 h (Bishop
& Maki, 1980). In another study, a steady state was reached within
seven days; the bioconcentration factor in whole body using a
kinetic method was reported to be 104; and the half-time for
clearance was two to five days during a depuration period of 14
days. The authors postulated that fish excrete LAS in the urine and
excrete shorter-chain carboxylates with the benzene ring intact
across the gill membranes. Both forms may also be excreted in the
faeces (Kimerle et al., 1981).
A4.2.3.2 Terrestrial plants
Foliar uptake of the calcium and sodium salts of 14C-LAS
(chain length not specified) by peanuts was studied seven and 30
days after application. No movement of LAS was detected: 70-80%
remained within the same leaf to which the compound was applied, and
no LAS were detected in other parts of the plant (Kawashima &
Takeno, 1982).
Aqueous solutions of 14C-LAS (chain length not specified) were
applied to soil (orthic luvisol), and ryegrass (Lolium perenne)
was grown under laboratory conditions for up to seven days. Uptake
of LAS after three days was 80 mg/kg at an application rate of
1 mg/kg dry weight, 370 mg/kg at a rate of 5 mg/kg, and 18 900 mg/kg
at 50 mg/kg. After seven days, levels of 600, 5000, and 19 300 mg/kg
were measured at the three dose levels, respectively (Litz et al.,
1987).
14C-LAS (chain length not specified) were applied under field
conditions to both loamy orthic luvisol and sandy dystric cambisol
soils irrigated with wastewater at rates of 5 and 50 g/m2. After
49 days, rye grass grown in the loamy soil contained residues of 130
and 1000 mg/kg dry weight at the two exposure rates, respectively.
Plants grown in the sandy soil contained 230 and 470 mg/kg,
respectively, after 54 days (Litz et al., 1987).
Two plant-soil microcosms were exposed to 14C-LAS (chain
length not specified), and LAS degradation and percolation were
followed for up to 109 days. The initial soil concentrations were
16.2 µg/g dry soil in potato soil (sandy) and 27.2 µg/g in grass,
bean, and radish soil (clay-like). The concentrations of
radiolabelled compounds in the plants decreased rapidly: at the end
of exposure, 39.1-65.8 µg LAS equivalents per g fresh weight of
plant were found in potatoes (study duration, 76 days) and
62.1-213.3 µg/g in grass, radishes, and beans (study duration, 109
days) (Figge & Schoberl, 1989).
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
The concentrations of LAS have been quantified by means of a
specific, sensitive analytical method in almost every environmental
compartment in which they might be present. The concentrations
decrease progressively from wastewater to treated effluent and
surface waters, and low concentrations are found in the sea.
The environmental concentrations of LAS are directly dependent
on use patterns, the type and efficiency of sewage treatment, and
the characteristics of the receiving environment. In areas where LAS
are the predominant surfactants used, typical concentrations are
1-10 mg/litre in wastewater, 0.05-0.1 mg/litre in effluents that
have undergone biological treatment, 0.05-0.6 mg/litre in effluents
passed through a percolating filter, 0.005-0.050 mg/litre in surface
waters below sewage outfalls (with concentrations decreasing rapidly
to 0.01 mg/litre downstream from the outfall), < 1-10 mg/kg in
river sediments (up to 100 mg/kg in highly polluted sediments near
discharge zones), 1-10 g/kg in sewage sludge, and < 1-5 mg/kg in
sludge-amended soils. The initial concentration of LAS in
sludge-amended soils is 5-10 mg/kg, but up to 50 mg/kg have been
reported after atypically heavy appli-cations. The concentration of
LAS in estuarine waters is 0.001-0.010 mg/litre but is higher where
wastewater is discharged directly. The concentrations in offshore
marine waters are < 0.001-0.002 mg/litre.
A wide range of environmental concentrations has been reported,
owing to use of different analytical methods; differences in
characteristics of sampling sites, which range from highly polluted
areas with inadequate sewage treatment to areas where sewage
undergoes extensive treatment; seasonal differences, which can
account for a twofold variation; and differences in the use of LAS.
Monitoring has shown no accumulation of LAS in environmental
compartments over time. The concentrations in soil do not increase
with time but are diminished due to mineralization. As LAS are not
degraded under strictly anaerobic condition, they are not
mineralized in anaerobic sediments. With current use of LAS, the
rates of their assimilation in all receiving environmental
compartments is equal to their rate of input, implying a steady
state.
A5.1 Environmental levels
LAS have been measured in most environmental compartments,
including discharge (raw sewage), sewers, sewage treatment plants,
sludge-amended soils and land fill, river water, river sediments,
subsurface soils, groundwater, and estuaries (Berna et al., 1991).
A decline in the concentrations of anionic surfactants in the
environment, as assessed by measurement of MBAS, was seen in Europe,
Japan, and the United States after ABS was replaced by LAS (Sullivan
& Evans, 1968; Sullivan & Swisher, 1969; Gerike et al., 1989).
Similar declines have been observed more recently in countries such
as Thailand, where the change to LAS detergents is also more recent
(Berna et al., 1991).
A5.1.1 Wastewater, sewage effluent, and sludge
The concentrations of LAS in sewage influent and effluent at
sewage treatment plants are shown in Table 9; those in sewage sludge
are given in Table 10.
The efficiency of wastewater treatment plants in removing LAS is
reported to exceed that of removal of biochemical oxygen demand.
Activated sludge removed an average of 98% LAS, trickling filters
removed 80%, and primary clarification, 27%. The average
concentration in raw sewage was 3.5 mg/litre, and those in effluent
were 2.1 mg/litre after primary treatment and 0.06 mg/litre in
activated sludge. The average chain length of LAS was C12.5 in
sewage sludge and C12 in influent sewage (Rapaport & Eckhoff,
1990).
The amount of LAS removed in a sewage treatment plant was 93% on
the basis of total organic carbon and 98.1% on the basis of a
specific method. The contribution of LAS to the total organic carbon
was estimated to be 0.93% in treated water and 3.0% in digested
sludge; 75.9% of LAS present in the raw sewage was mineralized
during treatment and 7% was in the form of sulfoxyphenyl-
carboxylates, a product of the biodegradation of LAS, suggesting
that biodegradation of LAS had reached a steady state. These figures
were obtained by analysis for sulfoxyphenylcarboxylates (Berna et
al., 1993b).
In another study, 40% of LAS was removed in a wastewater
treatment plant. The half-life for removal from the sewer pipe was
calculated to be 11 h (Moreno et al., 1990).
A5.1.2 Sediment
The concentrations of LAS in sediment are shown in Table 11, and
those in sediment samples collected at various distances from sites
of effluent outfall are shown in Table 12.
Table 9. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sewage
influent and effluent
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
Switzerland (29 sites, 1986 Raw sewage 0.95-3.9 Brunner et al. (1988)
1 sampling) Effluent 0.007-0.33
Germany (11 sites, 1985 Influent (activated sludge) 5.1 (1-13.3) 4 (0.54-12.4) Matthijs & De Henau
1 sampling) Influent (trickling filter) 8.8 (8.1-9.9) 7.4 (6.8-8.4) (1987)
Effluent (activated sludge) 0.19 (0.09-0.28) 0.07 (0.05-0.11)
Effluent (trickling filter) 1.1 (0.84-1.5) 0.76 (0.61-0.94)
United Kingdom
(several samples) 1982 Effluent 0.69 (0.58-0.81) 0.31 (0.21-0.42) Gilbert & Pettigrew
(1984)
River Thames area 1987 Sludge 15.1-341 Holt et al. (1989)
(5 sites, several
samples)
Israel (4 sites) 1983 Influent 9.6-10.6a Zoller (1985)
Effluent 0.3-11.0a
United States 1979 Effluent 0.078-0.303 Eganhouse et al. (1983)
(4 sites, 45 samples 1976-86 Influent 3.7 ± 1.1 Rapaport & Eckhoff
Effluent (activated sludge) 0.05 ± 0.04 (1990)
Effluent (trickling filter) 0.6 ± 0.3
Effluent (primary) 2.2 ± 0.4
Table 9 (contd)
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
United States Influent 5.9-6.5 5.7-6.5 Osburn (1986)
(1 sampling) Influent 3.7-5.2 3.8-4.9
Effluent 0.39-1.02 0.14-0.60
(2 sites, 9 samples) 1983 Raw influent 4.17 3.73 Sedlak & Booman
Primary influent 3.18 2.97 (1986)
Primary effluent 1.66-2.82 1.73-2.51
Final effluent 0.03-0.06 0.02-0.05
Canada (4 sites, 1976-86 Influent 2.0 ± 0.6 Rapaport & Eckhoff
45 samples yearly) Effluent (activated sludge) 0.09 ± 0.05 (1990)
Effluent (primary) 1.7-2.3
Japan
(5 sites, 60 samples) 1972-73 Influent 5.1-14.0 Oba et al. (1976)
Effluent 0.3-4.7
(6 sites, 1-2 samples) 1984 Influent (suspended particles) 0.236-1.504 Takada & Ishiwatari
Effluent (suspended particles) 0.0001-0.001 (1987)
a Total anionic surfactants (mainly LAS)
Table 10. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sewage sludge
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
Switzerland
(8 and 12 sites, Digested sludge 2900-11 900 McEvoy & Giger
(1985, 1986)
1 sampling)
(29 sites, 1986 50-13 800a Brunner et al.
1 sampling) (1988)
Spain
(5 sites, several Activated sludge 7000-30 200a Berna et al. (1989)
samplings) (anaerobic digestion)
Aerated, settling system 400-700a
Finland (12 sites, Digested sludge 3400-6300a McEvoy & Giger
1 sampling) (1986)
Belgium (11 sites, 1985 Aerobic sludge 5399 (3042-8133) 281 (182-432) Matthiijs & De
1 sampling) Digested sludge 9017 (3632-17 006) 4917 (1327-9927) Henau (1987)
Germany (4 sites, 1981-86 4920 (1330-9930) Rapaport &
45 samples yearly) Eckhoff (1990)
Table 10 (contd.)
Location
Year Material Concentration (mg/litre) Reference
MBAS LAS
United States
(4 sites, 45 1981-86 4660 ± 1540 Rapaport &
samples yearly) Eckhoff (1990)
12 sites, NY, Digested sludge 6900a McEvoy & Giger
(1 sampling) (1986)
(12 sites, CA, Digested sludge 5200a
1 sampling)
(1 sampling) Primary sludge 110-126 107-127 Osburn (1986)
(2 sites, OK, 1983 Primary sludge 4610-6120 5340-6310 Sedlak & Booman
9 samples) Secondary sludge 520-990 410-860 (1986)
Anaerobic digester 6860 6660
Aerobic digester 3820 4250
Drying bed (anaerobic) 170 160
Drying bed (aerobic) 230 150
Southern California 1981 Effluent particulates 1342 Eganhouse et al.
(marine) (1983)
a Dry weight
Table 11. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sediments in
the United States and Japan
Location Year Concentration (mg/kg) Reference
MBAS LAS
United States
Rivers (activated sludge) 0.3-3.8 McAvoy et al. (1993)
Rivers (trickling filter) 0.2-340
Mississippi River 1991-92 < 0.01-5 Tabor et al. (1993)
Japan
Tokyo Bay (1 sampling, few samples) 1969 35 (33-37) Ambe (1973)
River (1 sampling, few samples) 61 (55-65)
River Sagami estuary (16 sites, 1 sampling) 7.9-39a ND-17 Utsunomiya et al. (1980)
Sagami Bay (16 sites, 1 sampling) 5.1-15 ND
Rivers 1977 < 1-260 Environment Agency Japan
(1978)
Lake Suwa (1 site, 3 samples) 1977 1.0-7.0
Rivers (9 sites, 7 samples, 1 year); 1982-83 107 (ND-567) Takada & Ishiwatari (1987);
(1 site 52 samples) Takada et al. (1992b)
Estuaries (1 site, 52 samples) 1983-84 4.82 (0.12-36.6) Takada et al. (1992b)
Tokyo Bay (9 sites, 7 samples, 1 year) 1980 71.0 Takada & Ishiwatari (1987)
Tokyo Bay 1984 0.02 (ND-0.06) Takada et al. (1992a)
Sumida River (12 sites, 1 sampling) 1982 0.069 Kikuchi et al. (1986)
Tama River (3 sites, 8 samples) 1977 3.5-86.3 Hon-Nami & Hanya (1980b)
Tama River (10-12 sites) 1982 0.141 Kikuchi et al. (1986)
Tokyo Bay (10-12 sites) 1982 < 0.001-0.002
Table 11 (contd)
Location Year Concentration (mg/kg) Reference
MBAS LAS
Japan (contd).
Tsurumi River (7 sites, 12 samples) 1984 17-45a Yoshikawa et al. (1985)
Tama River 1981 2.79-10.72 Yoshimura et al. (1984b)
Ports and coast 1977 < 1-2.9 Environment Agency Japan
(1978)
ND, not determined
a Dry weight
Table 12. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sediment of rivers in
Germany and the United States at various distances from effluent outfalls
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
German rivers (14 sites, several 1978-82 Below outfall 1.5-174a De Henau et al.
samples) (1986)
United States
Rivers (4 sites, 45 samples) 1978-82 Below outfall 190 Rapaport &
yearly < 5 miles (8.0 km) 11.9 Eckhoff
> 5 miles (8.0 km) 5.3 (1990)
(1 sampling) 0.5 miles (0.8 km) 118-317 100-322 Osburn (1986)
4.4 miles (7.1 km) 4.1-19 2.0-5.1
7.4 miles (11.9 km) 7.5-10.6 1.3-4.4
Rapid Creek, South Dakota 1979-80 0.8 km 44.6-275 Games (1983)
7 km 3.2-9.1
11.7 km 2.1-8.4
25.3 km 2.7-10.1
48 km 1.4
87.2 km 1.5
Little Miami River, Ohio Downstream from sewage ND-1.2 Hand et al. (1990)
4 sites, 1 sampling) treatment plants 24.7-290b
Rivers (4 sites, 45 samples) 1978-82 Below outfall 190 Rapaport &
Above outfallc 1.0-1.2 Eckhoff (1990);
Below outfall (left)c 0.3-1.6 McAvoy et al.
Below outfall (middle)c 0.6-3.8 (1993)
Table 12 (contd)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United States (contd)
Rivers (4 sites, 45 samples) 1978-82 Below outfall (right)c 0.8-3.4
(contd). Above outfalld 0.2-0.9
Below outfall (left)d 0.2-130
Below outfall (middle)d 0.6-124
Below outfall (right)d 9-340
a 13 of the 14 samples contained < 25 mg/kg and 10 contained < 10 mg/kg
b Suspended solids
c Activated sludge
d Trickling filter
Concentrations of LAS > 10 mg/kg were measured in sediments
from the upper estuaries near Tokyo Bay and < 1 mg/kg in the lower
estuaries. The concentrations of LAS in sediments decreased
offshore, falling below 0.01 mg/kg in sediments sampled 10 km from
the mouths of the rivers. The authors suggested that loss of LAS was
due to rapid degradation in the coastal zone (Takada et al., 1992a).
It was reported in one study that C13 was the most abundant
homologue of LAS in river sediment (Yoshikawa et al., 1985); another
group found that C12 was the most abundant of the LAS in estuarine
sediments and that no C10 were present (Utsunomiya et al., 1980).
C12 and C13 LAS predominated in sediment and C10 and C11
homologues were the most abundant in water (Hon-Nami & Hanya,
1980b). The average chain length of LAS in Japanese river sediments
was C11.8-C12.2 (Hon-Nami & Hanya, 1980b; Yoshimura et al.,
1984a).
In a study of marine sediments from an area adjacent to the
point of discharge from a submarine sewer, LAS were detected only in
the vicinity of the discharge, at a concentration of 0.1 mg/kg, and
not in sediment sampled 50 m outside this area. The average chain
length was C11.7. In a comparison of the chain lengths of LAS
detected in various environmental compartments and those used in
detergent products, the LAS detected in sludge and sediment were
relatively higher homologues and those in the water phase were
lighter (Prats et al., 1993).
The average concentration of LAS in river sediments sampled
upstream of an activated sludge treatment plant outfall was
1.1 mg/kg, and those in sediments downstream of the plant were
0.3-3.8 mg/kg (McAvoy et al., 1993).
A5.1.3 Surface water
The concentrations of LAS in water are shown in Table 13 and
those in samples taken at various distances from sites of effluent
outfall in Table 14.
After replacement of branched-chain ABS, which are only
sparingly biodegradable, with the straight-chain LAS, the
concentrations of MBAS decreased in many rivers. ABS were replaced
by LAS in Japan in the late 1960s; the ratio of LAS to total ABS in
river water rose from 20 to 70% in 1967-70 and had reached 90% by
1973 (Miura et al., 1968; Ihara et al., 1970; Oba et al., 1975). The
levels of MBAS were monitored in the Illinois River, United States,
from 1959 to 1966; those in 1965 and 1966 reflected the change in
surfactant usage (Sullivan & Evans, 1968), and this trend continued
in 1967 and 1968 (Sullivan & Swisher, 1969). In the River Rhine, the
level of anionic detergents, measured as MBAS, fell steadily between
1971 and 1977 (Hellmann, 1978). In water samples from 140 sites on
Table 13. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in water
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Freshwater
United States
Rivers (4 sites, 45 samples yearly) 1978-86 0.041-0.115 Rapaport & Eckhoff
(1990)
Little Miami River, Ohio (4 sites, < 0.05 Hand et al. (1990)
one sampling) Interstitial ND-0.08
Illinois River (one sampling)a 1959-65 0.54 Sullivan & Swisher
1965-66 0.22 (1969)
1968 0.05-0.06
Rapid Creek, South Dakota 1979-80 0.01-0.270 Games (1983)
Mississippi River (36 sites) 1991-92 < 0.01-0.3 McAvoy et al. (1993)
(350 samples) < 0.01-0.046 < 0.005 Tabor et al. (1993)
Japan
Rivers (23 sites, 51 samples) 1977 < 0.01-2.9 Environment
Agency Japan (1978)
Rivers (1 sampling) 0.018-0.59 Tsukioka &
Murakami (1983)
Oohori River (6 sites monthly) 1987-88 approx. 0.5-1.6 Amano et al. (1991)
Lake Teganuma (6 sites monthly) 1987-88 ND-approx. 0.7
Tama River (3 sites, 8 samples) 1977-78 0.24-1.24 0.108-0.491 Hon-Nami & Hanya
(1980a)
Table 13 (contd.)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Japan (contd)
Rivers, Hyogo Prefecture (70 sites) 0.004-2.5 Kobuke (1985)
Tama River (3 sites, 1 sampling) 0.035-0.219 Yoshikawa et al.
(1984)
Tama River (10-12 sites) 1982 0.128 Kikuchi et al. (1986)
Sumida River (10-12 sites) 1982 0.005-0.01 Kikuchi et al. (1986)
Rivers (1 sampling) 0.06-0.12 Saito & Hagiwara
(1982)
Rivers, Niigata Prefecture (6 sites, 0.02-2.63 0.18 (max) Motoyama & Mukai
1 sampling)
(1981)
Rivers, coastal area, Hiroshima 0.019 Okamoto & Shirane
Prefecture (20 sites) (0.001-0.06) (1982)
Inland Sea, Eastern Seto (4 sites, 1975 0.016-0.077 Yoshida & Takeshita
1 sampling) (1978)
(17 sites, 1 sampling) 1976 0.01-0.048
Tsurumi River, Kanagawa 1984-76 Surface 0-0.8 0.01-0.29 Yoshikawa et al.
(7 sites once) (1985)
Yodo River, Osaka (several sites) 1989 Surface 0.043-0.089 Nonaka et al. (1990)
Tama River, Tokyo (2 sites, 1981 Surface 0.2 Yoshimura et al.
4 samples) (1984b)
Sumidogawa River (2 samples) 1983 Suspended 0.0048-0.054 Takada & Ishiwatari
Tomogawa River (5 samples) particles 0.0005-0.0025 (1987)
Teshiro River, Nagoya (4 sites, 1989 Surface 0.01-0.27 Kojima (1989)
4 samples)
Table 13 (contd)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Japan (contd)
Lake Biwa, Shiga 1988 Surface 0.00 Shiga Prefecture
(1988)
Teganuma, Chiba (1 site, 1988 Surface ND-0.423 Amano et al. (1989)
12 samples)
River (several sites) 1988 Surface 0.019-1.4 Nonaka et al. (1989)
Nagoya Bay 1989 Surface 0.00 Kojima (1989)
Rivers, Fukuoka City ND-1.6 Ohkuma (1981)
Europe
River Rhine (several sites) 1971-72 0.08-0.24 Hellmann (1978)
Saar River (11 sites, 1 sampling) 1985 0.13 0.04 Matthijs & De Henau
(0.03-0.25) (0.01-0.09) (1987)
German rivers (several sites) 1976-79 0.075-0.5 Fischer (1980)
Dutch river (Amsterdam drinking- 0.004-0.141 0.003-0.037 Waters (1976)
water supply) (8 sites)
Florence, Italy (several samples) 1983 Aqueduct 0 .01-0.1 Mancini et al. (1984)
(several sites) 1982 Well water 0.00-0.01
United Kigdom
Rivers 1982 0.04-0.26 0.012-0.08 Gilbert & Pettigrew
(1984)
Rivers (8 sites) 0.035-0.217 0.009-0.097 Waters (1976)
Rivers (4 sites) 1977-78 0.022-0.473 0.007-0.173 Waters & Garrigan
(1983)
Table 13 (contd)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Groundwater 1992 < 0.01-0.02 Field et al. (1992)
Estuarine and marine water
North Sea (19 sites) 1989 < 0.0005-0.0012 Stalmans et al.
(1991)
Krka River estuary, Croatia 1990 Wastewater 0.42-0.78 Terzic & Ahel (1993)
(below 50 m > 50 m) Estuarine water 0.003-0.007
0.001-0.002
Tokyo Bay, Japan (8 samples) 1978 0.03-0.07 < 0.003-0.014 Hon-Nami & Hanya
(1980a)
Tokyo Bay, Japan (10-12 samples) 1982 0.001-0.03 Kikuchi et al. (1986)
Osaka Bay, Japan (several sites) 1988 Surface ND-0.0072 Nonaka et al. (1989)
ND, not detected
a 10-20% of MBAS were LAS
Table 14. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in water at various
distances from effluent outfalls
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United States
Rivers (4 sites, 45 samples 1978-86 Below outfall 0.115 Rapaport & Eckhoff
yearly) < 5 miles (8 km) 0.079 (1990)
> 5 miles (8 km) 0.041
(1 sampling) 0.5 miles (0.8 km) 0.400 0.270 Osburn (1986)
4.4 miles (7.1 km) 0.300 0.150
7.4 miles (11.9 km) 0.250 0.120
15.8 miles (25.4 km) 0.240 0.100
30.0 miles (48.3 km) 0.130 0.040
55.0 miles (88.5 km) 0.100 0.010
Rapid Creek, South Dakota 1979-80 0.8 km 0.270 Games (1983)
7 km 0.150-0.190
11.7 km 0.120
25.3 km 0.080
48 km 0.040
87.2 km 0.010
Rivers Above outfall < 0.01-0.9 McAvoy et al. (1993)
Below outfall (left) < 0.01-0.33
Below outfall (middle) < 0.01-0.3
Below outfall (right) < 0.01-0.3
Table 14 (contd)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
Canadian rivers (4 sites, 45 1978-86 Below outfall 0.053 Rapaport & Eckhoff
samples yearly) (1990)
Rio Grande, Brazil 1979 90 m 0.05-4.5 Kantin et al. (1981)
(1 sampling, 50 samples)
German rivers (several sites) 1976-79 Unpolluted 0.075 Fischer (1980)
Polluted 0.2-0.5
(4 sites, 45 samples yearly) 1978-86 Below outfall 0.01-0.09 Rapaport & Eckhoff
(1990)
United Kingdom
Rivers (several samples) 1982 Above discharge 0.04 0.012 Gilbert & Pettigrew
(0.02-0.07) (0.008-0.019) (1984)
Close to discharge 0.26 0.08
(0.11-0.47) (0.01-0.17)
5-16 km 0.16 0.04
(0.08-0.23) (0.008-0.095)
Avon River (4 sites) 1977-78 Head water 0.03-0.039 0.009-0.015 Waters & Garrigan
0.5 km 0.21-0.371 0.056-0.173 (1983)
6 km 0.095-0.22 0.011-0.095
Tean River (4 sites) 1977-78 Head water 0.035-0.073 0.008-0.019
Directly below sewage 0.208-0.473 0.067-0.144
treatment
5 km 0.145-0.234 0.019-0.07
Table 14 (contd.)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United Kingdom (contd)
Trent River (4 sites) 1977-78 Head water 0.022-0.052 0.01-0.011
20-35 km below head 0.08-0.227 0.007-0.072
water
Nene River tributary 1978 0.104 0.011
(4 sites)
In the vicinity of 0.206-0.216 0.035-0.037
sewage effluent disharge
3.5 km 0.184 0.035
13.5 km 0.06 0.007
four German rivers, MBAS concentrations fell by 90% between 1964 and
1987 (Gerike et al., 1989).
The mean level of MBAS in rivers in the United Kingdom was
0.15 mg/litre. On average, only 26% was attributable to LAS (by
microdesulfonation and gas-liquid chromatography), but the levels of
LAS and their contribution to the total MBAS concentration varied
according to the sampling site, with a higher proportion of LAS in
samples from sites near sewage effluent discharge points (Waters &
Garrigan, 1983). Similar findings were reported by Gilbert &
Pettigrew (1984), who found that LAS represented 45% of total MBAS
in actual sewage. Sites immediately below sewage outfalls were found
to have higher MBAS:LAS ratios than sites further downstream
(Osburn, 1986).
In Lake Biwa basin, Japan, during the summer months of 1983, LAS
were found in a wide range of concentrations. The highest, measured
as MBAS, were > 0.2 mg/litre at river mouths. The levels in rivers
flowing from densely populated areas were 0.05-0.2 mg/litre MBAS and
those flowing from less populated areas were < 0.05 mg/litre. The
middle stream zone of the River Isasa, in a densely populated area,
contained levels of 0.36-1.91 mg/litre, and surfactant levels in
residential areas showed daily fluctuations related to discharge
(Sueishi et al., 1988). Several observations apply to these studies.
Firstly, the fact that daily fluctuations were observed indicates
that the samples may have been taken from the actual discharge
plume, so that the wastewater effluent may not have been completely
mixed with the recipient surface water. Secondly, in several
Japanese studies of heavy discharge zones, anionic surfactants could
not be detected in surface waters, although the analytical detection
limit of MBAS in the mid-1980s was 0.05-0.1 mg/litre. Thirdly,
sewage treatment at several of the sites has improved considerably
over the last decade.
Seasonal trends in the concentrations of LAS were observed in
the Oohori River and Lake Teganuma, Japan, in 1987 and 1988, with
low levels in summer and high levels in winter (Amano et al., 1991).
The concentrations of LAS were measured in the Tamagawa River,
Japan, at two-week intervals for two years, by sampling water from
the boundary between freshwater and brackish zones. The
concentrations measured in winter were about five times higher than
those measured in summer, when long-chain homologues tended to be
depleted. The distribution of isomers also showed a clear seasonal
trend, with a greater loss of external isomers in summer. The
seasonal changes are thought to be the result of differences in
water temperature and microbial activity. The flux of LAS in the
river was estimated to be 320 tons/year (293 tonnes/year), which
exceeds the total amount of LAS accumulated in the bay sediment,
indicating that > 99.9% of LAS in the estuary and the bay was
degraded (Takada et al., 1992b).
The concentrations of LAS in suspended particles from
tributaries of Tokyo Bay, Japan, were 0.5-53.8 µg/litre. Those in
suspended particles from a wastewater influent were 297-504 µg/litre
and those in the effluent, 0.1-1.22 µg/litre (Takada & Ishiwatari,
1987).
The concentrations of LAS in the estuary of the Krka River,
Croatia, were 420-780 µg/litre near municipal wastewater outlets; 50
m from the wastewater outlets, the concentrations were 7.2 µg/litre
at a depth of 0.5 m and 3.2 µg/litre at a depth of 6 m. The
concentrations in water sampled more than 50 m from the input area
were 1-2 µg/litre. The Krka River estuary was reported to be highly
stratified, with vertical transport of pollutants reduced by the
freshwater-saline boundary. The concentrations of LAS were
negatively correlated with salinity; the maximum concentration,
24 µg/litre, was detected in the surface monolayer. An increase in
the relative abundance of lower homologues of LAS (C10 and C11)
was reported in comparison with the original distribution of
homologues in the wastewater, indicating more rapid depletion of
higher homologues, possibly by biodegradation and fast settling with
particles from sewage (Terzic & Ahel, 1993).
In a comparison of the distribution of homologues of LAS in the
Tama River, Japan, with those established for active substances used
in commercial detergents, the levels of C12 and C13 LAS were
found to decrease over time and those of C10 and C11 to increase
(Hon-Nami & Hanya, 1980a). C11 was the commonest LAS homologue in
river water (Kobuke, 1985; Yoshikawa et al., 1985), and no C13 LAS
were present (Utsunomiya et al., 1980). The average chain length of
LAS in Japanese rivers was C10.9-C11.2 (Nakae et al., 1980;
Yoshimura et al., 1984a; Kobuke, 1985).
Several research groups have confirmed that such changes in
chain length occur during the environmental passage of LAS. In a
study in which the concentration of homologues of LAS was measured
quantitatively by HPLC during activated sludge treatment and lagoon
treatment of wastewater in Spain, the average chain length decreased
from C11.7 in raw material, to C11.3 in the dissolved phase of
raw wastewater, and to C10.3 in the dissolved phase of treated
effluent. A slight increase in average chain length was reported for
the solids compartment in each of these systems, adding to
laboratory findings that the longest homologues adsorb most strongly
to sediment. The reduction in average chain length in the water
compartments was environmentally significant, since shorter
homologues of LAS are less toxic to aquatic organisms. Thus, the
LC50 values for daphnia were higher for shorter homologues (>
20 mg/litre for C11 and 10 mg/litre for C11.7) (Prats et al.,
1993).
The Japanese Soap & Detergent Association (1992) reported a
decrease in LAS concentrations in the Tama River near Tokyo, Japan,
from 2.3 mg/litre in 1967 to 0.2 mg/litre in 1991. The decrease was
attributed to the development of sewage systems along the river:
sewage coverage was 26% in 1974 and 89% in 1990. This information
can be used to estimate concentrations of LAS in developing
countries with inadequate sewage systems but where detergent use is
increasing.
Low levels of LAS were reported in water from the Scheldt River
estuary and in a series of samples from the North Sea (see Table
13). The concentrations in the estuary decreased rapidly from about
0.010-0.012 mg/litre to values below the limit of analytical
detection (0.5 µg/litre) concurrently with an increase in salinity.
The concentrations decreased more rapidly than on the basis of
dilution alone, indicating that removal occurred rapidly. The
authors did not report whether the removal of LAS was related to
adsorption onto settling solids, to biodegradation, or to a
combination of the two. The concentration of LAS in samples taken
offshore was consistently below the limit of detection (Stalmans et
al., 1991).
A5.1.4 Soil and groundwater
The levels of LAS in sludge-amended soil were 0.9-1.3 mg/kg in
German soils used for agriculture. A level of 2.2 mg/kg was found in
the United Kingdom in soil that was used only for the disposal of
sludge (De Henau et al., 1986). MBAS were found at a level of
24.7 mg/kg (14.4-37.5 mg/kg) and LAS at 1.4 mg/kg (0.9-2.2 mg/kg) in
German agricultural soils that had been amended with sludge
(Matthijs & De Henau, 1987). The levels of LAS in soils near the
River Thames, United Kingdom, in 1987 to which sludge had been
applied previously were < 0.2-2.5 mg/kg. Soils that had received an
application of sludge during 1987 had levels of LAS of <
0.2-19.8 mg/kg (Holt et al., 1989).
Levels of 13-47 mg/kg were found on the surface of sludge-
amended soil in the United States in 1979; < 5 mg/kg were found at
a depth of 15-90 cm (Rapaport & Eckhoff, 1990).
A concentration of 22.4 mg/kg LAS was measured in agricultural
soil that had recently been amended with anaerobically digested
sludge. The concentration was 3.1 mg/kg six months after application
of the sludge and 0.7 mg/kg after 12 months (Prats et al., 1993).
HPLC, fluorescence detection, and mass spectrometry were used to
analyse samples of a groundwater plume which originated from an
underground discharge of sewage. It was found that 96% of the LAS
was removed from the aqueous phase during sewage treatment and an
additional 3% during infiltration with groundwater. The
concentrations in ground-water were below the detection limit of
0.01-0.02 mg/litre. The disappearance of LAS during groundwater
infiltration was calculated to follow first-order kinetics. LAS were
detected (by mass spectrometry) at only trace levels in groundwater
sampled 20-500 m down the gradient from the infiltration zone (Field
et al., 1992).
A5.1.5 Drinking-water
The concentration of LAS reported in Dutch tap-water was
0.003 mg/litre; MBAS levels were about three times higher. In
tap-water in the United Kingdom, the concentration of LAS was
0.007 mg/litre; that of MBAS was again three times higher (Waters,
1976). The concentrations of LAS in Italian well-water were below
the analytical limit of detection of 0.0084 mg/litre (Mancini et
al., 1984). LAS were not detected in Japanese drinking-water in the
1970s at a limit of detection of 0.001 mg/litre (Yushi, 1978).
A5.1.6 Biota
The concentrations of LAS in biota are shown in Table 15.
A5.2 Environmental processes that influence concentrations of
linear alkylbenzene sulfonates
A shift towards LAS of lower chain lengths has been reported in
environmental samples in comparison with the distribution of chain
lengths in raw materials. It has also been reported that about 50%
of the total LAS in samples of water is associated with either
suspended particles or dissolved organic matter. Reductions in both
the chain length and the concentration of dissolved LAS will result
in decreased aquatic toxicity (see also section 9).
A5.2.1 Changes in chain length distribution during environmental
removal of linear alkylbenzene sulfonates
The concentrations of LAS and related compounds were measured in
350 samples of water and sediment from the Mississippi River, United
States. Those in surface water were < 0.005 mg/litre. LAS in
sediment had longer chains than those in the overlying water column
(Tabor et al., 1993).
A gradual reduction in the average chain length of homologues
was observed as they passed through a wastewater treatment plant:
untreated wastewater, C12.1; treated effluent, C12; surface
water below a sewage outfall, C11.7 (Castles et al., 1989).
Isomers of C13 LAS have partition coefficients that are typically
one order of magnitude higher than those of the corresponding
isomers of the C12 LAS homologues (Amano et al., 1991).
Table 15. Total body concentrations of linear alkylbenzene sulfonates
in biota in Japan
Organism Year Location Concentration Reference
(mg/kg dry
weight)
Algae 1980-81 River < 1-368 Katsuno et al. (1983)
Pond snail 1979 River 0.4-1.81 Tanaka & Nakanishi
(Sinotaia (1981)
quadratus
histrica)
Gizzard shad 1982 Bay < 1 or < 2 Tokai et al. (1990)
(Konosirus 1983 < 0.1-0.3
punctatus)
A5.2.2 Specification of linear alkylbenzene sulfonates in
surface waters
In most programmes for monitoring LAS in the environment, the
total sample of waste or surface water is analysed, and separate
concentrations of LAS in the fractions of dissolved and suspended
solids are not determined. In a study in which these concentrations
were reported, the mean levels of dissolved LAS were 8.4 mg/litre in
raw wastewater (range, 5.6-11.4 mg/litre) and 5.5 mg/litre in the
suspended solid fraction. In the seven wastewaters studied, an
average of about 65% was present in the filtered (filtration, <
1 µm) 'dissolved' fraction and 35% in the 'solids-associated'
fraction. In treated effluent, 85% of LAS was in the dissolved
fraction and 15% in the solids-associated fraction (Berna et al.,
1993b). In wastewater treatment works, 49-63% of the LAS was in the
dissolved phase and 37-51% in the solids-associated phase (Berna et
al., 1989). In filtered (0.7 µm) wastewater containing LAS at
2.55-2.95 mg/litre, 25-30% LAS was dissolved, and the remaining
70-75% was associated with the solid phase (Cavalli et al., 1991).
The average chain length of homologues of LAS in raw wastewater
was lower in the dissolved phase (C11.2-C11.4) than in the
solids-associated phase (C11.9-C12.0). The authors reported that
39-43% of LAS was present in the dissolved phase and 57-61% in the
solids phase (Prats et al., 1993).
Humic acids extracted from sediments and soils formed strong
association complexes with LAS under environmental conditions, as
observed with fluorescence quenching techniques. The bioavailabilty
of LAS to aquatic organisms is reduced as a result of these
complexes (McAvoy et al., 1993).
A5.3 Estimation of human intake
Human daily intake has been estimated on the assumption that LAS
are taken up from drinking-water and from washing food, vegetables,
dishes, and the skin. The estimates vary from 4.5 to 14.5 mg/day
(Ikeda, 1965; Tokyo Metropolitan Government, 1974; Sterzel, 1992).
The higher figure is based on dubious assumptions about the
concentrations of LAS on vegetables, and the lower value is probably
a more realistic estimate.
The human intake of all anionic surfactants is estimated to be
0.044-0.944 mg/kg per day (Sterzel, 1992), and the maximum daily
intake of ABS, 0.14 mg/kg per day (Ikeda, 1965).
A6. KINETICS
Section summary
LAS are readily absorbed by experimental animals in the
gastrointestinal tract, are distributed throughout the body, and are
extensively metabolized. The parent compound and metabolites are
excreted primarily via the urine and faeces, although there are
marked differences between the isomers in the route of excretion.
The main urinary metabolites identified in rats are
sulfophenylbutanoic acid and sulfophenylpentanoic acid, which are
probably formed through omega-oxidation followed by ß-oxidation of
LAS, although the metabolic pathways in primates may differ.
Although few data are available, it would appear that dermally
applied LAS are not readily absorbed through the skin, although
prolonged contact may compromise the epidermal barrier and permit
more extensive absorption.
A6.1 Absorption, distribution, and excretion
After oral administration of 2 mg/animal of the calcium or
sodium salt of 14C-LAS (chain length, C12) to Wistar rats,
radiolabel was detected in plasma after 0.25 h, reaching maxima at
2 h (0.86 and 1.00 µg/g of the two salts, respectively), and then
decreasing gradually with time; the mean biological half-lives were
calculated to be 10.9 and 10.8 h, respectively. Four hours after
oral administration of the calcium or sodium salt, the concentration
of radiolabel was high in the digestive tract (especially in the
stomach: 22.56 and 31.67 µg/g as the parent compound or metabolites;
and large intestine: 43.24 and 27.26 µg/g) and in the urinary
bladder (34.89 and 16.58 µg/g). The concentrations were also high in
the liver (2.73 and 2.13 µg/g), kidney (1.19 and 1.35 µg/g), testis
(0.08 and 0.11 µg/g), spleen (1.63 and 0.16 µg/g), and lung (0.49
and 0.44 µg/g). At 48 and 168 h, there was little further change.
During the 168-h period after administration, 50% of the radiolabel
on the calcium salt was excreted in urine and 51% in faeces, and 47%
of that on the sodium salt was excreted in urine and 50% in the
faeces (Sunakawa et al., 1979).
Doses of 1 mg per 200 g body weight of two radiolabelled LAS
isomers (chain length, C12) with the benzene sulfonate moieties at
the 2 and 6 positions were administered orally and intravenously to
rats; the same dose was also administered to anaesthetized rats with
bile-duct cannulas by intravenous or intraduodenal injection.
Forty-eight hours after oral or intravenous administration, there
were marked differences in the disposition of the isomers in the
urine and faeces: most of the radiolabel associated with the 2
isomer (75.3%) was in the urine, whereas most of that on the 6
isomer (77.9%) was present in the faeces. After intravenous
administration to bile duct-cannulated rats, 88.6% of the 2 isomer
was recovered in the urine, whereas 83.1% of the 6 isomer was in the
bile. Studies of absorption after intraduodenal administration
showed that both isomers were extensively absorbed within 6 h
(Rennison et al., 1987).
After a dose of 1.2 mg 35S-LAS in aqueous solution was
administered by gavage to bile duct-ligated rats, 89% was absorbed
from the gastro-intestinal tract, as seen by the presence of
radiolabel recovered in urine. Absorption probably occurred mainly
via portal venous blood, since only 1.6% was recovered in the
lymphatic system. When the same dose was administered to bile
duct-cannulated rats, 46% of the radiolabel was recovered in urine,
29% in faeces, and 25% in bile after 90 h. Enterohepatic circulation
was determined in a study in which the bile from one rat was
transmitted to the intestine of another through a cannula; all of
the radioactive LAS excreted in the bile was reabsorbed. In a
separate study, 40-58% of single oral doses of 35S-LAS ranging
from 0.6 to 40.0 mg was excreted in the urine and 39-56% in the
faeces within 72 h of administration (Michael, 1968).
The excretory pattern of 14C-sodium dodecylbenzene sulfonate
was examined in male rats administered a concentration of 1.4 mg/kg
of diet daily for five weeks. The total intake was 1213 µg/rat, of
which 81.8% was excreted during the dosing period, with 52.4% in the
faeces and 29.4% in the urine. After a further week on a normal
diet, however, only 7.8% of the estimated residual amount was found
in excreta. Of a single intraperitoneal injection of 0.385 mg
14C-sodium dodecylbenzene sulfonate/rat (2.26 mg/kg body weight),
84.7% was eliminated within the first 24 h and 94.5% within 10 days
(Lay et al., 1983).
LAS were not detected in the uterus of pregnant ICR mice
administered a single oral dose of 350 mg/kg body weight on day 3 of
gestation (Koizumi et al., 1985).
14C-LAS (chain length, C10-C14, predominantly C11,
C12, and C13) were applied at 250 µg/7.5 cm2 in water to
clipped dorsal skin of rats; the treated area was washed after
15 min, and the animals were restrained from grooming. Most of the
radiolabel was rinsed off, but some of the 14C-LAS
(11 ± 4 µg/cm2) were detected on the treated area; none were
detected in urine or faeces 24 h after the application. In an
accompanying study in vitro, there was no measurable penetration of
14C-LAS (chain length, C12) through isolated human epidermis or
rat skin 24 or 48 h after application (Howes, 1975).
A mixture of 35S-LAS and white petrolatum (29 mg/0.3 ml) was
applied to a 4-cm2 area of the dorsal skin of guinea-pigs, and 24
h after the application about 0.1% of the applied dose was found in
urine and about 0.01% in blood and the main organs. After dermal
application of the same dose to rats and guinea-pigs, the
concentration of 35S in the liver was 9.7 µg/g equivalent of LAS
in rats and about 0.4 µg/g in guinea-pigs (Hasegawa & Sato, 1978).
After a single oral administration of 150 mg/kg 14C-LAS (mean
relative molecular mass, 349) in aqueous solution to rhesus monkeys
(Macaca mulatta), plasma concentrations of radiolabel reached a
maximum equivalent to 41.2 µg/ml at 4 h and then declined over
6-24 h, with a biological half-life of about 6.5 h. The observed
peak plasma concentration of radioactivity (33.6 µg/ml) and the
biological half-life (about 5 h) after seven consecutive daily oral
administrations of 30 mg/kg body weight were similar to those found
after a single administration. The highest concentration of 14C
(238.6 µg/g) was found in the stomach 2 h after the last dose.
Concentrations were also high in the intestinal tract (108 µg/g),
kidney (135.6 µg/g), and liver (64.8 µg/g) and were moderately high
in the lung (19.8 µg/g), pancreas (17.7 µg/g), adrenal glands
(20.6 µg/g), and pituitary gland (17 µg/g). At 24 h, the
concentrations were higher in the intestinal tract (255.4 µg/g) and
liver (10.5 µg/g) than in plasma (2.4 µg/g), whereas those in most
tissues were lower than those in plasma, indicating that there is no
specific accumulation or localization of LAS and their metabolites
in these tissues. After seven subcutaneous doses of 1 mg/kg per day
of 14C-LAS, most of the radiolabel remained in the skin; the
concentration was generally highest at the injection site
(113.96 µg/g). The levels of radiolabel were also high in the
intestinal tract (2.41 µg/g), kidney (1.83 µg/g), lung (2.45 µg/g),
spleen (2.43 µg/g), thyroid (1.24 µg/g), and pituitary (1.00 µg/g)
at 2 h. The concentration in most tissues was generally lower at
4 h, except in the intestinal tract (3.50 µg/g), liver (1.74 µg/g),
and kidney (1.92 µg/g). The high level of radiolabel in the
intestinal tract probably indicates biliary excretion. The average
rates of excretion of radiolabel in urine and faeces during 120 h
after administration of single oral or subcutaneous doses of
14C-LAS to male and female rhesus monkeys are shown in Table 16.
In animals of each sex, radiolabel was excreted primarily in the
urine after either route of administration (Cresswell et al., 1978).
When sodium 35S-dodecylbenzenesulfonate (3.3 mmol/kg body
weight) was administered in the diet to young pigs, at least 35% of
the dose was absorbed through the intestinal tract. After 40 h,
30-40% of the dose had been excreted in urine and > 60% in faeces.
The concentration of radiolabel after 200 h was relatively high in
bristles and bones and low in liver, kidney, and spleen
(quantitative data not presented). After 10 weeks, traceable amounts
of 35S (0.05% of the administered dose) were found in bristles,
bones, skin, lung, and brain (Havermann & Menke, 1959).
Table 16. Excretion of 14C-linear alkyl benzene sulfonates in
rhesus monkeys
Route of administration Sex Concentration (%)
Urine Faeces
Oral (30 mg/kg body weight) Male 68.3 25.9
Female 74.0 20.3
Subcutaneous (1 mg/kg) Male 63.8 12.5
Female 64.3 9.2
From Cresswell et al. (1978); values are average rates of excreted
radioactivity during the 120-h period after a single dose.
A6.2 Biotransformation
The main metabolites isolated from the urine of rats
administered 35S-LAS orally were probably a mixture of sulfophenyl
butanoic (I) and sulfophenyl pentanoic acids (II):
CH3-CH-CH2-COOH CH3-CH-CH2-CH2-COOH
| |
O O
| |
SO3H SO3H
(I) (II)
The material used in the experiment was a mixture of C10-C14 LAS
(mainly C11, C12, and C13). The compounds in this mixture are
probably degraded by omega-oxidation, followed by catabolism through
a ß-oxidation mechanism to form the above metabolites, with
excretion of four or five carbons in the urine (Michael, 1968).
After oral administration of the calcium or sodium salt of
14C-LAS to rats, two metabolites were detected in urine and four
in faeces by thin-layer chromatography. The two urinary and two of
the faecal metabolites were believed to be compounds similar to
metabolites (I) and (II) previously identified by Michael (1968)
(Sunakawa et al., 1979).
Thin-layer chromatography of urine extracts after oral or
sub-cutaneous administration of 14C-LAS to rhesus monkeys showed
only trace amounts of the unchanged compound, and five metabolites
more polar than LAS were detected. These metabolites have not been
identified. Incubation of urine samples with ß-glucuronidase or
sulfatase did not affect the components, which were therefore
probably not present as the corresponding conjugates (Cresswell et
al., 1978).
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 values for sodium salts of LAS are
404-1470 mg/kg body weight in rats and 1259-2300 mg/kg body weight
in mice. LAS irritate skin and eyes.
Minimal effects, including biochemical alterations and
histopathological changes in the liver, were reported in subchronic
studies in rats administered LAS in the diet or drinking-water at
concentrations equivalent to a dose of about 120 mg/kg body weight
per day. Although ultrastructural changes in liver cells were
observed at lower doses in one study, these changes appeared to be
reversible. Effects have not been seen at similar doses in other
studies, but the organs may have been examined more closely in this
study. Reproductive effects, including decreased pregnancy rate and
litter loss, have been reported in animals administered doses >
300 mg/kg body weight per day. Histopathological and biochemical
changes have been observed following long-term dermal application on
rats of solutions of LAS at concentrations > 5% and after 30 days'
dermal application on guinea-pigs of 60 mg/kg body weight. Repeated
dermal application of solutions containing > 0.3% LAS induced
fetotoxic and reproductive effects, although these doses also
induced maternal toxicity.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of LAS in experimental animals, owing to the
small number of animals used, low or insufficient doses tested, the
absence of a maximal tolerated dose, and limited histopathological
examination. The limited studies available in which animals were
administered LAS orally, however, provide no evidence of
carcinogenicity.
Limited data also indicate that LAS are not genotoxic in vivo
or in vitro.
A7.1 Single exposures
The LD50 values for the sodium and magnesium salts of LAS
given orally, subcutaneously, or intravenously are summarized in
Table 17. Rats appear to be more sensitive than mice to LAS,
regardless of the route of exposure. The LD50 values for LAS given
orally were 1259-3400 mg/kg body weight in mice and 404-1900 mg/kg
body weight in rats. Differences were seen according to the sex,
strain, and age of the animals and the test material.
Table 17. Acute toxicity of linear alkylbenzene sulfonates
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
Mouse
NR NR Oral 2170 60% active ingredient Yanagisawa et
al. (1964)
DD M Oral 2300 34.55% solution Tiba (1972)
ddY M Oral 1665 Purified Kobayashi et
ICR-JCL F Oral 1950 Purified al. (1972)
M Oral 1250 Commercial soln, 19.0% Kuwano et al.
F Oral 1540 Commercial soln, 19.0% (1976)
M Oral 1370 Commercial soln, 17.1%
F Oral 1560 Commercial soln, 17.1%
M Oral 2160 99.5% active ingredient Ito et al. (1978)
of C10-C13
F Oral 2250 99.5% active ingredient
of C10-C13
M Oral 2600 Magnesium salt of above
F Oral 3400 Magnesium salt of above
M s.c. 1250 99% active ingredient Ito et al. (1978)
of C10-C13
F s.c. 1400 99% active ingredient
of C10-C13
M s.c. 1529 Magnesium salt of above
F s.c. 1550 Magnesium salt of above
Table 17 (contd)
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
ICR-JCL M i.v. 207 99% active ingredient
(contd) of C10-C13
F i.v. 298 99% active ingredient
of C10-C13
M i.v. 98 Magnesium salt of above
F i.v. 151 Magnesium salt of above
NR NR i.v. 120 Yanagisawa et al.
(1964)
Rat
FDRL M,F Oral 650 Nominal chain length, Oser & Morgareidge
C12 (range C9-C15) (1965)
Wistar
6 w M Oral 873 Purified Kobayashi et
6 w F Oral 760 al. (1972)
10 w M Oral 404
10 w F Oral 409
M Oral 1460 99.5% active ingredient Ito et al. (1978)
of C10-C13
F Oral 1470 99.5% active ingredient
of C10-C13
M Oral 1900 Magnesium salt of above
F Oral 1840 Magnesium salt of above
Table 17 (contd)
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
CRJ-SD M s.c. 840 99.5% active ingredient
of C10-C13
F s.c. 810 99.5% active ingredient
of C10-C13
M s.c. 710 Magnesium salt of above
F s.c. 730 Magnesium salt of above
M i.v. 119 99.5% active ingredient
of C10-C13
F i.v. 126 99.5% active ingredient
of C10-C13
M i.v. 27.2 Magnesium salt of above
F i.v. 35.0 Magnesium salt of above
NR, not reported; M, male; F, female; s.c., subcutaneous; i.v., intravenous;
w, weeks
a As active ingredient
b Sodium salt, unless specifically indicated
The main clinical signs observed after oral administration of
doses near or greater than the LD50 consisted of reduced voluntary
activity, piloerection, diarrhoea, and weakness. Diarrhoea was more
severe in rats than mice (Kobayashi et al., 1972). Convulsions,
torsion, and paralysis of the hind limbs were also observed in some
of mice (Kobayashi et al., 1972; Kuwano et al., 1976). Death usually
occurred within 24 h of administration. Transient cardiac arrest,
dyspnoea, cyanosis, respiratory collapse, and death occurred during
intravenous injection (Ito et al., 1978).
At autopsy, hyperaemia and haemorrhage of the stomach and
intestine, bloating of the intestine with thinning of its wall, and
congestion of some internal organs were the main macroscopic
findings; histological examination showed congestion and epithelial
degeneration of the gastrointestinal mucosa (Kobayashi et al., 1972;
Kuwano et al., 1976; Ito et al., 1978).
A7.2 Short-term exposure
A7.2.1 Mouse
In a study of the toxicity of a commercial preparation of LAS
(17.1% active ingredient), 44 male and 16 female C57Bl TW mice were
given subcutaneous injections according to the following schedule:
0.02 ml of 1% of the preparation for 10 consecutive days from the
day of birth, 0.04 ml of the same solution for the following 10
days, 0.02 ml of a 10% solution five times over the next 10 days,
and 0.04 ml of the same solution every other day for a further 30 or
60 days. Eight males and six females served as untreated controls.
Epilation and dermatitis usually occurred in animals given
continuous injections of the test material. Adhesions between some
organs, most frequently between the spleen and kidney, were observed
in those receiving injections from the day of birth. Neither the
growth nor the survival of the animals was affected. Although the
weights of the liver, kidney, and spleen were significantly
increased in animals receiving treatment for 60 days,
histopathological examination of the liver, kidney, adrenal glands,
and thyroid by light and electron microscopy showed no evidence of
toxicity (Kikuchi, 1978).
A7.2.2 Rat
A7.2.2.1 Administration in the diet
Groups of five male Wistar rats were fed diets containing LAS
(60% active ingredient; chain length distribution: 10.6% C10, 34.1%
C11, 27.7% C12, 19.0% C13, 8.7% C14) at a concentration
of 0, 0.6, 1.2, or 1.8% (equivalent to 180, 360, or 540 mg/kg body
weight per day) for two and four weeks, and lipids in serum and
liver were analysed. Body weight gain was suppressed in the group
receiving 1.8% at four weeks, and the relative liver weight was
increased at two weeks and thereafter in the groups receiving 1.2
and 1.8%. The levels of triglyceride and total lipids in the serum
had decreased markedly at two weeks in all the experimental groups,
and the levels of phospholipids and cholesterol in the serum had
decreased significantly at two weeks in the groups given 1.2 and
1.8%. These changes were less apparent at four weeks, but
triglyceride, phospholipid, and cholesterol levels in serum were
significantly decreased in the group given 1.8%. Significant
increases in triglyceride levels were seen in the liver after two
weeks in the groups receiving 0.6 and 1.8%, and in cholesterol
levels in the group given 0.6% (Yoneyama & Hiraga, 1977).
Technical-grade sodium LAS (87.9% active ingredient; chain
length distribution: 1.8% C10, 43.2% C11, 32.2% C12, 5.3%
C14, 1.5% C15) were fed to five groups of 10 weanling
Sprague-Dawley rats of each sex at a dietary level of 0, 0.02, 0.1,
or 0.5% (equivalent to 8.8, 44, or 220 mg/kg body weight per day)
for 90 days. No adverse effects were found on survival, growth, food
conversion efficiency, haematological values, urinary analytical
values, or absolute or relative organ weights. There were no gross
or microscopic histological changes attributable to ingestion of the
test material (Kay et al., 1965).
Technical-grade LAS (normal chain length, C12; range,
C9-C15; mean relative molecular mass, 346) were fed to three
groups of weanling FDRL rats, each consisting of 15 males and 15
females, at a dose of 0, 0.05, or 0.25 g/kg body weight per day for
12 weeks. No adverse effects were noted on survival, behaviour,
growth, food conversion efficiency, haematological measurements,
blood chemistry, urine analytical values, organ weights, or gross or
microscopic appearance, except for a slight increase in liver weight
in females given 0.25 g/kg body weight per day (Oser & Morgareidge,
1965).
A diet containing LAS at a concentration of 1.5% (equivalent to
750 mg/kg body weight per day) or a control diet was given to groups
of five male Wistar rats for 2, 4, or 12 weeks. LAS depressed body
weight gain, and the relative liver weight was significantly
increased after two weeks of treatment. The activities of alkaline
phosphatase and glutamate-pyruvate transaminase in serum were
significantly increased at each observation period, and cholesterol
and protein levels were significantly decreased by four weeks. In
the liver, the activities of glucose-6-phosphatase and
glucose-6-phosphate dehydrogenase were decreased, and the activity
of isocitrate dehydrogenase was increased at each observation point.
Enzymatic examination of the renal cortex showed decreased
activities of glucose-6-phosphatase and 5'-nucleotidase at each
observation period, an increase in the activity of lactate
dehydrogenase at 12 weeks, and increased activity of isocitrate
dehydrogenase at 2 and 4 weeks. In the renal medulla, the activity
of Na,K-ATPase was decreased, that of lactate dehydrogenase was
increased at 12 weeks, and that of isocitrate dehydrogenase was
decreased at 2 weeks but increased at 12 weeks (Ikawa et al., 1978).
Groups of five male Wistar rats were given a diet or
drinking-water containing LAS at a concentration of 0.4% (diet:
200 mg/kg body weight per day; drinking-water: 560 mg/kg per day)
for two weeks in order to determine the effects of LAS on the
synthesis of lipids in the liver. Lipids were thus measured in the
liver, and uptake of acetate-1-14C by the lipids was examined.
Decreases in the levels of total lipids and triglyceride were seen
in both groups, but there were no significant changes in
phospholipid or cholesterol levels. Uptake of acetate-1-14C by
lipids in the liver was increased in both groups; uptake of
phospholipids and triglycerides tended to increase, and that of
phospholipids increased significantly in rats given LAS in the diet
(Yoneyama et al., 1978).
A7.2.2.2 Administration by gavage
Groups of 12 male and 12 female Sprague-Dawley rats were given
the magnesium salt of LAS by gavage at a dose of 0, 155, 310, or
620 mg/kg body weight for one month. Body weight gain was depressed
in males and females at 620 mg/kg body weight; one male and two
females at this dose also had diarrhoea and loss of appetite and
subsequently died. Haematological examination revealed significant
decreases in haemoglobin concentration and haematocrit in males at
620 mg/kg body weight. A significant increase in the activity of
alkaline phosphatase and a significant decrease in calcium levels
were seen in males at 310 or 620 mg/kg body weight; and a
significant increase was seen in the activity of glutamate-oxalate
transminase and a significant decrease in protein levels in females
at those doses. Females at all doses had a significant decrease in
calcium levels. At the highest dose, females had a significant
increase in the activity of alkaline phosphatase, a significant
decrease in cholesterol level, and increased weight of the liver,
but the weight of the thymus decreased. The weight of the heart
decreased in females at 310 and 620 mg/kg body weight. Histological
examination of the liver revealed no abnormalities (Ito et al.,
1978).
Groups of 12 male and 12 female Sprague-Dawley rats were given
the sodium salt of LAS (chain length distribution: < 0.1% C9,
10.1% C10, 33.7% C11, 31.0% C12, 25.1% C13) at a dose of 0,
125, 250, or 500 mg/kg body weight by gavage once a day. Diarrhoea
was observed in the group receiving 500 mg/kg, and soft faeces were
observed in the other two groups. Body weight gain was depressed in
males of all groups and in females at 500 mg/kg. Haematological
examination revealed no abnormalities. Serum analysis revealed a
significant increase in the activity of alkaline phosphatase in
males at 500 mg/kg, a significant decrease in calcium levels in
males of all groups, significant increases in the activity of
gluatamate-oxalate transaminase and in blood-urea nitrogen in
females at 500 mg/kg, a significant decrease in calcium level in
females at 250 or 500 mg/kg, and significantly decreased protein and
albumin levels in females of all groups. At 500 mg/kg, the weights
of spleen and heart were significantly decreased in males; in
females, liver weights were increased but the weights of the heart
and thymus were decreased. No histological abnormalities were seen
in the liver (Ito et al., 1978).
A7.2.2.3 Dermal application
Continued, repeated, or extremely high doses of LAS, like other
detergents, compromise the integrity of the skin so that penetration
occurs, causing a variety of anomalies. As the design of the
following two studies was not adequate, the observations are not
considered to be relevant to human risk assessment.
Application of 2 ml of a commercial preparation of LAS (23.4%
active ingredient) to the thoracic skin of six male Wistar rats
resulted in redness and wrinkling of the skin after 24 h. The
redness then increased, the corium was lacerated, and bleeding
occurred. These effects were most severe after five to seven days,
but after a further 10 days the skin began to recover. Six rats died
after 19 days, probably because of the extremely high dose used. The
livers of three rats were examined by electron microscopy after
three and 30 days and the findings compared with those in the
control group. At three days, marked changes were seen in the
components of the liver parenchymal cells, such as separation of the
intracellular space, appearance of dark cells with high electron
density, dysmorphia of mitochondria, extracellular prolapse of
mitochondria, proliferation of rough-surfaced endoplasmic reticulum,
lysosome proliferation, and a decrease in the prevalence of fatty
droplets. At 30 days, many liver parenchymal cells were filled with
abnormally divided and proliferated mitochondria, and an abnormal
increase in smooth-surfaced endoplasmic reticula was noted. There
were no granules of glycogen or fatty droplets. Structures
resembling necrotic cells were also observed (Sakashita et al.,
1974).
A commercial preparation of LAS (23.4% active ingredient) was
applied dermally to male rats (number not given) at a dose of
5 mg/kg body weight active ingredient once a day for 30 days, and
the liver was examined by electron microscopy. Degeneration was seen
in part of the liver, in the form of atrophy and high density.
Intra-mitochondrial deposits and deformation of the Golgi apparatus
were also noted (Sakashita, 1979).
A7.2.2.4 Subcutaneous injection
A commercial preparation of LAS (27% active ingredient) was
given subcutaneously to groups of five male and five female Wistar
rats at a dose of 2 ml/kg body weight per day of a 0, 0.02, 0.2, or
2% solution of the preparation for 25 or 50 days. Rats receiving the
2% solution had reduced body weight gain, increased weights of
liver, kidney, and spleen, a low serum albumin:globulin ratio, low
serum protein, and reduced ornithine aminotransferase activity in
the liver (Hayashi, 1980).
A7.2.3 Guinea-pig
Twelve guinea-pigs were treated daily for 30 days with a
solution of LAS in distilled water equivalent to 60 mg/kg body
weight, which was applied to a 4-cm2 area of clipped dorsal skin.
Twelve controls received acetone at 0.5 ml. The animals were
sacrificed after 30 days, and samples were taken from liver and
kidney and homogenized for determination of enzymes, lipid
peroxidation, glutathione, and protein. The activities of
ß-glucuronidase, gamma-glutamyl transpeptidase, 5-nucleotidase, and
sorbitol dehydrogenase were increased in liver and kidney. Lipid
peroxidation was increased in kidney but not in liver, and the
glutathione content was unchanged in both organs. Extensive fatty
changes were found in hepatic lobules, with dilation of sinusoids;
tubular lesions were found in the kidney, predominantly in the
proximal and distal portions (Mathur et al., 1992).
A7.2.4 Monkey
LAS (chain length, C10-C13) were given to four groups of
three male and three female rhesus monkeys at a daily dose of 0, 30,
150, or 300 mg/kg body weight orally simultaneously with a dose of
0, 0.1, 0.5, or 1.0 mg/kg per day subcutaneously, for 28 days.
Monkeys that received 300 mg/kg orally and 1.0 mg/kg subcutaneously
vomited frequently, usually within 3 h of administration; these
animals and those given 150 mg/kg orally and 0.5 mg/kg
subcutaneously also had an increased frequency of loose or liquid
faeces. Fibrosis at the injection sites was reported in all test
animals, and the incidence and severity were related to dose.
Treatment had no effect on ophthalmoscopic, haematological, or
urinary parameters, on organ weight, or on histopathological
appearance (Heywood et al., 1978).
The studies of short-term exposure to LAS are summarized in
Table 18.
Table 18. Summary of studies of short-term exposure to linear alkylbenzene sulfonates (LAS)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Mouse, C57Bl TW LAS (a.i. 17.1%) s.c. 63 or 76 mg/kg Abdominal adhesions, increased Kikuchi (1978)
44 M, 16 bw/day, 60-90 days weights of liver, kidney, and
spleen after 60-day treatment;
no histopathological changes
in liver, kidney, adrenal or thyroid
glands
Rat, Wistar LAS, C10-C14 Diet 0, 0.6, 1.2, 1.8%, Decreased serum triglyceride, Yoneyama & Hiraga
5 M (a.i. 60%) 4 weeks total lipids, phospholipids, and (1977)
cholesterol; increased relative
liver weight at 1.2 and 1.8%;
suppression of body weight gain
at 1.8%
Rat, SD LAS, C10-C15 Diet 0, 0.02, 0.1, 0.5%, No adverse effects Kay et al. (1965)
10 M, 10 F (a.i. 8-9%) 90 days
Rat, FDRL LAS, C9-C15 Diet 0, 0.05, 0.25 g/kg bw Slight increase in liver weight in Oser & Morgareidge
15 M, 15 F (a.i. 39.5%) per day, 12 weeks females at high dose (1965)
Rat, Wistar LAS (NS) Diet 1.5%, 24 weeks Increased activities of serum, Ikawa et al. (1978)
4 M hepatic, and renal enzymes;
depressed body weight gain;
increased relative liver weight
Table 18 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, CRJ-SD LAS, Na, C10-C13 Gavage 125, 250, 500 mg/kg Altered serum enzyme activity Ito et al. (1978)
12 M, 12 F (a.i. 99.5%) bw per day, 1 month and calcium levels at high doses;
decreased serum protein and
albumin levels in all treated
females; decreased spleen and
heart weights in males at highest
dose; increased liver weight and
decreased heart and thymus
weights in females at highest dose;
no histopathological abnormalities
in liver
Rat, CRJ-SD LAS Mg, C10-C13 Gavage 155, 310, 620 mg/kg Altered haemoglobin, haematocrit, Ito et al. (1978)
12 M, 12 F (a.i. 96.9%) bw per day, 1 month serum enzyme activities, calcium
level at high doses; depressed
body weight gain at highest dose;
increased liver weight and
decreased heart and thymus
weights in females at highest dose;
no histopathological abnormalities
in liver
Rat, Wistar LAS detergent Dermal 2 ml/animal Skin irritation; liver parenchymal Sakashita et al.
6 M (a.i. 23.4%) 3.5 × 4.5 cm, 30 days changes with necrotic cells; no (1974)
glycogen granules or fat droplets
Rat, Wistar LAS detergent Dermal 5 mg/kg bw, once/ Degenerative changes in liver Sakashita (1979)
6 M (a.i. 23.4%) day, 30 days
Table 18 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar LAS detergent s.c. 0, 0.02, 0.2, 2%, Depressed body weight gain; Hayashi (1980)
5 M, 5 F (a.i. 27%) 2 ml/kg bw per day, increased weights of liver, kidney,
50 days and spleen; and altered hepatic
enzyme activities at highest dose
Rat, Wistar LAS Drinking- 0.4%, 2 weeks Decreased hepatic total lipids and Yoneyama et al.
8 M, 8 F (a.i. 60.2%) water triglycerides; increased uptake of (1978)
acetate-1-14C, phospholipids, and
triglycerides
Guinea-pig LAS (NS) Dermal 60 mg/kg bw, Altered hepatic and renal enzyme Mathur et al. (1992)
12 M, 12 F 30 days on 4 cm2 activities; fatty degeneration in
liver; renal tubular lesions
Rhesus monkey LAS C10-C13 Gavage 0.30, 150, 300 mg/kg Vomiting and diarrhoea; no Heywood et al.
3M, 3F (a.i. 20.5%) s.c. 0, 0.1, 0.5, 1.0 mg/kg ophthalmic, haematological or (1978)
bw per day, 28 days urinary changes; no effect on
organ weights; no histopatho-
logical changes
M, male; F, female; a.i., active ingredient; s.c., subcutaneous
A7.3 Long-term exposure; carcinogenicity
A7.3.1 Mouse
A7.3.1.1 Administration in the diet
Groups of eight or nine ICR mice were given diets containing LAS
at a concentration of 0.6 or 1.8% for nine months (corresponding to
intakes of 500 and 1000 mg/kg body weight per day). There was no
reduction in body weight gain at either dose, but the weight of the
liver was increased in both males and females. Significant decreases
were seen in the activities of hepatic lactate dehydrogenase and
renal acid phosphatase in male mice (Yoneyama et al., 1976).
A7.3.1.2 Administration in the drinking-water
Drinking-water containing 100 ppm LAS (corresponding to 20 mg/kg
body weight per day) was supplied to ddy mice (sex and number not
stated) for six months, and they were then allowed to recover for
two months. Mice were killed for electron microscopy of the liver at
one, two, three, and six months and after the two-month recovery
period. Hepatic damage was observed at one and six months,
consisting of the disappearance of the nucleolus, atrophy of the
Golgi apparatus, degranulation of rough-surfaced endoplasmic
reticulum, degeneration of mitochondria, and increased numbers of
primary and secondary lysosomes including autophagic vacuoles with a
myelinated core. In mice examined after the two-month recovery
period, some hepatic damage was seen, which was characterized by
changes in mitochondrial structure and the presence of numerous fat
droplets. Other cellular effects had reversed, indicating that the
liver cells had recovered (Watari et al., 1977). Because an
extremely high dose was used in this study, the observations have
little relevance to human risk.
Groups of eight or nine ICR mice were given water containing LAS
at a concentration of 0.07, 0.2, or 0.6% for nine months,
corresponding to intakes of about 0.1, 0.25, or 0.6 g/kg body weight
per day for males and 0.1, 0.25, or 0.9 g/kg body weight per day for
females. Body weight gain was depressed in males and females at
0.6%, and there were dose-related increases in liver weight in
females in all dose groups. In the group given 0.6% LAS, the
activity of hepatic glutamate-oxalate transaminase was significantly
decreased in males and the activity of renal glucose-6-phosphatase
was decreased in animals of each sex (Yoneyama et al., 1976).
A7.3.2 Rat
A7.3.2.1 Administration in the diet
LAS (98.1% active ingredient; chain length distribution,
C10-C14) were fed to four groups of Charles River weanling rats,
each consisting of 50 males and 50 females, at a dietary level of 0,
0.02, 0.1, or 0.5% (corresponding to 10, 50, or 250 mg/kg body
weight per day) for two years. No adverse effects on growth or feed
conversion efficiency were observed. Five males and females from
each group were killed at 8 and 15 months, and all survivors at 24
months; all animals were necropsied, haematological values were
determined, and tissues were taken for histological examination. No
consistent change was seen that could be considered a toxic
response. Animals that showed significant loss of weight,
development of tumours, or other evidence of abnormalities were also
sacrificed and their tissues preserved for study. The incidences of
tumours and of common incidental diseases were similar in all
dietary groups (Buehler et al., 1971).
Diets containing technical-grade LAS (chain length distribution:
10.6% C10, 34.1% C11, 27.7% C12, 19.0% C13, 8.7% C14; mean
relative molecular mass, 345.8) at a concentration of 0, 0.07, 0.2,
0.6, or 1.8% were given to groups of 10 Wistar rats of each sex for
six months. The group given 1.8% had diarrhoea, markedly depressed
growth, increased caecal weight, and marked degeneration of renal
tubules. The group given 0.6% had slightly depressed growth,
increased caecal weight, increased serum alkaline phosphatase
activity, decreased serum protein, and degeneration of renal
tubules. The group given 0.2% had increased caecal weight and slight
degeneration of renal tubules. The group given 0.07%, corresponding
to about 40 mg/kg body weight per day, showed no effects
attributable to treatment (Yoneyama et al., 1972).
Groups of eight male and eight female Wistar rats were given
diets containing LAS at a concentration of 0, 0.6, or 1.8% for nine
months, corresponding to intakes of 230 or 750 mg/kg body weight per
day for males and 290 or 1900 mg/kg body weight per day for females.
In rats given 1.8% LAS, body weight gain was reduced in both males
and females. Haematological examination revealed a significant
decrease in leukocytes in males at 0.6% and significant decreases in
mean corpuscular volume and mean corpuscular haemoglobin in females
at 1.8%. The activity of glutamate-oxalate transferase and the
levels of cholesterol and albumin in serum were significantly
decreased and the activity of alkaline phosphatase and the levels of
blood-urea nitrogen and cholinesterase were significant increased in
males at 1.8%; females at that dose had a significant decrease in
cholesterol level and a significant increase in alkaline phosphatase
activity. At 0.6%, males had a significant decrease in glucose
level, and females had a significant decrease in the activity of
glutamate-pyruvate transaminase. The caecal weight of male rats and
the liver and caecal weights of female rats at 1.8% were
significantly increased. Enzymatic examination of the liver revealed
dose-related decreases in the activities of glucose-6-phosphate
dehydrogenase and lactate dehydrogenase in male rats. At 1.8%, males
had significantly decreased activities of glucose-6-phosphatase,
glutamate-pyruvate transaminase, and glutamate-oxalate transaminase
and a dose-related decrease in the activity of glucose-6-phosphate
dehydrogenase; females had significantly decreased activities of
glucose-6-phosphatase and glutamate-oxalate transaminase. Enzymatic
examination of the kidneys of females at 1.8% showed significantly
decreased activities of glucose-6-phosphatase, Na,K-ATPase, and
lactate dehydrogenase (Yoneyama et al., 1976).
Groups of 50 male and 50 female Wistar weanling rats were given
diets containing LAS (10.6% C10, 34.1% C11, 27.7% C12, 19.0%
C13, 8.7% C14; mean relative molecular mass, 345.8) at a
concentration of 0, 0.04, 0.16, or 0.6%. In each group, five rats of
each sex were fed for one, three, six, or 12 months, and groups of
15 rats of each sex were fed for 24 months or more. The group fed
0.6% had slightly increased liver and caecal weights, and increased
activity of glutamate-pyruvate transaminase and alkaline phosphatase
in serum. The treatment had no adverse effect on the intake of food,
body weight gain, general condition, mortality, or mean survival. On
the basis of these results, it was concluded that a diet containing
LAS at a concentration of 0.6% (300 mg/kg body weight per day) had
no adverse effects on the rats (Yoneyama et al., 1977).
Groups of 50 male and 50 female Wistar rats were fed LAS
(C10-C14) in the diet at a concentration of 0, 0.04, 0.16, or
0.6% and were then submitted to a detailed histopathological
examination. After one month, proliferation of hepatic cells in the
liver, slight swelling of the renal tubules, and narrowing of the
tubular lumen were found in treated animals. Since these alterations
later disappeared, they were considered to represent adaptation to
the administration of LAS. No histological lesions were seen in the
organs of rats that were fed for 24 months or more that could be
attributed to treatment. Various types of tumour were observed in
both treated and control rats but did not appear to be due to LAS
(Fujii et al., 1977).
A7.3.2.2 Administration in the drinking-water
Groups of eight to nine male and eight to nine female Wistar
rats were given LAS at a concentration of 0, 0.07, 0.2, or 0.6% in
drinking-water for nine months. Body weight gain was suppressed in
males given 0.6%. Haematological examination revealed no significant
change in any of the experimental groups, but a dose-related
decrease in cholesterol level was seen in males. No change in organ
weight was seen that was due to administration of LAS. Significant
decreases in the activities of glutamate-oxalate transaminase and
lactate dehydrogenase were seen in males at 0.2% and a dose-related
increase in the activity of glutamate-oxalate transaminase in
females. A significant decrease in renal Na,K-ATPase was seen in the
group given 0.2%. The dose of 0.07% corresponded to intakes of LAS
of 50 and 120 mg/kg body weight per day in males and females, and
the dose of 0.2% to intakes of 120 and 170 mg/kg body weight per
day, respectively (Yoneyama et al., 1976).
A commercial preparation of LAS (27% active ingredient) was
given to groups of five male Wistar rats in drinking-water at a
concentration of 0, 0.3, 3, 30, or 300 ppm (corresponding to 0.007,
0.07, 0.7, or 7 mg/kg body weight per day) for 60, 124, or 181 days.
Although a reduction in body weight gain, changes in blood
biochemistry, and increased ornithine aminotransferase activity in
the liver were noted in some animals, they were not proportional to
dose or feeding period (Hayashi, 1980).
Groups of 20 male Wistar rats were given water containing LAS
(34.55% commercial solution) at a concentration of 0, 0.01, 0.05, or
0.1% for two years, the highest dose corresponding to an intake of
about 200 mg/kg body weight per day. No changes attributable to the
administration of LAS were seen in terms of growth, mortality, the
weights of major organs, or histopathological appearance (Tiba,
1972).
A group consisting of 62 male and 62 female Wistar rats was
given drinking-water containing LAS (mean relative molecular mass,
348; 38.74% active ingredient) at a concentration of 0.1%
(corresponding to 140 mg/kg body weight per day), and a control
group of 37 male and 37 females was given normal drinking-water.
Five to 12 rats in the experimental group and three to 12 rats in
the control group were killed at 3, 6, 12, and 18 months, and all
surviving animals were killed at 24-26 months. Administration of LAS
had no effect on the intake of water, mortality, body weight gain,
or general condition. Histopathological examination revealed
atrophy; fatty changes were found in hepatic cells in treated
animals at six months, when there were also significant increases in
the activities of glutamate-oxalate and glutamate-pyruvate
transaminases and in the level of bilirubin. LAS had no effect on
haematological parameters (Endo et al., 1980).
A group of 60 male and 60 female rats (strain not specified)
received drinking-water containing 0.01% of a preparation containing
51% LAS for 100 weeks; a similar group was untreated. No detrimental
effects on body weight and no pathological effects, including
tumours, were reported (Bornmann et al., 1963).
A7.3.2.3 Administration by gavage
Groups of 20 male and 20 female Sprague-Dawley rats were given a
solution of a magnesium salt of LAS at doses of 10, 75, 150, or
300 mg/kg body weight per day by gavage for six months. Body weight
gain was suppressed, and slight decreases were observed in serum
protein, albumin, and calcium ion level, but the changes were within
the physiological range (Ito et al., 1978).
A7.3.2.4 Dermal application
A dose of 0.1 ml/kg body weight of a 0.5, 1.0, or 5.0% solution
of magnesium LAS (in 3% polyethylene glycol) was applied to the
backs of 20 male and 20 female Sprague-Dawley rats six times a week
for six months. Slight redness at the application site was observed
transiently in males and occasionally in females at 5%. Body weight
was slightly suppressed in males at that dose, and one male in the
control group and one at 5.0% died of unknown causes. Treatment had
no definite effect in terms of food conversion efficiency, urinary,
haematological, serum biochemistry, or histopathological findings,
or organ weights (Ito et al., 1978). No systemic toxicity was
reported in this study. Sakashita et al. (1974) and Sakashita (1979)
(see section 7.2.2.3) may have obtained positive results because
they used a shorter period of exposure, during which skin integrity
may have been compromised, resulting in absorption of the
preparation of LAS through the skin to produce systemic effects.
LAS (19.7% active ingredient) were applied to the dorsal skin of
SLC-Wistar rats three times per week at a dose of 0.005, 0.025, or
0.125 ml/rat (equivalent to 1, 5, or 25 mg/rat) for 24 months. A
dose of 0.025 ml of an LAS-based detergent containing 19.9% LAS
(equivalent to 5 mg LAS per rat) and distilled water was given to
controls. Each application was washed from the skin with warm water
after 24 h. Treatment had no effect on organ weights or
histopathological appearance, and there was no evidence of toxicity
or carcinogenicity (Taniguchi et al., 1978).
Long-term studies of exposure to and the carcinogenicity of LAS
are summarized in Table 19.
A7.4 Skin and eye irritation; sensitization
The potential of LAS to irritate the skin depends on the
concentration applied. On the basis of the criteria of the European
Commission and the OECD test guideline, LAS were classified as
irritating to the skin at concentrations above 20% (European
Committee of Organic Surfactants and Their Intermediates, 1990).
Table 19. Summary of studies of long-term exposure to linear alkylbenzene sulfonates (LAS)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Mouse, SLC-ICR LAS (a.i. 60%) Diet 0, 0.6, 1.8%, Increased liver weight; Yoneyama et al.
8-9 M, 8-9 F 9 months decreased hepatic and renal (1976)
enzyme activities in males
Mouse, ddy (NR) LAS (NS) Drinking- 20 mg/kg bw per Degenerative changes in liver, Watari et al. (1977)
water day, 6 months with partial recovery after
end of treatment
Mouse, ICR LAS (a.i. 60%) Drinking- 0, 0.07, 0.2, 0.6, Depressed body weight gain at Yoneyama et al.
8-9 M, 8-9 F water 1.8%, 9 months high dose; dose-related increase (1976)
in liver weight in all treated
females; changes in hepatic
enzyme activities at high dose
Rat, Wistar LAS, C10-C14 Diet 0, 0.07, 0.2, 0.6, Dose-related depression of Yoneyama et al.
10 M, 10 F 1.8%, 6 months growth, caecal enlargement, (1972)
and renal tubular degeneration
at > 0.07%
Rat, Wistar LAS (a.i. 60%) Diet 0, 0.6, 1.8%, Depressed body weight gain Yoneyama et al.
8 M, 8 F 9 months at high dose; changes in (1976)
haematological parameters, in serum
and hepatic enzyme activities,
and in cholesterol levels at both
doses; changes in renal enzyme
activities in females at high dose
Table 19 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar LAS (a.i. 60%) Drinking- 0, 0.07, 0.2, 0.6%, Depressed body weight gain in Yoneyama et al.
8-9 M, 8-9 F water 9 months males at high dose; no changes (1976)
in haematological parameters or
organ weight; changes in serum
and renal enzyme activities at 0.2%
Rat, Wistar LAS, C10-C14 Diet 0, 0.04, 0.16, 0.6%, Slight increase in liver and Yoneyama et al.
50 M, 50 F (a.i. 60%) 24 months caecal weights and changes in (1977)
serum enzym activities at high
dose; no effect on body weight
gain
Rat, Charles River LAS, C10-C14 Diet 0, 0.02, 0.1, 0.5%, No treatment-related effects Buehler et al.
50 M, 50 F (a.i98.1%) 2 years (1971)
Rat, Wistar LAS, C10-C14 Diet 0, 0.04, 0.16, 0.6%, Transient changes in liver and Fujii et al. (1977)
50 M, 50 F (a.i. 60%) 2 years kidney; no treatment-related
histopathological abnormalities
at end of study
Rat, SD LAS Mg, C10-C13 Gavage 75, 150, 300 mg/kg Depressed body weight gain; no Ito et al. (1978)
20 M, 20 F (a.i. 96.9%) bw per day, 6 significant adverse effects
months
Rat, Wistar, 5 M LAS detergent Drinking- 0, 0.3, 3, 30, 300 Depressed body weight gain and Hayashi (1980)
(a.i. 27%) water ppm, 181 days changes in blood biochemistry
and liver enzyme activity considered
not to be related to treatment
Table 19 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar, 20 M LAS (a.i. 34.55%) Drinking- 0, 0.01, 0.05, 0.1%, No adverse effects Tiba (1972)
water 2 years
Rat, Wistar LAS (a.i. 38.74%) Drinking- 0, 0.1%, 26 months Fatty changes and atrophy in Endo et al. (1980)
62 M, 62 F water liver; changes in hepatic enzyme
activities; no effect on body
weight gain
Rat LAS (Marlon Drinking- 0, 0.01%, No adverse effects Bornmann et al.
60 M, 60 F BW 2043) water 100 weeks (1963)
Rat, SD LAS Mg, C10-C13 Dermal 0.5, 1.0, 5% in Slight reduction in body weight Ito et al. (1978)
20 M, 20 F (a.i. 96.9%) polyethylene glycol, gain of males at high dose; no
6 months other adverse effects
Rat, SLC-Wistar LAS (a.i. 19.7%) Dermal 0, 6.7, 33.3, 167.0 No adverse effects Taniguchi et al.
25 M, 25 F mg/kg bw, 3 × per (1978)
week, 2 years
Rat, SLC-Wistar LAS detergent Dermal 0, 33.3 mg/kg bw No adverse effects Taniguchi et al.
25 M, 25 F (a.i. 19.9%) 3 × per week, 2 years (1978)
M, male; F, female; NS, not specified; a.i., active ingredient; SD, Sprague-Dawley
A7.4.1 Studies of skin
Solutions of LAS (chain length distribution, C10-C13;
purity, 99.9%) were applied to the backs of groups of three male
Wistar rats at a rate of 0.5 g of a 20 or 30% solution once a day
for 15 days. On the sixteenth day of the experiment, the skin at the
application site and the tissues of the tongue and oral mucosa (to
examine the effects of licking) of the rats that received 30% were
examined histologically. Body weight gain was reduced in the group
exposed to 20%, and body weight was decreased in animals exposed to
30%. An infiltrating, yellow-red brown crust was observed after two
to three days at 20% and after one to two days at 30%; at four to
six days, the crust was abraded, and erosion was observed.
Histological examination of the application site revealed severe
necrosis of the region, from the epidermis cuticle to the upper
layer of the dermis, severe infiltration of leukocytes in the
necrotic site, diffuse inflammatory cell infiltration of all of the
layers of the corium, and swelling of collagenous fibres in the
dermis. Histological examination of the tongue showed no changes,
but examination of the oral mucosa revealed atrophy and slight
degeneration of the epithelium (Sadai & Mizuno, 1972).
Some batches of a paste of LAS (volume not stated) induced weak
to moderate sensitization in guinea-pig skin at induction
concentrations of 2-100% and challenge concentrations of 1-2%. A
prototype liquid laundry detergent (10% LAS) induced sensitization
at a challenge concentration of 1% (0.1% as LAS) (Nusair et al.,
1988).
The biochemical and pathomorphological effects of LAS on the
skin of four female albino CDRI guinea-pigs were investigated by
shaving the abdominal skin and immersing the animals up to the neck
in a 1% aqueous solution of neutralized LAS for 90 min daily for
seven consecutive days. A control group was immersed in water
according to the same schedule. After each immersion, the animals
were washed and their skin dried. The animals were killed after
seven days, and skin samples were taken. The skin of guinea-pigs
exposed to the solution of LAS had increased activity of histidine
decarboxylase, decreased sulfhydryl groups and histamine, and
decreased activity of lactic dehydrogenase. It appeared to be
shrunken, with thinner layers of dermis and epidermis than controls.
There were also areas of scarring in the epidermis and ridging of
epidermis and dermis (Misra et al., 1989a).
A7.4.2 Studies of the eye
A volume of 0.1 ml of a solution of LAS (relative molecular
mass, 346.5) at five concentrations ranging from 0.01 to 1.0% was
instilled into the eyes of rabbits (13 per group). The rabbits were
observed for 24 h after application. The group receiving 0.01% had
no abnormalities, but that given 0.05% had slight congestion.
Concentrations of 0.5% and more induced marked reactions, such as
severe congestion and oedema, increased secretion, opacity of the
cornea, and disappearance of the corneal reflex (Oba et al., 1968a).
Solutions of LAS (chain length distribution, C10-C14; 80.9%
C11-C13) at six concentrations ranging from 0.01 to 5.0% were
instilled into the eyes of rabbits (three per group). The rabbits
were observed for 168 h after application. The group given 0.01% had
no reaction, but within 2 h those given 0.05% had slight congestion
and those at 0.1% had considerable congestion or oedema, which had
disappeared by 24 h. Animals given 0.5% or more had marked
reactions, such as severe congestion and oedema, increased
secretion, opacity of the cornea, and disappearance of the corneal
reflex, for 24 h but then tended to recover; the signs had
disappeared completely within 120 h (Iimori et al., 1972).
A7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
The reproductive toxicity of LAS and formulations of LAS has
been evaluated in studies by oral (gavage, diet, drinking-water),
dermal (skin painting), and parenteral (subcutaneous)
administration. Similar effects were seen, regardless of the route
of application. The studies had a number of deficiencies, however,
which are summarized below.
In some studies, widely separated dose levels were used (Palmer
et al., 1975a; Takahashi et al., 1975; Tiba et al., 1976; Hamano et
al., 1976), so that it is difficult to assess dose-response
relationships and to interpret the results. Some of the studies
included only one dose (Bornmann et al., 1963; Sato et al., 1972;
Endo et al., 1980) and some two (Iimori et al., 1973; Nolen et al.,
1975; Takahashi et al., 1975; Hamano et al., 1976; Tiba et al.,
1976). The studies done on formulations are difficult to interpret,
as the effects seen may have been due to another component. In some
cases, the details of the formulation are not given, so that the
dose of LAS is also unknown. Certain studies of dermal exposure
(Sato et al., 1972; Masuda et al., 1973, 1974; Palmer et al., 1975a;
Nishimura, 1976; Daly et al., 1980) involved levels that compromised
the integrity of the skin and caused overt toxicity.
The teratogenic effects of some commercial formulations of LAS
reported by Mikami and co-workers (1969), mainly in mice, were not
reproduced in other studies. A number of studies indicated that LAS
have some reproductive toxicity, but the effects were seen only at
doses that caused maternal toxicity. No teratogenic effects were
observed. These studies are summarized in Tables 20-22.
Table 20. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and formulations of LAS,
administered orally
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
Diet Charles River 14, 70, 350 84 Combined study of reproduction Buehler et al. (1971)
rats (20) (0.02, 0.1, 0.5%) and teratogenicity (three generations);
no effects attributable to LAS
Diet SD rats (16) 78, 780 (0.1, 1.0%) 0-20 No abnormalities at either dose; few Tiba et al. (1976)
offspring at high dose
Gavage ICR mice (NS) 300, 600 6,8,10 High incidence of cleft palate and Mikami et al. (1969)
exencephaly in fetuses at high dose
Gavage ICR mice (14) 40, 400 (0.4, 4.0%) 0-6 No effects at low dose; reduced weight Takahashi et al.
7-13 gain and pregnancy rate at high dose (1975)
Gavage ICR mice (25-33) 10, 100, 300 6-15 Reduced weight gain at all levels, Shiobara & Imahori
particularly at highest dose; two (1976)
dams died at highest dose; all
fetuses of one dam died in utero;
decreased body weight and delayed
ossification in living fetuses but no
increase in incidence of malformations
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS (contd).
Gavage ICR mice 14, 20, 350 1-3 No effect on implantation rate at Koizumi et al. (1985)
any dose
Gavage CD rats (20) 0.2, 2.0, 300, 600 6-15, rats No effects on any species at two lower Palmer et al. (1975a)
CD-1 mice (20) and mice doses
NZW rabbits (13) 6-18, Rats: reduced weight gain and one
rabbits death at highest dose
Mice: reduced weight gain, seven
deaths, and four litter losses at 300 mg/kg
bw per day; 18 deaths, one litter loss
and one non-pregnancy at 600 mg/kg
bw per day
Rabbits: reduced weight gain, 11 deaths,
two litter losses at 300 mg/kg bw per
day; all animals died at highest dose
Gavage CD rats (30) 125, 500, 2000 6-15 Two-generation study of reproductive Robinson &
and developmental toxicity; delayed Schroeder (1992)
ossification significant at highest dose,
slight at middle dose; no reproductive
or developmental toxicity
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS (contd).
Drinking- Charles River 7 (0.01%) Three-generation study of fertility; no Bornmann et al.
water rats (10) teratogenic effects (1963)
Drinking- Wistar rats (20) 70 (0.1%) Four-generation study of reproductive Endo et al. (1980)
water toxicity; no effects attributable to LAS
Drinking- Wistar rats (20) 383 mg/rat (0.1%) 6-15 No effects in rats; rabbits had reduced Endo et al. (1980)
water NZW rabbit (11) 3030 mg/rabbit 6-18 weight gain and delayed ossification
(0.1%) but no malformations
17% LAS, 7% alcohol ethoxylate sulfate
Gavage CD rats (20) 0.8, 8, 1,200, 2400 6-15 No increase in major malformations Palmer et al. (1975a)
CD-1 mice (20) 1.064, 10.64, 6-15 or significant changes in anomalies
1600, 320
NZW rabbits (13) 0.8, 8, 1200, 2400 6-18
45% LAS
Diet CD rats (25) 80, 400, 800 6-15 No treatment-related effects on Nolen et al. (1975)
(0.1, 0.5, 1.0%) reproduction or embryonic
development
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
1% LAS
Gavage ICR mice (18-23) 800, 1200, 1500, 6-15 No increase in fetal malformations; Yamamoto et al.
3000 decreased body weight and delayed (1976)
ossification at 1200 mg/kg bw
19% LAS
Gavage IRC mice (9-13) 125, 4000 6 No effect on fetal viability or Hamano et al.
development (1976)
NS, not specified
Table 21. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and formulations of
LAS, administered dermally
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
CD rats (20) 0.6, 6.0, 60 1-15 Slight reduction in body weight gain at Palmer et al.
(0.03, 0.3, 3.0%) highest dose; no effect on litter parameters (1975a)
at any dose; no evidence of malformations
CD-1 mice (20) 5, 50 , 500 2-13 Reduced body weight gain, fewer pregnancies,
(0.03, 0.3, 3.0%) and total litter loss at highest dose; no
malformations
NZW rabbits (13) 0.9, 9, 90 1-16 Marked reduction in body weight gain, fewer
(0.03, 0.3, 3.0%) pregnancies, and two litter losses at highest
dose; reduced body weight gain at 9 mg/kg bw
per day; no malformations
Wistar rats (20) 20, 100, 400 0-20 Reduced body weight gain, decreased Nishimura (1976)
(1, 5, 20%) pregnancy rates and delayed ossification
at highest dose; no effects at lower doses
Wistar rats (20) 20, 100, 400 0-20 Irritation at site and reduced body weight Daly et al. (1980)
(1, 5, 20%); gain at two higher doses; no change in fetal
rinse-off parameters at any level
Table 21 (contd)
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
Wistar rats (contd)
0.1, 2, 10 0-20 No change in fetal parameters at any level
(0.05, 0.1, 0.5%);
leave on
ddy/s mice (16) 110 (2.22%) 0-13 No abnormalities in dams or fetuses Sato et al. (1972)
ddy mice (4-10) 0.084, 0.84, 8.4 2-14 No fetal or reproductive effects Masuda et al.
(0.017, 0.17, 1.7%) (1973, 1974)
ICR mice 4.2, 8.4, 12.0, 16.5 1-13 Delayed ossification at two highest
(25-30) (0.85, 1.7, 2.55, 3.4%) doses
ICR mice 15, 150, 1500 6-15 Clear decrease in pregnancy rate and Imahori et al.
(27-28) (0.03, 0.3, 3.0%) decrease in fetal weight at highest dose; (1976)
no increase in malformations in fetus
17% LAS, 7% ethanol, 15% urea
ICR mice 2.5, 25, 75 1-13 Decrease in pregnancy rate at Inoue & Masuda
(11-20) (0.5, 5, 15%) highest dose; no other effects (1976)
Table 21 (contd)
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
16.3% LAS
ICR mice 25, 50, 100 0-13 Reduced pregnancy rate and Nakahara et al.
(17-50) (5, 10, 20%) some total litter losses at (1976)
highest dose
Unknown formulation
ddy/s mice 65 (15%) 0-13 Decreased body weight gain, Sato et al. (1972)
(21) decreased pregnancy rate,
decreased fetal weight, and delayed
ossification
Unknown formulation
IRC mice 75, 100 0-12 Decreased pregnancy rates Iimori et al. (1973)
(27-39) (15, 20%) at both levels
Unknown formulation
IRC mice 30, 65, 85, 0-13 Decreased pregnancy rates at all Takahashi et al.
(15-19) 100, 125 doses; decreased fetal body (1975)
(13.0, 17.0, weight; delayed ossification at all
20.0, 25.0%) doses except 65 mg/kg bw per day
Table 22. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and LAS formulations,
administered subcutaneously
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
ICR mice 0.4, 2.0, 10% 7-13 No significant effects on dams; high Masuda & Inoue (1974)
(21-24) incidence of skeletal variations and
delayed ossification, not dose-related;
no abnormalities
ICR mice 20, 200 0-3 Irritation at injection site and reduced Takahashi et al. (1975)
(12-19) (0.35, 1.00%) 8-11 pregnancy rate at highest dose; no
malformations or anomalies
17% LAS, 7% ethanol, 15% urea
CR mice 30, 150 7-13 No increase in major malformations Inoue & Masuda (1976)
(16-17) 0-13 or minor anomalies; increase in
implantations at high dose given on
days 0-13
A7.6 Mutagenicity and related end-points
A7.6.1 Studies in vitro
Assays for mutagenicity were performed in vitro with two
commercial products containing 17.1 and 19% LAS, either undiluted or
diluted 10 and 100 times (Oda et al., 1977), 99.5% pure LAS (Fujita
et al., 1977), 95.5% pure sodium salt, or 96.2% pure calcium salt
(Inoue & Sunakawa, 1979), using Bacillus subtilis H17 (rec+) and
M45 (rec-), Salmonella typhimurium TA98 and TA100 (including
a metabolic activation system), and Escherichia coli WP2 uvrA.
All of the assays gave negative results. LAS 99.5% pure (Fujita et
al., 1977) were also tested in S. typhimurium TA1535 and TA1537,
again with negative results. Thesodium and calcium salts in the
presence of various liver homogenates (Sunakawa et al., 1981) and a
22.2% solution of LAS (C10-C14, 10-200 µg/plate) (Inoue et al.,
1980) were tested in S. typhimurium TA98 and TA100. No
mutagenicity was seen.
A7.6.2 Studies in vivo
Groups of male ICR:JCL mice were given LAS at a dose of 200,
400, and 800 mg/kg body weight per day by gavage for five days and
were killed 6 h after the final administration for examination of
chromosomal aberrations in bone-marrow cells. One commercial
preparation containing 19.0% LAS was also given, at a dose of 800,
1600, or 3200 mg/kg body weight, and another containing 17.1% LAS at
a dose of 1000, 2000, or 4000 mg/kg body weight once only by gavage.
The highest doses were 50% of the respective LD50 values. Bone
marrow was examined 6, 24 and 48 h after administration. There was
no significant difference between any of the groups given LAS and
the negative control group in the incidence of chromosomal
aberrations. Mitomycin C, used as a positive control at 5 mg/kg body
weight, induced severe chromosomal aberrations (Inoue et al., 1977).
Groups of five male Wistar rats, Sprague-Dawley rats, and ICR
mice were given a diet containing 0.9% LAS for nine months. The
equivalent doses were 450 mg/kg body weight per day in rats and
1170 mg/kg body weight per day in mice. There were no significant
differences in the incidence of chromosomal aberrations between the
experimental and control groups (Masubuchi et al., 1976).
After LAS (C10-C15) were fed to groups of six male and six
female Colworth/Wistar rats in the diet at concentrations of 0.56 or
1.13%, equivalent to 280 or 565 mg/kg body weight per day, for 90
days, no alteratuons were seen in chromosomes in bone marrow (Hope,
1977).
In three male ddY mice given LAS at 100 mg/kg body weight by
intraperitoneal injection, there was no differences between the
treated animals and a control group in the incidence of
polychromatic erythrocytes with micronuclei in bone-marrow cells
(Kishi et al., 1984).
An assay to detect dominant lethal mutations was performed in
seven male ICR:JCL mice given a diet containing 0.6% LAS at
300 mg/kg body weight per day for nine months. Each of the male mice
was then mated with two female mice that had not been given LAS, and
11 of the 14 females became pregnant. The pregnant mice were
laparotomized on day 13 of gestation to determine the numbers of
luteal bodies, implantations, surviving fetuses, and dead fetuses.
There were no significant differences in fertility, mortality of ova
and embryos, the number of surviving fetuses, or the index of
dominant lethal induction (Roehrborn) between the experimental and
control groups (Masubuchi et al., 1976).
LAS were administered as a single oral dose of 2 mg to pregnant
ICR mice on day 3 of gestation; on day 17 of gestation, each animal
received a subcutaneous dose of 1, 2, or 10 mg/mouse and was killed
24 h later. There was no difference among treated groups in the
incidence of polychromatic erythrocytes with micronuclei in maternal
bone marrow or fetal liver or blood. No mutagenic effect was found
in any of the groups (Koizumi et al., 1985).
A7.7 Special studies
A7.7.1 Studies in vitro
The haemolytic action of LAS was investigated by mixing red
blood cells from rabbits with solutions of LAS at concentrations of
1-1000 mg/litre at 38°C for 30 min. Haemolysis occurred at
concentrations > 5 mg/litre (Yanagisawa et al., 1964). Red blood
cells from rabbits were mixed with solutions of various
concentrations of LAS (relative molecular mass, 346.5) at room
temperature for 3 h. The 50% haemolytic concentration of LAS was
9 mg/litre (Oba et al., 1968a).
Purified LAS at various concentrations were added to 10 µl of
normal plasma obtained from male rats, and prothrombin time was
determined. Prothrombin time was prolonged; the 50% inhibitory
concentration was about 0.6 mmol/litre. When LAS at various
concentrations were added to a mixture of 1% fibrinogen and
thrombin, the time of formation of a mass of fibrin was prolonged by
inhibition of thrombin activity. The 50% inhibitory concentration
was about 0.05 mmol/litre (Takahashi et al., 1974).
LAS influenced the thermal denaturation and decreased the
fluorescence profile of bovine serum albumin in vitro, indicating
protein-LAS interaction (Javed et al., 1988).
Eggs from female B6C3F1 mice were fertilized in vitro and
incubated in culture medium containing LAS at concentrations between
0.015 and 0.03%; eggs grown in culture medium without LAS served as
controls. Eggs exposed for 1 h, washed, and then cultured for five
days developed normally to the blastocyst stage when the
concentration of LAS was less than 0.025%; at concentrations higher
than 0.03%, the eggs did not develop beyond the one-cell stage. With
continuous exposure to LAS for five days, a concentration of 0.01%
slightly impaired development to the blastocyst stage, and 0.025%
prevented development to the one-cell stage (Samejima, 1991).
LAS with a chain length distribution of C10-C14 did not
induce transformation of cryopreserved primary cultures of Syrian
golden hamster embryo cells in vitro (Inoue et al., 1979, 1980).
A7.7.2 Biochemical effects
The levels of amylase, alkaline phosphatase, glutamate-oxalate
transaminase, and glutamate-pyruvate transaminase and of the
electrolytes Ca, P, and Mg in serum were determined up to 24 h after
a single oral administration of 2, 5, 50, or 100 mg/kg body weight
of LAS (60% active ingredient) or dermal application of 5 ml of a 1,
5, 10, or 20% solution of LAS to rabbits (number not stated). The
levels of total Ca, Ca2+, Mg, and P were generally lower after
either type of administration than before. Although there was no
definite trend, the activities of the enzymes tended to decrease
regardless of the route of the administration or the dose
(Yanagisawa et al., 1964).
Groups of three male mice were given an intraperitoneal
injection of 0.3 g/kg body weight of LAS (C14) in order to study
the effects on the formation of methaemoglobin, determined 0.5, 1,
and 2 h afterinjection of LAS. The level of methaemoglobin in the
experimental groups was not significantly greater than that in the
control group at any time (Tamura & Ogura, 1969).
The effects of LAS (sodium dodecylbenzenesulfonate) on fasting
blood glucose level and glucose tolerance curves were investigated
in 40 male and 50 female albino rats pretreated with 0.25 g/kg body
weight per day of LAS for three months. At the end of this period,
the rats were divided into four groups and given distilled water,
6.1 g/kg body weight of glucose, 0.94 g/kg body weight of LAS, or
6.1 g/kg body weight of glucose plus 0.94 g/kg body weight of LAS by
gavage. Blood glucose was then estimated at 30-min intervals.
Administration of LAS in conjunction with glucose resulted in higher
initial levels of blood glucose in male rats and persistently higher
levels in females than did administration of glucose alone. Females
in control and pretreated groups generally had higher blood glucose
levels in response to administration of glucose or LAS plus glucose
than did male rats (Antal, 1972).
A8. EFFECTS ON HUMANS
Section summary
Human skin can tolerate contact with solutions of up to 1% LAS
for 24 h with only mild irritation. Like other surfactants, LAS can
delipidate the skin surface, elute natural moisturizing factor,
denature the proteins of the outer epidermal layer, and increase
permeability and swelling of the outer layer. LAS do not induce skin
sensitization in humans, and there is no conclusive evidence that
they induce eczema. No serious injuries or fatalities have been
reported following accidental ingestion of LAS-containing surfactant
preparations.
A8.1 Exposure of the general population
Surface-active agents are used in shampoos, dish-washing
products, household cleaners, laundry detergents, and other
applications such as industrial cleaners. LAS are major components
of such products. In general, the concentration of nonionic and
ionic surfactants is 10-20%.
A8.2 Clinical studies
A8.2.1 Skin irritation and sensitization
LAS are mildly to moderately irritating to human skin, depending
on the concentration. There is no evidence that they sensitize the
skin in humans.
The relative intensity of skin roughness induced on the surface
of the forearms of volunteers (a circulation method) due to contact
with LAS of different alkyl chain lengths (C8, C10, C11-C16)
was characterized mainly by gross visible changes. C12 LAS
produced more skin roughening than LAS with longer or shorter alkyl
chains. The degree of skin roughening in vivo correlated with the
extent of protein denaturation measured in vitro (Imokawa et al.,
1975a).
Primary skin irritation induced by an LAS formulation (average
chain length, C12; relative molecular mass, 346.5), by
alpha-olefin sulfonates (AOS) (27% C15, 25% C16, 28% C17, 8%
C18; relative molecular mass, 338.5), and by alkyl sulfates (AS)
(C12; relative molecular mass, 346.5) was compared in a 24-h
closed-patch test on the forearms of seven male volunteers. A 1%
aqueous solution (pH 6.8) of each substance was used, and the
relative intensity of skin irritation was scored by grading
erythema, fissuring, and scales. The average score for LAS was
similar to that for AOS but significantly lower than that for AS
( p < 0.05) (Oba et al., 1968a).
In another comparison, the intensity of skin irritation induced
by 1% aqueous solutions of LAS (C10-C13), AOS (C14, C16,
C18), and the sodium salt of AS (C12-C15) was studied in a
24-h closed-patch test on the forearm and in a test in which the
substance was dripped onto the interdigital surface for 40 min once
daily for two consecutive days at a rate of 1.2-1.5 ml/min. Skin
reactions were scored by grading erythema in the patch test and by
grading scaling in the drip test. In the patch test, the score for
LAS was similar to that for AOS but significantly lower than that
for AS. In the drip test, the score for LAS was similar to that for
AS but higher than that for AOS (Sadai et al., 1979).
Repeated patch tests with LAS at aqueous concentrations of 0.05
and 0.2% produced mild to moderate primary irritation. In a study on
the sensitization potential of LAS for human skin, a 0.1% aqueous
preparation caused no sensitization in 86 subjects (Procter & Gamble
Co., unpublished data).
No skin sensitization was seen in 2294 volunteers exposed to LAS
or in 17 887 exposed to formulations of LAS (Nusair et al., 1988).
A8.2.2 Effects on the epidermis
The main effects of surface-active agents on the epidermal
(stratum corneum) are:
-- delipidation of the skin surface or outer layer;
-- elution of natural moisturizing factor, which maintains the
water content of the outer layer;
-- denaturation of stratum corneum protein; and
-- increased permeability, swelling of the outer layer, and
inhibition of enzyme activities in the epidermis.
These effects and some others present a hazard to the skin; they
are described below.
In an investigation of the relationship between the irritating
potential of LAS in vivo and its ability to remove lipid from the
stratum corneum in vitro, LAS removed detectable levels of lipids
only at levels above the critical micelle concentration (0.04%). LAS
removed only small amounts of cholesterol, free fatty acids, the
esters of those materials, and possibly squalene. At concentrations
below that level, LAS can bind to and irritate the stratum corneum.
The clinical irritation produced by LAS is therefore unlikely to be
directly linked to extraction of lipid, and milder forms of
irritation may involve binding of LAS to and denaturation of keratin
as well as disruption of lipid (Froebe et al., 1990).
The results of the human arm immersion test with measurement of
eluted amino acids and protein, the skin permeation test, freeing of
sulfhydryl groups, and the patch test were compared for nine kinds
of surfactant, including LAS, ABS, AS, alcohol ethoxylate sulfate,
soap, nonionic surfactant, and amphoteric surfactant. LAS gave
intermediate reactions in the patch test and the permeation test and
showed a high level of sulfhydryl group freeing activity. The
results of the tests for evaluating surfactants did not agree with
those for the immersion test, which the author considered to provide
the best simulation of actual use (Polano, 1968).
In a number of studies, denaturation of outer layer proteins was
observed in vitro (Van Scott & Lyon, 1953; Harrold, 1959; Wood &
Bettley, 1971; Imokawa et al., 1974; Okamoto, 1974; Imokawa et al.,
1975b; Imokawa & Katsumi, 1976). Sodium dodecylbenzenesulfonate
stimulated penetration of sodium ions through isolated human
epidermis, partly because the detergent can denature proteins of the
epidermal stratum corneum (Wood & Bettley, 1971). Sodium laurate and
sodium lauryl sulfate were the most effective of several surfactants
in inducing swelling of the horny layer (Putterman et al., 1977).
The lysosome labilizing effects of surfactants, measured as the
release of enzyme from lysosomes, were shown to diminish in the
order cationic > anionic > nonionic surfactants (Imokawa &
Mishima, 1979). When ovalbumin was used as a simulated epidermis
protein, sodium lauryl sulfate was found to denature skin protein
extensively by exposing concealed sulfhydryl groups in LAS of alkyl
chain length C8-C16 (Blohm, 1957).
In immersion tests of the hand and the forearm up to 5 cm above
the wrist, falling off of skin scales diminished in the order:
sec-alkane sulfonate > LAS > AOS, alcohol ethoxylate sulfate
(Okamoto, 1974), but the distribution of carbon chain lengths among
the samples was not described. In a comparison of skin roughening by
a circulation method, the effects diminished in the order C12 AS
> C12 AOS > C12 sec-alkane sulfonate > C12 LAS (Imokawa
et al., 1974, 1975a,b). Skin roughening caused by several
surfactants that are components of commercial products was studied
by the method of Ito & Kakegawa (1972), in which various
concentrations are dripped onto the fingers. The effects diminished
in the order C10-C13 LAS = C12-C15 AS > C11, C13, C15
alcohol ethoxylate sulfate ( n = 0-3) > C14, C16, C18 AOS
> C11-C15 polyoxyethylene alkylether (Sadai et al., 1979).
A8.2.3 Hand eczema
The skin reaction to 0.04, 0.4, and 4.0% aqueous solutions of
LAS (10.0% C10, 34.3% C11, 31.5% C12, 24.7% C13) was tested
in a 24-h closed-patch test on the lower backs of 10 healthy
volunteers and 11 patients with hand eczema (progressive keratosis
palmaris). The incidence and intensity of skin reactions were
greater in the group with hand eczema, but the difference was not
statistically significant (Okamoto & Takase, 1976a,b).
In order to assess the possible etiological correlation between
exposure to LAS and hand eczema, 0.04, 0.4, and 4% aqueous solutions
of LAS were applied in 48-h closed-patch tests on the lower backs
of 20 women with hand eczema and 42 with other skin diseases. The
skin reaction was scored grossly from 0 to 5 on the basis of the
occurrence or intensity of erythema, papules, and vesicles. The
average score appeared to increase in parallel with the
concentration of LAS but did not differ between the groups with hand
eczema and other skin diseases (Sasagawa et al., 1978).
Nine proprietary household detergents were tested in 24-h
closed-patch tests on the lower backs of 160 women with hand eczema.
The surfactant concentrations in five of the products were: (i) 2%
ABS-Na, 15% LAS-Na; (ii) 2% ABS-Na, 14% LAS-Na; (iii) 17% LAS-Na,
12% alcohol ethoxylate sulfate; (iv) 11% ABS-Na, 11% LAS-Na; (v) 19%
LAS-Na. When the detergents were applied daily (for an unspecified
period) at an aqueous concentration of 0.175-0.8%, positive
responses were observed in 3.1% of the women, but they were
considered not to be allergic because the redness of the skin
disappeared completely within two days (Kawamura et al., 1970).
Three proprietary household detergents containing LAS were
tested in 24-h closed-patch tests on the forearms of 13 women with
'housewives' dermatitis' and 13 with other skin diseases. The
detergent was applied either undiluted or in a 0.2% aqueous
solution. Undiluted solutions of all three detergents caused mild to
moderate skin reactions, at incidences of 38.5, 48.1, and 73.1%,
which did not differ between the groups with housewives' dermatitis
and other skin diseases. The 0.2% aqueous solutions did not induce
skin reactions (Ishihara & Kinebuchi, 1967).
Two series of field tests were conducted to estimate if exposure
to a variety of synthetic detergent formulations was associated with
causation or aggravation of hand eczema in women. In the first
series, 162 female volunteers were divided into two groups and
instructed to wear a rubber glove on either the left or the right
hand while using the detergents. The test was conducted for one
month, and the gross appearance of hands before and after the test
period was compared. The relative intensity of noninflammatory
keratosis of the hands was increased in individuals in both groups
on hands that were covered and to a slightly greater extent on hands
that were uncovered. In the second series of tests, 881 housewives
were divided into three groups and instructed to use only one brand
of household detergent, containing LAS, AOS, or ABS during the test
period and to wear rubber gloves on both hands while using the
detergent. The test was conducted for 1.5 months, and the gross
appearance of hands before and after the test period was compared.
Skin roughness was not worsened in any of the three groups (Watanabe
et al., 1968).
A8.2.4 Occupational exposure
Sixty workers exposed at work to an atmosphere containing LAS at
8.64 mg/m3 were tested for serum lipid and sugar content and for
the activities of selected serum enzymes. The levels of total plasma
lipids and plasma cholesterol were slightly lower in the exposed
group than in controls, but no differences were noted for blood
sugar, plasma phospholipid, plasma lipoprotein, alpha-amylase,
leucine aminopeptidase, or pseudocholinesterase. The duration of
exposure before testing was not indicated (Rosner et al., 1973).
In an investigation of the asthmagenic properties of sodium
isononanoyl oxybenzene sulfonate, detergent industry workers were
also tested with LAS. Three workers previously exposed to sodium
isononanoyl oxybenzene sulfonate, three unexposed controls without
asthma, and three controls with asthma were challenged with
0.01-100 µg of LAS. No changes were seen after inhalation of LAS in
any of the subjects; but sodium isononanoyl oxybenzene sulfonate
induced asthmatic symptoms in the previously exposed workers and not
in the control groups (Stenton et al., 1990).
A8.2.5 Accidental or suicidal ingestion
No symptoms were seen in four cases of accidental ingestion of
unknown amounts of a household synthetic detergent containing LAS as
the main component (Hironaga, 1979).
A 32-year-old woman who had ingested 160 ml of a 21% aqueous
solution of LAS with suicidal intent showed transient, slight mental
confusion, vomiting, pharyngeal pain, hypotension, decreased plasma
cholinesterase activity, and increased urinary urobilinogen, but all
of these symptoms disappeared rapidly (Ichihara et al., 1967).
In a review of 1 581 540 cases of human exposure to a wide range
of chemicals reported by the United States Poison Control Centers in
1989, 7983 people had been exposed to household automatic dishwasher
preparations (alkali, anionic or nonionic, other or unknown) and 506
had required treatment in a health facility; 8950 had been exposed
to household cleansers, with 894 requiring treatment; 12 876 had
been exposed to laundry preparations, with 1542 treated; and 621 had
been exposed to industrial detergents (anionic, cationic, nonionic),
with 321 cases requiring treatment. There were no deaths, and only
12 of the treated cases were classified as 'major outcome'.
Virtually all the reports involved accidental exposure. The
compositions of the cleaning preparations, routes of exposure, and
clinical descriptions were not provided (Litovitz et al., 1990).
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
LAS have been tested extensively, both in the laboratory and
under field conditions, but the following aspects must be considered
in interpreting test results. Comparison of the results of tests
carried out on either mixtures of homologues of LAS or LAS of
specified chain length is restricted, because the toxicity of LAS is
influenced by the chain length, and homologues of lower chain length
are less toxic than those with longer chains; furthermore, chain
length was rarely specified in older studies. Studies of the effects
of formulations of LAS on environmental biota are not included in
this section.
Organisms are not exposed to a constant concentration of LAS in
water, owing to the high adsorptivity and biodegradability of LAS.
As LAS are adsorbed on suspended solids or food particles, they have
reduced bioavailability. The adsorption kinetics of LAS also depend
on the chain length of the homologues. Studies of aquatic toxicity
involving flow-through or static renewal (at least daily) should
therefore be given more prominence than studies based on static
conditions, although flow-through and static renewal cannot be used
in (semi-) chronic studies of lower organisms, such as daphnia.
Studies in which the actual concentration was measured should
likewise be given more consideration than those that rely on nominal
concentrations.
The effects of LAS on the aquatic environment have been studied
in short- and long-term studies in the laboratory and under more
realistic conditions: micro- and mesocosm and field studies. In
general, a decrease in alkyl chain length or a more internal
position of the phenyl group is accompanied by a decrease in
toxicity. Data on fish and daphnia indicate that a decrease in chain
length of one unit (e.g. C12 to C11) is accompanied by an
approximately 50% decrease in toxicity, but there is no linear
relationship between chain length and toxicity. In aquatic
microorganisms, the effects are strongly related to variables such
as the type of test system and use of mixed cultures as opposed to
individual species. EC50 values range from 0.5 mg/litre (single
species) to > 1000 mg/litre.
In freshwater fish, the acute LC50 values of C8-C15 LAS
are 0.1-125 mg/litre. The chronic L(E)C50 values of LAS (C11.7 and
not specified) in two species tested were 2.4 and 11 mg/litre, and
NOECs ranging from 0.11 to 8.4 mg/litre have been reported for
C11.2-C13 (or not specified). Marine fish appear to be more
sensitive, with acute LC50 values for C11.7 (or not specified)
in six species of 0.05-7 mg/litre, chronic LC50 values for LAS of
unspecified chain length in two species of 0.01-1 mg/litre, and an
NOEC for C12 in one species of < 0.02 mg/litre.
Results in aquatic plants are also species dependent. In
freshwater plants, the EC50 values for LAS (with chain lengths
shown in parentheses) were 10-235 mg/litre for green algae
(C10-C14), 5-56 mg/litre for blue algae (C11.1-C13),
1.4-50 mg/litre for diatoms (C11.6-C13), and 2.7-4.9 mg/litre
for macrophytes (C11.8). Marine algae appear to be even more
sensitive. There is probably no linear relationship between chain
length and toxicity to algae.
The effects of LAS on freshwater algae have also been tested
under realistic conditions in systems with various trophic levels,
comprising enclosures in lakes (lower organisms), model ecosystems
(sediment: water systems), a river below and above a wastewater
treatment plant outfall, and experimental streams. In general, C12
LAS were used. Algae were more sensitive in summer, when the 3-h
EC50 values with regard to photosynthesis were 0.2-8.1 mg/litre,
whereas studies of model ecosystems showed no effects on the
relative abundance of algal communities at 0.35 mg/litre. No effects
were seen in these studies at 0.24-5 mg/litre, depending on the
organism and parameter tested.
In aquatic invertebrates, the acute L(E)C50 values were
4.6-200 mg/litre for molluscs (either C13 or not specified),
0.12-27 mg/litre for crustaceans (C11.2-C18 or not specified),
1.7-16 mg/litre for worms (C11.8 or not specified), and
1.4-270 mg/litre for insects (C10-C15). The chronic L(E)C50
values were 2.2 mg/litre for insects (C11.8) and 1.1-2.3 mg/litre
for crustaceans (C11.8-C13). The chronic NOEC for crustaceans,
on the basis of lethality or reproduction, was 0.2-10 mg/litre
(C11.8 or not specified). Marine invertebrates are more sensitive,
with LC50 values of 1 to >100 mg/litre (almost all C12) and
NOEC values of 0.025-0.4 mg/litre (chain lengths not specified).
Biodegradation products and by-products of LAS are 10-100 times
less toxic than the parent compound.
Fewer data are available on the effects of LAS in the
terrestrial environment. For the plant species tested, the NOEC
values were < 10-20 mg/litre in nutrient solutions and 100 mg/kg
(C10-C13) for growth of plants in soils. The 14-day LC50 for
earthworms was > 1000 mg/kg.
One study in which chickens were treated in the diet resulted in
an NOEC based on egg quality of > 200 mg/kg.
A9.1 Effect of chain length on the toxicity of linear
alkylbenzene sulfonates
The ecotoxicity of homologues of LAS varies according to the
length of the alkyl chain and the position of the benzene ring on
this chain. In general, homologues with longer chains are more
ecotoxic than shorter ones, and ecotoxicity increases with the
proximity of the benzene ring to the end of the chain. The results
of studies on the effect of LAS chain length on acute toxicity to
fish are presented in Table 23.
The effect of chain length can also be seen on the basis of
quantitative structure-activity relationships (Roberts, 1989, 1991)
calculated from the octanol-water partition coefficients of
homologues of LAS. The slope of the relationship varied from 0.64 to
0.78; therefore, using an average slope of 0.70, it was calculated
that a decrease in chain length from C12 to C11 reduced the
aquatic toxicity of LAS by a factor of 2.4, with a corresponding
decrease in the octanol-water partition coefficient of 0.54.
Table 23. Effect of the chain length of linear alkylbenzene sulfonates (LAS)
on their acute toxicity to freshwater fish
Homologue Fathead minnow Goldfish Guppy Golden orfe
of LAS Pimephales Carassius Lebistes Idus idus
promelas auratus reticulatus melanotus
48-h LC50 6-h LC50 LC50 (mg/litre)c 96-h LC50
(mg/litre)a (mg/litre)b (mg/litre)d
C10 43.0 61.0 50 16.6
C11 16.0 22.5 6.5
C12 4.7 8.5 5 2.6
C13 0.4 3.3 0.57
C14 0.4 1 0.26
C16 0.087 1 0.68
C18 0.38 15
a From Kimerle & Swisher (1977)
b From Gafa (1974)
c From Borstlap (1967)
d From Hirsch (1963)
A9.2 Microorganisms
No adverse effects were seen on the performance of
laboratory-scale activated sludge units after addition of <
20 mg/litre LAS. At 50 mg/litre, nitrification was decreased in
extended aeration units that were treating synthetic sewage (Janicke
& Niemitz, 1973). A bacterium similar to Klebsiella pneumoniae
isolated from sewage degraded LAS at a concentration of 10 ml/litre,
but a concentration of 20 ml/litre inhibited the growth of the
bacterium by 39% (Hong et al., 1984).
The toxicity of microorganisms in activated sludge increases
with the length of the alkyl chain up to approximately C12 and
then decreases (Table 24), presumably because of decreased
bioavailability (e.g. greater sorption of these higher chain
lengths) (Verge et al., 1993).
Table 24. Results of tests for the inhibition of activated
sludge by the sodium salt of linear alkylbenzene
sulfonates (LAS)
LAS Chain length 3-h EC50 (mg/litre)
Pure homologues C10 1042-1200
C11 740-782
C12 500-723
C13 700-795
C14 900-1045
Commercial
formulations C11 760
C11.6 550
C13 650
From Verge et al. (1993)
A mixed bacterial culture was acclimatized to 10 mg/litre LAS
(C9-C14) and was then maintained in either river water, forest
soil, or wastewater from a detergent plant, the concentration of LAS
being increased every five days. At 20.8 and 46 mg/litre, no effect
was reported on the specific growth rate of the bacteria; however,
at 70 mg/litre, the growth rate was inhibited by 18%, and at
95 mg/litre growth was almost zero. Concentrations of 186 and
465 mg/litre LAS inhibited growth completely (Hrsak et al., 1981).
The acute toxicity of LAS (C9-C14) in naturally occurring
bacteria was studied in freshwater and seawater samples by measuring
3H-thymidine incorporation. The EC50 values were 0.5-1.66 mg/litre
for all samples. Toxicity was found to increase with an increasing
relative abundance of longer carbon chains (Martinez et al., 1989).
For bacteria collected from the Rhone River plume (an estuarine
area) and exposed to LAS, the EC50, based on 3H-thymidine
incorporation, was 11.9 mg/litre (Martinez et al., 1991).
The 8-h EC50, based on specific growth rate, of Pseudomonas
fluorescens in solutions of C11.1 LAS under static conditions
was 3200-5600 mg/litre (Canton & Slooff, 1982).
The effect of C11.6 LAS on the structure and function of
microbial communities was studied in a flow-through model ecosystem
containing several trophic levels at concentrations of 0.5 or
5 mg/litre. LAS had no effect on microbial structure at either dose
level, but at 5 mg/litre it inhibited the degradation of both
glucose and LAS. In an experiment in which LAS were supplied in
sewage, neither microbial structure nor function was affected
(Larson & Maki, 1982).
The effects of LAS on the microbial activity of soils were
studied on the basis of Fe[III] reduction. The no-effect-level was
found to be 250 mg/kg; the EC50 was about 500 mg/kg in a strongly
adsorbing soil and 33-55 mg/kg in a poorly adsorbing soil (Welp &
Brummer, 1985).
LAS at concentrations of 0.8-50 g/m2 had no effect on
respiration of loamy soil, sandy soil, or sandy soil irrigated with
wastewater for one or 14 days (Litz et al., 1987).
A9.3 Aquatic organisms
A9.3.1 Aquatic plants
A9.3.1.1 Freshwater algae and cyanobacteria
The 96-h EC50 values for C13 LAS on population growth were
116 mg/litre for the green alga Selenastrum capricornutum,
5 mg/litre for the blue-green alga Microcystis aeruginosa, and
1.4 mg/litre for the diatom Navicula pelliculosa. The EC50
values for C12 LAS were 29 mg/litre for Selenastrum and
0.9 mg/litre for Microcystis (Lewis & Hamm, 1986). The EC50 for
C11.7 LAS on growth of Selenastrum was reported to be 83
mg/litre (Konno & Wakabayashi, 1987). The EC50 values for C11.6
LAS were found to be 50-100 mg/litre for Selenastrum,
10-20 mg/litre for Mycrocystis, and 20-50 mg/litre for the diatom
Nitzschia fonticola (Yamane et al., 1984). The seven-day EC50
for C12 LAS in the green alga Chlorella pyrenoidosa, based on
growth, was 10 mg/litre (Kondo et al., 1983).
The 96-h EC50 values in algae grown in solutions of C11.1
LAS under static conditions, measured as biomass, were
32-56 mg/litre for Microcystis aeruginosa and 18-32 mg/litre for
Chlorella vulgaris (Canton & Slooff, 1982).
A study of the toxicity of various formulations of LAS to the
algae Scenedesmus subspicatus and Selenastrum capricornutum
(Table 25) indicated that commercial mixtures are as or slightly
less toxic than homologues. This finding may be due to a difference
in the sensitivity of the two algae, since those tested with the
homologues were of a different origin than those tested with
commercial LAS (Verge et al., 1993).
Table 25. Results of tests for the toxicity of the sodium salt
of linear alkylbenzene sulfonates (LAS) in algae
LAS Chain length 72-h EC50 (mg/litre)
Pure homologues C10 235
C11 118
C12 62
C13 33
C14 18
Commercial
formulations C11 80
C11.6 80
C13 62
From Verge et al. (1993)
LAS (chain length not specified) significantly reduced the
growth of the green alga Selenastrum capricornutum at a
concentration of 40 mg/litre or more. A significant decrease in
growth was also noted at 10 mg/litre, but no significant effect was
observed at 20 or 30 mg/litre (Nyberg, 1988).
A9.3.1.2 Marine algae
Growth of Gymnodinium breve was reduced by 69% rafter nine days'
exposure to C12 LAS (Kutt & Martin, 1977). These results were
confirmed in a study in which C13 LAS were introduced at the
bottom or surface of a water column: Exposure to LAS at
concentrations > 0.025 mg/litre inhibited growth completely within
two days (Hitchcock & Martin, 1977). These results suggest that
Gymnodinium breve is more sensitive to the effects of LAS than
other algae.
For C11.7 LAS, the seven-day EC50 for growth and the two-day
EC50 for ATP activity on the marine diatom Thalassiosira
pseudonana were both 10 mg/litre (Kondo et al., 1983).
Exposure of the alga Porhyra yezoensis, a standard test
species in Japan, to LAS (C10-C14) under semi-static conditions
gave a 10-day E50 (based on growth) of 0.56 mg/litre (Takita,
1985).
A9.3.1.3 Macrophytes
The seven-day EC50 values for C11.8 LAS on the duckweed
Lemna minor under flow-through conditions were 2.7 mg/litre for
frond count, 3.6 mg/litre for dry weight, and 4.9 mg/litre for root
length. The time-independent EC50 for growth rate and doubling
time was 4.8 mg/litre (Bishop & Perry, 1981).
A9.3.2 Aquatic invertebrates
A9.3.2.1 Acute toxicity
The acute toxicity of LAS to aquatic invertebrates is summarized
in Tables 26 and 27. For marine invertebrates, the 96-h LC50
values for C12 LAS range from 3 mg/litre for barnacles to >
100 mg/litre for several other species (Table 26). Freshwater
invertebrates show a range of 48-h LC50 values from 0.11 mg/litre
(C16) for a daphnid to 270 mg/litre (C11.8) for an isopod (Table
27). Several marine invertebrate species are more sensitivite to LAS
at the larval stage than as adults (Table 26).
Freshwater mussels (Anodonta cygnea) were more sensitive to
LAS during the reproductive period than during the non-reproductive
period, the 96-h LC50 being reduced from 200 to 50 mg/litre
(Bressan et al., 1989).
Studies with Daphnia magna revealed a correlation between
chain length and toxicity. The acute toxicity (24-h and 48-h LC50)
of LAS to Daphnia magna increased with chain length between C10
and C14 (Kimerle & Swisher, 1977) and with chain lengths between
C10 and C16 (Maki & Bishop, 1979), although similar values were
obtained for C16 and C18 homologues. No significant difference
in sensitivity was seen between Daphnia magna and Daphnia pulex.
A similar result was obtained with homologue mixtures (Martinez et
al., 1989): toxicity was correlated with the homologues in which
long chains were the most abundant.
Partial biodegradation of LAS significantly reduces the specific
toxicity (by unit weight) of the remaining LAS to Daphnia magna.
For example, LAS with a high relative molecular mass and a 48-h
LC50 of 2 mg/litre had an LC50 of 30-40 mg/litre after 80-85%
degradation (Kimerle & Swisher, 1977); the longer homologues and
more terminal isomers, which are the most toxic, are therefore also
the more readily biodegraded. Shorter carboxylates formed during the
degradation of LAS were three to four orders of magnitude less toxic
than LAS (Swisher et al., 1978). Other workers also found a
Table 26. Acute toxicity of linear alkylbenzene sulfonates (LAS) to estuarine and marine invertebrates
Organism Size or Static or Temp. Salinity LAS chain End-point Concentration Reference
age flow (°C) (%) length (mg/litre)a
Sea squirt Larva Static 20 NS 6-h LC50 1 Renzoni (1974)
(Ciona intestinalis)
Common mussel Static 6-8 32-34 C12 96-h LC50 > 100 Swedmark et
(Mytilus edulis) Static 15-17 32-34 C12 96-h LC50 50 al. (1971)
Mussel Staticr 18 35 NS 48-h LC50 39.8 Bressan et al.
(Mytilus galloprovincialis) Adult 18 35 NS 96-h LC50 1.66 (1989)
Cockle Static 6-8 32-34 C12 96-h LC50 5 Swedmark et
(Cardium edule) Juvenile Static 15-17 32-34 C12 96-h LC50 5 al. (1971)
Clam Static 6-8 32-34 C12 96-h LC50 70
(Mya arenaria) Static 15-17 32-34 C12 96-h LC50 < 25
Scallop Static 6-8 32-34 C12 96-h LC50 < 5
(Pecten maximus)
Scallop Static 15-17 32-34 C12 96-h LC50 < 5
Decapod Static 15-17 32-34 C12 96-h LC50 50
(Leander adspersus) Intermoult Static 6-8 32-34 C12 96-h LC50 50
Postmoult Static 6-8 32-34 C12 96-h LC50 25
Hermit crab Static 6-8 32-34 C12 96-h LC50 > 100
(Eupagurus bernhardus)
Table 26 (contd)
Organism Size or Static or Temp. Salinity LAS chain End-point Concentration Reference
age flow (°C) (%) length (mg/litre)a
Spider crab Larva Static 6-8 32-34 C12 96-h LC50 9
(Hyas araneus) Adult Static 6-8 32-34 C12 96-h LC50 > 100
Shore crab Static 6-8 32-34 C12 96-h LC50 > 100
(Carcinus maenus)
Barnacle Larva Static 6-8 32-34 C12 96-h LC50 3
(Balanus balanoides) Adult Static 6-8 32-34 C12 96-h LC50 50
Brine shrimp Static 25 C11-C13 24-h LC50 33 Price et al.
(Artemia salina) (1974)
Static: water unchanged for duration of test; NS, not specified; staticr, static renewal: water changed every 12 h; flow, flow-through
conditions: LAS concentration in water maintained continuously
a Based on nominal concentration
Table 27. Acute toxicity of linear alkylbenzene sulfonates (LAS) to freshwater invertebrates
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Bivalve mollusc 11 cm Staticr 18 8.0 NS 96-h LC50 200b Bressan et al.
(Anodonta cygnea) 18 8.0 NS 96-h LC50 50b,c (1989)
Bivalve mollusc 9 cm Staticr 18 8.0 NS 96-h LC50 182.5b
(Unio elongatulus)
Snail Static 21 62 7.3 av. C13 24-h LC50 4.6b Dolan &
(Gonobasis sp.) Hendricks (1976)
Snail (Physa integra) Flow 15 41-47 7.5-7.7 NS 96-h LC50 9b Arthur (1970)
Amphipod (Gammarus Flow 15 41-47 7.5-7.7 NS 96-h LC50 7b
pseudolimnaeus)
Amphipod 4.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 3.3b Lewis &
(Gammarus sp.) Suprenant (1983)
Campeloma decisum Flow 15 41-47 7.5-7.7 NS 96-h LC50 27b Arthur (1970)
Water flea < 24 h Static 20 25 C11.7 24-h LC50 17 Wakabayashi
(Daphnia magna) et al. (1988)
< 24 h Static 21 120 7.4 C10 48-h LC50 9.55d Maki &
Bishop (1979)
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Water flea (contd) < 24 h Static 21 120 7.4 C11 48-h LC50 1.15d
(Daphnia magna) < 24 h Static 21 120 7.4 C12 48-h LC50 5.88-6.84d
< 24 h Static 21 120 7.4 C13 48-h LC50 2.63d
< 24 h Static 21 120 7.4 C14 48-h LC50 0.68-0.8d
< 24 h Static 21 120 7.4 C16 48-h LC50 0.11-0.2d
< 24 h Static 21 120 7.4 C18 48-h LC50 0.12d
< 18 h Static C13.3 48-h LC50 2.3b Kimerle &
< 18 h Static C10 48-h LC50 12.3b Swisher (1977)
< 18 h Static C11 48-h LC50 5.7b
< 18 h Static C12 48-h LC50 3.5b
< 18 h Static C13 48-h LC50 2.0b
< 18 h Static C14 48-h LC50 0.7b
< 24 h Static 19 C11.2 48-h LC50 18-32b Canton &
Slooff (1982)
< 24 h Static 21 131 7.4-7.8 C11.8 48-h LC50 4.8d Lewis (1983)
< 24 h Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8-5.6b Lewis &
Suprenant
(1983)
< 24 h Static 21 295-310 7.3-8.4 C11.8 48-h LC50 3.6-4.7b Taylor (1985)
< 48 h Static 22 241 7.8 C11 48-h EC50 2.2b,e Barera &
Adams (1983)
Flow C11.8 48-h LC50 4.4d Bishop &
Perry (1981)
< 12 h Flow 21 120 7.4 C11.8 96-h LC50 23.94d Maki (1979a)
< 12 h Flow 21 120 7.4 C13 48-h LC50 2.19d
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Water flea < 24 h Static 20 25 C11.7 24-h LC50 18 Wakabayashi
(Daphnia pulex) et al. (1988)
< 24 h Static 21 120 7.4 C12 48-h LC50 8.62d Maki &
Bishop (1979)
< 24 h Static 21 120 7.4 C14 48-h LC50 0.59d
< 24 h Static 21 120 7.4 C16 48-h LC50 0.15d
Oligochaete (Dero sp.) 6.0 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.7b Lewis &
Suprenant
(1983)
Roundworm (nematode) 0.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.7b
(Rhabditis sp.)
Flatworm 3.4 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8b
(Dugesia sp.)
Branchiura sowerbyi Staticr 10 25 8.0 NS 96-h LC50 10.8b,f Bressan et al.
10 25 8.0 NS 96-h LC50 4.4b (1989)
Worm (Limnodrilus Staticr 10 25 8.0 NS 96-h LC50 7.8b,f
hoffmeisteri) 10 25 8.0 NS 96-h LC50 2.0b
Isopod (Asellus sp.) 5.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8b Lewis &
Suprenant
(1983)
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Midge (Chironomus Larva Flow 22 150 7.8-8.4 C11.8 72-h LC50 2.2d Pittinger
riparius) et al. (1989)
Midge (Paratanytarsus 3.6 mm Static 2 165 7.9-8.4 C11.8 48-h LC50 1.8d Lewis &
parthenogenica) Suprenant
(1983)
Mosquito (Aedes Larva Static C10-13 24-h LC50 6b Van Emden et
aegypti) Larva Static C10-15 24-h LC50 2b al. (1974)
3-4 d Static 23 C11.1 48-h LC50 56-100b Canton &
Slooff (1982)
Mayfly Larva Static 10 53 7.5-7.8 C11.6 24-h LC50 13.6b Dolan et al.
(Isonychia sp.) Larva Static 10 53 7.5-7.8 C11.6 48-h LC50 10.4b (1974)
Larva Static 10 53 7.5-7.8 C11.6 96-h LC50 5.33b
Larva Static 10 53 7.5-7.8 C13.1 24-h LC50 4.19b
Larva Static 10 53 7.5-7.8 C11.6 48-h LC50 12.47b
Larva Static 10 53 7.5-7.8 C11.6 96-h LC50 1.36b
Staticr, static renewal: water changed every 12 h; NS, not specified; flow, flow-through conditions: LAS concentration in water maintained
continuously; static: water unchanged for duration of test
a mg/litre CaCO3
b Based on nominal concentration
c Test performed during the reproductive period
d Based on measured concentrations
e Based on immobilization
f Organism exposed in the presence of sediment
reduction in the acute toxicity of LAS to Daphnia magna during
primary degradation (Gard-Terech & Palla, 1986).
Increasing hardness also increased the acute toxicity (48-h
LC50) of C11.8 LAS from a nominal concentration of 7.1 mg/litre
at 25 mg/litre CaCO3 to 4.0 mg/litre at 350 mg/litre CaCO3;
however, significant additional physiological stress was induced if
the hardness of the culture water was significantly different from
that of the test water. Pre-exposure to 0.4 mg/litre LAS (one-tenth
of the 48-h LC50) for up to seven generations (14 weeks) had no
significant effect on the susceptibility of daphnids to acute
exposures (Maki & Bishop, 1979).
Loading density, ranging from 10 daphnids per 20 ml to 20
daphnids per 1000 ml, had no significant effect on the acute
toxicity of C11.8 LAS for Daphnia magna (Lewis, 1983). Daphnids
fed a diet containing Selenastrum had a significant, twofold
decrease in acute toxicity due to C11.8 LAS in comparison with
unfed daphnids (Taylor, 1985). The presence of sediment reduced the
acute toxicity of LAS to the oligochaete worms Branchiura sowerbyi
and Limnodrilus hoffmeisteri. The NOEC and LOEC for B. sowerbyi
were 2.5 times higher in the presence of sediment, and those for
L. hoffmeisteri were 4-4.5 times higher (Bressan et al., 1989; see
also Table 27).
The 96-h EC50 values for duplicate studies of the effect of
LAS on attachment of the podia of the sea urchin Hemicentrotus
pulcherrimus were 3.7 and 3.8 mg/litre (Lee & Park, 1984).
The data from other studies (Lal et al., 1983, 1984a,b; Misra et
al., 1984; Chattopadhyay & Konar, 1985; Misra et al., 1985; Devi &
Devi, 1986; Misra et al., 1987, 1989a,b, 1991) could not be
adequately interpreted because of deficiencies in the data or
method, including inadequate characterization of the test material
with regard to chain-length distribution and use of test material in
an acidified form. The range of values for toxicity reported in
these studies was 10-100 times greater than that in numerous studies
of the same or similar species, and the high values have not been
verified by these or other researchers. As the toxic effects
reported are not considered to be representative of those of
commercial LAS, the data were not used in evaluating the
environmental effects of LAS.
A 72-h LC50 of 2.2 mg/litre was reported for C11.8 LAS in
newly hatched larvae of the midge (Chironomus riparius) (Pittinger
et al., 1989).
A9.3.2.2 Short-term and long-term toxicity
The 21-day LC50 for the water flea (Daphnia magna) was
18 mg/litre, and the NOEC, based on survival, was 10 mg/litre under
static renewal conditions. The 21-day EC50, based on reproduction,
was estimated to be > 10 mg/litre (Canton & Slooff, 1982). The
14-day EC50 for C12 LAS in Daphnia carinata, based on
reproduction, was 16.8 mg/litre (Hattori et al., 1984).
Diet had a significant effect on the sensitivity of Daphnia
magna to the chronic toxicity of C11.8 LAS. The NOEC values
showed a threefold variation of 1.2-3.2 mg/litre and the 21-day
LC50 values a twofold variation of 2.2-4.7 mg/litre with diet. A
threefold variation in toxicity in tests in Daphnia is not,
however, unusual (Taylor, 1985).
Under continuous-flow conditions, a 21-day LC50 value of
1.67 mg/litre was found for daphnids (Daphnia magna) exposed to
C11.8 LAS and 1.17 mg/litre for those exposed to C13 LAS. The
EC50 values for reproductive toxicity were 1.5 mg/litre for
C11.8 LAS and 11.1 mg/litre for C13 with respect to total young
production, 2.3 mg/litre for C11.8 and 1.4.1 mg/litre for C13
for average brood size, and 2.31 mg/litre for C11.8 and
1.29 mg/litre for C13 for percentage of days on which reproduction
occurred (Maki, 1979a).
Campeloma decisum, Gammarus pseudolimnaeus, and Physa integra
were exposed to LAS at concentrations of 0.2-4.4 mg/litre for six
weeks; amphipods were exposed for a further 15 weeks. Survival,
growth, reproduction, feeding, and mobility were studied. The
maximum acceptable concentrations of LAS were found to be
0.2-0.4 mg/litre for Gammarus and 0.4-1.0 mg/litre for Campeloma;
P. integra were not significantly affected (Arthur, 1970).
Fertilized eggs of sea urchins (Paracentrotus lividus) were
treated with LAS at concentrations of 0-0.5 mg/litre for 40 days.
The pattern of embryonic development was unaffected, but the mean
length of the somatic rods of the echinoplutei were reduced
successively with increasing LAS concentrations. A significant
reduction in growth occurred at doses between 0.35 and 0.4 mg/litre;
above 0.45 mg/litre, alterations in skeletal development were
induced (Bressan et al., 1989).
Oligochaete worms (B. sowerbyi) were maintained in LAS at a
concentration of 0.5, 2.5, or 5.0 mg/litre for up to 140 days in the
presence of sediment. Exposed worms laid fewer cocoons and eggs, but
the worms exposed to 5 mg/litre were the least affected. The
percentage of degenerated cocoons, the percentage of worms hatching,
the mean number of eggs per cocoon, and the mean embryonic
development time were all unaffected by treatment. Worms exposed via
the sediment only were not affected (Bressan et al., 1989).
Growth of mussels (Mytilus galloprovincialis) exposed to LAS
at a concentration of 0.25 or 0.5 mg/litre for 220 days, expressed
as mean length of the major axis of the shell, was significantly
slowed ( p < 0.001). The mean (± SE) increments in growth were:
control, 3.11 ± 0.34; 0.25 mg/litre, 1.71 ± 0.15; 0.5 mg/litre,
1.48 ± 0.16 (Bressan et al., 1989).
Eggs of the common mussel, M. edulis, were exposed from the
time of fertilization for 240 h. Fertility was decreased at the
lowest concentration of 0.05 mg/litre and fertilization did not take
place at concentrations in excess of 1 mg/litre. LAS at
concentrations > 0.3 mg/litre inhibited the development of mussel
larvae by delaying the transitory stages of larval development.
Reduced growth rates were observed at concentrations > 0.1 mg/litre
(Granmo, 1972).
Newly fertilized eggs of American oysters (Crassostrea
virginica) were exposed to LAS (chain length not specified, but
likely to be C13) for 48 h. The percentage of eggs that developed
normally was significantly reduced at concentrations greater than
0.025 mg/litre. The percentage survival of oyster larvae hatched in
'clean' water and exposed to LAS at a concentration of 1 mg/litre
for 10 days was significantly decreased, and growth (mean length)
was significantly reduced at 0.5 mg/litre (Calabrese & Davis, 1967).
Embryos of sea urchins (P. lividus) were exposed to LAS at
concentrations of 0.25-0.5 mg/litre from the time of fertilization
for 40 h. At concentrations > 0.45 mg/litre, skeletal development
was totally inhibited; a significant decrease was observed at
0.3 mg/litre. The effect of LAS was found to be maximal at the end
of gastrulation when calcium uptake is high (Bressan et al., 1991).
The effects of LAS were studied on the eggs and sperm of the sea
squirt Ciona intestinalis. Fertility and hatchability were
markedly reduced at 0.1 mg/litre when eggs and sperm were exposed
for the entire developmental period; however, if they were exposed
only before fertilization, fertility and hatchability were slightly
reduced at 0.1 mg/litre but markedly at 1 mg/litre. Male gametes
appeared to be particularly sensitive to the toxic effects of LAS
(Renzoni, 1974).
Two marine benthic filter feeders, the sea squirts Botryllus
schlosseri and Botrylloides leachi were exposed at different
periods of development to LAS. When larvae were exposed from
spawning for 6 h, the incidence of abnormal metamorphosis was
significantly increased at 1 mg/litre LAS for Botryllus and 2
mg/litre for Botrylloides. The frequency of spontaneously settled
larvae of both species also increased with exposure to LAS and
seemed to be a selective effect of LAS. The frequency was
significantly different from controls at 1 and 3 mg/litre for the
two species, respectively. In a second experiment, young colonies
were exposed to LAS for 15 days immediately after discharge by the
parental colony. Growth rates were significantly decreased at
0.5 mg/litre for Botryllus and at 0.25 mg/litre for Botrylloides.
When colonies were exposed from the end of metamorphosis, their
growth rates were similarly affected, but the mortality rate was
significantly lower. The effects of LAS thus appear to be exerted
mainly on the pelagic phase of the life cycle (Marin et al., 1991).
No significant reduction in egg hatching of midges (Chironomus
riparius) was seen at the highest concentration of C11.8 LAS
tested (18.9 mg/litre), but newly hatched larvae were more
sensitive, with a 72-h LC50 of 2.2 mg/litre. In bioassays of part
of the life cycle in a sediment and water system, the percentages of
winged adults emerging were monitored after continuous exposure of
larvae and pupae. The NOEC for sediment containing LAS was 319 mg/kg
(dry weight). In the absence of sediment, the NOEC was
2.40 mg/litre. Both tests were conducted for about 20 days
(Pittinger et al., 1989).
A9.3.2.3 Biochemical and physiological effects
Juvenile mussels (M. galloprovincialis) were exposed to LAS at
a concentration of 0.25 or 0.5 mg/litre for 220 days. Oxygen uptake
and the retention rate of neutral red (a measure of filtration rate)
were significantly decreased, but no effect was detected on nitrogen
excretion (measured as ammonia). When the experiment was repeated
over a seven-day period at a concentration of LAS of 1 or
1.5 mg/litre, no significant effect was seen on nitrogen metabolism
and the results for oxygen uptake were inconclusive. The filtration
rate was again significantly reduced when compared with that in
control mussels (Bressan et al., 1989).
The 48-h LC50 for lugworms (Arenicola marina) exposed to LAS
was calculated to be 12.5 mg/litre (95% confidence interval,
8.6-18.2). When tissues from a lugworm exposed to a concentration
close to that of the LC50 were examined for changes in morphology
by both light and electron microscopy, serious damage was reported
in the caudal epidermis, epidermic receptors, and gills; no effect
was reported in the thoracic epidermis or the intestine. In the
caudal epidermis, LAS destroyed the papillae, disrupting the
internal structure, occasionally displacing the musculature below
the papillae and thus giving it direct contact with seawater.
Deciliation of the epidermic receptors was also reported. These
effects were considered to indicate that the physiological response
of damaged epidermic receptors was reduced or blocked by exposure to
LAS. Changes in the morphology of the gills included destruction of
the epithelium and blood vessels, causing complete solubilization of
branch apexes, and development of holes at the base of the gills
(Conti, 1987).
A9.3.3 Fish
A9.3.3.1 Acute toxicity
The acute toxicity of LAS to fish is summarized in Tables 28 and
29. Only a few studies were available on marine fish, providing two
96-h LC50 values, 1 and 1.5 mg/litre LAS. Tests in various species
of freshwater fish gave a wide range of LC50 values: the 48-h
values ranged from 0.2 mg/litre for brown trout (Salmo trutta) to
125 mg/litre for the golden orfe (Idus idus memanotus), and the
96-h values ranged from 0.1 mg/litre for brown trout to 23 mg/litre
for white tilapia (Tilapia melanopleura).
The acute toxicity tended to increase with increasing carbon
chain length. Thus, C14 LAS were more acutely toxic to bluegill
(Lepomis macrochirus) than C12 compounds (Swisher et al., 1964);
the acute toxicity of LAS to the golden orfe increased with chain
length from C8 to C15 but decreased at C16 (Hirsch, 1963).;
and a similar trend was found for fathead minnows (Pimephalus
promelas) exposed to LAS with chain lengths of C10 to C14
(Kimerle & Swisher, 1977).
The 96-h LC50 values in bluegill (Lepomis macrochirus) were
0.64 mg/litre for C14 and 3 mg/litre for C12 LAS but
75 mg/litre for the intermediate degradation product,
sulfophenylundecanoic acid disodium salt (Swisher et al., 1964).
Biodegradation of LAS with a high relative molecular mass
progressively shifted the homologue distribution in favour of
shorter chain lengths and reduced the acute toxicity of the compound
to bluegill (Dolan & Hendricks, 1976). Similar findings were
reported for fathead minnow (Swisher et al., 1978), goldfish
(Carassius auratus) (Divo & Cardini, 1980) and zebra fish
(Brachydanio rerio) (Gard-Terech & Palla, 1986).
In rainbow trout (Oncorhynchus mykissi), addition of LAS
(C10-C15) to activated sludge plant effluent increased the
nominal 96-h LC50 from 0.36 to 29.5 mg/litre (Brown et al., 1978).
No deaths were observed among bluegill exposed for 4-11 days to
effluent from continuous-flow activated sludge units fed
100 mg/litre LAS (Swisher et al., 1964).
Water hardness was found to be the most significant
environmental factor in the acute toxicity of LAS to bluegill,
increasing with the level of hardness. At a water hardness of
15 mg/litre CaCO3, the mean LC50 was 4.25 mg/litre; at
290 mg/litre CaCO3, the LC50 was reduced to 2.85 mg/litre
(Hokanson & Smith, 1971). Similarly, when water hardness was
increased from 0 to 500 mg/litre CaCO3, the LC50 for C18 LAS
in goldfish was reduced from 15 to 5.7 mg/litre (Gafa, 1974).
Exposure of the freshwater bleeker (Puntius gonionotus) to LAS
gave 96-h LC50 values of 13.6 mg/litre at a water hardness of
50 mg/litre CaCO3, 11.8 mg/litre at 110 mg/litre CaCO3, and
11.4 mg/litre at 260 mg/litre CaCO3 (Eyanoer et al., 1985).
The toxicity of C11.7 LAS to the medaka (Oryzias latipes)
increased with increasing salinity, but the effect was less
pronounced than that of water hardness (Wakabayashi & Onizuka,
1986).
Temperature was reported to have no significant effect on the
acute toxicity of LAS (Hokanson & Smith, 1971), but in another study
increasing the water temperature from 28 to 35°C marginally
decreased the 96-h LC50 for the bleeker, from 11.8 to
11.5 mg/litre (Eyanoer et al., 1985).
A reduction in the dissolved oxygen concentration from 7.5 to
1.9 mg/litre reduced the 24-h LC50 in bluegill from 2.2 to
0.2 mg/litre. When the fish were first acclimatized to reduced
oxygen levels, the effect was less pronounced (Hokanson & Smith,
1971).
No significant effect on the acute toxicity of LAS to bluegill
was observed after a bentonite suspension was added to water at
concentrations of 0, 50, or 200 mg/litre (Hokanson & Smith, 1971).
Addition of gluten, however, reduced the 24-h and 48-h acute
toxicity of LAS to both himedaka (Oryzias latipes) and cobalt
suzume (Chrysiptera hollisi) (Iimori & Takita, 1979).
A9.3.3.2 Chronic toxicity
Exposure of the eggs of fathead minnows (Pimephales promelas) to
LAS from laying until all surviving eggs had hatched under
flow-through conditions gave a nine-day LC50 of 2.4 mg/litre,
which would result in an LC50 of 3.4 mg/litre after 24 h of
exposure (Pickering, 1966).
Eggs of cod (Gadus morhua) were exposed to LAS at a
concentration of 0.005, 0.02, 0.05, or 0.1 mg/litre from
fertilization until hatching under flow-through conditions. There
were no significant effects at 0.005 mg/litre. At a concentration of
0.02 mg/litre, only 42% of the embryos completely developed into
larvae, and there was an increased occurrence of tail malformations
in comparison with controls. At 0.05 mg/litre, few eggs developed to
embryos. No eggs developed to the blastula stage at a concentration
of 0.1 mg/litre. In a repetition of the test at 0.05 mg/litre, fewer
eggs and larvae died, but there was an increased frequency of
abnormal embryos and inactive and crippled larvae (Swedmark &
Granmo, 1981).
Eggs, larvae, and immature adult fathead minnows (Pimephales
promelas) were exposed to LAS at a concentration of 0.34, 0.63,
1.2, or 2.7 mg/litre for up to four months. No significant effect
was observed on the number of spawnings, the total number of eggs
produced, the mean number of spawnings per female, the mean number
of eggs per spawning, or the percentage hatchability; however, the
two highest concentrations significantly reduced the survival of fry
(Pickering & Thatcher, 1970).
The effects of C11.8 and C13 LAS on the number of females,
the number of spawnings, total number of eggs produced, and number
of eggs per female were also studied in the fathead minnow over a
period of one year. As C11.8 LAS had no significant effect on
these parameters at a concentration of 1.09 mg/litre and a water
hardness of 120 mg/litre CaCO3, the NOEC was 0.9 mg/litre; C13
LAS were more toxic, and the NOEC was 0.15 mg/litre. At a lower
water hardness (39 mg/litre), however, survival of larvae was
impaired at 0.74 mg/litre (Maki, 1979a). NOECs in the fathead minnow
in life-cycle and embryo-larval tests were dependent on mean alkyl
chain length: 5.1-8.4 mg/litre for C11.2, 0.48 mg/litre for
C11.7, and 0.11-0.25 mg/litre for C13.3 (Holman & Macek, 1980).
The LC50 value of LAS in the eggs of carp (Cyprinus carpio)
exposed from spawning to hatching was 11 mg/litre. In determinations
of the sensitivity of eggs at different stages of development after
spawning, the 24-h LC50 values were 15 mg/litre for eggs exposed
between 2 and 26 h, 25 mg/litre for exposure between 26 and 50 h,
and 32 mg/litre for exposure between 50 h and hatching (Kikuchi et
al., 1976).
Bluegill (Lepomis macrochirus) were exposed to LAS from
fertilization to yolk-sac resorption at a concentration of 1.8, 3.5,
4.6, or 5.5 mg/litre. The lowest concentrations did not affect
hatchability or survival. Survival among those exposed to
3.5 mg/litre which hatched successfully was significantly reduced
within two days of hatching, and 95% were dead by the end of the
experiment. Eggs exposed to 4.6 or 5.5 mg/litre failed to hatch
(Hokanson & Smith, 1971).
The NOEC of LAS in guppies (Poecilia reticulata), based on
mortality, behaviour, and growth over 28 days, was 3.2 mg/litre
(Canton & Slooff, 1982).
Studies of the short- and long-term toxicity of LAS to
freshwater and marine fish are summarized in Tables 28 and 29.
A9.3.3.3 Biochemical and physiological effects
The main injury to the gills of catfish (Heteropneustes
fossilis) exposed to LAS at 1 or 2.5 mg/litre was progressive
separation of the lamellae from their vascular components.
Table 28. Toxicity of linear alkylbenzene sulfonates (LAS) to freshwater fish
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Brown trout Flow 15 26-30 NS 48-h LC50 5.3 Reiff et al.
(Salmo trutta) Flow 15 26-30 NS 96-h LC50 4.6 (1979)
Flow 15 26-30 NS 48-h LC50 2.3
Flow 15 26-30 NS 96-h LC50 1.4
Flow 15 26-30 NS 48-h LC50 0.4
Flow 15 26-30 NS 96-h LC50 0.4
Flow 15 250 NS 48-h LC50 2
Flow 15 250 NS 96-h LC50 0.9
Flow 15 250 NS 48-h LC50 0.7-0.9
Flow 15 250 C10-C15 48-h LC50 0.2
Flow 15 250 C10-C15 96-h LC50 0.1
Masu trout 2 mo Staticr 8.5-9.6 30 C11.7 96-h LC50 4.4 Wakabayashi et
(Oncorhynchus masou) al. (1984)
Rainbow trout Flow 15 250 C12.6 96-h LC50 0.36b Brown et al.
(Oncorhynchus mykiss) (1978)
40 d Staticr 8.8-10.9 25 C11.7 96-h LC50 4.7 Wakabayashi et
al. (1984)
4 d Staticr 10 25 C11.7 96-h LC50 2.1 Wakabayashi &
19 d Staticr 10 25 C11.7 96-h LC50 3.4 Onizuka (1986)
Goldfish Static 20 C16 6-h LC50 61 Gafa (1974)
(Carassius auratus) Static 20 C17 6-h LC50 22.5
Static 20 C18 6-h LC50 8.5
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Goldfish (contd) Static 20 C19 6-h LC50 3.3
(Carassius auratus) Static 20 C16-C19 6-h LC50 7.6
Static 20 C16-C19 6-h LC50 10
Static 20 C16-C19 6-h LC50 12.2
Static 20 100 NS 6-h LC50 8.2 Reiff et al.
Static 20 100 NS 6-h LC50 7 (1979)
Static 20 100 NS 6-h LC50 4.3
3.1-6.0 Flow 20-23 45-96 7.1-9.3 24-h LC50 7.6 Tsai & McKee
cm Flow 20-23 45-96 7.1-9.3 48-h LC50 7.5 (1978)
Flow 20-23 45-96 7.1-9.3 72-h LC50 7.0
Flow 20-23 45-96 7.1-9.3 96-h LC50 6.2
Bluegill sunfish 1.6 g Static 23 76 7.5 av. C13 48-h LC50 0.72b Dolan &
(Lepomis macrochirus) 1.6 g Static 23 76 7.5 av. C13 96-h LC50 0.72b Hendricks
(1976)
Flow 23 50 7.5 av. C13 96-h LC50 4c Thatcher &
Santner (1967)
Finger Static 25 15 C11.2 48-h LC50 4.0-4.5b Hokanson &
Finger Static 25 290 C11.2 48-h LC50 2.8-2.9b Smith (1971)
Flow C11.8 96-h LC50 1.7c Bishop & Perry
(1981)
Fathead minnow Static C13.3 48-h LC50 1.7b Kimerle &
(Pimephales promelas) Static C10 48-h LC50 43b Swisher (1977)
Static C11 48-h LC50 16b
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Fathead minnow (contd) Static C12 48-h LC50 4.7b
Static C14 48-h LC50 0.4b
2-3 mo Static 40 C11.2 96-h LC50 12.3c Holman & Macek
2-3 mo Static 40 C11.7 96-h LC50 4.1c (1980)
2-3 mo Static 40 C13.3 96-h LC50 0.86
Static 25 48-h LC50 4.6 Pickering &
Static 25 96-h LC50 5.0 Thatcher (1970)
Flow 15 43 7.2-7.9 96-h LC50 3.4 McKim et al.
(1975)
Flow 23 50 7.5 96-h LC50 4.2 Thatcher &
Santner (1967)
Flow 25 96-h LC50 4.2-4.5 Pickering &
Thatcher (1970)
2.5 cm Flow 18 116 7.9 C12 96-h LC50 3.5 Solon et al.
(1969)
Harlequin fish Flow 20 20 NS 48-h LC50 7.6 Reiff et al.
(Rasbora heteromorpha) Flow 20 20 NS 96-h LC50 6.1 (1979)
Flow 20 20 NS 48-h LC50 5.1
Flow 20 20 NS 96-h LC50 4.6
Flow 20 20 C10-C15 48-h LC50 0.9
Flow 20 20 NS 96-h LC50 0.7
Carp (Cyprinus carpio) 4.4 mg Static 22 25 7 C11.7 12-h LC50 5.6 Kikuchi et al.
Static 22 25 7 C11.7 48-h LC50 5.6 (1976)
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Carp (contd) 3.5-5.5 Static 21 7.5-7.8 NS 48-h LC50 6.8 Lopez-Zavala
cm Static 21 7.5-7.8 NS 96-h LC50 5.0 et al. (1975)
7 d Staticr 22 25 7.0 C11.7 48-h LC50 5.6 Arima et al.
6 mo Staticr 22 25 6.5-7.1 C11.7 48-h LC50 10 (1981)
50 d Staticr 21 75 C11.7 96-h LC50 4.4 Wakabayashi et
al. (1984)
2 d Staticr 20 25 C11.7 96-h LC50 4.6 Wakabayashi &
15 d Staticr 20 25 C11.7 96-h LC50 2.6 Onizuka (1986)
White tilapia 5-7 cm Static 21 7.5-7.8 NS 48-h LC50 26 Lopez-Zavala
(Tilapia melanopleura) 5-7 cm Static 21 7.5-7.8 NS 96-h LC50 23 et al. (1975)
Guppy 3-4 wk Staticr 23 C8-C14 96-h LC50 5.6-10 Canton & Slooff
(Poecilia reticulata) (1982)
Northern pike Flow 15 43 7.2-7.9 96-h LC50 3.7 McKim et al.
(Esox lucius) (1975)
White sucker Flow 15 43 7.2-7.9 96-h LC50 4 McKim et al.
(Catostomus commersoni) (1975)
Golden orfe Static 18-20 C8 48-h LC50 125 Hirsch (1963)
(Idus idus melanotus) Static 18-20 C9 48-h LC50 88
Static 18-20 C10 48-h LC50 16.6
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Golden orfe (contd) Static 18-20 C11 48-h LC50 6.6
Static 18-20 C12 48-h LC50 2.6
Static 18-20 C13 48-h LC50 0.57
Static 18-20 C15 48-h LC50 0.23
Static 18-20 C16 48-h LC50 0.68
1.2-1.8 g Static 20 NS 48-h LC50 3.94 Mann (1976)
Static 20 NS 48-h LC50 1.85
Static 20 NS 48-h LC50 1.24
Flow 20 150 NS 48-h LC50 4.9 Reiff et al.
(1979)
Flow 20 150 NS 48-h LC50 2.4
Flow 20 150 NS 48-h LC50 1.2
Flow 20 268 NS 48-h LC50 2.1-2.9
Flow 20 268 NS 96-h LC50 1.9-2.9
Flow 20 268 NS 48-h LC50 1.3-1.7
Flow 20 268 NS 96-h LC50 1.2-1.3
Flow 20 268 NS 48-h LC50 0.8-0.9
Flow 20 268 NS 96-h LC50 0.4-0.6
Himedaka (killifish) 4-5 wk Staticr 23 C8-C14 96-h LC50 10-18 Canton & Slooff
(Oryzias latipes) (1982)
323 mg Staticr 23-24 5.6-6.1 C11.7 48-h LC50 15 Kikuchi et
al.
323 mg Staticr 23-24 5.6-6.1 C11.7 48-h LC50 10 (1976)
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 12 Kikuchi &
approx. 262 mg Staticr 21-22 6.7-7.1 NS 48-h LC50 10 Wakabayashi
(1984)
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Himedaka (contd) C161 48-h LC50 > 50 Tomiyama (1974)
C6 48-h LC50 > 50
C8 48-h LC50 > 50
C10 48-h LC50 > 50
C12 48-h LC50 4
C14 48-h LC50 4 Iimori &
Takita
(1979)
25 7.2-7.9 48-h LC50 7.6 Hidaka et al.
(1984)
25 7.2-7.9 96-h LC50 7.3
Adult Staticr 20 5 C11.7 96-h LC50 13 Wakabayashi &
Adult Staticr 20 25 C11.7 96-h LC50 8.8 Onizuka (1986)
Adult Staticr 20 125 C11.7 96-h LC50 4.8
Adult Staticr 20 625 C11.7 96-h LC50 3.2
Adult Staticr 20 0 C11.7 48-h LC50 6.7 Wakabayashi &
Adult Staticr 20 1 C11.7 48-h LC50 4.8 Onizuka (1986)
Adult Staticr 20 5 C11.7 48-h LC50 4.7
Adult Staticr 20 10 C11.7 48-h LC50 3.5
Adult Staticr 20 15 C11.7 48-h LC50 3.8
Adult Staticr 20 20 C11.7 48-h LC50 2.5
Adult Staticr 20 25 C11.7 48-h LC50 1.9
Adult Staticr 20 30 C11.7 48-h LC50 1.6
Adult Staticr 20 35 C11.7 48-h LC50 1.4
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Cobalt suzume 48-h LC50 1.3 Iimori &
(Chrysiptera hollisi) Takita (1979)
Smallmouth bass Flow 15 43 7.2-7.9 NS 96-h LC50 3.7 McKim et al.
(Micropterus dolomieu) (1975)
Black bullhead Flow 23 50 7.5 NS 96-h LC50 6.4 Thatcher &
(Ictalurus melas) Santner (1967)
Common shiner Flow 23 50 7.5 NS 96-h LC50 4.9 Thatcher &
(Notropis cornutus) Santner (1967)
Emerald shiner Flow 23 50 7.5 NS 96-h LC50 3.3 Thatcher &
(Notropis atherinoides) Santner (1967)
Bleeker 0.3 g Static 28 NS 96-h LC50 11.8 Eyanoer et al.
(Puntius gonionotus) 0.3 g Static 35 NS 96-h LC50 11.5 (1985)
0.3 g Static 50 NS 96-h LC50 13.6
0.3 g Static 110 NS 96-h LC50 11.8
0.3 g Static 260 NS 96-h LC50 11.4
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Ayu 0.26 mg 1 NS 48-h LC50 0.86 Sueishi et al.
(Plecoglossus altivelis) 0.29 g 1 NS 48-h LC50 0.53 (1988)
1.24 g 1 NS 48-h LC50 0.77
6.51 g 1 NS 48-h LC50 1.45
27.98 g 1 NS 48-h LC50 1.17
Flow, flow-through conditions: LAS concentration in water maintained continuously; NS, not specified; staticr, static renewal: water changed
periodically; static, water unchanged for the duration of test; finger, fingerling
a mg/litre CaCO3
b Based on nominal concentration
c Based on measured concentrations
Table 29. Toxicity of linear alkylbenzene sulfonates (LAS) to marine fish
Organism Size or Static or Temp. Salinity LAS chain End-point Concn Reference
age flow (°C) (%) length (mg/litre)
Cod (Gadus morhua) 30 cm Static 6-8 32-34 C12 96-h LC50 1a Swedmark et al.
30 cm Static 15-17 32-34 C12 96-h LC50 < 1a (1971)
Flounder Static 6-8 32-34 C12 96-h LC50 1.5a
(Pleuronectes flesus) Static 15-17 32-34 C12 96-h LC50 < 1a
Plaice (Pleuronectes Static 6-8 32-34 C12 96-h LC50 > 1 -< 5a
platessa)
Mosbled sole Newly NS 48-h LC50 0.5-1 Yasunaga (1976)
(Limanda yokohamae) hatched
10 days NS 48-h LC50 0.1-0.5
30 days NS 48-h LC50 0.5-1
40 days NS 48-h LC50 < 0.1
Newly NS 48-h LC50 0.05-0.1
hatched
Olive flounder 5 days NS 48-h LC50 < 0.1
(Paralichtys olivaceus) 10 days NS 48-h LC50 0.1-0.5
30 days NS 48-h LC50 0.1-0.5
Himedaka (killifish) Adult Staticr 20 0 C11.7 48-h LC50 6.7 Wakabayashi &
(Oryzias latipes) Adult Staticr 20 1 C11.7 48-h LC50 4.8 Onizuka (1986)
Adult Staticr 20 5 C11.7 48-h LC50 4.7
Table 29 (contd)
Organism Size or Static or Temp. Salinity LAS chain End-point Concn Reference
age flow (°C) (%) length (mg/litre)
Himedaka (contd) Adult Staticr 20 10 C11.7 48-h LC50 3.5
Adult Staticr 20 15 C11.7 48-h LC50 3.8
Adult Staticr 20 20 C11.7 48-h LC50 2.5
Adult Staticr 20 25 C11.7 48-h LC50 1.9
Adult Staticr 20 30 C11.7 48-h LC50 1.6
Adult Staticr 20 35 C11.7 48-h LC50 1.2
Static: water unchanged for duration of test; staticr, static renewal: water changed periodically; NS, not specified
a Based on nominal concentration
The activity of the enzymes of aerobic metabolism was decreased, and
that of lactate dehydrogenase was strongly increased (Zaccone et
al., 1985). Concentrations of 2.19 mg/litre C11.8 LAS and
0.39 mg/litre C13 LAS significantly increased the 24-h mean
ventilation rate (number of opercular closures per minute) of
bluegill (Lepomis macrochirus) (Maki, 1979b).
A concentration of 36 mg/litre LAS severely affected the
viability of the perfused gills of rainbow trout (Oncorhynchus
mykissi). Vascular resistance increased gradually during
perfusion, with a concomitant decrease in oxygen transfer. LAS at
0.05 mg/litre more than doubled cadmium transfer (0.8 µg/litre)
through the perfused gills; and at concentrations of 36 mg/litre LAS
and 0.9 mg/litre cadmium, there was a marked reduction in cadmium
transfer (Pärt et al., 1985).
A9.3.3.4 Behavioural effects
Hidaka and co-workers have reported several studies of the
avoidance of surfactants by fish (Hidaka et al., 1984; Hidaka &
Tatsukawa, 1989; Tatsukawa & Hidaka, 1978). The results should be
interpreted with caution, since the environmental relevance and the
reproducibility and sensitivity of these tests is unclear;
furthermore, no effect was seen after removal of the olfactory
organs. Another study (Maki, 1979a) showed no adverse toxicological
effects at concentrations two times greater than those reported to
cause avoidance reactions.
Hidaka et al. (1984) found that the minimal avoidance
concentration of LAS, i.e. the concentration at which fish spent 65%
of a 5-min period in clean water in order to avoid LAS, was
13.5 µg/litre for medakas (Oryzias latipes). Medakas exposed to
LAS at concentrations of 5-50 µg/litre for 10 min showed significant
avoidance to 10, 20, and 30 µg/litre. No significant avoidance of
concentrations of 10-50 µg/litre LAS was found after removal of the
olfactory organs (Hidaka & Tatsukawa, 1989).
The threshold concentrations for avoidance of LAS by ayu
(Plecoglossus altivelis) were 0.11 µg/litre of a formulation and
1.5 µg/litre of pure reagant LAS (Tatsukawa & Hidaka, 1978).
A9.3.3.5 Interactive effects with other chemicals
The chronic toxicity of para, para-DDT (50 mg/litre) to
goldfish (Carassius auratus) was increased by prior exposure to
LAS at 4 mg/litre for 37 days (Dugan, 1967).
The toxicity of 1 mg/litre LAS solution to mosquito fish exposed
under static conditions was not affected by allowing the LAS
solution to react with excess chlorine (Katz & Cohen, 1976).
A concentration of 1 mg/litre LAS significantly increased the
toxicity of fuel oil to bluegill (Lepomis macrochirus), reducing
the 24-h LC50 from 91 to 51 mg/litre. The authors concluded that
sublethal concentrations of LAS increased the toxicity of fuel oil
by increasing its penetration (Hokanson & Smith, 1971). The toxicity
of No. 2 and No. 4 fuel oils in six species of freshwater fish was
increased in the presence of 1-5 mg/litre LAS (Rehwoldt et al.,
1974).
The uptake of cadmium by freshwater trout (Salmo gairdneri)
exposed to 0.14 µmol/litre LAS was more than two times greater than
in controls. Reduced cadmium uptake was reported in fish exposed to
100 µmol/litre LAS. The authors reported that trout exposed to low
levels of both LAS and cadmium could take up lethal cadmium
concentrations. LAS were reported to interact with the gill proteins
involved in cadmium transport, resulting in increased permeability
to cadmium (Pärt et al., 1985).
Fathead minnows (Pimepheles promelas) were exposed to various
pesticides in the presence and absence of 1 mg/litre LAS. Parathion
acted synergistically with LAS, reducing the 96-h LC50 from 1410
to 720 µg/litre. Endrin and LAS showed no synergism, and no
consistent results were obtained for DDT (Solon et al., 1969).
Methyl parathion, ronnel, trithion, and trichloronat were also found
to act synergistically with LAS, but neither ortho-ethyl-ortho-4-
nitrophenyl phenylphos-phonothioate nor dicapthon exhibited
synergism. The synergistic relationship does not appear to be
exclusive to a general structural group (Solon & Nair, 1970).
Goldfish (Carassius auratus) were exposed to mixtures of LAS
and chloramines and LAS and copper at ratios of 1:1, 2:1, and 1:2,
and toxicity curves and 24-h and 96-h LC50 values were compared.
LAS and chloramines had an additive effect at a ratio of 1:1, but at
2:1 and 1:2 synergistic effects were seen. LAS and copper at ratios
of 1:1 and 2:1 had additive effects; however, at 1:2, high
concentrations and longer exposure times had additive effects, and
low concentrations and shorter exposure times had synergistic
effects (Tsai & McKee, 1978).
When eggs of cod (Gadus morhua) were exposed to mixtures of
LAS and zinc or copper from fertilization to hatching, zinc had a
weak synergistic affect on the toxicity of LAS, but LAS had a strong
synergistic affect on the toxicity of copper (Swedmark & Granmo,
1981).
In a study of the effect of polyoxyethylene (20) on the acute
toxicity of C12 LAS, red killifish (Oryzias latipes) and carp
(Cyprinus carpio) were exposed to the 48-h LC50 of LAS for the
respective species and to 5-40 mg/litre of either a polyoxyethylene
sorbitan ester, a polyethylene glycol, a polypropylene glycol, or a
protein (albumin, kaolin, or bentonite). Addition of most of these
substances decreased mortality. No mortality was observed in carp
exposed to LAS and 14 or 28 mg/litre polyoxyethylene (20) sorbitan
monooleate (SMOE20) or to other nonionic surfactants with a similar
polyoxyethylene sorbitan ester structure-polyoxyethylene (6)
sorbitan monolaurate, polyoxy-ethylene (6) sorbitan monooleate,
polyoxyethylene (20) sorbitan monolaurate, and polyoxyethylene (20)
sorbitan monostearate-or to albumin. No significant effect on
mortality induced by LAS was reported after simultaneous exposure to
polyoxyethylene (6) sorbitan monostearate, polyethylene glycol,
polypropylene glycol, kaolin, or bentonite. The authors also
examined the histological effects of these chemicals on the gills of
carp exposed to high concentrations of LAS, including the 48-h
LC50 of 3.5 mg/litre and the LC100 of 7 mg/litre. Histological
changes in fish exposed only to 3.5 mg/litre LAS included the
appearance of mucous cells and agglutination of the secondary
lamellae; carp exposed to a mixture of LAS and SMOE20 showed only
slight swelling of the secondary lamellae and slight proliferation
of the gill epidermal cells. Exposure only to LAS at 7 mg/litre
resulted in marked proliferation of the epidermal cells and
agglutination of secondary lamellae; exposure to both LAS and SMOE20
induced only swelling of the secondary lamellae. No effects were
reported on the gills of control fish or on other organs of the
exposed fish; and no significant differences from controls were
reported in haematological or serum biochemical parameters for fish
exposed to either LAS or the LAS:SMOE20 mixture. When the
distribution of LAS in the tissues and organs of carp was examined,
higher levels were found in the blood and most organs after exposure
to LAS only than after exposure to the mixture; the differences were
statistically significant in blood, muscle, and gill but not in
spleen or gall-bladder. Adsorption of the 5- and 6-phenyl isomers of
LAS was similar when they were given alone or in conjunction with
SMOE20, but more of the 4- and (especially) the 2-phenyl isomers was
adsorbed by fish receiving LAS alone, indicating that SMOE20
decreases the acute toxicity of LAS to fish by decreasing the
adsorption on the gills of the more toxic isomer (Toshima et al.,
1992).
A9.3.4 Amphibia
No reliable data were available.
A9.3.5 Studies of the mesocosm and communities
Diversity and similarity indices were used in many studies to
assess the effects of LAS on phytoplankton communities, usually on
the basis of taxonomy, mean number of species, and density. Mean
density and similarity indices were then compared with those of
controls. In general, these indices are not sensitive to change, as
the densities of some species may decrease while the indices do not.
The effects of C12 and C13 LAS on short-term photosynthetic
activity were studied in plankton sampled from Acton Lake, Ohio,
United States, during May-October in the laboratory and in situ.
Toxicity increased with the temperature of the water, the most
sensitive period being June-August, and LAS were less toxic during
periods of diatom dominance and low phytoplankton density. Thus the
density of diatoms decreased during June-August, and that of green
and blue algae increased. The comparison of the results of
laboratory and field tests was highly dependent on species and the
in-situ end-point. Short-term tests for photosynthetic activity in
situ gave 3-h EC50 values of 0.2-8.1 mg/litre (mean, 1.9 mg/litre)
for C13 LAS and 0.5-8.0 mg/litre (mean, 3.4 mg/litre) for C12
LAS (Lewis & Hamm, 1986). (See also section 9.3.1.1.)
In another study of the effect of LAS on phytoplankton
communities in Acton Lake, Ohio (Lewis, 1986), phytoplankton were
exposed in situ to LAS at a concentration of 0.01, 0.02, 0.24,
0.80, 27, or 108 mg/litre for 10 days. The LOEL for LAS, based on
community similarity indices and the mean number of species, was
108 mg/litre. The similarity index (coefficient of community)
decreased as the concentration of LAS increased, with calculated
values of 0.62 at 0.01 mg/litre and 0.43 at 108 mg/litre. No
significant effects were seen on the community diversity index or
phytoplankton density. Green algae were the species least affected,
on the basis of abundance, followed in order of decreasing tolerance
by blue-green algae and diatom species. Chlorophyta species were
the most abundant at higher concentrations of LAS, comprising 74% of
the total cell volume at 108 mg/litre; their abundance tended to
increase to a maximum at this concentration and then decrease to
values similar to those of the controls. Chlorophyta species of
the genera Chlamydomonas, Oocystis, and Sphaerocystis were not
found after exposure to higher concentrations of LAS. Chlamydomonas
was found only in waters with a concentration of LAS <
0.8 mg/litre, and Oocystis and Sphaerocystis were found only at
concentrations < 27 mg/litre. The peak density of blue-green
phytoplankton (56% of cell volume) was achieved at 0.24 mg/litre
LAS, declining to 17% at 108 mg/litre. The density of the major
species, Schizothrix calcicola, was greatest at 27 mg/litre LAS but
declined to a level below that of controls at 108 mg/litre LAS. The
abundance of diatoms was low at all concentrations of LAS. At
concentrations < 0.24 mg/litre, the average density of diatoms
was 23% of the total cell volume, similar to that of controls; at
concentrations of 0.24-0.8 mg/litre, the diatom density was 10% of
the cell volume. The mean densities of the major diatom species,
such as Cyclotella glomerata, Cyclotella pseudostelligera, and
Nitzschia frustulum v. perminuta, followed the overall trend for
diatoms, reaching a peak at low LAS concentrations and declining to
control values at higher concentrations.
In the same study, the laboratory-based 96-h EC50 values for
exposure to C11.8 LAS were calculated to be 29.0 mg/litre for
Selenastrum and 0.0096 mg/litre for Microcystis, on the basis of
population growth. The lowest concentration of LAS that produced a
significant effect on algal growth in the laboratory was
0.05-1.0 mg/litre, which is considerably lower than the
27-108 mg/litre value found to be the lowest that altered the
structure of a natural phytoplankton community. The differences
between the results of laboratory and field tests were smaller for a
laboratory-based EC50 than for an LOEL. Calculations based on the
EC50 give a 30-fold difference for Microcystis but essentially no
difference for Selenastrum (Lewis, 1986).
The toxic effects of LAS were also examined on periphyton
communities above and below the outfall of a wastewater treatment
plant on Little Miami River, Ohio, United States. The dominant
species at both test sites were diatoms, Amphora perpusilla and
Navicula minima accounting for at least 80% of the total cell
volume. The tests were conducted in situ, with 21-day
continous-flow exposure to LAS (average chain length, C11.9) in
river water entering submerged exposure tubes at a concentration of
0.2, 1.1, 9.8, or 28.1 mg/litre, after a four-week colonization
period. The delivery rate of LAS was adjusted daily according to
measurements of river flow in order to maintain the desired test
concentrations. The periphyton at the site below the treatment plant
outfall were exposed to LAS in the presence of 20-30% treated
municipal effluent. No effects on the structure or function of the
periphyton community above the outfall were reported after exposure
to an average concentration of LAS < 1 mg/litre. The lowest
concentration that had an effect on the upstream periphyton
community was 9.8 mg/litre, which reduced photosynthesis by 16%; a
concentration of 28.1 mg/litre reduced photosynthesis by 64%, with a
noticeable reduction in chlorophyll a. No effects on community
similarity or diversity were reported in comparison with control
communities. Mean cell densities were increased by 26% after
exposure to 0.2 mg/litre LAS and by 17% after exposure to
1.1 mg/litre; exposure to 28.1 mg/litre reduced mean cell density by
28%. Exposure to LAS had no significant effects on the abundance of
the three main species in the upstream community. Increased
photosynthesis (by 12-39%) and chlorophyll a (50-51%), were
reported after exposure to 1.1 or 9.8 mg/litre LAS, but exposure to
28.1 mg/litre resulted in a 52% decrease in photosynthesis and a 71%
decrease in chlorophyll a. No effects on the similarity or
diversity of the periphyton community were reported at any
concentration of LAS tested. Cell densities of periphyton were
increased by 34% after exposure to 9.8 mg/litre LAS and by 13% after
exposure to 28.1 mg/litre. The abundance of the three main species
in the downstream periphyton community was not affected. The lowest
concentration of LAS that induced an effect was 3.3 mg/litre for the
upstream periphyton community and 16.6 mg/litre for the downstream
community. The authors suggested that the difference between the two
values was due to the presence of 20-30% sewage downstream, which
reduced the bioavailability of LAS (Lewis et al., in press).
When an aquatic ecosystem was exposed to LAS at concentrations
of 0.25-1.1 mg/litre for 90 days, the numbers of phytoplankton were
unaffected but primary productivity was significantly reduced at all
concentrations. The zooplankton population showed a more variable
response: the number of rotifers was reduced at all concentrations,
and those of Diaptomus and Cyclops were reduced at >
0.51 mg/litre. The number of ostracods was decreased at
0.38 mg/litre but was increased at 0.51 and 1.1 mg/litre. The
chironomid population was significantly reduced at concentrations
> 0.38 mg/litre (Chattopadhyay & Konar, 1985). Exposure of an
aquatic ecosystem consisting of phytoplankton, zooplankton, and
benthic organisms to 1 mg/litre of a preparation of LAS for 90 days
significantly reduced the numbers of phytoplankton and zooplankton
per litre but did not significantly affect the numbers of chironomid
larvae (Panigrahi & Konar (1986).
The effect of C12 LAS on microbial communities was studied in
a model ecosystem consisting of a 19-litre glass tank containing
sediment from Winton Lake, Ohio, United States, and several trophic
levels, comprising bacteria, algae, macrophytes (Elodea canadensis,
Lemna minor), macroinvertebrates (Daphnia magna, Parantanytarsus
parthenogenica), and blugill sunfish (Lepomis macrochirus).
After a four-week equilibrium period, LAS were added at 0.5 or
5.0 mg/litre to a flow-through system with six to 10 replacements
per day for 26 days. The structure of the microbial communities was
not affected, and no differences were reported in mean biomass or
number of colony-forming units between the microorganisms exposed at
the two levels. The function of the microbial communities, assayed
by measuring the degradation of both LAS and D-glucose, was reduced
only at 5.0 mg/litre. In a similar system, in which the same
concentrations of LAS were added in the form of sewage effluent, no
effect was seen on the structure of the microbial community or on
their function, measured only as the degradation of LAS (Larson &
Maki, 1982).
Addition of LAS (average chain length, C11.8) at a measured,
relatively uniform concentration of 0.36 ± 0.05 mg/litre to 50-m
outdoor experimental streams had no effect on total density, species
richness, percentage similarity, or dominance of macroinvertebrates
or periphyton or on the processing of organic matter of leaf discs.
Fathead minnows (Pimephales promelas) and amphipods (Hyallela
azteca) were exposed in groups of 10 and 20 per box placed in the
streams at three locations. The mortality rates of the amphipods
were 17-25% after exposure to LAS and 47% among controls; no effects
were seen on the survival or weights of the fish, although minor
effects were found on length (Fairchild et al., 1993).
A study of the fate and effects of surfactants in outdoor
artificial streams addressed the effect of LAS on drift and
population densities of macroinverebrates, the reproductive
behaviour of an amphipod, the scud (Gammarus pulex), the survival
of a fish, the three-spined stickleback (Gasterosteus aculeatus),
and photosynthesis by the community. The concentration of LAS in
sediment was reported to increase with increasing water
concentration, and selective adsorption of longer-chain LAS
homologues to sediment was reported. The microbial populations of
both the water and the sediment adapted to LAS, resulting in a
reduction in its half-life during the test. LAS at concentrations
< 1.5 mg/litre did not affect macroinverebrate drift, population
density, or community photosynthesis. Survival of the fish and
reproduction by the amphipod were affected at concentrations of
1.5-3.0 mg/litre (Mitchell & Holt, 1993).
A9.3.6 Field studies
The effect of LAS downstream of a sewage outflow was studied by
monitoring sediment, water, and the distribution of invertebrates at
an upstream control site, a site near the discharge point, and a
site 200 m downstream of the outflow. The concentrations of LAS in
sediment were 1-40 mg/kg dry weight, with concentrations < 2 mg/kg
at the control site and 200 m downstream. No effect of LAS in the
effluent or in the streambed sediments could be discerned on the
invertebrate populations (Ladle et al., 1989).
A9.3.7 Toxicity of biodegradation intermediates and impurities
of linear alkylbenzene sulfonates
Tests of degradation products and impurities of LAS show that
they are less toxic than LAS themselves.
A9.3.7.1 Individual compounds
The 48-h LC50 values in Daphnia magna were 208 ± 85 mg/litre
for sulfophenylundecanoic acid, disodium salt (mixed isomers, 6-10
phenyl); about 6000 mg/litre for 3-(sulfophenyl) butyric acid,
disodium salt; and about 5000 mg/litre for 4-(sulfophenyl) valeric
acid, disodium salt. The equivalent 48-h LC50 values in the
fathead minnow (Pimephales promelas) were 77 ± 12, about 10 000,
and about 10 000 mg/litre, respectively (Kimerle & Swisher, 1977).
The 24-h LC50 values in Daphnia were about 22 000 mg/litre for
3-sulfophenylbutyric acid, disodium salt; about 12 000 mg/litre for
3-sulfophenylheptanoic acid, disodium salt; > 22 000 mg/litre for
3-sulfophenylbutyric acid, disodium salt; and 2 000 mg/litre for
sulfophenylundecanoic acid, disodium salt. Other tests were carried
out with the last two compounds, giving 96-h LC50 values of about
28 000 and 1200 mg/litre in fathead minnows (Pimephales promelas);
28-day NOELs of > 2000 and > 200 mg/litre for survival and
reproduction of Daphnia; and 30-day NOECs of > 1400 and >
52 mg/litre for survival of the fry of fathead minnows (egg
hatchability and fry growth were less sensitive) (Swisher et al.,
1978).
The 96-h LC50 for mixed isomers of sulfophenylundecanoic acid
disodium salt in bluegill (Leponis macrochirus) was 75 mg/litre
(Swisher et al., 1964). The 24-96-h LC50 values in fathead minnows
were 1000-1500 mg/litre for sulfophenylundecanoic acid (C11) and
25 000-32 000 mg/litre for sulfophenyl butyrate (C4) (Swisher et
al., 1978).
The 48-h LC50 for the alkanoic acid derivatives of
2-sulfophenyl C13 LAS and 4-sulfophenyl C13 LAS in nearly pure
form was > 800 mg/litre in goldfish (Carassius auratus) (Divo &
Cardini, 1980).
The 24-h LC50 values for Daphnia magna exposed to
dialkyltetralin sulfonates, which are trace contaminants of LAS,
were 420, 195, 110, 50, and 27 mg/litre for tetralin sodium
sulfonates of chain lengths C10, C11, C12, and C13,
respectively (Arthur D. Little Inc., 1991).
A9.3.7.2 Effluents
Interpretation of tests on effluents must take into account the
following:
-- As concentrations arwe often reported as MBAS, testing of
effluent from a sewage treatment plant may result in overestimation
of the actual concentrations of LAS, owing to interference (see
section 2.3).
-- The bioavailability of LAS is decreased by the presence of high
concentrations of suspended solids; thus, as effluents are diluted
in the environment, availability is usually increased, although
biodegradation occurs.
Addition of LAS (C10-C15) to detergent-free activated sludge
plant effluent (95% was removed as MBAS) gave a nominal 96-h LC50
in rainbow trout (Oncorhynchus mykiss) of 0.36 mg/litre. After
treatment, the 96-h LC50 was 29.5 mg/litre, expressed in terms of
the concentration of the surfactant in the influent (Brown et al.,
1978).
When bluegill were exposed to effluent from continuous-flow
activated sludge units fed 100 mg/litre LAS, none died during
4-11-day exposure (Swisher et al., 1964).
A9.4 Terrestrial organisms
A9.4.1 Terrestrial plants
Young seedlings of tomato, lettuce, radish, pea, cucumber, and
barley were grown in a soil-based compost and were watered and given
a foliar spray of a preparation of LAS. No effects were noted at
concentrations up to 100 mg/litre (Gilbert & Pettigrew, 1984). In
another study, barley, tomato, and bean plants were grown from seed
and watered with a solution containing LAS at a concentration of 10,
25, or 40 mg/litre. Plants that received the lowest dose germinated
at the same time as controls, but plants watered at 25 or
40 mg/litre germinated three days later. The growth of barley plants
was inhibited at all three concentrations; however, the dose of
25 mg/litre increased the growth rate of beans, and the highest dose
increased the growth rate of both tomatoes and beans (Lopez-Zavala
et al., 1975).
The 21-day EC50 values for LAS (C10-C13), based on the
emergence of seedlings and early stages of growth, were 167 mg/litre
in sorghum, 289 mg/litre in sunflower, and 316 mg/kg in mung bean.
The highest concentration that caused no significant reduction in
the growth of any of the three species was 100 mg/kg (Holt et al.,
1989; Mieure et al., 1990). In a second study, 407 mg/kg C11.36 or
393 mg/kg C13.13 LAS were mixed with sewage sludge, and nine
common plant species, including five crop plants, were exposed as
seed either at the same time or two weeks after application of the
sludge to soil at a rate of 9000 kg/ha. There was no significant
effect on seed germination and no significant inhibition of growth
(Mieure et al., 1990).
Orchid seedlings (Phalaenopsis or Epidendrum sp.) were grown
in culture media containing either the sodium or the ammonium salt
of LAS at a concentration of 10, 100, or 1000 mg/litre. The lowest
dose had no effect on growth, and that of ammonium LAS had no effect
on germination. At 100 mg/litre, survival was halved and germination
completely inhibited (Ernst et al., 1971). A concentration of
1000 mg/litre caused drastic changes in morphology, loss of
membranes, swelling of thylakoids, and the appearance of dense
osmophilic granules in chloroplasts (Healey et al., 1971).
The growth of pea seedlings grown for 26 days in quartz sand to
which 0.005% (50 mg/kg) LAS had been added was significantly
reduced, as measured by the fresh weight of roots and the length and
fresh weight of pea greens (Lichtenstein et al., 1967).
LAS were not toxic with respect to growth at the early life
stages of radish, Chinese cabbage, and rice when added in hydroponic
culture at concentrations of 10, 20, and 20 mg/litre, respectively;
concentrations of 20, 35, and 35 mg/litre were toxic (Takita, 1982).
When seeds of Pisum sativum and Crotolaria juncea were
exposed to LAS for 24 h before sowing, the percentage germination
was reduced at concentrations of 1 ml/litre for P. sativum and
10 ml/litre for C. juncea, although no statistical analysis was
presented. No germination occurred after exposure to LAS at
concentrations of 20 ml/litre for P. sativum and 40 ml/litre for
C. juncea. Radicle length was reduced at > 0.1 ml/litre in both
species (Sharma et al., 1985).
Application of LAS at 50 g/m2 under field conditions to loamy
and sandy soils (corresponding to 0.47-1 mg/kg dry weight,
respectively) led to considerable physiological damage, including
leaf necrosis, chlorosis, and turgescence, to ryegrass (Lolium
perenne) after 14 days; however, there was no difference in the
fresh weight yield after harvesting at 45-54 days (Litz et al.,
1987).
A9.4.2 Terrestrial invertebrates
When the earthworm Eisenia foetida was exposed to C11.36 LAS
incorporated into soil at nominal concentrations of 63-1000 mg/kg
dry weight, the 14-day LC50 was > 1000 mg/kg. On the basis of a
statistical analysis of body weights, the no-effect concentration
was 250 mg/kg; this was confirmed by HPLC to be 235 mg/kg. In a
second study, C11.36 and C13.13 LAS were incorporated into
sludge and applied to soil, and the earthworm Lumbricus terrestris
was exposed to the subsequent mixture, which contained LAS at
concentrations of 84-1333 mg/kg. The 14-day LC50 was again found
to be greater than the highest concentration (> 1333 mg/kg). The
no-effect concentration, based on weight and burrowing behaviour,
was the nominal concentration of 667 mg/kg, measured by HPLC as
613 mg/kg. The worms were exposed, however, to LAS under conditions
of continuous light, which would inhibit them from surfacing to feed
and thus increase their exposure to and the toxicity of the test
over that of the same concentration in the field (Mieure et al.,
1990).
Topical application to house flies (Musca domestica) of LAS at
the same time as parathion, diazinon, or dieldrin in ratios of 1:1
and 1:10 had no effect on the toxicity of the insecticides. When LAS
were added to soil treated with parathion or diazinon, however, a
significant synergistic effect was observed on the toxicity of the
insecticides to the fruit fly Drosophila melanogaster. The optimal
concentration of LAS that resulted in synergy was 23 mg/kg
(Lichtenstein, 1966).
A9.4.3 Birds
No significant effect on egg quality was found after Leghorn
chickens were fed a diet containing 200 mg/kg LAS for 45 days
(Lopez-Zavala et al., 1975).
B. alpha-Olefin sulfonates
B1. SUMMARY
See Overall Summary, Evaluation, and Recommendations (pp. 7-21).
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
B2.1 Identity
Chemical formula: CnH2nO3S Na, CnH2n+1O4S Na ( n = 14-18)
Chemical structure: CH3(CH2)jCH:CH(CH2)kSO3- Na+
CH3(CH2)mCH(CH2)nSO3- Na+
OH ( m,n, integers)
Common names: Sodium alpha-olefinsulfonate,
alpha-olefin-sulfonic acid sodium salt, AOS
sodium salt
Common trade names: Bioterge AS 40 F, Elfan OS 46, Geropon
MLS/A, Hostapur OS Brands, Lipolan, Lipomix
G, Lipon PB-800, Lutensit A-PS, Nansa
LSS38/AS, Sawaclean, Sermul EA 214,
Sulframin AOS, Witconate (McCutcheon, 1989)
Abbreviations: AOS, AOS-Na
CAS Registry numbers: 29963-33-5 Sodium 1-tetradecenesulfonate
29734-60-9: Sodium hexadecenesulfonate
13513-23-0: Sodium 3-hydroxyhexadecyl-1-
sulfonate
26446-92-4: Octadecene-1-sulfonic acid
sodium salt
13513-42-3: 3-Hydroxy-1-octadecanesulfonic
acid, sodium salt
Specifications: AOS are mixtures consisting of about 60-65%
alkene sulfonates, 30-35% hydroxylalkane
sulfonates, and 5-10% disulfonates. Various
positional isomers of alkene sulfonates and
hydroxyalkane sulfonates have been reported
(Gentempo et al., 1985; Williamson, 1993).
Sodium C14-C16 AOS are typically
shipped as solutions containing 35-40%
active matter in water. Sodium C16-C18
AOS are typically slurries containing
28-30% active matter in water at ambient
temperature.
B2.2 Physical and chemical properties
AOS are white crystalline solids consisting of various chemical
compounds and their isomers, with different properties. Typical
properties of AOS are given in Table 30. Two ranges are usually
offered; the commonest are based on C14-C16 olefin and the other
on C16-C18 olefin. Detergency is maximal with alkyl chain
lengths of C15-C18. Maximal detergency is also obtained with the
same range of alkyl chain lengths in a detergent formulation that
includes alkali builders and chelating agents (Yamane et al., 1970).
AOS are stable, even in hot acidic media.
Table 30. Relationship between alkyl chain length, Krafft point,
critical micelle concentration (CMC), and surface
tension of alpha-olefin sulfonates
Alkyl chain Krafft pointa CMCal Surface tension
length (°C) (g/litre) (dyne/cm)
12 - 4.0 -
14 - 1.0 30
16 10 0.3 33
18 30 0.1 35
20 40 - -
(25°C) (25°C)
From Ohki & Tokiwa (1970)
a The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature. For
surfactants, there is a distinct, sharp bend (break-point) in the
solubility-temperature curve. The steep increase in solubility
above the sharp bend is caused by micelle formation. The point of
intersection of the solubility and critical micelle curves, plotted
as a function of temperature, is referred to as the Krafft point.
This is a triple point at which surfactant molecules coexist as
monomers, micelles, and hydrated solids. The temperature
corresponding to the Krafft point is called the Krafft temperature.
At above the Krafft temperature and critical micelle concentration,
a micellar solution is formed. Under these conditions, higher levels
than the aqueous solubility may be obtained.
B2.3 Analytical methods
There is no officially recognized specific procedure for the
analysis of AOS in environmental samples. The methods commonly used
to analyse anionic surfactants are also used for AOS, except those
involving high-performance liquid chromatography (HPLC), which has
limited use in environmental analyses for AOS, because they do not
absorb ultra-violet radiation as effectively as do linear
alkylbenzene sulfonates (LAS). A modified version of the methylene
blue-active substance (MBAS)-HPLC method described in the monograph
on LAS has been developed (Takita & Oba, 1985).
Nonspecific methods used in the analysis of anionic surfactants
in general, such as the MBAS method, can be used to analyse
materials for AOS (see section 2.3 of the monograph on LAS).
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
AOS do not occur naturally.
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
AOS are synthesized industrially. Although they have been
available since the 1930s, production for use in commercial
surfactant formulations was somewhat limited until recently owing to
a lack of suitable feedstock. Development of continuous and
short-contact sulfur trioxide sulfonation processes and the
increased availability of highly pure Ziegler-derived alpha-olefin
feedstock has recently made AOS surfactants competitive with other
surfactants on the market (Arthur D. Little Inc., 1977, 1981).
The estimated world consumption of AOS in 1988 was 50 200 tonnes
(Colin A. Houston & Associates Inc., 1990). In 1990, that group
estimated that world consumption would be 51 900 tonnes; an
alternative estimate (Hewin International Inc. 1992) was 76 000
tonnes (Table 31).
Table 31. Estimated worldwide consumption of alpha-olefin
sulfonates (tonnes)
Region Household Personal Industrial and All uses
products care institutional
products use
North America 3 000 7 000 4 000 14 000
Western Europe 2 000 3 000 3 000 8 000
Japan 24 000 7 000 2 000 33 000
Rest of the 18 000 3 000 - 21 000
world
Total 57 000 20 000 9 000 76 000
From Hewin International Inc. (1992)
AOS are prepared commercially by direct sulfonation of linear
alpha-olefins with a dilute stream of vaporized sulfur trioxide in a
continuous thin-film reactor. The olefin is obtained by wax cracking
or ethylene polymerization with a Ziegler-type catalyst (Tomiyama,
1970). The reaction is complex and follows several paths, forming
large amounts of various sultones as intermediates which hydrolyse
during subsequent quenching and neutralization. Commercial AOS
products contain a mixture of two major components, alkene sulfonate
and hydroxyalkane sulfonate, with smaller amounts of alkene
disulfonates, hydroxyalkane disulfonates, and saturated sultones.
B3.2.2 Uses
AOS are good detergents, have good foaming characteristics in
hard water and are used in heavy-duty laundry detergents, light-duty
dishwashing detergents, shampoos, and cosmetics. Table 31 indicates
the use patterns for AOS.
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
It can be inferred that AOS are transported into the environment
in a similar manner to that established for LAS, alkyl sulfates, and
other detergent surfactants. Fewer data are available on the
environmental transport, distribution, and transformation of AOS
than for LAS. The environmental fate of AOS is similar to that of
LAS and alkyl sulfates: it is readily biodegraded under aerobic
conditions, and primary biodegradation is complete within 2-10 days,
depending on the temperature. At temperatures below 5-10°C,
biodegradation kinetics are reduced, owing to a reduction in
microbial activity. No data were available on abiotic degradation.
There was no evidence of bioaccumulation or bioconcentration in a
study of fish in which the uptake and distribution of AOS were
examined.
B4.1 Transport and distribution between media
In the same manner as other detergent compounds, AOS are
discharged into the environment in wastewater. The wastewater may
undergo sewage treatment if such facilities are available. In
countries where there are no adequate wastewater treatment
facilities, AOS released to the environment are removed by
biodegradation and adsorption mechanisms (see section 4.2 of the
monograph on LAS).
Limited studies of the adsorption of AOS are available. In a
study of the adsorption of C12 AOS on river sediments, the
equilibrium quantities adsorbed were proportional to the organic
carbon content of the sediments, with a sorption coefficient Koc
(dimensionless; normalized for the level of organic matter) of 0.65.
This indicates that adsorption of C12 AOS is slightly weaker,
than, for example, that of C12 LAS or C12 alkyl sulfonates
(Urano et al., 1984). Like other detergent chemicals, AOS are
adsorbed onto sewage sludge and river sediments in the environment.
B4.2 Biotransformation
B4.2.1 Biodegradation
B4.2.1.1 Aerobic biodegradation
Primary biodegradation of AOS, studied in die-away tests in
water from various sites on the Tama River, Japan, was complete
within three to five days when measured by the MBAS method; however,
total organic carbon was completely removed after an incubation time
of 20 days. In a study of AOS in seawater collected from the mouth
of the Tama River, 99% of MBAS was removed within one day, and 90%
of organic carbon was removed within five days (Sekiguchi et al.,
1975b).
In a comparison of the MBAS and total organic carbon methods for
measuring biodegradation with the shake-culture method, AOS lost 99%
of their activity as measured by the MBAS method and 90% of total
carbon within one day; 100% was lost within five days (Sekiguchi et
al., 1975a). In another study, complete loss of parent AOS (initial
concentration, 100 mg/litre) as determined by the MBAS method was
seen within 15 days, and 90% of total organic carbon was removed
within eight days (Miura et al., 1979). In a static die-away test
system, 90% biodegradation of three commercial AOS products,
comprising 100% C14-C16 AOS and > 95% C15-C18 AOS (determined
as MBAS), was reported within four days (Gafa & Lattanzi, 1974).
In a shake-culture test in Bunch-Cambers medium, C15-C18 AOS
were degraded by 99% (determined as MBAS) or 90% (removal of total
organic carbon) within one day; 100% total organic carbon was
removed within five days. The authors did not verify whether the
removal was the result of adsorption or mineralization (Sekiguchi et
al., 1972). The biodegradation of C15 AOS and three C15-C18
compounds with disulfonate contents of < 4, 15, and 50% in a
shake-flask culture system was reported to be 96% (determined as
MBAS), with no significant difference between compounds (Oba et al.,
1968b).
In a modified OECD screening test, 85% of C14-C18 AOS
(measured as chemical oxygen demand) was removed. Measurement of
MBAS in the same test indicated 99% removal (Gerike, 1987).
The aerobic biodegradation of 20 mg/litre AOS at 27°C was
followed during a 10-day incubation period. Primary degradation,
measured by the MBAS method, was complete within 10 days. The
theoretical CO2 production had reached 30-40% within that time
(Itoh et al., 1979).
The oxygen uptake of C14-C18 AOS was reported to be 85% of
the theoretical oxygen demand in a closed-bottle test (Gerike,
1987). The average biochemical oxygen demand for C12-C18 AOS
containing up to 40% hydroxylalkane sulfonates was 51.6% at five
days, while glucose under the same conditions had a biochemical
oxygen demand of 69.6% (Procter & Gamble Co, unpublished data).
The primary and ultimate biodegradability of a series of pure
AOS homologues (C12, C14, C16, and C18) was determined by
measuing CO2 production. Primary biodegradation was 98-99% within
three days, the rate of degradation varying with chain length.
Degradation of C12 and C14 AOS occurred at a similar rate (65%
within 30 days), but C18 AOS degraded more slowly. Mineralization
of all AOS samples was reported to be at least 50% within two weeks,
whereas mineralization of glucose during that time was 75-80%
(Kravetz et al., 1982). In a study of the biodegradation of the two
major breakdown products of AOS, alkene sulfonate and hydroxyalkane
sulfonate, AOS homologues (C15, C16, C17, C18) were degraded
to about 50%, and in each case the alkene sulfonate was degraded at
least twice as fast as the hydroxyalkane sulfonate (Sekiguchi et
al., 1975c).
The biodegradation of C18 AOS at a concentration of
28 mg/litre was studied in activated sludge (concentration, 100 mg
dry matter per litre) over 12 days: 90% was lost within eight days,
as measured by removal of chemical oxygen demand. The specific rate
of biodegradation was calculated to be 5.3 mg/g per h (Pitter &
Fuka, 1979).
In the OECD confirmatory test with activated sludge, 20 mg/litre
AOS were degraded, as follows: 97% C14 AOS within 17 days, 98%
C16 AOS within seven days, and 94% C14-C18 AOS within eight
days (Maag et al., 1975).
Primary biodegradation of C15-C18 AOS was dependent on
incubation temperature in die-away tests with water from the Tama
River, Japan. Primary biodegradation was complete within two days at
27°C, within five days at 15°C, and within two days at 21°C;
however, at a water temperature of 10°C about 20% of the AOS had
still not been degraded within the nine-day test (Kikuchi, 1985).
When C15-C18 AOS were added to seawater, no MBAS activity
was present after five days (Marquis et al., 1966).
B4.2.1.2 Anaerobic degradation
The primary anaerobic biodegradation of C15-C18 AOS
(measured as MBAS) by bacteria on sludge sampled from a sewage
treatment plant was 19% within 14 days and 31% within 28 days. More
parent AOS were degraded by bacteria from the bottom of a private
cesspool, with 34% lost within 14 days and 43% within 28 days. The
anaerobic degradation reported may have been due to the presence of
hydroxyalkane sulfonate compounds (Oba et al., 1967). AOS and LAS
were reported to be the two surfactants that were least degraded
anaerobically (Itoh et al., 1987).
B4.2.2 Abiotic degradation
No information was available.
B4.2.3 Bioaccumulation and biomagnification
Rapid, significant absorption of 14C-AOS by the gills of
goldfish (Carassius auratus) was seen after exposure to AOS at a
concentration of 10 mg/litre. The concentration of AOS in the gills
increased from 0.3 mg/kg after 0.5 h of exposure to 48.3 mg/kg after
3 h. AOS were not detected in the alimentary canal (Tomiyama, 1975).
Three hours is a relatively short exposure, and the authors did not
determine whether a steady state of adsorption had been achieved.
Tomiyama (1978) reported that AOS accumulated to the greatest extent
in the gills of exposed fish, with additional accumulation in the
gall-bladder. Only limited conclusions can be drawn from this study,
however, owing to the short exposure period.
B4.3 Interaction with other physical, chemical, and biological
factors
No information was available.
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Few data are available on environmental concentrations of AOS
because of the lack of an accepted analytical method for this
purpose. A modified analytical method based on MBAS-HPLC measurement
has been used to measure AOS (Takita & Oba, 1985). The concentration
in the Tama River, Japan, was calculated to be < 0.0016-0.002
mg/litre.
The annual average concentration of AOS in wastewater was
0.160-0.164 mg/litre on the basis of total MBAS concentrations of
8.4 and 8.2 mg/litre. AOS was not detected in the effluent from a
treatment plant outfall (Oba et al., 1976).
AOS can be expected to mineralize rapidly in all environmental
compartments and to be removed to a large extent during sewage
treatment. Environmental concentrations in receiving surface waters,
sediments, soils, estuaries, and the marine environment can also be
expected to be low.
B6. KINETICS
Section summary
AOS administered orally are readily absorbed by the
gastrointestinal tract of rats and are distributed throughout the
body; they are eliminated primarily in the urine and, to a lesser
extent, in the faeces within 24 h of administration. AOS applied
dermally are absorbed only minimally by intact skin. Several
metabolites have been isolated, but their chemical structures have
not been identified.
B6.1 Absorption, distribution, and excretion
14C-AOS were synthesized by sulfonation and hydrolysis of
tetradecene-1-14C. The labelled compound was composed of a
mixture of about 55% sodium 3-hydroxyalkane sulfonate
[C11H23CH(OH)-CH2SO3Na] and about 45% sodium 2-14C alkenyl sulfonate
[C11H23CH2CH214CH2SO3Na]. After oral administration of
100 mg/kg 14C-AOS (50 µCi/kg) in water to rats, the level of
radiolabel in blood reached a peak at 3 h (0.08% of the dose/ml) and
then rapidly decreased, since little radioactivity was detected 24 h
after the administration. At 4 h after administration, 0.45% of the
dose per gram of tissue was detected in liver and 0.65% in kidney,
but the levels in tissues other than the gastrointestinal tract were
< 0.1%. Thereafter, the radiolabel in organs and tissues decreased
rapidly, and 24 h after administration, about 0.8% was detected in
the caecal contents and < 0.02% in other tissues. No specific
accumulation was observed in any tissue. Within 24 h of
administration, 72% of the dose was excreted in urine and 22% in
faeces. At the end of the experiment, after four days, no 14C
residue (< 0.1% of the dose) was detected in urine or faeces.
Cumulative excretion in the bile within 12 h after administration
was about 4.3% of the radioactivity administered (Inoue et al.,
1982).
The biological half-lives of AOS and their metabolites in blood
after intravenous administration of 10 mg/kg 14C-AOS in rats were
15 and 1 h, respectively. The marked difference in half-life can be
accounted for by the fact that the binding of AOS to plasma
proteins, especially serum albumin, increased in proportion to its
concentration while that of the metabolites did not increase to any
appreciable extent. The volume of distribution of AOS was
8 litres/kg, and that of the metabolites was 0.5 litres/kg (Inoue et
al., 1982).
A dose of 0.5 ml of a 0.2% aqueous solution of 14C-AOS was
applied to the dorsal skin (4 × 3 cm) of rats with bile-duct and
bladder cannulae. The total amount absorbed through the skin was
estimated to be about 0.6% on the basis of the recoveries of 14C
in urine, bile, and the main organs over 24 h. At that time, the
level of radiolabel was higher in the liver (0.123% of dose) than in
the kidney (0.059%), spleen (0.004%), brain (0.01%), or lung
(0.012%). A total of about 0.24% of the applied dose was recovered
in these organs. After 24 h, 0.33% of the radiolabel was excreted in
the urine and 0.08% in the bile. When the solution was painted on
skin damaged by 20 applications of cellophane adhesive tape to
remove the stratum corneum, the rates of excretion were 36.3% in the
urine and 1.8% in the bile (Minegishi et al., 1977).
B6.2 Biotransformation
AOS and its metabolites were investigated in tissues and
excrement after oral administration of 100 mg/kg 14C-AOS to rats.
AOS and a metabolite more polar than AOS were detected in blood,
liver, kidney, bile, and urine by thin-layer chromatography. As most
of the 14C-labelled compounds in urine were alcoholic,
unsaturated, and of sulfonic functionality, the metabolite may be a
hydroxylated or polyhydroxylated sulfonic acid with a shorter chain
than AOS, although the precise chemical structure remains to be
elucidated (Inoue et al., 1982).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 for AOS sodium salt in mice was 3000 mg/kg. AOS
are skin and eye irritants. Data from studies in experimental
animals are limited, but no effects were observed in a long-term
study in which oral doses of 250 mg/kg body weight per day were
administered to rats. Fetotoxicity was observed in the progeny of
rabbits administered a maternally toxic dose of 300 mg/kg body
weight per day.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of AOS in experimental animals; however, in
the limited studies available in which animals were administered AOS
orally or on the skin, there was no evidence of carcinogenicity.
The limited data available also indicate that AOS are not
genotoxic in vivo or in vitro.
B7.1 Single exposures
The LD50 values for AOS (sodium salt of sulfonated C15-C18
n-olefin) in male ddy mice were 3000 mg/kg body weight by oral
administration, 1660 mg/kg by subcutaneous injection, 170 mg/kg by
intraperitoneal injection, and 90 mg/kg by intravenous injection.
The toxic effects seen at high oral doses were reduced voluntary
activity, diarrhoea, anaemia, dyspnoea, and respiratory collapse.
Clonic convulsions followed by respiratory collapse were seen in
animals given the material intravenously (Oba et al., 1968a).
B7.2 Short-term exposure
No data were available.
B7.3 Long-term exposure; carcinogenicity
B7.3.1 Mouse
The skin of Swiss-Webster mice was painted with 20% C14-C18
AOS, 25% C14-C18 AOS, 20% C14-C16 AOS, 25% C14-C16 AOS,
6.7 or 8.3% C16 1,4-sultone, water, or acetone, or remained
untreated. Animals were treated with 0.02 ml of test material on
about 1 cm2 of exposed skin three times per week for 92 weeks.
Final necropsies were conducted when the survival of each group
reached 30% (approx. 19 months). Histopathological examination
showed no evidence of carcinogenicity with any test material (Haar,
1983).
B7.3.2 Rat
AOS (97.93% of a 60.4:39.6% (w/w) mixture of alkenyl sulfonate
and hydroxyalkane sulfonate; chain-length distribution, 25% C14,
45% C16, 30% C18) were fed to four groups of 50 male and 50
female CFY rats at a dietary level of 0, 1000, 2500, or 5000 ppm,
corresponding to 49, 122, or 245 mg/kg body weight per day, for two
years. No adverse clinical signs were seen, and survival rates were
not affected by treatment. The rate of body weight gain was
marginally lower during the second trimester of the study in both
males and females receiving 5000 ppm, and food intake was marginally
lower during the first year among females receiving 5000 ppm. During
the remainder of the study, body weight gain and food consumption
were similar to those of the control animals. Investigation of the
eyes, blood, and urine of controls and of those receiving 5000 ppm
several times during the experiment revealed no reaction to
treatment; and no changes related to treatment were seen in gross
appearance or organ weights of rats in any group killed after 104
weeks. Histological examination of a limited range of tissues did
not provide evidence of toxicity or tumour induction that could be
attributed to treatment (Hunter & Benson, 1976).
Groups of 40 male and 40 female Wistar rats were fed the
following materials in the diet for 24-27 weeks: 1, 0.75, or 0.5%
C14-C18 AOS (corresponding to 500, 375, or 250 mg/kg body weight
per day); 1, 0.75, or 0.5% C14-C18 AOS (corresponding to 500,
375, or 250 mg/kg body weight per day); or 0.33, 0.25, or 0.16%
C161,4-sultone (corresponding to 165, 125, or 80 mg/kg body weight
per day). One control group consisted of 100 males and 100 females
and another of 40 males and 40 females. No excess of tumours over
that in controls was observed with any treatment (Haar, 1983).
In 70-week studies on Wistar rats, 0.5 ml of a 1.0, 10, or 30%
aqueous solution of AOS or 0.5 ml of a 50% aqueous solution of a
detergent based on AOS was applied dermally; 24 h after the
application, each site was washed with warm water. No abnormal gross
or histopathological findings were reported (Tomizawa, 1978).
These studies are summarized in Table 32.
B7.4 Skin and eye irritation; sensitization
AOS (C10; purity, 99.21%) were applied as 0.5 g of a 20 or 30%
solution once a day for 15 days to the backs of three male Wistar
rats. The skin at the application site and the tissues of the tongue
and oral mucosa of animals receiving the 30% solution were examined
histologically 16 days after application. Body weight gain was
reduced in the group given the 20% solution, and body weight was
decreased in the group at 30%. Macroscopically, there were no
abnormalities at the application site. Histologically, although
atrophy of the stratum spinosum was noted, neither necrosis nor
Table 32. Carcinogenicity of alpha-olefin sulfonates (AOS) after long-term exposure
Species, strain, Test material Route Dosage Results Reference
numbers per group (specification)
Mouse, Swiss-Webster AOS, C14-C18 Dermal 0, 200, 250 mg/kg No gross or histopathological Haar (1983)
40 M, 40 F (water, acetone) adverse effects on skin
3 times/week,
92 weeks
Mouse, Swiss-Webster AOS, C14-C16 Dermal 0, 200, 250 mg/kg No gross or histopathological Haar (1983)
40 M, 40 F (water, acetone) adverse effects on skin
3 times/week,
92 weeks
Rat, CFY, 50 M, 50 F AOS, C14-C18 Oral 0, 0.1, 0.25, 0.5%, No adverse effects Hunter & Benson
(a.i., 97-93%) (diet) 2 years (1976)
Rat, X-MRC, 40 M, 40 F AOS, C14-C18 Oral 0, 0.5, 0.75, 1.0%, No excess of tumours in Haar (1983)
(diet) 24-27 months comparison with controls
Rat, X-MRC, 40 M, 40 F AOS, C14-C16 Oral 0, 0.5, 0.75, 1.0%, No excess of tumours in Haar (1983)
(diet) 24-27 months comparison with controls
Rat, Wistar, 10 M, 10 F AOS, C16-C19 Dermal 0, 250, 2500, 7500 No gross or histopathological Tomizawa (1978)
mg/kg bw, 3 times abnormalities
per week, 70 weeks
Rat, Wistar, 10 M, 10 F AOS-based Dermal 12.5 g/kg bw No gross or histopathological Tomizawa (1978)
detergent 3 times/week, abnormalities
70 weeks
M, male; F, female; a.i., active ingredient
inflammatory cell infiltration was present. No abnormalities of the
tongue were observed, but severe atrophy was observed in the mucosa
of the oral cavity. The local lesions caused by application of AOS
were reported to be minimal in comparison with those induced by
application of linear dodecylbenzenesulfate or lauryl sulfate (Sadai
& Mizuno, 1972).
Solutions of 0.05-4% AOS (sodium salt of sulfonated C15-C18
n-alpha-olefin) were instilled at a dose of 0.1 ml into the eyes
of one to three rabbits, and the eyes were examined after 24 h. No
abnormal findings were observed with the 0.05% solution, but slight
congestion was observed with 0.1% and marked reactions, including
severe congestion and oedema, increased secretion, opacity of the
cornea, and absence of the corneal reflex, were observed at > 1%
(Oba et al., 1968a).
Solutions of C14-C19 olefin (84% C15-C17) and five other
solutions consisting mainly of C10, C12, C14, C16, or C18
were instilled into the eyes of three rabbits at one of six
concentrations ranging from 0.01 to 5%. The rabbits were examined
over a period of 168 h. The materials elicited similar reactions. No
abnormal reaction was seen with 0.05%; slight congestion was
observed with 0.1% within 2 h after application of the solution; and
marked congestion or oedema was observed with 0.5%, which
disappeared by 24 h. In the groups treated with 1 or 5%, marked
reactions, including severe congestion and oedema, increased
secretion, turbidity of the cornea, and disappearance of the corneal
reflex, continued for 24 h but had usually completely disappeared by
120 h (Iimori et al., 1972).
In 1973, the apparent sensitizing potential of AOS attracted
attention (Haar, 1983). AOS can contain unsaturated gamma-sultones
when manufactured under certain conditions, and these are strong
sensitizers in guinea-pigs (Haar, 1983; Roberts & Williams, 1983;
Roberts et al., 1990). When the levels of these sultones were
reduced to low levels by altering the manufacturing techniques, AOS
no longer caused sensitization (Haar, 1983; Oba et al., 1985;
Roberts et al., 1990).
Skin sensitization was studied in guinea-pigs with pastes made
of C14-C16 AOS, a light-duty dishwashing detergent containing
AOS, some consumer products containing AOS, and mixtures of these
products with alkyl unsaturated sultone in sodium lauryl sulfate or
hypochlorite bleach. The pastes, the dishwashing detergent, most of
the consumer products, and the mixtures with hypochlorite bleach
induced sensitization, the degree of response being related to the
amount of unsaturated gamma-sultone present in the material tested
(Bay & Danneman, 1985).
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
AOS (C14-C18) were administered at a concentration of 0.2,
2, 300, or 600 mg/kg body weight per day to CD rats, CD-1 mice, and
NZW rabbits orally once a day by gavage. Groups of 20 rats and mice
were given AOS on days 6-15 of pregnancy, and groups of 13 rabbits
were treated on days 6-18 of pregnancy. The doses of 0.2 and 2 mg/kg
were estimated to be equivalent to 1-2 and 10-20 times the maximal
amount of AOS to which humans are exposed. No adverse effects were
seen in rat dams, even at the maximal dose. Mouse dams given 300 or
600 mg/kg showed piloerection, decreased movement, and inhibition of
body weight gain; six dams at 600 mg/kg died. All rabbits given
600 mg/kg and one given 300 mg/kg died; anorexia and decreased body
weight were seen initially in surviving dams given 300 mg/kg. Both
mouse and rabbit dams given 0.2 or 2 mg/kg showed only initial
inhibition of body weight gain. No adverse effects were seen on
litter parameters of rats at any dose. In mice, total litter loss
was found in five dams given 600 mg/kg and in six dams given
300 mg/kg; however, the average number of live fetuses in the other
dams was no different from that in controls. The average body
weights of the fetuses of dams given 300 or 600 mg/kg was
significantly lower than that of controls. The incidence of major
malformations was not significantly increased in rats, mice, or
rabbits. There were no significant minor anomalies or skeletal
variations (extra ribs) in rats at any dose. The offspring of mice
at 600 mg/kg had a significant increase in delayed ossification.
Those of rabbits at 300 mg/kg had a significant increase in skeletal
anomalies and variations, although the incidence of skeletal
variations was within the normal background range, and there was no
delayed ossification. The effects of AOS on the fetuses, such as
changes in litter parameters and delayed ossification, were
considered to reflect the effects of AOS on the dams. There were no
adverse effects on fetuses of mouse or rabbit dams given 0.2 or
2 mg/kg or on fetuses of rat dams given 0.2, 2, 300, or 600 mg/kg,
where effects on the dams were either not observed or were minimal
(Palmer et al., 1975b).
AOS and AOS-S (a synthetic detergent with AOS as the main
ingredient) were applied to the shaven dorsal skin of mice at a dose
of 0.5 ml/mouse per day of a 0.1% (the concentration of AOS usually
found in detergents), 1%, or 5% aqueous solution of AOS or a 0.5%
(equivalent to 0.1% AOS), 5%, or 25% aqueous solution of AOS-S on
days 0-14 of pregnancy. Adverse effects on the dams and fetuses were
found in a few cases. None of the dams died; the viability, body
weight, and sex ratio of the fetuses did not differ from those of
controls; and there were no malformations (Sawano, 1978).
B7.6 Mutagenicity and related end-points
AOS did not cause differential toxicity in Bacillus subtilis
rec at a concentration of 20 µg/disc or reverse mutation in
Salmonella typhimurium TA98 or TA100 at 10-100 µg/disc, in the
presence or absence of metabolic activation (Oda et al., 1980).
One batch of AOS (C14-C16; 28.4% active ingredient) induced
host-mediated mutagenicity at 283 mg/kg body weight in rats
inoculated with S. typhimurium TA1530 but not in an assay with
TA1534 or in plate incorporation assays with either strain (Arthur
D. Little Inc., 1993).
B7.7 Special studies
Rabbit erythrocytes were mixed with solutions containing various
concentrations of AOS (sodium salt of sulfonated C15-C16
n-alpha-olefin; average relative molecular mass, 338.5) at room
temperature for 3 h. The 50% haemolytic concentration was
1.5 mg/litre (Oba et al., 1968a). The effects of AOS on
methaemoglobin formation were studied in groups of three male mice
given an oral or intraperitoneal dose of 0.3 or 3.0 g/kg body weight
C15-C18 AOS. The level of methaemoglobin in blood was measured
0.5, 1, 2, 3, and 24 h after administration of AOS. No significant
increase was observed (Tamura & Ogura, 1969).
In an immunological study of AOS, a complex (HA) prepared by
mixing AOS with human serum albumin (HSA) containing 30 mg of total
protein was injected subcutaneously or intravenously into rabbits
during a period of 2.5 months, and the anti-serum produced was
subjected to the ordinary precipitation reaction. As a control,
anti-AOS-serum, similarly prepared, was subjected to the same
reaction. Minor positive reactions were seen in the HA-anti-HA and
HSA-anti-HSA systems but not in the AOS-anti-HA or AOS-anti-AOS
systems (Iimori & Ushiyama, 1971).
B8. EFFECTS ON HUMANS
Section summary
In patch tests, human skin can tolerate contact to solutions
containing up to 1% AOS for 24 h with only mild irritation. AOS can
cause delipidation of the skin surface, elution of natural
moisturizing factor, denaturation of the outer epidermal layer
proteins, and increased permeability and swelling of the outer
layer. AOS did not induce skin sensitization in volunteers. There is
no conclusive evidence that AOS induce eczema. No serious injuries
or fatalities have been reported following accidental ingestion of
detergent formulations that could contain AOS.
B8.1 Exposure of the general population
AOS surface-active agents are found in shampoos, dishwashing
products, household cleaners, and laundry detergents. The
composition of nonionic and ionic surfactants in these products
varies between 10 and 30%. Surface-active agents can affect human
skin and eyes.
B8.2 Clinical studies
B8.2.1 Skin irritation and sensitization
AOS are mildly to moderately irritating to human skin, depending
on the concentration.
The relative intensity of skin roughness induced on the surface
of the forearm was evaluated in volunteers by a circulation method
consisting of contact with 1% solutions of C12, C14, C16, and
C18 AOS for 10 min. The skin response was characterized mainly on
the basis of gross visible changes. C12 AOS induced more skin
roughening than compounds with longer or shorter alkyl chains. The
relative degree of skin roughening in vivo was correlated with the
extent of protein denaturation measured in vitro (Imokawa et al.,
1975a).
Primary skin irritation induced by a 1% aqueous solution
(pH 6.8) of AOS containing 27% C15, 25% C16, 28% C17, and 18%
C18 (relative molecular mass, 338.5) was studied in a 24-h
closed-patch test on the forearms of seven male volunteers. The
intensity of skin irritation was scored by grading erythema,
fissuring, and scales. The average score for AOS was 3.97 and that
for a control (water) was 1.79. The same compound was evaluated at
0.3% for the relative intensity of skin lesions produced on the
surface of the hands by an immersion test involving 30 repetitions
of a 1-min dip and 1-min dry. The average score for AOS with regard
to erythema, irritation, fissuring, scaling, and loss of suppleness
was 5.75, while that for the water control was 2.5 (Oba et al.,
1968a).
Skin irritation induced by a 1% aqueous solution of C14,
C16, and C18 AOS was studied in a 24-h closed-patch test on the
forearm and in a test in which the compound was dripped onto the
interdigital surface for 40 min once daily for two consecutive days
at a rate of 1.2-1.5 ml/min. Skin reactions were scored by grading
erythema in the patch test and by grading scaling in the drip test.
The score for AOS was 1 (slight erythema) in the patch test and 0.35
(minimal scaling) in the drip test (Sadai et al., 1979).
In sensitization tests on volunteers, AOS in a paste or in a
detergent mixture containing up to 0.06% AOS and up to 0.002 ppm
unsaturated g-sultone did not produce sensitization, although one
subject had a strong dermal response, which was considered to be due
to pre-existing sensitization. Two out of 264 subjects using a
light-duty detergent containing AOS developed hand dermatitis and
had positive reactions to AOS paste and/or unsaturated gamma-sultone
in sodium lauryl sulfate in a patch test. Use of a hand dishwashing
liquid containing AOS did not cause sensitization provided the
level of unsaturated g-sultones was kept low (Bay & Danneman, 1985).
Patch tests on 790 volunteers after four months' use of a
dishwashing liquid showed no evidence of sensitization (Oba et al.,
1985).
B8.2.2 Effects on the epidermis
The effects of AOS on the epidermal outer layer (stratum
corneum) are similar to those of other surface-active agents (see
section 8.2.2 of the monograph on LAS), including delipidation of
the skin surface, elution of natural moisturizing factor,
denaturation of stratum corneum protein, increased permeability,
swelling of the stratum corneum, and inhibition of enzyme activities
in the epidermis (Wood & Bettley, 1971; Imokawa et al., 1974;
Okamoto, 1974; Imokawa et al., 1975a,b).
The effects of anionic surfactants on various types of proteins
were studied using skin keratin as a filamentous protein, bovine
serum albumin as a globular protein, acid phosphatase as an enzyme
protein, and membrane lysosome as a membrane protein. The denaturing
effects of surfactants were measured as liberation of sulfhydryl
groups and enzyme inhibition. AOS were less potent than LAS or alkyl
sulfates. A relationship was observed between denaturing potency,
skin irritant action, and alkyl chain length (Imokawa & Katsumi,
1976; Imokawa & Mishima, 1976).
B8.2.3 Hand eczema
Skin reactions to a 0.04, 0.4, or 4.0% aqueous solution of AOS
(25.0% C14, 45.0% C16, 30.0% C18)were evaluated in a 24-h
closed-patch test on the lower back of 10 healthy volunteers and 11
patients with hand eczema (progressive keratosis palmaris). The
incidence and intensity of skin reactions were significantly higher
in the group with hand eczema than in a control group with normal
skin (Okamoto & Takase, 1976a,b).
B8.2.4 Accidental or suicidal ingestion
No data were available that related specifically to AOS.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
Limited data are available on the effects of AOS on
environmental organisms. The 24-h LC50 values for daphnids were
19-26 mg/litre; the 48-h LC50 values ranged from 0.3 mg/litre for
brown trout (Salmo trutta) to 6.8 mg/litre for golden orfe (Idus
idus melanotus), and the 96-h LC50 was 0.5-5.0 mg/litre for
brown trout. One study suggested that AOS have little toxicity for
birds.
B9.1 Microorganisms
No information was available.
B9.2 Aquatic organisms
B9.2.1 Aquatic plants
The EC50 values for C16.4 AOS in the green alga Selenastrum
capricornutum exposed for two to three days, based on growth, fell
within the range 45-65 mg/litre (Yamane et al., 1984). The EC50
for C16-C18 AOS on the growth of S. capricornutum was >
20 mg/litre (Konno & Wakabayashi (1987).
B9.2.2 Aquatic invertebrates
Daphnia magna and Dapghnia pulex less than 24 h old were
exposed to C16-C18 AOS under static conditions, in which the
water was unchanged for the duration of the test, at a temperature
of 20°C and a water hardness of 25 mg/litre CaCO3. The 6-h LC50
values were > 64 and > 130 mg/litre, and the 24-h LC50 values
were 19 and 26 mg/litre, for the two species respectively
(Wakabayashi et al., 1988).
B9.2.3 Fish
The acute toxicity of AOS to fish is summarized in Table 33. The
48-h LC50 values ranged from 0.3 mg/litre for brown trout (Salmo
trutta) to 6.8 mg/litre for golden orfe (Idus idus melanotus);
the 96-h LC50 for brown trout was 0.5-5.0 mg/litre. Acute toxicity
tended to increase with carbon chain length.
When eggs of rainbow trout (Oncorhynchus mykiss) and carp
(Cyprinus carpio) were exposed to C16-C18 AOS, the EC50
values, based on hatchability, were 4.9 for rainbow trout and
3.0 mg/litre for carp (Wakabayashi & Onizuka, 1986). In one-month
old rainbow trout under semi-static conditions, the 14- and 28-day
LC50 values for C16-C18 AOS were 0.62 and 0.58 mg/litre. The
EC50 based on growth was 0.35 mg/litre (Wakabayashi & Mizorogi,
1989).
The time to lethality in goldfish (Carassius auratus) exposed
to AOS was 2 h at a concentration of 5 mg/litre and 1 h at
10 mg/litre. Addition of 2100 mg/litre egg albumin increased the
time to 100% lethality to 3 h and addition of 4200 mg/litre albumin
increased the time to 6 h (Tomiyama, 1974).
B9.3 Terrestrial organisms
B9.3.1 Terrestrial plants
AOS were not toxic with respect to growth at the early life
stages of radish, Chinese cabbage, and rice when added in hydroponic
culture at concentrations of 56, 56, and 32 mg/litre, respectively;
concentrations of 100, 100, and 56 mg/litre were toxic (Takita,
1982).
B9.3.2 Terrestrial invertebrates
No information was available.
B9.3.3 Birds
No significant effect on egg quality was found after Leghorn
chickens were fed a diet containing 200 mg/kg AOS for 45 days
(Lopez-Zavala et al., 1975).
Table 33. Toxicity of alpha-olefin sulfonates (AOS) to fish
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Masu trout 2 mo Staticr 8.5-9.6 30 NS C16-C18 96-h LC50 0.56
Wakabayashi
(Oncorhynchus et al. (1984)
masou)
Rainbow trout 40 d Staticr 8.8-10.9 25 NS 96-h LC50 0.78 Wakabayashi
(Oncorhynchus et al. (1984)
mykiss) 4 d Staticr 10 25 NS C16-C18 96-h LC50 0.61 Wakabayashi
19 d Staticr 10 25 NS C16-C18 96-h LC50 0.98 & Onizuka
(1986)
Brown trout 2.8-5.8 cm Flow 15 26-30 NS C14-C16 48-h LC50 2.5-5.0b Reiff et al.
(Salmo trutta) 2.8-5.8 cm Flow 15 26-30 NS C14-C16 96-h LC50 2.5-5.0b (1979)
2.8-5.8 cm Flow 15 26-30 NS C16-C18 48-h LC50 0.6b
2.8-5.8 cm Flow 15 26-30 NS C16-C18 96-h LC50 0.5b
2-4 cm Flow 15 250 NS C14-C16 48-h LC50 3.5b
2-4 cm Flow 15 250 NS C16-C18 96-h LC50 3.1b
2-4 cm Flow 15 250 NS C14-C16 48-h LC50 0.3-0.5b
Goldfish Static 20 NS C12-C16 6-h LC50 11.2c Gafa (1974)
(Carassius auratus) Static 20 NS C14-C18 6-h LC50 3.0c
Table 33 (contd)
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Golden orfe 1.2-1.8 g Static 20 NS C14-C16 48-h LC50 5.08 Mann (1976)
(Idus idus 1.2-1.8 g Static 20 NS C16-C18 48-h LC50 1.44
melanotus) 5-7 cm Flow 20 150 NS C14-C16 48-h LC50 5.7b Reiff et al.
5-7 cm Flow 20 150 NS C16-C18 48-h LC50 1.9b (1979)
Flow 20 268 NS C14-C16 48-h LC50 3.7-6.8b
Flow 20 268 NS C16-C18 48-h LC50 1.0b
Flow 20 268 NS C14-C16 96-h LC50 3.4-4.9b
Flow 20 268 NS C16-C18 96-h LC50 0.9b
Harlequin fish Flow 20 20 NS C14-C16 48-h LC50 4.8b Reiff et al.
(Rasbora Flow 20 20 NS C16-C18 48-h LC50 0.9b (1979)
heteromorpha) Flow 20 20 NS C14-C16 96-h LC50 3.3b
Flow 20 20 NS C16-C18 96-h LC50 0.5b
Medaka 175-332 mg Static 21-22 25 6.7-7.1 C14-C18 6-h LC50 6.2b Kikuchi &
(Oryzias latipes) 175-332 mg Static 21-22 25 6.7-7.1 C14-C18 48-h LC50 1.8b Wakabayashi
175-332 mg Static 21-22 25 6.7-7.1 C16-C18 6-h LC50 2.7b (1984)
175-332 mg Static 21-22 25 6.7-7.1 C16-C18 48-h LC50 0.81b
Carp 3.5-5.5 cm Static 21 7.5-7.8 Technical 24-h LC50 3.2c Lopez-Zavala
(Cyprinus carpio) 3.5-5.5 cm Static 21 7.5-7.8 Technical 96-h LC50 3.0c et al. (1975)
2 d Staticr 20 25 NS C16-C18 96-h LC50 > 1.4 Wakabayashi
15 d Staticr 20 25 NS C16-C18 96-h LC50 1.5 & Onizuka
(1986)
Table 33 (contd)
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Carp (contd). 50 d Staticr 21 75 NS 96-h LC50 1.0 Wakabayashi
(Cyprinus carpio) Staticr et al. (1984)
White tilapia 5-7 cm Static 21 7.5-7.8 Technical 24-h LC50 2.0c Lopez-Zavala
(Tilapia melan 5-7 cm Static 21 7.5-7.8 Technical 96-h LC50 2.0c et al. (1975)
opleura)
Grey mullet Static 20.6-22.0 96-h LC50 0.70 Wakabayashi
(Mugil cephalus) et al. (1984)
Staticr, static renewal: water changed at regular intervals; flow, flow-through conditions: concentration in water maintained
continuously; static: water unchanged for duration of test
a mg/litre CaCO3
b Measured concentration
c Nominal concentration
C. Alkyl sulfates
C1. SUMMARY
See Overall Summary, Evaluation, and Recommendations (pp. 7-21)
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
C2.1 Identity
Chemical formula: CnH2n+1O4S Na ( n = 10-8)
Chemical structure: CnH2n+1OSO3- Na+ ( n, integer)
Common names: Sodium alkylsulfate, sulfuric acid alkyl
ester sodium salt, alkylsulfate sodium salt,
alcohol sulfuric ester sodium salt, sodium
dodecyl sulfate, sodium lauryl sulfate
Common trade names: Akyporox SAL SAS, Akyposal, Alphenate TFC
76, Alscoap LN, Aremsol, Berol, Cosmopon,
Dehydag, Elfan, Emal, Empicol, Gardinol,
Genapol CRT 40, Manro, Marlinat KT 50,
Melanol LP 1, Monogen, Montopol CST,
Montovol, Neopon LT, Nikkol, Nissan Persoft
SK, Perlankrol ATL-40, Perlankrol, Polystep
B, Rewopol, Sactipon, Sactol, Sandopan KD,
Sermul, Stepanol WA 100, Sufatol, Sufetal,
Sulfopon, Sunnol, Surfax, Swascol, Teepol HB
7, Tensopol Tesapon, Texapon, Ufarol AM 70,
Zoharpon, Zorapol LS-30, (McCutcheon, 1993)
Abbreviations: AS, AS-Na, SDS
CAS Registry numbers: 151-21-3 (C12 AS), 1120-04-3 (C18 AS),
68130-43-8 (C8-C18 AS)
Specification: AS are higher alcohol sulfuric ester salt
types of anionic surfactants. Depending on
which precursor alcohol is used as the raw
material, the alkyl group is linear or
branched, may contain a single homologue or
a mixture of chain lengths, and is usually
primary. The data presented are applicable
mainly to linear alcohol sulfates and AS
with predominantly single or similar type of
branching.
C2.2 Physical and chemical properties
AS are white crystalline powders. Their physical properties differ
widely depending on their alkyl groups, and they are usually produced
and used as mixtures. The relationships between the critical micelle
concentration, solubility, and alkyl chain length are shown in Table
34.
Table 34. Relationships between alkyl chain length, critical
micelle concentration (CMC), and solubility
Alkyl chain CMC × 10-3 Solubility/°Cb,c
length mol/litrea,c
8 136 -
12 8.6 15
14 2.4 28
16 0.58 42
18 0.16 55
a From Evans (1956)
b Temperature at which 10 g of AS dissolve in 1 litre of water
(Gotte, 1954)
c The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature.
For surfactants, there is a distinct, sharp bend (break-point) in
the solubility-temperature curve. The steep increase in solubility
above the sharp bend is caused by micelle formation. At above the
critical micelle concentration, a micellar solution is formed.
Under these conditions, higher levels than the aqueous solubility
may be obtained.
AS are readily hydrolysed in hot acidic media. Compounds with an
alkyl chain length of C10 (27°C), C12 (25°C), C14 (40°C), or
C16 (40°C) have a surface tension of 40 dyne/cm at the temperatures
shown in parentheses at concentrations greater than the critical
micelle concentration, indicating a good ability to reduce surface
tension (Dreger et al., 1944).
Cleansing capacity at 25°C increases with alkyl chain length up to
C13 and then becomes constant up to C16. In an actual detergent
containing alkali builders and chelating agents, however, maximal
detergency was obtained with C14 compounds (Yamane et al., 1970).
C2.3 Analysis
C2.3.1 Isolation
Since AS are readily susceptible to hydrolysis in acidic media,
special attention is required.
C2.3.2 Analytical methods
There is no officially recognized, specific procedure for the
analysis of AS in environmental samples. The methods used for
analysing linear alkylbenzene sulfonates (LAS) are commonly used for
AS, except those involving high-performance liquid chromatography
(HPLC), which is of limited use for detecting AS in environmental
samples because AS do not effectively absorb ultra-violet radiation.
An HPLC method for the analysis of AS after its conversion by
derivatization into an ultra-violet-active species has been proposed
(Utsunomiya et al., 1982). A modified analytical method has been
developed that is based on measurement of methylene blue-active
substances (MBAS) by HPLC. This method permits determination of AS at
concentrations as low as 0.05 mg/litre (Takita & Oba, 1985). Trace
enrichment followed by gas chromatography and flame ionization
detection have been proposed for the sensitive determination of AS as
their trimethylsilyl ethers in environmental samples (Fendinger et
al., 1992a).
Non-specific methods used in the analysis of anionic surfactants
in general, such as the methylene blue method, may be used for the
analysis of AS (see also section 2.3 of the monograph on LAS).
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Section summary
Few quantitative data are available on AS in the environment, but
AS can be expected to mineralize rapidly in all environmental
compartments and to be removed to a large extent during sewage
treatment. Environmental concentrations in receiving surface waters,
sediments, soils, estuaries, and the marine environment can be
expected to be low.
C3.1 Natural occurrence
AS do not occur naturally.
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
AS are synthesized industrially. Worldwide consumption of AS in
1987 was about 117 000 tonnes in the United States, 56 000 tonnes in
western Europe, and 46 000 tonnes in Japan (Richtler & Knaut, 1988).
In western Germany in 1987, some 10 000 tonnes of AS and 87 000 tonnes
of LAS were used (Schöberl et al., 1988). Worldwide consumption was
estimated to be 289 000 tonnes in 1990 (Hewin International Inc.,
1992; see Table 35).
Table 35. Estimated worldwide use of alkyl sulfates in
1990 (tonnes)
Region Household Personal care Industrial and
products products institutional
use
North America 140 000 33 000 9 000
Western Europe 49 000 12 000 7 000
Japan 21 000 6 000 4 000
Rest of the - 4 000 4 000
world
Total 210 000 55 000 24 000
From Hewin International Inc. (1992)
AS were originally made by the sulfation of natural fatty
alcohols. They are currently produced from both natural and synthetic
fatty alcohols. Primary AS are usually manufactured by conventional
sulfation of the parent alcohol with either sulfur trioxide or
chlorosulfonic acid. The product of this reaction is then neutralized
with an appropriate base (NaOH, Na2CO3, NH4OH, or
triethanolamines).
C3.2.2 Uses
Initially, AS were used as washing agents for wool or as active
ingredients in heavy-duty laundry detergents. They are now used mainly
in personal care products (shampoos, toothpastes, toiletries),
household detergents (light-duty dishwashing detergents, heavy-duty
laundry detergents), and industrial applications.
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
AS can be expected to be transported into the environment by
mechanisms similar to those that operate for LAS and alpha-olefin
sulfonates (AOS). AS are readily biodegradable under aerobic
conditions, both in laboratory tests and under environmental
conditions, and primary biodegradation is complete within two to five
days. Less information is available on the effect of temperature on
the biodegradation of AS than for LAS. The biodegradation kinetics of
AS appear to be less affected by temperature than those of other
surfactants. The whole-body bioconcentration factors are 2-73,
depending on chain length. AS are taken up by fish mainly through the
gills and are subsequently distributed to the liver and gall-bladder.
After biotransformation, AS are excreted rapidly. They are not
bioconcentrated or biomagnified in aquatic organisms.
C4.1 Transport and distribution between media
After use, AS are discharged into the environment in wastewater,
like other detergent compounds, where they can undergo sewage
treatment if such facilities are available. In countries where
adequate wastewater treatment facilities are not available, AS
released to the environment are removed by biodegradation and
adsorption in the receiving surface water (see section 4.2 of the
monograph on AOS).
Sorption equilibria were obtained rapidly (within 20 min) for pure
homologues of AS (> 99%) with chain lengths of C8, C9, C10,
C11, C12, C13, and C14, suggesting that sorption is due to a
hydrophobic bonding mechanism, as has been observed for other
surfactants. Thus, sorption of AS to sediment is likely to be stronger
for longer chain homologues than for shorter ones. The KD values for
C12 AS were 70 and 100 for two river sediments, whereas for C12
LAS on the same sediments they were 310 and 330 (Marchesi et al.,
1991). Adsorption of AS therefore competes in kinetic terms with
biodegradation as a mechanism for removal of AS from the environment,
as is seen for surfactants in general.
C4.2 Biotransformation
C4.2.1 Biodegradation
C4.2.1.1 Biodegradation pathway; mechanism
Several species of bacteria have been found that can mineralize
AS. AS with chains longer than six carbons are degraded by the initial
action of a sulfatase enzyme, producing sulfate and the corresponding
alcohol. The alcohol is readily oxidized by formation of an aldehyde,
to produce carboxy acid, which can be further oxidized by ß-oxidation
and in the citric acid cycle. Secondary ketones and hydroxy ketones of
AS are produced as metabolites but have not been detected in simulated
activated sludge. Biodegradation of short-chain homologues of AS may
proceed by oxidation of the chain before hydrolysis of the ester bond
by the sulfatase enzyme.
The metabolic pathway for biodegradation of C12 AS by
Pseudomonas strains has been described (Hsu, 1965; Thomas & White,
1989). Initial liberation of the sulfate head produces dodecanol,
which is further transformed into more polar metabolites, including
dodecanal and dodecanoic acid. These products may be further
metabolized by ß-oxidation, or they may be elongated to C14, C16,
or C18 fatty acyl residues, which are then incorporated into lipid
fractions such as phospholipids (Thomas & White, 1989).
C4.2.1.2 Biodegradation in the environment
The aerobic biodegradation of 20 mg/litre AS at 27°C was followed
during a 10-day incubation period. Primary degradation, measured by
the MBAS method, was complete within five days. The theoretical
production of CO2 reached 60-90% within 10 days (Itoh et al., 1979).
The biodegradation of AS at a concentration of 30 mg/litre was
studied in a vessel containing activated sludge at a concentration of
100 mg/litre over a period of 12 days, by measuring chemical oxygen
demand. All of the AS were lost within two days; the specific rate of
biodegradation was calculated to be 20 mg/g per h (Pitter & Fuka,
1979).
The biodegradation of an initial concentration of 6 mg/litre C12
AS was studied by the die-away method, in which disappearance of the
compound is followed over a given period. Less than 10% of the
original amount remained in river water in the test vessel after 12
days' exposure, and complete degradation was reported within 21 days
(Okpokwasili & Nwabuzor, 1988).
The capacity of epilithic (sampled from the surface of pebbles)
and planktonic river bacterial populations to degrade C12 AS was
studied under simulated environmental conditions. Samples were
collected from four polluted sites and one clean site in a polluted
river in South Wales, United Kingdom. In die-away tests, AS were
degraded after an apparent lag at all four polluted sites, but
degradation by the bacterial populations at the clean site was
relatively slow. Quantification of the kinetic components that
contributed to the die-away curves demonstrated that biodegradation of
AS occurred at concentrations below its Km by bacteria with
exponential growth that are unaffected by addition of the test
substrate. Degradation of AS in the clean sample followed a
different pattern, but there was generally little or no growth on
endogenous carbon. The authors concluded that the capacity of
epilithic bacterial populations to degrade C12 AS is more stable
than that of planktonic populations (Anderson et al., 1990).
Riverine bacteria that can grow in the presence of
0.5 mmol/litre C12 AS are widespread, and a greater incidence of
isolates resistant to C12 AS was recorded at a polluted site than in
clean water. The ability of each culture to produce alkyl sulfatases,
the enzymes that initiate degradation of AS, was also determined.
Bacteria containing alkyl sulfatases were widespread, but a greater
alkyl sulfatase yield was obtained from polluted site. The authors
concluded that more strains at the polluted site had constitutive
rather than inducible enzymes. An increased incidence of strains
containing multiple alkyl sulfatases was also recorded at the polluted
site (White et al., 1985).
In another study in South Wales, the distribution of planktonic
bacteria capable of degrading 98.5% C12 was examined in water
samples at sites along a river. The annual mean prevalence of such
bacteria was 8.1-16.0% of the total number of isolates. The proportion
of isolates that degrade AS in clean water was no different from that
at polluted sites, and a lower density was recorded at the source
owing to a reduction in overall numbers. A higher percentage of
bacteria capable of degrading C12 AS was recorded in estuarine
samples than in samples from the middle of the polluted river;
however, when cell numbers were taken into account, the cell density
was similar at all polluted sites on the river, including the estuary.
The incidence of these isolates was not correlated with either
biochemical oxygen demand or oxygen concentration, but the incidence
tended to increase at the end of the summer. More than half of the
isolates contained constitutive alkyl sulfatase enzymes, while they
were induced or repressed in the remainder after exposure to AS. No
variation in the proportions of type of enzyme regulation was seen
between sampling sites or times (White et al., 1989).
The biodegradation of AS was also examined at three sites, above,
at, and below a sewage works outfall on the South Wales river. Samples
capable of degrading C12 AS after only one day's exposure were found
at each site. No biodegradation of AS was reported at a pristine
source site. The onset of biodegradation was more rapid following
longer exposure of the river, suggesting the existence of an adaptive
mechanism. A model of the die-away kinetics of degradation suggested
that C12 AS were biodegraded by a bacterial population growing at
the expense of endogenous carbon. The activity of the epilithic
samples in degrading AS increased during the first four days of
exposure at each site. The stabilized values (days 4-14) increased
from the upstream site to the outfall, decreasing to intermediate
values downstream. The sewage input had less effect on activities
in degrading AS than on bacterial cell densities. Little variation in
growth characteristics was seen throughout colonization at the three
sites. The authors concluded that the adaptation seen during exposure
in the river was attributable to colonization of the epilithon by an
existing population that degradedC12 AS and not to acquisition or
adaptation of biodegrading capacity (Russell et al., 1991).
The half-life for primary degradation of 20 mg/litre C12 AS in
seawater varied over a range of 0.26 to 0.34 days, and degradation was
reported to follow first-order kinetics. Primary degradation was
followed by an immediate increase in bacterial number and thymidine
incorporation (Vives-Rego et al., 1987). C12 AS was found to be
degraded rapidly in seawater, and 250 g/litre were found in sediment;
at 25°C, 90% was degraded within five days. No lag phase was reported,
and the degradation kinetics were reported to be first-order (Sales et
al., 1987).
C15-C16 AS were 98% removed at 15°C and 99% removed at 8°C
(Gilbert & Pettigrew, 1984). Similarly, C12-C15 and C12-C14 AS
were found (by the MBAS method) to be biodegraded during winter and
spring in a trickling filter sewage treatment plant (Mann & Reid,
1971). These results suggest that temperature has no major effect on
the removal of alkyl sulfates under environmental conditions.
Primary biodegradation of C12 AS was less affected by incubation
temperature than that of other anionic surfactants in die-away tests
with water from the Tama River, Japan. Primary biodegradation was
complete within one day at temperatures of 21 and 27°C, within two
days at 15°C, and within three days at 10°C (Kikuchi, 1985).
Over 99% of MBAS activity in activated sludge was lost in a 19-day
OECD screening test and in the 28-day OECD confirmatory test.
Mineralization of both C12-C14 and C16-C18 AS was complete,
with 90-95% degradation for C12-C14 AS and 77-88% for C16-C18
AS in the two test systems (Steber & Wierich, 1987).
C4.2.1.3 Anaerobic degradation
Biodegradation of AS under anaerobic conditions has been reported
in several studies, with 88% degradation of stearyl sulfate containing
C14 AS in an anaerobic screening test (Birch et al., 1989) and 95%
ultimate degradation of the same compound (Steber & Wierich, 1987).
C4.2.2 Abiotic degradation
No information was available.
C4.2.3 Bioaccumulation and biomagnification
Carp (Cyprinus carpio) were exposed to 35S-C12 AS at a
concentration of 0.85 mg/litre for up to 24 h. Within 1 h, AS was
concentrated in the gills, hepatopancreas, and kidneys with
concentration factors of 1.6, 1.4, and 1.5, respectively. After the
initial uptake in the gills, the levels of AS fell, and other organs
and tissues, such as the skin surface, muscle, brain, kidney,
hepatopancreas, and gall-bladder showed gradual uptake over the
exposure period. The concentration factors after 24 h ranged from 2.0
for the skin surface to 43 for gall-bladder. Blood and kidney also
showed uptake, but the levels after 24 h were less than those after 4
and 8 h, respectively. AS were lost rapidly from all tissues except
the gall-bladder when the fish were kept in 'clean' water for 48 h
(Kikuchi et al., 1978).
Carp maintained in water containing 0.5 mg/litre 35S-C12 AS
absorbed the compound within 1 h, and an equilibrium for the whole
body and gall-bladder was reached within 24 h, with concentration
factors of about 4 and 700, respectively. After 24 h, the levels of AS
in hepatopancreas had decreased from the initial level. When the fish
were transferred to 'clean' water, 50% of the AS was still present
after 72 h (Wakabayashi et al., 1978).
When carp were exposed to 35S-C12 AS at concentrations between
2.7 µg/litre and 40 mg/litre for up to 120 h, equilibrium was reached
within 72 h, at concentration factors of 3.9-5.3, which were
independent of the concentration of AS in solution (Wakabayashi et
al., 1981).
In a study of the effect of chain length on the uptake of AS, carp
were exposed to 0.5 mg/litre of 35S-C12, 35S-C14, or
35S-C16 AS for 24 h. Absorption of AS reached a maximum within the
exposure period. The whole-body concentration factors were 2.1, 11,
and 73 for the three surfactants respectively, and thus increased with
alkyl chain length. This tendency was also observed in gills and
hepatopancreas, but the factors in the gall-bladder were almost the
same for the three homologues. When fish were transferred to 'clean'
water, the elimination rate decreased with increasing carbon chain
length, and 50% of C16 AS was retained after 120 h (Wakabayashi et
al., 1980).
The absorption, tissue distribution, metabolism, and route of
excretion of 50 mg/litre C12 AS were studied in goldfish (Carassius
auratus) exposed for 24 h. AS was absorbed mainly through the gills
and was distributed rapidly throughout the body; it was absorbed to a
lesser extent (20% of total) by cutaneous absorption and orally (8%).
The highest concentration of AS was measured in the gall-bladder,
mainly because of its small size. The greatest proportion of the
absorbed AS was located in the body, gut, liver, and gall-bladder. The
level of AS in the tissues fell by 38% over 24 h in unfed fish and by
68% in fed fish. The high concentration of AS in the liver and
gall-bladder was thought to indicate metabolism of the compound in the
liver. The metabolites of AS that were identified included successive
products of ß-oxidation of the alkyl chain and butyric-4-sulfate
(Tovell et al., 1975).
C4.3 Interaction with other physical, chemical, and biological
factors
The presence of 1 mg/litre AS (chain length unspecified) had no
significant effect on the uptake of mercury by phytoplankton
( Diogenes sp.) or mussels ( Mytilus sp.) (Laumond et al., 1973).
Exposure of bacteria to 20 mg/litre phenol and 0.5 mg/litre C12
AS resulted in a directly additive effect. Exposure to 2.5 mg/litre
phenol and 0.5 mg/litre AS resulted in a synergistic effect. No
interactive effects were reported between sodium cyanide and AS in the
same test protocol (Dutka & Kwan, 1982).
C4.4 Ultimate fate following use
As no specific analytical method is available for AS, their
concentrations in environmental samples have not been established.
Like detergent compounds, AS are present in wastewater after use. A
large proportion is removed during treatment of wastewater, mainly as
a result of a combination of biodegradation and adsorption processes.
As for other surfactants, these processes continue when AS are
released into the environment.
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
Data on the environmental concentrations of AS are limited. At
sewage treatment plants where the influent concentrations of AS were
< 0.01-0.7 mg/litre, the effluent contained predominantly C12 AS,
at concentrations of < 0.005-0.1 mg/litre. Surface waters receiving
treated wastewater contained AS at concentrations below the detection
limit of 0.005 mg/litre.
Environmental levels
AS were measured at two sewage treatment plants in the United
States where the influent concentrations were < 0.01-0.7 mg/litre,
which were at least 2.4 times lower than those predicted on the basis
of use of AS and per-capita wastewater in the United States. The
predominant homologues of AS in untreated wastewater were C12,
C14, and C15. The effluent contained predominantly C12 AS, at
concentrations of < 0.005-0.1 mg/litre, showing that removal exceeded
98% during rotating biological contact and activated sludge treatment.
Surface waters receiving treated wastewater contained AS at
concentrations below the detection limit of 0.005 mg/litre (Fendinger
et al., 1992a,b).
C6. KINETICS
Section summary
AS are readily absorbed by the gastrointestinal tract after oral
administration and are excreted principally in the urine, only minor
amounts being eliminated in the faeces. Penetration of AS through
intact skin appears to be minimal. AS are extensively metabolized in
various species to several metabolites. Butyric acid-4-sulfate has
been identified as their major metabolite.
C6.1 Absorption, distribution, and excretion
In a study of the absorption of higher alcohol sulfates,
14C-hexadecyl sulfate salts were administered orally to humans and
dogs. After a single dose of 14.4 mg/kg bw of the salts to dogs, the
maximal plasma concentration of hexadecyl sulfate (1.22-2.45 µg/ml)
was reached within 30-60 min; 6 h later, the plasma concentration had
decreased to about one-tenth of the peak value. Within 72 h, 50-79% of
the administered dose had been excreted in the urine and 12-41% in the
faeces. After a single dose of 360 mg to humans, the maximal plasma
concentration was reached at 2 h, although there was marked variation
between individuals (range, about 3.1-23 µmol/ml) (Merits, 1975).
Potassium dodecyl 35S-sulfate was injected intravenously or
intraperitoneally at 1 mg/ml to male and female rats. The proportions
of the administered dose excreted in the urine and faeces and the
amounts retained in the carcass after 24 h are shown in Table 36. Most
of the radiolabel appeared in the urine of both male and female rats,
although some was present as inorganic 35S-sulfate. The intestinal
flora do not play a significant role in the metabolism of potassium
dodecyl 35S-sulfate, since the distribution of radiolabel in the
urine and faeces was similar in rats pretreated with antibiotics and
in untreated rats. Whole-body autoradiograms of rats killed 5 min
after administration of the compound by intraperitoneal injection
showed significant amounts of radiolabel in the liver; the
concentrations increased up to 30 min and then gradually declined,
only trace amounts remaining after 4 h. The kidney was the only other
organ in which any appreciable accumulation was reported (quantitative
data not presented) (Denner et al., 1969).
In order to investigate the percutaneous absorption of AS,
0.5 ml of 25 mmol/litre sodium 14C-dodecyl sulfate in water was
applied to the dorsal skin (10 cm2) of rats for 15 min. Heavy
deposition of the surfactant on the skin surface and in the upper
regions of the hair follicles was observed. The 14C level in urine
was calculated to be equivalent to a penetration of 0.26 µg/cm2 per
24 h (Howes, 1975).
Table 36. Excretion of 14C-alkyl sulfates by rats after injection
of 1 mg/ml
Route of Sex Excretion (%)
administration
Urine Faeces
(total 35S)
Inorganic 35S Total35S
Intraperitoneal Male 86.3 14.4 0.2
Female 93.2 18.1 0.9
Intravenous Male 95.6 23.5 -
Female 97.4 11.4 -
From Denner et al. (1969)
In young swine administered sodium dodecyl 35S-sulfate
(3.3 mmol/animal) orally, the labelled compound was well absorbed from
the intestine. Traces of radiolabelled sulfur were found only in
bristles, bones, and bone marrow. The total amounts of 35S retained
in organs and tissues were 1.7% of the dose at 82 h, 0.6% at 200 h,
and 0.18% at 10 weeks. About 90% of the sodium dodecyl sulfate was
recovered in urine and about 10% in faeces at 140 h (Havermann &
Menke, 1959).
Similar results were obtained in guinea-pigs in a study of the
percutaneous absorption of 3 µmol sodium lauryl 35S-sulfate in water
through skin in vivo. Less than 0.4% of the dose was found to have
penetrated the skin, on the basis of recovery of radiolabel in the
urine, faeces, and expired air. The permeability constant was
calculated to be 0.65 × 10-6 cm/min (Prottey & Ferguson, 1975).
In a study of the dermal absorption of some homologues of AS,
ranging from octyl to octadecyl sulfate, by isolated human abdominal
skin, no penetration of the dermis was detected (Blank & Gould, 1961).
The rates of excretion in urine and faeces after oral,
intravenous, or intraperitoneal administration of 14C- or
35S-labelled C10-C18 AS to rats, dogs, and humans are summarized
in Table 37.
C6.2 Biotransformation
Potassium dodecyl 35S-sulfate was extensively metabolized in
rats to yield a single ester sulfate, identified as butyric acid
4-35S-sulfate (III in scheme below), and inorganic 35S-sulfate.
Table 37. Excretion of alkyl sulfates (AS) in the urine and faeces of rats, dogs, and humans
ASa Species Treatment Length of Excretion (%) Reference
treatment
(h) Urine Faeces
35S-AS(C10)-K Rat 1 mg/rat ip 48 82.9 1.2 Burke et al. (1975)
79.5 1.0
35S-AS(C11)-K Rat 1 mg/200 g ip 48 98.2 2.5 Burke et al. (1976)
90.6 7.3
Rat 1 mg/200 g po 48 75.1 14.3
88.7 5.7
Rat 1 mg/200 g iv 48 85.9 5.9
74.8 18.5
35S-AS(C12)-K Rat 1 mg/rat ip 48 86.3 0.2 Denner et al. (1969)
93.2 0.9
Rat 1 mg/rat po 48 98.7 0.7
106.9 0.5
35S-AS(C16)-EM Rat 14.4 mg/kg po 96 94 5 Merits (1975)
14C-AS(C16)-EM Rat 14.4 mg/kg po 72 87 3
35S-AS(C16)-Na Dog 2.9 mg/kg iv 72 83 3
14C-AS(C16)-TMA Dog 4.4 mg/kg iv 48 50 41
Table 37 (contd)
ASa Species Treatment Length of Excretion (%) Reference
treatment
(h) Urine Faeces
35S-AS(C16)-EM Dog 14.4 mg/kg po 72 52 37
14C-AS(C16)-EM Dog 14.4 mg/kg po 72 65 26
14C-AS(C16)-EM Human 250 mg po 72 80 7
20 73
35S-AS(C18)-K Rat 1 mg/rat ip 48 77.1 1.1 Burke et al. (1975)
73.9 2.6
Rat 1 mg/200 g po 48 76.7 4.1
68.8 6.1
35S-AS(C18)-Na Rat 4 mg/rat po 48 95.3 2.2 Adachi et al. (1979)
a K, potassium salt; EM, erythromycin salt; TMA, trimethylammonium salt
These compounds were degraded by a process involving initial
omega-oxidation followed by ß-oxidation of fatty acids with successive
elimination of a C2 fragment. The final product of degradation of
potassium dodecyl 35S-sulfate was potassium butyric acid
4-35S-sulfate, which was excreted in urine. When this product was
injected intraperitoneally into rats, it was mostly eliminated
unchanged in the urine, but about 20% of the dose was present as an
inorganic 35S-sulfate. These findings suggest that the sulfate ester
is hydrolysed in vivo (Denner et al., 1969).
Butyric acid 4-sulfate was hydrolysed nonenzymatically in vitro
at pH 5.0 and above, and the 4-butyrolactone (IV) and inorganic
SO42- ion were liberated in approximately equimolar amounts (Ottery
et al., 1970).
After administration of 14C-hexadecyl sulfate to rats, dogs, and
humans, the main metabolite was identified as the sulfate ester of
4-hydroxybutyric acid. A minor metabolic product, tert-14C-
butyrolactone, was also isolated from the urine of rats, dogs, and
humans. The urine of dogs contained still another metabolite, which
was isolated and identified as glycollic acid sulfate (V) (Merits,
1975).
HOOC-CH2-CH2-CH2OSO3H OC-CH2-CH2CH2 HOOC-CH2OSO3H
Butyric acid 4-sulfate 4-Butyrolactone Glycollic acid sulfate
(III) (IV) (V)
Qualitative analysis of 35S in the urine of rats administered
potassium decyl 35-sulfate or potassium octadecyl 35-sulfate
intravenously showed that butyric acid 4-35-sulfate was the major
metabolite and inorganic 35-sulfate a minor metabolite; no unchanged
compound was detected (Burke et al., 1975).
Similar results were obtained when sodium octadecyl 35-sulfate
was administered orally to rats . It was suggested that alkylsulfates
with even-numbered carbons, like C10, C12, C16, and C18, are
degraded by a common pathway involving omega-oxidation followed by
ß-oxidation, and finally excreted in urine as metabolized forms with
C4 or C2 (Adachi et al., 1979).
The metabolism of surfactants with odd-numbered carbon chains,
like C11 potassium undecyl 35-sulfate, was also investigated in
rats. Propionic acid 3-35-sulfate was identified as the major
metabolite in urine; pentanoic acid 5-35-sulfate and inorganic
35-sulfate were identified as minor metabolites (Burke et al., 1975,
1976).
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 values for AS in rats ranged from 1000 to
4120 mg/kg bw. AS irritate the skin and eye at concentrations of about
0.5% or more. Although the effects of short- and long-term exposure to
AS in animals have been investigated, most of the studies are limited
by inadequate histopathological examination or small group size. Toxic
effects have been reported in rats administered AS in the diet or
drinking-water at concentrations equivalent to > 200 mg/kg per day.
Maternal toxicicity and fetotoxic effects have been observed at a
dose equivalent to 200 mg/kg per day.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of AS in experimental animals; however, in the
limited studies available, in which animals were administered AS in
the diet, there was no evidence of carcinogenicity.
On the basis of limited data, AS also do not appear to be
genotoxic in vivo or in vitro.
7.1 Single exposures
The oral, intraperitoneal, intravenous, and dermal LD50 values
for AS are summarized in Table 38. The acute oral toxicity of AS in
rats and guinea-pigs may vary with the length of the alkyl chain, and
compounds with shorter chains are less toxic. The low LD50 value for
sodium lauryl sulfate after dermal application to rabbits may indicate
rapid skin penetration.
There were no overt signs of poisoning, except diarrhoea in rats
given sodium coconut alcohol sulfate orally (Brown & Muir, 1970);
however, signs of central nervous stimulation, including tremors,
tonic-clonic convulsions, and respiratory collapse, were observed in
rabbits, guinea-pigs, and rats given lauryl sulfate dermally and in
rabbits given the compound intravenously (Carson & Oser, 1964).
In animals that died after receiving large doses of AS, the main
gross pathological findings were haemorrhage and congestion of the
stomach wall and bloodstained urine. Histopathological examination of
rats given the sodium sulfate derivative of 3,9-diethyltridecane-
4-ol orally revealed congestion, cloudy swelling of convoluted tubules
with marked toxic degeneration of the cells, and granular detritus in
the kidneys of animals killed by the LD50, whereas only congestion
and cloudy swelling were seen in the kidneys of animals that survived
the LD50. At larger doses, similar severe kidney injury and necrosis
of the intestinal villi of the entire mucosal surface of the small
intestine were observed. Only minor injury was seen in the liver, and
the other organs examined were normal (Smyth et al., 1941).
Table 38. Acute toxicity of alkyl sulfates (AS)
Species Sex Route LD50a Test material Reference
Mouse NS po 2900 C8 sodium AS Gloxhuber
2200 C10 sodium AS (1974)
2700 C12 sodium AS
3000 C14 sodium AS
> 8000 C16 sodium AS
> 8000 C18 sodium AS
Rat M po 4120 40% solution of sodium Smyth et
2-ethylhexanol sulfate al. (1941)
M po 1250 25% solution of sodium
7-ethyl-2-methyl
undecanol-4 sulfate
M po 1425 25% solution of sodium
3,9-diethyl tridecanol-6
sulfate
M po 2730 30% solution of sodium
lauryl sulfate
F,M po 1280 86% sodium laury sulfate Walker et
(C12-C15) al. (1967)
F,M po 1000-2000 Sodium coconut alcohol Brown &
sulfate (mainly C12) Muir (1970)
F,M ip 210 Sodium lauryl sulfate Epstein et
al. (1939)
F,M Dermal 2000 (100% 30% slurry of sodium lauryl Carson &
deaths) sulfate Oser (1964)
Table 38 (contd)
Species Sex Route LD50a Test material Reference
Guinea- F,M po 1520 40% solution of sodium Smyth et
pig 2-ethylhexanol sulfate al., (1941)
F,M po 650 25% solution of sodium
7-ethyl-2-methyl
undecanol-4 sulfate
F,M po 425 25% solution of sodium
3,9-diethyl tridecanol-6
sulfate
F,M Dermal 1200 33% slurry of sodium lauryl Carson &
(no deaths) sulfate Oser (1964)
F,M Dermal 2000 (100% 33% slurry of sodium lauryl
deaths) sulfate
Rabbit F,M Dermal 580 33% slurry of sodium lauryl
sulfate
F,M iv 121 (100% 33% slurry of sodium lauryl
deaths) sulfate
M, male; F, female
a As active ingredient
C7.2 Short-term exposure
The results of short-term tests for toxicity with repeated doses
are summarized in Table 39.
C7.2.1 Rat
C7.2.1.1 Administration in the diet
Groups of five male and five female Wistar rats were fed diets
containing technical-grade sodium lauryl sulfate (purity, 98%) at a
concentration of 0, 0.5, 1, or 2% (equivalent to 245, 490, or
980 mg/kg of diet per day) for two or four weeks. No abnormalities
were seen in behaviour or food intake; but body weight gain was
significantly suppressed in females at the highest dose, and
haematological examination revealed a significant decrease in red
blood cells at two weeks. Biochemical examination of the serum
revealed a significant increase in the glucose level at two weeks in
males given 2%, a significant increase in glutamate-oxalate
transaminase at two weeks in females given 1 or 2%, significant
increases in glutamate-pyruvate transaminase and alkaline phosphatase
activities at four weeks in all females, and a significant decrease in
cholinesterase activity at four weeks in females given 2%. Both the
absolute and relative weights of the liver and thyroid were increased
at two weeks in males and females given 2%, and those of the liver and
left kidney were increased at four weeks in all females; the weights
of the thymus were decreased in males given 2% at four weeks.
Histopathological examination of rats with increased liver weight
revealed slight swelling of liver cells and increased numbers of
dividing liver cells. This finding was considered to be an adaptation
to administration of the test material. Cylinders in the renal
tubules, vacuolar degeneration of the epithelial cells of the renal
tubules, periodic acid-Schiff stain-positive substances in the renal
tubules, and atrophy of the renal glomeruli were observed mainly in
rats given 1 or 2% (Oishi et al., 1974).
Groups of 25 albino rats (sex not specified) were given diets
containing a sodium lauryl sulfate formulation (Iriumr) at a dose of
0, 30, or 60 mg/animal per day for eight weeks. The only abnormal sign
in the experimental groups was soft stools. Histological examinations
of the livers of four rats in each group revealed swelling of liver
cells, compression of cellular cords, and prominent nuclei. These
effects were particularly marked in rats given the high dose (Hatton
et al., 1940).
Table 39. Results of short-term exposure of experimental animals to alkyl sulfates (AS)
Species, strain, Material Route Dosage Results Reference
numbers per group
Rat, Wistar, 10 AS, C12 (a.i. 98%) Diet 0, 0.5, 1.0, 2.0%, Changes in haematological Oishi et al.
4 weeks parameters, serum enzyme (1974)
activities, and liver; depressed
body weight gain in females at
highest dose; increased weights
of liver, thyroid and kidney at
highest dose; decreased thymus
weight in males
Rat, 25 AS, C12 (Irium(R) Diet 30, 60 mg/rat per Dose-related hepatic effects Hatton et al.
day, 5 weeks (1940)
Rat, Wistar, 5 AS, C12 Diet 1.5%, 12 weeks Changes in serum, renal, and Ikawa et al.
hepatic enzyme activities; (1978)
depressed body weight gain;
increased liver weight
Rat, Osborne- AS, C12 Diet 9, 2, 4, 8%, Diarrhoea, abdominal bloating; Fitzhugh &
Mendel, 5 M 4 months depressed body weight gain Nelson (1948)
Rat, Carworth, AS, C12-C15 Diet 9, 0.04, 0.02, 0.1, Increased liver weight in Walker et al.
24 (a.i. 86%) 0.5%, 13 weeks females at highest dose (1967)
Rat, Wistar, AS Drinking- 0, 0.25, 0.5, 1.0, Renal changes; proteinuria; Smyth et al.
5, 10 water 2.0, 4.0%, 30 days depressed body weight gain at 4% (1941)
sodium 2-ethylhexanol sulfate
Rat, Wistar AS (a.i.22.5%) Dermal 5 mg/kg per day, Dermal irritation; hepatic Sakashita et
15 M 30 days effects al. (1974)
Table 39 (contd)
Species, strain, Material Route Dosage Results Reference
numbers per group
Rat, Wistar AS (a.i. 22.5%) Dermal 5 mg/kg per day, Hepatic degeneration Sakashita
(NS) M 30 days (1979)
Rabbit AS, sodium lauryl Dermal 6, 60, 150 mg/kg, Dermal irritation Carson & Oser
3 M, 3 F sulfate 5 times/week, (1964)
3 months
a.i., active ingredient; M, male; F, female; NS, not specified
Groups of five male Wistar SPF rats were fed a diet containing
sodium dodecyl sulfate at a concentration of 1.5% (equivalent to
750 mg/kg of diet per day) for 2, 4, or 12 weeks, and were compared
with a control group. Body weight gain was suppressed and relative
liver weight significantly increased from two weeks. Biochemical
analysis of serum revealed increased activities of alkaline
phosphatase and glutamate-pyruvate transaminase and a decreased level
of cholesterol. Enzymatic examinations of the liver showed decreased
activity of glucose-6-phosphatase at 12 weeks, decreased activity of
glucose-6-phosphate dehydrogenase and increased activity of lactate
dehydrogenase at each observation time, and increased isocitrate
dehydrogenase activity at 4 and 12 weeks. Examination of the renal
cortex showed decreased activities of 5'-nucleotidase and Mg-ATPase at
12 weeks and increased isocitrate dehydrogenase activity at 4 and 12
weeks. Examination of the renal medulla showed decrease activities of
Mg- and Na,K-ATPases and increased isocitrate dehydrogenase activity
at 12 weeks (Ikawa et al., 1978).
Groups of five male Osborne-Mendel rats were given diets
containing sodium lauryl sulfate at a concentration of 0, 2, 4, or 8%
(equivalent to 1000, 2000, or 4000 mg/kg of diet per day) for four
months. Significant inhibition of growth was observed with 4%; severe
diarrhoea and marked abdominal bloating were noted at 8%, and all the
rats died within two weeks. Autopsy revealed irritation of the
gastrointestinal tract in rats fed 8% (Fitzhugh & Nelson, 1948).
Technical-grade sodium lauryl sulfate (86% w/w active ingredient;
chain length distribution, C12-C15) was fed to four groups of 12
male and 12 female Carworth Farm 'E' rats at a dietary level of 0, 40,
200, 1000, or 5000 ppm (corresponding to 2, 10, 50, or 250 mg/kg bw
per day) for 13 weeks. No abnormalities were observed in behaviour,
body weight, food intake, haematological parameters, urinary pH or
osmolality, serum urea or protein, or organ weights, except for a
significant increase in the absolute weight of the liver in females
fed 5000 ppm (Walker et al., 1967).
C7.2.1.2 Administration in the drinking-water
Groups of five or 10 male Wistar rats were given water containing
sodium 2-ethylhexanol sulfate, sodium 7-ethyl-2-methyl undecanol-4
sulfate, or sodium 3,9- diethyl tridecanol-6 sulfate at a
concentration of 0, 0.25, 0.5, 1, 2, or 4% for 30 days. Water intake
was decreased at concentrations > 2% of sodium 2-ethylhexanol
sulfate and sodium 7-ethyl-2-methyl undecanol-4 sulfate and at > 1%
sodium 3,9-diethyl tridecanol-6 sulfate. Body weight gain was
suppressed at 4% sodium 2-ethylhexanol sulfate. None of the rats died,
and no haematological abnormalities were observed during the
experiment. Proteinurea was seen at 2 and 4% sodium 2-ethylhexanol
sulfate. The major histopatho-logical findings were renal changes,
including light cloudy swelling and secretion in the renal tubules and
congestion or dilation of Bowman's capsule. The no-effect doses were
0.44 g/kg bw per day of sodium 2-ethylhexanol sulfate, 0.1 g/kg bw per
day of sodium 7-ethyl-2-methyl undecanol-4 sulfate, and 0.25 g/kg bw
per day of sodium 3,9- diethyl tridecanol-6 sulfate (Smyth et al.,
1941).
C7.2.1.3 Dermal application
A group of 15 male Wistar rats received 2 ml of a commercial
preparation of AS (22.5% active ingredient) on their backs, and the
livers of three rats were examined under the electron microscope three
and 30 days later; a control group was available. Redness of the skin
and wrinkles were observed in treated animals at 24 h; the redness
subsequently increased, the dermis became lacerated, and bleeding
occurred. These lesions reached a peak at 57 days but tended to
regress about 10 days later. Five rats died within the first 19 days.
Electron microscopy at three days revealed separation of the
intercellular space, cells with a high electron density, elongation of
mitochondria, swelling of the smooth-surfaced endoplasmic reticulum,
and a decreased prevalence of fatty droplets. Electron microscopy at
30 days showed liver parenchymal cells filled with mitochondria,
apparently abnormally divided and proliferated smooth-surfaced
endoplasmic reticulum, abnormally rough-surfaced cells, a typical
Golgi apparatus, myelin-like structures in bile canaliculi, and
extracellular prolapse of mitochondria (Sakashita et al., 1974).
Electron microscopy of the liver was also performed after dermal
application of a commercial preparation of AS (22.5% active
ingredient) to male Wistar rats (number not specified) at a dose of
5 mg/kg active ingredient once a day for 30 days. Hepatic
degeneration, seen as atrophy and a high density of liver cells, was
observed; in cells, there was deformation of nuclei, mitochondria, and
the Golgi apparatus, an increased number of lysosomes, and swelling of
endoplasmic reticula (Sakashita, 1979).
As no information was given on the method of application (occluded
or non-occluded), these results were not interpretable in terms of
risk to human health.
C7.2.2 Rabbit
Sodium lauryl sulfate was applied dermally to three groups,
consisting of two male and two female rabbits with intact skin and one
male and one female rabbit with abraded skin, at a dose of 6, 60, or
150 mg/kg bw five times per week for three months. A control group
consisted of one male and one female with intact skin and one male
with abraded skin. Dose-related irritation of the skin was observed in
all treated animals (Carson & Oser, 1964).
C7.3 Long-term exposure; carcinogenicity
C7.3.1 Mouse
In a study of the effects of AS on the carcinogenicity of
benzo[a]pyrene (BaP), a 10% AS solution, a 0.3% BaP solution, and a
10% AS:0.3% BaP solution were applied to the backs of groups of 10
male and 20 female mice twice a week for one year. Skin tumours
appeared in all mice treated with BaP or AS:BaP. The average ages at
the appearance of skin tumours were 119 days in the group exposed to
BaP and 102 days in that exposed to AS:BaP. It was concluded that AS
accelerated the induction of tumours by BaP ( p < 0.1). Untreated
mice and vehicle (acetone) controls had no skin tumours; one female
exposed to AS had a skin tumour, but this finding was not considered
to be related to treatment (Yamamoto, 1977).
C7.3.2 Rat
C7.3.2.1 Administration in the diet
Three groups of 12 weanling male Osborne-Mendel rats were given
food containing sodium lauryl sulfate at a concentration of 0.25, 0.5,
or 1.0% for two years; there was a similar sized control group. No
effects attributable to the test material were observed on growth,
mortality, or the macroscopic or histopathological appearance of
organs. No tumours were reported (Fitzhugh & Nelson, 1948). As there
were few animals per group and no toxic effects at any dose, the
observations are considered to be of limited value.
C7.3.2.2 Administration in the drinking-water
Groups of 4-11 white rats were given drinking-water containing
sodium lauryl sulfate at a concentration of 0, 0.1, 0.25, 0.5, 1, 5,
or 10% for 120 or 160 days. Dose-related increases in mortality
occurred at doses > 0.25%; at doses > 5%, all rats died.
Histological examination of rats exposed to doses > 0.25% revealed
marked inflammatory changes of the lumen of the oesophagus in those
that died, but the changes were slight in surviving animals. No
abnormalities were seen in the liver, kidney, or intestine. The intake
of the materials was about 30 mg/animal per day in those given 0.1%
and 150 mg/animal per day in those given 1.0% (Epstein et al., 1939).
Groups of 9 or 10 weanling male Wistar rats were given
drinking-water containing technical-grade sodium lauryl sulfate at a
concentration of 0, 0.05, or 0.25% for five months. Growth was not
suppressed, even at the higher concentration, and the activities of
serum enzymes, including glutamate-oxalate and glutamate-pyruvate
transaminases, alkaline phosphatase and cholinesterase, were not
affected. At 0.25%, the triglyceride level increased in the liver but
decreased in serum, while hepatic and serum levels of cholesterol,
phospholipids, and free fatty acids were unchanged. Increased weights
of spleen, lung, and kidney were noted at 0.25%. Histopathologically
diagnosed broncho-pneumonia, observed in all animals given 0.25% and
two animals given 0.05%, was considered to be a characteristic effect
of the test material (Fukazawa et al., 1978).
The results of long-term studies are shown in Table 40.
C7.4 Skin and eye irritation; sensitization
C7.4.1 Local irritation
C7.4.1.1 Skin
Groups of two to six white rats received a subcutaneous injection
of 1 ml of one of 10 solutions of sodium AS, ranging from 0.125 to 10%
and were observed for one week after the injection. No reactions
occurred at 0.125%, but sloughing and subcutaneous lumps in the skin
appeared in rats given doses > 0.19%. In a study in which the
diffusibility of trypan blue was used as an index of irritation,
groups of five to nine white rats were given subcutaneous injections
of 0.2 ml sodium AS at one of six concentrations ranging from 0.15 to
5%. Two hours after the injection, slight reactions were seen in
animals given 0.15% and marked reactions in those given 2.5 or 5%
(Epstein et al., 1939).
Groups of three albino rabbits received closed-patch applications
of 5 ml of 1, 5, or 25% sodium lauryl sulfate solution on intact and
abraded areas of shaven abdominal skin. Over a 14-day period, 10
applications were made to intact skin and three to abraded skin;
additionally, small amounts of the material were applied daily to the
intact ears of groups of three rabbits. Occluded application to the
abdomen produced erythema and blistering, which was more severe on
abraded skin. Application to the intact ear resulted in very slight
erythema at the 1% concentration, very slight to slight erythema at
5%, and slight erythema with moderate to severe burns at 25% (Olson et
al., 1962).
Sodium alcohol (coconut alcohol, mainly C12) sulfate solutions
of 0.1, 1.0, and 2.5% were applied in occluded tests in rabbits as
1 ml of each solution on the back three times on three days.
Macroscopic and histological examination seven days after application
revealed no abnormalities at 1.0% and moderate irritation at 2.5%. In
open tests, 1 ml of each of the solutions was applied to the backs of
rabbits and 0.5 ml to the backs of guinea-pigs five times a week for
4.5 weeks. No abnormal findings were seen in animals receiving 0.1 or
1.0% groups, but there was moderate irritation at 2.5% (Brown & Muir,
1970).
Table 40. Results of long-term exposure of experimental animals to alkyl sulfates (AS)
Species, strain, Material Route Dosage Results Reference
numbers per group
Mouse, ddy/SLC 10% AS, 3% Dermal Twice per Skin tumours Yamamoto (1977)
10 M, 20 F benzo[ a]pyrene week, 1 year
Rat, Osborne-Mendel 1.0%AS, C12 Diet 0, 0.25, 0.5, 1.0%, No effects Fitzhugh & Nelson
10-12 M 2 years (1948)
Rat, Wistar, 9-10 AS, C12 Diet 0, 0.05, 0.25%, Increased weights of Fukuzawa et al.
5 months spleen, lung, and liver (1978)
at highest dose
Rat, 4-11 AS, C12 Diet 0, 0.1, 0.25, 0.5 Oesophageal irritation Epstein et al.
1.0, 5.0%, 160 days (1939)
Groups of three male Wistar rats received applications of 0.5 g of
a 20 or 30% solution of linear lauryl sulfate (C12; purity, 98.91%)
on the back once a day for 15 days. The skin at the application site
and the tissues of the tongue and oral mucosa (to determine the
effects of licking) of animals receiving the 30% solution were
examined histologically 16 days after application. Body weight gain
was inhibited in the group given the 20% solution; body weight was
decreased in the group at 30%, and two rats had died by the end of the
experiment. A dry, thick, yellowish-white or reddish-brown crust was
observed after two to three days in animals given 20% and after one to
two days in those given 30%. When the crust was abraded several days
later, ulcers occurred at the abraded site, which remained unchanged
for 16 days in animals at 20% group and were aggravated in those at
30%. Histological examination of the application site revealed severe
necrosis extending from the epidermis to the upper layer of the
dermis, dense inflammatory-cell infiltration into the upper layer of
the dermis just below the necrotic area, diffuse inflammatory-cell
infiltration throughout the dermis, swelling of collagenous fibres in
the dermis, and sloughing. Histological examination of the tongue
revealed necrosis extending from the surface to the middle epithelial
layer of the mucosa, inflammatory-cell infiltration into the upper
layer of the dermis, and sloughing. Histological examination of the
mucosa of the oral cavity revealed thickening of the stratum corneum
and germinative and slight degeneration (pale staining) of epithelial
cells (Sadai & Mizuno, 1972).
The effects of sodium lauryl sulfate on oesophageal and gastric
mucosa were studied in cats by irrigation and pledget techniques. In
the irrigiation technique, the stomachs and oesophaguses of two cats
were filled with 10 and 20% solutions of sodium lauryl sulfate,
respectively, for 15 min, and then tissues were taken for histological
examination. Pledgets soaked in 10 or 20% sodium lauryl sulfate
solution were applied to the exposed oesophageal and gastric mucosa of
two other cats for 10 min, and specimens were taken 90 min later. The
10% solution produced moderate injury to the oesophagus, consisting of
intramucosal oedema and congestion and loss of superficial epithelial
layers; in the stomach, there was hydropic degeneration, loss of
surface mucosal cells, vascular congestion with submucosal oedema, and
occasional focal ulceration. Treatment with the 20% solution resulted
in more extensive damage, and particularly extensive submucosal oedema
and disruption and erosion of the superficial mucosa of both the
oesophagus and stomach (Berensen & Temple, 1976).
C7.4.1.2 Eye
Three drops of one of nine solutions of sodium lauryl sulfate
ranging from 0.019 to 5.0% were instilled into the eyes of rabbits
three times at 10-min intervals, and the rabbits were observed for
48 h. There were no abnormal findings at 0.038%, but slight chemosis
and redness were seen at 0.075% and marked chemosis and redness at 5%
(Epstein et al., 1939).
The minimal concentration of sodium lauryl sulfate that caused
corneal necrosis (detected by fluorescein staining) after instillation
into the eyes of rabbits was 0.1% (Smyth et al., 1941). In another
study, two drops of a 1, 5, or 25% solution of sodium lauryl sulfate
were instilled into both sides of the eyes of groups of three rabbits;
30 min later, one of the eyes was washed. Moderate corneal injury was
observed in unwashed eyes of animals receiving the 5 or 25% solution;
in washed eyes, either slight conjunctivitis or moderate corneal
injury was observed at 25%, slight conjunctivitis at 5%, and only very
slight conjunctivitis at 1% (Olson et al., 1962).
In an irritation test based on a method developed by the United
States Food and Drug Administration, 0.1, 1, or 25% solutions of
sodium coconut alcohol sulfate were instilled into the eyes of
rabbits. No reaction was seen at 0.1%; mild conjunctivitis lasting for
48 h was seen at 1%, and severe conjunctivitis lasting for 72 h was
observed at 25% group, but there was no permanent damage (Brown &
Muir, 1970). Solutions of a synthetic alkyl sulfate and five AS
consisting mainly of C10, C12, C14, C16, or C18, were instilled
at concentrations of 0.01-5% into the eyes of three rabbits, which
were observed for 168 h. The materials caused similar reactions. No
abnormalities were seen at 0.01%. Slight congestion and marked
congestion or oedema were observed at 0.05 and 0.1% within 2 h, but
these effects had disappeared 24 h later. In the groups given >
0.5%, marked reactions were seen for 24 h, including severe congestion
and oedema, increased lachrymal secretion, turbidity of the cornea,
and disappearance of the corneal reflex, but these tended to regress
and had disappeared completely by 120 h (Iimori et al., 1972).
C7.4.2 Skin sensitization
A 0.1% solution of a sodium lauryl sulfate derivative of coconut
alcohol was applied to the skin or injected intradermally into groups
of 10 guinea-pigs three times per week for three weeks. Ten days later
the animals received challenge doses and were observed for 48 h. No
reaction occurred in the group treated dermally, but a slight reaction
was observed 24 h after the challenge in some of the guinea-pigs
treated intradermally (Brown & Muir, 1970).
C7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Daily doses of 0.2, 2, 300, or 600 mg/kg bw of AS were
administered by gavage to CD rats, CD-1 mice, and NZW rabbits. Groups
of 20 rats and mice were given AS on days 6-15 of pregnancy, and
groups of 13 rabbits were treated on days 6-18 of pregnancy. The doses
of 0.2 and 2 mg/kg bw per day were estimated to be equivalent to 1-2
and 10-20 times the maximal amount of AS to which humans are exposed.
Three rats given 600 mg/kg bw died during the study, but the surviving
rats and those given 300 mg/kg bw had only mild to moderate inhibition
of body weight gain. Mice given 600 mg/kg bw showed severe effects,
including anorexia and inhibition of body weight gain, and four
animals died during the study; in those given 300 mg/kg bw, inhibition
of body weight gain was mild to moderate. Rabbits given 600 mg/kg bw
showed severe effects, including diarrhoea, anorexia, and reduced rate
of body weight gain, and 11 died during the study; those given
300 mg/kg bw showed mild to moderate reduction of body weight gain. No
toxic effects were seen in any of the animals given 0.2 or 2 mg/kg bw.
No adverse effects were seen on litters of rats at any dose. Some mice
and rabbits at each dose had total litter loss, but the other litter
parameters did not differ from those of controls. No major
malformations were seen at any dose in offspring of rats, mice, or
rabbits, and the incidence of skeletal variations in offspring of rats
given 600 mg/kg bw was significantly low. A high incidence of skeletal
anomalies was seen in litters of mice given 600 mg/kg bw, and those of
rabbits at 2.0 mg/kg bw had a significantly higher incidence of
skeletal variations; however, the incidences of anomalies and
variations were within the background range (Palmer et al., 1975a).
Groups of 21 ICR mice received applications of 15 mg/kg bw per day
of a 0.4, 4, or 6% aqueous solution of AS (98% sodium dodecyl sulfate,
0.5% N2SO4, 0.1% NaCl, and 0.1% H2O) to a 3 × 3-cm2 area of
shaven dorsal skin on days 6-13 of pregnancy. The 0.4% solution was
equivalent to about 10-12 times the specified concentration used by
humans, and the application area was equivalent to about one-seventh
of the total surface area of the mouse. The body weight gain of dams
exposed to the 4 or 6% solution was reduced; there were no deaths. The
numbers of dams with surviving young were 19/21 in the control group,
20/20 at 0.4%, 17/20 at 4%, and 11/21 at 6%; the decrease in dams at
6% was significant. Fetal weights were significantly lower in dams at
4 and 6%, but there were no other differences from the control values.
The incidence of cleft palate was fairly high in offspring of dams
exposed to the 4 or 6% solution, and a tendency to delayed
ossification was seen; however, none was significant (Takahashi et
al., 1976).
A dose of 0.1 ml/day of a 2% aqueous solution of AS was applied to
a 2 × 3-cm2 area of shaven dorsal skin in groups of 20-26 ICR mice
on days 1-17 of pregnancy. The same dose of a 20% solution was applied
to a similar group up to the 10th day of pregnancy, and implantation
was examined on the 11th day. In addition, 14 mice were injected
subcutaneously with 2 mg/kg bw per day of AS on days 8-10 of
pregnancy. The numbers of dams with implantations were 18/20 controls,
14/22 at 2%, 1/26 at 20%, and 13/14 at 2 mg/kg bw; the decrease at 20%
was significant. There were no significant changes in litter
parameters and no significant changes in the incidences of major
malformations, minor anomalies, or skeletal variations. AS thus
disturbed implantation and caused abortion at maternally toxic doses,
but in surviving litters it had no effect on the size or numbers of
fetuses, although low fetal weight and delayed ossification were
observed. At doses that had no or only mild effects on the dams, no
adverse effects were seen on the fetuses. The effects of AS on the
fetus therefore appear to be secondary to the toxic effects on the
dams (Nomura et al., 1980).
C7.6 Mutagenicity and related end-points
Sodium lauryl sulfate did not cause differential toxicity in
Bacillus subtilis H17 ( rec+) or M45 ( rec-) at concentrations
of 20-2000 µg/plate, and it did not induce reverse mutations in
Salmonella typhimurium TA98 or TA100 at 1-500 µg/plate or in
Escherichia coli WP2 trp at 10-1000 µg per plate (Inoue &
Sunakawa, 1979).
Sodium lauryl sulfate, Dobanol 25 sulfate LCU, and Dobanol 25
sulfate HCB (aliphatic alcohol sulfates with chain lengths of
C10-C15) were fed in the diet to groups of six male and six female
Colworth/Wistar rats for 90 days at a concentration of 0.56 or 1.13%,
the latter being the maximal tolerated dose. No effect was seen on
chromosomes in bone-marrow cells (Hope, 1977).
After dodecyl sulfate was administered to male ddY mice
intra-peritoneally at 50 mg/kg bw, the incidence of polychromatic
erythrocytes with micronuclei in the bone marrow was similar in
treated and control groups (Kishi et al., 1984).
C7.7 Special studies
Intravenous injection of 1 mg/min sodium decyl sulfate or
5.7 mg/min sodium dodecyl sulfate to cats increased pulmonary arterial
pressure, caused a small increase in systemic vascular resistance, and
reduced the ventilation volume per minute after about 5 min.
Intravenous injection of 4.6 mg/min sodium octyl sulfate or 6.3 mg/min
sodium tetradecyl sulfate had similar effects. The increase in
pulmonary arterial pressure was considered to be due to a direct
effect on the smooth muscle of blood vessels and bronchi. The blood
sugar level was unchanged (Schumacher et al., 1972).
The effects of sodium lauryl sulfate on histamine release from
mast cells were studied in vitro in peritoneal mast cells isolated
from rats. Histamine was released at a concentration of
0.03 mmol/litre, and the critical micelle concentration in buffer at
22°C was 1.0 mmol/litre. Sodium lauryl sulfate and its mono- and
tri-ethoxy derivatives had the most potent histamine releasing
capacity of nine surfactants with a chain length of C12 (Prottey &
Ferguson, 1975).
C8. EFFECTS ON HUMANS
Section summary
In patch tests, human skin can tolerate contact with solutions
containing up to 1% AS for 24 h with only mild irritation. AS caused
delipidation of the skin surface, elution of natural moisturizing
factor, denaturation of the proteins of the outer epidermal layer, and
increased permeability and swelling of the outer layer. They did not
induce skin sensitization in volunteers, and there is no evidence that
they induce eczema. No lasting injuries or fatalities have been
reported following accidental ingestion of detergent formulations
containing AS.
C8.1 Exposure of the general population
Surface-active agents are found in shampoos, dishwashing products,
household cleaners, and laundry detergents, and AS are major
components of these products. The composition of nonionic and ionic
surfactants varies between 10 and 30%. Surface-active agents can
affect human skin and eyes.
C8.2 Clinical studies
C8.2.1 Skin irritation and sensitization
AS can be mildly to moderately irritating to human skin. No data
were available on sensitization.
The relative intensity of skin erythema produced on the lower back
of volunteers was evaluated by applying concentrations of 0.2-5.4% of
C8, C10, C12, C14, or C16 AS under a closed patch for 24 h
or under a closed patch re-applied once daily for 10 days. C12 AS
were more potent than AS with other alkyl chain lengths (Kligman &
Wooding, 1967).
A circulation method was used to evaluate the relative intensity
of skin roughness induced on the surface of the forearms of volunteers
after application for 1 min of 1% aqueous solutions of AS with an
alkyl chain length of C8, C10, C12, or C14. The potential to
cause skin roughness increased with alkyl chain length, reaching
maximal intensity at C12 (Imokawa et al., 1974, 1975a). In other
studies, the relative degree of skin roughening was correlated with
the extent of protein denaturation but not with irritating potential
determined in a closed-patch test (Imokawa et al., 1975b).
Primary skin irritation induced by a 1% aqueous solution (pH 6.8)
of dodecyl sulfate (relative molecular mass, 288.5) was studied in a
24-h closed-patch test on the forearms of seven male volunteers. The
relative intensity of skin irritation was scored by grading erythema,
fissuring, and scaling. The average score for AS was 4.86, whereas
that for a water control was 1.79. Dodecyl sulfate was more irritating
than either LAS or AOS (Oba et al., 1968a).
The intensity of skin irritation produced by a 1% aqueous
solution of sodium AS was studied in a 24-h closed-patch test on the
forearm and in a 40-min drip test on the interdigital surface in which
the compound was dripped once daily for two consecutive days at a rate
of 1.2-1.5 ml/min. Skin reactions were scored by grading erythema in
the patch test and by grading scaling in the drip test. The average
scores were 2.5 for primary skin irritation at 24 h in the patch test
and 1 for scaling at two days in the drip test; in both tests, the
control value was 0. AS was more irritating than LAS or AOS in the
patch test, whereas the score of AS for skin scaling in the drip test
was similar to that of LAS but higher than that of AOS (Sadai et al.,
1979).
Moderate to intense erythema was produced on the forearms of
10 volunteers in a 24-h closed-patch test by a 10% aqueous solution
of AS with an average chain length of C12. The mean irritation
scores were significantly higher at 26 h (2.85 out of 8 possible
points) and at 28 h (2.88) than at 24 h (2.00), when the patches were
removed. Irritation had decreased by 48 h, and a significant decrease
in the intensity of inflammation was apparent at 96 h (Dahl & Trancik,
1977).
In a 48-h patch test on the upper arms of 100 pairs of twins
(54 monozygotic, 46 dizygotic) with a solution of 0.5% C12 AS, no
reaction was seen in 50% of the subjects, and slight reaction, ranging
from noninflammatory changes to mild erythema, in the other 50%. The
response was not related to the type of twin (Holst & Moller, 1975).
Application of aqueous 0.5, 1, or 2% solutions of AS with an
average chain length of C12 to the backs of healthy male volunteers
produced epidermal hyperplasia. Treatment with the 1% solution induced
an approximately 30-fold increase in mitotic activity, which peaked 48
h after treatment. Application of either the 0.5 or the 2% solution
induced similar but milder changes (Fisher & Maibach, 1975).
Skin permeability to C8, C10, C12, C14, C16, and C18
AS prepared as 0.02, 0.5, and 1% solutions (0.58% C8 and 0.74%
C18) was studied by a circulation method on the forearms of healthy
male and female volunteers. C12 AS attained maximum permeation,
whereas the permeation of C8 and C18 AS was of the same order as
that of water. The authors pointed out the close relationship between
permeation and irritation (Szakall & Schulz, 1960).
C8.2.2 Effects on the epidermis
The effects of AS on the stratum corneum include delipidation of
the skin surface, elution of natural moisturizing factor, denaturation
of protein of the stratum corneum, increased permeability, swelling of
the stratum corneum, and inhibition of enzyme activities in the
epidermis. These effects, and some others, constitute a potential
hazard to the epidermis.
The water-holding capacity of thin sheets of callus isolated from
the plantar surface of the human foot, with relative moisture contents
of 76, 88, and 97%, was compared before and after immersion in water,
AS, or soap solution. Water-holding capacity was measured as the
weight of water taken up from each solution. The relative moisture
content decreased after treatment with AS or soap solution (Blank &
Shappirio, 1955).
Elution of natural moisturizing factor was compared for nine kinds
of surfactants, including AS, in the arm immersion test, in patch
tests, and by measuring eluted amino acids and protein, skin
permeation, and freeing of sulfhydryl groups. AS induced a strong
reaction in the immersion test and relatively strong reactions in the
other tests. The author concluded that the immersion test was the best
simulation of actual use (Polano, 1968).
A detergent consisting of long-chain AS was shown to denature
stratum corneum protein and thus expose enclosed sulfhydryl groups
(Anson, 1941). AS readily released sulfhydryl groups from stratum
corneum obtained from abdominal skin taken at autopsy within 12 h of
death, but there was no correlation between changes in epidermal
permeability and the amounts of sulfhydryl released (Wood & Bettley,
1971). AS were the most effective surfactants with regard to
denaturation of protein, measured as inhibition of invertase activity
(Imokawa et al., 1974; Okamoto, 1974). AS were found to denature skin
keratin (a filamentous protein), bovine serum albumin (a globular
protein), acid phosphatase (an enzyme protein), and membrane lysozymes
(membrane protein) (Imokawa & Katsumi, 1976). Sodium laurate was
reported to produce swelling of the stratum corneum (Putterman et al.,
1977).
AS with a hydrophobic chain length of C12 were maximally
absorbed on human callus. Extraction of proteins from human callus was
also a function of chain length: C12 and C14 AS were much more
active than C8, C10, and C18 AS (Dominguez et al., 1977).
C8.2.3 Hand eczema
In a 24-h closed-patch test of 0.2-0.5% aqueous solutions of AS on
the fingers of nine women with hand eczema, skin lesions were not
exacerbated, although four women felt slight itching at the patch site
(Sasagawa, 1963).
C8.2.4 Accidental or suicidal ingestion
Four members of a family accidentally ingested unknown quantities
of a household detergent containing 24% lauryl sulfate, 60% sodium
tripolyphosphate, and 16% anhydrous soap. Shortly after ingestion, all
of the family members experienced abdominal pain and nausea. The
10-year-old daughter and 13-year-old son felt oropharyngeal pain, and
the son was found at endoscopic examination to have a 2.5 × 2 cm
oropharyngeal burn in the right posterior pharynx and first-degree
burns of the oesophagus. The mother had erythema, friability, erythema
and a few superficial erosions of the distal oesophagus, and gastritis
evidenced by exudate and petechial lesions on the mucosa. The father
had haematemesis on a few occasions. The mother, father, and son were
examined about one month after the incident by an X ray examination
after a barium meal; no strictures were found (Berenson & Temple,
1974).
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
AS have been studied in short- and long-term studies in the
laboratory and in one study carried out under more realistic
conditions. Their toxicity is dependent on alkyl chain length, but no
data were available on the differential toxicity of linear and
branched AS.
In aquatic organisms, the EC50 values for C12 AS in a
community of marine microorganisms were 2.1-4.1 mg/litre. The NOEC
values were 35-550 mg/litre (C16/C18) for Pseudomonas putida and
14-26 mg/litre (C12-C16/C18) for green algae; and the EC50
values were > 20-65 mg/litre (C12-C13) for green algae and 18-43
mg/litre (C12) for macrophytes.
In aquatic invertebrates, the L(E)C50 values were 4-140 mg/litre
(C12/C15-C16/C18) for freshwater species and 1.7-56 mg/litre
(all C12) for marine species. The long-term NOECs were 16.5 mg/litre
(C16-C18) for Daphnia magna and 0.29-0.73 mg/litre (chain length
not specified) for marine species.
In fish, the LC50 values were 0.5-5.1 mg/litre (C12-C16 or chain
length not specified) for freshwater species and 6.4-16 mg/litre
(all C12) for marine species. In a 48-h study of Oryzias latipes,
chain length influenced LC50 values, the measured concentrations being
46 mg/litre for C12, 2.5 mg/litre for C14, and 0.61 mg/litre
for C16. This and other studies indicate that toxicity differs by a
factor of five for two units of chain length.
In a flow-through study of the effect of C16-C18 AS on a
biocenosis, an NOEC of 0.55 mg/litre was observed. Many of the studies
of toxicity in aquatic environments were carried out under static
conditions. As AS are readily biodegraded, this design may result in
underestimates of toxicity.
Few data were available on the effects of AS on terrestrial
organisms. An NOEC of > 1000 mg/kg (C16-C18) was reported for
earthworms and turnips.
C9.1 Microorganisms
During tests of biodegradation, marine bacteria used 20 mg/litre
AS as a nutrient source. It was therefore concluded that its toxicity
for the bacterial community studied is nil or very low (Vives-Rego et
al., 1987). In a study of the effect of C12 AS on the metabolic
activity of a marine microbial community, the EC50 values for toxic
effects on thymidine incorporation and glucose metabolism were
reported to be 4.1 and 2.1 mg/litre, respectively. AS also increased
exoproteolytic activity (Vives-Rego et al., 1986).
The 30-min EC50 for C16-C18 AS, based on oxygen consumption,
was 35 mg/litre in Pseudomonas putida (Robra, 1976). The NOEC for
cell reproduction in Pseudomonas putida exposed to C16-C18 AS
was 550 mg/litre (Bringmann & Kühn, 1977).
C9.2 Aquatic organisms
C9.2.1 Aquatic plants
C9.2.1.1 Freshwater algae
The phytoflagellate alga Poterioochromonas malhamensis was
exposed to C12 AS at sublethal concentrations of 28.8, 57.6, 72,
86.4, 100.8, and 115.2 mg/litre (100, 200, 250, 300, 350, and
400 µmol/litre), being transferred every three to four days into fresh
medium with a higher test concentration. The initial cell density in
each medium was 0.1 × 106 cells/ml; the final cell density, after
exposure to the highest concentration of AS, was 0.05 × 106 cells/ml,
which was similar to that reached after exposure of unacclimatized
algal cultures to 200 µmol/litre AS. Exposure to AS at 57.6 mg/litre
(240 µmol/litre) was reported to affect mitosis and cytokinesis, with
the formation of cells containing up to 12 nuclei. Exposure of the
alga to 50.4 mg/litre (175 µmol/litre) AS resulted in a 24% increase
in telophases (binucleated cells). Cells with eight nuclei were also
reported in this culture (Röderer, 1987).
The green alga Selenastrum capricornutum was exposed to analytical
grade C12 AS at a concentration of 10, 20, 30, 40, 50, or
100 mg/litre in synthetic medium for three weeks. Growth was reduced
by 30% at the lowest concentration (Nyberg, 1988).
The green alga Chlamydomonas reinhardi was exposed to 0.02, 0.2,
or 2.0 mmol/litre of C10, C12, C14, C16, or C18 AS for 7-10
days. Photometric absorption (652 nm) by the exposed cultures was no
different from that by controls for the first six days of exposure,
although it was reduced slightly at 2 mmol/litre. The authors
concluded that the AS were present at below the critical micelle
concentration at all concentrations tested (Ernst et al., 1983).
The EC50 for growth of the green alga Selenastrum capricornutum
exposed to C12 AS for two to three days was within the range 45-65
mg/litre (Yamane et al., 1984). An EC50 of 9 mg/litre C14 AS was
found for growth of S. capricornutum (Konno & Wakabayashi, 1987).
C9.2.1.2 Macrophytes
The seven-day EC50 values for C12 AS in the duckweed Lemna
minor under flow-through conditions were 43 mg/litre for frond count,
29 mg/litre for dry weight, and 18 mg/litre for root length.
The time-independent EC50 for growth rate/doubling time was
44 mg/litre (Bishop & Perry, 1981).
C9.2.2 Aquatic invertebrates
The acute toxicity of AS to aquatic invertebrates is summarized in
Table 41. The 48-h LC50 values were 8-60 mg/litre for daphnids; the
96-h LC50 values ranged from 3.2 to 4.2 mg/litre for marine
invertebrates.
The 48-h LC50 for lugworms (Arenicola marina) exposed to AS
was calculated to be 15.2 mg/litre (95% confidence interval,
13.2-17.6). Tissues from lugworms exposed to AS at a concentration
close to that of the LC50 were examined for changes in morphology by
both light and electron microscopy: serious damage was found in the
epidermic receptors and less serious damage in the caudal epidermis
and gills. No morphological effects were reported on the thoracic
epidermis or intestine. AS caused separation inside the caudal
epithelial layer, resulting in holes in some caudal papillae.
Deciliation of the epidermic receptors was also reported. The authors
concluded that the physiological response of damaged epidermic
receptors was reduced or blocked after exposure to AS. AS also induced
fissures in the epithelial layer of the gills (Conti, 1987).
Caeriodaphnia dubia were exposed to C12 AS for three
generations under static renewal conditions, with the following mean
water parameters: temperature, 26.2°C; pH, 8.2; hardness,
94.4 mg/litre CaCO3; and alkalinity, 82.2 mg/litre CaCO3. The
water was changed every second day. The LC50 for survival of three
broods of C. dubia was calculated to be 41 ± 3.2 mg/litre. The mean
EC50, based on progeny produced, was calculated to be 36 ± 3.2
mg/litre. No statistically significant effects were reported after
exposure to 83 mg/litre AS, although the size of later broods was
reduced (Cowgill et al., 1990).
The effect of 0.25-10 mg/litre AS was studied on the growth and
survival of eggs and larvae of oysters (Crassostrea virginica) and
clams (Mercenaria mercenaria). The minimal concentrations that
caused a significant reduction in the number of fertilized eggs which
developed into normal larvae two days after hatching were
0.73 mg/litre for clams and 0.29 mg/litre for oysters. The minimal
concentration that caused a significant reduction in growth and
survival between two and 12 or 14 days after hatching was
1.46 mg/litre for both species. The EC50 values, based on the
development of fertilized clam and oyster eggs to normal
straight-hinge larvae after 48 h, were calculated to be
0.47 mg/litre for clams and 0.37 mg/litre for oysters (Hidu, 1965).
After snails (Lymnaea peregra) were exposed to C12 AS at
measured concentrations of 0.6-12 mg/litre for six days, a
significant, dose-related reduction in the dry weight of shells was
observed, but the organic content of shells was not significantly
affected at any concentration (Tarazona & Nunez, 1987).
C9.2.3 Fish
The acute toxicity of AS to fish is also summarized in Table 41.
The 48-h LC50 values were 0.5-51 mg/litre for medaka (Oryzias
latipes). A 96-h LC50 value of 1.7 mg/litre was reported for both
rainbow trout (Salmo gairdneri) and sheepshead minnow (Cyprinodon
variegatus). The acute toxicity of AS to fish tends to increase with
increasing carbon-chain length.
Rainbow trout (Oncorhynchus mykiss) and goldfish (Carrasius
auratus) were exposed to C12 AS at a concentration of 70 mg/litre
at different levels of water hardness. Trout treated in hard water
(300 mg/litre CaCO3) died within 40-45 min; those treated in soft
water (60 mg/litre CaCO3) died after 3 h. Goldfish treated in hard
water died within 90-110 min, whereas those treated in distilled water
(no CaCO3) were alive and apparently normal after 24 h (Tovell et
al., 1974). When yearling rainbow trout were maintained in water
containing C12 AS at a concentration of 100 mg/litre, the time to
50% lethality was calculated to be 4.9 h. The changes seen in the
gills were typical of an acute inflammatory reaction: The gill
epithelium was lifted away from the underlying tissue, and lymphocytes
and granulocytes invaded the subepithelial spaces. Large numbers of
epithelial cells died, but the epithelium was not punctured (Abel &
Skidmore, 1975).
After exposure of the eggs of carp (Cyprinus carpio) to AS of
various chain lengths from spawning to hatching, the LC50 values
were calculated to be 18 mg/litre for C12 AS, 2.9 mg/litre for C14
AS, and > 1.6 mg/litre for C16 AS (Kikuchi et al., 1976).
The minimal avoidance concentration of AS, i.e. the concentration
at which fish spend 65% of a 5-min period in clean water in order to
avoid AS, was 7.1 µg/litre for medakas (Oryzias latipes) (Hidaka et
al., 1984). The threshold concentrations for avoidance of AS by ayu
(Plecoglossus altivelis) were 4.0 µg/litre of a formulation and 8.4
µg/litre of pure reagant AS (Tatsukawa & Hidaka, 1978). The
environmental relevance of avoidance studies is questionable (see also
section A9.3.3.4 of the monograph on LAS).
Larvae of the fathead minnow (Pimephales promelas) were exposed to
C12 AS at a concentration of 1.2, 2.3, 4.6, 9.2, or 18.4 mg/litre
for seven days under static renewal conditions. Survival and final dry
weight were not significantly affected at concentrations up to and
including 4.6 mg/litre; however, at 9.2 and 18.4 mg/litre, no fish
Table 41. Toxicity of alkyl sulfates (AS) to aquatic organisms
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Reference
age flow (°C) or salinity length (mg/litre)
Eastern oyster Embryo Static 20 25a C12 48-h LC50 1.7b Mayer (1987)
(Crassostrea virginica)
Mysid shrimp Juvenile Static 25 30a C12 96-h LC50 3.2b
(Metamysidopsis swifti)
Mysid shrimp Juvenile Static 25 20a C12 96-h LC50 4.2b
(Mysidopsis bahia) Adult Static 22 C12 96-h LC50 6.62 Roberts et al.
(1982)
Shrimp Adult Static 22 20.0 ± 0.5a C12 96-h LC50 7.24
(Neomysis americana)
Copepod Adult Static 10a C12 96-h LC50 2.6
(Eurytemora affinis)
(Acartia tonsa) Adult Static C12 96-h LC50 0.55
Scud NS 72-h LC50 9-46 Gilbert &
(Gammarus pulex) Pettigrew
(1984)
Water flea Static 20 C12 24-h EC50 17.4 Snell &
(Daphnia magna) Persoone
(1989)
Static 20 C12 24-h EC50 27.5 Persoone et
al. (1989)
C16-C18 24-h EC50 27.5 Steber et al.
(1988)
C12 24-h EC50 10.5-24.3 Cowgill et
al. (1990)
Table 41 (contd)
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Reference
age flow (°C) or salinity length (mg/litre)
Water flea C12 24-h LC50 15.0 Snell &
(Daphnia pulex) Persoone
(1989)
C12 24-h LC50 9.5-20.5 Cowgill et
al. (1990)
Mosquito 2nd/3rd Static 25 C12-C15 24-h LC50 4 van Emden et
(Aedes aegypti) stage al. (1974)
Rainbow trout Flow 15 350-375c 8.3-8.5 NS 96-h LC50 4.62 Fogels &
(Salmo gairdneri) Sprague (1977)
NS 96-h LC50 1.7 Gilbert &
Pettigrew
(1984)
Atlantic silverside 59 mm Static 22 10a C12 96-h LC50 6.4 Roberts et al.
(Menidia menidia) (1982)
Medaka (killifish) 48-h LC50 10 Tomiyama
(Oryzias latipes) (1974)
323 mg Staticr 23-24 5.6-5.8 C12 24-h LC50 70b Kikuchi et al.
323 mg Staticr 23-24 5.6-5.8 C12 48-h LC50 51b (1976)
323 mg Staticr 19-21 5.6-5.8 C14 24-h LC50 5.9b
323 mg Staticr 19-21 5.6-5.8 C16 24-h LC50 0.78b
323 mg Staticr 19-21 5.6-5.8 C12 48-h LC50 0.5b
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 46d Kikuchi &
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 2.5d Wakabayashi
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 0.61d (1984)
Table 41 (contd)
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Referenceage
flow (°C) or salinity length (mg/litre)
Sheepshead minnow Juvenile Static 25 20a C12 96-h LC50 1.7b Mayer (1987)
(Cyprinodon variegatus)
Fathead minnow NS Static NS 80-400 7.4-8.2 NS 96-h LC50 5-6 Henderson et
(Pimephales promelas) al. (1959)
< 30 d Static 20 C15 48-h LC50 7.8 Cowgill et al.
<30 d 17 C12 24-h LC50 7.7-9.7 (1990)
<30 d 17 C12 96-h LC50 7.0-9.0
30±2 d 20 C12 48-/96-h LC50 38.0
Carp 4.4 mg Static 22 25 7 C10 12-h LC50 180b Kikuchi et al.
(Cyprinus carpio) 4.4 mg Static 22 25 7 C10 48-h LC50 13b (1976)
4.4 mg Static 22 25 7 C12 12-h LC50 46b
4.4 mg Static 22 25 7 C14 48-h LC50 5.0b
4.4 mg Static 22 25 7 C16 12-h LC50 0.69b
4.4 mg Static 22 25 7 C16 48-h LC50 0.69b
Static, water unchanged for duration of test; NS, not specified; flow, flow-through conditions: AS concentration in water maintained
continuously; staticr, static renewal: water changed at regular intervals
a Salinity (%)
b Based on nominal concentrations
c Hardness expressed as mg/litre CaCO3
d Based on measured concentrations
survived. When the test was repeated over an eight-day period,
significantly reduced survival was seen at 4.6 mg/litre, but this
result was variable, as some replicates did not show significant
effects. The mean of the geometric means of the NOEC and LOEC values
for the embryo-larval test was 3.8 mg/litre; the mean LC50 value was
5.5 mg/litre (Pickering, 1988).
An LC50 value of 38 mg/litre was reported for fathead minnows
exposed to C12 AS for either 48 or 96 h. The authors suggested that
the same value was obtained because the tests were not carried out
aseptically and the C12 AS had degraded completely within 48 h
(Cowgill et al., 1990).
C9.2.4 Tests in biocenoses
In a flow-through biocenosis test, 13 species of aquatic organisms
were exposed to C16-C18AS. The species used represented several
trophic levels: seven species of algae, four species of protozoa, and
two species of rotifers. An NOEC of 0.55 mg/litre was reported for
'biocenotic toxicity'. The lowest concentration at which biocenotic
toxicity was reported was 1.65 mg/litre (Guhl, 1987).
C9.3 Terrestrial organisms
No information was available.
APPENDIX I
APPENDIX I.
Reference values for intakes and body weights of laboratory animals, with conversion factors for
deriving no-observed-adverse-effect levels (NOAELs) in milligrams per kilogram per day from
doses administered as parts per million
Species Body Inhalation Water Food Dose conversiona
weight (kg) rate consumption consumption
Air Water Food
(m3/day) (litres/ (g/day)
day)
Mouse 0.03b 0.04b 0.006b 4b 1.33 0.20 0.13
Rat 0.35b 0.11d 0.05b 18b 0.31 0.14 0.05
Hamster 0.14b 0.13b 0.03b 12b 0.93 0.21 0.09
Guinea-pig 0.84b 0.40b 0.20b 34b 0.48 0.24 0.04
Rabbit 3.8b 2.0b 0.41b 186b 0.53 0.11 0.05
Rhesus monkey 8.0b 5.4c 0.53b 320b 0.68 0.07 0.04
Dog 12b 4.3b 0.61b 300b 0.36 0.05 0.03
Cat 1.5d 0.75d 0.15e 168e 0.50 0.10 0.11
Pig 80e - 5.5e 2250e 0.07 0.03
From Health Canada (in press); most values have been rounded to two significant figures.
a Air: 1 mg/m3 in air = x in mg/kg bw per day; water: 1 ppm (mg/litre) = x in mg/kg bw per day;
food: 1 ppm in food = x in mg/kg bw per day
b From Calabrese & Kenyon (1991)
c Calculated from the minute volume of 220 ml/kg bw reported by Flecknell (1987)
d From Flecknell (1987); values are average of the ranges reported.
e From Canadian Council on Animal Care (1980-84); values are average of the ranges reported.
REFERENCES
Abbott DC (1962) The colorimetric determination of anionic
surface-active materials in water. Analyst, 87: 286-293.
Abdel-Shafy HI, Azzam AM, & El-Gamal IM (1988) Studies on the
degradation of synthetic detergents by sewage. Bull Environ Contam
Toxicol, 41: 310-316.
Abe S (1984) [Biodegradation of sodium alkylbenzenesulfonates by a
soil perfusion method.] Nippon Kagaku Kaishi, 9: 1465-1470 (in
Japanese).
Abe S & Seno M (1987) Biodegradation of sodium linear
alkylbenzenesulfonates evaluated with a soil perfusion method. J Am
Oil Chem Soc, 64: 148-152.
Abel PD & Skidmore JF (1975) Toxic effects of an anionic detergent on
the gills of rainbow trout. Water Res, 9: 759-765.
Acher AJ & Yaron B (1977) Behavior of anionic surfactants in a
soil-sewage effluent system. J Environ Qual, 6: 418-420.
Adachi A & Kobayashi T (1982) [Atomic absorption spectrophotometric
determination of anionic surfactants in water.] Eisei Kagaku, 28 (2):
99-105 (in Japanese).
Adachi T, Tanaka A, & Yamaha T (1979) [Metabolism of octadecyl sulfate
in rats.] Shokuhin Eiseigaku Zasshi, 20 (2): 120-126 (in Japanese).
Amano K & Fukushima T (1993) Partitioning of linear
alkylbenzenesulfonates in natural water and sediment. J Environ Sci
Health, 28: 683-696.
Amano K, Fukushima T, Inaba K, & Nakasugi O (1989) Adsorption of
linear alkylbenzenesulfonates with the suspended solids in natural
aquatic systems. Jpn J Water Pollut Res, 12: 506-515.
Amano K, Fukushima T, & Nakasugi O (1991) Fate of linear alkylbenzene
sulfonates in a lake estuary. Water Sci Technol, 23: 497-506.
Ambe Y (1973) Determination of alkylbenzene sulfonate (ABS) in bottom
sediment. Environ Sci Technol, 7: 542-545.
Ambe Y & Hanya T (1972) [Determination of alkylbenzene sulfonate in
polluted water by a combined method of methylene blue colorimetry with
infrared spectrometry.] Bunseki Kagaku, 21: 252-256 (in Japanese).
Anderson DJ, Day MJ, Russell NJ, & White GF (1990) Die-away kinetic
analysis of the capacity of epilithic and planktonic bacteria from
clean and polluted river water to biodegrade sodium dodecyl sulfate.
Appl Environ Microbiol, 56: 758-763.
Anson ML (1941) The sulfhydryl groups of egg albumin. J Gen Physiol,
24: 399-421.
Antal M (1972) Changes in blood glucose level induced by sodium
dodecylbenzene sulfonate (SDBS) in rats. Z Ernährwiss, 12 (2):
144-151.
Arima T, Takahashi K, Kawana T, Wakabayashi M, & Kikuchi M (1981)
Toxicity of detergent to aquatic organisms. Aquaculture, 29: 30-37.
Arthur JW (1970) Chronic effects of linear alkylate sulfonate
detergent on Gammarus pseudolimnaeus, Campeloma decisum and Physa
integra. Water Res, 4: 251-257.
Arthur D Little Inc. (1977) Human safety and environmental aspects of
major surfactants. A Report to the Soap and Detergent Association, New
York, 31 May 1977. Cambridge, Massachusetts, Arthur D. Little Inc.,
201 pp.
Arthur D Little Inc. (1981) Human safety and environmental aspects of
major surfactants. A Report to the Soap and Detergent Association, New
York, 20 February 1981. Cambridge, Massachusetts, Arthur D. Little
Inc., 291 pp.
Arthur D Little Inc. (1991) Environmental and human safety of major
surfactants: Vol. 1. Anionic surfactants: Part 1. Linear alkylbenzene
sulfonates. Final Report to the Soap and Detergent Association, New
York, February 1991. Cambridge, Massachusetts, Arthur D. Little Inc.,
244 pp.
Arthur D Little Inc. (1993) Environmental and human safety of major
surfactants: Vol. 1. Anionic surfactants: Part 4. Alpha olefin
sulfonates. Final Report to the Soap and Detergent Association, New
York, August 1993. Cambridge, Massachusetts, Arthur D. Little Inc., 84
pp.
Bae KS, Lee HJ, Hah YC, & Hong SW (1982) [The distribution and annual
variation of detergent-degrading bacteria in the lower Han river.]
Korean J Microbiol, 20: 113-118 (in Korean).
Barera Y & Adams WJ (1983) Resolving some practical questions about
Daphnia acute toxicity tests. In: Bishop WE, Cardwell RD, & Heidolph
BB ed. Aquatic toxicology and hazard assessment. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 509-518
(ASTM STP 802).
Bay PHS & Danneman PJ (1985) Skin sensitization potential of
alpha-olefin sulfonate (AOS) and a prototype dishwashing detergent
containing AOS. J Am Oil Chem Soc, 62 (4): 646.
Berenson MM & Temple AR (1974) Detergent ingestion; unique experience
of a family. Clin Toxicol, 7 (1): 25-28.
Berenson MM & Temple AR (1976) Detergent toxicity: effects on
esophageal and gastric mucosa. Clin Toxicol, 8: 399-404.
Berna JL, Ferrer J, Moreno A, Prats D, & Ruiz Bevia F (1989) The fate
of LAS in the environment. Tenside Surfactants Deterg, 26: 101-107.
Berna JL, Moreno A, & Ferrer J (1991) The behaviour of LAS in the
environment. J Chem Technol Biotechnol, 50: 387-398.
Berna JL, Moreno A, Banerji A, Fritsch TR, & Vora BV (1993a) Growth
and developments in LAB technologies: 30 years of innovation and more
to come. Presentation at the 1993 World Surfactant Congress, 23
September 1993, Montreux, Switzerland. New York, Soap and Detergent
Association, 27 pp.
Berna JL, Moreno A, & Ferrer J (1993b) An assessment of the ultimate
biodegradation of LAS. Tenside Surfactants Deterg, 30 (3): 217-222.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, & Bentinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19: 1527-1550.
Bishop WE & Maki AW (1980) A critical comparison of two
bioconcentration test methods. In: Eaton JG, Parrish PR, & Hendricks
AC ed. Aquatic toxicology. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 61-77 (ASTM STP 707).
Bishop WE & Perry RL (1981) Development and evaluation of a
flow-through growth inhibition test with duckweed (Lemna minor). In:
Branson DR & Dickson KL ed. Aquatic toxicology and hazard assessment.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 421-435 (ASTM STP 737).
Blank IH & Gould E (1961) Penetration of anionic surfactants into
skin: (II) study of mechanisms which impede the penetration of
synthetic anionic surfactants into skin. J Invest Dermatol, 37 (6):
311-315.
Blank IH & Shappirio EB (1955) The water content of the stratum
corneum. J Invest Dermatol, 25: 391-401.
Blohm SG (1957) The connection between skin-irritating and
protein-denaturing effects of some surface-active agents. Acta
Dermatol Venereol, 37 (4): 269-275.
von Bock KJ & Mann H (1971) [Anionic surfactants in the coastal areas
of the North Sea.] Arch. Fischereiwiss, 22: 287-292 (in German).
Bornmann G, Loeser A, & Stanisic M (1963) [Study of a detergent based
on dodecylbenzene sulfonate.] Fette Seifen Anstrichm, 65 (10): 818-824
(in German).
Borstlap (1967) Intermediate biodegradation products of anionic
detergents; their toxicity and foaming properties. In: Proceedings of
the International Congress on Surface Active Substances, Brussels,
September 1964, Vol. 3, 891-901.
Brenner TE (1968) The impact of biodegradable surfactants on water
quality. J Am Oil Chem Soc, 45: 433-436.
Bressan M, Brunetti R, Casellato S, Fava GC, Giro P, Marin M,
Negrisolo P, Tallandini L, Thomann S, Tosoni L, Turchetto M, &
Campesan GC (1989) Effects of linear alkylbenzene sulfonate (LAS) on
benthic organisms. Tenside Surfactants Deterg, 26: 148-158.
Bressan M, Marin MG, & Brunetti R (1991) Effects of linear
alkylbenzene sulphonate (LAS) on skeletal development of sea urchin
embryos (Paracentrotus lividus Lmk). Water Res, 25: 613-616.
Bringmann G & Kühn R (1977) [Limiting values for noxious effects of
water pollutants on bacteria (Pseudomonas putida) and green algae
(Scendesmus quadricauda) in the cell multiplication test.] Z Wasser
Abwasser Forsch, 10 (3): 87-98 (in German).
Brown VKH & Muir CMC (1970) The toxicities of some coconut alcohol and
dobanol-23 derived surfactants. Tenside Surfactants Deterg, 7 (3):
137-139.
Brown VM, Abram FSH, & Collins LJ (1978) The acute lethal toxicity to
rainbow trout of an LAS surfactant and of its residues and degradation
products. Tenside Surfactants Deterg, 15 (2): 57-59.
Brunner PH, Capri S, Marcomini A, & Giger W (1988) Occurrence and
behaviour of linear alkylbenzene sulphonates, nonylphenol
diethoxylates in sewage and sewage sludge treatment. Water Res, 22:
1465-1472.
Buehler EV, Newmann EA, & King WR (1971) Two-year feeding and
reproduction study in rats with linear alkylbenzene sulfonate (LAS).
Toxicol Appl Pharmacol, 18: 83-91.
Burke B, Olavesen AH, Curtis CG, & Powell GM (1975) The biodegradation
of some anionic detergents in the rat: a common metabolic pathway.
Xenobiotica, 5 (9): 573-584.
Burke B, Olavesen AH, Curtis CG, & Powell GM (1976) The biodegradation
of the surfactant undecyl sulphate. Xenobiotica, 6 (11): 667-678.
Cain RB, Willats AJ, & Bird JA (1971) Surfactant biodegradation:
metabolism and enzymology. In: Biodegradation of materials. New York,
John Wiley and Sons, vol 2, pp 136-144.
Calabrese A & Davis HC (1967) Effects of 'soft' detergents on embryos
and larvae of the American oyster (Crassostrea virginica). Proc Natl
Shellfish Assoc, 57: 11-16.
Calabrese EJ & Kenyon EM (1991) Air toxics and risk assessment.
Chelsea, Michigan, Lewis Publishers Inc., 612 pp.
Canadian Council on Animal Care (1980-1984) Guide to the care and use
of experimental animals: Vols 1 and 2. Ottawa, Canadian Council on
Animal Care.
Canton JH & Slooff W (1982) Substitutes for phosphate containing
washing products: their toxicity and biodegradability in the aquatic
environment. Chemosphere, 11: 891-907.
Carson S & Oser BL (1964) Dermal toxicity of sodium lauryl sulfate. J
Soc Cosmet Chem, 15: 137-147.
Castles MA, Moore BL, & Ward SR (1989) Measurement of linear
alkylbenzenesulfonates in aqueous environmental matrices by liquid
chromatography with fluorescence detection. Anal Chem, 61 (22):
2534-2540.
Cavalli L, Gellera A, Lazzarin A, Nucci GC, Romano P, Ranzani M, &
Lorenzi E (1991) Linear alkylbenzene sulphonate removal and
biodegradation in a metropolitan plant for water treatment. Riv Ital
Sostanze Grasse, 68: 75-81.
Cavalli L, Divo C, Giuffrida G, Pellizzon T, Radici P, Valtorta L, &
Zatta A (1993a) Producing linear alkyl benzene (LAB) from linear
olefins using an AlCl3 catalyst. Spec Chem, August: 228-231.
Cavalli L, Gellera A, & Landone A (1993b) LAS removal and
biodegradation in a wastewater treatment plant. Environ Toxicol Chem,
12: 1777-1788.
CEFIC (1992) Linear alkylbenzene. Report of a working group. Brussels,
European Chemical Industry Council, 6 pp.
Chattopadhyay DN & Konar SK (1985) Chronic effects of linear alkyl
benzene sulfonate on aquatic ecosystem. Environ Ecol, 3: 428-433.
Colin A Houston & Associates Inc. (1990) Higher alcohols world markets
1988-2000. A multiclient study: January 1990. Mamaroneck, New York,
Colin A. Houston & Associates, 21 pp.
Comotto RM, Kimerle RA, & Swisher RD (1979) Bioconcentration and
metabolism of linear alkylbenzene sulphonate by daphnids and fathead
minnows. In: Marking LL & Kimerle RA ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 232-250 (ASTM STP 667).
Conti E (1987) Acute toxicity of three detergents and two insecticides
in the lugworm, Arenicola marina (L.): a histological and a scanning
electron microscopic study. Aquat Toxicol, 10: 325-334.
Cook TM & Goldman CK (1974) Degradation of anionic detergents in
Chesapeake Bay. Chesapeake Sci, 15: 52-55.
Cordon TC, Maurer EW, & Stirton AJ (1972) The biodegradation of some
sulfated alkanolamides. J Am Oil Chem Soc, 49: 174-177.
Cowgill UM, Milazzo DP, & Landenberger BD (1990) The reproducibility
of the three brood Ceriodaphnia test using the reference toxicant
sodium lauryl sulphate. Arch Environ Contam Toxicol, 19: 513-517.
Cresswell DG, Baldock GA, Chasseaud LF, & Hawkins DR (1978) Toxicology
studies of linear alkylbenzene sulphonate (LAS) in rhesus monkeys:
(II) The disposition of [14C]LAS after oral or subcutaneous
administration. Toxicology, 11: 5-17.
Dahl MV & Trancik RJ (1977) Sodium lauryl sulfate irritant patch
tests: degree of inflammation at various times. Contact Dermatitis, 3:
263-266.
Daly LW, Schroeder RE, & Killeen JC (1980) A teratology study of
topically applied linear alkylbenzene sulphonate in rats. Food
CosmetToxicol, 18: 55-58.
De Henau H, Matthijs E, & Hopping WD (1986) Linear alkylbenzene
sulphonates (LAS) in sewage sludges, soils and sediments: analytical
determination and environmental safety considerations. Int J Environ
Anal Chem, 26: 279-293.
De Henau H, Matthijs E, & Namkung E (1989) Trace analysis of LAS by
HPLC. Detailed results from two municipal sewage treatment plants. In:
Quaghebeur D, Temmerman I, & Angeletti G ed. Organic contaminants in
waste water, sludge and sediment: Occurrence, fate and disposal.
London, Elsevier Science Publishers, pp 5-18.
Denner WHB, Olavesen AH, Powell GM, & Dodgson KS (1969) The metabolism
of potassium dodecyl [35] sulphate in the rat. Biochem J, 111:
43-51.
Devi Y & Devi S (1986) Effect of synthetic detergents on germination
of fern spores. Bull Environ Contam Toxicol, 37: 837-843.
Di Corcia A, Marchetti M, Samperi R, & Marcomini A (1991) Liquid
chromatographic determination of linear alkylbenzenesulfonates in
aqueous environmental samples. Anal Chem, 63: 1179-1182.
Divo C & Cardini G (1980) Primary and total biodegradation of linear
alkylbenzene sulphonates. Tenside Surfactants Deterg, 17: 30-36.
Dolan JM & Hendricks AC (1976) The lethality of an intact and degraded
LAS mixture to bluegill sunfish and a snail. J Water Pollut Control
Fed, 48: 2570-2577.
Dolan JM, Gregg BC, Cairns J, Dickson KL, & Henricks AC (1974) The
acute toxicity of three new surfactant mixtures to a mayfly larvae.
Arch Hydrobiol, 74: 123-132.
Dominguez JG, Parra JL, Infante MR, Pelejero CM, Balaguer F, & Sastre
T (1977) A new approach to the theory of adsorption and permeability
of surfactants on keratinic proteins: the specific behaviour of
certain hydrophobic chains. J Soc Cosmet Chem (UK), 28: 165-182.
Dreger EE, Keim GI, Miles GD, Shedlovsky L, & Ross J (1944) Sodium
alcohol sulfates: properties involving surface activity. Ind Eng Chem,
36 (7): 610-617.
Dugan PR (1967) Influence of chronic exposure to anionic detergents on
toxicity of pesticides to goldfish. J Water Pollut Control Fed, 39:
63-71.
Dutka BJ & Kwan KK (1982) Application of four bacterial screening
procedures to assess changes in the toxicity of chemicals in mixtures.
Environ Pollut, A29: 125-134.
Eganhouse RP, Blumfield DL, & Kaplan IR (1983) Long-chain
alkylbenzenes as molecular tracers of domestic wastes in the marine
environment. Environ Sci Technol, 17: 523-530.
Eggert CR, Kaley RG, & Gledhill WE (1979) Application of a laboratory
freshwater lake model in the study of linear alkylbenzene sulfonate
(LAS) biodegradation. In: Bourquin AW & Pritchard PH ed. Proceedings
of the Workshop on Microbial Degradation of Pollutants in Marine
Environments, Pensacola Beach, Florida, 9-14 April 1978. Washington,
DC, US Environmental Protection Agency, pp 451-461 (EPA-600/9-79-012).
Endo T, Furuido Y, Namie K, Yamamoto N, Hasunuma H, & Ueda K (1980)
[Studies of the chronic toxicity and teratogenicity of synthetic
surfactants.] Ann Rep Tokyo Metrop Res Inst Environ Prot [Tokyo Kogai
Kenkyujo Nempo]: 236-246 (in Japanese).
Environment Agency Japan (1978) Chemicals in the environment. Tokyo,
81 pp.
Epstein S, Throndson AH, Dock W, & Tainter ML (1939) Possible
deleterious effects of using soap substitutes in dentifrices. J Am
Dental Assoc, 26: 1461-1471.
Ernst R, Arditti J, & Healey PL (1971) Biological effects of
surfactants l. Influence on the growth of orchid seedlings. New
Phytol, 70: 457-475.
Ernst R, Gonzales CJ, & Arditti J (1983) Biological effects of
surfactants: Part 6-- effects of anionic, non-ionic and amphoteric
surfactants on a green alga (Chlamydomonas). Environ Pollut, A31:
159-175.
European Committee of Organic Surfactants and Their Intermediates
(1990) Classification and labelling of surfactants. Brussels, 38 pp.
Eurostat (1991) Environment statistics. Annex to overview of the state
of the environment in the European Communities 1992: COM(92) 23
Final--Volume III. Brussels, Commission of the European Communities,
102 pp.
Evans HC (1956) Alkyl sulphates (Part 1); critical micelle
concentrations of the sodium salts. J Soc Cosmet Chem, 7: 579-586.
Eyanoer HF, Upatham ES, Duangsawasdi M, & Tridech S (1985) Effects of
water hardness and temperature on the toxicity of detergents to the
freshwater fish, Puntius gonionotus, bleeker. J Sci Soc Thailand,
11: 67-77.
Fairchild JF, Dwyer FJ, La Point T, Burch SA, & Ingersoll CG (1993)
Evaluation of a laboratory-generated NOEC for linear alkylbenzene
sulfonate in outdoor experimental streams. Environ Toxicol Chem, 12:
1763-1775.
Federle TW & Pastwa GM (1988) Biodegradation of surfactants in
saturated subsurface sediments: a field study. Ground Water, 26:
761-770.
Federle TW & Schwab BS (1989) Mineralization of surfactants by
microbiota of aquatic plants. Appl Environ Microbiol, 55: 2092-2094.
Federle TW & Schwab BS (1992) Mineralisation of surfactants in
anaerobic sediments of a laundromat wastewater pond. Water Res, 26:
123-127.
Federle TW & Ventullo RM (1990) Mineralization of surfactants by
microbiota of submerged plant detritus. Appl Environ Microbiol, 56:
333-339.
Fendinger NJ, Begley WM, McAvoy DC, & Eckhoff WS (1992a) Determination
of alkyl sulfate surfactants in natural waters. Environ Sci Technol,
26: 2493-2498.
Fendinger NJ, Begley WM, McAvoy DC, & Eckhoff WS (1992b) Monitoring of
alkyl sulfate in wastewater treatment systems and surface water.
Presented before the Division of Environmental Chemistry, American
Chemical Society, San Francisco, April 1992. Washington, DC, American
Chemical Society, p 2.
Field JA, Barber EM, Thurman EM, Moore BL, Lawrence DA, & Peake DA
(1992) Fate of alkylbenzenesulfonates and dialkyltetralinsulfonates in
sewage-contaminated groundwater. Environ Sci Technol, 26 (6):
1140-1148.
Figge K & Schoberl P (1989) LAS and the application of sewage sludge
in agriculture. Tenside Surfactants Deterg, 26: 122-128.
Fischer WK (1980) [Development of detergent concentration in German
rivers 1960-1980.] Tenside Surfactants Deterg, 17: 250-261 (in
German).
Fischer WK & Gerike P (1975) Biodegradability determinations via
unspecific analyses (chemical oxygen demand, dissolved organic carbon)
in coupled units of the OECD confirmatory test--II: Results. Water
Res, 9: 1137-1141.
Fisher LB & Maibach HI (1975) Effect of some irritants on human
epidermal mitosis. Contact Dermatitis, 1: 273-276.
Fitzhugh OG & Nelson AA (1948) Chronic oral toxicity of surface-active
agents. J Am Pharm Assoc, 37: 29-32.
Flecknell PA (1987) Laboratory animal anaesthesia. An introduction for
research workers and technicians. Toronto, Academic Press, pp 118-132.
Fogels A & Sprague JB (1977) Comparative short-term tolerance of
zebrafish, flagfish, and rainbow trout to five poisons including
potential reference toxicants. Water Res, 11: 811-817.
Froebe CL, Simion FA, Rhein LD, Cagan RH, & Kligman A (1990) Stratum
corneum lipid removal by surfactants: relation to in vivo irritation.
Dermatologica, 181 (4): 277-283.
Fujii T, Sakamoto Y, Abe Y, Mikurita H, Yuzawa K, & Hiraga K (1977)
[Pathological examination of rats fed with linear alkylbenzene
sulfonate for their lifespan.] Ann Rep Tokyo Metrop Res Lab Public
Health, 28 (2): 85-108 (in Japanese).
Fujita H, Kojima A, & Hiraga K (1977) [Screening tests of mutagenicity
of linear alkylbenzene sulfonate (LAS) in a microbial system.] Ann Rep
Tokyo Metrop Res Lab Public Health, 28 (2): 112-114 (in Japanese).
Fujiwara K, Sugiyama T, Koike K, & Oba K (1975) [Biodegradation of
linear alkylate sulfonates in a river model.] Jpn J Hyg, 29: 552-557
(in Japanese).
Fukuzawa K, Tokumura A, Yamada S, & Tsukatani H (1978) [Oral toxicity
of sodium dodecyl sulfate.] Eisei Kagaku, 24 (2): 107-110 (in
Japanese).
Gafa S (1974) Studies on relationship between acute toxicity to fish
and surface activity of anionic surfactants. Riv Ital Sostanze Grasse,
51: 182-192.
Gafa S & Lattanzi B (1974) Evaluation of some 'third generation'
anionic surfactants in comparison with a linear alkylbenzene
sulfonate. 12th World Congress on Soaps. Milan, International Society
for Fat Research.
Gagnon MJ (1979) Note on a rapid and sensitive method for the
determination of anionic detergents in natural waters at the ppb
level. Water Res, 13: 53-56.
Games LM (1983) Practical applications and comparisons of
environmental exposure assessment models. In: Bishop WE, Cardwell RD,
& Heidolph BB ed. Aquatic toxicity and hazard assessment: Sixth ASTM
Symposium. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 282-299.
Gard-Terech A & Palla JC (1986) Comparative kinetics study of the
evolution of freshwater aquatic toxicity and biodegradability of
linear and branched alkylbenzene sulfonates. Ecotoxicol Environ Saf,
12: 127-140.
Gentempo PG, Dickson MK, & Guin KF (1985) Analysis of alpha-olefin
sulfonates via quantitative carbon-13 NMR. J Am Oil Chem Soc, 62 (4):
645.
Gerike P (1987) Environmental impact. In: Falbe J ed. Surfactants in
consumer products. Berlin, Springer-Verlag, pp 450-474.
Gerike P, Winkler K, Schneider W, & Jakob W (1989) Residual LAS in
German rivers. Tenside Surfactants Deterg, 26: 136-140.
Giger W, Alder AC, Brunner PH, Marcomini A, & Siegrist H (1989)
Behaviour of LAS in sewage and sludge treatment and in sludge-treated
soil. Tenside Surfactants Deterg, 26: 95-100.
Gilbert PA & Pettigrew R (1984) Surfactants and the environment. Int
J Cosmet Sci, 6: 149-158.
Gledhill WE (1975) Screening test for assessment of ultimate
biodegradability: Linear alkylbenzene sulfonates. Appl Microbiol, 30:
922-929.
Gloxhuber C (1974) Toxicological properties of surfactants. Arch
Toxicol, 32: 245-269.
Gotte E (1954) [Properties of salts of primary unbranched
alkylsulfates and their solutions with regard to their technical use,
Part 1.] Fette Seifen Anstrichm, 56 (8): 583-587 (in German).
Granmo A (1972) Development and growth of eggs and larvae of Mytilus
edulis exposed to a linear dodecylbenzenesulphonate, LAS. Mar Biol,
15: 356-358.
Guhl W (1987) [Biotic similarity indices in the comparison of waters
from identical sources.] Limnologica (Berlin), 18 (1): 1-13 (in
German).
Haar GT (1983) Safety of alpha olefin sulfonates. J Am Oil Chem Soc,
60 (4): 876-881.
Halvorson H & Ishaque M (1969) Microbiology of domestic wastes; III.
Metabolism of LAS-type detergents by bacteria from a sewage lagoon.
Can J Microbiol, 15: 571-576.
Hamano Y, Yamamoto H, Inoue K, Oda Y, Kuwano A, Mitsuda B, & Kunita N
(1976) [Studies on the toxicity of commercial detergents (4th report);
fetal toxicity (fetal median lethal dose) in mice.] Ann Rep Osaka
Prefect Inst Public Health, 7: 147-152 (in Japanese).
Hand VC, Rapaport RA, & Pittinger CA (1990) First validation of a
model for the adsorption of linear alkylbenzenesulfonate (LAS) to
sediment and comparison to chronic effects data. Chemosphere, 21:
741-750.
Harrold SP (1959) Denaturation of epidermal keratin by surface active
agents. J Invest Dermatol, 32: 581-588.
Hasegawa H & Sato M (1978) [Permeation of LAS through rat skin.]
Tokyo, Science and Technology Agency, Research Cordination Bureau, pp
172-175 (in Japanese).
Hashimoto S, Sakurai K, & Nagai T (1976) [Determination of trace
amounts of alkylbenzene sulfonate in the river water by high
performance liquid chromatography.] Bunseki Kagaku, 25: 639-643 (in
Japanese).
Hatton EH, Fosdick LS, & Calandra J (1940) The toxicity and
rubefacient action of sulphated higher alcohols. J Dent Res, 19:
87-92.
Hattori M, Seno K, Harada S, Ishizu Y, & Goto M (1984) [Effects of
some environmental chemicals in the Daphnia reproduction test.] Jpn
Ecol Chem, 6: 23-27 (in Japanese).
Havermann H & Menke KH (1959) [Biological study of the water-soluble
surface-active substances.] Fette Seifen Anstrichm, 61 (6): 429-434
(in German).
Hayashi M (1980) [Toxicological studies on detergents containing
linear alkylbenzene sulfonate (LAS) in rats and mice.] Shikoku Med J,
36 (1): 37-44 (in Japanese).
Healey PL, Ernst R, & Arditti J (1971) Biological effects of
surfactants: II. Influence on the ultrastructure of orchid seedlings.
New Phytol, 70: 477-482.
Health Canada (in press) Canadian Environmental Protection Act. Human
health risk assessment for priority substances; Appendix E. Reference
values for intakes and body weights of laboratory animals. Ottawa,
Health and Welfare Canada.
Hellmann H (1978) [Anionic detergents in the Rhine 1971-1977, their
degradation and biodegradation potential of the river.] Tenside
Surfactants Deterg, 15: 291-294 (in German).
Henderson C, Pickering QH, & Cohen JM (1959) The toxicity of synthetic
detergents and soaps to fish. Sew Ind Wastes, 31: 295-306.
Hennes EC & Rapaport RA (1989) Calculation and analytical verification
of LAS concentrations in surface waters, sediment and soil. Tenside
Surfactants Deterg, 26: 141-147.
Hewin International Inc. (1992) The technology and markets for anionic
surfactants: North America, western Europe and Japan in the global
perspective 1991-1995 and scenario 2000. Amsterdam, Hewin
International Inc., 53 pp.
Heywood R, James RW, & Sortwell RJ (1978) Toxicology studies of linear
alkylbenzene sulphonate (LAS) in rhesus monkeys; (I) simultaneous oral
and subcutaneous administration for 28 days. Toxicology, 11: 245-250.
Hidaka H & Tatsukawa R (1989) Avoidance by olfaction in a fish, medaka
(Oryzias latipes), to aquatic contaminants. Environ Pollut, 56:
299-309.
Hidaka H, Suga M, & Tatsukawa R (1984) [Avoidance of anionic
surfactants in medakas (Oryzias latipes).] Nippon Nogeikagaku
Kaishi, 58: 1-7 (in Japanese).
Hidaka H, Kubota H, Gratzel M, Serpone N, & Pelizzetti E (1985)
Photodegradation of surfactants; I. Degradation of sodium
dodecylbenzene sulfonate in aqueous semiconductor dispersions. Nouv J
Chim, 9: 67-69.
Hidu H (1965) Effects of synthetic surfactants on the larvae of clams
(M. mercenaria) and oysters (C. virginica). J Water Pollut Control
Fed, 37: 262-270.
Higuchi K, Shimoishi Y, Miyata H, & Toei K (1982) Spectrophotometric
determination of sodium 4-dodecylbenzenesulfonate with
1-(N-methylpyridinium-4-yl-azo)-4-(4-diethylaminophenylazo)naphthale
ne iodide. Bull Chem Soc Jpn, 55 (2): 621-622.
Hironaga K (1979) [Case reports; 29 emergency cases of children
(non-fatal cases of drinking household products).] Yakuji Shinpo, 36:
27-29 (in Japanese).
Hirsch E (1963) [Structure of alkylbenzenesulfonates and impact on
behaviour of fish.] Vom Wasser, 30: 249-259 (in German).
Hitchcock WS & Martin DF (1977) Effects and fate of a surfactant in
cultures of the red tide organisms, Gymnodinium breve. Bull Environ
Contam Toxicol, 18: 291-296.
Hokanson KEF & Smith LL (1971) Some factors influencing toxicity of
linear alkylate sulfonate (LAS) to the bluegill. Trans Am Fish Soc,
100: 1-12.
Hollis CG, Phillips WK, & Johnson JE (1976) Thermal effects on
biodegradation of linear alkyl benzenesulfonate. Dev Ind Microbiol,
17: 345-350.
Holman WF & Macek KJ (1980) An aquatic safety assessment of linear
alkylbenzene sulfonate (LAS): chronic effects on fathead minnows.
Trans Am Fish Soc, 109: 122-131.
Holst R & Moller H (1975) One hundred twin pairs patch tested with
primary irritants. Br J Dermatol, 93: 145-149.
Holt MS, Matthijs E, & Waters J (1989) The concentrations and fate of
linear alkylbenzene sulphonate in sludge amended soils. Water Res, 23:
749-759.
Holysh M, Paterson S, Mackay D, & Bandurraga MM (1986) Assessment of
the environmental fate of linear alkylbenzenesulphonates. Chemosphere,
15: 3-20.
Hong SD, Lee SH, Hah SC, & Ha HP (1984) [Biodegradation of synthetic
detergents by Klebsiella pneumoniae L-127.] Korean J Appl Microbiol
Bioeng, 12: 91-97 (in Korean).
Hon-Nami H & Hanya T (1980a) Linear alkylbenzene sulfonates in river,
estuary and bay water. Water Res, 14: 1251-1256.
Hon-Nami H & Hanya T (1980b) Differences in the composition of linear
alkylbenzene sulfonate homologues in river sediment and river water.
Jpn J Limnol, 41: 1-4.
Hope J (1977) Absence of chromosome damage in the bone marrow of rats
fed detergent actives for 90 days. Mutat Res, 56: 47-50.
Howes D (1975) The percutaneous absorption of some anionic
surfactants. J Soc Cosmet Chem, 26: 47-63.
Hrsak D, Bosnjak M, & Johanides V (1981) Kinetics of linear
alkylbenzene sulphonate and secondary alkane sulphonate
biodegradation. Tenside Surfactants Deterg, 18: 137-140.
Hrsak D, Bosnjak M, & Johanides V (1982) Enrichment of linear
alkylbenzenesulphonate (LAS) degrading bacteria in continuous culture.
J Appl Bacteriol, 53: 413-422.
Hsu YC (1965) Detergent-splitting enzyme from pseudomonas. Nature,
207: 385-388.
Huber L (1989) Conclusions for an ecological evaluation of LAS.
Tenside Surfactants Deterg, 26 (2): 71-74.
Huddleston RL & Allred RC (1963) Microbial oxidation of sulfonated
alkylbenzenes. In: Vol 4. Developments in industrial microbiology.
Washington, DC, American Institute of Biological Sciences, pp 24-38.
Huddleston RL & Nielsen AM (1979) LAS biodegradation: the fate of the
aromatic ring. Soap Cosmet Chem Spec, March: 34-44.
Hunter B & Benson HG (1976) Long-term toxicity of the surfactant
alpha-olefin sulphonate (AOS) in the rat. Toxicology, 5: 359-370.
Husmann W (1968) Solving the detergent problem in Germany. Water
Pollut Control, 3: 80-90.
Ichihara Y, Yoshiue S, Omure K, Fujimoto T, Moritomo M, & Yoshikawa J
(1967) [A case study of anionic surfactant poisoning.] J Jpn Soc Int
Med [Nichi Naikaishi], 56 (11): 128 (in Japanese).
Ihara I, Ozaki M, Nakamura F, Yokota I, Nakaura H, Aso T, Ozaki M,
Kaneko Y, Miyauchi S, Miura K, Hayakawa Y, Moriyama S, Ishiguro T, &
Yoshida Y. (1970) [ABS in the water of the Tama River; V. Data from
February 1968 to May 1970.] J Jpn Oil Chem Soc, 19: 1043-1048 (in
Japanese).
Iimori M & Takita Y (1979) [A study of the effect of linear
alkylbenzenesulfonate on fish.] J Jpn Oil Chem Soc, 28: 185-189 (in
Japanese).
Iimori M & Ushiyama S (1971) [Serological studies on alpha-olefin
sulfonate protein complexes.] J Jpn Oil Chem Soc, 20 (2): 24-26 (in
Japanese).
Iimori M, Ogata T, & Kudo K (1972) [Eye irritation testing of surface
active agents in experimental animals.] J Jpn Oil Chem Soc, 21 (6):
334-337 (in Japanese).
Iimori M, Inoue S, & Yano K (1973) [Effects of skin application of
detergents on pregnant mice.] J Jpn Oil Chem Soc [Yukagaku], 22 (12):
807-813 (in Japanese).
Ikawa M, Yoneyama M, Nakao T, & Hiraga K (1978) [Uptake of organic
acid and organic base by renal cortical slices of rats treated with
LAS and ABS.] Ann Rep Tokyo Metrop Res Lab Public Health., 29 (2):
51-54 (in Japanese).
Ikeda Y (1965) [Food additives and synthetic detergents.] Sogo-Rinsyo,
14 (4): 621-626 (in Japanese).
Imahori A, Kitagawa T, & Shiobara S (1976) [Effects of linear
alkylbenzene sulfonate (LAS) applied dermally to pregnant mice on the
pregnant mice and their fetuses.] Jpn J Public Health [Nippon
Koshueisei Zasshi], 23 (2): 68-72 (in Japanese).
Imokawa G & Katsumi M (1976) [Denaturing action of typical anionic
surfactants on several proteins.] J Jpn Oil Chem Soc [Yukagaku], 25
(1): 24-30 (in Japanese).
Imokawa G & Mishima Y (1976) [The cumulative effect of surface active
agents on human cutaneous horny layers.] Nichihikaishi, 86 (8):
473-481 (in Japanese).
Imokawa G & Mishima Y (1979) Cumulative effect of surfactants on
cutaneous horny layers: lysosome labilizing action. Contact
Dermatitis, 5: 151-162.
Imokawa G, Sumura K, & Katsumi M (1974) [A correlation between
adsorption of a surfactant onto callus and resulting skin-roughness.]
J Jpn Oil Chem Soc [Yukagaku], 23 (11): 719-725 (in Japanese).
Imokawa G, Sumura K, & Katsumi M (1975a) A study of skin roughness
caused by surfactants: (I) A new method in vivo for evaluation of
skin roughness. J Am Oil Chem Soc, 52: 479-483.
Imokawa G, Sumura K, & Katsumi M (1975b) Study of skin roughness
caused by surfactants: (II) Correlation between protein denaturation
and skin roughness. J Am Oil Chem Soc, 52: 484-489.
Inaba K & Amano K (1988) HPLC determination of linear
alkylbenzenesulfonate (LAS) in the aquatic environment. Seasonal
changes in LAS concentration in polluted lake water and sediment. Int
J Environ Anal Chem, 34: 203-213.
Inoue K & Masuda F (1976) Effects of detergents on mouse fetuses. J
Jpn Food Hgy Soc [Shoku Eishi], 17 (2): 158-169.
Inoue K & Sunakawa T (1979) [Mutagenicity tests of surfactants.] Jpn
Fragr J, 38: 67-75 (in Japanese).
Inoue K, Shibata T, Hamano Y, Oda Y, Kuwano A, Yamamoto H, Mitsuda B,
& Kunita N. (1977) [ In vivo cytogenetic tests of some synthetic
detergents in mice.] Ann Rep Osaka Prefect Inst Public Health, 8:
17-24 (in Japanese).
Inoue K, Kaneko K, & Yoshida M (1978) Adsorption of
dodecylbenzenesulfonates by soil colloids and influence of soil
colloids on their degradation. Soil Sci Plant Nutr, 24: 91-102.
Inoue K, Sunakawa T, & Takayama S (1979) [Studies of in vitro cell
transformation and mutagenicity by surfactants and other compounds.]
J Jpn Soc Cosmet Chem [Shogishi], 13 (2): 50-60 (in Japanese).
Inoue K, Sunakawa T, & Takayama S (1980) Studies of in vitro cell
transformation and mutagenicity by surfactants and other compounds.
Food Cosmet Toxicol, 18: 289-296.
Inoue S, O'Grodnick JS, & Tomizawa S (1982) Metabolism of alpha-olefin
sulfonate (AOS) in rats. Fundam Appl Toxicol, 2: 130-138.
Ishihara M & Kinebuchi S (1967) [Causes of hand eczema, and their
examination methods.] Rinsho Hifuka, 21 (3): 241-251 (in Japanese).
Ito T & Kakegawa S (1972) [Development of mild detergent
formulations.] Baioteku, 3 (2): 157-164 (in Japanese).
Ito R, Kawamura H, Chang HS, Kudo K, Kajiwara S, Toida S, Seki Y,
Hashimoto M, & Fukushima A (1978) [Acute, subacute and chronic
toxicity of magnesium linear alkylbenzene sulfonate (LAS-Mg).] J Med
Soc Toho Univ [Toho Igakukai Zasshi], 25 (5-6): 850-875 (in Japanese).
Itoh S-I, Setsuda S, Utsonomiya A & Naito S (1979) [Studies on the
biodegradation test method for chemical substances: II. Ultimate
biodegradabilities of some anionic, nonionic, and cationic surfactants
estimated by CO2 production.] J Jpn Oil Chem Soc [Yukagaku], 28:
199-204 (in Japanese).
Itoh S, Naito S, & Unemoto T (1987) [Comparative studies on anaerobic
biodegradation of anionic and nonionic surfactants.] Eisei Kagaku, 33:
415-422 (in Japanese).
Janicke W (1971) On the behaviour of synthetic organic substances
during the treatment of waste liquors-- IV. Progress of the
biodegradation of `soft' anionic synthetic detergents. Water Res, 5:
917-931.
Janicke W & Hilge G (1979) [Biodegradability of anionic/cationic
surfactant complexes under the aerobic and anaerobic conditions of
effluent and sludge treatment.] Tenside Surfactants Deterg, 16:
117-122 (in German).
Janicke W & Niemitz W (1973) [Comparative investigations of detergent
raw materials and commercial detergents and biological sewage
treatments.] Vom Wasser, 40: 369-389 (in German).
Japanese Soap & Detergent Association (1992) [Annual report of water
quality in the environment.] Tokyo, Japanese Soap and Detergent
Association (in Japanese).
Javed S, Misra V, & Viswanathan PN (1988) In vitro studies on the
effect of linear alkyl benzene sulphonate on serum albumin. Int J
Cosmet Sci, 10: 241-246.
Jimenez L, Breen A, Thomas N, Federle TW, & Sayler GS (1991)
Mineralization of linear alkylbenzene sulfonate by a four-member
aerobic bacterial consortium. Appl Environ Microbiol, 57: 1566-1569.
Jones JH (1945) General colorimetric method for determination of small
quantities of sulfonated or sulfated surface active compounds. J Assoc
Off Agric Chem, 28 (2): 398-408.
Kantin R, Baumgarten MGZ, Cabeda M, Beaumord AC, & de Almeida TL
(1981) Concentration of anionic detergents in Rio Grande water (South
Brazil). Mar Pollut Bull, 12: 50-53.
Katsuno T, Tsukioka T, & Kono Y (1983) [LAS in river sediments, algae
and water plants.] Bull Nagano Res Inst Health Pollut, 6: 23-25 (in
Japanese).
Katz BM & Cohen GM (1976) Toxicities of excessively chlorinated
organic compounds. Bull Environ Contam Toxicol, 15 (6): 644-650.
Kawamura T, Sasagawa S, Masuda T, Honda S, Kinoshita M, Harada S,
Ishizaki T, Nagai R, Hirokawa K, Anzai T, Anekoji K, Hidano A, Kawano
T, Ikegami I, Sato S, & Aoyama T (1970) [Basic studies on the
standardization of a skin patch test.] Jpn J Dermatol [Nihon Hifu
Kagakukai Zasshi], 80 (5): 301-314 (in Japanese).
Kawashima K & Takeno T (1982) [Fate of linear alkylbenzenesulfonates
(14C-LAS-Ca, 14C-LAS-Na) in soils and plants.] J Jpn Oil Chem Soc
[Yukagaku], 31: 944-948 (in Japanese).
Kay JH, Kohn FE, & Calandra JC (1965) Subacute oral toxicity of a
biodegradable, linear alkylbenzene sulfonate. Toxicol Appl Pharmacol,
7: 812-818.
Kikuchi K (1978) Observations on some tissues of mice injected with
LAS synthetic detergent for varying periods from the day of birth.
Kawasaki Med J, 4 (3): 193-201.
Kikuchi M (1985) [Biodegradation of some surfactants in river water
with relation to chemical structure and water temperature.] Bull Jpn
Soc Sci Fish, 51: 1859-1864 (in Japanese).
Kikuchi M & Wakabayashi M (1984) Lethal response of some surfactants
to medaka (Oryzias latipes) with relation to chemical structure.
Bull Jpn Soc Sci Fish, 50: 1235-1240.
Kikuchi M, Wakabayashi M, Nakamura T, Inoue W, Takahashi K, Kawana T,
Kawahara H, & Koido Y (1976) A study of detergents. II. Acute toxicity
of anionic surfactants on aquatic organisms. Ann Rep Tokyo Metrop Res
Inst Environ Prot: 57-69.
Kikuchi M, Wakabayashi M, Kojima H, & Yoshida T (1978) Uptake,
distribution, and elimination of sodium alkyl sulfate in carp.
Ecotoxicol Environ Saf, 2: 115-127.
Kikuchi M, Tokai A, & Yoshida T (1986) Determination of trace levels
of linear alkylbenzenesulfonates in the marine environment by
high-performance liquid chromatography. Water Res, 20 (5): 643-650.
Kimerle RA & Swisher RD (1977) Reduction of aquatic toxicity of linear
alkylbenzene sulfonate (LAS) by biodegradation. Water Res, 11: 31-37.
Kimerle RA, Macek KJ, Sleight BH, & Burrows ME (1981) Bioconcentration
of linear alkylbenzene sulfonate (LAS) in bluegill (Lepomis
macrochirus). Water Res, 15: 251-256.
Kishi M, Satoh S, Horiguchi, Y, & Ito K (1984) [Effects of surfactants
on bone marrow cells.] Bull Kanagawa Public Health Lab, 14: 57-58 (in
Japanese).
Kligman AM & Wooding WM (1967) A method for the measurement and
evaluation of irritants on human skin. J Invest Dermatol, 49 (1):
78-94.
Knaebel DB, Federle TW, & Vestal JB (1990) Mineralization of linear
alkylbenzene sulfonate LAS) and linear alcohol ethoxylate (LAE) in 11
contrasting soils. Environ Toxicol Chem, 9: 981-988.
Knopp PV, Uhren LJ, Rohlich GA, & Nichols MS (1965) Field study of the
removal of linear alkylate sulfonate detergents by the activated
sludge process. J Am Oil Chem Soc, 42: 867-873.
Kobayashi H, Ichikawa H, Fujii T, Yano N, Konno T, Hiraga K, Nakamura
H, Watanabe Y, & Mimura S (1972) [Studies on toxicity of synthetic
detergents. (I) Acute oral toxicity of linear and branched alkyl
benzene sulfonate.] Ann Rep Tokyo Metrop Res Lab Public Health, 24:
397-408 (in Japanese).
Kobayashi T, Adachi A, Iida H, Oba K, Oda K, Okumura H, Tsuji K,
Numata H, & Morimoto Y (1986) [Collaborative studies on determination
of anionic surfactants in water using Co-PADAP.] Hyg Chem [Eisei
Kagaku], 32 (5): 391-396 (in Japanese).
Kobuke Y (1985) [Concentration of linear alkylbenzene sulfonates (LAS)
and its composition in the river waters of Hyogo Prefecture.] Jpn J
Limnol [Rikusuigaku Zasshi], 46: 279-286 (in Japanese).
Koizumi N, Ninomiya R, Inoue Y, Tsukamoto T, Fujii M, & Yamamoto Y
(1985) Implantation disturbance studies with linear alkylbenzene
sulphonate in mice. Arch Environ Contam Toxicol, 14: 73-81.
Kojima S (1989) [Pollution by fluorescent whitening agents (FWA) and
linear alkylbenzene sulfonates (LAS) in environmental water.] Ann Rep
Environ Pollut Res Inst Nagoya, 19: 61-68 (in Japanese).
Kondo M, Yamamoto H, & Arakawa Y (1983) [Effects of detergents on
algae growth and physiological activities.] Bull Aichi Environ Center,
11: 10-21 (in Japanese).
Konno R & Wakabayashi M (1987) [Effects of chemical compounds on the
growth of planktonic algae.] Ann Rep Tokyo Metrop Res Inst Environ
Protect [Tokyo Kogai Kenkyujo Nempo]: 113-116 (in Japanese).
Kravetz LH, Chung JC, & Rapean JC (1982) Ultimate biodegradation
studies of alpha olefin sulfonates. J Am Oil Chem Soc, 59 (4):
206-210.
Krishna Murti GSR, Volk VV, & Jackson ML (1966) Soil adsorption of
linear alkylate sulfonate. Soil Sci Soc Am Proc, 30: 685-688.
Kutt E & Martin DF (1977) Effect of selected surfactants on the growth
characteristics of Gymnodinium breve. Mar Biol, 28: 253-259.
Kuwano A, Yamamoto H, Inoue K, Hamano Y, Oda Y, Mitsuda B, & Kunita N
(1976) [Studies on toxicity of commercial detergents (2nd report).
Acute toxicity in mice.] Ann Rep Osaka Prefect Inst Public Health, 7:
137-140 (in Japanese).
Ladle M, House WA, Armitage PD, & Farr IS (1989) Faunal
characteristics of a site subject to sewage plant discharge. Tenside
Surfactants Deterg, 26: 159-168.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1983) Comparative
studies on ecotoxicology of synthetic detergents. Ecotoxicol Environ
Saf, 7: 538-545.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1984a) The water
flea (Daphnia magna) as a sensitive indicator for the assessment of
toxicity of synthetic detergents. Ecotoxicol Environ Saf, 8: 447-450.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1984b) Effect of
synthetic detergents on some of the behavioral patterns of fish
fingerlings (Cirrhina mrigala) and its relation to ecotoxicology.
Bull Environ Contam Toxicol, 32: 109-115.
Larson RJ (1990) Structure-activity relationships for biodegradation
of linear alkylbenzenesulfonates. Environ Sci Technol, 24: 1241-1246.
Larson RJ & Maki AW (1982) Effect of linear alkylbenzene sulfonate on
the structure and function of microbial communities in model
ecosystems. In: Pearson JG, Foster RB, & Bishop WE ed. Aquatic
toxicology and hazard assessment. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 120-136 (ASTM STP 766).
Larson RJ & Payne AG (1981) Fate of the benzene ring of linear
alkylbenzene sulphonate in natural waters. Appl Environ Microbiol, 41:
621-627.
Larson RJ, Federle TW, Shimp RJ, & Ventullo RM (1989) Behaviour of
linear alkylbenzene sulfonate (LAS) in soil infiltration and
groundwater. Tenside Surfactants Deterg, 26: 116-121.
Laumond F, Neuberger M, Oonier B, Fourey A, Bittel R, & Aubert M
(1973) Experimental investigations at the laboratory on the transfer
of mercury in marine trophic chains. Rev Int Oceanogr Med, 31/32:
47-53.
Lay JP, Klein W, & Korte F (1983) Elimination and biodistribution
studies of [14C]dodecylbenzene sulfonate in rats, following low
dosing in the daily diet and a single i.p. administration. Toxicol
Lett, 17: 187-192.
Lee S-M & Park C-K (1984) [Acute toxicity of onchean stream water to
the sea urchin, Hemicentrotus pulcherrimus.] Bull Korean Fish Soc,
17: 414-422 (in Korean).
Leidner H, Gloor R, & Wuhrmann K (1976) [The kinetics of degradation
of linear alyklbenzene sulfonate.] Tenside Surfactants Deterg, 13:
122-130 (in German).
Leo AJ & Hansch C (1979) Substituent constants for correlation.
Analysis in chemistry and biology. New York: Wiley, 98 pp.
Lewis MA (1983) Effect of loading density on the acute toxicities of
surfactants, copper, and phenol to Daphnia magna Straus. Arch
Environ Contam Toxicol, 12: 51-55.
Lewis MA (1986) Comparison of the effects of surfactants on freshwater
phytoplankton communities in experimental enclosures and on algal
population growth in the laboratory. Environ Toxicol Chem, 5: 319-332.
Lewis MA & Hamm BG (1986) Environmental modification of the
photosynthetic response of lake plankton to surfactants and
significance to a laboratory-field comparison. Water Res, 20:
1575-1582.
Lewis MA & Suprenant D (1983) Comparative acute toxicities of
surfactants to aquatic invertebrates. Ecotoxicol Environ Saf, 7:
313-322.
Lewis MA, Pittinger CA, Davidson DH, & Ritchie CJ (in press) In-situ
response of natural periphyton to an anionic surfactant and an
environmental risk assessment for phytotoxic effects. Environ Toxicol
Chem.
Lichtenstein EP (1966) Increase of persistence and toxicity of
parathion and diazinon in soils with detergents. J Econ Entomol, 59:
985-993.
Lichtenstein EP, Fuhremann TW, Scopes NEA, & Skrentny RF (1967)
Translocation of insecticides from soils into pea plants. Effects of
the detergent LAS on translocation and plant growth. J Agric Food
Chem, 15: 864-869.
Litovitz TL, Schmitz BF, & Bailey KM (1990) 1989 Annual Report of the
American Association of Poison Control Centers National Data
Collection System. Am J Emerg Med, 8: 394-445.
Litz N, Doering HW, Thiele M, & Blume H-P (1987) The behavior of
linear alkylbenzenesulfonate in different soils: A comparison between
field and laboratory studies. Ecotoxicol Environ Saf, 14: 103-116.
Longwell J & Maniece WD (1955) Determination of anionic detergents in
sewage, sewage effluents and river waters. Analyst, 80: 167-171.
Lopez-Zavala A, Aluja AS de, Elias BL, Manjarrez L, Buchmann A,
Mercado L, & Caltenco S (1975) The effects of the ABS, LAS and AOS
detergents on fish, domestic animals and plants. Prog Water Technol,
7: 73-82.
Maag H, Praun F, & Schoberl P (1975) [Recent discoveries in the olefin
sulfonate field. I. Effect of carbon number and branching of the alkyl
chain on the application and environmental characteristics of olefin
sulfonates.] Tenside Surfactants Deterg, 12: 11-15 (in German).
Maki AW (1979a) Correlations between Daphnia magna and fathead
minnow (Pimephales promelas) chronic toxicity values for several
classes of test substance. J Fish Res Board Can, 36: 411-421.
Maki AW (1979b) Respiratory activity of fish as a predictor of chronic
fish toxicity values for surfactants. In: Marking LL & Kimerle RA ed.
Aquatic toxicology. Philadelphia, Pennsylvania, American Society for
Testing and Materials, pp 77-95 (ASTM STP 667).
Maki AW & Bishop WE (1979) Acute toxicity studies of surfactants to
Daphnia magna and Daphnia pulex. Arch Environ Contam Toxicol, 8:
599-612.
Mancini P, Bellandi S, La Conca P, & Pantani F (1984)
[Microdetermination of anionic surface active agents in drinking water
and in waters that can be made potable.] Riv Merceol, 23: 143-149 (in
Italian).
Mann H (1976) [Golden orfe fish comparative testing of acute toxicity
of substances in water and wastewater: results of a ring test.] Wasser
Abwasser-Forsch, 9: 103-109 (in German).
Mann AH & Reid VW (1971) Biodegradation of synthetic detergents
evaluation by community trials: I. Linear alkylbenzene sulphonates. J
Am Oil Chem Soc, 48: 588-594.
Marchesi JR, House WA, White GF, Russell NJ, & Farr IS (1991) A
comparative study of the adsorption of linear alkyl sulphates and
alkylbenzene sulphonates on river sediments. Colloids Surf, 53: 63-78.
Marcomini A & Giger W (1987) Simultaneous determination of linear
alkylbenzenesulfonates, alkylphenol polyethoxylates, and nonylphenol
by high-performance liquid chromatography. Anal Chem, 59: 1709-1715.
Marin MG, Bressan M, & Brunetti R (1991) Effects of linear
alkylbenzene sulphonate (LAS) on two marine benthic organisms. Aquat
Toxicol, 19: 241-248.
Marquis DM, Sharman SH, House R, & Sweeney WA (1966) Alpha olefin
sulfonates from a commercial SO3-air reactor. J Am Oil Chem Soc, 43:
607-614.
Martinez J, Vives-Rego J, & Sanchez-Leal J (1989) The effect of
chemical structure and molecular weight of commercial alkylbenzenes on
the toxic response of Daphnia and naturally occurring bacteria in
fresh and seawater. Water Res, 23: 569-572.
Martinez J, Soto Y, Vives-Rego J, & Bianchi M (1991) Toxicity of Cu,
Ni and alkylbenzene sulfonate (LAS) on the naturally occurring
bacteria in the Rhone River plume. Environ Toxicol Chem, 10: 641-647.
Masubuchi M, Takahashi A, Takahashi O, & Hiraga K (1976) [Cytogenetic
studies and dominant lethal tests with long term administration of
butylated hydroxytoluene (BHT) and linear alkylbenzene sulfonate (LAS)
in mice and rats.] Ann Rep Tokyo Metrop Res Lab Public Health, 27 (2):
100-104 (in Japanese).
Masuda F & Inoue K (1974) [Effects on fetuses of linear alkylbenzene
sulfonate administered subcutaneously to pregnant mice.] Congenit Anom
[Senten Ijo], 14 (4): 309-318 (in Japanese).
Masuda F, Okamoto K, & Inoue K (1973) [Effects on fetuses of linear
alkylbenzene sulfonate applied to mouse maternal skin during
pregnancy.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 14 (6):
580-582 (in Japanese).
Masuda F, Okamoto K, & Inoue K (1974) [Effects of linear alkylbenzene
sulfonate applied dermally to pregnant mice on fetal development.] J
Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 15 (5): 349-355 (in
Japanese).
Mathur AK, Narang S, Gupta BN, Singh A, Singh S, & Shanker R (1992)
Effect of dermal exposure to LAS detergent and HCH pesticide in guinea
pigs: biochemical and histopathologic changes in liver and kidney. J
Toxicol Cutan Ocular Toxicol, 11 (1): 3-13.
Matsueda T, Osaki Y, & Shigee S (1982) [Determination of
alkylbenzenesulfonate in water by high-performance liquid
chromatography.] Jpn Anal (Bunseki Kagaku), 31: 59-62 (in Japanese).
Matsuura T & Smith JM (1970) Kinetics of photodecomposition of dodecyl
benzene sulfonate. Ind Eng Chem Fundam, 9: 252-260.
Matthijs E & De Henau H (1987) Determination of LAS. Tenside
Surfactants Deterg, 24: 193-199.
Maurer EW, Cordon TC, & Stirton AJ (1971) Microaerophilic
biodegradation of tallow-based anionic detergents in river water. J Am
Oil Chem Soc, 48: 163-165.
Mayer FL (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Washington, DC, US Environmental Protection Agency, p 178
(EPA/600/8-87/017; PB-87-188686).
McAvoy DC, Eckhoff WS, & Rapaport RA (1993) Fate of linear
alkylbenzene sulfonate in the environment. Environ Toxicol Chem, 12:
977-987.
McCutcheon (1989) McCutcheon's emulsifiers and detergents:
International edition. Glen Rock, New Jersey, McCutcheon's Division,
Manufacturing Confectioner Publishing Co., 240 pp.
McCutcheon (1993) Volume 1: McCutcheon's emulsifiers & detergents:
North American edition-International edition. Glen Rock, New Jersey,
McCutcheon's Division, Manufacturing Confectioner Publishing Co. 295
pp.
McEvoy J & Giger W (1985) Accumulation of linear
alkylbenzenesulphonate surfactants in sewage sludges.
Naturwissenschaften, 72: 429-431.
McEvoy J & Giger W (1986) Determination of linear
alkylbenzenesulfonates in sewage sludge by high-resolution gas
chromatography/mass spectrometry. Environ Sci Technol, 20: 376-383.
McKim JM, Arthur JW, & Thorslund TW (1975) Toxicity of a linear
alkylate sulfonate detergent to larvae of four species of freshwater
fish. Bull Environ Contam Toxicol, 14: 1-7.
Merits I (1975) The metabolism of labelled hexadecyl sulphate salts in
the rat, dog and human. Biochem J, 148: 219-228.
Michael WR (1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol Appl Pharmacol, 12: 473-485.
Mieure JP, Waters J, Holt MS, & Matthijs E (1990) Terrestrial safety
assessment of linear alkylbenzene sulfonate. Chemosphere, 21: 251-262.
Mikami Y, Nagai H, Sakai Y, Fukushima S, & Nishino T (1969) [Effects
of a detergent on the development of the mouse embryo.] Congenit Anom
(Senten Ijo), 9: 230 (in Japanese).
Minegishi K, Osawa M, & Yamaha T (1977) Percutaneous absorption of
alpha-olefin sulfonate (AOS) in rats. Chem Pharm Bull, 25 (4):
821-825.
Ministry of Health and Welfare (1992) [Trend analysis of water works
in Japan.] J Jpn Water Works Assoc, 61: 41-78 (in Japanese).
Misra V, Lal H, Viswanathan PN & Krishna Murti CR (1984) 45Ca uptake
from water by snails (Lymnaea vulgaris) in control and
detergent-polluted samples. Ecotoxicol Environ Saf, 8: 97-99.
Misra V, Lal H, Chawla G, & Viswanathan PN (1985) Pathomorphologic
changes in gills of fish fingerlings (Cirrhina mrigala) by linear
alkyl benzene sulfonate. Ecotoxicol Environ Saf, 10: 302-308.
Misra V, Chawla G, Kumar V, Lal H, & Viswanathan PN (1987) Effect of
linear alkyl benzene sulfonate in skin of fish fingerlings (Cirrhina
mrigala): Observations with scanning electron microscope. Ecotoxicol
Environ Saf, 13: 164-168.
Misra V, Javed S, Pandey DP, & Viswanathan PN (1989a) Biochemical and
pathomorphologic studies for safety evaluation of synthetic
detergents. J Toxicol Cutan Ocular Toxicol, 8: 271-278.
Misra V, Kumar V, Pandey SD, & Viswanathan PN (1989b) Uptake and
distribution of 203Hg by fish fingerlings, Cirrhina mrigala,
exposed to linear alkyl benzene sulphonate. Bull Environ Contam
Toxicol, 43: 139-143.
Misra V, Kumar V, Pandey SD, & Viswanathan PN (1991) Biochemical
alterations in fish fingerlings (Cyprinus carpio) exposed to
sublethal concentration of linear alkyl benzene sulphonate. Arch
Environ Contam Toxicol, 21: 514-517.
Mitchell GC & Holt MS (1993) The fate and effects of linear
alkylbenzene sulphonate (LAS) in outdoor artificial streams. Presented
at the First SETAC World Congress: Ecotoxicology and Environmental
Chemistry--A Global Perspective, Lisbon, Portugal, 28-31 March 1993.
New York, Society of Environmental Toxicology and Chemistry.
Miura KIV, Suzuki K, Tsuchikura A, Baba Y, Hayakana T, Hayashi S,
Moriyama T, Ishiguro T, & Yoshida Y (1968) [Alkylbenzene sulphonate in
the water of the Tama River.] J Jpn Oil Chem Soc [Yukagaku], 17:
635-637 (in Japanese).
Miura K, Yamanaka K, Sangai T, Yoshimura K, & Hayashi N (1979)
[Application of the biological oxygen consumption measurement
technique in the biodegradation testing of surfactants.] J Jpn Oil
Chem Soc [Yukagaku], 28 (5): 351-355 (in Japanese).
Moreno A, Ferrer J, & Berna JL (1990) Biodegradability of LAS in a
sewer system. Tenside Surfactants Deterg, 27 (5): 312-315.
Motomizu S, Fujiwara S, Fujiwara A, & Toei K (1982) Solvent
extraction-spectrophotometric determination of anionic surfactants
with ethyl violet. Anal Chem, 54: 392-397.
Motoyama S & Mukai H (1981) [Synthetic detergents in the environment.]
Niigota Rikagaku, 7: 21-29 (in Japanese).
Nakae A, Tsuji K, & Yamanaka M (1980) Determination of trace amounts
of alkylbenzenesulfonates by high-performance liquid chromatography
with fluorimetric detection. Anal Chem, 52 (14): 2275-2277.
Nakahara M, Ishibashi M, Asao T, Tsukamoto T, Shinagawa K, Kinoshita
Y, Yamamoto H, Mitsuda B, & Kunita N (1976) [Studies on toxicity of
commercial detergents; 1st report.] Ann Rep Osaka Prefect Inst Public
Health, 7: 131-135 (in Japanese).
Nakamura E, Fujisawa I, & Namiki H (1983) [Determination of anionic
surfactant in water by indirect atomic absorption spectrometry after
solvent extraction with potassium-dibenzo-18-crown-6.] Jpn Anal
[Bunseki Kagaku], 32: 332-336 (in Japanese).
Nielsen AM & Huddleston RL (1981) Ultimate biodegradation of linear
alkylbenzene sulfonate alkyl and ring carbon. Dev Ind Microbiol, 22:
415-424.
Nielsen AM, Meyers JD, Bleckmann CA, & Huddleston RL (1980) LAS
biodegradation: Ultimate fate of alkyl and ring carbon. Househ Pers
Prod Ind, March: 68-87.
Nishimura H (1976) [Could skin application of cleansing detergents be
teratogenic cause in successive joint studies and interpretation of
the results?] Congenit Anom (Senten Ijo), 16: 175-185 (in Japanese).
Nolen GA, Klusman LW, Patrick LF, & Geil RG (1975) Teratology studies
of a mixture of tallow alkyl ethoxylate and linear alkylbenzene
sulfonate in rats and rabbits. Toxicology, 4: 231-243.
Nomura T, Kimura S, Hata S, Kanzaki T, & Tanaka H (1980) The synthetic
surfactants AS and LAS interrupt pregnancy in mice. Life Sci, 26:
49-54.
Nonaka K, Hattori Y, & Nakamoto M (1989) [Determination of LAS in
environmental and domestic waste waters.] Suishitsu Odaku Kenkyo, 12
(3): 194-200 (in Japanese).
Nonaka K, Hayashi Y, & Nakamoto M (1990) [The distribution and fate of
LAS in some river waters of Osaka.] Ann Rep Environ Pollut Control
Cent Osaka Prefect Gov, 12: 68-79 (in Japanese).
Nusair TL, Danneman PJ, Stotte J, & Bay PHS (1988) Consumer products:
risk assessment process for contact sensitization. Toxicologist, 8:
258.
Nyberg H (1988) Growth of Selenastrum capricornutum in the presence
of synthetic surfactants. Water Res, 22: 217-223.
Oba K & Yoshida Y (1965) [Determination of ABS in well water.] J Jpn
Water Works Assoc [Suido Kyokai Zasshi], 371: 16-21 (in Japanese).
Oba K, Yoshida Y, & Tomiyama S (1967) [Studies on the biodegradation
of synthetic detergents. I. Biodegradation of anionic surfactants
under aerobic and anaerobic conditions.] J Jpn Oil Chem Soc
[Yukagaku], 16: 517-523 (in Japanese).
Oba K, Mori A, & Tomiyama S (1968a) [Biochemical studies of
n-alpha-olefin sulfonates: (II) Acute toxicity, skin and eye
irritation, and some other physiological properties.] J Jpn Oil Chem
Soc [Yukagaku], 17 (11): 628-634 (in Japanese).
Oba K, Mori A, & Tomiyama S (1968b) [Biochemical studies of
n-alpha-olefin sulfonate. (I) Biodegradability under aerobic
conditions.] J Jpn Oil Chem Soc [Yukagaku], 17 (9): 517-520 (in
Japanese).
Oba K, Yagi R, & Miura K (1975) [Conversion of detergents to
biodegradable compounds and their removal in municipal sewage
treatment plants.] Yosui To Haisui, 17: 1385-1391 (in Japanese).
Oba K, Miura K, Sekiguchi H, Yagi R, & Mori A (1976) Microanalysis of
anionic surfactants in waste water by infrared spectroscopy. Water
Res, 10: 149-155.
Oba K, Mori A, & Nagai T (1985) Safety in use of AOS materials in
Japan. J Am Oil Chem Soc, 62: 645.
Oda Y, Kuwano A, Inoue K, Hamano Y, Yamamoto H, Mitsuda B, & Kunita N
(1977) [Studies on the toxicity of commercial detergents. 6th report:
mutagenicity study in bacteria.] Ann Rep Osaka Prefect Inst Public
Health, 8: 11-16 (in Japanese).
Oda Y, Yamamoto H, & Kunita N (1980) [Studies on the mutagenicity of
surfactants.] Abstracts of 9th Annual Meeting of the Japanese
Environmental Mutagen Society. J Jpn Mutag Soc, 37: 11-16 (in
Japanese).
Ohki K & Tokiwa F (1969) [Physicochemical properties of sulfate-type
and ether sulfate-type surfactants: effects of alkyl chain structure.]
J Chem Soc Jpn Chem Ind Chem [Nihon Kagakuzasshi], 90 (11): 1114-1118
(in Japanese).
Ohki K & Tokiwa F (1970) [Physicochemical properties of
alpha-olefin-sulfonate sodium salts.] J Chem Soc Jpn Chem Ind Chem
[Nihon Kagakuzasshi], 91 (6): 534-539 (in Japanese).
Ohkuma T (1981) [Determination of sodium linear alkylbenzene sulfonate
in rivers of Fukuoka City.] Fukuoka-shi Eisei Skikenshoho, 7: 75-80
(in Japanese).
Oishi S, Takahashi O, Masubuchi M, Fukuda H, Kamiya N, Yoshida S,
Mikuriya H, Yuzawa K, & Hiraga K (1974) [The toxicity of the higher
alcoholic detergents; (II), Subacute toxicity of sodium dodecyl
sulfate.] Ann Rep Tokyo Metrop Res Lab Public Health, 25: 669-675 (in
Japanese).
Okamoto K (1974) [Action of the surfactants upon the skin,
desquamative change of the skin surface, irritation of the skin and
the inhibition of invertase activity by surfactants.] J Jpn Oil Chem
Soc [Yukagaku], 23 (11): 726-729 (in Japanese).
Okamoto T & Shirane Y (1982) [Pollution by linear alkylbenzene
sulfonates (LAS) in river and coastal areas in Hiroshima Prefecture.]
Hiroshima-Ken Kankyo Sata Kenkyu Hokoku, 3: 60-65 (in Japanese).
Okamoto K & Takase Y (1976a) [Studies on keratodermia tylodes palmaris
progressiva.] Shinshu Med J [Shinshu Ishi], 24 (2): 131-141 (in
Japanese).
Okamoto K & Takase Y (1976b) [Action of surfactants upon the skin;
(IV), Effect of sodium alkyl sulfates with lipophilic groups of
different alkyl chain length on the skin.] Shinshu Med J [Shinshu
Ishi], 24 (3): 243-250 (in Japanese).
Okpokwasili GC & Nwabuzor CN (1988) Primary biodegradation of anionic
surfactants in laundry detergents. Chemosphere, 17: 2175-2182.
Olson KJ, Dupree RW, Plomer ET, & Rowe VK (1962) Toxicological
properties of several commercially available surfactants. J Soc Cosmet
Chem (USA), 13: 469-482.
Osburn QW (1986) Analytical methodology for linear alkylbenzene
sulfonate (LAS) in waters and wastes. J Am Oil Chem Soc, 63: 257-263.
Oser BL & Morgareidge K (1965) Toxicologic studies with branched and
linear alkyl benzene sulfonates in rats. Toxicol Appl Pharmacol, 7:
819-825.
Ottery J, Olavesen AH, & Dodgson KS (1970) Metabolism of dodecyl
sulphate in the rat: non-enzymic liberation of sulphate and
gamma-butyrolactone from the major metabolite, butyric acid
4-sulphate. Life Sci, 9 (2): 1335-1340.
Painter HA (1992) Anionic surfactants. In: de Oude NT ed.The handbook
of environmental chemistry. Vol 3, Part F: Anthropogenic compounds.
Berlin, Springer-Verlag, pp 1-88.
Painter HA & Mobey FE (1992) The question of the anaerobic
biodegradability of linear alkylbenzene sulfonates (LAS). Proceedings
of the European Committee of Organic Surfactants and Their
Intermediates (CESIO) Surfactant Congress, London, June 1992.
Brussels, CESIO, pp 34-41.
Painter HA & Zabel TF (1988) Review of the environmental safety of
LAS. Report to the Council of European Federations of Chemical
Industry (CEFIC). Medmenham, Water Research Centre, 245 pp (CO
1659-M/1/EV 8658).
Painter HA & Zabel T (1989) The behaviour of LAS in sewage treatment.
Tenside Surfactants Deterg, 26: 108-115.
Palmer AK, Readshaw MA, & Neuff AM (1975a) Assessment of the
teratogenic potential of surfactants (Part I)--LAS, AS and CLD.
Toxicology, 3: 91-106.
Palmer AK, Readshaw MA, & Neuff AM (1975b) Assessment of the
teratogenic potential of surfactants (Part II)--AOS. Toxicology, 3:
107-113.
Palmisano AC, Schwab BS, Maruscik DA, & Ventullo RM (1991) Seasonal
changes in mineralisation of xenobiotics by stream microbial
communities. Can J Microbiol, 37: 939-948.
Panigrahi AK & Konar SK (1986) Effects of mixture of petroleum
refinery effluent and an anionic linear alkyl benzene sulfonate
detergent on aquatic ecosystem. Environ Ecol, 4: 434-438.
Pärt P, Svanberg O, & Bergstrom E (1985) The influence of surfactants
on gill physiology and cadmium uptake in perfused rainbow trout gills.
Ecotoxicol Environ Saf, 9: 135-144.
Persoone G, Van de Vel A, Van Steertegem M, & De Nayer B (1989)
Predictive value of laboratory tests with aquatic invertebrates:
influence of experimental conditions. Aquat Toxicol, 14: 149-167.
Pickering QH (1966) Acute toxicity of alkyl benzene sulfonate and
linear alkylate sulfonate to the eggs of the fathead minnow,
Pimephales promelas. Air Water Pollut Int J, 10: 385-391.
Pickering QH (1988) Evaluation and comparison of two short-term
fathead minnow tests for estimating chronic toxicity. Water Res, 22:
883-893.
Pickering QH & Thatcher TO (1970) The chronic toxicity of linear
alkylate sulfonate (LAS) to Pimephales promelas, Rafinesque. J Water
Pollut Control Fed, 42: 243-254.
Pitter P & Fuka T (1979) The problem of ultimate biodegradability of
linear alkylbenzene sulphonates. Tenside Surfactants Deterg, 16:
298-302.
Pittinger CA, Woltering DM, & Masters JA (1989) Bioavailability of
sediment-sorbed and aqueous surfactants to Chironomus riparius
(midge). Environ Toxicol Chem, 8: 1023-1033.
Polano MK (1968) The interaction of detergents and the human skin. J
Soc Cosmet Chem, 19: 3-20.
Prats D, Ruiz F, Vazquez B, Zarzo D, Berna JL, & Moreno A (1993) LAS
homolog distribution shift during wastewater treatment and composting:
ecological implications. Environ Toxicol Chem, 12: 1599-1608.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Prottey C & Ferguson T (1975) Factors which determine the skin
irritation potential of soaps and detergents. J Soc Cosmet Chem, 26:
29-46.
Putterman GT, Wolejsza NF, Wolfram MA, & Laden K (1977) The effect of
detergents on swelling of stratum corneum. J Soc Cosmet Chem (USA),
28: 521-532.
Rama Bhat S, Crisp PT, Eckert JM, & Gibson NA (1980) A
spectrophotometric method for the determination of anionic surfactants
at µg-l1 levels. Anal Chem Acta, 116: 191-193.
Rapaport RA & Eckhoff WS (1990) Monitoring linear alkyl benzene
sulfonate in the environment: 1973-1986. Environ Toxicol Chem, 9:
1245-1257.
Rapaport RA, Hopping WD, & Eckloff WS (1987) Monitoring LAS in the
environment: abstracts and programme. Eighth Annual Meeting of the
Society of Environmental Toxicology and Chemistry (SETAC), Pensacola,
Florida. New York, Society of Environmental Toxicology and Chemistry.
Rehwoldt R, Lasko L, Shaw C, & Wirhowski E (1974) Toxicity study of
two oil spill reagents toward Hudson River fish species. Bull Environ
Contam Toxicol, 11: 159-162.
Reiff B, Lloyd R, How MJ, Brown D, & Alabaster JS (1979) The acute
toxicity of eleven detergents to fish: results of an interlaboratory
exercise. Water Res, 13: 207-210.
Rennison PM, Powell GM, Olavesen AH, Howes D, & Black JG (1987)
Absorption and excretion of linear alkylbenzene sulphonates in rats.
Biochem Soc Trans, 15: 456.
Renzoni A (1974) Influence of toxicants on marine invertebrate larvae.
Thalassia Jugosl., 10: 197-211.
Richtler HJ & Knaut J (1988) World prospects for surfactants. In:
Proceedings of the Second World Surfactants Congress: Surfactants in
Our World Today and Tomorrow, Paris, 24-27 May 1988. Paris, Syndicat
National des Fabricants d'Agents de Surface et de Produits Auxiliaires
Industriels.
Richtler HJ & Knaut J (1991) Environment and oleochemicals--a
challenge. Fett Wissensch Technol, 93: 1-13.
Roberts DW (1989) Aquatic toxicity of linear alkyl benzene sulphonates
(LAS)--A QSAR analysis. In: Turner JE, England MW, Schultz TW, & Kwaak
NJ ed. QSAR 88. Proceedings of the Third International Workshop on
Quantitative Structure-Activity Relationships in Environmental
Toxicology, Knoxville, Tennessee, 22-26 May 1988. Springfield,
Virginia, US Department of Commerce, National Technical Information
Service, pp 91-98.
Roberts DW (1991) QSAR issues in aquatic toxicology of surfactants.
Sci Total Environ, 109/110: 557-568.
Roberts DW & Williams DL (1983) Sultones as by-products in anionic
surfactants. Tenside Surfactants Deterg, 20 (3): 109-111.
Roberts MH, Warinner JE, Tsai CF, Wright D, & Cronin E (1982)
Comparison of estuarine species sensitivities to three toxicants. Arch
Environ Contam Toxicol, 11: 681-692.
Roberts DW, Lawrence JG, Fairweather IA, Clemett CJ, & Saul CD (1990)
Alk-1-ene-1,3-sultones in alpha-olefin sulphonates. Tenside
Surfactants Deterg, 27 (2): 82-86.
Robinson EC & Schroeder RE (1992) Reproductive and developmental
toxicity studies of a linear alkylbenzene mixture in rats. Fundam Appl
Toxicol, 18 (4): 549-556.
Robra KH (1976) [Detection of toxic water contaminants by inhibition
of substrate oxidation in Pseudomonas using polarography.]
Wasser/Abwasser, 117: 80-86 (in German).
Röderer G (1987) Toxic effects of tetraethyl lead and its derivatives
on the chrysophyte Poterioochromonas malhamensis. VIII. Comparative
studies with surfactants. Arch Environ Contam Toxicol, 16: 291-301.
Rohrer DM & Woodbridge DD (1975) Reduction in surfactants by
irradiation. Bull Environ Contam Toxicol, 13: 31-36.
Rosner E, Sawinsky A, Molnar A, Pasztor G, & Mihak I (1973)
[Laboratory tests on workers exposed to dodecyl benzolsulfonate.]
Egeszsegtudomany, 17: 163-165 (in Hungarian).
Russell NJ, Anderson DJ, Day MJ, & White GF (1991) Colonization of
biofilms by bacteria capable of biodegrading sodium dodecyl sulphate
(SDS) at clean and polluted sites. Microbiol Ecol, 22: 85-98.
Sadai M & Mizuno N (1972) [Effect of long term topical application of
some anionic surfactants on the skin, oral mucous membrane, and
tongue.] Jpn J Dermatol [Nihon Hifuka Gakkaishi], 82 (4): 207-221 (in
Japanese).
Sadai M, Sato T, Arima M, Adachi K, & Tamai H (1979) [Effect of some
surfactants on the skin.] J Jpn Cosmet Sci Soc [Kousho Kaishi], 3 (1):
60-62 (in Japanese).
Saito T & Hagiwara K (1982) Analysis of traces of surfactants in water
with anion-exchange resin and polymeric adsorbent. Fresenius Z Anal
Chem, 312: 533-535.
Saito T, Higashi K, & Hagiwara K (1982) Determination of traces of
sodium alkylbenzenesulphonate by high-performance liquid
chromatography: application to water. Fresenius Z Anal Chem, 313:
21-23.
Sakashita S (1979) Electron microscopic study of liver tissue after
cutaneous administration of detergents: multiple application of 1%
solution. Jpn J Clin Electron Microsc [Nihon Rinsho Denshikenbikyo
Gakkaishi], 12 (1-2): 189-216.
Sakashita S, Agata I, & Akiyoshi S (1974) [An electron microscopic
study on the liver after the dermal application of various
detergents.] Mie Med J [Mie Igaku], 18: 124-143 (in Japanese).
Sales D, Quiroga JM, & Gomez-Parra A (1987) Primary biodegradation
kinetics of anionic surfactants in the marine environment. Bull
Environ Contam Toxicol, 39: 385-392.
Samejima Y (1991) [Effects of synthetic surfactants and natural soap
on the development of mouse embryos in vitro and the fertilizing
capacity of mouse and human sperm.] J Osaka Univ Med Sch, 3 (12):
675-682 (in Japanese).
Sasagawa S (1963) [The present status of housewives' hand dermatitis
in Japan and the role of detergents as possible causes.] Hifukano
Rinsho, 5: 47-55 (in Japanese).
Sasagawa S, Takase Y, & Ishihara M (1978) [A study on the irritation
effects of detergent components on human skin by the patch test: A
report of studies on synthetic detergents.] Tokyo, Science and
Technology Agency of Japan, Research Coordination Bureau pp 243-266
(in Japanese).
Sato K, Ando H, Yuzawa K, & Hiraga K (1972) [Studies on toxicity of
synthetic detergents; (III), Examination of teratogenic effects of
alkyl benzene sulfonate spread on the skin of mice.] Ann Rep Tokyo
Metrop Res Lab Public Health, 24: 441-448 (in Japanese).
Sawada K, Inomata S, Gobara B, & Suzuki T (1983) Extraction and
determination of anionic surfactants with copper (II) ethylenediamine
derivative complexes. Talanta, 30 (3): 155-159.
Sawano J (1978) [Teratogenicity studies of anionic surfactants (AOS,
AOS-S) absorbed through skin: A report of studies on synthetic
detergents.] Tokyo, Science and Technology Agency of Japan, Research
Coodination Bureau, pp 112-113 (in Japanese).
Schöberl P (1989) Basic principles of LAS biodegradation. Tenside
Surfactants Deterg, 26: 86-94.
Schöberl P, Bock KJ, & Huber L (1988) Surfactant biodegradation.
Tenside Surfactants Deterg, 25: 2-7.
Schumacher KA, Classen HG, Marquardt P, & Spath L (1972) [Acute
pulmonary hypertonus in cats after parenteral application of sodium
decylsulfate and sodium dodecyl sulfate.] Arzneim Forsch, 22 (9):
1489-1491 (in German).
Sedlak RI & Booman KA (1986) A study of LAS and alcohol ethoxylate
removal at a municipal wastewater treatment plant. Chim Oggi, 9:
21-24.
Sekiguchi HK, Miura K, Oba K, & Mori A (1972) Biodegradation of AOS
and other surfactants. J Jpn Oil Chem Soc, 21: 451-455.
Sekiguchi H, Miura K, Oba K, & Mori A (1975a) [Biodegradation of
alpha-olefin sulfonates (AOS) and other surfactants.] J Jpn Oil Chem
Soc [Yukagaku], 24: 145-148 (in Japanese).
Sekiguchi H, Miura K, & Oba K (1975b) [Biodegradation of some anionic
surfactants in the river die-away test.] J Jpn Oil Chem Soc
[Yukagaku], 24: 451-455 (in Japanese).
Sekiguchi H, Oba K, Masuda M, & Sugiyama T (1975c) [Studies on the
relative biodegradation rate of alkene vs hydroxyalkane sulfonates in
alpha-olefin sulfonate.] J Jpn Oil Chem Soc [Yukagaku], 24: 675-679
(in Japanese).
Setzkorn EA & Huddleston RL (1965) Ultraviolet spectroscopic analysis
for following the biodegradation of hydrotopes. J Am Oil Chem Soc, 42:
1081-1084.
Sharma AK, Bandre TR, Srinivasu T, & Chandra N (1985) Deleterious
effects of detergent on plants. Environ Ecol, 3: 444-445.
Shiga Prefecture (1988) [Water quality of Lake Biwa in 1988.] Shiga
Prefect Inst Public Health: 22 (in Japanese).
Shimp RJ (1989) LAS biodegradation in estuaries. Tenside Surfactants
Deterg, 26 (6): 390-393.
Shiobara S & Imahori A (1976) [Effects of linear alkylbenzene
sulfonate orally administered to pregnant mice on the pregnant mice
and their fetuses.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 17
(4): 295-301 (in Japanese).
Smyth HF, Seaton J, & Fischer L (1941) Some pharmacological properties
of the 'Tergitol' penetrants. J Ind Hyg Toxicol, 23 (10): 478-483.
Snell TW & Persoone G (1989) Acute toxicity bioassays using rotifers.
II. A freshwater test with Brachionus rubens. Aquat Toxicol, 14:
81-92.
Solon JM & Nair JH (1970) The effect of a sublethal concentration of
LAS on the acute toxicity of various phosphate pesticides to the
fathead minnow ( Pimephales promelas Rafinesque). Bull Environ Contam
Toxicol, 5: 408-413.
Solon JM, Lincer JL, & Nair JH (1969) The effect of sublethal
concentration of LAS on the acute toxicity of various insecticides to
the fathead minnow ( Pimephales promelas Rafinesque). Water Res, 3:
767-775.
Stalmans M, Matthijs E, & de Oude NT (1991) Fate and effects of
detergent chemicals in the marine and estuarine environment. Water Sci
Technol, 24: 115-126.
Standing Technical Committee on Synthetic Detergents (1978) Annual
report of the UK Standing Technical Committee on Synthetic Detergents.
London, Her Majesty's Stationery Office.
Standing Technical Committee on Synthetic Detergents (1989) Annual
report of the UK Standing Technical Committee on Synthetic Detergents.
London, Her Majesty's Stationery Office.
Steber J (1979) Investigations into the biodegradation of
14C-labelled linear alkyl benzene sulphonate in models of surface
waters and sewage purification plants. Tenside Surfactants Deterg, 16:
140-145.
Steber J & Wierich P (1987) The anaerobic degradation of detergent
range fatty alcohol ethoxylates. Studies with 14C-labelled model
surfactants. Water Res, 21: 661-667.
Steber J, Gode P, & Guhl W (1988) Fatty alcohol sulfates: The
ecological evaluation of a group of important detergent surfactants.
Fett Wiss Technol, 90: 32-38.
Stenton SC, Dennis JH, Walters EH, & Hendrick DJ (1990) Asthmagenic
properties of a newly developed detergent ingredient: sodium
isononanoyl oxybenzene sulphonate. Br J Ind Med, 47 (6): 405-410.
Sterzel W (1992) Daily human intake. In: Gloxhuber C & Künstler K ed.
Anionic surfactants; biochemistry, toxicology, dermatology, 2nd ed.
New York, Marcel Dekker, Inc., pp 411-417.
Sueishi T, Morioka T, Kaneko H, Kusaka M, Yagi S, & Chikami S (1988)
Environmental risk assessment of surfactants: fate and environmental
effects in Lake Biwa basin. Regul Toxicol Pharmacol, 8: 4-21.
Sullivan WT & Evans RL (1968) Major US river reflects surfactant
changes. Environ Sci Technol, 2: 194-200.
Sullivan WT & Swisher RD (1969) MBAS and LAS surfactants in the
Illinois River, 1968. Environ Sci Technol, 3: 481-483.
Sunakawa T, Ikida Y, & Okamoto K (1979) [Absorption, distribution,
metabolism, and xcretion of linear alkylbenzene sulfonate in rats.] J
Jpn Oil Chem Soc [Yukagaku], 39 (2): 59-68 (in Japanese).
Sunakawa T, Inoue K, & Okamoto K (1981) [Studies on the mutagenicity
of surfactants; mutagenicity of surfactants following activation with
various liver homogenates (S-9) and mutagenicity in the presence of
norharman.] Hyg Chem [Eisei Kagaku], 27 (4): 204-211 (in Japanese).
Swedmark M & Granmo A (1981) Effects of mixtures of heavy metals and
a surfactant on the development of cod ( Gadus morhua L.). Rapp P-V
Reun Cons Int Explor Mer, 178: 95-103.
Swedmark M, Braaten B, Emanuelsson E, & Granmo A (1971) Biological
effects of surface active agents on marine animals. Mar Biol, 9:
183-201.
Swisher RD (1963) The chemistry of surfactant biodegradation. J Am Oil
Chem Soc, 40: 648-656.
Swisher RD (1967) Biodegradation of LAS-benzene rings in activated
sludge. J Am Oil Chem Soc, 44: 717-724.
Swisher RD (1970) Surfactant biodegradation. New York, Marcel Dekker,
Inc., 469 pp (Surfactant Science Series, Vol 3).
Swisher RD (1972) Linear alkylbenzene sulfonate (LAS) benzene rings:
Biodegradation and acclimation studies. J Jpn Oil Chem Soc, 21:
130-142.
Swisher RD (1976) Surfactants: from recalcitrant to docile. In:
Proceedings of the 3rd International Biodegradation Symposium. London,
Applied Science Publishers, pp 853-865.
Swisher RD (1981) The problem of ultimate biodegradability of linear
alkylbenzene sulfonates: an extension. Tenside Surfactants Deterg, 18:
57-63.
Swisher RD (1987) Surfactant biodegradation. New York, Marcel Dekker,
Inc., 1085 pp (Surfactant Science Series, Vol 18).
Swisher RD, O'Rourke JT, & Tomlinson HD (1964) Fish bioassays of
linear alkylate sulfonates (LAS) and intermediate biodegradation
products. J Am Oil Chem Soc, 41: 746-752.
Swisher RD, Gledhill WE, Kimerle RA, & Taulli TA (1978) Carboxylated
intermediates in the biodegradation of linear alkylbenzene sulfonates
(LAS). In: Proceedings of the 7th International Congress on Surface
Active Substances, Moscow, June 1976. Moscow, Teknol Vody, Vol 4, pp
218-230.
Szakall A & Schulz KH (1960) [Permeation of fatty alcohol sulfate and
sodium soap of defined chain length (C8-C18) in intact human skin,
and its relation to irritant activity.] Fette Seifen Anstrichm, 62
(3): 170-175 (in German).
Tabor CF, Barber LB, & Runnells DD (1993) Anionic surfactants in the
Mississippi River: A detailed examination of the occurrence and fate
of linear alkylbenzene sulfonate. Presented at the Division of
Environmental Chemistry, American Chemical Society, Denver, Colorado,
28 March-2 April 1993.
Taguchi S, Tonoshima T, Kasahara I, & Goto K (1981) [Extraction and
spectrophotometric determination of anionic surfactants with
[2-(5-chloro-2-pyridylazo)-5-diethylaminophenolato] cobalt (III)
chloride.] Kogyo Yosui, 278: 23-27 (in Japanese).
Takada H & Ishiwatari R (1987) Linear alkylbenzenes in urban riverine
environments in Tokyo: distribution, source, and behavior. Environ Sci
Technol, 21: 875-883.
Takada H, Ishiwatari R, & Ogura N (1992a) Distribution of linear
alkylbenzenes (LABs) and linear alkylbenzene-sulphonates (LAS) in
Tokyo Bay sediments. Estuar Coast Shelf Sci., 35: 141-156.
Takada H, Ogura N, & Ishiwatari R (1992b) Seasonal variations and
modes of riverine input of organic pollutants to the coastal zone: 1.
Flux of detergent-derived pollutants to Tokyo Bay. Environ Sci
Technol, 26: 2517-2523.
Takahashi O, Shiba H, Nakao T, & Hiraga K (1974) [Inhibition of
thrombin by linear alkylbenzene sulfonate (LAS).] Ann Rep Tokyo Metrop
Res Lab Public Health, 25: 637-645 (in Japanese).
Takahashi A, Sato K, Ando H, Kubo Y, & Hiraga K (1975) [Teratogenicity
of some synthetic detergent and linear alkylbenzene sulfonate (LAS).]
Ann Rep Tokyo Metrop Res Lab Public Health, 26 (2): 67-78 (in
Japanese).
Takahashi A, Ando H, Kubo Y, & Hiaga K (1976) [Effects of dermal
application of sodium dodecyl sulfate (SDS) on pregnant mice and their
fetuses.] Ann Rep Tokyo Metrop Res Lab Public Health, 27 (2): 113-118
(in Japanese).
Takami K, Kamo T, Mochizuki K, Sugimae A, & Nakamoto M (1987) [Rapid
HPLC determination of linear alkylbenzenesulfonates in environmental
water by using concentration technique with a mini column.] Jpn Anal
[Bunseki Kagaku], 36: 276-281 (in Japanese).
Takano S, Yagi N, & Kunihiro K (1975) [Determination of trace amounts
of alkyl benzenesulfonates in water: determination by methylene blue
spectrophotometry and high performance liquid chromatography.] J Jpn
Oil Chem Soc [Yukagaku], 24 (6): 389-394 (in Japanese).
Takeshita R & Yoshida H (1975) [Studies of anion active surfactants;
(I), Determination of anion active surfactants in natural and waste
waters using cleanup by Amberlite XAD2 column chromatography.] J Hyg
Chem [Eisei Kagaku], 21 (4): 209-215 (in Japanese).
Takita Y (1982) [The effects of some detergents and anionic
surfactants on the early stages of growth of some agricultural
plants.] J Jpn Oil Chem Soc [Yukagaku], 31: 507-510 (in Japanese).
Takita Y (1985) [Effects of biodegradation intermediates of linear
alkylbenzenesulfonate on the growth of sporelings of Porphyra
yeroensis.] J Jpn Oil Chem Soc [Yukagaku], 34: 198-201 (in
Japanese).
Takita Y & Oba K (1985) [Determination of trace amounts of anionic
surfactants in the water of Tama River.] Jpn J Water Pollut Res, 8
(11): 752-754 (in Japanese).
Tamura J & Ogura Y (1969) [Pharmacological studies on surface active
agents. 9: Anionic surface active agents and methaemoglobin formation
in the mouse.] Med Biol [Igakuto Seibutugaku], 8 (2): 41-44 (in
Japanese).
Tanaka Y & Nakanishi H (1981) [Purification on Sephadex LH-20 and high
performance liquid chromatographic determination of
dodecylbenzenesulfonate in sediment and biological samples.] Bunseki
Kagaku, 30: 567-73 (in Japanese).
Tang NH (1974) Relationship between BOD removal and LAS detergent
removal. Springfield, Virginia, National Technical Information
Service, 66 pp (NTIS Report PB-232 977/7WP).
Taniguchi S, Yamada A, Morita S, Ogaki S, & Noda T (1978) Results of
studies on synthetic detergents. Tokyo, Science and Technology Agency,
Research Coordination Bureau, pp 18-54.
Tarazona JV & Nunez O (1987) Acute toxicity of synthetic detergents to
snails: Effect of sodium lauryl sulfate on Limnaea peregra shells.
Bull Environ Contam Toxicol, 39: 1036-1040.
Tatsukawa R & Hidaka H (1978) [The avoidance test of chemical
substances by fish: avoidance of detergents by ayu (Plecoglossus
altivelis).] Nippon Nosei Kasaku Kaishi, 52: 263-270 (in Japanese).
Taylor MJ (1985) Effect of diet on the sensitivity of Daphnia magna
to linear alkylbenzene sulfonate. In: Cardwell RD, Purdy R, & Bahner
RC ed. Aquatic toxicology and hazard assessment: Seventh Symposium.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 53-72 (ASTM STP 854).
Terzic S & Ahel M (1993) Determination of linear alkylbenzene
sulphonates in Krka River estuary. Bull Environ Contam Toxicol, 50:
241-246.
Thatcher TO & Santner JF (1967) Acute toxicity of LAS to various fish
species. Proceedings of 21st Industrial Waste Conference, Purdue
University. Washington, Industrial Waste Association, pp 996-1002.
Thomas ORT & White GF (1989) Metabolic pathway for the biodegradation
of sodium dodecyl sulfate by pseudomonas sp. C12B. Biotechnol Appl
Biochem, 11: 318-327.
Thurman EM, Barber LB, & LeBlanc D (1986) Movement and fate of
detergents in groundwater: a field study. J Contam Hydrol, 1: 143-161.
Tiba S (1972) [Studies on the acute and chronic toxicity of linear
alkylbenzene sulfonate.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku
Zasshi], 13 (6): 509-516 (in Japanese).
Tiba S, Shiobara S, Imahori A, & Kitagawa T (1976) [Effects of linear
alkylbenzene sulfonate on dam, fetus, and newborn rat.] J Food Hyg Soc
Jpn [Shokuhin Eiseigaku Zasshi], 17 (1): 66-71 (in Japanese).
Tokai A, Kikuchi M, Wakabayashi M, & Yoshida T (1990) [Environmental
behavior of selected chemicals in Tokyo Bay with relation to their
physicochemical and biochemical properties.] J Chem Soc Jpn Chem Ind
Chem [Nippon Kagaku Kaishi], 9: 982-991 (in Japanese).
Tokyo Metropolitan Government (1974) [A study on detergents.] Tokyo,
Tokyo Metropolitan Government, 10 pp (Report No. 49-5-29) (in
Japanese).
Tomiyama S (1970) [Alph-olefin sulfonate: commercial production and
performance.] J Jpn Oil Chem Soc [Yukagaku], 19 (6): 359-368 (in
Japanese).
Tomiyama S (1972) [Current problems of detergents, mainly related to
water pollution.] J Jpn Oil Chem Soc [Yukagaku], 21 (1): 2-8 (in
Japanese).
Tomiyama S (1974) [Effects of surfactants on fish.] Bull Jpn Soc Sci
Fish, 40: 1291-1296 (in Japanese).
Tomiyama S (1975) Fundamental study of biochemical behavior of anionic
sulfonate- and sulfate-type surfactants. J Am Oil Chem Soc, 52:
135-138.
Tomiyama S (1978) [Possible mechanisms of the effects on fish and
shellfish of household detergents.] J Jpn Oil Chem Soc [Yukagaku], 27:
347-353 (in Japanese).
Tomizawa S (1978) Results of studies on synthetic detergents. Tokyo,
Science and Technology Agency, Research Coordination Bureau, pp 55-88.
Toshima Y, Moriya T, & Yoshimura K (1992) Effects of polyoxyethylene
(20) sorbitan monooleate on the acute toxicity of linear
alkylbenzenesulfonate (C12LAS) to fish. Ecotoxicol Environ Saf, 24:
26-36.
Tovell PWA, Newsome C, & Howes D (1974) Effect of water hardness on
the toxicity of an anionic detergent to fish. Water Res, 8: 291-296.
Tovell PWA, Howes D, & Newsome CS (1975) Absorption, metabolism and
excretion by goldfish of the anionic detergent sodium lauryl sulphate.
Toxicology, 4: 17-29.
Trehy ML, Gledhill WE, & Orth RG (1990) Determination of linear
alkylbenzenesulfonates and dialkyltetrasulfonates in waters and
sediment by gas chromatography/mass spectrometry. Anal Chem, 62:
2581-2586.
Tsai CF & McKee RA (1978) The toxicity to goldfish of mixtures of
chloramines, and LAS (linear alkylate sulfonate). College Park,
Maryland, University of Maryland, Water Resources Research Center, 36
pp (Technical Report No. 44; PB-280-554).
Tsukioka T & Murakami T (1983) [Determination of
linear-alkylbenzenesulfonate by gas chromatography.] Jpn Anal [Bunseki
Kagaku], 32: 723-728 (in Japanese).
Uchiyama M (1977) Determination of alkyl benzenesulfonate in water by
the UV method. Water Res, 11: 205-207.
Urano K & Saito M (1985) Biodegradability of surfactants and
inhibition of surfactants to biodegradation of other pollutants.
Chemosphere, 14: 1333-1342.
Urano K, Saito M, & Murata C (1984) Adsorption of surfactants on
sediments. Chemosphere, 13: 293-300.
United States Environmental Protection Agency (1981) Mono (C10-16)
alkylbenzenesulfonate acids and their sodium salts. Draft hazard
information review for TSCA Interagency Testing Committee (EPA
Contract No. 68-01-5789). Rockville, Maryland, Enviro Control,
101 pp.
Utsunomiya A, Itoh SI, Setsuda S, Naito S, & Shimozato T (1980)
[Studies on sodium linear alkylbenzenesulfonate (LAS). I. Distribution
of LAS in the sediments of the Sagami River estuary.] Eisei Kagaku,
26: 159-166 (in Japanese).
Utsunomiya A, Ikeda T, Takamatsu K, & Naito S (1982) [Determination of
sodium alkylsulfates as 3,5-dinitrobenzoate derivatives.] Jpn Anal
[Bunseki Kagaku], 31: 115-119 (in Japanese).
Van Emden HM, Kroon CCM, Schoeman EN, & Van Seventer HA (1974) The
toxicity of some detergents tested on Aedes aegypti L., Lebistes
reticulatus Peters, and Biomphalaria glabrata (Say). Environ
Pollut, 6: 297-308.
Van Scott EJ & Lyon JB (1953) A chemical measure of the effect of
soaps and detergents on the skin. J Invest Dermatol, 21: 199-203.
Verge C, Moreno A, Bravo J, Ferrer J, & Bengoechea C (1993) Toxicity
of LAS vs activated sludge of a WWTP and microalgae (Scenedesmus
subspicatus). Presented at Society of Environmental Toxicology and
Chemistry (SETAC) 1st World Congress, Lisbon, March 1993. New York,
Society of Environmental Toxicology and Chemistry.
Vives-Rego J, Vaque D, & Martinez J (1986) Effect of heavy metals and
surfactants on glucose metabolism, thymidine incorporation and
exoproteolytic activity in sea water. Water Res, 20: 1411-1415.
Vives-Rego J, Vaque MD, Leal JS, & Parra J (1987) Surfactants
biodegradation in sea water. Tenside Surfactants Deterg, 24: 20-22.
Volk VV & Jackson ML (1968) Alkyl benzene sulfonate and linear
alkylate sulfonate adsorption by hydroxy iron and aluminium systems.
J Water Pollut Control Fed, 40: 205-213.
Wagner R (1978) [The content of MBAS and BiAS in a communal sewage
treatment plant.] Wasser/Abwasser, 119: 235-242 (in German).
Wakabayashi M & Mizorogi N (1989) [Subacute toxicity of chemical
substances on rainbow trout.] Ann Rep Tokyo Metrop Res Inst Environ
Prot [Tokyo Kogai Kenkyujo Nempo], 1988: 167-169 (in Japanese).
Wakabayashi M & Onizuka S (1986) [Some factors affecting toxicity of
chemicals to fishes.] Ann Rep Tokyo Metrop Res Inst Environ Prot
[Tokyo Kogai Kenkyujo Nempo], 1986: 102-104 (in Japanese).
Wakabayashi M, Kikuchi M, Kojima H, & Yoshida T (1978) Bioaccumulation
profile of sodium linear alkylbenzene sulfonate and sodium alkyl
sulfate in carp. Chemosphere, 11: 917-924.
Wakabayashi M, Kikuchi M, Kojima H, & Yoshida T (1980) Effect of alkyl
chain on the uptake, distribution, and excretion of 35-labelled
alkyl sulfates in carp. Ecotoxicol Environ Saf, 4: 195-206.
Wakabayashi M, Kikuchi M, Sato A, & Yoshida T (1981) [The relationship
between exposure concentration and bioaccumulation of surfactants.]
Bull Jpn Soc Sci Fish, 47: 1383-1387 (in Japanese).
Wakabayashi M, Kikuchi M, Naganuma Y, & Kawakara H (1984) [Acute
toxicity of some detergents in fishes.] Ann Rep Tokyo Metrop Res Inst
Environ Prot [Tokyo Kogai Kenkyujo Nempo], 1984: 114-118 (in
Japanese).
Wakabayashi M, Konno R, & Nishiido T (1988) [Relative lethal
sensitivity of two daphnid species to some chemicals.] Ann Rep Tokyo
Metrop Res Inst Environ Prot [Tokyo Kogai Kenkyujo Nempo], 1988:
126-128 (in Japanese).
Waldmeyer T (1968) Analytical records of synthetic detergent
concentrations, 1956-1966. Water Pollut Control, 67 (2): 66-79.
Walker AIT, Brown VKH, Ferrigan LW, Pickering RG, & Williams DA (1967)
Toxicity of sodium lauryl sulphate, sodium lauryl ethoxysulphate and
corresponding surfactants derived from synthetic alcohols. Food Cosmet
Toxicol, 5: 763-769.
Ward TE (1986) Biodegradation of LAS and model chemicals in aerobic
and anaerobic sewage treatment processes and sludge-amended soils.
Abstracts of the 7th Annual Meeting of the Society of Environmental
Toxicology and Chemistry (SETAC), Arlington, USA, 6-9 November 1983.
New York, Society of Environmental Toxicology and Chemistry (Abstract
300).
Ward TE & Larson RJ (1989) Biodegradation kinetics of linear
alkylbenzene sulfonate in sludge-amended agricultural soils.
Ecotoxicol Environ Saf, 17: 119-130.
Watanabe Y, Nagashima T, & Imai N (1968) [Effect of detergents on
human skin in relation to hand eczema.] Saigai Igaku, 11 (9): 933-940
(in Japanese).
Watari N, Torizawa K, Kanai M, & Suzuki Y (1977) Ultrastructural
observations of the protective effect of glycyrrhizin for mouse liver
injury caused by oral administration of detergent ingredient(LAS). Jpn
J Clin Electron Microsc [Nihon Rinsho Denshikenbikyo Kaishi]), 10
(1-2): 121-139.
Waters J (1976) The contribution of linear alkylbenzene sulphonate to
the MBAS level of some UK and Dutch surface waters. Vom Wasser, 47:
131-140.
Waters J & Garrigan JT (1983) An improved microdesulphonation/gas
liquid chromatography procedure for the determination of linear
alkylbenzene sulphonates in UK rivers. Water Res, 17: 1549-1562.
Waters J, Holt MS, & Matthijs E (1989) Fate of LAS in sludge amended
soils. Tenside Surfactants Deterg, 26: 129-135.
Welp G & Brummer G (1985) [The Fe(III)-reduction test: a simple
procedure to determine the effects of environmental chemicals on
microbial activity in soils.] Z Pflanzenernähr Bodenkd, 148: 10-23 (in
German).
White GF, Russell NJ, & Day MJ (1985) A survey of sodium dodecyl
sulphate (SDS) resistance and alkylsulphatase production in bacteria
from clean and polluted river sites. Environ Pollut, A37: 1-11.
White GF, Anderson DJ, Day MJ, & Russell NJ (1989) Distribution of
planktonic bacteria capable of degrading sodium dodecyl sulphate (SDS)
in a polluted South Wales river. Environ Pollut, 57: 103-115.
Williamson R (1993) Alpha olefin sulfonate composition. Chem Week, 27
January: 13.
Wood DCF & Bettley FR (1971) The effect of various detergents on human
epidermis. Br J Dermatol, 84: 320-325.
Yakabe Y, Etoh C, Matsunobo Y, Katsuura H, Miura K, & Yoshimura K
(1992) Kinetic study on the biodegradation of linear
alkybenzenesulfonates (LAS) in well water. Chemosphere, 24 (8):
969-977.
Yamamoto H (1977) [Carcinogenic and co-carcinogenic testing of
detergents (alkyl-sulfonic-natrium, polyoxy-ethylene-alkylether and
potassium soap) on the skin of mice.] J Nara Med Assoc [Nara Igaku
Zasshi], 28: 307-327 (in Japanese).
Yamamoto K & Motomizu S (1987) [Solvent extraction with
spectrophotometric determination of anionic surfactants in seawater.]
Jpn Anal [Bunseki Kagaku], 36: 335-338 (in Japanese).
Yamamoto H, Inoue K, Hamano Y, Oda Y, Kuwano A, Mitsuda B, & Kunita N
(1976) [Studies on toxicity of commercial detergents. 3rd Report.
Teratogenic effects in mice administered orally.] Ann Rep Osaka
Prefect Inst Public Heath, 7: 141-145 (in Japanese).
Yamane I, Nagayama M, Kashiwa I, Kuwamura H, Ando S, & Mori A (1970)
[Olefin sulfonates. IV. Correlation between carbon chain length and
surface-active properties of alpha-olefin sulfonates.] J Chem Soc Jpn
[Kogyo Kagaku Zasshi], 73 (4): 723-727 (in Japanese).
Yamane AN, Okada M, & Sudo R (1984) The growth inhibition of
planktonic algae due to surfactants used in washing agents. Water Res,
18: 1101-1105.
Yanagisawa F, Watanabe S, & Yamagishi T (1964) [Biochemical studies of
dodecylbenzene sulfonates; differences between soft and hard
detergents.] Jpn J Public Health [Nihon Koshueisei Zasshi], 11 (13):
859-864 (in Japanese).
Yasuda H (1980) [Determination of anionic surfactants in sea and river
waters by anion exchange method.] Jpn J Public Health [Nihon
Koshueisei Zasshi], 27 (8): 373-378 (in Japanese).
Yasunaga Y (1976) [The influence of some pollutants on the survival of
eggs and larvae of two species of flatfish, Limanda yokohamae and
Paralichtys olivaceus.] Bull Tokai Reg Fish Res Lab, 86: 81-111 (in
Japanese).
Yediler A, Zhang Y, Cai JP, & Korte F (1989) Effect of the microbial
population size on the degradation of linear alkylbenzene sulfonate in
lake water. Chemosphere, 18: 1589-1597.
Yokoyama Y & Sato H (1991) Reversed-phase high-performance liquid
chromatographic determination of linear alkylbenzenesulponates in
river water at ppb levels by precolumn concentration. J Chromatogr,
555: 155-162.
Yoneyama M & Hiraga K (1977) [Effect of linear alkylbenzene sulfonate
on serum lipid in rats.] Ann Rep Tokyo Metrop Res Lab Public Health,
28 (2): 109-111 (in Japanese).
Yoneyama M, Fujii T, Ikawa M, Shiba H, Sakamoto Y, Yano N, Kobayashi
H, Ichikawa H, & Hiraga K (1972) [Studies on the toxicity of synthetic
detergents. (II) Subacute toxicity of linear and branched alkyl
benzene sulfonates in rats.] Ann Rep Tokyo Metrop Res Lab Public
Health, 24: 409-440 (in Japanese).
Yoneyama M, Mabuchi Y, Ikawa M, Kobayashi H, & Ichikawa H (1976)
[Subacute toxicity of linear alkylbenzene sulfonate.] Ann Rep Tokyo
Metrop Res Lab Public Health, 27 (2): 105-112 (in Japanese).
Yoneyama M, Masubuchi M, Oishi S, Takahashi O, Ikawa M, Yoshida S,
Oishi H, Mikuriya H, Yuzawa K, & Hiraga K (1977) [Toxicity of linear
alkylbenzene sulfonate by dietary administration for life-span to
rats.] Ann Rep Tokyo Metrop Res Lab Public Heath, 28 (2): 73-84 (in
Japanese).
Yoneyama M, Ikawa M, Nakao T, & Hiraga K (1978) [Effects of LAS on
incorporation of acetate-1-14C in liver lipids in rats.] Ann Rep
Tokyo Metrop Res Lab Public Health, 29 (2): 55-57 (in Japanese).
Yoshida H & Takeshita R (1978) [Studies on anion active surfactants:
II; Contamination of anionic surfactants in the Inland Sea of eastern
Seto.] Eisei Kagaku, 24: 78-82 (in Japanese).
Yoshikawa S, Sano H, & Harada T (1984) [Determination of LAS in river
water by high-performance liquid chromatography.] Suishitsu Odaku
Kenkyu, 7: 191-194 (in Japanese).
Yoshikawa S, Sano H, & Harada T (1985) [Distribution of LAS in river
water and sediment at Tsurumi River.] Jpn J Water Pollut Res, 8:
755-758 (in Japanese).
Yoshimura H, Tanaka S, Fujiyama Y, Sugiyama T, & Nagai T (1984a)
[Determination of ionic surfactants by high performance liquid
chromatography.] J Chem Soc Jpn [Nihon Kagaku Kaishi], 3: 445-451 (in
Japanese).
Yoshimura K, Hayashi K, Kawase J, & Tsuji K (1984b) [Presence of
anionic surfactants in rivers.] Jpn J Limnol [Rikusuigaku Zasshi], 45:
51-60 (in Japanese).
Yushi (1978) Good decomposition of LAS together with detection of ABS.
Water quality and synthetic detergents from the standpoint of a 1977
study. Yushi, 31: 30-33 (in Japanese)
Zaccone G, Fasulo S, Lo Cascio P, & Licata A (1985) Patterns of enzyme
activities in the gills of the catfish Heteropneustes fossilis
(Bloch) exposed to the anion-active detergent
Na-alkyl-benzenesulphonate (LAS). Histochemistry, 82: 341-343.
Zoller U (1985) The 'hard' and 'soft' surfactant profile of Israel
municipal wastewaters. J Am Oil Chem Soc, 62: 1006-1009.
ALKYLBENZENESULFATES A CHAINE DROITE ET COMPOSES VOISINS
1. RECAPITULATION, EVALUATION ET RECOMMANDATIONS GENERALES
1.1 Identité, propriété et méthodes d'analyse
Les alkylbenzènesulfonates à chaîne droite (appelé aussi
alkylbenzènesulfonates linéaires ou ASL, les alpha-oléfine-
sulfonates (AOS) et les alkylsulfates (AS)) sont des tensio-actifs
anioniques dont les molécules sont caractérisées par la présence d'un
groupement hydrophobe et d'un groupement hydrophile (polaire). Les
produits du commerce sont des mélanges d'isomères et d'homologues de
produits voisins, qui different par leurs propriétés physicochimiques
et qui, sous leurs diverses formes, ont des applications variées.
L'analyse des ASL, des AOS et des AS peut se faire par des
méthodes non spécifiques. On utilise généralement l'essai au bleu de
méthylène, qui permet de mettre en évidence tout composé contenant un
groupement anionique et un groupement hydrophobe. On peut donc être
gêné par la présence d'autres substances lorsqu'on travaille sur des
échantillons prélevés dans l'environnement; en outre, la sensibilité
de la méthode n'est que de 0,02 mg/litre. On a mis au point d'autres
méthodes non spécifiques qui peuvent se substituer à celles-ci mais on
ne les utilise guère. En ce qui concerne les échantillons prélevés
dans l'environnement, il n'existe de méthodes spécifiques que pour les
ASL et les AS. En ce qui concerne les AOS, on dispose d'une méthode
améliorée qui repose sur la réaction au bleu de méthylène et la
chromatographie en phase liquide à haute performance (HPLC).
Les ASL sont des composés non volatils que l'on obtient par
sulfonation des alkylbenzènes à chaîne droite. Les produits du
commerce sont toujours constitués de mélanges d'homologues ayant des
chaînes alkylées de différentes longueurs (C10-C13 ou C14) et
d'isomères qui différent par la position du point d'attache de la
chaine sur le noyau phényle (positions 2 à 5). Tous les homologues et
isomères des ASL peuvent être dosés dans des échantillons
environnementaux ou d'autres matrices au moyen de méthodes d'analyse
spécifiques comme la HPLC, la chromatographie en phase gazeuse et la
chromatographie en phase gazeuse couplée à la spectrométrie de masse.
Les AOS sont également des dérivés non volatils produit par
sulfonation des alpha-oléfines. Ils consistent dans le mélange de deux
types de composés, les alcène-sulfonates de sodium et les
hydroxyalcane-sulfonates de sodium, avec une chaine alkylée en
C14-C18.
Egalement non volatils, les AS s'obtiennent en traitant par
l'acide sulfurique, les alcools d'origine oléochimique ou
pétrochimique. Ce sont des mélanges d'homologues avec une chaîne
alkylée en C10-C18. On met actuellement au point des méthodes
d'analyse spécifiques pour la surveillance de l'environnement.
1.2 Sources d'exposition humaine et environnementale
On utilise les ASL, les AOS et les AS comme principes actifs de
divers produits d'entretien ou d'hygiène personnelle, ou encore, pour
certaines applications spéciales. Après usage, ces détergents sont
rejetés dans l'environnement avec les eaux usées.
Il peut y avoir exposition professionnelle à ces composés. Quant
à l'exposition de la population humaine en général et des êtres
vivants dans leur milieu naturel, elle dépend du type d'application de
ces substances (ou d'autres tensio-actifs), des pratiques locales en
matière de traitement des effluents et des caractéristiques du milieu
récepteur.
En 1990, la consommation mondiale de ces produits d'établissait à
2 millions de tonnes pour les ASL, 86 000 tonnes pour le AOS et 289
000 tonnes pour les AS.
1.3 Concentrations dans l'environnement
1.3.1 Alkylbenzènesulfonates à chaîne droite
On peut doser les ASL à l'aide de méthodes spécifiques et
sensibles dans pratiquement tous les compartiments du milieu où ils
sont susceptibles de se trouver. Leur concentration diminue
progressivement selon la séquence suivante: eaux usées > effluents
traités > eaux de surface > mer.
Dans les zones où l'on utilise principalement des ASL comme
tensio-actifs, leur concentration est généralement de 1-10 mg/litre
dans les eaux usées, de 0,05-0,1 mg/litre dans les effluents traités
par voie biologique, de 0,05-0,6 mg/litre dans les effluents traités
sur lit filtrant, de 0,005-0,05 mg/litre dans les eaux de surface
situées au-dessous de déversoirs d'égouts (avec des concentrations qui
tombent rapidement à 0,01 mg/litre en aval du déversoir), de < 1-10
mg/kg dans les sédiments de cours d'eau (< 100 mg/kg dans les
sédiments très pollués à proximité des zones de décharge), de 1-10
g/kg dans les boues d'égouts, de < 1 5 mg/kg dans les sols amendés
à l'aide de boues d'égouts (initialement 5-10 mg/kg; on a trouvé des
concentrations < 50 mg/kg après d'importants épandages de boues,
d'ailleurs non représentatifs). La concentration des ASL dans les eaux
estuarielles varie de 0,001 à 0,01 mg/litre, mais elle peut être
beaucoup plus élevée là où il y a déversement direct d'eaux usées. En
mer, à distance du rivage, les concentrations vont de < 0,001 à
0,002 mg/litre.
Il est à noter que la concentration de ASL varie considérablement
dans l'environnement. Ces variations sont dues à la diversité des
méthodes d'analyse, des points de prélèvement (qui vont de zones très
polluées où sont déversés des effluents insuffisamment traités à des
secteurs où l'effluent a subi un traitement intensif), des périodes de
prélèvement (ce qui selon le cas peut signifier une différence du
simple au double) et enfin, des volumes de ASL consommés.
La surveillance de l'environnement montre que les ASL ne
s'accumulent pas au cours du temps dans les différents compartiments
du milieu. La concentration dans le sol, loin d'augmenter, diminue au
contraire par suite de la minéralisation. Comme les ASL ne se
décomposent pas en anaérobiose stricte (pour donner naissance à du
méthane), on ne peut pas en conclure qu'ils subissent une
minéralisation dans les sédiments anaérobies. Au taux actuel
d'utilisation, les ASL parviennent dans les différents compartiments
de l'environnement à un rythme sensiblement égal à celui de leur
assimilation, ce qui crée les conditions d'un état stationnaire.
1.3.2 Les alpha-oléfine-sulfonates et alkyle-sulfates
Les données dont on dispose sur la concentration des AOS dans
l'environnement sont limitées en raison de la difficulté à analyser
les échantillons prélevés dans le milieu. En général, on peut déceler
la présence des tensio-actifs anioniques au moyen de méthodes
colorimétriques non spécifiques (comme celles qui sont basées sur la
réaction au bleu de méthylène), mais la présence d'autres substances
est gênante et ces méthodes ne permettent pas de procéder à un dosage
spécifique des alpha-oléfine-sulfonates. Une méthode spécifique de
dosage des AS dans l'environnement est en cours de mise au point.
Les études effectuées en laboratoire indiquent que les AOS et les
AS sont rapidement minéralisés dans tous les compartiments de
l'environnement et presque totalement éliminés des effluents au cours
du traitement de ces derniers. Leur concentration dans les eaux de
surface, les sédiments, le sol, les eaux estuarielles et le milieu
marin est probablement faible. C'est précisément ce que l'on a
constaté pour la concentration des AOS dans l'eau des rivières.
1.4 Transport, distribution et transformation dans l'environnement
Aux températures inférieures à 5-10°C, la cinétique de
biodégradation des ASL, des AOS et des AS est ralentie en raison de la
réduction de l'activité microbienne.
1.4.1 Alkylbenzène-sulfonates à chaîne droite
Les voies de pénétration des ASL dans l'environnement varient
selon les pays, mais la porte d'entrée principale est constituée par
la décharge des stations d'épuration des eaux usées. Lorsque ces
stations sont inexistantes ou fonctionnent mal, il peut y avoir
décharge directe dans les rivières, les lacs et la mer. L'épandage de
boues d'égout sur les terrains agricoles peut également constituer une
voie de pénétration de ASL dans l'environnement.
A mesure qu'ils pénètrent dans l'environnement, les ASL en sont
éliminés par divers mécanismes qui vont de l'adsorption à la
biodégradation ultime. Les ASL sont adsorbés sur les surfaces
colloïdales et les particules en suspension, et l'on a mesuré des
coefficients d'adsorption de 40-5200 litres/kg selon le milieu et la
structure des ASL en cause. Ils subissent une biodécomposition dans
les eaux de surface (demi-vie 1-2 jours), dans les sédiments aérobies
(1-3 jours) ainsi que dans les écosystèmes marins et estuariels (5-10
jours).
Lors du traitement primaire des effluents, environ 25% des ASL (de
10-40%) s'adsorbent sur les boues résiduelles et sont rejetés avec
elles. Ils ne sont pas éliminés au cours de la digestion anaérobie des
boues mais au cours du traitement aérobie, leur demi-vie étant alors
de 10 jours. Après épandage des boues sur le sol, les ASL sont
générale-ment décomposés à hauteur de 90% en l'espace de trois mois,
la demi-vie étant de l'ordre de 5-30 jours.
Le facteur de concentration des ASL dans le corps entier varie de
100 à 300 pour l'ensemble des 14C-ASL et 14C-métabolites. Ils sont
captés par les poissons essentiellement à travers les branchies et se
répartissent ensuite dans le foie et la vésicule après
biotransformation. Les ASL sont rapidement excrétés et rien n'indique
par conséquent qu'ils subissent une bioamplification.
1.4.2 alpha-Oléfine-sulfonates
Les données relatives au transport, à la distribution et à la
transformation des AOS dans l'environnement sont encore moins
nombreuses que dans le cas des ASL. On peut toutefois penser que les
AOS sont transportées dans l'environnement à peu près comme les ASL,
les AS et les autres détergents tensio-actifs et que leur destinée y
est analogue à celles des ASL et des AS. En aérobiose, elles subissent
une biodécomposition rapide et cette biodécomposition primaire est
achevée en 2 à 10 jours, en fonction de la température. On ne
dispose que de données limitées sur la bioaccumulation des AOS; en
tout état de cause elles ne s'accumulent pas chez les poissons. On ne
dispose d'aucune donnée sur leur décomposition en milieu abiotique.
1.4.3 Alkylsulfates
Les AS sont transportés dans l'environnement par des mécanismes
analogues à ceux qui sont à l'oeuvre dans le cas des ASL et des AOS.
Ils sont facilement biodégradable en aérobiose ou en anaérobiose, que
ce soit au laboratoire ou dans l'environnement; la biodécomposition
primaire est achevée en l'espace de 2 à 5 jours. Les facteurs de
bioconcentration pour le corps entier varient de 2 à 73 ainsi qu'avec
la longueur de la chaîne des différents homologues. Chez les poissons,
les AS sont captés, distribués, biotransformés et excrétés de la même
manière que les ASL et ne se concentrent pas dans les autres
organismes aquatiques.
1.5 Cinétique
Les ASL, les AOS et le AS sont facilement résorbés dans les voies
digestives, après quoi ils se répartissent dans l'ensemble de
l'organisme où ils sont largement métabolisés. Les ASL subissent une
omega- et une ß-oxydation. Les composés initiaux et leurs métabolites
sont principalement excrétés par la voie rénale, encore qu'une
certaine proportion de la dose absorbée puisse l'être également pas la
voie fécale, après métabolisation et passage dans les voies biliaires.
Les ASL, les AOS et les AS ne sont absorbés qu'en quantités minimes
par voie percutanée lorsque la peau est intacte, mais un contact
prolongé peut altérer l'intégrité de la barrière épidermique, ce qui
permet une résorption plus importante; à fortes concentrations, il
peut y avoir réduction du temps de pénétration.
1.6 Effets sur les animaux de laboratoire et sur les systèmes
d'épreuve in vitro
On a relevé, pour la DL50 des sels de sodium des ASL, des
valeurs allant de 404 à 1470 mg/kg de poids corporel chez le rat et
de 1259 à 2300 mg/kg de poids corporel chez la souris, ce qui incite
à penser que les rats sont plus sensibles que les souris à l'action
toxique des ASL. Chez la souris, on a obtenu une DL50 de 3000 mg/kg
de poids corporel pour un sel de sodium d'AOS. Chez le rat, les
valeurs de la DL50 par voie orale allaient de 1000 à 4120 mg/kg
de poids corporel pour les AS. Les ASL, les AOS et les AS sont
irritants pour la peau et les yeux.
Lors d'études subchroniques au cours desquelles on a administré à
des rats des ASL dans leur nourriture ou leur eau de boisson à des
concentrations quotidiennes correspondant à plus de 120 mg/kg de
poids corporel, on a observé des effets minimes, qui consistaient
notamment en modifications des paramètres biochimiques et altérations
histopathologiques au niveau du foie. Bien que lors d'une étude, on
ait observé des modifications ultrastructurales dans les hépatocytes
à des doses plus faibles, ces modifications se sont révélées
réversibles. D'ailleurs, les autres études n'ont pas révélé de tels
effets aux mêmes doses, mais il n'est pas exclu que lors de l'étude
initiale, les organes aient fait l'objet d'un examen plus minutieux.
Des effets ont également été observés sur la fonction de reproduction
chez des animaux auxquels on avait administré des doses quotidiennes
> 300 mg/kg; il s'agissait d'une réduction du taux de grossesse et
d'une certaine mortalité dans les portées. Après application cutanée
de longue durée à des rats de solutions de ASL à plus de 5% et
application, également cutanée, du même type de solution à des cobayes
à raison de 60 mg/kg de poids corporel pendant 30 jours, on a observé
des modifications biochimiques et histopathologiques. Des applications
cutanées répétées de solutions de teneur > 0,3% de ASL ont
produit des effets toxiques sur les foetus ainsi que sur la
reproduction, mais les doses étaient également toxiques pour les
femelles gestantes.
On n'a guère de données résultant d'études sur des animaux de
laboratoire qui permettraient d'évaluer les effets potentiels des AOS
chez l'homme. Aucun effet n'a été observé sur des rats ayant reçu,
pendant une longue durée, des doses quotidiennes de 250 mg/kg de poids
corporel en administration orale; toutefois une dose quotidienne de
300 mg/kg de poids corporel, toxique pour les femelles gestantes, a
entraîné des effets foetotoxiques chez des lapins. L'application
topique d'AOS sur la peau et les yeux de divers animaux de laboratoire
a produit des effets localisés.
Les effets d'une exposition à long et à court terme aux AS ont été
étudiés à plusieurs occasions sur l'animal mais la plupart des études
en question pêchent par les insuffisances des examens
histopathologiques ou la trop petite taille des groupes; en outre, les
doses les plus élevées utilisées dans les études à long terme n'ont
pas produit le moindre effet toxique, de sorte qu'il n'a pas été
possible d'établir la valeur de la dose sans effets nocifs
observables. Cependant, lorsqu'on a administré à des rats des
concentrations quotidiennes de ces substances correspondant à
200 mg/kg de poids corporel ou davantage, par incorporation à leur
nourriture ou à leur eau de boisson, on a systématiquement observé un
certain nombre d'effets. En outre, l'application topique sur la peau
ou les yeux d'AS à des concentrations égales ou supérieures à environ
0,5%, a donné lieu à une irritation localisée. Par ailleurs à fortes
concentrations, on observe des effets toxiques sur les femelles
gestantes ainsi que sur les foetus.
La plupart des études à long terme ne se prêtent pas à
l'évaluation du pouvoir cancérogène des ASL, des AOS et des AS chez
l'animal de laboratoire en raison de facteurs tel que le nombre trop
faible d'animaux, un nombre de doses limité, la non détermination de
la dose tolérée maximale, et, en outre, un examen histopathologique
limité dans la majorité des cas. Dans les travaux où les effets
anatomo-pathologiques ont été convenablement étudiés, on n'a pas
déterminé la dose tolérée maximale et les doses employées n'ont pas
produit d'effets toxiques. Toutefois et compte tenu de ces réserves,
on peut retenir que les études au cours desquelles on a administré à
des animaux des ASL, des AOS et des AS par voie orale, n'ont pas
révélé de signes de cancérogénicité; quant aux études à long terme
consistant en applications topiques d'AOS par badigeonnage cutané,
elles n'ont pas non plus révélé la présence d'effets imputables à ces
substances.
Sur la base de ces données limitées, il ne semble pas que ces
composés soient génotoxiques in vivo ou in vitro.
1.7 Effets sur l'homme
L'application d'un timbre cutané imprégné de solution contenant
jusqu'à 1% de ASL, d'AOS ou d'AS pendant 24 heures montre que la peau
humaine supporte le contact avec cette substance au prix d'une légère
irritation. Ces tensio-actifs provoquent une délipidation de
l'épiderme, une élution du facteur d'humidification naturelle, ainsi
qu'une dénaturation des protéines de la couche épidermique externe,
dont ils augmentent la perméabilité et dont ils provoquent le
gonflement. Ni les ASL, ni les AOS, ni les AS n'ont provoqué de
sensibilisation cutanée chez les volontaires et rien n'indique de
façon concluante qu'ils puissent provoquer un eczéma. On n'a pas
signalé de lésions graves ou mortelles consécutives à l'ingestion
accidentelle de ces tensio-actifs.
1.8 Effets sur l'environnement
1.8.1 Alkylbenzène-sulfonates à chaîne droite
1.8.1.1 Milieu aquatique
Les ASL ont été très largement étudiés tant au laboratoire (études
à court et à long terme) que dans des conditions plus proches de la
réalité (études sur le micro- et le mésocosme et études en situation
réelle). En général, la diminution de la longueur de la chaîne alkylée
ou une plus grande intériorisation du groupement phényle
s'accompagnent d'une diminution de la toxicité. Les observations
effectuées sur des poissons et sur des daphnies montrent que lorsque
la longueur de la chaîne diminue d'une unité (par exemple lorsqu'elle
passe de C12 à C11), la toxicité est approximativement divisée par
deux.
Les résultats des tests en laboratoire sont les suivants:
-- Microorganismes: Les résultats sont très variables en raison
de l'utilisation de systèmes d'épreuve très divers (par exemple
inhibition des boues activées, cultures mixtes et espèces
individuelles). Les valeurs de la CE50 vont de 0,5 mg/litre (une
seul espèce) à > 1000 mg/litre. Dans le cas des microorganismes, il
n'existe pas de relation linéaire entre la longueur de la chaîne et la
toxicité.
-- Plantes aquatiques: Les résultats dépendent largement de
l'espèce. En ce qui concerne les plantes d'eau douce, les valeurs de
la CE50 se situent entre 10 et 235 mg/litre (C10-C14), dans le
cas des algues vertes; entre 5 et 56 mg/litre (C11,1-C13), dans le
cas des algues bleu-vert; entre 1,4 et 50 mg/litre (C11,6-C13)
pour les diatomées et entre 2,7 et 4,9 mg/litre (C11,8) pour les
macrophytes. Il semble que les algues marines soient même encore plus
sensibles. Dans le cas des algues, il n'y a probablement pas non plus
de relation linéaire entre la longueur de la chaine et la toxicité.
-- Invertébrés: Les valeurs de la CE50 et de la CL50
(exposition aiguë) pour au moins 22 espèces d'eau douce se situent
entre les limites suivantes: 4,6-200 mg/litre (longueur de chaine non
précisée; C13) dans le cas des mollusques; 0,12-27 mg/litre
(longueur de chaine non précisée; C11,2-C18) dans le cas des
crustacés; 1,7-16 mg/litre (longueur de chaine non précisée; C11,8)
dans le cas des vers et enfin 1,4-270 mg/litre (C10-C15) dans le
cas des insectes. Dans le cas d'une exposition chronique, les valeurs
de la CE50 et de la CL50 sont de 2,2 mg/litre (C11,8) pour les
insectes et de 1,1-2,3 mg/litre (C11,8-C13) pour les crustacés. La
concentration sans effets chroniques observables (basée sur la
mortalité ou des effets sur la fonction de reproduction) est de 0,2 à
10 mg/litre (longueur de chaîne non précisée; C11,8) pour les
crustacés. Il semble que les invertébrés marins soient plus sensibles,
avec des valeurs de la CL50 allant de 1 à plus de 100 mg/litre (dans
presque tous les cas, C12) pour 13 espèces et avec une concentration
sans effets observables de 0,025 à 0,4 mg/litre (longueur de chaine
non précisée dans l'ensemble des tests) dans le cas des sept espèces
étudiées
-- Poissons: Pour 21 espèces d'eau douce, les valeurs de la
CL50 aiguë se situent entre 0,1 et 125 mg/litre (C8-C15); les
valeurs de la CE50 et/ou de la CL50 pour une exposition chronique
sont, pour deux espèces, respectivement égales à 2,4 et à 11 mg/litre
(longueur de chaîne non spécifiée; C11,7); quant à la concentration
sans effets observables, elle va de 0,11-8,4 à 1,8 mg/litre (longueur
de chaine non précisée; C11,2-C13) pour deux espèces. Dans ce cas
encore, les poissons de mer se révèlent plus sensibles, avec des
valeurs de la CL50 aiguë allant de 0,05 à 7 mg/litre (longueur de
chaîne non spécifiée; C11,7) pour six espèces et des valeurs de la
CL50 chronique allant de 0,01 à 1 mg/litre (longueur de chaîne non
précisée) pour deux espèces. Dans la plupart des publications, la
longueur de la chaine n'est pas précisée. Pour des espèces marines, on
a également fait état d'une concentration sans effets observables <
0,02 mg/litre (C12).
Les produits communément utilisés dans le commerce ont en moyenne,
une chaîne latérale en C12. Des composés ayant diverses longueurs de
chaîne ont été étudiés sur Daphnia magna et sur des poissons, mais
dans le cas des autres organismes d'eau douce, c'est en général des
composés dont la longueur de chaine moyenne est de C11,8 qui ont été
utilisés. Les valeurs caractéristiques de la CE50 et de la CL50 aiguë
pour les ASL en C12 sont 3-6 mg/litre chez Daphnia magna et
2-15 mg/litre chez les poissons d'eau douce; celles de la
concentration sans effets observables pour une exposition chronique
sont de 1,2 à 3,2 mg/litre pour Daphnia magna et de 0,48-0,9
mg/litre pour les poissons d'eau douce. Chez les poissons de mer, les
valeurs caractéristiques de la CL50 aiguë pour des ASL en C12
sont de < 1-6,7 mg/litre.
Les organismes halophiles et en particulier les invertébrés, se
révèlent être plus sensibles aux ASL que les organismes d'eau douce.
Chez les invertébrés, l'action séquestrante des ASL sur le calcium
peut affecter la biodisponibilité de cet ion pour la morphogénèse. Les
ASL exercent un effet général sur le transport ionique. Les produits
de biodécomposition et les sous-produits des ASL sont 10 à 100 fois
plus toxiques que les composés de départ.
Les résultats obtenus dans des conditions plus proches de la
réalité sont les suivants: on a étudié les ASL au moyen de toute sorte
de tests en eau douce et à plusieurs niveaux trophiques, notamment
dans des enceintes lacustres (organismes inférieurs), dans des
écosystèmes modèles (sédiments et réseaux hydrographiques), des cours
d'eau en aval et en amont des déversoirs de stations d'épuration des
eaux usées et enfin, des cours d'eau expérimentaux. Dans presque tous
les cas on a utilisé des ASL en C12. Les algues se sont révélées
être plus sensibles en été qu'en hiver, les valeurs de la CL50 à 3
heures étant de 0,2 à 8,1 mg/litre après la photosynthèse, alors que
dans les écosystèmes modèles, on n'observait aucun effet sur
l'abondance relative des populations d'algues à la concentration de
0,35 mg/litre. Selon ces études, la valeur de la concentration sans
effets observables se situe de 0,24 à 5 mg/litre selon l'organisme et
le paramètre étudié. Ces résultats sont en assez bon accord avec ceux
des épreuves en laboratoire.
1.8.1.2 Milieu terrestre
On dispose de données sur les végétaux et les lombrics. Pour sept
espèces de plantes étudiées dans des solutions nutritives, on a obtenu
des valeurs de la concentration sans effets observables qui se situent
dans les limites < 10-20 mg/litre; pour trois espèces étudiées sur
sol d'après leur croissance, on a obtenu 100 mg/kg (C10-C13). Pour
les lombrics, la CL50 à 14 jours était > 1000 mg/kg.
1.8.1.3 Oiseaux
Une étude sur des poulets qui recevaient une nourriture contenant
de ces substances, a permis de fixer à > 200 mg/kg la dose sans
effets observables (d'après la qualité des oeufs).
1.8.2 alpha-Oléfine-sulfonates
On dispose de données limitées concernant les effets des AOS sur
les organismes aquatiques et terrestres.
1.8.2.1 Milieu aquatique
On ne dispose que des résultats des épreuves en laboratoire:
-- Algues: Valeur de la CE50 : > 20-65 mg/litre (C16-C18)
pour les algues vertes
-- Invertébrés: Valeur de la CL50 : 19 et 26 mg/litre
(C16-C18) pour la daphnie
-- Poissons: Pour neuf espèces de poissons on a obtenu des
valeurs de la CL50 aiguë de 0,3-6,8 mg/litre (C12-C18). Sur la
base d'études à court terme effectuées sur la truite de mer (Salmo
trutta), l'ide rouge (Idus melanotus) et le rasbora (Rasbora
heteromorpha), on peut conclure que la toxicité des composés en
C14-C16 est environ cinq fois plus faible que celle des composés
en C16-C18, avec des valeurs de la CL50 (à toutes les
concentrations mesurées) de 0,5-3,1 (C16-C18) et de
2,5-5,0 mg/litre (C14-C16). Deux études à long terme effectuées
sur la truite arc-en-ciel ont montré que le paramètre le plus sensible
était la croissance, et qu'il permettait d'obtenir une CE50 de
0,35 mg/litre. Pour ce qui est des poissons de mer, on a obtenu pour
le mulet gris ou muge (Mugal cephalus), une valeur de la CL50 à 96
heures de 0,70 mg/litre.
1.8.2.2 Milieu terrestre
Une étude portant sur des végétaux en solution nutritive a montré
que la concentration sans effets observables se situait dans les
limites 32-56 mg/litre. Dans une autre étude, portant cette fois sur
des poulets qui recevaient les AOS dans leur nourriture, on a obtenu
une valeur > 200 mg/kg pour la concentration sans effets observables
(d'après la qualité des oeufs).
1.8.3 Alkyl-sulfates
1.8.3.1 Organismes aquatiques
Les AS ont fait l'objet d'études à court et à long terme et d'une
étude dans des conditions plus proches de la réalité. On constate
encore que leur toxicité dépend de la longueur de la chaîne latérale
alkylée; par contre on ne dispose d'aucune donnée qui indiquerait
l'existence d'une différence de toxicité entre les AS à chaine droite
et les AS à chaîne ramifiée.
Les résultats des épreuves de laboratoire sont les suivants:
-- Microorganismes: Les valeurs de la CE50 dans une communauté
marine étaient de 2,1-4,1 mg/litre (C12). Pour Pseudomonas putida,
les concentrations sans effets observables étaient de 35-550 mg/litre
(C16-C18).
-- Végétaux aquatiques: Les valeurs de la CE50 s'établissaient
comme suit: > 20-65 mg/litre (C12-C13) pour les algues vertes et
18-43 mg/litre (C12) pour les macrophytes. Les concentrations sans
effets observables s'établissaient à 14-26 mg/litre (C12-C16/C18)
chez les algues vertes.
-- Invertébrés: Les valeurs de la CE50 et de la CL50 se
situaient entre 4 et 140 mg/litre (C12/C15-C16/C18) pour les
espèces d'eau douce et entre 1,7 et 56 mg/litre (tous les composés en
C12) chez les espèces marines. La concentration sans effets
observables pour Daphnia magna était de 16,5 mg/litre (C16/C18)
en exposition chronique, les valeurs se situant entre 0,29 et
0,73 mg/litre (longueur de chaine non précisée) pour les espèces marines.
-- Poissons: Les valeurs de la CL50 se situaient entre 0,5 et
5,1 mg/litre (longueur de chaine non précisée ou C12-C16) pour des
espèces d'eau douce et entre 6,4 et 16 mg/litre (tous les composés en
C12) pour les espèces marines. On n'a pas eu connaissance d'études
à long terme.
Il est à noter que nombre de ces travaux ont été effectués dans
des conditions statiques. Comme les AS sont facilement biodégradables,
il est possible qu'on en ait sous estimé la toxicité. Lors d'une étude
de 48 heures sur Oryzias latipes, on a obtenu pour la CL50 des
valeurs respectivement égales à 46, 2,5 et 0,61 mg/litre (mesures de
concentrations) pour des composés en C12, C14 et C16. Cette
étude et d'autres, montrent que la toxicité s'accroît d'un facteur 5
lorsque la longueur de la chaîne augmente de deux unités. Une étude
dynamique sur une biocénose, avec des composés en C16-C18 a permis
d'obtenir une concentration sans effets observables de 0,55 mg/litre.
1.8.3.2 Organismes terrestres
On a fait état, pour les lombrics et les navets, de concentrations
sans effets observables de valeur > 1000 mg/kg (C16-C18).
1.9 Evaluation des risques pour la santé humaine
Les ASL sont les tensio-actifs les plus largement utilisés pour la
fabrication de détergents et de produits de nettoyage; les AOS et les
AS entrent également dans la composition des détergents et des
produits destinés à l'hygiène personelle. La principale voie
d'exposition humaine est donc le contact cutané. Cependant de petites
quantités de ASL, d'AOS et d'AS peuvent être ingérées avec l'eau de
boisson ou sous forme de résidus subsistant sur les ustentsiles de
cuisine et dans les aliments. Bien que les données sur ce point soient
limitées, on peut estimer à environ 5 mg/personne la quantité de ASL
ingérée quotidiennement de cette manière. Quant à l'exposition
professionnelle à ces trois catégories de produits, elle peut
intervenir lors de la préparation des différentes substances qui en
contiennent, mais on ne dispose d'aucune donnée sur les effets qu'une
exposition chronique à ces composés pourrait avoir sur l'homme.
Les ASL, les AOS et les AS peuvent irriter la peau par suite d'un
contact répété ou prolongé aux concentrations qui sont celles des
produits non dilués. Chez le cobaye, les AOS peuvent provoquer une
sensibilisation cutanée lorsque la concentration en sultone
gamma-insaturée dépasse environ 10 ppm.
Les études à long terme sur animaux de laboratoire dont on connaît
les résultats sont insuffisantes pour permettre d'évaluer le pouvoir
cancérogène des ASL, des AOS et des AS, et ce, pour différentes
raisons: conception même de ces études, trop petit nombre d'animaux
utilisés et doses administrées trop faibles, enfin examens
histopathologiques trop succints. Compte tenu de ces réserves, les
résultats fournis par les études au cours desquelles les animaux ont
reçu des ASL, des AOS ou des AS par voie orale, ne comportent aucun
signe de cancérogénicité; par ailleurs l'application d'AOS aux animaux
par badigeonnage cutané, a également donné des résultats négatifs. Ces
composés ne se révèlent pas non plus génotoxiques in vivo ou in
vitro, encore que peu d'études aient été publiées sur ce point.
Des études sub-chroniques au cours desquelles des rats avaient
reçu des ASL dans leur nourriture ou leur eau de boisson à des
concentrations quotidiennes correspondant environ à 120 mg/kg de poids
corporel, ont révélé la présence d'effets minimes, notamment des
altérations biochimiques et des modifications histopathologiques au
niveau du foie; toutefois d'autres études au cours desquelles des
animaux avaient été exposés plus longtemps à des doses plus élevées
n'ont pas mis d'effets en évidence. L'application cutanée de ASL a
provoqué une intoxication générale ainsi que des effets localisés.
La dose journalière moyenne de ASL absorbée par la population
générale, telle qu'on peut l'évaluer sur la base d'estimations de
l'exposition de cette population par l'intermédiaire de l'eau de
boisson, des ustensiles de cuisine et des aliments, est probablement
beaucoup plus faible (de l'ordre de trois ordres de grandeur) que les
concentrations qui se révèlent produire des effets insignifiants sur
les animaux de laboratoire.
Les effets des AOS observés sur l'homme à l'occasion des quelques
études dont on a connaissance, rapellent ceux qui ont été mis en
évidence chez des animaux de laboratoire exposés aux ASL. Comme on ne
dispose pas de données suffisantes pour évaluer la dose journalière
moyenne d'AOS absorbée par la population générale ni sur les
concentrations susceptibles de produire des effets chez l'homme et
l'animal, il n'est pas possible de savoir avec certitude si
l'exposition aux AOS présentes dans l'environnement représente un
risque pour la santé humaine. Les concentrations d'AOS présentes dans
les milieux auxquels l'homme pourrait être exposé, sont de toute
manière plus faibles que celles des ASL, du fait de la moindre
utilisation des AOS.
Des effets ont été observés systématiquement à l'occasion de
quelques études à portée limitée effectuées sur des rats à qui l'on
avait fait ingérer quotidiennement des AOS soit avec leur nourriture,
soit dans leur eau de boisson à des concentrations supérieures ou
égales à 200 mg/kg de poids corporel. Des applications topiques
répétées ou prolongées produisent également des effets localisés sur
la peau et les yeux. On ne dispose pas non plus de données suffisantes
pour évaluer la dose journalière moyenne d'AS absorbée par la
population générale. Toutefois, étant donné que les tensio-actifs à
base d'AS ne sont pas utilisés aussi abondamment que ceux qui
contiennent des ASL, il est probable que la dose d'AS absorbée est au
moins mille fois plus faible que celle qui produit des effets sur
l'animal.
1.10 Evaluation des effets sur l'environnement
Les ASL, les AS et les AOS sont utilisés en grandes quantités et
rejetés dans l'environnement avec les eaux usées. Pour évaluer le
risque qui leur est attaché, il faut comparer les concentrations
auxquelles l'exposition peut se produire avec celles qui ne provoquent
aucun effet indésirable, cette comparaison pouvant être faite pour un
certain nombre de milieux présents dans l'environnement. En ce qui
concerne les tensio-actifs anioniques en général, les plus importants
de ces milieux sont constitués par les stations de traitement des eaux
usées, les eaux de surface, les sols amendés au moyen de sédiments et
de boues d'égout, ainsi que les eaux estuarielles et marines. Les
composés subissent une biodécomposition (depuis les premiers stades
jusqu'à leur dégradation ultime) ainsi qu'une adsorption, qui
réduisent leur concentration dans l'environnement ainsi que leur
biodisponibilité. Le racourcissement de la chaîne latérale alkylée et
la disparition de la structure du composé initial conduisent à des
composés qui sont moins toxiques que les molécules de départ. Il
importe d'en tenir compte lorsqu'on compare les résultats des épreuves
en laboratoire aux effets qui pourraient se produire dans
l'environnement. En outre, lorsqu'on évalue le risque associé à
l'exposition, dans l'environnement, à ces trois types de tensio-
actifs anioniques, il faut que les comparaisons entre les
différentes épreuves de toxicité portent sur des composés dont la
chaîne latérale à la même longueur.
Les effets des ASL sur les organismes aquatiques ont été très
largement étudiés. Lors des épreuves de laboratoire effectuées en eau
douce, ce sont les poissons qui se sont révélés les plus sensibles;
ainsi la concentration sans effets observables pour un cyprin
d'Amérique du Nord, Pimephales promelas, est d'environ
0,5 mg/litre (C12); tous ces résultats ont été confirmés lors
d'épreuves effectuées dans des conditions plus proches de la réalité.
Pour ce qui est du phytoplancton, des épreuves de toxicité aiguë
sur trois heures ont donné, pour la CE50, des valeurs de
0,2-0,1 mg/litre (C12-C13), alors qu'on n'a constaté aucun effet
sur l'abondance relative du plancton dans d'autres tests effectués à
la concentration de 0,24 mg/litre (C11,8). Il semble que les espèces
marines soient légèrement plus sensibles que la plupart des autres
groupes taxonomiques.
Ces trois types de composés anioniques se retrouvent dans
l'environnement à des concentrations qui varient dans de larges
limites. De ce fait, il n'est pas possible de procéder à une
évaluation du risque écologique qui soit d'une portée générale. Toute
évaluation du risque doit s'appuyer sur une connaissance suffisante de
l'exposition et des concentrations agissantes dans l'écosystème
étudié.
Pour ce qui est de l'évaluation du risque imputable à la présence
d'AS et d'AOS dans l'environnement, il faudra encore réunir des
données précises sur l'exposition à ces composés. C'est pourquoi on
utilise des modèles pour étudier l'exposition à ces produits dans les
différents milieux qui en sont les récepteurs. En ce qui concerne les
organismes aquatiques, les données toxicologiques sur les AS et les
AOS sont relativement rares, notamment dans les cas d'exposition
chronique à des concentrations constantes. Celles dont on dispose
montrent que cette toxicité est analogue à celle des autres
tensio-actifs anioniques.
Les organismes aquatiques halophiles se révèlent plus sensibles
que les organismes dulçaquicoles à ces composés; toutefois leur
concentration est plus faible dans l'eau de mer, sauf au débouché des
émissaires d'eaux usées. La destinée et les effets de ces composés,
qui sont présents dans les effluents déversés en mer, n'ont pas été
étudiés en détail.
Pour évaluer la sûreté écologique de tensio-actifs tels que les
ASL, les AOS et les AS, il faut comparer les concentrations effectives
dans l'environnement à celles qui ne produisent aucun effet. Les
besoins en matière de recherche sont déterminés non seulement par les
propriétés intrensèques de tel ou tel produit chimique mais aussi par
les modalités ou les tendances de sa consommation. Tous ces facteurs
peuvent varier fortement d'une région à l'autre, aussi l'appréciation
et l'évaluation des risques doivent-elles être effectuées région par
région.
1.11 Recommandations pour la protection de la santé humaine
et de l'environnement
1. Comme il peut y avoir exposition à des poussières sur les lieux de
travail (au cours de la fabrication et de la préparation des
différentes formules), il faut veiller à ce que les précautions
habituelles d'hygiène et sécurité du travail soient respectées afin
d'assurer la protection des travailleurs.
2. La composition des préparations destinées à la consommation des
ménages et à l'usage industriel doit être étudiée pour éviter tout
danger, en particulier lorsqu'il s'agit de produits destinés au
nettoyage ou au lavage du linge à la main.
3. L'exposition à ces produits dans l'environnement et les effets
qu'ils peuvent avoir doivent faire l'objet d'une surveillance
appropriée afin que l'on puisse reconnaître à temps la présence de
tout concentration excessive dans tel ou tel milieu.
1.12 Recommandations pour les recherches futures
Santé humaine
1. Etant donné que le contact cutané est la principale voie
d'exposition humaine aux ASL, aux AOS et aux AS et que l'on ne dispose
pas d'études à long terme suffisantes sur la toxicité cutanée ou la
cancérogénicité de ces produits chez les animaux de laboratoire, il
est recommandé de procéder à des études à long terme convenablement
conçues au cours desquelles il sera procédé à l'application de ces
composés sur la peau des animaux.
2. En raison de l'absence de données définitives sur la génotoxicité
des AOS et des AS, il conviendrait de procéder à des études
supplémentaires in vivo et in vitro.
3. En raison de l'insuffisance des études existantes concernant les
effets toxiques de ces produits sur la reproduction et le
développement, il conviendrait d'effectuer, sur des animaux de
laboratoire, des études qui permettent d'obtenir des résultats
définitifs sur la valeur des concentrations agissant ou au contraire,
sans effets des ASL, des AOS et des AS.
4. Etant donné que l'on ne connaît pas de façon suffisamment précise
l'exposition aux ASL, aux AOS et aux AS, il faudrait surveiller
l'exposition de la population générale à ces produits, en particulier
lorsque ces tensio-actifs sont utilisés pour le nettoyage et le lavage
du linge à la main.
5. Etant donné que les ASL, les AOS et les AS peuvent favoriser le
transport d'autres produits chimiques dans les différents milieux qui
composent l'environnement et en faire varier la biodisponibilité et la
toxicité dans les eaux de surface, les sédiments, les cours d'eau et
les sols auxquels l'être humain pourrait se trouver exposé, il
conviendrait d'étudier les interactions de ces produits avec d'autres
substances présentes dans l'environnement et les conséquences qui en
découlent pour la santé humaine.
Sûreté écologique
6. Des études supplémentaires devraient être effectuées afin
d'élucider les mécanismes de l'adsorption et de la désorption des AOS
et des AS. Elles devraient également porter sur le partage des ASL,
des AOS et des AS entre les particules colloïdales en solution ou en
suspension dans l'eau. Il faudrait effectuer une modélisation
mathématique des coefficients de sorption et valider les modèles
obtenus en fonction des paramètres physicochimiques.
7. En cas d'exposition à des sols amendés à l'aide de boues d'égout
ou à des sédiments de rivière, il faudrait étudier la biodécomposition
des AOS et des AS dans ces milieux. L'étude des sédiments (dans les
zones d'aérobiose et d'anaérobiose) devrait s'effectuer en aval des
points où sont rejetées des eaux traitées ou non traitées ou des
émissaires d'évacuation.
8. Il faudrait surveiller au niveau régional et national les
concentrations en ASL, AOS et AS dans l'environnement afin d'obtenir
des données sur l'exposition. Il faudrait également mettre au point
des méthodes d'analyse permettant de déceler la présence de faibles
teneurs en AOS et en AS dans les compartiments appropriés de
l'environnement.
9. Il faudrait établir des bases de données nationales sur la
concentration des ASL, AOS et AS dans les eaux usées et les cours
d'eau ainsi que sur les différents types de stations d'épuration, leur
implantation et leur efficacité, afin de mieux étudier l'impact des
décharges dans l'environnement.
10. Il faudrait effectuer des études à long terme sur la toxicité des
AOS et des AS pour les poissons (espèces d'eau douce et espèces
marines) et des invertébrés aquatiques, afin d'en établir la
sensibilité relative.
ALKILSULFONATOS LINEALES Y SUSTANCIAS RELACIONADOS
1. RESUMEN GENERAL, EVALUACION Y RECOMENDACIONES
1.1 Identidad y métodos analíticos
Los alkilsulfonatos lineales (ASL), los alpha-olefinsulfonatos
(AOS) y los alkilsulfatos (AS) son sustancias tensioactivas aniónicas
con moléculas que se caracterizan por tener un grupo hidrófobo y uno
hidrófilo (polar). Las mezclas comerciales están formadas por isómeros
y homólogos de compuestos relacionados entre sícon distintas
propiedades fisicoquímicas, obteniéndose formulaciones con diversas
aplicaciones.
Los ASL, los AOS y los AS se pueden analizar por métodos no
específicos. El ensayo que se suele utilizar es el de las sustancias
que reaccionan con el azul de metileno, es decir, todas las que
contienen un grupo aniónico e hidrófobo. Por consiguiente, si se
utiliza para muestras del medio ambiente se producen interferencias
analíticas; por otra parte, la sensibilidad de este método es de unos
0,02 mg/litro. Aunque se han buscado alternativas no específicas a
este método, su uso no es habitual. En el análisis del medio ambiente
sólo hay métodos específicos para los ASL y los AS. Para el análisis
de los AOS se dispone de un método mejorado basado en la reactividad
del azul de metileno y la cromatografía líquida de alto rendimiento
(HPLC).
Los ASL son sustancias no volátiles que se forman por la
sulfonación del alkilbenceno lineal. Los productos comerciales son
siempre mezclas de homólogos con la cadena alkilo de distintas
longitudes (C10-C13 o C14) e isómeros que difieren en las
posiciones del anillo de fenilo (2-5 fenil). En las muestras del medio
ambiente y en otras matrices se pueden determinar todos los homólogos
e isómeros de los ASL por medio de métodos analíticos específicos como
la HPLC, la cromatografía de gases y la cromatografía de
gases-espectrometría de masas.
Los AOS son sustancias no volátiles producidas por la sulfonación
de las alpha-olefinas. Son mezclas de dos compuestos, el
alkensulfonato de sodio y el sulfonato de hidroxialkano, con
longitudes de la cadena alkilo de C14-C18.
Los AS son compuestos no volátiles producidos por la sulfatación
de alcoholes oleoquímicos o petroquímicos. Son mezclas de homólogos
con longitudes de la cadena alkilo de C10-C18. Se están
perfeccionando métodos analíticos específicos para la vigilancia del
medio ambiente.
1.2 Fuentes de exposición humana y ambiental
Los ASL, los AOS y los AS se utilizan como ingredientes activos en
productos de uso doméstico y de aseo personal y en aplicaciones
especializadas. Una vez utilizadas, dichas sustancias detergentes
pasan al medio ambiente en las aguas residuales.
Se dan casos de exposición en el trabajo a estas sustancias. La
exposición de la población humana general y de los organismos del
medio ambiente depende de la aplicación de los ASL, los AOS y los AS
(y de otras sustancias tensioactivas), de las prácticas de tratamiento
de las aguas residuales y de las características del medio ambiente al
que llegan.
En 1990, el consumo mundial fue de unos dos millones de toneladas
de ASL, 86 000 toneladas de AOS y 289 000 toneladas de AS.
1.3 Concentraciones en el medio ambiente
1.3.1 Alkilsulfonatos lineales
Las concentraciones de ASL se han determinado cuantitativamente
por medio de un método analítico sensible específico en casi todos los
compartimentos del medio ambiente en los que pueden estar presentes.
Las concentraciones disminuyen progresivamente en el siguiente orden:
aguas residuales > efluente tratado > aguas superficiales > mar.
En las zonas donde los ASL son las sustancias tensioactivas
predominantes utilizadas, las concentraciones suelen ser de
1-10 mg/litro en las aguas residuales, 0,05-0,1 mg/litro en los
efluentes sometidos a un tratamiento biológico, 0,05-0,6 mg/litro en
los efluentes tratados con un filtro de goteo, 0,005-0,05 mg/litro en
las aguas superficiales por debajo de los desagües de aguas residuales
(con concentraciones que disminuyen con rapidez a 0,01 mg/litro
corriente abajo del desagüe), < 1-10 mg/kg en los sedimentos
fluviales (< 100 mg/kg en los sedimentos muy contaminados cerca de
las zonas de vertido), 1-10 g/kg en los fangos de alcantarillado y <
1-5 mg/kg en los suelos tratados con fangos (al principio 5-10 mg/kg;
se ha registrado una concentración de < 50 mg/kg después de
aplicaciones anormalmente elevadas de fangos). Las concentraciones de
ASL en las aguas de estuario son de 0,001-0,01 mg/litro, aunque hay
niveles más elevados en los lugares donde se vierten directamente
aguas residuales. Las concentraciones en el agua marina cercana a la
costa son < 0,001-0,002 mg/litro.
Hay que señalar que las concentraciones de ASL en el medio
ambiente varían mucho. Esto se debe a diferencias en los métodos
analíticos, las características de los lugares de muestreo (que van
desde zonas muy contaminadas con un tratamiento inadecuado de las
aguas residuales hasta zonas donde dichas aguas se someten a un
tratamiento a fondo), la estación (los valores pueden ser en una el
doble que en otra) y el consumo de ASL.
La vigilancia del medio ambiente pone de manifiesto que no se ha
producido acumulación de ASL en sus compartimentos a lo largo del
tiempo. Las concentraciones en el suelo no aumentan con el tiempo,
sino que disminuyen debido a la mineralización. Los ASL no se degradan
en condiciones estrictamente anaerobias (para formar metano), por lo
que no se puede concluir que estén mineralizados en sedimentos
anaerobios. Con la utilización presente, el ritmo de asimilación de
ASL en todos los compartimentos del medio ambiente que los reciben es
igual al ritmo de entrada, por lo que la situación es estable.
1.3.2 alpha-Olefinsulfonatos y alkilsulfatos
Los datos disponibles sobre las concentraciones de AOS en el medio
ambiente son limitados debido a la dificultad para analizarlos en las
muestras de dicho medio. Hay métodos colorimétricos no específicos
(como el basado en el azul de metileno) que permiten detectar
sustancias tensioactivas aniónicas en general, pero se ven afectados
por interferencias analíticas y no son idóneos para determinar
concentraciones determinadas de AOS. Se está preparando un método
específico para medir los AS en muestras del medio ambiente.
En estudios de laboratorio se ha observado que los AOS y los AS se
mineralizan con rapidez en todos los compartimentos del medio ambiente
y prácticamente se eliminan del todo de las aguas residuales durante
el tratamiento. Las concentraciones en el agua superficial, los
sedimentos, el suelo, el agua de estuario y el medio marino son
probablemente bajas. Se ha comprobado que la concentración de AOS en
el agua fluvial es pequeña.
1.4 Transporte, distribución y transformación en el medio ambiente
A temperaturas por debajo de 5-10°C, la cinética de la
biodegradación de los ASL, los AOS y los AS se reduce debido a la
disminución de la actividad microbiana.
1.4.1 Alkilsulfonatos lineales
Las vías de entrada de los ASL en el medio ambiente varían de un
país a otro, pero la principal es el vertido de las depuradoras de
aguas residuales. Cuando no hay depuradoras o son inadecuadas, las
aguas residuales se pueden verter directamente en los ríos, los lagos
o el mar. Otra vía de entrada de ASL en el medio ambiente es la
dispersión de fangos de alcantarillado en las tierras cultivadas.
Durante su recorrido hasta llegar al medio ambiente, los ASL se
eliminan mediante una combinación de adsorción y biodegradación
primaria y final. Los ASL se adsorben sobre superficies coloidales y
partículas en suspensión, con unos coeficientes medidos de adsorción
de 40-5200 litros/kg, en función de los medios y de la estructura de
los ASL. Se biodegradan en el agua superficial (semivida de 1-2 días),
los sedimentos aerobios (1-3 días) y los sistemas marinos y de
estuarios (5-10 días).
Durante el tratamiento primario de las aguas residuales se adsorbe
alrededor del 25% (intervalo, 10-40%) de los ASL en los fangos
residuales y se elimina con ellos. No se eliminan durante la digestión
anaerobia de los fangos, sino durante su tratamiento aerobio, con una
semivida de unos 10 días. Tras la aplicación de fangos al suelo, en
general se degrada el 90% de los ASL en tres meses, con una semivida
de 5-30 días.
Los factores de concentración en el organismo completo para los
ASL oscilan entre 100 y 300 para la suma de los ASL-14C y los
metabolitos de 14C. Los peces los absorben sobre todo por las
branquias, distribuyéndose después al hígado y la vesículas biliar
tras la biotransformación. Los ASL se excretan con rapidez, por lo que
no hay pruebas de que se produzca bioampliación.
1.4.2 alpha-Olefinsulfonatos
Los datos disponibles sobre el transporte, distribución y
transformación en el medio ambiente para los AOS son más escasos que
para los ASL. Cabe suponer que los AOS llegan al medio ambiente de
manera análoga a la establecida para los ASL, los AS y otras
sustancias tensioactivas detergentes, y su destino en él es semejante
al de los ASL y los AS. En condiciones aerobias se biodegradan
fácilmente y la biodegradación primaria se completa en 2-10 días, en
función de la temperatura. Son limitados los datos disponibles sobre
la bioacumulación de los AOS; en peces no se observó ninguna. No hay
datos relativos a la degradación abiótica.
1.4.3 Alkilsulfatos
Los AS llegan al medio ambiente por mecanismos análogos a los de
los ASL y los AOS.Son fácilmente biodegradables en condiciones
aerobias y anaerobias en el laboratorio y en el medio ambiente; la
biodegradación primaria se termina en 2-5 días. El factor de
bioconcentración en el organismo entero oscila entre 2 y 73 y varía
con la longitud de la cadena de los homólogos de los AS. Los peces
absorben, distribuyen, biotransforman y excretan los AS de la misma
manera que los ASL y no se produce bioconcentración en los organismos
acuáticos.
1.5 Cinética
Los ASL, los AOS y los AS se absorben fácilmente en el aparato
digestivo y se distribuyen ampliamente por todo el organismo, con una
metabolización extensa. En los ASL se produce omega- y ß-oxidación.
Las sustancias originales y los metabolitos se excretan sobre todo a
través de los riñones, aunque una parte de la cantidad absorbida se
puede excretar en forma de metabolitos en las heces por excreción
biliar. Parece que por la piel intacta solamente se absorben
cantidades mínimas de ASL, AOS y AS, aunque el contacto prolongado
puede poner en peligro la integridad de la barrera cutánea,
permitiendo así una mayor absorción; las concentraciones elevadas
pueden reducir el tiempo necesario para la penetración.
1.6 Efectos en los animales de laboratorio y en los sistemas
de prueba in vitro
Los valores de la DL50 por vía oral para las sales sódicas de
los ASL fueron de 404-1470 mg/kg de peso corporal en ratas y de
1259-2300 mg/kg en ratones, lo cual parece indicar que las ratas son
más sensibles que los ratones a la toxicidad de los ASL. En ratones se
midió para una sal sódica de AOS una DL50 por vía oral de
3000 mg/kg de peso corporal. Los valores de la DL50 de AS por vía
oral en ratas fueron de 1000-4120 mg/kg de peso corporal. Los ASL, AOS
y AS irritan la piel y los ojos.
Se han descrito efectos mínimos, entre ellos alteraciones
bioquímicas y cambios histopatológicos en el hígado, en estudios de
toxicidad subcrónica en los que se administraron ASL a ratas con los
alimentos o el agua de beber en concentraciones equivalentes a dosis
superiores a 120 mg/kg de peso corporal al día. Aunque en un estudio
se observaron cambios estructurales de las células hepáticas a dosis
menores, al parecer eran reversibles. En otros estudios no se
detectaron efectos con dosis análogas, pero tal vez el examen de los
órganos fuera más detenido en el primer estudio. Se han notificado
efectos en la reproducción, por ejemplo menor tasa de gestación y
pérdida de crías, en animales que recibieron dosis > 300 mg/kg al
día. Se observaron cambios histopatológicos y bioquímicos tras la
aplicación cutánea de larga duración a ratas de soluciones > 5% de
ASL y después de la aplicación durante 30 días de 60 mg/kg de peso
vivo en la piel de cobayas. La aplicación cutánea repetida de
soluciones > 0,3% de ASL indujo efectos fetotóxicos y en la
reproducción, pero también se observó toxicidad materna.
Son escasos los datos disponibles de estudios en animales
experimentales que permitan evaluar los posibles efectos de los AOS en
el ser humano. No se observó ningún efecto en ratas que recibieron por
vía oral dosis de 250 mg/kg de peso corporal al día en aplicación
crónica, pero se apreció fetotoxicidad en conejas a las que se
administró una dosis tóxica para la madre de 300 mg/kg de peso
corporal al día. La aplicación de AOS a la piel y los ojos de animales
experimentales indujo efectos locales.
Aunque se han investigado en varios estudios los efectos de la
exposición de corta y larga duración de animales a los AS, en la
mayoría de los casos el examen histopatológico fue inadecuado o el
tamaño de los grupos pequeño; por otra parte, las dosis más altas
utilizadas en los estudios de larga duración no produjeron ningún
efecto tóxico, de manera que no se pudo establecer un NOAEL. Sin
embargo, se han descrito habitualmente efectos en ratas que recibían
AS en los alimentos o el agua de beber a concentraciones equivalentes
a 200 mg/kg de peso corporal al día o más. Se han observado efectos
locales en la piel y los ojos tras la aplicación tópica de
concentraciones aproximadas del 0,5% de AS o más. A concentraciones
más elevadas se han registrado efectos de toxicidad materna y
fetotóxicos.
La mayoría de los estudios de larga duración son inadecuados para
evaluar el potencial carcinogénico de los ASL, los AOS y los AS en
animales experimentales, debido a factores como el pequeño número de
animales, el número limitado de dosis, la ausencia de una dosis
tolerada máxima y la limitación del examen histopatológico en la
mayoría de los estudios. En los casos en que se describieron de manera
apropiada los hallazgos patológicos no se utilizaron dosis toleradas
máximas y las dosis no produjeron efectos tóxicos. Teniendo presentes
estas limitaciones, sin embargo, en los estudios en los que se
administraron por vía oral ASL, AOS y AS no se obtuvo ninguna prueba
de carcinogenicidad; en estudios de larga duración de aplicación de
AOS en la piel con un pincel no se observó ningún efecto.
Según los limitados datos disponibles, no parece que estas
sustancias tengan efectos genotóxicos in vivo o in vitro.
1.7 Efectos en el ser humano
Los resultados obtenidos en pruebas de parche demuestran que la
piel humana puede tolerar el contacto con soluciones de ASL, AOS o AS
de hasta un 1% durante 24 horas con la única reacción de una
irritación leve. Estas sustancias tensioactivas provocaron la pérdida
de lípidos de la superficie de la piel, la elución del factor
hidratante natural y la desnaturalización de las proteínas de la capa
epidérmica externa y aumentaron la permeabilidad y la hinchazón de
esta capa. Los ASL, los AOS y los AS no indujeron sensibilización
cutánea en voluntarios y no se ha encontrado ninguna prueba definitiva
de que induzcan la formación de eczemas. No se han comunicado lesiones
graves ni muertes tras la ingestión accidental de estas sustancias
tensioactivas por personas.
1.8 Efectos en el medio ambiente
1.8.1 Alkilsulfonatos lineales
1.8.1.1 Medio acuático
Los ASL han sido objeto de amplios estudios tanto en el
laboratorio (estudios de corta y larga duración) como en condiciones
más naturales (microcosmos y mesocosmos y estudios sobre el terreno).
En general, la disminución de la cadena alkilo o la posición más
interna del grupo fenilo van acompañadas de una menor toxicidad. Las
observaciones en peces y en Daphnia indican que al disminuir la
longitud de la cadena en una unidad (por ejemplo de C12 a C11) la
toxicidad se reduce prácticamente a la mitad.
Los resultados de las pruebas de laboratorio han sido los
siguientes:
-- Microorganismos: Los resultados son muy variables debido al
uso de diversos sistemas de prueba (Por ejemplo, inhibición de fango
activado; cultivos mixtos y especies individuales). Los valores de la
CE50 oscilan entre 0,5 mg/litro (especie única) y > 1000 mg/litro.
Para los microorganismos no hay relación lineal entre la longitud de
la cadena y la toxicidad.
-- Plantas acuáticas: Los resultados dependen mucho de las
especies. Para los organismos de agua dulce, los valores de la
CE50 son de 10-235 mg/litro (C10-C14) en las algas verdes, de
5-56 mg/litro (C11,1-C13) en las algas cianofíceas, de
1,4-50 mg/litro (C11,6-C13) en las diatomeas y de
2,7-4,9 mg/litro (C11,8) en las macrofitas; al parecer las algas
marinas son aún más sensibles. En las algas es probable que no haya
relación lineal entre la longitud de la cadena y la toxicidad.
-- Invertebrados: Los valores de la CL(E)50 aguda por lo menos
en 22 especies de agua dulce son de 4,6-200 mg/litro (longitud de la
cadena sin especificar; C13) para los moluscos; 0,12-27 mg/litro
(sin especificar; C11,2-C18) para los crustáceos; 1,7-16 mg/litro
(sin especificar; C11,8) para los gusanos; y 1,4-270 mg/litro
(C10-C15) para los insectos. Los valores de la CL(E)50 crónica
son de 2,2 mg/litro (C11,8) para los insectos y 1,1-2,3 mg/litro
(C11,8-C13) para los crustáceos. La concentración crónica sin
efectos observados (NOEC; basada en la letalidad o los efectos en la
reproducción) es de 0,2-10 mg/litro (sin especificar; C11,8) para
los crustáceos. Parece que los invertebrados marinos son más
sensibles, con valores de la CL50 de 1 a > 100 mg/litro (casi
siempre C12) para 13 especies y NOEC de 0,025-0,4 mg/litro (sin
especificar en todas las pruebas) para siete especies ensayadas.
-- Peces: Los valores de la CL50 aguda son de 0,1-125 mg/litro
(C8-C15) para 21 especies de agua dulce; los valores de la
CL(E)50 crónica son de 2,4 y 11 mg/litro (sin especificar; C11,7)
para dos especies; y las NOEC de 0,11-8,4 y 1,8 mg/litro (sin
especificar; C11,2-C13) para dos especies. También en este caso
los peces marinos parecen ser más sensibles, con valores de la CL50
aguda de 0,05-7 mg/litro (sin especificar; C11,7) para seis especies
y de la CL50 crónica de 0,01-1 mg/litro (sin especificar) para
dos especies. En la mayoría de los informes no se indicaba la
longitud de la cadena. Para especies marinas se señaló una NOEC de <
0,02 mg/litro (C12).
La longitud media de la cadena de los productos utilizados
habitualmente en el comercio es C12. Se han probado compuestos de
numerosas longitudes de cadena en Daphnia magna y en peces, pero la
longitud utilizada en otros organismos de agua dulce ha solido ser la
de C11,8. Los valores típicos de la CL(E)50 para el ASL C12 son
de 3-6 mg/litro en Daphnia magna y de 2-15 mg/litro en peces de agua
dulce, y las NOEC crónicas típicas son de 1,2-3,2 mg/litro para
Daphnia y de 0,48-0,9 mg/litro para los peces de agua dulce. Los
valores típicos de la CL50 de los ASL con cadenas de esta longitud
son en los peces marinos < 1-6,7 mg/litro.
Los organismos de agua salada, en particular los invertebrados,
parecen ser más sensibles que los de agua dulce a los ASL. En los
invertebrados, la acción inhibidora de los ASL sobre el calcio puede
afectar a la disponibilidad de este ión para la morfogénesis. Los ASL
tienen un efecto general sobre el transporte iónico. Los productos de
la biodegradación y los subproductos de los ASL son 10-100 veces menos
tóxicos que las sustancias de las que proceden.
Los resultados obtenidos en condiciones más reales son los
siguientes: Se han utilizado ASL en todas las pruebas de agua dulce a
varios niveles tróficos, como recintos cerrados en lagos (organismos
inferiores), modelos de ecosistemas (sistemas de sedimentos y agua),
ríos por debajo y por encima del desagüe de depuradoras de aguas
residuales y corrientes experimentales. En casi todos los casos se
emplearon ASL C12. Al parecer las algas son más sensibles en verano
que en invierno, puesto que los valores de la CE50 en tres horas
fueron de 0,2-8,1 mg/litro después de la fotosíntesis, mientras que en
los modelos de ecosistemas no se observó ningún efecto en la
abundancia relativa de las comunidades de algas con 0,35 mg/litro. Los
niveles sin efecto en estos estudios fueron de 0,24-5 mg/litro, en
función del organismo y del parámetro ensayado. Estos resultados
prácticamente coinciden con los de las pruebas de laboratorio.
1.8.1.2 Medio terrestre
Se dispone de información acerca de las plantas y las lombrices de
tierra. Las NOEC para siete especies de plantas en pruebas realizadas
con soluciones de nutrientes son < 10-20 mg/litro; la correspondiente
a tres especies en suelo, mediante pruebas basadas en el crecimiento
fue de 100 mg/litro (C10-C13). La CL50 en 14 días fue para las
lombrices de tierra > 1000 mg/kg.
1.8.1.3 Aves
En un estudio con pollos tratados en la alimentación se obtuvo una
NOEC (basada en la calidad de los huevos) > 200 mg/kg.
1.8.2 alpha-Olefinsulfonatos
Los datos acerca de los efectos de los AOS en los organismos
acuáticos y terrestres son limitados.
1.8.2.1 Medio acuático
Solamente se dispone de datos de pruebas de laboratorio:
-- Algas: Los valores de la CE50 que se han descrito para las
algas verdes son > 20-65 mg/litro (C16-C18).
-- Invertebrados: Para Daphnia se han notificado valores de la
CL50 de 19 y 26 mg/litro (C16-C18).
-- Peces: Los valores de la CL50 son de 0,3-6,8 mg/litro
(C12-C18) para nueve especies de peces. De los estudios de corta
duración realizados en la trucha común (Salmo trutta), Idus
melanotus y Rasbora heteromorpha, cabe concluir que la toxicidad
de los compuestos C14-C16 es unas cinco veces inferior a la de los
C16-C18, con valores de la CL50 (todas las concentraciones
medidas) de 0,5-3,1 (C16-C18) y 2,5-5,0 mg/litro (C14-C16). En
dos estudios de larga duración en la trucha arcoiris se comprobó que
el crecimiento era el parámetro más sensible, con una CE50 de
0,35 mg/litro. En un pez marino, el pardete (Mugal cephalus), el
valor de la CL50 en 96 horas fue de 0,70 mg/litro.
1.8.2.2 Medio terrestre
En un estudio de plantas con soluciones de nutrientes, la NOEC fue
de 32-56 mg/litro. En un estudio con pollos tratados en la
alimentación, se notificó una NOEC (basada en la calidad de los
huevos) > 200 mg/kg.
1.8.3 Alkilsulfatos
1.8.3.1 Organismos acuáticos
Se han realizado estudios de los AS de corta y larga duración y
uno en condiciones más reales. Su toxicidad también depende de la
longitud de la cadena alkilo; no se disponía de información sobre
diferencias de toxicidad entre los AS lineales y ramificados.
Los resultados de las pruebas de laboratorio son los siguientes:
-- Microorganismos: Los valores de la CE50 en un conjunto de
microorganismos marinos fueron de 2,1-4,1 mg/litro (C12). Las NOEC
en Pseudomonas putida fueron de 35-550 mg/litro (C16-C18).
-- Plantas acuáticas: Los valores de la CE50 fueron >
20-65 mg/litro (C12-C13) en algas verdes y de 18-43 mg/litro
(C12) en macrofitas. Las NOEC fueron de 14-26 mg/litro
(C12-C16/C18) en algas verdes.
-- Invertebrados: Los valores de la CL(E)50 fueron de
4-140 mg/litro (C12/C15-C16/C18) en especies de agua dulce y
de 1,7-56 mg/litro (todos C12) en especies marinas. La NOEC crónica
en Daphnia magna fue de 16,5 mg/litro (C16/C18) y en especies
marinas de 0,29-0,73 mg/litro (longitud de la cadena sin especificar).
-- Peces: Los valores de la CL50 fueron de 0,5-5,1 mg/litro
(longitud de la cadena sin especificar o C12-C16) en especies de
agua dulce y de 6,4-16 mg/litro (todos C12) en especies marinas. No
había estudios de larga duración.
Hay que señalar que muchos de estos estudios se llevaron a cabo en
condiciones estáticas. Los AS son fácilmente biodegradables, por lo
que se puede haber infravalorado su toxicidad. En un estudio de 48
horas con Oryzias latipes, los valores de la CL50 fueron de 46, 2,5
y 0,61 mg/litro (concentraciones medidas) para los compuestos C12,
C14 y C16 respectivamente. Este y otros estudios indican que la
toxicidad difiere en un factor de cinco por cada dos unidades de
longitud de la cadena. En un estudio de biocenosis de paso de
corriente con compuestos de C16-C18 se observó una NOEC de
0,55 mg/litro.
1.8.3.2 Organismos terrestres
Se han notificado valores de la NOEC > 1000 mg/kg (C16-C18)
en lombrices de tierra y en nabos.
1.9 Evaluación del riesgo para la salud humana
Los ASL son los agentes tensioactivos más utilizados en
detergentes y productos de limpieza. También se utilizan AOS y AS en
detergentes y en productos de aseo personal. Por consiguiente, la
principal vía de exposición humana es el contacto cutáneo. Pueden
ingerirse pequeñas cantidades de ASL, AOS y AS con el agua potable y
debido a la presencia de residuos en utensilios y alimentos. Aunque la
información de que se dispone es limitada, la ingesta diaria de ASL
por esos medios se puede estimar en unos 5 mg/persona. Puede
producirse exposición en el trabajo a los ASL, AOS y AS durante la
formulación de diversos productos, pero no hay datos acerca de los
efectos de una exposición crónica a estas sustancias en el ser humano.
Los ASL, AOS y AS pueden irritar la piel después de un contacto
cutáneo repetido o prolongado con concentraciones análogas a las
presentes en los productos sin diluir. En los cobayas los AOS pueden
inducir sensibilización cutánea cuando el nivel de sulfona g
insaturada es superior a unas 10 ppm.
Los estudios disponibles de larga duración en animales
experimentales son insuficientes para evaluar el potencial
carcinogénico de los ASL, AOS y AS, debido a factores como el diseño
del estudio, el uso de un número pequeño de animales, el ensayo de
dosis insuficientes y lo limitado del examen histopatológico. En los
escasos estudios en los que se administró ASL, AOS o AS a animales por
vía oral no se observaron signos de carcinogenicidad; también fueron
negativos los resultados de los estudios de larga duración en los que
se administraron AOS mediante aplicación cutánea. Estas sustancias no
parecen ser genotóxicas in vivo o in vitro, aunque se tienen
noticias de pocos estudios.
Se han descrito efectos mínimos, entre ellos alteraciones
bioquímicas y cambios histopatológicos hepáticos, en estudios
subcrónicos en los que se administraron ASL a ratas en la alimentación
o el agua de beber a concentraciones equivalentes a una dosis
aproximada de 120 mg/kg de peso corporal al día, aunque no se observó
ningún efecto en estudios en los que los animales estuvieron expuestos
a dosis más elevadas durante períodos más largos. La aplicación
cutánea de ASL ocasionó tanto toxicidad sistémica como efectos
locales.
La ingesta diaria media de ASL de la población general, con
arreglo a estimaciones limitadas de la exposición por medio del agua
potable, utensilios y alimentos probablemente sea muy inferior (unas
tres veces menor) a los niveles con los que se ha observado que
inducen efectos leves en los animales experimentales.
Los efectos de los AOS observados en el ser humano en los escasos
estudios disponibles son parecidos a los descritos en los animales
expuestos a los ASL. Debido a que son insuficientes los datos para
estimar la ingesta diaria media de AOS de la población general y los
relativos a los niveles que inducen efectos en el ser humano y en los
animales, no es posible evaluar con suficiente confianza si la
exposición a los AOS en el medio ambiente representa un riesgo para la
salud humana. Sin embargo, los niveles de AOS en medios a los que
puede estar expuesto el ser humano probablemente sean inferiores a los
de ASL, puesto que se utilizan menos.
Se han descrito repetidamente efectos en un pequeño número de
estudios limitados en ratas que recibieron AS en la alimentación o en
el agua de beber en concentraciones equivalentes a dosis de
200 mg/kg de peso corporal al día o más. Se han observado efectos
locales en la piel y en los ojos tras una aplicación tópica repetida
o prolongada. Los datos disponibles son insuficientes para estimar la
ingesta diaria media de AS de la población general. Sin embargo, dado
que no se utilizan tanto agentes tensioactivos con AS como los que
contienen ASL, la ingesta de AS será probablemente como mínimo tres
veces inferior a las dosis que se ha demostrado que inducen efectos en
los animales.
1.10 Evaluación de los efectos en el medio ambiente
Los ASL, los AOS y los AS se utilizan en grandes cantidades y se
liberan en el medio ambiente por medio de las aguas residuales. Para
evaluar el riesgo es preciso comparar las concentraciones de
exposición con las que no producen efectos adversos, y esto se puede
hacer para varios compartimentos del medio ambiente. Para los agentes
tensioactivos aniónicos en general, los compartimentos más importantes
son las depuradoras de aguas residuales, las aguas superficiales, los
suelos tratados con sedimentos y fangos y los medios estuarinos y
marinos. Se produce tanto biodegradación (primaria y final) como
adsorción, por lo que disminuyen las concentraciones y la
biodisponibilidad en el medio ambiente. Con la reducción de la
longitud de la cadena y la pérdida de la estructura original se forman
sustancias menos tóxicas que la primera. Es importante tener en cuenta
estos aspectos a la hora de interpretar los resultados de laboratorio
con los posibles efectos en el medio ambiente. Por otra parte, al
evaluar el riesgo asociado con la exposición del medio ambiente a
estos tres compuestos aniónicos se debe establecer una comparación con
los resultados de las pruebas de toxicidad de sustancias cuya cadena
tenga la misma longitud.
Se han realizado abundantes pruebas de los efectos de los ASL en
los organismos acuáticos. En las pruebas de laboratorio con agua dulce
parece que los peces eran las especies más sensibles; la NOEC para
Pimephales promelas fue de unos 0,5 mg/litro (C12), y estos
resultados se confirmaron en pruebas realizadas en condiciones más
reales. en el fitoplancton se han observado diferencias: en ensayos
de toxicidad agua de tres horas los valores de la CE50 fueron de
0,2-0,1 mg/litro (C12-C13), mientras que no se detectaron efectos
en la abundancia relativa en otras pruebas con 0,24 mg/litro
(C11,8). Parece que las especies marinas eran ligeramente más
sensibles que la mayoría de los otros grupos taxonómicos.
En el medio ambiente hay una amplia gama de concentraciones de las
tres sustancias aniónicas, como se ha puesto de manifiesto en las
numerosas mediciones de los ASL. Debido a esta amplia gama, no se
puede hacer una evaluación del riesgo de estas sustancias para el
medio ambiente de aplicación general. Para evaluar el riesgo se deben
conocer de manera apropiada las concentraciones de exposición y las
que tienen efectos en el ecosistema que interesa.
Se necesitan datos precisos sobre la exposición a los AS y los AOS
si se quiere hacer una evaluación del riesgo para el medio ambiente.
Por consiguiente se están utilizando modelos a fin de evaluar las
concentraciones de exposición en los compartimentos del medio ambiente
que los reciben. Los datos sobre la toxicidad de los AS y los AOS para
los organismos acuáticos, especialmente después de una exposición
crónica a concentraciones estables, son relativamente escasos. Los
datos disponibles indican que la toxicidad de estos productos es
análoga a la de otras sustancias tensioactivas aniónicas.
Los organismos de agua salada parecen ser más sensibles que los de
agua dulce a estos compuestos; sin embargo, su concentración es menor
en el agua del mar, excepto cerca de los desagües de alcantarillados.
No se han investigado con detalle su destino y sus efectos en las
aguas residuales vertidas en el mar.
Si se desea evaluar la inocuidad para el medio ambiente de agentes
tensioactivos como los ASL, los AOS y los AS hay que comparar las
concentraciones reales en el medio ambiente con las que no tienen
ningún efecto. Las necesidades de investigación se determinan no sólo
por las propiedades intrínsecas de un producto químico, sino también
por sus características o la tendencia del consumo. Estos varían
considerablemente entre las distintas zonas geográficas, por lo que la
evaluación debe ser de ámbito regional.
1.11 Recomendaciones para la protección de la salud humana y
el medio ambiente
1. Puesto que en el lugar de trabajo se puede producir exposición al
polvo (durante la elaboración y formulación), deben utilizarse
prácticas normalizadas de higiene del trabajo a fin de asegurar la
protección de la salud de los trabajadores.
2. La composición de las formulaciones para uso privado e industrial
se debe diseñar de manera que se evite el riesgo, especialmente en las
formulaciones utilizadas para la limpieza o el lavado a mano.
3. La exposición y los efectos en el medio ambiente se deben vigilar
de manera apropiada con objeto de tener una indicación pronta de
cualquier acumulación excesiva en los compartimentos pertinentes del
medio ambiente.
1.12 Recomendaciones de nuevas investigaciones
Salud humana
1. Debido a que la piel es la principal vía de exposición humana a
los ASL, los AOS y los AS y a que no se dispone de estudios adecuados
de larga duración acerca de la toxicidad cutánea o la carcinogenicidad
en animales experimentales, se recomienda la realización de estudios
de larga duración debidamente diseñados de aplicación cutánea de estas
sustancias.
2. Ante la falta de datos definitivos sobre la genotoxicidad de los
AOS y los AS, deben llevarse a cabo nuevos estudios in vivo e in
vitro.
3. A la vista de la insuficiencia de los estudios disponibles sobre
la toxicidad en la reproducción y el desarrollo, se han de realizar
estudios definitivos en animales de laboratorio a fin de obtener datos
relativos a los efectos de los ASL, los AOS y los AS y los niveles con
efectos y sin ellos.
4. Puesto que la exposición a los ASL, los AOS y los AS no está
debidamente definida, se debe vigilar la exposición de la población
general, en particular cuando estas sustancias tensioactivas se
utilizan en la limpieza y el lavado a mano.
5. Debido a que los ASL, los AOS y los AS pueden potenciar el
transporte de otras sustancias químicas y regular su biodisponibilidad
y toxicidad en las aguas superficiales, los sedimentos fluviales y los
suelos a los que puede estar expuesto el ser humano, deben
investigarse las interacciones con otras sustancias químicas del medio
ambiente y las consecuencias para las personas.
Inocuidad para el medio ambiente
6. Deben realizarse nuevos estudios sobre los mecanismos de adsorción
y desorción de los AOS y los AS. También se debe estudiar el reparto
de los ASL, los AOS y los AS entre las partículas coloidales disueltas
y suspendidas en el agua. Hay que elaborar modelos matemáticos de los
coeficientes de sorción y validarlos con arreglo a parámetros
fisicoquímicos.
7. Se han de realizar estudios de la biodegradación de los AOS y los
AS en suelos tratados con fangos y en sedimentos fluviales (zonas
aerobias y anaerobias) corriente abajo de los vertidos de aguas
residuales tratadas y sin tratar.
8. Se deben vigilar en los ámbitos regional y nacional las
concentraciones de ASL, AOS y AS en el medio ambiente, a fin de
obtener información acerca de la exposición. Se han de preparar
métodos analíticos para la detección de niveles bajos de AOS y AS en
los compartimentos pertinentes del medio ambiente.
9. Hay que organizar bases de datos nacionales sobre las
concentraciones de ASL, AOS y AS en las aguas residuales y en los ríos
y sobre los tipos, la eficacia y el lugar de las depuradoras de aguas
residuales, con objeto de facilitar la evaluación de los efectos de
los vertidos de estas sustancias tensioactivas en el medio ambiente.
10. Deben realizarse estudios de la toxicidad de los AOS y los AS en
los peces (de agua dulce y marinos) y los invertebrados acuáticos para
establecer la sensibilidad relativa de estas especies.
j,k: integers ( j + k = 7-11)
Common name: Sodium linear alkylbenzenesulfonate
Common synonyms: LAS, LAS sodium salt, linear
alkylbenzene-sulfonic acid sodium salt,
linear dodecyl-benzenesulfonic acid
sodium salt, sodium straight chain
alkylbenzenesulfonate
CAS Registry number: 68411-30-3 (LAS sodium salt, C10-13
alkyl)
Common trade names: Ablusol DBC, Agrilan WP, Alkasurf CA,
Arylan, Atlas G-3300B, Atlox, Biosoft,
Berol, Calsoft, Demelan CB-30, Elecut
S-507, Elfan, Emulphor ECB, Emulsogen
Brands, Gardilene, Hexaryl, Idet,
Kllen, Lutopon SN, Manro, Marlopon,
Marlon A, Nacconol 90 F, Nansa HS 80,
Nansa Lutersit, Neopelex, Sandozin AM,
Sipex, Sulfamin, Sulframin, Surfax 495,
Teepol, Tersapol, Tersaryl, Ufaryl DL
80P, Witconate (McCutcheon, 1993)
Abbreviations: LAS, LAS-Na
Specification: LAS are anionic surfactants which were
introduced in the 1960s as more
biodegradable replacements for highly
branched alkyl-benzene sulfonates. LAS
are produced by sulfonation of linear
alkylbenzene (LAB) with sulfur trioxide
(SO3), usually on a falling film
reactor or with oleum in batch
reactors. The corresponding sulfonic
acid is subsequently neutralized with
an alkali such as caustic soda. The
hydrocarbon intermediate, LAB, is
currently produced mainly by alkylation
of benzene with n-olefins or
n-chloroparaffins using hydrogen
fluoride (HF) or aluminium chloride
(AlCl3) as a catalyst, and the LAS
derivatives are thus generally referred
to in that context (Cavalli et al.,
1993a). Currently, 74% of world
production of LAB is via HF and 26% via
AlCl3 (Berna et al., 1993a).
LAS are a mixture of homologues and phenyl positional isomers,
each containing an aromatic ring sulfonated at the para position
and attached to a linear alkyl chain of C10-C14 (in Europe,
predominantly C10-C13) at any position except the terminal one.
The product is generally used in detergents in the form of the
sodium salt.
Some of the typical characteristics of LAS, including the
distribution of alkyl chain lengths and the positions of the phenyl
rings in the two types of LAS used in laundry detergents, are shown
in the box below. The United States Toxic Substances Control Act
inventory lists LAS homologues with chain lengths up to C18
(Tables 1 and 2), but these products are not currently used for
commercial purposes.
A2.2 Physical and chemical properties
The properties of LAS differ greatly depending on the alkyl
chain length. Table 3 shows the Krafft points (temperature at which
1 g of LAS dissolve in 100 ml of water) and the relative critical
micelle concentrations of the single homologues.
Typical characteristics of linear alkylbenzene sulfonates used in laundry
detergents:
Appearance (commercial product): White paste (containing water)
Average length of alkyl carbon chain: 11.8
Average relative molecular mass: 342
Unsulfonated matter: 1-2%
Alkyl chain distribution:
C10 10-15%
C11 25-35%
C12 25-35%
C13 15-30%
C14 0-5%
Phenyl ring position LAS (LAB-HFa) LAS (LAB-AlCl3b)
2-phenyl 18 28
3-phenyl 16 19
4-phenyl 17 17
5-phenyl 24 18
6-phenyl 25 18
From Cavalli et al. (1993a)
a Hydrofluoric acid-catalysed process
b Aluminium chloride-catalysed process
Table 1. Mixtures of linear alkylbenzene sulfonates and their salts found in the
United States Toxic Substances Control Act inventory
Generic benzene- CAS number
sulfonic acid groups
Acid Salts
(C10-13)Alkyl-a 68411-30-3 (sodium salt)
(C10-16)Alkyl- 68584-22-5 68584-23-6 (calcium salt)
68584-26-9 (magnesium salt)
68584-27-0 (potassium salt)
Mono (C6-12)alkyl- 68608-87-7 (sodium salt)
Mono(C7-17)alkyl- 68953-91-3 (calcium salt)
68953-94-6 (potassium salt)
Mono(C9-12)alkyl- 68953-95-7 (sodium salt)
Mono(C10-16)alkyl- 68910-31-6 (ammonium salt)
68081-81-2 (sodium salt)
Mono(C12-18)alkyl- 68648-97-5 (potassium salt)
a There may be more than one alkyl substituent per benzene ring (United
States Environmental Protection Agency, 1981).
Table 2. Individual linear alkylbenzene sulfonates (LAS) found in the United States Toxic Substances Control Act inventory
Parent sulfonic acid Empirical CAS Registry number
(abbreviation) formula
Acids Sodium salts Other salts
Dodecylbenzene C16H26O3S 1322-98-1 1322-98-1
(C10 LAS) (140-60-3)a (2627-06-7)a
Undecylbenzene C17H28O3S 50854-94-9 27636-75-5 NH4 salt, 61931-75-7
(C11 LAS)
Dodecylbenzene C18H30O3S 27176-87-0 25155-30-0 Al salt, 29756-98-7; NH4 salt, 1331-61-9;
(C12 LAS) (2211-98-5)a Ca salt, 26264-06-2; K salt, 27177-77-1;
(68628-60-4)b also numerous salts with alkyl amines
(18777-54-3)c
Tridecylbenzene C19H32O3S 25496-01-9 26248-24-8 Also salts with alkyl amines
(C13 LAS)
Tetradecylbenzene C20H34O3S 30776-59-1 28348-61-0
(C14 LAS) (47377-10-2)a (1797-33-7)a
Pentadecylbenzene C21H36O3S 61215-89-2 K salt, 64716-02-5
(C15 LAS)
Hexadecylbenzene C22H38O3S (16722-32-0)a K salt, 64716-00-3
(C16 LAS)
Heptadecylbenzene C23H40O3S 39735-13-2
(C17 LAS)
From United States Environmental Protection Agency (1981)
a Specifies para substitution
b Specifies para substitution at second position on alkyl chain
Table 3. Relationship between alkyl chain length, Krafft point,
and critical micelle concentration (CMC) of linear
alkylbenzene sulfonates
Alkyl chain length Krafft point (°C) CMC × 10-3 (25°C)
10 -1 5.8
12 3 1.1
14 8 0.24
15 - 0.11
16 13 -
From Ohki & Tokiwa (1970)
The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature. For
surfactants, a distinct, sharp bend (break point) is observed in the
solubility/temperature curve. The steep rise in solubility above the
sharp bend is caused by micelle formation. The point of intersection
of the solubility and critical micelle curves plotted as a function
of temperature is referred to as the Krafft point, which is a triple
point at which surfactant molecules coexist as monomers, micelles,
and hydrated solids. The temperature corresponding to the Krafft
point is called the Krafft temperature. Above the Krafft temperature
and critical micelle concentration, a micellar solution is formed
and higher than aqueous solubility may be obtained.
As commercial LAS are a mixture of homologues and phenyl-
positional isomers, their properties may differ. Even some products
with the same alkyl chain distribution (same average carbon number)
have different properties, depending on the 2-phenyl isomer content.
The solubility in water of commercial LAS used for detergents
(average alkyl carbon length, 11.8), for example, which is important
for liquid formulations, is typically about 25% at 25°C for LAS (LAB
via HF) and about 38% at 25°C for LAS (LAB via AlCl3) (Cavalli et
al., 1993a).
As LAS are anionic surfactants, they lower the surface tension
of water so that it can wet and penetrate fabrics more easily to
loosen and remove soils and stains. Micelles, which are formed at
low concentrations, solubilize oil and stains effectively (Ohki &
Tokiwa, 1969). Other important properties of LAS are detergency,
foaming, sensitivity to Ca and Mg ions, wetting, and surface
tension, which reach their optimal values generally when the alkyl
chain length is about C12 (Yamane et al., 1970).
A physico-chemical property often used in environmental
modelling is the octanol-water partition coefficient (Kow).
Although it is impossible to measure the Kow for surface-active
compounds like LAS, it can be calculated. Roberts (1989) modified
the fragment method of Leo & Hansch (1979) in order to take the
branching of position into account. He thus defined a function, log
( CP + 1), where CP is found by pairing off carbon atoms along
the two branches up to the terminus of the shorter branch. (In the
case of LAS, CP is the carbon number of the shorter of the
integers j and k noted in section 2.1.) This gave the formula:
log Kow = ALK-1.44 log ( CP + 1),
where ALK is log Kow calculated without a branch factor.
In order to calculate log Kow for multicomponent materials
like LAS, the calculated Kow for each component is multiplied by
the mole fraction of the corresponding component, the products are
summed, and the logarithm is calculated to give log WAK ( WA,
weighted average).
A2.3 Analysis
A2.3.1 Isolation
A number of analytical methods are available for the
determination of LAS in water, but the primary method is assay as
methylene blue-active substances (MBAS). The methylene blue reaction
responds to any compound containing an anionic centre and a
hydrophobic centre, because such compounds tend to form an
extractable ion pair when they combine with cationic dyes such as
methylene blue; as only the oxidized form is blue, many positive
interferences may occur. Negative interference in MBAS analysis is
seen in the presence of cationic substances such as proteins and
amines (Swisher, 1970, 1987). Therefore, isolation of LAS from a
sample is one of the most important aspects of their analysis. Most
analytical methods include appropriate procedures for isolation.
A2.3.2 Analytical methods
The analytical methods available for determining LAS in water
include nonspecific methods, involving colorimetric, fluorimetric,
and atomic adsorption techniques, and specific methods involving
techniques such as high-performance liquid chromatography (HPLC),
gas chromatography (GC) and GC-mass spectrometry (MS).
A2.3.2.1 Nonspecific methods
The simplest procedure for the determination of LAS in aqueous
solution is a two-phase titration method. LAS are titrated in a
mixed aqueous chloroform medium with a standard solution of a
cationic reagent, such as benzethonium chloride (Hyamine 1622), and
a small amount of indicator, such as a mixture of dimidium bromide
and acid blue. The end-point is determined by a change in the colour
of the organic solvent (ISO 2271, 1972).
The main nonspecific analytical method used is assay for MBAS,
described above. Colorimetric techniques are routinely used to
determine low concentrations of anionic surfactants, including LAS,
in aqueous samples and have been used extensively in testing and
environmental monitoring of these materials. The colorimetric
methods have the same common analytical basis, that is, formation of
solvent extractable compounds between the anionic surfactant and an
intensely coloured cationic species. The most commonly used cationic
reagent for this purpose is methylene blue (Swisher, 1970, 1987).
The same principle has been used as the basis of many other
procedures for the determination of anionic surfactants.
It has been shown or predicted that organic sulfates,
sulfonates, carboxylates, phenols, and even simple inorganic anions
such as cyanide, nitrate, thiocyanate, and sulfate can be methylene
blue-reactive (Swisher, 1970, 1987). The negative interferences that
can occur as a result of direct competition of other 'cationic'
materials are generally considered to be less important than
positive interferences, and the entities detected by the analysis
are correctly referred to as MBAS.
The procedure developed by Longwell & Maniece (1955) and the
improved version of Abbott (1962) are considered to be the best
methods for the determination of MBAS in aqueous samples. The
sensitivity of these procedures is such that levels of
0.01-0.02 mg/litre MBAS can be determined.
The MBAS response can be used as an acceptable overestimate of
the synthetic anionics present in domestic wastewaters, but these
materials may comprise only a small proportion of the total MBAS in
surface waters (Waters & Garrigan, 1983; Matthijs & De Henau, 1987).
Berna et al. (1991) found that LAS contributed 75% of the MBAS in
integrated sewage and 50% in treated water. Direct methylene blue
analysis of extracts derived from sludge, sediment, and soil
invariably leads to highly inflated estimates of LAS (Matthijs & De
Henau, 1987). Numerous attempts have been made to improve the
specificity of methylene blue analysis, by using a variety of
separation steps before the usual colorimetric estimation. Such
indirect procedures are usually lengthy, difficult, and still
susceptible to interference. A number of analytical methods for the
determination of LAS involving extraction and methylene blue are
summarized in Table 4.
Table 4. Analytical methods for anionic surfactants in environmental water using methylene
blue and extraction
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Absorption Extract LAS in water 50-300 Urea, Jones (1945)
photometry into chloroform as thiocyanate,
ion-pair with MB; measure chloride
absorption of chloroform
solution at 650 nm
Extract from alkaline 10-100 As above Longwell &
solution, wash with Maniece
cidic MB (1955)
Remove impurities 0.1-1 As above Abbot (1962)
from MBreagent by
chloroformextraction
Remove MBAS by TLC 0.1-1 Oba & Yoshida
(1965)
Remove MBAS on Takeshita &
polymer bead column Yoshida
(1975)
Remove MBAS on ion 0.02 Yasuda
exchange column (1980)
UV absorption Re-extract LAS into 1 Uchiyama
photometry water; measure UV (1977)
absorption at 222 nm
Table 4 (contd)
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Infra-red Use to reduce 1000 Ambe &
spectometry interference from MBAS Hanya
(1972)
Gas Convert into fluorine 0.02 Tsukioka &
chromatography derivative; measure Murakami
by ECD (1983)
HPLC Remove MB by cation 0.1 Hashimoto et
exchange, HPLC al. (1976)
Remove MB by anion 0.02 Saito et al.
exchange, HPLC (1982)
LAS, linear alkylbenzene sulfonates; MB, methylene blue; MBAS, methylene blue-active
substances; TLC, thin-layer chromatography; UV, ultraviolet radiation; ECD, electron
capture detection; HPLC, high-performance liquid chromatography
Many other cationic dyes and metal chelates have been used as
colorimetric (and fluorimetric) reagents for the determination of
anionic surfactants, including LAS. Use of the cationic metal
chelates has also led to the development of sensitive atomic
absorption methods for indirect determination of anionic surfactants
in fresh, estuarine, and marine waters. Although these alternative
systems may offer some advantages over the methylene blue cation
method, they cannot match the wide experience gained with methylene
blue analysis. Some examples of analytical methods based on the use
of alternative cationic reagents are shown in Table 5.
A2.3.2.2 Specific methods
Good progress has been made towards developing methods for the
specific determination of the many homologues and phenyl-positional
isomers of LAS in almost all laboratory and environmental matrices
(liquid and solid) at concentrations down to micrograms per litre.
High-resolution GC techniques have allowed determination of all the
major components of LAS (homologues and phenyl-positional isomers)
in environmental samples. Waters & Garrigan (1983) and Osburn (1986)
reported improved microdesulfonation-GC procedures for the
determination of LAS in both liquid and solid matrices.
Derivatization techniques offer an alternative approach to
desulfonation for increasing the volatility of LAS for GC (or GC-MS)
analysis (Hon-nami & Hanya, 1980a; McEvoy & Giger, 1986; Trehy et
al., 1990. The GC-MS technique was also applied, after ion-pair,
supercritical fluid extraction and derivatization, to five sewage
sludges, and the LAS were found to occur at 3.83-7.51 g/kg on a
daily basis (Field et al., 1992). These GC procedures, however,
involve extensive sample pre-treatment and depend on conversion of
the isolated LAS into a suitably volatile form for GC determination;
they are therefore time-consuming.
HPLC offers a more convenient means for determining homologues
of LAS in all types of environmental matrices routinely. Several
researchers have reported HPLC procedures for LAS which involve
trace enrichment of the surfactant as the first step (Kikuchi et
al., 1986; Matthijs & De Henau, 1987; Castles et al., 1989; Di
Corcia et al., 1991). Takita & Oba (1985) developed a modified
analytical method based on MBAS-HPLC measurement. Further HPLC
methods, some requiring no sample preparation, are listed in
Table 6.
Table 5. Analytical methods involving reagents other than methylene blue
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Absorption 1-Methyl-4-(4-diethyl- 0.04 Fe[III] Higuchi et al.
photometry aminophenylazo)pyridinium (1982)
iodide; measure
chloroform solution at
564 nm
Bis[2-(5-chloro-2- 0.06 Taguchi et al.
pyridylazo)-5-diethyl- (1981);
aminophenolato]Co Kobayashi
[III] chloride; measure et al. (1986)
benzene solution at
560 nm
Ethylviolet; measure 0.01 Motomizu et
benzene or toluene al. (1982);
solution at 540 nm Yamamoto &
Motomizu (1987)
Atomic Bis[2-(5-chloro-2-
absorption pyridylazo)-5- 1 × 10-3 Hydro- Adachi &
spectrometry diethylaminophenolato] chlorite Kobayashi
Co [III] chloride; measure ion (1982)
Co by atomic absorption
spectrometry
Potassium dibenzo- 0.05 Alkali, Nakamura et al.
18-crown-6; measure K alkaline (1983)
earth
metals
Table 5 (contd)
Method Isolation method/ Limit of Interference Reference
procedure detection
(mg/litre)
Atomic Cu[II] ethylenediamine 0.03 × 10-3 Gagnon (1979);
absorption derivatives; measure Cu Sawada et al.
spectrometry (1983)
Absorption Bis(ethylenediamine)Cu; 5 × 10-3 Rama Bhat
photometry determine Cu after et al. (1980)
addition of 1-(2-
pyridylazo)-2-naphthol
at 560 nm
GC-MS Extract solid phase on 1 × 10-3 Trehy et al.
C8 column; derivatize (1990)
LAS with sulfonyl
chloride for GC-MS
LAS, linear alkylbenzene sulfonates; GC-MS, gas chromatography-mass spectrometry
Table 6. Analytical methods for linear alkylbenzene sulfonates (LAS) by specific analysees
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Recover LAS on column Column, silica gel, mobile phase 0.02-0.03 Takano et al. (1975)
chromatograph packed hexane:ethanol containing
with polymer beads sulfuric acid; UV at 225 nm
Extract LAS with methylisobutyl Column, ODS; mobile phase, 0.05 Matsueda et al.
ketone ethanol:water; UV at 225 nm (1982)
Recover LAS by ionexchange Column, cyanopropyl-modified silica; 0.04 Saito et al.
column mobile phase, ethanol:water; (1982)
chromatography UV at 225 nm
Direct analysis Column, ODS; mobile phase, methanol: 0.1 Nakae et al.
water with sodium perchlorate; (1980)
fluorescence detector capable of
determining alkyl homologue distribution
Extract LAS using Column, ODS; mobile phase, acetonitrile: 0.1 × 10-3 Kikuchi et al.
mini-column water with sodium perchlorate; (1986)
fluorescence detector
Concentrate LAS using Column, ODS; mobile phase, acetonitrile: 3 × 10-3 Takami et al.
mini-cartridge column water with sodium perchlorate; (1987)
connected in sequence with fluorescence detector
HPLC system
Table 6 (contd)
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Extract LAS with methylisobutyl Column, ODS; mobile phase, acetonitrile: NR Inaba & Amano
ketone water (gradient elution to sharpen peak) (1988)
with sodium perchlorate; UV at 222 nm
Extract from solids with Column, octyl-modified silica; 0.8 Marcomini &
methanol on Soxhlet mobile phase, 2-propanol:water: (injected Giger
acetonitrile (gradient elution) weight) (1987)
with sodium perchlorate; fluorescence
detector
Two-step solid phase Column, C1 Sphesorb; mobile phase, 7 × 10-3 Castles et al. (1989)
extraction with C2 and THF:water with sodium perchlorate;
SAX cartridges fluorescence detector
Extract LAS using Column, C8-DB (Supelco); mobile 0.8 × 10-3 Di Corcia et al.
Carbopack B (graphitized phase, methanol:water with sodium (1991)
carbon black) cartridge perchlorate; fluorescence detector
Concentrate LAS on Column, Wakosil 5C4; mobile phase, 10 × 10-3 Yokoyama & Sato
anion-exchange pre-column acetonitrile:water with sodium (1991)
connected to HPLC system perchlorate; UV at 220 nm
Table 6 (contd)
Extraction method Analytical conditions Limit of detection Reference
(mg/litre)
Ion-pair extraction under Column, capillary gas chromatography, NR Field et al.
SFE conditions using 20 m; mass spectrometry with electron (1992)
tetralhyl-ammonium ion impact ionization operating in
pair reagents, coupled with selected ion mode
ion-pair derivatization
Solid-phase extraction for HPLC column, Bandapat C18 10 × 10-3 Matthijs & De Henau
purification and gradient elution water:acetonitrile (water phase) (1987)
concentration and 0.15 mol/litre NaClOn 0.1 (solid phase)
UV, ultraviolet spectrometry; ODS, octadecyl silica; HPLC, high-performance liquid chromatography; NR, not reported;
SFE, supercritical fluid extraction
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
LAS do not occur naturally.
A3.2 Anthropogenic sources
LAS are synthetic surfactants that were introduced as prime
components of almost all types of household surfactant products in
the early 1960s to replace alkylbenzene sulfonates (ABS), which were
then in widespread use. The change-over from ABS to LAS took place
gradually, starting in the United Kingdom (1960) and then spreading
to Germany (1961), the United States of America (1963), Japan (1965)
and to other European countries (Brenner, 1968; Husmann, 1968;
Waldmeyer, 1968; Tomiyama, 1972).
After use, LAS are discharged into wastewater. As the surfactant
components of the detergent products are soluble, they eventually
reach raw sewage at concentrations of 1-7 mg/litre (Rapaport et al.,
1987). Unlike ABS, which has a branched alkyl chain structure, LAS
with a linear, straight alkyl chain structure are readily
biodegradable. Their use has alleviated significant environmental
hazards such as foaming and residual surfactant in water.
A3.2.1 Production levels and processes
Annual world production of surfactants, excluding soap, in 1990
was estimated to be about 7 million tonnes (Colin A. Houston &
Associates, Inc., 1990; Richtler & Knaut, 1991). World consumption
of LAS in 1989 was about 2.43 million tonnes, 50% of which was used
in North America, western Europe, and Japan (Hewin International
Inc., 1992). Worldwide consumption of LAS in 1990 was about 2
million tonnes, with the following geographical distribution:
western Europe, 23%; North America, 19%, eastern Asia, 16%,
South-east Asia, 12%; eastern Europe, 11%; western Asia, 7%; South
America, 7%; and Africa, 5% (CEFIC, 1992). Berna et al. (1993a)
reported that, in 1990, 380 000 tonnes were used in western Europe,
180 000 tonnes in eastern Europe, 110 000 in Africa, 100 000
tonnes in western Asia, 305 000 in eastern Asia, 180 000 in
South-east Asia, 295 000 in North America, and 140 000 in Latin
America. An additional demand of 650 000 tonnes is expected by the
year 2000. The estimates for 1990 show an increase over 1987, when
LAS production in the United States, Japan, and western Europe was
about 1.4 million tonnes, on the basis of global demand for linear
alkylbenzene (Painter & Zabel, 1988), and consumption of LAS was
about 307 500 tonnes in the United States, 485 000 tonnes in western
Europe, and 145 000 tonnes in Japan (Richtler & Knaut, 1988).
LAS are complex mixtures of isomers and homologues in
proportions dictated by the starting materials and reaction
conditions. LAS are manufactured by reacting the parent
alkylbenzenes with sulfuric acid or sulfur trioxide to give the
corresponding sulfonic acid, which is then neutralized to the
desired salt. This is usually the sodium salt but ammonium, calcium,
potassium, and triethanolamine salts are also made. The reactions
are smooth and the yields nearly quantitative. Commercial LAS
contain linear alkyl chains 10-14 carbons in length, with phenyl
groups placed at various internal positions on the alkyl chain, with
the exception of 1-phenyl (Painter & Zabel, 1988).
LAS are manufactured in an enclosed process; under normal
conditions, therefore, exposure can occur only at the stage of
detergent formulation, by inhalation or dermally. Dermal exposure is
generally short and accidental, whereas exposure by inhalation can
occur continually.
The concentration of surfactants in water from washing machines
is 0.2-0.6%. LAS are estimated to represent 5-25% of the total
surfactant mixture.
In Germany in 1988, when annual consumption of LAS in the
western states was about 85 000 tonnes, daily consumption was 3.8 g
per inhabitant per day. As consumption of drinking-water was 190
litres per inhabitant per day, the average LAS concentration in
sewage was 20 mg/litre. Consumption of LAS per capita in other
countries is shown in Table 7 (Huber, 1989).
A3.2.2 Uses
LAS are the most widely used surfactants in detergent and
cleaning products, in both liquid and powder preparations and for
household and industrial use. The amount of LAS in a product depends
on several factors, including the type of application (washing-up
products, light- and heavy-duty powders and liquids) and the
formulation, but is usually 5-25%. Small amounts of LAS are used in
non-detergent applications, but these represent less than 5% of
total world consumption.
Table 7. Specific consumption of linear alkylbenzene sulfonates (LAS)
in various countries
Country Water usage LAS usage Reference
(litres per capita (g per capita
per day) per day)
Germany - 3.8 Huber (1989)
185 2.2 Wagner (1978)
United States 560 2.6a, 2.1b Rapaport et al.
(1987)
United Kingdom 208 3.5c, 2.7c Standing Technical
Committee on
Synthetic Detergents
(1978, 1989)
Spain - 5.6a, 2.6b Berna et al. (1989)
Japan 493 2.7 Ministry of Health and
Welfare (1992);
Hewin International
Inc. (1992)
a Calculated from sales
b Calculated by analysis
c Methylene blue-active substances
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
The way in which LAS enter the environment varies between
countries, but the major route is via discharge from sewage
treatment works. Direct discharge of sewage to rivers, lakes, and
the sea occurs when wastewater treatment facilities are absent or
inadequate. Another route of entry of LAS into the environment is
via disposal of sewage sludge on agricultural land.
Throughout their journey into the environment, LAS are removed
by a combination of adsorption and primary or ultimate
biodegradation. LAS adsorb onto colloidal surfaces and suspended
particles, with measured adsorption coefficients of 40-5200
litres/kg, depending on the medium and structure of the LAS. LAS
undergo primary biodegradation in all environmentally relevant
compartments, such as raw sewage, sewage treatment water, surface
waters, sediments, and soils. They are readily and ultimately
mineralized under aerobic conditions in the laboratory and the
field. They tend not to be biodegraded under methanogenic conditions
or if the initial LAS concentration is so high that microbial
degradation is inhibited (> 20-30 mg/litre). Typical half-lives for
aerobic biodegradation of LAS are 1-8 days in river water, 1-2 days
in sediments, and 5-10 days in marine systems. The rate of
biodegradation depends on temperature: biodegradation is rapid
between 10 and 25°C; at lower temperatures, biodegradation kinetics
are reduced, in close association with microbial activity. During
primary sewage treatment, LAS are partially adsorbed onto and
removed with waste sludge to an extent of about 25% (range, 10-40%).
LAS are not removed during anaerobic sludge digestion but are
removed during aerobic treatment with a half-life of about 10 days.
Application of the sludge to soil generally results in 90%
degradation within three months, with a half-life of 5-30 days.
LAS are not bioconcentrated or biomagnified in aquatic
organisms. They are readily absorbed through the gills and body
surface of fish and are distributed via the blood to the systemic
organs. Most LAS-related compounds (parent compound and metabolites)
have been detected in the gall-bladder and hepatopancreas of fish.
They are usually cleared rapidly, with a half-life of two to three
days.
A4.1 Transport and distribution between media
Detergent chemicals such as LAS are normally discharged after
use into sewers in communal wastewater. The proportion of wastewater
that is subjected to sewage treatment varies widely between
countries. In most advanced countries, 50 to > 90% may be treated,
whereas in less developed countries the proportion may be as little
as 5-30% (Eurostat, 1991). In countries where there is no or
inadequate sewage treatment, LAS are removed from the environment
via adsorption and mineralization in the receiving surface waters.
Anionic surfactants such as LAS can adsorb onto the solid
substrates associated with sewage, sludge, sediment, and soil; the
extent of adsorption is dependent on the composition and physical
nature of the solid matrix. Measured values of the adsorption
constant (Kd) for LAS on a range of solid substrates were compiled
by Painter & Zabel (1989), who reported Kd values of 590-1400
litres/kg for primary sludge, 660-5200 1itres/kg for activated
sludge, and 40-360 1itres/kg for river water sediment.
A4.1.1 Wastewater treatment
Under certain conditions, up to 50% of the LAS present can be
biodegraded in sewers before entering sewage treatment (Moreno et
al., 1990). In large-volume batch biodegradation tests with
acclimatized sludge, the MBAS levels decreased to 10% of the initial
concentration within 15 days. During biodegradation, the toxicity of
the test solution decreased in parallel with the reduction in MBAS.
A relative enrichment of the shorter chain homologues was observed
by GC analysis concurrently with the decrease in MBAS levels,
indicating preferential removal of the higher homologues (Dolan &
Hendricks, 1976).
The distribution and fate of LAS have been established in the
course of mass balance studies at sewage treatment plants in Spain
(Berna et al., 1989), Italy (Cavalli et al., 1991), Switzerland
(Giger et al., 1989), Germany (De Henau et al., 1989), and the
United States (Rapaport & Eckhoff, 1990; McAvoy et al., 1993).
Efficient, well-operated activated sludge plants generally remove
most of the LAS during aerobic treatment, and the overall removal of
LAS in primary settlement and secondary aerobic treatment stages can
be < 98% (Berna et al., 1991). Smaller amounts of LAS were
removed (77 ± 15%) in less efficient, trickling filter plants
(McAvoy et al., 1993).
The main mechanism for removal of LAS during sewage treatment is
biodegradation (Berna et al., 1991), but a significant fraction (on
average, 20-30%) of the LAS entering sewage treatment plants may be
removed on primary sewage solids and do not undergo aerobic sewage
treatment (Giger et al., 1989). Instead, the sludge is digested
under anaerobic conditions, and in some countries a high proportion
may then be applied raw or digested to agricultural land as a source
of plant nutrients (Berna et al., 1991). In Germany and the United
Kingdom, 40-45% of sewage sludge is disposed of in this way (Waters
et al., 1989). Since LAS do not undergo significant anaerobic
biodegradation under methanogenic conditions, concentrations of
3-12 g/kg can be found on dried solids in sludge (see Section 5,
Table 10). Any LAS in sludge applied to agricultural soil should
then be rapidly biodegraded, since the receiving soil environment is
aerobic. In Germany and the United Kingdom a typical application of
digested sludge was estimated to add LAS at a rate of 7-16 mg/kg
soil (Waters et al., 1989).
Adsorption can account for 15-40% of the removal of LAS from raw
sewage during the primary settlement stage of treatment (Berna et
al., 1989; Giger et al., 1989). Berna et al. (1989) reported that
precipitation and adsorption were particularly important in removing
LAS from wastewater containing high concentrations of calcium and
magnesium ions.
The percentage adsorption of C10, C11, C12, and C13 LAS
onto activated sludge, Amazon clay, and various bacteria and algae
was directly related to the chain length and phenyl position: longer
homologues and more terminal phenyl isomers were adsorbed much more
readily than other forms. Adsorption of LAS at a concentration of
23 mg/litre was found to be pH-dependent, with adsorption increasing
as the pH decreased from 7 to 3 (Yoshimura et al., 1984a).
A4.1.2 Surface waters, sediments, and soils
The half-life for the removal of LAS by combined sorption and
settling < 12 km below a sewage outfall in Rapid Creek, South
Dakota, United States, was 0.25 days. The biodegradation half-life
was 1.5 days (Rapaport & Eckhoff, 1990). The partition coefficient
of LAS between natural water and sediment was reported to increase
with increasing chain length and with the position of the phenyl
nearer to the end of the chain. Adsorption was increased when either
the concentration of suspended solids or fractional organic carbon
was increased (Amano & Fukushima, 1993).
Freshwater pearl oysters are cultivated in Lake Nishinoko,
Japan, which has an area of 2.8 km2, an average depth of 1.5 m,
and a residence period of 27 days. The water of the lake was found
to contain total concentrations of MBAS of 0.01-0.02 mg/litre and
LAS concentrations of 0.005-0.018 mg/litre. The partition
coefficients of LAS (Kd) were 70 litres/kg for bottom
sediment:water and 11 litres/kg for oyster:water (Sueishi et al.,
1988). The authors concluded that when a river flows into a
semi-enclosed lagoon, the fate of the surfactants is dominated by
mass transfer between media and transformation due to degradation
rather than spatial transportation.
LAS were present in Swiss soils that had been treated with
sludge for 10 years; however, the application rates were six times
higher than normal. The reported half-lives were 5-80 days. The
authors noted that it is not entirely correct to use half-life to
describe the loss of LAS from soils, because there is competition
between biodegradation and sorption on and into soil particulates,
and LAS may persist at very low 'threshold' levels. During the
330-day study, the levels of LAS decreased from 45 mg/kg dry soil to
a residual level of 5 mg/kg (Giger et al., 1989).
A comparison of the measured concentration of LAS with detailed
records on the amount of sludge applied on 51 fields in England
indicated that loss of LAS exceeded 98% in fields that had not
recently been sprayed with sludge; losses from fields that had been
sprayed recently were calculated to be 70-99% of the estimated
cumulative load. The calculated half-lives for removal of LAS from
soil sprayed with sludge were 7-22 days. Examination of the
distribution of homologues suggested that loss of LAS is the result
of biodegradation and not leaching (Holt et al., 1989). In a study
of the disappearance of LAS from sludge-amended soils at two
locations, the average half-lives were 26 days when sludge was
applied at a rate of 1.6 kg dry sludge per m2 (giving a
concentration of LAS of 16.4 mg/kg soil) and 33 days when sludge was
applied at 5 kg wet sludge per m2 (concentration, 52.5 mg/kg soil)
(Berna et al., 1989). In another study, the half-life for LAS in
soil was more than three months; there was no evidence that they
accumulate in soil over time (Rapaport & Eckhoff, 1990).
When C13 LAS were applied to various soil (surface) types at a
concentration of 0.05 mg/kg under laboratory test conditions, the
half-lives were 1-5 days, with an average of two days. There was no
significant variation with regard to soil type. In a second
experiment, the average half-life of C12 LAS applied to subsurface
soil was 20 days (Larson et al., 1989).
After grass, radishes, and garden beans were grown for 76 days
in soil treated with 14C-LAS at a rate of approximately
1.2 g/m2, 98% of radioactive residues were recovered, with 63.6%
released to the atmosphere, 26.8% found in the soil, 6.6% in plant
biomass, and 0.9% leached out in percolated water. When potatoes
were grown on the soil for 106 days, 97.9% of the radioactivity was
recovered, and 72.3% was released to the atmosphere, 18.3% to the
soil, 5.9% in plant biomass, and 1.4% leached into percolated water
(Figge & Schoberl, 1989).
LAS in a plume of contaminated groundwater on Cape Cod, United
States, were degraded rapidly and was found only within 0.6 km of
the sewage disposal bed (Thurman et al., 1986).
Effects on the biodegradation of LAS applied at 50 mg/litre of
aqueous dispersion were studied in three Japanese soil types
inoculated with sewage. The rate of sludge application used in this
study was not typical of that found in the environment. Primary
degradation, as measured by the presence of MBAS, reached 70% within
16 days. Addition of andosol (allophane) and weathered granite
(kaolin and illite) both reduced primary degradation, and 40-50% of
the LAS was still present after 30 days, indicating that the rate of
microbial degradation of LAS adsorbed onto soils containing large
amounts of allophane and/or sesquioxides was reduced. A
montmorillonite soil did not affect the rate of degradation (Inoue
et al., 1978).
The behaviour of C10-C13 LAS and C12 LAS at concentrations
of 50 and 100 mg/litre was studied by HPLC in perfusion tests on two
types of soil, a clay loam and a sandy loam. The sandy loam, with a
lower content of humus and clay, adsorbed less LAS with a longer
lag. During the first three days of perfusion, only adsorption
occurred, 50% being adsorbed; after nine days, decomposition was
observed and only 16.6% of the LAS remained; after 15 days, the LAS
had almost completely disappeared (Abe & Seno, 1987).
LAS were applied at a rate of 5 g/m2 to three soil types:
loamy orthic luvisol under agricultural land, sandy acidic dystric
combisol under a pine forest, and combisol irrigated with
wastewater. The half-life in loamy soil was five days; 80% was
degraded after 12 days, and none was detectable after 28 days. With
45 mm of precipitation, about 8% of the LAS percolated to a depth of
10-30 cm. The LAS moved significantly more slowly than radioactively
labelled water. LAS were less mobile in the sandy soil, with a
maximal percolation depth of 5 cm after two weeks, whereas water
percolated 15 cm. The half-life in the sandy soil was 10 days, with
80% degradation after 19 days and total degradation after 28 days.
The LAS were bound to organic material in the humic litter, which
probably slowed degradation and reduced mobility. In combisol
irrigated with wastewater, the LAS were bound mainly in the upper
5 cm, with some percolation to 10-30 cm after an application of
180 mm of wastewater. The half-life was 12 days, and 80% was
degraded within 21 days; however, there was no further degradation
after 28 days, and the remaining LAS were tightly bound. Increasing
the application rate to 50 g/m2 had no effect on percolation;
however, the half-life was doubled. Samples collected during the
winter showed much slower degradation, with half-lives of 68-117
days. Percolation was also much deeper; the authors suggest this was
due to a higher rate of precipitation and extensive evaporation
(Litz et al., 1987).
In a study of the adsorption of LAS in aqueous solution onto
clay grumusol and sandy regosol soils, a linear adsorption isotherm
was obtained. The release of the surfactant was proportional to the
initial adsorption and the soil type, suggesting ready desorption.
More LAS was adsorbed by the clay soil than by the sandy soil (Acher
& Yaron, 1977).
Hydroxy aluminium and iron adsorbed LAS more readily and with a
much larger capacity than other soil constituents, such as organic
matter, silica gel, layer silicates, and calcium carbonate (Volk &
Jackson, 1968).
In a study of the adsorption of LAS applied at a concentration
of 2 mg/litre to a variety of Wisconsin (United States) soils, a
highly significant correlation was found between adsorption and
organic matter content (including the iron and aluminium
components), phosphate fixing capacity, and aluminium content. The
removal of sesquioxides reduced the adsorption of LAS to zero;
however treatment of montmorillonitic soils with H2O2 and
Na2S2O4 increased adsorption by oxidizing and removing the
organic matter, indicating that montmorillonite can adsorb LAS.
Treatment of soils with H2O2 increased adsorption because iron
and aluminium were released from organic chelates (Krishna Murti et
al., 1966).
Adsorption of LAS to microorganisms was found on the basis of
calculated adsorption isotherms to be more important than adsorption
to humic substances (Urano et al., 1984; Urano & Saito, 1985).
A4.1.3 Fate models
One model of the fate of LAS predicted the sorption coefficient
to within one order of magnitude. The sorption distribution
coefficient was consistently underpredicted, so that when the
concentrations of LAS in interstitial and overlying water were
predicted from concentrations in sediment they were overestimated.
The model thus provided conservative estimates for assessing safety
in aquatic media (Hand et al., 1990).
The reported concentrations of LAS in Rapid Creek, South Dakota,
United States, were compared with expected concentrations generated
by the quantitative water-air-sediment interaction fugacity model,
which is based on physical, chemical, reactive, and transport
properties and emission rates into rivers. In general, close
agreement was reached: in both cases, LAS had a residence time of
about two days. The authors pointed out that the results might
differ if the model were applied in situations that differed
hydrodynamically (Holysh et al., 1986).
A model to predict surface water concentrations of LAS in German
and American rivers included the following parameters: river flow
and velocity, sewage treatment plant location and type, discharge
volume, and connected population. The values obtained were in
general agreement with those measured. The authors also investigated
a septic tank discharge at a Canadian site by applying a groundwater
model, which was based on hydrogeological, biodegradation, and
sorption data. The predicted and measured concentrations were in
good agreement (Hennes & Rapaport, 1989).
A mathematical model was derived to explain a downstream
decrease in the concentration of LAS in the Lake Teganuma estuary,
Japan. The model included the adsorption coefficient, the
biodegradation rate constant, and the rate of transport (diffusive
and settling) flux of LAS between water and sediment. The model
predictions and laboratory findings were used to confirm that
biodegradation is the predominant mechanism for removal of LAS from
the estuary (Amano et al., 1991).
A model based on data from the Lake Biwa basin was devised to
predict the fate of LAS in Japanese rivers, assuming that complete
mixing occurs in any given cross-section of a river. The parameters
included the cross-sectional mean concentration of LAS, time
elapsed, flow velocity, longitudinal dispersion coefficient, decay
due to biodegradation and sedimentation, water depth, and river
width (Sueishi et al., 1988).
The measured concentrations of LAS in United States river water
under critical flow conditions were mirrored by the predictions of a
simple dilution model, which predicts chemical concentrations below
the mixing zone of wastewater treatment plants. The model is based
on three large databases, which link river flow, treatment type and
wastewater discharge volume; the output of the model is a frequency
distribution of concentrations just below the mixing zone of
treatment plant outfalls. The model predicted that 95% of the river
waters below that point would have concentrations of LAS of <
0.35 mg/litre during critical low-flow periods. The sampling sites
selected for this study were reported to have a low dilution factor
for mixing effluent with surface water, however. The predictions
therefore represent a 'worst-case scenario', since the 95 percentile
value represents critical low-flow periods, in which the lowest ever
recorded flow is used for a consecutive period of seven days within
10 years (McAvoy et al., 1993).
A4.2 Environmental transformation
A4.2.1 Biodegradation
A4.2.1.1 Aerobic degradation
Studies on aerobic biodegradation of LAS can be divided into
those of primary degradation and those of ultimate degradation.
Primary degradation of LAS occurs during the initial reactions in
the metabolic pathway, and the products are often shorter-chain
homologues. The ultimate degradation of LAS is that of the entire
molecule to its biodegradation end-products, CO2, H2O, and
NH4. These products are used in cell synthesis or, in the case of
CO2, excreted. The ultimate degradation of LAS normally requires
the action of several species of bacteria.
The degradation pathway of LAS has been described (Huddleston &
Allred, 1963; Swisher, 1963). The steps, shown in Figure 1, are:
omega-oxidation of the end of the alkyl chain, rapid ß-oxidation of
the chain, and oxidation of the ring.
Figure 1. Postulated metabolic pathway of linear alkylbenzene sulfonates
omega-oxidation
CH3(CH2)nCH(C6H4SO3H)(CH2)mCH ------------------> COOH(CH2)nCH(C6H4SO3H)CH2)mCH3
(n>m) |
|
| ß-oxidation
|
v
ß-oxidation
COOHCH(C6H4SO3H)(CH2)mCH3 <------------------ COOH(CH2)n-2CH(C6H4SO3H)(CH2)mCH3
|
| Ring
| dihydroxylation
|
v
COOHCH(C6H2SO3H)CH2CH3 ------------------> ring fission at the 1-2 position
of the ring, then desulfonation to
aliphatic products and sulphate.
From Painter (1992)
Swisher (1981) pointed out that ultimate biodegradation (at
least 80%) is achievable under the correct conditions, which
include:
(i) the presence of mixed bacterial species,
(ii) free access to new bacteria during the test,
(iii) acclimatization,
(iv) enough growth factors and food, and
(v) limitation of the LAS concentration to that found in the
environment.
Biodegradation of LAS begins at the terminus of the alkyl chain
with an omega-oxidation and is followed by successive cleavage of
C2 fragments (ß-oxidation) (Huddleston & Allred, 1963; Swisher,
1963). The resulting sulfocarboxylic acids have a chain length of
four to five carbon atoms (Schöberl, 1989). These intermediates are
further biodegraded by oxidative scission of the aromatic ring and
cleavage of the sulfonate group (Setzkorn & Huddleston, 1965;
Swisher, 1967). Catabolites of further oxidation steps are fed into
the central metabolic pathways, i.e. the Krebs cycle and glyoxylate
cycle (Schöberl, 1989).
LAS degradation begins at the longest end of the linear alkyl
chain, with omega- and ß-oxidation, and proceeds up to the
sulfophenylmono-carboxylic acids (one to two CH2 groups) (Divo &
Cardini, 1980). Under mild conditions, as in river water,
intermediates such as sulfo-phenylcarboxylic acids are often not
degraded, as the greater distance between sulfophenyl groups and the
far end of the hydrophobic group increases the speed of primary
biodegradation (Swisher, 1976). Once the terminal methyl group has
been attacked, primary biodegradation is rapid (Swisher, 1970;
Gledhill, 1975). Short-chain sulfophenylmonocarboxylic acids were
not degraded by Pseudomonas but were degraded by mixed cultures of
microorganisms (Leidner et al., 1976). The initial attack that opens
the aromatic ring is the rate limiting step for ultimate
biodegradation: once the ring is opened, degradation is rapid.
Enzymological methods were used to show that the same sequence
of steps occurs when ring degradation proceeds via the catechol
derivative. A variety of microorganisms isolated from soil, sewage,
and river water showed at least five distinct metabolic routes for
the degradation of LAS: omega- and ß-oxidation of the side-chain;
oxidation and desulfonation followed by cleavage of the aromatic
nucleus; reductive desulfonation of the ring; and metabolic
alpha-oxidation of the side-chain, followed by ß-oxidation and
desulfonation. Metabolism of alkyl chains shorter than four carbons
was initiated through the aromatic nucleus by hydrolytic or
reductive desulfonation of the ring (Cain et al., 1971). LAS may
also be cleaved by biochemical mechanisms (Schöberl, 1989).
Primary degradation
(i) Low levels of biomass
Measurement of MBAS was compared with measurement of total
organic carbon for detecting biodegradation in shake cultures. With
the MBAS method, LAS had lost 98% of their activity within five
days, whereas 34% of the total carbon had disappeared by that time,
and 70% was lost by the end of the 31-day test (Sekiguchi et al.,
1975a).
In a modification of the screening test of the Organisation for
Economic Co-operation and Development (OECD), accepted by the
European Commission, the percentage of dissolved organic carbon was
found to have decreased by more than 80% within four weeks. The
authors cautioned that the decrease in LAS may not have been due
solely to biological degradation, since 40-50% of organic carbon was
also removed from abiotic controls, suggesting that adsorption may
account for part of the removal of LAS (Canton & Slooff, 1982). When
aerobic biodegradation of 10 mg/litre LAS was followed during a
10-day incubation period at 27°C, primary degradation, measured by
the MBAS method, was complete within 8-10 days, and the theoretical
CO2 production reached 20-25% within 10 days. At a concentration
of 20 mg/litre, no degradation was observed, but this elevated
concenration may have inhibited the microbial inoculum (Itoh et al.,
1979).
The rate and degree of biodegradation of LAS are dependent on
temperature. In an unacclimatized microbial population, no more than
25% biodegradation was achieved at 5°C during a 28-day test,whereas
at 15, 25, and 35°C about 90% degradation was achieved within 7-14
days. At 45°C, the microbial population degraded 75% of the LAS
within 14 days, but this rate of degradation was not maintained,
probably because of loss of the acclimatized seed due to the high
temperature. A clearer effect of temperature was observed when the
microorganisms were acclimatized to LAS before the test. Under these
conditions, the rate of biodegradation increased steadily with
increasing temperature from 15 to 35°C (Hollis et al., 1976).
(ii) Wastewater treatment
In the OECD screening test, there was 95% loss of LAS, measured
by the MBAS method, and similar losses were measured in OECD
confirmatory test No. 1 with 20 mg/litre LAS. In the closed-bottle
test with a concentration of LAS of 2 mg/litre, there was 90-95%
analytical loss (by the MBAS method) and 60-65% loss of biochemical
oxygen demand. Coupled-unit tests with 10 mg/litre LAS and a mean
hydraulic retention time of 6 h showed 94% removal of chemical
oxygen demand (values > 73% indicate benzene ring opening) (Fischer
& Gerike, 1975). In activated sludge, 80-90% of dissolved organic
carbon and benzene rings disappeared within 6 h (Swisher, 1972). A
bacterium similar to Klebsiella pneumoniae, isolated from sewage,
degraded 93% of a concentration of LAS reported as 1% (10 g/litre),
as measured by the MBAS method (Hong et al., 1984). A direct
correlation was found between the rate of primary degradation of
1.5 mg/litre C11.7 LAS and the initial bacterial population size
(Yediler et al., 1989).
The biodegradation of C9-C13 LAS at concentrations of 25,
50, and 65 mg/litre was monitored in activated sludge at
100 mg/litre over a period of 12 days. Four methods were used: MBAS,
chemical oxygen demand, dissolved organic carbon, and ultra-violet
spectrophotometry. The results obtained with the MBAS method showed
a percentage loss of 94-97% for the three concentrations of LAS,
whereas the other methods showed losses of approx. 50% at
25 mg/litre LAS and approx. 70% at 50 and 65 mg/litre. The specific
rate of biodegradation was calculated to be 3.6 mg/g per h, on the
basis of loss of chemical oxygen demand (Pitter & Fuka, 1979).
The degradation ratio (biochemical oxygen demand:total oxygen
demand) for LAS by a synthetic sewage solution after five days was
0.81 for a concentration of 3 mg/litre and 0.14 for 10 mg/litre.
Concentrations of 30 and 100 mg/litre LAS were not degraded during
the 14-day test. Even after acclimatization to a concentration of
5 mg/litre LAS for one month, the two higher concentrations were not
degraded, probably because these levels inhibited the microbial
inoculum (Urano & Saito, 1985).
The percentage removal of biochemical oxygen demand and of LAS
were found to be significantly correlated in activated sludge and in
a trickling filter system under laboratory and field conditions,
implying that a well-functioning sewage treatment plant effectively
removes LAS (Tang, 1974).
LAS at a concentration of 150 mg/litre were inoculated into
sewage water collected from French water treatment plants. In six
out of eight experiments, primary degradation was almost complete
(90%) within seven days, but in the other two experiments only
45-55% degradation was achieved. The authors concluded that rapid
biodegradation of LAS requires the presence of a community of
several bacterial species, including Flavobacterium, Pseudomonas,
and Acinetobacter (Gard-Terech & Palla, 1986).
In an extended aeration activated sludge plant, 95-99% of LAS
was removed. Degradation of LAS and reduction of biochemical oxygen
demand were strongly correlated, in a 1:1 ratio (Knopp et al.,
1965). In long-term laboratory tests, 95-97% of LAS was removed by
activated sludge (Janicke & Hilge, 1979).
In a wastewater treatment plant where the input water had an
MBAS concentration of 6.2-9.4 mg/litre, at least 99% of the LAS
present was removed during treatment, biodegradation accounting for
85%. The relative composition of long-chain (C12-C13) homologues
adsorbed on the suspended solids was increased in comparison with
the relative incidence of short-chain (C10-C11) homologues
detected in the aqueous phases. Sulfophenylcarboxylates were
identified as intermediates of the biodegradation of LAS but were
detected only in the aqueous and not in the adsorbed phases (Cavalli
et al., 1993b).
Biodegradation of LAS in field trials with trickling filter
sewage was 86-95%, and average biochemical oxygen demand removal was
93.8%. Thus, the LAS appeared to be removed almost as rapidly as the
naturally occurring organic material. The linear correlation between
degradation and temperature (7.5-17.5°C) was highly significant.
Further degradation (94-99%) took place after additional aeration
(Mann & Reid, 1971).
MBAS degradation did not correspond to biodegradation of LAS
(20-200 mg/litre) in laboratory sludge units, because of the
presence of intermediates not accounted for by analysis of MBAS
(Janicke, 1971).
(iii) Surface waters
Primary degradation, measured by HPLC, of 5 mg/litre C11 LAS
in a static lake microcosm was complete within 18 days. The
sulfo-phenylcarboxylic acid intermediates produced were completely
degraded within 22 days (Eggert et al., 1979).
Aerobic degradation of 5 mg/litre LAS in river water, measured
by MBAS levels, was 100% after seven days at 25°C. Under
microaerophilic conditions at 25 and 35°C), no degradation took
place within 10 days (Maurer et al., 1971; Cordon et al., 1972).
In die-away tests with water from various sites on the Tama
River, Japan, primary biodegradation (measured by the MBAS method)
was complete within 7-15 days, but total organic carbon was
completely removed within an incubation time of 45 days. In a study
of LAS in seawater collected from the mouth of the Tama River,
degradation was only 50% complete within 60 days, as measured by
total organic carbon (Sekiguchi et al., 1975b). In a study to
monitor detergent-degrading bacteria from the Han River, Republic of
Korea, the lowest density was found in January and the highest in
July; the dominant group throughout the year was Pseudomonas (Bae
et al., 1982). Mixed and pure isomers of LAS were metabolized
readily (97.5%) by bacteria collected during the summer from a
sewage lagoon, but bacteria collected from under the ice during the
winter were not able to metabolize LAS (Halvorson & Ishaque, 1969).
Primary biodegradation of C10-C13 LAS was dependent on
incubation temperature in die-away tests with water from the Tama
River, Japan: primary biodegradation was complete within two days at
27°C, within six days at 15°C, and within three days at 21°C; at a
water temperature of 10°C, however, only 20% of the LAS had been
degraded within the nine-day test (Kikuchi, 1985). The optimal
temperature for the biodegradation of LAS in a river water die-away
test was found to be 25°C (Yoshimura et al., 1984b).
Degradation of 10 mg/litre LAS in a simulated river model was
found to be almost complete within 20 days, on the basis of MBAS
levels in water and sludge; however, ultra-violet spectrophotometry
showed that 40% of the LAS remained in the water and 25% in the
sludge. LAS with an alkyl chain length of C10 were degraded more
slowly than those with a chain length of C14, and LAS compounds
with sulfylphenyl groups near the terminal part of the alkyl chains
were degraded more easily than those with such groups further from
the end (Fujiwara et al., 1975).
In a study of the biodegradation of LAS (10 mg/litre) and a 1:1
LAS:ABS (10 mg/litre) mixture in canal water with an unaerated or
aerated system, LAS were rapidly degraded in the unaerated system,
by 14.9% within two days and 40.7% within seven days. Biodegradation
was more rapid in the aerated tanks, with 40.4% degraded within two
days and 74.5% after seven days. Addition of sewage to the test
system further increased the rate of degradation in the aerated
system: addition of 0.5 ml/litre sewage resulted in degradation of
78.2% after two days and 89.4% after seven days, and addition of
1.0 ml/litre sewage resulted in degradation of 89.7% after two days
and 99.8% within three days. No results were reported for the
unaerated system. The LAS-ABS mixture was degraded more slowly than
pure LAS: after two days, 12.3% was degraded without aeration, 36.4%
with aeration, 60.1% with addition of 0.5 ml/litre sewage, and 78.3%
after addition of 1.0 ml/litre sewage. The corresponding
degradations calculated after seven days were 32.5, 66.0, 80.7, and
87.3%. The authors concluded that degradation of these detergents
was increased by aerating the tank and by increasing the number of
microflora by adding sewage (Abdel-Shafy et al., 1988).
In the Lake Teganuma estuary (Japan), an average of 66% of LAS
is removed, with seasonal variability, ranging from 28% in winter to
100% in summer. Laboratory studies (based on HPLC methods) of
estuarine water showed that LAS degraded with a half-life of eight
days at 5°C and 0.2 days at 25°C. Model calculations and field
monitoring showed that biodegradation is 10 times more important in
the removal of LAS from the estuary during summer than is the
settling of solids or adsorption to bottom sediments. At lower
temperatures, biodegradation and the other removal mechanisms are of
equal importance (Amano et al., 1991).
In well water, biodegradation of all LAS homologues
(C10-C13) and isomers (maximal concentration, 2.5 mg/litre)
after an acclimatization period of one day was reported to follow
zero-order kinetics (Yakabe et al., 1992).
In seawater, primary biodegradation of 20 mg/litre LAS was 70%
after 10 days; the half-life was six to nine days (Vives-Rego et
al., 1987).
(iv) Soil
In soil degradation tests, levels of 2.5 mg/kg MBAS were reached
within 15 days of the addition of 20 mg/kg LAS (Cordon et al.,
1972). The biodegradation of LAS in soil was studied by measuring
the amounts of ferroin reagent-active substance and total organic
carbon. At 50 mg/litre LAS, total organic carbon disappeared within
50 days, whereas total ferroin reagent-active substance was
completely lost after only 10 days. Both chemical and physical
properties of the soils affected the loss of LAS: more LAS was
adsorbed onto clay loam than sandy loam, and biodegradation occurred
more readily in the clay loam (Abe, 1984). In a further study
(initial concentration not given), loss of C10-C13 and C12
LAS was complete within 15 days when measured as ferroin
reagent-active substances; however, when measured as total organic
carbon, residues remained until day 50 in the clay loam and beyond
day 60 in the sandy loam (Abe & Seno, 1987).
Ultimate degradation
A number of studies have been conducted of the biodegradation of
phenyl-radiolabelled LAS, in which 14CO2 production was measured.
(i) Screening tests
In a simple shake-flask system with LAS, CO2 evolution reached
60% or more of the theoretical value (Gledhill, 1975).
Four gram-negative bacteria synergistically mineralized
10 mg/litre 14C-LAS. After 13 days of incubation, 29% of the
14C-LAS was mineralized to 14CO2. Pure cultures were unable to
mineralize the LAS, although three of them carried out primary
biodegradation, measured by the MBAS method (Jimenez et al., 1991).
Pseudomonas, Alcaligenes, Necromonas, and Moraxella spp. isolated
from activated sludge and river water degraded the alkyl chains of
C12 LAS, while a group of unidentified Gram-negative bacteria cleaved
the benzene ring. A mixture of the two groups degraded LAS completely
(Yoshimura et al., 1984b).
(ii) Wastewater treatment
Mixed cultures of microorganisms found under natural conditions
or in sewage treatment plants can readily degrade LAS, to 95% of
MBAS and > 80% of dissolved organic carbon (Schöberl, 1989).
During a 19-day OECD screening test for the biodegradation of
14C-LAS, there was a high degree of ring mineralization, as seen
by the evolution of 55% as 14CO2. In a continuous system, 80% of
the LAS was evolved as CO2, with a mean retention time of 3 h;
2-3% remained as unaltered surfactant and 15-25% as the
sulfophenylcarboxylic acid intermediates (Steber, 1979).
Loss of MBAS (primary biodegradation) and ring cleavage were
found to be nearly complete (> 98%) during simulated waste
treatment of 14C-LAS. During simulated secondary waste treatment,
62% of alkyl and ring carbon was converted to CO2, 28-30% was
assimilated into biomass, and 8-10% remained as soluble residue. In
die-away tests, 85-100% of the substrates of LAS were converted to
CO2 within 91 days (Nielsen et al., 1980; Nielsen & Huddleston,
1981).
Continuous-flow experiments were conducted with mixed bacterial
cultures isolated from a detergent plant wastewater containing five
species of Achromobacter and two species of Acinetobacter. All
were more efficient at primary degradation than ultimate degradation
of LAS at concentrations of 20 and 50 mg/litre. One species of each
genus could effect primary degradation even after isolation (Hrsak
et al., 1982).
In a semi-continuous activated sludge method, 95% of the phenyl
ring of radiolabelled LAS was cleaved, indicating near complete
biodegradation of the whole molecule. Complete primary degradation
(MBAS method) of C10, C12, and C14 LAS was followed by 99-100%
ultimate degradation (HPLC and ultra-violet fluorescence). In
die-away tests with 10 mg/litre of C10, C12, and C14 LAS,
primary degradation was rapid and complete; 100% of C12 LAS was
removed within four days. Almost complete ultimate degradation was
observed within the 80-day test, with 90% ring cleavage of C10 LAS
and C11 LAS within 10 days and 70% ring cleavage of C14 LAS
within 30 days; however, no HPLC analysis was carried out on C14
LAS after day 30 (Huddleston & Nielsen, 1979).
The biodegradation of LAS (C9-C14) by a mixed bacterial
culture was studied in river water, forest soil, and wastewater from
a detergent plant. The bacteria were acclimatized to 10 mg/litre
LAS. Under continuous-flow conditions, LAS at a concentration of
20.8 mg/litre were 96% degraded, and a concentration of 46 mg/litre
was 64% degraded. Only 8-10% of the breakdown products were
completely mineralized; however, under the flow-through conditions
of this test, water-soluble compounds were usually removed via the
aqueous effluent and were not present long enough to allow
mineralization. Acclimatization considerably increased the kinetics
of mineralization (Hrsak et al., 1981).
(iii) Surface water and sediment
Detritus is a significant site of surfactant removal, and LAS
were found to be the most sorptive of the surfactants tested. In
wastewater from a pond containing submerged oak leaves, degradation
followed first-order kinetics, with a half-life of 12.6 days. LAS
were mineralized more slowly by leaves from a control pond, and an
S-shaped pattern of degradation was seen (Federle & Ventullo, 1990).
In river water in which the biomass levels were 10-100 times
higher below than above a sewage outfall, primary degradation of
added C11.6 LAS (11 mg/litre) and background LAS (0.37 mg/litre)
was rapid in water taken from below the outfall, with a half-life of
0.23 days (based on measurements of MBAS). Mineralization of the
benzene ring was rapid in water from below the outfall containing
sediment (500 mg/litre), with a half-life of 0.7 days. Water taken
above the sewage outfall also underwent ring mineralization, but the
rate of degradation was about 25% of that seen for water from below
the outfall, with a half-life of 2.7 days. When samples were
incubated in the absence of sediment, ring degradation was much
slower, with half-lives of 1.4 days in water taken from below the
outfall and approx.14 days in water taken above it. In all cases,
degradation was immediate in water taken below the outfall, but
occurred after a three-day lag in water taken above (Larson & Payne,
1981).
Degradation of C10-C14 homologues of LAS at concentrations
of 10 or 100 µg/litre followed first-order kinetics in both river
water and river water plus sediment; the half-time for
mineralization of the benzene ring was 15-33 h. The length of the
alkyl chain and the phenyl position had no significant effect, and
there was no effect of suspended sediment or competing homologues
(Larson, 1990).
LAS were degraded in leaf litter, creek water, periphyton, and
sediment at temperatures as low as 4°C, with half-lives of 6-11
days. Temperature changes altered the dependence of the
biodegradation of LAS: the half-lives increased by less than a
factor of two over an 18°C temperature range. Under realistic
conditions, temperature had less effect than was predicted on the
basis of classical thermodynamic studies in the laboratory
(Palmisano et al., 1991). The dependence of the biodegradation of
LAS follows a classical Arrhenius relationship down to about 12°C,
with a tenfold increase in reaction kinetics for every 2°C drop in
temperature (Larson, 1990).
Mineralization of LAS in saturated subsurface sediment from a
wastewater pond and in a pristine pond was monitored by amending the
sediment with 14C-LAS and measuring the evolution of 14CO2.
Mineralization in both sediments exhibited first-order kinetics. LAS
were mineralized without a lag in wastewater sediment, with
half-lives of 3.2-16.5 days. In the control pond, LAS were
mineralized much more slowly, with half-lives of 5.2-1540 days, and
only after a lag of 2-40 days; the lag tended to increase with
increasing depth. These findings confirm the assumption that
acclimatization considerably increases the kinetics of LAS
mineralization (Federle & Pastwa, 1988).
A study was conducted of the biodegradation of LAS by
microorganisms associated with the roots of two aquatic plants,
duckweed (Lemna minor) and cattail (Typha latifola).
Microorganisms from the roots of cattail mineralized 14C-LAS
without a lag, attaining 17% evolution of 14CO2 within the
35-day experiment. Microbiota associated with duckweed roots did not
mineralize LAS. The fact that the plants came from a pristine pond
or from a wastewater pond had no effect on the ability of the
microorganisms to mineralize LAS (Federle & Schwab, 1989).
More than 70% of parent LAS (20 mg/litre) in natural seawater at
22°C was biodegraded within 10 days, with an estimated half-life of
6-9 days (Vives-Rego et al., 1987). In an investigation of the
primary biodegradation kinetics of LAS (10 mg/litre) in natural
seawater in the presence of sediments (250 g/litre), 60% remained
after 20 days at 15°C and almost 100% of LAS at 5°C; however, at 20
and 25°C, only a small percentage of the original concentration
remained (Sales et al., 1987). In another study in seawater, 97% of
parent LAS (10 mg/litre) was biodegraded within two weeks (von Bock
& Mann, 1971).
More than 85% of LAS (C11.8) in estuarine water underwent
primary biodegradation, measured as MBAS removal, after 11 days
(Arthur D. Little Inc., 1991). In water from Chesapeake Bay, United
States, 75% of MBAS were removed within three days (Cook & Goldman,
1974). In a study of effluent-exposed estuarine waters, with
phenyl-radiolabelled C13 LAS, production of 14CO2 represented
42% of the added label. Addition of sediment from the site
(1 g/litre) increased the 14CO2 yield to 60%. In both tests, the
half-life for mineralization of LAS was about seven days. Up to 54%
of a radiolabelled control chemical, glucose, was mineralized. Thus,
mineralization of LAS occurs rapidly in pre-exposed estuarine
systems, with half-lives shorter than the typical hydraulic
residence times of such estuaries (Shimp, 1989).
(iv) Soils and groundwater
A simple shake-flask system was used to determine CO2
evolution in a test to assess the ultimate biodegradability of LAS
by microorganisms in soil and sewage. At 30 mg/litre, high
relative-molecular-mass LAS were biodegraded more slowly than those
with a low relative molecular mass. Ultimate biodegradation could
not be assessed precisely within the 28-day test period, but CO2
removal was 37-77% and dissolved organic carbon removal was 59-84%.
Ultimate biodegradation of the entire molecule (total CO2)
occurred concomitantly with biodegradation of the benzene ring
(14CO2). Ring desulfonation, measured as 35S-LAS, was rapid
and occurred mainly after primary biodegradation (MBAS method)
(Gledhill, 1975).
The kinetics of the ultimate biodegradation of C10-C14 LAS
to CO2 was studied in a sludge-amended soil at 0.1-10 times
environmental concentrations. All four homologues underwent rapid
degradation, with half-lives for the breakdown of the benzene ring
of 18-26 days (Ward & Larson, 1989).
Microbial mineralization of 50 µg/kg 14C-LAS was examined in
soil types ranging from a loamy sand impacted with sewage effluent
to a highly organic alpine soil, by monitoring the evolution of
14CO2. LAS were mineralized without a lag in all soils;
mineralization exhibited first-order kinetics in nine of the 11 soil
types. Asymptotic yields of CO2 ranged from 16 to 70%; the
half-lives were 1.1-3.7 days. The degradation rates were not
correlated with microbial activity, pH, total organic content, or
previous exposure (Knaebel et al., 1990).
After 14C calcium and sodium salts of LAS were applied to two
silty loam soils, the distribution of 14C was similar. After 60
days, 31-47% of the applied 14C had evolved as 14CO2 and
31-40% was present as soil residue, possibly as a combination of
parent and metabolized surfactant (Kawashima & Takeno, 1982).
A4.2.1.2 Anaerobic degradation
Degradation of LAS (measured as MBAS) was much slower under
anaerobic conditions in activated sludge than under aerobic
conditions. No degradation had taken place after one day; up to 20%
had been degraded between days 3 and 21, and 36% after 28 days. When
soil and wastewater were used, only 20% of the MBAS had disappeared
within 28 days (Oba et al., 1967). No significant removal of LAS was
reported in an anaerobic sludge digester at a Swiss sewage treatment
plant (Giger at al., 1989).
In a review of the fate of LAS in anaerobic and aerobic sewage
treatment plants, it was concluded that drying anaerobic sludge on
open beds considerably reduces the LAS content. Anaerobic
degradation of LAS is, however, limited, as the addition of LAS at
15 g/kg raw sewage (about 15 g/litre raw sewage) may inhibit
anaerobic degradation. In the laboratory, digestion of LAS was
impaired at concentrations of > 15-20 g/kg, and a concentration of
20 g/kg seriously inhibited gas production, especially when other
potentially inhibitory compounds were present. The concentration of
LAS normally found in sewage (5-10 g/kg) is, however, unlikely to
inhibit anaerobic degradation (Painter & Zabel, 1989). About 15-35%
of LAS in raw sewage is physically removed in primary settlers in
sewage treatment plants, accounting for most of the LAS found in
anaerobic sludge. Precipitation of LAS is correlated with water
hardness, since the solubilities of the calcium and magnesium salts
of LAS are very low; the solubility products ranged from
2.2 × 10-10 for C10 LAS to 6.2 × 10-13 for C13 LAS (Berna et
al., 1989). The effect of water hardness was confirmed by mass
balance analysis of Na+, Ca2+, and Mg2+ (Berna et al., 1993b).
The content of total calcium and magnesium in anaerobically digested
sludge was 43 times higher than that in water. High contents of LAS
in the sludge (up to 30 g/kg) did not inhibit the anaerobic
digestion process (Painter & Mobey, 1992), probably because LAS were
present as calcium and magnesium salts and therefore had reduced
bioavailability.
LAS were not degraded in an anaerobic sediment from a pond
receiving wastewater from a laundromat. Despite an exposure period
of 25 years, no anaerobic degradation was reported (Federle &
Schwab, 1992).
Pre-aerobic treatment of LAS may cause changes in the molecule
that permit subsequent degradation under anaerobic conditions (Ward,
1986).
A4.2.2 Abiotic degradation
The mechanisms of abiotic degradation of LAS reported below are
not of environmental significance, since biodegradation and sorption
are rapid, effective removal mechanisms.
A4.2.2.1 Photodegradation
In a study of the kinetics of the photodecomposition of C12
LAS, using a continuous-flow reactor, the initial concentrations
were 60-182 mg/litre and the radiation wavelength was 200-450 nm.
Conversion of LAS to intermediate products occurred within 1 min,
yielding 7 mol CO2 per mol LAS, and was complete within 20 min.
The reaction rate was increased by two orders of magnitude by ferric
perchlorate (Matsuura & Smith, 1970).
Rapid photodegradation of LAS (50 mg/litre) occurred within
1-2 h in an aqueous, aerated titanium dioxide suspension without
noble metal catalysts. There was rapid decomposition of the aromatic
ring and slower oxidation of the aliphatic ring. Photodegradation
was dependent on the simultaneous presence of titanium dioxide,
oxygen, and light (Hidaka et al., 1985).
A4.2.2.2 Cobalt-60 irradiation
The decomposition of LAS was studied in distilled water
irradiated with cobalt-60 gamma rays, which react with water to
produce oxygen, peroxide, hydrogen peroxide, and other strong
oxidizing agents. A concentration of 10 mg/litre LAS was reduced to
7.8 mg/litre by absorption of 10 Gy and to 0.9 mg/litre by
absorption of 100 Gy. The rate of irradiation was found to be less
important than the total absorbed energy (Rohrer & Woodbridge,
1975).
A4.2.3 Bioaccumulation and biomagnification
Studies of the bioaccumulation potential of LAS have all been
carried out with LAS labelled with 14C or 35S. It should be
noted that as these techniques do not usually allow consideration of
metabolic transformation the actual bioaccumulation of the parent
compound may be overestimated. Toxic concentrations of the breakdown
products of LAS are discussed in section A9.3.7.
A4.2.3.1 Aquatic organisms
Bioaccumulation has been studied in daphnids and fish (Table 8).
LAS are readily absorbed through the gills and body surface of fish
and are subsequently distributed via the blood to the organs and
tissues; most LAS accumulate in the gall-bladder and hepatopancreas.
Clearance is usually rapid, with a half-life of two to three days.
Short-chain LAS are accumulated to a lesser degree than long-chain
LAS.
Only 1% of 0.5 mg/litre LAS added to water was retained in
Daphnia magna within three or four days after transfer to 'clean'
water. Almost all of the chemical was in the form of intact LAS. In
fathead minnows (Pimephales promelas), metabolic transformation
occurred. All tissues monitored showed some uptake, with
concentration factors ranging from 79-372 in muscle to 21 000-70 000
in gall-bladder. Within four days of transfer to 'clean' water, 85%
of the LAS had been lost, and almost 100% was lost within 10 days
(Comotto et al., 1979).
Table 8. Bioconcentration factors for linear alkylbenzene sulfonates in aquatic invertebrates and fish
Organism Static/flow Exposure Duration Chain Steady Bioconcentration Tissue Reference
concentration of test length state factor
(mg/litre) (days)
Daphnia magna Flow 0.07 3 C12 ? 490 Comotto et al.
(1979)
0.11 560
044 720
0.09 3 C13 Yes 1250
0.11 1050
0.41 1325
Cyprinus carpio Static 61.1 1 C12 Yes 4.1 Skin surface Kikuchi et al.
(1978)
1000 Gall-bladder
Flow 0.5 4 C12 Yes 20 Whole body Wakabayashi
30 Hepatopancreas et al. (1978)
9000 Gall-bladder
0.0091 5 C12 Yes 16 Whole body Wakabayashi
et al. (1981)
0.3 400
1.0 300
Pimephales Flow 0.1 11 C12 Yes 551 Whole body Comotto et al.
promelas C13 1223 (1979)
C12, C13 269
Table 8 (contd)
Organism Static/flow Exposure Duration Chain Steady Bioconcentration Tissue Reference
concentration of test length state factor
(mg/litre) (days)
Lepomis Flow 0.063 28 C12 Yes 260 Whole body Bishop &
macrochinus 0.064 120 Maki (1980)
Flow 0.5 35 C11.7 Yes 107 Whole body Kimerle et al.
5000 Gall-bladder (1981)
Static, water unchanged for the duration of the test; flow, concentration in water maintained continuously
In an experiment in which the aqueous concentrations of an
initial concentration of 1.1 mg/litre LAS decreased by 20% during
the test, the compounds were concentrated in the gills of carp
(Cyprinus carpio) within 2 h of exposure, with a concentration
factor of 40. Skin surface, muscle, brain, kidney, hepatopancreas,
and gall-bladder showed more gradual uptake of LAS over the 24 h of
exposure, with concentration factors ranging from 4.1 for skin
surface to 1000 for gall-bladder. Blood, gonads, and spleen also
took up LAS but were not monitored throughout the period of
exposure. LAS was lost rapidly from all tissues except the
gall-bladder during 48 h in 'clean' water (Kikuchi et al., 1978).
In the bluegill (Lepomis macrochirus), a steady state was
reached within 120-168 h. The bioconcentration factor was calculated
by a kinetic method to be 286 for a concentration of LAS of
0.8 mg/litre and 132 for 0.08 mg/litre. LAS were cleared rapidly
after the fish were transferred to 'clean' water, with 99%
eliminated within 336 h; the time for clearance was 29-30 h (Bishop
& Maki, 1980). In another study, a steady state was reached within
seven days; the bioconcentration factor in whole body using a
kinetic method was reported to be 104; and the half-time for
clearance was two to five days during a depuration period of 14
days. The authors postulated that fish excrete LAS in the urine and
excrete shorter-chain carboxylates with the benzene ring intact
across the gill membranes. Both forms may also be excreted in the
faeces (Kimerle et al., 1981).
A4.2.3.2 Terrestrial plants
Foliar uptake of the calcium and sodium salts of 14C-LAS
(chain length not specified) by peanuts was studied seven and 30
days after application. No movement of LAS was detected: 70-80%
remained within the same leaf to which the compound was applied, and
no LAS were detected in other parts of the plant (Kawashima &
Takeno, 1982).
Aqueous solutions of 14C-LAS (chain length not specified) were
applied to soil (orthic luvisol), and ryegrass (Lolium perenne)
was grown under laboratory conditions for up to seven days. Uptake
of LAS after three days was 80 mg/kg at an application rate of
1 mg/kg dry weight, 370 mg/kg at a rate of 5 mg/kg, and 18 900 mg/kg
at 50 mg/kg. After seven days, levels of 600, 5000, and 19 300 mg/kg
were measured at the three dose levels, respectively (Litz et al.,
1987).
14C-LAS (chain length not specified) were applied under field
conditions to both loamy orthic luvisol and sandy dystric cambisol
soils irrigated with wastewater at rates of 5 and 50 g/m2. After
49 days, rye grass grown in the loamy soil contained residues of 130
and 1000 mg/kg dry weight at the two exposure rates, respectively.
Plants grown in the sandy soil contained 230 and 470 mg/kg,
respectively, after 54 days (Litz et al., 1987).
Two plant-soil microcosms were exposed to 14C-LAS (chain
length not specified), and LAS degradation and percolation were
followed for up to 109 days. The initial soil concentrations were
16.2 µg/g dry soil in potato soil (sandy) and 27.2 µg/g in grass,
bean, and radish soil (clay-like). The concentrations of
radiolabelled compounds in the plants decreased rapidly: at the end
of exposure, 39.1-65.8 µg LAS equivalents per g fresh weight of
plant were found in potatoes (study duration, 76 days) and
62.1-213.3 µg/g in grass, radishes, and beans (study duration, 109
days) (Figge & Schoberl, 1989).
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
The concentrations of LAS have been quantified by means of a
specific, sensitive analytical method in almost every environmental
compartment in which they might be present. The concentrations
decrease progressively from wastewater to treated effluent and
surface waters, and low concentrations are found in the sea.
The environmental concentrations of LAS are directly dependent
on use patterns, the type and efficiency of sewage treatment, and
the characteristics of the receiving environment. In areas where LAS
are the predominant surfactants used, typical concentrations are
1-10 mg/litre in wastewater, 0.05-0.1 mg/litre in effluents that
have undergone biological treatment, 0.05-0.6 mg/litre in effluents
passed through a percolating filter, 0.005-0.050 mg/litre in surface
waters below sewage outfalls (with concentrations decreasing rapidly
to 0.01 mg/litre downstream from the outfall), < 1-10 mg/kg in
river sediments (up to 100 mg/kg in highly polluted sediments near
discharge zones), 1-10 g/kg in sewage sludge, and < 1-5 mg/kg in
sludge-amended soils. The initial concentration of LAS in
sludge-amended soils is 5-10 mg/kg, but up to 50 mg/kg have been
reported after atypically heavy appli-cations. The concentration of
LAS in estuarine waters is 0.001-0.010 mg/litre but is higher where
wastewater is discharged directly. The concentrations in offshore
marine waters are < 0.001-0.002 mg/litre.
A wide range of environmental concentrations has been reported,
owing to use of different analytical methods; differences in
characteristics of sampling sites, which range from highly polluted
areas with inadequate sewage treatment to areas where sewage
undergoes extensive treatment; seasonal differences, which can
account for a twofold variation; and differences in the use of LAS.
Monitoring has shown no accumulation of LAS in environmental
compartments over time. The concentrations in soil do not increase
with time but are diminished due to mineralization. As LAS are not
degraded under strictly anaerobic condition, they are not
mineralized in anaerobic sediments. With current use of LAS, the
rates of their assimilation in all receiving environmental
compartments is equal to their rate of input, implying a steady
state.
A5.1 Environmental levels
LAS have been measured in most environmental compartments,
including discharge (raw sewage), sewers, sewage treatment plants,
sludge-amended soils and land fill, river water, river sediments,
subsurface soils, groundwater, and estuaries (Berna et al., 1991).
A decline in the concentrations of anionic surfactants in the
environment, as assessed by measurement of MBAS, was seen in Europe,
Japan, and the United States after ABS was replaced by LAS (Sullivan
& Evans, 1968; Sullivan & Swisher, 1969; Gerike et al., 1989).
Similar declines have been observed more recently in countries such
as Thailand, where the change to LAS detergents is also more recent
(Berna et al., 1991).
A5.1.1 Wastewater, sewage effluent, and sludge
The concentrations of LAS in sewage influent and effluent at
sewage treatment plants are shown in Table 9; those in sewage sludge
are given in Table 10.
The efficiency of wastewater treatment plants in removing LAS is
reported to exceed that of removal of biochemical oxygen demand.
Activated sludge removed an average of 98% LAS, trickling filters
removed 80%, and primary clarification, 27%. The average
concentration in raw sewage was 3.5 mg/litre, and those in effluent
were 2.1 mg/litre after primary treatment and 0.06 mg/litre in
activated sludge. The average chain length of LAS was C12.5 in
sewage sludge and C12 in influent sewage (Rapaport & Eckhoff,
1990).
The amount of LAS removed in a sewage treatment plant was 93% on
the basis of total organic carbon and 98.1% on the basis of a
specific method. The contribution of LAS to the total organic carbon
was estimated to be 0.93% in treated water and 3.0% in digested
sludge; 75.9% of LAS present in the raw sewage was mineralized
during treatment and 7% was in the form of sulfoxyphenyl-
carboxylates, a product of the biodegradation of LAS, suggesting
that biodegradation of LAS had reached a steady state. These figures
were obtained by analysis for sulfoxyphenylcarboxylates (Berna et
al., 1993b).
In another study, 40% of LAS was removed in a wastewater
treatment plant. The half-life for removal from the sewer pipe was
calculated to be 11 h (Moreno et al., 1990).
A5.1.2 Sediment
The concentrations of LAS in sediment are shown in Table 11, and
those in sediment samples collected at various distances from sites
of effluent outfall are shown in Table 12.
Table 9. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sewage
influent and effluent
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
Switzerland (29 sites, 1986 Raw sewage 0.95-3.9 Brunner et al. (1988)
1 sampling) Effluent 0.007-0.33
Germany (11 sites, 1985 Influent (activated sludge) 5.1 (1-13.3) 4 (0.54-12.4) Matthijs & De Henau
1 sampling) Influent (trickling filter) 8.8 (8.1-9.9) 7.4 (6.8-8.4) (1987)
Effluent (activated sludge) 0.19 (0.09-0.28) 0.07 (0.05-0.11)
Effluent (trickling filter) 1.1 (0.84-1.5) 0.76 (0.61-0.94)
United Kingdom
(several samples) 1982 Effluent 0.69 (0.58-0.81) 0.31 (0.21-0.42) Gilbert & Pettigrew
(1984)
River Thames area 1987 Sludge 15.1-341 Holt et al. (1989)
(5 sites, several
samples)
Israel (4 sites) 1983 Influent 9.6-10.6a Zoller (1985)
Effluent 0.3-11.0a
United States 1979 Effluent 0.078-0.303 Eganhouse et al. (1983)
(4 sites, 45 samples 1976-86 Influent 3.7 ± 1.1 Rapaport & Eckhoff
Effluent (activated sludge) 0.05 ± 0.04 (1990)
Effluent (trickling filter) 0.6 ± 0.3
Effluent (primary) 2.2 ± 0.4
Table 9 (contd)
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
United States Influent 5.9-6.5 5.7-6.5 Osburn (1986)
(1 sampling) Influent 3.7-5.2 3.8-4.9
Effluent 0.39-1.02 0.14-0.60
(2 sites, 9 samples) 1983 Raw influent 4.17 3.73 Sedlak & Booman
Primary influent 3.18 2.97 (1986)
Primary effluent 1.66-2.82 1.73-2.51
Final effluent 0.03-0.06 0.02-0.05
Canada (4 sites, 1976-86 Influent 2.0 ± 0.6 Rapaport & Eckhoff
45 samples yearly) Effluent (activated sludge) 0.09 ± 0.05 (1990)
Effluent (primary) 1.7-2.3
Japan
(5 sites, 60 samples) 1972-73 Influent 5.1-14.0 Oba et al. (1976)
Effluent 0.3-4.7
(6 sites, 1-2 samples) 1984 Influent (suspended particles) 0.236-1.504 Takada & Ishiwatari
Effluent (suspended particles) 0.0001-0.001 (1987)
a Total anionic surfactants (mainly LAS)
Table 10. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sewage sludge
Location Year Material Concentration (mg/litre) Reference
MBAS LAS
Switzerland
(8 and 12 sites, Digested sludge 2900-11 900 McEvoy & Giger
(1985, 1986)
1 sampling)
(29 sites, 1986 50-13 800a Brunner et al.
1 sampling) (1988)
Spain
(5 sites, several Activated sludge 7000-30 200a Berna et al. (1989)
samplings) (anaerobic digestion)
Aerated, settling system 400-700a
Finland (12 sites, Digested sludge 3400-6300a McEvoy & Giger
1 sampling) (1986)
Belgium (11 sites, 1985 Aerobic sludge 5399 (3042-8133) 281 (182-432) Matthiijs & De
1 sampling) Digested sludge 9017 (3632-17 006) 4917 (1327-9927) Henau (1987)
Germany (4 sites, 1981-86 4920 (1330-9930) Rapaport &
45 samples yearly) Eckhoff (1990)
Table 10 (contd.)
Location
Year Material Concentration (mg/litre) Reference
MBAS LAS
United States
(4 sites, 45 1981-86 4660 ± 1540 Rapaport &
samples yearly) Eckhoff (1990)
12 sites, NY, Digested sludge 6900a McEvoy & Giger
(1 sampling) (1986)
(12 sites, CA, Digested sludge 5200a
1 sampling)
(1 sampling) Primary sludge 110-126 107-127 Osburn (1986)
(2 sites, OK, 1983 Primary sludge 4610-6120 5340-6310 Sedlak & Booman
9 samples) Secondary sludge 520-990 410-860 (1986)
Anaerobic digester 6860 6660
Aerobic digester 3820 4250
Drying bed (anaerobic) 170 160
Drying bed (aerobic) 230 150
Southern California 1981 Effluent particulates 1342 Eganhouse et al.
(marine) (1983)
a Dry weight
Table 11. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sediments in
the United States and Japan
Location Year Concentration (mg/kg) Reference
MBAS LAS
United States
Rivers (activated sludge) 0.3-3.8 McAvoy et al. (1993)
Rivers (trickling filter) 0.2-340
Mississippi River 1991-92 < 0.01-5 Tabor et al. (1993)
Japan
Tokyo Bay (1 sampling, few samples) 1969 35 (33-37) Ambe (1973)
River (1 sampling, few samples) 61 (55-65)
River Sagami estuary (16 sites, 1 sampling) 7.9-39a ND-17 Utsunomiya et al. (1980)
Sagami Bay (16 sites, 1 sampling) 5.1-15 ND
Rivers 1977 < 1-260 Environment Agency Japan
(1978)
Lake Suwa (1 site, 3 samples) 1977 1.0-7.0
Rivers (9 sites, 7 samples, 1 year); 1982-83 107 (ND-567) Takada & Ishiwatari (1987);
(1 site 52 samples) Takada et al. (1992b)
Estuaries (1 site, 52 samples) 1983-84 4.82 (0.12-36.6) Takada et al. (1992b)
Tokyo Bay (9 sites, 7 samples, 1 year) 1980 71.0 Takada & Ishiwatari (1987)
Tokyo Bay 1984 0.02 (ND-0.06) Takada et al. (1992a)
Sumida River (12 sites, 1 sampling) 1982 0.069 Kikuchi et al. (1986)
Tama River (3 sites, 8 samples) 1977 3.5-86.3 Hon-Nami & Hanya (1980b)
Tama River (10-12 sites) 1982 0.141 Kikuchi et al. (1986)
Tokyo Bay (10-12 sites) 1982 < 0.001-0.002
Table 11 (contd)
Location Year Concentration (mg/kg) Reference
MBAS LAS
Japan (contd).
Tsurumi River (7 sites, 12 samples) 1984 17-45a Yoshikawa et al. (1985)
Tama River 1981 2.79-10.72 Yoshimura et al. (1984b)
Ports and coast 1977 < 1-2.9 Environment Agency Japan
(1978)
ND, not determined
a Dry weight
Table 12. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in sediment of rivers in
Germany and the United States at various distances from effluent outfalls
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
German rivers (14 sites, several 1978-82 Below outfall 1.5-174a De Henau et al.
samples) (1986)
United States
Rivers (4 sites, 45 samples) 1978-82 Below outfall 190 Rapaport &
yearly < 5 miles (8.0 km) 11.9 Eckhoff
> 5 miles (8.0 km) 5.3 (1990)
(1 sampling) 0.5 miles (0.8 km) 118-317 100-322 Osburn (1986)
4.4 miles (7.1 km) 4.1-19 2.0-5.1
7.4 miles (11.9 km) 7.5-10.6 1.3-4.4
Rapid Creek, South Dakota 1979-80 0.8 km 44.6-275 Games (1983)
7 km 3.2-9.1
11.7 km 2.1-8.4
25.3 km 2.7-10.1
48 km 1.4
87.2 km 1.5
Little Miami River, Ohio Downstream from sewage ND-1.2 Hand et al. (1990)
4 sites, 1 sampling) treatment plants 24.7-290b
Rivers (4 sites, 45 samples) 1978-82 Below outfall 190 Rapaport &
Above outfallc 1.0-1.2 Eckhoff (1990);
Below outfall (left)c 0.3-1.6 McAvoy et al.
Below outfall (middle)c 0.6-3.8 (1993)
Table 12 (contd)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United States (contd)
Rivers (4 sites, 45 samples) 1978-82 Below outfall (right)c 0.8-3.4
(contd). Above outfalld 0.2-0.9
Below outfall (left)d 0.2-130
Below outfall (middle)d 0.6-124
Below outfall (right)d 9-340
a 13 of the 14 samples contained < 25 mg/kg and 10 contained < 10 mg/kg
b Suspended solids
c Activated sludge
d Trickling filter
Concentrations of LAS > 10 mg/kg were measured in sediments
from the upper estuaries near Tokyo Bay and < 1 mg/kg in the lower
estuaries. The concentrations of LAS in sediments decreased
offshore, falling below 0.01 mg/kg in sediments sampled 10 km from
the mouths of the rivers. The authors suggested that loss of LAS was
due to rapid degradation in the coastal zone (Takada et al., 1992a).
It was reported in one study that C13 was the most abundant
homologue of LAS in river sediment (Yoshikawa et al., 1985); another
group found that C12 was the most abundant of the LAS in estuarine
sediments and that no C10 were present (Utsunomiya et al., 1980).
C12 and C13 LAS predominated in sediment and C10 and C11
homologues were the most abundant in water (Hon-Nami & Hanya,
1980b). The average chain length of LAS in Japanese river sediments
was C11.8-C12.2 (Hon-Nami & Hanya, 1980b; Yoshimura et al.,
1984a).
In a study of marine sediments from an area adjacent to the
point of discharge from a submarine sewer, LAS were detected only in
the vicinity of the discharge, at a concentration of 0.1 mg/kg, and
not in sediment sampled 50 m outside this area. The average chain
length was C11.7. In a comparison of the chain lengths of LAS
detected in various environmental compartments and those used in
detergent products, the LAS detected in sludge and sediment were
relatively higher homologues and those in the water phase were
lighter (Prats et al., 1993).
The average concentration of LAS in river sediments sampled
upstream of an activated sludge treatment plant outfall was
1.1 mg/kg, and those in sediments downstream of the plant were
0.3-3.8 mg/kg (McAvoy et al., 1993).
A5.1.3 Surface water
The concentrations of LAS in water are shown in Table 13 and
those in samples taken at various distances from sites of effluent
outfall in Table 14.
After replacement of branched-chain ABS, which are only
sparingly biodegradable, with the straight-chain LAS, the
concentrations of MBAS decreased in many rivers. ABS were replaced
by LAS in Japan in the late 1960s; the ratio of LAS to total ABS in
river water rose from 20 to 70% in 1967-70 and had reached 90% by
1973 (Miura et al., 1968; Ihara et al., 1970; Oba et al., 1975). The
levels of MBAS were monitored in the Illinois River, United States,
from 1959 to 1966; those in 1965 and 1966 reflected the change in
surfactant usage (Sullivan & Evans, 1968), and this trend continued
in 1967 and 1968 (Sullivan & Swisher, 1969). In the River Rhine, the
level of anionic detergents, measured as MBAS, fell steadily between
1971 and 1977 (Hellmann, 1978). In water samples from 140 sites on
Table 13. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in water
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Freshwater
United States
Rivers (4 sites, 45 samples yearly) 1978-86 0.041-0.115 Rapaport & Eckhoff
(1990)
Little Miami River, Ohio (4 sites, < 0.05 Hand et al. (1990)
one sampling) Interstitial ND-0.08
Illinois River (one sampling)a 1959-65 0.54 Sullivan & Swisher
1965-66 0.22 (1969)
1968 0.05-0.06
Rapid Creek, South Dakota 1979-80 0.01-0.270 Games (1983)
Mississippi River (36 sites) 1991-92 < 0.01-0.3 McAvoy et al. (1993)
(350 samples) < 0.01-0.046 < 0.005 Tabor et al. (1993)
Japan
Rivers (23 sites, 51 samples) 1977 < 0.01-2.9 Environment
Agency Japan (1978)
Rivers (1 sampling) 0.018-0.59 Tsukioka &
Murakami (1983)
Oohori River (6 sites monthly) 1987-88 approx. 0.5-1.6 Amano et al. (1991)
Lake Teganuma (6 sites monthly) 1987-88 ND-approx. 0.7
Tama River (3 sites, 8 samples) 1977-78 0.24-1.24 0.108-0.491 Hon-Nami & Hanya
(1980a)
Table 13 (contd.)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Japan (contd)
Rivers, Hyogo Prefecture (70 sites) 0.004-2.5 Kobuke (1985)
Tama River (3 sites, 1 sampling) 0.035-0.219 Yoshikawa et al.
(1984)
Tama River (10-12 sites) 1982 0.128 Kikuchi et al. (1986)
Sumida River (10-12 sites) 1982 0.005-0.01 Kikuchi et al. (1986)
Rivers (1 sampling) 0.06-0.12 Saito & Hagiwara
(1982)
Rivers, Niigata Prefecture (6 sites, 0.02-2.63 0.18 (max) Motoyama & Mukai
1 sampling)
(1981)
Rivers, coastal area, Hiroshima 0.019 Okamoto & Shirane
Prefecture (20 sites) (0.001-0.06) (1982)
Inland Sea, Eastern Seto (4 sites, 1975 0.016-0.077 Yoshida & Takeshita
1 sampling) (1978)
(17 sites, 1 sampling) 1976 0.01-0.048
Tsurumi River, Kanagawa 1984-76 Surface 0-0.8 0.01-0.29 Yoshikawa et al.
(7 sites once) (1985)
Yodo River, Osaka (several sites) 1989 Surface 0.043-0.089 Nonaka et al. (1990)
Tama River, Tokyo (2 sites, 1981 Surface 0.2 Yoshimura et al.
4 samples) (1984b)
Sumidogawa River (2 samples) 1983 Suspended 0.0048-0.054 Takada & Ishiwatari
Tomogawa River (5 samples) particles 0.0005-0.0025 (1987)
Teshiro River, Nagoya (4 sites, 1989 Surface 0.01-0.27 Kojima (1989)
4 samples)
Table 13 (contd)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Japan (contd)
Lake Biwa, Shiga 1988 Surface 0.00 Shiga Prefecture
(1988)
Teganuma, Chiba (1 site, 1988 Surface ND-0.423 Amano et al. (1989)
12 samples)
River (several sites) 1988 Surface 0.019-1.4 Nonaka et al. (1989)
Nagoya Bay 1989 Surface 0.00 Kojima (1989)
Rivers, Fukuoka City ND-1.6 Ohkuma (1981)
Europe
River Rhine (several sites) 1971-72 0.08-0.24 Hellmann (1978)
Saar River (11 sites, 1 sampling) 1985 0.13 0.04 Matthijs & De Henau
(0.03-0.25) (0.01-0.09) (1987)
German rivers (several sites) 1976-79 0.075-0.5 Fischer (1980)
Dutch river (Amsterdam drinking- 0.004-0.141 0.003-0.037 Waters (1976)
water supply) (8 sites)
Florence, Italy (several samples) 1983 Aqueduct 0 .01-0.1 Mancini et al. (1984)
(several sites) 1982 Well water 0.00-0.01
United Kigdom
Rivers 1982 0.04-0.26 0.012-0.08 Gilbert & Pettigrew
(1984)
Rivers (8 sites) 0.035-0.217 0.009-0.097 Waters (1976)
Rivers (4 sites) 1977-78 0.022-0.473 0.007-0.173 Waters & Garrigan
(1983)
Table 13 (contd)
Location Year Water Concentration (mg/litre) Reference
sample
MBAS LAS
Groundwater 1992 < 0.01-0.02 Field et al. (1992)
Estuarine and marine water
North Sea (19 sites) 1989 < 0.0005-0.0012 Stalmans et al.
(1991)
Krka River estuary, Croatia 1990 Wastewater 0.42-0.78 Terzic & Ahel (1993)
(below 50 m > 50 m) Estuarine water 0.003-0.007
0.001-0.002
Tokyo Bay, Japan (8 samples) 1978 0.03-0.07 < 0.003-0.014 Hon-Nami & Hanya
(1980a)
Tokyo Bay, Japan (10-12 samples) 1982 0.001-0.03 Kikuchi et al. (1986)
Osaka Bay, Japan (several sites) 1988 Surface ND-0.0072 Nonaka et al. (1989)
ND, not detected
a 10-20% of MBAS were LAS
Table 14. Concentrations of methylene blue-active substances (MBAS) and linear alkylbenzene sulfonates (LAS) in water at various
distances from effluent outfalls
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United States
Rivers (4 sites, 45 samples 1978-86 Below outfall 0.115 Rapaport & Eckhoff
yearly) < 5 miles (8 km) 0.079 (1990)
> 5 miles (8 km) 0.041
(1 sampling) 0.5 miles (0.8 km) 0.400 0.270 Osburn (1986)
4.4 miles (7.1 km) 0.300 0.150
7.4 miles (11.9 km) 0.250 0.120
15.8 miles (25.4 km) 0.240 0.100
30.0 miles (48.3 km) 0.130 0.040
55.0 miles (88.5 km) 0.100 0.010
Rapid Creek, South Dakota 1979-80 0.8 km 0.270 Games (1983)
7 km 0.150-0.190
11.7 km 0.120
25.3 km 0.080
48 km 0.040
87.2 km 0.010
Rivers Above outfall < 0.01-0.9 McAvoy et al. (1993)
Below outfall (left) < 0.01-0.33
Below outfall (middle) < 0.01-0.3
Below outfall (right) < 0.01-0.3
Table 14 (contd)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
Canadian rivers (4 sites, 45 1978-86 Below outfall 0.053 Rapaport & Eckhoff
samples yearly) (1990)
Rio Grande, Brazil 1979 90 m 0.05-4.5 Kantin et al. (1981)
(1 sampling, 50 samples)
German rivers (several sites) 1976-79 Unpolluted 0.075 Fischer (1980)
Polluted 0.2-0.5
(4 sites, 45 samples yearly) 1978-86 Below outfall 0.01-0.09 Rapaport & Eckhoff
(1990)
United Kingdom
Rivers (several samples) 1982 Above discharge 0.04 0.012 Gilbert & Pettigrew
(0.02-0.07) (0.008-0.019) (1984)
Close to discharge 0.26 0.08
(0.11-0.47) (0.01-0.17)
5-16 km 0.16 0.04
(0.08-0.23) (0.008-0.095)
Avon River (4 sites) 1977-78 Head water 0.03-0.039 0.009-0.015 Waters & Garrigan
0.5 km 0.21-0.371 0.056-0.173 (1983)
6 km 0.095-0.22 0.011-0.095
Tean River (4 sites) 1977-78 Head water 0.035-0.073 0.008-0.019
Directly below sewage 0.208-0.473 0.067-0.144
treatment
5 km 0.145-0.234 0.019-0.07
Table 14 (contd.)
Location Year Sampling site (distance Concentration (mg/litre) Reference
from effluent outfall)
MBAS LAS
United Kingdom (contd)
Trent River (4 sites) 1977-78 Head water 0.022-0.052 0.01-0.011
20-35 km below head 0.08-0.227 0.007-0.072
water
Nene River tributary 1978 0.104 0.011
(4 sites)
In the vicinity of 0.206-0.216 0.035-0.037
sewage effluent disharge
3.5 km 0.184 0.035
13.5 km 0.06 0.007
four German rivers, MBAS concentrations fell by 90% between 1964 and
1987 (Gerike et al., 1989).
The mean level of MBAS in rivers in the United Kingdom was
0.15 mg/litre. On average, only 26% was attributable to LAS (by
microdesulfonation and gas-liquid chromatography), but the levels of
LAS and their contribution to the total MBAS concentration varied
according to the sampling site, with a higher proportion of LAS in
samples from sites near sewage effluent discharge points (Waters &
Garrigan, 1983). Similar findings were reported by Gilbert &
Pettigrew (1984), who found that LAS represented 45% of total MBAS
in actual sewage. Sites immediately below sewage outfalls were found
to have higher MBAS:LAS ratios than sites further downstream
(Osburn, 1986).
In Lake Biwa basin, Japan, during the summer months of 1983, LAS
were found in a wide range of concentrations. The highest, measured
as MBAS, were > 0.2 mg/litre at river mouths. The levels in rivers
flowing from densely populated areas were 0.05-0.2 mg/litre MBAS and
those flowing from less populated areas were < 0.05 mg/litre. The
middle stream zone of the River Isasa, in a densely populated area,
contained levels of 0.36-1.91 mg/litre, and surfactant levels in
residential areas showed daily fluctuations related to discharge
(Sueishi et al., 1988). Several observations apply to these studies.
Firstly, the fact that daily fluctuations were observed indicates
that the samples may have been taken from the actual discharge
plume, so that the wastewater effluent may not have been completely
mixed with the recipient surface water. Secondly, in several
Japanese studies of heavy discharge zones, anionic surfactants could
not be detected in surface waters, although the analytical detection
limit of MBAS in the mid-1980s was 0.05-0.1 mg/litre. Thirdly,
sewage treatment at several of the sites has improved considerably
over the last decade.
Seasonal trends in the concentrations of LAS were observed in
the Oohori River and Lake Teganuma, Japan, in 1987 and 1988, with
low levels in summer and high levels in winter (Amano et al., 1991).
The concentrations of LAS were measured in the Tamagawa River,
Japan, at two-week intervals for two years, by sampling water from
the boundary between freshwater and brackish zones. The
concentrations measured in winter were about five times higher than
those measured in summer, when long-chain homologues tended to be
depleted. The distribution of isomers also showed a clear seasonal
trend, with a greater loss of external isomers in summer. The
seasonal changes are thought to be the result of differences in
water temperature and microbial activity. The flux of LAS in the
river was estimated to be 320 tons/year (293 tonnes/year), which
exceeds the total amount of LAS accumulated in the bay sediment,
indicating that > 99.9% of LAS in the estuary and the bay was
degraded (Takada et al., 1992b).
The concentrations of LAS in suspended particles from
tributaries of Tokyo Bay, Japan, were 0.5-53.8 µg/litre. Those in
suspended particles from a wastewater influent were 297-504 µg/litre
and those in the effluent, 0.1-1.22 µg/litre (Takada & Ishiwatari,
1987).
The concentrations of LAS in the estuary of the Krka River,
Croatia, were 420-780 µg/litre near municipal wastewater outlets; 50
m from the wastewater outlets, the concentrations were 7.2 µg/litre
at a depth of 0.5 m and 3.2 µg/litre at a depth of 6 m. The
concentrations in water sampled more than 50 m from the input area
were 1-2 µg/litre. The Krka River estuary was reported to be highly
stratified, with vertical transport of pollutants reduced by the
freshwater-saline boundary. The concentrations of LAS were
negatively correlated with salinity; the maximum concentration,
24 µg/litre, was detected in the surface monolayer. An increase in
the relative abundance of lower homologues of LAS (C10 and C11)
was reported in comparison with the original distribution of
homologues in the wastewater, indicating more rapid depletion of
higher homologues, possibly by biodegradation and fast settling with
particles from sewage (Terzic & Ahel, 1993).
In a comparison of the distribution of homologues of LAS in the
Tama River, Japan, with those established for active substances used
in commercial detergents, the levels of C12 and C13 LAS were
found to decrease over time and those of C10 and C11 to increase
(Hon-Nami & Hanya, 1980a). C11 was the commonest LAS homologue in
river water (Kobuke, 1985; Yoshikawa et al., 1985), and no C13 LAS
were present (Utsunomiya et al., 1980). The average chain length of
LAS in Japanese rivers was C10.9-C11.2 (Nakae et al., 1980;
Yoshimura et al., 1984a; Kobuke, 1985).
Several research groups have confirmed that such changes in
chain length occur during the environmental passage of LAS. In a
study in which the concentration of homologues of LAS was measured
quantitatively by HPLC during activated sludge treatment and lagoon
treatment of wastewater in Spain, the average chain length decreased
from C11.7 in raw material, to C11.3 in the dissolved phase of
raw wastewater, and to C10.3 in the dissolved phase of treated
effluent. A slight increase in average chain length was reported for
the solids compartment in each of these systems, adding to
laboratory findings that the longest homologues adsorb most strongly
to sediment. The reduction in average chain length in the water
compartments was environmentally significant, since shorter
homologues of LAS are less toxic to aquatic organisms. Thus, the
LC50 values for daphnia were higher for shorter homologues (>
20 mg/litre for C11 and 10 mg/litre for C11.7) (Prats et al.,
1993).
The Japanese Soap & Detergent Association (1992) reported a
decrease in LAS concentrations in the Tama River near Tokyo, Japan,
from 2.3 mg/litre in 1967 to 0.2 mg/litre in 1991. The decrease was
attributed to the development of sewage systems along the river:
sewage coverage was 26% in 1974 and 89% in 1990. This information
can be used to estimate concentrations of LAS in developing
countries with inadequate sewage systems but where detergent use is
increasing.
Low levels of LAS were reported in water from the Scheldt River
estuary and in a series of samples from the North Sea (see Table
13). The concentrations in the estuary decreased rapidly from about
0.010-0.012 mg/litre to values below the limit of analytical
detection (0.5 µg/litre) concurrently with an increase in salinity.
The concentrations decreased more rapidly than on the basis of
dilution alone, indicating that removal occurred rapidly. The
authors did not report whether the removal of LAS was related to
adsorption onto settling solids, to biodegradation, or to a
combination of the two. The concentration of LAS in samples taken
offshore was consistently below the limit of detection (Stalmans et
al., 1991).
A5.1.4 Soil and groundwater
The levels of LAS in sludge-amended soil were 0.9-1.3 mg/kg in
German soils used for agriculture. A level of 2.2 mg/kg was found in
the United Kingdom in soil that was used only for the disposal of
sludge (De Henau et al., 1986). MBAS were found at a level of
24.7 mg/kg (14.4-37.5 mg/kg) and LAS at 1.4 mg/kg (0.9-2.2 mg/kg) in
German agricultural soils that had been amended with sludge
(Matthijs & De Henau, 1987). The levels of LAS in soils near the
River Thames, United Kingdom, in 1987 to which sludge had been
applied previously were < 0.2-2.5 mg/kg. Soils that had received an
application of sludge during 1987 had levels of LAS of <
0.2-19.8 mg/kg (Holt et al., 1989).
Levels of 13-47 mg/kg were found on the surface of sludge-
amended soil in the United States in 1979; < 5 mg/kg were found at
a depth of 15-90 cm (Rapaport & Eckhoff, 1990).
A concentration of 22.4 mg/kg LAS was measured in agricultural
soil that had recently been amended with anaerobically digested
sludge. The concentration was 3.1 mg/kg six months after application
of the sludge and 0.7 mg/kg after 12 months (Prats et al., 1993).
HPLC, fluorescence detection, and mass spectrometry were used to
analyse samples of a groundwater plume which originated from an
underground discharge of sewage. It was found that 96% of the LAS
was removed from the aqueous phase during sewage treatment and an
additional 3% during infiltration with groundwater. The
concentrations in ground-water were below the detection limit of
0.01-0.02 mg/litre. The disappearance of LAS during groundwater
infiltration was calculated to follow first-order kinetics. LAS were
detected (by mass spectrometry) at only trace levels in groundwater
sampled 20-500 m down the gradient from the infiltration zone (Field
et al., 1992).
A5.1.5 Drinking-water
The concentration of LAS reported in Dutch tap-water was
0.003 mg/litre; MBAS levels were about three times higher. In
tap-water in the United Kingdom, the concentration of LAS was
0.007 mg/litre; that of MBAS was again three times higher (Waters,
1976). The concentrations of LAS in Italian well-water were below
the analytical limit of detection of 0.0084 mg/litre (Mancini et
al., 1984). LAS were not detected in Japanese drinking-water in the
1970s at a limit of detection of 0.001 mg/litre (Yushi, 1978).
A5.1.6 Biota
The concentrations of LAS in biota are shown in Table 15.
A5.2 Environmental processes that influence concentrations of
linear alkylbenzene sulfonates
A shift towards LAS of lower chain lengths has been reported in
environmental samples in comparison with the distribution of chain
lengths in raw materials. It has also been reported that about 50%
of the total LAS in samples of water is associated with either
suspended particles or dissolved organic matter. Reductions in both
the chain length and the concentration of dissolved LAS will result
in decreased aquatic toxicity (see also section 9).
A5.2.1 Changes in chain length distribution during environmental
removal of linear alkylbenzene sulfonates
The concentrations of LAS and related compounds were measured in
350 samples of water and sediment from the Mississippi River, United
States. Those in surface water were < 0.005 mg/litre. LAS in
sediment had longer chains than those in the overlying water column
(Tabor et al., 1993).
A gradual reduction in the average chain length of homologues
was observed as they passed through a wastewater treatment plant:
untreated wastewater, C12.1; treated effluent, C12; surface
water below a sewage outfall, C11.7 (Castles et al., 1989).
Isomers of C13 LAS have partition coefficients that are typically
one order of magnitude higher than those of the corresponding
isomers of the C12 LAS homologues (Amano et al., 1991).
Table 15. Total body concentrations of linear alkylbenzene sulfonates
in biota in Japan
Organism Year Location Concentration Reference
(mg/kg dry
weight)
Algae 1980-81 River < 1-368 Katsuno et al. (1983)
Pond snail 1979 River 0.4-1.81 Tanaka & Nakanishi
(Sinotaia (1981)
quadratus
histrica)
Gizzard shad 1982 Bay < 1 or < 2 Tokai et al. (1990)
(Konosirus 1983 < 0.1-0.3
punctatus)
A5.2.2 Specification of linear alkylbenzene sulfonates in
surface waters
In most programmes for monitoring LAS in the environment, the
total sample of waste or surface water is analysed, and separate
concentrations of LAS in the fractions of dissolved and suspended
solids are not determined. In a study in which these concentrations
were reported, the mean levels of dissolved LAS were 8.4 mg/litre in
raw wastewater (range, 5.6-11.4 mg/litre) and 5.5 mg/litre in the
suspended solid fraction. In the seven wastewaters studied, an
average of about 65% was present in the filtered (filtration, <
1 µm) 'dissolved' fraction and 35% in the 'solids-associated'
fraction. In treated effluent, 85% of LAS was in the dissolved
fraction and 15% in the solids-associated fraction (Berna et al.,
1993b). In wastewater treatment works, 49-63% of the LAS was in the
dissolved phase and 37-51% in the solids-associated phase (Berna et
al., 1989). In filtered (0.7 µm) wastewater containing LAS at
2.55-2.95 mg/litre, 25-30% LAS was dissolved, and the remaining
70-75% was associated with the solid phase (Cavalli et al., 1991).
The average chain length of homologues of LAS in raw wastewater
was lower in the dissolved phase (C11.2-C11.4) than in the
solids-associated phase (C11.9-C12.0). The authors reported that
39-43% of LAS was present in the dissolved phase and 57-61% in the
solids phase (Prats et al., 1993).
Humic acids extracted from sediments and soils formed strong
association complexes with LAS under environmental conditions, as
observed with fluorescence quenching techniques. The bioavailabilty
of LAS to aquatic organisms is reduced as a result of these
complexes (McAvoy et al., 1993).
A5.3 Estimation of human intake
Human daily intake has been estimated on the assumption that LAS
are taken up from drinking-water and from washing food, vegetables,
dishes, and the skin. The estimates vary from 4.5 to 14.5 mg/day
(Ikeda, 1965; Tokyo Metropolitan Government, 1974; Sterzel, 1992).
The higher figure is based on dubious assumptions about the
concentrations of LAS on vegetables, and the lower value is probably
a more realistic estimate.
The human intake of all anionic surfactants is estimated to be
0.044-0.944 mg/kg per day (Sterzel, 1992), and the maximum daily
intake of ABS, 0.14 mg/kg per day (Ikeda, 1965).
A6. KINETICS
Section summary
LAS are readily absorbed by experimental animals in the
gastrointestinal tract, are distributed throughout the body, and are
extensively metabolized. The parent compound and metabolites are
excreted primarily via the urine and faeces, although there are
marked differences between the isomers in the route of excretion.
The main urinary metabolites identified in rats are
sulfophenylbutanoic acid and sulfophenylpentanoic acid, which are
probably formed through omega-oxidation followed by ß-oxidation of
LAS, although the metabolic pathways in primates may differ.
Although few data are available, it would appear that dermally
applied LAS are not readily absorbed through the skin, although
prolonged contact may compromise the epidermal barrier and permit
more extensive absorption.
A6.1 Absorption, distribution, and excretion
After oral administration of 2 mg/animal of the calcium or
sodium salt of 14C-LAS (chain length, C12) to Wistar rats,
radiolabel was detected in plasma after 0.25 h, reaching maxima at
2 h (0.86 and 1.00 µg/g of the two salts, respectively), and then
decreasing gradually with time; the mean biological half-lives were
calculated to be 10.9 and 10.8 h, respectively. Four hours after
oral administration of the calcium or sodium salt, the concentration
of radiolabel was high in the digestive tract (especially in the
stomach: 22.56 and 31.67 µg/g as the parent compound or metabolites;
and large intestine: 43.24 and 27.26 µg/g) and in the urinary
bladder (34.89 and 16.58 µg/g). The concentrations were also high in
the liver (2.73 and 2.13 µg/g), kidney (1.19 and 1.35 µg/g), testis
(0.08 and 0.11 µg/g), spleen (1.63 and 0.16 µg/g), and lung (0.49
and 0.44 µg/g). At 48 and 168 h, there was little further change.
During the 168-h period after administration, 50% of the radiolabel
on the calcium salt was excreted in urine and 51% in faeces, and 47%
of that on the sodium salt was excreted in urine and 50% in the
faeces (Sunakawa et al., 1979).
Doses of 1 mg per 200 g body weight of two radiolabelled LAS
isomers (chain length, C12) with the benzene sulfonate moieties at
the 2 and 6 positions were administered orally and intravenously to
rats; the same dose was also administered to anaesthetized rats with
bile-duct cannulas by intravenous or intraduodenal injection.
Forty-eight hours after oral or intravenous administration, there
were marked differences in the disposition of the isomers in the
urine and faeces: most of the radiolabel associated with the 2
isomer (75.3%) was in the urine, whereas most of that on the 6
isomer (77.9%) was present in the faeces. After intravenous
administration to bile duct-cannulated rats, 88.6% of the 2 isomer
was recovered in the urine, whereas 83.1% of the 6 isomer was in the
bile. Studies of absorption after intraduodenal administration
showed that both isomers were extensively absorbed within 6 h
(Rennison et al., 1987).
After a dose of 1.2 mg 35S-LAS in aqueous solution was
administered by gavage to bile duct-ligated rats, 89% was absorbed
from the gastro-intestinal tract, as seen by the presence of
radiolabel recovered in urine. Absorption probably occurred mainly
via portal venous blood, since only 1.6% was recovered in the
lymphatic system. When the same dose was administered to bile
duct-cannulated rats, 46% of the radiolabel was recovered in urine,
29% in faeces, and 25% in bile after 90 h. Enterohepatic circulation
was determined in a study in which the bile from one rat was
transmitted to the intestine of another through a cannula; all of
the radioactive LAS excreted in the bile was reabsorbed. In a
separate study, 40-58% of single oral doses of 35S-LAS ranging
from 0.6 to 40.0 mg was excreted in the urine and 39-56% in the
faeces within 72 h of administration (Michael, 1968).
The excretory pattern of 14C-sodium dodecylbenzene sulfonate
was examined in male rats administered a concentration of 1.4 mg/kg
of diet daily for five weeks. The total intake was 1213 µg/rat, of
which 81.8% was excreted during the dosing period, with 52.4% in the
faeces and 29.4% in the urine. After a further week on a normal
diet, however, only 7.8% of the estimated residual amount was found
in excreta. Of a single intraperitoneal injection of 0.385 mg
14C-sodium dodecylbenzene sulfonate/rat (2.26 mg/kg body weight),
84.7% was eliminated within the first 24 h and 94.5% within 10 days
(Lay et al., 1983).
LAS were not detected in the uterus of pregnant ICR mice
administered a single oral dose of 350 mg/kg body weight on day 3 of
gestation (Koizumi et al., 1985).
14C-LAS (chain length, C10-C14, predominantly C11,
C12, and C13) were applied at 250 µg/7.5 cm2 in water to
clipped dorsal skin of rats; the treated area was washed after
15 min, and the animals were restrained from grooming. Most of the
radiolabel was rinsed off, but some of the 14C-LAS
(11 ± 4 µg/cm2) were detected on the treated area; none were
detected in urine or faeces 24 h after the application. In an
accompanying study in vitro, there was no measurable penetration of
14C-LAS (chain length, C12) through isolated human epidermis or
rat skin 24 or 48 h after application (Howes, 1975).
A mixture of 35S-LAS and white petrolatum (29 mg/0.3 ml) was
applied to a 4-cm2 area of the dorsal skin of guinea-pigs, and 24
h after the application about 0.1% of the applied dose was found in
urine and about 0.01% in blood and the main organs. After dermal
application of the same dose to rats and guinea-pigs, the
concentration of 35S in the liver was 9.7 µg/g equivalent of LAS
in rats and about 0.4 µg/g in guinea-pigs (Hasegawa & Sato, 1978).
After a single oral administration of 150 mg/kg 14C-LAS (mean
relative molecular mass, 349) in aqueous solution to rhesus monkeys
(Macaca mulatta), plasma concentrations of radiolabel reached a
maximum equivalent to 41.2 µg/ml at 4 h and then declined over
6-24 h, with a biological half-life of about 6.5 h. The observed
peak plasma concentration of radioactivity (33.6 µg/ml) and the
biological half-life (about 5 h) after seven consecutive daily oral
administrations of 30 mg/kg body weight were similar to those found
after a single administration. The highest concentration of 14C
(238.6 µg/g) was found in the stomach 2 h after the last dose.
Concentrations were also high in the intestinal tract (108 µg/g),
kidney (135.6 µg/g), and liver (64.8 µg/g) and were moderately high
in the lung (19.8 µg/g), pancreas (17.7 µg/g), adrenal glands
(20.6 µg/g), and pituitary gland (17 µg/g). At 24 h, the
concentrations were higher in the intestinal tract (255.4 µg/g) and
liver (10.5 µg/g) than in plasma (2.4 µg/g), whereas those in most
tissues were lower than those in plasma, indicating that there is no
specific accumulation or localization of LAS and their metabolites
in these tissues. After seven subcutaneous doses of 1 mg/kg per day
of 14C-LAS, most of the radiolabel remained in the skin; the
concentration was generally highest at the injection site
(113.96 µg/g). The levels of radiolabel were also high in the
intestinal tract (2.41 µg/g), kidney (1.83 µg/g), lung (2.45 µg/g),
spleen (2.43 µg/g), thyroid (1.24 µg/g), and pituitary (1.00 µg/g)
at 2 h. The concentration in most tissues was generally lower at
4 h, except in the intestinal tract (3.50 µg/g), liver (1.74 µg/g),
and kidney (1.92 µg/g). The high level of radiolabel in the
intestinal tract probably indicates biliary excretion. The average
rates of excretion of radiolabel in urine and faeces during 120 h
after administration of single oral or subcutaneous doses of
14C-LAS to male and female rhesus monkeys are shown in Table 16.
In animals of each sex, radiolabel was excreted primarily in the
urine after either route of administration (Cresswell et al., 1978).
When sodium 35S-dodecylbenzenesulfonate (3.3 mmol/kg body
weight) was administered in the diet to young pigs, at least 35% of
the dose was absorbed through the intestinal tract. After 40 h,
30-40% of the dose had been excreted in urine and > 60% in faeces.
The concentration of radiolabel after 200 h was relatively high in
bristles and bones and low in liver, kidney, and spleen
(quantitative data not presented). After 10 weeks, traceable amounts
of 35S (0.05% of the administered dose) were found in bristles,
bones, skin, lung, and brain (Havermann & Menke, 1959).
Table 16. Excretion of 14C-linear alkyl benzene sulfonates in
rhesus monkeys
Route of administration Sex Concentration (%)
Urine Faeces
Oral (30 mg/kg body weight) Male 68.3 25.9
Female 74.0 20.3
Subcutaneous (1 mg/kg) Male 63.8 12.5
Female 64.3 9.2
From Cresswell et al. (1978); values are average rates of excreted
radioactivity during the 120-h period after a single dose.
A6.2 Biotransformation
The main metabolites isolated from the urine of rats
administered 35S-LAS orally were probably a mixture of sulfophenyl
butanoic (I) and sulfophenyl pentanoic acids (II):
CH3-CH-CH2-COOH CH3-CH-CH2-CH2-COOH
| |
O O
| |
SO3H SO3H
(I) (II)
The material used in the experiment was a mixture of C10-C14 LAS
(mainly C11, C12, and C13). The compounds in this mixture are
probably degraded by omega-oxidation, followed by catabolism through
a ß-oxidation mechanism to form the above metabolites, with
excretion of four or five carbons in the urine (Michael, 1968).
After oral administration of the calcium or sodium salt of
14C-LAS to rats, two metabolites were detected in urine and four
in faeces by thin-layer chromatography. The two urinary and two of
the faecal metabolites were believed to be compounds similar to
metabolites (I) and (II) previously identified by Michael (1968)
(Sunakawa et al., 1979).
Thin-layer chromatography of urine extracts after oral or
sub-cutaneous administration of 14C-LAS to rhesus monkeys showed
only trace amounts of the unchanged compound, and five metabolites
more polar than LAS were detected. These metabolites have not been
identified. Incubation of urine samples with ß-glucuronidase or
sulfatase did not affect the components, which were therefore
probably not present as the corresponding conjugates (Cresswell et
al., 1978).
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 values for sodium salts of LAS are
404-1470 mg/kg body weight in rats and 1259-2300 mg/kg body weight
in mice. LAS irritate skin and eyes.
Minimal effects, including biochemical alterations and
histopathological changes in the liver, were reported in subchronic
studies in rats administered LAS in the diet or drinking-water at
concentrations equivalent to a dose of about 120 mg/kg body weight
per day. Although ultrastructural changes in liver cells were
observed at lower doses in one study, these changes appeared to be
reversible. Effects have not been seen at similar doses in other
studies, but the organs may have been examined more closely in this
study. Reproductive effects, including decreased pregnancy rate and
litter loss, have been reported in animals administered doses >
300 mg/kg body weight per day. Histopathological and biochemical
changes have been observed following long-term dermal application on
rats of solutions of LAS at concentrations > 5% and after 30 days'
dermal application on guinea-pigs of 60 mg/kg body weight. Repeated
dermal application of solutions containing > 0.3% LAS induced
fetotoxic and reproductive effects, although these doses also
induced maternal toxicity.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of LAS in experimental animals, owing to the
small number of animals used, low or insufficient doses tested, the
absence of a maximal tolerated dose, and limited histopathological
examination. The limited studies available in which animals were
administered LAS orally, however, provide no evidence of
carcinogenicity.
Limited data also indicate that LAS are not genotoxic in vivo
or in vitro.
A7.1 Single exposures
The LD50 values for the sodium and magnesium salts of LAS
given orally, subcutaneously, or intravenously are summarized in
Table 17. Rats appear to be more sensitive than mice to LAS,
regardless of the route of exposure. The LD50 values for LAS given
orally were 1259-3400 mg/kg body weight in mice and 404-1900 mg/kg
body weight in rats. Differences were seen according to the sex,
strain, and age of the animals and the test material.
Table 17. Acute toxicity of linear alkylbenzene sulfonates
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
Mouse
NR NR Oral 2170 60% active ingredient Yanagisawa et
al. (1964)
DD M Oral 2300 34.55% solution Tiba (1972)
ddY M Oral 1665 Purified Kobayashi et
ICR-JCL F Oral 1950 Purified al. (1972)
M Oral 1250 Commercial soln, 19.0% Kuwano et al.
F Oral 1540 Commercial soln, 19.0% (1976)
M Oral 1370 Commercial soln, 17.1%
F Oral 1560 Commercial soln, 17.1%
M Oral 2160 99.5% active ingredient Ito et al. (1978)
of C10-C13
F Oral 2250 99.5% active ingredient
of C10-C13
M Oral 2600 Magnesium salt of above
F Oral 3400 Magnesium salt of above
M s.c. 1250 99% active ingredient Ito et al. (1978)
of C10-C13
F s.c. 1400 99% active ingredient
of C10-C13
M s.c. 1529 Magnesium salt of above
F s.c. 1550 Magnesium salt of above
Table 17 (contd)
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
ICR-JCL M i.v. 207 99% active ingredient
(contd) of C10-C13
F i.v. 298 99% active ingredient
of C10-C13
M i.v. 98 Magnesium salt of above
F i.v. 151 Magnesium salt of above
NR NR i.v. 120 Yanagisawa et al.
(1964)
Rat
FDRL M,F Oral 650 Nominal chain length, Oser & Morgareidge
C12 (range C9-C15) (1965)
Wistar
6 w M Oral 873 Purified Kobayashi et
6 w F Oral 760 al. (1972)
10 w M Oral 404
10 w F Oral 409
M Oral 1460 99.5% active ingredient Ito et al. (1978)
of C10-C13
F Oral 1470 99.5% active ingredient
of C10-C13
M Oral 1900 Magnesium salt of above
F Oral 1840 Magnesium salt of above
Table 17 (contd)
Species/ Sex Route LD50a Test materialb Reference
strain (mg/kg
body
weight)
CRJ-SD M s.c. 840 99.5% active ingredient
of C10-C13
F s.c. 810 99.5% active ingredient
of C10-C13
M s.c. 710 Magnesium salt of above
F s.c. 730 Magnesium salt of above
M i.v. 119 99.5% active ingredient
of C10-C13
F i.v. 126 99.5% active ingredient
of C10-C13
M i.v. 27.2 Magnesium salt of above
F i.v. 35.0 Magnesium salt of above
NR, not reported; M, male; F, female; s.c., subcutaneous; i.v., intravenous;
w, weeks
a As active ingredient
b Sodium salt, unless specifically indicated
The main clinical signs observed after oral administration of
doses near or greater than the LD50 consisted of reduced voluntary
activity, piloerection, diarrhoea, and weakness. Diarrhoea was more
severe in rats than mice (Kobayashi et al., 1972). Convulsions,
torsion, and paralysis of the hind limbs were also observed in some
of mice (Kobayashi et al., 1972; Kuwano et al., 1976). Death usually
occurred within 24 h of administration. Transient cardiac arrest,
dyspnoea, cyanosis, respiratory collapse, and death occurred during
intravenous injection (Ito et al., 1978).
At autopsy, hyperaemia and haemorrhage of the stomach and
intestine, bloating of the intestine with thinning of its wall, and
congestion of some internal organs were the main macroscopic
findings; histological examination showed congestion and epithelial
degeneration of the gastrointestinal mucosa (Kobayashi et al., 1972;
Kuwano et al., 1976; Ito et al., 1978).
A7.2 Short-term exposure
A7.2.1 Mouse
In a study of the toxicity of a commercial preparation of LAS
(17.1% active ingredient), 44 male and 16 female C57Bl TW mice were
given subcutaneous injections according to the following schedule:
0.02 ml of 1% of the preparation for 10 consecutive days from the
day of birth, 0.04 ml of the same solution for the following 10
days, 0.02 ml of a 10% solution five times over the next 10 days,
and 0.04 ml of the same solution every other day for a further 30 or
60 days. Eight males and six females served as untreated controls.
Epilation and dermatitis usually occurred in animals given
continuous injections of the test material. Adhesions between some
organs, most frequently between the spleen and kidney, were observed
in those receiving injections from the day of birth. Neither the
growth nor the survival of the animals was affected. Although the
weights of the liver, kidney, and spleen were significantly
increased in animals receiving treatment for 60 days,
histopathological examination of the liver, kidney, adrenal glands,
and thyroid by light and electron microscopy showed no evidence of
toxicity (Kikuchi, 1978).
A7.2.2 Rat
A7.2.2.1 Administration in the diet
Groups of five male Wistar rats were fed diets containing LAS
(60% active ingredient; chain length distribution: 10.6% C10, 34.1%
C11, 27.7% C12, 19.0% C13, 8.7% C14) at a concentration
of 0, 0.6, 1.2, or 1.8% (equivalent to 180, 360, or 540 mg/kg body
weight per day) for two and four weeks, and lipids in serum and
liver were analysed. Body weight gain was suppressed in the group
receiving 1.8% at four weeks, and the relative liver weight was
increased at two weeks and thereafter in the groups receiving 1.2
and 1.8%. The levels of triglyceride and total lipids in the serum
had decreased markedly at two weeks in all the experimental groups,
and the levels of phospholipids and cholesterol in the serum had
decreased significantly at two weeks in the groups given 1.2 and
1.8%. These changes were less apparent at four weeks, but
triglyceride, phospholipid, and cholesterol levels in serum were
significantly decreased in the group given 1.8%. Significant
increases in triglyceride levels were seen in the liver after two
weeks in the groups receiving 0.6 and 1.8%, and in cholesterol
levels in the group given 0.6% (Yoneyama & Hiraga, 1977).
Technical-grade sodium LAS (87.9% active ingredient; chain
length distribution: 1.8% C10, 43.2% C11, 32.2% C12, 5.3%
C14, 1.5% C15) were fed to five groups of 10 weanling
Sprague-Dawley rats of each sex at a dietary level of 0, 0.02, 0.1,
or 0.5% (equivalent to 8.8, 44, or 220 mg/kg body weight per day)
for 90 days. No adverse effects were found on survival, growth, food
conversion efficiency, haematological values, urinary analytical
values, or absolute or relative organ weights. There were no gross
or microscopic histological changes attributable to ingestion of the
test material (Kay et al., 1965).
Technical-grade LAS (normal chain length, C12; range,
C9-C15; mean relative molecular mass, 346) were fed to three
groups of weanling FDRL rats, each consisting of 15 males and 15
females, at a dose of 0, 0.05, or 0.25 g/kg body weight per day for
12 weeks. No adverse effects were noted on survival, behaviour,
growth, food conversion efficiency, haematological measurements,
blood chemistry, urine analytical values, organ weights, or gross or
microscopic appearance, except for a slight increase in liver weight
in females given 0.25 g/kg body weight per day (Oser & Morgareidge,
1965).
A diet containing LAS at a concentration of 1.5% (equivalent to
750 mg/kg body weight per day) or a control diet was given to groups
of five male Wistar rats for 2, 4, or 12 weeks. LAS depressed body
weight gain, and the relative liver weight was significantly
increased after two weeks of treatment. The activities of alkaline
phosphatase and glutamate-pyruvate transaminase in serum were
significantly increased at each observation period, and cholesterol
and protein levels were significantly decreased by four weeks. In
the liver, the activities of glucose-6-phosphatase and
glucose-6-phosphate dehydrogenase were decreased, and the activity
of isocitrate dehydrogenase was increased at each observation point.
Enzymatic examination of the renal cortex showed decreased
activities of glucose-6-phosphatase and 5'-nucleotidase at each
observation period, an increase in the activity of lactate
dehydrogenase at 12 weeks, and increased activity of isocitrate
dehydrogenase at 2 and 4 weeks. In the renal medulla, the activity
of Na,K-ATPase was decreased, that of lactate dehydrogenase was
increased at 12 weeks, and that of isocitrate dehydrogenase was
decreased at 2 weeks but increased at 12 weeks (Ikawa et al., 1978).
Groups of five male Wistar rats were given a diet or
drinking-water containing LAS at a concentration of 0.4% (diet:
200 mg/kg body weight per day; drinking-water: 560 mg/kg per day)
for two weeks in order to determine the effects of LAS on the
synthesis of lipids in the liver. Lipids were thus measured in the
liver, and uptake of acetate-1-14C by the lipids was examined.
Decreases in the levels of total lipids and triglyceride were seen
in both groups, but there were no significant changes in
phospholipid or cholesterol levels. Uptake of acetate-1-14C by
lipids in the liver was increased in both groups; uptake of
phospholipids and triglycerides tended to increase, and that of
phospholipids increased significantly in rats given LAS in the diet
(Yoneyama et al., 1978).
A7.2.2.2 Administration by gavage
Groups of 12 male and 12 female Sprague-Dawley rats were given
the magnesium salt of LAS by gavage at a dose of 0, 155, 310, or
620 mg/kg body weight for one month. Body weight gain was depressed
in males and females at 620 mg/kg body weight; one male and two
females at this dose also had diarrhoea and loss of appetite and
subsequently died. Haematological examination revealed significant
decreases in haemoglobin concentration and haematocrit in males at
620 mg/kg body weight. A significant increase in the activity of
alkaline phosphatase and a significant decrease in calcium levels
were seen in males at 310 or 620 mg/kg body weight; and a
significant increase was seen in the activity of glutamate-oxalate
transminase and a significant decrease in protein levels in females
at those doses. Females at all doses had a significant decrease in
calcium levels. At the highest dose, females had a significant
increase in the activity of alkaline phosphatase, a significant
decrease in cholesterol level, and increased weight of the liver,
but the weight of the thymus decreased. The weight of the heart
decreased in females at 310 and 620 mg/kg body weight. Histological
examination of the liver revealed no abnormalities (Ito et al.,
1978).
Groups of 12 male and 12 female Sprague-Dawley rats were given
the sodium salt of LAS (chain length distribution: < 0.1% C9,
10.1% C10, 33.7% C11, 31.0% C12, 25.1% C13) at a dose of 0,
125, 250, or 500 mg/kg body weight by gavage once a day. Diarrhoea
was observed in the group receiving 500 mg/kg, and soft faeces were
observed in the other two groups. Body weight gain was depressed in
males of all groups and in females at 500 mg/kg. Haematological
examination revealed no abnormalities. Serum analysis revealed a
significant increase in the activity of alkaline phosphatase in
males at 500 mg/kg, a significant decrease in calcium levels in
males of all groups, significant increases in the activity of
gluatamate-oxalate transaminase and in blood-urea nitrogen in
females at 500 mg/kg, a significant decrease in calcium level in
females at 250 or 500 mg/kg, and significantly decreased protein and
albumin levels in females of all groups. At 500 mg/kg, the weights
of spleen and heart were significantly decreased in males; in
females, liver weights were increased but the weights of the heart
and thymus were decreased. No histological abnormalities were seen
in the liver (Ito et al., 1978).
A7.2.2.3 Dermal application
Continued, repeated, or extremely high doses of LAS, like other
detergents, compromise the integrity of the skin so that penetration
occurs, causing a variety of anomalies. As the design of the
following two studies was not adequate, the observations are not
considered to be relevant to human risk assessment.
Application of 2 ml of a commercial preparation of LAS (23.4%
active ingredient) to the thoracic skin of six male Wistar rats
resulted in redness and wrinkling of the skin after 24 h. The
redness then increased, the corium was lacerated, and bleeding
occurred. These effects were most severe after five to seven days,
but after a further 10 days the skin began to recover. Six rats died
after 19 days, probably because of the extremely high dose used. The
livers of three rats were examined by electron microscopy after
three and 30 days and the findings compared with those in the
control group. At three days, marked changes were seen in the
components of the liver parenchymal cells, such as separation of the
intracellular space, appearance of dark cells with high electron
density, dysmorphia of mitochondria, extracellular prolapse of
mitochondria, proliferation of rough-surfaced endoplasmic reticulum,
lysosome proliferation, and a decrease in the prevalence of fatty
droplets. At 30 days, many liver parenchymal cells were filled with
abnormally divided and proliferated mitochondria, and an abnormal
increase in smooth-surfaced endoplasmic reticula was noted. There
were no granules of glycogen or fatty droplets. Structures
resembling necrotic cells were also observed (Sakashita et al.,
1974).
A commercial preparation of LAS (23.4% active ingredient) was
applied dermally to male rats (number not given) at a dose of
5 mg/kg body weight active ingredient once a day for 30 days, and
the liver was examined by electron microscopy. Degeneration was seen
in part of the liver, in the form of atrophy and high density.
Intra-mitochondrial deposits and deformation of the Golgi apparatus
were also noted (Sakashita, 1979).
A7.2.2.4 Subcutaneous injection
A commercial preparation of LAS (27% active ingredient) was
given subcutaneously to groups of five male and five female Wistar
rats at a dose of 2 ml/kg body weight per day of a 0, 0.02, 0.2, or
2% solution of the preparation for 25 or 50 days. Rats receiving the
2% solution had reduced body weight gain, increased weights of
liver, kidney, and spleen, a low serum albumin:globulin ratio, low
serum protein, and reduced ornithine aminotransferase activity in
the liver (Hayashi, 1980).
A7.2.3 Guinea-pig
Twelve guinea-pigs were treated daily for 30 days with a
solution of LAS in distilled water equivalent to 60 mg/kg body
weight, which was applied to a 4-cm2 area of clipped dorsal skin.
Twelve controls received acetone at 0.5 ml. The animals were
sacrificed after 30 days, and samples were taken from liver and
kidney and homogenized for determination of enzymes, lipid
peroxidation, glutathione, and protein. The activities of
ß-glucuronidase, gamma-glutamyl transpeptidase, 5-nucleotidase, and
sorbitol dehydrogenase were increased in liver and kidney. Lipid
peroxidation was increased in kidney but not in liver, and the
glutathione content was unchanged in both organs. Extensive fatty
changes were found in hepatic lobules, with dilation of sinusoids;
tubular lesions were found in the kidney, predominantly in the
proximal and distal portions (Mathur et al., 1992).
A7.2.4 Monkey
LAS (chain length, C10-C13) were given to four groups of
three male and three female rhesus monkeys at a daily dose of 0, 30,
150, or 300 mg/kg body weight orally simultaneously with a dose of
0, 0.1, 0.5, or 1.0 mg/kg per day subcutaneously, for 28 days.
Monkeys that received 300 mg/kg orally and 1.0 mg/kg subcutaneously
vomited frequently, usually within 3 h of administration; these
animals and those given 150 mg/kg orally and 0.5 mg/kg
subcutaneously also had an increased frequency of loose or liquid
faeces. Fibrosis at the injection sites was reported in all test
animals, and the incidence and severity were related to dose.
Treatment had no effect on ophthalmoscopic, haematological, or
urinary parameters, on organ weight, or on histopathological
appearance (Heywood et al., 1978).
The studies of short-term exposure to LAS are summarized in
Table 18.
Table 18. Summary of studies of short-term exposure to linear alkylbenzene sulfonates (LAS)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Mouse, C57Bl TW LAS (a.i. 17.1%) s.c. 63 or 76 mg/kg Abdominal adhesions, increased Kikuchi (1978)
44 M, 16 bw/day, 60-90 days weights of liver, kidney, and
spleen after 60-day treatment;
no histopathological changes
in liver, kidney, adrenal or thyroid
glands
Rat, Wistar LAS, C10-C14 Diet 0, 0.6, 1.2, 1.8%, Decreased serum triglyceride, Yoneyama & Hiraga
5 M (a.i. 60%) 4 weeks total lipids, phospholipids, and (1977)
cholesterol; increased relative
liver weight at 1.2 and 1.8%;
suppression of body weight gain
at 1.8%
Rat, SD LAS, C10-C15 Diet 0, 0.02, 0.1, 0.5%, No adverse effects Kay et al. (1965)
10 M, 10 F (a.i. 8-9%) 90 days
Rat, FDRL LAS, C9-C15 Diet 0, 0.05, 0.25 g/kg bw Slight increase in liver weight in Oser & Morgareidge
15 M, 15 F (a.i. 39.5%) per day, 12 weeks females at high dose (1965)
Rat, Wistar LAS (NS) Diet 1.5%, 24 weeks Increased activities of serum, Ikawa et al. (1978)
4 M hepatic, and renal enzymes;
depressed body weight gain;
increased relative liver weight
Table 18 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, CRJ-SD LAS, Na, C10-C13 Gavage 125, 250, 500 mg/kg Altered serum enzyme activity Ito et al. (1978)
12 M, 12 F (a.i. 99.5%) bw per day, 1 month and calcium levels at high doses;
decreased serum protein and
albumin levels in all treated
females; decreased spleen and
heart weights in males at highest
dose; increased liver weight and
decreased heart and thymus
weights in females at highest dose;
no histopathological abnormalities
in liver
Rat, CRJ-SD LAS Mg, C10-C13 Gavage 155, 310, 620 mg/kg Altered haemoglobin, haematocrit, Ito et al. (1978)
12 M, 12 F (a.i. 96.9%) bw per day, 1 month serum enzyme activities, calcium
level at high doses; depressed
body weight gain at highest dose;
increased liver weight and
decreased heart and thymus
weights in females at highest dose;
no histopathological abnormalities
in liver
Rat, Wistar LAS detergent Dermal 2 ml/animal Skin irritation; liver parenchymal Sakashita et al.
6 M (a.i. 23.4%) 3.5 × 4.5 cm, 30 days changes with necrotic cells; no (1974)
glycogen granules or fat droplets
Rat, Wistar LAS detergent Dermal 5 mg/kg bw, once/ Degenerative changes in liver Sakashita (1979)
6 M (a.i. 23.4%) day, 30 days
Table 18 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar LAS detergent s.c. 0, 0.02, 0.2, 2%, Depressed body weight gain; Hayashi (1980)
5 M, 5 F (a.i. 27%) 2 ml/kg bw per day, increased weights of liver, kidney,
50 days and spleen; and altered hepatic
enzyme activities at highest dose
Rat, Wistar LAS Drinking- 0.4%, 2 weeks Decreased hepatic total lipids and Yoneyama et al.
8 M, 8 F (a.i. 60.2%) water triglycerides; increased uptake of (1978)
acetate-1-14C, phospholipids, and
triglycerides
Guinea-pig LAS (NS) Dermal 60 mg/kg bw, Altered hepatic and renal enzyme Mathur et al. (1992)
12 M, 12 F 30 days on 4 cm2 activities; fatty degeneration in
liver; renal tubular lesions
Rhesus monkey LAS C10-C13 Gavage 0.30, 150, 300 mg/kg Vomiting and diarrhoea; no Heywood et al.
3M, 3F (a.i. 20.5%) s.c. 0, 0.1, 0.5, 1.0 mg/kg ophthalmic, haematological or (1978)
bw per day, 28 days urinary changes; no effect on
organ weights; no histopatho-
logical changes
M, male; F, female; a.i., active ingredient; s.c., subcutaneous
A7.3 Long-term exposure; carcinogenicity
A7.3.1 Mouse
A7.3.1.1 Administration in the diet
Groups of eight or nine ICR mice were given diets containing LAS
at a concentration of 0.6 or 1.8% for nine months (corresponding to
intakes of 500 and 1000 mg/kg body weight per day). There was no
reduction in body weight gain at either dose, but the weight of the
liver was increased in both males and females. Significant decreases
were seen in the activities of hepatic lactate dehydrogenase and
renal acid phosphatase in male mice (Yoneyama et al., 1976).
A7.3.1.2 Administration in the drinking-water
Drinking-water containing 100 ppm LAS (corresponding to 20 mg/kg
body weight per day) was supplied to ddy mice (sex and number not
stated) for six months, and they were then allowed to recover for
two months. Mice were killed for electron microscopy of the liver at
one, two, three, and six months and after the two-month recovery
period. Hepatic damage was observed at one and six months,
consisting of the disappearance of the nucleolus, atrophy of the
Golgi apparatus, degranulation of rough-surfaced endoplasmic
reticulum, degeneration of mitochondria, and increased numbers of
primary and secondary lysosomes including autophagic vacuoles with a
myelinated core. In mice examined after the two-month recovery
period, some hepatic damage was seen, which was characterized by
changes in mitochondrial structure and the presence of numerous fat
droplets. Other cellular effects had reversed, indicating that the
liver cells had recovered (Watari et al., 1977). Because an
extremely high dose was used in this study, the observations have
little relevance to human risk.
Groups of eight or nine ICR mice were given water containing LAS
at a concentration of 0.07, 0.2, or 0.6% for nine months,
corresponding to intakes of about 0.1, 0.25, or 0.6 g/kg body weight
per day for males and 0.1, 0.25, or 0.9 g/kg body weight per day for
females. Body weight gain was depressed in males and females at
0.6%, and there were dose-related increases in liver weight in
females in all dose groups. In the group given 0.6% LAS, the
activity of hepatic glutamate-oxalate transaminase was significantly
decreased in males and the activity of renal glucose-6-phosphatase
was decreased in animals of each sex (Yoneyama et al., 1976).
A7.3.2 Rat
A7.3.2.1 Administration in the diet
LAS (98.1% active ingredient; chain length distribution,
C10-C14) were fed to four groups of Charles River weanling rats,
each consisting of 50 males and 50 females, at a dietary level of 0,
0.02, 0.1, or 0.5% (corresponding to 10, 50, or 250 mg/kg body
weight per day) for two years. No adverse effects on growth or feed
conversion efficiency were observed. Five males and females from
each group were killed at 8 and 15 months, and all survivors at 24
months; all animals were necropsied, haematological values were
determined, and tissues were taken for histological examination. No
consistent change was seen that could be considered a toxic
response. Animals that showed significant loss of weight,
development of tumours, or other evidence of abnormalities were also
sacrificed and their tissues preserved for study. The incidences of
tumours and of common incidental diseases were similar in all
dietary groups (Buehler et al., 1971).
Diets containing technical-grade LAS (chain length distribution:
10.6% C10, 34.1% C11, 27.7% C12, 19.0% C13, 8.7% C14; mean
relative molecular mass, 345.8) at a concentration of 0, 0.07, 0.2,
0.6, or 1.8% were given to groups of 10 Wistar rats of each sex for
six months. The group given 1.8% had diarrhoea, markedly depressed
growth, increased caecal weight, and marked degeneration of renal
tubules. The group given 0.6% had slightly depressed growth,
increased caecal weight, increased serum alkaline phosphatase
activity, decreased serum protein, and degeneration of renal
tubules. The group given 0.2% had increased caecal weight and slight
degeneration of renal tubules. The group given 0.07%, corresponding
to about 40 mg/kg body weight per day, showed no effects
attributable to treatment (Yoneyama et al., 1972).
Groups of eight male and eight female Wistar rats were given
diets containing LAS at a concentration of 0, 0.6, or 1.8% for nine
months, corresponding to intakes of 230 or 750 mg/kg body weight per
day for males and 290 or 1900 mg/kg body weight per day for females.
In rats given 1.8% LAS, body weight gain was reduced in both males
and females. Haematological examination revealed a significant
decrease in leukocytes in males at 0.6% and significant decreases in
mean corpuscular volume and mean corpuscular haemoglobin in females
at 1.8%. The activity of glutamate-oxalate transferase and the
levels of cholesterol and albumin in serum were significantly
decreased and the activity of alkaline phosphatase and the levels of
blood-urea nitrogen and cholinesterase were significant increased in
males at 1.8%; females at that dose had a significant decrease in
cholesterol level and a significant increase in alkaline phosphatase
activity. At 0.6%, males had a significant decrease in glucose
level, and females had a significant decrease in the activity of
glutamate-pyruvate transaminase. The caecal weight of male rats and
the liver and caecal weights of female rats at 1.8% were
significantly increased. Enzymatic examination of the liver revealed
dose-related decreases in the activities of glucose-6-phosphate
dehydrogenase and lactate dehydrogenase in male rats. At 1.8%, males
had significantly decreased activities of glucose-6-phosphatase,
glutamate-pyruvate transaminase, and glutamate-oxalate transaminase
and a dose-related decrease in the activity of glucose-6-phosphate
dehydrogenase; females had significantly decreased activities of
glucose-6-phosphatase and glutamate-oxalate transaminase. Enzymatic
examination of the kidneys of females at 1.8% showed significantly
decreased activities of glucose-6-phosphatase, Na,K-ATPase, and
lactate dehydrogenase (Yoneyama et al., 1976).
Groups of 50 male and 50 female Wistar weanling rats were given
diets containing LAS (10.6% C10, 34.1% C11, 27.7% C12, 19.0%
C13, 8.7% C14; mean relative molecular mass, 345.8) at a
concentration of 0, 0.04, 0.16, or 0.6%. In each group, five rats of
each sex were fed for one, three, six, or 12 months, and groups of
15 rats of each sex were fed for 24 months or more. The group fed
0.6% had slightly increased liver and caecal weights, and increased
activity of glutamate-pyruvate transaminase and alkaline phosphatase
in serum. The treatment had no adverse effect on the intake of food,
body weight gain, general condition, mortality, or mean survival. On
the basis of these results, it was concluded that a diet containing
LAS at a concentration of 0.6% (300 mg/kg body weight per day) had
no adverse effects on the rats (Yoneyama et al., 1977).
Groups of 50 male and 50 female Wistar rats were fed LAS
(C10-C14) in the diet at a concentration of 0, 0.04, 0.16, or
0.6% and were then submitted to a detailed histopathological
examination. After one month, proliferation of hepatic cells in the
liver, slight swelling of the renal tubules, and narrowing of the
tubular lumen were found in treated animals. Since these alterations
later disappeared, they were considered to represent adaptation to
the administration of LAS. No histological lesions were seen in the
organs of rats that were fed for 24 months or more that could be
attributed to treatment. Various types of tumour were observed in
both treated and control rats but did not appear to be due to LAS
(Fujii et al., 1977).
A7.3.2.2 Administration in the drinking-water
Groups of eight to nine male and eight to nine female Wistar
rats were given LAS at a concentration of 0, 0.07, 0.2, or 0.6% in
drinking-water for nine months. Body weight gain was suppressed in
males given 0.6%. Haematological examination revealed no significant
change in any of the experimental groups, but a dose-related
decrease in cholesterol level was seen in males. No change in organ
weight was seen that was due to administration of LAS. Significant
decreases in the activities of glutamate-oxalate transaminase and
lactate dehydrogenase were seen in males at 0.2% and a dose-related
increase in the activity of glutamate-oxalate transaminase in
females. A significant decrease in renal Na,K-ATPase was seen in the
group given 0.2%. The dose of 0.07% corresponded to intakes of LAS
of 50 and 120 mg/kg body weight per day in males and females, and
the dose of 0.2% to intakes of 120 and 170 mg/kg body weight per
day, respectively (Yoneyama et al., 1976).
A commercial preparation of LAS (27% active ingredient) was
given to groups of five male Wistar rats in drinking-water at a
concentration of 0, 0.3, 3, 30, or 300 ppm (corresponding to 0.007,
0.07, 0.7, or 7 mg/kg body weight per day) for 60, 124, or 181 days.
Although a reduction in body weight gain, changes in blood
biochemistry, and increased ornithine aminotransferase activity in
the liver were noted in some animals, they were not proportional to
dose or feeding period (Hayashi, 1980).
Groups of 20 male Wistar rats were given water containing LAS
(34.55% commercial solution) at a concentration of 0, 0.01, 0.05, or
0.1% for two years, the highest dose corresponding to an intake of
about 200 mg/kg body weight per day. No changes attributable to the
administration of LAS were seen in terms of growth, mortality, the
weights of major organs, or histopathological appearance (Tiba,
1972).
A group consisting of 62 male and 62 female Wistar rats was
given drinking-water containing LAS (mean relative molecular mass,
348; 38.74% active ingredient) at a concentration of 0.1%
(corresponding to 140 mg/kg body weight per day), and a control
group of 37 male and 37 females was given normal drinking-water.
Five to 12 rats in the experimental group and three to 12 rats in
the control group were killed at 3, 6, 12, and 18 months, and all
surviving animals were killed at 24-26 months. Administration of LAS
had no effect on the intake of water, mortality, body weight gain,
or general condition. Histopathological examination revealed
atrophy; fatty changes were found in hepatic cells in treated
animals at six months, when there were also significant increases in
the activities of glutamate-oxalate and glutamate-pyruvate
transaminases and in the level of bilirubin. LAS had no effect on
haematological parameters (Endo et al., 1980).
A group of 60 male and 60 female rats (strain not specified)
received drinking-water containing 0.01% of a preparation containing
51% LAS for 100 weeks; a similar group was untreated. No detrimental
effects on body weight and no pathological effects, including
tumours, were reported (Bornmann et al., 1963).
A7.3.2.3 Administration by gavage
Groups of 20 male and 20 female Sprague-Dawley rats were given a
solution of a magnesium salt of LAS at doses of 10, 75, 150, or
300 mg/kg body weight per day by gavage for six months. Body weight
gain was suppressed, and slight decreases were observed in serum
protein, albumin, and calcium ion level, but the changes were within
the physiological range (Ito et al., 1978).
A7.3.2.4 Dermal application
A dose of 0.1 ml/kg body weight of a 0.5, 1.0, or 5.0% solution
of magnesium LAS (in 3% polyethylene glycol) was applied to the
backs of 20 male and 20 female Sprague-Dawley rats six times a week
for six months. Slight redness at the application site was observed
transiently in males and occasionally in females at 5%. Body weight
was slightly suppressed in males at that dose, and one male in the
control group and one at 5.0% died of unknown causes. Treatment had
no definite effect in terms of food conversion efficiency, urinary,
haematological, serum biochemistry, or histopathological findings,
or organ weights (Ito et al., 1978). No systemic toxicity was
reported in this study. Sakashita et al. (1974) and Sakashita (1979)
(see section 7.2.2.3) may have obtained positive results because
they used a shorter period of exposure, during which skin integrity
may have been compromised, resulting in absorption of the
preparation of LAS through the skin to produce systemic effects.
LAS (19.7% active ingredient) were applied to the dorsal skin of
SLC-Wistar rats three times per week at a dose of 0.005, 0.025, or
0.125 ml/rat (equivalent to 1, 5, or 25 mg/rat) for 24 months. A
dose of 0.025 ml of an LAS-based detergent containing 19.9% LAS
(equivalent to 5 mg LAS per rat) and distilled water was given to
controls. Each application was washed from the skin with warm water
after 24 h. Treatment had no effect on organ weights or
histopathological appearance, and there was no evidence of toxicity
or carcinogenicity (Taniguchi et al., 1978).
Long-term studies of exposure to and the carcinogenicity of LAS
are summarized in Table 19.
A7.4 Skin and eye irritation; sensitization
The potential of LAS to irritate the skin depends on the
concentration applied. On the basis of the criteria of the European
Commission and the OECD test guideline, LAS were classified as
irritating to the skin at concentrations above 20% (European
Committee of Organic Surfactants and Their Intermediates, 1990).
Table 19. Summary of studies of long-term exposure to linear alkylbenzene sulfonates (LAS)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Mouse, SLC-ICR LAS (a.i. 60%) Diet 0, 0.6, 1.8%, Increased liver weight; Yoneyama et al.
8-9 M, 8-9 F 9 months decreased hepatic and renal (1976)
enzyme activities in males
Mouse, ddy (NR) LAS (NS) Drinking- 20 mg/kg bw per Degenerative changes in liver, Watari et al. (1977)
water day, 6 months with partial recovery after
end of treatment
Mouse, ICR LAS (a.i. 60%) Drinking- 0, 0.07, 0.2, 0.6, Depressed body weight gain at Yoneyama et al.
8-9 M, 8-9 F water 1.8%, 9 months high dose; dose-related increase (1976)
in liver weight in all treated
females; changes in hepatic
enzyme activities at high dose
Rat, Wistar LAS, C10-C14 Diet 0, 0.07, 0.2, 0.6, Dose-related depression of Yoneyama et al.
10 M, 10 F 1.8%, 6 months growth, caecal enlargement, (1972)
and renal tubular degeneration
at > 0.07%
Rat, Wistar LAS (a.i. 60%) Diet 0, 0.6, 1.8%, Depressed body weight gain Yoneyama et al.
8 M, 8 F 9 months at high dose; changes in (1976)
haematological parameters, in serum
and hepatic enzyme activities,
and in cholesterol levels at both
doses; changes in renal enzyme
activities in females at high dose
Table 19 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar LAS (a.i. 60%) Drinking- 0, 0.07, 0.2, 0.6%, Depressed body weight gain in Yoneyama et al.
8-9 M, 8-9 F water 9 months males at high dose; no changes (1976)
in haematological parameters or
organ weight; changes in serum
and renal enzyme activities at 0.2%
Rat, Wistar LAS, C10-C14 Diet 0, 0.04, 0.16, 0.6%, Slight increase in liver and Yoneyama et al.
50 M, 50 F (a.i. 60%) 24 months caecal weights and changes in (1977)
serum enzym activities at high
dose; no effect on body weight
gain
Rat, Charles River LAS, C10-C14 Diet 0, 0.02, 0.1, 0.5%, No treatment-related effects Buehler et al.
50 M, 50 F (a.i98.1%) 2 years (1971)
Rat, Wistar LAS, C10-C14 Diet 0, 0.04, 0.16, 0.6%, Transient changes in liver and Fujii et al. (1977)
50 M, 50 F (a.i. 60%) 2 years kidney; no treatment-related
histopathological abnormalities
at end of study
Rat, SD LAS Mg, C10-C13 Gavage 75, 150, 300 mg/kg Depressed body weight gain; no Ito et al. (1978)
20 M, 20 F (a.i. 96.9%) bw per day, 6 significant adverse effects
months
Rat, Wistar, 5 M LAS detergent Drinking- 0, 0.3, 3, 30, 300 Depressed body weight gain and Hayashi (1980)
(a.i. 27%) water ppm, 181 days changes in blood biochemistry
and liver enzyme activity considered
not to be related to treatment
Table 19 (contd)
Species, strain, Test material Route Dosage Results Reference
numbers (specification)
per group
Rat, Wistar, 20 M LAS (a.i. 34.55%) Drinking- 0, 0.01, 0.05, 0.1%, No adverse effects Tiba (1972)
water 2 years
Rat, Wistar LAS (a.i. 38.74%) Drinking- 0, 0.1%, 26 months Fatty changes and atrophy in Endo et al. (1980)
62 M, 62 F water liver; changes in hepatic enzyme
activities; no effect on body
weight gain
Rat LAS (Marlon Drinking- 0, 0.01%, No adverse effects Bornmann et al.
60 M, 60 F BW 2043) water 100 weeks (1963)
Rat, SD LAS Mg, C10-C13 Dermal 0.5, 1.0, 5% in Slight reduction in body weight Ito et al. (1978)
20 M, 20 F (a.i. 96.9%) polyethylene glycol, gain of males at high dose; no
6 months other adverse effects
Rat, SLC-Wistar LAS (a.i. 19.7%) Dermal 0, 6.7, 33.3, 167.0 No adverse effects Taniguchi et al.
25 M, 25 F mg/kg bw, 3 × per (1978)
week, 2 years
Rat, SLC-Wistar LAS detergent Dermal 0, 33.3 mg/kg bw No adverse effects Taniguchi et al.
25 M, 25 F (a.i. 19.9%) 3 × per week, 2 years (1978)
M, male; F, female; NS, not specified; a.i., active ingredient; SD, Sprague-Dawley
A7.4.1 Studies of skin
Solutions of LAS (chain length distribution, C10-C13;
purity, 99.9%) were applied to the backs of groups of three male
Wistar rats at a rate of 0.5 g of a 20 or 30% solution once a day
for 15 days. On the sixteenth day of the experiment, the skin at the
application site and the tissues of the tongue and oral mucosa (to
examine the effects of licking) of the rats that received 30% were
examined histologically. Body weight gain was reduced in the group
exposed to 20%, and body weight was decreased in animals exposed to
30%. An infiltrating, yellow-red brown crust was observed after two
to three days at 20% and after one to two days at 30%; at four to
six days, the crust was abraded, and erosion was observed.
Histological examination of the application site revealed severe
necrosis of the region, from the epidermis cuticle to the upper
layer of the dermis, severe infiltration of leukocytes in the
necrotic site, diffuse inflammatory cell infiltration of all of the
layers of the corium, and swelling of collagenous fibres in the
dermis. Histological examination of the tongue showed no changes,
but examination of the oral mucosa revealed atrophy and slight
degeneration of the epithelium (Sadai & Mizuno, 1972).
Some batches of a paste of LAS (volume not stated) induced weak
to moderate sensitization in guinea-pig skin at induction
concentrations of 2-100% and challenge concentrations of 1-2%. A
prototype liquid laundry detergent (10% LAS) induced sensitization
at a challenge concentration of 1% (0.1% as LAS) (Nusair et al.,
1988).
The biochemical and pathomorphological effects of LAS on the
skin of four female albino CDRI guinea-pigs were investigated by
shaving the abdominal skin and immersing the animals up to the neck
in a 1% aqueous solution of neutralized LAS for 90 min daily for
seven consecutive days. A control group was immersed in water
according to the same schedule. After each immersion, the animals
were washed and their skin dried. The animals were killed after
seven days, and skin samples were taken. The skin of guinea-pigs
exposed to the solution of LAS had increased activity of histidine
decarboxylase, decreased sulfhydryl groups and histamine, and
decreased activity of lactic dehydrogenase. It appeared to be
shrunken, with thinner layers of dermis and epidermis than controls.
There were also areas of scarring in the epidermis and ridging of
epidermis and dermis (Misra et al., 1989a).
A7.4.2 Studies of the eye
A volume of 0.1 ml of a solution of LAS (relative molecular
mass, 346.5) at five concentrations ranging from 0.01 to 1.0% was
instilled into the eyes of rabbits (13 per group). The rabbits were
observed for 24 h after application. The group receiving 0.01% had
no abnormalities, but that given 0.05% had slight congestion.
Concentrations of 0.5% and more induced marked reactions, such as
severe congestion and oedema, increased secretion, opacity of the
cornea, and disappearance of the corneal reflex (Oba et al., 1968a).
Solutions of LAS (chain length distribution, C10-C14; 80.9%
C11-C13) at six concentrations ranging from 0.01 to 5.0% were
instilled into the eyes of rabbits (three per group). The rabbits
were observed for 168 h after application. The group given 0.01% had
no reaction, but within 2 h those given 0.05% had slight congestion
and those at 0.1% had considerable congestion or oedema, which had
disappeared by 24 h. Animals given 0.5% or more had marked
reactions, such as severe congestion and oedema, increased
secretion, opacity of the cornea, and disappearance of the corneal
reflex, for 24 h but then tended to recover; the signs had
disappeared completely within 120 h (Iimori et al., 1972).
A7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
The reproductive toxicity of LAS and formulations of LAS has
been evaluated in studies by oral (gavage, diet, drinking-water),
dermal (skin painting), and parenteral (subcutaneous)
administration. Similar effects were seen, regardless of the route
of application. The studies had a number of deficiencies, however,
which are summarized below.
In some studies, widely separated dose levels were used (Palmer
et al., 1975a; Takahashi et al., 1975; Tiba et al., 1976; Hamano et
al., 1976), so that it is difficult to assess dose-response
relationships and to interpret the results. Some of the studies
included only one dose (Bornmann et al., 1963; Sato et al., 1972;
Endo et al., 1980) and some two (Iimori et al., 1973; Nolen et al.,
1975; Takahashi et al., 1975; Hamano et al., 1976; Tiba et al.,
1976). The studies done on formulations are difficult to interpret,
as the effects seen may have been due to another component. In some
cases, the details of the formulation are not given, so that the
dose of LAS is also unknown. Certain studies of dermal exposure
(Sato et al., 1972; Masuda et al., 1973, 1974; Palmer et al., 1975a;
Nishimura, 1976; Daly et al., 1980) involved levels that compromised
the integrity of the skin and caused overt toxicity.
The teratogenic effects of some commercial formulations of LAS
reported by Mikami and co-workers (1969), mainly in mice, were not
reproduced in other studies. A number of studies indicated that LAS
have some reproductive toxicity, but the effects were seen only at
doses that caused maternal toxicity. No teratogenic effects were
observed. These studies are summarized in Tables 20-22.
Table 20. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and formulations of LAS,
administered orally
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
Diet Charles River 14, 70, 350 84 Combined study of reproduction Buehler et al. (1971)
rats (20) (0.02, 0.1, 0.5%) and teratogenicity (three generations);
no effects attributable to LAS
Diet SD rats (16) 78, 780 (0.1, 1.0%) 0-20 No abnormalities at either dose; few Tiba et al. (1976)
offspring at high dose
Gavage ICR mice (NS) 300, 600 6,8,10 High incidence of cleft palate and Mikami et al. (1969)
exencephaly in fetuses at high dose
Gavage ICR mice (14) 40, 400 (0.4, 4.0%) 0-6 No effects at low dose; reduced weight Takahashi et al.
7-13 gain and pregnancy rate at high dose (1975)
Gavage ICR mice (25-33) 10, 100, 300 6-15 Reduced weight gain at all levels, Shiobara & Imahori
particularly at highest dose; two (1976)
dams died at highest dose; all
fetuses of one dam died in utero;
decreased body weight and delayed
ossification in living fetuses but no
increase in incidence of malformations
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS (contd).
Gavage ICR mice 14, 20, 350 1-3 No effect on implantation rate at Koizumi et al. (1985)
any dose
Gavage CD rats (20) 0.2, 2.0, 300, 600 6-15, rats No effects on any species at two lower Palmer et al. (1975a)
CD-1 mice (20) and mice doses
NZW rabbits (13) 6-18, Rats: reduced weight gain and one
rabbits death at highest dose
Mice: reduced weight gain, seven
deaths, and four litter losses at 300 mg/kg
bw per day; 18 deaths, one litter loss
and one non-pregnancy at 600 mg/kg
bw per day
Rabbits: reduced weight gain, 11 deaths,
two litter losses at 300 mg/kg bw per
day; all animals died at highest dose
Gavage CD rats (30) 125, 500, 2000 6-15 Two-generation study of reproductive Robinson &
and developmental toxicity; delayed Schroeder (1992)
ossification significant at highest dose,
slight at middle dose; no reproductive
or developmental toxicity
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS (contd).
Drinking- Charles River 7 (0.01%) Three-generation study of fertility; no Bornmann et al.
water rats (10) teratogenic effects (1963)
Drinking- Wistar rats (20) 70 (0.1%) Four-generation study of reproductive Endo et al. (1980)
water toxicity; no effects attributable to LAS
Drinking- Wistar rats (20) 383 mg/rat (0.1%) 6-15 No effects in rats; rabbits had reduced Endo et al. (1980)
water NZW rabbit (11) 3030 mg/rabbit 6-18 weight gain and delayed ossification
(0.1%) but no malformations
17% LAS, 7% alcohol ethoxylate sulfate
Gavage CD rats (20) 0.8, 8, 1,200, 2400 6-15 No increase in major malformations Palmer et al. (1975a)
CD-1 mice (20) 1.064, 10.64, 6-15 or significant changes in anomalies
1600, 320
NZW rabbits (13) 0.8, 8, 1200, 2400 6-18
45% LAS
Diet CD rats (25) 80, 400, 800 6-15 No treatment-related effects on Nolen et al. (1975)
(0.1, 0.5, 1.0%) reproduction or embryonic
development
Table 20 (contd)
Route Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
1% LAS
Gavage ICR mice (18-23) 800, 1200, 1500, 6-15 No increase in fetal malformations; Yamamoto et al.
3000 decreased body weight and delayed (1976)
ossification at 1200 mg/kg bw
19% LAS
Gavage IRC mice (9-13) 125, 4000 6 No effect on fetal viability or Hamano et al.
development (1976)
NS, not specified
Table 21. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and formulations of
LAS, administered dermally
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
CD rats (20) 0.6, 6.0, 60 1-15 Slight reduction in body weight gain at Palmer et al.
(0.03, 0.3, 3.0%) highest dose; no effect on litter parameters (1975a)
at any dose; no evidence of malformations
CD-1 mice (20) 5, 50 , 500 2-13 Reduced body weight gain, fewer pregnancies,
(0.03, 0.3, 3.0%) and total litter loss at highest dose; no
malformations
NZW rabbits (13) 0.9, 9, 90 1-16 Marked reduction in body weight gain, fewer
(0.03, 0.3, 3.0%) pregnancies, and two litter losses at highest
dose; reduced body weight gain at 9 mg/kg bw
per day; no malformations
Wistar rats (20) 20, 100, 400 0-20 Reduced body weight gain, decreased Nishimura (1976)
(1, 5, 20%) pregnancy rates and delayed ossification
at highest dose; no effects at lower doses
Wistar rats (20) 20, 100, 400 0-20 Irritation at site and reduced body weight Daly et al. (1980)
(1, 5, 20%); gain at two higher doses; no change in fetal
rinse-off parameters at any level
Table 21 (contd)
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
Wistar rats (contd)
0.1, 2, 10 0-20 No change in fetal parameters at any level
(0.05, 0.1, 0.5%);
leave on
ddy/s mice (16) 110 (2.22%) 0-13 No abnormalities in dams or fetuses Sato et al. (1972)
ddy mice (4-10) 0.084, 0.84, 8.4 2-14 No fetal or reproductive effects Masuda et al.
(0.017, 0.17, 1.7%) (1973, 1974)
ICR mice 4.2, 8.4, 12.0, 16.5 1-13 Delayed ossification at two highest
(25-30) (0.85, 1.7, 2.55, 3.4%) doses
ICR mice 15, 150, 1500 6-15 Clear decrease in pregnancy rate and Imahori et al.
(27-28) (0.03, 0.3, 3.0%) decrease in fetal weight at highest dose; (1976)
no increase in malformations in fetus
17% LAS, 7% ethanol, 15% urea
ICR mice 2.5, 25, 75 1-13 Decrease in pregnancy rate at Inoue & Masuda
(11-20) (0.5, 5, 15%) highest dose; no other effects (1976)
Table 21 (contd)
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
16.3% LAS
ICR mice 25, 50, 100 0-13 Reduced pregnancy rate and Nakahara et al.
(17-50) (5, 10, 20%) some total litter losses at (1976)
highest dose
Unknown formulation
ddy/s mice 65 (15%) 0-13 Decreased body weight gain, Sato et al. (1972)
(21) decreased pregnancy rate,
decreased fetal weight, and delayed
ossification
Unknown formulation
IRC mice 75, 100 0-12 Decreased pregnancy rates Iimori et al. (1973)
(27-39) (15, 20%) at both levels
Unknown formulation
IRC mice 30, 65, 85, 0-13 Decreased pregnancy rates at all Takahashi et al.
(15-19) 100, 125 doses; decreased fetal body (1975)
(13.0, 17.0, weight; delayed ossification at all
20.0, 25.0%) doses except 65 mg/kg bw per day
Table 22. Studies of the reproductive toxicity and teratogenicity of linear alkylbenzene sulfonates (LAS) and LAS formulations,
administered subcutaneously
Species (no. of Dose (mg/kg Length of Comments and results Reference
animals/group) bw per day) treatment
(days)
LAS
ICR mice 0.4, 2.0, 10% 7-13 No significant effects on dams; high Masuda & Inoue (1974)
(21-24) incidence of skeletal variations and
delayed ossification, not dose-related;
no abnormalities
ICR mice 20, 200 0-3 Irritation at injection site and reduced Takahashi et al. (1975)
(12-19) (0.35, 1.00%) 8-11 pregnancy rate at highest dose; no
malformations or anomalies
17% LAS, 7% ethanol, 15% urea
CR mice 30, 150 7-13 No increase in major malformations Inoue & Masuda (1976)
(16-17) 0-13 or minor anomalies; increase in
implantations at high dose given on
days 0-13
A7.6 Mutagenicity and related end-points
A7.6.1 Studies in vitro
Assays for mutagenicity were performed in vitro with two
commercial products containing 17.1 and 19% LAS, either undiluted or
diluted 10 and 100 times (Oda et al., 1977), 99.5% pure LAS (Fujita
et al., 1977), 95.5% pure sodium salt, or 96.2% pure calcium salt
(Inoue & Sunakawa, 1979), using Bacillus subtilis H17 (rec+) and
M45 (rec-), Salmonella typhimurium TA98 and TA100 (including
a metabolic activation system), and Escherichia coli WP2 uvrA.
All of the assays gave negative results. LAS 99.5% pure (Fujita et
al., 1977) were also tested in S. typhimurium TA1535 and TA1537,
again with negative results. Thesodium and calcium salts in the
presence of various liver homogenates (Sunakawa et al., 1981) and a
22.2% solution of LAS (C10-C14, 10-200 µg/plate) (Inoue et al.,
1980) were tested in S. typhimurium TA98 and TA100. No
mutagenicity was seen.
A7.6.2 Studies in vivo
Groups of male ICR:JCL mice were given LAS at a dose of 200,
400, and 800 mg/kg body weight per day by gavage for five days and
were killed 6 h after the final administration for examination of
chromosomal aberrations in bone-marrow cells. One commercial
preparation containing 19.0% LAS was also given, at a dose of 800,
1600, or 3200 mg/kg body weight, and another containing 17.1% LAS at
a dose of 1000, 2000, or 4000 mg/kg body weight once only by gavage.
The highest doses were 50% of the respective LD50 values. Bone
marrow was examined 6, 24 and 48 h after administration. There was
no significant difference between any of the groups given LAS and
the negative control group in the incidence of chromosomal
aberrations. Mitomycin C, used as a positive control at 5 mg/kg body
weight, induced severe chromosomal aberrations (Inoue et al., 1977).
Groups of five male Wistar rats, Sprague-Dawley rats, and ICR
mice were given a diet containing 0.9% LAS for nine months. The
equivalent doses were 450 mg/kg body weight per day in rats and
1170 mg/kg body weight per day in mice. There were no significant
differences in the incidence of chromosomal aberrations between the
experimental and control groups (Masubuchi et al., 1976).
After LAS (C10-C15) were fed to groups of six male and six
female Colworth/Wistar rats in the diet at concentrations of 0.56 or
1.13%, equivalent to 280 or 565 mg/kg body weight per day, for 90
days, no alteratuons were seen in chromosomes in bone marrow (Hope,
1977).
In three male ddY mice given LAS at 100 mg/kg body weight by
intraperitoneal injection, there was no differences between the
treated animals and a control group in the incidence of
polychromatic erythrocytes with micronuclei in bone-marrow cells
(Kishi et al., 1984).
An assay to detect dominant lethal mutations was performed in
seven male ICR:JCL mice given a diet containing 0.6% LAS at
300 mg/kg body weight per day for nine months. Each of the male mice
was then mated with two female mice that had not been given LAS, and
11 of the 14 females became pregnant. The pregnant mice were
laparotomized on day 13 of gestation to determine the numbers of
luteal bodies, implantations, surviving fetuses, and dead fetuses.
There were no significant differences in fertility, mortality of ova
and embryos, the number of surviving fetuses, or the index of
dominant lethal induction (Roehrborn) between the experimental and
control groups (Masubuchi et al., 1976).
LAS were administered as a single oral dose of 2 mg to pregnant
ICR mice on day 3 of gestation; on day 17 of gestation, each animal
received a subcutaneous dose of 1, 2, or 10 mg/mouse and was killed
24 h later. There was no difference among treated groups in the
incidence of polychromatic erythrocytes with micronuclei in maternal
bone marrow or fetal liver or blood. No mutagenic effect was found
in any of the groups (Koizumi et al., 1985).
A7.7 Special studies
A7.7.1 Studies in vitro
The haemolytic action of LAS was investigated by mixing red
blood cells from rabbits with solutions of LAS at concentrations of
1-1000 mg/litre at 38°C for 30 min. Haemolysis occurred at
concentrations > 5 mg/litre (Yanagisawa et al., 1964). Red blood
cells from rabbits were mixed with solutions of various
concentrations of LAS (relative molecular mass, 346.5) at room
temperature for 3 h. The 50% haemolytic concentration of LAS was
9 mg/litre (Oba et al., 1968a).
Purified LAS at various concentrations were added to 10 µl of
normal plasma obtained from male rats, and prothrombin time was
determined. Prothrombin time was prolonged; the 50% inhibitory
concentration was about 0.6 mmol/litre. When LAS at various
concentrations were added to a mixture of 1% fibrinogen and
thrombin, the time of formation of a mass of fibrin was prolonged by
inhibition of thrombin activity. The 50% inhibitory concentration
was about 0.05 mmol/litre (Takahashi et al., 1974).
LAS influenced the thermal denaturation and decreased the
fluorescence profile of bovine serum albumin in vitro, indicating
protein-LAS interaction (Javed et al., 1988).
Eggs from female B6C3F1 mice were fertilized in vitro and
incubated in culture medium containing LAS at concentrations between
0.015 and 0.03%; eggs grown in culture medium without LAS served as
controls. Eggs exposed for 1 h, washed, and then cultured for five
days developed normally to the blastocyst stage when the
concentration of LAS was less than 0.025%; at concentrations higher
than 0.03%, the eggs did not develop beyond the one-cell stage. With
continuous exposure to LAS for five days, a concentration of 0.01%
slightly impaired development to the blastocyst stage, and 0.025%
prevented development to the one-cell stage (Samejima, 1991).
LAS with a chain length distribution of C10-C14 did not
induce transformation of cryopreserved primary cultures of Syrian
golden hamster embryo cells in vitro (Inoue et al., 1979, 1980).
A7.7.2 Biochemical effects
The levels of amylase, alkaline phosphatase, glutamate-oxalate
transaminase, and glutamate-pyruvate transaminase and of the
electrolytes Ca, P, and Mg in serum were determined up to 24 h after
a single oral administration of 2, 5, 50, or 100 mg/kg body weight
of LAS (60% active ingredient) or dermal application of 5 ml of a 1,
5, 10, or 20% solution of LAS to rabbits (number not stated). The
levels of total Ca, Ca2+, Mg, and P were generally lower after
either type of administration than before. Although there was no
definite trend, the activities of the enzymes tended to decrease
regardless of the route of the administration or the dose
(Yanagisawa et al., 1964).
Groups of three male mice were given an intraperitoneal
injection of 0.3 g/kg body weight of LAS (C14) in order to study
the effects on the formation of methaemoglobin, determined 0.5, 1,
and 2 h afterinjection of LAS. The level of methaemoglobin in the
experimental groups was not significantly greater than that in the
control group at any time (Tamura & Ogura, 1969).
The effects of LAS (sodium dodecylbenzenesulfonate) on fasting
blood glucose level and glucose tolerance curves were investigated
in 40 male and 50 female albino rats pretreated with 0.25 g/kg body
weight per day of LAS for three months. At the end of this period,
the rats were divided into four groups and given distilled water,
6.1 g/kg body weight of glucose, 0.94 g/kg body weight of LAS, or
6.1 g/kg body weight of glucose plus 0.94 g/kg body weight of LAS by
gavage. Blood glucose was then estimated at 30-min intervals.
Administration of LAS in conjunction with glucose resulted in higher
initial levels of blood glucose in male rats and persistently higher
levels in females than did administration of glucose alone. Females
in control and pretreated groups generally had higher blood glucose
levels in response to administration of glucose or LAS plus glucose
than did male rats (Antal, 1972).
A8. EFFECTS ON HUMANS
Section summary
Human skin can tolerate contact with solutions of up to 1% LAS
for 24 h with only mild irritation. Like other surfactants, LAS can
delipidate the skin surface, elute natural moisturizing factor,
denature the proteins of the outer epidermal layer, and increase
permeability and swelling of the outer layer. LAS do not induce skin
sensitization in humans, and there is no conclusive evidence that
they induce eczema. No serious injuries or fatalities have been
reported following accidental ingestion of LAS-containing surfactant
preparations.
A8.1 Exposure of the general population
Surface-active agents are used in shampoos, dish-washing
products, household cleaners, laundry detergents, and other
applications such as industrial cleaners. LAS are major components
of such products. In general, the concentration of nonionic and
ionic surfactants is 10-20%.
A8.2 Clinical studies
A8.2.1 Skin irritation and sensitization
LAS are mildly to moderately irritating to human skin, depending
on the concentration. There is no evidence that they sensitize the
skin in humans.
The relative intensity of skin roughness induced on the surface
of the forearms of volunteers (a circulation method) due to contact
with LAS of different alkyl chain lengths (C8, C10, C11-C16)
was characterized mainly by gross visible changes. C12 LAS
produced more skin roughening than LAS with longer or shorter alkyl
chains. The degree of skin roughening in vivo correlated with the
extent of protein denaturation measured in vitro (Imokawa et al.,
1975a).
Primary skin irritation induced by an LAS formulation (average
chain length, C12; relative molecular mass, 346.5), by
alpha-olefin sulfonates (AOS) (27% C15, 25% C16, 28% C17, 8%
C18; relative molecular mass, 338.5), and by alkyl sulfates (AS)
(C12; relative molecular mass, 346.5) was compared in a 24-h
closed-patch test on the forearms of seven male volunteers. A 1%
aqueous solution (pH 6.8) of each substance was used, and the
relative intensity of skin irritation was scored by grading
erythema, fissuring, and scales. The average score for LAS was
similar to that for AOS but significantly lower than that for AS
( p < 0.05) (Oba et al., 1968a).
In another comparison, the intensity of skin irritation induced
by 1% aqueous solutions of LAS (C10-C13), AOS (C14, C16,
C18), and the sodium salt of AS (C12-C15) was studied in a
24-h closed-patch test on the forearm and in a test in which the
substance was dripped onto the interdigital surface for 40 min once
daily for two consecutive days at a rate of 1.2-1.5 ml/min. Skin
reactions were scored by grading erythema in the patch test and by
grading scaling in the drip test. In the patch test, the score for
LAS was similar to that for AOS but significantly lower than that
for AS. In the drip test, the score for LAS was similar to that for
AS but higher than that for AOS (Sadai et al., 1979).
Repeated patch tests with LAS at aqueous concentrations of 0.05
and 0.2% produced mild to moderate primary irritation. In a study on
the sensitization potential of LAS for human skin, a 0.1% aqueous
preparation caused no sensitization in 86 subjects (Procter & Gamble
Co., unpublished data).
No skin sensitization was seen in 2294 volunteers exposed to LAS
or in 17 887 exposed to formulations of LAS (Nusair et al., 1988).
A8.2.2 Effects on the epidermis
The main effects of surface-active agents on the epidermal
(stratum corneum) are:
-- delipidation of the skin surface or outer layer;
-- elution of natural moisturizing factor, which maintains the
water content of the outer layer;
-- denaturation of stratum corneum protein; and
-- increased permeability, swelling of the outer layer, and
inhibition of enzyme activities in the epidermis.
These effects and some others present a hazard to the skin; they
are described below.
In an investigation of the relationship between the irritating
potential of LAS in vivo and its ability to remove lipid from the
stratum corneum in vitro, LAS removed detectable levels of lipids
only at levels above the critical micelle concentration (0.04%). LAS
removed only small amounts of cholesterol, free fatty acids, the
esters of those materials, and possibly squalene. At concentrations
below that level, LAS can bind to and irritate the stratum corneum.
The clinical irritation produced by LAS is therefore unlikely to be
directly linked to extraction of lipid, and milder forms of
irritation may involve binding of LAS to and denaturation of keratin
as well as disruption of lipid (Froebe et al., 1990).
The results of the human arm immersion test with measurement of
eluted amino acids and protein, the skin permeation test, freeing of
sulfhydryl groups, and the patch test were compared for nine kinds
of surfactant, including LAS, ABS, AS, alcohol ethoxylate sulfate,
soap, nonionic surfactant, and amphoteric surfactant. LAS gave
intermediate reactions in the patch test and the permeation test and
showed a high level of sulfhydryl group freeing activity. The
results of the tests for evaluating surfactants did not agree with
those for the immersion test, which the author considered to provide
the best simulation of actual use (Polano, 1968).
In a number of studies, denaturation of outer layer proteins was
observed in vitro (Van Scott & Lyon, 1953; Harrold, 1959; Wood &
Bettley, 1971; Imokawa et al., 1974; Okamoto, 1974; Imokawa et al.,
1975b; Imokawa & Katsumi, 1976). Sodium dodecylbenzenesulfonate
stimulated penetration of sodium ions through isolated human
epidermis, partly because the detergent can denature proteins of the
epidermal stratum corneum (Wood & Bettley, 1971). Sodium laurate and
sodium lauryl sulfate were the most effective of several surfactants
in inducing swelling of the horny layer (Putterman et al., 1977).
The lysosome labilizing effects of surfactants, measured as the
release of enzyme from lysosomes, were shown to diminish in the
order cationic > anionic > nonionic surfactants (Imokawa &
Mishima, 1979). When ovalbumin was used as a simulated epidermis
protein, sodium lauryl sulfate was found to denature skin protein
extensively by exposing concealed sulfhydryl groups in LAS of alkyl
chain length C8-C16 (Blohm, 1957).
In immersion tests of the hand and the forearm up to 5 cm above
the wrist, falling off of skin scales diminished in the order:
sec-alkane sulfonate > LAS > AOS, alcohol ethoxylate sulfate
(Okamoto, 1974), but the distribution of carbon chain lengths among
the samples was not described. In a comparison of skin roughening by
a circulation method, the effects diminished in the order C12 AS
> C12 AOS > C12 sec-alkane sulfonate > C12 LAS (Imokawa
et al., 1974, 1975a,b). Skin roughening caused by several
surfactants that are components of commercial products was studied
by the method of Ito & Kakegawa (1972), in which various
concentrations are dripped onto the fingers. The effects diminished
in the order C10-C13 LAS = C12-C15 AS > C11, C13, C15
alcohol ethoxylate sulfate ( n = 0-3) > C14, C16, C18 AOS
> C11-C15 polyoxyethylene alkylether (Sadai et al., 1979).
A8.2.3 Hand eczema
The skin reaction to 0.04, 0.4, and 4.0% aqueous solutions of
LAS (10.0% C10, 34.3% C11, 31.5% C12, 24.7% C13) was tested
in a 24-h closed-patch test on the lower backs of 10 healthy
volunteers and 11 patients with hand eczema (progressive keratosis
palmaris). The incidence and intensity of skin reactions were
greater in the group with hand eczema, but the difference was not
statistically significant (Okamoto & Takase, 1976a,b).
In order to assess the possible etiological correlation between
exposure to LAS and hand eczema, 0.04, 0.4, and 4% aqueous solutions
of LAS were applied in 48-h closed-patch tests on the lower backs
of 20 women with hand eczema and 42 with other skin diseases. The
skin reaction was scored grossly from 0 to 5 on the basis of the
occurrence or intensity of erythema, papules, and vesicles. The
average score appeared to increase in parallel with the
concentration of LAS but did not differ between the groups with hand
eczema and other skin diseases (Sasagawa et al., 1978).
Nine proprietary household detergents were tested in 24-h
closed-patch tests on the lower backs of 160 women with hand eczema.
The surfactant concentrations in five of the products were: (i) 2%
ABS-Na, 15% LAS-Na; (ii) 2% ABS-Na, 14% LAS-Na; (iii) 17% LAS-Na,
12% alcohol ethoxylate sulfate; (iv) 11% ABS-Na, 11% LAS-Na; (v) 19%
LAS-Na. When the detergents were applied daily (for an unspecified
period) at an aqueous concentration of 0.175-0.8%, positive
responses were observed in 3.1% of the women, but they were
considered not to be allergic because the redness of the skin
disappeared completely within two days (Kawamura et al., 1970).
Three proprietary household detergents containing LAS were
tested in 24-h closed-patch tests on the forearms of 13 women with
'housewives' dermatitis' and 13 with other skin diseases. The
detergent was applied either undiluted or in a 0.2% aqueous
solution. Undiluted solutions of all three detergents caused mild to
moderate skin reactions, at incidences of 38.5, 48.1, and 73.1%,
which did not differ between the groups with housewives' dermatitis
and other skin diseases. The 0.2% aqueous solutions did not induce
skin reactions (Ishihara & Kinebuchi, 1967).
Two series of field tests were conducted to estimate if exposure
to a variety of synthetic detergent formulations was associated with
causation or aggravation of hand eczema in women. In the first
series, 162 female volunteers were divided into two groups and
instructed to wear a rubber glove on either the left or the right
hand while using the detergents. The test was conducted for one
month, and the gross appearance of hands before and after the test
period was compared. The relative intensity of noninflammatory
keratosis of the hands was increased in individuals in both groups
on hands that were covered and to a slightly greater extent on hands
that were uncovered. In the second series of tests, 881 housewives
were divided into three groups and instructed to use only one brand
of household detergent, containing LAS, AOS, or ABS during the test
period and to wear rubber gloves on both hands while using the
detergent. The test was conducted for 1.5 months, and the gross
appearance of hands before and after the test period was compared.
Skin roughness was not worsened in any of the three groups (Watanabe
et al., 1968).
A8.2.4 Occupational exposure
Sixty workers exposed at work to an atmosphere containing LAS at
8.64 mg/m3 were tested for serum lipid and sugar content and for
the activities of selected serum enzymes. The levels of total plasma
lipids and plasma cholesterol were slightly lower in the exposed
group than in controls, but no differences were noted for blood
sugar, plasma phospholipid, plasma lipoprotein, alpha-amylase,
leucine aminopeptidase, or pseudocholinesterase. The duration of
exposure before testing was not indicated (Rosner et al., 1973).
In an investigation of the asthmagenic properties of sodium
isononanoyl oxybenzene sulfonate, detergent industry workers were
also tested with LAS. Three workers previously exposed to sodium
isononanoyl oxybenzene sulfonate, three unexposed controls without
asthma, and three controls with asthma were challenged with
0.01-100 µg of LAS. No changes were seen after inhalation of LAS in
any of the subjects; but sodium isononanoyl oxybenzene sulfonate
induced asthmatic symptoms in the previously exposed workers and not
in the control groups (Stenton et al., 1990).
A8.2.5 Accidental or suicidal ingestion
No symptoms were seen in four cases of accidental ingestion of
unknown amounts of a household synthetic detergent containing LAS as
the main component (Hironaga, 1979).
A 32-year-old woman who had ingested 160 ml of a 21% aqueous
solution of LAS with suicidal intent showed transient, slight mental
confusion, vomiting, pharyngeal pain, hypotension, decreased plasma
cholinesterase activity, and increased urinary urobilinogen, but all
of these symptoms disappeared rapidly (Ichihara et al., 1967).
In a review of 1 581 540 cases of human exposure to a wide range
of chemicals reported by the United States Poison Control Centers in
1989, 7983 people had been exposed to household automatic dishwasher
preparations (alkali, anionic or nonionic, other or unknown) and 506
had required treatment in a health facility; 8950 had been exposed
to household cleansers, with 894 requiring treatment; 12 876 had
been exposed to laundry preparations, with 1542 treated; and 621 had
been exposed to industrial detergents (anionic, cationic, nonionic),
with 321 cases requiring treatment. There were no deaths, and only
12 of the treated cases were classified as 'major outcome'.
Virtually all the reports involved accidental exposure. The
compositions of the cleaning preparations, routes of exposure, and
clinical descriptions were not provided (Litovitz et al., 1990).
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
LAS have been tested extensively, both in the laboratory and
under field conditions, but the following aspects must be considered
in interpreting test results. Comparison of the results of tests
carried out on either mixtures of homologues of LAS or LAS of
specified chain length is restricted, because the toxicity of LAS is
influenced by the chain length, and homologues of lower chain length
are less toxic than those with longer chains; furthermore, chain
length was rarely specified in older studies. Studies of the effects
of formulations of LAS on environmental biota are not included in
this section.
Organisms are not exposed to a constant concentration of LAS in
water, owing to the high adsorptivity and biodegradability of LAS.
As LAS are adsorbed on suspended solids or food particles, they have
reduced bioavailability. The adsorption kinetics of LAS also depend
on the chain length of the homologues. Studies of aquatic toxicity
involving flow-through or static renewal (at least daily) should
therefore be given more prominence than studies based on static
conditions, although flow-through and static renewal cannot be used
in (semi-) chronic studies of lower organisms, such as daphnia.
Studies in which the actual concentration was measured should
likewise be given more consideration than those that rely on nominal
concentrations.
The effects of LAS on the aquatic environment have been studied
in short- and long-term studies in the laboratory and under more
realistic conditions: micro- and mesocosm and field studies. In
general, a decrease in alkyl chain length or a more internal
position of the phenyl group is accompanied by a decrease in
toxicity. Data on fish and daphnia indicate that a decrease in chain
length of one unit (e.g. C12 to C11) is accompanied by an
approximately 50% decrease in toxicity, but there is no linear
relationship between chain length and toxicity. In aquatic
microorganisms, the effects are strongly related to variables such
as the type of test system and use of mixed cultures as opposed to
individual species. EC50 values range from 0.5 mg/litre (single
species) to > 1000 mg/litre.
In freshwater fish, the acute LC50 values of C8-C15 LAS
are 0.1-125 mg/litre. The chronic L(E)C50 values of LAS (C11.7 and
not specified) in two species tested were 2.4 and 11 mg/litre, and
NOECs ranging from 0.11 to 8.4 mg/litre have been reported for
C11.2-C13 (or not specified). Marine fish appear to be more
sensitive, with acute LC50 values for C11.7 (or not specified)
in six species of 0.05-7 mg/litre, chronic LC50 values for LAS of
unspecified chain length in two species of 0.01-1 mg/litre, and an
NOEC for C12 in one species of < 0.02 mg/litre.
Results in aquatic plants are also species dependent. In
freshwater plants, the EC50 values for LAS (with chain lengths
shown in parentheses) were 10-235 mg/litre for green algae
(C10-C14), 5-56 mg/litre for blue algae (C11.1-C13),
1.4-50 mg/litre for diatoms (C11.6-C13), and 2.7-4.9 mg/litre
for macrophytes (C11.8). Marine algae appear to be even more
sensitive. There is probably no linear relationship between chain
length and toxicity to algae.
The effects of LAS on freshwater algae have also been tested
under realistic conditions in systems with various trophic levels,
comprising enclosures in lakes (lower organisms), model ecosystems
(sediment: water systems), a river below and above a wastewater
treatment plant outfall, and experimental streams. In general, C12
LAS were used. Algae were more sensitive in summer, when the 3-h
EC50 values with regard to photosynthesis were 0.2-8.1 mg/litre,
whereas studies of model ecosystems showed no effects on the
relative abundance of algal communities at 0.35 mg/litre. No effects
were seen in these studies at 0.24-5 mg/litre, depending on the
organism and parameter tested.
In aquatic invertebrates, the acute L(E)C50 values were
4.6-200 mg/litre for molluscs (either C13 or not specified),
0.12-27 mg/litre for crustaceans (C11.2-C18 or not specified),
1.7-16 mg/litre for worms (C11.8 or not specified), and
1.4-270 mg/litre for insects (C10-C15). The chronic L(E)C50
values were 2.2 mg/litre for insects (C11.8) and 1.1-2.3 mg/litre
for crustaceans (C11.8-C13). The chronic NOEC for crustaceans,
on the basis of lethality or reproduction, was 0.2-10 mg/litre
(C11.8 or not specified). Marine invertebrates are more sensitive,
with LC50 values of 1 to >100 mg/litre (almost all C12) and
NOEC values of 0.025-0.4 mg/litre (chain lengths not specified).
Biodegradation products and by-products of LAS are 10-100 times
less toxic than the parent compound.
Fewer data are available on the effects of LAS in the
terrestrial environment. For the plant species tested, the NOEC
values were < 10-20 mg/litre in nutrient solutions and 100 mg/kg
(C10-C13) for growth of plants in soils. The 14-day LC50 for
earthworms was > 1000 mg/kg.
One study in which chickens were treated in the diet resulted in
an NOEC based on egg quality of > 200 mg/kg.
A9.1 Effect of chain length on the toxicity of linear
alkylbenzene sulfonates
The ecotoxicity of homologues of LAS varies according to the
length of the alkyl chain and the position of the benzene ring on
this chain. In general, homologues with longer chains are more
ecotoxic than shorter ones, and ecotoxicity increases with the
proximity of the benzene ring to the end of the chain. The results
of studies on the effect of LAS chain length on acute toxicity to
fish are presented in Table 23.
The effect of chain length can also be seen on the basis of
quantitative structure-activity relationships (Roberts, 1989, 1991)
calculated from the octanol-water partition coefficients of
homologues of LAS. The slope of the relationship varied from 0.64 to
0.78; therefore, using an average slope of 0.70, it was calculated
that a decrease in chain length from C12 to C11 reduced the
aquatic toxicity of LAS by a factor of 2.4, with a corresponding
decrease in the octanol-water partition coefficient of 0.54.
Table 23. Effect of the chain length of linear alkylbenzene sulfonates (LAS)
on their acute toxicity to freshwater fish
Homologue Fathead minnow Goldfish Guppy Golden orfe
of LAS Pimephales Carassius Lebistes Idus idus
promelas auratus reticulatus melanotus
48-h LC50 6-h LC50 LC50 (mg/litre)c 96-h LC50
(mg/litre)a (mg/litre)b (mg/litre)d
C10 43.0 61.0 50 16.6
C11 16.0 22.5 6.5
C12 4.7 8.5 5 2.6
C13 0.4 3.3 0.57
C14 0.4 1 0.26
C16 0.087 1 0.68
C18 0.38 15
a From Kimerle & Swisher (1977)
b From Gafa (1974)
c From Borstlap (1967)
d From Hirsch (1963)
A9.2 Microorganisms
No adverse effects were seen on the performance of
laboratory-scale activated sludge units after addition of <
20 mg/litre LAS. At 50 mg/litre, nitrification was decreased in
extended aeration units that were treating synthetic sewage (Janicke
& Niemitz, 1973). A bacterium similar to Klebsiella pneumoniae
isolated from sewage degraded LAS at a concentration of 10 ml/litre,
but a concentration of 20 ml/litre inhibited the growth of the
bacterium by 39% (Hong et al., 1984).
The toxicity of microorganisms in activated sludge increases
with the length of the alkyl chain up to approximately C12 and
then decreases (Table 24), presumably because of decreased
bioavailability (e.g. greater sorption of these higher chain
lengths) (Verge et al., 1993).
Table 24. Results of tests for the inhibition of activated
sludge by the sodium salt of linear alkylbenzene
sulfonates (LAS)
LAS Chain length 3-h EC50 (mg/litre)
Pure homologues C10 1042-1200
C11 740-782
C12 500-723
C13 700-795
C14 900-1045
Commercial
formulations C11 760
C11.6 550
C13 650
From Verge et al. (1993)
A mixed bacterial culture was acclimatized to 10 mg/litre LAS
(C9-C14) and was then maintained in either river water, forest
soil, or wastewater from a detergent plant, the concentration of LAS
being increased every five days. At 20.8 and 46 mg/litre, no effect
was reported on the specific growth rate of the bacteria; however,
at 70 mg/litre, the growth rate was inhibited by 18%, and at
95 mg/litre growth was almost zero. Concentrations of 186 and
465 mg/litre LAS inhibited growth completely (Hrsak et al., 1981).
The acute toxicity of LAS (C9-C14) in naturally occurring
bacteria was studied in freshwater and seawater samples by measuring
3H-thymidine incorporation. The EC50 values were 0.5-1.66 mg/litre
for all samples. Toxicity was found to increase with an increasing
relative abundance of longer carbon chains (Martinez et al., 1989).
For bacteria collected from the Rhone River plume (an estuarine
area) and exposed to LAS, the EC50, based on 3H-thymidine
incorporation, was 11.9 mg/litre (Martinez et al., 1991).
The 8-h EC50, based on specific growth rate, of Pseudomonas
fluorescens in solutions of C11.1 LAS under static conditions
was 3200-5600 mg/litre (Canton & Slooff, 1982).
The effect of C11.6 LAS on the structure and function of
microbial communities was studied in a flow-through model ecosystem
containing several trophic levels at concentrations of 0.5 or
5 mg/litre. LAS had no effect on microbial structure at either dose
level, but at 5 mg/litre it inhibited the degradation of both
glucose and LAS. In an experiment in which LAS were supplied in
sewage, neither microbial structure nor function was affected
(Larson & Maki, 1982).
The effects of LAS on the microbial activity of soils were
studied on the basis of Fe[III] reduction. The no-effect-level was
found to be 250 mg/kg; the EC50 was about 500 mg/kg in a strongly
adsorbing soil and 33-55 mg/kg in a poorly adsorbing soil (Welp &
Brummer, 1985).
LAS at concentrations of 0.8-50 g/m2 had no effect on
respiration of loamy soil, sandy soil, or sandy soil irrigated with
wastewater for one or 14 days (Litz et al., 1987).
A9.3 Aquatic organisms
A9.3.1 Aquatic plants
A9.3.1.1 Freshwater algae and cyanobacteria
The 96-h EC50 values for C13 LAS on population growth were
116 mg/litre for the green alga Selenastrum capricornutum,
5 mg/litre for the blue-green alga Microcystis aeruginosa, and
1.4 mg/litre for the diatom Navicula pelliculosa. The EC50
values for C12 LAS were 29 mg/litre for Selenastrum and
0.9 mg/litre for Microcystis (Lewis & Hamm, 1986). The EC50 for
C11.7 LAS on growth of Selenastrum was reported to be 83
mg/litre (Konno & Wakabayashi, 1987). The EC50 values for C11.6
LAS were found to be 50-100 mg/litre for Selenastrum,
10-20 mg/litre for Mycrocystis, and 20-50 mg/litre for the diatom
Nitzschia fonticola (Yamane et al., 1984). The seven-day EC50
for C12 LAS in the green alga Chlorella pyrenoidosa, based on
growth, was 10 mg/litre (Kondo et al., 1983).
The 96-h EC50 values in algae grown in solutions of C11.1
LAS under static conditions, measured as biomass, were
32-56 mg/litre for Microcystis aeruginosa and 18-32 mg/litre for
Chlorella vulgaris (Canton & Slooff, 1982).
A study of the toxicity of various formulations of LAS to the
algae Scenedesmus subspicatus and Selenastrum capricornutum
(Table 25) indicated that commercial mixtures are as or slightly
less toxic than homologues. This finding may be due to a difference
in the sensitivity of the two algae, since those tested with the
homologues were of a different origin than those tested with
commercial LAS (Verge et al., 1993).
Table 25. Results of tests for the toxicity of the sodium salt
of linear alkylbenzene sulfonates (LAS) in algae
LAS Chain length 72-h EC50 (mg/litre)
Pure homologues C10 235
C11 118
C12 62
C13 33
C14 18
Commercial
formulations C11 80
C11.6 80
C13 62
From Verge et al. (1993)
LAS (chain length not specified) significantly reduced the
growth of the green alga Selenastrum capricornutum at a
concentration of 40 mg/litre or more. A significant decrease in
growth was also noted at 10 mg/litre, but no significant effect was
observed at 20 or 30 mg/litre (Nyberg, 1988).
A9.3.1.2 Marine algae
Growth of Gymnodinium breve was reduced by 69% rafter nine days'
exposure to C12 LAS (Kutt & Martin, 1977). These results were
confirmed in a study in which C13 LAS were introduced at the
bottom or surface of a water column: Exposure to LAS at
concentrations > 0.025 mg/litre inhibited growth completely within
two days (Hitchcock & Martin, 1977). These results suggest that
Gymnodinium breve is more sensitive to the effects of LAS than
other algae.
For C11.7 LAS, the seven-day EC50 for growth and the two-day
EC50 for ATP activity on the marine diatom Thalassiosira
pseudonana were both 10 mg/litre (Kondo et al., 1983).
Exposure of the alga Porhyra yezoensis, a standard test
species in Japan, to LAS (C10-C14) under semi-static conditions
gave a 10-day E50 (based on growth) of 0.56 mg/litre (Takita,
1985).
A9.3.1.3 Macrophytes
The seven-day EC50 values for C11.8 LAS on the duckweed
Lemna minor under flow-through conditions were 2.7 mg/litre for
frond count, 3.6 mg/litre for dry weight, and 4.9 mg/litre for root
length. The time-independent EC50 for growth rate and doubling
time was 4.8 mg/litre (Bishop & Perry, 1981).
A9.3.2 Aquatic invertebrates
A9.3.2.1 Acute toxicity
The acute toxicity of LAS to aquatic invertebrates is summarized
in Tables 26 and 27. For marine invertebrates, the 96-h LC50
values for C12 LAS range from 3 mg/litre for barnacles to >
100 mg/litre for several other species (Table 26). Freshwater
invertebrates show a range of 48-h LC50 values from 0.11 mg/litre
(C16) for a daphnid to 270 mg/litre (C11.8) for an isopod (Table
27). Several marine invertebrate species are more sensitivite to LAS
at the larval stage than as adults (Table 26).
Freshwater mussels (Anodonta cygnea) were more sensitive to
LAS during the reproductive period than during the non-reproductive
period, the 96-h LC50 being reduced from 200 to 50 mg/litre
(Bressan et al., 1989).
Studies with Daphnia magna revealed a correlation between
chain length and toxicity. The acute toxicity (24-h and 48-h LC50)
of LAS to Daphnia magna increased with chain length between C10
and C14 (Kimerle & Swisher, 1977) and with chain lengths between
C10 and C16 (Maki & Bishop, 1979), although similar values were
obtained for C16 and C18 homologues. No significant difference
in sensitivity was seen between Daphnia magna and Daphnia pulex.
A similar result was obtained with homologue mixtures (Martinez et
al., 1989): toxicity was correlated with the homologues in which
long chains were the most abundant.
Partial biodegradation of LAS significantly reduces the specific
toxicity (by unit weight) of the remaining LAS to Daphnia magna.
For example, LAS with a high relative molecular mass and a 48-h
LC50 of 2 mg/litre had an LC50 of 30-40 mg/litre after 80-85%
degradation (Kimerle & Swisher, 1977); the longer homologues and
more terminal isomers, which are the most toxic, are therefore also
the more readily biodegraded. Shorter carboxylates formed during the
degradation of LAS were three to four orders of magnitude less toxic
than LAS (Swisher et al., 1978). Other workers also found a
Table 26. Acute toxicity of linear alkylbenzene sulfonates (LAS) to estuarine and marine invertebrates
Organism Size or Static or Temp. Salinity LAS chain End-point Concentration Reference
age flow (°C) (%) length (mg/litre)a
Sea squirt Larva Static 20 NS 6-h LC50 1 Renzoni (1974)
(Ciona intestinalis)
Common mussel Static 6-8 32-34 C12 96-h LC50 > 100 Swedmark et
(Mytilus edulis) Static 15-17 32-34 C12 96-h LC50 50 al. (1971)
Mussel Staticr 18 35 NS 48-h LC50 39.8 Bressan et al.
(Mytilus galloprovincialis) Adult 18 35 NS 96-h LC50 1.66 (1989)
Cockle Static 6-8 32-34 C12 96-h LC50 5 Swedmark et
(Cardium edule) Juvenile Static 15-17 32-34 C12 96-h LC50 5 al. (1971)
Clam Static 6-8 32-34 C12 96-h LC50 70
(Mya arenaria) Static 15-17 32-34 C12 96-h LC50 < 25
Scallop Static 6-8 32-34 C12 96-h LC50 < 5
(Pecten maximus)
Scallop Static 15-17 32-34 C12 96-h LC50 < 5
Decapod Static 15-17 32-34 C12 96-h LC50 50
(Leander adspersus) Intermoult Static 6-8 32-34 C12 96-h LC50 50
Postmoult Static 6-8 32-34 C12 96-h LC50 25
Hermit crab Static 6-8 32-34 C12 96-h LC50 > 100
(Eupagurus bernhardus)
Table 26 (contd)
Organism Size or Static or Temp. Salinity LAS chain End-point Concentration Reference
age flow (°C) (%) length (mg/litre)a
Spider crab Larva Static 6-8 32-34 C12 96-h LC50 9
(Hyas araneus) Adult Static 6-8 32-34 C12 96-h LC50 > 100
Shore crab Static 6-8 32-34 C12 96-h LC50 > 100
(Carcinus maenus)
Barnacle Larva Static 6-8 32-34 C12 96-h LC50 3
(Balanus balanoides) Adult Static 6-8 32-34 C12 96-h LC50 50
Brine shrimp Static 25 C11-C13 24-h LC50 33 Price et al.
(Artemia salina) (1974)
Static: water unchanged for duration of test; NS, not specified; staticr, static renewal: water changed every 12 h; flow, flow-through
conditions: LAS concentration in water maintained continuously
a Based on nominal concentration
Table 27. Acute toxicity of linear alkylbenzene sulfonates (LAS) to freshwater invertebrates
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Bivalve mollusc 11 cm Staticr 18 8.0 NS 96-h LC50 200b Bressan et al.
(Anodonta cygnea) 18 8.0 NS 96-h LC50 50b,c (1989)
Bivalve mollusc 9 cm Staticr 18 8.0 NS 96-h LC50 182.5b
(Unio elongatulus)
Snail Static 21 62 7.3 av. C13 24-h LC50 4.6b Dolan &
(Gonobasis sp.) Hendricks (1976)
Snail (Physa integra) Flow 15 41-47 7.5-7.7 NS 96-h LC50 9b Arthur (1970)
Amphipod (Gammarus Flow 15 41-47 7.5-7.7 NS 96-h LC50 7b
pseudolimnaeus)
Amphipod 4.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 3.3b Lewis &
(Gammarus sp.) Suprenant (1983)
Campeloma decisum Flow 15 41-47 7.5-7.7 NS 96-h LC50 27b Arthur (1970)
Water flea < 24 h Static 20 25 C11.7 24-h LC50 17 Wakabayashi
(Daphnia magna) et al. (1988)
< 24 h Static 21 120 7.4 C10 48-h LC50 9.55d Maki &
Bishop (1979)
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Water flea (contd) < 24 h Static 21 120 7.4 C11 48-h LC50 1.15d
(Daphnia magna) < 24 h Static 21 120 7.4 C12 48-h LC50 5.88-6.84d
< 24 h Static 21 120 7.4 C13 48-h LC50 2.63d
< 24 h Static 21 120 7.4 C14 48-h LC50 0.68-0.8d
< 24 h Static 21 120 7.4 C16 48-h LC50 0.11-0.2d
< 24 h Static 21 120 7.4 C18 48-h LC50 0.12d
< 18 h Static C13.3 48-h LC50 2.3b Kimerle &
< 18 h Static C10 48-h LC50 12.3b Swisher (1977)
< 18 h Static C11 48-h LC50 5.7b
< 18 h Static C12 48-h LC50 3.5b
< 18 h Static C13 48-h LC50 2.0b
< 18 h Static C14 48-h LC50 0.7b
< 24 h Static 19 C11.2 48-h LC50 18-32b Canton &
Slooff (1982)
< 24 h Static 21 131 7.4-7.8 C11.8 48-h LC50 4.8d Lewis (1983)
< 24 h Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8-5.6b Lewis &
Suprenant
(1983)
< 24 h Static 21 295-310 7.3-8.4 C11.8 48-h LC50 3.6-4.7b Taylor (1985)
< 48 h Static 22 241 7.8 C11 48-h EC50 2.2b,e Barera &
Adams (1983)
Flow C11.8 48-h LC50 4.4d Bishop &
Perry (1981)
< 12 h Flow 21 120 7.4 C11.8 96-h LC50 23.94d Maki (1979a)
< 12 h Flow 21 120 7.4 C13 48-h LC50 2.19d
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Water flea < 24 h Static 20 25 C11.7 24-h LC50 18 Wakabayashi
(Daphnia pulex) et al. (1988)
< 24 h Static 21 120 7.4 C12 48-h LC50 8.62d Maki &
Bishop (1979)
< 24 h Static 21 120 7.4 C14 48-h LC50 0.59d
< 24 h Static 21 120 7.4 C16 48-h LC50 0.15d
Oligochaete (Dero sp.) 6.0 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.7b Lewis &
Suprenant
(1983)
Roundworm (nematode) 0.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.7b
(Rhabditis sp.)
Flatworm 3.4 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8b
(Dugesia sp.)
Branchiura sowerbyi Staticr 10 25 8.0 NS 96-h LC50 10.8b,f Bressan et al.
10 25 8.0 NS 96-h LC50 4.4b (1989)
Worm (Limnodrilus Staticr 10 25 8.0 NS 96-h LC50 7.8b,f
hoffmeisteri) 10 25 8.0 NS 96-h LC50 2.0b
Isopod (Asellus sp.) 5.3 mm Static 22 165 7.9-8.4 C11.8 48-h LC50 1.8b Lewis &
Suprenant
(1983)
Table 27 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Midge (Chironomus Larva Flow 22 150 7.8-8.4 C11.8 72-h LC50 2.2d Pittinger
riparius) et al. (1989)
Midge (Paratanytarsus 3.6 mm Static 2 165 7.9-8.4 C11.8 48-h LC50 1.8d Lewis &
parthenogenica) Suprenant
(1983)
Mosquito (Aedes Larva Static C10-13 24-h LC50 6b Van Emden et
aegypti) Larva Static C10-15 24-h LC50 2b al. (1974)
3-4 d Static 23 C11.1 48-h LC50 56-100b Canton &
Slooff (1982)
Mayfly Larva Static 10 53 7.5-7.8 C11.6 24-h LC50 13.6b Dolan et al.
(Isonychia sp.) Larva Static 10 53 7.5-7.8 C11.6 48-h LC50 10.4b (1974)
Larva Static 10 53 7.5-7.8 C11.6 96-h LC50 5.33b
Larva Static 10 53 7.5-7.8 C13.1 24-h LC50 4.19b
Larva Static 10 53 7.5-7.8 C11.6 48-h LC50 12.47b
Larva Static 10 53 7.5-7.8 C11.6 96-h LC50 1.36b
Staticr, static renewal: water changed every 12 h; NS, not specified; flow, flow-through conditions: LAS concentration in water maintained
continuously; static: water unchanged for duration of test
a mg/litre CaCO3
b Based on nominal concentration
c Test performed during the reproductive period
d Based on measured concentrations
e Based on immobilization
f Organism exposed in the presence of sediment
reduction in the acute toxicity of LAS to Daphnia magna during
primary degradation (Gard-Terech & Palla, 1986).
Increasing hardness also increased the acute toxicity (48-h
LC50) of C11.8 LAS from a nominal concentration of 7.1 mg/litre
at 25 mg/litre CaCO3 to 4.0 mg/litre at 350 mg/litre CaCO3;
however, significant additional physiological stress was induced if
the hardness of the culture water was significantly different from
that of the test water. Pre-exposure to 0.4 mg/litre LAS (one-tenth
of the 48-h LC50) for up to seven generations (14 weeks) had no
significant effect on the susceptibility of daphnids to acute
exposures (Maki & Bishop, 1979).
Loading density, ranging from 10 daphnids per 20 ml to 20
daphnids per 1000 ml, had no significant effect on the acute
toxicity of C11.8 LAS for Daphnia magna (Lewis, 1983). Daphnids
fed a diet containing Selenastrum had a significant, twofold
decrease in acute toxicity due to C11.8 LAS in comparison with
unfed daphnids (Taylor, 1985). The presence of sediment reduced the
acute toxicity of LAS to the oligochaete worms Branchiura sowerbyi
and Limnodrilus hoffmeisteri. The NOEC and LOEC for B. sowerbyi
were 2.5 times higher in the presence of sediment, and those for
L. hoffmeisteri were 4-4.5 times higher (Bressan et al., 1989; see
also Table 27).
The 96-h EC50 values for duplicate studies of the effect of
LAS on attachment of the podia of the sea urchin Hemicentrotus
pulcherrimus were 3.7 and 3.8 mg/litre (Lee & Park, 1984).
The data from other studies (Lal et al., 1983, 1984a,b; Misra et
al., 1984; Chattopadhyay & Konar, 1985; Misra et al., 1985; Devi &
Devi, 1986; Misra et al., 1987, 1989a,b, 1991) could not be
adequately interpreted because of deficiencies in the data or
method, including inadequate characterization of the test material
with regard to chain-length distribution and use of test material in
an acidified form. The range of values for toxicity reported in
these studies was 10-100 times greater than that in numerous studies
of the same or similar species, and the high values have not been
verified by these or other researchers. As the toxic effects
reported are not considered to be representative of those of
commercial LAS, the data were not used in evaluating the
environmental effects of LAS.
A 72-h LC50 of 2.2 mg/litre was reported for C11.8 LAS in
newly hatched larvae of the midge (Chironomus riparius) (Pittinger
et al., 1989).
A9.3.2.2 Short-term and long-term toxicity
The 21-day LC50 for the water flea (Daphnia magna) was
18 mg/litre, and the NOEC, based on survival, was 10 mg/litre under
static renewal conditions. The 21-day EC50, based on reproduction,
was estimated to be > 10 mg/litre (Canton & Slooff, 1982). The
14-day EC50 for C12 LAS in Daphnia carinata, based on
reproduction, was 16.8 mg/litre (Hattori et al., 1984).
Diet had a significant effect on the sensitivity of Daphnia
magna to the chronic toxicity of C11.8 LAS. The NOEC values
showed a threefold variation of 1.2-3.2 mg/litre and the 21-day
LC50 values a twofold variation of 2.2-4.7 mg/litre with diet. A
threefold variation in toxicity in tests in Daphnia is not,
however, unusual (Taylor, 1985).
Under continuous-flow conditions, a 21-day LC50 value of
1.67 mg/litre was found for daphnids (Daphnia magna) exposed to
C11.8 LAS and 1.17 mg/litre for those exposed to C13 LAS. The
EC50 values for reproductive toxicity were 1.5 mg/litre for
C11.8 LAS and 11.1 mg/litre for C13 with respect to total young
production, 2.3 mg/litre for C11.8 and 1.4.1 mg/litre for C13
for average brood size, and 2.31 mg/litre for C11.8 and
1.29 mg/litre for C13 for percentage of days on which reproduction
occurred (Maki, 1979a).
Campeloma decisum, Gammarus pseudolimnaeus, and Physa integra
were exposed to LAS at concentrations of 0.2-4.4 mg/litre for six
weeks; amphipods were exposed for a further 15 weeks. Survival,
growth, reproduction, feeding, and mobility were studied. The
maximum acceptable concentrations of LAS were found to be
0.2-0.4 mg/litre for Gammarus and 0.4-1.0 mg/litre for Campeloma;
P. integra were not significantly affected (Arthur, 1970).
Fertilized eggs of sea urchins (Paracentrotus lividus) were
treated with LAS at concentrations of 0-0.5 mg/litre for 40 days.
The pattern of embryonic development was unaffected, but the mean
length of the somatic rods of the echinoplutei were reduced
successively with increasing LAS concentrations. A significant
reduction in growth occurred at doses between 0.35 and 0.4 mg/litre;
above 0.45 mg/litre, alterations in skeletal development were
induced (Bressan et al., 1989).
Oligochaete worms (B. sowerbyi) were maintained in LAS at a
concentration of 0.5, 2.5, or 5.0 mg/litre for up to 140 days in the
presence of sediment. Exposed worms laid fewer cocoons and eggs, but
the worms exposed to 5 mg/litre were the least affected. The
percentage of degenerated cocoons, the percentage of worms hatching,
the mean number of eggs per cocoon, and the mean embryonic
development time were all unaffected by treatment. Worms exposed via
the sediment only were not affected (Bressan et al., 1989).
Growth of mussels (Mytilus galloprovincialis) exposed to LAS
at a concentration of 0.25 or 0.5 mg/litre for 220 days, expressed
as mean length of the major axis of the shell, was significantly
slowed ( p < 0.001). The mean (± SE) increments in growth were:
control, 3.11 ± 0.34; 0.25 mg/litre, 1.71 ± 0.15; 0.5 mg/litre,
1.48 ± 0.16 (Bressan et al., 1989).
Eggs of the common mussel, M. edulis, were exposed from the
time of fertilization for 240 h. Fertility was decreased at the
lowest concentration of 0.05 mg/litre and fertilization did not take
place at concentrations in excess of 1 mg/litre. LAS at
concentrations > 0.3 mg/litre inhibited the development of mussel
larvae by delaying the transitory stages of larval development.
Reduced growth rates were observed at concentrations > 0.1 mg/litre
(Granmo, 1972).
Newly fertilized eggs of American oysters (Crassostrea
virginica) were exposed to LAS (chain length not specified, but
likely to be C13) for 48 h. The percentage of eggs that developed
normally was significantly reduced at concentrations greater than
0.025 mg/litre. The percentage survival of oyster larvae hatched in
'clean' water and exposed to LAS at a concentration of 1 mg/litre
for 10 days was significantly decreased, and growth (mean length)
was significantly reduced at 0.5 mg/litre (Calabrese & Davis, 1967).
Embryos of sea urchins (P. lividus) were exposed to LAS at
concentrations of 0.25-0.5 mg/litre from the time of fertilization
for 40 h. At concentrations > 0.45 mg/litre, skeletal development
was totally inhibited; a significant decrease was observed at
0.3 mg/litre. The effect of LAS was found to be maximal at the end
of gastrulation when calcium uptake is high (Bressan et al., 1991).
The effects of LAS were studied on the eggs and sperm of the sea
squirt Ciona intestinalis. Fertility and hatchability were
markedly reduced at 0.1 mg/litre when eggs and sperm were exposed
for the entire developmental period; however, if they were exposed
only before fertilization, fertility and hatchability were slightly
reduced at 0.1 mg/litre but markedly at 1 mg/litre. Male gametes
appeared to be particularly sensitive to the toxic effects of LAS
(Renzoni, 1974).
Two marine benthic filter feeders, the sea squirts Botryllus
schlosseri and Botrylloides leachi were exposed at different
periods of development to LAS. When larvae were exposed from
spawning for 6 h, the incidence of abnormal metamorphosis was
significantly increased at 1 mg/litre LAS for Botryllus and 2
mg/litre for Botrylloides. The frequency of spontaneously settled
larvae of both species also increased with exposure to LAS and
seemed to be a selective effect of LAS. The frequency was
significantly different from controls at 1 and 3 mg/litre for the
two species, respectively. In a second experiment, young colonies
were exposed to LAS for 15 days immediately after discharge by the
parental colony. Growth rates were significantly decreased at
0.5 mg/litre for Botryllus and at 0.25 mg/litre for Botrylloides.
When colonies were exposed from the end of metamorphosis, their
growth rates were similarly affected, but the mortality rate was
significantly lower. The effects of LAS thus appear to be exerted
mainly on the pelagic phase of the life cycle (Marin et al., 1991).
No significant reduction in egg hatching of midges (Chironomus
riparius) was seen at the highest concentration of C11.8 LAS
tested (18.9 mg/litre), but newly hatched larvae were more
sensitive, with a 72-h LC50 of 2.2 mg/litre. In bioassays of part
of the life cycle in a sediment and water system, the percentages of
winged adults emerging were monitored after continuous exposure of
larvae and pupae. The NOEC for sediment containing LAS was 319 mg/kg
(dry weight). In the absence of sediment, the NOEC was
2.40 mg/litre. Both tests were conducted for about 20 days
(Pittinger et al., 1989).
A9.3.2.3 Biochemical and physiological effects
Juvenile mussels (M. galloprovincialis) were exposed to LAS at
a concentration of 0.25 or 0.5 mg/litre for 220 days. Oxygen uptake
and the retention rate of neutral red (a measure of filtration rate)
were significantly decreased, but no effect was detected on nitrogen
excretion (measured as ammonia). When the experiment was repeated
over a seven-day period at a concentration of LAS of 1 or
1.5 mg/litre, no significant effect was seen on nitrogen metabolism
and the results for oxygen uptake were inconclusive. The filtration
rate was again significantly reduced when compared with that in
control mussels (Bressan et al., 1989).
The 48-h LC50 for lugworms (Arenicola marina) exposed to LAS
was calculated to be 12.5 mg/litre (95% confidence interval,
8.6-18.2). When tissues from a lugworm exposed to a concentration
close to that of the LC50 were examined for changes in morphology
by both light and electron microscopy, serious damage was reported
in the caudal epidermis, epidermic receptors, and gills; no effect
was reported in the thoracic epidermis or the intestine. In the
caudal epidermis, LAS destroyed the papillae, disrupting the
internal structure, occasionally displacing the musculature below
the papillae and thus giving it direct contact with seawater.
Deciliation of the epidermic receptors was also reported. These
effects were considered to indicate that the physiological response
of damaged epidermic receptors was reduced or blocked by exposure to
LAS. Changes in the morphology of the gills included destruction of
the epithelium and blood vessels, causing complete solubilization of
branch apexes, and development of holes at the base of the gills
(Conti, 1987).
A9.3.3 Fish
A9.3.3.1 Acute toxicity
The acute toxicity of LAS to fish is summarized in Tables 28 and
29. Only a few studies were available on marine fish, providing two
96-h LC50 values, 1 and 1.5 mg/litre LAS. Tests in various species
of freshwater fish gave a wide range of LC50 values: the 48-h
values ranged from 0.2 mg/litre for brown trout (Salmo trutta) to
125 mg/litre for the golden orfe (Idus idus memanotus), and the
96-h values ranged from 0.1 mg/litre for brown trout to 23 mg/litre
for white tilapia (Tilapia melanopleura).
The acute toxicity tended to increase with increasing carbon
chain length. Thus, C14 LAS were more acutely toxic to bluegill
(Lepomis macrochirus) than C12 compounds (Swisher et al., 1964);
the acute toxicity of LAS to the golden orfe increased with chain
length from C8 to C15 but decreased at C16 (Hirsch, 1963).;
and a similar trend was found for fathead minnows (Pimephalus
promelas) exposed to LAS with chain lengths of C10 to C14
(Kimerle & Swisher, 1977).
The 96-h LC50 values in bluegill (Lepomis macrochirus) were
0.64 mg/litre for C14 and 3 mg/litre for C12 LAS but
75 mg/litre for the intermediate degradation product,
sulfophenylundecanoic acid disodium salt (Swisher et al., 1964).
Biodegradation of LAS with a high relative molecular mass
progressively shifted the homologue distribution in favour of
shorter chain lengths and reduced the acute toxicity of the compound
to bluegill (Dolan & Hendricks, 1976). Similar findings were
reported for fathead minnow (Swisher et al., 1978), goldfish
(Carassius auratus) (Divo & Cardini, 1980) and zebra fish
(Brachydanio rerio) (Gard-Terech & Palla, 1986).
In rainbow trout (Oncorhynchus mykissi), addition of LAS
(C10-C15) to activated sludge plant effluent increased the
nominal 96-h LC50 from 0.36 to 29.5 mg/litre (Brown et al., 1978).
No deaths were observed among bluegill exposed for 4-11 days to
effluent from continuous-flow activated sludge units fed
100 mg/litre LAS (Swisher et al., 1964).
Water hardness was found to be the most significant
environmental factor in the acute toxicity of LAS to bluegill,
increasing with the level of hardness. At a water hardness of
15 mg/litre CaCO3, the mean LC50 was 4.25 mg/litre; at
290 mg/litre CaCO3, the LC50 was reduced to 2.85 mg/litre
(Hokanson & Smith, 1971). Similarly, when water hardness was
increased from 0 to 500 mg/litre CaCO3, the LC50 for C18 LAS
in goldfish was reduced from 15 to 5.7 mg/litre (Gafa, 1974).
Exposure of the freshwater bleeker (Puntius gonionotus) to LAS
gave 96-h LC50 values of 13.6 mg/litre at a water hardness of
50 mg/litre CaCO3, 11.8 mg/litre at 110 mg/litre CaCO3, and
11.4 mg/litre at 260 mg/litre CaCO3 (Eyanoer et al., 1985).
The toxicity of C11.7 LAS to the medaka (Oryzias latipes)
increased with increasing salinity, but the effect was less
pronounced than that of water hardness (Wakabayashi & Onizuka,
1986).
Temperature was reported to have no significant effect on the
acute toxicity of LAS (Hokanson & Smith, 1971), but in another study
increasing the water temperature from 28 to 35°C marginally
decreased the 96-h LC50 for the bleeker, from 11.8 to
11.5 mg/litre (Eyanoer et al., 1985).
A reduction in the dissolved oxygen concentration from 7.5 to
1.9 mg/litre reduced the 24-h LC50 in bluegill from 2.2 to
0.2 mg/litre. When the fish were first acclimatized to reduced
oxygen levels, the effect was less pronounced (Hokanson & Smith,
1971).
No significant effect on the acute toxicity of LAS to bluegill
was observed after a bentonite suspension was added to water at
concentrations of 0, 50, or 200 mg/litre (Hokanson & Smith, 1971).
Addition of gluten, however, reduced the 24-h and 48-h acute
toxicity of LAS to both himedaka (Oryzias latipes) and cobalt
suzume (Chrysiptera hollisi) (Iimori & Takita, 1979).
A9.3.3.2 Chronic toxicity
Exposure of the eggs of fathead minnows (Pimephales promelas) to
LAS from laying until all surviving eggs had hatched under
flow-through conditions gave a nine-day LC50 of 2.4 mg/litre,
which would result in an LC50 of 3.4 mg/litre after 24 h of
exposure (Pickering, 1966).
Eggs of cod (Gadus morhua) were exposed to LAS at a
concentration of 0.005, 0.02, 0.05, or 0.1 mg/litre from
fertilization until hatching under flow-through conditions. There
were no significant effects at 0.005 mg/litre. At a concentration of
0.02 mg/litre, only 42% of the embryos completely developed into
larvae, and there was an increased occurrence of tail malformations
in comparison with controls. At 0.05 mg/litre, few eggs developed to
embryos. No eggs developed to the blastula stage at a concentration
of 0.1 mg/litre. In a repetition of the test at 0.05 mg/litre, fewer
eggs and larvae died, but there was an increased frequency of
abnormal embryos and inactive and crippled larvae (Swedmark &
Granmo, 1981).
Eggs, larvae, and immature adult fathead minnows (Pimephales
promelas) were exposed to LAS at a concentration of 0.34, 0.63,
1.2, or 2.7 mg/litre for up to four months. No significant effect
was observed on the number of spawnings, the total number of eggs
produced, the mean number of spawnings per female, the mean number
of eggs per spawning, or the percentage hatchability; however, the
two highest concentrations significantly reduced the survival of fry
(Pickering & Thatcher, 1970).
The effects of C11.8 and C13 LAS on the number of females,
the number of spawnings, total number of eggs produced, and number
of eggs per female were also studied in the fathead minnow over a
period of one year. As C11.8 LAS had no significant effect on
these parameters at a concentration of 1.09 mg/litre and a water
hardness of 120 mg/litre CaCO3, the NOEC was 0.9 mg/litre; C13
LAS were more toxic, and the NOEC was 0.15 mg/litre. At a lower
water hardness (39 mg/litre), however, survival of larvae was
impaired at 0.74 mg/litre (Maki, 1979a). NOECs in the fathead minnow
in life-cycle and embryo-larval tests were dependent on mean alkyl
chain length: 5.1-8.4 mg/litre for C11.2, 0.48 mg/litre for
C11.7, and 0.11-0.25 mg/litre for C13.3 (Holman & Macek, 1980).
The LC50 value of LAS in the eggs of carp (Cyprinus carpio)
exposed from spawning to hatching was 11 mg/litre. In determinations
of the sensitivity of eggs at different stages of development after
spawning, the 24-h LC50 values were 15 mg/litre for eggs exposed
between 2 and 26 h, 25 mg/litre for exposure between 26 and 50 h,
and 32 mg/litre for exposure between 50 h and hatching (Kikuchi et
al., 1976).
Bluegill (Lepomis macrochirus) were exposed to LAS from
fertilization to yolk-sac resorption at a concentration of 1.8, 3.5,
4.6, or 5.5 mg/litre. The lowest concentrations did not affect
hatchability or survival. Survival among those exposed to
3.5 mg/litre which hatched successfully was significantly reduced
within two days of hatching, and 95% were dead by the end of the
experiment. Eggs exposed to 4.6 or 5.5 mg/litre failed to hatch
(Hokanson & Smith, 1971).
The NOEC of LAS in guppies (Poecilia reticulata), based on
mortality, behaviour, and growth over 28 days, was 3.2 mg/litre
(Canton & Slooff, 1982).
Studies of the short- and long-term toxicity of LAS to
freshwater and marine fish are summarized in Tables 28 and 29.
A9.3.3.3 Biochemical and physiological effects
The main injury to the gills of catfish (Heteropneustes
fossilis) exposed to LAS at 1 or 2.5 mg/litre was progressive
separation of the lamellae from their vascular components.
Table 28. Toxicity of linear alkylbenzene sulfonates (LAS) to freshwater fish
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Brown trout Flow 15 26-30 NS 48-h LC50 5.3 Reiff et al.
(Salmo trutta) Flow 15 26-30 NS 96-h LC50 4.6 (1979)
Flow 15 26-30 NS 48-h LC50 2.3
Flow 15 26-30 NS 96-h LC50 1.4
Flow 15 26-30 NS 48-h LC50 0.4
Flow 15 26-30 NS 96-h LC50 0.4
Flow 15 250 NS 48-h LC50 2
Flow 15 250 NS 96-h LC50 0.9
Flow 15 250 NS 48-h LC50 0.7-0.9
Flow 15 250 C10-C15 48-h LC50 0.2
Flow 15 250 C10-C15 96-h LC50 0.1
Masu trout 2 mo Staticr 8.5-9.6 30 C11.7 96-h LC50 4.4 Wakabayashi et
(Oncorhynchus masou) al. (1984)
Rainbow trout Flow 15 250 C12.6 96-h LC50 0.36b Brown et al.
(Oncorhynchus mykiss) (1978)
40 d Staticr 8.8-10.9 25 C11.7 96-h LC50 4.7 Wakabayashi et
al. (1984)
4 d Staticr 10 25 C11.7 96-h LC50 2.1 Wakabayashi &
19 d Staticr 10 25 C11.7 96-h LC50 3.4 Onizuka (1986)
Goldfish Static 20 C16 6-h LC50 61 Gafa (1974)
(Carassius auratus) Static 20 C17 6-h LC50 22.5
Static 20 C18 6-h LC50 8.5
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Goldfish (contd) Static 20 C19 6-h LC50 3.3
(Carassius auratus) Static 20 C16-C19 6-h LC50 7.6
Static 20 C16-C19 6-h LC50 10
Static 20 C16-C19 6-h LC50 12.2
Static 20 100 NS 6-h LC50 8.2 Reiff et al.
Static 20 100 NS 6-h LC50 7 (1979)
Static 20 100 NS 6-h LC50 4.3
3.1-6.0 Flow 20-23 45-96 7.1-9.3 24-h LC50 7.6 Tsai & McKee
cm Flow 20-23 45-96 7.1-9.3 48-h LC50 7.5 (1978)
Flow 20-23 45-96 7.1-9.3 72-h LC50 7.0
Flow 20-23 45-96 7.1-9.3 96-h LC50 6.2
Bluegill sunfish 1.6 g Static 23 76 7.5 av. C13 48-h LC50 0.72b Dolan &
(Lepomis macrochirus) 1.6 g Static 23 76 7.5 av. C13 96-h LC50 0.72b Hendricks
(1976)
Flow 23 50 7.5 av. C13 96-h LC50 4c Thatcher &
Santner (1967)
Finger Static 25 15 C11.2 48-h LC50 4.0-4.5b Hokanson &
Finger Static 25 290 C11.2 48-h LC50 2.8-2.9b Smith (1971)
Flow C11.8 96-h LC50 1.7c Bishop & Perry
(1981)
Fathead minnow Static C13.3 48-h LC50 1.7b Kimerle &
(Pimephales promelas) Static C10 48-h LC50 43b Swisher (1977)
Static C11 48-h LC50 16b
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Fathead minnow (contd) Static C12 48-h LC50 4.7b
Static C14 48-h LC50 0.4b
2-3 mo Static 40 C11.2 96-h LC50 12.3c Holman & Macek
2-3 mo Static 40 C11.7 96-h LC50 4.1c (1980)
2-3 mo Static 40 C13.3 96-h LC50 0.86
Static 25 48-h LC50 4.6 Pickering &
Static 25 96-h LC50 5.0 Thatcher (1970)
Flow 15 43 7.2-7.9 96-h LC50 3.4 McKim et al.
(1975)
Flow 23 50 7.5 96-h LC50 4.2 Thatcher &
Santner (1967)
Flow 25 96-h LC50 4.2-4.5 Pickering &
Thatcher (1970)
2.5 cm Flow 18 116 7.9 C12 96-h LC50 3.5 Solon et al.
(1969)
Harlequin fish Flow 20 20 NS 48-h LC50 7.6 Reiff et al.
(Rasbora heteromorpha) Flow 20 20 NS 96-h LC50 6.1 (1979)
Flow 20 20 NS 48-h LC50 5.1
Flow 20 20 NS 96-h LC50 4.6
Flow 20 20 C10-C15 48-h LC50 0.9
Flow 20 20 NS 96-h LC50 0.7
Carp (Cyprinus carpio) 4.4 mg Static 22 25 7 C11.7 12-h LC50 5.6 Kikuchi et al.
Static 22 25 7 C11.7 48-h LC50 5.6 (1976)
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Carp (contd) 3.5-5.5 Static 21 7.5-7.8 NS 48-h LC50 6.8 Lopez-Zavala
cm Static 21 7.5-7.8 NS 96-h LC50 5.0 et al. (1975)
7 d Staticr 22 25 7.0 C11.7 48-h LC50 5.6 Arima et al.
6 mo Staticr 22 25 6.5-7.1 C11.7 48-h LC50 10 (1981)
50 d Staticr 21 75 C11.7 96-h LC50 4.4 Wakabayashi et
al. (1984)
2 d Staticr 20 25 C11.7 96-h LC50 4.6 Wakabayashi &
15 d Staticr 20 25 C11.7 96-h LC50 2.6 Onizuka (1986)
White tilapia 5-7 cm Static 21 7.5-7.8 NS 48-h LC50 26 Lopez-Zavala
(Tilapia melanopleura) 5-7 cm Static 21 7.5-7.8 NS 96-h LC50 23 et al. (1975)
Guppy 3-4 wk Staticr 23 C8-C14 96-h LC50 5.6-10 Canton & Slooff
(Poecilia reticulata) (1982)
Northern pike Flow 15 43 7.2-7.9 96-h LC50 3.7 McKim et al.
(Esox lucius) (1975)
White sucker Flow 15 43 7.2-7.9 96-h LC50 4 McKim et al.
(Catostomus commersoni) (1975)
Golden orfe Static 18-20 C8 48-h LC50 125 Hirsch (1963)
(Idus idus melanotus) Static 18-20 C9 48-h LC50 88
Static 18-20 C10 48-h LC50 16.6
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Golden orfe (contd) Static 18-20 C11 48-h LC50 6.6
Static 18-20 C12 48-h LC50 2.6
Static 18-20 C13 48-h LC50 0.57
Static 18-20 C15 48-h LC50 0.23
Static 18-20 C16 48-h LC50 0.68
1.2-1.8 g Static 20 NS 48-h LC50 3.94 Mann (1976)
Static 20 NS 48-h LC50 1.85
Static 20 NS 48-h LC50 1.24
Flow 20 150 NS 48-h LC50 4.9 Reiff et al.
(1979)
Flow 20 150 NS 48-h LC50 2.4
Flow 20 150 NS 48-h LC50 1.2
Flow 20 268 NS 48-h LC50 2.1-2.9
Flow 20 268 NS 96-h LC50 1.9-2.9
Flow 20 268 NS 48-h LC50 1.3-1.7
Flow 20 268 NS 96-h LC50 1.2-1.3
Flow 20 268 NS 48-h LC50 0.8-0.9
Flow 20 268 NS 96-h LC50 0.4-0.6
Himedaka (killifish) 4-5 wk Staticr 23 C8-C14 96-h LC50 10-18 Canton & Slooff
(Oryzias latipes) (1982)
323 mg Staticr 23-24 5.6-6.1 C11.7 48-h LC50 15 Kikuchi et
al.
323 mg Staticr 23-24 5.6-6.1 C11.7 48-h LC50 10 (1976)
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 12 Kikuchi &
approx. 262 mg Staticr 21-22 6.7-7.1 NS 48-h LC50 10 Wakabayashi
(1984)
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Himedaka (contd) C161 48-h LC50 > 50 Tomiyama (1974)
C6 48-h LC50 > 50
C8 48-h LC50 > 50
C10 48-h LC50 > 50
C12 48-h LC50 4
C14 48-h LC50 4 Iimori &
Takita
(1979)
25 7.2-7.9 48-h LC50 7.6 Hidaka et al.
(1984)
25 7.2-7.9 96-h LC50 7.3
Adult Staticr 20 5 C11.7 96-h LC50 13 Wakabayashi &
Adult Staticr 20 25 C11.7 96-h LC50 8.8 Onizuka (1986)
Adult Staticr 20 125 C11.7 96-h LC50 4.8
Adult Staticr 20 625 C11.7 96-h LC50 3.2
Adult Staticr 20 0 C11.7 48-h LC50 6.7 Wakabayashi &
Adult Staticr 20 1 C11.7 48-h LC50 4.8 Onizuka (1986)
Adult Staticr 20 5 C11.7 48-h LC50 4.7
Adult Staticr 20 10 C11.7 48-h LC50 3.5
Adult Staticr 20 15 C11.7 48-h LC50 3.8
Adult Staticr 20 20 C11.7 48-h LC50 2.5
Adult Staticr 20 25 C11.7 48-h LC50 1.9
Adult Staticr 20 30 C11.7 48-h LC50 1.6
Adult Staticr 20 35 C11.7 48-h LC50 1.4
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Cobalt suzume 48-h LC50 1.3 Iimori &
(Chrysiptera hollisi) Takita (1979)
Smallmouth bass Flow 15 43 7.2-7.9 NS 96-h LC50 3.7 McKim et al.
(Micropterus dolomieu) (1975)
Black bullhead Flow 23 50 7.5 NS 96-h LC50 6.4 Thatcher &
(Ictalurus melas) Santner (1967)
Common shiner Flow 23 50 7.5 NS 96-h LC50 4.9 Thatcher &
(Notropis cornutus) Santner (1967)
Emerald shiner Flow 23 50 7.5 NS 96-h LC50 3.3 Thatcher &
(Notropis atherinoides) Santner (1967)
Bleeker 0.3 g Static 28 NS 96-h LC50 11.8 Eyanoer et al.
(Puntius gonionotus) 0.3 g Static 35 NS 96-h LC50 11.5 (1985)
0.3 g Static 50 NS 96-h LC50 13.6
0.3 g Static 110 NS 96-h LC50 11.8
0.3 g Static 260 NS 96-h LC50 11.4
Table 28 (contd)
Organism Size or Static or Temp. Hardness pH LAS chain End-point Concn Reference
age flow (°C) (mg/litre)a length (mg/litre)
Ayu 0.26 mg 1 NS 48-h LC50 0.86 Sueishi et al.
(Plecoglossus altivelis) 0.29 g 1 NS 48-h LC50 0.53 (1988)
1.24 g 1 NS 48-h LC50 0.77
6.51 g 1 NS 48-h LC50 1.45
27.98 g 1 NS 48-h LC50 1.17
Flow, flow-through conditions: LAS concentration in water maintained continuously; NS, not specified; staticr, static renewal: water changed
periodically; static, water unchanged for the duration of test; finger, fingerling
a mg/litre CaCO3
b Based on nominal concentration
c Based on measured concentrations
Table 29. Toxicity of linear alkylbenzene sulfonates (LAS) to marine fish
Organism Size or Static or Temp. Salinity LAS chain End-point Concn Reference
age flow (°C) (%) length (mg/litre)
Cod (Gadus morhua) 30 cm Static 6-8 32-34 C12 96-h LC50 1a Swedmark et al.
30 cm Static 15-17 32-34 C12 96-h LC50 < 1a (1971)
Flounder Static 6-8 32-34 C12 96-h LC50 1.5a
(Pleuronectes flesus) Static 15-17 32-34 C12 96-h LC50 < 1a
Plaice (Pleuronectes Static 6-8 32-34 C12 96-h LC50 > 1 -< 5a
platessa)
Mosbled sole Newly NS 48-h LC50 0.5-1 Yasunaga (1976)
(Limanda yokohamae) hatched
10 days NS 48-h LC50 0.1-0.5
30 days NS 48-h LC50 0.5-1
40 days NS 48-h LC50 < 0.1
Newly NS 48-h LC50 0.05-0.1
hatched
Olive flounder 5 days NS 48-h LC50 < 0.1
(Paralichtys olivaceus) 10 days NS 48-h LC50 0.1-0.5
30 days NS 48-h LC50 0.1-0.5
Himedaka (killifish) Adult Staticr 20 0 C11.7 48-h LC50 6.7 Wakabayashi &
(Oryzias latipes) Adult Staticr 20 1 C11.7 48-h LC50 4.8 Onizuka (1986)
Adult Staticr 20 5 C11.7 48-h LC50 4.7
Table 29 (contd)
Organism Size or Static or Temp. Salinity LAS chain End-point Concn Reference
age flow (°C) (%) length (mg/litre)
Himedaka (contd) Adult Staticr 20 10 C11.7 48-h LC50 3.5
Adult Staticr 20 15 C11.7 48-h LC50 3.8
Adult Staticr 20 20 C11.7 48-h LC50 2.5
Adult Staticr 20 25 C11.7 48-h LC50 1.9
Adult Staticr 20 30 C11.7 48-h LC50 1.6
Adult Staticr 20 35 C11.7 48-h LC50 1.2
Static: water unchanged for duration of test; staticr, static renewal: water changed periodically; NS, not specified
a Based on nominal concentration
The activity of the enzymes of aerobic metabolism was decreased, and
that of lactate dehydrogenase was strongly increased (Zaccone et
al., 1985). Concentrations of 2.19 mg/litre C11.8 LAS and
0.39 mg/litre C13 LAS significantly increased the 24-h mean
ventilation rate (number of opercular closures per minute) of
bluegill (Lepomis macrochirus) (Maki, 1979b).
A concentration of 36 mg/litre LAS severely affected the
viability of the perfused gills of rainbow trout (Oncorhynchus
mykissi). Vascular resistance increased gradually during
perfusion, with a concomitant decrease in oxygen transfer. LAS at
0.05 mg/litre more than doubled cadmium transfer (0.8 µg/litre)
through the perfused gills; and at concentrations of 36 mg/litre LAS
and 0.9 mg/litre cadmium, there was a marked reduction in cadmium
transfer (Pärt et al., 1985).
A9.3.3.4 Behavioural effects
Hidaka and co-workers have reported several studies of the
avoidance of surfactants by fish (Hidaka et al., 1984; Hidaka &
Tatsukawa, 1989; Tatsukawa & Hidaka, 1978). The results should be
interpreted with caution, since the environmental relevance and the
reproducibility and sensitivity of these tests is unclear;
furthermore, no effect was seen after removal of the olfactory
organs. Another study (Maki, 1979a) showed no adverse toxicological
effects at concentrations two times greater than those reported to
cause avoidance reactions.
Hidaka et al. (1984) found that the minimal avoidance
concentration of LAS, i.e. the concentration at which fish spent 65%
of a 5-min period in clean water in order to avoid LAS, was
13.5 µg/litre for medakas (Oryzias latipes). Medakas exposed to
LAS at concentrations of 5-50 µg/litre for 10 min showed significant
avoidance to 10, 20, and 30 µg/litre. No significant avoidance of
concentrations of 10-50 µg/litre LAS was found after removal of the
olfactory organs (Hidaka & Tatsukawa, 1989).
The threshold concentrations for avoidance of LAS by ayu
(Plecoglossus altivelis) were 0.11 µg/litre of a formulation and
1.5 µg/litre of pure reagant LAS (Tatsukawa & Hidaka, 1978).
A9.3.3.5 Interactive effects with other chemicals
The chronic toxicity of para, para-DDT (50 mg/litre) to
goldfish (Carassius auratus) was increased by prior exposure to
LAS at 4 mg/litre for 37 days (Dugan, 1967).
The toxicity of 1 mg/litre LAS solution to mosquito fish exposed
under static conditions was not affected by allowing the LAS
solution to react with excess chlorine (Katz & Cohen, 1976).
A concentration of 1 mg/litre LAS significantly increased the
toxicity of fuel oil to bluegill (Lepomis macrochirus), reducing
the 24-h LC50 from 91 to 51 mg/litre. The authors concluded that
sublethal concentrations of LAS increased the toxicity of fuel oil
by increasing its penetration (Hokanson & Smith, 1971). The toxicity
of No. 2 and No. 4 fuel oils in six species of freshwater fish was
increased in the presence of 1-5 mg/litre LAS (Rehwoldt et al.,
1974).
The uptake of cadmium by freshwater trout (Salmo gairdneri)
exposed to 0.14 µmol/litre LAS was more than two times greater than
in controls. Reduced cadmium uptake was reported in fish exposed to
100 µmol/litre LAS. The authors reported that trout exposed to low
levels of both LAS and cadmium could take up lethal cadmium
concentrations. LAS were reported to interact with the gill proteins
involved in cadmium transport, resulting in increased permeability
to cadmium (Pärt et al., 1985).
Fathead minnows (Pimepheles promelas) were exposed to various
pesticides in the presence and absence of 1 mg/litre LAS. Parathion
acted synergistically with LAS, reducing the 96-h LC50 from 1410
to 720 µg/litre. Endrin and LAS showed no synergism, and no
consistent results were obtained for DDT (Solon et al., 1969).
Methyl parathion, ronnel, trithion, and trichloronat were also found
to act synergistically with LAS, but neither ortho-ethyl-ortho-4-
nitrophenyl phenylphos-phonothioate nor dicapthon exhibited
synergism. The synergistic relationship does not appear to be
exclusive to a general structural group (Solon & Nair, 1970).
Goldfish (Carassius auratus) were exposed to mixtures of LAS
and chloramines and LAS and copper at ratios of 1:1, 2:1, and 1:2,
and toxicity curves and 24-h and 96-h LC50 values were compared.
LAS and chloramines had an additive effect at a ratio of 1:1, but at
2:1 and 1:2 synergistic effects were seen. LAS and copper at ratios
of 1:1 and 2:1 had additive effects; however, at 1:2, high
concentrations and longer exposure times had additive effects, and
low concentrations and shorter exposure times had synergistic
effects (Tsai & McKee, 1978).
When eggs of cod (Gadus morhua) were exposed to mixtures of
LAS and zinc or copper from fertilization to hatching, zinc had a
weak synergistic affect on the toxicity of LAS, but LAS had a strong
synergistic affect on the toxicity of copper (Swedmark & Granmo,
1981).
In a study of the effect of polyoxyethylene (20) on the acute
toxicity of C12 LAS, red killifish (Oryzias latipes) and carp
(Cyprinus carpio) were exposed to the 48-h LC50 of LAS for the
respective species and to 5-40 mg/litre of either a polyoxyethylene
sorbitan ester, a polyethylene glycol, a polypropylene glycol, or a
protein (albumin, kaolin, or bentonite). Addition of most of these
substances decreased mortality. No mortality was observed in carp
exposed to LAS and 14 or 28 mg/litre polyoxyethylene (20) sorbitan
monooleate (SMOE20) or to other nonionic surfactants with a similar
polyoxyethylene sorbitan ester structure-polyoxyethylene (6)
sorbitan monolaurate, polyoxy-ethylene (6) sorbitan monooleate,
polyoxyethylene (20) sorbitan monolaurate, and polyoxyethylene (20)
sorbitan monostearate-or to albumin. No significant effect on
mortality induced by LAS was reported after simultaneous exposure to
polyoxyethylene (6) sorbitan monostearate, polyethylene glycol,
polypropylene glycol, kaolin, or bentonite. The authors also
examined the histological effects of these chemicals on the gills of
carp exposed to high concentrations of LAS, including the 48-h
LC50 of 3.5 mg/litre and the LC100 of 7 mg/litre. Histological
changes in fish exposed only to 3.5 mg/litre LAS included the
appearance of mucous cells and agglutination of the secondary
lamellae; carp exposed to a mixture of LAS and SMOE20 showed only
slight swelling of the secondary lamellae and slight proliferation
of the gill epidermal cells. Exposure only to LAS at 7 mg/litre
resulted in marked proliferation of the epidermal cells and
agglutination of secondary lamellae; exposure to both LAS and SMOE20
induced only swelling of the secondary lamellae. No effects were
reported on the gills of control fish or on other organs of the
exposed fish; and no significant differences from controls were
reported in haematological or serum biochemical parameters for fish
exposed to either LAS or the LAS:SMOE20 mixture. When the
distribution of LAS in the tissues and organs of carp was examined,
higher levels were found in the blood and most organs after exposure
to LAS only than after exposure to the mixture; the differences were
statistically significant in blood, muscle, and gill but not in
spleen or gall-bladder. Adsorption of the 5- and 6-phenyl isomers of
LAS was similar when they were given alone or in conjunction with
SMOE20, but more of the 4- and (especially) the 2-phenyl isomers was
adsorbed by fish receiving LAS alone, indicating that SMOE20
decreases the acute toxicity of LAS to fish by decreasing the
adsorption on the gills of the more toxic isomer (Toshima et al.,
1992).
A9.3.4 Amphibia
No reliable data were available.
A9.3.5 Studies of the mesocosm and communities
Diversity and similarity indices were used in many studies to
assess the effects of LAS on phytoplankton communities, usually on
the basis of taxonomy, mean number of species, and density. Mean
density and similarity indices were then compared with those of
controls. In general, these indices are not sensitive to change, as
the densities of some species may decrease while the indices do not.
The effects of C12 and C13 LAS on short-term photosynthetic
activity were studied in plankton sampled from Acton Lake, Ohio,
United States, during May-October in the laboratory and in situ.
Toxicity increased with the temperature of the water, the most
sensitive period being June-August, and LAS were less toxic during
periods of diatom dominance and low phytoplankton density. Thus the
density of diatoms decreased during June-August, and that of green
and blue algae increased. The comparison of the results of
laboratory and field tests was highly dependent on species and the
in-situ end-point. Short-term tests for photosynthetic activity in
situ gave 3-h EC50 values of 0.2-8.1 mg/litre (mean, 1.9 mg/litre)
for C13 LAS and 0.5-8.0 mg/litre (mean, 3.4 mg/litre) for C12
LAS (Lewis & Hamm, 1986). (See also section 9.3.1.1.)
In another study of the effect of LAS on phytoplankton
communities in Acton Lake, Ohio (Lewis, 1986), phytoplankton were
exposed in situ to LAS at a concentration of 0.01, 0.02, 0.24,
0.80, 27, or 108 mg/litre for 10 days. The LOEL for LAS, based on
community similarity indices and the mean number of species, was
108 mg/litre. The similarity index (coefficient of community)
decreased as the concentration of LAS increased, with calculated
values of 0.62 at 0.01 mg/litre and 0.43 at 108 mg/litre. No
significant effects were seen on the community diversity index or
phytoplankton density. Green algae were the species least affected,
on the basis of abundance, followed in order of decreasing tolerance
by blue-green algae and diatom species. Chlorophyta species were
the most abundant at higher concentrations of LAS, comprising 74% of
the total cell volume at 108 mg/litre; their abundance tended to
increase to a maximum at this concentration and then decrease to
values similar to those of the controls. Chlorophyta species of
the genera Chlamydomonas, Oocystis, and Sphaerocystis were not
found after exposure to higher concentrations of LAS. Chlamydomonas
was found only in waters with a concentration of LAS <
0.8 mg/litre, and Oocystis and Sphaerocystis were found only at
concentrations < 27 mg/litre. The peak density of blue-green
phytoplankton (56% of cell volume) was achieved at 0.24 mg/litre
LAS, declining to 17% at 108 mg/litre. The density of the major
species, Schizothrix calcicola, was greatest at 27 mg/litre LAS but
declined to a level below that of controls at 108 mg/litre LAS. The
abundance of diatoms was low at all concentrations of LAS. At
concentrations < 0.24 mg/litre, the average density of diatoms
was 23% of the total cell volume, similar to that of controls; at
concentrations of 0.24-0.8 mg/litre, the diatom density was 10% of
the cell volume. The mean densities of the major diatom species,
such as Cyclotella glomerata, Cyclotella pseudostelligera, and
Nitzschia frustulum v. perminuta, followed the overall trend for
diatoms, reaching a peak at low LAS concentrations and declining to
control values at higher concentrations.
In the same study, the laboratory-based 96-h EC50 values for
exposure to C11.8 LAS were calculated to be 29.0 mg/litre for
Selenastrum and 0.0096 mg/litre for Microcystis, on the basis of
population growth. The lowest concentration of LAS that produced a
significant effect on algal growth in the laboratory was
0.05-1.0 mg/litre, which is considerably lower than the
27-108 mg/litre value found to be the lowest that altered the
structure of a natural phytoplankton community. The differences
between the results of laboratory and field tests were smaller for a
laboratory-based EC50 than for an LOEL. Calculations based on the
EC50 give a 30-fold difference for Microcystis but essentially no
difference for Selenastrum (Lewis, 1986).
The toxic effects of LAS were also examined on periphyton
communities above and below the outfall of a wastewater treatment
plant on Little Miami River, Ohio, United States. The dominant
species at both test sites were diatoms, Amphora perpusilla and
Navicula minima accounting for at least 80% of the total cell
volume. The tests were conducted in situ, with 21-day
continous-flow exposure to LAS (average chain length, C11.9) in
river water entering submerged exposure tubes at a concentration of
0.2, 1.1, 9.8, or 28.1 mg/litre, after a four-week colonization
period. The delivery rate of LAS was adjusted daily according to
measurements of river flow in order to maintain the desired test
concentrations. The periphyton at the site below the treatment plant
outfall were exposed to LAS in the presence of 20-30% treated
municipal effluent. No effects on the structure or function of the
periphyton community above the outfall were reported after exposure
to an average concentration of LAS < 1 mg/litre. The lowest
concentration that had an effect on the upstream periphyton
community was 9.8 mg/litre, which reduced photosynthesis by 16%; a
concentration of 28.1 mg/litre reduced photosynthesis by 64%, with a
noticeable reduction in chlorophyll a. No effects on community
similarity or diversity were reported in comparison with control
communities. Mean cell densities were increased by 26% after
exposure to 0.2 mg/litre LAS and by 17% after exposure to
1.1 mg/litre; exposure to 28.1 mg/litre reduced mean cell density by
28%. Exposure to LAS had no significant effects on the abundance of
the three main species in the upstream community. Increased
photosynthesis (by 12-39%) and chlorophyll a (50-51%), were
reported after exposure to 1.1 or 9.8 mg/litre LAS, but exposure to
28.1 mg/litre resulted in a 52% decrease in photosynthesis and a 71%
decrease in chlorophyll a. No effects on the similarity or
diversity of the periphyton community were reported at any
concentration of LAS tested. Cell densities of periphyton were
increased by 34% after exposure to 9.8 mg/litre LAS and by 13% after
exposure to 28.1 mg/litre. The abundance of the three main species
in the downstream periphyton community was not affected. The lowest
concentration of LAS that induced an effect was 3.3 mg/litre for the
upstream periphyton community and 16.6 mg/litre for the downstream
community. The authors suggested that the difference between the two
values was due to the presence of 20-30% sewage downstream, which
reduced the bioavailability of LAS (Lewis et al., in press).
When an aquatic ecosystem was exposed to LAS at concentrations
of 0.25-1.1 mg/litre for 90 days, the numbers of phytoplankton were
unaffected but primary productivity was significantly reduced at all
concentrations. The zooplankton population showed a more variable
response: the number of rotifers was reduced at all concentrations,
and those of Diaptomus and Cyclops were reduced at >
0.51 mg/litre. The number of ostracods was decreased at
0.38 mg/litre but was increased at 0.51 and 1.1 mg/litre. The
chironomid population was significantly reduced at concentrations
> 0.38 mg/litre (Chattopadhyay & Konar, 1985). Exposure of an
aquatic ecosystem consisting of phytoplankton, zooplankton, and
benthic organisms to 1 mg/litre of a preparation of LAS for 90 days
significantly reduced the numbers of phytoplankton and zooplankton
per litre but did not significantly affect the numbers of chironomid
larvae (Panigrahi & Konar (1986).
The effect of C12 LAS on microbial communities was studied in
a model ecosystem consisting of a 19-litre glass tank containing
sediment from Winton Lake, Ohio, United States, and several trophic
levels, comprising bacteria, algae, macrophytes (Elodea canadensis,
Lemna minor), macroinvertebrates (Daphnia magna, Parantanytarsus
parthenogenica), and blugill sunfish (Lepomis macrochirus).
After a four-week equilibrium period, LAS were added at 0.5 or
5.0 mg/litre to a flow-through system with six to 10 replacements
per day for 26 days. The structure of the microbial communities was
not affected, and no differences were reported in mean biomass or
number of colony-forming units between the microorganisms exposed at
the two levels. The function of the microbial communities, assayed
by measuring the degradation of both LAS and D-glucose, was reduced
only at 5.0 mg/litre. In a similar system, in which the same
concentrations of LAS were added in the form of sewage effluent, no
effect was seen on the structure of the microbial community or on
their function, measured only as the degradation of LAS (Larson &
Maki, 1982).
Addition of LAS (average chain length, C11.8) at a measured,
relatively uniform concentration of 0.36 ± 0.05 mg/litre to 50-m
outdoor experimental streams had no effect on total density, species
richness, percentage similarity, or dominance of macroinvertebrates
or periphyton or on the processing of organic matter of leaf discs.
Fathead minnows (Pimephales promelas) and amphipods (Hyallela
azteca) were exposed in groups of 10 and 20 per box placed in the
streams at three locations. The mortality rates of the amphipods
were 17-25% after exposure to LAS and 47% among controls; no effects
were seen on the survival or weights of the fish, although minor
effects were found on length (Fairchild et al., 1993).
A study of the fate and effects of surfactants in outdoor
artificial streams addressed the effect of LAS on drift and
population densities of macroinverebrates, the reproductive
behaviour of an amphipod, the scud (Gammarus pulex), the survival
of a fish, the three-spined stickleback (Gasterosteus aculeatus),
and photosynthesis by the community. The concentration of LAS in
sediment was reported to increase with increasing water
concentration, and selective adsorption of longer-chain LAS
homologues to sediment was reported. The microbial populations of
both the water and the sediment adapted to LAS, resulting in a
reduction in its half-life during the test. LAS at concentrations
< 1.5 mg/litre did not affect macroinverebrate drift, population
density, or community photosynthesis. Survival of the fish and
reproduction by the amphipod were affected at concentrations of
1.5-3.0 mg/litre (Mitchell & Holt, 1993).
A9.3.6 Field studies
The effect of LAS downstream of a sewage outflow was studied by
monitoring sediment, water, and the distribution of invertebrates at
an upstream control site, a site near the discharge point, and a
site 200 m downstream of the outflow. The concentrations of LAS in
sediment were 1-40 mg/kg dry weight, with concentrations < 2 mg/kg
at the control site and 200 m downstream. No effect of LAS in the
effluent or in the streambed sediments could be discerned on the
invertebrate populations (Ladle et al., 1989).
A9.3.7 Toxicity of biodegradation intermediates and impurities
of linear alkylbenzene sulfonates
Tests of degradation products and impurities of LAS show that
they are less toxic than LAS themselves.
A9.3.7.1 Individual compounds
The 48-h LC50 values in Daphnia magna were 208 ± 85 mg/litre
for sulfophenylundecanoic acid, disodium salt (mixed isomers, 6-10
phenyl); about 6000 mg/litre for 3-(sulfophenyl) butyric acid,
disodium salt; and about 5000 mg/litre for 4-(sulfophenyl) valeric
acid, disodium salt. The equivalent 48-h LC50 values in the
fathead minnow (Pimephales promelas) were 77 ± 12, about 10 000,
and about 10 000 mg/litre, respectively (Kimerle & Swisher, 1977).
The 24-h LC50 values in Daphnia were about 22 000 mg/litre for
3-sulfophenylbutyric acid, disodium salt; about 12 000 mg/litre for
3-sulfophenylheptanoic acid, disodium salt; > 22 000 mg/litre for
3-sulfophenylbutyric acid, disodium salt; and 2 000 mg/litre for
sulfophenylundecanoic acid, disodium salt. Other tests were carried
out with the last two compounds, giving 96-h LC50 values of about
28 000 and 1200 mg/litre in fathead minnows (Pimephales promelas);
28-day NOELs of > 2000 and > 200 mg/litre for survival and
reproduction of Daphnia; and 30-day NOECs of > 1400 and >
52 mg/litre for survival of the fry of fathead minnows (egg
hatchability and fry growth were less sensitive) (Swisher et al.,
1978).
The 96-h LC50 for mixed isomers of sulfophenylundecanoic acid
disodium salt in bluegill (Leponis macrochirus) was 75 mg/litre
(Swisher et al., 1964). The 24-96-h LC50 values in fathead minnows
were 1000-1500 mg/litre for sulfophenylundecanoic acid (C11) and
25 000-32 000 mg/litre for sulfophenyl butyrate (C4) (Swisher et
al., 1978).
The 48-h LC50 for the alkanoic acid derivatives of
2-sulfophenyl C13 LAS and 4-sulfophenyl C13 LAS in nearly pure
form was > 800 mg/litre in goldfish (Carassius auratus) (Divo &
Cardini, 1980).
The 24-h LC50 values for Daphnia magna exposed to
dialkyltetralin sulfonates, which are trace contaminants of LAS,
were 420, 195, 110, 50, and 27 mg/litre for tetralin sodium
sulfonates of chain lengths C10, C11, C12, and C13,
respectively (Arthur D. Little Inc., 1991).
A9.3.7.2 Effluents
Interpretation of tests on effluents must take into account the
following:
-- As concentrations arwe often reported as MBAS, testing of
effluent from a sewage treatment plant may result in overestimation
of the actual concentrations of LAS, owing to interference (see
section 2.3).
-- The bioavailability of LAS is decreased by the presence of high
concentrations of suspended solids; thus, as effluents are diluted
in the environment, availability is usually increased, although
biodegradation occurs.
Addition of LAS (C10-C15) to detergent-free activated sludge
plant effluent (95% was removed as MBAS) gave a nominal 96-h LC50
in rainbow trout (Oncorhynchus mykiss) of 0.36 mg/litre. After
treatment, the 96-h LC50 was 29.5 mg/litre, expressed in terms of
the concentration of the surfactant in the influent (Brown et al.,
1978).
When bluegill were exposed to effluent from continuous-flow
activated sludge units fed 100 mg/litre LAS, none died during
4-11-day exposure (Swisher et al., 1964).
A9.4 Terrestrial organisms
A9.4.1 Terrestrial plants
Young seedlings of tomato, lettuce, radish, pea, cucumber, and
barley were grown in a soil-based compost and were watered and given
a foliar spray of a preparation of LAS. No effects were noted at
concentrations up to 100 mg/litre (Gilbert & Pettigrew, 1984). In
another study, barley, tomato, and bean plants were grown from seed
and watered with a solution containing LAS at a concentration of 10,
25, or 40 mg/litre. Plants that received the lowest dose germinated
at the same time as controls, but plants watered at 25 or
40 mg/litre germinated three days later. The growth of barley plants
was inhibited at all three concentrations; however, the dose of
25 mg/litre increased the growth rate of beans, and the highest dose
increased the growth rate of both tomatoes and beans (Lopez-Zavala
et al., 1975).
The 21-day EC50 values for LAS (C10-C13), based on the
emergence of seedlings and early stages of growth, were 167 mg/litre
in sorghum, 289 mg/litre in sunflower, and 316 mg/kg in mung bean.
The highest concentration that caused no significant reduction in
the growth of any of the three species was 100 mg/kg (Holt et al.,
1989; Mieure et al., 1990). In a second study, 407 mg/kg C11.36 or
393 mg/kg C13.13 LAS were mixed with sewage sludge, and nine
common plant species, including five crop plants, were exposed as
seed either at the same time or two weeks after application of the
sludge to soil at a rate of 9000 kg/ha. There was no significant
effect on seed germination and no significant inhibition of growth
(Mieure et al., 1990).
Orchid seedlings (Phalaenopsis or Epidendrum sp.) were grown
in culture media containing either the sodium or the ammonium salt
of LAS at a concentration of 10, 100, or 1000 mg/litre. The lowest
dose had no effect on growth, and that of ammonium LAS had no effect
on germination. At 100 mg/litre, survival was halved and germination
completely inhibited (Ernst et al., 1971). A concentration of
1000 mg/litre caused drastic changes in morphology, loss of
membranes, swelling of thylakoids, and the appearance of dense
osmophilic granules in chloroplasts (Healey et al., 1971).
The growth of pea seedlings grown for 26 days in quartz sand to
which 0.005% (50 mg/kg) LAS had been added was significantly
reduced, as measured by the fresh weight of roots and the length and
fresh weight of pea greens (Lichtenstein et al., 1967).
LAS were not toxic with respect to growth at the early life
stages of radish, Chinese cabbage, and rice when added in hydroponic
culture at concentrations of 10, 20, and 20 mg/litre, respectively;
concentrations of 20, 35, and 35 mg/litre were toxic (Takita, 1982).
When seeds of Pisum sativum and Crotolaria juncea were
exposed to LAS for 24 h before sowing, the percentage germination
was reduced at concentrations of 1 ml/litre for P. sativum and
10 ml/litre for C. juncea, although no statistical analysis was
presented. No germination occurred after exposure to LAS at
concentrations of 20 ml/litre for P. sativum and 40 ml/litre for
C. juncea. Radicle length was reduced at > 0.1 ml/litre in both
species (Sharma et al., 1985).
Application of LAS at 50 g/m2 under field conditions to loamy
and sandy soils (corresponding to 0.47-1 mg/kg dry weight,
respectively) led to considerable physiological damage, including
leaf necrosis, chlorosis, and turgescence, to ryegrass (Lolium
perenne) after 14 days; however, there was no difference in the
fresh weight yield after harvesting at 45-54 days (Litz et al.,
1987).
A9.4.2 Terrestrial invertebrates
When the earthworm Eisenia foetida was exposed to C11.36 LAS
incorporated into soil at nominal concentrations of 63-1000 mg/kg
dry weight, the 14-day LC50 was > 1000 mg/kg. On the basis of a
statistical analysis of body weights, the no-effect concentration
was 250 mg/kg; this was confirmed by HPLC to be 235 mg/kg. In a
second study, C11.36 and C13.13 LAS were incorporated into
sludge and applied to soil, and the earthworm Lumbricus terrestris
was exposed to the subsequent mixture, which contained LAS at
concentrations of 84-1333 mg/kg. The 14-day LC50 was again found
to be greater than the highest concentration (> 1333 mg/kg). The
no-effect concentration, based on weight and burrowing behaviour,
was the nominal concentration of 667 mg/kg, measured by HPLC as
613 mg/kg. The worms were exposed, however, to LAS under conditions
of continuous light, which would inhibit them from surfacing to feed
and thus increase their exposure to and the toxicity of the test
over that of the same concentration in the field (Mieure et al.,
1990).
Topical application to house flies (Musca domestica) of LAS at
the same time as parathion, diazinon, or dieldrin in ratios of 1:1
and 1:10 had no effect on the toxicity of the insecticides. When LAS
were added to soil treated with parathion or diazinon, however, a
significant synergistic effect was observed on the toxicity of the
insecticides to the fruit fly Drosophila melanogaster. The optimal
concentration of LAS that resulted in synergy was 23 mg/kg
(Lichtenstein, 1966).
A9.4.3 Birds
No significant effect on egg quality was found after Leghorn
chickens were fed a diet containing 200 mg/kg LAS for 45 days
(Lopez-Zavala et al., 1975).
B. alpha-Olefin sulfonates
B1. SUMMARY
See Overall Summary, Evaluation, and Recommendations (pp. 7-21).
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
B2.1 Identity
Chemical formula: CnH2nO3S Na, CnH2n+1O4S Na ( n = 14-18)
Chemical structure: CH3(CH2)jCH:CH(CH2)kSO3- Na+
CH3(CH2)mCH(CH2)nSO3- Na+
OH ( m,n, integers)
Common names: Sodium alpha-olefinsulfonate,
alpha-olefin-sulfonic acid sodium salt, AOS
sodium salt
Common trade names: Bioterge AS 40 F, Elfan OS 46, Geropon
MLS/A, Hostapur OS Brands, Lipolan, Lipomix
G, Lipon PB-800, Lutensit A-PS, Nansa
LSS38/AS, Sawaclean, Sermul EA 214,
Sulframin AOS, Witconate (McCutcheon, 1989)
Abbreviations: AOS, AOS-Na
CAS Registry numbers: 29963-33-5 Sodium 1-tetradecenesulfonate
29734-60-9: Sodium hexadecenesulfonate
13513-23-0: Sodium 3-hydroxyhexadecyl-1-
sulfonate
26446-92-4: Octadecene-1-sulfonic acid
sodium salt
13513-42-3: 3-Hydroxy-1-octadecanesulfonic
acid, sodium salt
Specifications: AOS are mixtures consisting of about 60-65%
alkene sulfonates, 30-35% hydroxylalkane
sulfonates, and 5-10% disulfonates. Various
positional isomers of alkene sulfonates and
hydroxyalkane sulfonates have been reported
(Gentempo et al., 1985; Williamson, 1993).
Sodium C14-C16 AOS are typically
shipped as solutions containing 35-40%
active matter in water. Sodium C16-C18
AOS are typically slurries containing
28-30% active matter in water at ambient
temperature.
B2.2 Physical and chemical properties
AOS are white crystalline solids consisting of various chemical
compounds and their isomers, with different properties. Typical
properties of AOS are given in Table 30. Two ranges are usually
offered; the commonest are based on C14-C16 olefin and the other
on C16-C18 olefin. Detergency is maximal with alkyl chain
lengths of C15-C18. Maximal detergency is also obtained with the
same range of alkyl chain lengths in a detergent formulation that
includes alkali builders and chelating agents (Yamane et al., 1970).
AOS are stable, even in hot acidic media.
Table 30. Relationship between alkyl chain length, Krafft point,
critical micelle concentration (CMC), and surface
tension of alpha-olefin sulfonates
Alkyl chain Krafft pointa CMCal Surface tension
length (°C) (g/litre) (dyne/cm)
12 - 4.0 -
14 - 1.0 30
16 10 0.3 33
18 30 0.1 35
20 40 - -
(25°C) (25°C)
From Ohki & Tokiwa (1970)
a The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature. For
surfactants, there is a distinct, sharp bend (break-point) in the
solubility-temperature curve. The steep increase in solubility
above the sharp bend is caused by micelle formation. The point of
intersection of the solubility and critical micelle curves, plotted
as a function of temperature, is referred to as the Krafft point.
This is a triple point at which surfactant molecules coexist as
monomers, micelles, and hydrated solids. The temperature
corresponding to the Krafft point is called the Krafft temperature.
At above the Krafft temperature and critical micelle concentration,
a micellar solution is formed. Under these conditions, higher levels
than the aqueous solubility may be obtained.
B2.3 Analytical methods
There is no officially recognized specific procedure for the
analysis of AOS in environmental samples. The methods commonly used
to analyse anionic surfactants are also used for AOS, except those
involving high-performance liquid chromatography (HPLC), which has
limited use in environmental analyses for AOS, because they do not
absorb ultra-violet radiation as effectively as do linear
alkylbenzene sulfonates (LAS). A modified version of the methylene
blue-active substance (MBAS)-HPLC method described in the monograph
on LAS has been developed (Takita & Oba, 1985).
Nonspecific methods used in the analysis of anionic surfactants
in general, such as the MBAS method, can be used to analyse
materials for AOS (see section 2.3 of the monograph on LAS).
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
AOS do not occur naturally.
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
AOS are synthesized industrially. Although they have been
available since the 1930s, production for use in commercial
surfactant formulations was somewhat limited until recently owing to
a lack of suitable feedstock. Development of continuous and
short-contact sulfur trioxide sulfonation processes and the
increased availability of highly pure Ziegler-derived alpha-olefin
feedstock has recently made AOS surfactants competitive with other
surfactants on the market (Arthur D. Little Inc., 1977, 1981).
The estimated world consumption of AOS in 1988 was 50 200 tonnes
(Colin A. Houston & Associates Inc., 1990). In 1990, that group
estimated that world consumption would be 51 900 tonnes; an
alternative estimate (Hewin International Inc. 1992) was 76 000
tonnes (Table 31).
Table 31. Estimated worldwide consumption of alpha-olefin
sulfonates (tonnes)
Region Household Personal Industrial and All uses
products care institutional
products use
North America 3 000 7 000 4 000 14 000
Western Europe 2 000 3 000 3 000 8 000
Japan 24 000 7 000 2 000 33 000
Rest of the 18 000 3 000 - 21 000
world
Total 57 000 20 000 9 000 76 000
From Hewin International Inc. (1992)
AOS are prepared commercially by direct sulfonation of linear
alpha-olefins with a dilute stream of vaporized sulfur trioxide in a
continuous thin-film reactor. The olefin is obtained by wax cracking
or ethylene polymerization with a Ziegler-type catalyst (Tomiyama,
1970). The reaction is complex and follows several paths, forming
large amounts of various sultones as intermediates which hydrolyse
during subsequent quenching and neutralization. Commercial AOS
products contain a mixture of two major components, alkene sulfonate
and hydroxyalkane sulfonate, with smaller amounts of alkene
disulfonates, hydroxyalkane disulfonates, and saturated sultones.
B3.2.2 Uses
AOS are good detergents, have good foaming characteristics in
hard water and are used in heavy-duty laundry detergents, light-duty
dishwashing detergents, shampoos, and cosmetics. Table 31 indicates
the use patterns for AOS.
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
It can be inferred that AOS are transported into the environment
in a similar manner to that established for LAS, alkyl sulfates, and
other detergent surfactants. Fewer data are available on the
environmental transport, distribution, and transformation of AOS
than for LAS. The environmental fate of AOS is similar to that of
LAS and alkyl sulfates: it is readily biodegraded under aerobic
conditions, and primary biodegradation is complete within 2-10 days,
depending on the temperature. At temperatures below 5-10°C,
biodegradation kinetics are reduced, owing to a reduction in
microbial activity. No data were available on abiotic degradation.
There was no evidence of bioaccumulation or bioconcentration in a
study of fish in which the uptake and distribution of AOS were
examined.
B4.1 Transport and distribution between media
In the same manner as other detergent compounds, AOS are
discharged into the environment in wastewater. The wastewater may
undergo sewage treatment if such facilities are available. In
countries where there are no adequate wastewater treatment
facilities, AOS released to the environment are removed by
biodegradation and adsorption mechanisms (see section 4.2 of the
monograph on LAS).
Limited studies of the adsorption of AOS are available. In a
study of the adsorption of C12 AOS on river sediments, the
equilibrium quantities adsorbed were proportional to the organic
carbon content of the sediments, with a sorption coefficient Koc
(dimensionless; normalized for the level of organic matter) of 0.65.
This indicates that adsorption of C12 AOS is slightly weaker,
than, for example, that of C12 LAS or C12 alkyl sulfonates
(Urano et al., 1984). Like other detergent chemicals, AOS are
adsorbed onto sewage sludge and river sediments in the environment.
B4.2 Biotransformation
B4.2.1 Biodegradation
B4.2.1.1 Aerobic biodegradation
Primary biodegradation of AOS, studied in die-away tests in
water from various sites on the Tama River, Japan, was complete
within three to five days when measured by the MBAS method; however,
total organic carbon was completely removed after an incubation time
of 20 days. In a study of AOS in seawater collected from the mouth
of the Tama River, 99% of MBAS was removed within one day, and 90%
of organic carbon was removed within five days (Sekiguchi et al.,
1975b).
In a comparison of the MBAS and total organic carbon methods for
measuring biodegradation with the shake-culture method, AOS lost 99%
of their activity as measured by the MBAS method and 90% of total
carbon within one day; 100% was lost within five days (Sekiguchi et
al., 1975a). In another study, complete loss of parent AOS (initial
concentration, 100 mg/litre) as determined by the MBAS method was
seen within 15 days, and 90% of total organic carbon was removed
within eight days (Miura et al., 1979). In a static die-away test
system, 90% biodegradation of three commercial AOS products,
comprising 100% C14-C16 AOS and > 95% C15-C18 AOS (determined
as MBAS), was reported within four days (Gafa & Lattanzi, 1974).
In a shake-culture test in Bunch-Cambers medium, C15-C18 AOS
were degraded by 99% (determined as MBAS) or 90% (removal of total
organic carbon) within one day; 100% total organic carbon was
removed within five days. The authors did not verify whether the
removal was the result of adsorption or mineralization (Sekiguchi et
al., 1972). The biodegradation of C15 AOS and three C15-C18
compounds with disulfonate contents of < 4, 15, and 50% in a
shake-flask culture system was reported to be 96% (determined as
MBAS), with no significant difference between compounds (Oba et al.,
1968b).
In a modified OECD screening test, 85% of C14-C18 AOS
(measured as chemical oxygen demand) was removed. Measurement of
MBAS in the same test indicated 99% removal (Gerike, 1987).
The aerobic biodegradation of 20 mg/litre AOS at 27°C was
followed during a 10-day incubation period. Primary degradation,
measured by the MBAS method, was complete within 10 days. The
theoretical CO2 production had reached 30-40% within that time
(Itoh et al., 1979).
The oxygen uptake of C14-C18 AOS was reported to be 85% of
the theoretical oxygen demand in a closed-bottle test (Gerike,
1987). The average biochemical oxygen demand for C12-C18 AOS
containing up to 40% hydroxylalkane sulfonates was 51.6% at five
days, while glucose under the same conditions had a biochemical
oxygen demand of 69.6% (Procter & Gamble Co, unpublished data).
The primary and ultimate biodegradability of a series of pure
AOS homologues (C12, C14, C16, and C18) was determined by
measuing CO2 production. Primary biodegradation was 98-99% within
three days, the rate of degradation varying with chain length.
Degradation of C12 and C14 AOS occurred at a similar rate (65%
within 30 days), but C18 AOS degraded more slowly. Mineralization
of all AOS samples was reported to be at least 50% within two weeks,
whereas mineralization of glucose during that time was 75-80%
(Kravetz et al., 1982). In a study of the biodegradation of the two
major breakdown products of AOS, alkene sulfonate and hydroxyalkane
sulfonate, AOS homologues (C15, C16, C17, C18) were degraded
to about 50%, and in each case the alkene sulfonate was degraded at
least twice as fast as the hydroxyalkane sulfonate (Sekiguchi et
al., 1975c).
The biodegradation of C18 AOS at a concentration of
28 mg/litre was studied in activated sludge (concentration, 100 mg
dry matter per litre) over 12 days: 90% was lost within eight days,
as measured by removal of chemical oxygen demand. The specific rate
of biodegradation was calculated to be 5.3 mg/g per h (Pitter &
Fuka, 1979).
In the OECD confirmatory test with activated sludge, 20 mg/litre
AOS were degraded, as follows: 97% C14 AOS within 17 days, 98%
C16 AOS within seven days, and 94% C14-C18 AOS within eight
days (Maag et al., 1975).
Primary biodegradation of C15-C18 AOS was dependent on
incubation temperature in die-away tests with water from the Tama
River, Japan. Primary biodegradation was complete within two days at
27°C, within five days at 15°C, and within two days at 21°C;
however, at a water temperature of 10°C about 20% of the AOS had
still not been degraded within the nine-day test (Kikuchi, 1985).
When C15-C18 AOS were added to seawater, no MBAS activity
was present after five days (Marquis et al., 1966).
B4.2.1.2 Anaerobic degradation
The primary anaerobic biodegradation of C15-C18 AOS
(measured as MBAS) by bacteria on sludge sampled from a sewage
treatment plant was 19% within 14 days and 31% within 28 days. More
parent AOS were degraded by bacteria from the bottom of a private
cesspool, with 34% lost within 14 days and 43% within 28 days. The
anaerobic degradation reported may have been due to the presence of
hydroxyalkane sulfonate compounds (Oba et al., 1967). AOS and LAS
were reported to be the two surfactants that were least degraded
anaerobically (Itoh et al., 1987).
B4.2.2 Abiotic degradation
No information was available.
B4.2.3 Bioaccumulation and biomagnification
Rapid, significant absorption of 14C-AOS by the gills of
goldfish (Carassius auratus) was seen after exposure to AOS at a
concentration of 10 mg/litre. The concentration of AOS in the gills
increased from 0.3 mg/kg after 0.5 h of exposure to 48.3 mg/kg after
3 h. AOS were not detected in the alimentary canal (Tomiyama, 1975).
Three hours is a relatively short exposure, and the authors did not
determine whether a steady state of adsorption had been achieved.
Tomiyama (1978) reported that AOS accumulated to the greatest extent
in the gills of exposed fish, with additional accumulation in the
gall-bladder. Only limited conclusions can be drawn from this study,
however, owing to the short exposure period.
B4.3 Interaction with other physical, chemical, and biological
factors
No information was available.
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Few data are available on environmental concentrations of AOS
because of the lack of an accepted analytical method for this
purpose. A modified analytical method based on MBAS-HPLC measurement
has been used to measure AOS (Takita & Oba, 1985). The concentration
in the Tama River, Japan, was calculated to be < 0.0016-0.002
mg/litre.
The annual average concentration of AOS in wastewater was
0.160-0.164 mg/litre on the basis of total MBAS concentrations of
8.4 and 8.2 mg/litre. AOS was not detected in the effluent from a
treatment plant outfall (Oba et al., 1976).
AOS can be expected to mineralize rapidly in all environmental
compartments and to be removed to a large extent during sewage
treatment. Environmental concentrations in receiving surface waters,
sediments, soils, estuaries, and the marine environment can also be
expected to be low.
B6. KINETICS
Section summary
AOS administered orally are readily absorbed by the
gastrointestinal tract of rats and are distributed throughout the
body; they are eliminated primarily in the urine and, to a lesser
extent, in the faeces within 24 h of administration. AOS applied
dermally are absorbed only minimally by intact skin. Several
metabolites have been isolated, but their chemical structures have
not been identified.
B6.1 Absorption, distribution, and excretion
14C-AOS were synthesized by sulfonation and hydrolysis of
tetradecene-1-14C. The labelled compound was composed of a
mixture of about 55% sodium 3-hydroxyalkane sulfonate
[C11H23CH(OH)-CH2SO3Na] and about 45% sodium 2-14C alkenyl sulfonate
[C11H23CH2CH214CH2SO3Na]. After oral administration of
100 mg/kg 14C-AOS (50 µCi/kg) in water to rats, the level of
radiolabel in blood reached a peak at 3 h (0.08% of the dose/ml) and
then rapidly decreased, since little radioactivity was detected 24 h
after the administration. At 4 h after administration, 0.45% of the
dose per gram of tissue was detected in liver and 0.65% in kidney,
but the levels in tissues other than the gastrointestinal tract were
< 0.1%. Thereafter, the radiolabel in organs and tissues decreased
rapidly, and 24 h after administration, about 0.8% was detected in
the caecal contents and < 0.02% in other tissues. No specific
accumulation was observed in any tissue. Within 24 h of
administration, 72% of the dose was excreted in urine and 22% in
faeces. At the end of the experiment, after four days, no 14C
residue (< 0.1% of the dose) was detected in urine or faeces.
Cumulative excretion in the bile within 12 h after administration
was about 4.3% of the radioactivity administered (Inoue et al.,
1982).
The biological half-lives of AOS and their metabolites in blood
after intravenous administration of 10 mg/kg 14C-AOS in rats were
15 and 1 h, respectively. The marked difference in half-life can be
accounted for by the fact that the binding of AOS to plasma
proteins, especially serum albumin, increased in proportion to its
concentration while that of the metabolites did not increase to any
appreciable extent. The volume of distribution of AOS was
8 litres/kg, and that of the metabolites was 0.5 litres/kg (Inoue et
al., 1982).
A dose of 0.5 ml of a 0.2% aqueous solution of 14C-AOS was
applied to the dorsal skin (4 × 3 cm) of rats with bile-duct and
bladder cannulae. The total amount absorbed through the skin was
estimated to be about 0.6% on the basis of the recoveries of 14C
in urine, bile, and the main organs over 24 h. At that time, the
level of radiolabel was higher in the liver (0.123% of dose) than in
the kidney (0.059%), spleen (0.004%), brain (0.01%), or lung
(0.012%). A total of about 0.24% of the applied dose was recovered
in these organs. After 24 h, 0.33% of the radiolabel was excreted in
the urine and 0.08% in the bile. When the solution was painted on
skin damaged by 20 applications of cellophane adhesive tape to
remove the stratum corneum, the rates of excretion were 36.3% in the
urine and 1.8% in the bile (Minegishi et al., 1977).
B6.2 Biotransformation
AOS and its metabolites were investigated in tissues and
excrement after oral administration of 100 mg/kg 14C-AOS to rats.
AOS and a metabolite more polar than AOS were detected in blood,
liver, kidney, bile, and urine by thin-layer chromatography. As most
of the 14C-labelled compounds in urine were alcoholic,
unsaturated, and of sulfonic functionality, the metabolite may be a
hydroxylated or polyhydroxylated sulfonic acid with a shorter chain
than AOS, although the precise chemical structure remains to be
elucidated (Inoue et al., 1982).
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 for AOS sodium salt in mice was 3000 mg/kg. AOS
are skin and eye irritants. Data from studies in experimental
animals are limited, but no effects were observed in a long-term
study in which oral doses of 250 mg/kg body weight per day were
administered to rats. Fetotoxicity was observed in the progeny of
rabbits administered a maternally toxic dose of 300 mg/kg body
weight per day.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of AOS in experimental animals; however, in
the limited studies available in which animals were administered AOS
orally or on the skin, there was no evidence of carcinogenicity.
The limited data available also indicate that AOS are not
genotoxic in vivo or in vitro.
B7.1 Single exposures
The LD50 values for AOS (sodium salt of sulfonated C15-C18
n-olefin) in male ddy mice were 3000 mg/kg body weight by oral
administration, 1660 mg/kg by subcutaneous injection, 170 mg/kg by
intraperitoneal injection, and 90 mg/kg by intravenous injection.
The toxic effects seen at high oral doses were reduced voluntary
activity, diarrhoea, anaemia, dyspnoea, and respiratory collapse.
Clonic convulsions followed by respiratory collapse were seen in
animals given the material intravenously (Oba et al., 1968a).
B7.2 Short-term exposure
No data were available.
B7.3 Long-term exposure; carcinogenicity
B7.3.1 Mouse
The skin of Swiss-Webster mice was painted with 20% C14-C18
AOS, 25% C14-C18 AOS, 20% C14-C16 AOS, 25% C14-C16 AOS,
6.7 or 8.3% C16 1,4-sultone, water, or acetone, or remained
untreated. Animals were treated with 0.02 ml of test material on
about 1 cm2 of exposed skin three times per week for 92 weeks.
Final necropsies were conducted when the survival of each group
reached 30% (approx. 19 months). Histopathological examination
showed no evidence of carcinogenicity with any test material (Haar,
1983).
B7.3.2 Rat
AOS (97.93% of a 60.4:39.6% (w/w) mixture of alkenyl sulfonate
and hydroxyalkane sulfonate; chain-length distribution, 25% C14,
45% C16, 30% C18) were fed to four groups of 50 male and 50
female CFY rats at a dietary level of 0, 1000, 2500, or 5000 ppm,
corresponding to 49, 122, or 245 mg/kg body weight per day, for two
years. No adverse clinical signs were seen, and survival rates were
not affected by treatment. The rate of body weight gain was
marginally lower during the second trimester of the study in both
males and females receiving 5000 ppm, and food intake was marginally
lower during the first year among females receiving 5000 ppm. During
the remainder of the study, body weight gain and food consumption
were similar to those of the control animals. Investigation of the
eyes, blood, and urine of controls and of those receiving 5000 ppm
several times during the experiment revealed no reaction to
treatment; and no changes related to treatment were seen in gross
appearance or organ weights of rats in any group killed after 104
weeks. Histological examination of a limited range of tissues did
not provide evidence of toxicity or tumour induction that could be
attributed to treatment (Hunter & Benson, 1976).
Groups of 40 male and 40 female Wistar rats were fed the
following materials in the diet for 24-27 weeks: 1, 0.75, or 0.5%
C14-C18 AOS (corresponding to 500, 375, or 250 mg/kg body weight
per day); 1, 0.75, or 0.5% C14-C18 AOS (corresponding to 500,
375, or 250 mg/kg body weight per day); or 0.33, 0.25, or 0.16%
C161,4-sultone (corresponding to 165, 125, or 80 mg/kg body weight
per day). One control group consisted of 100 males and 100 females
and another of 40 males and 40 females. No excess of tumours over
that in controls was observed with any treatment (Haar, 1983).
In 70-week studies on Wistar rats, 0.5 ml of a 1.0, 10, or 30%
aqueous solution of AOS or 0.5 ml of a 50% aqueous solution of a
detergent based on AOS was applied dermally; 24 h after the
application, each site was washed with warm water. No abnormal gross
or histopathological findings were reported (Tomizawa, 1978).
These studies are summarized in Table 32.
B7.4 Skin and eye irritation; sensitization
AOS (C10; purity, 99.21%) were applied as 0.5 g of a 20 or 30%
solution once a day for 15 days to the backs of three male Wistar
rats. The skin at the application site and the tissues of the tongue
and oral mucosa of animals receiving the 30% solution were examined
histologically 16 days after application. Body weight gain was
reduced in the group given the 20% solution, and body weight was
decreased in the group at 30%. Macroscopically, there were no
abnormalities at the application site. Histologically, although
atrophy of the stratum spinosum was noted, neither necrosis nor
Table 32. Carcinogenicity of alpha-olefin sulfonates (AOS) after long-term exposure
Species, strain, Test material Route Dosage Results Reference
numbers per group (specification)
Mouse, Swiss-Webster AOS, C14-C18 Dermal 0, 200, 250 mg/kg No gross or histopathological Haar (1983)
40 M, 40 F (water, acetone) adverse effects on skin
3 times/week,
92 weeks
Mouse, Swiss-Webster AOS, C14-C16 Dermal 0, 200, 250 mg/kg No gross or histopathological Haar (1983)
40 M, 40 F (water, acetone) adverse effects on skin
3 times/week,
92 weeks
Rat, CFY, 50 M, 50 F AOS, C14-C18 Oral 0, 0.1, 0.25, 0.5%, No adverse effects Hunter & Benson
(a.i., 97-93%) (diet) 2 years (1976)
Rat, X-MRC, 40 M, 40 F AOS, C14-C18 Oral 0, 0.5, 0.75, 1.0%, No excess of tumours in Haar (1983)
(diet) 24-27 months comparison with controls
Rat, X-MRC, 40 M, 40 F AOS, C14-C16 Oral 0, 0.5, 0.75, 1.0%, No excess of tumours in Haar (1983)
(diet) 24-27 months comparison with controls
Rat, Wistar, 10 M, 10 F AOS, C16-C19 Dermal 0, 250, 2500, 7500 No gross or histopathological Tomizawa (1978)
mg/kg bw, 3 times abnormalities
per week, 70 weeks
Rat, Wistar, 10 M, 10 F AOS-based Dermal 12.5 g/kg bw No gross or histopathological Tomizawa (1978)
detergent 3 times/week, abnormalities
70 weeks
M, male; F, female; a.i., active ingredient
inflammatory cell infiltration was present. No abnormalities of the
tongue were observed, but severe atrophy was observed in the mucosa
of the oral cavity. The local lesions caused by application of AOS
were reported to be minimal in comparison with those induced by
application of linear dodecylbenzenesulfate or lauryl sulfate (Sadai
& Mizuno, 1972).
Solutions of 0.05-4% AOS (sodium salt of sulfonated C15-C18
n-alpha-olefin) were instilled at a dose of 0.1 ml into the eyes
of one to three rabbits, and the eyes were examined after 24 h. No
abnormal findings were observed with the 0.05% solution, but slight
congestion was observed with 0.1% and marked reactions, including
severe congestion and oedema, increased secretion, opacity of the
cornea, and absence of the corneal reflex, were observed at > 1%
(Oba et al., 1968a).
Solutions of C14-C19 olefin (84% C15-C17) and five other
solutions consisting mainly of C10, C12, C14, C16, or C18
were instilled into the eyes of three rabbits at one of six
concentrations ranging from 0.01 to 5%. The rabbits were examined
over a period of 168 h. The materials elicited similar reactions. No
abnormal reaction was seen with 0.05%; slight congestion was
observed with 0.1% within 2 h after application of the solution; and
marked congestion or oedema was observed with 0.5%, which
disappeared by 24 h. In the groups treated with 1 or 5%, marked
reactions, including severe congestion and oedema, increased
secretion, turbidity of the cornea, and disappearance of the corneal
reflex, continued for 24 h but had usually completely disappeared by
120 h (Iimori et al., 1972).
In 1973, the apparent sensitizing potential of AOS attracted
attention (Haar, 1983). AOS can contain unsaturated gamma-sultones
when manufactured under certain conditions, and these are strong
sensitizers in guinea-pigs (Haar, 1983; Roberts & Williams, 1983;
Roberts et al., 1990). When the levels of these sultones were
reduced to low levels by altering the manufacturing techniques, AOS
no longer caused sensitization (Haar, 1983; Oba et al., 1985;
Roberts et al., 1990).
Skin sensitization was studied in guinea-pigs with pastes made
of C14-C16 AOS, a light-duty dishwashing detergent containing
AOS, some consumer products containing AOS, and mixtures of these
products with alkyl unsaturated sultone in sodium lauryl sulfate or
hypochlorite bleach. The pastes, the dishwashing detergent, most of
the consumer products, and the mixtures with hypochlorite bleach
induced sensitization, the degree of response being related to the
amount of unsaturated gamma-sultone present in the material tested
(Bay & Danneman, 1985).
B7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
AOS (C14-C18) were administered at a concentration of 0.2,
2, 300, or 600 mg/kg body weight per day to CD rats, CD-1 mice, and
NZW rabbits orally once a day by gavage. Groups of 20 rats and mice
were given AOS on days 6-15 of pregnancy, and groups of 13 rabbits
were treated on days 6-18 of pregnancy. The doses of 0.2 and 2 mg/kg
were estimated to be equivalent to 1-2 and 10-20 times the maximal
amount of AOS to which humans are exposed. No adverse effects were
seen in rat dams, even at the maximal dose. Mouse dams given 300 or
600 mg/kg showed piloerection, decreased movement, and inhibition of
body weight gain; six dams at 600 mg/kg died. All rabbits given
600 mg/kg and one given 300 mg/kg died; anorexia and decreased body
weight were seen initially in surviving dams given 300 mg/kg. Both
mouse and rabbit dams given 0.2 or 2 mg/kg showed only initial
inhibition of body weight gain. No adverse effects were seen on
litter parameters of rats at any dose. In mice, total litter loss
was found in five dams given 600 mg/kg and in six dams given
300 mg/kg; however, the average number of live fetuses in the other
dams was no different from that in controls. The average body
weights of the fetuses of dams given 300 or 600 mg/kg was
significantly lower than that of controls. The incidence of major
malformations was not significantly increased in rats, mice, or
rabbits. There were no significant minor anomalies or skeletal
variations (extra ribs) in rats at any dose. The offspring of mice
at 600 mg/kg had a significant increase in delayed ossification.
Those of rabbits at 300 mg/kg had a significant increase in skeletal
anomalies and variations, although the incidence of skeletal
variations was within the normal background range, and there was no
delayed ossification. The effects of AOS on the fetuses, such as
changes in litter parameters and delayed ossification, were
considered to reflect the effects of AOS on the dams. There were no
adverse effects on fetuses of mouse or rabbit dams given 0.2 or
2 mg/kg or on fetuses of rat dams given 0.2, 2, 300, or 600 mg/kg,
where effects on the dams were either not observed or were minimal
(Palmer et al., 1975b).
AOS and AOS-S (a synthetic detergent with AOS as the main
ingredient) were applied to the shaven dorsal skin of mice at a dose
of 0.5 ml/mouse per day of a 0.1% (the concentration of AOS usually
found in detergents), 1%, or 5% aqueous solution of AOS or a 0.5%
(equivalent to 0.1% AOS), 5%, or 25% aqueous solution of AOS-S on
days 0-14 of pregnancy. Adverse effects on the dams and fetuses were
found in a few cases. None of the dams died; the viability, body
weight, and sex ratio of the fetuses did not differ from those of
controls; and there were no malformations (Sawano, 1978).
B7.6 Mutagenicity and related end-points
AOS did not cause differential toxicity in Bacillus subtilis
rec at a concentration of 20 µg/disc or reverse mutation in
Salmonella typhimurium TA98 or TA100 at 10-100 µg/disc, in the
presence or absence of metabolic activation (Oda et al., 1980).
One batch of AOS (C14-C16; 28.4% active ingredient) induced
host-mediated mutagenicity at 283 mg/kg body weight in rats
inoculated with S. typhimurium TA1530 but not in an assay with
TA1534 or in plate incorporation assays with either strain (Arthur
D. Little Inc., 1993).
B7.7 Special studies
Rabbit erythrocytes were mixed with solutions containing various
concentrations of AOS (sodium salt of sulfonated C15-C16
n-alpha-olefin; average relative molecular mass, 338.5) at room
temperature for 3 h. The 50% haemolytic concentration was
1.5 mg/litre (Oba et al., 1968a). The effects of AOS on
methaemoglobin formation were studied in groups of three male mice
given an oral or intraperitoneal dose of 0.3 or 3.0 g/kg body weight
C15-C18 AOS. The level of methaemoglobin in blood was measured
0.5, 1, 2, 3, and 24 h after administration of AOS. No significant
increase was observed (Tamura & Ogura, 1969).
In an immunological study of AOS, a complex (HA) prepared by
mixing AOS with human serum albumin (HSA) containing 30 mg of total
protein was injected subcutaneously or intravenously into rabbits
during a period of 2.5 months, and the anti-serum produced was
subjected to the ordinary precipitation reaction. As a control,
anti-AOS-serum, similarly prepared, was subjected to the same
reaction. Minor positive reactions were seen in the HA-anti-HA and
HSA-anti-HSA systems but not in the AOS-anti-HA or AOS-anti-AOS
systems (Iimori & Ushiyama, 1971).
B8. EFFECTS ON HUMANS
Section summary
In patch tests, human skin can tolerate contact to solutions
containing up to 1% AOS for 24 h with only mild irritation. AOS can
cause delipidation of the skin surface, elution of natural
moisturizing factor, denaturation of the outer epidermal layer
proteins, and increased permeability and swelling of the outer
layer. AOS did not induce skin sensitization in volunteers. There is
no conclusive evidence that AOS induce eczema. No serious injuries
or fatalities have been reported following accidental ingestion of
detergent formulations that could contain AOS.
B8.1 Exposure of the general population
AOS surface-active agents are found in shampoos, dishwashing
products, household cleaners, and laundry detergents. The
composition of nonionic and ionic surfactants in these products
varies between 10 and 30%. Surface-active agents can affect human
skin and eyes.
B8.2 Clinical studies
B8.2.1 Skin irritation and sensitization
AOS are mildly to moderately irritating to human skin, depending
on the concentration.
The relative intensity of skin roughness induced on the surface
of the forearm was evaluated in volunteers by a circulation method
consisting of contact with 1% solutions of C12, C14, C16, and
C18 AOS for 10 min. The skin response was characterized mainly on
the basis of gross visible changes. C12 AOS induced more skin
roughening than compounds with longer or shorter alkyl chains. The
relative degree of skin roughening in vivo was correlated with the
extent of protein denaturation measured in vitro (Imokawa et al.,
1975a).
Primary skin irritation induced by a 1% aqueous solution
(pH 6.8) of AOS containing 27% C15, 25% C16, 28% C17, and 18%
C18 (relative molecular mass, 338.5) was studied in a 24-h
closed-patch test on the forearms of seven male volunteers. The
intensity of skin irritation was scored by grading erythema,
fissuring, and scales. The average score for AOS was 3.97 and that
for a control (water) was 1.79. The same compound was evaluated at
0.3% for the relative intensity of skin lesions produced on the
surface of the hands by an immersion test involving 30 repetitions
of a 1-min dip and 1-min dry. The average score for AOS with regard
to erythema, irritation, fissuring, scaling, and loss of suppleness
was 5.75, while that for the water control was 2.5 (Oba et al.,
1968a).
Skin irritation induced by a 1% aqueous solution of C14,
C16, and C18 AOS was studied in a 24-h closed-patch test on the
forearm and in a test in which the compound was dripped onto the
interdigital surface for 40 min once daily for two consecutive days
at a rate of 1.2-1.5 ml/min. Skin reactions were scored by grading
erythema in the patch test and by grading scaling in the drip test.
The score for AOS was 1 (slight erythema) in the patch test and 0.35
(minimal scaling) in the drip test (Sadai et al., 1979).
In sensitization tests on volunteers, AOS in a paste or in a
detergent mixture containing up to 0.06% AOS and up to 0.002 ppm
unsaturated g-sultone did not produce sensitization, although one
subject had a strong dermal response, which was considered to be due
to pre-existing sensitization. Two out of 264 subjects using a
light-duty detergent containing AOS developed hand dermatitis and
had positive reactions to AOS paste and/or unsaturated gamma-sultone
in sodium lauryl sulfate in a patch test. Use of a hand dishwashing
liquid containing AOS did not cause sensitization provided the
level of unsaturated g-sultones was kept low (Bay & Danneman, 1985).
Patch tests on 790 volunteers after four months' use of a
dishwashing liquid showed no evidence of sensitization (Oba et al.,
1985).
B8.2.2 Effects on the epidermis
The effects of AOS on the epidermal outer layer (stratum
corneum) are similar to those of other surface-active agents (see
section 8.2.2 of the monograph on LAS), including delipidation of
the skin surface, elution of natural moisturizing factor,
denaturation of stratum corneum protein, increased permeability,
swelling of the stratum corneum, and inhibition of enzyme activities
in the epidermis (Wood & Bettley, 1971; Imokawa et al., 1974;
Okamoto, 1974; Imokawa et al., 1975a,b).
The effects of anionic surfactants on various types of proteins
were studied using skin keratin as a filamentous protein, bovine
serum albumin as a globular protein, acid phosphatase as an enzyme
protein, and membrane lysosome as a membrane protein. The denaturing
effects of surfactants were measured as liberation of sulfhydryl
groups and enzyme inhibition. AOS were less potent than LAS or alkyl
sulfates. A relationship was observed between denaturing potency,
skin irritant action, and alkyl chain length (Imokawa & Katsumi,
1976; Imokawa & Mishima, 1976).
B8.2.3 Hand eczema
Skin reactions to a 0.04, 0.4, or 4.0% aqueous solution of AOS
(25.0% C14, 45.0% C16, 30.0% C18)were evaluated in a 24-h
closed-patch test on the lower back of 10 healthy volunteers and 11
patients with hand eczema (progressive keratosis palmaris). The
incidence and intensity of skin reactions were significantly higher
in the group with hand eczema than in a control group with normal
skin (Okamoto & Takase, 1976a,b).
B8.2.4 Accidental or suicidal ingestion
No data were available that related specifically to AOS.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
Limited data are available on the effects of AOS on
environmental organisms. The 24-h LC50 values for daphnids were
19-26 mg/litre; the 48-h LC50 values ranged from 0.3 mg/litre for
brown trout (Salmo trutta) to 6.8 mg/litre for golden orfe (Idus
idus melanotus), and the 96-h LC50 was 0.5-5.0 mg/litre for
brown trout. One study suggested that AOS have little toxicity for
birds.
B9.1 Microorganisms
No information was available.
B9.2 Aquatic organisms
B9.2.1 Aquatic plants
The EC50 values for C16.4 AOS in the green alga Selenastrum
capricornutum exposed for two to three days, based on growth, fell
within the range 45-65 mg/litre (Yamane et al., 1984). The EC50
for C16-C18 AOS on the growth of S. capricornutum was >
20 mg/litre (Konno & Wakabayashi (1987).
B9.2.2 Aquatic invertebrates
Daphnia magna and Dapghnia pulex less than 24 h old were
exposed to C16-C18 AOS under static conditions, in which the
water was unchanged for the duration of the test, at a temperature
of 20°C and a water hardness of 25 mg/litre CaCO3. The 6-h LC50
values were > 64 and > 130 mg/litre, and the 24-h LC50 values
were 19 and 26 mg/litre, for the two species respectively
(Wakabayashi et al., 1988).
B9.2.3 Fish
The acute toxicity of AOS to fish is summarized in Table 33. The
48-h LC50 values ranged from 0.3 mg/litre for brown trout (Salmo
trutta) to 6.8 mg/litre for golden orfe (Idus idus melanotus);
the 96-h LC50 for brown trout was 0.5-5.0 mg/litre. Acute toxicity
tended to increase with carbon chain length.
When eggs of rainbow trout (Oncorhynchus mykiss) and carp
(Cyprinus carpio) were exposed to C16-C18 AOS, the EC50
values, based on hatchability, were 4.9 for rainbow trout and
3.0 mg/litre for carp (Wakabayashi & Onizuka, 1986). In one-month
old rainbow trout under semi-static conditions, the 14- and 28-day
LC50 values for C16-C18 AOS were 0.62 and 0.58 mg/litre. The
EC50 based on growth was 0.35 mg/litre (Wakabayashi & Mizorogi,
1989).
The time to lethality in goldfish (Carassius auratus) exposed
to AOS was 2 h at a concentration of 5 mg/litre and 1 h at
10 mg/litre. Addition of 2100 mg/litre egg albumin increased the
time to 100% lethality to 3 h and addition of 4200 mg/litre albumin
increased the time to 6 h (Tomiyama, 1974).
B9.3 Terrestrial organisms
B9.3.1 Terrestrial plants
AOS were not toxic with respect to growth at the early life
stages of radish, Chinese cabbage, and rice when added in hydroponic
culture at concentrations of 56, 56, and 32 mg/litre, respectively;
concentrations of 100, 100, and 56 mg/litre were toxic (Takita,
1982).
B9.3.2 Terrestrial invertebrates
No information was available.
B9.3.3 Birds
No significant effect on egg quality was found after Leghorn
chickens were fed a diet containing 200 mg/kg AOS for 45 days
(Lopez-Zavala et al., 1975).
Table 33. Toxicity of alpha-olefin sulfonates (AOS) to fish
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Masu trout 2 mo Staticr 8.5-9.6 30 NS C16-C18 96-h LC50 0.56
Wakabayashi
(Oncorhynchus et al. (1984)
masou)
Rainbow trout 40 d Staticr 8.8-10.9 25 NS 96-h LC50 0.78 Wakabayashi
(Oncorhynchus et al. (1984)
mykiss) 4 d Staticr 10 25 NS C16-C18 96-h LC50 0.61 Wakabayashi
19 d Staticr 10 25 NS C16-C18 96-h LC50 0.98 & Onizuka
(1986)
Brown trout 2.8-5.8 cm Flow 15 26-30 NS C14-C16 48-h LC50 2.5-5.0b Reiff et al.
(Salmo trutta) 2.8-5.8 cm Flow 15 26-30 NS C14-C16 96-h LC50 2.5-5.0b (1979)
2.8-5.8 cm Flow 15 26-30 NS C16-C18 48-h LC50 0.6b
2.8-5.8 cm Flow 15 26-30 NS C16-C18 96-h LC50 0.5b
2-4 cm Flow 15 250 NS C14-C16 48-h LC50 3.5b
2-4 cm Flow 15 250 NS C16-C18 96-h LC50 3.1b
2-4 cm Flow 15 250 NS C14-C16 48-h LC50 0.3-0.5b
Goldfish Static 20 NS C12-C16 6-h LC50 11.2c Gafa (1974)
(Carassius auratus) Static 20 NS C14-C18 6-h LC50 3.0c
Table 33 (contd)
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Golden orfe 1.2-1.8 g Static 20 NS C14-C16 48-h LC50 5.08 Mann (1976)
(Idus idus 1.2-1.8 g Static 20 NS C16-C18 48-h LC50 1.44
melanotus) 5-7 cm Flow 20 150 NS C14-C16 48-h LC50 5.7b Reiff et al.
5-7 cm Flow 20 150 NS C16-C18 48-h LC50 1.9b (1979)
Flow 20 268 NS C14-C16 48-h LC50 3.7-6.8b
Flow 20 268 NS C16-C18 48-h LC50 1.0b
Flow 20 268 NS C14-C16 96-h LC50 3.4-4.9b
Flow 20 268 NS C16-C18 96-h LC50 0.9b
Harlequin fish Flow 20 20 NS C14-C16 48-h LC50 4.8b Reiff et al.
(Rasbora Flow 20 20 NS C16-C18 48-h LC50 0.9b (1979)
heteromorpha) Flow 20 20 NS C14-C16 96-h LC50 3.3b
Flow 20 20 NS C16-C18 96-h LC50 0.5b
Medaka 175-332 mg Static 21-22 25 6.7-7.1 C14-C18 6-h LC50 6.2b Kikuchi &
(Oryzias latipes) 175-332 mg Static 21-22 25 6.7-7.1 C14-C18 48-h LC50 1.8b Wakabayashi
175-332 mg Static 21-22 25 6.7-7.1 C16-C18 6-h LC50 2.7b (1984)
175-332 mg Static 21-22 25 6.7-7.1 C16-C18 48-h LC50 0.81b
Carp 3.5-5.5 cm Static 21 7.5-7.8 Technical 24-h LC50 3.2c Lopez-Zavala
(Cyprinus carpio) 3.5-5.5 cm Static 21 7.5-7.8 Technical 96-h LC50 3.0c et al. (1975)
2 d Staticr 20 25 NS C16-C18 96-h LC50 > 1.4 Wakabayashi
15 d Staticr 20 25 NS C16-C18 96-h LC50 1.5 & Onizuka
(1986)
Table 33 (contd)
Species Length, Static or Temp. Hardness pH AOS chain End-point Concn Reference
weight, flow (°C) (mg/litre)a length (mg/litre)
or age
Carp (contd). 50 d Staticr 21 75 NS 96-h LC50 1.0 Wakabayashi
(Cyprinus carpio) Staticr et al. (1984)
White tilapia 5-7 cm Static 21 7.5-7.8 Technical 24-h LC50 2.0c Lopez-Zavala
(Tilapia melan 5-7 cm Static 21 7.5-7.8 Technical 96-h LC50 2.0c et al. (1975)
opleura)
Grey mullet Static 20.6-22.0 96-h LC50 0.70 Wakabayashi
(Mugil cephalus) et al. (1984)
Staticr, static renewal: water changed at regular intervals; flow, flow-through conditions: concentration in water maintained
continuously; static: water unchanged for duration of test
a mg/litre CaCO3
b Measured concentration
c Nominal concentration
C. Alkyl sulfates
C1. SUMMARY
See Overall Summary, Evaluation, and Recommendations (pp. 7-21)
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
C2.1 Identity
Chemical formula: CnH2n+1O4S Na ( n = 10-8)
Chemical structure: CnH2n+1OSO3- Na+ ( n, integer)
Common names: Sodium alkylsulfate, sulfuric acid alkyl
ester sodium salt, alkylsulfate sodium salt,
alcohol sulfuric ester sodium salt, sodium
dodecyl sulfate, sodium lauryl sulfate
Common trade names: Akyporox SAL SAS, Akyposal, Alphenate TFC
76, Alscoap LN, Aremsol, Berol, Cosmopon,
Dehydag, Elfan, Emal, Empicol, Gardinol,
Genapol CRT 40, Manro, Marlinat KT 50,
Melanol LP 1, Monogen, Montopol CST,
Montovol, Neopon LT, Nikkol, Nissan Persoft
SK, Perlankrol ATL-40, Perlankrol, Polystep
B, Rewopol, Sactipon, Sactol, Sandopan KD,
Sermul, Stepanol WA 100, Sufatol, Sufetal,
Sulfopon, Sunnol, Surfax, Swascol, Teepol HB
7, Tensopol Tesapon, Texapon, Ufarol AM 70,
Zoharpon, Zorapol LS-30, (McCutcheon, 1993)
Abbreviations: AS, AS-Na, SDS
CAS Registry numbers: 151-21-3 (C12 AS), 1120-04-3 (C18 AS),
68130-43-8 (C8-C18 AS)
Specification: AS are higher alcohol sulfuric ester salt
types of anionic surfactants. Depending on
which precursor alcohol is used as the raw
material, the alkyl group is linear or
branched, may contain a single homologue or
a mixture of chain lengths, and is usually
primary. The data presented are applicable
mainly to linear alcohol sulfates and AS
with predominantly single or similar type of
branching.
C2.2 Physical and chemical properties
AS are white crystalline powders. Their physical properties differ
widely depending on their alkyl groups, and they are usually produced
and used as mixtures. The relationships between the critical micelle
concentration, solubility, and alkyl chain length are shown in Table
34.
Table 34. Relationships between alkyl chain length, critical
micelle concentration (CMC), and solubility
Alkyl chain CMC × 10-3 Solubility/°Cb,c
length mol/litrea,c
8 136 -
12 8.6 15
14 2.4 28
16 0.58 42
18 0.16 55
a From Evans (1956)
b Temperature at which 10 g of AS dissolve in 1 litre of water
(Gotte, 1954)
c The solubility of surfactants in water, defined as the
concentration of dissolved molecules in equilibrium with a
crystalline surfactant phase, increases with rising temperature.
For surfactants, there is a distinct, sharp bend (break-point) in
the solubility-temperature curve. The steep increase in solubility
above the sharp bend is caused by micelle formation. At above the
critical micelle concentration, a micellar solution is formed.
Under these conditions, higher levels than the aqueous solubility
may be obtained.
AS are readily hydrolysed in hot acidic media. Compounds with an
alkyl chain length of C10 (27°C), C12 (25°C), C14 (40°C), or
C16 (40°C) have a surface tension of 40 dyne/cm at the temperatures
shown in parentheses at concentrations greater than the critical
micelle concentration, indicating a good ability to reduce surface
tension (Dreger et al., 1944).
Cleansing capacity at 25°C increases with alkyl chain length up to
C13 and then becomes constant up to C16. In an actual detergent
containing alkali builders and chelating agents, however, maximal
detergency was obtained with C14 compounds (Yamane et al., 1970).
C2.3 Analysis
C2.3.1 Isolation
Since AS are readily susceptible to hydrolysis in acidic media,
special attention is required.
C2.3.2 Analytical methods
There is no officially recognized, specific procedure for the
analysis of AS in environmental samples. The methods used for
analysing linear alkylbenzene sulfonates (LAS) are commonly used for
AS, except those involving high-performance liquid chromatography
(HPLC), which is of limited use for detecting AS in environmental
samples because AS do not effectively absorb ultra-violet radiation.
An HPLC method for the analysis of AS after its conversion by
derivatization into an ultra-violet-active species has been proposed
(Utsunomiya et al., 1982). A modified analytical method has been
developed that is based on measurement of methylene blue-active
substances (MBAS) by HPLC. This method permits determination of AS at
concentrations as low as 0.05 mg/litre (Takita & Oba, 1985). Trace
enrichment followed by gas chromatography and flame ionization
detection have been proposed for the sensitive determination of AS as
their trimethylsilyl ethers in environmental samples (Fendinger et
al., 1992a).
Non-specific methods used in the analysis of anionic surfactants
in general, such as the methylene blue method, may be used for the
analysis of AS (see also section 2.3 of the monograph on LAS).
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Section summary
Few quantitative data are available on AS in the environment, but
AS can be expected to mineralize rapidly in all environmental
compartments and to be removed to a large extent during sewage
treatment. Environmental concentrations in receiving surface waters,
sediments, soils, estuaries, and the marine environment can be
expected to be low.
C3.1 Natural occurrence
AS do not occur naturally.
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
AS are synthesized industrially. Worldwide consumption of AS in
1987 was about 117 000 tonnes in the United States, 56 000 tonnes in
western Europe, and 46 000 tonnes in Japan (Richtler & Knaut, 1988).
In western Germany in 1987, some 10 000 tonnes of AS and 87 000 tonnes
of LAS were used (Schöberl et al., 1988). Worldwide consumption was
estimated to be 289 000 tonnes in 1990 (Hewin International Inc.,
1992; see Table 35).
Table 35. Estimated worldwide use of alkyl sulfates in
1990 (tonnes)
Region Household Personal care Industrial and
products products institutional
use
North America 140 000 33 000 9 000
Western Europe 49 000 12 000 7 000
Japan 21 000 6 000 4 000
Rest of the - 4 000 4 000
world
Total 210 000 55 000 24 000
From Hewin International Inc. (1992)
AS were originally made by the sulfation of natural fatty
alcohols. They are currently produced from both natural and synthetic
fatty alcohols. Primary AS are usually manufactured by conventional
sulfation of the parent alcohol with either sulfur trioxide or
chlorosulfonic acid. The product of this reaction is then neutralized
with an appropriate base (NaOH, Na2CO3, NH4OH, or
triethanolamines).
C3.2.2 Uses
Initially, AS were used as washing agents for wool or as active
ingredients in heavy-duty laundry detergents. They are now used mainly
in personal care products (shampoos, toothpastes, toiletries),
household detergents (light-duty dishwashing detergents, heavy-duty
laundry detergents), and industrial applications.
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Section summary
AS can be expected to be transported into the environment by
mechanisms similar to those that operate for LAS and alpha-olefin
sulfonates (AOS). AS are readily biodegradable under aerobic
conditions, both in laboratory tests and under environmental
conditions, and primary biodegradation is complete within two to five
days. Less information is available on the effect of temperature on
the biodegradation of AS than for LAS. The biodegradation kinetics of
AS appear to be less affected by temperature than those of other
surfactants. The whole-body bioconcentration factors are 2-73,
depending on chain length. AS are taken up by fish mainly through the
gills and are subsequently distributed to the liver and gall-bladder.
After biotransformation, AS are excreted rapidly. They are not
bioconcentrated or biomagnified in aquatic organisms.
C4.1 Transport and distribution between media
After use, AS are discharged into the environment in wastewater,
like other detergent compounds, where they can undergo sewage
treatment if such facilities are available. In countries where
adequate wastewater treatment facilities are not available, AS
released to the environment are removed by biodegradation and
adsorption in the receiving surface water (see section 4.2 of the
monograph on AOS).
Sorption equilibria were obtained rapidly (within 20 min) for pure
homologues of AS (> 99%) with chain lengths of C8, C9, C10,
C11, C12, C13, and C14, suggesting that sorption is due to a
hydrophobic bonding mechanism, as has been observed for other
surfactants. Thus, sorption of AS to sediment is likely to be stronger
for longer chain homologues than for shorter ones. The KD values for
C12 AS were 70 and 100 for two river sediments, whereas for C12
LAS on the same sediments they were 310 and 330 (Marchesi et al.,
1991). Adsorption of AS therefore competes in kinetic terms with
biodegradation as a mechanism for removal of AS from the environment,
as is seen for surfactants in general.
C4.2 Biotransformation
C4.2.1 Biodegradation
C4.2.1.1 Biodegradation pathway; mechanism
Several species of bacteria have been found that can mineralize
AS. AS with chains longer than six carbons are degraded by the initial
action of a sulfatase enzyme, producing sulfate and the corresponding
alcohol. The alcohol is readily oxidized by formation of an aldehyde,
to produce carboxy acid, which can be further oxidized by ß-oxidation
and in the citric acid cycle. Secondary ketones and hydroxy ketones of
AS are produced as metabolites but have not been detected in simulated
activated sludge. Biodegradation of short-chain homologues of AS may
proceed by oxidation of the chain before hydrolysis of the ester bond
by the sulfatase enzyme.
The metabolic pathway for biodegradation of C12 AS by
Pseudomonas strains has been described (Hsu, 1965; Thomas & White,
1989). Initial liberation of the sulfate head produces dodecanol,
which is further transformed into more polar metabolites, including
dodecanal and dodecanoic acid. These products may be further
metabolized by ß-oxidation, or they may be elongated to C14, C16,
or C18 fatty acyl residues, which are then incorporated into lipid
fractions such as phospholipids (Thomas & White, 1989).
C4.2.1.2 Biodegradation in the environment
The aerobic biodegradation of 20 mg/litre AS at 27°C was followed
during a 10-day incubation period. Primary degradation, measured by
the MBAS method, was complete within five days. The theoretical
production of CO2 reached 60-90% within 10 days (Itoh et al., 1979).
The biodegradation of AS at a concentration of 30 mg/litre was
studied in a vessel containing activated sludge at a concentration of
100 mg/litre over a period of 12 days, by measuring chemical oxygen
demand. All of the AS were lost within two days; the specific rate of
biodegradation was calculated to be 20 mg/g per h (Pitter & Fuka,
1979).
The biodegradation of an initial concentration of 6 mg/litre C12
AS was studied by the die-away method, in which disappearance of the
compound is followed over a given period. Less than 10% of the
original amount remained in river water in the test vessel after 12
days' exposure, and complete degradation was reported within 21 days
(Okpokwasili & Nwabuzor, 1988).
The capacity of epilithic (sampled from the surface of pebbles)
and planktonic river bacterial populations to degrade C12 AS was
studied under simulated environmental conditions. Samples were
collected from four polluted sites and one clean site in a polluted
river in South Wales, United Kingdom. In die-away tests, AS were
degraded after an apparent lag at all four polluted sites, but
degradation by the bacterial populations at the clean site was
relatively slow. Quantification of the kinetic components that
contributed to the die-away curves demonstrated that biodegradation of
AS occurred at concentrations below its Km by bacteria with
exponential growth that are unaffected by addition of the test
substrate. Degradation of AS in the clean sample followed a
different pattern, but there was generally little or no growth on
endogenous carbon. The authors concluded that the capacity of
epilithic bacterial populations to degrade C12 AS is more stable
than that of planktonic populations (Anderson et al., 1990).
Riverine bacteria that can grow in the presence of
0.5 mmol/litre C12 AS are widespread, and a greater incidence of
isolates resistant to C12 AS was recorded at a polluted site than in
clean water. The ability of each culture to produce alkyl sulfatases,
the enzymes that initiate degradation of AS, was also determined.
Bacteria containing alkyl sulfatases were widespread, but a greater
alkyl sulfatase yield was obtained from polluted site. The authors
concluded that more strains at the polluted site had constitutive
rather than inducible enzymes. An increased incidence of strains
containing multiple alkyl sulfatases was also recorded at the polluted
site (White et al., 1985).
In another study in South Wales, the distribution of planktonic
bacteria capable of degrading 98.5% C12 was examined in water
samples at sites along a river. The annual mean prevalence of such
bacteria was 8.1-16.0% of the total number of isolates. The proportion
of isolates that degrade AS in clean water was no different from that
at polluted sites, and a lower density was recorded at the source
owing to a reduction in overall numbers. A higher percentage of
bacteria capable of degrading C12 AS was recorded in estuarine
samples than in samples from the middle of the polluted river;
however, when cell numbers were taken into account, the cell density
was similar at all polluted sites on the river, including the estuary.
The incidence of these isolates was not correlated with either
biochemical oxygen demand or oxygen concentration, but the incidence
tended to increase at the end of the summer. More than half of the
isolates contained constitutive alkyl sulfatase enzymes, while they
were induced or repressed in the remainder after exposure to AS. No
variation in the proportions of type of enzyme regulation was seen
between sampling sites or times (White et al., 1989).
The biodegradation of AS was also examined at three sites, above,
at, and below a sewage works outfall on the South Wales river. Samples
capable of degrading C12 AS after only one day's exposure were found
at each site. No biodegradation of AS was reported at a pristine
source site. The onset of biodegradation was more rapid following
longer exposure of the river, suggesting the existence of an adaptive
mechanism. A model of the die-away kinetics of degradation suggested
that C12 AS were biodegraded by a bacterial population growing at
the expense of endogenous carbon. The activity of the epilithic
samples in degrading AS increased during the first four days of
exposure at each site. The stabilized values (days 4-14) increased
from the upstream site to the outfall, decreasing to intermediate
values downstream. The sewage input had less effect on activities
in degrading AS than on bacterial cell densities. Little variation in
growth characteristics was seen throughout colonization at the three
sites. The authors concluded that the adaptation seen during exposure
in the river was attributable to colonization of the epilithon by an
existing population that degradedC12 AS and not to acquisition or
adaptation of biodegrading capacity (Russell et al., 1991).
The half-life for primary degradation of 20 mg/litre C12 AS in
seawater varied over a range of 0.26 to 0.34 days, and degradation was
reported to follow first-order kinetics. Primary degradation was
followed by an immediate increase in bacterial number and thymidine
incorporation (Vives-Rego et al., 1987). C12 AS was found to be
degraded rapidly in seawater, and 250 g/litre were found in sediment;
at 25°C, 90% was degraded within five days. No lag phase was reported,
and the degradation kinetics were reported to be first-order (Sales et
al., 1987).
C15-C16 AS were 98% removed at 15°C and 99% removed at 8°C
(Gilbert & Pettigrew, 1984). Similarly, C12-C15 and C12-C14 AS
were found (by the MBAS method) to be biodegraded during winter and
spring in a trickling filter sewage treatment plant (Mann & Reid,
1971). These results suggest that temperature has no major effect on
the removal of alkyl sulfates under environmental conditions.
Primary biodegradation of C12 AS was less affected by incubation
temperature than that of other anionic surfactants in die-away tests
with water from the Tama River, Japan. Primary biodegradation was
complete within one day at temperatures of 21 and 27°C, within two
days at 15°C, and within three days at 10°C (Kikuchi, 1985).
Over 99% of MBAS activity in activated sludge was lost in a 19-day
OECD screening test and in the 28-day OECD confirmatory test.
Mineralization of both C12-C14 and C16-C18 AS was complete,
with 90-95% degradation for C12-C14 AS and 77-88% for C16-C18
AS in the two test systems (Steber & Wierich, 1987).
C4.2.1.3 Anaerobic degradation
Biodegradation of AS under anaerobic conditions has been reported
in several studies, with 88% degradation of stearyl sulfate containing
C14 AS in an anaerobic screening test (Birch et al., 1989) and 95%
ultimate degradation of the same compound (Steber & Wierich, 1987).
C4.2.2 Abiotic degradation
No information was available.
C4.2.3 Bioaccumulation and biomagnification
Carp (Cyprinus carpio) were exposed to 35S-C12 AS at a
concentration of 0.85 mg/litre for up to 24 h. Within 1 h, AS was
concentrated in the gills, hepatopancreas, and kidneys with
concentration factors of 1.6, 1.4, and 1.5, respectively. After the
initial uptake in the gills, the levels of AS fell, and other organs
and tissues, such as the skin surface, muscle, brain, kidney,
hepatopancreas, and gall-bladder showed gradual uptake over the
exposure period. The concentration factors after 24 h ranged from 2.0
for the skin surface to 43 for gall-bladder. Blood and kidney also
showed uptake, but the levels after 24 h were less than those after 4
and 8 h, respectively. AS were lost rapidly from all tissues except
the gall-bladder when the fish were kept in 'clean' water for 48 h
(Kikuchi et al., 1978).
Carp maintained in water containing 0.5 mg/litre 35S-C12 AS
absorbed the compound within 1 h, and an equilibrium for the whole
body and gall-bladder was reached within 24 h, with concentration
factors of about 4 and 700, respectively. After 24 h, the levels of AS
in hepatopancreas had decreased from the initial level. When the fish
were transferred to 'clean' water, 50% of the AS was still present
after 72 h (Wakabayashi et al., 1978).
When carp were exposed to 35S-C12 AS at concentrations between
2.7 µg/litre and 40 mg/litre for up to 120 h, equilibrium was reached
within 72 h, at concentration factors of 3.9-5.3, which were
independent of the concentration of AS in solution (Wakabayashi et
al., 1981).
In a study of the effect of chain length on the uptake of AS, carp
were exposed to 0.5 mg/litre of 35S-C12, 35S-C14, or
35S-C16 AS for 24 h. Absorption of AS reached a maximum within the
exposure period. The whole-body concentration factors were 2.1, 11,
and 73 for the three surfactants respectively, and thus increased with
alkyl chain length. This tendency was also observed in gills and
hepatopancreas, but the factors in the gall-bladder were almost the
same for the three homologues. When fish were transferred to 'clean'
water, the elimination rate decreased with increasing carbon chain
length, and 50% of C16 AS was retained after 120 h (Wakabayashi et
al., 1980).
The absorption, tissue distribution, metabolism, and route of
excretion of 50 mg/litre C12 AS were studied in goldfish (Carassius
auratus) exposed for 24 h. AS was absorbed mainly through the gills
and was distributed rapidly throughout the body; it was absorbed to a
lesser extent (20% of total) by cutaneous absorption and orally (8%).
The highest concentration of AS was measured in the gall-bladder,
mainly because of its small size. The greatest proportion of the
absorbed AS was located in the body, gut, liver, and gall-bladder. The
level of AS in the tissues fell by 38% over 24 h in unfed fish and by
68% in fed fish. The high concentration of AS in the liver and
gall-bladder was thought to indicate metabolism of the compound in the
liver. The metabolites of AS that were identified included successive
products of ß-oxidation of the alkyl chain and butyric-4-sulfate
(Tovell et al., 1975).
C4.3 Interaction with other physical, chemical, and biological
factors
The presence of 1 mg/litre AS (chain length unspecified) had no
significant effect on the uptake of mercury by phytoplankton
( Diogenes sp.) or mussels ( Mytilus sp.) (Laumond et al., 1973).
Exposure of bacteria to 20 mg/litre phenol and 0.5 mg/litre C12
AS resulted in a directly additive effect. Exposure to 2.5 mg/litre
phenol and 0.5 mg/litre AS resulted in a synergistic effect. No
interactive effects were reported between sodium cyanide and AS in the
same test protocol (Dutka & Kwan, 1982).
C4.4 Ultimate fate following use
As no specific analytical method is available for AS, their
concentrations in environmental samples have not been established.
Like detergent compounds, AS are present in wastewater after use. A
large proportion is removed during treatment of wastewater, mainly as
a result of a combination of biodegradation and adsorption processes.
As for other surfactants, these processes continue when AS are
released into the environment.
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Section summary
Data on the environmental concentrations of AS are limited. At
sewage treatment plants where the influent concentrations of AS were
< 0.01-0.7 mg/litre, the effluent contained predominantly C12 AS,
at concentrations of < 0.005-0.1 mg/litre. Surface waters receiving
treated wastewater contained AS at concentrations below the detection
limit of 0.005 mg/litre.
Environmental levels
AS were measured at two sewage treatment plants in the United
States where the influent concentrations were < 0.01-0.7 mg/litre,
which were at least 2.4 times lower than those predicted on the basis
of use of AS and per-capita wastewater in the United States. The
predominant homologues of AS in untreated wastewater were C12,
C14, and C15. The effluent contained predominantly C12 AS, at
concentrations of < 0.005-0.1 mg/litre, showing that removal exceeded
98% during rotating biological contact and activated sludge treatment.
Surface waters receiving treated wastewater contained AS at
concentrations below the detection limit of 0.005 mg/litre (Fendinger
et al., 1992a,b).
C6. KINETICS
Section summary
AS are readily absorbed by the gastrointestinal tract after oral
administration and are excreted principally in the urine, only minor
amounts being eliminated in the faeces. Penetration of AS through
intact skin appears to be minimal. AS are extensively metabolized in
various species to several metabolites. Butyric acid-4-sulfate has
been identified as their major metabolite.
C6.1 Absorption, distribution, and excretion
In a study of the absorption of higher alcohol sulfates,
14C-hexadecyl sulfate salts were administered orally to humans and
dogs. After a single dose of 14.4 mg/kg bw of the salts to dogs, the
maximal plasma concentration of hexadecyl sulfate (1.22-2.45 µg/ml)
was reached within 30-60 min; 6 h later, the plasma concentration had
decreased to about one-tenth of the peak value. Within 72 h, 50-79% of
the administered dose had been excreted in the urine and 12-41% in the
faeces. After a single dose of 360 mg to humans, the maximal plasma
concentration was reached at 2 h, although there was marked variation
between individuals (range, about 3.1-23 µmol/ml) (Merits, 1975).
Potassium dodecyl 35S-sulfate was injected intravenously or
intraperitoneally at 1 mg/ml to male and female rats. The proportions
of the administered dose excreted in the urine and faeces and the
amounts retained in the carcass after 24 h are shown in Table 36. Most
of the radiolabel appeared in the urine of both male and female rats,
although some was present as inorganic 35S-sulfate. The intestinal
flora do not play a significant role in the metabolism of potassium
dodecyl 35S-sulfate, since the distribution of radiolabel in the
urine and faeces was similar in rats pretreated with antibiotics and
in untreated rats. Whole-body autoradiograms of rats killed 5 min
after administration of the compound by intraperitoneal injection
showed significant amounts of radiolabel in the liver; the
concentrations increased up to 30 min and then gradually declined,
only trace amounts remaining after 4 h. The kidney was the only other
organ in which any appreciable accumulation was reported (quantitative
data not presented) (Denner et al., 1969).
In order to investigate the percutaneous absorption of AS,
0.5 ml of 25 mmol/litre sodium 14C-dodecyl sulfate in water was
applied to the dorsal skin (10 cm2) of rats for 15 min. Heavy
deposition of the surfactant on the skin surface and in the upper
regions of the hair follicles was observed. The 14C level in urine
was calculated to be equivalent to a penetration of 0.26 µg/cm2 per
24 h (Howes, 1975).
Table 36. Excretion of 14C-alkyl sulfates by rats after injection
of 1 mg/ml
Route of Sex Excretion (%)
administration
Urine Faeces
(total 35S)
Inorganic 35S Total35S
Intraperitoneal Male 86.3 14.4 0.2
Female 93.2 18.1 0.9
Intravenous Male 95.6 23.5 -
Female 97.4 11.4 -
From Denner et al. (1969)
In young swine administered sodium dodecyl 35S-sulfate
(3.3 mmol/animal) orally, the labelled compound was well absorbed from
the intestine. Traces of radiolabelled sulfur were found only in
bristles, bones, and bone marrow. The total amounts of 35S retained
in organs and tissues were 1.7% of the dose at 82 h, 0.6% at 200 h,
and 0.18% at 10 weeks. About 90% of the sodium dodecyl sulfate was
recovered in urine and about 10% in faeces at 140 h (Havermann &
Menke, 1959).
Similar results were obtained in guinea-pigs in a study of the
percutaneous absorption of 3 µmol sodium lauryl 35S-sulfate in water
through skin in vivo. Less than 0.4% of the dose was found to have
penetrated the skin, on the basis of recovery of radiolabel in the
urine, faeces, and expired air. The permeability constant was
calculated to be 0.65 × 10-6 cm/min (Prottey & Ferguson, 1975).
In a study of the dermal absorption of some homologues of AS,
ranging from octyl to octadecyl sulfate, by isolated human abdominal
skin, no penetration of the dermis was detected (Blank & Gould, 1961).
The rates of excretion in urine and faeces after oral,
intravenous, or intraperitoneal administration of 14C- or
35S-labelled C10-C18 AS to rats, dogs, and humans are summarized
in Table 37.
C6.2 Biotransformation
Potassium dodecyl 35S-sulfate was extensively metabolized in
rats to yield a single ester sulfate, identified as butyric acid
4-35S-sulfate (III in scheme below), and inorganic 35S-sulfate.
Table 37. Excretion of alkyl sulfates (AS) in the urine and faeces of rats, dogs, and humans
ASa Species Treatment Length of Excretion (%) Reference
treatment
(h) Urine Faeces
35S-AS(C10)-K Rat 1 mg/rat ip 48 82.9 1.2 Burke et al. (1975)
79.5 1.0
35S-AS(C11)-K Rat 1 mg/200 g ip 48 98.2 2.5 Burke et al. (1976)
90.6 7.3
Rat 1 mg/200 g po 48 75.1 14.3
88.7 5.7
Rat 1 mg/200 g iv 48 85.9 5.9
74.8 18.5
35S-AS(C12)-K Rat 1 mg/rat ip 48 86.3 0.2 Denner et al. (1969)
93.2 0.9
Rat 1 mg/rat po 48 98.7 0.7
106.9 0.5
35S-AS(C16)-EM Rat 14.4 mg/kg po 96 94 5 Merits (1975)
14C-AS(C16)-EM Rat 14.4 mg/kg po 72 87 3
35S-AS(C16)-Na Dog 2.9 mg/kg iv 72 83 3
14C-AS(C16)-TMA Dog 4.4 mg/kg iv 48 50 41
Table 37 (contd)
ASa Species Treatment Length of Excretion (%) Reference
treatment
(h) Urine Faeces
35S-AS(C16)-EM Dog 14.4 mg/kg po 72 52 37
14C-AS(C16)-EM Dog 14.4 mg/kg po 72 65 26
14C-AS(C16)-EM Human 250 mg po 72 80 7
20 73
35S-AS(C18)-K Rat 1 mg/rat ip 48 77.1 1.1 Burke et al. (1975)
73.9 2.6
Rat 1 mg/200 g po 48 76.7 4.1
68.8 6.1
35S-AS(C18)-Na Rat 4 mg/rat po 48 95.3 2.2 Adachi et al. (1979)
a K, potassium salt; EM, erythromycin salt; TMA, trimethylammonium salt
These compounds were degraded by a process involving initial
omega-oxidation followed by ß-oxidation of fatty acids with successive
elimination of a C2 fragment. The final product of degradation of
potassium dodecyl 35S-sulfate was potassium butyric acid
4-35S-sulfate, which was excreted in urine. When this product was
injected intraperitoneally into rats, it was mostly eliminated
unchanged in the urine, but about 20% of the dose was present as an
inorganic 35S-sulfate. These findings suggest that the sulfate ester
is hydrolysed in vivo (Denner et al., 1969).
Butyric acid 4-sulfate was hydrolysed nonenzymatically in vitro
at pH 5.0 and above, and the 4-butyrolactone (IV) and inorganic
SO42- ion were liberated in approximately equimolar amounts (Ottery
et al., 1970).
After administration of 14C-hexadecyl sulfate to rats, dogs, and
humans, the main metabolite was identified as the sulfate ester of
4-hydroxybutyric acid. A minor metabolic product, tert-14C-
butyrolactone, was also isolated from the urine of rats, dogs, and
humans. The urine of dogs contained still another metabolite, which
was isolated and identified as glycollic acid sulfate (V) (Merits,
1975).
HOOC-CH2-CH2-CH2OSO3H OC-CH2-CH2CH2 HOOC-CH2OSO3H
Butyric acid 4-sulfate 4-Butyrolactone Glycollic acid sulfate
(III) (IV) (V)
Qualitative analysis of 35S in the urine of rats administered
potassium decyl 35-sulfate or potassium octadecyl 35-sulfate
intravenously showed that butyric acid 4-35-sulfate was the major
metabolite and inorganic 35-sulfate a minor metabolite; no unchanged
compound was detected (Burke et al., 1975).
Similar results were obtained when sodium octadecyl 35-sulfate
was administered orally to rats . It was suggested that alkylsulfates
with even-numbered carbons, like C10, C12, C16, and C18, are
degraded by a common pathway involving omega-oxidation followed by
ß-oxidation, and finally excreted in urine as metabolized forms with
C4 or C2 (Adachi et al., 1979).
The metabolism of surfactants with odd-numbered carbon chains,
like C11 potassium undecyl 35-sulfate, was also investigated in
rats. Propionic acid 3-35-sulfate was identified as the major
metabolite in urine; pentanoic acid 5-35-sulfate and inorganic
35-sulfate were identified as minor metabolites (Burke et al., 1975,
1976).
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Section summary
The oral LD50 values for AS in rats ranged from 1000 to
4120 mg/kg bw. AS irritate the skin and eye at concentrations of about
0.5% or more. Although the effects of short- and long-term exposure to
AS in animals have been investigated, most of the studies are limited
by inadequate histopathological examination or small group size. Toxic
effects have been reported in rats administered AS in the diet or
drinking-water at concentrations equivalent to > 200 mg/kg per day.
Maternal toxicicity and fetotoxic effects have been observed at a
dose equivalent to 200 mg/kg per day.
The available long-term studies are inadequate to evaluate the
carcinogenic potential of AS in experimental animals; however, in the
limited studies available, in which animals were administered AS in
the diet, there was no evidence of carcinogenicity.
On the basis of limited data, AS also do not appear to be
genotoxic in vivo or in vitro.
7.1 Single exposures
The oral, intraperitoneal, intravenous, and dermal LD50 values
for AS are summarized in Table 38. The acute oral toxicity of AS in
rats and guinea-pigs may vary with the length of the alkyl chain, and
compounds with shorter chains are less toxic. The low LD50 value for
sodium lauryl sulfate after dermal application to rabbits may indicate
rapid skin penetration.
There were no overt signs of poisoning, except diarrhoea in rats
given sodium coconut alcohol sulfate orally (Brown & Muir, 1970);
however, signs of central nervous stimulation, including tremors,
tonic-clonic convulsions, and respiratory collapse, were observed in
rabbits, guinea-pigs, and rats given lauryl sulfate dermally and in
rabbits given the compound intravenously (Carson & Oser, 1964).
In animals that died after receiving large doses of AS, the main
gross pathological findings were haemorrhage and congestion of the
stomach wall and bloodstained urine. Histopathological examination of
rats given the sodium sulfate derivative of 3,9-diethyltridecane-
4-ol orally revealed congestion, cloudy swelling of convoluted tubules
with marked toxic degeneration of the cells, and granular detritus in
the kidneys of animals killed by the LD50, whereas only congestion
and cloudy swelling were seen in the kidneys of animals that survived
the LD50. At larger doses, similar severe kidney injury and necrosis
of the intestinal villi of the entire mucosal surface of the small
intestine were observed. Only minor injury was seen in the liver, and
the other organs examined were normal (Smyth et al., 1941).
Table 38. Acute toxicity of alkyl sulfates (AS)
Species Sex Route LD50a Test material Reference
Mouse NS po 2900 C8 sodium AS Gloxhuber
2200 C10 sodium AS (1974)
2700 C12 sodium AS
3000 C14 sodium AS
> 8000 C16 sodium AS
> 8000 C18 sodium AS
Rat M po 4120 40% solution of sodium Smyth et
2-ethylhexanol sulfate al. (1941)
M po 1250 25% solution of sodium
7-ethyl-2-methyl
undecanol-4 sulfate
M po 1425 25% solution of sodium
3,9-diethyl tridecanol-6
sulfate
M po 2730 30% solution of sodium
lauryl sulfate
F,M po 1280 86% sodium laury sulfate Walker et
(C12-C15) al. (1967)
F,M po 1000-2000 Sodium coconut alcohol Brown &
sulfate (mainly C12) Muir (1970)
F,M ip 210 Sodium lauryl sulfate Epstein et
al. (1939)
F,M Dermal 2000 (100% 30% slurry of sodium lauryl Carson &
deaths) sulfate Oser (1964)
Table 38 (contd)
Species Sex Route LD50a Test material Reference
Guinea- F,M po 1520 40% solution of sodium Smyth et
pig 2-ethylhexanol sulfate al., (1941)
F,M po 650 25% solution of sodium
7-ethyl-2-methyl
undecanol-4 sulfate
F,M po 425 25% solution of sodium
3,9-diethyl tridecanol-6
sulfate
F,M Dermal 1200 33% slurry of sodium lauryl Carson &
(no deaths) sulfate Oser (1964)
F,M Dermal 2000 (100% 33% slurry of sodium lauryl
deaths) sulfate
Rabbit F,M Dermal 580 33% slurry of sodium lauryl
sulfate
F,M iv 121 (100% 33% slurry of sodium lauryl
deaths) sulfate
M, male; F, female
a As active ingredient
C7.2 Short-term exposure
The results of short-term tests for toxicity with repeated doses
are summarized in Table 39.
C7.2.1 Rat
C7.2.1.1 Administration in the diet
Groups of five male and five female Wistar rats were fed diets
containing technical-grade sodium lauryl sulfate (purity, 98%) at a
concentration of 0, 0.5, 1, or 2% (equivalent to 245, 490, or
980 mg/kg of diet per day) for two or four weeks. No abnormalities
were seen in behaviour or food intake; but body weight gain was
significantly suppressed in females at the highest dose, and
haematological examination revealed a significant decrease in red
blood cells at two weeks. Biochemical examination of the serum
revealed a significant increase in the glucose level at two weeks in
males given 2%, a significant increase in glutamate-oxalate
transaminase at two weeks in females given 1 or 2%, significant
increases in glutamate-pyruvate transaminase and alkaline phosphatase
activities at four weeks in all females, and a significant decrease in
cholinesterase activity at four weeks in females given 2%. Both the
absolute and relative weights of the liver and thyroid were increased
at two weeks in males and females given 2%, and those of the liver and
left kidney were increased at four weeks in all females; the weights
of the thymus were decreased in males given 2% at four weeks.
Histopathological examination of rats with increased liver weight
revealed slight swelling of liver cells and increased numbers of
dividing liver cells. This finding was considered to be an adaptation
to administration of the test material. Cylinders in the renal
tubules, vacuolar degeneration of the epithelial cells of the renal
tubules, periodic acid-Schiff stain-positive substances in the renal
tubules, and atrophy of the renal glomeruli were observed mainly in
rats given 1 or 2% (Oishi et al., 1974).
Groups of 25 albino rats (sex not specified) were given diets
containing a sodium lauryl sulfate formulation (Iriumr) at a dose of
0, 30, or 60 mg/animal per day for eight weeks. The only abnormal sign
in the experimental groups was soft stools. Histological examinations
of the livers of four rats in each group revealed swelling of liver
cells, compression of cellular cords, and prominent nuclei. These
effects were particularly marked in rats given the high dose (Hatton
et al., 1940).
Table 39. Results of short-term exposure of experimental animals to alkyl sulfates (AS)
Species, strain, Material Route Dosage Results Reference
numbers per group
Rat, Wistar, 10 AS, C12 (a.i. 98%) Diet 0, 0.5, 1.0, 2.0%, Changes in haematological Oishi et al.
4 weeks parameters, serum enzyme (1974)
activities, and liver; depressed
body weight gain in females at
highest dose; increased weights
of liver, thyroid and kidney at
highest dose; decreased thymus
weight in males
Rat, 25 AS, C12 (Irium(R) Diet 30, 60 mg/rat per Dose-related hepatic effects Hatton et al.
day, 5 weeks (1940)
Rat, Wistar, 5 AS, C12 Diet 1.5%, 12 weeks Changes in serum, renal, and Ikawa et al.
hepatic enzyme activities; (1978)
depressed body weight gain;
increased liver weight
Rat, Osborne- AS, C12 Diet 9, 2, 4, 8%, Diarrhoea, abdominal bloating; Fitzhugh &
Mendel, 5 M 4 months depressed body weight gain Nelson (1948)
Rat, Carworth, AS, C12-C15 Diet 9, 0.04, 0.02, 0.1, Increased liver weight in Walker et al.
24 (a.i. 86%) 0.5%, 13 weeks females at highest dose (1967)
Rat, Wistar, AS Drinking- 0, 0.25, 0.5, 1.0, Renal changes; proteinuria; Smyth et al.
5, 10 water 2.0, 4.0%, 30 days depressed body weight gain at 4% (1941)
sodium 2-ethylhexanol sulfate
Rat, Wistar AS (a.i.22.5%) Dermal 5 mg/kg per day, Dermal irritation; hepatic Sakashita et
15 M 30 days effects al. (1974)
Table 39 (contd)
Species, strain, Material Route Dosage Results Reference
numbers per group
Rat, Wistar AS (a.i. 22.5%) Dermal 5 mg/kg per day, Hepatic degeneration Sakashita
(NS) M 30 days (1979)
Rabbit AS, sodium lauryl Dermal 6, 60, 150 mg/kg, Dermal irritation Carson & Oser
3 M, 3 F sulfate 5 times/week, (1964)
3 months
a.i., active ingredient; M, male; F, female; NS, not specified
Groups of five male Wistar SPF rats were fed a diet containing
sodium dodecyl sulfate at a concentration of 1.5% (equivalent to
750 mg/kg of diet per day) for 2, 4, or 12 weeks, and were compared
with a control group. Body weight gain was suppressed and relative
liver weight significantly increased from two weeks. Biochemical
analysis of serum revealed increased activities of alkaline
phosphatase and glutamate-pyruvate transaminase and a decreased level
of cholesterol. Enzymatic examinations of the liver showed decreased
activity of glucose-6-phosphatase at 12 weeks, decreased activity of
glucose-6-phosphate dehydrogenase and increased activity of lactate
dehydrogenase at each observation time, and increased isocitrate
dehydrogenase activity at 4 and 12 weeks. Examination of the renal
cortex showed decreased activities of 5'-nucleotidase and Mg-ATPase at
12 weeks and increased isocitrate dehydrogenase activity at 4 and 12
weeks. Examination of the renal medulla showed decrease activities of
Mg- and Na,K-ATPases and increased isocitrate dehydrogenase activity
at 12 weeks (Ikawa et al., 1978).
Groups of five male Osborne-Mendel rats were given diets
containing sodium lauryl sulfate at a concentration of 0, 2, 4, or 8%
(equivalent to 1000, 2000, or 4000 mg/kg of diet per day) for four
months. Significant inhibition of growth was observed with 4%; severe
diarrhoea and marked abdominal bloating were noted at 8%, and all the
rats died within two weeks. Autopsy revealed irritation of the
gastrointestinal tract in rats fed 8% (Fitzhugh & Nelson, 1948).
Technical-grade sodium lauryl sulfate (86% w/w active ingredient;
chain length distribution, C12-C15) was fed to four groups of 12
male and 12 female Carworth Farm 'E' rats at a dietary level of 0, 40,
200, 1000, or 5000 ppm (corresponding to 2, 10, 50, or 250 mg/kg bw
per day) for 13 weeks. No abnormalities were observed in behaviour,
body weight, food intake, haematological parameters, urinary pH or
osmolality, serum urea or protein, or organ weights, except for a
significant increase in the absolute weight of the liver in females
fed 5000 ppm (Walker et al., 1967).
C7.2.1.2 Administration in the drinking-water
Groups of five or 10 male Wistar rats were given water containing
sodium 2-ethylhexanol sulfate, sodium 7-ethyl-2-methyl undecanol-4
sulfate, or sodium 3,9- diethyl tridecanol-6 sulfate at a
concentration of 0, 0.25, 0.5, 1, 2, or 4% for 30 days. Water intake
was decreased at concentrations > 2% of sodium 2-ethylhexanol
sulfate and sodium 7-ethyl-2-methyl undecanol-4 sulfate and at > 1%
sodium 3,9-diethyl tridecanol-6 sulfate. Body weight gain was
suppressed at 4% sodium 2-ethylhexanol sulfate. None of the rats died,
and no haematological abnormalities were observed during the
experiment. Proteinurea was seen at 2 and 4% sodium 2-ethylhexanol
sulfate. The major histopatho-logical findings were renal changes,
including light cloudy swelling and secretion in the renal tubules and
congestion or dilation of Bowman's capsule. The no-effect doses were
0.44 g/kg bw per day of sodium 2-ethylhexanol sulfate, 0.1 g/kg bw per
day of sodium 7-ethyl-2-methyl undecanol-4 sulfate, and 0.25 g/kg bw
per day of sodium 3,9- diethyl tridecanol-6 sulfate (Smyth et al.,
1941).
C7.2.1.3 Dermal application
A group of 15 male Wistar rats received 2 ml of a commercial
preparation of AS (22.5% active ingredient) on their backs, and the
livers of three rats were examined under the electron microscope three
and 30 days later; a control group was available. Redness of the skin
and wrinkles were observed in treated animals at 24 h; the redness
subsequently increased, the dermis became lacerated, and bleeding
occurred. These lesions reached a peak at 57 days but tended to
regress about 10 days later. Five rats died within the first 19 days.
Electron microscopy at three days revealed separation of the
intercellular space, cells with a high electron density, elongation of
mitochondria, swelling of the smooth-surfaced endoplasmic reticulum,
and a decreased prevalence of fatty droplets. Electron microscopy at
30 days showed liver parenchymal cells filled with mitochondria,
apparently abnormally divided and proliferated smooth-surfaced
endoplasmic reticulum, abnormally rough-surfaced cells, a typical
Golgi apparatus, myelin-like structures in bile canaliculi, and
extracellular prolapse of mitochondria (Sakashita et al., 1974).
Electron microscopy of the liver was also performed after dermal
application of a commercial preparation of AS (22.5% active
ingredient) to male Wistar rats (number not specified) at a dose of
5 mg/kg active ingredient once a day for 30 days. Hepatic
degeneration, seen as atrophy and a high density of liver cells, was
observed; in cells, there was deformation of nuclei, mitochondria, and
the Golgi apparatus, an increased number of lysosomes, and swelling of
endoplasmic reticula (Sakashita, 1979).
As no information was given on the method of application (occluded
or non-occluded), these results were not interpretable in terms of
risk to human health.
C7.2.2 Rabbit
Sodium lauryl sulfate was applied dermally to three groups,
consisting of two male and two female rabbits with intact skin and one
male and one female rabbit with abraded skin, at a dose of 6, 60, or
150 mg/kg bw five times per week for three months. A control group
consisted of one male and one female with intact skin and one male
with abraded skin. Dose-related irritation of the skin was observed in
all treated animals (Carson & Oser, 1964).
C7.3 Long-term exposure; carcinogenicity
C7.3.1 Mouse
In a study of the effects of AS on the carcinogenicity of
benzo[a]pyrene (BaP), a 10% AS solution, a 0.3% BaP solution, and a
10% AS:0.3% BaP solution were applied to the backs of groups of 10
male and 20 female mice twice a week for one year. Skin tumours
appeared in all mice treated with BaP or AS:BaP. The average ages at
the appearance of skin tumours were 119 days in the group exposed to
BaP and 102 days in that exposed to AS:BaP. It was concluded that AS
accelerated the induction of tumours by BaP ( p < 0.1). Untreated
mice and vehicle (acetone) controls had no skin tumours; one female
exposed to AS had a skin tumour, but this finding was not considered
to be related to treatment (Yamamoto, 1977).
C7.3.2 Rat
C7.3.2.1 Administration in the diet
Three groups of 12 weanling male Osborne-Mendel rats were given
food containing sodium lauryl sulfate at a concentration of 0.25, 0.5,
or 1.0% for two years; there was a similar sized control group. No
effects attributable to the test material were observed on growth,
mortality, or the macroscopic or histopathological appearance of
organs. No tumours were reported (Fitzhugh & Nelson, 1948). As there
were few animals per group and no toxic effects at any dose, the
observations are considered to be of limited value.
C7.3.2.2 Administration in the drinking-water
Groups of 4-11 white rats were given drinking-water containing
sodium lauryl sulfate at a concentration of 0, 0.1, 0.25, 0.5, 1, 5,
or 10% for 120 or 160 days. Dose-related increases in mortality
occurred at doses > 0.25%; at doses > 5%, all rats died.
Histological examination of rats exposed to doses > 0.25% revealed
marked inflammatory changes of the lumen of the oesophagus in those
that died, but the changes were slight in surviving animals. No
abnormalities were seen in the liver, kidney, or intestine. The intake
of the materials was about 30 mg/animal per day in those given 0.1%
and 150 mg/animal per day in those given 1.0% (Epstein et al., 1939).
Groups of 9 or 10 weanling male Wistar rats were given
drinking-water containing technical-grade sodium lauryl sulfate at a
concentration of 0, 0.05, or 0.25% for five months. Growth was not
suppressed, even at the higher concentration, and the activities of
serum enzymes, including glutamate-oxalate and glutamate-pyruvate
transaminases, alkaline phosphatase and cholinesterase, were not
affected. At 0.25%, the triglyceride level increased in the liver but
decreased in serum, while hepatic and serum levels of cholesterol,
phospholipids, and free fatty acids were unchanged. Increased weights
of spleen, lung, and kidney were noted at 0.25%. Histopathologically
diagnosed broncho-pneumonia, observed in all animals given 0.25% and
two animals given 0.05%, was considered to be a characteristic effect
of the test material (Fukazawa et al., 1978).
The results of long-term studies are shown in Table 40.
C7.4 Skin and eye irritation; sensitization
C7.4.1 Local irritation
C7.4.1.1 Skin
Groups of two to six white rats received a subcutaneous injection
of 1 ml of one of 10 solutions of sodium AS, ranging from 0.125 to 10%
and were observed for one week after the injection. No reactions
occurred at 0.125%, but sloughing and subcutaneous lumps in the skin
appeared in rats given doses > 0.19%. In a study in which the
diffusibility of trypan blue was used as an index of irritation,
groups of five to nine white rats were given subcutaneous injections
of 0.2 ml sodium AS at one of six concentrations ranging from 0.15 to
5%. Two hours after the injection, slight reactions were seen in
animals given 0.15% and marked reactions in those given 2.5 or 5%
(Epstein et al., 1939).
Groups of three albino rabbits received closed-patch applications
of 5 ml of 1, 5, or 25% sodium lauryl sulfate solution on intact and
abraded areas of shaven abdominal skin. Over a 14-day period, 10
applications were made to intact skin and three to abraded skin;
additionally, small amounts of the material were applied daily to the
intact ears of groups of three rabbits. Occluded application to the
abdomen produced erythema and blistering, which was more severe on
abraded skin. Application to the intact ear resulted in very slight
erythema at the 1% concentration, very slight to slight erythema at
5%, and slight erythema with moderate to severe burns at 25% (Olson et
al., 1962).
Sodium alcohol (coconut alcohol, mainly C12) sulfate solutions
of 0.1, 1.0, and 2.5% were applied in occluded tests in rabbits as
1 ml of each solution on the back three times on three days.
Macroscopic and histological examination seven days after application
revealed no abnormalities at 1.0% and moderate irritation at 2.5%. In
open tests, 1 ml of each of the solutions was applied to the backs of
rabbits and 0.5 ml to the backs of guinea-pigs five times a week for
4.5 weeks. No abnormal findings were seen in animals receiving 0.1 or
1.0% groups, but there was moderate irritation at 2.5% (Brown & Muir,
1970).
Table 40. Results of long-term exposure of experimental animals to alkyl sulfates (AS)
Species, strain, Material Route Dosage Results Reference
numbers per group
Mouse, ddy/SLC 10% AS, 3% Dermal Twice per Skin tumours Yamamoto (1977)
10 M, 20 F benzo[ a]pyrene week, 1 year
Rat, Osborne-Mendel 1.0%AS, C12 Diet 0, 0.25, 0.5, 1.0%, No effects Fitzhugh & Nelson
10-12 M 2 years (1948)
Rat, Wistar, 9-10 AS, C12 Diet 0, 0.05, 0.25%, Increased weights of Fukuzawa et al.
5 months spleen, lung, and liver (1978)
at highest dose
Rat, 4-11 AS, C12 Diet 0, 0.1, 0.25, 0.5 Oesophageal irritation Epstein et al.
1.0, 5.0%, 160 days (1939)
Groups of three male Wistar rats received applications of 0.5 g of
a 20 or 30% solution of linear lauryl sulfate (C12; purity, 98.91%)
on the back once a day for 15 days. The skin at the application site
and the tissues of the tongue and oral mucosa (to determine the
effects of licking) of animals receiving the 30% solution were
examined histologically 16 days after application. Body weight gain
was inhibited in the group given the 20% solution; body weight was
decreased in the group at 30%, and two rats had died by the end of the
experiment. A dry, thick, yellowish-white or reddish-brown crust was
observed after two to three days in animals given 20% and after one to
two days in those given 30%. When the crust was abraded several days
later, ulcers occurred at the abraded site, which remained unchanged
for 16 days in animals at 20% group and were aggravated in those at
30%. Histological examination of the application site revealed severe
necrosis extending from the epidermis to the upper layer of the
dermis, dense inflammatory-cell infiltration into the upper layer of
the dermis just below the necrotic area, diffuse inflammatory-cell
infiltration throughout the dermis, swelling of collagenous fibres in
the dermis, and sloughing. Histological examination of the tongue
revealed necrosis extending from the surface to the middle epithelial
layer of the mucosa, inflammatory-cell infiltration into the upper
layer of the dermis, and sloughing. Histological examination of the
mucosa of the oral cavity revealed thickening of the stratum corneum
and germinative and slight degeneration (pale staining) of epithelial
cells (Sadai & Mizuno, 1972).
The effects of sodium lauryl sulfate on oesophageal and gastric
mucosa were studied in cats by irrigation and pledget techniques. In
the irrigiation technique, the stomachs and oesophaguses of two cats
were filled with 10 and 20% solutions of sodium lauryl sulfate,
respectively, for 15 min, and then tissues were taken for histological
examination. Pledgets soaked in 10 or 20% sodium lauryl sulfate
solution were applied to the exposed oesophageal and gastric mucosa of
two other cats for 10 min, and specimens were taken 90 min later. The
10% solution produced moderate injury to the oesophagus, consisting of
intramucosal oedema and congestion and loss of superficial epithelial
layers; in the stomach, there was hydropic degeneration, loss of
surface mucosal cells, vascular congestion with submucosal oedema, and
occasional focal ulceration. Treatment with the 20% solution resulted
in more extensive damage, and particularly extensive submucosal oedema
and disruption and erosion of the superficial mucosa of both the
oesophagus and stomach (Berensen & Temple, 1976).
C7.4.1.2 Eye
Three drops of one of nine solutions of sodium lauryl sulfate
ranging from 0.019 to 5.0% were instilled into the eyes of rabbits
three times at 10-min intervals, and the rabbits were observed for
48 h. There were no abnormal findings at 0.038%, but slight chemosis
and redness were seen at 0.075% and marked chemosis and redness at 5%
(Epstein et al., 1939).
The minimal concentration of sodium lauryl sulfate that caused
corneal necrosis (detected by fluorescein staining) after instillation
into the eyes of rabbits was 0.1% (Smyth et al., 1941). In another
study, two drops of a 1, 5, or 25% solution of sodium lauryl sulfate
were instilled into both sides of the eyes of groups of three rabbits;
30 min later, one of the eyes was washed. Moderate corneal injury was
observed in unwashed eyes of animals receiving the 5 or 25% solution;
in washed eyes, either slight conjunctivitis or moderate corneal
injury was observed at 25%, slight conjunctivitis at 5%, and only very
slight conjunctivitis at 1% (Olson et al., 1962).
In an irritation test based on a method developed by the United
States Food and Drug Administration, 0.1, 1, or 25% solutions of
sodium coconut alcohol sulfate were instilled into the eyes of
rabbits. No reaction was seen at 0.1%; mild conjunctivitis lasting for
48 h was seen at 1%, and severe conjunctivitis lasting for 72 h was
observed at 25% group, but there was no permanent damage (Brown &
Muir, 1970). Solutions of a synthetic alkyl sulfate and five AS
consisting mainly of C10, C12, C14, C16, or C18, were instilled
at concentrations of 0.01-5% into the eyes of three rabbits, which
were observed for 168 h. The materials caused similar reactions. No
abnormalities were seen at 0.01%. Slight congestion and marked
congestion or oedema were observed at 0.05 and 0.1% within 2 h, but
these effects had disappeared 24 h later. In the groups given >
0.5%, marked reactions were seen for 24 h, including severe congestion
and oedema, increased lachrymal secretion, turbidity of the cornea,
and disappearance of the corneal reflex, but these tended to regress
and had disappeared completely by 120 h (Iimori et al., 1972).
C7.4.2 Skin sensitization
A 0.1% solution of a sodium lauryl sulfate derivative of coconut
alcohol was applied to the skin or injected intradermally into groups
of 10 guinea-pigs three times per week for three weeks. Ten days later
the animals received challenge doses and were observed for 48 h. No
reaction occurred in the group treated dermally, but a slight reaction
was observed 24 h after the challenge in some of the guinea-pigs
treated intradermally (Brown & Muir, 1970).
C7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Daily doses of 0.2, 2, 300, or 600 mg/kg bw of AS were
administered by gavage to CD rats, CD-1 mice, and NZW rabbits. Groups
of 20 rats and mice were given AS on days 6-15 of pregnancy, and
groups of 13 rabbits were treated on days 6-18 of pregnancy. The doses
of 0.2 and 2 mg/kg bw per day were estimated to be equivalent to 1-2
and 10-20 times the maximal amount of AS to which humans are exposed.
Three rats given 600 mg/kg bw died during the study, but the surviving
rats and those given 300 mg/kg bw had only mild to moderate inhibition
of body weight gain. Mice given 600 mg/kg bw showed severe effects,
including anorexia and inhibition of body weight gain, and four
animals died during the study; in those given 300 mg/kg bw, inhibition
of body weight gain was mild to moderate. Rabbits given 600 mg/kg bw
showed severe effects, including diarrhoea, anorexia, and reduced rate
of body weight gain, and 11 died during the study; those given
300 mg/kg bw showed mild to moderate reduction of body weight gain. No
toxic effects were seen in any of the animals given 0.2 or 2 mg/kg bw.
No adverse effects were seen on litters of rats at any dose. Some mice
and rabbits at each dose had total litter loss, but the other litter
parameters did not differ from those of controls. No major
malformations were seen at any dose in offspring of rats, mice, or
rabbits, and the incidence of skeletal variations in offspring of rats
given 600 mg/kg bw was significantly low. A high incidence of skeletal
anomalies was seen in litters of mice given 600 mg/kg bw, and those of
rabbits at 2.0 mg/kg bw had a significantly higher incidence of
skeletal variations; however, the incidences of anomalies and
variations were within the background range (Palmer et al., 1975a).
Groups of 21 ICR mice received applications of 15 mg/kg bw per day
of a 0.4, 4, or 6% aqueous solution of AS (98% sodium dodecyl sulfate,
0.5% N2SO4, 0.1% NaCl, and 0.1% H2O) to a 3 × 3-cm2 area of
shaven dorsal skin on days 6-13 of pregnancy. The 0.4% solution was
equivalent to about 10-12 times the specified concentration used by
humans, and the application area was equivalent to about one-seventh
of the total surface area of the mouse. The body weight gain of dams
exposed to the 4 or 6% solution was reduced; there were no deaths. The
numbers of dams with surviving young were 19/21 in the control group,
20/20 at 0.4%, 17/20 at 4%, and 11/21 at 6%; the decrease in dams at
6% was significant. Fetal weights were significantly lower in dams at
4 and 6%, but there were no other differences from the control values.
The incidence of cleft palate was fairly high in offspring of dams
exposed to the 4 or 6% solution, and a tendency to delayed
ossification was seen; however, none was significant (Takahashi et
al., 1976).
A dose of 0.1 ml/day of a 2% aqueous solution of AS was applied to
a 2 × 3-cm2 area of shaven dorsal skin in groups of 20-26 ICR mice
on days 1-17 of pregnancy. The same dose of a 20% solution was applied
to a similar group up to the 10th day of pregnancy, and implantation
was examined on the 11th day. In addition, 14 mice were injected
subcutaneously with 2 mg/kg bw per day of AS on days 8-10 of
pregnancy. The numbers of dams with implantations were 18/20 controls,
14/22 at 2%, 1/26 at 20%, and 13/14 at 2 mg/kg bw; the decrease at 20%
was significant. There were no significant changes in litter
parameters and no significant changes in the incidences of major
malformations, minor anomalies, or skeletal variations. AS thus
disturbed implantation and caused abortion at maternally toxic doses,
but in surviving litters it had no effect on the size or numbers of
fetuses, although low fetal weight and delayed ossification were
observed. At doses that had no or only mild effects on the dams, no
adverse effects were seen on the fetuses. The effects of AS on the
fetus therefore appear to be secondary to the toxic effects on the
dams (Nomura et al., 1980).
C7.6 Mutagenicity and related end-points
Sodium lauryl sulfate did not cause differential toxicity in
Bacillus subtilis H17 ( rec+) or M45 ( rec-) at concentrations
of 20-2000 µg/plate, and it did not induce reverse mutations in
Salmonella typhimurium TA98 or TA100 at 1-500 µg/plate or in
Escherichia coli WP2 trp at 10-1000 µg per plate (Inoue &
Sunakawa, 1979).
Sodium lauryl sulfate, Dobanol 25 sulfate LCU, and Dobanol 25
sulfate HCB (aliphatic alcohol sulfates with chain lengths of
C10-C15) were fed in the diet to groups of six male and six female
Colworth/Wistar rats for 90 days at a concentration of 0.56 or 1.13%,
the latter being the maximal tolerated dose. No effect was seen on
chromosomes in bone-marrow cells (Hope, 1977).
After dodecyl sulfate was administered to male ddY mice
intra-peritoneally at 50 mg/kg bw, the incidence of polychromatic
erythrocytes with micronuclei in the bone marrow was similar in
treated and control groups (Kishi et al., 1984).
C7.7 Special studies
Intravenous injection of 1 mg/min sodium decyl sulfate or
5.7 mg/min sodium dodecyl sulfate to cats increased pulmonary arterial
pressure, caused a small increase in systemic vascular resistance, and
reduced the ventilation volume per minute after about 5 min.
Intravenous injection of 4.6 mg/min sodium octyl sulfate or 6.3 mg/min
sodium tetradecyl sulfate had similar effects. The increase in
pulmonary arterial pressure was considered to be due to a direct
effect on the smooth muscle of blood vessels and bronchi. The blood
sugar level was unchanged (Schumacher et al., 1972).
The effects of sodium lauryl sulfate on histamine release from
mast cells were studied in vitro in peritoneal mast cells isolated
from rats. Histamine was released at a concentration of
0.03 mmol/litre, and the critical micelle concentration in buffer at
22°C was 1.0 mmol/litre. Sodium lauryl sulfate and its mono- and
tri-ethoxy derivatives had the most potent histamine releasing
capacity of nine surfactants with a chain length of C12 (Prottey &
Ferguson, 1975).
C8. EFFECTS ON HUMANS
Section summary
In patch tests, human skin can tolerate contact with solutions
containing up to 1% AS for 24 h with only mild irritation. AS caused
delipidation of the skin surface, elution of natural moisturizing
factor, denaturation of the proteins of the outer epidermal layer, and
increased permeability and swelling of the outer layer. They did not
induce skin sensitization in volunteers, and there is no evidence that
they induce eczema. No lasting injuries or fatalities have been
reported following accidental ingestion of detergent formulations
containing AS.
C8.1 Exposure of the general population
Surface-active agents are found in shampoos, dishwashing products,
household cleaners, and laundry detergents, and AS are major
components of these products. The composition of nonionic and ionic
surfactants varies between 10 and 30%. Surface-active agents can
affect human skin and eyes.
C8.2 Clinical studies
C8.2.1 Skin irritation and sensitization
AS can be mildly to moderately irritating to human skin. No data
were available on sensitization.
The relative intensity of skin erythema produced on the lower back
of volunteers was evaluated by applying concentrations of 0.2-5.4% of
C8, C10, C12, C14, or C16 AS under a closed patch for 24 h
or under a closed patch re-applied once daily for 10 days. C12 AS
were more potent than AS with other alkyl chain lengths (Kligman &
Wooding, 1967).
A circulation method was used to evaluate the relative intensity
of skin roughness induced on the surface of the forearms of volunteers
after application for 1 min of 1% aqueous solutions of AS with an
alkyl chain length of C8, C10, C12, or C14. The potential to
cause skin roughness increased with alkyl chain length, reaching
maximal intensity at C12 (Imokawa et al., 1974, 1975a). In other
studies, the relative degree of skin roughening was correlated with
the extent of protein denaturation but not with irritating potential
determined in a closed-patch test (Imokawa et al., 1975b).
Primary skin irritation induced by a 1% aqueous solution (pH 6.8)
of dodecyl sulfate (relative molecular mass, 288.5) was studied in a
24-h closed-patch test on the forearms of seven male volunteers. The
relative intensity of skin irritation was scored by grading erythema,
fissuring, and scaling. The average score for AS was 4.86, whereas
that for a water control was 1.79. Dodecyl sulfate was more irritating
than either LAS or AOS (Oba et al., 1968a).
The intensity of skin irritation produced by a 1% aqueous
solution of sodium AS was studied in a 24-h closed-patch test on the
forearm and in a 40-min drip test on the interdigital surface in which
the compound was dripped once daily for two consecutive days at a rate
of 1.2-1.5 ml/min. Skin reactions were scored by grading erythema in
the patch test and by grading scaling in the drip test. The average
scores were 2.5 for primary skin irritation at 24 h in the patch test
and 1 for scaling at two days in the drip test; in both tests, the
control value was 0. AS was more irritating than LAS or AOS in the
patch test, whereas the score of AS for skin scaling in the drip test
was similar to that of LAS but higher than that of AOS (Sadai et al.,
1979).
Moderate to intense erythema was produced on the forearms of
10 volunteers in a 24-h closed-patch test by a 10% aqueous solution
of AS with an average chain length of C12. The mean irritation
scores were significantly higher at 26 h (2.85 out of 8 possible
points) and at 28 h (2.88) than at 24 h (2.00), when the patches were
removed. Irritation had decreased by 48 h, and a significant decrease
in the intensity of inflammation was apparent at 96 h (Dahl & Trancik,
1977).
In a 48-h patch test on the upper arms of 100 pairs of twins
(54 monozygotic, 46 dizygotic) with a solution of 0.5% C12 AS, no
reaction was seen in 50% of the subjects, and slight reaction, ranging
from noninflammatory changes to mild erythema, in the other 50%. The
response was not related to the type of twin (Holst & Moller, 1975).
Application of aqueous 0.5, 1, or 2% solutions of AS with an
average chain length of C12 to the backs of healthy male volunteers
produced epidermal hyperplasia. Treatment with the 1% solution induced
an approximately 30-fold increase in mitotic activity, which peaked 48
h after treatment. Application of either the 0.5 or the 2% solution
induced similar but milder changes (Fisher & Maibach, 1975).
Skin permeability to C8, C10, C12, C14, C16, and C18
AS prepared as 0.02, 0.5, and 1% solutions (0.58% C8 and 0.74%
C18) was studied by a circulation method on the forearms of healthy
male and female volunteers. C12 AS attained maximum permeation,
whereas the permeation of C8 and C18 AS was of the same order as
that of water. The authors pointed out the close relationship between
permeation and irritation (Szakall & Schulz, 1960).
C8.2.2 Effects on the epidermis
The effects of AS on the stratum corneum include delipidation of
the skin surface, elution of natural moisturizing factor, denaturation
of protein of the stratum corneum, increased permeability, swelling of
the stratum corneum, and inhibition of enzyme activities in the
epidermis. These effects, and some others, constitute a potential
hazard to the epidermis.
The water-holding capacity of thin sheets of callus isolated from
the plantar surface of the human foot, with relative moisture contents
of 76, 88, and 97%, was compared before and after immersion in water,
AS, or soap solution. Water-holding capacity was measured as the
weight of water taken up from each solution. The relative moisture
content decreased after treatment with AS or soap solution (Blank &
Shappirio, 1955).
Elution of natural moisturizing factor was compared for nine kinds
of surfactants, including AS, in the arm immersion test, in patch
tests, and by measuring eluted amino acids and protein, skin
permeation, and freeing of sulfhydryl groups. AS induced a strong
reaction in the immersion test and relatively strong reactions in the
other tests. The author concluded that the immersion test was the best
simulation of actual use (Polano, 1968).
A detergent consisting of long-chain AS was shown to denature
stratum corneum protein and thus expose enclosed sulfhydryl groups
(Anson, 1941). AS readily released sulfhydryl groups from stratum
corneum obtained from abdominal skin taken at autopsy within 12 h of
death, but there was no correlation between changes in epidermal
permeability and the amounts of sulfhydryl released (Wood & Bettley,
1971). AS were the most effective surfactants with regard to
denaturation of protein, measured as inhibition of invertase activity
(Imokawa et al., 1974; Okamoto, 1974). AS were found to denature skin
keratin (a filamentous protein), bovine serum albumin (a globular
protein), acid phosphatase (an enzyme protein), and membrane lysozymes
(membrane protein) (Imokawa & Katsumi, 1976). Sodium laurate was
reported to produce swelling of the stratum corneum (Putterman et al.,
1977).
AS with a hydrophobic chain length of C12 were maximally
absorbed on human callus. Extraction of proteins from human callus was
also a function of chain length: C12 and C14 AS were much more
active than C8, C10, and C18 AS (Dominguez et al., 1977).
C8.2.3 Hand eczema
In a 24-h closed-patch test of 0.2-0.5% aqueous solutions of AS on
the fingers of nine women with hand eczema, skin lesions were not
exacerbated, although four women felt slight itching at the patch site
(Sasagawa, 1963).
C8.2.4 Accidental or suicidal ingestion
Four members of a family accidentally ingested unknown quantities
of a household detergent containing 24% lauryl sulfate, 60% sodium
tripolyphosphate, and 16% anhydrous soap. Shortly after ingestion, all
of the family members experienced abdominal pain and nausea. The
10-year-old daughter and 13-year-old son felt oropharyngeal pain, and
the son was found at endoscopic examination to have a 2.5 × 2 cm
oropharyngeal burn in the right posterior pharynx and first-degree
burns of the oesophagus. The mother had erythema, friability, erythema
and a few superficial erosions of the distal oesophagus, and gastritis
evidenced by exudate and petechial lesions on the mucosa. The father
had haematemesis on a few occasions. The mother, father, and son were
examined about one month after the incident by an X ray examination
after a barium meal; no strictures were found (Berenson & Temple,
1974).
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND THE FIELD
Section summary
AS have been studied in short- and long-term studies in the
laboratory and in one study carried out under more realistic
conditions. Their toxicity is dependent on alkyl chain length, but no
data were available on the differential toxicity of linear and
branched AS.
In aquatic organisms, the EC50 values for C12 AS in a
community of marine microorganisms were 2.1-4.1 mg/litre. The NOEC
values were 35-550 mg/litre (C16/C18) for Pseudomonas putida and
14-26 mg/litre (C12-C16/C18) for green algae; and the EC50
values were > 20-65 mg/litre (C12-C13) for green algae and 18-43
mg/litre (C12) for macrophytes.
In aquatic invertebrates, the L(E)C50 values were 4-140 mg/litre
(C12/C15-C16/C18) for freshwater species and 1.7-56 mg/litre
(all C12) for marine species. The long-term NOECs were 16.5 mg/litre
(C16-C18) for Daphnia magna and 0.29-0.73 mg/litre (chain length
not specified) for marine species.
In fish, the LC50 values were 0.5-5.1 mg/litre (C12-C16 or chain
length not specified) for freshwater species and 6.4-16 mg/litre
(all C12) for marine species. In a 48-h study of Oryzias latipes,
chain length influenced LC50 values, the measured concentrations being
46 mg/litre for C12, 2.5 mg/litre for C14, and 0.61 mg/litre
for C16. This and other studies indicate that toxicity differs by a
factor of five for two units of chain length.
In a flow-through study of the effect of C16-C18 AS on a
biocenosis, an NOEC of 0.55 mg/litre was observed. Many of the studies
of toxicity in aquatic environments were carried out under static
conditions. As AS are readily biodegraded, this design may result in
underestimates of toxicity.
Few data were available on the effects of AS on terrestrial
organisms. An NOEC of > 1000 mg/kg (C16-C18) was reported for
earthworms and turnips.
C9.1 Microorganisms
During tests of biodegradation, marine bacteria used 20 mg/litre
AS as a nutrient source. It was therefore concluded that its toxicity
for the bacterial community studied is nil or very low (Vives-Rego et
al., 1987). In a study of the effect of C12 AS on the metabolic
activity of a marine microbial community, the EC50 values for toxic
effects on thymidine incorporation and glucose metabolism were
reported to be 4.1 and 2.1 mg/litre, respectively. AS also increased
exoproteolytic activity (Vives-Rego et al., 1986).
The 30-min EC50 for C16-C18 AS, based on oxygen consumption,
was 35 mg/litre in Pseudomonas putida (Robra, 1976). The NOEC for
cell reproduction in Pseudomonas putida exposed to C16-C18 AS
was 550 mg/litre (Bringmann & Kühn, 1977).
C9.2 Aquatic organisms
C9.2.1 Aquatic plants
C9.2.1.1 Freshwater algae
The phytoflagellate alga Poterioochromonas malhamensis was
exposed to C12 AS at sublethal concentrations of 28.8, 57.6, 72,
86.4, 100.8, and 115.2 mg/litre (100, 200, 250, 300, 350, and
400 µmol/litre), being transferred every three to four days into fresh
medium with a higher test concentration. The initial cell density in
each medium was 0.1 × 106 cells/ml; the final cell density, after
exposure to the highest concentration of AS, was 0.05 × 106 cells/ml,
which was similar to that reached after exposure of unacclimatized
algal cultures to 200 µmol/litre AS. Exposure to AS at 57.6 mg/litre
(240 µmol/litre) was reported to affect mitosis and cytokinesis, with
the formation of cells containing up to 12 nuclei. Exposure of the
alga to 50.4 mg/litre (175 µmol/litre) AS resulted in a 24% increase
in telophases (binucleated cells). Cells with eight nuclei were also
reported in this culture (Röderer, 1987).
The green alga Selenastrum capricornutum was exposed to analytical
grade C12 AS at a concentration of 10, 20, 30, 40, 50, or
100 mg/litre in synthetic medium for three weeks. Growth was reduced
by 30% at the lowest concentration (Nyberg, 1988).
The green alga Chlamydomonas reinhardi was exposed to 0.02, 0.2,
or 2.0 mmol/litre of C10, C12, C14, C16, or C18 AS for 7-10
days. Photometric absorption (652 nm) by the exposed cultures was no
different from that by controls for the first six days of exposure,
although it was reduced slightly at 2 mmol/litre. The authors
concluded that the AS were present at below the critical micelle
concentration at all concentrations tested (Ernst et al., 1983).
The EC50 for growth of the green alga Selenastrum capricornutum
exposed to C12 AS for two to three days was within the range 45-65
mg/litre (Yamane et al., 1984). An EC50 of 9 mg/litre C14 AS was
found for growth of S. capricornutum (Konno & Wakabayashi, 1987).
C9.2.1.2 Macrophytes
The seven-day EC50 values for C12 AS in the duckweed Lemna
minor under flow-through conditions were 43 mg/litre for frond count,
29 mg/litre for dry weight, and 18 mg/litre for root length.
The time-independent EC50 for growth rate/doubling time was
44 mg/litre (Bishop & Perry, 1981).
C9.2.2 Aquatic invertebrates
The acute toxicity of AS to aquatic invertebrates is summarized in
Table 41. The 48-h LC50 values were 8-60 mg/litre for daphnids; the
96-h LC50 values ranged from 3.2 to 4.2 mg/litre for marine
invertebrates.
The 48-h LC50 for lugworms (Arenicola marina) exposed to AS
was calculated to be 15.2 mg/litre (95% confidence interval,
13.2-17.6). Tissues from lugworms exposed to AS at a concentration
close to that of the LC50 were examined for changes in morphology by
both light and electron microscopy: serious damage was found in the
epidermic receptors and less serious damage in the caudal epidermis
and gills. No morphological effects were reported on the thoracic
epidermis or intestine. AS caused separation inside the caudal
epithelial layer, resulting in holes in some caudal papillae.
Deciliation of the epidermic receptors was also reported. The authors
concluded that the physiological response of damaged epidermic
receptors was reduced or blocked after exposure to AS. AS also induced
fissures in the epithelial layer of the gills (Conti, 1987).
Caeriodaphnia dubia were exposed to C12 AS for three
generations under static renewal conditions, with the following mean
water parameters: temperature, 26.2°C; pH, 8.2; hardness,
94.4 mg/litre CaCO3; and alkalinity, 82.2 mg/litre CaCO3. The
water was changed every second day. The LC50 for survival of three
broods of C. dubia was calculated to be 41 ± 3.2 mg/litre. The mean
EC50, based on progeny produced, was calculated to be 36 ± 3.2
mg/litre. No statistically significant effects were reported after
exposure to 83 mg/litre AS, although the size of later broods was
reduced (Cowgill et al., 1990).
The effect of 0.25-10 mg/litre AS was studied on the growth and
survival of eggs and larvae of oysters (Crassostrea virginica) and
clams (Mercenaria mercenaria). The minimal concentrations that
caused a significant reduction in the number of fertilized eggs which
developed into normal larvae two days after hatching were
0.73 mg/litre for clams and 0.29 mg/litre for oysters. The minimal
concentration that caused a significant reduction in growth and
survival between two and 12 or 14 days after hatching was
1.46 mg/litre for both species. The EC50 values, based on the
development of fertilized clam and oyster eggs to normal
straight-hinge larvae after 48 h, were calculated to be
0.47 mg/litre for clams and 0.37 mg/litre for oysters (Hidu, 1965).
After snails (Lymnaea peregra) were exposed to C12 AS at
measured concentrations of 0.6-12 mg/litre for six days, a
significant, dose-related reduction in the dry weight of shells was
observed, but the organic content of shells was not significantly
affected at any concentration (Tarazona & Nunez, 1987).
C9.2.3 Fish
The acute toxicity of AS to fish is also summarized in Table 41.
The 48-h LC50 values were 0.5-51 mg/litre for medaka (Oryzias
latipes). A 96-h LC50 value of 1.7 mg/litre was reported for both
rainbow trout (Salmo gairdneri) and sheepshead minnow (Cyprinodon
variegatus). The acute toxicity of AS to fish tends to increase with
increasing carbon-chain length.
Rainbow trout (Oncorhynchus mykiss) and goldfish (Carrasius
auratus) were exposed to C12 AS at a concentration of 70 mg/litre
at different levels of water hardness. Trout treated in hard water
(300 mg/litre CaCO3) died within 40-45 min; those treated in soft
water (60 mg/litre CaCO3) died after 3 h. Goldfish treated in hard
water died within 90-110 min, whereas those treated in distilled water
(no CaCO3) were alive and apparently normal after 24 h (Tovell et
al., 1974). When yearling rainbow trout were maintained in water
containing C12 AS at a concentration of 100 mg/litre, the time to
50% lethality was calculated to be 4.9 h. The changes seen in the
gills were typical of an acute inflammatory reaction: The gill
epithelium was lifted away from the underlying tissue, and lymphocytes
and granulocytes invaded the subepithelial spaces. Large numbers of
epithelial cells died, but the epithelium was not punctured (Abel &
Skidmore, 1975).
After exposure of the eggs of carp (Cyprinus carpio) to AS of
various chain lengths from spawning to hatching, the LC50 values
were calculated to be 18 mg/litre for C12 AS, 2.9 mg/litre for C14
AS, and > 1.6 mg/litre for C16 AS (Kikuchi et al., 1976).
The minimal avoidance concentration of AS, i.e. the concentration
at which fish spend 65% of a 5-min period in clean water in order to
avoid AS, was 7.1 µg/litre for medakas (Oryzias latipes) (Hidaka et
al., 1984). The threshold concentrations for avoidance of AS by ayu
(Plecoglossus altivelis) were 4.0 µg/litre of a formulation and 8.4
µg/litre of pure reagant AS (Tatsukawa & Hidaka, 1978). The
environmental relevance of avoidance studies is questionable (see also
section A9.3.3.4 of the monograph on LAS).
Larvae of the fathead minnow (Pimephales promelas) were exposed to
C12 AS at a concentration of 1.2, 2.3, 4.6, 9.2, or 18.4 mg/litre
for seven days under static renewal conditions. Survival and final dry
weight were not significantly affected at concentrations up to and
including 4.6 mg/litre; however, at 9.2 and 18.4 mg/litre, no fish
Table 41. Toxicity of alkyl sulfates (AS) to aquatic organisms
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Reference
age flow (°C) or salinity length (mg/litre)
Eastern oyster Embryo Static 20 25a C12 48-h LC50 1.7b Mayer (1987)
(Crassostrea virginica)
Mysid shrimp Juvenile Static 25 30a C12 96-h LC50 3.2b
(Metamysidopsis swifti)
Mysid shrimp Juvenile Static 25 20a C12 96-h LC50 4.2b
(Mysidopsis bahia) Adult Static 22 C12 96-h LC50 6.62 Roberts et al.
(1982)
Shrimp Adult Static 22 20.0 ± 0.5a C12 96-h LC50 7.24
(Neomysis americana)
Copepod Adult Static 10a C12 96-h LC50 2.6
(Eurytemora affinis)
(Acartia tonsa) Adult Static C12 96-h LC50 0.55
Scud NS 72-h LC50 9-46 Gilbert &
(Gammarus pulex) Pettigrew
(1984)
Water flea Static 20 C12 24-h EC50 17.4 Snell &
(Daphnia magna) Persoone
(1989)
Static 20 C12 24-h EC50 27.5 Persoone et
al. (1989)
C16-C18 24-h EC50 27.5 Steber et al.
(1988)
C12 24-h EC50 10.5-24.3 Cowgill et
al. (1990)
Table 41 (contd)
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Reference
age flow (°C) or salinity length (mg/litre)
Water flea C12 24-h LC50 15.0 Snell &
(Daphnia pulex) Persoone
(1989)
C12 24-h LC50 9.5-20.5 Cowgill et
al. (1990)
Mosquito 2nd/3rd Static 25 C12-C15 24-h LC50 4 van Emden et
(Aedes aegypti) stage al. (1974)
Rainbow trout Flow 15 350-375c 8.3-8.5 NS 96-h LC50 4.62 Fogels &
(Salmo gairdneri) Sprague (1977)
NS 96-h LC50 1.7 Gilbert &
Pettigrew
(1984)
Atlantic silverside 59 mm Static 22 10a C12 96-h LC50 6.4 Roberts et al.
(Menidia menidia) (1982)
Medaka (killifish) 48-h LC50 10 Tomiyama
(Oryzias latipes) (1974)
323 mg Staticr 23-24 5.6-5.8 C12 24-h LC50 70b Kikuchi et al.
323 mg Staticr 23-24 5.6-5.8 C12 48-h LC50 51b (1976)
323 mg Staticr 19-21 5.6-5.8 C14 24-h LC50 5.9b
323 mg Staticr 19-21 5.6-5.8 C16 24-h LC50 0.78b
323 mg Staticr 19-21 5.6-5.8 C12 48-h LC50 0.5b
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 46d Kikuchi &
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 2.5d Wakabayashi
approx. 262 mg Staticr 21-22 6.7-7.1 C12 48-h LC50 0.61d (1984)
Table 41 (contd)
Species Size or Static or Temp. Hardness pH AS chain End-point Concn Referenceage
flow (°C) or salinity length (mg/litre)
Sheepshead minnow Juvenile Static 25 20a C12 96-h LC50 1.7b Mayer (1987)
(Cyprinodon variegatus)
Fathead minnow NS Static NS 80-400 7.4-8.2 NS 96-h LC50 5-6 Henderson et
(Pimephales promelas) al. (1959)
< 30 d Static 20 C15 48-h LC50 7.8 Cowgill et al.
<30 d 17 C12 24-h LC50 7.7-9.7 (1990)
<30 d 17 C12 96-h LC50 7.0-9.0
30±2 d 20 C12 48-/96-h LC50 38.0
Carp 4.4 mg Static 22 25 7 C10 12-h LC50 180b Kikuchi et al.
(Cyprinus carpio) 4.4 mg Static 22 25 7 C10 48-h LC50 13b (1976)
4.4 mg Static 22 25 7 C12 12-h LC50 46b
4.4 mg Static 22 25 7 C14 48-h LC50 5.0b
4.4 mg Static 22 25 7 C16 12-h LC50 0.69b
4.4 mg Static 22 25 7 C16 48-h LC50 0.69b
Static, water unchanged for duration of test; NS, not specified; flow, flow-through conditions: AS concentration in water maintained
continuously; staticr, static renewal: water changed at regular intervals
a Salinity (%)
b Based on nominal concentrations
c Hardness expressed as mg/litre CaCO3
d Based on measured concentrations
survived. When the test was repeated over an eight-day period,
significantly reduced survival was seen at 4.6 mg/litre, but this
result was variable, as some replicates did not show significant
effects. The mean of the geometric means of the NOEC and LOEC values
for the embryo-larval test was 3.8 mg/litre; the mean LC50 value was
5.5 mg/litre (Pickering, 1988).
An LC50 value of 38 mg/litre was reported for fathead minnows
exposed to C12 AS for either 48 or 96 h. The authors suggested that
the same value was obtained because the tests were not carried out
aseptically and the C12 AS had degraded completely within 48 h
(Cowgill et al., 1990).
C9.2.4 Tests in biocenoses
In a flow-through biocenosis test, 13 species of aquatic organisms
were exposed to C16-C18AS. The species used represented several
trophic levels: seven species of algae, four species of protozoa, and
two species of rotifers. An NOEC of 0.55 mg/litre was reported for
'biocenotic toxicity'. The lowest concentration at which biocenotic
toxicity was reported was 1.65 mg/litre (Guhl, 1987).
C9.3 Terrestrial organisms
No information was available.
APPENDIX I
APPENDIX I.
Reference values for intakes and body weights of laboratory animals, with conversion factors for
deriving no-observed-adverse-effect levels (NOAELs) in milligrams per kilogram per day from
doses administered as parts per million
Species Body Inhalation Water Food Dose conversiona
weight (kg) rate consumption consumption
Air Water Food
(m3/day) (litres/ (g/day)
day)
Mouse 0.03b 0.04b 0.006b 4b 1.33 0.20 0.13
Rat 0.35b 0.11d 0.05b 18b 0.31 0.14 0.05
Hamster 0.14b 0.13b 0.03b 12b 0.93 0.21 0.09
Guinea-pig 0.84b 0.40b 0.20b 34b 0.48 0.24 0.04
Rabbit 3.8b 2.0b 0.41b 186b 0.53 0.11 0.05
Rhesus monkey 8.0b 5.4c 0.53b 320b 0.68 0.07 0.04
Dog 12b 4.3b 0.61b 300b 0.36 0.05 0.03
Cat 1.5d 0.75d 0.15e 168e 0.50 0.10 0.11
Pig 80e - 5.5e 2250e 0.07 0.03
From Health Canada (in press); most values have been rounded to two significant figures.
a Air: 1 mg/m3 in air = x in mg/kg bw per day; water: 1 ppm (mg/litre) = x in mg/kg bw per day;
food: 1 ppm in food = x in mg/kg bw per day
b From Calabrese & Kenyon (1991)
c Calculated from the minute volume of 220 ml/kg bw reported by Flecknell (1987)
d From Flecknell (1987); values are average of the ranges reported.
e From Canadian Council on Animal Care (1980-84); values are average of the ranges reported.
REFERENCES
Abbott DC (1962) The colorimetric determination of anionic
surface-active materials in water. Analyst, 87: 286-293.
Abdel-Shafy HI, Azzam AM, & El-Gamal IM (1988) Studies on the
degradation of synthetic detergents by sewage. Bull Environ Contam
Toxicol, 41: 310-316.
Abe S (1984) [Biodegradation of sodium alkylbenzenesulfonates by a
soil perfusion method.] Nippon Kagaku Kaishi, 9: 1465-1470 (in
Japanese).
Abe S & Seno M (1987) Biodegradation of sodium linear
alkylbenzenesulfonates evaluated with a soil perfusion method. J Am
Oil Chem Soc, 64: 148-152.
Abel PD & Skidmore JF (1975) Toxic effects of an anionic detergent on
the gills of rainbow trout. Water Res, 9: 759-765.
Acher AJ & Yaron B (1977) Behavior of anionic surfactants in a
soil-sewage effluent system. J Environ Qual, 6: 418-420.
Adachi A & Kobayashi T (1982) [Atomic absorption spectrophotometric
determination of anionic surfactants in water.] Eisei Kagaku, 28 (2):
99-105 (in Japanese).
Adachi T, Tanaka A, & Yamaha T (1979) [Metabolism of octadecyl sulfate
in rats.] Shokuhin Eiseigaku Zasshi, 20 (2): 120-126 (in Japanese).
Amano K & Fukushima T (1993) Partitioning of linear
alkylbenzenesulfonates in natural water and sediment. J Environ Sci
Health, 28: 683-696.
Amano K, Fukushima T, Inaba K, & Nakasugi O (1989) Adsorption of
linear alkylbenzenesulfonates with the suspended solids in natural
aquatic systems. Jpn J Water Pollut Res, 12: 506-515.
Amano K, Fukushima T, & Nakasugi O (1991) Fate of linear alkylbenzene
sulfonates in a lake estuary. Water Sci Technol, 23: 497-506.
Ambe Y (1973) Determination of alkylbenzene sulfonate (ABS) in bottom
sediment. Environ Sci Technol, 7: 542-545.
Ambe Y & Hanya T (1972) [Determination of alkylbenzene sulfonate in
polluted water by a combined method of methylene blue colorimetry with
infrared spectrometry.] Bunseki Kagaku, 21: 252-256 (in Japanese).
Anderson DJ, Day MJ, Russell NJ, & White GF (1990) Die-away kinetic
analysis of the capacity of epilithic and planktonic bacteria from
clean and polluted river water to biodegrade sodium dodecyl sulfate.
Appl Environ Microbiol, 56: 758-763.
Anson ML (1941) The sulfhydryl groups of egg albumin. J Gen Physiol,
24: 399-421.
Antal M (1972) Changes in blood glucose level induced by sodium
dodecylbenzene sulfonate (SDBS) in rats. Z Ernährwiss, 12 (2):
144-151.
Arima T, Takahashi K, Kawana T, Wakabayashi M, & Kikuchi M (1981)
Toxicity of detergent to aquatic organisms. Aquaculture, 29: 30-37.
Arthur JW (1970) Chronic effects of linear alkylate sulfonate
detergent on Gammarus pseudolimnaeus, Campeloma decisum and Physa
integra. Water Res, 4: 251-257.
Arthur D Little Inc. (1977) Human safety and environmental aspects of
major surfactants. A Report to the Soap and Detergent Association, New
York, 31 May 1977. Cambridge, Massachusetts, Arthur D. Little Inc.,
201 pp.
Arthur D Little Inc. (1981) Human safety and environmental aspects of
major surfactants. A Report to the Soap and Detergent Association, New
York, 20 February 1981. Cambridge, Massachusetts, Arthur D. Little
Inc., 291 pp.
Arthur D Little Inc. (1991) Environmental and human safety of major
surfactants: Vol. 1. Anionic surfactants: Part 1. Linear alkylbenzene
sulfonates. Final Report to the Soap and Detergent Association, New
York, February 1991. Cambridge, Massachusetts, Arthur D. Little Inc.,
244 pp.
Arthur D Little Inc. (1993) Environmental and human safety of major
surfactants: Vol. 1. Anionic surfactants: Part 4. Alpha olefin
sulfonates. Final Report to the Soap and Detergent Association, New
York, August 1993. Cambridge, Massachusetts, Arthur D. Little Inc., 84
pp.
Bae KS, Lee HJ, Hah YC, & Hong SW (1982) [The distribution and annual
variation of detergent-degrading bacteria in the lower Han river.]
Korean J Microbiol, 20: 113-118 (in Korean).
Barera Y & Adams WJ (1983) Resolving some practical questions about
Daphnia acute toxicity tests. In: Bishop WE, Cardwell RD, & Heidolph
BB ed. Aquatic toxicology and hazard assessment. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 509-518
(ASTM STP 802).
Bay PHS & Danneman PJ (1985) Skin sensitization potential of
alpha-olefin sulfonate (AOS) and a prototype dishwashing detergent
containing AOS. J Am Oil Chem Soc, 62 (4): 646.
Berenson MM & Temple AR (1974) Detergent ingestion; unique experience
of a family. Clin Toxicol, 7 (1): 25-28.
Berenson MM & Temple AR (1976) Detergent toxicity: effects on
esophageal and gastric mucosa. Clin Toxicol, 8: 399-404.
Berna JL, Ferrer J, Moreno A, Prats D, & Ruiz Bevia F (1989) The fate
of LAS in the environment. Tenside Surfactants Deterg, 26: 101-107.
Berna JL, Moreno A, & Ferrer J (1991) The behaviour of LAS in the
environment. J Chem Technol Biotechnol, 50: 387-398.
Berna JL, Moreno A, Banerji A, Fritsch TR, & Vora BV (1993a) Growth
and developments in LAB technologies: 30 years of innovation and more
to come. Presentation at the 1993 World Surfactant Congress, 23
September 1993, Montreux, Switzerland. New York, Soap and Detergent
Association, 27 pp.
Berna JL, Moreno A, & Ferrer J (1993b) An assessment of the ultimate
biodegradation of LAS. Tenside Surfactants Deterg, 30 (3): 217-222.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, & Bentinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19: 1527-1550.
Bishop WE & Maki AW (1980) A critical comparison of two
bioconcentration test methods. In: Eaton JG, Parrish PR, & Hendricks
AC ed. Aquatic toxicology. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 61-77 (ASTM STP 707).
Bishop WE & Perry RL (1981) Development and evaluation of a
flow-through growth inhibition test with duckweed (Lemna minor). In:
Branson DR & Dickson KL ed. Aquatic toxicology and hazard assessment.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 421-435 (ASTM STP 737).
Blank IH & Gould E (1961) Penetration of anionic surfactants into
skin: (II) study of mechanisms which impede the penetration of
synthetic anionic surfactants into skin. J Invest Dermatol, 37 (6):
311-315.
Blank IH & Shappirio EB (1955) The water content of the stratum
corneum. J Invest Dermatol, 25: 391-401.
Blohm SG (1957) The connection between skin-irritating and
protein-denaturing effects of some surface-active agents. Acta
Dermatol Venereol, 37 (4): 269-275.
von Bock KJ & Mann H (1971) [Anionic surfactants in the coastal areas
of the North Sea.] Arch. Fischereiwiss, 22: 287-292 (in German).
Bornmann G, Loeser A, & Stanisic M (1963) [Study of a detergent based
on dodecylbenzene sulfonate.] Fette Seifen Anstrichm, 65 (10): 818-824
(in German).
Borstlap (1967) Intermediate biodegradation products of anionic
detergents; their toxicity and foaming properties. In: Proceedings of
the International Congress on Surface Active Substances, Brussels,
September 1964, Vol. 3, 891-901.
Brenner TE (1968) The impact of biodegradable surfactants on water
quality. J Am Oil Chem Soc, 45: 433-436.
Bressan M, Brunetti R, Casellato S, Fava GC, Giro P, Marin M,
Negrisolo P, Tallandini L, Thomann S, Tosoni L, Turchetto M, &
Campesan GC (1989) Effects of linear alkylbenzene sulfonate (LAS) on
benthic organisms. Tenside Surfactants Deterg, 26: 148-158.
Bressan M, Marin MG, & Brunetti R (1991) Effects of linear
alkylbenzene sulphonate (LAS) on skeletal development of sea urchin
embryos (Paracentrotus lividus Lmk). Water Res, 25: 613-616.
Bringmann G & Kühn R (1977) [Limiting values for noxious effects of
water pollutants on bacteria (Pseudomonas putida) and green algae
(Scendesmus quadricauda) in the cell multiplication test.] Z Wasser
Abwasser Forsch, 10 (3): 87-98 (in German).
Brown VKH & Muir CMC (1970) The toxicities of some coconut alcohol and
dobanol-23 derived surfactants. Tenside Surfactants Deterg, 7 (3):
137-139.
Brown VM, Abram FSH, & Collins LJ (1978) The acute lethal toxicity to
rainbow trout of an LAS surfactant and of its residues and degradation
products. Tenside Surfactants Deterg, 15 (2): 57-59.
Brunner PH, Capri S, Marcomini A, & Giger W (1988) Occurrence and
behaviour of linear alkylbenzene sulphonates, nonylphenol
diethoxylates in sewage and sewage sludge treatment. Water Res, 22:
1465-1472.
Buehler EV, Newmann EA, & King WR (1971) Two-year feeding and
reproduction study in rats with linear alkylbenzene sulfonate (LAS).
Toxicol Appl Pharmacol, 18: 83-91.
Burke B, Olavesen AH, Curtis CG, & Powell GM (1975) The biodegradation
of some anionic detergents in the rat: a common metabolic pathway.
Xenobiotica, 5 (9): 573-584.
Burke B, Olavesen AH, Curtis CG, & Powell GM (1976) The biodegradation
of the surfactant undecyl sulphate. Xenobiotica, 6 (11): 667-678.
Cain RB, Willats AJ, & Bird JA (1971) Surfactant biodegradation:
metabolism and enzymology. In: Biodegradation of materials. New York,
John Wiley and Sons, vol 2, pp 136-144.
Calabrese A & Davis HC (1967) Effects of 'soft' detergents on embryos
and larvae of the American oyster (Crassostrea virginica). Proc Natl
Shellfish Assoc, 57: 11-16.
Calabrese EJ & Kenyon EM (1991) Air toxics and risk assessment.
Chelsea, Michigan, Lewis Publishers Inc., 612 pp.
Canadian Council on Animal Care (1980-1984) Guide to the care and use
of experimental animals: Vols 1 and 2. Ottawa, Canadian Council on
Animal Care.
Canton JH & Slooff W (1982) Substitutes for phosphate containing
washing products: their toxicity and biodegradability in the aquatic
environment. Chemosphere, 11: 891-907.
Carson S & Oser BL (1964) Dermal toxicity of sodium lauryl sulfate. J
Soc Cosmet Chem, 15: 137-147.
Castles MA, Moore BL, & Ward SR (1989) Measurement of linear
alkylbenzenesulfonates in aqueous environmental matrices by liquid
chromatography with fluorescence detection. Anal Chem, 61 (22):
2534-2540.
Cavalli L, Gellera A, Lazzarin A, Nucci GC, Romano P, Ranzani M, &
Lorenzi E (1991) Linear alkylbenzene sulphonate removal and
biodegradation in a metropolitan plant for water treatment. Riv Ital
Sostanze Grasse, 68: 75-81.
Cavalli L, Divo C, Giuffrida G, Pellizzon T, Radici P, Valtorta L, &
Zatta A (1993a) Producing linear alkyl benzene (LAB) from linear
olefins using an AlCl3 catalyst. Spec Chem, August: 228-231.
Cavalli L, Gellera A, & Landone A (1993b) LAS removal and
biodegradation in a wastewater treatment plant. Environ Toxicol Chem,
12: 1777-1788.
CEFIC (1992) Linear alkylbenzene. Report of a working group. Brussels,
European Chemical Industry Council, 6 pp.
Chattopadhyay DN & Konar SK (1985) Chronic effects of linear alkyl
benzene sulfonate on aquatic ecosystem. Environ Ecol, 3: 428-433.
Colin A Houston & Associates Inc. (1990) Higher alcohols world markets
1988-2000. A multiclient study: January 1990. Mamaroneck, New York,
Colin A. Houston & Associates, 21 pp.
Comotto RM, Kimerle RA, & Swisher RD (1979) Bioconcentration and
metabolism of linear alkylbenzene sulphonate by daphnids and fathead
minnows. In: Marking LL & Kimerle RA ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 232-250 (ASTM STP 667).
Conti E (1987) Acute toxicity of three detergents and two insecticides
in the lugworm, Arenicola marina (L.): a histological and a scanning
electron microscopic study. Aquat Toxicol, 10: 325-334.
Cook TM & Goldman CK (1974) Degradation of anionic detergents in
Chesapeake Bay. Chesapeake Sci, 15: 52-55.
Cordon TC, Maurer EW, & Stirton AJ (1972) The biodegradation of some
sulfated alkanolamides. J Am Oil Chem Soc, 49: 174-177.
Cowgill UM, Milazzo DP, & Landenberger BD (1990) The reproducibility
of the three brood Ceriodaphnia test using the reference toxicant
sodium lauryl sulphate. Arch Environ Contam Toxicol, 19: 513-517.
Cresswell DG, Baldock GA, Chasseaud LF, & Hawkins DR (1978) Toxicology
studies of linear alkylbenzene sulphonate (LAS) in rhesus monkeys:
(II) The disposition of [14C]LAS after oral or subcutaneous
administration. Toxicology, 11: 5-17.
Dahl MV & Trancik RJ (1977) Sodium lauryl sulfate irritant patch
tests: degree of inflammation at various times. Contact Dermatitis, 3:
263-266.
Daly LW, Schroeder RE, & Killeen JC (1980) A teratology study of
topically applied linear alkylbenzene sulphonate in rats. Food
CosmetToxicol, 18: 55-58.
De Henau H, Matthijs E, & Hopping WD (1986) Linear alkylbenzene
sulphonates (LAS) in sewage sludges, soils and sediments: analytical
determination and environmental safety considerations. Int J Environ
Anal Chem, 26: 279-293.
De Henau H, Matthijs E, & Namkung E (1989) Trace analysis of LAS by
HPLC. Detailed results from two municipal sewage treatment plants. In:
Quaghebeur D, Temmerman I, & Angeletti G ed. Organic contaminants in
waste water, sludge and sediment: Occurrence, fate and disposal.
London, Elsevier Science Publishers, pp 5-18.
Denner WHB, Olavesen AH, Powell GM, & Dodgson KS (1969) The metabolism
of potassium dodecyl [35] sulphate in the rat. Biochem J, 111:
43-51.
Devi Y & Devi S (1986) Effect of synthetic detergents on germination
of fern spores. Bull Environ Contam Toxicol, 37: 837-843.
Di Corcia A, Marchetti M, Samperi R, & Marcomini A (1991) Liquid
chromatographic determination of linear alkylbenzenesulfonates in
aqueous environmental samples. Anal Chem, 63: 1179-1182.
Divo C & Cardini G (1980) Primary and total biodegradation of linear
alkylbenzene sulphonates. Tenside Surfactants Deterg, 17: 30-36.
Dolan JM & Hendricks AC (1976) The lethality of an intact and degraded
LAS mixture to bluegill sunfish and a snail. J Water Pollut Control
Fed, 48: 2570-2577.
Dolan JM, Gregg BC, Cairns J, Dickson KL, & Henricks AC (1974) The
acute toxicity of three new surfactant mixtures to a mayfly larvae.
Arch Hydrobiol, 74: 123-132.
Dominguez JG, Parra JL, Infante MR, Pelejero CM, Balaguer F, & Sastre
T (1977) A new approach to the theory of adsorption and permeability
of surfactants on keratinic proteins: the specific behaviour of
certain hydrophobic chains. J Soc Cosmet Chem (UK), 28: 165-182.
Dreger EE, Keim GI, Miles GD, Shedlovsky L, & Ross J (1944) Sodium
alcohol sulfates: properties involving surface activity. Ind Eng Chem,
36 (7): 610-617.
Dugan PR (1967) Influence of chronic exposure to anionic detergents on
toxicity of pesticides to goldfish. J Water Pollut Control Fed, 39:
63-71.
Dutka BJ & Kwan KK (1982) Application of four bacterial screening
procedures to assess changes in the toxicity of chemicals in mixtures.
Environ Pollut, A29: 125-134.
Eganhouse RP, Blumfield DL, & Kaplan IR (1983) Long-chain
alkylbenzenes as molecular tracers of domestic wastes in the marine
environment. Environ Sci Technol, 17: 523-530.
Eggert CR, Kaley RG, & Gledhill WE (1979) Application of a laboratory
freshwater lake model in the study of linear alkylbenzene sulfonate
(LAS) biodegradation. In: Bourquin AW & Pritchard PH ed. Proceedings
of the Workshop on Microbial Degradation of Pollutants in Marine
Environments, Pensacola Beach, Florida, 9-14 April 1978. Washington,
DC, US Environmental Protection Agency, pp 451-461 (EPA-600/9-79-012).
Endo T, Furuido Y, Namie K, Yamamoto N, Hasunuma H, & Ueda K (1980)
[Studies of the chronic toxicity and teratogenicity of synthetic
surfactants.] Ann Rep Tokyo Metrop Res Inst Environ Prot [Tokyo Kogai
Kenkyujo Nempo]: 236-246 (in Japanese).
Environment Agency Japan (1978) Chemicals in the environment. Tokyo,
81 pp.
Epstein S, Throndson AH, Dock W, & Tainter ML (1939) Possible
deleterious effects of using soap substitutes in dentifrices. J Am
Dental Assoc, 26: 1461-1471.
Ernst R, Arditti J, & Healey PL (1971) Biological effects of
surfactants l. Influence on the growth of orchid seedlings. New
Phytol, 70: 457-475.
Ernst R, Gonzales CJ, & Arditti J (1983) Biological effects of
surfactants: Part 6-- effects of anionic, non-ionic and amphoteric
surfactants on a green alga (Chlamydomonas). Environ Pollut, A31:
159-175.
European Committee of Organic Surfactants and Their Intermediates
(1990) Classification and labelling of surfactants. Brussels, 38 pp.
Eurostat (1991) Environment statistics. Annex to overview of the state
of the environment in the European Communities 1992: COM(92) 23
Final--Volume III. Brussels, Commission of the European Communities,
102 pp.
Evans HC (1956) Alkyl sulphates (Part 1); critical micelle
concentrations of the sodium salts. J Soc Cosmet Chem, 7: 579-586.
Eyanoer HF, Upatham ES, Duangsawasdi M, & Tridech S (1985) Effects of
water hardness and temperature on the toxicity of detergents to the
freshwater fish, Puntius gonionotus, bleeker. J Sci Soc Thailand,
11: 67-77.
Fairchild JF, Dwyer FJ, La Point T, Burch SA, & Ingersoll CG (1993)
Evaluation of a laboratory-generated NOEC for linear alkylbenzene
sulfonate in outdoor experimental streams. Environ Toxicol Chem, 12:
1763-1775.
Federle TW & Pastwa GM (1988) Biodegradation of surfactants in
saturated subsurface sediments: a field study. Ground Water, 26:
761-770.
Federle TW & Schwab BS (1989) Mineralization of surfactants by
microbiota of aquatic plants. Appl Environ Microbiol, 55: 2092-2094.
Federle TW & Schwab BS (1992) Mineralisation of surfactants in
anaerobic sediments of a laundromat wastewater pond. Water Res, 26:
123-127.
Federle TW & Ventullo RM (1990) Mineralization of surfactants by
microbiota of submerged plant detritus. Appl Environ Microbiol, 56:
333-339.
Fendinger NJ, Begley WM, McAvoy DC, & Eckhoff WS (1992a) Determination
of alkyl sulfate surfactants in natural waters. Environ Sci Technol,
26: 2493-2498.
Fendinger NJ, Begley WM, McAvoy DC, & Eckhoff WS (1992b) Monitoring of
alkyl sulfate in wastewater treatment systems and surface water.
Presented before the Division of Environmental Chemistry, American
Chemical Society, San Francisco, April 1992. Washington, DC, American
Chemical Society, p 2.
Field JA, Barber EM, Thurman EM, Moore BL, Lawrence DA, & Peake DA
(1992) Fate of alkylbenzenesulfonates and dialkyltetralinsulfonates in
sewage-contaminated groundwater. Environ Sci Technol, 26 (6):
1140-1148.
Figge K & Schoberl P (1989) LAS and the application of sewage sludge
in agriculture. Tenside Surfactants Deterg, 26: 122-128.
Fischer WK (1980) [Development of detergent concentration in German
rivers 1960-1980.] Tenside Surfactants Deterg, 17: 250-261 (in
German).
Fischer WK & Gerike P (1975) Biodegradability determinations via
unspecific analyses (chemical oxygen demand, dissolved organic carbon)
in coupled units of the OECD confirmatory test--II: Results. Water
Res, 9: 1137-1141.
Fisher LB & Maibach HI (1975) Effect of some irritants on human
epidermal mitosis. Contact Dermatitis, 1: 273-276.
Fitzhugh OG & Nelson AA (1948) Chronic oral toxicity of surface-active
agents. J Am Pharm Assoc, 37: 29-32.
Flecknell PA (1987) Laboratory animal anaesthesia. An introduction for
research workers and technicians. Toronto, Academic Press, pp 118-132.
Fogels A & Sprague JB (1977) Comparative short-term tolerance of
zebrafish, flagfish, and rainbow trout to five poisons including
potential reference toxicants. Water Res, 11: 811-817.
Froebe CL, Simion FA, Rhein LD, Cagan RH, & Kligman A (1990) Stratum
corneum lipid removal by surfactants: relation to in vivo irritation.
Dermatologica, 181 (4): 277-283.
Fujii T, Sakamoto Y, Abe Y, Mikurita H, Yuzawa K, & Hiraga K (1977)
[Pathological examination of rats fed with linear alkylbenzene
sulfonate for their lifespan.] Ann Rep Tokyo Metrop Res Lab Public
Health, 28 (2): 85-108 (in Japanese).
Fujita H, Kojima A, & Hiraga K (1977) [Screening tests of mutagenicity
of linear alkylbenzene sulfonate (LAS) in a microbial system.] Ann Rep
Tokyo Metrop Res Lab Public Health, 28 (2): 112-114 (in Japanese).
Fujiwara K, Sugiyama T, Koike K, & Oba K (1975) [Biodegradation of
linear alkylate sulfonates in a river model.] Jpn J Hyg, 29: 552-557
(in Japanese).
Fukuzawa K, Tokumura A, Yamada S, & Tsukatani H (1978) [Oral toxicity
of sodium dodecyl sulfate.] Eisei Kagaku, 24 (2): 107-110 (in
Japanese).
Gafa S (1974) Studies on relationship between acute toxicity to fish
and surface activity of anionic surfactants. Riv Ital Sostanze Grasse,
51: 182-192.
Gafa S & Lattanzi B (1974) Evaluation of some 'third generation'
anionic surfactants in comparison with a linear alkylbenzene
sulfonate. 12th World Congress on Soaps. Milan, International Society
for Fat Research.
Gagnon MJ (1979) Note on a rapid and sensitive method for the
determination of anionic detergents in natural waters at the ppb
level. Water Res, 13: 53-56.
Games LM (1983) Practical applications and comparisons of
environmental exposure assessment models. In: Bishop WE, Cardwell RD,
& Heidolph BB ed. Aquatic toxicity and hazard assessment: Sixth ASTM
Symposium. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 282-299.
Gard-Terech A & Palla JC (1986) Comparative kinetics study of the
evolution of freshwater aquatic toxicity and biodegradability of
linear and branched alkylbenzene sulfonates. Ecotoxicol Environ Saf,
12: 127-140.
Gentempo PG, Dickson MK, & Guin KF (1985) Analysis of alpha-olefin
sulfonates via quantitative carbon-13 NMR. J Am Oil Chem Soc, 62 (4):
645.
Gerike P (1987) Environmental impact. In: Falbe J ed. Surfactants in
consumer products. Berlin, Springer-Verlag, pp 450-474.
Gerike P, Winkler K, Schneider W, & Jakob W (1989) Residual LAS in
German rivers. Tenside Surfactants Deterg, 26: 136-140.
Giger W, Alder AC, Brunner PH, Marcomini A, & Siegrist H (1989)
Behaviour of LAS in sewage and sludge treatment and in sludge-treated
soil. Tenside Surfactants Deterg, 26: 95-100.
Gilbert PA & Pettigrew R (1984) Surfactants and the environment. Int
J Cosmet Sci, 6: 149-158.
Gledhill WE (1975) Screening test for assessment of ultimate
biodegradability: Linear alkylbenzene sulfonates. Appl Microbiol, 30:
922-929.
Gloxhuber C (1974) Toxicological properties of surfactants. Arch
Toxicol, 32: 245-269.
Gotte E (1954) [Properties of salts of primary unbranched
alkylsulfates and their solutions with regard to their technical use,
Part 1.] Fette Seifen Anstrichm, 56 (8): 583-587 (in German).
Granmo A (1972) Development and growth of eggs and larvae of Mytilus
edulis exposed to a linear dodecylbenzenesulphonate, LAS. Mar Biol,
15: 356-358.
Guhl W (1987) [Biotic similarity indices in the comparison of waters
from identical sources.] Limnologica (Berlin), 18 (1): 1-13 (in
German).
Haar GT (1983) Safety of alpha olefin sulfonates. J Am Oil Chem Soc,
60 (4): 876-881.
Halvorson H & Ishaque M (1969) Microbiology of domestic wastes; III.
Metabolism of LAS-type detergents by bacteria from a sewage lagoon.
Can J Microbiol, 15: 571-576.
Hamano Y, Yamamoto H, Inoue K, Oda Y, Kuwano A, Mitsuda B, & Kunita N
(1976) [Studies on the toxicity of commercial detergents (4th report);
fetal toxicity (fetal median lethal dose) in mice.] Ann Rep Osaka
Prefect Inst Public Health, 7: 147-152 (in Japanese).
Hand VC, Rapaport RA, & Pittinger CA (1990) First validation of a
model for the adsorption of linear alkylbenzenesulfonate (LAS) to
sediment and comparison to chronic effects data. Chemosphere, 21:
741-750.
Harrold SP (1959) Denaturation of epidermal keratin by surface active
agents. J Invest Dermatol, 32: 581-588.
Hasegawa H & Sato M (1978) [Permeation of LAS through rat skin.]
Tokyo, Science and Technology Agency, Research Cordination Bureau, pp
172-175 (in Japanese).
Hashimoto S, Sakurai K, & Nagai T (1976) [Determination of trace
amounts of alkylbenzene sulfonate in the river water by high
performance liquid chromatography.] Bunseki Kagaku, 25: 639-643 (in
Japanese).
Hatton EH, Fosdick LS, & Calandra J (1940) The toxicity and
rubefacient action of sulphated higher alcohols. J Dent Res, 19:
87-92.
Hattori M, Seno K, Harada S, Ishizu Y, & Goto M (1984) [Effects of
some environmental chemicals in the Daphnia reproduction test.] Jpn
Ecol Chem, 6: 23-27 (in Japanese).
Havermann H & Menke KH (1959) [Biological study of the water-soluble
surface-active substances.] Fette Seifen Anstrichm, 61 (6): 429-434
(in German).
Hayashi M (1980) [Toxicological studies on detergents containing
linear alkylbenzene sulfonate (LAS) in rats and mice.] Shikoku Med J,
36 (1): 37-44 (in Japanese).
Healey PL, Ernst R, & Arditti J (1971) Biological effects of
surfactants: II. Influence on the ultrastructure of orchid seedlings.
New Phytol, 70: 477-482.
Health Canada (in press) Canadian Environmental Protection Act. Human
health risk assessment for priority substances; Appendix E. Reference
values for intakes and body weights of laboratory animals. Ottawa,
Health and Welfare Canada.
Hellmann H (1978) [Anionic detergents in the Rhine 1971-1977, their
degradation and biodegradation potential of the river.] Tenside
Surfactants Deterg, 15: 291-294 (in German).
Henderson C, Pickering QH, & Cohen JM (1959) The toxicity of synthetic
detergents and soaps to fish. Sew Ind Wastes, 31: 295-306.
Hennes EC & Rapaport RA (1989) Calculation and analytical verification
of LAS concentrations in surface waters, sediment and soil. Tenside
Surfactants Deterg, 26: 141-147.
Hewin International Inc. (1992) The technology and markets for anionic
surfactants: North America, western Europe and Japan in the global
perspective 1991-1995 and scenario 2000. Amsterdam, Hewin
International Inc., 53 pp.
Heywood R, James RW, & Sortwell RJ (1978) Toxicology studies of linear
alkylbenzene sulphonate (LAS) in rhesus monkeys; (I) simultaneous oral
and subcutaneous administration for 28 days. Toxicology, 11: 245-250.
Hidaka H & Tatsukawa R (1989) Avoidance by olfaction in a fish, medaka
(Oryzias latipes), to aquatic contaminants. Environ Pollut, 56:
299-309.
Hidaka H, Suga M, & Tatsukawa R (1984) [Avoidance of anionic
surfactants in medakas (Oryzias latipes).] Nippon Nogeikagaku
Kaishi, 58: 1-7 (in Japanese).
Hidaka H, Kubota H, Gratzel M, Serpone N, & Pelizzetti E (1985)
Photodegradation of surfactants; I. Degradation of sodium
dodecylbenzene sulfonate in aqueous semiconductor dispersions. Nouv J
Chim, 9: 67-69.
Hidu H (1965) Effects of synthetic surfactants on the larvae of clams
(M. mercenaria) and oysters (C. virginica). J Water Pollut Control
Fed, 37: 262-270.
Higuchi K, Shimoishi Y, Miyata H, & Toei K (1982) Spectrophotometric
determination of sodium 4-dodecylbenzenesulfonate with
1-(N-methylpyridinium-4-yl-azo)-4-(4-diethylaminophenylazo)naphthale
ne iodide. Bull Chem Soc Jpn, 55 (2): 621-622.
Hironaga K (1979) [Case reports; 29 emergency cases of children
(non-fatal cases of drinking household products).] Yakuji Shinpo, 36:
27-29 (in Japanese).
Hirsch E (1963) [Structure of alkylbenzenesulfonates and impact on
behaviour of fish.] Vom Wasser, 30: 249-259 (in German).
Hitchcock WS & Martin DF (1977) Effects and fate of a surfactant in
cultures of the red tide organisms, Gymnodinium breve. Bull Environ
Contam Toxicol, 18: 291-296.
Hokanson KEF & Smith LL (1971) Some factors influencing toxicity of
linear alkylate sulfonate (LAS) to the bluegill. Trans Am Fish Soc,
100: 1-12.
Hollis CG, Phillips WK, & Johnson JE (1976) Thermal effects on
biodegradation of linear alkyl benzenesulfonate. Dev Ind Microbiol,
17: 345-350.
Holman WF & Macek KJ (1980) An aquatic safety assessment of linear
alkylbenzene sulfonate (LAS): chronic effects on fathead minnows.
Trans Am Fish Soc, 109: 122-131.
Holst R & Moller H (1975) One hundred twin pairs patch tested with
primary irritants. Br J Dermatol, 93: 145-149.
Holt MS, Matthijs E, & Waters J (1989) The concentrations and fate of
linear alkylbenzene sulphonate in sludge amended soils. Water Res, 23:
749-759.
Holysh M, Paterson S, Mackay D, & Bandurraga MM (1986) Assessment of
the environmental fate of linear alkylbenzenesulphonates. Chemosphere,
15: 3-20.
Hong SD, Lee SH, Hah SC, & Ha HP (1984) [Biodegradation of synthetic
detergents by Klebsiella pneumoniae L-127.] Korean J Appl Microbiol
Bioeng, 12: 91-97 (in Korean).
Hon-Nami H & Hanya T (1980a) Linear alkylbenzene sulfonates in river,
estuary and bay water. Water Res, 14: 1251-1256.
Hon-Nami H & Hanya T (1980b) Differences in the composition of linear
alkylbenzene sulfonate homologues in river sediment and river water.
Jpn J Limnol, 41: 1-4.
Hope J (1977) Absence of chromosome damage in the bone marrow of rats
fed detergent actives for 90 days. Mutat Res, 56: 47-50.
Howes D (1975) The percutaneous absorption of some anionic
surfactants. J Soc Cosmet Chem, 26: 47-63.
Hrsak D, Bosnjak M, & Johanides V (1981) Kinetics of linear
alkylbenzene sulphonate and secondary alkane sulphonate
biodegradation. Tenside Surfactants Deterg, 18: 137-140.
Hrsak D, Bosnjak M, & Johanides V (1982) Enrichment of linear
alkylbenzenesulphonate (LAS) degrading bacteria in continuous culture.
J Appl Bacteriol, 53: 413-422.
Hsu YC (1965) Detergent-splitting enzyme from pseudomonas. Nature,
207: 385-388.
Huber L (1989) Conclusions for an ecological evaluation of LAS.
Tenside Surfactants Deterg, 26 (2): 71-74.
Huddleston RL & Allred RC (1963) Microbial oxidation of sulfonated
alkylbenzenes. In: Vol 4. Developments in industrial microbiology.
Washington, DC, American Institute of Biological Sciences, pp 24-38.
Huddleston RL & Nielsen AM (1979) LAS biodegradation: the fate of the
aromatic ring. Soap Cosmet Chem Spec, March: 34-44.
Hunter B & Benson HG (1976) Long-term toxicity of the surfactant
alpha-olefin sulphonate (AOS) in the rat. Toxicology, 5: 359-370.
Husmann W (1968) Solving the detergent problem in Germany. Water
Pollut Control, 3: 80-90.
Ichihara Y, Yoshiue S, Omure K, Fujimoto T, Moritomo M, & Yoshikawa J
(1967) [A case study of anionic surfactant poisoning.] J Jpn Soc Int
Med [Nichi Naikaishi], 56 (11): 128 (in Japanese).
Ihara I, Ozaki M, Nakamura F, Yokota I, Nakaura H, Aso T, Ozaki M,
Kaneko Y, Miyauchi S, Miura K, Hayakawa Y, Moriyama S, Ishiguro T, &
Yoshida Y. (1970) [ABS in the water of the Tama River; V. Data from
February 1968 to May 1970.] J Jpn Oil Chem Soc, 19: 1043-1048 (in
Japanese).
Iimori M & Takita Y (1979) [A study of the effect of linear
alkylbenzenesulfonate on fish.] J Jpn Oil Chem Soc, 28: 185-189 (in
Japanese).
Iimori M & Ushiyama S (1971) [Serological studies on alpha-olefin
sulfonate protein complexes.] J Jpn Oil Chem Soc, 20 (2): 24-26 (in
Japanese).
Iimori M, Ogata T, & Kudo K (1972) [Eye irritation testing of surface
active agents in experimental animals.] J Jpn Oil Chem Soc, 21 (6):
334-337 (in Japanese).
Iimori M, Inoue S, & Yano K (1973) [Effects of skin application of
detergents on pregnant mice.] J Jpn Oil Chem Soc [Yukagaku], 22 (12):
807-813 (in Japanese).
Ikawa M, Yoneyama M, Nakao T, & Hiraga K (1978) [Uptake of organic
acid and organic base by renal cortical slices of rats treated with
LAS and ABS.] Ann Rep Tokyo Metrop Res Lab Public Health., 29 (2):
51-54 (in Japanese).
Ikeda Y (1965) [Food additives and synthetic detergents.] Sogo-Rinsyo,
14 (4): 621-626 (in Japanese).
Imahori A, Kitagawa T, & Shiobara S (1976) [Effects of linear
alkylbenzene sulfonate (LAS) applied dermally to pregnant mice on the
pregnant mice and their fetuses.] Jpn J Public Health [Nippon
Koshueisei Zasshi], 23 (2): 68-72 (in Japanese).
Imokawa G & Katsumi M (1976) [Denaturing action of typical anionic
surfactants on several proteins.] J Jpn Oil Chem Soc [Yukagaku], 25
(1): 24-30 (in Japanese).
Imokawa G & Mishima Y (1976) [The cumulative effect of surface active
agents on human cutaneous horny layers.] Nichihikaishi, 86 (8):
473-481 (in Japanese).
Imokawa G & Mishima Y (1979) Cumulative effect of surfactants on
cutaneous horny layers: lysosome labilizing action. Contact
Dermatitis, 5: 151-162.
Imokawa G, Sumura K, & Katsumi M (1974) [A correlation between
adsorption of a surfactant onto callus and resulting skin-roughness.]
J Jpn Oil Chem Soc [Yukagaku], 23 (11): 719-725 (in Japanese).
Imokawa G, Sumura K, & Katsumi M (1975a) A study of skin roughness
caused by surfactants: (I) A new method in vivo for evaluation of
skin roughness. J Am Oil Chem Soc, 52: 479-483.
Imokawa G, Sumura K, & Katsumi M (1975b) Study of skin roughness
caused by surfactants: (II) Correlation between protein denaturation
and skin roughness. J Am Oil Chem Soc, 52: 484-489.
Inaba K & Amano K (1988) HPLC determination of linear
alkylbenzenesulfonate (LAS) in the aquatic environment. Seasonal
changes in LAS concentration in polluted lake water and sediment. Int
J Environ Anal Chem, 34: 203-213.
Inoue K & Masuda F (1976) Effects of detergents on mouse fetuses. J
Jpn Food Hgy Soc [Shoku Eishi], 17 (2): 158-169.
Inoue K & Sunakawa T (1979) [Mutagenicity tests of surfactants.] Jpn
Fragr J, 38: 67-75 (in Japanese).
Inoue K, Shibata T, Hamano Y, Oda Y, Kuwano A, Yamamoto H, Mitsuda B,
& Kunita N. (1977) [ In vivo cytogenetic tests of some synthetic
detergents in mice.] Ann Rep Osaka Prefect Inst Public Health, 8:
17-24 (in Japanese).
Inoue K, Kaneko K, & Yoshida M (1978) Adsorption of
dodecylbenzenesulfonates by soil colloids and influence of soil
colloids on their degradation. Soil Sci Plant Nutr, 24: 91-102.
Inoue K, Sunakawa T, & Takayama S (1979) [Studies of in vitro cell
transformation and mutagenicity by surfactants and other compounds.]
J Jpn Soc Cosmet Chem [Shogishi], 13 (2): 50-60 (in Japanese).
Inoue K, Sunakawa T, & Takayama S (1980) Studies of in vitro cell
transformation and mutagenicity by surfactants and other compounds.
Food Cosmet Toxicol, 18: 289-296.
Inoue S, O'Grodnick JS, & Tomizawa S (1982) Metabolism of alpha-olefin
sulfonate (AOS) in rats. Fundam Appl Toxicol, 2: 130-138.
Ishihara M & Kinebuchi S (1967) [Causes of hand eczema, and their
examination methods.] Rinsho Hifuka, 21 (3): 241-251 (in Japanese).
Ito T & Kakegawa S (1972) [Development of mild detergent
formulations.] Baioteku, 3 (2): 157-164 (in Japanese).
Ito R, Kawamura H, Chang HS, Kudo K, Kajiwara S, Toida S, Seki Y,
Hashimoto M, & Fukushima A (1978) [Acute, subacute and chronic
toxicity of magnesium linear alkylbenzene sulfonate (LAS-Mg).] J Med
Soc Toho Univ [Toho Igakukai Zasshi], 25 (5-6): 850-875 (in Japanese).
Itoh S-I, Setsuda S, Utsonomiya A & Naito S (1979) [Studies on the
biodegradation test method for chemical substances: II. Ultimate
biodegradabilities of some anionic, nonionic, and cationic surfactants
estimated by CO2 production.] J Jpn Oil Chem Soc [Yukagaku], 28:
199-204 (in Japanese).
Itoh S, Naito S, & Unemoto T (1987) [Comparative studies on anaerobic
biodegradation of anionic and nonionic surfactants.] Eisei Kagaku, 33:
415-422 (in Japanese).
Janicke W (1971) On the behaviour of synthetic organic substances
during the treatment of waste liquors-- IV. Progress of the
biodegradation of `soft' anionic synthetic detergents. Water Res, 5:
917-931.
Janicke W & Hilge G (1979) [Biodegradability of anionic/cationic
surfactant complexes under the aerobic and anaerobic conditions of
effluent and sludge treatment.] Tenside Surfactants Deterg, 16:
117-122 (in German).
Janicke W & Niemitz W (1973) [Comparative investigations of detergent
raw materials and commercial detergents and biological sewage
treatments.] Vom Wasser, 40: 369-389 (in German).
Japanese Soap & Detergent Association (1992) [Annual report of water
quality in the environment.] Tokyo, Japanese Soap and Detergent
Association (in Japanese).
Javed S, Misra V, & Viswanathan PN (1988) In vitro studies on the
effect of linear alkyl benzene sulphonate on serum albumin. Int J
Cosmet Sci, 10: 241-246.
Jimenez L, Breen A, Thomas N, Federle TW, & Sayler GS (1991)
Mineralization of linear alkylbenzene sulfonate by a four-member
aerobic bacterial consortium. Appl Environ Microbiol, 57: 1566-1569.
Jones JH (1945) General colorimetric method for determination of small
quantities of sulfonated or sulfated surface active compounds. J Assoc
Off Agric Chem, 28 (2): 398-408.
Kantin R, Baumgarten MGZ, Cabeda M, Beaumord AC, & de Almeida TL
(1981) Concentration of anionic detergents in Rio Grande water (South
Brazil). Mar Pollut Bull, 12: 50-53.
Katsuno T, Tsukioka T, & Kono Y (1983) [LAS in river sediments, algae
and water plants.] Bull Nagano Res Inst Health Pollut, 6: 23-25 (in
Japanese).
Katz BM & Cohen GM (1976) Toxicities of excessively chlorinated
organic compounds. Bull Environ Contam Toxicol, 15 (6): 644-650.
Kawamura T, Sasagawa S, Masuda T, Honda S, Kinoshita M, Harada S,
Ishizaki T, Nagai R, Hirokawa K, Anzai T, Anekoji K, Hidano A, Kawano
T, Ikegami I, Sato S, & Aoyama T (1970) [Basic studies on the
standardization of a skin patch test.] Jpn J Dermatol [Nihon Hifu
Kagakukai Zasshi], 80 (5): 301-314 (in Japanese).
Kawashima K & Takeno T (1982) [Fate of linear alkylbenzenesulfonates
(14C-LAS-Ca, 14C-LAS-Na) in soils and plants.] J Jpn Oil Chem Soc
[Yukagaku], 31: 944-948 (in Japanese).
Kay JH, Kohn FE, & Calandra JC (1965) Subacute oral toxicity of a
biodegradable, linear alkylbenzene sulfonate. Toxicol Appl Pharmacol,
7: 812-818.
Kikuchi K (1978) Observations on some tissues of mice injected with
LAS synthetic detergent for varying periods from the day of birth.
Kawasaki Med J, 4 (3): 193-201.
Kikuchi M (1985) [Biodegradation of some surfactants in river water
with relation to chemical structure and water temperature.] Bull Jpn
Soc Sci Fish, 51: 1859-1864 (in Japanese).
Kikuchi M & Wakabayashi M (1984) Lethal response of some surfactants
to medaka (Oryzias latipes) with relation to chemical structure.
Bull Jpn Soc Sci Fish, 50: 1235-1240.
Kikuchi M, Wakabayashi M, Nakamura T, Inoue W, Takahashi K, Kawana T,
Kawahara H, & Koido Y (1976) A study of detergents. II. Acute toxicity
of anionic surfactants on aquatic organisms. Ann Rep Tokyo Metrop Res
Inst Environ Prot: 57-69.
Kikuchi M, Wakabayashi M, Kojima H, & Yoshida T (1978) Uptake,
distribution, and elimination of sodium alkyl sulfate in carp.
Ecotoxicol Environ Saf, 2: 115-127.
Kikuchi M, Tokai A, & Yoshida T (1986) Determination of trace levels
of linear alkylbenzenesulfonates in the marine environment by
high-performance liquid chromatography. Water Res, 20 (5): 643-650.
Kimerle RA & Swisher RD (1977) Reduction of aquatic toxicity of linear
alkylbenzene sulfonate (LAS) by biodegradation. Water Res, 11: 31-37.
Kimerle RA, Macek KJ, Sleight BH, & Burrows ME (1981) Bioconcentration
of linear alkylbenzene sulfonate (LAS) in bluegill (Lepomis
macrochirus). Water Res, 15: 251-256.
Kishi M, Satoh S, Horiguchi, Y, & Ito K (1984) [Effects of surfactants
on bone marrow cells.] Bull Kanagawa Public Health Lab, 14: 57-58 (in
Japanese).
Kligman AM & Wooding WM (1967) A method for the measurement and
evaluation of irritants on human skin. J Invest Dermatol, 49 (1):
78-94.
Knaebel DB, Federle TW, & Vestal JB (1990) Mineralization of linear
alkylbenzene sulfonate LAS) and linear alcohol ethoxylate (LAE) in 11
contrasting soils. Environ Toxicol Chem, 9: 981-988.
Knopp PV, Uhren LJ, Rohlich GA, & Nichols MS (1965) Field study of the
removal of linear alkylate sulfonate detergents by the activated
sludge process. J Am Oil Chem Soc, 42: 867-873.
Kobayashi H, Ichikawa H, Fujii T, Yano N, Konno T, Hiraga K, Nakamura
H, Watanabe Y, & Mimura S (1972) [Studies on toxicity of synthetic
detergents. (I) Acute oral toxicity of linear and branched alkyl
benzene sulfonate.] Ann Rep Tokyo Metrop Res Lab Public Health, 24:
397-408 (in Japanese).
Kobayashi T, Adachi A, Iida H, Oba K, Oda K, Okumura H, Tsuji K,
Numata H, & Morimoto Y (1986) [Collaborative studies on determination
of anionic surfactants in water using Co-PADAP.] Hyg Chem [Eisei
Kagaku], 32 (5): 391-396 (in Japanese).
Kobuke Y (1985) [Concentration of linear alkylbenzene sulfonates (LAS)
and its composition in the river waters of Hyogo Prefecture.] Jpn J
Limnol [Rikusuigaku Zasshi], 46: 279-286 (in Japanese).
Koizumi N, Ninomiya R, Inoue Y, Tsukamoto T, Fujii M, & Yamamoto Y
(1985) Implantation disturbance studies with linear alkylbenzene
sulphonate in mice. Arch Environ Contam Toxicol, 14: 73-81.
Kojima S (1989) [Pollution by fluorescent whitening agents (FWA) and
linear alkylbenzene sulfonates (LAS) in environmental water.] Ann Rep
Environ Pollut Res Inst Nagoya, 19: 61-68 (in Japanese).
Kondo M, Yamamoto H, & Arakawa Y (1983) [Effects of detergents on
algae growth and physiological activities.] Bull Aichi Environ Center,
11: 10-21 (in Japanese).
Konno R & Wakabayashi M (1987) [Effects of chemical compounds on the
growth of planktonic algae.] Ann Rep Tokyo Metrop Res Inst Environ
Protect [Tokyo Kogai Kenkyujo Nempo]: 113-116 (in Japanese).
Kravetz LH, Chung JC, & Rapean JC (1982) Ultimate biodegradation
studies of alpha olefin sulfonates. J Am Oil Chem Soc, 59 (4):
206-210.
Krishna Murti GSR, Volk VV, & Jackson ML (1966) Soil adsorption of
linear alkylate sulfonate. Soil Sci Soc Am Proc, 30: 685-688.
Kutt E & Martin DF (1977) Effect of selected surfactants on the growth
characteristics of Gymnodinium breve. Mar Biol, 28: 253-259.
Kuwano A, Yamamoto H, Inoue K, Hamano Y, Oda Y, Mitsuda B, & Kunita N
(1976) [Studies on toxicity of commercial detergents (2nd report).
Acute toxicity in mice.] Ann Rep Osaka Prefect Inst Public Health, 7:
137-140 (in Japanese).
Ladle M, House WA, Armitage PD, & Farr IS (1989) Faunal
characteristics of a site subject to sewage plant discharge. Tenside
Surfactants Deterg, 26: 159-168.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1983) Comparative
studies on ecotoxicology of synthetic detergents. Ecotoxicol Environ
Saf, 7: 538-545.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1984a) The water
flea (Daphnia magna) as a sensitive indicator for the assessment of
toxicity of synthetic detergents. Ecotoxicol Environ Saf, 8: 447-450.
Lal H, Misra V, Viswanathan PN, & Krishna Murti CR (1984b) Effect of
synthetic detergents on some of the behavioral patterns of fish
fingerlings (Cirrhina mrigala) and its relation to ecotoxicology.
Bull Environ Contam Toxicol, 32: 109-115.
Larson RJ (1990) Structure-activity relationships for biodegradation
of linear alkylbenzenesulfonates. Environ Sci Technol, 24: 1241-1246.
Larson RJ & Maki AW (1982) Effect of linear alkylbenzene sulfonate on
the structure and function of microbial communities in model
ecosystems. In: Pearson JG, Foster RB, & Bishop WE ed. Aquatic
toxicology and hazard assessment. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 120-136 (ASTM STP 766).
Larson RJ & Payne AG (1981) Fate of the benzene ring of linear
alkylbenzene sulphonate in natural waters. Appl Environ Microbiol, 41:
621-627.
Larson RJ, Federle TW, Shimp RJ, & Ventullo RM (1989) Behaviour of
linear alkylbenzene sulfonate (LAS) in soil infiltration and
groundwater. Tenside Surfactants Deterg, 26: 116-121.
Laumond F, Neuberger M, Oonier B, Fourey A, Bittel R, & Aubert M
(1973) Experimental investigations at the laboratory on the transfer
of mercury in marine trophic chains. Rev Int Oceanogr Med, 31/32:
47-53.
Lay JP, Klein W, & Korte F (1983) Elimination and biodistribution
studies of [14C]dodecylbenzene sulfonate in rats, following low
dosing in the daily diet and a single i.p. administration. Toxicol
Lett, 17: 187-192.
Lee S-M & Park C-K (1984) [Acute toxicity of onchean stream water to
the sea urchin, Hemicentrotus pulcherrimus.] Bull Korean Fish Soc,
17: 414-422 (in Korean).
Leidner H, Gloor R, & Wuhrmann K (1976) [The kinetics of degradation
of linear alyklbenzene sulfonate.] Tenside Surfactants Deterg, 13:
122-130 (in German).
Leo AJ & Hansch C (1979) Substituent constants for correlation.
Analysis in chemistry and biology. New York: Wiley, 98 pp.
Lewis MA (1983) Effect of loading density on the acute toxicities of
surfactants, copper, and phenol to Daphnia magna Straus. Arch
Environ Contam Toxicol, 12: 51-55.
Lewis MA (1986) Comparison of the effects of surfactants on freshwater
phytoplankton communities in experimental enclosures and on algal
population growth in the laboratory. Environ Toxicol Chem, 5: 319-332.
Lewis MA & Hamm BG (1986) Environmental modification of the
photosynthetic response of lake plankton to surfactants and
significance to a laboratory-field comparison. Water Res, 20:
1575-1582.
Lewis MA & Suprenant D (1983) Comparative acute toxicities of
surfactants to aquatic invertebrates. Ecotoxicol Environ Saf, 7:
313-322.
Lewis MA, Pittinger CA, Davidson DH, & Ritchie CJ (in press) In-situ
response of natural periphyton to an anionic surfactant and an
environmental risk assessment for phytotoxic effects. Environ Toxicol
Chem.
Lichtenstein EP (1966) Increase of persistence and toxicity of
parathion and diazinon in soils with detergents. J Econ Entomol, 59:
985-993.
Lichtenstein EP, Fuhremann TW, Scopes NEA, & Skrentny RF (1967)
Translocation of insecticides from soils into pea plants. Effects of
the detergent LAS on translocation and plant growth. J Agric Food
Chem, 15: 864-869.
Litovitz TL, Schmitz BF, & Bailey KM (1990) 1989 Annual Report of the
American Association of Poison Control Centers National Data
Collection System. Am J Emerg Med, 8: 394-445.
Litz N, Doering HW, Thiele M, & Blume H-P (1987) The behavior of
linear alkylbenzenesulfonate in different soils: A comparison between
field and laboratory studies. Ecotoxicol Environ Saf, 14: 103-116.
Longwell J & Maniece WD (1955) Determination of anionic detergents in
sewage, sewage effluents and river waters. Analyst, 80: 167-171.
Lopez-Zavala A, Aluja AS de, Elias BL, Manjarrez L, Buchmann A,
Mercado L, & Caltenco S (1975) The effects of the ABS, LAS and AOS
detergents on fish, domestic animals and plants. Prog Water Technol,
7: 73-82.
Maag H, Praun F, & Schoberl P (1975) [Recent discoveries in the olefin
sulfonate field. I. Effect of carbon number and branching of the alkyl
chain on the application and environmental characteristics of olefin
sulfonates.] Tenside Surfactants Deterg, 12: 11-15 (in German).
Maki AW (1979a) Correlations between Daphnia magna and fathead
minnow (Pimephales promelas) chronic toxicity values for several
classes of test substance. J Fish Res Board Can, 36: 411-421.
Maki AW (1979b) Respiratory activity of fish as a predictor of chronic
fish toxicity values for surfactants. In: Marking LL & Kimerle RA ed.
Aquatic toxicology. Philadelphia, Pennsylvania, American Society for
Testing and Materials, pp 77-95 (ASTM STP 667).
Maki AW & Bishop WE (1979) Acute toxicity studies of surfactants to
Daphnia magna and Daphnia pulex. Arch Environ Contam Toxicol, 8:
599-612.
Mancini P, Bellandi S, La Conca P, & Pantani F (1984)
[Microdetermination of anionic surface active agents in drinking water
and in waters that can be made potable.] Riv Merceol, 23: 143-149 (in
Italian).
Mann H (1976) [Golden orfe fish comparative testing of acute toxicity
of substances in water and wastewater: results of a ring test.] Wasser
Abwasser-Forsch, 9: 103-109 (in German).
Mann AH & Reid VW (1971) Biodegradation of synthetic detergents
evaluation by community trials: I. Linear alkylbenzene sulphonates. J
Am Oil Chem Soc, 48: 588-594.
Marchesi JR, House WA, White GF, Russell NJ, & Farr IS (1991) A
comparative study of the adsorption of linear alkyl sulphates and
alkylbenzene sulphonates on river sediments. Colloids Surf, 53: 63-78.
Marcomini A & Giger W (1987) Simultaneous determination of linear
alkylbenzenesulfonates, alkylphenol polyethoxylates, and nonylphenol
by high-performance liquid chromatography. Anal Chem, 59: 1709-1715.
Marin MG, Bressan M, & Brunetti R (1991) Effects of linear
alkylbenzene sulphonate (LAS) on two marine benthic organisms. Aquat
Toxicol, 19: 241-248.
Marquis DM, Sharman SH, House R, & Sweeney WA (1966) Alpha olefin
sulfonates from a commercial SO3-air reactor. J Am Oil Chem Soc, 43:
607-614.
Martinez J, Vives-Rego J, & Sanchez-Leal J (1989) The effect of
chemical structure and molecular weight of commercial alkylbenzenes on
the toxic response of Daphnia and naturally occurring bacteria in
fresh and seawater. Water Res, 23: 569-572.
Martinez J, Soto Y, Vives-Rego J, & Bianchi M (1991) Toxicity of Cu,
Ni and alkylbenzene sulfonate (LAS) on the naturally occurring
bacteria in the Rhone River plume. Environ Toxicol Chem, 10: 641-647.
Masubuchi M, Takahashi A, Takahashi O, & Hiraga K (1976) [Cytogenetic
studies and dominant lethal tests with long term administration of
butylated hydroxytoluene (BHT) and linear alkylbenzene sulfonate (LAS)
in mice and rats.] Ann Rep Tokyo Metrop Res Lab Public Health, 27 (2):
100-104 (in Japanese).
Masuda F & Inoue K (1974) [Effects on fetuses of linear alkylbenzene
sulfonate administered subcutaneously to pregnant mice.] Congenit Anom
[Senten Ijo], 14 (4): 309-318 (in Japanese).
Masuda F, Okamoto K, & Inoue K (1973) [Effects on fetuses of linear
alkylbenzene sulfonate applied to mouse maternal skin during
pregnancy.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 14 (6):
580-582 (in Japanese).
Masuda F, Okamoto K, & Inoue K (1974) [Effects of linear alkylbenzene
sulfonate applied dermally to pregnant mice on fetal development.] J
Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 15 (5): 349-355 (in
Japanese).
Mathur AK, Narang S, Gupta BN, Singh A, Singh S, & Shanker R (1992)
Effect of dermal exposure to LAS detergent and HCH pesticide in guinea
pigs: biochemical and histopathologic changes in liver and kidney. J
Toxicol Cutan Ocular Toxicol, 11 (1): 3-13.
Matsueda T, Osaki Y, & Shigee S (1982) [Determination of
alkylbenzenesulfonate in water by high-performance liquid
chromatography.] Jpn Anal (Bunseki Kagaku), 31: 59-62 (in Japanese).
Matsuura T & Smith JM (1970) Kinetics of photodecomposition of dodecyl
benzene sulfonate. Ind Eng Chem Fundam, 9: 252-260.
Matthijs E & De Henau H (1987) Determination of LAS. Tenside
Surfactants Deterg, 24: 193-199.
Maurer EW, Cordon TC, & Stirton AJ (1971) Microaerophilic
biodegradation of tallow-based anionic detergents in river water. J Am
Oil Chem Soc, 48: 163-165.
Mayer FL (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Washington, DC, US Environmental Protection Agency, p 178
(EPA/600/8-87/017; PB-87-188686).
McAvoy DC, Eckhoff WS, & Rapaport RA (1993) Fate of linear
alkylbenzene sulfonate in the environment. Environ Toxicol Chem, 12:
977-987.
McCutcheon (1989) McCutcheon's emulsifiers and detergents:
International edition. Glen Rock, New Jersey, McCutcheon's Division,
Manufacturing Confectioner Publishing Co., 240 pp.
McCutcheon (1993) Volume 1: McCutcheon's emulsifiers & detergents:
North American edition-International edition. Glen Rock, New Jersey,
McCutcheon's Division, Manufacturing Confectioner Publishing Co. 295
pp.
McEvoy J & Giger W (1985) Accumulation of linear
alkylbenzenesulphonate surfactants in sewage sludges.
Naturwissenschaften, 72: 429-431.
McEvoy J & Giger W (1986) Determination of linear
alkylbenzenesulfonates in sewage sludge by high-resolution gas
chromatography/mass spectrometry. Environ Sci Technol, 20: 376-383.
McKim JM, Arthur JW, & Thorslund TW (1975) Toxicity of a linear
alkylate sulfonate detergent to larvae of four species of freshwater
fish. Bull Environ Contam Toxicol, 14: 1-7.
Merits I (1975) The metabolism of labelled hexadecyl sulphate salts in
the rat, dog and human. Biochem J, 148: 219-228.
Michael WR (1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol Appl Pharmacol, 12: 473-485.
Mieure JP, Waters J, Holt MS, & Matthijs E (1990) Terrestrial safety
assessment of linear alkylbenzene sulfonate. Chemosphere, 21: 251-262.
Mikami Y, Nagai H, Sakai Y, Fukushima S, & Nishino T (1969) [Effects
of a detergent on the development of the mouse embryo.] Congenit Anom
(Senten Ijo), 9: 230 (in Japanese).
Minegishi K, Osawa M, & Yamaha T (1977) Percutaneous absorption of
alpha-olefin sulfonate (AOS) in rats. Chem Pharm Bull, 25 (4):
821-825.
Ministry of Health and Welfare (1992) [Trend analysis of water works
in Japan.] J Jpn Water Works Assoc, 61: 41-78 (in Japanese).
Misra V, Lal H, Viswanathan PN & Krishna Murti CR (1984) 45Ca uptake
from water by snails (Lymnaea vulgaris) in control and
detergent-polluted samples. Ecotoxicol Environ Saf, 8: 97-99.
Misra V, Lal H, Chawla G, & Viswanathan PN (1985) Pathomorphologic
changes in gills of fish fingerlings (Cirrhina mrigala) by linear
alkyl benzene sulfonate. Ecotoxicol Environ Saf, 10: 302-308.
Misra V, Chawla G, Kumar V, Lal H, & Viswanathan PN (1987) Effect of
linear alkyl benzene sulfonate in skin of fish fingerlings (Cirrhina
mrigala): Observations with scanning electron microscope. Ecotoxicol
Environ Saf, 13: 164-168.
Misra V, Javed S, Pandey DP, & Viswanathan PN (1989a) Biochemical and
pathomorphologic studies for safety evaluation of synthetic
detergents. J Toxicol Cutan Ocular Toxicol, 8: 271-278.
Misra V, Kumar V, Pandey SD, & Viswanathan PN (1989b) Uptake and
distribution of 203Hg by fish fingerlings, Cirrhina mrigala,
exposed to linear alkyl benzene sulphonate. Bull Environ Contam
Toxicol, 43: 139-143.
Misra V, Kumar V, Pandey SD, & Viswanathan PN (1991) Biochemical
alterations in fish fingerlings (Cyprinus carpio) exposed to
sublethal concentration of linear alkyl benzene sulphonate. Arch
Environ Contam Toxicol, 21: 514-517.
Mitchell GC & Holt MS (1993) The fate and effects of linear
alkylbenzene sulphonate (LAS) in outdoor artificial streams. Presented
at the First SETAC World Congress: Ecotoxicology and Environmental
Chemistry--A Global Perspective, Lisbon, Portugal, 28-31 March 1993.
New York, Society of Environmental Toxicology and Chemistry.
Miura KIV, Suzuki K, Tsuchikura A, Baba Y, Hayakana T, Hayashi S,
Moriyama T, Ishiguro T, & Yoshida Y (1968) [Alkylbenzene sulphonate in
the water of the Tama River.] J Jpn Oil Chem Soc [Yukagaku], 17:
635-637 (in Japanese).
Miura K, Yamanaka K, Sangai T, Yoshimura K, & Hayashi N (1979)
[Application of the biological oxygen consumption measurement
technique in the biodegradation testing of surfactants.] J Jpn Oil
Chem Soc [Yukagaku], 28 (5): 351-355 (in Japanese).
Moreno A, Ferrer J, & Berna JL (1990) Biodegradability of LAS in a
sewer system. Tenside Surfactants Deterg, 27 (5): 312-315.
Motomizu S, Fujiwara S, Fujiwara A, & Toei K (1982) Solvent
extraction-spectrophotometric determination of anionic surfactants
with ethyl violet. Anal Chem, 54: 392-397.
Motoyama S & Mukai H (1981) [Synthetic detergents in the environment.]
Niigota Rikagaku, 7: 21-29 (in Japanese).
Nakae A, Tsuji K, & Yamanaka M (1980) Determination of trace amounts
of alkylbenzenesulfonates by high-performance liquid chromatography
with fluorimetric detection. Anal Chem, 52 (14): 2275-2277.
Nakahara M, Ishibashi M, Asao T, Tsukamoto T, Shinagawa K, Kinoshita
Y, Yamamoto H, Mitsuda B, & Kunita N (1976) [Studies on toxicity of
commercial detergents; 1st report.] Ann Rep Osaka Prefect Inst Public
Health, 7: 131-135 (in Japanese).
Nakamura E, Fujisawa I, & Namiki H (1983) [Determination of anionic
surfactant in water by indirect atomic absorption spectrometry after
solvent extraction with potassium-dibenzo-18-crown-6.] Jpn Anal
[Bunseki Kagaku], 32: 332-336 (in Japanese).
Nielsen AM & Huddleston RL (1981) Ultimate biodegradation of linear
alkylbenzene sulfonate alkyl and ring carbon. Dev Ind Microbiol, 22:
415-424.
Nielsen AM, Meyers JD, Bleckmann CA, & Huddleston RL (1980) LAS
biodegradation: Ultimate fate of alkyl and ring carbon. Househ Pers
Prod Ind, March: 68-87.
Nishimura H (1976) [Could skin application of cleansing detergents be
teratogenic cause in successive joint studies and interpretation of
the results?] Congenit Anom (Senten Ijo), 16: 175-185 (in Japanese).
Nolen GA, Klusman LW, Patrick LF, & Geil RG (1975) Teratology studies
of a mixture of tallow alkyl ethoxylate and linear alkylbenzene
sulfonate in rats and rabbits. Toxicology, 4: 231-243.
Nomura T, Kimura S, Hata S, Kanzaki T, & Tanaka H (1980) The synthetic
surfactants AS and LAS interrupt pregnancy in mice. Life Sci, 26:
49-54.
Nonaka K, Hattori Y, & Nakamoto M (1989) [Determination of LAS in
environmental and domestic waste waters.] Suishitsu Odaku Kenkyo, 12
(3): 194-200 (in Japanese).
Nonaka K, Hayashi Y, & Nakamoto M (1990) [The distribution and fate of
LAS in some river waters of Osaka.] Ann Rep Environ Pollut Control
Cent Osaka Prefect Gov, 12: 68-79 (in Japanese).
Nusair TL, Danneman PJ, Stotte J, & Bay PHS (1988) Consumer products:
risk assessment process for contact sensitization. Toxicologist, 8:
258.
Nyberg H (1988) Growth of Selenastrum capricornutum in the presence
of synthetic surfactants. Water Res, 22: 217-223.
Oba K & Yoshida Y (1965) [Determination of ABS in well water.] J Jpn
Water Works Assoc [Suido Kyokai Zasshi], 371: 16-21 (in Japanese).
Oba K, Yoshida Y, & Tomiyama S (1967) [Studies on the biodegradation
of synthetic detergents. I. Biodegradation of anionic surfactants
under aerobic and anaerobic conditions.] J Jpn Oil Chem Soc
[Yukagaku], 16: 517-523 (in Japanese).
Oba K, Mori A, & Tomiyama S (1968a) [Biochemical studies of
n-alpha-olefin sulfonates: (II) Acute toxicity, skin and eye
irritation, and some other physiological properties.] J Jpn Oil Chem
Soc [Yukagaku], 17 (11): 628-634 (in Japanese).
Oba K, Mori A, & Tomiyama S (1968b) [Biochemical studies of
n-alpha-olefin sulfonate. (I) Biodegradability under aerobic
conditions.] J Jpn Oil Chem Soc [Yukagaku], 17 (9): 517-520 (in
Japanese).
Oba K, Yagi R, & Miura K (1975) [Conversion of detergents to
biodegradable compounds and their removal in municipal sewage
treatment plants.] Yosui To Haisui, 17: 1385-1391 (in Japanese).
Oba K, Miura K, Sekiguchi H, Yagi R, & Mori A (1976) Microanalysis of
anionic surfactants in waste water by infrared spectroscopy. Water
Res, 10: 149-155.
Oba K, Mori A, & Nagai T (1985) Safety in use of AOS materials in
Japan. J Am Oil Chem Soc, 62: 645.
Oda Y, Kuwano A, Inoue K, Hamano Y, Yamamoto H, Mitsuda B, & Kunita N
(1977) [Studies on the toxicity of commercial detergents. 6th report:
mutagenicity study in bacteria.] Ann Rep Osaka Prefect Inst Public
Health, 8: 11-16 (in Japanese).
Oda Y, Yamamoto H, & Kunita N (1980) [Studies on the mutagenicity of
surfactants.] Abstracts of 9th Annual Meeting of the Japanese
Environmental Mutagen Society. J Jpn Mutag Soc, 37: 11-16 (in
Japanese).
Ohki K & Tokiwa F (1969) [Physicochemical properties of sulfate-type
and ether sulfate-type surfactants: effects of alkyl chain structure.]
J Chem Soc Jpn Chem Ind Chem [Nihon Kagakuzasshi], 90 (11): 1114-1118
(in Japanese).
Ohki K & Tokiwa F (1970) [Physicochemical properties of
alpha-olefin-sulfonate sodium salts.] J Chem Soc Jpn Chem Ind Chem
[Nihon Kagakuzasshi], 91 (6): 534-539 (in Japanese).
Ohkuma T (1981) [Determination of sodium linear alkylbenzene sulfonate
in rivers of Fukuoka City.] Fukuoka-shi Eisei Skikenshoho, 7: 75-80
(in Japanese).
Oishi S, Takahashi O, Masubuchi M, Fukuda H, Kamiya N, Yoshida S,
Mikuriya H, Yuzawa K, & Hiraga K (1974) [The toxicity of the higher
alcoholic detergents; (II), Subacute toxicity of sodium dodecyl
sulfate.] Ann Rep Tokyo Metrop Res Lab Public Health, 25: 669-675 (in
Japanese).
Okamoto K (1974) [Action of the surfactants upon the skin,
desquamative change of the skin surface, irritation of the skin and
the inhibition of invertase activity by surfactants.] J Jpn Oil Chem
Soc [Yukagaku], 23 (11): 726-729 (in Japanese).
Okamoto T & Shirane Y (1982) [Pollution by linear alkylbenzene
sulfonates (LAS) in river and coastal areas in Hiroshima Prefecture.]
Hiroshima-Ken Kankyo Sata Kenkyu Hokoku, 3: 60-65 (in Japanese).
Okamoto K & Takase Y (1976a) [Studies on keratodermia tylodes palmaris
progressiva.] Shinshu Med J [Shinshu Ishi], 24 (2): 131-141 (in
Japanese).
Okamoto K & Takase Y (1976b) [Action of surfactants upon the skin;
(IV), Effect of sodium alkyl sulfates with lipophilic groups of
different alkyl chain length on the skin.] Shinshu Med J [Shinshu
Ishi], 24 (3): 243-250 (in Japanese).
Okpokwasili GC & Nwabuzor CN (1988) Primary biodegradation of anionic
surfactants in laundry detergents. Chemosphere, 17: 2175-2182.
Olson KJ, Dupree RW, Plomer ET, & Rowe VK (1962) Toxicological
properties of several commercially available surfactants. J Soc Cosmet
Chem (USA), 13: 469-482.
Osburn QW (1986) Analytical methodology for linear alkylbenzene
sulfonate (LAS) in waters and wastes. J Am Oil Chem Soc, 63: 257-263.
Oser BL & Morgareidge K (1965) Toxicologic studies with branched and
linear alkyl benzene sulfonates in rats. Toxicol Appl Pharmacol, 7:
819-825.
Ottery J, Olavesen AH, & Dodgson KS (1970) Metabolism of dodecyl
sulphate in the rat: non-enzymic liberation of sulphate and
gamma-butyrolactone from the major metabolite, butyric acid
4-sulphate. Life Sci, 9 (2): 1335-1340.
Painter HA (1992) Anionic surfactants. In: de Oude NT ed.The handbook
of environmental chemistry. Vol 3, Part F: Anthropogenic compounds.
Berlin, Springer-Verlag, pp 1-88.
Painter HA & Mobey FE (1992) The question of the anaerobic
biodegradability of linear alkylbenzene sulfonates (LAS). Proceedings
of the European Committee of Organic Surfactants and Their
Intermediates (CESIO) Surfactant Congress, London, June 1992.
Brussels, CESIO, pp 34-41.
Painter HA & Zabel TF (1988) Review of the environmental safety of
LAS. Report to the Council of European Federations of Chemical
Industry (CEFIC). Medmenham, Water Research Centre, 245 pp (CO
1659-M/1/EV 8658).
Painter HA & Zabel T (1989) The behaviour of LAS in sewage treatment.
Tenside Surfactants Deterg, 26: 108-115.
Palmer AK, Readshaw MA, & Neuff AM (1975a) Assessment of the
teratogenic potential of surfactants (Part I)--LAS, AS and CLD.
Toxicology, 3: 91-106.
Palmer AK, Readshaw MA, & Neuff AM (1975b) Assessment of the
teratogenic potential of surfactants (Part II)--AOS. Toxicology, 3:
107-113.
Palmisano AC, Schwab BS, Maruscik DA, & Ventullo RM (1991) Seasonal
changes in mineralisation of xenobiotics by stream microbial
communities. Can J Microbiol, 37: 939-948.
Panigrahi AK & Konar SK (1986) Effects of mixture of petroleum
refinery effluent and an anionic linear alkyl benzene sulfonate
detergent on aquatic ecosystem. Environ Ecol, 4: 434-438.
Pärt P, Svanberg O, & Bergstrom E (1985) The influence of surfactants
on gill physiology and cadmium uptake in perfused rainbow trout gills.
Ecotoxicol Environ Saf, 9: 135-144.
Persoone G, Van de Vel A, Van Steertegem M, & De Nayer B (1989)
Predictive value of laboratory tests with aquatic invertebrates:
influence of experimental conditions. Aquat Toxicol, 14: 149-167.
Pickering QH (1966) Acute toxicity of alkyl benzene sulfonate and
linear alkylate sulfonate to the eggs of the fathead minnow,
Pimephales promelas. Air Water Pollut Int J, 10: 385-391.
Pickering QH (1988) Evaluation and comparison of two short-term
fathead minnow tests for estimating chronic toxicity. Water Res, 22:
883-893.
Pickering QH & Thatcher TO (1970) The chronic toxicity of linear
alkylate sulfonate (LAS) to Pimephales promelas, Rafinesque. J Water
Pollut Control Fed, 42: 243-254.
Pitter P & Fuka T (1979) The problem of ultimate biodegradability of
linear alkylbenzene sulphonates. Tenside Surfactants Deterg, 16:
298-302.
Pittinger CA, Woltering DM, & Masters JA (1989) Bioavailability of
sediment-sorbed and aqueous surfactants to Chironomus riparius
(midge). Environ Toxicol Chem, 8: 1023-1033.
Polano MK (1968) The interaction of detergents and the human skin. J
Soc Cosmet Chem, 19: 3-20.
Prats D, Ruiz F, Vazquez B, Zarzo D, Berna JL, & Moreno A (1993) LAS
homolog distribution shift during wastewater treatment and composting:
ecological implications. Environ Toxicol Chem, 12: 1599-1608.
Price KS, Waggy GT, & Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-77.
Prottey C & Ferguson T (1975) Factors which determine the skin
irritation potential of soaps and detergents. J Soc Cosmet Chem, 26:
29-46.
Putterman GT, Wolejsza NF, Wolfram MA, & Laden K (1977) The effect of
detergents on swelling of stratum corneum. J Soc Cosmet Chem (USA),
28: 521-532.
Rama Bhat S, Crisp PT, Eckert JM, & Gibson NA (1980) A
spectrophotometric method for the determination of anionic surfactants
at µg-l1 levels. Anal Chem Acta, 116: 191-193.
Rapaport RA & Eckhoff WS (1990) Monitoring linear alkyl benzene
sulfonate in the environment: 1973-1986. Environ Toxicol Chem, 9:
1245-1257.
Rapaport RA, Hopping WD, & Eckloff WS (1987) Monitoring LAS in the
environment: abstracts and programme. Eighth Annual Meeting of the
Society of Environmental Toxicology and Chemistry (SETAC), Pensacola,
Florida. New York, Society of Environmental Toxicology and Chemistry.
Rehwoldt R, Lasko L, Shaw C, & Wirhowski E (1974) Toxicity study of
two oil spill reagents toward Hudson River fish species. Bull Environ
Contam Toxicol, 11: 159-162.
Reiff B, Lloyd R, How MJ, Brown D, & Alabaster JS (1979) The acute
toxicity of eleven detergents to fish: results of an interlaboratory
exercise. Water Res, 13: 207-210.
Rennison PM, Powell GM, Olavesen AH, Howes D, & Black JG (1987)
Absorption and excretion of linear alkylbenzene sulphonates in rats.
Biochem Soc Trans, 15: 456.
Renzoni A (1974) Influence of toxicants on marine invertebrate larvae.
Thalassia Jugosl., 10: 197-211.
Richtler HJ & Knaut J (1988) World prospects for surfactants. In:
Proceedings of the Second World Surfactants Congress: Surfactants in
Our World Today and Tomorrow, Paris, 24-27 May 1988. Paris, Syndicat
National des Fabricants d'Agents de Surface et de Produits Auxiliaires
Industriels.
Richtler HJ & Knaut J (1991) Environment and oleochemicals--a
challenge. Fett Wissensch Technol, 93: 1-13.
Roberts DW (1989) Aquatic toxicity of linear alkyl benzene sulphonates
(LAS)--A QSAR analysis. In: Turner JE, England MW, Schultz TW, & Kwaak
NJ ed. QSAR 88. Proceedings of the Third International Workshop on
Quantitative Structure-Activity Relationships in Environmental
Toxicology, Knoxville, Tennessee, 22-26 May 1988. Springfield,
Virginia, US Department of Commerce, National Technical Information
Service, pp 91-98.
Roberts DW (1991) QSAR issues in aquatic toxicology of surfactants.
Sci Total Environ, 109/110: 557-568.
Roberts DW & Williams DL (1983) Sultones as by-products in anionic
surfactants. Tenside Surfactants Deterg, 20 (3): 109-111.
Roberts MH, Warinner JE, Tsai CF, Wright D, & Cronin E (1982)
Comparison of estuarine species sensitivities to three toxicants. Arch
Environ Contam Toxicol, 11: 681-692.
Roberts DW, Lawrence JG, Fairweather IA, Clemett CJ, & Saul CD (1990)
Alk-1-ene-1,3-sultones in alpha-olefin sulphonates. Tenside
Surfactants Deterg, 27 (2): 82-86.
Robinson EC & Schroeder RE (1992) Reproductive and developmental
toxicity studies of a linear alkylbenzene mixture in rats. Fundam Appl
Toxicol, 18 (4): 549-556.
Robra KH (1976) [Detection of toxic water contaminants by inhibition
of substrate oxidation in Pseudomonas using polarography.]
Wasser/Abwasser, 117: 80-86 (in German).
Röderer G (1987) Toxic effects of tetraethyl lead and its derivatives
on the chrysophyte Poterioochromonas malhamensis. VIII. Comparative
studies with surfactants. Arch Environ Contam Toxicol, 16: 291-301.
Rohrer DM & Woodbridge DD (1975) Reduction in surfactants by
irradiation. Bull Environ Contam Toxicol, 13: 31-36.
Rosner E, Sawinsky A, Molnar A, Pasztor G, & Mihak I (1973)
[Laboratory tests on workers exposed to dodecyl benzolsulfonate.]
Egeszsegtudomany, 17: 163-165 (in Hungarian).
Russell NJ, Anderson DJ, Day MJ, & White GF (1991) Colonization of
biofilms by bacteria capable of biodegrading sodium dodecyl sulphate
(SDS) at clean and polluted sites. Microbiol Ecol, 22: 85-98.
Sadai M & Mizuno N (1972) [Effect of long term topical application of
some anionic surfactants on the skin, oral mucous membrane, and
tongue.] Jpn J Dermatol [Nihon Hifuka Gakkaishi], 82 (4): 207-221 (in
Japanese).
Sadai M, Sato T, Arima M, Adachi K, & Tamai H (1979) [Effect of some
surfactants on the skin.] J Jpn Cosmet Sci Soc [Kousho Kaishi], 3 (1):
60-62 (in Japanese).
Saito T & Hagiwara K (1982) Analysis of traces of surfactants in water
with anion-exchange resin and polymeric adsorbent. Fresenius Z Anal
Chem, 312: 533-535.
Saito T, Higashi K, & Hagiwara K (1982) Determination of traces of
sodium alkylbenzenesulphonate by high-performance liquid
chromatography: application to water. Fresenius Z Anal Chem, 313:
21-23.
Sakashita S (1979) Electron microscopic study of liver tissue after
cutaneous administration of detergents: multiple application of 1%
solution. Jpn J Clin Electron Microsc [Nihon Rinsho Denshikenbikyo
Gakkaishi], 12 (1-2): 189-216.
Sakashita S, Agata I, & Akiyoshi S (1974) [An electron microscopic
study on the liver after the dermal application of various
detergents.] Mie Med J [Mie Igaku], 18: 124-143 (in Japanese).
Sales D, Quiroga JM, & Gomez-Parra A (1987) Primary biodegradation
kinetics of anionic surfactants in the marine environment. Bull
Environ Contam Toxicol, 39: 385-392.
Samejima Y (1991) [Effects of synthetic surfactants and natural soap
on the development of mouse embryos in vitro and the fertilizing
capacity of mouse and human sperm.] J Osaka Univ Med Sch, 3 (12):
675-682 (in Japanese).
Sasagawa S (1963) [The present status of housewives' hand dermatitis
in Japan and the role of detergents as possible causes.] Hifukano
Rinsho, 5: 47-55 (in Japanese).
Sasagawa S, Takase Y, & Ishihara M (1978) [A study on the irritation
effects of detergent components on human skin by the patch test: A
report of studies on synthetic detergents.] Tokyo, Science and
Technology Agency of Japan, Research Coordination Bureau pp 243-266
(in Japanese).
Sato K, Ando H, Yuzawa K, & Hiraga K (1972) [Studies on toxicity of
synthetic detergents; (III), Examination of teratogenic effects of
alkyl benzene sulfonate spread on the skin of mice.] Ann Rep Tokyo
Metrop Res Lab Public Health, 24: 441-448 (in Japanese).
Sawada K, Inomata S, Gobara B, & Suzuki T (1983) Extraction and
determination of anionic surfactants with copper (II) ethylenediamine
derivative complexes. Talanta, 30 (3): 155-159.
Sawano J (1978) [Teratogenicity studies of anionic surfactants (AOS,
AOS-S) absorbed through skin: A report of studies on synthetic
detergents.] Tokyo, Science and Technology Agency of Japan, Research
Coodination Bureau, pp 112-113 (in Japanese).
Schöberl P (1989) Basic principles of LAS biodegradation. Tenside
Surfactants Deterg, 26: 86-94.
Schöberl P, Bock KJ, & Huber L (1988) Surfactant biodegradation.
Tenside Surfactants Deterg, 25: 2-7.
Schumacher KA, Classen HG, Marquardt P, & Spath L (1972) [Acute
pulmonary hypertonus in cats after parenteral application of sodium
decylsulfate and sodium dodecyl sulfate.] Arzneim Forsch, 22 (9):
1489-1491 (in German).
Sedlak RI & Booman KA (1986) A study of LAS and alcohol ethoxylate
removal at a municipal wastewater treatment plant. Chim Oggi, 9:
21-24.
Sekiguchi HK, Miura K, Oba K, & Mori A (1972) Biodegradation of AOS
and other surfactants. J Jpn Oil Chem Soc, 21: 451-455.
Sekiguchi H, Miura K, Oba K, & Mori A (1975a) [Biodegradation of
alpha-olefin sulfonates (AOS) and other surfactants.] J Jpn Oil Chem
Soc [Yukagaku], 24: 145-148 (in Japanese).
Sekiguchi H, Miura K, & Oba K (1975b) [Biodegradation of some anionic
surfactants in the river die-away test.] J Jpn Oil Chem Soc
[Yukagaku], 24: 451-455 (in Japanese).
Sekiguchi H, Oba K, Masuda M, & Sugiyama T (1975c) [Studies on the
relative biodegradation rate of alkene vs hydroxyalkane sulfonates in
alpha-olefin sulfonate.] J Jpn Oil Chem Soc [Yukagaku], 24: 675-679
(in Japanese).
Setzkorn EA & Huddleston RL (1965) Ultraviolet spectroscopic analysis
for following the biodegradation of hydrotopes. J Am Oil Chem Soc, 42:
1081-1084.
Sharma AK, Bandre TR, Srinivasu T, & Chandra N (1985) Deleterious
effects of detergent on plants. Environ Ecol, 3: 444-445.
Shiga Prefecture (1988) [Water quality of Lake Biwa in 1988.] Shiga
Prefect Inst Public Health: 22 (in Japanese).
Shimp RJ (1989) LAS biodegradation in estuaries. Tenside Surfactants
Deterg, 26 (6): 390-393.
Shiobara S & Imahori A (1976) [Effects of linear alkylbenzene
sulfonate orally administered to pregnant mice on the pregnant mice
and their fetuses.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku Zasshi], 17
(4): 295-301 (in Japanese).
Smyth HF, Seaton J, & Fischer L (1941) Some pharmacological properties
of the 'Tergitol' penetrants. J Ind Hyg Toxicol, 23 (10): 478-483.
Snell TW & Persoone G (1989) Acute toxicity bioassays using rotifers.
II. A freshwater test with Brachionus rubens. Aquat Toxicol, 14:
81-92.
Solon JM & Nair JH (1970) The effect of a sublethal concentration of
LAS on the acute toxicity of various phosphate pesticides to the
fathead minnow ( Pimephales promelas Rafinesque). Bull Environ Contam
Toxicol, 5: 408-413.
Solon JM, Lincer JL, & Nair JH (1969) The effect of sublethal
concentration of LAS on the acute toxicity of various insecticides to
the fathead minnow ( Pimephales promelas Rafinesque). Water Res, 3:
767-775.
Stalmans M, Matthijs E, & de Oude NT (1991) Fate and effects of
detergent chemicals in the marine and estuarine environment. Water Sci
Technol, 24: 115-126.
Standing Technical Committee on Synthetic Detergents (1978) Annual
report of the UK Standing Technical Committee on Synthetic Detergents.
London, Her Majesty's Stationery Office.
Standing Technical Committee on Synthetic Detergents (1989) Annual
report of the UK Standing Technical Committee on Synthetic Detergents.
London, Her Majesty's Stationery Office.
Steber J (1979) Investigations into the biodegradation of
14C-labelled linear alkyl benzene sulphonate in models of surface
waters and sewage purification plants. Tenside Surfactants Deterg, 16:
140-145.
Steber J & Wierich P (1987) The anaerobic degradation of detergent
range fatty alcohol ethoxylates. Studies with 14C-labelled model
surfactants. Water Res, 21: 661-667.
Steber J, Gode P, & Guhl W (1988) Fatty alcohol sulfates: The
ecological evaluation of a group of important detergent surfactants.
Fett Wiss Technol, 90: 32-38.
Stenton SC, Dennis JH, Walters EH, & Hendrick DJ (1990) Asthmagenic
properties of a newly developed detergent ingredient: sodium
isononanoyl oxybenzene sulphonate. Br J Ind Med, 47 (6): 405-410.
Sterzel W (1992) Daily human intake. In: Gloxhuber C & Künstler K ed.
Anionic surfactants; biochemistry, toxicology, dermatology, 2nd ed.
New York, Marcel Dekker, Inc., pp 411-417.
Sueishi T, Morioka T, Kaneko H, Kusaka M, Yagi S, & Chikami S (1988)
Environmental risk assessment of surfactants: fate and environmental
effects in Lake Biwa basin. Regul Toxicol Pharmacol, 8: 4-21.
Sullivan WT & Evans RL (1968) Major US river reflects surfactant
changes. Environ Sci Technol, 2: 194-200.
Sullivan WT & Swisher RD (1969) MBAS and LAS surfactants in the
Illinois River, 1968. Environ Sci Technol, 3: 481-483.
Sunakawa T, Ikida Y, & Okamoto K (1979) [Absorption, distribution,
metabolism, and xcretion of linear alkylbenzene sulfonate in rats.] J
Jpn Oil Chem Soc [Yukagaku], 39 (2): 59-68 (in Japanese).
Sunakawa T, Inoue K, & Okamoto K (1981) [Studies on the mutagenicity
of surfactants; mutagenicity of surfactants following activation with
various liver homogenates (S-9) and mutagenicity in the presence of
norharman.] Hyg Chem [Eisei Kagaku], 27 (4): 204-211 (in Japanese).
Swedmark M & Granmo A (1981) Effects of mixtures of heavy metals and
a surfactant on the development of cod ( Gadus morhua L.). Rapp P-V
Reun Cons Int Explor Mer, 178: 95-103.
Swedmark M, Braaten B, Emanuelsson E, & Granmo A (1971) Biological
effects of surface active agents on marine animals. Mar Biol, 9:
183-201.
Swisher RD (1963) The chemistry of surfactant biodegradation. J Am Oil
Chem Soc, 40: 648-656.
Swisher RD (1967) Biodegradation of LAS-benzene rings in activated
sludge. J Am Oil Chem Soc, 44: 717-724.
Swisher RD (1970) Surfactant biodegradation. New York, Marcel Dekker,
Inc., 469 pp (Surfactant Science Series, Vol 3).
Swisher RD (1972) Linear alkylbenzene sulfonate (LAS) benzene rings:
Biodegradation and acclimation studies. J Jpn Oil Chem Soc, 21:
130-142.
Swisher RD (1976) Surfactants: from recalcitrant to docile. In:
Proceedings of the 3rd International Biodegradation Symposium. London,
Applied Science Publishers, pp 853-865.
Swisher RD (1981) The problem of ultimate biodegradability of linear
alkylbenzene sulfonates: an extension. Tenside Surfactants Deterg, 18:
57-63.
Swisher RD (1987) Surfactant biodegradation. New York, Marcel Dekker,
Inc., 1085 pp (Surfactant Science Series, Vol 18).
Swisher RD, O'Rourke JT, & Tomlinson HD (1964) Fish bioassays of
linear alkylate sulfonates (LAS) and intermediate biodegradation
products. J Am Oil Chem Soc, 41: 746-752.
Swisher RD, Gledhill WE, Kimerle RA, & Taulli TA (1978) Carboxylated
intermediates in the biodegradation of linear alkylbenzene sulfonates
(LAS). In: Proceedings of the 7th International Congress on Surface
Active Substances, Moscow, June 1976. Moscow, Teknol Vody, Vol 4, pp
218-230.
Szakall A & Schulz KH (1960) [Permeation of fatty alcohol sulfate and
sodium soap of defined chain length (C8-C18) in intact human skin,
and its relation to irritant activity.] Fette Seifen Anstrichm, 62
(3): 170-175 (in German).
Tabor CF, Barber LB, & Runnells DD (1993) Anionic surfactants in the
Mississippi River: A detailed examination of the occurrence and fate
of linear alkylbenzene sulfonate. Presented at the Division of
Environmental Chemistry, American Chemical Society, Denver, Colorado,
28 March-2 April 1993.
Taguchi S, Tonoshima T, Kasahara I, & Goto K (1981) [Extraction and
spectrophotometric determination of anionic surfactants with
[2-(5-chloro-2-pyridylazo)-5-diethylaminophenolato] cobalt (III)
chloride.] Kogyo Yosui, 278: 23-27 (in Japanese).
Takada H & Ishiwatari R (1987) Linear alkylbenzenes in urban riverine
environments in Tokyo: distribution, source, and behavior. Environ Sci
Technol, 21: 875-883.
Takada H, Ishiwatari R, & Ogura N (1992a) Distribution of linear
alkylbenzenes (LABs) and linear alkylbenzene-sulphonates (LAS) in
Tokyo Bay sediments. Estuar Coast Shelf Sci., 35: 141-156.
Takada H, Ogura N, & Ishiwatari R (1992b) Seasonal variations and
modes of riverine input of organic pollutants to the coastal zone: 1.
Flux of detergent-derived pollutants to Tokyo Bay. Environ Sci
Technol, 26: 2517-2523.
Takahashi O, Shiba H, Nakao T, & Hiraga K (1974) [Inhibition of
thrombin by linear alkylbenzene sulfonate (LAS).] Ann Rep Tokyo Metrop
Res Lab Public Health, 25: 637-645 (in Japanese).
Takahashi A, Sato K, Ando H, Kubo Y, & Hiraga K (1975) [Teratogenicity
of some synthetic detergent and linear alkylbenzene sulfonate (LAS).]
Ann Rep Tokyo Metrop Res Lab Public Health, 26 (2): 67-78 (in
Japanese).
Takahashi A, Ando H, Kubo Y, & Hiaga K (1976) [Effects of dermal
application of sodium dodecyl sulfate (SDS) on pregnant mice and their
fetuses.] Ann Rep Tokyo Metrop Res Lab Public Health, 27 (2): 113-118
(in Japanese).
Takami K, Kamo T, Mochizuki K, Sugimae A, & Nakamoto M (1987) [Rapid
HPLC determination of linear alkylbenzenesulfonates in environmental
water by using concentration technique with a mini column.] Jpn Anal
[Bunseki Kagaku], 36: 276-281 (in Japanese).
Takano S, Yagi N, & Kunihiro K (1975) [Determination of trace amounts
of alkyl benzenesulfonates in water: determination by methylene blue
spectrophotometry and high performance liquid chromatography.] J Jpn
Oil Chem Soc [Yukagaku], 24 (6): 389-394 (in Japanese).
Takeshita R & Yoshida H (1975) [Studies of anion active surfactants;
(I), Determination of anion active surfactants in natural and waste
waters using cleanup by Amberlite XAD2 column chromatography.] J Hyg
Chem [Eisei Kagaku], 21 (4): 209-215 (in Japanese).
Takita Y (1982) [The effects of some detergents and anionic
surfactants on the early stages of growth of some agricultural
plants.] J Jpn Oil Chem Soc [Yukagaku], 31: 507-510 (in Japanese).
Takita Y (1985) [Effects of biodegradation intermediates of linear
alkylbenzenesulfonate on the growth of sporelings of Porphyra
yeroensis.] J Jpn Oil Chem Soc [Yukagaku], 34: 198-201 (in
Japanese).
Takita Y & Oba K (1985) [Determination of trace amounts of anionic
surfactants in the water of Tama River.] Jpn J Water Pollut Res, 8
(11): 752-754 (in Japanese).
Tamura J & Ogura Y (1969) [Pharmacological studies on surface active
agents. 9: Anionic surface active agents and methaemoglobin formation
in the mouse.] Med Biol [Igakuto Seibutugaku], 8 (2): 41-44 (in
Japanese).
Tanaka Y & Nakanishi H (1981) [Purification on Sephadex LH-20 and high
performance liquid chromatographic determination of
dodecylbenzenesulfonate in sediment and biological samples.] Bunseki
Kagaku, 30: 567-73 (in Japanese).
Tang NH (1974) Relationship between BOD removal and LAS detergent
removal. Springfield, Virginia, National Technical Information
Service, 66 pp (NTIS Report PB-232 977/7WP).
Taniguchi S, Yamada A, Morita S, Ogaki S, & Noda T (1978) Results of
studies on synthetic detergents. Tokyo, Science and Technology Agency,
Research Coordination Bureau, pp 18-54.
Tarazona JV & Nunez O (1987) Acute toxicity of synthetic detergents to
snails: Effect of sodium lauryl sulfate on Limnaea peregra shells.
Bull Environ Contam Toxicol, 39: 1036-1040.
Tatsukawa R & Hidaka H (1978) [The avoidance test of chemical
substances by fish: avoidance of detergents by ayu (Plecoglossus
altivelis).] Nippon Nosei Kasaku Kaishi, 52: 263-270 (in Japanese).
Taylor MJ (1985) Effect of diet on the sensitivity of Daphnia magna
to linear alkylbenzene sulfonate. In: Cardwell RD, Purdy R, & Bahner
RC ed. Aquatic toxicology and hazard assessment: Seventh Symposium.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 53-72 (ASTM STP 854).
Terzic S & Ahel M (1993) Determination of linear alkylbenzene
sulphonates in Krka River estuary. Bull Environ Contam Toxicol, 50:
241-246.
Thatcher TO & Santner JF (1967) Acute toxicity of LAS to various fish
species. Proceedings of 21st Industrial Waste Conference, Purdue
University. Washington, Industrial Waste Association, pp 996-1002.
Thomas ORT & White GF (1989) Metabolic pathway for the biodegradation
of sodium dodecyl sulfate by pseudomonas sp. C12B. Biotechnol Appl
Biochem, 11: 318-327.
Thurman EM, Barber LB, & LeBlanc D (1986) Movement and fate of
detergents in groundwater: a field study. J Contam Hydrol, 1: 143-161.
Tiba S (1972) [Studies on the acute and chronic toxicity of linear
alkylbenzene sulfonate.] J Food Hyg Soc Jpn [Shokuhin Eiseigaku
Zasshi], 13 (6): 509-516 (in Japanese).
Tiba S, Shiobara S, Imahori A, & Kitagawa T (1976) [Effects of linear
alkylbenzene sulfonate on dam, fetus, and newborn rat.] J Food Hyg Soc
Jpn [Shokuhin Eiseigaku Zasshi], 17 (1): 66-71 (in Japanese).
Tokai A, Kikuchi M, Wakabayashi M, & Yoshida T (1990) [Environmental
behavior of selected chemicals in Tokyo Bay with relation to their
physicochemical and biochemical properties.] J Chem Soc Jpn Chem Ind
Chem [Nippon Kagaku Kaishi], 9: 982-991 (in Japanese).
Tokyo Metropolitan Government (1974) [A study on detergents.] Tokyo,
Tokyo Metropolitan Government, 10 pp (Report No. 49-5-29) (in
Japanese).
Tomiyama S (1970) [Alph-olefin sulfonate: commercial production and
performance.] J Jpn Oil Chem Soc [Yukagaku], 19 (6): 359-368 (in
Japanese).
Tomiyama S (1972) [Current problems of detergents, mainly related to
water pollution.] J Jpn Oil Chem Soc [Yukagaku], 21 (1): 2-8 (in
Japanese).
Tomiyama S (1974) [Effects of surfactants on fish.] Bull Jpn Soc Sci
Fish, 40: 1291-1296 (in Japanese).
Tomiyama S (1975) Fundamental study of biochemical behavior of anionic
sulfonate- and sulfate-type surfactants. J Am Oil Chem Soc, 52:
135-138.
Tomiyama S (1978) [Possible mechanisms of the effects on fish and
shellfish of household detergents.] J Jpn Oil Chem Soc [Yukagaku], 27:
347-353 (in Japanese).
Tomizawa S (1978) Results of studies on synthetic detergents. Tokyo,
Science and Technology Agency, Research Coordination Bureau, pp 55-88.
Toshima Y, Moriya T, & Yoshimura K (1992) Effects of polyoxyethylene
(20) sorbitan monooleate on the acute toxicity of linear
alkylbenzenesulfonate (C12LAS) to fish. Ecotoxicol Environ Saf, 24:
26-36.
Tovell PWA, Newsome C, & Howes D (1974) Effect of water hardness on
the toxicity of an anionic detergent to fish. Water Res, 8: 291-296.
Tovell PWA, Howes D, & Newsome CS (1975) Absorption, metabolism and
excretion by goldfish of the anionic detergent sodium lauryl sulphate.
Toxicology, 4: 17-29.
Trehy ML, Gledhill WE, & Orth RG (1990) Determination of linear
alkylbenzenesulfonates and dialkyltetrasulfonates in waters and
sediment by gas chromatography/mass spectrometry. Anal Chem, 62:
2581-2586.
Tsai CF & McKee RA (1978) The toxicity to goldfish of mixtures of
chloramines, and LAS (linear alkylate sulfonate). College Park,
Maryland, University of Maryland, Water Resources Research Center, 36
pp (Technical Report No. 44; PB-280-554).
Tsukioka T & Murakami T (1983) [Determination of
linear-alkylbenzenesulfonate by gas chromatography.] Jpn Anal [Bunseki
Kagaku], 32: 723-728 (in Japanese).
Uchiyama M (1977) Determination of alkyl benzenesulfonate in water by
the UV method. Water Res, 11: 205-207.
Urano K & Saito M (1985) Biodegradability of surfactants and
inhibition of surfactants to biodegradation of other pollutants.
Chemosphere, 14: 1333-1342.
Urano K, Saito M, & Murata C (1984) Adsorption of surfactants on
sediments. Chemosphere, 13: 293-300.
United States Environmental Protection Agency (1981) Mono (C10-16)
alkylbenzenesulfonate acids and their sodium salts. Draft hazard
information review for TSCA Interagency Testing Committee (EPA
Contract No. 68-01-5789). Rockville, Maryland, Enviro Control,
101 pp.
Utsunomiya A, Itoh SI, Setsuda S, Naito S, & Shimozato T (1980)
[Studies on sodium linear alkylbenzenesulfonate (LAS). I. Distribution
of LAS in the sediments of the Sagami River estuary.] Eisei Kagaku,
26: 159-166 (in Japanese).
Utsunomiya A, Ikeda T, Takamatsu K, & Naito S (1982) [Determination of
sodium alkylsulfates as 3,5-dinitrobenzoate derivatives.] Jpn Anal
[Bunseki Kagaku], 31: 115-119 (in Japanese).
Van Emden HM, Kroon CCM, Schoeman EN, & Van Seventer HA (1974) The
toxicity of some detergents tested on Aedes aegypti L., Lebistes
reticulatus Peters, and Biomphalaria glabrata (Say). Environ
Pollut, 6: 297-308.
Van Scott EJ & Lyon JB (1953) A chemical measure of the effect of
soaps and detergents on the skin. J Invest Dermatol, 21: 199-203.
Verge C, Moreno A, Bravo J, Ferrer J, & Bengoechea C (1993) Toxicity
of LAS vs activated sludge of a WWTP and microalgae (Scenedesmus
subspicatus). Presented at Society of Environmental Toxicology and
Chemistry (SETAC) 1st World Congress, Lisbon, March 1993. New York,
Society of Environmental Toxicology and Chemistry.
Vives-Rego J, Vaque D, & Martinez J (1986) Effect of heavy metals and
surfactants on glucose metabolism, thymidine incorporation and
exoproteolytic activity in sea water. Water Res, 20: 1411-1415.
Vives-Rego J, Vaque MD, Leal JS, & Parra J (1987) Surfactants
biodegradation in sea water. Tenside Surfactants Deterg, 24: 20-22.
Volk VV & Jackson ML (1968) Alkyl benzene sulfonate and linear
alkylate sulfonate adsorption by hydroxy iron and aluminium systems.
J Water Pollut Control Fed, 40: 205-213.
Wagner R (1978) [The content of MBAS and BiAS in a communal sewage
treatment plant.] Wasser/Abwasser, 119: 235-242 (in German).
Wakabayashi M & Mizorogi N (1989) [Subacute toxicity of chemical
substances on rainbow trout.] Ann Rep Tokyo Metrop Res Inst Environ
Prot [Tokyo Kogai Kenkyujo Nempo], 1988: 167-169 (in Japanese).
Wakabayashi M & Onizuka S (1986) [Some factors affecting toxicity of
chemicals to fishes.] Ann Rep Tokyo Metrop Res Inst Environ Prot
[Tokyo Kogai Kenkyujo Nempo], 1986: 102-104 (in Japanese).
Wakabayashi M, Kikuchi M, Kojima H, & Yoshida T (1978) Bioaccumulation
profile of sodium linear alkylbenzene sulfonate and sodium alkyl
sulfate in carp. Chemosphere, 11: 917-924.
Wakabayashi M, Kikuchi M, Kojima H, & Yoshida T (1980) Effect of alkyl
chain on the uptake, distribution, and excretion of 35-labelled
alkyl sulfates in carp. Ecotoxicol Environ Saf, 4: 195-206.
Wakabayashi M, Kikuchi M, Sato A, & Yoshida T (1981) [The relationship
between exposure concentration and bioaccumulation of surfactants.]
Bull Jpn Soc Sci Fish, 47: 1383-1387 (in Japanese).
Wakabayashi M, Kikuchi M, Naganuma Y, & Kawakara H (1984) [Acute
toxicity of some detergents in fishes.] Ann Rep Tokyo Metrop Res Inst
Environ Prot [Tokyo Kogai Kenkyujo Nempo], 1984: 114-118 (in
Japanese).
Wakabayashi M, Konno R, & Nishiido T (1988) [Relative lethal
sensitivity of two daphnid species to some chemicals.] Ann Rep Tokyo
Metrop Res Inst Environ Prot [Tokyo Kogai Kenkyujo Nempo], 1988:
126-128 (in Japanese).
Waldmeyer T (1968) Analytical records of synthetic detergent
concentrations, 1956-1966. Water Pollut Control, 67 (2): 66-79.
Walker AIT, Brown VKH, Ferrigan LW, Pickering RG, & Williams DA (1967)
Toxicity of sodium lauryl sulphate, sodium lauryl ethoxysulphate and
corresponding surfactants derived from synthetic alcohols. Food Cosmet
Toxicol, 5: 763-769.
Ward TE (1986) Biodegradation of LAS and model chemicals in aerobic
and anaerobic sewage treatment processes and sludge-amended soils.
Abstracts of the 7th Annual Meeting of the Society of Environmental
Toxicology and Chemistry (SETAC), Arlington, USA, 6-9 November 1983.
New York, Society of Environmental Toxicology and Chemistry (Abstract
300).
Ward TE & Larson RJ (1989) Biodegradation kinetics of linear
alkylbenzene sulfonate in sludge-amended agricultural soils.
Ecotoxicol Environ Saf, 17: 119-130.
Watanabe Y, Nagashima T, & Imai N (1968) [Effect of detergents on
human skin in relation to hand eczema.] Saigai Igaku, 11 (9): 933-940
(in Japanese).
Watari N, Torizawa K, Kanai M, & Suzuki Y (1977) Ultrastructural
observations of the protective effect of glycyrrhizin for mouse liver
injury caused by oral administration of detergent ingredient(LAS). Jpn
J Clin Electron Microsc [Nihon Rinsho Denshikenbikyo Kaishi]), 10
(1-2): 121-139.
Waters J (1976) The contribution of linear alkylbenzene sulphonate to
the MBAS level of some UK and Dutch surface waters. Vom Wasser, 47:
131-140.
Waters J & Garrigan JT (1983) An improved microdesulphonation/gas
liquid chromatography procedure for the determination of linear
alkylbenzene sulphonates in UK rivers. Water Res, 17: 1549-1562.
Waters J, Holt MS, & Matthijs E (1989) Fate of LAS in sludge amended
soils. Tenside Surfactants Deterg, 26: 129-135.
Welp G & Brummer G (1985) [The Fe(III)-reduction test: a simple
procedure to determine the effects of environmental chemicals on
microbial activity in soils.] Z Pflanzenernähr Bodenkd, 148: 10-23 (in
German).
White GF, Russell NJ, & Day MJ (1985) A survey of sodium dodecyl
sulphate (SDS) resistance and alkylsulphatase production in bacteria
from clean and polluted river sites. Environ Pollut, A37: 1-11.
White GF, Anderson DJ, Day MJ, & Russell NJ (1989) Distribution of
planktonic bacteria capable of degrading sodium dodecyl sulphate (SDS)
in a polluted South Wales river. Environ Pollut, 57: 103-115.
Williamson R (1993) Alpha olefin sulfonate composition. Chem Week, 27
January: 13.
Wood DCF & Bettley FR (1971) The effect of various detergents on human
epidermis. Br J Dermatol, 84: 320-325.
Yakabe Y, Etoh C, Matsunobo Y, Katsuura H, Miura K, & Yoshimura K
(1992) Kinetic study on the biodegradation of linear
alkybenzenesulfonates (LAS) in well water. Chemosphere, 24 (8):
969-977.
Yamamoto H (1977) [Carcinogenic and co-carcinogenic testing of
detergents (alkyl-sulfonic-natrium, polyoxy-ethylene-alkylether and
potassium soap) on the skin of mice.] J Nara Med Assoc [Nara Igaku
Zasshi], 28: 307-327 (in Japanese).
Yamamoto K & Motomizu S (1987) [Solvent extraction with
spectrophotometric determination of anionic surfactants in seawater.]
Jpn Anal [Bunseki Kagaku], 36: 335-338 (in Japanese).
Yamamoto H, Inoue K, Hamano Y, Oda Y, Kuwano A, Mitsuda B, & Kunita N
(1976) [Studies on toxicity of commercial detergents. 3rd Report.
Teratogenic effects in mice administered orally.] Ann Rep Osaka
Prefect Inst Public Heath, 7: 141-145 (in Japanese).
Yamane I, Nagayama M, Kashiwa I, Kuwamura H, Ando S, & Mori A (1970)
[Olefin sulfonates. IV. Correlation between carbon chain length and
surface-active properties of alpha-olefin sulfonates.] J Chem Soc Jpn
[Kogyo Kagaku Zasshi], 73 (4): 723-727 (in Japanese).
Yamane AN, Okada M, & Sudo R (1984) The growth inhibition of
planktonic algae due to surfactants used in washing agents. Water Res,
18: 1101-1105.
Yanagisawa F, Watanabe S, & Yamagishi T (1964) [Biochemical studies of
dodecylbenzene sulfonates; differences between soft and hard
detergents.] Jpn J Public Health [Nihon Koshueisei Zasshi], 11 (13):
859-864 (in Japanese).
Yasuda H (1980) [Determination of anionic surfactants in sea and river
waters by anion exchange method.] Jpn J Public Health [Nihon
Koshueisei Zasshi], 27 (8): 373-378 (in Japanese).
Yasunaga Y (1976) [The influence of some pollutants on the survival of
eggs and larvae of two species of flatfish, Limanda yokohamae and
Paralichtys olivaceus.] Bull Tokai Reg Fish Res Lab, 86: 81-111 (in
Japanese).
Yediler A, Zhang Y, Cai JP, & Korte F (1989) Effect of the microbial
population size on the degradation of linear alkylbenzene sulfonate in
lake water. Chemosphere, 18: 1589-1597.
Yokoyama Y & Sato H (1991) Reversed-phase high-performance liquid
chromatographic determination of linear alkylbenzenesulponates in
river water at ppb levels by precolumn concentration. J Chromatogr,
555: 155-162.
Yoneyama M & Hiraga K (1977) [Effect of linear alkylbenzene sulfonate
on serum lipid in rats.] Ann Rep Tokyo Metrop Res Lab Public Health,
28 (2): 109-111 (in Japanese).
Yoneyama M, Fujii T, Ikawa M, Shiba H, Sakamoto Y, Yano N, Kobayashi
H, Ichikawa H, & Hiraga K (1972) [Studies on the toxicity of synthetic
detergents. (II) Subacute toxicity of linear and branched alkyl
benzene sulfonates in rats.] Ann Rep Tokyo Metrop Res Lab Public
Health, 24: 409-440 (in Japanese).
Yoneyama M, Mabuchi Y, Ikawa M, Kobayashi H, & Ichikawa H (1976)
[Subacute toxicity of linear alkylbenzene sulfonate.] Ann Rep Tokyo
Metrop Res Lab Public Health, 27 (2): 105-112 (in Japanese).
Yoneyama M, Masubuchi M, Oishi S, Takahashi O, Ikawa M, Yoshida S,
Oishi H, Mikuriya H, Yuzawa K, & Hiraga K (1977) [Toxicity of linear
alkylbenzene sulfonate by dietary administration for life-span to
rats.] Ann Rep Tokyo Metrop Res Lab Public Heath, 28 (2): 73-84 (in
Japanese).
Yoneyama M, Ikawa M, Nakao T, & Hiraga K (1978) [Effects of LAS on
incorporation of acetate-1-14C in liver lipids in rats.] Ann Rep
Tokyo Metrop Res Lab Public Health, 29 (2): 55-57 (in Japanese).
Yoshida H & Takeshita R (1978) [Studies on anion active surfactants:
II; Contamination of anionic surfactants in the Inland Sea of eastern
Seto.] Eisei Kagaku, 24: 78-82 (in Japanese).
Yoshikawa S, Sano H, & Harada T (1984) [Determination of LAS in river
water by high-performance liquid chromatography.] Suishitsu Odaku
Kenkyu, 7: 191-194 (in Japanese).
Yoshikawa S, Sano H, & Harada T (1985) [Distribution of LAS in river
water and sediment at Tsurumi River.] Jpn J Water Pollut Res, 8:
755-758 (in Japanese).
Yoshimura H, Tanaka S, Fujiyama Y, Sugiyama T, & Nagai T (1984a)
[Determination of ionic surfactants by high performance liquid
chromatography.] J Chem Soc Jpn [Nihon Kagaku Kaishi], 3: 445-451 (in
Japanese).
Yoshimura K, Hayashi K, Kawase J, & Tsuji K (1984b) [Presence of
anionic surfactants in rivers.] Jpn J Limnol [Rikusuigaku Zasshi], 45:
51-60 (in Japanese).
Yushi (1978) Good decomposition of LAS together with detection of ABS.
Water quality and synthetic detergents from the standpoint of a 1977
study. Yushi, 31: 30-33 (in Japanese)
Zaccone G, Fasulo S, Lo Cascio P, & Licata A (1985) Patterns of enzyme
activities in the gills of the catfish Heteropneustes fossilis
(Bloch) exposed to the anion-active detergent
Na-alkyl-benzenesulphonate (LAS). Histochemistry, 82: 341-343.
Zoller U (1985) The 'hard' and 'soft' surfactant profile of Israel
municipal wastewaters. J Am Oil Chem Soc, 62: 1006-1009.
ALKYLBENZENESULFATES A CHAINE DROITE ET COMPOSES VOISINS
1. RECAPITULATION, EVALUATION ET RECOMMANDATIONS GENERALES
1.1 Identité, propriété et méthodes d'analyse
Les alkylbenzènesulfonates à chaîne droite (appelé aussi
alkylbenzènesulfonates linéaires ou ASL, les alpha-oléfine-
sulfonates (AOS) et les alkylsulfates (AS)) sont des tensio-actifs
anioniques dont les molécules sont caractérisées par la présence d'un
groupement hydrophobe et d'un groupement hydrophile (polaire). Les
produits du commerce sont des mélanges d'isomères et d'homologues de
produits voisins, qui different par leurs propriétés physicochimiques
et qui, sous leurs diverses formes, ont des applications variées.
L'analyse des ASL, des AOS et des AS peut se faire par des
méthodes non spécifiques. On utilise généralement l'essai au bleu de
méthylène, qui permet de mettre en évidence tout composé contenant un
groupement anionique et un groupement hydrophobe. On peut donc être
gêné par la présence d'autres substances lorsqu'on travaille sur des
échantillons prélevés dans l'environnement; en outre, la sensibilité
de la méthode n'est que de 0,02 mg/litre. On a mis au point d'autres
méthodes non spécifiques qui peuvent se substituer à celles-ci mais on
ne les utilise guère. En ce qui concerne les échantillons prélevés
dans l'environnement, il n'existe de méthodes spécifiques que pour les
ASL et les AS. En ce qui concerne les AOS, on dispose d'une méthode
améliorée qui repose sur la réaction au bleu de méthylène et la
chromatographie en phase liquide à haute performance (HPLC).
Les ASL sont des composés non volatils que l'on obtient par
sulfonation des alkylbenzènes à chaîne droite. Les produits du
commerce sont toujours constitués de mélanges d'homologues ayant des
chaînes alkylées de différentes longueurs (C10-C13 ou C14) et
d'isomères qui différent par la position du point d'attache de la
chaine sur le noyau phényle (positions 2 à 5). Tous les homologues et
isomères des ASL peuvent être dosés dans des échantillons
environnementaux ou d'autres matrices au moyen de méthodes d'analyse
spécifiques comme la HPLC, la chromatographie en phase gazeuse et la
chromatographie en phase gazeuse couplée à la spectrométrie de masse.
Les AOS sont également des dérivés non volatils produit par
sulfonation des alpha-oléfines. Ils consistent dans le mélange de deux
types de composés, les alcène-sulfonates de sodium et les
hydroxyalcane-sulfonates de sodium, avec une chaine alkylée en
C14-C18.
Egalement non volatils, les AS s'obtiennent en traitant par
l'acide sulfurique, les alcools d'origine oléochimique ou
pétrochimique. Ce sont des mélanges d'homologues avec une chaîne
alkylée en C10-C18. On met actuellement au point des méthodes
d'analyse spécifiques pour la surveillance de l'environnement.
1.2 Sources d'exposition humaine et environnementale
On utilise les ASL, les AOS et les AS comme principes actifs de
divers produits d'entretien ou d'hygiène personnelle, ou encore, pour
certaines applications spéciales. Après usage, ces détergents sont
rejetés dans l'environnement avec les eaux usées.
Il peut y avoir exposition professionnelle à ces composés. Quant
à l'exposition de la population humaine en général et des êtres
vivants dans leur milieu naturel, elle dépend du type d'application de
ces substances (ou d'autres tensio-actifs), des pratiques locales en
matière de traitement des effluents et des caractéristiques du milieu
récepteur.
En 1990, la consommation mondiale de ces produits d'établissait à
2 millions de tonnes pour les ASL, 86 000 tonnes pour le AOS et 289
000 tonnes pour les AS.
1.3 Concentrations dans l'environnement
1.3.1 Alkylbenzènesulfonates à chaîne droite
On peut doser les ASL à l'aide de méthodes spécifiques et
sensibles dans pratiquement tous les compartiments du milieu où ils
sont susceptibles de se trouver. Leur concentration diminue
progressivement selon la séquence suivante: eaux usées > effluents
traités > eaux de surface > mer.
Dans les zones où l'on utilise principalement des ASL comme
tensio-actifs, leur concentration est généralement de 1-10 mg/litre
dans les eaux usées, de 0,05-0,1 mg/litre dans les effluents traités
par voie biologique, de 0,05-0,6 mg/litre dans les effluents traités
sur lit filtrant, de 0,005-0,05 mg/litre dans les eaux de surface
situées au-dessous de déversoirs d'égouts (avec des concentrations qui
tombent rapidement à 0,01 mg/litre en aval du déversoir), de < 1-10
mg/kg dans les sédiments de cours d'eau (< 100 mg/kg dans les
sédiments très pollués à proximité des zones de décharge), de 1-10
g/kg dans les boues d'égouts, de < 1 5 mg/kg dans les sols amendés
à l'aide de boues d'égouts (initialement 5-10 mg/kg; on a trouvé des
concentrations < 50 mg/kg après d'importants épandages de boues,
d'ailleurs non représentatifs). La concentration des ASL dans les eaux
estuarielles varie de 0,001 à 0,01 mg/litre, mais elle peut être
beaucoup plus élevée là où il y a déversement direct d'eaux usées. En
mer, à distance du rivage, les concentrations vont de < 0,001 à
0,002 mg/litre.
Il est à noter que la concentration de ASL varie considérablement
dans l'environnement. Ces variations sont dues à la diversité des
méthodes d'analyse, des points de prélèvement (qui vont de zones très
polluées où sont déversés des effluents insuffisamment traités à des
secteurs où l'effluent a subi un traitement intensif), des périodes de
prélèvement (ce qui selon le cas peut signifier une différence du
simple au double) et enfin, des volumes de ASL consommés.
La surveillance de l'environnement montre que les ASL ne
s'accumulent pas au cours du temps dans les différents compartiments
du milieu. La concentration dans le sol, loin d'augmenter, diminue au
contraire par suite de la minéralisation. Comme les ASL ne se
décomposent pas en anaérobiose stricte (pour donner naissance à du
méthane), on ne peut pas en conclure qu'ils subissent une
minéralisation dans les sédiments anaérobies. Au taux actuel
d'utilisation, les ASL parviennent dans les différents compartiments
de l'environnement à un rythme sensiblement égal à celui de leur
assimilation, ce qui crée les conditions d'un état stationnaire.
1.3.2 Les alpha-oléfine-sulfonates et alkyle-sulfates
Les données dont on dispose sur la concentration des AOS dans
l'environnement sont limitées en raison de la difficulté à analyser
les échantillons prélevés dans le milieu. En général, on peut déceler
la présence des tensio-actifs anioniques au moyen de méthodes
colorimétriques non spécifiques (comme celles qui sont basées sur la
réaction au bleu de méthylène), mais la présence d'autres substances
est gênante et ces méthodes ne permettent pas de procéder à un dosage
spécifique des alpha-oléfine-sulfonates. Une méthode spécifique de
dosage des AS dans l'environnement est en cours de mise au point.
Les études effectuées en laboratoire indiquent que les AOS et les
AS sont rapidement minéralisés dans tous les compartiments de
l'environnement et presque totalement éliminés des effluents au cours
du traitement de ces derniers. Leur concentration dans les eaux de
surface, les sédiments, le sol, les eaux estuarielles et le milieu
marin est probablement faible. C'est précisément ce que l'on a
constaté pour la concentration des AOS dans l'eau des rivières.
1.4 Transport, distribution et transformation dans l'environnement
Aux températures inférieures à 5-10°C, la cinétique de
biodégradation des ASL, des AOS et des AS est ralentie en raison de la
réduction de l'activité microbienne.
1.4.1 Alkylbenzène-sulfonates à chaîne droite
Les voies de pénétration des ASL dans l'environnement varient
selon les pays, mais la porte d'entrée principale est constituée par
la décharge des stations d'épuration des eaux usées. Lorsque ces
stations sont inexistantes ou fonctionnent mal, il peut y avoir
décharge directe dans les rivières, les lacs et la mer. L'épandage de
boues d'égout sur les terrains agricoles peut également constituer une
voie de pénétration de ASL dans l'environnement.
A mesure qu'ils pénètrent dans l'environnement, les ASL en sont
éliminés par divers mécanismes qui vont de l'adsorption à la
biodégradation ultime. Les ASL sont adsorbés sur les surfaces
colloïdales et les particules en suspension, et l'on a mesuré des
coefficients d'adsorption de 40-5200 litres/kg selon le milieu et la
structure des ASL en cause. Ils subissent une biodécomposition dans
les eaux de surface (demi-vie 1-2 jours), dans les sédiments aérobies
(1-3 jours) ainsi que dans les écosystèmes marins et estuariels (5-10
jours).
Lors du traitement primaire des effluents, environ 25% des ASL (de
10-40%) s'adsorbent sur les boues résiduelles et sont rejetés avec
elles. Ils ne sont pas éliminés au cours de la digestion anaérobie des
boues mais au cours du traitement aérobie, leur demi-vie étant alors
de 10 jours. Après épandage des boues sur le sol, les ASL sont
générale-ment décomposés à hauteur de 90% en l'espace de trois mois,
la demi-vie étant de l'ordre de 5-30 jours.
Le facteur de concentration des ASL dans le corps entier varie de
100 à 300 pour l'ensemble des 14C-ASL et 14C-métabolites. Ils sont
captés par les poissons essentiellement à travers les branchies et se
répartissent ensuite dans le foie et la vésicule après
biotransformation. Les ASL sont rapidement excrétés et rien n'indique
par conséquent qu'ils subissent une bioamplification.
1.4.2 alpha-Oléfine-sulfonates
Les données relatives au transport, à la distribution et à la
transformation des AOS dans l'environnement sont encore moins
nombreuses que dans le cas des ASL. On peut toutefois penser que les
AOS sont transportées dans l'environnement à peu près comme les ASL,
les AS et les autres détergents tensio-actifs et que leur destinée y
est analogue à celles des ASL et des AS. En aérobiose, elles subissent
une biodécomposition rapide et cette biodécomposition primaire est
achevée en 2 à 10 jours, en fonction de la température. On ne
dispose que de données limitées sur la bioaccumulation des AOS; en
tout état de cause elles ne s'accumulent pas chez les poissons. On ne
dispose d'aucune donnée sur leur décomposition en milieu abiotique.
1.4.3 Alkylsulfates
Les AS sont transportés dans l'environnement par des mécanismes
analogues à ceux qui sont à l'oeuvre dans le cas des ASL et des AOS.
Ils sont facilement biodégradable en aérobiose ou en anaérobiose, que
ce soit au laboratoire ou dans l'environnement; la biodécomposition
primaire est achevée en l'espace de 2 à 5 jours. Les facteurs de
bioconcentration pour le corps entier varient de 2 à 73 ainsi qu'avec
la longueur de la chaîne des différents homologues. Chez les poissons,
les AS sont captés, distribués, biotransformés et excrétés de la même
manière que les ASL et ne se concentrent pas dans les autres
organismes aquatiques.
1.5 Cinétique
Les ASL, les AOS et le AS sont facilement résorbés dans les voies
digestives, après quoi ils se répartissent dans l'ensemble de
l'organisme où ils sont largement métabolisés. Les ASL subissent une
omega- et une ß-oxydation. Les composés initiaux et leurs métabolites
sont principalement excrétés par la voie rénale, encore qu'une
certaine proportion de la dose absorbée puisse l'être également pas la
voie fécale, après métabolisation et passage dans les voies biliaires.
Les ASL, les AOS et les AS ne sont absorbés qu'en quantités minimes
par voie percutanée lorsque la peau est intacte, mais un contact
prolongé peut altérer l'intégrité de la barrière épidermique, ce qui
permet une résorption plus importante; à fortes concentrations, il
peut y avoir réduction du temps de pénétration.
1.6 Effets sur les animaux de laboratoire et sur les systèmes
d'épreuve in vitro
On a relevé, pour la DL50 des sels de sodium des ASL, des
valeurs allant de 404 à 1470 mg/kg de poids corporel chez le rat et
de 1259 à 2300 mg/kg de poids corporel chez la souris, ce qui incite
à penser que les rats sont plus sensibles que les souris à l'action
toxique des ASL. Chez la souris, on a obtenu une DL50 de 3000 mg/kg
de poids corporel pour un sel de sodium d'AOS. Chez le rat, les
valeurs de la DL50 par voie orale allaient de 1000 à 4120 mg/kg
de poids corporel pour les AS. Les ASL, les AOS et les AS sont
irritants pour la peau et les yeux.
Lors d'études subchroniques au cours desquelles on a administré à
des rats des ASL dans leur nourriture ou leur eau de boisson à des
concentrations quotidiennes correspondant à plus de 120 mg/kg de
poids corporel, on a observé des effets minimes, qui consistaient
notamment en modifications des paramètres biochimiques et altérations
histopathologiques au niveau du foie. Bien que lors d'une étude, on
ait observé des modifications ultrastructurales dans les hépatocytes
à des doses plus faibles, ces modifications se sont révélées
réversibles. D'ailleurs, les autres études n'ont pas révélé de tels
effets aux mêmes doses, mais il n'est pas exclu que lors de l'étude
initiale, les organes aient fait l'objet d'un examen plus minutieux.
Des effets ont également été observés sur la fonction de reproduction
chez des animaux auxquels on avait administré des doses quotidiennes
> 300 mg/kg; il s'agissait d'une réduction du taux de grossesse et
d'une certaine mortalité dans les portées. Après application cutanée
de longue durée à des rats de solutions de ASL à plus de 5% et
application, également cutanée, du même type de solution à des cobayes
à raison de 60 mg/kg de poids corporel pendant 30 jours, on a observé
des modifications biochimiques et histopathologiques. Des applications
cutanées répétées de solutions de teneur > 0,3% de ASL ont
produit des effets toxiques sur les foetus ainsi que sur la
reproduction, mais les doses étaient également toxiques pour les
femelles gestantes.
On n'a guère de données résultant d'études sur des animaux de
laboratoire qui permettraient d'évaluer les effets potentiels des AOS
chez l'homme. Aucun effet n'a été observé sur des rats ayant reçu,
pendant une longue durée, des doses quotidiennes de 250 mg/kg de poids
corporel en administration orale; toutefois une dose quotidienne de
300 mg/kg de poids corporel, toxique pour les femelles gestantes, a
entraîné des effets foetotoxiques chez des lapins. L'application
topique d'AOS sur la peau et les yeux de divers animaux de laboratoire
a produit des effets localisés.
Les effets d'une exposition à long et à court terme aux AS ont été
étudiés à plusieurs occasions sur l'animal mais la plupart des études
en question pêchent par les insuffisances des examens
histopathologiques ou la trop petite taille des groupes; en outre, les
doses les plus élevées utilisées dans les études à long terme n'ont
pas produit le moindre effet toxique, de sorte qu'il n'a pas été
possible d'établir la valeur de la dose sans effets nocifs
observables. Cependant, lorsqu'on a administré à des rats des
concentrations quotidiennes de ces substances correspondant à
200 mg/kg de poids corporel ou davantage, par incorporation à leur
nourriture ou à leur eau de boisson, on a systématiquement observé un
certain nombre d'effets. En outre, l'application topique sur la peau
ou les yeux d'AS à des concentrations égales ou supérieures à environ
0,5%, a donné lieu à une irritation localisée. Par ailleurs à fortes
concentrations, on observe des effets toxiques sur les femelles
gestantes ainsi que sur les foetus.
La plupart des études à long terme ne se prêtent pas à
l'évaluation du pouvoir cancérogène des ASL, des AOS et des AS chez
l'animal de laboratoire en raison de facteurs tel que le nombre trop
faible d'animaux, un nombre de doses limité, la non détermination de
la dose tolérée maximale, et, en outre, un examen histopathologique
limité dans la majorité des cas. Dans les travaux où les effets
anatomo-pathologiques ont été convenablement étudiés, on n'a pas
déterminé la dose tolérée maximale et les doses employées n'ont pas
produit d'effets toxiques. Toutefois et compte tenu de ces réserves,
on peut retenir que les études au cours desquelles on a administré à
des animaux des ASL, des AOS et des AS par voie orale, n'ont pas
révélé de signes de cancérogénicité; quant aux études à long terme
consistant en applications topiques d'AOS par badigeonnage cutané,
elles n'ont pas non plus révélé la présence d'effets imputables à ces
substances.
Sur la base de ces données limitées, il ne semble pas que ces
composés soient génotoxiques in vivo ou in vitro.
1.7 Effets sur l'homme
L'application d'un timbre cutané imprégné de solution contenant
jusqu'à 1% de ASL, d'AOS ou d'AS pendant 24 heures montre que la peau
humaine supporte le contact avec cette substance au prix d'une légère
irritation. Ces tensio-actifs provoquent une délipidation de
l'épiderme, une élution du facteur d'humidification naturelle, ainsi
qu'une dénaturation des protéines de la couche épidermique externe,
dont ils augmentent la perméabilité et dont ils provoquent le
gonflement. Ni les ASL, ni les AOS, ni les AS n'ont provoqué de
sensibilisation cutanée chez les volontaires et rien n'indique de
façon concluante qu'ils puissent provoquer un eczéma. On n'a pas
signalé de lésions graves ou mortelles consécutives à l'ingestion
accidentelle de ces tensio-actifs.
1.8 Effets sur l'environnement
1.8.1 Alkylbenzène-sulfonates à chaîne droite
1.8.1.1 Milieu aquatique
Les ASL ont été très largement étudiés tant au laboratoire (études
à court et à long terme) que dans des conditions plus proches de la
réalité (études sur le micro- et le mésocosme et études en situation
réelle). En général, la diminution de la longueur de la chaîne alkylée
ou une plus grande intériorisation du groupement phényle
s'accompagnent d'une diminution de la toxicité. Les observations
effectuées sur des poissons et sur des daphnies montrent que lorsque
la longueur de la chaîne diminue d'une unité (par exemple lorsqu'elle
passe de C12 à C11), la toxicité est approximativement divisée par
deux.
Les résultats des tests en laboratoire sont les suivants:
-- Microorganismes: Les résultats sont très variables en raison
de l'utilisation de systèmes d'épreuve très divers (par exemple
inhibition des boues activées, cultures mixtes et espèces
individuelles). Les valeurs de la CE50 vont de 0,5 mg/litre (une
seul espèce) à > 1000 mg/litre. Dans le cas des microorganismes, il
n'existe pas de relation linéaire entre la longueur de la chaîne et la
toxicité.
-- Plantes aquatiques: Les résultats dépendent largement de
l'espèce. En ce qui concerne les plantes d'eau douce, les valeurs de
la CE50 se situent entre 10 et 235 mg/litre (C10-C14), dans le
cas des algues vertes; entre 5 et 56 mg/litre (C11,1-C13), dans le
cas des algues bleu-vert; entre 1,4 et 50 mg/litre (C11,6-C13)
pour les diatomées et entre 2,7 et 4,9 mg/litre (C11,8) pour les
macrophytes. Il semble que les algues marines soient même encore plus
sensibles. Dans le cas des algues, il n'y a probablement pas non plus
de relation linéaire entre la longueur de la chaine et la toxicité.
-- Invertébrés: Les valeurs de la CE50 et de la CL50
(exposition aiguë) pour au moins 22 espèces d'eau douce se situent
entre les limites suivantes: 4,6-200 mg/litre (longueur de chaine non
précisée; C13) dans le cas des mollusques; 0,12-27 mg/litre
(longueur de chaine non précisée; C11,2-C18) dans le cas des
crustacés; 1,7-16 mg/litre (longueur de chaine non précisée; C11,8)
dans le cas des vers et enfin 1,4-270 mg/litre (C10-C15) dans le
cas des insectes. Dans le cas d'une exposition chronique, les valeurs
de la CE50 et de la CL50 sont de 2,2 mg/litre (C11,8) pour les
insectes et de 1,1-2,3 mg/litre (C11,8-C13) pour les crustacés. La
concentration sans effets chroniques observables (basée sur la
mortalité ou des effets sur la fonction de reproduction) est de 0,2 à
10 mg/litre (longueur de chaîne non précisée; C11,8) pour les
crustacés. Il semble que les invertébrés marins soient plus sensibles,
avec des valeurs de la CL50 allant de 1 à plus de 100 mg/litre (dans
presque tous les cas, C12) pour 13 espèces et avec une concentration
sans effets observables de 0,025 à 0,4 mg/litre (longueur de chaine
non précisée dans l'ensemble des tests) dans le cas des sept espèces
étudiées
-- Poissons: Pour 21 espèces d'eau douce, les valeurs de la
CL50 aiguë se situent entre 0,1 et 125 mg/litre (C8-C15); les
valeurs de la CE50 et/ou de la CL50 pour une exposition chronique
sont, pour deux espèces, respectivement égales à 2,4 et à 11 mg/litre
(longueur de chaîne non spécifiée; C11,7); quant à la concentration
sans effets observables, elle va de 0,11-8,4 à 1,8 mg/litre (longueur
de chaine non précisée; C11,2-C13) pour deux espèces. Dans ce cas
encore, les poissons de mer se révèlent plus sensibles, avec des
valeurs de la CL50 aiguë allant de 0,05 à 7 mg/litre (longueur de
chaîne non spécifiée; C11,7) pour six espèces et des valeurs de la
CL50 chronique allant de 0,01 à 1 mg/litre (longueur de chaîne non
précisée) pour deux espèces. Dans la plupart des publications, la
longueur de la chaine n'est pas précisée. Pour des espèces marines, on
a également fait état d'une concentration sans effets observables <
0,02 mg/litre (C12).
Les produits communément utilisés dans le commerce ont en moyenne,
une chaîne latérale en C12. Des composés ayant diverses longueurs de
chaîne ont été étudiés sur Daphnia magna et sur des poissons, mais
dans le cas des autres organismes d'eau douce, c'est en général des
composés dont la longueur de chaine moyenne est de C11,8 qui ont été
utilisés. Les valeurs caractéristiques de la CE50 et de la CL50 aiguë
pour les ASL en C12 sont 3-6 mg/litre chez Daphnia magna et
2-15 mg/litre chez les poissons d'eau douce; celles de la
concentration sans effets observables pour une exposition chronique
sont de 1,2 à 3,2 mg/litre pour Daphnia magna et de 0,48-0,9
mg/litre pour les poissons d'eau douce. Chez les poissons de mer, les
valeurs caractéristiques de la CL50 aiguë pour des ASL en C12
sont de < 1-6,7 mg/litre.
Les organismes halophiles et en particulier les invertébrés, se
révèlent être plus sensibles aux ASL que les organismes d'eau douce.
Chez les invertébrés, l'action séquestrante des ASL sur le calcium
peut affecter la biodisponibilité de cet ion pour la morphogénèse. Les
ASL exercent un effet général sur le transport ionique. Les produits
de biodécomposition et les sous-produits des ASL sont 10 à 100 fois
plus toxiques que les composés de départ.
Les résultats obtenus dans des conditions plus proches de la
réalité sont les suivants: on a étudié les ASL au moyen de toute sorte
de tests en eau douce et à plusieurs niveaux trophiques, notamment
dans des enceintes lacustres (organismes inférieurs), dans des
écosystèmes modèles (sédiments et réseaux hydrographiques), des cours
d'eau en aval et en amont des déversoirs de stations d'épuration des
eaux usées et enfin, des cours d'eau expérimentaux. Dans presque tous
les cas on a utilisé des ASL en C12. Les algues se sont révélées
être plus sensibles en été qu'en hiver, les valeurs de la CL50 à 3
heures étant de 0,2 à 8,1 mg/litre après la photosynthèse, alors que
dans les écosystèmes modèles, on n'observait aucun effet sur
l'abondance relative des populations d'algues à la concentration de
0,35 mg/litre. Selon ces études, la valeur de la concentration sans
effets observables se situe de 0,24 à 5 mg/litre selon l'organisme et
le paramètre étudié. Ces résultats sont en assez bon accord avec ceux
des épreuves en laboratoire.
1.8.1.2 Milieu terrestre
On dispose de données sur les végétaux et les lombrics. Pour sept
espèces de plantes étudiées dans des solutions nutritives, on a obtenu
des valeurs de la concentration sans effets observables qui se situent
dans les limites < 10-20 mg/litre; pour trois espèces étudiées sur
sol d'après leur croissance, on a obtenu 100 mg/kg (C10-C13). Pour
les lombrics, la CL50 à 14 jours était > 1000 mg/kg.
1.8.1.3 Oiseaux
Une étude sur des poulets qui recevaient une nourriture contenant
de ces substances, a permis de fixer à > 200 mg/kg la dose sans
effets observables (d'après la qualité des oeufs).
1.8.2 alpha-Oléfine-sulfonates
On dispose de données limitées concernant les effets des AOS sur
les organismes aquatiques et terrestres.
1.8.2.1 Milieu aquatique
On ne dispose que des résultats des épreuves en laboratoire:
-- Algues: Valeur de la CE50 : > 20-65 mg/litre (C16-C18)
pour les algues vertes
-- Invertébrés: Valeur de la CL50 : 19 et 26 mg/litre
(C16-C18) pour la daphnie
-- Poissons: Pour neuf espèces de poissons on a obtenu des
valeurs de la CL50 aiguë de 0,3-6,8 mg/litre (C12-C18). Sur la
base d'études à court terme effectuées sur la truite de mer (Salmo
trutta), l'ide rouge (Idus melanotus) et le rasbora (Rasbora
heteromorpha), on peut conclure que la toxicité des composés en
C14-C16 est environ cinq fois plus faible que celle des composés
en C16-C18, avec des valeurs de la CL50 (à toutes les
concentrations mesurées) de 0,5-3,1 (C16-C18) et de
2,5-5,0 mg/litre (C14-C16). Deux études à long terme effectuées
sur la truite arc-en-ciel ont montré que le paramètre le plus sensible
était la croissance, et qu'il permettait d'obtenir une CE50 de
0,35 mg/litre. Pour ce qui est des poissons de mer, on a obtenu pour
le mulet gris ou muge (Mugal cephalus), une valeur de la CL50 à 96
heures de 0,70 mg/litre.
1.8.2.2 Milieu terrestre
Une étude portant sur des végétaux en solution nutritive a montré
que la concentration sans effets observables se situait dans les
limites 32-56 mg/litre. Dans une autre étude, portant cette fois sur
des poulets qui recevaient les AOS dans leur nourriture, on a obtenu
une valeur > 200 mg/kg pour la concentration sans effets observables
(d'après la qualité des oeufs).
1.8.3 Alkyl-sulfates
1.8.3.1 Organismes aquatiques
Les AS ont fait l'objet d'études à court et à long terme et d'une
étude dans des conditions plus proches de la réalité. On constate
encore que leur toxicité dépend de la longueur de la chaîne latérale
alkylée; par contre on ne dispose d'aucune donnée qui indiquerait
l'existence d'une différence de toxicité entre les AS à chaine droite
et les AS à chaîne ramifiée.
Les résultats des épreuves de laboratoire sont les suivants:
-- Microorganismes: Les valeurs de la CE50 dans une communauté
marine étaient de 2,1-4,1 mg/litre (C12). Pour Pseudomonas putida,
les concentrations sans effets observables étaient de 35-550 mg/litre
(C16-C18).
-- Végétaux aquatiques: Les valeurs de la CE50 s'établissaient
comme suit: > 20-65 mg/litre (C12-C13) pour les algues vertes et
18-43 mg/litre (C12) pour les macrophytes. Les concentrations sans
effets observables s'établissaient à 14-26 mg/litre (C12-C16/C18)
chez les algues vertes.
-- Invertébrés: Les valeurs de la CE50 et de la CL50 se
situaient entre 4 et 140 mg/litre (C12/C15-C16/C18) pour les
espèces d'eau douce et entre 1,7 et 56 mg/litre (tous les composés en
C12) chez les espèces marines. La concentration sans effets
observables pour Daphnia magna était de 16,5 mg/litre (C16/C18)
en exposition chronique, les valeurs se situant entre 0,29 et
0,73 mg/litre (longueur de chaine non précisée) pour les espèces marines.
-- Poissons: Les valeurs de la CL50 se situaient entre 0,5 et
5,1 mg/litre (longueur de chaine non précisée ou C12-C16) pour des
espèces d'eau douce et entre 6,4 et 16 mg/litre (tous les composés en
C12) pour les espèces marines. On n'a pas eu connaissance d'études
à long terme.
Il est à noter que nombre de ces travaux ont été effectués dans
des conditions statiques. Comme les AS sont facilement biodégradables,
il est possible qu'on en ait sous estimé la toxicité. Lors d'une étude
de 48 heures sur Oryzias latipes, on a obtenu pour la CL50 des
valeurs respectivement égales à 46, 2,5 et 0,61 mg/litre (mesures de
concentrations) pour des composés en C12, C14 et C16. Cette
étude et d'autres, montrent que la toxicité s'accroît d'un facteur 5
lorsque la longueur de la chaîne augmente de deux unités. Une étude
dynamique sur une biocénose, avec des composés en C16-C18 a permis
d'obtenir une concentration sans effets observables de 0,55 mg/litre.
1.8.3.2 Organismes terrestres
On a fait état, pour les lombrics et les navets, de concentrations
sans effets observables de valeur > 1000 mg/kg (C16-C18).
1.9 Evaluation des risques pour la santé humaine
Les ASL sont les tensio-actifs les plus largement utilisés pour la
fabrication de détergents et de produits de nettoyage; les AOS et les
AS entrent également dans la composition des détergents et des
produits destinés à l'hygiène personelle. La principale voie
d'exposition humaine est donc le contact cutané. Cependant de petites
quantités de ASL, d'AOS et d'AS peuvent être ingérées avec l'eau de
boisson ou sous forme de résidus subsistant sur les ustentsiles de
cuisine et dans les aliments. Bien que les données sur ce point soient
limitées, on peut estimer à environ 5 mg/personne la quantité de ASL
ingérée quotidiennement de cette manière. Quant à l'exposition
professionnelle à ces trois catégories de produits, elle peut
intervenir lors de la préparation des différentes substances qui en
contiennent, mais on ne dispose d'aucune donnée sur les effets qu'une
exposition chronique à ces composés pourrait avoir sur l'homme.
Les ASL, les AOS et les AS peuvent irriter la peau par suite d'un
contact répété ou prolongé aux concentrations qui sont celles des
produits non dilués. Chez le cobaye, les AOS peuvent provoquer une
sensibilisation cutanée lorsque la concentration en sultone
gamma-insaturée dépasse environ 10 ppm.
Les études à long terme sur animaux de laboratoire dont on connaît
les résultats sont insuffisantes pour permettre d'évaluer le pouvoir
cancérogène des ASL, des AOS et des AS, et ce, pour différentes
raisons: conception même de ces études, trop petit nombre d'animaux
utilisés et doses administrées trop faibles, enfin examens
histopathologiques trop succints. Compte tenu de ces réserves, les
résultats fournis par les études au cours desquelles les animaux ont
reçu des ASL, des AOS ou des AS par voie orale, ne comportent aucun
signe de cancérogénicité; par ailleurs l'application d'AOS aux animaux
par badigeonnage cutané, a également donné des résultats négatifs. Ces
composés ne se révèlent pas non plus génotoxiques in vivo ou in
vitro, encore que peu d'études aient été publiées sur ce point.
Des études sub-chroniques au cours desquelles des rats avaient
reçu des ASL dans leur nourriture ou leur eau de boisson à des
concentrations quotidiennes correspondant environ à 120 mg/kg de poids
corporel, ont révélé la présence d'effets minimes, notamment des
altérations biochimiques et des modifications histopathologiques au
niveau du foie; toutefois d'autres études au cours desquelles des
animaux avaient été exposés plus longtemps à des doses plus élevées
n'ont pas mis d'effets en évidence. L'application cutanée de ASL a
provoqué une intoxication générale ainsi que des effets localisés.
La dose journalière moyenne de ASL absorbée par la population
générale, telle qu'on peut l'évaluer sur la base d'estimations de
l'exposition de cette population par l'intermédiaire de l'eau de
boisson, des ustensiles de cuisine et des aliments, est probablement
beaucoup plus faible (de l'ordre de trois ordres de grandeur) que les
concentrations qui se révèlent produire des effets insignifiants sur
les animaux de laboratoire.
Les effets des AOS observés sur l'homme à l'occasion des quelques
études dont on a connaissance, rapellent ceux qui ont été mis en
évidence chez des animaux de laboratoire exposés aux ASL. Comme on ne
dispose pas de données suffisantes pour évaluer la dose journalière
moyenne d'AOS absorbée par la population générale ni sur les
concentrations susceptibles de produire des effets chez l'homme et
l'animal, il n'est pas possible de savoir avec certitude si
l'exposition aux AOS présentes dans l'environnement représente un
risque pour la santé humaine. Les concentrations d'AOS présentes dans
les milieux auxquels l'homme pourrait être exposé, sont de toute
manière plus faibles que celles des ASL, du fait de la moindre
utilisation des AOS.
Des effets ont été observés systématiquement à l'occasion de
quelques études à portée limitée effectuées sur des rats à qui l'on
avait fait ingérer quotidiennement des AOS soit avec leur nourriture,
soit dans leur eau de boisson à des concentrations supérieures ou
égales à 200 mg/kg de poids corporel. Des applications topiques
répétées ou prolongées produisent également des effets localisés sur
la peau et les yeux. On ne dispose pas non plus de données suffisantes
pour évaluer la dose journalière moyenne d'AS absorbée par la
population générale. Toutefois, étant donné que les tensio-actifs à
base d'AS ne sont pas utilisés aussi abondamment que ceux qui
contiennent des ASL, il est probable que la dose d'AS absorbée est au
moins mille fois plus faible que celle qui produit des effets sur
l'animal.
1.10 Evaluation des effets sur l'environnement
Les ASL, les AS et les AOS sont utilisés en grandes quantités et
rejetés dans l'environnement avec les eaux usées. Pour évaluer le
risque qui leur est attaché, il faut comparer les concentrations
auxquelles l'exposition peut se produire avec celles qui ne provoquent
aucun effet indésirable, cette comparaison pouvant être faite pour un
certain nombre de milieux présents dans l'environnement. En ce qui
concerne les tensio-actifs anioniques en général, les plus importants
de ces milieux sont constitués par les stations de traitement des eaux
usées, les eaux de surface, les sols amendés au moyen de sédiments et
de boues d'égout, ainsi que les eaux estuarielles et marines. Les
composés subissent une biodécomposition (depuis les premiers stades
jusqu'à leur dégradation ultime) ainsi qu'une adsorption, qui
réduisent leur concentration dans l'environnement ainsi que leur
biodisponibilité. Le racourcissement de la chaîne latérale alkylée et
la disparition de la structure du composé initial conduisent à des
composés qui sont moins toxiques que les molécules de départ. Il
importe d'en tenir compte lorsqu'on compare les résultats des épreuves
en laboratoire aux effets qui pourraient se produire dans
l'environnement. En outre, lorsqu'on évalue le risque associé à
l'exposition, dans l'environnement, à ces trois types de tensio-
actifs anioniques, il faut que les comparaisons entre les
différentes épreuves de toxicité portent sur des composés dont la
chaîne latérale à la même longueur.
Les effets des ASL sur les organismes aquatiques ont été très
largement étudiés. Lors des épreuves de laboratoire effectuées en eau
douce, ce sont les poissons qui se sont révélés les plus sensibles;
ainsi la concentration sans effets observables pour un cyprin
d'Amérique du Nord, Pimephales promelas, est d'environ
0,5 mg/litre (C12); tous ces résultats ont été confirmés lors
d'épreuves effectuées dans des conditions plus proches de la réalité.
Pour ce qui est du phytoplancton, des épreuves de toxicité aiguë
sur trois heures ont donné, pour la CE50, des valeurs de
0,2-0,1 mg/litre (C12-C13), alors qu'on n'a constaté aucun effet
sur l'abondance relative du plancton dans d'autres tests effectués à
la concentration de 0,24 mg/litre (C11,8). Il semble que les espèces
marines soient légèrement plus sensibles que la plupart des autres
groupes taxonomiques.
Ces trois types de composés anioniques se retrouvent dans
l'environnement à des concentrations qui varient dans de larges
limites. De ce fait, il n'est pas possible de procéder à une
évaluation du risque écologique qui soit d'une portée générale. Toute
évaluation du risque doit s'appuyer sur une connaissance suffisante de
l'exposition et des concentrations agissantes dans l'écosystème
étudié.
Pour ce qui est de l'évaluation du risque imputable à la présence
d'AS et d'AOS dans l'environnement, il faudra encore réunir des
données précises sur l'exposition à ces composés. C'est pourquoi on
utilise des modèles pour étudier l'exposition à ces produits dans les
différents milieux qui en sont les récepteurs. En ce qui concerne les
organismes aquatiques, les données toxicologiques sur les AS et les
AOS sont relativement rares, notamment dans les cas d'exposition
chronique à des concentrations constantes. Celles dont on dispose
montrent que cette toxicité est analogue à celle des autres
tensio-actifs anioniques.
Les organismes aquatiques halophiles se révèlent plus sensibles
que les organismes dulçaquicoles à ces composés; toutefois leur
concentration est plus faible dans l'eau de mer, sauf au débouché des
émissaires d'eaux usées. La destinée et les effets de ces composés,
qui sont présents dans les effluents déversés en mer, n'ont pas été
étudiés en détail.
Pour évaluer la sûreté écologique de tensio-actifs tels que les
ASL, les AOS et les AS, il faut comparer les concentrations effectives
dans l'environnement à celles qui ne produisent aucun effet. Les
besoins en matière de recherche sont déterminés non seulement par les
propriétés intrensèques de tel ou tel produit chimique mais aussi par
les modalités ou les tendances de sa consommation. Tous ces facteurs
peuvent varier fortement d'une région à l'autre, aussi l'appréciation
et l'évaluation des risques doivent-elles être effectuées région par
région.
1.11 Recommandations pour la protection de la santé humaine
et de l'environnement
1. Comme il peut y avoir exposition à des poussières sur les lieux de
travail (au cours de la fabrication et de la préparation des
différentes formules), il faut veiller à ce que les précautions
habituelles d'hygiène et sécurité du travail soient respectées afin
d'assurer la protection des travailleurs.
2. La composition des préparations destinées à la consommation des
ménages et à l'usage industriel doit être étudiée pour éviter tout
danger, en particulier lorsqu'il s'agit de produits destinés au
nettoyage ou au lavage du linge à la main.
3. L'exposition à ces produits dans l'environnement et les effets
qu'ils peuvent avoir doivent faire l'objet d'une surveillance
appropriée afin que l'on puisse reconnaître à temps la présence de
tout concentration excessive dans tel ou tel milieu.
1.12 Recommandations pour les recherches futures
Santé humaine
1. Etant donné que le contact cutané est la principale voie
d'exposition humaine aux ASL, aux AOS et aux AS et que l'on ne dispose
pas d'études à long terme suffisantes sur la toxicité cutanée ou la
cancérogénicité de ces produits chez les animaux de laboratoire, il
est recommandé de procéder à des études à long terme convenablement
conçues au cours desquelles il sera procédé à l'application de ces
composés sur la peau des animaux.
2. En raison de l'absence de données définitives sur la génotoxicité
des AOS et des AS, il conviendrait de procéder à des études
supplémentaires in vivo et in vitro.
3. En raison de l'insuffisance des études existantes concernant les
effets toxiques de ces produits sur la reproduction et le
développement, il conviendrait d'effectuer, sur des animaux de
laboratoire, des études qui permettent d'obtenir des résultats
définitifs sur la valeur des concentrations agissant ou au contraire,
sans effets des ASL, des AOS et des AS.
4. Etant donné que l'on ne connaît pas de façon suffisamment précise
l'exposition aux ASL, aux AOS et aux AS, il faudrait surveiller
l'exposition de la population générale à ces produits, en particulier
lorsque ces tensio-actifs sont utilisés pour le nettoyage et le lavage
du linge à la main.
5. Etant donné que les ASL, les AOS et les AS peuvent favoriser le
transport d'autres produits chimiques dans les différents milieux qui
composent l'environnement et en faire varier la biodisponibilité et la
toxicité dans les eaux de surface, les sédiments, les cours d'eau et
les sols auxquels l'être humain pourrait se trouver exposé, il
conviendrait d'étudier les interactions de ces produits avec d'autres
substances présentes dans l'environnement et les conséquences qui en
découlent pour la santé humaine.
Sûreté écologique
6. Des études supplémentaires devraient être effectuées afin
d'élucider les mécanismes de l'adsorption et de la désorption des AOS
et des AS. Elles devraient également porter sur le partage des ASL,
des AOS et des AS entre les particules colloïdales en solution ou en
suspension dans l'eau. Il faudrait effectuer une modélisation
mathématique des coefficients de sorption et valider les modèles
obtenus en fonction des paramètres physicochimiques.
7. En cas d'exposition à des sols amendés à l'aide de boues d'égout
ou à des sédiments de rivière, il faudrait étudier la biodécomposition
des AOS et des AS dans ces milieux. L'étude des sédiments (dans les
zones d'aérobiose et d'anaérobiose) devrait s'effectuer en aval des
points où sont rejetées des eaux traitées ou non traitées ou des
émissaires d'évacuation.
8. Il faudrait surveiller au niveau régional et national les
concentrations en ASL, AOS et AS dans l'environnement afin d'obtenir
des données sur l'exposition. Il faudrait également mettre au point
des méthodes d'analyse permettant de déceler la présence de faibles
teneurs en AOS et en AS dans les compartiments appropriés de
l'environnement.
9. Il faudrait établir des bases de données nationales sur la
concentration des ASL, AOS et AS dans les eaux usées et les cours
d'eau ainsi que sur les différents types de stations d'épuration, leur
implantation et leur efficacité, afin de mieux étudier l'impact des
décharges dans l'environnement.
10. Il faudrait effectuer des études à long terme sur la toxicité des
AOS et des AS pour les poissons (espèces d'eau douce et espèces
marines) et des invertébrés aquatiques, afin d'en établir la
sensibilité relative.
ALKILSULFONATOS LINEALES Y SUSTANCIAS RELACIONADOS
1. RESUMEN GENERAL, EVALUACION Y RECOMENDACIONES
1.1 Identidad y métodos analíticos
Los alkilsulfonatos lineales (ASL), los alpha-olefinsulfonatos
(AOS) y los alkilsulfatos (AS) son sustancias tensioactivas aniónicas
con moléculas que se caracterizan por tener un grupo hidrófobo y uno
hidrófilo (polar). Las mezclas comerciales están formadas por isómeros
y homólogos de compuestos relacionados entre sícon distintas
propiedades fisicoquímicas, obteniéndose formulaciones con diversas
aplicaciones.
Los ASL, los AOS y los AS se pueden analizar por métodos no
específicos. El ensayo que se suele utilizar es el de las sustancias
que reaccionan con el azul de metileno, es decir, todas las que
contienen un grupo aniónico e hidrófobo. Por consiguiente, si se
utiliza para muestras del medio ambiente se producen interferencias
analíticas; por otra parte, la sensibilidad de este método es de unos
0,02 mg/litro. Aunque se han buscado alternativas no específicas a
este método, su uso no es habitual. En el análisis del medio ambiente
sólo hay métodos específicos para los ASL y los AS. Para el análisis
de los AOS se dispone de un método mejorado basado en la reactividad
del azul de metileno y la cromatografía líquida de alto rendimiento
(HPLC).
Los ASL son sustancias no volátiles que se forman por la
sulfonación del alkilbenceno lineal. Los productos comerciales son
siempre mezclas de homólogos con la cadena alkilo de distintas
longitudes (C10-C13 o C14) e isómeros que difieren en las
posiciones del anillo de fenilo (2-5 fenil). En las muestras del medio
ambiente y en otras matrices se pueden determinar todos los homólogos
e isómeros de los ASL por medio de métodos analíticos específicos como
la HPLC, la cromatografía de gases y la cromatografía de
gases-espectrometría de masas.
Los AOS son sustancias no volátiles producidas por la sulfonación
de las alpha-olefinas. Son mezclas de dos compuestos, el
alkensulfonato de sodio y el sulfonato de hidroxialkano, con
longitudes de la cadena alkilo de C14-C18.
Los AS son compuestos no volátiles producidos por la sulfatación
de alcoholes oleoquímicos o petroquímicos. Son mezclas de homólogos
con longitudes de la cadena alkilo de C10-C18. Se están
perfeccionando métodos analíticos específicos para la vigilancia del
medio ambiente.
1.2 Fuentes de exposición humana y ambiental
Los ASL, los AOS y los AS se utilizan como ingredientes activos en
productos de uso doméstico y de aseo personal y en aplicaciones
especializadas. Una vez utilizadas, dichas sustancias detergentes
pasan al medio ambiente en las aguas residuales.
Se dan casos de exposición en el trabajo a estas sustancias. La
exposición de la población humana general y de los organismos del
medio ambiente depende de la aplicación de los ASL, los AOS y los AS
(y de otras sustancias tensioactivas), de las prácticas de tratamiento
de las aguas residuales y de las características del medio ambiente al
que llegan.
En 1990, el consumo mundial fue de unos dos millones de toneladas
de ASL, 86 000 toneladas de AOS y 289 000 toneladas de AS.
1.3 Concentraciones en el medio ambiente
1.3.1 Alkilsulfonatos lineales
Las concentraciones de ASL se han determinado cuantitativamente
por medio de un método analítico sensible específico en casi todos los
compartimentos del medio ambiente en los que pueden estar presentes.
Las concentraciones disminuyen progresivamente en el siguiente orden:
aguas residuales > efluente tratado > aguas superficiales > mar.
En las zonas donde los ASL son las sustancias tensioactivas
predominantes utilizadas, las concentraciones suelen ser de
1-10 mg/litro en las aguas residuales, 0,05-0,1 mg/litro en los
efluentes sometidos a un tratamiento biológico, 0,05-0,6 mg/litro en
los efluentes tratados con un filtro de goteo, 0,005-0,05 mg/litro en
las aguas superficiales por debajo de los desagües de aguas residuales
(con concentraciones que disminuyen con rapidez a 0,01 mg/litro
corriente abajo del desagüe), < 1-10 mg/kg en los sedimentos
fluviales (< 100 mg/kg en los sedimentos muy contaminados cerca de
las zonas de vertido), 1-10 g/kg en los fangos de alcantarillado y <
1-5 mg/kg en los suelos tratados con fangos (al principio 5-10 mg/kg;
se ha registrado una concentración de < 50 mg/kg después de
aplicaciones anormalmente elevadas de fangos). Las concentraciones de
ASL en las aguas de estuario son de 0,001-0,01 mg/litro, aunque hay
niveles más elevados en los lugares donde se vierten directamente
aguas residuales. Las concentraciones en el agua marina cercana a la
costa son < 0,001-0,002 mg/litro.
Hay que señalar que las concentraciones de ASL en el medio
ambiente varían mucho. Esto se debe a diferencias en los métodos
analíticos, las características de los lugares de muestreo (que van
desde zonas muy contaminadas con un tratamiento inadecuado de las
aguas residuales hasta zonas donde dichas aguas se someten a un
tratamiento a fondo), la estación (los valores pueden ser en una el
doble que en otra) y el consumo de ASL.
La vigilancia del medio ambiente pone de manifiesto que no se ha
producido acumulación de ASL en sus compartimentos a lo largo del
tiempo. Las concentraciones en el suelo no aumentan con el tiempo,
sino que disminuyen debido a la mineralización. Los ASL no se degradan
en condiciones estrictamente anaerobias (para formar metano), por lo
que no se puede concluir que estén mineralizados en sedimentos
anaerobios. Con la utilización presente, el ritmo de asimilación de
ASL en todos los compartimentos del medio ambiente que los reciben es
igual al ritmo de entrada, por lo que la situación es estable.
1.3.2 alpha-Olefinsulfonatos y alkilsulfatos
Los datos disponibles sobre las concentraciones de AOS en el medio
ambiente son limitados debido a la dificultad para analizarlos en las
muestras de dicho medio. Hay métodos colorimétricos no específicos
(como el basado en el azul de metileno) que permiten detectar
sustancias tensioactivas aniónicas en general, pero se ven afectados
por interferencias analíticas y no son idóneos para determinar
concentraciones determinadas de AOS. Se está preparando un método
específico para medir los AS en muestras del medio ambiente.
En estudios de laboratorio se ha observado que los AOS y los AS se
mineralizan con rapidez en todos los compartimentos del medio ambiente
y prácticamente se eliminan del todo de las aguas residuales durante
el tratamiento. Las concentraciones en el agua superficial, los
sedimentos, el suelo, el agua de estuario y el medio marino son
probablemente bajas. Se ha comprobado que la concentración de AOS en
el agua fluvial es pequeña.
1.4 Transporte, distribución y transformación en el medio ambiente
A temperaturas por debajo de 5-10°C, la cinética de la
biodegradación de los ASL, los AOS y los AS se reduce debido a la
disminución de la actividad microbiana.
1.4.1 Alkilsulfonatos lineales
Las vías de entrada de los ASL en el medio ambiente varían de un
país a otro, pero la principal es el vertido de las depuradoras de
aguas residuales. Cuando no hay depuradoras o son inadecuadas, las
aguas residuales se pueden verter directamente en los ríos, los lagos
o el mar. Otra vía de entrada de ASL en el medio ambiente es la
dispersión de fangos de alcantarillado en las tierras cultivadas.
Durante su recorrido hasta llegar al medio ambiente, los ASL se
eliminan mediante una combinación de adsorción y biodegradación
primaria y final. Los ASL se adsorben sobre superficies coloidales y
partículas en suspensión, con unos coeficientes medidos de adsorción
de 40-5200 litros/kg, en función de los medios y de la estructura de
los ASL. Se biodegradan en el agua superficial (semivida de 1-2 días),
los sedimentos aerobios (1-3 días) y los sistemas marinos y de
estuarios (5-10 días).
Durante el tratamiento primario de las aguas residuales se adsorbe
alrededor del 25% (intervalo, 10-40%) de los ASL en los fangos
residuales y se elimina con ellos. No se eliminan durante la digestión
anaerobia de los fangos, sino durante su tratamiento aerobio, con una
semivida de unos 10 días. Tras la aplicación de fangos al suelo, en
general se degrada el 90% de los ASL en tres meses, con una semivida
de 5-30 días.
Los factores de concentración en el organismo completo para los
ASL oscilan entre 100 y 300 para la suma de los ASL-14C y los
metabolitos de 14C. Los peces los absorben sobre todo por las
branquias, distribuyéndose después al hígado y la vesículas biliar
tras la biotransformación. Los ASL se excretan con rapidez, por lo que
no hay pruebas de que se produzca bioampliación.
1.4.2 alpha-Olefinsulfonatos
Los datos disponibles sobre el transporte, distribución y
transformación en el medio ambiente para los AOS son más escasos que
para los ASL. Cabe suponer que los AOS llegan al medio ambiente de
manera análoga a la establecida para los ASL, los AS y otras
sustancias tensioactivas detergentes, y su destino en él es semejante
al de los ASL y los AS. En condiciones aerobias se biodegradan
fácilmente y la biodegradación primaria se completa en 2-10 días, en
función de la temperatura. Son limitados los datos disponibles sobre
la bioacumulación de los AOS; en peces no se observó ninguna. No hay
datos relativos a la degradación abiótica.
1.4.3 Alkilsulfatos
Los AS llegan al medio ambiente por mecanismos análogos a los de
los ASL y los AOS.Son fácilmente biodegradables en condiciones
aerobias y anaerobias en el laboratorio y en el medio ambiente; la
biodegradación primaria se termina en 2-5 días. El factor de
bioconcentración en el organismo entero oscila entre 2 y 73 y varía
con la longitud de la cadena de los homólogos de los AS. Los peces
absorben, distribuyen, biotransforman y excretan los AS de la misma
manera que los ASL y no se produce bioconcentración en los organismos
acuáticos.
1.5 Cinética
Los ASL, los AOS y los AS se absorben fácilmente en el aparato
digestivo y se distribuyen ampliamente por todo el organismo, con una
metabolización extensa. En los ASL se produce omega- y ß-oxidación.
Las sustancias originales y los metabolitos se excretan sobre todo a
través de los riñones, aunque una parte de la cantidad absorbida se
puede excretar en forma de metabolitos en las heces por excreción
biliar. Parece que por la piel intacta solamente se absorben
cantidades mínimas de ASL, AOS y AS, aunque el contacto prolongado
puede poner en peligro la integridad de la barrera cutánea,
permitiendo así una mayor absorción; las concentraciones elevadas
pueden reducir el tiempo necesario para la penetración.
1.6 Efectos en los animales de laboratorio y en los sistemas
de prueba in vitro
Los valores de la DL50 por vía oral para las sales sódicas de
los ASL fueron de 404-1470 mg/kg de peso corporal en ratas y de
1259-2300 mg/kg en ratones, lo cual parece indicar que las ratas son
más sensibles que los ratones a la toxicidad de los ASL. En ratones se
midió para una sal sódica de AOS una DL50 por vía oral de
3000 mg/kg de peso corporal. Los valores de la DL50 de AS por vía
oral en ratas fueron de 1000-4120 mg/kg de peso corporal. Los ASL, AOS
y AS irritan la piel y los ojos.
Se han descrito efectos mínimos, entre ellos alteraciones
bioquímicas y cambios histopatológicos en el hígado, en estudios de
toxicidad subcrónica en los que se administraron ASL a ratas con los
alimentos o el agua de beber en concentraciones equivalentes a dosis
superiores a 120 mg/kg de peso corporal al día. Aunque en un estudio
se observaron cambios estructurales de las células hepáticas a dosis
menores, al parecer eran reversibles. En otros estudios no se
detectaron efectos con dosis análogas, pero tal vez el examen de los
órganos fuera más detenido en el primer estudio. Se han notificado
efectos en la reproducción, por ejemplo menor tasa de gestación y
pérdida de crías, en animales que recibieron dosis > 300 mg/kg al
día. Se observaron cambios histopatológicos y bioquímicos tras la
aplicación cutánea de larga duración a ratas de soluciones > 5% de
ASL y después de la aplicación durante 30 días de 60 mg/kg de peso
vivo en la piel de cobayas. La aplicación cutánea repetida de
soluciones > 0,3% de ASL indujo efectos fetotóxicos y en la
reproducción, pero también se observó toxicidad materna.
Son escasos los datos disponibles de estudios en animales
experimentales que permitan evaluar los posibles efectos de los AOS en
el ser humano. No se observó ningún efecto en ratas que recibieron por
vía oral dosis de 250 mg/kg de peso corporal al día en aplicación
crónica, pero se apreció fetotoxicidad en conejas a las que se
administró una dosis tóxica para la madre de 300 mg/kg de peso
corporal al día. La aplicación de AOS a la piel y los ojos de animales
experimentales indujo efectos locales.
Aunque se han investigado en varios estudios los efectos de la
exposición de corta y larga duración de animales a los AS, en la
mayoría de los casos el examen histopatológico fue inadecuado o el
tamaño de los grupos pequeño; por otra parte, las dosis más altas
utilizadas en los estudios de larga duración no produjeron ningún
efecto tóxico, de manera que no se pudo establecer un NOAEL. Sin
embargo, se han descrito habitualmente efectos en ratas que recibían
AS en los alimentos o el agua de beber a concentraciones equivalentes
a 200 mg/kg de peso corporal al día o más. Se han observado efectos
locales en la piel y los ojos tras la aplicación tópica de
concentraciones aproximadas del 0,5% de AS o más. A concentraciones
más elevadas se han registrado efectos de toxicidad materna y
fetotóxicos.
La mayoría de los estudios de larga duración son inadecuados para
evaluar el potencial carcinogénico de los ASL, los AOS y los AS en
animales experimentales, debido a factores como el pequeño número de
animales, el número limitado de dosis, la ausencia de una dosis
tolerada máxima y la limitación del examen histopatológico en la
mayoría de los estudios. En los casos en que se describieron de manera
apropiada los hallazgos patológicos no se utilizaron dosis toleradas
máximas y las dosis no produjeron efectos tóxicos. Teniendo presentes
estas limitaciones, sin embargo, en los estudios en los que se
administraron por vía oral ASL, AOS y AS no se obtuvo ninguna prueba
de carcinogenicidad; en estudios de larga duración de aplicación de
AOS en la piel con un pincel no se observó ningún efecto.
Según los limitados datos disponibles, no parece que estas
sustancias tengan efectos genotóxicos in vivo o in vitro.
1.7 Efectos en el ser humano
Los resultados obtenidos en pruebas de parche demuestran que la
piel humana puede tolerar el contacto con soluciones de ASL, AOS o AS
de hasta un 1% durante 24 horas con la única reacción de una
irritación leve. Estas sustancias tensioactivas provocaron la pérdida
de lípidos de la superficie de la piel, la elución del factor
hidratante natural y la desnaturalización de las proteínas de la capa
epidérmica externa y aumentaron la permeabilidad y la hinchazón de
esta capa. Los ASL, los AOS y los AS no indujeron sensibilización
cutánea en voluntarios y no se ha encontrado ninguna prueba definitiva
de que induzcan la formación de eczemas. No se han comunicado lesiones
graves ni muertes tras la ingestión accidental de estas sustancias
tensioactivas por personas.
1.8 Efectos en el medio ambiente
1.8.1 Alkilsulfonatos lineales
1.8.1.1 Medio acuático
Los ASL han sido objeto de amplios estudios tanto en el
laboratorio (estudios de corta y larga duración) como en condiciones
más naturales (microcosmos y mesocosmos y estudios sobre el terreno).
En general, la disminución de la cadena alkilo o la posición más
interna del grupo fenilo van acompañadas de una menor toxicidad. Las
observaciones en peces y en Daphnia indican que al disminuir la
longitud de la cadena en una unidad (por ejemplo de C12 a C11) la
toxicidad se reduce prácticamente a la mitad.
Los resultados de las pruebas de laboratorio han sido los
siguientes:
-- Microorganismos: Los resultados son muy variables debido al
uso de diversos sistemas de prueba (Por ejemplo, inhibición de fango
activado; cultivos mixtos y especies individuales). Los valores de la
CE50 oscilan entre 0,5 mg/litro (especie única) y > 1000 mg/litro.
Para los microorganismos no hay relación lineal entre la longitud de
la cadena y la toxicidad.
-- Plantas acuáticas: Los resultados dependen mucho de las
especies. Para los organismos de agua dulce, los valores de la
CE50 son de 10-235 mg/litro (C10-C14) en las algas verdes, de
5-56 mg/litro (C11,1-C13) en las algas cianofíceas, de
1,4-50 mg/litro (C11,6-C13) en las diatomeas y de
2,7-4,9 mg/litro (C11,8) en las macrofitas; al parecer las algas
marinas son aún más sensibles. En las algas es probable que no haya
relación lineal entre la longitud de la cadena y la toxicidad.
-- Invertebrados: Los valores de la CL(E)50 aguda por lo menos
en 22 especies de agua dulce son de 4,6-200 mg/litro (longitud de la
cadena sin especificar; C13) para los moluscos; 0,12-27 mg/litro
(sin especificar; C11,2-C18) para los crustáceos; 1,7-16 mg/litro
(sin especificar; C11,8) para los gusanos; y 1,4-270 mg/litro
(C10-C15) para los insectos. Los valores de la CL(E)50 crónica
son de 2,2 mg/litro (C11,8) para los insectos y 1,1-2,3 mg/litro
(C11,8-C13) para los crustáceos. La concentración crónica sin
efectos observados (NOEC; basada en la letalidad o los efectos en la
reproducción) es de 0,2-10 mg/litro (sin especificar; C11,8) para
los crustáceos. Parece que los invertebrados marinos son más
sensibles, con valores de la CL50 de 1 a > 100 mg/litro (casi
siempre C12) para 13 especies y NOEC de 0,025-0,4 mg/litro (sin
especificar en todas las pruebas) para siete especies ensayadas.
-- Peces: Los valores de la CL50 aguda son de 0,1-125 mg/litro
(C8-C15) para 21 especies de agua dulce; los valores de la
CL(E)50 crónica son de 2,4 y 11 mg/litro (sin especificar; C11,7)
para dos especies; y las NOEC de 0,11-8,4 y 1,8 mg/litro (sin
especificar; C11,2-C13) para dos especies. También en este caso
los peces marinos parecen ser más sensibles, con valores de la CL50
aguda de 0,05-7 mg/litro (sin especificar; C11,7) para seis especies
y de la CL50 crónica de 0,01-1 mg/litro (sin especificar) para
dos especies. En la mayoría de los informes no se indicaba la
longitud de la cadena. Para especies marinas se señaló una NOEC de <
0,02 mg/litro (C12).
La longitud media de la cadena de los productos utilizados
habitualmente en el comercio es C12. Se han probado compuestos de
numerosas longitudes de cadena en Daphnia magna y en peces, pero la
longitud utilizada en otros organismos de agua dulce ha solido ser la
de C11,8. Los valores típicos de la CL(E)50 para el ASL C12 son
de 3-6 mg/litro en Daphnia magna y de 2-15 mg/litro en peces de agua
dulce, y las NOEC crónicas típicas son de 1,2-3,2 mg/litro para
Daphnia y de 0,48-0,9 mg/litro para los peces de agua dulce. Los
valores típicos de la CL50 de los ASL con cadenas de esta longitud
son en los peces marinos < 1-6,7 mg/litro.
Los organismos de agua salada, en particular los invertebrados,
parecen ser más sensibles que los de agua dulce a los ASL. En los
invertebrados, la acción inhibidora de los ASL sobre el calcio puede
afectar a la disponibilidad de este ión para la morfogénesis. Los ASL
tienen un efecto general sobre el transporte iónico. Los productos de
la biodegradación y los subproductos de los ASL son 10-100 veces menos
tóxicos que las sustancias de las que proceden.
Los resultados obtenidos en condiciones más reales son los
siguientes: Se han utilizado ASL en todas las pruebas de agua dulce a
varios niveles tróficos, como recintos cerrados en lagos (organismos
inferiores), modelos de ecosistemas (sistemas de sedimentos y agua),
ríos por debajo y por encima del desagüe de depuradoras de aguas
residuales y corrientes experimentales. En casi todos los casos se
emplearon ASL C12. Al parecer las algas son más sensibles en verano
que en invierno, puesto que los valores de la CE50 en tres horas
fueron de 0,2-8,1 mg/litro después de la fotosíntesis, mientras que en
los modelos de ecosistemas no se observó ningún efecto en la
abundancia relativa de las comunidades de algas con 0,35 mg/litro. Los
niveles sin efecto en estos estudios fueron de 0,24-5 mg/litro, en
función del organismo y del parámetro ensayado. Estos resultados
prácticamente coinciden con los de las pruebas de laboratorio.
1.8.1.2 Medio terrestre
Se dispone de información acerca de las plantas y las lombrices de
tierra. Las NOEC para siete especies de plantas en pruebas realizadas
con soluciones de nutrientes son < 10-20 mg/litro; la correspondiente
a tres especies en suelo, mediante pruebas basadas en el crecimiento
fue de 100 mg/litro (C10-C13). La CL50 en 14 días fue para las
lombrices de tierra > 1000 mg/kg.
1.8.1.3 Aves
En un estudio con pollos tratados en la alimentación se obtuvo una
NOEC (basada en la calidad de los huevos) > 200 mg/kg.
1.8.2 alpha-Olefinsulfonatos
Los datos acerca de los efectos de los AOS en los organismos
acuáticos y terrestres son limitados.
1.8.2.1 Medio acuático
Solamente se dispone de datos de pruebas de laboratorio:
-- Algas: Los valores de la CE50 que se han descrito para las
algas verdes son > 20-65 mg/litro (C16-C18).
-- Invertebrados: Para Daphnia se han notificado valores de la
CL50 de 19 y 26 mg/litro (C16-C18).
-- Peces: Los valores de la CL50 son de 0,3-6,8 mg/litro
(C12-C18) para nueve especies de peces. De los estudios de corta
duración realizados en la trucha común (Salmo trutta), Idus
melanotus y Rasbora heteromorpha, cabe concluir que la toxicidad
de los compuestos C14-C16 es unas cinco veces inferior a la de los
C16-C18, con valores de la CL50 (todas las concentraciones
medidas) de 0,5-3,1 (C16-C18) y 2,5-5,0 mg/litro (C14-C16). En
dos estudios de larga duración en la trucha arcoiris se comprobó que
el crecimiento era el parámetro más sensible, con una CE50 de
0,35 mg/litro. En un pez marino, el pardete (Mugal cephalus), el
valor de la CL50 en 96 horas fue de 0,70 mg/litro.
1.8.2.2 Medio terrestre
En un estudio de plantas con soluciones de nutrientes, la NOEC fue
de 32-56 mg/litro. En un estudio con pollos tratados en la
alimentación, se notificó una NOEC (basada en la calidad de los
huevos) > 200 mg/kg.
1.8.3 Alkilsulfatos
1.8.3.1 Organismos acuáticos
Se han realizado estudios de los AS de corta y larga duración y
uno en condiciones más reales. Su toxicidad también depende de la
longitud de la cadena alkilo; no se disponía de información sobre
diferencias de toxicidad entre los AS lineales y ramificados.
Los resultados de las pruebas de laboratorio son los siguientes:
-- Microorganismos: Los valores de la CE50 en un conjunto de
microorganismos marinos fueron de 2,1-4,1 mg/litro (C12). Las NOEC
en Pseudomonas putida fueron de 35-550 mg/litro (C16-C18).
-- Plantas acuáticas: Los valores de la CE50 fueron >
20-65 mg/litro (C12-C13) en algas verdes y de 18-43 mg/litro
(C12) en macrofitas. Las NOEC fueron de 14-26 mg/litro
(C12-C16/C18) en algas verdes.
-- Invertebrados: Los valores de la CL(E)50 fueron de
4-140 mg/litro (C12/C15-C16/C18) en especies de agua dulce y
de 1,7-56 mg/litro (todos C12) en especies marinas. La NOEC crónica
en Daphnia magna fue de 16,5 mg/litro (C16/C18) y en especies
marinas de 0,29-0,73 mg/litro (longitud de la cadena sin especificar).
-- Peces: Los valores de la CL50 fueron de 0,5-5,1 mg/litro
(longitud de la cadena sin especificar o C12-C16) en especies de
agua dulce y de 6,4-16 mg/litro (todos C12) en especies marinas. No
había estudios de larga duración.
Hay que señalar que muchos de estos estudios se llevaron a cabo en
condiciones estáticas. Los AS son fácilmente biodegradables, por lo
que se puede haber infravalorado su toxicidad. En un estudio de 48
horas con Oryzias latipes, los valores de la CL50 fueron de 46, 2,5
y 0,61 mg/litro (concentraciones medidas) para los compuestos C12,
C14 y C16 respectivamente. Este y otros estudios indican que la
toxicidad difiere en un factor de cinco por cada dos unidades de
longitud de la cadena. En un estudio de biocenosis de paso de
corriente con compuestos de C16-C18 se observó una NOEC de
0,55 mg/litro.
1.8.3.2 Organismos terrestres
Se han notificado valores de la NOEC > 1000 mg/kg (C16-C18)
en lombrices de tierra y en nabos.
1.9 Evaluación del riesgo para la salud humana
Los ASL son los agentes tensioactivos más utilizados en
detergentes y productos de limpieza. También se utilizan AOS y AS en
detergentes y en productos de aseo personal. Por consiguiente, la
principal vía de exposición humana es el contacto cutáneo. Pueden
ingerirse pequeñas cantidades de ASL, AOS y AS con el agua potable y
debido a la presencia de residuos en utensilios y alimentos. Aunque la
información de que se dispone es limitada, la ingesta diaria de ASL
por esos medios se puede estimar en unos 5 mg/persona. Puede
producirse exposición en el trabajo a los ASL, AOS y AS durante la
formulación de diversos productos, pero no hay datos acerca de los
efectos de una exposición crónica a estas sustancias en el ser humano.
Los ASL, AOS y AS pueden irritar la piel después de un contacto
cutáneo repetido o prolongado con concentraciones análogas a las
presentes en los productos sin diluir. En los cobayas los AOS pueden
inducir sensibilización cutánea cuando el nivel de sulfona g
insaturada es superior a unas 10 ppm.
Los estudios disponibles de larga duración en animales
experimentales son insuficientes para evaluar el potencial
carcinogénico de los ASL, AOS y AS, debido a factores como el diseño
del estudio, el uso de un número pequeño de animales, el ensayo de
dosis insuficientes y lo limitado del examen histopatológico. En los
escasos estudios en los que se administró ASL, AOS o AS a animales por
vía oral no se observaron signos de carcinogenicidad; también fueron
negativos los resultados de los estudios de larga duración en los que
se administraron AOS mediante aplicación cutánea. Estas sustancias no
parecen ser genotóxicas in vivo o in vitro, aunque se tienen
noticias de pocos estudios.
Se han descrito efectos mínimos, entre ellos alteraciones
bioquímicas y cambios histopatológicos hepáticos, en estudios
subcrónicos en los que se administraron ASL a ratas en la alimentación
o el agua de beber a concentraciones equivalentes a una dosis
aproximada de 120 mg/kg de peso corporal al día, aunque no se observó
ningún efecto en estudios en los que los animales estuvieron expuestos
a dosis más elevadas durante períodos más largos. La aplicación
cutánea de ASL ocasionó tanto toxicidad sistémica como efectos
locales.
La ingesta diaria media de ASL de la población general, con
arreglo a estimaciones limitadas de la exposición por medio del agua
potable, utensilios y alimentos probablemente sea muy inferior (unas
tres veces menor) a los niveles con los que se ha observado que
inducen efectos leves en los animales experimentales.
Los efectos de los AOS observados en el ser humano en los escasos
estudios disponibles son parecidos a los descritos en los animales
expuestos a los ASL. Debido a que son insuficientes los datos para
estimar la ingesta diaria media de AOS de la población general y los
relativos a los niveles que inducen efectos en el ser humano y en los
animales, no es posible evaluar con suficiente confianza si la
exposición a los AOS en el medio ambiente representa un riesgo para la
salud humana. Sin embargo, los niveles de AOS en medios a los que
puede estar expuesto el ser humano probablemente sean inferiores a los
de ASL, puesto que se utilizan menos.
Se han descrito repetidamente efectos en un pequeño número de
estudios limitados en ratas que recibieron AS en la alimentación o en
el agua de beber en concentraciones equivalentes a dosis de
200 mg/kg de peso corporal al día o más. Se han observado efectos
locales en la piel y en los ojos tras una aplicación tópica repetida
o prolongada. Los datos disponibles son insuficientes para estimar la
ingesta diaria media de AS de la población general. Sin embargo, dado
que no se utilizan tanto agentes tensioactivos con AS como los que
contienen ASL, la ingesta de AS será probablemente como mínimo tres
veces inferior a las dosis que se ha demostrado que inducen efectos en
los animales.
1.10 Evaluación de los efectos en el medio ambiente
Los ASL, los AOS y los AS se utilizan en grandes cantidades y se
liberan en el medio ambiente por medio de las aguas residuales. Para
evaluar el riesgo es preciso comparar las concentraciones de
exposición con las que no producen efectos adversos, y esto se puede
hacer para varios compartimentos del medio ambiente. Para los agentes
tensioactivos aniónicos en general, los compartimentos más importantes
son las depuradoras de aguas residuales, las aguas superficiales, los
suelos tratados con sedimentos y fangos y los medios estuarinos y
marinos. Se produce tanto biodegradación (primaria y final) como
adsorción, por lo que disminuyen las concentraciones y la
biodisponibilidad en el medio ambiente. Con la reducción de la
longitud de la cadena y la pérdida de la estructura original se forman
sustancias menos tóxicas que la primera. Es importante tener en cuenta
estos aspectos a la hora de interpretar los resultados de laboratorio
con los posibles efectos en el medio ambiente. Por otra parte, al
evaluar el riesgo asociado con la exposición del medio ambiente a
estos tres compuestos aniónicos se debe establecer una comparación con
los resultados de las pruebas de toxicidad de sustancias cuya cadena
tenga la misma longitud.
Se han realizado abundantes pruebas de los efectos de los ASL en
los organismos acuáticos. En las pruebas de laboratorio con agua dulce
parece que los peces eran las especies más sensibles; la NOEC para
Pimephales promelas fue de unos 0,5 mg/litro (C12), y estos
resultados se confirmaron en pruebas realizadas en condiciones más
reales. en el fitoplancton se han observado diferencias: en ensayos
de toxicidad agua de tres horas los valores de la CE50 fueron de
0,2-0,1 mg/litro (C12-C13), mientras que no se detectaron efectos
en la abundancia relativa en otras pruebas con 0,24 mg/litro
(C11,8). Parece que las especies marinas eran ligeramente más
sensibles que la mayoría de los otros grupos taxonómicos.
En el medio ambiente hay una amplia gama de concentraciones de las
tres sustancias aniónicas, como se ha puesto de manifiesto en las
numerosas mediciones de los ASL. Debido a esta amplia gama, no se
puede hacer una evaluación del riesgo de estas sustancias para el
medio ambiente de aplicación general. Para evaluar el riesgo se deben
conocer de manera apropiada las concentraciones de exposición y las
que tienen efectos en el ecosistema que interesa.
Se necesitan datos precisos sobre la exposición a los AS y los AOS
si se quiere hacer una evaluación del riesgo para el medio ambiente.
Por consiguiente se están utilizando modelos a fin de evaluar las
concentraciones de exposición en los compartimentos del medio ambiente
que los reciben. Los datos sobre la toxicidad de los AS y los AOS para
los organismos acuáticos, especialmente después de una exposición
crónica a concentraciones estables, son relativamente escasos. Los
datos disponibles indican que la toxicidad de estos productos es
análoga a la de otras sustancias tensioactivas aniónicas.
Los organismos de agua salada parecen ser más sensibles que los de
agua dulce a estos compuestos; sin embargo, su concentración es menor
en el agua del mar, excepto cerca de los desagües de alcantarillados.
No se han investigado con detalle su destino y sus efectos en las
aguas residuales vertidas en el mar.
Si se desea evaluar la inocuidad para el medio ambiente de agentes
tensioactivos como los ASL, los AOS y los AS hay que comparar las
concentraciones reales en el medio ambiente con las que no tienen
ningún efecto. Las necesidades de investigación se determinan no sólo
por las propiedades intrínsecas de un producto químico, sino también
por sus características o la tendencia del consumo. Estos varían
considerablemente entre las distintas zonas geográficas, por lo que la
evaluación debe ser de ámbito regional.
1.11 Recomendaciones para la protección de la salud humana y
el medio ambiente
1. Puesto que en el lugar de trabajo se puede producir exposición al
polvo (durante la elaboración y formulación), deben utilizarse
prácticas normalizadas de higiene del trabajo a fin de asegurar la
protección de la salud de los trabajadores.
2. La composición de las formulaciones para uso privado e industrial
se debe diseñar de manera que se evite el riesgo, especialmente en las
formulaciones utilizadas para la limpieza o el lavado a mano.
3. La exposición y los efectos en el medio ambiente se deben vigilar
de manera apropiada con objeto de tener una indicación pronta de
cualquier acumulación excesiva en los compartimentos pertinentes del
medio ambiente.
1.12 Recomendaciones de nuevas investigaciones
Salud humana
1. Debido a que la piel es la principal vía de exposición humana a
los ASL, los AOS y los AS y a que no se dispone de estudios adecuados
de larga duración acerca de la toxicidad cutánea o la carcinogenicidad
en animales experimentales, se recomienda la realización de estudios
de larga duración debidamente diseñados de aplicación cutánea de estas
sustancias.
2. Ante la falta de datos definitivos sobre la genotoxicidad de los
AOS y los AS, deben llevarse a cabo nuevos estudios in vivo e in
vitro.
3. A la vista de la insuficiencia de los estudios disponibles sobre
la toxicidad en la reproducción y el desarrollo, se han de realizar
estudios definitivos en animales de laboratorio a fin de obtener datos
relativos a los efectos de los ASL, los AOS y los AS y los niveles con
efectos y sin ellos.
4. Puesto que la exposición a los ASL, los AOS y los AS no está
debidamente definida, se debe vigilar la exposición de la población
general, en particular cuando estas sustancias tensioactivas se
utilizan en la limpieza y el lavado a mano.
5. Debido a que los ASL, los AOS y los AS pueden potenciar el
transporte de otras sustancias químicas y regular su biodisponibilidad
y toxicidad en las aguas superficiales, los sedimentos fluviales y los
suelos a los que puede estar expuesto el ser humano, deben
investigarse las interacciones con otras sustancias químicas del medio
ambiente y las consecuencias para las personas.
Inocuidad para el medio ambiente
6. Deben realizarse nuevos estudios sobre los mecanismos de adsorción
y desorción de los AOS y los AS. También se debe estudiar el reparto
de los ASL, los AOS y los AS entre las partículas coloidales disueltas
y suspendidas en el agua. Hay que elaborar modelos matemáticos de los
coeficientes de sorción y validarlos con arreglo a parámetros
fisicoquímicos.
7. Se han de realizar estudios de la biodegradación de los AOS y los
AS en suelos tratados con fangos y en sedimentos fluviales (zonas
aerobias y anaerobias) corriente abajo de los vertidos de aguas
residuales tratadas y sin tratar.
8. Se deben vigilar en los ámbitos regional y nacional las
concentraciones de ASL, AOS y AS en el medio ambiente, a fin de
obtener información acerca de la exposición. Se han de preparar
métodos analíticos para la detección de niveles bajos de AOS y AS en
los compartimentos pertinentes del medio ambiente.
9. Hay que organizar bases de datos nacionales sobre las
concentraciones de ASL, AOS y AS en las aguas residuales y en los ríos
y sobre los tipos, la eficacia y el lugar de las depuradoras de aguas
residuales, con objeto de facilitar la evaluación de los efectos de
los vertidos de estas sustancias tensioactivas en el medio ambiente.
10. Deben realizarse estudios de la toxicidad de los AOS y los AS en
los peces (de agua dulce y marinos) y los invertebrados acuáticos para
establecer la sensibilidad relativa de estas especies.
 j,k: integers ( j + k = 7-11)
Common name: Sodium linear alkylbenzenesulfonate
Common synonyms: LAS, LAS sodium salt, linear
alkylbenzene-sulfonic acid sodium salt,
linear dodecyl-benzenesulfonic acid
sodium salt, sodium straight chain
alkylbenzenesulfonate
CAS Registry number:
j,k: integers ( j + k = 7-11)
Common name: Sodium linear alkylbenzenesulfonate
Common synonyms: LAS, LAS sodium salt, linear
alkylbenzene-sulfonic acid sodium salt,
linear dodecyl-benzenesulfonic acid
sodium salt, sodium straight chain
alkylbenzenesulfonate
CAS Registry number: 