
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 168
CRESOLS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr L. Papa, US Environmental Protection
Agency, Cincinnati, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Cresols
(Environmental health criteria ; 168)
1.Cresols - adverse effects
2. Environmental exposure I.Series
ISBN 92 4 157168 1 (NLM Classification: QV 223)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
1. SUMMARY
1.1. Identity, properties and analytical methods
1.2. Uses, sources and levels of exposure
1.3. Kinetics and metabolism
1.4. Effects on laboratory mammals; in vitro systems
1.5. Effects on humans
1.6. Effects on other organisms
1.7. Conclusion and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factorsl
2.4. Analytical methods
2.4.1. Sampling
2.4.2. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.2. Transformation
4.2.1. Abiotic transformation
4.2.2. Biodegradation
4.3. Bioaccumulation and biomagnification
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food and beverages
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Endogenous cresols
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Inhalation route
7.1.2. Oral route
7.1.3. Dermal route
7.2. Short-term exposure
7.2.1. Inhalation route
7.2.2. Oral route
7.3. Long-term exposure
7.3.1. Inhalation route
7.3.2. Oral route
7.4. Skin and eye irritation
7.5. Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Other special studies
7.8.1. Neurological effects
7.8.2. Effects on the skin
7.9. Mechanism of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Poisoning incidents
8.1.2. Controlled human studies
8.1.3. Cancer
8.2. Occupational exposure
8.2.1. Poisoning incidents
8.2.2. Epidemiological studies
8.3. Subpopulations at special risk
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.1.1. Aquatic
9.1.1.1 Laboratory studies
9.1.1.2 Field studies
9.1.2. Terrestrial
9.1.2.1 Laboratory studies
9.1.2.2 Field studies
9.2. Plants
9.2.1. Aquatic
9.2.1.1 Laboratory studies
9.2.1.2 Field studies
9.2.2. Terrestrial
9.2.2.1 Laboratory studies
9.2.2.2 Field studies
9.3. Invertebrates
9.3.1. Aquatic
9.3.1.1 Laboratory studies
9.3.1.2 Field investigations
9.3.2. Terrestrial
9.3.2.1 Laboratory studies
9.3.2.2 Field studies
9.4. Vertebrates
9.4.1. Aquatic
9.4.1.1 Laboratory studies
9.4.1.2 Field studies
9.4.2. Terrestrial
9.4.2.1 Laboratory studies
9.4.2.2 Field studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of environmental risks
10.3. Guidance value
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1. Conclusions
11.2. Recommendations
12. FURTHER RESEARCH
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC,
JECFA, JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
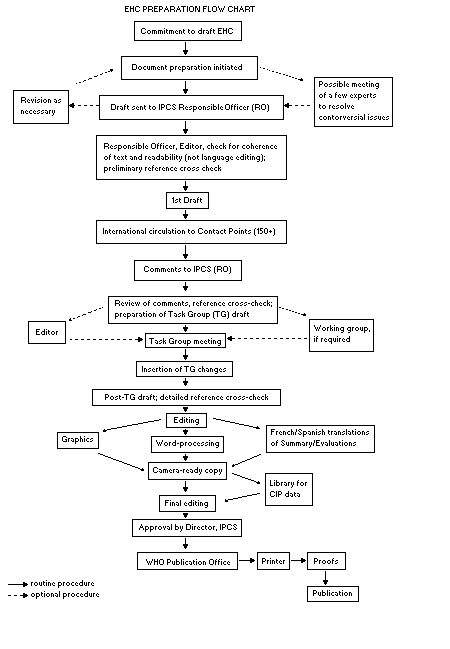
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
Members
Dr D. Anderson, British Industrial Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr M.R. Elwell, National Institute of Health, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr A. Meharg, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, United Kingdom
Dr C.-N. Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
(Vice-Chairman)
Dr Y. Pang, Division of Standard Setting, Chinese Academy of
Preventive Medicine, Beijing, China
Dr L. Papa, System Toxicants Assessment Branch, Office of Research and
Development, Environmental Criteria and Assessment Office, US
Environmental Protection Agency, Cincinnati, Ohio, USA
(Rapporteur)
Dr A. Pinter, National Institute of Hygiene, Budapest, Hungary
Dr S. Soliman, Pesticide Chemistry and Toxicology, College of
Agriculture and Veterinary Medicine, Bureidah, Saudi Arabia
Dr F.M. Sullivan, Division of Pharmacology and Toxicology, St Thomas's
Hospital, London, United Kingdom (Chairman)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
A WHO Task Group on Environmental Health Criteria for Cresols met
at the British Industrial Biological Research Association (BIBRA)
Toxicology International, Carshalton, Surrey, United Kingdom, from 27
June to 1 July 1994. Dr D. Anderson opened the meeting and welcomed
the participants on behalf of the host institution. Dr B.H. Chen,
IPCS, welcomed the participants on behalf of the Director, IPCS, and
the three cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to cresols.
Drs N.N. Molodkina, L.P. Kuzmina and A.L. Germanova, Centre for
International Projects, Moscow, Russian Federation, prepared a
preliminary draft. The first draft of this monograph was prepared by
Dr L. Papa, US Environmental Protection Agency, Cincinnati, USA. The
second draft was also prepared by Dr L. Papa, incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
1. SUMMARY
1.1 Identity, properties and analytical methods
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para position relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols.
However, commercial products contain up to 30% xylenol and 60%
C9-phenols and are known as "cresylic acids". Physically, cresols
consist either of a white crystalline solid or a yellowish liquid and
have a strong, phenol-like odour. They are highly flammable and are
soluble in water, ethanol, ether, acetone and alkali hydroxides.
Cresols undergo electrophilic substitution reactions at the vacant
ortho or para position relative to the hydroxyl group. They also
undergo condensation reactions with aldehydes, ketones or dienes.
Several methods can be used for determining the presence of
cresols in both environmental and biological media. The most commonly
used methods are gas chromatography with flame ionization detection
(GC-FID), gas chromatography with mass spectrophotometry (GC-MS) and
high-performance liquid chromatography (HPLC). Sampling of cresols in
air can be done by passing air through absorption cells using sodium
hydroxide or solid adsorbents.
1.2 Uses, sources and levels of exposure
Cresols have a wide variety of uses as solvents or disinfectants
or as intermediates in the production of numerous other substances.
These compounds are most commonly used in the production of
fragrances, antioxidants, dyes, pesticides and resins. Ortho- and
para-cresols are used in the production of lubricating oils, motor
fuels and rubber polymers, while meta-cresol is used in the
manufacture of explosives.
Cresols and cresol derivatives occur naturally in oils of various
plants, including Yucca gloriosa flowers, jasmine, Easter lily,
conifers, oaks and sandalwood trees, and are also a product of
combustion from natural fires and volcanic activity. Para-cresol is
found in the urine of animals and humans. Commercially cresols are
produced as by-products in the fractional distillation of crude oil
and coal tars. Small amounts are produced in vehicle exhaust,
municipal waste incinerators and from coal and wood combustion.
Cigarette smoke also contains cresols. The worldwide production of
cresols is unknown; annual production in the USA in 1990 was reported
to be 38 300 tonnes.
Environmental transport of cresols occurs through the vapour
phase of the atmosphere and from the atmosphere to surface water and
soil by rain-scavenging. Due to their volatilization, binding to
sediment and biodegradation, only small amounts of cresols are found
in water. In soils, cresols are slightly to highly mobile depending on
the sorption coefficient (Koc) of the soil. Cresols have been
detected in ground water, and so leaching must occur in soil.
Exposure to cresols can occur through air, water or food. The
median air concentration of o-cresols was 1.59 µg/m3 (0.359 ppb)
for 32 source-dominated sites in the USA. Surface water
concentrations in the USA range from below the detection limit to
77 µg/litre (STORET, 1993). Levels of 204 µg/litre were reported in
Japan in a river polluted by industrial effluents. Concentrations as
high as 2100 µg/litre for o-cresol and 1200 µg/litre for mixed
m- and p-cresols have been detected in waste waters. Rainwater
concentrations range from 240 to 2800 ng/litre for o-cresol and 380
to 2000 ng/litre for p- and m-cresol combined. Cresols have been
detected in foods and beverages. Concentrations in spirit beverages
were found to be within the range of 0.01-0.2 mg/litre. The amount in
tobacco smoke is 75 µg in a nonfilter American cigarette (85 mm). The
general population can be exposed to cresols from air inhalation,
drinking-water, food and beverage ingestion and dermal contact. In
general, the lack of adequate monitoring data makes the quantitative
estimates of daily intakes of cresol from these sources impossible.
Occupational exposure levels as high as 5.0 mg/m3 have been
reported.
1.3 Kinetics and metabolism
Cresols are absorbed across the respiratory and gastrointestinal
tracts and through the skin. The rate and extent of absorption of
cresols has not been studied specifically. However, studies have
shown that gastrointestinal and dermal absorption are rapid and
extensive. Cresols are distributed to all the major organs. The
primary metabolic pathway for cresols is conjugation with glucuronic
acid and inorganic sulfate. Minor metabolic pathways for cresols
include hydroxylation of the benzene ring and side-chain oxidation.
The main route for elimination of cresols from the body is renal
excretion in the form of conjugates.
1.4 Effects on laboratory mammals; in vitro systems
Acute poisoning with cresol vapours is unlikely due to the low
vapour pressure of these compounds. Mean lethal concentrations of
cresols in rats have been reported to be 29 mg/m3 for o- and
p-cresols and 58 mg/m3 for m-cresol. Oral LD50 values in rats
have been reported to be 121, 207 and 242 mg/kg body weight for o-,
p- and m-cresols, respectively. Interspecies comparisons show
that all three isomers are more toxic to mice than to rats and that
toxicity increases with concentration. Systemic toxicity and death
can result from dermal exposure. Dermal LD50 values in rabbits were
890, 2830, 300 and 2000 mg/kg body weight for o-, m-, p-and
mixed cresols, respectively. In rats dermal LD50 values were 620,
1100, 750 and 825 mg/kg body weight for o-, m-, p- and dicresol,
respectively.
Cresols are highly irritating to the skin and eyes of rabbits,
rats and mice.
Short-term exposure to inhaled mixtures of o-cresol aerosol and
vapour resulted in irritation of the respiratory tract, small
haemorrhages in the lung, body weight reduction and degeneration of
heart muscle, liver, kidney and nerve cells. Short-term (28-days)
oral exposure to daily doses of approximately 800 mg/kg body weight or
more resulted in reduced body weights, organ weight changes and
histopathological changes in the respiratory and gastrointestinal
tracts of rats. In mice, similarly exposed at 1500 mg/kg body weight,
more severe effects were reported, and at the highest concentrations
death resulted from exposure to o-, m- and p-cresols but not
from exposures to mixtures of isomers.
Longer-term exposure of rats to vapours of o-, m- or
p-cresol for up to 4 months resulted in weight loss, reduced
locomotor activity, inflammation of nasal membranes and skin, and
changes in the liver. Oral exposures for up to 13 weeks of mice,
rats and hamsters resulted in mortality, tremor, reduced body weights,
haematological effects, increase in organ weight, and hyperplasia of
nasal and forestomach epithelium.
Oral and inhalation exposure to cresol isomers result in
lengthened estrus cycle and histopathological changes in the uterus
and ovaries of rats and mice. No adverse effects on spermatogenesis
were observed in rats or mice. Mild fetotoxic effects have been
reported in rats and rabbits exposed to o- and p-cresols, but only
minor treatment-related developmental effects have been reported.
Some evidence of genotoxicity has been reported to result in vitro
from treatment with o- and p- cresols but not m-cresol. No
positive results were obtained in in vivo studies. However, some
evidence of promotive activities in skin has been reported. No
studies of carcinogenicity have been reported for any cresol isomers.
1.5 Effects on humans
Ingestion of cresols results in burning of the mouth and throat,
abdominal pain and vomiting. The target tissues/organs of ingested
cresols in humans are the blood and kidneys, and effects on the lungs,
liver, heart and central nervous system have also been reported. In
severe cases, coma and death may result. Dermal exposure has been
reported to cause severe skin burns, scarring, systemic toxicity and
death.
Occupational exposure to cresols usually results from dermal
contact. Acute exposures can result in severe burns, anuria, coma and
death. Very few data are available regarding reproductive effects and
there are no data on carcinogenicity in humans.
1.6 Effects on other organisms
Observations on microorganisms, invertebrates and fish have shown
that cresols may represent a risk to non-mammalian organisms at point
sources with high cresol concentration but not in the general
environment.
1.7 Conclusion and recommendations
At concentrations normally found in the environment, cresols do
not pose any significant risk for the general population. However,
the potential for adverse health effects exists in the case of people
with renal insufficiency or specific enzymic deficiency and under
conditions of high exposure.
Cresols may represent a risk to microorganisms, invertebrates and
fish at point sources with high cresol concentrations but not in the
general environment.
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess the carcinogenic hazard of cresols. Based on the results of
subchronic studies, an NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, an acceptable daily intake (ADI) of
0.17 mg/kg body weight per day can be established for cresols.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para positions relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols
(Deichmann & Keplinger, 1981). Mixtures of m- and p-cresol and of
o-, m- and p-cresol are occasionally called dicresol and
tricresol, respectively (Fiege & Bayer, 1987). Pure and commercial
cresol or cresylic acid is different from the commercial products
called "cresylic acids". The substance "cresylic acids" is a mixture
of phenolic compounds with a typical composition as follows: 0-1% m-
and p-cresol; 0-3% 2,4- and 2,6-xylenols; 10-20% 2,3- and
3,5-xylenols; 20-30% 3,4-xylenol; and 50-60% C9-phenols (Sax & Lewis,
1987). The chemical identity of cresols is shown in Table 1.
Commercial cresols are manufactured in a wide range of
grades and purities to suit the user's requirements. Typically,
technical grade cresol available in the USA contains about 20%
o-cresol, 40% m-cresol, 30% p-cresol, and 10% phenol and
xylenols (Deichmann & Keplinger, 1981). The individual isomers are
available at purity levels as low as 85% and as high as > 99% from
chemical suppliers in the USA.
2.2 Physical and chemical properties
The physical properties of the three individual isomers and the
mixture are given in Table 2.
Chemically, cresols behave similarly to phenol. These compounds
undergo electrophilic substitution reactions at the vacant ortho or
para position relative to the hydroxyl group. Chlorination,
bromination, sulfonation and nitration are examples of such
substitution reactions. Cresols can undergo condensation reactions
with aldehydes, ketones and dienes (Fiege & Bayer, 1987).
2.3 Conversion factors
Air at 25°C: 1 ppm = 4.42 mg/m3
1 mg/m3 = 0.23 ppm
Table 1. Chemical identity of cresols
o-Cresol p-Cresol m-Cresol Mixture
Chemical structure:
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
Members
Dr D. Anderson, British Industrial Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr M.R. Elwell, National Institute of Health, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr A. Meharg, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, United Kingdom
Dr C.-N. Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
(Vice-Chairman)
Dr Y. Pang, Division of Standard Setting, Chinese Academy of
Preventive Medicine, Beijing, China
Dr L. Papa, System Toxicants Assessment Branch, Office of Research and
Development, Environmental Criteria and Assessment Office, US
Environmental Protection Agency, Cincinnati, Ohio, USA
(Rapporteur)
Dr A. Pinter, National Institute of Hygiene, Budapest, Hungary
Dr S. Soliman, Pesticide Chemistry and Toxicology, College of
Agriculture and Veterinary Medicine, Bureidah, Saudi Arabia
Dr F.M. Sullivan, Division of Pharmacology and Toxicology, St Thomas's
Hospital, London, United Kingdom (Chairman)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
A WHO Task Group on Environmental Health Criteria for Cresols met
at the British Industrial Biological Research Association (BIBRA)
Toxicology International, Carshalton, Surrey, United Kingdom, from 27
June to 1 July 1994. Dr D. Anderson opened the meeting and welcomed
the participants on behalf of the host institution. Dr B.H. Chen,
IPCS, welcomed the participants on behalf of the Director, IPCS, and
the three cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to cresols.
Drs N.N. Molodkina, L.P. Kuzmina and A.L. Germanova, Centre for
International Projects, Moscow, Russian Federation, prepared a
preliminary draft. The first draft of this monograph was prepared by
Dr L. Papa, US Environmental Protection Agency, Cincinnati, USA. The
second draft was also prepared by Dr L. Papa, incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
1. SUMMARY
1.1 Identity, properties and analytical methods
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para position relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols.
However, commercial products contain up to 30% xylenol and 60%
C9-phenols and are known as "cresylic acids". Physically, cresols
consist either of a white crystalline solid or a yellowish liquid and
have a strong, phenol-like odour. They are highly flammable and are
soluble in water, ethanol, ether, acetone and alkali hydroxides.
Cresols undergo electrophilic substitution reactions at the vacant
ortho or para position relative to the hydroxyl group. They also
undergo condensation reactions with aldehydes, ketones or dienes.
Several methods can be used for determining the presence of
cresols in both environmental and biological media. The most commonly
used methods are gas chromatography with flame ionization detection
(GC-FID), gas chromatography with mass spectrophotometry (GC-MS) and
high-performance liquid chromatography (HPLC). Sampling of cresols in
air can be done by passing air through absorption cells using sodium
hydroxide or solid adsorbents.
1.2 Uses, sources and levels of exposure
Cresols have a wide variety of uses as solvents or disinfectants
or as intermediates in the production of numerous other substances.
These compounds are most commonly used in the production of
fragrances, antioxidants, dyes, pesticides and resins. Ortho- and
para-cresols are used in the production of lubricating oils, motor
fuels and rubber polymers, while meta-cresol is used in the
manufacture of explosives.
Cresols and cresol derivatives occur naturally in oils of various
plants, including Yucca gloriosa flowers, jasmine, Easter lily,
conifers, oaks and sandalwood trees, and are also a product of
combustion from natural fires and volcanic activity. Para-cresol is
found in the urine of animals and humans. Commercially cresols are
produced as by-products in the fractional distillation of crude oil
and coal tars. Small amounts are produced in vehicle exhaust,
municipal waste incinerators and from coal and wood combustion.
Cigarette smoke also contains cresols. The worldwide production of
cresols is unknown; annual production in the USA in 1990 was reported
to be 38 300 tonnes.
Environmental transport of cresols occurs through the vapour
phase of the atmosphere and from the atmosphere to surface water and
soil by rain-scavenging. Due to their volatilization, binding to
sediment and biodegradation, only small amounts of cresols are found
in water. In soils, cresols are slightly to highly mobile depending on
the sorption coefficient (Koc) of the soil. Cresols have been
detected in ground water, and so leaching must occur in soil.
Exposure to cresols can occur through air, water or food. The
median air concentration of o-cresols was 1.59 µg/m3 (0.359 ppb)
for 32 source-dominated sites in the USA. Surface water
concentrations in the USA range from below the detection limit to
77 µg/litre (STORET, 1993). Levels of 204 µg/litre were reported in
Japan in a river polluted by industrial effluents. Concentrations as
high as 2100 µg/litre for o-cresol and 1200 µg/litre for mixed
m- and p-cresols have been detected in waste waters. Rainwater
concentrations range from 240 to 2800 ng/litre for o-cresol and 380
to 2000 ng/litre for p- and m-cresol combined. Cresols have been
detected in foods and beverages. Concentrations in spirit beverages
were found to be within the range of 0.01-0.2 mg/litre. The amount in
tobacco smoke is 75 µg in a nonfilter American cigarette (85 mm). The
general population can be exposed to cresols from air inhalation,
drinking-water, food and beverage ingestion and dermal contact. In
general, the lack of adequate monitoring data makes the quantitative
estimates of daily intakes of cresol from these sources impossible.
Occupational exposure levels as high as 5.0 mg/m3 have been
reported.
1.3 Kinetics and metabolism
Cresols are absorbed across the respiratory and gastrointestinal
tracts and through the skin. The rate and extent of absorption of
cresols has not been studied specifically. However, studies have
shown that gastrointestinal and dermal absorption are rapid and
extensive. Cresols are distributed to all the major organs. The
primary metabolic pathway for cresols is conjugation with glucuronic
acid and inorganic sulfate. Minor metabolic pathways for cresols
include hydroxylation of the benzene ring and side-chain oxidation.
The main route for elimination of cresols from the body is renal
excretion in the form of conjugates.
1.4 Effects on laboratory mammals; in vitro systems
Acute poisoning with cresol vapours is unlikely due to the low
vapour pressure of these compounds. Mean lethal concentrations of
cresols in rats have been reported to be 29 mg/m3 for o- and
p-cresols and 58 mg/m3 for m-cresol. Oral LD50 values in rats
have been reported to be 121, 207 and 242 mg/kg body weight for o-,
p- and m-cresols, respectively. Interspecies comparisons show
that all three isomers are more toxic to mice than to rats and that
toxicity increases with concentration. Systemic toxicity and death
can result from dermal exposure. Dermal LD50 values in rabbits were
890, 2830, 300 and 2000 mg/kg body weight for o-, m-, p-and
mixed cresols, respectively. In rats dermal LD50 values were 620,
1100, 750 and 825 mg/kg body weight for o-, m-, p- and dicresol,
respectively.
Cresols are highly irritating to the skin and eyes of rabbits,
rats and mice.
Short-term exposure to inhaled mixtures of o-cresol aerosol and
vapour resulted in irritation of the respiratory tract, small
haemorrhages in the lung, body weight reduction and degeneration of
heart muscle, liver, kidney and nerve cells. Short-term (28-days)
oral exposure to daily doses of approximately 800 mg/kg body weight or
more resulted in reduced body weights, organ weight changes and
histopathological changes in the respiratory and gastrointestinal
tracts of rats. In mice, similarly exposed at 1500 mg/kg body weight,
more severe effects were reported, and at the highest concentrations
death resulted from exposure to o-, m- and p-cresols but not
from exposures to mixtures of isomers.
Longer-term exposure of rats to vapours of o-, m- or
p-cresol for up to 4 months resulted in weight loss, reduced
locomotor activity, inflammation of nasal membranes and skin, and
changes in the liver. Oral exposures for up to 13 weeks of mice,
rats and hamsters resulted in mortality, tremor, reduced body weights,
haematological effects, increase in organ weight, and hyperplasia of
nasal and forestomach epithelium.
Oral and inhalation exposure to cresol isomers result in
lengthened estrus cycle and histopathological changes in the uterus
and ovaries of rats and mice. No adverse effects on spermatogenesis
were observed in rats or mice. Mild fetotoxic effects have been
reported in rats and rabbits exposed to o- and p-cresols, but only
minor treatment-related developmental effects have been reported.
Some evidence of genotoxicity has been reported to result in vitro
from treatment with o- and p- cresols but not m-cresol. No
positive results were obtained in in vivo studies. However, some
evidence of promotive activities in skin has been reported. No
studies of carcinogenicity have been reported for any cresol isomers.
1.5 Effects on humans
Ingestion of cresols results in burning of the mouth and throat,
abdominal pain and vomiting. The target tissues/organs of ingested
cresols in humans are the blood and kidneys, and effects on the lungs,
liver, heart and central nervous system have also been reported. In
severe cases, coma and death may result. Dermal exposure has been
reported to cause severe skin burns, scarring, systemic toxicity and
death.
Occupational exposure to cresols usually results from dermal
contact. Acute exposures can result in severe burns, anuria, coma and
death. Very few data are available regarding reproductive effects and
there are no data on carcinogenicity in humans.
1.6 Effects on other organisms
Observations on microorganisms, invertebrates and fish have shown
that cresols may represent a risk to non-mammalian organisms at point
sources with high cresol concentration but not in the general
environment.
1.7 Conclusion and recommendations
At concentrations normally found in the environment, cresols do
not pose any significant risk for the general population. However,
the potential for adverse health effects exists in the case of people
with renal insufficiency or specific enzymic deficiency and under
conditions of high exposure.
Cresols may represent a risk to microorganisms, invertebrates and
fish at point sources with high cresol concentrations but not in the
general environment.
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess the carcinogenic hazard of cresols. Based on the results of
subchronic studies, an NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, an acceptable daily intake (ADI) of
0.17 mg/kg body weight per day can be established for cresols.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para positions relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols
(Deichmann & Keplinger, 1981). Mixtures of m- and p-cresol and of
o-, m- and p-cresol are occasionally called dicresol and
tricresol, respectively (Fiege & Bayer, 1987). Pure and commercial
cresol or cresylic acid is different from the commercial products
called "cresylic acids". The substance "cresylic acids" is a mixture
of phenolic compounds with a typical composition as follows: 0-1% m-
and p-cresol; 0-3% 2,4- and 2,6-xylenols; 10-20% 2,3- and
3,5-xylenols; 20-30% 3,4-xylenol; and 50-60% C9-phenols (Sax & Lewis,
1987). The chemical identity of cresols is shown in Table 1.
Commercial cresols are manufactured in a wide range of
grades and purities to suit the user's requirements. Typically,
technical grade cresol available in the USA contains about 20%
o-cresol, 40% m-cresol, 30% p-cresol, and 10% phenol and
xylenols (Deichmann & Keplinger, 1981). The individual isomers are
available at purity levels as low as 85% and as high as > 99% from
chemical suppliers in the USA.
2.2 Physical and chemical properties
The physical properties of the three individual isomers and the
mixture are given in Table 2.
Chemically, cresols behave similarly to phenol. These compounds
undergo electrophilic substitution reactions at the vacant ortho or
para position relative to the hydroxyl group. Chlorination,
bromination, sulfonation and nitration are examples of such
substitution reactions. Cresols can undergo condensation reactions
with aldehydes, ketones and dienes (Fiege & Bayer, 1987).
2.3 Conversion factors
Air at 25°C: 1 ppm = 4.42 mg/m3
1 mg/m3 = 0.23 ppm
Table 1. Chemical identity of cresols
o-Cresol p-Cresol m-Cresol Mixture
Chemical structure:
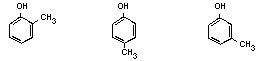 Empirical formula: C7H8O C7H8O C7H8O C7H8O
Relative molecular mass: 108.14 108.14 108.14 108.14
Common synonyms: 2-methyl phenol 4-methyl phenol 3-methyl phenol methyl phenol
2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
o-cresylic acid p-cresylic acid m-cresylic acid cresylic acid
acide cresylique (French)
cresoli (Italian)
kresolen (Dutch)
krezol (Polish)
kresol (German)
IUPAC name: 2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
CAS registry number: 95-48-7 106-44-5 108-39-4
RTECS: G06300000 G06475000 G06125000 G05950000
EEC number: 604-004-00-9 604-004-00-9 604-004-00-9 604-004-00-9
Table 2. Physical and chemical properties of cresolsa
o-Cresol m-Cresol p-Cresol Mixturef
Physical state and colour: white crystalline solid colourless to yellowish crystalline solid or colourless to yellowish
or yellowish liquid liquid yellowish liquid liquid
Odour: phenol-like phenol-like phenol-like phenol-like
Air odour thresholdb: 1.4 mg/m3 0.007 mg/m3 0.004 mg/m3 ND
Melting point (°C): 30.94 12.22 34.74 11-35
Boiling point at 1 atm (°C): 191.0 202.32 201.94 191-203
Flash point, closed cup (°C): 81 86 86 82
Ignition point (°C): 598 558 558 ND
Vapour pressure at 25°C (mmHg): 0.31 0.143 0.13 0.975 (at 38-53°C)g
Relative density at 25°C (g/cm3): 1.135 1.030 1.154 1.03-1.038
Refractive index at 25°C: 1.544 1.540 1.539 ND
Vapour density (air = 1 at 20°C): 3.7 3.72 3.72 NDe
Solubility in water at 25°C
(g/litre)c: 25.95 22.70 21.52 ND
Solubility in other solvents: soluble in ethanol, soluble in ethanol, soluble in ethanol, soluble in ethanol,
ethyl ether, acetone, ethyl ether, acetone, ethyl ether, acetone, glycol, aqueous
benzene, aqueous benzene, aqueous benzene, aqueous alkali hydroxides
alkali hydroxides alkali hydroxides alkali hydroxides
Table 2 (contd).
o-Cresol m-Cresol p-Cresol Mixturef
Sorption coefficient,
Koc (all isomers)d 22-3420
Log n-octanol/water partition
coefficiente (log Ko/w): 1.95 1.96 1.94 ND
pKa (25°C): 10.287 10.09 10.26 ND
Bioconcentration factorsh 14.1 19.9 ND ND
Odour threshold in water
(mg/litre)i,j 1.4 0.8 0.2 ND
Taste threshold concentration
in water (mg/litre)j 0.003 0.002 0.002 ND
Saturation concentration
in air (g/m3)j at 20°C 1.2 0.24 0.24 ND
at 30°C 2.8 0.68 0.74 ND
a Adapted from: Weast et al. (1988); Sax & Lewis (1987); Windholz et al. (1983); Riddick et al. (1986), unless otherwise specified
b Amoore & Hautala (1983)
c Yalkowsky et al. (1987)
d Boyd (1982); Southworth & Keller (1986); Koch & Nagel (1988)
e Hansch & Leo (1985)
f No data
g Parrish (1983)
h Freitag et al., (1982)
i Dietz & Traud (1978)
j Verschuesen (1983)
2.4 Analytical methods
2.4.1 Sampling
As is the case with any other analyte, sample loss and
contamination should be avoided during the collection, storage and
analysis of samples for cresol determination. Glass bottles, vials or
tubes have been used for the collection of environmental samples (US
EPA, 1982). Polyethylene containers are suitable for the collection
of biological samples (US NIOSH, 1989). Environmental aqueous samples
can be stored for a limited time (28 days) by adding sulfuric acid to
a pH < 2 (US EPA, 1982). Thymol has been used as a preservative for
biological samples (US NIOSH, 1989). Environmental and biological
samples that are to be shipped from the collection site to the
laboratory are cooled in ice.
Cresols in air can be sampled by passing air through an
absorption cell containing 0.1 N sodium hydroxide solution (Manita,
1966). More recent methods use solid adsorbents such as XAD-2 or
silica gel for trapping cresols from air (Neiminen & Heikkila, 1986;
US NIOSH, 1989). In a novel system, a miniaturized enrichment unit
has been used to concentrate cresols and other water-soluble analytes
in air by a water mist (Vecera & Janak, 1987). Aqueous samples can be
collected either by manual grab methods or by automated samplers.
Composite samples can be obtained by combining random samples
collected manually or by automated samplers (US EPA, 1982). Several
mechanical devices are available for collecting random or composite
semi-solid and solid samples either by grab or automated methods (US
EPA, 1982, 1986).
2.4.2 Analytical methods
Some of the methods used in measuring cresols in various
environmental and biological media are given in Table 3 along with
their corresponding references. The problem with the determination of
cresols by gas chromatography arises as a result of non-reproducible
elution from the gas chromatography column due to the polar and
volatile nature of cresols. Special columns or derivatization of the
cresols may alleviate the problem. Cresols are present in biological
samples as conjugates, and a hydrolysis method is used to release free
cresols. There is no consensus on the reliability of total hydrolysis
of the cresol conjugates (Balikova & Kohlicek, 1989).
Chudyk et al. (1985) tested a remote fluorescence technique using
ultraviolet laser fibre optics to analyse groundwater contaminants,
including o-cresol, in artificially prepared solutions. No data were
given on the detection limits or on the use of this technique in the
field. However, the authors speculated that the sensitivity is at or
below parts per billion levels at an instrument/analyte distance of
25 m.
Hoshika & Muto (1978) described a simple and rapid
gas-liquid-solid chromatographic (GLSC) method for the determination
of trace concentrations of 11 phenols including all isomers of cresol
in air. This method has been adopted and recommended by many other
investigators for measuring cresols in air samples. To overcome
interference by certain acidic compounds such as lower fatty acids and
mercaptans, the method uses two precolumns, a Tenax-GC and a Tenax-GC
plus alkaline. The gas chromatograph used was equipped with a flame
ionization detector (FID), a digital integrator and a glass analytical
column. With the Tenax-GC plus alkaline precolumn the phenol peaks
disappeared completely in the chromatograms, enabling phenols to be
identified by comparison with the chromatograms from the ordinary
Tanex-GC precolumn. The detection limit for cresols by this method
was reported to be at the ppb level.
Table 3. Sampling and analytical methods for determining cresols in environmental and biological samples
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Air
Air pump air through adsorbent tube; HPLC/UV o, m, p 0.3 ppt 90-110% Kuwata & Tanaka
desorb with methanol (1988)
Air aerodispersive enrichment into HPLC/ED o no data no data Vecera & Janak
water (1987)
Air pump air through silica gel tube; GC-FID o, m, p no data 98% at US NIOSH (1989)
desorb with acetone 22 mg/m3
Air pump air through mixed cellulose HPLC-UV o, m, p 0.5 ppb 52.4% Risner (1993)
ester membrane connected to silica
Sep-Pak, desorp with 1% acetic
acid in acetonitrile
Auto exhaust vapour collected in fritted bubbler HPLC-UV o, m, p 0.1-0.5 no data Kuwata et al.
and tobacco with aqueous NaOH buffered to pH 11.5; ng/sample (1981)
smoke add p-nitrobenzene-diazonium
tetra-fluoroborate; extract with CCl4
Air and water
Air and water mix NaOH solution from bubbler in case spectrophotometry o, m, p 0.005-0.03 no data Druyan (1974)
of air and distillate of water samples (TLC) µg/sample
in 1 N NaOH solution with
p-nitrophenyl-diazonium at pH 7-9;
extract with ether; spot on TLC plate
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Water adjust pH to 11; extract with GC/MS o, p 10 µg/litre no data US EPA (1988)
CH2Cl2; concentrate
Water solvent extraction, liquid GC/MS not no data no data Hites (1979)
chromatography prefractionation specified
Water adjust pH to 8-9; extract with spectrophotometry o, m 4 µg/litre 99-100.1% Hassan et al.
chloroform-ether; back extract (VIS) at 5-120 (1987)
in 0.1 N aqueous NaOH; add NaNO2 µg/litre
and H2SO4; remove excess NO;
add resorcinol
Water direct flow and spectrophotometry o, m 10-30 µg/litre 90-115% Khalaf et al.
stopped-flow injection, then (VIS) (1993)
derivatization with p-aminophenol
Rainwater direct injection onto ion exchange HPLC/CD o, m, p no data no data Hoffman &
column Tanner (1986)
Rainwater acidify; extract with CH2Cl2; GC/MS o, m, p no data > 50% Kawamura &
concentrate, methylate Kaplan (1986)
Soil
Soil, extract sample with CH2Cl2 using GC/MS o, p 330 ppb no data US EPA (1988)
sediment ultrasonic probe
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Sediment extract rapidly stirred sediment GC/MS not no data no data Goodley & Gordon
slurry with CH2Cl2 or ether, specified (1976)
concentrate
Biological samples
Expired draw air through XAD-2 adsorbent HPLC/ED o, m, p 8 µg/m3 no data Neiminen &
air tube; acetonitrile desorbtion Heikkila (1986)
Expired collect breath in Teflon bag; GC/MS not no data no data Krotoszynski &
air concentrate on Tenax GC absorbent; specified O'Neill (1982)
thermal desorption
Beef steam distil; extract distillate HRGC/MS o, m, p 0.2 mg/kg 83-98% at Matsumoto et al.
with ether 20-100 µg (1989)
per sample
Urine hydrolyse with sulfuric acid; GC/FID o, m, p no data 78-97% Needham et al.
extract with ethyl acetate (1984)
Urine hydrolyse with HCl and extract with HPLC/UV o, m, p 1 mg/litre 97-102% Yoshikawa et al.
isopropyl ether; remove solvent; (1986)
dissolve residue in water; add
ß-cyclodextrin
Urine acidify; steam distil; extract with GC/MS o no data no data Angerer & Wulf
methylene chloride (1985)
Urine hydrolyse with sulfuric acid; extract HPLC/UV o no data no data DeRosa et al.
with CH2Cl2; concentrate (1987)
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Urine hydrolyse with HCl or HClO4; extract GC-FID p 0.5 mg/litre 95% at US NIOSH (1989)
with ether 50 µg/ml
Urine and hydrolyse with H3PO4; extract with GC-FID o, m, p 1 mg/litre 69.4-73.3% Balikova &
serum n-hexane, acetylate extract at 50 Kohlicek (1989)
mg/litre
Faeces and homogenize faeces and hydrolyse HPLC-fluorescence p < 1 µg/kg for 99.4-101.9% Murray & Adams
urine urine buffered to pH 5.5, steam detector faeces; (1988)
distil < 1 µg/litre
for urine
a 0.01 nmol = 1.08 ng
b CD = conductivity detector; ED = electrochemical detector; FID = flame ionization detector; GC = gas chromatography;
HPLC = high-performance liquid chromatography; HRGC = high-resolution gas chromatography; m = meta-cresol; MS = mass spectrometry;
o = ortho-cresol; p = para-cresol; UV = ultraviolet detector
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Cresols and cresol derivatives occur naturally in various plants.
They are present in oils from jasmine, cassia, Easter lily, ylang
ylang, and Yucca gloriosa flowers and in peppermint, eucalyptus and
camphor. Oils from conifers, oaks and sandalwood trees also contain
cresol (Fiege & Bayer, 1987). Mammalian urine and faeces naturally
contain p-cresol (section 6.5). Poultry manure reportedly contains
p-cresol at an average concentration of 11.7 mg/kg (Yasuhara, 1987).
Cresols are frequently produced as metabolic intermediates in the
degradation of bound phenols by soil microorganisms. They are also
products of combustion and can be released to the atmosphere from
natural fires associated with lightning, spontaneous combustion and
volcanic activity (McKnight et al., 1982).
3.2 Anthropogenic sources
Cresols are contained in crude oil and coal tar. Therefore, the
dominant anthropogenic sources of cresols are accidental and process
discharge during the manufacture, use, transport and storage of
cresols or associated products of the coal tar and petroleum
industries. Cresols are also produced during coal gasification
(Giabbai et al., 1985; Neufeld et al., 1985), coal liquefaction
(Fedorak & Hrudey, 1986) and shale oil production (Snider & Manning,
1982; Dobson et al., 1985). Low levels of cresols are present in the
exhaust of vehicles powered with petroleum-based fuels (Hampton et
al., 1982; Johnson et al., 1989), stack emissions from municipal waste
incinerators (Junk & Ford, 1980; James et al., 1984), and emissions
from the incineration of vegetable materials (Liberti et al., 1983).
Cresols are also found in fly ash from coal and wood combustion (Junk
& Ford, 1980; Hawthorne et al., 1988, 1989). Cigarette smoke contains
cresols (Wynder & Hoffmann, 1967). In addition, the atmospheric
reaction of toluene with photochemically generated hydroxyl radicals
(HO*) produces cresols (Leone et al., 1985).
3.2.1 Production levels and processes
The oldest cresol production method used in the USA is fractional
distillation of coal tar. Most cresols in the USA are obtained via
catalytic and thermal cracking of naphtha fractions during petroleum
distillation. Since 1965, quantities of coal tar and petroleum
isolates have been insufficient to meet the rising demand for cresols
in the USA. Consequently, several processes for the manufacture of
the various isomers have been developed. One method of producing
o-cresol is by the methylation of phenol in the presence of
catalysts. Another method uses toluene sulfonation followed by
alkaline hydrolysis to produce p-cresol. Until 1972, cresols were
also produced by the cymene-cresol process, where cymene
( p-isopropyltoluene) is oxidized to cymene hydroperoxide, which
decomposes to cresols and acetone. This method is capable of
producing p- or m-cresol from the corresponding cymene isomer.
Alkaline chlorotoluene hydrolysis is used to produce a cresol mixture
with a high m-cresol content (Fiege & Bayer, 1987). The total
production of cresols in the USA, excluding production from coke oven
and gas-retort ovens, was 34 400 tonnes in 1989 and 38 300 tonnes in
1990 (USITC, 1990, 1991).
According to the Toxic Release Inventory (TRI) database,
maintained by the US EPA, manufacturing and processing industries in
the USA in 1987 released or transferred 52 tonnes of cresols to air,
water and land, 172.5 tonnes to wastewater treatment plants, and 20.45
tonnes to off-site locations for disposal (US EPA, 1989). The TRI
data may have under-estimated the actual release since only certain
types of facilities were required to report.
3.2.2 Uses
A considerable amount of o-cresol is consumed directly as
either a solvent or disinfectant. o-Cresol is also used as a
chemical intermediate for a variety of products, including deodorizing
and odour-enhancing compounds, pharmaceuticals, fragrances,
antioxidants, dye and dye intermediates, pesticides and resins.
Recently, an increasing proportion of o-cresol has been devoted to
the formulation of epoxy- o-cresol novolak resins (sealing materials
for integrated circuits silicon chips). o-Cresol is also used as an
additive to phenol-formaldehyde resins (Windholz et al., 1983; Fiege &
Bayer, 1987; Sax & Lewis, 1987).
p-Cresol is mainly used in the formulation of antioxidants such
as 2,6-di- tert-butyl- p-cresol for lubricating oil and motor fuels,
rubber, polymers, elastomers and food products. It is also used as an
intermediate in the fragrance and dye industries (Windholz et al.,
1983; Fiege & Bayer, 1987; Sax & Lewis, 1987).
m-Cresol, either pure or mixed with p-cresol, is important in
the production of contact herbicides and insecticides. Furthermore,
many flavour and fragrance compounds and several important
antioxidants are produced from m-cresol. It is also used in the
manufacture of explosives (Fiege & Bayer, 1987).
Mixtures of m- and p-cresol are used as disinfectants and
preservatives. Crude cresols are used as wood preservatives.
Tricresyl phosphate and diphenyl cresyl phosphate produced from m-
and p-cresol mixtures are used as flame-retardant plasticizers for
polyvinyl chloride and other plastics, fire-resistant hydraulic
fluids, additives for lubricants and air filter oils. Cresol mixtures
condensed with formaldehyde are important for modifying phenolic
resins. Cresols are also used in paints and textiles. Mixtures of
cresols are used as solvents for synthetic resin coatings such as wire
enamels, metal degreasers, cutting oils and agents to remove carbon
deposits from combustion engines. They are also used in ore
flotation, fibre treatment and photography (Deichmann & Keplinger,
1981; Windholz, 1983; Fiege & Bayer, 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The levels of cresols in the atmosphere will be regulated by the
physical properties of the compounds, their chemical reactivity and by
prevailing weather conditions (wind speed, precipitation, temperature
inversions, etc.). The vapour pressures of cresols range from 0.13 to
0.31 mmHg (Table 2); compounds with values greater than 0.0001 mmHg
should exist predominantly in the vapour phase (Eisenreich et al.,
1981) as opposed to the particulate-bound phase (Cautreels & van
Cauwenberghe, 1978). Photochemical attack (section 4.2) and rain
scavenging (Leuenberger et al., 1985; Czuczwa et al., 1987) rapidly
remove cresols from the vapour phase, counteracting the tendency of
compounds that exist in the vapour phase to be transported over long
distances.
4.1.2 Water
The processes that control the transport of cresols from water
and their distribution in water are volatility, values for the
sorption coefficient (Koc) to suspended solids and sediment, and
bioaccumulation in aquatic organisms. The bioaccumulation of cresols
in aquatic organisms is discussed in section 4.3. The volatility of a
compound can be qualitatively predicted from its Henry's Law constant
(H). The rate of volatilization from water is high for compounds with
H values ranging from 10-2 to 10-3 atm-m3/mol, and it is very
low for compounds with H values of 10-7 atm-m3/mol or less (Lyman et
al., 1990). Therefore, transport of cresols with H values of 1.26 ×
10-6 to 7.92 × 10-7 atm-m3/mol from water to the atmosphere will
not be significant. Furthermore, the ability of these phenolic
compounds to dissociate and to form hydrogen bonds, leading to binding
with both suspended solids or sediments, will decrease the rate of
volatilization even further. Since the cresols are soluble in water
(see Table 2), the small amounts of cresols typically found in the
aquatic environment will be present mostly in the aqueous phase.
However, transport of cresols from water to bottom sediment is
possible as a result of sorption and subsequent precipitation. For
hydrophobic compounds, the importance of the sorption process can
usually be predicted from the Koc values. Details of Koc levels are
given in section 4.1.3.
4.1.3 Soil
Koc values in soil of between 22 and 3420 have been reported
(Boyd, 1982; Southworth & Keller, 1986; Koch & Nagel, 1988). The
sorption of cresols to several soils correlates well with both pH and
clay mineral content in soil (Artiola-Fortuny & Fuller, 1982), and
several investigators reported that hydrogen bonding plays an
important role in the sorption of cresols to soil (Boyd, 1982;
Southworth & Keller, 1986).
The transport of cresols from soil to the atmosphere will occur
as a result of volatilization. The volatilization of cresols from
soil will be directly proportional to H values and inversely
proportional to Koc. Since the H values for cresols are low and the
Koc in soils capable of hydrogen bonding can be as high as 3420,
volatilization will not be significant in such soils. However, some
volatilization may occur due to the relatively high vapour pressure of
cresols (Table 2) and to the diffusion gradient between the soil and
the atmosphere. Loss of cresols by volatilization has been shown to
occur from highly contaminated soils (Evangelista et al., 1990).
Another process that may transport cresols from soil to ground water
is leaching. The leaching of cresols from soil will depend on the
Koc. This is variable so that with values near 3000, cresols will
be slightly mobile, whereas cresols in soil with Koc values in the
lowest range will be highly mobile (Swann et al., 1983). The
horizontal transport of cresols from one land area to another or to
surface water as a result of run-off will also occur to a certain
extent, dependent among other factors on the soil Koc value.
4.2 Transformation
4.2.1 Abiotic transformation
Two abiotic transformation processes, namely reaction with
hydroxyl HO* and nitrate NO3* radicals, are most important for
determining the fate of cresols in air. The rate constants for the
reaction with HO* are 4.2 × 10-11, 6.4 × 10-11 and 4.7 × 10-11
cm3/molecule-sec for o-, m- and p-cresol, respectively
(Atkinson et al., 1992). It may be estimated from the range of HO*
concentrations in the lower troposphere (from below the limits of
detection at 1 × 106 radicals/cm3 to a maximum of 5 × 106
radicals/cm3) (Atkinson, 1985), that the half-lives for the cresols
during the daytime may range from 3 to 5 h. The major products of the
reactions of HO* with cresols in the presence of nitrogen oxides are
pyruvic acid, acetaldehyde, formaldehyde, peroxyacetylnitrate and
nitrocresols (Atkinson et al., 1980; Grosjean, 1984, 1985). NO3* is
formed in the atmosphere as a result of the reaction of nitrogen oxide
with ozone and is photodecomposed quickly by sunlight (Carter et al.,
1981). Therefore, the reaction of atmospheric pollutants with NO3*
can be significant only during the night. The determined rate
constants for the reaction of NO3* with vapour-phase cresols are
1.37 × 10-11, 9.74 × 10-12 and 1.07 × 10-11 cm3/molecule-sec
for o-, m- and p-cresol, respectively (Carter et al., 1981;
Atkinson et al., 1992). Assuming that the average concentration of
NO3* in a typical night-time urban atmosphere is 2.4 × 108
molecules/cm3, cresols are estimated to be removed from the
atmosphere with half-lives of 5-10 min (Atkinson, 1985).
Abiotic reactions, such as photolysis, hydrolysis and oxidation
by photolytically produced HO* and singlet oxygen, play a minor role
in determining the fate of cresols in water (Smith et al., 1978; Faust
& Hoigné, 1987). However, the photolysis of o- and p-cresol is
accelerated in the presence of fulvic and humic materials present in
water. The estimated half-life for the disappearance of p-cresol in
pure water containing humic acid (9.5 mg/litre) and exposed to April
sunlight at 37.5°N latitude was 3 days (Smith et al., 1978). In a
polluted eutrophic Swiss lake with a dissolved organic matter
concentration of 3.1 mg/litre, the estimated natural half-lives for
p- and o-cresol in the top metre as a result of exposure to June
sunlight were 4.4 and 11 days, respectively (Faust & Hoigné, 1987).
The investigators concluded that photochemically produced organic
peroxide radicals generated from dissolved organic matter controlled
the sensitized photooxidation of cresols in the Swiss lake. In
addition, laboratory experiments have shown that iron (FeOOH) and
manganese (III/IV) oxides (MnOOH and MnO2), commonly found in
surface water particulate and soil, can oxidize cresols in solution
particularly at low pH (< 4) (Stone, 1987). However, oxidation of
cresols occurs more readily in fog and rain water due to the higher
concentration of manganese and iron oxide and low pH of these waters
(Stone, 1987).
Direct attack of cresols by ozone may also occur in water and
follows first-order reaction kinetics: 3 moles of ozone are required
to cause ring-opening of 1 mole of cresol (Zheng et al., 1993a,b). The
overall rate constant for the reaction increases with increasing pH
and temperature. Ozonation may be a possible remediation treatment for
cresol-contaminated waters.
Photochemical reactions will only occur in the upper few
millimetres of the soil surface, and it is unlikely that photochemical
attack will be an important pathway for cresol removal from soil. As
in the case of water, the abiotic hydrolysis of cresols in moist soil
may not be significant since there is no evidence that any soil
component is capable of accelerating this reaction. The oxidation of
cresols by iron(III) and manganese (III/IV) is likely in soils that
have low pH; however, laboratory or field data assessing the
importance of this reaction in determining the fate of cresols in soil
are not available.
4.2.2 Biodegradation
Biotic processes, namely biodegradation, may be more important
than other processes in determining the fate of cresols in water
(Smith et al., 1978). Cresols degraded rapidly in aerobic
biodegradation screening and sewage treatment plant simulation studies
(McKinney et al., 1956; Ludzack & Ettinger, 1960; Malaney, 1960;
Chambers et al., 1963; Tabak et al., 1964; Alexander & Lustigman,
1966; Malaney & McKinney, 1966; Young et al., 1968; Pauli & Franke,
1971; Baird et al., 1974; Pitter, 1976; Singer et al., 1979; Lund &
Rodriguez, 1984; Babeu & Vaishnav, 1987; Brown & Grady, 1990; Klecka
et al., 1990). According to one screening study, the rate of aerobic
biodegradation of the three isomeric cresols increased in the
following order: p- > m- > o-. While no lag time for
biodegradation was observed for m- and p-cresol, o-cresol showed
a lag time of 6 days (Liu & Pacepavicius, 1990). Aerobic
biodegradation in salt water (estuarine and sea water) is slower than
in fresh water, but the decrease in the rate is not enough to preclude
biodegradation as an important removal pathway in salt water (Palumbo
et al., 1988). Mixed and pure culture studies indicate that aerobic
biodegradation of cresols proceeds by initial formation of
hydroxylation products followed by ring-opening reactions (Bayly &
Wigmore, 1973; Masunaga et al., 1983, 1986).
Biodegradation reaction rates are widely variable and depend on a
number of interrelated factors or conditions of the source waters.
Results of several investigations have shown that factors such as
substrate and nutrient concentration, spatial and temporal sampling,
bacterial growth, biofilm formation, pH and temperature all influence
reaction rates. In general, higher nutrient concentrations and
temperatures (summer versus winter) increase the biodegradation of
cresols. However, degradation will decrease with increased humic acid
content (Visser et al., 1977; Smith et al., 1978; Paris et al., 1983,
Spain & van Veld 1983; Rogers et al., 1984; Lewis et al. 1984,1986;
Shimp & Pfaender, 1985a,b; Kollig et al., 1987; Gantzer et al., 1988;
Hwang et al. 1989).
The anaerobic biodegradation potential of cresols in aquatic
media has been observed in the presence of an electron acceptor, as
occurs in nitrate reduction, methanogenesis and sulfate reduction
conditions (Shelton & Tiedje, 1981; Horowitz et al., 1982; Boyd et
al., 1983; Fedorak & Hrudey, 1984; Bak & Widdel, 1986; Roberts et al.,
1987; Battersby & Wilson, 1988, 1989; Wang et al., 1988, 1989).
Cresols biodegrade more slowly under anaerobic conditions than under
aerobic conditions. While several investigators observed a lag period
before the onset of anaerobic biodegradation (Suflita et al., 1988;
Battersby & Wilson, 1989; Liu & Pacepavicius, 1990), Young & Rivera
(1985) observed no significant increase in the rate of p-cresol
metabolism as a result of acclimation. The anaerobic biodegradation
rate for cresols was p- > m- > o- (Suflita et al., 1988; Wang
et al., 1988; Battersby & Wilson, 1989). Other investigators have
reported that o-cresol is more biodegradable under anaerobic
conditions than p-cresol. The m-cresol isomer was found to be
the least biodegradable (Liu & Pacepavicius, 1990). The anaerobic
biodegradation of o- and p-cresol appears to proceed metabolically
by oxidation of the methyl group to produce first the corresponding
hydroxybenzaldehyde and then hydroxy-benzoic acid. The hydroxybenzoic
acid is then decarboxylated or dehydroxylated to produce phenol or
benzaldehyde, respectively (Smolenski & Suflita, 1987; Kühn et al.,
1988; Suflita et al., 1988, 1989). The metabolic pathway for
anaerobic biodegradation of m-cresol may be different from the
pathway for o- and p-cresols (Suflita et al., 1989).
Pseudomonads and other bacteria contain a flavocytochrome enzyme,
p-cresol methylhydroxylase (PCMH), which is capable of oxidizing
p-cresol without the participation of exogenous oxygen (Hopper,
1976, 1978; Hopper & Taylor, 1977; Keat & Hopper, 1978). This enzyme
catalyses the dehydrogenation and hydration of p-cresol and its
homologues to the corresponding alcohols and their further
dehydrogenation to the corresponding aldehydes or ketones. Thus,
p-cresol is oxidized under this condition to p-hydroxybenzyl
alcohol and then to p-hydroxybenzaldehyde. Isolation and then
resolution of the flavocytochrome PCMH into subunits and
reconstitution of the enzyme were studied by Keat & Hopper (1978),
McIntire et al. (1981, 1984, 1985, 1986), McIntire & Singer (1982),
Shamala et al. (1985, 1986) and Koerber et al. (1985).
The biodegradation of cresols in soil under aerobic conditions is
rapid. However, complete metabolism (to CO2 and H2O) of the
intermediate metabolites is slower (Medvedev & Davidov, 1981a,b;
Dobbins & Pfaender, 1988; Namkoong et al., 1988). Biodegradation is
likely to control the fate of cresols in soils. In surface soils from
an uncultivated grassland site, the estimated half-life for the
pseudo-first-order disappearance of the parent compound was 1.6 days
for o-cresol and 0.6 days for m-cresol. It could not be
calculated for p-cresols as the concentration had fallen below the
detection limits at the first sampling, which was 24 h after
initiation of the experiment (Namkoong et al., 1988). The half-lives
for complete metabolism in different soils ranged from 39 days to
about 1 year (Dobbins & Pfaender, 1988; Swindoll et al., 1988).
4.3 Bioaccumulation and biomagnification
The measured bioconcentration factors for o-cresol and
m-cresol in aquatic organisms were 14.1 and 19.9, respectively
(Freitag et al., 1982; Sabljic, 1987). There is no evidence in the
literature to indicate that biotransfer of cresols via the food chain
causes biomagnification of these compounds.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air monitoring data for cresols are sparse. These
compounds are short-lived in the air (see section 4.2.1) unless large
amounts are released over a short period of time. According to the
National Ambient Volatile Organic Compounds (VOCs) Data Base, a
compilation of published and unpublished air monitoring data in the
USA from 1970 to 1987, the median air concentration of o-cresol at
source-dominated sites was 1.59 µg/m3 (0.359 ppb) (range from below
detection limit to 10.58 µg/m3, 2.394 ppb) for 32 samples (Shah &
Heyerdahl, 1989). According to the same data base, o-cresol was not
detected in air samples from one urban, one rural and one remote area,
and m-cresol was also not detected in air samples from one urban,
one suburban, and one remote area in the USA. This data base does not
contain any monitoring data for p-cresol. The concentration of
o-cresol in one sample of the ambient air near a phenolic resin
factory in Japan was 179 µg/m3 (40 ppb) (Hoshika & Muto, 1978). In
air samples from rooms with a fireplace, cresol concentrations around
5 mg/m3 have been detected (Risner, 1993).
5.1.2 Water
In general, cresols will degrade in surface waters very rapidly.
The STORET data base, a computerized data base maintained by US EPA,
contains water quality data. According to STORET (1993), the mean,
minimum and maximum concentrations of ocresol in surface water were
10.89, below the detection limit and 68 µg/litre, respectively, out of
315 samples reported; for p- or m-cresol they were 12.5, 3.4 and
25 µg/litre out of 52 samples; and for p-cresol they were 12.45,
below the detection limit and 77 µg/litre out of 285 samples. In
addition, the three isomers of cresol were qualitatively detected in
Spirit Lake, a freshwater lake in the state of Washington, USA.
o-Cresol was also detected in two other freshwater bodies in the
same state. The presence of cresols was attributed to the Mount St.
Helens eruption (McKnight et al., 1982). Whether or not the cresols
originated from woodfires or the actual eruption was not clarified in
this study. p-Cresol was detected at a concentration of
200 µg/litre in water samples from the lower Tennessee River near
Calvert City, Kentucky, USA (Goodley & Gordon, 1976). m-Cresol was
qualitatively detected in St. Joseph River of the Lake Michigan Basin
(Great Lakes Water Quality Board, 1983). Cresols (isomers
unseparated) were not detected in Delaware River water samples taken
between Marcus Hook, Pennsylvania, and Trenton, New Jersey, USA,
during summer months, but were detected at 2 µg/litre in winter
(Sheldon & Hites, 1978). Concentrations of p-cresol as high as
204 µg/litre have been detected in a river in Japan polluted by
effluents from a leather factory (Yasuhara et al., 1981).
Although o-cresol has been qualitatively detected in
drinking-water in the USA (Clark et al., 1986), quantitative data
regarding cresol levels in drinking-water are not available.
Cresols have been qualitatively detected in effluent from sewage
treatment plants in the USA (Ellis et al., 1982). Concentrations of
70-150 µg/litre (isomer unidentified) have been measured in the
wastewater from a chemical manufacturing plant (Jungclaus et al.,
1978), and concentrations as high as 2100 µg/litre for o-cresol and
1200 µg/litre for mixed m- and p-cresol have been measured in
wastewater from a shale oil plant (Hawthorne & Sievers, 1984).
Cresols were detected at 20 µg/litre in the treated secondary effluent
from Philadelphia Northeast Sewage Treatment Plant, but were not
detected in Delaware River water near the discharge point of the
effluents or further downstream (Hites, 1979; Sheldon & Hites, 1979).
Furthermore, cresols have been detected in treated coke oven aqueous
condensates, wastewater from petroleum refineries and wood-preserving
plants, and aqueous effluents from synfuel processing (US EPA, 1982).
Cresols may persist in groundwater due to a lack of
microorganisms. Very little information regarding the concentration
of individual isomers has been reported in the literature.
Cresol concentrations measured in groundwater from hazardous
waste and landfill sites are shown in Table 4. Although the
concentration of p-cresol was below the detection limit
(30 µg/litre), o- and m-cresol concentrations of around
1400 µg/litre have been detected in creosote-contaminated groundwater
in Denmark (Flyvbjerg et al., 1993). According to STORET (1993), the
mean, minimum and maximum levels in groundwater from undefined sources
for o-cresol were 234.3, 0.9 and 100 000 µg per litre out of 1848
samples collected; for m-cresol were 1421.3, below the detection
limit and 100 000 µg/litre out of 712 samples; and for p-cresol were
15.79, 0.09 and 4800 µg/litre out of 1147 samples, respectively.
Rainwater from Portland, Oregon, collected during seven falls of
rain in 1984, contained o-cresol concentrations of 0.24-2.8 µg per
litre (mean of 1.02 µg/litre) and combined p- and m-cresol
concentrations of 0.38-2.0 µg/litre (mean of >1.1 µg/litre)
(Leuenberger et al., 1985). The concentration of o-cresol in
rainwater at a rural site in Switzerland (Greppen) ranged from
undetectable to 1.3 µg/litre. The combined concentration range of
m- and p-cresols in the same rainwater was 0.65-9.3 µg/litre
(Czuczwa et al., 1987).
Table 4. Cresol concentrations in the ground water of hazardous waste sites and landfills in the USA
No. of samples/ Concentration
Type/location Sampling date no. detecteda Isomer (mg/litre) Reference
Hazardous waste, no data 1/1 o 2.3 Weber & Matsumoto (1987)
Buffalo, New York 1/1 p 15.0
Former pine-tar manufacturing, no data 11/10 o 0.002-5.2 McCreary et al. (1983)
Gainesville, Florida 11/10 m and p 0.0004-11.1
Former wood preserving, 1984 19/6 o 0.04-7.1 Goerlitz et al. (1985)
Pensacola, Florida 19/3 p 0.02-6.2
19/4 m 0.05-13.7
Former coal gasification, no data 3/3 o 0.063-6.6 Stuermer et al. (1982)
Hoe Creek, Wyoming 3/3 m and p 0.096-16.0
Municipal landfill, 1982-1983 1/1 p 1.5 Sawhney & Kozloski (1984)
Southington, Connecticut 1982-1983 1/1 m 0.6
Underground solvent 1983 10/1 unseparated 0.04 Oliveira & Sitar (1985)
storage tanks,
Santa Clara, California
Hazardous waste, 1979-1984 4/1 unseparated 0.11 Ram et al. (1985)
Coventry, Rhode Island
a Number of samples compared with number in which cresols were detected
5.1.3 Soil
Cresols have been detected in about 1% of soil samples from 1300
Superfund (hazardous waste sites listed by US EPA in the National
Priority List) sites. The geometric mean concentrations of o- and
p-cresols in these samples were 409 and 677 µg/kg, respectively
(HAZDAT, 1992).
5.1.4 Food and beverages
Cresols have been detected in certain foods and beverages, such
as tomatoes, tomato ketchup, cooked asparagus, various cheeses,
butter, oil, red wine, spirits, raw and roasted coffee, black tea,
smoked foods and tobacco (Fiege & Bayer, 1987). Cresols were
identified as volatile components of fried chicken (Ho et al., 1983).
Quantitative data regarding cresols in food and beverages are limited.
Cresols have been detected in various beverages including Scotch
whisky (0.01-0.20 mg/litre), whiskies made outside of Scotland
(0.01-0.07 mg/litre), brandies including cognac and armagnac (trace to
0.02 mg/litre), and white and dark rums (trace to 0.20 mg/litre)
(Lehtonen, 1983). The total amount of cresols in the smoke from a
nonfilter American cigarette (85 mm) is about 75 µg (Wynder &
Hoffmann, 1967).
5.2 General population exposure
The general population can be exposed to cresols from air
inhalation, drinking-water and food ingestion, and dermal contact with
water or consumer products that contain cresols. Due to the lack of
adequate monitoring data regarding cresol levels in ambient air and
drinking-water, it is not possible to estimate quantitatively the
daily intake of cresols from these sources. Similarly, to estimate
the daily intake of cresol from food for a member of the general
population requires data concerning the level of these compounds in
total diet samples (various categories and quantities of food consumed
daily by a typical individual), and these data are not available.
Dermal contact to cresols may also result from use of certain consumer
products, since cresols may be used as disinfectants in some soap and
as wood preservatives. It is likely that people who live near certain
kinds of emission sources (e.g., heavy vehicular traffic, certain
incinerators, and landfill sites, such as abandoned coal tar or
creosote producer/user sites) will be exposed to higher levels of
cresols than the general population. Since both mainstream and
sidestream smoke of cigarettes contain cresols (Wynder & Hoffmann,
1967), smokers and those who inhale sidestream smoke may be exposed to
a higher level of cresols.
5.3 Occupational exposure
Occupational exposure to cresols is likely among workers
involved in the production of cresols or processes that produce
cresols (coal gasification, shale oil retorting) and those who use
cresols or products containing cresols (such as creosote). Little
information regarding occupational exposure to cresols is available.
The concentration of cresols in the workroom air of a pilot coal
gasification plant in the USA was < 0.44 mg/m3 (< 0.1 ppm)
(Dreibelbis et al., 1985). The extent of worker exposure to cresols
and other pollutants was measured in a facility in Finland that used
creosote for impregnation of wood. The highest observed mean
concentration of cresols in the air was 0.6 mg/m3 during periods in
which the cylinder used for impregnation was opened, followed by a
concentration of 0.2 mg/m3 during periods in which the cylinder was
closed (Heikkila et al., 1987).
All 14 countries listed in ILO Occupational Exposure Limits for
Airborne Toxic Substances (1991) have set an environmental
concentration of 22.1 mg/m3 (5 ppm) for time-weighted average (TWA)
exposure for all isomers of cresol.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Cresols are absorbed across the respiratory and gastrointestinal
linings and through the intact skin. Absorption of cresols through
the lungs has not been studied quantitatively. However, the
occurrence of mortality and other systemic effects in animals exposed
to cresol aerosols and vapours in air shows that absorption through
the lungs does occur (Uzhdavini et al., 1972; Pereima, 1975). The
rate and extent of gastrointestinal absorption of cresols have not
been studied specifically. However, they are suggested by data
showing that rabbits exposed orally to cresols excreted 65-84%
(depending on the isomer) of the administered dose in the urine within
24 h (Bray et al., 1950), indicating that at least that amount was
absorbed within that time period. The occurrence of coma, death and
systemic effects in humans after dermal exposure to cresols (see
section 8) indicates that these compounds can be absorbed through the
skin. In the case of an infant who had coal tar fluid (90% cresols in
water) spilled on his head, unconsciousness occurred within 5 min and
death within 4 h, showing that absorption was rapid (Green, 1975). An
in vitro study of the permeability of human skin to cresols showed
that these substances have permeability coefficients greater than that
of phenol, which is known to be readily absorbed across the human skin
(Roberts et al., 1977). Permeability coefficients (Kp) were estimated
from the steady-state slopes of the relation between the cumulative
amount of cresol isomer per unit area of membrane with time. The
following Kp values were determined: m-cresol = 2.54 × 10-4
cm/minute; o-cresol = 2.6 × 10-4 cm/minute; and p-cresol = 2.92 ×
10-4 cm/minute (Roberts et al., 1977).
In a similar study, Hinz et al. (1991) showed rapid percutaneous
transport of p-cresol across mouse skin in vitro. Approximately
70% of the dose was transported within 6 h.
6.2 Distribution
Very few data are available regarding the distribution of cresols
into various tissues. Oral exposure studies in dogs indicate that
cresols in the body concentrate in the blood, liver and brain
initially, but soon become more widespread, appearing in the lungs,
kidneys and other organs (Gadaskina & Filov, 1971). Cresols were
detected in the blood (120 mg/litre), liver, brain and urine of a
human infant who died 4 h after 20 ml of a cresol derivative was
spilled on his head (Green, 1975).
6.3 Metabolic transformation
The primary metabolic pathway for cresols is conjugation with
glucuronic acid and inorganic sulfate. At physiological pH, the
conjugated metabolites are ionized, thus reducing renal reabsorption
and aiding urinary excretion. After oral administration of cresols to
rabbits, 60-72% of the dose was recovered as ether glucuronide, and an
additional 10-15% was recovered as ethereal sulfate in the urine (Bray
et al., 1950). Similarly, in an earlier study in rabbits, 14.5-23.5%
of orally administered cresols was found to be conjugated with sulfate
in the urine (Williams, 1938). By analogy with other phenols, it may
be expected that the relative amounts of glucuronide and sulfate
conjugates will differ between species and will also vary with dose.
Minor metabolic pathways for cresols include hydroxylation of the
benzene ring (primarily for o- and m-cresols) and side-chain
oxidation (only for p-cresol). In orally dosed rabbits, 3% of the
administered dose was recovered in the urine as conjugated
2,5-dihydroxytoluene for both o- and m-cresols (Bray et al.,
1950). For p-cresol, only a trace amount of 3,4-dihydroxytoluene
was found, but 10% of the dose was recovered as p-hydroxybenzoic
acid. After cresols were administered to rabbits, only 1-2% of the
dose was found as unconjugated free cresol in the urine (Bray et al.,
1950). Thompson et al. (1994) studied the metabolism of
[14C]- p-cresol in rat liver slices and a microsomal fraction.
They found that [14C]- p-cresol is metabolized to a reactive
intermediate which co-valently binds to proteins in the liver slices
and that the binding is inhibited by n-acetylcysteine. In
microsomal incubations and a NADPH-generating system, covalent binding
of [14C]- p-cresol metabolites was also observed. This binding was
inhibited by glutathione (GSH) resulting in the formation of a
glutathione conjugate. In the absence of GSH, p-hydroxybenzyl
alcohol was the major microsomal metabolite formed from p-cresol.
Yashiki et al. (1989) reported the recovery of conjugated cresols in
the biological fluids of a 46-year-old man following the ingestion of
100 ml saponated cresol soap solution (42%). Conjugated and free m-
and p-cresols were measured in both the serum and urine 2 h after
ingestion. Of the total recovered in the serum, 79% p-cresol and
75% m-cresols were in the conjugated form while over 99% of m- and
p-cresols recovered in the urine was conjugated.
6.4 Elimination and excretion
Significant amounts of cresols are excreted in the bile, but most
of the cresols excreted in this manner are reabsorbed from the
intestine following hydrolysis by gut bacteria (Deichmann & Keplinger,
1981). The main route for removing cresols from the body is renal
elimination.
6.5 Endogenous cresols
Healthy humans excrete an average of about 50 mg (range 16-74 mg)
of p-cresol in the urine daily (Bone et al., 1976; Renwick et al.,
1988). Endogenous p-cresol is produced from tyrosine, an amino acid
present in most proteins, by anaerobic bacteria in the intestine (Bone
et al., 1976). Free p-cresol formed in this way is absorbed from
the intestine and eliminated in the urine as conjugates.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation route
Acute poisoning with cresol vapour is unlikely due to the low
vapour pressure of these compounds. However, inhalation of an aerosol
and vapour mixture may cause death. Uzhdavini et al. (1972) conducted
studies into the acute toxicity of o-cresol in mice. The mean lethal
concentration of the vapour/aerosol mixture was 178 mg/m3 (duration of
exposure not specified). Clinical signs of toxicity included
irritation of mucous membranes and neuromuscular excitation that
progressed from twitching of individual muscles to clonic convulsions.
Haematuria was reported at very high concentrations. Microscopic
examination revealed oedematous changes in the lung and necrotic and
degenerative changes in the liver (fatty degeneration, centrilobular
necrosis) and kidneys (oedema, swelling of the glomeruli, degeneration
of the tubular epithelium, and perivascular haemorrhage). Mean lethal
concentrations of cresols in rats were reported to be 29 mg/m3 for
o- and p-cresols and 58 mg/m3 for m-cresol (Pereima, 1975).
7.1.2 Oral route
Oral LD50 values for cresols are shown in Table 5. A
comparison of the LD50 values for all three cresol isomers from
these studies (e.g., Deichmann & Witherup, 1944; Bio-Fax, 1969) shows
that o-cresol is the most toxic isomer, followed by p-cresol and
then m-cresol. Interspecies comparisons reveal that all three
isomers are more toxic to mice than to rats, by this route of
administration, the LD50 values being 3-4 times higher in rats than
in comparably treated mice (Uzhdavini et al., 1972; Pereima, 1975).
The data also show that for all three isomers toxicity increases with
concentration; undiluted cresols were more toxic than cresols
delivered as 10% solutions in oil. In addition, there is some
evidence that the delivery vehicle affects toxicity; the LD50 value
for m-cresol was lower in rats given a 10% solution in water than in
rats given a 10% solution in oil.
Clinical signs of toxicity that preceded death in acute oral
lethality studies of all three cresol isomers were hypoactivity and
lethargy, excess salivation, dyspnoea, haemorrhagic rhinitis
( p-cresol only), incoordination, prostration, muscle twitches and
tremors, convulsions and coma (Deichmann & Witherup, 1944; Mellon
Institute, 1949; Bio-Fax, 1969; Hornshaw et al., 1986). Necropsy of
rats that died revealed gastrointestinal inflammation and haemorrhage,
as well as hyperaemia of the lungs, liver and kidney (Mellon
Institute, 1949; Bio-Fax, 1969). Necropsy of survivors after 14 days
of observation revealed only gastro-intestinal tract inflammation in
rats treated with p-cresol and no gross lesions in rats treated with
o- or m-cresol (Bio-Fax, 1969).
Table 5. Oral LD50 values for cresols
LD50
Cresol Species Vehicle (mg/kg) Reference
o-Cresol Rat 10% in oil 1470 Uzhdavini et al. (1976)
10% in oil 1350 Deichmann & Witherup (1944)
50% in oil 360 FDRL (1975)
Undiluted 121 Bio-Fax (1969)
Mouse 10% in oil 344 Uzhdavini et al. (1976)
Rabbit 10% in oil 940 Uzhdavini et al. (1976)
m-Cresol Rat 10% in oil 2010 Pereima (1975)
10% in oil 2020 Deichmann & Witherup (1944)
10% in water 520 Mellon Institute (1949)
Undiluted 242 Bio-Fax (1969)
Mouse 10% in oil 600 Pereima (1975)
10% in oil 828 Uzhdavini et al. (1976)
p-Cresol Rat 10% in oil 1430 Pereima (1975)
10% in oil 1460 Uzhdavini et al. (1976)
10% in oil 1800 Deichmann & Witherup (1944)
Undiluted 207 Bio-Fax (1969)
Mouse 10% in oil 440 Pereima (1975)
10% in oil 344 Uzhdavini et al. (1976)
Dicresol Rat 10% in oil 1625 Uzhdavini et al. (1976)
Mouse 10% in oil 651 Uzhdavini et al. (1976)
7.1.3 Dermal route
Cresols may cause death when applied to the skin. Dermal LD50
values in rabbits were 890, 2830, 300 and 2000 mg/kg for o-, m-,
p- and mixed cresols, respectively, following 24-h dermal exposure
(Vernot et al., 1977). In rats, the dermal LD50 values were 620,
1100, 750 and 825 mg/kg for o-cresol, m-cresol, p-cresol and
dicresol (a mixture of m- and p-cresols), respectively (Uzhdavini
et al., 1974, 1976).
7.2 Short-term exposure
7.2.1 Inhalation route
Uzhdavini et al. (1972) exposed mice to a mixture of o-cresol
aerosol and vapour 2 h/day, 6 days/week for 1 month; exposure
concentrations varied from 26 to 76 mg/m3, with an average of
50 mg/m3. No mortality was recorded. Clinical signs of toxicity
during the daily exposure periods were limited to signs of
respiratory irritation at the start of the exposure, followed by a
period of hypoactivity lasting until the end of the exposure. The
tails of some animals mummified and fell off after 18-20 days. Body
weight gain was slightly reduced compared to controls. Microscopic
examination revealed signs of irritation in the respiratory tract;
these included oedema, cellular proliferation, and small haemorrhages
in the lung. Other lesions included degeneration of heart muscle,
liver, kidney and nerve cells and glial elements of the central
nervous system.
7.2.2 Oral route
Female B6C3Fl mice (8-10 weeks of age) were exposed to
o-cresol at concentrations of 0, 6.5, 32.5, 65 or 130 mg/kg per day
ad libitum in the drinking water) for 14 days (CIIT, 1983).
Immunotoxicity or altered host resistance was measured as changes in
haematological values, lymphoid organ weights, altered lymphoid cell
morphology and cell or humoral-mediated immune function. No evidence
of immunotoxicity was seen in any of the parameters tested. No
changes in immune functions were reported at any dose level.
Therefore the threshold for immune response in these studies is above
130 mg/kg per day (see Table 6).
US NTP (1992) conducted 28-day studies in which Fischer 344/N
rats and B6C3F1 mice were exposed to o-, m-, p- or
m-/ p-cresol (60:40 mixture of the m- and p-) in the feed. For
each substance, groups of five animals of each sex and each species
were fed ad libitum diets containing 0, 300, 1000, 3000,
10 000 or 30 000 mg/kg. Estimated daily doses (mg/kg body weight per
day) in males and females of each species exposed to each test
substance are shown in Table 7. None of the cresols caused mortality
in rats. All cresols reduced feed consumption during the first week
of the study and body weight gain throughout the study in rats exposed
at the highest level. However, feed consumption of all dosed groups
was comparable to that of controls after the first week. Clinical
signs of toxicity were not observed in rats treated with o- or
m-cresol, but rats exposed to 30 000 mg p-cresol/kg had hunched
posture, rough hair coat and thin appearance. Thin appearance was
also noted in rats exposed to the highest dose of m-/ p-cresol.
Organ weight changes in rats included increases in absolute and
relative liver weight and kidney weight compared to brain weight.
Increases in several other organ weights, relative to body weight were
reported, but as there was a very marked decrease in body weight at
the highest dose levels, only the increased liver and kidney weights,
relative to brain weight, were regarded as being of biological
significance. No gross or microscopic lesions were found in rats
exposed to o-cresol. m-Cresol caused minimal-t o-mild atrophy of
the uterus in females exposed to 30 000 mg/kg. p-Cresol also caused
uterine atrophy in females exposed to 30 000 mg/kg, as well as bone
marrow hypo-cellularity and nasal lesions (atrophy of olfactory
epithelium and hyperplasia and squamous metaplasia of respiratory
epithelium) in rats exposed to > 3000 mg/kg. m-/ p-Cresol caused
hyperplasia of the respiratory epithelium in the nasal cavity at >
1000 mg/kg, increased colloid within thyroid follicles at >
3000 mg/kg, mild hyperplasia and hyperkeratosis of the oesophageal
epithelium and forestomach at > 3000 and > 10 000 mg/kg,
respectively, and mild bone marrow hypocellularity at >
10 000 mg/kg. A no-observed-adverse-effect level (NOAEL) of
3000 mg/kg was established for o, m and m/p cresols and a NOAEL of
1000 mg/kg for p-cresol based on organ weight and body weight
changes at higher doses.
In the mice exposed in this study death was caused by o-,
m-and p-cresol at 30 000 mg/kg and only by m- or p-cresol at
10 000 mg/kg. The m-/ p-mixture was not lethal to mice at any
concentration. For all cresols, high-dose mice that survived exposure
lost weight during the study, and body weight gain was generally
decreased in the 10 000 mg/kg groups as well. Clinical signs of
toxicity seen at > 10 000 mg/kg in mice exposed to m-and
p-cresols and 30 000 mg/kg in mice exposed to o- and
m-/ p-cresols included hunched posture, thin appearance, rough hair
coat, lethargy, hypothermia, rapid breathing and tremors. Organ
weight changes in mice were increased in absolute and relative liver
Table 6. Short-term toxicity of cresolsa
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ NR/F (8-10 o-cresol oral 0, 6.5, 32.5, 14 days No effects noted in haematology or CIIT (1983)
B6C3Fl weeks old) (drinking) 65 or 130 immune functions
water mg/kg/day
Mice/ 5/sex f/m o-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg death, (2 males & 1 US NTP
B6C3Fl 1000, 3000, female) tremors, rough hair coat, (1992)
10 000 or ovarian atrophy; > 10 000 mg/kg
30 000 body weight decreased, uterine
mg/kg diet atrophy; > 3000 mg/kg increased
relative liver weight
Mice/ 5/sex f/m m-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg increased brain weight, US NTP
B6C3Fl 1000, 3000, ovarian, uterine and mammary gland (1992)
10 000 or atrophy; 10 000 mg/kg (1 female)
30 000 and 30 000 mg/kg (2 male, 2 female)
mg/kg diet death, decreased body weight, clinical
signs of toxicity; > 3000 mg/kg
increased kidney weight; > 300 mg/kg
increased liver weight
Mice/ 5/sex f/m p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg death all animals; US NTP
B6C3Fl 1000, 3000, 10 000 mg/kg (1 male) death, clinical (1992)
10 000 or signs of toxicity, reduced body
30 000 weight; > 3000 mg/kg increased liver
mg/kg diet weight; > 300 mg/kg nasal respiratory
lesions
Table 6 (cont'd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ 5/sex f/m m-/p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg clinical toxicity, and US NTP
B6C3Fl (60:40 ratio) 1000, 3000, respiratory metaplasia and atrophy of (1992)
10 000 or nasal epithelium; > 3000 mg/kg
30 000 hyperplasia lungs, oesophagus and
mg/kg diet forestomach, uterine and ovarium
atrophy
Mink 5/sex f/m o-cresol oral (diet) 0, 240, 432, 28 days 2520 mg/kg reduced body weight Hornshaw
178, 1400 gain, increased relative heart weight, et al.,
or 2520 decreased haemoglobin; > 1400 (1986)
mg/kg diet mg/kg decreased RBC count; > 432
mg/kg increase relative liver weight
Ferrets 5/sex f/m o-cresol oral (diet) 0, 432, 778, 28 days 4536 mg/kg decreased RBC count; > Hornshaw
1400, 2520, 1400 mg/kg increased relative liver et al.,
4536 and kidney weight (1986)
mg/kg diet
Rats/ 5/sex f/m o-cresol oral (diet) 0, 300, 28 days > 3000 mg/kg increased relative liver US NTP
Fischer-344 1000, 3000, and kidney weight; 30 000 mg/kg (1992)
10 000, decreased body weight
30 000
mg/kg diet
Rats/ 5/sex f/m m-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg decreased body US NTP
Fischer-344 1000, 3000, weight; increased relative kidney (1992)
10 000, weight; mild atrophy of uterus; >
30 000 10 000 mg/kg increased relative liver
mg/kg diet weight
Table 6 (cont'd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rats/ 5/sex f/m p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg reduced body weight, US NTP
Fischer-344 1000, 3000, rough coat, thin appearance, uterine (1992)
10 000 or atrophy, bone marrow and nasal
30 000 lesions; > 10 000 mg/kg increased
mg/kg diet relative kidney weight; > 3000 mg/kg
increased relative liver weight
Rats/ 5/sex f/m m-/p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg reduced body weight, US NTP
Fischer-344 (60:40 1000, 3000, thin appearance, > 10 000 mg/kg (1992)
mixture) 10 000 or increased kidney weight, > 1000
30 000 mg/kg histopathogenic changes, and
mg/kg diet increased relative liver weight
Mice/NR NR/NR o-cresol inhalation 50 mg/m3 2 h/day inactivity, reduced body weight gain, Uzhdavini
6 days/ CNS effects; histopathological et al.
week for changes of lungs, kidney, liver, heart (1972)
1 month and CNS
a NR = not reported
Table 7. Comparative mean compound consumption by rats and mice in US NTP (1992) 28-day studiesa
Dose o-Cresol m-Cresol p-Cresol m-/p-Cresolb
Species (mg/kg diet) M F M F M F M F
Rats 0 0 0 0 0 0 0 0 0
300 27 27 25 25 25 25 26 27
1000 87 89 85 83 87 83 90 95
3000 266 271 252 252 256 242 261 268
10 000 861 881 870 862 835 769 877 886
30 000 2610 2510 2470 2310 2180 2060 2600 2570
Mice 0 0 0 0 0 0 0 0 0
300 66 82 53 66 50 60 50 65
1000 193 280 193 210 163 207 161 200
3000 558 763 521 651 469 564 471 604
10 000 1650 1670 1730 2080 1410 1590 1490 1880
30 000 4480 5000 4710 4940 no datac no data 4530 4730
a Compound consumption given in mg/kg body weight per day; M = male, F = female
b 60% m-cresol/40% p-cresol
c No data calculated due to 100% mortality
weight. Increases in several organ weights, relative to body weight,
were observed, but as there was a very marked decrease in body weight
at the highest dose level, only the increased liver weight, relative
to brain weight, was regarded as being of biological significance.
o-Cresol caused uterine atrophy in mice exposed to > 10 000 mg/kg
and ovarian atrophy in those exposed to 30 000 mg/kg diet. m-Cresol
caused uterine and ovarian lesions and mammary gland atrophy in mice
exposed to 30 000 mg/kg diet. These changes could have been secondary
to the marked loss of body weight. p-Cresol caused nasal lesions in
mice at all concentrations tested; these lesions consisted mostly of
mild hyperplasia and squamous metaplasia of the respiratory
epithelium. Effects on the olfactory epithelium (atrophy, necrosis)
were generally observed only in mice in the 30 000 mg/kg diet group.
Other lesions in the 30 000 mg/kg diet mice, which all died early in
the study, were renal tubular and hepatic necrosis together with
lymphoid depletion and necrosis in several organs. m-/ p-Cresol
caused hyperplasia of the respiratory epithelium at > 3000 mg/kg
diet. Atrophy and metaplasia of the olfactory epithelium were
observed in mice exposed to 30 000 mg/kg diet. Other lesions observed
at this level were mild bronchiolar hyperplasia, bone marrow
hypocellularity, minimal hyperplasia of the oesophagus and
forestomach, and uterine and ovarian atrophy. A NOAEL of 1000 mg/kg
diet could be identified for m-/pcresol and o- or p-cresol,
respectively, based on organ weight and body weight and
histopathological changes at higher doses. For m-cresol, the lowest
dose tested (300 mg/kg diet) resulted in a small increase in relative
liver weight in females only, and so could be regarded as a NOAEL.
Hornshaw et al. (1986) conducted 28-day feeding studies using
mink and ferrets. Groups of five mink of each sex were fed diets
containing 0, 240, 432, 778, 1400 or 2520 mg/kg diet of o-cresol.
Doses were calculated to be 0, 35, 80, 125, 200 and 320 mg/kg body
weight per day in males and 0, 55, 120, 190, 300 and 480 mg/kg body
weight per day in females. No deaths occurred during the study, and
no clinical signs of toxicity were observed. Consumption of feed by
mink exposed to 2520 mg/kg diet was reduced during the first week of
the study, and body weight gain was depressed in this group over the
course of the study. Haematological analyses revealed decreases in red
blood cell count at > 1400 mg/kg diet and in haemoglobin at
2520 mg/kg diet. No lesions were detected by gross necropsy, but
liver:body weight ratio was increased at > 432 mg/kg diet and
heart:body weight ratio was increased at 2520 mg/kg diet. An NOAEL of
240 mg/kg diet was identified in this study for male and female mink
exposed to o-cresol.
A similar study in ferrets was also conducted using groups of
five animals of each sex exposed to dietary concentrations of 0, 432,
778, 1400, 2520 and 4536 mg/kg diet of o-cresol. Doses were
calculated to be 0, 45, 85, 140, 290 and 400 mg/kg body weight per day
in males and 0, 80, 150, 240, 530 and 720 mg/kg body weight per day in
females. No mortality was recorded and no clinical signs of toxicity
were observed. Feed consumption was slightly reduced at 4536 mg/kg
diet, but no effect on body weight gain was noted. Red blood cell
count was decreased at 4536 mg/kg diet. Increases in liver:brain
weight ratio and kidney: brain weight ratio were reported at
> 1400 mg/kg diet and 4536 mg/kg diet, respectively. No lesions
were found by gross necropsy. A NOAEL of 778 mg/kg diet was
identified for male and female ferrets based on increased relative
liver weight at doses > 1400 mg/kg diet.
7.3 Long-term exposure
7.3.1 Inhalation route
Rats were exposed to an average concentration of 9 mg/m3 of
o-cresol vapour 4-6 h/day, 5 days/week for 4 months (Uzhdavini et
al., 1972). The number of animals and strain was not reported in the
study. Effects of o-cresol exposure in rats included accelerated
loss of conditioned defensive reflex, leukocytosis, decreased
erythroid/myeloid ratio in the bone marrow, increased duration of
hexanol narcosis (indicating possible impaired liver function) and
morphological changes in respiratory tissues (inflammation and
irritation of the upper respiratory tract, oedema, and perivascular
sclerosis in the lungs) (see Table 8).
In three other studies rats were administered individual cresol
isomers or a mixture of isomers by the inhalation route for 3 to 4
months at doses ranging from 0.05 to 10 mg/m3 (Uzhdavini & Gilev,
1976; Pereima, 1975; Uzhdavini et al., 1976). In each study a
decrease in body weight gain was reported for rats exposed to cresols.
Organ weight changes and histological alterations in the liver and
kidney were also reported in all three studies. Because of the
limited reporting of data regarding the exposure methods, number of
animals and results, these studies could not be adequately evaluated.
7.3.2 Oral route
In 13-week feed studies, groups of 20 male and 20 female Fischer
344/N rats were fed diets containing 0 to 30 000 mg/kg diet of
o-cresol or a 60:40 mixture of m-/ p-cresol (US NTP, 1992).
Estimated daily doses (mg/kg body weight per day) are shown in Table
9. No treatment-related deaths were caused by either isomer (Table
8). For both isomers, food consumption during the first week was
decreased at 30 000 mg/kg diet, and body weight gain was reduced at
> 15 000 mg/kg diet. Clinical signs of toxicity were not observed
in rats fed o-cresol, but rough hair coat and thin appearance were
noted in rats fed m-/ p-cresol at 30 000 mg/kg diet. Organ weight
changes in both males and females administered cresols included
increases in absolute and relative liver weight (> 7500 mg/kg diet
for both cresols) and kidney weight (> 7500 mg/kg diet for
m-/ p-cresol). Increases for several other organ weights (relative
to body weight) were reported but as there was a marked decrease in
body weight at the highest dose levels, only the increases in liver
and kidney weight relative to brain weight were regarded as
biologically significant. Haematology analyses were not significantly
affected by treatment with cresols. Results of urinalysis did not
indicate any significant renal damage. The most noteworthy finding of
clinical chemistry analyses of plasma was a dose-related increase in
total bile acids in males and females exposed to both isomers
(significant at dose > 1880 mg/kg diet for m-/ p-cresol and > 15 000
mg/kg diet for o-cresol), indicating decreased hepatocellular
function. Transitory increases in alanine aminotransferase and/or
sorbitol dehydrogenase near the start of the study in rats exposed to
both isomers suggest that hepatocellular injury may have occurred and
regressed. Histopathological changes included a dose-related increase
in the incidence and severity of hyperplasia in the nasal respiratory
epithelium of rats exposed to m-/ p-cresol in the feed (> 1880 mg/kg
diet), increased colloid within thyroid follicles (> 3750 mg/kg
diet), uterine atrophy (> 15 000 mg/kg diet), and bone
marrow hypocellularity (> 15 000 mg/kg diet). The only lesion in
rats treated with o-cresol was bone marrow hypocellu-larity at
> 7500 mg/kg diet. Both isomers appeared to lengthen the estrus
cycle in treated female rats. An NOAEL in rats of 3750 mg/kg diet was
identified for o-cresol. However, for m-/ p-cresol the lowest
dose tested resulted in changes in clinical chemistry and hyperplasia,
and so a threshold dose for m/ p-cresol could not be determined.
Table 8. Long-term toxicity of cresols
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rat/NR NR/NR o-cresol inhalation 9 ± 0.9 4 months: 2 decreased reflexes, leukocytosis, Uzhdavini
mg/m3 months at 6 h/ bone marrow loss, histopathological et al.,
day, 5 days/week; changes and increased narcosis (1972)
2 months at 4 h/
day, 5 days/week
Rat/ 20 of each o-cresol oral (diet) 0, 1880, 13 weeks 30 000 mg/kg diet: reduced body US NTP
Fischer- sex F/M 3750, 7500, weight increase; > 15 000 mg/kg diet: (1992)
344N 15 000, increased kidney weight, bile acids;
30 000 > 7500 mg/kg diet: increased liver
mg/kg diet weight, length of estrus cycle and
altered bone marrow
Rat/ 20 of each m-/p-cresol oral (diet) 0, 1880, 13 weeks 30 000 mg/kg diet: reduced body US NTP
Fischer- sex F/M (60:40 3750, 7500, weight and clinical toxicity; (1992)
344N mixture) 15 000, > 15 000 mg/kg diet: bone marrow
30 000 changes, uterine atrophy; > 7500 mg/kg
mg/kg diet diet: lengthened estrous cycle,
liver and kidney weight increased; >
3750 mg/kg diet: thyroid changes; >
1880 mg/kg diet: increased bile salts,
histological changes in nasal
epithelium
Table 8 (contd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ 10 of each o-cresol oral (diet) 0, 1250, 13 weeks > 20 000 mg/kg diet: lengthened US NTP
B6C3F1 sex F/M 2500, 5000 estrus cycle, hyperplasia forestomach; (1992)
10 000, 10 000 mg/kg diet: clinical toxicity;
20 000 > 5000 mg/kg diet: reduced body weight;
mg/kg diet > 2500 mg/kg diet: increased relative
and absolute liver and kidney weight
Mice/ 10 of each m-/p- oral (diet) 0, 625, 13 weeks 10 000 mg/kg diet: reduced body US NTP
B6C3F1 sex F/M cresols 1250, 2500, weight, clinical toxicity; (1992)
(60/40 5000, 10 000 > 7500 mg/kg diet: hyperplasia in
mixtures) mg/kg diet respiratory tract;
> 2500 mg/kg diet: increased relative
and absolute liver and kidney weight
Rat/ 30 of each o-cresol oral (diet) 0, 50, 175 13 weeks 600 mg/kg: death, coma, tremors, MBA
Sprague- sex F/M and 600 reduced body weight; (1988a)
Dawley mg/kg body 175 mg/kg: tremors (females)
weight per
day
Rat/ 30 of each m-cresol oral (diet) 0, 50, 150 13 weeks 450 mg/kg: tremors and lethargy; MBA
Sprague- sex F/M and 450 > 150 mg/kg: reduced body weight (1988b)
Dawley mg/kg body
weight per
day
Table 8 (contd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rat/ 30 of each p-cresol oral (diet) 0, 50, 175, 13 weeks 600 mg/kg: death, coma, tremors MBA
Sprague- sex F/M 600 mg/kg and reduced body weight; altered (1988c)
Dawley body weight clinical chemistry > 175 mg/kg:
per day decreases in erythrocyte count,
haemoglobin and haemocrit,
increased kidney weight (males) > 50
mg/kg: mild nephropathy (males only)
Table 9. Cresols consumption in the US NTP (1992) 13-week feed
studiesa
Cresol Concentration Males (mg/kg Females
(mg/kg diet) body weight) (mg/kg body
weight)
Rats
o-Cresol 0 0 0
1880 126 129
3750 247 256
7500 510 513
15 000 1017 1021
30 000 2028 2024
m-/p-Cresol 0 0 0
1880 123 131
3750 241 254
7500 486 509
15 000 991 1024
30 000 2014 2050
Mice
o-Cresol 0 0 0
1250 199 237
2500 400 469
5000 790 935
10 000 1460 1663
20 000 2723 3205
m/p-Cresol 0 0 0
625 96 116
1250 194 239
2500 402 472
5000 776 923
10 000 1513 1693
a Doses given in mg/kg body weight/day; food consumption was
measured twice weekly and averaged over the 13-week period
to give a daily average dose based on body weight.
US NTP (1992) also conducted 13-week studies in groups of 10
B6C3F1 mice of each sex fed diets containing 0 to 20 000 mg/kg diet
of o-cresol or 0 to 10 000 mg/kg diet of 60:40 m-/ p-cresol.
Estimated daily doses (mg/kg body weight per day) are shown in Table
9. No deaths were recorded in treated mice. Feed consumption was
reduced during the first week of the study for mice exposed to
20 000 mg/kg diet of o-cresol or 10 000 mg/kg diet of
m-/ p-cresol. Reduced body weight occurred at 5000 mg/kg diet for
o-cresol and 10 000 mg/kg diet for m-/ p-cresol. Hunched posture
and rough hair coat were observed in mice exposed to > 10 000 mg/kg
diet of either isomer. At doses of 2500 and 5000 mg/kg diet both
relative and absolute liver weights were significantly increased
(p < 0.01) for both o- and m-/ p-cresols, respectively.
Increases in other organ weights (relative to body weight) were
reported but as there was a marked decrease in body weight at the
highest dose levels, they were not regarded as being biologically
significant. No significant effects were detected in haematology,
urinalysis or clinical chemistry analyses. Histo-pathological
examination revealed mild hyperplasia of the respiratory epithelium of
the nose in mice fed m-/ p-cresol at > 2500 mg/kg diet and
minimal forestomach epithelial hyperplasia in mice fed o-cresol at
20 000 mg/kg diet. Exposure to o-cresol resulted in a lengthened
estrus cycle in treated mice in the 20 000 mg/kg diet group. Based on
these results, an NOAEL of 1250 mg/kg diet and 625 mg/kg diet can be
identified for mice exposed to o-cresol and m-/ p-cresols,
respectively.
Several 13-week studies of gavage exposure were conducted.
Groups of 30 male and 30 female Sprague-Dawley rats were treated with
0, 50, 175 and 600 mg/kg body weight ( o- and p-cresols) or 0, 50,
150 and 450 mg/kg body weight ( m-cresol) daily for 13 weeks by
gavage in corn oil in a volume of 5 ml/kg (MBA, 1988a,b,c). Both o-
and p-cresols caused mortality at the high dose of 600 mg/kg;
m-cresol was not lethal at the high dose of 450 mg/kg. All three
isomers caused lethargy and tremors in high-dose rats. In many of the
rats exposed to o- and p-cresols these signs were followed by
convulsions and coma. Although clinical signs of toxicity were mostly
limited to the high-dose groups, two female rats exposed to 175 mg/kg
of o-cresol also developed tremors, and one became comatose. In the
case of rats that survived, clinical signs disappeared one hour after
dosing. Body weight gain was reduced in high-dose rats exposed to all
three isomers and also in rats exposed to 150 mg/kg of m-cresol. No
other treatment-related effects were observed for o- and
m-cresols. However, a number of effects were detected in rats
treated with p-cresol. Mild reductions in red blood cell count,
haemoglobin, and haematocrit were noted in females treated with
> 175 mg/kg. Serum glutamic oxaloacetic transaminase (SGOT) and serum
glutamic pyruvic transaminase (SGPT) levels were increased in 4/10
females exposed to 600 mg/kg. Other changes in clinical chemistry
parameters were increased serum cholesterol in females at 600 mg/kg
and increased serum protein (mostly globulins) in males at
> 175 mg/kg. Increases in some organ weights (relative to body
weight) were reported, but as there was a marked decrease in body
weight at the highest dose levels, they were not regarded as
biologically significant. Epithelial metaplasia of the trachea
occurred in high-dose males and females. In male rats there was a
slight but statistically significant (p < 0.5) increase in the
incidence of nephropathy in the 50 mg/kg (11/20) and 600 mg/kg (12/20)
dose groups compared to the controls (4/20). However there was no
significant increase in the incidence of nephropathy at the 150 mg/kg
dose level (7/20) and the average severity of nephropathy was not
increased in any dose group. In the control groups of male rats from
the o-cresol and m-cresol studies, which were conducted
concurrently at the same laboratory, the incidences of nephropathy
were 10/20 and 7/20, respectively. Because of the variable incidence
of this spontaneously occurring lesion even among control groups, the
absence of a dose-related increased incidence or severity and the
absence of an effect on the kidney of female rats, it was considered
that nephropathy was a questionable treatment-related effect in male
rats. For this reason 50 mg/kg was regarded as a NOAEL for
p-cresol, based on the presence of haematological effects at
175 mg/kg. A NOAEL of 50 mg/kg body weight per day was identified for
o- and m-cresols. The NOAEL for o-cresol was based on reduced
body weight and tremors in female at doses of > 175 mg/kg; for
m-cresol the NOAEL of 50 mg/kg body weight per day was based on
reduced body weight in females and males at doses > 150 mg/kg.
Hamsters exposed to 1.5% p-cresol in the feed for 20 weeks
developed an increased incidence of mild-t o-moderate forestomach
hyperplasia compared to controls (Hirose et al., 1986).
Results of longer-term studies are summarized in Table 8.
7.4 Skin and eye irritation
Dermal application of cresols (0.5 ml of o-, m- or p-cresol
or a technical mixture of all three isomers) for 4 h caused visible
and irreversible tissue destruction in rabbits (Vernot et al., 1977).
Severe skin and eye irritation was reported in other laboratory tests
(Mellon Institute, 1949; Bio-Fax, 1969; Younger Labs, 1974; FDRL,
1975; Scientific Associates, 1976; Dow Chemical, 1978). Eye
irritation was also observed in rats and mice briefly exposed to high
concentrations of cresols ( o-cresol and technical cresol mixtures)
in the air (Campbell 1941; FDRL, 1975; Dow Chemical, 1978).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Reproduction
BRRC (1989a,b,c) conducted 2-generation reproduction studies on
rats using o-, m- and p-cresols. For each isomer, groups of 25
male and 25 female Sprague-Dawley CD rats were given 0, 30, 175 or
450 mg cresol/kg body weight daily by gavage in corn oil for 10 weeks
prior to breeding. Dosing of females was continued through a 3-week
mating period, gestation and lactation. After weaning, male and
female pups were given the same doses as their parents for 11 weeks.
As was the case for the F0 females, dosing of F1 females was
continued through a 3-week mating period, gestation and lactation.
All F2 pups were sacrificed at weaning. All three cresol isomers
caused toxic effects in the parental animals. In the F0 rats, toxic
effects were mostly limited to the 450 mg/kg groups and included
death, reduced body weight gain and clinical signs such as
hypoactivity, ataxia, twitches, tremors, prostration, rapid and
laboured respiration, urine stains and perioral wetness. In the F1
rats, some clinical signs of toxicity occurred in the 175 mg/kg groups
as well. However, effects on reproductive function or the morphology
of reproductive tissues were not detected in these studies, even at
doses producing overt parental toxicity. Decreased numbers of
spermatozoa and atrophy of seminal vesicles in some F0 males treated
with 450 mg m-cresol/kg was attributed to postmortem changes or
nonspecific stress; decreased spermatozoa in some F1 males treated
with 450 mg p-cresol/kg was also considered not to be
treatment-related. Similarly, Hornshaw et al. (1986) did not observe
reproductive effects in mink in a 1-generation study in which male and
female mink were fed a diet containing 0, 100, 400 or 1600 mg
o-cresol/kg diet for 2 months before mating and through weaning.
Estimated daily doses were 0, 5, 25 and 105 mg/kg body weight for
males and 0, 10, 40 and 190 mg/kg body weight for females. Parental
toxicity occurred in the mink fed 1600 mg/kg diet (reduced body weight
gain in males, increased relative liver weight and increased
erythrocyte count).
The US NTP (1992) study, discussed in detail in section 7.3.2,
included determination of sperm motility and concentration in male
F344/N rats and B6C3Fl mice after treatment with o-cresol and
m-/ p-cresol for 13 weeks. The length and stages of the estrus
cycles were also determined in female rats and mice. For both o-and
m-/ p-cresol, the rats were treated with 1880, 7500 or 30 000 mg/kg
in the diets. For o-cresol, the mice were treated with 1250, 5000
or 20 000 mg/kg in the diet, and for m-/ p-cresol mice were treated
with 625, 2500 or 10 000 mg/kg in the diet. No adverse effects on
sperm motility or concentration were observed at any dose level in
rats or mice with either o- or m-/ p-cresol. o-Cresol caused
an increased length in the estrus cycle in mice (increased time in
estrus) at 30 000 mg/kg only. A similar, but nonsignificant trend was
observed in rats. The decrease in body weight by itself was thought
not to be the cause for this effect. m-/ p-Cresol caused an
increased estrus cycle length in rats at 7500 and 30 000 mg/kg (all
stages affected) which was not related to body weight changes; there
were no effects on the estrus cycle in mice.
Increased testis weight was observed in ferrets dosed with
o-cresol 2520 and 4536 mg/kg in the diet (Hornshaw et al., 1986).
No adverse effects on the testis were observed in rats treated daily
with 600 mg o- or p-cresol per kg body weight or 450 mg mcresol
per kg body weight by gavage for 13 weeks (MBA, 1988a,b,c).
Pashkova (1972, 1973) studied the reproductive effects of
tricresol (a mixture of o-, m- and p-cresols) in white rats.
The rats were exposed to tricresol concentrations of 0, 0.6 or
4.0 mg/m3 in air for 4 months (daily exposure not specified).
Tricresol at a concentration of 4 mg/m3 had a detrimental effect on
the function and structure of the ovaries. The functional change
observed was a prolongation of both the estrus cycle and the estrus
stage of the cycle, accompanied by a shortening of the diestrus stage
of the cycle. Morphological analysis of the ovaries revealed a
decreased number of primary follicles and increased atresia. Similar,
but less pronounced morphological changes were produced by
0.6 mg/m3.
Izard et al. (1992) (abstract only) evaluated the reproductive
toxicity of o-cresol and a mixture of m- and p-cresol (59% +41%)
in CD-1 Swiss mice using the continuous breeding protocol. Mice
received cresols in feed at 0.25, 1.0 and 1.5% (2500, 10 000 and
15 000 mg/kg diet) of m- plus p-cresol or 0.05, 0.2 and 0.5% (500,
2000 and 5000 mg/kg diet) of o-cresol for 14 weeks. The authors
found that the m- plus p-cresol mixture at 1.5% in the feed
(equivalent to 2100 mg/kg body weight per day) significantly reduced
litter size (80% of control) and adjusted pup weight and increased
cumulative days to litter in the 2nd to 5th litters by 3 to 4 days.
Cross-over breeding of control and 1.5% m- plus p-cresol mixture
Fo animals resulted in decreased adjusted live pup weight of litters
with a treated parent of either sex. At necropsy, high-dose Fo
males had decreased body weight (90%) and relative seminal vesicle
weight. Relative kidney and liver weight increased at 1.0 and 1.5% in
males. In females, relative liver weight increased at all doses; this
was accompanied by decreased (94%) body weight at 1.5%.
The cresol mixture at levels of 1.0 and 1.5% adversely affected
pre- and post-weaning growth and survival. In the F1 generation,
the m- plus p-cresol mixture had no effect on reproductive
competence, but F1 postnatal growth and survival and F2 live pup
weight were decreased at 1.5% of the mixture. At necropsy, F1 males
had reduced body weight and relative seminal vesicle and prostate
weights at the 1.0 and 1.5% tested levels of the cresol mixture.
Females had reduced body weight at 1.0 and 1.5% levels of the mixture,
and relative liver and kidney weights were increased at all doses and
for both sexes. o-Cresol at doses up to 0.5% (equivalent to
550 mg/kg body weight per day) did not affect reproductive or general
toxicity parameters in either generation. They concluded that the
m- plus p-cresol mixture at > 1.0% caused minimal adult
reproductive toxicity but significant postnatal toxicity was observed.
o-Cresol was negative at the doses tested.
7.5.2 Embryotoxicity and teratogenicity
Developmental toxicity studies were conducted for o-, m- and
p-cresols in rats and rabbits (BRRC, 1988a,b). For each isomer,
groups of 25 inseminated female rats were given doses of 0, 30, 175 or
450 mg cresol/kg in corn oil by gavage on days 6-15 of gestation.
Maternal toxicity was evident at 450 mg/kg for all three isomers;
effects included death, reduced food consumption, decreased body
weight gain, and clinical signs such as audible respiration,
hypoactivity, ataxia and tremors. m-Cresol caused no effects on the
developing embryos at any dose, but o- and p-cresols both caused
mild fetotoxic effects at 450 mg/kg (increased incidences of dilated
lateral ventricles in the brain and minor skeletal variations,
respectively), which could have been secondary to maternal toxicity.
In the rabbit studies, groups of 14 inseminated females were given
cresol (0, 5, 50 or 100 mg/kg body weight daily) in corn oil by gavage
on days 6-18 of gestation. Maternal effects, including audible
respiration, ocular discharge, hypoactivity and death ( p-cresol
only), were seen after exposure to > 50 mg/kg. o-Cresol caused
fetotoxicity (increased incidences of subepidermal haematoma on the
head and poorly ossified sternebrae) in rabbits treated with
100 mg/kg. Neither m- nor p-cresol caused any developmental
effects in rabbits at any dose.
Developmental end-points were also monitored in the 2-generation
reproduction studies on rats discussed in section 7.5.1 (BRRC,
1989a,b,c). All three cresol isomers caused effects on pup body
weight at some time during development in these studies. Most of the
deficiencies in pup body weight or growth occurred in rats exposed to
450 mg/kg body weight per day, a dose that also caused overt toxicity
in parental rats. There were occasional body weight changes in
lower-dose groups (especially those treated with m-cresol), but it
is not clear that these changes were treatment-related. In addition
to its effect on pup body weight, m-cresol reduced F2 pup
survival from birth through lactation in the 450 mg/kg group.
In a developmental toxicity screening study, p-cresol was found
to cause maternal toxicity (reduced body weight gain) at a dose of
410 mg/kg body weight, but failed to elicit effects on
post-implantation loss or litter weight at any dose tested (Kavlock,
1990). In a study conducted on cultured rat embryos in vitro,
p-cresol caused dose-related effects on growth (reduced crown-rump
length, somite number and DNA content) and structural abnormalities
(increased hind limb bud absence and total tail defects). The
significance of these results is not clear (Oglesby et al., 1992).
7.6 Mutagenicity and related end-points
Data regarding the genotoxicity of cresols are presented in
Tables 10-14. Most of these data are for individual isomers, but some
information is also available for mixed isomers ( m/p and o/m/p
mixtures).
In vitro DNA repair assays (unscheduled DNA synthesis) were
negative in rat hepatocytes treated with o- or m-cresol, but a
weakly positive result was obtained with human lymphocytes treated
with p-cresol.
There is no evidence that cresols are mutagenic to Salmonella
typhimurium. None of the individual isomers induced mutations at
the tk locus of L5178Y mouse lymphoma cells, whereas the o/m/p
mixture of isomers was active in the presence of S9 mix. In Drosophila
melanogaster, sex-linked recessive lethal mutations were not induced
by either o- or p-cresol.
Chromosomal aberrations were induced in Chinese hamster (CHO)
cells in both the presence and absence of S9 mix, following treatment
with o- and p-cresols, but not with m-cresol. In mice in vivo,
there was no induction of chromosomal aberrations in bone marrow cells
by m-cresol or of micronuclei in peripheral blood erythrocytes by
o-cresol or the m/p isomer mixture.
Sister-chromatid exchanges (SCE) were induced in CHO cells by
o-cresol and by the o/m/p isomer mixture, but were not induced by
o-, m- or p-cresol in cultured human fibroblasts, after testing
only in the absence of S9 mix. In mice, in vivo tests for SCE
induction were inconclusive with o-cresol and negative with m- and
p-cresol.
No dominant lethal effects were observed following treatment of
male mice with either o- or p-cresol.
Table 10. Genotoxicity of o-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Litton Bionetics
(1981); Pool & Lin (1982);
Haworth et al. (1983)
Forward mutation L5178Y mouse lymphoma cells - - Litton Bionetics (1981)
Unscheduled DNA synthesis primary rat hepatocytes ND - Litton Bionetics (1981)
Chromosomal aberrations Chinese hamster ovary cells + + Hazleton Labs (1988a)
Sister-chromatid exchange Chinese hamster ovary cells + + Litton Bionetics (1981)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells - - Hazleton Labs (1988b);
Litton Bionetics (1981)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 10 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Sex-linked recessive lethal Drosophila melanogaster - Hazleton Labs (1989d)
Sister-chromatid exchange mouse ? Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
Micronucleus, peripheral mouse - US NTP (1992)
blood erythrocytes
Dominant lethal mouse - Hazleton Labs (1989a)
a - = negative result; + = positive result; ND = no data; ? = inconclusive
Table 11. Genotoxicity of m-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Haworth et al. (1983);
Pool & Lin (1982)
Forward mutation L5178Y mouse lymphoma cells - - Hazleton Labs (1988c)
Unscheduled DNA synthesis freshly cultured rat hepatocytes ND - Hazleton Labs (1988e)
Chromosomal aberrations Chinese hamster ovary cells - - Hazleton Labs (1988a)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells - - Hazleton Labs (1988d,f)
SV40 induction Syrian hamster kidney cells ND (+) Moore & Coohill (1983)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 11 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Chromosomal aberrations mouse - Hazleton Labs (1989c)
(bone marrow)
Sister-chromatid exchange mouse - Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
a - = negative result; (+) = weakly positive; ND = no data
Table 12. Genotoxicity of p-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Pool & Lin (1982);
Haworth et al. (1983)
Forward mutation L5178Y mouse lymphoma cells - - Hazleton Labs (1988c)
Semiconservative/repair DNA human peripheral lymphocytes ND (+) Daugherty & Franks (1986)
synthesis
Chromosomal aberrations Chinese hamster ovary cells + + Hazleton Labs (1988a)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells ND + Hazleton Labs (1988d)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 12 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Sex-linked recessive lethal Drosophila melanogaster - Hazleton Labs (1989e)
Sister-chromatid exchange mouse - Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
Dominant lethal mouse - Hazleton Labs (1989b)
a - = negative result; + = positive result; (+) = weakly positive; ND = no data
Table 13. In vitro genotoxicity of a 1:1:1 mixture of o-, m- and p-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
Reverse mutation Salmonella typhimurium - - Litton Bionetics (1980)
(on plates)
Forward mutation L5187Y mouse lymphoma cells + ? Litton Bionetics (1980)
Sister-chromatid exchange Chinese hamster ovary cells + + Litton Bionetics (1980)
Cell transformation mouse BALBc/3T3 cells + ND Litton Bionetics (1980)
a - = negative result; + = positive result; ? = inconclusive; ND = no data
Table 14. Genotoxicity of 60:40 m/p-cresol
Results
With Without
Assay Indicator organism activation activation Reference
Reverse mutation (on plates) Salmonella typhimurium - - US NTP (1992)
Micronuclei, peripheral blood mouse - US NTP (1992)
erythrocytes
BALBc/3T3 cells were transformed by p-cresol and the o/m/p
mixture, but not by o- or m-cresol. A weakly positive result was
obtained, however, in a viral enhancement assay with m-cresol.
Viral DNA amplification did not occur in SV-40-transformed
Chinese hamster embryo cells treated with o-, m- or p-cresol.
Antimutagenic effects of o- and p-cresol, but not of
m-cresol, have been demonstrated in methylnitrosoguanidine-induced
mutagenesis in Escherichia coli when given after the MNNG treatment
(Kushi & Yoshida, 1987).
In summary, these data indicate that m-cresol has little or no
genotoxic potential, and whereas both o- and p-cresol can induce
chromosomal aberrations in vitro and o-cresol can increase SCE
in vitro, they do not do so in vivo.
7.7 Carcinogenicity
There are no adequate bioassays or chronic studies available to
assess the carcinogenic potential of cresols. Two studies (Boutwell &
Bosch, 1959; Yanysheva et al., 1993) have indicated that cresols have
potential tumour-promoting activity. However, no conclusions can yet
be made regarding the carcinogenic potential of these compounds.
Boutwell & Bosch (1959) investigated the tumour-promoting ability
of cresols using a mouse skin-painting model. Groups of 27-29 mice
were given a single dermal application of 9,10-dimethyl-
1,2-benzanthracene, a cancer initiator, followed by application of 20%
solutions of o-, m- or p-cresol in benzene, twice a week for 12
weeks. Significant non-tumour-related mortality was produced by all
three cresol isomers. Among the survivors at 12 weeks, both the
average number of skin papillomas per mouse and the percentage of
exposed mice with at least one papilloma were increased by treatment
with cresols. o-Cresol was the most potent isomer and p-cresol
the least. No carcinomas were observed following treatment with
cresols. It should be noted that the vehicle used for cresols in this
study was benzene, a known carcinogen. The presence of benzene did
not appear to affect the results, however, since no papillomas were
observed in benzene-treated controls. This study suggests that
cresols may act as promoters.
Yanysheva et al. (1993) reported that o-cresol administered
orally (1 mg) twice weekly for up to 30 weeks to mice simultaneously
with benzo[ a]pyrene (1 mg) increased the incidence and malignancy of
tumours produced by benzo[ a]pyrene and shortened the latency period
for tumour development. These effects were not seen when higher
(10 mg) or lower (0.02 mg) doses of o-cresol and benzo[ a]pyrone
were administered. Administration of o-cresol before or after
benzo[ a]pyrene had the opposite effect, decreasing the
carcinogenicity of that chemical.
7.8 Other special studies
7.8.1 Neurological effects
A neurotoxicity study was performed on CD rats using all three
cresol isomers (TRL, 1986). Groups of 10 rats of each sex were
treated with o-cresol (0, 50, 175, 450 or 600 mg/kg body weight),
m-cresol (0, 50, 150 or 450 mg/kg), or p-cresol (0, 50, 175 or
600 mg/kg) in corn oil by gavage daily for 13 weeks. Both o- and
p-cresol caused death in the groups exposed to 600 mg/kg.
Convulsions were seen only in the groups treated with > 450 mg/kg.
Hypoactivity, rapid laboured respiration and excessive salivation were
observed sporadically at doses of > 50 mg/kg for all three isomers.
In spite of the observed clinical signs, few significant changes were
found in performance on neurobehavioural test batteries, no brain
weight changes were noted, and no gross or histopathological lesions
in the brain or other nervous tissues were found for any isomer.
Savolainen (1979) studied the effect on biochemical parameters in
the brain of Wistar rats (40 males) after exposure to o-cresol in
the drinking-water for 20 weeks. The administered concentration was
300 mg/litre, which provided daily doses of about 36 mg/kg body
weight. Increased activity of 2',3'-cyclic nucleotide
3'-phosphohydrolase in glial cells, and reductions in azoreductase and
glutathione in brain homogenate were found. No other
treatment-related effects were detected.
o-Cresol produced excitation of both the somatosensory evoked
potential and electroencephalogram in male Fischer-344 rats given a 1%
solution intravenously at the rate of 0.9 mg/min for 15 min (total
dose of 13.5 mg) (Mattsson et al., 1989). The rats were conscious and
responsive to stimuli. Muscle tremors developed if exposure was
sufficiently long.
7.8.2 Effects on the skin
Application of 0.5% p-cresol to the skin for 6 weeks resulted
in permanent depigmentation of the skin and hair in black and agouti
mice (Shelley, 1974). Depigmentation was accompanied by a caustic
effect in one black strain of mice, but not in another. In identical
trials, neither o- nor m-cresol caused depigmentation in mice.
7.9 Mechanisms of toxicity - mode of action
The main effects of cresols at the area of first contact are
irritancy or corrosivity, depending upon the concentration. These
primary effects are followed after absorption by haematoxicity,
hepatotoxicity and neurotoxicity. The mechanisms by which these
effects occur have not been specifically studied with respect to
cresols. It is frequently assumed that the basis for cresol toxicity
is similar to that for phenol. However, phenol has certain unique
properties, e.g., cardiac toxicity, which has not been reported for
cresols except in one human case of acute poisoning involving a very
high exposure (Arthur et al., 1977).
Cresols are relatively soluble in water and have been shown
in vitro to have high permeability coefficients for human skin
(Thompson et al., 1994). Consequently, they can be rapidly absorbed
and distributed throughout the body. Cresol metabolism is mainly
conjugation followed by urinary excretion as glucuronides and sulfates
(Bray et al., 1950). In addition, in vitro studies have shown that
microsome-dependent covalent binding to proteins occurs, but the
importance of this process is unknown (Thompson et al., 1994).
In an in vitro study, cresol caused inhibition of K-dependent
phosphatase activity of Na/K ATPase in erythrocyte membrane (Wardle,
1978). Dermally applied p-cresol inhibits ATPase activity, as
measured in both erythrocytes and brain. This could contribute to
toxicity by disturbing electrolyte balance across cell membranes,
which could result in haemolysis. Another factor leading to
erythrocyte damage could be the binding of cresols to iron complexes
(analogous to the process described in clay soils, see section 4.2.1).
If, indeed, this should occur, then it may contribute to
methaemoglobin formation, haemolysis and, in the liver, a compensatory
response leading to enlargement.
Neurotoxic mechanisms have received little study. At a
concentration of approximately 0.25 mM, p-cresol inactivates
dopamine ß-hydroxylase (DeWolf et al., 1988), and could thereby affect
neurotransmission by interfering with noradrenaline biosynthesis.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Poisoning incidents
The most commonly reported cases of cresol poisoning involve
accidental or intentional ingestion of cresol-containing substances.
Cresols are strong irritants, and their ingestion results in burning
of the mouth and throat, abdominal pain and vomiting (Isaacs, 1922;
Jouglard et al., 1971; Wiseman et al., 1980). The primary targets of
ingested cresols in humans appear to be the central nervous system,
blood and kidneys. Some effects on the lungs, heart and liver have
also been reported (Isaacs, 1922; Labram & Gervais, 1968; Chan et al.,
1971; Jouglard et al., 1971; Cote et al., 1984; Minami et al., 1990).
Chan et al. (1971) described two cases of oral cresol poisoning.
In one case, a woman swallowed about 250 ml disinfectant containing
50% mixed cresols. The patient was in a deep coma when admitted to
the hospital 2 h after ingestion, but regained consciousness 10 h
later. Haematological changes were remarkable. Within 7 h of
admission, erythrocyte glutathione levels were markedly reduced, and
methaemoglobinaemia was detected. Within 3 days, severe
haemoglobinaemia and haemoglobinuria were evident, along with
extensive Heinz body formation, indicating that massive intravascular
haemolysis had occurred. The patient died the next day, apparently
from thrombus formation and kidney failure secondary to acute
intravascular haemolysis. Autopsy revealed moderate fatty
degeneration in the liver and, in the kidney, fibrin clumps in the
glomeruli and moderate tubular degeneration consistent with
intravascular thrombosis. The authors also described the case of a
second woman who recovered after drinking about 100 ml of the same
cresol-containing disinfectant. The patient was semiconscious when
admitted to the hospital 1.5 h after ingestion. Methaemoglobin was
detected in the blood at admission, but not 6 h later. Heinz bodies
were observed 6 h after admission, but disappeared within 2 days.
Haemolytic anaemia and associated changes have been described in
other case reports. Heinz body formation, haemoglobinaemia and
haemoglobinuria were evidence of haemolytic anaemia in a man who drank
100 ml of "penetrating oil", a petroleum distillate containing 12%
mixed cresols (Cote et al., 1984). Severe haemolytic anaemia
developed during the second week following cresol ingestion in a man
who swallowed approximately 250 ml of a concentrated cresol mixture
(Jouglard et al., 1971). Dark urine and methaemoglobinaemia were
observed upon hospital admission in a man who had swallowed a
commercial disinfectant containing cresols 2 h earlier (Minami et al.,
1990). The concentration of methaemoglobin in the blood was monitored
regularly and was seen to increase markedly 15 h after admission to
the hospital. The patient was then given a blood transfusion, after
which methaemoglobin levels decreased to normal and the patient
recovered.
Labram & Gervais (1968) reported the case of a woman who
swallowed between 500 and 750 ml of a concentrated cresol mixture.
Upon admission to the hospital 45 min later, the patient was in a deep
coma and exhibited tachycardia with polymorphic ventricular
extrasystoles. A transient episode of ventricular fibrillation was
followed by cardiac arrest 24 h after admission to the hospital. At
autopsy, the most notable finding was massive eosinophilic necrosis in
the proximal tubule of the kidney. The investigators considered it
likely that this lesion occurred prior to death and represented a
target organ effect of cresol. Diffuse necrosis of the bronchial
epithelium was also thought to have occurred prior to death.
Pulmonary oedema and haemorrhage were also observed, but may have been
secondary to death. Diffuse lesions in other organs were also
considered to be secondary to death.
Isaacs (1922) reported symptoms of cresol poisoning in 52
patients who ingested 4-120 ml of disinfectant containing 25-50%
cresols. Mouth and throat burns, abdominal pain and vomiting were
common symptoms of cresol poisoning. Coma was also a frequent
occurrence; in some cases, unconsciousness occurred very soon after
exposure and lasted 14 h or more. Renal irritation and reduced
phenolsulfonephthalein output indicated the occurrence of kidney
effects in some patients. Darkly coloured urine was produced in most
cases and may have been due to haemoglobinuria. Blood abnormalities
were not detected, but details regarding blood analyses were not
reported; it is possible that some haematological changes (e.g.,
methaemoglobinaemia, Heinz body formation) may have been overlooked.
Only two of the 52 patients died; both deaths occurred within 30 min
of cresol ingestion.
Arthurs et al. (1977) reported a case of a 32-year-old man who
was admitted after he had taken more than 45 ml of cresols. This
patient was conscious upon admission. However, he became increasingly
dyspnoeic and developed tachycardia and systolic hypotension within
the next 12 h. The total serum phenol levels were elevated 24 h after
admission. The patient died 4 days later of myocardial failure and
pulmonary oedema.
Not all poisoning incidents with cresols involve oral exposure.
Accidental dermal exposure has also been reported, usually causing
corrosive damage to the skin (Herwick & Treweek, 1933; Green, 1975;
Wiseman et al., 1980; Pegg & Campbell, 1985). In one patient,
disfiguring scars remained visible a year after exposure (Herwick &
Treweek, 1933). Systemic effects of dermal exposure were reported by
Green (1975), who described the case of a 1-year-old baby who had
20 ml of a cresol derivative (90% mixed cresols in water) spilled on
his head. The spill area, shown by burning on the face and scalp,
covered about 7% of his body surface. The baby fell into a coma after
5 min and died within 4 h. Autopsy revealed haemorrhagic oedema in
the lungs, extensive centrilobular to mid-zonal necrosis in the liver,
congestion, swelling and tubular necrosis in the kidneys, and
congestion and swelling in the brain.
In some cases, cresols have been injected intentionally into the
vagina and uterus for the illegal purpose of inducing abortion. Signs
and symptoms in women exposed to cresols in this manner include
vaginal bleeding, abdominal cramps, severe burning pain, coma, massive
haemolysis, severe kidney nephrosis and failure, severe pulmonary
oedema with oil emboli, and death (Vance, 1945; Presley & Brown, 1956;
Finzer, 1961).
8.1.2 Controlled human studies
Uzhdavini et al. (1972) found that 6 mg/m3 was the threshold
concentration for the production of mucosal irritation by o-cresol
(vapour/aerosol mixture) in humans. At this concentration, 8 out of
10 subjects complained of symptoms such as dryness, nasal constriction
and throat irritation. However, the duration of exposure or the
composition of the compound (i.e. purity) were not specified in the
report. No reaction to cresol was noted in humans when the compound
was applied to the skin of the elbow as a 1% solution in alcohol
(Reimann, 1933).
8.1.3 Cancer
Epidemiological data regarding cancer and cresol exposure in
humans are not available in the literature. Two studies have examined
the production of endogenous p-cresol in cancer patients. Bone et
al. (1976) compared urinary p-cresol levels in six patients with
large bowel cancer with levels in 10 healthy patients and found no
difference in average daily urinary excretion of p-cresol.
Similarly, Renwick et al. (1988) found no difference in average daily
urinary excretion of p-cresol among 32 patients with transitional
cell carcinoma of the bladder and matched controls.
8.2 Occupational exposure
8.2.1 Poisoning incidents
Acute cresol poisoning during occupational exposure is usually a
result of dermal contact. In one case, a man fell into a vat
containing a cresylic acid derivative (Cason, 1959). The man suffered
burns on 15% of his body surface and developed anuria 36 h after
exposure. Blood urea levels increased steadily over the following
days. He fell into a coma 9 days after admission to the hospital and
died on the following day due to congestive heart failure.
Anuria was also observed in a man who worked with an antiseptic
solution containing concentrated mixed cresols for 2 days before
becoming ill (Larcan et al., 1974). Other significant observations in
this patient were haematological changes similar to those observed
after oral exposure, including methaemoglobinaemia, Heinz body
formation and massive haemolysis. The man died 3 days after admission
to the hospital.
Klinger & Norton (1945) reported the case of a man who had his
hands immersed in a 6% cresylic acid solution for 5-6 h. The man
survived, but experienced persistent eye-watering, followed by pain on
the side of his face and, ultimately, marked facial paralysis.
Thirteen cases of accidental burns were reported in workers
exposed to cresol (2), dichlorophenol (1) and phenol (10) (Ma et al.,
1982). Burns were diagnosed as first and second degree, small in area
and covered 0.5-10% of the body surface. The patients generally
demonstrated (in the following order) white, red, brown and black skin
colour, and then crusting, necrosis and sloughing. Patients were
treated immediately by washing affected areas. Twelve of 13 patients
fully recovered within 15 days with no scarring of skin.
Wu & Kwan (1984) reported a case of acute renal failure in a
healthy 50-year-old male technician accidentally exposed to a mixture
of cresols. The burned area was immediately irrigated with water.
The patient experienced dizziness, pain and numbness of burnt skin and
abdominal pain followed by oliguria and vomiting 8 h later. He
developed severe abdominal pain and vomiting and lesser oliguria
1 day after the exposure. The patient was admitted to hospital
3 days after exposure. A follow-up examination revealed decreased
pulse rate, urinary volume (70-190 ml/24 h), blood urea nitrogen
(440-1240 mg/litre), combining power of CO2 (40-42 vol %). Burnt
skin was light brown in colour and there was slight swelling and
tactile pain. The patient was treated for acute renal failure,
and complete recovery occurred within 27 days following exposure.
Ma & Wang (1989) reported a case of acute cresol burn and
poisoning in a 18-year-old woman accidentally exposed to cresol.
Exposure of face, hand, feet, thighs and perineum occurred. Face,
hand and feet were immediately irrigated with water, but contaminated
trousers were not taken off and thighs and perineum were not washed.
Burns were first and second degree in severity and covered 20% of the
body. After 10 min she exhibited erythema, discoloration of the skin,
and became delirious followed by coma. Pulmonary oedema and
haemoglobinuria were reported after treatment with a diuretic and
intravenous infusion. Additional therapy included peritoneal dialysis,
strict control of intravenous fluids, intravenous rogitine, oral
nitroglycerin and nifedipine etc. Peritoneal dialysis was continued
for 20 days. The patient was discharged 38 days later. No scarring
of the skin occurred.
8.2.2 Epidemiological studies
Molodkina et al. (1985) studied 174 female workers aged between
20 and 50 in an enamel wire production plant using mainly tricresol.
Seventy percent of the study population had been exposed to this
compound for at least 10 years. Tricresol concentrations averaged
1.4 mg/m3 per shift, with maximum recorded levels of 3.6-5.0 mg/m3.
Reported effects included circulatory disturbances and minor
haematological changes (decreases in red blood cell count, white blood
cell count and platelets). There were also reported to be decreases
in the activity of glucose-6-phosphate dehydrogenase and the
concentration of sulfhydryl groups within erythrocytes. Erythrocytes
were reported to have a shorter lifespan.
Syrovadko & Malysheva (1977) studied reproductive endpoints in
female workers exposed to tricresol and chlorobenzene during the
manufacture of enamel-insulated wire. Several reproductive disorders
were reported, including hormonal shifts, menstrual problems and
elevated incidences of perinatal mortality and abnormal development of
offspring.
A group of 58 women (without pre-existing genital disease),
exposed to tricresol and phosphoryl chloride in the production of
tricresylphosphate, comprised the study population investigated by
Pashkova (1973). There was an elevated incidence of menstrual
disturbances in the study population, with changes in the cycle
accompanied by difficult and painful menstruation. Changes in the
cycle were found to be the result of increased estrogen and decreased
progesterone activity, indicating ovarian dysfunction.
In neither of the above two studies was there any documentation
of the degree of exposure to cresols or any quantification of the
physiological changes mentioned. Therefore, the significance of these
studies cannot be assessed.
8.3 Subpopulations at special risk
Several populations have been identified that may be at special
risk from cresol exposure. For instance, in persons with renal
insufficiency, the renal clearance of phenol and p-cresol is
impaired, leading to accumulation of cresol in the blood (Niwa, 1993).
Individuals with glucose-6-phosphate dehydrogenase (G6DP) deficiency
may also have heightened sensitivity to the haematological effects of
cresols. In experiments in which blood was exposed to a disinfectant
containing 50% cresols in vitro, increased methaemoglobin formation
and decreased glutathione levels were more pronounced in blood from
subjects with glucose-6-phosphate dehydrogenase deficiency than blood
from normal subjects (Chan et al., 1971).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Aquatic
9.1.1.1 Laboratory studies
Growth studies have shown that cresols are moderately toxic to
aquatic bacteria, cyanobacteria (blue-green algae) and protozoa.
Growth inhibition thresholds for the bacterium Pseudomonas putida,
were 33 and 53 mg/litre for o- and m-cresol, respectively
(Bringmann & Kühn, 1976; Bringmann & Kühn, 1980), 6.8 and 13 mg/litre
for the cyanobacterium Microcystis aeruginosa, 17 and 31 mg/litre
for the bacteriovorous flagellate protozoan Entosiphon sulcatum
(Bringmann & Kühn, 1978a,b, 1980) and 132 and 114 mg/litre for the
saprozoic flagellate protozoan Chilomonas paramecium (Bringmann &
Kühn, 1980). The ciliate protozoan Tetrahymena pyriformis showed
low sensitivity to o-cresol and p-cresol, 48-h EC50 values for
growth inhibition being 203 and 168 mg/litre, respectively (Schultz &
Riggin, 1985; Schultz, 1987). The yeasts Pichia sp. and Rhodotorula
rubra had 50% growth reduction following 12 h of incubation with 400
and 200 mg p-cresol/litre, respectively (Kwasniewski & Kaiser,
1983).
Bacterial luminescence assays, which provide an indirect measure
of population inhibition, have been conducted in Photobacterium
phosphoreum. For p-cresol, 5-min EC50 (50% reduction of light)
values ranged from 1.5 to 1.72 mg/litre (Bulich & Isenberg, 1980,
1981; Bulich et al., 1981; Ribo & Kaiser, 1983).
The potential impact of m-cresol on wastewater treatment
systems appears to be minimal; the measured I50 (50% inhibition of
activated sludge respiration rates) is 458.1 mg/litre (Dow Chemical,
1984).
9.1.1.2 Field studies
Field studies on the degradation of fresh poplar leaves in
experimental streams dosed with 8 mg p-cresol/litre had decreased
rates of decomposition compared to control streams (Stout & Cooper,
1983). Although, treatment of the stream with p-cresol changed the
dynamics of invertebrate communities on the leaves (see section
9.3.1.2), the authors suggested that the principal factor in
decreasing decomposition was inhibition of aerobic microbial
degraders, due to the drop in dissolved oxygen concentrations below
1 mg/litre, which followed p-cresol addition.
9.1.2 Terrestrial
9.1.2.1 Laboratory studies
Although there have been many investigations regarding the
degradation of cresols by soil isolates in laboratory culture systems
(section 4.2.2), laboratory studies regarding the toxicity of cresols
to soil microorganisms are rare. One study using Pseudomonas putida,
a species common to both aquatic and terrestrial environments, is
discussed in section 9.1.1.1.
9.1.2.2 Field studies
Reports are available on cresol decomposition in soils (section
4.2.2), but field studies on the impact of cresols on the soil
microbial community have not been reported in the available
literature.
9.2 Plants
9.2.1 Aquatic
9.2.1.1 Laboratory studies
Laboratory investigations into the toxicity of cresols to plants
are summarized and referenced in Table 15. Studies have been
conducted in five species of algae and one vascular plant. Growth
inhibition threshold levels in algae ranged from 7.8 to 65 mg/litre,
indicating that cresols are moderately toxic to the species tested.
Similar levels of toxicity for algae have been observed for the three
isomers. EC50 values (mortality, reproduction and dry weight) from
static and flow-through tests on duckweed are similar and demonstrate
a low level of sensitivity of this species to o-cresol.
9.2.1.2 Field studies
The effects of p-cresol on respiration and photosynthesis in
the filamentous green alga Spyrogyra sp. were studied in a set of
open channel experimental streams (Stout & Kilham, 1983). Continuous
dosing of one channel for 48 h with 8 mg p-cresol/litre led to a
decrease in the oxygen concentration of the stream (to below
1 mg/litre). The large decrease in dissolved oxygen in the channel
resulting from p-cresol addition could only be partially accounted
for by inhibition of algal function and was mainly attributed to
microbial heterotrophs utilizing p-cresol as a substrate. This was
substantiated when laboratory results showed that exposure of
Spyrogyra sp. for 1 h to p-cresol (0, 0.9, 4.6, 14, 34 or 71 mg
per litre) resulted in dissolved oxygen concentrations of 7.0, 6.2,
6.8, 5.4, 5.2 and 5.6 mg/litre, respectively, while oxygen levels in
the dosed stream (field study) often fell well below these levels.
The algae turned brown at the three highest exposure levels.
Table 15. Toxicity of cresols to aquatic plants under static conditionsa
Test species Test chemical Age/size Temperature pH Test duration Effectb Concentration Reference
(°C) (days) (mg/litre)
Green alga o-cresol 10-day-old 27 7 7 NOEL 11 Bringmann & Kühn
(Scenedesmus culture (1978a,b, 1980)
quadricauda) m-cresol 8 15
Green alga p-cresol log phase NR 8 2 NOEL 7.8 Kuhn & Pattard
(S. subspicatus) (1990)
Green alga o-cresol log phase 25 7 2 NOEL 36 Slooff et al.
(S. pannonicus) (1983)
Green alga o-cresol log phase 26 7 4 NOEL 65 Slooff et al.
(Selenastrum (1983)
capricornutum)
Green alga (S. o-, m-, 14-day-old NR NR 14 EC50 for growth 137 Gaur (1988)
capricornutum) p-cresol culture inhibition
Green alga o-cresol log phase 25 7 2 growth 34 Slooff et al.
(Chlorella inhibition (1983)
pyrenoidosa)
Table 15 (contd).
Test species Test chemical Age/size Temperature pH Test duration Effectb Concentration Reference
(°C) (days) (mg/litre)
o-cresol steady state 25 8.8 3 EC50 for 100 Huang & Gloyna
m-cresol chlorophyl (1968)
p-cresol inhibition
(72 h)
Duckweed o-cresol 4.8-5.2 EC50 for Davis (1981)
(Lemna gibba) mortality 540
reproduction 245
dry weight 16.98
a Water was unchanged for the duration of the test; NR = not recorded
b EC50 = Concentration effecting 50% of the population; NOEL = no-observed-effect level
9.2.2 Terrestrial
9.2.2.1 Laboratory studies
Laboratory investigations regarding the effects of cresols on
terrestrial plants were not located in the available literature.
9.2.2.2 Field studies
No data relating to the impact of cresols on terrestrial plants
under field conditions could be located in the available literature.
9.3 Invertebrates
9.3.1 Aquatic
9.3.1.1 Laboratory studies
Laboratory investigations on the acute toxicity of cresols to
invertebrate species are presented in Table 16. Fifteen freshwater
and four marine species from a wide range of taxonomic groups have
been studied. LC50 values range from 1.4 to 165 mg/litre,
representing moderate to low levels of toxicity. Kühn et al. (1989a)
reported acute and 21-day NOEL values for reproductive effects in
Daphnia magna of 2.5 and 1.0 mg/litre, respectively, following
exposure to p-cresol. Devillers (1988) studied the relative acute
toxicity to Daphnia magna of phenols and three cresol isomers. The
results showed that, following 24 h of exposure at pH 7.8-8.2 and
under static condition, the immobilization concentrations (IC50) for
the 3 isomers were 18, 19 and 12 mg/litre. There were no significant
differences in the magnitude of toxicity of the three cresol isomers,
p-cresol being only slightly more toxic than o- or m-cresol.
In a laboratory study by Emery (1970), cresol (isomers not
specified) solutions were used to determine the relative toxic
responses of three immature phases and three mature states of
Gammarus faoccatus and Asellus militasis. Exposures of 48 h
revealed that adults were more tolerant than immature animals.
Asellids were about twice as tolerant as gammarids and mature
gammarids were 4 times more tolerant than immature animals. The most
susceptible phase of these crustaceans' life cycle was the first
instar.
Table 16. Acute toxicity of cresols to aquatic invertebrates
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterflea o-cresol stat NR NR NR NR 48 LC50 9.5 Slooff et al.
(Daphnia magna) (1983)
NOEC 2.9 Bringmann & Kühn
(1977)
o-cresol stat 24 h 20-22 7.6-7.7 70 24 LC50 19
o-cresol NR NR NR NR NR 48 LC50 5 Parkhurst et al.
(1979)
m-cresol stat 24 h 20-22 7.6-7.7 70 24 LC50 8.9 Bringmann & Kühn
(1977)
m-cresol NR NR NR NR NR 48 LC50 18.8 Parkhurst et al.
(1979)
p-cresol NR NR NR NR NR 48 LC50 1.4 Parkhurst et al.
(1979)
p-cresol stat 6-24 20 8.0 NR 24 EC50 14 Kühn et al.
(1989b)
Waterflea o-cresol stat NR NR 7 NR 48 LC50 9.6 Slooff et al.
(D. pulex) NOEC 5.2 (1983)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterflea o-cresol flow NR 14 7.6-8.3 569-865 48 LC50 > 94 Degraeve et al.
(D. pulicaria) (1980)
m-cresol flow NR 14 7.6-8.3 569-865 48 LC50 > 99.5 Degraeve et al.
(1980)
p-cresol flow NR 14 7.6-8.3 569-865 48 LC50 22.7 Degraeve et al.
(1980)
Aquatic sowbug o-cresol stat NR 20 7 NR 48 LC50 23 Slooff (1983)
(Asellus
aquaticus)
Scud o-cresol stat NR 20 7 NR 48 LC50 21 Slooff (1983)
Gammarus pulex)
Marine scud o-cresol stat adult 23 NR NR 96 LC50 10.2 Lee & Nicol
(Elasmopus (1978)
pectinicrus)
Marine sand o-cresol SR 3.8 cm 10 NR NR 59 LC50 14.2 McLeese et al.
shrimp (Crangon (1979)
septemspinosa)
Mayfly o-cresol stat NR 20 7 NR 48 LC50 50 Slooff (1983)
(Cloeon
dipterum)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterbug o-cresol stat NR 20 7 NR 48 LC50 80 Slooff (1983)
(Corixa
punctatum)
Mosquito o-cresol stat 3rd 26 7 NR NR LC50 80 Slooff (1983)
(Aedes aegypti) instar
Midge o-cresol stat NR 20 7 NR 48 LC50 34 Slooff (1983)
(Chironomus
thumni)
Dragonfly o-cresol stat NR 20 7 NR 48 LC50 46 Slooff (1983)
(Ischnura
elegans)
Stonefly o-cresol stat NR 20 7 NR 48 LC50 10 Slooff (1983)
(Nemoura
cinerea)
Hydra o-cresol stat budless 17 7 NR 48 LC50 75 Slooff (1983);
(Hydra Slooff et al.
oligactis) (1983)
Pond Snail o-cresol NR 3-4 20 7 NR 48 LC50 160 Slooff (1983);
(Lymnaea weeks Slooff et al.
stagnalis) (1983)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Flatworm o-cresol stat NR 20 7 NR 48 LC50 24 Slooff (1983)
(Dugesia
lugubris)
Oligochaete o-cresol stat 20 7 NR NR 48 LC50 165 Slooff (1983)
family
(Tubificidae)
Marine o-cresol stat 20 7 NR NR 48 LC50 135 Slooff (1983)
polychaete
(Ophryotrocha
diadema) o-, m-, stat NR NR NR NR 48 LC50 33-100 Parker (1984)
p-cresol
Marine green o-cresol stat eggs 5 NR NR 96 EC50 30 Falk-Petersen
sea urchin development et al. (1985)
(Strongylocentrotus
droebachien) m-cresol stat eggs 5 NR NR 96 EC50 30 Falk-Petersen
development et al. (1985)
p-cresol stat eggs 5 NR NR 96 EC50 5 Falk-Petersen
development et al. (1985)
a stat = static conditions (water unchanged for the duration of the test); flow = intermittent flow-through conditions; NR = not reported
b LC50 = concentration resulting in lethality of 50% of the test animals; NOEC = no-observed-effect concentration; EC50 = concentration
resulting in effects among 50% of the test animals
9.3.1.2 Field investigations
The effect of p-cresol on invertebrate colonization of
leafpacks was studied in open channel experimental streams (Stout &
Cooper, 1983). Two types of dosing regimes were used in an attempt to
distinguish between the direct toxicity of p-cresol and the indirect
effect of decreased dissolved oxygen concentration caused by the
stream microorganisms utilizing p-cresol as a substrate (section
9.1.1.2), previously shown to occur in p-cresol-treated streams.
Channels were either continuously dosed for 96 h with 8 mg
p-cresol/litre or intermittently dosed at the same level, with
temporary cessations of dosing when dissolved oxygen concentrations
decreased considerably compared to the control. Leafpacks containing
fresh Populus deltoides (poplar) leaves were added to the streams and
monitored for invertebrate colonization. Changes in colonization
patterns in the treated streams significantly altered the biomass of
invertebrates over time, and responses were less severe in the
intermittent-dose channels than in the continuous-dose ones. Leeches
and isopods, normally found among root mats, entered the water column
and became highly abundant leafpack colonists in the continuous-dose
channels compared to intermittent-treated and control streams. Snails
and flatworms, which are normal colonists on leafpacks, increased
dramatically in numbers, while freshwater scuds, also normal leafpack
colonists, decreased markedly and dead scuds were found floating in
the stream following dosing. Prior tests with scud showed a 44%
mortality rate in aerated water dosed with 5 mg/litre for 96-h, while
the 96-h LC50 in unaerated water was 2 mg/litre. These data suggest
the aquatic invertebrate community may be damaged more from decreased
available oxygen, an indirect effect of p-cresol in the water, than
by a direct toxic action of the substance.
9.3.2 Terrestrial
9.3.2.1 Laboratory studies
Laboratory investigations into the impact of cresols on
terrestrial invertebrates were not located in the available
literature.
9.3.2.2 Field studies
Field investigations concerning cresols were also not located in
the available literature.
9.4 Vertebrates
9.4.1 Aquatic
9.4.1.1 Laboratory studies
The acute toxicity of cresols to vertebrates has been studied in
nine species of fish (eight freshwater and one marine species) and two
species of freshwater amphibians (Table 17). LC50 values range from
7.9 to 40 mg/litre, indicating that the test materials are similar in
their level of toxicity and that they are moderately toxic to aquatic
vertebrates. Studies conducted in fathead minnows exposed to
o-cresol show that the toxicity of the compound is not affected by
water hardness.
9.4.1.2 Field studies
The effect of p-cresol on five fish species (smallmouth bass,
largemouth bass, fathead minnows, walleyed pike and bluegill sunfish)
was determined in an outdoor experimental stream (Cooper & Stout,
1982). Exposure over a 24-h period to a concentration of 8 mg/litre
caused no mortality, but examination of fish guts showed that they had
ceased feeding. There was no serious reduction in dissolved oxygen
during the experiment.
Total body burden measurements showed that the fish had a
bioaccumulation factor of 2.1 for p-cresol at the end of the
exposure period. Body burdens showed a rapid decrease on removal of
the contaminant.
During a 48-h pulsed exposure to 8 mg p-cresol/litre, the
mortality of walleyed pike was very high. Smallmouth bass showed
visible stress; largemouth bass showed no visible stress but had
stopped feeding. There were large decreases in dissolved oxygen
during the experiment. Bioaccumulation was determined in specific
body parts for bluegill sunfish. p-Cresol levels in eyes, mussels
and gills were low (2.8, 4.7 and 7.3 mg/kg, respectively) but were
high in liver and intestines (76 and 97 mg/kg, respectively). Again,
p-cresol was rapidly eliminated from the fish.
In the case of a 96-h exposure to 8 mg p-cresol/litre, the
mortality was very high in all species. Large, sustained drops in
dissolved oxygen also occurred.
Table 17. Acute toxicity of cresols to aquatic vertebrates
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Rainbow trout o-cresol NR 5-8 15 7-8 NR 48 LC50 13 Slooff et al.
(Oncorhynchus weeks NOEC 3.8 (1983)
mykiss)
o-cresol flow 7.9 cm 14 7.6-8.3 569-865 96 LC50 8.4 DeGraeve et al.
(1980)
m-cresol flow 7.9 cm 14 7.6-8.3 569-865 96 LC50 8.9 DeGraeve et al.
(1980)
p-cresol flow 7.3 cm 14 7.6-8.3 569-865 96 LC50 7.9 DeGraeve et al.
(1980)
Fathead minnow o-cresol NR 3-4 20 NR NR 48 LC50 34 Slooff et al.
(Pimephales weeks NOEC 30 (1983)
promelas)
o-cresol stat 17.9 mm; 25 7.7 47 96 LC50 14 Geiger et al.
29 days (1990)
o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 12.55 Pickering &
cm Henderson (1966)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
o-cresol stat 3.8-6.4 25 8.2 360 96 LC50 13.42 Pickering &
cm Henderson (1966)
o-cresol flow 5.0 cm 14 7.6-8.3 569-865 96 LC50 18.2 DeGraeve et al.
(1980)
m-cresol flow 4.9 cm 14 7.6-8.3 569-865 96 LC50 55.9 DeGraeve et al.
(1980)
p-cresol flow 5.2 cm 14 7.6-8.3 569-865 96 LC50 28.6 DeGraeve et al.
(1980)
p-cresol stat 4-8 18-22 < 5.9 soft 96 LC50 19 Mattson et al.
weeks (artificial) (1976)
1.1-3.1
cm
Fathead minnow p-cresol flow 28 days 24 7.8 48 96 LC50 16.5 Geiger et al.
(P. promelas) 20.9 mm (1986)
o-, m-, flow 29 days 25 7.6 46 96 LC50 12.8 Geiger et al.
p-cresol 20.8 mm (1990)
Bluegill o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 20.8 Pickering &
sunfish cm Henderson (1966)
(Lepomis
macrochirus)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Goldfish o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 23.25 Pickering &
(Carassius cm Henderson (1966)
auratus)
o-, m-, stat 60-90 mm 18-23 7.8 hard 5 days mortality 1.0 Ellis (1937)
p-cresol < 5 days NOEC 0.1
Mosquitofish o-, m-, stat adult 17-20 7.3-7.7 NR 96 LC50 22 Wallen et al.
(Gambusia p-cresol females (1957)
affinis)
Guppy o-cresol NR NR NR NR NR 48 LC50 38 Slooff et al.
(Poecilia NOEC 27 (1983)
reticulata)
o-cresol stat 1.9-2.5 25 7.5 20 96 LC50 18.85 Pickering &
cm Henderson (1966)
Golden orfe o-cresol NR NR NR NR NR 48 LC50 18 Slooff et al.
(Leuciscus (1983)
idus)
Japanese o-cresol NR 4-5 24 NR NR 48 LC50 41 Slooff et al.
killifish weeks NOEC 32 (1983)
(Oryzias
latipes)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Atlantic cod o-cresol stat eggs 5 NR NR 96 EC50 12 Falk-Petersen
(Gadus morhus) (development) et al. (1985)
m-cresol stat eggs 5 NR NR 96 EC50 > 30 Falk-Petersen
(development) et al. (1985)
Atlantic cod p-cresol stat eggs 5 NR NR 96 EC50 5 Falk-Petersen
(G. morhus) (development) et al. (1985)
Clawed toad o-cresol stat 3-4 20 NR NR 48 LC50 38 Slooff et al.
(Xenopus weeks NOEC 24 (1983);
laevis) Slooff &
Baerselman
(1980)
Salamander o-cresol stat 3-4 20 NR NR 48 LC50 40 Slooff et al.
(Ambystoma weeks NOEC 32 (1983);
mexicanum) Slooff &
Baerselman
(1980)
a Stat = static conditions (water unchanged for the duration of the test); flow = intermittent flow through conditions; NR = not reported
b LC50 = concentration resulting in lethality of 50% of the test animals; NOEC = no-observed-effect concentration; EC50 = concentration
resulting in effects among 50% of the test animals
Laboratory dose-response data for the species, investigated under
aerated conditions, showed that all species could withstand high
levels of p-cresol (up to 40 mg/litre) over a 96-h exposure period.
Thus the very high mortality observed in the experimental streams at a
p-cresol concentration of 8 mg/litre was due to the decreases in
dissolved oxygen and/or the synergistic effect of p-cresol and
dissolved oxygen on fish function.
9.4.2 Terrestrial
9.4.2.1 Laboratory studies
The acute oral toxicity for cresols was determined in
wild-trapped redwinged blackbirds (Agelaius phoeniceus)
preconditioned to captivity for 2-6 weeks and then dosed by gavage
with cresols formulated with propylene glycol over an 18-h period.
The LD50 values were calculated to be > 113 and > 96 mg/kg for
m- and p-cresol, respectively (Schafer et al., 1983).
9.4.2.2 Field studies
No data concerning field observations of the effects of cresols
on terrestrial vertebrates were present in the available literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Cresols consist either of a white crystalline solid or a
yellowish liquid. They have a phenolic-like odour and are freely
soluble in water. Cresols are found naturally in various plants and
oils and can be produced as combustion by-products from environmental
fires. They are also produced synthetically. Cresols have a wide
variety of uses as solvents and disinfectants or chemical
intermediates for pharmaceuticals, fragrances, antioxidants, dyes,
pesticides and resins.
Cresols have been detected in ambient air, surface- and
groundwater, and wastewater. They have also been detected in food and
beverages.
Cresols are rapidly absorbed by inhalation, ingestion and dermal
contact. They are readily distributed throughout the body. The
primary route of elimination is through the urine following
conjugation with glucuronides and sulfates.
The acute toxicity of cresols is mainly a consequence of their
strong irritant and corrosive activity. Toxic effects and clinical
signs following ingestion are burning of the mouth and throat,
abdominal pain and vomiting. More severe reactions may result in coma
and death. Target tissues and organs of ingested cresols in humans
are the blood, kidneys, lungs, heart, central nervous system and
liver. Inhalation of cresol vapour produces irritation of the nasal
membranes, throat and lungs. Acute poisoning during occupational
exposure is usually a result of dermal contact, which may result in
severe burns and scarring of the skin, haematological changes, kidney
failure, coma and death. There are very little data regarding
potential reproductive effects and no data on carcinogenicity in
humans. There is limited evidence to suggest that special populations
may be at greater risk from cresol exposure, e.g., individuals with
glucose-6-phosphate dehydrogenase deficiency or renal insufficiency
and the very young.
10.2 Evaluation of environmental risks
Cresols are present in the air, water and soil. They undergo a
number of chemical and biological reactions such as photolysis,
hydrolysis, oxidation and biodegradation. It appears, therefore, that
cresols will be relatively labile in the environment and will not
bioaccumulate to any significant extent. There are limited data
available on the levels of cresols in the ambient environment. A
median air concentration of 1.59 µg/m3 (0.359 ppb) has been reported
in the USA for source-dominated sites. Cresols have also been
detected in contaminated groundwater and surface water and at
hazardous waste sites. Observations on microorganisms, invertebrates
and fish are available and show that cresols may represent a risk to
non-mammalian organisms at point sources with high cresol
concentrations but not in the general environment.
10.3 Guidance value
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess carcinogenic hazard of cresols. Based on the results of
subchronic studies, a NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, applying the uncertainty factor of
300, an acceptable daily intake (ADI) of 0.17 mg/kg body weight per
day can be established for cresols.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
There is clear evidence in humans that, during dermal or oral
exposure, high concentrations of cresols are corrosive, absorbed
rapidly and produce severe toxicity that may result in death.
Inhalation may result in irritation of the respiratory tract. There
is no information regarding the chronic toxicity of cresols and no
adequate data regarding the carcinogenic potential of these compounds.
11.2 Recommendations
a) Cresols and mixtures containing cresols, including household
products, should be clearly labelled, warning of the acute
toxicity and corrosivity.
b) Cresols can readily penetrate the skin. Following direct
contact, contaminated areas should be washed immediately with
water and all contaminated clothing removed. Workers using
cresols or solutions containing cresols should wear protective
clothing.
c) Occupational exposure should be minimized.
12. FURTHER RESEARCH
There is a need for the following types of studies:
a) chronic toxicity and carcinogenicity studies in animals
b) studies on the toxic mechanisms of cresols
c) studies on workers occupationally exposed to cresols.
REFERENCES
Alexander M & Lustigman BK (1966) Effect of chemical structure on
microbial degradation of substituted benzenes. J Agric Food Chem,
14: 410-413.
Altmann HJ, Grunow W, Mohr U, Richter-Reichhelm HB, & Wester PW (1986)
Effects of BHA and related phenols on the forestomach of rats. Food
Chem Toxicol, 24(10/11): 1183-1188.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: Effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Arthurs GJ, Wise CC & Coles GA (1977) Poisoning by cresols.
Anesthesia, 32:642-643.
Artiola-Fortuny J & Fuller WH (1982) Adsorption of some
monohydroxybenzene derivatives by soils. Soil Sci, 133: 18-26.
Atkinson R (1985) Kinetics and mechanisms of the gas phase reactions
of the hydroxyl radical with organic compounds under atmospheric
condition. Chem Rev, 85: 69-201.
Atkinson R, Carter WPL, Darnall KR, Winer AM, & Pitts JN Jr (1980) A
smog chamber and modeling study of the gas phase NOx air
photo-oxidation of toluene and the cresols. Int J Chem Kinet,
12: 779-836.
Atkinson R, Carter WPL, Plum CN, Winer AM, & Pitts JN Jr (1984)
Kinetics of the gas-phase reactions of NO3 radicals with a series of
aromatics at 296±2 K. Int J Chem Kinet, 16: 887-898.
Atkinson R, Aschmann SM, & Avery J (1992) Reaction of OH and NO3
radicals with phenol, cresols and 2-nitrophenol at 296±K. Environ Sci
Technol, 26: 1397-1403.
Babeu L & Vaishnav DD (1987) Prediction of biodegradability for
selected organic chemicals. J Ind Microbiol, 2: 107-115.
Baird RB, Kuo CL, Shapiro JS, & Yanko WA (1974) The fate of phenolics
in wastewater - determination by direct-injection GLC and Warburg
respirometry. Arch Environ Contam Toxicol, 2: 165-178.
Bak F & Widdel F (1986) Anaerobic degradation of phenol and phenol
derivatives by Desulfobacterium phenolicum new species. Arch
Microbiol, 146(2): 177-180.
Balikova M & Kohlicek J (1989) Gas chromatography of simple phenolics
in biological fluids. J Chromatogr, 497: 159-167.
Bartholomew GW & Pfaender FK (1983) Influence of spatial and temporal
variations on organic pollutant biodegradation rates in an estuarine
environment. Appl Environ Microbiol, 45(1): 103-109.
Battersby NS (1990) A review of biodegradation kinetics in the aquatic
environment. Chemosphere, 21(10-11): 1243-1284.
Battersby NS & Wilson V (1988) Evaluation of a serum bottle technique
for assessing the anaerobic biodegradability of organic chemicals
under methanogenic conditions. Chemosphere, 17: 2441-2460.
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 55: 433-439.
Bayly RC & Wigmore GJ (1973) Metabolism of phenol and cresols by
mutants of Pseudomonas putida. J Bacteriol, 113: 1112-1120.
Bidleman TF (1988) Atmospheric processes. Environ Sci Technol,
22: 361-367.
Bio-Fax (1969) Toxicity data sheets for o-, p-, and m-cresol.
Northbrook, Illinois, Industrial Bio-Fax Laboratories, Inc.
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS205862).
Bone E, Tamm A, & Hill M (1976) The production of urinary phenols by
gut bacteria and their possible role in the causation of large bowel
cancer. Am J Clin Nutr, 29: 1448-1454.
Boutwell RK & Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer Res, 19: 413-424.
Boyd SA (1982) Adsorption of substituted phenols by soil. Soil Sci,
134: 337-343.
Boyd SA, Shelton DR, Berry D, & Tiedje JM (1983) Anaerobic
biodegradation of phenolic compounds in digested sludge. Appl Environ
Microbiol, 46: 50-54.
Bray HG, Thrope WV, & White K (1950) Metabolism of derivatives of
toluene. Biochem J, 46: 275-278.
Bringmann G & Kühn R (1976) Comparative results of the damaging
effects of water pollutants against bacteria (Pseudomonas putida)
and blue algae (Microcystis aeruginosa). Gas Wasserfach
Wasser-Abwasser, 117(9): 410-413.
Bringmann G & Kühn (1977) [Results of the damaging effect of water
pollutants on Daphnia magna.] Z Wasser Abwasser Forsch,
10(5): 161-166 (in German).
Bringmann G & Kühn R (1978a) [Limiting values for the noxious effects
of water pollutant material to blue algae (Microcystis aeruginosa) and
green algae (Scenedesmus).] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1978b) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) seruginosa and
Scenedesmus quadricauda. Mitt Int Ver Theor Angew Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14(3): 231-241.
Brown SC and Grady CPL Jr (1990) Biodegradation kinetics of
substituted phenolics: Demonstration of a protocol based on
electrolytic respirometry. Water Res, 24(7): 853-862.
BRRC (1988a) Developmental toxicity evaluation of o-, m-, or
p-cresol administered by gavage to Sprague Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Unpublished data submitted to
the US Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517695).
BRRC (1988b) Developmental toxicity evaluation of o-, m-, or
p-cresol administered by gavage to New Zealand white rabbits.
Export, Pennsylvania, Bushy Run Research Center (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517695).
BRRC (1989a) Two-generation reproduction study of o-cresol (CAS No.
95-48-7) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 51-614)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
BRRC (1989b) Two-generation reproduction study of p-cresol (CAS No.
106-44-5) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 52-512)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
BRRC (1989c) Two-generation reproduction study of m-cresol (CAS No.
108-39-4) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 51-634)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
Bulich AA & Isenberg DL (1980) Use of the luminescent bacterial system
for the rapid assessment of aquatic toxicity. Adv Instrum,
35(2): 35-40.
Bulich AA & Isenberg DL (1981) Use of the luminescent bacterial system
for the rapid assessment of aquatic toxicity. ISA Trans, 20(1): 29-33.
Bulich AA, Greene MW, & Isenberg DL (1981) Reliability of the
bacterial luminescence assay for determination of the toxicity of pure
compounds and complex effluents. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 338-347 (ASTM Special Technical
Publication No. 737).
Campbell I (1941) Petroleum cresylic acids: A study of their toxicity
and the toxicity of cresylic disinfectants. Soap Sanit Chem,
17(4): 103.
Carter WPL, Winer AM, & Pitts JN Jr (1981) Major atmospheric sink for
phenol and the cresols: Reaction with the nitrate radical. Environ Sci
Technol, 15(7): 829-831.
Cason JS (1959) Report on three extensive industrial chemical burns.
Br Med J, 1: 827-829.
Cautreels W & van Cauwenberghe K (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos Environ, 12: 1133-1141.
Chambers CW, Tabak HH, & Kabler PW (1963) Degradation of aromatic
compounds by phenol-adapted bacteria. J Water Pollut Contr Fed,
35: 1517-1528.
Chan TK, Mak LW, & Ng RP (1971) Methemoglobinemia, Heinz bodies and
acute massive intravascular hemolysis in Lysol poisoning. Blood,
38: 739-744.
Cheng M & Kligerman AD (1984) Evaluation of the genotoxicity of
cresols using sister-chromatid exchange (SCE). Mutat Res,
137(1): 51-55.
Chudyk WA, Carrabba MN, & Kenny JE (1985) Remote detection of
groundwater contaminants using far-UV laser-induced fluorescence. Anal
Chem 57(7): 1237-1242.
CIIT (1983) Preliminary results of in vivo and in vitro sister
chromatid exchange assays on cresol isomers and of an immunological
evaluation of o-cresol. Research Triangle Park, North Carolina,
Chemical Industry Institute of Toxicology (Report to the US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances) (CIIT Docket No. 12283).
Clark RM, Goodrich JA, & Deininger RA (1986) Drinking water and cancer
mortality. Sci Total Environ, 53(3): 153-172.
Cooper WE & Stout RJ (1982) Assessment of transport and fate of toxic
materials in an experimental stream ecosystem. In: Dickson KL ed.
Modelling the fate of chemicals in the aquatic environment. Pellston
Conference III. Ann Arbor, Michigan, Ann Arbor Science Publications,
pp 347-378.
Cote MA, Lyonnais J, & Leblond PF (1984) Acute Heinz-body anemia due
to severe cresol poisoning: Successful treatment with
erythrocytapheresis. Can Med Assoc J, 130(10): 1319-1322.
Czuczwa J, Leuenberger C, Tremp J, & Giger W (1987) Determination of
trace levels of phenol and cresols in rain by continuous liquid-liquid
extraction and high-performance liquid chromatography. J Chromatogr,
403: 233-241.
Daugherty JP & Franks H (1986) Effect of monocyclic derivatives on DNA
repair in human lymphocytes. Res Commun Chem Pathol Pharmacol,
54(1): 133-136.
Davis JA (1981) Comparison of static-replacement and flow-through
bioassays using duckweed, lemna gibba G-3. Washington, DC, US
Environmental Protection Agency (EPA 560/6-81-003; NTIS PB81-187650).
DeGraeve GM, Geiger DL, Meyer JS, & Bergman HL (1980) Acute and
embryo-larval toxicity of phenolic compounds to aquatic biota. Arch
Environ Contam Toxicol, 9(5): 557-568.
DeWolf WE Jr, Carr SA, Varrichio A, Goodhart PJ, Mentzer MA, Roberts
GD, Southan C, Dolle RE, & Kruse LI (1988) Inactivation of dopamine
beta-hydroxylase by p-cresol: Isolation and characterisation of
covalently modified active site peptides. Biochemistry, 27: 9093-9102.
Deichmann W & Keplinger ML (1981) Phenols and phenolic compounds. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, vol 2A, pp 2567-2627.
Deichmann WB & Witherup S (1944) Phenolic studies. VI: The acute and
comparative toxicity of phenol and o-, m-, and p-cresols for
experimental animals. J Pharmacol Exp Ther, 80: 233-240.
DeRosa E, Bartolucci GB, Sigon M, Callegaro R, Perbellini L, &
Brugnone F (1987) Hippuric acid and ortho-cresol as biological
indicators of occupational exposure to toluene. Am J Ind Med,
11(5): 529-537.
Devillers J (1988) Acute toxicity of cresols, xylenols and trimethyl
phenols to Daphnia magna straus 1820. Sci Total Environ, 76: 79-83.
Dietz F & Traud J (1978) [Odour and taste thresholds. Concentration of
phenol bodies.] GWF-Wasser/Abwasser 119(6) (in German).
Dobbins DC & Pfaender FK (1988) Methodology for assessing respiration
and cellular incorporation of radiolabeled substrates by soil
microbial communities. Microbiol Ecol, 15: 257-273.
Dobson KR, Stephenson M, Greenfield PF, & Bell PRF (1985)
Identification and treatability of organics in oil shale retort water.
Water Res 19: 849-856.
Douglas GR, Nestmann ER, Betts JL, Mueller JC, Lee EGH, Stich HF, San
RHC, Brouzes RJP, Chmelauskas AL, Paavile HD, & Walden CC (1980)
Mutagenic activity in pulp mill effluents. Water Chlorination: Environ
Impact Health Eff, 3: 865-880.
Dow Chemical (1978) Acute toxicological properties and industrial
handling hazards of cresol (ortho, meta, para isomers). Midland,
Michigan, Dow Chemical Company (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS206146).
Dreibelbis WG, Ealy JA, & Porter WE (1985) Industrial hygiene
monitoring for evaluation of employee exposure and control measures in
coal conversion program at Oak Ridge National Laboratory. In: Cooke M
& Dennis AJ ed. Polynuclear aromatic hydrocarbons: Mechanisms,
methods and metabolism. Columbus, Ohio, Battelle Press, pp 351-363.
Druyan EA (1974) [Separate determination of phenol and o-, m-, and
p-cresol in air with the aid of thin-layer chromatography.] Gig i
Sanit, 8: 51-53 (in Russian).
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol,
15: 30-38.
Ellis MM (1937) Detection and measurement of stream pollution.
Washington, DC, US Department of Commerce, Government Printing Office,
pp 365-437 (Bureau of Fisheries Bulletin No. 22).
Ellis DD, Jone CM, Larson RA, & Schaeffer DJ (1982) Organic
constituents of mutagenic secondary effluents from wastewater
treatment plants. Arch Environ Contam Toxicol, 11: 373-382.
Emery RM (1970) The comparative acute toxicity of cresol to two
benthic crustaceans. Water Res, 4: 485-491.
Evangelista RA, Allen HL, & Mandel RM (1990) Treatment of phenol and
cresol contaminated soil. In: Characterization and cleanup of chemical
waste sites. Symposium of the 197th National Meeting of the American
Chemical Society, Dallas, TX, April 10, 1989. J Hazard Mater,
25(3): 343-360.
Falk-Petersen IB, Kjorsvik E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Faust BC & Hoigné J (1987) Sensitized photooxidation of phenols by
fulvic acid in natural waters. Environ Sci Technol, 21: 957-964.
FDRL (1975) Acute toxicity studies of ortho-cresol in rats and
rabbits. Saddle Brook, New Jersey, Food and Drug Research Laboratories
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS206095).
Fedorak PM & Hrudey SE (1984) The effects of phenol and some alkyl
phenolics on batch anaerobic methanogenesis. Water Res, 18: 361-367.
Fedorak PM & Hrudey SE (1986) Nutrient requirements for the
methanogenic degradation of phenol and p-cresol in anaerobic draw
and feed cultures. Water Res, 20(7): 929-933.
Fiege H & Bayer AG (1987) Cresols and xylenols. In: Gerharte W ed.
Ullman's encyclopedia of industrial chemistry, 5th ed. New York, VCH
Publishers, vol 8A, pp 25-59.
Finzer KH (1961) Lower nephron nephrosis due to concentrated Lysol
vaginal douches: A report of two cases. Can Med Assoc J, 84: 549.
Fiserova-Bergerova V, Pierce JT, & Droz PO (1990) Dermal absorption
potential of industrial chemicals: Criteria for skin notation. Am J
Ind Med, 17(5): 617-636.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15(3): 219-232.
Flyvbjerg J, Arvin E, Jensen BK, & Olsen SK (1993) Microbial
degradation of phenols and aromatic hydrocarbons in
creosote-contaminated groundwater under nitrate-reducing conditions. J
Contam Hydrol, 12 :133-150.
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, & Korte F (1982) Ecotoxicological profile analysis: VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicol Environ Saf, 60: 60-81.
Gadaskina ID & Filov VA (1971) Transformations and determination of
industrial poisons in the human body. Leningrad, Meditsina,
pp 202-205.
Gaffney JS, Streit GE, Spall WD, & Hall JH (1987) Beyond acid rain: Do
soluble oxidants toxins interact with SO2 and NOx to increase
ecosystem effects? Environ Sci Technol, 21(6): 519-523.
Gantzer CJ, Kollig HP, Rittmann BE, & Lewis DL (1988) Predicting the
rate of trace-organic compound removal by natural biofilms. Water Res,
22: 191-200.
Garrett JS (1975) Association between bladder tumors and chronic
exposure to cresols and cresote (letter). J Occup Med, 17: 492.
Gaur JP (1988) Toxicity of some oil constituents to Selenastrum
capricornutum. Acta Hydrochim Hydrobiol, 16(6): 617-620.
Geiger DL, Poirier SH, Brooke LT, & Call DJ (1986) Acute toxicities of
organic chemicals to fathead minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 3, pp 1-24, 165-166.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 5, pp 1-24, 157-160.
Giabbai MF, Cross WH, Chian ESK, & DeWalle FB (1985) Characterization
of major and minor organic pollutants in wastewaters from coal
gasification processes. Int J Environ Anal Chem, 20: 113-129.
Goerlitz DF, Troutman DE, Godsy EM, & Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environ Sci Technol, 19: 955-961.
Goodley PC & Gordon M (1976) Characterization of industrial organic
compounds in water. Trans Ky Acad Sci, 37: 11-15.
Gosselin RE, Hodge HC, Smith RP, & Gleason MN (1976) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams
Wilkins & Co.
Great Lakes Water Quality Board (1983) An inventory of chemical
substances identified in the Great Lakes ecosystem. Volume 1: Summary,
pp 1-195 (Report to the Great Lakes Water Quality Board, Windsor,
Ontario, Canada).
Green MA (1975) A household remedy misused-fatal cresol poisoning
following cutaneous absorption (a case report). Med Sci Law,
15: 65-66.
Grosjean D (1984) Atmospheric reactions of ortho cresol: Gas phase and
aerosol products. Atmos Environ, 18: 1641-1652.
Grosjean D (1985) Reactions of o-cresol and nitrocresol with NOx
in sunlight and with ozone-nitrogen dioxide mixtures in the dark.
Environ Sci Technol, 19(10): 968-974.
Hampton CV, Pierson WR, Harvey TM, Upolegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road: I. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol,
16: 287-298. Hansch C & Leo AJ (1985) Medchem Project: Issue 26.
Claremont, California, Pomona College.
Hassan SM, Salem FB, & El-Salam NA (1987) Colorimatric determination
of phenols in water samples. Anal Lett, 20(5): 677-688.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil wastewaters. Environ Sci Technol, 18: 483-490.
Hawthorne SB, Miller DJ, Barkley RM, & Krieger MS (1988)
Identification of methoxylated phenols as candidate tracers for
atmospheric wood smoke pollution. Environ Sci Technol,
22(10): 1191-1196.
Hawthorne SB, Krieger MS, Miller DJ, & Mathiason MB (1989) Collection
and quantitation of methoxylated phenol tracers for atmospheric
pollution from residential wood stoves. Environ Sci Technol,
23(4): 470-475.
HAZDAT (1992) Hazardous substances database. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry, Exposure and Disease
Registry Branch, Office of External Affairs.
Hazleton Labs (1988a) Mutagenicity tests on o-, m-, and p-cresol
in an in vitro cytogenetic assay measuring chromosomal aberration
frequencies in CHO cells. Kensington, Maryland, Hazleton Laboratories
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS0517691).
Hazleton Labs (1988b) Mutagenicity tests on o-cresol in the
in vitro transformation of BALB/C-3T3 cells assay in the presence of
rat liver cell activation system. Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the US Environmental
Protection Agency, Office of Toxic Substances) (Fiche No. OTS0517697).
Hazleton Labs (1988c) Mutagenicity tests of p-cresol and m-cresol
in a mouse lymphoma mutation assay. Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the US Environmental
Protection Agency, Office of Toxic Substances) (Fiche No. OTS0517693).
Hazleton Labs (1988d) Mutagenicity tests on meta-cresol and
para-cresol in the in vitro transformation of BALB/C-3T3 cells
assay. Kensington, Maryland, Hazleton Laboratories (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517694).
Hazleton Labs (1988e) Mutagenicity tests on meta-cresol in a rat
primary hepatocyte unscheduled DNA synthesis assay. Kensington,
Maryland, Hazleton Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517692).
Hazleton Labs (1988f) Mutagenicity tests on m-cresol in the
in vitro transformation of BALB/C-3T3 cells assay. Kensington,
Maryland, Hazleton Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517698).
Hazleton Labs (1989a) Dominant lethal assay in mice: ortho-Cresol
CRE-9.1-DL-HLA (HLA study No. 10004-0-471). Kensington, Maryland,
Hazleton Laboratories (Unpublished data submitted to the Chemical
Manufacturers Association, Washington).
Hazleton Labs (1989b) Dominant lethal assay in mice: para-Cresol
CP945 (HLA study No. 10003-0-471). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Hazleton Labs (1989c) Mutagenicity test on cresol program panel sample
#2 meta-cresol in the mouse bone marrow cytogenetic assay (HLA study
No. 10002-0-451). Kensington, Maryland, Hazleton Laboratories
(Unpublished data submitted to the Chemical Manufacturers Association,
Washington).
Hazleton Labs (1989d) Mutagenicity test on ortho-cresol (lot number
RC645A) Drosophila melanogaster sex-linked recessive lethal test
(HLA study No. 10004-0-461). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Hazleton Labs (1989e) Mutagenicity test on para-cresol (lot number
1206, batch 807) Drosophila melanogaster sex-linked recessive lethal
test (HLA study No. 10003-0-461). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Heikkila PR, Hameila M, Pyy L, & Raunu P (1987) Exposure to creosote
in the impregnation and handling of impregnated wood. Scand J Work
Environ Health, 13: 431-437.
Herwick RP & Treweek DN (1933) Burns from anesthesia mask sterilized
in compound solution of cresol. J Am Med Assoc, 100: 407-408.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds: Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinz RS, Lorence CR, Hodson CD, Hansch C, Hall LL, & Guy RH (1991)
Percutaneous penetration of para-substituted phenols in vitro. Fundam
Appl Toxicol, 17: 575-583.
Hirose M, Inoue T, Asamoto M, Tagawa Y, & Ito N (1986) Comparison of
the effects of 13 phenolic compounds in induction of proliferative
lesions of the forestomach and increase in the labelling indices of
the glandular stomach and urinary bladder epithelium of Syrian golden
hamsters. Carcinogenesis, 7(8): 1285-1289.
Hites RA (1979) Sources and fates of industrial organic chemicals: A
case study. In: Proceedings of the 8th National Conference on
Municipal Sludge Management. Silver Spring, Maryland, Information
Transfer, Inc., pp 107-119.
Ho CT, Lee KN, & Jin QZ (1983) Isolation and identification of
volatile flavor compounds in fried bacon. J Agric Food Chem,
31: 336-342.
Hoffman WA Jr & Tanner RL (1986) Detection of organic acids in
atmosphere precipitation. Upton, New York, Brookhaven National
Laboratory, Environmental Chemistry Division, Department of Applied
Science (Report to the US Department of Energy) (BNL-51922;
NTIS DE8 005294).
Hopper DJ (1976) The hydroxylation of p-cresols and its conversion
to p-hydroxybenzaldehydreu in Pseudomonas putida. Biochem Biophys
Res Commun, 69(2): 462-468.
Hopper DJ (1978) Incorporation of [18O] Water in the formation of
p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from
Pseudomonas putida. Biochem J, 75: 345-347.
Hopper DJ & Taylor DG (1977) The purification and properties of
p-cresol-(acceptor) oxidoreductase (hydroxylating), a
flavocytochrome from Pseudomonas putida. Biochem J, 167(1): 155-162.
Hornshaw TC, Aulerich RJ, & Ringer RK (1986) Toxicity of o-cresol to
mink and European ferrets. Environ Toxicol Chem, 5(8): 713-720.
Horowitz A, Shelton DR, Cornell CP, & Tiedje JM (1982) Anaerobic
degradation of aromatic compounds in sediments and digested sludge.
Dev Ind Microbiol, 23: 435-444.
Hoshika Y & Muto G (1978) Gas-liquid-solid chromatographic
determination of phenols in air using Tenax-GC and alkaline
precolumns. J Chromatogr, 187: 277-284.
Huang JC & Gloyna EF (1968) Effect of organic compounds on
photosynthetic oxygenation: I. Chlorophyll destruction and suppression
of photosynthetic oxygen production. Water Res, 2: 347-366.
Hwang HM, Hodson RE, & Lewis DL (1989) Microbial degradation kinetics
of toxic organic chemicals over a wide range of concentrations in
natural aquatic systems. Environ Toxicol Chem, 8: 65-74.
ILO (1991) Occupational exposure limits for airborne toxic substances.
Geneva, International Labour Office.
Isaacs R (1922) Phenol and cresol poisoning. Ohio State Med J,
18: 558-561.
Izard PA, Fail PA, George JD, Grizzle TB, & Heindel JJ (1992)
Reproductive toxicity of cresol isomers administered in feed to mouse
breeding pairs. Toxicologist, 12: 198 (Abstract).
James RH, Adams RE, Finkel JM, Miller HC, & Johnson LD (1984)
Evaluation of analytical methods for the determination of POHC on
combustion products. In: Proceedings of the 77th Annual Meeting of
the Air Pollution Control Association, San Francisco, California,
24-29 June. Research Triangle Park, North Carolina, Industrial
Research Laboratory, pp 1-25 (NTIS/PB84-246289).
Johnson LD, Midgett MR, James RH, Thomason MM, & Manier ML (1989)
Screening approach for principal organic hazardous constituents and
products of incomplete combustion. J Air Pollut Control Assoc,
39(5): 709-713.
Jouglard J, Aquaron R, Gatua-Pelanchon J, Trigano A, & Bel JG (1971)
Intoxications aiguës par un antiseptique ménager: le crésyl. Mars Méd,
108: 425-431.
Jungclaus GA, Lopez-Avila, & Hites RA (1978) Organic compounds in an
industrial waste water: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Junk GA & Ford CS (1980) A review of organic emissions from selected
combustion processes. Chemosphere, 9: 187-230.
Kavlock RJ (1990) Structure-activity relationships in the
developmental toxicity of substituted phenols: In vivo effects.
Teratology, 41: 43-49.
Kawamura K & Kaplan IR (1986) Compositional change of organic matter
in rainwater during precipitation events. Atmos Environ,
20(3): 527-536.
Keat MJ & Hopper DJ (1978) The aromatic alcohol dehydrogenase in
Pseudomonas putida N.C.I.B. 9869 grown on 3,5-xylenol and p-cresol.
Biochem J, 175(2): 959-967.
Khalal KD, Hasan BA, Moralesrubio A, & Delaguardia M (1993)
Flow-injection spectrophotometric determination of cresol compounds in
water by raction with p-aminophenol. Mikrochim Acta,
112(1-4): 99-111.
Klecka GM, Davis JW, Gray DR, & Madsen SS (1990) Natural
bioremediation of organic contaminants in ground water: Cliffs-Dow
Superfund Site. Ground Water, 28(4): 534-543.
Klinger ME & Norton JF (1945) Toxicity of cresylic acid-containing
solvent. US Navy Med Bull, 44(2): 438-439.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77(2-3): 269-276.
Koerber SC, McIntire W, Bohmont C, & Singer TP (1985) Resolution of
the flavocytochrome p-cresol methylhydroxylase in subunits and
reconstitution of the enzyme. Biochemistry, 24: 5276-5280.
Kollig HP, Parrish RS, & Holm HW (1987) An estimate of the variability
in biotransformation kinetics of xenobiotics in natural waters by
Aufwuchs communities. Chemosphere, 16: 49-60.
Krotoszynski BK & O'Neill HJ (1982) Involuntary bioaccumulation of
environmental pollutants in nonsmoking heterogenous human population.
J Environ Sci Health Part A Environ Sci Eng, 17(6): 855-883.
Kühn R & Pattard M (1990) Results of the harmful effects of water
pollutants to green algae (Scenedesmus subspicatus) in the cell
multiplication inhibition test. Water Res, 24(1): 31-38.
Kühn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kühn R, Pattard M, Pernak KD, & Winter A (1989a) Results of the
harmful effects of water pollutants to Daphnia magna in the 21 day
reproduction test. Water Res, 23(4): 501-510.
Kühn R, Pattard M, Pernak KD, & Winter A (1989b) Results of the
harmful effects of selected water pollutants (anilines, phenols,
alphatic compounds) to Daphnia magna. Water Res, 23(4): 495-499.
Kurlyandsky BA, Partsef DP, & Chernomorskiy AR (1975) [A procedure for
determining the mean daily maximum permissible concentration of
tricresol in atmospheric air.] Gig i Sanit, 5: 85-87 (in Russian).
Kushi A & Yoshida D (1987) Antimutagenic effects of phenols on MNNG -
induced mutagenesis in Escherichia coli. Agric Biol Chem,
51: 1439-1440.
Kuwata K & Tanaka S (1988) Liquid chromatographic determination of
traces of phenols in air. J Chromatogr, 442: 407-411.
Kuwata K, Uebori M, & Yamazaki Y (1981) Reversed-phase liquid
chromatographic determination of phenols in auto exhaust and tobacco
smoke as p-nitrobenzeneazophenol derivatives. Anal Chem,
53(9): 1531-1534.
Kwasniewski K & Kaiser KLE (1983) Toxicities of selected phenols to
fermentative and oxidative yeasts. Bull Environ Contam Toxicol,
31(2): 188-194.
Labram C & Gervais P (1968) Un cas d'intoxication massive par le
crésyl. Sem Hop Paris, 44: 3029-3031.
Larcan A, Lambert H, & Laprevote-Heully MC (1974) Intoxication aiguë
par le crésyl, à propos d'une observation avec hémolyse aiguë massive,
méthémoglobinémie et corps de Heinz. Eur J Toxicol, 7: 5-8.
Lee WY & Nicol JAC (1978) Individual and combined toxicity of some
petroleum aromatics to the marine amphipod Elasmopus pectenicrus.
Mar Biol, 48(3): 215-222.
Lehtonen M (1983) Gas-liquid chromatographic determination of volatile
phenols in matured distilled alcoholic beverages. J Assoc Off Anal
Chem, 66(1): 62-70.
Leone JA, Flagan RC, Grosjean D, & Seinfeld JH (1985) An outdoor smog
chamber and modeling study of toluene-NOx photooxidation. Int J Chem
Kinet, 17(2): 177-216.
Leuenberger C, Ligocki MP, & Pankow JF (1985) Trace organic compounds
in rain. 4. Identities, concentrations, and scavenging mechanisms
for phenols in urban air and rain. Environ Sci Technol,
19(11): 1053-1058.
Lewis DL, Holm HW, & Hodson RE (1984) Application of single and
multiphasic Michaelis-Menten kinetics to predictive modeling for
aquatic ecosystems. Environ Toxicol Chem, 3: 563-574.
Lewis DL, Kollig HP, & Hodson RE (1986) Nutrient limitation and
adaptation of microbial populations to chemical transformations. Appl
Environ Microbiol, 51: 598-603.
Liberti A, Goretti G, & Russo MV (1983) PCDD and PCDF formation in the
combustion of vegetable wastes. Chemosphere, 12: 661-663.
Litton Bionetics (1980) Sister chromatid exchange assay, Ames assay,
mouse lymphoma forward mutation assay, and transformation assay for a
sample containing 33-1/3% each ortho-, meta-, and para-cresol.
Kensington, Maryland, Litton Bionetics, Inc. (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517528).
Litton Bionetics (1981) Sister chromatid exchange assay, Ames assay,
mouse lymphoma forward mutation assay, and cell transformation on
o-cresol. Unpublished data submitted to EPA/OTS
(Fiche no. OTS0517531).
Liu D & Pacepavicius G (1990) A systematic study of the aerobic and
anaerobic biodegradation of 18 chlorophenols and 3 cresols. Toxic
Assess Int J, 5(4): 367-388.
Ludzack FJ & Ettinger MB (1960) Chemical structures resistant to
aerobic biochemical stabilization. J Water Pollut Control Fed,
32: 1173-2000.
Lund FA & Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acids. J Gen Appl
Microbiol, 30: 53-61.
Lyman WJ, Reehl WF, & Rosenblatt DH ed (1982) Handbook of chemical
property estimation methods. New York, McGraw Hill Book Co.,
chapter 15.
Lyman WJ, Reehl WF, & Rosenblatt DH ed (1990) Handbook of chemical
property estimation methods: environmental behavior of organic
compounds. Washington, DC, American Chemical Society, pp 15-16.
Ma L & Wang CC (1989) [Case report of cresol burn and poisoning.] Chin
J Ind Hyg Occup Dis, 7: 219-220 (in Chinese).
Ma SH, Ma CS & Lin RC (1982) [Clinical studies 83 cases of chemical
burns.] Chin J Dermatol, 15(1): 9-10 (in Chinese).
McCreary JJ, Jackson JG, & Zoltek J Jr (1983) Toxic chemicals in an
abandoned phenolic waste site. Chemosphere, 12: 1619-1632.
McIntire W & Singer TP (1982) Resolution of p-cresol
methylhydroxylase into catalytically active subunits and
reconstitution of the flavocytochrome. FEBS Lett, 143(2): 316-318.
McIntire W, Edmonson DE, Hopper DJ, & Singer TP (1981) 8
alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group
of bacterial p-cresol methylhydroxylase. Biochemistry,
20(11): 3068-3075.
McIntire W, Hopper DJ, Craig JC, Everhart ET, Webster RV, Causer MJ, &
Singer TP (1984) Stereochemistry of 1-(4'-hydroxyphenyl)ethanol
produced by hydroxylation of 4-ethylphenol by p-cresol
methylhydroxylase. Biochem J, 224(2): 617-621.
McIntire W, Hopper DJ, & Singer TP (1985) p-Cresol
methylhydroxylase. Assay and general properties. Biochem J,
228(2): 325-335.
McKinney RE, Tomlinson HD, & Wilcox RL (1956) Metabolism of aromatic
compounds by activated sludge. Sew Ind Wastes, 28: 547-557.
McKnight DM, Pereira WE, Ceazan ML, & Wissmar RC (1982)
Characterization of dissolved organic materials in surface waters
within the blast zone of Mount St. Helens, Washington. Org Geochem,
4: 85-92.
McIntire W, Singer TP, Smith AJ, & Matthews FS (1986) Amino acid and
sequence analysis of the cytochrome and flavoprotein subunits of
p-cresol methylhydroxylase. Biochemistry, 25:5975-5981.
McLeese DW, Zitko V, & Peterson MR (1979) Structure-lethality
relationships for phenols, anilines and other aromatic compounds in
shrimp and clams. Chemosphere, 8(2): 53-57.
Malaney GW (1960) Oxidative abilities of aniline-acclimated activated
sludge. J Water Pollut Control Fed, 32: 1300-1311.
Malaney GW & McKinney RE (1966) Oxidative abilities of
benzene-acclimated activated sludge. Water Sew Works, 113: 302-309.
Manita MD (1966) [Analysis of the vapors of monohydric phenols
(phenol, o-, m-, and p-cresol) in atmospheric air by the
ultraviolet spectrophotometric method.] Gig i Sanit 8: 60-63
(in Russian).
Masunaga S, Urushigawa Y, & Yonezawa Y (1983) Microbial transformation
of o-cresol to dihydroxytoluenes by phenol acclimated activated
sludge. Chemosphere, 12: 1075-1082.
Masunaga S, Urishigawa Y, & Yonezawa Y (1986) Biodegradation pathway
of o-cresol by heterogeneous culture. Phenol activated sludge.
Water Res, 20: 477-484.
Matsumoto H, Kuwabara K, Murakami Y, Nishimune T, & Sueki K (1989)
Cresol isomers contaminating beef on the market. J Food Hyg Soc Jpn,
30(3): 250-253.
Mattsson JL, Albee RR, & Gorzinski SJ (1989) Similarities of toluene
and o-cresol neuroexcitation in rats. Neurotoxicol Teratol,
11(1): 71-75.
Mattson VR, Arthur JW, Walbridge CT (1976) Acute toxicity of selected
organic compounds to fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-76-097).
MBA (1988a) Subchronic toxicity of ortho-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to the US Environmental Protection Agency).
MBA (1988b) Subchronic toxicity of para-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to US Environmental Protection Agency).
MBA (1988c) Subchronic toxicity of meta-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to the US Environmental Protection Agency).
Medvedev VA & Davidov VD (1981a) The influence of isomers on the
transformation rate of phenols in Chernozem soil. In: Overcash MR ed.
Decomposition of toxic and nontoxic organic compounds in soil. Ann
Arbor, Michigan, Ann Arbor Scientific Publishers, pp 175-181.
Medvedev VA & Davidov VD (1981b) The transformation of various coke
industry products in Chernozem soil. In: Overcash MR ed. Decomposition
of toxic and nontoxic organic compounds in soil. Ann Arbor, Michigan,
Ann Arbor Scientific Publishers, pp 245-254.
Mellon Institute (1949) The acute toxicity of m-cresol. Pittsburgh,
Pennsylvania, Mellon Institute of Industrial Research (Unpublished
data submitted to the US Environmental Protection Agency, Office of
Toxic Substances) (Fiche No. OTS0517523).
Minami M, Katzumata M, & Tomoda A (1990) Methemoglobinemia with
oxidized hemoglobins and modified hemoglobins found in bloods of
workers handling aromatic compounds and in those of man who drank
cresol solution. Biomed Biochim Acta, 49(2-3): 5327-5333.
Molodkina NN, Gabulgalimova RR, Umarova SI, & Matveev AA (1985)
Hygienic evaluation of the combined effect of some organic solvents
(chlorobenzene and tricresol). In: Kasparova AA ed. Methodological
principles for ensuring healthier working conditions at industrial
plants with a leading chemical factor. Moscow, Research Institute of
Labor Hygiene and Occupational Diseases, Academy of Medical Sciences,
pp 82-88.
Moore SP & Coohill TP (1983) An SV40 mammalian inductest for putative
carcinogens. Prog Nucleic Acid Res Mol Biol, 29: 149-153.
Murray KE & Adams RF (1988) Determination of simple phenols in feces
and urine by high performance liquid chromatography. J Chromatogr
Biomed Appl, 431(1): 143-149.
Namkoong W, Loehr RC, & Malina JF Jr (1988) Kinetics of phenolic
compounds removal in soil. Hazard Waste Hazard Mater, 5(4): 321-328.
Needham LL, Head SL, & Cline RE (1984) Determination of phenols and
cresols in urine by gas chromatography. Anal Lett, 17(B14): 1555-1565.
Neiminen E & Heikkila P (1986) Simultaneous determination of phenol,
cresols and xylenols in workplace air, using a
polystyrene-divinylbenzene column and electrochemical detection. J
Chromatogr, 360(1): 271-278.
Neufeld RD, Debes MR, Moretti C, Mayernik J, Keleti G, Sykora J, &
Bender J (1985) Cooling tower evaporation of treated coal gasification
wastewaters. J Water Pollut Control Fed, 57(9): 955-964.
Niwa T (1993) Phenol and p-cresol accumulated in uremic serum
measured by HPLC with fluorescence detection. Clin Chem, 39:108-111.
Oglesby LA, Ebron-McCoy MT, Logsdon TR, Copeland F, Beyer PE, &
Kavlock RJ (1992) In vitro embryotoxicity of a series of
para-substituted phenols: Structure, activity, and correlation with
in vivo data. Teratology, 45(1): 11-34.
Oliveira DP & Sitar N (1985) Ground water contamination from
underground solvent storage tanks, Santa Clara, California. In:
Proceedings of the 5th National Symposium on Aquifer Restoration and
Ground Water Monitoring. Richmond, California, Cooper Engineers, Inc.
and Berkeley, California, Department of Civil Engineering, University
of California, pp 691-708.
Palumbo AV, Pfaender FK, & Paerl HW (1988) Biodegradation of NTA and
m-cresol in coastal environments. Environ Toxicol Chem, 7: 573-585.
Paris DF, Wolfe NL, Steen WC, & Baughman GL (1983) Effect of phenol
molecular structure on bacterial transformation rate constants in pond
and river samples. Appl Environ Microbiol, 45: 1153-1155.
Parker JG (1984) The effects of selected chemicals and water quality
on the marine polychaete Ophryotrocha diadema. Water Res,
18(7): 865-868.
Parkhurst BR, Bradshaw AS, Forte JL, & Wright GP (1979) An evaluation
of the acute toxicity to aquatic biota of a coal conversion effluent
and its major components. Bull Environ Contam Toxicol, 23(3): 349-356.
Parrish CF (1983) Solvents, industrial. In: Grayson M ed.
Kirk-Othmer's encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 21, pp 386-387.
Pashkova GA (1972) [Special effects of cresol and phosphoryl chloride
on the endocrine glands.] Kuibyshev, USSR, Research Institute of
Epidemiology, Microbiology and Hygiene, pp 203-204 (Scientific
Publication No. 7) (in Russian).
Pashkova GA (1973) [Comparative evaluation of the gonadotrophic and
general toxic effect of tricresol, phosophoryl chloride and
tricresylphosphate.] In: [Problems in labour hygiene, occupational
pathology and toxicology in the production and testing of
phosphor o-organic plasticizers.] Moscow, pp 86-90.
Pauli O & Franke G (1971) Behaviour and degradation of technical
preservatives in the biological purification of sewage. Biodeterior
Mater Proc Int Biodeterior Symp 2nd, 1971: 52-60.
Pegg SP & Campbell DC (1985) Children's burns due to cresol. Burns,
11(4): 294-296.
Pereima VL (1975) Inhalational effect of cresol isomers at low
concentrations and means for improving detoxication processes in
experiments on white rats. Lvov Univesity, pp 86-90 (Dissertaton).
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38(9): 1419-1429.
Pitter P (1976) Determination of biological degradability of organic
substances. Water Res, 10: 231-235.
Pool BL & Lin PZ (1982) Mutagenicity testing in the Salmonella
typhimurium assay of phenolic compounds and phenolic fractions
obtained from smokehouse smoke condensates. Food Chem Toxicol,
20(4): 383-391.
Pool BL, Yalkinoglu AO, Klein P, & Schlehofer JR (1989) DNA
amplification in genetic toxicology. Mutat Res, 213:61-72.
Presley JA & Brown WE (1956) Lysol-induced criminal abortion. Obstet
Gynecol, 8: 368-370.
Ram NM, Exner P, Bell R, & Santos S (1985) Feasibility of treating
contaminated ground water at a hazardous waste site. In: Proceedings
of the NWWA/API Conference on Petroleum Hydrocarbons and Organic
Chemicals in Ground Water Prevention, Detection and Restoration,
Houston, Texas, 13-15 November 1985. Dublin, Ohio, National Water Well
Association, pp 513-534.
Reimann SP (1933) "Sensitivity" to sylphydryl. Am J Clin Pathol,
3(2): 167-170.
Renwick AG, Thakrar A, Lawrie CA, & George CF (1988) Microbial amino
acid metabolites and bladder cancer: No evidence of promoting activity
in man. Hum Toxicol, 7(3): 267-272.
Ribo JM, Kaiser KLE. 1983. Effects of selected chemicals to
photoluminescent bacteria and their correlations with acute and
sublethal effects on other organisms. Chemosphere,
12(11-12): 1421-1442.
Riddick JA, Bunger WB, & Sakano TK (1986) Organic solvents. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Inc., pp 224-229.
Risner CH (1993) The quantification of hydroquinone, catechol, phenol,
3-methylcatechol, scopoletin, m+p-cresol and o-cresol in indoor
air sample by high performance liquid chromatography. J Liq
Chromatogr, 16:4117-4140.
Roberts MS, Anderson RA, & Swabrick J (1977) Permeability of human
epidermis to the phenolic compounds. J Pharm Pharmacol, 29: 677-683.
Roberts DJ, Fedorak PM, & Hrudey SE (1987) Comparison of the fates of
the methyl carbons of m-cresol and p-cresol in methanogenic
consortia. Can J Microbiol, 33: 335-338.
Rogers JE, Li SW, & Felice LJ (1984) Microbiological transformation
kinetics of xenobiotics in aquatic environment. Richland, Washington,
Battelle Pacific Northwest Laboratories, p 105 (NTIS PB84-162866).
Sabljic A (1987) [The prediction of fish bioconcentration factors of
organic pollutants from the molecular connectivity model.] Z Gesamte
Hyg Grenzgeb, 33: 493-496 (in Yugoslavian).
Savolainen H (1979) Toxic effects of peroral o-cresol intake on rat
brain. Res Commun Chem Pathol Pharmacol, 25(2): 357-364.
Sawahata T & Neal NA (1983) Biotransformation of phenol t-hydroquinone
and catechol by rat liver microsomes. Mol Pharmacol, 23: 453-460.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Sax NI & Lewis RJ (1987) Hawley's condensed chemical dictionary, 11th
ed. New York, Van Nostrand Reinhold Co, pp 320-321.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12(3): 355-382.
Schultz TW (1987) The use of the ionization constant (pKa) in
selecting models of toxicity in phenols. Ecotoxicol Environ Saf,
14(2): 178-813.
Schultz TW & Riggin GW (1985) Predictive correlations for the toxicity
of allkyl- and halogen-substituted phenols. Toxicol Lett, 25: 47-54.
Scientific Associates (1976) Skin corrosiveness test in rabbits. St.
Louis, Missouri, Scientific Associates, Inc. (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS206095).
Shah JJ & Heyerdahl EK (1989) National ambient volatile organic
compound (VOCs) data base update. Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Science
Research Laboratory (EPA 600/3-88/010(a)).
Shamala N, Lim LW, Mathews FS, McIntire W, Singer TP, & Hopper DJ
(1985) Preliminary X-ray study of p-cresol methylhydroxylase
(flavocytochrome c) from Pseudomonas putida N.C.I.B. 9869. J Mol
Biol, 183(3): 517-518.
Shamala N, Lim LW, Mathews FS, McIntire W, Singer TP, & Hopper DJ
(1986) Structure of an intermolecular electron-transfer complex:
p-cresol methylhydroxylase at 6.0-A resolution. Proc Natl Acad Sci
(USA), 83(13): 4626-4630.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12: 1188-1194.
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Shelley WB (1974) p-Cresol: Cause of ink-induced hair depigmentation
in mice. Br J Dermatol, 90: 169-174.
Shelton DR & Tiedje JM (1981) Development of test for determining
anaerobic biodegradation potential. Washington, DC, US Environmntal
Protection Agency, Office of Toxic Substances.
Shimp RJ & Pfaender FK (1985a) Influence of easily degradable
naturally occurring carbon substrates on biodegradation of
monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol,
49: 394-401.
Shimp RJ & Pfaender FK (1985b) Influence of naturally occurring humic
acids on biodegradation of monosubstituted phenols by aquatic
bacteria. Appl Environ Microbiol, 49: 402-407.
Singer PC, Lamb JC III, Pfaender FK, & Goodman R (1979) Treatability
and assessment of coal conversion wastewaters: Phase I. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Industrial Environmental Research Laboratory.
Slooff W (1983) Benthic macroinvertebrates and water quality
assessment: Some toxicological considerations. Aquat Toxicol,
4: 73-82.
Slooff W & Baerselman R (1980) Comparison of the usefulness of the
Mexican Axolol (Ambystoma mexicanum) and the clawed toad (Xenopus
laevis) in toxicological bioassays. Bull Environ Contam Toxicol,
24(3): 439-443.
Slooff W, Canton JH, & Hermens JLM (1983) Comparison of the
susceptibility of 22 freshwater species to 15 chemical compounds: I.
(Sub)acute toxicity tests. Aquat Toxicol, 4(2): 113-128.
Smith JH, Mabey WR, Bohonos N, Holt BR, Lee SS, Chou T-W, Bomberger
DC, & Mill T (1978) Environmental pathways of selected chemicals in
freshwater systems. Part II: Laboratory studies. Research Triangle
Park, North Carolina, US Environmental Protection Agency,
Environmental Research Laboratory.
Smolenski WJ & Suflita JM (1987) Biodegradation of cresol isomers in
anoxic aquifers. Appl Environ Microbiol, 58: 710-716.
Snider EH & Manning FS (1982) A survey of pollutant emission levels in
waste waters and residuals from the petroleum refining industry.
Environ Int, 7: 237-258.
Southworth GR & Keller JL (1986) Hydrophobic sorption of polar
organics by low organic carbon soils. Water Air Soil Pollut,
28(3-4): 239-248.
Spain JC & van Veld PA (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum, and chemical structure. Appl
Environ Microbiol, 45(2): 428-435.
Stone AT (1987) Reductive dissolution of manganese (III/IV) oxides by
substituted phenols. Environ Sci Technol, 21: 979-988.
STORET (1993) Online retrieval from data base. Washington, DC, US
Environmental Protection Agency.
Stout J & Kilham SS (1983) Effects of p-cresol on photosynthetic and
respiration rates of a filamentous green alga (Spirogyra). Bull
Environ Contam Toxicol, 30(1): 1-5.
Stout RJ & Cooper WE (1983) Effect of p-cresol on leaf decomposition
and invertebrate colonization in experimental outdoor streams. Can J
Fish Aquat Sci, 40(10): 1647-1657.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Suflita JM, Gibson SA, & Beeman RE (1988) Anaerobic biotransformations
of pollutant chemicals in aquifers. J Ind Microbiol, 3(3): 179-194.
Suflita JM, Liang LN, & Saxena A (1989) The anaerobic biodegradation
of o-, m-, and p-cresol by sulfate-reducing bacterial
enrichment cultures obtained from a shallow anoxic aquifer. J Ind
Microbiol, 4(4): 255-266.
Swann RL, Laskowski DA, McCall PJ, & Vander Kuy K (1983) A rapid
method for the estimation of the environmental parameters
octanol/water partition coefficient, soil sorption constant, water to
air ratio, and water solubility. Residue Rev, 85: 17-28.
Swindoll CM, Aelion CM, Dobbins DC, Jiang O, Long SC, & Pfaender FK
(1988) Aerobic biodegradation of natural and xenobiotic organic
compounds by subsurface microbial communities. Environ Toxicol Chem,
7(4): 291-299.
Syrovadko ON & Malysheva ZV (1977) Working conditions and their effect
on some specific functions of women engaged in the manufacture of
enamel-insulated wires. Gig Tr Prof Zabol, 4: 25-28.
Tabak HH, Chambers CW, & Kabler PW (1964) Microbial metabolism of
aromatic compounds: I. Decomposition of phenolic compounds and
aromatic hydrocarbons by phenol-adapted bacteria. J Bacteriol,
87: 910-919.
Thompson DC, Perera K, Fisher R, & Brendel K (1994) Cresol isomers:
comparison of toxic potency in rat liver slices. Toxicol Appl
Pharmacol, 125:51-58.
TRL (1986) Subchronic neurotoxicity study in rats of orth o-, meta-,
and para-cresol. Research Triangle Park, North Carolina, Toxicity
Research Laboratories (Unpublished data submitted to the US
Environmental Protection Agency).
US EPA (1982) Assessment of testing needs: Cresols support document.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances, Test Rule Development Branch, Assessment Division, p 373.
US EPA (1986) Test methods for evaluating solid waste: SW-846. Vol.
II: Field manual physical/chemical methods, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and Emergency
Response.
US EPA (1988) US EPA contract laboratory program statement of work for
organic analysis: Semi-volatile compounds. Washington, DC, US
Environmental Protection Agency, pp D1/SV-D45/SV.
US EPA (1989) Toxics release inventory. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
USITC (1990) Synthetic organical chemicals, United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, p 3-2 (Publication No 2338).
USITC (1991) Synthetic organical chemicals, United States production
and sales, 1990. Washington, DC, United States International Trade
Commission, p 3-2 (Publication No. 2470).
US NIOSH (1989) Manual of analytical methods, 3rd ed. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
US NTP (1992) Toxicity studies of cresols (CAS nos. 95-48-7, 108-39-4,
106-44-5) in F344/N rats and B6C3F1 mice (feed studies). Research
Triangle Park, Noth Carolina, National Toxicology Program.
Uzhdavini ER, Astaf'yeva IK, Mamayeva AA, & Bakhtizina GZ (1972)
[Inhalation toxicity of o-cresol.] Tr Ufimskogo
Nauchno-Issledovatel'skogo Inst Gig Prof Zabol, 7: 115-119
(in Russian).
Uzhdavini ER & Gilev VG (1976) Toxicity of the lower phenols in the
case of epicutaneous applications. Tr Bashkir Med Inst, 19: 162-167.
Uzhdavini ER, Astafeva IK, & Mamaeva AA (1974) Acute toxicity of the
lower phenols. Gig Tr Prof Zabol, 2: 58-59.
Uzhdavini ER, Astafeva IK, Mamaeva AA, & Gilev VG (1976) Materials for
establishing the limiting dose of dicresol in the air at production
premises. Gig Tr Prof Zabol, 9: 53-55.
Vance BM (1945) Intrauterine injection of Lysol as an abortifacient:
Report of a fatal case complicated by oil embolism and Lysol
poisoning. Arch Pathol, 40: 395-398.
Vecera Z & Janak J (1987) Continuous aerodispersive enrichment unit
for trace determination of pollutants in air. Anal Chem,
59(11): 1494-1498.
Verchuesen K (1983) Handbook of environmental data on organic
chemicals. 2nd edition. New York, Van Nostrand Reinhold Company,
pp 403-410.
Vernot EH, MacEwen JD, Haun CC, & Kinkead ER (1977) Acute toxicity and
skin corrosion data from some organic and inorganic compounds and
aqueous solutions. Toxicol Appl Pharmacol, 42: 417-423.
Visser SA, LaMontagne G, Zoulalian V, & Tessier A (1977) Bacteria
active in the degradation of phenols in polluted waters of the St.
Lawrence River. Arch Environ Contam Toxicol, 6: 455-469.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sew Ind Wastes,
29(6): 695-711.
Wang YT, Suidan MT, Pfeffer JT, & Najm I (1988) Effects of some alkyl
phenols on methanogenic degradation of phenol. Appl Environ Microbiol,
54(5): 1277-1279.
Wang YT, Suidan MT, Pfeffer JT, & Najm I (1989) The effect of
concentration of phenols on their batch methanogenesis. Biotechnol
Bioeng J, 33(10): 1353-1357.
Weast RC, Astle MJ, & Beyer WH (1988) CRC handbook of chemistry and
physics, 69th ed. Boca Raton, Florida, CRC Press, Inc, pp C/218-C/220.
Weber AS & Matsumoto MR (1987) Feasibility of intermittent biological
treatment for hazardous wastes. Environ Prog, 6(3): 166-171.
Williams RA, MacRae R, & Shepherd MJ (1989) Non-aqueous size-exclusion
chromatography coupled online to reversed-phase high-performance
liquid chromatography interface development and applications to the
analysis of low-molecular-weight contaminants and additives in foods.
J Chromatogr, 477(2): 315-326.
Williams RT (1938) CXVIII: Studies in detoxication: I. The influence
of (a) dose and (b) o-, m- and p-substitution on the sulfate
detoxication of phenol in the rabbit. Biochem J, 32: 878-887.
Windholz M, Budavini S, Blumetti RF & Otterbein ES ed. (1983) The
Merck index: an encyclopedia of chemicals, drugs, and biologicals,
10th ed. Rahway, New Jersey, Merck and Co., Inc.
Wiseman HW, Turner WH, & Volans GW (1980) Acute poisoning due to
Wright's vaporizing fluid. Postgrad Med J, 56(653): 166-168.
Wu HY & Kwan Y (1984) [Case report of an acute renal failure
complicated by cresol burns.] Chin J Prev Med, 18:145-149
(in Chinese).
Wynder EL & Hoffmann D ed. (1967) Tobacco and tobacco smoke. New York,
London, San Francisco, Academic Press, pp 387, 499-500.
Yalkowsky SH, Valvani SC, & Kun W (1987) Arizona database of aqueous
solutions.
Yanysheva NYA, Balenko NV, Chernichenko IA, & Babiy VF (1993)
Peculiarities of carcinogenesis under simultaneous oral administration
of benzo( a)pyrene and o-cresol in mice. Environ Health Perspect,
101(Suppl. 3): 341-344.
Yashiki M, Kojima T, Miyazaki T, Ohikasne F, & Ohtani M (1989) Gas
chromatographic determination of cresols in the biological fluids of a
non-fatal case of cresol intoxication. Forensic Sci Int, 47: 21-29.
Yasuhara A (1987) Identification of volatile compounds in poultry
manure by gas chromatography-mass spectrometry. J Chromatogr,
387: 371-378.
Yasuhara AO, Shiraishi H, Tsuji M, & Okuno T (1981) Analysis of
organic substances in highly polluted river water by mass
spectrometry. Environ Sci Technol, 15: 570-573.
Yoshikawa M, Arashidani K, Kodama Y (1986) [A simple determination of
phenol in urine by high performance liquid chromatography.] San Igaku,
27(2): 83-89 (in Japanese, with English abstract).
Young LY & Rivera MD (1985) Methanogenic degradation of four phenolic
compounds. Water Res, 19: 1325-1332.
Young RHF, Ryckman DW, & Buzzell JC Jr (1968) An improved tool for
measuring biodegradability. J Water Pollut Control Fed, 40: R354-R367.
Younger Labs (1974) Skin irritation in albino rabbits after
application of o-, m-, and p-cresol. St. Louis, Missouri,
Younger Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517499).
Zheng Y, Hill DO, & Kuo CH (1993a) Destruction of cresols by chemical
oxidation. J Hazard Mater, 34:245-260.
Zheng Y, Hill DO, & Kuo CH (1993b) Rates of ozonation of cresol
isomers in aqueous solutions. Ozone Sci Eng, 15: 267-278.
RESUME
1. Identité, propriétés et méthodes d'analyse
Les crésols sont des phénols isomères substitués par un
groupement méthyle en position ortho, méta ou para par rapport au
groupement hydroxyle. Le crésol du commerce, également connu sous le
nom d'acide crésylique, contient les trois isomères à côté d'un peu de
phénol et de xylènes. Toutefois certains produits du commerce
contiennent jusqu'à 30% de xylénol et 60% de phénols en C9 et sont
également désignés sous le nom "d'acides crésyliques". Physiquement,
les crésols se présentent sous la forme d'un solide cristallin blanc
ou d'un liquide jaunâtre à forte odeur phénolique. Ils sont très
inflammables et sont solubles dans l'eau, l'éthanol, l'éther,
l'acétone et les hydroxydes alcalins. Les crésols subissent des
réactions de substitution électrophiles en position ortho ou para
du groupement hydroxyle. Ils donnent également lieu à des réactions
de condensation avec les aldéhydes, les cétones ou les diènes.
On peut utiliser plusieurs méthodes pour rechercher la présence
de crésols dans l'environnement ou les milieux biologiques. Les plus
couramment utilisées sont la chromatographie en phase gazeuse avec
détection par ionisation de flamme, la chromatographie en phase
gazeuse couplée à la spectrophotométrie de masse et enfin la
chromatographie liquide à haute performance. Pour l'échantillonnage
dans l'air, on peut faire passer celui-ci dans un absorbeur contenant
de l'hydroxyde de sodium ou des adsorbants solides.
2. Usages, sources et niveaux d'exposition
Les crésols sont très largement utilisés comme solvants ou
désinfectants ou encore comme intermédiaires dans la production d'un
grand nombre d'autres substances. Ces composés sont le plus souvent
utilisés pour la production d'arômes, d'antioxydants, de colorants, de
pesticides et de résines. L' ortho- et le para-crésol sont
utilisés pour la production d'huiles lubrifiantes, de combustibles
pour véhicules à moteur et d'élastomères, le meta-crésol étant
utilisé à la fabrication d'explosifs.
Les crésols et leurs dérivés existent à l'état naturel dans les
huiles essentielles de diverses plantes telles que les fleurs de
Yucca gloriosa, dans le jasmin, le lys, les conifères, les chênes et
le santal, et ils constituent également un produit de la combustion
naturelle de certaines substances et de l'activité volcanique. On
trouve du para-crésol dans l'urine des animaux et de l'homme. Les
crésols du commerce sont des sous-produits de la distillation
fractionnée du pétrole brut et du goudron de houille. Ils se
retrouvent en petites quantités dans les échappements des véhicules à
moteur, lors de l'incinération des déchets municipaux et de la
combustion de la houille et du bois. La fumée de cigarettes contient
également des crésols. On ignore quelle est la production mondiale
totale de crésols; pour les Etats-Unis d'Amérique, on indiquait en
1990 une production annuelle totale de 38 300 tonnes.
Le transport des crésols dans le milieu s'effectue dans la phase
gazeuse de l'atmosphère, et de l'atmosphère aux eaux de surface et au
sol par entraînement avec les précipitations. En raison de leur
volatilité, de leur fixation aux sédiments et de leur biodégradation,
les crésols ne se retrouvent dans l'eau qu'en petites quantités. Dans
le sol, ils peuvent être légèrement ou fortement mobiles en fonction
du coefficient d'adsorption du sol (Koc). On a décelé la présence
de crésols dans les eaux souterraines, de sorte qu'un lessivage doit
se produire.
Il peut y avoir exposition aux crésols par l'intermédiaire de
l'air, de l'eau ou de la nourriture. La concentration médiane dans
l'air des o-crésols a été trouvée égale à 1,59 µg/m3 (0,359 ppm)
sur 32 sites des Etats-Unis d'Amérique dominés par des sources de
pollution. Dans ce même pays, les concentrations dans les eaux de
surface vont de valeurs inférieures à la limite de détection jusqu'à
77 µg/litre (STORET, 1993). Au Japon, on a trouvé une concentration
de 204 µg/litre dans une rivière polluée par des effluents
industriels. Des teneurs pouvant atteindre 2100 µg/litre dans le cas
de l' o-crésol et 1200 µg/litre dans le cas d'un mélange de m- et
de p-crésols ont été mesurées dans des eaux usées. Dans l'eau de
pluie, les concentrations vont de 240 à 2800 ng/litre dans le cas de
l' o-crésol et de 380 à 2000 ng/litre pour le mélange de p- et de
m-crésols. On a décelé la présence de crésols dans des denrées
alimentaires et des boissons. Dans les spiritueux, des concentrations
se situant entre les limites 0,01-0,02 mg/litre ont été mesurées.
Dans la fumée émise par une cigarette américaine sans bout-filtre
(85 mm), la teneur est de 75 µg. La population générale peut être
exposée aux crésols par suite de l'inhalation d'air, de l'ingestion
d'eau, de nourriture et de boissons diverses ainsi que par contact
cutané. En général il est impossible d'évaluer quantitativement la
dose de crésols absorbée à partir de ces sources par suite de
l'absence de données de surveillance suffisantes. En ce qui concerne
l'exposition professionnelle, on a fait état de concentrations
atteignant 5,0 mg/m3.
3. Cinétique et métabolisme
Les crésols sont résorbés au niveau des voies respiratoires et
digestives ainsi que de l'épiderme. La vitesse et l'ampleur de cette
absorption n'ont pas fait l'objet d'études particulières. Cependant
certains travaux montrent qu'au niveau des voies digestives et de
l'épiderme, l'absorption est rapide et importante. Les crésols se
répartissent dans l'ensemble des principaux viscères. La principale
voie métabolique des crésols consiste dans une conjugaison avec
l'acide glucuronique et les sulfates inorganiques. Il existe des
voies métaboliques secondaires comportant une hydroxylation du cycle
benzénique ainsi qu'une oxydation de la chaîne latérale. Les crésols
sont principalement éliminés par les reins sous forme de conjugués.
4. Effets sur les mammifères de laboratoire et les systèmes in vitro
Une intoxication aiguë par les vapeurs de crésols est peu
probable en raison de la faible tension de vapeur de ces composés.
Chez le rat, on a observé des concentrations létales moyennes de
29 mg/m3 pour l' o- et le p-crésol et de 58 mg/m3 pour le
m-crésol. Chez le même animal, les valeurs de la DL50 par voie
orale sont respectivement de 121, 207 et 242 mg/kg de poids corporel
pour l' o-, le p- et le m-crésol respectivement. Les
comparaisons interspécifiques montrent que les trois isomères sont
tous plus toxiques pour la souris que pour le rat et que leur toxicité
croît avec la concentration. L'exposition cutanée peut entraîner une
intoxication générale mortelle. Chez le lapin on a relevé pour la
DL50 des valeurs respectivement égales à 890, 2830, 300 et
2000 mg/kg de poids corporel pour l' o-, le m- et le p-crésol et
leurs mélanges. Chez le rat, la DL50 cutanée se situait
respectivement à 620, 1100, 750 et 825 mg/kg de poids corporel pour
l' o-, le m- et le p-crésol ainsi que pour le dicrésol.
Chez le lapin, le rat et la souris, les crésols sont extrêmement
irritants pour la peau et les yeux.
Chez des animaux à qui l'on avait fait respirer pendant une
courte durée des mélanges d'aérosols et de vapeurs d' o-crésol, on a
observé une irritation des voies respiratoires, de petites hémorragies
au niveau des poumons, une réduction du poids corporel ainsi qu'une
dégénérescence cellulaire du myocarde, du foie, du rein et des nerfs.
En faisant absorber à des rats pendant une courte durée (28 jours) des
doses quotidiennes d'environ 800 mg ou plus de crésols par kg de poids
corporel, on a constaté une réduction du poids corporel, une
modification du poids des organes ainsi que des anomalies
histopathologiques au niveau des voies respiratoires et digestives.
Chez des souris exposées de la même manière à des doses de 1500 mg/kg
de poids corporel, les effets ont été plus sévères et les animaux sont
morts aux concentrations les plus élevées d' o-, de m- et de
p-crésol, à l'exclusion des mélanges de ces isomères.
L'exposition prolongée de rats à des vapeurs d' o-, de m- ou
de p-crésol (pendant une durée allant jusqu'à 4 mois) a eu pour
effets une perte de poids, une réduction de l'activité locomotrice,
une inflammation des muqueuses nasales et de la peau ainsi que des
anomalies au niveau du foie. Exposés par voie orale à des crésols
pendant 13 semaines, des souris, des rats et des hamsters ont
présentés les effets suivants: mortalité, tremblements, réduction du
poids corporel, effets hématologiques, accroissement du poids des
organes, hyperplasie de l'épithélium nasal et de celui de la portion
cardiaque de l'estomac.
L'exposition de rats et de souris à des crésols isomères par voie
orale ou par voie respiratoire provoque un allongement du cycle
oestral ainsi que des modifications histopathologiques au niveau de
l'utérus et des ovaires. On n'a en revanche pas observé d'effets
indésirables sur la spermatogénèse. De légers effets cytotoxiques ont
été signalés chez des rats et des lapins exposés à de l' o- et du
p-crésol, mais on n'a observé sur le développement que des effets
mineurs qui puissent être attribuables à ce traitement. Le traitement
in vitro par de l' o- et du p-crésol, à l'exclusion du
m-crésol, entraînerait une certaine génotoxicité. En revanche les
études in vivo n'ont pas fait ressortir de résultats positifs.
Pourtant, il existe des signes d'activité promotrice au niveau cutané.
Aucune étude de cancérogénicité n'a été publiée sur l'un quelconque
des isomères du crésol.
5. Effets sur l'homme
L'ingestion de crésols provoque des brûlures de la bouche et de
la gorge, des douleurs abdominales et des vomissements. Après
ingestion, les tissus et les organes-cibles sont, chez l'homme, le
sang et les reins et l'on a également fait état d'effets sur les
poumons, le foie, le coeur et le système nerveux central. Dans les
cas graves, on peut observer un coma mortel. Après exposition
cutanée, on a signalé de graves brûlures laissant subsister des
cicatrices, une intoxication générale et la mort.
En général, l'exposition professionnelle aux crésols résulte d'un
contact cutané. Une exposition aiguë peut provoquer de graves
brûlures, une anurie et un coma mortel. On ne dispose que de très peu
de données sur les effets au niveau de l'appareil reproducteur et on
n'a aucun renseignement sur la cancérogénicité de ces produits pour
l'homme.
6. Effets sur les autres êtres vivants
Les observations effectuées sur des microorganismes, des
invertébrés et des poissons montrent que les crésols peuvent
constituer un risque pour les organismes non-mammaliens là où des
sources ponctuelles de pollution déterminent de fortes concentrations,
mais ce n'est pas le cas dans l'environnement en général.
7. Conclusions et recommandations
Aux concentrations généralement présentes dans l'environnement,
les crésols ne constituent pas un risque important pour la population
générale. Toutefois il y a possibilité d'effets indésirables pour les
insuffisants rénaux ainsi que pour les personnes présentant certains
déficits enzymatiques; ce risque existe également en cas de forte
exposition.
Les crésols peuvent constituer un risque pour les
microorganismes, les invertébrés et les poissons là où des sources
ponctuelles de pollution déterminent de fortes concentrations, mais ce
n'est pas le cas dans l'environnement en général.
On ne dispose d'aucune donnée concernant les effets de
l'exposition chronique aux crésols. Dans ces conditions, il n'est pas
possible d'évaluer le risque cancérogène imputable à ces produits. Si
on s'appuie sur les résultats d'études sub-chroniques, on peut estimer
à 50 mg/kg de poids corporel par jour la dose sans effets indésirables
observables. Il a été recommandé d'appliquer un facteur d'incertitude
de 300 qui est établi comme suit: 10 pour tenir compte des variations
interspécifiques; 10 pour tenir compte de l'absence de données de
toxicité chronique ainsi que de l'activité génotoxique et promotrice
possible des crésols et enfin 3 pour tenir compte des variations
intraspécifiques tenant à un métabolisme plus ou moins rapide et
complet. Dans ces conditions, on peut fixer à 0,17 mg/kg de poids
corporel par jour la dose journalière acceptable (DJA) de crésols.
RESUMEN
1. Identidad, propiedades y métodos analíticos
Los cresoles son fenoles sustituidos isoméricos con un
sustituyente metilo en una de las posiciones orto, para o meta
respecto al grupo hidróxilo. El cresol comercial, conocido también
como ácido cresílico, contiene los tres isómeros con pequeñas
cantidades de fenol y xilenoles. Sin embargo, los productos
comerciales, conocidos como "ácidos cresílicos", contienen hasta un
30% de xilenol y un 60% de C9-fenoles. Físicamente, los cresoles
consisten en un sólido cristalino blanco o un líquido amarillento y
tienen un fuerte olor a fenol. Son altamente inflamables y se
disuelven en agua, etanol, éter, acetona e hidróxidos alcalinos. Los
cresoles sufren reacciones de sustitución electrofílica en la posición
orto o para libre en relación con el grupo hidróxilo. Asimismo,
experimentan reacciones de condensación con aldehídos, cetonas o
dienos.
La presencia de cresoles tanto en medios naturales como
biológicos puede ser determinada usando varios métodos. Los más
corrientes son la cromatografía de gases con detector de ionización de
llama (GC-FID), la cromatografía de gases con espectrofotometría de
masas (GC-MS) y la cromatografía líquida de alta resolución (HPLC).
El muestreo de los cresoles en la atmósfera puede realizarse pasando
aire a través de células de absorción, utilizando hidróxido de sodio o
adsorbentes sólidos.
2. Usos, fuentes y niveles de exposición
Los cresoles ofrecen gran variedad de usos como solventes o
desinfectantes, o como intermedios en la producción de muchas otras
sustancias. Estos compuestos son utilizados principalmente en la
producción de perfumes, antioxidantes, tintes, plaguicidas y resinas.
Los cresoles orto- y para- se utilizan en la producción de aceites
lubricantes, combustibles para motores y polímeros de caucho, mientras
que el meta-cresol interviene en la fabricación de explosivos.
Los cresoles y sus derivados se encuentran naturalmente en los
aceites de diversas plantas (tales como flores de Yucca gloriosa,
jazmín, Lilium longiflorum var. eximium, coníferas, roble y sándalo) y
como producto de combustión de los incendios naturales y la actividad
volcánica. El para-cresol está presente en la orina de animales y
del hombre. Comercialmente, los cresoles son generados como
subproductos de la destilación fraccional de petróleo crudo y
alquitranes de hulla. Asimismo, se producen pequeñas cantidades en
caños de escape de vehículos, incineradores municipales de desechos y
mediante la combustión del carbón y de la madera. El humo de
cigarrillo también contiene cresoles. No se conoce la producción
mundial de cresoles; el total registrado en los Estados Unidos en 1990
ascendió a 38 300 toneladas.
El transporte de cresoles en el medio ambiente se realiza en la
fase vapor de la atmósfera, y de la atmósfera a las aguas
superficiales y al suelo por el lavado de las lluvias. Debido a su
volatilización, su adherencia a sedimentos y su biodegradación, los
cresoles se encuentran sólo en pequeñas cantidades en el agua. Su
movilidad en suelos va de leve a alta, según el coeficiente (Koc) de
sorción del suelo. Se han detectado cresoles en aguas subterráneas,
lo cual sugiere que algún grado de lixiviación debe ocurrir en el
suelo.
La exposición a los cresoles puede ocurrir a través del aire, del
agua o de los alimentos. En 32 sitios identificados de los Estados
Unidos se halló una concentración atmosférica mediana de o-cresoles
de 1,59 µg/m3 (0,359 partes por mil millones). En los Estados
Unidos, las concentraciones en aguas superficiales van de niveles
inferiores al umbral de detección a 77 µg/litro (STORET, 1993). En el
Japón, se hallaron niveles de 204 µg/litro en un río contaminado por
efluentes industriales. En aguas residuales, ha sido posible detectar
concentraciones de hasta 2100 µg/litro para el o-cresol y de
1200 µg/litro para m- y p-cresoles combinados. En el agua de
lluvia, las concentraciones van de 240 a 2800 ng/litro para el
o-cresol y de 380 a 2000 ng/litro para p- y m-cresoles
combinados. También se han detectado cresoles en alimentos y bebidas.
En bebidas alcohólicas se determinaron concentraciones del orden de
0,01-0,2 mg/litro. En el humo de tabaco, esta cantidad es de 75 µg
para un cigarrillo americano sin filtro (85 mm). La exposición de la
población general a los cresoles puede darse por inhalación, por el
agua potable, por la ingestión de alimentos y bebidas, y por contacto
cutáneo. En general, la falta de datos adecuados de seguimiento
impide realizar estimaciones cuantitativas de la ingesta diaria de
cresol por estas vías. Se han señalado niveles de exposición
ocupacional de hasta 5,0 mg/m3.
3. Cinética y metabolismo
Los cresoles se absorben a través de los tractos respiratorio y
gastrointestinal y por contacto con la piel. Si bien el índice y la
magnitud de la absorción no han sido estudiados específicamente,
diversos estudios han probado que la absorción gastrointestinal y
dérmica es rápida y extensa. Los cresoles se distribuyen a todos los
principales órganos. Su principal vía metabólica es la conjugación
con ácido glucurónico y sulfato inorgánico; las vías metabólicas
secundarias incluyen la hidroxilación del anillo de benceno y la
oxidación de cadena lateral. La principal vía de eliminación es la
excreción renal en forma de conjugados.
4. Efectos en mamíferos de laboratorio; sistemas in vitro
La intoxicación aguda con cresoles es poco probable, debido a la
baja presión de vapor de estos compuestos. Se han señalado
concentraciones letales medias en ratas de 29 mg/m3 para o- y
p-cresoles y de 58 mg/m3 para m-cresoles. En ratas, los valores
orales DL50 notificados han sido de 121, 207 y 242 mg/kg de peso
corporal para o-, p- y m-cresoles, respectivamente. Las
comparaciones entre especies revelan que los tres isómeros son más
tóxicos en ratones que en ratas y que la toxicidad aumenta con la
concentración. La exposición cutánea puede provocar toxicidad
sistémica y muerte. Los valores dérmicos DL50 en conejos fueron de
890, 2830, 300 y 2000 mg/kg de peso corporal para cresoles o-, m-,
p- y combinados, respectivamente. En ratas, se registraron valores
dérmicos DL50 de 620, 1100, 750 y 825 mg/kg de peso corporal para
o-, m-, p- cresoles y dicresol, respectivamente.
Los cresoles ocasionan graves irritaciones dérmicas y oculares en
conejos, ratas y ratones.
La exposición por breves periodos a mezclas inhaladas de
aerosoles y vapores de o-cresol provocó irritación del tracto
respiratorio, pequeñas hemorragias pulmonares, pérdida de peso
corporal y degeneración de músculo cardiaco, hígado, riñón y células
nerviosas. La exposición oral por breves periodos (28 días) a dosis
diarias de unos 800 mg/kg de peso corporal o más produjo pérdida de
peso corporal, alteración del peso de los órganos y cambios
histopatológicos en los tractos respiratorio y gastrointestinal de las
ratas. Más graves fueron los efectos constatados en ratones expuestos
a una administración similar de dosis de 1500 mg/kg de peso corporal;
a concentraciones más elevadas, la muerte fue causada por la
exposición a o-, m- y p-cresoles, mas no por exposiciones a
mezclas de isómeros.
Una exposición más prolongada de ratas, de hasta 4 meses, a
vapores de o-, m- y p-cresol provocó pérdida de peso, reducción
de la actividad locomotriz, cambios hepáticos e inflamación de
membranas nasales y piel. La exposición oral de ratones, ratas y
hámsters por periodos de hasta 13 semanas ocasionó muerte, temblores,
pérdida de peso corporal, alteraciones hematológicas, aumento del peso
de los órganos e hiperplasia del epitelio nasal y del cardias.
La exposición oral y por inhalación a isómeros del cresol da
lugar a ciclos estruales prolongados y modificaciones histopatológicas
del útero y los ovarios de ratas y ratones. No se observaron efectos
sobre la espermatogénesis en ratas y ratones. En ratas y conejos
expuestos a o- y p-cresoles se registraron efectos embriotóxicos
moderados; no obstante, sólo se han señalado anomalías menores del
desarrollo relacionadas con el tratamiento. Ciertos indicios de
genotoxicidad han sido constatados in vitro como consecuencia del
tratamiento con o- y p-cresoles, pero no con m-cresol. No se
obtuvieron resultados positivos en estudios in vivo; sin embargo, se
observaron indicios de actividad promotora en la piel. No se han
notificado estudios de carcinogenicidad para ninguno de los isómeros
del cresol.
5. Efectos en la especie humana
La ingestión de cresoles provoca quemaduras de boca y esófago,
dolores abdominales y vómitos. Los tejidos y órganos afectados por la
ingestión de cresoles son la sangre y los riñones, aunque también se
han señalado efectos en los pulmones, el hígado, el corazón y el
sistema nervioso central. En casos graves, puede producirse coma y
muerte. La exposición cutánea ha ocasionado graves quemaduras de
piel, cicatrices, toxicidad sistémica y muerte.
En el medio laboral, la exposición a los cresoles suele
producirse por contacto cutáneo. La exposición aguda puede dar por
resultado graves quemaduras, anuria, coma y muerte. Existen muy pocos
datos sobre sus efectos en la reproducción, y ninguno sobre la
carcinogenicidad en el ser humano.
6. Efectos en otros organismos
Las observaciones en microorganismos, invertebrados y peces han
revelado que los cresoles pueden suponer un riesgo para organismos
diferentes de los mamíferos en puntos específicos con altas
concentraciones de cresol, pero no en el medio ambiente en general.
7. Conclusión y recomendaciones
En las concentraciones normalmente halladas en el medio ambiente,
los cresoles no presentan un riesgo significativo para la población
general. Con todo, pueden darse efectos adversos en personas que
padecen de insuficiencia renal o de una deficiencia enzimática
específica, así como en condiciones de alta exposición.
Los cresoles pueden suponer un riesgo para microorganismos,
invertebrados y peces en puntos específicos con una alta concentración
de cresoles, pero no en el medio ambiente en general.
Al no disponerse de datos sobre las consecuencias de una
exposición crónica, no existe una información adecuada que permita
evaluar el riesgo carcinogénico de los cresoles. Partiendo de los
resultados de estudios subcrónicos, puede establecerse un nivel sin
efectos adversos observados (NOAEL) de 50 mg/kg de peso corporal al
día para los tres isómeros del cresol. Se ha recomendado un factor de
incertidumbre 300, que se descompone así: 10 por la variación entre
especies; 10 por la falta de estudios de toxicidad crónica y por la
posible actividad genotóxica y promotora de los cresoles; y 3 por la
variación dentro de la misma especie basada en el metabolismo completo
y rápido. Por consiguiente, puede establecerse para los cresoles una
ingesta diaria admisible de 0,17 mg/kg de peso corporal.
Empirical formula: C7H8O C7H8O C7H8O C7H8O
Relative molecular mass: 108.14 108.14 108.14 108.14
Common synonyms: 2-methyl phenol 4-methyl phenol 3-methyl phenol methyl phenol
2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
o-cresylic acid p-cresylic acid m-cresylic acid cresylic acid
acide cresylique (French)
cresoli (Italian)
kresolen (Dutch)
krezol (Polish)
kresol (German)
IUPAC name: 2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
CAS registry number: 95-48-7 106-44-5 108-39-4
RTECS: G06300000 G06475000 G06125000 G05950000
EEC number: 604-004-00-9 604-004-00-9 604-004-00-9 604-004-00-9
Table 2. Physical and chemical properties of cresolsa
o-Cresol m-Cresol p-Cresol Mixturef
Physical state and colour: white crystalline solid colourless to yellowish crystalline solid or colourless to yellowish
or yellowish liquid liquid yellowish liquid liquid
Odour: phenol-like phenol-like phenol-like phenol-like
Air odour thresholdb: 1.4 mg/m3 0.007 mg/m3 0.004 mg/m3 ND
Melting point (°C): 30.94 12.22 34.74 11-35
Boiling point at 1 atm (°C): 191.0 202.32 201.94 191-203
Flash point, closed cup (°C): 81 86 86 82
Ignition point (°C): 598 558 558 ND
Vapour pressure at 25°C (mmHg): 0.31 0.143 0.13 0.975 (at 38-53°C)g
Relative density at 25°C (g/cm3): 1.135 1.030 1.154 1.03-1.038
Refractive index at 25°C: 1.544 1.540 1.539 ND
Vapour density (air = 1 at 20°C): 3.7 3.72 3.72 NDe
Solubility in water at 25°C
(g/litre)c: 25.95 22.70 21.52 ND
Solubility in other solvents: soluble in ethanol, soluble in ethanol, soluble in ethanol, soluble in ethanol,
ethyl ether, acetone, ethyl ether, acetone, ethyl ether, acetone, glycol, aqueous
benzene, aqueous benzene, aqueous benzene, aqueous alkali hydroxides
alkali hydroxides alkali hydroxides alkali hydroxides
Table 2 (contd).
o-Cresol m-Cresol p-Cresol Mixturef
Sorption coefficient,
Koc (all isomers)d 22-3420
Log n-octanol/water partition
coefficiente (log Ko/w): 1.95 1.96 1.94 ND
pKa (25°C): 10.287 10.09 10.26 ND
Bioconcentration factorsh 14.1 19.9 ND ND
Odour threshold in water
(mg/litre)i,j 1.4 0.8 0.2 ND
Taste threshold concentration
in water (mg/litre)j 0.003 0.002 0.002 ND
Saturation concentration
in air (g/m3)j at 20°C 1.2 0.24 0.24 ND
at 30°C 2.8 0.68 0.74 ND
a Adapted from: Weast et al. (1988); Sax & Lewis (1987); Windholz et al. (1983); Riddick et al. (1986), unless otherwise specified
b Amoore & Hautala (1983)
c Yalkowsky et al. (1987)
d Boyd (1982); Southworth & Keller (1986); Koch & Nagel (1988)
e Hansch & Leo (1985)
f No data
g Parrish (1983)
h Freitag et al., (1982)
i Dietz & Traud (1978)
j Verschuesen (1983)
2.4 Analytical methods
2.4.1 Sampling
As is the case with any other analyte, sample loss and
contamination should be avoided during the collection, storage and
analysis of samples for cresol determination. Glass bottles, vials or
tubes have been used for the collection of environmental samples (US
EPA, 1982). Polyethylene containers are suitable for the collection
of biological samples (US NIOSH, 1989). Environmental aqueous samples
can be stored for a limited time (28 days) by adding sulfuric acid to
a pH < 2 (US EPA, 1982). Thymol has been used as a preservative for
biological samples (US NIOSH, 1989). Environmental and biological
samples that are to be shipped from the collection site to the
laboratory are cooled in ice.
Cresols in air can be sampled by passing air through an
absorption cell containing 0.1 N sodium hydroxide solution (Manita,
1966). More recent methods use solid adsorbents such as XAD-2 or
silica gel for trapping cresols from air (Neiminen & Heikkila, 1986;
US NIOSH, 1989). In a novel system, a miniaturized enrichment unit
has been used to concentrate cresols and other water-soluble analytes
in air by a water mist (Vecera & Janak, 1987). Aqueous samples can be
collected either by manual grab methods or by automated samplers.
Composite samples can be obtained by combining random samples
collected manually or by automated samplers (US EPA, 1982). Several
mechanical devices are available for collecting random or composite
semi-solid and solid samples either by grab or automated methods (US
EPA, 1982, 1986).
2.4.2 Analytical methods
Some of the methods used in measuring cresols in various
environmental and biological media are given in Table 3 along with
their corresponding references. The problem with the determination of
cresols by gas chromatography arises as a result of non-reproducible
elution from the gas chromatography column due to the polar and
volatile nature of cresols. Special columns or derivatization of the
cresols may alleviate the problem. Cresols are present in biological
samples as conjugates, and a hydrolysis method is used to release free
cresols. There is no consensus on the reliability of total hydrolysis
of the cresol conjugates (Balikova & Kohlicek, 1989).
Chudyk et al. (1985) tested a remote fluorescence technique using
ultraviolet laser fibre optics to analyse groundwater contaminants,
including o-cresol, in artificially prepared solutions. No data were
given on the detection limits or on the use of this technique in the
field. However, the authors speculated that the sensitivity is at or
below parts per billion levels at an instrument/analyte distance of
25 m.
Hoshika & Muto (1978) described a simple and rapid
gas-liquid-solid chromatographic (GLSC) method for the determination
of trace concentrations of 11 phenols including all isomers of cresol
in air. This method has been adopted and recommended by many other
investigators for measuring cresols in air samples. To overcome
interference by certain acidic compounds such as lower fatty acids and
mercaptans, the method uses two precolumns, a Tenax-GC and a Tenax-GC
plus alkaline. The gas chromatograph used was equipped with a flame
ionization detector (FID), a digital integrator and a glass analytical
column. With the Tenax-GC plus alkaline precolumn the phenol peaks
disappeared completely in the chromatograms, enabling phenols to be
identified by comparison with the chromatograms from the ordinary
Tanex-GC precolumn. The detection limit for cresols by this method
was reported to be at the ppb level.
Table 3. Sampling and analytical methods for determining cresols in environmental and biological samples
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Air
Air pump air through adsorbent tube; HPLC/UV o, m, p 0.3 ppt 90-110% Kuwata & Tanaka
desorb with methanol (1988)
Air aerodispersive enrichment into HPLC/ED o no data no data Vecera & Janak
water (1987)
Air pump air through silica gel tube; GC-FID o, m, p no data 98% at US NIOSH (1989)
desorb with acetone 22 mg/m3
Air pump air through mixed cellulose HPLC-UV o, m, p 0.5 ppb 52.4% Risner (1993)
ester membrane connected to silica
Sep-Pak, desorp with 1% acetic
acid in acetonitrile
Auto exhaust vapour collected in fritted bubbler HPLC-UV o, m, p 0.1-0.5 no data Kuwata et al.
and tobacco with aqueous NaOH buffered to pH 11.5; ng/sample (1981)
smoke add p-nitrobenzene-diazonium
tetra-fluoroborate; extract with CCl4
Air and water
Air and water mix NaOH solution from bubbler in case spectrophotometry o, m, p 0.005-0.03 no data Druyan (1974)
of air and distillate of water samples (TLC) µg/sample
in 1 N NaOH solution with
p-nitrophenyl-diazonium at pH 7-9;
extract with ether; spot on TLC plate
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Water adjust pH to 11; extract with GC/MS o, p 10 µg/litre no data US EPA (1988)
CH2Cl2; concentrate
Water solvent extraction, liquid GC/MS not no data no data Hites (1979)
chromatography prefractionation specified
Water adjust pH to 8-9; extract with spectrophotometry o, m 4 µg/litre 99-100.1% Hassan et al.
chloroform-ether; back extract (VIS) at 5-120 (1987)
in 0.1 N aqueous NaOH; add NaNO2 µg/litre
and H2SO4; remove excess NO;
add resorcinol
Water direct flow and spectrophotometry o, m 10-30 µg/litre 90-115% Khalaf et al.
stopped-flow injection, then (VIS) (1993)
derivatization with p-aminophenol
Rainwater direct injection onto ion exchange HPLC/CD o, m, p no data no data Hoffman &
column Tanner (1986)
Rainwater acidify; extract with CH2Cl2; GC/MS o, m, p no data > 50% Kawamura &
concentrate, methylate Kaplan (1986)
Soil
Soil, extract sample with CH2Cl2 using GC/MS o, p 330 ppb no data US EPA (1988)
sediment ultrasonic probe
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Sediment extract rapidly stirred sediment GC/MS not no data no data Goodley & Gordon
slurry with CH2Cl2 or ether, specified (1976)
concentrate
Biological samples
Expired draw air through XAD-2 adsorbent HPLC/ED o, m, p 8 µg/m3 no data Neiminen &
air tube; acetonitrile desorbtion Heikkila (1986)
Expired collect breath in Teflon bag; GC/MS not no data no data Krotoszynski &
air concentrate on Tenax GC absorbent; specified O'Neill (1982)
thermal desorption
Beef steam distil; extract distillate HRGC/MS o, m, p 0.2 mg/kg 83-98% at Matsumoto et al.
with ether 20-100 µg (1989)
per sample
Urine hydrolyse with sulfuric acid; GC/FID o, m, p no data 78-97% Needham et al.
extract with ethyl acetate (1984)
Urine hydrolyse with HCl and extract with HPLC/UV o, m, p 1 mg/litre 97-102% Yoshikawa et al.
isopropyl ether; remove solvent; (1986)
dissolve residue in water; add
ß-cyclodextrin
Urine acidify; steam distil; extract with GC/MS o no data no data Angerer & Wulf
methylene chloride (1985)
Urine hydrolyse with sulfuric acid; extract HPLC/UV o no data no data DeRosa et al.
with CH2Cl2; concentrate (1987)
Table 3 (contd).
Sample Analytical Sample detection Percentage
matrix Preparation method methodb Isomer limit recovery Reference
Urine hydrolyse with HCl or HClO4; extract GC-FID p 0.5 mg/litre 95% at US NIOSH (1989)
with ether 50 µg/ml
Urine and hydrolyse with H3PO4; extract with GC-FID o, m, p 1 mg/litre 69.4-73.3% Balikova &
serum n-hexane, acetylate extract at 50 Kohlicek (1989)
mg/litre
Faeces and homogenize faeces and hydrolyse HPLC-fluorescence p < 1 µg/kg for 99.4-101.9% Murray & Adams
urine urine buffered to pH 5.5, steam detector faeces; (1988)
distil < 1 µg/litre
for urine
a 0.01 nmol = 1.08 ng
b CD = conductivity detector; ED = electrochemical detector; FID = flame ionization detector; GC = gas chromatography;
HPLC = high-performance liquid chromatography; HRGC = high-resolution gas chromatography; m = meta-cresol; MS = mass spectrometry;
o = ortho-cresol; p = para-cresol; UV = ultraviolet detector
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Cresols and cresol derivatives occur naturally in various plants.
They are present in oils from jasmine, cassia, Easter lily, ylang
ylang, and Yucca gloriosa flowers and in peppermint, eucalyptus and
camphor. Oils from conifers, oaks and sandalwood trees also contain
cresol (Fiege & Bayer, 1987). Mammalian urine and faeces naturally
contain p-cresol (section 6.5). Poultry manure reportedly contains
p-cresol at an average concentration of 11.7 mg/kg (Yasuhara, 1987).
Cresols are frequently produced as metabolic intermediates in the
degradation of bound phenols by soil microorganisms. They are also
products of combustion and can be released to the atmosphere from
natural fires associated with lightning, spontaneous combustion and
volcanic activity (McKnight et al., 1982).
3.2 Anthropogenic sources
Cresols are contained in crude oil and coal tar. Therefore, the
dominant anthropogenic sources of cresols are accidental and process
discharge during the manufacture, use, transport and storage of
cresols or associated products of the coal tar and petroleum
industries. Cresols are also produced during coal gasification
(Giabbai et al., 1985; Neufeld et al., 1985), coal liquefaction
(Fedorak & Hrudey, 1986) and shale oil production (Snider & Manning,
1982; Dobson et al., 1985). Low levels of cresols are present in the
exhaust of vehicles powered with petroleum-based fuels (Hampton et
al., 1982; Johnson et al., 1989), stack emissions from municipal waste
incinerators (Junk & Ford, 1980; James et al., 1984), and emissions
from the incineration of vegetable materials (Liberti et al., 1983).
Cresols are also found in fly ash from coal and wood combustion (Junk
& Ford, 1980; Hawthorne et al., 1988, 1989). Cigarette smoke contains
cresols (Wynder & Hoffmann, 1967). In addition, the atmospheric
reaction of toluene with photochemically generated hydroxyl radicals
(HO*) produces cresols (Leone et al., 1985).
3.2.1 Production levels and processes
The oldest cresol production method used in the USA is fractional
distillation of coal tar. Most cresols in the USA are obtained via
catalytic and thermal cracking of naphtha fractions during petroleum
distillation. Since 1965, quantities of coal tar and petroleum
isolates have been insufficient to meet the rising demand for cresols
in the USA. Consequently, several processes for the manufacture of
the various isomers have been developed. One method of producing
o-cresol is by the methylation of phenol in the presence of
catalysts. Another method uses toluene sulfonation followed by
alkaline hydrolysis to produce p-cresol. Until 1972, cresols were
also produced by the cymene-cresol process, where cymene
( p-isopropyltoluene) is oxidized to cymene hydroperoxide, which
decomposes to cresols and acetone. This method is capable of
producing p- or m-cresol from the corresponding cymene isomer.
Alkaline chlorotoluene hydrolysis is used to produce a cresol mixture
with a high m-cresol content (Fiege & Bayer, 1987). The total
production of cresols in the USA, excluding production from coke oven
and gas-retort ovens, was 34 400 tonnes in 1989 and 38 300 tonnes in
1990 (USITC, 1990, 1991).
According to the Toxic Release Inventory (TRI) database,
maintained by the US EPA, manufacturing and processing industries in
the USA in 1987 released or transferred 52 tonnes of cresols to air,
water and land, 172.5 tonnes to wastewater treatment plants, and 20.45
tonnes to off-site locations for disposal (US EPA, 1989). The TRI
data may have under-estimated the actual release since only certain
types of facilities were required to report.
3.2.2 Uses
A considerable amount of o-cresol is consumed directly as
either a solvent or disinfectant. o-Cresol is also used as a
chemical intermediate for a variety of products, including deodorizing
and odour-enhancing compounds, pharmaceuticals, fragrances,
antioxidants, dye and dye intermediates, pesticides and resins.
Recently, an increasing proportion of o-cresol has been devoted to
the formulation of epoxy- o-cresol novolak resins (sealing materials
for integrated circuits silicon chips). o-Cresol is also used as an
additive to phenol-formaldehyde resins (Windholz et al., 1983; Fiege &
Bayer, 1987; Sax & Lewis, 1987).
p-Cresol is mainly used in the formulation of antioxidants such
as 2,6-di- tert-butyl- p-cresol for lubricating oil and motor fuels,
rubber, polymers, elastomers and food products. It is also used as an
intermediate in the fragrance and dye industries (Windholz et al.,
1983; Fiege & Bayer, 1987; Sax & Lewis, 1987).
m-Cresol, either pure or mixed with p-cresol, is important in
the production of contact herbicides and insecticides. Furthermore,
many flavour and fragrance compounds and several important
antioxidants are produced from m-cresol. It is also used in the
manufacture of explosives (Fiege & Bayer, 1987).
Mixtures of m- and p-cresol are used as disinfectants and
preservatives. Crude cresols are used as wood preservatives.
Tricresyl phosphate and diphenyl cresyl phosphate produced from m-
and p-cresol mixtures are used as flame-retardant plasticizers for
polyvinyl chloride and other plastics, fire-resistant hydraulic
fluids, additives for lubricants and air filter oils. Cresol mixtures
condensed with formaldehyde are important for modifying phenolic
resins. Cresols are also used in paints and textiles. Mixtures of
cresols are used as solvents for synthetic resin coatings such as wire
enamels, metal degreasers, cutting oils and agents to remove carbon
deposits from combustion engines. They are also used in ore
flotation, fibre treatment and photography (Deichmann & Keplinger,
1981; Windholz, 1983; Fiege & Bayer, 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The levels of cresols in the atmosphere will be regulated by the
physical properties of the compounds, their chemical reactivity and by
prevailing weather conditions (wind speed, precipitation, temperature
inversions, etc.). The vapour pressures of cresols range from 0.13 to
0.31 mmHg (Table 2); compounds with values greater than 0.0001 mmHg
should exist predominantly in the vapour phase (Eisenreich et al.,
1981) as opposed to the particulate-bound phase (Cautreels & van
Cauwenberghe, 1978). Photochemical attack (section 4.2) and rain
scavenging (Leuenberger et al., 1985; Czuczwa et al., 1987) rapidly
remove cresols from the vapour phase, counteracting the tendency of
compounds that exist in the vapour phase to be transported over long
distances.
4.1.2 Water
The processes that control the transport of cresols from water
and their distribution in water are volatility, values for the
sorption coefficient (Koc) to suspended solids and sediment, and
bioaccumulation in aquatic organisms. The bioaccumulation of cresols
in aquatic organisms is discussed in section 4.3. The volatility of a
compound can be qualitatively predicted from its Henry's Law constant
(H). The rate of volatilization from water is high for compounds with
H values ranging from 10-2 to 10-3 atm-m3/mol, and it is very
low for compounds with H values of 10-7 atm-m3/mol or less (Lyman et
al., 1990). Therefore, transport of cresols with H values of 1.26 ×
10-6 to 7.92 × 10-7 atm-m3/mol from water to the atmosphere will
not be significant. Furthermore, the ability of these phenolic
compounds to dissociate and to form hydrogen bonds, leading to binding
with both suspended solids or sediments, will decrease the rate of
volatilization even further. Since the cresols are soluble in water
(see Table 2), the small amounts of cresols typically found in the
aquatic environment will be present mostly in the aqueous phase.
However, transport of cresols from water to bottom sediment is
possible as a result of sorption and subsequent precipitation. For
hydrophobic compounds, the importance of the sorption process can
usually be predicted from the Koc values. Details of Koc levels are
given in section 4.1.3.
4.1.3 Soil
Koc values in soil of between 22 and 3420 have been reported
(Boyd, 1982; Southworth & Keller, 1986; Koch & Nagel, 1988). The
sorption of cresols to several soils correlates well with both pH and
clay mineral content in soil (Artiola-Fortuny & Fuller, 1982), and
several investigators reported that hydrogen bonding plays an
important role in the sorption of cresols to soil (Boyd, 1982;
Southworth & Keller, 1986).
The transport of cresols from soil to the atmosphere will occur
as a result of volatilization. The volatilization of cresols from
soil will be directly proportional to H values and inversely
proportional to Koc. Since the H values for cresols are low and the
Koc in soils capable of hydrogen bonding can be as high as 3420,
volatilization will not be significant in such soils. However, some
volatilization may occur due to the relatively high vapour pressure of
cresols (Table 2) and to the diffusion gradient between the soil and
the atmosphere. Loss of cresols by volatilization has been shown to
occur from highly contaminated soils (Evangelista et al., 1990).
Another process that may transport cresols from soil to ground water
is leaching. The leaching of cresols from soil will depend on the
Koc. This is variable so that with values near 3000, cresols will
be slightly mobile, whereas cresols in soil with Koc values in the
lowest range will be highly mobile (Swann et al., 1983). The
horizontal transport of cresols from one land area to another or to
surface water as a result of run-off will also occur to a certain
extent, dependent among other factors on the soil Koc value.
4.2 Transformation
4.2.1 Abiotic transformation
Two abiotic transformation processes, namely reaction with
hydroxyl HO* and nitrate NO3* radicals, are most important for
determining the fate of cresols in air. The rate constants for the
reaction with HO* are 4.2 × 10-11, 6.4 × 10-11 and 4.7 × 10-11
cm3/molecule-sec for o-, m- and p-cresol, respectively
(Atkinson et al., 1992). It may be estimated from the range of HO*
concentrations in the lower troposphere (from below the limits of
detection at 1 × 106 radicals/cm3 to a maximum of 5 × 106
radicals/cm3) (Atkinson, 1985), that the half-lives for the cresols
during the daytime may range from 3 to 5 h. The major products of the
reactions of HO* with cresols in the presence of nitrogen oxides are
pyruvic acid, acetaldehyde, formaldehyde, peroxyacetylnitrate and
nitrocresols (Atkinson et al., 1980; Grosjean, 1984, 1985). NO3* is
formed in the atmosphere as a result of the reaction of nitrogen oxide
with ozone and is photodecomposed quickly by sunlight (Carter et al.,
1981). Therefore, the reaction of atmospheric pollutants with NO3*
can be significant only during the night. The determined rate
constants for the reaction of NO3* with vapour-phase cresols are
1.37 × 10-11, 9.74 × 10-12 and 1.07 × 10-11 cm3/molecule-sec
for o-, m- and p-cresol, respectively (Carter et al., 1981;
Atkinson et al., 1992). Assuming that the average concentration of
NO3* in a typical night-time urban atmosphere is 2.4 × 108
molecules/cm3, cresols are estimated to be removed from the
atmosphere with half-lives of 5-10 min (Atkinson, 1985).
Abiotic reactions, such as photolysis, hydrolysis and oxidation
by photolytically produced HO* and singlet oxygen, play a minor role
in determining the fate of cresols in water (Smith et al., 1978; Faust
& Hoigné, 1987). However, the photolysis of o- and p-cresol is
accelerated in the presence of fulvic and humic materials present in
water. The estimated half-life for the disappearance of p-cresol in
pure water containing humic acid (9.5 mg/litre) and exposed to April
sunlight at 37.5°N latitude was 3 days (Smith et al., 1978). In a
polluted eutrophic Swiss lake with a dissolved organic matter
concentration of 3.1 mg/litre, the estimated natural half-lives for
p- and o-cresol in the top metre as a result of exposure to June
sunlight were 4.4 and 11 days, respectively (Faust & Hoigné, 1987).
The investigators concluded that photochemically produced organic
peroxide radicals generated from dissolved organic matter controlled
the sensitized photooxidation of cresols in the Swiss lake. In
addition, laboratory experiments have shown that iron (FeOOH) and
manganese (III/IV) oxides (MnOOH and MnO2), commonly found in
surface water particulate and soil, can oxidize cresols in solution
particularly at low pH (< 4) (Stone, 1987). However, oxidation of
cresols occurs more readily in fog and rain water due to the higher
concentration of manganese and iron oxide and low pH of these waters
(Stone, 1987).
Direct attack of cresols by ozone may also occur in water and
follows first-order reaction kinetics: 3 moles of ozone are required
to cause ring-opening of 1 mole of cresol (Zheng et al., 1993a,b). The
overall rate constant for the reaction increases with increasing pH
and temperature. Ozonation may be a possible remediation treatment for
cresol-contaminated waters.
Photochemical reactions will only occur in the upper few
millimetres of the soil surface, and it is unlikely that photochemical
attack will be an important pathway for cresol removal from soil. As
in the case of water, the abiotic hydrolysis of cresols in moist soil
may not be significant since there is no evidence that any soil
component is capable of accelerating this reaction. The oxidation of
cresols by iron(III) and manganese (III/IV) is likely in soils that
have low pH; however, laboratory or field data assessing the
importance of this reaction in determining the fate of cresols in soil
are not available.
4.2.2 Biodegradation
Biotic processes, namely biodegradation, may be more important
than other processes in determining the fate of cresols in water
(Smith et al., 1978). Cresols degraded rapidly in aerobic
biodegradation screening and sewage treatment plant simulation studies
(McKinney et al., 1956; Ludzack & Ettinger, 1960; Malaney, 1960;
Chambers et al., 1963; Tabak et al., 1964; Alexander & Lustigman,
1966; Malaney & McKinney, 1966; Young et al., 1968; Pauli & Franke,
1971; Baird et al., 1974; Pitter, 1976; Singer et al., 1979; Lund &
Rodriguez, 1984; Babeu & Vaishnav, 1987; Brown & Grady, 1990; Klecka
et al., 1990). According to one screening study, the rate of aerobic
biodegradation of the three isomeric cresols increased in the
following order: p- > m- > o-. While no lag time for
biodegradation was observed for m- and p-cresol, o-cresol showed
a lag time of 6 days (Liu & Pacepavicius, 1990). Aerobic
biodegradation in salt water (estuarine and sea water) is slower than
in fresh water, but the decrease in the rate is not enough to preclude
biodegradation as an important removal pathway in salt water (Palumbo
et al., 1988). Mixed and pure culture studies indicate that aerobic
biodegradation of cresols proceeds by initial formation of
hydroxylation products followed by ring-opening reactions (Bayly &
Wigmore, 1973; Masunaga et al., 1983, 1986).
Biodegradation reaction rates are widely variable and depend on a
number of interrelated factors or conditions of the source waters.
Results of several investigations have shown that factors such as
substrate and nutrient concentration, spatial and temporal sampling,
bacterial growth, biofilm formation, pH and temperature all influence
reaction rates. In general, higher nutrient concentrations and
temperatures (summer versus winter) increase the biodegradation of
cresols. However, degradation will decrease with increased humic acid
content (Visser et al., 1977; Smith et al., 1978; Paris et al., 1983,
Spain & van Veld 1983; Rogers et al., 1984; Lewis et al. 1984,1986;
Shimp & Pfaender, 1985a,b; Kollig et al., 1987; Gantzer et al., 1988;
Hwang et al. 1989).
The anaerobic biodegradation potential of cresols in aquatic
media has been observed in the presence of an electron acceptor, as
occurs in nitrate reduction, methanogenesis and sulfate reduction
conditions (Shelton & Tiedje, 1981; Horowitz et al., 1982; Boyd et
al., 1983; Fedorak & Hrudey, 1984; Bak & Widdel, 1986; Roberts et al.,
1987; Battersby & Wilson, 1988, 1989; Wang et al., 1988, 1989).
Cresols biodegrade more slowly under anaerobic conditions than under
aerobic conditions. While several investigators observed a lag period
before the onset of anaerobic biodegradation (Suflita et al., 1988;
Battersby & Wilson, 1989; Liu & Pacepavicius, 1990), Young & Rivera
(1985) observed no significant increase in the rate of p-cresol
metabolism as a result of acclimation. The anaerobic biodegradation
rate for cresols was p- > m- > o- (Suflita et al., 1988; Wang
et al., 1988; Battersby & Wilson, 1989). Other investigators have
reported that o-cresol is more biodegradable under anaerobic
conditions than p-cresol. The m-cresol isomer was found to be
the least biodegradable (Liu & Pacepavicius, 1990). The anaerobic
biodegradation of o- and p-cresol appears to proceed metabolically
by oxidation of the methyl group to produce first the corresponding
hydroxybenzaldehyde and then hydroxy-benzoic acid. The hydroxybenzoic
acid is then decarboxylated or dehydroxylated to produce phenol or
benzaldehyde, respectively (Smolenski & Suflita, 1987; Kühn et al.,
1988; Suflita et al., 1988, 1989). The metabolic pathway for
anaerobic biodegradation of m-cresol may be different from the
pathway for o- and p-cresols (Suflita et al., 1989).
Pseudomonads and other bacteria contain a flavocytochrome enzyme,
p-cresol methylhydroxylase (PCMH), which is capable of oxidizing
p-cresol without the participation of exogenous oxygen (Hopper,
1976, 1978; Hopper & Taylor, 1977; Keat & Hopper, 1978). This enzyme
catalyses the dehydrogenation and hydration of p-cresol and its
homologues to the corresponding alcohols and their further
dehydrogenation to the corresponding aldehydes or ketones. Thus,
p-cresol is oxidized under this condition to p-hydroxybenzyl
alcohol and then to p-hydroxybenzaldehyde. Isolation and then
resolution of the flavocytochrome PCMH into subunits and
reconstitution of the enzyme were studied by Keat & Hopper (1978),
McIntire et al. (1981, 1984, 1985, 1986), McIntire & Singer (1982),
Shamala et al. (1985, 1986) and Koerber et al. (1985).
The biodegradation of cresols in soil under aerobic conditions is
rapid. However, complete metabolism (to CO2 and H2O) of the
intermediate metabolites is slower (Medvedev & Davidov, 1981a,b;
Dobbins & Pfaender, 1988; Namkoong et al., 1988). Biodegradation is
likely to control the fate of cresols in soils. In surface soils from
an uncultivated grassland site, the estimated half-life for the
pseudo-first-order disappearance of the parent compound was 1.6 days
for o-cresol and 0.6 days for m-cresol. It could not be
calculated for p-cresols as the concentration had fallen below the
detection limits at the first sampling, which was 24 h after
initiation of the experiment (Namkoong et al., 1988). The half-lives
for complete metabolism in different soils ranged from 39 days to
about 1 year (Dobbins & Pfaender, 1988; Swindoll et al., 1988).
4.3 Bioaccumulation and biomagnification
The measured bioconcentration factors for o-cresol and
m-cresol in aquatic organisms were 14.1 and 19.9, respectively
(Freitag et al., 1982; Sabljic, 1987). There is no evidence in the
literature to indicate that biotransfer of cresols via the food chain
causes biomagnification of these compounds.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air monitoring data for cresols are sparse. These
compounds are short-lived in the air (see section 4.2.1) unless large
amounts are released over a short period of time. According to the
National Ambient Volatile Organic Compounds (VOCs) Data Base, a
compilation of published and unpublished air monitoring data in the
USA from 1970 to 1987, the median air concentration of o-cresol at
source-dominated sites was 1.59 µg/m3 (0.359 ppb) (range from below
detection limit to 10.58 µg/m3, 2.394 ppb) for 32 samples (Shah &
Heyerdahl, 1989). According to the same data base, o-cresol was not
detected in air samples from one urban, one rural and one remote area,
and m-cresol was also not detected in air samples from one urban,
one suburban, and one remote area in the USA. This data base does not
contain any monitoring data for p-cresol. The concentration of
o-cresol in one sample of the ambient air near a phenolic resin
factory in Japan was 179 µg/m3 (40 ppb) (Hoshika & Muto, 1978). In
air samples from rooms with a fireplace, cresol concentrations around
5 mg/m3 have been detected (Risner, 1993).
5.1.2 Water
In general, cresols will degrade in surface waters very rapidly.
The STORET data base, a computerized data base maintained by US EPA,
contains water quality data. According to STORET (1993), the mean,
minimum and maximum concentrations of ocresol in surface water were
10.89, below the detection limit and 68 µg/litre, respectively, out of
315 samples reported; for p- or m-cresol they were 12.5, 3.4 and
25 µg/litre out of 52 samples; and for p-cresol they were 12.45,
below the detection limit and 77 µg/litre out of 285 samples. In
addition, the three isomers of cresol were qualitatively detected in
Spirit Lake, a freshwater lake in the state of Washington, USA.
o-Cresol was also detected in two other freshwater bodies in the
same state. The presence of cresols was attributed to the Mount St.
Helens eruption (McKnight et al., 1982). Whether or not the cresols
originated from woodfires or the actual eruption was not clarified in
this study. p-Cresol was detected at a concentration of
200 µg/litre in water samples from the lower Tennessee River near
Calvert City, Kentucky, USA (Goodley & Gordon, 1976). m-Cresol was
qualitatively detected in St. Joseph River of the Lake Michigan Basin
(Great Lakes Water Quality Board, 1983). Cresols (isomers
unseparated) were not detected in Delaware River water samples taken
between Marcus Hook, Pennsylvania, and Trenton, New Jersey, USA,
during summer months, but were detected at 2 µg/litre in winter
(Sheldon & Hites, 1978). Concentrations of p-cresol as high as
204 µg/litre have been detected in a river in Japan polluted by
effluents from a leather factory (Yasuhara et al., 1981).
Although o-cresol has been qualitatively detected in
drinking-water in the USA (Clark et al., 1986), quantitative data
regarding cresol levels in drinking-water are not available.
Cresols have been qualitatively detected in effluent from sewage
treatment plants in the USA (Ellis et al., 1982). Concentrations of
70-150 µg/litre (isomer unidentified) have been measured in the
wastewater from a chemical manufacturing plant (Jungclaus et al.,
1978), and concentrations as high as 2100 µg/litre for o-cresol and
1200 µg/litre for mixed m- and p-cresol have been measured in
wastewater from a shale oil plant (Hawthorne & Sievers, 1984).
Cresols were detected at 20 µg/litre in the treated secondary effluent
from Philadelphia Northeast Sewage Treatment Plant, but were not
detected in Delaware River water near the discharge point of the
effluents or further downstream (Hites, 1979; Sheldon & Hites, 1979).
Furthermore, cresols have been detected in treated coke oven aqueous
condensates, wastewater from petroleum refineries and wood-preserving
plants, and aqueous effluents from synfuel processing (US EPA, 1982).
Cresols may persist in groundwater due to a lack of
microorganisms. Very little information regarding the concentration
of individual isomers has been reported in the literature.
Cresol concentrations measured in groundwater from hazardous
waste and landfill sites are shown in Table 4. Although the
concentration of p-cresol was below the detection limit
(30 µg/litre), o- and m-cresol concentrations of around
1400 µg/litre have been detected in creosote-contaminated groundwater
in Denmark (Flyvbjerg et al., 1993). According to STORET (1993), the
mean, minimum and maximum levels in groundwater from undefined sources
for o-cresol were 234.3, 0.9 and 100 000 µg per litre out of 1848
samples collected; for m-cresol were 1421.3, below the detection
limit and 100 000 µg/litre out of 712 samples; and for p-cresol were
15.79, 0.09 and 4800 µg/litre out of 1147 samples, respectively.
Rainwater from Portland, Oregon, collected during seven falls of
rain in 1984, contained o-cresol concentrations of 0.24-2.8 µg per
litre (mean of 1.02 µg/litre) and combined p- and m-cresol
concentrations of 0.38-2.0 µg/litre (mean of >1.1 µg/litre)
(Leuenberger et al., 1985). The concentration of o-cresol in
rainwater at a rural site in Switzerland (Greppen) ranged from
undetectable to 1.3 µg/litre. The combined concentration range of
m- and p-cresols in the same rainwater was 0.65-9.3 µg/litre
(Czuczwa et al., 1987).
Table 4. Cresol concentrations in the ground water of hazardous waste sites and landfills in the USA
No. of samples/ Concentration
Type/location Sampling date no. detecteda Isomer (mg/litre) Reference
Hazardous waste, no data 1/1 o 2.3 Weber & Matsumoto (1987)
Buffalo, New York 1/1 p 15.0
Former pine-tar manufacturing, no data 11/10 o 0.002-5.2 McCreary et al. (1983)
Gainesville, Florida 11/10 m and p 0.0004-11.1
Former wood preserving, 1984 19/6 o 0.04-7.1 Goerlitz et al. (1985)
Pensacola, Florida 19/3 p 0.02-6.2
19/4 m 0.05-13.7
Former coal gasification, no data 3/3 o 0.063-6.6 Stuermer et al. (1982)
Hoe Creek, Wyoming 3/3 m and p 0.096-16.0
Municipal landfill, 1982-1983 1/1 p 1.5 Sawhney & Kozloski (1984)
Southington, Connecticut 1982-1983 1/1 m 0.6
Underground solvent 1983 10/1 unseparated 0.04 Oliveira & Sitar (1985)
storage tanks,
Santa Clara, California
Hazardous waste, 1979-1984 4/1 unseparated 0.11 Ram et al. (1985)
Coventry, Rhode Island
a Number of samples compared with number in which cresols were detected
5.1.3 Soil
Cresols have been detected in about 1% of soil samples from 1300
Superfund (hazardous waste sites listed by US EPA in the National
Priority List) sites. The geometric mean concentrations of o- and
p-cresols in these samples were 409 and 677 µg/kg, respectively
(HAZDAT, 1992).
5.1.4 Food and beverages
Cresols have been detected in certain foods and beverages, such
as tomatoes, tomato ketchup, cooked asparagus, various cheeses,
butter, oil, red wine, spirits, raw and roasted coffee, black tea,
smoked foods and tobacco (Fiege & Bayer, 1987). Cresols were
identified as volatile components of fried chicken (Ho et al., 1983).
Quantitative data regarding cresols in food and beverages are limited.
Cresols have been detected in various beverages including Scotch
whisky (0.01-0.20 mg/litre), whiskies made outside of Scotland
(0.01-0.07 mg/litre), brandies including cognac and armagnac (trace to
0.02 mg/litre), and white and dark rums (trace to 0.20 mg/litre)
(Lehtonen, 1983). The total amount of cresols in the smoke from a
nonfilter American cigarette (85 mm) is about 75 µg (Wynder &
Hoffmann, 1967).
5.2 General population exposure
The general population can be exposed to cresols from air
inhalation, drinking-water and food ingestion, and dermal contact with
water or consumer products that contain cresols. Due to the lack of
adequate monitoring data regarding cresol levels in ambient air and
drinking-water, it is not possible to estimate quantitatively the
daily intake of cresols from these sources. Similarly, to estimate
the daily intake of cresol from food for a member of the general
population requires data concerning the level of these compounds in
total diet samples (various categories and quantities of food consumed
daily by a typical individual), and these data are not available.
Dermal contact to cresols may also result from use of certain consumer
products, since cresols may be used as disinfectants in some soap and
as wood preservatives. It is likely that people who live near certain
kinds of emission sources (e.g., heavy vehicular traffic, certain
incinerators, and landfill sites, such as abandoned coal tar or
creosote producer/user sites) will be exposed to higher levels of
cresols than the general population. Since both mainstream and
sidestream smoke of cigarettes contain cresols (Wynder & Hoffmann,
1967), smokers and those who inhale sidestream smoke may be exposed to
a higher level of cresols.
5.3 Occupational exposure
Occupational exposure to cresols is likely among workers
involved in the production of cresols or processes that produce
cresols (coal gasification, shale oil retorting) and those who use
cresols or products containing cresols (such as creosote). Little
information regarding occupational exposure to cresols is available.
The concentration of cresols in the workroom air of a pilot coal
gasification plant in the USA was < 0.44 mg/m3 (< 0.1 ppm)
(Dreibelbis et al., 1985). The extent of worker exposure to cresols
and other pollutants was measured in a facility in Finland that used
creosote for impregnation of wood. The highest observed mean
concentration of cresols in the air was 0.6 mg/m3 during periods in
which the cylinder used for impregnation was opened, followed by a
concentration of 0.2 mg/m3 during periods in which the cylinder was
closed (Heikkila et al., 1987).
All 14 countries listed in ILO Occupational Exposure Limits for
Airborne Toxic Substances (1991) have set an environmental
concentration of 22.1 mg/m3 (5 ppm) for time-weighted average (TWA)
exposure for all isomers of cresol.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Cresols are absorbed across the respiratory and gastrointestinal
linings and through the intact skin. Absorption of cresols through
the lungs has not been studied quantitatively. However, the
occurrence of mortality and other systemic effects in animals exposed
to cresol aerosols and vapours in air shows that absorption through
the lungs does occur (Uzhdavini et al., 1972; Pereima, 1975). The
rate and extent of gastrointestinal absorption of cresols have not
been studied specifically. However, they are suggested by data
showing that rabbits exposed orally to cresols excreted 65-84%
(depending on the isomer) of the administered dose in the urine within
24 h (Bray et al., 1950), indicating that at least that amount was
absorbed within that time period. The occurrence of coma, death and
systemic effects in humans after dermal exposure to cresols (see
section 8) indicates that these compounds can be absorbed through the
skin. In the case of an infant who had coal tar fluid (90% cresols in
water) spilled on his head, unconsciousness occurred within 5 min and
death within 4 h, showing that absorption was rapid (Green, 1975). An
in vitro study of the permeability of human skin to cresols showed
that these substances have permeability coefficients greater than that
of phenol, which is known to be readily absorbed across the human skin
(Roberts et al., 1977). Permeability coefficients (Kp) were estimated
from the steady-state slopes of the relation between the cumulative
amount of cresol isomer per unit area of membrane with time. The
following Kp values were determined: m-cresol = 2.54 × 10-4
cm/minute; o-cresol = 2.6 × 10-4 cm/minute; and p-cresol = 2.92 ×
10-4 cm/minute (Roberts et al., 1977).
In a similar study, Hinz et al. (1991) showed rapid percutaneous
transport of p-cresol across mouse skin in vitro. Approximately
70% of the dose was transported within 6 h.
6.2 Distribution
Very few data are available regarding the distribution of cresols
into various tissues. Oral exposure studies in dogs indicate that
cresols in the body concentrate in the blood, liver and brain
initially, but soon become more widespread, appearing in the lungs,
kidneys and other organs (Gadaskina & Filov, 1971). Cresols were
detected in the blood (120 mg/litre), liver, brain and urine of a
human infant who died 4 h after 20 ml of a cresol derivative was
spilled on his head (Green, 1975).
6.3 Metabolic transformation
The primary metabolic pathway for cresols is conjugation with
glucuronic acid and inorganic sulfate. At physiological pH, the
conjugated metabolites are ionized, thus reducing renal reabsorption
and aiding urinary excretion. After oral administration of cresols to
rabbits, 60-72% of the dose was recovered as ether glucuronide, and an
additional 10-15% was recovered as ethereal sulfate in the urine (Bray
et al., 1950). Similarly, in an earlier study in rabbits, 14.5-23.5%
of orally administered cresols was found to be conjugated with sulfate
in the urine (Williams, 1938). By analogy with other phenols, it may
be expected that the relative amounts of glucuronide and sulfate
conjugates will differ between species and will also vary with dose.
Minor metabolic pathways for cresols include hydroxylation of the
benzene ring (primarily for o- and m-cresols) and side-chain
oxidation (only for p-cresol). In orally dosed rabbits, 3% of the
administered dose was recovered in the urine as conjugated
2,5-dihydroxytoluene for both o- and m-cresols (Bray et al.,
1950). For p-cresol, only a trace amount of 3,4-dihydroxytoluene
was found, but 10% of the dose was recovered as p-hydroxybenzoic
acid. After cresols were administered to rabbits, only 1-2% of the
dose was found as unconjugated free cresol in the urine (Bray et al.,
1950). Thompson et al. (1994) studied the metabolism of
[14C]- p-cresol in rat liver slices and a microsomal fraction.
They found that [14C]- p-cresol is metabolized to a reactive
intermediate which co-valently binds to proteins in the liver slices
and that the binding is inhibited by n-acetylcysteine. In
microsomal incubations and a NADPH-generating system, covalent binding
of [14C]- p-cresol metabolites was also observed. This binding was
inhibited by glutathione (GSH) resulting in the formation of a
glutathione conjugate. In the absence of GSH, p-hydroxybenzyl
alcohol was the major microsomal metabolite formed from p-cresol.
Yashiki et al. (1989) reported the recovery of conjugated cresols in
the biological fluids of a 46-year-old man following the ingestion of
100 ml saponated cresol soap solution (42%). Conjugated and free m-
and p-cresols were measured in both the serum and urine 2 h after
ingestion. Of the total recovered in the serum, 79% p-cresol and
75% m-cresols were in the conjugated form while over 99% of m- and
p-cresols recovered in the urine was conjugated.
6.4 Elimination and excretion
Significant amounts of cresols are excreted in the bile, but most
of the cresols excreted in this manner are reabsorbed from the
intestine following hydrolysis by gut bacteria (Deichmann & Keplinger,
1981). The main route for removing cresols from the body is renal
elimination.
6.5 Endogenous cresols
Healthy humans excrete an average of about 50 mg (range 16-74 mg)
of p-cresol in the urine daily (Bone et al., 1976; Renwick et al.,
1988). Endogenous p-cresol is produced from tyrosine, an amino acid
present in most proteins, by anaerobic bacteria in the intestine (Bone
et al., 1976). Free p-cresol formed in this way is absorbed from
the intestine and eliminated in the urine as conjugates.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation route
Acute poisoning with cresol vapour is unlikely due to the low
vapour pressure of these compounds. However, inhalation of an aerosol
and vapour mixture may cause death. Uzhdavini et al. (1972) conducted
studies into the acute toxicity of o-cresol in mice. The mean lethal
concentration of the vapour/aerosol mixture was 178 mg/m3 (duration of
exposure not specified). Clinical signs of toxicity included
irritation of mucous membranes and neuromuscular excitation that
progressed from twitching of individual muscles to clonic convulsions.
Haematuria was reported at very high concentrations. Microscopic
examination revealed oedematous changes in the lung and necrotic and
degenerative changes in the liver (fatty degeneration, centrilobular
necrosis) and kidneys (oedema, swelling of the glomeruli, degeneration
of the tubular epithelium, and perivascular haemorrhage). Mean lethal
concentrations of cresols in rats were reported to be 29 mg/m3 for
o- and p-cresols and 58 mg/m3 for m-cresol (Pereima, 1975).
7.1.2 Oral route
Oral LD50 values for cresols are shown in Table 5. A
comparison of the LD50 values for all three cresol isomers from
these studies (e.g., Deichmann & Witherup, 1944; Bio-Fax, 1969) shows
that o-cresol is the most toxic isomer, followed by p-cresol and
then m-cresol. Interspecies comparisons reveal that all three
isomers are more toxic to mice than to rats, by this route of
administration, the LD50 values being 3-4 times higher in rats than
in comparably treated mice (Uzhdavini et al., 1972; Pereima, 1975).
The data also show that for all three isomers toxicity increases with
concentration; undiluted cresols were more toxic than cresols
delivered as 10% solutions in oil. In addition, there is some
evidence that the delivery vehicle affects toxicity; the LD50 value
for m-cresol was lower in rats given a 10% solution in water than in
rats given a 10% solution in oil.
Clinical signs of toxicity that preceded death in acute oral
lethality studies of all three cresol isomers were hypoactivity and
lethargy, excess salivation, dyspnoea, haemorrhagic rhinitis
( p-cresol only), incoordination, prostration, muscle twitches and
tremors, convulsions and coma (Deichmann & Witherup, 1944; Mellon
Institute, 1949; Bio-Fax, 1969; Hornshaw et al., 1986). Necropsy of
rats that died revealed gastrointestinal inflammation and haemorrhage,
as well as hyperaemia of the lungs, liver and kidney (Mellon
Institute, 1949; Bio-Fax, 1969). Necropsy of survivors after 14 days
of observation revealed only gastro-intestinal tract inflammation in
rats treated with p-cresol and no gross lesions in rats treated with
o- or m-cresol (Bio-Fax, 1969).
Table 5. Oral LD50 values for cresols
LD50
Cresol Species Vehicle (mg/kg) Reference
o-Cresol Rat 10% in oil 1470 Uzhdavini et al. (1976)
10% in oil 1350 Deichmann & Witherup (1944)
50% in oil 360 FDRL (1975)
Undiluted 121 Bio-Fax (1969)
Mouse 10% in oil 344 Uzhdavini et al. (1976)
Rabbit 10% in oil 940 Uzhdavini et al. (1976)
m-Cresol Rat 10% in oil 2010 Pereima (1975)
10% in oil 2020 Deichmann & Witherup (1944)
10% in water 520 Mellon Institute (1949)
Undiluted 242 Bio-Fax (1969)
Mouse 10% in oil 600 Pereima (1975)
10% in oil 828 Uzhdavini et al. (1976)
p-Cresol Rat 10% in oil 1430 Pereima (1975)
10% in oil 1460 Uzhdavini et al. (1976)
10% in oil 1800 Deichmann & Witherup (1944)
Undiluted 207 Bio-Fax (1969)
Mouse 10% in oil 440 Pereima (1975)
10% in oil 344 Uzhdavini et al. (1976)
Dicresol Rat 10% in oil 1625 Uzhdavini et al. (1976)
Mouse 10% in oil 651 Uzhdavini et al. (1976)
7.1.3 Dermal route
Cresols may cause death when applied to the skin. Dermal LD50
values in rabbits were 890, 2830, 300 and 2000 mg/kg for o-, m-,
p- and mixed cresols, respectively, following 24-h dermal exposure
(Vernot et al., 1977). In rats, the dermal LD50 values were 620,
1100, 750 and 825 mg/kg for o-cresol, m-cresol, p-cresol and
dicresol (a mixture of m- and p-cresols), respectively (Uzhdavini
et al., 1974, 1976).
7.2 Short-term exposure
7.2.1 Inhalation route
Uzhdavini et al. (1972) exposed mice to a mixture of o-cresol
aerosol and vapour 2 h/day, 6 days/week for 1 month; exposure
concentrations varied from 26 to 76 mg/m3, with an average of
50 mg/m3. No mortality was recorded. Clinical signs of toxicity
during the daily exposure periods were limited to signs of
respiratory irritation at the start of the exposure, followed by a
period of hypoactivity lasting until the end of the exposure. The
tails of some animals mummified and fell off after 18-20 days. Body
weight gain was slightly reduced compared to controls. Microscopic
examination revealed signs of irritation in the respiratory tract;
these included oedema, cellular proliferation, and small haemorrhages
in the lung. Other lesions included degeneration of heart muscle,
liver, kidney and nerve cells and glial elements of the central
nervous system.
7.2.2 Oral route
Female B6C3Fl mice (8-10 weeks of age) were exposed to
o-cresol at concentrations of 0, 6.5, 32.5, 65 or 130 mg/kg per day
ad libitum in the drinking water) for 14 days (CIIT, 1983).
Immunotoxicity or altered host resistance was measured as changes in
haematological values, lymphoid organ weights, altered lymphoid cell
morphology and cell or humoral-mediated immune function. No evidence
of immunotoxicity was seen in any of the parameters tested. No
changes in immune functions were reported at any dose level.
Therefore the threshold for immune response in these studies is above
130 mg/kg per day (see Table 6).
US NTP (1992) conducted 28-day studies in which Fischer 344/N
rats and B6C3F1 mice were exposed to o-, m-, p- or
m-/ p-cresol (60:40 mixture of the m- and p-) in the feed. For
each substance, groups of five animals of each sex and each species
were fed ad libitum diets containing 0, 300, 1000, 3000,
10 000 or 30 000 mg/kg. Estimated daily doses (mg/kg body weight per
day) in males and females of each species exposed to each test
substance are shown in Table 7. None of the cresols caused mortality
in rats. All cresols reduced feed consumption during the first week
of the study and body weight gain throughout the study in rats exposed
at the highest level. However, feed consumption of all dosed groups
was comparable to that of controls after the first week. Clinical
signs of toxicity were not observed in rats treated with o- or
m-cresol, but rats exposed to 30 000 mg p-cresol/kg had hunched
posture, rough hair coat and thin appearance. Thin appearance was
also noted in rats exposed to the highest dose of m-/ p-cresol.
Organ weight changes in rats included increases in absolute and
relative liver weight and kidney weight compared to brain weight.
Increases in several other organ weights, relative to body weight were
reported, but as there was a very marked decrease in body weight at
the highest dose levels, only the increased liver and kidney weights,
relative to brain weight, were regarded as being of biological
significance. No gross or microscopic lesions were found in rats
exposed to o-cresol. m-Cresol caused minimal-t o-mild atrophy of
the uterus in females exposed to 30 000 mg/kg. p-Cresol also caused
uterine atrophy in females exposed to 30 000 mg/kg, as well as bone
marrow hypo-cellularity and nasal lesions (atrophy of olfactory
epithelium and hyperplasia and squamous metaplasia of respiratory
epithelium) in rats exposed to > 3000 mg/kg. m-/ p-Cresol caused
hyperplasia of the respiratory epithelium in the nasal cavity at >
1000 mg/kg, increased colloid within thyroid follicles at >
3000 mg/kg, mild hyperplasia and hyperkeratosis of the oesophageal
epithelium and forestomach at > 3000 and > 10 000 mg/kg,
respectively, and mild bone marrow hypocellularity at >
10 000 mg/kg. A no-observed-adverse-effect level (NOAEL) of
3000 mg/kg was established for o, m and m/p cresols and a NOAEL of
1000 mg/kg for p-cresol based on organ weight and body weight
changes at higher doses.
In the mice exposed in this study death was caused by o-,
m-and p-cresol at 30 000 mg/kg and only by m- or p-cresol at
10 000 mg/kg. The m-/ p-mixture was not lethal to mice at any
concentration. For all cresols, high-dose mice that survived exposure
lost weight during the study, and body weight gain was generally
decreased in the 10 000 mg/kg groups as well. Clinical signs of
toxicity seen at > 10 000 mg/kg in mice exposed to m-and
p-cresols and 30 000 mg/kg in mice exposed to o- and
m-/ p-cresols included hunched posture, thin appearance, rough hair
coat, lethargy, hypothermia, rapid breathing and tremors. Organ
weight changes in mice were increased in absolute and relative liver
Table 6. Short-term toxicity of cresolsa
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ NR/F (8-10 o-cresol oral 0, 6.5, 32.5, 14 days No effects noted in haematology or CIIT (1983)
B6C3Fl weeks old) (drinking) 65 or 130 immune functions
water mg/kg/day
Mice/ 5/sex f/m o-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg death, (2 males & 1 US NTP
B6C3Fl 1000, 3000, female) tremors, rough hair coat, (1992)
10 000 or ovarian atrophy; > 10 000 mg/kg
30 000 body weight decreased, uterine
mg/kg diet atrophy; > 3000 mg/kg increased
relative liver weight
Mice/ 5/sex f/m m-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg increased brain weight, US NTP
B6C3Fl 1000, 3000, ovarian, uterine and mammary gland (1992)
10 000 or atrophy; 10 000 mg/kg (1 female)
30 000 and 30 000 mg/kg (2 male, 2 female)
mg/kg diet death, decreased body weight, clinical
signs of toxicity; > 3000 mg/kg
increased kidney weight; > 300 mg/kg
increased liver weight
Mice/ 5/sex f/m p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg death all animals; US NTP
B6C3Fl 1000, 3000, 10 000 mg/kg (1 male) death, clinical (1992)
10 000 or signs of toxicity, reduced body
30 000 weight; > 3000 mg/kg increased liver
mg/kg diet weight; > 300 mg/kg nasal respiratory
lesions
Table 6 (cont'd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ 5/sex f/m m-/p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg clinical toxicity, and US NTP
B6C3Fl (60:40 ratio) 1000, 3000, respiratory metaplasia and atrophy of (1992)
10 000 or nasal epithelium; > 3000 mg/kg
30 000 hyperplasia lungs, oesophagus and
mg/kg diet forestomach, uterine and ovarium
atrophy
Mink 5/sex f/m o-cresol oral (diet) 0, 240, 432, 28 days 2520 mg/kg reduced body weight Hornshaw
178, 1400 gain, increased relative heart weight, et al.,
or 2520 decreased haemoglobin; > 1400 (1986)
mg/kg diet mg/kg decreased RBC count; > 432
mg/kg increase relative liver weight
Ferrets 5/sex f/m o-cresol oral (diet) 0, 432, 778, 28 days 4536 mg/kg decreased RBC count; > Hornshaw
1400, 2520, 1400 mg/kg increased relative liver et al.,
4536 and kidney weight (1986)
mg/kg diet
Rats/ 5/sex f/m o-cresol oral (diet) 0, 300, 28 days > 3000 mg/kg increased relative liver US NTP
Fischer-344 1000, 3000, and kidney weight; 30 000 mg/kg (1992)
10 000, decreased body weight
30 000
mg/kg diet
Rats/ 5/sex f/m m-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg decreased body US NTP
Fischer-344 1000, 3000, weight; increased relative kidney (1992)
10 000, weight; mild atrophy of uterus; >
30 000 10 000 mg/kg increased relative liver
mg/kg diet weight
Table 6 (cont'd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rats/ 5/sex f/m p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg reduced body weight, US NTP
Fischer-344 1000, 3000, rough coat, thin appearance, uterine (1992)
10 000 or atrophy, bone marrow and nasal
30 000 lesions; > 10 000 mg/kg increased
mg/kg diet relative kidney weight; > 3000 mg/kg
increased relative liver weight
Rats/ 5/sex f/m m-/p-cresol oral (diet) 0, 300, 28 days 30 000 mg/kg reduced body weight, US NTP
Fischer-344 (60:40 1000, 3000, thin appearance, > 10 000 mg/kg (1992)
mixture) 10 000 or increased kidney weight, > 1000
30 000 mg/kg histopathogenic changes, and
mg/kg diet increased relative liver weight
Mice/NR NR/NR o-cresol inhalation 50 mg/m3 2 h/day inactivity, reduced body weight gain, Uzhdavini
6 days/ CNS effects; histopathological et al.
week for changes of lungs, kidney, liver, heart (1972)
1 month and CNS
a NR = not reported
Table 7. Comparative mean compound consumption by rats and mice in US NTP (1992) 28-day studiesa
Dose o-Cresol m-Cresol p-Cresol m-/p-Cresolb
Species (mg/kg diet) M F M F M F M F
Rats 0 0 0 0 0 0 0 0 0
300 27 27 25 25 25 25 26 27
1000 87 89 85 83 87 83 90 95
3000 266 271 252 252 256 242 261 268
10 000 861 881 870 862 835 769 877 886
30 000 2610 2510 2470 2310 2180 2060 2600 2570
Mice 0 0 0 0 0 0 0 0 0
300 66 82 53 66 50 60 50 65
1000 193 280 193 210 163 207 161 200
3000 558 763 521 651 469 564 471 604
10 000 1650 1670 1730 2080 1410 1590 1490 1880
30 000 4480 5000 4710 4940 no datac no data 4530 4730
a Compound consumption given in mg/kg body weight per day; M = male, F = female
b 60% m-cresol/40% p-cresol
c No data calculated due to 100% mortality
weight. Increases in several organ weights, relative to body weight,
were observed, but as there was a very marked decrease in body weight
at the highest dose level, only the increased liver weight, relative
to brain weight, was regarded as being of biological significance.
o-Cresol caused uterine atrophy in mice exposed to > 10 000 mg/kg
and ovarian atrophy in those exposed to 30 000 mg/kg diet. m-Cresol
caused uterine and ovarian lesions and mammary gland atrophy in mice
exposed to 30 000 mg/kg diet. These changes could have been secondary
to the marked loss of body weight. p-Cresol caused nasal lesions in
mice at all concentrations tested; these lesions consisted mostly of
mild hyperplasia and squamous metaplasia of the respiratory
epithelium. Effects on the olfactory epithelium (atrophy, necrosis)
were generally observed only in mice in the 30 000 mg/kg diet group.
Other lesions in the 30 000 mg/kg diet mice, which all died early in
the study, were renal tubular and hepatic necrosis together with
lymphoid depletion and necrosis in several organs. m-/ p-Cresol
caused hyperplasia of the respiratory epithelium at > 3000 mg/kg
diet. Atrophy and metaplasia of the olfactory epithelium were
observed in mice exposed to 30 000 mg/kg diet. Other lesions observed
at this level were mild bronchiolar hyperplasia, bone marrow
hypocellularity, minimal hyperplasia of the oesophagus and
forestomach, and uterine and ovarian atrophy. A NOAEL of 1000 mg/kg
diet could be identified for m-/pcresol and o- or p-cresol,
respectively, based on organ weight and body weight and
histopathological changes at higher doses. For m-cresol, the lowest
dose tested (300 mg/kg diet) resulted in a small increase in relative
liver weight in females only, and so could be regarded as a NOAEL.
Hornshaw et al. (1986) conducted 28-day feeding studies using
mink and ferrets. Groups of five mink of each sex were fed diets
containing 0, 240, 432, 778, 1400 or 2520 mg/kg diet of o-cresol.
Doses were calculated to be 0, 35, 80, 125, 200 and 320 mg/kg body
weight per day in males and 0, 55, 120, 190, 300 and 480 mg/kg body
weight per day in females. No deaths occurred during the study, and
no clinical signs of toxicity were observed. Consumption of feed by
mink exposed to 2520 mg/kg diet was reduced during the first week of
the study, and body weight gain was depressed in this group over the
course of the study. Haematological analyses revealed decreases in red
blood cell count at > 1400 mg/kg diet and in haemoglobin at
2520 mg/kg diet. No lesions were detected by gross necropsy, but
liver:body weight ratio was increased at > 432 mg/kg diet and
heart:body weight ratio was increased at 2520 mg/kg diet. An NOAEL of
240 mg/kg diet was identified in this study for male and female mink
exposed to o-cresol.
A similar study in ferrets was also conducted using groups of
five animals of each sex exposed to dietary concentrations of 0, 432,
778, 1400, 2520 and 4536 mg/kg diet of o-cresol. Doses were
calculated to be 0, 45, 85, 140, 290 and 400 mg/kg body weight per day
in males and 0, 80, 150, 240, 530 and 720 mg/kg body weight per day in
females. No mortality was recorded and no clinical signs of toxicity
were observed. Feed consumption was slightly reduced at 4536 mg/kg
diet, but no effect on body weight gain was noted. Red blood cell
count was decreased at 4536 mg/kg diet. Increases in liver:brain
weight ratio and kidney: brain weight ratio were reported at
> 1400 mg/kg diet and 4536 mg/kg diet, respectively. No lesions
were found by gross necropsy. A NOAEL of 778 mg/kg diet was
identified for male and female ferrets based on increased relative
liver weight at doses > 1400 mg/kg diet.
7.3 Long-term exposure
7.3.1 Inhalation route
Rats were exposed to an average concentration of 9 mg/m3 of
o-cresol vapour 4-6 h/day, 5 days/week for 4 months (Uzhdavini et
al., 1972). The number of animals and strain was not reported in the
study. Effects of o-cresol exposure in rats included accelerated
loss of conditioned defensive reflex, leukocytosis, decreased
erythroid/myeloid ratio in the bone marrow, increased duration of
hexanol narcosis (indicating possible impaired liver function) and
morphological changes in respiratory tissues (inflammation and
irritation of the upper respiratory tract, oedema, and perivascular
sclerosis in the lungs) (see Table 8).
In three other studies rats were administered individual cresol
isomers or a mixture of isomers by the inhalation route for 3 to 4
months at doses ranging from 0.05 to 10 mg/m3 (Uzhdavini & Gilev,
1976; Pereima, 1975; Uzhdavini et al., 1976). In each study a
decrease in body weight gain was reported for rats exposed to cresols.
Organ weight changes and histological alterations in the liver and
kidney were also reported in all three studies. Because of the
limited reporting of data regarding the exposure methods, number of
animals and results, these studies could not be adequately evaluated.
7.3.2 Oral route
In 13-week feed studies, groups of 20 male and 20 female Fischer
344/N rats were fed diets containing 0 to 30 000 mg/kg diet of
o-cresol or a 60:40 mixture of m-/ p-cresol (US NTP, 1992).
Estimated daily doses (mg/kg body weight per day) are shown in Table
9. No treatment-related deaths were caused by either isomer (Table
8). For both isomers, food consumption during the first week was
decreased at 30 000 mg/kg diet, and body weight gain was reduced at
> 15 000 mg/kg diet. Clinical signs of toxicity were not observed
in rats fed o-cresol, but rough hair coat and thin appearance were
noted in rats fed m-/ p-cresol at 30 000 mg/kg diet. Organ weight
changes in both males and females administered cresols included
increases in absolute and relative liver weight (> 7500 mg/kg diet
for both cresols) and kidney weight (> 7500 mg/kg diet for
m-/ p-cresol). Increases for several other organ weights (relative
to body weight) were reported but as there was a marked decrease in
body weight at the highest dose levels, only the increases in liver
and kidney weight relative to brain weight were regarded as
biologically significant. Haematology analyses were not significantly
affected by treatment with cresols. Results of urinalysis did not
indicate any significant renal damage. The most noteworthy finding of
clinical chemistry analyses of plasma was a dose-related increase in
total bile acids in males and females exposed to both isomers
(significant at dose > 1880 mg/kg diet for m-/ p-cresol and > 15 000
mg/kg diet for o-cresol), indicating decreased hepatocellular
function. Transitory increases in alanine aminotransferase and/or
sorbitol dehydrogenase near the start of the study in rats exposed to
both isomers suggest that hepatocellular injury may have occurred and
regressed. Histopathological changes included a dose-related increase
in the incidence and severity of hyperplasia in the nasal respiratory
epithelium of rats exposed to m-/ p-cresol in the feed (> 1880 mg/kg
diet), increased colloid within thyroid follicles (> 3750 mg/kg
diet), uterine atrophy (> 15 000 mg/kg diet), and bone
marrow hypocellularity (> 15 000 mg/kg diet). The only lesion in
rats treated with o-cresol was bone marrow hypocellu-larity at
> 7500 mg/kg diet. Both isomers appeared to lengthen the estrus
cycle in treated female rats. An NOAEL in rats of 3750 mg/kg diet was
identified for o-cresol. However, for m-/ p-cresol the lowest
dose tested resulted in changes in clinical chemistry and hyperplasia,
and so a threshold dose for m/ p-cresol could not be determined.
Table 8. Long-term toxicity of cresols
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rat/NR NR/NR o-cresol inhalation 9 ± 0.9 4 months: 2 decreased reflexes, leukocytosis, Uzhdavini
mg/m3 months at 6 h/ bone marrow loss, histopathological et al.,
day, 5 days/week; changes and increased narcosis (1972)
2 months at 4 h/
day, 5 days/week
Rat/ 20 of each o-cresol oral (diet) 0, 1880, 13 weeks 30 000 mg/kg diet: reduced body US NTP
Fischer- sex F/M 3750, 7500, weight increase; > 15 000 mg/kg diet: (1992)
344N 15 000, increased kidney weight, bile acids;
30 000 > 7500 mg/kg diet: increased liver
mg/kg diet weight, length of estrus cycle and
altered bone marrow
Rat/ 20 of each m-/p-cresol oral (diet) 0, 1880, 13 weeks 30 000 mg/kg diet: reduced body US NTP
Fischer- sex F/M (60:40 3750, 7500, weight and clinical toxicity; (1992)
344N mixture) 15 000, > 15 000 mg/kg diet: bone marrow
30 000 changes, uterine atrophy; > 7500 mg/kg
mg/kg diet diet: lengthened estrous cycle,
liver and kidney weight increased; >
3750 mg/kg diet: thyroid changes; >
1880 mg/kg diet: increased bile salts,
histological changes in nasal
epithelium
Table 8 (contd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Mice/ 10 of each o-cresol oral (diet) 0, 1250, 13 weeks > 20 000 mg/kg diet: lengthened US NTP
B6C3F1 sex F/M 2500, 5000 estrus cycle, hyperplasia forestomach; (1992)
10 000, 10 000 mg/kg diet: clinical toxicity;
20 000 > 5000 mg/kg diet: reduced body weight;
mg/kg diet > 2500 mg/kg diet: increased relative
and absolute liver and kidney weight
Mice/ 10 of each m-/p- oral (diet) 0, 625, 13 weeks 10 000 mg/kg diet: reduced body US NTP
B6C3F1 sex F/M cresols 1250, 2500, weight, clinical toxicity; (1992)
(60/40 5000, 10 000 > 7500 mg/kg diet: hyperplasia in
mixtures) mg/kg diet respiratory tract;
> 2500 mg/kg diet: increased relative
and absolute liver and kidney weight
Rat/ 30 of each o-cresol oral (diet) 0, 50, 175 13 weeks 600 mg/kg: death, coma, tremors, MBA
Sprague- sex F/M and 600 reduced body weight; (1988a)
Dawley mg/kg body 175 mg/kg: tremors (females)
weight per
day
Rat/ 30 of each m-cresol oral (diet) 0, 50, 150 13 weeks 450 mg/kg: tremors and lethargy; MBA
Sprague- sex F/M and 450 > 150 mg/kg: reduced body weight (1988b)
Dawley mg/kg body
weight per
day
Table 8 (contd).
Species/ Number/ Compound Route Dose Length of Effects References
strain sex exposure
Rat/ 30 of each p-cresol oral (diet) 0, 50, 175, 13 weeks 600 mg/kg: death, coma, tremors MBA
Sprague- sex F/M 600 mg/kg and reduced body weight; altered (1988c)
Dawley body weight clinical chemistry > 175 mg/kg:
per day decreases in erythrocyte count,
haemoglobin and haemocrit,
increased kidney weight (males) > 50
mg/kg: mild nephropathy (males only)
Table 9. Cresols consumption in the US NTP (1992) 13-week feed
studiesa
Cresol Concentration Males (mg/kg Females
(mg/kg diet) body weight) (mg/kg body
weight)
Rats
o-Cresol 0 0 0
1880 126 129
3750 247 256
7500 510 513
15 000 1017 1021
30 000 2028 2024
m-/p-Cresol 0 0 0
1880 123 131
3750 241 254
7500 486 509
15 000 991 1024
30 000 2014 2050
Mice
o-Cresol 0 0 0
1250 199 237
2500 400 469
5000 790 935
10 000 1460 1663
20 000 2723 3205
m/p-Cresol 0 0 0
625 96 116
1250 194 239
2500 402 472
5000 776 923
10 000 1513 1693
a Doses given in mg/kg body weight/day; food consumption was
measured twice weekly and averaged over the 13-week period
to give a daily average dose based on body weight.
US NTP (1992) also conducted 13-week studies in groups of 10
B6C3F1 mice of each sex fed diets containing 0 to 20 000 mg/kg diet
of o-cresol or 0 to 10 000 mg/kg diet of 60:40 m-/ p-cresol.
Estimated daily doses (mg/kg body weight per day) are shown in Table
9. No deaths were recorded in treated mice. Feed consumption was
reduced during the first week of the study for mice exposed to
20 000 mg/kg diet of o-cresol or 10 000 mg/kg diet of
m-/ p-cresol. Reduced body weight occurred at 5000 mg/kg diet for
o-cresol and 10 000 mg/kg diet for m-/ p-cresol. Hunched posture
and rough hair coat were observed in mice exposed to > 10 000 mg/kg
diet of either isomer. At doses of 2500 and 5000 mg/kg diet both
relative and absolute liver weights were significantly increased
(p < 0.01) for both o- and m-/ p-cresols, respectively.
Increases in other organ weights (relative to body weight) were
reported but as there was a marked decrease in body weight at the
highest dose levels, they were not regarded as being biologically
significant. No significant effects were detected in haematology,
urinalysis or clinical chemistry analyses. Histo-pathological
examination revealed mild hyperplasia of the respiratory epithelium of
the nose in mice fed m-/ p-cresol at > 2500 mg/kg diet and
minimal forestomach epithelial hyperplasia in mice fed o-cresol at
20 000 mg/kg diet. Exposure to o-cresol resulted in a lengthened
estrus cycle in treated mice in the 20 000 mg/kg diet group. Based on
these results, an NOAEL of 1250 mg/kg diet and 625 mg/kg diet can be
identified for mice exposed to o-cresol and m-/ p-cresols,
respectively.
Several 13-week studies of gavage exposure were conducted.
Groups of 30 male and 30 female Sprague-Dawley rats were treated with
0, 50, 175 and 600 mg/kg body weight ( o- and p-cresols) or 0, 50,
150 and 450 mg/kg body weight ( m-cresol) daily for 13 weeks by
gavage in corn oil in a volume of 5 ml/kg (MBA, 1988a,b,c). Both o-
and p-cresols caused mortality at the high dose of 600 mg/kg;
m-cresol was not lethal at the high dose of 450 mg/kg. All three
isomers caused lethargy and tremors in high-dose rats. In many of the
rats exposed to o- and p-cresols these signs were followed by
convulsions and coma. Although clinical signs of toxicity were mostly
limited to the high-dose groups, two female rats exposed to 175 mg/kg
of o-cresol also developed tremors, and one became comatose. In the
case of rats that survived, clinical signs disappeared one hour after
dosing. Body weight gain was reduced in high-dose rats exposed to all
three isomers and also in rats exposed to 150 mg/kg of m-cresol. No
other treatment-related effects were observed for o- and
m-cresols. However, a number of effects were detected in rats
treated with p-cresol. Mild reductions in red blood cell count,
haemoglobin, and haematocrit were noted in females treated with
> 175 mg/kg. Serum glutamic oxaloacetic transaminase (SGOT) and serum
glutamic pyruvic transaminase (SGPT) levels were increased in 4/10
females exposed to 600 mg/kg. Other changes in clinical chemistry
parameters were increased serum cholesterol in females at 600 mg/kg
and increased serum protein (mostly globulins) in males at
> 175 mg/kg. Increases in some organ weights (relative to body
weight) were reported, but as there was a marked decrease in body
weight at the highest dose levels, they were not regarded as
biologically significant. Epithelial metaplasia of the trachea
occurred in high-dose males and females. In male rats there was a
slight but statistically significant (p < 0.5) increase in the
incidence of nephropathy in the 50 mg/kg (11/20) and 600 mg/kg (12/20)
dose groups compared to the controls (4/20). However there was no
significant increase in the incidence of nephropathy at the 150 mg/kg
dose level (7/20) and the average severity of nephropathy was not
increased in any dose group. In the control groups of male rats from
the o-cresol and m-cresol studies, which were conducted
concurrently at the same laboratory, the incidences of nephropathy
were 10/20 and 7/20, respectively. Because of the variable incidence
of this spontaneously occurring lesion even among control groups, the
absence of a dose-related increased incidence or severity and the
absence of an effect on the kidney of female rats, it was considered
that nephropathy was a questionable treatment-related effect in male
rats. For this reason 50 mg/kg was regarded as a NOAEL for
p-cresol, based on the presence of haematological effects at
175 mg/kg. A NOAEL of 50 mg/kg body weight per day was identified for
o- and m-cresols. The NOAEL for o-cresol was based on reduced
body weight and tremors in female at doses of > 175 mg/kg; for
m-cresol the NOAEL of 50 mg/kg body weight per day was based on
reduced body weight in females and males at doses > 150 mg/kg.
Hamsters exposed to 1.5% p-cresol in the feed for 20 weeks
developed an increased incidence of mild-t o-moderate forestomach
hyperplasia compared to controls (Hirose et al., 1986).
Results of longer-term studies are summarized in Table 8.
7.4 Skin and eye irritation
Dermal application of cresols (0.5 ml of o-, m- or p-cresol
or a technical mixture of all three isomers) for 4 h caused visible
and irreversible tissue destruction in rabbits (Vernot et al., 1977).
Severe skin and eye irritation was reported in other laboratory tests
(Mellon Institute, 1949; Bio-Fax, 1969; Younger Labs, 1974; FDRL,
1975; Scientific Associates, 1976; Dow Chemical, 1978). Eye
irritation was also observed in rats and mice briefly exposed to high
concentrations of cresols ( o-cresol and technical cresol mixtures)
in the air (Campbell 1941; FDRL, 1975; Dow Chemical, 1978).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Reproduction
BRRC (1989a,b,c) conducted 2-generation reproduction studies on
rats using o-, m- and p-cresols. For each isomer, groups of 25
male and 25 female Sprague-Dawley CD rats were given 0, 30, 175 or
450 mg cresol/kg body weight daily by gavage in corn oil for 10 weeks
prior to breeding. Dosing of females was continued through a 3-week
mating period, gestation and lactation. After weaning, male and
female pups were given the same doses as their parents for 11 weeks.
As was the case for the F0 females, dosing of F1 females was
continued through a 3-week mating period, gestation and lactation.
All F2 pups were sacrificed at weaning. All three cresol isomers
caused toxic effects in the parental animals. In the F0 rats, toxic
effects were mostly limited to the 450 mg/kg groups and included
death, reduced body weight gain and clinical signs such as
hypoactivity, ataxia, twitches, tremors, prostration, rapid and
laboured respiration, urine stains and perioral wetness. In the F1
rats, some clinical signs of toxicity occurred in the 175 mg/kg groups
as well. However, effects on reproductive function or the morphology
of reproductive tissues were not detected in these studies, even at
doses producing overt parental toxicity. Decreased numbers of
spermatozoa and atrophy of seminal vesicles in some F0 males treated
with 450 mg m-cresol/kg was attributed to postmortem changes or
nonspecific stress; decreased spermatozoa in some F1 males treated
with 450 mg p-cresol/kg was also considered not to be
treatment-related. Similarly, Hornshaw et al. (1986) did not observe
reproductive effects in mink in a 1-generation study in which male and
female mink were fed a diet containing 0, 100, 400 or 1600 mg
o-cresol/kg diet for 2 months before mating and through weaning.
Estimated daily doses were 0, 5, 25 and 105 mg/kg body weight for
males and 0, 10, 40 and 190 mg/kg body weight for females. Parental
toxicity occurred in the mink fed 1600 mg/kg diet (reduced body weight
gain in males, increased relative liver weight and increased
erythrocyte count).
The US NTP (1992) study, discussed in detail in section 7.3.2,
included determination of sperm motility and concentration in male
F344/N rats and B6C3Fl mice after treatment with o-cresol and
m-/ p-cresol for 13 weeks. The length and stages of the estrus
cycles were also determined in female rats and mice. For both o-and
m-/ p-cresol, the rats were treated with 1880, 7500 or 30 000 mg/kg
in the diets. For o-cresol, the mice were treated with 1250, 5000
or 20 000 mg/kg in the diet, and for m-/ p-cresol mice were treated
with 625, 2500 or 10 000 mg/kg in the diet. No adverse effects on
sperm motility or concentration were observed at any dose level in
rats or mice with either o- or m-/ p-cresol. o-Cresol caused
an increased length in the estrus cycle in mice (increased time in
estrus) at 30 000 mg/kg only. A similar, but nonsignificant trend was
observed in rats. The decrease in body weight by itself was thought
not to be the cause for this effect. m-/ p-Cresol caused an
increased estrus cycle length in rats at 7500 and 30 000 mg/kg (all
stages affected) which was not related to body weight changes; there
were no effects on the estrus cycle in mice.
Increased testis weight was observed in ferrets dosed with
o-cresol 2520 and 4536 mg/kg in the diet (Hornshaw et al., 1986).
No adverse effects on the testis were observed in rats treated daily
with 600 mg o- or p-cresol per kg body weight or 450 mg mcresol
per kg body weight by gavage for 13 weeks (MBA, 1988a,b,c).
Pashkova (1972, 1973) studied the reproductive effects of
tricresol (a mixture of o-, m- and p-cresols) in white rats.
The rats were exposed to tricresol concentrations of 0, 0.6 or
4.0 mg/m3 in air for 4 months (daily exposure not specified).
Tricresol at a concentration of 4 mg/m3 had a detrimental effect on
the function and structure of the ovaries. The functional change
observed was a prolongation of both the estrus cycle and the estrus
stage of the cycle, accompanied by a shortening of the diestrus stage
of the cycle. Morphological analysis of the ovaries revealed a
decreased number of primary follicles and increased atresia. Similar,
but less pronounced morphological changes were produced by
0.6 mg/m3.
Izard et al. (1992) (abstract only) evaluated the reproductive
toxicity of o-cresol and a mixture of m- and p-cresol (59% +41%)
in CD-1 Swiss mice using the continuous breeding protocol. Mice
received cresols in feed at 0.25, 1.0 and 1.5% (2500, 10 000 and
15 000 mg/kg diet) of m- plus p-cresol or 0.05, 0.2 and 0.5% (500,
2000 and 5000 mg/kg diet) of o-cresol for 14 weeks. The authors
found that the m- plus p-cresol mixture at 1.5% in the feed
(equivalent to 2100 mg/kg body weight per day) significantly reduced
litter size (80% of control) and adjusted pup weight and increased
cumulative days to litter in the 2nd to 5th litters by 3 to 4 days.
Cross-over breeding of control and 1.5% m- plus p-cresol mixture
Fo animals resulted in decreased adjusted live pup weight of litters
with a treated parent of either sex. At necropsy, high-dose Fo
males had decreased body weight (90%) and relative seminal vesicle
weight. Relative kidney and liver weight increased at 1.0 and 1.5% in
males. In females, relative liver weight increased at all doses; this
was accompanied by decreased (94%) body weight at 1.5%.
The cresol mixture at levels of 1.0 and 1.5% adversely affected
pre- and post-weaning growth and survival. In the F1 generation,
the m- plus p-cresol mixture had no effect on reproductive
competence, but F1 postnatal growth and survival and F2 live pup
weight were decreased at 1.5% of the mixture. At necropsy, F1 males
had reduced body weight and relative seminal vesicle and prostate
weights at the 1.0 and 1.5% tested levels of the cresol mixture.
Females had reduced body weight at 1.0 and 1.5% levels of the mixture,
and relative liver and kidney weights were increased at all doses and
for both sexes. o-Cresol at doses up to 0.5% (equivalent to
550 mg/kg body weight per day) did not affect reproductive or general
toxicity parameters in either generation. They concluded that the
m- plus p-cresol mixture at > 1.0% caused minimal adult
reproductive toxicity but significant postnatal toxicity was observed.
o-Cresol was negative at the doses tested.
7.5.2 Embryotoxicity and teratogenicity
Developmental toxicity studies were conducted for o-, m- and
p-cresols in rats and rabbits (BRRC, 1988a,b). For each isomer,
groups of 25 inseminated female rats were given doses of 0, 30, 175 or
450 mg cresol/kg in corn oil by gavage on days 6-15 of gestation.
Maternal toxicity was evident at 450 mg/kg for all three isomers;
effects included death, reduced food consumption, decreased body
weight gain, and clinical signs such as audible respiration,
hypoactivity, ataxia and tremors. m-Cresol caused no effects on the
developing embryos at any dose, but o- and p-cresols both caused
mild fetotoxic effects at 450 mg/kg (increased incidences of dilated
lateral ventricles in the brain and minor skeletal variations,
respectively), which could have been secondary to maternal toxicity.
In the rabbit studies, groups of 14 inseminated females were given
cresol (0, 5, 50 or 100 mg/kg body weight daily) in corn oil by gavage
on days 6-18 of gestation. Maternal effects, including audible
respiration, ocular discharge, hypoactivity and death ( p-cresol
only), were seen after exposure to > 50 mg/kg. o-Cresol caused
fetotoxicity (increased incidences of subepidermal haematoma on the
head and poorly ossified sternebrae) in rabbits treated with
100 mg/kg. Neither m- nor p-cresol caused any developmental
effects in rabbits at any dose.
Developmental end-points were also monitored in the 2-generation
reproduction studies on rats discussed in section 7.5.1 (BRRC,
1989a,b,c). All three cresol isomers caused effects on pup body
weight at some time during development in these studies. Most of the
deficiencies in pup body weight or growth occurred in rats exposed to
450 mg/kg body weight per day, a dose that also caused overt toxicity
in parental rats. There were occasional body weight changes in
lower-dose groups (especially those treated with m-cresol), but it
is not clear that these changes were treatment-related. In addition
to its effect on pup body weight, m-cresol reduced F2 pup
survival from birth through lactation in the 450 mg/kg group.
In a developmental toxicity screening study, p-cresol was found
to cause maternal toxicity (reduced body weight gain) at a dose of
410 mg/kg body weight, but failed to elicit effects on
post-implantation loss or litter weight at any dose tested (Kavlock,
1990). In a study conducted on cultured rat embryos in vitro,
p-cresol caused dose-related effects on growth (reduced crown-rump
length, somite number and DNA content) and structural abnormalities
(increased hind limb bud absence and total tail defects). The
significance of these results is not clear (Oglesby et al., 1992).
7.6 Mutagenicity and related end-points
Data regarding the genotoxicity of cresols are presented in
Tables 10-14. Most of these data are for individual isomers, but some
information is also available for mixed isomers ( m/p and o/m/p
mixtures).
In vitro DNA repair assays (unscheduled DNA synthesis) were
negative in rat hepatocytes treated with o- or m-cresol, but a
weakly positive result was obtained with human lymphocytes treated
with p-cresol.
There is no evidence that cresols are mutagenic to Salmonella
typhimurium. None of the individual isomers induced mutations at
the tk locus of L5178Y mouse lymphoma cells, whereas the o/m/p
mixture of isomers was active in the presence of S9 mix. In Drosophila
melanogaster, sex-linked recessive lethal mutations were not induced
by either o- or p-cresol.
Chromosomal aberrations were induced in Chinese hamster (CHO)
cells in both the presence and absence of S9 mix, following treatment
with o- and p-cresols, but not with m-cresol. In mice in vivo,
there was no induction of chromosomal aberrations in bone marrow cells
by m-cresol or of micronuclei in peripheral blood erythrocytes by
o-cresol or the m/p isomer mixture.
Sister-chromatid exchanges (SCE) were induced in CHO cells by
o-cresol and by the o/m/p isomer mixture, but were not induced by
o-, m- or p-cresol in cultured human fibroblasts, after testing
only in the absence of S9 mix. In mice, in vivo tests for SCE
induction were inconclusive with o-cresol and negative with m- and
p-cresol.
No dominant lethal effects were observed following treatment of
male mice with either o- or p-cresol.
Table 10. Genotoxicity of o-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Litton Bionetics
(1981); Pool & Lin (1982);
Haworth et al. (1983)
Forward mutation L5178Y mouse lymphoma cells - - Litton Bionetics (1981)
Unscheduled DNA synthesis primary rat hepatocytes ND - Litton Bionetics (1981)
Chromosomal aberrations Chinese hamster ovary cells + + Hazleton Labs (1988a)
Sister-chromatid exchange Chinese hamster ovary cells + + Litton Bionetics (1981)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells - - Hazleton Labs (1988b);
Litton Bionetics (1981)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 10 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Sex-linked recessive lethal Drosophila melanogaster - Hazleton Labs (1989d)
Sister-chromatid exchange mouse ? Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
Micronucleus, peripheral mouse - US NTP (1992)
blood erythrocytes
Dominant lethal mouse - Hazleton Labs (1989a)
a - = negative result; + = positive result; ND = no data; ? = inconclusive
Table 11. Genotoxicity of m-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Haworth et al. (1983);
Pool & Lin (1982)
Forward mutation L5178Y mouse lymphoma cells - - Hazleton Labs (1988c)
Unscheduled DNA synthesis freshly cultured rat hepatocytes ND - Hazleton Labs (1988e)
Chromosomal aberrations Chinese hamster ovary cells - - Hazleton Labs (1988a)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells - - Hazleton Labs (1988d,f)
SV40 induction Syrian hamster kidney cells ND (+) Moore & Coohill (1983)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 11 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Chromosomal aberrations mouse - Hazleton Labs (1989c)
(bone marrow)
Sister-chromatid exchange mouse - Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
a - = negative result; (+) = weakly positive; ND = no data
Table 12. Genotoxicity of p-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vitro
Reverse mutation Salmonella typhimurium - - Douglas et al. (1980); Florin et
(on plates) al. (1980); Pool & Lin (1982);
Haworth et al. (1983)
Forward mutation L5178Y mouse lymphoma cells - - Hazleton Labs (1988c)
Semiconservative/repair DNA human peripheral lymphocytes ND (+) Daugherty & Franks (1986)
synthesis
Chromosomal aberrations Chinese hamster ovary cells + + Hazleton Labs (1988a)
Sister-chromatid exchange cultured human fibroblasts ND - Cheng & Kligerman (1984)
Cell transformation mouse BALBc/3T3 cells ND + Hazleton Labs (1988d)
Viral DNA amplification SV-40 transformed Chinese ND - Pool et al. (1989)
hamster cell line
Table 12 (contd).
Resultsa
With Without
Assay Indicator organism activation activation Reference
In vivo
Sex-linked recessive lethal Drosophila melanogaster - Hazleton Labs (1989e)
Sister-chromatid exchange mouse - Cheng & Kligerman (1984)
(bone marrow, alveolar
macrophages, and
regenerating liver cells)
Dominant lethal mouse - Hazleton Labs (1989b)
a - = negative result; + = positive result; (+) = weakly positive; ND = no data
Table 13. In vitro genotoxicity of a 1:1:1 mixture of o-, m- and p-cresol
Resultsa
With Without
Assay Indicator organism activation activation Reference
Reverse mutation Salmonella typhimurium - - Litton Bionetics (1980)
(on plates)
Forward mutation L5187Y mouse lymphoma cells + ? Litton Bionetics (1980)
Sister-chromatid exchange Chinese hamster ovary cells + + Litton Bionetics (1980)
Cell transformation mouse BALBc/3T3 cells + ND Litton Bionetics (1980)
a - = negative result; + = positive result; ? = inconclusive; ND = no data
Table 14. Genotoxicity of 60:40 m/p-cresol
Results
With Without
Assay Indicator organism activation activation Reference
Reverse mutation (on plates) Salmonella typhimurium - - US NTP (1992)
Micronuclei, peripheral blood mouse - US NTP (1992)
erythrocytes
BALBc/3T3 cells were transformed by p-cresol and the o/m/p
mixture, but not by o- or m-cresol. A weakly positive result was
obtained, however, in a viral enhancement assay with m-cresol.
Viral DNA amplification did not occur in SV-40-transformed
Chinese hamster embryo cells treated with o-, m- or p-cresol.
Antimutagenic effects of o- and p-cresol, but not of
m-cresol, have been demonstrated in methylnitrosoguanidine-induced
mutagenesis in Escherichia coli when given after the MNNG treatment
(Kushi & Yoshida, 1987).
In summary, these data indicate that m-cresol has little or no
genotoxic potential, and whereas both o- and p-cresol can induce
chromosomal aberrations in vitro and o-cresol can increase SCE
in vitro, they do not do so in vivo.
7.7 Carcinogenicity
There are no adequate bioassays or chronic studies available to
assess the carcinogenic potential of cresols. Two studies (Boutwell &
Bosch, 1959; Yanysheva et al., 1993) have indicated that cresols have
potential tumour-promoting activity. However, no conclusions can yet
be made regarding the carcinogenic potential of these compounds.
Boutwell & Bosch (1959) investigated the tumour-promoting ability
of cresols using a mouse skin-painting model. Groups of 27-29 mice
were given a single dermal application of 9,10-dimethyl-
1,2-benzanthracene, a cancer initiator, followed by application of 20%
solutions of o-, m- or p-cresol in benzene, twice a week for 12
weeks. Significant non-tumour-related mortality was produced by all
three cresol isomers. Among the survivors at 12 weeks, both the
average number of skin papillomas per mouse and the percentage of
exposed mice with at least one papilloma were increased by treatment
with cresols. o-Cresol was the most potent isomer and p-cresol
the least. No carcinomas were observed following treatment with
cresols. It should be noted that the vehicle used for cresols in this
study was benzene, a known carcinogen. The presence of benzene did
not appear to affect the results, however, since no papillomas were
observed in benzene-treated controls. This study suggests that
cresols may act as promoters.
Yanysheva et al. (1993) reported that o-cresol administered
orally (1 mg) twice weekly for up to 30 weeks to mice simultaneously
with benzo[ a]pyrene (1 mg) increased the incidence and malignancy of
tumours produced by benzo[ a]pyrene and shortened the latency period
for tumour development. These effects were not seen when higher
(10 mg) or lower (0.02 mg) doses of o-cresol and benzo[ a]pyrone
were administered. Administration of o-cresol before or after
benzo[ a]pyrene had the opposite effect, decreasing the
carcinogenicity of that chemical.
7.8 Other special studies
7.8.1 Neurological effects
A neurotoxicity study was performed on CD rats using all three
cresol isomers (TRL, 1986). Groups of 10 rats of each sex were
treated with o-cresol (0, 50, 175, 450 or 600 mg/kg body weight),
m-cresol (0, 50, 150 or 450 mg/kg), or p-cresol (0, 50, 175 or
600 mg/kg) in corn oil by gavage daily for 13 weeks. Both o- and
p-cresol caused death in the groups exposed to 600 mg/kg.
Convulsions were seen only in the groups treated with > 450 mg/kg.
Hypoactivity, rapid laboured respiration and excessive salivation were
observed sporadically at doses of > 50 mg/kg for all three isomers.
In spite of the observed clinical signs, few significant changes were
found in performance on neurobehavioural test batteries, no brain
weight changes were noted, and no gross or histopathological lesions
in the brain or other nervous tissues were found for any isomer.
Savolainen (1979) studied the effect on biochemical parameters in
the brain of Wistar rats (40 males) after exposure to o-cresol in
the drinking-water for 20 weeks. The administered concentration was
300 mg/litre, which provided daily doses of about 36 mg/kg body
weight. Increased activity of 2',3'-cyclic nucleotide
3'-phosphohydrolase in glial cells, and reductions in azoreductase and
glutathione in brain homogenate were found. No other
treatment-related effects were detected.
o-Cresol produced excitation of both the somatosensory evoked
potential and electroencephalogram in male Fischer-344 rats given a 1%
solution intravenously at the rate of 0.9 mg/min for 15 min (total
dose of 13.5 mg) (Mattsson et al., 1989). The rats were conscious and
responsive to stimuli. Muscle tremors developed if exposure was
sufficiently long.
7.8.2 Effects on the skin
Application of 0.5% p-cresol to the skin for 6 weeks resulted
in permanent depigmentation of the skin and hair in black and agouti
mice (Shelley, 1974). Depigmentation was accompanied by a caustic
effect in one black strain of mice, but not in another. In identical
trials, neither o- nor m-cresol caused depigmentation in mice.
7.9 Mechanisms of toxicity - mode of action
The main effects of cresols at the area of first contact are
irritancy or corrosivity, depending upon the concentration. These
primary effects are followed after absorption by haematoxicity,
hepatotoxicity and neurotoxicity. The mechanisms by which these
effects occur have not been specifically studied with respect to
cresols. It is frequently assumed that the basis for cresol toxicity
is similar to that for phenol. However, phenol has certain unique
properties, e.g., cardiac toxicity, which has not been reported for
cresols except in one human case of acute poisoning involving a very
high exposure (Arthur et al., 1977).
Cresols are relatively soluble in water and have been shown
in vitro to have high permeability coefficients for human skin
(Thompson et al., 1994). Consequently, they can be rapidly absorbed
and distributed throughout the body. Cresol metabolism is mainly
conjugation followed by urinary excretion as glucuronides and sulfates
(Bray et al., 1950). In addition, in vitro studies have shown that
microsome-dependent covalent binding to proteins occurs, but the
importance of this process is unknown (Thompson et al., 1994).
In an in vitro study, cresol caused inhibition of K-dependent
phosphatase activity of Na/K ATPase in erythrocyte membrane (Wardle,
1978). Dermally applied p-cresol inhibits ATPase activity, as
measured in both erythrocytes and brain. This could contribute to
toxicity by disturbing electrolyte balance across cell membranes,
which could result in haemolysis. Another factor leading to
erythrocyte damage could be the binding of cresols to iron complexes
(analogous to the process described in clay soils, see section 4.2.1).
If, indeed, this should occur, then it may contribute to
methaemoglobin formation, haemolysis and, in the liver, a compensatory
response leading to enlargement.
Neurotoxic mechanisms have received little study. At a
concentration of approximately 0.25 mM, p-cresol inactivates
dopamine ß-hydroxylase (DeWolf et al., 1988), and could thereby affect
neurotransmission by interfering with noradrenaline biosynthesis.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Poisoning incidents
The most commonly reported cases of cresol poisoning involve
accidental or intentional ingestion of cresol-containing substances.
Cresols are strong irritants, and their ingestion results in burning
of the mouth and throat, abdominal pain and vomiting (Isaacs, 1922;
Jouglard et al., 1971; Wiseman et al., 1980). The primary targets of
ingested cresols in humans appear to be the central nervous system,
blood and kidneys. Some effects on the lungs, heart and liver have
also been reported (Isaacs, 1922; Labram & Gervais, 1968; Chan et al.,
1971; Jouglard et al., 1971; Cote et al., 1984; Minami et al., 1990).
Chan et al. (1971) described two cases of oral cresol poisoning.
In one case, a woman swallowed about 250 ml disinfectant containing
50% mixed cresols. The patient was in a deep coma when admitted to
the hospital 2 h after ingestion, but regained consciousness 10 h
later. Haematological changes were remarkable. Within 7 h of
admission, erythrocyte glutathione levels were markedly reduced, and
methaemoglobinaemia was detected. Within 3 days, severe
haemoglobinaemia and haemoglobinuria were evident, along with
extensive Heinz body formation, indicating that massive intravascular
haemolysis had occurred. The patient died the next day, apparently
from thrombus formation and kidney failure secondary to acute
intravascular haemolysis. Autopsy revealed moderate fatty
degeneration in the liver and, in the kidney, fibrin clumps in the
glomeruli and moderate tubular degeneration consistent with
intravascular thrombosis. The authors also described the case of a
second woman who recovered after drinking about 100 ml of the same
cresol-containing disinfectant. The patient was semiconscious when
admitted to the hospital 1.5 h after ingestion. Methaemoglobin was
detected in the blood at admission, but not 6 h later. Heinz bodies
were observed 6 h after admission, but disappeared within 2 days.
Haemolytic anaemia and associated changes have been described in
other case reports. Heinz body formation, haemoglobinaemia and
haemoglobinuria were evidence of haemolytic anaemia in a man who drank
100 ml of "penetrating oil", a petroleum distillate containing 12%
mixed cresols (Cote et al., 1984). Severe haemolytic anaemia
developed during the second week following cresol ingestion in a man
who swallowed approximately 250 ml of a concentrated cresol mixture
(Jouglard et al., 1971). Dark urine and methaemoglobinaemia were
observed upon hospital admission in a man who had swallowed a
commercial disinfectant containing cresols 2 h earlier (Minami et al.,
1990). The concentration of methaemoglobin in the blood was monitored
regularly and was seen to increase markedly 15 h after admission to
the hospital. The patient was then given a blood transfusion, after
which methaemoglobin levels decreased to normal and the patient
recovered.
Labram & Gervais (1968) reported the case of a woman who
swallowed between 500 and 750 ml of a concentrated cresol mixture.
Upon admission to the hospital 45 min later, the patient was in a deep
coma and exhibited tachycardia with polymorphic ventricular
extrasystoles. A transient episode of ventricular fibrillation was
followed by cardiac arrest 24 h after admission to the hospital. At
autopsy, the most notable finding was massive eosinophilic necrosis in
the proximal tubule of the kidney. The investigators considered it
likely that this lesion occurred prior to death and represented a
target organ effect of cresol. Diffuse necrosis of the bronchial
epithelium was also thought to have occurred prior to death.
Pulmonary oedema and haemorrhage were also observed, but may have been
secondary to death. Diffuse lesions in other organs were also
considered to be secondary to death.
Isaacs (1922) reported symptoms of cresol poisoning in 52
patients who ingested 4-120 ml of disinfectant containing 25-50%
cresols. Mouth and throat burns, abdominal pain and vomiting were
common symptoms of cresol poisoning. Coma was also a frequent
occurrence; in some cases, unconsciousness occurred very soon after
exposure and lasted 14 h or more. Renal irritation and reduced
phenolsulfonephthalein output indicated the occurrence of kidney
effects in some patients. Darkly coloured urine was produced in most
cases and may have been due to haemoglobinuria. Blood abnormalities
were not detected, but details regarding blood analyses were not
reported; it is possible that some haematological changes (e.g.,
methaemoglobinaemia, Heinz body formation) may have been overlooked.
Only two of the 52 patients died; both deaths occurred within 30 min
of cresol ingestion.
Arthurs et al. (1977) reported a case of a 32-year-old man who
was admitted after he had taken more than 45 ml of cresols. This
patient was conscious upon admission. However, he became increasingly
dyspnoeic and developed tachycardia and systolic hypotension within
the next 12 h. The total serum phenol levels were elevated 24 h after
admission. The patient died 4 days later of myocardial failure and
pulmonary oedema.
Not all poisoning incidents with cresols involve oral exposure.
Accidental dermal exposure has also been reported, usually causing
corrosive damage to the skin (Herwick & Treweek, 1933; Green, 1975;
Wiseman et al., 1980; Pegg & Campbell, 1985). In one patient,
disfiguring scars remained visible a year after exposure (Herwick &
Treweek, 1933). Systemic effects of dermal exposure were reported by
Green (1975), who described the case of a 1-year-old baby who had
20 ml of a cresol derivative (90% mixed cresols in water) spilled on
his head. The spill area, shown by burning on the face and scalp,
covered about 7% of his body surface. The baby fell into a coma after
5 min and died within 4 h. Autopsy revealed haemorrhagic oedema in
the lungs, extensive centrilobular to mid-zonal necrosis in the liver,
congestion, swelling and tubular necrosis in the kidneys, and
congestion and swelling in the brain.
In some cases, cresols have been injected intentionally into the
vagina and uterus for the illegal purpose of inducing abortion. Signs
and symptoms in women exposed to cresols in this manner include
vaginal bleeding, abdominal cramps, severe burning pain, coma, massive
haemolysis, severe kidney nephrosis and failure, severe pulmonary
oedema with oil emboli, and death (Vance, 1945; Presley & Brown, 1956;
Finzer, 1961).
8.1.2 Controlled human studies
Uzhdavini et al. (1972) found that 6 mg/m3 was the threshold
concentration for the production of mucosal irritation by o-cresol
(vapour/aerosol mixture) in humans. At this concentration, 8 out of
10 subjects complained of symptoms such as dryness, nasal constriction
and throat irritation. However, the duration of exposure or the
composition of the compound (i.e. purity) were not specified in the
report. No reaction to cresol was noted in humans when the compound
was applied to the skin of the elbow as a 1% solution in alcohol
(Reimann, 1933).
8.1.3 Cancer
Epidemiological data regarding cancer and cresol exposure in
humans are not available in the literature. Two studies have examined
the production of endogenous p-cresol in cancer patients. Bone et
al. (1976) compared urinary p-cresol levels in six patients with
large bowel cancer with levels in 10 healthy patients and found no
difference in average daily urinary excretion of p-cresol.
Similarly, Renwick et al. (1988) found no difference in average daily
urinary excretion of p-cresol among 32 patients with transitional
cell carcinoma of the bladder and matched controls.
8.2 Occupational exposure
8.2.1 Poisoning incidents
Acute cresol poisoning during occupational exposure is usually a
result of dermal contact. In one case, a man fell into a vat
containing a cresylic acid derivative (Cason, 1959). The man suffered
burns on 15% of his body surface and developed anuria 36 h after
exposure. Blood urea levels increased steadily over the following
days. He fell into a coma 9 days after admission to the hospital and
died on the following day due to congestive heart failure.
Anuria was also observed in a man who worked with an antiseptic
solution containing concentrated mixed cresols for 2 days before
becoming ill (Larcan et al., 1974). Other significant observations in
this patient were haematological changes similar to those observed
after oral exposure, including methaemoglobinaemia, Heinz body
formation and massive haemolysis. The man died 3 days after admission
to the hospital.
Klinger & Norton (1945) reported the case of a man who had his
hands immersed in a 6% cresylic acid solution for 5-6 h. The man
survived, but experienced persistent eye-watering, followed by pain on
the side of his face and, ultimately, marked facial paralysis.
Thirteen cases of accidental burns were reported in workers
exposed to cresol (2), dichlorophenol (1) and phenol (10) (Ma et al.,
1982). Burns were diagnosed as first and second degree, small in area
and covered 0.5-10% of the body surface. The patients generally
demonstrated (in the following order) white, red, brown and black skin
colour, and then crusting, necrosis and sloughing. Patients were
treated immediately by washing affected areas. Twelve of 13 patients
fully recovered within 15 days with no scarring of skin.
Wu & Kwan (1984) reported a case of acute renal failure in a
healthy 50-year-old male technician accidentally exposed to a mixture
of cresols. The burned area was immediately irrigated with water.
The patient experienced dizziness, pain and numbness of burnt skin and
abdominal pain followed by oliguria and vomiting 8 h later. He
developed severe abdominal pain and vomiting and lesser oliguria
1 day after the exposure. The patient was admitted to hospital
3 days after exposure. A follow-up examination revealed decreased
pulse rate, urinary volume (70-190 ml/24 h), blood urea nitrogen
(440-1240 mg/litre), combining power of CO2 (40-42 vol %). Burnt
skin was light brown in colour and there was slight swelling and
tactile pain. The patient was treated for acute renal failure,
and complete recovery occurred within 27 days following exposure.
Ma & Wang (1989) reported a case of acute cresol burn and
poisoning in a 18-year-old woman accidentally exposed to cresol.
Exposure of face, hand, feet, thighs and perineum occurred. Face,
hand and feet were immediately irrigated with water, but contaminated
trousers were not taken off and thighs and perineum were not washed.
Burns were first and second degree in severity and covered 20% of the
body. After 10 min she exhibited erythema, discoloration of the skin,
and became delirious followed by coma. Pulmonary oedema and
haemoglobinuria were reported after treatment with a diuretic and
intravenous infusion. Additional therapy included peritoneal dialysis,
strict control of intravenous fluids, intravenous rogitine, oral
nitroglycerin and nifedipine etc. Peritoneal dialysis was continued
for 20 days. The patient was discharged 38 days later. No scarring
of the skin occurred.
8.2.2 Epidemiological studies
Molodkina et al. (1985) studied 174 female workers aged between
20 and 50 in an enamel wire production plant using mainly tricresol.
Seventy percent of the study population had been exposed to this
compound for at least 10 years. Tricresol concentrations averaged
1.4 mg/m3 per shift, with maximum recorded levels of 3.6-5.0 mg/m3.
Reported effects included circulatory disturbances and minor
haematological changes (decreases in red blood cell count, white blood
cell count and platelets). There were also reported to be decreases
in the activity of glucose-6-phosphate dehydrogenase and the
concentration of sulfhydryl groups within erythrocytes. Erythrocytes
were reported to have a shorter lifespan.
Syrovadko & Malysheva (1977) studied reproductive endpoints in
female workers exposed to tricresol and chlorobenzene during the
manufacture of enamel-insulated wire. Several reproductive disorders
were reported, including hormonal shifts, menstrual problems and
elevated incidences of perinatal mortality and abnormal development of
offspring.
A group of 58 women (without pre-existing genital disease),
exposed to tricresol and phosphoryl chloride in the production of
tricresylphosphate, comprised the study population investigated by
Pashkova (1973). There was an elevated incidence of menstrual
disturbances in the study population, with changes in the cycle
accompanied by difficult and painful menstruation. Changes in the
cycle were found to be the result of increased estrogen and decreased
progesterone activity, indicating ovarian dysfunction.
In neither of the above two studies was there any documentation
of the degree of exposure to cresols or any quantification of the
physiological changes mentioned. Therefore, the significance of these
studies cannot be assessed.
8.3 Subpopulations at special risk
Several populations have been identified that may be at special
risk from cresol exposure. For instance, in persons with renal
insufficiency, the renal clearance of phenol and p-cresol is
impaired, leading to accumulation of cresol in the blood (Niwa, 1993).
Individuals with glucose-6-phosphate dehydrogenase (G6DP) deficiency
may also have heightened sensitivity to the haematological effects of
cresols. In experiments in which blood was exposed to a disinfectant
containing 50% cresols in vitro, increased methaemoglobin formation
and decreased glutathione levels were more pronounced in blood from
subjects with glucose-6-phosphate dehydrogenase deficiency than blood
from normal subjects (Chan et al., 1971).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Aquatic
9.1.1.1 Laboratory studies
Growth studies have shown that cresols are moderately toxic to
aquatic bacteria, cyanobacteria (blue-green algae) and protozoa.
Growth inhibition thresholds for the bacterium Pseudomonas putida,
were 33 and 53 mg/litre for o- and m-cresol, respectively
(Bringmann & Kühn, 1976; Bringmann & Kühn, 1980), 6.8 and 13 mg/litre
for the cyanobacterium Microcystis aeruginosa, 17 and 31 mg/litre
for the bacteriovorous flagellate protozoan Entosiphon sulcatum
(Bringmann & Kühn, 1978a,b, 1980) and 132 and 114 mg/litre for the
saprozoic flagellate protozoan Chilomonas paramecium (Bringmann &
Kühn, 1980). The ciliate protozoan Tetrahymena pyriformis showed
low sensitivity to o-cresol and p-cresol, 48-h EC50 values for
growth inhibition being 203 and 168 mg/litre, respectively (Schultz &
Riggin, 1985; Schultz, 1987). The yeasts Pichia sp. and Rhodotorula
rubra had 50% growth reduction following 12 h of incubation with 400
and 200 mg p-cresol/litre, respectively (Kwasniewski & Kaiser,
1983).
Bacterial luminescence assays, which provide an indirect measure
of population inhibition, have been conducted in Photobacterium
phosphoreum. For p-cresol, 5-min EC50 (50% reduction of light)
values ranged from 1.5 to 1.72 mg/litre (Bulich & Isenberg, 1980,
1981; Bulich et al., 1981; Ribo & Kaiser, 1983).
The potential impact of m-cresol on wastewater treatment
systems appears to be minimal; the measured I50 (50% inhibition of
activated sludge respiration rates) is 458.1 mg/litre (Dow Chemical,
1984).
9.1.1.2 Field studies
Field studies on the degradation of fresh poplar leaves in
experimental streams dosed with 8 mg p-cresol/litre had decreased
rates of decomposition compared to control streams (Stout & Cooper,
1983). Although, treatment of the stream with p-cresol changed the
dynamics of invertebrate communities on the leaves (see section
9.3.1.2), the authors suggested that the principal factor in
decreasing decomposition was inhibition of aerobic microbial
degraders, due to the drop in dissolved oxygen concentrations below
1 mg/litre, which followed p-cresol addition.
9.1.2 Terrestrial
9.1.2.1 Laboratory studies
Although there have been many investigations regarding the
degradation of cresols by soil isolates in laboratory culture systems
(section 4.2.2), laboratory studies regarding the toxicity of cresols
to soil microorganisms are rare. One study using Pseudomonas putida,
a species common to both aquatic and terrestrial environments, is
discussed in section 9.1.1.1.
9.1.2.2 Field studies
Reports are available on cresol decomposition in soils (section
4.2.2), but field studies on the impact of cresols on the soil
microbial community have not been reported in the available
literature.
9.2 Plants
9.2.1 Aquatic
9.2.1.1 Laboratory studies
Laboratory investigations into the toxicity of cresols to plants
are summarized and referenced in Table 15. Studies have been
conducted in five species of algae and one vascular plant. Growth
inhibition threshold levels in algae ranged from 7.8 to 65 mg/litre,
indicating that cresols are moderately toxic to the species tested.
Similar levels of toxicity for algae have been observed for the three
isomers. EC50 values (mortality, reproduction and dry weight) from
static and flow-through tests on duckweed are similar and demonstrate
a low level of sensitivity of this species to o-cresol.
9.2.1.2 Field studies
The effects of p-cresol on respiration and photosynthesis in
the filamentous green alga Spyrogyra sp. were studied in a set of
open channel experimental streams (Stout & Kilham, 1983). Continuous
dosing of one channel for 48 h with 8 mg p-cresol/litre led to a
decrease in the oxygen concentration of the stream (to below
1 mg/litre). The large decrease in dissolved oxygen in the channel
resulting from p-cresol addition could only be partially accounted
for by inhibition of algal function and was mainly attributed to
microbial heterotrophs utilizing p-cresol as a substrate. This was
substantiated when laboratory results showed that exposure of
Spyrogyra sp. for 1 h to p-cresol (0, 0.9, 4.6, 14, 34 or 71 mg
per litre) resulted in dissolved oxygen concentrations of 7.0, 6.2,
6.8, 5.4, 5.2 and 5.6 mg/litre, respectively, while oxygen levels in
the dosed stream (field study) often fell well below these levels.
The algae turned brown at the three highest exposure levels.
Table 15. Toxicity of cresols to aquatic plants under static conditionsa
Test species Test chemical Age/size Temperature pH Test duration Effectb Concentration Reference
(°C) (days) (mg/litre)
Green alga o-cresol 10-day-old 27 7 7 NOEL 11 Bringmann & Kühn
(Scenedesmus culture (1978a,b, 1980)
quadricauda) m-cresol 8 15
Green alga p-cresol log phase NR 8 2 NOEL 7.8 Kuhn & Pattard
(S. subspicatus) (1990)
Green alga o-cresol log phase 25 7 2 NOEL 36 Slooff et al.
(S. pannonicus) (1983)
Green alga o-cresol log phase 26 7 4 NOEL 65 Slooff et al.
(Selenastrum (1983)
capricornutum)
Green alga (S. o-, m-, 14-day-old NR NR 14 EC50 for growth 137 Gaur (1988)
capricornutum) p-cresol culture inhibition
Green alga o-cresol log phase 25 7 2 growth 34 Slooff et al.
(Chlorella inhibition (1983)
pyrenoidosa)
Table 15 (contd).
Test species Test chemical Age/size Temperature pH Test duration Effectb Concentration Reference
(°C) (days) (mg/litre)
o-cresol steady state 25 8.8 3 EC50 for 100 Huang & Gloyna
m-cresol chlorophyl (1968)
p-cresol inhibition
(72 h)
Duckweed o-cresol 4.8-5.2 EC50 for Davis (1981)
(Lemna gibba) mortality 540
reproduction 245
dry weight 16.98
a Water was unchanged for the duration of the test; NR = not recorded
b EC50 = Concentration effecting 50% of the population; NOEL = no-observed-effect level
9.2.2 Terrestrial
9.2.2.1 Laboratory studies
Laboratory investigations regarding the effects of cresols on
terrestrial plants were not located in the available literature.
9.2.2.2 Field studies
No data relating to the impact of cresols on terrestrial plants
under field conditions could be located in the available literature.
9.3 Invertebrates
9.3.1 Aquatic
9.3.1.1 Laboratory studies
Laboratory investigations on the acute toxicity of cresols to
invertebrate species are presented in Table 16. Fifteen freshwater
and four marine species from a wide range of taxonomic groups have
been studied. LC50 values range from 1.4 to 165 mg/litre,
representing moderate to low levels of toxicity. Kühn et al. (1989a)
reported acute and 21-day NOEL values for reproductive effects in
Daphnia magna of 2.5 and 1.0 mg/litre, respectively, following
exposure to p-cresol. Devillers (1988) studied the relative acute
toxicity to Daphnia magna of phenols and three cresol isomers. The
results showed that, following 24 h of exposure at pH 7.8-8.2 and
under static condition, the immobilization concentrations (IC50) for
the 3 isomers were 18, 19 and 12 mg/litre. There were no significant
differences in the magnitude of toxicity of the three cresol isomers,
p-cresol being only slightly more toxic than o- or m-cresol.
In a laboratory study by Emery (1970), cresol (isomers not
specified) solutions were used to determine the relative toxic
responses of three immature phases and three mature states of
Gammarus faoccatus and Asellus militasis. Exposures of 48 h
revealed that adults were more tolerant than immature animals.
Asellids were about twice as tolerant as gammarids and mature
gammarids were 4 times more tolerant than immature animals. The most
susceptible phase of these crustaceans' life cycle was the first
instar.
Table 16. Acute toxicity of cresols to aquatic invertebrates
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterflea o-cresol stat NR NR NR NR 48 LC50 9.5 Slooff et al.
(Daphnia magna) (1983)
NOEC 2.9 Bringmann & Kühn
(1977)
o-cresol stat 24 h 20-22 7.6-7.7 70 24 LC50 19
o-cresol NR NR NR NR NR 48 LC50 5 Parkhurst et al.
(1979)
m-cresol stat 24 h 20-22 7.6-7.7 70 24 LC50 8.9 Bringmann & Kühn
(1977)
m-cresol NR NR NR NR NR 48 LC50 18.8 Parkhurst et al.
(1979)
p-cresol NR NR NR NR NR 48 LC50 1.4 Parkhurst et al.
(1979)
p-cresol stat 6-24 20 8.0 NR 24 EC50 14 Kühn et al.
(1989b)
Waterflea o-cresol stat NR NR 7 NR 48 LC50 9.6 Slooff et al.
(D. pulex) NOEC 5.2 (1983)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterflea o-cresol flow NR 14 7.6-8.3 569-865 48 LC50 > 94 Degraeve et al.
(D. pulicaria) (1980)
m-cresol flow NR 14 7.6-8.3 569-865 48 LC50 > 99.5 Degraeve et al.
(1980)
p-cresol flow NR 14 7.6-8.3 569-865 48 LC50 22.7 Degraeve et al.
(1980)
Aquatic sowbug o-cresol stat NR 20 7 NR 48 LC50 23 Slooff (1983)
(Asellus
aquaticus)
Scud o-cresol stat NR 20 7 NR 48 LC50 21 Slooff (1983)
Gammarus pulex)
Marine scud o-cresol stat adult 23 NR NR 96 LC50 10.2 Lee & Nicol
(Elasmopus (1978)
pectinicrus)
Marine sand o-cresol SR 3.8 cm 10 NR NR 59 LC50 14.2 McLeese et al.
shrimp (Crangon (1979)
septemspinosa)
Mayfly o-cresol stat NR 20 7 NR 48 LC50 50 Slooff (1983)
(Cloeon
dipterum)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Waterbug o-cresol stat NR 20 7 NR 48 LC50 80 Slooff (1983)
(Corixa
punctatum)
Mosquito o-cresol stat 3rd 26 7 NR NR LC50 80 Slooff (1983)
(Aedes aegypti) instar
Midge o-cresol stat NR 20 7 NR 48 LC50 34 Slooff (1983)
(Chironomus
thumni)
Dragonfly o-cresol stat NR 20 7 NR 48 LC50 46 Slooff (1983)
(Ischnura
elegans)
Stonefly o-cresol stat NR 20 7 NR 48 LC50 10 Slooff (1983)
(Nemoura
cinerea)
Hydra o-cresol stat budless 17 7 NR 48 LC50 75 Slooff (1983);
(Hydra Slooff et al.
oligactis) (1983)
Pond Snail o-cresol NR 3-4 20 7 NR 48 LC50 160 Slooff (1983);
(Lymnaea weeks Slooff et al.
stagnalis) (1983)
Table 16 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Flatworm o-cresol stat NR 20 7 NR 48 LC50 24 Slooff (1983)
(Dugesia
lugubris)
Oligochaete o-cresol stat 20 7 NR NR 48 LC50 165 Slooff (1983)
family
(Tubificidae)
Marine o-cresol stat 20 7 NR NR 48 LC50 135 Slooff (1983)
polychaete
(Ophryotrocha
diadema) o-, m-, stat NR NR NR NR 48 LC50 33-100 Parker (1984)
p-cresol
Marine green o-cresol stat eggs 5 NR NR 96 EC50 30 Falk-Petersen
sea urchin development et al. (1985)
(Strongylocentrotus
droebachien) m-cresol stat eggs 5 NR NR 96 EC50 30 Falk-Petersen
development et al. (1985)
p-cresol stat eggs 5 NR NR 96 EC50 5 Falk-Petersen
development et al. (1985)
a stat = static conditions (water unchanged for the duration of the test); flow = intermittent flow-through conditions; NR = not reported
b LC50 = concentration resulting in lethality of 50% of the test animals; NOEC = no-observed-effect concentration; EC50 = concentration
resulting in effects among 50% of the test animals
9.3.1.2 Field investigations
The effect of p-cresol on invertebrate colonization of
leafpacks was studied in open channel experimental streams (Stout &
Cooper, 1983). Two types of dosing regimes were used in an attempt to
distinguish between the direct toxicity of p-cresol and the indirect
effect of decreased dissolved oxygen concentration caused by the
stream microorganisms utilizing p-cresol as a substrate (section
9.1.1.2), previously shown to occur in p-cresol-treated streams.
Channels were either continuously dosed for 96 h with 8 mg
p-cresol/litre or intermittently dosed at the same level, with
temporary cessations of dosing when dissolved oxygen concentrations
decreased considerably compared to the control. Leafpacks containing
fresh Populus deltoides (poplar) leaves were added to the streams and
monitored for invertebrate colonization. Changes in colonization
patterns in the treated streams significantly altered the biomass of
invertebrates over time, and responses were less severe in the
intermittent-dose channels than in the continuous-dose ones. Leeches
and isopods, normally found among root mats, entered the water column
and became highly abundant leafpack colonists in the continuous-dose
channels compared to intermittent-treated and control streams. Snails
and flatworms, which are normal colonists on leafpacks, increased
dramatically in numbers, while freshwater scuds, also normal leafpack
colonists, decreased markedly and dead scuds were found floating in
the stream following dosing. Prior tests with scud showed a 44%
mortality rate in aerated water dosed with 5 mg/litre for 96-h, while
the 96-h LC50 in unaerated water was 2 mg/litre. These data suggest
the aquatic invertebrate community may be damaged more from decreased
available oxygen, an indirect effect of p-cresol in the water, than
by a direct toxic action of the substance.
9.3.2 Terrestrial
9.3.2.1 Laboratory studies
Laboratory investigations into the impact of cresols on
terrestrial invertebrates were not located in the available
literature.
9.3.2.2 Field studies
Field investigations concerning cresols were also not located in
the available literature.
9.4 Vertebrates
9.4.1 Aquatic
9.4.1.1 Laboratory studies
The acute toxicity of cresols to vertebrates has been studied in
nine species of fish (eight freshwater and one marine species) and two
species of freshwater amphibians (Table 17). LC50 values range from
7.9 to 40 mg/litre, indicating that the test materials are similar in
their level of toxicity and that they are moderately toxic to aquatic
vertebrates. Studies conducted in fathead minnows exposed to
o-cresol show that the toxicity of the compound is not affected by
water hardness.
9.4.1.2 Field studies
The effect of p-cresol on five fish species (smallmouth bass,
largemouth bass, fathead minnows, walleyed pike and bluegill sunfish)
was determined in an outdoor experimental stream (Cooper & Stout,
1982). Exposure over a 24-h period to a concentration of 8 mg/litre
caused no mortality, but examination of fish guts showed that they had
ceased feeding. There was no serious reduction in dissolved oxygen
during the experiment.
Total body burden measurements showed that the fish had a
bioaccumulation factor of 2.1 for p-cresol at the end of the
exposure period. Body burdens showed a rapid decrease on removal of
the contaminant.
During a 48-h pulsed exposure to 8 mg p-cresol/litre, the
mortality of walleyed pike was very high. Smallmouth bass showed
visible stress; largemouth bass showed no visible stress but had
stopped feeding. There were large decreases in dissolved oxygen
during the experiment. Bioaccumulation was determined in specific
body parts for bluegill sunfish. p-Cresol levels in eyes, mussels
and gills were low (2.8, 4.7 and 7.3 mg/kg, respectively) but were
high in liver and intestines (76 and 97 mg/kg, respectively). Again,
p-cresol was rapidly eliminated from the fish.
In the case of a 96-h exposure to 8 mg p-cresol/litre, the
mortality was very high in all species. Large, sustained drops in
dissolved oxygen also occurred.
Table 17. Acute toxicity of cresols to aquatic vertebrates
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Rainbow trout o-cresol NR 5-8 15 7-8 NR 48 LC50 13 Slooff et al.
(Oncorhynchus weeks NOEC 3.8 (1983)
mykiss)
o-cresol flow 7.9 cm 14 7.6-8.3 569-865 96 LC50 8.4 DeGraeve et al.
(1980)
m-cresol flow 7.9 cm 14 7.6-8.3 569-865 96 LC50 8.9 DeGraeve et al.
(1980)
p-cresol flow 7.3 cm 14 7.6-8.3 569-865 96 LC50 7.9 DeGraeve et al.
(1980)
Fathead minnow o-cresol NR 3-4 20 NR NR 48 LC50 34 Slooff et al.
(Pimephales weeks NOEC 30 (1983)
promelas)
o-cresol stat 17.9 mm; 25 7.7 47 96 LC50 14 Geiger et al.
29 days (1990)
o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 12.55 Pickering &
cm Henderson (1966)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
o-cresol stat 3.8-6.4 25 8.2 360 96 LC50 13.42 Pickering &
cm Henderson (1966)
o-cresol flow 5.0 cm 14 7.6-8.3 569-865 96 LC50 18.2 DeGraeve et al.
(1980)
m-cresol flow 4.9 cm 14 7.6-8.3 569-865 96 LC50 55.9 DeGraeve et al.
(1980)
p-cresol flow 5.2 cm 14 7.6-8.3 569-865 96 LC50 28.6 DeGraeve et al.
(1980)
p-cresol stat 4-8 18-22 < 5.9 soft 96 LC50 19 Mattson et al.
weeks (artificial) (1976)
1.1-3.1
cm
Fathead minnow p-cresol flow 28 days 24 7.8 48 96 LC50 16.5 Geiger et al.
(P. promelas) 20.9 mm (1986)
o-, m-, flow 29 days 25 7.6 46 96 LC50 12.8 Geiger et al.
p-cresol 20.8 mm (1990)
Bluegill o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 20.8 Pickering &
sunfish cm Henderson (1966)
(Lepomis
macrochirus)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Goldfish o-cresol stat 3.8-6.4 25 7.5 20 96 LC50 23.25 Pickering &
(Carassius cm Henderson (1966)
auratus)
o-, m-, stat 60-90 mm 18-23 7.8 hard 5 days mortality 1.0 Ellis (1937)
p-cresol < 5 days NOEC 0.1
Mosquitofish o-, m-, stat adult 17-20 7.3-7.7 NR 96 LC50 22 Wallen et al.
(Gambusia p-cresol females (1957)
affinis)
Guppy o-cresol NR NR NR NR NR 48 LC50 38 Slooff et al.
(Poecilia NOEC 27 (1983)
reticulata)
o-cresol stat 1.9-2.5 25 7.5 20 96 LC50 18.85 Pickering &
cm Henderson (1966)
Golden orfe o-cresol NR NR NR NR NR 48 LC50 18 Slooff et al.
(Leuciscus (1983)
idus)
Japanese o-cresol NR 4-5 24 NR NR 48 LC50 41 Slooff et al.
killifish weeks NOEC 32 (1983)
(Oryzias
latipes)
Table 17 (contd).
Test species Test Test Age/ Temperature pH Hardness Test Parameterb Concentration Reference
chemical typea size (°C) (mg CaCO3/ duration (mg/litre)
litre) (h)
Atlantic cod o-cresol stat eggs 5 NR NR 96 EC50 12 Falk-Petersen
(Gadus morhus) (development) et al. (1985)
m-cresol stat eggs 5 NR NR 96 EC50 > 30 Falk-Petersen
(development) et al. (1985)
Atlantic cod p-cresol stat eggs 5 NR NR 96 EC50 5 Falk-Petersen
(G. morhus) (development) et al. (1985)
Clawed toad o-cresol stat 3-4 20 NR NR 48 LC50 38 Slooff et al.
(Xenopus weeks NOEC 24 (1983);
laevis) Slooff &
Baerselman
(1980)
Salamander o-cresol stat 3-4 20 NR NR 48 LC50 40 Slooff et al.
(Ambystoma weeks NOEC 32 (1983);
mexicanum) Slooff &
Baerselman
(1980)
a Stat = static conditions (water unchanged for the duration of the test); flow = intermittent flow through conditions; NR = not reported
b LC50 = concentration resulting in lethality of 50% of the test animals; NOEC = no-observed-effect concentration; EC50 = concentration
resulting in effects among 50% of the test animals
Laboratory dose-response data for the species, investigated under
aerated conditions, showed that all species could withstand high
levels of p-cresol (up to 40 mg/litre) over a 96-h exposure period.
Thus the very high mortality observed in the experimental streams at a
p-cresol concentration of 8 mg/litre was due to the decreases in
dissolved oxygen and/or the synergistic effect of p-cresol and
dissolved oxygen on fish function.
9.4.2 Terrestrial
9.4.2.1 Laboratory studies
The acute oral toxicity for cresols was determined in
wild-trapped redwinged blackbirds (Agelaius phoeniceus)
preconditioned to captivity for 2-6 weeks and then dosed by gavage
with cresols formulated with propylene glycol over an 18-h period.
The LD50 values were calculated to be > 113 and > 96 mg/kg for
m- and p-cresol, respectively (Schafer et al., 1983).
9.4.2.2 Field studies
No data concerning field observations of the effects of cresols
on terrestrial vertebrates were present in the available literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Cresols consist either of a white crystalline solid or a
yellowish liquid. They have a phenolic-like odour and are freely
soluble in water. Cresols are found naturally in various plants and
oils and can be produced as combustion by-products from environmental
fires. They are also produced synthetically. Cresols have a wide
variety of uses as solvents and disinfectants or chemical
intermediates for pharmaceuticals, fragrances, antioxidants, dyes,
pesticides and resins.
Cresols have been detected in ambient air, surface- and
groundwater, and wastewater. They have also been detected in food and
beverages.
Cresols are rapidly absorbed by inhalation, ingestion and dermal
contact. They are readily distributed throughout the body. The
primary route of elimination is through the urine following
conjugation with glucuronides and sulfates.
The acute toxicity of cresols is mainly a consequence of their
strong irritant and corrosive activity. Toxic effects and clinical
signs following ingestion are burning of the mouth and throat,
abdominal pain and vomiting. More severe reactions may result in coma
and death. Target tissues and organs of ingested cresols in humans
are the blood, kidneys, lungs, heart, central nervous system and
liver. Inhalation of cresol vapour produces irritation of the nasal
membranes, throat and lungs. Acute poisoning during occupational
exposure is usually a result of dermal contact, which may result in
severe burns and scarring of the skin, haematological changes, kidney
failure, coma and death. There are very little data regarding
potential reproductive effects and no data on carcinogenicity in
humans. There is limited evidence to suggest that special populations
may be at greater risk from cresol exposure, e.g., individuals with
glucose-6-phosphate dehydrogenase deficiency or renal insufficiency
and the very young.
10.2 Evaluation of environmental risks
Cresols are present in the air, water and soil. They undergo a
number of chemical and biological reactions such as photolysis,
hydrolysis, oxidation and biodegradation. It appears, therefore, that
cresols will be relatively labile in the environment and will not
bioaccumulate to any significant extent. There are limited data
available on the levels of cresols in the ambient environment. A
median air concentration of 1.59 µg/m3 (0.359 ppb) has been reported
in the USA for source-dominated sites. Cresols have also been
detected in contaminated groundwater and surface water and at
hazardous waste sites. Observations on microorganisms, invertebrates
and fish are available and show that cresols may represent a risk to
non-mammalian organisms at point sources with high cresol
concentrations but not in the general environment.
10.3 Guidance value
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess carcinogenic hazard of cresols. Based on the results of
subchronic studies, a NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, applying the uncertainty factor of
300, an acceptable daily intake (ADI) of 0.17 mg/kg body weight per
day can be established for cresols.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
There is clear evidence in humans that, during dermal or oral
exposure, high concentrations of cresols are corrosive, absorbed
rapidly and produce severe toxicity that may result in death.
Inhalation may result in irritation of the respiratory tract. There
is no information regarding the chronic toxicity of cresols and no
adequate data regarding the carcinogenic potential of these compounds.
11.2 Recommendations
a) Cresols and mixtures containing cresols, including household
products, should be clearly labelled, warning of the acute
toxicity and corrosivity.
b) Cresols can readily penetrate the skin. Following direct
contact, contaminated areas should be washed immediately with
water and all contaminated clothing removed. Workers using
cresols or solutions containing cresols should wear protective
clothing.
c) Occupational exposure should be minimized.
12. FURTHER RESEARCH
There is a need for the following types of studies:
a) chronic toxicity and carcinogenicity studies in animals
b) studies on the toxic mechanisms of cresols
c) studies on workers occupationally exposed to cresols.
REFERENCES
Alexander M & Lustigman BK (1966) Effect of chemical structure on
microbial degradation of substituted benzenes. J Agric Food Chem,
14: 410-413.
Altmann HJ, Grunow W, Mohr U, Richter-Reichhelm HB, & Wester PW (1986)
Effects of BHA and related phenols on the forestomach of rats. Food
Chem Toxicol, 24(10/11): 1183-1188.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: Effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Arthurs GJ, Wise CC & Coles GA (1977) Poisoning by cresols.
Anesthesia, 32:642-643.
Artiola-Fortuny J & Fuller WH (1982) Adsorption of some
monohydroxybenzene derivatives by soils. Soil Sci, 133: 18-26.
Atkinson R (1985) Kinetics and mechanisms of the gas phase reactions
of the hydroxyl radical with organic compounds under atmospheric
condition. Chem Rev, 85: 69-201.
Atkinson R, Carter WPL, Darnall KR, Winer AM, & Pitts JN Jr (1980) A
smog chamber and modeling study of the gas phase NOx air
photo-oxidation of toluene and the cresols. Int J Chem Kinet,
12: 779-836.
Atkinson R, Carter WPL, Plum CN, Winer AM, & Pitts JN Jr (1984)
Kinetics of the gas-phase reactions of NO3 radicals with a series of
aromatics at 296±2 K. Int J Chem Kinet, 16: 887-898.
Atkinson R, Aschmann SM, & Avery J (1992) Reaction of OH and NO3
radicals with phenol, cresols and 2-nitrophenol at 296±K. Environ Sci
Technol, 26: 1397-1403.
Babeu L & Vaishnav DD (1987) Prediction of biodegradability for
selected organic chemicals. J Ind Microbiol, 2: 107-115.
Baird RB, Kuo CL, Shapiro JS, & Yanko WA (1974) The fate of phenolics
in wastewater - determination by direct-injection GLC and Warburg
respirometry. Arch Environ Contam Toxicol, 2: 165-178.
Bak F & Widdel F (1986) Anaerobic degradation of phenol and phenol
derivatives by Desulfobacterium phenolicum new species. Arch
Microbiol, 146(2): 177-180.
Balikova M & Kohlicek J (1989) Gas chromatography of simple phenolics
in biological fluids. J Chromatogr, 497: 159-167.
Bartholomew GW & Pfaender FK (1983) Influence of spatial and temporal
variations on organic pollutant biodegradation rates in an estuarine
environment. Appl Environ Microbiol, 45(1): 103-109.
Battersby NS (1990) A review of biodegradation kinetics in the aquatic
environment. Chemosphere, 21(10-11): 1243-1284.
Battersby NS & Wilson V (1988) Evaluation of a serum bottle technique
for assessing the anaerobic biodegradability of organic chemicals
under methanogenic conditions. Chemosphere, 17: 2441-2460.
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 55: 433-439.
Bayly RC & Wigmore GJ (1973) Metabolism of phenol and cresols by
mutants of Pseudomonas putida. J Bacteriol, 113: 1112-1120.
Bidleman TF (1988) Atmospheric processes. Environ Sci Technol,
22: 361-367.
Bio-Fax (1969) Toxicity data sheets for o-, p-, and m-cresol.
Northbrook, Illinois, Industrial Bio-Fax Laboratories, Inc.
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS205862).
Bone E, Tamm A, & Hill M (1976) The production of urinary phenols by
gut bacteria and their possible role in the causation of large bowel
cancer. Am J Clin Nutr, 29: 1448-1454.
Boutwell RK & Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer Res, 19: 413-424.
Boyd SA (1982) Adsorption of substituted phenols by soil. Soil Sci,
134: 337-343.
Boyd SA, Shelton DR, Berry D, & Tiedje JM (1983) Anaerobic
biodegradation of phenolic compounds in digested sludge. Appl Environ
Microbiol, 46: 50-54.
Bray HG, Thrope WV, & White K (1950) Metabolism of derivatives of
toluene. Biochem J, 46: 275-278.
Bringmann G & Kühn R (1976) Comparative results of the damaging
effects of water pollutants against bacteria (Pseudomonas putida)
and blue algae (Microcystis aeruginosa). Gas Wasserfach
Wasser-Abwasser, 117(9): 410-413.
Bringmann G & Kühn (1977) [Results of the damaging effect of water
pollutants on Daphnia magna.] Z Wasser Abwasser Forsch,
10(5): 161-166 (in German).
Bringmann G & Kühn R (1978a) [Limiting values for the noxious effects
of water pollutant material to blue algae (Microcystis aeruginosa) and
green algae (Scenedesmus).] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1978b) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) seruginosa and
Scenedesmus quadricauda. Mitt Int Ver Theor Angew Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14(3): 231-241.
Brown SC and Grady CPL Jr (1990) Biodegradation kinetics of
substituted phenolics: Demonstration of a protocol based on
electrolytic respirometry. Water Res, 24(7): 853-862.
BRRC (1988a) Developmental toxicity evaluation of o-, m-, or
p-cresol administered by gavage to Sprague Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Unpublished data submitted to
the US Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517695).
BRRC (1988b) Developmental toxicity evaluation of o-, m-, or
p-cresol administered by gavage to New Zealand white rabbits.
Export, Pennsylvania, Bushy Run Research Center (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517695).
BRRC (1989a) Two-generation reproduction study of o-cresol (CAS No.
95-48-7) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 51-614)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
BRRC (1989b) Two-generation reproduction study of p-cresol (CAS No.
106-44-5) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 52-512)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
BRRC (1989c) Two-generation reproduction study of m-cresol (CAS No.
108-39-4) administered by gavage to Sprague-Dawley (CD) rats. Export,
Pennsylvania, Bushy Run Research Center (Project report 51-634)
(Unpublished data submitted to the Chemical Manufacturers Association
Cresols Panel, Washington).
Bulich AA & Isenberg DL (1980) Use of the luminescent bacterial system
for the rapid assessment of aquatic toxicity. Adv Instrum,
35(2): 35-40.
Bulich AA & Isenberg DL (1981) Use of the luminescent bacterial system
for the rapid assessment of aquatic toxicity. ISA Trans, 20(1): 29-33.
Bulich AA, Greene MW, & Isenberg DL (1981) Reliability of the
bacterial luminescence assay for determination of the toxicity of pure
compounds and complex effluents. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 338-347 (ASTM Special Technical
Publication No. 737).
Campbell I (1941) Petroleum cresylic acids: A study of their toxicity
and the toxicity of cresylic disinfectants. Soap Sanit Chem,
17(4): 103.
Carter WPL, Winer AM, & Pitts JN Jr (1981) Major atmospheric sink for
phenol and the cresols: Reaction with the nitrate radical. Environ Sci
Technol, 15(7): 829-831.
Cason JS (1959) Report on three extensive industrial chemical burns.
Br Med J, 1: 827-829.
Cautreels W & van Cauwenberghe K (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos Environ, 12: 1133-1141.
Chambers CW, Tabak HH, & Kabler PW (1963) Degradation of aromatic
compounds by phenol-adapted bacteria. J Water Pollut Contr Fed,
35: 1517-1528.
Chan TK, Mak LW, & Ng RP (1971) Methemoglobinemia, Heinz bodies and
acute massive intravascular hemolysis in Lysol poisoning. Blood,
38: 739-744.
Cheng M & Kligerman AD (1984) Evaluation of the genotoxicity of
cresols using sister-chromatid exchange (SCE). Mutat Res,
137(1): 51-55.
Chudyk WA, Carrabba MN, & Kenny JE (1985) Remote detection of
groundwater contaminants using far-UV laser-induced fluorescence. Anal
Chem 57(7): 1237-1242.
CIIT (1983) Preliminary results of in vivo and in vitro sister
chromatid exchange assays on cresol isomers and of an immunological
evaluation of o-cresol. Research Triangle Park, North Carolina,
Chemical Industry Institute of Toxicology (Report to the US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances) (CIIT Docket No. 12283).
Clark RM, Goodrich JA, & Deininger RA (1986) Drinking water and cancer
mortality. Sci Total Environ, 53(3): 153-172.
Cooper WE & Stout RJ (1982) Assessment of transport and fate of toxic
materials in an experimental stream ecosystem. In: Dickson KL ed.
Modelling the fate of chemicals in the aquatic environment. Pellston
Conference III. Ann Arbor, Michigan, Ann Arbor Science Publications,
pp 347-378.
Cote MA, Lyonnais J, & Leblond PF (1984) Acute Heinz-body anemia due
to severe cresol poisoning: Successful treatment with
erythrocytapheresis. Can Med Assoc J, 130(10): 1319-1322.
Czuczwa J, Leuenberger C, Tremp J, & Giger W (1987) Determination of
trace levels of phenol and cresols in rain by continuous liquid-liquid
extraction and high-performance liquid chromatography. J Chromatogr,
403: 233-241.
Daugherty JP & Franks H (1986) Effect of monocyclic derivatives on DNA
repair in human lymphocytes. Res Commun Chem Pathol Pharmacol,
54(1): 133-136.
Davis JA (1981) Comparison of static-replacement and flow-through
bioassays using duckweed, lemna gibba G-3. Washington, DC, US
Environmental Protection Agency (EPA 560/6-81-003; NTIS PB81-187650).
DeGraeve GM, Geiger DL, Meyer JS, & Bergman HL (1980) Acute and
embryo-larval toxicity of phenolic compounds to aquatic biota. Arch
Environ Contam Toxicol, 9(5): 557-568.
DeWolf WE Jr, Carr SA, Varrichio A, Goodhart PJ, Mentzer MA, Roberts
GD, Southan C, Dolle RE, & Kruse LI (1988) Inactivation of dopamine
beta-hydroxylase by p-cresol: Isolation and characterisation of
covalently modified active site peptides. Biochemistry, 27: 9093-9102.
Deichmann W & Keplinger ML (1981) Phenols and phenolic compounds. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, vol 2A, pp 2567-2627.
Deichmann WB & Witherup S (1944) Phenolic studies. VI: The acute and
comparative toxicity of phenol and o-, m-, and p-cresols for
experimental animals. J Pharmacol Exp Ther, 80: 233-240.
DeRosa E, Bartolucci GB, Sigon M, Callegaro R, Perbellini L, &
Brugnone F (1987) Hippuric acid and ortho-cresol as biological
indicators of occupational exposure to toluene. Am J Ind Med,
11(5): 529-537.
Devillers J (1988) Acute toxicity of cresols, xylenols and trimethyl
phenols to Daphnia magna straus 1820. Sci Total Environ, 76: 79-83.
Dietz F & Traud J (1978) [Odour and taste thresholds. Concentration of
phenol bodies.] GWF-Wasser/Abwasser 119(6) (in German).
Dobbins DC & Pfaender FK (1988) Methodology for assessing respiration
and cellular incorporation of radiolabeled substrates by soil
microbial communities. Microbiol Ecol, 15: 257-273.
Dobson KR, Stephenson M, Greenfield PF, & Bell PRF (1985)
Identification and treatability of organics in oil shale retort water.
Water Res 19: 849-856.
Douglas GR, Nestmann ER, Betts JL, Mueller JC, Lee EGH, Stich HF, San
RHC, Brouzes RJP, Chmelauskas AL, Paavile HD, & Walden CC (1980)
Mutagenic activity in pulp mill effluents. Water Chlorination: Environ
Impact Health Eff, 3: 865-880.
Dow Chemical (1978) Acute toxicological properties and industrial
handling hazards of cresol (ortho, meta, para isomers). Midland,
Michigan, Dow Chemical Company (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS206146).
Dreibelbis WG, Ealy JA, & Porter WE (1985) Industrial hygiene
monitoring for evaluation of employee exposure and control measures in
coal conversion program at Oak Ridge National Laboratory. In: Cooke M
& Dennis AJ ed. Polynuclear aromatic hydrocarbons: Mechanisms,
methods and metabolism. Columbus, Ohio, Battelle Press, pp 351-363.
Druyan EA (1974) [Separate determination of phenol and o-, m-, and
p-cresol in air with the aid of thin-layer chromatography.] Gig i
Sanit, 8: 51-53 (in Russian).
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol,
15: 30-38.
Ellis MM (1937) Detection and measurement of stream pollution.
Washington, DC, US Department of Commerce, Government Printing Office,
pp 365-437 (Bureau of Fisheries Bulletin No. 22).
Ellis DD, Jone CM, Larson RA, & Schaeffer DJ (1982) Organic
constituents of mutagenic secondary effluents from wastewater
treatment plants. Arch Environ Contam Toxicol, 11: 373-382.
Emery RM (1970) The comparative acute toxicity of cresol to two
benthic crustaceans. Water Res, 4: 485-491.
Evangelista RA, Allen HL, & Mandel RM (1990) Treatment of phenol and
cresol contaminated soil. In: Characterization and cleanup of chemical
waste sites. Symposium of the 197th National Meeting of the American
Chemical Society, Dallas, TX, April 10, 1989. J Hazard Mater,
25(3): 343-360.
Falk-Petersen IB, Kjorsvik E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Faust BC & Hoigné J (1987) Sensitized photooxidation of phenols by
fulvic acid in natural waters. Environ Sci Technol, 21: 957-964.
FDRL (1975) Acute toxicity studies of ortho-cresol in rats and
rabbits. Saddle Brook, New Jersey, Food and Drug Research Laboratories
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS206095).
Fedorak PM & Hrudey SE (1984) The effects of phenol and some alkyl
phenolics on batch anaerobic methanogenesis. Water Res, 18: 361-367.
Fedorak PM & Hrudey SE (1986) Nutrient requirements for the
methanogenic degradation of phenol and p-cresol in anaerobic draw
and feed cultures. Water Res, 20(7): 929-933.
Fiege H & Bayer AG (1987) Cresols and xylenols. In: Gerharte W ed.
Ullman's encyclopedia of industrial chemistry, 5th ed. New York, VCH
Publishers, vol 8A, pp 25-59.
Finzer KH (1961) Lower nephron nephrosis due to concentrated Lysol
vaginal douches: A report of two cases. Can Med Assoc J, 84: 549.
Fiserova-Bergerova V, Pierce JT, & Droz PO (1990) Dermal absorption
potential of industrial chemicals: Criteria for skin notation. Am J
Ind Med, 17(5): 617-636.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15(3): 219-232.
Flyvbjerg J, Arvin E, Jensen BK, & Olsen SK (1993) Microbial
degradation of phenols and aromatic hydrocarbons in
creosote-contaminated groundwater under nitrate-reducing conditions. J
Contam Hydrol, 12 :133-150.
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, & Korte F (1982) Ecotoxicological profile analysis: VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicol Environ Saf, 60: 60-81.
Gadaskina ID & Filov VA (1971) Transformations and determination of
industrial poisons in the human body. Leningrad, Meditsina,
pp 202-205.
Gaffney JS, Streit GE, Spall WD, & Hall JH (1987) Beyond acid rain: Do
soluble oxidants toxins interact with SO2 and NOx to increase
ecosystem effects? Environ Sci Technol, 21(6): 519-523.
Gantzer CJ, Kollig HP, Rittmann BE, & Lewis DL (1988) Predicting the
rate of trace-organic compound removal by natural biofilms. Water Res,
22: 191-200.
Garrett JS (1975) Association between bladder tumors and chronic
exposure to cresols and cresote (letter). J Occup Med, 17: 492.
Gaur JP (1988) Toxicity of some oil constituents to Selenastrum
capricornutum. Acta Hydrochim Hydrobiol, 16(6): 617-620.
Geiger DL, Poirier SH, Brooke LT, & Call DJ (1986) Acute toxicities of
organic chemicals to fathead minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 3, pp 1-24, 165-166.
Geiger DL, Brooke LT, & Call DJ (1990) Acute toxicities of organic
chemicals to fathead minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 5, pp 1-24, 157-160.
Giabbai MF, Cross WH, Chian ESK, & DeWalle FB (1985) Characterization
of major and minor organic pollutants in wastewaters from coal
gasification processes. Int J Environ Anal Chem, 20: 113-129.
Goerlitz DF, Troutman DE, Godsy EM, & Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environ Sci Technol, 19: 955-961.
Goodley PC & Gordon M (1976) Characterization of industrial organic
compounds in water. Trans Ky Acad Sci, 37: 11-15.
Gosselin RE, Hodge HC, Smith RP, & Gleason MN (1976) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams
Wilkins & Co.
Great Lakes Water Quality Board (1983) An inventory of chemical
substances identified in the Great Lakes ecosystem. Volume 1: Summary,
pp 1-195 (Report to the Great Lakes Water Quality Board, Windsor,
Ontario, Canada).
Green MA (1975) A household remedy misused-fatal cresol poisoning
following cutaneous absorption (a case report). Med Sci Law,
15: 65-66.
Grosjean D (1984) Atmospheric reactions of ortho cresol: Gas phase and
aerosol products. Atmos Environ, 18: 1641-1652.
Grosjean D (1985) Reactions of o-cresol and nitrocresol with NOx
in sunlight and with ozone-nitrogen dioxide mixtures in the dark.
Environ Sci Technol, 19(10): 968-974.
Hampton CV, Pierson WR, Harvey TM, Upolegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road: I. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol,
16: 287-298. Hansch C & Leo AJ (1985) Medchem Project: Issue 26.
Claremont, California, Pomona College.
Hassan SM, Salem FB, & El-Salam NA (1987) Colorimatric determination
of phenols in water samples. Anal Lett, 20(5): 677-688.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil wastewaters. Environ Sci Technol, 18: 483-490.
Hawthorne SB, Miller DJ, Barkley RM, & Krieger MS (1988)
Identification of methoxylated phenols as candidate tracers for
atmospheric wood smoke pollution. Environ Sci Technol,
22(10): 1191-1196.
Hawthorne SB, Krieger MS, Miller DJ, & Mathiason MB (1989) Collection
and quantitation of methoxylated phenol tracers for atmospheric
pollution from residential wood stoves. Environ Sci Technol,
23(4): 470-475.
HAZDAT (1992) Hazardous substances database. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry, Exposure and Disease
Registry Branch, Office of External Affairs.
Hazleton Labs (1988a) Mutagenicity tests on o-, m-, and p-cresol
in an in vitro cytogenetic assay measuring chromosomal aberration
frequencies in CHO cells. Kensington, Maryland, Hazleton Laboratories
(Unpublished data submitted to the US Environmental Protection Agency,
Office of Toxic Substances) (Fiche No. OTS0517691).
Hazleton Labs (1988b) Mutagenicity tests on o-cresol in the
in vitro transformation of BALB/C-3T3 cells assay in the presence of
rat liver cell activation system. Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the US Environmental
Protection Agency, Office of Toxic Substances) (Fiche No. OTS0517697).
Hazleton Labs (1988c) Mutagenicity tests of p-cresol and m-cresol
in a mouse lymphoma mutation assay. Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the US Environmental
Protection Agency, Office of Toxic Substances) (Fiche No. OTS0517693).
Hazleton Labs (1988d) Mutagenicity tests on meta-cresol and
para-cresol in the in vitro transformation of BALB/C-3T3 cells
assay. Kensington, Maryland, Hazleton Laboratories (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517694).
Hazleton Labs (1988e) Mutagenicity tests on meta-cresol in a rat
primary hepatocyte unscheduled DNA synthesis assay. Kensington,
Maryland, Hazleton Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517692).
Hazleton Labs (1988f) Mutagenicity tests on m-cresol in the
in vitro transformation of BALB/C-3T3 cells assay. Kensington,
Maryland, Hazleton Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517698).
Hazleton Labs (1989a) Dominant lethal assay in mice: ortho-Cresol
CRE-9.1-DL-HLA (HLA study No. 10004-0-471). Kensington, Maryland,
Hazleton Laboratories (Unpublished data submitted to the Chemical
Manufacturers Association, Washington).
Hazleton Labs (1989b) Dominant lethal assay in mice: para-Cresol
CP945 (HLA study No. 10003-0-471). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Hazleton Labs (1989c) Mutagenicity test on cresol program panel sample
#2 meta-cresol in the mouse bone marrow cytogenetic assay (HLA study
No. 10002-0-451). Kensington, Maryland, Hazleton Laboratories
(Unpublished data submitted to the Chemical Manufacturers Association,
Washington).
Hazleton Labs (1989d) Mutagenicity test on ortho-cresol (lot number
RC645A) Drosophila melanogaster sex-linked recessive lethal test
(HLA study No. 10004-0-461). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Hazleton Labs (1989e) Mutagenicity test on para-cresol (lot number
1206, batch 807) Drosophila melanogaster sex-linked recessive lethal
test (HLA study No. 10003-0-461). Kensington, Maryland, Hazleton
Laboratories (Unpublished data submitted to the Chemical Manufacturers
Association, Washington).
Heikkila PR, Hameila M, Pyy L, & Raunu P (1987) Exposure to creosote
in the impregnation and handling of impregnated wood. Scand J Work
Environ Health, 13: 431-437.
Herwick RP & Treweek DN (1933) Burns from anesthesia mask sterilized
in compound solution of cresol. J Am Med Assoc, 100: 407-408.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds: Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinz RS, Lorence CR, Hodson CD, Hansch C, Hall LL, & Guy RH (1991)
Percutaneous penetration of para-substituted phenols in vitro. Fundam
Appl Toxicol, 17: 575-583.
Hirose M, Inoue T, Asamoto M, Tagawa Y, & Ito N (1986) Comparison of
the effects of 13 phenolic compounds in induction of proliferative
lesions of the forestomach and increase in the labelling indices of
the glandular stomach and urinary bladder epithelium of Syrian golden
hamsters. Carcinogenesis, 7(8): 1285-1289.
Hites RA (1979) Sources and fates of industrial organic chemicals: A
case study. In: Proceedings of the 8th National Conference on
Municipal Sludge Management. Silver Spring, Maryland, Information
Transfer, Inc., pp 107-119.
Ho CT, Lee KN, & Jin QZ (1983) Isolation and identification of
volatile flavor compounds in fried bacon. J Agric Food Chem,
31: 336-342.
Hoffman WA Jr & Tanner RL (1986) Detection of organic acids in
atmosphere precipitation. Upton, New York, Brookhaven National
Laboratory, Environmental Chemistry Division, Department of Applied
Science (Report to the US Department of Energy) (BNL-51922;
NTIS DE8 005294).
Hopper DJ (1976) The hydroxylation of p-cresols and its conversion
to p-hydroxybenzaldehydreu in Pseudomonas putida. Biochem Biophys
Res Commun, 69(2): 462-468.
Hopper DJ (1978) Incorporation of [18O] Water in the formation of
p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from
Pseudomonas putida. Biochem J, 75: 345-347.
Hopper DJ & Taylor DG (1977) The purification and properties of
p-cresol-(acceptor) oxidoreductase (hydroxylating), a
flavocytochrome from Pseudomonas putida. Biochem J, 167(1): 155-162.
Hornshaw TC, Aulerich RJ, & Ringer RK (1986) Toxicity of o-cresol to
mink and European ferrets. Environ Toxicol Chem, 5(8): 713-720.
Horowitz A, Shelton DR, Cornell CP, & Tiedje JM (1982) Anaerobic
degradation of aromatic compounds in sediments and digested sludge.
Dev Ind Microbiol, 23: 435-444.
Hoshika Y & Muto G (1978) Gas-liquid-solid chromatographic
determination of phenols in air using Tenax-GC and alkaline
precolumns. J Chromatogr, 187: 277-284.
Huang JC & Gloyna EF (1968) Effect of organic compounds on
photosynthetic oxygenation: I. Chlorophyll destruction and suppression
of photosynthetic oxygen production. Water Res, 2: 347-366.
Hwang HM, Hodson RE, & Lewis DL (1989) Microbial degradation kinetics
of toxic organic chemicals over a wide range of concentrations in
natural aquatic systems. Environ Toxicol Chem, 8: 65-74.
ILO (1991) Occupational exposure limits for airborne toxic substances.
Geneva, International Labour Office.
Isaacs R (1922) Phenol and cresol poisoning. Ohio State Med J,
18: 558-561.
Izard PA, Fail PA, George JD, Grizzle TB, & Heindel JJ (1992)
Reproductive toxicity of cresol isomers administered in feed to mouse
breeding pairs. Toxicologist, 12: 198 (Abstract).
James RH, Adams RE, Finkel JM, Miller HC, & Johnson LD (1984)
Evaluation of analytical methods for the determination of POHC on
combustion products. In: Proceedings of the 77th Annual Meeting of
the Air Pollution Control Association, San Francisco, California,
24-29 June. Research Triangle Park, North Carolina, Industrial
Research Laboratory, pp 1-25 (NTIS/PB84-246289).
Johnson LD, Midgett MR, James RH, Thomason MM, & Manier ML (1989)
Screening approach for principal organic hazardous constituents and
products of incomplete combustion. J Air Pollut Control Assoc,
39(5): 709-713.
Jouglard J, Aquaron R, Gatua-Pelanchon J, Trigano A, & Bel JG (1971)
Intoxications aiguës par un antiseptique ménager: le crésyl. Mars Méd,
108: 425-431.
Jungclaus GA, Lopez-Avila, & Hites RA (1978) Organic compounds in an
industrial waste water: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Junk GA & Ford CS (1980) A review of organic emissions from selected
combustion processes. Chemosphere, 9: 187-230.
Kavlock RJ (1990) Structure-activity relationships in the
developmental toxicity of substituted phenols: In vivo effects.
Teratology, 41: 43-49.
Kawamura K & Kaplan IR (1986) Compositional change of organic matter
in rainwater during precipitation events. Atmos Environ,
20(3): 527-536.
Keat MJ & Hopper DJ (1978) The aromatic alcohol dehydrogenase in
Pseudomonas putida N.C.I.B. 9869 grown on 3,5-xylenol and p-cresol.
Biochem J, 175(2): 959-967.
Khalal KD, Hasan BA, Moralesrubio A, & Delaguardia M (1993)
Flow-injection spectrophotometric determination of cresol compounds in
water by raction with p-aminophenol. Mikrochim Acta,
112(1-4): 99-111.
Klecka GM, Davis JW, Gray DR, & Madsen SS (1990) Natural
bioremediation of organic contaminants in ground water: Cliffs-Dow
Superfund Site. Ground Water, 28(4): 534-543.
Klinger ME & Norton JF (1945) Toxicity of cresylic acid-containing
solvent. US Navy Med Bull, 44(2): 438-439.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77(2-3): 269-276.
Koerber SC, McIntire W, Bohmont C, & Singer TP (1985) Resolution of
the flavocytochrome p-cresol methylhydroxylase in subunits and
reconstitution of the enzyme. Biochemistry, 24: 5276-5280.
Kollig HP, Parrish RS, & Holm HW (1987) An estimate of the variability
in biotransformation kinetics of xenobiotics in natural waters by
Aufwuchs communities. Chemosphere, 16: 49-60.
Krotoszynski BK & O'Neill HJ (1982) Involuntary bioaccumulation of
environmental pollutants in nonsmoking heterogenous human population.
J Environ Sci Health Part A Environ Sci Eng, 17(6): 855-883.
Kühn R & Pattard M (1990) Results of the harmful effects of water
pollutants to green algae (Scenedesmus subspicatus) in the cell
multiplication inhibition test. Water Res, 24(1): 31-38.
Kühn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kühn R, Pattard M, Pernak KD, & Winter A (1989a) Results of the
harmful effects of water pollutants to Daphnia magna in the 21 day
reproduction test. Water Res, 23(4): 501-510.
Kühn R, Pattard M, Pernak KD, & Winter A (1989b) Results of the
harmful effects of selected water pollutants (anilines, phenols,
alphatic compounds) to Daphnia magna. Water Res, 23(4): 495-499.
Kurlyandsky BA, Partsef DP, & Chernomorskiy AR (1975) [A procedure for
determining the mean daily maximum permissible concentration of
tricresol in atmospheric air.] Gig i Sanit, 5: 85-87 (in Russian).
Kushi A & Yoshida D (1987) Antimutagenic effects of phenols on MNNG -
induced mutagenesis in Escherichia coli. Agric Biol Chem,
51: 1439-1440.
Kuwata K & Tanaka S (1988) Liquid chromatographic determination of
traces of phenols in air. J Chromatogr, 442: 407-411.
Kuwata K, Uebori M, & Yamazaki Y (1981) Reversed-phase liquid
chromatographic determination of phenols in auto exhaust and tobacco
smoke as p-nitrobenzeneazophenol derivatives. Anal Chem,
53(9): 1531-1534.
Kwasniewski K & Kaiser KLE (1983) Toxicities of selected phenols to
fermentative and oxidative yeasts. Bull Environ Contam Toxicol,
31(2): 188-194.
Labram C & Gervais P (1968) Un cas d'intoxication massive par le
crésyl. Sem Hop Paris, 44: 3029-3031.
Larcan A, Lambert H, & Laprevote-Heully MC (1974) Intoxication aiguë
par le crésyl, à propos d'une observation avec hémolyse aiguë massive,
méthémoglobinémie et corps de Heinz. Eur J Toxicol, 7: 5-8.
Lee WY & Nicol JAC (1978) Individual and combined toxicity of some
petroleum aromatics to the marine amphipod Elasmopus pectenicrus.
Mar Biol, 48(3): 215-222.
Lehtonen M (1983) Gas-liquid chromatographic determination of volatile
phenols in matured distilled alcoholic beverages. J Assoc Off Anal
Chem, 66(1): 62-70.
Leone JA, Flagan RC, Grosjean D, & Seinfeld JH (1985) An outdoor smog
chamber and modeling study of toluene-NOx photooxidation. Int J Chem
Kinet, 17(2): 177-216.
Leuenberger C, Ligocki MP, & Pankow JF (1985) Trace organic compounds
in rain. 4. Identities, concentrations, and scavenging mechanisms
for phenols in urban air and rain. Environ Sci Technol,
19(11): 1053-1058.
Lewis DL, Holm HW, & Hodson RE (1984) Application of single and
multiphasic Michaelis-Menten kinetics to predictive modeling for
aquatic ecosystems. Environ Toxicol Chem, 3: 563-574.
Lewis DL, Kollig HP, & Hodson RE (1986) Nutrient limitation and
adaptation of microbial populations to chemical transformations. Appl
Environ Microbiol, 51: 598-603.
Liberti A, Goretti G, & Russo MV (1983) PCDD and PCDF formation in the
combustion of vegetable wastes. Chemosphere, 12: 661-663.
Litton Bionetics (1980) Sister chromatid exchange assay, Ames assay,
mouse lymphoma forward mutation assay, and transformation assay for a
sample containing 33-1/3% each ortho-, meta-, and para-cresol.
Kensington, Maryland, Litton Bionetics, Inc. (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS0517528).
Litton Bionetics (1981) Sister chromatid exchange assay, Ames assay,
mouse lymphoma forward mutation assay, and cell transformation on
o-cresol. Unpublished data submitted to EPA/OTS
(Fiche no. OTS0517531).
Liu D & Pacepavicius G (1990) A systematic study of the aerobic and
anaerobic biodegradation of 18 chlorophenols and 3 cresols. Toxic
Assess Int J, 5(4): 367-388.
Ludzack FJ & Ettinger MB (1960) Chemical structures resistant to
aerobic biochemical stabilization. J Water Pollut Control Fed,
32: 1173-2000.
Lund FA & Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acids. J Gen Appl
Microbiol, 30: 53-61.
Lyman WJ, Reehl WF, & Rosenblatt DH ed (1982) Handbook of chemical
property estimation methods. New York, McGraw Hill Book Co.,
chapter 15.
Lyman WJ, Reehl WF, & Rosenblatt DH ed (1990) Handbook of chemical
property estimation methods: environmental behavior of organic
compounds. Washington, DC, American Chemical Society, pp 15-16.
Ma L & Wang CC (1989) [Case report of cresol burn and poisoning.] Chin
J Ind Hyg Occup Dis, 7: 219-220 (in Chinese).
Ma SH, Ma CS & Lin RC (1982) [Clinical studies 83 cases of chemical
burns.] Chin J Dermatol, 15(1): 9-10 (in Chinese).
McCreary JJ, Jackson JG, & Zoltek J Jr (1983) Toxic chemicals in an
abandoned phenolic waste site. Chemosphere, 12: 1619-1632.
McIntire W & Singer TP (1982) Resolution of p-cresol
methylhydroxylase into catalytically active subunits and
reconstitution of the flavocytochrome. FEBS Lett, 143(2): 316-318.
McIntire W, Edmonson DE, Hopper DJ, & Singer TP (1981) 8
alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group
of bacterial p-cresol methylhydroxylase. Biochemistry,
20(11): 3068-3075.
McIntire W, Hopper DJ, Craig JC, Everhart ET, Webster RV, Causer MJ, &
Singer TP (1984) Stereochemistry of 1-(4'-hydroxyphenyl)ethanol
produced by hydroxylation of 4-ethylphenol by p-cresol
methylhydroxylase. Biochem J, 224(2): 617-621.
McIntire W, Hopper DJ, & Singer TP (1985) p-Cresol
methylhydroxylase. Assay and general properties. Biochem J,
228(2): 325-335.
McKinney RE, Tomlinson HD, & Wilcox RL (1956) Metabolism of aromatic
compounds by activated sludge. Sew Ind Wastes, 28: 547-557.
McKnight DM, Pereira WE, Ceazan ML, & Wissmar RC (1982)
Characterization of dissolved organic materials in surface waters
within the blast zone of Mount St. Helens, Washington. Org Geochem,
4: 85-92.
McIntire W, Singer TP, Smith AJ, & Matthews FS (1986) Amino acid and
sequence analysis of the cytochrome and flavoprotein subunits of
p-cresol methylhydroxylase. Biochemistry, 25:5975-5981.
McLeese DW, Zitko V, & Peterson MR (1979) Structure-lethality
relationships for phenols, anilines and other aromatic compounds in
shrimp and clams. Chemosphere, 8(2): 53-57.
Malaney GW (1960) Oxidative abilities of aniline-acclimated activated
sludge. J Water Pollut Control Fed, 32: 1300-1311.
Malaney GW & McKinney RE (1966) Oxidative abilities of
benzene-acclimated activated sludge. Water Sew Works, 113: 302-309.
Manita MD (1966) [Analysis of the vapors of monohydric phenols
(phenol, o-, m-, and p-cresol) in atmospheric air by the
ultraviolet spectrophotometric method.] Gig i Sanit 8: 60-63
(in Russian).
Masunaga S, Urushigawa Y, & Yonezawa Y (1983) Microbial transformation
of o-cresol to dihydroxytoluenes by phenol acclimated activated
sludge. Chemosphere, 12: 1075-1082.
Masunaga S, Urishigawa Y, & Yonezawa Y (1986) Biodegradation pathway
of o-cresol by heterogeneous culture. Phenol activated sludge.
Water Res, 20: 477-484.
Matsumoto H, Kuwabara K, Murakami Y, Nishimune T, & Sueki K (1989)
Cresol isomers contaminating beef on the market. J Food Hyg Soc Jpn,
30(3): 250-253.
Mattsson JL, Albee RR, & Gorzinski SJ (1989) Similarities of toluene
and o-cresol neuroexcitation in rats. Neurotoxicol Teratol,
11(1): 71-75.
Mattson VR, Arthur JW, Walbridge CT (1976) Acute toxicity of selected
organic compounds to fathead minnows. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-76-097).
MBA (1988a) Subchronic toxicity of ortho-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to the US Environmental Protection Agency).
MBA (1988b) Subchronic toxicity of para-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to US Environmental Protection Agency).
MBA (1988c) Subchronic toxicity of meta-cresol in Sprague Dawley
rats. Bethesda, Maryland, Microbiological Associates (Unpublished
data submitted to the US Environmental Protection Agency).
Medvedev VA & Davidov VD (1981a) The influence of isomers on the
transformation rate of phenols in Chernozem soil. In: Overcash MR ed.
Decomposition of toxic and nontoxic organic compounds in soil. Ann
Arbor, Michigan, Ann Arbor Scientific Publishers, pp 175-181.
Medvedev VA & Davidov VD (1981b) The transformation of various coke
industry products in Chernozem soil. In: Overcash MR ed. Decomposition
of toxic and nontoxic organic compounds in soil. Ann Arbor, Michigan,
Ann Arbor Scientific Publishers, pp 245-254.
Mellon Institute (1949) The acute toxicity of m-cresol. Pittsburgh,
Pennsylvania, Mellon Institute of Industrial Research (Unpublished
data submitted to the US Environmental Protection Agency, Office of
Toxic Substances) (Fiche No. OTS0517523).
Minami M, Katzumata M, & Tomoda A (1990) Methemoglobinemia with
oxidized hemoglobins and modified hemoglobins found in bloods of
workers handling aromatic compounds and in those of man who drank
cresol solution. Biomed Biochim Acta, 49(2-3): 5327-5333.
Molodkina NN, Gabulgalimova RR, Umarova SI, & Matveev AA (1985)
Hygienic evaluation of the combined effect of some organic solvents
(chlorobenzene and tricresol). In: Kasparova AA ed. Methodological
principles for ensuring healthier working conditions at industrial
plants with a leading chemical factor. Moscow, Research Institute of
Labor Hygiene and Occupational Diseases, Academy of Medical Sciences,
pp 82-88.
Moore SP & Coohill TP (1983) An SV40 mammalian inductest for putative
carcinogens. Prog Nucleic Acid Res Mol Biol, 29: 149-153.
Murray KE & Adams RF (1988) Determination of simple phenols in feces
and urine by high performance liquid chromatography. J Chromatogr
Biomed Appl, 431(1): 143-149.
Namkoong W, Loehr RC, & Malina JF Jr (1988) Kinetics of phenolic
compounds removal in soil. Hazard Waste Hazard Mater, 5(4): 321-328.
Needham LL, Head SL, & Cline RE (1984) Determination of phenols and
cresols in urine by gas chromatography. Anal Lett, 17(B14): 1555-1565.
Neiminen E & Heikkila P (1986) Simultaneous determination of phenol,
cresols and xylenols in workplace air, using a
polystyrene-divinylbenzene column and electrochemical detection. J
Chromatogr, 360(1): 271-278.
Neufeld RD, Debes MR, Moretti C, Mayernik J, Keleti G, Sykora J, &
Bender J (1985) Cooling tower evaporation of treated coal gasification
wastewaters. J Water Pollut Control Fed, 57(9): 955-964.
Niwa T (1993) Phenol and p-cresol accumulated in uremic serum
measured by HPLC with fluorescence detection. Clin Chem, 39:108-111.
Oglesby LA, Ebron-McCoy MT, Logsdon TR, Copeland F, Beyer PE, &
Kavlock RJ (1992) In vitro embryotoxicity of a series of
para-substituted phenols: Structure, activity, and correlation with
in vivo data. Teratology, 45(1): 11-34.
Oliveira DP & Sitar N (1985) Ground water contamination from
underground solvent storage tanks, Santa Clara, California. In:
Proceedings of the 5th National Symposium on Aquifer Restoration and
Ground Water Monitoring. Richmond, California, Cooper Engineers, Inc.
and Berkeley, California, Department of Civil Engineering, University
of California, pp 691-708.
Palumbo AV, Pfaender FK, & Paerl HW (1988) Biodegradation of NTA and
m-cresol in coastal environments. Environ Toxicol Chem, 7: 573-585.
Paris DF, Wolfe NL, Steen WC, & Baughman GL (1983) Effect of phenol
molecular structure on bacterial transformation rate constants in pond
and river samples. Appl Environ Microbiol, 45: 1153-1155.
Parker JG (1984) The effects of selected chemicals and water quality
on the marine polychaete Ophryotrocha diadema. Water Res,
18(7): 865-868.
Parkhurst BR, Bradshaw AS, Forte JL, & Wright GP (1979) An evaluation
of the acute toxicity to aquatic biota of a coal conversion effluent
and its major components. Bull Environ Contam Toxicol, 23(3): 349-356.
Parrish CF (1983) Solvents, industrial. In: Grayson M ed.
Kirk-Othmer's encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 21, pp 386-387.
Pashkova GA (1972) [Special effects of cresol and phosphoryl chloride
on the endocrine glands.] Kuibyshev, USSR, Research Institute of
Epidemiology, Microbiology and Hygiene, pp 203-204 (Scientific
Publication No. 7) (in Russian).
Pashkova GA (1973) [Comparative evaluation of the gonadotrophic and
general toxic effect of tricresol, phosophoryl chloride and
tricresylphosphate.] In: [Problems in labour hygiene, occupational
pathology and toxicology in the production and testing of
phosphor o-organic plasticizers.] Moscow, pp 86-90.
Pauli O & Franke G (1971) Behaviour and degradation of technical
preservatives in the biological purification of sewage. Biodeterior
Mater Proc Int Biodeterior Symp 2nd, 1971: 52-60.
Pegg SP & Campbell DC (1985) Children's burns due to cresol. Burns,
11(4): 294-296.
Pereima VL (1975) Inhalational effect of cresol isomers at low
concentrations and means for improving detoxication processes in
experiments on white rats. Lvov Univesity, pp 86-90 (Dissertaton).
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38(9): 1419-1429.
Pitter P (1976) Determination of biological degradability of organic
substances. Water Res, 10: 231-235.
Pool BL & Lin PZ (1982) Mutagenicity testing in the Salmonella
typhimurium assay of phenolic compounds and phenolic fractions
obtained from smokehouse smoke condensates. Food Chem Toxicol,
20(4): 383-391.
Pool BL, Yalkinoglu AO, Klein P, & Schlehofer JR (1989) DNA
amplification in genetic toxicology. Mutat Res, 213:61-72.
Presley JA & Brown WE (1956) Lysol-induced criminal abortion. Obstet
Gynecol, 8: 368-370.
Ram NM, Exner P, Bell R, & Santos S (1985) Feasibility of treating
contaminated ground water at a hazardous waste site. In: Proceedings
of the NWWA/API Conference on Petroleum Hydrocarbons and Organic
Chemicals in Ground Water Prevention, Detection and Restoration,
Houston, Texas, 13-15 November 1985. Dublin, Ohio, National Water Well
Association, pp 513-534.
Reimann SP (1933) "Sensitivity" to sylphydryl. Am J Clin Pathol,
3(2): 167-170.
Renwick AG, Thakrar A, Lawrie CA, & George CF (1988) Microbial amino
acid metabolites and bladder cancer: No evidence of promoting activity
in man. Hum Toxicol, 7(3): 267-272.
Ribo JM, Kaiser KLE. 1983. Effects of selected chemicals to
photoluminescent bacteria and their correlations with acute and
sublethal effects on other organisms. Chemosphere,
12(11-12): 1421-1442.
Riddick JA, Bunger WB, & Sakano TK (1986) Organic solvents. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Inc., pp 224-229.
Risner CH (1993) The quantification of hydroquinone, catechol, phenol,
3-methylcatechol, scopoletin, m+p-cresol and o-cresol in indoor
air sample by high performance liquid chromatography. J Liq
Chromatogr, 16:4117-4140.
Roberts MS, Anderson RA, & Swabrick J (1977) Permeability of human
epidermis to the phenolic compounds. J Pharm Pharmacol, 29: 677-683.
Roberts DJ, Fedorak PM, & Hrudey SE (1987) Comparison of the fates of
the methyl carbons of m-cresol and p-cresol in methanogenic
consortia. Can J Microbiol, 33: 335-338.
Rogers JE, Li SW, & Felice LJ (1984) Microbiological transformation
kinetics of xenobiotics in aquatic environment. Richland, Washington,
Battelle Pacific Northwest Laboratories, p 105 (NTIS PB84-162866).
Sabljic A (1987) [The prediction of fish bioconcentration factors of
organic pollutants from the molecular connectivity model.] Z Gesamte
Hyg Grenzgeb, 33: 493-496 (in Yugoslavian).
Savolainen H (1979) Toxic effects of peroral o-cresol intake on rat
brain. Res Commun Chem Pathol Pharmacol, 25(2): 357-364.
Sawahata T & Neal NA (1983) Biotransformation of phenol t-hydroquinone
and catechol by rat liver microsomes. Mol Pharmacol, 23: 453-460.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Sax NI & Lewis RJ (1987) Hawley's condensed chemical dictionary, 11th
ed. New York, Van Nostrand Reinhold Co, pp 320-321.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12(3): 355-382.
Schultz TW (1987) The use of the ionization constant (pKa) in
selecting models of toxicity in phenols. Ecotoxicol Environ Saf,
14(2): 178-813.
Schultz TW & Riggin GW (1985) Predictive correlations for the toxicity
of allkyl- and halogen-substituted phenols. Toxicol Lett, 25: 47-54.
Scientific Associates (1976) Skin corrosiveness test in rabbits. St.
Louis, Missouri, Scientific Associates, Inc. (Unpublished data
submitted to the US Environmental Protection Agency, Office of Toxic
Substances) (Fiche No. OTS206095).
Shah JJ & Heyerdahl EK (1989) National ambient volatile organic
compound (VOCs) data base update. Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Science
Research Laboratory (EPA 600/3-88/010(a)).
Shamala N, Lim LW, Mathews FS, McIntire W, Singer TP, & Hopper DJ
(1985) Preliminary X-ray study of p-cresol methylhydroxylase
(flavocytochrome c) from Pseudomonas putida N.C.I.B. 9869. J Mol
Biol, 183(3): 517-518.
Shamala N, Lim LW, Mathews FS, McIntire W, Singer TP, & Hopper DJ
(1986) Structure of an intermolecular electron-transfer complex:
p-cresol methylhydroxylase at 6.0-A resolution. Proc Natl Acad Sci
(USA), 83(13): 4626-4630.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12: 1188-1194.
Sheldon LS & Hites RA (1979) Sources and movement of organic chemicals
in the Delaware River. Environ Sci Technol, 13: 574-579.
Shelley WB (1974) p-Cresol: Cause of ink-induced hair depigmentation
in mice. Br J Dermatol, 90: 169-174.
Shelton DR & Tiedje JM (1981) Development of test for determining
anaerobic biodegradation potential. Washington, DC, US Environmntal
Protection Agency, Office of Toxic Substances.
Shimp RJ & Pfaender FK (1985a) Influence of easily degradable
naturally occurring carbon substrates on biodegradation of
monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol,
49: 394-401.
Shimp RJ & Pfaender FK (1985b) Influence of naturally occurring humic
acids on biodegradation of monosubstituted phenols by aquatic
bacteria. Appl Environ Microbiol, 49: 402-407.
Singer PC, Lamb JC III, Pfaender FK, & Goodman R (1979) Treatability
and assessment of coal conversion wastewaters: Phase I. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Industrial Environmental Research Laboratory.
Slooff W (1983) Benthic macroinvertebrates and water quality
assessment: Some toxicological considerations. Aquat Toxicol,
4: 73-82.
Slooff W & Baerselman R (1980) Comparison of the usefulness of the
Mexican Axolol (Ambystoma mexicanum) and the clawed toad (Xenopus
laevis) in toxicological bioassays. Bull Environ Contam Toxicol,
24(3): 439-443.
Slooff W, Canton JH, & Hermens JLM (1983) Comparison of the
susceptibility of 22 freshwater species to 15 chemical compounds: I.
(Sub)acute toxicity tests. Aquat Toxicol, 4(2): 113-128.
Smith JH, Mabey WR, Bohonos N, Holt BR, Lee SS, Chou T-W, Bomberger
DC, & Mill T (1978) Environmental pathways of selected chemicals in
freshwater systems. Part II: Laboratory studies. Research Triangle
Park, North Carolina, US Environmental Protection Agency,
Environmental Research Laboratory.
Smolenski WJ & Suflita JM (1987) Biodegradation of cresol isomers in
anoxic aquifers. Appl Environ Microbiol, 58: 710-716.
Snider EH & Manning FS (1982) A survey of pollutant emission levels in
waste waters and residuals from the petroleum refining industry.
Environ Int, 7: 237-258.
Southworth GR & Keller JL (1986) Hydrophobic sorption of polar
organics by low organic carbon soils. Water Air Soil Pollut,
28(3-4): 239-248.
Spain JC & van Veld PA (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum, and chemical structure. Appl
Environ Microbiol, 45(2): 428-435.
Stone AT (1987) Reductive dissolution of manganese (III/IV) oxides by
substituted phenols. Environ Sci Technol, 21: 979-988.
STORET (1993) Online retrieval from data base. Washington, DC, US
Environmental Protection Agency.
Stout J & Kilham SS (1983) Effects of p-cresol on photosynthetic and
respiration rates of a filamentous green alga (Spirogyra). Bull
Environ Contam Toxicol, 30(1): 1-5.
Stout RJ & Cooper WE (1983) Effect of p-cresol on leaf decomposition
and invertebrate colonization in experimental outdoor streams. Can J
Fish Aquat Sci, 40(10): 1647-1657.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Suflita JM, Gibson SA, & Beeman RE (1988) Anaerobic biotransformations
of pollutant chemicals in aquifers. J Ind Microbiol, 3(3): 179-194.
Suflita JM, Liang LN, & Saxena A (1989) The anaerobic biodegradation
of o-, m-, and p-cresol by sulfate-reducing bacterial
enrichment cultures obtained from a shallow anoxic aquifer. J Ind
Microbiol, 4(4): 255-266.
Swann RL, Laskowski DA, McCall PJ, & Vander Kuy K (1983) A rapid
method for the estimation of the environmental parameters
octanol/water partition coefficient, soil sorption constant, water to
air ratio, and water solubility. Residue Rev, 85: 17-28.
Swindoll CM, Aelion CM, Dobbins DC, Jiang O, Long SC, & Pfaender FK
(1988) Aerobic biodegradation of natural and xenobiotic organic
compounds by subsurface microbial communities. Environ Toxicol Chem,
7(4): 291-299.
Syrovadko ON & Malysheva ZV (1977) Working conditions and their effect
on some specific functions of women engaged in the manufacture of
enamel-insulated wires. Gig Tr Prof Zabol, 4: 25-28.
Tabak HH, Chambers CW, & Kabler PW (1964) Microbial metabolism of
aromatic compounds: I. Decomposition of phenolic compounds and
aromatic hydrocarbons by phenol-adapted bacteria. J Bacteriol,
87: 910-919.
Thompson DC, Perera K, Fisher R, & Brendel K (1994) Cresol isomers:
comparison of toxic potency in rat liver slices. Toxicol Appl
Pharmacol, 125:51-58.
TRL (1986) Subchronic neurotoxicity study in rats of orth o-, meta-,
and para-cresol. Research Triangle Park, North Carolina, Toxicity
Research Laboratories (Unpublished data submitted to the US
Environmental Protection Agency).
US EPA (1982) Assessment of testing needs: Cresols support document.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances, Test Rule Development Branch, Assessment Division, p 373.
US EPA (1986) Test methods for evaluating solid waste: SW-846. Vol.
II: Field manual physical/chemical methods, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and Emergency
Response.
US EPA (1988) US EPA contract laboratory program statement of work for
organic analysis: Semi-volatile compounds. Washington, DC, US
Environmental Protection Agency, pp D1/SV-D45/SV.
US EPA (1989) Toxics release inventory. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
USITC (1990) Synthetic organical chemicals, United States production
and sales, 1989. Washington, DC, United States International Trade
Commission, p 3-2 (Publication No 2338).
USITC (1991) Synthetic organical chemicals, United States production
and sales, 1990. Washington, DC, United States International Trade
Commission, p 3-2 (Publication No. 2470).
US NIOSH (1989) Manual of analytical methods, 3rd ed. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
US NTP (1992) Toxicity studies of cresols (CAS nos. 95-48-7, 108-39-4,
106-44-5) in F344/N rats and B6C3F1 mice (feed studies). Research
Triangle Park, Noth Carolina, National Toxicology Program.
Uzhdavini ER, Astaf'yeva IK, Mamayeva AA, & Bakhtizina GZ (1972)
[Inhalation toxicity of o-cresol.] Tr Ufimskogo
Nauchno-Issledovatel'skogo Inst Gig Prof Zabol, 7: 115-119
(in Russian).
Uzhdavini ER & Gilev VG (1976) Toxicity of the lower phenols in the
case of epicutaneous applications. Tr Bashkir Med Inst, 19: 162-167.
Uzhdavini ER, Astafeva IK, & Mamaeva AA (1974) Acute toxicity of the
lower phenols. Gig Tr Prof Zabol, 2: 58-59.
Uzhdavini ER, Astafeva IK, Mamaeva AA, & Gilev VG (1976) Materials for
establishing the limiting dose of dicresol in the air at production
premises. Gig Tr Prof Zabol, 9: 53-55.
Vance BM (1945) Intrauterine injection of Lysol as an abortifacient:
Report of a fatal case complicated by oil embolism and Lysol
poisoning. Arch Pathol, 40: 395-398.
Vecera Z & Janak J (1987) Continuous aerodispersive enrichment unit
for trace determination of pollutants in air. Anal Chem,
59(11): 1494-1498.
Verchuesen K (1983) Handbook of environmental data on organic
chemicals. 2nd edition. New York, Van Nostrand Reinhold Company,
pp 403-410.
Vernot EH, MacEwen JD, Haun CC, & Kinkead ER (1977) Acute toxicity and
skin corrosion data from some organic and inorganic compounds and
aqueous solutions. Toxicol Appl Pharmacol, 42: 417-423.
Visser SA, LaMontagne G, Zoulalian V, & Tessier A (1977) Bacteria
active in the degradation of phenols in polluted waters of the St.
Lawrence River. Arch Environ Contam Toxicol, 6: 455-469.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sew Ind Wastes,
29(6): 695-711.
Wang YT, Suidan MT, Pfeffer JT, & Najm I (1988) Effects of some alkyl
phenols on methanogenic degradation of phenol. Appl Environ Microbiol,
54(5): 1277-1279.
Wang YT, Suidan MT, Pfeffer JT, & Najm I (1989) The effect of
concentration of phenols on their batch methanogenesis. Biotechnol
Bioeng J, 33(10): 1353-1357.
Weast RC, Astle MJ, & Beyer WH (1988) CRC handbook of chemistry and
physics, 69th ed. Boca Raton, Florida, CRC Press, Inc, pp C/218-C/220.
Weber AS & Matsumoto MR (1987) Feasibility of intermittent biological
treatment for hazardous wastes. Environ Prog, 6(3): 166-171.
Williams RA, MacRae R, & Shepherd MJ (1989) Non-aqueous size-exclusion
chromatography coupled online to reversed-phase high-performance
liquid chromatography interface development and applications to the
analysis of low-molecular-weight contaminants and additives in foods.
J Chromatogr, 477(2): 315-326.
Williams RT (1938) CXVIII: Studies in detoxication: I. The influence
of (a) dose and (b) o-, m- and p-substitution on the sulfate
detoxication of phenol in the rabbit. Biochem J, 32: 878-887.
Windholz M, Budavini S, Blumetti RF & Otterbein ES ed. (1983) The
Merck index: an encyclopedia of chemicals, drugs, and biologicals,
10th ed. Rahway, New Jersey, Merck and Co., Inc.
Wiseman HW, Turner WH, & Volans GW (1980) Acute poisoning due to
Wright's vaporizing fluid. Postgrad Med J, 56(653): 166-168.
Wu HY & Kwan Y (1984) [Case report of an acute renal failure
complicated by cresol burns.] Chin J Prev Med, 18:145-149
(in Chinese).
Wynder EL & Hoffmann D ed. (1967) Tobacco and tobacco smoke. New York,
London, San Francisco, Academic Press, pp 387, 499-500.
Yalkowsky SH, Valvani SC, & Kun W (1987) Arizona database of aqueous
solutions.
Yanysheva NYA, Balenko NV, Chernichenko IA, & Babiy VF (1993)
Peculiarities of carcinogenesis under simultaneous oral administration
of benzo( a)pyrene and o-cresol in mice. Environ Health Perspect,
101(Suppl. 3): 341-344.
Yashiki M, Kojima T, Miyazaki T, Ohikasne F, & Ohtani M (1989) Gas
chromatographic determination of cresols in the biological fluids of a
non-fatal case of cresol intoxication. Forensic Sci Int, 47: 21-29.
Yasuhara A (1987) Identification of volatile compounds in poultry
manure by gas chromatography-mass spectrometry. J Chromatogr,
387: 371-378.
Yasuhara AO, Shiraishi H, Tsuji M, & Okuno T (1981) Analysis of
organic substances in highly polluted river water by mass
spectrometry. Environ Sci Technol, 15: 570-573.
Yoshikawa M, Arashidani K, Kodama Y (1986) [A simple determination of
phenol in urine by high performance liquid chromatography.] San Igaku,
27(2): 83-89 (in Japanese, with English abstract).
Young LY & Rivera MD (1985) Methanogenic degradation of four phenolic
compounds. Water Res, 19: 1325-1332.
Young RHF, Ryckman DW, & Buzzell JC Jr (1968) An improved tool for
measuring biodegradability. J Water Pollut Control Fed, 40: R354-R367.
Younger Labs (1974) Skin irritation in albino rabbits after
application of o-, m-, and p-cresol. St. Louis, Missouri,
Younger Laboratories (Unpublished data submitted to the US
Environmental Protection Agency, Office of Toxic Substances)
(Fiche No. OTS0517499).
Zheng Y, Hill DO, & Kuo CH (1993a) Destruction of cresols by chemical
oxidation. J Hazard Mater, 34:245-260.
Zheng Y, Hill DO, & Kuo CH (1993b) Rates of ozonation of cresol
isomers in aqueous solutions. Ozone Sci Eng, 15: 267-278.
RESUME
1. Identité, propriétés et méthodes d'analyse
Les crésols sont des phénols isomères substitués par un
groupement méthyle en position ortho, méta ou para par rapport au
groupement hydroxyle. Le crésol du commerce, également connu sous le
nom d'acide crésylique, contient les trois isomères à côté d'un peu de
phénol et de xylènes. Toutefois certains produits du commerce
contiennent jusqu'à 30% de xylénol et 60% de phénols en C9 et sont
également désignés sous le nom "d'acides crésyliques". Physiquement,
les crésols se présentent sous la forme d'un solide cristallin blanc
ou d'un liquide jaunâtre à forte odeur phénolique. Ils sont très
inflammables et sont solubles dans l'eau, l'éthanol, l'éther,
l'acétone et les hydroxydes alcalins. Les crésols subissent des
réactions de substitution électrophiles en position ortho ou para
du groupement hydroxyle. Ils donnent également lieu à des réactions
de condensation avec les aldéhydes, les cétones ou les diènes.
On peut utiliser plusieurs méthodes pour rechercher la présence
de crésols dans l'environnement ou les milieux biologiques. Les plus
couramment utilisées sont la chromatographie en phase gazeuse avec
détection par ionisation de flamme, la chromatographie en phase
gazeuse couplée à la spectrophotométrie de masse et enfin la
chromatographie liquide à haute performance. Pour l'échantillonnage
dans l'air, on peut faire passer celui-ci dans un absorbeur contenant
de l'hydroxyde de sodium ou des adsorbants solides.
2. Usages, sources et niveaux d'exposition
Les crésols sont très largement utilisés comme solvants ou
désinfectants ou encore comme intermédiaires dans la production d'un
grand nombre d'autres substances. Ces composés sont le plus souvent
utilisés pour la production d'arômes, d'antioxydants, de colorants, de
pesticides et de résines. L' ortho- et le para-crésol sont
utilisés pour la production d'huiles lubrifiantes, de combustibles
pour véhicules à moteur et d'élastomères, le meta-crésol étant
utilisé à la fabrication d'explosifs.
Les crésols et leurs dérivés existent à l'état naturel dans les
huiles essentielles de diverses plantes telles que les fleurs de
Yucca gloriosa, dans le jasmin, le lys, les conifères, les chênes et
le santal, et ils constituent également un produit de la combustion
naturelle de certaines substances et de l'activité volcanique. On
trouve du para-crésol dans l'urine des animaux et de l'homme. Les
crésols du commerce sont des sous-produits de la distillation
fractionnée du pétrole brut et du goudron de houille. Ils se
retrouvent en petites quantités dans les échappements des véhicules à
moteur, lors de l'incinération des déchets municipaux et de la
combustion de la houille et du bois. La fumée de cigarettes contient
également des crésols. On ignore quelle est la production mondiale
totale de crésols; pour les Etats-Unis d'Amérique, on indiquait en
1990 une production annuelle totale de 38 300 tonnes.
Le transport des crésols dans le milieu s'effectue dans la phase
gazeuse de l'atmosphère, et de l'atmosphère aux eaux de surface et au
sol par entraînement avec les précipitations. En raison de leur
volatilité, de leur fixation aux sédiments et de leur biodégradation,
les crésols ne se retrouvent dans l'eau qu'en petites quantités. Dans
le sol, ils peuvent être légèrement ou fortement mobiles en fonction
du coefficient d'adsorption du sol (Koc). On a décelé la présence
de crésols dans les eaux souterraines, de sorte qu'un lessivage doit
se produire.
Il peut y avoir exposition aux crésols par l'intermédiaire de
l'air, de l'eau ou de la nourriture. La concentration médiane dans
l'air des o-crésols a été trouvée égale à 1,59 µg/m3 (0,359 ppm)
sur 32 sites des Etats-Unis d'Amérique dominés par des sources de
pollution. Dans ce même pays, les concentrations dans les eaux de
surface vont de valeurs inférieures à la limite de détection jusqu'à
77 µg/litre (STORET, 1993). Au Japon, on a trouvé une concentration
de 204 µg/litre dans une rivière polluée par des effluents
industriels. Des teneurs pouvant atteindre 2100 µg/litre dans le cas
de l' o-crésol et 1200 µg/litre dans le cas d'un mélange de m- et
de p-crésols ont été mesurées dans des eaux usées. Dans l'eau de
pluie, les concentrations vont de 240 à 2800 ng/litre dans le cas de
l' o-crésol et de 380 à 2000 ng/litre pour le mélange de p- et de
m-crésols. On a décelé la présence de crésols dans des denrées
alimentaires et des boissons. Dans les spiritueux, des concentrations
se situant entre les limites 0,01-0,02 mg/litre ont été mesurées.
Dans la fumée émise par une cigarette américaine sans bout-filtre
(85 mm), la teneur est de 75 µg. La population générale peut être
exposée aux crésols par suite de l'inhalation d'air, de l'ingestion
d'eau, de nourriture et de boissons diverses ainsi que par contact
cutané. En général il est impossible d'évaluer quantitativement la
dose de crésols absorbée à partir de ces sources par suite de
l'absence de données de surveillance suffisantes. En ce qui concerne
l'exposition professionnelle, on a fait état de concentrations
atteignant 5,0 mg/m3.
3. Cinétique et métabolisme
Les crésols sont résorbés au niveau des voies respiratoires et
digestives ainsi que de l'épiderme. La vitesse et l'ampleur de cette
absorption n'ont pas fait l'objet d'études particulières. Cependant
certains travaux montrent qu'au niveau des voies digestives et de
l'épiderme, l'absorption est rapide et importante. Les crésols se
répartissent dans l'ensemble des principaux viscères. La principale
voie métabolique des crésols consiste dans une conjugaison avec
l'acide glucuronique et les sulfates inorganiques. Il existe des
voies métaboliques secondaires comportant une hydroxylation du cycle
benzénique ainsi qu'une oxydation de la chaîne latérale. Les crésols
sont principalement éliminés par les reins sous forme de conjugués.
4. Effets sur les mammifères de laboratoire et les systèmes in vitro
Une intoxication aiguë par les vapeurs de crésols est peu
probable en raison de la faible tension de vapeur de ces composés.
Chez le rat, on a observé des concentrations létales moyennes de
29 mg/m3 pour l' o- et le p-crésol et de 58 mg/m3 pour le
m-crésol. Chez le même animal, les valeurs de la DL50 par voie
orale sont respectivement de 121, 207 et 242 mg/kg de poids corporel
pour l' o-, le p- et le m-crésol respectivement. Les
comparaisons interspécifiques montrent que les trois isomères sont
tous plus toxiques pour la souris que pour le rat et que leur toxicité
croît avec la concentration. L'exposition cutanée peut entraîner une
intoxication générale mortelle. Chez le lapin on a relevé pour la
DL50 des valeurs respectivement égales à 890, 2830, 300 et
2000 mg/kg de poids corporel pour l' o-, le m- et le p-crésol et
leurs mélanges. Chez le rat, la DL50 cutanée se situait
respectivement à 620, 1100, 750 et 825 mg/kg de poids corporel pour
l' o-, le m- et le p-crésol ainsi que pour le dicrésol.
Chez le lapin, le rat et la souris, les crésols sont extrêmement
irritants pour la peau et les yeux.
Chez des animaux à qui l'on avait fait respirer pendant une
courte durée des mélanges d'aérosols et de vapeurs d' o-crésol, on a
observé une irritation des voies respiratoires, de petites hémorragies
au niveau des poumons, une réduction du poids corporel ainsi qu'une
dégénérescence cellulaire du myocarde, du foie, du rein et des nerfs.
En faisant absorber à des rats pendant une courte durée (28 jours) des
doses quotidiennes d'environ 800 mg ou plus de crésols par kg de poids
corporel, on a constaté une réduction du poids corporel, une
modification du poids des organes ainsi que des anomalies
histopathologiques au niveau des voies respiratoires et digestives.
Chez des souris exposées de la même manière à des doses de 1500 mg/kg
de poids corporel, les effets ont été plus sévères et les animaux sont
morts aux concentrations les plus élevées d' o-, de m- et de
p-crésol, à l'exclusion des mélanges de ces isomères.
L'exposition prolongée de rats à des vapeurs d' o-, de m- ou
de p-crésol (pendant une durée allant jusqu'à 4 mois) a eu pour
effets une perte de poids, une réduction de l'activité locomotrice,
une inflammation des muqueuses nasales et de la peau ainsi que des
anomalies au niveau du foie. Exposés par voie orale à des crésols
pendant 13 semaines, des souris, des rats et des hamsters ont
présentés les effets suivants: mortalité, tremblements, réduction du
poids corporel, effets hématologiques, accroissement du poids des
organes, hyperplasie de l'épithélium nasal et de celui de la portion
cardiaque de l'estomac.
L'exposition de rats et de souris à des crésols isomères par voie
orale ou par voie respiratoire provoque un allongement du cycle
oestral ainsi que des modifications histopathologiques au niveau de
l'utérus et des ovaires. On n'a en revanche pas observé d'effets
indésirables sur la spermatogénèse. De légers effets cytotoxiques ont
été signalés chez des rats et des lapins exposés à de l' o- et du
p-crésol, mais on n'a observé sur le développement que des effets
mineurs qui puissent être attribuables à ce traitement. Le traitement
in vitro par de l' o- et du p-crésol, à l'exclusion du
m-crésol, entraînerait une certaine génotoxicité. En revanche les
études in vivo n'ont pas fait ressortir de résultats positifs.
Pourtant, il existe des signes d'activité promotrice au niveau cutané.
Aucune étude de cancérogénicité n'a été publiée sur l'un quelconque
des isomères du crésol.
5. Effets sur l'homme
L'ingestion de crésols provoque des brûlures de la bouche et de
la gorge, des douleurs abdominales et des vomissements. Après
ingestion, les tissus et les organes-cibles sont, chez l'homme, le
sang et les reins et l'on a également fait état d'effets sur les
poumons, le foie, le coeur et le système nerveux central. Dans les
cas graves, on peut observer un coma mortel. Après exposition
cutanée, on a signalé de graves brûlures laissant subsister des
cicatrices, une intoxication générale et la mort.
En général, l'exposition professionnelle aux crésols résulte d'un
contact cutané. Une exposition aiguë peut provoquer de graves
brûlures, une anurie et un coma mortel. On ne dispose que de très peu
de données sur les effets au niveau de l'appareil reproducteur et on
n'a aucun renseignement sur la cancérogénicité de ces produits pour
l'homme.
6. Effets sur les autres êtres vivants
Les observations effectuées sur des microorganismes, des
invertébrés et des poissons montrent que les crésols peuvent
constituer un risque pour les organismes non-mammaliens là où des
sources ponctuelles de pollution déterminent de fortes concentrations,
mais ce n'est pas le cas dans l'environnement en général.
7. Conclusions et recommandations
Aux concentrations généralement présentes dans l'environnement,
les crésols ne constituent pas un risque important pour la population
générale. Toutefois il y a possibilité d'effets indésirables pour les
insuffisants rénaux ainsi que pour les personnes présentant certains
déficits enzymatiques; ce risque existe également en cas de forte
exposition.
Les crésols peuvent constituer un risque pour les
microorganismes, les invertébrés et les poissons là où des sources
ponctuelles de pollution déterminent de fortes concentrations, mais ce
n'est pas le cas dans l'environnement en général.
On ne dispose d'aucune donnée concernant les effets de
l'exposition chronique aux crésols. Dans ces conditions, il n'est pas
possible d'évaluer le risque cancérogène imputable à ces produits. Si
on s'appuie sur les résultats d'études sub-chroniques, on peut estimer
à 50 mg/kg de poids corporel par jour la dose sans effets indésirables
observables. Il a été recommandé d'appliquer un facteur d'incertitude
de 300 qui est établi comme suit: 10 pour tenir compte des variations
interspécifiques; 10 pour tenir compte de l'absence de données de
toxicité chronique ainsi que de l'activité génotoxique et promotrice
possible des crésols et enfin 3 pour tenir compte des variations
intraspécifiques tenant à un métabolisme plus ou moins rapide et
complet. Dans ces conditions, on peut fixer à 0,17 mg/kg de poids
corporel par jour la dose journalière acceptable (DJA) de crésols.
RESUMEN
1. Identidad, propiedades y métodos analíticos
Los cresoles son fenoles sustituidos isoméricos con un
sustituyente metilo en una de las posiciones orto, para o meta
respecto al grupo hidróxilo. El cresol comercial, conocido también
como ácido cresílico, contiene los tres isómeros con pequeñas
cantidades de fenol y xilenoles. Sin embargo, los productos
comerciales, conocidos como "ácidos cresílicos", contienen hasta un
30% de xilenol y un 60% de C9-fenoles. Físicamente, los cresoles
consisten en un sólido cristalino blanco o un líquido amarillento y
tienen un fuerte olor a fenol. Son altamente inflamables y se
disuelven en agua, etanol, éter, acetona e hidróxidos alcalinos. Los
cresoles sufren reacciones de sustitución electrofílica en la posición
orto o para libre en relación con el grupo hidróxilo. Asimismo,
experimentan reacciones de condensación con aldehídos, cetonas o
dienos.
La presencia de cresoles tanto en medios naturales como
biológicos puede ser determinada usando varios métodos. Los más
corrientes son la cromatografía de gases con detector de ionización de
llama (GC-FID), la cromatografía de gases con espectrofotometría de
masas (GC-MS) y la cromatografía líquida de alta resolución (HPLC).
El muestreo de los cresoles en la atmósfera puede realizarse pasando
aire a través de células de absorción, utilizando hidróxido de sodio o
adsorbentes sólidos.
2. Usos, fuentes y niveles de exposición
Los cresoles ofrecen gran variedad de usos como solventes o
desinfectantes, o como intermedios en la producción de muchas otras
sustancias. Estos compuestos son utilizados principalmente en la
producción de perfumes, antioxidantes, tintes, plaguicidas y resinas.
Los cresoles orto- y para- se utilizan en la producción de aceites
lubricantes, combustibles para motores y polímeros de caucho, mientras
que el meta-cresol interviene en la fabricación de explosivos.
Los cresoles y sus derivados se encuentran naturalmente en los
aceites de diversas plantas (tales como flores de Yucca gloriosa,
jazmín, Lilium longiflorum var. eximium, coníferas, roble y sándalo) y
como producto de combustión de los incendios naturales y la actividad
volcánica. El para-cresol está presente en la orina de animales y
del hombre. Comercialmente, los cresoles son generados como
subproductos de la destilación fraccional de petróleo crudo y
alquitranes de hulla. Asimismo, se producen pequeñas cantidades en
caños de escape de vehículos, incineradores municipales de desechos y
mediante la combustión del carbón y de la madera. El humo de
cigarrillo también contiene cresoles. No se conoce la producción
mundial de cresoles; el total registrado en los Estados Unidos en 1990
ascendió a 38 300 toneladas.
El transporte de cresoles en el medio ambiente se realiza en la
fase vapor de la atmósfera, y de la atmósfera a las aguas
superficiales y al suelo por el lavado de las lluvias. Debido a su
volatilización, su adherencia a sedimentos y su biodegradación, los
cresoles se encuentran sólo en pequeñas cantidades en el agua. Su
movilidad en suelos va de leve a alta, según el coeficiente (Koc) de
sorción del suelo. Se han detectado cresoles en aguas subterráneas,
lo cual sugiere que algún grado de lixiviación debe ocurrir en el
suelo.
La exposición a los cresoles puede ocurrir a través del aire, del
agua o de los alimentos. En 32 sitios identificados de los Estados
Unidos se halló una concentración atmosférica mediana de o-cresoles
de 1,59 µg/m3 (0,359 partes por mil millones). En los Estados
Unidos, las concentraciones en aguas superficiales van de niveles
inferiores al umbral de detección a 77 µg/litro (STORET, 1993). En el
Japón, se hallaron niveles de 204 µg/litro en un río contaminado por
efluentes industriales. En aguas residuales, ha sido posible detectar
concentraciones de hasta 2100 µg/litro para el o-cresol y de
1200 µg/litro para m- y p-cresoles combinados. En el agua de
lluvia, las concentraciones van de 240 a 2800 ng/litro para el
o-cresol y de 380 a 2000 ng/litro para p- y m-cresoles
combinados. También se han detectado cresoles en alimentos y bebidas.
En bebidas alcohólicas se determinaron concentraciones del orden de
0,01-0,2 mg/litro. En el humo de tabaco, esta cantidad es de 75 µg
para un cigarrillo americano sin filtro (85 mm). La exposición de la
población general a los cresoles puede darse por inhalación, por el
agua potable, por la ingestión de alimentos y bebidas, y por contacto
cutáneo. En general, la falta de datos adecuados de seguimiento
impide realizar estimaciones cuantitativas de la ingesta diaria de
cresol por estas vías. Se han señalado niveles de exposición
ocupacional de hasta 5,0 mg/m3.
3. Cinética y metabolismo
Los cresoles se absorben a través de los tractos respiratorio y
gastrointestinal y por contacto con la piel. Si bien el índice y la
magnitud de la absorción no han sido estudiados específicamente,
diversos estudios han probado que la absorción gastrointestinal y
dérmica es rápida y extensa. Los cresoles se distribuyen a todos los
principales órganos. Su principal vía metabólica es la conjugación
con ácido glucurónico y sulfato inorgánico; las vías metabólicas
secundarias incluyen la hidroxilación del anillo de benceno y la
oxidación de cadena lateral. La principal vía de eliminación es la
excreción renal en forma de conjugados.
4. Efectos en mamíferos de laboratorio; sistemas in vitro
La intoxicación aguda con cresoles es poco probable, debido a la
baja presión de vapor de estos compuestos. Se han señalado
concentraciones letales medias en ratas de 29 mg/m3 para o- y
p-cresoles y de 58 mg/m3 para m-cresoles. En ratas, los valores
orales DL50 notificados han sido de 121, 207 y 242 mg/kg de peso
corporal para o-, p- y m-cresoles, respectivamente. Las
comparaciones entre especies revelan que los tres isómeros son más
tóxicos en ratones que en ratas y que la toxicidad aumenta con la
concentración. La exposición cutánea puede provocar toxicidad
sistémica y muerte. Los valores dérmicos DL50 en conejos fueron de
890, 2830, 300 y 2000 mg/kg de peso corporal para cresoles o-, m-,
p- y combinados, respectivamente. En ratas, se registraron valores
dérmicos DL50 de 620, 1100, 750 y 825 mg/kg de peso corporal para
o-, m-, p- cresoles y dicresol, respectivamente.
Los cresoles ocasionan graves irritaciones dérmicas y oculares en
conejos, ratas y ratones.
La exposición por breves periodos a mezclas inhaladas de
aerosoles y vapores de o-cresol provocó irritación del tracto
respiratorio, pequeñas hemorragias pulmonares, pérdida de peso
corporal y degeneración de músculo cardiaco, hígado, riñón y células
nerviosas. La exposición oral por breves periodos (28 días) a dosis
diarias de unos 800 mg/kg de peso corporal o más produjo pérdida de
peso corporal, alteración del peso de los órganos y cambios
histopatológicos en los tractos respiratorio y gastrointestinal de las
ratas. Más graves fueron los efectos constatados en ratones expuestos
a una administración similar de dosis de 1500 mg/kg de peso corporal;
a concentraciones más elevadas, la muerte fue causada por la
exposición a o-, m- y p-cresoles, mas no por exposiciones a
mezclas de isómeros.
Una exposición más prolongada de ratas, de hasta 4 meses, a
vapores de o-, m- y p-cresol provocó pérdida de peso, reducción
de la actividad locomotriz, cambios hepáticos e inflamación de
membranas nasales y piel. La exposición oral de ratones, ratas y
hámsters por periodos de hasta 13 semanas ocasionó muerte, temblores,
pérdida de peso corporal, alteraciones hematológicas, aumento del peso
de los órganos e hiperplasia del epitelio nasal y del cardias.
La exposición oral y por inhalación a isómeros del cresol da
lugar a ciclos estruales prolongados y modificaciones histopatológicas
del útero y los ovarios de ratas y ratones. No se observaron efectos
sobre la espermatogénesis en ratas y ratones. En ratas y conejos
expuestos a o- y p-cresoles se registraron efectos embriotóxicos
moderados; no obstante, sólo se han señalado anomalías menores del
desarrollo relacionadas con el tratamiento. Ciertos indicios de
genotoxicidad han sido constatados in vitro como consecuencia del
tratamiento con o- y p-cresoles, pero no con m-cresol. No se
obtuvieron resultados positivos en estudios in vivo; sin embargo, se
observaron indicios de actividad promotora en la piel. No se han
notificado estudios de carcinogenicidad para ninguno de los isómeros
del cresol.
5. Efectos en la especie humana
La ingestión de cresoles provoca quemaduras de boca y esófago,
dolores abdominales y vómitos. Los tejidos y órganos afectados por la
ingestión de cresoles son la sangre y los riñones, aunque también se
han señalado efectos en los pulmones, el hígado, el corazón y el
sistema nervioso central. En casos graves, puede producirse coma y
muerte. La exposición cutánea ha ocasionado graves quemaduras de
piel, cicatrices, toxicidad sistémica y muerte.
En el medio laboral, la exposición a los cresoles suele
producirse por contacto cutáneo. La exposición aguda puede dar por
resultado graves quemaduras, anuria, coma y muerte. Existen muy pocos
datos sobre sus efectos en la reproducción, y ninguno sobre la
carcinogenicidad en el ser humano.
6. Efectos en otros organismos
Las observaciones en microorganismos, invertebrados y peces han
revelado que los cresoles pueden suponer un riesgo para organismos
diferentes de los mamíferos en puntos específicos con altas
concentraciones de cresol, pero no en el medio ambiente en general.
7. Conclusión y recomendaciones
En las concentraciones normalmente halladas en el medio ambiente,
los cresoles no presentan un riesgo significativo para la población
general. Con todo, pueden darse efectos adversos en personas que
padecen de insuficiencia renal o de una deficiencia enzimática
específica, así como en condiciones de alta exposición.
Los cresoles pueden suponer un riesgo para microorganismos,
invertebrados y peces en puntos específicos con una alta concentración
de cresoles, pero no en el medio ambiente en general.
Al no disponerse de datos sobre las consecuencias de una
exposición crónica, no existe una información adecuada que permita
evaluar el riesgo carcinogénico de los cresoles. Partiendo de los
resultados de estudios subcrónicos, puede establecerse un nivel sin
efectos adversos observados (NOAEL) de 50 mg/kg de peso corporal al
día para los tres isómeros del cresol. Se ha recomendado un factor de
incertidumbre 300, que se descompone así: 10 por la variación entre
especies; 10 por la falta de estudios de toxicidad crónica y por la
posible actividad genotóxica y promotora de los cresoles; y 3 por la
variación dentro de la misma especie basada en el metabolismo completo
y rápido. Por consiguiente, puede establecerse para los cresoles una
ingesta diaria admisible de 0,17 mg/kg de peso corporal.
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
Members
Dr D. Anderson, British Industrial Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr M.R. Elwell, National Institute of Health, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr A. Meharg, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, United Kingdom
Dr C.-N. Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
(Vice-Chairman)
Dr Y. Pang, Division of Standard Setting, Chinese Academy of
Preventive Medicine, Beijing, China
Dr L. Papa, System Toxicants Assessment Branch, Office of Research and
Development, Environmental Criteria and Assessment Office, US
Environmental Protection Agency, Cincinnati, Ohio, USA
(Rapporteur)
Dr A. Pinter, National Institute of Hygiene, Budapest, Hungary
Dr S. Soliman, Pesticide Chemistry and Toxicology, College of
Agriculture and Veterinary Medicine, Bureidah, Saudi Arabia
Dr F.M. Sullivan, Division of Pharmacology and Toxicology, St Thomas's
Hospital, London, United Kingdom (Chairman)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
A WHO Task Group on Environmental Health Criteria for Cresols met
at the British Industrial Biological Research Association (BIBRA)
Toxicology International, Carshalton, Surrey, United Kingdom, from 27
June to 1 July 1994. Dr D. Anderson opened the meeting and welcomed
the participants on behalf of the host institution. Dr B.H. Chen,
IPCS, welcomed the participants on behalf of the Director, IPCS, and
the three cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to cresols.
Drs N.N. Molodkina, L.P. Kuzmina and A.L. Germanova, Centre for
International Projects, Moscow, Russian Federation, prepared a
preliminary draft. The first draft of this monograph was prepared by
Dr L. Papa, US Environmental Protection Agency, Cincinnati, USA. The
second draft was also prepared by Dr L. Papa, incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
1. SUMMARY
1.1 Identity, properties and analytical methods
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para position relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols.
However, commercial products contain up to 30% xylenol and 60%
C9-phenols and are known as "cresylic acids". Physically, cresols
consist either of a white crystalline solid or a yellowish liquid and
have a strong, phenol-like odour. They are highly flammable and are
soluble in water, ethanol, ether, acetone and alkali hydroxides.
Cresols undergo electrophilic substitution reactions at the vacant
ortho or para position relative to the hydroxyl group. They also
undergo condensation reactions with aldehydes, ketones or dienes.
Several methods can be used for determining the presence of
cresols in both environmental and biological media. The most commonly
used methods are gas chromatography with flame ionization detection
(GC-FID), gas chromatography with mass spectrophotometry (GC-MS) and
high-performance liquid chromatography (HPLC). Sampling of cresols in
air can be done by passing air through absorption cells using sodium
hydroxide or solid adsorbents.
1.2 Uses, sources and levels of exposure
Cresols have a wide variety of uses as solvents or disinfectants
or as intermediates in the production of numerous other substances.
These compounds are most commonly used in the production of
fragrances, antioxidants, dyes, pesticides and resins. Ortho- and
para-cresols are used in the production of lubricating oils, motor
fuels and rubber polymers, while meta-cresol is used in the
manufacture of explosives.
Cresols and cresol derivatives occur naturally in oils of various
plants, including Yucca gloriosa flowers, jasmine, Easter lily,
conifers, oaks and sandalwood trees, and are also a product of
combustion from natural fires and volcanic activity. Para-cresol is
found in the urine of animals and humans. Commercially cresols are
produced as by-products in the fractional distillation of crude oil
and coal tars. Small amounts are produced in vehicle exhaust,
municipal waste incinerators and from coal and wood combustion.
Cigarette smoke also contains cresols. The worldwide production of
cresols is unknown; annual production in the USA in 1990 was reported
to be 38 300 tonnes.
Environmental transport of cresols occurs through the vapour
phase of the atmosphere and from the atmosphere to surface water and
soil by rain-scavenging. Due to their volatilization, binding to
sediment and biodegradation, only small amounts of cresols are found
in water. In soils, cresols are slightly to highly mobile depending on
the sorption coefficient (Koc) of the soil. Cresols have been
detected in ground water, and so leaching must occur in soil.
Exposure to cresols can occur through air, water or food. The
median air concentration of o-cresols was 1.59 µg/m3 (0.359 ppb)
for 32 source-dominated sites in the USA. Surface water
concentrations in the USA range from below the detection limit to
77 µg/litre (STORET, 1993). Levels of 204 µg/litre were reported in
Japan in a river polluted by industrial effluents. Concentrations as
high as 2100 µg/litre for o-cresol and 1200 µg/litre for mixed
m- and p-cresols have been detected in waste waters. Rainwater
concentrations range from 240 to 2800 ng/litre for o-cresol and 380
to 2000 ng/litre for p- and m-cresol combined. Cresols have been
detected in foods and beverages. Concentrations in spirit beverages
were found to be within the range of 0.01-0.2 mg/litre. The amount in
tobacco smoke is 75 µg in a nonfilter American cigarette (85 mm). The
general population can be exposed to cresols from air inhalation,
drinking-water, food and beverage ingestion and dermal contact. In
general, the lack of adequate monitoring data makes the quantitative
estimates of daily intakes of cresol from these sources impossible.
Occupational exposure levels as high as 5.0 mg/m3 have been
reported.
1.3 Kinetics and metabolism
Cresols are absorbed across the respiratory and gastrointestinal
tracts and through the skin. The rate and extent of absorption of
cresols has not been studied specifically. However, studies have
shown that gastrointestinal and dermal absorption are rapid and
extensive. Cresols are distributed to all the major organs. The
primary metabolic pathway for cresols is conjugation with glucuronic
acid and inorganic sulfate. Minor metabolic pathways for cresols
include hydroxylation of the benzene ring and side-chain oxidation.
The main route for elimination of cresols from the body is renal
excretion in the form of conjugates.
1.4 Effects on laboratory mammals; in vitro systems
Acute poisoning with cresol vapours is unlikely due to the low
vapour pressure of these compounds. Mean lethal concentrations of
cresols in rats have been reported to be 29 mg/m3 for o- and
p-cresols and 58 mg/m3 for m-cresol. Oral LD50 values in rats
have been reported to be 121, 207 and 242 mg/kg body weight for o-,
p- and m-cresols, respectively. Interspecies comparisons show
that all three isomers are more toxic to mice than to rats and that
toxicity increases with concentration. Systemic toxicity and death
can result from dermal exposure. Dermal LD50 values in rabbits were
890, 2830, 300 and 2000 mg/kg body weight for o-, m-, p-and
mixed cresols, respectively. In rats dermal LD50 values were 620,
1100, 750 and 825 mg/kg body weight for o-, m-, p- and dicresol,
respectively.
Cresols are highly irritating to the skin and eyes of rabbits,
rats and mice.
Short-term exposure to inhaled mixtures of o-cresol aerosol and
vapour resulted in irritation of the respiratory tract, small
haemorrhages in the lung, body weight reduction and degeneration of
heart muscle, liver, kidney and nerve cells. Short-term (28-days)
oral exposure to daily doses of approximately 800 mg/kg body weight or
more resulted in reduced body weights, organ weight changes and
histopathological changes in the respiratory and gastrointestinal
tracts of rats. In mice, similarly exposed at 1500 mg/kg body weight,
more severe effects were reported, and at the highest concentrations
death resulted from exposure to o-, m- and p-cresols but not
from exposures to mixtures of isomers.
Longer-term exposure of rats to vapours of o-, m- or
p-cresol for up to 4 months resulted in weight loss, reduced
locomotor activity, inflammation of nasal membranes and skin, and
changes in the liver. Oral exposures for up to 13 weeks of mice,
rats and hamsters resulted in mortality, tremor, reduced body weights,
haematological effects, increase in organ weight, and hyperplasia of
nasal and forestomach epithelium.
Oral and inhalation exposure to cresol isomers result in
lengthened estrus cycle and histopathological changes in the uterus
and ovaries of rats and mice. No adverse effects on spermatogenesis
were observed in rats or mice. Mild fetotoxic effects have been
reported in rats and rabbits exposed to o- and p-cresols, but only
minor treatment-related developmental effects have been reported.
Some evidence of genotoxicity has been reported to result in vitro
from treatment with o- and p- cresols but not m-cresol. No
positive results were obtained in in vivo studies. However, some
evidence of promotive activities in skin has been reported. No
studies of carcinogenicity have been reported for any cresol isomers.
1.5 Effects on humans
Ingestion of cresols results in burning of the mouth and throat,
abdominal pain and vomiting. The target tissues/organs of ingested
cresols in humans are the blood and kidneys, and effects on the lungs,
liver, heart and central nervous system have also been reported. In
severe cases, coma and death may result. Dermal exposure has been
reported to cause severe skin burns, scarring, systemic toxicity and
death.
Occupational exposure to cresols usually results from dermal
contact. Acute exposures can result in severe burns, anuria, coma and
death. Very few data are available regarding reproductive effects and
there are no data on carcinogenicity in humans.
1.6 Effects on other organisms
Observations on microorganisms, invertebrates and fish have shown
that cresols may represent a risk to non-mammalian organisms at point
sources with high cresol concentration but not in the general
environment.
1.7 Conclusion and recommendations
At concentrations normally found in the environment, cresols do
not pose any significant risk for the general population. However,
the potential for adverse health effects exists in the case of people
with renal insufficiency or specific enzymic deficiency and under
conditions of high exposure.
Cresols may represent a risk to microorganisms, invertebrates and
fish at point sources with high cresol concentrations but not in the
general environment.
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess the carcinogenic hazard of cresols. Based on the results of
subchronic studies, an NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, an acceptable daily intake (ADI) of
0.17 mg/kg body weight per day can be established for cresols.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para positions relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols
(Deichmann & Keplinger, 1981). Mixtures of m- and p-cresol and of
o-, m- and p-cresol are occasionally called dicresol and
tricresol, respectively (Fiege & Bayer, 1987). Pure and commercial
cresol or cresylic acid is different from the commercial products
called "cresylic acids". The substance "cresylic acids" is a mixture
of phenolic compounds with a typical composition as follows: 0-1% m-
and p-cresol; 0-3% 2,4- and 2,6-xylenols; 10-20% 2,3- and
3,5-xylenols; 20-30% 3,4-xylenol; and 50-60% C9-phenols (Sax & Lewis,
1987). The chemical identity of cresols is shown in Table 1.
Commercial cresols are manufactured in a wide range of
grades and purities to suit the user's requirements. Typically,
technical grade cresol available in the USA contains about 20%
o-cresol, 40% m-cresol, 30% p-cresol, and 10% phenol and
xylenols (Deichmann & Keplinger, 1981). The individual isomers are
available at purity levels as low as 85% and as high as > 99% from
chemical suppliers in the USA.
2.2 Physical and chemical properties
The physical properties of the three individual isomers and the
mixture are given in Table 2.
Chemically, cresols behave similarly to phenol. These compounds
undergo electrophilic substitution reactions at the vacant ortho or
para position relative to the hydroxyl group. Chlorination,
bromination, sulfonation and nitration are examples of such
substitution reactions. Cresols can undergo condensation reactions
with aldehydes, ketones and dienes (Fiege & Bayer, 1987).
2.3 Conversion factors
Air at 25°C: 1 ppm = 4.42 mg/m3
1 mg/m3 = 0.23 ppm
Table 1. Chemical identity of cresols
o-Cresol p-Cresol m-Cresol Mixture
Chemical structure:
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
Members
Dr D. Anderson, British Industrial Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr M.R. Elwell, National Institute of Health, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr A. Meharg, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, United Kingdom
Dr C.-N. Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
(Vice-Chairman)
Dr Y. Pang, Division of Standard Setting, Chinese Academy of
Preventive Medicine, Beijing, China
Dr L. Papa, System Toxicants Assessment Branch, Office of Research and
Development, Environmental Criteria and Assessment Office, US
Environmental Protection Agency, Cincinnati, Ohio, USA
(Rapporteur)
Dr A. Pinter, National Institute of Hygiene, Budapest, Hungary
Dr S. Soliman, Pesticide Chemistry and Toxicology, College of
Agriculture and Veterinary Medicine, Bureidah, Saudi Arabia
Dr F.M. Sullivan, Division of Pharmacology and Toxicology, St Thomas's
Hospital, London, United Kingdom (Chairman)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CRESOLS
A WHO Task Group on Environmental Health Criteria for Cresols met
at the British Industrial Biological Research Association (BIBRA)
Toxicology International, Carshalton, Surrey, United Kingdom, from 27
June to 1 July 1994. Dr D. Anderson opened the meeting and welcomed
the participants on behalf of the host institution. Dr B.H. Chen,
IPCS, welcomed the participants on behalf of the Director, IPCS, and
the three cooperating organizations (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft monograph and made an evaluation of the
risks for human health and the environment from exposure to cresols.
Drs N.N. Molodkina, L.P. Kuzmina and A.L. Germanova, Centre for
International Projects, Moscow, Russian Federation, prepared a
preliminary draft. The first draft of this monograph was prepared by
Dr L. Papa, US Environmental Protection Agency, Cincinnati, USA. The
second draft was also prepared by Dr L. Papa, incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
1. SUMMARY
1.1 Identity, properties and analytical methods
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para position relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols.
However, commercial products contain up to 30% xylenol and 60%
C9-phenols and are known as "cresylic acids". Physically, cresols
consist either of a white crystalline solid or a yellowish liquid and
have a strong, phenol-like odour. They are highly flammable and are
soluble in water, ethanol, ether, acetone and alkali hydroxides.
Cresols undergo electrophilic substitution reactions at the vacant
ortho or para position relative to the hydroxyl group. They also
undergo condensation reactions with aldehydes, ketones or dienes.
Several methods can be used for determining the presence of
cresols in both environmental and biological media. The most commonly
used methods are gas chromatography with flame ionization detection
(GC-FID), gas chromatography with mass spectrophotometry (GC-MS) and
high-performance liquid chromatography (HPLC). Sampling of cresols in
air can be done by passing air through absorption cells using sodium
hydroxide or solid adsorbents.
1.2 Uses, sources and levels of exposure
Cresols have a wide variety of uses as solvents or disinfectants
or as intermediates in the production of numerous other substances.
These compounds are most commonly used in the production of
fragrances, antioxidants, dyes, pesticides and resins. Ortho- and
para-cresols are used in the production of lubricating oils, motor
fuels and rubber polymers, while meta-cresol is used in the
manufacture of explosives.
Cresols and cresol derivatives occur naturally in oils of various
plants, including Yucca gloriosa flowers, jasmine, Easter lily,
conifers, oaks and sandalwood trees, and are also a product of
combustion from natural fires and volcanic activity. Para-cresol is
found in the urine of animals and humans. Commercially cresols are
produced as by-products in the fractional distillation of crude oil
and coal tars. Small amounts are produced in vehicle exhaust,
municipal waste incinerators and from coal and wood combustion.
Cigarette smoke also contains cresols. The worldwide production of
cresols is unknown; annual production in the USA in 1990 was reported
to be 38 300 tonnes.
Environmental transport of cresols occurs through the vapour
phase of the atmosphere and from the atmosphere to surface water and
soil by rain-scavenging. Due to their volatilization, binding to
sediment and biodegradation, only small amounts of cresols are found
in water. In soils, cresols are slightly to highly mobile depending on
the sorption coefficient (Koc) of the soil. Cresols have been
detected in ground water, and so leaching must occur in soil.
Exposure to cresols can occur through air, water or food. The
median air concentration of o-cresols was 1.59 µg/m3 (0.359 ppb)
for 32 source-dominated sites in the USA. Surface water
concentrations in the USA range from below the detection limit to
77 µg/litre (STORET, 1993). Levels of 204 µg/litre were reported in
Japan in a river polluted by industrial effluents. Concentrations as
high as 2100 µg/litre for o-cresol and 1200 µg/litre for mixed
m- and p-cresols have been detected in waste waters. Rainwater
concentrations range from 240 to 2800 ng/litre for o-cresol and 380
to 2000 ng/litre for p- and m-cresol combined. Cresols have been
detected in foods and beverages. Concentrations in spirit beverages
were found to be within the range of 0.01-0.2 mg/litre. The amount in
tobacco smoke is 75 µg in a nonfilter American cigarette (85 mm). The
general population can be exposed to cresols from air inhalation,
drinking-water, food and beverage ingestion and dermal contact. In
general, the lack of adequate monitoring data makes the quantitative
estimates of daily intakes of cresol from these sources impossible.
Occupational exposure levels as high as 5.0 mg/m3 have been
reported.
1.3 Kinetics and metabolism
Cresols are absorbed across the respiratory and gastrointestinal
tracts and through the skin. The rate and extent of absorption of
cresols has not been studied specifically. However, studies have
shown that gastrointestinal and dermal absorption are rapid and
extensive. Cresols are distributed to all the major organs. The
primary metabolic pathway for cresols is conjugation with glucuronic
acid and inorganic sulfate. Minor metabolic pathways for cresols
include hydroxylation of the benzene ring and side-chain oxidation.
The main route for elimination of cresols from the body is renal
excretion in the form of conjugates.
1.4 Effects on laboratory mammals; in vitro systems
Acute poisoning with cresol vapours is unlikely due to the low
vapour pressure of these compounds. Mean lethal concentrations of
cresols in rats have been reported to be 29 mg/m3 for o- and
p-cresols and 58 mg/m3 for m-cresol. Oral LD50 values in rats
have been reported to be 121, 207 and 242 mg/kg body weight for o-,
p- and m-cresols, respectively. Interspecies comparisons show
that all three isomers are more toxic to mice than to rats and that
toxicity increases with concentration. Systemic toxicity and death
can result from dermal exposure. Dermal LD50 values in rabbits were
890, 2830, 300 and 2000 mg/kg body weight for o-, m-, p-and
mixed cresols, respectively. In rats dermal LD50 values were 620,
1100, 750 and 825 mg/kg body weight for o-, m-, p- and dicresol,
respectively.
Cresols are highly irritating to the skin and eyes of rabbits,
rats and mice.
Short-term exposure to inhaled mixtures of o-cresol aerosol and
vapour resulted in irritation of the respiratory tract, small
haemorrhages in the lung, body weight reduction and degeneration of
heart muscle, liver, kidney and nerve cells. Short-term (28-days)
oral exposure to daily doses of approximately 800 mg/kg body weight or
more resulted in reduced body weights, organ weight changes and
histopathological changes in the respiratory and gastrointestinal
tracts of rats. In mice, similarly exposed at 1500 mg/kg body weight,
more severe effects were reported, and at the highest concentrations
death resulted from exposure to o-, m- and p-cresols but not
from exposures to mixtures of isomers.
Longer-term exposure of rats to vapours of o-, m- or
p-cresol for up to 4 months resulted in weight loss, reduced
locomotor activity, inflammation of nasal membranes and skin, and
changes in the liver. Oral exposures for up to 13 weeks of mice,
rats and hamsters resulted in mortality, tremor, reduced body weights,
haematological effects, increase in organ weight, and hyperplasia of
nasal and forestomach epithelium.
Oral and inhalation exposure to cresol isomers result in
lengthened estrus cycle and histopathological changes in the uterus
and ovaries of rats and mice. No adverse effects on spermatogenesis
were observed in rats or mice. Mild fetotoxic effects have been
reported in rats and rabbits exposed to o- and p-cresols, but only
minor treatment-related developmental effects have been reported.
Some evidence of genotoxicity has been reported to result in vitro
from treatment with o- and p- cresols but not m-cresol. No
positive results were obtained in in vivo studies. However, some
evidence of promotive activities in skin has been reported. No
studies of carcinogenicity have been reported for any cresol isomers.
1.5 Effects on humans
Ingestion of cresols results in burning of the mouth and throat,
abdominal pain and vomiting. The target tissues/organs of ingested
cresols in humans are the blood and kidneys, and effects on the lungs,
liver, heart and central nervous system have also been reported. In
severe cases, coma and death may result. Dermal exposure has been
reported to cause severe skin burns, scarring, systemic toxicity and
death.
Occupational exposure to cresols usually results from dermal
contact. Acute exposures can result in severe burns, anuria, coma and
death. Very few data are available regarding reproductive effects and
there are no data on carcinogenicity in humans.
1.6 Effects on other organisms
Observations on microorganisms, invertebrates and fish have shown
that cresols may represent a risk to non-mammalian organisms at point
sources with high cresol concentration but not in the general
environment.
1.7 Conclusion and recommendations
At concentrations normally found in the environment, cresols do
not pose any significant risk for the general population. However,
the potential for adverse health effects exists in the case of people
with renal insufficiency or specific enzymic deficiency and under
conditions of high exposure.
Cresols may represent a risk to microorganisms, invertebrates and
fish at point sources with high cresol concentrations but not in the
general environment.
No information is available regarding the effects of chronic
exposure to cresols. Therefore, there is inadequate information to
assess the carcinogenic hazard of cresols. Based on the results of
subchronic studies, an NOAEL of 50 mg/kg body weight per day can be
established for all three cresol isomers. An uncertainty factor of
300 was recommended, composed as follows: 10 to account for
interspecies variation; 10 to account for the lack of chronic toxicity
studies and possible genotoxic and promoting activity of cresols, and
3 to account for intraspecies variation based on the rapid and
complete metabolism. Therefore, an acceptable daily intake (ADI) of
0.17 mg/kg body weight per day can be established for cresols.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Cresols are isomeric substituted phenols with a methyl
substituent at either the ortho, meta or para positions relative to
the hydroxyl group. Commercial cresol, also known as cresylic acid,
contains all three isomers with small amounts of phenol and xylenols
(Deichmann & Keplinger, 1981). Mixtures of m- and p-cresol and of
o-, m- and p-cresol are occasionally called dicresol and
tricresol, respectively (Fiege & Bayer, 1987). Pure and commercial
cresol or cresylic acid is different from the commercial products
called "cresylic acids". The substance "cresylic acids" is a mixture
of phenolic compounds with a typical composition as follows: 0-1% m-
and p-cresol; 0-3% 2,4- and 2,6-xylenols; 10-20% 2,3- and
3,5-xylenols; 20-30% 3,4-xylenol; and 50-60% C9-phenols (Sax & Lewis,
1987). The chemical identity of cresols is shown in Table 1.
Commercial cresols are manufactured in a wide range of
grades and purities to suit the user's requirements. Typically,
technical grade cresol available in the USA contains about 20%
o-cresol, 40% m-cresol, 30% p-cresol, and 10% phenol and
xylenols (Deichmann & Keplinger, 1981). The individual isomers are
available at purity levels as low as 85% and as high as > 99% from
chemical suppliers in the USA.
2.2 Physical and chemical properties
The physical properties of the three individual isomers and the
mixture are given in Table 2.
Chemically, cresols behave similarly to phenol. These compounds
undergo electrophilic substitution reactions at the vacant ortho or
para position relative to the hydroxyl group. Chlorination,
bromination, sulfonation and nitration are examples of such
substitution reactions. Cresols can undergo condensation reactions
with aldehydes, ketones and dienes (Fiege & Bayer, 1987).
2.3 Conversion factors
Air at 25°C: 1 ppm = 4.42 mg/m3
1 mg/m3 = 0.23 ppm
Table 1. Chemical identity of cresols
o-Cresol p-Cresol m-Cresol Mixture
Chemical structure:
 Empirical formula: C7H8O C7H8O C7H8O C7H8O
Relative molecular mass: 108.14 108.14 108.14 108.14
Common synonyms: 2-methyl phenol 4-methyl phenol 3-methyl phenol methyl phenol
2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
o-cresylic acid p-cresylic acid m-cresylic acid cresylic acid
acide cresylique (French)
cresoli (Italian)
kresolen (Dutch)
krezol (Polish)
kresol (German)
IUPAC name: 2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
CAS registry number:
Empirical formula: C7H8O C7H8O C7H8O C7H8O
Relative molecular mass: 108.14 108.14 108.14 108.14
Common synonyms: 2-methyl phenol 4-methyl phenol 3-methyl phenol methyl phenol
2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
o-cresylic acid p-cresylic acid m-cresylic acid cresylic acid
acide cresylique (French)
cresoli (Italian)
kresolen (Dutch)
krezol (Polish)
kresol (German)
IUPAC name: 2-hydroxy toluene 4-hydroxy toluene 3-hydroxy toluene hydroxy toluene
CAS registry number: 